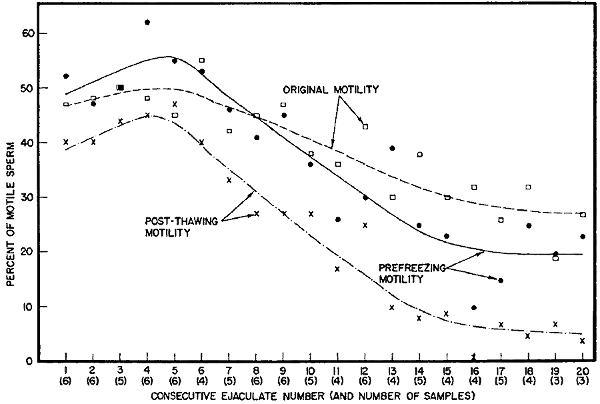
| Percent of motile sperm before and after freezing consecutive ejaculates collected within a 4-hour period | |
| from each of 6 bulls | (Fig. 1) |
Title: Preservation of Bull Semen at Sub-Zero Temperatures
Author: N. L. VanDemark
M. E. Friedman
W. C. Kinney
W. J. Miller
Carlos Rodriguez
Release date: August 11, 2011 [eBook #37041]
Language: English
Credits: Produced by Bryan Ness, Harry Lamé and the Online
Distributed Proofreading Team at http://www.pgdp.net (This
file was produced from images generously made available
by The Internet Archive/American Libraries.)
Please refer to the Transcriber's Notes at the end of this document.
| By | N. L. VanDemark |
| W. J. Miller | |
| W. C. Kinney, Jr. | |
| Carlos Rodriguez | |
| M. E. Friedman |
Bulletin 621
UNIVERSITY OF ILLINOIS
AGRICULTURAL EXPERIMENT STATION
| Urbana, Illinois | October, 1957 |
| Publications in the Bulletin series report the results of investigations made or sponsored by the Experiment Station | |
The authors gratefully acknowledge the financial assistance given to the Department of Dairy Science in support of these investigations by the Southern Illinois Breeding Association of Breese, Illinois, and the Northern Illinois Breeding Co-op of Hampshire, Illinois, through the Illinois Dairy Breeding Federation.
By N. L. VanDemark, W. J. Miller, W. C. Kinney, Jr., Carlos Rodriguez, and M. E. Friedman[A]
[A] This publication was prepared by N. L. VanDemark, Professor of Dairy Physiology. Research reported herein was carried out cooperatively by the senior author and W. J. Miller, W. C. Kinney, Jr., Carlos Rodriguez, and M. E. Friedman, formerly members of the Department of Dairy Science.
The first announcement in 1951 of the successful impregnation of a cow with bull semen that had been frozen stimulated much interest and research in freezing as a method of preserving bull semen. Research during the years following 1951 resulted in considerable progress and success in using freezing as a means of holding semen for long periods of time without loss of fertility.
Between 1952 and 1957, research on many aspects of the preservation of bull semen at sub-zero temperatures was carried out in the Department of Dairy Science at the University of Illinois. Many of these investigations have aided in perfecting the freezing technique that has been adapted for practical use. Some of these findings have been published, but many items have gone unreported except for general references at scientific meetings.
It is the purpose of this bulletin to bring together the results of several experiments carried out in connection with the freezing of bull semen and to present a practical freezing procedure based on the results of these experiments and findings at other institutions. Persons interested in the development of the procedures and the reasons why certain steps are necessary in freezing semen will find the details in the first part of this bulletin. Those interested only in the freezing procedure may turn to page 35 where a practical method of freezing semen is described.
While it has been known for a long time that various types of tissues and organisms can withstand freezing and are even preserved by freezing, the first attempts at freezing sperm cells were made before the turn of the century. In 1897, Davenport[1] [B] found that human sperm would withstand freezing. For thirty to forty years after that, little[6] attention was paid to freezing as a possible means of preserving semen. An excellent review of the early attempts to freeze sperm has been assembled by Polge and Parkes.[2] These investigators also gave a good account of their work at the British National Institute of Medical Research in London, where in 1949 they demonstrated that glycerol would protect fowl sperm so that it would survive freezing. The next year they found that bull sperm and the sperm of several other species were protected by glycerol during freezing. During the same year, Emmens and Blackshaw[3] showed that ram and bull sperm would survive freezing. In 1951 frozen semen was used to produce a calf in England and a lamb in Australia.
The highlights in the development of frozen semen have been covered by other reviews and reports. Interested persons will find the articles of Polge and Parkes[2] and Smith[4] especially good on the early history and theoretical aspects of freezing sperm. Later progress on the freezing procedure has been reviewed and covered in a number of detailed reports.[5], [6], [7] Many items not covered in those articles have been assembled here.
One of the first considerations in freezing semen is that of deciding which semen samples are to be frozen. Since preservation of the semen—the maintenance of the potential motility and especially the fertility of the sperm—is the primary aim, some attention should be directed to the kind of semen sample that will withstand freezing. Do the initial characteristics of the sample indicate whether the sperm will withstand freezing? Does maturity of the sperm affect their freezability?
Predicting freezability. Estimates of semen quality in the past have been based in part on the numbers of sperm present in a fresh sample and on the percentage and rate of motility shown by the sperm. These characteristics were used to determine the relationship between the original concentration of sperm (in the fresh, undiluted sample), the percentage and rate of sperm motility in the diluted samples just prior to freezing, and the percentage and rate of sperm motility following freezing and thawing. From data collected before and after freezing and thawing 54 ejaculates, it was found that there was not a significant correlation between the number of sperm present in the original sample and the percent of motile sperm present after freezing and thawing (r = 0.03). A highly significant correlation (r = 0.45) was found,[7] however, between the percentages before freezing and after thawing. While this correlation coefficient was highly significant, its magnitude indicates that only about one fifth of the variation in percentage of motile sperm observed after freezing was accounted for by the motility of the sperm prior to freezing.
Freezability of first and second ejaculates. In the early days of artificial breeding in this country, it was commonly believed that a second ejaculate collected a few minutes after the first resulted in a larger ejaculate containing more sperm. With the development of the procedure of stimulating sexual excitement by restraint prior to collecting semen, this difference between first and second ejaculates has been greatly reduced. Still it was noted that second ejaculates frequently withstood freezing better than first ejaculates, even though restraint and stimulation of the bull occurred prior to collection of the first ejaculate. During the course of a number of experiments, it was possible to compare the freezability of 2 ejaculates that were collected a few minutes apart from the same bull. Two consecutive ejaculates were obtained one or more times from 24 bulls so that a total of 58 comparisons could be made. The mean prefreezing and post-thawing percentages of motile sperm in first and second ejaculates are presented in Table 1.
An analysis of variance showed that in this comparison the differences between first and second ejaculates in sperm survival during freezing were highly significant. A later comparison of 27 first and second ejaculates from 26 bulls did not show as great a difference between first and second ejaculates in their ability to withstand freezing (Table 1).
| Number of bulls |
Number of ejaculates |
Ejaculate | Prefreezing motility (percent) |
Post-thawing motility (percent) |
Survival (percent) |
| 24 | 58 | 1st | 60 | 39 | 65 |
| 2d | 62 | 45 | 74 | ||
| 26 | 27 | 1st | 60 | 36 | 60 |
| 2d | 65 | 40 | 61 | ||
Freezability of several consecutive ejaculates. The fact that second ejaculates sometimes withstood freezing better than first ejaculates suggested that the maturity of the sperm might be a factor affecting freezability. An opportunity to check this idea came when 20 consecutive[8] ejaculates were collected from each of 6 bulls within a 4-hour period. The sperm in samples collected in this manner might be expected to be less mature with each additional collection.
The results obtained in freezing several consecutive ejaculates are shown in Figure 1 as averages for the ejaculates from 6 bulls. In same instances, there was an insufficient quantity of semen available to test the freezability. (Procedure: Diluted to 30 × 106 sperm per ml. with 1:1 yolk-citrate, then cooled and glycerolated with an equal volume of 14 percent glycerol (percent by volume) in 2.9 percent sodium citrate. Final sperm concentration, 15 × 106. Equilibration time, 15 hours. Freezing rate, 2° C. per minute from +5° to -19° C. then 4° C. per minute from -19° to -79° C. Held frozen for 5 or more hours then thawed in water at 5° C. and checked for motility.)

| Percent of motile sperm before and after freezing consecutive ejaculates collected within a 4-hour period | |
| from each of 6 bulls | (Fig. 1) |
In general, the motility before freezing improved slightly from the first to the fourth to sixth ejaculate and then declined until about the 12th or 14th ejaculate, at which point the prefreezing motility seemed to level off through the 20th ejaculate (Fig. 1). The percentage of motile sperm found after freezing and thawing followed the same trend at an average level 10 to 15 percent lower than the prefreezing level. As is readily seen from the trend lines in Fig. 1, the difference between the prefreezing motility and the post-thawing motility increased[9] gradually after about the fifth ejaculate. Although the absolute difference did not increase greatly, the percentage of survival after freezing dropped from 81 percent on the first 5 ejaculates to 26.5 percent on the last 5 (Table 2).
(Weighted averages for 6 bulls)
| Ejaculate | Number of ejaculates |
Prefreezing motility (percent) |
Post-thawing motility (percent) |
Survival (Percent) |
| 1st to 5th | 29 | 53.3 | 43.2 | 81.0 |
| 6th to 10th | 26 | 43.8 | 30.2 | 69.0 |
| 11th to 15th | 23 | 28.6 | 14.5 | 50.7 |
| 16th to 20th | 19 | 18.1 | 4.8 | 26.5 |
Freezability of epididymal sperm. Since the freezability of bull semen seemed to be better in second than in first ejaculates and some improvement in freezability was evident through the first 4 to 6 ejaculates taken consecutively, the question of whether epididymal sperm would withstand freezing seemed to be important. Although when 20 collections were made, the later ejaculates no doubt contained fewer mature sperm, the lowered freezability could have been due to accessory gland secretions rather than changes in the sperm themselves. Removing sperm directly from the epididymis would eliminate any effect that the accessory gland secretions could be exerting. Further, if epididymal sperm could be frozen, obtaining and using semen from a bull shortly after his death should be possible.
| Bull | Prefreezing motility (percent) |
Post-thawing motility (percent) |
Survival (percent) |
| 1 | 50 | 40 | 80 |
| 2 | 40 | 13 | 32 |
| 3 | 60 | 15 | 25 |
| 4 | 30 | 15 | 50 |
| 5+6 | 40 | 25 | 62 |
| Average | 44 | 22 | 50 |
To determine whether epididymal sperm would withstand freezing, the 12 epididymides (cauda only) of 6 slaughtered bulls were flushed[10] with saline (0.9 percent) and the sperm obtained were frozen using the same procedure as was used with the 20 consecutive ejaculates discussed earlier. Averages of the 2 epididymides from each bull are given in Table 3; the samples from bulls 5 and 6 were combined. From the data in Table 3, it is obvious that motile sperm were present after freezing and thawing epididymal samples. It is likely that further experience in handling epididymal sperm may lead to improved results. Using frozen epididymal sperm from 2 bulls, Canadian workers have produced confirmed pregnancies in 8 out of 12 cows.[8]
Freezability of washed sperm. In the laboratory it is frequently desirable to study sperm free of the seminal plasma in which they are ejaculated. Sperm can be separated from the seminal plasma by centrifugation, removal of the supernatant plasma, and resuspension in a salt solution of known composition. Sometimes it is desirable to repeat the process. This tends to wash the sperm with the salt solution and sperm handled in this way are called washed sperm.
Sperm cells centrifuged three times and washed twice in 0.9 percent sodium chloride solution withstood freezing well when finally resuspended and frozen in yolk-citrate diluent. The percentage of survival in three samples subjected to this treatment was 60 percent. Thus it appears that the seminal plasma itself is not essential for ejaculated sperm to survive the rigors of freezing. This is not surprising, since it had already been found that epididymal sperm, which also are free of accessory gland secretions, can withstand freezing and thawing.
Both of the extenders that are widely used in routine storage of bull semen at 5° C. are used for freezing semen. These are the egg yolk-sodium citrate and whole or skimmilk extenders. Most of the research with extenders for freezing bull semen in this laboratory has been done with the yolk-citrate diluents.
Proportion of egg yolk in the final diluent. Some early experiences with a diluent consisting of one part yolk and one part 2.9 percent sodium citrate dihydrate in distilled water showed poor sperm survival following freezing. The final mixture with this diluent consisted of about 45 percent yolk. In other attempts at adding glycerol in order to freeze semen, the final proportion of yolk was diminished and better sperm survival was obtained. Several experiments were carried out to test the effect of varying levels of egg yolk.
In the first efforts to find the optimum level of egg yolk, the level[11] of yolk in the final frozen mixture was varied from about 6 to 46 percent. These levels were obtained by varying the proportion of yolk to 2.9 percent citrate solution in the original extending media and also in the media added in glycerolating the samples.
Split portions of 20 semen samples were frozen in each of the extender combinations indicated in Table 4. The mean percentages of motile sperm found before and after freezing and thawing are shown also. The highest percentages were found with extenders containing 23 and 24 percent yolk. The highest percentage of yolk, resulting when a 1:1 (yolk to citrate) extender was used for both extending and glycerolating, proved to be most detrimental to sperm survival during freezing. The lowest percentage of yolk used (6 percent) was not as effective in protecting sperm during freezing as the intermediate levels tested (Fig. 2).
(Average of 20 semen samples)
| Diluent | Medium | Yolk in final mixture[D] (percent) |
Pre- freezing motility (percent) |
Post- thawing motility (percent) |
Survival (percent) |
||||||
| Extending | Glycerolating[C] | ||||||||||
| yolk | : | citrate | yolk | : | citrate | ||||||
| 1 | 1 | : | 1 | 1 | : | 1 | 45.7 | 65 | 5 | 8 | |
| 2 | 1 | : | 1 | 1 | : | 3 | 34.9 | 64 | 27 | 42 | |
| 3 | 1 | : | 3 | 1 | : | 1 | 33.6 | 63 | 33 | 52 | |
| 4 | 1 | : | 1 | 0 | : | 1 | 24.2 | 64 | 39 | 61 | |
| 5 | 1 | : | 3 | 1 | : | 3 | 22.8 | 63 | 37 | 59 | |
| 6 | 1 | : | 3 | 0 | : | 1 | 12.1 | 59 | 33 | 56 | |
| 7 | 1 | : | 7 | 1 | : | 7 | 11.4 | 56 | 35 | 62 | |
| 8 | 1 | : | 7 | 0 | : | 1 | 6.0 | 52 | 26 | 50 | |
| 9 | 1 | : | 15 | 1 | : | 15 | 5.7 | 49 | 25 | 51 | |
[C] This mixture included 14 percent glycerol.
[D] The average initial sperm concentration was 900 × 106/ml. Sufficient extender was added to give 30 × 106/ml. at the first extension. Thus the final concentration was 15 × 106 sperm/ml. after glycerolization.
Since rather large changes in the percentages of yolk were used in this experiment, two further trials were conducted in which 16, 24, and 32 percent yolk in the final mixture were compared, with the final citrate percentages held constant. In these tests, 16 and 24 percent yolk maintained sperm better at all citrate levels tried than 32 percent yolk. The 16 percent level was slightly better at most of the levels of citrate tested (Fig. 3).
Citrate level in the final diluent. The early work of the British indicated that a final citrate level near 2 percent in the diluent was[12] satisfactory for freezing bull sperm. Later, in a personal communication, Polge of the British group suggested that a citrate level of about 2.35 percent might be best with a final glycerol concentration of 7 percent. Some of the first attempts in this laboratory at establishing the optimum yolk-to-citrate ratios are shown in Fig. 3. In these experiments, the optimum levels of citrate appeared to be lower than anticipated from the British work. Thus a more complex experiment was set up to test a wider range of citrate levels using 16 and 24 percent egg yolk in the final freezing mixture. The average percentages of motile sperm found after freezing 10 semen samples at each of the citrate and yolk levels in this experiment are shown also in Fig. 3. Little difference in freezability was found between citrate percentages of 1.55 and 1.95. When the rate of sperm motility following freezing and thawing was considered along with the percent of motile sperm, a slight advantage was found with 16 percent yolk and a citrate concentration of 1.55 percent.
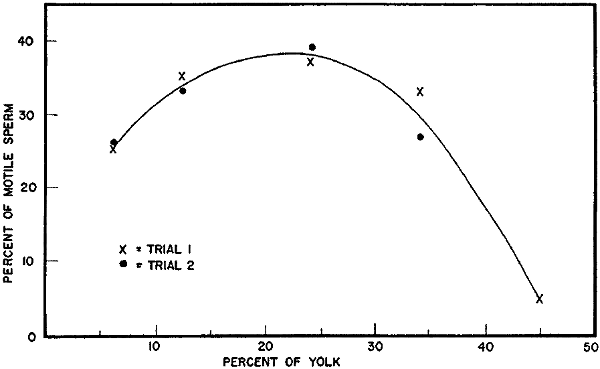
| Percent of motile sperm after freezing and thawing semen in diluents containing various levels of egg | |
| yolk | (Fig. 2) |
From the results of these experiments, and from several reports in the literature, [5], [6], [7], [9], [10] it appears that a diluting medium resulting in a final concentration of 16 to 25 percent yolk and 1.55 to 2.2 percent sodium citrate dihydrate is highly satisfactory for freezing.
Storing and freezing diluent. In some instances it would be advantageous to have prepared diluent on hand for use at any time. The[13] suitability of stored diluent was tested with a yolk-citrate (equal parts yolk and citrate without antibiotics added) diluent prepared and stored at 5° C. for 0, 2, 5, 7, and 9 days. Seven semen samples were diluted and frozen in these diluents. No difference was noted in the survival of sperm that could be attributed to the age of the diluent.
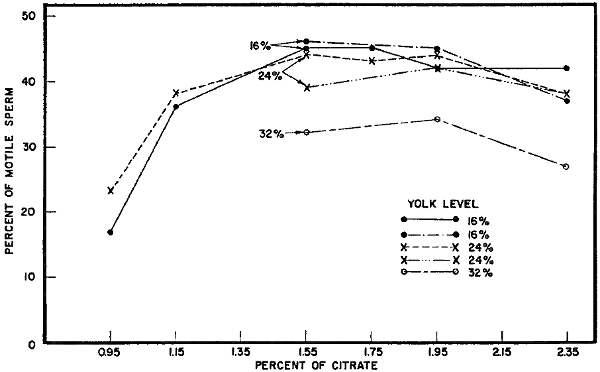
| Percent of motile sperm after freezing and thawing semen in diluents containing various levels of egg | |
| yolk and various percentages of sodium citrate | (Fig. 3) |
In another trial, a similar diluent (1:1 yolk to citrate with 1000 units of penicillin and 5000 units of streptomycin) was prepared and stored in the freezer compartment of a refrigerator at -15° C. Upon thawing, it was whitish in color and more viscous than freshly prepared diluent. Except for the fact that the viscosity seemed to reduce the rate of sperm motility, this frozen diluent stored for 65 days compared favorably with freshly prepared diluent for freezing semen.
Other diluents. Without the protective action of egg yolk or milk, few bull sperm will survive freezing. Several diluents were compared on a limited scale for freezing bull sperm. The results of these trials are compiled in Table 5. In this trial the yolk-citrate extender served best in maintaining sperm motility during freezing. Yolk-phosphate and homogenized whole milk were slightly less protective and yolk-saline seemed to furnish the least protection to sperm during freezing.
A number of investigations in other laboratories have now proven that milk can be used as effectively as the yolk-citrate diluent for freezing bull sperm.[6], [7]
| Extender | Dilution rate (semen: extender) |
Pre- freezing motility (percent) |
Post- thawing motility (percent) |
Survival (percent) |
Motility after storage[E] (percent) |
| Yolk-citrate | 1:1 | 60 | 49 | 82 | 46 |
| 1:10 | 53 | 45 | 85 | 36 | |
| Yolk-saline | 1:1 | 57 | 29 | 51 | 28 |
| 1:10 | 60 | 31 | 52 | 24 | |
| Yolk-phosphate | 1:1 | 55 | 33 | 64 | 25 |
| 1:10 | 60 | 43 | 72 | 25 | |
| Whole milk | 1:1 | 60 | 40 | 67 | 35 |
| 1:10 | 60 | 35 | 58 | 16 |
[E] Stored at 5° C. for 7 hours after thawing.
The first trials by the British at freezing bull semen were made with samples containing many millions of sperm cells. In routine artificial breeding, it is common to add extenders to semen so that one milliliter of diluted semen may contain only 10 million living sperm cells. (This number still insures optimal fertility.) Frequently the addition of 100 or more parts of the yolk extender to each part of the original semen sample is possible without reducing the sperm numbers below 10 million per milliliter. No one knew if this process of dilution would affect the resistance of bull sperm to freezing. The effect of various rates of dilution on the freezability of bull sperm was tested with 10 semen samples. The results, presented in Table 6, show that the numbers of sperm between 10 and 90 million per milliliter did not influence the percentage of sperm that survived freezing.
In a later trial it was found that sperm survival was slightly better at lower dilution rates than in the same samples frozen following dilution to 15 million sperm per milliliter. However, field trials with frozen semen carried out by others, using sperm numbers as low as 15 million per milliliter of semen inseminated or even lower, have been highly satisfactory.[11], [12]
During the early studies in the Illinois laboratory, the effects of glycerol level were also tested.[13] These effects are discussed in the section on glycerol additions beginning on page 17.
Effect of further dilution and refreezing after the initial freezing. Under some circumstances it might be advantageous to freeze semen[15] with a high concentration of sperm cells and then extend it further after thawing. With such a procedure less storage space is needed than when dilution is carried to the maximum before freezing. Two experiments were conducted to test the effects of dilution and storage at 5° C. and dilution and refreezing following an initial freezing of concentrated samples.
(Average of 10 ejaculates)
| Glycerol level (percent) |
Post-thawing motility (percent)[F] | |||
| Number of sperm (millions/ml.) | Average | |||
| 90 | 30 | 10 | ||
| 5 | 36.0 | 34.0 | 36.0 | 35.0 |
| 10 | 22.0 | 24.0 | 23.0 | 23.0 |
| 15 | 3.2 | 0.9 | 0.2 | 1.4 |
| Average | 20.3 | 19.8 | 19.9 | 20.0 |
[F] Mean initial motility of sperm before freezing was 55 percent.
Four semen samples were split and extended at rates of 1:1 (semen to extender) and 1:10. These were frozen, then thawed and halved. One half was further extended to a level of 15 million sperm per milliliter; the sperm numbers in the other remained unchanged. Each of these halves was split again, and one portion of each was stored at 5° C. for 3 to 7 hours. The other two portions were refrozen.
| Dilution of semen (semen: extender) |
Pre- freezing motility (percent) |
Post-thawing motility | |||||
| After first freezing |
After storage[G] | After refreezing[H] | |||||
| No further dilution |
Diluted to 15 million/ml |
No further dilution |
Diluted to 15 million/ml |
||||
| First trial: 4 samples | |||||||
| 1:1 | 60 | 49 | 46 | 34 | 31 | 6 | |
| 1:10 | 53 | 45 | 36 | 30 | 25 | 5 | |
| Second trial: 7 samples | |||||||
| 1:9 | 67 | 47 | 41 | 35 | 28 | 11 | |
| 15 million/ml | 67 | 30 | 32 | .. | 18 | .. | |
| Glycerol level (percent) |
Sperm motility | |||||||||||||||||||
| Post- thawing |
After storage at 5° C. | Average | ||||||||||||||||||
| 1 day | 3 days | 7 days | ||||||||||||||||||
| percent | rate | percent | rate | percent | rate | percent | rate | percent | rate | |||||||||||
| Control[I] | 56 | 2.5 | 55 | 1.9 | 46 | 1.8 | 38 | 1.4 | 48 | 1.90 | ||||||||||
| 0 | 54 | 2.4 | 44 | 1.9 | 46 | 1.8 | 36 | 1.4 | 45 | 1.87 | ||||||||||
| 5 | 52 | 2.2 | 50 | 1.9 | 46 | 1.7 | 32 | 1.4 | 45 | 1.80 | ||||||||||
| 1 | 0 | 52 | 2.3 | 46 | 1.8 | 42 | 1.7 | 28 | 1.6 | 42 | 1.85 | |||||||||
| 2 | 0 | 52 | 2.1 | 50 | 1.7 | 44 | 1.6 | 38 | 1.1 | 46 | 1.62 | |||||||||
| 3 | 0 | 50 | 0.7 | 44 | 0.5 | 42 | 0.4 | 30 | 0.4 | 42 | 0.51 | |||||||||
| Average | 53 | 2.0 | 3 | 47 | 1.6 | 2 | 44 | 1.5 | 0 | 34 | 1.2 | 2 | .. | .... | ||||||
[I] The control differed from the 0-glycerol treatment in that no additional citrate or glycerol solution was added.
[17]A similar trial was carried out with seven samples; one portion was diluted 1:9; the other was extended at the outset to 15 million sperm per milliliter. Results for both tests are summarized in Table 7.
From Table 7 it can be seen that refreezing following an initial freezing further reduced the number of surviving sperm. The second freezing was more detrimental to the portion of the samples extended to 15 million sperm per milliliter than to the portion that was refrozen at a higher sperm concentration. The percentage of motile sperm remained fairly high in the portions that were diluted to 15 million sperm and stored at 5° C. However, in all cases, survival was best in the samples at the lower dilution levels.
When the British procedure for freezing bull semen was first tried in this country, many of the refinements of the technique still had not been defined. It was known that glycerol worked well in protecting sperm during freezing. The effects of glycerol on sperm at 5° C., the appropriate levels to use in freezing, and the manner of adding it were not well established. Therefore, a number of trials were conducted in an attempt to establish the best procedures.
Effect of glycerol on sperm survival at 5° C. Since early work indicated the need for adding glycerol to diluted semen in order to protect the sperm during freezing, it was considered important to determine the levels of glycerol that sperm would tolerate at 5° C. Ten semen samples were extended 1:9 (semen to diluent) in a 1:1 yolk-citrate diluent (yolk to 2.9 percent sodium citrate dihydrate). Each sample was then split into 6 portions and an equal volume of citrate solution containing glycerol was added slowly to each to bring the glycerol in the final mixture to 0, 5, 10, 20, or 30 percent (by volume). These samples were stored at 5° C. and examined for motile sperm after 1, 3, and 7 days. The effects of glycerol levels on the percentage of sperm surviving and the rate (or speed) of their forward motion (0 = no forward motion; 4 = extremely rapid progressive motility) are presented in Table 8.
The percentage of motile sperm decreased slightly at the higher levels of glycerol. The most noticeable effect of the increase in glycerol level was the reduction in the rate of forward motion of the sperm. At the 30-percent level, the sperm moved slowly and could be seen to[18] rotate as they moved forward. Some samples were checked after slowly bringing the diluent up to a level of 40 percent glycerol; the sperm seemed to be immobilized completely in this solution.
Glycerol levels for freezing semen. The British procedure called for the use of 10 percent glycerol in the final mixture of semen and extender prior to freezing. Yet, as shown in Table 6, in our laboratory 5 percent glycerol resulted in the survival of a higher percentage of sperm than did 10 or 15 percent. In order to define more clearly the optimum glycerol level, several ejaculates of semen were subsampled and portions were frozen after the addition of yolk-citrate extender and glycerol in varying quantities. From Table 9 it can be seen that glycerol levels of 6 and 8 percent in the final mixture resulted in maximum sperm survival during freezing. These results were confirmed in tests on the survival of sperm at 5° C. storage for 3 days following freezing and thawing with varying glycerol levels (see Table 10).
The results shown in Tables 9 and 10 were confirmed also in later experiments. Thirty-six samples were subjected to various levels of glycerol and no significant difference in freezability was found between 6 and 8 percent. Based on these findings, a glycerol level of 7 percent was adopted for use in all experiments described in this bulletin, unless otherwise indicated. Results in a number of other laboratories have agreed with our findings regarding the use of approximately 7 percent glycerol with the yolk-citrate diluent.[5], [6], [7], [9], [10] With milk as the extender, 10 to 13 percent glycerol has been preferred by some.[5], [6], [7]
| Glycerol level (percent) |
Number of samples |
Pre- freezing motility (percent) |
Post- thawing motility (percent) |
Survival (percent) |
|
| 2 | 10 | 53 | 2 | 4 | |
| 4 | 19 | 55 | 29 | 53 | |
| 6 | 19 | 55 | 34 | 62 | |
| 8 | 19 | 55 | 35 | 64 | |
| 1 | 0 | 19 | 55 | 24 | 44 |
| 1 | 2 | 10 | 53 | 13 | 25 |
(Average of 13 ejaculates)
| Glycerol level (percent) |
Sperm motility (percent) | |||
| Post- thawing |
After storage at 5° C. | |||
| 1 day | 3 days | |||
| 4 | 29 | 22 | 20 | |
| 6 | 38 | 34 | 24 | |
| 8 | 42 | 33 | 17 | |
| 1 | 0 | 33 | 18 | 6 |
(Average of 12 ejaculates)
| Temperature during addition of glycerol (° C.) |
Equilibration time (hours) |
Post-thawing motility (percent) | ||||||||
| Glycerol additions | ||||||||||
| 5 | 3 | 1 | Average | |||||||
| 4.5 | 2 | 48 | 48 | 45 | 47.4 | |||||
| 6 | 49 | 51 | 47 | 48.8 | ||||||
| 18 | 46 | 47 | 46 | 46.3 | ||||||
| Average | 47 | .8 | 48 | .6 | 46 | .0 | 47.5 | |||
| 1 | 0.0 | 2 | 44 | 43 | 45 | 43.9 | ||||
| 6 | 48 | 50 | 46 | 47.9 | ||||||
| 18 | 43 | 46 | 42 | 44.0 | ||||||
| Average | 45 | .0 | 46 | .5 | 44 | .3 | 45.3 | |||
| 1 | 5.5 | 2 | 41 | 38 | 38 | 39.1 | ||||
| 6 | 42 | 45 | 43 | 43.6 | ||||||
| 18 | 42 | 43 | 42 | 42.5 | ||||||
| Average | 42 | .0 | 41 | .8 | 41 | .4 | 41.7 | |||
Rate, temperature, and method of adding glycerol. Closely associated with the question of how much glycerol should be added is that of how the additions should be made. Originally it was believed that the glycerol should be added in stages so that changes would occur gradually. However, there would be a saving in time if the entire amount could be added at once. Also, if the glycerol addition could be made soon after the dilution with egg yolk-citrate extender at room temperature, time would be gained in processing the semen for use. Since aging in vitro is known to reduce the fertilizing ability of sperm, every effort should be made to keep the processing time at a minimum. The results of an experiment involving these items, along with that of how much time should be allowed after the additions before freezing (equilibration time), are presented in Table 11. One can see that sperm survived freezing better when the diluted semen was cooled to 4.5° C. before the glycerol was added. The survival at 10° and 15.5° C. was[20] reduced with each rise in temperature. Thus, it appears that cooling to refrigerator temperature (4-5° C.) before adding the glycerol should be a part of the routine procedure.
A comparison of the results from adding the glycerol in 5, 3, and 1 equal portions is given also in Table 11. Little difference in survival during freezing was noted between the three rates of addition. Using 3 equal additions resulted in slightly better results, but the advantage was not statistically significant. While little difference was evident from adding the glycerol in 3 portions as compared to 1, many still use 3 additions in the hope of obtaining a slightly better sperm survival. In fact, some have gone to a procedure of adding the glycerol dropwise with constant gentle agitation. This method has not been tested in this laboratory.
Allowing sperm to equilibrate with the glycerol. Allowing sperm to stand in the presence of glycerol is considered by some to be necessary in order that the glycerol penetrate the sperm heads before freezing. From the first successful attempts at freezing bull sperm came the practice of allowing 12 to 20 hours for this process of equilibration. A long equilibration time results in aging the sperm. Data from a number of sources indicate that a drop of approximately 5 percent in fertility in the field occurs with each 24 hours of aging in the test tube. Thus it would seem desirable to reduce the equilibration time to a minimum commensurate with good freezability in order to reduce the effects of aging (at 5° C.). Results of attempting to reduce equilibration time are given in Table 11. At 4.5° C., little variation in motility following freezing and thawing was found after equilibration times of 2, 6, and 18 hours. At the higher temperatures of 10° and 15.5° C., the shortest equilibration time—2 hours—was slightly more detrimental with the differences significant at the 5-percent level at 15.5° C. For all temperatures combined, 6 hours was significantly better than 2 or 18 hours.
Sugar additions and equilibration time. Early in their experiences in freezing semen, the Australian workers found a short equilibration time—30 minutes—to be satisfactory if sugars were added to the diluent.[5] This protective action of sugars during the equilibration period was confirmed in our investigations. The results of one phase of this study are shown in Table 12. From these data it can be seen that the presence of glucose or rhamnose at a level of 1.25 percent improved sperm survival during the period of equilibration. In another trial these sugars and two others, arabinose and xylose, were tested for their protective action in freezing semen. The percentages of surviving sperm remaining after the various steps in the freezing procedure with and without the presence of these sugars are shown in Table 13.
| Stage when observed | Sperm motility (percent) | ||||
| Glycerol only |
Glycerol and glucose |
Glycerol and rhamnose |
|||
| Fresh diluted semen | 56 | 56 | 56 | ||
| After glycerolization | 54 | 54 | 54 | ||
| After equilibration | |||||
| 2 | hours | 51 | 53 | 53 | |
| 6 | hours | 48 | 52 | 53 | |
| 12 | hours | 46 | 50 | 51 | |
| 18 | hours | 40 | 46 | 46 | |
[J] Glycerol level in the final frozen mixture was 7 percent. Sugars were added to a level of 1.25 percent.
Three of the sugars—glucose, arabinose, and rhamnose—protected the sperm during equilibration and freezing. Xylose was less effective, but its addition resulted in slightly better sperm survival than glycerol alone. It was found also that the methylene-blue reduction time (metabolic test for semen quality) was faster in samples to which the sugars had been added—after glycerolization, after equilibration, and after freezing the samples. This is confirming evidence for the presence of more living and actively metabolizing sperm in the portions to which sugars had been added.
(Average of 10 ejaculates)
| Stage of observation |
Sperm motility (percent) | ||||
| Glycerol only |
Glycerol and glucose |
Glycerol and arabinose |
Glycerol and xylose |
Glycerol and rhamnose |
|
| Fresh diluted semen | 63 | 63 | 63 | 63 | 63 |
| After glycerolization | 54 | 55 | 54 | 57 | 60 |
| After 18 hours equilibration | 39 | 43 | 44 | 39 | 46 |
| After freezing to -79° C. and immediate thawing |
28 | 34 | 34 | 29 | 24 |
| After 4 days at -79° C. | 23 | 26 | 26 | 25 | 27 |
[K] Glycerol level in the final frozen mixture was 7 percent. Sugars were added to a level of 1.25 percent.
[22]Substitutes for glycerol. Since glycerol was so effective in protecting sperm during freezing, many have assumed that related compounds might be even better. Several compounds, some related to glycerol and some not, have been tried as substitutes for glycerol in the freezing procedure. They include ethylene glycol, propylene glycol, trimethylene glycol, mannitol, sorbitol, dextrans, and seminal-plasma proteins. None of these materials has been as effective as glycerol in protecting sperm during freezing. In fact, several of the materials proved to be injurious to sperm prior to attempts to freeze the samples. While the work in our laboratory with these substances as glycerol substitutes was by no means finally conclusive, because of the many possible interactions of experimental conditions, sufficient data were gathered to lead us to abandon further study until greater promise of success might be evident.
Effect of freezing rate on sperm survival. Reports by one group of British workers in early trials on freezing bull semen indicated that the rate of cooling in freezing should not exceed 2° C. per minute between +5° and -15° C., although below -15° C. the rate could be faster. Another group expressed the view that semen could be plunged into dry ice at -79° C. after it had been cooled to -15° C. To clarify this part of the freezing procedure, 11 samples of semen were subdivided and portions of each were frozen at rates of 0.25°, 0.5°, 1.0°, 2.0°, and 4.0° C. drop per minute between +5° and -20° C. and then twice these rates between -20° and -79° C. Vials of each ejaculate at +5° C. were also plunged directly into an alcohol bath at -79° C. The samples which were cooled at the rates of 0.25°, 0.5°, 1.0°, 2.0°, and 4.0° C. per minute had the following percentages of motile sperm after thawing: 30, 40, 46, 44, and 44. A mean of 32 percent of the sperm in the samples that were plunged directly into an alcohol bath at -79° C. were motile after thawing. There were no statistically significant differences among the samples frozen at 1.0°, 2.0° or 4.0° C. per minute. All of the others had significantly lower survival rates. Thus, it is obvious that too slow a cooling rate and plunging the samples directly into a -79° C. bath from a temperature of +5° C. cause greater harm to the sperm than cooling at a rate between 1.0° and 4.0° C. per minute.
Some investigators have suggested that rapid cooling below -20° C. is not detrimental to frozen semen. This idea was tested in conjunction with other experiments. Twenty-five samples cooled slowly (2°[23] C. per minute to -28° C., then 4° C. per minute to -79° C.) showed 62 percent sperm survival compared with only 45 percent when cooled rapidly below -28° C. (2° C. per minute to -28° C. then plunged into bath at -79° C.). Thus, rapid cooling was detrimental even after the critical temperature range of +5° C. to -20° C. had been passed.
Rate of cooling in plastic and in glass. Plastic vials do not conduct the cold as rapidly as glass ampules do. The temperature in both glass and plastic containers tends to lag behind the change in the bath in which they are immersed as is shown in Figure 4.
Temperatures in the immersion bath were recorded in a 2-milliliter glass ampule containing 1 milliliter diluted semen and in an 8-milliliter plastic vial containing 2.5 milliliters of diluted semen. A second plastic vial and glass ampule filled to capacity with diluted semen showed a cooling rate almost identical to that shown in Figure 4. It was obvious from the comparison that samples in the plastic vials cooled slower than those in glass and that the volume of semen (at least the small volumes used) in the vials had little effect on the rate of cooling. In another experiment, it was shown that the volume of diluted semen in the ampule to be frozen (0.2, 1.0 or 5.0 ml.) had little or no effect on the survival of the sperm.
In freezing and storing bull sperm, an alcohol bath containing dry ice at a temperature of -79° C. has been used as a cooling agent. In many areas, the availability of dry ice is limited and the cost is rather high. Mechanical means are available for obtaining temperatures as low as, or lower than, -79° C. but for the most part they are expensive. If warmer temperatures were suitable for storing frozen semen, the ordinary deep-freeze, which operates at -15° to -25° C., might be used.
Storage at temperatures from -23° to -79° C. In testing the effects of storage temperatures on the survival of frozen bull sperm (in a diluent containing 7 percent glycerol), 9 ejaculates were frozen and kept at -23°, -37°, -51°, -65°, and -79° C. The desired temperatures were maintained by dropping pieces of dry ice into ethyl alcohol baths as needed. Samples were thawed after 1 hour, 1 day, 3 days, and 5 days. After 1 hour, the samples maintained at the various temperatures exhibited approximately equal motility (Fig. 5).
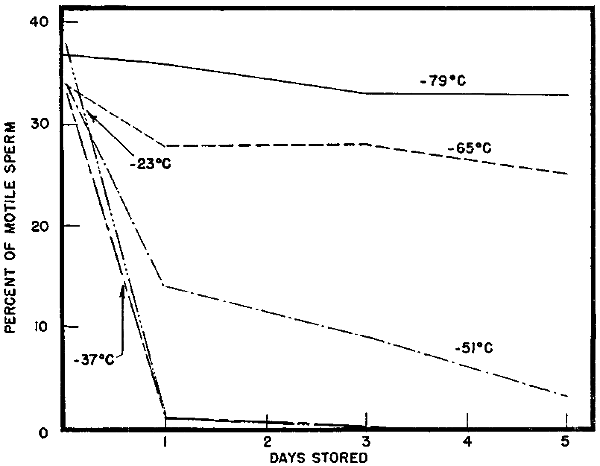
| Effect of freezing and storing bull sperm at various temperatures on the sperm motility at thawing | |
| (average of 9 ejaculates) | (Fig. 5) |
[25]At the end of 1 day, samples stored at -79° C. exhibited approximately the same motility as did similar samples stored for 1 hour. The samples stored at -65° C. had declined slightly in motility and those maintained at -51° C. had only one-third the motility which they had displayed at 1 hour. The samples at -23° and -37° C. exhibited practically no motility after 1 day in storage. After 5 days, only 3 of the 8 ejaculates stored at -51° C. showed motility upon thawing. Apparently detrimental changes take place more rapidly when the samples are stored at temperatures warmer than -65° C. The nature of these changes has not been determined. Reports from other laboratories indicate that storage temperatures much lower than -79° C. are just as satisfactory as -79° C.
No tests of the effects of storage at -79° C. for periods longer than 51 days have been conducted in this laboratory. Portions of 12 ejaculates were frozen and stored at -79° C. for various periods. One portion of each of these was examined on the second, ninth, 16th and 51st day of storage. The percent of motile sperm and rate of motility at each of these examinations were as follows:
| Day | 2 | 9 | 16 | 51 | ||||
| Percent of motile sperm | 49 | 46 | 40 | 38 | ||||
| Rate of motility | 2 | .5 | 2 | .3 | 2 | .2 | 2 | .2 |
The average prefreezing motility percentage for the above samples was 58, with an average rate of motility of 2.9. It is apparent from these results that the loss in motility was greatest due to the initial freezing, and after that the drop was most pronounced during the first 16 days of storage.
The British and the Australians have both reported the successful maintenance of fertility in frozen semen stored at -79° C. for over two years.[5]
Use of higher glycerol levels and a -20° C. storage temperature. In 1953, a report from Arkansas suggested that warmer storage temperatures could be used if a high percentage of glycerol were included in the freezing mixture.[7] To test the effectiveness of various glycerol levels on protecting sperm stored at deep-freeze temperatures, glycerol levels of 3.5, 5.5, 7.5, and 9.5 percent were used with portions of 4 semen samples. Survival in the portions frozen and stored at -20° C. was poor compared with the portions reduced and held at -79° C. In a second experiment, 4 samples were subdivided and frozen with a final concentration of 7, 11, 15, and 19 percent glycerol in the semen-diluent mixture. In this trial, poor results were obtained at -20° C.[26] except that glycerol at a level of 19 percent protected the sperm more effectively than at lower levels. Maximal survival at -79° C. was obtained at the 7-percent glycerol level. A final trial was run, using glycerol levels of 7, 11, 15, 19, 23, 27, and 31 percent. The percentages of motile sperm present after storage at -79° C. and -20° C. are shown in Table 14.
(Average of 8 ejaculates)
| Storage temperature (°C.) |
Glycerol level (percent) |
Sperm motility after storage (percent) | |
| 18 hours | 42 hours | ||
| -79 | 7 | 61 | 61 |
| -20 | 7 | 2 | 1 |
| 11 | 3 | 1 | |
| 15 | 14 | 10 | |
| 19 | 30 | 22 | |
| 23 | 29 | 19 | |
| 27 | 25 | 18 | |
| 31 | 21 | 12 | |
While survival was fair over a short period of time with 19 percent glycerol at -20° C., deterioration was rapid during storage. After 18 hours of storage, the samples at -20° C. (19 percent glycerol) contained only one half as many motile sperm as were still present in the samples at -79° C. (7 percent glycerol). After 42 hours of storage, the best samples at -20° C. contained only one-third the number of motile sperm still present in the samples stored at -79° C. These trials leave little doubt that under the present system of freezing and storing, storage at ordinary deep-freeze temperatures is far inferior to storage at dry-ice temperatures.
The importance of carefully controlled cooling and storage has been emphasized in the foregoing sections. The need for controlling thawing rates and the temperature of thawing was not clearly defined in the early work on freezing bull semen. The British used a thawing temperature of 40° C., which was satisfactory. If there is a need to hold the semen for a time after thawing, then a lower thawing temperature might be more desirable so that cooling again will not be necessary.[27]
Comparison of thawing temperatures of 5° C. and 38° C. The effects of thawing at temperatures of 38° (body temperature) and 5° C. (refrigerator temperature) were investigated. The first trial involved thawing as rapidly as possible by dropping glass ampules of frozen semen into water baths at the two temperatures. The frozen semen samples contained glycerol levels of 4, 6, 8, and 10 percent. The mean percentages of motile sperm found after thawing thirteen diluted semen samples treated in this manner are shown in Figure 6.
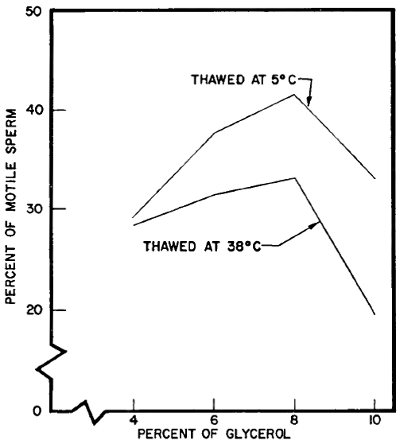
| Effect of glycerol percentage and thawing temperature on sperm | |
| motility after freezing and thawing | (Fig. 6) |
The 5° C. thawing temperature resulted in a higher percentage of sperm survival at all the glycerol levels than 38° C., with the difference in favor of 5° C. becoming greater as the glycerol level increased. The reason for the interaction between glycerol level and thawing temperature is not known. It may be that the presence of the higher levels of glycerol at 38° C. brought about harmful metabolic activity. The difference in survival of sperm in semen thawed at 5° C. and at 38° C. continued during storage at 5° C. (Table 15). It was also evident that the interaction between glycerol level and thawing temperature continued during storage (Fig. 7).
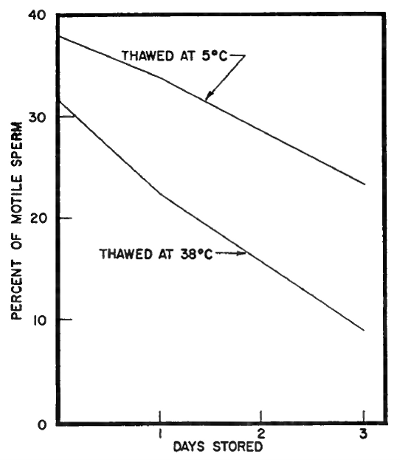
| Effect of thawing temperature on sperm motility during storage at 5° | |
| C. following freezing and thawing | (Fig. 7) |
(Average of 13 ejaculates)
| Thawing temperature (° C.) |
Glycerol level (percent) |
Sperm motility (percent) | |||
| Post- thawing |
After storage at 5° C. | Average | |||
| 1 day | 3 days | ||||
| 38 | 4 | 28.5 | 17.3 | 5.1 | 17.0 |
| 6 | 31.5 | 22.4 | 9.2 | 21.1 | |
| 8 | 33.1 | 15.0 | 4.6 | 17.6 | |
| 10 | 19.5 | 3.6 | 0.8 | 8.0 | |
| Average | 28.2 | 14.6 | 4.9 | 12.2 | |
| 5 | 4 | 29.2 | 21.7 | 19.8 | 23.9 |
| 6 | 37.7 | 33.8 | 23.5 | 31.7 | |
| 8 | 41.5 | 33.1 | 17.3 | 30.6 | |
| 10 | 33.1 | 18.5 | 6.0 | 19.2 | |
| Average | 35.4 | 26.8 | 16.6 | 20.6 | |
It is obvious that motility falls off rapidly after the semen is thawed. In a field trial in which the initial intent was to test the effect of glycerol levels on fertility of frozen semen, the semen was thawed in the morning and used during the same day. Survival of the sperm with 4 percent glycerol was so poor that only a few breedings were made with these samples. Even at 7 and 10 percent, the fertility results were much lower than with semen that had not been subjected to freezing. At that time it was felt that thawing the samples and using them throughout the day may have caused the low fertility results. Since[29] then, a large-scale experiment by Cornell University investigators, in cooperation with the New York Artificial Breeders’ Cooperative, has shown definitely that thawing should be delayed until a few minutes prior to breeding.[11] If the semen is used immediately, a thawing temperature of either 5° or 38° C. appears to be suitable. However, there is less danger of cold shock due to recooling if 5° C is used.
Thawing rate in plastic and in glass. Glass ampules transmit cold or heat more readily than plastic ones. The temperature rise is rapid in both glass and plastic when samples are taken from the storage box at -79° C. and placed in water at 5° C. However, complete thawing occurs more rapidly in glass than in plastic ampules. The changes in temperature that occurred when glass and plastic ampules were thawed in a water bath at 5° C. are shown in Figure 8. The initial temperature rise for the first minute or two was about the same, then the rate of warming in the plastic slowed and actual melting of the frozen sample occurred a little over a minute later in the plastic than it did in the glass. Both were thawed in less than four minutes.
By finding how methods of handling affect the sperm cells, one can sometimes improve the procedures to avoid harmful effects. Some attempts have been made in this laboratory to determine the effects of the freezing procedures on the metabolic activity of bull spermatozoa. These investigations have been limited in scope, involving the measurement of oxygen-consumption and estimates of sperm motility during and after incubation at 37° C. in a Warburg apparatus.
Effect of glycerol additions on oxygen uptake of diluted semen at 37° C. The effect of adding glycerol to diluted semen on oxygen consumption of the sperm was tested in a Warburg apparatus, using semen diluted with an extender consisting of one part egg yolk and one part 2.9 percent sodium citrate dihydrate. The yolk-citrate extender was added to the semen at a rate which brought the sperm concentration in 0.5 milliliter to 200 million to 500 million. An exact count was used to calculate the oxygen uptake per 108 sperm per hour (ZO2).
Glycerol in various percentages in 2.9 percent sodium citrate dihydrate solution was placed in the sidearm of the Warburg flasks.[31] The diluted semen was held in the main compartment. After a 60-minute preliminary run, in which the rate of oxygen uptake of the sperm in yolk-citrate diluent was determined, the contents of the sidearm were tipped into the main compartment. The resulting glycerol percentages after mixing the sidearm and main compartment contents were 0, 4, 8, and 12 percent. Ten samples of semen were subsampled and the oxygen uptake of each was determined at all four levels of glycerol.
Oxygen uptake was increasingly stimulated during the first 20-minute interval by each increase in the amount of glycerol added (Fig. 9). After the first 20 minutes, the rate of oxygen utilization decreased at the two higher levels of glycerol but persisted at 4 percent. The rate of oxygen consumption for the first 20-minute period at the 4-percent glycerol level was 130 percent that of the control to which only sodium citrate had been added. At 8 and 12 percent the values for the period were 144 and 192 percent, respectively, of the control rate.
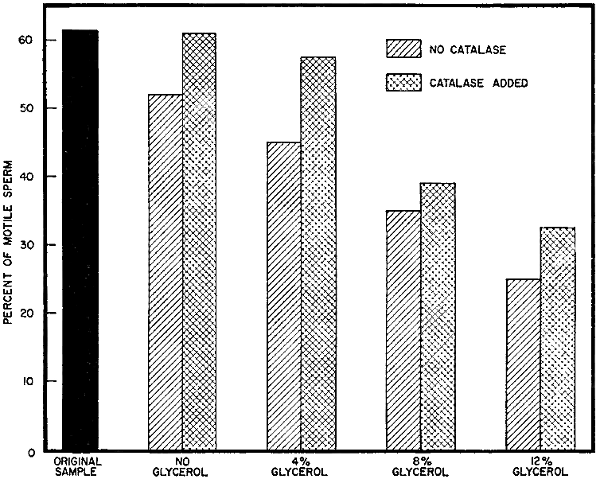
| Effect of glycerol and glycerol-plus-catalase additions on sperm motility during incubation at 37° C. | |
| (Fig. 10) | |
[32]With each increase in glycerol level, motility was reduced during the incubation period. This is shown in Figure 10 along with the effect on motility of adding catalase, which is discussed in the next section.
Effect of glycerol-plus-catalase on oxygen uptake of diluted semen. Certain bacteria have been shown to break glycerol down, forming hydrogen peroxide as follows:
Glycerol + oxygen → lactic acid + hydrogen peroxide.
Hydrogen peroxide is known to be detrimental to sperm. The addition of glycerol to diluted semen first increased oxygen uptake and then reduced it. Since a reduction in sperm survival followed, some harmful action must have taken place with the addition of glycerol at 37° C. To test whether this action could be due to the release of hydrogen peroxide as occurs in certain bacteria, glycerol with catalase—the enzyme which breaks down hydrogen peroxide—was added to a portion of 8 diluted semen samples and the oxygen uptake was recorded. Comparison of the resulting oxygen uptake with glycerol and with glycerol plus catalase is shown in Figure 11.
Oxygen consumption was increased by the presence of added[33] catalase at all glycerol levels and in the control. Sperm survival during the 3-hour period at 37° C. also was improved by the presence of catalase (Fig. 10). However, the general trend in oxygen consumption produced by the addition of glycerol was not changed greatly. The higher levels of glycerol still stimulated oxygen uptake during the first 20-minute period after the additions and then slowed the rate of oxygen utilization. The rate of utilization was generally higher during the test period in the presence of catalase than without added catalase. It appeared that a part of the harmful effect of glycerol might be due to the formation of hydrogen peroxide. Still, the detrimental effects of the higher levels of glycerol were not completely removed.
(Average of 5 ejaculates)
| Semen sample tested | Microliters of oxygen utilized per 108 sperm |
|||
| First hour | Second hour | |||
| Fresh diluted semen | 10.3 | 8.1 | ||
| Fresh diluted semen glycerol tipped in at end of first hour | 9.7 | [L] | 12.9 | [L] |
| Aged 20 to 24 hours at 5° C. | 11.2 | 8.3 | ||
| Aged 20 to 24 hours at 5° C. glycerol tipped in at end of first hour | 11.8 | [L] | 12.9 | [L] |
| After 20 hours equilibration with glycerol | 11.7 | [L] | 7.8 | [L] |
| After freezing and thawing | 9.7 | 6.3 | ||
Effect of freezing procedures on oxygen utilization by sperm. Limited data have been obtained on the effects of some of the freezing procedures on the oxygen utilization of bull sperm. The results obtained in these experiments confirmed the earlier findings that tipping glycerol directly into the diluted semen at 37° C. caused an increase in oxygen consumption (Table 16). All other steps in the freezing procedure had little effect on oxygen consumption by the sperm. Except where glycerol was added during the determination, the rate of oxygen utilization was lower the second hour than during the first. The oxygen uptake of semen that had been frozen and thawed seemed to drop faster than that of unfrozen samples.
Effect of freezing procedures on methylene-blue reduction time. The methylene-blue reduction test has been used as a means of measuring semen quality and is dependent on the metabolic activity of the[34] sperm. The effects of various freezing procedures on the ability of samples to decolorize methylene blue were determined with 10 semen samples. Sperm numbers were standardized to 300 × 106 cells per milliliter and the time required for these cells to reduce a 1:40,000 solution of methylene blue was determined on freshly diluted semen, after the addition of glycerol, after equilibration, and after freezing and thawing. Portions of each diluted sample were tested at these stages of the procedure with glycerol alone added and with glycerol and various sugars added.
A marked increase in the time required for the sperm to reduce methylene blue occurred when the glycerol was added (Table 17). This increase was greatest in the portions with glycerol alone and with glycerol and glucose. The time increase was less pronounced in the presence of the three pentose sugars used. Following equilibration, the samples regained the ability to reduce methylene blue at a rate only slightly slower than when they were fresh. Freezing and storage of semen resulted in slower reduction of the methylene blue than was shown after equilibration with glycerol. Since freezing usually kills some of the sperm, a slowing of the reduction time after freezing would be expected.
(Average of 10 ejaculates)
| Methylene-blue reduction time (minutes) | |||||
| Glycerol only |
Glycerol and glucose |
Glycerol and arabinose |
Glycerol and xylose |
Glycerol and rhamnose |
|
| Fresh semen | 5.2 | 5.2 | 5.2 | 5.2 | 5.2 |
| After glycerolization | 26.4 | 25.2 | 17.3 | 14.3 | 19.4 |
| After 18 hours equilibration | 7.4 | 6.5 | 6.4 | 5.3 | 6.2 |
| Thawed immediately after freezing | 11.5 | 10.5 | 9.4 | 9.0 | 9.4 |
| Thawed 48 hours after freezing | 14.3 | 10.2 | 11.3 | 10.1 | 9.5 |
[M] Glycerol level in the final frozen mixture was 7 percent. Sugars were added to a level of 1.25 percent.
Good results usually can be obtained in freezing bull semen if care is taken in collecting, diluting and processing the semen. Occasionally the semen from certain bulls will not withstand freezing well. The reason for this is not understood at present. However, carefully following the directions and suggestions given below will usually produce satisfactory results with semen samples that are of good quality at the start.
Experience in the field has shown that fertility results with frozen semen are usually slightly lower during the first few months than with liquid semen stored at 5° C. (41° F.). Most units that have worked with frozen semen over a period of a few months are able to improve and do get fertility results as good as, or better than, obtained in their liquid semen program.
Collection of the semen. In order to obtain the best possible semen for freezing, care and cleanliness should be exercised in making the collection. The artificial vagina, and the glassware used should be clean and dry. The underline of the bull should also be clean and dry. The bull should be restrained near the teaser cow for a minute or two prior to collection in order to excite the flow of secretions prior to ejaculation. Allowing the bull to mount the teaser once without serving the artificial vagina is a good practice to use in properly stimulating the bull before collection of the semen.
If the bull has not been used for three or four days, the collection of a second ejaculate for freezing may be advisable. The second ejaculate seems to withstand freezing better than the first in many instances. A clean, dry artificial vagina should be used for each ejaculate collected. Repeated collections in the same artificial vagina may result in contamination of the semen with bacteria, lubricating jelly and minute particles of dirt. The semen sample should be protected from contamination and from sudden temperature drops (cold shock).
Preparation of extender. A suitable egg yolk-citrate extender for freezing bull semen can be prepared by the following procedure. One part egg yolk (free of egg white and the membrane surrounding the yolk) is mixed with 4 parts 2.4 to 2.9 percent sodium citrate dihydrate solution. The citrate is prepared with distilled water and[36] then boiled or autoclaved. The citrate solution should be cooled before it is mixed with the egg yolk. After the egg and citrate are mixed, 1000 units of penicillin and 1000 micrograms of streptomycin are added per milliliter of extender. Sulfanilamide should not be added. This extender can be prepared 12 to 24 hours before use if it is stored at refrigerator temperature. The portion of the extender needed for the original dilution of the semen should be warmed to room temperature before it is mixed with the semen.
Dilution after collection. As soon as possible after collection, the semen sample should be diluted with the extender. The extender must be at the same temperature as the semen (room temperature) when the two are mixed together. At this time the semen can be partially diluted (1 part semen to 4 parts of extender) or diluted to a sperm concentration twice the final desired concentration (later in adding the glycerol for freezing, the semen is diluted further with an equal volume of glycerol containing extender). The diluted semen is slowly cooled (11⁄2 to 21⁄2 hours) to 5° C. (41° F.). Some units using frozen semen now allow the semen to stand at 5° C. for 5 to 6 hours before glycerolization to allow the antibiotics to be more effective against any vibrio fetus organisms that may be present. This step is taken because it has been shown that glycerol inhibits the effectiveness of the antibiotics.[6] After cooling, semen can be further diluted to twice the desired sperm concentration if that were not done at the start. (Caution: Be sure semen and diluent are at the same temperature.)
Adding the glycerol. The glycerol solution is prepared by adding 14 volumes of glycerol (reagent grade) to 86 volumes of yolk-citrate diluent (same as yolk-citrate used for original dilution). This solution may be added dropwise with constant gentle mixing to the already diluted semen, or one-third at a time at 10-minute intervals with gentle mixing during each addition. Either method should take about 20 to 30 minutes. The total volume of glycerol-yolk-citrate solution added should be equal to the volume of the original diluted semen. In this way a concentration of 7 percent glycerol is obtained in the final mixture that is to be frozen. Care must be taken to keep the temperature at 5° C. (41° F.) during the time the glycerol is being added. (A cold room is best for maintaining a temperature of 5° C., but with care the operation can be carried out at room temperature by using pans of ice water and a refrigerator.)
Equilibration. The results presented in this bulletin suggest that[37] little or no time need be allowed after the glycerol is added before freezing. However, results obtained by other workers show improved fertility with at least 12 hours equilibration. Some units getting good fertility results with frozen semen also are allowing the semen to stand at 5° C. for 12 to 18 hours before freezing. After the semen has equilibrated with the glycerol, 1-milliliter portions of the mixture are placed in 1.2- to 2-milliliter vials or ampules which are then sealed. Ampuling can be done with an automatic syringe or pipette, provided a large gage needle is used. Also, it is important not to force the fluid mixture rapidly through the syringe or the sperm may be injured.
Freezing. The vials or ampules of diluted semen are placed in a bath of isopropyl alcohol which has been cooled to 5° C. (41° F.). This bath can be a wide-mouth thermos bottle or an insulated container of almost any sort with a large opening at the top. The size needed depends on the number of ampules being frozen. Some sort of convenient tray for holding the ampules in an orderly fashion and enabling the samples to be completely submerged is desirable. A few ampules can be kept together easily by placing them in a polyethylene freezer bag that has had many small holes cut in it to let the alcohol of the bath contact the ampules. The ampules must be completely covered by the alcohol to insure uniform cooling.
The alcohol of the bath and the ampules of semen are cooled by adding chipped or ground dry ice in sufficient amounts to lower the temperature of the bath 2° C. (3.6° F.) per minute from +5° to -20° C. From -20° down to -79° C., the rate of cooling can be doubled (4° C. or 7.2° F.). Electrical equipment that regulates the cooling rate to the desired temperatures is available commercially, but the cost may be too high for some small operations. The samples should be held at -79° C. (-110° F.) until they are thawed. This can be done by using an alcohol bath and dry ice or by special mechanical refrigerating equipment. At no time prior to thawing should the samples be exposed to warmer temperatures.
Thawing. The ampules of frozen semen can be thawed by removing them from the dry ice storage box and dropping them into a water bath at 5° C. (41° F.). Thawing temperatures up to body temperature, 38° C. (100° F.), can be used but extreme care must then be taken not to pass the semen through a cold inseminating tube; for this would subject the sperm to cold shock. The semen should be used for breeding within a few minutes after thawing.
[1] Davenport, C. B. Effect of chemical and physical agents upon protoplasm. Macmillan and Co., New York. 1897.
[2] Polge, C., and Parkes, A. S. Possibilities of long-term storage of spermatozoa at low temperatures. Anim. Breeding Abs. 20:1-5. 1952.
[3] Emmens, C. W., and Blackshaw, A. W. The low temperature storage of ram, bull, and rabbit spermatozoa. Austral. Vet. Jour. 26:226. 1950.
[4] Smith, Audrey W. Effects of low temperatures on living cells and tissues. In biological applications of freezing and drying. Ed. R. J. C. Harris. Academic Press, Inc., New York, 1954.
[7] Proceedings of the American Dairy Science Association, 1953, 1954, and 1955. Published in the June issue of the Journal of Dairy Science for each year.
[8] Barker, C. A. V. Low temperature preservation of bovine epididymal spermatozoa. Canad. Jour. Comp. Med. 18:390-393. 1954.
[9] Saroff, Jack, and Mixner, J. P. The relationship of egg yolk and glycerol content of diluters and glycerol equilibration time to survival of bull spermatozoa after low temperature freezing. Jour. Dairy Sci. 38:292-297. 1955.
[10] Cragle, R G., Myers, R. M., Waugh, R. K., Hunter, J. S., and Anderson, R. L. The effects of various levels of sodium citrate, glycerol, and equilibration time on survival of bovine spermatozoa after storage at -79° C. Jour. Dairy Sci. 38:508-514. 1955.
[11] Bratton, R. W., Foote, R. H., and Cruthers, Joan C. Preliminary fertility results with frozen bovine spermatozoa. Jour. Dairy Sci. 38:40-46. 1955.
[12] Hafs, H. D., and Elliott, F. I. The effects of methods of adding egg yolk and monosaccharides on the survival of frozen bull spermatozoa. Jour. Dairy Sci. 38:811-815. 1955.
[13] Miller, W. J., and VanDemark, N. L. The influence of glycerol level, various temperature aspects, and certain other factors on the survival of bull spermatozoa at sub-zero temperatures. Jour. Dairy Sci. 37:45-51. 1954.
| °C. | °F. |
| +38 | +100 |
| +35 | +95 |
| +30 | +86 |
| +25 | +77 |
| +20 | +68 |
| +15 | +59 |
| +10 | +50 |
| +5 | +41 |
| 0 | +32 |
| -5 | +23 |
| -10 | +14 |
| -15 | +5 |
| -18 | 0 |
| -20 | -4 |
| -25 | -13 |
| -30 | -22 |
| -35 | -31 |
| -40 | -40 |
| -45 | -49 |
| -50 | -58 |
| -55 | -67 |
| -60 | -76 |
| -65 | -85 |
| -70 | -94 |
| -75 | -103 |
| -79 | -110 |
Transcriber's Notes:
The original text has not been modified, except that some minor typographical errors have been corrected silently.
Lettered footnotes (with anchors [A], [B], etc.), explaining the text, have been moved to directly below the paragraph or table they refer to. Numbered footnotes ([1], [2], etc.) refer to references, that are listed towards the end of the text.
Where a single footnote is referenced more than once, the backwards link to the anchor has not been implemented.
Back to top of document.