The cover above is simulated. Typographical corrections listed at the end of this version are highlighted in light green. The list of publications has been compiled after the article's text.
(Family Colubridae, from Middle America)
Lawrence
1963
Editors: E. Raymond Hall, Chairman, Henry S. Fitch,
Theodore H. Eaton, Jr.
Volume 15, No. 6, pp. 251-295, 9 figs.
Published October 18, 1963
University of Kansas
Lawrence, Kansas
JEAN M. NEIBARGER, STATE PRINTER
TOPEKA, KANSAS
1963
29-5936
| PAGE | |
| Introduction | 253 |
| Acknowledgments | 254 |
| Materials and Methods | 254 |
| Genus Conophis Peters | 255 |
| Key to the Species and Subspecies | 257 |
| Analysis of Characters | 257 |
| Scutellation | 258 |
| Size and Proportions | 258 |
| Color Pattern | 260 |
| Sexual Dimorphism | 260 |
| C. lineatus | 262 |
| C. lineatus dunni | 262 |
| C. lineatus lineatus | 267 |
| C. lineatus concolor | 270 |
| C. nevermanni | 272 |
| C. pulcher | 274 |
| C. vittatus | 277 |
| Skull | 282 |
| Dentition | 288 |
| Vertebrae | 288 |
| Hemipenes | 289 |
| Food and Feeding | 289 |
| Effect of Poison | 290 |
| 29 | |
| 292 | |
| 293 | |
Need for a comprehensive systematic review of the snakes of the genus Conophis was pointed out by Stuart (1954a, b). Since these snakes appeared to be of zoogeographic importance in the Central American region, I undertook the review as set forth on the following pages.[Pg_254]
For permission to examine specimens, and for information concerning specimens in their care, I am grateful to Mr. L. C. Battersby and Miss Alice G. C. Grandison, British Museum (Natural History); Mr. Charles M. Bogert and Dr. Richard G. Zweifel, American Museum of Natural History; Dr. Doris M. Cochran, United States National Museum; Prof. William B. Davis, Agricultural and Mechanical College of Texas; Dr. Josef Eiselt, Naturhistorisches Museums, Vienna; Prof. Norman Hartweg and Prof. Laurence C. Stuart, Museum of Zoology, University of Michigan; Dr. Robert F. Inger, Chicago Natural History Museum; Dr. Alan E. Leviton, California Academy of Sciences; Mr. Edmond V. Malnate, Academy of Natural Sciences, Philadelphia; Prof. George S. Myers, Stanford University Natural History Museum; Mr. Wilfred T. Neill, Ross Allen's Reptile Institute; Mr. Neil D. Richmond, Carnegie Museum; Dr. William J. Riemer, University of Florida Collections; Prof. Robert C. Stebbins, Museum of Vertebrate Zoology, University of California; Prof. Hobart M. Smith, University of Illinois Natural History Museum; and Dr. Ernest E. Williams, Museum of Comparative Zoology, Harvard.
Prof. William E. Duellman supplied invaluable information and guidance in my study. I am grateful to Prof. E. Raymond Hall for use of facilities of the Museum of Natural History and editorial assistance. I thank Prof. Laurence C. Stuart and Prof. Edward H. Taylor for information and suggestions. My own field experience in Middle America came as a result of assisting Professor Duellman in his own researches supported by a grant from the National Science Foundation (NSF-G 9827). For these things I am deeply grateful. Specimens that I have seen alive were collected by field companions Dale L. Hoyt and Jerome B. Tulecke. Finally, I am grateful to my wife, Margaret L. Wellman, for much help including typing much of the manuscript.
Of the 325 specimens of the genus Conophis available to me, representing most of those in museum collections, scale counts were made in the usual manner on 309. Ventrals were counted following the system proposed by Dowling (1951:97-99); the anal plate was not included. The anteroposterior position of the place where reduction occurs in the number of the dorsal rows of scales is designated by citing the number of the ventral scale directly beneath that place.
Measurements were taken to the nearest millimeter by means of a millimeter stick. Body length is the distance from the tip of the snout to the posterior edge of the anal plate; tail length, from the latter point to the tip of the tail; and total length, the sum of the body plus tail.
Descriptions of color are based on preserved specimens. Where descriptions of the color of living individuals are given, the data were taken from Kodachrome slides made available to me by William E. Duellman. Due to the transient nature of the longitudinal dark stripes in these snakes, no standard terminology has been devised, except that the posterior continuations of the stripes which on the head pass through the eye are termed lateral stripes; the posterior continuations of the median stripe of the head are termed dorsolateral [Pg_255] stripes. A paravertebral stripe is one that is present on the scale-row on either side of, but not including, the mid-dorsal (vertebral) scale-row.
In order to reduce confusion in the discussion of variation, the numbers designating the rows of dorsal scales are written as 1st, 2nd, whereas the numbers designating the stripes are written as first, second.
Except in three dried skeletons, teeth were counted on dentigerous bones in situ. Since teeth are often missing, the sockets were counted in order to obtain an accurate count.
In accounts of the species and subspecies, the observed range of variation is followed by the mean in parentheses; in some instances the mean is followed by the standard deviation, also in parentheses. An example is 65-79 (70.6 ± 3.93).
Each synonymy includes all generic and specific combinations known to me that have been used for the genus, and, in addition, references to catalogues, checklists, and reports of collections.
Localities of occurrence that are not plotted on the distribution maps are recorded in italic type under Specimens Examined. In the list of Specimens Examined the localities and specimens are listed in the following order: countries in alphabetical order; states or departments in alphabetical order in each country; localities in alphabetical order in each state or department; museum numbers in numerical order after the abbreviations of names of museums. When more than one specimen bears a single catalogue number, the number of specimens is given in parentheses following the museum catalogue number. Specimens for which data are given only as to country or to state or department are listed first after the name of that political unit under "no specific locality."
The abbreviations for the museum collections are:
| AMNH | American Museum of Natural History |
| ANSP | Academy of Natural Sciences of Philadelphia |
| BMNH | British Museum (Natural History) |
| CAS | California Academy of Sciences |
| CNHM | Chicago Natural History Museum |
| ERA-WTN | E. Ross Allen-Wilfred T. Neill, Ross Allen's Reptile Institute |
| KU | University of Kansas Museum of Natural History |
| MCZ | Museum of Comparative Zoology, Harvard |
| MVZ | Museum of Vertebrate Zoology, University of California |
| NMW | Naturhistorisches Museums Wien, Vienna |
| SU | Stanford University Natural History Museum |
| TCWC | Texas Cooperative Wildlife Collection, Agricultural and Mechanical College of Texas |
| UF | University of Florida Collections |
| UIMNH | University of Illinois Museum of Natural History |
| UMMZ | University of Michigan Museum of Zoology |
| USNM | United States National Museum |
Historical summary.—In 1854 Duméril, Bibron and Duméril described and figured Tomodon lineatum from America. In 1860 Peters described and figured as a new genus and species, Conophis vittatus, based on a specimen that he had obtained from a dealer in Hamburg. The provenance of this specimen is not known, for it was discovered aboard a ship near the mouth of the Mississippi River. It was not until 1871 that Cope included lineatus in the genus Conophis. Cope (1861) proposed the name Conophis vittatus (nec Peters, 1860). Later (1900) he changed its name to Conophis lineaticeps. Early uncertainty of the relationships of the species lineatus caused Günther (1858) to place it in the genus Psammophis. With the exception of Garman (1884a and 1884b) who placed lineatus in the genus Tachymenis, and Wettstein (1934) who reported five specimens of Conophis nevermanni as Coniophanes i. imperialis, all specimens reported after 1876 were placed in the genus Conophis.
The only previous attempt to review the systematics of this genus was made by Smith (1941) who based his study primarily on specimens in the United States National Museum. He examined only 28 specimens, including none of one species (nevermanni).
Description.—Hemipenis slightly bifurcate having forked sulcus spermaticus, large spines near base, and smaller spines or papillae on flounces nearer apices; prediastemal maxillary teeth 8-12, subequal in length, and followed by short diastema and one enlarged fang or two; fangs grooved, only one functional at any one time, unless snake is in process of shedding teeth; teeth 6-10 on palatine, 15 to 19 on pterygoid, 15 to 21 on dentary; teeth on dentary decreasing in size posteriorly; large parotid (venom) gland on either side of head in temporal region; head shields of basically unmodified colubrid type excepting decurved rostral; rostral concave below and therein modified for burrowing; internasals and prefrontals paired; nasals divided; loreal single; preocular one, rarely two; postoculars, two; supralabials, 7-8, 3rd and 4th or 4th and 5th under eye; infralabials, 8-11, usually 9 or 10; temporals, normally 1 plus 2 plus 3; chin-shields subequal in length; ventrals, 149-183, rounded and overlapping; caudals, 55-89, paired and imbricate; anal divided; dorsal [Pg_257] scales smooth and in 19 rows at mid-body with no apical pits or keels; scale reduction normally involving fusion of 3rd and 4th rows, resulting in 17 scale-rows near tail; tail length more than 20 per cent of body length; maximum total length exceeding 1.1 meters; dorsal color pattern consisting of dark stripes, or no darkening, on paler ground-color; ventral surfaces immaculate pale yellowish or white, except on specimens having single lateral dark spots on some or all ventrals; pupil round; diurnal or crepuscular; feeding primarily on small lizards, sometimes on small mammals or other snakes.
Distribution.—Semi-arid regions of southern México and Central America as far south as Costa Rica.
Although many juveniles differ greatly in general coloration from the adults, both the juveniles and the adults of any species or subspecies can be identified from the following key; juveniles differ from adults in extent and intensity of dark pigmentation but not in rows of scales involved.
| 1. | Seven supralabials (3rd and 4th below orbit); 3 to 8 dark stripes along body |
| 2 | |
| Eight supralabials (4th and 5th below orbit); unstriped or with more than 4 dark stripes along body, or dark with 2 or 4 pale stripes | |
| 3 | |
| 2. | Dark stripes involving no more than one longitudinal scale-row |
| C. lineatus lineatus (part), p. 267 | |
| Dark stripes involving at least two adjacent scale-rows | |
| C. vittatus, p. 277 | |
| 3. | Supralabials having black borders above; head and body generally black with 2 or 4 white lines running length of body |
| C. nevermanni, p. 272 | |
| Supralabials immaculate or having dark borders below; head and body usually pale with dark stripes, or without stripes | |
| 4 | |
| 4. | Lateral dark stripe through eye involving upper half of second scale-row; dark stripe on paravertebral row, at least posteriorly |
| C. pulcher, p. 274 | |
| Lateral dark stripe becoming indistinct on body, or restricted to 4th or 3rd and 4th rows anteriorly, not involving 2nd scale-row on anterior 1/3 of body (an auxiliary lateral stripe sometimes present involving 2nd row); no paravertebral stripes | |
| 5 | |
| 5. | Stripes disappearing posteriorly (except for small spots of pigment on scale-row 4 or 7); 1st scale-row unpigmented |
| C. lineatus concolor, p. 270 | |
| Stripes present posteriorly; 1st scale-row pigmented | |
| 6 | |
| 6. | Lateral stripes narrow on nape, restricted to 4th scale-row on body |
| C. lineatus lineatus (part), p. 267 | |
| Lateral stripes involving 3rd and 4th rows, at least on nape | |
| C. lineatus dunni, p. 262 | |
Characters showing inter-specific and intra-specific variation and that have a wide range of variation were analyzed statistically, when possible, in order to determine extent of variation. One character (see table 3) was analyzed for sexual dimorphism, and for it the coefficient of difference is also given. The statistical terms and formulae have been adopted from Mayr, Linsley and Usinger (1953). Dorsal head shields varied individually and were of no taxonomic importance. Osteological and hemipeneal characters did not show enough variation to be considered here.
Labials, dorsals, ventrals, and subcaudals were the most useful scales.
Labials.—All species usually have eight supralabials except C. vittatus, which has seven. The only other population having a relatively high frequency of occurrence of seven supralabials is C. l. lineatus. In specimens having eight supralabials, the fourth and fifth enter the orbit; in specimens having seven supralabials, the third and fourth enter the orbit (the second and third are fused). Usually there are ten infralabials, sometimes nine or eleven; specimens having seven supralabials usually have nine infralabials, sometimes eight, rarely ten.
Dorsals.—Although there is no variation in the number of rows of dorsal scales, there is some in the method of scale reduction. There are 19 rows of dorsal scales from close behind the head to about midway on the body where two rows are lost, leaving 17 rows from there to near the base of the tail. This reduction is accomplished by fusion of the scales of the 3rd and 4th rows or sometimes by the dropping out of the 3rd row. The place at which reduction occurs in number of dorsal scales in relation to the ventral (scale) directly below is highly variable and of little taxonomic importance (table 1).
| Taxon | Number of Specimens | Range | Mean | Standard Deviation | Standard Error | Coefficient of Variation |
| l. concolor | 45 | 89-114 | 102.5 | 5.57 | 0.83 | 5.43 |
| l. dunni | 36 | 91-111 | 102.1 | 4.59 | 0.77 | 4.50 |
| l. lineatus | 26 | 91-107 | 100.2 | 3.59 | 0.72 | 3.58 |
| nevermanni | 6 | 84- 97 | 93.2 | 4.71 | 1.92 | 5.05 |
| pulcher | 26 | 94-119 | 104.6 | 4.90 | 0.96 | 4.68 |
| vittatus | 170 | 84-118 | 102.3 | 6.60 | 0.16 | 6.45 |
Ventrals.—The number of ventral scutes varies from 149-183, and shows no significant variation in the means (table 2).
Subcaudals.—The number of subcaudal scutes varies from 55 to 89. In some populations there is no overlap in the range of variation of males and females. The total variation and sexual dimorphism are analyzed in table 3.
Although considerable variation in size is observable, little taxonomic use is made of size since sufficient series are not available to determine age classes. The subspecies attaining the largest size is C. lineatus concolor; all others are smaller and of about the same size and proportions. The longest specimen, a male of C. l. concolor, has a body length of 893 mm., a tail length of 274 mm., and a total length of 1167 mm.
| Taxon | Number of Specimens | Range | Mean | Standard Deviation | Standard Error | Coefficient of Variation |
| l. concolor | 45 | 158-170 | 163.7 | 1.56 | 0.23 | 0.95 |
| l. dunni | 36 | 159-178 | 167.2 | 4.56 | 0.76 | 2.72 |
| l. lineatus | 26 | 157-169 | 163.5 | 3.59 | 0.72 | 2.20 |
| nevermanni | 6 | 173-183 | 176.5 | 4.00 | 1.63 | 2.27 |
| pulcher | 26 | 149-180 | 169.5 | 5.31 | 1.04 | 3.13 |
| vittatus | 171 | 149-180 | 163.7 | 6.33 | 0.15 | 3.87 |
of Subcaudals in Conophis
| Taxon | Sex | Number of Specimens | Range | Mean | Standard Deviation | Standard Error | Coefficient of Variation | Coefficient of Difference |
| lineatus concolor | ♂ | 22 | 68-74 | 70.3 | 2.14 | 0.46 | 3.04 | |
| 1.97 | ||||||||
| ♀ | 16 | 56-65 | 61.8 | 2.18 | 0.55 | 3.53 | ||
| lineatus dunni | ♂ | 14 | 67-80 | 74.5 | 3.86 | 1.03 | 5.18 | |
| 0.95 | ||||||||
| ♀ | 16 | 60-72 | 67.1 | 3.91 | 0.97 | 5.82 | ||
| lineatus lineatus | ♂ | 11 | 67-73 | 69.8 | 6.17 | 1.85 | 8.84 | |
| 0.60 | ||||||||
| ♀ | 9 | 60-66 | 62.4 | 6.17 | 2.06 | 9.89 | ||
| nevermanni | ♂ | 3 | 82-89 | 85.3 | …… | …… | …… | |
| …… | ||||||||
| ♀ | 2 | 71-76 | 73.5 | …… | …… | …… | ||
| pulcher | ♂ | 7 | 70-79 | 74.3 | 3.11 | 1.17 | 4.19 | |
| 0.93 | ||||||||
| ♀ | 11 | 65-71 | 68.2 | 3.42 | 1.08 | 5.01 | ||
| vittatus | ♂ | 95 | 59-76 | 67.8 | 3.33 | 0.34 | 4.91 | |
| 1.28 | ||||||||
| ♀ | 58 | 55-66 | 60.0 | 2.75 | 0.36 | 4.58 |
This is the primary feature used to separate species and subspecies in this genus. The color pattern consists of three black or deep brown stripes on the dorsal part of the head, one mid-dorsally, and one on each side of the head passing through the eye. On the body, there are usually dark longitudinal stripes on a pale tan or white background. There may be as few as three in vittatus, and as many as 13 in l. dunni; except that there is none in C. l. concolor. There are two pairs of primary dark stripes. The first is the body stripe that is the posterior extension of the stripe which on the head passes through the eye and is termed the lateral stripe. The other primary stripe is the posterior continuation of the mid-dorsal head stripe. Usually it is split into two dorsolateral stripes on the body. Stripes may be present on the scale-row to either side of the primary stripe. These stripes are usually dark brown or black and are the secondary stripes. Finally, additional stripes may be present that are paler brown and bear no direct relationship to the primary stripes. These are auxiliary stripes.
Every stripe originates either as broad continuous stripe or as a row of spots or dashes, forming a discontinuous stripe, which in some specimens becomes continuous posteriorly. The stripes are usually black or deep brown, although auxiliary stripes are sometimes paler. The dorsal ground color is pale brown, tan, olive, or white; usually the ground color is palest ventrally and darkest dorsally.
In some specimens of Conophis the lateral tips of the ventrals are spotted, one spot on each end of each ventral. Otherwise, the ventrals are immaculate white.
In some species there is considerable ontogenetic change in color pattern, although the juveniles bear the basic color characteristics of the adults. For example, juveniles of the sympatric species C. lineatus dunni and C. pulcher can be separated on the basis of which scale-rows are darkly pigmented. C. l. dunni has eight stripes in juveniles and as many as 13 in adults. Juveniles show a greater contrast between the black stripes and the pale ground color than do adults. With increased age (size) the stripes in some populations become paler and are split; simultaneously the ground color becomes darker.
Sexual dimorphism is evident in all species and subspecies of Conophis. Differences always exist in the number of subcaudals and in the tail/body ratio; males have more subcaudals and relatively longer tails than do females (table 3). Otherwise, there is little sexual dimorphism in these snakes. Males and females cannot be differentiated by any feature of coloration.
Formulation of a biological concept of the species as defined by Mayr (1942) is difficult when most of the data primarily relied upon are from preserved specimens. Nevertheless, a total view of variation was attempted so that differences within and between populations could be recognized. Differences, between populations, [Pg_261] that seem to be part of a continuous or internal cline (Huxley, 1942) are not used for characterizing subspecies.
Tomodon lineatum (in part) Duméril, Bibron and Duméril, Erpétologie Générale, 7(pt. 2):936-938, February 25, 1854.
Diagnosis.—No dark pigmentation posterior to nape; lateral dark stripe anteriorly passing through eye and posteriorly involving 4th or 3rd and 4th scale-rows only; first scale-row darkly pigmented; no paravertebral dark stripe; six to thirteen (or no) dark stripes at mid-body; usually eight (sometimes seven) supralabials immaculate white or having dark ventral margins.
Variation.—The variation in this species is discussed more completely in the descriptions of the subspecies. One hundred and seven specimens have 157 to 178 (164.8) ventrals. Eighty-eight of these snakes having complete tails have 56 to 80 (68.0) subcaudals; the number of ventrals plus subcaudals varies from 222 to 247 (233.5) in 87 of these. On 107 specimens the reduction from 19 to 17 dorsal scale-rows takes place between ventrals 89 and 114 (101.8). Sexual dimorphism is evident in the number of subcaudals; there are, on the average, fewer subcaudals in females than in males of each subspecies. The largest specimen is a male C. l. concolor (USNM 46345) from Chichén Itzá, Yucatán, México, having a body length of 893 mm., a tail length of 274 mm. and a total length of 1167 mm. The smallest is a juvenile C. l. dunni (MCZ 49749) from Tegucigalpa, Honduras, having a body length of 162 mm., a tail length of 51 mm. and a total length of 213 mm.
The greatest variation is in coloration. Dark color, or lack thereof, has been used to separate the subspecies of C. lineatus. The ground-color is pale brown, pale olive or white, either with no stripes on the body or with eight to thirteen dark stripes at mid-body. Specimens having dark stripes on the body always have black or dark brown pigmentation on the first, 4th and 7th dorsal scale-rows. In some there is dark pigmentation on the 2nd, 3rd, 8th and 10th rows of scales. The stripes appear on the nape or farther posteriorly, usually on the anterior third of the body, either as a series of spots or dashes that form a continuous stripe farther posteriorly or as a continuous stripe.
The ventrals usually have more or less conspicuous dark spots laterally on those specimens having dark stripes present on the dorsum; spots are absent on all specimens having no dorsal stripes and on some specimens having dorsal stripes. Except for the dark lateral spots (when present) the ventrals are immaculate white. Usually the dorsal ground-color is pale tan, especially on the striped forms. The ground-color is usually palest on the lower dorsal scale rows and darkest dorsally.
Three populations are separable as subspecies; one has no stripes on the body and occurs in the Yucatán Peninsula. The other two have stripes on the dorsum and vary clinally in coloration from the north (Veracruz, México) to south (Costa Rica) (Fig. 2). Reasons for separating these widespread, variable snakes into two subspecies are that they are discontinuous in distribution (the population in Veracruz is disjunct from the one that extends from Guatemala to Costa Rica), and that these populations have distinctly different color patterns.

Conophis lineatus, Cope, 3rd Ann. Rept. Peabody Acad. Sci., p. 82, 1871; Proc. Acad. Nat. Sci. Philadelphia, 23:204, October 24, 1871; Journ. Acad. Nat. Sci. Philadelphia, ser. 2, 8:137, 1876; Bull. U. S. Natl. Mus., 32:77, 1887; Günther, Biologia Centrali-Americana, p. 165, March, 1895; Boulenger, Catalogue of the Snakes in the British Museum (Natural History), 3:122-123, 1896; Werner, Arch. Naturges., 90, abt. A, 12:143, 1925; Schmidt, Zool. Ser. Field Mus. Nat. Hist., 12:199-200, November 21, 1928; Amaral, Mem. Inst. Butantan, 4:212, 1929; Werner, Zool. Jahrb., 57:184, 1929; Stuart, Occas. Papers Mus. Zool. Univ. Michigan, 292:5, June 29, 1934; Dunn, Copeia, no. 4:214, December 31, 1937.
Conophis lineatus similis Smith, Journ. Washington Acad. Sci., 31:123-124, March 15, 1941 (Type.—United States National Museum, No. 79963; type locality.—Managua, Nicaragua; nec Bocourt in Duméril, Bibron and Mocquard, Mission Scientifique au Mexique et dans l'Amerique Centrale, 2:647-648, 1886); Cochran, Bull. U. S. Natl. Mus., 220:167, 1961.
Conophis lineatus dunni Smith, Proc. U. S. Natl. Mus. 92:394-395, November 5, 1942; Savage, Trans. Kansas Acad. Sci., 50:483-486, December 31, 1949; Taylor, Univ. Kansas Sci. Bull., 34(pt. 1):145, October 1, 1951; Neill and Allen, Publ. Res. Div. Ross Allen's Rept. Inst., 2:56, November 10, 1959; Herpetologica, 16:146-148, fig. 2, September 23, 1960.
Conophis pulcher pulcher, Stuart, Misc. Publ. Mus. Zool. Univ. Michigan, 69:79, June 12, 1948; Contr. Lab. Vert. Biol. Univ. Michigan, 45:24, [Pg_264] May, 1950; Contr. Lab. Vert. Biol. Univ. Michigan, 49:14, August, 1951; Contr. Lab. Vert. Biol. Univ. Michigan, 65:19-20 (part), March, 1954.
Conophis pulcher plagosus, Mertens, Zool. Anz., 148:93, February, 1952; Abhand. Senken. Naturw. Gesell., 487:61-62, December 1, 1952.
Conophis lineatus nevermanni, Taylor, Univ. Kansas Sci. Bull., 37(pt. 1):563-565, fig. 16, October 15, 1955.
Type.—United States National Museum, no. 79963, obtained by Lt. H. C. Kellers. Type locality: Managua, Nicaragua. There are also three paratypes; one a topotype (USNM 79964), one from "Nicaragua" (USNM 25237), and one from Esparta, Costa Rica (USNM 37758).
Diagnosis.—Lateral dark stripe anteriorly passing through eye and posteriorly involving 3rd and 4th scale-rows; 1st scale-row darkly pigmented; no paravertebral dark stripe, although vertebral row sometimes darkly pigmented; six to thirteen stripes at mid-body; eight supralabials immaculate or having dark ventral margins.
Variation.—Thirty-six specimens have 159 to 178 (167.2 ± 4.56) ventrals. Thirty of these snakes having complete tails have 60 to 80 (70.5 ± 5.36) subcaudals; the number of ventrals plus subcaudals varies from 224 to 247 (237.6). In 36 specimens the reduction from 19 to 17 dorsal scales takes place between ventrals 91 and 111 (102.1 ± 4.59). Sexual dimorphism is evident in the number of subcaudals; 16 females have 60 to 72 (67.1), and 14 males have 67 to 80 (74.5) subcaudals. The largest specimen (ERA-WTN BH-300) is a female from Augustine, British Honduras, having a body length of 732 mm., a tail length of 183 mm. and a total length of 915 mm. A juvenile (MCZ 49794) from Tegucigalpa, Honduras, has a body length of 162 mm., a tail length of 51 mm. and a total length of 213 mm.
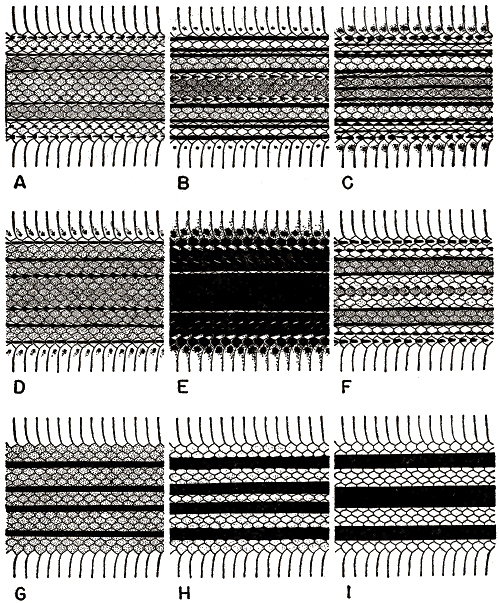
The greatest variation is in coloration. The ground-color is pale brown or white with dark stripes of black or deep brown present dorsally and laterally. Some specimens from Costa Rica have as many as 13 dark stripes at mid-body (fig. 1, C). In these snakes the first row of dorsal scales bears a series of large, slightly elongated, dark spots; on the 2nd row a narrow dark brown stripe on the middle of the scales; on the 3rd a black stripe on the dorsal one-third to one-half of the scales; on the 4th and the 7th rows black stripes on the medial half of the scales of each row; on the 8th and 10th (vertebral) rows dark brown stripes on the medial third of the scales of each row. A specimen from Guatemala (UMMZ 107339) shows the greatest reduction of stripes and dark pigmentation (fig. 1, A); it has only eight stripes at mid-body: on the first row of dorsal scales a discontinuous stripe is formed by a series of dashes; the 3rd row bears a series of small black spots near the base and tip of each scale; the 4th and 7th rows bear continuous black stripes on the medial third to fourth of the scales of each row; the 8th row has extremely small dark spots near the tips of some scales.
The primary stripes, characteristic of the species lineatus, are those on the 1st, 4th and 7th rows of dorsal scales; these are the most prominent stripes. In some specimens these primary stripes begin as spots or dashes on the nape and become continuous stripes posteriorly; in others they are continuous for the length of the body. The stripe on the 1st row is most variable; usually it consists of only a discontinuous series of dashes for most of its length. The secondary stripes are those on the 3rd and 8th rows; of these, only the one on the 3rd scale-row is present on the nape. The stripe on the 3rd row in [Pg_265] combination with the dark stripe on the 4th row is the posterior continuation of the dark stripe that on the head passes through the eye; this stripe is characteristic of C. lineatus dunni. Both secondary stripes usually begin anteriorly as a series of spots or dashes and become continuous stripes posteriorly; occasionally near the base of the tail they fuse with the primary stripes on the 4th and 7th rows. In some specimens in Costa Rica indistinct stripes are present on the 10th (posteriorly the 9th) rows, and in some specimens in Honduras, Nicaragua, and Costa Rica similar indistinct stripes are present on the 2nd row.
Usually there are more or less conspicuous dark spots laterally on the ventrals, but in some specimens there are no spots. Except for the dark lateral spots (when present) the ventrals are immaculate white. The dorsal ground-color is a pale brown or brownish white in preserved specimens on the 1st, 2nd, 3rd and 4th rows of scales where dark stripes or spots are not present. The ground-color of the dorsum between the 5th rows on each side is a somewhat darker shade of pale to medium brown.
Never is more than the lower one-third of each of the supralabials brown. In many specimens little or no brown is present on the lower margins of these scales. Some of the specimens having brown on the supralabials also have dusky markings of tan or gray on the chin and infralabials. Specimens from the northern part of the range (Guatemala) less frequently have dark chins and supralabials than do specimens from the southern part of the range (Costa Rica). There is, nevertheless, at any one locality considerable variation in the amount of dark pigmentation present on the chin and supralabials, thereby indicating that the slight geographic trend in this character is not significant.
Probably the most common pattern of dorsal coloration consists of eight or ten dark stripes (fig. 1, B). In snakes having this pattern the stripes on the 1st, 3rd, 4th and 7th rows are always present and prominent, although those on the 1st and 3rd rows sometimes are present as discontinuous rows of dashes. The ground-color from the venter to the 7th row is usually pale brown, and that dorsally between the 7th rows on each side is usually a darker, medium brown. A series of spots or dashes or a continuous stripe is sometimes present on the 8th row of scales.
Snakes having a larger number of dark stripes and more dark pigmentation occur in the southern part of the range. There seems to be a cline from paler snakes having fewer stripes in the north to darker snakes in the south.
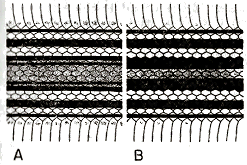 |
Fig. 3. Patterns of dorsal coloration at mid-body of juveniles of two sympatric species of Conophis. A. C. lineatus dunni (MCZ 49794) from Tegucigalpa, Honduras. B. C. pulcher (MCZ 49791) from Tegucigalpa, Honduras. Approximately × 1. |
In juveniles, there are six or eight black stripes boldly contrasting with a white or pale tan ground-color (fig. 3, A). The first pair of stripes is on the [Pg_266] 1st scale-row; the second pair, on the 3rd and 4th scale-rows; the third pair, on the 7th row; the fourth pair (when present), on the 8th row. Ontogenetic change in coloration consists of the splitting of the second pair of dark stripes in the juvenile. Additional stripes may form later on the 2nd and/or 10th rows of dorsal scales.
Remarks.—Savage (1949:483-486) stated that his specimen of C. l. dunni (from Honduras) resembled l. lineatus in having secondary stripes on the 2nd and 8th rows and dark pigmentation throughout the length of the 2nd row. As can be seen from the preceding discussion of variation, a specimen having this color pattern is clearly within the observed range of variation of l. dunni. The specimen in no way represents an intergrade between C. l. dunni and l. lineatus.
A specimen in the British Museum (Natural History), catalogued in 1853 (no. 53.2.4.16), has the locality listed as "México." Since this specimen is of C. l. dunni and this subspecies occurs only south of México, the locality must be considered erroneous; possibly the locality as recorded referred only to the fact that the specimen came from tropical Middle America.
The absence of paravertebral stripes, the presence of a lateral dark stripe on the nape involving the 3rd and 4th rows of scales, and the darkly pigmented 1st scale-row, in combination with the characteristics of the genus, distinguish C. l. dunni from all other snakes in México and Central America. The only sympatric species of this genus, C. pulcher, differs in that it has paravertebral stripes (though never a vertebral dark stripe). Conophis pulcher has a lateral dark stripe that includes the upper half of the second scale-row on the anterior part of the body; stripes of C. l. dunni never include more than the 3rd and 4th rows. Even as juveniles the paravertebral row is not darkly pigmented in C. l. dunni as it is in C. pulcher.
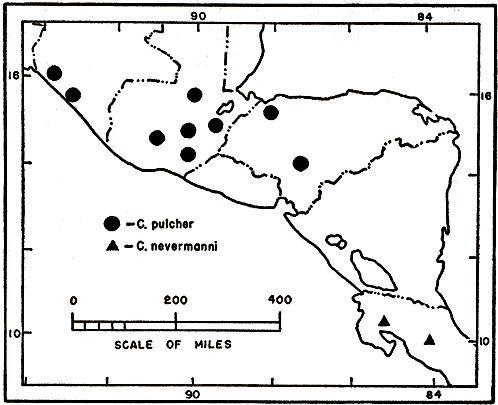
Distribution.—Semi-arid habitats from sea level to elevations of 1000 m. from the Cuilco Valley in western Guatemala, El Peten and British Honduras southeastward to northeastern and southern Honduras, western Nicaragua and northwestern Costa Rica (fig. 2).
Specimens examined.—Total of 41 specimens, as follows: British Honduras: Cayo District: Augustine, ERA-WTN BH-300; Mountain Pine Ridge, 10 mi. E Augustine, ERA-WTN BH-298.
Costa Rica: no specific locality, AMNH 17309. "Cartago," BMNH 71.11.22.15. Puntarenas: 32 km. N Barranca, KU 35630; Esparta, USNM 37758. "San José," ANSP 3480, 12232.
El Salvador: Morazan: El Divisadero, CNHM 10999. San Miguel: San Pedro, MCZ 57061.
Guatemala: El Petén: Sojio (Toocog), AMNH 69969, 69986. Huehuetenango: flood plain Río Cuilco, W of Finca Canibal, 18 km. N Tacaná, UMMZ 98283. Santa Rosa: Santa Rosa, UMMZ 107339.
[Pg_267] Honduras: no specific locality, AMNH 32814, UF 7657. Cortes: Cofradía, SU 8422; Gracias, CNHM 28560; Hacienda de Santa Ana, W San Pedro Sula, CNHM 5297; San Pedro Sula, UMMZ 68695(2); near San Pedro Sula, MCZ 27563. Francisco Morazan: Potrero de Melio, Escuela Agricola Pan-Americana, MCZ 49987; Tegucigalpa, MCZ 49784, 49786, 49789-90, 49792, 49794.
Mexico: no specific locality, BMNH 53.2.4.16.
Nicaragua: no specific locality, UMMZ 65633, USNM 25237. Leon: El Polvón, MCZ 5645, 5696. Managua: Managua, USNM 79963-64; 3 mi. SW Managua, KU 42315; 8 mi. WNW Managua, KU 42314; 1 mi. N Sabana Grande, KU 42311-13. Matagalpa: 1.5 mi. N Matagalpa, UMMZ 116537.
Tomodon lineatum (in part) Duméril, Bibron and Duméril, Erpétologie Générale, 7(pt. 2):936-938, atlas, pl. 73, February 25, 1854; Bocourt, Journ. de Zool., 5:406-407, 1876.
Tomodon lineatus, Jan, Arch. Zool. Anat. Fis., Genoa, 2(2):234, March 1863; Elenco sistematico degli ofidi. Milano, p. 57, 1863; Muller, Reisen in den Vereinigten Staaten, Canada, und Mexico. Bd. 3. Beitrage zur Geschichte, Statistik, und Zoologie von Mexiko. 3:607, 1865; Jan and Sordelli, Iconographie Generale des Ophidiens, Milano. liv. 19, pl. 6, fig. 3, December, 1866; liv. 50, pl. 2, fig. 34, November, 1881.
Tachymenis lineata (in part), Garman, Bull. Essex Inst., 16: 33, January 9, 1884; Mem. Mus. Comp. Zool., 8:60-61, July, 1884.
Conophis lineatus, Bocourt in Duméril, Bocourt and Mocquard, Mission Scientifique au Mexique et dans l'Amerique Centrale, 2:643-644, pl. 38, fig. 5, 1886; Cope, Trans. Amer. Philos. Soc., 18:218, pl. 28, fig. 2, (hemipenis), April 15, 1895; Boulenger, Catalogue of the Snakes in the British Museum (Natural History), 3:122-123 (part), 1896; Cope, Ann. Rept. U. S. Natl. Mus. for 1898, pp. 1094-1095, 1242, pl. 26, fig. 2, (hemipenis), 1900; Amaral, Mem. Inst. Butantan, 4:212, 1929; Mittleman, Copeia, no. 2:122, June 30, 1944.
Conophis lineatus lineatus, Smith, Journ. Washington Acad. Sci., 31:122, March 15, 1941; Proc. U. S. Natl. Mus., 92:395, November 5, 1942; Proc. U. S. Natl. Mus., 93:407, October 29, 1943; Smith and Taylor, Bull. U. S. Natl. Mus., 187:43, October 5, 1945; Shannon and Smith, Trans. Kansas Acad. Sci., 52:505, December 31, 1949; Smith and Taylor, Univ. Kansas Sci. Bull., 33(pt. 2):351, March 20, 1950; Werler and Smith, Texas Journ. Sci. 4(4):565, December 30, 1952; Fugler and Dixon, Herpetologica, 14:186, December 1, 1958.
Type.—Museum National d'Histoire Naturelle, Paris, no. 3738. Type locality.—"México," restricted to Veracruz, Veracruz, México, by Smith and Taylor (1950:351). Little is known about the type specimen, and nothing, concerning its collector or the locality at which it was collected. Smith (1941:122) assumed that the specimen illustrated by Bocourt in Duméril, Bocourt, and Mocquard (1886:pl. 38, fig. 5) was the type of C. l. lineatus. I have also made this assumption concerning the identity of the type specimen of this species, especially because of the many inconsistencies appearing in the plate accompanying the description by Duméril, Bibron and Duméril (1854:pl. 73), and by Jan and Sordelli (1866:pl. 6). Neither show the nape nor a regular number of dorsal scales by which accurate determination of color pattern can be made and by means of which C. l. dunni and C. l. lineatus can be separated.
Diagnosis.—Lateral dark stripe anteriorly passing through eye and posteriorly involving fourth scale-row only; first scale-row darkly pigmented; no [Pg_268] paravertebral stripe; no dark pigment on vertebral row; six or eight dark stripes at mid-body, secondary stripes often present posteriorly; usually eight (sometimes seven) supralabials immaculate or having dark ventral margins.
Variation.—Twenty-six specimens have 157 to 169 (163.5 ± 3.59) ventrals. Twenty of these snakes having complete tails have 60 to 73 (66.5 ± 4.26) subcaudals; the number of ventrals plus subcaudals varies from 224 to 238 (230.1) in nineteen of these. In 26 specimens the reduction from 19 to 17 dorsal scale-rows takes place between ventrals 91 and 107 (100.2 ± 3.59). Sexual dimorphism is evident in the number of subcaudals; nine females have 60 to 66 (62.4), and 11 males have 68 to 73 (69.8) subcaudals. The largest specimen (AMNH 19643) is a male from "México," having a body length of 626 mm., a tail length of 168 mm. and a total length of 786 mm. No small juveniles have been examined; the smallest specimen (AMNH 19618) is a male from Veracruz, México, having a body length of 325 mm., a tail length of 90 mm. and a total length of 415 mm.
The greatest variation is in coloration. In preserved specimens the ground-color is white, tannish-white, or often pale blue, with dark stripes of black or deep brown present dorsolaterally and laterally. Secondary stripes of paler brown are sometimes present, but the pale browns have faded badly on many specimens. Normally four black stripes are present at mid-body—a lateral pair on the 4th row of dorsal scales and a dorsolateral pair on the 7th row (fig. 1, D). The lateral pair is the posterior continuation of the stripe that on the head passes through the eye; it continues on the nape as a narrow stripe on the 4th row only. In a few specimens the lateral stripe broadens to include the upper third of the 3rd row posterior to the nape. In some specimens both the dorsolateral and lateral dark stripes are present on the nape as a row of elongated spots or dashes that become continuous stripes of even width one-third to one-half of the distance posteriorly along the body; in other specimens the stripes are continuous on the nape. Posterior to the place of dorsal scale-reduction from 19 to 17 rows by the fusion of the 3rd and 4th rows, the lateral and dorsolateral stripes are moved downward by one row. In some specimens secondary black or dark brown stripes are present in the form of a series of dashes on the 5th and 8th rows; posterior to the place of scale reduction, these dashes are on the 4th and 7th rows. These dashes form a continuous stripe near the base of the tail. On the tail the secondary and primary stripes on adjacent rows sometimes fuse into a single broader stripe.
Usually the 1st row of dorsal scales is dark brown; in some specimens the brown on the 1st or 7th row has faded in preservative. A few specimens have small black spots on the moderate brown background of the 1st row; in others the 1st row is only a somewhat darker brown than the ground-color. The 2nd row sometimes is a medium brown, and appears to be an additional stripe.
The ventrals usually have more or less conspicuous dark spots laterally; in some specimens there are no spots. Except for the lateral spots (when present) the ventrals are immaculate white. The dorsal ground-color is pale brownish-white, white or pale blue between the 4th and 7th rows of dorsal scales and dorsally between the 7th rows on each side. Stripes are never present on the uniformly pale colored 8th, 9th and vertebral scale-rows.
Usually there are eight supralabials on each side; however, seven of the 27 specimens examined have seven supralabials on each side, and three others [Pg_269] have seven on one side, and eight on the other. Never is more than the lower third of the supralabials dark brown. In many specimens little or no brown is on the supralabials. There is little or no brown on the chin.
Variation in coloration and in number of supralabials appears to be of no geographic significance.
Although no juveniles have been collected, I expect that juveniles resemble adults in coloration. Probably there would be a greater contrast between the dark stripes and the pale ground-color in juveniles.
In life an adult from three miles northwest of Lerdo de Tejada, Veracruz, México (UMMZ 114484), had black stripes on the 4th and 7th rows of dorsal scales, and black spots on a brown background on the 1st row. The 2nd row had a medial, pale to medium brown auxiliary stripe on a brownish-white background. Posterior to the nape the 3rd row was medium brown. The area between the 4th and 7th rows and the dorsum between the 7th row of scales on each side was a pale brownish-white. Posterior to the place of scale-reduction the primary stripes were displaced downward by one row to the 3rd and 6th rows and secondary stripes originated as elongated spots on the 4th and 7th rows. Near the tail the secondary stripes were broad and continuous. The head was white or tannish-white with three dark brown or black stripes.
Remarks.—In his diagnosis of C. l. lineatus, Smith (1941:122) states: "lateral dark stripe … very narrow posterior to nape, extending along fourth scale row; posteriorly a stripe along third and eighth (farther posteriorly the seventh) scale rows; a narrow dark stripe along sixth scale row, continuous throughout length of body…." I fail to find a dark stripe on the 6th row throughout the length of the body. In all specimens that I have seen, there is a dark stripe on the 7th row anteriorly and on the 6th row posteriorly. In many specimens the stripes on the 3rd and 8th (posteriorly the 7th) scale-rows are absent or present so far posteriorly that the 8th row is never involved.
The dark brown on the first scale-row and the presence of a lateral dark stripe on the 4th row of dorsal scales only, in combination with the characteristics of the genus, distinguish C. l. lineatus from all other snakes in México.
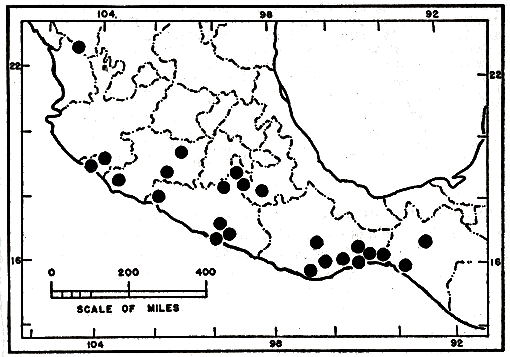
Distribution.—Semi-arid habitats on the coastal plain of Veracruz, México, from Tecolutla to Lerdo de Tejada and Piedras Negras (fig. 2).
Specimens examined.—Total of 27, as follows: México: no specific locality, AMNH 19614-15, 19621-24, 19642-43, NMW 16827. Veracruz: no specific locality, AMNH 19618-20, CAS 73640, NMW 16829; 4 km. S Alvarado, KU 58124; 14 mi. N Alvarado, UIMNH 46978; 6 mi. SE Boca del Río, UIMNH 28023; Etiopa, 2 mi. S Tecolutla, UIMNH 3847; ca. 30 mi. E Jalapa, AMNH 81948; 3 mi. NW Lerdo de Tejada, UMMZ 114484-85; Paso del Macho, USNM 109708; Río Blanco, 20 km. WNW Piedras Negras, KU 23253; Veracruz, AMNH 19612, UF 8990; W side Veracruz, AMNH 19616; 2 mi. W Veracruz, AMNH 19617, 19619.
Types.—Two in the United States National Museum, no. 12368 (two specimens). Type locality: "Yucatán," restricted to Chichén Itzá, Yucatán, México by Smith and Taylor (1950:352).
Diagnosis.—Dark stripes either absent posterior to the nape, or present as a row of small spots on fourth or seventh scale-row; no dark stripe on first scale-row; eight supralabials having dark ventral margins.
Variation.—Forty-five specimens have 158 to 170 (163.7 ± 1.56) ventrals. Thirty-eight of these snakes having complete tails have 56 to 74 (66.7 ± 4.77) subcaudals; the number of ventrals plus subcaudals varies from 222 to 245 (230.6). In 45 specimens the reduction from 19 to 17 dorsal scales takes place between ventrals 89 and 114 (102.5 ± 5.57). Sexual dimorphism is evident in the number of subcaudals; 16 females have 56 to 65 (61.8), and 22 males have 68 to 74 (70.3) subcaudals. The longest specimen (USNM 46395) is a male from Chichén Itzá, Yucatán, having a body length of 893 mm., a tail length of 274 mm., and a total length of 1167 mm. A juvenile (AMNH 38833) from Chichén Itzá, Yucatán, has a body length of 194 mm., a tail length of 50 mm., and a total length of 244 mm.
The venter is immaculate white or pale yellow and the dorsum of the body is immaculate pale gray to pale olive. Some specimens have small dark brown spots on the tips of the scales of the 4th or of the 7th row, but never on both. Only on the nape are spots present on both the 4th and the 7th rows; these spots are the posterior continuations of the dark stripes on the head and on many specimens do not reach the nape. Posterior to the place of scale reduction from 19 to 17 rows by the fusion of the 3rd and 4th rows of scales, the dark spots (when present) are on the 3rd or 6th row of scales.
[Pg_271] The coloration of juveniles is the same as that of adults. Color in life is thought not too different from that of preserved specimens, for notes on the color of living individuals (Neill and Allen, 1961:44) agree with what I have observed on preserved snakes.
Remarks.—The specimen from "Petén" (USNM, no. 4941) is the only specimen that has a controversial history. As can be seen from the synonymy of the species, the relationship of this specimen with the rest of the genus has been interpreted in several ways. Smith (1941:122-123) stated that the above specimen was catalogued as being from El Salvador; however, the locality was presumed by him to be El Petén, Guatemala, due to the presence in the bottle of a piece of paper inscribed "Conophis vittatus, Petén, J. M. Dow." This specimen is the one mentioned by Cope (1861:300, 1876:76, and 1900:1094-95), and in the first paper is ascribed to Guatemala. In 1900 this specimen was named C. lineaticeps by Cope who thought the specimen differed significantly from C. concolor (Cope, 1867:318-319). This specimen has the coloration normal for C. l. concolor as far posteriorly as mid-body; beyond mid-body the dark lines, typical of C. l. lineatus or of C. l. dunni, are present. It is likely that this specimen is an intergrade between C. l. concolor and C. l. dunni, the other subspecies present in Guatemala.
The only specimen not from the Yucatán Peninsula is allegedly from Patuca, Honduras (USNM 20271). It was obtained in the 1870's. Possibly more collecting will verify the presence of C. l. concolor in northern Honduras. This individual may be merely a genetically aberrant specimen from an area where normal specimens are C. l. dunni. Neill and Allen (1961:44-45) suggested that the specimen from Patuca implies widely overlapping distributions for C. l. dunni and C. concolor. The occurrence of C. l. concolor in Honduras needs to be verified before this assumption is made. There can, therefore, at present be no objection to the view that intergradation between the subspecies C. l. dunni and C. l. concolor could occur through a relatively broad area of El Petén and British Honduras.
Neill and Allen (1961:44-45) further suggest that the present range of C. l. dunni extends "presumably still farther northward toward the Méxican state of Veracruz where C. l. lineatus exists." Actually the presence of the subspecies C. l. dunni and C. l. lineatus as presently disjunct populations implies merely that they were presumably a continuous population at some time in the past.
The characteristics of the genus in combination with the reduction [Pg_272] of dark coloration posterior to the head distinguish this snake from all other snakes in México and Central America.
Distribution.—The Yucatán Peninsula: eastern Campeche, all of Yucatán, probably in Quintana Roo, and the northern third of British Honduras. A record for northeastern Honduras is questioned (fig. 2).
Specimens examined.—Total of 48, as follows: British Honduras: Belize District: 13.0 mi. W, 1.5 mi. S Belize, ERA-WTN BH-1562.
Guatemala: El Petén, no specific locality, USNM 4941.
Honduras: Colón: Patuca, USNM 20271.
México: Campeche: Champotón, UMMZ 73063-66; Encarnación, CNHM 106462. Yucatán: no specific locality, BMNH 80.7.13.30; Chichén Itzá, AMNH 38826, 38833, CNHM 20610-11, 26986-87, 36299-300, 36303-04, 36307, 36316, MCZ 7422, 28748, UMMZ 68236, 73060-62, 80806, USNM 46395; Kantunil, CNHM 36301, 36305-06, 36308-09, 36312-13; Libré Union, CNHM 36298, 36302, 36310-11, 36314; Mayapán, CNHM 40720; Mérida, CNHM 19411, 19413, NMW 16828; Progreso, CNHM 40721; Tekom, CNHM 49374; Yokdzonot, CNHM 36315.
Type.—Academy of Natural Sciences of Philadelphia, no. 22423, obtained by Emmet R. Dunn from Prof. Manuel Valerio. Type locality: Río Poas de Aserri (a few miles south of San José), Costa Rica.
Diagnosis.—Head and body dark brown or black above with two or four white stripes along body; usually two white lines on head immediately above eye passing from canthus rosetralis posteriorly to connect with white stripe on 6th row of dorsal scales; eight supralabials with black margins above.
Variation.—Six specimens have 173 to 183 (176.5 ± 4.00) ventrals. Five of these snakes having complete tails have 71 to 89 (80.6 ± 7.15) subcaudals; the number of ventrals plus subcaudals varies from 250 to 263 (257.0). In the six specimens the reduction from 19 to 17 dorsal scales takes place between ventrals 84 and 97 (93.2 ± 4.71). Sexual dimorphism is evident in the number of subcaudals; two females have 71 and 76 (73.5), and three males have 82 to 89 (85.3) subcaudals. The longest specimen (ANSP 22424) is a female from San José, Costa Rica, having a body length of 660 mm., a tail length of 168 mm. and a total length of 828 mm.
The dorsal coloration (fig. 1, E) varies from a black ground-color with two or four narrow white stripes to a dark brown ground-color with a series of black stripes and four white stripes. In the black specimens there are no dark stripes. The darkest specimen (NMW 16838:1) has only two white stripes; these more or less continuous stripes are on the ventral third of the 2nd row of scales and occasionally on the dorsalmost part of the first scale-row. The venter is immaculate white except for black on the tips of the ventral scales. The dorsum above the 2nd scale-row is uniform black. There are no white stripes on the head.
[Pg_273] The palest specimen (NMW 16838:2) has four dorsal white stripes; the lateral pair of these stripes is on the ventral half of the 2nd and the dorsal third of the 1st scale-rows; the dorsolateral pair is on the dorsal two-thirds of the 6th and the ventral third of the 7th rows of scales. This latter stripe is the posterior continuation of the white stripe on the head, which originates immediately posterior to the rostral scale and passes posteriorly along the canthus rostralis and along the lateral margin of the supraocular scale to the nape. Posterior to the place of scale reduction, the dorsolateral white stripe is displaced ventrally one scale-row. Except for black flecks or spots on the lateral margins of the ventrals, the venter is immaculate white. The dorsum above the lateral white stripes is brown and black; there is a pair of dorsolateral white stripes. The dorsal half of the 2nd, most of the 3rd, 4th and 5th rows of scales are black; the dorsal margin of the 3rd, both margins of the 4th, and the ventral margin of the 5th rows are paler brown. The dorsal two-thirds of the 7th, all but the dorsal most part of the 8th, and the middle two-thirds of the 10th scale-rows are black; the areas between are a medium brown.
Only six specimens are available on which to base a description of the variation in this species. Furthermore, there are no juveniles, notes on the colors of living individuals, or photographs of this species.
Remarks.—Taylor (1955:563-565) hesitantly referred a specimen (KU 35630) from 32 kilometers north of Barranca, Puntarenas [Pg_274] Province, Costa Rica, to Conophis lineatus nevermanni. This specimen, a female, has 169 ventrals and ventral scale-reduction taking place opposite the 109th ventral; both of these characters are well out of the range of C. nevermanni. Furthermore, the ventral margins of the supralabials are brown, and the pale dorsal stripes are tan and too wide for C. nevermanni (compare figs. 1, C and E). The specimen definitely is C. lineatus dunni, and corresponds well with another specimen from Costa Rica (ANSP 12232).
The dark brown or black dorsum with two or four white stripes and the presence of eight supralabials having dark brown dorsal margins, in combination with the characters of the genus, serve to distinguish Conophis nevermanni from other Central American snakes.
Distribution.—Pacific coastal plain of northwestern Costa Rica and the Meseta Central of central Costa Rica (fig. 4).
Specimens examined.—Total of six, as follows: Costa Rica: Guanacaste: Bebedero, Río Tenorio, NMW 16838(5). "San José," ANSP 22424.
Types.—Three in the United States National Museum, nos. 6751 (2 specimens) and 6803, obtained by Henery Hague. Type locality: "Petén," or "Verapaz," Guatemala. There is much doubt about localities for many of Hague's specimens collected in the 1860's (Stuart, 1948:10). Since Conophis pulcher is found predominantly in semi-arid environments, the types might have come from the semi-arid Cahabón, Negro, or Salamá river basins—all places near the sugar plantation that Hague managed at San Jerónimo, Baja Verapaz. Possibly the types were obtained from as far away as the Motagua Valley or the southeastern highlands of Guatemala, both of which areas Hague is known to have visited.
Diagnosis.—Paravertebral stripes present at least posteriorly (fig. 1, F); eight or ten stripes at mid-body; lateral dark stripe passing through eye anteriorly and including at least upper one-half of second scale-row from neck region posteriorly to place of scale reduction near mid-body; eight supralabials immaculate or having dark ventral margins.
Variation.—Twenty-six specimens have 161 to 182 (169.5 ± 5.31) ventrals. Eighteen of these snakes with complete tails have 65 to 79 (70.6 ± 3.93) subcaudals; the number of ventrals plus subcaudals varies from 231 to 251 (239.3). In 26 specimens the reduction from 19 to 17 dorsal scales takes place between ventrals 94 and 119 (104.6 ± 4.90). Sexual dimorphism is evident in the number of subcaudals; eleven females have 65 to 71 (68.2), and seven males have 70 to 79 (74.3) subcaudals. The longest specimen (AMNH 58364) is a female from El Zamarano, Honduras, having a body length of 703 mm., a tail length of 164 mm. and a total length of 867 mm. The smallest juvenile (MCZ 49793) from Tegucigalpa, Honduras, has a body length of 162 mm., a tail length of 46 mm. and a total length of 208 mm.
The dorsal ground-color is pale brown or white; black or dark brown stripes are present dorsally and laterally. Normally ten stripes are present at mid-body; the first pair on the first row of dorsal scales; the second pair on the upper half of 2nd and lower part of 3rd rows; the third pair on 4th row; the fourth pair on 7th and sometimes part of 8th rows; the fifth pair (paravertebral stripes) on the 9th row. Posterior to the place of reduction from 19 to 17 rows by the fusion of the 3rd and 4th rows, the third, fourth and fifth pairs of stripes are displaced downward one row. Sometimes the second and third pairs of stripes are fused resulting in only eight stripes at mid-body. On some specimens the fourth and fifth pairs of stripes are close together, but in none are they fused so as to result in a pattern of six stripes at mid-body.
The paravertebral stripes begin anteriorly on the nape or at any point on the anterior one-third of the body and continue as discrete stripes onto the base of the tail. Anteriorly these stripes are always broken into a series of dashes; posteriorly the stripes are continuous. In specimens in which the paravertebral stripes do not begin on the anterior-most part of the body, there is no paravertebral pigmentation anteriorly.
In addition to the paravertebrals, the other dorsal dark stripes are variable. In some specimens the stripes are present anteriorly and gradually disappear near mid-body (the first dark stripe only on three specimens). In other [Pg_276] specimens the stripes are present anteriorly as dashes and become continuous at mid-body; in others the stripes are continuous throughout. Posteriorly continuous stripes are of uniform width; anteriorly sometimes they are wide on the tip of each scale and narrow on the base (fig. 1, F). The variation in continuity and width described above is found in all of the dorsal dark stripes.
The ventrals usually have more or less conspicuous dark spots laterally; in some specimens there are no spots. Except for the dark lateral spots, when present, the ventrals are immaculate white. Usually the dorsal ground-color is a pale tan, especially between the first and second, and the third and fourth dark stripes. The areas between the second and third dark stripes and across the dorsum between the fourth stripes on each side are pale brown. In some specimens the dorsum between the paravertebral stripes is still paler brown.
Never is more than the lower third of the supralabials brown. Many specimens have little brown, and others none. In most of those specimens having brown on the supralabials, the chin and infralabials are dusky tan or gray. There is little or no brown on the supralabials or the chin in the northern part of the range (Chiapas), whereas the greatest amount of brown on the labials and chin is found on some specimens from the southern part of the range (Honduras). Since there is considerable variation in the amount of brown on the chin and labials of specimens from single localities, the slight geographic trend in this character seemingly is not significant.
In juveniles six black or dark brown stripes boldly contrast with a white or pale tan ground-color. At mid-body the first pair of dark stripes is on the 1st scale row; the second pair on the 3rd and 4th rows; the third pair on the 7th, 8th and at least the lower half of the 9th rows (fig. 3, B). Ontogenetic change in coloration consists of the splitting of the second and third pairs of dark stripes in the juvenile. The first stripe does not split. Consequently adults have ten dark stripes.
In life an adult from Tonalá, Chiapas, had black stripes. The ground-color below the second stripe, and between the third and fourth dark stripes was tan. The area between the second and third dark stripes was reddish-brown, as was the dorsum between the fourth pair of dark stripes, except that the 10th scale-row was paler.
Three excellent photographs of this species have been published under the name Conophis lineatus (Ditmars, 1931:pls. 26 and 27).
Remarks.—Smith (1941:121-122) described C. pulcher plagosus from Tonalá, Chiapas, and characterized the subspecies by its having "(1) the ventrals completely unspotted; (2) secondary lines on paravertebral rows not continuous posteriorly; (3) all other lines on body also somewhat spotted in appearance; (4) dusky markings on chin and supralabial border very dim (less distinct than in p. pulcher or any member of the lineatus series)." Although all Chiapan specimens lack ventral spots, specimens from Guatemala have no spots, small spots, or large spots. Even in specimens from Tegucigalpa, Honduras, the southernmost limit of the range, [Pg_277] the spotting varies from a few inconspicuous spots to many large spots. Paravertebral rows were continuous posteriorly in alimens examined by me. Likewise, all other stripes were continuous bands of uniform width posteriorly, having appeared anteriorly as rows of spots or dashes. The amount of brown on the chin and labials has been shown previously not to be geographically significant. The absence of characters of adequate significance to separate populations precludes the naming of subspecies in this species.
Mertens (1952a:93, and 1952b:61-62) designated three specimens from El Salvador as C. pulcher plagosus. In the latter paper, Mertens, on the basis of a description of a specimen of "C. lineatus" from Divisadero, El Salvador, given by Schmidt (1928:200), referred that specimen also to C. pulcher plagosus. I have examined this specimen and refer it to C. lineatus dunni. Although I have not seen Merten's specimens, on the basis of the excellent descriptions given by Mertens (1952b:61-62), I refer the three Salvadoranean specimens to C. lineatus dunni.
The presence of paravertebral stripes in combination with the characteristics of the genus distinguish Conophis pulcher from all other snakes in southern México and Central America. The only sympatric species of this genus, C. lineatus dunni, differs in that it lacks paravertebral stripes, although it may have a single vertebral stripe. Conophis lineatus dunni has lateral dark stripes that are present on the 3rd and 4th scale-rows, never on the anterior third of the body as in C. pulcher. Even in juveniles the third pair of dark stripes includes the lower part of the 9th scale-row in C. pulcher, whereas the dorsal most dark stripe of C. lineatus dunni never includes more than the lower part of the 8th scale-row.
Distribution.—Pacific coastal region of Chiapas, México, southeastward into Guatemala; southeastern highlands and the dry valley of central and eastern Guatemala; Caribbean lowlands of Honduras southward to the region of Tegucigalpa, Honduras (fig. 4).
Specimens examined.—Total of 27, as follows: Guatemala: no specific locality, CNHM 22912, NMW 16830. Jutiapa: Hacienda Mongoy, UMMZ 106725. El Progreso: El Progreso, CAS 67000; El Rancho, UMMZ 106724; San Antonio, CAS 66999. "Peten," USNM 6751(2), 6803. Sacatepequez: Dueñas, BMNH 64.1.26.17, 64.1.26.126-127. Zacapa: Pepesca, AMNH 72555-56.
Honduras: no specific locality, AMNH 58364. Cortes: San Pedro Sula, CNHM 5295-96. Francisco Morazan: El Zamarano, AMNH 70189; Tegucigalpa, MCZ 49785, 49787-88, 49791, 49793, 49795.
México: Chiapas: Soconusco, UIMNH 33646-47; Tonalá, USNM 109707.
Type.—Zoologisches Museum Berlin. Type locality not given, for the specimen was purchased from a dealer in Hamburg. The type locality was first restricted to "Acapulco," Guerrero, by Smith (1941:119), then to Laguna Coyuca, Guerrero, México, by Smith and Taylor (1950:331).
Diagnosis.—Three or four dorsal dark stripes, each involving two or more adjacent scale-rows; never having brown or black on the 1st scale-row; seven supralabials immaculate white or pale tannish-white.
Variation.—One hundred seventy-one specimens have 149 to 181 (163.7 ± 6.33) ventrals. One hundred fifty-three of these having complete tails have 55 to 76 (64.8 ± 4.90) subcaudals; the number of ventrals plus subcaudals varies from 214 to 245 (228.5). In 170 specimens the reduction from 19 to 17 dorsal scales takes place between ventrals 84 and 118 (102.3 ± 6.60). Sexual dimorphism is evident in the number of subcaudals; 58 females have 55 to 66 (60.0) and 95 males have 59 to 76 (67.8) subcaudals. The longest specimen (AMNH 68004) is a male from Escurano, Oaxaca, México, having a body length of 668 mm., a tail length of 182 mm. and a total length of 850 mm. A juvenile (CNHM 40435) from Tehuantepec, Oaxaca, México, has a body length of 133 mm., a tail length of 31 mm. and a total length of 164 mm.
Variation in coloration is of such magnitude that it has been used as the basis for recognition of subspecies. Unfortunately, until this time, most specimens reported upon in the literature represented the two extremes of variation. After examining the coloration of 174 specimens with respect to geographic distribution, I conclude that only one highly variable species is represented. Specimens from the northern and western parts of the range (Michoacán, Colima, and Durango) have the color pattern of C. vittatus as described by Peters (1860:518-521); these snakes have four narrow black stripes on a white or pale tan background, and an immaculate white venter. The lateral dark stripe, which on the head passes through the eye, is present on the dorsal half of the 3rd and the ventral half of the 4th scale-rows; the dorsolateral dark stripe, which passes along the middle of the head and splits on the nape, is present on the middle of the 8th scale-row. The other extreme in color pattern consists of three broad stripes; the two dorsolateral stripes are fused. This pattern is prevalent in specimens from the area around Tehuantepec, Oaxaca. The lateral stripes include the dorsal half to two-thirds of the 2nd, all of the 3rd and 4th, and half of the 5th scale-rows; the fused dorsolateral stripes sometimes cover all of the area dorsal to and including the dorsal third of the 7th scale-row.
Snakes from areas between Tehuantepec and the margins of the distribution [Pg_280] of this species are variously intermediate between the extremes described above. In some snakes from these areas the lateral stripes are broad and include either the dorsal half of the 2nd scale-row or the ventral half of the 5th scale-row, but not both on the same specimen. Also, the dorsolateral stripes are broad and include most of the 9th and a part of the 10th scale-rows. Many specimens from the area around Tehuantepec, where the three-striped pattern is prevalent, have an intermediate pattern. Some have white on the center of the 10th scale-row or lateral stripes that are not so broad as to include the 3rd and 4th and half of each of the 2nd and 5th scale-rows.
The supralabials are immaculate white or pale tan, except that in some specimens the dorsalmost part of some supralabials are dark brown or black as they are included in the ventral boundary of the dark stripe that passes through the eye. There are no dusky markings on the chin or on any of the ventral scales.
There is no ontogenetic change in color pattern; juveniles have the same coloration as adults from the same geographic area.
Color in life is not greatly different from that of preserved specimens. One specimen (UMMZ 114483) from 10.8 miles south of Oaxaca, had in life black stripes, a pale yellowish tan dorsal ground-color and a pale off-white venter.
An excellent photograph of this species appears in Schmidt and Inger (1957:230) under the name Conophis lineatus.
Remarks.—I have been unable to find variation of geographic importance in scutellation in this species. A wide range of variation in the characters of scutellation is present in specimens from most localities; it shows no significant clinal or geographic trends. As I have stated previously, in the discussion of variation, coloration has been the feature primarily used by previous workers to distinguish two "subspecies" for this species; C. vittatus vittatus having four black stripes and C. vittatus viduus having three black stripes. Most of the three-striped snakes occur in the vicinity of Tehuantepec, Oaxaca, whereas the four-striped snakes are found near the margins of the range of the species in Durango, Colima, Michoacán, Morelos and Puebla. Specimens that would have to be considered intergrades between the "subspecies" are found in Michoacán, Guerrero, Oaxaca and Chiapas. At the time the subspecies were proposed only specimens from Tehuantepec or from marginal areas were known. Utilizing the large number of specimens of this species presently available, geographic variation is found to be clinal, from those with three stripes from near Tehuantepec, through several intermediate patterns present on specimens from single localities in Guerrero, Oaxaca and Chiapas, to those with four dark stripes in areas farthest removed to the north and west from Tehuantepec. Since only coloration shows geographic variation, and since this variation represents a continuous cline, subspecies cannot be recognized for this species.
[Pg_281] The presence and position of the three or four dark stripes on the body and the absence of brown on the 1st scale-row or on the ventral scales, in combination with the generic characters, distinguish Conophis vittatus from all other Méxican snakes. The only other snake that occurs in western México that has been confused with C. vittatus is Coniophanes piceivittus taylori, which has 25, instead of 19, scale-rows.
Distribution.—Semi-arid habitats on Pacific slopes from extreme southern Durango southeastward to Tuxtla Gutierrez, Chiapas, and inland in the eastern Balsas Basin to Morelos and western Puebla (fig. 5).
Specimens examined.—Total of 174, as follows: México: no specific locality, AMNH 66150-52, SU 9465. Chiapas: Piedra Parada, USNM 121453. Pizo de Oro, UIMNH 40821. Tuxtla Gutierrez, Parque Madero, UIMNH 37992-93, 38036-37. Colima: no specific locality, MCZ 46860, USNM 31394, 31396-97. 1 mi. SW Colima, AMNH 12783. S of Manzanillo, AMNH 19641. Durango: Hacienda de Gabriel, AMNH 14217. Guerrero: Acahuizotla, TCWC 7419, 9469. 1 mi. W Acahuizotla, TCWC 7418. 3 mi. W Acapulco, AMNH 71626. 6 mi. E Acapulco, TCWC 9476-77. 10 mi. S Acapulco, TCWC 8578. Agua del Obispo, CNHM 104948, TCWC 11586. near Chilpancingo, MVZ 45067, UMMZ 85722-23. 1 mi. SW Colotlipa, TCWC 9471-74. 2 mi. SW Colotlipa, TCWC 9475. 14 mi. S Ixtapán de la Sal, KU 67648. Laguna Coyuca, CNHM 25881, UMMZ 80942. near La Unión, AMNH 66337. Magueyes, Laguna Coyuca, AMNH 66149. Playa Encantada, TCWC 9470. 1 mi. S Tierra Colorada, KU 67649. near Xaltinanguis, km. 405, CNHM 104947. Michoacán: Coalcomán, UMMZ 104693. 1/2 mi. SE Coalcomán, UMMZ 104492. 1 mi. N. Coalcomán, UMMZ 112543. 1 mi. NE Coalcomán, UMMZ 104692. Puerta de la Playa, UMMZ 105155. 12 mi. S [Pg_282] Tzitzio (by road), UMMZ 99153. Morelos: 12 km. NW Axochiapan, TCWC 7311, UIMNH 17613, 25924. 7 mi. SE Cuernavaca, MVZ 32258. Huajintlán, km. 133, CNHM 103270. 12 km. S Puente de Ixtla, km. 133, CNHM 104949. Oaxaca: Bisiliana, AMNH 68010. near Caoba, foot of Cerro Arenal, AMNH 68009. Cerro Arenal, AMNH 68000-03. Cerro de Laollaga, UIMNH 36213. Cerro de San Pedro, UIMNH 17616. Cerro Palma de Oro, UIMNH 37116. "C. Madrena, Sto. T. Quieri," UIMNH 46904. near Chivela, MCZ 25021. Cinco Cerros, UIMNH 37114. Dami Liesa, AMNH 66877, UIMNH 6158, 37115. Escuranos, AMNH 66873-74, 68004-06. Finca Santa Teresa, 2 km. NW Tehuantepec, UMMZ 82648. Huilotepec, AMNH 66878, UIMNH 40820. between Huilotepec and Tehuantepec, AMNH 65106, UMMZ 82644-45. Las Tejas, UIMNH 6151-54. Mixtequilla, UIMNH 6157, 36211. between Mixtequilla Mountains and Tehuantepec, UMMZ 82652. between Niltepec and "Carixxal," AMNH 68876. 10.8 mi. SE Oaxaca, UMMZ 114483. Quiengola, UIMNH 17617. between Quiengola Mountains and Tehuantepec, UMMZ 82647. Rancho Poso Río, 6 km. S Tehuantepec, UIMNH 6144-49, 37117-19, UMMZ 82649-51. Rincón Bamba, CNHM 105129-30, UIMNH 17615. Salazar, AMNH 66875. vicinity of Salina Cruz, UMMZ 82653. San Gerónimo, AMNH 4306, CNHM 1457. San Lucas Ixtepec, UIMNH 36206. San Juan Lajarcia, UIMNH 36212. San Mateo del Mar, AMNH 65914. San Pablo, UIMNH 36207. Santa María (Cerro de Liesa), AMNH 68011. Tapanatepec, MCZ 27806-11. Tehuantepec, AMNH 19644, 65107-09, 65907-13 plus 7, 66871-72, 66879, 68007-08, CNHM 40435-36, 105126-28, MCZ 46403, UIMNH 6150, 17614, 17618, 29692, 36208, 37120-21, UMMZ 82642-43, 82646, USNM 109709-14, 1-2 leagues SSE Tehuantepec, UMMZ 82639-41. Tenango, UIMNH 36209-10. between Tlacolulita and Tequisistlán, CNHM 105125. Yerba Santa, UIMNH 6155-56. Puebla: Atencingo, KU 39626.
In studying the osteology of the genus Conophis, I have examined two complete skeletons (one C. vittatus and one C. lineatus); two additional skulls of C. vittatus and C. lineatus; and 24 sets of dentigerous bones, representing all of the species. Terminology of the skeletal elements is that of Duellman (1958), Parker (1878), Radovanovic (1937) and Szunyoghy (1932). The drawing of the right side of the skull of a specimen of Tomodon lineatus that appears in Jan and Sordelli (1881:liv. 50, pl. 2, fig. 34) is of little value due to its small size and lack of detail.
The skull of Conophis is typical of a relatively unspecialized colubrid snake. Skulls of Conophis lineatus concolor and C. vittatus closely resemble each other. The following description is based primarily on the skull of C. lineatus concolor (UMMZ S-778).
The elements are discussed in the following order: nasal region, cranium and associated elements, maxillo-palatal-pterygoid arch, mandible, dentition, and vertebrae.
Nasal region.—The premaxillary is relatively heavy and has a concavity posteroventrally. The lateral processes slope downward, but remain fairly thick, and do not project far laterally. This shape (fig. 6) tends to strengthen the nasal region; this anterior strengthening may be a reflection of the fossorial habits of these snakes. There are no posterior processes of the premaxillary; thus the line of fusion with the nasals and septo-maxillaries is broad. The [Pg_283] nasal plate is more than twice as long as wide. The nasals are relatively flat above, although each curves slightly downward medially and fuses into the medial nasal septum; laterally each nasal is narrower and deflected downward, forming a small dorsal shield over the nasal cavity. The septo-maxillaries are closely associated with the vomers and form the cavity in which the organ of Jacobson is situated. The broad medial part of the septo-maxillary forms the roof and anterior border of the cavity, whereas the anterior part of the vomer contains the main part of the capsule and forms the posterior and most of the lateral borders of the cavity. The vomer has a thin anterior ridge that gradually disappears before it reaches the border of the premaxillary. The vomer is approximately U-shaped, when viewed from below. It has no posterior process and does not articulate with the parasphenoid; there is a sizeable gap between the two bones. The septo-maxillary has a lateral process that terminally is directed slightly anteriorly.

[Pg_284] Cranium and associated elements.—The frontal is almost three times as long as it is wide; it is flat above with an emarginate dorsolateral margin that forms the upper limit of the optic capsule. Ventrally the frontal is concave and forms the median limits of the optic cavity. Farther ventrally the frontal joins with the parasphenoid, which at this place forms the ventral extent of the skull, and together with the basisphenoid forms the ventral part of the posterior three-fourths of the skull. In ventral aspect, the parasphenoid is a long, thin bone, slightly expanded anteriorly. It forms the anterior floor of the optic foramen; whereas the frontal forms the anterior roof of the same opening. The frontal and its septo-maxillary process surround the olfactory fenestra. The prefrontal articulates with the anterolateral process of the frontal. The posterior surface of the prefrontal forms the anterior wall of the orbit of the eye. The articulating surface upon which the median process of the maxillary bone rests is situated ventrally. The anterior dorsal surface of the prefrontal, together with the anterolateral edge of the frontal, extends slightly over the nasal cavity, affording some degree of protection for the contained organs and forming the posterior border of the cavity. A small nasal process also extends anteriorly from the ventrolateral surface of the prefrontal. The orbital-nasalis foramen is located in the anterior surface of the prefrontal. The parietals are fused into one large bone that forms the roof and sides of the middle part of the cranial cavity. From its suture with the frontal, the dorsal surface of the parietal is relatively flat in the area bounded laterally by the parietal crests, which extend posteromedially from the anterolateral corners of the bone and converge medially at a point near its posterior margin. A slight posterior extension of the parietal crests forms the supratemporal crest, which is present on the posterior part of the parietal and on the anterior part of the supraoccipital. The postfrontals are attached to the anterolateral processes of the parietal. Together the anterior surfaces of these two bones form the posterior rim of the orbit of the eye. The postfrontal extends laterally and ventrally and has a terminal extension that projects anterolaterally. In an articulated skull the trans-palatine articulates with the ventrolateral articulating surface of the postfrontal. Anteromedially, the parietal forms the roof and posterior margin of the optic foramen. The basisphenoid, which is fused with the parasphenoid, also forms a small part of the posteroventral margin of the optic foramen. The basisphenoid forms the floor of the middle part of the cranial cavity and the ventromedial down-pouching that contains the pituitary body. Posterolateral to the parietal and dorsal to the posterior part of the basisphenoid is the prootic. Laterally this bone is deeply emarginate; posteriorly it forms a large part of the otic notch, through which the columella passes. The columella is a long, thin bony rod that terminates posteriorly in cartilage. It is the cartilagenous part of the columella that connects with the external sound detecting mechanism. There are several foramina on the lateral surface of the prootic. On the anterolateral surface of the prootic, branches of the trigeminal nerve pass through three foramina whereas the facial nerve passes through the single posterior foramen [Pg_285] near the otic notch. The squamosal is attached dorsoventrally to the posterior part of the parietal and to the lateral part of the prootic. At this place of attachment there is on the prootic a relatively heavy crest that forms a rather broad articulating base. The squamosal is long, flat, and curves slightly in a dorsal direction throughout its length; it becomes thinner and narrower posteriorly. The posterior third of the squamosal forms a broad base by means of which the squamosal articulates with the quadrate. The columella and the squamosal extend posteriorly beyond the limits of the braincase. Posteriorly the skull consists of four bones: an unpaired median dorsal supraoccipital, an unpaired median ventral basioccipital and two lateral exoccipitals. The basioccipital does not have noticeable pterygoid processes, but is rather smooth ventrally and only slightly emarginate on its posterolateral margins. Posteriorly, this bone forms the ventral part of the occipital condyle. The rest of the condyle, on each side, is formed by the exoccipitals. The exoccipitals also form part of the base to which the squamosal is attached. The exoccipitals extend around the sides of the foramen magnum and meet dorsally. Each exoccipital also forms the posterior part of the otic notch, which traverses the exoccipital. The exoccipitals bear moderate occipital crests that extend [Pg_286] posterolaterally across the supraoccipital as branches from the supratemporal crest. The supraoccipital also has a medial crest that extends a short distance posteriorly from the supratemporal crest onto the exoccipitals at their dorsal line of fusion.

Maxillo-palatal-pterygoid arch.—In an articulated skull, the anterior edge of the maxillary is immediately posterior to the lateral tip of the premaxillary (fig. 7). The maxillary is curved moderately laterally and is not robust at its tip, but it becomes heavier about one-third of its length posteriorly. A dorsomedian process begins at about one-third of its distance from the anterior end; the prefrontal articulates with this process. The process is broad and almost flat, except that at its medial end, an elongate, rounded knob extends ventrally. The dorsomedian process of the maxillary extends toward, but does not meet, a lateral process from the palatine. The maxillary teeth are set in sockets on the ventral surface of the bone. Just posterior to the level of the last prediastemal tooth is the median trans-palatine process that articulates with the anteromedian part of the trans-palatine. Immediately posterior to this process, the maxillary narrows slightly; then it broadens to form an obliquely oriented knob. The posteroventral surface of the posterior knob of the maxillary bears one or two enlarged maxillary teeth. (These teeth are discussed further in the section on Dentition.) The anterolateral part of the trans-palatine articulates with the dorsal surface of the posterior knob of the maxillary. Toward the middle of its length, the trans-palatine narrows considerably; then it broadens again and articulates with the pterygoid. The palatine is slightly rounded at its anterior end, which extends anteriorly to the posterior margin of the vacuity containing Jacobson's organ. The palatine extends posteriorly to the trans-palatine process of the maxilla, where the palatine articulates with the pterygoid. A posterior pterygoid process from the palatine projects posteromedially from the end of the palatine and overlaps the anterior end of the pterygoid. Just less than one-half the distance from the anterior end of the palatine, there is a lateral process that curves ventrolaterally forming a blunt tip posteriorly. Slightly more posteriorly and on the medial side of the palatine, is a medial sphenoid process, which is thin, rather broad, and curves ventromedially; ultimately it comes to lie near the anterior part of the parasphenoid. The palatine teeth are set in shallow sockets on the ventral edge of the bone. Of the bones of the maxillo-palatal-pterygoid arch, those on the pterygoid extend farthest posteriorly. The pterygoid is broad medially and posteriorly, although pointed at its posterior tip. The trans-palatine articulates in a broad line at about one-third of the distance along the lateral margin of the pterygoid. Immediately posterior to this articulation, there is a median ridge on the pterygoid; lateral to the pterygoid ridge is an abrupt hollow, the pterygoid groove. Posteromedially, this groove becomes gradually more shallow and disappears. The dorsal surface of the pterygoid is rounded anteriorly and somewhat flattened posteriorly, whereas the ventral surface is gently rounded along its length, except that there is a high median crest. The pterygoid teeth are situated in shallow sockets along this crest. The teeth diminish in size posteriorly.

Mandible.—The dentary (fig. 8) is compressed laterally and rounded below. The teeth, which are longest about one-third of the way from the anterior end of the dentary, are set in sockets on the medial side of the bone. [Pg_287] The posterior half of the dentary overlies the fused surangular-prearticular part of the articular. Ventrally, the posterior part of the dentary underlies the splenial, which is set in a median trench within the dentary. Near the common suture of the dentary and the splenial is the large inferior alveolar foramen; completely within the splenial and ventral to the inferior alveolar foramen is the anterior mylohyoid foramen. Posterior to the splenial and also forming a part of the ventral surface of the mandible is the wedge-shaped angular, which lies directly beneath the fused surangular-prearticular. As has been implied, the articular, the surangular, and the prearticular are fused. The prearticular part of this bone forms a part of Meckel's canal. In the surangular part, immediately posterior to the end of the dentary, is the large surangular foramen. Lying in a longitudinal axis along the medial surface of the articular is a high crest, dorsal to which is a deep hollow. The lateral wall of the articular above this hollow is thin and rounded dorsally; the ventral surface is uniformly round and slightly curved dorsally, except that it ends with a short tympanic crest, which projects beyond the articulation with the quadrate. Where the quadrate articulates with the dorsolateral surface of the posterior portion of the squamosal, the former is broad and has a high mid-lateral crest, which extends about one-third of the distance down the quadrate before disappearing. The columellar process (the place of fusion of the [Pg_288] columella) is about two-thirds of the way down the medial surface of the quadrate. Ventrally the quadrate has a narrow neck dorsal to its articulation with the articular. The articulation is formed by two lateral flanges of the quadrate that fit over a medial ridge formed by the articular.
Teeth on the maxillary and pterygoid decrease in size posteriorly, whereas those of the dentary do likewise except for the first one or two that are usually slightly smaller than those immediately posterior. The palatine teeth are subequal in size. The enlarged, grooved teeth on the maxillary are in shallow sockets on the posteroventral surface of the posterior knob of the maxillary. These teeth point posteriorly. The grooves are deep and are situated anterolaterally. One or two enlarged grooved teeth are present on a given maxillary. There seems to be a correlation between the type of preservation, the age of the snake, and the number of grooved teeth. Old (large) individuals always have only one grooved tooth that is rooted and functional, whereas some of the younger animals have two in place. Usually replacement teeth are present in alcoholic specimens, but these unrooted teeth are lost in the preparation of dried skeletons. Thus, it seems that in Conophis only one pair of grooved teeth is functional at any one time, although usually replacement teeth are present behind and beside the functional one. Some specimens have one tooth in the medial socket on one side and one in the lateral socket on the other. Replacement teeth on the maxillary and dentary are present in the buccal tissue on the medial side of the bones, whereas on the palatines and pterygoids, the replacement teeth are present laterally. Apparently there are no significant differences in dentition among the members of the genus Conophis.
The fiftieth vertebra of Conophis vittatus (UMMZ 82642) can be described as follows: The neural spine is elongate, thin and low; the posterior edge is sharply emarginate, and the anterior edge is only slightly emarginate. The zygosphene is thin dorsoventrally; in a ventral or dorsal view the zygosphene has a slightly concave anterior edge, the flat surface of which is oriented ventrolaterally. The centrum is elongate and triangular from below; it is widest at the paradiapophyses and narrowest at the short condylar neck. The condylus is directed posteriorly. The centrum, when viewed laterally, is slightly concave and has prominent subcentral ridges that extend from the median side of the paradiapophysial articular surfaces posteriorly to the neck of the condylus. The paradiapophysial articular surfaces are well developed and have two facets. The diapophysial surface is larger and more spherical than the parapophysial one. The parapophysial process projects beyond the parapophysial articular surface and is nearly even with the lip of the cotyle, which is slightly oval. The neural arch is slightly depressed; its width is somewhat less than the width of the cotyle. The articular surfaces of the postzygapophyses are oval and are directed posterolaterally. There is a strongly developed concave interzygapophysial ridge. A well-developed accessory spine extends laterally beyond the oval articular facets of the pre-zygapophysis and forms a slightly flattened, blunt spine. Excellent drawings [Pg_289] of the middle thoracic vertebra of Conophis lineatus dunni from Honduras were published by Auffenberg (1958:6).
The hemipenes of Conophis are moderately caliculate, having spines covering the surface from the base to near the apex (fig. 9). These spines are largest near the base and are reduced to small papillate projections near the apex. The apex terminates in a small disc having three to five laminae in C. vittatus and one lamina in C. lineatus concolor. The sulcus is bifurcate; the fork is near the base and almost gives the appearance of two sulci on some specimens. Distally the apices are widely separated, and the intervening space gives the hemipenis a slightly bilobed appearance in some species (especially C. vittatus) or a deeply bilobed appearance in others (especially C. lineatus concolor).
The everted hemipenis reaches posteriorly to the eighth subcaudal scale. The sulcus bifurcates at the third subcaudal scale. The situation is similar in situ (Cope, 1895:pl. 28, fig. 2).
There are no apparent hemipenial differences among the species of the genus Conophis. As can be seen in the above description, the hemipenis of C. vittatus is less bilobed and has a more pronounced disc at the apex than the others. The hemipenis of C. lineatus concolor is most bilobed, but has the smallest apical disc. The other species and subspecies vary widely within these extremes.
Conophis eats mostly small lizards, especially Cnemidophorus. In México Conophis occurs in semi-arid habitat where Cnemidophorus is common. A specimen each of Conophis vittatus and C. lineatus lineatus were obtained while I was collecting Cnemidophorus. The only record of Conophis having fed on a warm-blooded vertebrate was obtained in the course of this study, when I recovered from the stomach of a Conophis lineatus concolor (CNHM 36299) from Chichén Itzá, Yucatán, a heteromyid rodent (Heteromys gaumeri).
[Pg_290] Ralph Axtell (personal communication) observed Conophis actively searching for food at dusk. His observations were made near Tehuantepec, Oaxaca, and the snakes were seen to chase lizards of the genus Cnemidophorus. Near Alvarado, Veracruz, in the late afternoon, I watched a Conophis lineatus lineatus follow a lizard into a hole.
Mittleman (1944:122) presents the only discussion of the mode of feeding of a captive specimen of Conophis lineatus ssp. When presented with a Thamnophis slightly smaller than itself, the Conophis struck, and within eight minutes immobilized the Thamnophis. Within one-half hour the Thamnophis was swallowed. Three days later the Conophis ate another Thamnophis, though still distended from its first meal; nine days later it ate a Storeria. In the course of several months, the Conophis ate various toads and hylids and two more Storeria. Apparently members of the genus Conophis do not constrict their prey, but rely upon a combination of loss of blood and action of the venom to completely immobilize their prey.
Ditmars (1931:pls. 26-27) showed three photographs of "Conophis lineatus" (actually Conophis pulcher) ingesting another snake, identified by him as a young Ophis (= Xenodon) colubrinus.
The rear fangs of these snakes are large for the size of the snake. Various collectors have been bitten, and several reports of the effect of the poison have been published. The snakes are aggressive and bite constantly while being handled. A field companion, Dale L. Hoyt, was bitten on the forefinger by a specimen of C. l. lineatus and immediately felt a burning sensation. The finger swelled, much as it would if stung by a wasp, but it returned to normal size in about twenty-four hours. Ditmars (1931:legend pl. 27) reported immediate burning pain and a localized swelling, an inch in diameter and half an inch high, which lasted for several hours. Mertens (1952b:83) reported merely that the hand of the gardener at the Instituto Tropical in San Salvador bled strongly for a full hour. Edward H. Taylor was bitten by a specimen of Conophis vittatus (Taylor and Smith, 1939:252); pain and swelling lasted for some time. Taylor (personal communication) is still troubled by damage incurred by that bite, which apparently resulted in mechanical damage to the second joint of the middle finger, for the joint swells when the finger is used or exercised. William E. Duellman (personal communication) was bitten on the hand in July, [Pg_291] 1956. There was immediate pain and localized swelling, both of which disappeared several hours later.
The genus Conophis is known only from the Recent. Except that Conophis belongs to the subfamily xenodontinae and probably is of New World origin, little is known about the relationships of the genus. Auffenberg (1958) described a new genus and species of fossil colubrid snake from the Miocene of Montana as Dryinoides oxyrhachis and compared it with several recent genera. This specimen, of which there is a relatively complete skull and a series of vertebrae, seems most closely to resemble a specimen of Conophis lineatus dunni (UF 7657) from Honduras, with which it was compared in basic osteology. The two genera could be related, for the progenitors of Conophis possibly inhabited much of North America in the Miocene.
Another possibility is that the main stock of the xenodontines reached South America in earliest Tertiary times, and that the formation of the Panamanian and Colombian seaways that separated South America and Central America from the Late Paleocene to the middle of the Pliocene left the Conophis stock isolated in Middle America where members of the genus dispersed through semi-arid habitats.
Turning our attention now to the species within the genus, instead of the genus as a whole, Conophis vittatus is readily set apart from other members of the genus on the basis of the universal presence of seven supralabials. In basic coloration it also differs, having no stripe on the 1st scale-row, or spots on the venter, and a maximum of four broad stripes on the body. The other species appear to be more closely related; these make up the C. lineatus-group. Conophis nevermanni differs so much from the other species that it might be placed in a separate group. Nevertheless, the basic striped pattern, which is masked by the increased melanism of many specimens, indicates that nevermanni is more closely related to the lineatus-group than to vittatus. The lineatus-group, thus, consists of pulcher, nevermanni and the three subspecies of lineatus. In this group the color pattern is characterized by the high frequency of ventral spotting, darkening of part of the supralabials, dark pigmentation on the 1st scale-row, and more than four dark stripes on the body of adults. Conophis lineatus concolor, on which the dark pigmentation on the body apparently is secondarily lost, is an exception.
[Pg_292] If differences in color pattern be used as an indication of the relationships between the species and subspecies of the genus Conophis, I would consider C. vittatus the most divergent unit. The subspecies of lineatus closely resemble one another and, as a unit, resemble pulcher from which they differ primarily in the position of the dorsalmost stripes. Conophis nevermanni is more divergent than is pulcher from the species lineatus, but probably is not so far removed from lineatus as is vittatus.
In the light of what has been pointed out immediately above with respect to resemblances of, and differences between, the species, an hypothesis to account for their formation and for their presence in the areas where they are today is the following: Concurrent with climatic fluctuations in the Late Pliocene and Pleistocene, the northernmost population differentiated into the species vittatus, and has subsequently spread north and west from the region of Tehuantepec, México. During the same period nevermanni became isolated in northern Costa Rica.
The species pulcher probably differentiated from the remaining lineatus stock during the Early Pleistocene orogenic upheaval in Guatemala. The pulcher stock was isolated on the Pacific Coastal slopes of Guatemala, while lineatus moved through the subhumid corridor of northern Middle America into México and southward toward Costa Rica (Stuart, 1954a). In the Late Pleistocene and Recent, pulcher moved back across the central Guatemalan highlands occupying its present range in northern Middle America. Primarily because of the formation of unsuitable habitat (wet forest) that presently separates the geographic ranges of populations of lineatus, this species differentiated into three subspecies.
The genus Conophis Peters, 1860, contains four species. Three are monotypic and the fourth has three subspecies, making a total of six taxa.
The genus is characterized by maxillary teeth of equal size followed by a diastema and two enlarged grooved fangs. The scales are smooth, in 19 rows at mid-body, and 17 nearer the tail. The anal is divided, apical pits are lacking, the head shields are normal for a colubrid, and the hemipenis is bilobed having many large basal spines.
The six taxa are separated primarily on the basis of color pattern, but characters of scutellation, including numbers of dorsals, ventrals, [Pg_293] caudals, and places of reduction of the number of dorsal scale-rows, were analyzed.
Snakes of this genus are distributed throughout semi-arid environments from southern México southward into Costa Rica. They feed upon lizards, primarily of the genus Cnemidophorus; in addition they are known to eat small rodents and other snakes.
Conophis is a member of the subfamily Xenodontinae and, as presently understood, has no known living close relatives. A single specimen of Dryinoides from the Miocene of Montana has been compared with this genus. The genus Conophis is thought to have evolved in Middle America. The present distribution and differentiation probably are primarily the result of climatic fluctuations in Middle America, which produced the areas of subhumid environment where Conophis presently lives.
Transmitted November 30, 1962.
29-5936
Institutional libraries interested in publications exchange may obtain this series by addressing the Exchange Librarian, University of Kansas Library, Lawrence, Kansas. Copies for individuals, persons working in a particular field of study, may be obtained by addressing instead the Museum of Natural History, University of Kansas, Lawrence, Kansas. There is no provision for sale of this series by the University Library, which meets institutional requests, or by the Museum of Natural History, which meets the requests of individuals. However, when individuals request copies from the Museum, 25 cents should be included, for each separate number that is 100 pages or more in length, for the purpose of defraying the costs of wrapping and mailing.
* An asterisk designates those numbers of which the Museum's supply (not the Library's supply) is exhausted. Numbers published to date, in this series, are as follows:
| Vol. 1. | Nos. 1-26 and index. Pp. 1-638, 1946-1950. | |
| *Vol. 2. | (Complete) Mammals of Washington. By Walter W. Dalquest. Pp. 1-444, 140 figures in text. April 9, 1948. | |
| Vol. 3. | *1. | The avifauna of Micronesia, its origin, evolution, and distribution. By Rollin H. Baker. Pp. 1-359, 16 figures in text. June 12, 1951. |
| *2. | A quantitative study of the nocturnal migration of birds. By George H. Lowery, Jr. Pp. 361-472, 47 figures in text. June 29, 1951. | |
| 3. | Phylogeny of the waxwings and allied birds. By M. Dale Arvey. Pp. 473-530, 530, 49 figures in text, 13 tables. October 10. 1951. | |
| *4. | Birds from the state of Veracruz, Mexico. By George H. Lowery, Jr., and Walter W. Dalquest. Pp. 531-649, 7 figures in text, 2 tables. October 10, 1951. | |
| Index. Pp. 651-681. | ||
| *Vol. 4. | (Complete) American weasels. By E. Raymond Hall. Pp. 1-466, 41 plates, 31 figures in text. December 27, 1951. | |
| Vol. 5. | Nos. 1-37 and index. Pp. 1-676, 1951-1953. | |
| *Vol. 6. | (Complete) Mammals of Utah, taxonomy and distribution. By Stephen D. Durrant. Pp. 1-549, 91 figures in text, 30 tables. August 10, 1952. | |
| Vol. 7. | Nos. 1-15 and index. Pp. 1-651, 1952-1955. | |
| Vol. 8. | Nos. 1-10 and index. Pp. 1-675, 1954-1956. | |
| Vol. 9. | *1. | Speciation of the wandering shrew. By James S. Findley. Pp. 1-68, 18 figures in text. December 10, 1955. |
| 2. | Additional records and extension of ranges of mammals from Utah. By Stephen D. Durrant, M. Raymond Lee, and Richard M. Hansen. Pp. 69-80. December 10, 1955. | |
| 3. | A new long-eared myotis (Myotis evotis) from northeastern Mexico. By Rollin H. Baker and Howard J. Stains. Pp. 81-84. December 10, 1955. | |
| 4. | Subspeciation in the meadow mouse, Microtus pennsylvanicus, in Wyoming. By Sydney Anderson. Pp. 85-104, 2 figures in text. May 10, 1956. | |
| 5. | The condylarth genus Ellipsodon. By Robert W. Wilson. Pp. 105-116, 6 figures in text. May 19, 1956. | |
| 6. | Additional remains of the multituberculate genus Eucosmodon. By Robert W. Wilson. Pp. 117-123, 10 figures in text. May 19, 1956. | |
| 7. | Mammals of Coahulia, Mexico. By Rollin H. Baker. Pp. 125-335, 75 figures in text. June 15, 1956. | |
| 8. | Comments on the taxonomic status of Apodemus peninsulae, with description of a new subspecies from North China. By J. Knox Jones, Jr. Pp. 337-346, 1 figure in text, 1 table. August 15, 1956. | |
| 9. | Extensions of known ranges of Mexican bats. By Sydney Anderson. Pp. 347-351. August 15, 1956. | |
| 10. | A new bat (Genus Leptonycteris) from Coahulia. By Howard J. Stains. Pp. 353-356. January 21, 1957. | |
| 11. | A new species of pocket gopher (Genus Pappogeomys) from Jalisco, Mexico. By Robert J. Russell. Pp. 357-361. January 21, 1957. | |
| 12. | Geographic variation in the pocket gopher, Thomomys bottae, in Colorado. By Phillip M. Youngman. Pp. 363-384, 7 figures in text. February 21, 1958. | |
| 13. | New bog lemming (genus Synaptomys) from Nebraska. By J. Knox Jones, Jr. Pp. 385-388. May 12, 1958. | |
| 14. | Pleistocene bats from San Josecito Cave, Nuevo León, México. By J. Knox Jones, Jr. Pp. 389-396. December 19, 1958. | |
| 15. | New subspecies of the rodent Baiomys from Central America. By Robert L. Packard. Pp. 397-404. December 19, 1958. | |
| 16. | Mammals of the Grand Mesa, Colorado. By Sydney Anderson. Pp. 405-414, 1 figure in text, May 20, 1959. | |
| 17. | Distribution, variation, and relationships of the montane vole, Microtus montanus. By Sydney Anderson. Pp. 415-511, 12 figures in text, 2 tables. August 1, 1959. | |
| 18. | Conspecificity of two pocket mice, Perognathus goldmani and P. artus. By E. Raymond Hall and Marilyn Bailey Ogilvie. Pp. 513-518, 1 map, January 14, 1960. [Pg_ii] | |
| 19. | Records of harvest mice, Reithrodontomys, from Central America, with description of a new subspecies from Nicaragua. By Sydney Anderson and J. Knox Jones, Jr. Pp. 519-529. January 14, 1960. | |
| 20. | Small carnivores from San Josecito Cave (Pleistocene), Nuevo León, México. By E. Raymond Hall. Pp. 531-538, 1 figure in text. January 14, 1960. | |
| 21. | Pleistocene pocket gophers from San Josecito Cave, Nuevo León, México. By Robert J. Russell. Pp. 539-548, 1 figure in text. January 14, 1960. | |
| 22. | Review of the insectivores of Korea. By J. Knox Jones, Jr., and David H. Johnson. Pp. 549-578. February 23, 1960. | |
| 23. | Speciation and evolution of the pygmy mice, genus Baimoys. By Robert L. Packard. Pp. 579-670, 4 plates, 12 figures in text. June 16, 1960. | |
| Index. Pp. 671-690 | ||
| Vol. 10. | 1. | Studies of birds killed in nocturnal migration. By Harrison B. Tordoff and Robert M. Mengel. Pp. 1-44, 6 figures in text, 2 tables. September 12, 1956. |
| 2. | Comparative breeding behavior of Ammospiza caudacuta and A. maritima. By Glen E. Woolfenden. Pp. 45-75, 6 plates, 1 figure. December 20, 1956. | |
| 3. | The forest habitat of the University of Kansas Natural History Reservation. By Henry S. Fitch and Ronald R. McGregor. Pp. 77-127, 2 plates, 7 figures in text, 4 tables. December 31, 1956. | |
| 4. | Aspects of reproduction and development in the prairie vole (Microtus ochrogaster). By Henry S. Fitch. Pp. 129-161, 8 figures in text, 4 tables. December 19, 1957. | |
| 5. | Birds found on the Arctic slope of northern Alaska. By James W. Bee. Pp. 163-211, plates 9-10, 1 figure in text. March 12, 1958. | |
| *6. | The wood rats of Colorado: distribution and ecology. By Robert B. Finley, Jr. Pp. 213-552, 34 plates, 8 figures in text, 35 tables. November 7, 1958. | |
| 7. | Home ranges and movements of the eastern cottontail in Kansas. By Donald W. Janes. Pp. 553-572, 4 plates, 3 figures in text. May 4, 1959. | |
| 8. | Natural history of the salamander, Aneides hardyi. By Richard F. Johnston and Gerhard A. Schad. Pp. 573-585. October 8, 1959. | |
| 9. | A new subspecies of lizard, Cnemidophorus sacki, from Michoacán, México. By William E. Duellman. Pp. 587-598, 2 figures in text. May 2, 1960. | |
| 10. | A taxonomic study of the middle American snake, Pituophis deppei. By William E. Duellman. Pp. 599-610. 1 plate, 1 figure in text. May 2, 1960. | |
| Index. Pp. 611-626. | ||
| Vol. 11. | Nos. 1-10 and index. Pp. 1-703, 1958-1960. | |
| Vol. 12. | 1. | Functional morphology of three bats: Sumops, Myotis, Macrotus. By Terry A. Vaughan. Pp. 1-153, 4 plates, 24 figures in text. July 8, 1959. |
| *2. | The ancestry of modern Amphibia: a review of the evidence. By Theodore H. Eaton, Jr. Pp. 155-180, 10 figures in text. July 10, 1959. | |
| 3. | The baculum in microtine rodents. By Sydney Anderson. Pp. 181-216, 49 figures in text. February 19, 1960. | |
| *4. | A new order of fishlike Amphibia from the Pennsylvanian of Kansas. By Theodore H. Eaton, Jr., and Peggy Lou Stewart. Pp. 217-240, 12 figures in text. May 2, 1960. | |
| 5. | Natural history of the bell vireo. By Jon C. Barlow. Pp. 241-296, 6 figures in text. March 7, 1962. | |
| 6. | Two new pelycosaurs from the lower Permian of Oklahoma. By Richard C. Fox. Pp. 297-307, 6 figures in text. May 21, 1962. | |
| 7. | Vertebrates from the barrier island of Tamaulipas, México. By Robert K. Selander, Richard F. Johnston, B. J. Wilks, and Gerald G. Raun. Pp. 309-345, pls. 5-8. June 18, 1962. | |
| 8. | Teeth of Edestid sharks. By Theodore H. Eaton, Jr. Pp. 347-362, 10 figures in text. October 1, 1962. | |
| More numbers will appear in volume 12. | ||
| Vol. 13. | 1. | Five natural hybrid combinations in minnows (Cyprinidae). By Frank B. Cross and W. L. Minckley. Pp. 1-18. June 1, 1960. |
| 2. | A distributional study of the amphibians of the Isthmus of Tehuantepec, México. By William E. Duellman. Pp. 19-72, pls. 1-8, 3 figures in text. August 16, 1960. | |
| 3. | A new subspecies of the slider turtle (Pseudemys scripta) from Coahulia, México. By John M. Legler. Pp. 73-84, pls. 9-12, 3 figures in text. August 16, 1960. | |
| 4. | Autecology of the copperhead. By Henry S. Fitch. Pp. 85-288, pls. 13-20, 26 figures in text. November 30, 1960. | |
| 5. | Occurrence of the garter snake, Thamnophis sirtalis, in the Great Plains and Rocky Mountains. By Henry S. Fitch and T. Paul Maslin. Pp. 289-308, 4 figures in text. February 10, 1961. | |
| 6. | Fishes of the Wakarusa river in Kansas. By James E. Deacon and Artie L. Metcalf. Pp. 309-322, 1 figure in text. February 10, 1961. | |
| 7. | Geographic variation in the North American cyprinid fish, Hybopsis gracilis. By Leonard J. Olund and Frank B. Cross. Pp. 323-348, pls. 21-24, 2 figures in text. February 10, 1961. [Pg_iii] | |
| 8. | Descriptions of two species of frogs, genus Ptychohyla; studies of American hylid frogs, V. By William E. Duellman. Pp. 349-357, pl. 25, 2 figures in text. April 27, 1961. | |
| 9. | Fish populations, following a drought, in the Neosho and Marais des Cygnes rivers of Kansas. By James Everett Deacon. Pp. 359-427, pls. 26-30, 3 figs. August 11, 1961. | |
| 10. | Recent soft-shelled turtles of North America (family Trionychidae). By Robert G. Webb. Pp. 429-611, pls. 31-54, 24 figures in text. February 16, 1962. | |
| Index. Pp. 613-624. | ||
| Vol. 14. | 1. | Neotropical bats from western México. By Sydney Anderson. Pp. 1-8. October 24, 1960. |
| 2. | Geographic variation in the harvest mouse. Reithrodontomys megalotis, on the central Great Plains and in adjacent regions. By J. Knox Jones, Jr., and B. Mursaloglu. Pp. 9-27, 1 figure in text. July 24, 1961. | |
| 3. | Mammals of Mesa Verde National Park, Colorado. By Sydney Anderson. Pp. 29-67, pls. 1 and 2, 3 figures in text. July 24, 1961. | |
| 4. | A new subspecies of the black myotis (bat) from eastern Mexico. By E. Raymond Hall and Ticul Alvarez. Pp. 69-72, 1 figure in text. December 29, 1961. | |
| 5. | North American yellow bats, "Dasypterus," and a list of the named kinds of the genus Lasiurus Gray. By E. Raymond Hall and J. Knox Jones, Jr. Pp. 73-98, 4 figures in text. December 29, 1961. | |
| 6. | Natural history of the brush mouse (Peromyscus boylii) in Kansas with description of a new subspecies. By Charles A. Long. Pp. 99-111, 1 figure in text. December 29, 1961. | |
| 7. | Taxonomic status of some mice of the Peromyscus boylii group in eastern Mexico, with description of a new subspecies. By Ticul Alvarez. Pp. 113-120, 1 figure in text. December 29, 1961. | |
| 8. | A new subspecies of ground squirrel (Spermophilus spilosoma) from Tamaulipas, Mexico. By Ticul Alvarez. Pp. 121-124. March 7, 1962. | |
| 9. | Taxonomic status of the free-tailed bat, Tadarida yucatanica Miller. By J. Knox Jones, Jr., and Ticul Alvarez. Pp. 125-133, 1 figure in text. March 7, 1962. | |
| 10. | A new doglike carnivore, genus Cynaretus, from the Clarendonian Pliocene, of Texas. By E. Raymond Hall and Walter W. Dalquest. Pp. 135-138, 2 figures in text. April 30, 1962. | |
| 11. | A new subspecies of wood rat (Neotoma) from northeastern Mexico. By Ticul Alvarez. Pp. 139-143. April 30, 1962. | |
| 12. | Noteworthy mammals from Sinaloa, Mexico. By J. Knox Jones, Jr., Ticul Alvarez, and M. Raymond Lee. Pp. 145-159, 1 figure in text. May 18, 1962. | |
| 13. | A new bat (Myotis) from Mexico. By E. Raymond Hall. Pp. 161-164, 1 figure in text. May 21, 1962. | |
| 14. | The mammals of Veracruz. By E. Raymond Hall and Walter W. Dalquest. Pp. 165-362, 2 figures. May 20, 1963. | |
| 15. | The recent mammals of Tamaulipas, México. By Ticul Alvarez. Pp. 363-473, 5 figures in text. May 20, 1963. | |
| More numbers will appear in volume 14. | ||
| Vol. 15. | 1. | The amphibians and reptiles of Michoacán, México. By William E. Duellman. Pp. 1-148, pls. 1-6, 11 figures in text. December 20, 1961. |
| 2. | Some reptiles and amphibians from Korea. By Robert G. Webb, J. Knox Jones, Jr., and George W. Byers. Pp. 149-173. January 31, 1962. | |
| 3. | A new species of frog (Genus Tomodactylus) from western México. By Robert G. Webb. Pp. 175-181, 1 figure in text. March 7, 1962. | |
| 4. | Type specimens of amphibians and reptiles in the Museum of Natural History, the University of Kansas. By William E. Duellman and Barbara Berg. Pp. 183-204. October 26, 1962. | |
| 5. | Amphibians and Reptiles of the Rainforests of Southern El Petén, Guatemala. By William E. Duellman. Pp. 205-249, pls. 7-10, 6 figures in text. October 4, 1963. | |
| 6. | A revision of snakes of the genus Conophis (Family Colubridae, from Middle America). By John Wellman. Pp. 251-295, 9 figures in text. October 4, 1963. | |
| More numbers will appear in volume 15. | ||
For consistancy, a number of word which had alternate spellings were altered to match the most prevalent version used. For example, where the word Mexico was used in the body of the article, the more frequent spelling (México) was substituted. However, in the reference sections, the spelling was not altered as that may have been the spelling used by the article's author. All occurrances of Érpétologie Genérale were correcteded to Erpétologie Générale (Pp. 255, 262, 267, 277, and 278).
On page 279 under Variation there appears to be a miscalculation:
| Page | Correction | |
| 264 | immaculaate ⇒ immaculate | |
| 264 | chacteristic ⇒ characteristic | |
| 266 | elevatons ⇒ elevations | |
| 267 | Dumeril ⇒ Duméril | |
| 277 | Duméil ⇒ Duméril | |
| 279 | Tehauntepec ⇒ Tehuantepec | |
| 280 | Deleted repeated "Oaxaca," | |
| 292 | primarly ⇒ primarily | |
| 295 | hertetofaunal ⇒ herpetofaunal | |
| i | V. 9 No. 12 - Pp. 363-387 ⇒ Pp. 363-384 | |
| iii | V. 13 No. 8 - Decriptions ⇒ Descriptions | |
| iii | V. 14 No. 8 - anad ⇒ and | |
| iii | V. 14 No. 14 - anad ⇒ and |