
Title: Life History and Ecology of the Five-Lined Skink, Eumeces fasciatus
Author: Henry S. Fitch
Release date: June 10, 2012 [eBook #39958]
Language: English
Credits: Produced by Chris Curnow, Tom Cosmas, Joseph Cooper and
the Online Distributed Proofreading Team at
http://www.pgdp.net

University of Kansas Publications
Museum of Natural History
Volume 8, No. 1, pp. 1-156, 2 pls., 26 figs. in text, 17 tables
BY
University of Kansas
Lawrence
University of Kansas Publications, Museum of Natural History
Editors: E. Raymond Hall, Chairman, A. Byron Leonard, Robert W. Wilson
Volume 8, No. 1, pp. 1-156, 2 pls., 26 figs. in text, 17 tables
Published September 1, 1954
University of Kansas
Lawrence, Kansas
PRINTED BY
FERD VOILAND, JR., STATE PRINTER
TOPEKA, KANSAS
1954
25-3559
By
The common five-lined skink (or common blue-tailed skink) is a small woodland lizard, abundantly and widely distributed over the eastern United States. Many authors have casually discussed this lizard or have treated in detail some phase of its biology. Excellent brief summaries of the known facts concerning its life history have been published by Smith (1946:349-350 and 1950:187-188) and Pope (1947:153-157). Nevertheless, no thoroughgoing study of its life history and ecology has heretofore been made.
In 1932, taxonomic studies by Dr. Edward H. Taylor revealed that the lizards previously referred to in the literature as Eumeces fasciatus, actually were three closely related and similar, partly sympatric species. Although Taylor’s work was careful and detailed, and indicated numerous minor differences by which the three species could be distinguished, many herpetologists were reluctant to accept his findings for nearly a decade thereafter. Consequently a large amount of literature concerning five-lined skinks is either obviously composite in the sense that it is based upon two or three species, or is not definitely assignable to any one species. In the study here reported upon, all pertinent literature available to me has been examined, and evaluated, and important findings of other authors have been incorporated in the discussion. However, mine was primarily a field study, and in one small part of the geographic range of the one species.
The University of Kansas Natural History Reservation is a tract of 590 acres preserved as a natural area, available for the pursuit of ecological studies. The studies undertaken include intensive investigations of selected species of vertebrate animals. The main criteria used in selecting these species have been whether or not they were sufficiently abundant and generally enough distributed to play an important role in the over-all ecology of the area, and whether a species was sufficiently accessible for study with available techniques. Among the 300 species of vertebrate animals recorded [4] from the Reservation, the five-lined skink is one of those most frequently noticed in the field. In actual numbers it is probably exceeded only by the cricket frog (Acris gryllus), the leopard frog (Rana pipiens), the ring-necked snake (Diadophis punctatus), the prairie vole (Microtus ochrogaster) and perhaps the white-footed mouse (Peromyscus leucopus). Although numerous, the skink is not easy to study because it is secretive in its behavior, and is inactive in inaccessible shelters during the greater part of the year.
The five-lined skink generally occurs along with a characteristic set of community associates in a particular type of situation. It is a predator on various small animals, mostly invertebrates. For some of the many prey species the effect is certainly negligible, but for others its predation may be a major ecological factor. In areas where optimum habitat conditions exist its biomass may exceed that of any other insectivorous animal, and in such situations it assumes a major role as a predator and as a competitor with other insectivorous types. In turn it provides part of the food source of various larger predators, including reptiles, birds and mammals. It is a host and carrier of various parasites, including at least one species that regularly attacks humans—the common chigger. It is not evident on the basis of the present findings that the skink is either harmful or beneficial to any perceptible degree, in its over-all effect on human affairs and economy. Nevertheless, there probably are various unsuspected relationships.
In the course of my field study many workers on the University of Kansas Natural History Reservation helped by capturing skinks; especially Sydney Anderson, Richard Freiburg, John Hawken, Dennis G. Rainey and Lewis L. Sandidge. Mr. Robert Gordon very kindly furnished information on specimens in the Tulane University collection, which served as a basis for comparing the breeding schedule of the southern population with that of E. fasciatus in northeastern Kansas. Dr. W. J. Breckenridge kindly permitted examination of material in the University of Minnesota Museum of Natural History. Dr. Edward H. Taylor has made helpful suggestions from time to time. Mr. Richard B. Loomis helped me in various ways with the field work, and made available his personal field notes with records of predation on Eumeces by various snakes. Dr. E. Raymond Hall, Director of the Museum of Natural History, has critically examined the manuscript, and has been helpful in various ways. The line drawings and graphs, with the exception of Figures 8 and 9, were made or completed by Mrs. Louise Brunk, artist for the Museum.
The study here reported on was initiated in May 1949, and was continued through 1950, 1951 and 1952. A few observations made in 1948 have been included. Various separate items of information obtained in 1953 have likewise been incorporated especially where histories of individual skinks are presented, but the manuscript was completed in essentially its present form in the fall of 1952.
Skinks were obtained by active search; rocks and boulders were lifted up and the skinks thus exposed were seized by hand before they had time to escape. This method was effective when the skinks were using rocks for shelter and when temperatures were low enough so that they were slow and sluggish, but in hot weather the skinks were so quick and active that those exposed usually escaped. Usually skinks could be obtained much more easily by trapping. At the pond rock pile (Fig. 26), for instance, shelter was so readily available that the skinks could seldom be caught by hand. Gallon cans buried with the tops open flush with the surface of the ground served as pitfalls and were effective when they were carefully placed, at the bases of rock ledges or logs or stumps, where the skinks were most likely to fall into them. Most of the skinks recorded at the rock pile were caught by this method, and sometimes several were caught together in the same pitfall. Ordinarily each pitfall was covered with a large flat rock, propped against a nearby object to leave ample space for the skink to enter beneath it. The rocks provided protection from direct sunlight, from rain, and from predators. Still another method of catching skinks was with wire screen funnel traps (Fitch 1951:77). These funnel traps were of different sizes, and were made of different kinds of wire mesh. They were set for reptiles that were mostly larger than five-lined skinks, and those having quarter-inch wire mesh permitted many of the immature skinks to escape. Most of these funnel traps were from about one foot long and five inches in diameter, to about twice these dimensions, with funnel openings about 1.5 inches in diameter. Some made of 1⁄8 inch wire mesh, six or seven inches long, and three or four inches in diameter, with funnel openings only a little larger than the body diameter of an adult skink, were found to be suitable for skinks of all sizes, and were used successfully at the pond rock pile. Most of the skinks trapped were adult males, and they were taken chiefly in May. The funnel traps were generally placed at the edges of rock outcrops, boulders or logs, where skinks were likely to be intercepted in their usual travel [6] routes. Each method of collecting skinks resulted in occasional mortality to them but most losses were in those caught in funnel traps. In these traps they sustained rapid loss of moisture, and were usually somewhat desiccated. Two or more adult males were often caught together, and in most of these instances the first one caught probably served as bait attracting another and arousing his pugnacious interest. Injuries were frequent, and some deaths occurred because in the close confines of a trap the loser in a fight was unable to escape further attacks.
Most of the skinks caught were examined, and released within a few minutes. Snout-vent length was measured by holding the skink against a rigid transparent plastic millimeter ruler and exerting a slight pull on each end of the lizard until it tired and relaxed its muscles, eliminating bends and kinks. Even with such precaution, precise measurements could not be obtained and the readings often varied a millimeter or more for the same skink measured two or more times on the same day. Tail length was similarly recorded with separate readings for the original and regenerated portions. Also recorded were sex (when discernible), color and pattern, breeding data, injuries, general condition, and sometimes temperature. Many of the skinks were brought to the laboratory, and were weighed to the nearest tenth of a gram.
Occasional trips were made to localities away from the Reservation to collect skinks. Some of those obtained were kept under observation in terraria where their behavior was studied. Most were preserved and were used for data on habitat preferences, seasonal changes in the gonads, size group, stomach contents, and various other items of information.
The scutellation and osteology have been described in detail by Taylor (1936:39-48 and 199-206) and others, and need not be repeated. The five-lined skink is slender and elongate, somewhat snake-like (though much less so than many other skinks) as the head, neck, body, and tail are not well set off from each other, and the sleek, streamlined contours are broken only by the small limbs protruding from the sides of the body. The body is slightly flattened laterally, tending toward quadrangular shape in cross section. The head is wedge-shaped, with a short, rounded snout. The nostrils are laterally placed, well back from the tip of the snout. The eyes are small and deep set; the iris is dark. The neck is thick and strong, nearly as long as the head. The torso is 31⁄2 to 4 times as long as it is wide. The tail is almost square in cross section at its base, but [7] is circular in cross section for most of its length. The limbs are moderately developed; when adpressed along the sides of the body, the forelimb and hind limb overlap by a length about equal to the longest toes of the forelimb. The limbs are pentadactyl and all the toes are well developed and have claws (Figures 1 and 2). The claws are short, and are curved in such a manner that their tips are directed downward, each approximately at right angles to the axis of the toe (Figure 2b). The limbs are moderately thick and muscular. The upper arm and forearm segments are of approximately equal length, as are the femoral and tibio-fibular segments of the hind limb.
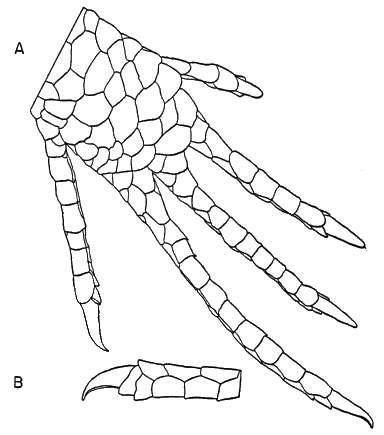
Fig. 2.
A. Antiplantar view of right hind foot, × 9.
B. Terminal part of second toe of left hind foot, and its claw, in lateral
view, × 9.
[8] The five-lined pattern is characteristic of the hatchling, but gradual ontogenetic change results in its dulling, suppression, and eventual loss. In the hatchling the ground color of the head and body is black or dark brown, with five milky white longitudinal stripes extending the length of the head and body, and on the basal one-fourth of the tail. The five light lines are of approximately equal width, and are separated by dark interspaces 11⁄2 to 2 times as wide. The mid-dorsal stripe includes most of the two mid-dorsal scale rows. Posteriorly it extends onto the base of the tail, where it becomes increasingly suffused with the blue color of the tail, widens, and loses its identity. In the nuchal region, this dorsal stripe narrows and splits into left and right branches, which diverge anteriorly to form a lyrate pattern on the head. On either side of the dorsal stripe are the dark interspaces, nearly twice as wide as the stripe itself and tapering to a point posteriorly on the tail, likewise tapering anteriorly to a point immediately above and in front of the eye. Lateral to these dark areas are the dorsolateral stripes; they extend from the basal one-fourth of the tail anteriorly onto the head along the superciliary region, tapering to a point on the anterior superciliary. Below these stripes are the dark lateral areas which extend from the basal part of the tail anteriorly along the sides of body and neck region (including the upper half of the aperture of the ear), eye region, and loreal region. Below this dark area on each side is the lateral stripe. It extends along the sides just above the level of the limb insertions (broken or pinched to a fraction of its average width above the hind limb insertion), broken by the ear opening, and extending anteriorly to include all the supralabial scales (with the exception of their upper edges) and the rostral. Here the left and right lateral stripes may be said to join; however in the facial region these stripes are not well defined, partly because the dark areas that border their lower edges do not extend so far forward. This lowermost dark area is about equal in width to the lateral stripe. It extends from the posterior infralabials posteriorly, to include the fore- and hind-limbs, and onto the basal part of the tail. The ventral surface of the head and body is dull white or pearly gray.
Thus, there are 12 longitudinal bands of color on the body: the five narrow, subequal, pale lines separated by the six dark areas, of which the dorsal and dorsolateral are broad and of approximately equal width, while the ventrolateral is narrower; and lastly the broad, pale ventral area.
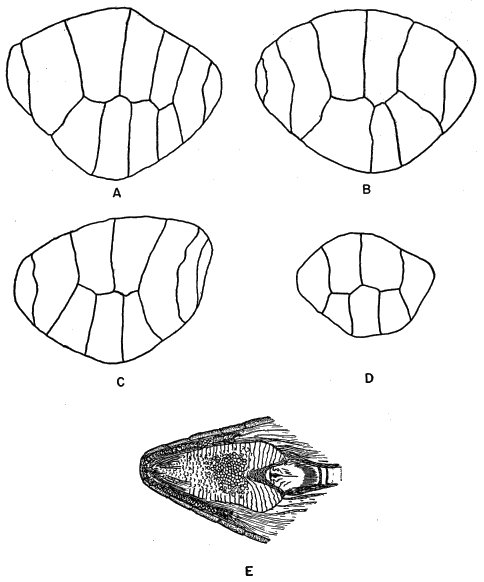
Fig. 3. A. Osteoderm of an old adult male, from near the midline of the back,
× 25.
B. Another osteoderm from same male, from belly near midline, × 25.
C. Another osteoderm from side of same male, at a point approximately
halfway between foreleg and hind leg, × 25.
D. Osteoderm of a juvenile obtained in April, from near midline of
back, × 25.
E. Tongue from dorsal view, shown in its normal position in the lower
jaw, × 21⁄2.
The tail in young individuals is bright blue. In Eumeces the tail characteristically has a color different from that of the body, and is usually more conspicuous; in many species it is blue, but in others it may be purple, greenish-blue, red, pink, or orange. Hatchlings have the most brightly colored tails, and as growth proceeds the colors gradually become duller. In E. fasciatus the bright colors of the tail are mostly or entirely lost in old adults, especially in males, and in individuals of either sex that have lost their original tails [10] and regenerated new tails. Young which lose their tails and regenerate them at an early age have the regenerated portions colored almost as brightly as the originals at first.
The skin is tight fitting and relatively thick, stiffened by a bony armor. A small bony plate or osteoderm underlies each scale. Oliver (1951:127) has called attention to the pattern of ornamentation on the osteoderms, which becomes more complex with advancing age. He has suggested the possibility that age might be accurately determined on the basis of extent of osteodermal ornamentation. I have compared osteodermal ornamentation in marked individuals of known age, but have found it to be of limited applicability as a method of age determination; size and pattern are probably more satisfactory bases for estimating age, even though they do not permit definite aging of old adults and are not infallible for skinks short of adult size. In adult E. fasciatus the pattern of ornamentation is closely similar to that figured for E. laticeps by Oliver (op. cit.) and also resembles the pattern shown for an Old World skink, Mabuya multifasciata, as figured by Smith (1935: 2). The pattern differs somewhat in osteoderms on different parts of the body, and is most nearly symmetrical in those near the midline on either dorsal or ventral surface (Figure 3).
Eumeces is a widespread genus occurring in the New World in southern Canada and southward into Costa Rica. The greatest number of forms is in Mexico. In the Old World numerous species occur in southeastern Asia and on adjacent islands, and other species occur westward across southern Asia, and across North Africa to Morocco, with a major break in the continuity of distribution in the Himalayan region. Taylor in his revision recognized 57 forms with fifty full species, belonging to 15 major groups within the genus. Since then only relatively minor changes in classification have been proposed. Several new species and subspecies have been named, and several species have been relegated to the status of subspecies.
Within the genus there are several groups that have representatives in both the New World and the Old World. Smith and Etheridge (1953:159) point out that the most primitive line of Eumeces is best represented in the Old World, where there are two groups and nine species, while in the New World this line has only three tropical relict forms. For this reason, Smith and Etheridge concur with Taylor (1936:67) in considering the genus to be of Old World [11] origin; but the two main lines of the genus (the four-lined and five-lined stocks) are both regarded as being of New World origin. According to this idea, the Asiatic members of these two groups migrated from the New World. In the early Tertiary, warm temperate climates extended north to the Arctic Circle, and Eumeces, or at least some of its species, may have had a distribution straddling migration routes to both North America and Asia.
Of the 15 groups within the genus, the fasciatus group, with a dozen species, has more representatives than any other. The fasciatus group is characterized by having the tail bright blue with dorsal body pattern of five light lines on a darker ground color; mid-dorsal line bifurcating on head to form lyrate markings (this striped pattern and bright color of the tail becoming dull or obsolete in the adults); medial preanal scales overlapped by those lateral to them; two pairs of nuchals; no postfemoral pocket; four supraoculars; scales on sides of body in parallel rows. The characters that separate members of the fasciatus group from each other are minor. The width and position of the light lines differ somewhat among them. The mid-dorsal light line bifurcates either on the nuchals or on the parietals. The complex of scales in the temporal region differ in shape and relative size.
The following table, compiled mostly from information set forth by Taylor (1936:186-283), indicates some of the main differences and similarities between species in the chief characters upon which the classification is based.
The close resemblance between E. fasciatus and its Asiatic relatives is remarkable considering the great distance separating them and the long time that must have elapsed since their isolation began. Some of the Asiatic forms differ from each other almost as much as they differ from fasciatus. Of the Asiatic species, elegans, tamdaoensis, oshimensis, and marginatus differ from fasciatus in markedly larger size; elegans, marginatus, oshimensis, and stimsonii differ in lacking a postnasal; all but tamdaoensis tunganus and xanthi differ in having only a single postmental; all but tunganus, E. latiscutatus okadae (and sometimes oshimensis and elegans) differ in reduced number of scale rows; all but tunganus differ in having a lateral postanal scale differentiated, and usually keeled; tunganus, xanthi and elegans differ in having a patch of enlarged scales on the posterior side of the thigh; and in all, the primary temporals and upper and lower secondary temporals differ in size and proportions. Although some of the Asiatic forms seem to be directly derived from others, fasciatus is somewhat intermediate between the more divergent forms, and fulfills most of the conditions to be looked for in an ancestral type.
Table 1. Distribution, Pattern, Size, and Lepidosis of the “Five-lined” Skinks (Fasciatus Group of the Genus Eumeces)
| fasciatus | laticeps | inexpectatus | tunganus | xanthi | elegans | tamadoensis | oshimensis | stimsonii | barbouri | marginatus | latiscutatus | |
| Distribution | E U. S., except Fla. and N New England |
Most of E U. S., except N tier of states |
SE U. S. | W Szechwan (in N China) |
SE China | SW China, Formosa, Pesca- dores I. |
Indo- China |
Amami- gunto I. |
Ishigaki- jima, Riu Kiu I. |
Amami- shima |
Okinawa | Japan, (main I.) |
| Juvenal Pattern | 5 lined | 5 or 7 lined | 5 or 7 lined | 5 lined | 5 lined | 5 lined | 5 lined | 5 lined | 7 lined | ...... | 5 lined | 5 lined |
| Max. snout-vent
length in mm. |
80 | 130 | 89 | 81 | 76 | 96 | ...... | 99 | 63 | 66 | 93 | 80 |
| Postnasal | present | present | present | present | present | absent | present | absent | absent | present | absent | present |
| Postmental | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 |
| No. scale rows | 28-30 | 30-32 | 30-32 | 28 | 22-24 | 26-28 | ...... | 26-28 | 26 | 22 | 26 | 26 (or 24) |
| Lateral postanal scales |
undiffer- entiated |
undiffer- entiated |
undiffer- entiated |
undiffer- entiated |
differ- entiated |
keeled | ...... | keeled | keeled | ..... | keeled | keeled |
| Large scales on back of thigh |
absent | absent | absent | present | present | present irregular |
...... | absent | enlarged; regular |
absent | absent | absent |
| Median subcaudals |
widened | widened | not widened |
widened | widened | widened | ...... | widened | not widened |
widened | widened | widened |
The American Eumeces laticeps and E. inexpectatus seem to be more specialized than E. fasciatus and might have been derived from it or from a common ancestor differing but little from the modern fasciatus. Both differ from fasciatus in having more scale rows. E. laticeps also differs in having eight instead of seven supralabials and in having the median subcaudal scales greatly widened, in having intercalated plates on the outer side of the fourth toe nearly to the ultimate phalanx, posterior supralabial low and elongate, young sometimes seven-lined instead of five-lined, and especially in much larger size, stocky build, and in early loss of striped pattern. E. inexpectatus differs in having the median subcaudals not at all enlarged, and in having the dorsolateral stripes a little more widely separated from the midline.
Eumeces fasciatus and its relatives present a curious exception to Jordan’s Rule, which states that the nearest relatives of any given species are to be found neither in the same area nor in a remote one, but in an adjacent region separated by a barrier. E. fasciatus is absent from almost all of Florida; otherwise its range overlaps most of the ranges of both laticeps and inexpectatus, the former including the southeastern United States south of about latitude 40°, and the latter being mainly in the Atlantic and Gulf states from Chesapeake Bay into eastern Louisiana. Presumably both of these species began their differentiation as southern populations of an ancestral fasciatus and later became isolated from it and continued their differentiation until they overlapped it again as distinct species. The differentiation of laticeps, being much greater, presumably took place at an earlier time than did that of inexpectatus, and at present it overlaps fasciatus more extensively. E. laticeps probably diverged to such an extent that competition with fasciatus is greatly reduced where the two species occur together.
Since Eumeces laticeps was recognized by Taylor as a species distinct from fasciatus, numerous authors have accumulated field observations that demonstrate ecological divergence between the two. Conant (1951:33) wrote that in Ohio laticeps prefers a dry habitat of bare rocks, cliffs, dry hillsides, and trees. He summed up the habitat difference as follows: “Fasciatus appears to be essentially terrestrial, to prefer a moist environment and to be at home in ravines in southern Ohio. Laticeps on the other hand, is largely arboreal (particularly adults), prefers dry cliffs, sunny hillsides and hilltops and lives in general above the habitat of fasciatus.” Netting [14] (1939:127) likewise states that in Pennsylvania E. laticeps inhabits drier places than does fasciatus, and is largely arboreal. Other authors with few exceptions agree that laticeps is largely arboreal, but most describe it as at home in forest swamps and bottomlands. My own field experience with it is limited. In the Pigeon Lake area of Miami County, Kansas, the northwesternmost known locality of occurrence for laticeps, the habitat relations described by Conant for Ohio were almost reversed. Eumeces laticeps was relatively scarce, and confined to the vicinity of the swamp chiefly in areas that are flooded in time of high water. All those seen were on or near massive snags of dead trees still standing, but decayed and honeycombed with cavities. Slabs of bark clinging loosely to the tree trunks, with spaces beneath, provided shelter for the skinks and for the abundant arthropod fauna which probably constituted their chief food source. This is one of the few places in Kansas where a remnant of the original bottomland forest remains. In central Louisiana, in 1947 and 1948, persons living on the Kisatchie National Forest told me of large, red-headed skinks living in hollow trees, which must have been E. laticeps. In the literature E. laticeps is frequently referred to as red-headed, although the reddish suffusion on the head of the adult male is ephemeral in this species as it is in E. fasciatus and others. The heightened activity of the adult males in the breeding season seems to have drawn attention to this conspicuous temporary coloration while its absence at other seasons has scarcely been mentioned.
Mansueti (1948:213) describing the habits of laticeps in Maryland, Louisiana and elsewhere in the southern states, emphasizes its arboreal habits, referring to it as “‘scorpion’ of the treetops.” He describes it as dashing up and down tree trunks, along fences, and in abandoned buildings. However, he states that it also spends much time on the ground, and may take refuge in holes and cracks near ground level, and gravid females are less arboreal, making their nests in decayed logs of chestnut or oak. He mentions individuals having been found living far above ground in tall trees, in nests of birds of prey. One old male that was frequently seen by him always retreated far up a dead chestnut tree that towered above the surrounding forest of scrub pine. Mansueti also mentions arboreal combats between males and implies that they are territorial. Taylor (1936:59) described laticeps as typically an arboreal form, almost invariably found in trees, and he indicated that it has claws more curved than in other species—an obvious arboreal adaptation. Parker (1948:25), however, stated that “E. laticeps is reputed to [15] be rather arboreal, but field work in western Tennessee has not borne out this belief. A few of the specimens have been found in tall, dead trees, as has E. fasciatus.” This statement evidently was based on a small number of observations.
Cook (1943:15) mentions a female laticeps found in a nest with a clutch of 27 eggs (hence certainly a communal nest of two or more females) in a burrow under a log, on July 8, 1941, in Lee County, Mississippi. This account is under the name Eumeces fasciatus but the large size of the female precludes the possibility of it being either fasciatus or inexpectatus. The remainder of Cook’s account is evidently based on a composite of observations on all three species.
Goin and Goin (1951:29-33) have given an excellent brief account of behavior and seasonal schedule in a small colony of E. laticeps near Gainesville, Florida, based on almost daily observations over a period of years. In view of the greatly different climatic conditions, the seasonal schedule is remarkably similar to that of E. fasciatus in Kansas, and it seems that the minimum threshold temperatures required for activity are much higher in laticeps. Temperatures of 80° F. or above for several consecutive days seemed to be a necessary stimulus for emergence from hibernation; emergence was in the last week in March or the first week in April in Florida. Hatching was found to take place in late June or early July. Adults were last seen before retiring into dormancy in the latter half of September and young of the year remained active into October some two or three weeks later. The skinks observed all lived in hollow water-oaks. When the population was at an especially high level, in the late summer of 1949, each hollow oak was inhabited by one young and one adult. Territoriality and mutual exclusiveness of adults and even of young seems to be implied. The skinks were seen eating spiders, ants, and cockroaches.
Neill (1950:115) mentions one sizable colony of E. laticeps living in a treeless urban area, in Georgia and depending for shelter on piles of metal drums and other industrial equipment. Evidently, however, this was an exceptional situation. In another paper, Neill (1948b:109) described the specialized hibernation site requirements of laticeps in Georgia; the skink retires inside large, rotting pine stumps, especially those that are leaning. He states (1948a:157) that in Georgia, laticeps is most common in the Coastal Plain and is much less numerous above the Fall Line (the line between the Coastal Plain and the Piedmont). Deckert (1918:31) wrote of “Plestiodon fasciatus” in the vicinity of Jacksonville, [16] Florida, where only E. laticeps and E. inexpectatus occur: “Inhabits hollow trees, always near water. Blue-tailed ones often live around human habitations.”
With regard to the ecological traits of E. inexpectatus that distinguish it from fasciatus, authors are much less definite, and evidence is somewhat conflicting as the differences are relatively minor. Engels (1949:269) noted the occurrence of E. inexpectatus on two low islands of submarine origin, off the North Carolina Coast, Harkers Island and Shackelford Banks, and he surmised that the absence from them of E. fasciatus and E. laticeps must have some ecological significance, since all three species occur on the adjacent mainland. Most of the island inexpectatus were taken from beneath loose bark of standing trees, while mainland fasciatus was taken from beneath loose bark of fallen trees.
Barbour and Carr (1940:129) wrote of inexpectatus in the vicinity of Miami, Florida: “… it seems to be the only one [of the five-lined skinks] which has adapted itself to life under the rather specialized environmental conditions existing in its rocky and decidedly tropical habitat. It is one of the very few forms which have established themselves on some of the waterless and poorly vegetated islands on both coasts of the peninsula. E. inexpectatus is much less arboreal than either laticeps or fasciatus. Although it climbs trees when pressed, it is usually found on the ground among leaves or about fallen logs, and particularly about stone walls or old buildings made of cut rock.”
On the other hand, Neill (1948a:157) states that in Georgia, inexpectatus is often observed basking on tree trunks, and though adults often forage on the ground, they dash for the nearest tree when disturbed, usually climbing to a considerable height before halting. The juveniles, however, are said to climb only rarely; they hide beneath objects on the ground when they are pursued. Neill stated that E. inexpectatus occurs in dry pine forests where laticeps and fasciatus are lacking, as well as in moist or even swampy woods. E. inexpectatus often forages on the sides of old buildings.
Hoffman (1953:172), in discussing means of differentiating between inexpectatus and fasciatus in Virginia, states that there are ample differences in color and behavior as well as in scalation. He describes the color difference (blue color of tail of juveniles extending anteriorly beyond pelvis; light stripes reddish-orange on head, sublateral line present, in inexpectatus) but he does not describe the differences in behavior. He states that inexpectatus is the most abundant lizard in southeastern Virginia. Carr (1940:76) also [17] states that inexpectatus is less arboreal than laticeps and is often found under logs and boards in dry sand.
E. inexpectatus thus seems to be adapted to a somewhat drier, more open, habitat than that typical of fasciatus, but it is not clear whether either species is more arboreal in habits. It is to be hoped that the present inconclusive summary will draw attention to the problem and will lead to more critical comparisons of the habitats and behavior of the two species by herpetologists in the southeastern states. The differences, both ecological and morphological, that distinguish inexpectatus from fasciatus are of a degree usually found between subspecies of the same species. The extensive geographic overlap between them is indeed remarkable in view of the slight degree of differentiation, morphologically and ecologically. They are, however, complementary in part in their ranges, while laticeps shares all parts of its range with either one or the other, or both of them (see Figures 4 and 5).
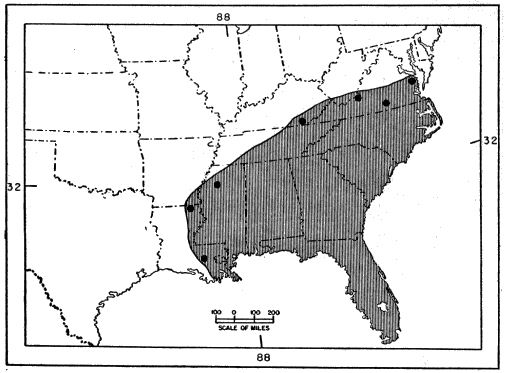
Fig. 4. Geographic distribution of Eumeces inexpectatus, as indicated by published records; only marginal and near-marginal records are shown, excluding those of doubtful validity.
Under present conditions, with these three species so similar in habits and so extensively overlapping in geographic range, it is difficult to visualize a barrier such as would have been required for allopatric speciation of the type, usual in vertebrates, to have occurred. One might be tempted to postulate sympatric speciation, with the parent form, presumably fasciatus, giving rise to the other [18] two by abrupt mutations. However, the demonstrable antiquity of the five-lined skinks would allow ample time for divergence, allopatric speciation, and subsequent disappearance of the barrier and intermingling of populations. The displacement of floras and faunas that occurred in the Pleistocene, with the successive advances and retreats of the continental ice sheets might have had some part in bringing about the present overlapping distribution, after the disappearance of the original barrier. Such a barrier might have been an eastward extension of the central grasslands to the Atlantic Coast at a time when the climate of the continent was warmer and drier.
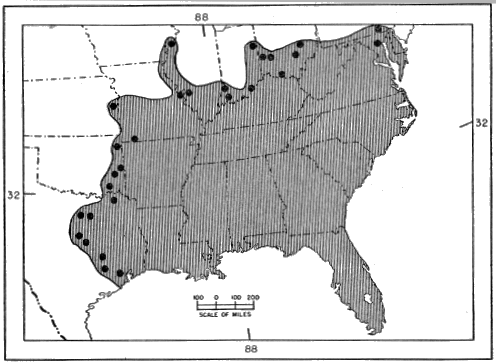
Fig. 5. Geographic distribution of Eumeces laticeps, as indicated by published records; only marginal and near-marginal records are shown, excluding those of doubtful validity.
For approximately half the year, at the latitude of northeastern Kansas, five-lined skinks are dormant. In early fall, even before the advent of cold weather, they are hard to find apparently having begun their retirement into the sheltered situations where they spend the winter, even though they may not be fully dormant at that time.
Remarkably little is known of the hibernation habits of this species or of reptiles in general for that matter. The limit of tolerance [19] to low temperatures, the type of insulating medium, the moisture relationships, the specific stimuli which cause the animal to retire to its hibernation site or to emerge from it have not been determined. On only a few occasions have natural hibernating sites or the dormant skinks in them been observed by zoologists. Linsdale (1927:78) recorded one found in a sawdust pile late in the winter of 1924 in Doniphan County, Kansas. Hamilton (1948:211) found skinks of this species hibernating in Grant Parish, Louisiana, in hollow logs 18-20 inches in diameter, five in one log and three in another, on January 23, 1943. Frost in the damp wood almost reached the lizards, which were in a torpid condition. These observations were made when the temperature was 36°F. after the weather had begun to moderate following an unprecedented four-day cold wave when temperatures dropped to within a few degrees of 0°F. In both logs the skinks were accompanied by hibernating anoles (Anolis carolinensis). Neill (1948b:109) in Richmond County, Georgia, found E. fasciatus hibernating in old stumps, fallen timber, piles of debris, or beneath rocks and ground litter. Beneath scraps of rotting wood he often found dead, frost-rimmed specimens which apparently had frozen to death. Hibernating skinks of this species were found singly or in pairs. Some were not fully dormant when found but could only gape and twist when uncovered.
Of hibernating E. laticeps, Neill wrote, “Many examples are covered with a waxy exudation, which I believe to be a secretion of the lizard itself, rather than of the surrounding medium. This exudation has been noted in other species also.” Scott and Sheldahl (1937:192) described a hibernating aggregation of Eumeces septentrionalis found in Palo Alto County, Iowa, on February 15, 1937, as follows: “The skinks were found beneath a ledge of yellow clay about four and one-half feet below the surface. The lizards, 52 in number, were assembled in a compact group about the size and shape of a football. A soft, web-like material surrounded the mass and adhered to the bodies of the animals. Upon being uncovered some of them exhibited signs of life; others were dead.” Breckenridge (1943:595) reported that a gravel digging crew found hibernating E. septentrionalis in late October and in January at depths of two feet (one), and three feet (groups of three and eight). Tihen (1937:405) recorded that two five-lined skinks found on January 13, 1948, were hibernating eight feet underground at Ranson, Ness County, Kansas. This locality is far to the west of the main range of fasciatus. Conant (1951:30) mentions the finding [20] in Ohio of a young blue-tailed skink under a log where it seemed to be hibernating, on January 22. The spot where it was resting was soggy, and surrounding areas were covered with several inches of water.
In the course of the present study, no five-lined skinks were found hibernating under natural conditions, but on numerous occasions in early spring, two or three or four skinks were found together under massive flat rocks in semi-torpid condition, beside deep holes or crevices which presumably led to their hibernation sites in better insulated cavities. In the winter none could be found in such situations under large rocks, nor in the superficial types of hibernation sites described by Neill and Hamilton in the southern states. In the more severe winter climate of Kansas better protected hibernation sites are required. In the rock ledge situations where skinks were studied, excavation for the purpose of finding hibernating individuals was not practical.
On several occasions when skinks were put in the freezing compartment of a refrigerator and frozen solid, at temperatures several degrees below freezing, they failed to revive when warmed. However, they can survive temperatures a little below freezing. On April 1, 1953, one was placed in the freezing compartment with a thermometer inserted rectally. After 21⁄2 hours when the compartment was opened, this thermometer showed a temperature of -2.5°C, after a delay of several seconds in obtaining a reading because of condensed moisture on the thermometer obscuring the mercury column. Another thermometer that rested beside the skink in the compartment showed 27°F. The skink was limp and immobile. It was placed on a table top at normal room temperature, and it warmed rapidly. When it had reached 1.5°C, it contracted its muscles in response to a light pinch. At 9.5°C it raised its head and had its eyes partly open. Twenty minutes after its removal from the freezing compartment, it was still lying in the same position, its temperature having reached 13.5°C. When handled it seemed dazed for several seconds as if just awaking. Then it crawled away briskly.
On March 28, 1953, a skink was placed in the freezing compartment for about 10 minutes, and upon removal its temperature was recorded as -0.5°C. It was not frozen, but was limp and unresponsive to such stimuli as pinching or pricking. At 1.5°C feeble movements of the legs were noticed. The eyes were still closed. At 3.4°C the legs moved as if in walking. At 6.0°C the skink raised its head and took several steps forward. At 7.5°C it protruded its [21] tongue and dragged itself about for several steps. At 9.0°C movements of the sides indicated an inspiration approximately every three seconds. At 12.2°C it opened its eyes.
On March 25, 1953, a skink that I had caught the day before and left overnight in an unheated room, was found to have burrowed into loose earth in its container. When exposed, its temperature was 1.8°C and it was unable to crawl normally, but took only one step at a time, and progressed with slow lateral squirming motions. Placed on the ground outside the building, in the shade where there was still a little frost, it moved forward persistently for several inches trying to burrow into the surface litter. After a few minutes, its eyes were shut and it seemed incapable of further locomotion. Its temperature was 1.4°C. When placed on its back it was able to turn over slowly after several seconds. A few minutes later its temperature was 0°C, and it was totally helpless, although still capable of feeble movement. When stimulated by touch, it flexed its body a little, or moved each limb slowly in an arc as if walking, the movement taking several seconds. Placed on its back or side it was unable to right itself.
Less than three hours later I saw a skink that was active in the field. Slight movement at the edge of a rock that was exposed to sunshine attracted my attention and turning the rock I found the skink underneath, lively enough to scramble for shelter but slow and stiff compared to those that are fully active. Its temperature was 13.5°C and air temperature was 7.5°C. In damp soil beneath the rock where the lizard was found, temperature was only 5.7°C. It seemed that the skink had been sufficiently warmed by contact with the undersurface of the rock to move into the open, and was just emerging when I approached. After capturing the skink, I set it on a rock in the sunshine, and in five minutes its temperature had risen to 26°C.
As compared with its reptilian associates in northeastern Kansas, Eumeces fasciatus is outstanding in its ability to become active and carry on normal activities at relatively low air temperatures. In spring it is usually seen in the open before any other kind of reptile, because it has the capacity to move about sluggishly at temperatures so low that some other reptiles are numbed and completely immobilized, and because it has small size enabling it to make rapid adjustment upward by insolation, or contact with sunshine-warmed surfaces. By virtue of this ability it has been able to extend its range farther northward than most other reptiles, and it has gained the advantage of a longer growing season. This advantage [22] was especially apparent in the spring of 1953. A mid-March warm spell with seven out of eight successive days having maximum temperatures in the sixties culminated on March 20, with a maximum air temperature of 82°F. This warmth was sufficient to activate most of the five-lined skinks, and a few reptiles of other kinds. After the unseasonably high temperature of March 20, there was rapid return to cooler weather with temperatures frequently below normal throughout April. As a result there was little activity of other kinds of reptiles that month, but five-lined skinks were active on most days. On only a few days, those with temperatures in the low forties or those on which the sky remained overcast, did the skinks remain inactive. On most days maximum temperatures were in the fifties and sky was clear. Under these conditions the skinks were able to emerge and bask, rapidly raising their body temperatures far above those of the air and substrate.
By the end of April some kinds of deciduous trees have not yet begun to leaf out, and in most other kinds the leaves are still in an early stage of development. Absence of a leaf canopy during April permits the skinks to utilize the spring sunshine to maintain their body temperatures at almost the same high level that they maintain in the same situations in hot summer weather.
Table 2. Temperatures (in Degrees Centigrade) of Skinks Found Under Flat Rocks Exposed to Sunshine, Contrasted With Air Temperatures; Spring of 1953.
| Date | Age and sex |
Skink temperature |
Air temperature |
| March 23 | Ad. ♀ | 20.8 | 12.4 |
| March 23 | young | 24.7 | 12.4 |
| March 25 | Ad. ♂ | 22.8 | 12.5 |
| March 25 | young | 21.0 | 12.5 |
| March 25 | young | 25.7 | 14.5 |
| March 25 | young | 22.5 | 14.5 |
| March 27 | Ad. ♂ | 26.6 | 16.5 |
| March 27 | young | 22.0 | 16.5 |
| March 27 | Ad. ♀ | 22.5 | 16.5 |
| March 27 | Ad. ♀ | 20.5 | 16.2 |
| March 27 | Ad. ♀ | 26.5 | 19.3 |
| March 27 | Ad. ♀ | 30.7 | 19.3 |
| April 4 | young | 22.0 | 18.1 |
| April 5 | Ad. ♀ | 26.0 | 13.0 |
| April 6 | Ad. ♂ | 31.5 | 13.5 |
| April 6 | Ad. ♂ | 23.7 | 16.0 |
| April 6 | Ad. ♀ | 22.2 | 16.0 |
| April 6 | Ad. ♂ | 20.0 | 16.0 |
| April 6 | Ad. ♀ | 20.0 | 16.0 |
| April 6 | Ad. ♀ | 26.5 | 20.3 |
| April 20 | Ad. ♀ | 29.7 | 17.2 |
| April 20 | Ad. ♀ | 25.8 | 17.2 |
Recent studies by Cowles and Bogert (1944:288-289) and Bogert (1949:198) have brought out the fact that terrestrial poikilotherms, and especially lizards, maintain fairly high and constant body temperatures through behavioral thermoregulation, during their periods of activity. For genera and species of lizards, there are optimum body temperatures, which the individual tends to maintain, fluctuating within a range of only a few degrees while it is active. Forms that are not closely related may differ notably in their optimum temperatures, although within any one genus the range is slight. For example in the iguanid genus, Sceloporus, Bogert found that different species from such distant regions as Arizona and Florida agreed in having body temperatures approximating 35° or 36°C., while different members of the teiid genus Cnemidophorus in the same two regions were found to approximate 41°C. in mean temperatures. In commenting on the distribution of North American lizards as affected by opportunity for behavioral thermoregulation by direct insolation, Bogert (op. cit.:205) wrote: “Such secretive lizards as skinks (principally Eumeces in North America) with low body temperature preferences approximating 30°C. are dominant in Florida and the Gulf Coast, in contrast to the Teiidae and Iguanidae (several genera in the United States), which are far more abundant in the arid regions of the Southwest.” Bogert and Cowles (1947:19) record that in a large individual of Eumeces inexpectatus taken near the Archbold Biological Station in Florida, the body temperature was 33.2°C.
In the 1952 season, a small thermometer of the type described by Bogert (op. cit.:197) was frequently carried on collecting trips, and cloacal temperatures were recorded for the lizards collected. For those found in traps the opportunity for behavioral thermoregulation was limited, and temperatures usually approximated those of the air. The circumstances of capture, and the air temperatures were recorded for most of the skinks taken. For those found under rocks or in other shelter, the temperature usually approximated that of the immediate surroundings, and averaged much lower than for those taken in the open, but some found in such shelters had temperatures many degrees higher than their surroundings, and were fully active, having evidently just taken to cover to escape notice as the collector approached. As soon as a lizard was secured it was held in a leather glove or heavy cloth to prevent conduction of heat from the collector’s hand, and a reading was taken within a few seconds. Most of the skinks found in the open could not be caught immediately but were secured only after minutes of maneuvering on the part of both collector and lizard. In most instances this [24] maneuvering probably entailed some loss of heat by the lizard, as it interrupted its thermoregulatory behavior to run to a place of concealment, usually in shadow on a tree trunk, or in or beneath ground litter. Excluding all those not found active in the open, the mean temperature, in a sample of 41, was 31.5°C. ± .60. This figure is thought to be slightly too low because of heat loss by many of the skinks in the time required to capture them.
In order to test the range of tolerance and verify the preferred optimum temperature of the five-lined skink, an experimental terrarium was set up providing extremes of temperature at each end. A false floor of 1⁄8 inch wire screen was provided, with a seven-inch strip of galvanized sheet metal beneath it at each end. Beneath the screen and sheet metal at one end the space was filled with chopped ice, and “dry ice.” Observations were made on hot, clear summer days, with the terrarium arranged so that the half of it containing ice, was in shadow, and the other half was in sunshine. The strip of metal, warmed by direct sunlight, became uncomfortably hot to the touch while at the other end the sheet metal and overlying screen were cooled by the ice. A narrow zone across the middle of the terrarium had screen but no underlying sheet metal and was the only part within which the lizard could maintain normal temperature, one end being uncomfortably hot and the other end too cool. A large dead skink left on the metal strip in direct sunlight for five minutes had a cloacal temperature of 45.3°C., and after five minutes on the screen at the cool end, its temperature had dropped to 25.5°C. On several occasions a number of skinks were put in the terrarium and their temperatures taken at brief intervals. Temperatures ranged from 21.6°C. to 37.7°C. but were mostly within a much narrower range, from 28° to 36°C. One skink that seemed to be sick was sluggish in behavior, not responding to the extremes of temperatures as readily as the other individuals and his temperature fluctuated widely and irregularly. Eliminating this individual, 66 temperature readings taken, from five other skinks, gave a mean of 32.6°C. ± .235. While nearly all the temperature readings were within a range of ten degrees, two of the readings were outstandingly low and perhaps should be discarded. If this is done, a mean of 33.8°C. ± .19 is obtained for the remaining 64. There is distinct bimodality in this series however, with a mean of 34.2° for the 49 higher readings, and a mean of 28.8°C. for the 15 lower temperatures. A similar bimodality is evident in the readings obtained from skinks caught in the open under natural conditions. It seems that the lower readings result from lags in the skinks’ response when [25] body temperature drops slightly below the optimum. The skink is quick to make adjustment whenever its temperature appreciably exceeds this optimum level, and is in extreme discomfort at only a few degrees higher temperature. At slightly lower temperatures, however, the skink experiences no discomfort, and only slightly decreased efficiency in its various functions, and its thermoregulatory behavior in making readjustment toward the optimum is likely to be leisurely and interrupted unless its temperature drops below 28°C.
Catching the skinks in the experimental terrarium at frequent intervals to take their temperatures involved some disturbance to them, interrupting their thermoregulatory behavior. The experimenter’s first attempt to grasp a skink sometimes failed, and it then dashed about the terrarium for several seconds, probably altering its temperature somewhat. Nevertheless most of the lizards’ movements were motivated by thermoregulation. This was especially evident when they were left undisturbed, and is illustrated by the following notes on behavior of an adult female and half-grown young of fasciatus and a young E. obsoletus on the afternoon of July 21, 1952.
| 2:58 | All resting over cooled metal. |
| 3:01 | Female runs to line of sunshine and shadow, coming to rest with approximately half her body in sunshine, the other half in shadow over the cooled metal. |
| 3:03 | Female reverses position so that hindquarters previously in shadow are now in sunshine, and forequarters are in shadow. |
| 3:031⁄2 | Young runs to middle coming to rest in sunshine on screen. |
| 3:04 | Female moves back to the cool end. |
| 3:05 | Young moves to edge of cooled metal but not over it, in a narrow m |
| 3:051⁄2 | E. obsoletus moves from cool end to middle, partly in sunshine. |
| 3:07 | E. obsoletus adjusts its position in narrow middle strip of shadow just off the cold end. |
| 3:08 | Boards used for shading adjusted back slightly so that E. obsoletus is in sunshine. |
| 3:081⁄2 | E. obsoletus moves back to cold end. |
| 3:10 | Young still at middle, but resting mainly over cooled metal with tail partly in sunshine. |
| 3:101⁄2 | Young moves out into sunshine at middle. |
| 3:11 | Female moves out into sunshine at middle. E. obsoletus moves over cooled metal to its edge, coming to rest partly in sunshine. |
| 3:12 | Female moves back over cooled metal. |
| 3:131⁄2 | Air temperature 33.3°C. E. obsoletus shifts a short distance so that it is resting entirely over the cooled metal, with only part of its tail receiving sunshine. |
| 3:17 | Young moves about in sunshine, then comes to rest in shadow with half its body over cooled metal. [26] |
| 3:19 | Young shifts so that more than half its body is in sunlight in middle section. |
| 3:20 | Young shifts away from sunlight, coming to rest with most of its body over the cooled metal. |
| 3:211⁄2 | Female moves from cooled metal to sunshine in middle strip. |
| 3:23 | Female moves out of sunshine, partly over edge of cooled metal. |
| 3:30 | Young moves off cooled metal, coming to rest over edge of warmed metal in narrow middle strip that is in shadow. |
| 3:301⁄2 | Young moves back away from warmed metal, pauses briefly, and then moves over cooled metal coming to rest there. |
| 3:31 | Female shifts so that about half her body is in sunshine in the middle. |
| 3:32 | Female shifts back into shadow, partly over cooled metal. |
| 3:33 | Boards providing shade readjusted so that female is in sunshine. |
| 3:331⁄2 | Female moves back into shadow over cooled metal. |
| 3:38 | Female moves to edge of cooled metal, resting partly in sunshine; sky is becoming slightly overcast. |
| 3:40 | Temperature of female 33.4°C. |
| 3:41 | Temperature of young 32.8°C. |
| 3:43 | Temperature of E. obsoletus 32.4°C. |
| 3:45 | Young moves to shaded edge of warmed metal. Finds a dead spider dropped there and eats it. |
| 3:47 | Temperature of female 32.3°C. |
| 3:48 | Temperature of young 36.4°C. |
| 3:50 | Temperature of E. obsoletus 33.8°C. |
| 3:52 | Sky partly overcast with thin layer of clouds; observations concluded. |
Having once emerged from its hiding place a skink becomes more or less independent of the temperature of the air and substrate, as it is capable of thermoregulation through insolation. However, after a period of cooling and inactivity in dormancy, or merely resting for the night in temporary shelter, the skink is dependent on warmth from the air or substrate or both to become sufficiently activated so that it can emerge and take advantage of direct sunlight. About 10:00 a. m. on April 13, 1951, when the air temperature was a little less than 10°C., a large adult male rustling among dry leaves attracted my attention. Obviously recently emerged from hibernation, he was caked with dried mud and his eyelids were nearly sealed shut. He had been sunning, however, and was active enough to elude my attempts to catch him, as he scurried into a deep crevice under the ledge. On the morning of March 24, 1951, while the temperature was still between 10° and 15°C., a subadult skink, the first one of the season, was seen sunning [27] itself at the entrance of a deep crevice under the ledge. This skink was still not fully active, and its movements were stiff, yet it was alert and wary, and it quickly retreated back into the crevice. During the first week of May, 1952, skinks were active in abundance and numbers were caught daily in funnel traps and pitfalls. On May 9, however, the maximum air temperature was 16.5°C. with cloudy sky and occasional showers. Under these conditions skinks stayed under cover; none was seen in the open nor caught in a trap, and several found under rocks were slow and sluggish. On May 10 a terrarium with several adults was placed in dilute sunshine beside a window in an unheated room. After a period of basking the skinks were stimulated to activity, but were unable to attain normally high temperatures, and as a result their movements were like slow motion caricatures of the normal behavior. Males approached each other with menacing demeanor, with heads turned, snouts depressed, and forequarters standing high. Frequently one would edge up to another and bite hard at its flanks. The several males were sexually aroused by the presence of the two females, but were capable of only the preliminary phases of courtship, in delayed and protracted form. The temperature of one was 18.2°C. when the sun had nearly set and activity was tapering off, at an air temperature of 16.2°C. At 16°C. skinks in a terrarium with no access to sunshine for the most part showed no interest in food and kept out of sight under cover. When exposed their activity was directed almost entirely toward burrowing into the substrate or searching for objects beneath which to hide. One adult female was partly exposed by scraping away loose soil into which she had burrowed. A mealworm was then dropped just in front of her head. She tested it several times with her tongue and then ate it without emerging, her movements being much less brisk than they normally are in feeding. Probably this approximates the threshhold temperature for feeding behavior. At 19.5°C. the several skinks in this terrarium were moving about in the open although they were not exposed to sunshine, and they accepted food avidly when it was offered, but were much slower than at optimum temperatures. On May 16, 1951, when a pair of skinks were put together in a terrarium in the laboratory at 21°C., copulation ensued but it was of longer duration than in other observed instances, seemingly because of the relatively low temperature.
Relatively few temperature readings on gravid or brooding females under natural conditions were obtained as they were easily disturbed and tended to desert their nests at slight provocation. [28] To avoid desertions handling was kept to a minimum. Occasionally gravid females were caught in the open, but most of them were in nest burrows under flat rocks. These females found in nests were mostly cold to the touch, and the temperature readings taken on some of them usually approximated the air temperature, being either higher or lower (depending on whether the air was cooling or warming and whether the lizards were warmed by contact with rock or soil receiving sunshine). On May 23, 1952, 22 skinks were seen, four adult males, seven adult gravid females, and 11 young. Of these the adult females all were in nest burrows, and were cold and slow; consequently all of them were caught without difficulty. The males and young, however, were either fully warmed or warm enough to escape rapidly, so that only three of the young and no adult males were caught. Temperatures of the females tested were 25.6°, 23.6°, 23.5°, 22.3°, and 19.4°, and for the three young, 32.8°, 28.4°, and 28.4°. Air temperature varied from 20.5° to 24.8°. For the total of 30 females in nest burrows whose temperatures were taken in 1952, the average was 26.3°C, ranging from 16° to 34°. Gravid females, and those with nests and eggs were rarely seen in the open.
The five-lined skink is confined to a region where summer rains are frequent. It is evident that a regular supply of drinking water is one of the most critical ecological requirements. Bogert and Cowles (1947:19) found that an E. inexpectatus experimentally kept at high temperature lost moisture at a more rapid rate than any other reptile tested (including two other kinds of lizards, four kinds of turtles, an alligator, and three kinds of snakes). They remarked that this rapid moisture loss presumably accounts for the inability of skinks to survive in containers when no moisture is readily available, and also accounts for their absence in truly arid habitats. The Natural History Reservation is situated near the western edge of the species’ range in a climate that may be near the limit of its range of tolerance. However, on most summer mornings low woodland vegetation is copiously laden with dew, and this evidently fulfills the need for drinking water. Diminution of surface activity and retirement to underground retreats seem to be closely correlated with cessation of rains in late summer. After rainless periods in August and September, when morning dew is no longer available these skinks, especially the adults, are no longer regularly seen in the open. They have retreated to underground shelters where they spend nearly all their time. The time of disappearance varies from year to year and the correlation with varying weather [29] conditions seems obvious. While no actual experiments were performed to determine the moisture requirements, it is evident that the need for moisture rises sharply with increased temperature. Skinks that are dormant in hibernation survive for periods of months without drinking, with but little loss of weight. In their underground shelters temperature is low and presumably relative humidity is high. At temperatures above their optimum of approximately 34°C. the skinks are especially subject to rapid moisture loss, since evaporation of body moisture is resorted to as a device to keep the temperature below the lethal level. The skinks subjected to extremes of temperature in an experimental terrarium were seen to lap up condensed moisture on the cooled metal plate at intervals of a few minutes. After an hour or more in the experimental terrarium they seemed somewhat debilitated. Skinks brought from the study areas to the laboratory for weighing and other records, were ordinarily returned on the following day. When circumstances prevented adherence to this schedule in hot summer weather, mortality could be expected in the skinks kept in cloth bags or glass containers, unless water was provided. Dramatic weight loss of up to more than 30 per cent was recorded in some individuals, kept at the high temperatures which usually prevailed in the laboratory, over periods of days in the summer. Skinks having access to drinking water often ingest amounts far beyond their immediate requirements, which may be stored in the bladder and drawn upon over periods of days as it is needed, or may be utilized to dampen the soil of the underground shelter and raise the humidity, as incubating females seem to do.
Eumeces fasciatus corresponds in its distribution with the original hardwood forests of eastern North America, as mapped by Braun (1950:cover folder) and the “Oak-Wild Turkey Biome” of Shelford (1945:240). Few species of vertebrate animals have ranges that coincide more closely with this extensive area (exclusive of the northern edge, that part characterized by Braun as the Hemlock-White Pine-Northern Hardwoods). This latter is a mixed forest which actually is transitional between the more typical deciduous forest farther south and the Taiga Biome (or Formation) to the north, which is dominated entirely by conifers. At the northern edge of its range Eumeces fasciatus is much less generally distributed than it is farther south. Although it is well established and even may be locally numerous in South Dakota, Minnesota, Wisconsin, [30] northern Michigan, Ontario, northern New York, and Connecticut, the locality records from these states are few, and seemingly represent isolated and widely separated colonies that are able to persist because of favorable combinations of environmental factors not of general occurrence in the surrounding regions. Figure 6 shows the extent of the hardwood forests as mapped by Braun (excluding the transitional Hemlock-White Pine-Northern Hardwoods Association) with specific locality records of E. fasciatus included in all outlying portions of the range. The locality records are those published by Taylor (1936:206-212) supplemented by other marginal records, more recently published, by Hamilton (1947:64) for New York, Breckenridge (1944:97) for Minnesota, Hudson (1942:42) for Nebraska, Smith (1950:185) for Kansas, Brown (1950:116) for Texas, Neill (1948:156) for Georgia, and Neill and Allen (1950:156) for Florida. Along the northern edge of its range, the skink invades the Hemlock-White Pine-Northern Hardwoods Association, in Massachusetts, New York, Pennsylvania, Ontario, Michigan, and Wisconsin, but does not penetrate far into it anywhere. Correspondence of its northern limits with those of the Oak-Chestnut, Maple-Basswood, Beech-Maple and Oak-Hickory associations is remarkably close, considering the fact that the boundaries of these climax associations are not sharply defined; rather they merge by gradual stages into the northern coniferous forests, with outlying peninsulas and islands where conditions are favorable.
The outlying northern localities where E. fasciatus occurs within the Hemlock-White Pine-Northern Hardwoods Association are all within the region of Pleistocene glaciation, which 20,000 years ago, or even more recently, were covered with the continental ice mass during Wisconsinan time. Yet the localized northern populations of skinks evidently are relicts from a time when favorable conditions were more widespread in the general region. Braun (op. cit.:464-465) indicates five successive postglacial stages in the trends of climate up to the present, as revealed by bog pollen profiles: (1) Cool and moist; (2) warm and dry; (3) warm and humid; (4) warm and dry; (5) cool and moist. Stages 2 and 4 would have been most favorable for encroachment of the skink into glaciated regions, whereas stages 3 and 5 might have caused retrenchment of its populations. In view of the localized habits of individuals, and the lack of any mechanism for rapid dispersal, the time available seems no more than adequate for the distance of 200 miles or more northward [31] that the skinks must have moved since the final retreat of the ice sheet. This northward movement involved crossing of formidable barriers such as the Great Lakes. Even minor barriers such us small rivers and creeks, might be expected to halt population movements for long periods.
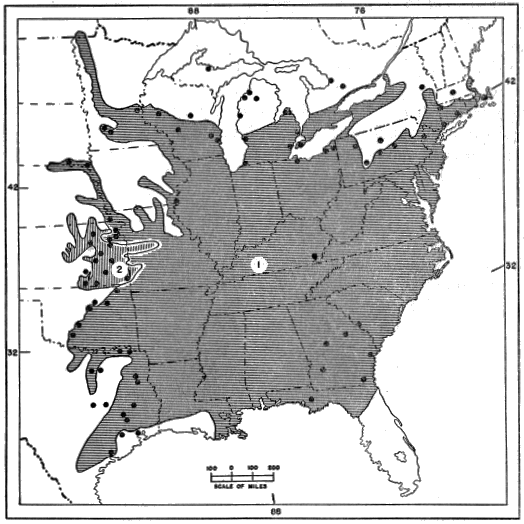
Fig. 6. Geographic distribution of Eumeces fasciatus as indicated by published records (marginal and near-marginal records shown, excluding those of doubtful validity). (1) Distribution of the Deciduous Forest Formation of eastern North America, as mapped by Braun (1950), but excluding the Hemlock-White Pine-Northern Hardwoods Association that is transitional to the more northern coniferous forests. (2) The shaded area in Kansas that is outside the Deciduous Forest Formation comprises the Kaw River District, Cherokee Prairie District, and southern Osage Savannah Biotic District (Cockrum, 1952).
The over-all geographic range is approximately square, roughly a thousand miles across, from north to south and from east to west. On the east and south it is limited by the Atlantic Ocean and the Gulf of Mexico. On the north and west its limits correspond with those of the hardwood forests. On the northwest, it reaches southwestern Minnesota and the southeastern corner of South Dakota, [32] extending far out into peninsular extensions of the Oak-Hickory Association which penetrate westward into the prairies along the main river valleys.
In Kansas it occurs over the eastern one-fourth, west to the Flint Hills, and a little farther west in peninsular extensions of the forest along some of the main river valleys. In Braun’s map the Deciduous Forest Biome is shown to reach only the eastern edge of Kansas along the Kaw River and Missouri River at and near their junction, the Osage (or Marais des Cygnes) River valley near the Missouri border, and the southeastern corner of Kansas. However, for almost 100 miles farther west from the Missouri border, the country has the aspect of a savannah with scattered groves of trees on hillsides and along streams, providing suitable habitat. The distribution of the five-lined skink in eastern Kansas corresponds well with certain “Biotic Districts” as mapped by Cockrum (1952:12), namely the Kaw River, Osage Savannah (southern part), and Cherokee Prairie. Conversely the skink is excluded from the Short Grass Plains and Mixed Grass Plains Biotic Districts which occupy nearly all of the western three-fourths of the state. There are two specimens in the University of Kansas Natural History Museum, labelled Ranson, Ness County. This locality, in the western third of the state, more than 150 miles from any other recorded station, may represent an isolated colony; however Smith (1950:185) states that the record needs verification, and it is not included in the map, Figure 6.
In Oklahoma the distribution records fit fairly well the portion of the state mapped by Braun as the Oak-Hickory Association of the Deciduous Forest, but extends a little farther west in the northeastern part of the state. A game type map published by the Oklahoma Game and Fish Department, Division of Wildlife Restoration, in 1943 shows in more detail distribution of the main vegetation types within the state. The locality records for the skink fall almost entirely within three of the fifteen vegetation types mapped, namely, the oak-pine, and oak-hickory forest of the state’s eastern edge and the post oak-blackjack oak type of the eastern and central parts. The locality records extend almost throughout the area occupied by these three types but not in attenuate westward extensions of the post oak-blackjack type that occur along several of the main stream courses. In Texas likewise the recorded localities fall mainly within the area mapped as deciduous forest, but with several slightly beyond its boundaries. In a detailed map of the “game regions” of Texas (Anonymous, 1945:1), some of these outlying localities fall into the coastal prairie area, and the remainder into the post oak [33] and blackland prairie belts, which grade into each other and the oak-hickory forest.
The former distribution of the five-lined skink may be postulated on the basis of the fossil record of its community associates since it is a primitive and conservative type. Taylor (1936:56) explained the present discontinuous distribution of the genus on opposite sides of the world on the basis of a former northern connection of the continents. He wrote: “I regard migration from North America to Asia as having taken place via land bridges joining the Alaskan peninsula with Asia either at Bering Straits or via the Aleutian Island arc to Kamchatka, or both. One would need postulate but slight climatic changes since the present climate of this coastal region is probably no more rigorous than that of southern Canada which has three species of the genus.” However, such former northward distribution, while entirely probable, would have been possible only in a climate much milder than that which prevails at present. In Asia, tunganus on the mainland and latiscutatus on the island of Hokkaido extend north to about latitude 43°, and in North America, fasciatus extends slightly farther north. In order to have crossed between Alaska and Asia on presumed land bridges these skinks would have had to extend their ranges about 20 degrees north of their present limits, into what is now a cool climate. The winter climate of the Bering Sea is perhaps not much beyond the range of tolerance of the more cold-adapted forms of Eumeces, but the cold, cloudy, wet, and changeable summer climate is far beyond the range of tolerance of Eumeces or any other lizard.
It is highly improbable that the fossil record will yield direct evidence for the existence of a northern ancestral Eumeces of the fasciatus group. The characters by which the various forms are recognized are to be found mainly in details of pattern and scalation; the skeleton is so conservative that specific characters are ill defined or lacking even in well preserved fossil material. This hypothetical ancestor probably was a member of a deciduous forest community having components in common with the modern forests where the American and Asiatic species occur, along with types now extinct, and others which, though existing at the present time, have become separated from their original associates and occur in other regions.
Hollick (1936:11) has described a rich early Tertiary Alaskan flora strikingly different from that of the same region at the present time. Composed of genera now characteristic of warm-temperate to subtropical climates, it was remarkable in having many types of [34] plants that are now most characteristic of the North American hardwood forests in the southeastern part of the continent. Besides such widespread genera as Fagus, Betula, Ulmus, Platanus, Castanea, Corylus, Carpinus, Crataegus, Spiraea, Myrica, Smilax, Pinus, Picea, and Abies, this flora included others now characteristic of both warm-temperate southeastern North America and Eastern Asia, as Magnolia, Nyssa, Sassafras, Persea, Benzoin, Hamamelis, Liquidambar, Celastrus, Nelumbo, and Onoclea. It included genera Carya, Taxodium and Comptonia that now are limited to SE North America, Sequoia, now limited to western North America, and also included several genera which at present are limited to southeastern Asia: Ginkgo, Glyptostrobus, Cinnamomum, Hausmannia, Artocarpus, Dillenia and Koelreuteria. This fossil flora provides strong evidence that in the early Tertiary climatic and habitat conditions as far north as Alaska were favorable for the existence of an ancestral Eumeces similar to the modern E. fasciatus, which might have given rise to both North American and Asiatic members of the fasciatus group.
There is abundant evidence for the existence of an Eocene land connection between Alaska and northeastern Siberia, permitting free interchange of faunas between the two continents, as shown by the almost simultaneous appearance of various mammalian groups in the fossil records of Asia and North America. Simpson (1947:627) has summarized the evidence that such intermigrations were occurring throughout most of the Tertiary, with occasional interruptions as in middle Eocene, and in middle and late Oligocene, and with increasing selectivity, chiefly a progressive tendency toward screening out of the groups less tolerant of cold (judged on the basis of their modern representatives). In the late Tertiary, and especially in the Pleistocene, animals known to have made migrations between North America and Asia were types now characteristic of boreal climates (e. g. pika, hare, vole, lemmings, marmot, jumping mouse, fox, wolverine, bear, moose, caribou, sheep, bison, camels, mammoth). Simpson believes that there was fairly strong climatic selectivity even in the Miocene interchanges, and he indicates several important groups that were non-migrants in the Miocene, most of them remaining so through the Pliocene and Pleistocene—the primates, Rhizomyidae, Gliridae, Viverridae, Hyaenidae, Dicerorhininae, Suidae, late Anthracotheriidae, Hippopotamidae, Tragulidae, Muntiacinae, Lagomerycidae, Giraffidae, and Bovidae. He states that there is good evidence that these are all mainly warm-climate animals which are not likely to have ranged in any [35] force into a cold-temperate or boreal environment. In view of these conclusions it seems doubtful whether Eumeces or other reptiles could have crossed the Alaskan-Siberian land connection so late as the Miocene.
On the contrary, the climate and habitat conditions with which Eumeces might have been associated, although present as far north as Alaska in the Eocene, evidently had shifted far to the south by mid-Tertiary time. Axelrod (1950:230) has described a Miocene forest of the Columbia Plateau and northern Great Basin indicative of a uniform temperate climate and an average rainfall of thirty-five to sixty inches. This forest included: (a) various genera now characteristic of the southeastern hardwood forest or confined to it—Carya, Castanea, Comptonia, Fagus, Liquidambar, Nyssa, Taxodium; (b) other genera at present more characteristic of the western United States—Sequoia, Lithocarpus, Pseudotsuga, Mahonia, Thuja, Gaultheria, Amelanchier; (c) wide-ranging genera including Alnus, Acer, Betula, Populus, Quercus, Picea, Pinus, Tsuga, Cornus, Ribes, Rosa, Hydrangea; (d) modern east Asian genera, including Ginkgo, Ailanthus, Glyptostrobus, Keteleria, Koelreuteria, Metasequoia, Pseudolarix, Pterocarya, Zelkova, which were eliminated from the North American flora in the latter part of the Tertiary. In short, this western Miocene forest was remarkably similar in many respects both to the presumably ancestral early Tertiary Alaskan forest and the modern southeastern hardwood forest. The extent of this Miocene forest is unknown but judging from the sites where it has been recorded, it had progressed about halfway, both in latitude and in actual distance, from Alaska to the area occupied by the modern southeastern deciduous forests. Several other reptilian genera have distributions similar to that of the fasciatus group, with representatives in southeastern Asia and southeastern North America that probably have parallel histories of distributional divergence from early Tertiary northern ancestors similar to contemporary species (Schmidt, 1946:148-150). Alligator, Natrix, Ancistrodon, Scincella, Elaphe, Opheodrys, and within the genus Eumeces, the obsoletus group, all provide excellent examples.
Accounts of the habits and habitat, by various authors, indicate versatility in behavior, and adaptation to a variety of habitat conditions in different climates and plant associations. Some of the differences evidently result from the skink’s tendency to maintain itself in surroundings of favorable temperature and humidity, which [36] obviously are to be found in different types of situations at different extremes of the range. Hence even though the skink itself may remain unchanged, it tends to behave somewhat differently under diverse environmental conditions. Such environmentally enforced differences in habits would be difficult to distinguish from those having a genetic basis. Although no subspecies of Eumeces fasciatus have been recognized, local populations undoubtedly differ somewhat in size and other characters that have a genetic basis.
At the northern edge of its geographic range, fasciatus occurs in isolated colonies and seems to be restricted to open, rocky situations which receive the maximum amount of sunlight. Breckenridge (1944:96) wrote that at the two Minnesota localities representing the northwestern corner of the known range, the skinks were found at granite outcrops, and he mentions one found in western Wisconsin, at Taylor Falls, under an 18-inch slab of a basalt outcrop in sparse oak woods. Patch (1934:51) described a habitat at Arden, Ontario, among massive granite-gneiss domes, with sparse vegetation. At Point Pelee, Ontario, the species is common in the drier, more sparsely wooded situations, hiding beneath loose bark of stumps and logs.
Ruthven (1911:264) found E. fasciatus in the vicinity of sandy beaches in the Saginaw Bay region of Michigan. Elsewhere in its range it is more characteristically an inhabitant of hardwood forests, preferring the better drained and more rocky situations, according to the testimony of numerous authors. In eastern Illinois, Smith (1947:33) found it confined to the area south of the Shelbyville moraine, and not ranging into a prairie habitat. Near Elkville, Illinois, Cagle found the species abundant in higher and drier areas within sparse stands of oak in second growth woods, but it was absent from the low swampy areas adjacent to streams. Conant (1951:30, 210), describing the habitat in Ohio, stated that the species does not occur in swamps and areas that are subject to spring floods nor on dry hillsides, but is abundant in some areas where there are rotting stumps and logs remaining from former patches of swamp forest, and usually is found in low, moist situations, in wooded valleys or even at the edges of swamps and bogs. Lynn (1936:49) wrote that in Virginia, it is most often seen on steep, boulder-strewn hillsides and old sawdust piles. In the central Ozarks of Missouri, Owen (1949:49) found it abundant and saw it almost daily on rocky ledges, fallen timber, and fence rails, while E. laticeps was seen only once. Taylor (1936:59) wrote that E. fasciatus occurs where there is timber and is often found about fallen [37] trees and rotting stumps, or about old sawmills where wood refuse has accumulated. Smith (1950:187) wrote that in Kansas the species is commonly found in wooded areas in moist situations about stones, leaves and rotten logs. Gloyd (1928:120) wrote that in Franklin County, Kansas, E. fasciatus occurred in upland situations and was the most abundant lizard where there were rocks, brush, or decaying wood. Gloyd (1932:401) also recorded it as abundant in the Pigeon Lake area, Miami County, Kansas, in wooded areas of sufficient elevation to be out of the river flood-plain.
In northeastern Kansas I have collected or observed this skink in several dozen localities, and searched unsuccessfully in numerous other localities. Absence of this skink, in some situations and its presence and relative abundance in others, provided a basis for appraising the environmental factors that are of critical importance. River valleys, of the Kaw and Wakarusa and their tributaries, with deep alluvial soil, alternate with flat or rolling upland some two hundred feet higher in elevation, and having shallow, rocky soil. Where the uplands slope to the valley floors, there are steep hillsides, usually with extensive limestone outcrops along their upper slopes. The alluvial plains formerly supported hardwood forests, while the hill slopes and uplands were largely prairie. At the present time the bottomland forest has been almost completely destroyed, as it grew on the most fertile and potentially productive soil, and has been replaced by cultivated crops. There are still trees along streambanks, and in occasional woodlots, but I have failed to find any skinks in such situations. I doubt that they ever have been numerous in the bottomland woods; lack of rocks for shelter, and periodic flooding are unfavorable factors. In the Kaw flood of June and July, 1951, for instance, the entire valley was inundated, and in smaller tributary valleys such as that of the Wakarusa, flooding is frequent at the season when skinks are incubating their eggs. The uplands, formerly prairie, now are used partly for cultivated crops and partly for pasture. The soil is poor and rocky, and now heavily eroded. The pastures mostly have a weedy type of vegetation indicative of overgrazing. Five-lined skinks are absent from most of this upland.
The steep slopes from the upland to the valley floor are now mostly wooded, and the population of skinks is chiefly in this band of woodland. Some of the hillsides that have relatively gentle slopes are treeless and are used for pasture, or are even under [38] cultivation. Where second growth forest is present its aspect differs depending upon slope, exposure, and past treatment. Osage orange and honey locust are aggressive invaders on some dry hillside pastures, and in this type of woods the skinks are scarce or absent. Some hillside areas, especially on moist north slopes have thick second-growth woods, in which elm is usually the principal tree, with several oaks and hickories, walnut, hackberry, coffee tree, locust and osage orange, and with a dense understory vegetation of dogwood, gooseberry and coralberry, with vine tangles of grape, poison oak, and greenbrier. Such woodlands provide little food for livestock, and are often fenced off from adjacent pastures. The shading creates conditions unfavorable for skinks and they are relatively scarce in the denser woods. They are much more numerous in woodlands that are fenced in with pastures heavily grazed by cattle or horses, with understory vegetation kept cropped back, and with more open ground and patches of sunlight. However, they are absent or scarce in woods that have been subjected over periods of years to browsing, by sheep or goats, so heavily that hardly any herbaceous vegetation remains and so heavily that the soil is packed from trampling. Along the upper slopes, especially about heads of gullies, in areas strewn with flat rocks, in fairly open mixed woods, with some decaying wood on the ground, habitat conditions are most nearly optimum for the skinks. Artificial habitat features, such as rock piles, stone walls, wood piles, rail fences, or old deserted buildings and sheds, with loose boards lying about on the ground may support unusually high concentrations of skinks when the surrounding habitat is favorable.
The University of Kansas Natural History Reservation where most of the field work for this study was done, has been described in a recent publication (Fitch 1952:8). While records were obtained from scattered points throughout the 590-acre Reservation and elsewhere in northeastern Kansas, field study of this skink was concentrated on four relatively small areas totalling only about ten acres in extent (Figure 26). These areas were selected on the basis of abundance and availability of the skinks, and of variety of habitat conditions represented.
One of these sites was a deserted quarry on a southward projecting spur of the plateau-like cuesta top, where the upper layers of the Oread limestone are prominently exposed. In the course of operations, begun about 1937, the area was denuded of trees and [39] shrubs, and the upper layers of limestone were removed from a strip about 50 feet wide and more than 100 yards long. The exposed outcrop presented a vertical rock face five to ten feet high, with south and southeast exposure. Numerous jagged seams and fissures in the rock hastened its disintegration. Quarrying had been discontinued several years before the present study was begun in 1948. At that time there were talus-like accumulations of rock and soil several feet wide along the base of the rock face, supporting a luxuriant pioneer vegetation especially, sweet clover, stickleaf, ragweed and elm seedlings.
The habitat conditions provided by the exposed rock outcrop at the border of woods and open land, proved unusually favorable for reptiles in general, and it was one of the most productive sites on the Reservation for Sonoran skinks, collared lizards, racerunners, ring-necked snakes, blue-racers, bull snakes, pilot blacksnakes, scarlet king snakes, slender tantillas, copperheads, and timber rattlesnakes. For the five-lined skink, however, this disturbed area was marginal, and supported only a sparse population. Several decaying two-inch boards were preferred hiding places where the skinks were found most frequently, and remains of collapsed rock walls, one in the center of the area and one at the edge of the woods, were also occupied. Skinks may have tended to wander away to more favorable situations or may have been more subject to predation than those elsewhere, since the incidence of recaptures was relatively low. Most of the records from this general area were from a ledge in adjacent woods rather than from the quarry itself. Another site was a rock fill in a ravine below a pond made in 1937. This rock fill was 70 feet long, up to 30 feet wide, and three feet deep. East and north of the rock pile was a grassy dike, and beyond it the pond. On the west open grassland extended approximately 200 feet to the edge of the woods, with a diversion ditch at its border. On the south end, the rock pile was adjacent to woodland at the base of a steep slope with north exposure. On this slope the dense stand of second growth oak and hickory with an almost continuous leaf canopy was a poor habitat. The rock pile was thus partly isolated and surrounded by areas that were either uninhabitable to the skinks or supported only sparse populations of them. By 1948 the rock pile was partly covered by grape vines. Dead leaves and other debris had accumulated in the deeper interstices between the rocks. Spiders, beetles, snails and other small animals were extremely numerous in the vicinity of the rock pile and provided an abundant food supply. A large sycamore on the west side [40] of the rocks provided some afternoon shade. This rock pile provided shelter for reptiles other than the five-lined skink—especially the garter snake, water snake, copperhead, and brown skink. Another area of about two and a fourth acres (“Skink Woods,” Figure 21) was the one most productive of skinks. It is a wooded upper slope adjacent to a hilltop pasture. Along the hilltop rim the upper stratum of the Oread limestone presents a rock face as much as four feet high at the north end, but less exposed at the south end where it was partly covered by deposited soil. Approximately 100 feet down the slope a second outcropping is present, with many loose rocks and boulders throughout the whole area. Soil is light and loamy. The slope has a west exposure. The stand of trees is fairly open, with several large elms, walnuts, and yellow oaks, and occasional hackberries, ailanthus and red haws. This area was included in a narrow strip of woodland fenced about 1940 as a runway connecting a hilltop pasture with a valley pasture where water was available at a time when both pastures were heavily grazed by horses and cattle. As a result of trampling, browsing and grazing by livestock, understory vegetation of this area presented a different aspect from that in most other parts of the woodland. Saplings of the dominant tree species and shrubs, notably dogwood, gooseberry and crabapple, were relatively scarce. Herbaceous vegetation, especially muhly grass, was conspicuous. By 1953 in the fifth growing season after livestock were removed, the area still contrasted with other parts of the woodland in sparseness of shrubby vegetation. Old stock trails were still discernible, and some sheet erosion and gullying had occurred. The effect of livestock in holding back woody undergrowth seemed to be an important factor in improving the habitat as the skinks were much scarcer in adjacent woodlands on either side that were similar in species composition, size, and numbers of the larger trees, but different in having much thicker underbrush. These adjacent woodlands were not entirely comparable, however, because they had more north-facing exposures. Reptile associates in the Skink Woods area include the brown skink, Sonoran skink, glass-snake, worm snake, ring-necked snake, blue-racer, garter snake, pilot blacksnake, copperhead and timber rattlesnake, but only the worm snake and ring-necked snake were abundant.
Rat Woods, an area of approximately four acres, was like Skink Woods, formerly the upper part of a connecting strip between hilltop and valley pastures and was altered by the effect of concentrated trampling and browsing by livestock. It is V-shaped, with [41] the apex at the north end, and the slope exposures southwest and southeast. The area is bisected from north to south by a small gully, and remains of an old rock wall. To the east of this gully the lower outcrop is prominent but west of the gully, it is but little developed. As compared with other wooded areas, this one was relatively dry. Trees, and other vegetation in general, are somewhat more xeric in aspect than are those in Skink Woods. Along the upper ledge are elms and hackberries, with many thick clumps of fragrant sumac. The trees are mainly elm, walnut, honey locust, and osage orange with hardly any oaks or hickories and, with shrubby undergrowth of dogwood, gooseberry, and coralberry sparser than in adjacent woodlands. Herbaceous vegetation consists largely of muhly grass, geum, and avens. On the hilltop edge above the ledge are many flat rocks of varying sizes, and the slope is thickly strewn with rocks, some of the larger ones deeply embedded in the soil. The population of five-lined skinks was relatively sparser than in Skink Woods. Other reptiles including the Sonoran skink, racerunner, glass-snake, worm snake, ring-necked snake, blue-racer, bull snake, pilot blacksnake, garter snake, scarlet king snake, slender tantilla, and copperhead, were more numerous in this area than in most other parts of the Reservation. The comparatively scarce prairie skink was found only in this area, and the scarlet king snake and slender tantilla were found only here and at the quarry.
Collectors and other observers have often noted that reptiles, in general, are not found in equal abundance throughout the entire season of their activity. Many kinds are most in evidence within a period of weeks after emergence from hibernation, which corresponds with the time of breeding and later they become much scarcer. In skinks of the genus Eumeces this tendency is perhaps even more pronounced than in most other kinds of reptiles. By midsummer or considerably earlier their period of greatest activity is passed, and in some kinds, adults, or individuals of any size can rarely be found in the latter half of the growing season, even by a skilled collector familiar with their habitats and habits. Thus, Taylor (1936:5) in the preface of his revision of Eumeces, describing the difficulties involved in assembling needed series of the many Mexican species by collecting on summer field trips, wrote: "In 1934 in western Mexico … I met with most disheartening [42] results … (although more than 1500 specimens were collected) only a single specimen of Eumeces was taken. Hobart Smith, in 1934, accompanied by David Dunkle, made a journey into northwestern Mexico … and while generally successful, likewise obtained only a single specimen of Eumeces."
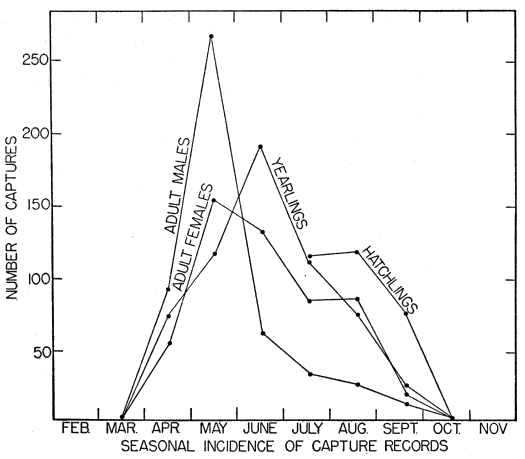
Fig. 7. Seasonal occurrence of five-lined skinks, based on data collected in 1949, 1950, 1951, and 1952; adult males and adult females are taken in greatest numbers in May, and in progressively smaller numbers through the summer and autumn; yearlings are found in increasing numbers through March, April, May, and June, then in decreasing numbers through the summer and autumn.
In the present study the tendency of E. fasciatus to concentrate its surface activity in early spring was clearly shown. In unseasonably warm weather in early spring, even in February in one instance, individual skinks have been found active on the surface or beneath flat rocks warmed by the sun; but general emergence ordinarily does not occur until sometime in April, depending on the weather. Unless the weather is much warmer than the seasonal norm, the skinks spend much of April in a torpid condition, either not becoming fully active until late in the month, or lapsing into torpidity with the return of cool weather after their first emergence from hibernation. During warm periods in April, [43] however, activity is at or near its annual maximum for all individuals regardless of sex or age.
In May, with the advent of much warmer weather, daytime temperatures are usually high enough for the skinks to be active. Adult males travel about more actively and persistently than females or young, and as a result they are found so much more frequently that the numbers taken approximate those for adult females and young combined. Many of the adult males recorded in May were taken in funnel traps or pitfalls. Active males in the open were difficult to catch, and a high percentage of them escaped. To the casual collector or observer, these skinks are much more in evidence in May than at any other time of year, and most of those seen are adult males. By June, the numbers of skinks seen in the open decline abruptly. The adult males become relatively scarce, with reduction from more than half to about one-sixth of the total, and the young, about half-grown at that season, make up approximately half of the total. The adult females make up approximately one-third of the total June sample, but few of them were found active on the ground surface. Most were found in nest burrows beneath flat rocks. Under such conditions they tended to be sluggish in behavior, and were caught much more easily than were males and young. July was characterized by progressive decrease in the numbers of adult males, adult females, and second year young, whereby the numbers of each group were little more than half of those for June; and by appearance of a new crop of hatchlings which made up about one-third of the month’s sample. Hatchlings first appeared from early July to late July in different years; few were recorded in July in some years. Females were much less commonly found in nests in July than in June because many nesting attempts were terminated before the beginning of July or early in the month, and probably because those that remained were often more deeply buried and better concealed. By August the adult males, and the second year young (by then approaching adult size) were found in still smaller numbers, but the number of hatchlings and of adult females approximated those recorded in July. In the females there is evidently some resumption of activity after the incubation period is terminated. The females are then hungry and sometimes emaciated, weighing less, on the average, than the year-old young of shorter snout-vent length. The numbers of hatchlings are augmented through early August in some years, as late broods continue to hatch. By early September few skinks except hatchlings are to be found, and activity continues to wane throughout the month. [44] In October skinks of any age or sex group are a rarity, even though temperature is about the optimum for their activity. Little is known concerning where and how they spend the fall months. Probably they are not actually dormant, but retreat underground where temperature is moderate and humidity is high. Individuals kept in captivity at this season were listless showing but little inclination to feed. The only five-lined skink taken on the Reservation in November was found in a funnel trap after a rain at the end of a long drought. It may have been attracted to the surface by moisture.
The following table shows the dates on which various events of the annual cycle were observed in each of five different years. Owing, to the secretive habits of the skinks, these events generally were not observed until somewhat after their earliest occurrence in any one season. The lag was greater in some instances than in others.
Table 3. Phenology of the Annual Cycle in Five Different Years.
| 1949 | 1950 | 1951 | 1952 | 1953 | |
| Earliest emergence from hibernation | Mar. 30 | ....... | Mar. 24 | Mar. 29 | Mar. 20 |
| General emergence from hibernation | ....... | Apr. 7 | Apr. 14 | Apr. 17 | Mar. 27 |
| Breeding coloration appearing in males | ....... | Apr. 15 | Apr. 25 | Apr. 28 | Apr. 16 |
| Peak of breeding season | May 3 | May 12 | May 16 | May 10 | May 7 |
| Females starting nest burrows | May 26 | May 24 | May 19 | May 19 | May 24 |
| Last appearance of gravid females | June 10 | June 17 | June 29 | June 9 | ....... |
| Earliest appearance of eggs | June 10 | June 13 | June 24 | June 22 | June 16 |
| Earliest appearance of hatchlings | July 5 | July 15 | July 23 | July 3 | July 13 |
| Latest hatching date | July 15 | Aug. 8 | Aug. 8 | July 14 | ....... |
| Latest fall record | Oct. 15 | Sept. 19 | Sept. 26 | Nov. 9 | Oct. 12 |
Reynolds (1943:370 and 1947:191) studied the histological and gross seasonal changes in the reproductive organs of the adult male Eumeces fasciatus. There is a well defined annual cycle. "Early seasonal increase in seminiferous epithelial heights and in diameter of lumina and tubules reached a maximum in April followed by regression reaching complete involution by August. Late seasonal revival of activity results, by November, in size of testicular elements comparable to those seen in January. Primary spermatocytes predominate in the germinal epithelium in January, secondary [45] spermatocytes and spermatids in February, with spermatids and metamorphosing sperm dominating from March until late June when the germinal material of the current season is exhausted." Fifty-three adult males were used as a basis for his study. These were of diverse origins from Arkansas, Florida, Missouri, Tennessee, and Indiana. Since sexual cycles in such widely ranging species tend to be synchronized with local phenology, and change somewhat from one region to another, the seasonal cycle may have been somewhat obscured by the diverse origins of the material. The Florida specimens may have been of the species E. inexpectatus. Apparently Reynolds’ experimental skinks were kept in captivity for varying lengths of time before their reproductive organs were examined. The normal cycle would almost certainly be altered in captivity, especially in skinks kept at high temperatures during the time that they would normally be hibernating.
The seasonal change in gross appearance of the testes is not great. In the breeding season the testes are slightly enlarged and are firm and engorged, with pinkish or orange tinge. In immature males, and adults that are not in breeding condition, the testes are smaller, attenuate, paler colored, and flaccid. Sizes of testes in some males killed in the breeding season are recorded in Table 4.
Table 4. Sizes of Testes in Spring and Early Summer in Sexually Mature and Juvenal Males.
| Date | Snout-vent length in mm. |
Sizes of testes in mm. |
Age class | |
| May 6, | 1951 | 76 | 7.0 × 4.0 | old adult |
| May 20, | 1951 | 77 | 5.0 × 2.8 | old adult |
| May 20, | 1951 | 74 | 6.2 × 3.2 | old adult |
| May 20, | 1951 | 74 | 5.5 × 3.0 | old adult |
| May 20, | 1951 | 66 | 5.0 × 2.8 | young adult |
| May 20, | 1951 | 65 | 4.2 × 3.2 | young adult |
| May 20, | 1951 | 64 | 5.3 × 3.1 | young adult |
| May 20, | 1951 | 45 | 2.5 × 1.0 | juvenile |
| May 20, | 1951 | 40 | 1.5 × .3 | juvenile |
| June 3, | 1951 | 65 | 5.0 × 2.5 | young adult |
| June 10, | 1951 | 67 | 4.0 × 1.8 | young adult |
| June 25, | 1951 | 75 | 4.0 × 2.0 | old adult |
| June 25, | 1951 | 70 | 3.5 × 1.8 | young adult |
| June 25, | 1951 | 51 | 2.0 × .5 | juvenile |
From the time of emergence in spring, males show some tendency to seek out females, and frequently a pair may be found together under the same rock, weeks before the onset of the breeding season. There is no satisfactory evidence that such associations have any [46] permanence. At the time of emergence from hibernation the males rarely have even a trace of reddish coloration on their heads, and more than a month normally elapses before attainment of breeding coloration. Each year that observations were made activity of the skinks was interrupted by cold weather in April, so that the lizards were fully active for only part of the time between their earliest emergence and their attainment of breeding condition five to eight weeks later. The reddish suffusion of the breeding season, hardly showing in the first few weeks after emergence, appears suddenly within a few days in all adult males of the population. The best indication of the time necessary to attain breeding condition was provided by an adult male whose hibernation was interrupted on December 15 by bringing him into a warm room where he was kept at 80° F. or more in the daytime, and approximately 70° F. at night. Thirteen days later, on December 28, the male had developed a noticeable reddish suffusion. On January 3, nineteen days after hibernation terminated, the suffusion was near its maximum. When an adult female was placed with the male on this date, he showed sexual interest but the courtship was not consummated. On January 6, the 22nd day, the male’s colors had reached their maximum, and when the female was placed with him, pursuit and copulation occurred promptly.
In the spring of 1952, the first skink of the season was found on March 29, still only partly activated, and under a large flat rock. Skinks were not caught or seen in any numbers until April 17, however, and general emergence probably occurred only a day or two earlier than this. On May 10, 1952, breeding activity was estimated to be at its peak. By May 28, the reddish suffusion was conspicuously faded in several males taken. By June 10 it was no longer discernible.
In the immature female the oviducts are small and threadlike, and the ovaries have grapelike clusters of pale whitish eggs, which are minute, often less than .5 mm. in diameter (Figure 8A). In sexually mature females ova enlarge rapidly after emergence from hibernation in the spring. While eggs are still in the ovary, they are approximately spherical. In late April and early May the developing ova enlarge rapidly. Approximate average sizes (dimensions in mm.) of developing ovarian ova in each of 22 mature females on different dates were as follows: April 17, 1949: 2.6, 2.3, 2.2, 2.2, 1.9, 1.9; April 18, 1949: 2.2, 1.9, 1.8, 1.1, 1.1; April 24, 1949: 4.6, 3.2, 2.5, 2.3; May 6, 1951: 2.5, 2.3; May 20, 1951: 7.0, 6.2; May 25, 1951: 8.0; June 3, 1951: 6.0, 5.5.
The two females containing ovarian eggs on June 3, 1951, were retarded individuals, taken along with several others that had already ovulated. Copulation takes place in early May before the ova have grown to their full size. In the following weeks both the ova and the oviducts enlarge rapidly. Upon passing into the oviducts, the ova assume an oval shape and are approximately 9 by 6 mm. before the albumen and shell are added. Deposition of a clutch of eggs probably extends over only a day or two at most, as clutches appear abruptly in the nest cavities. On only a few occasions were the females found in nest cavities with their clutches partly laid.
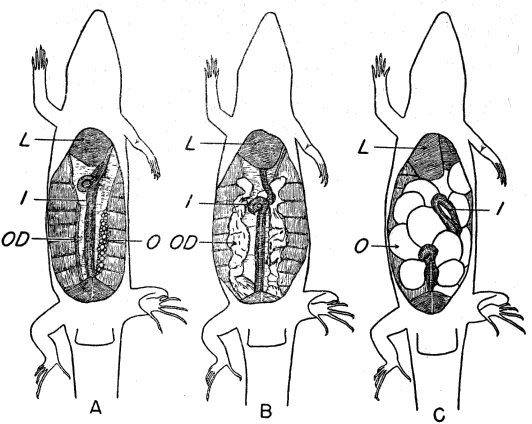
Fig. 8. Adult female skinks with ventral body walls removed to show reproductive organs. A. Condition in April shortly before the breeding season; the ovary (O) is still small and elongate, with the small ova forming a grapelike cluster; right ovary removed to expose the small bandlike oviduct (OD) beneath it. B. Condition in late May shortly before ovulation; the greatly enlarged ovaries are removed to expose the oviducts (OD) now enlarged and convoluted for reception of the ova. C. Same stage as B, with mature ova (O) filling most of the body cavity and concealing other internal organs, I—intestine; L—liver; approximately natural size.
Sexual behavior is for the most part limited to a short period of weeks in spring. In an average year in the area of the study the first two weeks of May would include the peak and the greater part [48] of the breeding season. The “courtship,” such as it is, and mating have been described by many observers. However none of the published accounts seems to include all the essential features in their usual sequence as observed in the present study. It has been brought out by the studies of Noble and Bradley (1933:94), Noble and Teale (1930:54) and Schmidt (1933:71-76) that the sexual behavior of lizards has phylogenetic significance. Certain basic patterns in mating behavior are characteristic of saurian families, other traits are characteristic of genera, while certain details may be characteristic of species, or perhaps even of subspecies.
In the breeding season the adult male directs the greater part of his activities to a search for females, and finds them by both sight and scent. Observations on searching males suggest that they trail females by scent to some extent, or at least detect their presence in the general vicinity by this means. Upon discovering a female, the male pursues her with vigor and determination unless the temperature is too low, or unless he is not at the height of breeding condition. The female makes no positive response but reacts to the male’s presence by fleeing, either frantically or perfunctorily, but if she is physiologically ready to breed the reaction is usually somewhat intermediate between these extremes. The first reaction of the male as he approaches the female is to touch her with his tongue, apparently receiving olfactory stimuli which are essential to the mating pattern. Rushing in pursuit of the female he then attempts to seize her in his jaws. Most often a preliminary grasp is secured on the female’s tail. The female may resist vigorously, wriggling and clawing, turning upon the male to bite or to threaten with her gaping jaws. At the first opportunity the male deftly shifts his grip from the female’s tail or hindquarters to a more anterior position, which may be as far forward as the forelimbs or may be as much as an inch behind them, a little to one side of the mid-dorsal line. The male secures his hold by pinching loose skin into a small fold. Having gained this position the male is more or less out of reach of the female’s jaws, and after a brief struggle both rest quietly except for their rapid breathing, usually for a minute or more, the ventral surface of the male resting on the female’s dorsal surface. The male suddenly thrusts his tail beneath that of the female. His hind leg then rests over the base of her tail and the right angle formed by the laterally projecting hind leg and the tail in each lizard aids to guide their hindquarters into position so that cloacal contact is established. Copulation then begins immediately. The male’s body may be bent in a semicircle, to one side of the female, or may be in an S-shaped loop, depending on whether or not the hemipenis employed is on the side opposite to that on which the female is grasped. Only one hemipenis is inserted, but occasionally the other may be everted also. As copulation begins the male’s hind leg, flexed over the female’s tail base quivers, but otherwise there is hardly any movement during approximately the first one-third of the copulatory period, and this phase may last for from one to three minutes. Then, abruptly, the male begins rhythmic, jerky flexions of the proximal portion of the tail, at the rate of approximately one per second. These tail movements are in a dorsoventral plane, and there is no perceptible movement of the body. Shortly after these movements cease, contact is broken usually at the initiative of the female, as she suddenly struggles to escape and is released either immediately or after a few seconds by the male. She then moves away, pressing her cloacal region against the ground. Her movements have become unhurried, with little or no attempt to avoid the male’s attention. The male usually follows, either close behind, or straddling the female’s tail or body. He may nip at her tail or body repeatedly, but without securing a grip. When the female pauses, he may come to rest with his chin or forequarters resting on her. Usually the association does not last more than a few minutes.

Fig. 1. Habitat of Eumeces fasciatus near the center of the "Skink Woods" study area on the University of Kansas Natural History Reservation, a glade with loose rocks that were used as nesting sites and shelter by many five-lined skinks.

Fig. 2. A log on rocky slope in open woods with sparse undergrowth, fifty feet from center of glade shown in Fig. 1. The trees are mostly oaks (Quercus Muehlenbergii). The decaying log in middle foreground is much frequented by the skinks as a shelter and source of insect food.

Fig. 1. Old adult male, year-old young and hatchling in July, showing differences in size and pattern.
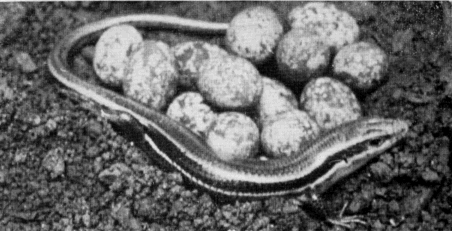
Fig. 2. Adult female skink in a natural nest, with her clutch of eggs late in incubation. The nest cavity is excavated in loose soil beneath a flat rock, which was raised momentarily to expose the nest to view.
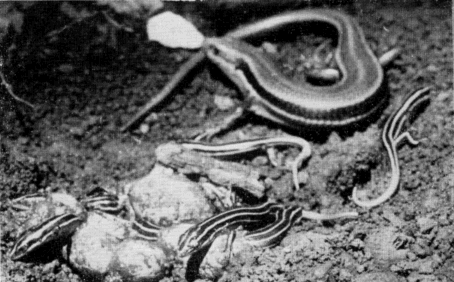
Fig. 3. The same female and nest, with eggs in process of hatching.
Noble and Bradley (1933:77) mention frequent homosexual matings between captive males. However, I observed no homosexual matings, either under natural conditions or in confinement. The pugnacious behavior of males that are in breeding condition ordinarily would prevent homosexual mating. Males in such weakened condition as to be unable to defend themselves effectively might evoke sexual attack, instead of the usual fighting response in other males. Although no actual experiments were performed in the present study in connection with the courtship and mating behavior, accounts of some workers seem misleading. My own observations indicate that the capacity for sex discrimination in this particular kind of lizard, and probably in others, has been underrated. For example, it has been stated that the male rushes with open mouth at the neck of any other skink that happens to be around, and he identifies it as a male if it fights back, or as a female if it does not. On the contrary my observations indicate that sex recognition occurs almost as soon as the male is aware of another skink’s presence. The red head of the breeding male is an excellent example of a social releaser in the sense that this term was used by Tinbergen (1948:8). Like the red belly of the breeding [50] male stickleback, it facilitates sex recognition and evokes hostile behavior on the part of other males. Courtship, mating, and fighting reactions however, seem to be evoked by the interaction of a complex of social releasers. Whereas males and females are strikingly different in appearance in the breeding season, visual sex recognition is complicated by ontogenetic changes. The body stripes characteristic of the female pattern, become dull or even disappear in some old females, which then approximate the typical male pattern. On the other hand newly matured males in their first breeding season retain distinct body stripes of the female pattern. Their sex is evidenced mainly by their reddish facial suffusion, which is not quite so extensively developed as it is in older individuals. Also, in these newly matured males the temporal region is not so swollen as it is in old males.
The male whose dormancy was terminated in early winter by bringing him into a warm room causing him to assume breeding coloration and to breed some four months earlier than those under natural conditions has already been mentioned. By the time the regular breeding season arrived, this male had long since undergone sexual regression and retained no trace of the red suffusion. In this condition, placed in a terrarium with a mixed group of breeding adults, his social status was of unusual interest. He exhibited no interest in the females and was less pugnacious toward other males than were the individuals in breeding condition. Although he seemed somewhat more nervous and timid, his hostile behavior was not entirely suppressed, as from time to time he moved up to other males and bit them viciously. His color pattern resembled those of certain old adult females in which the body stripes have been suppressed, but the breeding males evidenced no uncertainty as to his sex and were uniformly hostile. Their reactions were not noticeably different toward him than they were toward breeding males. The importance of an olfactory stimulus as a social releaser in sexual behavior of lizards has not been appreciated, although Noble and Mason (1933:10) did demonstrate its importance in the behavior of the female toward her eggs.
It is evident from published accounts, and from my own limited experience with fasciatus in parts of its range other than northeastern Kansas, that the phenology of the breeding cycle is subject to geographic variation, synchronizing with the somewhat different climatic conditions under which the species occurs. However, the difference is less than might be expected, in view of the species’ extensive range. As a result of the early spring, and the warm [51] summer climate in the southern states, dates of laying and hatching may be several weeks advanced. On April 12, 1952, Dr. Wilfred T. Neill showed me several live E. fasciatus, collected a few days before along the Trinity River in southeastern Texas, which appeared to be at the height of breeding condition. In northeastern Kansas on that date, general emergence had not yet occurred, and it was not until about May 10 that the population attained the peak of breeding condition. On May 8, 1948, near Burr Ferry, Vernon Parish, Louisiana, I caught an adult female in her nest burrow, and she contained eggs ready to be laid. Data with which Mr. Robert Gordon kindly provided me for specimens from southern Louisiana and southeastern Texas, in the Tulane University collection, indicate gravid females on June 4, 1952, and June 17, 1948 (3), and females with their egg clutches on June 16, 1948, June 17, 1948, June 23, 1950; and hatching dates in captivity of July 19, 1949, July 19, 1950, July 25-26, 1949. These dates correspond well with those for specimens obtained in northeastern Kansas in the same years. In the northern part of the range, Ruthven (1911:264) recorded that in the Saginaw Bay region, females taken on June 19 had eggs nearly ready to be laid, and after July 2 clutches were found frequently; young of the year were first observed on July 31. A juvenal specimen in the University of Minnesota Natural History Museum, collected on August 11, 1938, at Dresser Junction, Wisconsin, is 301⁄2 mm. in snout-vent length—approximately the size of juveniles in northeastern Kansas at the same season. Evans and Roecker (1951:6) record hatching as occurring in the first week of September at Arden, Ontario, indicating that at the northern edge of the range hatching may be delayed as much as two months. With such delayed hatching, but little time remains for the young to grow before they are forced into retirement for hibernation.
Territoriality in the usual sense is lacking in the five-lined skink, and could scarcely exist in an animal of its habits. To defend a definite area (territory) against intruders of its own species, the animal would have to detect such intruders promptly. The skink, however, is so secretive in habits that at any given time the individual is likely to be hiding and inactive, even when conditions are favorable for it to be in the open, and other individuals therefore can then wander onto its home range unopposed. Even when an individual is active, it lacks the ability to detect others, except within a radius which would encompass only a small fraction of [52] the entire home range. The senses are inadequate to inform one lizard of the presence of another until the two are only a few yards, or even a few inches apart. Usually the lizard is on the ground, where even small objects obstruct its view, and vision is probably effective for only a few yards. Hearing is probably effective for about the same radius in detecting animals of approximately its own size. Scent is effective in detecting prey near at hand or on contact, but probably does not serve for detection of other lizards that are not in the immediate vicinity. Therefore, the area covered by one in the course of its normal activities may harbor many others, and individuals most of the time are unaware of the others on their home ranges.
Under most circumstances these skinks behave toward each other with tolerance or indifference, but during the breeding season adult males become hostile, and fight on sight. Their reddish facial suffusion serves as a social releaser which elicits hostile behavior and facilitates sex recognition. As the breeding season wanes, the reddish suffusion fades rapidly and male hostility, probably controlled by the same hormonal complex, is likewise suppressed. Hostile behavior is rare in adult females or young at any time.
Combats and pursuits have been observed most frequently the last week of April and especially in the first two weeks of May. At this season funnel traps set along rock ledges often caught two adult male skinks together. In almost every instance one of the two confined males was mutilated, with pieces of skin and flesh bitten from the tail and with chin, snout, and neck scarred; most serious wounds were usually in the sacral region or base of the tail or both. Often the wounds were so severe that the skink died in a short time in captivity and presumably others that were released died also.
On April 28, 1949, a large adult male skink, chased by another, ran out in the middle of a trail and stopped. The pursuer stopped a few inches from it, then after a long pause, retreated in the direction from which it had come. For the five minutes that the pursued skink was watched, it lay motionless, partly hidden by dry leaves, evidently seeking to avoid further pursuit by concealment. I caught it without difficulty, and it seemed weak and dazed, as if injured in the fight. Its reddish suffusion was conspicuous, but not fully developed.
On May 3, 1949, an adult male having bright red facial suffusion was observed searching persistently in ground litter; he was seen to find and pursue a female, and to copulate. A few minutes after mating was completed and the pair separated, a second male also searching in the vicinity came within sight of the first one. The two noticed each other at a distance of about 18 inches, indicating their awareness by their more alert, jerky movements, and spasmodic vibrating of their tails. The newcomer darted at the other, and for a moment [53] they dodged and sparred. As one broke away to run, the other seized it by the tail. They were on an exposed tree root about an inch in diameter. The skink that was caught twisted its body around underneath the root and seized its adversary by the tail likewise, so that their linked bodies encircled the root, each squirming to disengage itself from the other’s jaws. After a few seconds they did break apart, and then maneuvered briefly menacing each other at close quarters, but they gradually moved away and lost contact.
On May 10, 1949, two adult males were seen to approach each other slowly, pausing for perhaps a minute when they were a little more than one foot apart. Then one edged up to the other, and with a sudden lunge seized it by the head. The one seized broke away with a vigorous jerk, and promptly retaliated by biting the first one’s head. After a few seconds of rapid sparring and thrashing, they broke apart, and one chased the other for several feet until it eluded further pursuit by dodging and hiding.
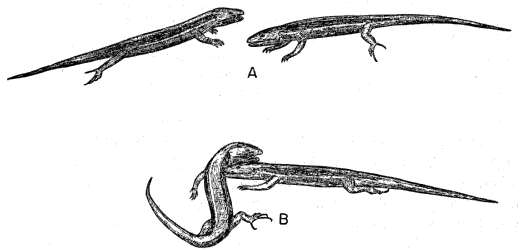
Fig. 9. Adult male skinks fighting. A. Menacing approach. B. One has lunged and secured a grip on the other’s side, holding it at right angle. The one caught is unable to flex its body and neck enough to secure a retaliatory grip on the attacker, and must break away by violent thrashing.
On May 12, 1950, my attention was attracted by a rustling in dry leaves. Within a few inches of my foot two adult males were struggling fiercely with jaws interlocked. Sudden violent twisting and thrashing alternated with quiet periods of a few seconds duration, in which the lizards scarcely moved except for heavy panting and twitching of their tails. After perhaps two minutes of fighting, one broke away and ran. For a distance of several feet it was closely pursued by the other, which, however, soon lost contact with it in the rough terrain and surface litter.
On May 12, 1951, rustling in dry leaves attracted my attention to two large adult males fighting. For about fifteen minutes that they were observed, they struggled, with neither yielding ground, though they thrashed and rolled about over an area of several square feet. Sometimes they were disengaged for short intervals. Then facing in opposite directions, with their heads side by side, they would snap at each other’s necks and shoulders (Figure 9). Part of the time both males had grips and were biting each other simultaneously, but more frequently one or the other had a temporary advantage. When one secured a grip it would strain to the utmost, biting as hard as it could and lunging forward with frequent short jerks, meanwhile striving to keep out of reach of the [54] other’s jaws. The one caught in the attacker’s grip was usually unable to flex its body sharply enough to reach its opponent at all, or could barely reach it at such an oblique angle that its jaws slipped off the smooth body. Sometimes the one held did succeed in catching the other’s front foot. The one caught in the other’s jaws always succeeded in tearing loose after a short time. In the interval while the attacker rested with jaws partly relaxed, the victim had an opportunity to break away. Even when both were free, they did not obtain grips easily, but often made several unsuccessful lunges and bites, the jaws of each slipping off the firm, smooth sides of its opponent. Sometimes the attacker seized a fold of skin, or sometimes obtained a wide grip on its body. One which had obtained a grip sometimes rolled rapidly, spinning the other around and dashing it against the ground. As these rotations stopped, the victim might come to rest on its back in such a position that it was temporarily helpless, but always broke loose after further struggles. Neither showed any inclination to retreat until finally, when they were interlocked, rolling about almost at my feet, I attempted to catch them. Then they instantly disengaged and rushed away, and one escaped. The one caught had suffered but little injury in the fight. Numerous tooth marks were discernible as minute abrasions on the surface of the scales, but the bony dermal armor had not been perceptibly penetrated during the prolonged and violent struggle.
The eggs of Eumeces fasciatus are like diminutive chicken eggs in appearance. They are white when first laid, slightly translucent when held to the light. Within a day or two after they are laid, these eggs are soiled to a dull tan color, somewhat mottled, as a result of being rolled and dragged about in contact with the floor and wall of the nest burrow. Like the eggs of most other reptiles, those of Eumeces fasciatus have parchmentlike shells. These shells are thin and easily punctured. As incubation proceeds, the egg enlarges by gradual absorption of moisture and the somewhat elastic shell is stretched. An egg left in water for as much as a day does not gain in weight appreciably. Except for occasional abnormal ones, the eggs of any one clutch are notably uniform in size and shape at the time they are laid. As incubation proceeds, some eggs enlarge more rapidly than others, and attain larger ultimate size. Differences in shape also appear, some eggs becoming relatively elongate and thin, while others are thick and blunt. Some become distorted to asymmetrical shapes. In nests that have been deserted by the females, eggs of irregular shape are especially noticeable. It seems probable that the frequent shifting of the eggs by the female prevents unequal drying or stretching in different areas of the shell. Normal young were observed to hatch from grossly misshapen eggs. Under conditions of drought, the eggs may not enlarge normally during the latter part of incubation, and may become indented or partly collapsed, and yet apparently normal young [55] hatch from them. Both in the field, and in laboratory experiments, eggs were found to have remarkable tolerance for excess moisture. After heavy rains of summer thunderstorms, nests were sometimes found to have water trickling through them, and on occasion eggs were found to be partly submerged in water in the nest cavity. Exposed rocks at the heads of small gullies often were chosen by the female skinks as the shelter for their nests. In these situations the nests were exposed to run-off water. In July, 1951, especially, unusually heavy precipitation resulted in the flooding of many nests. In some instances desertion by the females and destruction of the eggs seemed to have been caused by this flooding, even in the well-drained hillside situations where this study was made.
Table 5.—Measurements in Millimeters and Weights in Grams of Eggs in the Same Clutch at Different Stages During Their Incubation, Showing Gradual Increase in Size.
| June 17 (laid) |
June 18 |
June 24 |
June 28 |
July 17 |
July 20 |
July 28 |
July 30 (hatched) |
|||||||
| Average length (for 7) |
..... | .... | .... | .... | 13.7 | 14.3 | 14.7 | ....... | ||||||
| Average width (for 7) |
..... | .... | .... | .... | 10.5 | 10.9 | 11.1 | ....... | ||||||
| Typical length | ..... | 11.1 | .... | 12.5 | 14.0 | 14.3 | 14.8 | ....... | ||||||
| Typical width | ..... | 7.5 | .... | 9.9 | 11.0 | 11.2 | 11.0 | ....... | ||||||
| Maximum length | ..... | 11.5 | .... | .... | 14.5 | 15.0 | 15.5 | ....... | ||||||
| Maximum width | ..... | 7.5 | .... | .... | 10.9 | 11.1 | 11.4 | ....... | ||||||
| Minimum length | ..... | 10.5 | .... | .... | 12.5 | 12.8 | 13.5 | ....... | ||||||
| Minimum width | ..... | 7.0 | .... | .... | 9.9 | 10.0 | 10.5 | ....... | ||||||
| Average weight | ..... | .38 | 10 | .58 | 5 | .63 | 9 | .82 | 8 | .90 | 7 | 1.0 | 7 | ....... |
| Typical weight | ..... | .4 | .... | .... | .... | .9 | 1.0 | ....... | ||||||
| Maximum weight | ..... | .... | .... | .... | .... | 1.0 | 1.1 | ....... | ||||||
| Minimum weight | ..... | .... | .... | .... | .... | .7 | .7 | ....... | ||||||
| Superior number indicates the number of individuals averaged. | ||||||||||||||
The extent of tolerance to immersion in water probably depends on the stage of development, the temperature, the oxygen content of the water and other factors. One egg was fully immersed for ten minutes on July 20, 1951, then returned to a container with damp soil in the laboratory, where it seemed to develop normally. On July 30 it was opened and found to have a living fetus, [56] which was a week short of hatching. On July 22 another egg of the same clutch was immersed and left in water for 23 hours. On July 30 it was ruptured in handling and found to contain a living fetus. On July 31 two eggs were placed in a dish of water in a refrigerator. On August 5 they were removed and opened. Fetuses were dead and were not appreciably larger than the one of the same clutch in the egg opened on July 31. On August 5 two of the remaining eggs of this clutch were placed in a Petri dish, partly immersed in water, with approximately one-fourth of the surface of each protruding and exposed to the air. Forty-eight hours later it was found that both eggs had hatched. Evaporation had reduced the water in the dish to an amount sufficient to cover only about the lower one-third of each egg. One hatchling was missing, evidently having climbed out of the shallow dish and escaped to the floor. The other was found still standing in the water with its head protruding, and it was lively and in good condition. The remaining four eggs in this clutch, which had been kept in a container of damp earth, were also hatching on this date. On July 10, 1952, an egg in a late stage of incubation was immersed in water in the laboratory. On July 14 when removed, it had fungus growing on it, and was found to have a dead fetus, nearly full-sized.
The range of temperature tolerance of the embryo is wide, probably comparable to that of the adult. Time required for incubation is dependent on temperature. Persistently wet and cloudy weather in the summer of 1951, keeping temperatures relatively low in nests, was a contributing cause to late hatching that summer. As compared with 1952, hatching was about one month delayed in 1951, but later emergence and breeding accounts for part of the difference. The extent to which low temperature may delay incubation was indicated by the effect of refrigeration on several experimental eggs, as recorded below.
| 1. | July 8, 1952 | Egg transferred from natural nest to jar of damp soil in refrigerator at 13.8°C. |
| July 14, 1952 | Seems to be in good condition. | |
| July 19, 1952 | Partly collapsed. Weight and measurements same as on July 8; opened and found to contain a dead fetus. Snout-vent length 23 mm., forehead bulging, skin delicate and membranous. Colors somewhat dull, indicating that it was not quite fully developed, although it had attained the minimum hatching size. | |
| 2. | July 10, 1952 | Egg from natural nest (15.0 × 10.5 mm., .95 gm.) put in refrigerator at 11.6°C. Control (14.5 × 10.6 mm., .8 gm.) from the same clutch kept in hatching medium in laboratory. |
| July 13, 1952 | Control egg hatching; refrigerated egg shows no indication of hatching. | |
| July 14, 1952 | Experimental egg 15.8 × 10.8 mm., 1.0 gm., seems to be in good condition. Nest from which it was taken found to have all remaining eggs hatching today. | |
| July 19, 1952 | Experimental egg 15.0 × 10.0 mm., 1.0 gm., removed from refrigerator and transferred to container in damp rotten wood in laboratory. Seems to be in good condition. [57] | |
| July 23, 1952 | Experimental egg found to be hatched this morning, and hatching must have occurred either in the night or late yesterday. Eggshell still damp and pliable. | |
| 3. | July 10, 1952 | Egg from natural nest (14.0 × 10.5 mm., .8 gm.) put in refrigerator at 11.2°C., in container with damp decayed wood. Control egg (14.2 × 10.1 mm., .8 gm.) from the same clutch kept in the same hatching medium in the laboratory. |
| July 12, 1952 | Nest from which experimental and control eggs were taken has started to hatch, and two hatchlings were seen there. | |
| July 13, 1952 | Control egg hatched. | |
| July 14, 1952 | Experimental egg 14.2 × 10.1 mm., .8 gm., seems to be in good condition. Nest in field examined and all eggs were hatched, with only three of the hatchlings remaining, the others having dispersed. | |
| July 19, 1952 | Experimental egg 14.0 × 10.0 mm., .95 gm., still appears to be in good condition; removed from refrigerator and kept in laboratory. | |
| July 23, 1952 | Experimental egg found to be hatched, and hatchling active although still in hiding beneath rotten wood. Probably it hatched early in the day of July 22; the empty shell is still moist. |
These experiments seem to show that, in the later stages of incubation at least, lowering of temperature to 11° or 12°C. almost halts development of the fetus. Harm does not necessarily result, however, and when again warmed to normal incubation temperatures, the eggs eventually hatch, the incubation period being lengthened by a time approximately equivalent to the interval of refrigeration.
Under natural conditions the time required for incubation probably varies within wide limits, controlled mainly by temperature. No two clutches receive the same amount of heat, as sites differ greatly in extent of insulation, and exposure to sunlight. Each year, earliest appearance of hatchlings is in a warm, sunny situation, and in cooler, well shaded places hatchlings appear somewhat later. Their incubation is evidently somewhat protracted, although later emergence from hibernation and later breeding of adults in these situations might also contribute to the delay.
Widely different incubation periods have been recorded in the literature and the variation probably is not due to temperature alone. Noble and Mason (1933:4) recorded incubation periods for six females from the same locality, and evidently kept under the same laboratory conditions, as 47, 41, 36, 29, 29, and 27 days. Despite [58] the wide difference in incubation time, all six clutches hatched within a 12-day period from July 5-17. It seems improbable that differences in temperature account for the 20-day disparity between maximum and minimum incubation time, in these females kept under similar conditions. Cagle (1940:229) recorded an even shorter incubation period for one kept in the laboratory, which laid eggs on June 30; hatching occurred on July 23 and 24. Retention of eggs in the oviduct by females kept under unnatural conditions would partly explain their late laying and the short incubation period of their clutches. Such ability to retain eggs in the oviduct while their development proceeds would not be especially surprising in E. fasciatus since its congener E. lynxe of the highlands in southern Mexico is normally ovoviviparous (Hartweg, 1931:61; Taylor, 1936:171). Cagle did not determine incubation time for any of the natural nests found, but evidently in all of them laying occurred earlier than in the single female brought to the laboratory while still gravid. All the eggs in natural nests found by him were brought to the laboratory and most of them were hatched. Cagle remarked: “The fact that these 26 nests hatched within a period of nine days seemingly indicates that the egg laying period extends over not more than two weeks.”
In the present study no incubation periods so short as those recorded by Noble and Mason, and Cagle, were observed. Incubation times were recorded for clutches both in the laboratory and in the field, but for most of the clutches only approximate incubation periods were recorded. Failure to record the exact date of laying or of hatching, or both resulted from attempts to avoid frequent disturbance of females in their nests, which might have caused them to desert.
One clutch of eggs laid in a terrarium probably on June 17, 1951—possibly a day or two earlier—hatched on July 30, after an incubation of about 44 days. Another clutch, found in a terrarium on July 17, 1951, was estimated to have been laid about a week earlier, judging from the average length (11.8 mm.) and average weight (.55 gm.) of the eggs. These eggs hatched on August 9, a little more than three weeks after their discovery. A clutch found in the field on June 25, 1951, evidently recently laid (average length 12 mm., weight .45 gm.), hatched 41 days later, on August 5. Another clutch found in a terrarium on July 17, 1951, was estimated to have been laid ten days or two weeks before, as the average length was 12.7 mm. The eggs hatched on August 7, three weeks after their discovery. On June 25, 1951, an incomplete clutch of three eggs was found with a female which still had an unlaid egg. The three eggs probably had been laid the same day or the day before. They were kept in the laboratory and weighed and measured at intervals until July 28, 33 days after their discovery when both those that remained were accidentally punctured [59] and found to have nearly full term fetuses. In the field a nest which contained only a gravid female on June 24, 1951, had a clutch of eggs already mud stained and slightly enlarged on June 29. The most probable date of laying was June 26. On August 6 the eggs had all hatched but several young were still in the nest. Probably most hatched on August 5. The incubation time was hence approximately 40 days.
On June 21, 1951, a natural nest was found with eggs already somewhat enlarged (12.5 × 8 mm.) and mud stained. This nest was checked from time to time in the next few weeks, and after 39 days, on July 30, it was found that all the eggs had recently hatched, but six young were still in the nest cavity.
Another nest was found on June 24, 1951, with the eggs already markedly enlarged (14 × 8 mm.) indicating that laying must have been several days earlier—probably well over a week. Hatching occurred approximately 34 days later, probably on July 28, since on July 26 there was no sign that hatching was imminent, and on July 30 only the empty dried eggshells remained in the nest.
The incubation time approximated six weeks for those nests with most complete records. Under wet and stormy weather conditions such as prevailed in 1951, this may have been the normal incubation period, but in warmer and drier years incubation time is shortened.
In the five-lined skink each adult female normally produces one clutch of eggs annually. The size of the clutch produced is subject to individual variation, and is influenced by the age, size and condition of the female. Geographic variation in clutch size might also be expected. Data were obtained from breeding females killed and dissected, from counts of eggs found in natural nests in the field, and from clutches of eggs laid by females kept in captivity. For the total of 115 recorded clutches represented by the combined data from all these sources, the average number of eggs per clutch was 9.5.
In many females dissected for the purpose of obtaining egg counts, ovulation had not yet occurred. The ovarian eggs present in each of these females included two main size groups, the larger ones in process of maturing and evidently destined for deposition in the current season, and minute, immature ones. A few of intermediate size were always present, however, resulting in uncertainty as to the size of the clutch being produced, especially when development had not proceeded far. Even when the larger eggs formed a fairly distinct size group, some usually were well below maximum size. Relatively high counts of clutches were obtained from these examinations of enlarged ovarian eggs. Evidently development frequently is arrested, and resorption may occur before ovulation. As a result the numbers of ovarian eggs developing are a poor indication of actual clutch size. A series of gravid females were obtained [60] and examined after ovulation; the numbers of eggs in their oviducts probably indicates accurately the sizes of their clutches. Gravid females taken from their nest burrows and kept in the laboratory in containers with loose damp soil soon excavated new burrows and deposited clutches. Many natural nests were found in the field, and the egg counts obtained from them provided further data concerning clutch size. Although most of these clutches probably had their full complements of eggs, others certainly had sustained losses to predators, or to the females themselves, which may eat some of the eggs. Therefore the average number found is erroneously low. Some of the natural nests found may have contained two or more clutches or parts of them, and the higher counts obtained from natural nests therefore are also questionable.
For different sets of data on clutch size, numbers were as follows:
| Source of Sample | Number of clutches |
Mean | Standard deviation |
Maximum | Minimum |
| Early ovarian | 25 | 11.4 ± .46 | 2.28 | 20 | 5 |
| Late ovarian, uterine, or laid in captivity |
56 | 9.16 ± .21 | 1.85 | 15 | 4 |
| In natural nests | 34 | 8.82 ± .32 | 1.85 | 16 | 4 |
On the average, larger females produce more eggs per clutch than do smaller females. Of 49 females for which measurements were recorded, and which had uterine or large ovarian eggs, 31 were 70 mm. or more in snout-vent length. These 31, mostly or entirely old adults, averaged 9.9 eggs per clutch, whereas 18 others that were 69 mm. or less in snout-vent length, and that must have been mainly or entirely newly matured adults in their first breeding season, averaged only 7.8 eggs per clutch.
Smith (1946:350) states that in the northern part of the range of this skink there is some indication of decrease in size of clutches. This is not well shown by published records. For the southern states, most of the published records of clutch size are by authors who did not clearly distinguish between the three kinds of five-lined skinks, and there is some doubt as to which species is involved in each record. For 56 clutches reported upon from north of approximately latitude 37°, I obtain a slightly higher figure than for 11 clutches from south of this line. Geographic trends are, of [61] course, obscured by individual variation, and perhaps by abnormal clutches produced by individuals kept in captivity.
In Table 7, the figures marked with asterisks pertain to clutches that might have belonged to skinks of the species E. laticeps or E. inexpectatus since they were recorded in regions where laticeps and in some cases, inexpectatus also, occurs along with fasciatus. If these questionable clutches are excluded the remaining 55, definitely of fasciatus, average 8.48 eggs per clutch, whereas the 12 questionable clutches average 8.42. Both figures are close to the average of 8.82 ± .32 eggs for the 34 natural nests recorded in the present study. For the total of 1661 eggs of 182 clutches, from the combined sample of all available records for clutches found in the present study or reported upon in the literature, the average egg number is 9.13.
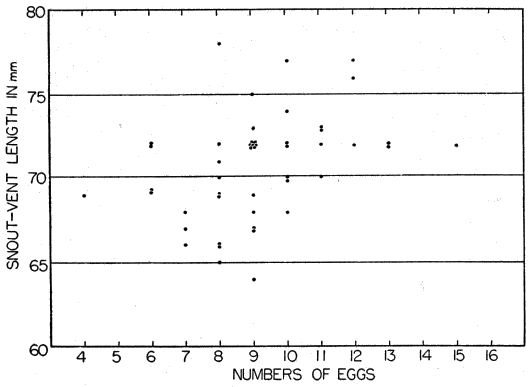
Fig. 10. Correlation between size of female and number of eggs in clutch; females in their first breeding season, mostly less than 72 mm. in snout-vent length, produce smaller clutches, on the average, than do larger and older females, but there is extensive overlap.
To sum up the available information on clutch size, the number of eggs is most typically 9, 10, or 11 and is more in large old females, than in small, newly matured females. In natural nests, even in those that are successful, there is often some loss of eggs, which are eaten by predators, or by the female herself, with the [62] result that the egg counts made by various observers average somewhat lower than the numbers actually produced. The loss during incubation cannot be measured readily since it is almost certainly sharply increased by the disturbance entailed in observing nests. Exposing nests, even momentarily, for observation, may result in compacting of the surrounding soil, desiccation, temporary or permanent desertion by the female, and exposure to predation. Some indication of the incidence of loss during incubation might be obtained by counting and measuring the eggs in newly found nests and correlating numbers with size (indicating the length of time incubated).
Table 7.—Numbers of Eggs Per Clutch, Time of Occurrence, Laying Dates and Hatching Dates, as Reported in the Literature by Various Authors.
| Author | Numbers of eggs per clutch |
Date recorded | Natural nest |
Laying date | Hatching date |
Locality |
| Allard | 7* | .... | Yes | .... | .... | Northern Georgia |
| Bishop | 8* | .... | Yes | .... | .... | Breathitt Co., Kentucky |
| Blanchard | 9* | .... | .... | .... | .... | Tennessee |
| Burt | 6; 11 | May, and June 18, 1926 |
Yes | June 12, 1926 | .... | Douglas Co., Kansas |
| Burt | 9*, 9*, 9*, 10* |
June 25 to July 13, 1926 |
Yes | .... | .... | Arkansas |
| Burt | 8* | June 6, 1933 | Yes | .... | .... | Ashville, North Carolina |
| Burt | 8* | June 28, 1934 | Yes | .... | .... | Scott, Mississippi |
| Burt | 6* | July 7, 1933 | Yes | .... | .... | Emma, Georgia |
| Burt | 6* | July 8, 1933 | Yes | .... | .... | Elk River, Alabama |
| Cagle | Average 9.16 in 26 nests (6-15) |
June-July | Yes | June 30 | July 23-24 | Elkville, Illinois |
| Conant | 7, 9, 10, 11, 13 |
.... | .... | .... | July 27, July 27 |
Ohio |
| Dunn | 12* | .... | .... | .... | Aug. 9 | |
| Evans and Roecker |
6, 7 | .... | Yes | .... | First week of Sept. |
Arden, Ontario |
| Fitch (field notes) |
9 | July 22, 1947 | Yes | .... | .... | Vernon Parish, Louisiana |
| McCauley | 3; 20 in 3 other nests combined |
.... | Yes | July 5 and 6 | August 30 | Maryland |
| Noble and Mason |
2, 5, 5, 6, 7, 8, 8 |
.... | No | May 23, 27, 31; June 6, 6, 13, 20 |
July 5, 5, 6, 7, 9, 17 |
Anderson Co., Kansas |
| Ruthven | 6, 6, 8, 9, 11, 13, 14 |
.... | Yes | .... | .... | Michigan |
| Smith | 9 | .... | Yes | .... | .... | Ohio |
Lizards and snakes of several different families, are known to brood their clutches of eggs, although the great majority of oviparous forms do not do so. The brooding habit is perhaps best known in Eumeces fasciatus, and has been described by many authors. By far the most thorough account is that of Noble and Mason (1933) who observed and experimented upon seven females that laid clutches of eggs in captivity. These females, kept in separate terraria, excavated nest burrows for reception of their clutches, and remained with them throughout the time of incubation. There were three characteristic brooding postures; curved in a semicircle around the clutch, in an S-shaped figure extending among them, or lying straight, either over or among the eggs. The brooding females, taken quietly from their nests without disturbing them, were found to have temperatures averaging .4°C. higher than the nests. Evidently normal room temperatures were maintained in the laboratory where the terraria were kept. The females occasionally left their nests, especially in late afternoon, to wander about the terraria, and to bask in sunlight. While basking, their temperatures averaged 2.7°C. higher than the nest temperatures. The authors suggested that an important function of the brooding female was to transfer warmth from absorbed sunlight to the eggs. They state: “In nature the importance of the mother’s body heat in the incubation of the eggs probably varies greatly with the type of nesting site selected.” They suggest that in clutches deposited in logs or stumps beneath a thin layer of bark exposed to direct sunlight the need for warming by the female would be less.
My own observations do not support the idea that brooding by the female serves to hasten the development of the eggs. Both in the laboratory and in natural nests, clutches deserted by disturbed females hatched and the hatching was not unduly delayed. In the field, females were never observed to bask in the sun beside their nest burrows, and seemingly left them infrequently even to feed. When a female was caught in her nest burrow, her temperature nearly always approximated that of the surrounding earth with which she was in contact. The temperature in each nest depends primarily upon its situation. When the immediate vicinity of the nest receives direct sunlight, the eggs are warmed without the aid of the female, but when there is no sunlight the temperature is much lower. In order to maintain an appreciably higher nest temperature the female would have to make frequent trips to spots perhaps several feet or several yards away to find sunlight. Upon [64] returning to the nest, her body heat would be quickly dissipated into the eggs and the surrounding damp soil. She would need to shuttle back and forth almost continually between the nest and a spot exposed to sunshine. Cloudy weather often preventing the warming of the eggs by absorption of solar heat prevails during much of the incubation season, in the region of the present study, and probably to an even greater extent throughout the range as a whole.
Noble and Mason state (op. cit.:9) that while in some non-brooding kinds of lizards the eggs are actually damaged by turning, the female fasciatus frequently turns her eggs and moves the whole clutch about in the nest cavity. On returning to their nests the experimental females each invariably touched one or more eggs with their tongues as an olfactory test. Eggs of other kinds of lizards not of the genus Eumeces, and shellacked eggs of fasciatus, or paraffin models of them, ordinarily were discarded immediately after a single touch of the tongue. Eggs of other individuals of the species, and even the eggs of Eumeces laticeps were accepted as part of the brood. Any of the experimental females would quickly retrieve one of her eggs moved a short distance outside the nest cavity. Even if the whole clutch of eggs were scattered about, the female would, over a period of hours, gather the eggs and return them to the nest cavity. This movement of the eggs is accomplished by rolling or pushing them in a loop of the body or tail, or, less frequently, by grasping an egg in the jaws, lifting it, and gently placing it in a new position. Even if the females were blindfolded, they were still able to retrieve scattered eggs, but one in which the tongue tip was experimentally removed showed no further interest in its eggs, presumably having lost the capacity to recognize them by olfactory test.
In the present study clutches unattended by females were observed to sustain heavy losses, both in the laboratory and in the field, and no doubt the attending female performs important functions other than that of warming the eggs. In the damp or wet nest cavity, the eggs tend to adhere to each other and to the earth walls and floor, and become sealed to such surfaces as a result of partial drying, reducing the amount of surface exposed to the air and probably hindering respiration. An eggshell sealed in prolonged contact with the soil tends to rot with the result that it is easily ruptured, and even if it is not broken there is the likelihood of fungi or microorganisms gaining entry and killing the embryo. In many of the eggs that were handled to obtain measurements and [65] weight, rupturing of shells occurred. The shells are tough and elastic to the extent that even when eggs being handled were accidentally dropped on the floor on several occasions, no damage to them resulted. However, slight friction on the shell was sometimes sufficient to puncture one. Particles of sharp rock from the nest cavity may adhere to the shell, and result in rupturing, perhaps at weak spots where prolonged contact with the soil has caused deterioration. The female tends to keep her eggs in a compact cluster, shifting their position frequently so that no part of an eggshell adheres to its surroundings long enough for rotting to occur, and most of the surface of each egg is exposed to the air.
Another important function of the brooding female seems to be that of altering the nest burrow and shifting the eggs so that the effects of unfavorable weather are minimized. The usual response to warm and dry weather is deepening of the nest burrow. A cavity originally in loose soil on the underside of a flat rock, having the eggs in contact with the rock surface, may be displaced downward. The female excavates loose soil from the floor of the burrow and packs it on the top and sides, until the eggs are two or even three inches underground, in a cavity different in position and shape from the original one, although derived from it by gradual stages. In many instances, however, no such response to drying was observed. Probably extensive alteration of the nest burrow no longer is possible after drying of the soil has progressed beyond a certain stage as these skinks are not strong diggers. In some nests that were examined frequently, with resulting desertions by the attending females, the outlines of the cavities became indistinct and the soil around them became dry and packed. In heavy rains, when nest burrows are partly flooded, the females move the eggs to avoid their being submerged. The extent of the female’s activity within the nest burrow is suggested by the glazed condition of the earth walls and floor, and by the mottled appearance which the eggshells soon acquire as a result of being slid and dragged about in the nest cavity.
Still another important function of the female is to dampen the nest burrow to prevent desiccation of the eggs. Even in dry weather, females taken from nests almost invariably voided water in relatively large quantities. They drink dew or other available water, and may void the contents of the bladder to moisten the nest cavity, as on numerous occasions, when nests were exposed by raising [66] flat rocks covering them, part of the chamber was seen to be recently watered, and distinctly moister than the surrounding soil.
Noble and Mason (op. cit.:16-19) found that brooding females, in the laboratory, would vigorously defend their eggs against small enemies, including mice and lizards and the smaller kinds of snakes that were tested. The female watched alertly as the intruder approached, and attempted to bite it if it came too near or touched an egg. The females failed to defend their nests against persons and against a large blacksnake; when confronted with such a threat, the female would run from her nest cavity to hide. Cagle (1940:228) stated that the brooding females found by him stayed in the nests even when the logs in which they were situated were chopped open with an ax, and that the skinks would attempt to bite when touched with the finger.
In the present study, females whose nests were exposed never made any active attempt to defend them. Many darted away and hid as soon as they were exposed. In other instances, especially when the nest cavity was only partly exposed, from one side, the female cowered back against the inner wall, opening her mouth in threat if closely approached. If further molested she might then attempt to escape. In brooding females a tendency to sluggishness, and an affinity for the eggs delayed the usually speedy escape reactions. The temperature of the female was ordinarily lower than it would have been in the open or on the underside of a flat rock, and this also tended to slow her reactions. Gravid females when exposed in nest cavities that still contain no eggs are similarly sluggish and reluctant to leave differing little or none in behavior from those that have laid their clutches. Usually the female was found with her body encircling the eggs, holding them together in a compact cluster in the center of the nest cavity. The eggs rest in contact with the loose soil on the floor of the cavity, with each other, and with the female’s body in the case of the outer ones of the cluster.
Normal brooding habits proved to be difficult to follow because the females were easily disturbed. In many instances those that had excavated nest burrows, but had not yet laid, deserted the nests after the disturbance involved in raising the sheltering rock. Females that had already laid before discovery of their nests were somewhat less inclined to desert, but many did so.
On numerous occasions, at the time of year when most females are gravid and are staying in nest burrows, I have discovered well formed nest burrows empty and seemingly deserted, with no female [67] in evidence nearby. In some instances the female may have been out foraging or basking although she was not seen, and in other instances the female may have been killed by a predator or eliminated by some other accident. However, it seems that gravid females frequently do desert their original nest burrows, for one cause or another, and excavate new ones. Such desertions were noted many times in the females observed on the study area, where the disturbance from my own activities in raising the sheltering rocks may have caused shifts, but it was probably not the sole motivation. One female shifted approximately 120 feet, to excavate her second nest burrow in a site that was damper and more heavily shaded than the first site. This was in the notably dry summer of 1952. Most of the favorite sites under flat rocks in open situations, that were used in 1950 and 1951, were not occupied in 1952 or 1953, although several females did use them for original excavations, which were deserted before laying, as drought conditions developed. In the summers of 1952 and 1953 nests were difficult to find, and those discovered were on the average deeper and better protected than those found in other years.
As compared with other North American lizards in general, Eumeces fasciatus is notable for the relatively exposed and superficial situations chosen as nesting sites. However, it occurs in a climate of high humidity; in contrast, the great majority of our lizards live in arid climates where the eggs are in much greater danger of desiccation, and require better shelter to maintain the humidity at a sufficiently high level. Accounts in the literature and observations in the present study indicate that these skinks exercise a wide range of choice of nesting sites. Ruthven (1911:264) stated that in northern Michigan nests were usually in decaying logs; occasional nests were found in burrows in sand, but invariably decaying wood was present in or around at least part of the nest.
Blanchard (1922) mentions a nest in Tennessee that may have been made by either this species or E. laticeps “in a hollow in a dead willow tree about fifteen feet from the ground buried in the loose, damp, rotted wood.” Noble and Mason (op. cit.:16) quote Blanchard (in litt.) that in northern Michigan fasciatus nests in logs that are exposed to sunlight. Conant (1951:31) stated that several clutches of eggs found in Ohio were an inch to six inches beneath the upper surface of the log or stump which sheltered them. Evans and Roecker (1951:70) record finding two incubating females inside rotten pine logs, in Ontario. Cagle, studying this species near Elkville, Illinois, in oak-hickory woods, found 25 natural nests of [68] which three were in loose soil among the roots of a fallen tree, another was under loose bark of a log, and the remainder were all in cavities of partly decayed logs. Bishop (1926:119) recorded finding a female with a clutch of eggs beneath damp boards at Quicksand, Breathitt County, Kentucky.
In the present study, more than one hundred natural nests were found, of which just one (containing two clutches of eggs) was in decaying wood beneath the bark of an old log. All other nests were beneath rocks. On the University of Kansas Natural History Reservation, where most of the nests were found, the policy is not to tear apart decaying logs; therefore the nests probably present in such situations were not ordinarily found. On several occasions groups of hatchlings were seen on logs within which they probably had hatched. In the area of the study, however, decaying logs are scarce. The hardwood forests consist mostly of young trees that are second growth on cutover areas or pioneer on areas that were previously grassland. Because of frequent cutting there are few old mature trees, and logs have not accumulated on the forest floor. In northeastern Kansas, nesting in logs is comparatively rare. On wooded slopes and the edges of level hilltops, the flat limestone rocks that are often abundant provide preferred nesting sites. Even on collecting trips off the Reservation, where stumps and logs could be torn apart and searched, flat rocks were found to provide the main source of nesting sites. These nest rocks varied from less than an inch in thickness to nine inches or more, and from a few inches in diameter to three feet or more. Some were resting loosely on the surface of the soil and others were deeply sunken, on one side. Some were in situations exposing them to nearly the maximum amount of sunshine whereas others were in sites nearly always shaded. The varied character of the nesting sites chosen demonstrated a wide range of tolerance for temperature, moisture, and other factors, in the gravid and brooding female and in the developing embryo.
As already mentioned, Noble and Mason (op. cit.:9-10) noted that females would accept and brood the eggs of other individuals just as readily as their own, and several writers have reported gregarious nesting habits, with two or more females occupying either the same nest cavity, or separate cavities that were in close proximity. For instance, Cagle wrote that among the small logs he found to contain nests, four logs each contained one nest, five each contained two nests, and two each contained three nests, while three other nests were found within an eight inch square area in loose [69] soil among tree roots. McCauley (1939:93) in Maryland found three females brooding clutches of eggs, which totaled 20, and which were so near together that there was uncertainty as to which clutch certain eggs belonged in.
The gregarious nesting habit may be of benefit in permitting maximum utilization of choice nesting sites, where such sites are in short supply in an environment otherwise favorable. Also, the gregarious tendencies make possible more continuous guarding of the eggs against such natural enemies as can be repulsed by the female, since each female occasionally interrupts her brooding to bask or forage.
Many of the nests that I found were in close proximity to others. Often two nests, and sometimes even three, were found beneath the same rock, and sometimes a distance of only two or three inches intervened between the separate clutches. It seemed, however, that in almost every instance each female had excavated a separate nest chamber originally. In some instances adjacent nest chambers communicated with each other.
On July 13, 1948, a communal nest was discovered beneath loose bark of a decaying elm log. There were 22 eggs in the combined clutch, and there were two females in the vicinity. The bark was raised on several different days to examine the eggs, and one or both females always were found with the eggs.
On June 10, 1949, at the pond rock pile, a flat rock was turned and an unusual nesting aggregation consisting of a minimum of eight females, and probably more than ten, was found. The nests were somewhat disturbed by movement of the rock. The ground beneath was honeycombed with tunnels connecting the flask-shaped nest cavities, which were in part open to the rock surface on their upper sides. Clutches of eggs numbered 13, 12, 11, 8, and 6 (the last attended by a female which appeared to be still distended with several more unlaid eggs). Of five other females taken, two had laid and three were still gravid. Of the five clutches, two had eggs noticeably larger than those in the other three, and with their shells mottled brown from adhering earth. These nest cavities were about half an inch deep and two to three inches wide. The females were released as soon as they had been examined. One female moved about over the nest areas exposed, and evinced interest in a lone egg which had become separated from the others. She moved up to it, standing high off the ground, with her head turned at right angles to her body as if preparing to push the egg forward in [70] the angle thus formed, and tested it with her tongue, but then she became alarmed and left the vicinity. The flat rock was lowered over the nests again with a minimum of disturbance.
On July 9, 1949, the flat rock covering the nests was raised again. Most of the eggs had hatched. Two broods of hatchlings were still in their respective nest cavities, and one entire clutch had not begun to hatch although its incubation was nearly completed. Three eggs of Scincella laterale were found mixed with the Eumeces eggs. One of these was opened to verify their identity; the other two hatched a few days later in the laboratory.
The following selected excerpts from my field notes, setting forth histories of several nests, so far as they were known, give some idea of the types of nesting sites chosen, the behavior of the females, and the hazards to which the eggs are exposed.
No. 1. At corner of pond rock pile.
June 21, 1951. Female escaped when rock was turned. One egg measured 12.5 × 8 mm., mud-stained.
June 22, 1951. Nest not in evidence when rock was turned; digging into loose soil beneath to a depth of about an inch I exposed the eggs but did not disturb them further.
July 23, 1951. When rock was turned, female did not attempt to escape, but withdrew to far corner of nest cavity; when caught she voided a large scat which seemed to consist mainly of Ceuthophilus remains. Largest eggs in the clutch were 18 × 10 mm. but two were noticeably smaller, and all were heavily coated with dried mud.
July 30, 1951. Six young in the nest cavity, still not fully active; all of them were heavily coated with dried mud.
No. 2. At hilltop ledge, under flat rock 13 × 10 × 1 inches, with one edge sunken in soil; exposed to sunshine for most of day.
June 24, 1951. Female, snout-vent length 70 mm., tail 27-51, weight 5 gms. Nine eggs, one of which measured 14 × 8 mm.
July 18, 1951. Nine eggs still in their original nest cavity, attended by the female; she escaped into crevice behind the rock. The eggs were in slightly damp soil, and in contact with the undersurface of the rock on their upper sides; one egg was 17 × 10 mm.
July 26, 1951. Eggs caked with dried mud; still attended by female.
July 30, 1951. Dry and empty eggshells in nest cavity, evidently all the eggs had hatched; no other trace of female nor of young; July 28th seems most probable hatching date—if, on the 27th, some of eggs almost certainly would have shown signs of hatching on the 26th when they were examined, and if on the 29th some stragglers almost surely would have remained at the nest on July 30.
No. 3. In small gully, on lower slope in hickory woods, beneath rock 9 × 9 × 1 inches, shaded by trees on south side for much of the day, especially during latter part of morning.
June 24, 1951. The gravid female was deep in nest burrow.
June 29, 1951. When rock was lifted no trace of nest was visible except for slightly disturbed loose soil at the point where it had been. When some of this loose soil was cleared away, nest was revealed, with 11 eggs, mud-stained, approximately 12.5 × 8 mm. The female was cold and sluggish, and did not attempt to escape, but cowered in the back of the nest burrow, with jaws gaping; she was caught and marked.
July 20, 1951. Eight eggs remaining in the nest—two were accidentally destroyed in moving them. These two were fertile and contained live embryos, one of which measured 29 mm. in over-all length. One of the remaining eggs was 16.5 × 10 mm. Female was present with the eggs.
July 25, 1951. Eggs still present in the nest cavity; female not in evidence, but might have been concealed in corner of nest chamber as it was not disturbed.
July 28, 1951. Female was again found with the eggs. One or more of the seven remaining eggs were punctured in moving them during their examination. Eggs about 16 × 10 mm.
August 3, 1951. Female was in nest with the eggs some of which are slightly indented from drying.
August 6, 1951. When rock was turned, female darted out and ran to cover about ten feet away. The eggs had hatched but two young remained in the nest cavity, still rather slow and feeble in their movements and not yet fully active. When routed from cover a second time, the female ran back to the nest rock and took shelter beneath it.
No. 4. On upper slope above ledge, under a rock 18 × 9 inches, in site shaded most of day; burrow nearly concealed beneath rock.
June 24, 1951. Nest occupied by a gravid female, apparently ready to lay.
June 30, 1951. Rock covering this nest has been undermined by a mole tunnel, and many nearby rocks are undermined also. The eggs were almost certainly destroyed by the mole’s tunneling and may have been eaten by it, since no remains are in evidence.
No. 5. At hilltop ledge beside old abandoned road, beneath flat rock nine inches in diameter and about 11⁄2 inches thick, shaded for first half of morning and most of afternoon, but exposed to mid-day sunshine.
June 29, 1951. Standing water in bottom of nest chamber 11⁄2 inches below underside of the rock. Some of the eggs are more than half submerged. One egg is 14 × 8 mm.
July 21, 1951. Entrance of abandoned nest burrow has been enlarged by running water channelled through in run-off during and after heavy rains; shrivelled remains of eggs present at the bottom of the burrow.
No. 6. On grassy hilltop a few yards from ledge under flat rock, 9 × 6 × 2 inches.
July 23, 1951. Large female (snout-vent length 75 mm.) with three eggs, 16 × 22 mm.
July 27, 1951. Female escaped from nest cavity as rock was raised. Three eggs were still in the nest, and a young skink was partly emerged from one. A second egg not yet hatching was somewhat flaccid, 16 mm. long, heavily coated with dried mud. The third egg much shrivelled, was opened and found to have a dead fetus, perhaps a week short of hatching.
July 28, 1951. The flat rock which formerly covered the nest cavity was found to have been raised and displaced, and no trace of the female, eggs or young remained. Of possible predators that might have moved the rock and destroyed the nest, skunk and opossum seemed the most likely, but there was no definite clue as to the predator’s identity.
No. 7. Two feet northeast of pond rock pile, under rock about one foot square on upper surface with maximum thickness of about eight inches, lying with upper side at 45-degree angle. The nest was under one edge, with approximately three inches of rock over it. The rock was exposed to sunshine throughout the day, except for grass shading its edges.
July 23, 1951. When rock was turned, the female darted out of the nest cavity, but in her dash to escape she dropped into a nearby pitfall. When handled, she voided feces which contained the nearly intact shell of a skink egg. Six eggs present in the nest; one selected as typical was 111⁄2 × 8 mm. The eggs were slightly misshapen and might have been damaged from drying.
July 26, 1951. When rock was raised, female darted out and escaped. The six eggs still remained in the nest.
August 2, 1951. When rock was raised the female was not in evidence, and only three eggs could be found; they had fallen from the nest cavity to the bottom of the depression where the rock was imbedded and were somewhat dried and indented.
No. 8. North slope, beneath rock approximately 18 × 15 × 4 inches, at edge of small gully, where shaded most of the time including mid-day hours.
July 20, 1951. Female attempted to escape from the nest. Four eggs visible in nest, one 151⁄2 × 10 mm.
July 25, 1951. When rock was raised the female ran from the nest.
July 27, 1951. When rock was raised the female was in the nest with the eggs; she ran and hid beneath a boulder five feet away. After a few minutes she emerged and ran 15 feet to a hickory sapling and climbed it.
July 28, 1951. Female was not in the nest but the four eggs were still present.
July 30, 1951. Female found dead and partly eaten by ants beside rock one foot from nest; eggs still present in the nest.
July 31, 1951. Eggs still present in the nest.
August 3, 1951. Eggs still present, including some deep in the nest cavity which apparently were overlooked previously.
August 6, 1951. One much indented egg found outside the nest cavity was opened and found to contain a live fetus, seemingly fully developed and normal. The opened egg was placed on damp soil in a shady place near the nest, but two hours later the hatchling had been killed and partly eaten by swarms of ants.
August 9, 1951. The remaining eggs had disappeared, evidently taken by a predator as no empty shells remained to indicate that the young had hatched.
Cagle (1940:229 and 232) has graphically described and illustrated the hatching of the five-lined skink, and numerous observations in the present study have served to corroborate his description. The first indication that the time of hatching is at hand is a twitching [73] or jerking movement within the egg which continues until the shell is slit. According to Noble and Mason (1933:5) the shell is slit with the elongate premaxillary egg tooth which has its distal third bent forward nearly at right angles to its base. Some young remain for an hour or more with only the snout visible, however, once the head is extruded it is not again withdrawn unless the lizard is badly startled. The eyes are opened and blinked slowly, closed for a few minutes, and opened again. After the eyes have become adjusted, the fore-body emerges and the front legs are freed. In one clutch, observed by Cagle, hatching time for individual eggs varied from 45 minutes to five and three-fourths hours. If startled by visual or tactile stimuli, the little skink may lunge forward through the slit shell, with a sudden straightening of its body, and rush away for several inches. Its movements are slow, stiff and clumsy as compared with those of a skink that is a few days old and fully active. Hatching of a clutch ordinarily extends over 24 hours or more. Some of the young may be fully hatched and active before others from the same clutch have slit their eggshells.
Eggs ready to hatch ordinarily weigh somewhat more than one gram, up to at least as much as 1.7 grams, but much of this weight is made up of water absorbed during incubation. The hatchlings usually weigh from .2 to .45 grams. For each of two eggshells recently vacated, that were washed and squeezed dry, weights were approximately .125 grams. Hatchlings of the same brood differ perceptibly in size with several per cent variation in total length, and weight. Some seem to be less fully developed than others. On July 8, 1952, hatching of the last young in a clutch was observed. Upon emergence, it differed in appearance from the others of the brood hatched a few hours earlier. The top of its head bulged slightly as in fetuses. The umbilicus was not yet closed, and the protruding yolk mass hindered the hatchling’s movements and made crawling difficult. In order to progress it had to stand high off the ground to prevent its ventral surface from dragging. Protrusion of the yolk mass has been described in newly emerged hatchlings for the closely related E. anthracinus (Clausen, 1938:3-7) as well as in fasciatus. Cagle (loc. cit.) states that the mass of yolk is at first about 3 mm. in diameter, but is completely used at the end of the third day. A group of young retained by him, without food, died the sixth day after hatching, seemingly from starvation. Three of five recently hatched young were found by Cagle to have eaten ant pupae placed in a box with them on the preceding day, even though the skinks still retained the yolk masses. One hatchling of this group [74] ate its own tail that had been broken off in handling. Cagle described a color change taking place during the first few hours after hatching; the ground color, dull greenish at first, darkens to an iridescent black, the pale stripes are altered from an original tan color to bronze, with a tinge of reddish on the head, and the ventral surface which is partially transparent showing the outlines of the internal organs at first, soon becomes opaque white.
Contrary to the statement by Noble and Mason (1933:5) that in captivity the hatchlings seldom stayed together more than a few hours, litters of young fully active, a day or two after hatching were found in the nests with the females still looped around them on several occasions. On one such occasion, although the brood scattered immediately into surrounding vegetation where they hid, I succeeded in catching the female and six of the young, and put them all together in a nylon bag to carry them back to the laboratory. Several hours after the bag had been placed on a table it was noticed that the family had again gathered into a compact cluster in the bag with the female’s body looped around the young in the characteristic brooding position seen in those with young or eggs in their nest cavities. When hatching is complete, the female may leave before the young have dispersed. On August 5, 1950, a nest under observation was found to have all of the young or most of them still clustered in the cavity, but the female was not in evidence. The young were active, and immediately took alarm as the rock was raised exposing them. Almost instantly, they scattered and vanished. Subsequent search revealed five of the young, each poorly concealed in tufts of grass or under dry leaves or other ground litter at the edges of the depression where the rock had lain. Once hidden, these young were reluctant to run again and depended on concealment.
Having once left the nest, the young probably do not return to it, as many nests examined within a few days after hatching were never found occupied either by females or young after their original dispersal. As soon as the dispersal occurs family ties are permanently severed. On July 19, 1950, a group of active hatchlings was observed moving about over a log, on what was probably the first day of activity away from the nest. The log was in the bottom of a steep-walled gully, where it had come to rest the night before. It had been an erect but dead and partly undermined snag on the edge of the gully, and was blown down that night in a violent thunderstorm. Most of the log was held clear of the rushing water in the bottom of the gully by projecting limbs. The little skinks [75] were darting in and out of holes and crevices in the log, pausing frequently to bask. As many as four were in sight simultaneously, but probably the total included several more, as it was difficult to keep track of individuals. An adult female, presumably the mother of the litter was also present, but she took no interest in the young, and they showed no evidence of dependence on her. On the contrary, several times when one or another of the young happened to come near the female in the course of its wandering, and noticed her, it was seen to shy away in sudden alarm.
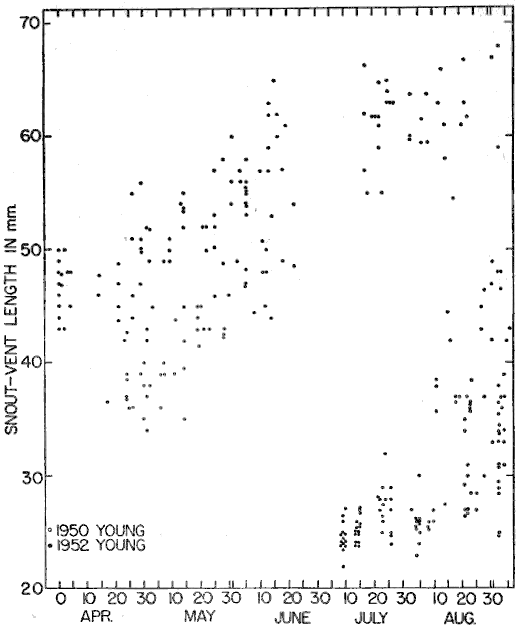
Fig. 11. Sizes on specific dates of young hatched in 1950 and 1952. Approximate size ranges at different times of year, and differences in trend between the two years are brought out.
The young were much more active than the female. These and other young observed in the open were almost constantly in motion. Pauses to bask at any one spot were of only a few seconds duration. A certain log in Skink Woods evidently was the site of one or more successful skink nests each year that observations were made, although a nest was actually found in it only in 1948. On July 26, 1950, recently hatched young were active on this log. Temperature was about 22°C. and the young were alternating frequently between shade and sunshine to maintain their body temperature. Collectively they seemed to cover every square inch of the log surface, poking and probing into niches, crevices and insect borings. They had a tendency to seek out the highest points on the log as resting places.
In moving about, foraging or sunning, the young often carry the tail arched high, and keep it in motion with slow squirming undulations. These undulations may be continued even when the lizard itself has come to rest momentarily. The movements of the tail together with its vivid blue color serve to attract attention to it. Such behavior has not been observed in adults or partly grown young. Jopson (1938:90) observed an instance in which two dogs cornered a young five-lined skink (either the present species or E. laticeps) but were distracted by the wriggling of its bright blue tail “either dropped by autotomy or knocked off” so that the skink itself was allowed to escape. On another occasion these same two dogs attacking an adult male skink, were not distracted by the wriggling but dull colored broken tail, and they killed the lizard.
The subject of growth in Eumeces was briefly discussed by Taylor (1936:66) in his revision of the genus. Sorting fairly large series of museum specimens into seeming age-size groups, Taylor concluded that skinks require as much as 9 or 10 years to attain adult size. For fasciatus, for instance, the snout-vent length of 65.7 mm. (small adult size) was considered typical of individuals in their ninth year of life, with yearly gain of only 6 or 7 mm. in length in the young. I have seen the original data on which this conclusion was based, and the age groupings, as assigned by Taylor, seemed plausible. However, in the light of present knowledge, it is certain that the seeming intervals between his assumed age groups would have disappeared with a still larger series of specimens. The eight or nine size groups that Taylor recognized as distinct annual age groups actually comprise only two age groups, each having such [77] wide dispersion of individuals (by retardation of some and acceleration of others) that there is overlapping in size between them.
Growth in reptiles is now much better understood. Many species have been studied by a variety of methods, including observation of growth in captives, recording of growth in marked individuals living under natural conditions, and sorting of large series into age-size groups. Two species of Eumeces have been studied in some detail. Breckenridge (1943:601-602) marked all the individuals of septentrionalis that could be found in a small colony in Minnesota and he concluded from the growth recorded in several that were recaptured, that these skinks grow to mature size (65 mm. and larger) at the end of their second year of life and are ready to breed the following spring. Rodgers and Memmler (1943:61) plotted the size distribution of a large year-round collection of skiltonianus from near Berkeley, California. They found that in this species hatching occurs in July and August, hatchlings are about 25 mm. in snout-vent length, and grow to about 50 mm. by the time they are one year old, and to about 65 mm. at two years of age, but most of them breed at the end of their third year. Within the genus the species septentrionalis and skiltonianus belong to groups separate from each other and from that including fasciatus. While septentrionalis and skiltonianus resemble each other in their growth pattern and in the time required to reach sexual maturity, fasciatus is notably different in its more rapid growth and the shorter time it requires to reach breeding maturity. This would scarcely be expected, as all three are of similar size. Furthermore, skiltonianus in the region of Rodgers’ and Memmler’s study has a longer growing season than fasciatus in northeastern Kansas, while septentrionalis in Minnesota has a growing season markedly shorter than either. It is noteworthy that each of these three skinks is the northernmost lizard in the section of the country where it occurs.
In the present study growth was investigated by measuring and marking large numbers of young, many of which were recaptured for subsequent records, and by sorting into age-size groups all available measurements. An understanding of the latter set of data was facilitated by correlating it with the growth records of marked individuals. Changes in the phenology of growth from year to year according to weather conditions were noted.
As already indicated, hatching occurs from early July to mid-August in northeastern Kansas. Unseasonably cool weather with frequent rains may cause cumulative delay in breeding and incubation so that hatching may average several weeks later than it does [78] in years with relatively warm and dry weather during the breeding season. Within any one year hatching time is concentrated, so that the majority of the young hatch within a period of two weeks, but microclimates in the situations where the nests are made may differ enough to cause this much spread. Individuals living on north slopes in thick woods, and receiving the minimum amount of sunlight may have their emergence from hibernation and attainment of breeding condition delayed. Later, nesting in the same situations, they may have incubation of their clutches similarly delayed.
Newly hatched young average just under an inch in snout-vent length (23-27 mm.) and weigh .2 to .45 grams. Most rapid growth occurs in the period of weeks following hatching. The growth rate during this late summer period cannot be well shown by comparing average size of series taken on successive dates, because each series is likely to include some newly hatched young.
In 1949, a series of recently hatched young averaged 26.7 mm. on July 10. By August 26, average length in a series collected was 42.9 mm., indicating an average gain of at least .35 mm. per day. One that may be considered typical was marked on July 23, 1950, soon after hatching, and it had a snout-vent length of 26.5 mm. and weighed .25 grams. It was recaptured just a month later when it had grown to 36 mm. snout-vent length, and weighed .8 grams. Potential growth rate under favorable conditions is shown by the fact that some individuals have attained a snout-vent length of 50 mm. by the third week of August, thus approximately doubling their hatching length. A maximum growth rate of about .5 mm. per day is indicated for these accelerated individuals, but on the average, young are considerably less than 50 mm. in length even when they enter hibernation. At the other extreme, representing retarded growth, is an individual having a snout-vent length of only 34 mm. on May 1. It must have been approximately nine months old on that date, but of course had spent at least six months in hibernation. Even if it made rapid growth subsequently, this yearling could scarcely have attained by midsummer the pre-hibernation length of the most accelerated individuals.
During the growing season following their first hibernation period, the young grow to small adult size in most instances. After emerging from a second hibernation they mature sexually and constitute an important part of the breeding population.
Many of the skinks marked before their first hibernation, as hatchlings, when they were a few days or a few weeks old, were subsequently recaptured as well-grown yearlings or small adults, [79] affording ample information as to the usual growth rate and the extremes of acceleration or retardation that occasionally occur. Records of selected individuals in this group of skinks, marked early in life and recaptured after a hibernation, are recorded below.
Table 8. Records of Individual Skinks Marked as Hatchlings (Before the First Hibernation) and Recaptured the Following Year. Rapid Rate of Early Growth Is Shown.
| Date | Snout-vent length in mm. | Tail length in mm. | Weight in grams | Remarks | |||
| No. 1. | August 8, | 1951 | 23 | 1⁄2 | 301⁄2 | .25 | Had just hatched when first recorded; second capture was made soon after emergence from hibernation. All three captures within a 50-foot diameter. |
| April 28, | 1952 | 39 | 55 + 1⁄2 | 1.3 | |||
| June 7, | 1952 | 48 | 69 + 1 | .... | |||
| No. 2. | July 8, | 1952 | 25 | 25 (broken stub) | .3 | ||
| April 23, | 1953 | 42 | 17 + 26 | .... | |||
| June 23, | 1953 | 56 | 22 + 36 | .... | |||
| No. 3. | July 16, | 1948 | 26 | 1⁄2 | 37 | .... | Caught at the same place on both occasions; in a little less than a year this female grew to small adult size. |
| July 5, | 1949 | 68 | 1011⁄2 | .... | |||
| No. 4. | August 23, | 1950 | 36 | 55 | .9 | The interval between captures included about two months of active life, plus the hibernation period; caught at the same place on both occasions. | |
| May 19, | 1951 | 46 | 691⁄2 | 1.7 | |||
| No. 5. | September 2, | 1950 | 34 | 1⁄2 | 33 (broken stub) | .... | Tail broken at first capture; recaptured 40 feet from original location. |
| June 12, | 1951 | 45 | 48 + 3 | 2.0 | |||
| No. 6. | July 28, | 1949 | 36 | 56 | .... | Recaptured 75 feet from original location. | |
| April 21, | 1950 | 49 | 83 | 2.5 | |||
| No. 7. | August 31, | 1951 | 38 | 58 | .... | All three captures within a 70-foot diameter. | |
| May 25, | 1952 | 48 | 82 | .... | |||
| June 30, | 1952 | 63 | 1⁄2 | 57 + 26 | .... | ||
| No. 8. | August 23, | 1950 | 36 | 44 (broken stub) | .7 | Tail broken at first capture. Capture sites 150 feet apart. | |
| July 23, | 1951 | 69 | 37 + 49 | .... | |||
| No. 9. | August 23, | 1949 | 39 | 531⁄2 (regenerated) | .... | This male was retarded in growth, being still well short of small adult size as its second hibernation period approached; all four captures recorded within a few yards. | |
| June 7, | 1950 | 46 | 701⁄2 (regenerated) | 2.1 | |||
| July 23, | 1950 | 58 | 88 (regenerated) | 3.7 | |||
| September 3, | 1950 | 62 | 91 (regenerated) | 4.9 | |||
| No. 10. | July 31, | 1949 | 38 | 23 (broken stub) | .... | Capture sites 20 feet apart. | |
| June 17, | 1950 | 58 | 43 + 36 | 3.6 | |||
| No. 11. | August 13, | 1949 | 40 | 66 | .... | Approximately a year after its original record this skink was recaptured 80 feet away, still short of small adult size. | |
| August 8, | 1950 | 63 | 90 (regenerated) | 5.0 | |||
| No. 12. | August 19, | 1949 | 42 | 40 (broken stub) | .... | All three captures within a 50-foot diameter. |
|
| June 13, | 1950 | 58 | 1⁄2 | 58 + 28 | 4.1 | ||
| July 5, | 1950 | 63 | 62 + 31 | 5.9 | |||
Many other young were not caught and marked until the growing season following their first hibernation, and were recaptured within this second growing season weeks or months after they were originally marked, and after they had made substantial growth. Those recaptured near the end of this second growing season, when they were a year old, or a little more, usually had attained small adult size or were nearing it. Selected records of these yearlings are presented below.
Table 9. Selected Records of Individual Skinks Marked as Yearlings (After Emergence From the First Hibernation) and Recaptured One or More Times the Same Year. Rapid Growth Is Shown.
| Date | Snout-vent length in mm. |
Tail length in mm. |
Weight in grams |
Remarks | |||
| No. 1. | May 2, | 1951 | 38 | 531⁄2 | .... | Capture sites 30 feet apart. | |
| September 25, | 1951 | 62 | 25 + 31 | .... | |||
| No. 2. | May 8, | 1951 | 39 | 57 | .... | Capture sites 150 feet apart. | |
| August 2, | 1951 | 60 | 67 + 25 | .... | |||
| No. 3. | April 17, | 1952 | 39 | 55 | 1.1 | Capture sites 30 feet apart. | |
| June 23, | 1952 | 57 | 73 (regenerated) | .... | |||
| No. 4. | May 20, | 1952 | 45 | 67 | .... | Capture sites 15 feet apart. | |
| May 28, | 1952 | 47 | 71 | .... | |||
| June 9, | 1952 | 53 | 82 | .... | |||
| No. 5. | May 22, | 1952 | 48 | 1⁄2 | 771⁄2 | 2.0 | Capture sites 10 feet apart. |
| July 20, | 1952 | 63 | 106 | 5.3 | |||
| No. 6. | June 11, | 1950 | 49 | 49 (broken stub) | 2.4 | Capture sites 20 feet apart. | |
| September 2, | 1950 | 63 | 63 + 31 | 4.9 | |||
| No. 7. | April 14, | 1950 | 47 | 72 | 1.9 | Capture sites 50 feet apart. | |
| May 29, | 1950 | 50 | 821⁄2 | 2.5 | |||
| No. 8. | May 12, | 1952 | 49 | 77 | .... | Capture sites 60 feet apart. | |
| June 18, | 1952 | 61 | 1⁄2 | 98 | .... | ||
| No. 9. | June 4, | 1950 | 54 | 89 | 2.8 | Both captures at same site. | |
| August 1, | 1950 | 64 | 1⁄2 | 101 (broken stub) | 5.7 | ||
| No. 10. | June 11, | 1950 | 49 | 49 (broken stub) | 2.4 | Capture sites 20 feet apart. | |
| September 2, | 1950 | 63 | 63 + 31 | 4.9 | |||
| No. 11. | June 13, | 1949 | 57 | 68 (regenerated) | .... | ||
| August 8, | 1949 | 70 | 37 + 11 | .... | |||
Adult skinks can be found in greatest numbers in the breeding season and many of the young that were marked were recaptured as newly matured breeding adults soon after their second hibernation, often still short of average adult size. Selected records of such individuals are presented below.
Table 10. Records of Individual Skinks Marked as Young and Recaptured as Adults.
| Date | Snout-vent length in mm. |
Tail length in mm. |
Weight in grams |
Remarks | ||
| No. 1. | Male | Probably less than a month old at first capture 21 months later and 185 feet away, he had red facial suffusion already somewhat faded as the breeding season waned. | ||||
| August 21, 1950 | 34 | 48 | .7 | |||
| May 30, 1952 | 69 | 37 + 49 | .... | |||
| No. 2. | Male | All three captures within a 70-foot diameter. | ||||
| July 31, 1949 | 39 | 64 | .... | |||
| August 22, 1949 | 47 | 75 | .... | |||
| May 19, 1951 | 73 | 69 (regenerated) | .... | |||
| No. 3. | Male | Capture sites 10 feet apart. | ||||
| August 5, 1949 | 36 | 57 | .... | |||
| May 3, 1951 | 67 | 103 | 5.1 | |||
| No. 4. | Male | Capture sites 535 feet apart. | ||||
| June 16, 1951 | 44 | 41 (broken stub) | .... | |||
| May 28, 1952 | 63 | 77 (regenerated) | .... | |||
| No. 5. | Male | Capture sites 100 feet apart. | ||||
| April 12, 1950 | 45 | 73 | 1.9 | |||
| May 1, 1951 | 67 | 17 + 48 | .... | |||
| No. 6. | Male | This individual had attained approximately average adult size by the 1951 breeding season; all three captures were within a distance of 90 feet. | ||||
| April 12, 1950 | 46 | 4 + 15 | 1.3 | |||
| August 10, 1950 | 67 | 75 (regenerated) | 5.3 | |||
| May 12, 1951 | 71 | 77 (regenerated) | .... | |||
| No. 7. | Male | |||||
| April 30, 1950 | 48 | 1⁄2 | 781⁄2 | 2.4 | ||
| June 15, 1950 | 56 | 94 | 2.9 | |||
| May 19, 1951 | 67 | 90 (broken stub) | .... | |||
| No. 8. | Male | Capture sites 450 feet apart. | ||||
| May 3, 1950 | 47 | 51 + 4 | 1.7 | |||
| May 29, 1951 | 75 | 115 (regenerated) | .... | |||
| No. 9. | Male | Capture sites 90 feet apart. | ||||
| June 2, 1949 | 51 | 46 (broken stub) | .... | |||
| May 2, 1950 | 66 | 1⁄2 | 311⁄2 + 51 | 7.0 | ||
| No. 10. | Male | Capture sites within 40 feet. | ||||
| May 20, 1950 | 58 | 921⁄2 | 4.0 | |||
| June 21, 1950 | 61 | 95 | 4.7 | |||
| August 21, 1950 | 70 | 108 (broken stub) | 7.2 | |||
| No. 11. | Male | |||||
| June 25, 1950 | 62 | 100 | 5.1 | |||
| May 1, 1951 | 71 | 113 | 7.1 | |||
| No. 12. | Female | Capture sites 160 feet apart. | ||||
| April 15, 1950 | 46 | 1⁄2 | 731⁄2 | 1.5 | ||
| May 20, 1951 | 72 | 113 | .... | |||
| No. 13. | Female | Capture sites 20 feet apart. | ||||
| June 11, 1950 | 51 | 69 | 2.5 | |||
| May 25, 1951 | 66 | 40 | .... | |||
| No. 14. | Female | Capture sites 20 feet apart. | ||||
| June 6, 1949 | 52 | 47 (regenerated) | .... | |||
| May 20, 1950 | 68 | 1⁄2 | 69 (regenerated) | 7.5 | ||
| June 9, 1950 | 71 | 71 (regenerated) | .... | |||
| No. 15. | Female | Capture sites 20 feet apart. [82] | ||||
| July 2, 1950 | 60 | 100 | 4.2 | |||
| May 21, 1951 | 74 | 33 + 35 | .... | |||
| No. 16. | Female | Capture sites 35 feet apart. |
||||
| June 12, 1950 | 57 | 83 | 3.1 | |||
| May 1, 1951 | 71 | 1⁄2 | 53 (broken stub) | 6.4 | ||
| No. 17. | Female | This female probably hatched in July 1948 and was nearing adult size when first caught at an age of a little less than a year. By the next breeding season it was an average sized adult; both captures at same site. | ||||
| June 22, 1949 | 62 | 24 (broken stub) | .... | |||
| May 22, 1950 | 72 | 27 + 7 | 9.0 | |||
| No. 18. | Female | This female probably was approximately a year old when first caught, and she grew to average adult size by the next spring; both captures at same site. | ||||
| July 4, 1950 | 64 | 30 + 55 | 4.3 | |||
| May 23, 1951 | 73 | 31 + 62 | .... | |||
| No. 19. | Female | This female was about a year old when first captured; loss of weight in July 1951 was caused by its laying a clutch of eggs. All three captures were within a 15-foot diameter. | ||||
| July 5, 1950 | 61 | 1⁄2 | 921⁄2 (regenerated) | 4.7 | ||
| June 14, 1951 | 73 | 111 (regenerated) | 8.2 | |||
| June 29, 1951 | 74 | 106 (regenerated) | 5.0 | |||
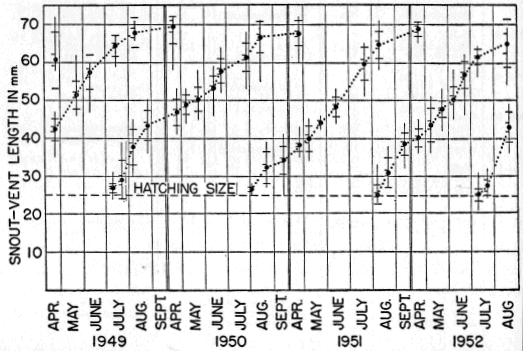
Fig. 12. Sizes of immature skinks of successive annual broods, grouped in biweekly or monthly intervals, with mean, standard error, standard deviation, and extremes shown for each group.
A certain small percentage fail to attain minimum adult size or breeding maturity by the time of emergence from their second hibernation. Among 77 individuals marked as young either soon after hatching or in spring and early summer, and recaptured the following spring, only one had failed to grow to adult size. It was 46.5 mm. in length when marked on June 13. When recaptured on April 25 of the following year, it had grown to a length of 59 mm., still short of minimum adult length. During the interval between captures it had maintained about the average growth rate. Its failure to attain maturity was obviously the result of its early retardation, and probably late hatching was primarily responsible. Although this is the only individual with known history, which failed to attain breeding maturity after its second hibernation, occasional specimens are taken in spring which are somewhat below adult size but seem too large to be young hatched the preceding summer. Obviously, the incidence of such failure from year to year would be influenced by weather conditions, and an unusually cool summer may result in such delayed laying and hatching that an unusually large proportion of young might fail to attain sexual maturity at the usual time. At more northern localities, the percentage of such failures might be expected to increase. At the northern edge of the range attainment of breeding maturity may normally require more than two years. Such delayed development would result in a drastic reduction of the reproductive potential which might be critically limiting to the species, even in an otherwise favorable environment, as the population would be unable to replace rapidly enough the individuals eliminated by normal mortality factors.
In contrast to the delayed development of those that have failed to attain maturity at an age of two years, is the accelerated development of those that have already more than doubled in length before the first hibernation, and continue to grow rapidly after emergence. By late spring they are already approaching adult size, perhaps even before laying has occurred, and while breeding is still in progress. It is certain that in northeastern Kansas there is no breeding by such accelerated individuals approaching adult size at an age of nine or ten months. Farther south in the species’ range with a much longer growing season, there is perhaps some possibility of such early breeding by first-year individuals. This would reduce by more than half the length of time required for a generation, and would tremendously increase the reproductive potential. With such added impetus to its reproduction the species might be able to withstand greatly increased predation pressure, or other mortality factors.
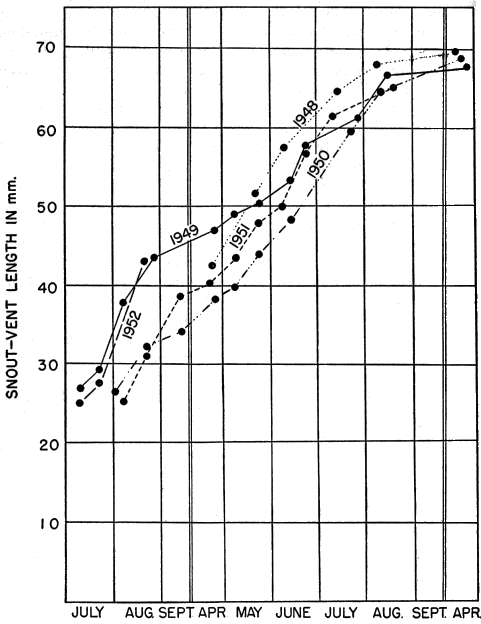
Fig. 13. Growth curves of successive annual broods (designated by the year of hatching), superimposed to bring out differences in trends resulting from changes in weather from year to year.
Extremes of acceleration or retardation are relatively rare in the population studied. Nevertheless, in April there are some individuals between 50 and 60 mm. in snout-vent length which cannot be classified with certainty as to their age group, and might be either accelerated individuals about nine months old or retarded individuals about 21 months old.
The spread in size for any given age group is especially large, if data from different years are combined. A typical individual, having a snout-vent length of 25 mm. at hatching in mid-July may have attained 30 mm. by early August, 35 mm. by late August, and 45 mm. by the time it hibernates late in September. Emerging shortly before the middle of April it may grow to 50 mm. by the end of May, 58 mm. by the end of June, and more than 60 mm. by the end of July when it is a little more than a year old. By the time of its second hibernation it may have attained a length of from 65 mm. to 70 mm., and emerges from this hibernation as a breeding adult.
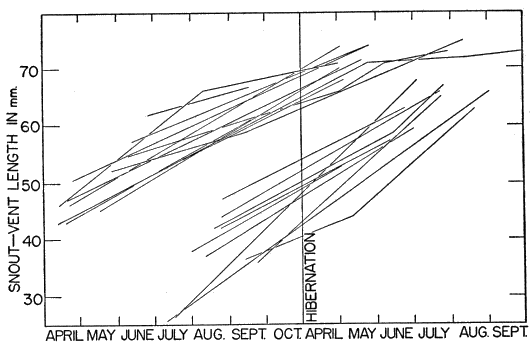
Fig. 14. Records of growth of immature individual skinks, both hatchlings and yearlings, that were marked in one year and recaptured the next.
In reptiles in general there is a wide range in adult size, and the extent and rapidity of continued growth after attainment of sexual maturity and minimum adult size is still insufficiently understood. Information bearing on this problem was obtained in the present study from the recapture of marked skinks already measured as adults. It is evident that the growth rate of the young, amounts to as much as 15 mm. per month in snout-vent length in the late summer period from hatching until hibernation, averages perhaps three or four mm. per month in the summer after emergence from the first hibernation, and tapers off rapidly as adult size is approached.
One hundred of the skinks marked as adults or subadults and recaptured after intervals of months, including, in most instances, one or more hibernation periods, represent in the aggregate, 87 years of [86] growth. These records show that after minimum adult size of 65 mm. is attained, growth slows abruptly, and that by the time a length of approximately 75 mm. is attained in most instances growth has become extremely slow in males and has virtually stopped in females. Males attain a maximum size several millimeters larger than that of females. Individuals differ greatly in their growth, however; some adults continue to grow rapidly till they near the maximum size, whereas others apparently stop growing when they are still below average adult size. Unusually large specimens are not necessarily old, but may have attained their size only a year or two after reaching maturity through the accelerated growth resulting from abundant food and predisposing genetic factors. Likewise, unusually old individuals are not necessarily the largest, but may be only a little above average adult size. It may be assumed that no growth occurs during the period of winter dormancy, which occupies approximately half the year in the population studied. To compute growth rates, in those recaptured after an intervening hibernation, periods of hibernation, arbitrarily estimated as six months, were subtracted from the time elapsed between captures.
Table 11. Average Growth Rate in a Selected Sample of Skinks of Adult Size.
| Size Group | Males | Females | ||
| Average growth mm. per month | Number of skinks in sample | Average growth mm. per month | Number of skinks in sample | |
| 65-68 mm. | 1.4 | 11 | .8 | 12 |
| 69-72 mm. | .7 | 12 | .4 | 21 |
| 73-76 mm. | .7 | 13 | .3 | 21 |
| 77-80 mm. | .4 | 7 | .... | .... |
Opportunity to compare the rapid growth of young during their first year of life with the relatively slow continued growth after attainment of sexual maturity is afforded by the records of skinks caught and marked while yet immature and recaptured in two or more successive years after their attainment of sexual maturity. The records of selected individuals of this group are presented below. With the exception of number three, all in this series are of the 1949 brood, and probably all hatched within a two-week period.
Table 12.—Records of Individual Skinks Marked as Young and Recaptured Repeatedly After Attainment of Adult Size, Showing Trend of Progressively Slowing Growth.
| Date | Snout-vent length in mm. | Tail length in mm. | Weight in grams | Remarks | |||
| No. 1. | Male | At an age of 33 months this male was far short of maximum size, and smaller than some males a year younger; all four captures within a 65-foot diameter. | |||||
| April 12, | 1950 | 43 | 71 | 1.5 | |||
| August 30, | 1950 | 56 | 56 + 21 | 5.4 | |||
| May 23, | 1951 | 68 | 59 + 32 | .... | |||
| April 28, | 1952 | 73 | 62 + 38 | 6.6 | |||
| No. 2. | Male | At an age of approximately one year this male was approaching small adult size; when last captured at an age of 34 months, he was a large adult. All five records within a 190-foot diameter. | |||||
| July 5, | 1950 | 61 | 921⁄2 (regenerated) | 5.2 | |||
| July 28, | 1950 | 64 | 97 (regenerated) | 5.4 | |||
| May 3, | 1951 | 68 | 96 (broken stub) | 5.8 | |||
| June 21, | 1951 | 72 | 1011⁄2 (regenerated) | .... | |||
| May 1, | 1952 | 78 | 101 (regenerated) | .... | |||
| No. 3. | Male | This skink was nearly a year old and nearing adult size when first captured; recaptured in each of the four succeeding years, he showed slowing growth. He was near the maximum size at the time of his last capture when he was about 57 months old, and evidently had stopped growing (for movement see No. 2, p. 110). | |||||
| June 22, | 1949 | 65 | 111 | .... | |||
| May 4, | 1950 | 72 | 1⁄2 | 115 | 7.3 | ||
| June 17, | 1950 | 73 | 116 | 7.8 | |||
| May 15, | 1951 | 80 | 125 | .... | |||
| May 13, | 1952 | 82 | 125 | .... | |||
| April 6, | 1953 | 82 | 104 (regenerated) | .... | |||
| No. 4. | Female | This individual, marked when less than two weeks old, had grown to nearly the maximum female size at an age of 34 months; all four captures within a 175-foot diameter. | |||||
| July 13, | 1949 | 27 | 341⁄2 | .... | |||
| June 1, | 1950 | 54 | 1⁄2 | 931⁄2 | 3.1 | ||
| August 21, | 1951 | 74 | 119 | .... | |||
| May 1, | 1952 | 76 | 123 | 10.0 | |||
| No. 5. | Female | All six records within a 65-foot diameter (See Figure 21). |
|||||
| April 15, | 1950 | 43 | 70 | 1.4 | |||
| June 5, | 1950 | 52 | 1⁄2 | 87 | 2.8 | ||
| May 25, | 1951 | 71 | 82 + 29 | .... | |||
| September 28, | 1951 | 73 | 111 (regenerated) | .... | |||
| April 26, | 1952 | 74 | 113 (regenerated) | 7.4 | |||
| April 24, | 1953 | 76 | 114 (regenerated) | .... | |||
| No. 6. | Female | Hatched in July 1949, this skink had attained the maximum female size at an age of a little more than three years; (for movement see Figure 25). | |||||
| April 21, | 1950 | 46 | 75 | 2.1 | |||
| May 7, | 1950 | 48 | 15 (broken stub) | 2.0 | |||
| May 3, | 1951 | 74 | 29 + 57 | 8.5 | |||
| May 2, | 1952 | 78 | 25 + 64 | .... | |||
| August 27, | 1952 | 79 | 1⁄2 | 95 (regenerated) | 8.3 | ||
| No. 7. | Female | Hatched in July 1949, this skink was 11 months old and about half-grown when it was marked. When last caught at an age of 35 months it was of average adult female size, having grown less than numbers 4 and 6 at the same age. All five captures were within a 60-foot diameter (Fig. 24). | |||||
| June 5, | 1950 | 51 | 82 | 2.5 | |||
| July 13, | 1950 | 59 | 93 | 3.9 | |||
| July 29, | 1950 | 64 | 98 | 4.4 | |||
| August 21, | 1951 | 69 | 80 (broken stub) | 5.0 | |||
| May 28, | 1952 | 73 | 83 + 91⁄2 | .... [88] | |||
| No. 8. | Female | Hatched in July 1949, this skink was of average adult female size and was breeding in May 1951; it grew nearly to maximum female size in the next 11 months. All captures | |||||
| April 26, | 1950 | 50 | 1⁄2 | 781⁄2 | 2.7 | ||
| May 24, | 1951 | 74 | 107 (regenerated) | .... | |||
| April 28, | 1952 | 78 | 93 (regenerated) | 8.5 | |||
| April 23, | 1953 | 80 | 93 (regenerated) | .... | |||
| No. 9. | Female | All three captures at the same site. | |||||
| July 5, | 1950 | 60 | 95 | 4.5 | |||
| August 6, | 1951 | 71 | 1061⁄2 | 5.6 | |||
| May 28, | 1952 | 72 | 110 | 8.5 | |||
| No. 10. | Male | Hatched in July 1949, this male grew less rapidly than most, and in the spring of 1953 was smaller than some others that were a year younger, or even two | |||||
| April 23, | 1950 | 46 | 1⁄2 | 66 (regenerated) | 1.8 | ||
| June 13, | 1950 | 52 | 1⁄2 | 26 + 3 | 2.7 | ||
| September 2, | 1950 | 66 | 32 + 51 | 6.2 | |||
| May 29, | 1951 | 67 | 33 + 58 | .... | |||
| August 3, | 1951 | 70 | 94 (regenerated) | .... | |||
| March 27, | 1953 | 74 | 78 (regenerated) | 7.1 | |||
| No. 11. | Female | This skink had attained maximum female size when she was a little less than four years old. | |||||
| April 26, | 1950 | 50 | 1⁄2 | 781⁄2 | 2.7 | ||
| May 24, | 1951 | 74 | 87 | .... | |||
| April 28, | 1952 | 78 | 72 + 21 | 8.5 | |||
| April 23, | 1953 | 80 | 73 + 20 | .... | |||
Differences in their growth rates therefore reflect differences in sex, individual vigor, and local situation, in individuals living at the same time and within the same general environment.
Changing weather, and other factors that vary from year to year cause marked differences in the dates of important events in the annual cycle, and in the stage of development at any given date. Data are available for five successive annual broods of young, those of 1948, 1949, 1950, 1951, and 1952, and each brood differs from the others to some extent, as shown in Figures 11 to 13. In 1949, for instance, young hatched relatively early, and probably most of them were active by the middle of July. They made rapid growth in August, averaging larger than young hatched in other years on any given date in late summer. However, they retired into dormancy early in the fall. Cool and dry weather in early September ended their activity for the season. In 1950, young hatched, on the average, at least three weeks later, about the first of August, but they remained active until late in September, and by hibernation time had partly caught up to the stage of development attained by the young of 1949. Most young of 1951 hatched late in the first half of [89] August, and at first were smaller than those of 1950 and much smaller than those of 1949 on corresponding dates, but favorable weather in the early fall hastened their development. By early September they had caught up and passed the stage of development of young of 1950 and by the time they retired to dormancy in late September, they had reduced by half the size-advantage of the young of 1949 at the time these latter retired into hibernation. The young of 1951 appeared to be few in numbers, and a lack of competition may have been a factor in their rapid early development.
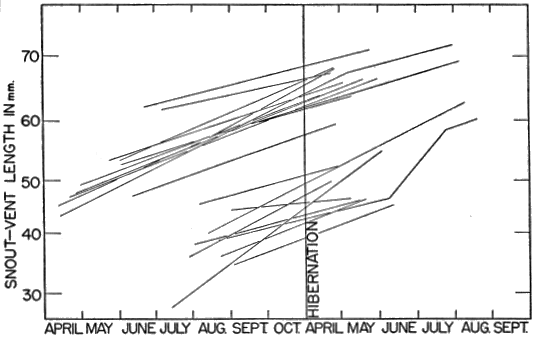
Fig. 15. Records of growth in another group of recaptured young that grew less rapidly than those of Fig. 14.
The young of 1948, first sampled after their emergence from their first hibernation in mid-April of 1949, were then somewhat intermediate in size as compared with those of 1949 and 1950 at the same times of year. Their subsequent development was rapid; by late May they had caught up and passed the stage reached by the 1949 young at the same time of year. The young of 1950 after having a late start, were further set back by cold weather in April 1951 delaying their emergence from hibernation. As a result they were still unusually small in late April and May. Even though they grew rapidly subsequently, they were consistently smaller than those of other broods on corresponding dates. Favorable fall weather prolonging the 1951 growing season into late September beyond the time of retirement in other years may have permitted many of them to attain adult size.
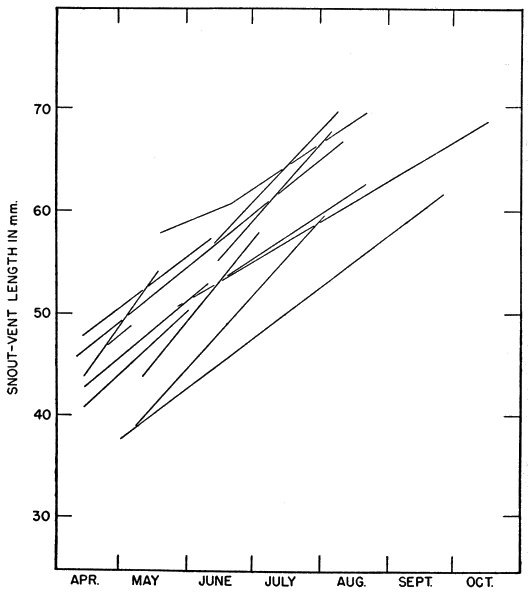
Fig. 16. Records of immature individual skinks marked and recaptured within the same growing season, showing the trend of rapid growth, and differences in growth rate between individuals.
The varying fortunes of the several annual broods studied were closely correlated with weather trends, and suggest possible effects of slight changes in climate. An unfavorable sequence of weather might bring about drastic reduction of the population without causing any direct mortality. A late spring in two successive years would have cumulative effect in delaying emergence and breeding of adults the first year, and delaying in the second year emergence of the young, already retarded by the lateness of their hatching. If this sequence were followed by onset of unusually cool and dry weather in early September, or even in late August, the young might [91] be “caught short,” and forced to hibernate while still in the 50-60 mm. size class. Emerging the following spring, they might have failed to mature sexually, reducing by perhaps half the number of productive adults. At the northern extreme of the species’ range, length of growing season may be more critical than extremes of temperature in limiting the numbers and distribution. Growing seasons that average long enough and warm enough to permit attainment of maturity by onset of the second hibernation period may be essential to the species. While no two annual broods of young in the same locality come under exactly the same weather influences, extremes of retardation or acceleration continuing throughout development are relatively rare. Retarding effects of unfavorable weather causing delayed breeding and hatching, may be offset by prolongation of warm weather in the fall thus delaying hibernation, or by warm spring weather hastening emergence from hibernation.
Under favorable conditions an adult female produces about ten offspring annually of which about half are females. It is calculated that if all survived, after ten breeding seasons, the progeny of an original female might have increased to a population of more than 97,000, under the climatic conditions of eastern Kansas, permitting attainment of breeding maturity late in the second year of life. In the same ten year period under climatic conditions delaying maturity until late in the third year of life (as seems normally to occur in E. septentrionalis and E. skiltonianus, and probably in E. fasciatus at the northern edge of its range) the original female would have produced a population of somewhat less than 7,800 assuming that all survived. With a long growing season such as occurs in the southern part of the range, it seems theoretically possible (though not probable) that individuals might mature before the end of their first year, in time to participate in the next breeding season. If this should occur the original female might produce a population of more than 120 million by the end of the tenth breeding season.
Progressive alteration of the color pattern is more rapid in males than in females and is synchronized with growth. During the first year of life changes in the pattern are gradual, and consist chiefly of loss in vividness. The blue of the tail is slightly dulled. The light lines become suffused with brown and the dorsolateral dark areas become paler, with light brown areas appearing on the corners of the scales and gradually spreading to replace the original black. In skinks that are in the second year of life the striped pattern although [92] still conspicuous is made up of two shades of brown instead of the earlier black and white markings.
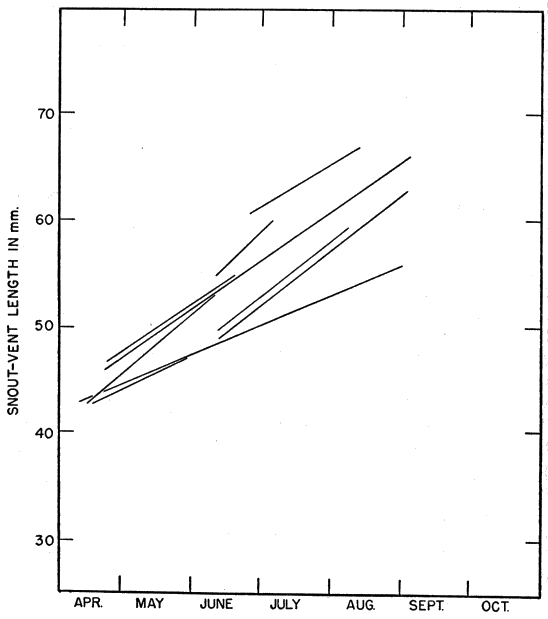
Fig. 17. Records of another group of immature skinks marked and recaptured within the same growing season.
Even in hatchlings, the dorsal part of the rostrum and the inter-nasals are of a somewhat neutral brownish color, matching neither the light lines nor the dark interspaces of the striped body pattern. With advancing age this neutral brown color gradually spreads posteriorly on the head so that the striking lyrate marking of the bifurcated dorsal stripe on the head in the juvenile become obscured by the time the skink has grown to small adult size, at 21 months. The top of the head is then dull brown, with a slightly mottled appearance caused by the different intensity of pigmentation in different areas. The stripes though still discernible, are faint and [93] inconspicuous. Dorsally, on the body, the stripes are still conspicuous, but are dull and lacking in contrast. At this stage, the dark lateral area is retained with intensity of pigmentation scarcely diminished.
Table 13. Normal Range of Variation in Dorsal Striping of Head and Body, and in Color of Tail According to Age and Sex.
| Age, Size and Sex | Condition of stripes | Color of tail | |||||
| Sharp | Distinct | Dull | Faint | Absent | Original | Regenerated | |
| Small young | bright blue | bright blue | |||||
| body | X | ||||||
| head | X | ||||||
| Larger young | bright blue | duller blue | |||||
| body | X | ||||||
| head | X | X | |||||
| Young adult female | dull blue | gray-blue | |||||
| body | X | X | to brown | ||||
| head | X | X | X | ||||
| Young adult male | mostly dull | gray-blue | |||||
| body | X | X | blue | to brown | |||
| head | X | X | X | ||||
| 3 year adult female | mostly | mostly | |||||
| body | X | X | brown and | brown and | |||
| head | X | X | gray with | gray | |||
| scattered | |||||||
| blue scales | |||||||
| 3 year adult male | mostly | brown | |||||
| body | X | brown; | |||||
| head | X | occasional | |||||
| bluish scales | |||||||
| Old adult female | brown; | brown | |||||
| body | X | X | X | occasional | |||
| head | X | bluish scales | |||||
| Old adult male | brown | brown | |||||
| body | X | ||||||
| head | X | ||||||
In tracing the gradual ontogenetic changes in the striped pattern, from the vividly contrasting colors of hatchlings to the dull, patternless coloration of old adult males, five descriptive terms have been applied to the successive stages: “sharp,” “distinct,” “dull,” “faint,” and “absent.” To most individuals below minimum adult size, the term “sharp” is applicable, although there is some loss in vividness in the larger young, as compared with hatchlings. Fading of the original striped pattern proceeds more rapidly on the head than on the body. Upon emergence from their second hibernation at an age of about 21 months, the skinks, mostly grown to adult size, and ready to mature sexually, still show but little sexual difference. They retain the hatchling pattern essentially unchanged, but with colors dulled and contrasts reduced. Within a few weeks the newly matured males undergo relatively rapid color change as the breeding [94] season progresses. The stripes tend to fade and blend into the dark areas adjacent to them. In the two-year-old males stripes are distinct to dull on the body and faint or absent on the head, while in females of the same age group, body stripes are sharp or distinct.
Table 13 refers to adult pattern and coloration as they appear in the breeding season. After the breeding season, in late spring and early summer, when the red suffusion of the head and neck has faded in adult males, the original striped pattern, after having been almost completely suppressed may again become discernible. Individuals of the same size differ in extent of pattern change, and the color descriptions made of individuals were not sufficiently detailed to show fully the changes occurring between successive dates of capture. However, most large adult males taken later than mid-June had at least some trace of the striped body pattern and many of them had become so much like females in appearance that close scrutiny was necessary to determine their sex. They were especially like females in having the dark lateral area extending forward onto the cheek and setting it off sharply from the paler temporal region above it. In breeding males the head has no such dark markings and is suffused with red.
Even among those skinks which have never broken their tails there is a wide range of variation in relative length of tail. This is partly a matter of relative growth since the proportions change during the course of development. Also there may be slight sexual difference and there is much individual variation. In fetuses still well below hatching size, the tail length is less than the snout-vent length. For instance, an egg in a natural nest 12 days short of hatching contained a fetus that had a snout-vent length of 14 mm. and tail length of 12 mm. (Figure 18). In the late stages of fetal development the tail growth is relatively rapid. At hatching, the tail is considerably more than half the total length. In a large series of young with snout-vent lengths from 30 mm. down to hatching size of 25 mm. or less, the tail length averaged 130.8 per cent of snout-vent length. In larger young, up to a snout-vent length of 40 mm. or more, the tail continues to lengthen more rapidly than the body. In skinks that are about two thirds grown, the tails average relatively longer than in either larger or smaller individuals. In the sample representing the size class 50-54 mm. snout-vent length, the tails average 163.3 per cent of the snout-vent lengths, whereas in groups of adults of various sizes and both sexes, the tail length [95] is near 155 or 156 per cent of the snout-vent length. Sexual dimorphism in tail length is slight if it exists at all; in adult males, tails averaged a little longer than in adult females.
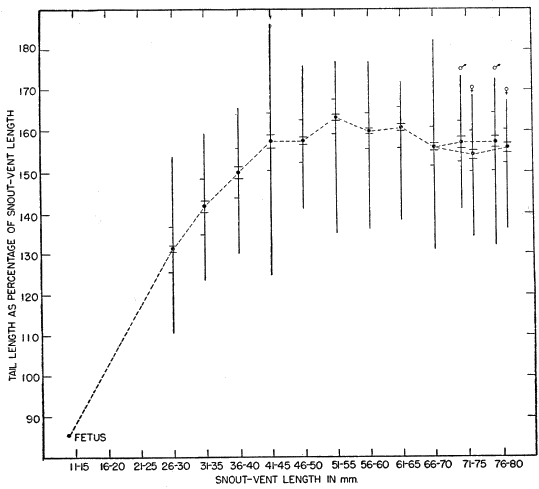
Fig. 18. Diagram showing relative tail-length (as a percentage of snout-vent length) in skinks of different size groups that retain their original tails unbroken; in the early stages of growth the tail becomes relatively longer as size increases, but the trend is reversed before adult size is attained. For each series the mean, standard error, standard deviation, and extremes are shown.
When a skink’s tail is broken, there is almost no loss of blood. The fractured surface is rough and irregular, with exposed muscle masses protruding on the detached end and corresponding concavities on the end of the stump tail retained by the lizard. The concavities are soon filled with oozing blood, and a thick scab forms. As healing begins, the broken end presents a flat, slightly irregular surface. When the scab is sloughed off, a slightly convex surface of delicate, pale-colored new skin of the regenerating tail, is exposed. At first, no scale structure is discernible. As growth proceeds, the new tail takes on a bluntly conical shape. During the early stages of growth, it is well set off from the original portion by the abrupt [96] taper at the point of contact and by its paler coloration and different texture, with no scales discernible at first, and later with fine and granular scalation. The new tail elongates until the more abrupt taper beyond the point of the break is no longer noticeable, and the coloration, surface texture and scalation match that of the original portion so closely that it is difficult to determine where the break occurred or even to ascertain that there has been one. On the regenerated tail, however, the scales are less uniform in size and less regular in shape. The regenerated tail, being different from the original in internal structure, with a cartilaginous rod replacing the vertebral column, is less fragile and subsequent fractures are most likely to be on the part proximal to the regeneration. Nevertheless, fractures of regenerated tails occur occasionally. In old skinks especially, the tail eventually may consist of three or more distinct segments including the basal remnant of the original tail and the successive regenerations. When a break in the regenerated tail occurs, the detached portion is relatively inert, and is capable of only feeble twitching movements in contrast with the lively wriggling normally displayed in a newly detached tail that includes part of the central nervous system.
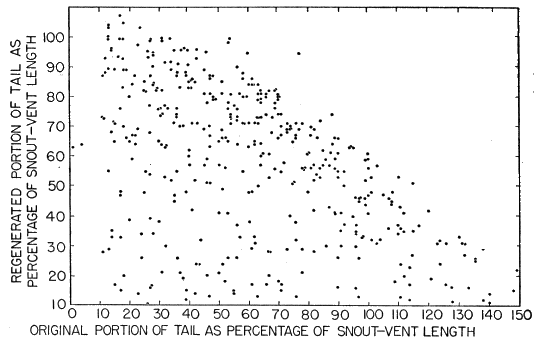
Fig. 19. Relative lengths of original and regenerated portions of tails in skinks which have had their tails broken and regenerated; for each individual, length of each part of the tail is expressed as a percentage of the snout-vent length.
Rate of growth in the regenerating tail is controlled by a variety of factors, such as age, condition, and activity of the individual, and [97] site of the fracture. A break occurring early in the skink’s lifetime results in regeneration more complete than occurs in an adult sustaining the same type of injury. The regenerated tail eventually may be longer and thicker than the lost part if the lizard is young and still growing. But the regenerated tail is never so long as the original one would have been. Regeneration is most extensive in those tails broken near the base. The farther from the base the break occurs the shorter is the part regenerated. As a result, tails that have had time to regenerate do not differ greatly in total length regardless of where the break occurred. However, the nearer the break is to the base, the shorter is the total tail-length after regeneration (Figures 19 and 20). If only the tip of the tail is lost, regeneration may not occur. In the skinks examined that had regenerated tails the proportions varied over a wide range. Presumably, in many, growth of the regenerated portion was still incomplete.
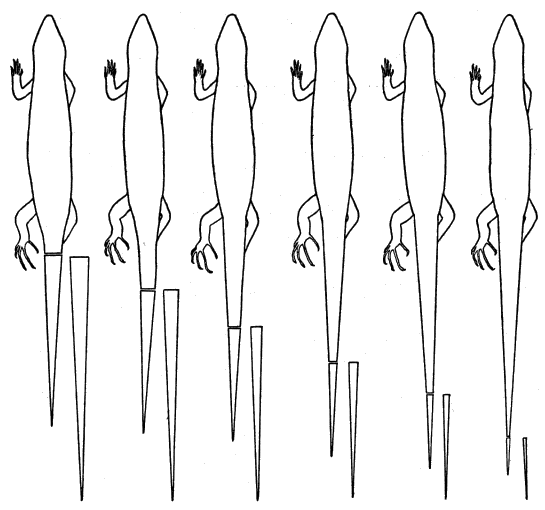
Fig. 20. Diagrams showing typical extent of tail regeneration in skinks having tails broken at different points, × approximately 1⁄2. Original parts of the tails are at the right.
Table 14. Records of Regeneration of the Tail in Individual Skinks Marked and Recaptured.
| Age and Sex | Date | Snout-vent length in mm. | Tail length in mm. | |
| No. 1. | June 1, | 1951 | 73 | 38 (newly broken) |
| Adult male | June 26, | 1951 | 73 | 38 + 14 |
| August 17, | 1951 | 76 | 40 + 45 | |
| April 29, | 1952 | 76 | 40 + 45 | |
| No. 2. | May 28, | 1949 | 51 | 46 (newly broken) |
| Immature | October 15, | 1949 | 69 | 51 + 21 |
| No. 3. | June 11, | 1950 | 49 | 49 (newly broken) |
| Immature | September 2, | 1950 | 63 | 63 + 31 |
| No. 4. | August 10, | 1950 | 621⁄2 | 60 (newly broken) |
| Immature female | June 14, | 1951 | 68 | 62 + 17 |
| No. 5. | August 14, | 1950 | 671⁄2 | 77 + 41⁄2 |
| Adult male | September 3, | 1950 | 681⁄2 | 77 + 61⁄2 |
| April 27, | 1951 | 69 | 78 + 9 | |
| No. 6. | April 7, | 1950 | 67 | 38 (newly broken; separated end 66) |
| Adult male | July 27, | 1950 | 71 | 39 + 471⁄2 |
| No. 7. | May 28, | 1951 | 70 | 18 (newly broken; separated end 100) |
| Adult male | June 14, | 1951 | 71 | 18 + 1 |
| September 22, | 1951 | 76 | 19 + 29 + 71⁄2 | |
| No. 8. | June 12, | 1951 | 72 | 33 (recently broken) |
| Adult male | July 28, | 1951 | 76 | 36 + 31 |
| No. 9. | May 2, | 1951 | 45 | 47 + 1 |
| Juvenile | May 14, | 1951 | 45 | 47 + 3 |
| May 19, | 1951 | 45 | 47 + 5 | |
| No. 10. | June 7, | 1952 | 64 | 51 (recently broken) |
| Subadult female | June 24, | 1952 | 64 | 51 + 11 |
| No. 11. | August 19, | 1949 | 42 | 40 (newly broken) |
| Juvenile | June 13, | 1950 | 581⁄2 | 58 + 28 |
| August 5, | 1950 | 63 | 62 + 31 | |
| No. 12. | May 17, | 1951 | 78 | 20 (newly broken) |
| Adult male | June 12, | 1951 | 78 | 20 + 13 |
Under favorable conditions regeneration occurs at a relatively rapid rate. After a period of healing the new tail grows with a sudden spurt, making most of its gain in length within a few weeks. Then growth abruptly slows or ceases altogether. In young similarly rapid growth of the regenerating tail occurs, but subsequently [99] the increase is more gradual corresponding to the over-all growth of the lizard. In numerous adult skinks marked, and recorded as having well-regenerated tails, the proportions recorded at subsequent captures months or years later were still just the same, demonstrating that extent of regeneration is not proportional to elapsed time. Those adult skinks having unusually long regenerated tails presumably are individuals in which the original tail was lost early in life, and the potentiality for regeneration is probably somewhat less in older individuals, especially those that have stopped growing.
Successive records of selected individuals are listed in Table 14 to illustrate trends in regeneration of the tail. In those instances in which the tail is referred to as “newly broken” the separation usually occurred as an accident at the time the lizard was captured, while in those designated as “recently broken” separation had already occurred in some earlier accident but regeneration was not yet perceptibly underway. In the “Tail length” column, plus signs separate the original portion of tail, on the left, from the regenerated portion, on the right.
As in many other kinds of lizards, the tail in the five-lined skink serves as a reservoir for fat, which may be drawn upon for nutrition in time of food scarcity. An individual that is in good condition has a plump and rounded tail. Fat comprises much of its bulk. Upon emergence from hibernation this fat supply is not noticeably depleted. Brooding females in the latter part of the incubation period have the supply of caudal fat most noticeably depleted, and their tails may appear emaciated, with kinks on the terminal portion. It is my impression that in adults the capacity for storage of fat is most developed in the females, and that their tails vary in proportions more than do those of males. The capacity to shed the tail easily seems somewhat inconsistent with this function of fat storage. Loss of the tail sometimes involves loss of a large amount of reserve fat. Many detached tails that were broken accidentally at the time of the skinks’ captures were weighed. In those that were broken off near the base and were not previously regenerated, weights were usually 16 to 20 percent of the lizards’ total weights.
Data obtained concerning the movements of these skinks demonstrated that individuals tend to limit their activities to small areas thoroughly familiar to them, and wander but little. Although the nature and extent of movements in reptiles in general, and in lizards especially, are poorly known, my findings are perhaps what might [100] be expected from the studies of earlier workers on various other species of reptiles.
Goin and Goin (1951:29) observed that Eumeces laticeps in Florida lives in hollow stumps, each individual excluding other adults from its stump but tolerating young. Movements have not been studied in detail in any member of the Scincidae, however. The observations of Goin and Goin, and those of other authors, seem to indicate that E. laticeps is territorial, and that each individual centers its activities about a tree or snag, regularly using the same hollow as a shelter and home base. In contrast, E. fasciatus is not territorial and has no regular home base.
The iguanid genus Sceloporus is perhaps better known than any other kind of lizard as regards its movements. Studies by Newman and Patterson (1909), Stebbins and Robinson (1946), and Fitch (1940) on three different species have shown that individuals of Sceloporus keep to small individual areas, and that territoriality is well developed, in some species at least.
Among other reptiles, turtles are much better known, as detailed studies of movements have been made on several species, of which the life histories and ecology have been thoroughly investigated (Nichols, 1939; Cagle, 1942 and 1944; Woodbury and Hardy, 1948; Stickel, 1950). They have been found to have well-defined and fairly extensive home ranges, which are not defended as territories. Studies of movements in several different kinds of snakes, by Blanchard and Finster (1933), Stickel and Cope (1947), Fitch (1949), Lowe and Norris (1950), and Carpenter (1952) have shown that these reptiles usually have definite home ranges, which may be several or many acres in extent. Their home ranges are not defended as territories against other members of the species. In general, turtles and snakes have been found to occupy home ranges that are much larger than those of lizards.
Most information concerning movements of Eumeces fasciatus has been obtained from the recapture of marked individuals. Actual distances of travel, and the time, frequency and motivation of movement was uncertain. A skink marked, recorded, and subsequently recaptured at a second location may have wandered widely in the meantime, visiting points relatively remote from either location of capture. The two points of capture may be within a home range regularly or occasionally covered by the individual in the course of its routine activities; or the second point may have been recorded only after a permanent shift of activities away from the area within [101] which the original point was located. Various types of movements probably were involved.
Interpretation of the records is difficult because of the paucity of direct observations on the behavior and movements of skinks under natural conditions. Often when one is alarmed, it will run as much as 30 feet, in a fairly direct course, to a tree or bush or rock where it can find refuge. Undisturbed individuals move about slowly and circuitously. It is difficult to keep one under observation for any length of time because of the secretive habits causing it to keep under cover, as much as possible while moving about, and to hide in response to any slight disturbance.
It is obvious that individuals shift their activities from time to time, occupying new areas either abruptly or by gradual stages. Even though a successful skink has a life span of several or many years, the populations on the small study areas were found to be much altered from one year to the next. Presumably this change was brought about largely by shifts in home ranges. Several shifts of hundreds of feet were recorded, but the chances of recovering marked individuals that moved so far were relatively poor because their movements generally took them beyond the limits of the study area to locations where recapture was unlikely. Skinks often were caught at their hiding places beneath rocks or other sheltering objects. In many of these instances it was evident from the position, temperature and state of activity of the lizard that it had been in the open but had become alarmed as the collector drew near and had retreated unnoticed to its shelter just before capture, whereas in other instances it was obviously at rest in its chosen shelter. Except for females in their nest burrows individuals were not ordinarily recaptured regularly at the same hiding places. They may seek new hiding places after each period of activity.
However many of the skinks captured were taken again, after long intervals, near the same places. Time elapsed between successive captures for different individuals ranged from one day to 47 months. Of the total of 323 recaptured by September, 1952, approximately half, 162, were taken after intervals including one or more hibernation periods. In appraising home ranges and detecting the occasional shifts over a relatively long time span, chronology of the records needs to be taken into account. Records clustering about the same center seem to indicate continued occupancy of an established home range. However, when one or more early records are well separated from one or more later records, a shift in range [102] seems probable. In some instances successive records were progressively farther from the starting point suggesting two or more shifts in the same direction from an original home range.
Although recorded movements varied from a few inches to hundreds of yards, the most noteworthy feature in general was the short distance between points of capture (considered in relation to the potential mobility of the lizards) after days, weeks, months or years. In many instances no movement was demonstrable, even though successive points of capture were not exactly the same. Named natural landmarks, mostly trees, boulders and logs, well distributed over the study area, were used as a basis for locating points on the map. Direction and distance in feet to the nearest landmark was recorded for each site of capture, but for distances of more than 25 feet estimates were made to the nearest ten feet. Usually at least one landmark was available within a 50-foot radius from any point where a capture was made. Occasional estimates made for distances of more than 50 feet, or even more than 100 feet, in the absence of suitable landmarks nearby, were sources of inaccuracy. For such estimates errors of up to ten feet were common, and some errors of greater magnitude were made.
For most individuals successive sites of capture tended to cluster within a small area, but the occasional outlying capture sites indicate that each individual does range outside the area in which its activities are concentrated. These occasional excursions cannot be consistently attributed to any one ecologic requirement, nor are they limited to any particular time within the season of activity. Adult males, however, tend to make longer movements in the brief period of concentrated sexual activity, thereby increasing their chances of finding mates. Similarly, adult females may wander beyond their usual ranges in search of suitable nesting sites. The home range may be thought of as consisting of a small central portion where activities are largely concentrated, and an outer area several times as large, familiar to the animal but used to a lesser extent by it. The activities gradually become more diffuse farther from the central part of the home range. In the five-lined skink, home ranges are unlikely to approximate the circular shape because they are molded with respect to environmental features that are not uniformly distributed. A rotting log, an old tree with decayed hollow base and nearby fallen slabs of bark and dead limbs, a rock outcrop with numerous deep holes and crevices, or a group of flat rocks in a forest glade fulfill requirements not met in the surrounding habitat with the result that home ranges are built around them. [103] Consequently a home range may be long and narrow, with maximum diameter several times the minimum diameter.
The usual concept of home range, as a finite area with well defined boundaries is not entirely satisfactory for an animal with the habits of the five-lined skink. The skink spends much of its time in inactivity underground or otherwise concealed and sheltered, and when it does move about it takes advantage of natural travel-ways over rock surfaces, tree trunks, and logs. If a log happens to be the home range center, the skink may travel the length of the log many times without making a comparable trip at right angles to this axis of travel, although it may make short side dashes to secure food. On more extended forays, the directional sequence of movements is largely controlled by the distribution of suitable cover and travel routes, as the skink avoids both open areas and dense vegetation. Outlying portions of the home range probably are not uniformly covered but are reached only occasionally as the lizard is led along some natural travel route, or after it has visited, in succession, a series of locations attractive in providing shelter or food.
Marked skinks were recaptured at distances up to 680 feet from points of original capture. Considering only the most remote points of capture for those individuals recaptured more than once, the average recorded movement for the entire group of 323 recaptured skinks was 58 feet. This figure provides a basis for comparing vagility of this species with others. Eliminating some individuals of indefinite status, the average movement for 75 adult males was 69 feet; for 102 adult females, 45 feet; and for 112 young, 61 feet. For the adult females, home range data are biased by the fact that many were caught repeatedly at or near their nests. It is not clear whether females that do not have nests range less widely than males.
Only 15 individuals, less than five per cent, had moved more than 250 feet. These longest movements were: 680 feet, adult female, 26 months; 680 feet, adult female, 10 months; 680 feet, subadult male, one year; 650 feet, young to adult male, 22 months; 640 feet, subadult to adult female, two years; 535 feet, young male, 11 months; 510 feet, adult male, 11 months; 490 feet, young (sex undetermined), 10 months; 450 feet, young male, 13 months; 350 feet, young (sex undetermined), 101⁄2 months; 335 feet, adult female 131⁄2 months; 275 feet, adult male, 35 months; 275 feet, adult male, 24 months; 270 feet, young to adult male, 121⁄2 months.
For those skinks caught on only two occasions, at different places, the single movement record provides some clue as to the location and size of the home range. No evidence was obtained to indicate [104] that the activities of these lizards center at fixed home bases. It may be assumed that any two successive captures of the same individual separated by a substantial time interval, will be distributed at random to each other within the area to which the animal’s activities are confined. The varied techniques of capture, by hand and with different types of traps, would help to secure random distribution of capture sites. If the home range were covered uniformly by the animal in the course of its activities, any two random capture sites would be on the average separated by a distance equal to half the home range diameter. If the animal tends to concentrate its activities in the central part of the home range, as seems to be the case, the capture sites will be correspondingly closer together. For the 196 skinks that were caught on only two occasions, average movement was 62 feet. Within this group the 42 adult males that were recaptured only once had averaged movements of 58 feet. One had made an exceptionally long movement of 510 feet, which obviously was not entirely within its home range. Excluding this one long movement, the remaining 41 had moved on the average, approximately 47 feet (Table 15). Among the other skinks caught only twice one of 61 females and 8 of 93 young had likewise made such long shifts that it seemed inadvisable to include them in computing the size of the home range.
Distance between points of capture showed little correlation with elapsed time. For 24 of the adult males that were recaptured in the same year they were originally marked, the average distance was 49 feet, whereas in the 17 others recaptured after one or more hibernations the average movement was 45 feet. For adult females, the corresponding figures were, respectively, 22 feet and 29 feet; and for young, 33 feet and 66 feet.
For those individuals recaptured twice, at different locations, the three points of capture show to a greater or lesser degree the position, and, in part, the extent of the home range. Of course, all three points may be concentrated near the center of the home range, or they all may be scattered along its edges. In general, however, each point will lie somewhere between the center and edge of the home range, separated from each of the other two points by a distance of, on the average, approximately a home range radius.
Table 15 shows that adult males and young tend to range more widely than adult females, and that young tend to shift to new areas more frequently than do adults. Many of the recorded movements (in addition to the long ones that were excluded from the home [105] range computations) may have involved short shifts in ranges. If all such shifts could be definitely identified and eliminated from the computations, actual home ranges might be considerably smaller than those indicated by the present set of data. Home ranges approximately 90 feet across for adult males and young, and a little more than 30 feet across for females are indicated. Actual area of a home range would amount to only a fraction of an acre—from about one-seventh to less than one-fiftieth. The dash of an alarmed skink to a place of refuge, though involving at most only a few seconds, may traverse a large part of its home range. Through long association the lizard is thoroughly familiar with the terrain, so that it can take full advantage of the peculiar features in escaping, hunting, traveling or resting.
Table 15. Distances Between Successive Sites of Capture for Marked Five-lined Skinks on Study Areas, Indicating Home Range Sizes.
| Age, Sex and Number of Captures | Average maximum distance in feet between points of capture, and extremes | Number of skinks included in sample | Number of skinks discarded from sample because of relatively long movements, indicative of shifts of range |
| Adult males | |||
Individuals captured just twice |
47 (225-0) | 41 | 1 |
Individuals captured just three times |
47 (130-0) | 18 | 0 |
Individuals captured four or more times |
91 (200-0) | 17 | 2 |
| Adult females | |||
Individuals captured just twice |
16 (90-0) | 56 | 4 |
Individuals captured just three times |
25 (90-0) | 25 | 3 |
Individuals captured four or more times |
28 (90-0) | 15 | 1 |
| Young | |||
Individuals captured just twice |
45 (160-0) | 85 | 8 |
Individuals captured just three times |
46 (150-0) | 14 | 0 |
Individuals captured four or more times |
82 (175-0) | 14 | 2 |
Relatively few marked individuals were caught four or more times at different sites. For these individuals listed below the distribution of the sites is more or less indicative of shape and size of the home range in some instances. For some of them successive locations of capture are shown and possible home ranges are outlined in Figures 21-25.
Fig. 21. Map of Skink Woods study-area, showing chief physiographic features and landmarks, and showing also successive sites and dates of capture of a marked male skink and two marked females, suggesting extent of home ranges.
No. 1: Seven captures in two years, on May 13, 1950, May 12, 1951, and in 1952 on April 28, May 1, 2, 4 and 6, these seven locations well distributed over a stretch of rocky slope 275 feet in greatest diameter. The fifth location was only 20 feet from the original, whereas the last, only four days later, was the most remote, suggesting that the whole area covered may have been within a home range.
No. 2: Seven captures in 46 months, skink not fully grown when first captured on June 22, 1949; 275 feet south on May 4, 1950; had moved from this second location 150 feet west northwest on June 17, 1950, and this third location together with the last four, on May 15, 1951, and May 13 and 15, 1952, and April 6, 1953, were all within a 20 foot diameter. Evidently two shifts in range were involved.
No. 3: Six captures, all at different locations, in 22 months, on July 5 and 28, 1950, May 3 and 23, and June 21, 1951, and May 1, 1952. The 190-foot-wide area was probably all within a home range, as the fourth and fifth sites were those most remote from each other.
No. 4: Six captures in 21 months, in 1950 on August 14 and September 3, in 1951 on April 27 and August 21, and in 1952, on May 28 and 30. The four 1950 and 1951 locations were within a 30-foot diameter, whereas the two 1952 locations were 150 feet farther east, and even nearer together, suggesting a shift in range.
No. 5: Five captures in five months, all within a 40-foot diameter, on April 24, May 7 and 28, June 14, and September 22, 1951. The first and third locations were at almost the same spot.
No. 6: Five captures all at different locations, in 23 months; in 1950 on July 27, in 1951 on April 30 and May 25, and in 1952, on May 1 and June 28. The second, third and fourth locations were all within 45 feet of each other and of the first, but the last was 110 feet from the first, possibly representing a shift.
No. 7: Four captures in two months, at approximately the same place on May 1 and 5, 1950; on May 30 had moved 35 feet farther north along ledge, and on July 1, 25 feet farther in the same direction.
No. 8: Four captures in one year, all at approximately the same place along rock ledge, on June 17, 1949, and April 21, May 3 and June 15, 1950; trapped three times and once caught by hand.
No. 9: Four captures in one year, on April 7 and 11, and July 27, 1950, and April 14, 1951, the four different locations all within a 30-foot diameter.
No. 10: Four captures in 22 months, in 1950 on July 7, and again on July 23, 175 feet farther north; on May 25, 1951, 200 feet east of second location, and on May 2, 1952, 30 feet from third location. At least one shift in range probably occurred, from 1950 to 1951.
No. 11: Four captures in 36 days, in 1951 on April 30, May 8 and 15, and June 5. The last two captures were made in the same trap and were only 15 feet from the original location, but the second location was 130 feet from both. Because the time span was short and the lizard returned from the most remote point, it seems probable that all four records were within its home range.
Fig. 24. Sites of successive captures of marked skinks, a male and two females, in the Skink Woods study-area.
No. 12: Four captures in 11 months, all within a 50-foot diameter, in 1951, on June 1 and 26, and August 27, and in 1952, on April 29.
No. 13: Four captures in 15 days, all in July 1949 within a 10-foot diameter.
No. 14: Four captures in 22 months, July 22, 1950 (as subadult), in 1951, on May 8 and June 5, and on May 13, 1952. Second location 295 feet southwest [110] of first, third 30 feet north of second, and fourth 650 feet east of second and third. Probably two shifts of range were involved.
No. 1: Six captures in 26 months; in 1950 at the same place on June 4 and 13, in 1951 on May 26 it had moved from the original quarry ledge location 680 feet south southeast down the slope to the pond rock pile, where recaptured on June 9, and in 1952 on May 21 and July 22.
No. 2: Six captures at four locations all within a 25-foot diameter, in 13 months; June 5, 1950, and May 25, June 18, 26 and 29, 1952. On each occasion this female was hiding in a nest burrow, but she shifted to new nest sites as a result of disturbance by the investigator or flooding when there were unusually heavy rains.
Fig. 25. Sites of successive captures of a marked male and a marked female, each taken in three different years in the Skink Woods study-area.
No. 3: Five captures in 34 months, all within a radius of a few yards, at the pond rock pile, on August 8, 1949, June 5 and July 23, 1951, and May 15 and June 4, 1952.
No. 4: Four captures in 34 months, all within a radius of a few yards at the pond rock pile, on August 8, 1949, June 7, 1950, May 30, 1951 and May 21, [111] 1952. It is notable that this female was taken only once in each of four different years, her occupancy of this rock pile seemingly continuing throughout the duration of the study.
No. 5: Four captures in two months, in 1950 on April 15, and on April 26 had moved 50 feet south; on May 23 she was approximately 50 feet from both second and third locations, and on June 5 was between second and third locations.
No. 6: Four captures in 23 months, all within a 20-foot stretch of ledge, in 1950 on June 5 and 17, in 1951 on August 22, and in 1952 on May 1.
No. 7: Four captures in one year, in 1951 on May 19, June 12, June 24, and in 1952 on May 21, all four locations within a 15-foot diameter.
No. 8: Four captures in 23 months, in 1950 on July 5 (as a subadult), in 1951 on August 6 and 15, and in 1952 on May 28, all within a radius of a few yards at the pond rock pile.
No. 9: Four captures in 13 months, on August 2 and 3, 1951, and May 28 and August 31, 1952. From the original location successive sites were 30 feet southwest, 20 feet south southwest, and 30 feet north.
No. 1: (male) Five captures in 331⁄2 months; marked as hatchling on July 13, 1949, and recaptured on June 1, 1950, 175 feet northwest down slope. Subsequent locations of this lizard, as an adult, were, in 1951, on August 21 and 24, and 1952 on May 1, 80 feet east, 80 feet east, and 70 feet northeast from the second location.
No. 2: (male) Five captures in a little more than one year, all within a radius of a few yards at the pond rock pile, in 1949 on August 23, and in 1950 on June 7, July 23, August 19, and September 3.
No. 4: (male) Four captures in 11 months all within a 30-foot stretch along the ledge, in 1950 on July 4, and in 1951 on May 6, 14, and 25.
No. 5: (male) Four captures in one year, in 1950 on September 4, and in 1951 on May 11, June 14, and August 21; the first and last locations were together separated from the second and third, also together, by about 20 feet.
No. 6: (male) Four captures in 13 months, in 1950 on April 19, June 5 and June 6, and in 1951 on May 14. All four locations were linearly distributed along the ledge, the second and third near together 30 feet north of the first and the fourth 30 feet south of the first.
No. 7: (sex undetermined) Four captures in one month, on April 24, and May 2, 4, and 21, 1952, well scattered within a 70-foot diameter.
No. 8: (female) Eight captures in 25 months, in 1950 on June 5 and 9, and in 1951 on May 25, August 15, and September 28, and in 1952 on April 24 and 26. All were within a 150-foot diameter, the first three all within 40 feet, the fifth and sixth near together but 35 feet north northeast from the first group, the last three all within a 90-foot diameter and all to the north of the first five. At least one shift probably was involved.
No. 9: (female) Five captures in 28 months, in 1950 on April 21 and May 7, in 1951 on May 3, and in 1952 on May 2 and August 27. The first three captures were all at approximately the same location, from which the fourth was 60 feet north and the fifth was 130 feet east.
No. 10: (female) Five captures in 24 months; in 1950 on June 5 and 13, and July 29, in 1951 on August 21, and in 1952 on May 28. From the original [112] location successive captures were 50 feet west, 35 feet west northwest, 40 feet west, and 50 feet west.
Less complete records of the movements of other individuals are included along with growth data, on pages 79 to 82 and 87 to 88.
Sizes of home ranges are affected by the type of habitat. For instance, the pond rock pile approximately 70 × 30 feet, must have constituted the entire home range for the many individuals living in it, since it was surrounded by areas that did not provide suitable habitat. No less than 212 five-lined skinks were taken in this small rock pile area in four seasons, and it is obvious that many of these were occupying it simultaneously since a substantial proportion of the total were caught there in more than one year. This rock pile provided in particularly concentrated form the essential habitat requirements, such as an abundant and varied arthropod food supply, an almost infinitely large number of hiding places beneath and between the rocks, basking sites, and flat rocks with damp soil beneath, suitable for nests. In open woods home ranges tend to be larger or, at least, more elongate. Scattered distribution of such habitat features as flat rocks and outcrops, stumps, logs, and glades with patches of sunlight, may induce an individual to extend its activities over a more extensive area. For some of the adult males for which largest numbers of records are available, showing repeated movements back and forth within a definite area which seemingly constituted a home range, movements of 275 feet, 225 feet, 170 feet, 165 feet, 150 feet and 130 feet, respectively, have been recorded. For one young which grew to the size of a subadult during the period covered by the records, movements within a 150-foot diameter were recorded. These individuals all had home ranges substantially larger than the average. It seems that in the five-lined skink there is no fixed size or shape for a home range, but that it varies within rather wide limits depending on age, sex, and perhaps individual peculiarities and on the presence and distribution of essential habitat features within the general area.
Most of the young that were recaptured had grown to subadult or adult size, so that the movements they made as young cannot be separated from those made when they were full grown or nearly so. For 40, however, recapture records are available while they were still less than 56 mm. long. One of those was an exceptionally long movement of 215 feet, obviously involving a shift of range. For the other 39, the average movement was 34 feet, almost intermediate between the average movements of adult males and females. Observations on recently hatched young have given the impression that they keep to narrowly limited areas probably only a few yards in [113] extent at first. For instance, at various times several members of a brood of young have been observed foraging simultaneously but independently on the same 10-foot log, within a few feet of each other. For periods of up to more than a week they had failed to disperse any farther than this from the nest, although probably never returning to the nest itself after having left. In subsequent weeks, however, the young are likely to shift their activities from the immediate vicinity of the nest site to more favorable nearby areas, and gradually extend their ranges. By the time they are one-fourth grown they are ranging over areas larger than those used by adult females.
Some of the shifts in range are probably forced upon individual skinks by changes in seasonal distribution of food, shelter and other requirements, causing them to abandon certain areas and invade others by gradual stages, without venturing far, at any time, into unfamiliar surroundings. Occasional individuals apparently get lost and undergo a period of wandering before they re-establish a home range. An individual venturing slightly beyond the border of its home range might lose its orientation and fail to return, especially if it left under conditions of stress, as when pursued by an enemy, or a rival of its own species. Several individuals originally captured in the vicinity of the quarry or nearby ledges, were subsequently recaptured at the pond rock pile more than 200 yards away. In these instances it may be that the lizard wandered from its home range along the ledge, and finding itself in thick woods, with nearly continuous canopy permitting insufficient sunlight, and with few rocks for shelter, it continued down the slope to the lower edge of the woods, crossed a ditch, and a 100-foot stretch of grassland, and finally reached the exceptionally favorable habitat provided by the rock pile.
The extent to which memory persists through the season of dormancy is little known, but great change takes place in the habitat during the colder half of the year when the lizard’s activity is suspended. Even if the area is one that is free from gross disturbance by man or large animals, the changes occurring are so great that the area might be scarcely recognizable from the lizard’s viewpoint. Herbaceous vegetation mantling the soil, at the height of its development in late summer, will have died, dried out and the leaves and stalks will have been matted down by wind, rain, and snow, and incorporated in the surface layer of soil by the next spring. Shrubs and trees having shed their leaves, present contours quite [114] different from those in autumn. Holes and crevices familiar as avenues of escape, will have been sealed, by the weather collecting and compacting surface debris. Less extensive changes are involved in the occasional blowing down of trees and dead snags, erosion of gullies, deposition of sediment and drift wood, and disintegration of logs. Many of the invertebrates which are the main food sources in late summer, are unavailable in early spring, being at different stages in the life cycle or annual cycle of abundance; and those kinds which make up the bulk of the spring diet likewise are often unavailable in fall. These changes in location of food supply, shelter, and other needs, and the seasonal change in microhabitat, breaking the established routine of conditioned responses to habitat features would seem to promote shifts in range after emergence from hibernation. The available records tend to bear out this supposition. Of the 15 skinks recorded as making long movements of more than 250 feet that almost certainly involved shift in range, only one was recaptured the same season; the other fourteen had passed one or more hibernations.
In the course of the study approximately 30 individuals were released or accidentally escaped at places other than the locations where they were originally taken. Some of these were young hatched in the laboratory, some were of unknown origin, their locality tags having been lost before release while they were being handled in the laboratory, or escaped from defective cloth bags while they were awaiting processing or release, and some taken on remote parts of the Reservation or nearby land were deliberately released on one of the study areas with the idea that they would replace skinks of the same sex and age, recently eliminated through an accident of trapping or handling. Ten were released in Skink Woods, ten at the pond rock pile, eight at the laboratory building, and two near Rat Ledge. In no instance was a transferred skink known to have found its way back to an original home range, although some might have done so with fairly short trips of only a few hundred feet, and the chances of recapturing them would have been good. Therefore it seems that homing instinct is either wholly lacking or but feebly developed. The incidence of recaptures was low, only four for the entire group, suggesting a tendency to wander away from the area of release before settling down on a home range. One young found on May 11, 1950, in the laboratory where it probably had escaped, was released in Skink Woods, and was recaptured three times in the summer of 1951, in what seemed to be a home range within 80 feet of the point of release. Another young [115] of unknown origin released in Skink Woods on May 18, 1950, was recaptured six days later 160 feet away. Five hatchlings from a clutch of eggs incubated and hatched in the laboratory, were released in Skink Woods on August 8, 1952. The following April two of them were recaptured, only 20 feet and 25 feet respectively, from the point of release. The movements and dispersal of this group from the point of release probably paralleled that of a typical brood dispersing from its nest after hatching under natural conditions. An adult male captured just off the Reservation was released at the pond rock pile on May 15, 1952, and was recaptured there on June 2 and June 4. In general, skinks transferred from their original location seem soon to settle down in a new range if the habitat is favorable, but establishment of a home range may or may not be preceded by an initial period of wandering.
McCauley (1939:151) examined contents of 25 alimentary tracts of E. fasciatus collected in Maryland as the basis for the most extensive account of the food habits yet published. One tract contained a broken Eumeces tail, possibly that of the lizard that ate it, which had a recently broken stump tail. A half-grown skink contained numerous Eumeces scales, and McCauley interpreted this as indicating that it had fed on another of its own species or of E. laticeps. As no other hard parts of the assumed victim were in evidence, these scales may have been the lizard’s own slough. (In the present study it was found that eating of the slough was far more frequent than cannibalism.) Arthropod prey included: 11 orthopterans (4 undetermined, 3 unspecified grasshoppers, 2 gryllids, 1 blattid, 1 acridid); 10 coleopterans (7 undetermined, 1 each of rhynchophoran, cerambycid, carabid, staphylinid larva, elaterid adult and larva); 8 spiders; 5 pulmonate snails; 5 flies; 3 undetermined; and one each of lepidopteran larva and adult, ant, dragonfly, thysanuran, and sow bug.
In Ohio, Conant (1940:31) noted food items consisting largely of grasshopper nymphs and small beetles. He found that in captivity these skinks would eat mealworms, crickets, grasshoppers, spiders, roaches, and newborn mice, and a few individuals would lap egg from a mixture of chopped meat and eggs. One large male killed and ate a small common swift (Sceloporus undulatus). Netting (1939:162) mentioned newborn mice, birds’ eggs and small lizards as possible prey, although stating that this species is mainly insectivorous.
Taylor (1936:61) describing the feeding habits of lizards of this genus wrote: “The food consists of a very extensive variety of insects and insect larvae, Arachnida and occasionally small crustaceans. In a few specimens traces of plant material have been observed, but I regard this as being most probably of accidental introduction in the diet. Probably the most surprising fact about the diet of the forms examined is that ants are absent.” In the present study of E. fasciatus, the trends in general bore out Taylor’s findings concerning absence of ants from the diet, although three ants were found among more than 600 other food items. These three, one of them a larva, were of the two largest species among the many kinds of ants found in the area of the study. Most of these local kinds of ants are below the minimum size of prey ordinarily taken by the skinks. Colonies of small ants, Aphenogaster sp., for instance, are abundant in the soil beneath flat rocks in the same situations where the skinks are found, and constitute most of the food of the small toads, Microhyla olivacea, which were abundant in the same habitat and microhabitat as the skinks, especially in the Skink Woods study area (Freiburg, 1951:383).
Burt (1928:56) without citing specific records, stated that “The food of E. fasciatus consists largely of insects and spiders,” but in another paper (1928:62) he listed contents of two stomachs, including a wood roach (Parcoblatta), a cricket (Gryllus pennsylvanicus), a grasshopper, and 2 spiders (attid and lycosid). Smith, summarizing the findings of other authors (1946:350), stated that “The food consists of various small insects, insect larvae, earthworms, spiders, etc. Small vertebrates such as young lizards and mice are sometimes eaten.” In a later work Smith (1950:188) altered this statement slightly: “The food consists of almost any small moving animal, including many kinds of arthropods and even small vertebrates.”
Many authors have mentioned predation on mammals by these skinks, but without citing specific instances, which must be rare indeed, for the smallest newborn mice seem to be near the maximum size of objects that could possibly be swallowed by the largest adults of the common five-lined skink. Various early records and statements pertaining to predation on small vertebrates by five-lined skinks probably pertain in most cases to E. laticeps, which is much larger than E. fasciatus, and more powerful.
Barbour (1950:102) recorded stomach contents of an E. fasciatus collected in Harlan County, Kentucky, as consisting of 60 per cent Arachnida, 30 per cent adult Lepidoptera, and 10 per cent ants, by [117] volume. Werler and McCallion (1951:250) mentioned that on two occasions these skinks in Virginia were seen to eat tenebrionid beetles and larvae.
Webb (1949:294) fed captive skinks with field crickets (Gryllus) and noted that the lizards tended to seize them by the pronotum, and then worked forward to the head, chewing vigorously to disable them. The seized crickets attempted to defend themselves by striking the lizards’ faces and eyes with the cerci and tibial spines. Webb also offered his skinks newly hatched snails, Helix aspersa, which were noticed and fed upon when they moved. In one instance, he noted that a skink found a quiescent snail, and swallowed it after testing it with the tongue a few times.
McIlhenny (1937:232) has published a remarkable account of observations on the foraging behavior of a large adult male skink (stated to be E. fasciatus but almost certainly E. laticeps) in southern Louisiana, which climbed among vines on the side of a house and attacked nests of wasps, Polistes pallipes and P. bellicosus, shaking out the larvae, pausing to crush and swallow the few adults that lit on it and attempted, unsuccessfully, to sting. After many larvae had been shaken to the ground the skink descended and made a leisurely search, eating them in seemingly prodigious quantities. Several times it climbed back into the vines to shake out more larvae, and each time retrieved from the ground those it could find. After feeding to repletion it returned to its habitual shelter in a hollow live oak fifty feet from the house. In a two-week period, however, it returned frequently to raid the wasp nests in the vines, and eventually it had attacked all of the 32 nests that were originally present, completely destroying many of them.
In the course of the present study direct observations on the food habits of skinks rarely could be made in the field. Most of those seen had been alarmed by the presence of the observer, and already had begun a dash for shelter. Others not sufficiently alarmed to take cover, were affected by an observer’s presence, so that usually they ceased their normal activities and crouched attempting to conceal themselves or slithered nervously from one vantage point to another, on the alert for any sign of danger.
On September 1, 1951, a young skink (30-35 mm. snout-vent length) was discovered on the cement walk just outside the laboratory building, holding a cricket (Nemobius) which evidently it had just caught. When I came out of the building, the skink, alarmed, ran about ten feet, holding the cricket by one leg. The cricket was still alive but was nearly immobilized, except for twitching of its [118] antennae and mandibles, and evidently it had already been shaken and battered. After maneuvering about the cement walk the skink ran through the open door into the building. Though seeming to be uneasy at my proximity it was still mainly intent on subduing and swallowing its prey. Following, I caused the skink to take alarm. It dashed back through the door to the walk outside and still carrying the cricket, it ran along the walk to the steps leading up to another building and climbed onto the first step where its uneasiness soon subsided. The cricket was remarkably large in proportion to the skink itself, being of approximately the same diameter, with a length nearly half that of the skink’s snout-vent length. Nevertheless, in about five minutes the skink had swallowed it entire. As swallowing began, on the cement step, the skink was in bright sunshine of early afternoon. In less than a minute it seemed to become overheated, and dragged the prey back several inches into shadow. While swallowing was still in progress, it again ran forward till its anterior half was in sunshine, seemingly regulating its body temperature by these frequent shifts.
A similar encounter between a larger juvenile and a cricket (Ceuthophilus) was observed on May 9, 1953. After I had stood for several minutes beside a rock ledge in woods, my attention was attracted by a rustling sound in dry leaves. The skink, emerging onto the ledge from a cavity beneath exposed hackberry roots had its head raised high and was darting about, peering into crevices and examining its surroundings with unusual animation. After several seconds the cricket hopped into view. Possibly it had been injured already, as it moved deliberately, with short hops. Instantly the skink darted in pursuit, following its erratic course persistently, as it made several hops. In a few seconds the skink caught the cricket, bit it vigorously, and battered it against the rock ledge with violent lateral shaking. Several times the cricket was knocked from the skink’s jaws, but each time it was quickly retrieved. In a few seconds its struggles were subdued, but the skink continued to worry it, dropping it and retrieving it dozens of times. The skink seized the cricket by one of the large rear legs, which was snapped off with a sudden vigorous shake. The skink then dropped and lost the detached leg, and ran back to seize the cricket again. The performance was repeated with several other legs and the antennae, until most of the appendages were eliminated and the body was softened by continued biting and chewing. Then although the cricket was of body diameter almost as great as the skink itself, the lizard swallowed it head first, engulfing it with violent gulping [119] movements. After the front end of the prey had entered the gullet, muscles of the throat and neck were brought into play in forcing it farther down. Swallowing movements were snake-like, the lizard turning its head at right angles to the body to squeeze the morsel down.
At the pond rock pile on May 7, 1952, a small adult male was watched as it moved about over the rocks. A lycosid spider (Pardosa lapidicina) carrying an egg sac was basking on an inclined rock surface. When the skink had come within a few inches, it made a sudden rush at the spider which escaped easily. As this common rock-living spider can move with almost incredible speed, skinks probably do not often catch them in the open.
Captive skinks, in taking their food, seem to rely much less than some other lizards on movement of the prey as a means of detecting it. An active and hungry skink often failed to notice a spider or insect moving about on the opposite side of the terrarium a foot or more away. However, on many occasions, skinks moving about the terrarium and coming upon a motionless prey item have been seen to stop and examine it intently for several seconds, then grasp it, often in a tentative and hesitant manner, after testing it with the tongue. Sight and scent seem to be about equally important in prey recognition, each supplementing the other, and often functioning simultaneously. As many of the animals preyed upon are secretive and would seldom be found in the open by day, it seems that much of the prey is found in hiding places—in leaf litter on the forest floor, beneath flat rocks or at their edges, and in chinks and crannies of decaying logs, stumps, and tree trunks. Some of the prey animals taken are of types that are more active and swift than the skinks themselves. Presumably the olfactory sense is the more important in detecting prey that is motionless or concealed. Stebbins (1948:202) studied the nasal structure of Eumeces, and compared it with that of other lizards. He concluded that the extensive mucus-secreting and olfactory surfaces suggest relatively efficient humidification of inspired air and efficient olfaction in lizards of this genus. In captivity five-lined skinks thrived when provided with ample moisture and shelter and food and kept within the proper temperature range. The reactions of these captive skinks to various small animals introduced into their terraria provided clues as to their food preferences, but also were misleading in some instances. On many occasions hatchlings and young of various sizes were kept with adults of both sexes and subadults, but no instances of cannibalism were ever recorded in captivity. No [120] hostility was seen except between adults, mainly in the breeding season. Young of the little brown skink, Scincella laterale, kept with adult E. fasciatus, and small enough to be eaten by them, likewise were unmolested. Small snakes such as Diadophis, Carphophis, and Storeria placed in terraria with the skinks evoked no strong reaction. Occasionally mild avoidance reactions were aroused but the skinks were never seen to display any hostility and readily became accustomed to such cage mates. Mealworms, the most readily available food for the captive skinks, were generally accepted by those that were hungry and sufficiently warm, but were taken with little enthusiasm. They were seldom noticed unless the skinks were within a few inches. Skinks sometimes tested them with their tongues and examined them intently then moved away without eating them. Earthworms, offered on several occasions, were not eaten. Harvestmen, seemingly of the same kind as those found in scats, were ignored by some captive individuals and taken by others but with some signs of distaste. Ants were ignored. Scarabaeid beetles, that seemed small enough to be eaten, were attacked unsuccessfully, as they were too heavily armored to be crushed in the skinks’ jaws. Wasps (Polistes) placed in terraria were avoided, as were carabid beetles and reduviid bugs. A spider placed in the terrarium usually aroused one or more skinks to animated pursuit, as soon as it moved. Even spiders that seemed to be too large to be swallowed were sometimes pursued and attacked. Occasionally freshly killed prey was taken, especially spiders and wasp larvae. Of invertebrates minute forms are not taken, while certain ants, and various others of the kinds of insects most common on the study areas and often found rather closely associated with the skinks and using the same shelters, were never represented among the recorded food items. Carabid beetles (Brachinus, Calosoma, Lebia, Harpalus, Pasimachus), and reduviid bugs (Melanolestes, etc.) seemed to be especially abundant and available, but habitually avoided possibly because of their noxious qualities. Diptera were entirely absent from the sample in the present study—they and many other insects are so much quicker than the skinks that ordinarily these insects cannot be caught. Foliage-living insects and those that are strong and persistent fliers, are rarely available as prey.
A total of 738 food items were recorded in the present study. Arachnids with 360 items, and insects with 319, together made up 92 percent of these food items. There were 334 spiders (most were not definitely identified, but four were thomisids, 40 were lycosids, and 79 were salticids, the latter group including 27 of the genus [121] Phidippus); 26 harvestmen (Leiobunum vittatum and others); 149 orthopterans (51 ceuthophilid crickets, 31 gryllid crickets, 27 tettigoniid locusts; 17 unspecified, 14 roaches, 9 locustid grasshoppers); 80 indeterminate insects; 39 beetles (mostly carabids and scarabaeids within a narrow size range); 19 larvae (13 lepidopteran, 2 coleopteran, 1 ant, 3 indeterminate); 2 ants (Camponotus herculaneus and C. castaneus); 2 wasps; 1 moth; 1 centipede; 59 snails (31 indeterminate, 18 Gastrocopta armifera, 8 Retinella electrina, 1 Strobilops labyrynthica, 1 Hawaia minuscula); 23 sloughed skins of the skinks themselves; 2 skink eggs; and 2 skink hatchlings.
This sample is based on combined sets of data from analysis of stomach contents and of “scats.” The two sets of data present somewhat divergent trends, and perhaps neither is adequately representative of the food habits in the geographic area represented. A total of 620 food items found in scats represented an average of 1.67 items per scat, whereas in 80 stomachs containing food the average was 1.44 items per stomach. Of the skinks killed and dissected more than half had empty stomachs. Many of them were, however, found inactive in shelter so that it was obvious that they had not foraged recently. Many were not killed immediately and they may have had time to digest any food in their stomachs.
Determinations of the prey down to species were possible in relatively few instances; usually only the family or the order could be determined. Those who have attempted food habits studies of insectivorous small vertebrates will appreciate the obstacles encountered. The invertebrates available to the skinks in the area of the study included many thousands of species. A large number of these species, perhaps the majority, belong to groups still not thoroughly studied, so that their taxonomy is in a state of confusion. Ordinarily the prey is crushed in the jaws and battered on the ground before ingestion; diagnostic structures are often broken or lost, making identification far more difficult. Prey animals taken are often in immature or larval stages which lack the distinguishing features presented by adults. Even the combined efforts of a team of specialists on each of the prey groups involved probably would not have sufficed to obtain generic and specific identification of every item found. In the present study, however, all determinations were made by the writer, with the aid of the small reference collection at the University of Kansas Natural History Reservation.
The 80 specimens used for stomach contents analysis nearly all came from localities off the Reservation, but all within a ten-mile radius thereof. A dozen localities were represented by these specimens, [122] and within each locality specimens were taken in somewhat different situations. Therefore the stomach contents analyzed represents a wide range of ecological conditions, including many different microhabitats. All the stomach contents were collected in late April, May, and June—within the first half of the skinks’ active season. Trends might be expected to differ in late summer and fall.
The food items from stomachs included: 38 spiders (8 of the salticid genus Phidippus, 5 lycosids, 4 thomisids, and the remainder unspecified); 15 insect larvae (7 of them lepidopteran and one tentatively identified as an ant, Camponotus castaneus, the rest unspecified); 13 unspecified insects; 10 crickets; 9 roaches; 9 snails (5 of them Gastrocopta armifera); 7 beetles; 4 sloughs of skinks; 3 grasshoppers; 2 grouse locusts; and one each of cave cricket (Ceuthophilus?), ant (Camponotus castaneus), moth, centipede, sow bug, and egg of a skink. The egg was probably laid by the female that ate it, since she was found brooding an unusually small clutch of only three eggs.
The condition of food items found in stomachs varied greatly. Some were nearly intact, while others were fragmentary and represented by only a few of the more durable and indigestible parts. The larvae of various insects found in stomachs examined are especially noteworthy, since but little comparable material was found in the much larger group of items identified from scats.
The scatological material was even less satisfactory than the stomach material in providing determinable food items. The scats of these skinks are, roughly, 10 to 20 mm. long and two to four mm. in diameter, usually cylindrical and almost straight, and capped at one end with a white chalky deposit of uric acid. Superficially they have some resemblance to bird droppings, but are different in texture. The uric acid deposit is loose and crumbly, and much less compact than that with bird feces, and the food residue is much less completely disintegrated than is similar material in feces of birds. Common small snakes which might produce feces of similar size, include the ring-necked snake (Diadophis punctatus), the worm snake (Carphophis amoenus), and DeKay’s snake (Storeria dekayi), but their feces have a much higher moisture content, lack the definite shape of the skink scats, and ordinarily do not contain readily recognizable residue of the prey. The six other species of lizards on the Reservation, the collared lizard (Crotaphytus collaris), brown skink (Scincella laterale), prairie skink (Eumeces septentrionalis), Sonoran skink (E. obsoletus), six-lined racerunner (Cnemidophorus sexlineatus) and glass “snake” (Ophisaurus attenuatus) might produce [123] scats indistinguishable from those of the five-lined skink. However, none of these lizards except the relatively rare and secretive brown skink, occurred in either of the two situations where most of the scats were collected and it is highly improbable that the scat collection included any material from species other than the five-lined skink.
The scats consist mainly of chitinous fragments of arthropod prey. Usually the prey fragments are so well comminuted, mixed, and scattered that reconstruction is difficult. Degree of disintegration differs greatly, depending not only on the type of prey eaten, but probably also on the condition and temperature of the lizard, and the amount of other food in its digestive tract. Arthropods which have recently undergone ecdysis and have the exoskeleton still thin and soft are no doubt digested much more completely than those that have more heavily sclerotized parts. In spiders the chelicerae are more resistant to digestion than are other parts of the exoskeleton, and frequently appear, intact or nearly so, in the scat contents. The fangs being even more resistant, were sometimes found separately when no other cheliceral parts were recognizably preserved. Frequently large fragments of the carapace, with some of the eyes or all of them, were found. Spider abdomens sometimes were distinguishable, but were collapsed and compressed. Spider legs conspicuous in most of the scats, were so broken, tangled, and distorted that they were of little diagnostic value. In harvestmen, dorsal shields were nearly always fairly intact; but only small fragments of the elongate slender legs were found and they were mostly broken off when the attacking skinks battered the phalangid against the ground before swallowing it. The horny outer wings of crickets, roaches, and beetles usually were in recognizable though fragmentary condition. Occasional heads of insects often were found fairly intact. Insect legs were sometimes intact, sometimes broken into sections or crushed and fragmented. The thorax was usually represented by scattered fragments of chitin, and the abdomen by the separate chitin bands of each body segment.
Shells of snails were sometimes found nearly intact in the scats, although showing the effect of the digestive juices in their extreme brittleness. In other instances all that remained of the shell was the inner columella, and small scattered fragments.
Certain of the items eaten were probably so thoroughly digested as to leave either no hard parts at all, or minute and nondescript parts that were not recognized. The common small slug Deroceras laeve, for instance, would seem to be just as suitable and available [124] for food as the various kinds of snails, but it was not recorded in either stomachs or scats. Having no hard parts except the vestigial internal shell, it probably would not be recognized in scats, even though it had been eaten. Various insect larvae, having thin outer cuticles and virtually no hard sclerotized structures except in the head, likewise probably would leave no recognizable parts. Molted skin of the skinks themselves seemed to be little altered by the digestive processes.
Table 16. Frequency of Occurrence by Months of Various Types of Prey in a Collection of 371 Scats of Eumeces fasciatus.
| May (and April) | June | July | Aug. | Sept. | Total | |
| Spider | ||||||
| unspecified | 32 | 32 | 16 | 100 | 10 | 190 |
| salticid | 10 | 18 | 5 | 31 | 7 | 71 |
| lycosid | 7 | 1 | 3 | 18 | 4 | 33 |
| Harvestman (phalangid) | .... | 7 | 1 | 16 | 2 | 26 |
| Orthopteran | ||||||
| unspecified | .... | 4 | .... | 14 | .... | 18 |
| cricket (ceuthophilid) | .... | 6 | 11 | 31 | 2 | 50 |
| cricket (gryllid) | 2 | 1 | .... | 16 | 1 | 20 |
| grouse locust | 3 | 2 | .... | 17 | 2 | 24 |
| grasshopper | .... | .... | .... | 6 | .... | 6 |
| roach | 1 | 2 | .... | 2 | .... | 5 |
| Beetle | 4 | 13 | 1 | 11 | 2 | 31 |
| Ant | .... | .... | 1 | .... | .... | 1 |
| Wasp | 1 | .... | .... | 1 | .... | 2 |
| Caterpillar | 1 | .... | .... | 1 | 1 | 3 |
| Other insects | 3 | 8 | 6 | 45 | 5 | 67 |
| Five-lined skink | ||||||
| slough | 2 | 1 | 3 | 12 | 1 | 19 |
| hatchling | .... | .... | .... | 1 | 1 | 2 |
| Snail | ||||||
| unspecified | 3 | 6 | 6 | 8 | 6 | 29 |
| Gastrocopta | .... | 2 | .... | 8 | 1 | 11 |
| Retinella | 1 | 1 | .... | 6 | .... | 8 |
| Total | 70 | 104 | 53 | 344 | 45 | 616 |
The collection of 371 skink scats originated mainly from two places on the Reservation nearly three-quarters of a mile apart, the pond rock pile and an old wooden bridge across a ravine. On the weathered planks of the bridge, the scats were conspicuous and could be easily gathered in quantity. At the pond rock pile, where skinks were especially abundant and were intensively studied, their scats were frequently noticed on the large rocks where they hunted and basked. A third smaller collection of scats was made in the vicinity of the laboratory buildings and adjacent rock walk frequented [125] by a few skinks. A small number of additional scats were collected elsewhere on the Reservation, but ordinarily the scats were so inconspicuous in the woodland situations where skinks occurred under typical habitat conditions, that few were found. The rock pile, bridge, and vicinity of buildings are not typical of the species’ habitat and might offer somewhat different choices of prey items.
The 30 scat collections were made in 1951 and 1952. Seasonally, the sample of scats overlapped but little the sample of stomach contents, and was concentrated in the latter half of the growing season. The distribution by months was as follows: April-2; May-38; June-60; July-29; August-213; September-26. Most of the scats probably were deposited within a few days of the time they were collected, because scats disintegrate and disappear rapidly in the field where they are exposed to rain, wind and dung-feeding insects.
No clearly defined seasonal trends are revealed in Table 16 but the monthly samples, except that for August, are scarcely adequate for this purpose. Approximately equal numbers of scats were collected at the two main stations, the pond rock pile and the bridge, but some kinds of items were unequally represented in the two samples.
Table 17. Comparison of Frequency of Occurrence of Various Food Items in Two Different Small Areas, Based on Scat Analysis.
| Total from both collecting stations | Percentage of total in bridge sample | Percentage of total in pond rock pile sample | |
| Spiders (all) | 292 | 63.3 | 36.7 |
| salticids | 67 | 79.2 | 20.8 |
| Phidippus audax | 16 | 100.0 | |
| Phidippus sp | 3 | 100.0 | |
| lycosids | 33 | 36.3 | 63.7 |
| harvestmen | 28 | 57.1 | 42.9 |
| ceuthophilids | 39 | 30.8 | 69.2 |
| grouse locusts | 25 | 92.0 | 8.0 |
| crickets | 26 | 42.3 | 57.7 |
| snail | 34 | 61.9 | 38.1 |
| Gastrocopta | 11 | 91.0 | 9.0 |
| Retinella | 6 | 50.0 | 50.0 |
Spiders, harvestmen, and snails were well represented in both samples. In the bridge sample, salticids (especially Phidippus audax), grouse locusts, and the snail Gastrocopta were more numerous. In the rock pile sample lycosids, and especially ceuthophilid crickets were more abundant. The ceuthophilids were notably [126] numerous among the rocks, and many of them were caught in the wire funnel traps placed there for skinks.
Little is known concerning the quantitative food requirement of any kind of lizard. Five-lined skinks fast for at least half the year during the period of dormancy, from September to April. When they emerge from dormancy in spring most of them are plump and appear to have lost little weight in the course of their long fast. In the season of activity, obviously the quantity of food consumed fluctuates according to temperature and activity of the lizard. Most of the prey taken falls within a fairly narrow size range. The prey ordinarily is swallowed entire or nearly so. This imposes a definite upper size limit. The skink of course lacks the ophidian capacity to ingest relatively enormous objects. The mental symphysis and pectoral girdle would prevent ingestion of an object much larger than the skink’s body diameter, but soft-bodied and flexible arthropods of body diameter approximately equal to that of the skink may be ingested. Typical food items are of such size that from one to three of them fill the stomach to capacity. On one occasion, in an attempt to feed a brood of young recently hatched in the laboratory, I dropped into their jar a mass of newly hatched house spiders (Theridion tepidariorum). As these minute spiders swarmed over and around the skinks, the lizards gave little heed to them except occasionally to jerk or scratch in irritation. One skink, however, was seen to snap up a spider which ran near its snout. The adult female Theridion from the same web was then introduced into the skinks’ jar, although it seemed too large prey for these small lizards, as its abdomen was fully as large as their body diameter. When it ran, the hatchling skinks immediately became alert and several chased it biting at it in frantic excitement. They had difficulty in grasping its smooth rounded surface, but eventually one did catch it and eat it. Full-grown mealworms averaging 26 mm. in length, and approximately .11 grams, are somewhat smaller than the usual prey of adults. In captivity hungry adult skinks took from one to nine such mealworms at a meal. However, they could not be induced to feed daily over periods of weeks, even when kept at high temperatures. Over a period of 64 days an adult male kept at approximately 80° F. in the daytime and 10 to 15 degrees lower at night, ate a total of 30 mealworms, which, in the aggregate, weighed approximately 42 per cent of his body weight. In 35 days under the same conditions an adult female ate 24 mealworms, approximately 32 per cent of her body weight.
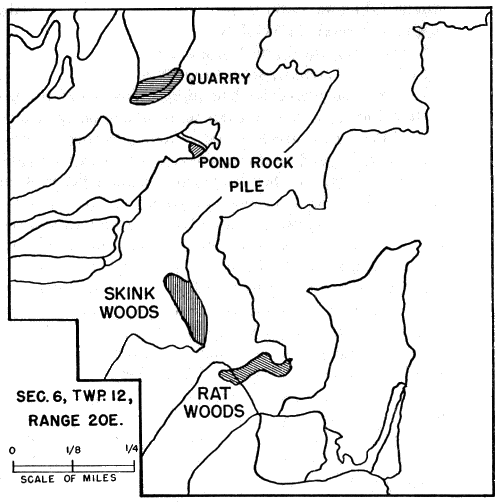
Fig. 26. Map of University of Kansas Natural History Reservation showing locations of the four study areas (shaded) where most data on five-lined skinks were obtained.
Like other members of the family Scincidae, Eumeces fasciatus tends to be secretive in its habits and it depends on concealment rather than speed, aggressive behavior, or noxious qualities to escape its enemies. As compared with lizards in general, or with other members of the genus Eumeces, five-lined skinks are relatively unspecialized in their behavior, and retain a good deal of versatility. While primarily terrestrial, they are able to burrow and climb. Their reactions toward prey and natural enemies vary greatly according to circumstances.
They are less secretive than many other kinds of skinks. Nevertheless the numbers active on the ground surface at any one time, even under the most favorable weather conditions, probably are [128] only a fraction of the total population. For instance, in two or three hours of intensive search in Skink Woods, in which almost every square yard of the area was inspected, a dozen skinks constituted an unusually good catch. Seldom were as many as 20 seen—and most of these only when uncovered in their hiding places. At the pond rock pile, often half a dozen or even more could be seen simultaneously or within the course of a few minutes, as they basked or darted about over the rock surface. These, however, represented only a small part of the number known to occur in the rock pile, which could be observed in its entirety from one spot. At other times, especially in late summer and early fall, even when weather seemed favorable, cursory search of each of the study areas failed to reveal a single individual. Presumably at such times the majority of individuals of the dense population were sheltered deep underground in relatively inaccessible hiding places. Others which escaped attention may have been climbing on tree trunks or logs, or may have been foraging on the ground but close to hiding places into which they darted undetected. The habit of “freezing” in response to a potential danger is commonly noticed in these skinks, and usually it is effective in concealing them.
Having elongate bodies and short limbs, five-lined skinks are not especially swift of foot, but the jerkiness of their movements provides compensatory elusiveness. One sufficiently warm to be fully active is nervous in its actions. Even when resting or basking it is likely to shift its position frequently, fidgeting, blinking, and panting, obviously on the alert for any sign of danger. In moving about, it usually progresses only a few steps at a time, with frequent pauses sometimes only a fraction of a second in duration. These numerous stops allow the animal to examine the terrain immediately ahead of it, and perhaps avoid blundering within reach of a lurking enemy.
Ontogenetic change in the color pattern is of significance in connection with the secretive habits. The red facial suffusion of the breeding male renders him more conspicuous in his natural surroundings, but this bright color is ephemeral. It is developed as a warning, for display to other males. Otherwise, in the adult male the color of dull brown is inconspicuous in its natural surroundings, usually against a background of leaf litter, dead stems, and soil. In the juvenile the contrasting pattern of dark brown ground-color, five longitudinal light stripes, and a vivid blue tail, is far more conspicuous. The young skink might seem to be handicapped in its chances for survival by this conspicuousness. However, [129] in snakes it has been shown that a vivid striped pattern, characteristic of forms that are fast moving and live in dense vegetation, serves to conceal motion, and aid its possessor in confusing and eluding pursuers. The young skinks, being far more active than the adults, may use the striped pattern more effectively in this way. Often when a young skink is startled in its natural surroundings, and takes to cover, the observer does not see its outlines at all, and is conscious of it only as a flash of blue. On many occasions, while walking in the woods, I have had my attention attracted by a faint rustling of dry leaves, and have received such a fleeting impression of the flashing blue tail as to be uncertain whether or not I had actually seen a skink, until, raising a flat rock or other shelter, I found that one actually was present, concealing itself in the nearby hiding place. The erratic movements of a frightened skink that is warm and fully active, make it exceedingly elusive. With sudden lashing movements of its heavy tail and hindquarters, it may flip its body about, facing first in one direction and then in another, as it pauses before or after a rush for shelter. The sudden reversals of direction are so confusing to the pursuer that the skink may often escape by hiding after a few seconds of pursuit, even though the situation provides no shelter where the lizard is entirely secure. The tail-flip described is characteristically given at the instant the lizard reaches shelter such as a crevice, or hole, and just before it disappears. By the instantaneous pivoting of its body, throwing its tail in an arc, in the direction of its original course, the lizard creates the optical illusion of having moved beyond the point where it has taken to shelter. The peculiar writhing movements of the tail of juveniles that are moving about in the open accentuate the conspicuousness of the vividly colored tail, and suggest that this conspicuousness may be advantageous to the lizard in serving as a decoy to catch the attention of predators and distract them from the lizard itself.
In hatchlings the mortality rate is high. Tails are broken frequently in those that survive, suggesting that the tail may be useful in diverting enemies from the lizard itself. Among 121 young of the smallest sizes, (snout-vent lengths in the range of 23 to 29 mm.) 7.4 percent already had broken tails (not including, of course, those in which the tails were broken while the skinks were being captured). In slightly larger young, those in the 30-34 mm. range, perhaps averaging one month old, nearly one-fourth had lost their original tails. In those in the 35-55 mm. size class, mostly one to three [130] months old, about half have already lost parts of their original tails. In those that are in the size group 65-69 mm. normally attained at an age of a year, approximately three-fourths have regenerated tails, and in adults the proportion with unbroken tails is even smaller—down to 16.5 percent in females of more than 75 mm. snout-vent length. In adults the incidence of broken and regenerated tails is slightly higher in females than in males. Defense of nests and sluggishness in the females during the time that they are excavating the nest burrows and guarding their eggs may result in their tails being broken more frequently.
Tree-climbing is a common means of escape and it is curious that many of those who have described the habits of E. fasciatus have either failed to note it at all or have minimized arboreal habits. Taylor (1936:59) cited two instances of tree-climbing but stated: “Only rarely is this form seen in trees, at least in the western part of its range.” Conant (1951:30) stated: “They seldom climb trees, contrary to the habit of laticeps and inexpectatus.” Hudson (1942:42) mentioned seeing an adult that escaped by climbing the side of a hollow tree in southeastern Nebraska.
In the present study, tree-climbing as a means of escape was observed frequently, probably more than two hundred times in all. It was characteristic of both sexes and all ages, and was one of the commonest responses to danger. In summer when skinks were fully active, they usually moved too rapidly to be caught by hand either in the open or where they were uncovered when I turned over rocks or other shelter. To obtain specimens in any numbers at such times, an understanding was essential of the somewhat stereotyped behavior pattern involved in their escape by tree-climbing. A skink that was alarmed in the course of its foraging or basking on the ground litter was likely to run directly to the nearest tree trunk, often a distance of several or many yards, and start up it, instantly disappearing to the far side of it. The trees climbed were usually small, two to eight inches in trunk diameter; however, in the second growth forest where the study was made, large mature trees were relatively scarce. Having started up the tree trunk and concealed itself on the side opposite from its pursuer, the skink usually stopped one to five feet from the ground and waited quietly for the danger to pass. A vine of Virginia creeper, poison ivy, grape or moonseed, or a shrub such as gooseberry, providing screening foliage at the base of the tree trunk, furnished the type of sheltered situation that the skink was most likely to choose as a stopping place. The most effective technique for catching the lizard was to [131] move slowly around the tree trunk at a distance of at least 20 or 30 feet and look for the lizard clinging to it. Having located the lizard, the collector might take careful note of its position, then return to the opposite side of the tree and approach, unseen, to close range to make a sudden grab around the trunk. This ruse often succeeded; more frequently it failed, because of the lizard’s adroitness in dodging, or failure of the collector to gauge its position accurately, or a slight shifting of its position between the time it was seen and the time when an attempt was made to catch it. The response of the lizard to the unsuccessful attempt to seize it depended on whether or not it was touched, and in which direction it was driven. It might drop to the ground and burrow into leaf litter or dash away to other shelter, or it might stay on the tree trunk and spiral rapidly upward out of reach. Because of the squirrel-like tendency to keep the tree trunk between it and the pursuer, the skink usually could be relocated only after some maneuvering. Having climbed the tree trunk to the bases of the main branches, the skink usually showed little inclination to move out along them but tended to hide in the crotches or to spiral back down the trunk. Often a long stick or pole was used effectively to drive a skink back down the trunk by touching or pushing it on the upper side. A skink maneuvered to the lower part of the tree trunk was never loath to leave it in a dash for other shelter, which might be another tree trunk nearby. In moving downward or horizontally on a tree trunk or limb, a skink allows its heavy tail to bend downward from its own weight. The tail probably handicaps the lizard’s climbing to some extent, and those with short regenerated tails have an advantage.
The following extracts from my field notes are selected as typical illustrations of the climbing habit as a response to danger.
September 15, 1948. A skink darted across the trail in front of me, to a tree 18 inches in diameter and climbed to a height of five feet where it stopped. Each time that I moved to approach and examine it, the skink was disturbed, and darted jerkily higher up the trunk until it was well out of reach at a height of about ten feet.
May 2, 1949. Seeing an adult male skink lying in the open, I attempted to stalk it, but it became alarmed, ran to a shagbark hickory about six inches in diameter, and soon had climbed to a height of 25 feet.
June 4, 1949. Juvenile, basking a few inches above ground on trunk of an elm ten inches in diameter, took alarm at my approach, and climbed rapidly out of reach, where it concealed itself in thick foliage.
June 22, 1949. Movement two feet above ground on an elm sapling attracted my attention; an adult male and a juvenal skink were clinging to the trunk only a few inches apart, and neither moved as I approached and examined [132] them from a distance of less than three feet. The concealment afforded by numerous short twigs with thick foliage apparently caused them to feel secure.
September 21, 1949. A juvenile was noticed climbing eight feet above the ground on a locust trunk. As I approached the skink continued upward to a height of approximately 15 feet above the ground where it disappeared around the trunk and could not be relocated.
July 7, 1950. A nearly grown juvenile ran to an elm sapling four inches in diameter, and climbed up out of reach. When the skink reached the main crotch, it turned facing downward alertly. By reaching up with a long stick and poking it on the hindquarters, I succeeded several times in chasing it part way down the trunk, but each time it ran back up to the crotch and returned to the same position.
July 26, 1950. A hatchling uncovered beneath a flat rock ran to a nearby oak tree about four inches in diameter and climbed to a height of five feet before it was caught. An adult female seen foraging in the open ran to a dead shrub and climbed one of the stems, inclined at an angle of about 45°. Ascending this stem she was unable to get more than three feet above the ground, and was easily captured. Another adult female seen foraging in the open ran to an oak about three inches in diameter, climbed rapidly to a height a little more than a foot above the ground, and concealed herself under the stem of a poison ivy vine twined about the tree trunk.
July 27, 1951. A female brooding her eggs dashed out of the nest when the flat rock covering it was lifted, ran 15 feet to a hickory sapling and climbed it.
May 1, 1952. An adult male found beneath a rock ran to a small tree ten feet away, climbed up on the opposite side, and stopped about a foot above the ground. My first attempt to seize it failed and it ran around the trunk and stopped at a height of four feet. The next try was likewise unsuccessful, and the skink dropped to the ground and burrowed into leaf litter.
May 15, 1952. An adult male startled as it basked in a patch of sunlight in thick woods, dashed 25 feet without stopping, to an osage orange tree and disappeared behind the base of the trunk. Moving to the far side of the tree I located the skink clinging to the trunk two feet above the ground. My attempt to catch it failed and it spiralled up the trunk to a height of ten feet. When I poked at it with a stick, it crouched close to the trunk allowing the stick almost to touch it, then it spiralled down the trunk and could not be relocated.
June 23, 1952. When I struck the trunk of a partly dead ailanthus tree with a brush knife to determine whether it was hollow, a juvenile darted out of a cavity five feet above the ground, ran farther up the trunk, and disappeared into another small hole. An adult male was seen running across the vertical wall of a building, clinging to the rough asphalt siding. When it was alarmed it ran to a crevice and hid.
A more unusual escape-reaction was observed on May 25, 1952, at Tonganoxie State Lake, by Sydney Anderson, who recorded that a skink, alarmed by him at the edge of the water dived and hid among submerged rocks. Similarly, Boyer and Heinze (1934:194) record of this species, in Jefferson County, Missouri: "When pursued [133] they do not hesitate to take to the water and are very agile swimmers over short distances at least." Parker (1948:25) wrote that in western Tennessee fasciatus sometimes showed a preference for habitat in the vicinity of water, and, if other concealment was not available, it would usually take refuge in the water.
Little is known concerning the kinds of predators that destroy five-lined skinks, or their importance in its ecology. In studies of the food habits of various predatory birds and mammals, workers often have been interested chiefly in items of direct economic bearing, and have tended to lump as “lizard” or “reptile” material that might have included Eumeces fasciatus. I have been able to find only a few specific references to predation on it. Nevertheless many kinds of predators probably utilize it as food, at least occasionally. Owls probably seldom have opportunity to prey on these skinks, which are not known to be active after dark. Nestling broad-winged hawks observed in 1954 were found to have eaten an adult and a subadult five-lined skink on June 13 and June 23. The Cooper’s hawk and red-shouldered hawk also are probable predators as both are known to feed upon small reptiles. Mammalian predators which might be expected to take skinks occasionally include the red fox, gray fox, bobcat, mink, weasels, skunks, opossum, armadillo, moles, and shrews. Snakes, especially those of the genera Elaphe, Lampropeltis, Cemophora, Micrurus and Ancistrodon, may include some of the chief predators on the skink. Certain larger lizards also may prey upon it.
Of these several potential predators, only the opossum, armadillo, and snakes (Elaphe obsoleta, E. guttata, Lampropeltis triangulum, L. calligaster, L. getulus, and Ancistrodon contortrix), Sonoran skink and the greater five-lined skink (in confinement) have actually been recorded as preying on Eumeces fasciatus but circumstantial evidence has been obtained for the mole (Scalopus aquaticus) and short-tailed shrew (Blarina brevicauda). The short-tailed shrew may be one of the major predators on the skink. This shrew prefers the same habitats and occurs throughout the skink’s extensive range. Like the skink, it is a characteristic inhabitant of the hardwood forests of the eastern United States, but its range extends farther north and west. A high proportion of the skinks examined had scars, usually on the sides or dorsal surface of the body, or of the tail near its base—wounds which must have been made by a small, sharp-toothed animal. For example, in May [134] 1951, eighteen per cent of 155 skinks captured on the study areas had such scars. The incidence seemed to vary according to age and possibly sex; the scars were present in 22.9 per cent of the adult males, 25.5 per cent of the adult females, and only 9.8 per cent of the yearlings (these three groups being represented by approximately equal numbers in the sample). As the scars are more or less permanent, adults could be expected to show a much higher incidence than young. Females, being inclined to stay in their nest burrows and defend them against small predators, may receive more wounds than the males, which are quicker to escape. None of the invertebrates present on the study area is sufficiently large or powerful to inflict such wounds, and none of the birds, reptiles, or amphibians has a dentition capable of producing them. The wood mouse (Peromyscus leucopus) is the most abundant small mammal in the skink’s habitat; other rodents present in relatively small numbers include the prairie vole (Microtus ochrogaster), harvest mouse (Reithrodontomys megalotis) and pine vole (Microtus pinetorum). Both voles and harvest mice have been known to kill skinks caught in the same traps with them, but individuals experimentally placed with skinks in captivity have failed to molest them and it seems likely that the attacks in traps were motivated by extreme hunger or self defense. The irregular scars from lacerated wounds characteristic of the skinks bear little similarity to rodent bites, in which the long, sharp-edge incisors make slit-like punctures. Other small mammals abundant in the places where skinks were studied were the insectivores: the common mole, short-tailed shrew, and least shrew (Cryptotis parva).
On one occasion when a large five-lined skink was put in a terrarium with a recently captured short-tailed shrew, each displayed strong aversion for the other. The skink crouched, attempting to conceal itself in the end of the terrarium farthest from the shrew, and resisted efforts to drive it toward the shrew. In exploring the terrarium the shrew several times sensed the skink’s presence, and then scampered away in frantic haste. The skink also rushed away several times when the shrew came close enough to disturb it. Three days later, when the shrew had become accustomed to the terrarium, the test was repeated, with different results. The shrew, having finished the food left for it, was noticed moving about the terrarium, sniffing and testing objects with its tactile snout, obviously hungry and searching for more food. The skink was then dropped near it. In a few seconds the shrew sensed the skink’s presence and pounced upon it, and bit hard on its back. The skink [135] reacted with a violent flexure of its body which caused the shrew to release it instantly, and both rushed away in opposite directions. After a few seconds the shrew located the skink again, and moved up to it with little hesitation but with nervous alert sniffing, and delivered another quick bite after which the two separated as before, the skink showing signs of injury. Soon the shrew attacked a third time, and bit the skink’s tail severing it near the base. As the skink rushed away, the detached tail performed lively squirming movements, but the shrew seized it, held it down, and began to eat the exposed flesh on the broken end as the tail writhed. After rapid nibbling it would drop the tail, and leaving it temporarily would explore the terrarium. Several times on these trips it encountered the skink and renewed its attack. As death of the skink seemed imminent, it was then removed, and it survived with no apparent ill effects. The wounds inflicted by the shrew bore close resemblance to those noticed on skinks in the wild. It seemed almost certain that Blarina had inflicted most of these wounds or all of them. On subsequent occasions several other captive shrews that were tested, quickly killed and ate skinks that were introduced into their containers. The least shrew, Cryptotis, likewise occurred in all situations where skinks were taken, and in some localities was more abundant than the larger Blarina. Bites inflicted by these two kinds of shrews might be indistinguishable, but because of its larger size, Blarina would seem by far the more formidable enemy.
Reynolds (1945:367) found E. fasciatus to be the most frequent reptile in a collection of opossum scats from Missouri, with two occurrences in 100 fall scats and ten occurrences in 100 spring scats. Sandidge (1953:98 and 101) recorded one of these skinks among numerous other items identified from stomach contents of sixty-six opossums. Probably the opossum is a frequent predator on this skink. Although no specific instances were obtained on the area of the study, flat rocks a few inches in diameter frequently have been found flipped over, larger ones and those solidly anchored in the ground have been found partly undermined by opossums scratching away the loose dirt at their edges. The rocks found disturbed by opossums were typical of those used as shelter by the skink. On many occasions wire funnel traps set for skinks and other reptiles along hilltop rock ledges were found to have been disturbed, either shifted in position or with their rock shelters removed, or rolled downhill or broken open. Similarly, heavy flat rocks used to cover pitfalls, to protect the small animals falling into them from predators, often were found [136] to have been shifted somewhat, or completely removed. When such raids became frequent and troublesome, steel traps were set beside the reptile traps to discourage the raiders or catch them and determine their identity. On several occasions opossums were caught and somewhat less frequently, spotted skunks (Spilogale interrupta). These skunks probably prey regularly on lizards including the five-lined skink. However no definite records were obtained. Crabb (1941:356-358) in his food habits study of the spotted skunk in southeastern Iowa, did not record this or any other species of reptile among the items identified in 834 scats. On the Reservation both opossums and skunks were, in many instances, attracted to the reptile traps by the insects and other arthropods in them, rather than by lizards. The striped skunk (Mephitis mephitis) is another of the predators which probably feeds upon the five-lined skink occasionally on this area.
In the contents of 103 armadillo stomachs collected in west-central Louisiana, in 1947 and 1948 I found the broken tail of one Eumeces fasciatus. The lizard itself evidently had escaped (Fitch, 1949a:88). Many clutches of lizard eggs were found in the contents of the armadillo stomachs and some of these probably were eggs of Eumeces, which are similar to those of other small lizards in the same region (Anolis carolinensis, Sceloporus undulatus) in size, shape, and color.
Among 217 identified prey items from stomachs and scats of Sonoran skinks (Eumeces obsoletus) from northeastern Kansas were remains of three hatchling five-lined skinks. Taylor (1953b:212) recorded that a Eumeces laticeps shipped from Arkansas to Kansas ate an E. fasciatus that was with it in the container. Several authors have recorded predation on Eumeces fasciatus by snakes of various kinds in captivity. Conant (1951:211) recorded that one was eaten by a blacksnake (Coluber constrictor) placed in the collecting sack with it. Anderson (1942:211 and 216) recorded that a king snake (Lampropeltis getulus holbrooki) and a young copperhead (Ancistrodon contortrix) each fed upon them. Hurter (1911:184) recorded that a milk snake, Lampropeltis triangulum syspila, placed in a bucket with a Eumeces fasciatus was found swallowing it a short time later and its tail had been broken off.
Ruthven (1911:268) mentioned that stomachs of milk snakes, L. t. triangulum, collected in Michigan contained remains of five-lined skinks. Ditmars (1907:352) wrote that stomachs of several L. t. elapsoides contained Eumeces, and Wright and Bishop (1915:167) wrote of the same kind of king snake in the Okefinokee Swamp [137] region: “It feeds on ground lizards, skinks, swifts, and other snakes and lizards.”
Mr. Richard B. Loomis is of the opinion that the five-lined skink is one of the chief food sources for the milk snake (L. t. syspila). Having kept many of these snakes in captivity and experimentally offered them different types of prey, he found that individuals inclined to feed would avidly seize and eat skinks and young mice, but other proffered prey, small adult rodents, snakes, or lizards other than Eumeces were either rejected or were taken with some hesitation. These milk snakes have habitat preferences similar to the skink, which would seem to be one of the most available food sources. Loomis recorded in his field notes that a juvenal blotched king snake (L. calligaster) 310 mm. in total length, taken on April 8, 1950, seven miles southwest of Tulsa, Oklahoma, had eaten a large adult E. fasciatus. Another juvenal blotched king snake that he found under a flat rock near Sunflower, Johnson County, Kansas, regurgitated an adult five-lined skink. Loomis also recorded a juvenal rat snake (Elaphe guttata emoryi) and a juvenal pilot black snake (E. obsoleta) each feeding on individuals of Eumeces fasciatus in captivity. Uhler, Cottam and Clarke (1939:622) in a study of the contents of the alimentary tracts of 893 snakes of 18 species, from the George Washington National Forest, Virginia, found among the prey items only one skink (species undetermined but most probably E. fasciatus). It had been eaten by one of the two corn snakes (Elaphe guttata) that were examined in the study.
On June 11, 1950, in Skink Woods, a young copperhead 335 mm. in snout-vent length and weighing 27.6 grams, had a gravid female skink in its stomach. Another young copperhead (335 mm., 36.1 grams) trapped near Rat Woods on August 28, 1953, had in its stomach a bob-tailed adult five-lined skink. Many copperheads collected on the Reservation were kept in captivity for short periods, and from them a total of 44 scats were obtained, each scat containing the remains of one or more prey animals eaten in the wild. Of this total, five scats contained remains of Eumeces fasciatus, which was one of the more frequent items, although small mammals collectively made up the bulk of the scat contents.
Skinks, like many other lizards, are likely to be infested with parasites. Little attention was devoted to the endoparasites in the present study, but they were noted from time to time. On several occasions small nematodes and flukes were seen in feces voided by [138] lizards which were handled. Small white cysts were seen in the body cavities of several that were dissected.
Harwood (1932:65) examined for endoparasites nine E. fasciatus along with many other reptiles and amphibians collected near Houston, Texas. Most of them were infested and five kinds of helminths were identified. Two of the skinks were infested with Oswaldocruzia pipiens, a spirurid nematode that was also present in various other lizards, snakes, toads and frogs from the same region; four had Comocercoides dukae, an oxyurid nematode also present in various lizards, snakes, turtles, and frogs; one had in its intestine Oochoristica eumecis, named as a new species by Harwood, and found only in Eumeces; one contained Cysticercus sp. in its body cavity, present in great abundance as white globular structures .6 mm. in diameter (Harwood states that possibly these were larvae of Oochoristica). One skink contained Mesocoelium americanum, a dicrocoelid trematode which was found also in the brown skink (Scincella laterale) and DeKay’s snake (Storeria dekayi).
The ectoparasites of these skinks consist mainly of chiggers. Wharton (1952:135) lists three species; Trombicula alfreddugesi, T. splendens, and T. gurneyi. The first species is the common pest chigger of humans and domestic animals in the United States, and south through tropical America. Wharton lists 136 known hosts which are fairly evenly divided among mammals, birds and reptiles; he lists four kinds of frogs and toads. Trombicula splendens is a similar and closely related species which has been recorded from thirty-eight vertebrate hosts including mammals, birds, reptiles, and a tree-toad. Trombicula gurneyi belongs to a separate subgenus and it was originally recorded from Eumeces fasciatus which seems to be one of the principal hosts.
Two of these mites, T. alfreddugesi and T. gurneyi, were on skinks collected on the Reservation, and nearby areas. A four year study of the chiggers in this general region by Loomis (MS), Wolfenbarger (1953) and Kardos (MS) has clarified the ecological relationships of the several kinds of chiggers present, including their local distribution with respect to vegetation, soil type, moisture and temperature, host preferences, and seasonal occurrence. At the quarry, Rat Woods and the pond rock pile, the chigger population consisted chiefly of T. alfreddugesi, while at Skink Woods T. gurneyi was also abundant. In some local situations where they are among the most abundant of vertebrates the skinks probably are important [139] as hosts of T. gurneyi. An individual skink may have dozens of chiggers on it at one time but usually there are fewer.
There are several favorable sites of attachment. The most favored site is in the axilla. There the scales are minute and granular with exposed areas of thin and tender skin, and the chiggers are well protected from dessication and are not likely to be rubbed off as the skink moves about. Other favorite sites of attachment are: about the insertion of the hind limb, about the cloacal opening, on the eyelids and on the toes. Only occasionally are chiggers found attached on the dorsal surface. When attached in protected spots in the tender skin of the axilla or groin, they are often in dense clusters of a dozen or more. Damage to the skin resulting from the attachment of the first chiggers renders conditions more favorable for the attachment of others. At Rat Ledge and at the quarry, many of the larger Sonoran skinks (Eumeces obsoletus) were captured, and individuals were far more heavily infested than were E. fasciatus from the same places. A single Sonoran skink might be found to have hundreds of chiggers, widely distributed over its body with concentrations at the axillae, groins, lateral neck region, and any injured spots where the protective armor of scales was broken. The reasons for the greater susceptibility of E. obsoletus are not entirely clear. It is a larger, less active species with coarser scalation, and is more subterranean in its habits.
The chiggers that attach to skinks seem to occasion but little discomfort. There is no local swelling and inflammation such as occurs in humans. The infestations observed in five-lined skinks were not sufficiently severe to cause debilitation or any noticeable symptoms. There is, however, a possibility that chiggers are vectors of microorganisms causing diseases in reptiles, just as they are for certain mammals (including humans) in some parts of the world.
Bishopp and Trembley (1935:42) record a single kind of tick, Ixodes ricinus scapularis Say, the black-legged tick, as parasitic in its immature stages on Eumeces fasciatus. This tick, however, has been recorded principally from mammals, of which many kinds serve as hosts for its larval, nymphal, and adult stages.
Population structure obviously differs from place to place and from time to time. Because of the differences in secretiveness and elusiveness between young and adults and between males and females, true sex ratios and age ratios are obscured. In the period of [140] weeks between the emergence from hibernation and the onset of the breeding season, these skinks tend to be less secretive than at other times, and secondary sexual and age differences in behavior are minimized. A sample at this season should be more representative of the true population composition than samples taken at other times of year. In a sample of 308 skinks available for the month of April, including the collections made on the Reservation and on nearby areas, in 1949, 1950, 1951, and 1952, 36.7 per cent were adult males, 28.3 per cent were adult females, and 35.0 per cent were young. That these figures cannot, however, be accepted as an accurate indication of the population composition is shown by the data from the areas where intensive population studies were made. Data are most complete from Skink Woods. For 292 adults taken there over a four year period, the sex ratio was 100:122.6. On this area after the first year of study a substantial proportion of the individuals recorded were repeaters from one year to the next, and in some cases for three or even four successive years. Many could be definitely assigned to a known age group. By analogy the majority of others could be tentatively assigned with some assurance on the basis of measurements, and relatively few were of indeterminate status. By assigning each of these indeterminate individuals to one or another age group, on the basis of greatest probability, the approximate composition of the population could be determined. Of 611 adults, 55 per cent were “two-year olds” (in the season between their second and third hibernations, which was their first breeding season). The percentage was not significantly different in the two sexes.
On the average, a pair of adults produces somewhat more than nine eggs per year. From the time individuals of a brood start their development in the egg until they are breeding adults two years later, they undergo such drastic reduction in numbers that, on the average, approximately only one per brood survives. Most of the mortality probably occurs early, especially before hatching, also in the inexperienced hatchlings, and in the first hibernation. In spring, after emergence from hibernation, young are generally taken in smaller numbers than are adults. Their relative scarcity is only apparent, owing to greater secretiveness, and greater elusiveness when found. In spring, newly matured adults (age class about 21 months) may be taken in somewhat larger numbers than young (age class about 9 months). The latter obviously must be more numerous, in a stable population however, as the 21 month age class necessarily has sustained some loss since it was 9 months old.
Success of the annual brood varies greatly from year to year, depending on the weather and various other factors. In 1949 evidently conditions were near optimum; young hatched early and were especially numerous in late summer. In 1950 these young hatched in 1949 made up 40 per cent of the total catch (excluding hatchlings) in Skink Woods and were relatively more numerous than young of the corresponding age group in other years. In 1951, these young of the 1949 brood, grown to adults, made up 70 per cent of the breeding populations, as against 36 per cent for the corresponding class in 1950 and 58 per cent for the corresponding class in 1952.
Even after attainment of adulthood, any given age group evidently is subject to annual reduction amounting to at least half its numbers. Within six or seven years, at the most, the original numbers would be reduced to an insignificant percentage. At an age of four or five years individuals probably have attained their maximum size, with obscured pattern and changed proportions suggestive of advanced age. Occasional individuals possibly attain much greater age, but certainly few live more than five years. Like most small animals, the five-lined skink has a short life expectancy and a rapid population turnover. As compared with mammals of comparable sizes, the small rodents and insectivores that are this lizard’s community associates and are subject to many of the same hazards, the skink is notably successful, with a much longer life expectancy. For these small mammals the life span is seldom as long as a year. Most kinds of small birds likewise have a life expectancy less than that of the five-lined skink, although somewhat greater than that of small mammals.
The population density changes constantly, following an annual cycle with gradual reduction to its lowest ebb in late June or early July, then rapid increase to a high point a few weeks later when hatching of the single annual brood has been completed. In a normally successful breeding season the population is at least doubled, but reproductive success varies from year to year, as the population responds to weather conditions that are favorable or unfavorable, even where the environment remains fairly stable. In most places, however, local populations continue upward or downward trends for periods of years in response to successional changes which cause progressive improvement or deterioration of local habitats. Local populations are likely to be more or less isolated from others by areas where the habitat does not exist. Even in an area [142] of favorable habitat such as a wooded hillside of several acres, the population is not at all evenly distributed, but concentrations occur along rock outcrops, and about decaying logs, or stone piles. In intervening areas lacking such abundant shelter, and less productive of food, the population is sparse, or there may be no permanent residents.
In view of these traits, and the difficulty of obtaining a representative sample, no precise measurements of population density can be made. During the time required to secure a sample, the population undergoes change. At the pond rock pile, an area of approximately .05 acre, the skinks were found in remarkably high concentrations, 57 in 1949, 85 in 1950, 37 in 1951, and 51 in 1952. These numbers represent population densities of, respectively, 1120 per acre, 1960 per acre, 746 per acre, and 1000 per acre. No such concentrations were found elsewhere, and probably do not occur in natural habitat. The Skink Woods study area of 21⁄4 acres is typical of favorable habitat in the region of the study, and the numbers taken there are more significant. For 1949 the 74 skinks recorded comprise an incomplete sample, and the population density of 33 per acre represented is certainly somewhat too low. For other years the following population densities (exclusive of hatchlings) are indicated: 1950, 92 per acre; 1951, 61 per acre; 1952, 49 per acre. These figures are only approximate, of course, and it is difficult to judge how accurately they reflect the true numbers. Even the most intensive collecting may be insufficient to obtain every individual on a small area. Within each season there are shifts of range by some individuals, off the study area and corresponding shifts onto it by others, so that the numbers caught in the course of an entire season are somewhat too high. The individuals taken on the study area may regularly range beyond its boundaries to some extent, so that the seeming population density is somewhat too high. Actually this was probably a minor source of error for the Skink Woods study area, as nearly half its perimeter was bordered by an open field uninhabitable for the skinks, and the remaining perimeter adjoined areas much less favorable than the central portion.
Census of the population of the study area by a ratio such as the “Lincoln Index” used in game management studies was scarcely practicable because of the changing seasonal habits distorting the recorded ratios of the sexes and of age groups somewhat differently at different stages of the season. These changing ratios tend to produce an erroneously high population figure, unless separate computations are made from the data for adult males, adult females, and [143] young. Census figures obtained by this method were erratic but seemed to bear out in a general way, the population figures based on total numbers of individuals taken.
In favorable habitat where they occur in high populations of 50 to 100 per acre in spring, these lizards must attain a biomass of a pound or more per acre. Biomass in a population probably fluctuates but little during the course of the annual cycle, even though the number of individuals changes greatly. The steady elimination of individuals through various mortality factors, is compensated for by rapid growth of the young.
Five-lined skinks were studied for four consecutive years in four small areas, totalling approximately ten acres, on the University of Kansas Natural History Reservation, Douglas County, Kansas. The information gained from intensive study on these areas has been supplemented by data from skinks collected elsewhere in northeastern Kansas, and from an extensive literature pertaining to this species.
The genus Eumeces, to which the common five-lined skink belongs, has more than 50 species, occurring throughout Central America, North America to the latitude of southern Canada, and, in the Old World, across southern Asia and North Africa. Within the genus, the five-lined skinks, comprising a dozen species, form a natural group of closely related forms. In this “fasciatus group” nine of the species occur in the Orient, Japan and neighboring islands and the adjacent mainland. The remaining three, including E. fasciatus, occur in the eastern United States. Specific differences are to be found in details of pattern, scalation, and size, and, in some instances, they were long unrecognized. E. fasciatus coincides closely in its distribution with the Deciduous Forest Biome of southeastern North America. An early Tertiary deciduous forest in Alaska and probably in the Bering Strait area, evidently growing in a humid, mild-temperate climate, included genera of plants that are now most characteristic of southeastern North America along with other kinds now characteristic of forest remnants in southeastern Asia, and still others characteristic of the western United States. The fasciatus group seemingly dispersed from a northern center that may have coincided with the early Tertiary deciduous forest of Alaska.
Eumeces laticeps almost coincides in distribution with E. fasciatus, but does not occur quite so far north, and unlike fasciatus it occurs [144] throughout Florida. Young are similar in appearance but laticeps is a larger, more powerful species, notably arboreal in its habits. E. inexpectatus much more closely resembles fasciatus, and ecological divergence is slight. It is characteristic of hot and dry rocky areas in open woods, and is more southern in distribution, although there is extensive overlap with fasciatus and inexpectatus shares nearly all of its range with laticeps.
Eumeces fasciatus is most abundant in well-drained, open, rocky situations within its forest habitat. It is scarce or absent in bottomland forest that is subject to flooding and requires a forest with openings in the leaf canopy so that sunshine patches for basking are available. In northeastern Kansas, at least, woodlands that are browsed by livestock, and have scanty undergrowth, provide better habitat than those that are protected. E. fasciatus is likely to be most abundant in cutover woodland, and may reach greatest numbers in artificial situations, such as old rock piles, or the vicinity of deserted sawmills. In the north, the species is increasingly confined to open situations, while in the south it may inhabit heavily wooded areas. An abundant supply of moisture is a necessity and the species is limited to a climate of high humidity. Dew normally supplies the source of drinking water, without which the skinks rapidly become emaciated and die. Optimum body temperature was determined to be near 34°C., from a series of temperature readings taken both under natural conditions and in confinement under conditions permitting behavioral thermoregulation. By thermoregulatory behavior, active skinks in the wild tend to maintain their body temperatures near this level over a wide range of environmental temperatures. They can tolerate body temperatures only a few degrees higher, but, within a range of several degrees below 34°C., efficiency is little impaired and incentive to make readjustment is slight. At progressively lower temperatures skinks become slower and less efficient. They are, however, capable of copulation at temperatures down to 21°C, and of feeding at 16°C. At 10°C. they are slow and clumsy, barely capable of normal locomotion. At temperatures near freezing they are torpid; they can survive temperatures a little below freezing, but cannot survive being frozen solid. More than half the year is spent in hibernation in northeastern Kansas. Weight loss is slight during hibernation.
Normally the skinks emerge from hibernation in early April in northeastern Kansas, several weeks earlier in the southern states and correspondingly later in the northern part of the range. Maximum activity occurs in the period of weeks following emergence; interrupted [145] from time to time by cold weather which necessitates return to torpidity. After approximately three weeks of activity the adults attain breeding condition. Breeding males acquire a salmon red suffusion of the head region. They become pugnacious and fight on sight. Fighting does not involve territorial defense. In confinement males may mutilate or kill each other. In their search for females, and fighting, the breeding males are so much more active and conspicuous than they are at other times of year that published descriptions usually refer to males as red-headed, with no cognizance of the fact that this condition exists for only a few weeks in the annual cycle. Old adult males lose the striped pattern and blue color of the tail of the young, and are golden brown, usually a little darker on the sides. Males find females by a combination of sight and scent. Sexual relations are promiscuous, and there is but little courtship behavior. The male pursues the female and grasps in his jaws loose skin at or behind her shoulder region, and maintains this hold during copulation which lasts about five minutes. Within a few days after insemination, usually in early May, females become actively hostile to males. In late May or early June the gravid females become unusually secretive and excavate nest burrows in damp soil under flat rocks, or in rotten wood of decaying logs and stumps. The single annual clutch of eggs is laid in June. The average clutch is somewhat more than nine eggs, with larger and older females slightly exceeding younger and smaller females in average productivity. The female remains in the nest burrow with her clutch most of the time, from laying until after hatching. She alters the nest burrow, dampens it in time of drought, keeps the cavity from being filled with loose soil, prevents the eggs from adhering to the sides or floor of the cavity, and she may repulse certain small predators capable of destroying the eggs if they were left undefended. When they are laid, the eggs are approximately 11 × 71⁄2 mm. and weigh .4 grams or a little less. By hatching time they have enlarged to 15 × 11 mm. and each weighs about a gram. Recorded incubation periods vary from 27 days to 47 days; development of the embryo is slowed at low temperatures, and eggs experimentally kept in a refrigerator at 11° to 12°C. for periods of days hatched later than others of the same clutch that developed under normal conditions, indicating that development was almost halted in the eggs kept at such low temperatures. Eggs are, however, tolerant of a wide range of temperature, and can develop in nearly dry soil, or can survive partial submersion in water for at least two days. Under weather conditions prevailing in 1951, incubation periods of about six weeks were recorded. Incubation may be shortened [146] by retention of ova in the oviducts in early stages of embryonic development. Hatching may occur from the first week of July to mid-August, but in any one year most clutches hatch within two weeks of each other. Hatching of eggs in a clutch extends over a day or two. The hatchling gradually becoming active inside the egg, slits the leathery shell with its egg tooth, and spends several hours in the early stages of emergence. After resting with head and shoulders protruding, becoming adjusted to the outside environment and gaining strength, it lunges from the egg. For a day or two after hatching, the young remains in the nest, being slow and feeble, and handicapped in its movements by the protruding belly distended by the yolk mass. The female usually remains in the nest cavity for a day or two after the eggs hatch, showing affinity for the young by curling around them protectively. Family ties are broken as soon as the young leave the nest, and they do not return. Hatchlings average a little less than an inch in snout-vent length, and have a sharply defined five-lined pattern on a black ground color, and vivid blue tail. Hatchlings make rapid growth in late summer, and by the time of their retirement into hibernation, the more successful may have doubled in length, and may have increased their original weight, of approximately .3 or .4 grams, more than eight-fold. After emergence from their first hibernation the young continue their rapid growth. When they are a year old, some of them are as large as small adults. However, they can usually be distinguished from adults by the more sharply defined pattern. These grown young retain the hatchling pattern but the contrast between stripes and ground color, and between body and tail is not quite so sharp. Especially in those with regenerated tails, the vivid blue of the hatchling’s tail has become much dulled. By the time they retire to their second hibernation, the young have mostly grown to small adult size. A small percentage are retarded in their growth and fail to mature. Upon emergence from their second hibernation, the grown young mature sexually and participate in the annual breeding season, in early May, and they may comprise the majority of breeding adults. The ratio of new adults to old adults however varies from year to year depending on the varying fortunes of successive annual broods. The new adults overlap older ones in size, but are usually distinguishable on the basis of their coloration, as they retain the striped body pattern (dulled, especially on the head) and with distinctly blue color on the tail. In skinks that are three years old or more, the dorsal stripes have become obscured and partly blended with the ground color, which becomes progressively paler with advancing age. Metamorphosis is most complete in old [147] males, which retain no trace of the stripes or of the blue color on the tail. Old females usually retain the dark lateral area, and the tail is usually bluish gray, with a blue scale remaining here and there if the tail has not been regenerated.
Most individuals lose their original tails, however. By the time the young are approximately two months old, about half have had their tails broken, and by the time they are a year old and have grown to small adult size, three-fourths have regenerated tails. Some individuals may have had their tails broken and regenerated many times. Tails regenerate rapidly and most of the growth is made within the first few weeks. The regenerated tail is not so long as the original lost portion.
Individuals tend to stay within small areas which are their regular home ranges. These ranges are only a fraction of an acre in extent, but vary considerably in size and shape according to the individual and the situation. Home ranges of approximately 90-foot diameter for adult males and young, and a little more than 30-foot diameter for adult females are indicated. A home range generally centers about some environmental feature providing shelter and food, such as a log, hollow tree, or rock outcrop. Activity tends to be concentrated in the central part of the home range. An individual may continue to occupy the same home range throughout its lifetime, or it may gradually alter its range, shifting by slow stages into a new area. Some individuals seem to “get lost” or voluntarily shift, and settle in a new area which may be hundreds of feet removed from the original range. Shifts are most likely to occur after emergence from hibernation, when the lizard finds its habitat somewhat altered. Individuals released in areas strange to them settled down and established new home ranges, either immediately or after brief wandering.
The five-lined skink is a predator, occasionally taking small vertebrates (lizards and possibly newborn mice) but depending for most of its food on invertebrates. Of these it takes a wide variety. Spiders are the mainstay of the diet, and various salticids and lycosids are the kinds most frequently preyed upon. Phalangids are also eaten. Of insects, orthopterans (including roaches, ceuthophilid and gryllid crickets, grouse locusts, and small grasshoppers) are most important in the food. Larvae of moths and both larvae and adults of beetles are also taken in quantity. Small snails make up an important part of the diet, and the skinks often eat their own sloughed skins. Less frequently taken food items include certain large ants, centipedes, moths, and miscellaneous insects. Rarely the adult skinks may even eat eggs or young of their own species. [148] Prey is found by sight and scent, and consists of almost any small animals within a certain size range (small enough to be swallowed entire, but large enough to make up a substantial part of a meal) of types which are not too heavily armored, lack noxious defensive secretions, and live on or in the ground or decaying wood. They are ordinarily crushed in the jaws or battered against the ground, and then swallowed entire.
Natural enemies of the five-lined skink certainly include the broad-winged hawk and probably other kinds of hawks and also include various predatory mammals such as the opossum, armadillo, skunks, moles and shrews; snakes (the copperhead, milk snake, king snake, corn snake, and probably others), the Sonoran skink and even the closely related but larger Eumeces laticeps. On the Reservation, the short-tailed shrew was certainly the commonest, and probably by far the most important natural enemy. A high proportion of the skinks examined had scars resembling those inflicted by shrews experimentally confined with skinks in captivity. In time of danger escape reactions vary according to the type of enemy and the attending circumstances. Frequently an alarmed skink may escape into a hole or crevice, running directly to it from a distance of several yards. Under other circumstances a skink may burrow into ground litter of dry leaves and other debris, or may even dive and hide underwater. One of the commonest escape reactions is climbing tree trunks. It occurs even in gravid females that are slow and clumsy, being weighed down with eggs. Generally the skink stays on the main trunk of the tree, attempting to conceal itself by utilizing the screening vegetation that is available. In the young, especially, the bright blue tail seems to be used as a decoy, for it is carried, arched high and waved conspicuously as the lizard moves about. Rapid lashing movements of the conspicuous tail as the animal darts erratically for shelter may serve to confuse a pursuer, at least as to the direction that the skink has taken.
This skink is parasitized by various helminths, both cestodes and nematodes, which inhabit the digestive tract and body cavity. Some of these infest many kinds of amphibian and reptilian hosts, but others may be confined to the five-lined skink. Their life cycles, and effect upon the host are not well known. Ectoparasites consist principally of chiggers. Three kinds have been recorded on the skinks; Trombicula alfreddugesi, T. splendens, and T. gurneyi. The first two are common pest chiggers which attack humans as well as a wide variety of other mammalian, avian and reptilian hosts. T. gurneyi is a less common species found mainly on Eumeces fasciatus and confined to its woodland habitat.
Allard, H. A.
1909. Notes on some salamanders and lizards of North Georgia. Science, 30:122-124.
Anderson, P.
1942. Amphibians and reptiles of Jackson County, Missouri. Bull. Chicago Acad. Sci., 6 (no. 11):203-220.
Anonymous.
1945. Principal game birds and mammals of Texas. Texas Game Fish and Oyster Commission, Von Boeckman-Jones Co. Press, Austin, Texas. 149 pp.
Axelrod, D. I.
1950. Studies in late Tertiary paleobotany. Carnegie Inst. Wash., Publ. 590, 323 pp.
Barbour, R. W.
1950. The reptiles of Big Black Mountains, Harlan County, Kentucky. Copeia, 1950:100-107.
Barbour, T., and Carr, A. F.
1940. Eumeces in the Miami area. Copeia, 1940:129.
Bishop, C.
1926. Records of some amphibians and reptiles from Kentucky. Copeia, 152:118-120.
Bishopp, F. C., and Trembley, H. L.
1945. Distribution and hosts of certain North American ticks. Journ. Parasit., 31:1-54, 18 figs.
Blanchard, F. W.
1922. The amphibians and reptiles of western Tennessee. Occ. Pap. Mus. Zool. Univ. Michigan, 117:1-18.
Blanchard, F. N., and Finster, E. B.
1933. A method of marking snakes for future recognition, with a discussion of some problems and results. Ecology, 13:334-347, 7 figs.
Bogert, C. M.
1949. Thermoregulation in reptiles, a factor in evolution. Evolution, 3:195-211.
Bogert, C. M., and Cowles, R. B.
1947. Moisture loss in relation to habitat selection in some Floridian reptiles. Amer. Mus. Nov., 1358:1-34, 23 figs.
Boyer, D. A., and Heinze, A. A.
1934. An annotated list of the amphibians and reptiles from Jefferson County, Missouri. Trans. Acad. Sci., St. Louis, 28 (no. 4):183-200.
Brady, M.
1927. Notes on the amphibians and reptiles of the Dismal Swamp. Copeia, 162:26-29.
Braun, E. Lucy.
1950. Deciduous forests of eastern North America. The Blakiston Co., Philadelphia, xiv + 596 pp.
Breckenridge, W. J.
1943. The life history of the black-banded skink Eumeces septentrionalis (Baird). Amer. Midl. Nat., 29:591-606, 7 figs.
1944. Reptiles and amphibians of Minnesota. Univ. Minnesota Press, pp. 1-202.
Brimley, C. S.
1903. Notes on the reproduction of certain reptiles. Amer. Nat., 37:261-266.
Brown, B. C.
1950. An annotated check list of the reptiles and amphibians of Texas. Baylor Univ. Studies, Baylor Univ. Press, Waco, Texas, xii + 257 pp.
Brumwell, M. J.
1951. An ecological survey of the Fort Leavenworth Military Reservation. Amer. Midl. Nat., 45:187-231, 6 pls.
Burt, C. E.
1928a. The lizards of Kansas. Trans. Acad. Sci. St. Louis, 26:1-81.
1928b. Insect food of Kansas lizards with notes on feeding habits. Journ. Kansas Ent. Soc., 1:50-68.
1937. The lizards of the southeastern United States. Trans. Kansas Acad. Sci., 40:349-366.
Cagle, F. R.
1940. Eggs and natural nests of Eumeces fasciatus. Amer. Midl. Nat., 23:227-233, 2 figs.
1942. Turtle populations in southern Illinois. Copeia, 1942:155-162.
1944. Home range, homing behavior, and migration in turtles. Misc. Publ. Mus. Zool., Univ. Michigan, 61:1-34, 2 pls.
Carpenter, C. R.
1952. Comparative ecology of the common garter snake (Thamnophis s. sirtalis), the ribbon snake (Thamnophis s. sauritus) and Butler’s garter snake (Thamnophis butleri) in mixed populations. Ecol. Monogr., 22:235-258, 11 figs.
Carr, A. F.
1940. A contribution to the herpetology of Florida. Univ. Florida Publ. Biol. Ser., 3:iv + 118 pp.
Clausen, R. T.
1938. Notes on Eumeces anthracinus in central New York. Copeia, 1938:3-7.
Cockrum, E. L.
1952. Mammals of Kansas. Univ. Kansas Publ., Mus. of Nat. Hist., 7:303 pp., 73 figs.
Conant, R.
1951. The reptiles of Ohio. (Reprinted with supplement) Amer. Midl. Nat., 20:1-200, 26 pls., 38 maps.
Cook, F. A.
1942. Alligators and lizards in Mississippi. Bull. Mississippi State Fish and Game Comm., v + 20 pp.
Corrrington, J. D.
1929. Herpetology of the Columbia, South Carolina, region. Copeia, 172:58-83.
Cowles, R. B., and Bogert, C. M.
1944. A preliminary study of the thermal requirements of desert reptiles. Bull. Amer. Mus. Nat. Hist., 83:261-296, pls. 19-29, figs. 1-3.
Crabb, W. D.
1941. Food habits of the prairie spotted skunk in southeastern Iowa. Journ. Mamm., 22:349-364.
Deckert, R. F.
1918. A list of reptiles from Jacksonville, Florida. Copeia, 54:30-33.
Ditmars, R. L.
1907. The reptile book. Doubleday, Page and Co., New York, xxxii + 472p pp., 136 pls.
Dunn, E. R.
1920. Some reptiles and amphibians from Virginia, North Carolina, Tennessee and Alabama. Proc. Biol. Soc. Washington, 33:129-138.
Edgren, R. A., and Stille, W. T.
1948. Checklist of Chicago area amphibians and reptiles. Nat. Hist. Misc., 26:1-7.
Engels, W. L.
1949. The blue-tailed skinks (Eumeces) of two North Carolina coastal islands. Copeia, 1949 (4):269-271.
Evans, H. E., and Roecker, R. M.
1951. Notes on the herpetology of Ontario, Canada. Herpetologica, 7:69-71.
Fitch, H. S.
1940. A field study of growth and behavior in the fence lizard. Univ. California Publ. Zool., 44:151-172.
1949a. Road counts of snakes in western Louisiana. Herpetologica, 5:87-90.
1949b. Studies of snake populations in central California. Amer. Midl. Nat., 41:513-579.
1951. A simplified type of funnel trap for reptiles. Herpetologica, 7:77-80.
1952. The University of Kansas Natural History Reservation. Univ. Kansas Mus. Nat. Hist. Misc. Publ., 4:1-38, 4 pls., 3 figs.
Force, E. R.
1930. The amphibians and reptiles of Tulsa County, Oklahoma, and vicinity. Copeia, 1930:25-39.
Freiburg, R. E.
1951. An ecological study of the narrow-mouthed toad (Microhyla olivacea) in northeastern Kansas. Trans. Kansas Acad. Sci., 54:374-386.
Gloyd, H. K.
1928. The amphibians and reptiles of Franklin County, Kansas. Trans. Kansas Acad. Sci., 31:115-141.
1932. The herpetological fauna of the Pigeon Lake region, Miami County, Kansas. Occ. Pap. Michigan Acad. Sci. Arts and Letters, 15:389-409.
Goin, O. B., and Goin, C. J.
1951. Notes on the natural history of the lizard, Eumeces laticeps in northern Florida. Joun. Florida Acad. Sci., 14:29-33.
Goin, C. J., and Richmond, N. D.
1938. Notes on a collection of amphibians and reptiles from New Kent County, Virginia. Ann. Carnegie Mus., 27:301-310.
Goodman, J. D.
1948. A report on the reptiles collected by J. M. Shaffer from the Keokuk Area, 1863-1895. Proc. Iowa Acad. Sci., 55:365-366.
Hamilton, W. J., Jr.
1947. Eumeces fasciatus in northern New York. Copeia, 1947:64.
1948. Hibernation site of the lizards Eumeces and Anolis in Louisiana. Copeia, 1948:211.
Hartweg, N.
1931. Apparent ovoviviparity in the Mexican skink, Eumeces lynxe Wiegmann. Copeia, 1931:61.
Harwood, P. D.
1932. The helminths parasitic in the Amphibia and Reptilia of Houston, Texas, and vicinity. Proc. U. S. Nat. Mus., 81, 17:1-71.
Hoffman, R. L.
1945. Range extension for Eumeces laticeps Taylor. Proc. Biol. Soc. Washington, 58:131-132.
1953. Interesting herpesian records from Camp Pickett, Virginia. Herpetologica, 8:171-174.
Hollick, A.
1936. The Tertiary floras of Alaska. U. S. D. I. Professional Papers, 182:171 pp., 122 pls.
Hoyle, W. L.
1937. Notes on faunal collecting in Kansas. Trans. Kansas Acad. Sci., 39:283-293.
Hudson, G. E.
1942. The amphibians and reptiles of Nebraska. Nebraska Cons. Bull., 24:iv + 146 pp., 20 pls., 32 figs.
Hurter, J. H.
1911. Herpetology of Missouri. Trans. Acad. Sci. St. Louis, 20:59-274.
Jopson, H.
1938. Observation of the survival value of the character of the blue tail in Eumeces. Copeia, 1938:90.
1940. Reptiles and amphibians from Georgetown, South Carolina. Herpetologica, 2:39-43.
Kardos, E. H.
1952. Biological and systematic studies on the subgenus Neotrombicula (genus Trombicula) in the United States (Acarina, Trombiculidae). Unpublished thesis, University of Kansas Library.
Klots, A. B.
1930. Notes on Amphibia and Lacertilia collected at Weymouth, N. J. Copeia, 173:107-111.
Leonard, A. B., and Goble, C. R.
1952. Mollusca on the University of Kansas Natural History Reservation. Univ. Kansas Sci. Bull., 34:1013-1055, 2 pls.
Linsdale, J. M.
1927. Amphibians and reptiles of Doniphan County, Kansas. Copeia, 1927:75-81.
Logier, E. B.
1939. The reptiles of Ontario. Royal Ontario Mus. Handbook, 4:63 pp., 7 pls.
Lowe, C. H., Jr., and Norris, K. S.
1950. Aggressive behavior in male sidewinders Crotalus cerastes, with a discussion of aggressive behavior and territoriality in snakes. Nat. Hist. Misc., 66:1-13.
Lynn, W. G.
1936. Reptile records from Stafford County, Virginia. Copeia, 1936:169-170.
Mansueti, R.
1948. “Scorpion” of the tree tops. Natural History, 57:213-215 and 240.
McCauley, R. H., Jr.
1939. Differences in the young of Eumeces fasciatus and Eumeces laticeps. Copeia, 1939:93-95.
McIlhenny, E. A.
1937. Notes on the five-lined skink. Copeia, 1937:232-233.
Mills, C.
1948. A check list of the amphibians and reptiles of Canada. Herpetologica, 4 (second supplement):1-15.
Neill, W. T.
1948a. The lizards of Georgia. Herpetologica, 4:153-158.
1948b. Hibernation habits of amphibians and reptiles in Richmond County, Georgia. Herpetologica, 4:107-114.
1950. Reptiles and amphibians in urban areas of Georgia. Herpetologica, 6:113-116.
Neill, W. T., and Allen, R.
1950. Eumeces fasciatus in Florida. Copeia, 1950:156.
Netting, M. G.
1939a. The reptiles of Pennsylvania. Biennial Rept. Pennsylvania Fish. Comm., 1936-1938:122-132.
1939b. Reptiles killed on a “Vermin” campaign in Mercer County, West Virginia. Proc. West Virginia Acad. Sci., 13:162-166.
Newmann, H. H., and Patterson, J. T.
1909. Field studies of the behavior of the lizard, Sceloporus spinosus floridanus. Bull. Univ. Texas Sci. Ser., 15:1-24, 13 figs.
Nichols, J. T.
1939. Range and homing of individual box turtles. Copeia, 1939:125-127.
Noble, G. K., and Bradley, H. T.
1933. The mating behavior of lizards; its bearing on the theory of sexual selection. Ann. New York Acad. Sci., 35:25-100.
Noble, G. K., and Kumpf, K. F.
1936. The function of Jacobson’s organ in lizards. Journ. Gen. Psych., 48:371-382.
Noble, G. K., and Mason, E. R.
1933. Experiments on the brooding habits of the lizards Eumeces and Ophisaurus. Amer. Mus. Nov., 619:1-29.
Noble, G. K., and Teale, H. K.
1930. The courtship of some iguanid and teiid lizards. Copeia, 1930:54-56.
Oliver, J.
1951. Ontogenetic changes in osteodermal ornamentation in skinks. Copeia, 1951:127-130.
Owen, V.
1949. New snake records and notes from Morgan County, Missouri. Herpetologica, 5:49-50.
Parker, M. V.
1942. Notes on the herpetology of Clay and Greene counties, Arkansas. Proc. Arkansas Acad. Sci., 2:15-30.
1948. A contribution to the herpetology of western Tennessee. Journ. Tennessee Acad. Sci., 22:20-30.
Patch, C. L.
1934. Eumeces in Canada. Copeia, 1934:50-51.
Pope, C. H.
1944. Amphibians and reptiles of the Chicago area. Chicago Nat. Hist. Mus. Press, 275 pp., 12 pls.
Reese, R. W.
1949. The occurrence of Eumeces inexpectatus in Kentucky. Nat. Hist. Misc., 39:1-2.
Reynolds, A. E.
1943. The normal seasonal reproductive cycle in the male Eumeces fasciatus together with some observations on the effect of castration and hormone administration. Journ. Morph., 72:331-375, 2 pls.
1947. Sex hormones responses of the hemipenis of Eumeces fasciatus as reflected by organ weight. Proc. Indiana Acad. Sci., 57:191-198.
Reynolds, H. C.
1945. Some aspects of the life history and ecology of the opossum in central Missouri. Journ. Mamm., 26:361-379.
Rodgers, T. L., and Memmler, V. H.
1943. Growth in the western blue-tailed skink. Trans. San Diego Soc. Nat. Hist., 10:61-68, 1 fig.
Sandidge, L. L.
1953. Food and dens of the opossum (Didelphis virginiana) in northeastern Kansas. Trans. Kansas Acad. Sci., 56:97-106.
Schmidt, K. P.
1933. Notes on the breeding habits of lizards. Zool. Ser. Field Mus. Nat. Hist., 20:71-76.
1946. On the zoogeography of the Holarctic region. Copeia, 1946:144-152, 1 fig.
1950. The concept of geographic range, with illustrations from amphibians and reptiles. The Texas Journal of Sciences, 1950, No. 3:326-334.
Schroeder, R. C.
1951. A range extension for Eumeces laticeps. Herpetologica, 7:172.
Scott, T. G., and Sheldahl, R. B.
1937. Black-banded skink in Iowa. Copeia, 1937:192.
Seibert, H. C., and Hagen, C. W., Jr.
1947. Studies on a population of snakes in Illinois. Copeia, 1947:2-22.
Simpson, G. G.
1947. Holarctic mammalian faunas and continental relationships during the Cenozoic. Bull. Geol. Soc. Am., 58:613-688, 6 figs.
Shelford, V. E.
1945. The relative merits of the life zone and biome concepts. Wilson Bull., 57:248-252, 1 map.
Smith, H. M.
1946. Handbook of lizards. Comstock Publishing Co., Ithaca, N. Y. xxi + 557 pp., 135 pls., 136 figs., 41 maps.
1950. Handbook of amphibians and reptiles of Kansas. Univ. Kansas Publ., Mus. Nat. Hist., Misc. Publ., 2:1-336 pp., 233 figs.
Smith, H. M., and Etheridge, R.
1953. Resurrection of Plestiodon Bellii Gray (Reptilia: Squamata: Lacertilia) for a Mexican Skink. Herpetologica, 8:153-161.
Smith, H. M., and Leonard, A. B.
1934. Distributional records of amphibians and reptiles in Oklahoma. Am. Midl. Nat., 15:190-196.
Smith, M. A.
1935. Reptilia and Amphibia. Vol. II-Sauria in The fauna of British India including Ceylon and Burma. Taylor and Francis, London, xiii + 440 pp., 92 figs.
Smith, P. W.
1947. The reptiles and amphibians of western and central Illinois. Bull. Chicago Acad. Sci., 8:21-40.
Stebbins, R. C.
1948. Nasal structure in lizards with reference to olfaction and conditioning of inspired air. Am. Journ. Anat., 83:183-221.
Stebbins, R. C., and Robinson, H. B.
1946. Further analysis of a population of the lizard Sceloporus graciosus gracilis. Univ. California Publ. Zool., 48:149-168, 7 pls., 2 figs.
Stickel, L. F.
1950. Populations and home range relationships of the box turtle, Terrapene c. carolina (Linnaeus). Ecol. Monogr., 20:351-378.
Stickel, W. H., and Cope, J. B.
1947. The home ranges and wanderings of snakes. Copeia, 1947:127-136.
Swanson, P.
1939. Herpetological notes from Indiana. Amer. Midl. Nat., 22:684-696.
Taylor, E. H.
1932a. Eumeces inexpectatus: a new American lizard of the family Scincidae. Univ. Kansas Sci. Bull., 20:251-258, pls. 17-18.
1932b. Eumeces laticeps: a neglected species of skink. Univ. Kansas Sci. Bull., 20:263-272, pls. 19-20.
1935. Arkansas amphibians and reptiles in the Kansas University Museum. Univ. Kansas Sci. Bull., 22:207-218.
1936. A taxonomic study of the cosmopolitan scincoid lizards of the genus Eumeces with an account of the distribution and relationships of its species. Univ. Kansas Sci. Bull., 23:1-643.
1943. Mexican lizards of the genus Eumeces, with comments on recent literature of the genus. Univ. Kansas Sci. Bull., 29:269-300.
Tihen, J. A.
1937. Additional records of amphibians and reptiles in Kansas counties. Trans. Kansas Acad. Sci., 40:401-409.
Tinbergen, N.
1948. Social releasers and the experimental method required in their study. Wilson Bull., 60:6-51.
Trowbridge, A. H.
1937. Ecological observations on amphibians and reptiles collected in southeastern Oklahoma. Amer. Midl. Nat., 18:285-303.
Uhler, F. M., Cottam, C., and Clarke, T. E.
1939. Food of snakes of the George Washington National Forest, Virginia. Trans. 4th Amer. Wildlife Conf., Amer. Wildlife Inst., Washington, D. C., 1939:605-622.
Webb, G. R.
1949. Notes on the coition and feeding of the blue-tailed skink. Copeia, 1949:294.
Werler, J. E., and McCallion, J.
1951. Notes on a collection of amphibians and reptiles from Princess Anne County, Virginia. Amer. Midl. Nat., 45:245-252.
Wharton, G. W., and Fuller, H. S.
1952. A manual of the chiggers. Memoirs Ent. Soc. Washington, 4:185 pp., 17 figs.
Williams, J. B.
1903. A further note on the blue-tailed lizard. Ottawa Naturalist, 17:60.
Wolfenbarger, K.
1953. Systematic and Biological Studies on North American Chiggers of the genus Trombicula, subgenus Eutrombicula (Acarina, Trombiculidae). Ann. Ent. Soc. Amer., 45:645-677.
Wright, A. H., and Bishop, S. C.
1915. A biological reconnaissance of the Okefinokee Swamp in Georgia. II. Snakes. Proc. Acad. Nat. Sci. Philadelphia, 67:139-192.
Transmitted March 25, 1954.
![]()
25-3559
Institutional libraries interested in publications exchange may obtain this series by addressing the Exchange Librarian, University of Kansas Library, Lawrence, Kansas. Copies for individuals, persons working in a particular field of study, may be obtained by addressing instead the Museum of Natural History, University of Kansas, Lawrence, Kansas. There is no provision for sale of this series by the University Library, which meets institutional requests, or by the Museum of Natural History, which meets the requests of individuals. Nevertheless, when individuals request copies from the Museum, 25 cents should be included, for each separate number that is 100 pages or more in length, for the purpose of defraying the costs of wrapping and mailing.
* An asterisk designates those numbers of which the Museum’s supply (not the Library’s supply) is exhausted. Numbers published to date, in this series, are as follows:
| Vol. 1. | 1. | The pocket gophers (Genus Thomomys) of Utah. By Stephen D. Durrant. Pp. 1-82, 1 figure in text; August 15, 1946. |
| 2. | The systematic status of Eumeces pluvialis Cope, and noteworthy records of other amphibians and reptiles from Kansas and Oklahoma. By Hobart M. Smith. Pp. 85-89. August 15, 1946. | |
| 3. | The tadpoles of Bufo cognatus Say. By Hobart M. Smith. Pp. 93-96, 1 figure in text. August 15, 1946. | |
| 4. | Hybridization between two species of garter snakes. By Hobart M. Smith. Pp. 97-100. August 15, 1946. | |
| 5. | Selected records of reptiles and amphibians from Kansas. By John Breukelman and Hobart M. Smith. Pp. 101-112. August 15, 1946. | |
| 6. | Kyphosis and other variations in soft-shelled turtles. By Hobart M. Smith. Pp. 117-124, 3 figures in text. July 7, 1947. | |
| *7. | Natural history of the prairie vole (Mammalian Genus Microtus). By E. W. Jameson, Jr. Pp. 125-151, 4 figures in text. October 6, 1947. | |
| 8. | The postnatal development of two broods of great horned owls (Bubo virginianus). By Donald F. Hoffmeister and Henry W. Setzer. Pp. 157-173, 5 figures in text. October 6, 1947. | |
| 9. | Additions to the list of the birds of Louisiana. By George H. Lowery, Jr. Pp. 177-192. November 7, 1947. | |
| 10. | A checklist of the birds of Idaho. By M. Dale Arvey. Pp. 193-216. November 29, 1947. | |
| 11. | Subspeciation in pocket gophers of Kansas. By Bernardo Villa R. and E. Raymond Hall. Pp. 217-236, 2 figures in text. November 29, 1947. | |
| 12. | A new bat (Genus Myotis) from Mexico. By Walter W. Dalquest and E. Raymond Hall. Pp. 237-244, 6 figures in text. December 10, 1947. | |
| 13. | Tadarida femorosacca (Merriam) in Tamaulipas, Mexico. By Walter W. Dalquest and E. Raymond Hall. Pp. 245-248, 1 figure in text. December 10, 1947. | |
| 14. | A new pocket gopher (Thomomys) and a new spiny pocket mouse (Liomys) from Michoacán, Mexico. By E. Raymond Hall and Bernardo Villa R. Pp. 249-256, 6 figures in text. July 26, 1948. | |
| 15. | A new hylid frog from eastern Mexico. By Edward H. Taylor. Pp. 257-264, 1 figure in text. August 16, 1948. | |
| 16. | A new extinct emydid turtle from the Lower Pliocene of Oklahoma. By Edwin C. Galbreath. Pp. 265-280, 1 plate. August 16, 1948. | |
| 17. | Pliocene and Pleistocene records of fossil turtles from western Kansas and Oklahoma. By Edwin C. Galbreath. Pp. 281-284. August 16, 1948. | |
| 18. | A new species of heteromyid rodent from the Middle Oligocene of northeastern Colorado with remarks on the skull. By Edwin C. Galbreath. Pp. 285-300, 2 plates. August 16, 1948. | |
| 19. | Speciation in the Brazilian spiny rats (Genus Proechimys, Family Echimyidae). By João Moojen. Pp. 301-406, 140 figures in text. December 10, 1948. | |
| 20. | Three new beavers from Utah. By Stephen D. Durrant and Harold S. Crane. Pp. 407-417, 7 figures in text. December 24, 1948. | |
| 21. | Two new meadow mice from Michoacán, Mexico. By E. Raymond Hall. Pp. 423-427, 6 figures in text. December 24, 1948. | |
| 22. | An annotated check list of the mammals of Michoacán, Mexico. By E. Raymond Hall and Bernardo Villa R. Pp. 431-472, 2 plates, 1 figure in text. December 27, 1949. | |
| 23. | Subspeciation in the kangaroo rat, Dipodomys ordii. By Henry W. Setzer. Pp. 473-573, 27 figures in text, 7 tables. December 27, 1949. | |
| 24. | Geographic range of the hooded skunk, Mephitis macroura, with description of a new subspecies from Mexico. By E. Raymond Hall and Walter W. Dalquest. Pp. 575-580, 1 figure in text. January 20, 1950. [ii] | |
| 25. | Pipistrellus cinnamomeus Miller 1902 referred to the Genus Myotis. By E. Raymond Hall and Walter W. Dalquest. Pp. 581-590, 5 figures in text. January 20, 1950. | |
| 26. | A synopsis of the American bats of the Genus Pipistrellus. By E. Raymond Hall and Walter W. Dalquest. Pp. 591-602, 1 figure in text. January 20, 1950. | |
| Index. Pp. 605-638. | ||
| *Vol. 2. | (Complete) Mammals of Washington. By Walter W. Dalquest. Pp. 1-444, 140 figures in text. April 9, 1948. | |
| Vol. 3. | *1. | The avifauna of Micronesia, its origin, evolution, and distribution. By Rollin H. Baker. Pp. 1-359, 16 figures in text. June 12, 1951. |
| *2. | A quantitative study of the nocturnal migration of birds. By George H. Lowery, Jr. Pp. 361-472, 47 figures in text. June 29, 1951. | |
| 3. | Phylogeny of the waxwings and allied birds. By M. Dale Arvey. Pp. 473-530, 49 figures in text, 13 tables. October 10, 1951. | |
| 4. | Birds from the state of Veracruz, Mexico. By George H. Lowery, Jr., and Walter W. Dalquest. Pp. 531-649, 7 figures in text, 2 tables. October 10, 1951. | |
| Index. Pp. 651-681. | ||
| *Vol. 4. | (Complete) American weasels. By E. Raymond Hall. Pp. 1-466, 41 plates, 31 figures in text. December 27, 1951. | |
| Vol. 5. | 1. | Preliminary survey of a Paleocene faunule from the Angels Peak area, New Mexico. By Robert W. Wilson. Pp. 1-11, 1 figure in text. February 24, 1951. |
| 2. | Two new moles (Genus Scalopus) from Mexico and Texas. By Rollin H. Baker. Pp. 17-24. February 28, 1951. | |
| 3. | Two new pocket gophers from Wyoming and Colorado. By E. Raymond Hall and H. Gordon Montague. Pp. 25-32. February 28, 1951. | |
| 4. | Mammals obtained by Dr. Curt von Wedel from the barrier beach of Tamaulipas, Mexico. By E. Raymond Hall. Pp. 33-47, 1 figure in text. October 1, 1951. | |
| 5. | Comments on the taxonomy and geographic distribution of some North American rabbits. By E. Raymond Hall and Keith R. Kelson. Pp. 49-58. October 1, 1951. | |
| 6. | Two new subspecies of Thomomys bottae from New Mexico and Colorado. By Keith R. Kelson. Pp. 59-71, 1 figure in text. October 1, 1951. | |
| 7. | A new subspecies of Microtus montanus from Montana and comments on Microtus canicaudus Miller. By E. Raymond Hall and Keith R. Kelson. Pp. 73-79. October 1, 1951. | |
| 8. | A new pocket gopher (Genus Thomomys) from eastern Colorado. By E. Raymond Hall. Pp. 81-85. October 1, 1951. | |
| 9. | Mammals taken along the Alaskan Highway. By Rollin H. Baker. Pp. 87-117, 1 figure in text. November 28, 1951. | |
| *10. | A synopsis of the North American Lagomorpha. By E. Raymond Hall. Pp. 119-202, 68 figures in text. December 15, 1951. | |
| 11. | A new pocket mouse (Genus Perognathus) from Kansas. By E. Lendell Cockrum. Pp. 203-206. December 15, 1951. | |
| 12. | Mammals from Tamaulipas, Mexico. By Rollin H. Baker. Pp. 207-218. December 15, 1951. | |
| 13. | A new pocket gopher (Genus Thomomys) from Wyoming and Colorado. By E. Raymond Hall. Pp. 219-222. December 15, 1951. | |
| 14. | A new name for the Mexican red bat. By E. Raymond Hall. Pp. 223-226. December 15, 1951. | |
| 15. | Taxonomic notes on Mexican bats of the Genus Rhogeëssa. By E. Raymond Hall. Pp. 227-232. April 10, 1952. | |
| 16. | Comments on the taxonomy and geographic distribution of some North American woodrats (Genus Neotoma). By Keith R. Kelson. Pp. 233-242. April 10, 1952. | |
| 17. | The subspecies of the Mexican red-bellied squirrel, Sciurus aureogaster. By Keith R. Kelson. Pp. 243-250, 1 figure in text. April 10, 1952. | |
| 18. | Geographic range of Peromyscus melanophrys, with description of new subspecies. By Rollin H. Baker. Pp. 251-258, 1 figure in text. May 10, 1952. | |
| 19. | A new chipmunk (Genus Eutamias) from the Black Hills. By John A. White. Pp. 259-262. April 10, 1952. | |
| 20. | A new piñon mouse (Peromyscus truei) from Durango, Mexico. By Robert B. Finley, Jr. Pp. 263-267. May 23, 1952. | |
| 21. | An annotated checklist of Nebraskan bats. By Olin L. Webb and J. Knox Jones, Jr. Pp. 269-279. May 31, 1952. | |
| 22. | Geographic variation in red-backed mice (Genus Clethrionomys) of the southern Rocky Mountain region. By E. Lendell Cockrum and Kenneth L. Fitch. Pp. 281-292, 1 figure in text. November 15, 1952. | |
| 23. | Comments on the taxonomy and geographic distribution of North American microtines. By E. Raymond Hall and E. Lendell Cockrum. Pp. 293-312. November 17, 1952. | |
| [iii] | 24. | The subspecific status of two Central American sloths. By E. Raymond Hall and Keith R. Kelson. Pp. 313-337. November 21, 1952. |
| 25. | Comments on the taxonomy and geographic distribution of some North American marsupials, insectivores, and carnivores. By E. Raymond Hall and Keith R. Kelson. Pp. 319-341. December 5, 1952. | |
| 26. | Comments on the taxonomy and geographic distribution of some North American rodents. By E. Raymond Hall and Keith R. Kelson. Pp. 343-371. December 15, 1952. | |
| 27. | A synopsis of the North American microtine rodents. By E. Raymond Hall and E. Lendell Cockrum. Pp. 373-498, 149 figures in text. January 15, 1953. | |
| 28. | The pocket gophers (Genus Thomomys) of Coahuila, Mexico. By Rollin H. Baker. Pp. 499-514, 1 figure in text. June 1, 1953. | |
| 29. | Geographic distribution of the pocket mouse, Perognathus fasciatus. By J. Knox Jones, Jr. Pp. 515-526, 7 figures in text. August 1, 1953. | |
| 30. | A new subspecies of wood rat (Neotoma mexicana) from Colorado. By Robert B. Finley, Jr. Pp. 527-534, 2 figures in text. August 15, 1953. | |
| 31. | Four new pocket gophers of the genus Cratogeomys from Jalisco, Mexico. By Robert J. Russell. Pp. 535-542. October 15, 1953. | |
| 32. | Genera and subgenera of chipmunks. By John A. White. Pp. 543-561, 12 figures in text. December 1, 1953. | |
| 33. | Taxonomy of the chipmunks, Eutamias quadrivittatus and Eutamias umbrinus. By John A. White. Pp. 563-582, 6 figures in text. December 1, 1953. | |
| 34. | Geographic distribution and taxonomy of the chipmunks of Wyoming. By John A. White. Pp. 584-610, 3 figures in text. December 1, 1953. | |
| 35. | The baculum of the chipmunks of western North America. By John A. White. Pp. 611-631, 19 figures in text. December 1, 1953. | |
| 36. | Pleistocene Soricidae from San Josecito Cave, Nuevo Leon, Mexico. By James S. Findley. Pp. 633-639. December 1, 1953. | |
| 37. | Seventeen species of bats recorded from Barro Colorado Island, Panama Canal Zone. By E. Raymond Hall and William B. Jackson. Pp. 641-646. December 1, 1953. | |
| Index. Pp. 647-676. | ||
| *Vol. 6. | (Complete) Mammals of Utah, taxonomy and distribution. By Stephen D. Durrant. Pp. 1-549, 91 figures in text, 30 tables. August 10, 1952. | |
| Vol. 7. | *1. | Mammals of Kansas. By E. Lendell Cockrum. Pp. 1-303, 73 figures in text, 37 tables. August 25, 1952. |
| 2. | Ecology of the opossum on a natural area in northeastern Kansas. By Henry S. Fitch and Lewis L. Sandidge. Pp. 305-338, 5 figures in text. August 24, 1953. | |
| 3. | The silky pocket mice (Perognathus flavus) of Mexico. By Rollin H. Baker. Pp. 339-347, 1 figure in text. February 15, 1954. | |
| 4. | North American jumping mice (Genus Zapus). By Philip H. Krutzsch. Pp. 349-472, 47 figures in text, 4 tables. April 21, 1954. | |
| 5. | Mammals from Southeastern Alaska. By Rollin H. Baker and James S. Findley. Pp. 473-477. April 21, 1954. | |
| 6. | Distribution of Some Nebraskan Mammals. By J. Knox Jones, Jr. Pp. 479-487. April 21, 1954. | |
| 7. | Subspeciation in the montane meadow mouse, Microtus montanus, in Wyoming and Colorado. By Sydney Anderson. Pp. 489-506, 2 figures in text. July 23, 1954. | |
| 8. | A new subspecies of bat (Myotis velifer) from southeastern California and Arizona. By Terry A. Vaughn. Pp. 507-512. July 23, 1954. | |
| More numbers will appear in volume 7. | ||
| Vol. 8. | 1. | Life history and ecology of the five-lined skink, Eumeces fasciatus. By Henry S. Fitch. Pp. 1-156, 26 figs. in text. September 1, 1954. |
| More numbers will appear in volume 8. | ||
Although both juvenal and juvenile both appear in the text. The term juvenal appears to represent any animal in first year skin, feathers or fur. Therefore, both words were retained as in the original printed version. No significant typographical error were found in the text. For the illustrations, the scale is indicated (i.e., × 9); but is to be taken as a relative scale here as monitor resolutions vary. The list of Publications was compiled at the end.