
Title: Scientific American Supplement, No. 711, August 17, 1889
Author: Various
Release date: October 31, 2005 [eBook #16972]
Most recently updated: December 12, 2020
Language: English
Credits: Produced by Juliet Sutherland and the Online Distributed
Proofreading Team at www.pgdp.net
A novel and interesting series of operations was carried out at Gibraltar a few weeks ago, with a view to test the promptitude with which the garrison of the famous Rock could turn out to resist a sudden attack by a powerful iron-clad fleet. The supposed enemy was represented by the Channel Squadron, under the command of Vice-Admiral Baird, and consisting of H.M.S. Northumberland (flag ship), the Agincourt, Monarch, Iron Duke, and Curlew. The "general idea" of the operations was that a hostile fleet was known to be cruising in the vicinity, and that an attack on the Rock might be made. The squadron left Gibraltar and proceeded to the westward, returning to the eastward through the Straits under cover of the night.
The Governor of Gibraltar, General the Hon. Sir Arthur Hardinge, issued orders for the whole garrison to stand to their arms at dawn, and subsequent days, until the attack should be made; but by his express command no batteries were to be manned, or any troops moved from their alarm posts, until the signal was given that an attack was imminent. The alarm signal ordered was that of three guns fired in rapid succession from the Upper Signal Station on the summit of the Rock, to be followed, after a short pause, by two more shots. It was a matter of complete uncertainty as to the direction from which the attack would be made.
Every detail was carefully carried out, as if the impending attack was a real affair. The telegraphic communication between the various parts of the Rock was supplemented by signalers; arrangements were made for the ready supply of reserve ammunition for all arms; and the medical authorities established dressing stations, at numerous points of the Rock, to render "first aid" to those who might chance to be numbered among the "wounded." Day broke with a "Levanter," and the heavy clouds hanging about rendered any distant view a matter of difficulty. However, before it had become actually daylight the alarm guns gave notice that the enemy had been sighted. The troops turned out with great promptitude, being all at their assigned stations in less than a quarter of an hour, and were shortly ordered to various points commanding the east side of the Rock. As day broke, the hostile ships were to be discerned steaming in single line ahead, from the northeast, along the back of the Rock, and about 5,000 yards from it. The flag ship, followed by the Monarch and the Agincourt, proceeded toward Europa Point, while the Iron Duke and the Curlew stood close in to the eastern beach, so as to engage the northern defenses of the fortress. The first shot was fired by the flag ship, shortly before six o'clock in the morning, at the southern defenses. It was replied to, in less than three minutes, by the Europa batteries, and very shortly the engagement became general. The plan of tactics employed by the squadron was that of steaming rapidly up and down, and concentrating their fire in turn on the various shore batteries. Later on, the whole squadron assembled off Europa Point, and fired broadsides by electricity as they steamed past at speed. The spectacle at this moment was a very fine one, the roar of the heavy guns of the ships being supplemented by the sharp, rapid report of the quick-firing guns, which were supposed to be sending a storm of small shell among the defenders of the Rock. The incessant rattle of the ships' machine guns was also heard in the intervals between the thundering broadsides of heavy ordnance. All the ships were, of course, cleared for action, with topmasts and yards sent down, and it is needless to say they looked exceedingly workmanlike and formidable.
The various batteries on the Rock replied with great vivacity, and the general effect produced as gun after gun was brought to bear on the ships, and the white smoke wreathed itself round the many crags and precipices of the grim old Rock, was a sight long to be remembered. The exercise afforded to both branches of the service was undoubtedly most instructive. Our illustration is a sketch by Captain Willoughby Verner from one of the batteries above the Europa Flats, at which point the governor took up his position to watch the operations.—Illustrated London News.
Notwithstanding that the political situation of Europe seems to be less threatening among its leading powers, still the uncertainty prevalent among those who are generally considered the arbiters of public affairs has had its influence in contracting the limits of speculative adventure, thereby circumscribing the general course of trade throughout the Mediterranean.
In renewing to the department my reports upon the navigation and general commerce of Gibraltar, I beg to state that there has been a tolerably fair current business prevailing in American produce during the past quarter, consisting chiefly in flour, tobacco, and refined petroleum in cases, imported direct from New York.
The steady demand for American petroleum confirms the fact that Russian petroleum so far receives but little attention in this market from the regular traders and consumers, so long as supplies from the United States can be regularly imported at reasonable prices. It, however, remains an open question, in the event of lower prices ruling in the Russian petroleum regions, whether American supplies may not later on experience some greater competitive foreign interference.
According to the statistical data, steam vessels of all nationalities have continued to make Gibraltar their port of call, not only for orders, but also for replenishing their stock of fuel and provisions, and in larger numbers than ever before, the number in 1888 having reached 5,712 steam vessels, measuring in all 5,969,563 tons, while in 1887 the number was only 5,187 steam vessels, with an aggregate tonnage of 5,372,962. This increase cannot but result in considerable benefit to the coal and maritime traffic, which now forms the most important portion of the general commerce of Gibraltar, in spite of the keen competition it experiences from other British and foreign coaling ports.
Freights have also advanced in favor of steamship interests, which, with higher prices in England for coal, have also caused an advance in the price of coal at this port, to the benefit of the coal merchants and others interested in this important trade. At present the ruling price for steam coal is 24s. per ton, deliverable from alongside of coal hulks moored in the bay. As near as I have been able to ascertain, the quantity of coal sold in this market during the past year for supplying merchant steam vessels has amounted to about 508,000 tons, which is an increase of about 20,000 tons over the year 1887.
Notwithstanding that plans have already been submitted to the British government for the construction of a dry dock in Gibraltar, the matter remains somewhat in suspense, since it meets with some opposition on the part of the British government, which, in face of the European fever for general arming, seems more inclined to utilize in another form the expense which such a work would entail upon the imperial government, by replacing the obsolete ordnance recently removed from this fortress and substituting new defenses and guns of the most approved patterns, a matter which has evidently been receiving, for some time past, the special attention of the British military authorities, not doubting that the recent visit to the fortress of the Duke of Cambridge has had some connection with it. In fact, it is reported that the duke has already expressed the opinion that this fortress requires a larger number of artillerymen than are quartered here at present to man its batteries, and it would seem that this recommendation is likely to be carried out.
It is yet somewhat too early to venture an opinion regarding the growing crops of cereals in this Spanish neighborhood, but the agricultural and manufacturing interests in Spain have suffered so much in the past years that the general feeling in Spain continues to tend toward establishing increased restrictions against foreign competition in her home markets. There is every probability that the provinces of Malaga and Granada may shortly be granted the privilege of cultivating the tobacco plant under government supervision, as an essay. If properly managed, it may form an important and lucrative business for those interested in land and agricultural pursuits.
After many consecutive years of heavy outlays, difficulties, and constant disappointments, a new English company has recently succeeded in commencing the construction of a railway from the neighboring Spanish town of Algeciras to join, via Ronda, the railway station of Bobadilla, on the railroad line toward Malaga. It is presumed that when this railroad will be in running order it will greatly benefit this community, especially if the Spanish government should decide to establish custom houses at Algeciras and the Spanish lines outside the gates of this fortress, similar to those existing on the frontiers of France and Portugal.
That some idea may be formed of the constant important daily intercourse which exists between this fortress and Spain, I may state that late police statistics show that 1,887,617 passes were issued to visitors entering this fortress on daily permits during the year 1888, 1,608,004 entering by the land route and 279,613 by sea. I must, however, observe that the larger portion of these visitors consists of laborers, coal heavers, market people, and others engaged in general traffic.
A new industry in cork has lately sprung up, in which leading Spanish and native commercial firms in Gibraltar are directly interested to a considerable extent. Extensive warehouses for the storing of cork wood and machinery for the manufacture of bottle corks have recently been established at the Spanish lines, about a mile distant from this fortress, in Spanish territory, where large quantities of cork have already been stored. The cork is obtained and collected from the valuable trees, which are owned by the representatives of some of the oldest nobility of Spain, who have sold the products of their extensive woods to private individuals for periods reaching as far on as ten years, for which concession large cash advances have already been made. The woods commence at a distance of about twelve miles from Gibraltar, and are of considerable extent.
The railway now in course of construction passes through these woods, which may ere long offer quite picturesque scenery for travelers, especially when the cork trees are bearing acorns, which form the principal food for the fattening of large herds of swine during certain seasons of the year, in this way, also, contributing to the value of this tree, which, like the other kinds of oak trees, is of long and tardy growth. The tree from which the cork is obtained is somewhat abundant in the mountainous districts of Andalusia. It grows to a height of about 30 feet, and resembles the Quercus ilex, or evergreen oak, and attains to a great age. After arriving at a certain state of maturity it periodically sheds its bark, but this bark is found to be of better quality when artificially removed from the tree, which may be effected without injury to the tree itself. After the tree has attained twenty-five years it may be barked, and the operation is afterward repeated once in every seven years. The quality of the cork seems to improve with the increasing age of the tree, which is said to live over one hundred and fifty years. The bark is taken off during July and August.
Cork dust is also obtained from this cork wood, and is much used in the packing of grapes, which fruit is largely shipped from the eastern coast of Spain, especially from Almeria, during the vintage seasons, for the American and British markets.—Reports of U.S. Consuls.
The point or rock known as Gibraltar is a promontory two and one-half miles long and from a quarter to three-quarters of a mile wide. It rises abruptly from the sandy shore to a height at its highest point of 1,408 ft. It is composed of gray limestone, honeycombed with caves and subterranean passages, some of which contain most beautiful stalactites in the form of massive pillars.
Gibraltar is emphatically a fortress, and in some respects its fortifications are unique. On the eastern side the rock needs no defense beyond its own precipitous cliffs, and in all other directions it has been rendered practically impregnable. Besides a sea wall extending at intervals round the western base of the rock, and strengthened by curtains and bastions and three formidable forts, there are batteries in all available positions from the sea wall up to the summit, 1,350 feet above the sea, and a remarkable series of galleries has been hewn out of the solid face of the rock toward the north and northwest. These galleries have an aggregate length of between two and three miles, and their breadth is sufficient to let a carriage pass. Portholes are cut at intervals of twelve yards, so contrived that the gunners are safe from the shot of any possible assailants. At the end of one of the galleries hollowed out in a prominent part of the cliff is St. George's Hall, 50 feet long by 85 feet wide, in which the governor was accustomed to give fetes. Alterations, extensions, and improvements are continually taking place in the defensive system, and new guns of the most formidable sort are gradually displacing or supplementing the old fashioned ordnance.
The whole population of Gibraltar, whether civil or military, is subjected to certain stringent rules. For even a day's sojourn the alien must obtain a pass from the town major, and if he wish to remain longer, a consul or householder must become security for his good behavior. Licenses of residence are granted only for short periods—ten, fifteen, or twenty days—but they can be renewed if occasion require. Military officers may introduce a stranger for thirty days. A special permit is necessary if the visitor wishes to sketch.
Though the town of Gibraltar may be said to date from the fourteenth century, it has preserved very little architectural evidence of its antiquity. Rebuilt on an enlarged and improved plan after its almost complete destruction during the great siege, it is still, on the whole, a mean-looking town, with narrow streets and lanes and an incongruous mixture of houses after the English and the Spanish types. As a proprietor may at any moment be called upon to give up his house and ground at the demand of the military authorities, he is naturally deterred from spending his money on substantial or sumptuous erections. The area of the town is about one hundred acres.
Gibraltar was known to the Greek and Roman geographers as Calpe or Alybe, the two names being probably corruptions of the same local (perhaps Phenician) word. The eminence on the African coast near Ceuta, which bears the modern English name of Apes' Hill, was then designated Abyla; and Calpe and Abyla, at least according to an ancient and widely current interpretation, formed the renowned pillars of Hercules (Herculis columnæ), which for centuries were the limits of enterprise to the seafaring peoples of the Mediterranean world.
The strategic importance of the rock appears to have been first discovered by the Moors, who, when they crossed over from Africa in the eighth century, selected it as the site of a fortress. From their leader, Tarik Ibn Zeyad, it was called Gebel Tarik or Tarik's Hill; and, though the name had a competitor in Gebel af Futah, or Hill of the Entrance, it gradually gained acceptance, and still remains sufficiently recognizable in the corrupted form of the present day. The first siege of the rock was in 1309, when it was taken by Alonzo Perez de Guzman for Ferdinand IV. of Spain, who, in order to attract inhabitants to the spot, offered an asylum to swindlers, thieves, and murderers, and promised to levy no taxes on the import or export of goods. The attack of Ismail Ben Ferez, in 1315 (second siege), was frustrated; but in 1333 Vasco Paez de Meira, having allowed the fortifications and garrison to decay, was obliged to capitulate to Mahomet IV. (third siege). Alphonso's attempts to recover possession (fourth siege) were futile, though pertinacious and heroic, and he was obliged to content himself with a tribute for the rock from Abdul Melek of Granada; but after his successful attack on Algeciras in 1344 he was encouraged to try his fortune again at Gibraltar. In 1349 he invested the rock, but the siege (fifth siege) was brought to an untimely close by his death from the plague in February, 1350. The next or sixth siege resulted simply in the transference of the coveted position from the hands of the King of Morocco to those of Yussef III. of Granada; and the seventh, undertaken by the Spanish Count of Niebla, Enrico de Guzman, proved fatal to the besieger and his forces. In 1462, however, success attended the efforts of Alphonso de Arcos (eighth siege), and in August the rock passed once more under Christian sway. The Duke of Medina Sidonia, a powerful grandee who had assisted in its capture, was anxious to get possession of the fortress, and though Henry IV. at first managed to maintain the claims of the crown, the duke ultimately made good his ambition by force of arms (ninth siege), and in 1469 the king was constrained to declare his son and his heirs perpetual governors of Gibraltar. In 1479 Ferdinand and Isabella made the second duke Marquis of Gibraltar, and in 1492 the third duke, Don Juan, was reluctantly allowed to retain the fortress. At length, in 1501, Garcilaso de la Vega was ordered to take possession of the place in the king's name, and it was formally incorporated with the domains of the crown. After Ferdinand and Isabella were both dead the duke, Don Juan, tried in 1506 to recover possession, and added a tenth to the list of sieges. Thirty-four years afterward the garrison had to defend itself against a much more formidable attack (eleventh siege)—the pirates of Algiers having determined to recover the rock for Mahomet and themselves. The conflict was severe, but resulted in the repulse of the besiegers. After this the Spaniards made great efforts to strengthen the place, and they succeeded so well that throughout Europe Gibraltar was regarded as impregnable.
In the course of the war of the Spanish succession, however, it was taken by a combined English and Dutch fleet under Sir George Rooke, assisted by a body of troops under Prince George of Hesse-Darmstadt. The captors had ostensibly fought in the interests of Charles Archduke of Austria (afterward Charles III.), but, though his sovereignty over the rock was proclaimed on July 24, 1704, Sir George Rooke on his own responsibility caused the English flag to be hoisted, and took possession in name of Queen Anne. It is hardly to the honor of England that it was both unprincipled enough to sanction and ratify the occupation and ungrateful enough to leave unrewarded the general to whose unscrupulous patriotism the acquisition was due. The Spaniards keenly felt the injustice done to them, and the inhabitants of the town of Gibraltar in great numbers abandoned their homes rather than recognize the authority of the invaders. In October, 1704, the rock was invested by sea and land; but the Spanish ships were dispersed by Sir John Leake, and the Marquis of Villadarias fared so ill with his forces that he was replaced by Marshal Tesse, who was at length compelled to raise the siege in April, 1705. During the next twenty years there were endless negotiations for the peaceful surrender of the fortress, and in 1726 the Spaniards again appealed to arms. But the Conde de la Torres, who had the chief command, succeeded no better than his predecessors, and the defense of the garrison under General Clayton and the Earl of Portmore was so effectual that the armistice of June 23 practically put a close to the siege, though two years elapsed before the general pacification ensued. The most memorable siege of Gibraltar, indeed one of the most memorable of all sieges, was that which it sustained from the combined land and sea forces of France and Spain during the years 1779-1783. The grand attack on the place was made on the 13th September, 1782, and all the resources of power and science were exhausted by the assailants in the fruitless attempt. On the side of the sea they brought to bear against the fortress forty-six sail of the line and a countless fleet of gun and mortar boats. But their chief hope lay in the floating batteries planned by D'Arcon, an eminent French engineer, and built at the cost of half a million sterling. They were so constructed as to be impenetrable by the red hot shot which it was foreseen the garrison would employ; and such hopes were entertained of their efficiency that they were styled invincible. The Count D'Artois (afterward Charles X.) hastened from Paris to witness the capture of the place. He arrived in time to see the total destruction of the floating batteries and a considerable portion of the combined fleet by the English fire. Despite this disaster, however, the siege continued till brought to a close by the general pacification, February 2, 1783. The history of the four eventful years' siege is fully detailed in the work of Drinkwater, who himself took part in the defense, and in the life of its gallant defender Sir George Augustus Eliott, afterward Lord Heathfield, whose military skill and moral courage place him among the best soldiers and noblest men whom Europe produced during the 18th century.
Since 1783 the history of Gibraltar has been comparatively uneventful. In the beginning of 1801 there were rumors of a Spanish and French attack, but the Spanish ships were defeated off Algeciras in June by Admiral Saumarez. Improvements in the fortifications, maintenance of military discipline, and legislation in regard to trade and smuggling are the principal matters of recent interest.
Another addition was made to the Austrian navy by the launching on May 18 of the ram cruiser Franz Josef I. from the yards of S. Rocco in the Stabilimento Tecnico Triestino. Her dimensions are: Length (over all), 103.7 meters; length (between perpendiculars), 97.9 meters; greatest breadth (outside), 14.8 meters; draught (bow), 5.28 meters; draught (stern), 6.05 meters; displacement on the construction water line, 4,000 tons. The armament consists of two 24-centimeter and six 15-centimeter Krupp breech loaders of 35 caliber length, two 7-centimeter Uchatius guns as an armament for the boats and for landing purposes, eleven Hotchkiss quick-firing guns, and several torpedo-launching ports; indicated horse power with natural draught 6,400, speed 17.5 knots; with forced draught 9,800, speed 19 knots.
The ship is built of steel, and constructed according to the "double bottom" system along the engine, boiler, and ammunition rooms. The vaulted armor deck, extending 1.25 meters below the water line and protecting the most vital parts of the ship, is 0.057 meter thick. There are more than 100 water tight compartments below and above the deck. A protecting belt of "cellulose" is provided for the engines and boilers, extending from the armor deck downward.
The two main guns, placed on Krupp's hydraulic carriages, occupy positions in front and rear, and are protected by stands 0.09 meter thick and 1.60 meters high. They fire en barbette with a lateral range each of 260 degrees at bow and stern—i.e., 130 degrees on either of the broadsides. The weight of the barrel of the gun is 25 tons, that of the steel shell 215 kilogrammes (about 430 lb.), that of the brown powder charge 100 kilogrammes; initial velocity of projectile, 610 meters; penetration, 0.524 meter iron; longest range, 17 kilometers (about 10½ English miles); range at 15 deg. elevation, 10 kilometers. The six 15-centimeter guns are placed in a kind of machicouli arrangement in two tiers on each of the broadsides, so that always four guns can fire in the direction of the keel to the front and rear. The weight of the barrel of the gun is each six tons, that of the steel shells 51 kilogrammes, that of the charge 22 kilogrammes; initial velocity, 610 meters.
The 11 quick-firing guns are partly placed along the broadsides, partly in the masts, of which there are two. The triple expansion engines, having each a bronze screw of 4.42 meters diameter, with three blades and a rise of 6.3 meters, make with natural draught 105 revolutions, and with forced draught 120. The pumping apparatus are able to lift in one hour 400 tons of water. The front boiler room contains a special cylindrical boiler for the working of the electrical apparatus, for hydraulic pumps of the artillery service, for anchor windlasses, ventilators, fire engines, etc. The whole engines weigh 890 tons. The bunkers have a capacity for 660 tons of coal, which allows for a run of 4,500 sea miles.
Figs. 1 and 2 represent, upon a scale of about 1/10, two types of torpedoes, the greatest number possible of the parts of which are made revolvable, so as to render the torpedoes as dirigible as the gyrating motion permits of.
Fig. 1 represents an electric torpedo actuated by accumulators, A A, keyed upon the shaft, and revolving along with the gearings. At the beginning of the running, the accumulators are not all coupled, but under the action of a clockwork movement which is set in motion at the moment of starting, metallic brushes descend one after another upon the collectors, B, and set in action new batteries for keeping constant or, if need be, accelerating the speed at the end of the travel.

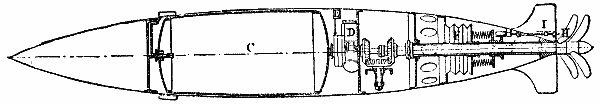
Fig. 2 represents an air torpedo proposed by the same inventor. The air reservoir, C, revolves along with the gearings under the action of the pneumatic machine, D. The central shaft is hollow, so as to serve as a conduit. The admission of air into the slide valve of the machine is regulated by a clockwork which actuates a slide in an aperture whose form and dimensions are so calculated that the speed remains as constant as possible toward the end of the travel.
The trajectory of the two torpedoes is regulated by a cylindrical bellows, F, which gives entrance to the sea water. The springs shown in the figure balance the hydraulic pressure. The tension of these springs is regulated by the rod, H, according to the indications of the scale of depths, I.
When the torpedo reaches too great a depth, the action of the springs can no longer balance the increase of the hydraulic pressure, and the accumulation of the charge in the rear causes the front to rise toward the surface. When the torpedo reaches the surface, a contrary action is produced.—Revue Industrielle.
The accompanying engraving represents the remarkable steamboat that the unfortunate Marquis de Jouffroy constructed at Paris in 1816, after organizing a company for the carriage of passengers on the Seine. De Jouffroy, as well known, made the first experiment in steam navigation at Lyons in 1783, but the inventor's genius was not recognized, and he met with nothing but deception and hostility. With the obstinacy of men of conviction, he did not cease to prosecute his task. He assuredly had an inkling of the future in store for the invention that he was offering to humanity.
The paddle wheel boat that he constructed at Paris in 1816 did not succeed any better than its predecessors; it was remarkable nevertheless in appearance and structure.
The engine was forward, as shown in the engraving, which is copied from a composition of Dubucourt's.
The company organized by the marquis was ruined, and, as well known, the unfortunate inventor himself died in poverty in 1832, at the age of eighty-one years.—La Nature.
The American Institute of Electrical Engineers at its last meeting of the season, held June 25, again considered the subject of electrical traction, the paper presented by Mr. Leo Daft being based upon some recent electrical work on the elevated railroads and its bearing on the rapid transit problem. The Railroad Gazette gives the following abstract:
He introduced the subject with a tribute to the efficiency of the elevated railroad system as it is now operated by steam, with special reference to that section of it known as the Ninth Avenue line, upon which his experiments with the electric motor have been conducted, over which passengers are now conveyed a distance of five miles in 26 minutes for five cents, which he considered the best and cheapest municipal rapid transit in the world, and which is operated with a higher degree of safety than any other railroad in the world making an equal number of stops per 100 miles. On a recent holiday, April 30 last, 835,720 passengers were carried upon the entire system without noticeable detention or accident. The rapidly increasing traffic makes the demand for better facilities a pressing one, and as the average half million now carried daily will soon become a million, it appears doubtful if any method can be devised of providing for the growth by the use of steam motors on the present structures, which are now taxed to their utmost. To the mind of the mechanical engineer, having in view the ordinary coefficients of tractive ability, there is no remedy for this. The speaker stated that these coefficients were not entirely trustworthy. He reiterated his previously expressed opinion, based on frequent experiments, that there is a decided increase in traction gained by the passage of the electric current from the wheels to the rails, giving the details of one test where a motor with a load making a total of 600 lb. climbed a gradient of 2,900 ft. per mile, starting from a state of rest. He stated that some of those people who had ridiculed his statements had finally admitted that they were true.
The motor Ben Franklin, which had been used in making these tests on the elevated roads, weighed 10 tons, and performed service nearly equal to the steam motors weighing 18 tons. The object of these tests was the determination of coal economy. Tests with a Prony brake showed that the motor developed 128 H.P. The piece of track on which the experiments were conducted embraced 2,200 ft. of level track and 1-8/10 miles of gradients, varying from 11-3/10 to 98-7/10 ft. per mile, while at Thirtieth street the station is at the foot of the steepest grade, thus testing to the utmost the tractive capacity of the motor. The experiments were begun in October, 1888, and carried on between the hours of 9 P.M. and 4 A.M., beginning with one or two cars, the load being increased nightly until it was finally made up of eight coaches of 12 tons each, which were hauled up the 98 ft. grade at a speed of 7½ miles per hour, the entire distance being covered at the rate of 14-6/10 miles per hour. The maximum speed obtained on level with that train was 16.36 miles per hour. Seventy trips were subsequently made with a 70 ton train operated between the steam trains under 3 minutes headway, but the work was considered too critical on account of the absence of suitable brakes. A number of experiments made about this time showed that the mean speed with a three-car train running express on the up-town track was about 24 miles per hour, although the ability of the motor on a level with a similar train was nearly 28 miles per hour. This, however, was not the maximum speed, as the level track was not long enough to permit of its attaining the highest rate. It was the opinion of the speaker, however, that the speed attained could not be exceeded with prudence on the elevated structure.
The measurements of speed were made by dividing the track into 19 sections of 500 ft., each section being provided with a circuit-closing plate connected with a chronograph which was carefully tested. The indicator cards were taken at the central station by Mr. Idell and his assistants, and the dynamometer used was of the liquid type made by Mr. Shaw, of Philadelphia. The diagrams prepared from the data obtained were then explained by the speaker, who stated that there was not a marked difference between the 10 ton motor and the 18 ton locomotive in the initial effort on the level, as will be seen by comparing a run observed by a railroad officer on March 9 with a steam motor and a load of about 57½ tons. The steam motor required 1 min. and 29 sec. to make the distance from 14th to 23d streets, while the electric motor with a train of 70 tons made the same trip in 1 min. and 50 sec.; the absence of power brakes compelled the current to be taken off at 19th street, while it was probable that the throttle of the steam locomotive was not closed until it reached 23d street, this being the usual practice. The data obtained in these experiments shows that 29,940 h.p. is required to operate the Ninth avenue railroad for the 16 hours' service, or an average of 1,871 h.p. per hour, or 2,181 h.p., adding station friction. The varying requirements of the traffic during the day shows that the service could be advantageously divided up between four stationary engines of 800 h.p. each, there being but five hours of the day when all of them would be required. The fuel consumption per day, allowing 22 lb. of coal per h.p. per hour at $2.25 per ton, would make a total of $92.25 per diem for fuel, the coal being a mixture deliverable at the dock for about $1.80 per ton. The weight of coal used for the present locomotives is about the same, viz., 40 tons per day, but practice has shown it to be most economical to use coal of the best quality, costing $5 per ton, making the cost of fuel about double that required for the electric system. Without entering into other economies which the speaker claimed were in favor of electricity, and ignoring the plan suggested by Sir William Siemens of braking the train by converting the motor into a dynamo and thus utilizing the energy of momentum, he believed that the economy in fuel alone was sufficient to prove that the application of power by electricity was preferable to direct steam propulsion for the elevated railroad service.
There is perhaps no subject which at the present time can have a greater interest to the physicist, the electrician, and the electrical engineer than the one which heads this paper. The advances which have been made in the study from its purely theoretical or scientific side, and the great technical progress in the utilization of the known facts and principles concerning magnetic inductions, can but deepen and strengthen that interest.
On the side of pure theory we find the eager collection of experimental data to be submitted to the scrutiny of the ablest and brightest minds, to be examined and reasoned upon with the hope of finding some clew to satisfying explanations, and on the side of practice we find the search for new facts and relations no less diligent, though often stimulated by practical problems presented for solution. Indeed, the urgency for results is often the greater on the practical side, for theory can wait, practice cannot, at least in the United States.
We must look for continued triumphs in both directions, and the most welcome of all will be the framing of a theory or explanation which will enable us to interpret magnetic and electric phenomena. The recent beautiful experiments of Hertz on magnetic waves have opened a fertile region for investigation.
It would seem that the study of magnetism and electricity will give us the ability to investigate the ether of space, which medium has been theorized upon at great length, with the result of leaving it very much where it was before, a mysterious necessity.
Faraday says, speaking of magnetism:
"Such an action may be a function of the ether, for it is not at all unlikely that if there be an ether it should have other uses than simply the conveyance of radiations." 3,075. Vol. III., Exp. Res.
"It may be a vibration of the hypothetical ether, or a state of tension of that ether equivalent to either a dynamic or a static condition," etc. 3,263. Vol. III., Exp. Res.
Faraday again says, speaking of the magnetic power of a vacuum:
Modern views would seem to point that through a study of magnetic phenomena we may take a feeble hold upon the universal ether. Magnetism is an action or condition of that medium, and it may be that electrical actions are the expression of molecular disturbances brought about by ether strains or interferences. The close relations which are shown to exist between magnetism and light tend to strengthen such views. Indeed, it would not be too much to expect that if the mechanics of the ether are ever worked out, we should find the relation between sensible heat and electric currents to be as close as that of light to magnetism, perhaps find ultimately the forms of matter, the elements and compounds to be the more complex manifestations of the universal medium—aggregations in stable equilibrium. It is a difficult conception, I confess, and a most shadowy and imperfect one, yet facts and inferences which favor such views are not wanting.
Our science of electricity seems almost to be in the same condition that chemistry was before the work of Lavoisier had shed its light on chemical theory. Our store of facts is daily increasing, and apparently disconnected phenomena are being brought into harmonious relation. Perhaps the edifice of complete theory will not be more than begun in our time, perhaps the building process will be a very gradual one, but I cannot refrain from the conviction that the intelligence of man will, if it has time, continue its advance until such a structure exists.
I have been led to make these general allusions to electrical theory in order to emphasize the fact that in the present paper no unraveling of the mystery is to be attempted, but rather the presentation of some few considerations upon a subject of absorbing interest.
The conception of Faraday in regard to the existence of lines of magnetic force representing directions of magnetic strain or tension in a medium has not only lost nothing of its usefulness up to the present time, but has continually been of great service in the understanding of magnetic phenomena. We need spend no time in showing, as Faraday and others have done, that these lines are always closed circuits, polarized so that the direction of the lines cannot be reversed without reversal of the actions. Nor need we take time to show that in any medium the lines are mutually repellent laterally if of the same direction of polarization. Opposing this tendency to separation or lateral diffusion of magnetic force is the strong apparent tendency of the lines to shorten themselves in any medium. These actions are distributed by the presentation of a better medium, as iron instead of space or air. Lines of force will move into the better medium, having apparently the constant tendency to diminish the resistance in their paths.
The peculiar and mysterious nature of media, such as iron, is to permit an extraordinary crowding of lines on account of slight resistance to their passage through it. We need not, in addition, do more than refer to the other well-known facts of an electric current developing magnetic lines encircling the conductor, as being the general type, which includes all forms of magnetic field or electro-magnets, sustained by currents, and the fact of a development when magnetic lines or circuits and material masses are in relative movement of electromotive forces transversely to the direction of the lines of magnetism, and also transversely to the direction of relative movement, as in the case of electric conductors traversing or cutting through a field, or of a field traversing or being moved across a conductor. We must not forget that even insulators, as well as conductors, cutting lines of force, have the electromotive force developed in them. The action simply develops potential difference, and this generates the current where a circuit exists. While we are in the habit of saying that a conductor moved across a field of lines, or vice versa, generates electric current, I think the statement incomplete. The movement only sets up a potential difference, and the power expended in effecting the movement generates C × E. The current is energy less the potential, or the energy expended gives the two effects of potential or pressure and current or rate of movement. Consequently an insulator, or an open-circuited conductor, traversing a field, consumes no energy, potential difference only being produced. Nevertheless, as will be shown, the magnetic circuits or lines themselves may furnish the energy for their own movement across a conductor, and so develop current as well as potential.
This occurs in the effort of lines to shorten their paths, to lessen their density, to pass to better media. Indeed, a close examination will show that wherever power is expended in developing current in a circuit, cutting lines of force, the energy expended is first employed in stretching the lines, which thus receive the energy required to permit them, in shortening, to cut the conductor and set up currents in the electric circuit in accordance with the potential difference developed in that circuit and its resistance.
I think we may also say, though I do not remember to have seen the statement so put, that whenever electric potential is set up inductively, as in self-induction, mutual induction, induction from one circuit to another, and induction from magnets or magnetic field, it is set up by the movement of lines of force laterally across the body, mass or conductor in which the potential is developed, and that whenever current is set up in a wire or an existing current prolonged, or an existing current checked by induction, self-induction, or induction from magnets, the action is a transfer of energy, represented by strained lines of force shortening or lessening their resistance, or lengthening and increasing the resistance in their paths. The magnetic field is like an elastic spring—it can in one condition represent stored energy—it can be strained and will store energy—it can be made to relieve its strain and impart energy.
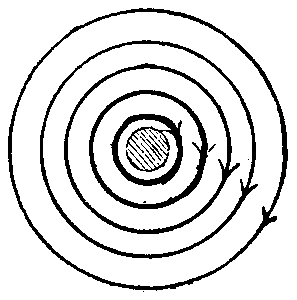 Fig. 1.
Fig. 1.
Let us examine some known phenomena in this light. Take the case of a simple wire, conveying current, say, in a line away from observer, Fig. 1. There exists a free field of circular magnetism (so called), shading off away from the wire, and which is represented by concentric circles of increased diameter. The superior intensity or strength of the lines near the wire may also be represented by their thickness. This is often shown also by crowding the lines near the wire, though I am disposed to regard Fig. 1 as more nearly expressing the condition, unless we are to regard the lines as simply indicating a sort of atmosphere of magnetic effect whose density becomes less as we proceed outward from the wire, in which case either form of symbol suffices. The direction of polarization of the lines may be indicated by an arrow head pointing in a direction of right-handed rotation in the path of the lines. This is the typical figure or expression for all forms of simple magnetic circuit—the form of the lines, their length, position, density, will depend on the shape of the conductor or conductors (when more than one) and the materials surrounding or in proximity to the wire or wires.
If the current traversing the conductor is constant, the magnetic field around it is stable and static, unless other influences come in to modify it. The cutting off of the current is followed by instability of the field whereby it can and must produce dynamic effects. I say must because the field represents stored energy, and in disappearing must give out that energy. To throw light on this part of the subject is one of the objects of the present paper. Cutting off the current supply in the case assumed leaves the developed magnetic lines or strains unsupported. They at once shorten their paths or circuits, collapsing upon the conductor as it were, and continuing this action, cut the section of the conductor, and apparently disappear in magnetic closed circuits of infinitesimal diameter but of great strength of polarization. It appears to me that we must either be prepared to give up the idea of lines of force or take the position that the magnetic circuits precipitate themselves in shortening their circuits and disappearing upon and cut the conductor. It was Hughes who put forward the idea that an iron bar in losing its apparent magnetism really short-circuits the lines in itself as innumerable strongly magnetized closed circuits among the molecules. In becoming magnetic once more these short circuits are opened or extended into the air by some source of energy applied to strain the lines, such as a current in a conductor around the bar.
May not this idea be extended, then, to include the magnetic medium, the ether itself? Does it contain intensely polarized closed circuits of magnetism which are ready to be stretched or extended under certain conditions by the application of energy, which energy is returned by the collapse of the extended circuits? This is doubtless but a crude expression of the real condition of things, for the lines are only symbols for a condition of strain in a medium which cannot be represented in thought, as we know nothing of its real nature. There is one point in this connection which I must emphasize. The strained lines, Fig. 1, are indications of stored energy in the ether, and the lines cannot disappear without giving out that energy. Ordinarily, it makes its appearance as the extra current, and adds itself so as to prolong the current which extended the lines when an attempt is made to cut off such current. Were it conceivable that the current could be cut off and the wire put on open circuit while the lines still remained open or strained, the energy must still escape when the field disappears. It would then produce such a high potential as to be able to discharge from the ends of the conductor, and if the conductor were of some section, part of the energy would be expended in setting up local currents in it. The field could not disappear without an outlet for the energy it represents. But we cannot cut off a current in a wire so as to leave the wire on open circuit with the lines of the magnetic circuit remaining around it without iron or steel or the like in the magnetic circuit. We can approach that condition, however, by breaking the circuit very quickly with a condenser of limited capacity around the break. This is done in the Ruhmkorff coil primary; the condenser forms a sort of blind alley for the extra current on its beginning to flow out of the primary coil. But the condenser charges and backs up and stops the discharge from the primary, even giving a reverse current. The lines of magnetic force collapse, however, and have their effect in the enormous potential set up in the secondary coil.
Take away the secondary coil so as to stop that outlet, the energy expends itself on the iron core and the primary coil. Take away the iron core, and the energy of magnetization of the air or ether core expends itself on the wire of the primary and, possibly, also on the dielectric of the condenser to some extent. The extra current becomes in this instance an oscillatory discharge of very high period back and forth through the primary coil from the condenser, until the energy is lost in the heat of C2 × R. This conversion is doubtless rendered all the more rapid by uneven distribution of current and eddy current set up in the wire of the coil.
The considerations just given concern the loss of field or the shortening and apparent disappearance of the magnetic lines or circuits, as giving rise to the self-induction or increased potential on breaking. Where the energizing current is slowly cut off or diminished the energy is gradually transferred to the wire in producing elevation of potential during the decrease; and the collapse and cutting of the wire by the collapsing circuits or lines is then only more gradual.
Let the current be returned to the wire after disappearance of magnetism, and the lines again seem to emanate from the wire and at the same time cut it and produce a counter potential in it, which is the index of the abstraction of energy from the circuit, and its storing up in the form of elastically strained lines of magnetism around the conductor. The effect is that of self-induction on making or upon increase of current, the measure of the amount being the energy stored in the magnetic circuits which have been extended or opened up by the current. The greater the current and the shorter the path for the lines developed around the axis of the conductor, the greater the energy stored up. Hence, a circular section conductor has the highest self-induction, a tube of same section less as its diameter increases, a flat strip has less as its width increases and thickness diminishes, a divided conductor much less than a single conductor of same shape and section. Separating the strands of a divided conductor increases the length of magnetic paths around it, and so diminishes the self-induction. A striking instance of this latter fact was developed in conveying very heavy alternating currents of a very low potential a distance of about three feet by copper conductors, the current being used in electric welding operations.
The conductors were built up of flat thin strips of copper for flexibility. When the strips were allowed to lie closely together, the short conductor showed an enormous self-induction, which cut down the effective potential at its ends near the work. By spreading apart the strips so as to lengthen a line around the conductor, the self-induction could be easily made less than 35 per cent. of what it had been before. The interweaving of the outgoing and return conductor strands as one compound conductor gets rid almost entirely of the self-inductive effects, because neither conductor has any free space in which to develop strong magnetic forces, but is opposed in effect everywhere by the opposite current in its neighbor.
Where a number of conductors are parallel, and have the same direction of current, as in a coil or in a strand, it is evident that statically the conductor may be considered as replaceable by a single conductor with the same external dimensions and same total current in the area occupied, the magnetic forces or lines surrounding them being of same intensity. But with changing current strength the distribution of current in the conductor has also a powerful effect on the energy absorbed or given out in accordance with the magnetism produced. Hence the self-induction of a strand, coil or conductor of the same section varies with the rapidity of current changes, owing to the conduction being uneven.
The uneven distribution of current, or its tendency to flow on the outer parts of a conductor when the rate of variation or alternation is made great, is in itself a consequence of the fact that less energy is transferred into magnetism in this case than when the current flows uniformly over the section, or is concentrated at the center. In other words, when a uniform current traverses a conductor of the same section, the circular magnetism, or surrounding magnetic lines, are to be found not only outside the conductor, but also beneath its exterior. Since in forming these lines on passage of current the middle of section would be surrounded by more lines than any other part of the conductor, the current tends to keep out of that part and move nearer the exterior in greater amount. Hence, in rapidly alternating currents the conductor section is practically lessened, being restricted largely to the outer metal of the conductor. If the round conductor, Fig. 2, were made of iron, the magnetism interior to it and set up by a current in it would be very much greater, the section of the conductor being filled with magnetic circuits or lines around the center. The total magnetism, external and internal, would be much greater in this case for a given current flow, and the energy absorbed and given out in formation and loss of field or the self-induction would be much increased. This could, however, be greatly diminished by slitting the conductor radially or making it of a number of separate wires out of lateral magnetic contact one with the other, Fig. 3. In these cases the resistance of the interior magnetic circuits would be increased, as there would be several breaks in the continuity around the center of the conductor. The total magnetism which could be set up by a current would be lessened, and the self-induction, therefore, lessened.
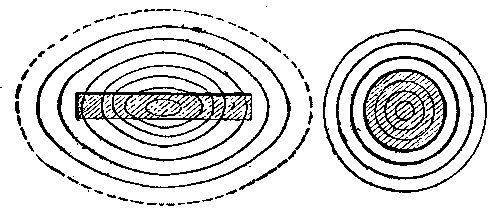
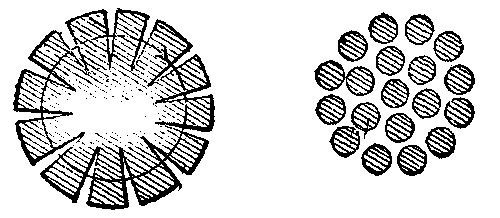
The moment we begin the bringing of iron into proximity with an electric conductor conveying current, we provide a better medium for the flow or development of magnetic lines or circuits. In other words, the lines may then be longer, yet equally intense, or more lines may be crowded into a section of this metal than in air or space. Figs. 4a, 4b, 4c show the effect brought about by bringing iron of different forms near to the conductor.
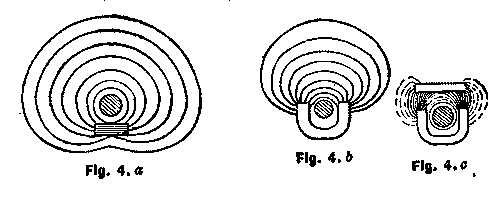
It shows, in other words, the development of the ordinary electro-magnet of the horseshoe form, and the concentration of the lines in the better medium. The lines also tend to shorten and diminish the resistance to their passage, so that attraction of the iron to the conductor takes place, and if there is more than one piece of iron, they tend to string themselves around the conductor in magnetic contact with one another.
When copper bars of 1 inch diameter are traversed by currents of 40,000 to 60,000 amperes, as in welding them, the magnetic forces just referred to become so enormous that very heavy masses of iron brought up to the bar are firmly held, even though the current be of an alternating character, changing direction many times a second.
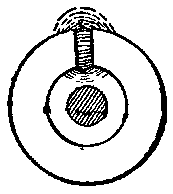 Fig. 5.
Fig. 5.
When a conductor is surrounded by a cast iron ring, as in Fig. 5, the current in such conductor has an excellent magnetic medium surrounding it. A large amount of energy is then abstracted on the first impulse of current, which goes to develop strong and dense magnetic lines through the iron ring and across the gap in it. On taking off the current the energy is returned as extra current, and its force is many times what would be found with air alone surrounding the conductor. We have then greatly increased the self-induction, the storing of energy and opposition to current flow at the beginning, the giving back of energy and assistance to the current flow on attempting to remove or stop the current. Let us now complete the ring, by making it of iron, endless, Fig. 6, with the conductor in the middle.
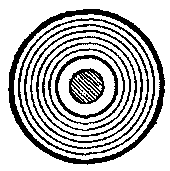 Fig. 6.
Fig. 6.
We now find that on passing current through the conductor it meets with a very strong opposing effect or counter potential. The evolution of magnetic lines, or the opening out of magnetic circuits, goes on at a very rapid rate. Each line or magnetic circuit evolved, and cutting the conductor, flies at once outward, and locates itself in the iron ring. This ring can carry innumerable lines, and they do not crowd one another. It permits the lines even to lengthen in reaching it, and yet, on account of its low resistance to their passage, the lengthening is equivalent to their having shortened in other media. We will suppose the current not sufficient to exhaust this peculiar capacity for lines which the iron has. Equilibrium is reached, the conductor has opened up innumerable closed circuits, and caused them to exist in the ring still closed; but in iron, not space or ether merely. The current passing has continued its action and storage of energy until to emit another line in view of the resistance now found in the crowded iron ring is impossible.
Now let us cut off the current. We are surprised to find a very weak extra current, a practical absence of self-induction on breaking, or, at least, a giving out of energy in nowise comparable to that on making. Let us put on the current as it was before. Another curious result. But little self-induction now on making energy not absorbed.
Now cut off the current again. Same effect as before. Now let us put on the current reversed in direction. At once we find a very strong counter potential or opposing self-induction developed.
The ring had been polarized, or retained its magnetic energy, and we are now taking out one set of lines and putting in reversely polarized lines of force. This done, we break the reversed current without much effect of self-induction. The ring remains polarized and inert until an opposite flow of current be sent through. Iron is then a different medium from the ether.
The ring once magnetized must, in losing its magnetism, permit a closure of the lines by shortening. This involves their passage from the iron across the space in the center of the ring, notwithstanding its great resistance to the lines of force. As passage from iron to air is equivalent to lengthening of the lines, it is readily seen that such lengthening may oppose more effect than a slight shortening due to leaving iron, for air or space may give in provoking a closure and disappearance of the lines. Looked at from another standpoint, the lines on the iron may actually require a small amount of initial energy to dislodge them therefrom, so that after being dislodged they may collapse and yield whatever energy they represent.
I must reserve for the future further consideration of the iron ring, but in thinking upon this matter I am led to think that the production of a magnetic line in an iron ring around a conductor may represent a sort of wave of energy, an absorption of energy on the evolution of the line from the conductor, and a slight giving out of energy on the line reaching that position of proximity to the iron ring, that its passage thereto may be said to be a shortening process or a lessening of its resistance.
The magnetism in air, gases, and non-magnetic bodies, being assumed to be that of the ether, this medium shows no such effects as those we get with the ring. It does not become permanently polarized, as does even soft iron under the condition of a closed ring. The iron possesses coercive force, or magnetic rigidity, and a steel ring would show more of it. The molecules of the iron or steel take a set. If we were to cut the soft iron ring, or separate it in any way, this introduction of resistance of air for ether in the magnetic circuit would cause the lines to collapse and set up a current in the conductor. The energy of the ring would have been restored to the latter. The curious thing is that physically the polarized ring does not present any different appearance or ordinary properties different from those of a plain ring, and will not deflect a compass needle. Its condition is discoverable, however, by the test of self-induction to currents of different direction. As a practical consideration, we may mention in this connection that a self-inductive coil for currents of one direction must be constructed differently from one to be used with alternating currents. The former must have in its magnetic circuit a section of air or the like, or be an imperfectly closed circuit, as it were. The latter should have as perfectly closed a magnetic circuit as can be made. We see here also the futility of constructing a Ruhmkorff core coil on the closed iron magnetic circuit plan, because the currents in the primary are interrupted, not reversed.
The considerations just put forward in relation to the closed iron ring, and its passive character under the condition of becoming polarized, are more important than at first appears. It has been found that the secondary current wave of a closed iron circuit induction coil or transformer, whose primary circuit receives alternating current, is lagged from its theoretical position of 90 degrees behind the primary wave an additional 90 degrees, so that the phases of the two currents are directly opposed; or the secondary current working lamps only in its circuit is one half a wave length behind a primary, instead of a quarter wave length, as might have been expected.
But when it is understood that the iron core polarized in one direction by the primary impulse does not begin to lose its magnetism when that impulse simply weakens, but waits until an actual reversal of current has taken place, it will be seen that the secondary current, which can only be produced when magnetic lines are leaving the core and cutting the secondary coil, or when the lines are being evolved and passing into the core from the primary coil, will have a beginning at the moment the primary reverses, will continue during the flow of that impulse, and will end at substantially the same time with the primary impulse, provided the work of the secondary current is not expended in overcoming self-induction, which would introduce a further lag. Moreover, the direction of the secondary current will be opposite to that of the primary, because the magnetic circuits which are opened up by the primary current in magnetizing the core, or which are closed or collapsed by it in demagnetizing the core, will always cut the secondary coil in the direction proper for this result. Transformers of the straight core type with very soft iron in the cores and not too high rates of alternation should approximate more nearly the theoretical relation of primary and secondary waves, because the magnetic changes in the core are capable of taking place almost simultaneously with the changes of strength of the primary current. This fact also has other important practical and theoretical bearings.
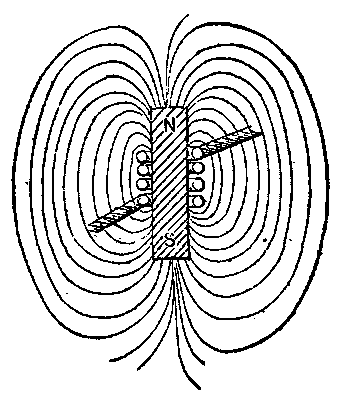 Fig. 7.
Fig. 7.
Let us assume a plain iron core, Fig. 7, magnetized as indicated, so that its poles, N, S, complete their magnetic circuits by what is called free field or lines in space around it. Let a coil of wire be wound thereon as indicated. Now assume that the magnetism is to be lost or cease, either suddenly or slowly. An electric potential will be set up in the coil, and if it has a circuit, work or energy will be produced or given out in that circuit, and in any other inductively related to it. Hence the magnetic field represents work or potential energy. But to develop potential in the wire the lines must cut the wire. This they can do by collapsing or closing on themselves. The bar seems, therefore, to lose its magnetism by gaining it all, and in doing so all the external lines of force moving inward cut the wire. The magnetic circuits shorten and short-circuit themselves in the bar, perhaps as innumerable molecular magnetic circuits interior to the iron medium. To remagnetize the bar we may pass an electric current through the coil. The small closed circuits are again distended, the free field appears, and the lines moving outward cut across the wire coil opposite to the former direction and produce a counter potential in the wire, and consequent absorption of the energy represented in the free field produced. As before studied, the magnetism cannot disappear without giving out the energy it represents, even though the wire coil be on open circuit, and therefore unable to discharge that energy. The coil open-circuited is static, not dynamic. In such assumed case the lines in closing cut the core and heat it. Let us, however, laminate the core or subdivide it as far as possible, and we appear to have cut off this escape for the energy. This is not really so, however. We have simply increased the possible rate of speed of closure, or movement of the lines, and so have increased for the divided core the intensity of the actions of magnetic friction and local currents in the core, the latter still receiving the energy of the magnetic circuit. This reasoning is based on the possibility in this case of cutting off the current in the magnetizing coil and retaining the magnetic field. This is of itself probably impossible with soft iron. That the core receives the energy when the coil cannot is shown in the well known fact that in some dynamos with armatures of bobbins on iron cores, the running of the armature coils on open circuit gives rise to dangerous heating of the cores, and that under normal work the heating is less. In the former case the core accumulates the energy represented in the magnetic changes. In the latter the external circuit of the machine and its wire coils take the larger part of the energy which is expended in doing the work in the circuit. In this case, also, the current in the coils causes a retardation of the speed of change and extent of change of magnetism in the iron cores, which keeps down the intensity of the magnetic reaction. In fact, this retardation or lag and reduction of range of magnetic change may in some machines be made so great by closing the circuit of the armature coils themselves or short-circuiting them that the total heat developed in the cores is much less than under normal load.
I wish now, in closing, to refer briefly to phenomena of moving lines of force, and to the effects of speed of movement. In order to generate a given potential in a length of conductor we have choice of certain conditions. We can vary the strength of field and we can vary the velocity. We can use a strong field and slow movement of conductor, or we can use a weak field and rapid movement of the conductor. But we find also that where the conductor has large section it is liable to heat from eddy currents caused by one part of its section being in a stronger field than another at the same time. One part cuts the lines where they are dense and the other where they are not dense, with the result of difference of potential and local currents which waste energy in heat. We cannot make the conductor move in a field of uniform density, because it must pass into and out of the field. The conditions just stated are present in dynamos for heavy current work, where the speed of cutting of lines is low and the armature conductor large in section.
But we find that in a transformer secondary we can use very large section of conductor, even (as in welding machines) 12 to 15 square inches solid copper, without meeting appreciable difficulty from eddy currents in it. The magnetic lines certainly cut the heavy conductor and generate the heavy current and potential needed. What difference, if any, exists? In the transformer the currents are generated by magnetic field of very low density, in which the lines are moving across the conductor with extreme rapidity. The velocity of emanation of lines around the primary coil is probably near that of light, and each line passes across the section secondary conductor in a practically inappreciable time. There is no cause then for differences of potential at different parts of the section heavy secondary. Then to avoid eddy currents in large conductors and generate useful currents in them, we may cause the conductor to be either moved into and out of a low density field with very great speed, or better, we must cause the lines of a very low or diffused field to traverse or cut across the conductor with very high velocity.
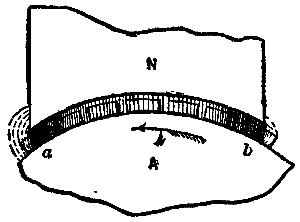 Fig. 8.
Fig. 8.
It is a known fact that, in dynamos with large section armature conductors, there are less eddy currents produced in the conductors when they are provided with iron cores or wound upon iron cores than when the conductors are made into flat bobbins moved in front of field poles. Projections existing on the armature between which the conductors are placed have a like effect, and enable us to employ heavy bars or bundles of wire without much difficulty from local currents. The reason is simple. In the armatures with coils without iron in them, or without projections extending between the turns, the conductor moves into and out of a very dense field at comparatively low velocity, so that any differences of potential developed in the parts of the section of conductor have full effect and abundant time to act in setting up harmful local currents. In the cases in which iron projects through the coil or conductor, the real action is that the lines of the magnetic circuits move at high speeds across the conductor, and the conductor is at all times in a field of very low density. Figs. 8 and 9 will make this plain. In Fig. 8 we have shown a smooth armature surface, having a heavy conductor laid thereon, and which is at a just entering a dense field at the edge of the pole, N, and at b leaving such field. It will be seen that when in such position the conductor, if wide, is subjected to varying field strength, and moves at a low speed for the generation of the working potential as it passes through the field, thus giving rise to eddy currents in the conductor.
 Fig. 9.
Fig. 9.
In Fig. 9 the conductors are set down between projections, in which case both armature and field poles are laminated or subdivided. As each projection leaves the edge of field pole, N, the lines which it had concentrated on and through it snap backward at an enormous speed, and cross the gap to the next succeeding projection on the armature, cutting the whole section of the heavy armature conductor at practically the same instant. This brisk transfer of lines goes on from each projection to the succeeding one in front of the field pole, leaving a very low density of field at any time between the projections. The best results would be obtained when the armature conductor does not project beyond or quite fill the depth of groove between the projections. Of course there are other remedies for the eddy current difficulty, notably the stranding and twisting of the conductor on the armatures so as to average the position of the parts of the compound conductor.
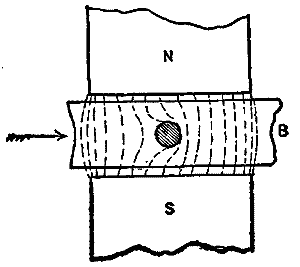 Fig. 10.
Fig. 10.
Perhaps the most extreme case of what may be called dilution of field by projections and by closed magnetic circuits in transformers would be that of a block of iron, B, Fig. 10, moved between poles, N and S, and having a hole through it, into and through which a conductor is carried. The path through the iron is so good that we can scarcely consider that any lines cross the hole from N to S; yet as B moves forward there is a continual snapping transfer of lines from the right forward side of the hole to the left or backward side, cutting the conductor as they fly across, and developing an electromotive force in it. I have described this action more in detail because we have in it whatever distinction in the manner of cutting the lines of the field is to be found between wire on smooth armatures and on projection armatures and modifications thereof; and also between flat, open coils passing through a field and bobbins with cores of iron. The considerations advanced also bring out the relation which exists between closed iron circuit transformers and closed iron circuit (projection) dynamos, as we may call them.
I had intended at the outset of this paper to deal to some extent with the propagation of lines of magnetism undergoing retardation in reference to alternating current motor devices, transformers with limited secondary current, or constant average current, an alternating motor working with what I may term a translation lag, etc.; but it was soon found that these matters must remain over for a continuation of this paper at some future time. My endeavor has been in the present paper to deal with the lines of force theory as though it were a symbol of the reality, but I confess that it is done with many misgivings that I may have carried it too far. Yet, if we are to use the idea at all it has seemed but right to apply it wherever it may throw any light on the subject or assist in our understanding of phenomena.
A paper read before the American Institute of Electrical Engineers, New York, May 22, 1889.
Immediately on entering the Machinery Hall by the galerie leading from the central dome, and occupying a prominent position at the commencement of the Swiss section, is a very important plant of dynamos, motors, and steam engines, put down by the Oerlikon Works, of Zurich. During the time the machinery is kept running in the hall, power is supplied electrically to drive the whole of the main shafting in the Swiss section and part of that in the Belgian section, amounting in all to some 200 ft., a large number of machines of various industries deriving their power from these lines of shafting, while during the evening a portion of the upper and lower galleries adjoining this section is lit by some twenty-five arc lamps run from this exhibit. Steam is supplied from the Roser boilers in the motive power court. The whole of the generating plant is illustrated in one view, and a separate view is given of the motor employed to drive the main shafting, this latter view showing the details of connection to the same. On the extreme right hand side of the first view is a direct coupled engine and dynamo of 20 horse power, a separate cut of which is given in Fig. 3. The engine is of the vertical single cylinder type, standing 5 ft. high, and fitted, as are the other two engines exhibited, with centrifugal governor gear on the fly wheel, acting directly on the throw of the cutoff valve eccentric. The two standards, supporting the cylinder and forming the guide bars, together with the entire field magnets and pole pieces of the dynamo, and the bed plate common to both, are cast in one piece.
The machine is specially designed for ship lighting, and with the view of preventing any magnetic effect upon the ship's compass, the field is arranged so that the armature, pole pieces, and coils are entirely inclosed by iron. Any tendency to leakage of magnetic lines will therefore be within the machine, the iron acting as a shield. This build of field—shown in Fig. 3a—is also advantageous as a mechanical shield to the parts of the machine most likely to suffer from rough handling in transport, and it will be seen that the field coils are easily slipped on before the armature is mounted in its bearings.
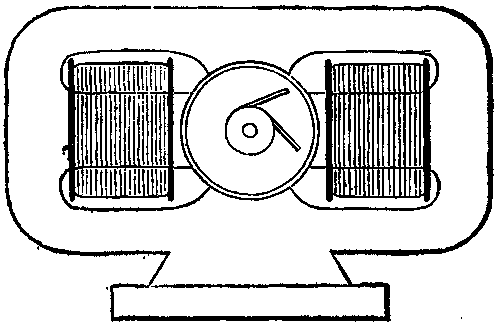 FIG. 3A
FIG. 3A
The winding is compound, and in such a direction that the two opposite horizontal poles have the same polarity; it follows from this that there will be two consequent poles in the iron, these being opposite in name to the horizontal poles and at right angles to them, viz., above and below the armature. Opposite sections of the commutator are connected together internally as in most four-pole machines, so that only two brushes are necessary, at 90 deg. apart.
The section of iron in the field is 60 square inches and rectangular in form, and the whole machine measures 4 ft. 3 in. in length, and 2 ft. in height, without including the height of the bed plate. The armature is 17 in. in length and the same in diameter, measured over the winding, and develops at the machine terminals 70 volts and 200 amperes at 480 revolutions. The moving parts of the engine are well balanced, and run remarkably well and without noise at this high rate of speed.
This dynamo serves to develop power to run a motor in an adjoining inclosure, containing some fine specimens of lathes and machine tools constructed by the Oerlikon Works. These are driven by the motor through the medium of a countershaft, and the power and speed are controlled from the switch board seen at the left of the exhibit, and in Fig. 11. The resistance, R1, serves to vary the intensity of the shunt field of the dynamo, the volts being indicated by the voltmeter V1, and a resistance separate from the switch board is inserted in the main circuit of the two machines. The ammeter, A2, is directly connected to the dynamo, and therefore indicates the current, whatever circuit this machine is running.
A larger combined engine and dynamo, seen in the center of the stand, serves to run the lighting of the galleries. The engine is a 60 horse power compound, running at 350 revolutions, and fitted with a governor on the fly wheel, like that described above.
The dynamo is a two-pole machine, the upper pole and yoke being cast in one, and the lower pole, yoke, and combined bed plate forming a separate casting. The two vertical cores, over which the field bobbins are slipped, are of wrought iron, and are turned with a shoulder at either end, the yokes being recessed to fit them exactly. The cores are then bolted to the yokes vertically from the top and horizontally below. The field of this machine is shunt-wound, and in order to maintain the potential constant a hand-regulated resistance—R2 on the switch board—is added in circuit with the shunt field. The voltmeter, V2, immediately above this resistance, serves to indicate the difference of potential at the machine terminals. Both voltmeters are fitted with keys, so that they are only put in circuit when the readings are taken.
The main terminals of this machine are fitted on substantial insulating bases, fixed one at each end of the top yoke. These connect to the external circuit by a heavy cable—the machine being capable of developing 500 amperes—and to the shunt circuit, and regulating resistance by small wires; while the two connections to the brushes are by four covered wires in parallel on each side. This mode of connection is more flexible than a short length of heavy cable, and looks well, the wires being held neatly together by vulcanized fiber bridges. The dynamo is a low tension machine, the field being regulated to give 65 volts when running the lamp circuits.
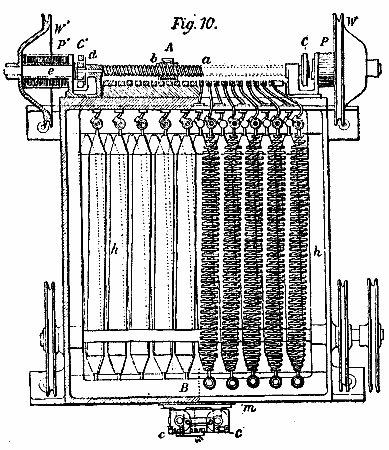 Fig. 10.
Fig. 10.
The illustration, Fig. 10, represents the automatic re-regulator—C.E.L. Brown's patent. Motion is imparted to the cores of two electro-magnets at the ends by the pulleys, W W1. The cores have a projection opposite to the spindle, a b, which latter is screw-threaded. By a relay one or other electro-magnet is put in action, and the rotating core, which is magnetized, causes rotation of the spindle by attraction, resulting in the movement of the contact along the resistance stops. The relay is acted upon directly by the potential of the dynamo, and the variable resistance is included in the shunt field of the machine, so that changes in the potential, resulting from changes in load or speed, are compensated for.
The arrangements of the lamp circuits and the lamp itself may now be described. The lamps are all run in parallel circuit, but are divided into groups of five, each group being controlled by a separate switch on the board—Figs. 11 and 11a. These switches are not in direct communication with the dynamo, but make that connection through a large central switch, S2, which therefore carries the whole current. The returns from each group are brought to the connections seen between the two resistances, where the circuits may be disconnected if desired, and the main current then passes through the ammeter, A3, to the other terminal of the machine. One of the smaller switches at the top, Fig. 11a, is directly connected with one terminal of the 20 horse power dynamo before mentioned, and the other side of the switch to the motor in the machine tool exhibit. Also one of the switches in connection with the central switch, S2, is connected to the same motor, and therefore the latter may be run by either machine, or, in fact, any combination of machines, lamps, and motor be made as required.
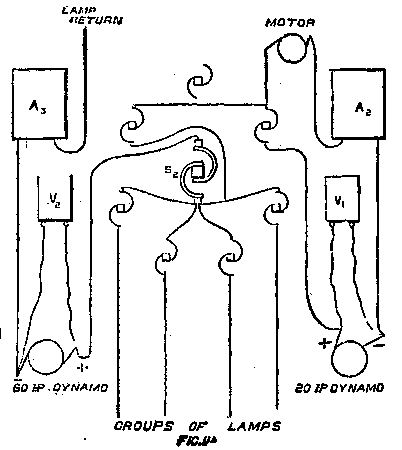 Fig. 11a
Fig. 11a
The form of switch made by the Oerlikon Works is illustrated in Fig. 7. Two thick semicircular bands of copper are screwed at one end to opposite sides of a square block which is turned round by the switch handle. The block has a projection at each corner, and two strong, flat, stationary springs are attached to the framework of the switch and press on opposite sides of the block. The ends of the springs engage in the projections and prevent the switch being turned round the wrong way, while the pressure of the springs on opposite sides forces the copper bands to take up a position exactly in line with the terminal contacts when the switch is closed, or at right angles to them when it is opened.
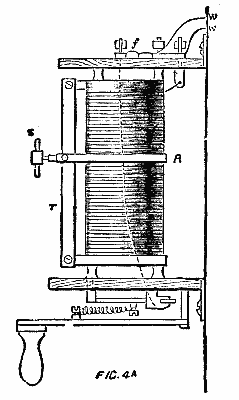 Fig. 4A
Fig. 4A
Further, each lamp has its own separate adjustable resistance, fuse, and switch. These are of special construction, combined in one, and are illustrated in Figs. 4 and 4a; the other figures, 4b and 4c, showing some of the details of the same. The wires, W W, lead from and to one lamp. The current enters at one wire, passes through the fuse, f—Figs. 4c and 4a—down the center of the cylinder to a divided contact, into which a switch arm can be shot. When this is so, a connection is made to the upright brass rod, T, which serves to grip the band, R, passing round the body of the cylinder. The current then passes through all the turns of wire above the band, and out at the other terminal. The resistance can be varied by raising or lowering the band. Fig. 4b shows the manner of tightening the band against the wires on the cylinder. The upright rod, T, is seen in section, and is fixed in one position to the frame of the apparatus. Abutting against this, and working in the block to which the two ends of the band are screwed, is a thumb screw, S, by turning which the band may be loosened for adjusting, and tightened when the right position is found. The cylinder is covered with asbestos sheet, and the wire, which is of nickel, and measures altogether from 3 to 4 ohms, is wound helically round this. The switch arm, to which the handle is attached below, does not itself make and break the circuit, but carries a spring, as shown, which, when the arm is at the end of its movement, pulls over the contact lever with a rapid action, shooting the same between the divided contact piece, and making a perfect contact. The switchboard forms one side of a closed wooden case or cupboard, with sufficient room for a man to enter and adjust the resistances or switches for each lamp. These are screwed to the inside of the case in rows, to the number of twenty-five. The greatest care has been taken in the fixing of the connections to the inside of this case, and no leading wires of different potential are allowed to cross each other.
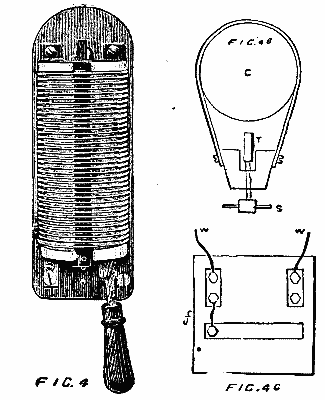 Figs. 4, 4B and 4C
Figs. 4, 4B and 4C
The Oerlikon lamp, which is designed to work with constant potential, is shown partly in section in Fig. 8. There is only one solenoid, A, through which all the current passes, and whose action is to strike the arc and maintain the current constant. The soft iron core, C, is suspended from the inside of the tube, T, in which it has an up and down movement checked by an air piston in the tube. An end elevation of the brake wheels and solenoid is given in Fig. 9, where it will be seen that the spindle carrying these wheels also carries between them a pinion engaging with the rack rod, R. The top carbon attached to the rack rod falls by its own weight, and is therefore in contact with the lower carbon before the lamp is switched in circuit. When this is done the core is instantly magnetized, and attracted to the soft iron brake wheels, which it holds firmly. The air cushion in the tube prevents the core being drawn up until it has fairly gripped the sides of the wheels. The subsequent raising of the core therefore turns the wheels, raises the rack rod, and strikes the arc. The feed is operated by the weakening of the magnetic field of the coil, which causes the core to lose its grip of the wheels, and allows the top carbon to descend. The catch, L, Fig. 8, has a lateral play, and serves to engage in the teeth of the rack rod, so as to prevent its falling when being trimmed. Each carbon when in position is held against two rectangular guide bars by the pressure of a wire spring—see figure. In this way the carbon is pressed against two parallel knife edges, and is therefore always in true alignment. The action of the lamp is very simple, the working parts are few and solidly constructed, and the regulation, as exhibited by the lamps running in the galleries, is exceptionally steady.
The transmission of power plant consists of two 250 horse power dynamos—C.E.L. Brown's patent—the generator being driven by a vertical compound condensing engine of the same power, running at 180 revolutions. The dynamo generator is a four-pole 600 volt direct current machine, series wound, and may be distinguished in the engraving next to the switch board; while the motor receiver connected to it, and erected in another portion of the Swiss section, is of exactly the same size and type. The field, which is hexagonal in shape, is cast in two pieces, bolted together horizontally, the cross-sectional area of iron being 170 square inches. The armature is cylindrical, and built up of flat rings stamped out of soft sheet iron, eight notches in the same being provided to fit over the arms of the spider keyed to the shaft. The spider is in halves, which are bolted together longitudinally after the rings are in position. It is Gramme wound, and measures over the winding 7 in. radial depth, 37 in. outside diameter, and 22 in. in length. The current is collected by four brushes. The fitting and mechanical build of the dynamos leaves nothing to be desired. All the working parts of the dynamos and engines are turned up to gauge and template, so as to be interchangeable. As an instance of this, the armature of the generator was built in the works, while the field magnets were being erected in the exhibition, and, on arrival, fitted in position perfectly, and ran at once without trouble.
The energy taken off on the motor shaft is close on 200 horse power, but varies according to the machines at work; the speed of the motor does not, however, vary more than 3 per cent., and the brushes need no adjustment. About 6 ft. of shafting is coupled on in line with the motor shaft, and an extra plummer block fixed at the end. This shafting carries at its extremity an additional 2 ft. pulley, the power being delivered by belting from these pulleys to two large pulleys on the main shaft.
The machines run by this transmission consist of the looms of Rieter & Co., of Winterthur; the large flour mill and lift of A. Millot & Co.; the flour milling machinery of Frederick Wegmann & Co., of Zurich; the brick and tile making machines of the Rorschach foundries; and the looms of Messrs. Houget & Teston, of Verviers, in the Belgian section. A 15 horse power two-pole Oerlikon dynamo is also run by a belt from the main shaft, and generates power to drive a motor of similar type in the Swiss section of the upper gallery. This runs a length of countershafting supplying power to three silk-weaving machines constructed by Benninger Frères; six weaving machines from the Ruti works, near Zurich; and one knitting machine exhibited by Edward Dubied & Co., of Couvet.
The dynamo and motor are connected to the main cable by switches of the type shown in Fig. 5. These are specially designed to destroy the extra current on breaking circuit by the formation of an arc which gradually increases the resistance till the break occurs, rendering it less sudden. One wire passes through the handle and makes contact with the springs, and the other is attached to the clamp in which the carbon rod is held. The current is made to enter at the carbon rod, so that the arcs formed cause consumption of the carbon. A magnetic cut-out—Fig. 6—is also provided to each machine; this consists of an electro-magnet, through which the main current passes, provided with side pole pieces. A flat soft iron plate armature is hinged so as to come up against the pole pieces when attracted. When the current is not sufficiently strong to cause the plate to be attracted, a hole in the center of the latter engages over a small projection in the top of a weighted arm hinged in the center of the board, and keeps it upright. If now the current exceeds the limits of safety to the machine, due to a too heavy load being thrown on, the armature is attracted and releases the vertical arm, which falls over and enters with considerable force between the two spring contacts below. These contacts are connected to the field terminals, which are, therefore, short-circuited, and prevent the dynamo generating any current. A retractile spring can be adjusted to cause cut-off at any required current. These details are indicated in our illustrations mounted on their respective switch boards.
Since the erection of plant by these works at Solothurn for transmitting 50 horse power five miles distant, which attracted so much interest some time ago, several important works have been carried out. Among these we may mention a 280 horse power transmission at 1½ kilom. distance to a cotton mill at Derendingen in Switzerland, a 250 horse power transmission at ½ kilom. distance, carried out for Gaetano Rossi at Piovene in Italy, and a 300 horse transmission at 6 kilom. distance installed for Giovanni Rossi, in which the power is given off at two different stations.—The Engineer.
Although telephonic novelties are not numerous at the Universal Exposition, telephony—that quite young branch of electric science—is daily the object of curious and interesting experiments which we must make known to our readers, a large number of whom were not yet born to scientific life when the experiments were made for the first time at Paris in 1881; and it is proper to congratulate the Société Générale des Téléphones on having repeated them in 1889 to the great satisfaction of the rising generation.
We allude to the Ader system of telephonic transmissions of sounds in such a way that they can be heard by an audience.
The essential parts of this mode of transmission consist of two distinct systems—transmitters and receivers.
 Fig. 1.—THE ADER FLOURISH OF TRUMPETS
Fig. 1.—THE ADER FLOURISH OF TRUMPETS
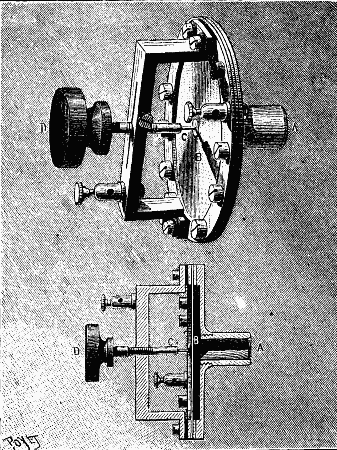 Fig. 2.—DETAILS OF THE TRANSMITTER.
Fig. 2.—DETAILS OF THE TRANSMITTER.
The transmitters are four in number, and are actuated by the same number of musicians, each humming into them his part of the quartet (Fig. 1). This transmitter, represented apart in elevation and section in Fig. 2, is identical with the one used in the curious experiment with the singing condenser. At A is a mouthpiece before which the musician hums his part as upon a reed pipe. He causes the plate, B, to vibrate in unison with the sound that he emits, and this produces periodical interruptions of varying rapidity between the disk, B, and the point, C. The button, D, serves to regulate the distance in such a way that the breakings of the circuit shall be very complete and produce sounds in the receivers as pure as allowed by this special mode of transmission, in which all the harmonics are systematically suppressed in order to re-enforce the fundamental.
This transmitter interrupter is interposed in the circuit of a battery of accumulators, with the five receivers that it actuates, in such a way that the four transmitters and five receivers form in reality four groups of distinct autonomous transmission, the accordance of which is absolutely dependent upon that of the artists who make them vibrate.
The five receivers are arranged over the front door of the telephone pavilion, near the Eiffel tower (Fig. 3). Each consists of a horseshoe magnet provided, between its branches, with two small iron cores having a space of a few millimeters between them (Fig. 4). Each of these soft iron cores carries a copper wire bobbin, N, the number of spirals of which is properly calculated for the effect to be produced. Opposite the vacant space left by the two cores, there is a small piece, t, of rectangular form, and also of soft iron, fixed to a vibrating strip of firwood, L, of about 4 inches section. The periodical breaking of the circuit produced by the transmitter causes a variation in the magnetization of the iron cores of the five receivers and makes the firwood strips vibrate energetically. These vibrations are received and poured forth as it were in front of the telephone pavilion, by large brass trumpets arranged in front of each receiver, as shown in Fig. 3.
It would be difficult for us to pass any judgment whatever upon the musical and artistic value of these transmissions of trumpet music to a distance; we prefer to confess our incompetency in the matter. But it is none the less certain that these experiments are having the same success that they had at their inception in 1881 at the Universal Exposition of Electricity, and they allow us to foresee that there is a time coming in which it will be possible to transmit speech to a distance with the same intensity that the present trumpet flourishes have. Although all the tentatives hitherto made in this direction have not given very brilliant results, we must not despair of attaining the end some day or other. Less than fifteen years ago the telephone did not exist; now it covers the world with its lines.—La Nature.
During the last ten years there has been an enormous increase in the production of these preparations, and the time will come when their application in dyeing and calico printing will become so general as to completely supersede the employment of the raw materials. The manufacture of these extracts, to be thoroughly successful, requires to be so conducted as to secure the perfect exhaustion of the dyewoods without the slightest destruction or deterioration of the coloring matters contained in them; and though nothing like perfection has been reached in the attainment of these objects, it is certain that the processes of extraction and evaporation now employed by the best makers are a very great improvement on the older methods. Indeed, there is no difficulty nowadays in procuring dyewood extracts of high excellence if the consumer is willing to pay a price for them corresponding to their quality, and knows how to avail himself of the aid of chemical skill to control his purchases. Unfortunately, however, there is so much hankering after cheap articles, and so little care is taken to ascertain their real quality, that every scope is afforded to the malpractices of the adulterer. There are many dye and print works in which large quantities of these extracts are used without being subjected to trustworthy tests. Moreover, much of the testing is done by fallacious methods and often by biased hands. So fallacious, indeed, are some of these tests, that grossly adulterated extracts are often declared superior to the purer ones, the cause of this being the application of an insufficient proportion of mordant in the dyeing or printing trials, and the consequent waste of the excess of coloring matter in the case of the purer preparation.
Professional analytical chemists have hitherto given but little attention to these preparations, and the employment of experienced chemists in works is as yet far from general. The testing of dyewood extracts in such a manner as to throw full light on their purity, the quality of raw material from which they are prepared, their exact commercial value their suitability for special purposes, and the proportion and nature of any adulterants they may contain, is of course a difficult and tedious task, and must be left to the expert who is in possession of authentic specimens prepared by himself of all the different extracts made from every variety and quality of raw materials, and who combines a thorough knowledge of experimental dyeing and printing with a large experience in the chemical investigation of these preparations. But when the object of the testing is merely careful comparison of the sample in question with an original sample or previous deliveries, the case is much simplified, and comes within the scope of the general chemist or the laboratory attached to works. A few years ago I recommended carefully conducted dyeing trials on woolen cloth mordanted with bichromate of potash as the best and simplest mode adapted to such cases, and my subsequent experience enables me to confirm that observation to the fullest extent. Most of these extracts contain the coloring matter in two states, the developed and the undeveloped, and an oxidizing mordant such as bichromate of potash causes the latter as well as the former to enter completely into combination with a metallic base; whereas many of the other mordants, such as alumina or tin compounds, merely take up the developed portion of the coloring matter together with such small and variable proportions of the undeveloped as might undergo oxidation during the process of dyeing. I would therefore suggest dyeing trials with alumina, tin, iron, etc., only as subsidiary tests indicating the suitability of an extract for certain special purposes, while recommending the trial with bichromate of potash as the one giving the best information respecting the actual strength of the extract in relation to the raw material from which it was obtained, and as giving a fair idea of the money value of the sample. Cotton dyeing does not, as a general rule, afford a good means of assaying extracts, as it is generally done under conditions which do not admit of complete exhaustion of the dye bath, but it might often with advantage be resorted to as an additional trial throwing further light on the degree of oxidation or development of the coloring matter. Printing trials are apt to give fallacious results unless the proportion of mordant is carefully adjusted to the amount of coloring matter present, and several trials with different proportions would be necessary to prevent erroneous conclusions. For the trials with bichromate of potash on wool I would recommend pieces of cloth weighing about 150 grains, and the most suitable proportion of bichromate of potash is 3 per cent. of the weight of the cloth. The requisite number of pieces (equal to the number of samples to be tested) should be thoroughly scoured and then heated in the bichromate solution at or near the boiling point for not less than 1½ hours, after which they should be well washed and then dyed separately in the solutions of equal weights of the extracts at the same temperature and for the same length of time; 15 grains of extract is a suitable quantity for a first trial under these conditions. These trials can then be repeated with different relative proportions of extract in order to ascertain what weight of a sample would give the same depth of color as 15 grains of the standard example. Many precautions are required both in the mordanting and dyeing processes in order to obtain trustworthy results; and though the trials with bichromate of potash give the most reliable information of any single test, they should be supplemented by the subsidiary tests already alluded to, and also by a chemical examination, in order to obtain a knowledge, not merely of the wood strength, but also of the general nature of the extract. An adulteration with molasses or glucose can be best determined by fermentation in comparison with a pure sample. Mineral adulterants may, of course, be detected by an estimation and analysis of the ash, after making due allowances for variations due to differences in different kinds of the same dyewoods. The estimation of the individual coloring matters in these extracts by means of a chemical analysis is under all circumstances a task requiring much experience, especially as the coloring principles are associated in different qualities of each class of dyewood with different proportions of other constituents which often give much trouble to the unpracticed experimenter. Extracts made from logwood roots are now largely manufactured and often substituted or mixed with the extracts of real logwood, and have in some instances been palmed of as logwood extracts of high quality. The correct determination of such admixtures, like the fixing of anything like the exact commercial value of dyewood extracts, requires nothing less than a complete chemical investigation coupled with numerous dyeing trials in comparison with standard preparations, and should be left to an expert.
The presence in dyewood extracts of coloring matters in various stages of development has hitherto militated against their use in place of the raw materials by many dyers and printers who are still employing inherited and antiquated processes in which the whole of the coloring matter is not rendered available. It is often asserted by these that even the best of extracts fail to give anything like the results attained by the use of well-prepared woods, and that, indeed, their application proves a complete failure. Such failure, however, is simply due to the want of chemical knowledge on the part of the dyers, for there is no real difficulty in making any good and pure extract serve all the purposes for which the woods were used. It is to be hoped that in this branch of industry, as well as in many others, the employment of chemists will become more general than at present, and not be restricted, as is often the case, to young men without experience and without the trained intellect so essential to success in chemical investigations. High class chemical skill is of course available to the manufacturer, but the man of science who brings matured knowledge and valuable brain work into the business required social as well as pecuniary recognition, and the sooner and more fuller this fact is appreciated the better it will be for the maintenance and progress of our industries.
With regard to the astringent extracts, such as sumac, myrabolam, divi, valonia, quebracho, oak, etc., it is the aim of the manufacturer, whenever such extracts are intended for the purposes of dyeing and printing, to obtain the tannin in a form in which it is best calculated to fix itself upon the fiber. The case is somewhat different when the same extracts are required for tanning. For this purpose it is necessary that the extract shall have considerable permeating power, and that the tannin contained in it shall readily yield leather of the desired texture, color, and permanency. Extracts specially suited for this purpose are by no means always the most suitable for the dyer, and vice versa.
A brief description of the processes by which the astringent extracts may be tested with particular reference to their fitness for definite purposes concluded the paper.
With regard to the question as to whether experimental dyeing with bichromate of potash should be employed as a test even in works where all the dyeing was done with other mordants, he was decidedly of opinion that it should always be resorted to as one of the tests, inasmuch as it was the only simple and expeditious method giving a fair idea of the actual wood strength and money value of the extract. The test should, in such cases, be supplemented by dyeing trials with the mordants used at the works, and, if necessary, also by a chemical analysis. Printing trials were not necessarily bad tests, since oxidizing was usually added in these where it was necessary, and any undeveloped coloring matter would thus be oxidized during the steaming process: but, as he had stated before, it was essentially necessary in such cases to have a fair idea of the amount of actual coloring matter in the extract and to adjust the proportion of mordant accordingly. Such trials should therefore be preceded by carefully conducted dyeing trials with bichromate of potash. Mr. Thomson had raised the question whether it would not be well for the manufacturer to prepare these extracts in such a manner that they would contain all the coloring matter in one condition only, in order to insure greater uniformity in their quality and mode of application. This would, no doubt, be a desirable step to take if the owners of dye and print works were more in the habit of availing themselves of the service of competent chemists experienced in this branch, for then they would be able to make any extract do its full work irrespective of the state of development of the coloring matter. Such, however, was not the case, and it was a very common thing for the consumer of dyewood extracts to require the manufacturer to prepare them specially for him so as to suit his own dyeing recipes, or in other words to give exactly the same shades, weight for weight, by his own method of dyeing as the article he was in the habit of using. The manufacturer was thus often compelled to make many different qualities of the same extract to suit different customers. For the same reason adulterated articles were often preferred to the pure ones. There was, perhaps, no branch of industry in which chemical skill of a high order could be applied with greater advantage than in dyeing, and nowhere was this fact less recognized. Some of the processes of dyeing were exceedingly wasteful and stood in much need of improvement. He (Mr. Siebold) knew a large works in which a ton of logwood extract was used daily for black dyeing only, and he might safely assert that of this enormous quantity only a very small proportion would be fixed on the fiber, while by far the greater proportion was utterly wasted. Such a waste could only be prevented by a searching investigation of its causes by trained skill. Mr. Thomson had further alluded to the color obtained with logwood or logwood extract and wool mordanted with bichromate of potash, and seemed to be under the impression that the color thus obtained was not black, but blue. This was undoubtedly the case in dyeing trials performed as tests, as these were conducted purposely with a very small proportion of coloring matter in order to admit of a better comparison of the resulting depth of shades. But with larger proportions of logwood the color obtained was a fine bluish-black, and with the addition of a small proportion of fustic or quercitron bark to the logwood a jet black was readily produced. With regard to Mr. Watson Smith's observation as to fractional dyeing, he (Mr. Siebold) did not regard this method as a suitable trial for ascertaining the strength of an extract, but he admitted it was occasionally very valuable for detecting an admixture of extracts of other dyewoods, such as quercitron bark extract in logwood extract. It was also a good method of ascertaining the speed of dyeing and hence the relative proportion of fully developed coloring matter of an extract.—Jour. Soc. Chem. Industry.
What I want to show is the manner in which the process has been tested. My employer, Mr. Bierstadt, has given me permission to show you some samples, and also his chart containing the spectrum colors: violet, indigo blue, green, yellow, orange, red, and black. This chart has been photographed in the orthochromatic and also in the ordinary way.
There are many ways of producing an orthochromatic effect; one is the use of a glass tank placed behind or in front of the lens, in which a coloring matter from either a vegetable or mineral product is placed; this tank or cell is, however, only for use in the studio, as for outdoor photography we have a colored glass screen, so as not to be bothered with carrying colored solution.
The tank is constructed as follows: Procure two pieces of best white plate glass, about 6 inches square; between these place a piece of rubber of the same size square, and about 3/8 of an inch thick. In the center of this rubber cut out a circle about 4 inches diameter, and from one of the corners to the center of the circle cut out a narrow strip ¼ inch wide; this serves as the mouth of the tank. The two pieces of glass and the rubber are cemented together with rubber cement; then, to hold it firmly together, two brass flanges are used as a clamp, with four screws at an equal distance apart; a thin sheet of rubber is on the glass side of the flanges to prevent direct contact with the glass, the center remaining clear for the rays of light to pass through solution and glass.
One of the best orthochromatic effects made through this tank is with a three-grains-to-the-ounce solution of bichromatic of ammonia or bichromate of potassium. In this method there is no preparation used on the plate. A common rapid dry plate is exposed through this solution; the exposure, however, is about twenty times longer than it would be if you removed the tank with the yellow solution, or, in other words, if a dry-plate is exposed one minute without the yellow solution it would have to be exposed twenty minutes through a three-grain solution of bichromate of potassium or ammonia. It produces wonderful results on an oil painting or any highly colored object.
Another method, and the one best adapted for landscapes, is to bathe the plate in erythrosine and then expose it through a yellow glass screen.
As an illustration, suppose we have before us a beautiful landscape. In the foreground beautiful foliage, in the center a lake, in the distance hills, with a bluish haze appearing pleasing to the eye, also a nice sky with light clouds. Now make a plain negative, and see what has become of your clouds, hills, and the distance—not visible! Some photographers have been led to think that by underexposing they retain the distance, but they sacrifice the foreground; besides, it does not produce an orthochromatic effect.
But it is a good idea to expose longer on the foreground than you do on the distance. This can be done by raising the cap of the lens skyward and gradually shut off, giving the foreground more exposure.
Plates are prepared for orthochromatic work as follows: Take any ordinary rapid dry plate, place it in a bath containing
| Distilled water | 200 c.c. |
| Strong liquid ammonia | 2 c.c. |
Rock it for two minutes, work as dark as you possibly can. Now take it out, and place it in the second bath for one and one-fourth minutes and keep it rocking. Have on hand for use a stock solution of
| Distilled water | 1,000 parts. |
| Erythrosine "Y" brand | 1 part. |
Prepare second bath as follows:
| Erythrosine stock solution | 25 c.c. |
| Distilled water | 175 c.c. |
| Strong water ammonia | 4 c.c. |
After removing the plate, dip it again face down to rinse off any particles of scum, etc., that may get in the bath accidentally. This bath may be used for one dozen 8 by 10, when it should be thrown away and fresh bath used.
After the plates come out of the last bath, they should be stood on clean blotting paper to absorb the excess of solution. I would also advise to use clean fingers. Pyro. or hypo. on the fingers is a drawback to success.
After plates have been drained, place them in a cleaned rack in an absolutely light-tight closet, with air holes so constructed as to admit air but no light; the plates will dry in from eight to twelve hours. They are best prepared in the evening, and, if the closet is good, will be dry in the morning.
After the plates are dry they may be packed face to face with nothing between them, in a double-cover paper box, and put in a dark closet free from sulphureted hydrogen gas, until ready for use. I have kept plates for three months in this way, and they were in good condition. Great care should be used in developing these plates, as they are sensitive to the red; get used to developing in a dark part of the dark room; occasionally you may look at the process of development in a little stronger light.
The exposure through the yellow screen with an erythrosine plate is about the same as if you had no orthochromatic plate—a plain plate instead—provided you are not using too dark a yellow on your screen. This can only be determined by experience. I will give to a common plate about four seconds, an orthochromatic plate under the same conditions five seconds.
The yellow glass screen is prepared as follows: Take a piece of best plate glass—common cannot be used—clean it nicely; take another large plate glass, or anything that is level and true, level it with a small spirit-level. Now take the cleaned piece of glass and coat it with
| AURENTIA COLLODION. | ||
|---|---|---|
| Ether | 5 | oz. |
| Alcohol | 5 | oz. |
| Cotton | 60 | grs. |
The aurentia to be added to suit your judgment; it takes a very small quantity to make an intense yellowish-red collodion. Pour it on the center of the glass, flow it to the edges, and before it sets place it on the level glass and allow it to set; when set put it in a rack to dry.
Should it dry in ridges, the collodion may be too thick, and it must be thinned down with equal parts of alcohol and ether. A single piece of plate glass, about one-eighth inch thick, coated with aurentia collodion, is all that is required with an erythrosine plate. Or, after a piece has been successfully coated, another piece of the same plate glass, and the same size, may be cemented together with balsam, having the coated aurentia side between the two glasses; the edges may then be bound with paper.
In using different colored solutions, collodion, etc., I have found that one will change the focus and the other not. With some screens you must focus with them in their positions; take away the screen, and the picture appears out of focus. I cannot fully explain why it is, and for this reason will not make the attempt; experience alone can teach it.
Another thing that has been tried lately is to do away with the yellow screen by substituting a yellow coating direct on the plate. No doubt the focus on an object that requires absolute sharpness is somewhat affected by the use of a glass. We have been successful, on a small scale, to coat the plate with the following yellow solution:
Place in a tray enough of a saturated solution of tropæolin in wood alcohol to cover the plate; allow it to remain ten seconds. It is necessary that the plate should be bathed previously in erythrosine and dried. Before applying the tropæolin, which, being in alcohol, dries in a few minutes, have some blotting paper on hand, as the solution gathers in a pool and leaves bad marks on the end of the plate.
The plate can be developed in the usual way. Try it and see the results.—Reported in the Beacon.
Read before the Photographic Association of Brooklyn.
Platinotype, which may be considered to be the most artistic of photographic printing processes, may be separated into its three modifications—the hot bath and cold bath, in which a faintly visible image is developed, and the Pizzighelli printing-out paper. The hot bath process, again, may be divided into the black and white and sepia papers. I intend to give you a rough outline of the preparation of the paper and working of these modifications, concluding by demonstrating the hot bath method, and handing around prints by it.
Platinotype may almost be styled an iron printing process, for, while no trace of iron or its salts is found in the finished print, certain salts of iron are mixed with the platinum salt, which is platinum combined with two atoms of chlorine (PtCl2), as a means for readily reducing it; this, however, cannot be effected without the presence of neutral oxalate of potash, hence the use of the oxalate bath. There is no platinum in the paper for the cold bath process, it being coated with ferric oxalate mixed with a very small quantity of chloride of mercury—somewhere about one grain to an ounce of ferric oxalate solution. When dry it is ready for exposure, which is about three times less than with silver printing.
It is absolutely necessary to store all papers for platinum printing in an air-tight tin containing chloride of calcium, which must be dried by heating from time to time. For the cold bath, however, it is important to have moisture present during printing, or it may be after printing and before development. If the paper is left in a dampish room for fifteen minutes, it should be sufficient. Prints made by exposing damp paper, or damping dry paper just before development, must be developed within one hour if the maximum of vigor is desired; by delaying the development some hours, the prints in the meantime being stored in a drawer so that they may retain their moisture, an increase of half tone and warmth of color will be obtained. If it should be necessary to delay development for a day or two, the prints must be dried before a fire soon after being removed from the frames, and then stored in a calcium tube until wanted for development.
While printing, the lemon color of the paper receives a grayish colored image, which, although faint, can, with practice, be judged as easily as silver printing.
The developer consists of oxalate of potash and potassic chloro-platinite—about thirty grains of the platinum salt to half an ounce of oxalate forming about six ounces of solution; a great many variations, however, may be made in the proportions of platinum salt and oxalate, and different effects secured. Development is effected by sliding the print face downward on to the developer, which must be rocked after the development of each print to avoid scum marks. To clear the prints they are washed in three or four baths of a weak solution of hydrochloric acid after leaving the developer, to remove all traces of the iron salts, and finally washed for a quarter of an hour in three changes of water; they are then finished, and may be dried between clean blotting paper.
Pizzighelli's process differs from the above in being one that prints fully out in the frame without development; the paper contains the platinum and iron salts as well as the developer, and so prints and develops at the same time. Although excellent prints can be produced with it, for general work the results of the paper, as at present made, will not compare with the hot and cold bath processes. It is, however, excellent for printing from very dense negatives, and occasional negatives that seem extremely suitable for it. The paper should be breathed on before printing, as if it is quite dry the printing will be very slow and irregular. The best conditions for the preparation of the paper have scarcely been decided upon yet, and it is not quite fair to judge the process. The prints are cleared in the acid baths and washed for about a quarter of an hour.
The sepia and black hot bath processes are much alike in the general treatment. There are, however, some special precautions to be observed with the sepia paper, the chief being to protect it from any but the faintest rays of light; the prints, unlike the black ones, may be affected by light when in the acid bath. A special solution must be added to the developer to keep the lights pure. Over-exposure cannot be corrected by using a cooler bath, as is the case with the black prints, and the paper does not remain good so long.
The paper for the black prints by the hot bath process is washed with a mixture of potassic platinous chloride and ferric oxalate, the proportion being about sixty grains of the platinum salt to one ounce of the iron solution. It will not keep good longer than twenty minutes or so, and must be applied to the paper directly after mixing. The ferric oxalate in the paper is reduced by the action of light to ferrous oxalate, which forms the faint visible image; this, when the paper is floated on the oxalate of potash bath, is capable of reducing the platinum salt in contact with it into metallic platinum; but the ferric salt, which remains unaltered, has no action on the platinum salt, leaving these parts, which represent the high lights of the print, untouched. The ferric oxalate is removed by the acid baths which follow the development. A good temperature for development is 150° Fahr., and when using this so much detail should not be apparent as when printing for the cold bath process, in which all the detail desired should be very faintly visible. There are, however, many methods of exposing the paper and developing it, and no fixed rule can be made, but the development must in every case be suited to the exposure or the result will be a failure. For instance, the paper may be printed until all detail is visible, but a very much cooler development must be used, say 80° or 90°; on the other hand, a slightly short exposure may be given, and a temperature of 180° to 200° used. 150° should be taken as the normal temperature, and kept to until some experience has been gained, as employing all temperatures will lead to confusion, and nothing will be learned. Some negatives require a special treatment, and both printing and development must be altered, while for a very dense negative the paper may be left out in a dampish room for some time. It will then print with less contrast and more half tone. A thin negative is better printed by the cold bath process, but negatives should be good and brilliant for platinotype printing. Any one taking up platinotype and getting only weak prints would do well to look to his negatives instead of blaming the paper, as the high lights should be fairly dense, and the deep shadows nearly clear glass.
Time for complete development should always be allowed; with a hot bath fifteen seconds will be sufficient, but if a cooler development is used, or the prints are solarized in the shadows, more time should be allowed. When the deep shadows are solarized, or appear lighter than surrounding parts, a hot and prolonged development is required to obtain sufficient blackness, as they have a tendency to look like brown paper. I have found breathing on solarized shadows useful, as in the presence of slight moisture they begin to print out and become dark before development, getting black almost directly the print is floated on the oxalate. Three or four acid baths of about ten minutes each are used, and the prints are washed as before. The process throughout takes much less time than silver printing, and can be kept on all the winter, when it is nearly impossible to print in silver. Prints can be developed in weak daylight or gaslight, and prolonged washing is dispensed with.—N.P. Fox, reported in Br. Jour. of Photo.
A communication to the North London Photographic Society.
In the first part of this paper were described certain forms of silver; among them a lilac blue substance, very soluble in water, with a deep red color. After undergoing purification, it was shown to be nearly pure silver. During the purification by washing it seemed to change somewhat, and, consequently, some uncertainty existed as to whether or not the purified substance was essentially the same as the first product; it seemed possible that the extreme solubility of the product in its first condition might be due to a combination in some way with citric acid, the acid separating during the washing. Many attempts were made to get a decisive indication, and two series of analyses, one a long one, to determine the ratio between the silver and the citric acid present, without obtaining a wholly satisfactory result, inasmuch as even these determinations of mere ratio involved a certain degree of previous purification which might have caused a separation.
This question has since been settled in an extremely simple way, and the fact established that the soluble blue substance contains not a trace of combined citric acid.
The precipitated lilac blue substance (obtained by reducing silver citrate by ferrous citrate) was thrown on a filter and cleared of mother water as far as possible with a filter pump. Pure water was then poured on in successive portions until more than half the substance was dissolved. The residue, evidently quite unchanged, was, of course, tolerably free from mother water. It was found that by evaporating it to dryness over a water bath, most of the silver separated out as bright white normal silver; by adding water and evaporating a second time, the separation was complete, and water added dissolved no silver. The solution thus obtained was neutral. It must have been acid had any citric acid been combined originally with the silver. This experiment, repeated with every precaution, seems conclusive. The ferrous solution, used for reducing the silver citrate, had been brought to exact neutrality with sodium hydroxide. After the reduction had been effected, the mother water over the lilac blue precipitate was neutral or faintly acid.
A corroborating indication is the following: The portions of the lilac blue substance which were dissolved on the filter (see above) were received into a dilute solution of magnesium sulphate, which throws down insoluble allotropic silver of the form I have called B (see previous paper). This form has already been shown to be nearly pure silver. The magnesia solution, neutral before use, was also neutral after it had effected the precipitation, indicating that no citric acid had been set free in the precipitation of the silver.
It seems, therefore, clear that the lilac blue substance contains no combined citric acid. Had the solubility of the silver been due to combination with either acid or alkali, the liquid from which it was separated by digestion at or below 100° C. must have been acid or alkaline; it could not have been neutral.
We have, therefore, this alternative: In the lilac blue substance we have either pure silver in a soluble form or else a compound of silver, with a perfectly neutral substance generated from citric acid in the reaction which leads to the formation of the lilac blue substance. If this last should prove the true explanation, then we have to do with a combination of silver of a quite different nature from any silver compounds hitherto known. A neutral substance generated from citric acid must have one or more atoms of hydrogen replaced by silver. This possibility recalls the recent observations of Ballo, who, by acting with a ferrous salt on tartaric acid, obtained a neutral colloid substance having the constitution of arabin, C6H10O6.
To appreciate the difficulty of arriving at a correct conclusion, it must be remembered that the silver precipitate is obtained saturated with strong solutions of ferric and ferrous citrate, sodium citrate, sulphate, etc. These cannot be removed by washing with pure water, in which the substance itself is very soluble, but must be got rid of by washing with saline solutions, under the influence of which the substance itself slowly but continually changes. Next, the saline solution used for washing must be removed by alcohol. During this treatment, the substance, at first very soluble, gradually loses its solubility, and, when ready for analysis, has become wholly insoluble. It is impossible at present to say whether it may not have undergone other change; this is a matter as to which I hope to speak more positively later. It is to be remarked, however, that these allotropic forms of silver acquire and lose solubility from very slight causes, as an instance of which may be mentioned the ease with which the insoluble form B recovers its solubility under the influence of sodium sulphate and borate, and other salts, as described in the previous part of this paper.
The two insoluble forms of allotropic silver which I have described as B and C—B, bluish green; C, rich golden color—show the following curious reaction. A film of B, spread on glass and heated in a water stove to 100° C. for a few minutes becomes superficially bright yellow. A similar film of the gold colored substance, C, treated in the same way, acquires a blue bloom. In both cases it is the surface only that changes.
Sensitiveness to Light.—All these forms of silver are acted upon by light. A and B acquire a brownish tinge by some hours' exposure to sunlight. With C the case is quite different, the color changes from that of red gold to that of pure yellow gold. The experiment is an interesting one. The exposed portion retains its full metallic brilliancy, giving an additional proof that the color depends upon molecular arrangement, and this with the allotropic forms of silver is subject to change from almost any influence.
Stability.—These substances vary greatly in stability under influences difficult to appreciate. I have two specimens of the gold yellow substance, C, both made in December, 1886, with the same proportions, under the same conditions. One has passed to dazzling white, normal silver, without falling to powder, or undergoing disaggregation of any sort; the fragments have retained their shape, simply changing to a pure frosted white, remaining apparently as solid as before; the other is unchanged, and still shows its deep yellow color and golden luster. Another specimen made within a few months and supposed to be permanent has changed to brown. Complete exclusion of air and light is certainly favorable to permanence.
Physical Condition.—The brittleness of the substances B and C, the facility with which they can be reduced to the finest powder, makes a striking point of difference between allotropic and normal silver. It is probable that normal silver, precipitated in fine powder and set aside moist to dry gradually, may cohere into brittle lumps, but these would be mere aggregations of discontinuous material. With allotropic silver the case is very different, the particles dry in optical contact with each other, the surfaces are brilliant, and the material evidently continuous. That this should be brittle indicates a totally different state of molecular constitution from that of normal silver.
Specific Gravities.—The allotropic forms of silver show a lower specific gravity than that of normal silver.
In determining the specific gravities it was found essential to keep the sp. gr. bottle after placing the material in it for some hours under the bell of an air pump. Films of air attach themselves obstinately to the surfaces, and escape but slowly even in vacuo.
Taken with this precaution, the blue substance, B, gave specific gravity 9.58, and the yellow substance, C, specific gravity 8.51. The specific gravity of normal silver, after melting, was found by G. Rose to be 10.5. That of finely divided silver obtained by precipitation is stated to be 10.62.1
I believe these determinations to be exact for the specimens employed. But the condition of aggregation may not improbably vary somewhat in different specimens. It seems, however, clear that these forms of silver have a lower specific gravity than the normal, and this is what would be expected.
Chestnut Hill, Philadelphia, May, 1889.—Amer. Jour. of Science.
Watts' Dict., orig. ed., v. 277.
In treating this subject it is necessary to limit it within comparatively narrow bounds, for bodies of the turpentine class are exceedingly numerous and not well understood. In this definite class turpentine means the exudation from various trees of the natural order Coniferæ, consisting of a hydrocarbon, C10H16, and a resin. The constitution of the hydrocarbons in turpentine from different sources, though identical chemically, varies physically, the boiling point ranging from 156° C. to 163° C., the density from 0.855 to 0.880, and the action on polarized light from -40.3 to +21.5. They are very unstable bodies in their molecular constitution, heat, sulphuric acid, and other reagents modifying their properties. The resins are also very variable bodies formed probably by oxidation of the hydrocarbons, and as this oxidation is more or less complete, mixtures are formed very difficult to separate and study.
Turpentine as met with in commerce is mainly derived from Pinus maritima, yielding French turpentine, and Pinus australis, furnishing most of the American turpentine. The latter is obtained from North and South Carolina, Georgia and Alabama. In Hanbury and Fluckiger's Pharmacographia there is a full description of the manner in which the trees are wounded to obtain the turpentine. Besides these there are Venice turpentine from the larch, Pinus Larix, Strassburg turpentine from Abies pectinata, and Canada balsam from Pinus balsamea.
The crude American turpentine is a viscid liquid of about the consistence of honey, but varying to a soft solid, known as gum, thus, according to the amount of exposure which it has undergone, it contains about 10 to 25 per cent. of "spirits," to which the name of turpentine is commonly given, the rest being resin, or as it is usually called, rosin.
In Liverpool almost all the spirits of turpentine comes from America, so that it is almost impossible to get a sample of French.
The terpene from American turpentine is called austraterebenthene. It possesses dextro-rotatory polarization of +21.5. Its density is 0.864. Boiling point 156° C.
In taking the boiling point of a commercial sample of spirits it is necessary to wait until the thermometer becomes steady. Not more than 5 per cent. should pass over before this takes place, and then there is not more than two or three degrees of rise until almost all is distilled over.
The liquids of lower boiling point do not appear to have been much studied. In French spirits they seem to be of the same composition as the main product, but with more action on polarized light.
French spirits of turpentine is mainly composed of terebenthene. The boiling point and sp. gr. are the same as those of the austraterebenthene, but the polarization is left handed and amounts to -40.5.
Isomeric modifications. Heated to 300° C. in a sealed tube for two hours, it becomes an isomeric compound, boiling at 175° C., while the density is lowered, being only 0.8586 at 0° C. The rotatory power is only -9°. It oxidizes much more rapidly. It is called isoterebenthene and has a smell of essential oil of lemons.
By the action of a small quantity of sulphuric acid, among other products terebene is formed. It has the same boiling point and sp. gr. as terebenthene, but is without action on polarized light. Austraterebenthene forms similar if not identical bodies.
Polymers. One part of boron fluoride BF3 instantly converts 160 parts of terebenthene into polymers boiling above 300° C., and optically inactive. H2SO4 does the same on heating and forms diterebene C20H32.
Terchloride of antimony does the same, and also produces tetraterebene C40H64, a solid brittle compound formed by the union of four molecules of C10H16. It does not boil below 350° C. and decomposes on heating.
Compound with H2O. Terpin C10H182HO is formed when 1 volume of spirits of turpentine is mixed with 6 of nitric acid and 1 of alcohol, and exposed to air for some weeks. Crystals are formed which are pressed, decolorized by animal charcoal, and recrystallized from boiling water.
Compounds with HCl. When a slow current of HCl is passed through cooled spirits of turpentine, two isomeric compounds are formed, one solid, and one liquid. The lower the temperature is kept, the more of the solid body is produced. To obtain the solid body pure it is pressed and recrystallized from ether or alcohol. It is volatile and has the odor of camphor. It is called artificial camphor, and has the composition C10H16HCl. There is also a compound with 2HCl.
Oxidation products. By passing air into spirits of turpentine oxygen is absorbed. It was thought at one time that ozone was produced, but Kingzett's view is that camphoric peroxide is formed C10H14O4, and that in presence of water it decomposes into camphoric acid and H2O2. This liquid constitutes the disinfectant known as "sanitas," which possesses the advantages of a pleasant smell and non-poisonous properties. C10H18O2 may be obtained by exposing spirits of turpentine in a flask full of oxygen with a little water.
Camphor C16H16O has been made in small quantity by oxidizing spirits of turpentine. Terebenthene belongs to the benzene or aromatic series, which can be shown from its connection with cymene. Cymene is methylpropyl-benzene, and can be made from terpenes by removing two atoms of H. It has not yet been converted again into terpene, but the connection is sufficiently proved. The presence of CH3 in terpenes is shown by their yielding chloroform when distilled with bleaching powder and water. The resin is imperfectly known. It was supposed to consist of picric and sylvic acids. It is also stated to contain abietic anhydride C44H62O4, but it is difficult to understand how a compound containing C44 can be produced from C10H16. The most probable view is that it is the anhydride of sylvic acid, which is probably C20H30O2.
The dark colored resin which is obtained when the turpentine is distilled without water can be converted into a transparent slightly yellow body by distillation with superheated steam. A small portion is decomposed, but the greater part distills unchanged. It is used in making soap which will lather with sea water.
When distilled alone, various hydrocarbons, resin oil and resin pitch, are obtained.
I find that commercial spirits of turpentine varies in sp. gr. from 0.865 to 0.869 at 15° C. The higher sp. gr. appears to be connected with the presence of resinous bodies, the result of oxidation. The boiling point is very uniform, ranging from 155° C. to 157° C. at 760 mm. Taking these two points together, it is hardly possible to adulterate spirits of turpentine without detection. I give the figures for a few imitations or adulterations:
| Sp. gr. | B.P. | |
| No. 1 | 0.821 | 137° C. |
| No. 2 | 0.884 | 165° C. |
| No. 3 | 0.815 | 150° C. |
| No. 4 | 0.895 | 156° C. |
There is a considerable difference in the flashing point, no doubt due to the longer or shorter exposure of the crude turpentine, by which more or less of the volatile portion escapes.
Read at a meeting of the Liverpool Chemists' Association.
It is well known that the paraffine obtained by the distillation of petroleum residues is crystalline, while that obtained directly (as in the filtration of residuum) is amorphous. Ozokerite or ceresine differs but slightly from paraffine, the principal distinction being want of crystalline structure in it as found. Other characteristics, such as the melting point, specific gravity, etc., vary in both, and so are not of importance in a comparison. Hence it has been asked, Is the paraffine occurring in petroleum and ozokerite identical with that which is produced by their distillation? As crystalline paraffine could be obtained from ozokerite by distillation alone, many persons have supposed that it was engendered in the process. Recently, however, crystalline paraffine has been obtained from ozokerite by dissolving the latter in warm amyl alcohol; on cooling the greater part separates out in crystals having the luster of mother-of-pearl. By repetition of this process, a substance is obtained that is scarcely to be distinguished from the paraffine obtained by distillation. Apparently there exists then in ozokerite, together with paraffine, other substances not capable of crystallization which keep the paraffine from crystallizing. These colloids appear to be separated by amyl alcohol in virtue of their greater solubility in that menstruum. It is also reasonable to suppose that they undergo change or decomposition by distillation.
So as petroleum residues are amorphous, and the crystalline paraffine is first produced by distillation, it has been argued that the paraffine present in crude petroleum is approximately the same thing as ozokerite.
This, however, is not sufficient to establish the pyrogenic origin of all crystallized paraffine, as crystals can be obtained from the amorphous residues by distillation at normal or reduced pressure or in a current of steam. To explain these facts two assumptions are possible. Either the chemical and physical properties of all or some of the solid constituents are changed by the distillation, and the paraffine is changed from the amorphous into the crystalline variety, or the change produced by the distillation takes place in the medium (i.e., the mother liquid) in which the paraffine exists. The change effected in ozokerite and in petroleum residues when crystalline paraffine is obtained by distillation is to be regarded as a purification, and can be effected partially by treatment with amyl alcohol. In the same way, by repeated treatment of petroleum residuum with amyl alcohol, a substance of melting point 59° C. can be obtained, which cannot be distinguished from ordinary paraffine.
The treatment with amyl alcohol has therefore accomplished the same results as was obtained by distillation, and the action is probably the same, i.e., a partial separation of colloid substance. These facts point to the conclusion that crystallizable paraffine exists ready formed in both petroleum and in ozokerite, but in both cases other colloidal substances prevent its crystallization. By distillation, these colloids appear to be destroyed or changed so as to allow the paraffine to crystallize.
It is a generally known fact that liquids always appear among the products of the distillation of paraffine, no matter in what way the distillation be conducted. This shows that some paraffine is decomposed in the operation.
The name proto-paraffine has been given to ozokerite and to the paraffine of petroleum in contradistinction to pyro-paraffine, the name that has been applied to the paraffine obtained by distillation from any source.
According to Reichenbach, paraffine may crystallize in three forms: needles, angular grains, and leaflets having the luster of mother-of-pearl. Hofstadter, in an article on the identity of paraffine from different sources, confirmed this statement, and added further that at first needles, then the angular forms, and then the leaflets are formed. Fritsche found, by means of the microscope, in the ethereal solution of ozokerite, very fine and thin crystal leaflets concentrically grouped, and in the alcoholic solution fine irregular leaflets. Zaloziecki has recently developed these microscopic investigations to a much greater extent. According to this observer, the principal part of paraffine, as seen under the microscope, consists of shining stratified leaflets with a darker edge. The most characteristic and well developed crystals are formed by dissolving paraffine in a mixture of ethyl and amyl alcohols and chilling. The crystals are rhombic or hexagonal tablets or leaves, and are quite regularly formed. They are unequally developed in different varieties of paraffine. The best developed are those obtained from ceresine. Their relative size and appearance give an indication as to the purity of the paraffine, and, as they are always present, they are to be counted among the characteristic tests for paraffine. Reichenbach observed that mere traces of empyreumatic oil prevented their formation.
The old method of determining the amount of paraffine in petroleum was to carry out the refining process on a small scale; that is, to distill the residue from the kerosene oils to coking, chill out the paraffine, press it thoroughly between filter paper, and weigh the residue. The sources of error in this procedure are manifold; the principal one is the solubility of paraffine in oils, which depends upon the character of both the paraffine and the oil, and also upon the temperature. The next greatest source of error is variation in the process of distillation and the difference between working on the small scale and on the large scale.
In most cases, where a paraffine determination is to be carried out, one has to deal with a mixture of paraffine with liquid oils. Now, paraffine is not a substance defined by characteristic physical properties which distinguish it from the liquid portions of petroleum. It consists of a mixture of homologous hydrocarbons, which form a solid under ordinary conditions. The hydrocarbons of this mixture show a gradation in their properties, and gradually approximate to those which are liquid at ordinary temperatures. It is a well known fact that a separation of these homologues is entirely impossible by distillation. It has also been ascertained that the liquid constituents of petroleum do not always possess boiling points that are lower than those of the solid constituents. This shows that we have to deal not merely with hydrocarbons of one, but of several series.
When determinations of the amount of paraffine are to be made, then it becomes necessary to specify with exactness what is to be called paraffine. The most definite property that can be made use of for this purpose is the melting point. For several reasons it is convenient to include under this name hydrocarbons of melting point as low as 35°-40° C.
The method proposed by Zaloziecki for the determination of paraffine is the following: The most volatile portions of the petroleum are separated by distillation, until the thermometer shows 200° C. These portions are separated, as they exert great solvent action upon paraffine. At the same time he finds that no pyro-paraffine is formed under this temperature. A weighed portion of the residue is taken and mixed with ten parts by weight of amyl alcohol and ten parts of seventy-five per cent. ethyl alcohol: the mixture is then chilled for twelve hours to 0° C. It is then filtered cold, washed first with a mixture of amyl and ethyl alcohols, and then with ethyl alcohol alone. The paraffine is transferred to a small porcelain evaporating dish and dried at 110° C. It is then heated with concentrated sulphuric acid to 150°-160° C. for fifteen to thirty minutes with constant stirring. The acid is then neutralized and the paraffine extracted by petroleum ether. On evaporation of the solvent, the paraffine is dried at 100° C. and weighed. Zaloziecki found, according to this method, in three samples of Galician petroleums, 4.6, 5.8 and 6.5 per cent., respectively, of proto-paraffine. The method was carried out as above with four samples of American petroleums, Colorado oil from Florence, Col.; Warren County oil from Wing Well, Warren, Pa.; Washington oil from Washington County, Pa.; Middle District oil from Butler County, Pa., all furnished by Professor Sadtler.
They were very different in physical properties and in appearance, the Colorado oil being a much heavier oil than the others and the Washington oil being an amber oil, while the other two were of the ordinary dark green color and consistence. The losses on distillation to 200° C. were very different, being about one-tenth in the case of the Colorado oil and nearly one-half in the case of the others. The percentages of partially refined proto-paraffine in the four reduced oils (all below 200° C. off) were as follows: for the Colorado oil, 23.9 per cent.; for the Warren oil, 26.5 per cent.; for the Washington oil, 26.6 per cent.; and for the Middle District oil, 28.2 per cent.
The question now arises, What value has this determination of the proto-paraffine which may exist in an oil? As before said, a portion of the paraffine is always decomposed in distillation at temperatures sufficiently high to drive over the paraffine oils, so the yield of pyro-paraffine is always less than the proto-paraffine shown to be present originally. Zaloziecki found this in the case of the several Galician oils he examined. Corresponding to the 4.6, 5.8 and 6.5 per cent. of proto-paraffine in the several oils he obtained 2.18, 2.65 and 2.35 per cent., respectively, of pyro-paraffine.
For the present, however, the extraction of proto-paraffine on a large scale by means of such solvents as amyl and ethyl alcohols is out of the question on account of their cost. A distillation, under reduced pressure and with superheated steam, would, however, prevent much of the decomposition of the original proto-paraffine and increase the yield of pyro-paraffine.
This study of Zaloziecki's method and the examination of American oils was suggested by Professor Sadtler and carried out in his laboratory.
An abstract of thesis by E.A. Partridge, class of '89, Univ. of Pa. Read before the Chemical Section of the Franklin Institute by Prof. S.P. Sadtler.
The young student of physics occasionally has difficulty in grasping the laws of pressure in fluids. His every day experience has taught him that a push against a solid body causes it to push in the same direction, and he often receives with some doubt the statement that pressure applied to a fluid is transmitted equally in every direction. The experiments ordinarily shown in illustration of this principle prove that pressure is transmitted in all directions, but do not prove the equality of transmission, and in spite of all the text books may tell him, the student is apt to cling to the idea that a downward pressure applied to a liquid is more apt to burst the bottom than the side of the containing vessel.
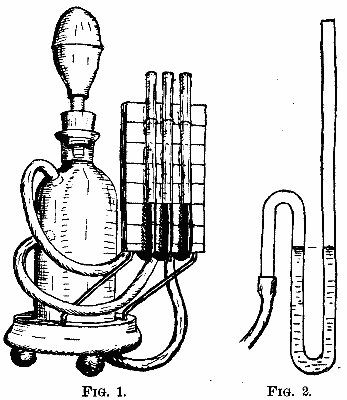
The little piece of apparatus shown in Fig. 1 was designed to furnish a clear demonstration of the principle under consideration. It is essentially an arrangement by which a downward pressure is applied to a confined mass of air or water, and the resultant pressures measured in the three directions, down, up, and sideways. By means of a broken rat tail file kept wet with turpentine three holes are bored through a bottle, one through the bottom, one through the side, and one through the shoulder, as near the neck as may be convenient. The operation is quick and easy, the only precaution to be observed being to work very slowly and use but a slight pressure when the glass is nearly perforated. The holes may be enlarged to any size required by careful filing with the wet file. From each of the holes a rubber tube leads to one of the glass manometer tubes at the right in the figure, the joints being made air tight by slipping into each rubber tube a piece of glass tubing about half an inch long in order to swell it to the size of the hole it is to fit. The ends of these glass tubes must be well rounded by partial fusion in a gas flame, that there may be no sharp edges to cut the rubber. The bottle rests in a depression in the turned wood base, the lower rubber tube passing out through a hole in the wood. Fig. 2 shows the shape of the manometer tubes. They are made of quarter inch glass tubing bent to shape in a flame and left open at both ends. They are mounted on a scale board which has several equidistant horizontal lines running across it. The two bent wires which support the scale board fit loosely in holes in it and in the base. This method of mounting is very handy, since it permits the scale board to be swung to right or left as may be convenient, or turned round so as to show the fittings on its back, without moving the bottle. The three manometers are filled to the same level with mercury, the quantity being adjusted by means of a pipette. A perforated rubber stopper, fitted with a glass tube on which is slipped a rubber syringe bulb, completes the apparatus.
When the bulb is pinched between the fingers, the mercury is forced up to the same height in each of the manometers, thus proving that the pressure is exerted equally in the three directions, up, down, and sideways. With the bottle filled with water the same effect follows, the law being the same for liquids and gases. When using water in the apparatus it is essential that the rubber tubes, as well as the bottle, be filled, and when used in the class room it is better to show the experiment with water first, it being easier and quicker to empty the bottle and tubes than to fill them.
Although well known to fruit growers and generally represented in all parts of Britain, this noble French pear has not become a universal favorite. If the quality of the fruit, independently of its fine, handsome appearance, was bad, or even indifferent, it might be exterminated from our lists, but this we know is not the case, as any one who has tasted good samples grown in France, the Channel Islands, and upon favorable soils in this country will bear out the statement that the flavor is superb. Some fruits, we know, are quite incapable of being good, as they have no quality in them; but here we have one of the hardiest of trees, capable of giving us quantity as well as quality, provided we cultivate properly. Pears, no doubt, are capricious, like our seasons, but given a good average year, soils and stocks which suit them, a light, warm, airy aspect, and good culture, a great number of varieties formerly only good enough for stewing are now elevated, and most deservedly so, to the dessert table. But, assuming that some sorts known to be good do not reach their highest standard of excellence every year, they are infinitely superior to many of the old stewers, as they carry their own sugar, a quality which fits them for consumption by the most delicate invalids. Indeed, so prominently have choice dessert pears, and apples too for that matter, come to the front for cooking purposes, that a new demand is now established, and although Duchesse d'Angoulême, always juicy and sweet, from bad situations does not always come up to the fine quality met within Covent Garden in November, it is worthy of our skill, as we know it has all the good points of a first rate pear when properly ripened.
The original tree of this pear was observed by M. Anne Pierre Andusson, a nurseryman at Angers, growing in a farm garden near Champigne, in Anjou, and having procured grafts of it, he sold the trees, in 1812, under the name of Poire des Eparannais. In 1820, he sent a basket of the fruit to the Duchesse d'Angoulême, with a request to be permitted to name the pear in honor of her. The request was granted, and the pear has since borne its present name.
That such a fine pear, which does so well in France, would soon find its way to England there exists little doubt, as we find that within a few years it became established and well known throughout the United Kingdom. All the earliest trees would be worked upon the pear or free stock, and as root pruning until recently was but little practiced, we may reasonably suppose that the majority of them are deeply anchored in clay, marl, and other subsoils calculated to force a crude, gross growth from which high flavored fruit could not be expected. These defects under modern culture upon the quince and double grafting are giving way, as we find, on reference to the report of the committee of the pear conference, held at Chiswick in 1885, that twenty counties in England, also Scotland, Ireland, and Wales, contributed no less than 121 dishes to the tables, and thirty-eight growers voted in favor of the Duchesse being recognized as one of our standard dessert varieties. This step looks like progress, as it is a record of facts which cannot be gainsaid, and it now remains to be seen whether the English grower, whose indomitable will has brought him to the front in the subjugation of other fruits, will be successful with the fine Duchesse d'Angoulême. Although this remarkable pear cannot easily be mistaken, for the benefit of those who do not know it, the following description may not be out of place. Fruit large, often very large, 3½ inches wide and 3 inches to 4 inches high, roundish obovate, uneven, and bossed in its outline. Skin greenish yellow, changing to pale dull yellow, covered with veins and freckles of pale brown russet, and when grown against a south wall it acquires a brown cheek. Eye open, with erect dry segments, set in a deep irregular basin. Stalk 1 inch long, inserted in a deep irregular cavity. Flesh white, buttery, and melting, with a rich flavor when well ripened; otherwise rather coarse grained and gritty.
As to culture, experienced fruitists say the tree grows vigorously and well. It bears abundantly, and succeeds either on the pear or quince stock, forming handsome pyramids, but is better on the quince. Here, then, we have the key to the secret of success: The cordon on the quince; roots near the surface; loam, sound, sandy, and good; and good feeding. Aspect, a good wall facing south or west—the latter, perhaps, the best. Those who have not already done so, should try trees on the quince as pyramids and bushes, as this, like some other capricious pears, although the fruit be smaller, may put in better flavor than is met with in fruit from hot walls.—The Garden.
The following is from an address delivered by Mr. Robert Douglas before the Association of American Nurserymen at the meeting in Chicago recently.
It is the prevailing and almost universal belief that when native forests are destroyed they will be replaced by other kinds, for the simple reason that the soil has been impoverished of the constituents required for the growth of that particular tree or trees. This I believe to be one of the fallacies handed down from past ages, taken for granted, and never questioned. Nowhere does the English oak grow better than where it grew when William the Conqueror found it at the time he invaded Britain. Where do you find white pines growing better than in parts of New England where this tree has grown from time immemorial? Where can you find young redwoods growing more thriftily than among their giant ancestors, nearly or quite as old as the Christian era?
The question why the original growth is not reproduced can best be answered by some illustrations. When a pine forest is burned over, both trees and seeds are destroyed, and as the burned trees cannot sprout from the stump like oaks and many other trees, the land is left in a condition well suited for the germination of tree seeds, but there are no seeds to germinate. It is an open field for pioneers to enter, and the seeds which arrive there first have the right of possession. The aspen poplar (Populus tremuloides) has the advantage over all other trees. It is a native of all our northern forests, from the Atlantic to the Pacific. Even fires cannot eradicate it, as it grows in moist as well as dry places, and sprouts from any part of the root. It is a short-lived tree, consequently it seeds when quite young and seeds abundantly; the seeds are light, almost infinitesimal, and are carried on wings of down. Its seeds ripen in spring, and are carried to great distances at the very time when the ground is in the best condition for them. Even on the dry mountain sides in Colorado, the snows are just melting and the ground is moist where they fall.
To grow this tree from seed would require the greatest skill of the nurseryman, but the burnt land is its paradise. Wherever you see it on high, dry land you may rest assured that a fire has been there. On land slides you will not find its seeds germinating, although they have been deposited there as abundantly as on the burned land.
Next to the aspen and poplars comes the canoe birch, and further north the yellow birch, and such other trees as have provision for scattering their seeds. I have seen acorns and nuts germinating in clusters on burned lands in a few instances. They had evidently been buried there by animals and had escaped the fires. I have seen the red cherry (Prunus Pennsylvanica) coming up in great quantities where they might never have germinated had not the fires destroyed the debris which covered the seed too deeply.
A careful examination around the margin of a burned forest will show the trees of surrounding kinds working in again. Thus by the time the short-lived aspens (and they are very short-lived on high land) have made a covering on the burned land, the surrounding kinds will be found re-established in the new forest, the seeds of the conifers, carried in by the winds, the berries by the birds, the nuts and acorns by the squirrels, the mixture varying more or less from the kinds which grew there before the fire.
It is wonderful how far the seeds of berries are carried by birds. The waxwings and cedar birds carry seeds of our tartarean honeysuckles, purple barberries and many other kinds four miles distant, where we see them spring up on the lake shore, where these birds fly in flocks to feed on the juniper berries. It seems to be the same everywhere. I found European mountain ash trees last summer in a forest in New Hampshire; the seed must have been carried over two miles as the crow flies.
While this alternation is going on in the East, and may have been going on for thousands of years, the Rocky Mountain district is not so fortunate. When a forest is burned down in that dry region, it is doubtful if coniferous trees will ever grow again, except in some localities specially favored. I have seen localities where short-lived trees were dying out and no others taking their places. Such spots will hereafter take their places above the timber line, which seems to me to be a line governed by circumstances more than by altitude or quality of soil.
There are a few exceptions where pines will succeed pines in a burned-down forest. Pinus Murrayana grows up near the timber line in the Rocky Mountains. This tree has persistent cones which adhere to the trees for many years. I have counted the cones of sixteen years on one of these trees, and examined burned forests of this species, where many of the cones had apparently been bedded in the earth as the trees fell. The heat had opened the cones and the seedlings were growing up in myriads; but not a conifer of any other kind could be seen as far as the fire had reached.
In the Michigan Peninsula, northern Wisconsin and Minnesota, P. Banksiana, a comparatively worthless tree, is replacing the valuable red pine (P. resinosa), and in the Sierras P. Murrayana and P. tuberculata are replacing the more valuable species by the same process.
In this case, also, the worthless trees are the shortest lived. So we see that nature is doing all that she can to remedy the evil. Man only is reckless, and especially the American man. The Mexican will cut large limbs off his trees for fuel, but will spare the tree. Even the poor Indian, when at the starvation point, stripping the bark from the yellow pine (P. ponderosa), for the mucilaginous matter being formed into sap wood, will never take a strip wider than one third the circumference of the tree, so that its growth may not be injured.
We often read that oaks are springing up in destroyed forests where oaks had never grown before. The writers are no doubt sincere, but they are careless. The only pine forests where oaks are not intermixed are either in land so sandy that oaks cannot be made to grow on them at all, or so far north that they are beyond their northern limit. In the Green Mountains and in the New England forests, in the pine forests in Pennsylvania, in the Adirondacks, in Wisconsin and Michigan—except in sand—I have found oaks mixed with the pines and spruces. In northwestern Minnesota and in northern Dakota the oaks are near their northern limit, but even there the burr oak drags on a bare existence among the pines and spruces. In the Black Hills, in Dakota, poor, forlorn, scrubby burr oaks are scattered through the hills among the yellow pines. In Colorado we find them as shrubs among the pines and Douglas spruces. In New Mexico we find them scattered among the piñons. In Arizona they grow like hazel bushes among the yellow pines. On the Sierra Nevada the oak region crosses the pine region, and scattering oaks reach far up into the mountains. Yet oaks will not flourish between the one hundredth meridian and the eastern base of the Sierras, owing to the aridity of the climate. I recently found oaks scattered among the redwoods on both sides of the Coast Range Mountains.
Darwin has truly said, "The oaks are driving the pines to the sands." Wherever the oak is established—and we have seen that it is already established whereever it can endure the soil and climate—there it will remain and keep on advancing. The oak produces comparatively few seeds. Where it produces a hundred, the ash and maple will yield a thousand, the elm ten thousand, and many other trees a hundred thousand. The acorn has no provision for protection and transportation like many tree seeds. Many kinds are furnished with wings to float them on the water and carry them in the air. Nearly every tree seed, except the acorn, has a case to protect it while growing, either opening and casting the seeds off to a distance when ripe or falling with them to protect them till they begin to germinate. Even the equally large seeds of other kinds are protected in some way. The hickory nut has a hard shell, which shell itself is protected by a strong covering until ripe. The black walnut has both a hard shell and a fleshy covering. The acorn is the only seed I can think of which is left by nature to take care of itself. It matures without protection, falls heavily and helplessly to the ground, to be eaten and trodden on by animals, yet the few which escape and those which are trodden under are well able to compete in the race for life. While the elm and maple seeds are drying up on the surface, the hickories and the walnuts waiting to be cracked, the acorn is at work with its coat off. It drives its tap root into the earth in spite of grass, and brush, and litter. No matter if it is shaded by forest trees so that the sun cannot penetrate, it will manage to make a short stem and a few leaves the first season, enough to keep life in the root, which will drill in deeper and deeper. When age or accident removes the tree which has overshadowed it, then it will assert itself. Fires may run over the land, destroying almost everything else, the oak will be killed to the ground, but it will throw up a new shoot the next spring, the root will keep enlarging, and when the opportunity arrives it will make a vigorous growth, in proportion to the strength of the root, and throw out strong side roots, and after that care no more for its tap root, which has been its only support, than the frog cares for the tail of the tadpole after it has got on its own legs.
There is no mystery about the succession of forest growths, nothing in nature is more plain and simple. We cannot but admire her wisdom, economy, and justness, compensating in another direction for any disadvantage a species may have to labor under. Every kind of tree has an interesting history in itself. Seeds with a hard shell, or with a pulpy or resinous covering which retards their germination, are often saved from becoming extinct by these means.
The red cedar (Juniperus Virginiana) reaches from Florida to and beyond Cape Cod; it is among the hills of Tennessee, through the Middle States and New England. It is scattered through the Western States and Territories, at long distances apart, creeping up the Platte River, in Nebraska. (I found only three in the Black Hills, in Dakota, in an extended search for the different trees which grow there. Found only one in a long ramble in the hills at Las Vegas, New Mexico.) Yet this tree has crept across the continent, and is found here and there in a northwesterly direction between the Platte and the Pacific Coast. It is owing to the resinous coating which protects its seeds that this tree is found to-day scattered over that immense region.
I have thought that an example of the intelligence (instinct?) of a class of fish which has come under my observation during my excursions into the Adirondack region of New York State might possibly be of interest to your readers, especially as I am not aware that any one except myself has noticed it, or, at least, has given it publicity.
The female sun-fish (called, I believe, in England, the roach or bream) makes a "hatchery" for her eggs in this wise. Selecting a spot near the banks of the numerous lakes in which this region abounds, and where the water is about 4 inches deep, and still, she builds, with her tail and snout, a circular embankment 3 inches in height and 2 thick. The circle, which is as perfect a one as could be formed with mathematical instruments, is usually a foot and a half in diameter; and at one side of this circular wall an opening is left by the fish of just sufficient width to admit her body, thus:
The mother sun-fish, having now built or provided her "hatchery," deposits her spawn within the circular inclosure, and mounts guard at the entrance until the fry are hatched out and are sufficiently large to take charge of themselves. As the embankment, moreover, is built up to the surface of the water, no enemy can very easily obtain an entrance within the inclosure from the top; while there being only one entrance, the fish is able, with comparative ease, to keep out all intruders.
I have, as I say, noticed this beautiful instinct of the sun-fish for the perpetuity of her species more particularly in the lakes of this region; but doubtless the same habit is common to these fish in other waters.
William L. Stone.
Jersey City Heights, N.J.
Among the many traces which man has left of his existence in long past ages on the face of the earth, says a correspondent of the Scotsman, none are more interesting and instructive than the lake dwellings of Switzerland and other countries, which have been discovered within the last fifty years or so. Although these relics of the past are far more modern than those which we referred to in a late article on "Primeval Man," and are probably included within the range of Egyptian and other chronologies, yet they stretch far beyond the historic period, so far as Europe is concerned, and throw a flood of light on the habits of our ancestors, or at any rate predecessors, in these regions. We are tolerably well acquainted with the history of the Jews when David worked his way up from the shepherd's staff to the royal scepter, or when Joshua drove out the Canaanites and took possession of their land, but of what was going on in Europe in these times we have hitherto had no knowledge whatever. These lake dwellings, however, were in all probability inhabited by human beings somewhere about the time when the events we have referred to took place, and may have been inhabited before the earlier of them.
The first hint we had of the existence of these remarkable dwellings was obtained in 1829, when an excavation was being made on the shore of a Swiss lake. Some wooden piles, apparently very old, and other antiquities were found by the workmen. Not much attention, however, was paid to this discovery till 1854, when a Mr. Aeppli drew attention to some remains of human handiwork found near his house, in part of the bed of a lake which had been left dry during a season of great drought. The workmen employed in recovering some land from the lake found the heads of a great many wooden piles protruding through the mud, and also a number of stags' horns, and implements of various descriptions. Stimulated by this discovery, search was made in various lakes, and the result was truly astonishing. In every direction remains of the habitations of prehistoric man were discovered, and relics were found in such abundance that the history of this unknown past could be traced through long ages, and the habits of the people ascertained with a very considerable amount of probability. The details are so numerous that it would be impossible in the space at our disposal to go into them all.
Of course, during the long time that has elapsed since these structures were erected, their remains have been reduced to mere ruins, and it is only by comparing one with another that we are able to picture to ourselves what they were originally like and what sort of life was led by the men who inhabited them. The oldest of these dwellings belong to the stone age, when man had not acquired any knowledge of the use of metal; when all his instruments were merely sharpened stones, fixed in wooden handles, or pieces of bone, horn, or other natural material. They are therefore somewhat roughly finished, but at the same time exhibit considerable ingenuity and skill. The method of construction seems to have been somewhat as follows: A suitable situation, not far from the shore, where the water was not very deep, having been fixed upon, these prehistoric builders drove into the muddy bottom of the lake a number of piles or long stakes, arranged generally pretty close together, and in some sort of regular order. These piles were formed generally from stems of trees, with the bark on, but occasionally from split wood. The ends were sharpened to a point by the aid of fire or by cutting with stone axes. On a sufficient number being driven in, and their upper ends brought to a level above the surface of the water, platform beams were laid across, fastened by wooden pegs, or in some cases fixed into notches cut in the heads of the vertical piles. The platform was generally very roughly made, just a series of unbarked stems placed side by side and covered with layers of earth or clay, with numerous openings through which refuse of all kinds fell into the water beneath. In many cases connection with the shore was made by means of a narrow bridge or gangway, constructed in the same manner. On this rude platform huts were erected by driving small piles or stakes which projected above the floor, and to these were fastened boards standing edgeways like the skirting of our ordinary rooms, and marking out the size of each building. The walls of the huts were formed of small branches of twigs interwoven and plastered over with clay. The roof was made of straw or reeds like a thatched cottage. In size these huts were probably eighteen to twenty feet long, eight or ten feet broad, and about six feet high. They may have been divided into rooms, but there is no evidence of this. Each was provided with a hearth formed of three or four slabs of stone. The number of huts in each settlement must have been considerable, in fact, they must have formed villages of no mean extent, for as many as forty, fifty, or even a hundred thousand piles have been found spread over a large extent of ground, forming the foundation of one such settlement. It is probable, however, that these were not so numerous when first erected, but were gradually added to as the population increased. This fact, along with many others, shows that these dwellings were inhabited for long periods of time, during which the population pursued their ordinary life in comparative peace and quietness in their island homes.
Such is, in brief, a general account of these remarkable structures. Of course there were several variations in the methods of fixing these piles, one of which may be mentioned as showing the ingenuity of the builders. Where the piles did not get a firm hold of the lake bottom, they carried out in boats or rafts loads of stones, which they threw down between the piles, thus firmly fixing them, just as modern engineers sometimes do for a similar purpose. As to the habits of the people who dwelt in these lake dwellings, we get a considerable amount of information from the various implements, refuse, etc., which fell through the imperfectly closed platforms into the lake, and which have been preserved in the mud at the bottom. They were fishers, hunters, shepherds, and agriculturists. Skeletons of fish are found in large abundance, and in some settlements even the fishing nets, and hooks made of boar's tusks, have been discovered. Then again there is an abundance of remains of the hunter's feast; bones of the stag, wild boar, bear, wolf, otter, squirrel, and many other wild animals are found in rich profusion, and often these are split and the marrow extracted. These ancient men, however, did not entirely rely on such precarious provision for their wants, but were so far advanced in civilization that they kept cattle and domestic animals of various kinds. They possessed dogs in great numbers, as well as cows, sheep, goats, and pigs, and in winter time had these housed on their settlements, as among the remains found are litters of straw, etc., which had evidently served as bedding for these animals. This, of course, necessitated the gathering of grass or other material for their food. They also cultivated wheat, barley, flax, and a number of other vegetable products. Their methods of cultivation were no doubt very rude, consisting of a mere scratching of the ground with crooked branches of trees or with simple instruments made of stags' horn; but, nevertheless, they succeeded in getting very good results. Among the relics which they have left are found stones for crushing corn, the grain which they used, and even the very cakes or bread which they made. There are also fruits, such as the apple, pear, nut, etc.; so that the bill of fare of prehistoric man was by no means contemptible. He had fish, game, beef, mutton, pork, bread, and fruit, besides a plentiful supply of water from the lake at his door. He was acquainted with the potter's art, and manufactured earthen vessels of various kinds. He seems to have produced two kinds—a coarser and a finer; the former made from clay mixed with a quantity of grains of stone, and the latter of washed loam. These he ornamented in an elementary fashion with certain lines and marks. Some of the vessels he used have been found with a burnt crust of the porridge which he had been making adhering. As to his clothes, these were probably formed in great part from the skins of wild or domestic animals, but he also used fabrics made from flax, which he had learned to weave, as remains of cloth, twine, rope, etc., are not infrequently found in his dwellings.
One prominent feature in the history of these lake dwellers is their gradual advance in the arts of civilization. While the main features of their settlements remain very much the same during the whole period of their residence, there is a gradual improvement in the details; the settlements become larger, and the implements, etc., better finished. And this is especially observable in the change of material which the dweller uses. In the earlier stages of his existence stone is the predominant feature, all his knives, saws, chisels, axes, etc., are made from this substance; but as time rolls on, one or two implements are found made of bronze, which is a mixture of tin and copper, and requires for its production a certain amount of knowledge and mechanical skill. Gradually the number of bronze implements increases until eventually stone is superseded altogether, and improved forms of weapons of war make their appearance, and his work has a more finished look, arising from his improved implements. Whether the manufacture of bronze was an original discovery of his own, or whether it was an importation from some more advanced race, is not certainly known; but as he undoubtedly had intercourse with the East, it is probable that the first bronze was imported, and that afterward he discovered the way to manufacture it himself. However this may be, it seems evident that the introduction of this material greatly aided his development. As stone gave place to bronze, so in the course of time this latter gave place to iron, probably introduced in the same manner some considerable time before the dawn of history; and this metal held its place until these habitations were finally abandoned.
With regard to the religion of these lake dwellers, if they had any, nothing is known. From some curious objects formed somewhat like the crescent of the moon, which are found in considerable numbers, it has been supposed that they worshiped that body; but there seems to be really no evidence for this supposition, and these objects may only have been ornaments, or perhaps charms, fixed above the doors of their huts something after the manner of the horse shoe nailed over the door in modern times to keep away evil spirits. So far as can be inferred from the remains that have been examined, the same race seems to have inhabited these dwellings from their commencement to their end. There is no appearance of invasion from without; all seems continuous. Probably his race came in early time from the East, and were a pastoral people, with flocks, herds, and domestic animals, and built their peculiar habitations to protect themselves from human enemies. Certainly the arrangements were well fitted for the purpose in those days, when the club and the spear were almost the only weapons of offense. Dr. Keller, who has investigated this subject with great care, is of the opinion that these lake dwellers were a branch of the great Celtic race.
The best feed for young turkeys and ducks is yelks of hard-boiled eggs, and after they are several days old the white may be added. Continue this for two or three weeks, occasionally chopping onions fine and sometimes sprinkling the boiled eggs with black pepper; then give rice, a teacupful with enough milk to just cover it, and boil slowly until the milk is evaporated. Put in enough more to cover the rice again, so that when boiled down the second time it will be soft if pressed between the fingers. Milk must not be used too freely, as it will get too soft and the grains will adhere together. Stir frequently when boiling. Do not use water with the rice, as it forms a paste and the chicks cannot swallow it. In cold, damp weather, a half teaspoonful of Cayenne pepper in a pint of flour, with lard enough to make it stick together, will protect them from diarrhea. This amount of food is sufficient for two meals for seventy-five chicks. Give all food in shallow tin pans. Water and boiled milk, with a little lime water in each occasionally, is the best drink until the chicks are two or three months old, when loppered and buttermilk may take the place of the boiled milk. Turkeys like best to roost on trees, and in their place artificial roots may be made by planting long forked locust poles and laying others across the forks.—American Agriculturist.
Keep the turkey hens tame by feeding them close to the house. Have two or three barrels in sheltered corners containing plenty of straw or leaves for them to lay in. Gather the eggs every evening, as turkey eggs are very easily chilled. Keep the eggs in a woolen cloth on end and turn them every three days. Set the first seven eggs under a chicken hen, as they get too old before the turkey hen will go to sitting. Make a board pen ten or twelve feet square and twelve or fourteen inches high. Put a coop in it and put your hen and turkeys in it. Feed the hen with corn and the turkeys soaked wheat bread (corn meal will kill them), until they are a week old (I feed five or six times a day). Then feed wheat until they are big enough to eat corn. Give plenty of fresh water in a shallow vessel. Keep the mother in the pen until they are large enough to fly over the top of the boards. Let them out awhile about the middle of the day. Shut them in at night. A turkey hen does not like to be shut up, but have a good big coop for her and she will go in. Don't let the little turkeys get their backs wet until they are feathered. The turkey hen will sit down when night comes just where she happens to be, but if you drive her home a few times she will come herself after that. Always feed them when they come home, no matter if they are full of "hoppers." Have your No. 2 pen in the orchard under an apple tree where it is shady. Have the turkey hen's pen close to the chicken hen's pen, so that when the chicken hen weans her turkeys, they will soon learn to go with the turkey hen. Give them a dose of black pepper in their feed every cold rain. And never, no never, get excited and in a hurry while working with turkeys if you don't want them to get wild and fly all over the plantation. Three or four weeks before selling, feed all the corn they will eat.
Restrain your desire to count your young turkeys, and let them alone for twenty-four hours after they get into this world. Remove them to a clean, airy, roomy coop, and give them boiled eggs, stale wheat bread crumbs just moistened with milk or water, "Dutch" cheese, or a mixture of all these.
For the first two weeks feed entirely with the eggs, bread, curds, cooked rice and cooked oatmeal. About the third week commence feeding cooked cornmeal; and from that on they may have any cooked food that would be suitable for chickens of the same age. Season all food slightly with salt and pepper, and twice a week add a level tablespoonful of bone meal to a pint of feed. Never feed any sour food or sloppy food of any kind, except sour milk, and never feed any uncooked food of any kind until after they have thrown out the red on their heads. Feed often, five or six times a day, until after they are three months old; then, if insects are numerous, you may gradually reduce the number of meals per day to three or even two.
After they are three months old they may be given wheat, cracked corn, etc., but not whole corn until they are five months old. Keep the coops dry and clean, and the turkeys out of the dew and rain until they are fully feathered, and have thrown out the red. Dampness and filth will kill young turkeys as surely as a dose of poison. For the first few days confine the poults to the limits of the coop and safety run; then, if all appear strong and well, give the mother hen and her brood liberty on pleasant days after the dew is off.
If they get caught out in a shower, get them to shelter as soon as possible; and if they are chilled take them to the house and thoroughly dry and warm them. See that the little turkeys come home every night. The turkey mother must, for the first few nights, be hunted up and driven home. After they are three months old, turkeys are quite hardy, and may be allowed range at all times. If turkeys that are well cared for, and have always seemed all right, show signs of drooping when about six weeks or two months old, give Douglas mixture in the drink or food, and add a little cooked meat to the food once a day.—The Practical Farmer.
For an ordinary place, select from a good breed (I prefer the bronze) a large gobbler and two or three hens. As soon as the warm weather comes, place about the barn in sheltered places two or three barrels on their sides, and in them make nice nests. In these the hens will lay. Gather the eggs every day, keeping them in a cool place. When a box contains 23 eggs mark it No. 1 and begin to fill a second box, and when it contains 23 eggs mark it No. 2 and so continue. It is well to leave turkey hens on the nest two or three days, for they often lay one or two eggs after they begin to show signs of sitting.
When you have decided to sit a hen, give her a good nest and 15 eggs and at the same time give a common hen eight eggs. These, when hatched, are all to be given to the turkey hen. Never try to raise turkeys with a domestic fowl. If you have no place free of grass, you can start turkeys with difficulty. Feeding is of the greatest importance. For the first week I have found wheat bread moistened in water the most satisfactory. If you can feed them by sunrise for the first three or four weeks, you need lose hardly a bird. Each evening try and call them nearer and nearer home, so that you will not be troubled with their wandering to the neighbors'. As early as possible train them to roost high, so as to be out of danger at night. Bird dogs are often very destructive to turkeys, at times destroying a whole flock in a single night. Fatten with corn. The turkey crop ought to be one of the most profitable on our farms.
Dr. G.G. Groff.
Pennsylvania.
Turkeys want care, especially for the first two or three weeks. I feed graham and wheat bread, made by scalding the flour, making a very stiff dough, and baking in a hot oven; soak over night in cold water. I also give them plenty of young onions, cutting them up with scissors. Be careful not to let young turkeys out in the morning while the grass is wet. After the birds are two weeks old I feed wheat, but no corn until they are about a month old. I like hen mothers best, for turkey mothers are rangers, and do not take kindly to being kept in a coop. The bread will keep a week if made right, but do not soak more than will be wanted in a day, as it soon sours. I feed scraps from the table, such as potatoes and bits of meat cut very fine, but not much of the latter to young birds. I rarely lose a bird.—Mrs. E. Reith, in Homestead.
In turkey raising the one who is the most careful and attentive to the small things is the most successful. The first laying of eggs should be set under a chicken hen. The turkey hen will, after a few days' confinement, lay another batch of eggs. A good-sized hen will cover and care for ten eggs; a turkey hen, seventeen. Make a large, roomy nest of soft, fine hay—straw is too brittle and slippery. If there is danger of lice in the nest-box, sprinkle with water in which carbolic acid has been mixed in the proportion of eight drops to a half gallon of water. Don't wet the eggs with this. After the eggs have been sat on one week, sprinkle with warm water every other day, until the last week; then every day, until they hatch. Have the water clear, and use a flower or fine rose sprinkler. Let the water be of the same temperature as the eggs, which can be ascertained by slipping a thermometer under the hen for a few minutes. This softens the shells, and as a little turkey is very weak, it is helped out easily, and is stronger than if working long to get out.
Let the little turkeys get well dried and strong enough to climb around the edges of their nest before taking them off. Have a pen, say six feet square, built for them, and made tight at the sides clear down to the ground, to keep them from getting out and being chilled. Put sand and fine gravel over the ground, and cover enough of it to afford shelter at night and when it rains. They may be kept in this pen the first four or five days, then let out after dew is off, and shut up before night.
For the first few days' feed, nothing is better than clabber cheese or curd made by scalding clabbered milk until the curd separates and is cooked, then skimmed out and fed. Mix a little black pepper with this every other day. Meal must not be fed raw for several weeks, and then should be mixed with sour milk instead of water. Bake the meal into bread by mixing it, unsifted, with sour milk, and adding a little soda and pepper. Spinach, lettuce, onion tops and any other tender greens, chopped fine, are excellent food. From the time a turkey is hatched until it is ready for market it should have plenty of milk. Give them clear water to drink, for milk is a food. See that the very young ones have milk and water in quite shallow dishes, for they are in danger of getting wet if the dish is deep.
at the first signs of rain, and they will soon learn to run and fly to their coop at the first drops. Always shut them up at night, for they are early risers and will be out long before the dew is dried off. Don't pen them too near the house. Feed them at or near the same place all the time and they will learn to go there when hungry. Give them a good feed at night and they will remember to come home for it. If the morning is dry, feed lightly and let them hunt the rest in the orchard and fields. Keep the grass and weeds mowed around their pen and feeding places. Mix slaked lime in the dust for them to take their dust bath in, and sprinkle the carbolic acid and water over and around their roosting pen. Keep pails and kettles covered, for they will get drowned if they have half a chance, as they begin to fly so young. Of course a turkey hen will take her young off, and care for them after a fashion, but the safest way to make them tame is to raise them where they may be cared for. Even if the turkey hen hatches her last batch of eggs, it is a good plan to have a hen ready to take the little turkeys and slip them away at night. If she still stays on her nest give her 20 or 25 hen's eggs, and if she hatches them let her run with the chickens. They are not so tender or so easily led astray as turkeys are, nor as valuable.—Mrs. Jas. R. Hinds, in Orange Judd Farmer.
My experience in the use of water in almost every disease occurring in this climate has long since satisfied me that it is less objectionable and produces quicker and better results than any other treatment, and can be used when all other medication is contra-indicated. Drinking water should be pure, uncontaminated by animal or vegetable impurities, and given ad libitum, unless, in rare instances, it should cause vomiting or interfere with the capability of digesting food. If children are comatose or delirious, as they frequently are in typhoid fever, give water to them regularly, or force it upon them, if they refuse to take it, as I was obliged to do with a child of six years just recovering from that fever.
It is my custom to allow cold drinks of water in all cases of measles whenever patients desire it, and I am satisfied that it aids the early appearance of the rash, and certainly is cooling and grateful to the patient. Hot drinks or vile and nauseous teas are unnecessary in this disease, and should be discarded as useless, odious, and disgusting. If congestion of the lungs or any intercurrent inflammation occurs, or the rash is much delayed, a hot water bath or the old reliable corn sweat will break up the complication with amazing rapidity, and if the head is kept cool, will not generally be unacceptable to the patient.
Hot baths reduce temperature by causing free perspiration afterward, and cold packs reduce it by cooling the surface sufficiently long to reduce the heat of the blood, and, if used judiciously, seldom fail of success. I have reduced the temperature four degrees in two hours by wrapping around a child a sheet wet with tepid water, and no other covering. Cold packs are sometimes objectionable, because of their depressing effects, and should only be used to reduce high temperature and when there is no congestion or inflammation of any of the vital organs of the body.
Cold water poured in a small stream from a pitcher upon the head for five or ten minutes will often relieve headache, and is a benefit in all inflammatory brain diseases, if, at the same time, you can put the feet into hot water containing mustard or pepper.
Large enemas of warm water will care for spasmodic colic, and I have, in one instance, relieved strangulated hernia by the same method, and at another time the same result was accomplished by a large injection of warm linseed oil. I have often applied a cloth wet with cold water upon the throats of children suffering with spasmodic croup, with satisfactory results.
I have seen infants suffering with diarrhea or summer complaint, sleepless, worrying, fretting, or crying from thirst, begging for water, and the mother or nurse afraid to give it more than a teaspoonful or two at a time, saying that it vomited everything it drank as soon as taken. I have often, when visiting such cases, called for a glass of cold water, and, to the surprise of the mother, would allow it to take all it could drink, which usually would be retained, and the child would soon be wrapped in a refreshing sleep. Without medicine, a proper regulation of the child's diet would soon restore it to health again.
The spasms of children, from whatever causes, or the eclampsia from uræmic poisoning, are often readily controlled when immersed in hot water or given a hot vapor bath or corn sweat. If the convulsions of children are accompanied by a high temperature, put them into water of 100° and then gradually cool it down to 68° or 70°, and then keep them in a room of the same temperature, with little covering. If the temperature rises, repeat the treatment as frequently as necessary, and I think you will not be disappointed in the results.
Scarlet fever and diphtheria, two of the most dreaded and formidable diseases of children, are largely shorn of their terrors when, in addition to an early and thorough medicinal treatment, the little patients are bathed in as warm water as the surface will allow frequently, or for thirty minutes wrapped in a warm, wet blanket, followed by warm, dry coverings, to maintain the perspiration that such treatment usually produces. It has proved to me a valuable aid in eliminating from the blood the specific poison which causes these diseases, and I can safely recommend it to your notice and trial.
There is no disease more favorably influenced by this treatment than pneumonia, and in mild cases one daily warm bath or sweat, without medicine, will be sufficient to arrest this disease, and it is among the first things I usually order. If I find a child or infant with a temperature of 103° to 105°, short, dry, and painful cough, dyspnœa, rapid pulse, great thirst, or vomiting, with dry crepitation in any part of the lung tissue, I order it rolled up in a blanket or sheet coming out of hot water, and in thirty minutes change it to warm, dry blankets, and soon the little fretful, worrying sufferer would rest in a quiet, peaceful sleep.—Peoria Med. Mo.
With perhaps the exception of heredity, the question of stimulants and narcotics in their relation to the physical welfare of the race is second to none in importance. With trifling exceptions, the whole world is addicted to their use. The universality of such use has led many to consider them a necessity to man, and that they are God's gifts to him, and, if rightly used, are of physical benefit. It may not be a perversion of judgment to consider that their widespread popular use is greatly due to the efforts of the race to gain anæsthesia for, and distraction from, those pains and punishments that are the inevitable sequence of departure from hygienic and social law on the part of the individual, his ancestry, and society in general.
The taste for these things is acquired, not natural, though the acquisition may be through hereditary influence. An idea is held by a majority of even fairly intelligent individuals that there is a justifiable, harmless, and even beneficial use of these substances by the general public, though acknowledging that beyond a certain indefinite line this use becomes an abuse.
I believe that there may occasionally be cases in which the physical benefits derived from their use outweigh the injury they inflict, but I think this use is very much less than is generally supposed, and if we can judge from the preponderance of evil effected by such use, these substances ought to be considered as the materialized curses of God rather than as beneficent gifts. The prevalent idea as to the beneficent nature of these substances I consider to be a delusion that can only be explained upon the hypothesis that there is a widespread lack of appreciation of the fact that, though they may have an immediate pleasant and agreeable effect upon the body, their injurious effects are cumulative, and are usually ultimate, and so distant as to be difficult of direct connection with their cause to ordinary observation. The more moderate the use of these substances, the more remotely is the effect removed from the cause and more difficult of detection. That the ordinary habitual, so-called moderate use of stimulants and narcotics, such as tea, coffee, tobacco, and alcohol, is, in the vast majority of cases, really an abuse, is a proposition that I think should be admitted by all who have given the subject an unbiased study.
The idea that the user of tobacco and other injurious substances will be cognizant of the injury inflicted by habitual use in moderate or even excessive amounts is an undoubted fallacy. The daily, weekly, or monthly injurious effect may be entirely unobservable to even trained physicians, and yet the ultimate cumulative effect may be fatal. I can instance numerous cases of physicians directly fatally injured by the use of alcohol, who have never had the slightest cognizance of the fact; and I can also instance cases of grave disease from the use of tobacco where the patients never have believed that tobacco has been the cause of their troubles, even after a unanimous opinion to that effect has been expressed by a number of competent medical advisers. The habitual consumption of opium, in doses of any amount, is generally admitted by most people to be physically injurious outside of its strict medicinal application. Moderate indulgence in alcohol as a beverage is beginning to acquire a very widespread evil reputation. But how about tobacco? Tea and coffee we can confidently leave to the consideration of a somewhat remote posterity of a considerably advanced intelligence and elevated hygienic ideals.
The relation of tobacco to the physical welfare of man can only be fairly estimated by viewing the subject in its broadest aspect; by considering its effects upon the race as a whole rather than in individual cases; by taking into consideration economical and other social conditions that at first sight might be considered as having little relevancy to the medical side of the subject. But there can be no just consideration of the matter otherwise. The direct deleterious effects of the immoderate use of tobacco are readily observable; but the great bulk of the evil physical effects due to the moderate use of this plant are of an intermediate nature and not directly noticeable; nevertheless, they are real, and worthy of medical attention. The plainly marked results following the use of tobacco in relatively large amounts seem to be due to quick and extreme interference with nutrition, and a diminution of function of all kinds, which may be represented by anything from a slight decrease of appetite and digestive ability up to a complete loss of function of almost any important organ. Tobacco has stimulating as well narcotic properties, but as ordinarily used its stimulating effect appears to be slight as compared with its narcotic influence. In this respect it differs from alcohol, the use of which, owing to the usual method of introduction in large amounts through the stomach, produces directly, by stimulation, readily noticeable structural changes. But with tobacco the direct evil results are mostly of a functional character, and are more generally diffused, owing to the usual slow manner of introduction into the body. These two properties have an effect upon the body in moderate use as well as in immoderate use, the effect being simply in proportion to the quantity used, though the effects of moderate use may not be measurable by ordinary means. It is easy to see the effects of large amounts of tobacco in the stunted growth of adolescents; in functional cardiac disorders; in intellectual sluggishness, loss of memory, and color blindness; in loss of appetite, and other neuroses of motion, and marked blunting of various functions of sensation, and in degeneracy of descendants; but that lesser evils are produced must be proved mostly by inference, circumstantial collateral evidence, and analogy.
The greater evils that are the outcome of a moderate use of tobacco are probably due to prolonged slight interference with nutrition, and consequent general decrease of vitality, which renders the individual more susceptible through indirect influence to the invasion of disease, and which lessens the capacity for productive effort.
It is of course difficult, and perhaps even impossible, to accurately estimate the value of tobacco to the race; but let us glance at the pros and cons, and then each one can roughly estimate for himself. Tobacco may be used medicinally, but it is a dangerous and uncertain remedy, and it probably has not one medicinal use that cannot be more suitably met by other remedies. One can readily imagine easier digestion as the result of the sedative influence of the after-dinner cigar upon a disquieted nervous system, especially if the coincident irritation of alcohol and coffee have need of correction; but it can also be imagined that in most of such cases the remedy has been the cause of and will further increase the disordered condition, and that nutrition of deficiently nourished nerve tissue is rationally indicated rather than partial narcotization. There then remains, so far as I can see, the solace of moderate anæsthesia and, occasionally, of occupation for idlers, as the only items that can be placed to the credit of tobacco. There certainly are individual cases where such usage may be more provocative of physical benefit than evil, but, before judging for the race as a whole, compute the other side of the question.
Tobacco injures the general health of the public through the economic loss caused by its consumption. The people of our country spend annually over seven hundred millions of dollars for tobacco—twenty per cent. more than is spent for bread. This sum represents only a minor part of the cost of the tobacco habit to the country. The crop is immensely exhaustive to the soil. Its culture has blighted whole sections of fertile territory. In the time consumed by the producer and the trader in its production, manufacture, and sale, and by the consumer in its use, and by the general interference with vital activity and consequent decreased productive capacity, there is represented an almost unimaginable sum of money. Certainly the people at large are not so well fed both as to quantity and quality, or so thoroughly clothed, or so hygienically housed that they can afford this gigantic economic waste.
There can be little doubt that if the people had sufficient intelligence and moral strength to taboo tobacco, this comparatively senseless outgo would be largely devoted to supplying these and other necessities of an exalted health status.
Tobacco injures health through its moral effects. The tobacco habit is certainly a dirty and frequently a disgusting habit, and encourages other dirty practices. Its use tends to make men cowardly, irritable in temper, and low in spirits. It blunts ideas of purity and courtesy, leading to invasion of the rights of others. It is presumed that few medical men would visit a delicate, sensitive patient after saturation with the "fragrant" effluvia of onions, but thousands whose systems are saturated with nicotine and who reek with nauseating odor do not hesitate to inflict their presence on sick or well. The time will come when the tobacco user will not be allowed to poison the atmosphere that is the common property of the public—will not be allowed to force the inhalation of nicotine upon the general public, to say nothing of being allowed to poison the infants and women in his own family. What would be said of a man who introduced poison in any degree into the food or drink of his child? Is the poisoning of the household atmosphere by the ignorant, thoughtless, or selfish smoker morally more defensible? Tobacco injures health through hereditary influence. The tobacco user begets, more certainly than the non-user, puny children with disordered nervous conditions. Luckily for our race, the women, who have the most important prenatal influence in guarding its physical well-being, are practically non-users of the plant. The general health status of the race is improving, not because the use of tobacco or the indulgence in other questionable practices is harmless, but because, among other things, of the great advance in general intelligence and knowledge of hygienic law.
A person, or the public in general, may practice an injurious habit, and yet more than counteract its influence by opposing beneficial practices.
Horace Greeley said, "Show me a drunkard who does not use tobacco, and I will show you a white blackbird." In this country, where dietetic drinking habits are not common in the family, the weakening of moral fiber by indulgence in tobacco is usually the introduction into the round of vicious indulgences, and thus directly or indirectly affects health. Smoking induces dryness of the mucous membrane of the mouth and consequent thirst. The partially paralyzed nerve terminals want something more stimulating than water to afford relief. Furthermore, blunted appetite induces deficient nutrition, and consequently there is a call for some "pick-me-up;" hence we find that the use of tobacco tends to the habitual use of alcoholic beverages, and there are very few habitual users of alcohol who escape without structural injuries to the body as well as perversion of its functions. Decrease of vital activity in all the tissues of the body marks the use of tobacco. The tendency is toward functional paralysis, though occasional signs of stimulative irritation are to be noticed, especially in the respiratory passages. The interference with intellectual activity is marked. It is said that during a period of fifty years no tobacco user stood at the head of his class in Harvard. The accumulated testimony of investigating observers is conclusive that, other things being equal, users of tobacco, in schools of all grades, never do so well in their studies as non-users.
One head of a public school said he could always tell when a boy commenced to use tobacco by the record of his recitations. Professor Oliver, of the Annapolis Academy, said he could indicate the boy who used tobacco by his absolute inability to draw a clean, straight line. The deleterious effects of tobacco have become so clearly apparent that we find its sale to minors is prohibited in France, Germany, and various sections of this country. It is somewhat a question if, at the present time, the race is not doing itself more injury by its use of tobacco than it is with alcohol, because of its more universal use, particularly by youth, and because of the respectability of the habit, which comes of its use by a certain intelligent part of the race, including teachers of morals and physics, and even temperance reformers. There is a widespread sentiment in existence that it is not a respectable thing to be even partly paralyzed by alcohol, but how few there are who consider narcosis as in any way connected with the use of tobacco. Its effect is more diffused and masked, and is not so acutely serious in individual cases, but through its interference with vital activity, tobacco is probably more generally injurious to the race than alcohol.
The editorial fiat of "too long" prevents a full exposition of the subject, but, in closing, let me say I hear millions of tobacco users ask, "Why, then, was this plant given to man, if its general effects are so decidedly evil?" The question presupposes design in creation. Without subscribing to this theory, or pretending to have solved the mystery of the presence of evil in the world, the answer may be suggested that the overcoming of many seductive evils becomes to man a means of his progressive higher development. Of one thing I am convinced, that the physical development and welfare of man is interfered with in strict sequence to his consumption of substances that are unnecessary for his nutrition—stimulants and narcotics inclusive.—Medical Record.
Dr. F. Engelmann, in Cent. f. Gyn., claims that acetic acid possesses equally as good antiseptic properties as carbolic acid; in fact, that it is to be preferred, as it is completely harmless, even if used in concentrated solutions, and that it is a valuable hæmostatic, an advantageous addition particularly in obstetrics. Another important property is its ease of transition into the tissues, which, according to Engelmann's experiments, is by far greater than that of all the other antiseptics. Of bichloride it is well known that it forms an insoluble combination with albumen, and can therefore act only on the surface, while acetic acid extends into the deeper tissues with ease.
Acetic acid also affects the metal of the instruments, but not as severely as the bichloride; the forceps, for instance, may be placed for a quarter of an hour in an irrigator filled with a three per cent. solution of acetic acid without being injured.
A pleasant effect of acetic acid is that it softens and lubricates the skin. The author generally used a three per cent. solution; at times he has made use of a five per cent. solution, which would easily cause a painful burning at sore places, so that he only used the latter strength in septic cases, as the three per cent. solution proved to be a satisfactory antiseptic for general purposes.
To combat this often distressing disease I have tried the administration of several medicines, namely, bromide of potassium, asafœtida, valerian, morphine, belladonna, etc., and I have very closely watched their effects, but none of them proved of much use. Having observed, however, that during the late cholera epidemic some of the patients admitted into the hospital under my medical charge slept well, had their anxiety improved, and some of them ultimately recovered, after the application of a strong counter-irritation of the pneumogastric nerves in the neck, namely, between the mastoid process and the angle of the lower jaw, I tried the same treatment on whooping patients, and I have no hesitation in stating that the result was very satisfactory. I may quote one single case of the many I have had under treatment.
A boy, aged twelve years, of weak constitution, was suffering from frequent and intense attacks of whooping cough. At a time the fits were so vehement that blood came out of his eyes and mouth. The case was a severe one, and I thought it would very likely end fatally. I prescribed several medicines, and even subcutaneous injections of morphine, but without any avail. I then tried for the first time the counter-irritation on both sides of the neck, and this means acted like magic. In four or five days the patient recovered, and was able to go to school. Since that time I have been applying the same treatment, either on the right side only or on both, with the greatest benefit.—Br. Med. Jour.
At a recent meeting of the Physical Society, Berlin, Prof. Preyer spoke on reflexes in the embryo. His researches extended over many classes of animals. As representing mammals, guinea pigs were chiefly used; and for reptiles, snakes; while in addition the embryos of fishes, frogs, mollusks, and other lower animals were also employed. But of all animals birds are most suitable for embryological observations, inasmuch as with due precautions the development of one and the same individual can be followed for a considerable time. Birds' eggs can be incubated in a warm chamber, and by removing a portion of the shell and replacing it by an unbroken piece from another egg, it becomes possible to follow the daily development of the chick and to experiment upon it. As early as the ninetieth hour of incubation, spontaneous "impulsive" movements may be observed, taking place apparently without any external stimulus as a cause, and at a time when no muscles or nerves have as yet been developed. After the occurrence of these spontaneous movements, and at the earliest on the fifth day of incubation, movements are observed to result from the application of mechanical, chemical, and electrical stimuli. In order to observe these the eggs must be allowed to cool down until all spontaneous movements have ceased. From the tenth to the thirteenth day more complicated and reflex actions occur on the application of stimuli, as, for instance, movements of the eyelids, beak, and limbs; and if the stimuli are strong, reflex respiratory movements. These reflexes make their appearance before any ganglia have become differentiated. Prof. Preyer considered himself justified in concluding from this that ganglia are not essential for the liberation of reflex actions. He intends, on some future occasion, to give a more detailed account of these experiments, and of the conclusions which may be drawn from them. In the discussion which ensued the conclusions of the speaker were contested from many sides.
The principal subject of the lecture is the peculiar colored reflection observed in certain specimens of chlorate of potash. Reflection implies a high degree of discontinuity. In some cases, as in decomposed glass, and probably in opals, the discontinuity is due to the interposition of layers of air; but, as was proved by Stokes, in the case of chlorate crystals the discontinuity is that known as twinning. The seat of the color is a very thin layer in the interior of the crystal and parallel to its faces.
The following laws were discovered by Stokes:
(1) If one of the crystalline plates be turned round in its own plane, without alteration of the angle of incidence, the peculiar reflection vanishes twice in a revolution, viz., when the plane of incidence coincides with the plane of symmetry of the crystal. [Shown.]
(2) As the angle of incidence is increased, the reflected light becomes brighter and rises in refrangibility. [Shown.]
(3) The colors are not due to absorption, the transmitted light being strictly complementary to the reflected.
(4) The colored light is not polarized. It is produced indifferently, whether the incident light be common light or light polarized in any plane, and is seen whether the reflected light be viewed directly or through a Nicol's prism turned in any way. [Shown.]
(5) The spectrum of the reflected light is frequently found to consist almost entirely of a comparatively narrow band. When the angle of incidence is increased, the band moves in the direction of increasing refrangibility, and at the same time increases rapidly in width. In many cases the reflection appears to be almost total.
In order to project these phenomena a crystal is prepared by cementing a smooth face to a strip of glass whose sides are not quite parallel. The white reflection from the anterior face of the glass can then be separated from the real subject of the experiment.
A very remarkable feature in the reflected light remains to be noticed. If the angle of incidence be small, and if the incident light be polarized in or perpendicularly to the plane of incidence, the reflected light is polarized in the opposite manner. [Shown.]
Similar phenomena, except that the reflection is white, are exhibited by crystals prepared in a manner described by Madan. If the crystal be heated beyond a certain point the peculiar reflection disappears, but returns upon cooling. [Shown.]
In all these cases there can be little doubt that the reflection takes place at twin surfaces, the theory of such reflection (Phil. Mag., Sept., 1888) reproducing with remarkable exactness most of the features above described. In order to explain the vigor and purity of the color reflected in certain crystals, it is necessary to suppose that there are a considerable number of twin surfaces disposed at approximate equal intervals. At each angle of incidence there would be a particular wave length for which the phases of the several reflections are in agreement. The selection of light of a particular wave length would thus take place upon the same principle as in diffraction spectra, and might reach a high degree of perfection.
In illustration of this explanation an acoustical analogue is exhibited. The successive twin planes are imitated by parallel and equidistant disks of muslin (Figs. 1 and 2) stretched upon brass rings and mounted (with the aid of three lazy-tongs arrangements) so that there is but one degree of freedom to move, and that of such a character as to vary the interval between the disks without disturbing their equidistance and parallelism.
The source of sound is a bird call, giving a pure tone of high pitch (inaudible), and the percipient is a high-pressure flame issuing from a burner so oriented that the direct waves are without influence upon the flame (see Nature, xxxviii., 208; Proc. Roy. Inst., January, 1888). But the waves reflected from the muslin arrive in the effective direction, and if of sufficient intensity induce flaring. The experiment consists in showing that the action depends upon the distance between the disks. If the distance be such that the waves reflected from the several disks co-operate,2 the flame flares, but for intermediate adjustments recovers its equilibrium. For full success it is necessary that the reflective power of a single disk be neither too great nor too small. A somewhat open fabric appears suitable.
It was shown by Brewster that certain natural specimens of Iceland spar are traversed by thin twin strata. A convergent beam, reflected at a nearly grazing incidence from the twin planes, depicts upon the screen an arc of light, which is interrupted by a dark spot corresponding to the plane of symmetry. [Shown.] A similar experiment may be made with small rhombs in which twin layers have been developed by mechanical force after the manner of Reusch.
The light reflected from fiery opals has been shown by Crookes to possess in many cases a high degree of purity, rivaling in this respect the reflection from chlorate of potash.
The explanation is to be sought in a periodic stratified structure. But the other features differ widely in the two cases. There is here no semicircular evanescence, as the specimen is rotated in azimuth. On the contrary, the colored light transmitted perpendicularly through a thin plate of opal undergoes no change when the gem is turned round in its own plane. This appears to prove that the alternate states are not related to one another as twin crystals. More probably the alternate strata are of air, as in decomposed glass. The brilliancy of opals is said to be readily affected by atmospheric conditions.
Abstract of the Friday evening lecture delivered by Lord Rayleigh, F.R.S., at the Royal Institution, on April 12, 1889.
If the reflection were perpendicular, the interval between successive disks would be equal to the half wave-length, or to some multiple of this.
Contained in Scientific American Supplement during the past ten years, sent free of charge to any address. MUNN & CO., 361 Broadway, New York.
$2.50 a Year. Single Copies, 25 cts.
This is a Special Edition of the Scientific American, issued monthly—on the first day of the month. Each number contains about forty large quarto pages, equal to about two hundred ordinary book pages, forming, practically, a large and splendid Magazine of Architecture, richly adorned with elegant plates in colors and with fine engravings, illustrating the most interesting examples of modern Architectural Construction and allied subjects.
A special feature is the presentation in each number of a variety of the latest and best plans for private residences, city and country, including those of very moderate cost as well as the more expensive. Drawings in perspective and in color are given, together with full Plans, Specifications, Costs, Bills of Estimate, and Sheets of Details.
No other building paper contains so many plans, details, and specifications regularly presented as the Scientific American. Hundreds of dwellings have already been erected on the various plans we have issued during the past year, and many others are in process of construction.
Architects, Builders, and Owners will find this work valuable in furnishing fresh and useful suggestions. All who contemplate building or improving homes, or erecting structures of any kind, have before them in this work an almost endless series of the latest and best examples from which to make selections, thus saving time and money.
Many other subjects, including Sewerage, Piping, Lighting, Warming, Ventilating, Decorating, Laying out of Grounds, etc., are illustrated. An extensive Compendium of Manufacturers' Announcements is also given, in which the most reliable and approved Building Materials, Goods, Machines, Tools, and Appliances are described and illustrated, with addresses of the makers, etc.
The fullness, richness, cheapness, and convenience of this work have won for it the Largest Circulation of any Architectural publication in the world.
A Catalogue of valuable books on Architecture, Building, Carpentry, Masonry, Heating, Warming, Lighting, Ventilation, and all branches of industry pertaining to the art of Building, is supplied free of charge, sent to any address.
MUNN & CO., Publishers,
361 Broadway, New York.
In connection with the publication of the Building Edition of the Scientific American, Messrs. Munn & Co. furnish plans and specifications for buildings of every kind, including Churches, Schools, Stores, Dwellings, Carriage Houses, Barns, etc.
In this work they are assisted by able and experienced architects. Full plans, details, and specifications for the various buildings illustrated in this paper can be supplied.
Those who contemplate building, or who wish to alter, improve, extend, or add to existing buildings, whether wings, porches, bay windows, or attic rooms, are invited to communicate with the undersigned. Our work extends to all parts of the country. Estimates, plans, and drawings promptly prepared. Terms moderate. Address
MUNN & CO., 361 Broadway, New York.
Terms of Subscription, $5 a year.
Sent by mail, postage prepaid, to subscribers in any part of the United States or Canada. Six dollars a year, sent, prepaid, to any foreign country.
All the back numbers of The Supplement, from the commencement, January 1, 1876, can be had. Price, 10 cents each.
All the back volumes of The Supplement can likewise be supplied. Two volumes are issued yearly. Price of each volume, $2.50 stitched in paper, or $3.50 bound in stiff covers.
Combined Rates.—One copy of Scientific American and one copy of Scientific American Supplement, one year, postpaid, $7.00.
A liberal discount to booksellers, news agents, and canvassers.
MUNN & CO., Publishers,
361 Broadway, New York, N.Y.
Manufacturers, Agriculturists, Chemists, Engineers, Mechanics, Builders, men of leisure, and professional men, of all classes, need good books in the line of their respective callings. Our post office department permits the transmission of books through the mails at very small cost. A comprehensive catalogue of useful books by different authors, on more than fifty different subjects, has recently been published, for free circulation, at the office of this paper. Subjects classified with names of author. Persons desiring a copy have only to ask for it, and it will be mailed to them. Address,
MUNN & CO., 361 Broadway, New York.
In connection with the Scientific American, Messrs. Munn & Co. are solicitors of American and Foreign Patents, have had 42 years' experience, and now have the largest establishment in the world. Patents are obtained on the best terms.
A special notice is made in the Scientific American of all inventions patented through this Agency, with the name and residence of the Patentee. By the immense circulation thus given, public attention is directed to the merits of the new patent, and sales or introduction often easily effected.
Any person who has made a new discovery or invention can ascertain, free of charge, whether a patent can probably be obtained, by writing to Munn & Co.
We also send free our Hand Book about the Patent Laws, Patents, Caveats, Trade Marks, their costs and how procured. Address
MUNN & CO.,
361 Broadway, New York.
Branch Office, 622 and 624 F St., Washington, D.C.