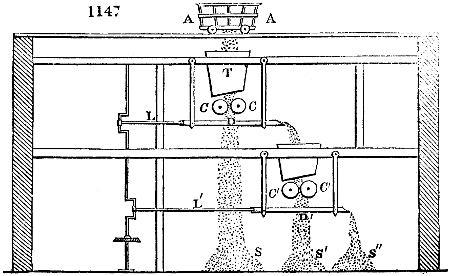
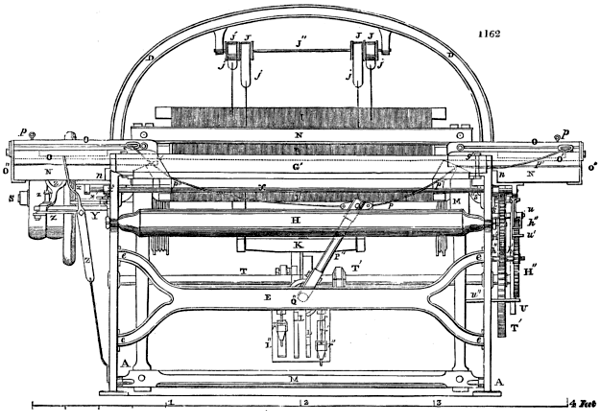
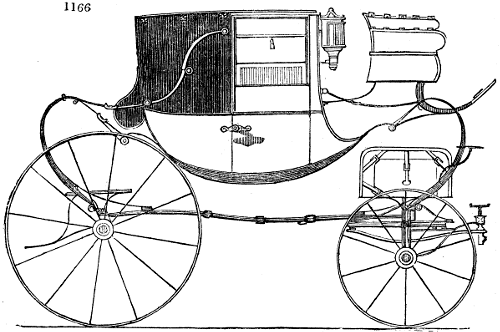
A
DICTIONARY
OF
ARTS, MANUFACTURES,
AND
MINES:
CONTAINING
A CLEAR EXPOSITION
OF THEIR PRINCIPLES
AND PRACTICE.
BY
ANDREW URE, M.D.
F.R.S. M.G.S. M.A.S. LOND.; M. ACAD. N.S. PHILAD.; S. PH. SOC. N. GERM.
HANOV.; MULII. ETC. ETC.
ILLUSTRATED WITH TWELVE HUNDRED AND FORTY
ENGRAVINGS ON WOOD.
Second Edition.
LONDON:
PRINTED FOR
LONGMAN, ORME, BROWN, GREEN, & LONGMANS,
PATERNOSTER-ROW.
1840.
London:
Printed by A. Spottiswoode,
New-Street-Square.
[iii]
PREFACE.
It is the business of operative industry to produce, transform, and distribute
all such material objects as are suited to satisfy the wants of
mankind. The primary production of these objects is assigned to the
husbandman, the fisherman, and the miner; their transformation to the
manufacturer and artisan; and their distribution to the engineer, shipwright,
and sailor.[1] The unworked or raw materials are derived,—1.
from the organic processes of vegetables and animals, conducted
either without or with the fostering care of man; 2. from the boundless
stores of mineral and metallic wealth, arranged upon or within the
surface of the earth by the benignant Parent of our being, in the fittest
condition to exercise our physical and intellectual powers in turning
them to the uses of life.
The task which I have undertaken in the present work, is to describe
and explain the transformations of these primary materials, by mechanical
and chemical agencies, into general objects of exchangeable value;
leaving, on the one hand, to the mechanical engineer, that of investigating
the motive powers of transformation and transport; and, on the other
hand, to the handicraftsman, that of tracing their modifications into objects
of special or local demand. Contemplated in this view, an art or manufacture
may be defined to be that species of industry which effects a
certain change in a substance, to suit it for the general market, by combining
its parts in a new order and form, through mechanical or chemical
means. Iron will serve the purpose of illustrating the nature of the distinctions
here laid down, between mechanical engineering; arts and
manufactures; and handicraft trades. The engineer perforates the ground
with a shaft, or a drift, to the level of the ore, erects the pumps for
drainage, the ventilating, and hoisting apparatus, along with the requisite[iv]
steam or water power; he constructs the roads, the bridges, canals,
railways, harbours, docks, cranes, &c., subservient to the transport of the
ore and metal; he mounts the steam or water power, and bellows for
working the blast-furnaces, the forges, and the cupolas; his principal
end and aim on all occasions being to overcome the forces of inertia,
gravity, and cohesion. The ores extracted and sorted by the miner,
and transported by the engineer to the smelting station, are there skilfully
blended by the iron-master (manufacturer), who treats them in a
furnace appropriately constructed, along with their due proportions of
flux and fuel, whereby he reduces them to cast iron of certain quality,
which he runs off at the right periods into rough pigs or regular
moulds; he then transforms this crude metal, by mechanical and chemical
agencies, into bar and plate iron of various sizes and shapes, fit for
the general market; he finally converts the best of the bars into steel,
by the cementation furnace, the forge, and the tilt-hammer; or the best
of the plates into tin-plate. When farther worked by definite and
nearly uniform processes into objects of very general demand in all
civilized countries, these iron and steel bars still belong to the domain
of manufactures; as, for example, when made into anchors, chain-cables,
files, nails, needles, wire, &c.; but when the iron is fashioned,
into ever varying and capricious forms, they belong either to the
general business of the founder and cutler, or to the particular calling
of some handicraft, as the locksmith, gratesmith, coachsmith, gunsmith,
tinman, &c.
Such are the principles which have served to guide me in selecting
articles for the present volume. By them, as a clue, I have endeavoured
to hold a steady course through the vast and otherwise perplexing labyrinth
of arts, manufactures, and mines; avoiding alike engineering and
mechanical arts, which cause no change in the texture or constitution of
matter,—and handicraft operations, which are multiform, capricious, and
hardly susceptible of scientific investigation. In fact, had such topics
been introduced into the volume, it would have presented a miscellaneous
farrago of incongruous articles, too numerous to allow of their being
expounded in a manner either interesting or instructive to the manufacturer
and the metallurgist. I readily acknowledge, however, that I have
not been able to adhere always so rigorously as I could have wished to
the above rule of selection; having been constrained by intelligent and
influential friends to introduce a few articles which I would gladly have
left to the mechanical engineer. Of these Printing is one, which, having
had no provision made for it in my original plan, was too hastily
compiled to admit of my describing, with suitable figures, the flat-printing
automatic machine of Mr. Spottiswoode, wherewith the pages of this
volume were worked off; a mechanism which I regard as the most
elegant, precise, and productive, hitherto employed to execute the best
style of letter press.
I have embodied in this work the results of my long experience as a
Professor of Practical Science. Since the year 1805, when I entered at
an early age upon the arduous task of conducting the schools of chemistry[v]
and manufactures in the Andersonian Institution, up to the
present day, I have been assiduously engaged in the study and improvement
of most of the chemical and many of the mechanical arts.
Consulted professionally by proprietors of factories, workshops, and
mines, of various descriptions, both in this country and abroad, concerning
derangements in their operations, or defects in their products, I have
enjoyed peculiar opportunities of becoming familiar with their minutest
details, and have frequently had the good fortune to rectify what was
amiss, or to supply what was wanting. Of the stores of information
thus acquired, I have availed myself on the present occasion; careful,
meanwhile, to neglect no means of knowledge which my extensive
intercourse with foreign nations affords.
I therefore humbly hope that this work will prove a valuable contribution
to the literature of science, serving—
In the first place, to instruct the Manufacturer, Metallurgist, and
Tradesman, in the principles of their respective processes, so as to render
them in reality the masters of their business, and to emancipate them
from a state of bondage to operatives, too commonly the slaves of blind
prejudice and vicious routine.
Secondly, to afford to Merchants, Brokers, Drysalters, Druggists, and
Officers of the Revenue, characteristic descriptions of the commodities
which pass through their hands.
Thirdly, by exhibiting some of the finest developments of chemistry
and physics, to lay open an excellent practical school to students of these
kindred sciences.
Fourthly, to teach Capitalists, who may be desirous of placing their
funds in some productive bank of industry, to select judiciously among
plausible claimants.
Fifthly, to enable Gentlemen of the Law to become well acquainted
with the nature of those patent schemes which are so apt to give rise to
litigation.
Sixthly, to present to our Legislators such a clear exposition of our
staple manufactures, as may dissuade them from enacting laws which
obstruct industry, or cherish one branch of it to the injury of many
others: and,
Lastly, to give the General Reader, intent chiefly on intellectual cultivation,
a view of many of the noblest achievements of science, in effecting
those grand transformations of matter to which Great Britain owes her
paramount wealth, rank, and power among the kingdoms.
The latest statistics of every important object of manufacture is given,
from the best, and, usually, from official authority, at the end of each
article.[2]
The following summary of our manufactures is extracted from
Mr. Macqueen’s General Statistics of the British Empire, published
in 1836. It shows the amount of capital embarked in the various[vi]
departments of manufacturing industry, and of the returns of that
capital:—
| |
Capital. |
Produce. |
| |
£ |
£ |
| Cotton |
manufactures |
40,973,872 |
52,513,586 |
| Woollen |
ditto |
36,000,000 |
44,250,000 |
| Silk |
ditto |
8,000,000 |
10,000,000 |
| Linen |
ditto |
12,000,000 |
15,421,186 |
| Leather |
ditto |
13,000,000 |
16,000,000 |
| Iron |
ditto, to making pig iron |
10,000,000 |
7,098,000 |
| Ditto, |
hardware, cutlery, &c. |
25,000,000 |
31,072,600 |
| Copper and brass |
3,600,000 |
4,673,186 |
| China, glass, &c. |
8,600,000 |
10,892,794 |
| Paper, furniture, books, &c. |
10,000,000 |
14,000,000 |
| Spirits (British), ales, soap, &c. |
37,600,000 |
47,163,847 |
| Sundries additional |
|
9,000,000 |
| Totals |
204,773,872 |
262,085,199 |
In consequence of an arrangement with Mr. William Newton,
patent agent, and proprietor of the London Journal of Arts, Sciences,
and Manufactures, I have been permitted to enrich this Dictionary
with many interesting descriptions and illustrative figures of modern
patent inventions and improvements, which I could not otherwise
have presented to my readers. Mr. Newton has lately enhanced the
value of his Journal by annexing to it a catalogue raisonnée, entitled
“An Analytical Index to the Subjects contained in the 23 Volumes,”
which constitute the first and second series. The subsequent 13 volumes,
of his Conjoined Series, are of still superior interest; and the whole form
a vast storehouse of Mechanical and Chemical Invention.
Although I am conscious of having used much diligence for many
years in collecting information for this work, from every quarter within
my reach, the utmost pains in preparing it for publication, and incessant
vigilance during its passage through the press, yet I am fully aware that
it must contain several errors and defects. These I shall study to
rectify, should the Public deem this volume worthy of a supplement.
In this hope, I earnestly solicit the suggestions of my readers; trusting
that ere long our Post Office system will cease to be such an obstacle
as it has long been to the collection and diffusion of useful knowledge,
and a tax upon science which the remuneration of its literature cannot
by any means bear.
Since this book is not a Methodical Treatise, but a Dictionary, one
extensive subject may be necessarily dispersed through many articles.
Thus, for example, information upon the manufacture of Colours will be
found under azure; black pigment; bone-black; bronze; brown dye;
calico-printing; carmine; carthamus; chromium; cochineal; crayons;
dyeing; enamels; gold; gilding; gamboge; gray dye; green dye;
green paints; indigo; kermes; lac dye; lakes; madder; massicot;
mercury, periodide of; Naples yellow; orange dye; orpiment; paints,
grinding of; ochres; paper-hangings; pastes; pearl white; Persian
berries; pottery pigments; Prussian blue; purple of Cassius; red lead;[vii]
rouge; Scheele’s green; Schweinfurth green; stained glass; terra di
Sienna; ultramarine; umber; verditer; vermilion; vitrifiable colours,
weld, white lead; woad; yellow, king’s.
A casual consulter of the Dictionary, who did not advert to this distribution,
might surmise it to be most deficient, where it is in reality
most copious.
The elaborate and costly Encyclopedias, and Dictionaries of Arts,
which have appeared from time to time in this country, and abroad,
have, for the most part, treated of the mechanical manufactures, more
fully and correctly than of the chemical. The operations of the former
are, in fact, tolerably obvious and accessible to the inspection of the
curious; nor are they difficult to transfer into a book, with the aid of a
draughtsman, even by a person but moderately versed in their principles.
But those of the latter are not unfrequently involved in complicated
manipulations, and depend, for their success, upon a delicate play of
affinities, not to be understood without an operative familiarity with the
processes themselves. Having enjoyed the best opportunities of studying
the chemical arts upon the greatest scale in this kingdom and on the
Continent, I may venture, without the imputation of arrogance, to claim
for my work, in this respect, more precision and copiousness than its
predecessors possess. I have gone as far in describing several curious
processes, hitherto veiled in mystery, as I felt warranted, without breach
of confidence, to go; regarding it as a sacred duty never to publish any
secret whatever, without the consent of its proprietor. During my
numerous tours through the factory districts of Great Britain, France,
&c., many suggestions, however, have been presented to my mind,
which I am quite at liberty to communicate in private, or carry into
execution, in other districts too remote to excite injurious competition
against the original inventors. I am also possessed of many plans
of constructing manufactories, of which the limits of this volume did
not permit me to avail myself, but which I am ready to furnish, upon
moderate terms, to proper applicants. I conclude by pointing attention
to the very insecure tenure by which patents for chemical or chemico-mechanical
inventions are held; of which there is hardly one on record
which may not be readily evaded by a person skilled in the resources of
practical chemistry, or which could stand the ordeal of a court of law
directed by an experienced chemist. The specifications of such patents
stand in need of a thorough reform; being for the most part not only
discreditable and delusive to the patentees, but calculated to involve them
in one of the greatest of evils—a chancery suit.
London:
13. Charlotte Street, Bedford Square,
March 1. 1839.
Dr. URE is preparing for publication, in one large volume, 8vo., Chemistry in
Theory and Practice; embodying a New System of Research, of such facility and
precision, as will enable chemical manufacturers of every class, medical practitioners,
metallurgists, farmers, merchants, brokers, druggists, drysalters, officers of the revenue,
as well as general students, to analyze their respective objects in much less time than
is usually required at present by professional chemists. A descriptive Index will be
annexed for converting this systematic work into a Dictionary of Chemical Science.
[1]
A
DICTIONARY
OF
ARTS, MANUFACTURES, AND MINES.
A.
ABB-WOOL. Among clothiers, this term signifies the woof or weft.
ACETATE. (Acétate, Fr.; Essigsäure, Germ.) Any saline compound of which
the acetic is the acid constituent; as acetate of soda, of iron, of copper, &c.
ACETATE OF ALUMINA, see Red Liquor and Mordant; of Copper, see
Copper; of Iron, see Iron; of Lead, see Lead; of Lime, see Pyrolignous Acid.
ACETIC ACID (Acide Acétique, Fr.; Essigsäure, Germ.) is the name of the sour
principle which exists in vinegar. It occurs, ready formed, in several products of
the vegetable kingdom, and is generated during the spontaneous fermentation of many
vegetable and animal juices. The sambucus nigra, or black elder, the phœnix dactilifera,
and the rhus typhinus are plants which afford a notable quantity of vinegar. It
is found, likewise, in the sweat, urine, milk, and stomach of animals. All infusions of
animal or vegetable matters in water, when exposed for some time to the air, at a moderate
temperature, ferment into vinegar; and most vegetables, when subjected to
decomposition by fire, give off condensable vapours of acetic acid. All liquids containing
alcohol are susceptible of passing into the state of vinegar; but the pre-existence
of alcohol is not necessary to this change, as we learn from the acetification of vegetable
soups, infusion of cabbage, starch—paste, &c.
Vinegar may be distinguished into four varieties, according to the mode of its production,
though all of them are capable of being converted, by chemical means, into
one identical acetic acid. 1. Wine vinegar. 2. Malt vinegar. 3. Sugar vinegar.
4. Wood vinegar, or pyrolignous acid. Fermentation is the source of the acid in the
first three varieties. Here alcohol is first generated, and is next converted into vinegar
by the influence of the air at a genial temperature; a change which will be investigated
under Fermentation. But the conversion of spirit of wine into acetic acid may be demonstrated
by direct experiment. When the vapour of alcohol is brought into contact
in the atmosphere with the black powder obtained by mixing muriate of platina, potash,
and alcohol, vinegar is rapidly formed at the expense of the alcohol. In Germany, where
crude alcohol bears a low price, the manufacture of vinegar has been arranged upon that
principle, which, as throwing some light on the process of acetification, I shall briefly
describe. See Platinum for the mode of preparing the above powder.
Under a large case, which for experimental purposes may be made of glass, several
saucer-shaped dishes of pottery or wood are to be placed in rows, upon shelves over
each other, a few inches apart. A portion of the black platina powder moistened being
suspended over each dish, let as much vinous spirits be put into them as the oxygen of
the included air shall be adequate to acidify. This quantity may be inferred from
the fact, that 1000 cubic inches of air can oxygenate 110 grains of absolute alcohol, converting
them into 122 grains of absolute acetic acid, and 641⁄2 grains of water.
The above simple apparatus is to be set in a light place (in sunshine, if convenient),
at a temperature of from 68° to 86° Fahr., and the evaporation of the alcohol is to be
promoted by hanging several leaves of porous paper in the case, with their bottom edges
dipped in the spirit. In the course of a few minutes, a most interesting phenomenon
will be perceived. The mutual action of the platina and the alcohol will be displayed
by an increase of temperature, and a generation of acid vapours, which, condensing on
the sides of the glass-case, trickle in streams to the bottom. This striking transformation
continues till all the oxygen of the air be consumed. If we wish, then, to renew
the process, we must open the case for a little, and replenish it with air. With a box
of 12 cubic feet in capacity, and with a provision of 7 or 8 ounces of the platina powder[2]
we can, in the course of a day, convert one pound of alcohol into pure acetic acid, fit for
every purpose, culinary or chemical. With from 20 to 30 pounds of the platina powder
(which does not waste), we may transform, daily, nearly 300 pounds of bad spirits into
the finest vinegar. Though our revenue laws preclude the adoption of this elegant
process upon the manufacturing scale in this country, it may be regarded as one of the
greatest triumphs of chemistry, where art has rivalled nature in one of her most mysterious
operations.
To readers acquainted with chemical symbols, the following numerical representation
of the conversion of alcohol into acetic acid may be acceptable:—
| 580·64 |
parts by weight |
of alcohol |
= |
H12 C4 O2 |
consist of |
| 74·88 |
|
of hydrogen |
= |
H12 |
| 305·76 |
|
of carbon |
= |
C4 |
| 200·00 |
|
of oxygen |
= |
O2 |
If we combine with this mixture, 400 parts of oxygen = O4, we have,—
| of water |
= |
337·44 |
= |
H6 O3 |
| acetic acid |
= |
643·20 |
= |
H6 C4 O3 |
Hence, in this formation of vinegar, 100 parts by weight of alcohol take 68·89 parts of
oxygen; and there are produced 58·11 parts of water, and 110·78 of acetic acid.
These beautiful experiments prove, that when in a mere mixture of alcohol and water,
under the influence of the atmospheric air and heat, some vinegar comes to be formed
after a considerable time, the same formation of vinegar takes place in a similar, but
more effective, manner, when a ferment is present, which acts here in a somewhat
analogous way to the platina powder in the preceding case. Several azotized substances
serve as re-agents towards the acetous fermentation,—such as vinegar ready-made,
vinegar-yeast, or lees, barley bread, leaven, beer barm, and similar vegetable
matters, which contain gluten. The best and purest ferment is, however, vinegar itself.
With this ferment we must conjoin, as an essential condition of acetification, the free
access of atmospheric air.
It is a well-known fact, that spirituous liquors, as weak brandy, wine, and beer, &c.,
may be preserved for years in close vessels, without undergoing the acetous fermentation,
even when they repose upon a layer of lees. It is equally well known, that these very
liquors, if they stand for some time in open vessels, become readily sour, especially if exposed,
also, to a somewhat high temperature. If we fill a flask with common brandy,
and subject it, without a stopper, to the influence of air and warmth, the contained
liquor may, at the end of many weeks, discover no sensible acidity: if we add to the
same brandy a ferment, and stop the flask air-tight, everything will still remain unchanged;
but if we leave a portion of air in the flask, or leave it uncorked, vinegar will
soon make its appearance in the brandy.
If we investigate the nature of the air which remains over brandy in the act of acetification,
we shall find that it consists entirely of carbonic acid and azote, the oxygen being
absorbed and combined in the acetic acid and water formed.
Since this absorption of oxygen from the air can take place only at the surface of the
fermenting liquors, we thus see the necessity and the practical importance of amplifying
that surface, in order to accelerate and complete the acetification, by multiplying the
points of contact between the alcohol and the oxygen. The essence of the new German
method of rapid acetification depends upon this principle.
Temperature has also a remarkable influence on the formation of vinegar. The acid
fermentation proceeds very feebly in the cold, but takes an accelerated pace as the heat
is raised. It would even appear that spirituous vapours brought by themselves in
contact with atmospheric air, without the aid of any ferment, are capable of being converted
into acetic acid, since it has happened in the rectification of brandy, in a still
furnished with a large capital and adopter pipe into which air was allowed to enter, that
vinegar made its appearance. Hence, warmth does not seem to act as a promoter of the
combination of alcohol with oxygen in a merely chemical point of view, but it acts, so to
speak, physically. Over the warm liquor a stratum of spirit vapour appears to float,
which, coming there into conflict with the atmospherical oxygen, probably causes the
generation of some acetic acid, and thus accelerates the operation, much more than by
the mere contact of the oxygen with the liquid surface.
When we expose any spirituous liquors, as wine, beer, &c., with the requisite
ferment, to the external air, at a temperature of from 64° to 68° Fahr., the fluid, however
clear before, becomes soon turbid; filamentous slimy particles begin to appear
moving in the middle and on the sides of the vessel, and then form a scum on the top of
the liquor. When this scum has acquired a certain thickness and consistence, it falls in
a sediment to the bottom. The Germans call it the vinegar mother, as it serves to excite
acetification in fresh liquors. Meanwhile, the liquor has become warmer than the surrounding[3]
air, and the vinegar process betrays itself by diffusing a peculiar aroma in the
apartment. Whenever all the alcohol present has been converted into acetic acid, the
liquor comes into a state of repose; its temperature sinks to the pitch of the atmosphere;
it becomes bright, and is the article well known by its taste and smell under the name
of vinegar.
Genuine wine or raisin vinegar differs from that formed either from apples, or sugar,
beer, &c., in containing wine-stone or tartar; by which peculiarity it may be distinguished,
except in those cases where crude tartar has been artificially added to the
other vinegars, as a disguise. Barley-malt vinegar contains some phosphoric acid, in
the state of phosphate of lime or magnesia, derived from the grain.
After these general observations upon acetification, we shall now proceed to describe
the processes for manufacturing vinegar on the commercial scale.
1. Wine vinegar.—The first consideration with a vinegar maker is a good fermenting
room, in which the wines may be exposed to a steady temperature, with an adequate
supply of atmospherical air. As this air is soon deprived of its oxygenous constituent,
facilities ought to be provided for a renewal of it by moderate ventilation. The air
holes for this purpose ought to be so contrived that they may be shut up when the
temperature begins to fall too low, or in windy weather. The best mode of communicating
the proper warmth to a chamber of this kind is by means of fire-flues or hot
water pipes, running along its floor at the sides and ends, as in a hothouse; the fireplace
being on the outside, so that no dust may be created by it within. The flue is best made
of bricks, and may have a cross section of 10 or 12 inches by 15 deep. The soot deposited,
even when coals are burned, will find ample space in the bottom of the flue, without
interfering essentially with the draught, for a very long period, if it be made of the
above dimensions. Low-roofed apartments are preferable to high ones; and those built
with thick walls, of imperfectly conducting materials, such as bricks, lined with lath
and plaster work. Should the chamber, however, have a high ceiling, the fermenting
tuns must be raised to a suitable height on scaffolding, so as to benefit by the warmest
air. Sometimes the vinegar vessels are placed at different levels; in which case the
upper ones acetify their contents much sooner than the under, unless they are emptied
and filled alternately, which is a good plan.
Orleans is the place most famous for vinegars. The building there destined to their
manufacture is called a vinaigrerie, and is placed, indifferently, either on the ground floor
or the floor above it; but it has always a southern exposure, to receive the influence
of the sunbeams. The vessels employed for carrying on the fermentation are casks,
called mothers. Formerly they were of a large capacity, containing about 460 litres
(115 gallons, Eng.); but at the present day they are barrels of half that capacity, or
somewhat less than an old English hogshead. It is now known that the wine passes
sooner into vinegar the smaller the mass operated upon, the more extensive its contact
with the air, and the more genial its warmth. These casks were formerly arranged in
three ranks by means of massive scaffolding; they are now set in four ranks, but they
rest on much smaller rafters, sustained by uprights, and can be packed closer together.
The casks, which are laid horizontally, are pierced at the upper surface of their front end
with two holes: one, to which the name of eye is given, is two inches in diameter; it
serves for putting in the charge, and drawing off the vinegar when it is made; the
other hole is much smaller, and is placed immediately alongside; it is merely an air
hole, and is necessary to allow the air to escape, because the funnel completely fills the
other hole in the act of filling the cask.
When new vessels are mounted in a vinegar work, they must be one third filled with
the best vinegar that can be procured, which becomes the true mother of the vinegar
to be made; because it is upon this portion that the wine to be acidified is successively
added. At the ordinary rate of work, they put at first upon the mother, which occupies
one third of the vessel, a broc of 10 litres of red or white wine; eight days afterwards
they add a second broc; then a third, and a fourth, always observing the same interval of
time, 8 days. After this last charge, they draw off about 40 litres of vinegar, and then
recommence the successive additions.
It is necessary that the vessel be always one third empty if we wish the acetification
to go on steadily; but as a portion of the tartar and the lees forms and accumulates in
the lower part of the cask, so as eventually to counteract the fermentation, the time
arrives when it is requisite to interrupt it, in order to remove this residuum, by clearing
out all the contents. The whole materials must be renovated every 10 years; but the
casks, if well made and repaired, will serve for 25 years.
We have mentioned a definite period at which the vinegar may be drawn off; but that
was on the supposition that the process had all the success we could wish: there are
circumstances, difficult to appreciate, which modify its progress, as we shall presently
show. We ought, therefore, before discharging the vinegar, to test and see if the fermentation[4]
has been complete. We proceed as follows: we plunge into the liquor a
white stick or rod, bent at one end, and then draw it out in a horizontal direction: if it
be covered with a white thick froth, to which is given the name of work (travail), we
judge that the operation is terminated; but if the work, instead of being white and
pearly, be red, the manufacturers regard the fermentation to be unfinished, and they
endeavour to make it advance, by adding fresh wine, or by increasing the heat of the
apartment.
It is not always easy to explain why the fermentation does not go on as rapidly in one
case as in another. There are even certain things which seem at present to be entirely
inexplicable. It happens sometimes, for example, that although all the vessels have been
equally charged, and with the same wine, yet the fermentation does not form in the same
manner in the whole; it will move rapidly in some, be languid, or altogether inert,
in others. This is a very puzzling anomaly; which has been ascribed to electrical and
other obscure causes, because it is not owing to want of heat, the casks in the warmest
positions being frequently in fault; nor to the timber of the cask. It, however, paralyses
the process so completely that the most expert vinegar makers have nothing else
for it, when this accident happens, than to empty entirely what they call the lazy
cask, and to fill it with their best vinegar. The fermentation now begins, and proceeds
as well in it as in the others. See Fermentation.
We must here make an important remark, relatively to the temperature which should
prevail in the fermentation room. In many chemical works we find it stated, that the
heat should not exceed 18° R., or 65° Fahr., for fear of obtaining bad products. But
the vinegar makers constantly keep up the heat at from 24° to 25° R., 75° to 77° F.;
when the acetification advances much more rapidly, and the vinegar is equally strong.
The best proof of this heat not being too high is, that under it, the vessels in the upper
part of the room, work best and quickest. In Orleans, cast-iron stoves and wood fuel are
used for communicating the requisite warmth.
Before pouring the wine into the mothers, it is clarified in the following manner.
There are tuns which can contain from 12 to 15 pieces of wine. Their upper end has at
its centre an opening of four or five inches diameter, which may be closed afterwards
with a wooden cover; this opening is for the purpose of receiving a large funnel.
The inside of the tun is filled with chips of beechwood, well pressed down. The wine
is poured upon these chips, allowed to remain for some time, and then gently drawn off
by a pipe in the lower part of the vessel. The lees are deposited upon the chips, and
the wine runs off quite clear. However, it happens sometimes, notwithstanding this
precaution, that the vinegar, after it is made, requires to be clarified, more particularly if
the wine employed had been weak. The vinegar must be filtered in the same way; and
it derives an advantage from it, as the products of different casks get thereby mixed and
made uniform.
By this Orleans method several weeks elapse before the acetification is finished; but
a plan has been lately devised in Germany to quicken greatly the acid fermentation by
peculiar constructions. This system is called, the quick vinegar work, because it will
complete the process in the course of 2 or 3 days, or even in a shorter time. It depends,
chiefly, upon the peculiar construction of the fermenting vessels, whereby the vinous
liquor is exposed on a vastly expanded surface to the action of the atmospheric air.
An oaken tub, somewhat narrower at the bottom than the top, from 6 to 7 feet high
and 3 feet in diameter, is furnished with a well-fitted grooved, but loose, cover. About
half a foot from its mouth, the tub has a strong oak or beech hoop fitted to its inside
surface, sufficiently firm to support a second cover, also well fitted, but moveable. The
space under this second cover is destined to contain the vinous liquor, and in order to
bring it very amply into contact with the atmosphere, the following contrivances have
been resorted to: This cover is perforated, like a sieve, with small holes, of from 1 to 2
lines in diameter, and about 11⁄2 inch apart. Through each of these holes a wick of pack-thread
or cotton is drawn, about 6 inches long, which is prevented from falling through
by a knot on its upper end, while its under part hangs free in the lower space. The
wicks must be just so thick as to allow of the liquor poured above the cover passing
through the holes in drops. The edges of the lid must be packed with tow or hemp to
prevent the liquor running down through the interval.
The whole lower compartment is now to be filled with chips of beechwood up to
nearly the perforated cover. The liquor, as it trickles through the holes, diffuses itself
over the chips, and, sinking slowly, collects at the bottom of the tub. The chips should
be prepared for this purpose by being repeatedly scalded in boiling water, then dried,
and imbued with hot vinegar. The same measures may also be adopted for the tub.
To provide for the renewal of the air, the tub is perforated at about a foot from its
bottom with eight holes, set equally apart round the circumference, two thirds of an inch
wide, and sloping down, through which the air may enter into this lower compartment,
without the trickling liquor being allowed to flow out. In order that the foul air which has[5]
become useless may escape, four large holes are pierced in the sieve cover, at equal distances
asunder and from the centre, whose united areas are rather smaller than the total areas
of the holes in the side of the tub. Into these four holes open glass tubes must be
inserted, so as to stand some inches above the cover, and to prevent any of the liquor
from running through them. The proper circulation of the air takes place through
these draught holes. This air may afterwards pass off through a hole of 21⁄2 inches
diameter in the uppermost cover, in which a funnel is placed for the supply of liquor as
it is wanted to keep up the percolation.
The temperature of the fermenting compartment is ascertained by means of a thermometer,
whose bulb is inserted in a hole through its side, and fastened by a perforated
cork. The liquor collected in the under vessel runs off by a syphon inserted near its
bottom, the leg of which turns up to nearly the level of the ventilating air pipes before it
is bent outwards and downwards. Thus the liquor will begin to flow out of the under
compartment only when it stands in it a little below the sieve cover, and then it will run
slowly off at the inclined mouth of the syphon, at a level of about 3 inches below the
lower end of the glass tubes. There is a vessel placed below, upon the ground, to
receive it. The tub itself is supported upon a wooden frame, or a pier of brickwork, a
foot or 18 inches high.
A tub constructed like the above is called a GRADUATION VESSEL, which see. It is worked
in the following way:—The vinegar room must be, in the first place, heated to from 100°
to 110° F., or till the thermometer in the graduation vessel indicates at least 77°. The
heat may then be modified. We now pour through the uppermost cover of the tub a
mixture, warmed to 144° F., of 8 parts proof spirits, 25 parts soft water, 15 parts of
good vinegar, and as much clear wine or beer. The water should be first heated, and then
the vinegar, spirits, and wine may be added to it. Of this mixture, so much should
be poured in as is necessary to cover over the second lid, 2 or 3 inches deep, with the
liquor; after which, the rest may be poured slowly in, as it is wanted.
When the liquor has run for the first time through the graduation vessel, it is not yet
sufficiently acidified; but the weak vinegar collected in the exterior receiving cistern
must be a second time, and, if need be, a third time, passed through the graduation tub,
in order to convert all the alcohol into acetic acid. In general, we may remark, that
the stronger the vinous liquor the more difficult and tedious is its conversion into
vinegar, but it is so much the stronger. To lessen this difficulty somewhat, it would be
well not to put all the spirits at first into the wash, or mixed liquors, but to add a little
more of it at the second and the third running, especially when we desire to have very strong
vinegar.
After the graduation vessel has been some days at work, it is no longer necessary to
add vinegar to the mixture of spirits and water, since the sides of the graduation tub, the
beech chips, and the packthreads, are all impregnated with the ferment, and supply its
place. The mixture must, however, be always maintained at the temperature of 100°.
Instead of the above mixture of brandy, water, and wine, we may employ, according to
Dingler, a clear fermented wort of malt, mixed with a little spirits. The perfect vinegar,
which collects in the receiving cistern, may be immediately racked off into the store
casks for sale.
It has been objected to this process, that, in consequence of the mixture of saccharine
and glutinous materials, which are contained in beer or worts, along with the acetous
fermentation, there is also, partially, a vinous fermentation, and much carbonic acid,
thereby disengaged, so as to obstruct the acetification. This obstruction may be remedied
by a freer circulation of air, or by the exposure of quicklime in the chamber. It is a
more substantial objection, that, from the addition of beer, &c., more lees, or dregs, are
deposited in the graduation tub, whereby a more frequent cleansing of it, and of the
beech chips, with a loss of time and vinegar, becomes necessary. The only mode of
obviating this difficulty is, to take well-clarified fermented wash.
Another evil attendant on the quick process is, the evaporation of the spirituous
liquors. Since, in the graduation tub, there is a temperature of 110°, it is impossible to
avoid a loss of spirit from the circulation and efflux of the air. The air, indeed, that
issues from the top hole in the uppermost cover, might be conducted over an extensive
surface of fresh water, where its spirit would be condensed in a great measure. But,
after all, this fear of great loss is, I believe, groundless; because the spirit is rapidly
acidified by the oxygen of the air, and thereby rapidly loses its volatility.
The supply of the warm wash should be drawn from a cistern placed near the ceiling,
where the temperature of the apartment is hottest; and it may be replenished from the
partly acetified liquor in the cistern on the floor. With this view, two cisterns should
be placed above, so that one of them may always contain liquor sufficiently hot, and thus
the process will suffer no interruption.
When malt wash is used for this quick process, the resulting vinegar must be clarified[6]
in a tun with beech chips, as above described. In two or three days the impurities will
be deposited, and the fine vinegar may be racked off.
The following prescription, for preparing what he calls malt wine, is given by Dr.
Kastner. Eighty pounds of pale barley malt, and 40 pounds of pale wheat malt, are to
be crushed together. These 120 pounds are to be infused with 150 quarts of water, at
the temperature of 122° Fahr., afterwards with 300 quarts of boiling water, and the
whole body is to be mashed thoroughly, till all the lumps disappear. It is then to
be left at rest in a large covered tub, for two or three hours, to allow the grains to
settle down, from which the wort is to be drawn off. When it has fallen to the temperature
of 64° Fahr., 15 pounds of good yeast are to be stirred in, and it must now be
left for two or three days to ferment, in a loosely covered tun. When the vinous fermentation
has taken place, the clear liquor must be drawn off by a tap hole, a little above
the bottom, so as to leave the lees and scum in the tun. This malt wine, he adds, may
be kept for a long time in close vessels, and is always ready for making quick vinegar.
2. Malt Vinegar.—The greater part of British vinegar is made from malt, by the
following process:—1 boll of good barley malt, properly crushed, is to be mashed with
water at 160° Fahr. The first water should have that temperature; the second must be
hotter than 160°, and the third water, for the extraction of all the soluble matter, may
be boiling hot. Upon the whole, not more than 100 gallons of wort should be extracted.
After the liquor has cooled to 75° Fahr., 3 or 4 gallons of beer yeast are poured in, and
well mixed with a proper stirrer. In 36 or 40 hours, according to the temperature of
the air, and the fermenting quality of the wash, it is racked off into casks, which are
laid upon their sides in the fermenting apartment of the vinegar work, which should be
kept at a temperature of 70° at least; in summer partly by the heat of the sun, but in
general by the agency of proper stoves, as above described. The bung-holes should be
left open, and the casks should not be full, in order that the air may act over an extensive
surface of the liquor. It would be proper to secure a freer circulation to the air, by
boring a hole in each end of the cask, near its upper edge. As the liquor, by evaporation,
would be generally a few degrees colder than the air of the apartment, a circulation of
air would be established in at the bung-hole, and out by the end holes. By the ordinary
methods, three months are required to make this vinegar marketable, or fit for the
manufacture of sugar of lead.
In making vinegar for domestic purposes, the casks are usually set on their ends; and
they have, sometimes, a false bottom, pierced with holes, placed about a foot above the
true one. On this bottom, a quantity of rape, or the refuse raisins, &c. from the making
of British wines, is laid. The malt liquor has a proper quantity of yeast added to it. In
about 24 hours it becomes warm, and is then racked off into another similar cask.
After some time, this racking process is discontinued, and the vinegar is allowed to complete
its fermentation quietly. The proper temperature must always be kept up, by
placing the cask in a warm situation. A little wine-stone (argal) added to the malt
wash, would make the vinegar liker that made from wine. Sometimes a little isinglass
is employed to clarify vinegar. A portion of sulphuric acid is often added to it.
3. Sugar vinegar.—By pursuing the following plan, an excellent sugar vinegar may be
made. In 158 quarts of boiling water dissolve 10 pounds of sugar, and 6 pounds of
wine-stone; put the solution into a fermenting cask, and when it is cooled to the temperature
of from 75° to 80°, add 4 quarts of beer yeast to it. Stir the mixture well,
then cover the vessel loosely, and expose it for 6 or 8 days to the vinous fermentation, at
a temperature of from 70° to 75° Fahr. When it has become clear, draw off the vinous
liquor, and either acetify it in the graduation tub above described, or by the common
vinegar process. Before it is finished, we should add to it 12 quarts of strong spirits
(brandy), and 15 quarts of good vinegar, to complete the acetous fermentation. With
a graduation tub which has been used, this addition of vinegar is unnecessary.
The following simpler prescription for making sugar vinegar deserves attention. For
every gallon of hot water take 18 ounces of sugar; and when the syrup has cooled to
75°, add 4 per cent., by measure, of yeast. When the vinous fermentation is pretty well
advanced, in the course of 2 or 3 days, rack off the clear wash from the lees into a proper
cask, and add 1 ounce of wine-stone, and 1 of crushed raisins, for every gallon of water.
Expose it in a proper manner, and for a proper time, to the acetifying process; and then
rack off the vinegar, and fine it upon beech chips. It should be afterwards put into
bottles, which are to be well corked.
Vinegar obtained by the preceding methods has always a yellowish or brownish
colour. It may be rendered colourless by distillation. For nicer chemical purposes,
this is done in a glass retort; but on a large scale, it is usually performed in a clean
copper still, furnished with a capital and worm-refrigeratory, either of silver or block
tin. It is volatile at the boiling temperature of water; and if the process be carried on
briskly, it will not sensibly corrode the copper. But we can never obtain, in this way,
a strong article; for, as soon as the vinegar gets concentrated to a certain degree, we[7]
cannot force off the remainder by heat, for fear of giving it an empyreumatic odour;
because the gluten, colouring matter, &c. begin to adhere to the bottom of the still. We
are, therefore, obliged to suspend the operation at the very time when the acid is acquiring
strength. It has been also proposed to concentrate vinegar by the process of congelation;
but much of it remains entangled among the frozen water; and common distilled vinegar
is so weak, that it congeals in one mass.
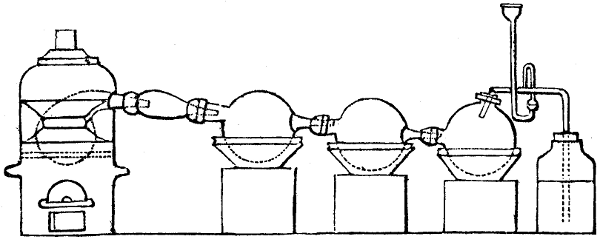
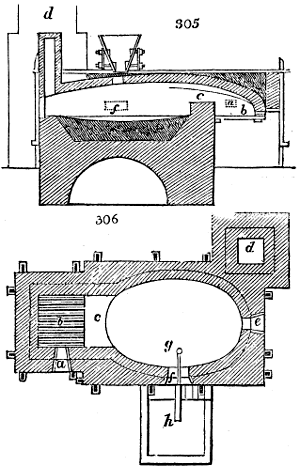
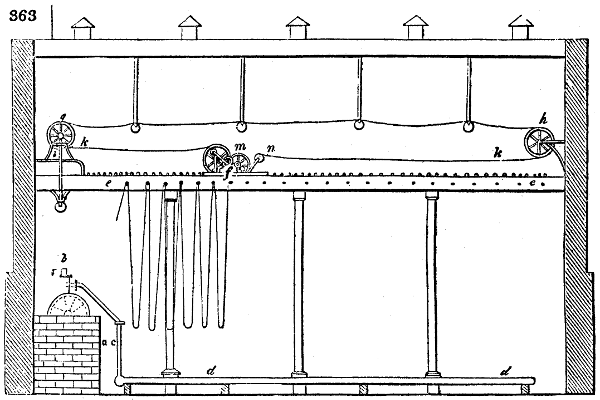
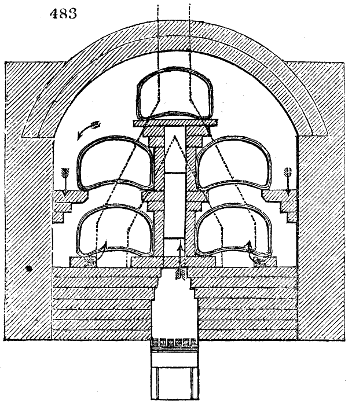
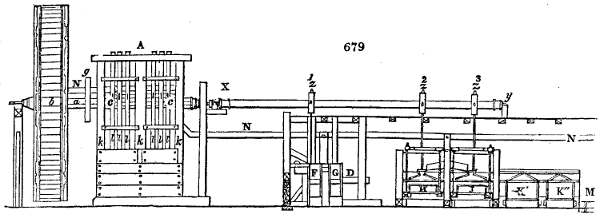
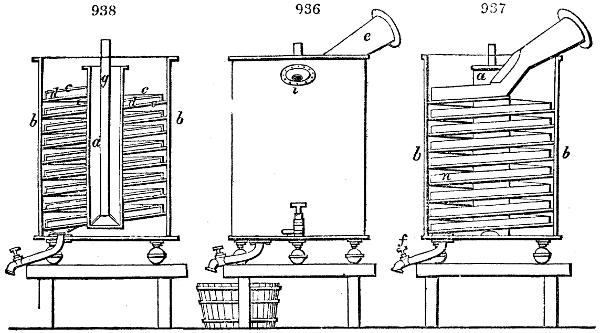
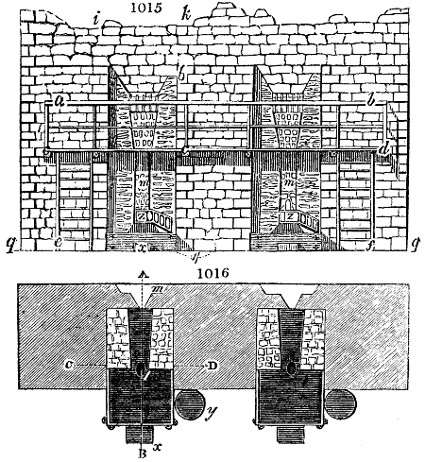
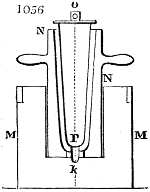
Fig. 1.
Before the process for pyrolignous acid, or wood vinegar, was known, there was only
one method of obtaining strong vinegar practised by chemists; and it is still followed by
some operators, to prepare what is called radical or aromatic vinegar. This consists in
decomposing, by heat alone, the crystallised binacetate of copper, commonly, but improperly,
called distilled verdigris. With this view, we take a stoneware retort, (fig. 1.)
of a size suited to the
quantity we wish to operate
upon; and coat it with
a mixture of fire clay and
horsedung, to make it
stand the heat better.
When this coating is dry,
we introduce into the retort
the crystallised acetate
slightly bruised, but
very dry; we fill it as far
as it will hold without
spilling when the beak is considerably inclined. We then set it in a proper furnace.
We attach to its neck an adopter pipe, and two or three globes with opposite tubulures,
and a last globe with a vertical tubulure. The apparatus is terminated by a
Welter’s tube, with a double branch; the shorter issues from the last globe, and the
other dips into a flask filled with distilled vinegar. Every thing being thus arranged,
we lute the joinings with a putty made of pipeclay and linseed oil, and cover them with
glue paper. Each globe is placed in a separate basin of cold water, or the whole may
be put into an oblong trough, through which a constant stream of cold water is made to
flow. The tubes must be allowed a day to dry. Next day we proceed to the distillation,
tempering the heat very nicely at the beginning, and increasing it by very slow
degrees till we see the drops follow each other pretty rapidly from the neck of the retort, or
the end of the adopter tube. The vapours which pass over are very hot, whence a series
of globes are necessary to condense them. We should renew, from time to time, the
water of the basins, and keep moist pieces of cloth upon the globes; but this demands
great care, especially if the fire be a little too brisk, for the vessels become, in that case,
so hot, that they would infallibly be broken, if touched suddenly with cold water. It is
always easy for us to regulate this operation, according to the emission of gas from the
extremity of the apparatus. When the air bubbles succeed each other with great
rapidity, we must damp the fire.
The liquor which passes in the first half hour is weakest; it proceeds, in some
measure, from a little water sometimes left in the crystals, which when well made,
however, ought to be anhydrous. A period arrives towards the middle of the process
when we see the extremity of the beak of the retort, and of the adopter, covered with
crystals of a lamellar or needle shape, and of a pale green tint. By degrees these
crystals are carried into the condensed liquid by the acid vapours, and give a colour to
the product. These crystals are merely some of the cupreous salt forced over by the
heat. As the process approaches its conclusion, we find more difficulty in raising the
vapours; and we must then augment the intensity of the heat, in order to continue their
disengagement. Finally, we judge that the process is altogether finished, when the
globes become cold, notwithstanding the furnace is at the hottest, and when no more
vapours are evolved. The fire may then be allowed to go out, and the retort to cool.
As the acid thus obtained is slightly tinged with copper, it must be rectified before
bringing it into the market. For this purpose we may make use of the same apparatus,
only substituting for the stoneware retort a glass one, placed in a sand bath. All the
globes ought to be perfectly clean and dry. The distillation is to be conducted in the
usual way. If we divide the product into thirds, the first yields the feeblest acid, and
the third the strongest. We should not push the process quite to dryness, because
there remains in the last portions certain impurities, which would injure the flavour of
the acid.
The total acid thus obtained forms nearly one half of the weight of the acetate
employed, and the residuum forms three tenths; so that about two tenths of the acid
have been decomposed by the heat, and are lost. As the oxide of copper is readily
reduced to the metallic state, its oxygen goes to the elements of one part of the acid, and
forms water, which mingles with the products of carbonic acid, carburetted hydrogen, and[8]
carbonic oxide gases which are disengaged; and there remains in the retort some charcoal
mixed with metallic copper. These two combustibles are in such a state of division, that
the residuum is pyrophoric. Hence it often takes fire the moment of its being removed
from the cold retort. The very considerable loss experienced in this operation has
induced chemists to try different methods to obtain all the acid contained in the acetate.
Thus, for example, a certain addition of sulphuric acid has been prescribed; but, besides
that the radical vinegar obtained in this way always contains sulphurous acid, from
which it is difficult to free it, it is thereby deprived of that spirit called the pyro-acetic,
which tempers the sharpness of its smell, and gives it an agreeable aroma. It is to be
presumed, therefore, that the preceding process will continue to be preferred for making
aromatic vinegar. Its odour is often further modified by essential oils, such as those of
rosemary, lavender, &c.
4. Pyrolignous Acid, or Wood Vinegar.—The process for making this acid is founded
upon the general property of heat, to separate the elements of vegetable substances, and
to unite them anew in another order, with the production of compounds which did not
exist in the bodies subjected to its action. The respective proportion of these products
varies, not only in the different substances, but also in the same substance, according as
the degree of heat has been greater or less, or conducted with more or less skill. When
we distil a vegetable body in a close vessel, we obtain at first the included water, or that
of vegetation; there is next formed another portion of water, at the expense of the
oxygen and hydrogen of the body; a proportional quantity of charcoal is set free, and,
with the successive increase of the heat, a small portion of charcoal combines with the
oxygen and hydrogen to form acetic acid. This was considered, for some time, as a
peculiar acid, and was accordingly called pyrolignous acid. As the proportion of carbon
becomes preponderant, it combines with the other principles, and then some empyreumatic
oil is volatilised, of little colour, but which becomes thicker, and of a darker tint,
always getting more loaded with carbon.
Several elastic fluids accompany these different products. Carbonic acid comes over,
but in small quantity, much carburetted hydrogen, and, towards the end, a considerable
proportion of carbonic oxide. The remainder of the charcoal, which could not be carried
off in these several combinations, is found in the retort, and preserves, usually, the form
of the vegetable body which furnished it. Since mankind have begun to reason on the
different operations of the arts, and to raise them to a level with scientific researches
they have introduced into several branches of manufacture a multitude of improvements, of
which, formerly, they would hardly have deemed them susceptible. Thus, in particular,
the process for carbonising wood has been singularly meliorated, and in reference to the
preceding observations, advantage has been derived from several products that formerly
were not even collected.
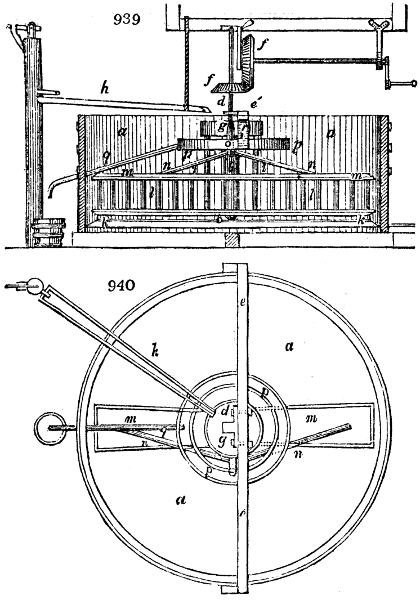
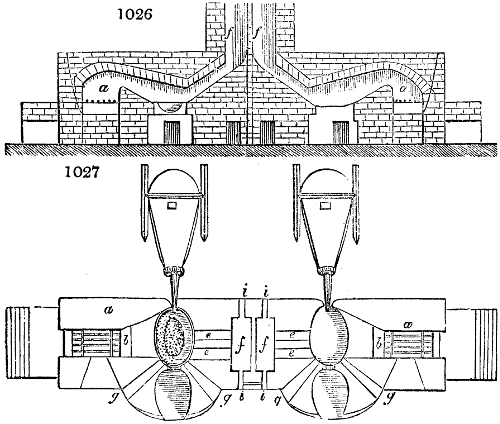
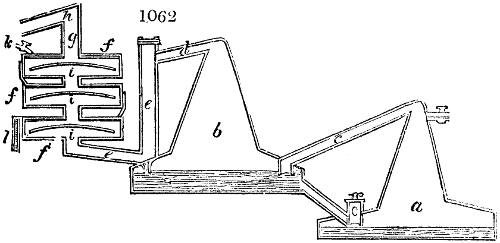
Fig. 2.
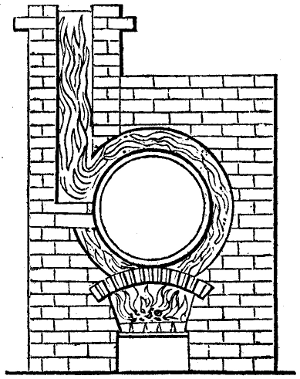
The apparatus employed for obtaining crude vinegar from wood, by the agency of heat,
are large iron cylinders. In this country they are made
of cast iron, and are laid horizontally in the furnace; in
France, they are made of sheet iron riveted together, and
they are set upright in the fire. Fig. 2. will give an accurate
idea of the British plan, which is much the same as
that adopted for decomposing pit coal in gas works, only
that the cylinders for the pyrolignous acid manufacture are
generally larger, being frequently 4 feet in diameter, and
6 or 8 feet long, and built horizontally in brickwork, so
that the flame of one furnace may play around two of
them. It would, probably, answer better, if their size
were brought nearer the dimensions of the gas-light retorts,
and if the whole system of working them were assimilated
to that of coal gas.
The following arrangement is adopted in an excellent establishment
in Glasgow, where the above large cylinders are
6 feet long, and both ends of them project a very little beyond
the brickwork. One end has a disc or round plate of cast iron, well fitted, and firmly bolted
to it, from the centre of which disc an iron tube, about 6 inches diameter, proceeds and
enters, at a right angle, the main tube of refrigeration. The diameter of this tube may be
from 9 to 14 inches, according to the number of cylinders. The other end of the cylinder
is called the mouth of the retort; this is closed by a disc of iron, smeared round its edge by
clay lute, and secured in its place by fir wedges. The charge of wood for such a cylinder
is about 8 cwt. The hard woods—oak, ash, birch, and beech—are alone used; fir does
not answer. The heat is kept up during the day-time, and the furnace is allowed to cool
during the night. Next morning, the door is opened, the charcoal removed, and a new
charge of wood is introduced. The average product of crude vinegar called pyrolignous
acid, is 35 gallons. It is much contaminated with tar, is of a deep brown colour,[9]
and has a sp. gr. of 1·025. Its total weight is therefore about 300 lbs., but the
residuary charcoal is found to weigh no more than one fifth of the wood employed;
hence nearly one half of the ponderable matter of the wood is dissipated in incondensable
gases. Count Rumford states, that the charcoal is equal in weight to more than
four tenths of the wood from which it is made. The count’s error seems to have arisen
from the slight heat of an oven to which his wood was exposed in a glass cylinder. The
result now given, is the experience of an eminent manufacturing chemist.
The crude pyrolignous acid is rectified by a second distillation in a copper still, in the
body of which about 20 gallons of viscid tarry matter are left from every 100. It has
now become a transparent brown vinegar, having a considerably empyreumatic smell,
and a sp. gr. of 1·013. Its acid powers are superior to those of the best household
vinegar, in the proportion of three to two. By redistillation, saturation with quicklime,
evaporation of the liquid acetate to dryness, and conversion into acetate of soda by
sulphate of soda, the empyreumatic matter is so completely dissipated, that on decomposing
the pure acetate of soda by sulphuric acid, a perfectly colourless and grateful
vinegar rises in distillation. Its strength will be proportionable to the concentration of
the decomposing acid.
The acetic acid of the chemist may be prepared also in the following modes:—1. Two
parts of fused acetate of potash, with one of the strongest oil of vitriol, yield, by slow
distillation from a glass retort into a refrigerated receiver, concentrated acetic acid. A
small portion of sulphurous acid, which contaminates it, may be removed by redistillation
from a little acetate of lead. 2. Or four parts of good sugar of lead, with one part of
sulphuric acid, treated in the same way, afford a slightly weaker acetic acid. 3. Gently
calcined sulphate of iron, or green vitriol, mixed with sugar of lead, in the proportion of 1
of the former to 21⁄2 of the latter, or with acetate of copper, and carefully distilled from a
porcelain retort into a cool receiver, may be also considered an economical process. But
that with binacetate of copper above described, is preferable to any of these.
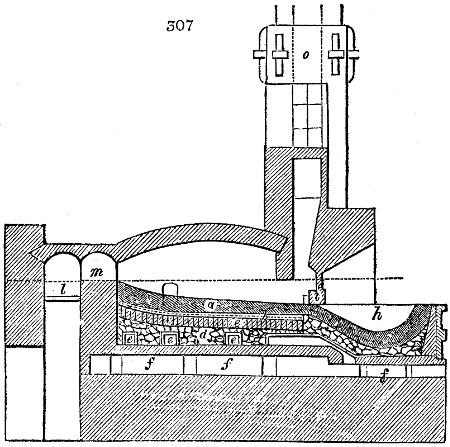
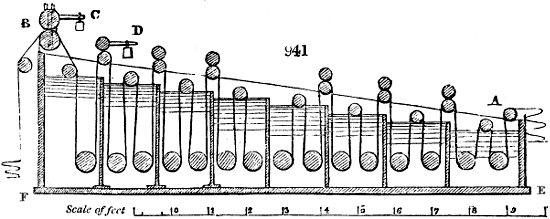
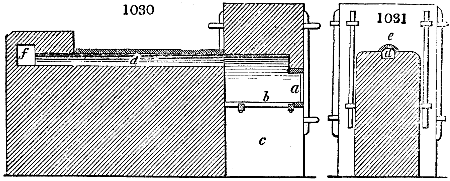
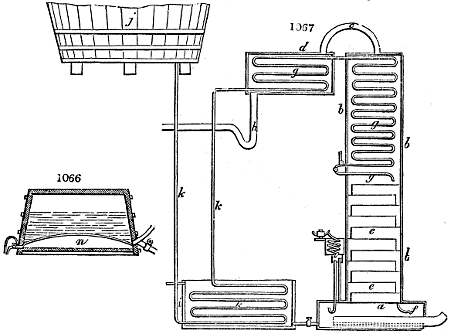
Fig. 3.
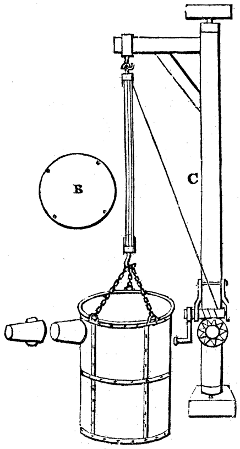
The manufacture of pyrolignous acid is conducted in
the following way in France. Into large cylindrical
vessels (fig. 3.) made of rivetted sheet iron, and having
at their top and side a small sheet iron cylinder, the wood
intended for making charcoal is introduced. To the
upper part of this vessel a cover of sheet iron, B, is
adapted, which is fixed with bolts. This vessel, thus
closed, represents, as we see, a vast retort. When it is
prepared, as we have said, it is lifted by means of a swing
crane, C, and placed in a furnace, D, (fig. 4.) of a
form relative to that of the vessel, and the opening of the
furnace is covered with a dome, E, made of masonry or
brickwork. The whole being thus arranged, heat is applied
in the furnace at the bottom. The moisture of
the wood is first dissipated, but by degrees the liquor
ceases to be transparent, and becomes sooty. An adopter
tube, A, is then fitted to the lateral cylinder. This adopter
enters into another tube at the same degree of inclination
which commences the condensing apparatus. The means
of condensation vary according to the localities. In certain
works they cool by means of air, by making the
vapour pass through a long series of cylinders, or sometimes,
even, through a series of casks connected together;
but most usually water is used for condensing, when it
can be easily procured in abundance. The most simple
apparatus employed for
this purpose consists of
two cylinders, F, F, (fig. 4.)
the one within the other,
and which leave between
them a sufficient space to
allow a considerable body
of water to circulate along
and cool the vapours. This
double cylinder is adapted
to the distilling vessel, and
placed at a certain inclination.
To the first double
tube, F, F, a second, and[10]
sometimes a third, entirely similar, are connected, which, to save space, return upon themselves
in a zigzag fashion. The water is set in circulation by an ingenious means
now adopted in many different manufactories. From the lower extremity, G, of the
system of condensers, a perpendicular tube rises, whose length should be a little more
than the most elevated point of the system. The water, furnished by a reservoir, L,
enters by means of the perpendicular tube through the lower part of the system, and
fills the whole space between the double cylinders. When the apparatus is in action,
the vapours, as they condense, raise the temperature of the water, which, by the column
in L G, is pressed to the upper part of the cylinders, and runs over by the spout K.
To this point a very short tube is attached, which is bent towards the ground, and
serves as an overflow.
Fig. 4.
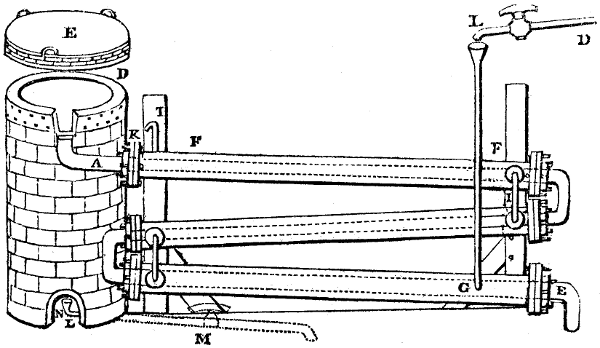
The condensing apparatus is terminated by a conduit in bricks covered and sunk in
the ground. At the extremity of this species of gutter is a bent tube, E, which
discharges the liquid product into the first cistern. When it is full, it empties itself, by
means of an overflow pipe, into a great reservoir; the tube which terminates the gutter
plunges into the liquid, and thus intercepts communication with the inside of the apparatus.
The disengaged gas is brought back by means of pipes M L, from one of the
sides of the conduit to the under part of the ash pit of the furnace. These pipes are
furnished with stopcocks M, at some distance in front of the furnace, for the purpose of
regulating the jet of the gas, and interrupting, at pleasure, communication with the inside of
the apparatus. The part of the pipes which terminates in the furnace rises perpendicularly
several inches above the ground, and is expanded like the rose of a watering can, N. The
gas, by means of this disposition, can distribute itself uniformly under the vessel, without
suffering the pipe which conducts it to be obstructed by the fuel or the ashes.
The temperature necessary to effect the carbonisation is not considerable: however, at
the last it is raised so high as to make the vessels red hot; and the duration of the process is
necessarily proportional to the quantity of wood carbonised. For a vessel which shall
contain about 5 meters cube (nearly 6 cubic yds.), 8 hours of fire is sufficient. It is
known that the carbonisation is complete by the colour of the flame of the gas: it is
first of a yellowish red; it becomes afterwards blue, when more carbonic oxide than
carbonic hydrogen is evolved; and towards the end it becomes entirely white,—a circumstance
owing, probably, to the furnace being more heated at this period, and the
combustion being more complete. There is still another means of knowing the state of
the process, to which recourse is more frequently had; that is the cooling of the first
tubes, which are not surrounded with water: a few drops of this fluid are thrown upon
their surface, and if they evaporate quietly, it is judged that the calcination is sufficient.
The adopter tube is then unluted, and is slid into its junction pipe; the orifices are
immediately stopped with plates of iron and plaster loam. The brick cover, E, of the
furnace is first removed by means of the swing crane, then the cylinder itself is lifted
out and replaced immediately by another one previously charged. When the cylinder which
has been taken out of the furnace is entirely cooled, its cover is removed, and the charcoal
is emptied. Five cubic meters of wood furnish about 7 chaldrons (voies) and a half of
charcoal. (For modifications of the wood-vinegar apparatus, see Charcoal and
Pyrolignous Acid.)
The different qualities of wood employed in this operation give nearly similar product
in reference to the acid; but this is not the case with the charcoal, for it is better the
harder the wood; and it has been remarked, that wood long exposed to the air furnishes
a charcoal of a worse quality than wood carbonised soon after it is cut.
Having described the kind of apparatus employed to obtain pyrolignous acid, I shall
now detail the best mode of purifying it. This acid has a reddish brown colour; it holds
in solution a portion of empyreumatic oil and of the tar which were formed at the same
time; another portion of these products is in the state of a simple mixture; the latter
may be separated by repose alone. It is stated, above, that the distilling apparatus
terminates in a subterranean reservoir, where the products of all the vessels are mixed.
A common pump communicates with the reservoir, and sinks to its very bottom, in order
that it may draw off only the stratum of tar, which, according to its greater density,
occupies the lower part. From time to time the pump is worked to remove the
tar as it is deposited. The reservoir has at its top an overflow pipe, which discharges
the clearest acid into a cistern, from which it is taken by means of a second pump.
The pyrolignous acid thus separated from the undissolved tar is transferred from this
cistern into large sheet iron boilers, where its saturation is effected either by quicklime
or by chalk; the latter of which is preferable, as the lime is apt to take some of the tar
into combination. The acid parts by saturation with a new portion of the tar, which is
removed by skimmers. The neutral solution is then allowed to rest for a sufficient time
to let its clear parts be drawn off by decantation.
The acetate of lime thus obtained indicates by the hydrometer, before being mixed with
the waters of edulcoration, a degree corresponding to the acidimetric degree of the acid[11]
employed. This solution must be evaporated till it reaches a specific gravity of 1·114
(15° Baumé), after which there is added to it a saturated solution of sulphate of soda.
The acids exchange bases; sulphate of lime precipitates, and acetate of soda remains in
solution. In some manufactures, instead of pursuing the above plan, the sulphate of
soda is dissolved in the hot pyrolignous acid, which is afterwards saturated with chalk
or lime. By this means no water need be employed to dissolve the sulphate, and
accordingly the liquor is obtained in a concentrated form without evaporation. In both
modes the sulphate of lime is allowed to settle, and the solution of acetate of soda is decanted.
The residuum is set aside to be edulcorated, and the last waters are employed
for washing fresh portions.
The acetate of soda which results from this double decomposition is afterwards
evaporated till it attains to the density of 1·225 or 1·23, according to the season. This
solution is poured into large crystallising vessels, from which, at the end of 3 or 4 days,
according to their capacity, the mother waters are decanted, and a first crystallisation is
obtained of rhomboidal prisms, which are highly coloured and very bulky. Their
facettes are finely polished, and their edges very sharp. The mother waters are submitted
to successive evaporations and crystallisations till they refuse to crystallise, and they are
then burnt to convert them into carbonate of soda.
To avoid guesswork proportions, which are always injurious, by the loss of time which
they occasion, and by the bad results to which they often lead, we should determine
experimentally, beforehand, the quantities absolutely necessary for the reciprocal decomposition,
especially when we change the acid or the sulphate. But it may be remarked
that, notwithstanding all the precautions we can take, there is always a notable quantity
of sulphate of soda and acetic acid, which disappear totally in this decomposition. This
arises from the circumstance that sulphate of soda and acetate of lime do not completely
decompose each other, as I have ascertained by experiments on a very considerable scale;
and thus a portion of each of them is always lost with the mother waters. It might be
supposed that by calcining the acetate of lime we could completely destroy its empyreumatic
oil; but, though I have made many experiments, with this view I never could
obtain an acetate capable of affording a tolerable acid. Some manufacturers prefer to
make the acetate of soda by direct saturation of the acid with the alkali, and think
that the higher price of this substance is compensated by the economy of time and fuel
which it produces.
The acetate of soda is easily purified by crystallisations and torrefaction; the latter
process, when well conducted, freeing it completely from every particle of tar. This
torrefaction, to which the name of fusion may be given, requires great care and dexterity.
It is usually done in shallow cast iron boilers of a hemispherical shape. During all the
time that the heat of about 500° Fahr. is applied, the fused mass must be diligently
worked with rakes; an operation which continues about 24 hours for half a ton of materials.
We must carefully avoid raising the temperature so high as to decompose the
acetate, and be sure that the heat is equally distributed; for if any point of the mass
enters into decomposition, it is propagated with such rapidity, as to be excessively difficult
to stop its progress in destroying the whole. The heat should never be so great as
to disengage any smoke, even when the whole acetate is liquefied. When there is no
more frothing up, and the mass flows like oil, the operation is finished. It is now
allowed to cool in a body, or it may be ladled out into moulds, which is preferable.
When the acetate is dissolved in water, the charcoaly matter proceeding from the
decomposition of the tar must be separated by filtration, or by boiling up the liquor to
the specific gravity 1·114, when the carbonaceous matter falls to the bottom. On evaporating
the clear liquor, we obtain an acetate perfectly fine, which yields beautiful crystals
on cooling. In this state of purity it is decomposed by sulphuric acid, in order to separate
its acetic acid.
This last operation, however simple it appears, requires no little care and skill. The
acetate of soda crystallised and ground is put into a copper, and the necessary quantity
of sulphuric acid of 1·842 (about 35 per cent. of the salt) to decompose almost, but not
all, the acetate, is poured on. The materials are left to act on each other; by degrees
the acetic acid quits its combination, and swims upon the surface; the greater part of
the resulting sulphate of soda falls in a pulverulent form, or in small granular crystals, to
the bottom. Another portion remains dissolved in the liquid, which has a specific
gravity of 1·08. By distillation we separate this remainder of the sulphate, and finally
obtain acetic acid, having a specific gravity of 1·05, an agreeable taste and smell, though
towards the end it becomes a little empyreumatic, and coloured; for which reason, the
last portions must be kept apart. The acid destined for table use ought to be distilled
in an alembic whose capital and condensing worm are of silver; and to make it very
fine, it may be afterwards infused over a little washed bone-black. It is usually obtained
in a pretty concentrated state; but when we wish to give it the highest degree of concentration,
we mix with it a quantity of dry muriate of lime, and distil anew. This acid may[12]
be afterwards exposed to congelation, when the strongest will crystallise. It is decanted,
and the crystals are melted by exposing them to a temperature of from 60° to 70° Fahr.;
this process is repeated till the acid congeals without remainder, at the temperature of 55°
Fahr. It has then attained its maximum strength, and has a specific gravity of 1·063.
We shall add an observation on the above mode of decomposing the acetate of soda by
sulphuric acid. Many difficulties are experienced in this process, if the sulphuric acid
be poured on in small quantities at a time; for then such acrid fumes of acetic acid
are disengaged, that the workmen are obliged to retire. This inconvenience may be
saved by adding all the sulphuric acid at once; it occupies the lower part of the vessel,
and decomposes only the portion of the acetate in contact with it; the heat evolved in
consequence of this reaction is diffused through a great mass, and produces no sensible
effect. When the sulphuric acid forms an opening, or a species of little crater, the
workman, by means of a rake, depresses the acetate into it by degrees, and then the
decomposition proceeds as slowly as he desires.
The acetic acid, like the nitric, chloric, and some others, has not hitherto been
obtained free from water, and the greatest degree of concentration which we have been
able to give it is that in which it contains only the quantity of water equivalent to the
atomic weight of another oxidized body; a quantity which amounts to 14·89 per cent.
The processes prescribed for preparing concentrated acetic acid sometimes tend to
deprive it of that water without which it could not exist: hence, in all such cases,
there is a part of the acid itself decomposed to furnish the water necessary to the constitution
of the remainder. The constituent principles of the decomposed portion then
form a peculiar, intoxicating, highly inflammatory liquid, called the PYRO-ACETIC SPIRIT.
The most highly concentrated acid of 1·063 becomes denser by the addition of a
certain quantity of water up to a certain point. According to Berzelius, the prime
equivalent of this acid is 643·189, oxygen being reckoned 100. Now, the above
strongest acid consists of one prime of acid, and one of water = 1124·79. When it
contains three atoms of water, that is, 337·437 parts to 643·189, or 34·41 to 65·59 in
100, it then has taken its maximum density of 1·075; after which the further addition
of water diminishes its specific gravity, as the following table of Mollerat shows. His
supposed anhydrous or dry acid contains, at 1·0630, 0·114 parts of water.
Table of Acetic Acid.
Water in
100 parts. |
Specific
gravity. |
| 0·00 |
1 |
·0630 |
| 8·37 |
1 |
·0742 |
| 17·00 |
1 |
·0770 |
| 23·00 |
1 |
·0791 |
| 28·10 |
1 |
·0763 |
| 33·83 |
1 |
·0742 |
| 37·60 |
1 |
·0728 |
| 47·00 |
1 |
·0658 |
| 50·00 |
1 |
·0637 |
| 51·80 |
1 |
·063 |
Acetic acid readily takes fire when it is heated in open vessels to the boiling point,
and it burns with a blue flame, nearly like alcohol. It must be kept in close vessels,
otherwise it loses its strength, by attracting humidity from the air. When concentrated,
it is used only as a scent, or pungent exciter of the olfactory organs, in sickness and
fainting fits. Its anti-epidemic qualities are apocryphal. What is met with in the
shops under the name of salts of vinegar is nothing but sulphate of potash, put up in
small phials, and impregnated with acetic acid, sometimes rendered aromatic with oil of
rosemary or lavender.
Acetic acid, in its dry state, as it exists in fused acetate of potash or soda, is composed
of
| 47·536 |
carbon |
| 5·822 |
hydrogen |
| 46·642 |
oxygen |
| 100·000 |
|
And its symbol by Berzelius is H6 C4 O3 = A. We must bear in mind that his
atomic weight for hydrogen is only one half of the number usually assigned to it by
British chemists, in consequence of his making water a compound of two atoms of
hydrogen and one of oxygen.
When the vapour of acetic acid is made to traverse a red-hot tube of iron, it is converted
into water, carbonic acid, carburetted hydrogen, but chiefly pyro-acetic spirit.[13]
Acetic acid is a solvent of several organic products; such as camphor, gluten, gum-resins,
resins, the fibrine of blood, the white of egg, &c.
It is an important problem to ascertain the purity and strength of vinegar. Spurious
acidity is too often given to it by cheaper acids, such as the sulphuric and the nitric.
The former, may most surely be detected by the nitrate of baryta, or even by acetate of
lead, which occasion a white precipitate in such adulterated vinegar. For the case of
nitric, which is more insidious, the proper test is, a bit of gold leaf, wetted with a few
drops of muriatic acid. If the leaf dissolves, on heating the mixture in a watch glass, we
may be sure that nitric acid is present.
Specific gravity, if determined by a sensible hydrometer, is a good test of the strength
of the genuine vinegar; and the following table of Messrs. Taylor is nearly correct, or
sufficiently so for commercial transactions.
Revenue proof vinegar, called by the English manufacturer No. 24., has a specific
gravity of
| 1·0085 |
and contains of real acid in 100— |
5 |
| 1·0170 |
|
10 |
| 1·0257 |
|
15 |
| 1·0320 |
|
20 |
| 1·0470 |
|
30 |
| 1·0580 |
|
40 |
An excise duty of 2d. is levied on every gallon of the above proof vinegar. Its
strength is not, however, estimated directly by its specific gravity, but by the specific
gravity which it assumes when saturated with quicklime. The decimal fraction of the
specific gravity of the calcareous acetate is very nearly the double of that of the pure
vinegar; or, 1·009 in vinegar becomes 1·018 in acetate of lime. The vinegar of malt
contains so much mucilage or gluten, that when it has only the same acid strength as
the above, it has a density of 1·0014, but it becomes only 1·023 when converted into
acetate of lime: indeed, 0·005 of its density is due to mucilaginous matter. This fact
shows the fallacy of trusting to the hydrometer for determining the strength of vinegars,
which may be more or less loaded with vegetable gluten. The proper test of this, as of
all other acids, is, the quantity of alkaline matter which a given weight or measure of it
will saturate. For this purpose the bicarbonate of potash, commonly called, in the
London shops, carbonate, may be employed very conveniently. As it is a very uniform
substance, and its atomic weight, by the hydrogen radix, is 100·584, while the atomic
weight of acetic acid, by the same radix, is 51·563, if we estimate 2 grains of the
bicarbonate as equivalent to 1 of the real acid, we shall commit no appreciable error.
Hence, a solution of the carbonate containing 200 grains in 100 measures, will form an
acetimeter of the most perfect and convenient kind; for the measures of test liquid expended
in saturating any measure,—for instance, an ounce or 1000 grains of acid,—will indicate
the number of grains of real acetic acid in that quantity. Thus, 1000 grains of the
above proof, would require 50 measures of the acetimetrical alkaline solution, showing that
it contains 50 grains of real acetic acid in 1000, or 5 per cent.
It is common to add to purified wood vinegar, a little acetic ether, or caramelised
(burnt) sugar to colour it, also, in France, even wine, to flavour it. Its blanching effect
upon red cabbage, which it has been employed to pickle, is owing to a little sulphurous acid.
This may be removed by redistillation with peroxide of manganese. Indeed, Stoltze
professes to purify the pyrolignous acid solely by distilling it with peroxide of manganese,
and then digesting it with bruised wood charcoal; or by distilling it with a mixture of
sulphuric acid and manganese. But much acid is lost in this case by the formation of
acetate of that metal.
Birch and beech afford most Pyrolignous acid, and pine the least. It is exclusively
employed in the arts, for most purposes of which it need not be very highly
purified. It is much used in calico printing, for preparing acetate of iron called
Iron Liquor, and acetate of alumina, called Red Liquor; which see. It serves also to
make sugar of lead; yet when it contains its usual quantity, after rectification, of tarry
matter, the acetate of lead will hardly crystallise, but forms cauliflower concretions. This
evil may be remedied, I believe, by boiling the saline solution with a very little nitric
acid, which causes the precipitation of a brown granular substance, and gives the liquor
a reddish tinge. The solution being afterwards treated with bruised charcoal, becomes
colourless, and furnishes regular crystals of acetate or sugar of lead.
Pyrolignous acid possesses, in a very eminent degree, anti-putrescent properties. Flesh
steeped in it for a few hours may be afterwards dried in the air without corrupting; but
it becomes hard, and somewhat leather-like: so that this mode of preservation does not
answer well for butcher’s meat. Fish are sometimes cured with it. See Pyro-acetic
Spirit; Pyroxilic Ether; Pyroxolic Spirit; Pyrolignous Acid and Vinegar.
[14]
ACETIMETER. An apparatus for determining the strength of vinegar. See the
conclusion of the preceding article for a description of my simple method of acetimetry.
ACETONE. The new chemical name of pyro-acetic spirit.
ACID OF ARSENIC. (Acide Arsenique, Fr.; Arseniksäure, Germ.)
ACIDS. A class of chemical substances characterised by the property of combining
with and neutralising the alkaline and other bases, and of thereby forming a peculiar
class of bodies called salts. The acids which constitute objects of special manufacture
for commercial purposes are the following:—acetic, arsenious, carbonic, chromic, citric,
malic, muriatic, nitric, oxalic, phosphoric, sulphuric, tartaric, which see.
ACROSPIRE. (Plumule, Fr.; Blattkeim, Germ.) That part of a germinating
seed which botanists call the plumula, or plumes. See Beer and Malt.
ADDITIONS. Such articles as are added to the fermenting wash of the distiller
are distinguished by this trivial name.
ADIPOCIRE. Fr. (Fettwachs, Germ.) The fatty matter generated in dead
bodies buried under peculiar circumstances. In 1786 and 1787, when the churchyard
of the Innocents, at Paris, was cleaned out, and the bones transported to the catacombs,
it was discovered that not a few of the cadavres were converted into a saponaceous
white substance, more especially many of those which had been interred for fifteen
years in one pit, to the amount of 1500, in coffins closely packed together. These
bodies were flattened, in consequence of their mutual pressure; and, though they generally
retained their shape, there was deposited round the bones of several a grayish
white, somewhat soft, flexible substance. Fourcroy presented to the Academy of
Sciences, in 1789, a comprehensive memoir upon this phenomenon, which appeared to
prove that the fatty body was an ammoniacal soap, containing phosphate of lime; that
the fat was similar to spermaceti, as it assumed on slow cooling a foliated crystalline
structure; as also to wax, as, when rapidly cooled, it became granular: hence he called it
Adipocire. Its melting point was 52·5° C. (126·5° Fahr.). He likewise compared
this soap to the fat of gall-stones, and supposed it to be a natural product of the slow
decomposition of all animal matter, except bones, nails, and hairs.
This substance was again examined by Chevreul in 1812, and was found by him to
contain margaric acid, oleic acid, combined with a yellow colouring, odorous matter,
besides ammonia, a little lime, potash, oxide of iron, salts of lactic acid, an azotized
substance; and was therefore considered as a combination of margaric and oleic acids,
in variable proportions (whence arose its variable fusibility), but that it was not
analogous with either spermaceti or cholesterine (gallstones). These fat acids are
obviously generated by the reaction of the ammonia upon the margarine and oleine,
though they eventually lose the greater part of that volatile alkali.
According to the views of both Gay Lussac and Chevreul, this adipocire proceeds
solely from the pre-existing fat of the dead body, and not from the flesh, tendons, or
cartilages, as had been previously imagined; which had led to some expensive and
abortive attempts, upon the great scale of manufacture, to convert the dead bodies of
cattle into adipocire, for the purposes of the candle-maker or soap-boiler, by exposing
them for some time to the action of moisture.
Von Hartkol made experiments during 25 years upon this subject, from which he
inferred, that there is no formation of adipocire in bodies buried in dry ground; that
in moist earth the fat of the dead body does not increase, but changes into a fetid
saponaceous substance, incapable of being worked into either soap or candles; that the
dead bodies of mammalia immersed in running water, leave behind after 3 years a pure
fat, which is more abundant from young than from old animals; that the intestines
afford more fat than the muscles; that from this fat, without any purification, candles
may be made, as void of smell, as hard, and as white, as from bleached wax; that from
cadavers immersed for 3 years in stagnant water, more fat is procured than from those
in running water, but that it needs to be purified before it can be made into soap or
candles.
The cause of the difference between Hartkol’s and Chevreul’s results cannot be
assigned, as the latter has not published his promised remarks upon the subject. At
any rate, dead animal matter can be worked up more profitably than in making artificial
adipocire.
ADIT. The horizontal entrance of a mine. It is sometimes called the drift. See
Mining and Metallurgy.
ADULTERATION. The debasing any product of manufacture, especially
chemical, by the introduction of cheap materials. The art of ascertaining the genuineness
of the several products will be taught under the specific objects of manufacture.
AFFINITY. The chemical term denoting the peculiar attractive force which produces
the combination of dissimilar substances; such as of an alkali with an acid, or of
sulphur with a metal.
[15]
AGARIC. A species of boletus or fungus, which grows in dunghills; with the
salts of iron it affords a black dye. It is said to be convertible into a kind of
china ink.
AGATE. A siliceous mineral which is cut into seals and other forms for the coarser
kinds of jewellery. See Gem.
ALABASTER, is a stone usually white, and soft enough to be scratched by iron.
There are two kinds of it: the gypseous, which is merely a natural semi-crystalline
sulphate of lime; and the calcareous alabaster, which is a carbonate of lime. The
oriental alabaster is always of the latter kind, and is most esteemed, because it is
agreeably variegated with lively colours, and especially with zones of honey-yellow,
yellow-brown, red, &c.; it is, moreover, susceptible of taking a marble polish.
The fineness of the grain of alabaster, the uniformity of its texture, the beauty of its
polished surface, and its semi-transparency, are the qualities which render it valuable to
the sculptor and to the manufacturer of ornamental toys.
The limestone alabaster is frequently found as a yellowish-white deposit in certain
fountains. The most celebrated spring of this kind is that of the baths of San Filippo,
in Tuscany. The water, almost boiling hot, runs over an enormous mass of stalactites,
which it has formed, and holds the carbonate of lime in solution by means of sulphuretted
hydrogen (according to M. Alexandre Brongniart), which escapes by contact of the
atmosphere. Advantage has been taken of this property to make basso relievos of considerable
hardness, by placing moulds of sulphur very obliquely, or almost upright, in
wooden tubs open at the bottom. These tubs are surmounted at the top with a large
wooden cross. The water of the spring, after having deposited in an external conduit
or cistern the coarser sediment, is made to flow upon this wooden cross, where it is
scattered into little streamlets, and thence lets fall, upon the sulphur casts, a precipitate
so much the finer the more nearly vertical the mould. From one to four months are
required for this operation, according to the thickness of the deposited crust. By
analogous processes, the artists have succeeded in moulding vases, figures of animals, and
other objects, in relief, of every different form, which require only to be trimmed a little,
and afterwards polished.
The common alabaster is composed of sulphuric acid and lime, though some kinds of
it effervesce with acids, and therefore contain some carbonate of lime. This alabaster
occurs in many different colours, and of very different degrees of hardness, but it is
always softer than marble. It forms, usually, the lowest beds of the gypsum quarries.
The sculptors prefer the hardest, the whitest, and those of a granular texture, like Carrara
marble, and so like that they can only be distinguished by the hardness.
The alabaster is worked with the same tools as marble; and as it is many degrees
softer, it is so much the more easily cut; but it is more difficult to polish, from its little
solidity. After it has been fashioned into the desired form, and smoothed down with
pumice stone, it is polished with a pap-like mixture of chalk, soap, and milk; and, last of
all, finished by friction with flannel. It is apt to acquire a yellowish tinge.
Besides the harder kinds, employed for the sculpture of large figures, there is a softer
alabaster, pure white and semi-transparent, from which small ornamental objects are made,
such as boxes, vases, lamps, stands of time-pieces, &c. This branch of business is much
prosecuted in Florence, Leghorn, Milan, &c., and employs a great many turning lathes.
Of all the alabasters the Florentine merits the preference, on account of its beauty and
uniformity, so that it may be fashioned into figures of considerable size; for which purpose
there are large work-shops where it is cut with steel saws into blocks and masses of various
shapes. Other sorts of gypsum, such as that of Salzburg and Austria, contain sand veins,
and hard nodules, and require to be quarried by cleaving and blasting operations, which are
apt to crack it, and unfit it for all delicate objects of sculpture. It is, besides, of a gray
shade, and often stained with darker colours.
The alabaster best adapted for the fine arts is pretty white when newly broken, and
becomes whiter on the surface by drying. It may be easily cut with the knife or chisel,
and formed into many pleasing shapes by suitable steel tools. It is worked either by
the hand alone, or with the aid of a turning lathe. The turning tools should not be too
thin or sharp-edged; but such as are employed for ivory and brass are most suitable for
alabaster, and are chiefly used to shave and to scratch the surface. The objects which
cannot be turned may be fashioned by the rasping tools, or with minute files, such as
variegated foliage. Fine chisels and graving tools are also used for the better pieces of
statuary.
For polishing such works, a peculiar process is required: pumice stone, in fine
powder, serves to smooth down the surfaces very well, but it soils the whiteness of the
alabaster. To take away the unevennesses and roughnesses dried shave-grass (equisetum)
answers best. Frictions with this plant and water polish down the asperities left by
the chisel: the fine streaks left by the grass may be removed by rubbing the pieces with
slaked lime, finely pulverised and sifted, made into a paste, or putty, with water. The[16]
polish and satin-lustre of the surface are communicated by friction, first with soap-water
and lime, and finally with powdered and elutriated talc or French chalk.
Such articles as consist of several pieces are joined by a cement composed of quicklime
and white of egg, or of well-calcined and well-sifted Paris plaster, mixed with the least
possible quantity of water.
Alabaster objects are liable to become yellow by keeping, and are especially injured
by smoke, dust, &c. They may be in some measure restored by washing with soap and
water, then with clear water, and again polished with shave-grass. Grease spots may
be removed either by rubbing with talc powder, or with oil of turpentine.
The surface of alabaster may be etched by covering over the parts that are not to
be touched with a solution of wax in oil of turpentine, thickened with white lead,
and immersing the articles in pure water after the varnish has set. The action of
the water is continued from 20 to 50 hours, more or less, according to the depth to
which the etching is to be cut. After removing the varnish with oil of turpentine, the
etched places, which are necessarily deprived of their polish, should be rubbed with a
brush dipped in finely-powdered gypsum, which gives a kind of opacity, contrasting well
with the rest of the surface.
Alabaster may be stained either with metallic solutions, with spirituous tinctures of
dyeing plants, or with coloured oils, in the same way as marbles.
This substance has been hardened, it is said, by exposing it to the heat of a baker’s
oven for 10 or 20 hours, after taking it out of the quarry, and giving it the figure, roughly,
which it is intended to have. After this exposure, it must be dipped for two minutes in
running water; when it is cold, it must be dipped a second time for the same period.
On being exposed to the air for a few days, alabaster so treated acquires a marble-like
hardness. I doubt the truth of this statement.
ALBUM GRÆCUM. The white dung of dogs, sometimes used to soften leather
in the process of dressing it after the depilatory action of lime.
ALCARAZZAS. A species of porous earthenware, made in Spain, for cooling
liquors. See Pottery.
ALCOHOL. The well-known intoxicating liquor procured by distillation from
various vegetable juices, and infusions of a saccharine nature, which have undergone the
vinous fermentation. Common alcohol, or proof spirit, as it is called, contains about
one half its weight of water. It may be concentrated till its specific gravity becomes so
low as 0·825, by simple redistillation at a steam or water-bath heat; but to make it
stronger, we must mix with it, in the still or retort, dry carbonate of potash, muriate of
lime, or some other substances strongly attractive of water, and then it may be obtained
of a specific gravity so low as 0·791 at 16° Reaumur (68° Fahr.), water being 1·000.
At 0·825, it contains, still, 11 per cent. of water; and in this state it is as volatile as
absolute alcohol, on account of the inferior density of the aqueous vapour, compared to
the alcoholic. Indeed, according to Yelin and Fuchs, the boiling point of anhydrous
alcohol is higher than of that which contains 2 or 3 per cent. of water; hence, in the
distillation of alcohol of 94 per cent., the first portions that come over are more aqueous
than the following. Absolute alcohol has its boiling point at 1681⁄2° Fahr.: but when
it holds more than 6 per cent. of water, the first portions that come over are richest in
alcohol, and the temperature of the boiling point, or of the spirituous vapour, is always
higher the longer the distillation continues. According to Gröning’s researches, the
following temperatures of the alcoholic vapours correspond to the accompanying contents
of alcohol in per centage of volume, which are disengaged in the boiling of the spirituous
liquid.
| Temperature. |
Alcoholic
content of
the
vapour. |
Alcoholic
content of
the boiling
liquid. |
| Fahr. |
170 |
·0 |
93 |
|
92 |
| |
171 |
·8 |
92 |
|
90 |
| |
172 |
|
91 |
|
85 |
| |
172 |
·8 |
90 |
1⁄2 |
80 |
| |
174 |
|
90 |
|
70 |
| |
174 |
·6 |
89 |
|
70 |
| |
176 |
|
87 |
|
65 |
| |
178 |
·3 |
85 |
|
50 |
| |
180 |
·8 |
82 |
|
40 |
| |
183 |
|
80 |
|
35 |
| |
185 |
|
78 |
|
30 |
| |
187 |
·4 |
76 |
|
25 |
| |
189 |
·8 |
71 |
|
20 |
| |
192 |
·0 |
68 |
|
18 |
| |
164 |
|
66 |
|
15 |
| |
196 |
·4 |
61 |
|
12 |
| |
198 |
·6 |
55 |
|
10 |
| |
201 |
|
50 |
|
7 |
| |
203 |
|
42 |
|
5 |
| |
205 |
·4 |
36 |
|
3 |
| |
207 |
·7 |
28 |
|
2 |
| |
210 |
|
13 |
|
1 |
| |
212 |
|
0 |
|
0 |
[17]
Gröning undertook this investigation in order to employ the thermometer as an alcoholmeter
in the distillation of spirits; for which purpose he thrust the bulb of the thermometer
through a cork, inserted into a tube fixed in the capital of the still. The state of the
barometer ought also to be considered in making comparative experiments of this kind.
Since, by this method, the alcoholic content may be compared with the temperature of
the vapour that passes over at any time, so, also, the contents of the whole distillation
may be found approximately; and the method serves as a convenient means of making
continual observations on the progress of the distillation.
The temperature, corresponding to a certain per centage of alcohol in vapour, suggests
the employment of a convenient method for obtaining, at one process, a spirit as free from
water as it can be made by mere distillation. We place over the top of the capital a
water-bath, and lead up through it a spiral pipe from the still, which there passes obliquely
downwards, and proceeds to the refrigeratory. If this bath be maintained, by a constant
influx of cold water, at a certain temperature, only the alcoholic vapour corresponding to
that temperature will pass over, and the rest will be recondensed and returned into the
still. If we keep the temperature of the water at 174°, for example, the spirituous
vapour which passes over will contain 90 per cent. of absolute alcohol, according to the
preceding table. The skilful use of this principle constitutes the main improvement
in modern distilleries. See Distillation and Still.
Another method for concentrating alcohol is that discovered by Sömmering, founded
upon the property of ox bladders to allow water to pass through and evaporate out of
them, but not to permit alcohol to transpire, or only in a slight degree. Hence, if an
ox’s bladder is filled with spirit of wine, well tied at the mouth, and suspended in a warm
place, the water will continually exhale, and the alcohol will become nearly anhydrous;
for in this way alcohol of 97 or 98 per cent. may be obtained.
According to Sömmering, we should take for this purpose the bladder of an ox or a calf,
soak it for some time in water, then inflate it and free it from the fat and the attached
vessels; which is to be also done to the other surface, by turning it inside out. After it is
again inflated and dried, we must smear over the outer side twice, and the inner side four
times, with a solution of isinglass, by which its texture is made closer, and the concentration
of the alcohol goes on better. A bladder so prepared may serve more than a
hundred times. It must be charged with the spirits to be concentrated, leaving a small space
vacant, it is then to be tightly bound at the mouth, and suspended in a warm situation,
at a temperature of 122° Fahr., over a sand-bath, or in the neighbourhood of an oven.
The surface of the bladder remains moist with the water, as long as the sp. gr. of the
contained spirit is greater than 0·952. Weak spirit loses its water quicker than strong;
but in from 6 to 12 hours the alcohol may be concentrated, when a suitable heat is employed.
This economical method is particularly applicable in obtaining alcohol for the
preparation of varnishes. When the alcohol is to serve for other purposes, it must be
freed, by distillation, from certain matters dissolved out of the bladder. Alcohol may
likewise be strengthened, as Sömmering has ascertained when the vessel that contains
the spirit is bound over with a bladder which does not come into contact with the
liquid. Thus, too, all other liquors containing alcohol and water, as wine, cider, &c.,
may be made more spirituous.
To procure absolute alcohol, we must take chloride of calcium recently fused, reduce
it to coarse powder, and mix it with its own weight of spirit of wine, of sp. gr. 0·833,
in a bottle, which is to be well stoppered, and to be agitated till the salt is dissolved.
The clear solution is to be poured into a retort, and half of the volume of the alcohol
employed, or so much as has the sp. gr. 0·791 at 68° Fahr., is to be distilled off at a
gentle heat. Quicklime has also been employed for the same purpose, but it is less
powerful and convenient. Alcohol, nearly free from water, may be obtained without
distillation, by adding dry carbonate of potash to a spirit of wine, of sp. gr. 0·825. The
water combines with the potash, and falls to the bottom in a dense liquid, while the pure
spirit floats on the surface. This contains however a little alkali, which can only be separated
by distillation.
Anhydrous alcohol is composed by weight of 52·66 carbon, 12·90 hydrogen, and
34·44 of oxygen. It has a very powerful attraction for water, and absorbs it from the
atmosphere; therefore it must be kept in well-closed vessels. It also robs vegetable and
animal bodies of their moisture; and hence common alcohol is employed for preserving
anatomical preparations. Alcohol is a solvent for many substances: resins, essential oils,
camphor, are abundantly dissolved by it, forming varnishes, perfumed spirits, &c. The
solution of a resin or essential oil in alcohol becomes milky on the addition of
water, which, by its attraction for alcohol, separates these substances. Several salts,
especially the deliquescent, are dissolved by it, and some of them give a colour to its
flame; thus, the solutions of the salts of strontia in alcohol burn with a crimson flame,
those of copper and borax green, lime reddish, and baryta yellow.
When water is mixed with alcohol, heat and a condensation of volume are the result;[18]
these effects being greatest with 54 per cent. of alcohol and 46 of water, and thence
decreasing with a greater proportion of water. For alcohol which contains 90 per cent. of
water, this condensation amounts to 1·94 per cent. of the volume; for 80 per cent.,
2·87; for 70 per cent., 3·44; for 60 per cent., 3·73; for 40 per cent., 3·44; for 30 per
cent., 2·72; for 20 per cent., 1·72; for 10 per cent., 0·72. Hence, to estimate the
quantity of alcohol in any spirit it is necessary that the specific gravity be ascertained
for each determinate proportion of alcohol and water that are mixed together. When
this is done, we may, by means of an areometer constructed for liquids lighter than
water, determine the strength of the spirit, either by a scale of specific gravities or by an
arbitrary graduation corresponding to certain commercial objects, and thus we may
determine the per centage of alcohol in whisky or brandy of any strength or purity. An
areometer intended for this use has been called an alcoholmeter, in particular when the
scale of it is so graduated that, instead of the specific gravity, it indicates immediately the
per centage of anhydrous alcohol in a given weight or volume of the liquid. The scale
graduated according to the per centage of pure alcohol by weight, constitutes the
alcoholmeter of Richter; and that by the per centage in volume, the alcoholmeter of
Tralles and Gay Lussac.
As liquors are sold in general by the measure, not by the weight, it is convenient,
therefore, to know the alcoholic content of the mixtures in the per centage by volume.
Tralles has constructed new tables upon the principles of those of Gilpin, in which the
proportion is given by volume, and anhydrous alcohol is assumed for the basis; which,
at 60° Fahr., has a specific gravity of 0·7939 compared with water at its maximum
density, or a specific gravity 0·7946 compared with water of the temperature of 60°
Fahr. Gilpin’s alcohol of 0·825 contains 92·6 per cent. by volume of anhydrous alcohol.
The following table exhibits the per centage of anhydrous alcohol by volume, at a
temperature of 60° Fahr., in correspondence with the specific gravities of the spirits,
water being considered at 60° Fahr. to have a specific gravity of 0·9991.
Alcoholmetrical Table of Tralles.
Alcohol
in 100
measures
of spirit. |
Specific
gravity
at 60° Fahr. |
Difference
of the sp. gr. |
| 0 |
9991 |
|
| 1 |
9976 |
15 |
| 2 |
9961 |
15 |
| 3 |
9947 |
14 |
| 4 |
9933 |
14 |
| 5 |
9919 |
14 |
| 6 |
9906 |
13 |
| 7 |
9893 |
13 |
| 8 |
9881 |
12 |
| 9 |
9869 |
12 |
| 10 |
9857 |
12 |
| 11 |
9845 |
12 |
| 12 |
9834 |
11 |
| 13 |
9823 |
11 |
| 14 |
9812 |
11 |
| 15 |
9802 |
10 |
| 16 |
9791 |
11 |
| 17 |
9781 |
10 |
| 18 |
9771 |
10 |
| 19 |
9761 |
10 |
| 20 |
9751 |
10 |
| 21 |
9741 |
10 |
| 22 |
9731 |
10 |
| 23 |
9720 |
11 |
| 24 |
9710 |
10 |
| 25 |
9700 |
10 |
| 26 |
9689 |
11 |
| 27 |
9679 |
10 |
| 28 |
9668 |
11 |
| 29 |
9657 |
11 |
| 30 |
9646 |
11 |
| 31 |
9634 |
12 |
| 32 |
9622 |
12 |
| 33 |
9609 |
13[19] |
| 34 |
9596 |
13 |
| 35 |
9583 |
13 |
| 36 |
9570 |
13 |
| 37 |
9556 |
14 |
| 38 |
9541 |
15 |
| 39 |
9526 |
15 |
| 40 |
9510 |
16 |
| 41 |
9494 |
16 |
| 42 |
9478 |
16 |
| 43 |
9461 |
17 |
| 44 |
9444 |
17 |
| 45 |
9427 |
17 |
| 46 |
9409 |
18 |
| 47 |
9391 |
18 |
| 48 |
9373 |
18 |
| 49 |
9354 |
19 |
| 50 |
9335 |
19 |
| 51 |
9315 |
20 |
| 52 |
9295 |
20 |
| 53 |
9275 |
20 |
| 54 |
9254 |
21 |
| 55 |
9234 |
20 |
| 56 |
9213 |
21 |
| 57 |
9192 |
21 |
| 58 |
9170 |
22 |
| 59 |
9148 |
22 |
| 60 |
9126 |
22 |
| 61 |
9104 |
22 |
| 62 |
9082 |
22 |
| 63 |
9059 |
23 |
| 64 |
9036 |
23 |
| 65 |
9013 |
23 |
| 66 |
8989 |
24 |
| 67 |
8965 |
24 |
| 68 |
8941 |
24 |
| 69 |
8917 |
24 |
| 70 |
8892 |
25 |
| 71 |
8867 |
25 |
| 72 |
8842 |
25 |
| 73 |
8817 |
25 |
| 74 |
8791 |
26 |
| 75 |
8765 |
26 |
| 76 |
8739 |
26 |
| 77 |
8712 |
27 |
| 78 |
8685 |
27 |
| 79 |
8658 |
27 |
| 80 |
8631 |
27 |
| 81 |
8603 |
28 |
| 82 |
8575 |
28 |
| 83 |
8547 |
28 |
| 84 |
8518 |
29 |
| 85 |
8488 |
30 |
| 86 |
8458 |
30 |
| 87 |
8428 |
30 |
| 88 |
8397 |
31 |
| 89 |
8365 |
32 |
| 90 |
8332 |
33 |
| 91 |
8299 |
33 |
| 92 |
8265 |
34 |
| 93 |
8230 |
35 |
| 94 |
8194 |
36 |
| 95 |
8157 |
37 |
| 96 |
8118 |
39 |
| 97 |
8077 |
41 |
| 98 |
8034 |
43 |
| 99 |
7988 |
46 |
| 100 |
7939 |
49 |
Remarks on the preceding Table of Alcohol.
The third column of this table exhibits the differences of the specific gravities, which
give the denominator of the fraction for such densities as are not found sufficiently near
in the table; and the difference of their numerators is the next greatest to the density
found in the table. For example: if the specific gravity of the liquor found for
60° Fahr. = 9605 (the per centage will be between 33 and 34), the difference from
9609 (which is the next greatest number in the table) = 4, and the fraction is 4⁄13;
therefore the true per centage is 334⁄13. From the construction of this table the per
centage of alcohol by weight may also be found. For instance: we multiply the number
representing the volumes of alcohol (given in the table for any determinate specific
gravity of the mixture) by the specific gravity of the pure alcohol, that is, by 7939,
and the product is the number of pounds of alcohol in so many pounds as the specific
gravity multiplied by 100 gives. Thus, in the mixture of 9510 specific gravity, there are
40 measures of alcohol; hence there are also in 95,100 pounds of this spirit
7939 + 40 = 31·756 pounds of alcohol; and in 100 pounds of the spirits of 0·9510
specific gravity, 33·39 pounds of alcohol are contained.
As the preceding table gives the true alcoholic content when the portion of spirit under
trial has the normal temperature of 60° Fahr., the following table gives the per centage of
alcohol for the specific gravities corresponding to the accompanying temperatures.
For example: if we have a spirituous liquor at 80° Fahr., whose specific gravity is
0·9342, the alcohol present is 45 per cent. of the volume, or that specific gravity at that
temperature is equal to the specific gravity 0·9427 at the normal temperature of
60° Fahr. This table may also be employed for every degree of the thermometer and
every per centage, so as to save computation for the intervals. It is evident from
inspection that a difference of 5° Fahr. in the temperature changes the specific gravity of
the liquor by a difference nearly equal to 1 volume per cent. of alcohol; thus at
35° and 85° Fahr. the very same specific gravity of the liquor shows nearly 10 volumes
per cent. of alcohol more or less; the same, for example, at 60 and 40 per cent.
Alco-
hol
per
cent. |
Temperature. |
Alco-
hol
per
cent. |
Temperature. |
| 30° F. |
35° F. |
40° F. |
45° F. |
50° F. |
55° F. |
60° F. |
65° F. |
70° F. |
75° F. |
80° F. |
85° F. |
| 0 |
9994 |
9997 |
9997 |
9998 |
9997 |
9994 |
0 |
9991 |
9987 |
9991 |
9976 |
9970 |
9962 |
| 5 |
9924 |
9926 |
9926 |
9926 |
9925 |
9922 |
5 |
9919 |
9915 |
9909 |
9903 |
9897 |
9889 |
| 10 |
9868 |
9869 |
9868 |
9867 |
9865 |
9861 |
10 |
9857 |
9852 |
9845 |
9839 |
9831 |
9823 |
| 15 |
9823 |
9822 |
9820 |
9817 |
9813 |
9807 |
15 |
9802 |
9796 |
9788 |
9779 |
9771 |
9761 |
| 20 |
9786 |
9782 |
9777 |
9772 |
9766 |
9759 |
20 |
9751 |
9743 |
9733 |
9722 |
9711 |
9700 |
| 25 |
9753 |
9746 |
9738 |
9729 |
9720 |
9709 |
25 |
9700 |
9690 |
9678 |
9665 |
9652 |
9638 |
| 30 |
9717 |
9707 |
9695 |
9684 |
9672 |
9659 |
30 |
9646 |
9632 |
9618 |
9603 |
9588 |
9572 |
| 35 |
9671 |
9658 |
9644 |
9629 |
9614 |
9599 |
35 |
9583 |
9566 |
9549 |
9532 |
9514 |
9495 |
| 40 |
9615 |
9598 |
9581 |
9563 |
9546 |
9528 |
40 |
9510 |
9491 |
9472 |
9452 |
9433 |
9412 |
| 45 |
9544 |
9525 |
9506 |
9486 |
9467 |
9447 |
45 |
9427 |
9406 |
9385 |
9364 |
9342 |
9320 |
| 50 |
9460 |
9440 |
9420 |
9399 |
9378 |
9356 |
50 |
9335 |
9313 |
9290 |
9267 |
9244 |
9221 |
| 55 |
9368 |
9347 |
9325 |
9302 |
9279 |
9256 |
55 |
9234 |
9211 |
9187 |
9163 |
9139 |
9114 |
| 60 |
9267 |
9245 |
9222 |
9198 |
9174 |
9150 |
60 |
9126 |
9102 |
9076 |
9051 |
9026 |
9000 |
| 65 |
9162 |
9138 |
9113 |
9088 |
9063 |
9038 |
65 |
9013 |
8988 |
8962 |
8936 |
8909 |
8882 |
| 70 |
9046 |
9021 |
8996 |
8970 |
8944 |
8917 |
70 |
8892 |
8866 |
8839 |
8812 |
8784 |
8756 |
| 75 |
8925 |
8899 |
8873 |
8847 |
8820 |
8792 |
75 |
8765 |
8738 |
8710 |
8681 |
8652 |
8622 |
| 80 |
8798 |
8771 |
8744 |
8716 |
8688 |
8659 |
80 |
8631 |
8602 |
8573 |
8544 |
8514 |
8483 |
| 85 |
8663 |
8635 |
8606 |
8577 |
8547 |
8517 |
85 |
8488 |
8458 |
8427 |
8396 |
8365 |
8333 |
| 90 |
8517 |
8486 |
8455 |
8425 |
8395 |
8363 |
90 |
8322 |
8300 |
8268 |
8236 |
8204 |
8171 |
Alco-
hol
per
cent. |
Temperature. |
| 30° F. |
35° F. |
40° F. |
45° F. |
50° F. |
55° F. |
60° F. |
65° F. |
70° F. |
75° F. |
80° F. |
85° F. |
| 0 |
9994 |
9997 |
9997 |
9998 |
9997 |
9994 |
9991 |
9987 |
9991 |
9976 |
9970 |
9962 |
| 5 |
9924 |
9926 |
9926 |
9926 |
9925 |
9922 |
9919 |
9915 |
9909 |
9903 |
9897 |
9889 |
| 10 |
9868 |
9869 |
9868 |
9867 |
9865 |
9861 |
9857 |
9852 |
9845 |
9839 |
9831 |
9823 |
| 15 |
9823 |
9822 |
9820 |
9817 |
9813 |
9807 |
9802 |
9796 |
9788 |
9779 |
9771 |
9761 |
| 20 |
9786 |
9782 |
9777 |
9772 |
9766 |
9759 |
9751 |
9743 |
9733 |
9722 |
9711 |
9700 |
| 25 |
9753 |
9746 |
9738 |
9729 |
9720 |
9709 |
9700 |
9690 |
9678 |
9665 |
9652 |
9638 |
| 30 |
9717 |
9707 |
9695 |
9684 |
9672 |
9659 |
9646 |
9632 |
9618 |
9603 |
9588 |
9572 |
| 35 |
9671 |
9658 |
9644 |
9629 |
9614 |
9599 |
9583 |
9566 |
9549 |
9532 |
9514 |
9495 |
| 40 |
9615 |
9598 |
9581 |
9563 |
9546 |
9528 |
9510 |
9491 |
9472 |
9452 |
9433 |
9412 |
| 45 |
9544 |
9525 |
9506 |
9486 |
9467 |
9447 |
9427 |
9406 |
9385 |
9364 |
9342 |
9320 |
| 50 |
9460 |
9440 |
9420 |
9399 |
9378 |
9356 |
9335 |
9313 |
9290 |
9267 |
9244 |
9221 |
| 55 |
9368 |
9347 |
9325 |
9302 |
9279 |
9256 |
9234 |
9211 |
9187 |
9163 |
9139 |
9114 |
| 60 |
9267 |
9245 |
9222 |
9198 |
9174 |
9150 |
9126 |
9102 |
9076 |
9051 |
9026 |
9000 |
| 65 |
9162 |
9138 |
9113 |
9088 |
9063 |
9038 |
9013 |
8988 |
8962 |
8936 |
8909 |
8882 |
| 70 |
9046 |
9021 |
8996 |
8970 |
8944 |
8917 |
8892 |
8866 |
8839 |
8812 |
8784 |
8756 |
| 75 |
8925 |
8899 |
8873 |
8847 |
8820 |
8792 |
8765 |
8738 |
8710 |
8681 |
8652 |
8622 |
| 80 |
8798 |
8771 |
8744 |
8716 |
8688 |
8659 |
8631 |
8602 |
8573 |
8544 |
8514 |
8483 |
| 85 |
8663 |
8635 |
8606 |
8577 |
8547 |
8517 |
8488 |
8458 |
8427 |
8396 |
8365 |
8333 |
| 90 |
8517 |
8486 |
8455 |
8425 |
8395 |
8363 |
8322 |
8300 |
8268 |
8236 |
8204 |
8171 |
[20]
The importance of extreme accuracy in determining the density of alcoholic mixtures
in the United Kingdom, on account of the great revenue derived from them to the State,
and their consequent high price in commerce, induced the Lords of the Treasury a few
years ago to request the Royal Society to examine the construction and mode of applying
the instrument now in use for ascertaining and charging the duty on spirits. This instrument,
which is known and described in the law as Sikes’s hydrometer, possesses, in many
respects, decided advantages over those formerly in use. The committee of the Royal
Society state, that a definite mixture of alcohol and water is as invariable in its value as
absolute alcohol can be; and can be more readily, and with equal accuracy, identified
by that only quality or condition to which recourse can be had in practice, namely,
specific gravity. The committee further proposed, that the standard spirit be that which,
consisting of alcohol and water alone, shall have a specific gravity of 0·92 at the
temperature of 62° Fahr., water being unity at the same temperature; or, in other
words, that it shall at 62° weigh 92⁄100 or 23⁄25 of an equal bulk of water at the same
temperature.
This standard is rather weaker than the old proof, which was 12⁄13, or 0·923; or in the
proportion of nearly 1·1 gallon of the present proof spirit per cent. The proposed
standard will contain nearly one half by weight of absolute alcohol. The hydrometer
ought to be so graduated as to give the indication of strength; not upon an arbitrary
scale, but in terms of specific gravity at the temperature of 62°.
The committee recommend the construction of an equation table, which shall indicate
the same strength of spirit at every temperature. Thus in standard spirit at 62° the
hydrometer would indicate 920, which in this table would give proof spirit. If that
same spirit were cooled to 40°, the hydrometer would indicate some higher number; but
which, being combined in the table with the temperature as indicated by the thermometer,
should still give proof or standard spirit as the result.
It is considered advisable, in this and the other tables, not to express the quality of the
spirit by any number over or under proof, but to indicate at once the number of gallons of
standard spirit contained in, or equivalent to, 100 gallons of the spirit under examination.
Thus, instead of saying 23 over proof, it is proposed to insert 123; and in place of
35·4 under proof, to insert its difference to 100, or 64·6.
It has been considered expedient to recommend a second table to be constructed, so
as to show the bulk of spirit of any strength at any temperature, relative to a standard
bulk of 100 gallons at 62°. In this table a spirit which had diminished in volume, at
any given temperature, 0·7 per cent., for example, would be expressed by 99·3; and a
spirit which had increased at any given temperature 0·7 per cent., by 100·7.
When a sample of spirit, therefore, has been examined by the hydrometer and
thermometer, these tables will give first the proportion of standard spirit at the observed
temperature, and next the change of bulk of such spirit from what it would be at the
standard temperature. Thus, at the temperature of 51°, and with an indication
(sp. gr.) of 8240, 100 gallons of the spirit under examination would be shown by the
first table to be equal to 164·8 gallons of standard spirit of that temperature; and by the
second table it would appear that 99·3 gallons of the same spirit would become 100 at
62°, or in reality contain the 164·8 gallons of spirit in that state only in which it is to
be taxed.
But as it is considered that neither of these tables can alone be used for charging the
duty (for neither can express the actual quantity of spirit of a specific gravity of 0·92 at
62° in 100 gallons of stronger or weaker spirit at temperatures above or below 62°), it is
considered essential to have a third table, combining the two former, and expressing this
relation directly, so that upon mere inspection it shall indicate the proportion of standard
spirit in 100 gallons of that under examination in its then present state. In this table
the quantities should be set down in the actual number of gallons of standard spirit at
62°, equivalent to 100 of the spirit under examination; and the column of quantities
may be expressed by the term value, as it in reality expresses the proportion of the only
valuable substance present. As this will be the only table absolutely necessary to be
used with the instrument for the purposes of the excise, it may, perhaps, be thought
unnecessary to print the former two.
[21]
The following specimen table has been given by the committee:—
| Temperature 45°. |
Temperature 75°. |
Indica-
tion.[3] |
Strength. |
Value. |
Indica-
tion. |
Strength. |
Value. |
| 9074 |
114·5 |
|
8941 |
114·5 |
|
| 7 |
114·3 |
|
4 |
114·3 |
|
| 9 |
114·2 |
|
5 |
114·2 |
|
| 81 |
114·0 |
|
8 |
114·0 |
|
| 3 |
113·9 |
|
9 |
113·9 |
|
| 5 |
113·7 |
|
52 |
113·7 |
|
| 6 |
113·6 |
|
3 |
113·6 |
|
| 9 |
113·4 |
|
6 |
113·4 |
|
| 90 |
113·3 |
|
7 |
113·3 |
|
| 3 |
113·1 |
|
9 |
113·1 |
|
The mixture of alcohol and water, taken as spirit in Mr. Gilpin’s tables, is that of
which the specific gravity is 0·825 at 60° Fahr., water being unity at the same temperature.
The specific gravity of water at 60° being 1000, at 62° it is 99,981. Hence,
in order to compare the specific gravities given by Mr. Gilpin with those which would
result when the specific gravity of water at 62° is taken at unity, all the former numbers
must be divided by 99,981.
Table of the Specific Gravities of different Mixtures, by Weight, of Alcohol and Water,
at different Temperatures; constructed by Mr. Gilpin, for the use of the British
Revenue on Spirits.
Tem-
pera-
ture,
Fahr. |
Pure
Alco-
hol. |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
5
Water. |
10
Water. |
15
Water. |
20
Water. |
25
Water. |
30
Water. |
35
Water. |
40
Water. |
45
Water. |
50
Water. |
55
Water. |
60
Water. |
65
Water. |
70
Water. |
75
Water. |
80
Water. |
85
Water. |
90
Water. |
95
Water. |
100
Water. |
| Deg. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 30 |
0 |
·83896 |
0 |
·84995 |
0 |
·85957 |
0 |
·86825 |
0 |
·87585 |
0 |
·88282 |
0 |
·88921 |
0 |
·89511 |
0 |
·90054 |
0 |
·90558 |
0 |
·91023 |
0 |
·91449 |
0 |
·91847 |
0 |
·92217 |
0 |
·92563 |
0 |
·92889 |
0 |
·93191 |
0 |
·93474 |
0 |
·93741 |
0 |
·93991 |
0 |
·94222 |
| 35 |
|
·83672 |
|
·84769 |
|
·85729 |
|
·86587 |
|
·87357 |
|
·88059 |
|
·88701 |
|
·89294 |
|
·89839 |
|
·90345 |
|
·90811 |
|
·91241 |
|
·91640 |
|
·92009 |
|
·92355 |
|
·92680 |
|
·92986 |
|
·93274 |
|
·93541 |
|
·93790 |
|
·94025 |
| 40 |
|
·83445 |
|
·84539 |
|
·85507 |
|
·86361 |
|
·87184 |
|
·87838 |
|
·88481 |
|
·89073 |
|
·89617 |
|
·90127 |
|
·90596 |
|
·91026 |
|
·91428 |
|
·91799 |
|
·92151 |
|
·92476 |
|
·92783 |
|
·93072 |
|
·93341 |
|
·93592 |
|
·93827 |
| 45 |
|
·83214 |
|
·84310 |
|
·85277 |
|
·86131 |
|
·86905 |
|
·87613 |
|
·88255 |
|
·88849 |
|
·89396 |
|
·89909 |
|
·90380 |
|
·90812 |
|
·91211 |
|
·91584 |
|
·91937 |
|
·92264 |
|
·92570 |
|
·92859 |
|
·93131 |
|
·93382 |
|
·93621 |
| 50 |
|
·82977 |
|
·84076 |
|
·85042 |
|
·85902 |
|
·86676 |
|
·87384 |
|
·88030 |
|
·88626 |
|
·89174 |
|
·89684 |
|
·90160 |
|
·90596 |
|
·90997 |
|
·91370 |
|
·91723 |
|
·92051 |
|
·92358 |
|
·92647 |
|
·92919 |
|
·93177 |
|
·93419 |
| 55 |
|
·82736 |
|
·83834 |
|
·84802 |
|
·85664 |
|
·86441 |
|
·87150 |
|
·87796 |
|
·88393 |
|
·88945 |
|
·89458 |
|
·89933 |
|
·90367 |
|
·90768 |
|
·91144 |
|
·91502 |
|
·91837 |
|
·92145 |
|
·92436 |
|
·92707 |
|
·92963 |
|
·93208 |
| 60 |
|
·82500 |
|
·83599 |
|
·84568 |
|
·85430 |
|
·86208 |
|
·86918 |
|
·87569 |
|
·88169 |
|
·88720 |
|
·89232 |
|
·89707 |
|
·90144 |
|
·90549 |
|
·90927 |
|
·91287 |
|
·91622 |
|
·91933 |
|
·92225 |
|
·92499 |
|
·92758 |
|
·93002 |
| 65 |
|
·82262 |
|
·83362 |
|
·84334 |
|
·85193 |
|
·85976 |
|
·86686 |
|
·87337 |
|
·87938 |
|
·88490 |
|
·89006 |
|
·89479 |
|
·89920 |
|
·90328 |
|
·90707 |
|
·91066 |
|
·91400 |
|
·91715 |
|
·92010 |
|
·92283 |
|
·92546 |
|
·92794 |
| 70 |
|
·82023 |
|
·83124 |
|
·84092 |
|
·84951 |
|
·85736 |
|
·86451 |
|
·87105 |
|
·87705 |
|
·88254 |
|
·88773 |
|
·89252 |
|
·89695 |
|
·90104 |
|
·90484 |
|
·90847 |
|
·91181 |
|
·91493 |
|
·91793 |
|
·92069 |
|
·92333 |
|
·92580 |
| 75 |
|
·81780 |
|
·82878 |
|
·83851 |
|
·84710 |
|
·85496 |
|
·86212 |
|
·86864 |
|
·87466 |
|
·88018 |
|
·88538 |
|
·89018 |
|
·89464 |
|
·89872 |
|
·90252 |
|
·90617 |
|
·90952 |
|
·91270 |
|
·91569 |
|
·91849 |
|
·92111 |
|
·92364 |
| 80 |
|
·81530 |
|
·82631 |
|
·83603 |
|
·84467 |
|
·85248 |
|
·85966 |
|
·86622 |
|
·87228 |
|
·87776 |
|
·88301 |
|
·88781 |
|
·89225 |
|
·89639 |
|
·90021 |
|
·90385 |
|
·90723 |
|
·91046 |
|
·91340 |
|
·91622 |
|
·91891 |
|
·92142 |
| 85 |
|
·81291 |
|
·82396 |
|
·83371 |
|
·84243 |
|
·85036 |
|
·85757 |
|
·86411 |
|
·87021 |
|
·87590 |
|
·88120 |
|
·88609 |
|
·89043 |
|
·89460 |
|
·89843 |
|
·90209 |
|
·90558 |
|
·90882 |
|
·91186 |
|
·91465 |
|
·91729 |
|
·91969 |
| 90 |
|
·81044 |
|
·82150 |
|
·83126 |
|
·84001 |
|
·84797 |
|
·85518 |
|
·86172 |
|
·86787 |
|
·87360 |
|
·87889 |
|
·88376 |
|
·88817 |
|
·89230 |
|
·89617 |
|
·89988 |
|
·90342 |
|
·90688 |
|
·90967 |
|
·91248 |
|
·91511 |
|
·91751 |
| 95 |
|
·80794 |
|
·81900 |
|
·82877 |
|
·83753 |
|
·84550 |
|
·85272 |
|
·85928 |
|
·86542 |
|
·87114 |
|
·87654 |
|
·88146 |
|
·88588 |
|
·89003 |
|
·89390 |
|
·89763 |
|
·90119 |
|
·90443 |
|
·90747 |
|
·91029 |
|
·91290 |
|
·91531 |
| 100 |
|
·80548 |
|
·81657 |
|
·82630 |
|
·83513 |
|
·84038 |
|
·85031 |
|
·85688 |
|
·86302 |
|
·86879 |
|
·87421 |
|
·87915 |
|
·883671 |
|
·88769 |
|
·89158 |
|
·89536 |
|
·89889 |
|
·90215 |
|
·90522 |
|
·90805 |
|
·91066 |
|
·91310 |
Tem-
pera-
ture,
Fahr. |
Pure
Alco-
hol. |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
5
Water. |
10
Water. |
15
Water. |
20
Water. |
25
Water. |
30
Water. |
35
Water. |
40
Water. |
45
Water. |
50
Water. |
| Deg. |
|
|
|
|
|
|
|
|
|
|
|
| 30 |
0 |
·83896 |
0 |
·84995 |
0 |
·85957 |
0 |
·86825 |
0 |
·87585 |
0 |
·88282 |
0 |
·88921 |
0 |
·89511 |
0 |
·90054 |
0 |
·90558 |
0 |
·91023 |
| 35 |
|
·83672 |
|
·84769 |
|
·85729 |
|
·86587 |
|
·87357 |
|
·88059 |
|
·88701 |
|
·89294 |
|
·89839 |
|
·90345 |
|
·90811 |
| 40 |
|
·83445 |
|
·84539 |
|
·85507 |
|
·86361 |
|
·87184 |
|
·87838 |
|
·88481 |
|
·89073 |
|
·89617 |
|
·90127 |
|
·90596 |
| 45 |
|
·83214 |
|
·84310 |
|
·85277 |
|
·86131 |
|
·86905 |
|
·87613 |
|
·88255 |
|
·88849 |
|
·89396 |
|
·89909 |
|
·90380 |
| 50 |
|
·82977 |
|
·84076 |
|
·85042 |
|
·85902 |
|
·86676 |
|
·87384 |
|
·88030 |
|
·88626 |
|
·89174 |
|
·89684 |
|
·90160 |
| 55 |
|
·82736 |
|
·83834 |
|
·84802 |
|
·85664 |
|
·86441 |
|
·87150 |
|
·87796 |
|
·88393 |
|
·88945 |
|
·89458 |
|
·89933 |
| 60 |
|
·82500 |
|
·83599 |
|
·84568 |
|
·85430 |
|
·86208 |
|
·86918 |
|
·87569 |
|
·88169 |
|
·88720 |
|
·89232 |
|
·89707 |
| 65 |
|
·82262 |
|
·83362 |
|
·84334 |
|
·85193 |
|
·85976 |
|
·86686 |
|
·87337 |
|
·87938 |
|
·88490 |
|
·89006 |
|
·89479 |
| 70 |
|
·82023 |
|
·83124 |
|
·84092 |
|
·84951 |
|
·85736 |
|
·86451 |
|
·87105 |
|
·87705 |
|
·88254 |
|
·88773 |
|
·89252 |
| 75 |
|
·81780 |
|
·82878 |
|
·83851 |
|
·84710 |
|
·85496 |
|
·86212 |
|
·86864 |
|
·87466 |
|
·88018 |
|
·88538 |
|
·89018 |
| 80 |
|
·81530 |
|
·82631 |
|
·83603 |
|
·84467 |
|
·85248 |
|
·85966 |
|
·86622 |
|
·87228 |
|
·87776 |
|
·88301 |
|
·88781 |
| 85 |
|
·81291 |
|
·82396 |
|
·83371 |
|
·84243 |
|
·85036 |
|
·85757 |
|
·86411 |
|
·87021 |
|
·87590 |
|
·88120 |
|
·88609 |
| 90 |
|
·81044 |
|
·82150 |
|
·83126 |
|
·84001 |
|
·84797 |
|
·85518 |
|
·86172 |
|
·86787 |
|
·87360 |
|
·87889 |
|
·88376 |
| 95 |
|
·80794 |
|
·81900 |
|
·82877 |
|
·83753 |
|
·84550 |
|
·85272 |
|
·85928 |
|
·86542 |
|
·87114 |
|
·87654 |
|
·88146 |
| 100 |
|
·80548 |
|
·81657 |
|
·82630 |
|
·83513 |
|
·84038 |
|
·85031 |
|
·85688 |
|
·86302 |
|
·86879 |
|
·87421 |
|
·87915 |
Tem-
pera-
ture,
Fahr. |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
100
Alco-
hol |
55
Water. |
60
Water. |
65
Water. |
70
Water. |
75
Water. |
80
Water. |
85
Water. |
90
Water. |
95
Water. |
100
Water. |
| Deg. |
|
|
|
|
|
|
|
|
|
|
| 30 |
0 |
·91449 |
0 |
·91847 |
0 |
·92217 |
0 |
·92563 |
0 |
·92889 |
0 |
·93191 |
0 |
·93474 |
0 |
·93741 |
0 |
·93991 |
0 |
·94222 |
| 35 |
|
·91241 |
|
·91640 |
|
·92009 |
|
·92355 |
|
·92680 |
|
·92986 |
|
·93274 |
|
·93541 |
|
·93790 |
|
·94025 |
| 40 |
|
·91026 |
|
·91428 |
|
·91799 |
|
·92151 |
|
·92476 |
|
·92783 |
|
·93072 |
|
·93341 |
|
·93592 |
|
·93827 |
| 45 |
|
·90812 |
|
·91211 |
|
·91584 |
|
·91937 |
|
·92264 |
|
·92570 |
|
·92859 |
|
·93131 |
|
·93382 |
|
·93621 |
| 50 |
|
·90596 |
|
·90997 |
|
·91370 |
|
·91723 |
|
·92051 |
|
·92358 |
|
·92647 |
|
·92919 |
|
·93177 |
|
·93419 |
| 55 |
|
·90367 |
|
·90768 |
|
·91144 |
|
·91502 |
|
·91837 |
|
·92145 |
|
·92436 |
|
·92707 |
|
·92963 |
|
·93208 |
| 60 |
|
·90144 |
|
·90549 |
|
·90927 |
|
·91287 |
|
·91622 |
|
·91933 |
|
·92225 |
|
·92499 |
|
·92758 |
|
·93002 |
| 65 |
|
·89920 |
|
·90328 |
|
·90707 |
|
·91066 |
|
·91400 |
|
·91715 |
|
·92010 |
|
·92283 |
|
·92546 |
|
·92794 |
| 70 |
|
·89695 |
|
·90104 |
|
·90484 |
|
·90847 |
|
·91181 |
|
·91493 |
|
·91793 |
|
·92069 |
|
·92333 |
|
·92580 |
| 75 |
|
·89464 |
|
·89872 |
|
·90252 |
|
·90617 |
|
·90952 |
|
·91270 |
|
·91569 |
|
·91849 |
|
·92111 |
|
·92364 |
| 80 |
|
·89225 |
|
·89639 |
|
·90021 |
|
·90385 |
|
·90723 |
|
·91046 |
|
·91340 |
|
·91622 |
|
·91891 |
|
·92142 |
| 85 |
|
·89043 |
|
·89460 |
|
·89843 |
|
·90209 |
|
·90558 |
|
·90882 |
|
·91186 |
|
·91465 |
|
·91729 |
|
·91969 |
| 90 |
|
·88817 |
|
·89230 |
|
·89617 |
|
·89988 |
|
·90342 |
|
·90688 |
|
·90967 |
|
·91248 |
|
·91511 |
|
·91751 |
| 95 |
|
·88588 |
|
·89003 |
|
·89390 |
|
·89763 |
|
·90119 |
|
·90443 |
|
·90747 |
|
·91029 |
|
·91290 |
|
·91531 |
| 100 |
|
·883671 |
|
·88769 |
|
·89158 |
|
·89536 |
|
·89889 |
|
·90215 |
|
·90522 |
|
·90805 |
|
·91066 |
|
·91310 |
Tem-
pera-
ture,
Fahr. |
95
Alco-
hol |
90
Alco-
hol |
85
Alco-
hol |
80
Alco-
hol |
75
Alco-
hol |
70
Alco-
hol |
65
Alco-
hol |
60
Alco-
hol |
55
Alco-
hol |
50
Alco-
hol |
45
Alco-
hol |
40
Alco-
hol |
35
Alco-
hol |
30
Alco-
hol |
25
Alco-
hol |
20
Alco-
hol |
15
Alco-
hol |
10
Alco-
hol |
5
Alco-
hol |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
| Deg. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 30 |
0 |
·94447 |
0 |
·94675 |
0 |
·94920 |
0 |
·95173 |
0 |
·95429 |
0 |
·95681 |
0 |
·95944 |
0 |
·96209 |
0 |
·96470 |
0 |
·96719 |
0 |
·96967 |
0 |
·97200 |
0 |
·97418 |
0 |
·97635 |
0 |
·97860 |
0 |
·98108 |
0 |
·98412 |
0 |
·98804 |
0 |
·99334 |
| 35 |
|
·94249 |
|
·94484 |
|
·94734 |
|
·94988 |
|
·95246 |
|
·95502 |
|
·95772 |
|
·96048 |
|
·96315 |
|
·96579 |
|
·96840 |
|
·97086 |
|
·97319 |
|
·97556 |
|
·97801 |
|
·98076 |
|
·98397 |
|
·98804 |
|
·99344 |
| 40 |
|
·94058 |
|
·94295 |
|
·94547 |
|
·94802 |
|
·95060 |
|
·95328 |
|
·95602 |
|
·95879 |
|
·96159 |
|
·96434 |
|
·96706 |
|
·96967 |
|
·97220 |
|
·97472 |
|
·97737 |
|
·98033 |
|
·98373 |
|
·98795 |
|
·99345 |
| 45 |
|
·93860 |
|
·94096 |
|
·94348 |
|
·94605 |
|
·94871 |
|
·95143 |
|
·95423 |
|
·95703 |
|
·95993 |
|
·96280 |
|
·96563 |
|
·96840 |
|
·97110 |
|
·97384 |
|
·97666 |
|
·97980 |
|
·98338 |
|
·98774 |
|
·99338 |
| 50 |
|
·93658 |
|
·93897 |
|
·94149 |
|
·94414 |
|
·94683 |
|
·94958 |
|
·95243 |
|
·95534 |
|
·95831 |
|
·96126 |
|
·96420 |
|
·96708 |
|
·96995 |
|
·97284 |
|
·97589 |
|
·97920 |
|
·98293 |
|
·98745 |
|
·99316 |
| 55 |
|
·93452 |
|
·93696 |
|
·93948 |
|
·94213 |
|
·94486 |
|
·94767 |
|
·95057 |
|
·95357 |
|
·95662 |
|
·95966 |
|
·96272 |
|
·96575 |
|
·96877 |
|
·97181 |
|
·97500 |
|
·97847 |
|
·98239 |
|
·98702 |
|
·99284 |
| 60 |
|
·93247 |
|
·93493 |
|
·93749 |
|
·94018 |
|
·94296 |
|
·94579 |
|
·94876 |
|
·95181 |
|
·95493 |
|
·95804 |
|
·96122 |
|
·96437 |
|
·96752 |
|
·97074 |
|
·97410 |
|
·97771 |
|
·98176 |
|
·98654 |
|
·99244 |
| 65 |
|
·93040 |
|
·93285 |
|
·93546 |
|
·93822 |
|
·94099 |
|
·94388 |
|
·94689 |
|
·95000 |
|
·95318 |
|
·95635 |
|
·95962 |
|
·96288 |
|
·96620 |
|
·96959 |
|
·97309 |
|
·97688 |
|
·98106 |
|
·98594 |
|
·99194 |
| 70 |
|
·92828 |
|
·93076 |
|
·93337 |
|
·93616 |
|
·93898 |
|
·94193 |
|
·94500 |
|
·94813 |
|
·95139 |
|
·95469 |
|
·95802 |
|
·96143 |
|
·96484 |
|
·96836 |
|
·97203 |
|
·97596 |
|
·98028 |
|
·98527 |
|
·99134 |
| 75 |
|
·92613 |
|
·92865 |
|
·93132 |
|
·93413 |
|
·93695 |
|
·93989 |
|
·94301 |
|
·94623 |
|
·94957 |
|
·95292 |
|
·95638 |
|
·95987 |
|
·96344 |
|
·96708 |
|
·97086 |
|
·97495 |
|
·97943 |
|
·98454 |
|
·99066 |
| 80 |
|
·92393 |
|
·92646 |
|
·92917 |
|
·93201 |
|
·93488 |
|
·93785 |
|
·94102 |
|
·94431 |
|
·94768 |
|
·95111 |
|
·95467 |
|
·95826 |
|
·96192 |
|
·96568 |
|
·96963 |
|
·97385 |
|
·97845 |
|
·98367 |
|
·98991 |
Tem-
pera-
ture,
Fahr. |
95
Alco-
hol |
90
Alco-
hol |
85
Alco-
hol |
80
Alco-
hol |
75
Alco-
hol |
70
Alco-
hol |
65
Alco-
hol |
60
Alco-
hol |
55
Alco-
hol |
50
Alco-
hol |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
| Deg. |
|
|
|
|
|
|
|
|
|
|
| 30 |
0 |
·94447 |
0 |
·94675 |
0 |
·94920 |
0 |
·95173 |
0 |
·95429 |
0 |
·95681 |
0 |
·95944 |
0 |
·96209 |
0 |
·96470 |
0 |
·96719 |
| 35 |
|
·94249 |
|
·94484 |
|
·94734 |
|
·94988 |
|
·95246 |
|
·95502 |
|
·95772 |
|
·96048 |
|
·96315 |
|
·96579 |
| 40 |
|
·94058 |
|
·94295 |
|
·94547 |
|
·94802 |
|
·95060 |
|
·95328 |
|
·95602 |
|
·95879 |
|
·96159 |
|
·96434 |
| 45 |
|
·93860 |
|
·94096 |
|
·94348 |
|
·94605 |
|
·94871 |
|
·95143 |
|
·95423 |
|
·95703 |
|
·95993 |
|
·96280 |
| 50 |
|
·93658 |
|
·93897 |
|
·94149 |
|
·94414 |
|
·94683 |
|
·94958 |
|
·95243 |
|
·95534 |
|
·95831 |
|
·96126 |
| 55 |
|
·93452 |
|
·93696 |
|
·93948 |
|
·94213 |
|
·94486 |
|
·94767 |
|
·95057 |
|
·95357 |
|
·95662 |
|
·95966 |
| 60 |
|
·93247 |
|
·93493 |
|
·93749 |
|
·94018 |
|
·94296 |
|
·94579 |
|
·94876 |
|
·95181 |
|
·95493 |
|
·95804 |
| 65 |
|
·93040 |
|
·93285 |
|
·93546 |
|
·93822 |
|
·94099 |
|
·94388 |
|
·94689 |
|
·95000 |
|
·95318 |
|
·95635 |
| 70 |
|
·92828 |
|
·93076 |
|
·93337 |
|
·93616 |
|
·93898 |
|
·94193 |
|
·94500 |
|
·94813 |
|
·95139 |
|
·95469 |
| 75 |
|
·92613 |
|
·92865 |
|
·93132 |
|
·93413 |
|
·93695 |
|
·93989 |
|
·94301 |
|
·94623 |
|
·94957 |
|
·95292 |
| 80 |
|
·92393 |
|
·92646 |
|
·92917 |
|
·93201 |
|
·93488 |
|
·93785 |
|
·94102 |
|
·94431 |
|
·94768 |
|
·95111 |
Tem-
pera-
ture,
Fahr. |
45
Alco-
hol |
40
Alco-
hol |
35
Alco-
hol |
30
Alco-
hol |
25
Alco-
hol |
20
Alco-
hol |
15
Alco-
hol |
10
Alco-
hol |
5
Alco-
hol |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
100
Water. |
| Deg. |
|
|
|
|
|
|
|
|
|
| 30 |
0 |
·96967 |
0 |
·97200 |
0 |
·97418 |
0 |
·97635 |
0 |
·97860 |
0 |
·98108 |
0 |
·98412 |
0 |
·98804 |
0 |
·99334 |
| 35 |
|
·96840 |
|
·97086 |
|
·97319 |
|
·97556 |
|
·97801 |
|
·98076 |
|
·98397 |
|
·98804 |
|
·99344 |
| 40 |
|
·96706 |
|
·96967 |
|
·97220 |
|
·97472 |
|
·97737 |
|
·98033 |
|
·98373 |
|
·98795 |
|
·99345 |
| 45 |
|
·96563 |
|
·96840 |
|
·97110 |
|
·97384 |
|
·97666 |
|
·97980 |
|
·98338 |
|
·98774 |
|
·99338 |
| 50 |
|
·96420 |
|
·96708 |
|
·96995 |
|
·97284 |
|
·97589 |
|
·97920 |
|
·98293 |
|
·98745 |
|
·99316 |
| 55 |
|
·96272 |
|
·96575 |
|
·96877 |
|
·97181 |
|
·97500 |
|
·97847 |
|
·98239 |
|
·98702 |
|
·99284 |
| 60 |
|
·96122 |
|
·96437 |
|
·96752 |
|
·97074 |
|
·97410 |
|
·97771 |
|
·98176 |
|
·98654 |
|
·99244 |
| 65 |
|
·95962 |
|
·96288 |
|
·96620 |
|
·96959 |
|
·97309 |
|
·97688 |
|
·98106 |
|
·98594 |
|
·99194 |
| 70 |
|
·95802 |
|
·96143 |
|
·96484 |
|
·96836 |
|
·97203 |
|
·97596 |
|
·98028 |
|
·98527 |
|
·99134 |
| 75 |
|
·95638 |
|
·95987 |
|
·96344 |
|
·96708 |
|
·97086 |
|
·97495 |
|
·97943 |
|
·98454 |
|
·99066 |
| 80 |
|
·95467 |
|
·95826 |
|
·96192 |
|
·96568 |
|
·96963 |
|
·97385 |
|
·97845 |
|
·98367 |
|
·98991 |
Experiments were made, by direction of the committee, to verify Gilpin’s tables,
which showed that the error introduced in ascertaining the strength of spirits by tables
founded on Gilpin’s numbers must be quite insensible in the practice of the revenue.
The discrepancies thus detected, on a mixture of a given strength, did not amount in any
one instance to unity in the fourth place of decimals. From a careful inspection of such
documents the committee are of opinion, that Gilpin’s tables possess a degree of accuracy
far surpassing what could be expected, and sufficiently perfect for all practical or
scientific purposes.
The following table is given by Mr. Lubbock, for converting the apparent specific
gravity, or indication, into true specific gravity:—
Indi-
ca-
tion. |
-Temperature+ |
Indi-
ca-
tion. |
| 30° |
32° |
37° |
42° |
47° |
52° |
57° |
62° |
67° |
72° |
77° |
80° |
| ·82 |
·00083 |
·00078 |
·00065 |
·00052 |
·00039 |
·00025 |
·00012 |
|
·00011 |
·00024 |
·00035 |
·00042 |
·82 |
| ·83 |
·00084 |
·00079 |
·00066 |
·00052 |
·00039 |
·00026 |
·00012 |
|
·00012 |
·00024 |
·00036 |
·00042 |
·83 |
| ·84 |
·00085 |
·00080 |
·00066 |
·00053 |
·00039 |
·00026 |
·00013 |
|
·00012 |
·00024 |
·00036 |
·00043 |
·84 |
| ·85 |
·00086 |
·00081 |
·00067 |
·00054 |
·00040 |
·00026 |
·00013 |
|
·00012 |
·00025 |
·00037 |
·00043 |
·85 |
| ·86 |
·00087 |
·00082 |
·00068 |
·00054 |
·00040 |
·00027 |
·00013 |
|
·00012 |
·00025 |
·00037 |
·00044 |
·86 |
| ·87 |
·00088 |
·00083 |
·00069 |
·00055 |
·00041 |
·00027 |
·00013 |
|
·00012 |
·00025 |
·00037 |
·00044 |
·87 |
| ·88 |
·00089 |
·00084 |
·00070 |
·00055 |
·00041 |
·00027 |
·00013 |
|
·00012 |
·00026 |
·00038 |
·00045 |
·88 |
| ·89 |
·00090 |
·00085 |
·00070 |
·00055 |
·00042 |
·00028 |
·00013 |
|
·00012 |
·00026 |
·00038 |
·00045 |
·89 |
| ·90 |
·00091 |
·00085 |
·00071 |
·00056 |
·00042 |
·00028 |
·00014 |
|
·00013 |
·00026 |
·00039 |
·00046 |
·90 |
| ·91 |
·00092 |
·00086 |
·00072 |
·00057 |
·00043 |
·00028 |
·00014 |
|
·00013 |
·00026 |
·00039 |
·00046 |
·91 |
| ·92 |
·00093 |
·00087 |
·00073 |
·00058 |
·00043 |
·00029 |
·00014 |
|
·00013 |
·00027 |
·00040 |
·00047 |
·92 |
| ·93 |
·00094 |
·00088 |
·00073 |
·00059 |
·00044 |
·00029 |
·00014 |
|
·00013 |
·00027 |
·00040 |
·00047 |
·93 |
| ·94 |
·00095 |
·00089 |
·00074 |
·00059 |
·00044 |
·00029 |
·00014 |
|
·00013 |
·00027 |
·00040 |
·00048 |
·94 |
| ·95 |
·00096 |
·00090 |
·00075 |
·00060 |
·00045 |
·00029 |
·00014 |
|
·00013 |
·00028 |
·00041 |
·00048 |
·95 |
| ·96 |
·00097 |
·00091 |
·00076 |
·00060 |
·00045 |
·00030 |
·00014 |
|
·00013 |
·00028 |
·00041 |
·00049 |
·96 |
| ·97 |
·00098 |
·00092 |
·00077 |
·00061 |
·00046 |
·00030 |
·00015 |
|
·00014 |
·00028 |
·00042 |
·00049 |
·97 |
| ·98 |
·00099 |
·00093 |
·00077 |
·00062 |
·00046 |
·00030 |
·00015 |
|
·00014 |
·00028 |
·00042 |
·00050 |
·98 |
| ·99 |
·00100 |
·00094 |
·00078 |
·00062 |
·00047 |
·00031 |
·00015 |
|
·00014 |
·00029 |
·00043 |
·00050 |
·99 |
| 1·00 |
·00101 |
·00095 |
·00079 |
·00063 |
·00047 |
·00031 |
·00015 |
|
|
|
|
|
1·00 |
[23]
Fig. 5.
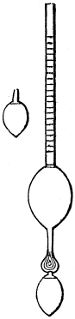
The hydrometer constructed, under the directions of the Commissioners of Excise, by
Mr. Bate, has a scale of 4 inches in length divided into 100 parts,
and 9 weights. It has thus a range of 900 divisions, and expresses
specific gravities at the temperature of 62° Fahr. In order to render
this instrument so accurate a measurer of the specific gravity, at the
standard temperature, as to involve no error of an appreciable amount,
Mr. Bate has constructed the weights (which in this instrument are immersed
in the fluid of different specific gravities) so that each successive
weight should have an increase of bulk over the preceding weight
equal to that part of the stem occupied by the scale, and an increase
of weight sufficient to take the whole of the scale, and no more, down
to the liquid. This arrangement requires great accuracy of workmanship,
and enhances the price of the instrument. But it allows of increased
strength in the ball, where it is very much required, and it
gives, upon inspection only, the indication (apparent specific gravity)
by which the general table is to be examined and the result ascertained.
Fig. 5. represents this instrument and two of its nine ballast weights.
It comprehends all specific gravities between 820 and 1000. It indicates
true specific gravity with almost perfect accuracy at the temperature
of 62° Fahr.; but it does not exclude other instruments from
being used in conjunction with tables. The latter are, in fact, independent
of the instrument, and may be used with gravimeters, or
any instrument affording indications by specific gravity at a given
temperature.
The commercial value of spirituous liquors being much lower in
France than in England, a less sensible instrument becomes sufficient
for the wants of that country. Baumé’s and Cartier’s hydrometers,
with short arbitrary scales, are very much employed, but they have been lately superseded
by an ingenious and ready instrument contrived by M. Gay Lussac, and called
by him an alcoomètre. He takes for the term of comparison pure alcohol by volume,
at the temperature of 15° Cent., and represents the strength of it by 100 centimes,
or by unity. Consequently, the strength of a spirituous liquid is the number of centimes
in volume of pure alcohol which that liquid contains at the temperature of 15°
Cent. The instrument is formed like a common hydrometer, and is graduated for the
temperature of 15° Cent. Its scale is divided into 100 parts or degrees, each of which
denotes a centime of alcohol; the division 0 at the bottom of the stem corresponds
to pure water, and the division 100 at its top, to pure alcohol. When immersed in a
spirituous liquor at 15° Cent. (59° Fahr.) it announces its strength directly. For
example: if in spirits supposed at the temperature of 15° Cent. it sinks to the division 50,
it indicates that the strength of this liquor is 50 per cent., or that it contains 50 centimes
of pure alcohol. In our new British proof spirit, it would sink to nearly 57, indicating
57 by volume of pure alcohol, allowing for condensation, or 50 by weight. A table of
correction is given for temperature, which he calls “Table of real strength of spirituous
liquors.” The first vertical column of this table contains the temperatures, from 0° to
30° Cent., and the first horizontal line the indications of the alcoomètre. In the same
table we have most ingeniously inserted a correction for the volume of the spirits when
the temperature differs from 15° Cent. If we take 1000 litres or gallons, measured at
the temperature of 2°, of a spirituous liquor whose apparent strength is 44c; its
real strength at 15° will from the preceding mode of correction be 49c. On heating this
liquid to 15°, in order to find its real specific gravity or strength, its bulk will become
greater; and, instead of 1000 litres or gallons, which it measured at 2°, we shall
have 1009 at 15° C. This number is inscribed in smaller characters in the same
square cell with the real force, precisely under 49c. All the numbers in small
characters, printed under each real strength, indicate the volume which 1000 litres of a
spirituous liquor would have, when measured at the temperature at which its apparent
strength is taken. In the above example, the quantity in litres or gallons of pure
alcohol contained in 1000 litres or gallons of the spirits, measured at the temperature of
2°, will be, therefore,—1009 lit. × 0·49 = 494 lit. 41.
This quantity of pure alcohol, thus estimated, is called richness of spirit in alcohol, or
simply richness.
Let us take an example similar to the preceding, but at a higher temperature than
15° Cent. Suppose we have 1000 litres measured, at the temperature of 25°, of spirits
whose apparent strength is 53c, what is the real quantity of pure alcohol which this
spirit contains at the temperature of 15°? We shall find in the table, first of all, that
the real strength of the spirits is 49c·3. As to its bulk or volume, it is very clear
that the 1000 litres in cooling from 25° to 15°, will occupy a smaller space. This
[24]volume will be 993 litres; it is inscribed directly below 49c·3, the real strength. We
shall therefore have of pure alcohol, contained in the 1000 litres of spirits, measured at
the temperature of 25°, or their richness, 993 lit. × 0·493 = 489 lit. 55.
[25-
27]
Alcometrical Table of real Strength, by M. Gay Lussac.
Tem-
pera-
ture
C. |
31c |
32c |
33c |
34c |
35c |
36c |
37c |
38c |
39c |
40c |
41c |
42c |
43c |
44c |
45c |
46c |
47c |
48c |
49c |
50c |
51c |
52c |
53c |
54c |
55c |
56c |
57c |
58c |
59c |
60c |
61c |
62c |
63c |
64c |
65c |
66c |
67c |
68c |
69c |
70c |
71c |
72c |
73c |
74c |
75c |
76c |
77c |
78c |
79c |
80c |
81c |
82c |
83c |
84c |
85c |
86c |
87c |
88c |
89c |
90c |
| Deg. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10 |
33·0 |
34 |
35 |
36 |
37 |
38 |
39 |
40 |
41 |
42 |
43 |
44 |
45 |
46 |
46·9 |
47·9 |
48·9 |
49·9 |
50·9 |
51·8 |
52·8 |
53·8 |
54·8 |
55·8 |
56·8 |
57·8 |
58·8 |
59·7 |
60·7 |
61·7 |
62·7 |
63·7 |
64·7 |
65·7 |
66·7 |
67·6 |
68·6 |
69·6 |
70·6 |
71·6 |
72·6 |
73·5 |
74·5 |
75·5 |
76·5 |
77·5 |
78·5 |
79·5 |
80·5 |
81·5 |
82·4 |
83·4 |
84·4 |
85·4 |
86·4 |
87·4 |
88·3 |
89·3 |
90·2 |
91·2 |
| 1002 |
1002 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
| 11 |
32·6 |
33·6 |
34·6 |
35·6 |
36·6 |
37·6 |
38·6 |
39·6 |
40·6 |
41·6 |
42·6 |
43·6 |
44·6 |
45·6 |
46·6 |
47·6 |
48·6 |
49·5 |
50·5 |
51·5 |
52·5 |
53·5 |
54·4 |
55·4 |
56·4 |
57·4 |
58·4 |
59·4 |
60·4 |
61·4 |
62·4 |
63·4 |
64·4 |
65·4 |
66·4 |
67·3 |
68·3 |
69·3 |
70·3 |
71·3 |
72·3 |
73·2 |
74·2 |
75·2 |
76·2 |
77·2 |
78·2 |
79·2 |
80·2 |
81·2 |
82·2 |
83·1 |
84·1 |
85·1 |
86·1 |
87·1 |
88 |
89 |
90 |
91 |
| 1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
| 12 |
32·2 |
33·2 |
34·2 |
35·2 |
36·2 |
37·2 |
38·2 |
39·2 |
40·2 |
41·2 |
42·2 |
43·2 |
44·2 |
45·2 |
46·2 |
47·2 |
48·2 |
49·2 |
50·2 |
51·1 |
52·1 |
53·1 |
54·1 |
55 |
56 |
57 |
58 |
59 |
60 |
61 |
62 |
63 |
64 |
65 |
66 |
67 |
68 |
69 |
70 |
71 |
72 |
72·9 |
73·9 |
74·9 |
75·9 |
76·9 |
77·9 |
78·9 |
79·9 |
80·9 |
81·9 |
82·9 |
83·9 |
84·8 |
85·8 |
86·8 |
87·8 |
88·7 |
89·7 |
90·7 |
| 1001 |
1001 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
| 13 |
31·8 |
32·8 |
33·8 |
34·8 |
35·8 |
36·8 |
37·8 |
38·8 |
39·8 |
40·8 |
41·8 |
42·8 |
43·8 |
44·8 |
45·8 |
46·8 |
47·8 |
48·8 |
49·8 |
50·8 |
51·8 |
52·7 |
53·7 |
54·7 |
55·7 |
56·7 |
57·7 |
58·7 |
59·7 |
60·7 |
61·7 |
62·7 |
63·7 |
64·7 |
65·7 |
66·7 |
67·7 |
68·7 |
69·6 |
70·6 |
71·6 |
72·6 |
73·6 |
74·6 |
75·6 |
76·6 |
77·6 |
78·6 |
79·6 |
80·6 |
81·6 |
82·6 |
83·6 |
84·6 |
85·5 |
86·5 |
87·5 |
88·5 |
89·5 |
90·5 |
| 1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1092 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
| 14 |
31·4 |
32·4 |
33·4 |
34·4 |
35·4 |
36·4 |
37·4 |
38·4 |
39·4 |
40·4 |
41·4 |
42·4 |
43·4 |
44·4 |
45·4 |
46·4 |
47·4 |
48·4 |
49·4 |
50·4 |
51·4 |
52·3 |
53·3 |
54·3 |
55·3 |
56·3 |
57·3 |
58·3 |
59·3 |
60·3 |
61·3 |
62·3 |
63·3 |
64·3 |
65·3 |
66·3 |
67·3 |
68·3 |
69·3 |
70·3 |
71·3 |
72·3 |
73·3 |
74·3 |
75·3 |
76·3 |
77·3 |
78·3 |
79·3 |
80·3 |
81·3 |
82·3 |
83·3 |
84·3 |
85·3 |
86·3 |
87·3 |
88·2 |
89·2 |
90·2 |
| 1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1000 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
| 15 |
31 |
32 |
33 |
34 |
35 |
36 |
37 |
38 |
39 |
40 |
41 |
42 |
43 |
44 |
45 |
46 |
47 |
48 |
49 |
50 |
51 |
52 |
53 |
54 |
55 |
56 |
57 |
58 |
59 |
60 |
61 |
62 |
63 |
64 |
65 |
66 |
67 |
68 |
69 |
70 |
71 |
72 |
73 |
74 |
75 |
76 |
77 |
78 |
79 |
80 |
81 |
82 |
83 |
84 |
85 |
86 |
87 |
88 |
89 |
90 |
| 1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
| 16 |
30·6 |
31·6 |
32·5 |
33·5 |
34·5 |
35·5 |
36·5 |
37·5 |
38·5 |
39·5 |
40·6 |
41·6 |
42·6 |
43·6 |
44·6 |
45·6 |
46·6 |
47·6 |
48·6 |
49·6 |
50·6 |
51·6 |
52·6 |
53·6 |
54·6 |
55·6 |
56·6 |
57·6 |
58·6 |
59·6 |
60·6 |
61·7 |
62·7 |
63·7 |
64·7 |
65·7 |
66·7 |
67·7 |
68·7 |
69·7 |
70·7 |
71·7 |
72·7 |
73·7 |
74·7 |
75·7 |
76·7 |
77·7 |
78·7 |
79·7 |
80·7 |
81·7 |
82·7 |
83·7 |
84·7 |
85·7 |
86·7 |
87·7 |
88·7 |
89·7 |
| 1000 |
1000 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
| 17 |
30·2 |
31·2 |
32·1 |
33·1 |
34·1 |
35·1 |
36·1 |
37·1 |
38·1 |
39·1 |
40·2 |
41·2 |
42·2 |
43·2 |
44·9 |
45·2 |
46·2 |
47·2 |
48·2 |
49·2 |
50·3 |
51·3 |
52·3 |
53·3 |
54·3 |
55·3 |
56·3 |
57·3 |
58·3 |
59·3 |
60·3 |
61·3 |
62·3 |
63·3 |
64·3 |
65·3 |
66·3 |
67·3 |
68·3 |
69·3 |
70·3 |
71·3 |
72·3 |
73·3 |
74·3 |
75·4 |
76·4 |
77·4 |
78·4 |
79·4 |
80·4 |
81·4 |
82·4 |
83·4 |
84·4 |
85·4 |
86·4 |
87·4 |
88·4 |
89·5 |
| 999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
| 18 |
29·8 |
30·8 |
31·7 |
32·7 |
33·7 |
34·7 |
35·7 |
36·7 |
37·7 |
38·7 |
39·8 |
40·8 |
41·8 |
42·8 |
43·8 |
44·9 |
45·9 |
46·9 |
47·9 |
48·9 |
49·9 |
50·9 |
51·9 |
52·9 |
53·9 |
54·9 |
55·9 |
56·9 |
57·9 |
58·9 |
59·9 |
61 |
62 |
63 |
64 |
65 |
66 |
67 |
68 |
69 |
70 |
71 |
72 |
73 |
74 |
75·1 |
76·1 |
77·1 |
78·1 |
79·1 |
80·1 |
81·1 |
82·1 |
83·1 |
84·1 |
85·2 |
86·2 |
87·2 |
88·2 |
89·2 |
| 999 |
999 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
| 19 |
29·4 |
30·4 |
31·3 |
32·3 |
33·3 |
34·3 |
35·3 |
36·3 |
37·3 |
38·3 |
39·4 |
40·4 |
41·4 |
42·5 |
43·5 |
44·5 |
45·5 |
46·5 |
47·5 |
48·5 |
49·5 |
50·6 |
51·6 |
52·6 |
53·6 |
54·6 |
55·6 |
56·6 |
57·6 |
58·6 |
59·6 |
60·6 |
61·6 |
62·7 |
63·7 |
64·7 |
65·7 |
66·7 |
67·7 |
68·7 |
69·7 |
70·7 |
71·7 |
72·7 |
73·7 |
74·7 |
75·8 |
76·8 |
77·8 |
78·8 |
79·8 |
80·8 |
81·9 |
82·9 |
83·9 |
84·9 |
85·9 |
86·9 |
87·9 |
88·9 |
| 998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
| 20 |
29 |
30 |
30·9 |
31·9 |
32·9 |
33·9 |
34·9 |
35·9 |
36·9 |
37·9 |
39 |
40 |
41 |
42·1 |
43·1 |
44·1 |
45·1 |
46·1 |
47·2 |
48·2 |
49·2 |
50·2 |
51·2 |
52·2 |
53·2 |
54·2 |
55·2 |
56·2 |
57·2 |
58·2 |
59·2 |
60·3 |
61·3 |
62·3 |
63·3 |
64·3 |
65·4 |
66·4 |
67·4 |
68·4 |
69·4 |
70·4 |
71·4 |
72·4 |
73·4 |
74·4 |
75·5 |
76·5 |
77·5 |
78·5 |
79·5 |
80·5 |
81·6 |
82·6 |
83·6 |
84·6 |
85·6 |
86·6 |
87·7 |
88·7 |
| 998 |
998 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
| 21 |
28·6 |
29·6 |
30·5 |
31·5 |
32·5 |
33·5 |
34·5 |
35·5 |
36·5 |
37·5 |
38·6 |
39·6 |
40·6 |
41·7 |
42·7 |
43·7 |
44·8 |
45·8 |
46·8 |
47·8 |
48·8 |
49·8 |
50·8 |
51·8 |
52·9 |
53·9 |
54·9 |
55·9 |
56·9 |
57·9 |
58·9 |
59·9 |
61 |
62 |
63 |
64 |
65 |
66 |
67 |
68·1 |
69·1 |
70·1 |
71·1 |
72·1 |
73·1 |
74·1 |
75·2 |
76·2 |
77·2 |
78·2 |
79·2 |
80·2 |
81·3 |
82·3 |
83·3 |
84·3 |
85·3 |
86·4 |
87·4 |
88·4 |
| 997 |
997 |
997 |
997 |
997 |
997 |
997 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
| 22 |
28·2 |
29·2 |
30·1 |
31·1 |
32·1 |
33·1 |
34·1 |
35·1 |
36·1 |
37·1 |
38·2 |
39·2 |
40·2 |
41·3 |
42·3 |
43·3 |
44·3 |
45·3 |
46·4 |
47·4 |
48·4 |
49·4 |
50·4 |
51·4 |
52·5 |
53·5 |
54·5 |
55·5 |
56·5 |
57·5 |
58·5 |
59·5 |
60·6 |
61·6 |
62·7 |
63·7 |
64·7 |
65·7 |
66·7 |
67·7 |
68·8 |
69·8 |
70·8 |
71·8 |
72·8 |
73·8 |
74·8 |
75·9 |
76·9 |
77·9 |
78·9 |
79·9 |
81 |
82 |
83 |
84 |
85 |
86·1 |
87·1 |
88·2 |
| 997 |
997 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
| 23 |
27·8 |
28·8 |
29·7 |
30·7 |
31·7 |
32·7 |
33·7 |
34·7 |
35·7 |
36·7 |
37·8 |
38·8 |
39·8 |
40·9 |
41·9 |
42·9 |
43·9 |
44·9 |
46 |
47 |
48 |
49·1 |
50·1 |
51·1 |
52·1 |
53·1 |
54·1 |
55·1 |
56·1 |
57·1 |
58·1 |
59·2 |
60·2 |
61·3 |
62·3 |
63·3 |
64·3 |
65·4 |
66·4 |
67·4 |
68·4 |
69·4 |
70·5 |
71·5 |
72·5 |
73·5 |
74·5 |
75·5 |
76·6 |
77·6 |
78·6 |
79·6 |
80·7 |
81·7 |
82·7 |
83·8 |
84·8 |
85·8 |
86·8 |
87·9 |
| 996 |
996 |
996 |
996 |
996 |
996 |
996 |
995 |
995 |
995 |
995 |
995 |
995 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
| 24 |
27·4 |
28·4 |
29·3 |
30·3 |
31·3 |
32·3 |
33·3 |
34·3 |
35·3 |
36·3 |
37·4 |
38·4 |
39·4 |
40·5 |
41·5 |
42·5 |
43·6 |
44·6 |
45·6 |
46·6 |
47·6 |
48·7 |
49·7 |
50·7 |
51·8 |
52·8 |
53·8 |
54·8 |
55·8 |
56·8 |
57·8 |
58·9 |
59·9 |
61 |
62 |
63 |
64 |
65 |
66 |
67·1 |
68·1 |
69·1 |
70·1 |
71·2 |
72·2 |
73·2 |
74·2 |
75·2 |
76·3 |
77·3 |
78·3 |
79·3 |
80·4 |
81·4 |
82·4 |
83·5 |
84·5 |
85·5 |
86·5 |
87·6 |
| 996 |
996 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
993 |
993 |
993 |
993 |
993 |
693 |
993 |
993 |
993 |
993 |
993 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
| 25 |
27 |
28 |
28·9 |
29·9 |
30·9 |
31·9 |
32·9 |
33·9 |
34·9 |
35·9 |
37 |
38 |
39 |
40·1 |
42·1 |
42·2 |
43·2 |
44·2 |
45·2 |
46·3 |
47·3 |
48·3 |
49·3 |
50·3 |
51·4 |
52·4 |
53·4 |
54·4 |
55·5 |
56·5 |
57·5 |
58·5 |
59·5 |
60·6 |
61·6 |
62·6 |
63·7 |
64·7 |
65·7 |
66·7 |
67·8 |
68·8 |
69·8 |
70·8 |
71·8 |
72·8 |
73·9 |
74·9 |
76 |
77 |
78 |
79 |
80·1 |
81·1 |
82·1 |
83·2 |
84·2 |
85·2 |
86·3 |
87·4 |
| 995 |
995 |
995 |
995 |
995 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
990 |
990 |
990 |
990 |
990 |
990 |
990 |
990 |
Tem-
pera-
ture
C. |
31c |
32c |
33c |
34c |
35c |
36c |
37c |
38c |
39c |
40c |
41c |
42c |
| Deg. |
|
|
|
|
|
|
|
|
|
|
|
|
| 10 |
33·0 |
34 |
35 |
36 |
37 |
38 |
39 |
40 |
41 |
42 |
43 |
44 |
| 1002 |
1002 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1004 |
| 11 |
32·6 |
33·6 |
34·6 |
35·6 |
36·6 |
37·6 |
38·6 |
39·6 |
40·6 |
41·6 |
42·6 |
43·6 |
| 1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1003 |
1003 |
1003 |
1003 |
| 12 |
32·2 |
33·2 |
34·2 |
35·2 |
36·2 |
37·2 |
38·2 |
39·2 |
40·2 |
41·2 |
42·2 |
43·2 |
| 1001 |
1001 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
| 13 |
31·8 |
32·8 |
33·8 |
34·8 |
35·8 |
36·8 |
37·8 |
38·8 |
39·8 |
40·8 |
41·8 |
42·8 |
| 1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
| 14 |
31·4 |
32·4 |
33·4 |
34·4 |
35·4 |
36·4 |
37·4 |
38·4 |
39·4 |
40·4 |
41·4 |
42·4 |
| 1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
| 15 |
31 |
32 |
33 |
34 |
35 |
36 |
37 |
38 |
39 |
40 |
41 |
42 |
| 1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
| 16 |
30·6 |
31·6 |
32·5 |
33·5 |
34·5 |
35·5 |
36·5 |
37·5 |
38·5 |
39·5 |
40·6 |
41·6 |
| 1000 |
1000 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
| 17 |
30·2 |
31·2 |
32·1 |
33·1 |
34·1 |
35·1 |
36·1 |
37·1 |
38·1 |
39·1 |
40·2 |
41·2 |
| 999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
| 18 |
29·8 |
30·8 |
31·7 |
32·7 |
33·7 |
34·7 |
35·7 |
36·7 |
37·7 |
38·7 |
39·8 |
40·8 |
| 999 |
999 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
| 19 |
29·4 |
30·4 |
31·3 |
32·3 |
33·3 |
34·3 |
35·3 |
36·3 |
37·3 |
38·3 |
39·4 |
40·4 |
| 998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
997 |
997 |
997 |
997 |
| 20 |
29 |
30 |
30·9 |
31·9 |
32·9 |
33·9 |
34·9 |
35·9 |
36·9 |
37·9 |
39 |
40 |
| 998 |
998 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
| 21 |
28·6 |
29·6 |
30·5 |
31·5 |
32·5 |
33·5 |
34·5 |
35·5 |
36·5 |
37·5 |
38·6 |
39·6 |
| 997 |
997 |
997 |
997 |
997 |
997 |
997 |
996 |
996 |
996 |
996 |
996 |
| 22 |
28·2 |
29·2 |
30·1 |
31·1 |
32·1 |
33·1 |
34·1 |
35·1 |
36·1 |
37·1 |
38·2 |
39·2 |
| 997 |
997 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
995 |
| 23 |
27·8 |
28·8 |
29·7 |
30·7 |
31·7 |
32·7 |
33·7 |
34·7 |
35·7 |
36·7 |
37·8 |
38·8 |
| 996 |
996 |
996 |
996 |
996 |
996 |
996 |
995 |
995 |
995 |
995 |
995 |
| 24 |
27·4 |
28·4 |
29·3 |
30·3 |
31·3 |
32·3 |
33·3 |
34·3 |
35·3 |
36·3 |
37·4 |
38·4 |
| 996 |
996 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
994 |
994 |
994 |
| 25 |
27 |
28 |
28·9 |
29·9 |
30·9 |
31·9 |
32·9 |
33·9 |
34·9 |
35·9 |
37 |
38 |
| 995 |
995 |
995 |
995 |
995 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
Tem-
pera-
ture
C. |
43c |
44c |
45c |
46c |
47c |
48c |
49c |
50c |
51c |
52c |
53c |
54c |
| Deg. |
|
|
|
|
|
|
|
|
|
|
|
|
| 10 |
45 |
46 |
46·9 |
47·9 |
48·9 |
49·9 |
50·9 |
51·8 |
52·8 |
53·8 |
54·8 |
55·8 |
| 1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
| 11 |
44·6 |
45·6 |
46·6 |
47·6 |
48·6 |
49·5 |
50·5 |
51·5 |
52·5 |
53·5 |
54·4 |
55·4 |
| 1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
| 12 |
44·2 |
45·2 |
46·2 |
47·2 |
48·2 |
49·2 |
50·2 |
51·1 |
52·1 |
53·1 |
54·1 |
55 |
| 1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
| 13 |
43·8 |
44·8 |
45·8 |
46·8 |
47·8 |
48·8 |
49·8 |
50·8 |
51·8 |
52·7 |
53·7 |
54·7 |
| 1001 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
| 14 |
43·4 |
44·4 |
45·4 |
46·4 |
47·4 |
48·4 |
49·4 |
50·4 |
51·4 |
52·3 |
53·3 |
54·3 |
| 1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1000 |
1001 |
1001 |
1001 |
1001 |
| 15 |
43 |
44 |
45 |
46 |
47 |
48 |
49 |
50 |
51 |
52 |
53 |
54 |
| 1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
| 16 |
42·6 |
43·6 |
44·6 |
45·6 |
46·6 |
47·6 |
48·6 |
49·6 |
50·6 |
51·6 |
52·6 |
53·6 |
| 999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
| 17 |
42·2 |
43·2 |
44·9 |
45·2 |
46·2 |
47·2 |
48·2 |
49·2 |
50·3 |
51·3 |
52·3 |
53·3 |
| 999 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
| 18 |
41·8 |
42·8 |
43·8 |
44·9 |
45·9 |
46·9 |
47·9 |
48·9 |
49·9 |
50·9 |
51·9 |
52·9 |
| 998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
| 19 |
41·4 |
42·5 |
43·5 |
44·5 |
45·5 |
46·5 |
47·5 |
48·5 |
49·5 |
50·6 |
51·6 |
52·6 |
| 997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
| 20 |
41 |
42·1 |
43·1 |
44·1 |
45·1 |
46·1 |
47·2 |
48·2 |
49·2 |
50·2 |
51·2 |
52·2 |
| 997 |
997 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
| 21 |
40·6 |
41·7 |
42·7 |
43·7 |
44·8 |
45·8 |
46·8 |
47·8 |
48·8 |
49·8 |
50·8 |
51·8 |
| 996 |
996 |
996 |
996 |
996 |
996 |
995 |
995 |
995 |
995 |
995 |
995 |
| 22 |
40·2 |
41·3 |
42·3 |
43·3 |
44·3 |
45·3 |
46·4 |
47·4 |
48·4 |
49·4 |
50·4 |
51·4 |
| 995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
994 |
| 23 |
39·8 |
40·9 |
41·9 |
42·9 |
43·9 |
44·9 |
46 |
47 |
48 |
49·1 |
50·1 |
51·1 |
| 995 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
| 24 |
39·4 |
40·5 |
41·5 |
42·5 |
43·6 |
44·6 |
45·6 |
46·6 |
47·6 |
48·7 |
49·7 |
50·7 |
| 994 |
994 |
994 |
994 |
994 |
994 |
993 |
993 |
993 |
993 |
993 |
693 |
| 25 |
39 |
40·1 |
42·1 |
42·2 |
43·2 |
44·2 |
45·2 |
46·3 |
47·3 |
48·3 |
49·3 |
50·3 |
| 993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
992 |
Tem-
pera-
ture
C. |
55c |
56c |
57c |
58c |
59c |
60c |
61c |
62c |
63c |
64c |
65c |
66c |
| Deg. |
|
|
|
|
|
|
|
|
|
|
|
|
| 10 |
56·8 |
57·8 |
58·8 |
59·7 |
60·7 |
61·7 |
62·7 |
63·7 |
64·7 |
65·7 |
66·7 |
67·6 |
| 1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
| 11 |
56·4 |
57·4 |
58·4 |
59·4 |
60·4 |
61·4 |
62·4 |
63·4 |
64·4 |
65·4 |
66·4 |
67·3 |
| 1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
| 12 |
56 |
57 |
58 |
59 |
60 |
61 |
62 |
63 |
64 |
65 |
66 |
67 |
| 1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
| 13 |
55·7 |
56·7 |
57·7 |
58·7 |
59·7 |
60·7 |
61·7 |
62·7 |
63·7 |
64·7 |
65·7 |
66·7 |
| 1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
| 14 |
55·3 |
56·3 |
57·3 |
58·3 |
59·3 |
60·3 |
61·3 |
62·3 |
63·3 |
64·3 |
65·3 |
66·3 |
| 1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
| 15 |
55 |
56 |
57 |
58 |
59 |
60 |
61 |
62 |
63 |
64 |
65 |
66 |
| 1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
| 16 |
54·6 |
55·6 |
56·6 |
57·6 |
58·6 |
59·6 |
60·6 |
61·7 |
62·7 |
63·7 |
64·7 |
65·7 |
| 999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
| 17 |
54·3 |
55·3 |
56·3 |
57·3 |
58·3 |
59·3 |
60·3 |
61·3 |
62·3 |
63·3 |
64·3 |
65·3 |
| 998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
| 18 |
53·9 |
54·9 |
55·9 |
56·9 |
57·9 |
58·9 |
59·9 |
61 |
62 |
63 |
64 |
65 |
| 998 |
998 |
998 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
| 19 |
53·6 |
54·6 |
55·6 |
56·6 |
57·6 |
58·6 |
59·6 |
60·6 |
61·6 |
62·7 |
63·7 |
64·7 |
| 997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
| 20 |
53·2 |
54·2 |
55·2 |
56·2 |
57·2 |
58·2 |
59·2 |
60·3 |
61·3 |
62·3 |
63·3 |
64·3 |
| 996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
| 21 |
52·9 |
53·9 |
54·9 |
55·9 |
56·9 |
57·9 |
58·9 |
59·9 |
61 |
62 |
63 |
64 |
| 995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
| 22 |
52·5 |
53·5 |
54·5 |
55·5 |
56·5 |
57·5 |
58·5 |
59·5 |
60·6 |
61·6 |
62·7 |
63·7 |
| 994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
| 23 |
52·1 |
53·1 |
54·1 |
55·1 |
56·1 |
57·1 |
58·1 |
59·2 |
60·2 |
61·3 |
62·3 |
63·3 |
| 994 |
994 |
994 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
| 24 |
51·8 |
52·8 |
53·8 |
54·8 |
55·8 |
56·8 |
57·8 |
58·9 |
59·9 |
61 |
62 |
63 |
| 993 |
993 |
993 |
993 |
993 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
| 25 |
51·4 |
52·4 |
53·4 |
54·4 |
55·5 |
56·5 |
57·5 |
58·5 |
59·5 |
60·6 |
61·6 |
62·6 |
| 992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
991 |
991 |
991 |
Tem-
pera-
ture
C. |
67c |
68c |
69c |
70c |
71c |
72c |
73c |
74c |
75c |
76c |
77c |
78c |
| Deg. |
|
|
|
|
|
|
|
|
|
|
|
|
| 10 |
68·6 |
69·6 |
70·6 |
71·6 |
72·6 |
73·5 |
74·5 |
75·5 |
76·5 |
77·5 |
78·5 |
79·5 |
| 1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
| 11 |
68·3 |
69·3 |
70·3 |
71·3 |
72·3 |
73·2 |
74·2 |
75·2 |
76·2 |
77·2 |
78·2 |
79·2 |
| 1003 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
| 12 |
68 |
69 |
70 |
71 |
72 |
72·9 |
73·9 |
74·9 |
75·9 |
76·9 |
77·9 |
78·9 |
| 1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
| 13 |
67·7 |
68·7 |
69·6 |
70·6 |
71·6 |
72·6 |
73·6 |
74·6 |
75·6 |
76·6 |
77·6 |
78·6 |
| 1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1092 |
1002 |
1002 |
1002 |
| 14 |
67·3 |
68·3 |
69·3 |
70·3 |
71·3 |
72·3 |
73·3 |
74·3 |
75·3 |
76·3 |
77·3 |
78·3 |
| 1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
| 15 |
67 |
68 |
69 |
70 |
71 |
72 |
73 |
74 |
75 |
76 |
77 |
78 |
| 1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
| 16 |
66·7 |
67·7 |
68·7 |
69·7 |
70·7 |
71·7 |
72·7 |
73·7 |
74·7 |
75·7 |
76·7 |
77·7 |
| 999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
| 17 |
66·3 |
67·3 |
68·3 |
69·3 |
70·3 |
71·3 |
72·3 |
73·3 |
74·3 |
75·4 |
76·4 |
77·4 |
| 998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
| 18 |
66 |
67 |
68 |
69 |
70 |
71 |
72 |
73 |
74 |
75·1 |
76·1 |
77·1 |
| 997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
| 19 |
65·7 |
66·7 |
67·7 |
68·7 |
69·7 |
70·7 |
71·7 |
72·7 |
73·7 |
74·7 |
75·8 |
76·8 |
| 997 |
997 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
| 20 |
65·4 |
66·4 |
67·4 |
68·4 |
69·4 |
70·4 |
71·4 |
72·4 |
73·4 |
74·4 |
75·5 |
76·5 |
| 996 |
996 |
996 |
996 |
996 |
996 |
995 |
995 |
995 |
995 |
995 |
995 |
| 21 |
65 |
66 |
67 |
68·1 |
69·1 |
70·1 |
71·1 |
72·1 |
73·1 |
74·1 |
75·2 |
76·2 |
| 995 |
995 |
995 |
995 |
995 |
995 |
995 |
994 |
994 |
994 |
994 |
994 |
| 22 |
64·7 |
65·7 |
66·7 |
67·7 |
68·8 |
69·8 |
70·8 |
71·8 |
72·8 |
73·8 |
74·8 |
75·9 |
| 994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
993 |
993 |
993 |
993 |
| 23 |
64·3 |
65·4 |
66·4 |
67·4 |
68·4 |
69·4 |
70·5 |
71·5 |
72·5 |
73·5 |
74·5 |
75·5 |
| 993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
992 |
992 |
992 |
992 |
| 24 |
64 |
65 |
66 |
67·1 |
68·1 |
69·1 |
70·1 |
71·2 |
72·2 |
73·2 |
74·2 |
75·2 |
| 992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
991 |
| 25 |
63·7 |
64·7 |
65·7 |
66·7 |
67·8 |
68·8 |
69·8 |
70·8 |
71·8 |
72·8 |
73·9 |
74·9 |
| 991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
Tem-
pera-
ture
C. |
79c |
80c |
81c |
82c |
83c |
84c |
85c |
86c |
87c |
88c |
89c |
90c |
| Deg. |
|
|
|
|
|
|
|
|
|
|
|
|
| 10 |
80·5 |
81·5 |
82·4 |
83·4 |
84·4 |
85·4 |
86·4 |
87·4 |
88·3 |
89·3 |
90·2 |
91·2 |
| 1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
1005 |
| 11 |
80·2 |
81·2 |
82·2 |
83·1 |
84·1 |
85·1 |
86·1 |
87·1 |
88 |
89 |
90 |
91 |
| 1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
1004 |
| 12 |
79·9 |
80·9 |
81·9 |
82·9 |
83·9 |
84·8 |
85·8 |
86·8 |
87·8 |
88·7 |
89·7 |
90·7 |
| 1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
1003 |
| 13 |
79·6 |
80·6 |
81·6 |
82·6 |
83·6 |
84·6 |
85·5 |
86·5 |
87·5 |
88·5 |
89·5 |
90·5 |
| 1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
1002 |
| 14 |
79·3 |
80·3 |
81·3 |
82·3 |
83·3 |
84·3 |
85·3 |
86·3 |
87·3 |
88·2 |
89·2 |
90·2 |
| 1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
1001 |
| 15 |
79 |
80 |
81 |
82 |
83 |
84 |
85 |
86 |
87 |
88 |
89 |
90 |
| 1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
1000 |
| 16 |
78·7 |
79·7 |
80·7 |
81·7 |
82·7 |
83·7 |
84·7 |
85·7 |
86·7 |
87·7 |
88·7 |
89·7 |
| 999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
999 |
| 17 |
78·4 |
79·4 |
80·4 |
81·4 |
82·4 |
83·4 |
84·4 |
85·4 |
86·4 |
87·4 |
88·4 |
89·5 |
| 998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
998 |
| 18 |
78·1 |
79·1 |
80·1 |
81·1 |
82·1 |
83·1 |
84·1 |
85·2 |
86·2 |
87·2 |
88·2 |
89·2 |
| 997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
997 |
| 19 |
77·8 |
78·8 |
79·8 |
80·8 |
81·9 |
82·9 |
83·9 |
84·9 |
85·9 |
86·9 |
87·9 |
88·9 |
| 996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
996 |
| 20 |
77·5 |
78·5 |
79·5 |
80·5 |
81·6 |
82·6 |
83·6 |
84·6 |
85·6 |
86·6 |
87·7 |
88·7 |
| 995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
995 |
| 21 |
77·2 |
78·2 |
79·2 |
80·2 |
81·3 |
82·3 |
83·3 |
84·3 |
85·3 |
86·4 |
87·4 |
88·4 |
| 994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
994 |
| 22 |
76·9 |
77·9 |
78·9 |
79·9 |
81 |
82 |
83 |
84 |
85 |
86·1 |
87·1 |
88·2 |
| 993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
993 |
| 23 |
76·6 |
77·6 |
78·6 |
79·6 |
80·7 |
81·7 |
82·7 |
83·8 |
84·8 |
85·8 |
86·8 |
87·9 |
| 992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
992 |
| 24 |
76·3 |
77·3 |
78·3 |
79·3 |
80·4 |
81·4 |
82·4 |
83·5 |
84·5 |
85·5 |
86·5 |
87·6 |
| 991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
991 |
| 25 |
76 |
77 |
78 |
79 |
80·1 |
81·1 |
82·1 |
83·2 |
84·2 |
85·2 |
86·3 |
87·4 |
| 991 |
991 |
991 |
991 |
990 |
990 |
990 |
990 |
990 |
990 |
990 |
990 |
[28]
I consider the preceding table, which I have extracted from the longer tables of M.
Gay Lussac, as an important addition to the resources of British dealers and manufacturing
chemists. With the aid of his little instrument, which may be got for a trifle
from its ingenious maker, M. Collardeau, Rue Faubourg St. Martin, at Paris, or constructed
by one of the London hydrometer artists, the per centage of real alcohol, and
the real value of any spirituous liquor, may be determined to sufficient nicety for most
purposes, in a far easier manner than by any instruments now used in this country. It
has been adopted by the Swedish government, with M. Gay Lussac’s tables.
M. Gay Lussac’s table gives, by inspection, the true bulk of the spirits as corrected for
temperature; that is, their volume, if of the normal temperature of 15° Cent. (59° Fahr.).
Now this is important information; for, if a person buys 1000 gallons of spirits in hot
weather, and pays for them exactly according to their strength corrected for temperature,
he will not have 1000 gallons when the weather is in its mean state. He
may lose, in this way, several gallons without being aware of it from his hydrometer.
Sometimes, after moist autumns, when damaged grain abounds, the alcohol distilled
from its fermented wash contains a peculiar volatile body. When we apply our nose
to this species of spirits in its hot state, the volatile substance dissolved in it irritates
the eyes and nostrils: it has very nearly the same smell as an alcoholic solution of
cyanogen, as any chemist may discover by standing near the discharge pipe of the
refrigeratory worm of a raw-grain whisky still. Such spirits intoxicate more strongly than
pure spirits of the same strength, and excite, in many persons, even temporary frenzy.
It is a volatile fatty matter, of a very fetid odour, when obtained by itself, as I have procured
it in cold weather at some of the great distilleries in Scotland. It does not combine
with bases. At the end of a few months, it spontaneously decomposes in the spirits,
and leaves them in a less nauseous and noxious state. By largely diluting the spirits
with water, and distilling at a moderate temperature, the greater part of this oil may be
separated. Part of it comes over with the strongest alcohol, and part with the latter
runnings, which are called by the distillers strong and weak feints. The intermediate
portion is purer spirit. The feints are always more or less opalescent, or become so
on dilution with water, and then throw up an oily pellicle upon their surface. The
charcoals of light wood, such as pine or willow, well calcined, and infused in sufficient
quantity with the spirits prior to rectification, will deprive them of the greater part of
that oily contamination. Animal charcoal, well calcined, has also been found useful;
but it must be macerated for some time with the empyreumatic spirits, before distillation.
Another mode of separating that offensive oil is, to agitate the impure spirits
with a quantity of a fat oil, such as olive oil, or oil of almonds, to decant off the oil, and
re-distil the spirits with a little water.
Some foreign chemists direct empyreumatic or rank spirits, to be rectified with the
addition of chloride of lime. I have tried this method in every way, and on a considerable
scale, but never found the spirits to be improved by it. They were rather deteriorated.
See Brandy, Distillation, Fermentation, Gin, Rum, Whisky.
Anhydrous or absolute alcohol, when swallowed, acts as a mortal poison, not only by
its peculiar stimulus on the nervous system, but by its abstracting the aqueous particles
from the soft tissue of the stomach, with which it comes in contact, so as to destroy its
organisation. Alcohol of 0·812 consists, by experiments, of 3 atoms of carbon, 6 of
hydrogen, and 2 of oxygen; absolute alcohol consists, probably, of 2 of carbon, 3 of
hydrogen, and 1 of oxygen.
ALE. The fermented infusion of pale malted barley, usually combined with infusion
of hops. See Beer.
ALEMBIC, a Still; which see.
ALEMBROTH, salt of. The salt of wisdom, of the alchemists; a compound of bichloride
of mercury and sal ammoniac, from which the old white precipitate of mercury
is made.
ALGAROTH, powder of. A compound of oxide and chloride of antimony, being
a precipitate obtained by pouring water into the acidulous chloride of that metal.
ALKALI. A class of chemical bodies, distinguished chiefly by their solubility in
water, and their power of neutralising acids, so as to form saline compounds. The
alkalis of manufacturing importance are, ammonia, potash, soda, and quinia. These
alkalis change the purple colour of red cabbage and radishes to a green, the reddened
tincture of litmus to a purple, and the colour of turmeric and many other yellow dyes
to a brown. Even when combined with carbonic acid, the first three alkalis exercise
this discolouring power, which the alkaline earths, lime and barytes, do not. The same
three alkalis have an acrid, and somewhat urinous taste; the first two are energetic
solvents of animal matter; and the three combine with oils, so as to form soaps. They
unite with water in every proportion, and also with alcohol; and the first three combine
with water after being carbonated.
[29]
ALKALIMETER. An instrument for measuring the alkaline force or purity of
any of the alkalis of commerce. It is founded on the principle, that the quantity of
real alkali present in any sample, is proportional to the quantity of acid which a given
weight of it can neutralize. See the individual alkalis, Potash, and Soda.
ALKANA, is the name of the root and leaves of Lausania inermis, which have been
long employed in the East, to dye the nails, teeth, hair, garments, &c. The leaves,
ground and mixed with a little limewater, serve for dyeing the tails of horses in Persia
and Turkey.
ALKANET, the root of. (Anchusa tinctoria.) A species of bugloss, cultivated
chiefly in the neighbourhood of Montpellier. It affords a fine red colour to alcohol
and oils; but a dirty red to water. Its principal use is for colouring ointments,
cheeses, and pommades. The spirituous tincture gives to white marble a beautiful deep
stain.
ALLIGATION. An arithmetical formula, useful, on many occasions, for ascertaining
the proportion of constituents in a mixture, when they have undergone no change of
volume by chemical action. When alcoholic liquors are mixed with water, there is a
condensation of bulk, which renders that arithmetical rule inapplicable. The same thing
holds, in some measure, in the union of metals by fusion. See Alloy.
ALLOY. (Alliage, Fr.; Legirung, Germ.) This term formerly signified a compound
of gold and silver, with some metal of inferior value, but it now means any compound
of any two or more metals whatever. Thus, bronze is an alloy of copper and tin;
brass, an alloy of copper and zinc; and type metal, an alloy of lead and antimony. All
the alloys possess metallic lustre, even when cut or broken to pieces; they are opaque; are
excellent conductors of heat and electricity; are frequently susceptible of crystallising;
are more or less ductile, malleable, elastic, and sonorous. An alloy which consists of
metals differently fusible is usually malleable in the cold, and brittle when hot, as is
exemplified with brass and gong metal.
Many alloys consist of definite or equivalent proportions of the simple component
metals, though some alloys seem to form in any proportion, like combinations of salt or
sugar with water. It is probable that peculiar properties belong to the equivalent or
atomic ratio, as is exemplified in the superior quality of brass made in that proportion.
One metal does not alloy indifferently with every other metal, but it is governed in
this respect by peculiar affinities; thus, silver will hardly unite with iron, but it combines
readily with gold, copper, and lead. In comparing the alloys with their constituent
metals, the following differences may be noted; in general, the ductility of the alloy is
less than that of the separate metals, and sometimes in a very remarkable degree; on
the contrary, the alloy is usually harder than the mean hardness of its constituents. The
mercurial alloys or amalgams are, perhaps, exceptions to this rule.
The specific gravity is rarely the mean between that of each of its constituents, but is
sometimes greater and sometimes less, indicating, in the former case, an approximation,
and in the latter, a recedure, of the particles from each other in the act of their
union. The following tables of binary alloys exhibit this circumstance in experimental
detail:—
Alloys having a density greater than
the mean of their constituents. |
Alloys having a density less than
the mean of their constituents. |
| Gold and zinc |
Gold and silver |
| Gold and tin |
Gold and iron |
| Gold and bismuth |
Gold and lead |
| Gold and antimony |
Gold and copper |
| Gold and cobalt |
Gold and iridium |
| Silver and zinc |
Gold and nickel |
| Silver and lead |
Silver and copper |
| Silver and tin |
Silver and lead |
| Silver and bismuth |
Iron and bismuth |
| Silver and antimony |
Iron and antimony |
| Copper and zinc |
Iron and lead |
| Copper and tin |
Tin and lead |
| Copper and palladium |
Tin and palladium |
| Copper and bismuth |
Tin and antimony |
| Lead and antimony |
Nickel and arsenic |
| Platinum and molybdenum |
Zinc and antimony. |
| Palladium and bismuth. |
|
It would be hardly possible to infer the melting point of an alloy from that of each of
its constituent metals; but, in general, the fusibility is increased by mutual affinity
in their state of combination. Of this, a remarkable instance is afforded in the
fusible metal consisting of 8 parts of bismuth, 5 of lead, and 3 of tin, which melts at the[30]
heat of boiling water or 212° Fahr., though the melting point deduced from the mean of
its components should be 514°. This alloy may be rendered still more fusible by
adding a very little mercury to it, when it forms an excellent material for certain
anatomical injections, and for filling the hollows of carious teeth. Nor do the colours of
alloys depend, in any considerable degree, upon those of the separate metals; thus, the
colour of copper, instead of being rendered paler by a large addition of zinc, is thereby
converted into the rich-looking pinchbeck metal.
By means of alloys, we multiply, as it were, the numbers of useful metals, and sometimes
give usefulness to such as are separately of little value. Since these compounds
can be formed only by fusion, and since many metals are apt to oxidise readily at their
melting temperature, proper precautions must be taken in making alloys to prevent this
occurrence, which is incompatible with their formation. Thus, in combining tin and lead,
rosin or grease is usually put on the surface of the melting metals, the carbon produced
by the decomposition of which protects them, in most cases, sufficiently from oxidisement.
When we wish to combine tin with iron, as in the tinning of cast-iron tea kettles, we
rub sal ammoniac upon the surfaces of the hot metals in contact with each other, and thus
exclude the atmospheric oxygen by means of its fumes. When there is a notable
difference in the specific gravities of the metals which we wish to combine, we often find
great difficulties in obtaining homogeneous alloys; for each metal may tend to assume
the level due to its density, as is remarkably exemplified in alloys of gold and silver
made without adequate stirring of the melting metals. If the mass be large, and slow
of cooling after it is cast in an upright cylindrical form, the metals sometimes separate,
to a certain degree, in the order of their densities. Thus, in casting large bells and
cannons with copper alloys, the bottom of the casting is apt to contain too much copper and
the top too much tin, unless very dexterous manipulation in mixing the fused materials
have been employed immediately before the instant of pouring out the melted mass.
When such inequalities are observed, the objects are broken and re-melted, after which
they form a much more homogeneous alloy. This artifice of a double melting is often
had recourse to, and especially in casting the alloys for the specula of telescopes.
When we wish to alloy three or more metals, we often experience difficulties, either
because one of the metals is more oxidable, or denser, or more fusible, than the others,
or because there is no direct affinity between two of the metals. In the latter predicament,
we shall succeed better by combining the three metals, first in pairs, for example,
and then melting the two pairs together. Thus, it is difficult to unite iron with bronze
directly; but if, instead of iron, we use tin plate, we shall immediately succeed, and the
bronze, in this manner, acquires valuable qualities from the iron. Thus, also, to render
brass better adapted for certain purposes, a small quantity of lead ought to be added to
it, but this cannot be done directly with advantage: it is better to melt the lead first
along with the zinc, and then to add this alloy to the melting copper, or the copper to
that alloy, and fuse them together.
We have said that the difference of fusibility was often an obstacle to metallic combination;
but this circumstance may also be turned to advantage in decomposing certain
alloys by the process called eliquation. By this means silver may be separated from
copper, if a considerable quantity of lead be first alloyed with the said copper; this
alloy is next exposed to a heat just sufficient to melt the lead, which then sweats out, so
to speak, from the pores of the copper, and carries along with it the greater part of the
silver, for which it has a strong affinity. The lead and the silver are afterwards
separated from each other, in virtue of their very different oxidability, by the action of
heat and air.
One of the alloys most useful to the arts is brass; it is more ductile and less easily
oxidised than even its copper constituent, notwithstanding the opposite nature of the
zinc. This alloy may exist in many different proportions, under which it has different
names, as tombac, similor, pinchbeck, &c. Copper and tin form, also, a compound of
remarkable utility, known under the names of hard brass, for the bushes, steps, and
bearings of the axles, arbours, and spindles in machinery; and of bronze, bell-metal, &c.
Gold and silver, in their pure state, are too soft and flexible to form either vessels
or coins of sufficient strength and durability; but when alloyed with a little copper, they
acquire the requisite hardness and stiffness for these and other purposes.
When we have occasion to unite several pieces of the same or of different metals, we
employ the process called soldering, which consists in fixing together the surfaces by
means of an interposed alloy, which must be necessarily more fusible than the metal or
metals to be joined. That alloy must also consist of metals which possess a strong
affinity for the substances to be soldered together. Hence each metal would seem to
require a particular kind of solder, which is, to a certain extent, true. Thus, the solder
for gold trinkets and plate is an alloy of gold and silver, or gold and copper; that of
silver trinkets, is an alloy of silver and copper; that of copper is either fine tin, for
pieces that must not be exposed to the fire, or a brassy alloy called hard solder, of which[31]
the zinc forms a considerable proportion. The solder of lead and tinplate is an alloy of
lead and tin, and that of tin is the same alloy with a little bismuth. Tinning, gilding,
and silvering may also be reckoned a species of alloys, since the tin, gold, and silver are
superficially united in these cases to other metals.
Metallic alloys possess usually more tenacity than could be inferred from their
constituents; thus, an alloy of twelve parts of lead with one of zinc has a tenacity
double that of zinc. Metallic alloys are much more easily oxidised than the separate
metals, a phenomenon which may be ascribed to the increase of affinity for oxygen which
results from the tendency of the one of the oxides to combine with the other. An
alloy of tin and lead heated to redness takes fire, and continues to burn for some time
like a piece of bad turf.
Every alloy is, in reference to the arts and manufactures, a new metal, on account of its
chemical and physical properties. A vast field here remains to be explored. Not above
sixty alloys have been studied by the chemists out of many hundred which may be
made; and of these very few have yet been practically employed. Very slight modifications
often constitute very valuable improvements upon metallic bodies. Thus, the
brass most esteemed by turners at the lathe contains from two to three per cent. of lead;
but such brass does not work well under the hammer; and, reciprocally, the brass which
is best under the hammer is too tough for turning.
That metallic alloys tend to be formed in definite proportions of their constituents is
clear from the circumstance that the native gold of the auriferous sands is an alloy with
silver, in the ratios of 1 atom of silver united to 4, 5, 6, 12 atoms of gold, but never with
a fractional part of an atom. Also, in making an amalgam of 1 part of silver with 12
or 15 of mercury, and afterwards squeezing the mixture through chamois leather, the
amalgam separates into 2 parts: one, containing a small proportion of silver and much
mercury, passes through the skin; and the other, formed of 1 of silver and 8 of
mercury, is a compound in definite proportions, which crystallises readily, and remains
in the knot of the bag. An analogous separation takes place in the tinning of mirrors;
for on loading them with the weights, a liquid amalgam of tin is squeezed out, while
another amalgam remains in a solid form composed of tin and mercury in uniform atomic
proportions. But, as alloys are generally soluble, so to speak, in each other, this definiteness
of combination is masked and disappears in most cases.
M. Chaudet has made some experiments on the means of detecting the metals of
alloys by the cupelling furnace, and they promise useful applications. The testing
depends upon the appearances exhibited by the metals and their alloys when heated on a
cupel. Pure tin, when heated this way, fuses, becomes of a greyish black colour, fumes
a little, exhibits incandescent points on its surface, and leaves an oxide, which, when
withdrawn from the fire, is at first lemon-yellow, but when cold, white. Antimony
melts, preserves its brilliancy, fumes, and leaves the vessel coloured lemon-yellow when
hot, but colourless when cold, except a few spots of a rose tint. Zinc burns brilliantly,
forming a cone of oxide; and the oxide, much increased in volume, is, when hot, greenish,
but when cold, perfectly white. Bismuth fumes, becomes covered with a coat of melted
oxide, part of which sublimes, and the rest enters the pores of the cupel; when cold, the
cupel is of a fine yellow colour, with spots of a greenish hue. Lead resembles bismuth
very much; the cold cupel is of a lemon-yellow colour. Copper melts, and
becomes covered with a coat of black oxide; sometimes spots of a rose tint remain on
the cupel.
Alloys.—Tin 75, antimony 25, melt, become covered with a coat of black oxide,
have very few incandescent points; when cold, the oxide is nearly black, in consequence
of the action of the antimony: a 1⁄400 part of antimony may be ascertained in
this way in the alloy. An alloy of antimony, containing tin, leaves oxide of tin in the
cupel: a 1⁄100 part of tin may be detected in this way. An alloy of tin and zinc gives an
oxide which, whilst hot, is of a green tint, and resembles philosophic wool in appearance.
An alloy containing 99 tin, 1 zinc, did not present the incandescent points of pure tin,
and gave an oxide of greenish tint when cold. Tin 95, bismuth 5 parts, gave an oxide
of a grey colour. Tin and lead give an oxide of a rusty brown colour. An alloy of lead
and tin, containing only 1 per cent. of the latter metal, when heated, does not expose a
clean surface, like lead, but is covered at times with oxide of tin. Tin 75, and copper
25, did not melt, gave a black oxide: if the heat be much elevated, the under part of
the oxide is white, and is oxide of tin; the upper is black, and comes from the copper.
The cupel becomes of a rose colour. If the tin be impure from iron, the oxide produced
by it is marked with spots of a rust colour.
The degree of affinity between metals may be in some measure estimated by the
greater or less facility with which, when of different degrees of fusibility or volatility,
they unite, or with which they can, after union, be separated by heat. The greater or
less tendency to separate into differently proportioned alloys, by long-continued fusion,
may also give some information upon this subject. Mr. Hatchett remarked, in his[32]
elaborate researches on metallic alloys, that gold made standard with the usual precautions,
by silver, copper, lead, antimony, &c., and then cast, after long fusion, into vertical
bars, was by no means an uniform compound; but that the top of the bar, corresponding
to the metal at the bottom of the crucible, contained the larger proportion of gold.
Hence, for a more thorough combination, two red-hot crucibles should be employed, and
the liquefied metals should be alternately poured from the one into the other. To
prevent unnecessary oxidisement from the air, the crucibles should contain, besides the
metal, a mixture of common salt and pounded charcoal. The metallic alloy should also be
occasionally stirred up with a rod of pottery ware.
The most direct evidence of a chemical change having been effected in alloys is,
when the compound melts at a lower temperature than the mean of its ingredients.
Iron, which is nearly infusible, acquires almost the fusibility of gold when alloyed with
this precious metal. The analogy is here strong with the increase of solubility which
salts acquire by mixture, as is exemplified in the difficulty of crystallising residuums of
saline solutions, or mother waters, as they are called.
In common cases the specific gravity affords a good criterion whereby to judge of the
proportion of two metals in an alloy. But a very fallacious rule has been given in some
respectable works for computing the specific gravity that should result from the alloying
of given quantities of two metals of known densities, supposing no chemical condensation
or expansion of volume to take place. Thus, it has been taught, that if gold and copper
be united in equal weights, the computed specific gravity is merely the arithmetical
mean between the numbers denoting the two specific gravities. Whereas, the specific
gravity of any alloy must be computed by dividing the sum of the two weights by the
sum of the two volumes, compared, for conveniency sake, to water reckoned unity. Or,
in another form, the rule may be stated thus:—Multiply the sum of the weights into
the products of the two specific-gravity numbers for a numerator; and multiply each
specific gravity-number into the weight of the other body, and add the two products
together for a denominator. The quotient obtained by dividing the said numerator by
the denominator, is the truly computed mean specific gravity of the alloy. On comparing
with that density, the density found by experiment, we shall see whether expansion
or condensation of volume has attended the metallic combination. Gold having a
specific gravity of 19·36, and copper of 8·87, when they are alloyed in equal weights, give,
by the fallacious rule of the arithmetical mean of the densities 19·36 + 8·872 = 14·11;
whereas the rightly computed mean density is only 12·16. It is evident that, on comparing
the first result with experiment, we should be led to infer that there had been a
prodigious condensation of volume, though expansion has actually taken place. Let
W, w be the two weights; P, p the two specific gravities, then M, the mean specific
gravity, is given by the formula—
(W + w)PpPw + pW
∴ 2Δ = - (P - p)2P + p =
twice
the error of the arithmetical mean; which is therefore always in excess.
ALMOND. (Amande, Fr.; Mandel, Germ.) There are two kinds of almond which
do not differ in chemical composition, only that the bitter, by some mysterious reaction
of its constituents, generates in the act of distillation a quantity of a volatile oil, which
contains hydrocyanic acid. Vogel obtained from bitter almonds 8·5 per cent. of husks.
After pounding the kernels, and heating them to coagulate the albumen, he procured,
by expression, 28 parts of an unctuous oil, which did not contain the smallest particle
of hydrocyanic acid. The whole of the oil could not be extracted in this way. The
expressed mass, treated with boiling water, afforded sugar and gum, and, in consequence
of the heat, some of that acid. The sugar constitutes 6·5 per cent. and the gum 3. The vegetable
albumen extracted, by means of caustic potash, amounted to 30 parts: the vegetable
fibre to only 5. The poisonous aromatic oil, according to Robiquet and Boutron-Charlard,
does not exist ready-formed in the bitter almond, but seems to be produced under
the influence of ebullition with water. These chemists have shown that bitter almonds
deprived of their unctuous oil by the press, when treated first by alcohol, and then by
water, afford to neither of these liquids any volatile oil. But alcohol dissolves out a
peculiar white crystalline body, without smell, of a sweetish taste at first, and afterwards
bitter, to which they gave the name of amygdaline. This substance does not seem
convertible into volatile oil.
Sweet almonds by the analysis of Boullay, consist of 54 parts of the bland almond
oil, 6 of uncrystallisable sugar, 3 of gum, 24 of vegetable albumen, 24 of woody fibre,
5 of husks, 3·5 of water, 0·5 of acetic acid, including loss. We thus see that sweet
almonds contain nearly twice as much oil as bitter almonds do.
ALMOND OIL. A bland fixed oil, obtained usually from bitter almonds by the
action of a hydraulic press, either in the cold, or aided by hot iron plates. See Oil.
[33]
ALOE. A series of trials has been made within a few years at Paris to ascertain the
comparative strength of cables made of hemp and of the aloe from Algiers; and they
are said to have all turned to the advantage of the aloe. Of cables of equal size, that
made of aloe raised a weight of 2,000 kilogrammes (2 tons nearly); that made of hemp,
a weight of only 400 kilogrammes. At the exposition of objects of national industry,
two years ago, in Brussels, I saw aloe cordage placarded, as being far preferable to hempen.
See Rope.
ALUDEL. A pear-shaped vessel open at either end, of which a series are joined
for distilling mercury in Spain. See Mercury.
ALUM. (Alun, Fr.; Alaum, Germ.) A saline body, consisting of the earth of clay,
called alumina by the chemists, combined with sulphuric acid and potash, or sulphuric
acid and ammonia, into a triple compound. It occurs in the crystallised form of octahedrons,
has an acerb subacid taste, and reddens the blue colour of litmus or red cabbage.
Alum works existed many centuries ago at Roccha, formerly called Edessa, in Syria,
whence the ancient name of Roch alum given to this salt. It was afterwards made at
Foya Nova, near Smyrna, and in the neighbourhood of Constantinople. The Genoese,
and other trading people of Italy, imported alum from these places into western Europe,
for the use of the dyers of red cloth. About the middle of the fifteenth century, alum began
to be manufactured at La Tolfa, Viterbo, and Volaterra, in Italy; after which time the
importation of oriental alum was prohibited by the pope, as detrimental to the interests
of his dominions. The manufacture of this salt was extended to Germany at the beginning
of the sixteenth century, and to England at a somewhat later period, by Sir
Thomas Chaloner, in the reign of Elizabeth. In its pure state, it does not seem to have
been known to the ancients; for Pliny, in speaking of something like plumose alum,
says, that it struck a black colour with pomegranate juice, which shows that the green
vitriol was not separated from it. The stypteria of Dioscorides, and the alumen of
Pliny, comprehended, apparently, a variety of saline substances, of which sulphate of
iron, as well as alumina, was probably a constituent part. Pliny, indeed, says, that a
substance called in Greek Ὑγρα, or watery, probably from its very soluble nature, which
was milk-white, was used for dyeing wool of bright colours. This may have been the
mountain butter of the German mineralogists, which is a native sulphate of alumina, of
a soft texture, waxy lustre, and unctuous to the touch.
The only alum manufactories now worked in Great Britain, are those of Whitby, in
England, and of Hurlett and Campsie, near Glasgow, in Scotland; and these derive the
acid and earthy constituents of the salt from a mineral called alum slate. This mineral
has a bluish or greenish-black colour, emits sulphurous fumes when heated, and acquires
thereby an aluminous taste. The alum manufactured in Great Britain contains potash
as its alkaline constituent; that made in France contains, commonly, ammonia, either
alone, or with variable quantities of potash. Alum may in general be examined by water
of ammonia, which separates from its watery solutions its earthy basis, in the form of a
light flocculent precipitate. If the solution be dilute, this precipitate will float long as
an opalescent cloud.
If we dissolve alum in 20 parts of water, and drop this solution slowly into water or
caustic ammonia till this be nearly, but not entirely, saturated, a bulky white precipitate
will fall down, which, when properly washed with water, is pure aluminous earth or
clay, and dried forms 10·82 per cent. of the weight of the alum. If this earth, while
still moist, be dissolved in dilute sulphuric acid, it will constitute, when as neutral
as possible, the sulphate of alumina, which requires only two parts of cold water for its
solution. If we now decompose this solution, by pouring into it water of ammonia,
there appears an insoluble white powder, which is subsulphate of alumina, or basic alum;
and contains three times as much earth as exists in the neutral sulphate. If, however,
we pour into the solution of the neutral sulphate of alumina a solution of sulphate of
potash, a white powder will fall if the solutions be concentrated, which is true alum; but
if the solutions be dilute, by evaporating their mixture, and cooling it, crystals of alum
will be obtained.
When newly precipitated alumina is boiled in a solution of alum, a portion of the
earth enters into combination with the salt, constituting an insoluble compound, which
falls in the form of a white powder. The same combination takes place, if we decompose
a boiling hot solution of alum with a solution of potash, till the mixture appears
nearly neutral by litmus paper. This insoluble or basic alum exists native in the
alum-stone of Tolfa, near Civita Vecchia, and it consists in 100 parts of 19·72 parts of
sulphate of potash, 61·99 basic sulphate of alumina, and 18·29 water. When this
mineral is treated with a due quantity of sulphuric acid, it dissolves, and is converted
into the crystallisable alum of commerce.
These experimental facts develope the principles of the manufacture of alum, which
is prosecuted under various modifications, for its important uses in the arts. Alum
seldom occurs ready-formed in nature; occasionally, as an efflorescence on stones, and in[34]
certain mineral waters in the East Indies. The alum of European commerce is fabricated
artificially, either from the alum schists or stones, or from clay. The mode of manufacture
differs according to the nature of these earthy compounds. Some of them, such as the
alum stone, contain all the elements of the salt, but mixed with other matters, from
which it must be freed. The schists contain only the elements of two of the constituents,
namely, clay and sulphur, which are convertible into sulphate of alumina, and this may
be then made into alum by adding the alkaline ingredient. To this class belong the
alum slates, and other analogous schists, containing brown coal.
1. Manufacture of Alum from the Alum Stone.—The alum-stone is a rare mineral, being
found in moderate quantity at Tolfa, and in larger in Hungary, at Bereghszasz, and Muszag,
where it forms entire beds in a hard substance, partly characterised by numerous
cavities, containing drusy crystallisations of alum-stone or basic alum. The larger
lumps contain more or fewer flints disseminated through them, and are, according to
their quality, either picked out to make alum, or are thrown away. The sorted pieces
are roasted or calcined, by which operation apparently the hydrate of alumina, associated
with the sulphate of alumina, loses its water, and, as burnt clay, loses its affinity for
alum. It becomes, therefore, free; and during the subsequent exposure to the weather
the stone gets disintegrated, and the alum becomes soluble in water.
The calcination is performed in common lime-kilns in the ordinary way. In the
regulation of the fire it is requisite, here, as with gypsum, to prevent any fusion or
running together of the stones, or even any disengagement of sulphuric or sulphurous
acids, which would cause a corresponding defalcation in the product of alum. For this
reason the contact of the ignited stones with carbonaceous matter ought to be avoided.
The calcined alum-stones, piled in heaps from 2 to 3 feet high, are to be exposed to
the weather, and meanwhile they must be continually kept moist by sprinkling them
with water. As the water combines with the alum the stones crumble down, and fall,
eventually, into a pasty mass, which must be lixiviated with warm water, and allowed to
settle in a large cistern. The clear supernatant liquor, being drawn off, must be
evaporated, and then crystallised. A second crystallisation finishes the process, and
furnishes a marketable alum. Thus the Roman alum is made, which is covered with a
fine red film of peroxide of iron.
2. Alum Manufacture from Alum Schist.—The greater portion of the alum found in
British commerce is made from alum-slate and analogous minerals. This slate contains
more or less iron pyrites, mixed with coaly or bituminous matter, which is occasionally
so abundant as to render them somewhat combustible. In the strata of brown coal and
bituminous wood, where the upper layers lie immediately under clay beds, they consist
of the coaly substance rendered impure with clay and pyrites. This triple mixture
constitutes the essence of all good alum schists, and it operates spontaneously towards
the production of sulphate of alumina. The coal serves to make the texture open, and
to allow the air and moisture to penetrate freely, and to change the sulphur and iron present
into acid and oxide. When these schists are exposed to a high temperature in
contact with air, the pyrites loses one half of its sulphur, in the form of sublimed
sulphur or sulphurous acid, and becomes a black sulphuret of iron, which speedily
attracts oxygen, and changes to sulphate of iron, or green vitriol. The brown coal schists
contain, commonly, some green vitriol crystals spontaneously formed in them. The
sulphate of iron transfers its acid to the clay, progressively, as the iron, by the action
of the air with a little elevation of temperature, becomes peroxidised; whereby sulphate
of alumina is produced. A portion of the green vitriol remains, however, undecomposed,
and so much the more as there may happen to be less of other salifiable bases present in
the clay slate. Should a little magnesia or lime be present, the vitriol gets more
completely decomposed, and a portion of Epsom salt and gypsum is produced.
The manufacture of alum from alum schists may be distributed under the six following
heads:—1. The preparation of the alum slate. 2. The lixiviation of the slate. 3. The
evaporation of the lixivium. 4. The addition of the saline ingredients, or the precipitation
of the alum. 5. The washing of the aluminous salts; and 6. The crystallisation.
1. Preparation of the Alum Slate.—Some alum slates are of such a nature that,
being piled in heaps in the open air, and moistened from time to time, they get spontaneously
hot, and by degrees fall into a pulverulent mass, ready to be lixiviated. The
greater part, however, require the process of ustulation, from which they derive many
advantages. The cohesion of the dense slates is thereby so much impaired that their decomposition
becomes more rapid; the decomposition of the pyrites is quickened by the
expulsion of a portion of the sulphur; and the ready-formed green vitriol is partly
decomposed by the heat, with a transference of its sulphuric acid to the clay, and
the production of sulphate of alumina.
Such alum-slates as contain too little bitumen or coal for the roasting process must be
interstratified with layers of small coal or brushwood over an extensive surface. At[35]
Whitby the alum rock, broken into small pieces, is laid upon a horizontal bed of fuel,
composed of brushwood; but at Hurlett small coal is chiefly used for the lower bed.
When about four feet of the rock is piled on, fire is set to the bottom in various parts; and
whenever the mass is fairly kindled, more rock is placed over the top. At Whitby this
piling process is continued till the calcining heap is raised to the height of 90 or 100 feet.
The horizontal area is also augmented at the same time till it forms a great bed nearly
200 feet square, having therefore about 100,000 yards of solid measurement. The
rapidity of the combustion is tempered by plastering up the crevices with small schist
moistened. When such an immense mass is inflamed, the heat is sure to rise too high,
and an immense waste of sulphur and sulphuric acid must ensue. This evil has been
noticed at the Whitby works. At Hurlett the height to which the heap is piled is only
a few feet, while the horizontal area is expanded; which is a much more judicious
arrangement. At Whitby 130 tons of calcined schist produce on an average 1 ton of
alum. In this humid climate it would be advisable to pile up on the top of the
horizontal strata of brushwood or coal, and schist, a pyramidal mass of schist, which
having its surface plastered smooth, with only a few air-holes, will protect the mass from
the rains, and at the same time prevent the combustion from becoming too vehement.
Should heavy rains supervene, a gutter must be scooped out round the pile for receiving
the aluminous lixivium, and conducting it into the reservoir.
It may be observed, that certain alum schists contain abundance of combustible
matter, to keep up a suitable calcining heat after the fire is once kindled; and therefore
nothing is needed but the first layer of brushwood, which, in this case, may be laid over
the first bed of the bituminous schist.
A continual, but very slow, heat, with a smothered fire, is most beneficial for the
ustulation of alum slate. When the fire is too brisk, the sulphuret of iron may run
with the earthy matters into a species of slag, or the sulphur will be dissipated in vapour,
by both of which accidents the product of alum will be impaired. Those bituminous
alum schists which have been used as fuel under steam boilers have suffered such a
violent combustion that their ashes yield almost no alum. Even the best regulated
calcining piles are apt to burn too briskly in high winds, and should have their draught-holes
carefully stopped under such circumstances. It may be laid down as a general
rule, that the slower the combustion the richer the roasted ore will be in sulphate of
alumina. When the calcination is complete, the heap diminishes to one half its original
bulk; it is covered with a light reddish ash, and is open and porous in the interior, so
that the air can circulate freely throughout the mass. To favour this access of air, the
masses should not be too lofty; and in dry weather a little water should be occasionally
sprinkled on them, which, by dissolving away some of the saline matter, will make the
interior more open to the atmosphere.
When the calcined mineral becomes thoroughly cold, we may proceed to the lixiviation.
But as, from the first construction of the piles or beds till their complete calcination,
many weeks, or even months, may elapse, care ought to be taken to provide a sufficient
number or extent of them, so as to have an adequate supply of material for carrying on
the lixiviating and crystallising processes during the course of the year, or at least during
the severity of the winter season, when the calcination may be suspended, and the
lixiviation becomes unsatisfactory. The beds are known to be sufficiently decomposed
by the efflorescence of the salt which appears upon the stones, from the strong aluminous
taste of the ashes, and from the appropriate chemical test of lixiviating an aliquot
average portion of the mass, and seeing how much alum it will yield to solution of
muriate or sulphate of potash.
2. The Lixiviation.—The lixiviation is best performed in stone-built cisterns; those of
wood, however strong at first, are soon decomposed, and need repairs. They ought to be
erected in the neighbourhood of the calcining heaps, to save the labour of transport, and so
arranged that the solutions from the higher cisterns may spontaneously flow into the lower.
In this point of view, a sloping terrace is the best situation for an alum work. In the
lowest part of this terrace, and in the neighbourhood of the boiling-house, there ought
to be two or more large deep tanks, for holding the crude lixivium, and they should be
protected from the rain by a proper shed. Upon a somewhat higher level the cisterns
of the clear lixivium may be placed. Into the highest range of cisterns the calcined
mineral is to be put, taking care to lay the largest lumps at the bottom, and to cover
them with lighter ashes. A sufficient quantity of water is now to be run over it, and
allowed to rest for some time. The lixivium may then be drawn off, by a stopcock
connected with a pipe at the bottom of the cistern, and run into another cistern at a
somewhat lower level. Fresh water must now be poured on the partly exhausted
schist, and allowed to remain for a sufficient time. This lixivium, being weak, should
be run off into a separate tank. In some cases a third addition of fresh water may be
requisite, and the weak lixivium which is drawn off may be reserved for a fresh portion of
calcined mineral. In order to save evaporation, it is always requisite to strengthen weak[36]
leys by employing them instead of water for fresh portions of calcined schist. Upon the
ingenious disposition and form of these lixiviating cisterns much of the economy and
success of an alum work depend. The hydrometer should be always used to determine
the degree of concentration which the solutions acquire.
The lixiviated stone being thus exhausted of its soluble ingredients, is to be removed
from the cisterns, and piled up in a heap in any convenient place, where it may be left
either spontaneously to decompose, or, after drying, may be subjected to another calcination.
The density of the solution may be brought, upon an average, up to the sp. gr.
of from 1·09 to 1·15. The latter density may always be obtained by pumping up the
weaker solutions upon fresh calcined mine. This strong liquor is then drawn off, when
the sulphate of lime, the oxide of iron, and the earths are deposited. It is of advantage
to leave the liquor exposed for some time, whereby the green vitriol may pass into a
persulphate of iron with the deposition of some oxide, while the liberated acid may
combine with some of the clay present, so as to increase the quantity of sulphate of
alumina. The manufacture of alum is the more imperfect, as the quantity of sulphate
of iron left undecomposed is greater, and therefore every expedient ought to be tried to
convert the sulphate of iron into sulphate of alumina.
3. The evaporation of the Schist Lixivium.—As the aluminous liquors, however
well settled at first, are apt, on the great scale, to deposit earthy matters in the
course of their concentration by heat, they are best evaporated by a surface fire,
such as that employed at Hurlett and Campsie. A water-tight stone cistern must
be built, having a layer of well rammed clay behind the flags or tiles which line
its bottom and sides. This cistern may be 4 or 6 feet wide, 2 or 3 feet deep, and
30 or 40 feet long, and it is covered in by an arch of stone or brickwork. At one
extremity of this tunnel, or covered canal, a fire-grate is set, and at the other a lofty
chimney is erected. The cistern being filled to the brim with the alum ley, a strong
fire is kindled in the reverberatory grate, and the flame and hot air are forced to sweep
along the surface of the liquor, so as to keep it in constant ebullition, and to carry off
the aqueous parts in vapour. The soot which is condensed in the process falls to the
bottom, and leaves the body of the liquor clear. As the concentration goes on, more of
the rough lixivium is run in from the settling cistern, placed on a somewhat higher level,
till the whole gets charged with a clear liquor of a specific gravity sufficiently high for
transferring into the proper lead boilers.
At Whitby, the lead pans are 10 feet long, 4 feet 9 inches wide, 2 feet 2 inches
deep at the one end, and 2 feet 8 inches deep at the other. This increase of depth and
corresponding slope, facilitates the decantation of the concentrated lixivium by means of
a syphon, applied at the lower end. The bottom of the pan is supported by a series of
parallel iron bars, placed very near each other. In these lead pans the liquor is concentrated,
at a brisk boiling heat, by means of the flame of a flue beneath them. Every
morning the pans are emptied into a settling cistern of stone or lead. The specific
gravity of the liquor should be about 1·4 or 1·5, being a saturated solution of the saline
matters present. The proper degree of density must vary, however, with different kinds
of lixivia, and according to the different views of the manufacturer. For a liquor which
consists of two parts of sulphate of alumina, and one part of sulphate of iron, a specific
gravity of 1·25 may be sufficient; but for a solution which contains two parts of sulphate
of iron to one of sulphate of alumina, so that the green vitriol must be withdrawn first of
all by crystallisation, a specific gravity of 1·4 may be requisite.
The construction of an evaporating furnace well adapted to the concentration of
aluminous and other crude lixivia, is described under Soda. The liquor basin may
be made of tiles or flags puddled in clay, and secured at the seams with a good
hydraulic cement. A mortar made of quicklime mixed with the exhausted schist in
powder, and iron turnings, is said to answer well for this purpose. Sometimes over the
reverberatory furnace a flat pan is laid, instead of the arched top, into which the crude liquor
is put for neutralisation and partial concentration. In Germany, such a pan is made of
copper, because iron would waste too fast, and lead would be apt to melt. From this
preparation basin the under evaporating trough is gradually supplied with hot liquor. At
one side of this lower trough, there is sometimes a door, through which the sediment may
be raked out as it accumulates upon the bottom. Such a contrivance is convenient for
this mode of evaporation, and it permits, also, any repairs to be readily made; but, indeed,
an apparatus of this kind, well mounted at first, will serve for many years.
In the course of the final concentration of the liquors, it is customary to add some of
the mother waters of a former process, the quantity of which must be regulated by a
proper analysis and knowledge of their contents. If these mother waters contain much
free sulphuric acid, from the peroxidation of their sulphate of iron, they may prove useful
in dissolving a portion of the alumina of the sediment which is always present in greater
or less quantity.
[37]
4. The precipitation of the Alum by adding Alkaline Salts.—As a general rule,
it is most advantageous to separate, first of all, from the concentrated clear liquors,
the alum in the state of powder or small crystals, by addition of the proper alkaline
matter, and to leave the mingled foreign salts, such as the sulphate of iron or magnesia,
in solution, instead of trying to abstract these salts by a previous crystallisation.
In this way we not only simplify and accelerate the manufacture of alum, and leave the
mother waters to be worked up at any convenient season, but we also avoid the risk of
withdrawing any of the sulphate of alumina with the sulphate of iron or magnesia.
On this account, the concentration of the liquor ought not to be pushed so far as that,
when it gets cold, it should throw out crystals, but merely to the verge of this point.
This density may be determined by suitable experiments.
The clear liquor should now be run off into the precipitation cistern, and have the
proper quantity of sulphate or muriate of potash, or impure sulphate or carbonate of
ammonia added to it. The sulphate of potash, which is the best precipitant, forms 18·34
parts out of 100 of crystallised alum; and therefore that quantity of it, or its equivalent
in muriate of potash, or other potash or ammoniacal salts, must be introduced into the
aluminous liquor. Since sulphate of potash takes 10 parts of cold water to dissolve it,
but is much more soluble in boiling water, and since the precipitation of alum is more
abundant the more concentrated the mingled solutions are, it would be prudent to add
the sulphate solution as hot as may be convenient; but, as muriate of potash is fully
three times more soluble in cold water, it is to be preferred as a precipitant, when it can
be procured at a cheap rate. It has, also, the advantage of decomposing the sulphate of
iron present into a muriate, a salt very difficult of crystallisation, and, therefore, less
apt to contaminate the crystals of alum. The quantity of alkaline salts requisite to
precipitate the alum, in a granular powder, from the lixivium, depends on their richness
in potash or ammonia, on the one hand, and on the richness of the liquors in
sulphate of alumina on the other; and it must be ascertained, for each large quantity of
product, by a preliminary experiment in a precipitation glass. Here, an aliquot measure
of the aluminous liquor being taken, the liquid precipitant must be added in successive
portions, as long as it causes any cloud, when the quantity added will be indicated by
the graduation of the vessel. A very exact approximation is not practicable upon the
great scale; but, as the mother waters are afterwards mixed together in one cistern, any
excess of the precipitant, at one time, is corrected by excess of aluminous sulphate
at another, and the resulting alum meal is collected at the bottom. When the precipitated
saline powder is thoroughly settled and cooled, the supernatant mother water
must be drawn off by a pump, or rather a syphon or stopcock, into a lower cistern.
The more completely this drainage is effected, the more easily and completely will the
alum be purified.
This mother liquor has, generally, a specific gravity of 1·4 at a medium temperature
of the atmosphere, and consists of a saturated solution of sulphate or muriate of black
and red oxide of iron, with sulphate of magnesia, in certain localities, and muriate of
soda, when the soaper’s salt has been used as a precipitant, as also a saturated solution of
sulphate of alumina. By adding some of it, from time to time, to the fresh lixivia, a
portion of that sulphate is converted into alum; but, eventually, the mother water must
be evaporated, so as to obtain from it a crop of ferruginous crystals; after which it
becomes capable, once more, of giving up its alum to the alkaline precipitants.
When the aluminous lixivia contain a great deal of sulphate of iron, it may be good
policy to withdraw a portion of it by crystallisation before precipitating the alum. With
this view, the liquors must be evaporated to the density of 1·4, and then run off into
crystallising stone cisterns. After the green vitriol has concreted, the liquor should be
pumped back into the evaporating pan, and again brought to the density of 1·4. On
adding to it, now, the alkaline precipitants, the alum will fall down from this concentrated
solution, in a very minute crystalline powder, very easy to wash and purify. But this
method requires more vessels and manipulation than the preceding, and should only be
had recourse to from necessity; since it compels us to carry on the manufacture of both
the valuable alum and the lower priced salts at the same time; moreover, the copperas
extracted at first from the schist liquors carries with it, as we have said, a portion of the
sulphate of alumina, and acquires thereby a dull aspect; whereas the copperas obtained
after the separation of the alum is of a brilliant appearance.
5. The washing, or edulcoration, of the Alum Powder.—This crystalline pulverulent
matter has a brownish colour, from the admixture of the ferruginous liquors; but
it may be freed from it by washing with very cold water, which dissolves not more
than one sixteenth of its weight of alum. After stirring the powder and the water
well together, the former must be allowed to settle, and then the washing must be
drawn off. A second washing will render the alum nearly pure. The less water
is employed, and the more effectually it is drained off, the more complete is the
process. The second water may be used in the first washing of another portion of[38]
alum powder, in the place of pure water. These washings may be added to the schist
lixivia.
6. The crystallisation.—The washed alum is put into a lead pan, with just enough water
to dissolve it at a boiling heat; fire is applied, and the solution is promoted by stirring.
Whenever it is dissolved in a saturated state, it is run off into the crystallising vessels, which
are called roching casks. These casks are about five feet high, three feet wide in the middle,
somewhat narrower at the ends; they are made of very strong staves, nicely fitted to
each other, and held together by strong iron hoops, which are driven on pro tempore, so
that they may be easily knocked off again, in order to take the staves asunder. The
concentrated solution, during its slow cooling in these close vessels, forms large regular
crystals, which hang down from the top, and project from the sides, while a thick layer or
cake lines the whole interior of the cask. At the end of eight or ten days, more or less,
according to the weather, the hoops and staves are removed, when a cask of apparently
solid alum is disclosed to view. The workman now pierces this mass with a pickaxe at
the side near the bottom, and allows the mother water of the interior to run off on the
sloping stone floor into a proper cistern, whence it is taken and added to another quantity
of washed powder to be crystallised with it. The alum is next broken into lumps,
exposed in a proper place to dry, and is then put into the finished bing for the market.
There is sometimes a little insoluble basic alum (subsulphate) left at the bottom of the
cask. This being mixed with the former mother liquors, gets sulphuric acid from them;
or, being mixed with a little sulphuric acid, it is equally converted into alum.
When, instead of potash or its salts, the ammoniacal salts are used, or putrid urine,
with the aluminous lixivia, ammoniacal alum is produced, which is perfectly similar to
the potash alum in its appearance and properties. At a gentle heat both lose their
water of crystallisation, amounting to 451⁄2 per cent. for the potash alum, and 48 for the
ammoniacal. The quantity of acid is the same in both, as, also, very nearly the
quantity of alumina, as the following analyses will show:
| Potash alum. |
Ammonia alum. |
| Sulphate of potash |
18·34 |
Sulphate of ammonia |
12·88 |
| Sulphate of alumina |
36·20 |
Sulphate of alumina |
38·64 |
| Water |
45·46 |
Water |
48·48 |
| 100·00 |
100·00 |
| Or otherwise, Potash alum. |
Ammonia alum. |
| 1 atom sulphate of potash |
1089·07 |
1 atom sulphate of ammonia |
716·7 |
| 1 atom sulphate of alumina |
2149·80 |
1 atom sulphate of alumina |
2149·8 |
| 24 water |
2669·52 |
24 water |
2699·5 |
| 5938·39 |
5566·0 |
| Or, Potash alum. |
Ammonia alum. |
| Alumina |
10·82 |
Alumina |
11·90 |
| Potash |
9·94 |
Ammonia |
3·89 |
| Sulphuric Acid |
33·77 |
Sulphuric acid |
36·10 |
| Water |
45·47 |
Water |
48·11 |
| 100·00 |
100·00 |
When heated pretty strongly, the ammoniacal alum loses its sulphuric acid and
ammonia, and only the earth remains. This is a very convenient process for procuring
pure alumina. Ammoniacal alum is easily distinguished from the other by the smell of
ammonia which it exhales when triturated with quicklime. The Roman alum, made
from alum stone, possesses most of the properties of the schist-made alums, but it has a
few peculiar characters: it crystallises always in opaque cubes, whereas the common
alum crystallises in transparent octahedrons. It is probable that Roman alum is a
sulphate of alumina and potash, with a slight excess of the earthy ingredient. It is
permanent when dissolved in cold water; for after a slow evaporation it is recovered in
a cubical form. But when it is dissolved in water heated to 110° Fahr. and upwards, or
when its solution is heated above this pitch, subsulphate of alumina falls, and on
evaporation octahedral crystals of common alum are obtained. The exact composition
of the Roman alum has not been determined, as far as I know. It probably differs
from the other also in its water of crystallisation. The Roman alum contains, according to
MM. Thenard and Roard, only 1⁄2200 of sulphate of iron, while the common commercial[39]
alums contain 1⁄1000. It may be easily purified by solution, granulation, crystallisation, and
washing, as has been already explained.
Alum is made extensively in France from an artificial sulphate of alumina. For this
purpose clays are chosen as free as possible from carbonate of lime and oxide of iron.
They are calcined in a reverberatory furnace, in order to expel the water, to peroxidise
the iron, and to render the alumina more easily acted on by the acid. The expulsion of
the water renders the clay porous and capable of absorbing the sulphuric acid by
capillary attraction. The peroxidation of the iron renders it less soluble in the
sulphuric acid; and the silica of the clay, by reacting on the alumina, impairs its
aggregation, and makes it more readily attracted by the acid. The clay should, therefore,
be moderately calcined; but not so as to indurate it like pottery ware, for it would then
suffer a species of siliceous combination which would make it resist the action of acids.
The clay is usually calcined in a reverberatory furnace, the flame of which serves
thereafter to heat two evaporating pans and a basin for containing a mixture of the
calcined clay and sulphuric acid. As soon as the clay has become friable in the furnace
it is taken out, reduced to powder, and passed through a fine sieve. With 100 parts of
the pulverised clay, 45 parts of sulphuric acid, of sp. gr. 1·45, are well mixed, in a
stone basin, arched over with brickwork. The flame and hot air of a reverberatory
furnace are made to play along the mixture, in the same way as described for evaporating
the schist liquors. See Soda. The mixture, being stirred from time to time, is, at the
end of a few days, to be raked out, and to be set aside in a warm place, for the acid to
work on the clay, during six or eight weeks. At the end of this time it must be washed,
to extract the sulphate of alumina. With this view, it may be treated like the roasted
alum ores above described. If potash alum is to be formed, this sulphate of alumina is
evaporated to the specific gravity of 1·38; but if ammonia alum, to the specific gravity
of only 1·24; because the sulphate of ammonia, being soluble in twice its weight of
water, will cause a precipitation of pulverulent alum from a weaker solution of sulphate
of alumina than the less soluble sulphate of potash could do.
The alum stone, from which the Roman alum is made, contains potash. The
following analysis of alunite, by M. Cordier, places this fact in a clear light:—
| Sulphate of potash |
18·53 |
| Sulphate of alumina |
38·50 |
| Hydrate of alumina |
42·97 |
| 100·00 |
To transform this compound into alum, it is merely necessary to abstract the hydrate
of alumina. The ordinary alum stone, however, is rarely so pure as the above analysis
would seem to show; for it contains a mixture of other substances; and the above are in
different proportions.
Alum is very extensively employed in the arts, most particularly in dyeing, lake
making, dressing sheep-skins, pasting paper, in clarifying liquors, &c. Its purity for
the dyer may be tested by prussiate of potash, which will give solution of alum a blue
tint in a few minutes if it contain even a very minute portion of iron. A bit of nut-gall
is also a good test of iron.
AMADOU. The French name of the spongy combustible substance, called in German
zunderschwamm, prepared from a species of agaric, the boletus igniarius, a kind of
mushroom, which grows on the trunks of old oaks, ashes, beeches, &c. It must be
plucked in the months of August and September. It is prepared by removing the
outer bark with a knife, and separating carefully the spongy substance of a yellow
brown colour, which lies within it, from the ligneous matter below. This substance is
cut into thin slices, and beat with a mallet to soften it, till it can be easily pulled
asunder between the fingers. In this state the boletus is a valuable substance for stopping
oozing hemorrhages, and some other surgical purposes. To convert it into tinder
it must receive a finishing preparation, which consists in boiling it in a strong solution
of nitre; drying it, beating it anew, and putting it a second time into the solution.
Sometimes, indeed, to render it very inflammable, it is imbued with gunpowder, whence
the distinction of black and brown amadou.
All the puff balls of the lycopodium genus of plants, which have a fleshy or filamentous
structure, yield a tinder quite ready for soaking in gunpowder water. The
Hindoos employ a leguminous plant, which they call solu, for the same purpose. Its
thick spongy stem, being reduced to charcoal, takes fire like amadou.
AMALGAM. When mercury is alloyed with any metal, the compound is called an
amalgam of that metal; as, for example, an amalgam of tin, bismuth, &c.
AMALGAMATION. This is a process used extensively in extracting silver and
gold from certain of their ores, founded on the property which mercury has to dissolve these[40]
metals as disseminated in the minerals, and thus to separate them from the earthy
matters. See Mercury, Metallurgy, and Silver.
AMBER. (Succin, Fr.; Bernstein, Germ.) A mineral solid, of a yellow colour
of various shades, which burns quite away with flame, and consists of carbon, hydrogen,
and oxygen, in nearly the same proportions, and the same state of combination, as vegetable
resin. Its specific gravity varies, by my trials, from 1·080 to 1·085. It becomes
negatively and powerfully electrical by friction. When applied to a lighted candle it
takes fire, swells considerably, and exhales a white smoke of a pungent odour; but
does not run into drops. Copal, which resembles it in several respects, differs in being
softer, and in melting into drops at the flame; and mellite, or honey-stone, which is a
mineral of a similar colour, becomes white when laid on a red-hot coal.
The texture of amber is resino-vitreous, its fracture conchoidal, and lustre glassy. It
is perfectly homogeneous; sufficiently hard to scratch gypsum, and to take a fine polish.
It is, however, scratched by calcareous spar. When amber is distilled in a retort,
crystalline needles of succinic acid sublime into the dome, and oil of amber drops from
the beak into the receiver. Fossil resins, such as that of Highgate, found in the London
clay formation, do not afford succinic acid by heat; nor does copal. Amber is
occasionally found of a whitish and brownish colour.
The most interesting fact relative to this vegeto-mineral is its geological position,
which is very characteristic and well determined. It is found almost uniformly in separate
nodules, disseminated in the sand, clay, or fragments of lignite of the plastic clay,
and lignite formation, situated between the calcaire grossier (crag limestone) of the
tertiary strata above, and the white chalk below. The size of these nodules varies from
a nut to a man’s head; but this magnitude is very rare in true amber. It does not
occur either in continuous beds, like the chalk flints, nor in veins; but it lies at one
time in the earthy or friable strata, which accompany or include the lignites; at another,
entangled in the lignites themselves; and is associated with the minerals which constitute
this formation, principally the pyrites, the most abundant of all. The pieces of amber
found in the sands, and other formations evidently alluvial, those met with on the sea-coasts
of certain countries, and especially Pomerania, come undoubtedly from the above
geological formation; for the organic matters found still adhering to the amber leave
no doubt as to its primitive place. Amber does not, therefore, belong to any postdiluvian
or modern soil, since its native bed is covered by three or four series of strata, often
of considerable thickness, and well characterised; proceeding upwards from the plastic
clay which includes the amber: these are, the crag limestone, the bone gypsum, with
its marls, the marly limestone, the upper marl sandstone, which covers it, and, lastly,
the freshwater or lacustrine formation, often so thick, and composed of calcareous and
siliceous rocks.
The amber bed is not, however, always covered with all these strata; and it is even
rare to see a great mass of one of them above the ground which contains it; because,
were it buried under such strata, it would be difficult to meet with such circumstances
as would lay it spontaneously open to the day. But by comparing observations made
in different places, relatively to the patches of these formations, which cover the amber
deposits, we find that no other mineral formations have been ever seen among them
except those above detailed, and thus learn that its geological locality is completely determined.
The proper yellow amber, therefore, or the Borussic, from the country where it has been
most abundantly found, belongs to the plastic clay formation, intermediate, in England,
between the chalk and the London clay. It is sometimes interposed in thin plates between
the layers of the lignites, but more towards the bark of the fibrous lignites, which retain
the form of the wood, than towards the middle of the trunk of the tree; a position analogous
to that of the resinous matters in our existing ligneous vegetables. The fibrous
lignites which thus contain amber belong to the dicotyledinous woods. Hence that
substance seems to have been formed during the life of the vegetable upon which it is
now encrusted. It must be remembered that the grounds containing the amber are
often replete with the sulphates of iron, alumina, and lime, or at least with the pyritous
elements of these salts. Some specimens of amber have a surface figured with irregular
meshes, indicating a sort of shrinkage from consolidation, and consequently a matter
that was at one time fluid, viscid, or merely soft. From optical examination, Dr.
Brewster has concluded amber to be of vegetable origin.
The different bodies included in the amber, distinguishable from its transparence, demonstrate,
indeed, in the most convincing manner, its primitive state of liquidity or softness.
These bodies have long exercised the skill of naturalists. They are generally insects,
or remains of insects, and sometimes leaves, stalks, or other portions of vegetables.
Certain families of insects occur more abundantly than others. Thus the hymenoptera,
or insects with four naked membranaceous wings, as the bee and wasp, and the diptera,
or insects with two wings, as gnats, flies, gadflies, &c.; then come the spider tribe;[41]
some coleoptera (insects with crustaceous shells or elytra, which shut together, and form
a longitudinal suture down the back,) or beetles, principally those which live on trees;
such as the elaterides, or leapers, and the chrysomelida. The lepidoptera, or insects with
four membranaceous wings, and pterigostea covered with mail-like scales, are very rare
in amber. We perceive from this enumeration, which results from the labours of
Germar, Schweiger, &c., that the insects enveloped in this resinous matter are in
general such as sit on the trunks of trees, or live in the fissures of their bark. Hitherto,
it has not been found possible to refer them to any living species; but it has been observed
in general that they resemble more the insects of hot climates than those of the
temperate zones.
The districts where amber occurs in a condition fit for mining operations are not
numerous; but those in which it is met with in small scattered bits are very abundant.
Its principal exploitation is in Eastern Prussia, on the coasts of the Baltic sea, from
Memel to Dantzick, particularly in the neighbourhood of Konigsberg, along the shore
which runs north and south from Grossdirschheim to Pillau, and in several other places
near Dantzick.
It is collected upon this coast in several ways; 1. In the beds of small streams
which run near the villages, and in rounded fragments without bark, or in the sand-banks
of rivers, in pieces thrown back by the sea, and rounded by the waves. 2. If
the pieces thrown up by the waters are not numerous, the fishers, clothed in a leather
dress, wade into the sea up to the neck, seek to discover the amber by looking along its
surface, and seize it with bag nets, hung at the end of very long poles. They conclude
that a great deal of amber has been detached from the cliffs by the sea, when many
pieces of lignite (wood coal) are seen afloat. This mode of collecting amber is not free
from danger, and the fishers, therefore, advance in troops, to lend each other aid in case
of accident; but their success, even thus, is most precarious. 3. The third method
of searching for amber is a real mining operation: it consists in digging pits upon the
borders of the sandy downs, sometimes to a depth of more than 130 feet. 4. The last
mode is by exploring the precipitous sea cliffs in boats, and detaching masses of loose
soil from them with long poles terminating in iron hooks; a very hazardous employment.
They search the cliffs with great care at the level, where the amber nodules
commonly lie, and loosen the seams with their hooks; in which business the boats are
sometimes broken against the precipices, or sunk by an avalanche of rubbish.
Amber occurs in Sicily, disseminated in beds of clay and marl, which lie below the
crag limestone. It is accompanied with bitumen; and, though a scanty deposit, it is
mined for sale. The pieces are coated with a kind of whitish bark, present a variety
of colours, and include many insects. Amber is found in a great many places in the
sandy districts of Poland, at a very great distance from the sea, where it is mixed with
cones of the pine. In Saxony it is met with in the neighbourhood of Pretsch and
Wittemberg, in a bituminous clay mingled with lignite. At the embouchure of the
Jenissey, in Siberia, it occurs likewise along with lignite; as also in Greenland.
Fine amber is considerably valued for making ornamental objects, and the coarser
kinds for certain uses in chemistry, medicine, and the arts. The oriental nations prize
more highly than the people of Europe trinkets made of amber; and hence the chief
commerce of the Pomeranian article is with Turkey. The Prussian government is
said to draw an annual revenue of 17,000 dollars from amber. A good piece of a pound
weight fetches 50 dollars. A mass weighing 13 pounds was picked up not long since
in Prussia, for which 5000 dollars were offered, and which would bring, in the opinion
of the Armenian merchants, from 30,000 to 40,000 dollars at Constantinople. At one
time it was customary to bake the opaque pieces of amber in sand, at a gentle heat, for
several hours, in order to make it transparent, or to digest it in hot rapeseed oil, with
the same view; but how far these processes were advantageous does not appear.
When amber is to be worked into trinkets, it is first split on a leaden plate at a lathe
(see Gems, Cutting of), and then smoothed into shape on a Swedish whetstone. It is
polished on the lathe with chalk and water, or vegetable oil, and finished by friction
with flannel. In these processes the amber is apt to become highly electrical, very hot,
and even to fly into fragments. Hence, the artists work the pieces time about, so as to
keep each of them cool, and feebly excited. The men are often seized with nervous
tremors in their wrists and arms from the electricity. Pieces of amber may be neatly
joined by smearing their edges with linseed oil, and pressing them strongly together,
while they are held over a charcoal fire. Solid specimens of amber, reported to have
been altogether fused by a particular application of heat, are now shown in the royal
cabinet of Dresden.
A strong and durable varnish is made by dissolving amber in drying linseed oil. For
this purpose, however, the amber must be previously heated in an iron pot, over a clear
red fire, till it soften and be semi-liquefied. The oil, previously heated, is to be now
poured in, with much stirring, in the proportion of 10 ounces to the pound of amber;[42]
and after the incorporation is complete, and the liquid somewhat cooled, a pound of oil
of turpentine must be added. Some persons prescribe 2 ounces of melted shellac,
though by this means they are apt to deepen the colour, already rendered too dark by
the roasting.
The fine black varnish of the coachmakers is said to be prepared by melting 16
ounces of amber in an iron pot, adding to it half a pint of drying linseed oil, boiling
hot, of powdered resin and asphaltum 3 ounces each: when the materials are well
united, by stirring over the fire, they are to be removed, and, after cooling for some
time, a pint of warm oil of turpentine is to be introduced.
The oil of amber enters into the composition of the old perfume called eau de luce;
and is convertible, by the action of a small quantity of strong nitric acid, into a viscid
mass like shoemakers’ rosin, which has a strong odour of musk, and, under the name
of artificial musk, has been prescribed, in alcoholic solution, as a remedy against hooping
cough, and other spasmodic diseases.
Acid of amber (succinic acid) is a delicate reagent, in chemistry, for separating red
oxide of iron from compound metallic solutions.
AMBERGRIS. (Ambregric, Fr.; Ambra, Germ.).—A morbid secretion of the liver
of the spermaceti whale (physeter macrocephalus), found usually swimming upon the
sea. It occurs upon the coasts of Coromandel, Japan, the Moluccas, and Madagascar,
and has sometimes been extracted from the rectum of whales in the South Sea fishery.
It has a gray-white colour, often with a black streak, or is marbled, yellow and black;
has a strong but rather agreeable smell, a fatty taste, is lighter than water, melts at 60°
C. (140° F.), dissolves readily in absolute alcohol, in ether, and in both fat and volatile
oils. It contains 85% of the fragrant substance called ambreine. This is extracted
from ambergris by digestion with alcohol of 0·827, filtering the solution, and leaving it
to spontaneous evaporation. It is thus obtained in the form of delicate white tufts:
which are convertible into ambreic acid by the action of nitric acid. Ambergris is used
in perfumery.
AMIANTHUS. A mineral in silky filaments, called also Asbestus.
AMMONIA. A chemical compound, called also volatile alkali. This substance,
in its purest state, is a highly pungent gas, possessed of all the mechanical properties of
the air, but very condensable with water. It consists of 3 volumes of hydrogen and 1
of azote condensed into two volumes; and hence its density is 0·591, atmospheric air
being 1·000. By strong compression and refrigeration it may be liquefied into a fluid,
whose specific gravity is 0·76 compared to water 1·000.
Ammonia gas is composed by weight of 82·53 azote and 17·47 hydrogen in 100
parts. It is obtained by mixing muriate of ammonia, commonly called sal ammoniac,
with quicklime, in a retort or still, applying a moderate heat, and receiving the gas either
over mercury for chemical experiments, or in water to make liquid ammonia for the
purposes of medicine and the arts. Woulfe’s apparatus is commonly employed for this
condensation.
Ammonia is generated in a great many operations, and especially in the decomposition
of many organic substances, by fire or fermentation. Urine left to itself for a few days is
found to contain much carbonate of ammonia, and hence this substance was at one time
collected in great quantities for the manufacture of certain salts of ammonia, and is still
used for its alkaline properties in making alum, scouring wool, &c. When woollen rags,
horns, bones, and other animal substances are decomposed in close vessels by fire, they
evolve a large quantity of ammonia, which distils over in the form of a carbonate. The
main source of ammonia now in this country, for commercial purposes, is the coal gas
works. A large quantity of watery fluid is condensed in their tar pits, which contains,
chiefly ammonia combined with sulphuretted hydrogen and carbonic acid. When this
water is saturated with muriatic acid and evaporated it yields muriate of ammonia, or
sal ammoniac, somewhat impure, which is afterwards purified by sublimation. See
Carbonate of Ammonia and Sal Ammoniac.
The soot of chimnies where coal is burned contains both sulphate and carbonate of
ammonia, and was extensively employed, at one time, to manufacture these salts.
In making water of ammonia on the great scale, a cast iron still should be preferred,
and equal weights of quicklime and sal ammoniac should be brought to the consistence
of a pap, with water, before the heat is applied. In this case, a refrigeratory worm or
globe should be interposed between the adopter tube of the capital of the still and the
bottles of Woulfe’s apparatus. The muriate of lime, or chloride of calcium, which is left
in the still when the whole ammonia is expelled, is of no value. Water is capable of
condensing easily about one third of its weight of ammonia gas, or 460 times its bulk.
The following table of the quantity of ammonia in 100 parts by weight of its aqueous
combinations, at successive densities, is the result of very careful experiments made by me,
and recorded in the Philosophical Magazine for March, 1821.
[43]
Table of Water of Ammonia or Volatile Alkali, by Dr. Ure.
Water
of
0·900. |
Ammo-
nia
in
100. |
Water
in
100. |
Specific
gravity
by
experi-
ment. |
Mean
specific
gravity. |
Equivalent primes. |
| 100 |
26·500 |
73·500 |
0·9000 |
|
|
| 95 |
25·175 |
74·825 |
0·9045 |
0·90452 |
|
Wat. Am. |
| 90 |
23·850 |
76·150 |
0·9090 |
0·90909 |
24 |
+ |
76, |
6 |
to |
1 |
| 85 |
22·525 |
77·475 |
0·9133 |
0·91370 |
|
| 80 |
21·200 |
78·800 |
0·9177 |
0·91838 |
21·25 |
+ |
78·75, |
7 |
to |
1 |
| 75 |
19·875 |
80·125 |
0·9227 |
0·92308 |
|
| 70 |
18·550 |
81·450 |
0·9275 |
0·92780 |
19·1 |
+ |
80·9, |
8 |
to |
1 |
| 65 |
17·225 |
82·775 |
0·9320 |
0·93264 |
17·35 |
+ |
82·65, |
9 |
to |
1 |
| 60 |
15·900 |
84·100 |
0·9363 |
0·93750 |
15·9 |
+ |
84·1, |
10 |
to |
1 |
| 55 |
14·575 |
85·425 |
0·9410 |
0·94241 |
14·66 |
+ |
85·34, |
11 |
to |
1 |
| 50 |
13·250 |
86·750 |
0·9455 |
0·94737 |
13·60 |
+ |
86·40, |
12 |
to |
1 |
| 45 |
11·925 |
88·075 |
0·9510 |
0·95238 |
11·9 |
+ |
88·1, |
14 |
to |
1 |
| 40 |
10·600 |
89·400 |
0·9564 |
0·95744 |
11·2 |
+ |
88·8, |
15 |
to |
1 |
| 35 |
9·275 |
90·725 |
0·9614 |
0·96256 |
|
| 30 |
7·950 |
92·050 |
0·9662 |
0·96774 |
8·63 |
+ |
91·37, |
20 |
to |
1 |
| 25 |
6·625 |
93·375 |
0·9716 |
0·97297 |
7 |
+ |
93, |
25 |
to |
1 |
| 20 |
5·300 |
94·700 |
0·9768 |
0·97826 |
6 |
+ |
94, |
30 |
to |
1 |
| 15 |
3·975 |
96·025 |
0·9828 |
0·98360 |
4·5 |
+ |
95·5, |
40 |
to |
1 |
| 10 |
2·650 |
97·350 |
0·9887 |
0·98900 |
3 |
+ |
97, |
60 |
to |
1 |
| 5 |
1·325 |
98·675 |
0·9945 |
0·99447 |
|
AMMONIAC, gum-resin. This is the inspissated juice of an umbelliferous plant
(the dorema armeniacum) which grows in Persia. It comes to us either in small
white tears clustered together, or in brownish lumps, containing many impurities. It
possesses a peculiar smell, somewhat like that of assafœtida, and a bitterish taste. It
is employed in medicine. Its only use in the arts is for forming a cement to join
broken pieces of china and glass, which may be prepared as follows: Take isinglass
1 ounce, distilled water 6 ounces, boil together down to 3 ounces, and add 11⁄2 ounce of
strong spirit of wine;—boil this mixture for a minute or two; strain it; add, while
hot, first, half an ounce of a milky emulsion of gum ammoniac, and then five drams of
an alcoholic solution of resin mastic. This resembles a substance sold in the London
shops, under the name of diamond cement. The recipe was given me by a respectable
dispensing chemist.
AMORPHOUS. Without shape. Said of mineral and other substances which
occur in forms not easy to be defined.
ANALYSIS. The art of resolving a compound substance or machine into its constituent
parts. Every manufacturer should so study this art, in the proper treatises, and
schools of Chemistry or Mechanics, as to enable him properly to understand and
regulate his business.
ANCHOR. (Ancre, Fr.; Anker, Germ.) An iron hook of considerable weight
and strength, for enabling a ship to lay hold of the ground, and fix itself in a certain
situation by means of a rope called the cable. It is an instrument of the greatest importance
to the navigator, since upon its taking and keeping hold depends his safety upon[44]
many occasions, especially near a lee shore, where he might be otherwise stranded or
shipwrecked. Anchors are generally made of wrought iron, except among nations who
cannot work this metal well, and who therefore use copper. The mode in which an
anchor operates will be understood from inspection of fig. 6., where, from the direction
of the strain, it is obvious that the anchor cannot move without ploughing up the
ground in which its hook or fluke is sunk. When this, however, unluckily takes
place, from the nature of the ground, from the mode of insertion of the anchor, or from
the violence of the winds or currents, it is called dragging the anchor. When the hold is
good, the cable or the buried arm will sooner break than the ship will drive. Anchors
are of different sizes, and have different names, according to the purposes they serve;
thus there are, sheet, best bower, small bower, spare, stream, and kedge anchors. Ships
of the first class have seven anchors, and smaller vessels, such as brigs and schooners,
three.
The manufacture of anchors requires great knowledge
of the structure of iron, and skill in the art of working
it. I shall give, here, a brief notice of the improved system
introduced by Mr. Perring, clerk of the cheque at Plymouth,
in which the proportions of the parts are admirably
adapted to the strains they are likely to suffer. In fig. 7.
A is the shank; B, the arm or fluke; C, the palm; D, the
blade; E, the square; F, the nut; G, the ring; H, the crown.
Formerly the shank was made of a number of square
iron rods, laid parallel together in a cylindrical form, and
bound by iron hoops. When they were welded into one
bar, the exterior rods could not fail to be partially burned
and wasted by the strong heat. Mr. Perring abated this
evil by using bars of the whole breadth of the shank, and
placing them right over each other, hooping them and
welding them together at two heats into one solid mass.
To any one who has seen the working of puddled iron, with
a heavy mill hammer, this operation will not appear difficult.
He formed the crown with bars similarly distributed with those of the shank. His
mode of uniting the flukes to the crown is probably the most valuable part of his
invention. The bars and half the breadth of the anchor are first welded separately, and
then placed side by side, where the upper half is worked into one mass, while the lower
part is left disunited, but has carrier iron bars, or porters, as these prolongation rods
are commonly called, welded to the extremity of each portion. The lower part is now
heated and placed in the clamping machine, which is merely an iron plate firmly bolted
to a mass of timber, and bearing upon its surface four iron pins. One end of the crown
is placed between the first of these pins, and passed under an iron strap; the other end
is brought between the other pins, and is bent by the leverage power of the elongated
rods or porters.
Thus a part of the arm being formed out of the crown gives much greater security
that a true union of fibres is effected, than when the junction was made merely by a
short scarf.
The angular opening upon the side opposite B H, fig. 7., is filled with the chock,
formed of short iron bars placed upright. When this has been firmly welded, the
truss-piece is brought over it. This piece is made of plates similar to the above, except
that their edges are here horizontal. The truss-piece is half the breadth of the arm; so
that when united to the crown, it constitutes, with the other parts, the total breadth of
the arms at those places.
The shank is now shut upon the crown; the square is formed, and the nuts welded to
it; the hole is punched out for the ring, and the shank is then fashioned.
The blade is made much in the way above described. In making the palm, an iron
rod is first bent into the approximate form, notching it so that it may more readily take
the desired shape. To one end a porter rod is fastened, by which the palm is carried
and turned round in the fire during the progress of the fabrication. Iron plates are
next laid side by side upon the rod, and the joint at the middle is broken by another
plate laid over it. When the mass is worked, its under side is filled up by similar
plates, and the whole is completely welded; pieces being added to the sides, if necessary,
to form the angles of the palm. The blade is then shut on to the palm, after which the
part of the arm attached to the blade is united to that which constitutes the crown. The
smith-work of the anchor is now finished.
The junction, or shutting on, as the workmen call it, of the several members of an
anchor, is effected by an instrument called a monkey, which is merely a mass of iron
raised to a certain height, between parallel uprights, as in the pile engine or vertical
ram, and let fall upon the metal previously brought to a welding heat.
[45]
The monkey and the hercules, both silly, trivial names, are similar instruments, and are
usually worked, like a portable pile engine, by the hands of several labourers, pulling
separate ropes. Many other modes of manufacturing anchors have been devised, in
which mechanical power is more extensively resorted to.
The upper end of the shank F (fig. 7.) is squared to receive and hold the stock
steadily, and keep it from turning. To prevent it shifting along, there are two knobs or
tenon-like projections. The point of the angle H, between the arms and the shank, is
sometimes called the throat. The arm B C generally makes an angle of 56° with the
shank A; it is either round or polygonal, and about half the length of the shank.
The stock of the anchor (fig. 6.) is made of oak. It consists of two beams which
embrace the square, and are firmly united by iron bolts and hoops, as shown in the
figure. The stock is usually somewhat longer than the shank, has in the middle a
thickness about one-twelfth of its length, but tapers at its under side to nearly one half
this thickness at the extremities. In small anchors the stock is frequently made of iron;
but in this case it does not embrace the anchor, but goes through a hole made in the
square, which is swelled out on purpose.
The weight of anchors for different vessels is proportioned to the tonnage; a good
rule being to make the anchor in hundredweights one-twentieth of the number of
tons of the burden. Thus a ship of 1000 tons would require a sheet anchor of 50 cwts.
Ships of war are provided with somewhat heavier anchors.
Several new forms and constructions of anchors were proposed under Mr. Piper’s
patent of November, 1822, by the adoption of which great advantages as to strength
were anticipated over every other form or construction previously made.
The particular object was to preserve such a disposition of the fibres of the metal as
should afford the greatest possible strength; in doing which the crossing or bending of
the fibres at the junctions of the shank, flukes, and crown, where great strength is required,
has been avoided as much as possible, so that the fibres are not disturbed or
injured.
In this respect most anchors are defective; for in connecting the shanks to the crown-pieces,
the grain of the metal is either crossed, or so much curved, as to strain the fibre,
and consequently induce a weakness where the greatest strength is required. And,
further, the very considerable thicknesses of metal which are to be brought into immediate
contact by means of the hammer in forging anchors upon the old construction,
render it highly probable that faulty places may be left within the mass, though they be
externally imperceptible. Mr. Piper’s leading principle was, that the fibre of the metal
should run nearly straight in all the parts where strength is particularly required.
Fig. 8. shows an anchor with one tumbling fluke, which
passes through the forked or branched part of the shank. The
lower part of this anchor, answering to the crown, has a spindle
through it, upon which the fluke turns, and a pin is there
introduced for the purpose of confining the fluke when in a
holding position. This shank is formed of a solid piece of
wrought iron, the fibres of which run straight, and at the
crown holes are pierced, which merely bulge the metal without
bending the fibres round so as to strain them. The arm and
fluke, also, are formed of one piece punched through without
curling or crossing the fibre, and the spindle which holds the
arm to the crown is likewise straight. This spindle extends some distance on each side
of the anchor, and is intended to answer the purpose of a stock; for when either of
the ends of the spindle comes in contact with the ground, the anchor will be thrown
over into a holding position; or an iron stock may be introduced near the shackle,
instead of these projecting ends. In the descent of the anchor, the fluke will fall over
towards that side which is nearest the ground, and will there be ready to take hold
when the anchor is drawn forward.
Fig. 9. is another anchor upon the same principle, but
slightly varied in form from the last. In this the forked part
of the shank is closer than in the former, and there are two
arms or flukes connected to the crown-pieces, one of which
falls into its holding position as the anchor comes to the
ground, and is held at its proper angle by the other fluke
stopping against the shank.
Fig. 10. represents another variation in the form of these
improved anchors, having two tumbling flukes, which are both
intended to take hold of the ground at the same time. The
shank is here, as before, made without crossing the grain of the iron, and the eyes for
admitting the bolt at the crown and at the shackle are punched out of the solid, not
formed by welding or turning the iron round. In this form a guard is introduced at[46]
the crown, to answer the purpose of a stock, by turning the flukes over into a holding
position. The arms and flukes are made, as before described, of the straight fibre of
the iron punched through, and the flukes are fixed to the spindle, which passes through
the crown-piece.

Fig. 11. has a shank without any fork, but formed straight throughout; the guard
here is an elongated frame of iron, for the same purpose as a stock, and is, with the
tumbling flukes, fastened to the spindle, which passes through the crown of the anchor,
and causes the flukes to fall into their holding position.
The principles of these new anchors are considered to consist in shanks which are made
of straight lengths of metal, and finished so that the fibres of the iron shall not be
injured by cross-shuts or uncertain welding; also each arm and palm is made in one
solid piece, and finished in straight lines, so that the fibres will not be altered, and the
shaft-pin or spindle will also be in one straight line; and this is the improvement
claimed. These anchors being made in separate pieces, give a great advantage to the
workman to execute each part perfectly; for he will not have such heavy weights to lift
when hot, which will render these anchors much stronger, with less weight; and if any
accident should happen to them, any part may be taken separate from the others to be
repaired, and several of those parts of the anchor which may be likely to break may be
carried on board, in case of accident. This anchor is so contrived that one of thirty
hundred weight may be taken to pieces and put together again, by one man, in twenty
minutes; it may also be dismounted, and stowed in any part of the ship, in as little
room as straight bars of iron, and speedily put together again.
The anchor (fig. 12.) patented by Mr. Brunton, in February,
1822, has its stock introduced at the crown part, for the purpose
of turning it over into a holding position. The shank is perforated
through the solid, in two places, with elliptical apertures,
for the purpose of giving it a greater stability, and more effectually
resisting the strain to which the anchor may be subjected.
The stock is a cylindrical iron rod, held at its extremities by
lateral braces, which are bolted to the shank.
Fig. 12. shows the form of the anchor. The shank is seen
upright, with one of the flukes projecting in its front; the horizontal iron stock is at
bottom; and the oblique braces are bolted to both shank and stock. The ends of the
stock, from the shoulder, are formed dove-tailed, and oval in the vertical direction, and
are protruded through apertures in the
braces, also oval, but in the horizontal direction,
and counter sunk. When the ends
of the stock have been thus introduced
through the holes, the braces are securely
bolted to the shank; the ends of the stock
are then spread, by hammering into the
counter-sunk holes of the braces, and by
that means they are made firm.
An anchor of this description is considered
by the patentee to possess considerable
advantage, particularly in point of
stability, over the ordinary construction of
anchors, and is economical, inasmuch as
a less weight of metal will give, upon
this plan, an equal degree of strength.
An ingenious form of anchor was made
the subject of a patent, by Lieutenant
Rodgers, of the Royal Navy, in 1828, and
was afterwards modified by him in a second
patent, obtained in August, 1829. The
whole of the parts of the anchor are to be
bound together by means of iron bands or
hoops, in place of bolts or pins.
Fig. 13. is a side view of a complete
anchor, formed upon his last improved
construction, and fig. 14., a plan of the
same; fig. 15., an end view of the crown
and flukes, or arms; fig. 16. represents
the two principal iron plates, a, a, of
which the shank is constructed, but so as to form parts of the stump arms to which the
flukes are to be connected.
The crown piece is to be welded to the stump piece, c c, fig. 16., as well as to the[47]
end l of the centre piece h h, and the scarfs m m are to be cut to receive the arms or
flukes. Previously, however, to uniting the arms or flukes with the stump arms, the
crown and throat of the anchor are to be strengthened, by the application of the crown
slabs n n, fig. 16., which are to be welded upon each side of the crown, overlapping
the end of the pillar h, and the throat or knees of the stump arms and the crown piece.
The stump arms are then to be strengthened in a similar manner, by the thin flat pieces
p p, which are to be welded upon each side. The palms are united to the flukes in
the usual way, and the flukes are also united to the stump arms by means of the long
scarfs m m. When the shank of the anchor has been thus formed, and united with
the flukes, the anchor smith’s work may be said to be complete.
Another of the improvements in the construction of anchors, claimed under this
patent, consists in a new method of affixing the stock upon the shank of the anchor,
which is effected in the following manner: in fig. 14. the stock is shown affixed to
the anchor; in fig. 17. it is shown detached. It may be made either of one or two
pieces of timber, as may be found most convenient. It is, however, to be observed that
the stock is to be completed before fitting on to the shank. After the stock is shaped,
a hole is to be made through the middle of it, to fit that part of the shank to which it is
to be affixed. Two stock plates are then to be let in, one on each side of the stock, and
made fast by counter sunk nails and straps, or hoops; other straps or hoops of iron are
also to be placed round the stock, as usual.
In place of nuts, formed upon the shank of the anchor, it is proposed to secure the
stock by means of a hoop and a key, shown above and below J, in fig. 14. By this
contrivance, the stock is prevented from going nearer to the crown of the anchor than it
ought to do, and the key prevents it from sliding towards the shackle.
Since fitting the stock to the shank of an anchor, by this method, prevents the use of
a ring, as in the ordinary manner, the patentee says that he in all cases substitutes a
shackle for the ring, and which is all that is required for a chain cable; but, when a
hempen cable is to be used, he connects a ring to the usual shackle, by means of a
joining shackle, as in figs. 13. and 14.
Mr. Rodgers proposes, under another patent, dated July, 1833, to alter the size and
form of the palms; having found from experience that anchors with small palms will
not only hold better than with large ones, but that the arms of the anchor, even without
any palms, have been found to take more secure hold of the ground than anchors of the
old construction, of similar weight and length. He has, accordingly, fixed upon one-fifth
of the length of the arm, as a suitable proportion for the length or depth of the
palm. He makes the palms, also, broader than they are long or deep.
ANIMÉ. A resin of a pale brown yellow colour, transparent and brittle. It
exudes from the courbaril of Cayenne, a tree which grows also in various parts of
South America. It occurs in pieces of various sizes, and it often contains so many insects
belonging to living species, as to have merited its name, as being animated. It contains
about a fifth of one per cent. of a volatile oil, which gives it an agreeable odour.
Alcohol does not dissolve the genuine animé, as I have ascertained by careful experiments;
nor does caoutchoucine; but a mixture of the two, in equal parts, softens it
into a tremulous jelly, though it will not produce a liquid solution. When reduced to
this state, the insects can be easily picked out, without injury to their most delicate parts.
The specific gravity of the different specimens of animé which I tried, varied from
1·054 to 1·057. When exposed to heat, in a glass retort over a spirit flame, it softens,
and, by careful management, it may be brought into liquid fusion, without discolouration.
It then exhales a few white vapours, of an ambrosiacal odour, which being condensed
in water, and the liquid being tested, is found to be succinic acid. Author.
It is extensively used by the varnish makers, who fuse it at a pretty high heat, and
in this state combine it with their oils, or other varnishes.
ANKER. A liquid measure of Amsterdam, which contains 32 gallons English.
ANNEALING or NEALING. (Le recuit, Fr.; das anlassen, Germ.) A process
by which glass is rendered less frangible; and metals, which have become brittle, either
in consequence of fusion, or long-continued hammering, are again rendered malleable.
When a glass vessel is allowed to cool immediately after being made, it will often sustain
the shock of a pistol-bullet, or any other blunt body falling into it from a considerable
height; while a small splinter of flint, or an angular fragment of quartz, dropped gently
into it, makes it sometimes immediately, sometimes after a few minutes, fly to pieces
with great violence. This extreme fragility is prevented by annealing, or placing the
vessels in an oven, where they take several hours or even some days to cool. Similar phenomena
are exhibited in a higher degree by glass-tears, or Prince Rupert’s drops. They
are procured by letting drops of melted glass fall into cold water. Their form resembles
that of a pear, rounded at one extremity, and tapering to a very slender tail at the other.
If a part of the tail be broken off, the whole drop flies to pieces with a loud explosion;
and yet the tail of a drop may be cut away by a glass-cutter’s wheel, or the thick end[48]
may be struck smartly with a hammer, without the fear of sustaining any injury. When
heated to redness, and permitted to cool gradually in the open air, they lose these
peculiarities, and do not differ sensibly from common glass.
The properties of unannealed glass depend on a peculiar structure, extending uniformly
through its whole substance; and the bursting of a glass drop by breaking off
the tail, or of an unannealed glass vessel, by dropping a piece of flint into it, arises from
a crack being thus begun, which afterwards extends its ramifications in different directions
throughout the glass.
When metals have been extended to a certain degree under the hammer, they become
brittle, and incapable of being further extended without cracking. In this case the
workman restores their malleability by annealing, or heating them red-hot. The
rationale of this process seems to be, that the hammering and extension of the metal
destroy the kind of arrangement which the particles of the metal had previous to the
hammering; and that the annealing, by softening the metal, enables it to recover its
original structure.
Of late years a mode has been discovered of rendering cast iron malleable, without
subjecting it to the action of puddling. The process is somewhat similar to that
employed in annealing glass. The metal is kept for several hours at a temperature a
little below its fusing point, and then allowed to cool slowly. In this manner vessels
are made of cast iron which can sustain considerable violence, without being broken.
See Steel, softening of.
ANNOTTO. (Rocou, or roucou, Fr.; orleans, Germ.) A somewhat dry and
hard paste, brown without, and red within. It is usually imported in cakes of two or
three pounds weight, wrapped up in leaves of large reeds, packed in casks, from America,
where it is prepared from the seeds of a certain tree, the bixa orellana, of Linnæus.
The pods of the tree being gathered, their seeds are taken out and bruised; they are
then transferred to a vat, which is called the steeper, where they are mixed with as much
water as covers them. Here the substance is left for several weeks, or even months;
it is now squeezed through sieves placed above the steeper, that the water containing
the colouring matter in suspension may return, into the vat. The residuum is preserved
under the leaves of the anana (pine-apple) tree, till it becomes hot by fermentation. It
is again subjected to the same operation, and this treatment is continued till no more
colour remains.
The substance thus extracted is passed through sieves, in order to separate the
remainder of the seeds, and the colour is allowed to subside. The precipitate is boiled
in coppers till it be reduced to a consistent paste; it is then suffered to cool, and dried
in the shade.
Instead of this long and painful labour, which occasions diseases by the putrefaction
induced, and which affords a spoiled product, Leblond proposes simply to wash the
seeds of annotto till they be entirely deprived of their colour, which lies wholly on their
surface; to precipitate the colour by means of vinegar or lemon juice, and to boil it up
in the ordinary manner, or to drain it in bags, as is practised with indigo.
The experiments which Vauquelin made on the seeds of annotto imported by Leblond,
confirmed the efficacy of the process which he proposed; and the dyers ascertained
that the annotto obtained in this manner was worth at least four times more than that
of commerce; that, moreover, it was more easily employed; that it required less solvent;
that it gave less trouble in the copper, and furnished a purer colour.
Annotto dissolves better and more readily in alcohol than in water, when it is introduced
into the yellow varnishes for communicating an orange tint.
The decoction of annotto in water has a strong peculiar odour, and a disagreeable
taste. Its colour is yellowish-red, and it remains a little turbid. An alkaline solution
renders its orange-yellow clearer and more agreeable, while a small quantity of a whitish
substance is separated from it, which remains suspended in the liquid. If annotto be
boiled in water along with an alkali, it dissolves much better than when alone, and the
liquid has an orange hue.
The acids form with this liquor an orange-coloured precipitate, soluble in alkalies,
which communicate to it a deep orange colour. The supernatant liquor retains only
a pale yellow hue.
When annotto is used as a dye, it is always mixed with alkali, which facilitates its
solution, and gives it a colour inclining less to red. The annotto is cut in pieces, and
boiled for some instants in a copper with its own weight of crude pearl ashes, provided
the shade wanted do not require less alkali. The cloths may be thereafter dyed in this
bath, either by these ingredients alone, or by adding others to modify the colour; but
annotto is seldom used for woollen, because the colours which it gives are too fugitive,
and may be obtained by more permanent dyes. Hellot employed it to dye a stuff,
prepared with alum and tartar; but the colour acquired had little permanence. It is
almost solely used for silks.
[49]
For silks intended to become aurora and orange, it is sufficient to scour them at the
rate of 20 per cent. of soap. When they have been well cleansed, they are immersed
in a bath prepared with water, to which is added a quantity of alkaline solution of
annotto, more or less considerable according to the shade that may be wanted. This
bath should have a mean temperature, between that of tepid and boiling water.
When the silk has become uniform, one of the hanks is taken out, washed, and wrung,
to see if the colour be sufficiently full; if it be not so, more solution of annotto is added,
and the silk is turned again round the sticks: the solution keeps without alteration.
When the desired shade is obtained, nothing remains but to wash the silk, and give
it two beetlings at the river, in order to free it from the redundant annotto, which would
injure the lustre of the colour.
When raw silks are to be dyed, those naturally white are chosen, and dyed in the
annotto bath, which should not be more than tepid, or even cold, in order that the
alkali may not attack the gum of the silk, and deprive it of the elasticity which it is
desirable for it to preserve.
What has been now said regards the silks to which the aurora shades are to be given;
but to make an orange hue, which contains more red than the aurora, it is requisite,
after dyeing with annotto, to redden the silks with vinegar, alum, or lemon juice. The
acid, by saturating the alkali employed for dissolving the annotto, destroys the shade of
yellow that the alkali had given, and restores it to its natural colour, which inclines a
good deal to red.
For the deep shades, the practice at Paris, as Macquer informs us, is to pass the silks
through alum; and if the colour be not red enough, they are passed through a faint
bath of brazil wood. At Lyons, the dyers who use carthamus, sometimes employ old
baths of this ingredient for dipping the deep oranges.
When the orange hues have been reddened by alum, they must be washed at the
river; but it is not necessary to beetle them, unless the colour turns out too red.
Shades may be obtained also by a single operation, which retain a reddish tint, employing
for the annotto bath a less proportion of alkali than has been pointed out.
Guhliche recommends to avoid heat in the preparation of annotto. He directs it to
be placed in a glass vessel, or in a glazed earthen one; to cover it with a solution of
pure alkali; to leave the mixture at rest for 24 hours; to decant the liquor, filter it, and
add water repeatedly to the residuum, leaving the mixture each time at rest for two or
three days, till the water is no longer coloured; to mix all these liquors, and preserve
the whole for use in a well-stopped vessel.
He macerates the silk for 12 hours in a solution of alum, at the rate of an eighth of
this salt for one part of silk, or in a water rendered acidulous by the aceto-citric acid
above described; and he wrings it well on its coming out of this bath.
Silk thus prepared is put into the annotto bath quite cold. It is kept in agitation
there till it has taken the shade sought for; or the liquor may be maintained at a heat
far below ebullition. On being taken out of the bath, the silk is to be washed and dried
in the shade.
For lighter hues, a liquor less charged with colour is taken; and a little of the acid
liquid which has served for the mordant may be added, or the dyed silk may be passed
through the acidulous water.
We have seen the following preparation employed for cotton velvet:—one part of
quicklime, one of potash, two of soda.
Of these a ley is formed, in which one part of annotto is dissolved; and the mixture
is boiled for an hour and a half. This bath affords the liveliest and most brilliant
auroras. The buff (chamois) fugitive dye is also obtained with this solution. For
this purpose only a little is wanted; but we must never forget, that the colours arising
from annotto are all fugitive.
Dr. John found in the pulp surrounding the unfermented fresh seeds, which are about
the size of little peas, 28 parts of colouring resinous matter, 26·5 of vegetable gluten,
20 of ligneous fibre, 20 of colouring extractive matter, 4 formed of matters analogous
to vegetable gluten and extractive, and a trace of spicy and acid matters.
The Gloucestershire cheese is coloured with annotto, in the proportion of one cwt. to
an ounce of the dye.
When used in calico-printing, it is usually mixed with potash or ammonia and starch.
It is an appropriate substance for tingeing varnishes, oils, spirits, &c.
The import duty upon annotto is 1s. per cwt. for flag, and 4s. for other sorts. In
1834, 252,981 lbs. were imported; and in 1835, 163,421 lbs. The revenue from this
drug in these two years, was 180l. and 98l. respectively.
ANTHRACITE, from ανθραξ, coal, is a species of coal found in the transition rock
formation, and is often called stone coal. It has a grayish black, or iron black colour,
an imperfectly metallic lustre, conchoidal fracture, and a specific gravity of from 1·4 to
1·6, being, therefore, much denser than the coal of the proper coal measures. It consists[50]
wholly of carbon, with a small and variable proportion of iron, silica, and alumina. It
is difficult to kindle in separate masses, and burns when in heaps or grates without
smell or smoke, leaving sometimes an earthy residuum. It has been little explored or
worked in the old world; but is extensively used in the United States of America,
and has become of late years a most valuable mineral to that country, where it is
burned in peculiar grates, adapted to its difficult combustion. In Pennsylvania the
anthracite coal formation has been traced through a tract many miles in width, and extending
across the two entire counties of Luzerne and Schuylkill. At Maunch Chunk,
upon the Lehigh, 800 men were employed so far back as 1825, in digging this coal. In
that year 750,000 bushels were dispatched for Philadelphia. It is worked there with
little cost or labour, being situated on hills from 300 to 600 feet above the level of
the neighbouring rivers and canals, and existing in nearly horizontal beds, of from 15 to
40 feet in thickness, covered by only a few feet of gravelly loam. At Portsmouth, in
Rhode Island, an extensive stratum of this coal has been worked, with some interruptions,
for 20 years; and more recently a mine of anthracite has been opened at Worcester,
in Massachusetts, at the head of the Blackstone canal. It has been of late employed
in South Wales, for smelting iron, and in a cupola blast furnace, by Mr. Crane.
ANTIGUGGLER. A small syphon of metal, which is inserted into the mouths of
casks, or large bottles, called carboys, to admit air over the liquor contained in them,
and thus to facilitate their being emptied without agitation or a guggling noise.
ANTIMONY. (Antimoine, Fr.; Spiessglanz, or Spiessglass, Ger.) The only ore
of this metal found in sufficient abundance to be smelted, is the sulphuret, formerly
called crude antimony. It occurs generally in masses, consisting of needles closely
aggregated, of a metallic lustre, a lead-gray colour, inclining to steel-gray, which is unchanged
in the streak. The needles are extremely brittle, and melt even in the flame
of a candle, with the exhalation of a sulphureous smell. The powder of this sulphuret
is very black, and was employed by women in ancient times to stain their eyebrows
and eyelids. This ore consists in 100 parts of 72·86 metal, and 27·14 sulphur. Specific
gravity from 4·13 to 4·6.
The veins of sulphuret of antimony occur associated with gangues of quartz, sulphate
of barytes, and carbonate of lime; those of Allémont occur in the numerous fissures of
a mica schist, evidently primitive.
In treating the ore to obtain the metal, the first object is to separate the gangue,
which was formerly done by filling crucibles with the mixed materials, placing them on
the hearth of an oven, and exposing them to a moderate heat. As the sulphuret easily
melts, it ran out through a hole in the bottom of the crucible into a pot placed beneath,
and out of the reach of the fire. But the great loss from breakage of the crucibles, has
caused another method to be adopted. In this the broken ore, being sorted, is laid on
the bottom of a concave reverberatory hearth, where it is reduced.
Figs. 18. 19. represent a wind or flame furnace, for the reduction of antimony. The
hearth is formed of sand
and clay solidly beat together,
and slopes from all
sides towards the middle,
where it is connected with
the orifice a, which is closed
with dense coal-ashes; b is
the air channel up through
the bridge; c, the door for
introducing the prepared ore, and running off the slags; d, the bridge; e, the grate; f, the
fire or fuel-door; g, the chimney. With 2 or 3 cwt. of ore, the smelting process is completed
in from 8 to 10 hours. The metal thus obtained is not pure enough, but must
be fused under coal dust, in portions of 20 or 30 pounds, in crucibles, placed upon a
reverberatory hearth.
To obtain antimony free from iron, it should be fused with some antimonic oxide in
a crucible, whereby the iron is oxidized and separated. The presence of arsenic in
antimony is detected by the garlic smell, emitted by such an alloy when heated at the
blow-pipe; or, better, by igniting it with nitre in a crucible; in which case, insoluble
antimonite and antimoniate of potash will be formed along with soluble arseniate.
Water digested upon the mixture, filtered, and then tested with nitrate of silver, will
afford the brown-red precipitate characteristic of arsenic acid.
According to Berthier, the following materials afford, in smelting, an excellent product
of antimony: 100 parts of sulphuret; 60 of hammerschlag (protoxide of iron from
the shingling or rolling mills); 45 to 50 of carbonate of soda; and 10 of charcoal powder.
From 65 to 70 parts of metallic antimony or regulus should be obtained. Glauber
salts may be used instead of soda. For another mode of smelting antimony, at Malbosc,
in the department of Ardèche, in France, see Liquation.
In the works where antimonial ores are smelted, by means of tartar (argol), the alkaline[51]
scoriæ, which cover the metallic ingots, are not rejected as useless, for they hold
a certain quantity of antimonial oxide in combination; a property of the potash flux,
which is propitious to the purity of the metal. These scoriæ, consisting of sulphuret
of potassium and antimonite of potash, being treated with water, undergo a reciprocal
decomposition; the elements of the water act on those of the sulphuret, and the
resulting alkaline hydro-sulphuret re-acts on the antimonial solution, so as to form a
species of kermes mineral, which precipitates. This is dried, and sold at a low price as
a veterinary medicine, under the name of kermes, by the dry way.
Metallic antimony, as obtained by the preceding process, is the antimony of commerce,
but is not absolutely pure; containing frequently minute portions of iron, lead,
and even arsenic; the detection and separation of which belong to the sciences of chemistry
and pharmacy. Antimony is a brittle metal, of a silvery white colour, with a
tinge of blue, a lamellar texture, and crystalline fracture. When heated at the blowpipe,
it melts with great readiness, and diffuses white vapours, possessing somewhat of a
garlic smell. If thrown in this melted state on a sheet of flat paper, the globule sparkles,
and bursts into a multitude of small spheroids, which retain their incandescence for a
long time, and run about on the paper, leaving traces of the white oxide produced
during the combustion. When this oxide is fused with borax, or other vitrifying matter,
it imparts a yellow colour to it. Metallic antimony, treated with hot nitric acid and
in a concentrated state, is converted into a powder, called antimonious acid, which is
altogether insoluble in the ordinary acid menstrua; a property by which the chemist
can separate that metal from lead, iron, copper, bismuth, and silver. According to
Bergman, the specific gravity of antimony is 6·86; but Haidinger makes the Swedish
native metal only 6·646. The alchemists had conceived the most brilliant hopes of this
metal; the facility with which it is alloyed with gold, since its fumes alone render this
most ductile metal immediately brittle, led them to assign to it a royal lineage, and to
distinguish it by the title of regulus, or the little king.
Its chief employment now is in medicine, and in making the alloys called type metal,
stereotype metal, music plates, and Britannia-metal; the first consisting of 6 of lead and
2 of antimony; the second of 6 of lead and 1 of antimony; the third of lead, tin,
and antimony; and the fourth also of lead, tin, and antimony, with occasionally a little
copper and bismuth.—For Glass of antimony, see Pastes.
ANTISEPTICS. Substances which counteract the spontaneous decomposition of
animal and vegetable substances. These are chiefly culinary salt, nitre, spices, and
sugar, which operate partly by inducing a change in the animal or vegetable fibres,
and partly by rendering the aqueous constituent unsusceptible of decomposition. See
Provisions, curing of.
ANVIL. A mass of iron, having a smooth and nearly flat top surface of steel; upon
which blacksmiths, and various other artificers, forge metals with the hammer. The common
anvil is usually made of seven pieces: 1, the core, or body; 2, 3, 4, 5, the four corner
pieces which serve to enlarge its base; 6, the projecting end, which has a square hole
for the reception of the tail or shank of a chisel on which iron bars may be cut through;
and 7, the beak, or horizontal cone round which rods or slips of metal may be turned
into a circular form, as in making rings. These 6 pieces are welded separately to the
first, or core, and then hammered into an uniform body. In manufacturing large
anvils two hearths are needed, in order to bring each of the two pieces to be welded,
to a proper heat by itself; and several men are employed in working them together
briskly in the welding state, by heavy swing hammers. The steel facing is applied by
welding in the same manner. The anvil is then hardened by heating it to a cherry red,
and plunging it into cold water; a running stream being preferable to a pool or cistern.
The facing should not be too thick a plate, for, when such, it is apt to crack in the
hardening. The face of the anvil is now smoothed upon a grindstone, and finally
polished with emery and crocus, for all delicate purposes of art.
The blacksmith, in general, sets his anvil loosely upon a wooden block, and in preference
on the root of an oak. But the cutlers and file-makers fasten their anvils to a
large block of stone; which is an advantage, for the more firmly and solidly this tool is
connected to the earth, the more efficacious will be the blows of the hammer on any
object placed upon it.
AQUAFORTIS. Nitric acid, somewhat dilute, was so named by the alchemists
on account of its strong solvent and corrosive operation upon many mineral, vegetable,
and animal substances. See Nitric Acid.
AQUA REGIA. The name given by the alchemists to that mixture of nitric and
muriatic acids which was best fitted to dissolve gold, styled by them the king of the
metals. It is now called nitro-muriatic acid.
AQUA VITÆ. The name very absurdly given to alcohol, when used as an
intoxicating beverage. It has been the aqua mortis to myriads of the human race; and
will, probably, ere long destroy all the native tribes of North America and Australia.
[52]
ARCHIL. A violet red paste used in dyeing, of which the substance called
cudbear in Scotland (from Cuthbert, its first preparer in that form), is a modification.
Two kinds of archil are distinguished in commerce, the archil plant of the Canaries, and
that of Auvergne. The first is most esteemed: it is prepared from the lichen rocellus,
which grows on rocks adjoining the sea in the Canary and Cape de Verde Islands, in Sardinia,
Minorca, &c., as well as on the rocks of Sweden. The second species is prepared
from the lichen parellus, which grows on the basaltic rocks of Auvergne.
There are several other species of lichen which might be employed in producing an
analogous dye, were they prepared, like the preceding, into the substance called archil.
Hellot gives the following method for discovering if they possess this property. A little
of the plant is to be put into a glass vessel; it is to be moistened with ammonia and
lime-water in equal parts; a little muriate of ammonia (sal ammoniac) is added; and
the small vessel is corked. If the plant be of a nature to afford a red dye, after three or
four days, the small portion of liquid, which will run off on inclining the vessel, now
opened, will be tinged of a crimson red, and the plant itself will have assumed this colour.
If the liquor or the plant does not take this colour, nothing need be hoped for; and it is
useless to attempt its preparation on the great scale. Lewis says, however, that he has
tested in this way a great many mosses, and that most of them afforded him a yellow or
reddish brown colour; but that he obtained from only a small number a liquor of a deep
red, which communicated to cloth merely a yellowish-red colour.
Prepared archil gives out its colour very readily to water, ammonia, and alcohol. Its
solution in alcohol is used for filling spirit-of-wine thermometers; and when these thermometers
are well freed from air, the liquor loses its colour in some years, as Abbé
Nollet observed. The contact of air restores the colour, which is destroyed anew, in
vacuo, in process of time. The watery infusion loses its colour, by the privation of air,
in a few days; a singular phenomenon, which merits new researches.
The infusion of archil is of a crimson bordering on violet. As it contains ammonia,
which has already modified its natural colour, the fixed alkalies can produce little change
on it, only deepening the colour a little, and making it more violet. Alum forms in it
a precipitate of a brown red; and the supernatant liquid retains a yellowish-red colour.
The solution of tin affords a reddish precipitate, which falls down slowly; the supernatant
liquid retains a feeble red colour. The other metallic salts produce precipitates
which offer nothing remarkable.
The watery solution of archil applied to cold marble, penetrates it, communicating a
beautiful violet colour, or a blue bordering on purple, which resists the air much longer
than the archil colours applied to other substances. Dufay says, that he has seen marble
tinged with this colour preserve it without alteration at the end of two years.
To dye with archil, the quantity of this substance deemed necessary, according to the
quantity of wool or stuff to be dyed, and according to the shade to which they are to be
brought, is to be diffused in a bath of water as soon as it begins to grow warm. The bath
is then heated till it be ready to boil, and the wool or stuff is passed through it without
any other preparation, except keeping that longest in, which is to have the deepest shade.
A fine gridelin, bordering upon violet, is thereby obtained; but this colour has no permanence.
Hence archil is rarely employed with any other view than to modify, heighten, and
give lustre to the other colours. Hellot says, that having employed archil on wool boiled
with tartar and alum, the colour resisted the air no more than what had received no
preparation. But he obtained from herb archil (l’orseille d’herbe) a much more durable
colour, by putting in the bath some solution of tin. The archil thereby loses its natural
colour, and assumes one approaching more or less to scarlet, according to the quantity
of solution of tin employed. This process must be executed in nearly the same manner
as that of scarlet, except that the dyeing may be performed in a single bath.
Archil is frequently had recourse to for varying the different shades and giving them
lustre; hence it is used for violets, lilacs, mallows, and rosemary flowers. To obtain a
deeper tone, as for the deep soupes au vin, sometimes a little alkali or milk of lime is
mixed with it. The suites of this browning may also afford agates, rosemary flowers,
and other delicate colours, which cannot be obtained so beautiful by other processes.
Alum cannot be substituted for this purpose; it not only does not give this lustre, but
it degrades the deep colours.
The herb-archil is preferable to the archil of Auvergne, from the greater bloom which
it communicates to the colours, and from the larger quantity of colouring matter. It
has, besides, the advantage of bearing ebullition. The latter, moreover, does not answer
with alum, which destroys the colour; but the herb archil has the inconvenience of
dyeing in an irregular manner, unless attention be given to pass the cloth through hot
water as soon as it comes out of the dye.
Archil alone is not used for dyeing silk, unless for lilacs; but silk is frequently
passed through a bath of archil, either before dyeing it in other baths or after it has been
dyed, in order to modify different colours, or to give them lustre. Examples of this[53]
will be given in treating of the compound colours. It is sufficient here to point out
how white silks are passed through the archil bath. The same process is performed
with a bath more or less charged with this colour, for silks already dyed.
Archil, in a quantity proportioned to the colour desired, is to be boiled in a copper.
The clear liquid is to be run off quite hot from the archil bath, leaving the sediment at
the bottom, into a tub of proper size, in which the silks, newly scoured with soap, are to
be turned round on the skein-sticks with much exactness, till they have attained the
wished-for shade. After this they must receive one beetling at the river.
Archil is in general a very useful ingredient in dyeing; but as it is rich in colour, and
communicates an alluring bloom, dyers are often tempted to abuse it, and to exceed the
proportions that can add to the beauty without at the same time injuring in a dangerous
manner the permanence of the colours. Nevertheless, the colour obtained when solution
of tin is employed, is less fugitive than without this addition: it is red, approaching
to scarlet. Tin appears to be the only ingredient which can increase its durability.
The solution of tin may be employed, not only in the dyeing bath, but for the preparation
of the silk. In this case, by mixing the archil with other colouring substances,
dyes may be obtained which have lustre with sufficient durability.
We have spoken of the colour of the archil as if it were natural to it; but it is, really,
due to an alkaline combination. The acids make it pass to red, either by saturating
the alkali, or by substituting themselves for the alkali.
The lichen which produces archil is subjected to another preparation, to make turnsole
(litmus). This article is made in Holland. The lichen comes from the Canary
Islands, and also from Sweden. It is reduced to a fine powder by means of a mill,
and a certain proportion of potash is mixed with it. The mixture is watered with
urine, and allowed to suffer a species of fermentation. When this has arrived at a
certain degree, carbonate of lime in powder is added, to give consistence and weight to
the paste, which is afterwards reduced into small parallelopipeds that are carefully
dried.
The latest researches on the lichens, as objects of manufacture, are those of Westring
of Stockholm. He examined 150 species, among which he found several which might
be rendered useful. He recommends that the colouring matter should be extracted in
the places where they grow, which would save a vast expense in curing, package,
carriage, and waste. He styles the colouring substance itself cutbear, persio, or turnsole;
and distributes the lichens as follows:—1st. Those which left to themselves,
exposed to moderate heat and moisture, may be fixed without a mordant upon wool or
silk; such are the L. cinereus, æmatonta, ventosus, corallinus, westringii, saxatilis, conspassus,
barbatus, plicatus, vulpinus, &c.
2. Those which develop a colouring matter fixable likewise without mordant, but
which require boiling and a complicated preparation; such are the lichens subcarneus,
dillenii, farinaceus, jubatus, furfuraceus, pulmonareus, cornigatus, cocciferus, digitatus,
ancialis, aduncus, &c. Saltpetre or sea-salt are requisite to improve the lustre and
fastness of the dye given by this group to silk.
3. Those which require a peculiar process to develop their colour; such as those
which become purple through the agency of stale urine or ammonia. Westring employed
the following mode of testing:—He put three or four drachms of the dried
and powdered lichen into a flask; moistened it with three or four measures of cold
spring water; put the stuff to be dyed into the mixture, and left the flask in a cool
place. Sometimes he added a little salt, saltpetre, quicklime, or sulphate of copper. If
no colour appeared, he then moistened the lichen with water containing one twentieth
of sal ammoniac, and one tenth of quicklime, and set the mixture aside in a cool place
from eight to fourteen days. There appeared in most cases a reddish or violet coloured
tint. Thus the lichen cinereus dyed silk a deep carmelite, and wool a light carmelite;
the l. physodes gave a yellowish-gray; the pustulatus, a rose red; sanguinarius, gray;
tartareus, found on the rocks of Norway, Scotland, and England, dyes a crimson-red.
In Jutland, cutbear is made from it, by grinding the dry lichen, sifting it, then setting
it to ferment in a close vessel with ammonia. The lichen must be of the third year’s
growth to yield an abundant dye; and that which grows near the sea is the best. It
loses half its weight by drying. A single person may gather from twenty to thirty
pounds a day in situations where it abounds. No less than 2,239,685 pounds were
manufactured at Christiansand, Flekkefiort, and Fakrsund, in Norway, in the course of
the six years prior to 1812. Since more solid dyes of the same shade have been
invented, the archil has gone much into disuse. Federigo, of Florence, who revived
its use at the beginning of the fourteenth century, made such an immense fortune by its
preparation, that his family became one of the grandees of that city, under the name of
Oricellarii, or Rucellarii. For more than a century Italy possessed the exclusive art of
making archil, obtaining the lichens from the islands of the Mediterranean. According
to an official report of 1831, Teneriffe furnished annually 500 quintals (cwts.) of lichen;[54]
the Canary Isles, 400; Fuerta Santura, 300; Lancerot, 300; Gomera, 300; isle of
Ferro, 800. This business belonged to the crown, and brought it a revenue of 1500
piastres. The farmers paid from 15 to 20 reals for the right to gather each quintal. At
that time the quintal fetched in the London market 4l. sterling.
Archil is perhaps too much used in some cloth factories of England, to the discredit of
our dyes. It is said, that by its aid one third of the indigo may be saved in the blue
vat; but the colour is so much the more perishable. The fine soft tint induced upon
much of the black cloth by means of archil is also deceptive. One half-pound of cutbear
will dye one pound of woollen cloth. A crimson red is obtained by adding to
the decoction of archil a little salt of tin (muriate), and passing the cloth through the
bath, after it has been prepared by a mordant of tin and tartar. It must be afterwards
passed through hot water.
ARDENT SPIRIT. Alcohol of moderate strength.
AREOMETER OF BAUMÉ. This scale is much used by the French authors.
Specific Gravity Numbers corresponding with Baumé’s Areometric Degrees.
| Liquids denser than Water. |
Less dense than Water. |
De-
grees. |
Spe-
cific
gravi-
ty. |
De-
grees. |
Spe-
cific
gravi-
ty. |
De-
grees. |
Spe-
cific
gravi-
ty. |
De-
grees. |
Spe-
cific
gravi-
ty. |
De-
grees. |
Spe-
cific
gravi-
ty. |
| 0 |
1·0000 |
26 |
1·2063 |
52 |
1·5200 |
10 |
1·0000 |
36 |
0·8488 |
| 1 |
1·0066 |
27 |
1·2160 |
53 |
1·5353 |
11 |
0·9932 |
37 |
0·8439 |
| 2 |
1·0133 |
28 |
1·2258 |
54 |
1·5510 |
12 |
0·9865 |
38 |
0·8391 |
| 3 |
1·0201 |
29 |
1·2358 |
55 |
1·5671 |
13 |
0·9799 |
39 |
0·8313 |
| 4 |
1·0270 |
30 |
1·2459 |
56 |
1·5833 |
14 |
0·9733 |
40 |
0·8295 |
| 5 |
1·0340 |
31 |
1·2562 |
57 |
1·6000 |
15 |
0·9669 |
41 |
0·8249 |
| 6 |
1·0411 |
32 |
1·2667 |
58 |
1·6170 |
16 |
0·9605 |
42 |
0·8202 |
| 7 |
1·0483 |
33 |
1·2773 |
59 |
1·6344 |
17 |
0·9542 |
43 |
0·8156 |
| 8 |
1·0556 |
34 |
1·2881 |
60 |
1·6522 |
18 |
0·9480 |
44 |
0·8111 |
| 9 |
1·0630 |
35 |
1·2992 |
61 |
1·6705 |
19 |
0·9420 |
45 |
0·8066 |
| 10 |
1·0704 |
36 |
1·3103 |
62 |
1·6889 |
20 |
0·9359 |
46 |
0·8022 |
| 11 |
1·0780 |
37 |
1·3217 |
63 |
1·7079 |
21 |
0·9300 |
47 |
0·7978 |
| 12 |
1·0857 |
38 |
1·3333 |
64 |
1·7273 |
22 |
0·9241 |
48 |
0·7935 |
| 13 |
1·0935 |
39 |
1·3451 |
65 |
1·7471 |
23 |
0·9183 |
49 |
0·7892 |
| 14 |
1·1014 |
40 |
1·3571 |
66 |
1·7674 |
24 |
0·9125 |
50 |
0·7849 |
| 15 |
1·1095 |
41 |
1·3694 |
67 |
1·7882 |
25 |
0·9068 |
51 |
0·7807 |
| 16 |
1·1176 |
42 |
1·3818 |
68 |
1·8095 |
26 |
0·9012 |
52 |
0·7766 |
| 17 |
1·1259 |
43 |
1·3945 |
69 |
1·8313 |
27 |
0·8957 |
53 |
0·7725 |
| 18 |
1·1343 |
44 |
1·4074 |
70 |
1·8537 |
28 |
0·8902 |
54 |
0·7684 |
| 19 |
1·1428 |
45 |
1·4206 |
71 |
1·8765 |
29 |
0·8848 |
55 |
0·7643 |
| 20 |
1·1515 |
46 |
1·4339 |
72 |
1·9000 |
30 |
0·8795 |
56 |
0·7604 |
| 21 |
1·1603 |
47 |
1·4476 |
73 |
1·9241 |
31 |
0·8742 |
57 |
0·7656 |
| 22 |
1·1692 |
48 |
1·4615 |
74 |
1·9487 |
32 |
0·8690 |
58 |
0·7526 |
| 23 |
1·1783 |
49 |
1·4758 |
75 |
1·9740 |
33 |
0·8639 |
59 |
0·7487 |
| 24 |
1·1875 |
50 |
1·4902 |
76 |
2·0000 |
34 |
0·8588 |
60 |
0·7449 |
| 25 |
1·1968 |
51 |
1·4951 |
|
|
35 |
0·8538 |
61 |
0·7411 |
ARGILLACEOUS EARTH. The earth of clay, called in chemistry alumina,
because it is obtained in greatest purity from alum.
ARGOL. Crude tartar; which see.
ARMS. Weapons of war. See Fire-Arms for an account of this manufacture.
ARRACK. A kind of intoxicating beverage made in India, by distilling the fermented
juice of the cocoa-nut, the palmyra tree, and rice in the husk.
ARROW ROOT. The root of the maranta arundinacea, a plant which grows in the
West Indies, furnishes, by pounding in mortars and elutriation through sieves, a peculiar
species of starch, commonly but improperly called arrow root. It is reckoned more
nourishing than the starch of wheat or potatoes, and is generally also freer from peculiar
taste or flavour. The fresh root consists, according to Benzon, of 0·07 of volatile oil;
26 of starch (23 of which are obtained in the form of powder, while the other 3 must
be extracted from the parenchyma in a paste by boiling water); 1·58 of vegetable albumen;
0·6 of a gummy extract; 0·25 of chloride of calcium; 6 of insoluble fibrine; and
65·6 of water.
The import duty upon arrow root from our own colonies, is 1s. per cwt.; from foreign
parts, 2d. per lib. In 1835, 987,966 lbs. were imported, of which only 6267 were
exported; leaving 895,406 for home consumption. The total revenue derived that year
from arrow root, was 518l. See Starch.
ARSENIC. This metal occurs native, in the state of oxide, and also combined with
sulphur under the improper name of yellow and red arsenic, or orpiment and realgar.
Arsenic is associated with a great many metallic ores; but it is chiefly extracted from
those of cobalt, by roasting, in which case the white oxide of arsenic, or, more correctly,
the arsenious acid is obtained. This acid is introduced occasionally in small quantities[55]
into the materials of flint glass, either before their fusion, or in the melting pot. It
serves to peroxidize the iron oxide in the sand, and thereby to purify the body of the
glass; but an excess of it makes the glass milky.
Scheele’s green is a combination of this arsenious acid with oxide of copper, or an
arsenite of copper, and is described under this metal.
Arseniate of potash is prepared, in the small way, by exposing to a moderate heat in a
crucible, a mixture of equal parts of white arsenic and nitre in powder. After fusion,
the crucible is to be cooled; the contents being dissolved in hot water, and the solution
filtered, will afford regular crystals on cooling. According to M. Berzelius, they are
composed of arsenic acid, 63·87; potash, 26·16; and water, 9·97. It is an acidulous
salt, and is hence usually called the binarseniate, to denote that its composition is 2
atoms of arsenic acid, and 1 of potash. This article is prepared upon the great scale, in
Saxony, by melting nitre and arsenious acid together in a cylinder of cast-iron. A
neutral arseniate also is readily formed, by saturating the excess of acid in the above salt
with potash; it does not crystallize. The acid arseniate is occasionally used in calico
printing, for preventing certain points of the cotton cloth from taking on the mordant;
with which view it is mixed up with gum water and pipe clay into a paste, which is
applied to such places with a block.
The extraction of arsenic from the cobalt ores, is performed at Altenberg and Reichenstein,
in Silesia, with an apparatus, excellently contrived to protect the health of the
smelters from the vapours of this most noxious metallic sublimate.
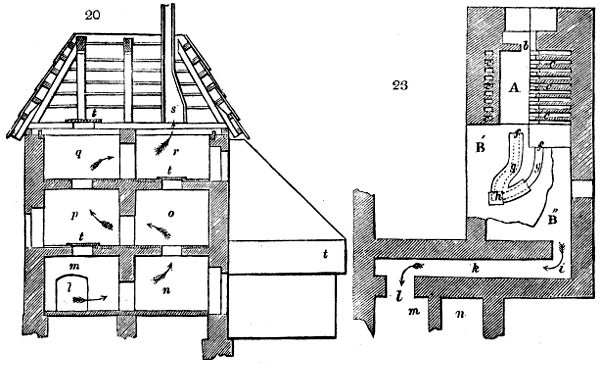
Figs. 20. to 23. represent the arsenical furnaces at Altenberg. Fig. 20. is a vertical
section of the poison tower; fig. 21., a longitudinal section of the subliming furnace A,
with the adjoining vault B, and the poison tower in part at n; fig. 22., the transverse
section of the furnace A, of fig. 21.; fig. 23., ground plan of the furnace A, where the
left half shows the part above, and the right the part below the muffle or oblong
retort; B′ is the upper view, B′′ the ground plan of the vault B, of fig. 21.; m, n, the
base of the poison tower. In the several figures the same letters denote the same objects:
a is the muffle; b is its mouth for turning over the arsenical schlich, or ground ore;
c c c, fire draughts or flues; d, an aperture for charging the muffle with fresh schlich;
e, the smoke chimney; f, two channels or flues for the ascent of the arsenious fumes,
which proceed to other two flues g, and then terminate both in h, which conducts the
fumes into the vault B. They issue by the door i, into the conduit k, thence by l into the
spaces m, n, o, p, q, r, of the tower. The incondensable gases escape by the chimney, s.[56]
The cover t, is removed after completion of the process, in order to push down the
precipitate into the lower compartments.
The arsenious schlichs, to the amount of 9 or 10 cwt. for one operation (1 roast-post,
or roasting round), are spread 2 or 3 inches thick upon the bottom of the muffle, heated
with a brisk fire to redness, then with a gentler heat, in order to oxidize completely,
before subliming, the arsenical ore. With this view the air must have free entrance, and
the front aperture of the muffle must be left quite open. After 11 or 12 hours, the
calcined materials are raked out by the mouth of the muffle, and fresh ones are introduced
by the openings indicated above, which are closed during the sublimation.
The arsenious acid found in these passages, is not marketable till it be re-sublimed
in large iron pots, surmounted with a series of sheet iron drums or cast-iron cylinders,
upon the sides of which the arsenic is condensed in its compact glassy form. The top
cylinder is furnished with a pipe, which terminates in a condensing chamber.
Figs. 24, 25. represent the arsenic refining furnaces at Reichenstein. Fig. 24. shows
at A, a vertical section of the furnace,
the kettle, and the surmounting drums
or cylinders; over B it is seen in elevation;
fig. 25. is a ground plan of the
four fireplaces. a is the grate; b, the
ash pit; c, the openings for firing; d,
the fire-place; e, iron pots or kettles
which are charged with the arsenious
powder; f, the fire flues proceeding to
the common chimney g; h, iron cylinders;
i, caps; k, pipes leading to
the poison vent l; m, openings in the
pipes for introducing the probing
wires.
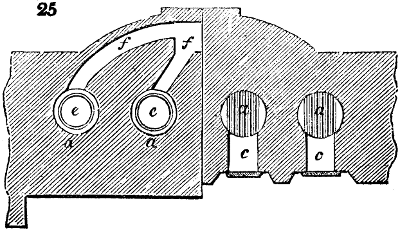
The conduct of the process is as
follows:—The pot is filled nearly to
its brim with 31⁄2 cwt. of the arsenic
meal, the cylinders are fitted on by
means of their handles, and luted together
with a mixture of loam, blood,
and hair; then is applied first a gentle,
and after half an hour, a strong fire,
whereby the arsenic is raised partly
in the form of a white dust, and partly
in crystals; which, by the continuance
of the heat, fuse together into a homogeneous
mass. If the fire be too feeble,
only a sublimate is obtained; but,
if too violent, much of the arsenic is
volatilized into the pipes. The workmen
judge by the heat of the cylinders
whether the operation be going
on well or not. After 12 hours the
furnace is allowed to cool, provided
the probe wires show that the sublimation
is over. The cylinders are
then lifted off, and the arsenious glass
is detached from their inner surface.
According to the quality of the poison-flour,
it yields from 3⁄4 to 7⁄8 of its weight
of the glass or enamel. Should any dark particles of metallic arsenic be intermixed
with the glass, a fresh sublimation must be had recourse to.
The following is the product in cwts. of arsenious acid, at Altenberg and Reichenstein,
in Silesia, in the years
| |
1825. |
1826. |
1827. |
1828. |
1829. |
1830. |
1831. |
1832. |
| White arsenic in a glassy state |
2632 |
1703 |
2686 |
1900 |
2070 |
2961 |
3337 |
2730 |
| Sublimed arsenic in powder |
- |
27 |
33 |
31 |
30 |
44 |
69 |
38 |
| Yellow arsenical glass |
112 |
11 |
56 |
- |
86 |
313 |
60 |
219 |
| Red arsenical glass |
3 |
- |
- |
- |
28 |
|
|
|
[57]
ARTESIAN WELLS. Under this name is designated a cylindrical perforation,
bored vertically down through one or more of the geological strata of the earth, till it
passes into a porous gravel bed containing water, placed under such incumbent pressure
as to make it mount up through the perforation, either to the surface or to a height
convenient for the operation of a pump. In the first case, these wells are called spouting
or overflowing. This property is not directly proportional to the depth, as might
at first sight be supposed, but to the subjacent pressure upon the water. We do not
know exactly the period at which the borer or sound was applied to the investigation of
subterranean fountains, but we believe the first overflowing wells were made in the
ancient French province of Artois, whence the name of Artesian. These wells, of such
importance to agriculture and manufactures, and which cost nothing to keep them in
condition, have been in use, undoubtedly, for several centuries in the northern departments
of France, and the north of Italy; but it is not more than 50 or 60 years since they
became known in England and Germany. There are now a great many such wells in
London and its neighbourhood, perforated through the immensely thick bed of the
London clay, and even through some portions of the subjacent chalk. The boring of
such wells has given much insight into the geological structure of many districts.
The formation of artesian wells depends on two things, essentially distinct from each
other: 1. On an acquaintance with the physical constitution, or nature, of the mineral
structure of each particular country; and, 2. On the skilful direction of the processes by
which we can reach the water level, and of those by which we can promote its ascent in
the tube. We shall first treat of the best method of making the well, and then offer
some general remarks on the other subjects.
The operations employed for penetrating the soil are entirely similar to those daily
practised by the miner, in boring to find metallic veins; but the well excavator must
resort to peculiar expedients to prevent the purer water, which comes from deep strata,
mingling with the cruder waters of the alluvial beds near the surface of the ground, as
also to prevent the small perforation getting eventually filled with rubbish.
The cause of overflowing wells has been ascribed to a variety of circumstances.
But, as it is now generally admitted that the numerous springs which issue from the
ground proceed from the infiltration of the waters progressively condensed in rain, dew,
snow, &c. upon the surface of our globe, the theory of these interior streamlets becomes
by no means intricate; being analogous to that of syphons and water jets, as expounded
in the treatises of physics. The waters are diffused, after condensation, upon the surface
of the soil, and percolate downwards, through the various pores and fissures of the
geological strata, to be again united subterraneously in veins, rills, streamlets, or expanded
films, of greater or less magnitude, or regularity. The beds traversed by
numerous disjunctions will give occasion to numerous interior currents in all directions,
which cannot be recovered, and brought to the day; but when the ground is composed
of strata of sand, or gravel very permeable to water, separated by other strata nearly
impervious to it, reservoirs are formed to our hand, from which an abundant supply of
water may be spontaneously raised. In this case, as soon as the upper stratum is
perforated, the waters may rise, in consequence of the hydrostatic pressure upon the
lower strata, and even overflow the surface in a constant stream, provided the level from
which they proceed be proportionally higher.
The sheets of water occur principally at the separation of two contiguous formations;
and, if the succession of the geological strata be considered, this distribution of the water
will be seen to be its necessary consequence. In fact, the lower beds are frequently
composed of compact sandstone or limestone, and the upper beds of clay. In level
countries, the formations being almost always in horizontal-beds, the waters which feed
the artesian wells must come from districts somewhat remote, where the strata are more
elevated, as towards the secondary and transition rocks. The copious streams condensed
upon the sides of these colder lands may be therefore regarded as the proper reservoirs
of our wells.
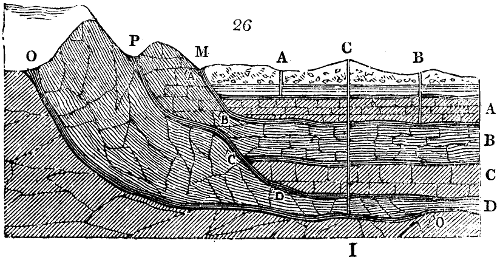
Fig. 26. represents the manner in which the condensed water of the heavens distributes
itself under the
surface of our globe.
Here we have a geological
section, showing
the succession of the
several formations, and
the sheets or laminæ of
water that exist at their
boundaries, as well as in
their sandy beds. The
figure shows also very
plainly that the height[58]
to which the water reascends in the bore of a well depends upon the height of the
reservoir which supplies the sheet of water to which the well is perforated. Thus the
well A, having gone down to the aqueous expanse A A, whose waters of supply are derived
from the percolation M, will afford rising waters, which will come to the surface;
whilst in the well B, supplied by the sheet P, the waters will spout above the surface,
and in the well C they will remain short of it. The same figure shows that these wells
often traverse sheets of water, which rise to different heights. Thus, in the well C
there are five columns of ascending waters, which rise to heights proportional to the
points whence they take their origin. Several of these will be spouting or overflowing,
but some will remain beneath the surface.
The situation of the intended well being determined upon, a circular hole is generally
dug in the ground, about 6 or 8 feet deep, and 5 or 6 feet wide. In the centre of this
hole the boring is carried on by two workmen below, assisted by a labourer above, as
shown in fig. 27.
The handle (fig. 28.) having a female screw in the bottom of its iron shank, with a
wooden bar or rail passing through the socket of the shank, and a ring at top, is the
general agent to which all the boring implements are to be attached. A chisel
(fig. 29.) is first employed, and connected
to this handle by its screw at top. If the
ground is tolerably soft, the weight of the
two workmen bearing upon the cross bar, and
occasionally forcing it round, will soon cause
the chisel to penetrate; but if the ground is
hard or strong, the workmen strike the chisel
down with repeated blows, so as to peck their
way, often changing their situation by walking
round, which breaks the stones, or other
hard substances, that may happen to obstruct
its progress.
The labour is very considerably reduced,
by means of an elastic wooden pole, placed
horizontally over the well, from which a chain is brought down, and attached to the ring
of the handle. This pole is usually made fast at one end, as a fulcrum, by being set
into a heap of heavy loose stones; at the other end the labourer above gives it a slight
up and down vibrating motion, corresponding to the beating motion of the workmen
below, by which means the elasticity of the pole in rising lifts the handle and pecker,
and thereby very considerably diminishes the labour of the workmen. See fig. 27.
When the hole has been thus opened by a chisel, as far as its strength would permit,
the chisel is withdrawn, and a sort of cylindrical auger (fig. 30.) attached to the handle
(fig. 28.), for the purpose of drawing up the dirt or broken stones which have been disturbed
by the chisel. A section of this auger is shown in fig. 31., by which the internal
valve will be seen. The auger being introduced into the hole, and turned round by the
workman, the dirt or broken stones will pass through the aperture at bottom (shown at[59]
fig. 32.), and fill the cylinder, which is then drawn up, and discharged at the top of
the auger, the valve preventing its escape at bottom.
In order to penetrate deeper into the ground, an iron rod, as a, fig. 33., is now to
be attached to the chisel, fig. 29., by screwing on to its upper end, and the rod is also
fastened to the handle, fig. 28., by screwing into its socket. The chisel having thus
become lengthened, by the addition of the rod, it is again introduced into the hole;
and the operation of pecking or forcing it down, is carried on by the workmen as before.
When the ground has been thus perforated, as far as the chisel and its rod will
reach, they must be withdrawn, in order again to introduce the auger, fig. 30., to collect
and bring up the rubbish; which is done by attaching it to the iron rod, in place
of the chisel. Thus as the hole becomes deepened, other lengths of iron rods are added,
by connecting them together, as a b are in fig. 34. The necessity of frequently withdrawing
the rods from the holes, in order to collect the mud, stones, or rubbish, and
the great friction produced by the rubbing of the tools against its sides, as well as the
lengths of rods augmenting in the progress of the operation, sometimes to the extent of
several hundred feet, render it extremely inconvenient, if not impossible, to raise them
by hand. A tripedal standard is, therefore, generally constructed by three scaffolding
poles tied together, over the hole, as shown fig. 27., from the centre of which a wheel and
axle, or a pair of pully blocks is suspended, for the purpose of hauling up the rods,
and from which hangs the fork, fig. 35. This fork is to be brought down under the shoulder,
near the top of each rod, and made fast to it by passing a pin through two little
holes in the claws. The rods are thus drawn up, about seven feet at a time, which is
the usual distance between each joint, and at every haul a fork, fig. 36., is laid horizontally
over the hole, with the shoulders of the lower rod resting between its claws,
by which means the rods are prevented from sinking down into the hole again, while
the upper length is unscrewed and removed. In attaching and detaching these lengths
of rod, a wrench, fig. 37., is employed, by which they are turned round, and the screws
forced up to their firm bearing.
The boring is sometimes performed for the first sixty or a hundred feet, by a chisel
of 21⁄2 inches wide, and cleared out by a gouge of 21⁄4 diameter, and then the hole is
widened by a tool, such as is shown at fig. 38. This is merely a chisel, as fig. 29., four
inches wide, but with a guide, a, put on at its lower part, for the purpose of keeping
it in a perpendicular direction; the lower part is not intended to peck, but to pass
down the hole previously made, while the sides of the chisel operate in enlarging the
hole to four inches. The process, however, is generally performed at one operation, by
a chisel of four inches wide, as fig. 29., and a gouge of three inches and three quarters, as
fig. 30.
It is obvious, that placing and displacing the lengths of rod, which is done every time
that the auger is required to be introduced or withdrawn, must, of itself, be extremely
troublesome, independent of the labour of boring, but yet the operation proceeds, when
no unpropitious circumstances attend it, with a facility almost incredible. Sometimes,
however, rocks intercept the way, which require great labour to penetrate; but this is
always effected by pecking, which slowly pulverises the stone. The most unpleasant
circumstance attendant upon this business is the occasional breaking of a rod into the
hole, which sometimes creates a delay of many days, and an incalculable labour in
drawing up the lower portion.
When the water is obtained in such quantities and of such quality as may be required,
the hole is dressed or finished by passing down it a diamond chisel, funnel mouthed,
with a triangular bit in its centre; this makes the sides smooth previous to putting in
the pipe. This chisel is attached to rods, and to the handle, as before described; and,
in its descent, the workmen continually walk round, by which the hole is made smooth
and cylindrical. In the progress of the boring, frequent veins of water are passed
through; but, as these are small streams, and perhaps impregnated with mineral substances,
the operation is carried on until an aperture is made into a main spring, which
will flow up to the surface of the earth. This must, of course, depend upon the level of
its source, which, if in a neighbouring hill, will frequently cause the water to rise up,
and produce a continued fountain. But if the altitude of the distant spring happens to
be below the level of the surface of the ground where the boring is effected, it sometimes
happens that a well of considerable capacity is obliged to be dug down to that level, in
order to form a reservoir, into which the water may flow, and whence it must be raised
by a pump; while, in the former instance, a perpetual fountain may be obtained.
Hence, it will always be a matter of doubt, in level countries, whether water can be
procured, which would flow near to or over the surface; if this cannot be effected, the
process of boring will be of little or no advantage, except as an experiment to ascertain
the fact.
In order to keep the strata pure, and uncontaminated with mineral springs, the hole
is cased, for a considerable depth, with a metallic pipe, about a quarter of an inch[60]
smaller than the bore. This is generally made of tin (though sometimes of copper or
lead) in convenient lengths; and, as each length is let down, it is held by a shoulder
resting in a fork, while another length is soldered to it; by which means a continuous
pipe is carried through the bore, as far as may be found necessary, to exclude land
springs, and to prevent loose earth or sand from falling in, and choking the aperture.
Mr. John Good, of Tottenham, who had been extensively employed in boring the
earth for water, obtained a patent, in Aug. 1823, for certain improved implements contrived
by him to facilitate his useful labours; a description of which cannot fail to be
interesting.
The figures annexed exhibit these ingenious tools; fig. 39. is an auger, to be connected
by the screw-head to the length of rods by which the boring is carried
on. This auger is for boring in soft clay or sand; it is cylindrical,
and has a slit or opening from end to end, and a bit, or
cutting-piece at bottom. When the earth is loose or wet, an auger
of the same form is to be employed, but the slit or opening reduced
in width, or even without a slit or opening. A similar auger is
used for cutting through chalk; but the point or bit at bottom
should then project lower, and, for that purpose, some of these
cylindrical augers are made with moveable bits, to be attached by
screws, which is extremely desirable in grinding them to cutting
edges. Fig. 40. is a hollow conical auger, for boring loose sandy
soils; it has a spiral cutting edge coiled round it, which, as it
turns, causes the loose soil to ascend up the inclined plane, and
deposit itself in the hollow within. Fig. 41. is a hollow cylinder
or tube, shown in section, with a foot-valve, and a bucket to be
raised by a rod and cord attached at the top; this is a pumping
tool, for the purpose of getting up water and sand that would not
rise by the auger. When this cylinder is lowered to the bottom of the bore, the bucket
is lifted up by the rod and cord, and descends again by its own gravity, having a valve
in the bucket, opening upwards, like other lift pumps; which, at every stroke, raises a
quantity of water and sand in the cylinder equal to the stroke; the ascent and descent of
the bucket being limited by a guide-piece at the top of the cylinder, and two small knobs
upon the rod, which stop against the cross-guide. Fig. 42. is a tool for getting up broken
rods. It consists of a small cylindrical piece at bottom, which the broken rod slips through
when it is lowered, and a small catch with a knife-edge, acted upon by a back-spring.
In rising, the tool takes hold of the broken rod, and thereby enables the workmen at
top to draw it up. Another tool for the same purpose, is shown at fig. 43., which is like
a pair of tongs; it is intended to be slidden down the bore, and for the broken rod to
pass between the two catches, which, pressed by back-springs, will, when drawn up, take
fast hold of the broken rod.
Fig. 44. is a tool for widening the hole, to be connected, like all the others, to the end
of the length of rods passed
down the bore; this tool has
two cutting-pieces extending on
the sides at bottom, by which,
as the tool is turned round in
the bore, the earth is peeled
away. Fig. 45. is a chisel, or
punch, with a projecting piece
to be used for penetrating
through stone; this chisel is,
by rising and falling, made to
peck the stone, and pulverize
it; the small middle part breaking
it away first, and afterwards
the broad part coming into action.
Fig. 46. is another chisel,
or punching tool, twisted on its
cutting edge, which breaks away
a greater portion of the stone as it beats against it.
The manner of forcing down lengths of cast-iron pipe, after the bore is formed, is
shown at fig. 47.; the pipe is seen below in the socket, at the end of which a block is
inserted; and from this block a rod extends upwards, upon which a weight at top
slides. To this weight cords are shown to be attached, reaching to the top of the bore;
where the workmen alternately raise the weight and let it fall, which, by striking upon
the block in its middle, beats down the pipe by a succession of strokes; and when one
length of pipe has, by these means, been forced down, another length is introduced into[61]
the socket of the former. Another tool for the same purpose is shown at fig. 48., which
is formed like an acorn; the raised part of the acorn strikes against the edge of the
pipe, and by that means, it is forced down the bore. When it happens that an auger
breaks in the hole, a tool similar to that shown at fig. 49. is introduced; on one side of
this tool a curved piece is attached, for the purpose of a guide, to conduct it past the
cylindrical auger; and at the end of the other side is a hook, which, taking hold of the
bottom edge of the auger, enables it to be drawn up.
Wrought iron, copper, tin, and lead pipes, are occasionally used for lining the bore;
and as these are subject to bends and bruises, it is necessary to introduce tools for the
purpose of straightening their sides. One of these tools is shown at fig. 50., which is a
bow, and is to be passed down the inside of the pipe, in order to press out any dents.
Another tool, for the same purpose, is shown at fig. 51., which is a double bow, and
may be turned round in the pipe for the purpose of straightening it all the way down;
at fig. 52., is a pair of clams, for turning the pipe round in the hole while driving.
When loose stones lie at the bottom of the hole, which are too large to be brought up
by the cylindrical auger, and cannot be conveniently broken, then it is proposed to
introduce a triangular claw, as fig. 53., the internal notches of which take hold of the
stone, and as the tool rises, bring it up. For raising broken rods, a tool like fig. 54.
is sometimes employed, which has an angular claw that slips under the shoulder of the
rod, and holds it fast while drawing up.
In raising pipes, it is necessary to introduce a tool into the inside of the pipe, by
which it will be held fast. Fig. 55. is a pine-apple tool for this purpose; its surface is
cut like a rasp, which passes easily down into the pipe, but catches as it is drawn up;
and by that means brings the pipe with it. Fig. 56. is a spear for the same purpose,
which easily enters the pipe by springing; at the ends of its prongs there are forks
which stick into the metal as it is drawn up, and thereby raise it.
These are the new implements, for which the patent was granted. In the process of
boring, there does not appear to be any thing new proposed; but that these several
tools are to be employed for boring, packing, and otherwise penetrating, raising the
earth, and extracting broken or injured tools. There are also suggestions for employing
long buckets, with valves opening upward in their bottoms, for the purpose of drawing
water from these wells when the water will not flow over the surface; also lift pumps,
with a succession of buckets for the same purpose. But as these suggestions possess
little if any novelty, it cannot be intended to claim them as parts of the patent.
ASPHALTUM. Native bitumen, so called from the lake Asphaltites.
ASSAY and ASSAYING. (Coupellation, Fr.; Abtreiben auf der capelle, Germ.)
This is the process by which the quality of gold and silver bullion, coin, plate, or
trinkets is ascertained with precision, or by which the quantity of either or both these
precious metals is determined in any given alloy. It is, therefore, a case of chemical
analysis, in which peculiar methods are employed to attain the object in view with
accuracy and dispatch. Assaying has been also extended of late years, to determine the
quantity of palladium and platina in certain bullion and gold dust brought from Brazil.
The art of assaying gold and silver by the cupel, is founded upon the feeble affinity
which these metals have for oxygen, in comparison with copper, tin, and the other
cheaper metals; and on the tendency which the latter metals have to oxidize rapidly in
contact with lead at a high temperature, and sink with it into any porous earthy vessel
in a thin glassy or vitriform state. The porous vessel may be made either of wood-ashes,
freed from their soluble matter by washing with water; or, preferably, of burned
bones reduced to a fine powder.
The lead added to the silver or gold to be assayed, serves chiefly to dissolve the
oxidized copper, whence it appears that the quantity of lead requisite for silver assays,
ought to be directly proportional to the quantity which the silver and copper would
separately require. It has been found by experiment, that 16 parts of lead are quite
sufficient to pass 1 of copper through the cupel; and that 3⁄10 of lead presents the most
suitable proportion for passing one of silver. From these principles, however, if we
should always regard the dose of lead to be employed for any alloy as being equal to
(16 × C) + (3⁄30 × S) we should certainly commit an error. The phenomena of cupellation
is of a more complex nature. Long practice and delicate trials alone can guide to the
proper quantity of lead to be employed for every various state of the alloy. The
following Table contains the results of M. D’Arcet’s elaborate experiments upon this
subject:—
[62]
| Alloy. |
Lead for 1
of Alloy. |
Ratio of the
Copper to
the Lead. |
| Silver. |
Copper. |
| 1000 |
0 |
3⁄10 |
0 |
| 950 |
50 |
3 |
1 : 60 |
| 900 |
100 |
7 |
1 : 70 |
| 800 |
200 |
10 |
1 : 50 |
| 700 |
300 |
12 |
1 : 40 |
| 600 |
400 |
14 |
1 : 35 |
| 500 |
500 |
16 or 17 |
1 : 32 |
| 400 |
600 |
16 — 17 |
1 : 26·7 |
| 300 |
700 |
16 — 17 |
1 : 22·9 |
| 200 |
800 |
16 — 17 |
1 : 20 |
| 100 |
900 |
16 — 17 |
1 : 17·8 |
| 0 |
1000 |
16 — 17 |
1 : 16 |
Bismuth may be used as a substitute for lead in cupellation; two parts of it being
nearly equivalent to three of lead. But its higher prices will prevent its general
introduction among assay masters.
We begin this assay process by weighing, in a delicate balance, a certain weight of
the metallic alloy; a gramme (= 15·444 gr.) is usually taken in France, and 12 grains
in this country. This weight is wrapped up in a slip of lead foil or paper, should it
consist of several fragments. This small parcel, thus enveloped, is then laid in a watch
glass or a capsule of copper, and there is added to it the proportion of lead suited to
the quality of alloy to be assayed; there being less lead, the finer the silver is presumed
to be. Those who are much in the habit of cupellation can make good guesses in this
way; though it is still guess work, and often leads to considerable error, for if too
much lead be used for the proportion of baser metal present, a portion of the silver
is wasted; but if too little, then the whole of the copper, &c. is not carried off, and
the button of fine silver remains more or less impure. The most expert and experienced
assayer by the cupel, produces merely a series of approximate conjectural results, which
fall short of chemical demonstration and certainty in every instance. The lead must be,
in all cases, entirely free from silver, being such as has been revived from pure litharge;
otherwise errors of the most serious kind would be occasioned in the assays.
The best cupels weigh 121⁄2 grammes, or 193 grains. The cupels allow the fused
oxides to flow through them as through a fine sieve, but are impermeable to the
particles of metals; and thus the former pass readily down into their substance while
the latter remain upon their surface; a phenomenon owing to the circumstance of the
glassy oxides moistening, as it were, the bone-ash powder, whereas the metals can
contract no adherence with it. Hence also the liquid metals preserve a hemispherical
shape in the cupels, as quicksilver does in a cup of glass, while the fused oxide spreads
over, and penetrates their substance, like water. A cupel may be regarded, in some
measure, as a filter permeable only to certain liquids.
If we put into a cupel, therefore, two metals, of which the one is unalterable in the
air, the other susceptible of oxidizement, and of producing a very fusible oxide, it is
obvious that, by exposing both to a proper degree of heat, we shall succeed in separating
them. We should also succeed, though the oxide were infusible, by placing it in contact
with another one, which may render it fusible. In both cases, however, the metal from
which we wish to part the oxides must not be volatile; it
should also melt, and form a button at the heat of cupellation;
for otherwise it would continue disseminated, attached
to the portion of oxide spread over the cupel, and
incapable of being collected.
The furnace and implements used for assaying in the
Royal Mint and the Goldsmiths’ Hall, in the city of London,
are the following:—
A A A A, fig. 58., is a front elevation of an assay furnace;
a a, a view of one of the two iron rollers on which the furnace
rests, and by means of which it is moved forward or
backward; b, the ash-pit; c c are the ash-pit dampers,
which are moved in a horizontal direction towards each
other for regulating the draught of the furnace; d, the
door, or opening, by which the cupels and assays are introduced
into the muffle; e, a moveable funnel or chimney
by which the draught of the furnace is increased.[63]
B B B B, fig. 59., is a perpendicular section of fig. 58.; a a, end view of the rollers;
b the ash-pit; c one of the ash-pit dampers; d the
grate, over which is the plate upon which the
muffle rests, and which is covered with loam nearly
one inch thick; f the muffle in section representing
the situation of the cupels; g the mouth-plate,
and upon it are laid pieces of charcoal, which during
the process are ignited, and heat the air that is
allowed to pass over the cupels, as will be more
fully explained in the sequel; h the interior of the
furnace, exhibiting the fuel.
The total height of the furnace is 2 feet 61⁄2 inches;
from the bottom to the grate, 6 inches; the grate,
muffle, plate, and bed of loam, with which it is
covered, 3 inches; from the upper surface of the
grate to the commencement of the funnel e, fig. 58.,
211⁄2 inches; the funnel e, 6 inches. The square
of the furnace which receives the muffle and fuel
is 113⁄4 inches by 15 inches. The external sides of the furnace are made of plates of
wrought iron, and are lined with a 2-inch fire-brick.
C C C C, fig. 60., is a horizontal section of the furnace over the grate, showing the width
of the mouth-piece, or plate
of wrought iron, which is
6 inches, and the opening which
receives the muffle-plate.
Fig. 61. represents the muffle
or pot, which is 12 inches
long, 6 inches broad inside;
in the clear 63⁄4: in height 41⁄2
inside measure, and nearly 51⁄2
in the clear.
Fig. 62., the muffle-plate, which is of the same size as the bottom of the muffle.
Fig. 63. is a representation of the sliding-door of the mouth-plate, as shewn at d,
in fig. 58.
Fig. 64., a front view of the mouth-plate or piece, d, fig. 58.
Fig. 65., a representation of the mode of making, or shutting up with pieces of
charcoal, the mouth of the furnace.
Fig. 66., the teaser for cleaning the grate.
Fig. 67., a larger teaser, which is introduced at the top of the furnace, for keeping a
complete supply of charcoal around the muffle.
Fig. 68., the tongs used for charging the assays into the cups.
Fig. 69. represents a board of wood used as a register, and is divided into 45 equal
compartments, upon which the assays are placed previously to their being introduced
into the furnace. When the operation is performed, the cupels are placed in the furnace
in situations corresponding to these assays on the board. By these means all confusion
is avoided, and without this regularity it would be impossible to preserve the accuracy
which the delicate operations of the assayer require.
I shall now proceed to a description of a small assay furnace, invented by Messrs.
Anfrye and d’Arcet, of Paris. They term it, Le Petit Fourneau à Coupelle. Fig. 70.
represents this furnace, and it is composed of a chimney or pipe of wrought iron a, and
of the furnace B. It is 171⁄2 inches high, and 71⁄4 inches wide. The furnace is formed
of three pieces; of a dome A; the body of the furnace B; and the ash-pit C, which is[64]
used as the base of the furnace, fig. 70. and 71. The principal piece, or body of the
furnace, B, has the form of a hollow tower, or of a hollow cylinder, flattened equally at
the two opposite sides parallel to the axis, in such a manner that the horizontal section
is elliptical. The foot which supports it is a hollow truncated cone, flattened in like
manner upon the two opposite sides, and having consequently for its basis two ellipses
of different diameters;
the smallest
ought to be equal to
that of the furnace,
so that the bottom of
the latter may exactly
fit it. The dome,
which forms an arch
above the furnace,
has also its base elliptical,
whilst that of
the superior orifice
by which the smoke
goes out preserves the
cylindrical form. The
tube of wrought iron
is 18 inches long and
21⁄2 inches diameter,
having one of its ends
a little enlarged, and
slightly conical, that
it may be exactly
fitted or jointed upon
the upper part of
the furnace dome d,
fig. 70. At the union
of the conical and
cylindrical parts of
the tube, there is
placed a small gallery
of iron, e, fig. 70,
71. See also a plan
of it, fig. 72. This
gallery is both ingenious
and useful.
Upon it are placed the cupels, which are thus annealed during the ordinary work of
the furnace, that they may be introduced into the muffle, when it is brought into its
proper degree of heat. A little above this gallery is a door f, by which, if thought
proper, the charcoal could be introduced into the furnace; above that there is placed at
g a throttle valve, which is used for regulating the draught of the furnace at pleasure.
Messrs. Anfrye and d’Arcet say, that, to give the furnace the necessary degree of heat
so as to work the assays of gold, the tube must be about 18 inches above the gallery,
for annealing or heating the cupels. The circular opening h, in the dome, fig. 70., and
as seen in the section, fig. 71., is used to introduce the charcoal into the furnace: it is
also used to inspect the interior of the furnace, and to arrange the charcoal round the
muffle. This opening is kept shut during the working of the furnace, with the mouth-piece,
of which the face is seen at n, fig. 71.
The section of the furnace, fig. 71., presents several openings, the principal of which is
that of the muffle; it is placed at i; it is shut with the semicircular door m, fig. 70., and
seen in the section m, fig. 71. In front of this opening, is the table or shelf, upon
which the door of the muffle is made to advance or recede; the letter q, fig. 71., shows
the face, side, and cross section of the shelf, which makes part of the furnace. Immediately
under the shelf, is a horizontal slit, l, which is pierced at the level of the
upper part of the grate, and used for the introduction of a slender rod of iron, that the
grate may be easily kept clean. This opening is shut at pleasure, by the wedge represented
at k, fig. 70. and 71.
Upon the back of the furnace is a horizontal slit p, fig. 71, which supports the fire-brick,
s, and upon which the end of the muffle, if necessary, may rest; u, fig. 71., is the
opening in the furnace where the muffle is placed.
The plan of the grate of the furnace is an ellipse: fig. 73. is a horizontal view of it.
The dimensions of that ellipsis determine the general form of the furnace, and thickness
of the grate. To give strength and solidity to the grate, it is encircled by a bar or hoop of[65]
iron. There is a groove in which the hoop of iron is fixed. The holes of the grate are
truncated cones, having the greater base below, that the ashes may more easily fall into
the ash-pit. The letter v, fig. 71., shows the form of these holes. The grate is supported
by a small bank or shelf, making part of the furnace, as seen at a, fig. 71.
The ash-pit, C, has an opening y in front, fig. 71.; and is shut when necessary by the
mouth-piece r, fig. 70. and 71.
To give strength and solidity to the furnace, it is bound with hoops of iron, at
b, b, b, b, fig. 70.
Figs. 74. 75. 76. are views of the muffle.
Fig. 77. is a view of a crucible for annealing gold.
Figs. 78. 79. 80. are cupels of various sizes, to be used in the furnace. They are the
same as those used by assayers in their ordinary furnaces.
Figs. 81. and 82. are views of the hand-shovels, used for filling the furnace with
charcoal; they should be made of such size and form as to fit the opening h, in
figs. 70. and 71.
The smaller pincers or tongs, by which the assays are charged into the cupels, and by
which the latter are withdrawn from the furnace, as well as the teaser for cleaning the
grate of the furnace, are similar to those used in the British Mint.
In the furnace of the Mint above described, the number of assays that can be made at
one time, is 45. The same number of cupels are put into the muffle. The furnace is
then filled with charcoal to the top, and upon this are laid a few pieces already ignited.
In the course of three hours, a little more or less, according to circumstances, the whole
is ignited; during which period, the muffle, which is made of fire-clay, is gradually
heated to redness, and is prevented from cracking; which a less regular or more sudden
increase of temperature would not fail to do: the cupels, also, become properly annealed.
All moisture being dispelled, they are in a fit state to receive the piece of
silver or gold to be assayed.
The greater care that is exercised in this operation, the less liable is the assayer to
accidents from the breaking of the muffle; which it is both expensive and troublesome to
fit properly into the furnace.
The cupels used in the assay process, are made of the ashes of burnt bones (phosphate
of lime). In the Royal Mint, the cores of ox-horn are selected for this purpose; and the
ashes produced are about four times the expense of the bone-ash, used in the process of
cupellation upon the large scale. So much depends upon the accuracy of an assay of
gold or silver, where a mass of 15lbs. troy in the first, and 60lbs. troy in the second
instance, is determined by the analysis of a portion not exceeding 20 troy grains, that
every precaution which the longest experience has suggested, is used to obtain an
accurate result. Hence the attention paid to the selection of the most proper materials
for making the cupels.
The cupels are formed in a circular mould made of cast steel, very nicely turned,
by which means they are easily freed from the mould when struck. The bone-ash is
used moistened with a quantity of water, sufficient to make the particles adhere firmly
together. The circular mould is filled, and pressed level with its surface; after which,
a pestle or rammer, having its end nicely turned, of a globular or convex shape, and of
a size equal to the degree of concavity wished to be made in the cupel for the reception
of the assay, is placed upon the ashes in the mould, and struck with a hammer until
the cupel is properly formed. These cupels are allowed to dry in the air for some time
before they are used. If the weather is fine, a fortnight will be sufficient.
An assay may prove defective for several reasons. Sometimes the button or bead
sends forth crystalline vegetations on its surface with such force, as to make one suppose
a portion of the silver may be thrown out of the cupel. When the surface of the bead
is dull and flat, the assay is considered to have been too hot, and it indicates a loss of
silver in fumes. When the tint of the bead is not uniform, when its inferior surface is
bubbly, when yellow scales of oxide of lead remain on the bottom of the cupel, and the
bead adheres strongly to it, by these signs it is judged that the assay has been too cold,
and that the silver retains some lead.
Lastly, the assay is thought to be good if the bead is of a round form, if its upper
surface is brilliant, if its lower surface is granular and of a dead white, and if it separates
readily from the cupel.
After the lead is put into the cupel, it gets immediately covered with a coat of oxide,
which resists the admission of the silver to be assayed into the melted metal; so that the
alloy cannot form. When a bit of silver is laid on a lead bath in this predicament, we
see it swim about for a long time without dissolving. In order to avoid this result, the
silver is wrapped up in a bit of paper; and the carburetted hydrogen generated by its
combustion, reduces the film of the lead oxide, gives the bath immediately a bright
metallic lustre, and enables the two metals readily to combine.
As the heat rises, the oxide of lead flows round about over the surface, till it is absorbed[66]
by the cupel. When the lead is wasted to a certain degree, a very thin film of
it only remains on the silver, which causes the iridescent appearance, like the colours of
soap-bubbles; a phenomenon, called by the old chemists, fulguration.
When the cupel cools in the progress of the assay, the oxygenation of the lead ceases;
and, instead of a very liquid vitreous oxide, an imperfectly melted oxide is formed, which
the cupel cannot absorb. To correct a cold assay, the temperature of the furnace ought
to be raised, and pieces of paper ought to be put into the cupel, till the oxide of lead
which adheres to it, be reduced. On keeping up the heat, the assay will resume its
ordinary train.
Pure silver almost always vegetates. Some traces of copper destroy this property,
which is obviously due to the oxygen which the silver can absorb while it is in fusion,
and which is disengaged the moment it solidifies. An excess of lead, by removing all
the copper at an early stage, tends to cause the vegetation.
The brightening is caused by the heat evolved, when the button passes from the
liquid to the solid state. Many other substances present the same phenomenon.
In the above operation it is necessary to employ lead which is very pure, or at least
free from silver. That kind is called poor lead.
It has been observed at all times, that the oxide of lead carries off with it, into the
cupel, a little silver in the state of an oxide. This effect becomes less, or even disappears,
when there is some copper remaining; and the more copper, the less chance
there is of any silver being lost. The loss of silver increases, on the other hand, with
the dose of lead. Hence the reason why it is so important to proportion the lead with
a precision which, at first sight, would appear to be superfluous. Hence, also, the
reason of the attempts which have, of late years, been made to change the whole system
of silver assays, and to have recourse to a method exempt from the above causes of error.
M. d’Arcet, charged by the Commission of the Mint in Paris, to examine into the
justice of the reclamations made by the French silversmiths against the public assays,
ascertained that they were well founded; and that the results of cupellation gave
for the alloys between 897 and 903 thousandths (the limits of their standard coin) an
inferior standard, by from 4 to 5 thousandth parts, from the standard or title which
should result from the absolute or actual alloy.
The mode of assay shows, in fact, that an ingot, experimentally composed of 900
thousandths of fine silver, and 100 thousandths of copper, appears, by cupellation, to be
only, at the utmost, 896 or 897 thousandths; whereas fine silver, of 1000 thousandths,
comes out nearly of its real standard. Consequently a director of the Mint, who should
compound his alloy with fine silver, would be obliged to employ 903 or 904 thousandths,
in order that, by the assay in the laboratory of the Mint, it should appear to have the
standard of 900 thousandths. These 3 or 4 thousandths would be lost to him, since
they would be disguised by the mode of assay, the definitive criterion of the quantity of
silver, of which the government keeps count from the coiner of the money.
From experiments subsequently made by M. d’Arcet, it appears that silver assays
always suffer a loss of the precious metal, which varies, however, with the standard of
the alloy. It is 1 thousandth for fine silver,
| 4·3 |
thousandths |
for |
silver |
of |
900 |
thousandths, |
| 4·9 |
— |
for |
— |
of |
800 |
— |
| 4·2 |
— |
for |
— |
of |
500 |
— |
and diminishes thereafter, progressively, till the alloy contains only 100 thousandths of
silver, at which point the loss is only 0·4.
Assays requested by the Commission of the Paris Mint, from the assayers of the
principal Royal Mints in Europe, to which the same alloys, synthetically compounded,
were sent, afforded the results inscribed in the following table.
| Names of the Assayers. |
Cities where
they reside. |
Standards found for
the Mathematical Alloys. |
| 950 mill. |
900 mill. |
800 mill. |
| F. de Castenhole, Mint Assayer |
Vienna |
946 |
·20 |
898 |
·40 |
795 |
·10 |
| A. R. Vervaëz, Ditto |
Madrid |
944 |
·40 |
893 |
·70 |
789 |
·20 |
| D. M. Cabrera, Assayer in Spain |
Ditto |
944 |
·40 |
893 |
·70 |
788 |
·60 |
| Assayer |
Amsterdam |
947 |
·00 |
895 |
·00 |
795 |
·00 |
| Mr. Bingley, Assay Master |
London |
946 |
·25 |
896 |
·25 |
794 |
·25 |
| Mr. Johnson, Assayer |
Ditto |
933 |
·33 |
883 |
·50 |
783 |
·33 |
| Inspector of the Mint |
Utrecht |
945 |
·00 |
896 |
·50 |
799 |
·00 |
| Assayer of the Mint |
Naples |
945 |
·00 |
891 |
·00 |
787 |
·00 |
| Assayer of Trade |
Ditto |
945 |
·00 |
891 |
·00 |
787 |
·00 |
| Assayer of the Mint |
Hamburgh |
946 |
·13⁄72 |
897 |
·41⁄72 |
798 |
·44⁄72 |
| Ditto |
Altona |
942 |
·1⁄4 |
894 |
·00 |
790 |
|
[67]
These results, as well as those in still greater numbers, obtained from the ablest
Parisian assayers, upon identical alloys of silver and copper, prove that the mode of
assay applied to them brings out the standard too low; and further, that the quantity
of silver masked or disguised, is not uniform for these different eminent assay masters.
An alloy, for example, at the standard of 900 thousandths is judged at
| |
M. |
| the Mint of |
Paris |
to have a standard of |
895·6 |
| At that of |
Vienna |
— |
898·4 |
| — |
Madrid |
— |
893·7 |
| — |
Naples |
— |
891·0 |
The fact thus so clearly made out of a loss in the standard of silver bullion and coin,
merits the most serious attention; and it will appear astonishing, perhaps, that a thing
recurring every day, should have remained for so long a time in the dark. In reality,
however, the fact is not new; as the very numerous and well-made experiments of
Tillet from 1760 to 1763, which are related in the memoirs of the Academy of
Sciences, show, in the silver assays, a loss still greater than that which was experienced
lately in the laboratory of the Commission of the French Mint. But he thought that,
as the error was common to the nations in general, it was not worth while or prudent
to introduce any innovation.
A mode of assaying, to give, with certainty, the standard of silver bullion, should be
entirely independent of the variable circumstances of temperature, and the unknown
proportions of copper, so difficult to regulate by the mere judgment of the senses. The
process by the humid way, recommended by me to the Royal Mint in 1829, and exhibited
as to its principles before the Right Honourable John Herries, then Master, in
1830, has all the precision and certainty we could wish. It is founded on the well-known
property which silver has, when dissolved in nitric acid, to be precipitated in a chloride of
silver quite insoluble, by a solution of sea salt, or by muriatic acid; but, instead of determining
the weight of the chloride of silver, which would be somewhat uncertain and
rather tedious, on account of the difficulty of drying it, we take the quantity of the
solution of sea salt which has been necessary for the precipitation of the silver. To
put the process in execution, a liquor is prepared, composed of water and sea salt in such
proportions that 1000 measures of this liquor may precipitate, completely, 12 grains of
silver, perfectly pure, or of the standard 1000, previously dissolved in nitric acid. The
liquor thus prepared, gives, immediately, the true standard of any alloy whatever, of
silver and copper, by the weight of it which may be necessary to precipitate 12 grains
of this alloy. If, for example 905 measures have been required to precipitate the 12
grains of alloy, its standard would be 905 thousandths.
The process by the humid way is, so to speak, independent of the operator. The
manipulations are so easy; and the term of the operation is very distinctly announced by
the absence of any sensible nebulosities on the affusion of sea salt into the silver solution,
while there remains in it 1⁄2 thousandth of metal. The process is not tedious, and in
experienced hands it may rival the cupel in rapidity; it has the advantage over the
cupel of being more within the reach of ordinary operators, and of not requiring a long
apprenticeship. It is particularly useful to such assayers as have only a few assays to
make daily, as it will cost them very little time and expense.
By agitating briskly during two minutes, or thereby, the liquid rendered milky by
the precipitation of the chloride of silver, it may be sufficiently clarified to enable us to
appreciate, after a few moments of repose, the disturbance that can be produced in it by
the addition of 1000 of a grain of silver. Filtration is more efficacious than agitation,
especially when it is employed afterwards; it may be sometimes used; but agitation,
which is much more prompt, is generally sufficient. The presence of lead and copper,
or any other metal, except mercury, has no perceptible influence on the quantity of sea
salt necessary to precipitate the silver; that is to say, the same quantity of silver, pure
or alloyed, requires for its precipitation a constant quantity of the solution of sea salt.
Supposing that we operate upon a gramme of pure silver, the solution of sea salt
ought to be such that 100 centimetres cube may precipitate exactly the whole silver.
The standard of an alloy is given by the number of thousandths of solution of sea salt
necessary to precipitate the silver contained in a gramme of the alloy.
When any mercury is accidentally present, which is, however, a rare occurrence, it
is made obvious by the precipitated chloride remaining white when exposed to daylight,
whereas when there is no mercury present, it becomes speedily first grey and then
purple. Silver so contaminated must be strongly ignited in fusion before being assayed,
and its loss of weight noted. In this case, a cupel assay must be had recourse to.
Preparation of the Normal Solution of Sea Salt, when it is measured by Weight.—Supposing
the sea salt pure as well as the water, we have only to take these two bodies in
the proportion of 0·5427 k. of salt to 99·4573 k. of water, to have 100 k. of solution,[68]
of which 100 grammes will precipitate exactly one gramme of silver. But instead of
pure salt, which is to be procured with difficulty, and which besides may be altered
readily by absorbing the humidity of the air, a concentrated solution of the sea salt of
commerce is to be preferred, of which a large quantity may be prepared at a time, to be
kept in reserve for use, as it is wanted. Instruction de Gay Lussac.
Preparation of the Normal Solution of Sea Salt, when measured by Volume.—The
measure by weight has the advantage of being independent of temperature, of having
the same degree of precision as the balance, and of standing in need of no correction.
The measure by volume has not all these advantages; but, by giving it sufficient
precision, it is more rapid, and is quite sufficient for the numerous daily assays of the
mint. This normal solution is so made, that a volume equal to that of 100 grammes
of water, or 100 centimetres cube, at a determinate temperature, may precipitate exactly
one gramme of silver. The solution may be kept at a constant temperature, and in
this case the assay stands in want of no correction; or if its temperature be variable,
the assay must be corrected according to its influence. These two circumstances make
no change in the principle of the process, but they are sufficiently important to occasion
some modifications in the apparatus. Experience has decided the preference in favour
of applying a correction to a variable temperature.
We readily obtain a volume of 100 cubic centimetres by means of a pipette, fig. 83.,
so gauged that when filled with water up to the
mark a, b, and well dried at its point, it will run
out, at a continuous efflux, 100 grammes of water
at the temperature of 15 C. (59 Fah.). We say
purposely at one efflux, because after the cessation
of the jet, the pipette may still furnish two or
three drops of liquid, which must not be counted
or reckoned upon. The weight of the volume of
the normal solution, taken in this manner with
suitable precautions, will be uniform from one
extreme to another, upon two centimetres and a
half, at most, or to a quarter of a thousandth, and
the difference from the mean will be obviously
twice less, or one half. Let us indicate the most
simple manner of taking a measure of the normal
solution of sea salt.
After having immersed the beak c of the pipette
in the solution, we apply suction by the mouth,
to the upper orifice, and thereby raise the liquid to
d above the circular line a b. We next apply
neatly the forefinger of one hand to this orifice,
remove the pipette from the liquid, and seize it
as represented in fig. 84. The mark a b being
placed at the level of the eye, we make the surface
of the solution become exactly a tangent to
the plane a b. At the instant it becomes a tangent, we leave the beak c of the pipette
open, by taking away the finger that had been applied to it, and without changing
any thing else in the position of the hands, we empty it into the bottle which should
receive the solution, taking care to remove it whenever the efflux has run out.
If after filling the pipette by suction, any one should find a difficulty in applying the
forefinger fast enough to the upper orifice, without letting the liquid run down below
the mark a b, he should remove the pipette from the solution with its top still closed
with his tongue, then apply the middle finger of one of his hands to the lower orifice;
after which he may withdraw his tongue, and apply the forefinger of the other hand to
the orifice previously wiped. This mode of obtaining a measure of normal solution of
sea salt is very simple, and requires no complex apparatus; but we shall indicate another
manipulation still easier, and also more exact.
In this new process the pipette is filled from the top like a bottle, instead of being
filled by suction, and it is moreover fixed. Fig. 85. represents the apparatus. D and
D′ are two sockets separated by a stop cock R. The upper one, tapped interiorly,
receives, by means of a cork stopper L, the tube T, which admits the solution of sea
salt. The lower socket is cemented on to the pipette; it bears a small air-cock R′, and
a screw plug V, which regulates a minute opening intended to let the air enter very
slowly into the pipette. Below the stop-cock R′, a silver tube N, of narrow diameter,
soldered to the socket, leads the solution into the pipette, by allowing the air, which it
displaces, to escape by the stop-cock R′. The screw plug, with the milled head V′,
replaces the ordinary screw by which the key of the stop-cock may be made to press,
with more or less force, upon its conical seat.
[69]
Fig. 86. represents, in a side view, the apparatus just described. We here remark
an air-cock R, and an opening m. At the extremity Q of the same figure, the conical
pipe T enters, with friction. It is by this pipe that the air is sucked into the pipette,
when it is to be filled from its beak.
The pipette is supported by two horizontal arms H K (fig. 87.) moveable about a
common axis A A, and capable of being drawn
out or shortened by the aid of two longitudinal
slits. They are fixed steadily by two
screw nuts e e′, and their distance may be varied
by means of round bits of wood or cork interposed,
or even by opposite screw nuts o o′. The
upper arm H is pierced with a hole, in which
is fixed, by the pressure of a wooden screw v,
the socket of the pipette. The corresponding
hole of the lower arm is larger; and the beak
of the pipette is supported in it by a cork stopper
L. The apparatus is fixed by its tail-piece
P, by means of a screw to the corner of a wall,
or any other prop.
The manner of filling the pipette is very simple.
We begin by applying the fore-finger of
the left hand to the lower aperture c; we then
open the two stop-cocks R and R′. Whenever
the liquor approaches the neck of the pipette, we
must temper its influx, and when it has arrived
at some millimetres above the mark a b, we close
the two stop-cocks, and remove our forefinger.
We have now nothing more to do than to
regulate the pipette; for which purpose the liquid
must touch the line a b, and must simply adhere
externally to the beak of the pipette.
This last circumstance is easily adjusted.
After taking away the finger which closed the aperture c of the pipette, we apply to this
orifice a moist sponge m, fig. 88., wrapped up in a linen rag, to absorb the superfluous
liquor as it drops out. This sponge
is called the handkerchief (mouchoir),
by M. Gay Lussac. The
pipette is said to be wiped when
there is no liquor adhering to its
point exteriorly.
For the convenience of operating,
the handkerchief is fixed by
friction in a tube of tin plate, terminated
by a cup, open at bottom
to let the droppings flow off into
the cistern C, to which the tube is
soldered. It may be easily removed
for the purpose of washing
it; and, if necessary, a little wedge
of wood, o, can raise it towards the pipette.
To complete the adjustment of the pipette, the liquid must be made merely to descend
to the mark a, b. With this view, and whilst the handkerchief is applied to the beak
of the pipette, the air must be allowed to enter very slowly by unscrewing the plug V,
fig. 85.; and at the moment of the contact the handkerchief must be removed, and the
bottle F, destined to receive the solution, must be placed below the orifice of the pipette,
fig. 88. As the motion must be made rapidly, and without hesitation, the bottle is
placed in a cylinder of tin-plate, of a diameter somewhat greater, and forming one body
with the cistern and the handkerchief. The whole of this apparatus has for a basis a
plate of tinned iron, moveable between two wooden rulers R R, one of which bears a
groove, under which the edge of the plate slips. Its traverses are fixed by two abutments
b b, placed so that when it is stopped by one of them, the beak of the pipette corresponds
to the centre of the neck of the bottle, or is a tangent to the handkerchief. This
arrangement, very convenient for wiping the pipette and emptying it, gives the apparatus
sufficient solidity, and allows of its being taken away, and replaced without deranging
any thing. It is obvious that it is of advantage, when once the entry of the air into
the pipette has been regulated by the screw V, to leave it constantly open, because the[70]
motion from the handkerchief to the bottle is performed with sufficient rapidity to
prevent a drop of the solution from collecting and falling down.
Temperature of the Solution.—After having described the manner of measuring by
volume the normal solution of the sea salt, we shall indicate the most convenient means
of taking the temperature. The thermometer is placed in a tube of glass T, fig. 89.,
which the solution traverses to arrive at the pipette. It is suspended in it by a piece of
cork, grooved on the four sides to afford passage to the liquid. The scale is engraved
upon the tube itself, and is repeated at the opposite side, to fix the eye by the coincidence
of this double division at the level of the thermometric column. The tube is joined
below to another narrower one, through which it is attached by means of a cork stopper
B, in the socket of the stop-cock of the pipette. At its upper part it is cemented into a
brass socket, screw-tapped in the inside, which is connected in its turn by a cock, with
the extremity, also tapped, of the tube above T, belonging to the reservoir of the normal
solution. The corks employed here as connecting links between the parts of the
apparatus, give them a certain flexibility, and allow of their being dismounted and remounted
in a very short time; but it is indispensable to make them be traversed by a
hollow tube of glass or metal, which will hinder them from being crushed by the pressure
they are exposed to. If the precaution be taken to grease them with a little suet and
to fill their pores, they will suffer no leakage.
Preservation of the Normal Solution of Sea Salt in metallic Vessels.—M. Gay Lussac
uses for this purpose a cylindrical vessel or drum of copper, of a capacity of about 110
litres, having its inside covered with a rosin and wax cement.
Preparation of the Normal Solution of Sea Salt, measuring it by Volume.—If the drum
contains 110 litres, we should put only 105 into it, in order that sufficient space may be
left for agitating the liquor without throwing it out. According to the principle that
100 centimetres cube, or 1⁄10 of a litre of the solution should contain enough of sea salt to
precipitate a gramme of pure silver; and, admitting moreover, 13·516 for the prime equivalent
of silver, and 7·335 for that of sea salt, we shall find the quantity of pure salt that
should be dissolved in the 105 litres of water, and which corresponds to 105 × 10 = 1050
grammes of silver, to be by the following proportion:—
13·516 : 7·335 ∷ 1050 gramm. : x = 569·83 gr.
And as the solution of the sea salt of commerce, formerly mentioned, contains approximately
250 grammes per kilogramme, we must take 2279·3 grammes of this
solution to have 569·83 gram. of salt. The mixture being perfectly made, the tubes
and the pipette must be several times washed by running the solution through them, and
putting it into the drum. The standard of the solution must be determined after it has
been well agitated, supposing the temperature to remain uniform.
To arrive more conveniently at this result, we begin by preparing two decimes solutions;
one of silver, and another of sea salt.
The decime solution of silver is obtained by dissolving 1 gramme of silver in nitric
acid, and diluting the solution with water till its volume become a litre.
The decime solution of sea salt may be obtained by dissolving 0·543 grammes of pure
sea salt in water, so that the solution shall occupy a litre; but we shall prepare it even
with the normal solution which we wish to test, by mixing a measure of it with 9
measures of water; it being understood that this solution is not rigorously equivalent to
that of silver, and that it will become so, only when the normal solution employed for
its preparation shall be finally of the true standard. Lastly, we prepare beforehand
several stoppered phials, in each of which we dissolve 1 gramme of silver in 8 or 10
grammes of nitric acid. For brevity’s sake we shall call these tests.
Now to investigate the standard of the normal solution, we must transfer a pipette of
it into one of these test phials; and we must agitate the liquors briskly to clarify them.
After some instants of repose, we must pour in 2 thousandths of the decime solution of
sea salt, which, we suppose, will produce a precipitate. The normal liquor is consequently
too feeble; and we should expect this, since the sea salt employed was not
perfectly pure. We agitate and add 2 fresh thousandths, which will also produce a precipitate.
We continue thus by successive additions of 2 thousandths, till the last produces
no precipitation. Suppose that we have added 16 thousandths: the last two should not
be reckoned, as they produced no precipitate; the preceding two were necessary, but
only in part; that is to say, the useful thousandths added are above 12 and below 14, or
otherwise they are on an average equal to 13.
Thus, in the condition of the normal solution, we require 1013 parts of it to precipitate
one gramme of silver, while we should require only 1000. We shall find the
quantity of concentrated solution of sea salt that we should add, by noting that the
quantity of solution of sea salt, at first employed, viz. 2279·3 grammes, produced a
standard of only 987 thousandths = 1000 - 13; and by using the following proportion:
987 : 2279·3 ∷ 13 : x = 30·02 grammes.
[71]
This quantity of the strong solution of salt, mixed with the normal solution in the
drum, will correct its standard, and we shall now see by how much.
After having washed the tubes and the pipette, with the new solution, we must
repeat the experiment upon a fresh gramme of silver. We shall find, for example, in
proceeding only by a thousandth at a time, that the first causes a precipitate, but not the
second. The standard of the solution is still too weak, and is comprised between 1000
and 1001; that is to say, it may be equal to 10001⁄2, but we must make a closer
approximation.
We pour into the test bottle 2 thousandths of the decime solution of silver, which
will destroy, perceptibly, two thousandths of sea salt, and the operation will have retrograded
by two thousandths; that is to say, it will be brought back to the point at
which it was first of all. If, after having cleared up the liquor, we add half a
thousandth of the decime solution, there will necessarily be a precipitate, as we knew
beforehand, but a second will cause no turbidity. The standard of the normal liquor
will be consequently comprehended between 1000 and 10001⁄2, or equal to 10001⁄4.
We should rest content with this standard, but if we wish to correct it, we may
remark that the two quantities of solution of salt added, viz. 2279·3 gr. + 30·02 gr. =
2309·32 gr. have produced only 999·75 thousandths, and that we must add a new
quantity of it corresponding to 1⁄4 of a thousandth. We make, therefore, the proportion
999·75 : 2309·32 ∷ 0·25 : x.
But since the first term differs very little from 1000, we may content ourselves to
have x by taking the 0·25⁄1000 of 2309·32, and we shall find 0·577 gr. for the quantity of
solution of sea salt to be added to the normal solution.
It is not convenient to take exactly so small a quantity of solution of sea salt by
the balance, but we shall succeed easily by the following process. We weigh 50
grammes of this solution, and we dilute it with water; so that it occupies exactly half
a litre, or 500 centimetres cube. A pipette of this solution, one centimetre cube in
volume, will give a decigramme of the primitive solution, and as such a small pipette is
divided into twenty drops, each drop, for example, will represent 5 milligrammes of the
solution. We should arrive at quantities smaller still by diluting the solution with a
proper quantity of water; but greater precision would be entirely needless.
The testing of the normal liquor just described, is, in reality, less tedious than might
be supposed. It deserves also to be remarked, that liquor has been prepared for more
than 1000 assays; and that, in preparing a fresh quantity, we shall obtain directly its
true standard, or nearly so, if we bear in mind the quantities of water and solution
of salt which had been employed.
Correction of the Standard of the Normal Solution of Sea Salt, when the Temperature changes.—We
have supposed, in determining the standard of the normal solution of sea salt,
that the temperature remained uniform. The assays made in such circumstances,
have no need of correction; but if the temperature should change, the same measure of
the solution will not contain the same quantity of sea salt. Supposing that we have
tested the solution of the salt at the temperature of 15° C.; if, at the time of making
the experiment, the temperature is 18° C., for example, the solution will be too weak
on account of its expansion, and the pipette will contain less of it by weight; if, on the
contrary, the temperature has fallen to 12°, the solution will be thereby concentrated
and will prove too strong. It is therefore proper to determine the correction necessary
to be made, for any variation of temperature.
To ascertain this point, the temperature of the solution of sea salt was made successively
to be 0°, 5°, 10°, 15°, 20°, 25°, and 30° C.; and three pipettes of the solution
were weighed exactly at each of these temperatures. The third of these weighings gave
the mean weight of a pipette. The corresponding weights of a pipette of the solution,
were afterwards graphically interpolated from degree to degree. These weights form
the second column of the following table, intitled, Table of Correction for the Variations
in the Temperature of the Normal Solution of the Sea Salt. They enable us to correct any
temperature between 0 and 30 degrees centigrade (32° and 86° Fahr.) when the solution
of sea salt has been prepared in the same limits.
Let us suppose, for example, that the solution has been made standard at 15°, and
that at the time of using it, the temperature has become 18°. We see by the second
column of the table, that the weight of a measure of the solution is 100·099 gr. at 15°,
and 100·065 at 18°; the difference 0·034 gr., is the quantity of solution less which has
been really taken; and of course we must add it to the normal measure, in order to
make it equal to one thousand millièmes. If the temperature of the solution had fallen
to 10 degrees, the difference of the weight of a measure from 10 to 15 degrees would be
0·019 gr. which we must on the contrary deduct from the measure, since it had been taken
too large. These differences of weight of a measure of solution at 15°, from that of a[72]
measure at any other temperature, form the column 15° of the table, where they are
expressed in thousandths; they are inscribed on the same horizontal lines as the temperatures
to which each of them relates with the sign + plus, when they must be added,
and with the sign - minus, when they must be subtracted. The columns 5°, 10°, 20°,
25°, 35°, have been calculated in the same manner for the cases in which the normal
solution may have been graduated to each of these temperatures. Thus, to calculate the
column 10, the number 100·118 has been taken of the column of weights for a term of
departure, and its difference from all the numbers of the same column has been
sought.
Table of Correction for the Variations in the Temperature of the Normal Solution of
the Sea Salt.
Tem-
pera-
ture. |
Weight. |
5° |
10° |
15° |
20° |
25° |
30° |
| |
gram. |
mill. |
mill. |
mill. |
mill. |
mill. |
mill. |
| 4 |
100,109 |
0·0 |
- 0·1 |
+ 0·1 |
+ 0·7 |
+ 1·7 |
+ 2·7 |
| 5 |
100,113 |
0·0 |
- 0·1 |
+ 0·1 |
+ 0·7 |
+ 1·7 |
+ 2·8 |
| 6 |
100,115 |
0·0 |
0·0 |
+ 0·2 |
+ 0·8 |
+ 1·7 |
+ 2·8 |
| 7 |
110,118 |
+ 0·1 |
0·0 |
+ 0·2 |
+ 0·8 |
+ 1·7 |
+ 2·8 |
| 8 |
100,120 |
+ 0·1 |
0·0 |
+ 0·2 |
+ 0·8 |
+ 1·8 |
+ 2·8 |
| 9 |
100,120 |
+ 0·1 |
0·0 |
+ 0·2 |
+ 0·8 |
+ 1·8 |
+ 2·8 |
| 10 |
100,118 |
+ 0·1 |
0·0 |
+ 0·2 |
+ 0·8 |
+ 1·7 |
+ 2·8 |
| 11 |
100,116 |
0·0 |
0·0 |
+ 0·2 |
+ 0·8 |
+ 1·7 |
+ 2·8 |
| 12 |
100,114 |
0·0 |
0·0 |
+ 0·2 |
+ 0·8 |
+ 1·7 |
+ 2·8 |
| 13 |
100,110 |
0·0 |
- 0·1 |
+ 0·1 |
+ 0·7 |
+ 1·7 |
+ 2·7 |
| 14 |
100,106 |
- 0·1 |
- 0·1 |
+ 0·1 |
+ 0·7 |
+ 1·6 |
+ 2·7 |
| 15 |
100,099 |
- 0·1 |
- 0·2 |
- 0·0 |
+ 0·6 |
+ 1·6 |
+ 2·6 |
| 16 |
100,090 |
- 0·2 |
- 0·3 |
- 0·1 |
+ 0·5 |
+ 1·5 |
+ 2·5 |
| 17 |
100,078 |
- 0·4 |
- 0·4 |
- 0·2 |
+ 0·4 |
+ 1·3 |
+ 2·4 |
| 18 |
100,065 |
- 0·5 |
- 0·5 |
- 0·3 |
+ 0·3 |
+ 1·2 |
+ 2·3 |
| 19 |
100,053 |
- 0·6 |
- 0·7 |
- 0·5 |
+ 0·1 |
+ 1·1 |
+ 2·2 |
| 20 |
100,039 |
- 0·7 |
- 0·8 |
- 0·6 |
0·0 |
+ 1·0 |
+ 2·0 |
| 21 |
100,021 |
- 0·9 |
- 1·0 |
- 0·8 |
- 0·2 |
+ 0·8 |
+ 1·9 |
| 22 |
100,001 |
- 1·1 |
- 1·2 |
- 1·0 |
- 0·4 |
+ 0·6 |
+ 1·7 |
| 23 |
99,983 |
- 1·3 |
- 1·4 |
- 1·2 |
- 0·6 |
+ 0·4 |
+ 1·5 |
| 24 |
99,964 |
- 1·5 |
- 1·5 |
- 1·4 |
- 0·8 |
+ 0·2 |
+ 1·3 |
| 25 |
99,944 |
- 1·7 |
- 1·7 |
- 1·6 |
- 1·0 |
0·0 |
+ 1·1 |
| 26 |
99,924 |
- 1·9 |
- 1·9 |
- 1·8 |
- 1·2 |
- 0·2 |
+ 0·9 |
| 27 |
99,902 |
- 2·1 |
- 2·2 |
- 2·0 |
- 1·4 |
- 0·4 |
+ 0·7 |
| 28 |
99,879 |
- 2·3 |
- 2·4 |
- 2·2 |
- 1·6 |
- 0·7 |
+ 0·4 |
| 29 |
99,858 |
- 2·6 |
- 2·6 |
- 2·4 |
- 1·8 |
- 0·9 |
+ 0·2 |
| 30 |
99,836 |
- 2·8 |
- 2·8 |
- 2·6 |
- 2·0 |
- 1·1 |
0·0 |
Several expedients have been employed to facilitate and abridge the manipulations. In
the first place, the phials for testing or assaying the specimens of silver should all be of
the same height and of the same diameter. They should be numbered at their top, as well
as on their stoppers, in the order 1, 2, 3, &c. They may be ranged successively in tens;
the stoppers of the same series being placed on a support in their proper order. Each two
phials should, in their turn, be placed in a japanned tin case (fig. 90.) with ten compartments
duly numbered. These compartments are cut out anteriorly to about half
their height, to allow the bottoms of the bottles to be seen. When each phial has received
its portion of alloy, through a wide-beaked funnel, there must be poured into it
about 10 grammes of nitric acid, of specific gravity 1·28, with a pipette, containing that
quantity; it is then exposed to the heat of a water bath, in order to facilitate the solution
of the alloy. The water bath is an oblong vessel made of tin plate, intended to
receive the phials. It has a moveable double bottom, pierced with small holes, for the
purpose of preventing the phials being broken, as it insulates them from the bottom to
which the heat is applied. The solution is rapid; and, since it emits nitrous vapours
in abundance, it ought to be carried on under a chimney.
The agitator.—Fig. 91. gives a sufficiently exact idea of it, and may dispense with a
lengthened description. It has ten cylindrical compartments, numbered from 1 to 10.
The phials, after the solution of the alloy, are arranged in it in the order of their numbers.
The agitator is then placed within reach of the pipette, intended to measure out
the normal solution of sea salt, and a pipette full of this solution is put into each phial.
Each is then closed with its glass stopper, previously dipped in pure water. They are
fixed in the cells of the agitator by wooden wedges. The agitator is then suspended[73]
to a spring R, and, seizing it with the two hands, the operator gives an alternating
rapid movement, which agitates the solution, and makes it, in less than a minute, as
limpid as water. This movement
is promoted by a spiral spring, B,
fixed to the agitator and the ground;
but this is seldom made use of, because
it is convenient to be able to
transport the agitator from one place
to another. When the agitation is
finished, the wedges are to be taken
out, and the phials are placed in order
upon a table furnished with round
cells destined to receive them, and to
screen them from too free a light.
When we place the phials upon this
table, we must give them a brisk circular
motion, to collect the chloride
of silver scattered round their sides;
we must lift out their stoppers, and
suspend them in wire rings, or pincers.
We next pour a thousandth of the
decime solution into each phial; and
before this operation is terminated,
there is formed in the first phials,
when there should be a precipitate, a
nebulous stratum, very well marked,
of about a centimetre in thickness.
At the back of the table there is a
black board divided into compartments
numbered from 1 to 10, upon
each of which we mark, with chalk,
the thousandths of the decime liquor
put into the correspondent phial. The
thousandths of sea salt, which indicate
an augmentation of standard, are preceded
by the sign +, and the thousandths
of nitrate of silver by the sign -.
When the assays are finished, the liquor of each phial is to be poured into a large
vessel, in which a slight excess of sea salt is kept; and when it is full, the supernatant
clear liquid must be run off with a syphon.
The chloride of silver may be reduced without any perceptible loss. After having
washed it well, we immerse pieces of iron or zinc into it, and add sulphuric acid in sufficient
quantity to keep up a feeble disengagement of hydrogen gas. The mass must not
be touched. In a few days the silver is completely reduced. This is easily recognised
by the colour and nature of the product; or by treating a small quantity of it with
water of ammonia, we shall see whether there be any chloride unreduced; for it will
be dissolved by the ammonia, and will afterwards appear upon saturating the ammonia
with an acid. The chlorine remains associated with the iron or the zinc in a state
of solution. The first washings of the reduced silver must be made with an acidulous
water, to dissolve the oxide of iron which may have been formed, and the other washings
with common water. After decanting the water of the last washing, we dry the mass,
and add a little powdered borax to it. It must be now fused. The silver being in a
bulky powder is to be put in successive portions into a crucible as it sinks down. The
heat should be at first moderate; but towards the end of the operation it must be pretty
strong to bring into complete fusion the silver and the scoriæ, and to effect their
complete separation. In case it should be supposed that the whole of the silver had
not been reduced by the iron or zinc, a little carbonate of potash should be added to the
borax. The silver may also be reduced by exposing the chloride to a strong heat, in
contact with chalk and charcoal.
The following remarks by M. Gay Lussac, the author of the above method, upon the
effect of a little mercury in the humid assay, are important:—
It is well known that chloride of silver blackens the more readily as it is exposed
to an intense light, and that even in the diffused light of a room, it becomes soon
sensibly coloured. If it contains four to five thousandths of mercury, it does not blacken;
it remains of a dead white: with three thousandths of mercury, there is no marked
discolouring in diffused light; with two thousandths it is slight; with one it is much
more marked, but still it is much less intense than with pure chloride. With half a[74]
thousandth of mercury the difference of colour is not remarkable, and is perceived only
in a very moderate light.
But when the quantity of mercury is so small that it cannot be detected by the
difference of colour in the chloride of silver, it may be rendered quite evident by a very
simple process of concentration. Dissolve one gramme of the silver supposed to contain
1⁄4 of a thousandth of mercury, and let only 1⁄4 of it be precipitated, by adding only 1⁄4 of
the common salt necessary to precipitate it entirely. In thus operating, the 1⁄4 thousandth
of mercury is concentrated in a quantity of chloride of silver four times smaller:
it is as if the silver having been entirely precipitated, four times as much mercury,
equal to two thousandths, had been precipitated with it.
In taking two grammes of silver, and precipitating only 1⁄4 by common salt, the
precipitate would be, with respect to the chloride of silver, as if it amounted to four
thousandths. By this process, which occupies only five minutes, because exact weighing
is not necessary, 1⁄10 of a thousandth of mercury may be detected in silver.
It is not useless to observe, that in making those experiments the most exact manner
of introducing small quantities of mercury into a solution of silver, is to weigh a minute
globule of mercury, and to dissolve it in nitric acid, diluting the solution so that it
may contain as many cubic centimetres as the globule weighs of centigrammes. Each
cubic centimetre, taken by means of a pipette, will contain one milligramme of
mercury.
If the ingot of silver to be assayed is found to contain a greater quantity of mercury,
one thousandth for example, the humid process ought either to be given up in this
case, or to be compared with cupellation.
When the silver contains mercury, the solution from which the mixed chlorides are
precipitated, does not readily become clear.
Silver containing mercury, put into a small crucible and mixed with lamp black,
to prevent the volatilization of the silver, was heated for three quarters of an hour
in a muffle, but the silver increased sensibly in weight. This process for separating the
mercury, therefore, failed. It is to be observed, that mercury is the only metal which
has thus the power of disturbing the analysis by the humid way.
Assaying of Gold.—In estimating or expressing the fineness of gold, the whole
mass spoken of is supposed to weigh 24 carats of 12 grains each, either real, or merely
proportional, like the assayer’s weights; and the pure gold is called fine. Thus, if
gold be said to be 23 carats fine, it is to be understood, that in a mass, weighing
24 carats, the quantity of pure gold amounts to 23 carats.
In such small work as cannot be assayed by scraping off a part and cupelling it,
the assayers endeavour to ascertain its fineness or quality by the touch. This is a
method of comparing the colour and other properties, of a minute portion of the
metal, with those of small bars, the composition of which is known. These bars are
called touch needles, and they are rubbed upon a smooth piece of black basaltes or
pottery, which, for this reason, is called the touchstone. Black flint slate will serve
the same purpose. Sets of gold needles may consist of pure gold; of pure gold, 231⁄2
carats with 1⁄2 carat of silver; 23 carats of gold with one carat of silver; 221⁄2 carats of
gold with 11⁄2 carat of silver; and so on, till the silver amounts to four carats; after
which the additions may proceed by whole carats. Other needles may be made in the
same manner, with copper instead of silver; and other sets may have the addition,
consisting either of equal parts of silver and copper, or of such proportions as the
occasions of business require. The examination by the touch may be advantageously
employed previous to quartation, to indicate the quantity of silver necessary to be
added.
In foreign countries, where trinkets and small work are required to be submitted to
the assay of the touch, a variety of needles is necessary; but they are not much used
in England. They afford, however, a degree of information which is more considerable
than might at first be expected. The attentive assayer compares not only the colour of
the stroke made upon the touchstone by the metal under examination, with that produced
by his needle, but will likewise attend to the sensation of roughness, dryness, smoothness,
or greasiness, which the texture of the rubbed metal excites, when abraded by the
stone. When two strokes perfectly alike in colour are made upon the stone, he may
then wet them with aquafortis, which will affect them very differently, if they be not
similar compositions; or the stone itself may be made red-hot by the fire, or by the blowpipe,
if thin black pottery be used; in which case the phenomena of oxidation will differ,
according to the nature and quantity of the alloy. Six principal circumstances appear
to affect the operation of parting; namely, the quantity of acid used in parting, or in
the first boiling; the concentration of this acid; the time employed in its application;
the quantity of acid made use of in the reprise, or second operation; its concentration;
and the time during which it is applied. From experiment it has been shown, that
each of these unfavourable circumstances might easily occasion a loss of from the half of[75]
a thirty-second part of a carat, to two thirty-second parts. The assayers explain their
technical language by observing, that in the whole mass consisting of twenty-four carats,
this thirty-second part denotes 1-768th part of the mass. It may easily be conceived,
therefore, that if the whole six circumstances were to exist, and be productive of errors,
falling the same way, the loss would be very considerable.
It is therefore indispensably necessary, that one uniform process should be followed
in the assays of gold; and it is a matter of astonishment, that such an accurate process
should not have been prescribed by government for assayers, in an operation of such
great commercial importance, instead of every one being left to follow his own
judgment. The process recommended in the old French official report is as follows:—twelve
grains of the gold intended to be assayed must be mixed with thirty grains of
fine silver, and cupelled with 108 grains of lead. The cupellation must be carefully
attended to, and all the imperfect buttons rejected. When the cupellation is ended, the
button must be reduced, by lamination, into a plate of 11⁄2 inches, or rather more, in
length, and four or five lines in breadth. This must be rolled up upon a quill, and
placed in a matrass capable of holding about three ounces of liquid, when filled up to
its narrow part. Two ounces and a half of very pure aquafortis, of the strength of 20
degrees of Baumé’s areometer, must then be poured upon it; and the matrass being
placed upon hot ashes, or sand, the acid must be kept gently boiling for a quarter of an
hour: the acid must then be cautiously decanted, and an additional quantity of 11⁄2
ounces must be poured upon the metal, and slightly boiled for twelve minutes. This
being likewise carefully decanted, the small spiral piece of metal must be washed with
filtered river water, or distilled water, by filling the matrass with this fluid. The
vessel is then to be reversed, by applying the extremity of its neck against the bottom
of a crucible of fine earth, the internal surface of which is very smooth. The annealing
must now be made, after having separated the portion of water which had fallen into
the crucible; and, lastly, the annealed gold must be weighed. For the certainty of
this operation, two assays must be made in the same manner, together with a third
assay upon gold of twenty-four carats, or upon gold the fineness of which is perfectly
and generally known.
No conclusion must be drawn from this assay, unless the latter gold should prove to
be of the fineness of twenty-four carats exactly, or of its known degree of fineness; for,
if there be either loss or surplus, it may be inferred, that the other two assays, having
undergone the same operation, must be subject to the same error. The operation being
made according to this process by several assayers, in circumstances of importance, such
as those which relate to large fabrications, the fineness of the gold must not be depended
upon, nor considered as accurately known, unless all the assayers have obtained an uniform
result, without communication with each other. This identity must be considered as
referring to the accuracy of half the thirty-second part of a carat. For, notwithstanding
every possible precaution or uniformity, it very seldom happens that an absolute agreement
is obtained between the different assays of one and the same ingot; because the
ingot itself may differ in its fineness in different parts of its mass.
The phenomena of the cupellation of gold are the same as of silver, only the
operation is less delicate, for no gold is lost by evaporation or penetration into the
bone-ash, and therefore it bears safely the highest heat of the assay furnace. The button
of gold never vegetates, and need not therefore be drawn out to the front of the muffle,
but may be left at the further end till the assay is complete. Copper is retained more
strongly by gold than it is by silver; so that with it 16 parts of lead are requisite to
sweat out 1 of copper; or, in general, twice as much lead must be taken for the copper
alloys of gold, as for those of silver. When the copper is alloyed with very small
quantities of gold, cupellation would afford very uncertain results; we must then have
recourse to liquid analysis.
M. Vauquelin recommends to boil 60 parts of nitric acid at 22° Baumé, on the spiral
slip or cornet of gold and silver alloy, for twenty-five minutes, and replace the liquid
afterwards by acid of 32°, which must be boiled on it for eight minutes. This process
is free from uncertainty when the assay is performed upon an alloy containing a considerable
quantity of copper. But this is not the case in assaying finer gold; for then
a little silver always remains in the gold. The surcharge which occurs here is 2 or 3
thousandths; this is too much, and it is an intolerable error when it becomes greater,
which often happens. This evil may be completely avoided by employing the following
process of M. Chaudet. He takes 0·500 of the fine gold to be assayed; cupels
it with 1·500 of silver, and 1·000 of lead; forms, with the button from the cupel, a
riband or strip three inches long, which he rolls into a cornet. He puts this into a
mattrass with acid at 22° B., which he boils for 3 or 4 minutes. He replaces this by
acid of 32° B., and boils for ten minutes; then decants off, and boils again with acid of
32°, which must be finally boiled for 8 or 10 minutes.
Gold thus treated is very pure. He washes the cornet, and puts it entire into a small[76]
crucible permeable to water; heats the crucible to dull redness under the muffle, when
the gold assumes the metallic lustre, and the cornet becomes solid. It is now taken out
of the crucible and weighed.
When the alloy contains platinum, the assay presents greater difficulties. In general,
to separate the platinum from the gold with accuracy, we must avail ourselves of a peculiar
property of platinum; when alloyed with silver, it becomes soluble in nitric
acid. Therefore, by a proper quartation of the alloy by cupellation, and boiling the
button with nitric acid, we may get a residuum of pure gold. If we were to treat the
button with sulphuric acid, however, we should dissolve nothing but the silver. The
copper is easily removed by cupellation. Hence, supposing that we have a quaternary
compound of copper, silver, platinum, and gold, we first cupel it, and weigh the button
obtained; the loss denotes the copper. This button, treated by sulphuric acid, will
suffer a loss of weight equal to the amount of silver present. The residuum, by
quartation with silver and boiling with nitric acid, will part with its platinum, and the
gold will remain pure. For more detailed explanations, see Platinum.
ATOMIC WEIGHTS or ATOMS, are the primal quantities in which the different
objects of chemistry, simple or compound, combine with each other, referred to a
common body, taken as unity. Oxygen is assumed by some philosophers, and hydrogen
by others, as the standard of comparison. Every chemical manufacturer should be
thoroughly acquainted with the combining ratios which are, for the same two substances,
not only definite, but multiple; two great truths, upon which are founded not merely the
rationale of his operations, but also the means of modifying them to useful purposes.
The discussion of the doctrine of atomic weights, or prime equivalents, belongs to pure
chemistry; but several of its happiest applications are to be found in the processes of art, as
pursued upon the greatest scale. For many instructive examples of this proposition,
the various chemical manufactures may be consulted in this Dictionary.
ATTAR OF ROSES. See Oils, Volatile, and Perfumery.
AURUM MUSIVUM. Mosaic gold, a preparation of tin; which see.
AUTOMATIC, a term which I have employed to designate such economic arts as
are carried on by self-acting machinery. The word “manufacture,” in its etymological
sense, means any system, or objects of industry, executed by the hands; but in the
vicissitude of language, it has now come to signify every extensive product of art which
is made by machinery, with little or no aid of the human hand, so that the most perfect
manufacture is that which dispenses entirely with manual labour.[4] It is in our modern
cotton and flax mills that automatic operations are displayed to most advantage; for
there the elemental powers have been made to animate millions of complex organs,
infusing into forms of wood, iron, and brass, an intelligent agency. And as the
philosophy of the fine arts, poetry, painting, and music, may be best studied in their
individual master-pieces, so may the philosophy of manufactures in these its noblest
creations.[5]
The constant aim and effect of these automatic improvements in the arts are philanthropic,
as they tend to relieve the workmen either from niceties of adjustment, which
exhaust his mind and fatigue his eyes, or from painful repetition of effort, which distort
and wear out his frame. A well arranged power-mill combines the operation of many
work-people, adult and young, in tending with assiduous skill, a system of productive
machines continuously impelled by a central force. How vastly conducive to the
commercial greatness of a nation, and the comforts of mankind, human industry can
become, when no longer proportioned in its results to muscular effort, which is by its
nature fitful and capricious, but when made to consist in the task of guiding the work
of mechanical fingers and arms regularly impelled, with equal precision and velocity,
by some indefatigable physical agent, is apparent to every visitor of our cotton, flax,
silk, wool, and machine factories. This great era in the useful arts is mainly due to
the genius of Arkwright. Prior to the introduction of his system, manufactures were
every where feeble and fluctuating in their development; shooting forth luxuriantly for
a season, and again withering almost to the roots like annual plants. Their perennial
growth then began, and attracted capital, in copious streams, to irrigate the rich domains
of industry. When this new career commenced, about the year 1770, the annual
consumption of cotton in British manufactures was under four millions of pounds’
weight, and that of the whole of Christendom was probably not more than ten millions.
Last year the consumption in Great Britain and Ireland was about two hundred and
seventy millions of pounds, and that of Europe and the United States together, four
hundred and eighty millions. In our spacious factory apartments the benignant power
of steam summons around him his myriads of willing menials, and assigns to each the
regulated task, substituting, for painful muscular effort upon their part, the energies
of his own gigantic arm, and demanding, in return, only attention and dexterity to
correct such little aberrations as casually occur in his workmanship. Under his auspices,[77]
and in obedience to Arkwright’s polity, magnificent edifices, surpassing far in number,
value, usefulness, and ingenuity of construction, the boasted monuments of Asiatic,
Egyptian, and Roman despotism, have, within the short period of fifty years, risen up
in this kingdom, to show to what extent capital, industry, and science, may augment
the resources of a state, while they meliorate the condition of its citizens. Such is the
automatic system, replete with prodigies in mechanics and political economy, which
promises, in its future growth, to become the great minister of civilisation to the
terraqueous globe, enabling this country, as its heart, to diffuse, along with its commerce,
the life-blood of knowledge and religion to myriads of people still lying “in the
region and shadow of death.”[6] Of these truths, the present work affords decisive evidence
in almost every page.
AUTOMATON. In the etymological sense, this word (self-working) signifies
every mechanical construction which, by virtue of a latent intrinsic force, not obvious
to common eyes, can carry on, for some time, certain movements more or less resembling
the results of animal exertion, without the aid of external impulse. In this
respect, all kinds of clocks and watches, planetariums, common and smoke jacks, with
a vast number of the machines now employed in our cotton, silk, flax, and wool
factories, as well as in our dyeing and calico printing works, may be denominated
automatic. But the term, automaton, is, in common language, appropriated to that
class of mechanical artifices in which the purposely concealed power is made to imitate
the arbitrary or voluntary motions of living beings. Human figures, of this kind, are
sometimes styled Androides, from the Greek term, like a man.
Although, from what we have said, clock-work is not properly placed under the
head automaton, it cannot be doubted that the art of making clocks in its progressive
improvement and extension, has given rise to the production of automata. The most
of these, in their interior structure, as well as in the mode of applying the moving
power, have a distinct analogy with clocks; and these automata are frequently mounted
in connection with watch work. Towards the end of the 13th century, several tower
clocks, such as those at Strasburg, Lubeck, Prague, Olmutz, had curious mechanisms
attached to them. The most careful historical inquiry proves that automata, properly
speaking, are certainly not older than wheel-clocks; and that the more perfect structures
of this kind are subsequent to the general introduction of spring clocks. Many
accounts of ancient automata, such as the flying doves of Archytas of Tarentum,
Regiomontanus’s iron flies, the eagle which flew towards the emperor Maximilian, in
Nurenberg, in the year 1470, were deceptions, or exaggerated statements; for, three such
masterpieces of art would form now, with every aid of our improved mechanisms, the
most difficult of problems. The imitation of flying creatures is extremely difficult, for
several reasons. There is very little space for the moving power, and the only material
possessed of requisite strength being metal, must have considerable weight. Two
automata, of the celebrated French mechanician, Vaucauson, first exhibited in the year
1738, have been greatly admired; namely a flute-player, five and a half feet high, with
its cubical pedestal, which played several airs upon the German flute; and that, not by
any interior tube-work, but through the actual blowing of air into the flute, the motion
of the tongue, and the skilful stopping of the holes with the fingers; as also a duck,
which imitated many motions of a natural kind in the most extraordinary manner.
This artist has had many imitators, of whom the brothers, Droz of Chaux de Fonds,
were the most distinguished. Several very beautiful clock mechanisms of theirs are
known. One of them with a figure which draws; another playing on the piano; a third
which writes, besides numerous other combined automata. Frederick Von Knauss
completed a writing machine at Vienna, in the year 1760. It is now in the model
cabinet of the Polytechnic Institute, and consists of a globe 2 feet in diameter, containing
the mechanism upon which a figure 7 inches high sits, and writes upon a sheet
of paper fixed to a frame, whatever has been placed beforehand upon a regulating
cylinder. At the end of every line, it rises and moves its hand sideways, in order to
begin a new line.
Very complete automata have not been made of late years, because they are very
expensive; and by soon satisfying curiosity, they cease to interest. Ingenious mechanicians
find themselves better rewarded by directing their talents to the self-acting
machinery of modern manufactures. We may notice here, however, the mechanical
trumpeter of Mälzl, at Vienna, and a similar work of Kauffmann, at Dresden. In
French Switzerland some artists continue to make minute automata which excite no
little wonder; such as singing canary birds, with various movements of a natural kind;
also little birds, sometimes hardly three quarters of an inch long, in snuff-boxes and
watches of enamelled gold. Certain artificial figures which have been denominated
automata, hardly deserve the name; since trick and confederacy are more or less concerned[78]
in their operation. To this head belong a number of figures apparently speaking
by mechanism; a clock which begins to strike, or to play, when a person makes a
sign of holding up his finger; this effect being probably produced by a concealed
green-finch, or other little bird, instructed to set off the détente of the wheel-work at a
signal. It is likely, also, that the chess player of Von Kempelen, which excited so much
wonder in the last century, had a concealed confederate. Likewise, the very ingenious
little figures of Tendler, father and son, which imitated English horsemen and rope-dancers,
constructed at Eisenerz, in Styria, are probably no more true automata than the
fantoccini, or figures of puppets which are exhibited in great perfection in many towns of
Italy, especially at Rome.
The moving power of almost all automata is a wound-up steel spring; because, in
comparison with other means of giving motion, it takes up the smallest room, is easiest
concealed, and set a going. Weights are seldom employed, and only in a partial way.
The employment of other moving powers is more limited; sometimes fine sand is made
to fall on the circumference of a wheel, by which the rest of the mechanism is moved.
For the same purpose water has been employed; and, when it is made to fall into an
air-chamber, it causes sufficient wind to excite musical sounds in pipes. In particular
cases quicksilver has been used, as, for example, in the Chinese tumblers, which is
only a physical apparatus to illustrate the doctrine of the centre of gravity.
Figures are frequently constructed for playthings, which move by wheels hardly
visible. An example of this simplest kind of automaton which may be introduced here,
as illustrating the self-acting principles of manufactures, is shown in the figure.
Fig. 92. exhibits the outlines of an automaton, representing a swan, with suitably
combined movements. The mechanism may be described, for the sake of clearness
of explanation, under distinct
heads. The first relates
to the motion of the whole
figure. By means of this
part it swims upon the water,
in directions changed from
time to time without exterior
agency. Another construction
gives to the figure the
faculty of bending its neck on
several occasions, and, to such
an extent, that it can plunge
the bill and a portion of the
head under water. Lastly,
it is made to move its head
and neck slowly from side to
side.
On the barrel of the spring,
exterior to the usual ratchet
wheel, there is a main-wheel, marked 1, which works into the pinion of the wheel 2.
The wheel 2 moves a smaller one, shown merely in dotted lines, and on the long axis
of the latter, at either end there is a rudder, or water-wheel, the paddles of which are
denoted by the letter a. Both of these rudder-wheels extend through an oblong
opening in the bottom of the figure down into the water. They turn in the direction
of the arrow, and impart a straight-forwards movement to the swan. The chamber,
in which these wheels revolve, is made water tight, to prevent moisture being thrown
upon the rest of the machinery. By the wheel 4, motion is conveyed to the fly-pinion 5;
the fly itself 6, serves to regulate the working of the whole apparatus, and it is provided
with a stop bar, not shown in the engraving, to bring it to rest, or set it a-going at
pleasure. Here, as we may imagine, the path pursued is rectilinear, when the rudder-wheels
are made to work in a square direction. An oblique bar, seen only in section
at b, movable about its middle point, carries at each end a web foot c, so that the direction
of the bar b, and of both feet towards the rudder wheels, determines the form of
the path which the figure will describe. The change of direction of that oblique bar
is effected without other agency. For this purpose, the wheel 1 takes into the pinion 7,
and this carries round the crown-wheel 8, which is fixed, with an eccentric disc 9, upon
a common axis. While the crown-wheel moves in the direction of the arrow, it turns
the smaller eccentric portion of the elliptic disc towards the lever m, which, pressed
upon incessantly by its spring, assumes, by degrees, the position corresponding with the
middle line of the figure, and afterwards an oblique position; then it goes back again,
and reaches its first situation; consequently through the reciprocal turning of the bar h,
and the swim-foot, is determined and varied the path which the swan must pursue.
This construction is available with all automata, which work by wheels; and it is obvious,[79]
that we may, by different forms of the disc 9, modify, at pleasure, the direction
and the velocity of the turnings. If the disc is a circle for instance, then the changes
will take place less suddenly; if the disc has an outward and inward curvature, upon
whose edge the end of the lever presses with a roller, the movement will take place in a
serpentine line.
The neck is the part which requires the most careful workmanship. Its outward case
must be flexible, and the neck itself should therefore be made of a tube of spiral wire,
covered with leather, or with a feathered bird-skin. The double line in the interior,
where we see the triangles e e e, denotes a steel spring made fast to the plate 10, which
forms the bottom of the neck; it stands loose, and needs to be merely so strong as to
keep the neck straight, or to bend it a little backwards. It should not be equally
thick in all points, but it should be weaker where the first graceful bend is to be made;
and, in general, its stiffness ought to correspond to the curvature of the neck of this bird.
The triangles e are made fast at their base to the front surface of the spring; in the
points of each there is a slit, in the middle of which a movable roller is set, formed of a
smoothly turned steel rod. A thin catgut string f, runs from the upper end of the
spring, where it is fixed over all these rollers, and passes through an aperture pierced
in the middle of 10, into the inside of the rump. If the catgut be drawn straight
back towards f, the spring, and consequently the neck, must obviously be bent,
and so much the more, the more tightly f is pulled, and is shortened in the hollow of
the neck. How this is accomplished by the wheel-work will presently be shown. The
wheel 11 receives its motion from the pinion s, connected with the main wheel 1.
Upon 11 there is, moreover, the disc 12, to whose circumference a slender chain is
fastened. When the wheel 11 turns in the direction of the arrow, the chain will be so
much pulled onwards through the corresponding advance at the point at 12, till this
point has come to the place opposite to its present situation, and, consequently, 11 must
have performed half a revolution. The other end of the chain is hung in the groove
of a very movable roller 14; and this will be turned immediately by the unwinding of
the chain upon its axis. There turns, in connection with it, however, the large roller 13,
to which the catgut f is fastened; and as this is pulled in the direction of the arrow,
the neck will be bent until the wheel 11 has made a half revolution. Then the drag
ceases again to act upon the chain and the catgut; the spring in the neck comes into
play: it becomes straight, erects the neck of the animal, and turns the rollers 13 and
14, back into their first position.
The roller 13 is of considerable size, in order that through the slight motion of the
roller 14, a sufficient length of the catgut may be wound off, and the requisite shortening
of the neck may be effected; which results from the proportion of the diameters
of the rollers 11, 13, and 14. This part of the mechanism is attached as near to the
side of the hollow body as possible, to make room for the interior parts, but particularly
for the paddle-wheels. Since the catgut, f, must pass downwards on the middle from
10, it is necessary to incline it sideways and outwards towards 13, by means of some
small rollers.
The head, constituting one piece with the neck, will be depressed by the complete
flexure of this; and the bill, being turned downwards in front of the breast, will touch
the surface of the water. The head will not be motionless; but it is joined on both
sides by a very movable hinge, with the light ring, which forms the upper part of the
clothing of the neck. A weak spring, g, also fastened to the end of the neck, tends to
turn the head backwards; but in the present position it cannot do so, because a chain
at g, whose other end is attached to the plate 10, keeps it on the stretch. On the bending
of the neck, this chain becomes slack; the spring g comes into operation, and throws
the head so far back, that, in its natural position, it will reach the water.
Finally, to render the turning of the head and the neck practicable, the latter is not
closely connected with the rump, while the plate 10 can turn in a cylindrical manner
upon its axis, but cannot become loose outwardly. Moreover, there is upon the axis of
the wheel 1, and behind it (shown merely as a circle in the engraving) a bevel wheel,
which works into a second similar wheel, 15, so as to turn it in a horizontal direction.
The pin 16, of the last wheel, works upon a two-armed lever 19, movable round the
point h, and this lever moves the neck by means of the pin 17. The shorter arm of the
lever 19 has an oval aperture in which the pin 16 stands. As soon as this, in consequence
of the movement of the bevel-wheel 15, comes into the dotted position, it pushes
the oval ring outwards on its smaller diameter, and thereby turns the lever upon the
point h, into the oblique direction shown by the dotted lines. The pin 16, having come
on its way right opposite to its present position, sets the lever again straight. Then the
lever, by the further progress of the pin in its circular path, is directed outwards to the
opposite side; and, at last, when 15 has made an entire revolution, it is quite straight.
The longer arm of the lever follows, of course, these alternating movements, so that it
turns the neck upon its plate 10, by means of the pin 17; and, as 18 denotes the bill,[80]
this comes into the dotted position. It may be remarked in conclusion, that the
drawing of fig. 92. represents about half the size of which the automaton may be constructed,
and that the body may be formed of thin sheet-copper or brass.
Fig. 93, 94, 95. show the plan of a third automaton. A horse which moves its feet
in a natural way, and draws a carriage with two figures sitting in it. The man appears
to drive the horse with a whip; the woman bends forwards from him in front. The
four wheels of the carriage have no connection with the moving mechanism. In fig. 95.,
some parts are represented upon a larger scale. The wheel 1, in fig. 93. operates
through the two carrier wheels upon the wheels marked 4 and 5. By means of the axis
of these two wheels, the feet are set in motion. The left fore-foot, a, then the right
hinder foot, move themselves backwards, and take hold of the ground with small tacks
in their hoofs, while the two other legs are bent and raised, but no motion of the body
takes place. The carriage, however, with which the horse is connected, advances upon
its wheels. By studying the mechanism of the foot, a, and the parts connected with it,
we can readily understand the principles of the movement. The axis of wheel 4 is
crank-shaped, on both sides, where it has to operate directly on the fore feet; but for
each foot, it is bent in an opposite direction, as is obvious in the front view fig. 94.
This crank, or properly its part furthest from the axis, serves instead of the pin 16, in
the swan, and moves like it in an oval spot, p, fig. 93. a two-armed lever, which gives
motion through tooth-work, but not as in the swan, by means of a second pin. This wheel-work
renders the motion smoother. The above lever has its fulcrum at n, fig. 93., about
which it turns alternately, to the one and the other side, by virtue of the rotation of the
wheel 4. The toothed arch, or the half-wheel on the under side, lays hold of a shorter
lever, in a similar arch, upon the upper joint of the foot, which is moved forwards and
backwards upon the pivot m. In virtue of the motions in the direction of the arrow,
the foot a will move itself first obliquely backwards, without bending, and the body
will thereby bend itself forwards. When the right hind foot makes the same motion,
both the other feet are raised and bent. The joints of the foot at d and e are formed
of hinges, which are so constructed that they can yield no farther than is necessary at
every oblique position of the foot. With the continued rotation of the wheel 4, the
lever turns itself about n, in an inverted direction inwards, and impels the uppermost
foot-joint forwards, so that it forms an acute angle with the body in front. The foot is
now twice bent upon its joints. This takes place by the traction of the chain t, which
is led over rollers (as the drawing shows) to the foot, and is there fastened. As its
upper end has its fixed point in the interior of the body, it is therefore drawn by
the eccentric pin r standing in the vicinity of m, and thus bends the foot at the
hinges. If there were space for it, a roller would answer better than a pin. By
the recedure of the uppermost joint into the first position, the tension of the chain t
ceases again of itself, while the pin r removes from it, and the foot is again extended in
a straight line by the small springs operating upon its two under parts, which were
previously bent stiffly by the chain. By the aid of the figures with this explanation, it
will be apparent that all the fore feet have a similar construction, that the proper
succession of motions will be effected through the toothed arcs, and the position of the
cranks on the axis of the wheels 4 and 5, and hence the advance of the figure must
follow. The wheel 6 puts the fly 7 in motion, by means of the small wheel marked 1;
on the fixed points of the 4 chains, by means of a ratchet-wheel and a catch, the[81]
necessary tension will again be produced when the chains have been drawn out a little.
There is sufficient room for a mechanism which could give motion to the head and ears,
were it thought necessary.
The proper cause of the motions may now be explained. In fig. 95., a, is a wheel
connected with the wound-up spring, by which the motion of the two human figures,
and also, if desired, that of the horse may be effected. The axis of the wheel b carries
a disc with pins, which operate upon the two-armed lever with its fulcrum e, and thus
cause the bending of the upper part of one of the figures, which has a hinge at f.
On the axis of that wheel there is a second disc c, for giving motion to the other
figure; which, for the sake of clearness, is shown separate, although it should sit alongside
of its fellow. On the upper end of the double-armed lever d, there is a cord
whose other end is connected with the moving arm, in the situation i, and raises it
whenever a pin in the disc presses the under part of the lever. A spring h brings the
arm back into the original position, when a pin has passed from the lever, and has left
it behind. The pins at c and d may be set at different distances from the middle of the
disc, whereby the motions of the figures by every contact of another pin, are varied, and
are therefore not so uniform, and consequently more natural.
For the connexion of both mechanisms, namely, the carriage with the horse, various
arrangements may be adopted. Two separate traction springs should be employed;
one at a, fig. 95., in the coach-seat; the other in the body of the horse. In the coach-seat
at b, the fly with its pinion, as well as a ratchet-wheel, is necessary. By means of
the shaft, the horse is placed in connexion with the waggon. It may, however, receive
its motion from the spring in the carriage, in which case one spring will be sufficient.
Upon the latter plan the following construction maybe adopted:—To the axis of b,
fig. 95., a bevel wheel is to be attached, and from this the motion is to be transmitted to
the bottom of the carriage with the help of a second bevel wheel s, connected with a
third bevel wheel t. This again turns the wheel u, whose long axis v goes to the middle
of the horse’s body, in an oblique direction, through the hollow shaft. This axis carries
an endless screw 9, fig. 93., with very oblique threads, which works into the little wheel
8, corresponding to the wheel 1, through an opening in the side of the horse, and in this
way sets the mechanism of the horse a-going. With this construction of fig. 95., a
spring of considerable strength is necessary, or if the height of the carriage-seat does
not afford sufficient room, its breadth will answer for placing two weaker springs alongside
of each other upon a common barrel.
AXE. A tool much used by carpenters for cleaving, and roughly fashioning, blocks
of wood. It is a flat iron wedge, with an oblong steel edge, parallel to which, in the
short base, is a hole for receiving and holding fast the end of a strong wooden handle.
In the cooper’s adze, the oblong edge is at right angles to the handle, and is slightly
curved up, or inflected towards it.
AXLES, of carriages; for their latest improvements, see Wheel Carriages.
AXUNGE. Hog’s lard; see Fat and Oils.
AZOTIZED, said of certain vegetable substances, which, as containing azote, were
supposed at one time to partake, in some measure, of the animal nature; most animal
bodies being characterised by the presence of much azote in their composition. The
vegetable products, indigo, cafeine, gluten, and many others, contain abundance of
azote.
AZURE, the fine blue pigment, commonly called smalt, is a glass coloured with
oxide of cobalt, and ground to an impalpable powder.
The manufacture of azure, or smalt, has been lately improved in Sweden, by the
adoption of the following process:—
The cobalt ore is first roasted till the greater part of the arsenic is driven off. The
residuary impure black oxide is mixed with as much sulphuric acid (concentrated) as
will make it into a paste, which is exposed at first to a moderate heat, then to a cherry-red
ignition for an hour. The sulphate thus obtained is reduced to powder, and
dissolved in water. To the solution, carbonate of potash is gradually added, in order
to separate the remaining portion of oxide of iron; the quantity of which depends
upon the previous degree of calcination. If it be not enough oxidized, the iron is
difficult to be got rid of.
When, from the colour of the precipitate, we find that the potash separates merely
carbonate of cobalt, it is allowed to settle, the supernatant liquor is decanted, and
precipitated, by means of a solution of silicate of potash, prepared as follows:—
Ten parts of potash are carefully mixed with fifteen parts of finely ground flints or
sand, and one part of pounded charcoal. This mixture is melted in a crucible of brick
clay, an operation which requires steady ignition during 5 or 6 hours. The mass,
when melted and pulverized, may be easily dissolved in boiling water, adding to it, by
little at a time, the glass previously ground. The filtered solution is colourless, and
keeps well in the air, if it contains one part of glass for 5 or 6 of water. The silicate[82]
of cobalt, which precipitates upon mixing the two solutions, is the preparation of cobalt
most suitable for painting upon porcelain, and for the manufacture of blue glass. See
Cobalt.
BABLAH. The rind or shell which surrounds the fruit of the mimosa cineraria;
it comes from the East Indies, as also from Senegal, under the name of Neb-neb. It
contains gallic acid, tannin, a red colouring matter, and an azotized substance; but the
proportion of tannin is smaller than in sumach, galls, and knoppern (gall-nuts of the
common oak) in reference to that of gallic acid, which is considerable in the bablah.
It has been used, in dyeing cotton, for producing various shades of drab; as a substitute
for the more expensive astringent die-stuffs.
BAGASSE. The sugar-cane, in its dry, crushed state, as delivered from the sugar-mill.
It is much employed for fuel in the colonial sugar-houses.
BAKING. (Cuire, Fr. Backen, Germ.) The exposure of any body to such a heat
as will dry and consolidate its parts without wasting them. Thus wood, pottery,
and porcelain, are baked, as well as bread.
BALANCE. To conduct arts, manufactures, and mines, with judgment and success,
recourse must be had, at almost every step, to a balance. Experience proves that all
material bodies, existing upon the surface of the earth, are constantly solicited by a
force which tends to bring them to its centre, and that they actually fall towards it
when they are free to move. This force is called gravity. Though the bodies be
not free, the effort of gravity is still sensible, and the resultant of all the actions which
it exercises upon their material points, constitutes what is popularly called their weight.
These weights are, therefore, forces which may be compared together, and by means of
machines may be made to correspond or be counterpoised.
To discover whether two weights be equal, we must oppose them to each other in a
machine where they act in a similar manner, and then see if they maintain an equilibrium;
for example, we fulfil this condition if we suspend them at the two extremities
of a lever, supported at its centre, and whose arms are equal. Such is the general idea
of a balance. The beam of a good balance ought to be a bar of well-tempered steel, of
such form as to secure perfect inflexibility under any load which may be fitly applied
to its extremities. Its arms should be quite equal in weight and length upon each side
of its point of suspension; and this point should be placed in a vertical line over the
centre of gravity; and the less distant it is from it, the more delicate will be the balance.
Were it placed exactly in that centre, the beam would not spontaneously recover the
horizontal position when it was once removed from it. To render its indications more
readily commensurable, a slender rod or needle is fixed to it, at right angles, in the line
passing through its centres of gravity and suspension. The point, or rather edge, of
suspension, is made of perfectly hard steel, and turns upon a bed of the same. For
common uses the arms of a balance can be made sufficiently equal to give satisfactory
results; but, for the more refined purposes of science, that equality should never be
presumed nor trusted to; and, fortunately, exact weighing is quite independent of that
equality. To weigh a body is to determine how many times the weight of that body
contains another species of known weight, as of grains or pounds, for example. In
order to find it out, let us place the substance, suppose a piece of gold, in the left hand
scale of the balance; counterpoise it with sand or shot in the other, till the index
needle be truly vertical, or stand in the middle of its scale, proving the beam to be
horizontal. Now remove gently the piece of gold, and substitute in its place standard
multiple weights of any graduation, English or French, till the needle again resumes the
vertical position, or till its oscillations upon either side of the zero point are equal. These
weights will represent precisely the weight of the gold, since they are placed in the same
circumstances precisely with it, and make the same equilibrium with the weight laid in
the other scale.
This method of weighing is obviously independent of the unequal length as well as
the unequal weight of the arms of the beam. For its perfection two requisites only are
indispensable. The first is that the points of suspension should be rigorously the same
in the two operations; for the power of a given weight to turn the beam being unequal,
according as we place it at different distances from the centre of suspension, did that
point vary in the two consecutive weighings, we would require to employ, in the second,
a different weight from that of the piece of gold, in order to form an equilibrium with
the sand or shot originally put in the opposite scale; and as there is nothing to indicate
such inequality in the states of the beam, great errors would result from it. The best
mode of securing against such inequality is to suspend the cords of the scales from
sharp-edged rings, upon knife edges, at the ends of the beam, both made of steel so[83]
hard tempered as to be incapable of indentation. The second condition is, that the
balance should be very sensible, that is, when in equilibrium and loaded, it may be
disturbed, and its needle may oscillate, by the smallest weight put into either of the
scales. This sensibility depends solely upon the centre or nail of suspension; and it
will be the more perfect the less friction there is between that knife-edge surface and the
plane which supports it. Both should therefore be as hard and highly polished as
possible; and should not be suffered to press against each other, except at the time of
weighing. Every delicate balance of moderate size, moreover, should be suspended
within a glass case, to protect it from the agitations of the air, and the corroding
influence of the weather. In some balances a ball is placed upon the index or needle,
(whether that index stand above or below the beam,) which may be made to approach
or recede from the beam by a fine-threaded screw, with the effect of varying the
centre of gravity relatively to the point of suspension, and thereby increasing, at
will, either the sensibility, or the stability of the balance. The greater the length
of the arms, the less distant the centre of gravity is beneath the centre of suspension,
the better polished its central knife-edge of 30°, the lighter the whole balance, and
the less it is loaded; the greater will be its sensibility. In all cases the arms must
be quite inflexible. A balance made by Ramsden for the Royal Society, is capable
of weighing ten pounds, and turns with one hundredth of a grain, which is the seven-millionth
part of the weight. In pointing out this balance to me one evening, Dr. Wollaston
told me it was so delicate, that Mr. Pond, then astronomer royal, when making
some observations with it, found its indications affected by his relative position before
it, although it was inclosed in a glass case. When he stood opposite the right arm,
that end of the beam preponderated, in consequence of its becoming expanded by the
radiation of heat from his body; and when he stood opposite the left arm, he made this
preponderate in its turn. It is probable that Mr. Pond had previously adjusted the
centres of gravity and suspension so near to each other as to give the balance its
maximum sensibility, consistent with stability. Were these centres made to coincide,
the beam, when the weights are equal, would rest in any position, and the addition of
the smallest weight would overset the balance, and place the beam in a vertical position,
from which it would have no tendency to return. The sensibility in this case would
be the greatest possible; but the other two requisites of level and stability would be
entirely lost. The case would be even worse if the centre of gravity were higher than
the centre of suspension, as the balance when deranged, if free, would make a revolution
of no less than a semi-circle. A balance may be made by a fraudulent dealer to weigh
falsely though its arms be equal, provided the suspension be as low as the centre of
gravity, for he has only to toss his tea, for instance, forcibly into one scale to cause
15 ounces of it, or thereby, to counterpoise a pound weight in the other. Inspectors
of weights, &c. are not au fait to this fruitful source of fraud among hucksters.
BALSAMS. (Baumes, Fr. Balsame, Germ.) Are native compounds of ethereal or
essential oils, with resin, and frequently benzoic acid. Most of them have the consistence
of honey; but a few are solid, or become so by keeping. They flow either
spontaneously, or by incisions made from trees and shrubs in tropical climates. They
possess peculiar powerful smells, aromatic hot tastes, but lose their odoriferous properties
by long exposure to the air. They are insoluble in water; soluble, to a considerable degree,
in ether; and completely in alcohol. When distilled with water,
ethereal oil comes over, and resin remains in the retort.
1. Balsams with benzoic acid:—
Balsam of Peru is extracted from the myroxylon peruiferum, a tree which grows in
Peru, Mexico, &c.; sometimes by incision, and sometimes by evaporating the decoction
of the bark and branches of the tree. The former kind is very rare, and is imported
in the husk of the cocoa nut, whence it is called balsam en coque. It is brown, transparent
only in thin layers, of the consistence of thick turpentine; an agreeable smell,
an acrid and bitter taste; formed of two matters, the one liquid, the other granular,
and somewhat crystalline. In 100 parts, it contains 12 of benzoic acid, 88 of resin,
with traces of a volatile oil.
The second sort, the black balsam of Peru, is much more common than the preceding,
translucent, of the consistence of well-boiled syrup, very deep red-brown
colour, an almost intolerably acrid and bitter taste, and a stronger smell than the other
balsam. Stoltze regards it as formed of 69 parts of a peculiar oil, 20·7 of a resin, little
soluble in alcohol, of 6·4 of benzoic acid, of 0·6 of extractive matter, and 0·9 of
water.
From its high price, balsam of Peru is often adulterated with copaiba, oil of turpentine,
and olive oil. One thousand parts of good balsam, should, by its benzoic
acid, saturate 75 parts of crystallised carbonate of soda. It is employed as a perfume
for pomatums, tinctures, lozenges, sealing-wax, and for chocolate and liqueurs, instead
of vanilla, when this happens to be very dear.
[84]
Liquid amber, Storax or Styrax, flows from the leaves and trunk of the liquid amber
styraciflua, a tree which grows in Virginia, Louisiana, and Mexico. It is brownish
ash-grey, of the consistence of turpentine, dries up readily, smells agreeably, like benzoin,
has a bitterish, sharp, burning taste; is soluble in 4 parts of alcohol, and contains
only 1·4 per cent. of benzoic acid.
Balsam of Tolu flows from the trunk of the myroxylon toluiferum, a tree which grows in
South America; it is, when fresh, of the consistence of turpentine, is brownish-red,
dries into a yellowish or reddish brittle resinous mass, of a smell like benzoin; is soluble
in alcohol and ether; affords, with water, benzoic acid.
Chinese varnish flows from the bark of the Augia sinensis; it is a greenish yellow
turpentine-like substance, smells aromatic, tastes strong and rather astringent, in thin
layers dries soon into a smooth shining lac, and consists of resin, ethereous oil, and benzoic
acid. It is soluble in alcohol and ether; and has been employed, immemorially,
in China, for lacquering and varnishing surfaces, either alone or coloured.
2. Balsams without benzoic acid:—
Copaiva balsam, balsam of copahu or capivi, is obtained from incisions made in the
trunk of the Copaifera officinalis, a tree which grows in Brazil and Cayenne. It is pale
yellow, middling liquid, clear transparent, has a bitter, sharp, hot taste; a penetrating
disagreeable smell; a specific gravity of from 0·950 to 0·996. It dissolves in absolute
alcohol, partially in spirit of wine, forms with alkalis, crystalline compounds. It consists
of 45·59 ethereous oil, 52·75 of a yellow brittle resin, and 1·66 of a brown viscid
resin. The oil contains no oxygen, has a composition like oil of turpentine, dissolves
caoutchouc (according to Durand), but becomes oxidised in the air, into a peculiar
species of resin. This balsam is used for making paper transparent, for certain lacquers,
and in medicine.
Mecca balsam, or opobalsam, is obtained both by incisions of, and by boiling, the
branches and leaves of the Balsamodendron Gileadense, a shrub which grows in Arabia
Felix, Lesser Asia and Egypt. When fresh it is turbid, whitish, becomes, by degrees,
transparent; yellow, thickish, and eventually solid. It smells peculiar, but agreeable;
tastes bitter and spicy; does not dissolve completely in hot spirit of wine, and contains
10 per cent. of ethereous oil, of the spec. grav. 0·876.
Japan lac varnish flows from incisions in the trunk of the Rhus Vernix (Melanorrhea
usitata) which is cultivated in Japan, and grows wild in North America. The juice
becomes black in the air; when purified, dissolves in very little oil; and, mixed with
colouring matter, it constitutes the celebrated varnish of the Japanese.
For Benzoin and Turpentine, see these articles in their alphabetical places.
BANDANNA. A style of calico printing, in which white or brightly coloured
spots are produced upon a red or dark ground. It seems to have been practised from
time immemorial in India, by binding up firmly with thread, those points of the cloth
which were to remain white or yellow, while the rest of the surface was freely subjected
to the dyeing operations.
The European imitations have now far surpassed, in the beauty and precision of
the design, the oriental patterns; having called into action the refined resources of
mechanical and chemical science. The general principles of producing bright figures
upon dark grounds, are explained in the article Calico-printing; but the peculiarities
of the Bandanna printing may be conveniently introduced here. In Brande’s Journal
for July 1823, I described the Bandanna gallery of Messrs. Monteith at Glasgow,
which, when in full action some years ago, might be reckoned the most magnificent and
profitable printing apartment in the world. The white spots were produced by a solution
of chlorine, made to percolate down through the Turkey red cotton cloth, in
certain points, defined and circumscribed by the pressure of hollow lead types in plates,
in a hydraulic press. Fig. 96., is an elevation of one press; A, the top or entablature;
B B, the cheeks or pillars; C, the upper block for fastening the upper lead perforated
pattern to; D, the lower block to which the fellow pattern is affixed, and which moves
up and down with the piston of the press; E, the piston or ram; F, the sole or
base; G, the water-trough, for the discharged or spotted calico to fall into; H, the
small cistern, for the aqueous chlorine or liquor-meter, with glass tubes for indicating
the height of liquor inside of the cistern; e e, glass stopcocks, for admitting the liquor
into that cistern from the general reservoir; f f, stopcocks for admitting water to wash
out the chlorine; g g, the pattern lead-plates, with screws for setting the patterns parallel
to each other; m m, projecting angular pieces at each corner, perforated with a half-inch
hole to receive the four guide-pins rising from the lower plate, which serve to secure
accuracy of adjustment between the two faces of the lead pattern plates; h h, two rollers
which seize and pull through the discharged pieces, and deliver them into the water-trough.
To the left of D there is a stopcock for filling the trough with water; l, is the
waste tube for chlorine liquor and water of washing. The contrivance for blowing a
stream of air across the cloth, through the pattern tubes, is not represented in the figure.
[85]
Sixteen engines, similar to the above, each possessing the power of pressing with
several hundred tons, are arranged in one line, in subdivisions of four; the spaces
between each subdivision serving as passages to allow the workmen to go readily
from the front to the back of the presses. Each occupies twenty-five feet, so that the
total length of the apartment is 100 feet.
To each press is attached a pair of patterns in lead, (or plates as they are called,) the
manner of forming which will be described in the sequel. One of these plates is fixed
to the upper block of the press. This block is so contrived, that it rests upon a kind of
universal joint, which enables this plate to apply more exactly to the under fellow-plate.
The latter sits on the moveable part of the press, commonly called the sill.
When this is forced up, the two patterns close on each other very nicely, by means of
the guide-pins at the corners, which are fitted with the utmost care.
The power which impels this great hydrostatic range is placed in a separate apartment,
called the machinery room. This machinery consists of two press cylinders
of a peculiar construction, having solid rams accurately fitted to them. To each of
these cylinders, three little force-pumps, worked by a steam-engine, are connected.
The piston of the large cylinder is eight inches in diameter, and is loaded with a
top-weight of five tons. This piston can be made to rise about two feet through a
leather-stuffing or collar. The other cylinder has a piston of only one inch in diameter,
which is also loaded with a top-weight of five tons. It is capable, like the other, of
being raised two feet through its collar.
Supposing the pistons to be at their lowest point, four of the six small force-pumps
are put in action by the steam-engine, two of them to raise the large piston, and two
the little one. In a short time, so much water is injected into the cylinders, that
the loaded pistons have arrived at their highest points. They are now ready for
working the hydrostatic discharge-presses, the water pressure being conveyed from
the one apartment to the other, under ground, through strong copper tubes, of small
calibre.
Two valves are attached to each press, one opening a communication between the large[86]
driving-cylinder and the cylinder of the press, the other between the small driving-cylinder
and the press. The function of the first is simply to lift the under-block of the press
into contact with the upper-block; that of the second, is to give the requisite compression
to the cloth. A third valve is attached to the press, for the purpose of discharging
the water from its cylinder, when the press is to be relaxed, in order to remove or draw
through the cloth.
From twelve to fourteen pieces of cloth, previously dyed Turkey-red, are stretched
over each other, as parallel as possible, by a particular machine. These parallel layers
are then rolled round a wooden cylinder, called by the workmen, a drum. This cylinder
is now placed in its proper situation at the back of the press. A portion of the fourteen
layers of cloth, equal to the area of the plates, is next drawn through between them,
by hooks attached to the two corners of the webs. On opening the valve connected with
the eight-inch driving-cylinder, the water enters the cylinder of the press, and instantly
lifts its lower block, so as to apply the under plate with its cloth, close to the upper one.
This valve is then shut, and the other is opened. The pressure of five tons in the one
inch prime-cylinder, is now brought to bear on the piston of the press, which is eight
inches in diameter. The effective force here will, therefore, be 5 tons × 82 = 320 tons;
the areas of cylinders being to each other, as the squares of their respective diameters.
The cloth is thus condensed between the leaden pattern-plates, with a pressure of 320
tons, in a couple of seconds;—a splendid example of automatic art.
The next step, is to admit the blanching or discharging liquor, (aqueous chlorine, obtained
by adding sulphuric acid to solution of chloride of lime,) to the cloth. This
liquor is contained in a large cistern, in an adjoining house, from which it is run at
pleasure into small lead cisterns H attached to the presses; which cisterns have graduated
index tubes, for regulating the quantity of liquor according to the pattern of discharge.
The stopcocks on the pipes and cisterns containing this liquor, are all made of glass.
From the measure-cistern H, the liquor is allowed to flow into the hollows in the upper
lead-plate, whence it descends on the cloth, and percolates through it, extracting in its
passage the Turkey-red dye. The liquor is finally conveyed into the waste pipe, from a
groove in the under block. As soon as the chlorine liquor has passed through, water is
admitted in a similar manner, to wash away the chlorine; otherwise, upon relaxing the
pressure, the outline of the figure discharged would become ragged. The passage of the
discharge liquor, as well as of the water through the cloth, is occasionally aided by a pneumatic
apparatus, or blowing machine; consisting of a large gasometer, from which air
subjected to a moderate pressure, may be allowed to issue, and act in the direction of the
liquid, upon the folds of the cloth. By an occasional twist of the air stopcock, the workman
also can ensure the equal distribution of the discharging liquor, over the whole excavations
in the upper plate. When the demand for goods is very brisk, the air apparatus
is much employed, as it enables the workman to double his product.
The time requisite for completing the discharging process in the first press is sufficient
to enable the other three workmen to put the remaining fifteen presses in play. The
discharger proceeds now from press to press, admits the liquor, the air, and the water;
and is followed at a proper interval by the assistants, who relax the press, move forwards
another square of the cloth, and then restore the pressure. Whenever the sixteenth press
has been liquored, &c., it is time to open the first press. In this routine, about ten minutes
are employed; that is 224 handkerchiefs (16 × 14) are discharged every ten minutes.
The whole cloth is drawn successively forward, to be successively treated in the above
method.
When the cloth escapes from the press, it is passed between the two rollers in front; from
which it falls into a trough of water placed below. It is finally carried off to the washing
and bleaching department, where the lustre of both the white and the red is considerably
brightened.
By the above arrangement of presses, 1600 pieces, consisting of 12 yards each = 19,200
yards, are converted into Bandannas in the space of ten hours, by the labour of four
workmen.
The patterns, or plates, which are put into the presses to determine the white figures
on the cloth, are made of lead in the following way. A trellis frame of cast-iron, one
inch thick, with turned-up edges, forming a trough rather larger than the intended lead
pattern, is used as the solid ground-work. Into this trough, a lead plate about one half
inch thick, is firmly fixed by screw nails passing up from below. To the edges of this lead
plate, the borders of the piece of sheet-lead are soldered, which covers the whole outer
surface of the iron frame. Thus a strong trough is formed, one inch deep. The upright
border gives at once great strength to the plate, and serves to confine the liquor. A thin
sheet of lead is now laid on the thick lead-plate, in the manner of a veneer on toilette-tables,
and is soldered to it round the edges. Both sheets must be made very smooth
beforehand, by hammering them on a smooth stone table, and then finishing with a plane:
the surface of the thin sheet (now attached), is to be covered with drawing paper, pasted[87]
on, and upon this the pattern is drawn. It is now ready for the cutter. The first thing
which he does, is to fix down with brass pins all the parts of the pattern which are to
be left solid. He now proceeds with the little tools generally used by block-cutters,
which are fitted to the different curvatures of the pattern, and he cuts perpendicularly
quite through the thin sheet. The pieces thus detached are easily lifted out; and thus
the channels are formed which design the white figures on the red cloth. At the bottom
of the channels, a sufficient number of small perforations are made through the
thicker sheet of lead, so that the discharging liquor may have free ingress and egress.
Thus, one plate is finished; from which, an impression is to be taken by means of printers’
ink, on the paper pasted upon another plate. The impression is taken in the hydrostatic
press. Each pair of plates constitutes a set, which may be put into the presses, and removed
at pleasure.
BARBERRY. The root of this plant contains a yellow colouring matter, which is
soluble in water and alcohol, and is rendered brown by alkalis. The solution is employed
in the manufacture of Morocco leather.
BARILLA. A crude soda, procured by the incineration of the salsola soda, a
plant cultivated for this purpose in Spain, Sicily, Sardinia, &c. Good barilla usually
contains, according to my analysis, 20 per cent. of real alkali, associated with muriates
and sulphates, chiefly of soda, some lime, and alumina, with very little sulphur. Caustic
lyes made from it, are used in the finishing process of the hard soap manufacture.
125,068 cwts. were imported in 1835, of which only 5,807 were exported. The duty
is 2s. per cwt. Of the above quantity, 64,174 came from Spain and the Balearic
islands, 39,943 from the Canaries, and 20,432 from Italy and the Italian islands.
BARIUM, the metallic basis of Baryta.
BARK OF OAK, for tanning. Unfortunately, the Tables of Revenue published
by the Board of Trade, mix up this bark and the dyeing barks together, and give the
sum of the whole for 1835, at 826,566 cwts., of which only 2,264 were re-exported.
The duty is 1d. per cwt. from British possessions, and 8d. from other parts.
BARLEY (Orge, Fr. Gerste, Germ.) English barley is that with two-rowed ears,
or the hordeum vulgare distichon of the botanists; the Scotch beer or bigg, is the hordeum
vulgare hexastichon. The latter has two rows of ears, but 3 corns come from the same
point, so that it seems to be six-eared. The grains of bigg are smaller than those
of barley, and the husks thinner. The specific gravity of English barley varies from
1·25 to 1·33; of bigg from 1·227 to 1·265; the weight of the husk of barley is 1⁄6,
that of bigg 2⁄9. 1000 parts of barley flour contain, according to Einhof, 720 of starch,
56 sugar, 50 mucilage, 36·6 gluten, 12·3 vegetable albumen, 100 water, 2·5 phosphate
of lime, 68 fibrous or ligneous matter. Sp. gravity of barley, is 1·235 by my trials.
BARM. The yeasty top of fermenting beer. See Beer, Distillation, Fermentation.
BARYTA or BARYTES, one of the simple earths. It may be obtained most
easily by dissolving the native carbonate of barytes (Witherite) in nitric acid, evaporating
the neutral nitrate till crystals be formed, draining and then calcining these in a
covered platina crucible, at a bright red heat. A less pure baryta may be obtained by
igniting strongly a mixture of the carbonate and charcoal, both in fine powder and
moistened. It is a grayish white earthy looking substance, fusible only at the jet of
the oxy-hydrogen blowpipe, has a sharp caustic taste, corrodes the tongue and all animal
matter, is poisonous even in small quantities, has a very powerful alkaline reaction;
a specific gravity of 4·0; becomes hot, and slakes violently when sprinkled with water,
falling into a fine white powder, called the hydrate of baryta, which contains 101⁄2 per
cent. of water, and dissolves in 10 parts of boiling water. This solution lets fall
abundant columnar crystals of hydrate of baryta as it cools; but it still retains one
twentieth its weight of baryta, and is called baryta water. The above crystals contain
61 per cent. of water, of which, by drying, they lose 50 parts. This hydrate may be
fused at a red heat without losing any more water. Of all the bases, baryta has the
strongest affinity for sulphuric acid, and is hence employed either in the state of the
above water, or in that of one of its neutral salts, as the nitrate or muriate, to detect the
presence, and determine the quantity of that acid present in any soluble compound.
Its prime equivalent, according to Berzelius, is 956,880, oxygen being 100; or 76,676,
hydrogen being 1,000. Native sulphate of baryta, or heavy spar, is fraudulently used
to adulterate white lead by the English dealers to a shameful extent.
BASSORINE. A constituent part of a species of gum which comes from Bassora,
as also of gum tragacanth, and of some gum resins. It is semi-transparent, difficult to
pulverise, swells considerably in cold or boiling water, and forms a thick mucilage
without dissolving. Treated with ten times its weight of nitric acid, it affords nearly
23 per cent. of its weight of mucic acid, being much more than is obtainable from gum
arabic or cherry-tree gum. Bassorine is very soluble in water slightly acidulated with[88]
nitric or muriatic acid. This principle is procured by soaking gum Bassora in a
great quantity of cold water, and in removing, by a filter, all the soluble parts.
BATHS. (Bains, Fr. Baden, Germ.) Warm baths have lately come into very
general use, and they are justly considered as indispensably necessary in all modern
houses of any magnitude, as also in club-houses, hotels, and hospitals. But the mode
of constructing these baths, and of obtaining the necessary supplies of hot and cold
water, does not appear to have undergone an improvement equal to the extension of
their employment.
The several points in regard to warm baths, are,
- The materials of which they are constructed.
- Their situation.
- The supply of cold water.
- The supply of hot water.
- Minor comforts and conveniences.
1. As to the materials of which they are constructed.—Of these the best are
slabs of polished marble, properly bedded with good water-tight cement, in a seasoned
wooden case, and neatly and carefully united at their respective edges. These, when
originally well constructed, form a durable, pleasant, and agreeable-looking bath; but
the expense is often objectionable, and, in upper chambers, the weight may prove
inconvenient. If of white or veined marble, they are also apt to get yellow or discoloured
by frequent use, and cannot easily be cleansed; so that large Dutch tiles, as
they are called, or square pieces of white earthenware, are sometimes substituted;
which, however, are difficultly kept water-tight; so that, upon the whole, marble is
preferable.
Where there are reasons for excluding marble, copper or tinned iron plate is the
usual material resorted to. The former is most expensive in the outfit, but far more
durable than the latter, which is, moreover, liable to leakage at the joints, unless most
carefully made. Either the one or the other should be well covered outside and inside,
with several coats of paint, which may then be marbled, or otherwise ornamented.
Wooden tubs, square or oblong, and oval, are sometimes used for warm baths; and
are cheap and convenient, but neither elegant nor cleanly. The wood always contracts
a mouldy smell; and the difficulty and nuisance of keeping them water-tight, and
preventing shrinkage, are such as to exclude them from all except extemporaneous
application.
2. As to the situation of the bath, or the part of the house in which it is to be
placed.—In hotels, and club-houses, this is a question easily determined: several baths
are usually here required, and each should have annexed to it, a properly warmed
dressing-room. Whether they are up stairs or down stairs, is a question of convenience,
but the basement story, in which they are sometimes placed, should always be avoided;
there is a coldness and dampness belonging to it, in almost all weathers, which is neither
agreeable nor salubrious.
In hospitals, there should be at least two or three baths on each side of the house,
(the men’s and women’s), and the supply of hot water should be ready at a moment’s
notice. The rooms in which the baths are placed should be light and comparatively
large and airy; and such conveniences for getting into and out of the bath should be
adopted, as the sick are well known to require. The dimensions of these baths should
also be larger than usual.
In private houses, the fittest places for warm baths are dressing-rooms annexed to
the principal bed-rooms; or, where such convenience cannot be obtained, a separate
bath-room, connected with the dressing-room, and always upon the bed-room floor. All
newly-built houses should be properly arranged for this purpose, and due attention
should be paid to the warming of the bath-room, which ought also to be properly ventilated.
A temperature of 70° may be easily kept up in it, and sufficient ventilation
is absolutely requisite, to prevent the deposition of moisture upon the walls and
furniture.
The objection which formerly prevailed, in respect to the difficulty of obtaining
adequate supplies of water, in the upper rooms, has been entirely obviated, by having
cisterns at or near the top of the house; and we would just hint that these should be
so contrived, as to be placed out of the reach of frost; a provision of the utmost importance
in every point of view, and very easily effected in a newly-built house, though
it unfortunately happens, that architects usually regard these matters as trifles, and
treat them with neglect, as indeed they do the warming and ventilation of buildings
generally.
3. The supply of water of proper quality and quantity, is a very important point,
as connected with the present subject. The water should be soft, clean, and pure; and
as free as possible from all substances mechanically suspended in it. In many cases,
it answers to dig a well for the exclusive supply of a large house with water. In most[89]
parts of London this may effectually be accomplished, at a comparatively moderate expense;
and, if the well be deep enough, the water will be abundant, soft, and pellucid.
The labour of forcing it by a pump to the top of the house, is the only drawback; this,
however, is very easily done by a horse-engine, or there are people enough about town,
glad to undertake it at a shilling a day. I am led to these remarks by observing the
filthy state of the water usually supplied, at very extravagant rates, by the water companies.
It often partakes more of the appearance of pea-soup than of the pure
element; fills our cisterns and pipes with mud and dirt, and, even when cleared by
subsidence, is extremely unpalatable. It deposits its nastiness in the pipes connected
with warm baths, and throws down a slippery deposit upon the bottom of the vessel
itself to such an extent, as often to preclude its being used, at least as a luxury, which
a clear and clean bath really is. This inconvenience may, in some measure, be avoided,
by suffering the water to throw down its extraneous matters upon the bottom of the
cistern, and drawing our supplies from pipes a little above it; there will, however, always
be more or less deposit in the pipes themselves; and every time the water runs
into the cistern, the grouts are stirred up, and diffused through its mass: this, from
some cause or other, has lately become an intolerable nuisance; and he who reflects
upon the miscellaneous contents of Thames water, will not have his appetite sharpened
by a draught of the Grand Junction beverage, nor feel reanimated and refreshed by
bathing in a compound so heterogeneous and unsavoury.
4. and 5. In public bathing establishments, where numerous and constant baths
are required, the simplest and most effective means of obtaining hot water for their
supply consists in drawing it directly into the baths from a large boiler, placed somewhere
above their level. This boiler should be supplied with proper feeding-pipes
and gauges; and, above all things, its dimensions should be ample; it should be of
wrought iron or copper, except where sea water is used, in which case the latter metal
is sometimes objectionable. The hot water should enter the bath by a pipe at least
an inch and a half in diameter; and the cold water by one of the same dimension, or
somewhat larger, so that the bath may not be long in filling. The relative proportions
of the hot and cold water are, of course, to be adjusted by a thermometer, and every bath
should have a two-inch waste-pipe, opening about two inches from the top of the bath,
and suffering the excess of water freely to run off; so that when a person is immersed
in the bath, or when the supplies of water are accidentally left open, there may be no
danger of an overflow.
Where there is a laundry in the upper story of the house, or other convenient place
for erecting a copper and its appurtenances, a plan similar to the above may often be
conveniently adopted in private houses, for the supply of a bath upon the principal bed-room
floor. An attempt is sometimes made to place boilers behind the fires of dressing-rooms,
or otherwise to erect them in the room itself, for the purpose of supplying
warm water; but this plan is always objectionable, from the complexity of the means
by which the supply of water is furnished to the boiler, and often dangerous from
the flues becoming choaked with soot, and taking fire. Steam is also apt, in such
cases, to escape in quantities into the room; so that it becomes necessary to search for
other methods of heating the bath; one or two of the least objectionable of which I
shall describe.
1. A contrivance of some ingenuity consists in suffering the water for the supply
of the bath to flow from a cistern above it, through a leaden pipe of about one inch
diameter, which is conducted into the kitchen or other convenient place where a large
boiler for the supply of hot water is required. The bath-pipe is immersed in this boiler,
in which it makes many convolutions, and, again emerging, ascends to the bath. The
operation is simply this:—the cold water passing through the convolutions of that
part of the pipe which is immersed in the boiling water, receives there sufficient heat for
the purpose required, and is delivered in that state by the ascending pipe into the bath,
which is also supplied with cold water and waste-pipes as usual. The pipe may be of
lead, as far as the descending and ascending parts are concerned, but the portion
forming the worm, or convolutions immersed in the boiler should be copper, in order
that the water within it may receive heat without impediment.
This plan is economical only where a large boiler is constantly kept at work in the
lower part of the house; otherwise, the trouble and expense of heating such a boiler,
for the mere purpose of the bath, render it unavailable. The worm-pipe is also apt to
become furred, upon the outside, by the deposition of the earthy impurities of the
water in which it is immersed; it then becomes a bad conductor of heat, is cleansed
with difficulty, and the plan is rendered ineffective. This system, however, has been
adopted, in some particular cases, with satisfaction.
2. A much more simple, economical, and independent mode of heating a warm
bath, by a fire placed at a distance from it, is the following, which is found to answer
perfectly in private houses, as well as upon a more extended scale in large establishments.[90]
It is certainly open to some objections, but these are overbalanced by its advantages.
A waggon-shaped boiler, holding about six gallons of water, is properly
placed over a small furnace, in any convenient and safe part of the house, as the kitchen,
scullery, servants’ hall, or wash-house. The bath itself, of the usual dimensions and
construction, is placed where it is wanted, with a due supply of cold water from
above. Two pipes issue from within an inch of the bottom of the bath at its opposite
extremities; one at the head of the bath, about one inch, and the other at the foot, an
inch and one eighth in diameter. These tubes descend to the boiler, the smaller one
entering it at the bottom, and the larger one issuing from its top.
Under these circumstances, supposing the pipes and boiler every where perfectly
tight, when the bath is filled, the water will descend into and expel the air from the
boiler, and completely fill it. Now, upon making a gentle fire under the boiler, an
ascending current of warm water will necessarily pass upwards through the larger pipe
which issues from its top, and cold water will descend by the pipe which enters at the
bottom; and thus, by the establishment of currents, the whole mass of water in the
bath will become heated to the desired point; or, if above it, the temperature may
easily be lowered by the admixture of cold water.
The advantages of this form of bath are numerous. The shorter the pipes of communication
the better, but they may extend forty or fifty feet without any inconvenience
beyond that of expense; so that there is no obstacle to the bath being near
the bed-room while the boiler is on the basement story. There is but little time required
for heating the bath; the water in which may, if requisite, be raised to about 100°
in about half an hour from the time of lighting the fire. The consumption of fuel
is also trifling.
The following are the chief disadvantages attendant upon this plan, and the means
of obviating them:—
It is necessary, when the water has acquired its proper temperature, to withdraw the
fire from the boiler, or not to use the bath immediately, as it may go on acquiring
some heat from the boiler, so that we may become inconveniently hot in the bath.
When, therefore, this bath is used, we may proceed as follows:—heat the water in it
an hour before it is wanted, to about 100°, and then extinguish the fire. The water
will retain its temperature, or nearly so, for three or four hours, especially if the bath
be shut up with a cover; so that when about to use it, cold water may be admitted till
the temperature is lowered to the required point, and thus all the above inconveniences
are avoided.
Another disadvantage of this bath arises from too fierce a fire being made under the
boiler, so as to occasion the water to boil within it, a circumstance which ought always
to be carefully avoided. In that case, the steam rising in the upper part of the boiler,
and into the top pipe, condenses there, and occasions violent concussions, the noise
of which often alarms the whole house, and leads to apprehensions of explosion, which,
however, is very unlikely to occur; but the concussions thus produced injure the pipes,
and may render them leaky: so that in regard to these, and all other baths, &c., we
may remark, that the pipes should pass up and down in such parts of the house as will
not be injured if some leakage takes place; and under the bath itself should be a sufficiently
large leaden tray with a waste-pipe, to receive and carry off any accidental
drippings, which might injure the ceilings of the rooms below. In all newly-built
houses, two or three flues should be left in proper places for the passage of ascending
and descending water-pipes; and these flues should in some way receive at their lower
part a little warm air in winter, to prevent the pipes freezing: the same attention should
also be paid to the situation of the cisterns of water in houses, which should be kept
within the house, and always supplied with a very ample waste-pipe, to prevent the
danger of overflow. Cisterns thus properly placed, and carefully constructed, should
be supplied from the water-mains by pipes kept under ground, till they enter the house,
and not carried across the area, or immediately under the pavement, where they are
liable to freeze.
3. Baths are sometimes heated by steam, which has several advantages: it may either
be condensed directly into the water of the bath, or, if the bath be of copper or tinned
iron, it may be conducted into a casing upon its outside, usually called a jacket; in
the latter case there must be a proper vent for the condensed water, and for the escape
of air and waste steam. Steam is also sometimes passed through a serpentine pipe,
placed at the bottom of the bath. But none of these methods are to be recommended
for adoption in private houses, and are only advisable in hospitals, or establishments
where steam boilers are worked for other purposes than the mere heating of baths.
Many copper and tin baths have been lately constructed in London, with a little
furnace attached to one end, and surrounded with a case or jacket, into which the water
flows and circulates backwards and forwards till the whole mass in the bath gets
heated to the due degree. One of the best of these is that constructed by Mr. Benham,[91]
of Wigmore Street. The bath must be placed near the fire-grate, and the smoke-pipe
of the attached furnace be conducted up the chimney a certain way to secure a sufficient
draught to maintain combustion. The above bath, well managed, heats the water from
50° to 98° in about 20 or 25 minutes, as I have experimentally proved. When the
proper temperature is attained, the fire must of course be extinguished.
BDELLIUM. A gum resin, produced by an unknown plant which grows in
Persia and Arabia. It comes to us in yellowish or reddish pieces, smells faintly,
like myrrh, and consists of 59 resin, 9·2 gum, 30·6 bassorine, and 1·2 ethereous oil.
BEER. (Bière, Fr. Bier, Germ.) The fermented infusion of malted barley, flavoured
with hops, constitutes the best species of beer; but there are many beverages of inferior
quality to which this name is given, such as spruce beer, ginger beer, molasses beer, &c.;
all of which consist of a saccharine liquor, partially advanced into the vinous fermentation,
and flavoured with peculiar substances.
The ancients were acquainted with beer, and the Romans gave it the appropriate
name of Cerevisia (quasi Ceresia), as being the product of corn, the gift of Ceres. The
most celebrated liquor of this kind in the old time, was the Pelusian potation, so called
from the town where it was prepared at the mouth of the Nile. Aristotle speaks of the
intoxication caused by beer; and Theophrastus very justly denominated it the wine of
barley. We may, indeed, infer from the notices found in historians, that drinks analogous
to our beer were in use among the ancient Gauls, Germans, and in fact almost every
people of our temperate zone; and they are still the universal beverage in every land
where the vine is not an object of rustic husbandry.
The manufacture of beer, or the art of brewing, may be conveniently considered under
five heads:—
1. An examination of the natural productions which enter into its composition; or of
barley and hops.
2. The changes which barley must undergo to fit it for making beer; or the processes
of malting and mashing.
3. The formation of a proper wort from the mashed malt and hops.
4. The fermentation of that wort; and
5. The fining, ripening, and preservation of the beer.
I. Of the materials.
1. Barley, wheat, maize, and several other kinds of corn are capable of undergoing those
fermentative changes, by which beer may be made; but the first substance is by far the
fittest. There are two species of barley, the hordeum vulgare or common barley, having
two seeds arranged in a row on its spikes; and the hordeum hexastichon, in which three
seeds spring from one point, so that its double row has apparently six seeds. The former
is the proper barley, and is much the larger sized grain; the latter is little known in
England, but is much cultivated in Scotland under the name of bear or big; being a
hardy plant adapted to a colder country. The finer the climate in which barley grows
the denser and larger its seed, and the thinner its husk; thus the Norfolk and Suffolk
barley is distinguished in these respects from that of Aberdeenshire. Big is a less compact
grain than barley; the weight of a Winchester bushel (2150·42 cubic inches) of
the former is only about 47 libs, while that of a bushel of the latter is nearly 51 libs.
Their constituents, however, bear much the same proportion to each other.
The quality of barley is proved not only by its density when dry, but by the increase
of volume which it acquires when steeped in water. Thus,
| |
100 |
measures |
of |
average |
English barley thereby |
swell into |
124. |
| |
100 |
— |
of |
— |
Scotch |
ditto, |
|
121. |
| |
100 |
— |
of |
— |
— |
bigg or bear, |
|
118. |
| Nay, |
100 |
of very fine Suffolk barley have swollen into |
183. |
| While |
100 |
of an inferior Scotch bigg became no more than |
109. |
This circumstance indicates so nearly the probable yield of malt, that it is carefully
attended to by the officers of excise, who gauge the steep cistern, and levy their duty in
conformity with the largest volume, 100 pounds of good barley become almost one half
heavier by the absorption of moisture; and weigh upon an average 147 pounds; the best
of course taking up most water.
By chemical analysis barley flour seems to consist of 67·18 parts of hordeine, or starch
and gluten intimately combined, 7·29 of vegetable fibre, 1·15 of coagulated albumen,
3·52 parts of gluten, 5·21 of sugar, 4·62 of gum, 0·24 of phosphate of lime, and 9·37 of
water. The loss amounted to 1·42. To these principles should be added a peculiar
volatile oil of a concrete nature, which is obtained during the process of distilling fermented
malt wash. (See Whiskey.) It may also be extracted from barley flour, by the
solvent action of alcohol; and never amounts to more than a few parts in the thousand.
The husk also contains some of that fetid oil. Proust thought that he had discovered in
barley a peculiar principle, to which he gave the name of hordeine, and which he separated
from starch by the action of both cold and boiling water. He found that by treating[92]
barley meal successively with water, he obtained from 89 to 90 parts of a farinaceous
substance, composed of from 32 to 33 of starch, and from 57 to 58 of hordeine. Einhof
obtained from barley seeds, 70·05 of flour, 18·75 of husks or bran, and 11·20 of water.
According to Proust hordeine is a yellowish powder, not unlike fine saw-dust. It
contains no azote, for it affords no ammonia by distillation, and is therefore very dissimilar
to gluten. In the germination of barley, which constitutes the process of malting,
the proportion of hordeine is greatly diminished by its conversion into sugar and starch.
Other chemists suppose that the hordeine of Proust is merely a mixture of the bran of
the barley with starch and gluten. It is obvious that the subject stands in need of new
chemical researches. In barley the husk constitutes from one fourth to one fifth of the
whole weight; in oats it constitutes one third; and in wheat, one tenth. From the analysis
of barley flour recently made, it appears to consist in 1000 parts: of water, 100; albumine,
22·3; sugar, 56; gum or mucilage, 50; gluten, 37·6; starch, 720; phosphate
of lime, 2·5.
2. The hop, humulus lupulus, the female flowers of the plant. Ives first directed attention
to a yellow pulverulent substance which invests the scales of the catkins, amounting to
about one eighth of their weight; and referred to it the valuable properties which hops impart
to beer. We may obtain this substance by drying the hops at a temperature of 86°
F., introducing them into a coarse canvass bag, and shaking it so that the yellow powder
shall pass through the pores of the canvass. This powder bears some resemblance to lycopodium.
Of the 13 parts in 100 of this powder, 4 parts are foreign matters, derived from
the scales of the cones; leaving 9 parts of a peculiar granular substance. When distilled
with water, this substance affords two per cent. of its weight (2⁄10 for 100 times the
weight of hops) of a volatile colourless oil, to which the plant owes its peculiar aroma.
This oil dissolves in water in considerable quantity. It appears to contain sulphur (for
it blackens solutions of silver), and also acetate of ammonia. No less than 65 per cent.
of the yellow dust is soluble in alcohol. This solution, treated with water and distilled,
leaves a resin, which amounts to 52·5 per cent. It has no bitter taste, and is soluble in
alcohol and ether. The watery solution from which the resin was separated contains the
bitter substance which has been called lupuline by Payen and Chevallier, mixed with a
little tannin and malic acid. To obtain this in a state of purity, the free acid must be
saturated with lime, the solution evaporated to dryness, and the residuum must be treated
with ether, which removes a little resin; after which the lupuline is dissolved out by alcohol,
which leaves the malate of lime. On evaporating away the alcohol, the lupuline
remains, weighing from 8·3 to 12·5 per cent. It is sometimes white, or slightly yellowish,
and opaque, sometimes orange yellow, and transparent. At ordinary temperatures
it is inodorous, but when heated strongly it emits the smell of hops. It possesses
the characteristic taste and bitterness of the hop. Water dissolves it only in the proportion
of 5 per cent., but it thereby acquires a pale yellow colour. Lupuline is neither
acid nor alkaline; it is acted upon neither by the dilute acids nor alkalies, nor by the
solutions of the metallic salts: it is quite soluble in alcohol, but hardly in ether. It
contains apparently no azote, for it affords no ammonia by destructive distillation; but
only an empyreumatic oil.
The yellow dust of hops contains, moreover, traces of a fatty matter, gum, a
small quantity of an azotised substance, and several saline combinations in minute
quantity. Boiling water dissolves from 19 to 31 per cent., of the contents of the dust, of
which a large proportion is resin. Ives thought that the scales of the catkins of hops, when
freed from the yellow powder, contained no principles analogous to it; but Payen and
Chevallier have proved the contrary. The cones of hop give up to boiling alcohol 36
per cent. of soluble matter; while the same cones, stripped of their yellow powder, yield
only 26 per cent.; and further, these chemists found the same principles in the different
parts of the hop, but in different proportions.
The packing of the hop catkins or cones is one of the most important operations
towards the preservation of this plant; and is probably the cause of the enormous difference
in value between the English and French hops after a few years’ keeping. The
former, at the end of six years, possess still great value, and may be sold as an article
only two or three years old; while the latter have lost the greater part of their value in
three years, and are no more saleable at the end of four. In France, it is packed merely
by tramping it with the feet in sacks. Under this slight pressure, large interstitial
spaces are left amid the mass of the hops, through which the air freely circulates, carrying
off the essential oil, and oxygenating some of the other proximate principles, so as
to render them inert. By the English method, on the contrary, the hops, after being
well rammed into strong sacks hung in frames, are next subjected to the action of a
hydraulic press. The valuable yellow powder thus inclosed on every side by innumerable
compact scales, is completely screened from the contact of the atmosphere, and
from all its vicissitudes of humidity. Its essential oil, in particular the basis of its flavour,
is preserved without decay.
[93]
According to the experiments of Chevallier and Payen upon the hops of England,
Flanders, the Netherlands, and the department of the Vosges, those of the county of
Kent afforded the largest cones, and were most productive in useful secreted and soluble
matters. Next to them were the hops of Alost.
The best hops have a golden yellow colour, large cones, an agreeable aroma; when
rubbed between the hands, they leave yellow traces, powerfully odoriferous, without any
broken portions of the plant, such as leaves, stems, and scaly fragments. When alcohol
is digested on good hops, from 9 to 12 per cent. of soluble yellow matter may be obtained
by evaporating it to dryness. This is a good test of their quality.
The best-flavoured and palest hops are packed in sacks of fine canvass, which are
called pockets, and weigh about 11⁄2 cwt. each. These are bought by the ale brewer.
The stronger-flavoured and darker-coloured hops are packed in bags of a very
coarse texture like door-mats, called hop bags: these contain generally about 3 cwt.,
and are sold to the porter and beer brewers. After the end of a year or two, hops
are reckoned to have lost much of their marketable value, and are then sold to the
second-rate porter brewers, under the name of old hops. The finest hops are grown in
the neighbourhood of Canterbury; but those of Worcester have an agreeable mildness of
flavour, greatly admired by many ale drinkers. When the bitter and aromatic principles
disappear, the hops are no better than so much chaff; therefore, an accurate chemical
criterion of their principles would be a great benefit to the brewer.
II. Malting.—This process consists of three successive operations; the steeping; the
couching, sweating, and flooring; and the kiln-drying.
The steeping is performed in large cisterns made of wood or stone, which being filled
with clear water up to a certain height, a quantity of barley is shot into them, and well
stirred about with rakes. The good grain is heavy, and subsides; the lighter grains,
which float on the surface, are damaged, and should be skimmed off; for they would
injure the quality of the malt, and the flavour of the beer made with it. They seldom
amount to more than two per cent. More barley is successively emptied into the steep
cistern, till the water stands only a few inches, about five, above its surface; when this
is levelled very carefully, and every light seed is removed. The steep lasts from forty
to sixty hours, according to circumstances; new barley requiring a longer period than
old, and bigg requiring much less time than barley.
During this steep, some carbonic acid is evolved from the grains, and combines with
the water, which, at the same time, acquires a yellowish tinge, and a strawy smell, from
dissolving some of the extractive matter of the barley husks. The grain imbibes about
one half its weight of water, and increases in size by about one-fifth. By losing this
extract, the husk becomes about one seventieth lighter in weight, and paler in colour.
The duration of the steep depends, in some measure, upon the temperature of the air,
and is shorter in summer than in winter. In general from 40 to 48 hours will be
found sufficient for sound dry grain. Steeping has for its object to expand the farina
of the barley with humidity, and thus prepare the seed for germination, in the same way
as the moisture of the earth prepares for the growth of the radicle and plumula in seed
sown in it. Too long continuance in the steep is injurious; because it prevents the
germination at the proper time, and thereby exhausts a portion of the vegetative power:
it causes also an abstraction of saccharine matter by the water. The maceration is known
to be complete when the grain may be easily transfixed with a needle, and is swollen
to its full size. The following is reckoned a good test:—If a barley-corn, when pressed
between the thumb and fingers, continues entire in its husk, it is not sufficiently steeped;
but if it sheds its flour upon the fingers, it is ready. When the substance exudes in the
form of a milky juice, the steep has been too long continued, and the barley is spoiled
for germination.
In warm weather it sometimes happens that the water becomes acescent before the
grain is thoroughly swelled. This accident, which is manifest to the taste and smell,
must be immediately obviated by drawing off the foul water through the tap at the
bottom of the cistern, and replacing it with fresh cold water. It does no harm to renew
it two or three times at one steep.
The couch.—The water being drawn off, and occasionally a fresh quantity passed
through, to wash away any slimy matter which may have been generated in warm
weather, the barley is now laid upon the couch floor of stone flags, in square heaps from
12 to 16 inches high, and left in that position for 24 hours. At this period, the bulk
of the grain being the greatest, it may be gauged by the revenue officers if they think
fit. The moisture now leaves the surface of the barley so completely, that it imparts
no dampness to the hand. By degrees, however, it becomes warm; the temperature
rising 10° above the atmosphere, while an agreeable fruity smell is evolved. At this time,
if the hand be thrust into the heap, it not only feels warm, but it gets bedewed with
moisture. At this sweating stage, the germination begins; the fibrils of the radicle
first sprout forth from the tip of every grain, and a white elevation appears, that soon[94]
separates into three or more radicles, which grow rapidly larger. About a day after
this appearance, the plumula peeps forth at the same point, proceeding thence beneath the
husk to the other end of the seed, in the form of a green leaflet.
The greatest heat of the couch is usually about 96 hours after the barley has been
taken out of the steep. In consequence, the radicles tend to increase in length with
very great rapidity, and must be checked by artificial means, which constitute the
chief art of the maltster. He now begins to spread the barley thinner on the floor,
and turns it over several times in the course of a day, bringing the portions of the
interior into the exterior surface. The depth, which was originally 15 or 16 inches,
is lowered a little at every turning over, till it be brought eventually down to three
or four inches. Two turnings a day are generally required. At this period of
spreading or flooring, the temperature in England is about 62°, and in Scotland 5 or 6
degrees lower.
About a day after the radicles appear, the rudiments of the stem, or of the plumula,
sprout forth, called by the English maltsters the acrospire. It issues from the same
end of the seed as the radicle, but turns round, and proceeds within the husk towards
the other end, and would there come forth as a green leaf, were its progress not
arrested. The malting, however, is complete before the acrospire becomes a leaf.
The barley couch absorbs oxygen and emits carbonic acid, just as animals do in
breathing, but to a very limited extent; for the grain loses only three per cent. of its
weight upon the malt floor, and a part of this loss is due to waste particles. As the
acrospire creeps along the surface of the seed, the farina within undergoes a remarkable
alteration. The gluten and mucilage disappear, in a great measure, the colour becomes
whiter, and the substance becomes so friable that it crumbles into meal between the
fingers. This is the great purpose of malting, and it is known to be accomplished
when the plumula or acrospire has approached the end of the seed. Now the further
growth must be completely stopped. Fourteen days may be reckoned the usual
duration of the germinating stage of the malting operations in England; but in
Scotland, where the temperature of the couch is lower, eighteen days or even twenty-one,
are sometimes required. The shorter the period within the above limits, the more
advantageous is the process to the maltster, as he can turn over his capital the sooner,
and his malt is also somewhat the better. Bigg is more rapid in its germination than
barley, and requires to be still more carefully watched. In dry weather it is sometimes
necessary to water the barley upon the couch.
Occasionally the odour disengaged from the couch is offensive, resembling that of
rotten apples. This is a bad prognostic, indicating either that the barley was of bad
quality, or that the workmen, through careless shovelling, have crushed a number of
the grains in turning them over. Hence when the weather causes too quick germination,
it is better to check it by spreading the heap out thinner than by turning it
too frequently over. On comparing different samples of barley, we shall find that the
best develope the germ or acrospire quicker than the radicles, and thus occasion a
greater production of the saccharine principle; this conversion advances along with the
acrospire, and keeps pace with it, so that the portion of the seed to which it has not
reached, is still in its unaltered starchy state. It is never complete for any single
barleycorn till the acrospire has come to the end opposite to that from which it sprung;
hence one part of the corn may be sugary, while the other is still insipid. If the
grain were allowed to vegetate beyond this term, the radicles being fully one third of
an inch long, the future stem would become visibly green in the exterior; it would
shoot forth rapidly, the interior of the grain would become milky, with a complete
exhaustion of all its useful constituents, and nothing but the husk would remain.
In France, the brewers, who generally malt their barley themselves, seldom leave it
on the couch more than 8 or 10 days, which, even taking into account the warmer
climate of their country, is certainly too short a period, and hence they make inferior
wort to the English brewer, from the same quantity of malt.
At the end of the germination, the radicles have become 11⁄2 longer than the barley,
and are contorted so that the corns hook into one another, but the acrospire is just
beginning to push through. A moderate temperature of the air is best adapted to
malting; therefore it cannot be carried on well during the heat of summer or the colds of
winter. Malt-floors should be placed in substantial thick-walled buildings, without
access of the sun, so that a uniform temperature of 59° or 60° may prevail inside.
Some recommend them to be sunk a little under the surface of the ground, if the
situation be dry.
During germination a remarkable change has taken place in the substance of the
grain. The glutinous constituent has almost entirely disappeared, and is supposed to
have passed into the matter of the radicles, while a portion of the starch is converted
into sugar and mucilage. The change is similar to what starch undergoes when
dissolved in water, and digested in a heat of about 160°F. along with a little gluten.[95]
The thick paste becomes gradually liquid, transparent, and sweet tasted, and the
solution contains now, sugar and gum, mixed with some unaltered starch. The gluten
suffers a change at the same time, and becomes acescent, so that only a certain quantity
of starch can be thus converted by a quantity of gluten. By the artificial growth upon
the malt-floor, all the gluten and albumen present in barley are not decomposed, and
only about one half of the starch is converted into sugar; the other half, by a continuance
of the germination, would only go to the growth of the roots and stems of the plant;
but it receives its nearly complete conversion into sugar without any notable waste of
substance in the brewer’s operation of mashing.
The kiln-drying.—When the malt has become perceptibly dry to the hand upon
the floor, it is taken to the kiln, and dried hard with artificial heat, to stop all further
growth, and enable it to be kept, without change, for future use, at any time. The
malt-kiln, which is particularly described in the next page, is a round or a square
chamber, covered with perforated plates of cast iron, whose area is heated by a stove or
furnace, so that not merely the plates on which the malt is laid are warmed, but the air
which passes up through the stratum of malt itself, with the effect of carrying off very
rapidly the moisture from the grains. The layer of malt should be about 3 or 4 inches
thick, and evenly spread, and its heat should be steadily kept at from the 90th to the
100th degree of Fahrenheit’s scale, till the moisture be mostly exhaled from it. During
this time the malt must be turned over at first frequently, and latterly every three or
four hours. When it is nearly dry, its temperature should be raised to from 145° to
165°F., and it must be kept at this heat till it has assumed the desired shade of colour,
which is commonly a brownish-yellow or a yellowish-brown. The fire is now allowed
to die out, and the malt is left on the plates till it has become completely cool; a result
promoted by the stream of cool air, which now rises up through the bars of the grate;
or the thoroughly dry browned malt may, by damping the fire, be taken hot from the
plates, and cooled upon the floor of an adjoining apartment. The prepared malt must
be kept in a dry loft, where it can be occasionally turned over till it is used. The
period of kiln-drying should not be hurried. Many persons employ two days in this
operation.
According to the colour and the degree of drying, malt is distributed into three sorts;
pale, yellow, and brown. The first is produced when the highest heat to which it has
been subjected is from 90° to 100° F.; the amber yellow, when it has suffered a heat
of 122°; and the brown when it has been treated as above described. The black malt
used by the porter brewer to colour his beer, has suffered a much higher heat, and is
partially charred. The temperature of the kiln should, in all cases, be most gradually
raised, and most equably maintained. If the heat be too great at the beginning, the
husk gets hard dried, and hinders the evaporation of the water from the interior substance;
and should the interior be dried by a stronger heat, the husk will probably split,
and the farina become of a horny texture, very refractory in the mash-tun. In general,
it is preferable to brown malt, rather by a long-continued moderate heat, than by a
more violent heat of shorter duration, which is apt to carbonise a portion of the mucilaginous
sugar, and to damage the article. In this way, the sweet is sometimes converted
into a bitter principle.
During the kiln-drying, the roots and acrospire of the barley become brittle, and
fall off; and are separated by a wire sieve whose meshes are too small to allow the
malt itself to pass through.
A quantity of good barley, which weighs 100 pounds, being judiciously malted, will
weigh, after drying and sifting, 80 pounds. Since the raw grain, dried by itself at
the same temperature as the malt, would lose 12 per cent. of its weight in water, the
malt process dissipates out of these remaining 88 pounds, only 8 pounds, or 8 per cent.
of the raw barley. This loss consists of—
| 1 |
1⁄2 |
per cent. |
dissolved out in the steep water, |
| 3 |
|
— |
dissipated in the kiln, |
| 3 |
|
— |
by the falling of the fibrils, |
| |
1⁄2 |
— |
of waste. |
The bulk of good malt exceeds that of the barley from which it was made, by about
8 or 9 per cent.
The operation of kiln-drying is not confined to the mere expulsion of the moisture
from the germinated seeds; but it serves to convert into sugar a portion of the starch
which remained unchanged, and that in a twofold way; first, by the action of the
gluten upon the fecula at an elevated temperature, as also by the species of roasting
which the starch undergoes, and which renders it of a gummy nature. (See Starch.)
We shall have a proof of this explanation, if we dry one portion of the malt in a
naturally dry atmosphere, and another in a moderately warm kiln; the former will
yield less saccharine extract than the latter. Moreover, the kiln-dried malt has a peculiar,
agreeable, and faintly burned taste, probably from a small portion of empyreumatic[96]
oil formed in the husk, and which not only imparts its flavour to the beer, but
also contributes to its preservation. It is therefore obvious, that the skilful preparation
of the malt must have the greatest influence both on the quantity and quality
of the worts to be made from it. If the germination be pushed too far, a part of the
extractible matter is wasted; if it has not advanced far enough, the malt will be too
raw, and too much of its substance will remain as an insoluble starch; if it is too
highly kiln-dried, a portion of its sugar will be caramelised, and become bitter; and if
the sweating was imperfect or irregular, much of the barley may be rendered lumpy
and useless. Good malt is distinguishable by the following characters:—
The grain is round and full, breaks freely between the teeth, and has a sweetish
taste, an agreeable smell, and is full of a soft flour from end to end. It affords no
unpleasant flavour on being chewed; it is not hard, so that when drawn along an oaken
table across the fibres, it leaves a white streak, like chalk. It swims upon water, while
unmalted barley sinks in it. Since the quality of the malt depends much on that of
the barley, the same sort only should be used for one malting. New barley germinates
quicker than old, which is more dried up; a couch of a mixture of the two would be
irregular, and difficult to regulate.
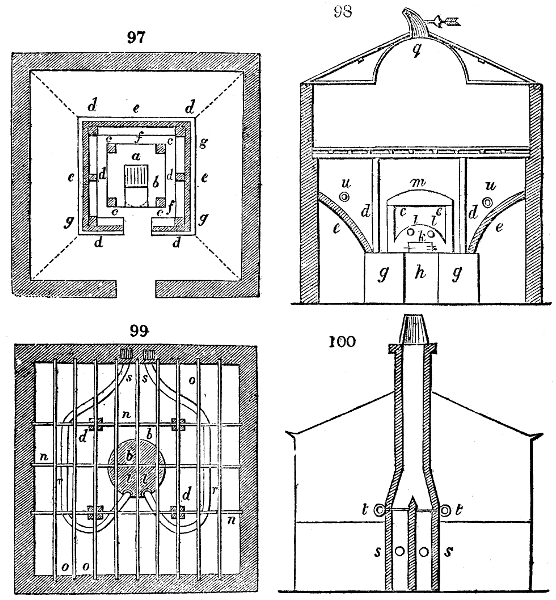
Description of the malt kiln.—Figs. 97, 98, 99, 100. exhibit the construction of a well-contrived
malt kiln. Fig. 97. is the ground plan; fig. 98. is the vertical section; and
figs. 99. and 100., a horizontal and vertical section in the line of the malt-plates. The same
letters denote the same parts in each of the figures. A cast-iron cupola-shaped oven is
supported in the middle, upon a wall of brickwork four feet high; and beneath it, are the
grate and its ash-pit. The smoke passes off through two equi-distant pipes into the chimney.
The oven is surrounded with four pillars, on whose top a stone lintel is laid: a is
the grate, 9 inches below the sole of the oven b; c c c c are the four nine-inch strong
pillars of brickwork which bear the lintel m; d d d d d d are strong nine-inch pillars,
which support the girder and joists upon which perforated plates repose; e denotes a
vaulted arch on each of the four sides of the oven; f is the space between the kiln
and the side arch, into which a workman may enter, to inspect and clean the kiln; g g,
the walls on either side of the kiln, upon which the arches rest, h, the space for the ashes
to fall; k, the fire-door of the kiln; l l, junction-pieces to connect the pipes r r with
the kiln; the mode of attaching them is shown in fig. 99. These smoke-pipes lie
about three feet under the iron plates, and at the same distance from the side walls;
they are supported upon iron props, which are made fast to the arches. In fig. 98., u[97]
shows their section; at s s, fig. 99., they enter the chimney, which is provided with two
register or damper plates, to regulate the draught through the pipes. These registers
are represented by t t, fig. 100., which shows a perpendicular section of the chimney.
m, fig. 98., is the lintel which causes the heated air to spread laterally instead of
ascending in one mass in the middle, and prevents any combustible particles from falling
upon the iron cupola. n n are the main girders of iron for the iron beams o o, upon
which the perforated plates p lie; q, fig. 98., is the vapour pipe in the middle of the roof,
which allows the steam of the drying malt to escape. The kiln may be heated either
with coal or wood.
The size of this kiln is about 20 feet square; but it may be made proportionally
either smaller or greater. The perforated floor should be large enough to receive the
contents of one steep or couch.
The perforated plate might be conveniently heated by steam pipes, laid zig-zag, or in
parallel lines under it; or a wire-gauze web might be stretched upon such pipes. The
wooden joists of a common floor would answer perfectly to support this steam-range, and
the heat of the pipes would cause an abundant circulation of air. For drying the pale
malt of the ale brewer, this plan is particularly well adapted.
The kiln-dried malt is sometimes ground between stones in a common corn mill, like
oatmeal; but it is more generally crushed between iron rollers, at least for the purposes
of the London brewers.
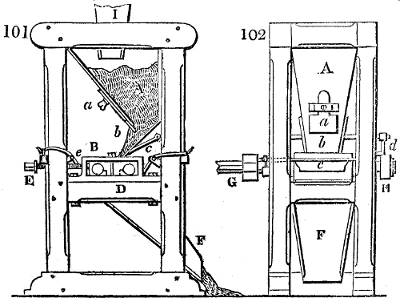
The crushing mill.—The cylinder malt-mill is constructed as shown in fig. 101, 102.
I is the sloping-trough, by which the malt is let down from its bin or floor to the
hopper A of the mill, whence
it is progressively shaken in
between the rollers B D. The
rollers are of iron, truly cylindrical,
and their ends rest in
bearers of hard brass, fitted into
the side frames of iron. A screw
E goes through the upright,
and serves to force the bearer
of the one roller towards that of
the other, so as to bring them
closer together when the crushing
effect is to be increased. G
is the square end of the axis,
by which one of the rollers
may be turned either by the
hand or by power; the other
derives its rotatory motion from a pair of equal-toothed wheels H, which are fitted to
the other end of the axes of the rollers. d is a catch which works into the teeth of
a ratchet-wheel on the end of one of the rollers (not shown in this view). The lever
c strikes the trough b at the bottom of the hopper, and gives it the shaking motion for
discharging the malt between the rollers, from the slide sluice a. e e, fig. 101., are
scraper-plates of sheet iron, the edges of which press by a weight against the surfaces
of the rollers, and keep them clean.
Instead of the cylinders, some employ a crushing mill of a conical-grooved form
like a coffee mill, upon a large scale. (See the general plan, infrà.)
The mashing and boiling.—Mashing is the operation by which the wort is extracted,
or eliminated from the malt, and whereby a saccharo-mucilaginous extract is made
from it. The malt should not in general be ground into a fine meal, for in that case
it would be apt to form a cohesive paste with hot water, or to set, as it is called, and to
be difficult to drain. In crushed malt, the husk remains nearly entire, and thus helps
to keep the farinaceous particles open and porous to the action of the water. The bulk
of the crushed malt is about one-fifth greater than that of the whole, or one bushel of
malt gives a bushel and a quarter of crushed malt. This is frequently allowed to lie
a few days in a cool place, in order that it may attract moisture from the air, which it
does very readily by its hygrometric power. Thus, the farinaceous substance which
had been indurated in the kiln, becomes soft, spongy, and fit for the ensuing process
of watery extraction.
Mashing has not for its object merely to dissolve the sugar and gum already present
in the malt, but also to convert into a sweet mucilage the starch which had remained
unchanged during the germination. We have already stated that starch, mixed with
gluten, and digested for some time with hot water, becomes a species of sugar. This
conversion takes place in the mash-tun. The malted barley contains not only a portion
of gluten, but diastase more than sufficient to convert the starch contained in it, by
this means, into sugar.
[98]
The researches of Payen and Persoz show, that the mucilage formed by the reaction
of malt upon starch, may either be converted into sugar, or be made into permanent
gum, according to the temperature of the water in which the materials are digested.
We take of pale barley malt, ground fine, from 6 to 10 parts, and 100 parts of starch; we
heat, by means of a water-bath, 400 parts of water in a copper, to about 80°F.; we
then stir in the malt, and increase the heat to 140°F., when we add the starch,
and stir well together. We next raise the temperature to 158°, and endeavour to
maintain it constantly at that point, or at least to keep it within the limits of 167° on
the one side, and 158° on the other. At the end of 20 or 30 minutes, the original milky
and pasty solution becomes thinner, and soon after as fluid nearly as water. This is the
moment in which the starch is converted into gum, or into that substance which the
French chemists call dextrine, from its power of polarising light to the right hand, whereas
common gum does it to the left. If this merely mucilaginous solution, which seems to
be a mixture of gum with a little liquid starch and sugar, be suitably evaporated, it may
serve for various purposes in the arts to which gum is applied, but with this view, it
must be quickly raised to the boiling point, to prevent the farther operation of the malt
upon it. If we wish, on the contrary, however, to promote the saccharine fermentation,
for the formation of beer, we must maintain the temperature at between 158° and 167°
for three or four hours, when the greatest part of the gum will have passed into sugar,
and by evaporation of the liquid at the same temperature, a starch syrup may be obtained
like that procured by the action of sulphuric acid upon starch. The substance, which operates
in the formation of sugar, or is the peculiar ferment of the sugar fermentation, may
be considered as a residuum of the gluten or vegetable albumen in the germinating
grain: it is reckoned by Payen and Persoz, a new proximate principle called diastase,
which is formed during malting, in the grains of barley, oats, and wheat, and may be
separated in a pure state, if we moisten the malt flour for a few minutes in cold water,
press it out strongly, filter the solution, and heat the clear liquid in a water bath, to the
temperature of 158°. The greater part of that albuminous azotised substance is thus
coagulated, and is to be separated by a fresh filtration; after which, the clear liquid is
to be treated with alcohol, when a flocky precipitate appears, which is diastase. To purify
it still further, especially from the azotised matter, we should dissolve it in
water, and precipitate again with alcohol. When dried at a low temperature, it appears
as a solid white substance, which contains no azote; is insoluble in alcohol, but dissolves
in water and proof spirit. Its solution is neutral and tasteless; when left to itself, it
changes with greater or less rapidity according to the temperature, and becomes sour at
a temperature of from 149° to 167°. It has the property of converting starch into gum
(dextrine) and sugar, and indeed, when sufficiently pure, with such energy that one
part of it disposes 2000 parts of dry starch to that change, but it operates the quicker the
greater its quantity. Whenever the solution of diastase with starch or with dextrine is
heated to the boiling point, it loses the sugar-fermenting property. One hundred parts
of well-malted starch appear to contain about one part of this substance.
We can now understand the theory of malting, and the limits between which the
temperature of the liquor, ought to be maintained in this operation; namely, the range
between 157° and 160°F. It has been ascertained as a principle in mashing, that
the best and soundest extract of the malt, is to be obtained, first of all, by beginning to
work with water at the lowest of these heats, and to conclude the mash with water at
the highest. Secondly, not to operate the extraction at once with the whole of the water
that is to be employed; but with separate portions and by degrees. The first portion is
added with the view of penetrating equally the crushed malt, and of extracting the
already formed sugar; the next for effecting the sugar fermentation by the action of the
diastase. By this means also, the starch is not allowed to run into a cohesive paste, and
the extract is more easily drained from the poorer mass, and comes off in the form of a
nearly limpid wort. The thicker moreover, or the less diluted the mash is, so much the
easier is the wort fined in the boiler or copper by the coagulation of the albuminous
matter: these principles illustrate, in every condition, the true mode of conducting
the mashing process; but different kinds of malt require a different treatment. Pale
and slightly kilned malt requires a somewhat lower heat than malt highly kilned, because
the former has more undecomposed starch, and is more ready to become pasty. The
former also, for the same reason, needs a more leisurely infusion than the latter, for its
conversion into mucilaginous sugar. The more sugar the malt contains, the more is its
saccharine fermentation accelerated by the action of the diastase. What has been here
said of pale malt, is still more applicable to the case of a mixture of raw grain with malt,
for it requires still gentler heats, and more cautious treatment.
III. The mash-tun is a large circular tub with a double bottom; the uppermost of
which is called a false bottom, and is pierced with many holes. There is a space of about
2 or 3 inches between the two, into which the stopcocks enter, for letting in the water
and drawing off the wort. The holes of the false bottom should be burned, and not bored,[99]
to prevent the chance of their filling up by the swelling of the wood, which would
obstruct the drainage: the holes should be conical, and largest below, being about 3⁄8 of
an inch there, and 1⁄8 at the upper surface. The perforated bottom must be fitted truly at
the sides of the mash-tun, so that no grains may pass through. The mashed liquor is let
off into a large back, from which it is pumped into the wort coppers. The mash-tun
is provided with a peculiar rotatory apparatus for agitating the crushed grains and water
together, which we shall presently describe. The size of the wort copper is proportional
to the amount of the brewing, and it must, in general, be at least so large as to
operate upon the whole quantity of wort made from one mashing; that is, for every
quarter of malt mashed, the copper should contain 140 gallons. The mash-tun ought to
be at least a third larger, and of a conical form, somewhat wider below than above.
The quantity of water to be employed for mashing, or the extraction of the wort, depends
upon the greater or less strength to be given to the beer. The seeds of the crushed
malt, after the wort is drawn off, retain still about 32 gallons of water for every quarter
of malt. In the boiling, and evaporation from the coolers, 40 gallons of water are dissipated
from one quarter of malt; constituting 72 gallons in all. If 13 quarters of
barley be taken to make 1500 gallons of beer, 2400 gallons of water must therefore be
required for the mashing. This example will give an idea of the proportions for an
ordinary quality of beer.
When the mash is to begin, the copper must be filled with water, and heated. As soon
as the water has attained the heat of 145° in summer, or 167° in winter, 600 gallons of it
are to be run off into the mash-tun, and the 13 quarters of crushed malt are to be gradually
thrown in and well intermixed by proper agitation, so that it may be uniformly
moistened, and no lumps may remain. After continuing the agitation in this way for one
half or three-quarters of an hour, the water in the copper will have approached to its boiling
point, when 450 gallons at the temperature of about 200° are to be run into the
mash-tun, and the agitation is to be renewed till the whole assumes an equally fluid
state: the tun is now to be well covered for the preservation of its heat, and to be
allowed to remain at rest for an hour, or an hour and a half. The mean temperature of
this mash may be reckoned at about 145°. The time which is necessary for the transmuting
heat of the remaining starch into sugar depends on the quality of the malt.
Brown malt requires less time than pale malt, and still less than a mixture with raw
grain, as already explained. After the mash has rested the proper time, the tap of the
tun is opened, and the clear wort is to be drawn out into the under back. If the wort
that first flows is turbid, it must be returned into the tun, till it runs clear. The amount
of this first wort may be about 675 gallons. Seven hundred and fifty gallons of water
at the temperature of 200° are now to be introduced up through the drained malt, into
the tun, and the mixture is to be agitated till it becomes uniform, as before. The mash-tun
is then to be covered, and allowed to remain at rest for an hour. The temperature
of this mash is from 167° to 174°. While the second mash is making, the worts of the
first are to be pumped into the wort copper, and set a-boiling as speedily as possible.
The wort of the second mash is to be drawn off at the proper time, and added to the
copper as fast as it will receive it, without causing the ebullition to stop.
A third quantity of water amounting to 600 gallons, at 200°, is to be introduced into
the mash-tun, and after half an hour, is to be drawn off, and either pumped into the wort
copper, or reserved for mashing fresh malt, as the brewer may think fit.
The quantity of extract, per barrel weight, which a quarter of malt yields to wort,
amounts to about 84 lbs. The wort of the first extract is the strongest; the second
contains, commonly, one-half the extract of the first; and the third, one-half of the
second; according to circumstances.
To measure the degrees of concentration of the worts drawn off from the tun, a particular
form of hydrometer, called a saccharometer, is employed, which indicates the
number of pounds weight of liquid contained in a barrel of 36 gallons imperial measure.
Now, as the barrel of water weighs 360 lbs., the indication of the instrument when
placed in any wort, shows by how many pounds a barrel of that wort is heavier than a
barrel of water; thus, if the instrument sinks with its poise till the mark 10 is upon a
line with the surface of the liquid, it indicates that a barrel of that wort weighs ten
pounds more than a barrel of water. See Saccharometer.
Or, supposing the barrel of wort weighs 396 lbs., to convert that number into specific
gravity, we have the following simple rule:—
360 : 396 ∷ 100 : 1·100;
at which density, by my experiments, the wort contains 25 per cent., of solid extract.
Having been employed to make experiments on the density of worts, and the fermentative
changes which they undergo, for the information of a committee of the House of
Commons, which sat in July and August, 1830, I shall here introduce a short abstract
of that part of my evidence which bears upon the present subject.
My first object was to clear up the difficulties which, to common apprehension, hung[100]
over the matter, from the difference in the scales of the saccharometers in use among the
brewers and distillers of England and Scotland. I found that one quarter of good malt
would yield to the porter brewer a barrel Imperial measure of wort, at the concentrated
specific gravity of 1·234. Now, if the decimal part of this number be multiplied by 360,
being the number of pounds weight of water in the barrel, the product will denote the
excess in pounds, of the weight of a barrel of such concentrated wort, over that of a barrel
of water, and that product is, in the present case, 84·24 pounds.
Mr. Martineau, jun., of the house of Messrs. Whitbread and Company, and a gentleman
connected with another great London brewery, had the kindness to inform me that
their average product from a quarter of malt was a barrel of 84 lbs. gravity. It is obvious,
therefore, that by taking the mean operation of two such great establishments, I
must have arrived very nearly at the truth.
It ought to be remarked that such a high density of wort as 1·234 is not the result of
any direct experiment in the brewery, for infusion of malt is never drawn off so strong;
that density is deduced by computation from the quantity and quality of several successive
infusions; thus, supposing a first infusion of the quarter of malt to yield a barrel of
specific gravity 1·112, a second to yield a barrel at 1·091, and a third a barrel at 1·031,
we shall have three barrels at the mean of these three numbers, or one barrel at their
sum, equal to 1·234.
I may here observe that the arithmetical mean or sum is not the true mean or sum of
the two specific gravities; but this difference is either not known or disregarded by the
brewers. At low densities this difference is inconsiderable, but at high densities it would
lead to serious errors. At specific gravity 1·231, wort or syrup contains one half of its
weight of solid pure saccharum, and at 1·1045 it contains one fourth of its weight; but
the brewer’s rule, when here applied, gives for the mean specific gravity 1·1155 =
1·231 + 1·0002.
The contents in solid saccharine matter at that density are however 271⁄4
per cent. showing the rule to be 21⁄4 lbs. wrong in excess on 100 lbs., or 9 lbs. per barrel.
The specific gravity of the solid dry extract of malt wort is 1·264; it was taken in oil
of turpentine, and the result reduced to distilled water as unity. Its specific volume is
0·7911, that is, 10 lbs. of it will occupy the volume of 7·911 lbs. of water. The mean
specific gravity, by computation of a solution of that extract in its own weight of water,
is 1·1166; but by experiment, the specific gravity of that solution is 1·216, showing
considerable condensation of volume in the act of combination with water.
The following Table shows the relation between the specific gravities of solutions of
malt extract, and the per-centage of solid extract they contain:
Extr.
Malt. |
Water. |
Malt
Extract
in 100. |
Sugar
in 100. |
Specific
gravity. |
| 600 |
+ |
600 |
50·00 |
47·00 |
1·2180 |
| 600 |
+ |
900 |
40·0 |
37·00 |
1·1670 |
| 600 |
+ |
1200 |
33·3 |
31·50 |
1·1350 |
| 600 |
+ |
1500 |
28·57 |
26·75 |
1·1130 |
| 600 |
+ |
1800 |
25·00 |
24·00 |
1·1000 |
The extract of malt was evaporated to dryness, at a temperature of about 250° F.,
without the slightest injury to its quality, or any empyreumatic smell. Bate’s tables
have been constructed on solutions of sugar, and not with solutions of extract of malt,
or they agree sufficiently well with the former, but differ materially from the latter.
Allan’s tables give the amount of a certain form of solid saccharine matter extracted
from malt, and dried at 175° F., in correspondence to the specific gravity of the solution;
but I have found it impossible to make a solid extract from infusions of malt, except at
much higher temperatures than 175° F. Indeed, the numbers on Allan’s saccharometer
scale clearly show that his extract was by no means dry: thus, at 1·100 of gravity he assigns
29·669 per cent. of solid saccharine matter; whereas there is at that density of solid extract
only 25 per cent. Again, at 1·135, Allan gives 40 parts per cent. of solid extract,
whereas there are only 331⁄3 present.
By the triple mashing operations above described, the malt is so much exhausted that
it can yield no further extract useful for strong beer or porter. A weaker wort might
no doubt still be drawn off for small beer, or for contributing a little to the strength of
the next mashing of fresh malt. But this I believe is seldom practised by respectable
brewers, as it impoverishes the grains which they dispose of for feeding cattle.
The wort should be transferred into the copper, and made to boil as soon as possible,
for if it remains long in the under-back it is apt to become acescent. The steam moreover
raised from it in the act of boiling serves to screen it from the oxygenating or acidifying
influence of the atmosphere. Until it begins to boil, the air should be excluded
by some kind of a cover.
[101]
Sometimes the first wort is brewed by itself into strong ale, the second by itself into
an intermediate quality; and the third into small beer; but this practice is not much
followed in this country.
We shall now treat of the boiling in of the hops. The wort drawn from the mash-tun,
whenever it is pumped into the copper, must receive its allowance of hops. Besides
evaporating off a portion of the water, and thereby concentrating the wort, boiling has a
twofold object. In the first place, it coagulates the albuminous matter, partly by the
heat, and partly by the principles in the hops, and thereby causes a general clarification
of the whole mass, with the effect of separating the muddy matters in a flocculent form.
Secondly, during the ebullition, the residuary starch and hordeine of the malt are converted
into a limpid sweetish mucilage, the dextrine above described; while some of the
glutinous stringy matter is rendered insoluble by the tannin principle of the hops, which
favours still further the clearing of the wort. By both operations the keeping quality
of the beer is improved. This boil must be continued during several hours; a longer
time for the stronger, and a shorter for the weaker beers. There is usually one seventh
or one sixth part of the water dissipated in the boiling copper. This process is known
to have continued a sufficient time, if the separation of the albuminous flocks is distinct,
and if these are found, by means of a proof gauge suddenly dipped to the bottom, to be
collected there, while the supernatant liquor has become limpid. Two or three hours’
boil is deemed long enough in many well-conducted breweries; but in some of those in
Belgium, the boiling is continued from 10 to 15 hours, a period certainly detrimental to
the aroma derived from the hop.
Many prefer adding the hops when the wort has just come to the boiling point.
Their effect is to repress the further progress of fermentation, and especially the passage
into the acetous stage, which would otherwise inevitably ensue in a few days. In this
respect, no other vegetable production hitherto discovered can be a substitute for the
hop. The odorant principle is not so readily volatilised as would at first be imagined;
for when hop is mixed with strong beer wort, and boiled for many hours, it can still
impart a very considerable degree of its flavour to weaker beer. By mere infusion in
hot beer or water, without boiling, the hop loses very little of its soluble principles.
The tannin of the hop combines, as we have said, with the vegetable albumen of the
barley, and helps to clarify the liquor. Should there be a deficiency of albumen and
gluten, in consequence of the mashing having been done at such a heat as to have
coagulated them beforehand, the defect may be remedied by the addition of a little
gelatine to the wort copper, either in the form of calf’s foot, or of a little isinglass.
If the hops be boiled in the wort for a longer period than 5 or 6 hours, they lose a
portion of their fine flavour; but if their natural flavour be rank, a little extra boiling
improves it. Many brewers throw the hops in upon the surface of the boiling wort,
and allow them to swim there for some time, that the steam may penetrate them, and
open their pores for a complete solution of their principles when they are pushed
down into the liquor. It is proper to add the hops in considerable masses, because
in tearing them asunder, some of the lupuline powder is apt to be lost.
The quantity of hop to be added to the wort varies according to the strength of the
beer, the length of time it is to be kept, or the heat of the climate where it is intended
to be sent. For strong beer, 41⁄2 lbs. of hops are required to a quarter of malt, when it
is to be highly aromatic and remarkably clear. For the stronger kinds of ale and
porter, the rule, in England, is to take a pound of hops for every bushel of malt, or 8 lbs.
to a quarter. Common beer has seldom more than a quarter of a pound of hops to the
bushel of malt.
It has been attempted to form an extract of hops by boiling in covered vessels, so as
not to lose the oil, and to add this instead of the hop itself to the beer. On the great
scale this method has no practical advantage, because the extraction of the hop is perfectly
accomplished during the necessary boiling of the wort, and because the hop operates
very beneficially, as we have explained, in clarifying the beer. Such an extract, moreover,
could be easily adulterated.
Of the Coolers.—The contents of the copper are run into what is called the
hop-back, on the upper part of which is fixed a drainer, to keep back the hops. The
pump is placed in the hop-back, for the purpose of raising the wort to the coolers,
usually placed in an airy situation upon the top of the brewery. Two coolers are
indispensable when we make two kinds of beer from the same brewing, and even in
single brewings, called gyles, if small beer is to be made. One of these coolers ought
to be placed above the level of the other. As it is of great consequence to cool the
worts down to the fermenting pitch as fast as possible, various contrivances have been
made for effecting this purpose. The common cooler is a square wooden cistern, about
6 inches deep, and of such an extent of surface that the whole of one boil may only
occupy 2 inches, or thereabouts, of depth in it. For a quantity of wort equal to about
1500 gallons its area should be at least 54 feet long and 20 feet wide. The seams of[102]
the cooler must be made perfectly water-tight and smooth, so that no liquor may lodge
in them when they are emptied. The utmost cleanliness is required, and an occasional
sweetening with lime-water.
The hot wort reaches the cooler at a temperature of from 200° to 208°, according to
the power of the pump. Here it should be cooled to the proper temperature for the
fermenting tun, which may vary from 54° to 64°, according to circumstances. The
refrigeration is accomplished by the evaporation of a portion of the liquor: it is more
rapid in proportion to the extent of the surface, to the low temperature, and the dryness
of the atmosphere surrounding the cooler. The renewal of a body of cool dry air by
the agency of a fan, may be employed with great advantage. The cooler itself must
be so placed that its surface shall be freely exposed to the prevailing wind of the district,
and be as free as possible from the eddy of surrounding buildings. It is thought by
many, that the agitation of the wort during its cooling, is hurtful. Were the roof
made moveable, so that the wort could be readily exposed, in a clear night, to the aspect
of the sky, it would cool rapidly by evaporation, on the principles explained by Dr.
Wells, in his “Essay on Dew.”
When the cooling is effected by evaporation alone, the temperature falls very slowly,
even in cold air, if it be loaded with moisture. But when the air is dry, the evaporation
is vigorous, and the moisture exhaled does not remain incumbent on the liquor, as in
damp weather, but is diffused widely in space. Hence we can understand how wort
cools so rapidly in the spring and autumn, when the air is generally dry, and even
more quickly than in winter, when the air is cooler, but loaded with moisture. In
fact, the cooling process goes on better when the atmosphere is from 50° to 55°, than
when it falls to the freezing point, because in this case, if the air be still, the vapours
generated remain on the surface of the liquor, and prevent further evaporation. In
summer the cooling can take place only during the night.
In consequence of the evaporation during this cooling process, the bulk of the worts
is considerably reduced; thus, if the temperature at the beginning was 208°, and if it
be at the end 64°, the quantity of water necessary to be evaporated to produce this
refrigeration would be nearly 1⁄8 of the whole, putting radiation and conduction of heat
out of the question. The effect of this will be a proportional concentration of
the beer.
The period of refrigeration in a well-constructed cooler, amounts to 6 or 7 hours in
favourable weather, but to 12 or 15 in other circumstances. The quality of the beer is
much improved by shortening this period; because, in consequence of the great surface
which the wort exposes to the air, it readily absorbs oxygen, and passes into the acetous
fermentation with the production of various mouldy spots; an evil to which ill-hopped
beer is particularly liable. Various schemes have been contrived to cool wort, by
transmitting it through the convolutions of a pipe immersed in cold water. The
best plan is to expose the hot wort for some hours freely to the atmosphere and the
cooler, when the loss of heat is most rapid by evaporation and other means, and when
the temperature falls to 100°, or thereby, to transmit the liquor through a zig-zag pipe,
laid almost horizontally in a trough of cold water. The various methods described
under Refrigerator are more complex, but they may be practised in many situations
with considerable advantage.
Whilst the wort reposes in the cooler, it lets fall a slight sediment, which consists
partly of fine flocks of coagulated albumen combined with tannin, and partly of starch,
which had been dissolved at the high temperature, and separates at the lower. The wort
should be perfectly limpid, for a muddy liquor never produces transparent beer. Such
beer contains, besides mucilaginous sugar and gum, usually some starch, which even
remains after the fermentation, and hinders its clarifying, and gives it a tendency to
sour. The wort contains more starch the hotter it has been mashed, the less hops have
been added, and the shorter time it has been boiled. The presence of starch in the
wort may be made manifest by adding a little solution of iodine in alcohol to it, when
it will become immediately blue. We thus see that the tranquil cooling of wort in a
proper vessel has an advantage over cooling it rapidly by a refrigeratory apparatus.
When the wort is sufficiently cool, it is let down into the fermenting tun. In this
transfer, the cooling might be carried several degrees lower, were the wort made to pass
down through a tube inclosed in another tube, along which a stream of cold water is
flowing in the opposite direction, as we have described in the sequel of Acetic Acid.
These fermenting tuns are commonly called gyle-tuns, or working tuns, and are either
square or circular, the latter being preferable on many accounts.
IV. Of the Fermentation.—In the great London breweries, the size of these fermenting
tuns is such that they contain from 1200 to 1500 barrels. The quantity of wort
introduced at a time must, however, be considerably less than the capacity of the vessel,
to allow room for the head of yeast which rises during the process; if the vessel be
cylindrical, this head is proportional to the depth of the worts. In certain kinds of[103]
fermentation, it may rise to a third of that depth. In general, the fermentation proceeds
more uniformly and constantly in large masses, because they are little influenced
by vicissitudes of temperature; smaller vessels, on the other hand, are more easily
handled. The general view of fermentation will be found under that title; I shall
here make a few remarks on what is peculiar to beer. During the fermentation of
wort, a portion of its saccharine matter is converted into alcohol, and wort thus
changed, is beer. It is necessary that this conversion of the sugar be only partial, for
beer which contains no undecomposed sugar would soon turn sour, and even in the
casks its alcohol undergoes a slow fermentation into vinegar. The amount of this
excess of sugar is greater in proportion to the strength of the wort, since a certain
quantity of alcohol, already formed, prevents the operation of the ferment on the remaining
wort. Temperature has the greatest influence upon the fermentation of wort.
A temperature of from 55° to 60° of the liquor, when that of the atmosphere is 55°,
is most advantageous for the commencement. The warmth of the wort as it comes
into the gyle-tun must be modified by that of the air in the apartment. In winter,
when this apartment is cold, the wort should not be cooled under 64° or 60°, as in that
case the fermentation would be tedious or interrupted, and the wort liable to spoil or
become sour. In summer, when the temperature of the place rises to above 75°, the
wort should be cooled, if possible, down to 55°, for which purpose it should be let in
by the system of double pipes, above mentioned. The higher the temperature of the
wort, the sooner will the fermentation begin and end, and the less is it in our power
to regulate its progress. The expert brewer must steer a middle course between these
two extremes, which threaten to destroy his labours. In some breweries a convoluted
pipe is made to traverse or go round the sides of the gyle-tun, through which warm
water is allowed to flow in winter, and cold in summer, so as to modify the temperature
of the mass to the proper fermenting pitch. If there be no contrivance of this
kind, the apartment may be cooled in summer, by suspending wet canvas opposite the
windows in warm weather, and kindling a small stove within it in cold.
When the wort is discharged into the gyle-tun, it must receive its dose of yeast,
which has been previously mixed with a quantity of the wort, and left in a warm place
till it has begun to ferment. This mixture, called lobb, is then to be put into the tun, and
stirred well through the mass. The yeast should be taken from similar beer. Its quantity
must depend upon the temperature, strength, and quantity of the wort. In general, one
gallon of yeast is sufficient to set 100 gallons of wort in complete fermentation. An excess
of yeast is to be avoided, lest the fermentation should be too violent, and be finished
in less than the proper period of 6 or 8 days. More yeast is required in winter than
summer; for, at a temperature of 50°, a double quantity may be used to that at 68°.
Six or eight hours after adding the yeast, the tun being meanwhile covered, the fermentation
becomes active: a white milky-looking froth appears, first on the middle, and
spreads gradually over the whole surface; but continues highest in the middle, forming
a frothy elevation, the height of which increases with the progress of the fermentation,
and whose colour gradually changes to a bright brown, the result, apparently, of the
oxidation of the extractive contained in this yeasty top. This covering screens the
wort from the contact of the atmospherical air. During this time, there is a perpetual
disengagement of carbonic acid gas, which is proportional to the quantity of sugar converted
into alcohol. The warmth of the fermenting liquid increases at the same time,
and is at a maximum when the fermentation has come to its highest point. This
increase of temperature amounts to from 9° to 14° or upwards, and is the greater the
more rapid the fermentation. But in general, the fermentation is not allowed to proceed
so far in the gyle-tun, for after it is advanced a little way, the beer is cleansed, that
is, drawn off into other vessels, which are large barrels set on end, with large openings in
their top, furnished with a sloping tray for discharging an excess of yeast into the wooden
trough, in which the stillions stand. These stillions are placed in communication with
a store-tub, which keeps them always full, by hydrostatic pressure, so that the head of
yeast may spontaneously flow over, and keep the body of liquor in the cask clean. This
apparatus will be explained in describing the brewery plant. See the figures, infrà.
It must be observed, that the quantity of yeast, and the heat of fermentation, differ
for every different quality of beer. For mild ale, when the fermentation has reached 75°,
its first flavour begins; at 80° the flavour increases; at 85° it approaches the high
flavour; at 90° it is high; but it may be carried to 100° and upwards, for particular
purposes. A wort of 30 lbs. per barrel (sp. gr. 1·088), ought to increase about 15°,
so that in order to arrive at 80°, it should be set at 65°. The quantity of yeast for such
an ale should be from 2 to 3 lbs. per barrel. The higher the heat, the less yeast is necessary.
If the heat of the fermentation should at any time fall, it must be raised by a
supply of fresh yeast, well stirred in; but this practice is not advisable in general,
because rousing the worts in the gyle-tun is apt to communicate a rank flavour of yeast
to the ale. It is the practice of many experienced brewers to look every 2 hours into the[104]
gyle-tun, chiefly with the view of observing the progress of the heat, which is low at
first, but afterwards often increases half a degree per hour, and subsequently declines, as
the fermentation approaches its conclusion, till at length the heat becomes uniform, or
sometimes decreases, before the fermentation is finished, especially where the quantity
operated upon is small.
Some brewers recommend, when the fermentation is carried to its utmost period, to
add about 7 lbs. of wheat or bean flour to a gyle-tun of 25 or 30 barrels, at the time of
cleansing, so as to quicken the discharge of the yeast, by disengagement of more carbonic
acid. The flour should be whisked up in a pail, with some of the beer, till the lumps
are broken, and then poured in. By early cleansing, the yeast is preserved longer in a
state proper for a perfect fermentation than by a contrary practice.
For old ale, which is to be long kept, the heat of the fermentation should not exceed
75°, but a longer time is required to complete the fermentation and ensure the future
good flavour of the ale.
For porter, the general practice is, to use from 4 to 41⁄2 lbs. of hops per barrel for keeping;
though what is termed mild or mixing porter, has not more than 3 or 31⁄2 lbs. The
heat of fermentation must not exceed 70°, and begin about 60°. If the heat tend to
increase much above that pitch in the gyle-tun, the porter should be cleansed, by means
of the stillions. At this period of the fermentation, care should be taken that the sweetness
of the malt be removed, for which purpose more yeast may be used than with any
other beer of the same strength. The quantity is from 3 to 4 lbs. per barrel, rousing the
wort in the gyle-tun every 2 hours in the day-time.
When the plan of cleansing casks is not employed, the yeast is removed from the
surface of the fermenting tun by a skimmer, and the clear beer beneath is then drawn
off into the ripening tuns, called store-vats, in which it is mixed up with different
brewings, to suit the taste of the customers. This transfer must take place whenever
the extrication of carbonic acid has nearly ceased; lest the alcohol formed should dissolve
some of the floating yeast, acquire thereby a disagreeable taste, and pass partially
into the acetous state.
In this process, during the formation of vinous spirit at the expense of the sugar, the
albumen and gluten diffused through the beer, being acted upon by the alcohol, become
insoluble; one portion of them is buoyed to the top with the carbonic acid gas, to form
the frothy yeast; and another portion falls to form the bottom barm. The former
consists of the same materials as the wort, with a large proportion of gluten, which forms
its active constituent; the latter is a peculiar deposit, consisting of the same gluten
mixed with the various dense impurities of the wort, and may be also used as a ferment,
but is cruder than the floating yeast. The amount of yeast is proportional to the activity
of the fermentation, or extrication of carbonic acid gas, as also to the heat of the mashing
process, and the quantity of starch or flour unaltered by germination. Pale malt affords
usually, more yeast than malt highly kilned. When the yeast becomes excessive, from
too violent fermentation, it should be skimmed off from time to time, which will tend to
cool the liquor and moderate the intestine changes.
After the beer is let down into the close store-tuns in the cellar, an obscure fermentation
goes on, for a considerable period in its body, which increases its spirituous
strength, and keeps up in it a constant impregnation of carbonic acid gas, so as to render
it lively and agreeable to the taste, when it is casked off for sale. It would appear, that
beer is never stationary in quality, while it is contained in the tuns; for the moment
when it ceases to improve by the decomposition of its residuary sugar, it begins to
degenerate into vinegar. This result may be produced either by the exhaustion of the
saccharine, or by the fermentative matter. The store cellar should therefore be under
ground, free from alternations of temperature, vibrations of carriages, and as cool as
possible. In the great London breweries, the fermentation is rendered very complete
in the cleansing butts; so that a slow and steady ripening is ensured in the great store-tuns.
The gyle-tuns are too capacious to permit the fermentation to be finished, with
either safety or sufficient dispatch in them.
V. Of ripening different kinds of Beer.—The varieties of beer depend either upon
the difference of their materials, or from a different management of the brewing processes.
With regard to the materials, beers differ in the proportion of their malt, hops, and
water; and in the different kinds of malt or other grain. To the class of table or
small beers, all those sorts may be referred whose specific gravity does not exceed 1·025,
which contain about 5 per cent. of malt extract, or nearly 18 pounds per barrel. Beers
of middling strength may be reckoned those between the density of 1·025 and 1·040;
which contain, at the average, 7 per cent., or 25 pounds per barrel. The latter may
be made with 400 quarters of malt to 1500 barrels of beer. Stronger beers have a
specific gravity of from 1·050 to 1·080, and take from 45 to 75 quarters of malt to
the same quantity of beer. The strongest beer found in the market is some of the
English and Scotch ales, for which from 18 to 27 quarters of malt are taken for 1500[105]
gallons of beer. Good porter requires from 16 to 18 quarters for that quantity. Beers
are sometimes made with the addition of other farinaceous matter to the malt; but
when the latter constitutes the main portion of the grain, the malting of the other
kinds of corn becomes unnecessary, for the diastase of the barley-malt changes the
starch into sugar during the mashing operation. Even with entirely raw grain, beer
is made in some parts of the Continent, the brewers trusting the conversion of the
starch into sugar to the action of the gluten alone, at a low mashing temperature, on
the principle of Saussure’s and Kirchoff’s researches.
The colour of the beer depends upon the colour of the malt, and the duration of the
boil in the copper. The pale ale is made, as we have stated, from steam or sun-dried
malt, and the young shoots of the hop; the deep yellow ale from a mixture of pale
yellow and brown malt; and the dark brown beer from well-kilned and partly carbonised
malt, mixed with a good deal of the pale, to give body. The longer and more
strongly heated the malt has been in the kiln, the less weight of extract, cæteris paribus,
does it afford. In making the fine mild ales, high temperatures ought to be avoided,
and the yeast ought to be skimmed off, or allowed to flow very readily from its top, by
means of the cleansing butt system, so that little ferment being left in it to decompose
the rest of the sugar, the sweetness may remain unimpaired. With regard to porter,
in certain breweries, each of the three kinds of malt employed for it is separately
mashed, after which the first and the half of the second wort is boiled along with the
whole of the hops, and thence cooled and set to ferment in the gyle-tun. The third
drawn wort, with the remaining half of the second, is then boiled with the same hops,
saved by the drainer, and, after cooling, added to the former in the gyle-tun, when the
two must be well roused together.
It is obvious, from the preceding development of principles, that all amylaceous and
saccharine materials, such as potatoes, beans, turnips, as well as cane and starch syrup,
molasses, &c., may be used in brewing beer. When, however, a superior quality of
brown beer is desired, malted barley is indispensable, and even with these substitutes
a mixture of it is most advantageous. The washed roots of the common carrot, of the
red and yellow beet, or of the potato, must be first boiled in water, and then mashed
into a pulp. This pulp must be mixed with water in the copper, along with wheaten
or oat meal, and the proper quantity of hops, then boiled during 8 or 9 hours. This
wort is to be cooled in the usual way, and fermented, with the addition of yeast.
A much better process is that now practised, on a considerable scale, at Strasbourg, in
making the ale, for which that city is celebrated. The mashed potatoes are mixed with
from a twentieth to a tenth of their weight of finely ground barley malt, and some
water. The mixture is exposed, in a water-bath, to a heat of 160° F. for four hours,
whereby it passes into a saccharine state, and may then be boiled with hops, cooled, and
properly fermented into good beer.
Maize, or Indian corn, has also been employed to make beer; but its malting is
somewhat difficult on account of the rapidity and vigour with which its radicles and
plumula sprout forth. The proper mode of causing it to germinate is to cover it, a few
inches deep, with common soil, in a garden or field, and to leave it there till the bed is
covered with green shoots of the plant. The corn must be then lifted, washed, and
exposed to the kiln.
The Difference of the Fermentation.—The greater or less rapidity with which the
worts are made to ferment has a remarkable influence upon the quality of the beer,
especially in reference to its fitness for keeping. The wort is a mucilaginous solution
in which the yeastly principles, eliminated by the fermentation, will, if favoured by
regular and slow intestine movements, completely rise to the surface, or sink to the
bottom, so as to leave the body fine. But, when the action is too violent, these barmy
glutinous matters get comminuted and dispersed through the liquor, and can never afterwards
be thoroughly separated. A portion of the same feculent matter becomes, moreover,
permanently dissolved, during this furious commotion, by the alcohol that is generated.
Thus the beer loses not merely its agreeable flavour and limpidity, but is apt to spoil
from the slightest causes. The slower, more regularly progressive, and less interrupted,
therefore, the fermentation is, so much better will the product be.
Beer, in its perfect condition, is an excellent and healthful beverage, combining, in
some measure, the virtues of water, of wine, and of food, as it quenches thirst, stimulates,
cheers, and strengthens. The vinous portion of it is the alcohol, proceeding from the
fermentation of the malt sugar. Its amount, in common strong ale or beer, is about
4 per cent., or four measures of spirits, specific gravity 0·825 in 100 measures of the
liquor. The best brown stout porter contains 6 per cent., the strongest ale even 8 per
cent.; but common beer only one. The nutritive part of the beer is the undecomposed
gum-sugar, and the starch-gum, not changed into sugar. Its quantity is very variable,
according to the original starch of the wort, the length of the fermentation, and the age
of the beer.
[106]
The main feature of good beer is fine colour and transparency; the production of
which is an object of great interest to the brewer. Attempts to clarify it in the cask
seldom fail to do it harm. The only thing that can be used with advantage for fining
foul or muddy beer, is isinglass. For porter, as commonly brewed, it is frequently had
recourse to. A pound of good isinglass will make about 12 gallons of finings. It is
cut into slender shreds, and put into a tub with as much vinegar or hard beer as will
cover it, in order that it may swell and dissolve. In proportion as the solution proceeds,
more beer must be poured upon it, but it need not be so acidulous as the first, because,
when once well softened by the vinegar, it readily dissolves. The mixture should be
frequently agitated with a bundle of rods, till it acquires the uniform consistence of thin
treacle, when it must be equalised still more by passing through a tammy cloth, or a
sieve. It may now be made up with beer to the proper measure of dilution. The
quantity generally used is from a pint to a quart per barrel, more or less, according to
the foulness of the beer. But before putting it into the butt, it should be diffused
through a considerable volume of the beer with a whisk, till a frothy head be raised
upon it. It is in this state to be poured into the cask, briskly stirred about; after
which the cask must be bunged down for at least 24 hours, when the liquor should be
limpid. Sometimes the beer will not be improved by this treatment; but this should
be ascertained beforehand, by drawing off some of the beer into a cylindric jar or phial,
and adding to it a little of the finings. After shaking and setting down the glass, we
shall observe whether the feculencies begin to collect in flocky parcels, which slowly
subside; or whether the isinglass falls to the bottom without making any impression
upon the beer. This is always the case when the fermentation is incomplete, or a secondary
decomposition has begun. Mr. Jackson has accounted for this clarifying effect
of isinglass in the following way.
The isinglass, he thinks, is first of all rather diffused mechanically, than chemically
dissolved, in the sour beer or vinegar, so that when the finings are put into the foul beer,
the gelatinous fibres, being set free in the liquor, attract and unite with the floating feculencies,
which before this union were of the same specific gravity with the beer, and
therefore could not subside alone; but having now acquired additional weight by the
coating of fish-glue, precipitate as a flocculent magma. This is Mr. Jackson’s explanation;
to which I would add, that if there be the slightest disengagement of carbonic
acid gas, it will keep up an obscure locomotion in the particles, which will prevent the said
light impurities, either alone or when coated with isinglass, from subsiding. The beer
is then properly enough called stubborn by the coopers. But the true theory of the action
of isinglass is, that the tannin of the hops combines with the fluid gelatine, and forms a
flocculent mass, which envelopes the muddy particles of the beer, and carries them to the
bottom as it falls, and forms a sediment. When after the finings are poured in, no
proper precipitate ensues, it may be made to appear by the addition of a little decoction
of hop.
Mr. Richardson, the author of the well-known brewer’s saccharometer, gives the
following as the densities of different kinds of beer:—
| Beer. |
Pounds
per
Barrel. |
Specific
Gravity. |
| Burton ale, 1st sort |
40 |
to |
43 |
1·111 to 1·120 |
| Burton ale, 2d ditto |
35 |
to |
40 |
1·097 to 1·111 |
| Burton ale, 3d ditto |
28 |
to |
33 |
1·077 to 1·092 |
| Common ale |
25 |
to |
27 |
1·070 to 1·073 |
| Ditto ditto |
|
21 |
|
1·058 |
| Porter, common sort |
|
18 |
|
1·050 |
| Ditto, double |
|
20 |
|
1·055 |
| Ditto, brown stout |
|
23 |
|
1·064 |
| Ditto, best brown stout |
|
26 |
|
1·072 |
| Common small beer |
|
6 |
|
1·014 |
| Good table beer |
12 |
to |
14 |
1·033 to 1·039 |
Of Returns or Malt Residuums.—When small beer is brewed after ale or porter, only
one mash is to be made; but where this is not done, there may be two mashes, in order
to economise malt to the utmost. We may let on the water at 160° or 165°, in any
convenient quantity, infuse for an hour or thereby, then run it off, and pump into the
copper, putting some hops into it, and causing it to boil for an instant; when it may be
transferred to the cooler. A second mash or return may be made in the same manner,
but at a heat 5° lower; and then disposed of in the boiler with some hops, which may
remain in the copper during the night at a scalding heat, and may be discharged into
the cooler in the morning. These two returns are to be let down into the under-back
immediately before the next brewing, and thence heated in the copper for the next[107]
mashing of fresh malt, instead of hot water, commonly called liquor, in the breweries.
But allowance must be made, in the calculation of the worts, for the quantity of fermentable
matter in these two returns. The nett aggregate saving is estimated from the
gravity of the return taken when cold in the cooler. A slight economy is also made in
the extra boiling of the used hops. The lapse of a day or two between the consecutive
brewings is no objection to the method of returns, because they are too weak in saccharine
matter to run any risk of fermentation.
In conclusion, it may be remarked that Mr. Richardson somewhat underrates the
gravity of porter, which is now seldom under 20 lbs. per barrel. The criterion for transferring
from the gyle-tun to the cleansing butts is the attenuation caused by the production
of alcohol in the beer: when that has fallen to 10 lbs. or 11 lbs., which it usually
does in 48 hours, the cleansing process is commenced. The heat is at this time generally
75°, if it was pitched at 65°; for the heat and the attenuation go hand in hand.
About thirty years ago, it was customary for the London brewers of porter, to keep
immense stocks of it for eighteen months or two years, with the view of improving its
quality. The beer was pumped from the cleansing butts into store-vats, holding from
twenty to twenty-five gyles or brewings of several hundred barrels each. The store-vats
had commonly a capacity of 5000 or 6000 barrels; and a few were double, and one was
treble, this size. The porter, during its long repose in these vats, became fine, and by
obscure fermentation its saccharine mucilage was nearly all converted into vinous liquor,
and dissipated in carbonic acid. Its hop-bitter was also in a great degree decomposed.
Good hard beer was the boast of the day. This was sometimes softened by the publican,
by the addition of some mild new-brewed beer. Of late years, the taste of the metropolis
has undergone such a complete revolution in this respect, that nothing but the
mildest porter will now go down. Hence, six weeks is a long period for beer to be
kept in London; and much of it is drunk when only a fortnight old. Ale is for the
same reason come greatly into vogue; and the two greatest porter houses, Messrs.
Barclay, Perkins, & Co., and Truman, Hanbury, & Co., have become extensive and successful
brewers of mild ale, to please the changed palate of their customers.
We shall add a few observations upon the brewing of Scotch ale. This beverage is
characterised by its pale amber colour, and its mild balsamic flavour. The bitterness of
the hop is so mellowed with the malt, as not to predominate. The ale of Preston Pans
is, in fact, the best substitute for wine which barley has hitherto produced. The low
temperature at which the Scotch brewer pitches his fermenting tun restricts his labours
to the colder months of the year. He does nothing during four of the summer months.
He is extremely nice in selecting his malt and hops; the former being made from the
best English barley, and the latter being the growth of Farnham or East Kent. The
yeast is carefully looked after, and measured into the fermenting tun in the proportion
of one gallon to 240 gallons of wort.
Only one mash is made by the Scotch ale brewer, and that pretty strong; but the
malt is exhausted by eight or ten successive sprinklings of liquor (hot water) over the
goods (malt), which are termed in the vernacular tongue, sparges. These waterings
percolate through the malt on the mash-tun bottom, and extract as much of the saccharine
matter as may be sufficient for the brewing. By this simple method much higher specific
gravities may be obtained than would be practicable by a second mash. With malt, the
infusion or saccharine fermentation of the diastase is finished with the first mash; and
nothing remains but to wash away from the goods the matter which that process
has rendered soluble. It will be found on trial that 20 barrels of wort drawn from a
certain quantity of malt, by two successive mashings, will not be so rich in fermentable
matter as 20 barrels extracted by ten successive sparges of two barrels each. The grains
always remain soaked with wort like that just drawn off, and the total residual quantity
is three fourths of a barrel for every quarter of malt. The gravity of this residual wort
will on the first plan be equal to that of the second mash; but on the second plan, it
will be equal only to that of the tenth sparge, and will be more attenuated in a very high
geometrical ratio. The only serious objection to the sparging system is the loss of time
by the successive drainages. A mash-tun with a steam jacket, promises to suit the
sparging system well; as it would keep up an uniform temperature in the goods, without
requiring them to be sparged with very hot liquor.
The first part of the Scotch process seems of doubtful economy; for the mash liquor is
heated so high as 180°. After mashing for about half an hour, or till every particle of
the malt is thoroughly drenched, the tun is covered, and the mixture left to infuse about
three hours; it is then drained off into the under-back, or preferably into the wort
copper.
After this wort is run off, a quantity of liquor (water), at 180° of heat, is sprinkled
uniformly over the surface of the malt; being first dashed on a perforated circular
board, suspended horizontally over the mash-tun, wherefrom it descends like a shower[108]
upon the whole of the goods. The percolating wort is allowed to flow off, by three or
more small stopcocks round the circumference of the mash-tun, to insure the equal
diffusion of the liquor.
The first sparge being run off in the course of twenty minutes, another similar one
is affused; and thus in succession till the whole of the drainage, when mixed with the
first mash-wort, constitutes the density adapted to the quality of the ale. Thus, the
strong worts are prepared, and the malt is exhausted either for table beer, or for a
return, as pointed out above. The last sparges are made 5° or 6° cooler than the
first.
The quantity of hops seldom exceeds four pounds to the quarter of malt. The
manner of boiling the worts is the same as that above described; but the conduct of
the fermentation is peculiar. The heat is pitched at 50°, and the fermentation continues
from a fortnight to three weeks. Were three brewings made in the week, seven
or eight working tuns would thus be in constant action; and, as they are usually in
one room, and some of them at an elevation of temperature of 15°, the apartment
must be propitious to fermentation, however low its heat may be at the commencement.
No more yeast is used than is indispensable: if a little more be needed, it is made
effective by rousing up the tuns twice a day from the bottom.
When the progress of the attenuation becomes so slack as not to exceed half a pound
in the day, it is prudent to cleanse, otherwise the top harm might re-enter the body of the
beer, and it would become yeast-bitten. When the ale is cleansed, the head, which has not
been disturbed for some days, is allowed to float on the surface till the whole of the then
pure ale is drawn off into the casks. This top is regarded as a sufficient preservative
against the contact of the atmosphere. The Scotch do not skim their tuns, as the
London ale brewers commonly do. The Scotch ale, when so cleansed, does not require
to be set upon close stillions. It throws off little or no yeast, because the fermentation
was nearly finished in the tun. The strength of the best Scotch ale ranges between 32
and 44 pounds to the barrel; or it has a specific gravity of from 1·088 to 1·122, according
to the price at which it is sold. In a good fermentation, seldom more than a fourth of
the original gravity of the wort remains at the period of the cleansing. Between one
third and one fourth is the usual degree of attenuation. Scotch ale soon becomes fine,
and is seldom racked for the home market. The following table will show the progress
of fermentation in a brewing of good Scotch ale:—
| 20 |
barrels of |
mash-worts of |
42 |
1⁄2 |
pounds gravity |
= |
860 |
·6 |
| 20 |
— |
returns |
6 |
1⁄10 |
= |
122 |
|
| 12 |
) |
982 |
·6 |
| pounds weight of extract per quarter of malt |
= |
81 |
|
Fermentation:—
| March |
24. |
pitched the |
tun at |
51°: yeast 4 gallons. |
| |
Temp. |
Gravity. |
| |
25. |
|
52° |
|
41 |
pounds. |
| |
28. |
|
56° |
|
39 |
|
| |
30. |
|
60° |
|
34 |
|
| April |
1. |
|
62° |
|
32 |
|
| |
4. |
|
65° |
|
29 |
added 1 lb. of yeast. |
| |
5. |
|
66° |
|
25 |
|
| |
6. |
|
67° |
|
23 |
|
| |
7. |
|
67° |
|
20 |
|
| |
8. |
|
66° |
|
18 |
|
| |
9. |
|
66° |
|
15 |
|
| |
10. |
|
64° |
|
14 |
·5 cleansed[7]. |
The following table shows the origin and the result of fermentation, in a number of
practical experiments:—
Original
Gravity
of the
Worts. |
Lbs. per
Barrel of
Saccharine
Matter. |
Specific
Gravity
of the Ale. |
Lbs. per
Barrel of
Saccharine
Matter. |
Attenuation,
or
Saccharum
decomposed. |
| 1 |
·0950 |
88 |
·75 |
1 |
·0500 |
40 |
·25 |
0 |
·478 |
| 1 |
·0918 |
85 |
·62 |
1 |
·0420 |
38 |
·42 |
0 |
·552 |
| 1 |
·0829 |
78 |
·125 |
1 |
·0205 |
16 |
·87 |
0 |
·787 |
| 1 |
·0862 |
80 |
·625 |
1 |
·0236 |
20 |
·00 |
0 |
·757 |
| 1 |
·0780 |
73 |
·75 |
1 |
·0280 |
24 |
·25 |
0 |
·698 |
| 1 |
·0700 |
65 |
·00 |
1 |
·0285 |
25 |
·00 |
0 |
·615 |
| 1 |
·1002 |
93 |
·75 |
1 |
·0400 |
36 |
·25 |
0 |
·613 |
| 1[109] |
·1025 |
95 |
·93 |
1 |
·0420 |
38 |
·42 |
0 |
·600 |
| 1 |
·0978 |
91 |
·56 |
1 |
·0307 |
27 |
·00 |
0 |
·705 |
| 1 |
·0956 |
89 |
·37 |
1 |
·0358 |
32 |
·19 |
0 |
·640 |
| 1 |
·1130 |
105 |
·82 |
1 |
·0352 |
31 |
·87 |
0 |
·661 |
| 1 |
·1092 |
102 |
·187 |
1 |
·0302 |
26 |
·75 |
0 |
·605 |
| 1 |
·1171 |
110 |
·00 |
1 |
·0400 |
36 |
·25 |
0 |
·669 |
| 1 |
·1030 |
96 |
·40 |
1 |
·0271 |
23 |
·42 |
0 |
·757 |
| 1 |
·0660 |
61 |
·25 |
1 |
·0214 |
17 |
·80 |
0 |
·709 |
The second column here does not represent, I believe, the solid extract, but the
pasty extract obtained as the basis of Mr. Allen’s saccharometer, and therefore each of
its numbers is somewhat too high. The last column, also, must be in some measure
erroneous, on account of the quantity of alcohol dissipated during the process of fermentation.
It must be likewise incorrect, because the density due to the saccharine matter
will be partly counteracted, by the effect of the alcohol present in the fermented liquor.
In fact, the attenuation does not correspond to the strength of the wort; being greatest in
the third brewing, and smallest in the first. The quantity of yeast for the above ale
brewings in the table was, upon an average, one gallon for 108 gallons; but it varied
with its quality, and with the state of the weather, which, when warm, permits much less
to be used with propriety.
The good quality of the malt, and the right management of the mashing, may be
tested by the quantity of saccharine matter contained in the successively drawn worts. With
this view, an aliquot portion of each of them should be evaporated by a safety-bath heat
to a nearly concrete consistence, and then mixed with twice its volume of strong spirit
of wine. The truly saccharine substance will be dissolved, while the starch and other
matters will be separated; after which the proportions of each may be determined by filtration
and evaporation. Or an equally correct, and much more expeditious, method of
arriving at the same result would be, after agitating the viscid extract with the alcohol
in a tall glass cylinder, to allow the insoluble fecula to subside, and then to determine
the specific gravity of the supernatant liquid by a hydrometer. The additional density
which the alcohol has acquired will indicate the quantity of malt sugar which it has
received. The following table, constructed by me, at the request of Henry Warburton,
Esq., M. P., chairman of the Molasses Committee of the House of Commons in 1830,
will show the brewer the principle of this important inquiry. It exhibits the quantity
in grains weight of sugar requisite to raise the specific gravity of a gallon of spirit of
different densities to the gravity of water = 1·000.
Specific Gravity
of Spirit. |
Grains, Weight
of Sugar in the
Gallon Imperial. |
| 0·995 |
0·980 |
| 0·990 |
1·890 |
| 0·985 |
2·800 |
| 0·980 |
3·710 |
| 0·975 |
4·690 |
| 0·970 |
5·600 |
| 0·965 |
6·650 |
| 0·960 |
7·070 |
| 0·955 |
8·400 |
| 0·950 |
9·310 |
The immediate purpose of this table was to show the effect of saccharine matter in
disguising the presence or amount of alcohol in the weak feints of the distiller. But
a similar table might easily be constructed, in which, taking a uniform quantity of alcohol
of 0·825, for example, the quantity of sugar in any wort-extract would be shown
by the increase of specific gravity which the alcohol received from agitation with a
certain weight of the wort, inspissated to a nearly solid consistence by a safety-pan,
made on the principle of my patent sugar-pan. (See Sugar.) Thus, the normal quantities
being 1000 grain measures of alcohol, and 100 grains by weight of inspissated
mash-extract, the hydrometer would at once indicate, by help of the table, first, the
quantity per cent. of truly saccharine matter, and next, by subtraction, that of farinaceous
matter present in it.
Plan, Machinery, and Utensils of a great Brewery.—Figs. 103. and 104. represent the
arrangement of the utensils and machinery in a porter brewery on the largest scale; in
which, however, it must be observed that the elevation fig. 103. is in a great degree imaginary
as to the plane upon which it is taken; but the different vessels are arranged so as[110]
to explain their uses most readily, and at the same time to preserve, as nearly as possible,
the relative positions which are usually assigned to each in works of this nature.
The malt for the supply of the brewery is stored in vast granaries or malt-lofts, usually
situated in the upper part of the buildings. Of these, I have been able to represent
only one, at A, fig. 103.: the others, which are supposed to be on each side of it, cannot[111]
be seen in this view. Immediately beneath the granary A, on the ground floor, is the
mill; in the upper story above it, are two pairs of rollers, fig. 101, 102, and 103, under
a, a, for bruising or crushing the grains of the malt. In the floor beneath the rollers are
the mill-stones b, b, where the malt is sometimes ground, instead of being merely bruised
by passing between the rollers, under a, a.
The malt, when prepared, is conveyed by a trough into a chest d, to the right of b,
from which it can be elevated by the action of a spiral screw, fig. 105., enclosed in the
sloping tube e, into the large chest or binn B, for holding ground malt, situated immediately
over the mash-tun D. The malt is reserved in this binn till wanted, and it is
then let down into the mashing-tun, where the extract is obtained by hot water supplied
from the copper G, seen to the right of B.
The water for the service of the brewery is obtained from the well E, seen beneath the
mill to the left, by a lifting pump worked by the steam engine; and the forcing-pipe f
of this pump conveys the water up to the large reservoir or water-back F, placed at the
top of the engine-house. From this cistern, iron pipes are laid to the copper G (on the
right-hand side of the figure), as also to every part of the establishment where cold water
can be wanted for cleaning and washing the vessels. The copper G can be filled with
cold water by merely turning a cock; and the water, when boiled therein, is conveyed
by the pipe g into the bottom of the mash-tun D. It is introduced beneath a false bottom,
upon which the malt lies, and, rising up through the holes in the false bottom, it
extracts the saccharine matter from the malt; a greater or less time being allowed for the
infusion, according to circumstances. The instant the water is drawn off from the copper,
fresh water must be let into it, in order to be ready for boiling the second mashing; because
the copper must not be left empty for a moment, otherwise the intense heat of the
fire would destroy its bottom. For the convenience of thus letting down at once as much
liquor as will fill the lower part of the copper, a pan or second boiler is placed over the
top of the copper, as seen in fig. 103.; and the steam rising from the copper communicates
a considerable degree of heat to the contents of the pan, without any expense of
fuel. This will be more minutely explained hereafter. (See fig. 107.)
During the process of mashing, the malt is agitated in the mash-tun, so as to expose
every part to the action of the water. This is done by a mechanism contained within
the mash-tun, which is put in motion by a horizontal shaft above it, H, leading from the
mill. The mash machine is shown separately in fig. 106. When the operation of mashing
is finished, the wort or extract is drained down from the malt into the vessel I, called
the under-back, immediately below the mash-tun, of like dimensions, and situated always
on a lower level, for which reason it has received this name. Here the wort does not remain
longer than is necessary to drain off the whole of it from the tun above. It is then
pumped up by the three-barrelled pump k, into the pan upon the top of the copper, by a
pipe which cannot be seen in this section. The wort remains in the pan until the water
for the succeeding mashes is discharged from the copper. But this delay is no loss of
time, because the heat of the copper, and the steam arising from it, prepare the wort,
which had become cooler, for boiling. The instant the copper is emptied, the first wort
is let down from the pan into the copper, and the second wort is pumped up from the
under-back into the upper pan. The proper proportion of hops is thrown into the copper
through the near hole, and then the door is shut down, and screwed fast, to keep in
the steam, and cause it to rise up through pipes into the pan. It is thus forced to blow
up through the wort in the pan, and communicates so much heat to it, or water, called liquor
by the brewers, that either is brought near to the boiling point. The different worts
succeed each other through all the different vessels with the greatest regularity, so that
there is no loss of time, but every part of the apparatus is constantly employed. When
the ebullition has continued a sufficient period to coagulate the grosser part of the extract,
and to evaporate part of the water, the contents of the copper are run off through a large
cock into the jack-back K, below G, which is a vessel of sufficient dimensions to contain
it, and provided with a bottom of cast-iron plates, perforated with small holes, through
which the wort drains and leaves the hops. The hot wort is drawn off from the jack-back
through the pipe h by the three-barrelled pump, which throws it up to the coolers
L, L, L; this pump being made with different pipes and cocks of communication, to serve
all the purposes of the brewery except that of raising the cold water from the well. The
coolers L, L, L, are very shallow vessels, built over one another in several stages: and that
part of the building in which they are contained is built with lattice-work or or shutter flaps,
on all sides, to admit free currents of air. When the wort is sufficiently cooled to be put to
the first fermentation, it is conducted in pipes from all the different coolers to the large
fermenting vessel or gyle-tun M, which, with another similar vessel behind it, is of sufficient
capacity to contain all the beer of one day’s brewings.
Whenever the first fermentation is concluded, the beer is drawn off from the great fermenting
vessel M, into the small fermenting casks or cleansing vessels N, of which there
are a great number in the brewery. They are placed four together, and to each four a common[112]
spout is provided to carry off the yeast, and conduct it into the troughs n, placed
beneath. In these cleansing vessels the beer remains till the fermentation is completed;
and it is then put into the store-vats, which are casks or tuns of an immense size, where
it is kept till wanted, and is
finally drawn off into barrels,
and sent away from the brewery.
The store-vats are not represented
in the figure: they are of a conical
shape, and of different dimensions,
from fifteen to twenty feet
diameter, and usually from fifteen
to twenty feet in depth. The
steam-engine which puts all the
machine in motion is exhibited
in its place, on the left side of
the figure. On the axis of the
large fly-wheel is a bevelled spur-wheel,
which turns another similar
wheel upon the end of a
horizontal shaft, which extends
from the engine-house to the
great horse-wheel, set in motion
by means of a spur-wheel. The
horse-wheel drives all the pinions
for the mill-stones b, b, and also
the horizontal axis which works
the three-barrelled pump k. The
rollers a, a, are turned by a bevel
wheel upon the upper end of the
axis of the horse-wheel, which is
prolonged for that purpose; and
the horizontal shaft H, for the
mashing engine, is driven by a
pair of bevel wheels. There is
likewise a sack-tackle, which is
not represented. It is a machine
for drawing up the sacks of malt
from the court-yard to the highest
part of the building, whence the
sacks are wheeled on a truck to
the malt-loft A, and the contents
of the sacks are discharged.
The horse-wheel is intended to
be driven by horses occasionally,
if the steam-engine should fail;
but these engines are now brought
to such perfection that it is very
seldom any recourse of this kind
is needed.
Fig. 104. is a representation
of the fermenting house at the
brewery of Messrs. Whitbread
and Company, Chiswell Street,
London, which is one of the most
complete in its arrangement in
the world: it was erected after the
plan of Mr. Richardson, who conducts
the brewing at those works.
The whole of fig. 104. is to be
considered as devoted to the same
object as the large vessel M and
the casks N, fig. 103. In fig. 104.,
r r is the pipe which leads from
the different coolers to convey
the wort to the great fermenting
vessels or squares M, of which there are two, one behind the other; f f represents a part
of the great pipe which conveys all the water from the well E, fig. 103, up to the water cistern[113]
F. This pipe is conducted purposely up the wall of the fermenting-house, fig. 104, and
has a cock in it, near r, to stop the passage. Just beneath this passage a branch-pipe p
proceeds, and enters a large pipe x x, which has the former pipe r withinside of it.
From the end of the pipe x, nearest to the squares M, another branch n n proceeds, and
returns to the original pipe f, with a cock to regulate it. The object of this arrangement
is to make all, or any part, of the cold water flow through the pipe x x, which surrounds
the pipe r, formed only of thin copper, and thus cool the wort passing through the pipe
r, until it is found by the thermometer to have the exact temperature which is desirable
before it is put to ferment in the great square M. By means of the cocks at n and p, the
quantity of cold water passing over the surface of the pipe r can be regulated at pleasure,
whereby the heat of the wort, when it enters into the square, may be adjusted within half
a degree.
When the first fermentation in the squares M M is finished, the beer is drawn off from
them by pipes marked c, and conducted by its branches W W W, to the different rows of
fermenting-tuns, marked N N, which occupy the greater part of the building. In the
hollow between every two rows are placed large troughs, to contain the yeast which
they throw off. The figure shows that the small tuns are all placed on a lower level
than the bottom of the great vessels M, so that the beer will flow into them, and, by hydrostatic
equilibrium, will fill them to the same level. When they are filled, the communication-cock
is shut; but, as the working off the yeast diminishes the quantity of
beer in each vessel, it is necessary to replenish them from time to time. For this
purpose, the two large vats O O are filled from the great squares M M, before any beer
is drawn off into the small casks N, and this quantity of beer is reserved at the higher
level for filling up. The two vessels O O are, in reality, situated between the two squares
M M; but I have been obliged to place them thus in the section, in order that they
may be seen. Near each filling-up tun O is a small cistern t communicating with the
tun O by a pipe, which is closed by a float-valve. The small cisterns t are always in
communication with the pipes which lead to the small fermenting vessels N; and therefore
the surface of the beer in all the tuns, and in the cisterns, will always be at the same
level; and as this level subsides by the working off of the yeast from the tuns, the float
sinks and opens the valve, so as to admit a sufficiency of beer from the filling-up tuns
O, to restore the surfaces of the beer in all the tuns, and also in the cistern t, to the
original level. In order to carry off the yeast which is produced by the fermentation of
the beer in the tuns O O, a conical iron dish or funnel is made to float upon the surface of
the beer which they contain; and from the centre of this funnel a pipe, o, descends, and
passes through the bottom of the tun, being packed with a collar of leather, so as to be
water-tight; at the same time that it is at liberty to slide down, as the surface of the beer
descends in the tun. The yeast flows over the edge of this funnel-shaped dish, and is
conveyed down the pipe to a trough beneath.
Beneath the fermenting-house are large arched vaults, P, built with stone, and lined
with stucco. Into these the beer is let down in casks when sufficiently fermented, and
is kept in store till wanted. These vaults are used at Mr. Whitbread’s brewery, instead
of the great store-vats of which we have before spoken, and are in some respects preferable,
because they preserve a great equality of temperature, being beneath the surface
of the earth.
The malt-rollers, or machines for bruising the grains of the malt, fig. 101. 102., have
been already described. The malt is shot down from A, fig. 103., the malt-loft, into the
hopper; and from this it is let out gradually through a sluice or sliding shuttle, a, fig. 103.
and falls between the rollers.
Fig. 105. is the screw by which the ground or bruised malt is raised up, or conveyed
from one part of the brewery to another. K is an inclined box or trough, in the centre
of which the axis of the screw H is placed; the spiral iron plate or worm, which is
fixed projecting from the axis, and which forms the screw, is made very nearly to
fill the inside of the box. By this means, when the screw is turned round by the
wheels E F, or by any other means, it raises up the malt from the box d, and delivers it
at the spout G.
This screw is equally applicable for conveying the malt horizontally in the trough K,
as slantingly; and similar machines are employed in various parts of breweries for conveying
the malt wherever the situation of the works require.
Fig. 106. is the mashing-machine. a a is the tun, made of wood staves, hooped together.
In the centre of it rises a perpendicular shaft, b, which is turned slowly round
by means of the bevelled wheels t u at the top. c c are two arms, projecting from
that axis, and supporting the short vertical axis d of the spur-wheel x, which is
turned by the spur-wheel w; so that, when the central axis b is made to revolve, it
will carry the thick short axle d round the tun in a circle. That axle d is furnished
with a number of arms, e e, which have blades placed obliquely to the plane of their[114]
motion. When the axis is turned round, these arms agitate the malt in the tun, and give
it a constant tendency to rise upwards from the bottom.
The motion of the axle d is produced by a wheel, x, on the upper end of it, which is
turned by a wheel, w, fastened on the middle of the tube b, which turns freely round
upon its central axis. Upon a higher point of the same tube b is a bevel wheel, o,
receiving motion from a bevel wheel, q, fixed upon the end of the horizontal axis n n,
which gives motion to the whole machine. This same axis has a pinion, p, upon
it, which gives motion to the wheel r, fixed near the middle of a horizontal axle,
which, at its left hand end, has a bevel pinion, t, working the wheel u, before mentioned.
By these means, the rotation of the central axis b will be very slow compared with the
motion of the axle d; for the latter will make seventeen or eighteen revolutions on its
own axis in the same space of time that it will be carried once round the tun by the
motion of the shaft b. At the beginning of the operation of mashing, the machine is
made to turn with a slow motion; but, after having wetted all the malt by one revolution,
it is driven quicker. For this purpose, the ascending-shaft f g, which gives[115]
motion to the machine, has two bevel wheels, h i, fixed upon a tube, f g, which is fitted
upon a central shaft. These wheels actuate the wheels m and o, upon the end of the
horizontal shaft n n; but the distance between the two wheels h and i is such, that they
cannot be engaged both at once with the wheels m and o; but the tube f g, to which
they are fixed, is capable of sliding up and down on its central axis sufficiently to bring
either wheel h or i into geer with its corresponding wheel o or m, upon the horizontal
shaft; and as the diameters of n o, and i m, are of very different proportions, the velocity
of the motion of the machine can be varied at pleasure, by using one or other. k and
k are two levers, which are forked at their extremities, and embrace collars at the ends
of the tube f g. These levers being united by a rod, l, the handle k gives the means of
moving the tube f g, and its wheels h i, up or down, to throw either the one or the
other wheel into geer.
The object of boiling the wort is not merely evaporation and concentration, but extraction,
coagulation, and, finally, combination with the hops; purposes which are better
accomplished in a deep confined copper, by a moderate heat, than in an open shallow
pan with a quick fire. The copper being encased above in brickwork, retains its digesting
temperature much longer than the pan could do. The waste steam of the close
kettle, moreover, can be economically employed in communicating heat to water or
weak worts; whereas the exhalations from an open pan would prove a nuisance, and
would need to be carried off by a hood. The boiling has a four-fold effect: 1. it concentrates
the wort; 2. during the earlier stages of heating, it converts the starch into
sugar, dextrine, and gum, by means of the diastase; 3. it extracts the substance of the
hops diffused through the wort; 4. it coagulates the albuminous matter present in the
grain, or precipitates it by means of the tannin of the hops.
The degree of evaporation is regulated by the nature of the wort, and the quality of
the beer. Strong ale and stout for keeping, require more boiling than ordinary porter
or table-beer brewed for immediate use. The proportion of the water carried off by
evaporation is usually from a seventh to a sixth of the volume. The hops are introduced
during the progress of the ebullition. They serve to give the beer not only a bitter
aromatic taste, but also a keeping quality, or they counteract its natural tendency to
become sour; an effect partly due to the precipitation of the albumen and starch, by
their resinous and tanning constituents, and partly to the antifermentable properties of
their lupuline, bitter principle, ethereous oil, and resin. In these respects, there is none of
the bitter plants which can be substituted for hops with advantage. For strong beer,
powerful fresh hops should be selected; for weaker beer, an older and weaker article
will suffice.
The hops are either boiled with the whole body of the wort, or extracted with a
portion of it; and this concentrated extract added to the rest. The stronger the hops
are, the longer time they require for extraction of their virtues; for strong hops, an hour
and a half or two hours boiling may be proper; for a weaker sort, half an hour or an
hour may be sufficient; but it is never advisable to push this process too far, lest a disagreeable
bitterness, without aroma, be imparted to the beer. In our breweries, it is the
practice to boil the hops with a part of the wort, and to filter the decoction through a
drainer, called the jack hop-back. The proportion of hops to malt is very various; but, in
general, from a pound and a quarter to a pound and a half of the former are taken for
100 lbs. of the latter in making good table-beer. For porter and strong ale, 2 pounds of
hops are used, or even more; for instance, one pound of hops to a bushel of malt, if the
beer be destined for the consumption of India.
During the boiling of the two ingredients, much coagulated albuminous matter, in
various states of combination, makes its appearance in the liquid, constituting what is
called the breaking or curdling of the wort, when numerous minute flocks are seen floating
in it. The resinous, bitter, and oily-ethereous principles of the hops combine with
the sugar and gum, or dextrine of the wort; but for this effect they require time and
heat; showing that the boil is not a process of mere evaporation, but one of chemical
reaction. A yellowish-green pellicle of hop-oil and resin appears upon the surface of
the boiling wort, in a somewhat frothy form: when this disappears, the boiling is presumed
to be completed, and the beer is strained off into the cooler. The residuary
hops may be pressed and used for an inferior quality of beer; or they may be boiled
with fresh wort, and be added to the next brewing charge.
Figs. 107, 108. represent the copper of a London brewery. Fig. 107. is a vertical section;
fig. 108., a ground-plan of the fire-grate and flue, upon a smaller scale: a is the close copper
kettle, having its bottom convex within; b is the open pan placed upon its top. From
the upper part of the copper, a wide tube, c, ascends, to carry off the steam generated
during the ebullition of the wort, which is conducted through four downwards-slanting
tubes, d d (two only are visible in this section), into the liquor of the pan b, in order to
warm its contents. A vertical iron shaft or spindle, e, passes down through the tube c,
nearly to the bottom of the copper, and is there mounted with an iron arm, called a[116]
rouser, which carries round a chain hung in loops, to prevent the hops from adhering to
the bottom of the boiler. Three bent stays, f, are stretched across the interior, to support
the shaft by a collet at their middle junction. The shaft carries at its upper end a bevel
wheel, g, working into a bevel pinion upon the axis h, which may be turned either by
power or by hand. The rouser shaft may be lifted by means of the chain i, which, going
over two pulleys, has its end passed round the wheel and axle k, and is turned by a
winch: l is a tube for conveying the waste steam into the chimney m.
The heat is applied as follows:—For heating the colossal coppers of the London
breweries, two separate fires are required, which are separated by a narrow wall of
brickwork, n, fig. 107M, 108. The dotted circle a′ a′ indicates the largest circumference of
the copper, and b′ b′ its bottom; o o are the grates upon which the coals are thrown,
not through folding doors (as of old), but through a short slanting iron hopper, shown at
p, fig. 107., built in the wall, and kept constantly filled with the fuel, in order to exclude
the air. Thus the lower stratum of coals gets ignited before it reaches the grate. Above
the hopper p, a narrow channel is provided for the admission of atmospherical air, in
such quantity merely as may be requisite to complete the combustion of the smoke of
the coals. Behind each grate there is a fire-bridge, r, which reflects the flame upwards,
and causes it to play upon the bottom of the copper. The burnt air then passes round
the copper in a semicircular flue, s s, from which it flows off into the chimney m, on
whose under end a sliding damper-plate, t, is placed, for tempering the draught. When
cold air is admitted at this orifice, the combustion of the fuel is immediately checked.
There is, besides, another slide-plate at the entrance of the slanting flue into the vertical
chimney, for regulating the play of the flame under and around the copper. If the plate
t be opened, and the other plate shut, the power of the fire is suspended, as it ought to
be, at the time of emptying the copper. Immediately over the grate is a brick arch, u,
to protect the front edge of the copper from the first impulsion of the flame. The
chimney is supported upon iron pillars, v v; w is a cavity closed with a slide-plate, through
which the ashes may be taken out from behind, by means of a long iron hook.
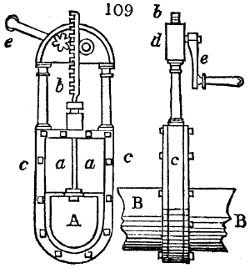
Fig. 109. represents one of the sluice-cocks, which are used to make the communications
of the pipes with the pumps, or other parts of the brewery. B B represents
the pipe in which the cock is placed. The two parts of this pipe are screwed to the
side of a box, C C, in which a slider, A, rises and falls, and intercepts, at pleasure, the
passage of the pipe. The slider is moved by the rod a. This passes through a stuffing-box,[117]
in the top of the box which contains the slider, and has the rack b fastened to it.
The rack is moved by a pinion fixed upon the axis of a handle e, and the rack and
pinion are contained in a frame d which is supported by two pillars. The frame
contains a small roller behind the rack, which bears it up towards the pinion, and keeps
its teeth up to the teeth of the pinion. The slider A is made to fit accurately against
the internal surface of the box C, and to bear against this surface by the pressure of a
spring, so as to make a perfectly close fitting.
Fig. 110. is a small cock to be placed in the side of the great store vats, for the
purpose of drawing off a small quantity of beer, to taste and try its quality. A is a
part of the stave or thickness of the great store vat; into this the tube B of the cock is
fitted, and is held tight in its place by a nut, a a, screwed on withinside. At the other
end of the tube B, a plug, c, is fitted, by grinding it into a cone, and it is kept in by a
screw. This plug has a hole up the centre of it, and from this a hole proceeds sidewise,
and corresponds with a hole made through the side of the tube when the cock is
open; but when the plug c is turned round, the hole will not coincide, and then the
cock will be shut. D is the handle or key of the cock, by which its plug is turned to
open or shut it: this handle is put up the bore of the tube (the cover E being first
unscrewed and removed), and the end of it is adapted to fit the end of the plug of the
cock. The handle has a tube or passage bored up it, to convey the beer away from the
cock when it is opened, and from this the passage f, through the handle, leads, to draw
the beer into a glass or tumbler. The hole in the side of the plug is so arranged, that,
when the handle is turned into a perpendicular direction, with the passage f downwards,
the cock will be open. The intention of this contrivance is, that there shall be no
considerable projection beyond the surface of the tun; because it sometimes happens
that a great hoop of the tun breaks, and, falling down, its great weight would strike out
any cock which had a projection; and, if this happened in the night, much beer might
be lost before it was discovered. The cock above described, being almost wholly withinside,
and having scarcely any projection beyond the outside surface of the tun, is secure
from this accident.
Fig. 111. is a small contrivance of a vent peg, to be screwed into the head of a common
cask when the beer is to be drawn off from it, and it is necessary to admit some air to
allow the beer to flow. A A represents a portion of the head
of the cask into which the tube B is screwed. The top of
this tube is surrounded by a small cup, from which project
the two small handles C C, by which the peg is turned round
to screw it into the cask. The cup round the other part of
the tube, is filled with water; into this a small cup, D, is inverted;
in consequence, the air can gain admission into the
cask when the pressure within is so far diminished, that the
air will bubble up through the water, and enter beneath the small cup D.
The most efficient substance for fining beer hitherto discovered is isinglass, which is
prepared by solution in vinegar or old stale beer, and this solution is afterwards reduced
with thin mild beer generally brewed for the purpose, in all large establishments, from
a raw or return wort. It must next be passed through a fine hair sieve, by means of
rubbing it down with a hard hair-brush, and brought to the proper consistency by thin
mild beer. If properly made, it will be clear, transparent, and free from feculencies.
Finings serve excellently to remove any extraneous matter that may be found floating
in the beer, and thus changes it from bright to brilliant. The common quantity used
is from a pint to a quart per barrel, according to the nature of the beer.
To ascertain whether the beer is in a fit state for fining, put it into a long glass
cylindric vessel, and add to it a teaspoonful, or thereby, of the fining; then give the
mixture a good shake, by turning the vessel up and down, after closing its mouth with[118]
the palm of the hand. If the beer has been well brewed, its aptitude to become bright
will be soon shown by the mixture getting thick and curdy; a bright portion will
generally show itself at the bottom or middle; after which the finings will gradually
mount to the top, taking up all the impurities along with them, till the whole becomes
brilliant. Some have said that the finings should carry the impurities down to the
bottom; but this, according to Mr. Black[8], takes place only with stubborn beer, which
would not become thoroughly bright with any quantity of finings which could be
introduced. Finings have usually a specific gravity of from 1·010 to 1·016, and, when
added to beer in a fit condition for fining, invariably go to the top, and not to the
bottom. In fining beer in a barrel laid on its side, if the finings do not make their
appearance at the bung-hole, the beer will not become bright. The isinglass must not
be dissolved with heat, nor in hot water.
Beer brewed from imperfectly malted grain, or from a mixture of malt and raw corn,
gives a fermentation quite different in flavour from that of beer from sound malt. The
nose is, in fact, the best guide to the experienced brewer for ascertaining whether his
process is going on well or ill.
Ropiness is a morbid state of beer, which is best remedied, according to Mr. Black,
by putting the beer into a vat with a false bottom, and adding, per barrel, 4 or 5 pounds
of hops, taken gradually away after the first boilings of the worts; and to them may be
added about half a pound per barrel of mustard-seed. Rouse the beer as the hops are
gradually introduced, and, in some months, the ropiness will be perfectly cured. The
beer should be drawn off from below the false bottom.
For theoretical views, see Fermentation; and for wort-cooling apparatus, see
Refrigerator.
BEET-ROOT SUGAR. See Sugar.
BELL-METAL, an alloy of copper and tin. See Copper.
BENGAL STRIPES. Ginghams; a kind of cotton cloth woven with coloured
stripes.
BENJAMIN or BENZOIN. (Benjoin, Fr.; Benzöe, Germ.) A species of resin
used chiefly in perfumery. It is extracted by incision from the trunk and branches of
the styrax benzoin, which grows in Java, Sumatra, Santa Fé, and in the kingdom of Siam.
The plant belongs to the decandria monogynia of Linnæus, and the natural family of
the ebenaceæ. It hardens readily in the air, and comes to us in brittle masses, whose
fracture presents a mixture of red, brown, and white grains of various sizes, which, when
white, and of a certain shape, have been called amygdaloid, from their resemblance to
almonds. The sorted benzoin is, on the other hand, very impure.
The fracture of benzoin is conchoidal, and its lustre greasy: its specific gravity varies
from 1·063 to 1·092. It has an agreeable smell, somewhat like vanilla, which is most
manifest when it is ground. It enters into fusion at a gentle heat, and then exhales a
white smoke, which may be condensed into the acicular crystals of benzoic acid, of which
it contains 18 parts in the hundred. Stoltze recommends the following process for extracting
the acid. The resin is to be dissolved in 3 parts of alcohol, the solution is to be
introduced into a retort, and a solution of carbonate of soda dissolved in dilute alcohol
is to be gradually added to it, till the free acid be neutralised; and then a bulk of water
equal to double the weight of the benzoin is to be poured in. The alcohol being drawn
off by distillation, the remaining liquor contains the acid, and the resin floating upon it
may be skimmed off and washed, when its weight will be found to amount to about 80
per cent. of the raw material. The benzoin contains traces of a volatile oil, and a substance
soluble in water, at least through the agency of carbonate of potash. Ether does
not dissolve benzoin completely. The fat and volatile oils dissolve very little of it.
Unverdorben has found in benzoin, besides benzoic acid, and a little volatile oil, no
less than three different kinds of resin, none of which has, however, been turned as yet
to any use in the arts.
Benzoin is of great use in perfumery, as it enters into a number of preparations;
among which may be mentioned fumigating pastilles, fumigating cloves (called also
nails), poudre à la maréchale, &c. The alcoholic tincture, mixed with water, forms
virginal milk. Benzoin enters also into the composition of certain varnishes employed
for snuff-boxes and walkingsticks, in order to give these objects an agreeable smell when
they become heated in the hand. It is likewise added to the spirituous solution of
isinglass with which the best court plaster is made.
BERLIN BLUE. Prussian blue. See Blue.
BERRIES OF AVIGNON, and Persian Berries. (Graines d’Avignon, Fr.;
Gelbbeeren, Germ.) A yellowish dye-drug, the fruit of the rhamnus infectorius, a plant[119]
cultivated in Provence, Languedoc, and Dauphiné, for the sake of its berries, which are
plucked before they are ripe, while they have a greenish hue. Another variety comes
from Persia, whence its trivial name; it is larger than the French kind, and has
superior properties. The principal substances contained in these berries are: 1. A
colouring matter, which is united with a matter insoluble in ether, little soluble in concentrated
alcohol, and very soluble in water: it appears to be volatile. 2. A matter
remarkable for its bitterness, which is soluble in water and alcohol. 3. A third principle,
in small quantity. A decoction of one part of the Avignon or Persian berry in ten of
water affords a brown-yellow liquor bordering upon green, having the smell of a vegetable
extract, and a slightly bitter taste.
With gelatine that decoction gives, after some time, a slight precipitate,—
| — |
alkalies |
a yellow hue, |
| — |
acids |
a slight muddiness, |
| — |
lime-water |
a greenish-yellow tint, |
| — |
alum |
a yellow colour, |
| — |
red sulphate of iron |
an olive-green colour, |
| — |
sulphate of copper |
an olive colour, |
| — |
proto-muriate of tin |
a greenish yellow with a slight precipitate. (See Calico Printing.) |
BERYL. A beautiful mineral or gem, of moderate price, usually of a green colour
of various shades, passing into honey-yellow and sky blue.
BEZOAR. The name of certain concretions found in the stomachs of animals, to
which many fanciful virtues were formerly ascribed. They are interesting only to the
chemical pathologist.
BILE. (Bile, Fr.; Galle, Germ.) The secreted liquor of the liver in animals. For
an account of the uses of animal bile in the arts, see Gall.
BIRDLIME. (Glu, Fr.; Vogelleim, Germ.) The best birdlime may be made from the
middle bark of the holly, boiled seven or eight hours in water, till it is soft and tender,
then laid by heaps in pits under ground, covered with stones after the water is
drained from it. There it must be left during two or three weeks, to ferment in the
summer season, and watered, if necessary, till it passes into a mucilaginous state. It is
then to be pounded in a mortar to a paste, washed in running water, and kneaded till
it be free from extraneous matters. It is next left for four or five days in earthen vessels
to ferment and purify itself, when it is fit for use. Birdlime may be made by the same
process from the mistletoe (viburnum lantana), young shoots of elder, and the barks of
other vegetables, as well as from most parasite plants.
Good birdlime is of a greenish colour, and sour flavour, somewhat resembling that of
linseed oil; gluey, stringy, and tenacious. By drying in the air it becomes brittle, and
may be powdered; but its viscosity may be restored by moistening it. It has an acid
reaction with litmus paper. It contains resin, mucilage, a little free acid, colouring
and extractive matter. The resin has been called Viscine.
BISMUTH. (Bismuth, Fr.; Wismuth, Germ.) Called also marcasite and tin-glass.
It was shown to be a metal somewhat different from lead, by G. Agricola, in 1546; Stahl
and Dufay proved its peculiarity; but it was more minutely distinguished by Pott and
Geoffroy, about the middle of the last century. It is a rare substance, occurring native,
as an oxide, under the name of bismuth ochre; as a sulphuret, called bismuth glance; as
a sulphuret with copper, called copper bismuth ore; as also with copper and lead, called
needle ore. It is found associated likewise with selenium and tellurium. The native metal
occurs in various forms and colours, as white, reddish, and variegated; in primitive and
floetz formations, along with the ores of cobalt, nickel, copper, silver, and bismuth ochre;
at the Saxon Erzgebirge, near Schneeberg, and Joh. Georgenstadt; also in Bohemia,
Baden, Wurtemberg, Hessia, Sweden, Norway, England, and France.
The production of this metal is but a limited object of the smelting-works of the
Saxon Erzgebirge at Schneeberg. It there occurs, mixed with cobalt speiss, in the
proportion of about 7 per cent. upon the average, and is procured by means of a peculiar
furnace of liquation, which is the most economical method, both as to saving fuel, and
oxidisement of the bismuth.
The bismuth eliquation furnace at Schneeberg is represented in figs. 112, 113, and 114.,
of which the first is a view from above, the second a view in front, and the third a transverse
section in the dotted line A B of fig. 112. a is the ash-pit; b, the fireplace; c, the eliquation
pipes; d, the grate of masonry or brickwork, upon which the fuel is thrown through
the fire-door e e. The anterior deeper lying orifice of the eliquation pipes is closed with
the clay-plate f; which has beneath a small circular groove, through which the liquefied
metal flows off. g is a wall extending from the hearth-sole nearly to the anterior
orifices of the eliquation pipes, in which wall there are as many fire-holes, h, as there are
pipes in the furnace; i are iron pans, which receive the fluid metal; h, a wooden water-trough,[120]
in which the bismuth is granulated and cooled; l, the posterior and higher
lying apertures of the eliquation pipes, shut merely with a sheet-iron cover. The
granulations of bismuth drained from the posterior openings fall upon the flat surfaces m,
and then into the water-trough. n n are draught-holes in the vault between the two
pipes, which serve for increasing or diminishing the heat at pleasure.
The ores to be eliquated (sweated) are sorted by hand from the gangue, broken into
pieces about the size of a hazel nut, and introduced into the ignited pipes; one charge
consisting of about 1⁄2 cwt.; so that the pipes are filled to half their diameter, and three
fourths of their length. The sheet-iron door is shut, and the fire strongly urged, whereby
the bismuth begins to flow in ten minutes, and falls through the holes in the clay-plates
into hot pans containing some coal-dust. Whenever it runs slowly, the ore is stirred
round in the pipes, at intervals during half an hour, in which time the liquation is
usually finished. The residuum, called bismuth barley (graupen), is scooped out with
iron rakes into a water trough; the pipes are charged afresh; the pans, when full, have
their contents cast into moulds, forming bars of from 25 to 50 pounds weight. About 20
cwt. of ore are smelted in 8 hours, with a consumption of 63 Leipzic cubic feet of wood.
The total production of Schneeberg, in 1830, was 9800 lbs. The bismuth thus procured
by liquation upon the great scale, contains no small admixture of arsenic, iron,
and some other metals, from which it may be freed by solution in nitric acid, precipitation
by water, and reduction of the subnitrated oxide by black flux. By exposing
the crude bismuth for some time to a dull red heat, under charcoal, arsenic is expelled.
Bismuth is white, and resembles antimony, but has a reddish tint; whereas the latter
metal has a bluish cast. It is brilliant, crystallises readily in small cubical facets, is
very brittle, and may be easily reduced to powder. Its specific gravity is 9·83; and by
hammering it with care, the density may be increased to 9·8827. It melts at 480° Fahr.,
and may be cooled 6 or 7 degrees below this point without fixing; but the moment it
begins to solidify, the temperature rises to 480°, and continues stationary till the whole
mass is congealed. When heated from 32° to 212°, it expands 1⁄710 in length. When
pure it affords a very valuable means of adjusting the scale of high-ranged thermometers.
At strong heats bismuth volatilises, may be distilled in close vessels, and is thus obtained
in crystalline laminæ.
The alloy of bismuth and lead in equal parts has a density of 10·709, being greater
than the mean of the constituents; it has a foliated texture, is brittle, and of the same
colour as bismuth. Bismuth, with tin, forms a compound more elastic and sonorous than
the tin itself, and is therefore frequently added to it by the pewterers. With 1 of bismuth
and 24 of tin, the alloy is somewhat malleable; with more bismuth, it is brittle.
When much bismuth is present, it may be easily parted by strong muriatic acid, which
dissolves the tin, and leaves the bismuth in a black powder. It has been said, that an
alloy of tin, bismuth, nickel, and silver, hinders iron from rusting. (Erdmann’s Journal.)
The alloy of bismuth with tin and lead was first examined by Sir I. Newton, and
has been called ever since fusible metal. Eight parts of bismuth, 5 of lead, and 3 of
tin, melt at the moderate temperature of 202° F.; but 2 of bismuth, 1 of lead, and 1 of
tin, melt at 200·75° F. according to Rose. A small addition of mercury of course aids
the fusibility. Such alloys serve to take casts of anatomical preparations. An alloy of 1
bismuth, 2 tin, and 1 lead, is employed as a soft solder by the pewterers; and the same
has been proposed as a bath for tempering steel instruments. Cake-moulds, for the
manufacturers of toilet soaps are made of the same metal; as also excellent clichés for
stereotype, of 3 lead, 2 tin, and 5 bismuth; an alloy which melts at 199° F. This compound
should be allowed to cool upon a piece of pasteboard, till it becomes of a doughy
consistence, before it is applied to the mould, to receive the impress of the stamp.
[121]
The employment of plates of fusible metal as safety rondelles, to apertures in the tops
of steam boilers has been proposed in France, because they would melt and give way
at elevations of temperature under those which would endanger the bursting of the
vessel; the fusibility of the alloy being proportioned to the quality of steam required
for the engine. It has been found, however, that boilers, apparently secured in this
way, burst, while the safety discs remained entire; the expansive force of the steam
causing explosion so suddenly, that the fusible alloy had not time to melt or give way.
There are two, perhaps three, oxides of bismuth; the first and the third, or the suboxide
and super-oxide, are merely objects of chemical curiosity. The oxide proper occurs
native, and may be readily formed by exposing the metal to a red-white heat in a muffle,
when it takes fire, burns with a faint blue flame, and sends off fumes which condense into
a yellow pulverulent oxide. But an easier process than that now mentioned is to
dissolve the bismuth in nitric acid, precipitate with water, and expose the precipitate to
a red heat. The oxide thus obtained has a straw yellow colour, and fuses at a high
heat into an opaque glass of a dark-brown or black colour; but which becomes less
opaque and yellow after it has cooled. Its specific gravity is so high as 8·211. It consists
of 89·87 of metal and 10·13 oxygen in 100 parts. The above precipitate, which
is a sub-nitrate of bismuth, is called pearl-white, and is employed as a flux for certain
enamels; as it augments their fusibility without imparting any colour to them. Hence,
it is used sometimes as a vehicle of the colours of other metallic oxides. When well
washed, it is employed in gilding porcelain; being added in the proportion of one
fifteenth to the gold. But pearl-white is most used by ladies, as a cosmetic for giving a
brilliant tint to a faded complexion. It is called blanc de fard, by the French. If it
contains, as bismuth often does, a little silver, it becomes grey or dingy coloured on exposure
to light. When the oxide is prepared, by dropping the nitric solution into an
alkaline lye in excess, if this precipitate is well washed and dried, it forms an excellent
medicine; and is given, mixed with gum tragacanth, for the relief of cardialgia,
or burning and spasmodic pains of the stomach.
Another sort of pearl-powder is prepared by adding a very dilute solution of common
salt to the above nitric solution of bismuth, whereby a pulverulent sub-chloride of the
metal is obtained in a light flocculent form. A similar powder of a mother-of-pearl
aspect may be formed by dropping dilute muriatic acid into the solution of nitrate of
bismuth. The arsenic always present in the bismuth of commerce is converted by
nitric acid into arsenic acid, which, forming an insoluble arseniate of bismuth, separates
from the solution, unless there be such an excess of nitric acid as to re-dissolve it.
Hence the medicinal oxide, prepared from a rightly-made nitrate, can contain no
arsenic. If we write with a pen dipped in that solution, the dry invisible traces will
become legible on plunging the paper in water.
It has been proposed to substitute bismuth for lead in assaying silver, as a smaller
quantity of it answers the purpose, and, as its oxide is more fluent, can therefore
penetrate the cupel more readily, and give a more rapid result. But, independently
of the objection from its high price, bismuth has the disadvantage of boiling up, as
well as of rocking or vegetating, with the silver, when the cupellation requires a high
heat. In extracting the silver from the galena found in the copper-mine of Yahlun,
it has happened sometimes that the silver concreted towards the end of the operation,
and produced a cauliflower excrescence, which had to be cupelled again with a fresh
dose of lead. It was observed that, in this case, a portion of the silver had passed into
the cupel. Berzelius detected in a sample of silver thus concreted the presence of
bismuth.
The nitrate of bismuth, mixed with solution of tin and tartar, has been employed as
a mordant for dyeing lilac and violet in calico printing.
BISTRE. (Bistre, Fr. bister, Germ.) A brown colour which is used in water
colours, in the same way as China ink. It is prepared from wood-soot, that of beech
being preferred. The most compact and best burned parcels of soot are collected
from the chimney, pulverised, and passed through a silk sieve. This powder is infused
in pure water, and stirred frequently with a glass ruler, then allowed to settle when the
water is decanted. If the salts are not all washed away, the process may be repeated
with warm water. The paste is now to be poured into a long narrow vessel filled with
water, stirred well, and left to settle for a few minutes, in order to let the grosser parts
subside. The supernatant part is then to be poured off into a similar vessel. This
process may be repeated twice or thrice, to obtain a very good bistre. At last the
settled deposit is sufficiently fine, and, when freed from its supernatant water, it is mixed
with gum-water, moulded into proper cakes, and dried. It is not used in oil painting,
but has the same effect in water-colours as brown pink has in oil.
BITUMEN, or ASPHALTUM. (Bitume, Fr. Erdpech, Germ.) A black substance
found in the earth, externally not dissimilar to pit-coal. It is composed of
carbon, hydrogen, and oxygen, like organic bodies; but its origin is unknown. It[122]
has not been observed among the primitive or older strata, but only in the secondary
and alluvial formations. It constitutes sometimes considerable beds, as in the isle of
Trinidad, where it occurs over an extensive district, in scattered masses. The greater
part of the asphaltum to be met with in commerce comes from the Dead Sea, on
whose shores it is cast up and gathered; whence it has got the name of Jewish bitumen.
In its black colour and fracture it resembles ordinary pitch. By friction it affords
negative electricity. Its average density is 1·16. It melts at the temperature of
boiling water, kindles very readily at the flame, burns brightly with a thick smoke,
and leaves little ashes. Distilled by itself, it yields a peculiar bituminous oil, very little
water, some combustible gases, and traces of ammonia. It leaves about one third of its
weight of charcoal after combustion, and ashes, containing silica, alumina, oxide of iron,
sometimes a little lime, and oxide of manganese. According to John, asphaltum may
be decomposed, by different solvents, into three distinct substances. Water dissolves
nothing; alcohol (anhydrous) dissolves out a yellow resin equal to 5 per cent. of the
weight of the asphaltum; that resin is soluble in dilute alcohol and in ether. The
portion not soluble in the alcohol gives up a brown resin to ether, amounting to 70 per
cent. of the weight of the asphaltum. On evaporating off the ether, the resin remains
of a brownish-black colour, which dissolves readily in the volatile oils and in the oil of
petroleum. The portion of asphaltum which does not dissolve in ether is very soluble
in oil of turpentine, and in oil of petroleum; but less so in oil of lavender. These
three resinous principles dissolve all together by digestion in the oils of anise, rosemary,
turpentine, olive, hemp-seed, nut, and linseed. Caustic potash dissolves a
notable quantity of asphaltum; but carbonate of potash has no effect upon it.
Asphaltum enters into the composition of hydraulic cements, and into that of black
varnishes, called japans, for coating iron trays, &c. A similar varnish may be prepared
by dissolving 12 parts of fused amber, 2 parts of rosin, and 2 parts of asphaltum, in 6
parts of linseed oil varnish, to which 12 parts of oil of turpentine have been added.
There is a kind of bitumen found at Aniches, in France, in the department of the
north, which is black, very fusible, and soft. It burns with flame. Alcohol, ether, and oil
of turpentine extract from it a fatty substance, which may be saponified with alkalis.
The bitumen of Murindò, near Choco, in Columbia, is of a brownish-black colour,
soft, and has an earthy fracture. It has an acrid taste, burns with a smell of vanilla,
and is said to contain a large quantity of benzoic acid. It appears to be the result of
the decomposition of trees containing benzoin.
Asphaltum occurs abundantly at the surface of the salt lake Asphaltites, in Judea,
produced from springs in the neighbourhood; it is floated down, gathers consistence,
and accumulates upon the surface of the lake; the winds drive it on the shores, and the
inhabitants collect it for sale. Its inspissation diffuses a disagreeable smell in the air of
that region, which is supposed by the natives to be powerful enough to kill birds when
they attempt to fly across the lake.
But probably the most remarkable locality of asphaltum in the world is the entire
basin, or rather plain of it, in the island of Trinidad, called the Tar Lake. It lies on the
highest land in the island, and emits a strong smell, sensible at ten miles’ distance. Its
first appearance is that of a lake of water, but, when viewed more nearly, it seems to be
a surface of glass. In hot weather its surface liquifies to the depth of an inch, and it
cannot then be walked upon. It is of a circular form, about three miles in circumference,
and of a depth not ascertained. Large fissures frequently open and close up in it,
whence the pitch has been supposed to float upon a body of water. The soil, for a considerable
distance round it, consists of cinders and burnt earth, and presents in many
points indications of convulsions by subterranean fire. In several parts of the neighbouring
woods, there are round holes and fissures in the ground, containing liquid
bitumen to the depth of two inches.
Mr. Hatchett examined some specimens from Trinidad, and concluded that what had
been heretofore supposed to be a pure mineral pitch was in reality only a porous stone
of the argillaceous kind, much impregnated with bitumen.
These various bitumens belong exclusively to the secondary and tertiary geological
formations, and are not found among primitive rocks, except very rarely in veins. They
occur most generally in calcareous, argillaceous, and sandy strata, and also in volcanic
districts. Petroleum frequently floats on the waters which issue from the volcanic
mountains, or which lie at their base; even the sea is at times covered with it near the
volcanic islands of Cape Verd. Mr. Breislak observed a petroleum spring rising from
the bottom of the sea near the southern base of Vesuvius.
The substance with which bitumen seems to have the most constant and most remarkable
relations, is sea-salt; so that almost all the countries most abundant in petroleum,
as Italy, Transylvania, Persia, the environs of Babylon, the region of the Dead Sea, &c.,
contain salt mines, or lakes, or exhibit saline efflorescences. Iron pyrites is often
impregnated with petroleum, or contains a bituminous nucleus.
[123]
The origin of bitumen is as little known as that of most of the productions of nature.
Some regard it as an empyreumatic oil, a matter analogous to liquid resin or essential
oil, resulting from the destruction of that astonishing multitude of animals and vegetables
buried in the earth, whose solid remains are daily brought to view in mineral researches.
It has been also supposed that naphtha and petroleum are the product of coals decomposed
either by the fire of volcanos, by the subterranean combustion of coal itself, or by the
decomposition of pyrites. The latter opinion is not supported by any direct evidence,
but the two former are sufficiently probable.
Elastic Bitumen is a rare substance, found hitherto only near Castleton, in Derbyshire,
in fissures of slaty clay.
Bituminous mastic, or cement, has been of late extensively employed in France for
covering roofs and terraces, and lining water cisterns. The mineral bitumen used for
the composition of this mastic is procured chiefly from the Obsann (Bas-Rhin), from
the Parc (department de l’Ain), and from the Puy-de-la-Poix (department of Puy-de-Dome).
But boiled coal-tar answers equally well. In the neighbourhood of these
localities, there is a limestone impregnated with bitumen, which suits for giving consistence
to the cement. This is well dried, ground to powder, sifted, and stirred while
hot, in about one fifth its weight of melted asphaltum, contained in a cast-iron boiler.
Dry chalk or bricks, ground and sifted, will suit equally well. As soon as this paste is
made quite homogeneous, it is lifted out with an iron shovel or spoon, and spread in
rectangular moulds, secured with pegs at the joints, fastened to a kind of platform of
smoothed planks, covered with strong sheet-iron. The sides of these moulds should be
previously smeared over with a thin coat of loam-paste, to prevent their adhesion to the
mastic. Whenever the cake is cold, the frame is taken asunder, and it is removed from
the iron plate by an oblong shovel, or strong spatula of iron. These cakes or bricks are
usually 18 inches long, 12 broad, and 4 thick, and weigh about 70 lbs.
BITTER PRINCIPLE. (Amère, Fr.; Bitterstoff, Germ.) This principle has not
been insulated hitherto by the chemist from the other proximate principles of plants, but
its existence is sufficiently recognised by the taste. The following list contains the
principal bitter substances, many of which have been used in the arts and in medicine.
| Name. |
Part employed. |
Country. |
Observations. |
| Quassia |
Wood |
Surinam, E. Indies |
Powerfully bitter |
| Wormwood |
Herb |
Great Britain |
Ditto |
| Aloe |
Inspissated juice |
South Africa |
Ditto |
| Angustura |
Bark |
South America |
Ditto |
| Orange |
Unripe fruit |
South of Europe |
|
- |
Aromatic bitter |
| Ditto |
Peel |
Ditto |
| Acorus |
Root |
Ditto |
Ditto |
| Carduus Benedictus |
Herb |
Greek Archipelago |
|
| Cascarilla |
Bark |
Jamaica |
Ditto |
| Centaury |
Herb |
Great Britain |
|
| Camomile |
Flowers |
|
|
| Colocynth |
Fruit |
Levant |
Intolerably bitter |
| Colombo |
Root |
East Africa |
Very bitter |
| Fumitory |
Herb |
Great Britain |
|
| Gentiana lutea |
Root |
Switzerland |
Very bitter |
| Ground Ivy |
Herb |
Great Britain |
|
| Walnut |
Peels |
|
With tannin |
| Island moss |
|
|
With starch |
| Hops |
Scales of the female flowers |
Great Britain |
Aromatic bitters |
| Milfoil |
Herb flowers |
Great Britain |
|
| Large-leaved Satyrion |
Herb |
Great Britain |
|
| Rhubarb |
Root |
China |
Disagreeable odour |
| Rue |
Herb |
Great Britain |
Bitter and sharp |
| Tansy |
Herb flowers |
Ditto |
Bitter and offensive |
| Bitter trefoil |
Herb |
Ditto |
|
| Simarouba |
Bark |
Guyana |
|
| Bryony |
Root |
Great Britain |
Sharp, bitter, nauseous |
| Coffee |
Seeds |
Arabia |
|
BLACK DYE. (Teinte noire, Fr. Schwartze farbe, Germ.) For 1 cwt. of cloth, there
are put into a boiler of middle size 18 lbs. of logwood, with as much Aleppo galls in powder,[124]
and the whole, being enclosed in a bag, is boiled in a sufficient quantity of water for
12 hours. One-third of this bath is transferred into another boiler with two pounds
of verdigris; and the stuff is passed through this solution, stirring it continually during
two hours, taking care to keep the bath very hot without boiling. The stuff is then
lifted out, another third of the bath is added to the boiler, along with eight pounds
of sulphate of iron or green vitriol. The fire is to be lowered while the sulphate dissolves,
and the bath is allowed to cool for half an hour, after which the stuff is introduced, and
well moved about for an hour, after which it is taken out to air. Lastly, the remaining
third of the bath is added to the other two, taking care to squeeze the bag well. 18 or
22 lbs. of sumach are thrown in; the whole is just brought to a boil, and then refreshed
with a little cold water; two pounds more of sulphate of iron are added, after which
the stuff is turned through for an hour. It is thereafter washed, aired, and put again
into the bath, stirring it continually for an hour. After this, it is carried to the river,
washed well, and then filled. Whenever the water runs off clear, a bath is prepared with
weld, which is made to boil for an instant; and after refreshing the bath the stuff is
turned in to soften, and to render the black more fast. In this manner, a very beautiful
black is obtained, without rendering the cloth too harsh.
Commonly more simple processes are employed. Thus the blue cloth is simply turned
through a bath of gall-nuts, where it is boiled for two hours. It is next passed through
a bath of logwood and sulphate of iron for two hours, without boiling, after which it is
washed and fulled.
Hellot has found that the dyeing might be performed in the following manner:—For
20 yards of dark blue-cloth, a bath is made of two pounds of fustic (morus
tinctoria), 41⁄4 lbs. of logwood, and 11 lbs. sumach. After boiling the cloth in it for three
hours it is lifted out, 11 lbs. of sulphate of iron are thrown into the boiler, and the cloth
is then passed through it during two hours. It is now aired, and put again in the bath
for an hour. It is, lastly, washed and scoured. The black is less velvety than that of the
preceding process. Experience convinced him that the maddering prescribed in the ancient
regulations only gives a reddish cast to the black, which is obtained finer and more
velvety without madder.
A black may be dyed likewise without having given a blue ground. This method is
employed for cloths of little value. In this case they are rooted; that is to say, they receive
a dun ground with walnut husks, or the root of the walnut tree, and are afterwards
made black in the manner above described, or in some other way; for it is obvious that
a black may be obtained by several processes.
According to Lewis, the proportions which the English dyers most generally adopt
are, for one hundred and twelve pounds of woollen cloth previously dyed of a dark
blue, about five pounds of sulphate of iron, as much gall-nuts, and thirty pounds of
logwood. They begin by galling the cloth, they then pass it through the decoction of
logwood, to which the sulphate of iron has been added.
When the cloth is completely dyed, it is washed in the river, and passed through the
fulling-mill till the water runs off clear and colourless. Some persons recommend, for
fine cloths, to full them with soap water. This operation requires an expert workman,
who can free the cloth thoroughly from the soap. Several recommend at its coming
from the fulling to pass the cloth through a bath of weld, with the view of giving softness
and solidity to the black. Lewis says, that passing the cloth through weld, after it
has been treated with soap, is absolutely useless, although it may be beneficial when this
operation has been neglected.
Different operations may be distinguished in dyeing silk black; the boiling of the silk,
its galling, the preparation of the bath, the operation of dyeing, the softening of the
black.
Silk naturally contains a substance called gum, which gives it the stiffness and elasticity
peculiar to it in its native state; but this adds nothing to the strength of the silk,
which is then styled raw; it rather renders it, indeed, more apt to wear out by the stiffness
which it communicates; and although raw silk more readily takes a black colour,
yet the black is not so perfect in intensity, nor does it so well resist the re-agents capable
of dissolving the colouring particles, as silk, which is scoured or deprived of its gum.
To cleanse silk intended for black, it is usually boiled four or five hours with one
fifth of its weight of white soap, after which it is carefully beetled and washed.
For the galling, nut-galls equal nearly to three fourths of the weight of the silk are
boiled during three or four hours; but on account of the price of Aleppo galls, more or
less of the white gall-nuts, or of even an inferior kind called galon, berry or apple galls,
are used. The proportion commonly employed at Paris is two parts of Aleppo galls to
from eight to ten parts of galon. After the boiling, the galls are allowed to settle for
about two hours. The silk is then plunged into the bath, and left in it from twelve to
thirty-six hours, after which it is taken out and washed in the river.
Silk is capable of combining with quantities, more or less considerable, of the astringent[125]
principle; whence results a considerable increase of weight, not only from the weight
of the astringent principle, but also from that of the colouring particles, which
subsequently fix themselves in proportion to the quantity of the astringent principle
which had entered into combination. Consequently, the processes are varied according
to the degree of weight which it is wished to communicate to the silk; a circumstance
requiring some illustration.
The commerce of silk goods is carried on in two ways; they are sold either by the
weight, or by the surface, that is, by measure. Thus the trade of Tours was formerly distinguished
from that of Lyons; the silks of the former being sold by weight, those of the
latter, by measure. It was therefore their interest to surcharge the weight at Tours, and,
on the contrary, to be sparing of the dyeing ingredients at Lyons; whence came the distinction
of light black and heavy black. At present, both methods of dyeing are practised
at Lyons, the two modes of sale having been adopted there.
Silk loses nearly a fourth of its weight by a thorough boiling, and it resumes, in the
light black dye, one half of this loss; but in the heavy black dye, it takes sometimes upwards
of a fifth more than its primitive weight; a surcharge injurious to the beauty of
the black, and the durability of the stuff. The surcharged kind is denominated English
black, because it is pretended that it was first practised in England. Since silk dyed with
a great surcharge has not a beautiful black, it is usually destined for weft, and is blended
with a warp dyed of a fine black.
The peculiarity of the process for obtaining the heavy black consists in leaving the
silk longer in the gall liquor, in repeating the galling, in passing the silk a greater number
of times through the dye, and even letting it lie in it for some time. The first galling
is usually made with galls which have served for a preceding operation, and fresh
gall-nuts are employed for the second. But these methods would not be sufficient for
giving a great surcharge, such as is found in what is called the English black. To give
it this weight, the silk is galled without being ungummed; and, on coming out of the
galls, it is rendered supple by being worked on the jack and pin.
The silk-dyers keep a black vat, and its very complex composition varies in different
dye-houses. These vats are commonly established for many years; and when their black
dye is exhausted it is renovated by what is called in France a brevet. When the deposit
which has accumulated in it is too great, it is taken out, so that at the end of a certain
time nothing remains of the several ingredients which composed the primitive bath, but
which are not employed in the brevet.
For the dyeing of raw silk black, it is galled in the cold, with the bath of galls which
has already served for the black of boiled silk. For this purpose, silk, in its native
yellow colour, is made choice of. It should be remarked, that when it is desired to
preserve a portion of the gum of the silk, which is afterwards made flexible, the galling
is given with the hot bath of gall-nuts in the ordinary manner. But here, where the
whole gum of the silk, and its concomitant elasticity, are to be preserved, the galling
is made in the cold. If the infusion of galls be weak, the silk is left in it for several
days.
Silk thus prepared and washed takes very easily the black dye, and the rinsing in a
little water, to which sulphate of iron may be added, is sufficient to give it. The dye is
made in the cold; but, according to the greater or less strength of the rinsings, it requires
more or less time. Occasionally three or four days are necessary; after which
it is washed, it is beetled once or twice, and it is then dried without wringing, to avoid
softening it.
Raw silk may be more quickly dyed, by shaking it round the rods in the cold bath
after the galling, airing it, and repeating these manipulations several times, after which
it is washed and dried as above.
Macquer describes a more simple process for the black by which velvet is dyed at
Genoa; and he says that this process, rendered still simpler, has had complete success
at Tours. The following is his description.
For 1 cwt. (50 kilogrammes) silk, (22 lbs. 11 kilogrammes) of Aleppo galls, in powder,
are boiled for an hour in a sufficient quantity of water. The bath is allowed to settle till
the galls have fallen to the bottom of the boiler, from which they are withdrawn; after
which 32 lbs. of English vitriol (or copperas) are introduced, with 13 lbs. of iron filings,
and 22 lbs. of country gum, put into a kind of two-handled cullender, pierced every
where with holes. This kettle is suspended by two rods in the boiler, so as not to reach
the bottom. The gum is left to dissolve for about an hour, stirring it from time to
time. If, after this time, some gum remains in the kettle, it is a proof that the bath,
which contains two hogsheads, has taken as much of it as is necessary. If, on the contrary,
the whole gum is dissolved, from one to 4 lbs. more may be added. This cullender
is left constantly suspended in the boiler, from which it is removed only when the
dyeing is going on; and thereafter it is replaced. During all these operations the boiler
must be kept hot, but without boiling. The galling of the silk is performed with one[126]
third of Aleppo galls. The silk is left in it for six hours the first time, then for twelve
hours. The rest, secundum artem.
Lewis states that he has repeated this process in the small way; and that, by adding
sulphate of iron progressively, and repeating the immersions of the silk a great number
of times, he eventually obtained a fine black.
Astringents differ from one another as to the quantity of the principle which enters
into combination with the oxide of iron. Hence, the proportion of the sulphate, or of
any other salt of iron, and that of the astringents, should vary according to the astringents
made use of, and according to their respective quantities. Gall-nut is the
substance which contains most astringent; sumach, which seems second to it in this
respect, throws down (decomposes), however, only half as much sulphate of iron.
The most suitable proportion of sulphate of iron appears to be that which corresponds
to the quantity of the astringent matter, so that the whole iron precipitable by
the astringent may be thrown down, and the whole astringent may be taken up in
combination with the iron. As it is not possible, however, to arrive at such precision,
it is better that the sulphate of iron should predominate, because the astringent, when
in excess, counteracts the precipitation of the black colouring particles, and has the property
of even dissolving them.
This action of the astringent is such that, if a pattern of black cloth be boiled with
gall-nuts, it is reducible to grey. An observation of Lewis may thence be explained.
If cloth be turned several times through the colouring bath, after it has taken a good
black colour, instead of acquiring more body, it is weakened, and becomes brownish.
Too considerable a quantity of the ingredients produces the same effect; to which the
sulphuric acid, set at liberty by the precipitation of the oxide of iron, contributes.
It is merely the highly oxidised sulphate which is decomposed by the astringent;
whence it appears, that the sulphate will produce a different effect according to its state
of oxidisement, and call for other proportions. Some advise, therefore, to follow the
method of Proust, employing it in the oxidised state; but in this case it is only partially
decomposed, and another part is brought, by the action of the astringent, into the lower
degree of oxidisement.
The particles precipitated by the mixture of an astringent and sulphate of iron have
not at first a deep colour; but they pass to a black by contact of air while they are
moist.
Under dyeing I shall show that the black dye is only a very condensed colour, and
that it assumes more intensity from the mixture of different colours likewise deep. It is
for this reason advantageous to unite several astringents, each combination of which
produces a different shade. But blue appears the colour most conducive to this effect,
and it corrects the tendency to dun, which is remarked in the black produced on stuffs
by the other astringents.
On this property is founded the practice of giving a blue ground to black cloths,
which acquire more beauty and solidity the deeper the blue. Another advantage of this
practice is to diminish the quantity of sulphuric acid which is necessarily disengaged by
the precipitation of the black particles, and which would not only counteract their fixation,
but would further weaken the stuff, and give it harshness.
For common stuffs, a portion of the effect of the blue ground is produced by the
rooting.
The mixture of logwood with astringents contributes to the beauty of the black in a
twofold way. It produces molecules of a hue different from what the astringents do,
and particularly blue molecules, with the oxide of copper, commonly employed in the
black dyes; which appears to be more useful the more acetate the verdigris made use
of contains.
The boil of weld, by which the dye of black cloth is frequently finished, may also
contribute to its beauty, by the shade peculiar to its combination. It has, moreover, the
advantage of giving softness to the stuffs.
The processes that are employed for wool, yield, according to the observation of
Lewis, only a rusty black to silk; and cotton is hardly dyed by the processes proper
for wool and silk. Let us endeavour to ascertain the conditions which these three
varieties of dyeing demand.
Wool has a great tendency to combine with colouring substances; but its physical
nature requires its combinations to be made in general at a high temperature. The
combination of the black molecules may therefore be directly effected in a bath, in proportion
as they form; and, if the operation be prolonged by subdividing it, it is only
with the view of changing the necessary oxidisement of the sulphate, and augmenting
that of the colouring particles themselves.
Silk has little disposition to unite with the black particles. It seems to be merely by
the agency of the tannin, with which it is previously impregnated, that these particles
can fix themselves on it, especially after it has been scoured. For this reason, silk baths[127]
should be old, and have the colouring particles accumulated in them, but so feebly suspended
as to yield to a weak affinity. Their precipitation is counteracted by the addition
of gum, or other mucilaginous substances. The obstacle which might arise from the
sulphuric acid set at liberty is destroyed by iron filings, or other basis. Thus, baths of
a very different composition, but with the essential condition of age, may be proper for
this dye. For cotton black dye, see Calico Printing.
BLACK PIGMENT. The finest light black is prepared principally for the
manufacturing of printers’ ink. In Messrs. Martin and Grafton’s patent process, the
black is obtained by burning common coal-tar, which should, however, be previously
divested, as much as possible, of the ammoniacal liquor and acid mixed with it in
the tank.
For this purpose, it is proposed that four casks should be employed, each capable of
holding 130 gallons, and into every one of them are to be put about 60 gallons of the
rough impure tar, to which an equal quantity of lime-water is to be added, and then
agitated by machinery or manual labour until the lime-water is completely mixed with
the tar. The vessels should next be suffered to rest for about six hours, by which
time the tar will settle at the bottom of the casks, and the water may be drawn off.
The casks containing the tar should now be filled with hot water, which may be
supplied from the boiler of a steam engine, and the whole again agitated as before.
This process may be repeated three times, suffering the tar to subside between each;
and twelve hours should be allowed for settling from the last water, so that the whole
of the tar and water may become separated, the water rising to the top of the cask, and
the tar being left at the bottom in a pure state.
But, as some of the water will yet remain mechanically combined with the tar, it is
proposed that the tar should be subjected to the process of distillation. For this
purpose, a still, capable of holding 120 gallons, may be employed, in which about
50 gallons, at one time, may be operated upon; when, by a gentle heat, the water, and
other impurities which the tar may have retained, will be driven off. As soon as the
water appears to have evaporated, and the spirit runs fine and clear, the process of
distillation should be stopped; and, when cold, the pure tar may be drawn off, and set
apart for the purpose of being employed as contemplated in the patent.
The tar thus purified may be now converted into black, or it may be subjected to
further rectification to divest it of the mineral pitch, or asphaltum, which is combined
with the oil and spirit: the latter is to be preferred, because the mineral pitch, or
asphaltum, is only inflammable at a high temperature, which renders it more troublesome
to use in the process here contemplated, and also would cause the apparatus to
require frequent cleaning from the carbonized pitch deposited. In order, therefore, to
get rid of the mineral pitch, or asphaltum, forty gallons of the tar are to be introduced
into a still, as before; and, instead of stopping the operation, as soon as the spirit begins
to come over, the distillation is continued with a strong heat, so as to force over the
whole of the oil and spirit, leaving the residuum of asphaltum in the still: this process,
however, is known to every chemist, and need not be further explained.
In fig. 115. is exhibited a rude representation of the apparatus employed in preparing
and collecting the fine light spirit black, produced by the combustion of the oil and
spirit of coal-tar, after it has been purified as above described. a is the brickwork
which supports a number of burners issuing from a tube, b, within, and here shown by[128]
dots, as passing along its whole length. Fig. 116. is a section of the brickwork, with
the tube, burner, and receiver, as will be described hereafter. The tube may be called
the tar main, as it is intended to be filled with tar: it is constructed of cast iron, and
from it issue several (in this figure twenty-four) jets or burners, c, c, c; any other
number may be employed. d is a furnace under the tar main, the flue of which
extends along, for the purpose of heating the tar to the boiling point, in order to
facilitate the process. From the main, b, the tar flows into the jets c; wicks are
introduced into the jets, and, when set fire to by a red-hot stick, will burn and emit a
very considerable quantity of smoke; which it is the object of this apparatus to conduct
through many passages, for the purpose of collecting its sooty particles.
There are a number of hoods, e, e, e, or bonnets, as they are termed, all of which,
through their pipes, have communication with, or lead into, a main chimney, f, f. Into
these hoods or bonnets the smoke of the burners ascends, and from thence passes into
the main chimney f, and thence through the smoke tubes into the box g: here the
heaviest particles of the black deposit themselves; but, as the smoke passes on through
the farther pipes, a deposit of the second, or finer, particles of black takes place in the
box h. From hence the smoke proceeds through other pipes into a series of canvass
bags, i, i, i, which are proposed to be about eighteen feet long, and three in diameter.
These bags are connected together at top and bottom alternately, and through the
whole series the smoke passes up one bag and down the next, depositing fine black,
called spirit black, upon the sides of the canvass. After the jets have continued burning
for several days, the bags are to be beaten with a stick, so that the black may fall to the
bottom; and, when a sufficient quantity has accumulated, the bags may be emptied and
swept out. Thus seventy or eighty bags may be employed; so that the smoke should
pass through a length of about 400 yards, the farthest of which will be found to contain
the finest black. The last bag should be left open, in order to allow the vapour to
escape into the open air.
The main tar tube will require to be emptied every four or five days, in order to
clear it from the pitchy matter that may have subsided from the burners, and they also
will require to be frequently poked with a wire, to clear off the black which forms upon
the edges, and to drive down the carbonized tar which attaches itself to the upper part
of the jets.
BLEACHING (Blanchiment, Fr.; Bleichen, Germ.) is the process by which the
textile filaments, cotton, flax, hemp, wool, silk, and the cloths made of them, as well as
various vegetable and animal substances, are deprived of their natural colour, and
rendered nearly or altogether white. The term bleaching comes from the French verb
blanchir, to whiten. The word blanch, which has the same origin, is applied to the
whitening of living plants by making them grow in the dark, as when the stems of
celery are covered over with mould.
The operations which the bleacher has recourse to differ according to the nature of
the bleaching means, the property of the stuff to be bleached, and local customs or circumstances;
and the result is also obtained with more or less rapidity, certainty,
economy, and perfection. The destruction of the colouring matters attached to the
bodies to be bleached is effected either by the action of the air and light, of chlorine, or
sulphurous acid; which may be considered the three bleaching powers employed for
manufacturing purposes.
Bleaching by the influence of air and sunshine is the most ancient, and still the most
common, method in several civilised countries; it is also supposed by many to be the
least injurious to the texture of yarn and cloth. The operations it involves are very
simple, consisting in the exposure of the goods upon a grass-plat to the sky, with their
occasional aspersion with moisture if necessary, in addition to the rain and dew. The
atmospheric air effects the bleaching by means of its oxygenous constituent, which combines
with the colouring matter, or its elements carbon and hydrogen, and either makes it
nearly white, or converts it into a substance easily soluble in water and alkaline solutions.
This natural process is too slow to suit the modern demands of the cotton and linen
manufacturers. Fortunately for them, a new bleaching agent, unknown to our forefathers,
has been discovered in chlorine, formerly called oxymuriatic acid, an agent modified by
chemistry so as to give an astonishing degree of rapidity, economy, and perfection, to this
important art. It is, however, not a little surprising, that the science which has so greatly
advanced its practical part should have left its theory far from complete, and should afford
no satisfactory answers to the two following questions.—What is the action of the solar
rays upon the colouring matter? How do air and chlorine operate upon this principle?
Some suppose that light predisposes the colouring matter to combine with oxygen; others
fancy that it acts merely in the manner of a high temperature, so as to determine a
reaction between the elements of that substance, and to cause a new combination
possessed of peculiar properties. It is generally admitted at the present day, that a
portion of the oxygen of the air passes into the colouring matter, and changes its constitution.[129]
This is, however, probably not the part which oxygen plays, nor is it the only
principle in the atmosphere which exercises a bleaching influence. Neither is the action
of chlorine such as has been commonly represented in our chemical systems.
But if authors offer us only vague hypotheses concerning the three principal agents,
light, oxygen, chlorine, they afford no information whatever concerning the phenomena
due to greasy spots so frequently found upon cotton cloth, and so very troublesome to
the bleacher. It has indeed been sometimes said in bleach-works, that fatty substances
are no longer soluble in alkalies when they are combined with oxygen. The
very reverse of this statement is probably nearer the truth.
The object of bleaching is to separate from the textile fibre, by suitable operations, all
the substances which mask its intrinsic whiteness; or which, in the course of ulterior
dyeing operations, may produce injurious effects. In this latter respect, cotton deserves
especial consideration. This substance is covered with a resinous matter, which obstructs
its absorption of moisture, and with a yellow colouring matter in very small quantity,
often so inconsiderable in some cottons, that it would be unnecessary to bleach them,
before submitting them to the dyer, were it not that the manipulations which they
undergo introduce certain impurities which are more or less injurious, and must be
removed. It is in fact a circumstance well known in the factories, that unbleached
cottons may be dyed any dark colour, provided they are deprived of that matter which
makes them difficult to moisten. The substances present in cotton goods are the
following:—
1. The resinous matter natural to the cotton filaments.
2. The proper colouring matter of this vegetable.
3. The paste of the weaver.
4. A fat matter.
5. A cupreous soap.
6. A calcareous soap.
7. The filth of the hands.
8. Iron, and some earthy substances.
1. The matter which prevents the moistening of cotton wool may be separated by
means of alcohol, which, when evaporated, leaves thin yellowish scales, soluble in alkalies,
in acids, and even in a large quantity of boiling water. For a long time the bleaching
process commenced with the removal of this resinous stuff, by passing the cloth or the
yarn through an alkaline ley. This was called scouring; it is now nearly laid aside.
2. The colouring matter of cotton seems to be superficial, and to have no influence
on the strength of the fibres; for the yarn is found to be as strong after it has been
stripped by caustic soda of its resinous and colouring matters, as it was before. The
colouring matter is slightly soluble in water, and perfectly in alkaline leys. When gray
calico is boiled in lime water, it comes out with a tint darker than it had before; whence
it might be supposed that the colouring matter was not dissolved out, even in part.
This, however, is not the case; for if we filter the liquor, and neutralise it with an acid,
we shall perceive light flocks, formed of the resinous substance, united with the colouring
matter. The dark colour of the cloth is to be ascribed solely to the property which lime
possesses of browning certain vegetable colours. This action is here exercised upon the
remaining colour of the cloth.
It may be laid down as a principle, that the colouring matter is not directly soluble
by the alkalies; but that it becomes so only after having been for some time exposed to
the joint action of air and light, or after having been in contact with chlorine. What
change does it thereby experience, which gives it this solubility? Experiments made
upon pieces of cloth placed in humid oxygen, in dry oxygen, in moist chlorine, and in dry
chlorine, tend to show that hydrogen is abstracted by the atmosphere; for in these
experiments proofs of dis-hydrogenation appeared, and of the production of carbonic acid.
In all cases of bleaching by chlorine, this principle combines immediately with the
hydrogen of the colouring matter, and forms muriatic acid, while the carbon is eliminated.
Undoubtedly water has an influence upon this phenomenon, since the bleaching
process is quicker with the humid chlorine than with the dry; but this liquid seems to
act here only mechanically, in condensing the particles of the gas into a solution. We
should also take into account the great affinity of muriatic acid for water.
3. The weaver’s dressing is composed of farinaceous matters, which are usually allowed
to sour before they are employed. It may contain glue, starch, gluten; which last is
very soluble in lime-water.
4. When the dressing gets dry, the hand-weaver occasionally renders his warp-threads
more pliant by rubbing some cheap kind of grease upon them. Hence it happens, that
the cloth which has not been completely freed from this fatty matter will not readily
imbibe water in the different bleaching operations; and hence, in the subsequent dyeing
or dunging, these greasy spots, under peculiar circumstances, somewhat like lithographic
stones, strongly attract the aluminous and iron mordants, as well as the dye stuffs, and[130]
occasion stains which it is almost impossible to discharge. The acids act differently
upon the fatty matters, and thence remarkable anomalies in bleaching take place. When
oil is treated with the acetic or muriatic acid, or with aqueous chlorine, it evolves no
gas, as it does with the sulphuric and nitric acids, but it combines with these substances
so as to form a compound which cannot be dissolved by a strong boiling ley of caustic
soda. Carbonic acid acts in the same way with oil. On the other hand, when the oils
and fats are sufficiently exposed to the air, they seize a portion of its oxygen, and
become thereby capable of saponification, that is, very soluble in the alkalies.
5. When the hand-weaver’s grease continues in contact for a night with the copper
dents of his reed, a kind of cupreous soap is formed, which is sometimes very difficult to
remove from the web. Lime-water does not dissolve it; but dilute sulphuric acid carries
off the metallic oxide, and liberates the margaric acid, in a state ready to be acted on by
alkalis.
6. When cloth is boiled with milk of lime, the grease which is uncombined unites
with that alkaline earth; and forms a calcareous soap, pretty soluble in a great excess of
lime-water, and still more so in caustic soda. But all fats and oils, as well as the soaps
of copper and lime, cease to be soluble in alkaline leys, when they have remained a considerable
time upon the goods, and have been in contact with acetic, carbonic, muriatic
acids, or chlorine. These results have been verified by experiment.
7. Cotton goods are sometimes much soiled, from being sewed or tamboured with
dirty hands; but they may be easily cleansed from this filth by hot water.
8. Any ferruginous or earthy matters which get attached to the goods in the course
of bleaching, are readily removable.
We are now prepared to understand the true principles of bleaching cotton goods, for
the most delicate operations of the calico printer.
1. The first process is steeping, or rather boiling, the goods in water, in order to remove
all the substances soluble in that liquid.
2. The next step is to wash or scour the goods by the dash-wheel or the stocks. This
is of great importance in the course of bleaching, and must be repeated several times; so
much so, that in winter, when the water of the dash-wheel is cold, the bleaching is
more tedious and difficult. Yarn and very open fabrics do not much need the dash-wheel.
By these first two operations, the woven goods lose about sixteen per cent. of their
weight, while they lose only two parts out of five hundred in all the rest of the
bleaching.
3. In the third place the calicoes are boiled with milk of lime, whereby they are
stripped of their gluten, and acquire a portion of calcareous soap. Formerly, and still
in many bleach-works, the gluten was got rid of by a species of fermentation of the
farinaceous dressing; but this method is liable to several objections in reference to the
calico-printer. 1. The fermentative action extends sometimes to the goods, and weakens
their texture, especially when they are piled up in a great heap without being previously
washed. 2. The spots of grease, or of the insoluble soaps, become thereby capable of
resisting the caustic alkalies, and are rendered in some measure indelible; an effect due
to the acetic and carbonic acids generated during fermentation, and which will be easily
understood from what has been said concerning the action of acids on fatty substances.
It is not, therefore, without good reason that many practical men throw some spent leys
into the fermenting vats, to neutralise the acids which are formed. Were it not for the
presence of fat, fermentation, skilfully conducted, would be an excellent means of carrying
off the gluten; and the steep is therefore applicable to power-loom goods, which
are not polluted with grease.
4. The goods are now subjected to a caustic soda ley, which dissolves out the soaps
of lime and copper, as well as that portion of the colouring matter which is sufficiently
dis-hydrogenated to be capable of combining with it. This bucking with ley, which is
repeated several times upon the goods, in order to purge them completely from the fatty
matter present in the hand-loom webs, and also partially introduced in the spinning, is
almost the only operation to which yarns for turkey red are subjected. After being
boiled in a caustic soda ley, they are passed through solutions of chloride of lime, and
afterwards through the acid steep.
5. When the goods are sufficiently bucked in the leys, they are either exposed to
chlorine, or laid out on the grass; sometimes both are had recourse to for delicate work.
These different modes of action have the same influence on the colouring matter, but
they give rise to different effects in reference to greasy stains.
The goods are dipped in a solution of chloride of lime, which should be kept tepid
by means of steam. Alongside of the chlorine cistern, there is another filled with dilute
sulphuric or muriatic acid. When the goods are taken out of the chlorine, they are
drained on the top of its cistern till no more liquid runs off them, and they are then
plunged into the sour. The action of the acid in the present case may be easily explained.[131]
In proportion as a salt of lime is formed, this base quits the chlorine, and
allows it to act freely upon the colouring matter. Thus we prevent the development of
too great a quantity of chlorine at once, which would be apt to injure the fibres; and
we pursue both a prudent and economical plan. Only so much chlorine as is strictly
necessary is called forth, and hence it excites no smell in the apartment.
The chlorine serves to acidify the colouring matter, by abstracting a portion of its
hydrogen; but we must take the greatest care that there is no grease upon the goods
before immersion in it, for the consequence would be, as above shown, very troublesome
spots. When the cloth is laid out upon the grass, it is the oxygen of the air which
acidifies the colouring matter; for which reason, the dew, which contains much air rich
in oxygen, singularly accelerates the bleaching process. It is likewise, by absorbing
oxygen from the atmosphere, that fats or oils pass to the state of margaric and oleic
acids, and become most easily saponified. Should the goods, however, be left too long
on the grass, the fats absorb carbonic acid, and become insoluble in leys.
6. The goods must now receive a new soda ley, to dissolve out that portion of the
colouring matter which has been dis-hydrogenated in the chlorine of the air, as well as
the grease, if any perchance remained in the soluble state. These last two operations
are to be several times repeated, because the colouring matter should be removed only
by degrees, for fear of injuring the texture of the goods, by subjecting them to too much
chlorine at a time.
7. We finish with the dilute sulphuric acid, which should be very weak and tepid.
It dissolves out the iron, and some earthy matters occasionally found upon cotton. The
goods must be most carefully washed at the dash-wheel, or in a stream of water on
quitting the sour bath, for if the acid were allowed to dry in them, it would infallibly
injure their texture by its concentration. In winter, if the goods are allowed to get
frozen with the acid upon them, they may likewise be damaged.
We may here observe, that when the goods are not to remain white, their bleaching
may be completed with a ley; for though it leaves a faint yellow tint, this is no inconvenience
to the dyer. But when they are to be finished with a starching after the last
ley, they must have another dip of the chlorine to render the white more perfect. An
immersion in the dilute acid has nearly the same effect.
The principles expounded above lead to this important consequence, that when we
wish to bleach goods that are free from greasy stains, as is the case generally with the
better kinds of muslins, or when we wish to bleach even greasy goods for the starch
finish, we may content ourselves with the following operations:—
1. Boiling in water.
2. Scouring by the stocks or the dash-wheel.
3. Bucking with milk of lime.
4. Passing through chlorine, or exposure on the grass.
5. Bucking, or bouking with milk of lime. These two latter operations require to
be alternated several times, till the whole of the colouring matter be removed.
6. Souring.
The bleaching of goods, which are never laid down on the green, and which are not
dried between two operations, may be completed in a couple of days. They answer as
well for the printer as the others, and they are as white. Cotton fibres or yarns suffer
no diminution of their strength, when the cloth has been properly treated in the above
described processes.
Accurate experiments have demonstrated that their strength is not impaired by being
boiled in milk of lime for two hours at the ordinary pressure, provided they be constantly
kept covered with liquid during the whole ebullition, and that they be well
washed immediately afterwards; or, by being boiled in pure water under the pressure
of ten atmospheres of steam; or by being boiled under the same pressure in a caustic
soda ley, marking 3° of Tweedale, or specific gravity 1·015, though it has increased to
double the density in the course of the boil, by the escape of the steam; or by being boiled
under the atmospheric pressure at 14° of Tweedale, or specific gravity 1·070; or by
being immersed for eight hours in chloride of lime, capable of decolouring three times
its bulk, of test solution of indigo (see Chlorine); and by being afterwards dipped
in sulphuric acid of specific gravity 1·067, Tweedale 14°; or by being steeped for
eighteen hours in sulphuric or muriatic acid of specific gravity 1·035, 7° Tweedale.
In other well-conducted bleach-works the following is the train of operations:—
1. Cleansing out the weaver’s dressing by steeping the cloth for twelve hours in cold
water, and then washing it at the stocks or the dash-wheel. 2. Boiling in milk of lime,
of a strength suited to the quality of the goods, but for a shorter time than with the
soda ley; two short operations with the lime, with intermediate washing, being preferable
to one of greater duration. 3 and 4. Two consecutive leys of ten or twelve hours’
boiling, with about two pounds of soda crystals for 1 cwt. of cloth. 5. Exposure to
the air for six or eight days, or the application of the chloride of lime and the sulphuric[132]
acid. 6. A ley of caustic soda, like the former, sometimes with less alkali. 7. Exposure
to the air for six or eight days, or chlorine and the sour, as above. 8. Caustic soda ley,
as before. 9. Chlorine and the sour. 10. Rinsing in hot water, or scouring at the
dash-wheel.
If the number of vessels to be heated exceeds four or five, there is an economy in
using steam as the medium of heat; but under this number there is an advantage in
the direct application of fire to a boiling or bucking apparatus; since when only two
vessels are in activity, there is a waste of fuel by the extra steam power. It deserves
to be remarked also, that the increase of the bulk of the liquid by the condensation of
the steam, does not permit the spent white ley to be turned to use for the green goods,
on account of its excessive dilution. With the milk of lime boil, however, this dilution
would be rather an advantage.
It has been found that the introduction of bran into the fermenting steep (when this
is used) endangers the texture of the goods, by causing a putrefactive fermentation in
some places.
When in the milk of lime boil there is too much of this caustic earth, or when it is
poured in on the top of the goods, they are apt to suffer damage. The milk of lime
should be introduced from beneath into the under compartment of the bucking apparatus.
For the same reason, after the caustic soda lye, the vessel should be filled up
with water, if the goods be not immediately transferred to the dash-wheel. When they
are allowed to become partially dry on the top, they are easily injured. The copper of
the bucking apparatus ought to be of a size proportioned to that of the surmounting
crib or vat; for when it is too small, the liquid is too long of being brought into
proper circulation, and the goods may be meanwhile injured. In a bucking apparatus,
which requires five or six hours to be brought into full play, those goods are very apt to
be injured, which lie immediately under the overflow pipe.
When the chloride of lime steep is too strong, sometimes small round holes are made
in the calico, just as if they had been cut out by a punch, especially in the borders or
thicker parts of the goods. This accident is owing to the presence of bubbles of
chlorine. From the saturated state of the liquid, they remain gaseous a sufficient
length of time for corroding the parts of the cloth with which they are in contact.
These will be obviously the denser parts, for they confine the gas most completely, or
prevent its diffusion through the mass. This evil is prevented by diluting the chloride
steep to the proper degree, and moving the goods through it.
The greasy spots, described above, show themselves in the maddering by attracting
the dye-stuff more copiously than the pure parts of the cloth, so as to mottle it; they
are also recognised in the white goods by being somewhat repulsive of moisture.
When the combination of fatty matters with chlorine takes place at the surface of cotton
goods, it is of a nature to resist the action of alkalies. It is the stearine, or the principle
of suet, particularly, which, by this means, acquires such a strong affinity for cottons;
the elaine, or the principle of oils, has no such remarkable affinity. Lime, in some
circumstances, seems to act as a mordant to greasy matters, and to fix them fast.
Hence the weaver should be prohibited, in all cases, from allowing candle-grease to
touch his web. Goods soiled with it should never be allowed to lie by in the warehouse,
but be immediately cleansed before the air has fixed the stearine by converting it
into margaric acid. Lime should, in these cases, be prudently employed; chlorine should
never be used till the greasy stains are thoroughly removed; and the bleacher should never
warrant his pieces for the printer till he has verified some of them by the water test.
I shall conclude this general analysis of the principles of bleaching by a few
precepts. Avoid lime, at the first ley, for goods which contain greasy spots; but use
it freely after one or two soda leys, and apply two soda leys after it. Do not apply
chlorine between these leys, but reserve it for the final operation. By this plan the
goods will be well bleached, and very little worn. Use the souring steeps freely,
giving them after each ley, whether of lime or soda, since the calcareous base, with
which the greasy spots get charged merely from hard water, is an obstacle to the further
action of the leys.
I shall now give some practical instructions concerning the several steps of the
bleaching process, as applied to cotton, linen, silk, and wool.
The first thing which the cotton bleacher does, is to mark the pieces with the initials
of the owner, by means of a stamp imbued with coal tar. The linen bleacher marks
with nitrate of silver, a far more expensive substance, but one which resists better the
severer treatment which his goods are destined to undergo.
The cotton goods are generally singed before they are sent to the bleacher, and this is
done either by passing them rapidly over a red-hot semi-cylinder of iron, or over a row
of gas flames, by Mr. Hall’s ingenious contrivance. (See Singeing.) Each piece is next
creased together lengthwise like a rope, folded into a bundle, and fixed by a noose at the
end. In this open state it is easily penetrated by the water of the soaking cistern into
which it is thrown. It is then scoured by the dash or wash-wheel. It is now ready for[133]
the bucking or steaming apparatus, where it is treated with milk of lime. The steam
chamber resembles the bucking vessel, without its bottom copper; that is to say, a few
inches below the grated bottom of the bucking tub, there is a close iron sole, through
the centre of which the steam is admitted by several small apertures, for the purpose of
diffusing it throughout the goods, and causing a liquid circulation by its pressure, as
the steam does in the proper bucking boiler. One pound of lime previously made into
a cream consistenced mixture, and passed through a sieve, is used for every thirty or forty
pounds of cloth, according to its colour and texture; and this cream mixed with more water
is interstratified with the pieces, as they are laid regularly in the vessel. Whenever this
is stocked with goods, all their interstices are filled up with water. After the lime
bucking, the cloth is transferred to the dash-wheel.
A pound of cloth requires for its whitening about half a pound of good average
chloride of lime or bleaching powder, as it is commonly called, and this ought to be
dissolved in about three gallons of water. Mr. Crum of Thorniebank, near Glasgow,
an extensive and excellent bleacher, has so modified Dr. Dalton’s ingenious plan of
testing the power of bleaching liquors by green sulphate of iron, as to give it much
greater precision for the bleacher’s use, than the discolouration of indigo originally proposed
by Berthollet. Mr. Crum dissolves four ounces of fresh green vitriol in hot water,
and then adds the solution of bleaching powder by small quantities at a time, till the
iron becomes wholly peroxidised, when the smell of chlorine will become perceptible.
When the bleacher has once found by trial the proper blanching power which his chlorine
steep ought to have, he can verify its standard, by seeing how much of it must be added
to an ounce, or any given weight of fresh copperas, dissolved in hot water, to cause the
peroxidisement and the exhalation of the peculiar odour. M. Gay Lussac’s new method
by arsenious acid will be described under chlorine. From the experiments which I
made some years ago[9], upon indigo, it will be seen that this dye stuff is so variable in
its quantity of colouring matter, that no two chemists operating with it independently,
as a test for chloride of lime, could arrive at the same result. They must provide
themselves with absolute indigo, by an expensive and troublesome process, not suited to
the busy bleacher. The vitriolage, as the French term it, or the souring of the English
bleacher, consists in immersing the goods for four hours in dilute sulphuric acid, containing
one gallon of oil of vitriol to from 25 to 30 of water, thoroughly intermixed by
stirring; for the density of the acid is an obstacle to its equal distribution through the
water. This dilute acid will have a density of from 1·047 to 1·040, and will contain
from 7 to 61⁄2 per cent. by weight of the oil of vitriol.
The goods are now washed, and then boiled for eight or nine hours in an alkaline
ley, containing about two pounds of crystals of soda, or their equivalent in soda ash or
pearl-ash, for every 100 lbs. of cloth. The ley must be made previously caustic by
quick lime. A washing in the wheel follows this boil; and then a chlorine steep for
five hours in a liquor two thirds of the strength of the former. It is next soured in the
dilute sulphuric acid, for two, three, or four hours, according to the colour and quality
of the cotton, and then thoroughly washed.
The cloth is now bleached white, but cannot be presented in the market till it
undergoes certain finishing processes. The piece is elongated from the folds which it
contracts during the rotation of the dash-wheel, by being thrown into a stream of water
in a cistern, terminated by the squeezing rollers, which take in the end of the piece, and
run it through between them, with the effect of making it nearly dry. Two pieces of
cloth pass simultaneously through the rollers, and are disentangled spontaneously, so to
speak, without the help of hands.
The squeezing rollers or squeezers, for discharging the greater part of the water from
the yarns and goods in the process of bleaching, are represented in figs. 117, 118., the[134]
former being a side-view, to show how the roller gudgeons lie in the slots of the frame,
and how the shaft of the upper roller is pressed downward by a weighted lever, through
a vertical junction road, jointed at the bottom to a nearly horizontal bar, on whose end
the proper weight is hung. In fig. 118. these rollers of birch-wood are shown in face;
the under one receiving motion through the toothed wheel on its shaft, from any suitable
power of water or steam. Upon the shaft of the latter, between the toothed wheel and
the roller, the lever and pulley for putting the machine into and out of geer is visible.
The under roller makes about 25 revolutions in the minute, in which time three pieces
of goods, stitched endwise, measuring 28 yards each, may be run through the machine,
from a water trough on one side, to a wooden grating upon the other.
When the goods are run through, they are carried off upon a grated wheelbarrow, in
a nearly dry state, and transferred to the spreading machine, called at Manchester a
candroy. In many bleach-works, however, the creased pieces are pulled straight by the
hands of women, and are then strongly beat against a wooden stock to smooth out the
edges. This being done, a number of pieces are stitched endwise together, preparatory to
being mangled.
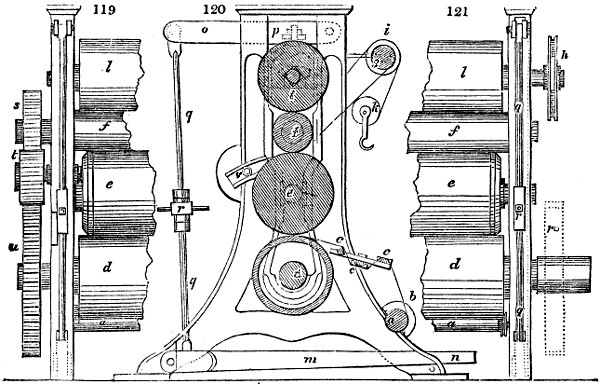
Calender.—Fig. 120. is a cross section of this machine, and figs. 119. 121. are front
views broken off. The goods are first rolled upon the wooden cylinder a, near the
ground; by the tension roller b, upon the same cylinder, the goods receive a proper
degree of stretching in the winding off. They then pass over the spreading bars c c c,
by which they are still more distended; next round the hollow iron cylinder d, 16 inches
diameter, and the paper cylinder e, of like dimensions; thence they proceed under the
second massive iron cylinder f, of 8 inches diameter, to be finally wound about the projecting
wooden roller g. This is set in motion by the pulleys h, fig. 121., and i, fig. 120.,
and receives its proper tension from the hanging roller k; l is a press cylinder, of 14
inches diameter, made of plane-tree wood. By its means we can at all times secure an
equal degree of pressure, which would be hardly possible did the weighted lever press
immediately upon two points of the calender rollers. The compression exercised by
the cylinders may be increased at pleasure by the bent lever m, weights being applied
to it at n. The upper branch of the lever o is made fast by screws and bolts at p, to
the upper press-cylinder. The junction leg q is attached to the intermediate piece r, by
left and right-handed screws, so that according as that piece is turned round to the right
or the left, the pressure of the weighted roller will be either increased or diminished.
By turning it still more, the piece will get detached, the whole pressure will be removed,
and the press-roller may be taken off; which is a main object of this mechanism.
The unequable movement of the cylinders is produced by the wheels s t u, of which
the undermost has 69, the uppermost has 20, and the carrier-wheel t, either 33, 32, or 20
teeth, according to the difference of speed required. The carrier-wheel is bolted on at
v, and adjusted in its proper place by means of a slot. To the undermost iron cylinder,
the first motion is communicated by any power, for which purpose either a rigger
(driving pulley) is applied to its shaft at u, or a crank motion. If it be desired to[135]
operate with a heated calender, the undermost hollow cylinder may be filled with hot
steam, admitted through a stuffing box at one end, and discharged through a stuffing
box at the other, or by a red-hot iron roller.
Pure starch would be too expensive a dressing for common calico shirtings, and therefore
an extemporaneous starch is made by mixing one pound of flour with one gallon of
water, and allowing the mixture to ferment in a warm place for twenty-four hours.
In this way, a portion of lactic acid is formed, which dissolves the gluten, or separates
it from the starch; so that when the whole is thrown upon a sieve, a liquid paste
passes through, which, being boiled, answers well for stiffening the goods, without giving
them a gray tinge. The paste is thinned with water to the desired degree, and faintly
tinged with solution of indigo. The starch, which is sometimes thickened with porcelain
clay, Paris plaster, or Spanish white, is put into a trough, and is evenly imparted to
the cloth as this is drawn down through it, by the traction of rollers. There is a
roller near the bottom of the trough, round which the cloth is made to run, to secure
its full impregnation; while the upper rollers serve to expel its excess of the starch, and
throw it back into the cistern. See Starching Apparatus.
The goods are next dried in an apartment heated by two, three, or more flues, running
along the floor, and covered usually with fire-tiles. At first the heat is moderate, but it
is gradually raised to upwards of 110° F.
The goods must now be passed again through the calender, in order to receive their
final smoothness and lustre. They are in the first place damped with a peculiar machine,
furnished with a circular brush, whose points revolve in contact with water in a
trough placed beneath them, and sprinkle drops of water upon the goods as they are
drawn forwards by a pair of cylinders. They are then subjected to the powerful pressure
of the calender rollers.
The calendered pieces are neatly folded into compact parcels, and stamped with the
marks of each particular manufacturer, or various devices to suit the markets for which
they are designed. They are finally piled on the sole of an hydraulic press, with a sheet
of pasteboard between each piece; but with occasional plates of iron to secure uniformity
of pressure throughout. When sufficiently condensed by the press, they are
taken out, and despatched to their respective manufacturers in a state ready for sale.
There are no less than 25 steps in the bleaching of calicoes, many of them effected
with expensive machinery; yet the whole do not produce to the bleacher more than
10 pence per piece, of 24 yards.
The following system was pursued a few years back, by a skilful bleacher of muslins
near Glasgow:—
“In fermenting muslin goods, we surround them with our spent leys, from the temperature
of 100° to 150° F., according to the weather, and allow them to ferment for
36 hours. In boiling 112 lbs. = 112 pieces of yard-wide muslin, we use 6 or 7 lbs. of
pearl-ashes, and 2 lbs. of soft soap, with 360 gallons of water, and allow them to boil for
6 hours; then wash them, and boil them again with 5 lbs. of pearl-ashes, and 2 lbs. of
soft soap, and allow them to boil 3 hours; then wash them with water, and immerse
them into the solution of oxymuriate of lime, at 5 on the test-tube, and allow them to
remain from 6 to 12 hours; next wash them, and immerse them into dilute sulphuric acid
at the specific gravity of 31⁄2 on Tweedale’s hydrometer = 1·0175, and allow them to remain
an hour. They are now well washed, and boiled with 21⁄2 lbs. of pearl-ashes, and
2 lbs. of soft soap for half an hour; afterwards washed and immersed into the oxymuriate
of lime as before, at the strength of 3 on the test-tube, which is stronger than the
former, and allowed to remain for 6 hours. They are again washed, and immersed in
diluted sulphuric acid at the specific gravity of 3 on Tweedale’s hydrometer = 1·015.
If the goods be strong, they will require another boil, steep, and sour. At any rate,
the sulphuric acid must be well washed out before they receive the finishing operation
with starch.
“With regard to the lime, which some use instead of alkali immediately after fermenting,
the same weight of it is employed as of pearl-ashes. The goods are allowed
to boil in it for 15 minutes, but not longer, otherwise the lime will injure the fabric.”
More recently the plan adopted is as follows; by which the purest whites are produced
for the London market.
“Lime is seldom used for our finer muslin goods, as it is found to injure their fabric,
and the colours do not keep for any length of time.
“An alkaline ley is made by boiling equal weights of lime and soda together for an
hour: this alkali is used for boiling goods the same as potash, but without soap.
“In finishing jacounets or muslins, after washing them from the sour, they are run
through spring-water containing a little fine smalts, which give them a clear shade;
if of a coarse fabric, a little well-boiled starch is added to the water. From this they
are wrung or pressed, and taken up by the selvage for the breadthing frame, and are
run off it upon a tin cylinder heated by steam, by which the piece is completely dried[136]
in 15 minutes: it is then stripped from the cylinder, neatly folded and pressed, which
finishes the piece for the market. From 6d. to 9d. per piece of 12 yards is obtained
for the bleaching and finishing of those goods.
“Book muslins, after being washed from the sour, are wrung or pressed; then they are
hung up to dry in a heated stove, previous to being put into starch, prepared by boiling
3 lbs. of it to every 5 gallons of water, with 20 ounces of smalts: they are wrung out of this
starch, and taken to a room heated to 110° F.; the starch is wrought into the piece till
clear, then taken into a cold room, and the selvages dressed or set, before being put on
the breadthing frame in the heated stove, where the piece is stretched to its length, while
three or four persons at each selvage keep the piece to its breadth. If a stiff finish is
wanted, they keep exactly opposite each other; but in breadthing the piece of elastic, they
cross the piece in breadthing, which gives it a springy elastic finish. From 9d. to 15d.
per piece of 12 yards is obtained for the bleaching and finishing of these goods.
“Sewed trimmings, flounces, and dresses are run through spring water containing fine
smalts with a little well-boiled starch. They are then taken to the drying stove, where
they are stented till dry, which finishes the piece for the market. From 6d. to 8d. per
piece is obtained for trimmings and flounces, and from 9d. to 1s. for dresses, bleaching
and finishing.”
In the bleaching of cotton cloth, where fixed colours are previously dyed in the yarn
before it is woven into cloth, such as the Turkey or Adrianople red, and its compounds of
lilac or purple, by the addition of iron bases, various shades of blue from indigo, together
with buff and gold colour, tinged with the oxides of iron, great care is necessary.
The common process of bleaching pulicates, into which permanent colours are woven,
is, to wash the dressing or starch well out in cold water; to boil them gently in soap,
and, after again washing, to immerse them in a moderately strong solution of the oxymuriate
of potash; and this process is followed until the white is good: they are then
soured in dilute sulphuric acid. If the goods are attended to in a proper manner, the
colours, in place of being impaired, will be found greatly improved, and to have acquired
a delicacy of tint which no other process can impart to them.
Pulicates, or ginghams, which have been woven along with yarn which has been previously
bleached, are first freed by washing from the starch or dressing: they are then
washed, or slightly boiled with soap. After which, they are completely rinsed in pure
spring water, and then soured.
Besides these common processes for bleaching, another was some time ago introduced,
which consisted in immersing the cotton or linen goods in pretty strong solution of caustic
alkali, and afterwards exposing them to the action of steam in a close vessel. It is
now generally abandoned.
The cotton or linen goods having been previously cleaned by steeping and washing, were,
after being well drained, steeped in a solution of caustic alkali of the specific gravity of
1020. After the superfluous alkaline ley had been drained from them, they were arranged
on a grating in a receiver. The cover was then placed on the vessel, and firmly
screwed down; and the steam was admitted by turning the stop-cock of the pipe
which communicated with a steam boiler of the common construction.
The stains which come out upon maddered goods, in consequence of defective bleaching,
are called in this country spangs. Their origin is such as I have described above, as
the following statement of facts will show. The weaver of calicoes receives frequently
a fine warp so tender from bad spinning or bad staple in the cotton, that it will not bear
the ordinary strain of the heddles, or friction of the shuttle and reed, and he is obliged
to throw in as much weft as will compensate for the weakness or thinness of the warp,
and make a good marketable cloth. He of course tries to gain his end at the least expense
of time and labour. Hence when his paste dressing becomes dry and stiff, he has
recourse to such greasy lubricants as he can most cheaply procure; which are commonly
either tallow or butter in a rancid state, but the former being the lowest priced is preferred.
Accordingly, the weaver, having heated a lump of iron, applies it to a piece of
tallow held over the warp in the loom, and causes the melted fat to drop in patches
upon the yarns, which he afterwards spreads more evenly by his brush. It is obvious,
however, that the grease must be very irregularly applied in this way, and be particularly
thick on certain spots. This irregularity seldom fails to appear when the goods are
bleached or dyed by the common routine of work. Printed calicoes examined by a skilful
eye, will be often seen to be stained with large blotches evidently occasioned by this
vile practice of the weaver. The ordinary workmen call these copper stains, believing
them to be communicated in the dyeing copper. Such stains on the cloth are extremely
injurious in dyeing with the indigo vat. The following plan is adopted by some Scotch
bleachers with the effect, it is said, of effectually counteracting spangs from grease.
The goods having been singed and steeped in pure water, as is customary in common
bleaching, they are passed through a pair of rollers to press out the impurities which
have been loosened by the steeping. It must here, however, be observed, that where the[137]
expense of one extra drying can be afforded, the process might be very much improved
by steeping the brown calicoes for thirty or forty hours before singeing, because this
would separate much of that impurity which usually becomes fixed in the stuff on its
being passed over the hot cylinders. When the pieces have been thus singed, steeped,
and pressed, they are boiled four times, ten or twelve hours at each time, in a solution of
caustic potash, of the specific gravity of from 1·0127 to 1·0156, washing them carefully
and thoroughly in pure water between each of these boilings. They are then immersed
in a solution of the chloride of potash, originally of the strength of 1·0625, and afterwards
reduced with twenty-four times its measure with water.
When the preparation is good, these proportions will whiten cotton goods completely
in eight hours. In this steep they are, however, generally suffered to remain twelve hours.
It has been supposed that the common bleaching liquor (chloride of lime) cannot, without
injury, be substituted for chloride of potash, but I believe this to be a mistake.
Some printers take the pieces from this solution, and, while wet, lay them upon the grass,
and there expose them to the sun and weather for two or three days. They are thence
removed to the sours, made of the specific gravity of about 1·0254 at the temperature of
110° of Fahrenheit. In bleaching common goods, and such as are not designed for the
best printing, the specific gravity of the sours is varied from that of 1·0146 to that of
1·0238, if weighed when they become of the temperature of the atmosphere. In these
they are suffered to lie for five or six hours, after which they are taken to the dash-wheel
and washed thoroughly. When this operation is finished, they are submitted to four more
boilings as before, with a solution of caustic potash; taking care to wash well between
each of these boilings. Sometimes pearl-ash, made caustic, is used for the last of these
boilings, lest the sulphur, which always exists in the potashes of commerce, should impair
the whites. They are next immersed in the diluted chloride of potash, of the strength
before mentioned; after which they are well washed in pure water, and then winched
for half an hour in common sours. The last process is that of careful washing in plenty
of clean water, after which they are not put into the stove, but are immediately hung
up in the airing sheds to dry gradually. The water must be good, and abundant.
The number of operations, as here described, is great; but I know of no other mode
of procedure by which perfect bleaching is so likely to be effected at all times and in all
seasons, without disappointment. It must here be remarked, that, for the best purposes
of printing, it would not be sufficient to take goods which have been bleached in the
common way and finish these by the better process; because the sulphate of lime deposited
in the cloth by that operation will be apt to spoil them for madder colours; at least,
a printer who is curious in his business would hesitate to work up such cloth.
Bucking or Bowking.—This is one of the most important operations in the bleaching
of both cotton and linen goods. There are several methods whereby this process is carried
on; but of these we shall select only two, distinguishing them as the old and new method
of bucking. In the former way, the cloths have been steeped in the alkaline lye, as
before described, and afterwards well washed, are regularly arranged in a large wooden
vat, or kieve; a boiler of sufficient capacity is then filled with caustic alkaline lye,
which is heated to the temperature of blood. The boiler is then emptied by a stop-cock
upon the linens in the kieve, until they are covered with the liquor. After having
remained on the cloth for some time, it is run off by a stop-cock, at the bottom of the
kieve, into an iron boiler sunk in the ground, from whence it is raised into the boiler
by a pump. The heat is now elevated to a higher temperature, and the lye again run
upon the goods in the kieve; from whence it is returned into the boiler, as before
described: and these operations are continued, always increasing the heat, until the
alkaline lye is completely saturated with the colouring matter taken from the cloth,
which is known by its having acquired a completely offensive smell, and losing its
causticity.
When we consider the effect which heated liquids have upon coloured vegetable
matter, we shall see the propriety of the temperature of the alkaline lye being gradually
increased. Thus, when vegetable substances are hastily plunged into boiling liquids,
the colouring matter, in place of being extracted, is, by this higher temperature, fixed
into them. It is on this principle that a cook acts in the culinary art, when the
green colour of vegetables is intended to be preserved: in place of putting them into
water when cold, they are kept back until the water is boiling; because it is well
known that, in the former case, the green colour would be entirely extracted, whereas,
when the vegetables are not infused until the water is boiling, the colour is completely
preserved or fixed. On the same principle, when the temperature of the alkaline lye is
gradually raised, the extractive and colouring matter is more effectually taken from the
cloth; and the case is reversed when the lye is applied at the boiling temperature: so
much so, that linen which has been so unfortunate as to meet with this treatment, can
never be brought to a good white.
When the alkaline lye is saturated with colouring matter, it is run off as unfit for[138]
further use in this operation; but, were the goods to be instantly taken out of the
kieve, and carried to be washed in the dash-wheel while hot, a certain portion of the
colouring matter would be again fixed into them, which is extremely difficult to
eradicate. In order to prevent this, the most approved bleachers run warm water upon
the cloth as soon as the impure lye is run off: this combines with and carries off part
of the remaining impurities. A stream of water is then allowed to run upon the cloth
in the kieve, until it comes off almost transparent. The goods are now to be taken
to the wash-stocks, or to the dash-wheel, to be further cleaned, with the greatest
efficacy.
The improved mode of bowking was the invention of Mr. John Laurie, a native
of Glasgow. It is now practised by many bleachers in Lancashire, some on more
perfect plans than others; but we shall give the description of the kind of apparatus
approved of by those whose experience and skill have rendered them the most competent
judges.
In fig. 122., A B C D is the wooden kieve, or kier, containing the cloth; C E F D
represents the cast-iron boiler; G G, the
pump; g K, the pipe of communication
between the kieve and the boiler. This
pipe has a valve on each of its extremities:
that on the upper extremity, when shut,
prevents the lye from running into the
boiler, and is regulated by the attendant
by means of the rod and handle g B.
The valve at K admits the lye; but, opening
inwards, it prevents the steam from
escaping through the pipe g K. The
boiler has a steam-tight iron cover, g L;
and at C D, in the kieve, is a wooden
grating, a small distance above the cover
of the boiler.
At M O is a broad plate of metal, in
order to spread the lye over the cloth.
It is hardly necessary to say that the
boiler has a furnace, as usual, for similar
purposes.
While the lye is at a low temperature,
the pump is worked by the mill or steam-engine.
When it is sufficiently heated, the elasticity of the steam forces it up through
the valves of the pump, in which case it is disjoined from the moving power.
N P is a copper spout, which is removed at the time of taking the cloth out of the
kieve.
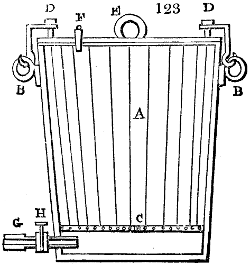
The boilers A, fig. 123., used in bleaching, are of the common form, having a stopcock,
H G, at bottom, for running off the waste lye.
They are commonly made of cast iron, and are capable
of containing from 300 to 600 gallons of water,
according to the extent of the business done. In
order that the capacity of the boilers may be enlarged,
they are formed so as to admit of a crib of wood,
strongly hooped, or, what is preferable, of cast iron,
to be fixed to the upper rim or edge of it. To keep
the goods from the bottom, where the heat acts most
forcibly, a strong iron ring, covered with netting
made of stout rope, C, is allowed to rest six or eight
inches above the bottom of the boiler. Four
double ropes are attached to the ring E, for withdrawing
the goods when sufficiently boiled, which
have each an eye for admitting hooks from the
running tackle of a crane. Where more boilers
than one are employed, the crane is so placed,
that, in the range of its sweep, it may withdraw the goods from any of them. For this
purpose, the crane turns on pivots at top and bottom; and the goods are raised or
lowered at pleasure, with double pulleys and sheaves, by means of a cylinder moved by
cast-iron wheels. The lid is secured by the screw bolts D D, and rings B B. F is a
safety valve.
The efficacy of Laurie’s bowking apparatus is remarkable. While the heat is
gradually rising, a current of fresh lye is constantly presented to the different surfaces
for saturating the goods, so as to increase its detersive powers. Besides, the manner in[139]
which the apparatus is worked, first by the water-wheel or steam-engine, and then by
its intrinsic operation, puts it completely out of the power of servants to slight the work;
not to speak of the great saving of alkali, which, in many cases, has been found to
amount to 25 per cent.
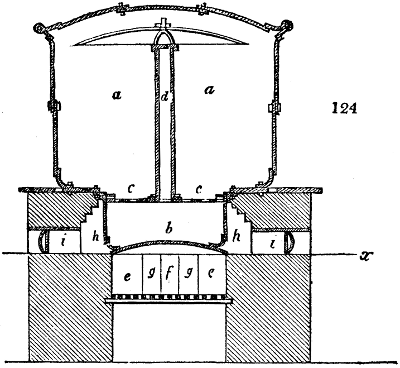
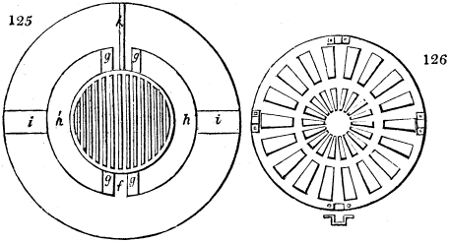
A simple modification of the bowking apparatus is shown in figs. 124, 125, 126.; the
first being a vertical section, the
second, a horizontal section in the
line x of the first. It consists
of two parts: the upper wide part,
a a, serves for the reception of the
goods, and the lower or pot, b, for
holding the lye; c c is an iron
grating, shown apart in fig. 126.
The grating has numerous square
apertures in the middle of the
disc, to which the rising pipe d is
screwed fast. The upper cylinder
is formed of cast iron, or of sheet
iron well rivetted at the edges; or
sometimes of wood, this being
secured at its under edge into a
groove in the top edge of the lye-pot.
The mouth of the cylinder
is constructed usually of sheet
iron. e e is the fire-grate, whose
upper surface is shown in fig. 125.:
it is made of cast iron, in three pieces. The flame is parted at f, and passes through
the two apertures g g, into the flues h h, so as to play round the pot, as is visible
in fig. 125.; and escapes by two outlets into the
chimney. The apertures i i serve for occasionally
cleaning out the flues h h, and are, at other times,
shut with an iron plate. In the partition f, which
separates the two openings g g, and the flues h h,
running round the pot, there is a circular space
at the point marked with k, fig. 125., in which the
large pipe for discharging the waste lye is lodged.
The upper large cylinder should be encased in
wood, with an intermediate space filled with sawdust,
to confine the heat. The action of this
apparatus is exactly the same as of that already
explained.
Besides the boiling, bucking, and other apparatus
above described, the machinery and utensils
used in bleaching are various, according to the
business done by the bleacher. When linen or
heavy cotton cloths are whitened, and the business
is carried on to a considerable extent, the
machines are both complicated and expensive.
They consist chiefly of a water-wheel, sufficiently
powerful for giving motion to the wash-stocks,
dash-wheels, squeezers, &c., with any other operations
where power is required.
Figs. 127, 128. represent a pair of wash-stocks.
A A are called the stocks, or feet. They are suspended
on iron pivots at B, and receive their motion
from wipers on the revolving-shaft C. The
cloth is laid in at D, and, by the alternate strokes of the feet, and the curved form of
the turnhead E, the cloth is washed and gradually turned. At the same time, an
abundant stream of water rushes on the cloth throughout holes in the upper part of
the turnhead. Wash-stocks are much used in Scotland and in Ireland. In the latter
country, they are often made with double feet, suspended above and below two turnheads,
and wrought with cranks instead of wipers. Wash-stocks, properly constructed,
make from 24 to 30 strokes per minute.
This mode of washing is now entirely given up in Lancashire, where a preference is
given to what are called dash-wheels and squeezers. The dash are small water-wheels,
the inside of which is divided into four compartments, and closed up, leaving only a
hole in each compartment for putting in the cloth.
[140]
There are, besides, smaller openings for the free admission and egress of the water
employed in cleansing. The cloth, by the motion of the wheel, is raised up in one part
of the revolution of the wheel; while, by its own weight, it falls in another. This kind
of motion is very effectual in washing the cloth, while, at the same time, it does not
injure its strength. The plan, however, where economy of water is of any importance,
is very objectionable; because the wheel must move at by far too great a velocity
to act to advantage as a water-wheel.
The wash or dash-wheel, now driven by power in all good bleach and print-works,
is represented in
fig. 129., upon the left side
in a back view, and upon
the right side in a front
view (the sketch being
halved). Fig. 130. is a
ground plan.
a a is the washing-wheel;
b b its shaft-ends; c c their
brass bearings or plummer-blocks,
supported upon the
iron pillars d d. The frame
is made of strong beams of
wood, e e, bound together
by cross bars with mortises.
f f, two of the circular
apertures, each leading to
a quadrantal compartment
within the dash-wheel. In
the back view (the left-hand half of the figure) the brass grating g g, of a curvilinear
form, is seen, through which the jets of water are admitted into the cavity of the
wheel; h h, are the round
orifices, through which the
foul water runs off, as each
quadrant passes the lower
part of its revolution; i, a
water-pipe, with a stop-cock
for regulating the washing-jets;
k k, the lever for throwing
the driving-crab l, or
coupling-box, into or out
of geer with the shaft of the
wheel. This machine is so
constructed, that the water-cock
is opened or shut by
the same leverage which
throws the wheel into or
out of geer. m, a wheel,
fixed upon the round extremity
of the shaft of the dash-wheel, which works into the toothed pinion connected[141]
with the prime mover. When the end of the lever k, whose fork embraces the
coupling-box upon the square part of the shaft, is pushed forwards or backwards, it
shifts the clutch into or out of geer with the toothed wheel m. In the latter case, this
wheel turns with its pinion without affecting the dash-wheel. n n, holdfasts fixed upon
the wooden frame, to which the boards o o are attached, for preventing the water from
being thrown about by the centrifugal force.
The dash-wheel is generally from 6 to 7 feet in diameter, about 30 inches wide, and
requires the power of about two horses to drive it.
From one to two pieces of calico may be done at once in each quadrantal compartment,
in the course of 8 or 10 minutes; hence, in a day of 13 hours, with two such
wheels 1200 pieces of yard-wide goods may be washed.
After the process of washing by the dash-wheel, the water is expressed from the cloth
by means of the squeezers already described.
Bleaching of Linen.—Linen contains much more colouring matter than cotton. The
former loses nearly a third of its weight, while the latter loses not more than a twentieth.
The fibres of flax possess, in the natural condition, a light gray, yellow, or blond colour.
By the operation of rotting, or, as it is commonly called, water-retting, which is employed
to enable the textile filaments to be separated from the boon, or woody matter, the colour
becomes darker, and, in consequence probably of the putrefaction of the green matter of
the bark, the colouring substance appears. Hence, flax prepared without rotting is much
paler, and its colouring matter may be in a great measure removed by washing with
soap, leaving the filaments nearly white. Mr. James Lee obtained a patent in 1812, as
having discovered that the process of steeping and dew-retting is unnecessary, and that
flax and hemp will not only dress, but will produce an equal if not greater quantity of
more durable fibre, when cleaned in the dry way. Mr. Lee stated that, when hemp or
flax plants are ripe, the farmer has nothing more to do than to pull, spread, and dry
them in the sun, and then to break them by proper machinery. This promising
improvement has apparently come to nought, having been many years abandoned by the
patentee himself, though he was favoured with a special act of parliament, which permitted
the specification of his patent to remain sealed up for seven years, contrary to
the general practice in such cases.
The substance which gives steeped flax its peculiar tint is insoluble in boiling water,
in acids, and in alkalies; but it possesses the property of dissolving in caustic or carbonated
alkaline lyes, when it has possessed the means of dehydrogenation by previous exposure to
oxygen. Hemp is, in this respect, analogous to flax. The bleaching of both depends
upon this action of oxygen, and upon the removal of the acidified dye, by means of an
alkali. This process is effected generally by the influence of air in combination with
light and moisture acting on the linen cloth laid upon the grass: but chlorine will effect
the same object more expeditiously. In no case, however, is it possible to acidify the colour
completely at once, but there must be many alternate exposures to oxygen or chlorine,
and alkali, before the flax becomes white. It is this circumstance alone which renders
the bleaching of linen an apparently complicated business.
Having made these preliminary observations with regard to the method of applying
the alkaline lyes used in bleaching linen cloth, I shall now bring the whole into one
point of view, by detailing the connection of these processes, as carried on at a bleach-field,
which has uniformly been successful in returning the cloth of a good white, and
has otherwise given satisfaction to its employers; and I shall only remark, that I by
no means hold it up as the best process which may be employed, as every experienced
bleacher knows that processes must be varied, not only according to existing circumstances,
but also according to the nature of the linens operated upon.
In order to avoid repetition, where washing is mentioned, it must always be understood
that the linen is taken to the wash-stocks or dash-wheel, and washed well in them
for some hours. This part of the work can never be overdone; and on its being
properly executed between every part of the bucking, boiling, steeping in the chloride
of lime solution, and souring, not a little of the success of bleaching depends. By
exposure is meant, that the linen cloth is taken and spread upon the bleach-green for
four, six, or eight days, according as the routine of business calls for the return of the
cloth, in order to undergo further operations.
A parcel of goods consists of 360 pieces of those linens which are called Britannias.
Each piece is 35 yards long; and they weigh, on an average, 10 lbs. each; the weight of
the parcel is, in consequence, about 3600 lbs. avoirdupois weight. The linens are first
washed, and then steeped in waste alkaline lye, as formerly described under these
processes; they then undergo the following operations:—
| 1st, |
Bucked with |
60 |
lbs. |
pearl-ashes, |
washed, |
exposed on the |
field. |
| 2d, |
Ditto |
80 |
|
ditto |
ditto |
ditto |
ditto. |
| 3d, |
Ditto |
90 |
|
potashes |
ditto |
ditto |
ditto. |
| 4th, |
Ditto |
80 |
|
ditto |
ditto |
ditto |
ditto. |
| 5th,[142] |
Ditto |
80 |
|
dito |
ditto |
ditto |
ditto. |
| 6th, |
Ditto |
50 |
|
ditto |
ditto |
ditto |
ditto. |
| 7th, |
Ditto |
70 |
|
ditto |
ditto |
ditto |
ditto. |
| 8th, |
Ditto |
70 |
|
ditto |
ditto |
ditto |
ditto. |
| 9th, |
Soured one night in dilute sulphuric acid, washed. |
| 10th, |
Bucked with |
50 |
lbs. |
pearl-ashes, |
washed, |
exposed on the |
field. |
| 11th, |
Immersed in the chloride of potash or lime 12 hours. |
| 12th, |
Boiled with |
30 |
lbs. |
pearl ashes, |
washed, |
exposed on the |
field. |
| 13th, |
Ditto |
30 |
|
ditto |
ditto |
ditto |
ditto. |
| 14th, |
Soured, washed. |
The linens are then taken to the rubbing-board, and well rubbed with a strong lather
of black soap, after which they are well washed in pure spring water. At this period
they are carefully examined, and those which are fully bleached are laid aside to be
blued, and made up for the market; while those which are not fully white are returned
to be boiled, and steeped in the chloride of lime or potash; then soured, until they are fully
white.
By the above process, 690 lbs. weight of alkali is taken to bleach 360 pieces of linen,
each piece consisting of 35 yards in length; so that the expenditure of alkali would be
somewhat less than 2 lbs. for each piece, were it not that some parts of the linens are
not fully whitened, as above noted. Two pounds of alkali may therefore be stated as
the average quantity employed for bleaching each piece of goods.
The method of bleaching linens in Ireland is similar to the foregoing; any alteration
in the process depending upon the judgment of the bleacher in increasing or diminishing
the quantity of alkali used. But it is common, at most bleach-fields, to steep the linens
in the chloride of lime or potash at an early stage of the process, or after the goods have
undergone the fifth or sixth operation of bucking. By this means those parts of the
flax which are most difficult to bleach are more easily acted upon by the alkali; and,
as before noticed, souring early in very dilute sulphuric acid, assists greatly in forwarding
the whitening of the linens. Mr. Grimshaw, calico-printer, near Belfast, was the first
who recommended early souring, which has since been very generally adopted.
The bleaching of Silk.—Silk in its raw state, as spun by the worm, is either white or
yellow of various shades, and is covered with a varnish, which gives it stiffness and a
degree of elasticity. For the greater number of purposes to which silk is applied, it
must be deprived of this native covering, which was long considered to be a sort of gum.
The operation by which this colouring matter is removed is called scouring, cleansing,
or boiling. A great many different processes have been proposed for freeing the silk
fibres from all foreign impurities, and for giving it the utmost whiteness, lustre, and
pliancy; but none of the new plans has superseded, with any advantage, the one practised
of old, which consists essentially in steeping the silk in a warm solution of soap; a
circumstance placed beyond all doubt by the interesting experiments of M. Roard.
The alkalies, or alkaline salts, act in a marked manner upon the varnish of silk, and effect
its complete solution; the prolonged agency of boiling water, alone answers the same
purpose; but nothing agrees so well with the nature of silk, and preserves its brilliancy
and suppleness so perfectly, as a rapid boil with soap-water. It would appear, however,
that the Chinese do not employ this method, but something that is preferable. Probably
the superior beauty of their white silk may be owing to the superiority of the raw material.
The most ancient method of scouring silk consists of three operations. For the first,
or the ungumming, thirty per cent. of soap is first of all dissolved in clean river water by
a boiling heat; then the temperature is lowered by the addition of a little cold water,
by withdrawing the fire, or at least by damping it. The hanks of silk suspended
upon horizontal poles over the boiler, are now plunged into the soapy solution, kept at
a heat somewhat under ebullition, which is an essential point; for if hotter, the soap
would attack the substance of the silk, and not only dissolve a portion of it, but deprive
the whole of its lustre. The portions of the hanks plunged in the bath get scoured by
degrees; the varnish and the colouring matter come away, and the silk assumes its proper
whiteness and pliancy. Whenever this point is attained, the hanks are turned round
upon the poles, so that the portion formerly in the air may be also subjected to the bath. As
soon as the whole is completely ungummed, they are taken out, wrung by the peg, and
shaken out; after which, the next step, called the boil, is commenced. Into bags of coarse
canvass, called pockets, about 25 lbs. or 35 lbs. of ungummed silk are enclosed, and put
into a similar bath with the preceding, but with a smaller proportion of soap, which may
therefore be raised to the boiling point without any danger of destroying the silk. The
ebullition is to be kept up for an hour and a half, during which time the bags must be
frequently stirred, lest those near the bottom should suffer an undue degree of heat.
The silk experiences in these two operations a loss of about 25 per cent. of its weight.
The third and last scouring operation is intended to give the silk a slight tinge, which[143]
renders the white more agreeable, and better adapted to its various uses in trade. In
this way we distinguish the China white, which has a faint cast of red, the silver white, the
azure white, and the thread white. To produce these different shades, we begin by
preparing a soap-water so strong as to lather by agitation; we then add to it, for the
China white, a little annotto, mixing it carefully in; and then passing the silk properly
through it, till it has acquired the wished for tint. As to the other shades, we need only
azure them more or less with a fine indigo, which has been previously washed several
times in hot water, and reduced to powder in a mortar. It is then diffused through
boiling water, allowed to settle for a few minutes, and the supernatant liquid, which
contains only the finer particles, is added to the soap bath, in such proportion as may be
requisite. The silk, on being taken out of this bath, must be wrung well, and stretched
upon perches to dry; after which it is introduced into the sulphuring chamber, if it is
to be made use of in the white state. At Lyons, however, no soap is employed at the
third operation: after the boil, the silk is washed, sulphured, and azured, by passing
through very clear river water properly blued.
The silks intended for the manufacture of blonds and gauzes are not subjected to the
ordinary scouring process, because it is essential, in these cases, for them to preserve their
natural stiffness. We must therefore select the raw silk of China, or the whitest raw
silks of other countries; steep them, rince them in a bath of pure water, or in one containing
a little soap; wring them, expose them to the vapour of sulphur, and then pass
them through the azure water. Sometimes this process is repeated.
Before the memoir of M. Roard appeared, extremely vague ideas were entertained
about the composition of the native varnish of silk. He has shown that this substance,
so far from being of a gummy nature, as had been believed, may be rather compared to
bees’ wax, with a species of oil, and a colouring matter, which exists only in raw silks.
It is contained in them to the amount of from 23 to 24 per cent., and forms the portion
of weight which is lost in the ungumming. It possesses, however, some of the properties
of vegetable gums, though it differs essentially as to others. In a dry mass, it is friable
and has a vitreous fracture; it is soluble in water, and affords a solution which lathers
like soap; but when thrown upon burning coals, it does not soften like gum, but burns
with the exhalation of a fetid odour. Its solution, when left exposed to the open air, at
first of a golden yellow, becomes soon greenish, and ere long putrefies, as a solution of
animal matter would do in similar circumstances. M. Roard assures us that the city of
Lyons alone could furnish several thousand quintals of this substance per annum, were
it applicable to any useful purpose.
The yellow varnish is of a resinous nature, altogether insoluble in water, very soluble
in alcohol, and contains a little volatile oil, which gives it a rank smell. The colour of
this resin is easily dissipated, either by exposure to the sun or by the action of chlorine:
it forms about one fifty-fifth of its weight.
Bees’ wax exists also in all the sorts of silk, even in that of China; but the whiter the
filaments, the less wax do they contain.
M. Roard has observed that, if the silk be exposed to the soap baths for some time
after it has been stripped of its foreign matters, it begins to lose body, and has its
valuable qualities impaired. It becomes dull, stiff, and coloured in consequence of the
solution more or less considerable of its substance; a solution which takes place in all
liquids, and even in boiling water. It is for this reason that silks cannot be alumed
with heat; and that they lose some of their lustre in being dyed brown, a colour which
requires a boiling hot bath. The best mode, therefore, of avoiding these inconveniences,
is to boil the silks in the soap-bath no longer than is absolutely necessary for the scouring
process, and to expose them in the various dyeing operations to as moderate temperature
as may be requisite to communicate the colour. When silks are to be dyed, much less
soap should be used in the cleansing, and very little for the dark colours. According
to M. Roard, raw silks, white or yellow, may be completely scoured, in one hour, with
15 lbs. of water for one of silk, and a suitable proportion of soap. The soap and the
silk should be put into the bath half an hour before its ebullition, and the latter should
be turned about frequently. The dull silks, in which the varnish has already undergone
some alteration, never acquire a fine white until they are exposed to sulphureous
acid gas. Exposure to light has also a very good effect in whitening silks, and is had
recourse to, it is said, with advantage by the Chinese.
Carbonate of soda has been proposed to be used instead of soap in scouring silk,
but it has never come into use. The Abbé Collomb, in 1785, scoured silk by eight
hours’ boiling in simple water, and he found the silks bleached in this way to be
stronger than by soap, but they are not nearly so white. A patent has been taken out
in England for bleaching them by steam, of which an account will be found under the
article Silk.
It appears that the Chinese do not use soap in producing those fine white silks which
are imported into Europe. Michel de Grubbens who resided long at Canton, saw and[144]
practised himself the operation there, which he published in the Memoirs of the
Academy of Stockholm in 1803. It consists in preparing the silk with a species of
white beans, smaller than the Turkey beans, with some wheat flour, common salt, and
water. The proportions are 5 parts of beans, 5 of salt, 6 of flour, and 25 of water, to
form this vegetable bath. The beans must be previously washed. It is difficult to discover
what chemical action can occur between that decoction and the varnish of raw
silk; possibly some acid may be developed, which may soften the gummy matter, and
facilitate its separation.
Baumé contrived a process which does not appear to have received the sanction of
experience, but which may put us in the right way. He macerates the yellow raw silk
in a mixture of alcohol at 36° (sp. gr. 0·837) and one thirty-second part of pure muriatic
acid. At the end of forty-eight hours, it is as white as possible, and the more so,
the better the quality of the silk. The loss which it suffers in this menstruum is only
one fortieth; showing that nothing but the colouring matter is abstracted. The expense
of this menstruum is the great obstacle to Baumé’s process. The alcohol, however,
might be in a very great measure recovered, by saturating the acid with chalk,
and redistillation.
Bleaching of Wool.—Wool, like the preceding fibrous matter, is covered with a peculiar
varnish, which impairs its qualities, and prevents it from being employed in the
raw state for the purposes to which it is well adapted when it is scoured. The English
give the name yolk, and the French suint, to that native coat: it is a fatty unctuous matter,
of a strong smell, which apparently has its chief origin in the cutaneous perspiration
of the sheep; but which, by the agency of external bodies, may have undergone some
changes which modify its constitution. It results from the experiments of M. Vauquelin,
that the yolk is composed of several substances; namely, 1. a soap with basis of
potash, which constitutes the greater part of it; 2. of a notable quantity of acetate of
potash; 3. of a small quantity of carbonate, and a trace of muriate, of potash; 4. of a
little lime in an unknown state of combination; 5. of a species of sebaceous matter,
and an animal substance to which the odour is due. There are several other accidental
matters present on sheep’s wool.
The proportion of yolk is variable in different kinds of wool, but in general it is
more abundant the finer the staple; the loss by scouring being 45 per cent. for the
finest wools, and 35 per cent. for the coarse.
The yolk, on account of its soapy nature, dissolves readily in water, with the exception
of a little free fatty matter, which easily separates from the filaments, and
remains floating in the liquor. It would thence appear sufficient to expose the wools to
simple washing in a stream of water; yet experience shows that this method never
answers so well as that usually adopted, which consists in steeping the wool for some
time in simple warm water, or in warm water mixed with a fourth of stale urine. From
15 to 20 minutes of contact are sufficient in this case, if we heat the bath as warm as
the hand can bear it, and stir it well with a rod. At the end of this time the wool may
be taken out, set to drain, then placed in large baskets, in order to be completely rinsed
in a stream of water.
It is generally supposed that putrid urine acts on the wool by the ammonia which it
contains, and that this serves to saponify the remainder of the fatty matter not combined
with the potash. M. Vauquelin is not of this opinion, because he found that
wool steeped in water, with sal ammoniac and quick lime, is not better scoured than an
equal quantity of wool treated with mere water. He was hence led to conclude that
the good effects of putrefied urine might be ascribed to any thing else besides the ammonia,
and probably to the urea. Fresh urine contains a free acid, which, by decomposing
the potash soap of the yolk, counteracts the scouring operation.
If wools are better scoured in a small quantity of water than in a great stream, we
can conceive that this circumstance must depend upon the nature of the yolk which, in
a concentrated solution, acts like a saponaceous compound, and thus contributes to remove
the free fatty particles which adhere to the filaments. It should also be observed
that too long a continuance of the wool in the yolk water, hurts its quality very much,
by weakening its cohesion, causing the filaments to swell, and even to split. It is said
then to have lost its nerve. Another circumstance in the scouring of wool, that should
always be attended to, is never to work the filaments together to such a degree as to
occasion their felting; but in agitating we must merely push them slowly round in the
vessel, or press them gently under the feet. Were it at all felted, it would neither card
nor spin well.
As the heat of boiling water is apt to decompose woollen fibres, we should be careful
never to raise the temperature of the scouring bath to near this point, nor, in fact, to
exceed 140° F. Some authors recommend the use of alkaline or soapy baths for
scouring wool, but practical people do not deviate from the method above described.
When the washing is completed, all the wool which is to be sent white into the market,[145]
must be exposed to the action of sulphurous acid, either in a liquid or a gaseous
state. In the latter case, sulphur is burned in a close chamber, in which the wools are
hung up or spread out; in the former, the wools are plunged into water, moderately
impregnated with the acid. (See Sulphuring.) Exposure on the grass may also contribute
to the bleaching of wool. Some fraudulent dealers are accused of dipping wools
in butter-milk, or chalk and water, in order to whiten them and increase their weight.
Wool is sometimes whitened in the fleece, and sometimes in the state of yarn; the
latter affording the best means of operating. It has been observed that the wool cut
from certain parts of the sheep, especially from the groins, never bleaches well.
After sulphuring, the wool has a harsh crispy feel, which may be removed by a weak
soap bath. To this also the wool comber has recourse when he wishes to cleanse and
whiten his wools to the utmost. He generally uses a soft or potash soap, and after the
wool is well soaked in the warm soap bath, with gentle pressure he wrings it well with
the help of a hook, fixed at the end of his washing tub, and hangs it up to dry.
Bleaching of rags, and paste for paper making.—After the rags are reduced to what is
called half stuff, they should have the greater part of the floating water run off, leaving
just enough to form a stir-about mass. Into this a clear solution of chloride of lime
should be poured, of such a strength as is suited to the colour of the rags, which should
have been previously sorted; and the engine is kept going so as to churn the rags
with the bleaching agent. After an hour, the water may be returned upon the engine,
and the washing of the paper resumed. From two to four pounds of good chloride of
lime are reckoned sufficient to bleach one hundred weight of rags.
When the rags consist of dyed or printed cottons, after being well washed and reduced
to half stuff, they should be put into a large cask or butt, supported horizontally
by iron axles upon cradle bearings, so that it may be made to revolve like a barrel-churn.
For each hundred weight of the coloured rags, take a solution containing from
four to eight pounds of chloride of lime; add it to the liquid mixture in the butt along
with half a pound of sulphuric acid for every pound of the chloride; and after inserting
the bung, or rather the square valve, set the vessel in slow revolution backwards and forwards.
In a short time the rags will be colourless. The rags and paper paste ought
to be very well washed, to expel all the chlorine, and perhaps a little muriatic acid
might be used with advantage to dissolve out all the calcareous matter, a portion of
which is apt to remain in the paper, and to operate injuriously upon both the pens and
the ink. Some of the French paper manufacturers bleach the paste with chlorine gas.
Paper prepared from such paste, well washed, is not apt to give a brown tint to maps, as
that carelessly bleached with chloride of lime is known to do.
BLENDE. (Fr. and Germ.) Sulphuret of zinc, so named from the German blenden
to dazzle, on account of its glistening aspect. It is called black jack from its usual
colour. Its lustre is pearly adamantine. Spec. gravity from 3·7 to 4·2. It contains
frequently iron, copper, arsenic, cadmium and silver, all associated with sulphur. It is
worked up partly into metallic zinc, and partly into the sulphate of zinc, or white vitriol.
It consists of 66·72 zinc, and 33·28 sulphur; being nearly by weight as two to one.
See Zinc.
BLOCK MANUFACTURE. Though the making of ships’ blocks belongs rather
to a dictionary of engineering than of manufactures, it may be expected that I should
give some account of the automatic machinery for making blocks, so admirably devised
and mounted by M. I. Brunel, Esq. for the British navy, in the dock-yard of Portsmouth.
The series of machines and operations are as follows:—
1. The straight cross cutting saw.—The log is placed horizontally on a very low bench
which is continued through the window of the mill into the yard. The saw is exactly
over the place where the log is to be divided. It is let down, and suffered to rest with
its teeth upon the log, the back still being in the cleft of the guide. The crank being
set in motion, the saw reciprocates backwards and forwards with exactly the same motion
as if worked by a carpenter, and quickly cuts through the tree. When it first begins to
cut, its back is in the cleft in the guide, and this causes it to move in a straight line;
but before it gets out of the guide, it is so deep in the wood as to guide itself: for in
cutting across the grain of the wood, it has no tendency to be diverted from its true line
by the irregular grain. When the saw has descended through the tree, its handle is
caught in a fixed stop, to prevent its cutting the bench. The machine is thrown out of
geer, the attendant lifts up the saw by a rope, removes the block cut off, and advances
the tree to receive a fresh cut.
2. The circular cross-cutting saw.—This saw possesses universal motion; but the axis
is always parallel to itself, and the saw in the same plane. It can be readily raised or
lowered, by inclining the upper frame on its axis; and to move it sidewise, the saw frame
must swing sidewise on its joints, which connect it with the upper frame. These movements
are effected by two winches, each furnished with a pair of equal pinions, working
a pair of racks fixed upon two long poles. The spindles of these winches are fixed in two[146]
vertical posts, which support the axis of the upper frame. One of these pairs of poles is
jointed to the extreme end of the upper frame; therefore by turning the handle belonging
to them, the frame and saw is elevated or depressed; in like manner, the other pair
is attached to the lower part of the saw frame, so that the saw can be moved sidewise by
means of their handles, which then swing the saw from its vertical position.
These two handles give the attendant a complete command of the saw, which we
suppose to be in rapid motion, the tree being brought forward and properly fixed. By
one handle, he draws the saw against one side of the tree, which is thus cut into, (perhaps
half through); now, by the other handle, he raises the saw up, and by the first-mentioned
handle he draws it across the top of the tree, and cuts it half through from the upper
side; he then depresses the saw and cuts half through from the next side; and lastly a
trifling cut of the saw, at the lower side, completely divides the tree, which is then advanced
to take another cut.
The great reciprocating saw is on the same principle as the saw mill in common use in
America.
3. The circular ripping saw is a thin circular plate of steel, with teeth similar to those
of a pit saw, formed in its periphery. It is fixed to a spindle placed horizontally, at a
small distance beneath the surface of a bench or table, so that the saw projects through
a crevice a few inches above the bench. The spindle being supported in proper collars,
has a rapid rotatory motion communicated to it by a pulley on the opposite end, round
which an endless strap is passed from a drum placed overhead in the mill. The block
cut by the preceding machine, from the end of the tree, is placed with one of the sides
flat upon the bench, and thus slides forward against the revolving saw which cuts the
wood with a rapidity incredible to any one who has not seen these or similar machines.
4. Boring machine.—The blocks, prepared by the foregoing saws, are placed in the
machine represented in fig. 131. This machine has an iron frame, A A, with three legs,
beneath which the block is introduced, and the screw near B being forced down upon it,
confines it precisely in the proper spot to receive the borers D and E. This spot is determined
by a piece of metal fixed perpendicularly just beneath the point of the borer E,
shown separately on the ground at X; this piece of metal adjusts the position for the
borer D, and its height is regulated by resting on the head of the screw x, which fastens
the piece X down to the frame. The sides of the block are kept in a parallel position, by
being applied against the heads of three screws tapped into the double leg of the frame[147]
A. The borer D is adapted to bore the hole for the centre pin in a direction exactly perpendicular
to the surface resting against the three screws; the other, at E, perforates the
holes for the commencement of the sheave holes. Both borers are constructed in nearly
the same manner; they are screwed upon the ends of small mandrels, mounted in frames
similar to a lathe. These frames, G and H, are fitted with sliders upon the angular edges
of the flat broad bars, I and K. The former of these is screwed fast to the frame; the
latter is fixed upon a frame of its own, moving on the centre screws, at L L, beneath the
principal frame of the machine. By this means the borer E can be moved within certain
limits, so as to bore holes in different positions. These limits are determined by two
screws, one of which is seen at a; the other being on the opposite side is invisible. They
are tapped through fixed pieces projecting up from the frame. A projecting piece of
metal, from the under side of the slider K of the borer E, stops against the ends of these
screws, to limit the excursion of the borer. The frames for both borers are brought up
towards the block by means of levers M and N. These are centered on a pin, at the
opposite sides of the frame of the machine, and have oblong grooves through them which
receive screw pins, fixed into the frames G and H, beneath the pulleys P P, which give
motion to the spindles.
5. The mortising machine is a beautiful piece of mechanism, but too complicated for
description within the limits prescribed to this article.
6. The corner saw, fig. 132., consists of a mandrel, mounted in a frame A, and,
carrying a circular saw L upon the extreme end of it. This mandrel and its frame
being exactly similar to those at G and H fig. 131., does not require a separate view,
although it is hid behind the saw, except the end of the screw, marked A. This frame
is screwed down upon the frame B B of the machine, which is supported upon four
columns. C C, D D, is an inclined bench, or a kind of trough, in which a block is laid,
as at E, being supported on its edge by the plane C C of this bench, and its end kept up
to its position by the other part of the bench D D.
By sliding the block along this bench, it is applied to the saw, which cuts off its
angles, as is evident from the figure, and prepares it for the shaping engine. All the
four angles are cut off in succession, by applying its different sides to the trough or
bench. In the figure, two of them are drawn as being cut, and the third is just marked
by the saw. This machine is readily adapted to different sizes of blocks, by the simple
expedient of laying pieces of wood of different thickness against the plane D D, so as to
fill it up, and keep the block nearer to or farther from the saw; for all the blocks are
required to be cut at the same angle, though, of course, a larger piece is to be cut
from large than from small blocks. The
block reduced to the state of E is now
taken to
7. The shaping machine.—A great
deal of the apparent complication of this
figure arises from the iron cage, which
is provided to defend the workmen, lest
the blocks, which are revolving in the
circles, or chuck, with an immense
velocity, should be loosened by the action
of the tool, and fly out by their
centrifugal force. Without this provision,
the consequences of such an accident
would be dreadful, as the blocks
would be projected in all directions, with
an inconceivable force.
8. The scoring engine receives two
blocks, as they come from the shaping
engine, and forms the groove round
their longest diameters for the reception
of their ropes or straps, as represented
in the two snatch blocks and double
block, under figs. 131, 132.
A, B, fig. 133., represent the above
two blocks, each held between two small
pillars a, (the other pillar is hid behind
the block) fixed in a strong plate D, and
pressed against the pillars by a screw b,
which acts on a clamp d. Over the
blocks a pair of circular planes or cutters
E E, are situated, both being fixed on the same spindle, which is turned by a pulley
in the middle of it. The spindle is fitted in a frame F F, moving in centres at e e, so[148]
as to rise and fall when moved by a handle f. This brings the cutters down upon
the blocks; and the depth to which they can cut is regulated by a curved shape g,
fixed by screws upon the plate D, between the blocks. Upon this rests a curved
piece of metal h, fixed to the frame F, and inclosing, but not touching, the pulley. To
admit the cutters to traverse the whole length of the blocks, the plate D, (or rather a
frame beneath it,) is sustained between the points of two centres. Screws are seen at
l, on these centres. The frame inclines when the handle L is depressed. At M is a
lever, with a weight at the end of it, counterbalancing the weight of the blocks, and
plate D, all which are above the centre on which they move. The frame F is also
provided with a counterpoise to balance the cutters, &c. The cutters E E are circular
wheels of brass, with round edges. Each has two notches in its circumference, at
opposite sides; and in these notches chisels are fixed by screws, to project beyond the
rim of the wheel, in the manner of a plane iron before its face.
This machine is used as follows:—In order to fix the block, it is pressed between
the two pins (only one of which at a, can be seen in this view), and the clamp d, screwed
up against it, so as just to hold the block, but no more. The clamp has two claws,
as is seen in the figure, each furnished with a ring entering the double prints previously
made, in the end of the block. These rings are partly cut away, leaving only
such a segment of each as will just retain the block, and the metal between them is
taken out to admit the cutter to operate between them, or nearly so. In putting the
blocks into this machine, the workman applies the double prints to the ends of the
claws of the clamps, but takes care that the blocks are higher between the pins a than
they should be; he then takes the handle f, and by it presses the cutters E E, (which
we suppose are standing still,) down upon the blocks, depressing them between their
pins at the same time, till the descent of the cutters is stopped by the piece h resting
on the shape g. He now turns the screws b b, to fix the blocks tight. The cutters
being put in motion cut the scores, which will be plainly seen by the mode of adjustment
just described, to be of no depth at the pin-hole; but by depressing the handle L,
so as to incline the blocks, and keeping the cutters down upon their shape g, by the
handle f, they will cut any depth towards the ends of the blocks, which the shape g
admits.
By this means one quarter of the score is formed; the other is done by turning both
blocks together half round in this manner. The centres l are not fitted into the plate D
itself, but into a frame seen at R beneath the plate, which is connected with it by a
centre pin, exactly midway between the two blocks A B. A spring catch, the end of
which is seen at r, confines them together; when this catch is pressed back, the plate D
can be turned about upon its centre pin, so as to change the blocks, end for end, and
bring the unscored quarters (i. e. over the clamps) beneath the cutters; the workman
taking the handles f and L, one in each hand, and pressing them down, cuts out the
second quarter. This might have been effected by simply lifting up the handle L; but
in that case the cutter would have struck against the grain of the wood, so as to cut
rather roughly; but by this ingenious device of reversing the blocks, it always cuts
clean and smooth, in the direction of the grain. The third and fourth quarters of the
score are cut by turning the other sides of the blocks upwards, and repeating the above
operation. The shape g can be removed, and another put in its place, for different
sizes and curves of block; but the same pins a, and holding clamps d, will suit many
different sizes.
By these machines the shells of the blocks are completely formed, and they are next
polished and finished by hand labour; but as this is performed by tools and methods
which are well known, it is needless to enter into any explanation: the finishing
required being only a smoothing of the surfaces. The machines cut so perfectly true
as to require no wood to be removed in the finishing; but as they cut without regard
to the irregularity of the grain, knots, &c., it happens that many parts are not so
smooth as might be wished, and for this purpose manual labour alone can be employed.
The lignum vitæ for the sheaves of the blocks, is cut across the grain of the wood by
two cross cutting saws, a circular and straight saw, as before mentioned. These
machines do not essentially differ in their principle from the great cross cutting saws
we have described, except that the wood revolves while it is cutting, so that a small saw
will reach the centre of a large tree, and at the same time cut it truly flat. The limits
prescribed for our plates will not admit of giving drawings of these machines, and the
idea which could be derived from a verbal description would not be materially different
from the cross cutting saws before mentioned. These machines cut off their plates for the
end of the tree, which are exactly the thickness for the intended sheave. These pieces
are of an irregular figure, and must be rounded and centered in the crown saw.
9. The crown saw is represented in fig. 134., where A is a pulley revolving by
means of an endless strap. It has the crown or trepan saw a fixed to it, by a screw cut
within the piece, upon which the saw is fixed, and which gives the ring or hoop of the[149]
saw sufficient stability to perform its office. Both the pulleys and saw revolve together
upon a truly cylindrical tube b, which is stationary, being attached by a flaunch c to a
fixed puppet B, and on this tube as an axis
the saw and pulley turn, and may be slid
endwise by a collar fitted round the centre
piece of the pulley, and having two iron
rods (only one of which can be seen at d
in the figure), passing through holes made
through the flaunch and puppet B. When
the saw is drawn back upon its central tube,
the end of the latter projects beyond the
teeth of the saw. It is by means of this fixed
ring or tube within the saw, that the piece
of wood e is supported during the operation
of sawing, being pressed forcibly against it
by a screw D, acting through a puppet fixed
to the frame of the machine. At the end of
this screw is a cup or bason which applies
itself to the piece of wood, so as to form a
kind of vice, one side being the end of the
fixed tube, the other the cup at the end of
the screw D. Within the tube b is a collar
for supporting a central axis, which is perfectly cylindrical. The other end of this axis,
(seen at f,) turns in a collar of the fixed puppet E. The central axis has a pulley F,
fixed on it, and giving it motion by a strap similar to the other. Close to the latter
pulley a collar g is fitted on the centre piece of the pulley, so as to slip round freely,
but at the same time confined to move endways with the pulley and its collar. This
collar receives the ends of the two iron rods d. The opposite ends of these rods are, as
above mentioned, connected by a similar collar, with the pulley A of the saw a. By
this connection, both the centre bit, which is screwed into the end of the central axis f,
and the saw sliding upon the fixed tube b, are brought forward to the wood at the same
time, both being in rapid motion by their respective pulleys.
10. The Coaking Engine.—This ingenious piece of machinery is used to cut the
three semicircular holes which surround the hole bored by the crown saw, so as to
produce a cavity in the centre of the disc.
11. Face-turning Lathe.—The sheave is fixed against a flat chuck at the end of a
mandrel, by an universal chuck, similar to that in the coaking engine, except that the
centre pin, instead of having a nut, is tapped into the flat chuck, and turned by a
screw-driver.
BLOOD. (Sang, Fr.; Blut, Germ.) The liquid which circulates in the arteries and
veins of animals; bright red in the former and purple in the latter, among all the tribes
whose temperature is considerably higher than that of the atmosphere. It consists 1. of
a colourless transparent solution of several substances in water; and 2. of red, undissolved
particles diffused through that solution. Its specific gravity varies with the nature and
health of the animal; being from 1·0527 to 1·0570 at 60° F. It has a saline sub-nauseous
taste, and a smell peculiar to each animal. When fresh drawn from the vessels, it rapidly
coagulates into a gelatinous mass, called the clot, cruor, or crassamentum, from which
after some time, a pale yellow fluid, passing into yellowish green, oozes forth, called the
serum. If the warm blood be stirred with a bundle of twigs, as it flows from the veins,
the fibrine concretes, and forms long fibres and knots, while it retains its usual appearance
in other respects. The clot contains fibrine and colouring matter in various proportions.
Berzelius found in 100 parts of the dried clot of blood, 35 parts of fibrine,
58 of colouring matter; 1·3 of carbonate of soda; 4 of an animal matter soluble in
water, along with some salts and fat. The specific gravity of the serum varies from
1·027 to 1·029. It forms about three fourths of the weight of the blood, has an alkaline
reaction, coagulates at 167° F. into a gelatinous mass, and has for its leading constituent
albumen to the amount of 8 per cent. besides fat, potash, soda, and salts of these bases.
Blood does not seem to contain any gelatine.
The red colouring matter called hematine, may be obtained from the cruor by washing
with cold water and filtering.
Blood was at one time largely employed for clarifying syrup, but it is very sparingly
used by the sugar refiners in Great Britain of the present day. It may be dried by evaporation
at a heat of 130° or 140°, and in this state has been transported to the colonies for purifying
cane juice. It is an ingredient in certain adhesive cements, coarse pigments for
protecting walls from the weather, for making animal charcoal in the Prussian blue
works, and by an after process, a decolouring carbon. It is used in some Turkey red
dye-works. Blood is a powerful manure.
[150]
BLOWING MACHINE. See Iron, Metallurgy, Ventilation.
BLOWPIPE. (Chalumeau, Fr.; Lothröhre, Germ.) Jewellers, mineralogists,
chemists, enamellers, &c. make frequent use of a tube, usually bent near the end, terminated
with a finely pointed nozzle, for blowing through the flame of a lamp, candle, or
gas-jet, and producing thereby a small conical flame possessing a very intense heat.
Modifications of blow pipes are made with jets of hydrogen, oxygen, or the two gases
mixed in due proportions.
BLUE DYES. (Teint, Germ. See Enamel.) The materials employed for
this purpose are indigo, Prussian blue, logwood, bilberry, (vaccinium myrtillus,) elder
berries, (sambucus nigra,) mulberries, privet berries, (ligustrum vulgare,) and some other
berries whose juice becomes blue by the addition of a small portion of alkali, or of the
salts of copper. For dyeing with the first three articles, see them in their alphabetical
places. I shall here describe the other or minor blue dyes.
To dye blue with such berries as the above, we boil one pound of them in water, adding
one ounce of alum, of copperas, and of blue vitriol, to the decoction, or in their stead
equal parts of verdegris and tartar, and pass the stuffs a sufficient time through the liquor.
When an iron mordant alone is employed, a steel blue tint is obtained; and when a tin
one, a blue with a violet cast. The privet berries which have been employed as sap
colours by the card painters, may be extensively used in the dyeing of silk. The berries
of the African night-shade (solanum guineense) have been of late years considerably applied
to silk on the continent in producing various shades of blue, violet, red, brown, &c.
but particularly violet. With alkalis and acids these berries have the same habitudes as
bilberries; the former turning them green, the latter red. They usually come from
Italy compressed in a dry cake, and are infused in hot water. The infusion is merely
filtered, and then employed without any mordant, for dyeing silk, being kept at a warm
temperature by surrounding the bath vessel with hot water. The goods must be winced
for six hours through it in order to be saturated with colour; then they are to be rinsed
in running water and dried. One pound of silk requires a pound and a half of the berry
cake. In the residuary bath, other tints of blue may be given. Sometimes the dyed
silk is finished by running it through a weak alum water. A colour approaching to indigo
in permanence, but which differs from it in being soluble in alkalis, though incapable
of similar disoxidizement, is the gardenia genipa and aculeata of South America whose
colourless juice becomes dark blue with contact of air; and dyes stuffs, the skin, and nails,
of an unchangeable deep blue colour, but the juice must be applied in the colourless
state.
BLUE PIGMENTS. Several metallic compounds possess a blue colour; especially
those of iron, cobalt, and molybdenum. The metallic pigments, little if at all employed,
but which may be found useful in particular cases, are the molybdate of mercury, the
hydro-sulphuret of tungsten, the prussiate of tungsten, the molybdate of tin, the oxide of
copper darkened with ammonia, the silicate of copper, and a fine violet colour formed
from manganese and molybdenum. The blues of vegetable origin, in common use, are indigo,
litmus, and blue cakes. The blue pigments of a metallic nature found in commerce
are the following: Prussian blue; mountain blue, a carbonate of copper mixed with
more or less earthy matter; Bremen blue, or verditer, a greenish blue colour obtained
from copper mixed with chalk or lime; iron blue, phosphate of iron, little employed;
cobalt blue, a colour obtained by calcining a salt of cobalt with alumina or oxide of tin;
smalt, a glass coloured with cobalt and ground to a fine powder; charcoal blue, a deep
shade obtained by triturating carbonized vine stalks with an equal weight of potash in a
crucible till the mixture ceases to swell, then pouring it upon a slab, putting it into water
and saturating the alkali with sulphuric acid. The liquor becomes blue, and lets fall a
dark blue precipitate, which becomes of a brilliant blue colour when heated.
Molybdenum blue is a combination of this metal, and oxide of tin or phosphate of lime.
It is employed both as a paint, and an enamel colour. A blue may also be obtained by
putting into molybdic acid, (made by digesting sulphuret of molybdenum with nitric
acid,) some filings of tin, and a little muriatic acid. The tin deoxidizes the molybdic
acid to a certain degree, and converts it into the molybdous, which when evaporated and
heated with alumina recently precipitated, forms this blue pigment. Ultramarine is a
beautiful blue pigment, which see.
BOMBAZINE. A worsted stuff, sometimes mixed with silk.
BONES. (Os, Fr.; Knochen, Germ.) They form the frame work of animal bodies,
commonly called the skeleton; upon which the soft parts are suspended, or in which they
are enclosed. Bones are invested with a membrane styled the periosteum, which is
composed of a dense tissue affording glue; whence it is convertible into jelly, by
ebullition with water. Bones are not equally compact throughout their whole substance;
the long ones have tubes in their centres lined with a kind of periosteum, of
more importance to the life of the bones than even their external coat. The flat, as[151]
well as the short and thick bones, exhibit upon their surface an osseous mass of a dense
nature, while their interior presents a cavity divided into small cellules by their bony
partitions.
In reference to the composition of bones, we have to consider two principal constituents;
the living portion or the osseous cartilage, and the inorganic or the earthy
salts of the bones.
The osseous cartilage is obtained by suspending bones in a large vessel full of dilute
muriatic acid, and leaving it in a cool place at about 50° Fahr. for example. The acid
dissolves the earthy salts of the bones without perceptibly attacking the cartilage, which,
at the end of a short time, becomes soft and translucid, retaining the shape of the bones;
whenever the acid is saturated, before it has dissolved all the earthy salts it should be
renewed. The cartilage is to be next suspended in cold water, which is to be frequently
changed till it has removed all the acidity. By drying, the cartilage shrinks a little,
and assumes a darker hue, but without losing its translucency. It becomes, at the
same time, hard and susceptible of breaking when bent, but it possesses great
strength.
This cartilage is composed entirely of a tissue passing into gelatine. By boiling with
water, it is very readily convertible into a glue, which passes clear and colourless through
the filter, leaving only a small portion of fibrous matter insoluble by further boiling.
This matter is produced by the vessels which penetrate the cartilage, and carry nourishment
to the bone. We may observe all these phenomena in a very instructive manner,
by macerating a bone in dilute muriatic acid, till it has lost about the half of its salts;
then washing it with cold water, next pouring boiling water upon it, leaving the whole
in repose for 24 hours, at a temperature a few degrees below 212° Fahr.
The cartilage, which has been stripped of its earthy salts dissolves, but the small
vessels which issue from the undecomposed portion of the bone remain under the form
of white plumes, if the water has received no movement capable of crushing or breaking
them. We may then easily recognise them with a lens, but the slightest touch tears
them, and makes them fall to the bottom of the vessel in the form of a precipitate; if
we digest bones with strong hot muriatic acid so as to accelerate their decomposition, a
portion of the cartilage dissolves in the acid with a manifest disengagement of carbonic
acid gas, which breaks the interior mass, and causes the half-softened bone to begin to
split into fibrous plates, separable in the direction of their length. According to Marx
these plates, when sufficiently thin, possess, like scales of mica, the property of polarising
light, a phenomenon which becomes more beautiful still when we soak them with
the essential oil of the bark of the Laurus Cassia. The osseous cartilage is formed
before the earthy part. The long bones are then solid, and they become hollow only
in proportion as the earthy salts appear. In the new-born infant, a large portion of the
bones is but partially filled with these salts, their deposition in cartilage takes place
under certain invariable points of ossification, and begins at a certain period after conception,
so that we may calculate the age of the foetus according to the progress which
ossification has made.
The earthy parts of bones are composed principally of the phosphate and carbonate
of lime in various proportions, variable in different animals, and mixed with small
quantities, equally variable, of phosphate of magnesia and fluate of lime. The easiest
means of procuring the earthy salts of bones consists in burning them to whiteness, but
the earthy residuum procured in this manner, contains substances which did not exist
beforehand in the bones, and which did not form a part of their earthy salts; as for example
sulphate of soda, produced at the expense of the sulphur of the bones and the
alkaline carbonate, proceeding from the cartilage with which it was combined. On the
other hand, the greater part of the lime has lost its carbonic acid. As the sulphuric acid
is the product of combustion, it is obvious that an acidulous solution of a fresh bone can
afford no precipitate with muriate of barytes. The phosphate of lime contained in the
bone-salts is a subphosphate, consisting, according to Berzelius, of three prime equivalents
of the acid, and 8 of the base; or of 2,677 parts of the former, and 2,848 of the latter.
It is always obtained when we precipitate the phosphate of lime by an excess of ammonia.
When calcined bones are distilled in a retort with their own weight of sulphuric
acid, a little fluoric acid is disengaged, and it acts on the surface of the glass. The following
analyses of the bones of men and horned cattle, are given by Berzelius. They
were dried after being stripped of their fat and periosteum till they lost no more
weight.
[152]
| |
Human bone. |
Ox bone. |
| |
|
|
| Cartilage completely soluble in water |
32 |
·17 |
|
- |
33 |
·3 |
| Vessels |
1 |
·13 |
| Subphosphate with a little fluate of lime |
53 |
·04 |
57 |
·35 |
| Carbonate of lime |
11 |
·3 |
3 |
·85 |
| Phosphate of magnesia |
1 |
·16 |
2 |
·05 |
| Soda with very little muriate of soda |
1 |
·20 |
3 |
·45 |
| |
100 |
·00 |
100 |
·00 |
The most essential difference in the composition of these bones is that those of man
contain three times as much carbonate of lime as those of the ox; and that the latter
are richer in phosphate of lime and magnesia in the same proportion. Fernandez de
Barros has established a comparison between the phosphate and carbonate of lime in
the bones of different animals. He found in 100 parts of earthy salt of the bones of the
following animals:—
| |
Phosphate
of lime. |
Carb.
lime. |
| Lion |
95·0 |
2·5 |
| Sheep |
80·0 |
19·3 |
| Hen |
88·9 |
10·4 |
| Frog |
95·2 |
2·4 |
| Fish |
91·9 |
5·3 |
The bones of fish are divided into those which contain earthy salts and those which
have none, called cartilaginous fishes. The enamel of the teeth is composed as
follows:—
| |
Human
enamel. |
Ox
enamel. |
| Phosphate of lime with fluate of lime |
88·5 |
85·0 |
| Carbonate of lime |
8·0 |
7·1 |
| Phosphate of magnesia |
1·5 |
3·0 |
| Soda |
0·0 |
1·4 |
| Brown membranes attached to the tooth, alkali, water |
2·0 |
3·5 |
| |
100·0 |
100·0 |
In the arts, the bones are employed by turners, cutlers, manufacturers of animal
charcoal; and, when calcined, by assayers for making cupels. In agriculture, they are
employed as a manure, for which purpose they should be ground in a mill, and the
powder sowed along with the seeds in a drill. It is supposed, in many cases, to increase
the crop in weight of grain and straw together, by from 40 to 50 per cent. In France,
soup is extensively made by dissolving bones in a steam-heat of two or three days’
continuance. The shavings of hartshorn, which is a species of bone, afford an elegant
jelly: the shavings of calves’ bones may be used in their stead.
Living bones acquire a red tinge when the animals receive madder with their food;
but they lose it when the madder is discontinued for some time.
BONE BLACK (Noir d’os, Fr.; Knochenschwartz, Germ.), or Animal charcoal, as
it is less correctly called, is the black carbonaceous substance into which bones are
converted by calcination in close vessels. This kind of charcoal has two principal
applications; to deprive various solutions, particularly syrups, of their colouring matters,
and to furnish a black pigment. The latter subject will be treated of under Ivory
Black.
The discovery of the antiputrescent and decolouring properties of charcoal in general,
is due to Lowitz, of Petersburg; but their modifications have occupied the attention of
many chemists since his time. Kels published, in 1798, some essays on the discolouring
of indigo, saffron, madder, syrup, &c. by means of charcoal, but he committed a mistake
in supposing bone black to have less power than the charcoal of wood. The first
useful application of charcoal to the purification of raw colonial sugar was made by
M. Guillon, who brought into the French markets considerable quantities of fine syrups,
which he discoloured by ground wood charcoal, and sold them to great advantage, as
much superior to the cassonades of that time. In 1811, M. Figuier, an apothecary at
Montpellier, published a note about animal charcoal, showing that it blanched vinegars
and wines with much more energy than vegetable charcoal; and, lastly, in 1812,[153]
M. Derosnes proposed to employ animal charcoal in the purification of syrups and
sugar refining. The quantities of bone black left in the retorts employed by
MM. Payen, for producing crude carbonate of ammonia, furnished abundant materials
for making the most satisfactory experiments, and enabled these gentlemen soon to
obtain ten per cent. more of refined sugar from the raw article than had been formerly
extracted, and to improve, at the same time, the characters of the lumps, bastards,
treacle, &c.
The calcination of bones is effected by two different systems of apparatus; by heating
them in a retort similar to that in which coal is decomposed in the gas works, or in
small pots piled up in a kiln. For the description of the former, see Gas-Light.
On the second plan, the bones, broken into pieces, are put into small cast-iron pots
of the form shown in fig. 135., about three eighths of an inch thick, two of which are
dexterously placed with their mouths in contact, and then luted together with loam.
The lip of the upper pot is made to slip inside of the under one. These double vessels,
containing together about fifty pounds of bones, are arranged alongside, and over each
other, in an oven, like a potter’s kiln, till it be filled. The oven or kiln may be either
oblong or upright. The latter is represented in fig. 136, 137, 138. A is the fireplace
or grate for the fuel; C C are the openings in the dome of the furnace through which the
flame flows; the divisions of these orifices are shown in fig. 138. B is the wall of
brick-work. D the space in which the pots are distributed. E is the door by which
the workman carries in the pots, which is afterwards built up with fire bricks, and
plastered over with loam. This door is seen in fig. 136. F F are the lateral flues for
conveying the disengaged gases into the air.
Fig. 139. is a longitudinal section, and fig. 140. a ground plan of a horizontal kiln
for calcining bones. a is the fire-chamber, lying upon a level with the sole of the
kiln; it is separated by a pillar b, from the calcining hearth c. In the pillar or wall,
several rows of holes d, are left at different heights; e is the entrance door; f, the outlet
vents for the gases, vapours, and smoke, into the chimney g; h, a sliding damper-plate
for regulating the admission of the air into the fire in the space a.
By this arrangement the offensive emanations are partly consumed, and partly carried
off with the smoke. To destroy the smell completely, the smoke should be made to
pass through a second small furnace.
The number of pots that may be put into a kiln of this kind depends, of course, upon
its dimensions; but, in general, from 100 to 150 are piled up over each other, in columns,
at once; the greatest heat being nearest the roof of the kiln; which resembles, in many
respects, that used for baking pottery ware.
In both kilns the interior walls are built of fire-bricks. In the oblong one, the
fiercest heat is near the vaulted roof; in the upright one, near the sole; and the pots,
containing the larger lumps of bones, should be placed accordingly near the top of the
former, and the bottom of the latter. Such a kiln may receive about seventy double
pots, containing in the whole thirty-five cwt. of bones.
After the earth is filled with the pots, and the entrance door is shut, the fire is
applied at first moderately, but afterwards it must be raised and maintained, at a brisk
heat, for eight or ten hours. The door of the ash-pit and the damper may now be
nearly closed, to moderate the draught, and to keep up a steady ignition for six or eight
hours longer, without additional firing; after which the doors must be all opened to
cool the furnace. When this is done, the brick-work of the entrance door must be
taken down, the kiln must be emptied, and immediately filled again with a set of pots
previously filled with bones, and luted together: the pots which have been ignited may,
in the course of a short time, be opened, and the contents put into the magazine. But[154]
in operating with the large decomposing cylinder retort, the bones being raked out hot,
must be instantly tossed into a receiver, which can be covered-in air-tight till they are cool.
The bones lose upon the average about one half of their weight in the calcination.
In reference to the quality of the black, experience has shown that it is so much more
powerful as a discolouring agent, as the bones from which it was made have been freer
from adhering fatty, fleshy, and tendinous matters.
The charcoal is ground in a mill, either to a fine powder and sifted; or into a coarse
granular state, like gunpowder, for the preparation of which two sieves are required,
one with moderately fine meshes, to allow the small dust to pass through, and one with
large meshes, to separate the proper sized grains from the coarser lumps. Either a corn-mill,
an edgestone mill, or a steel cylinder mill, may be employed for grinding bone-black,
and it is generally damped in the operation to keep down the fine dust.
Bone-black, as found in commerce, is very variable in its discolouring power, which
arises from its having been exposed either to too great a heat which has glazed its
carbon, or to too low a heat which has left its albumen imperfectly decomposed. A
steady ignition of due continuance is the proper decomposing temperature. Its composition
is generally as follows:—
Phosphate of lime, with carbonate of lime, and a little sulphuret of iron, or oxide of
iron, 88 parts; iron in the state of a silicated carburet, 2 parts; charcoal containing
about one fifteenth of azote, 10 parts. None of the substances present, except the charcoal,
possesses separately any discolouring power.
The quality may be tested by a solution of brown sugar, or molasses, or of indigo in
sulphuric acid. The last is generally preferred by the French chemists, who have occupied
themselves most with this subject, and it contains usually one thousandth part
of its weight of this dye-drug of the best quality. Other animal substances yield a
charcoal, possessed of very considerable discolouring properties. The following table by
M. Bussy exhibits an interesting comparison of almost every kind of charcoal in this
point of view.
Table of the discolouring powers of different charcoals.
| Species of Charcoal. |
Weight. |
Indigo
test
consumed. |
Molasses
test
consumed. |
Blanching
by
indigo. |
Power
by
molasses. |
| |
Gramme. |
Litres. |
|
|
|
| Blood calcined with potash |
1 |
1 |
·60 |
0 |
·18 |
50 |
|
20 |
|
| Ditto with chalk |
1 |
0 |
·57 |
0 |
·10 |
18 |
|
11 |
|
| Ditto with phosp. lime |
1 |
0 |
·38 |
0 |
·09 |
12 |
|
10 |
|
| Gelatine ditto with potash |
1 |
1 |
·15 |
0 |
·14 |
36 |
|
15 |
·5 |
| Albumen ditto ditto |
1 |
1 |
·08 |
0 |
·14 |
34 |
|
15 |
·5 |
| Starch ditto ditto |
1 |
0 |
·34 |
0 |
·08 |
10 |
·6 |
8 |
·8 |
| Charcoal from acet. potash |
1 |
0 |
·18 |
0 |
·04 |
5 |
·6 |
4 |
·4 |
| Ditto from carb. soda by phosphorus |
1 |
0 |
·38 |
0 |
·08 |
12 |
|
8 |
·8 |
| Calcined lamp black |
1 |
0 |
·128 |
0 |
·03 |
4 |
|
3 |
·3 |
| Ditto ditto potash |
1 |
0 |
·55 |
0 |
·09 |
15 |
·2 |
10 |
·6 |
| Bone black treated with mur. acid and potash |
1 |
1 |
·45 |
0 |
·18 |
45 |
|
20 |
|
| Bone black ditto with mur. acid |
1 |
0 |
·06 |
0 |
·015 |
1 |
·87 |
1 |
·6 |
| Oil calcined with phosp. of lime |
1 |
0 |
·064 |
0 |
·017 |
2 |
|
1 |
·9 |
| Crude bone black |
1 |
0 |
·032 |
0 |
·009 |
1 |
|
1 |
|
With regard to the mode of operation of bone black on coloured liquids, M. Payen
showed in his prize essay, 1. That the decolouring power of charcoal depends in
general upon its state of division; 2. That in the various charcoals, the carbonaceous
matter acts only upon the colouring matters, combining with and precipitating them; 3.
That in the application of charcoal to the refining of sugar, it acts also upon the gluten,
for it singularly promotes crystallisation; 4. That according to the above principles, the
decolouring action of charcoals may be so modified, as to make the most inert become
the most active; 5. That the distinction between animal and vegetable charcoals is improper,
and that we may substitute for it that of dull and brilliant charcoals; 6. That
of the substances present in charcoal besides carbon, and particularly animal charcoal,
those which favour the decolouring action, have an influence relative only to the carbon;
they serve as auxiliaries to it, by insulating its particles, and presenting them more
freely to the action of the colouring matter; 7. That animal charcoal, besides its decolouring
power, has the valuable property of taking lime in solution from water and[155]
syrup; 8. That neither vegetable, nor other charcoals, besides the animal, have this
power of abstracting lime; 9. That by the aid of the decolorimeter, or graduated tube
charged with test solution of indigo or molasses, it is easy to appreciate exactly the
decolouring properties of all kinds of charcoal.
Different varieties of lignite (fossilized wood) or even pit coal, when well carbonized
in close vessels, afford a decolouring charcoal of considerable value. By reducing 100
parts of clay into a thin paste with water, kneading into it 20 parts of tar, and 500 of
finely ground pit coal, drying the mixed mass, and calcining it out of contact of air, a
charcoally matter may be obtained not much inferior to bone-black in whitening syrups.
The restoration of animal charcoal from burnt bones, for the purpose of sugar refining,
has been long practised in France. Mr. W. Parker has lately made the following
process the subject of a patent. The charcoal, when taken from the vessels in which it
has been employed for the purposes of clarifying the sugar, is to be thoroughly washed
with the purest water that can be obtained, in order to remove all the saccharine matter
adhering to it. When the washing process has been completed, the charcoal is
laid out to dry, either in the open air or in a suitable stove, and when perfectly free
from moisture, it is to be separated into small pieces and sifted through a sieve, the
wires or meshes of which are placed at distances of about two and a half in every inch.
This sifting will not only divide the charcoal into small pieces, but will cause any
bits of wood or other improper matters to be separated from it.
The charcoal, thus prepared, is then to be packed lightly in cylindrical vessels called
crucibles, with some small quantity of bones, oil, or other animal matter mixed with it.
The crucibles are then to be closed by covers, and luted at the joints, leaving no other
opening but one small hole in the centre of the cover, through which any gas, generated
within the vessel when placed in the oven or furnace, may be allowed to escape.
The crucibles are now to be ranged round the oven, and placed, one upon another, in
vertical positions; and when the oven is properly heated, gas will be generated within
each crucible, and issue out from the central hole. The gas thus emitted, being of an
inflammable quality, will take fire, and assist in heating the crucibles; and the operation
being carried on until the crucibles become of a red heat, the oven is then to be closed,
and allowed to cool; after which the crucibles are to be removed, when the charcoal
will be found to have become perfectly renovated, and fit for use as before.
BORAX. A native saline compound of boracic acid and soda, found abundantly
in Thibet and in South America. The crude product from the former locality was
imported into Europe under the name of tincal, and was purified from some adhering
fatty matter by a process kept a long time secret by the Venetians and the Dutch, and
which consisted chiefly in boiling the substance in water with a little quicklime.
Gmelin found borax, in prismatic crystals, to contain 46·6 per cent. of water; and
Arvredson, in the calcined state, to consist of 68·9 of acid and 31·1 soda, in 100 parts.
M. Payen describes an octahedral borax, which contains only 30·64 per cent. of water,
and is therefore preferred by the braziers in their soldering processes.
Borax has a sweetish, somewhat lixivial taste, and affects vegetable colours like an
alkali; it is soluble in 12 parts of cold and 2 of boiling water. It effloresces and
becomes opaque in a dry atmosphere, and appears luminous, by friction, in the dark.
It melts at a heat a little above that of boiling water, and gives out its water of crystallization,
after which it forms a spongy mass, called calcined borax. The octahedral borax,
which is prepared by crystallization, in a solution of 1·256 sp. gr., kept up at 145° F., is
not efflorescent. When borax is ignited, it fuses into a glassy-looking substance.
The following is the improved mode of purifying borax. The crude crystals are to
be broken into small lumps, and spread upon a filter lined with a lead grating, under
which a piece of cloth is stretched upon a wooden frame. The lumps are piled up to
the height of 12 inches, and washed with small quantities of a caustic soda lye of 5° B.
(sp. gr. 1·033) until the liquor comes off nearly colourless; they are then drained, and
put into a large copper of boiling water, in such quantities that the resulting solution
stands 20° B. (sp. gr. 1·160). Carbonate of soda, equivalent to 12 per cent. of the
borax must now be added; the mixed solution is allowed to settle, and the clear liquid
syphoned off into crystallizing vessels. Whenever the mother waters get foul, they
must be evaporated to dryness in cast-iron pots, and roasted, to burn away the viscid
colouring matter.
Borax is sometimes adulterated with alum and common salt: the former addition
may be readily detected by a few drops of water of ammonia, which will throw down
its alumina; and the latter by nitrate of silver, which will give with it a precipitate
insoluble in nitric acid.
The native boracic acid obtained from the lakes of Tuscany, which has been manufactured
in France into borax, has greatly lowered the price of this article of commerce.
When MM. Payen and Cartier first began the business, they sold the crystals at the
same price as the Dutch, viz. 7 francs the kilogramme (21⁄5 lbs. avoird.); but, in a few[156]
years, they could obtain only 2 francs and 60 centimes, in consequence of the market
getting overstocked. The annual consumption of France in 1823 was 25,000 kilos.,
and the quantity produced in M. Payen’s works was 50,000. The mode of making
borax from the acid is as follows:—The lake water is evaporated in graduation
houses, and then concentrated in boilers till it crystallizes. In that state it is carried
to Marseilles. About 500 kilogrammes of water are made to boil in a copper, and
600 kilogrammes of crystallized carbonate of soda are dissolved in it by successive
additions of 20 kilogrammes. The solution being maintained at nearly the boiling
point, 500 kilogrammes of the crystallized boracic acid of Tuscany are introduced, in
successive portions. At each addition of about 10 kilogrammes, a lively effervescence
ensues, on which account the copper should be of much greater capacity than is
sufficient to contain the liquors. When the whole acid has been added, the fire must
be damped by being covered up with moist ashes, and the copper must be covered with
a tight lid and blankets, to preserve the temperature uniform. The whole is left in
this state during 30 hours; the clear liquor is then drawn off into shallow crystallizing
vessels of lead, in which it should stand no higher than 10 or 12 inches, to favour its
rapid cooling. At the end of three days in winter, and four in summer, the crystallization
is usually finished. The mother water is drawn off, and employed, instead of
simple water, for the purpose of dissolving fresh crystals of soda. The above crystals
are carefully detached with chisels, redissolved in boiling water, adding for each 100
kilos., 10 kilos. of carbonate of soda. This solution marks 20° B. (sp. gr. 1·160); and,
at least, one ton (1000 kilos.) of borax should be dissolved at once, in order to obtain
crystals of a marketable size. Whenever this solution has become boiling hot, it must
be run off into large crystallizing lead chests of the form of inverted truncated pyramids,
furnished with lids, enclosed in wooden frames, and surrounded with mats to confine
the heat. For a continuous business there should be at least 18 vessels of this kind;
as the solution takes a long time to complete its crystallization, by cooling to 30° C.
(86° F.). The borax crystals are taken out with chisels, after the liquor has been
drawn off, and the whole has become cold.
One hundred parts of the purest acid, usually extracted from the lakes of Tuscany,
contain only fifty parts of the real boracic acid, and yield no more, at the utmost, than
140 or 150 of good borax.
Dry borax acts on the metallic oxides at a high temperature, in a very remarkable
manner, melting and vitrifying them into beautiful coloured glasses. On this account
it is a most useful reagent for the blowpipe. Oxide of chrome tinges it of an emerald
green; oxide of cobalt, an intense blue; oxide of copper, a pale green; oxide of tin,
opal; oxide of iron, bottle green and yellow; oxide of manganese, violet; oxide of
nickel, pale emerald green. The white oxides impart no colour to it by themselves. In
the fusion of metals borax protects their surface from oxidizement, and even dissolves
away any oxides formed upon them; by which twofold agency it becomes an excellent
flux, invaluable to the goldsmith in soldering the precious metals, and to the brazier in
soldering copper and iron.
Borax absorbs muriatic and sulphurous acid gases, but no others, whereby it
becomes, in this respect, a useful means of analysis.
The strength or purity of borax may be tested by the quantity of sulphuric acid
requisite to neutralize a given weight of it, as indicated by tincture of litmus.
When mixed with shell-lac in the proportion of one part to five, borax renders that
resinous body soluble in water, and forms with it a species of varnish.
Boracic acid is a compound of 31·19 of boron and 68·81 oxygen, in 100 parts. Its
prime equivalent referred to oxygen 100, is 871·96.
The following process for refining the native Indian borax or tincal, has been
published by MM. Robiquet and Marchand:—
It is put into large tubs, covered with water for 3 or 4 inches above its surface, and
stirred through it several times during six hours. For 400 pounds of the tincal there
must now be added one pound of quicklime diffused through two quarts of water.
Next day the whole is thrown upon a sieve, to drain off the water with the impurities,
consisting, in some measure, of the fatty matter combined with the lime, as an insoluble
soap. The borax, so far purified, is to be dissolved in 21⁄2 times its weight of boiling
water, and eight pounds of muriate of lime are to be added for the above quantity of
borax. The liquor is now filtered, evaporated to the density of 18° or 20° B. (1·14
to 1·16 sp. grav.), and set to crystallize in vessels shaped like inverted pyramids, and
lined with lead. At the end of a few days, the crystallization being completed, the
mother waters are drawn off, the crystals are detached and dried. The loss of weight
in this operation is about 20 per cent.
The quantity of borax imported into the United Kingdom in 1835 was 335,224
pounds; whereof 122,022 pounds were exported. The duty is 10s. upon the refined,
and 4s. unrefined.
[157]
BOOKBINDING, is the art of sewing together the sheets of a book; and securing
them with a back and side boards. Binding is distinguished from stitching, which is
merely sewing the leaves without bands or backs; and from half-binding, which consists
in securing the back only with leather, the pasteboard sides being covered with
blue or marble paper; whereas in binding, both the back and sides are covered with
leather.
Bookbinding, according to the present mode, is performed in the following manner:—The
sheets are first folded into a certain number of leaves, according to the form in
which the book is to appear; viz. two leaves for folios, four for quartos, eight for
octavos, twelve for duodecimos, &c. This is done with a slip of ivory or boxwood,
called a folding stick; and in the arrangement of the sheets the workmen are directed
by the catch-words and signatures at the bottom of the pages. When the leaves are thus
folded and arranged in proper order, they are usually beaten upon a stone with a heavy
hammer, to make them solid and smooth, and are then condensed in a press. After
this preparation they are sewed in a sewing press, upon cords or packthreads called
bands, which are kept at a proper distance from each other, by drawing a thread through
the middle of each sheet, and turning it round each band, beginning with the first and
proceeding to the last. The number of bands is generally six for folios, and five for
quartos, or any smaller size. The backs are now glued, and the ends of the bands are
opened, and scraped with a knife, that they may be more conveniently fixed to the pasteboard
sides; after which the back is turned with a hammer, the book being fixed in a
press between boards, called backing boards, in order to make a groove for admitting the
pasteboard sides. When these sides are applied, holes are made in them for drawing
the bands through, the superfluous ends are cut off, and the parts are hammered
smooth. The book is next pressed for cutting; which is done by a particular machine
called the plough, to which is attached a knife. See the figures and descriptions infra.
It is then put into a press called the cutting press, betwixt two boards, one of which
lies even with the press, for the knife to run upon; and the other above for the knife
to cut against. After this the pasteboards are cut square with a pair of iron shears;
and last of all, the colours are sprinkled on the edges of the leaves, with a brush made
of hog’s bristles; the brush being held in the one hand, and the hair moved with the
other.
Different kinds of binding are distinguished by different names, such as law binding,
marble binding, French binding, Dutch binding, &c. In Dutch binding, the backs
are vellum. In French binding a slip of parchment is applied over the back between
each band, and the ends are pasted upon the inside of each pasteboard. This indorsing,
as it is called, is peculiar to the French binders; who are enjoined, by special ordonnance,
to back their books with parchment. The parchment is applied in the press,
after the back has been grated to make the paste take hold. The Italians still bind in
a coarse thick paper, and this they call binding alla rustica. It is extremely inconvenient,
as it is liable to wear without particular care.
A patent was obtained in 1799 by Messrs. John and Joseph Williams, stationers in
London, for an improved method of binding books of every description. The improvement
consists of a back, in any curved form, turned a little at the edges, and made of
iron, steel, copper, brass, tin, or of ivory, bone, wood, vellum, or, in short, any material
of sufficient firmness. This back is put on the book before it is bound, so as just to
cover without pressing the edges; and the advantage of it is that it prevents the book,
when opened, from spreading on either side, and causes it to rise in any part to nearly
a level surface. In this method of binding the sheets are prepared in the usual manner,
then sewed on vellum slips, glued, cut, clothed, and boarded, or half boarded; the
firm back is then fastened to the sides by vellum drawn through holes, or secured by
inclosing it in vellum or ferret wrappers, or other materials pasted down upon the
boards, or drawn through them.
A patent was likewise obtained in 1800 by Mr. Ebenezer Palmer, a London stationer,
for an improved way of binding books, particularly merchants’ account-books. This improvement
has been described as follows:—let several small bars of metal be provided
about the thickness of a shilling or more, according to the size and thickness of the
book; the length of each bar being from half an inch to several inches, in proportion to
the strength required in the back of the book. At each end of every bar let a pivot be
made of different lengths, to correspond to the thickness of two links which they are to
receive. Each link must be made in an oval form, and contain two holes proportioned
to the size of the pivots, these links to be the same metal as the hinge, and each of
them nearly equal in length to the width of two bars. The links are then to be riveted
on the pivots, each pivot receiving two of them, and thus holding the hinge together,
on the principle of a link-chain or hinge. There must be two holes or more of different
sizes, as may be required, on each bar of the hinge or chain; by means of these holes
each section of the book is strongly fastened to the hinge which operates with the back[158]
of the book, when bound, in such a manner as to make the different sections parallel
with each other, and thus admit writing without inconvenience on the ruled lines, close
to the back.
The leather used in covering books is prepared and applied as follows: being first
moistened in water, it is cut to the size of the book, and the thickness of the edge is
paired off on a marble stone. It is next smeared over with paste made of wheat flour,
stretched over the pasteboard on the outside, and doubled over the edges within. The
book is then corded, that is, bound firmly betwixt two boards, to make the cover stick
strongly to the pasteboard and the back; on the exact performance of which the neatness
of the book in a great measure depends. The back is then warmed at the fire to
soften the glue, and the leather is rubbed down with a bodkin or folding stick, to set
and fix it close to the back of the book. It is now set to dry, and when dry the boards
are removed; the book is then washed or sprinkled over with a little paste and water,
the edges and squares blacked with ink, and then sprinkled fine with a brush, by striking
it against the hand or a stick; or with large spots, by being mixed with solution of
green vitriol, which is called marbling. Two blank leaves are then pasted down to the
cover, and the leaves, when dry, are burnished in the press, and the cover rolled on the
edges. The cover is now glazed twice with the white of an egg, filleted, and last of all,
polished, by passing a hot iron over the glazed colour.
The employment in book binding of a rolling press for smoothing and condensing the
leaves, instead of the hammering which books have usually received, is an improvement introduced
several years ago into the trade by Mr. W. Burn. His press consists of two
iron cylinders about a foot in diameter, adjustable in the usual way, by means of a screw,
and put in motion by the power of one man or of two, if need be, applied to one or two
winch-handles. In front of the press sits a boy who gathers the sheets into packets, by
placing two, three, or four upon a piece of tin plate of the same size, and covering them
with another piece of tin plate, and thus proceeding by alternating tin plates and bundles
of sheets till a sufficient quantity have been put together, which will depend on the stiffness
and thickness of the paper. The packet is then passed between the rollers and received
by the man who turns the winch, and who has time to lay the sheets on one side,
and to hand over the tin plates by the time that the boy has prepared a second packet.
A minion bible may be passed through the press in one minute, whereas the time
necessary to beat it would be twenty minutes. It is not, however, merely a saving of
time that is gained by the use of the rolling press; the paper is made smoother than it
would have been by beating, and the compression is
so much greater, that a rolled book will be reduced
to about five-sixths of the thickness of the same book
if beaten. A shelf, therefore, that will hold fifty
books bound in the usual way would hold nearly
sixty of those bound in this manner, a circumstance
of no small importance, when it is considered how
large a space even a moderate library occupies, and
that book-cases are an expensive article of furniture.
The rolling press is now substituted for the hammer
by several considerable bookbinders.
Fig. 141. represents the sewing press, as it stands
upon the table, before which the bookbinder sits.
Fig. 142. is a ground plan without the parts a and
n in the former figure. A is the base-board, supported
upon the cross bars m n, marked with dotted
lines in fig. 142. Upon the screw rods r r fig. 141. the nuts t d serve to fix the flat upper
bar n, at any desired distance from the base. That bar has a slit along its middle, through
which the hooks below z z pass down for receiving the ends of the sewing cords p p,
fixed at y y, and stretched by the thumb-screws z z.
The bar y y is let into an oblong space cut out of the
front edge of the base board and fixed there by a
movable pin a, and a fixed pin at its other end round
which it turns.
Fig. 143. is the bookbinder’s cutting press, which is
set upright upon a sort of chest for the reception of
the paper parings; and consists of three sides, being
open above and to the left hand of the workman.
The pressbar, or beam a, has two holes n n upon its
under surface, for securing it to two pegs standing on
the top of the chest. The screw rods t t pass through
two tapped holes in the bar, marked with b c at its
upper end; their heads r r being held by the shoulders o o. The heads are pierced with[159]
holes into which lever pins are thrust for screwing the rods hard up. The heavy
beam a remains immovable, while the parallel bar with the book is brought home
towards it by the two screws. The two rulers s s serve as guides to preserve the
motions truly parallel; and the two parallel lath bars b c guide between them the end
bar e, of the plough, whose knife is shown at i, with its clamping screw z.
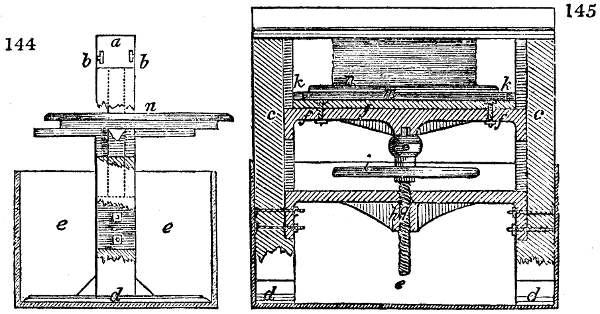
Mr. Oldham, printing engineer of the Bank of England, distinguished for mechanical
ingenuity, has contrived a convenient machine for cutting the edges of books, banknotes,
&c. either truly square or polygonal, with mathematical precision. Fig. 144. represents
an end elevation of the machine. Fig. 145. a side view of the same, the letters of
reference indicating the same parts of the machine in each of the figures.
a, is the top cross bar with rectangular grooves b b; c c, are side posts; d d, cross feet to
the same, with strengthening brackets; e e, a square box, in which the press stands, for
holding waste cuttings. Fig. 146. is a cross section of the upright posts, c c, taken horizontally.
There are rectangular grooves in the upright posts, for the projecting ends of
the cast iron cross bracket f, to slide up and down in. In the middle of the under-side
of this piece f, there is a boss, within which is a round recess, to receive the top of the
screw g, which works in the cast iron cross piece h, similarly made with the former, but
bolted firmly to the posts c c. Upon the screw g there is a circular handle or ring i, for
partially turning the screw, and immediately over it cross holes for tightening the press
by means of a lever bar. Upon the cross piece f, is bolted the board j, and upon each
end of this board is made fast the rabbetted pieces k k, for another board l, to slide in.
Across the middle of this board, and parallel to the pieces k k, the tongue piece m, is
made fast, which fits into a groove in the bottom of board l. A horizontal representation
of this is seen at fig. 147. and immediately under this view is also seen an end view of l,
and f, connected together, and a side view of f by itself. In the middle of the board l,[160]
is a pin for a circular board n, to turn upon, and upon this latter board is placed the
“material to be cut,” with a saving piece between it, and the circular piece which is to
be divided upon its edge into any number of parts required, with a stationary index on
the board l, to point to each.
It will now be understood that the “material to be cut,” may be turned round upon
the centre pin of the board n, and also that both it and the board can be shifted backward
and forward under the top cross piece a, and between the side slide slips k k, the
surfaces of which should also be divided into inches and tenths.
The plough, fig. 148., shown in several positions, is made to receive two knives or
cutters as the “material to be cut” may require, and which are situated in the plough
as I now describe. The plough is composed of three principal parts, namely, the top,
and its two sides. The top o, is made the breadth of the cross piece a, and with a
handle made fast thereon. The sides p p, are bolted thereto, with bolts and nuts through
corresponding holes in the top and sides. The figures below give inside views, and
cross sections of the details of the manner in which the cutters and adjustments are
mounted. A groove is cut down each cheek or side, in which are placed screws that
are held at top and bottom from moving up and down, but by turning they cause the
nuts upon them to do so; they are shown at q q. These nuts have each a pin projecting
inwards, that go into plain holes made in the top ends of cutters r r. The 148th. and
following figs. are 1⁄4 in scale.
The cutters, and the work for causing them to go up and down, are sunk into the
cheeks, so as to be quite level with their inner surfaces. Fig. 149. shows one of those
screws apart, how fixed, and with moveable nut and projecting pin. The top of each
screw terminates with a round split down, and above it a pinion wheel and boss thereon,
also similarly split. This pinion fits upon the split pin. Above, there is cross section
of a hollow coupling cap with steel tongue across, that fits into both the cuts of the screw
pin and pinion boss, so that when lowered upon each other, they must all turn together.
In the middle and on the top of the upper piece o, the larger wheel s, runs loose upon its
centre, and works into the two pinion-wheels t t. The wheel s has a fly-nut with wings
mounted upon it.
It will now be seen, when the plough is in its place as at fig. 150., that if it be pushed
to and fro by the right hand, and the nut occasionally turned by the left, the knives or
cutters will be protruded downwards at the same time, and these either will or will not
advance as the coupling caps u u are on or off. The ribs v v, run in the grooves b b,
fig. 144., and keep the cutters to their duty, working steadily. The top cross bar a, is the
exact breadth of a bank-note, by which means both knives are made to cut at the same
time. The paper is cut uniformly to one length, and accurately square.
By the use of this machine, the air-pump paper-wetting apparatus, and appendant
press, the paper of 45,000 notes is fully prepared in one hour and a half by one person,
and may then be printed. It is not so much injured by this process as by the ordinary
method of clipping by hand, soaking it, &c., which more or less opens and weakens the
fabric, especially of bank-note paper.
One of the greatest improvements ever made in the art of bookbinding is, apparently,
that for which Mr. William Hancock has very recently obtained a patent. After
folding the sheets in double leaves, he places them vertically, with the edges forming the
back of the book downwards in a concave mould, of such rounded or semi-cylindrical
shape as the back of the book is intended to have. The mould for this purpose consists
of two parallel upright boards, set apart upon a cradle frame, each having a portion or
portions cut out vertically, somewhat deeper than the breadth of the book, but of a width
nearly equal to its thickness before it is pressed. One of these upright boards may be
slidden nearer to or farther from its fellow, by means of a guide bar, attached to the sole
of the cradle. Thus the distance between the concave bed of the two vertical slots in
which the book rests, may be varied according to the length of the leaves. In all cases
about one-fourth of the length of the book at each end projects beyond the board, so
that one half rests between the two boards. Two or three packthreads are now bound
round the leaves thus arranged, from top to bottom of the page in different lines, in
order to preserve the form given to the back of the mould in which it lay. The book is
next subjected to the action of the press. The back, which is left projecting very slightly
in front, is then smeared carefully by the fingers with a solution of caoutchouc, whereby
each paper-edge receives a small portion of the cement. In a few hours it is sufficiently dry
to take another coat of a somewhat stronger caoutchouc solution. In 48 hours, 4 applications
of the caoutchouc may be made and dried. The back and the adjoining part of
the sides are next covered with the usual band or fillet of cloth, glued on with caoutchouc;
after which the book is ready to have the boards attached, and to be covered with leather
or parchment as may be desired.
We thus see that Mr. Hancock dispenses entirely with the operations of stitching,
sewing, sawing-in, hammering the back, or the use of paste and glue. Instead of leaves[161]
attached by thread stitches at 2 or 3 points, we have them agglutinated securely along
their whole length. Books bound in this way open so perfectly flat upon a table
without strain or resilience, that they are equally comfortable to the student, the musician,
and the merchant. The caoutchouc cement moreover being repulsive to insects, and not
affected by humidity, gives this mode of binding a great superiority over the old method
with paste or glue, which attracted the ravages of the moth, and in damp situations
allowed the book to fall to pieces. For engravings, atlasses, and ledgers, this binding is
admirably adapted, because it allows the pages to be displayed most freely, without the
risk of dislocating the volume; but for security, 3 or 4 stitches should be made. The leaves
of music-books bound with caoutchouc, when turned over, lie flat at their whole extent,
as if in loose sheets, and do not torment the musician like the leaves of the ordinary
books, which are so ready to spring back again. Manuscripts and collections of
letters which happen to have little or no margin left at the back for stitching them by,
may be bound by Mr. Hancock’s plan without the least encroachment upon the writing.
The thickest ledgers thus bound, open as easily as paper in quire, and may be written
on up to the innermost margin of the book without the least inconvenience.
Having inspected various specimens of Mr. Hancock’s workmanship, I willingly bear
testimony to the truth of the preceding statement. See Cloth Binding.
BOTTLE MANUFACTURE. The
following mechanism for moulding bottles,
forms the subject of a patent obtained by
Henry Rickets of Bristol in 1822. Fig. 155.
is a section of the apparatus, consisting of
a square frame, a a, of iron or wood; this
is fixed in a pit formed in the floor; b b
is the base of the frame, with an aperture
for knocking up the bottom of the
bottle; c c are four legs secured to the
frame-floor b, upon which the mould is
supported. The platform or stand of the
mould d d has an opening in its centre for
the introduction of the bottom of the
mould, which is raised against the bottom
of the bottle by the knocker up; e e are
the sides of the mould; and f f is the top
of the mould in two pieces, turning over
upon the joints at g g, so as to form the
neck of the bottle; h h are levers or arms
for raising and depressing the top pieces;
i i is a horizontal shaft or axle, turning in
bearings at each end, from which shaft two
levers, k k, extend; these levers are connected
by upright rods, l l, to the levers or
arms, h h, of the top pieces f f.
The weight of the arms h h, and rods l l, will, by their gravity, cause the top pieces
to open, as shown by the dotted lines; in this situation of the mould, the melted glass
is to be introduced by a tube as usual. The workman then steps with one foot upon
the knob m, which forces down the rod n, and by means of a short lever o, extending from
the shaft i, forces down the top pieces f, and closes the mould, as seen in the figure;
the glass is then made to extend itself to the shape of the mould, by blowing as usual,
so as to form the bottle, and the workman at this time putting his other foot upon the
knob p, depresses the rod q, and hence raises the bottom of the mould by means of the
knocker-up, r, so as to form the bottom of the bottle.
At the bottom of the mould a ring is introduced of any required thickness, for the
purpose of regulating the capacity of the bottle; upon which ring it is proposed to
raise letters and figures, as a mould to imprint the maker’s name and the size of the
bottle. These moulds can be removed and changed at pleasure. Under the knob p,
a collar or washer is to be introduced, of any required thickness, to regulate the
knocking up of the bottom, by which a perfect symmetry of form is presented. In
order to make bottles of different sizes or forms, the mould is intended to be removed,
and its place supplied by another mould of different dimensions and figure; the lower
parts of all the moulds being made to fit the same frame. Such a mould ought to
be prescribed by legislative enactment, with an excise stamp to define the capacity of
every bottle, and thereby put an end to the interminable frauds committed in the
measure of wine and all other liquors sold by the bottle.
BOUGIE. A smooth, flexible, elastic, slender cylinder, introduced into the urethra,
rectum, or œsophagus, for opening or dilating it, in cases of stricture and other diseases.[162]
The invention of this instrument is claimed by Aldereto, a Portuguese physician, but its
form and uses were first described by his pupil Amatus, in the year 1554. Some are
solid, and some hollow; some corrosive, and some mollifying. They generally owe their
elasticity to linseed oil, inspissated by long boiling, and rendered drying by litharge.
This viscid matter is spread upon a very fine cord or tubular web of cotton, flax, or silk,
which is rolled upon a slab when it becomes nearly solid by drying, and is finally
polished in the same way.
Pickel, a French professor of medicine, published the following recipe for the composition
of bougies. Take 3 parts of boiled linseed oil, one part of amber, and one
of oil of turpentine; melt and mix these ingredients well together, and spread the
compound at three successive intervals upon a silk cord or web. Place the pieces
so coated in a stove heated to 150° F.; leave them in it for 12 hours, adding 15
or 16 fresh layers in succession, till the instruments have acquired the proper size.
Polish them first with pumice-stone, and finally smooth with tripoli and oil. This process
is the one still employed in Paris, with some slight modifications; the chief of
which is dissolving in the oil one twentieth of its weight of caoutchouc to render the
substance more solid. For this purpose the caoutchouc must be cut into slender
shreds, and added gradually to the hot oil. The silk tissue must be fine and open, to
admit of the composition entering freely among its filaments. Each successive layer
ought to be dried first in a stove, and then in the open air, before another is applied.
This process takes two months for its completion, in forming the best bougies called
elastic; which ought to bear twisting round the finger without cracking or scaling, and
extension without giving way, but retracting when let go. When the bougies are to be
hollow, a mandril of iron wire, properly bent with a ring at one end, is introduced into
the axis of the silk tissue. Some bougies are made with a hollow axis of tin foil rolled
into a slender tube. Bougies are also made entirely of caoutchouc, by the intervention
of a solution of this substance in sulphuric ether, a menstruum sufficiently cheap in
France, on account of the low duty upon alcohol. There are medicated bougies, the
composition of which belongs to surgical pharmacy. The manufacture of these instruments
of various kinds forms a separate and not inconsiderable branch of industry at
Paris. MM. Feburger and Lamotte are eminent in this line.
BRACES. (Bretelles, Fr. Hosenträger, Germ.) Narrow fillets or bands of leather
or textile fabric, which pass over the shoulders, and are attached behind and before to
the waistbands of pantaloons and trowsers, in the act of wearing them, for supporting
their weight, and bracing them up to the body. It is a useful modern invention,
superseding the necessity of girding the belly with a tight girdle, as in former times.
BRAIDING MACHINE. (Machine à lacets, Fr.; Bortenwerkerstuhl, Germ.) This
being employed, not only to manufacture stay-laces, braid, and upholsterer’s cord, but
to cover the threads of caoutchouc for weaving brace-bands, deserves a description in
this work. Three threads at least are required to make such a knitted lace, but 11, 13,
or 17, and even 29 threads are often employed, the first three numbers being preferred.
They are made by means of a frame of a very ingenious construction, which moves by
a continuous rotation. We shall describe a frame with 13 threads, from which the
structure of the others may be readily conceived. The basis of the machine consists of
four strong wooden uprights, A, fig. 156, 157, 158., occupying the four angles of a
rectangle, of which one side is 14 inches long, the other 18 inches, and the height of the
rectangle about 40 inches. Fig. 156. is a section in a horizontal plane, passing through
the line a b of fig. 157. which is a vertical section in a plane passing through the centre of[163]
the machine C, according to the line c d, fig. 156. The side X is supposed to be the front
of the frame; and the opposite side, Y, the back. B, six spindles or skewers, numbered,
from 1 to 6, placed in a vertical position upon the circumference of a circle, whose centre
coincides with that of the machine at the point C. These six spindles are composed,
1. Of so many iron shafts or axes D, supported in brass collets E, (fig. 157.) and extended
downwards within 6 inches of the ground, where they rest in brass steps fixed
upon a horizontal beam. 2. Wooden heads, made of horn-beam or nut-tree, placed, the
first upon the upper end of each spindle, opposite the cut-out beam F, and the second
opposite the second beam G. 3. Wooden-toothed wheels, H, reciprocally working
together, placed between the beam G, and the collet-beam E. The toothed wheels and
the lower heads for each spindle are in one piece.
The heads and shafts of the spindles No. 1. and 6., are one fifth stronger than those of
the other spindles; their heads have five semi-circular grooves, and wheels of 60 teeth,
while the heads of the others have only four grooves, and wheels of 48 teeth; so that
the number of the grooves in the six spindles is 26, one half of which is occupied with
the stems of the puppets I, which carry the 13 threads from No. 1. to 13. The toothed
wheels, which give all the spindles a simultaneous movement, but in different directions,
are so disposed as to bring their grooves opposite to each other in the course of
rotation.
K, the middle winglet, triple at bottom and quintuple at top, which serves to guide
the puppets in the direction they ought to pursue.
L, three winglets, single at top and bottom, placed exteriorly, which serve a like
purpose.
M, two winglets, triple at bottom and single at top, placed likewise exteriorly, and
which serve the same purposes as the preceding; m, are iron pins inserted in the cut-out
beam G, which serve as stops or limits to the oscillations of the exterior winglets.
Now, if by any moving power (a man can drive a pair) rotation be impressed upon
the large spindle No. 1., in the direction of the arrow, all the other spindles will necessarily
pursue the rotatory movement indicated by the respective arrows. In this case,
the 13 puppets working in the grooves of the heads of the spindles will be carried round
simultaneously, and will proceed each in its turn, from one extremity of the machine to
the opposite point, crossing those which have a retrograde movement. The 13 threads
united at the point N, situated above the centre of the machine, will form at that point
the braid, which after having passed over the pulley o, comes between the
two rollers P Q, and is squeezed together, as in a flatting-mill, where the
braid is calendered at the same time that it is delivered. It is obvious
that the roller P, receives its motion from the toothed wheel of the
spindle No. 3., and from the intermediate wheels R, S, T, as well as from
the endless screw Z, which drives at proper speed the wheel W, fixed upon
the shaft of the roller P.
The braid is denser in proportion as the point N is less elevated above
the tops of the puppets; but in this case, the eccentric motion of these
puppets is much more sensible in reference to that point, towards
which all the threads converge, than when it is elevated. The threads
which must be always kept equally stretched by means of a weight, as
we shall presently see, are considerably strained by the traction, occasioned
by the constantly eccentric movement of the puppets. From
this cause, braiding machines must be worked at a moderate velocity.
In general, for fine work, 30 turns of the large spindle per minute are
the utmost that can safely be made.
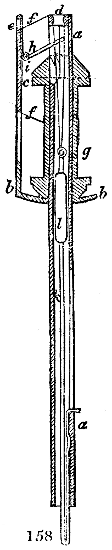
The puppet or spindle of this machine, being the most important
piece, I have represented it in section, upon a scale one fourth of its actual
size, fig. 158. It is formed of a tube, a, of strong sheet iron well
brazed; b is a disc, likewise of sheet iron, from which a narrow fillet, c,
rises vertically as high as the tube, where both are pierced with holes,
d e, through which the thread f is passed, as it comes from the bobbin,
g, which turns freely upon the tube a. The top of this bobbin is conical
and toothed. A small catch or detent, h, moveable in a vertical direction
round i, falls by its own weight into the teeth of the crown of
the bobbin, in which case this cannot revolve; but when the detent is
raised so far as to disengage the teeth, and at the same time to pull the
thread, the bobbin turns, and lets out thread till the detent falls back
into these same teeth.
A skewer of iron wire, k, is loaded with a small weight, l, melted upon
it. The top of this skewer has an eye in it, and the bottom is recurved as is shown in
fig. 158., so that supposing the thread comes to break, this skewer falls into the actual[164]
position in the figure, where we see its lower end extending beyond the tube a, by
about 1⁄4 of an inch; but as long as the thread is unbroken, the skewer k, which serves
to keep it always tense, during the eccentric movement of the puppet, does not pass
out below the tube.
This disposition has naturally furnished the means of causing the machine to stop,
whenever one of the threads breaks. This inferior protrusion of the skewer pushes in
its progress a detent, which instantly causes the band to slide from the driving pulley to
the loose pulley. Thus the machine cannot operate unless all the threads be entire. It
is the business of the operative, who has 3 or 4 under her charge, to mend the threads
as they break, and to substitute full bobbins for empty ones, whenever the machine is
stopped.
The braiding frame, though it does not move quickly, makes a great deal of noise,
and would make still more, were the toothed wheels made of metal instead of wood.
For them to act well, they should be made with the greatest precision, by means of
appropriate tools for forming the teeth of the wheels, and the other peculiar parts.
BRAN. (Son, Fr.; Kleie, Germ.) The husky portion of ground wheat, separated
by the boulter from the flour. It is advantageously employed by the calico printers, in
the clearing process, in which, by boiling in bran-water, the colouring matters adhering to
the non-mordanted parts of maddered goods, as well as the dun matters which cloud
the mordanted portions, are removed. A valuable series of researches concerning the
operation of bran in such cases was made a few years ago by that distinguished
chemist and calico printer, M. Daniel Kœchlin-Schouch, and published in the ninth
number of the Bulletin de la Société Industrielle de Mulhausen. Nine sets of experiments
are recorded, which justified the following conclusions.
1. The dose of two bushels of bran for 10 pieces of calico is the best, the ebullition
being kept up for an hour. A boil for the same time in pure water had no effect in
clearing either the grounds or the figures.
2. Fifteen minutes boiling are sufficient when the principal object is to clear white
grounds, but in certain cases thirty minutes are requisite to brighten the dyed parts. If,
by increasing the charge of bran, the time of the ebullition could be shortened, it would
be in some places, as Alsace, an economy; because for the passage of ten pieces through a
copper or vat heated with steam, 1 cwt. of coal is consumed in fuel which costs from
21⁄2 to 3 francs, while two bushels of bran are to be bought for one franc.
3. By increasing the quantity of water from 12 to 24 hectolitres with two bushels of
bran, the clearing effect upon the ten pieces was impaired. It is therefore advantageous
not to use too much water.
4. Many experiments concur to prove that flour is altogether useless for the clearing
boil, and that finer bran is inferior for this purpose to the coarser.
5. The white ground of the calicoes boiled with wheat bran, are distinguishable by
their superior brightness from that of those boiled with rye bran, and especially with
barley bran; the latter having hardly any effect.
6. There is no advantage in adding soap to the bran boil; though a little potash or
soda may be properly introduced when the water is calcareous.
7. The pellicle of the bran is the most powerful part, the flour and the starch are of
no use in clearing goods, but the mucilage which forms one third of the weight of the
bran has considerable efficacy, and seems to act in the following way. In proportion
as the mucilaginous substance dissolves the colouring and tawny matters upon the cloth,
the husky surface attracts and fixes upon itself the greater part of them. Accordingly,
when used bran is digested in a weak alkaline bath, it gives up the colour which it
had absorbed from the cloth.
The following chemical examination of bran is interesting. A pound of it was boiled
at successive times with water, the decoctions being filtered, let fall in cooling a greyish
deposit, which was separated by decantation. The clear liquor afforded by evaporation
to dryness four ounces of a brownish, brittle matter, composed chiefly of mucilage, a
little gluten, and starch. The gray deposit of the above filtered liquor amounted to half
an ounce. Nine ounces of the cortical portion of the bran were obtained. The loss
amounted to 21⁄2 ounces, being in some measure the hygrometric water of the bran
itself.
When boiled with distilled water, goods are cleared pretty well without bran. Certain
delicate dyes must be boiled only a few minutes in a strong decoction of bran
previously made.
BRANDY. The name given in this country to ardent spirits distilled from wine,
and possessed of a peculiar taste and flavour, due to a minute portion of a peculiar
volatile oil. Each variety of alcohol has an aroma characteristic of the fermented substance
from which it is procured; whether it be the grape, cherries, sugar-cane, rice,
corn, or potatoes; and it may be distinguished even as procured from different growths
of the vine. The brandies of Languedoc, Bordeaux, Armagnac, Cognac, Aunis,[165]
Saintonge, Rochelle, Orleans, Barcelona, Naples, &c. being each readily recognisable
by an experienced dealer.
Aubergier showed, by experiments, that the disagreeable taste of the spirits distilled
from the marc of the grape is owing to an essential oil contained in the skin of the
grape; and found that the oil, when insulated, is so energetic that a few drops are
sufficient to taint a pipe of 600 litres of fine-flavoured spirit.
The most celebrated of the French brandies, those of Cognac and Armagnac, are slightly
rectified to only from 0·935 to 0·922; they contain more than half their weight of water,
and come over therefore highly charged with the fragrant essential oil of the husk of the
grape. When, to save expense of carriage, the spirit is rectified to a much higher
degree, the dealer, on receiving it at Paris, reduces it to the market proof by the addition
of a little highly-flavoured weak brandy and water; but he cannot in this way produce
so finely-flavoured a spirit, as the weaker product of distillation of the Cognac wine.
If the best Cognac brandy be carefully distilled at a low heat, and the strong spirit be
diluted with water, it will be found to have suffered much in its flavour.
Genuine French brandy evinces an acid reaction with litmus paper, owing to a
minute portion of vinegar; it contains besides some acetic ether, and, when long kept
in oak casks, a little astringent matter. The following formula may be proposed for
converting a silent or flavourless corn spirit, into a factitious brandy. Dilute the pure
alcohol to the proof pitch, add to every hundred pounds weight of it from half a pound
to a pound of argol (crude winestone) dissolved in water, a little acetic ether, and
French-wine vinegar, some bruised French plums, and flavour-stuff from Cognac; then
distil the mixture with a gentle fire, in an alembic furnished with an agitator.
The spirit which comes over may be coloured with nicely burned sugar (caramel) to
the desired tint, and roughened in taste with a few drops of tincture of catechu or oak-bark.
The above recipe will afford a spirit free from the deleterious drugs too often used to
disguise and increase the intoxicating power of British brandies; one which may be
reckoned as wholesome as alcohol, in any shape, can ever be.
BRASS. (Laiton, cuivre jaune, Fr.; Messing, Germ.) An alloy of copper and
zinc. It was formerly manufactured by cementing granulated copper, called bean-shot,
or copper clippings, with calcined calamine (native carbonate of zinc) and charcoal, in
a crucible, and exposing them to bright ignition. Three parts of copper were used for
three of calamine and two of charcoal. The zinc reduced to the metallic state by the
agency of the charcoal, combined with the copper, into an alloy which formed, on
cooling, a lump at the bottom of the crucible. Several of these, being remelted and
cast into moulds, constituted ingots of brass for the market. James Emerson obtained
a patent, in 1781, for making brass by the direct fusion of its two metallic elements,
and it is now usually manufactured in this way.
It appears that the best proportion of the constituents to form fine brass is one prime
equivalent of copper = 631⁄2 + one of zinc = 32·3; or very nearly 2 parts of copper to 1 of
zinc. The bright gold coloured alloy, called Prince’s, or Prince Rupert’s metal, in this
country, consists apparently of two primes of zinc to one of copper, or of nearly equal
parts of each. Brass, or hard solder, consists of two parts of brass and one of zinc
melted together, to which a little tin is occasionally added; but when the solder must
be very strong, as for brass tubes that are to undergo drawing, two thirds of a part of
zinc are used for two parts of brass. Mosaic gold, according to the specification of
Parker and Hamilton’s patent consists of 100 parts of copper, and from 52 to 55 of
zinc; which is no atomic proportion. Bath metal is said to consist of 32 parts of brass
and 9 parts of zinc.
The button manufacturers of Birmingham make their platin with 8 parts of brass
and 5 of zinc; but their cheap buttons with an alloy of copper, tin, zinc, and lead.
Red brass, the Tombak of some, (not of the Chinese, for this is white copper,)
consists of more copper and less zinc than go to the composition of brass; being from
21⁄2 to 8 or 10 of the former to 1 of the latter. At the famous brass works of Hegermühl,
to be presently described, 11 parts of copper are alloyed with 2 of zinc into a red
brass, from which plates are made that are afterwards rolled into sheets. From such
an alloy the Dutch foil, as it is called, is manufactured at Nürnberg; Pinchbeck,
Similor, Mannheim gold, are merely different names of alloy similar to Prince’s metal.
The last consists of 3 of copper and 1 of zinc, separately melted, and suddenly incorporated
by stirring.—Wiegleb.
In the process of alloying two metals of such different fusibilities as copper and zinc,
a considerable waste of the latter metal by the combustion, to which it is so prone,
might be expected; but, in reality, their mutual affinities seem to prevent the loss, in
a great measure, by the speedy absorption of the zinc into the substance of the copper.
Indeed, copper plates and rods are often brassed externally by exposure, at a high
temperature, to the fumes of zinc, and afterwards laminated or drawn. The spurious[166]
gold wire of Lyons is made from such rods. Copper vessels may be superficially
converted into brass by boiling them in dilute muriatic acid, containing some winestone
and zinc amalgam.
The first step in making brass is to plunge slips of copper into melted zinc till an
alloy of somewhat difficult fusion be formed, to raise the heat, and add the remaining
proportion of the copper.
The brass of the first fusion is broken to pieces, and melted with a fresh quantity of
zinc, to obtain the finished brass. Each melting takes about 8 or 9 hours. The
metal is now cast into plates, about 40 inches long by 26 inches broad, and from one
third to one half inch thick. The moulds are, in this case also, slabs of granite
mounted in an iron frame. Granite appears to be preferred to every thing else as a
mould, because it preserves the heat long, and by the asperities of its surface, it keeps
hold of the clay lute applied to secure the joinings.
The cast plates are most usually rolled into sheets. For this purpose they are cut
into ribands of various breadths, commonly about 61⁄2 inches. The cylinders of the
brass rolling-press are generally 46 inches long, and 18 inches in diameter. The
ribands are first of all passed cold through the cylinders; but the brass soon becomes
too hard to laminate. It is then annealed in a furnace, and, after cooling, is passed
afresh through a rolling press. After paring off the chipped edges, the sheets are
laminated two at a time: and if they are to be made very thin, even eight plates are
passed through together. The brass in these operations must be annealed 7 or 8 times
before the sheet arrives at the required thinness. These successive heatings are very
expensive; and hence they have led the manufacturers to try various plans of economy.
The annealing furnaces are of two forms according to the size of the sheets
of brass. The smaller are about 12 feet long, with a fire place at each end, and about
13 inches wide. The arch of the furnace has a cylindrical shape, whose axis is
parallel to its small side. The hearth is horizontal, and is made of bricks set on edge.
In the front of the furnace there is a large door, which is raised by a lever, or chain,
and counterweight, and slides in a frame between two cheeks of cast iron. This
furnace has, in general, no chimney, except a vent slightly raised above the door, to
prevent the workmen being incommoded by the smoke. Sometimes the arch is perforated
with a number of holes. The sheets of brass are placed above each other, but
separated by parings, to allow the hot air to circulate among them, the lowest sheet
resting upon two bars of cast iron placed lengthwise.
The large furnaces are usually 32 feet long, by 61⁄2 feet wide, in the body, and 3 feet
at the hearth. A grate, 13 inches broad, extends along each side of the hearth,
through its whole length, and is divided from it by a small wall, 2 or 3 inches high.
The vault of the furnace has a small curvature, and is pierced with 6 or 8 openings,
which allow the smoke to pass off into a low bell-chimney above. At each end of the
furnace there is a cast-iron door, which slides up and down in an iron frame, and
is poised by a counterweight. On the hearth there is a kind of railway, composed
of two iron bars, on the grooves of which the carriage moves with its loads of sheets
of brass.
These sheets, being often 24 feet long, could not be easily moved in and out of the
furnace; but as brass laminates well in the cold state, they are all introduced and
moved out together. With this view, an iron carriage is framed with four bars, which
rest on four wheels. Upon this carriage, of a length nearly equal to that of the furnace,
the sheets are laid, with brass parings between them. The carriage is then raised by a
crane to a level with the furnace, and entered upon the grooved bars which lie upon the
hearth. That no heat may be lost, two carriages are provided, the one being ready to
put in as the other is taken out; the furnace is meanwhile uniformly kept hot. This
method, however convenient for moving the sheets in and out, wastes a good deal of fuel
in heating the iron carriage.
The principal places in which brass is manufactured on the great scale in England,
are Bristol, Birmingham, and Holywell, in North Wales.
The French writers affirm, that a brass, containing 2 per cent. of lead, works more
freely in the turning lathe, but does not hammer so well as the mere alloy of copper
and zinc.
At the brass manufactory of Hegermühl, upon the Finon canal near Potsdam, the
following are the materials of one charge; 41 pounds of old brass, 55 pounds refined
copper (gahrkupfer) granulated; and 24 pounds of zinc. This mixture, weighing 120
pounds, is distributed into four crucibles, and fused in a wind furnace with pitcoal fuel.
The waste varies from 21⁄2 to 4 pounds upon the whole.
Fig. 159. represents the furnace as it was formerly worked there with charcoal; a, the
laboratory in which the crucibles were placed. It was walled with fire bricks. The foundations
and the filling-in walls were formed of stone rubbish, as being bad conductors of
heat; sand and ashes may be also used; b, cast-iron circular grating plates pierced with[167]
12 holes (see fig. 160.), over them a sole of loam, c, is beat down, and perforated with holes
corresponding to those in the iron discs; d, the ash-pit; e, the bock, a draught flue which
conducts the air requisite to the combustion,
from a sunk tunnel, in communication
with several melting furnaces. The
terrace or crown of the furnace, f, lies on
a level with the foundry floor, h h, and is
shut with a tile of fire-clay, g, which may
be moved in any direction by means of
hooks and eyes in its binding iron ring.
Fig. 161. the tongs for putting in and taking
out the charges, as viewed from above and
from the side.
Figs. 162, 163. represent the furnaces
constructed more recently for the use of
pitcoal fuel; fig. 162. being an upright
section, and fig. 163. the ground plan. In
this furnace the crucibles are not surrounded
with the fuel, but they receive
the requisite melting heat from the flame
proceeding from the grate upon which it
is burned. The crucibles stand upon 7 binding arches, a, which unite in the middle at
the key-stone b, fig. 163. Between the arches are spaces through which the flame rises from
the grate c. d is the fire-door; e, a
sliding tile or damper for regulating
or shutting off the air-draught; f an
inclined plane, for carrying off the
cinders that fall through the grate,
along the draught tunnel g, so that
the air in entering below may not be
heated by them.
The crucibles are 16 inches deep,
91⁄2 wide at the mouth, 61⁄2 at the
bottom; with a thickness in the sides
of 1 inch and 11⁄2 below; they stand from 40 to 50 meltings. The old brass, which
fills their whole capacity, is first put in and melted down; the crucibles are now
taken out, and are charged with the half of the zinc in pieces of from 1 to 3
cubic inches in size, covered over with coal ashes; then one half of the copper charge is
introduced, again coal-dust; and thus the layers of zinc and copper are distributed
alternately with coal-ashes betwixt them, till the whole charge gets finally fused. Over
all, a thicker layer of carbonaceous matter is laid, to prevent oxidizement of the brass.
Eight crucibles filled in this way are put into the furnace between the 11 holes of the
grate shelf; and over them two empty crucibles are laid to be heated for the casting
operation. In from 31⁄2 to 4 hours the brass is ready to be poured out. Fifteen English
bushels of coals are consumed in one operation; of which six are used at the introduction
of the crucibles, and four gradually afterwards.
When sheet brass is to be made the following process is pursued:—
An empty crucible, called a caster (giesser), is taken out of the furnace through the
crown with a pair of tongs, and is kept red hot by placing it in a hollow hearth (mundal),
surrounded with burning coals; into this crucible the contents of four of the melting
pots are poured; the dross being raked out with an iron scraper. As soon as the melting
pot is emptied, it is immediately re-charged in the manner above described, and replaced
in the furnace. The surface of the melted brass in the caster is swept with the
stump of a broom, then stirred about with the iron rake, to bring up any light foreign
matter to the surface, which is then skimmed with a little scraper; the crucible is now
seized with the casting tongs, and emptied in the following way:—
The mould or form for casting sheet brass consists of two slabs of granite, a a, figs.
164, 165. They are 51⁄2 feet long; 3 feet broad, 1 foot thick, and, for greater security,
girt with iron bands, b b, 2 inches broad, 11⁄2 thick, and joined at the four corners with
bolts and nuts. The mould rests upon an oaken block, c, 31⁄2 feet long, 21⁄6 broad, and 11⁄4
thick, which is suspended at each end upon gudgeons, in bearing blocks, placed under the
foundery floor, d d, in the casting pit, e e. This is lined with bricks; and is 63⁄4 feet long,
51⁄2 broad, and 2 deep; upon the two long side walls of the pit, the bearing blocks are
laid, which support the gudgeons. The swing-blocks are 10 inches long, 18 inches
broad, 15 inches thick, and are somewhat rounded upon their back edge, so that the
casting frame may slope a little to the horizon. To these blocks two cross wooden arms,
f f, are mortised, upon which the under slab rests, freely, but so as to project about 5 inches[168]
backwards over the block, to secure an equipoise in the act of casting. g g are bars,
placed at both of the long sides, and one of the ends, between the slabs, to determine the
thickness of the brass-plate. Upon the other slab the gate h is fastened, a sheet of
iron 6 inches broad, which has nearly the shape of a parallel trapezium (lozenge), and
slopes a little towards the horizon. It serves for setting the casting pot upon in the act
of pouring out, and renders its emptying more convenient. That gate (steinmaul)
is coated with a mixture of loam and hair. The upper slab is secured to the under one
in its slanting position by an armour or binding. This consists of the tension bars of
wood, i k l m, of the iron bars n, (3 to 31⁄2 inches broad, 11⁄2 inch thick, see the top view,
fig. 165.) of a rod with holes and pins at its upper end, and of the iron screw spindle o.
The mode in which these parts act may be understood from inspection of the figure. In
order to lift the upper slab from the under one, which is effected by turning it round its
edge, a chain is employed, suspending two others, connected with the slab. The former
passes over a pulley, and may be pulled up and down by means of a wheel and axle, or
with the aid of a counterweight. Upon each of the two long sides of the slab there are
two iron rings, to which the ends of the chains may be hooked. The casting faces of the
slab must be coated with a layer of finely ground loam; the thinner the better.
When calamine is employed, 1⁄2 cwt. of copper, 3⁄4 cwt. of calamine, and 1⁄3 the volume
of both of charcoal mixed, are put into 7 crucibles, and exposed to heat during 11 or 12
hours; the product being from 70 to 72 lbs. of brass.
Brass-plate rolling.—At Hegermühl there are two re-heating or annealing furnaces,
one larger, 18 feet long, and another smaller, 81⁄2; the hot chamber is separated from the
fire place by iron beams, in such a way that the brass castings are played upon by the
flames on both their sides. After each passage through the laminating press (rolls) they
are heated anew, then cooled and laminated afresh, till they have reached the proper
length. The plates are besmeared with grease before rolling.
Fig. 166. shows the ground plan of the furnace and its railway; fig. 167. the cross section;
and fig. 168. the section lengthwise; a a, the iron way bars or rails upon the floor of the[169]
foundry, for enabling the wheels of the waggon-frame to move readily backwards and
forwards; b b, the two grates; c c, the ash pits; d d, the fire beams; e e e, vents in the
roof of the hot chamber f; g g, two plates for shutting the hot chamber; h, the flue;
i, the chimney. After the rolling, the sheets covered with a black oxide of copper, are
plunged into a mother water of the alum works for a few minutes, then washed in clean
water, and lastly, smeared with oil and scraped with a blunt knife.
In rough brass and brass wares, no less than 16,240 cwts. were manufactured in the
Prussian States in the year 1832.
For musical purposes, the brass wire made in Berlin, has acquired great and merited
celebrity; but that of Birmingham is now preferred even by foreigners.
Brass Colour, for staining glass, is prepared by exposing for several days thin plates
of brass upon tiles in the leer or annealing arch of the glass-house, till it be oxidized
into a black powder, aggregated in lumps. This being pulverized and sifted, is to be
again well calcined for several days more, till no particles remain in the metallic state;
when it will form a fine powder of a russet brown colour. A third calcination must now
be given, with a carefully regulated heat; its quality being tested from time to time by
fusion with some glass. If it makes the glass swell, and intumesce, it is properly prepared;
if not, it must be still farther calcined. Such a powder communicates to glass,
greens of various tints, passing into turquoise.
When thin narrow strips of brass are stratified with sulphur in a crucible, and calcined
at a red heat, they become friable, and may be reduced to powder. This being sifted
and exposed upon tiles in a reverberatory furnace for ten or twelve days, becomes fit for
use, and is capable of imparting a calcedony, red or yellow tinge to glass by fusion,
according to the mode and proportion of using it.
The glass-makers’ red colour may be prepared by exposing small plates of brass to a
moderate heat in a reverberatory furnace, till they are thoroughly calcined, when the
substance becomes pulverulent, and assumes a red colour. It is then ready for
immediate use.
Brass Colour, as employed by the colourmen to imitate brass, is of two tints, the
red or bronze, and the yellow like gilt brass. Copper filings mixed with red ochre or
bole, constitute the former; a powdered brass imported from Germany is used for the
latter. Both must be worked up with varnish after being dried with heat, and then spread
with a flat camel-hair brush evenly upon the surface of the object. The best varnish is
composed of 20 ounces of spirits of wine, 2 ounces of shellac, and 2 ounces of sandarach,
properly dissolved. See Varnish. Only so much of the brass powder and varnish
should be mixed at a time as is wanted for immediate use.
Brass Foil. Dutch leaf, called Knitter or Rauschgold in Germany, is made from
a very thin sheet brass, beat out under a hammer worked by water power, which gives
300 or 400 strokes per minute; from 40 to 80 leaves being laid over each other. By
this treatment it acquires its characteristic solidity and lustre. See above, the process
for converting the copper superficially into brass by the fumes of zinc.
BRAZING. (Braser, Fr.; Messing-lothung, Germ.) The soldering together of
edges of iron, copper, brass, &c., with an alloy consisting of brass and zinc, sometimes
with a little tin or silver. The surfaces to be thus united must be filed perfectly
bright, and not be soiled with the fingers or in any other way. The granular or nearly
pulverulent alloy is usually wetted with a paste of ground borax and water, applied in
this state, dried, and then exposed carefully to bright ignition at a clear forge fire.
Some workmen enclose the part to be soldered in a clay lute, but others prefer leaving
it uncovered, that they may see when the solder has flowed freely, and entered into all
the seams.
BRAZIL-WOOD. (Bois de Fernambouc, Fr.; Brasilienholz, Germ.) This dye-wood
derives its name from the part of America whence it was first imported. It has
also the names Fernambuca, wood of Saint Martha, and of Sapan, according to the
places which produce it. Linnæus distinguishes the tree which furnishes the Brazil
wood by the name of Cæsalpinia crista. It commonly grows in dry places among rocks.
Its trunk is very large, crooked, and full of knots. It is very hard, susceptible of a
fine polish, and sinks in water. It is pale when newly cleft, but becomes red on
exposure to the air.
It has different shades of red and orange. Its goodness is determined particularly by
its density. When chewed, a saccharine taste is perceived. It may be distinguished
from red saunders wood, as the latter does not yield its colour to water.
Boiling water extracts the whole colouring matter of Brazil-wood. If the ebullition
be long enough continued, it assumes a fine red colour. The residuum appears black.
In this case, an alkali may still extract much colouring matter. The solution in alcohol
or ammonia is still deeper than the preceding.
The decoction of Brazil-wood, called juice of Brazil, is observed to be less fit for
dyeing when recent, than when old or even fermented. By age it takes a yellowish-red[170]
colour. For making this decoction, Hellot recommends to use the hardest water;
but it should be remarked, that this water deepens the colour in proportion to the earthy
salts which it contains. After boiling this wood reduced to chips, or, what is preferable,
to powder, for three hours, this first decoction is poured into a cask. Fresh water is
poured on the wood, which is then made to boil for three hours, and mixed with the
former. When Brazil-wood is employed in a dyeing bath, it is proper to enclose it in
a thin linen bag, as well as all the dye woods in general.
Wool immersed in the juice of Brazil takes but a feeble tint, which is speedily
destroyed. It must receive some preparations.
The wool is to be boiled in a solution of alum, to which a fourth or even less of
tartar is added, for a larger proportion of tartar would make the colour yellowish. The
wool is kept impregnated with it for at least eight days, in a cool place. After this, it
is dyed in the Brazil juice with a slight boiling. But the first colouring particles that
are deposited, afford a less beautiful colour; hence it is proper to pass a coarser stuff
previously through the bath. In this manner a lively red is procured, which resists
pretty well the action of the air.
Brazil-wood is made use of for dyeing silk what is called false crimson, to distinguish
it from the crimson made by means of cochineal, which is much more permanent.
The silk should be boiled at the rate of 20 parts of soap per cent., and then alumed.
The aluming need not be so strong as for the fine crimson. The silk is refreshed at
the river, and passed through a bath more or less charged with Brazil juice, according to
the shade to be given. When water free from earthy salts is employed, the colour is
too red to imitate crimson; this quality is given it by passing the silk through a slight
alkaline solution, or by adding a little alkali to the bath. It might, indeed, be washed
in a hard water till it had taken the desired shade.
To make deeper false crimsons of a dark red, juice of logwood is put into the Brazil
bath after the silk has been impregnated with it. A little alkali may be added,
according to the shade that is wanted.
To imitate poppy or flame colour, an annotto ground is given to the silk, deeper even
than when it is dyed with carthamus. It is washed, alumed, and dyed with juice of
Brazil, to which a little soap water is usually added.
The colouring particles of Brazil wood are easily affected, and made yellow by the
action of acids.
They thus become permanent colours. But what distinguishes them from madder
and kermes, and approximates them to cochineal, is their reappearing in their natural
colour, when they are thrown down in a state of combination with alumina, or with
oxide of tin. These two combinations seem to be the fittest for rendering them durable.
It is requisite, therefore, to inquire what circumstances are best calculated to promote
the formation of these combinations, according to the nature of the stuff.
The astringent principle, likewise, seems to contribute to the permanence of the colouring
matter of Brazil wood; but it deepens its hue, and can only be employed for
light shades.
The colouring particles of Brazil wood are very sensible to the action of alkalies
which give them a purple hue; and there are several processes in which the alkalies,
either fixed or volatile, are used for forming violets and purples. But the colours obtained
by these methods, which may be easily varied according to the purpose, are
perishable, and possess but a transient bloom. The alkalies appear not to injure the
colours derived from madder, but they accelerate the destruction of most other
colours.
In England and Holland the dye-woods are reduced to powder by means of mills
erected for the purpose.
The bright fugitive red, called fancy red, is given to cotton by Nicaragua, or peachwood,
a cheap kind of Brazil wood.
The cotton being scoured and bleached, is boiled with sumach. It is then impregnated
with a solution of tin (at 5° Baumé, according to Vitalis). It should now be
washed slightly in a weak bath of the dyeing wood, and lastly, worked in a somewhat
stale infusion of the peach or Brazil wood. When the temperature of this is lukewarm,
the dye is said to take better. Sometimes two successive immersions in the bath are
given. It is now wrung out, aired, washed in water, and dried.
M. Vitalis says, that his solution of tin is prepared with two ounces of tin and a pound
of aqua regia made with two parts of nitric acid at 24° Baumé, and three parts of muriatic
acid at 22°.
For a rose colour, the cotton is alumed as usual, and washed from the alum. It then
gets the tin mordant, and is again washed. It is now turned through the dye-bath,
an operation which is repeated if necessary.
For purple a little alum is added to the Brazil bath.
1. For amaranth, the cotton is strongly galled, dried, and washed.
[171]
2. It is passed through the black cask (tonneau noir), see Black Dye, till it has
taken a strong grey shade.
3. It receives a bath of lime water.
4. Mordant of tin.
5. Dyeing in the Brazil wood bath.
6. The two last operations are repeated.
Dingler has endeavoured to separate the colouring matter of the different sorts of
Brazil wood, so as to obtain the same tint from the coarser as from the best Pernambuco.
His process consists in treating the wood with hot water or steam, in concentrating the
decoction so as to obtain 14 or 15 pounds of it from 4 pounds of wood, allowing it to
cool, and pouring into it two pounds of skim milk; agitating, then boiling for a few
minutes, and filtering. The dun colouring matters are precipitated by the coagulation
of the caseous substance. For dyeing, the decoctions must be diluted with water; for
printing they must be concentrated, so that 4 pounds of wood shall furnish only 5 or 6
pounds of decoction, and the liquor may be thickened in the ordinary way. These
decoctions may be employed immediately, as by this treatment they have acquired the
same property as they otherwise could get only by being long kept. A slight fermentation
is said to improve the colour of these decoctions; some ground wood is put into
the decoction to favour this process.
As gelatine produces no precipitate with these decoctions, they consequently contain
no tannin. Gall-nuts, however, sumach, the bark of birch or alder, render the colour of
Brazil wood more durable, upon alumed linen and cotton goods, but the shade is a
little darker.
In dyeing wool with Pernambuco, the temperature of the bath should never be above
150° Fahr., since higher heats impair the colour.
According to Dingler and Kurrer, bright and fast scarlet reds may be obtained upon
wool, by preparing a decoction of 50 pounds of Brazil wood in three successive boils,
and setting the decoction aside for 3 or 4 weeks in a cool place; 100 pounds of the wool
are then alumed in a bath of 22 pounds of alum and 11 pounds of tartar, and afterwards
rinsed in cold water. Meanwhile we fill two-thirds with water, a copper containing 30
pails, and heated to the temperature of 150° or 160° F. We pour in 3 pailfuls of the decoction,
heat to the same point again, and introduce 30 pounds of wool, which does not
take a scarlet, but rather a crimson tint. This being removed, 2 pails of decoction are
put in, and 30 pounds of wool which becomes scarlet, but not so fine as at the third
dip. If the dyer strengthens the colour a little at the first dip, a little more at the
second, and adds at the third and fourth the quantity of decoction merely necessary, he
will obtain an uniform scarlet tint. With 50 pounds of Pernambuco 1000 pounds of
wool may be dyed scarlet in this way, and with the deposits another 100 may be
dyed of a tile colour. An addition of weld renders the colour faster but less brilliant.
Karkutsch says the dye may be improved by adding some ox-gall to the bath.
In dyeing cotton the tannin and gallic acid are two necessary mordants, and the colour
is particularly bright and durable, when the cloth has been prepared with the oily process
of Turkey red.
It is said that stale urine heightens the colour of the Brazil dye when the ground wood
is moistened with it.
The quantity of Brazil or Nicaragua wood imported into the United Kingdom in 1835,
was 6,242 tons, whereof 1,811 were exported; of Brazilietto 230 tons. The duty upon
the first article is 5s. per ton.
BREAD (Pain, Fr.; Brod, Germ.) is the spongy mass produced by baking the
leavened or fermented dough of wheat or rye flour, at a proper heat. It is the principal
food of highly civilized nations. The skilful preparation of this indispensable article constitutes
the art of the Baker. Dough baked without being fermented constitutes cakes
or biscuits; but not bread strictly speaking.
Pliny informs us, that barley was the only species of corn at first used for food; and
even after the method of reducing it to flour had been discovered, it was long before
mankind learned the art of converting it into cakes.
Ovens were first invented in the East. Their construction was understood by the
Jews, the Greeks, and the Asiatics, among whom baking was practised as a distinct profession.
In this art, the Cappadocians, Lydians, and Phœnicians, are said to have particularly
excelled. It was not till about 580 years after the foundation of Rome, that
these artisans passed into Europe. The Roman armies, on their return from Macedonia,
brought Grecian bakers with them into Italy. As these bakers had handmills beside
their ovens, they still continued to be called pistores, from the ancient practice of bruising
the corn in a mortar; and their bakehouses were denominated pistoriæ. In the time
of Augustus there were no fewer than 329 public bakehouses in Rome; almost the whole
of which were in the hands of Greeks, who long continued the only persons in that city
acquainted with the art of baking good bread.
[172]
In nothing, perhaps, is the wise and cautious policy of the Roman government more
remarkably displayed, than in the regulations which it imposed on the bakers within
the city. To the foreign bakers who came to Rome with the army from Macedonia, a
number of freedmen were associated, forming together an incorporation from which
neither they nor their children could separate, and of which even those who married the
daughters of bakers were obliged to become members. To this incorporation were entrusted
all the mills, utensils, slaves, animals, every thing, in short, which belonged to
the former bakehouses. In addition to these, they received considerable portions of land;
and nothing was withheld, which could assist them in pursuing, to the best advantage,
their highly prized labours and trade. The practice of condemning criminals and slaves,
for petty offences, to work in the bakehouse, was still continued; and even the judges of
Africa were bound to send thither, every five years, such persons as had incurred that
kind of chastisement. The bakehouses were distributed throughout the fourteen divisions
of the city, and no baker could pass from one into another without special permission.
The public granaries were committed to their care; they paid nothing for the corn employed
in baking bread that was to be given in largess to the citizens; and the price of
the rest was regulated by the magistrates. No corn was given out of these granaries except
for the bakehouses, and for the private use of the prince. The bakers had besides
private granaries, in which they deposited the grain, which they had taken from the public
granaries for immediate use; and if any of them happened to be convicted of having
diverted any portion of the grain to another purpose, he was condemned to a ruinous
fine of five hundred pounds weight of gold.
Most of these regulations were soon introduced among the Gauls; but it was long
before they found their way into the more northern countries of Europe. Borrichius
informs us that in Sweden and Norway, the only bread known, so late as the middle of
the 16th century, was unleavened cakes kneaded by the women. At what period in our
own history the art of baking became a separate profession, we have not been able to ascertain;
but this profession is now common to all the countries in Europe, and the process
of baking is also nearly the same.
The French, who particularly excel in the art of baking, have a great many different
kinds of bread. Their pain bis, or brown bread, is the coarsest kind of all, and is made
of coarse groats mixed with a portion of white flour. The pain bis blanc, is a kind of
bread between white and brown, made of white flour and fine groats. The pain blanc,
or white bread, is made of white flour, shaken through a sieve after the finest flour has
been separated. The pain mollet, or soft bread, is made of the purest flour without any
admixture. The pain chaland, or customers’ bread, is a very white kind of bread, made
of pounded paste. Pain chapelé, is a small kind of bread, with a well-beaten and very
light paste, seasoned with butter or milk. This name is also given to a small bread, from
which the thickest crust has been removed by a knife. Pain cornu, is a name given by
the French bakers to a kind of bread made with four corners, and sometimes more. Of
all the kinds of small bread, this has the strongest and firmest paste. Pain à la reine,
queen’s bread, pain à la Sigovie, pain chapelé, and pain cornu, are all small kinds of bread,
differing only in the lightness or thickness of the paste. Pain gruau is a small very
white bread made now in Paris, from the flour separated after a slight grinding from
the best wheat. Such flour is in hard granular particles.
In this country we have fewer varieties of bread, and these differ chiefly in their degrees
of purity. Our white or fine bread is made of the purest flour; our wheaten
bread, of flour with a mixture of the finest bran; and our household bread, of the whole
substance of the grain without the separation either of the fine flour or coarse bran. We
have also symnel bread, manchet or roll bread, and French bread, which are all made of
the purest flour from the finest wheat; the roll bread being improved by the addition
of milk, and the French bread by the addition of eggs and butter. To these may be
added gingerbread, a cake made of flour, with almonds, liquorice, aniseed, rose-water,
and sugar or treacle; and mastlin bread, made of wheat and rye, or sometimes of wheat
and barley. We have various kinds of small bread, having various names, according to
their various forms. They are, in general, extremely light, and are sweetened with
sugar, currants, and other palatable ingredients. In Scotland there is a cake called
short bread, made from a pretty thick dough, enriched with butter, sweetened with sugar,
and seasoned with orange peel, or other kinds of spices.
The process of making bread is nearly the same in all the countries of modern Europe;
though the materials of which it as composed vary with the farinaceous productions of
different climates and soils. The flour of wheat is most generally employed for this purpose,
wherever that vegetable can be reared. This flour is composed of a small portion
of mucilaginous saccharine matter, soluble in cold water, from which it may be separated
by evaporation; of a great quantity of starch, which is scarcely soluble in cold water, but
capable of combining with that fluid by means of heat; and an adhesive gray substance
called gluten, insoluble in water, ardent spirit, oil, or ether, and resembling an animal[173]
substance in many of its properties. Flour kneaded with water, forms a tough rather indigestible
paste containing all the constituent parts which we have enumerated. Heat
produces a considerable change on the glutinous part of this compound, and renders it
more easy of mastication and digestion. Still, however, it continues heavy and tough, compared
with bread which is raised by leaven or yeast. Leaven is nothing more than a piece
of dough, kept in a warm place till it undergoes a process of fermentation; swelling, becoming
spongy, or full of air bubbles, at length disengaging an acidulo-spirituous vapour,
and contracting a sour taste. When this leaven is mingled in proper proportions with
fresh-made dough, it makes it rise more readily and effectually than it would do alone,
and gives it at the same time a greater degree of firmness. Upon the quality of the leaven
employed, the quality of the bread materially depends.
The principal improvement which has been made on bread in modern times, is the
substitution of yeast or barm in place of common leaven. This yeast is the viscid froth
that rises to the surface of beer, in the first stage of its fermentation. When mixed with
the dough, it makes it rise much more speedily and effectually than ordinary leaven,
and the bread is of course much lighter, and freer from that sour and disagreeable taste
which may often be perceived in bread raised with leaven, either because too much is
mingled with the paste, or because it has been allowed to advance too far in the process
of fermentation.
Bread properly raised and baked, differs materially from unleavened cakes, not only
in being less compact and heavy, and more agreeable to the taste, but in losing its
tenacious and glutinous qualities, and thus becoming more salutary and digestible.
We possess several analyses of wheat flour. Ordinary wheat (triticum hybernum
mixed with triticum turgidum) contains, according to the analyses made by Vauquelin of
several species of wheat flour, the following substances:—
| Species of Wheat. |
Water. |
Gluten. |
Starch. |
Sugar. |
Gum. |
Bran. |
Total. |
Water
of dough. |
| French wheat flour |
10·0 |
10·96 |
71·49 |
4·72 |
3·32 |
- |
100·49 |
50·3 |
Hard wheat of
Odessa flour |
12·0 |
14·55 |
56·50 |
8·48 |
4·90 |
2·3 |
98·73 |
51·2 |
Soft wheat of
Odessa flour |
10·0 |
12·00 |
62·00 |
7·56 |
5·80 |
1·2 |
98·42 |
54·8 |
| Same sort of flour |
8·0 |
12·10 |
70·84 |
4·90 |
4·60 |
- |
100·41 |
37·4 |
| Same sort of flour |
12·0 |
7·30 |
72·00 |
5·42 |
3·30 |
- |
100·02 |
37·2 |
Wheat of the
French bakers |
10·0 |
10·20 |
72·80 |
4·20 |
2·80 |
- |
100·00 |
40·6 |
Flour of the Paris
hospitals (2d quality) |
8·0 |
10·30 |
71·20 |
4·80 |
3·60 |
- |
97·90 |
37·8 |
| Ditto (3d quality) |
12·0 |
9·02 |
67·78 |
4·80 |
4·60 |
2·0 |
100·21 |
37·8 |
The following table of analyses merits also a place here.
| Species of Flour. |
Water. |
Gluten. |
Starch. |
Sugar. |
Gummi-
gluten. |
Albu-
men. |
Bran. |
| Flour of the triticum spelta |
1 |
22· |
|
74· |
|
5· |
50 |
1· |
|
1·50 |
|
| Ditto triticum hybernum |
1 |
24· |
|
68· |
|
5· |
0 |
1· |
|
1·50 |
|
| Ditto common wheat |
- |
12· |
5 |
74· |
5 |
12· |
|
2· |
|
|
|
| Ditto wheat and rye mixed (mastlin) |
6 |
9· |
80 |
75· |
50 |
4· |
22 |
3· |
28 |
- |
1·2 |
The first two of the above analyses were made by Vogel, the third by Proust, and the
fourth by Vauquelin.
Analyses of the flour of some other corns.
| Species of Flour. |
Starch. |
Mucilage. |
Gluten. |
Albumen. |
Sugar. |
Husk. |
Hordein. |
| White oatmeal |
59·00 |
2· |
5 |
- |
4·30 |
8·25 |
Of a fat oil, 2 |
|
| Barley meal |
32·00 |
9· |
|
3· |
- |
Of resin, 2 |
- |
55 |
The first analysis is by Vogel, the second by Proust.
It deserves to be remarked, that the flour of Odessa contains a much greater quantity
of sugar than the French flour. The substance indicated in the preceding table by the
name of gluten, is the gluten of Beccaria, that is to say, a mixture of gluten and vegetable[174]
albumen. The gum of wheat is not quite identical with ordinary gum. It is a brown
azotised substance, which, when treated by nitric acid, affords no mucic acid, but oxalic
acid and the bitter principle of Welter. It contains besides superphosphate of lime.
The last column of the first table exhibits the quantity of water necessary to convert
the flour into dough of the ordinary consistence, and it is usually proportional to the
quantity of gluten. The hard wheat of Odessa forms an exception in this respect; the
reason of the difference being that the starch contained in this flour is not as in ordinary
flour in a fine powder, but in small transparent grains, which resemble pounded gum,
and absorb less water than pulverulent starch.
The triticum monococcon, according to Zenneck, contains in its unsifted flour, 16·334
of gluten and vegetable albumen; 64·838 of starch; 11·347 of gum, sugar, and extractive;
7·481 of husks. The sifted flour affords 15·536 of gluten and vegetable albumen;
76·459 of starch; 7·198 of sugar, gum, and extractive; 0·807 of husky matter. It is
difficult to conceive how such great quantities of gluten, albumen, and extractive matter,
could disappear in the sifting. The triticum spelta contains in 100 parts of the finest
flour, 22·5 of a soft and humid gluten, mixed with vegetable albumen; 74 of starch,
and 5·5 of sugar. Here we have an excess of 2 parts in the 100.
Wheat furnishes very little ashes by incineration, not more than 0·15 per cent. of the
weight; containing superphosphates of soda, lime, and magnesia.
The object of baking is to combine the gluten and starch of the flour into a homogeneous
substance, and to excite such a vinous fermentative action, by means of its
saccharine matter, as shall disengage abundance of carbonic acid gas in it for making an
agreeable, soft, succulent, spongy, and easily digestible bread. The two evils to be
avoided in baking are, hardness on the one hand, and pastiness on the other. Well-made
bread is a chemical compound, in which the gluten and starch cannot be recognized
or separated, as before, by a stream of water. When flour is kneaded into a dough,
and spread into a cake, this cake, when baked, will be horny if it be thin, or if thick, will
be tough and clammy; whence we see the value of that fermentative process, which
generates thousands of little cells in the mass or crumb, each of them dry, yet tender
and succulent, through the intimate combination of the moisture. By this constitution
it becomes easily soluble in the juices of the stomach, or in other words, light of
digestion. It is moreover much less liable to turn sour than cakes made from unfermented
dough.
Rye, which also forms a true spongy bread, though inferior to that of wheat, consists
of similar ingredients; namely, 61·07 of starch; 9·48 of gluten; 3·28 of vegetable
albumen; 3·28 of uncrystallizable sugar; 11·09 of gum; 6·38 of vegetable fibre; the
loss upon the 100 parts amounted to 5·62, including an acid whose nature the analyst,
M. Einhof, did not determine. Rye flour contains also several salts, principally the
phosphates of lime and magnesia. This kind of grain forms a dark-coloured bread
reckoned very wholesome; comparatively little used in this country, but very much in
France, Germany, and Belgium.
Dough fermented with the aid either of leaven or yeast, contains little or none of the
saccharine matter of the flour, but in its stead a certain portion, nearly half its weight, of
spirit, which imparts to it a vinous smell, and is volatilized in the oven; whence it might
be condensed into a crude weak alcohol, on the plan of Mr. Hick’s patent, were it worth
while. But the increased complexity of the baking apparatus, will probably prove
an effectual obstacle to the commercial success of this project, upon which already
upwards of 20,000l. sterling have been squandered.
That the sugar of the flour is the true element of the fermentation preposterously
called panary, which dough undergoes, and that the starch and gluten have nothing to
do with it, may be proved by decisive experiments. The vinous fermentation continues
till the whole sugar is decomposed, and no longer; when if the process be not checked
by the heat of baking, the acetous fermentation will supervene. Therefore if a little
sugar be added to a flour which contains little or none, its dough will become susceptible
of fermenting, with extrication of gas, so as to make spongy succulent bread. But since
this sponginess is produced solely by the extrication of gas, and its expansion in the heat
of the oven, any substance capable of emitting gas, or of being converted into it under
these circumstances, will answer the same purpose. Were a solution of bicarbonate of
ammonia obtained by exposing the common sesqui-carbonate in powder for a day to the
air, incorporated with the dough, in the subsequent firing it will be converted into
vapour, and in its extrication render the bread very porous. Nay, if water highly
impregnated with carbonic acid gas be used for kneading the dough, the resulting bread
will be somewhat spongy. Could a light article of food be prepared in this way, then
as the sugar would remain undecomposed, the bread would be so much the sweeter, and
the more nourishing. How far a change propitious to digestion takes place in the
constitution of the starch and gluten, during the fermentative action of the dough, has
not been hitherto ascertained by precise experiments. Medical practitioners, who[175]
derive an enormous revenue from dyspepsia, should take some pains to investigate this
subject.
Dr. Colquhoun, in his able essay upon the art of making bread, has shown that its texture
when prepared by a sudden formation and disengagement of elastic fluid generated
within the oven, differs remarkably from that of a loaf which has been made after the
preparatory fermentation with yeast. Bread which has been raised with the common
carbonate of ammonia as used by the pastry-cooks, is porous no doubt, but not spongy
with vesicular spaces, like that made in the ordinary way. The former kind of bread
never presents that air-cell stratification which is the boast of the Parisian baker,
but which is almost unknown in London. I have found it moreover very difficult to
expel by the oven the last portion of the ammonia, which gives both a tinge and a taste
to the bread. I believe, however, that the bicarbonate would be nearly free from this
objection, which operates so much against the sesqui-carbonate of the shops.
In opposition to Mr. Edlin’s account of the excellent quality of bread made by impregnating
dough with carbonic acid gas[10], Dr. Colquhoun adduces Vogel’s experiments,
which show that such dough, when baked, after having been kept in a warm situation
during the usual time, afforded nothing better than a hard cake, which had no resemblance
to common bread. Vogel further states, as illustrative of the general necessity
of providing a sufficient supply of disengaged elastic fluid within the dough, before
baking it at all, that when he made various attempts to form a well-raised vesicular
loaf, within the oven, by mixing flour with carbonate of magnesia, or with zinc filings,
and then kneading it into a paste by means of water, acidulated with sulphuric acid, he
always met with complete failure and disappointment. Dr. Colquhoun performed a series
of well-devised experiments on this subject, which fully confirmed Vogel’s results, and
prove that a proper spongy bread cannot be made by the agency of either carbonic acid
water, or of mixtures of sesqui-carbonate of soda, and tartaric acid. The bread proved
doughy and dense in every case, though less so with the latter mixture than the former.
No loaf bread can, indeed, be well made by any of these two extemporaneous systems,
because they are inconsistent with the thorough kneading of the dough. It is this process
which renders dough at once elastic enough to expand when carbonic acid gas is
generated within it, and cohesive enough to confine the gas when it is generated. The
whole gas of the loaf is disengaged in its interior by a continuous fermentation, after
all the processes of kneading have been finished; for the loaf, after being kneaded,
weighed out, and shaped, is set aside till it expands gradually to double its bulk, before
it is put into the oven. But when a dough containing sesqui-carbonate of soda is mixed
with one containing muriatic acid, in due proportions to form the just dose of culinary
salt, the gas escapes during the necessary incorporation of the two, and the bread formed
from it is dense and hard. Dr. Whiting has, however, made this old chemical process
the subject of a new patent for baking bread.
When the baker prepares his dough, he takes a portion of the water needed for
the batch, having raised its temperature to from 70° to 100° F., dissolves a certain
proportion of his salt in it, then adds the yeast, and a certain quantity of his flour.
This mixture, called the sponge, is next covered up in the small kneading-trough,
alongside of the large one, and let alone for setting in a warm situation. In about an
hour, signs of vinous fermentation appear, by the swelling and heaving up of the sponge,
in consequence of the generation of carbonic acid; and if it be of a semi-liquid consistence,
large air bubbles will force their way to the surface, break, and disappear in
rapid succession. But when the sponge has the consistence of thin dough, it confines the
gas, becomes thereby equably and progressively inflated to double its original volume;
when no longer capable of containing the pent-up air, it bursts and subsides. This
process of rising and falling alternately might be carried on during twenty-four hours,
but the baker has learned by experience to guard against allowing full scope to the fermentative
principle. He generally interferes after the first, or at furthest after the
second or third dropping of the sponge; for were he not to do so, the bread formed with
such dough would invariably be found sour to the taste and the smell. Therefore he
adds at this stage to the sponge the reserved proportions of flour, salt, and water, which
are requisite to make the dough of the desired consistence and size; and next incorporates
the whole together by a long and laborious course of kneading. When this
operation has been continued till the fermenting and the fresh dough have been intimately
blended, and till the glutinous matter of both is worked into such union and consistence
that the mass becomes so tough and elastic as to receive the smart pressure of the
hand without adhering to it, the kneading is suspended for some time. The dough is
now abandoned to itself for a few hours, during which it continues in a state of active
fermentation throughout its entire mass. Then it is subjected to a second but much
less laborious kneading, in order to distribute the generated gas as evenly as possible[176]
among its parts, so that they may all partake equally of the vesicular structure. After
this second kneading, the dough is weighed out into the portions suitable to the size of
bread desired; which are of course shaped into the proper forms, and once more set
aside in a warm situation. The continuance of the fermentation soon disengages a fresh
quantity of carbonic acid gas, and expands the lumps to about double their pristine
volume. These are now ready for the oven, and when they finally quit it in the baked
state, are about twice the size they were when they went in. The generation of the
due quantity of gas should be complete before the lumps are transferred to the oven;
because whenever they encounter its heat, the process of fermentation is arrested; for
it is only the previously existing air which gets expanded throughout every part of the
loaf, swells out its volume, and gives it the piled and vesicular texture. Thus the well-baked
loaf is composed of an infinite number of cellules filled with carbonic acid gas,
and apparently lined with a glutinous membrane of a silky softness. It is this which
gives the light, elastic porous constitution to bread.
After suffering the fermentative process to exhaust itself in a mass of dough, and the
dough to be brought into that state in which the addition of neither yeast, nor starch,
nor gluten will produce any effect in restoring that action, if we mix in 4 per cent. of
saccharine matter, of any kind, with a little yeast, the process of fermentation will immediately
re-commence, and pursue a course as active and lengthened as at first, and
cease about the same period.[11]
This experiment, taken in connection with the facts formerly stated, proves that what was
called panary fermentation, is nothing but the ancient and well-known process of the vinous
fermentation of sugar, which generates alcohol. There seems to be but one objection to
the adoption of this theory. After the loaf is baked, there is found in its composition
nearly as much saccharine matter as existed in the flour before fermentation. M. Vogel
states that in the baked bread there remains 3·6 parts of sugar, out of the 5 parts which
it originally contained. Thus, in 100 parts of loaf bread prepared with wheaten flour,
distilled water, and yeast without the admixture of any common salt, he found the following
ingredients:—
| |
Sugar |
8· |
6 |
|
| Torrefied or gummy starch |
18· |
0 |
| Starch |
53· |
5 |
| Gluten, combined with a little starch, |
20· |
75 |
| Exclusive of carbonic acid, muriate of lime, phosphate of lime, &c. |
It must be borne in mind that in every loaf the process of fermentation has been prematurely
checked by the baker’s oven, and therefore the saccharine constituent can never
be wholly decomposed. It seems certain, also, that by the action of gluten upon the
starch in the early stage of the firing, a quantity of sugar will be formed by the saccharine
fermentation; which we have explained in treating of Beer.
Several masses of dough were prepared by Dr. Colquhoun in which pure wheat starch
was mixed with common flour, in various proportions. In some of the lumps this starch
had been gelatinized, with the minimum of hot water, before it was added to the flour. After
introducing the usual dose of salt, the dough was thoroughly kneaded, set apart for the
proper period, allowed to ferment in the accustomed way, and then baked in the oven.
In outward appearance, increase of bulk, and vesicular texture, none of them differed
materially from a common loaf, baked along with them for the sake of comparison;
except that when the starch considerably exceeded the proportion of flour in the lump,
the loaf, though whiter, had not risen so well, being somewhat less vesicular. But, on
tasting the bread of each loaf, those which contained most gelatinized starch were
unexpectedly found to be the sweetest. The other loaves, into which smaller quantities
of the gelatinized starch had been introduced, or only some dry starch, had no sweetish
taste whatever to distinguish them from ordinary bread. These facts seem to establish
the conclusion, that the presence of gelatinous starch in bread put into the oven, is a
means of forming a certain portion of saccharine matter within the loaf, during the
baking process. Now it is more than probable that gelatinized starch does exist, more
or less, in all loaves which have been fermented by our usual methods, and hence a
certain quantity of sugar will necessarily be generated at its expense, by the action of
heat. Thus the difficulty started by M. Vogel is sufficiently solved; and there remains
no doubt that, in the saccharine principle of flour, the fermentation has its origin and
end, while dough is under fermentation.
The source of the sourness which supervenes in bread, under careless or unskilful
hands, had been formerly ascribed to each of all the constituents of flour; to its gluten,
its starch, and its sugar; but erroneously, as we now see: for it is merely the result of
the second fermentation which always succeeds the vinous, when pushed improperly too
far. It has been universally taken for granted by authors, that the acid thus generated[177]
in dough is the acetic. But there appear good grounds to believe that it is frequently
a less volatile acid, probably the lactic, particularly when the process has been tardy,
from the imperfection of the yeast or the bad quality of the flour. The experiments of
Vogel, Braconnot, and others, prove that the latter acid is generated very readily, and
in considerable quantity during the spontaneous decomposition of a great many vegetable
substances, when in a state of humidity. The presence of lactic acid would account
for the curious fact, that the acidity of unbaked dough is much more perceptible to the
taste than to the smell; while the sourness of the same piece of bread, after coming out
of the oven, is, on the contrary, much more obvious to the olfactory organs than to the
palate. But this is exactly what ought to happen, if the lactic acid contributes, in
conjunction with the acetic, to produce the acescence of the dough. At the ordinary
temperature of a bakehouse, the former acid, though very perceptible in the mouth, is
not distinguishable by the nostrils; but as it is easily decomposed by heat, no sooner is
it exposed to the high temperature of the oven, than it is resolved, in a great measure,
into acetic acid[12], and thus becomes more manifest to the sense of smell, and less to that
of taste. This theory seems to explain satisfactorily all the phenomena accompanying
the progress of fermentation in baker’s dough, and also some of its results in the process
of baking which do not easily admit of any other solution.
There are extremely simple and effectual methods for enabling the baker to adopt
measures either to prevent or correct the evil of acescence, and these are to neutralize
the acid by the due exhibition of an alkali, such as soda; or an alkaline earth, such as
magnesia or chalk. And it affords a striking proof of how much the artisan has been
accustomed to plod, uninquiring and uninformed, over the same ground, that a remedy
so safe and so economical, should remain at this day unthought of and unemployed by
most of the manufacturers of bread in the United Kingdom. The introduction of a
small portion of carbonate of soda will rectify any occasional error in the result of the
so called panary fermentation, and will, in fact, restore the dough to its pristine sweetness.
The quantity of acetate of soda, which will be thus present in the bread, will be
altogether inconsiderable; and as it has no disagreeable taste, and is merely aperient to
the bowels in a very mild degree, it can form no objection in the eye of the public
police. The restoration of dough thus tainted with acid, and its conversion into
pleasant and wholesome bread, has been sufficiently verified by experiment. But,
according to Mr. Edmund Davy, carbonate of magnesia may be used with still greater
advantage, as during the slow action of the acid upon it, the carbonic acid evolved
serves to open up and lighten bread which would otherwise be dense and doughy from
the indifferent quality of the flour. Here, however, the dangerous temptation lies with
a sordid baker to use cheap or damaged flour, and to rectify the bread made of it by
chemical agents, innocent in themselves, but injurious as masks of a bad raw material.
When sour yeast must be used, as sometimes happens with the country bakers, or in
private houses at a distance from beer breweries, there can be no harm, but, on the
contrary, much propriety, in correcting its acidity, by the addition of as much carbonate
of soda to it as will effect its neutralization, but nothing more. When sour yeast has
been thus corrected, it has been found, in practice, to possess its fermentative power
unimpaired, and to be equally efficacious, with fresh formed yeast, in making good
palatable loaves.
We have seen that, in baking, about one fourth of the starch is converted into a
matter possessing the properties of British gum (see Starch), and also that the gluten,
though not decomposed, has its particles disunited, and is not so tough and adhesive as
it is in the flour. This principle is also, as we have said, useful in cementing all the
particles of the dough into a tenacious mass, capable of confining the elastic fluid
generated by the vinous fermentation of the sugar. Starch is the main constituent,
the basis of nourishment in bread, as well as in all farinaceous articles of food. The
albumen also of the wheat being coagulated by the heat of the oven, contributes to
the setting of the bread into a consistent elastic body.
In the mills in the neighbourhood of London, no less than seven distinct sorts
of flour are ground out of one quantity of wheat. These are for one quarter—
| Fine flour |
5 |
bushels |
3 |
|
pecks. |
| Seconds |
0 |
|
2 |
|
| Fine middlings |
0 |
|
1 |
|
| Coarse middlings |
0 |
|
0 |
·5 |
|
| Bran |
3 |
|
0 |
|
| Twenty-penny |
3 |
|
0 |
|
| Pollard |
2 |
|
|
| |
14 |
|
2 |
·5 |
|
[178]
So that we have nearly a double bulk of flour, or 14 bushels and 21⁄2 pecks from 8 bushels
of wheat. In the sifting of the flour through the bolter, there is a fine white angular meal
obtained called sharps, which forms the central part of the grain. It is consumed partly
by the fine biscuit bakers. The bakers of this country were formerly bound by law to bake
three kinds of bread, the wheaten, standard wheaten, and the household; marked respectively
with a W, S W, and H, and if they omitted to make these marks on their bread
they were liable to a penalty. The size of the loaves were usually peck, half-peck,
quartern, and half-quartern; the weights of which, within 48 hours of their being baked,
should have been respectively 17 lbs. 6 oz.; 8 lbs. 11 oz.; 4 lbs. 5 oz. 8 dr.; and 4 lbs.
2 oz. 14 dr. In general they weigh about one-seventh more before they enter the oven,
or they lose one-seventh of their weight in baking. The French bread loses fully one-sixth
in the oven, owing chiefly to its more oblong thin shape, as compared to the
cubical shape of the English bread. But this loss of weight is very variable, being
dependent upon the quality of the wheaten flour, and the circumstances of baking. The
present law in England defines the quartern loaf at 4 lbs., and subjects the baker to a
penalty if the bread be one ounce lighter than the standard. Hence it leaves the baker
in self-defence, to leave it in rather a damp and doughy state. But there is much light
bread sold in London. I have met with quartern loaves of 3 lbs. 10 ozs. A sack of
flour weighing 280 lbs. was presumed by the framers of our former parliamentary acts,
for the assize of bread, to be capable of being baked into 80 loaves. If this proportion
had been correct, one-fifth part of our quartern loaf must consist of water and salt, and
four-fifths of flour. But in general, of good wheaten flour, three parts will take up one
part of water; so that the sack of flour should have turned out, and actually did turn
out, more than 80 loaves. At present with 4 lb. bread it may well yield 92 loaves.
The following statement of the system of baking at Paris, I received in 1835 from a
very competent judge of the business.
1,000 kilogrammes of wheat = 5 quarters English, cost 200 fr., and yield 800 kilos
of flour of the best white quality, equivalent to 51⁄10 sacks French. Hence the sack of
flour costs 40 francs at the mill, and including the carriage to Paris, it costs 45 or 46
francs.
The profit of the flour dealer is about 31⁄2 francs, and the sale price becomes from 43
to 50 francs.
| Bread manufactured from the above. |
| |
£ |
s. |
d. |
|
£ |
s. |
d. |
| One day’s work of an ordinary baker, who makes four batches in a day, consists of 3 sacks at 50
francs, or 2l. sterling each |
|
6 |
0 |
0 |
|
| Salt 23⁄4 lbs. at 2d. per lb. |
|
0 |
0 |
5 |
1⁄2 |
| Yeast or leaven 3 lbs. at 5d. |
|
0 |
1 |
3 |
|
| Total cost of materials |
|
6 |
1 |
8 |
1⁄2 |
| Expenses of Baking. |
| Three workmen at different rates of wages, 15 francs |
0 |
12 |
0 |
|
| Fire-wood 0, as the charcoal produced pays for it |
| General expenses, such as rent, taxes, interest of capital, &c. |
0 |
12 |
0 |
|
| |
1 |
4 |
0 |
= |
1 |
4 |
0 |
|
| |
7 |
5 |
8 |
1⁄2 |
| For this sum 315 loaves are made, being 105 for every sack of flour weighing 156·66 kilos, or
3442⁄3 lbs. avoird. One loaf contains therefore 344·65105 = 3·282 lbs., and as 100 lbs. of flour in
Parisian baking are reckoned to produce 127 lbs. of bread, each loaf will weigh 4·168 lbs., avoird., and will cost 7l.
5s. 81⁄2d. divided by 315 = 51⁄2d. very nearly. The value
of 315 loaves at the sale price of 6d. will be |
|
7 |
17 |
6 |
|
| Upon this day’s work the clear profit is therefore |
|
0 |
11 |
9 |
1⁄2 |
A new baking establishment has been recently formed at the Royal Clarence
Victualling Establishment at Weevil, near Portsmouth, upon a scale of magnitude nearly
sufficient to supply the whole royal navy with biscuits, and that of a very superior
description. The following account of it is taken from the United Service Journal.
“It having been discovered that the flour supplied to government by contract, had in
many instances been most shamefully adulterated, the corn is ground at mills comprised
within the establishment, by which means the introduction of improper ingredients is
prevented, and precisely the proportion of bran which is requisite in the composition of
good sea-biscuit is retained, and no more. The flour-mill is furnished with 10 pairs of[179]
stones, by which 40 bushels of flour may be ground and dressed ready for baking, in an
hour. The baking establishment consists of 9 ovens, each 13 feet long by 11 feet wide,
and 171⁄2 inches in height. These are each heated by separate furnaces, so constructed
that a blast of hot air and fire sweeps through them, and gives to the interior the
requisite dose of heat in an incredible short space of time. The first operation in
making the biscuits, consists in mixing the flour or rather meal and water; 13 gallons
of water are first introduced into a trough, and then a sack of the meal, weighing 280 lbs.
When the whole has been poured in by a channel communicating with an upper room,
a bell rings, and the trough is closed. An apparatus consisting of two sets of what are
called knives, each set ten in number, are then made to revolve amongst the flour and
water by means of machinery. This mixing operation lasts one minute and a half,
during which time the double set of knives or stirrers makes twenty-six revolutions. The
next process is to cast the lumps of dough under what are called the breaking-rollers,—huge
cylinders of iron, weighing 14 cwt. each, and moved horizontally by the machinery
along stout tables. The dough is thus formed into large rude masses 6 feet long by 3
feet broad, and several inches thick. At this stage of the business, the kneading is still
very imperfect, and traces of dry flour may still be detected. These great masses of
dough are now drawn out, and cut into a number of smaller masses about a foot and a
half long by a foot wide, and again thrust under the rollers, which is repeated until the
mixture is so complete that not the slightest trace of any inequality is discoverable in any
part of the mass. It should have been stated that two workmen stand one at each side
of the rollers, and as the dough is flattened out, they fold it up, or double one part upon
another, so that the roller at its next passage squeezes these parts together, and forces
them to mix. The dough is next cut into small portions, and being placed upon large
flat boards, is, by the agency of machinery, conveyed from the centre to the extremity of
the baking-room. Here it is received by a workman, who places it under what is
called the sheet roller, but which, for size, colour, and thickness, more nearly resembles
a blanket. The kneading is thus complete, and the dough only requires to be cut into
biscuits before it is committed to the oven. The cutting is effected by what is called
the cutting-plate, consisting of a net-work of 52 sharp-edged hexagonal frames, each as
large as a biscuit. This frame is moved slowly up and down by machinery, and the
workman, watching his opportunity, slides under it the above-described blanket of
dough, which is about the size of a leaf of a dining table; and the cutting-frame in its
descent indents the sheet, but does not actually cut it through, but leaves sufficient
substance to enable the workman at the mouth of the oven to jerk the whole mass of
biscuits unbroken into it. The dough is prevented sticking to the cutting-frame by
the following ingenious device: between each of the cutter-frames is a small flat open
frame, movable up and down, and loaded with an iron ball, weighing several ounces.
When the great frame comes down upon the dough, and cuts out 52 biscuits, each of
these minor frames yields to the pressure, and is raised up; but as soon as the great
frame rises, the weight of the balls acting upon the little frames, thrusts the whole
blanket off, and allows the workmen to pull it out. One quarter of an hour is sufficient
to bake the biscuit, which is afterwards placed for three days in a drying room, heated
to 85° or 90°, which completes the process.” The following statement of the performance
of the machinery is taken from actual experiment; in 116 days, during 68 of
which, the work was continued for only 71⁄2 hours; and during 48, for only 53⁄4 hours
each day, in all 769 working hours, equal to 77 days of 10 hours each; the following
quantity of biscuit was baked in the 9 ovens; viz., 12,307 cwt. = 1,378,400 lbs. The
wages of the men employed in baking this quantity amounted to 273l. 10s. 91⁄2d.; if it
had been made by hand, the wages would have been 933l. 9s. 10d.; saving in the wages
of labour, 659l. 7s. 01⁄2d. In this, is not included any part of the interest of the sum
laid out upon the machine, or expended in keeping it in order. But in a very few years
at such an immense rate of saving, the cost of the engine and other machinery will be
repaid. This admirable apparatus is the invention of T. T. Grant, Esq. storekeeper of
the Royal Clarence Victualling Establishment, who, we believe, has been properly
rewarded, by a grant of 2,000l. from government.
The labour of incorporating the ingredients of bread, viz. flour, water and salt, or
kneading dough, is so great as to have led to the contrivance of various mechanical modes
of producing the same effect. One of the most ingenious is that for which a patent was
obtained in August, 1830, by Mr. Edwin Clayton. It consists of a rotatory kneading
trough, or rather barrel, mounted in bearings with a hollow axle, and of an interior
frame of cast iron made to revolve by a solid axle which passes through the hollow one;
in the frame there are cutters diagonally placed for kneading the dough. The revolving
frame and its barrel are made to turn in contrary directions, so as greatly to save time
and equalize the operation. This double action represents kneading by the two hands,
in which the dough is inverted from time to time, torn asunder, and reunited in every
different form. The mechanism will be readily understood from the following description.
[180]
Fig. 169. exhibits a front elevation of a rotatory kneading trough, constructed according
to improvements specified by the patentee, the barrel being shown in section: a is the
barrel, into which the several ingredients, consisting of flour, water, and yeast, are put,
which barrel is mounted in the frame-work b, with hollow axles c and d, which hollow axles
turn in suitable bearings at e; f is the revolving frame which is mounted in the interior of
the barrel a, by axles g and h. The ends of this revolving frame are fastened, or braced
together by means of the oblique cutters or braces i, which act upon the dough when
the machine is put in motion, and thus cause the operation of kneading.
Either the barrel may be made to revolve without the rotatory frame, or the rotatory
frame without the barrel, or both may be made to revolve together, but in opposite ways.
These several motions may be obtained by means of the geer-work, shown at k, l, and m,
as will be presently described.
If it be desired to have the revolving motion of the barrel and rotatory frame together,
but in contrary directions, that motion may be obtained by fastening the hollow axle of
the wheel m, by means of a screw n, to the axle h, of the rotatory frame f, tight, so as they
will revolve together, the other wheels k and l being used for the purpose of reversing the
motion of the barrel. It will then be found that by turning the handle o, the two motions
will be obtained.
If it be desired to put the rotatory frame f, only into motion, that action will be obtained
by loosening the screw n, upon the axle of the wheel m, when it will be found that
the axle h, will be made to revolve freely by means of the winch o, without giving motion
to the wheels k, l, and m, and thus the barrel will remain stationary. If the rotatory
action of the barrel be wanted, it will be obtained by turning the handle p, at the reverse
end of the machine, which, although it puts the geer at the opposite end of the barrel
into motion, yet as the hollow axle of the wheel m is not fastened to the axle h, by the
screw n, these wheels will revolve without carrying round the frame f.
M. Kuhlmann, Professor of Chemistry at Lille, having been called upon several times
by the courts of justice to examine by chemical processes bread suspected of containing
substances injurious to health, collected some interesting facts upon the subject, which
were published under the direction of the central council of salubrity of the department
du Nord.
For some time public attention had been drawn to an odious fraud committed by a
great many bakers in the north of France and in Belgium,—the introduction of a certain
quantity of sulphate of copper into their bread. When the flour was made from bad
grain this adulteration was very generally practised, as was proved by many convictions
and confessions of the guilty persons. When the dough does not rise well in the fermentation
(le pain pousse plat), this inconvenience was found to be obviated by the addition
of blue vitriol, which was supposed also to cause the flour to retain more water.
The quantity of blue water added is extremely small, and it is never done in presence of
strangers, because it is reckoned a valuable secret. It occasions no economy of yeast,
but rather the reverse. In a litre (about a quart) of water, an ounce of sulphate of copper
is dissolved; and of this solution a wine-glass full is mixed with the water necessary
for 50 quartern or 4 pound loaves.
M. Kuhlmann justly observes that there can be no safety whatever to the public when
such a practice is permitted, because ignorance and avarice are always apt to increase the
quantity of the poisonous water. In analyses made by him and his colleagues, portions
of bread were several times found so impregnated with the above salt that they had acquired
a blue colour, and presented occasionally even small crystals of the sulphate. By
acting on the poisoned bread with distilled water and testing the water with ferro-cyanate
(prussiate) of potash, the reddish brown precipitate or tint characteristic of copper
will appear even with small quantities. Should the noxious impregnation be still more
minute, the bread should be treated with a very dilute nitric-acid, either directly, or after
incineration in a platinum capsule, and the solution, when concentrated by evaporation,
should be tested by the ferro-cyanate of potash. In this way, a one seventy thousandth
part of sulphate of copper may be detected.
[181]
M. Kuhlmann deduces, from a series of experiments on baking with various small
quantities of sulphate of copper, that this salt exercises an extremely energetic action upon
the fermentation and rising of the dough, even when not above one seventy thousandth
part of the weight of the bread is employed; or one grain of sulphate for ten pounds of
bread. The proportion of the salt which makes the bread rise best is one twenty thousandth,
or one grain in three pounds of bread. If much more of the sulphate be added,
the bread becomes moist, less white, and acquires a peculiar disagreeable smell like that
of leaven. The increase of weight by increased moisture may amount to one sixteenth
without the bread appearing softer, in consequence of the solidifying quality of the copper;
for the acid does not seem to have any influence; as neither sulphate of soda, sulphate
of iron, nor sulphuric acid have any analogous power. Alum operates like blue
vitriol on bread, but larger quantities of it are required. It keeps water, and raises well,
to use the bakers’ terms.
When alum is present in bread it may be detected by treating the bread with distilled
water, filtering the water first through calico, and next through filtering paper, till it becomes
clear; then dividing it into two portions, and into the one pouring a few drops
of nitrate or muriate of barytes, and into the other a few drops of water of ammonia. In
the former a heavy white precipitate indicating sulphuric acid will appear, and in the
latter a light precipitate of alumina, redissoluble by a few drops of solution of caustic
potash.
When chalk or Paris plaster is used to sophisticate flour, they may be best detected
by incinerating the bread made of it, and examining the ashes with nitric acid which will
dissolve the chalk with effervescence, and the Paris plaster without. In both cases
the calcareous matter may be demonstrated in the solution, by oxalic acid, or better by
oxalate of ammonia.
In baking puff-paste the dough is first kneaded along with a certain quantity of butter,
then rolled out into a thin layer, which is coated over with butter, and folded face-wise
many times together, the upper and under surfaces being made to correspond. This
stratified mass is again rolled out into a thin layer, its surface is besmeared with butter,
and then it is folded face-wise as before. When this process is repeated ten or a dozen
times, the dough will consist of many hundred parallel laminæ, with butter interposed
between each pair of plates. When a moderately thick mass of this is put into the oven,
the elastic vapour disengaged from the water and the butter, diffuses itself between each
of the thin laminæ, and causes them to swell into what is properly called puff-paste,
being an assemblage of thin membranes, each dense in itself, but more or less distinct
from the other, and therefore forming apparently but not really light bread.
One of the most curious branches of the baker’s craft is the manufacture of gingerbread,
which contains such a proportion of molasses, that it cannot be fermented by
means of yeast. Its ingredients are flour, molasses or treacle, butter, common potashes,
and alum. After the butter is melted, and the potashes and alum are dissolved in a
little hot water, these three ingredients, along with the treacle, are poured among the
flour, which is to form the body of the bread. The whole is then incorporated by
mixture and kneading into a stiff dough. Of these five constituents the alum is
thought to be the least essential, although it makes the bread lighter and crisper, and
renders the process more rapid; for gingerbread dough requires to stand over several
days, sometimes 8 or 10, before it acquires that state of porosity which qualifies it for
the oven. The action of the treacle and alum on the potashes in evolving carbonic
acid, seems to be the gasefying principle of gingerbread; for if the carbonate of
potash is withheld from the mixture, the bread, when baked, resembles in hardness a
piece of wood.
Treacle is always acidulous. Carbonate of magnesia and soda may be used as substitutes
for the potashes. Dr. Colquhoun has found that carbonate of magnesia and
tartaric acid may replace the potashes and the alum with great advantage, affording a
gingerbread fully more agreeable to the taste, and much more wholesome than the
common kind, which contains a notable quantity of potashes. His proportions are one
pound of flour, a quarter of an ounce of carbonate of magnesia, and one eighth of an
ounce of tartaric acid; in addition to the treacle, butter, and aromatics as at present
used. The acid and alkaline earth must be well diffused through the whole dough.
The magnesia should, in fact, be first of all mixed with the flour. Pour the melted
butter, the treacle, and the acid dissolved in a little water all at once among the flour,
and knead into a consistent dough, which being set aside for half an hour or an hour will
be ready for the oven, and should never be kept unbaked more than 2 or 3 hours. The
following more complete recipe is given by Dr. Colquhoun, for making thin gingerbread
cakes:—
| |
Flour |
1 |
|
lb. |
|
| Treacle |
0 |
1⁄2 |
| Raw sugar |
0 |
1⁄4 |
|
| Butter[182] |
2 |
|
oz. |
| Carbon. magnesia |
0 |
1⁄4 |
|
| Tartaric acid |
0 |
1⁄8 |
|
| Ginger |
0 |
1⁄8 |
|
| Cinnamon |
0 |
1⁄8 |
|
| Nutmeg |
1 |
|
|
| This compound has rather more butter than common thin gingerbread. |
I shall here insert a passage from my Dictionary of Chemistry as published in 1821;
as it may prove interesting to many of my present readers.
“Under Process of Baking, in the Supplement to the Encyclopedia Britannica, we
have the following statement:—‘An ounce of alum is then dissolved over the fire in a
tin pot, and the solution poured into a large tub, called by the bakers the seasoning-tub.
Four pounds and a half of salt are likewise put into the tub, and a pailful of hot
water.’—Foot note on this passage.—‘In London, where the goodness of bread is estimated
entirely by its whiteness, it is usual with those bakers who employ flour of an
inferior quality, to add as much alum as common salt to the dough; or, in other words,
the quantity of salt added is diminished one half, and the deficiency supplied by an equal
weight of alum. This improves the look of the bread very much, rendering it much
whiter and firmer.’”
In a passage which we shall presently quote, our author represents the bakers of
London in a conspiracy to supply the citizens with bad bread. We may hence infer
that the full allowance he assigns of 21⁄4 pounds of alum for every 21⁄4 pounds of salt, will
be adopted in converting the sack of flour into loaves. But as a sack of flour weighs 280
pounds, and furnishes on an average 80 quartern loaves, we have 21⁄4 pounds divided by
80, or 15750 grains80 = 197 grains, for the quantity present, by this writer, in a London
quartern loaf. Yet in the very same page (39th of vol. ii.) we have the following
passage: “Alum is not added by all bakers. The writer of this article has been assured
by several bakers of respectability, both in Edinburgh and Glasgow, on whose
testimony he relies, and who made excellent bread, that they never employed any alum.
The reason for adding it given by the London bakers is, that it renders the bread whiter,
and enables them to separate readily the loaves from each other. This addition has been
alleged by medical men, and is considered by the community at large, as injurious to
the health, by occasioning constipation. But if we consider the small quantity of this
salt added by the baker, not quite 51⁄2 grains to a quartern loaf, we will not readily admit
these allegations. Suppose an individual to eat the seventh part of a quartern loaf a
day, he would only swallow eight-tenths of a grain of alum, or, in reality, not quite so
much as half a grain; for one half of this salt consists of water. It seems absurd to
suppose that half a grain of alum, swallowed at different times during the course of a
day, should occasion constipation.” Is it not more absurd to state 21⁄4 pounds or 36
ounces, as the alum adulteration of a sack of flour by the London bakers, and within
a few periods to reduce the adulteration to one ounce?
That this voluntary abstraction of 35⁄36 of the alum, and substitution of superior and
more expensive flour is not expected by him from the London bakers, is sufficiently
evident from the following story. It would appear that one of his friends had invented
a new yeast for fermenting dough, by mixing a quart of beer barm with a paste made of
ten pounds of flour and two gallons of boiling water, and keeping this mixture warm for
six or eight hours.
“Yeast made in this way,” says he, “answers the purposes of the baker much better
than brewers’ yeast, because it is clearer, and free from the hop mixture which sometimes
injures the yeast of the brewer. Some years ago the bakers of London, sensible of the
superiority of this artificial yeast, invited a company of manufacturers from Glasgow
to establish a manufactory of it in London, and promised to use no other. About
5,000l. accordingly was laid out on buildings and materials, and the manufactory was
begun on a considerable scale. The ale-brewers, finding their yeast, for which they had
drawn a good price, lie heavy on their hands, invited all the journeymen bakers to their
cellars, gave them their full of ale, and promised to regale them in that manner every
day, provided they would force their masters to take all their yeast from the ale-brewers.
The journeymen accordingly declared, in a body, that they would work no more for
their masters unless they gave up taking any more yeast from the manufactory. The
masters were obliged to comply; the new manufactory was stopped, and the inhabitants
of London were obliged to continue to eat worse bread, because it was the interest of
the ale-brewers to sell the yeast. Such is the influence of journeymen bakers in the
metropolis of England!”
This doleful diatribe seems rather extravagant; for surely beer yeast can derive
nothing noxious to a porter drinking people, from a slight impregnation of hops; while
it must form probably a more energetic ferment than the fermented paste of the new
company, which at any rate could be prepared in six or eight hours by any baker who[183]
found it to answer his purpose of making a pleasant-eating bread. But it is a very
serious thing for a lady or gentleman of sedentary habits, or infirm constitution, to
have their digestive process daily vitiated by damaged flour, whitened with 197 grains
of alum per quartern loaf. Acidity of stomach, indigestion, flatulence, headaches, palpitation,
costiveness, and urinary calculi may be the probable consequences of the
habitual introduction of so much acidulous and acescent matter.
I have made many experiments upon bread, and have found the proportion of alum very
variable. Its quantity seems to be proportional to the badness of the flour; and hence
when the best flour is used, no alum need be introduced. That alum is not necessary
for giving bread its utmost beauty, sponginess, and agreeableness of taste, is undoubted;
since the bread baked at a very extensive establishment in Glasgow, in which about 20
tons of flour were regularly converted into loaves in the course of a week, united
every quality of appearance with an absolute freedom from that acido-astringent drug.
Six pounds of salt were used for every sack of flour; which, from its good quality,
generally afforded 83 or 84 quartern loaves of the legal weight of four pounds five ounces
and a half each. The loaves lost nine ounces in the oven.
Every baker ought to be able to analyse his flour. He may proceed as follows:—A
ductile paste is to be made with a pound of the flour and a sufficient quantity of
water, and left at rest for an hour; then having tied across a bowl a piece of silken sieve-stuff,
a little below the surface of the water in the bowl, the paste is to be laid upon
the sieve on a level with the water, and kneaded tenderly with the hand, so as merely
to wash the starchy particles out of it. This portion of the flour gets immediately
diffused through the water, some of the other constituents dissolve, and the gluten
alone remains upon the filter. The water must be several times renewed till it ceases
to become milky. The last washings of the gluten are made out of the sieve.
The whole of the turbid washings are to be put into a tall conical glass or stoneware
vessel, and allowed to remain at rest, in a cool place, till they deposit the starch. The
clear supernatant liquor is then decanted off. The deposit consists of starch, with a
little gluten. It must be washed till the water settles over it quite clear, and then it
is to be dried.
The filtered waters being evaporated, at a boiling heat, discover flocks floating through
them, which have been supposed by some to be albumen, and by others gluten. At
last, phosphate of lime precipitates. When the residuum has assumed a syrupy consistence
in the cold, it is to be mixed with alcohol, in order to dissolve out its sugar.
Cold water being added to what remains, effects a solution of the mucilage, and leaves
the insoluble azotized matter with the phosphate of lime.
By this mode of analysis a minute portion of resin may remain in the gluten and
in the washing water; the gluten retains also a small proportion of a fixed oil, and a
volatile principle, which may be removed by alcohol. If we wish to procure the resin
alone, we must first of all treat the flour, well dried, with alcohol.
When corn flour, poor in gluten, is to be analyzed, the dough must be inclosed in a
linen bag, kneaded with water, and washed in that state.
In analyzing barley-meal by the above process, hordeine, mixed with common starch,
is obtained: they may be separated by boiling water, which dissolves the starch, and
leaves the hordeine under the aspect of saw-dust.
Fig. 171. is the plan of a London baker’s oven, fired with coal fuel.
Fig. 170. is the longitudinal section.
a, the body of the oven; b, the door; c, the fire-grate and furnace; d, the smoke
flue; e, the flue above the door, to carry off the steam and hot air, when taking out the
bread; f, recess below the door, for receiving the dust; g, damper plate to shut off the
steam flue; h, damper plate to shut off smoke flue, after the oven has come to its proper[184]
heat; i, a small iron pan over the fire-place c,
for heating water; k, ash-pit below the furnace.
Fig. 172. is the front view; the same letters refer
to the same objects in all the figures.
The flame and burnt air of the fire at c, sweep
along the bottom of the oven by the right hand
side, are reflected from the back to the left hand
side, and thence escape by the flue d; (see plan
fig. 171). Whenever the oven has acquired the
proper degree of heat, the fire is withdrawn, the
flues are closed by the damper plates, and the
lumps of fermented dough are introduced.
BRECCIA, an Italian term, used by mineralogists and architects to designate such
compound stony masses, natural or artificial, as consist of hard rocky fragments of
considerable size, united by a common cement. When these masses are formed of
small rounded pebbles, the conglomerate is called a pudding-stone, from a fancied
resemblance to plum pudding.
Concrete, now so much used for the foundations of large buildings, is a factitious
breccia, or pudding-stone. See Concrete.
BREWING. (Brasser, Fr.; Brauen, Germ.) The art of making beer, which see.
BRICK. (Brique, Fr.; Backsteine, ziegelsteine, Germ.) A solid, commonly rectangular,
composed of clay, hardened by heat, and intended for building purposes. The
natural mixture of clay and sand, called loam, as well as marl, which consists of lime
and clay, with little or no sand, constitutes also a good material for making bricks.
The poorer the marl is in lime, the worse adapted it is for agricultural purposes, and
the better for the brick manufacturer, being less liable to fuse in his kiln. When a
natural compound of silica and clay can be got nearly free from lime and magnesia, it
forms a kind of bricks very refractory in the furnace, hence termed fire-bricks. Such a
material is the slate-clay, schieferthon, of our coal measures, found abundantly, and of
excellent quality, at Stourbridge, and in the neighbourhood of Newcastle and Glasgow.
The London brickmakers add to the clay about one third of coal ashes obtained from
the kitchen dust-holes; so that when the bricks are put into the kiln, the quantity of
coaly matter attached to their surface, serves to economise fuel, and makes them less
apt to shrink in the fire; though they are less compact, and probably less durable, than
the bricks made in the coal districts of England.
The general process of brick-making consists in digging up the clay in autumn;
exposing it, during the whole winter, to the frost, and the action of the air, turning it
repeatedly, and working it with the spade; breaking down the clay lumps in spring,
throwing them into shallow pits, to be watered and soaked for several days. The next
step is to temper the clay, which is generally done by the treading of men or oxen.
In the neighbourhood of London, however, this process is performed in a horse-mill.
The kneading of the clay is, in fact, the most laborious but indispensable part of the
whole business; and that on which, in a great measure, the quality of the bricks
depends. All the stones, particularly the ferruginous, calcareous, and pyritous kinds,
should be removed, and the clay worked into a homogeneous paste, with as little water
as possible.
The earth, being sufficiently kneaded, is brought to the bench of the moulder, who
works the clay into a mould made of wood or iron, and strikes off the superfluous
matter. The bricks are next delivered from the mould, and ranged on the ground;
and when they have acquired sufficient firmness to bear handling, they are dressed with
a knife, and staked or built up in long dwarf walls, thatched over, and left to dry. An
able workman will make, by hand, 5,000 bricks in a day.
The different kinds of bricks made in England are principally place bricks, gray and
red stocks, marl facing bricks, and cutting bricks. The place bricks and stocks are used
in common walling. The marls are made in the neighbourhood of London, and used
in the outside of buildings; they are very beautiful bricks, of a fine yellow colour,
hard, and well burnt, and, in every respect, superior to the stocks. The finest kind of
marl and red bricks, called cutting bricks, are used in the arches over windows and
doors, being rubbed to a centre, and gauged to a height.
In France attempts were long ago made to substitute animals and machines for the
treading of men’s feet in the clay-kneading pit; but it was found that their schemes
could not replace, with advantage, human labour, where it is so cheap, particularly for
separating the stones and heterogeneous matters from the loam. The more it is worked,
the denser, more uniform, and more durable, the bricks which are made of it. A good
French workman, in a day’s labour of 12 or 13 hours, it has been said, is able to mould
from 9,000 to 10,000 bricks, 9 inches long, 41⁄2 inches broad, and 21⁄4 thick; but he must[185]
have good assistants under him. In many brickworks near Paris, screw-presses are
now used for consolidating the bricks and paving tiles in their moulds. M. Mollerat
employed the hydraulic press for the purpose of condensing pulverized clay, which,
after baking, formed beautiful bricks; but the process was too tedious and costly. An
ingenious contrivance for moulding bricks mechanically, is said to be employed near
Washington, in America. This machine moulds 30,000 in a day’s work of 12 hours,
with the help of one horse, yoked to a gin wheel, and the bricks are so dry when
discharged from their moulds, as to be ready for immediate burning. The machine is
described, with figures, in the Bulletin de la Société d’Encouragement for 1819, p. 361.
See further on, an account of our recent patents.
Bricks, in this country, are generally baked either in a clamp or in a kiln. The
latter is the preferable method, as less waste arises, less fuel is consumed, and the bricks
are sooner burnt. The kiln is usually 13 feet long, by 101⁄2 feet wide, and about 12 feet
in height. The walls are one foot two inches thick, carried up a little out of the
perpendicular, inclining towards each other at the top. The bricks are placed on flat
arches, having holes left in them resembling lattice-work; the kiln is then covered
with pieces of tiles and bricks, and some wood put in, to dry them with a gentle fire.
This continues two or three days before they are ready for burning, which is known by
the smoke turning from a darkish colour to transparent. The mouth or mouths of the
kiln are now dammed up with a shinlog, which consists of pieces of bricks piled one
upon another, and closed with wet brick earth, leaving above it just room sufficient to
receive a faggot. The faggots are made of furze, heath, brake, fern, &c., and the kiln
is supplied with these until its arches look white, and the fire appears at the top; upon
which the fire is slackened for an hour, and the kiln allowed gradually to cool. This
heating and cooling is repeated until the bricks be thoroughly burnt, which is generally
done in 48 hours. One of these kilns will hold about 20,000 bricks.
Clamps are also in common use. They are made of the bricks themselves, and
generally of an oblong form. The foundation is laid with place brick, or the driest of
those just made, and then the bricks to be burnt are built up, tier upon tier, as high as
the clamp is meant to be, with two or three inches of breeze or cinders strewed between
each layer of bricks, and the whole covered with a thick stratum of breeze. The fireplace
is perpendicular, about three feet high, and generally placed at the west end; and
the flues are formed by gathering or arching the bricks over, so as to leave a space
between each of nearly a brick wide. The flues run straight through the clamp, and
are filled with wood, coals, and breeze, pressed closely together. If the bricks are to
be burnt off quickly, which may be done in 20 or 30 days, according as the weather
may suit, the flues should be only at about six feet distance; but if there be no
immediate hurry, they may be placed nine feet asunder, and the clamp left to burn
off slowly.
Floating bricks are a very ancient invention: they are so light as to swim in water;
and Pliny tells us, that they were made at Marseilles; at Colento, in Spain; and at
Pittane, in Asia. This invention, however, was completely lost, until M. Fabbroni
published a discovery of a method to imitate the floating bricks of the ancients.
According to Posidonius, these bricks are made of a kind of argillaceous earth, which
was employed to clean silver plate. But as it could not be our tripoli, which is too
heavy to float in water, M. Fabbroni tried several experiments with mineral agaric,
guhr, lac-lunæ, and fossil meal, which last was found to be the very substance of which
he was in search. This earth is abundant in Tuscany, and is found near Casteldelpiano,
in the territories of Sienna. According to the analysis of M. Fabbroni, it consists of
55 parts of siliceous earth, 15 of magnesia, 14 of water, 12 of alumina, 3 of lime, and 1 of
iron. It exhales an argillaceous odour, and, when sprinkled with water, throws out a
light whitish smoke. It is infusible in the fire; and, though it loses about an eighth
part of its weight, its bulk is scarcely diminished. Bricks composed of this substance,
either baked or unbaked, float in water; and a twentieth part of clay may be added to
their composition without taking away their property of swimming. These bricks
resist water, unite perfectly with lime, are subject to no alteration from heat or cold,
and the baked differ from the unbaked only in the sonorous quality which they have
acquired from the fire. Their strength is little inferior to that of common bricks, but
much greater in proportion to their weight; for M. Fabbroni found, that a floating
brick, measuring 7 inches in length, 41⁄2 in breadth, and one inch eight lines in thickness,
weighed only 141⁄4 ounces; whereas a common brick weighed 5 pounds, 63⁄4 ounces.
The use of these bricks may be very important in the construction of powder magazines
and reverberatory furnaces, as they are such bad conductors of heat, that one end may
be made red hot while the other is held in the hand. They may also be employed for
buildings that require to be light; such as cooking-places in ships, and floating batteries,
the parapets of which would be proof against red-hot bullets.
The following plan of a furnace or kiln for burning tiles has been found very convenient:—
[186]
Fig. 173., front view, A A, B B, the solid walls of the furnace; a a a, openings to the
ash-pit, and the draught hole; b b b, openings for the supply of fuel, furnished with a
sheet-iron door. Fig. 174. Plan of the ash-pits and air channels c c c. The principal
branch of the ash-pit D D D, is also the opening for taking out the tiles, after removing
the grate; E the smoke flue. Fig. 175. Plan of the kiln seen from above. The grates
H H H. The tiles to be fired are arranged upon the spaces f f f f.
Fig. 176. is the plan and section of one of the grates upon a much larger scale than
in the preceding figures.
Mechanical brick moulding.—Messrs. Lyne and Stainford obtained
in August, 1825, a patent for a machine for making a
considerable number of bricks at one operation. It consists, in
the first place, of a cylindrical pug-mill of the kind usually employed
for comminuting clay for bricks and tiles, furnished with
rotatory knives, or cutters, for breaking the lumps and mixing
the clay with the other materials of which bricks are commonly
made. Secondly, of two movable moulds, in each of which
fifteen bricks are made at once; these moulds being made to
travel to and fro in the machine for the purpose of being alternately
brought under the pug-mill to be fitted with the clay,
and then removed to situations where plungers are enabled to
act upon them. Thirdly, in a contrivance by which the plungers
are made to descend, for the purpose of compressing the material
and discharging it from the mould in the form of bricks.
Fourthly, in the method of constructing and working trucks which carry the receiving
boards, and conduct the bricks away as they are formed.
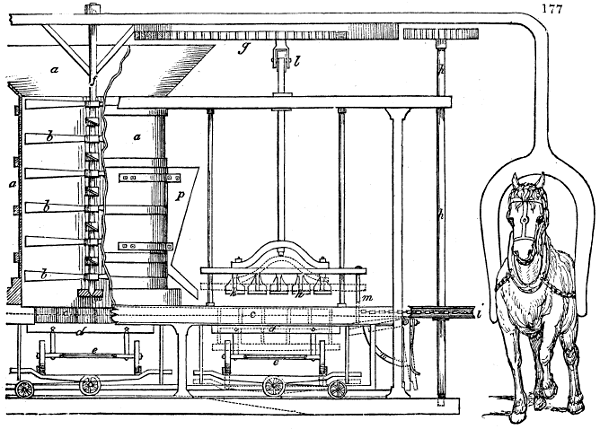
[187]
Fig. 177. exhibits the general construction of the apparatus; both ends of which
being exactly similar, little more than half of the machine is represented. a is the
cylindrical pug-mill, shown partly in section, which is supplied with the clay and other
materials from a hopper above; b b, are the rotatory knives or cutters, which are attached
to the vertical shaft, and being placed obliquely, press the clay down towards the
bottom of the cylinder, in the act of breaking and mixing it as the shaft revolves. The
lower part of the cylinder is open; and immediately under it the mould is placed in
which the bricks are to be formed. These moulds run to and fro upon ledges in the
side frames of the machine; one of the moulds only can be shown by dots in the figure,
the side rail intervening: they are situated at c c and are formed of bars of iron
crossing each other, and encompassed with a frame. The mould resembles an ordinary sash
window in its form, being divided into rectangular compartments (fifteen are proposed in
each) of the dimensions of the intended bricks, but sufficiently deep to allow the material,
after being considerably pressed in the mould, to leave it, when discharged, of the usual
thickness of a common brick.
The mould being open at top and bottom, the material is allowed to pass into it,
when situated exactly under the cylinder; and the lower side of the mould, when so
placed, is to be closed by a flat board d, supported by the truck e, which is raised by a
lever and roller beneath, running upon a plane rail with inclined ends.
The central shaft f, is kept in continual rotatory motion by the revolution of the
upper horizontal wheel g, of which it is the axis; and this wheel may be turned by a
horse yoked to a radiating arm, or by any other means. A part of the circumference
of the wheel g, has teeth which are intended at certain periods of its revolution to take
into a toothed pinion, fixed upon the top of a vertical shaft h h. At the lower part of
this vertical shaft, there is a pulley i, over which a chain is passed that is connected to
the two moulds c, and to the frame in which the trucks are supported; by the rotation
of the vertical shaft, the pulley winds a chain, and draws the moulds and truck frames
along.
The clay and other material having been forced down from the cylinder into the
mould, the teeth of the horizontal wheel g now come into geer with the pinion upon
h, and turn it and the shaft and pulley i, by which the chain is wound, and the mould
at the right hand of the machine brought into the situation shown in the figure; a
scraper or edge-bar under the pug-mill having levelled the upper face of the clay in
the mould, and the board d, supported by the truck e, formed the flat under side.
The mould being brought into this position, it is now necessary to compress the materials,
which is done by the descent of the plungers k k. A friction-roller l, pendant
from the under side of the horizontal wheel as that wheel revolves, comes in contact
with an inclined plane, at the top of the shaft of the plungers; and, as the friction-roller
passes over this inclined plane, the plungers are made to descend into the mould,
and to compress the material; the resistance of the board beneath causing the clay to
be squeezed into a compact state. When this has been effectually accomplished, the
further descent of the plungers brings a pin m, against the upper end of a quadrant
catch-lever n, and, by depressing this quadrant, causes the balance-lever upon which the
truck is now supported to rise at that end, and to allow the truck with the board d to
descend, as shown by dots; the plungers at the same time forcing out the bricks from
the moulds, whereby they are deposited upon the board d; when, by drawing the truck
forward out of the machine, the board with the bricks may be removed, and replaced by
another board. The truck may then be again introduced into the machine, ready to
receive the next parcel of bricks.
By the time that the discharge of the bricks from this mould has been effected, the
other mould under the pug cylinder has become filled with the clay, when the teeth of
the horizontal wheel coming round, take into a pinion upon the top of a vertical shaft
exactly similar to that at h, but at the reverse end of the machine, and cause the
moulds and the frame supporting the trucks to be slidden to the left end of the machine;
the upper surface of the mould being scraped level in its progress, in the way already
described. This movement brings the friction-wheel o, up the inclined plane, and
thereby raises the truck with the board to the under side of the mould, ready to receive
another supply of clay; and the mould at the left-hand side of the machine being now
in its proper situation under the plungers, the clay becomes compressed, and the bricks
discharged from the mould in the way described in the former instance; when this truck
being drawn out, the bricks are removed to be dried and baked, and another board is
placed in the same situation. There are boxes p, upon each side of the pug cylinder
containing sand, at the lower parts of which small sliders are to be opened (by contrivances
not shown in the figure) as the mould passes under them, for the purpose of
scattering sand upon the clay in the mould to prevent its adhering to the plungers.
There is also a rack and toothed sector, with a balance weight connected to the inclined
plane at the top of the plunger-rods, for the purpose of raising the plunger after the[188]
friction-roller has passed over it. And there is a spring acting against the back of the
quadrant-catch for the purpose of throwing it into its former situation, after the pin of
the plunger has risen.
One of the latest, and apparently most effective machines for brick-making, is that
patented by Mr. Edward Jones of Birmingham, in August 1835. His improvements
are described under four heads; the first applies to a machine for moulding the earth
into bricks in a circular frame-plate horizontally, containing a series of moulds or rectangular
boxes, standing radially round the circumference of the circular frame, into
which boxes successively the clay is expressed from a stationary hopper as the frame revolves,
and after being so formed, the bricks are successively pushed out of their boxes,
each by a piston, acted upon by an inclined plane below. The second head of the specification
describes a rectangular horizontal frame, having a series of moulding boxes
placed in a straight range, which are acted upon for pressing the clay by a corresponding
range of pistons fixed in a horizontal frame, worked up and down by rods extending
from a rotatory crank shaft, the moulding boxes being allowed to rise for the purpose of
enabling the pistons to force out the bricks when moulded, and leave them upon the bed
or board below. The third head applies particularly to the making of tiles, for the
flooring of kilns in which malt or grain is to be dried. There is in this contrivance a
rectangular mould, with pointed pieces standing up for the purpose of producing air-holes
through the tiles as they are moulded, which is done by pressing the clay into the
moulds upon the points, and scraping off the superfluous matter at top by hand. The
fourth or last head applies to moulding chimney pots in double moulds, which take
to pieces for the purpose of withdrawing the pot when the edges of the slabs or sides are
sufficiently brought into contact.
“The drawing which accompanies the specification very imperfectly represents some
parts of the apparatus, and the description is still more defective; but as we are acquainted
with the machinery, we will endeavour to give it an intelligible form, and
quote those parts of the specification which point the particular features of novelty
proposed to be claimed by the patentee as his invention, under the several heads.”[13]
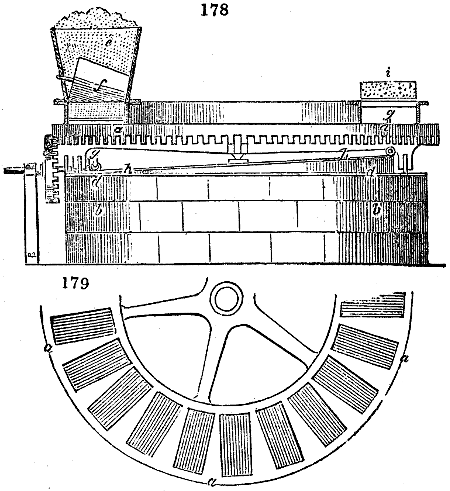
Fig. 178. represents, in elevation,
the first-mentioned machine
for moulding bricks.
The moulds are formed in the
face of a circular plate or wheel
a a, a portion of the upper
surface of which is represented
in the horizontal view, fig. 179.
Any convenient number of
these moulds are set radially in
the wheel, which is mounted
upon a central pivot, supported
by the masonry b b. There is
a rim of teeth round the outer
edge of the wheel a a, which
take into a pinion c, on a shaft
connected to the first mover;
and by these means the wheel
a, with the moulding boxes, is
made to revolve horizontally,
guided by arms with anti-friction
rollers, which run round a
horizontal plate a a, fixed upon
the masonry.
A hopper e, filled with the
brick earth shown with one of
the moulding boxes in section, is fixed above the face of the wheel in such a way, that
the earth may descend from the hopper into the several moulding boxes as the wheel
passes round under it; the earth being pressed into the moulds, and its surface scraped
off smooth by a conical roller f, in the bottom of the hopper.
Through the bottom of each moulding box there is a hole for the passage of a piston
rod g, the upper end of which rod carries a piston with a wooden pallet upon it, acting
within the moulding box; and the lower end of this rod has a small anti-friction roller
which, as the wheel a revolves, runs round upon the face of an oblique ring or inclined
way h h, fixed upon the masonry.
The clay is introduced into the moulding boxes from the hopper, fixed over the lowest[189]
part of the inclined way h, and it will be perceived that as the wheel revolves, the piston
rods g, in passing up the inclined way, will cause the pistons to force the new-moulded
bricks, with their pallet or board under them, severally up the mould, into the situation
shown at i, in fig. 178., from whence they are to be removed by hand. Fresh pallets
being then placed upon the several pistons, they, with the moulds, will be ready for
moulding fresh bricks, when, by the rotation of the wheel a, they are severally brought
under the hopper, the pistons having sunk to the bottoms of their boxes, as the piston
rods passed down the other side of the inclined way h.
The patentee says, after having described the first head of his invention, he would
have it understood that the same may be varied without departing from the main object
of the invention; viz. that of arranging a series of moulds when worked by means of an
inclined track, and in such manner that bricks, tiles, or other articles made of brick
earth, may be capable of being formed in a mould with pallets or boards laid within
the moulds, and constituting the bottoms thereof, the bricks being removed from out of
the moulds, with the pallets or boards under them, as above described. “I do not, therefore,
confine myself to the precise arrangement of the machine here shown, though it is
the best with which I am acquainted for the purpose.”
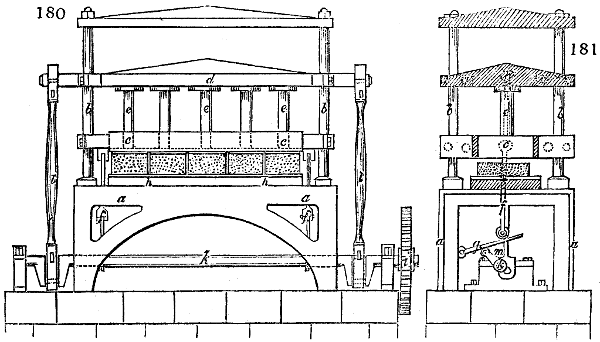
The second head of the invention is another construction of apparatus for moulding
bricks, in this instance, in a rectangular frame. Fig. 180. is a front elevation of the machine;
fig. 181., a section of the same taken transversely. a a is the standard frame-work
and bed on which the bricks are to be moulded. Near the corners of this standard
frame-work, four vertical pillars b b are erected, upon which pillars the frame of the
moulding boxes c, slides up and down, and also the bar d, carrying the rods of the pistons
e e e. These pistons are for the purpose of compressing the clay in the moulding
box, and therefore must stand exactly over and correspond with the respective moulds
in the frame c beneath.
The sliding frame c, constituting the sides and ends of the moulding boxes, is supported
at each end by an upright sliding rod f, which rods pass through guides fixed to
the sides of the standard frame a a, and at the lower end of each there is a roller, bearing
upon the levers g, on each side of the machine, but seen only in fig. 181., which levers,
when depressed, allow the moulding boxes to descend, and rest upon the bed or table of
the machine h h.
In this position of the machine resting upon the bed or table, the brick-earth is to be
placed upon, and spread over, the top of the frame c, by the hands of workmen, when
the descent of the plunger or pistons e e e, will cause the earth to be forced into the
moulds, and the bricks to be formed therein. To effect this, rotatory power is to be
applied to the toothed wheel i, fixed on the end of the main driving crank shaft k k,
which on revolving will, by means of the crank rods l l, bring down the bar a, with the
pistons or plungers e e e, and compress the earth compactly into the moulds, and thereby
form the bricks.
When this has been done, the bricks are to be released from the moulds by the
moulding frame c rising up from the bed, as shown in fig. 180., the pistons still remaining
depressed, and bearing upon the upper surfaces of the bricks. The moulding frame is
raised by means of cams m, upon the crank shaft, which at this part of the operation
are brought under the levers g, for the purpose of raising the cams and the sliding rods
f, into the position shown in fig. 181.
The bricks having been thus formed and released from their moulds, they are to be
removed from the bed of the machine by pushing forward, on the front side, fresh boards[190]
or pallets, which of course will drive the bricks out upon the other side, whence they
are to be removed by hand.
There is to be a small hole in the centre of each pallet, and also in the bed, for the
purpose of allowing any superfluous earth to be pressed through the moulding boxes
when the pistons descend. And in order to cut off the projecting piece of clay which
would be thus formed on the bottom of the brick, a knife-edge is in some way connected
to the bed of the machine; and as the brick slides over it, the knife separates the protuberant
lump: but the particular construction of this part of the apparatus is considered
to be of little importance; and the manner of effecting the object is not clearly stated
in the specification.
The patentee proposes a variation in this construction, which he describes in these
words: “It will be evident that in place of having the moulds to rise, they may, by
suitable arrangements, be made to descend below the bricks. In this case, in place of
the boards, stationary blocks to receive the pallets must be fixed on the bed of the
machine, and these blocks must be shaped in such a manner as to allow of the moulds
passing over them; and then it will be desirable to use the first part of my improvements,
that of having the pallets within the moulds at the time of moulding the bricks;
or in case of working with exceedingly stiff brick-earth, the pallets may be dispensed
with.” In 1835, 1,380,279,065 bricks paid duty in the United Kingdom; the revenue
from which was 405,580l. 6s. 3d.
BRIMSTONE. (Soufre, Fr.; Schwefel, Germ.) Sulphur, which see.
BRITISH GUM. The trivial name given to starch, altered by a slight calcination in
an oven, whereby it assumes the appearance and acquires the properties of gum, being
soluble in cold water, and forming in that state a paste well adapted to thicken the
colours of the calico printer. See Starch.
BROMINE, one of the archæal elements, which being developed from its combinations
at the positive pole of the voltaic circuit, has been therefore deemed to be idio-electro-positive
like oxygen and chlorine. It derives its name from its nauseous smell, Βρῶμος,
fœtor. It occurs in various saline springs on the continent of Europe, in those of Ashby
de la Zouche, and some others in England; in the lake Asphaltites, in sponges, in some
marine plants, in an ore of zinc, and in the cadmium of Silesia. At ordinary temperatures
it is liquid, of a dark brown colour in mass, but of a hyacinth-red in thin layers.
Its smell is rank and disagreeable, somewhat like that of chlorine. It has a very caustic
taste. Its specific gravity is 2·966. Applied to the skin it colours it deep yellow and
corrodes it. One drop put within the bill of a bird suffices to kill it. It combines with
oxygen with feeble affinity, forming bromic acid. Its attraction for hydrogen being far
more energetic, it forms therewith a strong acid, the hydrobromic.
Bromine dissolves very sparingly in water, but it is very soluble in alcohol and ether.
It combines with carbone, phosphorus, sulphur, and chlorine, as well as with most of the
metals. From its scarcity it has not hitherto been applied to any purpose in the arts,
but it is supposed to possess powerful discutient effects upon scrophulous and other
glandular tumours, whence the waters containing it are prescribed as an internal and
external remedy in such forms of disease.
BRONZE. A compound metal consisting of copper and tin, to which sometimes a
little zinc and lead are added. This alloy is much harder than copper, and was
employed by the ancients to make swords, hatchets, &c., before the method of working
iron was generally understood. The art of casting bronze statues may be traced to the
most remote antiquity, but it was first brought to a certain degree of refinement by
Theodoros and Rœcus of Samos, about 700 years before the Christian era, to whom the
invention of modelling is ascribed by Pliny. The ancients were well aware that by
alloying copper with tin, a more fusible metal was obtained, that the process of casting
was therefore rendered easier, and that the statue was harder and more durable; and
yet they frequently made them of copper nearly pure, because they possessed no means
of determining the proportions of their alloys, and because by their mode of managing
the fire, the copper became refined in the course of melting, as has happened to many
founders in our own days. It was during the reign of Alexander that bronze statuary
received its greatest extension, when the celebrated artist Lysippus succeeded by new
processes of moulding and melting to multiply groups of statues to such a degree that
Pliny called them the mob of Alexander. Soon afterwards enormous bronze colossuses
were made, to the height of towers, of which the isle of Rhodes possessed no less than one
hundred. The Roman consul Mutianus found 3,000 bronze statues at Athens, 3,000 at
Rhodes, as many at Olympia and at Delphi, although a great number had been previously
carried off from the last town.
In forming such statues, the alloy should be capable of flowing readily into all the
parts of the mould, however minute; it should be hard, in order to resist accidental
blows, be proof against the influence of the weather, and be of such a nature as to
acquire that greenish oxidized coat upon the surface which is so much admired in the[191]
antique bronzes, called patina antiqua. The chemical composition of the bronze alloy
is a matter therefore of the first moment. The brothers Keller, celebrated founders in
the time of Louis XIV., whose chefs d’œuvre are well known, directed their attention
towards this point, to which too little importance is attached at the present day. The
statue of Desaix in the Place Dauphine, and the column in the Place Vendôme are
noted specimens of most defective workmanship from mismanagement of the alloys of
which they are composed. On analyzing separately specimens taken from the bas-reliefs
of the pedestal of this column, from the shaft, and from the capital, it was found
that the first contained only six per cent. of alloy, and 94 of copper, the second much
less, and the third only 0·21. It was therefore obvious that the founder, unskilful in the
melting of bronze, had gone on progressively refining his alloy, by the oxidizement of
the tin, till he had exhausted the copper, and that he had then worked up the refuse
scoriæ in the upper part of the column. The cannons which the government furnished
him for casting the monument consisted of—
| Copper |
89·360 |
| Tin |
10·040 |
| Lead |
0·102 |
| Silver, zinc, iron, and loss |
0·498 |
| |
100·000 |
The moulding of the several bas-reliefs was so ill executed, that the chiselers employed
to repair the faults removed no less than 70 tons of bronze, which was given them,
besides 300,000 francs for their work. The statues made by the Kellers at Versailles
were found on chemical analysis to consist of—
| |
No. 1. |
No. 2. |
No. 3. |
The
mean. |
| Copper |
91·30 |
91·68 |
91·22 |
91·40 |
| Tin |
1·00 |
2·32 |
1·78 |
1·70 |
| Zinc |
6·09 |
4·93 |
5·57 |
5·53 |
| Lead |
1·61 |
1·07 |
1·43 |
1·37 |
| |
100·00 |
100·00 |
100·00 |
100·00 |
The analysis of the bronze of the statue of Louis XV. was as follows:—
| Copper |
82·45 |
Its specific gravity was 8·482. |
| Zinc |
10·30 |
|
| Tin |
4·10 |
|
| Lead |
3·15 |
|
| |
100·00 |
|
The alloy most proper for bronze medals which are to be afterwards struck, is composed
of from 8 to 12 parts of tin and from 92 to 88 of copper; to which if 2 or 3 parts
in the hundred of zinc be added they will make it assume a finer bronze tint. The alloy
of the Kellers is famous for this effect. The medal should be subjected to three or four
successive stamps of the press, and be softened between each blow by being heated and
plunged into cold water.
The bronze of bells or bell metal is composed in 100 parts of copper 78, tin 22. This
alloy has a fine compact grain, is very fusible and sonorous. The other metals sometimes
added are rather prejudicial, and merely increase the profit of the founders. Some
of the English bells consist of 80 copper, 10·1 tin, 5·6 zinc, and 4·3 lead; the latter
metal when in such large quantity is apt to cause insulated drops, hurtful to the uniformity
of the alloy.
The tam-tams and cymbals of bronze.—The Chinese make use of bronze instruments
forged by the hammer, which are very thin, and raised up in the middle; they are called
gongs, from the word tshoung which signifies a bell. Klaproth has shown that they contain
nothing but copper and tin; in the proportions of 78 of the former metal and 22 of
the latter. Their specific gravity is 8·815. This alloy when newly cast is as brittle as
glass, but by being plunged at a cherry-red heat into cold water and confined between
two discs of iron to keep it in shape, it becomes tough and malleable. The cymbals
consist of 80 parts copper and 20 tin.
Bronze vessels naturally brittle may be made tenacious by the same ingenious process,
for which the world is indebted to M. Darcet. Bronze mortars for pounding have their
lips tempered in the same way. Ancient warlike weapons of bronze were variously compounded;
swords were formed of 871⁄2 copper, and 121⁄2 tin in 100 parts; the springs of
balistæ consisted of 97 copper, and 3 tin.
Cannon metal consists of about 90 or 91 copper, and 10 or 9 of tin. From the experiments
of Papacino-d’Antony, made at Turin, in 1770, it appears that the most proper[192]
alloy for great guns is from 12 to 14 parts of tin to 100 of copper; but the Comte
Lamartilliere concluded from his experiments made at Douay, in 1786, that never less
than 8 nor more than 11 of tin should be employed in 100 parts of bronze.
Gilt ornaments of bronze.—This kind of bronze should be easy of fusion, and take perfectly
the impression of the mould. The alloy of copper and zinc is when fused of a
pasty consistence, does not make a sharp cast, is apt to absorb too much amalgam, is
liable to crack in cooling, and is too tough or too soft for the chaser or the turner. Were
the quantity of zinc increased to make the metal harder, it would lose the yellow colour
suitable to the gilder. A fourfold combination of copper, zinc, tin, and lead, is preferable
for making such ornamental bronze articles; and the following proportions are probably
the best, as they unite closeness of grain with the other good qualities. Copper 82, zinc
18, tin 3 or 1, lead 11⁄2 or 3. In the alloy which contains most lead, the tenacity is diminished
and the density is increased, which is preferable for pieces of small dimensions.
Another alloy which is said to require for its gilding only two thirds of the ordinary
quantity of gold, has the following composition: copper, 82·257; zinc, 17·481; tin,
0·238; lead, 0·024.
The antique bronze colour is given to figures and other objects made from these alloys
by the following process:—Two drams of sal-ammoniac, and half a dram of salt of sorrel,
(binoxalate of potash,) are to be dissolved in fourteen ounce measures (English) of colourless
vinegar. A hair pencil being dipped into this solution, and pressed gently between
the fingers, is to be rubbed equally over the clean surface of the object slightly warmed
in the sun or at a stove; and the operation is to be repeated till the wished for shade is
obtained. (See Gilding.)
The bronze founder ought to melt his metals rapidly, in order to prevent the loss of
tin, zinc, and lead, by their oxidizement. Reverberatory furnaces have been long used
for this operation; the best being of an elliptical form. The furnaces with dome tops
are employed by the bell founders, because their alloy being more fusible, they do not
require so intense a heat; but they also would find their advantage in using the most rapid
mode of fusion. The surface of the melting metals should be covered with small charcoal,
or coke; and when the zinc is added, it should be dextrously thrust to the bottom
of the melted copper. Immediately after stirring the melted mass so as to incorporate
its ingredients, it should be poured out into the moulds. In general the metals most
easily altered by the fire, as the tin, should be put in last. The cooling should be as
quick as possible in the moulds to prevent the risk of the metals separating from each
other in the order of their density, as they are very apt to do. The addition of a little
iron, in the form of tin-plate, to bronze, is reckoned to be advantageous.
One part of tin, and two parts of copper, (nearly one atom of tin and four of copper,
or more exactly 100 parts of tin, and 215 copper,) form the ordinary speculum metal of
reflecting telescopes, which is of all the alloys the whitest, the most brilliant, the hardest,
and the most brittle. The alloy of 1 part of tin, and 10 of copper, (or nearly one atom
of the former to eighteen of the latter,) is the strongest of the whole series.
Ornamental objects of bronze, after being cast, are commonly laid upon red-hot coals
till they take a dull red heat, and are then exposed for some time to the air. The surface
is thereby freed from any greasy matter, some portion of the zinc is dissipated, the
alloy assumes more of a coppery hue, which prepares for the subsequent gilding. The
black tinge which it sometimes gets from the fire may be removed by washing it with a
weak acid. It may be made very clean by acting upon it with nitric acid, of specific
gravity 1·324, to which a little common salt and soot have been added, the latter being of
doubtful utility; after which it must be well washed in water, and dried with rags or
saw dust.
Bronzing, is the art of giving to objects of wood, plaster, &c. such a surface as makes
them appear as if made of bronze. The term is sometimes extended to signify the production
of a metallic appearance of any kind upon such objects. They ought first to be
smeared over smoothly with a coat of size or oil varnish, and when nearly dry, the metallic
powder made from Dutch foil, gold leaf, mosaic gold, or precipitated copper, is to
be applied with a dusting bag, and then rubbed over the surface with a linen pad; or
the metallic powders may be mixed with the drying oil beforehand, and then applied
with a brush. Sometimes fine copper, or brass filings, or mosaic gold, are mixed previously
with some pulverized bone ash, and then applied in either way. A mixture of these
powders with mucilage of gum arabic is used to give paper or wood a bronze appearance.
The surface must be afterwards burnished. Copper powder precipitated by clean plates
of iron, from a solution of nitrate of copper, after being well washed and dried, has been
employed in this way, either alone or mixed with pulverized bone-ash. A finish is given
to works of this nature by a coat of spirit varnish.
A white metallic appearance is given to plaster figures by rubbing over them an amalgam
of equal parts of mercury, bismuth, and tin, and applying a coat of varnish over it.
The iron-coloured bronzing is given by black lead or plumbago, finely pulverized and[193]
washed. Busts and other objects made of cast iron acquire a bronze aspect by being
well cleaned and plunged in solution of sulphate of copper, whereby a thin film of this
metal is left upon the iron.
Copper acquires by a certain treatment a reddish or yellowish hue, in consequence of
a little oxide being formed upon its surface. Coins and medals may be handsomely
bronzed as follows: 2 parts of verdigris and 1 part of sal ammoniac are to be dissolved
in vinegar; the solution is to be boiled, skimmed, and diluted with water till it has
only a weak metallic taste, and upon further dilution lets fall no white precipitate. This
solution is made to boil briskly, and is poured upon the objects to be bronzed, which are
previously made quite clean, particularly free from grease, and set in another copper pan.
This pan is to be put upon the fire, that the boiling may be renewed. The pieces under
operation must be so laid that the solution has free access to every point of their surface.
The copper hereby acquires an agreeable reddish brown hue, without losing its lustre.
But if the process be too long continued, the coat of oxide becomes thick, and makes the
objects appear scaly and dull. Hence they must be inspected every 5 minutes, and
be taken out of the solution the moment their colour arrives at the desired shade. If
the solution be too strong, the bronzing comes off with friction, or the copper gets covered
with a white powder, which becomes green by exposure to air, and the labour is consequently
lost. The bronzed pieces are to be washed with many repeated waters, and
carefully dried, otherwise they would infallibly turn green. To give fresh-made bronze
objects an antique appearance, three quarters of an ounce of sal ammoniac, and a dram
and a half of binoxalate of potash (salt of sorrel) are to be dissolved in a quart of vinegar,
and a soft rag or brush moistened with this solution is to be rubbed over the clean bright
metal, till its surface becomes entirely dry by the friction. This process must be repeated
several times to produce the full effect; and the object should be kept a little
warm. Copper acquires very readily a brown colour by rubbing it with a solution of
the common liver of sulphur, or sulphuret of potash.
The Chinese are said to bronze their copper vessels by taking 2 ounces of verdigris,
2 ounces of cinnabar, 5 ounces of sal ammoniac and 5 ounces of alum, all in
powder, making them into a paste with vinegar, and spreading this pretty thick like a
pigment on the surfaces previously brightened. The piece is then to be held a little
while over a fire, till it becomes uniformly heated. It is next cooled, washed, and
dried; after which it is treated in the same way once and again till the wished-for
colour is obtained. An addition of sulphate of copper makes the colour incline more to
chesnut brown, and of borax more to yellow. It is obvious that the cinnabar produces
a thin coat of sulphuret of copper upon the surface of the vessel, and might probably be
used with advantage by itself.
To give the appearance of antique bronze to modern articles, we should dissolve 1
part of sal ammoniac, 3 parts of cream of tartar, and 6 parts of common salt in
12 parts of hot water, and mix with the solution 8 parts of a solution of nitrate
of copper of specific gravity 1·160. This compound, when applied repeatedly in a
moderately damp place to bronze, gives it in a short time a durable green coat,
which becomes by degrees very beautiful. More salt gives it a yellowish tinge, less
salt a bluish cast. A large addition of sal ammoniac accelerates the operation of the
mordant.
Browning of gun-barrels and other arms.—By this process, the surface of several
articles of iron acquires a shining brown colour. This preparation, which protects the
iron from rust, and also improves its appearance, is chiefly employed for the barrels of
fowling-pieces and soldier’s rifles, to conceal the fire-arms from the game and the enemy.
The finest kind of browning is the Damascus, in which dark and bright lines run through
the brown ground.
This operation consists in producing a very thin uniform film of oxide or rust upon
the iron, and giving a gloss to its surface by rubbing wax over it, or coating it with a
shell-lac varnish.
Several means may be employed to produce this rust speedily and well. The effect
may be obtained by inclosing the barrels in a space filled with the vapour of muriatic
acid. Moistening their surface with dilute muriatic or nitric acid, will answer the same
purpose. But the most common material used for browning, is the butter or chloride
of antimony, which, on account of its being subservient to this purpose, has been called
bronzing salt. It is mixed uniformly with olive oil, and rubbed upon the iron slightly
heated; which is afterwards exposed to the air, till the wished-for degree of browning is
produced. A little aquafortis is rubbed on after the antimony, to quicken its operation.
The brown barrel must be then carefully cleaned, washed with water, dried, and finally
polished, either by the steel burnisher, or rubbed with white wax, or varnished with a
solution of 2 ounces of shell-lac, and 3 drams of dragon’s blood, in 2 quarts of
spirit of wine.
The following process may also be recommended: Make a solution with half an[194]
ounce of aquafortis, half an ounce of sweet spirit of nitre, 1 ounce of spirit of wine,
2 ounces of sulphate of copper, and 1 ounce of tincture of iron, in so much water as
will fill altogether a quart measure. The gun barrel to be browned must first of all be
filed and polished bright, and then rubbed with unslaked lime and water to clear away
all the grease. Its two ends must now be stopped with wooden rods, which may serve
as handles, and the touch-hole must be filled with wax. The barrel is then to be rubbed
with that solution, applied to linen rags or a sponge, till the whole surface be equally
moistened; it is allowed to stand 24 hours, and is then scrubbed with a stiff
brush. The application of the liquid and the brushing may be repeated twice or oftener,
till the iron acquires a fine brown colour. After the last brushing, the barrel must be
washed with plenty of boiling water, containing a little potash; then washed with clean
water, dried, rubbed with polishing hard wood, and coated with shell-lac varnish, for
which purpose the barrel must be heated to the boiling point of water. It is finally polished
with a piece of hard wood.
Storch recommends to make a browning solution with 1 part of sulphate of copper,
one third of a part of sulphuric ether, and 4 parts of distilled water.
To give the damask appearance, the barrel must be rubbed over first with very dilute
aquafortis and vinegar, mixed with a solution of blue vitriol; washed and dried, and
rubbed with a hard brush to remove any scales of copper which may be precipitated
upon it from the sulphate.
Statues, vases, bas-reliefs, and other objects made of gypsum, may be durably bronzed,
and bear exposure to the weather better than after the ordinary oil-varnish, by the
following process:—Prepare a soap from linseed oil, boiled with caustic soda lye,
to which add a solution of common salt, and concentrate it by boiling, till it becomes
somewhat granular upon the surface. It is then thrown upon a piece of linen
cloth, and strained with moderate pressure. What passes through is to be diluted with
boiling water, and again filtered. On the other hand, 4 parts of blue vitriol and 1
part of copperas are to be dissolved separately in hot water. This solution is to be
poured slowly into the solution of soap, as long as it occasions any precipitate. This
flocculent matter is a mixture of cupreous soap and ferruginous soap, that is, a combination
of the oxides of copper and iron with the margaric acid of the soda soap. The
copper soap is green, the iron soap is reddish brown, and both together resemble that
green rust which is characteristic of the antique bronzes. When the precipitate is completely
separated, a fresh portion of the vitriol solution is to be poured upon it in a
copper pan, and is made to boil, in order to wash it. After some time, the liquid part
must be decanted, and replaced by warm water for the purpose of washing the metallic
soaps. They are finally treated with cold water, pressed in a linen bag, drained and
dried. In this state the compound is ready for use in the following way:—
Three pounds of pure linseed oil are to be boiled with twelve ounces of finely-powdered
litharge, then strained through a coarse canvass cloth, and allowed to stand in
a warm place till the soap turns clear. Fifteen ounces of this soap-varnish, mixed with
12 ounces of the above metallic soaps, and 5 ounces of fine white wax, are to be melted
together at a gentle heat in a porcelain basin, by means of a water bath. The mixture must
be kept for some time in a melted state, to expel any moisture which it may contain. It
must be then applied, by means of a painter’s brush, to the surface of the gypsum previously
heated to the temperature of about 200° F. By skilful management of the heat,
the colour may be evenly and smoothly laid on without filling up the minute lineaments
of the busts. When, after remaining in the cool air for a few days, the smell of the pigment
has gone off, the surface is to be rubbed with cotton wool, or a fine linen rag, and
variegated with a few streaks of metal powder or shell gold. Small objects may be
dipped in the melted mixture, and then exposed to the heat of a fire till they are thoroughly
penetrated and evenly coated with it.
BROWN DYE. Upon this subject some general views are given in the article Dyeing,
explanatory of the nature of this colour, to which I may in the first place refer. This
dye presents a vast variety of tints, from yellow and red to black brown, and is produced
either by mixtures of red, yellow, and blue with each other, or of yellow or red with
black, or by substantive colours, such as catechu or oxide of manganese, alone. We
shall here notice only the principal shades; leaving their modifications to the caprice or
skill of the dyer.
1. Brown from mixture of other colours.
Wool and woollen cloths must be boiled with one eighth their weight of alum and
sulpho-tartrate of iron (see this article); afterwards washed, and winced through the
madder bath, which dyes the portion of the stuff imbued with the alum red, and that
with the salt of iron black; the tint depending upon the proportion of each, and the
duration of the madder bath.
A similar brown is produced by boiling every pound of the stuff with two ounces of
alum, and one ounce of common salt, and then dyeing it in a bath of logwood containing[195]
either sulphotartrate, acetate, or sulphate of iron. Or the stuff may be boiled with
alum and tartar, dyed up in a madder bath, and then run through a black bath of
iron mordant and galls or sumach. Here the black tint is added to the red till the
proper hue be hit. The brown may be produced also by adding some iron liquor to
the madder bath, after the stuff has been dyed up in it with alum and tartar. A better
brown of this kind is obtained by boiling every pound of wool with 2 ounces of alum,
dyeing it up in cochineal, then changing the crimson thus given into brown, by turning
the stuff through the bath after acetate of iron has been added to it. Instead of the
cochineal, archil or cutbear, with a little galls or sumach, may be used.
Wool or silk may also receive a light blue ground from the indigo vat, then be mordanted
with alum, washed, and turned through a madder bath till the wished-for brown
be brought out. For the deeper shades, galls or sumach may be added to the paler
Brazil-wood, with more or less iron mordant. Instead of the indigo vat, Saxon blue
may be employed to ground the stuff before dyeing it with madder, or 5 pounds of
madder, with 1 pound of alum, a solution of one tenth of a pound of indigo in sulphuric
acid, may be used with the proper quantity of water for 20 pounds of wool;
for dark shades some iron mordant may be added. Or we may combine a bath of
cochineal or cutbear, fustic, and galls, and add to it sulphate of iron and sulphate of indigo,
blunted with a little potash.
If we boil woollen cloth with alum and tartar, then pass it through a madder bath,
and afterwards through one of weld or fustic, containing more or less iron mordant, we
obtain shades variable, according to the proportions of the materials, from mordoré and
cinnamon to chesnut brown.
After the same manner, bronze colours may be obtained from the union of olive dyes
with red. For 25 pounds of cloth, we take 4 pounds of fustic chips, boil them for 2
hours, turn the cloth in this bath for an hour, and drain it; then add to the bath from
4 to 6 ounces of sulphate of iron, and 1 pound of ordinary madder, or 2 pounds of
sandal wood; put the cloth again in this compound bath, and turn it through, till the
desired shade be obtained. By changing the proportions, and adding an iron mordant,
other tints may be produced.
This mode of dyeing is suitable for silk, but with three different baths, one of
logwood, one of Brazil-wood, and one of fustic. The silk, after being boiled with soap,
is to be alumed, and then dyed up in a bath compounded of these three decoctions,
mixed in the requisite proportions. By the addition of walnut peels, sulphate of copper,
and a little sulphate of iron, or by passing the silk through a bath of annotto, a variety
of brown shades may be had.
Or the silk may receive an annotto ground, and then be passed through a bath of
logwood or Brazil-wood. For 10 pounds of silk, 6 ounces of annotto are to be taken,
and dissolved with 18 ounces of potashes in boiling water. The silk must be winced
through this solution for 2 hours, then wrung out, dried, next alumed, passed through
a bath of Brazil-wood, and finally through a bath of logwood containing some sulphate
of iron. It is to be wrung out and dried.
Brown of different shades is imparted to cotton and linen, by impregnating them with
a mixed mordant of acetates of alumina and iron, and then dyeing them up, either with
madder alone, or with madder and fustic. When the aluminous mordant predominates,
the madder gives an amaranth tint. For horse-chesnut brown, the cotton must be
galled, plunged into a black bath, then into a bath of sulphate of copper, next dyed up
in a decoction of fustic, wrung out, passed through a strong madder bath, then through
the sulphate of copper solution, and finished with a soap boil. Different shades of cinnamon
are obtained, when cottons first dyed up with madder get an olive cast with iron
liquor in a fustic bath.
These cinnamon and mordoré shades are also produced by dyeing them first in a bath
of weld and verdigris, passing them through a solution of sulphate of iron, wringing
and drying them; next putting them through a bath containing 1 pound of galls for
10 pounds of stuff, again drying, next aluming, and maddering. They must be brightened
by a boil in soap water.
A superior brown is produced by like means upon cotton goods, which have undergone
the oiling process of the Turkey red dye. Such stuffs must be galled, mordanted
with alum (see Madder), sulphate of iron, and acetate of lead (equal to 2⁄3 of the alum);
after washing and drying, dyed in a madder bath, and cleared with a soap boil. The
tint of brown varies with the proportion of alum and sulphate of iron.
We perceive from these examples, in how many ways the browning of dyes may be
modified, upon what principles they are founded, and how we have it in our power to
turn the shade more or less towards red, black, yellow, blue, &c.
Brown may be produced by direct dyes. The decoction of oak bark dyes wool a
fast brown of different shades, according to the concentration of the bath. The colour
is more lively with the addition of alum.
[196]
The decoction of bastard marjoram (Origanum vulgare) dyes cotton and linen a reddish
brown, with acetate of alumina. Wool takes from it a dark brown.
The bark of the mangrove tree (Rizophora mangle) affords to wool boiled with alum
and tartar a fine red brown colour, which, with the addition of sulphate of iron, passes
into a fast chocolate.
The Bablah, the pods of the East Indian Mimosa cineraria, and the African Mimosa
nilotica, gives cotton a brown with acetate or sulphate of copper.
The root of the white sea rose (Nymphæa alba) gives to cotton and wool beautiful
shades of brown. A mordant of sulphate of iron and zinc is first given, and then the
wool is turned through the decoction of the root, till the wished-for shade is obtained.
The cotton must be mordanted with a mixture of the acetates of iron and zinc.
Walnut peels (Juglans regia), when ripe, contain a dark brown dye stuff, which communicates
a permanent colour to wool. The older the infusion or decoction of the peels,
the better dye does it make. The stuff is dyed in the lukewarm bath, and needs no
mordant, though it becomes brighter with alum. Or this dye may be combined with
the madder or fustic bath, to give varieties of shade. For dyeing silk, this bath should
be hardly lukewarm, for fear of causing inequality of colour.
The peelings of horse-chesnuts may be used for the same purpose. With muriate of
tin they give a bronze colour, and with acetate of lead a reddish brown.
Catechu gives cotton a permanent brown dye, as also a bronze, and mordoré, when its
solution in hot water is combined with acetate or sulphate of copper, or when the stuff
is previously mordanted with the acetates of copper and alumina mixed, sometimes with
a little iron liquor, rinsed, dried, and dyed up, the bath being at a boiling heat.
Ferrocyanate of copper gives a yellow brown or a bronze to cotton and silk.
The brown colour called carmelite by the French is produced by one pound of catechu
to four ounces of verdigris, with five ounces of muriate of ammonia.—The bronze
(solitaire) is given by passing the stuff through a solution of muriate or sulphate of
manganese, with a little tartaric acid, drying, passing through a potash lye at 4° Baumé,
brightening and fixing with solution of chloride of lime.
BRUSHES. (Brosses, Fr.; Bürsten, Germ.) Mr. T. Mason obtained a patent in
October, 1830, for an improvement in the manufacture of this article. It consists in a
firmer mode of fixing the knots or small bundles of hair into the stock or the handle of the
brush. This is done by forming grooves in the stocks of the brushes, for the purpose of
receiving the ends of the knots of hair, instead of the holes drilled into the wood, as in
brushes of the common constructions. These grooves are to be formed like a dovetail, or
wider at the bottom than the top; and when the ends of the knots of hair have been dipped
into cement, they are to be placed in the grooves and compressed into an oval form, by
which the ends of the hair will be pressed outwards into the recess or wider part of the
dovetailed groove, or the grooves may be formed with threads or teeth on the sides,
instead of being dovetailed; and the cement and hairs being pressed into the teeth or
threads, will cause them to adhere firmly to the stock or handle of the brush.
A metal ferrule may be placed on the outside of the stock of the brush, if necessary,
and secured by pins or rivets, or in any other convenient manner, which ferrule may
also form one side of the outer groove. Fig. 182. is
a plan view of the stock of a round brush; fig. 183. is a section of the same; a a are the dovetailed grooves,
which are turned out of the wood; b is the metal ferrule;
c c are knots or small bundles of hair, to form
the brush. After a number of the knots of hair are
prepared, the ends are to be dipped into proper cement,
and then placed into the grooves, when their ends are
to be squeezed by a pair of plyers, or other means,
which will compress them into the oval shape, as shown
in fig. 184., and cause the ends of the hairs to extend
outward under the dovetailed part of the recess.
The knots of hair are to be successively placed in
the grooves, and forced up by a tool against the last
knot put in, and so on, until the grooves are filled;
fig. 184. is a section taken through a brush with teeth or
threads of a screw formed upon the sides of the groove; into these teeth or threads the
cement and hairs will be forced by the compression, by which means they will be held
firmly in the stock of the brush.
BUTTER. (Beurre, Fr.; Butter, Germ.) Milk contains a fatty matter of more
or less consistency, modified very much according to the nature of the animals which
afford it. This substance is butter, held suspended in the milk by means of the caseous
matter and whey, with which it is intimately blended. Milk is a true emulsion
resulting from the mixture of these three ingredients, owing its opacity and white
colour to the diffusion through it of that butyraceous oil. When any circumstance[197]
dissolves this union, each component becomes insulated, and manifests its peculiar
properties. Milk, even left to itself, at a temperature of from 50° to 60° F., separates
spontaneously into several products. A layer of a fatter, more consistent, but lighter
nature, floats upon its surface, while the subjacent liquid forms a white magma, which
retains among its curdy flocks all the whey of the milk. The upper layer or cream
contains nearly the whole of the butter; but a portion remains entangled with the curd
and whey below.
It belongs to a work on husbandry or rural economy to treat fully of the operations
of the dairy; one of the principal of which is the extraction of butter from milk.
The Tartars and French have been long in the habit of preserving butter, by melting
it with a moderate heat, whereby are coagulated the albuminous and curdy matters
remaining in it, which are very putrescible. This fusion should be made by a heat of
a water bath, about 176° F., continued for some time, to effect the more complete
purification of the butter. If in this settled liquified state it be carefully decanted,
strained through a tammy cloth, and slightly salted, it may be kept for a long time
nearly fresh, without becoming in any degree rancid, more especially if it be put up in
small jars closely covered.
BUTTER OF CACAO. See Cacao and Oils.
BUTTON MANUFACTURE. This art is divided into several branches, constituting
so many distinct trades. Horn, leather, bone, and wood, are the substances
frequently employed for buttons, which are either plain, or covered with silk, mohair
thread, or other ornamental materials. The most durable and ornamental buttons are
made of various metals, polished, or covered with an exceedingly thin wash, as it is
termed, of some more valuable metal, chiefly tin, silver, and gold.
Those buttons intended to be covered with silk, &c. are termed, in general, moulds.
They are small circles, perforated in the centre, and made from those refuse chips of
bone which are too small for other purposes. These chips, which, for the large and
coarser buttons, are pieces of hard wood, are sawn into thin flakes, of an equal thickness;
from which, by a machine, the button moulds are cut out at two operations.
The shavings, sawdust, and more minute fragments, are used by manufacturers of
cutlery and iron toys, in the operations of case-hardening; so that not the smallest
waste takes place.
Metal buttons are formed of an inferior kind of brass, pewter, and other metallic
compositions: the shanks are made of brass or iron wire, the formation of which is a
distinct trade. The buttons are made by casting them round the shank. For this
purpose the workman has a pattern of metal, consisting of a great number of circular
buttons, connected together in one plane by very small bars from one to the next; and
the pattern contains from four to twelve dozen of buttons of the same size. An
impression from this pattern is taken in sand in the usual manner; and shanks are
pressed into the sand in the centre of each impression, the part which is to enter the
metal being left projecting above the surface of the sand. The buttons are now cast
from a mixture of brass and tin; sometimes a small proportion of zinc is added, which
is found useful in causing the metal to flow freely into the mould, and make a sharp
casting. When the buttons are cast, they are cleaned from the sand by brushing;
they are then broken asunder, and carried to a second workman at the lathe, who
inserts the shank of a button into a chuck of a proper figure, in which it is retained by
the back centre of the lathe being pressed against the button with a spring. The
circumference is now, by filing it as it turns round, reduced to a true circle; and the
button is instantly released by the workman’s holding back the centre, and is replaced
by another. A third workman now turns the back of the button smooth, in a chuck
lathe, and makes the projecting part round the shank true; and a fourth renders
the face of the button smooth, by placing it in a chuck, and applying the edge of a
square bar of steel across its centre.
Gilt buttons are stamped out from copper, (having sometimes a small alloy of zinc,)
laminated in the flatting mill to the proper thickness. The stamp is urged by a fly-press,
which cuts them out at one stroke. These circular pieces, called blanks, are
annealed in a furnace to soften them; and the maker’s name, &c. is struck on the back
by a monkey, which is a machine very similar to a pile-engine. This stamp also renders
the face very slightly convex, that the buttons may not stick together in the gilding
process. The shanks are next soldered on. The burnishing is performed by a
piece of hematites or blood-stone, fixed into a handle, and applied to the button as it
revolves by the motion of the lathe.
A great number of the buttons, thus prepared for gilding, are put into an earthen
pan, with the proper quantity of gold to cover them[14], amalgamated with mercury in the[198]
following manner:—The gold is put into an iron ladle, and a small quantity of
mercury added to it; the ladle is held over the fire, till the gold and mercury are
perfectly united. This amalgam being put into the pan with the buttons, as much
aquafortis, diluted with water, as will wet them all over, is thrown in, and they are
stirred up with a brush, till the acid, by its affinity to the copper, carries the amalgam
to every part of its surface, covering it with the appearance of silver. When this is
perfected, the acid is washed away with clean water. This process by the workman
is called quicking.
The old process in gilding buttons, called the drying off, was exceedingly pernicious
to the operator, as he inhaled the vapour of the mercury, which is well known to be
a violent poison. In order to obviate this, the following plan of apparatus has
been employed with success. The vapour, as it rises from the pan of buttons heated by
a charcoal fire, is conducted into an oblong iron flue or gallery, gently sloped downwards,
having at its end a small vertical tube dipping into a water cistern, for condensing the
mercury, and a large vertical pipe for promoting the draught of the products of the combustion.
Plated buttons are stamped by the fly-press, out of copper-plate, covered on one side
with silver at the flatting-mill. The copper side is placed upwards in stamping, and
the die or hole through which they are stamped, is rather chamfered at its edge, to
make the silver turn over the edge of the button. The backs are stamped in the same
manner as the gilt buttons. The shanks are soldered on with silver solder, and heated one
by one in the flame of a lamp, with a blow-pipe urged by bellows. The edges are now
filed smooth in the lathe, care being taken not to remove any of the silver which is
turned over the edge. They are next dipped in acid, to clean the backs, and boiled in
cream of tartar and silver, to whiten them; after which they are burnished, the
backs being first brushed clean by a brush held against them as they revolve in the
lathe. The mode of burnishing is the same as for gilt buttons.
Button shanks are made by hand from brass or iron wire, bent and cut by the following
means:—
The wire is lapped spirally round a piece of steel bar. The steel is turned round
by screwing it into the end of the spindle of a lathe, and the wire by this means lapped
close round it till it is covered. The coil of wire thus formed is slipped off, and a wire
fork or staple with parallel legs put into it. It is now laid upon an anvil, and by a
punch the coil of wire is struck down between the two prongs of the fork, so as to form
a figure 8, a little open in the middle. The punch has an edge which marks the middle
of the 8, and the coil being cut open by a pair of shears along this mark, divides
each turn of the coil into two perfect button shanks or eyes.
Mr. Holmes, of Birmingham, obtained in May, 1833, a patent for an improved construction
of buttons. Fig. 185. represents the outside appearance of one of his
improved shanks, as raised or formed out of the disc of metal which is to constitute the[199]
back of the button; fig. 186. an edge view, looking through the shank or loop; fig. 187. is
another edge view, looking at the raised shank or loop endways; fig. 188. is a section
taken through the shank and disc in the direction of the dotted line A B, in fig. 185.; and
fig. 189. another section taken in the direction of the dotted line C D, in fig. 185. All these
figures of his improved shanks, as well as those hereinafter described, together with the
tools used to form the same, are drawn at about half the real size, to show the parts
more distinctly. It will be seen that the shanks or loops a a are formed by partially
cutting and raising, or forcing up a portion of the metal disc or back b, and are compressed
or formed by the action of the tools, or punches and dies, so as to have a rounded
figure on the inside of the top part of the shank, as at c, the edges of the metal being
turned so as to prevent them cutting the threads by which the button is fastened to the
cloth or garment. It will be observed that, there being but one passage or way through
which the thread can be passed to sew on the button, and that opening being rounded
on all edges, will cause the threads to keep in the centre of the shanks, the form of the
shank allowing a much neater attachment to the garment, and keeping the threads from
the edges of the metal. The ends of the shank, or portions e e, which rise up from the disc
or back b, are made nearly circular, in order to avoid presenting any edges of the metal
to the sides of the button-hole; and when the shank is sewed on the cloth, it forms, in
conjunction with the threads, a round attachment, thereby preventing the shank from
cutting or wearing the button-hole: the threads, when the shank is properly sewed to
the garment, nearly filling up the opening through the shank, and completing that portion
of the circle which has been taken out of the shank by the dies in forming the crescented
parts of the loop. It will be therefore understood that the intention is, that the
inside edges of the shank should be turned as much as possible away from the threads
by which the button is sewed on the cloth, and that the outside of the shank should be
formed so as to present rounded surfaces to the button-hole, and that the thread should
fill up the opening through the shank, so as to produce a round attachment to the
garment. It should here be observed, that the backs of the buttons shown in these figures
are of the shape generally used for buttons covered with Florentine or other fabric, or
faced with plates of thin metal, and are intended to have the edges of a disc, or what is
termed a shell, forming the face, to be closed in upon the inclined or bevelled edges of
the backs. Having now described the peculiar form of the improved shanks which he
prefers, for buttons to be covered with Florentine or other fabric, or shells of thin metal
plate, he proceeds to describe some of the different variations from the same.
Fig. 190. is a representation of a shank, the cut through the disc or back being effected by
a parallel rib on the die, and corresponding groove in the shaping punch, instead of the semi-circular
or crescented cut shown in fig. 185.; fig. 191. is a view of another shank, the separation
of the sides of the loop being performed by straight edges in both punch and die.
He prefers finishing this shaped shank (that is, giving it the rounded form, to prevent its
cutting the threads), by detached punches, and dies, or pincers, as will be hereinafter described.
Fig. 192. is a representation of one of the improved shanks, which has merely
portions, f f, of the back of the button connected to its ends. This shank may be used
for buttons which have a metal shell to be closed in upon the bevelled edges of the ends,
or the shank piece may be otherwise connected to the face part of the button. Fig. 193.
is a representation of a shank raised out of a small disc of metal g g, intended to be
soldered to the disc of metal forming the button, or it may be otherwise fixed to the
back; fig. 194. is a representation of another shank for the same purpose, having only
portions of metal h h, for soldering or otherwise attaching it to the back of the button,
as by placing a ring or annular piece over it forming the back, which shall be confined
to the face, as before described; fig. 195. is a representation of a shank raised upon a dish
or bevelled piece of metal, and is intended to be used for buttons made from pearl-shell,
horn, wood, paper, or other substances. The back part of the button has a dovetailed
recess formed in it to receive the dish-shaped back, which is pressed into the recess, the
edges of the dish being expanded in the dovetailed parts of the recess by the ordinary
means, and thereby firmly fixing it to the button, as shown in fig. 196.
Having now explained the peculiar forms of his improved shanks, he proceeds to describe
the tools, or punches and dies, by which he cuts the disc or back from out of a sheet of metal,
and at the same operation produces and forms the shank complete. Fig. 197. is a longitudinal
section taken through a pair of dies and punches when separated; fig. 198. is a similar
section, taken when they are put together, and in the act of forming a shank after cutting
out the disc or back of the button from a sheet of metal; fig. 199. is a face view of the
punch; and fig. 200. is a similar representation of the counter die, with the tools complete,
a is the punch or cutter, and b the counter bed, by the circular edges of which the disc
of metal is cut out of the sheet; c is a die, fixed in the cutter a, (upon which the name
of the button-maker may be engraved). Fig. 201. is a face view of this die when removed
out of the punch; d is the counter die to the die c. It will be perceived that these dies
c and d, together with the punch and bed, compress the disc of metal into the form required[200]
for the back of the button; that shown in the figures, as before stated, is of the
shape used for buttons to be covered with Florentine or thin plate metal, in a round
shell closed in upon the inclined or bevelled edge of the back; e is the cutting and
shaping punch of the shank, which is fixed within the counter die; this punch cuts
through the metal of the disc, and forms the shank as the dies approach nearer together,
by raising or forcing it up into the recess or opening in the die c, where it is met by the
end of another shaping punch f, fixed in the punch a, which compresses the upper part
of the shank into the recess g, in the end of the punch e, thereby giving the shank its
rounded figure, and at the same time forming the other part of the shank into the required
shape, as described at figs. 185. to 189. The ends of these shaping punches fit into
and over each other, as will be seen by the detached figures of the punches designed for forming
the shank first described. Fig. 202. is a representation of the punches when apart
and removed out of the dies; fig. 203. is a longitudinal section of the same; fig. 204. is
another view of the punches as seen on the top. The sharp edge of the recess h, in the
punch e, comes in contact with the cutting edges of the projecting rib i, of the die c, and
thereby cuts through so much of the metal as is required. The edge k of this die keeps
the outside ends of the shank of a spherical figure, as before explained, while the punches
force up the metal, and form the elevated loop or shank: u u are holes made through the
counter die d, for the passage of clearing pins, which force out the shank or back piece
from the counter die when finished; the operation of which will be shown when describing
the machinery hereafter. There are adjusting screws at the back of the punches and
dies, by which they can be regulated and brought to their proper position one to the other.
Although he has shown the punches which form his improved shanks, fixed into and
working in conjunction with the punch and dies which cut out and shape the discs of
metal for the back of the button, yet he does not intend to confine himself to that mode
of using them, as flat blanks or discs for the backs of buttons may be cut out in a
separate stamping press, and afterwards shaped in the same press or in another, and then
brought under the operation of the punches which form his improved shanks, fixed in
any suitable press. This last-mentioned mode of producing button shanks and backs
he prefers when such metals are employed as require annealing between the operations
of shaping the backs and forming the shank. Fig. 205. is a section taken through a
pair of dies, in which the operation only of forming the shank is to be performed, the
backs being previously shaped in another press. In this instance the punches e and f
are mounted in guide-pieces m and n, which keep them in the proper position towards
each other, the die c being mounted in the piece n, and acting against the face of the
guide m. The blanks or backs of the buttons may be fed into these dies by hand or
any other means; and after the shank is formed, the finished back can be pushed out of
the lower die by clearing rods passed through the holes u u, and removed by hand, or in
any convenient manner.
When his improved shanks are formed out of iron or other metal which is too brittle to
allow of the shank being forced up and finished at one operation in the dies and punches,
he prefers cutting out and shaping the blank or back of the button first, and after annealing
it, to raise or force up the portion of metal to form the shank into the shape
shown in fig. 206., that is, without the edges of the metal being turned to prevent their
cutting the threads, and after again annealing it, to bend or turn the edges into the
shape shown in fig. 191. by means of suitable punches in another press, or by a pair of
pincers and punch as shown in fig. 207., which is a side view of a small apparatus to be
used for turning the edges of the shank by hand, with a partly formed shank seen under
operation. a, is the upper jaw of a pair of pincers, this jaw being fixed on to the head
of the standard b; the under jaw c, is formed by the end of the lever or handle d, which
has its fulcrum in the standard b. e, is a small punch, passed through a guide hole in
the head of the standard, one end projecting into the jaws of the pincers, the other
against a piece f, attached by a joint to the lever d, and working through a slot in the
head of the standard; this piece f, has an inclined plane on the side next the end of the
punch, which, in its descent, projects the punch forward against the top of the loop of
the shank, (placed at g,) as the pincers are closed by forcing down the lever d, and, in
conjunction with the jaws of the pincers, compresses the shank into the required form,
as shown at h, and in the enlarged fig. 191. A spring, i, acts against a pin fixed into the
punch e, for the purpose of bringing it back as the jaws open after forming a shank.
Figs. 208. and 209. represent the face and section of the dies mentioned before, for cutting
the slits in the discs, as at fig. 190.
Having explained the peculiar forms of his improved metallic shanks for buttons,
and the tools employed in making the same, he proceeds to describe the machinery or
apparatus by which he intends to carry his invention into effect. He proposes to take
a sheet of metal, say about 30 or 40 feet long, and of the proper width and thickness;
which thin sheet is to be wound upon a roller, and placed above the machine, so that it
can be easily drawn down into the machine as required for feeding the punches and[201]
dies. Fig. 210. is a plan view of a machine, intended to work any convenient number
of sets of punches and dies placed in rows. Eleven sets of punches and dies are represented,
each set being
constructed as described
under figs. 197 to 204;
fig. 211. is a side view,
and fig. 212. a longitudinal
section, taken through
the machine; figs. 213.
and 214. are transverse
sections taken through
the machine between the
punches and counter
dies, fig. 213. representing
its appearance at the
face of the punches, and
fig. 214. the opposite view
of the counter dies. a a,
are the punches; b b,
the counter dies; each
being mounted in rows
in the steel plates c c,
fixed upon two strong
bars d and e, by countersunk
screws and nuts,
the punches and dies
being retained in their
proper position by the
plates, which are screwed
on to the front of the
steel plates, and press
against the collars of the
punches and dies. The
bars d and e are both
mounted on the guide-pins
g g, fixed in the
heads h h of the frame,
which guide pins pass
through the bosses on
the ends of the bars.
The bar d is stationary
upon the guide pins,
being fixed to the heads
h h, by nuts and screws
passed through ears cast
on their bosses. The bar
e slides freely upon the
guide pins g g, as it is
moved backwards and forwards by the crank i i, and connecting-rods j j, as the crank shaft
revolves. The sheet of thin iron to be operated upon is placed, as before stated, above the
machine; its end being brought down as at a a, and passed between the guide rod and
clearing-plate k, and between the pair of feeding-rollers l l, which, by revolving, draw
down a further portion of the sheet of metal between the punches and dies, after each
operation of the punches.
[202]
As the counter dies advance towards the punches, they first come in contact with
the sheet of metal to be operated upon; and after having produced the pressure which
cuts out the discs, the perforations of the sheet are pushed on to the ends of the punches
by the counter dies; and in order that the sheet may be allowed to advance, the carriage
which supports the axles of the feeding-rollers, with the guide rod and clearing-plate,
are made to slide by means of the pin m, which works in a slot in the sliding-piece
n, bearing the axis of the feeding-roller l l, the slide n, being kept in its place on
the frame work by dovetailed guides shown in fig. 214.
When the counter dies have advanced near to the sheet of metal, the pin m comes
in contact with that end of the slot in the piece n, which is next to the punches, and
forces the carriage with feed-rollers and clearing-plate, and also the sheet of metal, onwards,
as the dies are advanced by the reaction of the cranks; and after they have cut
out the discs, and raised the shanks, the sheet of metal will remain upon the punches;
and when the bar e returns, the finished backs and shanks are forced out of the counter
dies, by the clearing-pins and rods o o, which project through the bar e, and through
the holes before mentioned in the counter dies; these clearing-pins being stationary between
the bars p p, mounted upon the standard q q, on the cross bar of the frame, as
shown in figs. 210., 212., 213. Immediately after this is done, the pins m come in contact
with the other ends of the slots in the pieces n, and draw back the feeding-rollers
l l, together with the clearing-plate k, and the sheet of metal, away from the punches into
the position represented in the figures.
At this time the feeding of the metal into the machine is effected by a crank-pin r, on
the end of the crank-shafts coming in contact with the bent end of the sliding-bar s,
supported in standards t t; and as the crank-shaft revolves, this pin r forces the bar s
forward, and causes the tooth or pall u, on its reverse end, to drive the racket-wheel v,
one or more teeth; and as the racket-wheel v is fixed on to the end of the axle of one
of the rollers l, it will cause that roller to revolve; and by means of the pair of spur-pinions
on the other ends of the axles of the feeding-rollers, they will both revolve
simultaneously, and thereby draw down the sheet of metal into the machine. It will
be perceived that the standards which support the clearing-plate and guide-bar are carried
by the axles of the feeding rollers, and partake of their sliding motion: also that
the clearing-pins o, are made adjustable between the bars p, to correspond with the
counter dies. There is an adjustable sliding stop x upon the bar s, which comes in
contact with the back standard t, and prevents the bar s sliding back too far, and consequently
regulates the quantity of sheet metal to be fed into the machine by the pall
and ratchet-wheel, in order to suit different sizes of punches and dies. In case the
weight of the bar c, carrying the counter dies, should wear upon its bearings, the guide
pins g g, have small friction-rollers y y, shown under the bosses of this bar, which friction-rollers
run upon adjustable beds or planes z z, by which means the guide pins may
be partially relieved from the weight of the bar c, and the friction consequently
diminished.
CABLE. (Cable, Fr.; Ankertau, Germ.) A strong rope or chain, connecting the
ship with the anchor for the purpose of mooring it to the ground. The sheet
anchor cable is the strongest, and is used at sea; the stream cable is more slender,
being used chiefly in rivers. A cable’s length is 120 fathoms. The greatest improvement
in mooring vessels has been the introduction of the chain cable, which,
when duly let out, affords in the weight of its long catenary curve, an elastic
tension and play to the ship under the pressure of wind. The dead strain upon
the anchor is thus greatly reduced, and the sudden pull by which the flukes or
arms are readily snapped is in a great measure obviated. The best iron cables are
chains made of links, bound and braced by rods across their middle. Experience has
taught that the ends of these links wear out much sooner than the sides. To remedy
this evil, Mr. Hawkes, iron manufacturer, obtained a patent in July, 1828, for constructing
these anchor chains with links considerably stouter at the ends than in the middle.
With this view, he forms the short rods of iron, of which the links are to be made, with
swells or protuberances about one third of their length from each of their ends, so that
when these are welded together, the slenderer parts are at the sides, and the thicker at
the ends of the elliptic links. Such rods as the above are formed at once by rolling,
swagging, or any other means. When the link is welded, it may be strengthened, by
a brace or stretcher fixed across the middle.
The first avowed proposal to substitute iron cables for cordage in the sea service, was
made by Mr. Slater, surgeon of the navy, who obtained a patent for the plan in 1808,
though he does not seem to have had the means of carrying it into effect; a very general
misfortune with ingenious projectors. It was Captain Brown of the West India[203]
merchant service who, in 1811, first employed chain cables in the vessel Penelope, of
400 tons burden, of which he was captain. He made a voyage in this ship from England
to Martinique and Guadaloupe and home again, in the course of four months, having
anchored many times in every variety of ground without any accident. He multiplied
his trials, and acquired certain proofs that iron might be substituted for hemp in making
cables, not only for mooring vessels, but for the standing rigging. Since this period
chain cables have been universally introduced into all the ships of the royal navy, but
the twisted links employed at first by Brown, have been replaced by straight ones, stayed
in the middle with a cross rod, the contrivance of Mr. Brunton, which was secured by
patent in this country and in France; but the latter patent was suffered to fall from not
being acted upon within the two years specified by law.
The first thing to be considered in the manufacture of iron cables is, to procure a
material of the best quality, and, in using it, always to keep in view the direction of the
strain, in order to oppose the maximum strength of the iron to it. The best form
of the links may be deduced from the following investigation.
Let A B fig. 215. be a circular link or ring, of one inch rod iron, the
outer circumference of the ring being 15 inches, and the inner 9.
If equal opposite forces be applied to the two points of the link
C D, pulling C towards E, and D towards F, the result will be, when
the forces are sufficiently intense, that the circular form of the link
will be changed into another form with two round ends and two
parallel sides, as seen in fig. 216. The ratio of the exterior to the
interior periphery which was originally as 15 to 9, or 5 to 3, is no
longer the same in fig. 216. Hence there will be a derangement in
the relative position of the component particles, and consequently
their cohesion will be progressively impaired, and eventually destroyed.
In fig. 215. the segment M N of the outside periphery being
equal to 3 inches, the corresponding inside segment will be 3⁄5 of it, or 14⁄5 inches. If
this portion of the link, in consequence of the stretching force, comes to be extended
into a straight line, as shown in fig. 216., the corresponding segments, interior
and exterior, must both be reduced to an equal length. The matter contained in the
3 inches of the outside periphery must therefore be either compressed, that is, condensed
into 14⁄5 inch, or the inside periphery, which is only 14⁄5 inch already, must be extended to
3 inches; that is to say, the exterior condensation and the interior expansion must take
place in a reciprocal proportion. But, in every case, it is impossible to effect this contraction
of one side of the rod, and extension of the other, without disrupture of the
link.
Let us imagine the outside periphery divided into an infinity of points, upon each of
which equal opposite forces act to straighten the curvature: they must undoubtedly occasion
the rupture of the corresponding part of the internal periphery. This is not the sole
injury which must result; others will occur, as we shall perceive in considering what
passes in the portion of the link which surrounds C D, fig. 216., whose length is 41⁄2 inches
outside, and 21⁄10 inside. The segments M P and N O, fig. 215., are actually reduced to semi-circumferences,
which are inside no more than half an inch, and outside as before.
There is thus contraction in the interior, with a quicker curvature or one of shorter
radius in the exterior. The derangement of the particles takes place here, in an order
inverse to that of the preceding case, but it no less tends to diminish the strength of that
portion of the link; whence we may certainly conclude that the circular form of cable
links is an extremely faulty one.
Leaving matters as we have supposed in fig. 215., but suppose that G is a rod introduced
into the mail, hindering its two opposite points A B from approximating. This circumstance
makes a remarkable change in the results. The link pulled as
above described, must assume the quadrilateral form shown in fig. 217.
It offers more resistance to deformation than before; but as it may
still suffer change of shape, it will lose strength in so doing, and cannot
therefore be recommended for the construction of cables which are
to be exposed to very severe strains.
Supposing still the link to be circular, if the ends of the stay comprehended a larger
portion of the internal periphery, so as to leave merely the space necessary for the plan
of the next link, there can be no doubt of its opposing more effectively the change of
form, and thus rendering the chain stronger. But, notwithstanding, the circular portions
which remain between the points of application of the strain and the stay, would tend
always to be straightened, and of consequence to be destroyed. Besides, though we
could construct circular links of sufficient strength to bear all strains, we ought still to
reject them, because they would consume more materials than links of a more suitable
form, as we shall presently see.
The effect of two opposite forces applied to the links of a chain, is, as we have seen,[204]
to reduce to a straight line or a straight plane every curved part which is not stayed;
whence it is obvious that twisted links, such as Brown first employed, even with a stay
in their middle, must of necessity be straightened out, because there is no resistance in
the direction opposed to the twist. A cable formed of twisted links, for a vessel of 400
tons stretches 30 feet, when put to the trial strain, and draws back only 10 feet. This
elongation of 20 feet proceeds evidently from the straightening of the twist in each link,
which can take place only by impairing the strength of the cable.
From the preceding remarks, it appears that the strongest links are such as present, in
their original form, straight portions between the points of tension; whence it is clear
that links with parallel sides and round ends, would be preferable to all others, did not
a good cable require to be able to resist a lateral force, as well as one in the direction of
its length.
Let us suppose that by some accident the link fig. 216. should have its two extremities
pulled towards Y and Z, whilst an obstacle X, placed right opposite to
its middle, resisted the effort. The side of the link which touches X,
would be bent inwards; but if as in fig. 218., there is a stay A G B, the
two sides would be bent at the same time; the link would notwithstanding
assume a faulty shape.
In thus rejecting all the vicious forms, we are naturally directed to that which deserves
the preference. It is shown in fig. 219. This link has a cast-iron stay with large ends,
it presents in all directions a great resistance to every
change of form; for let it be pulled in the direction a b,
against an obstacle c, it is evident that the portions d e
and d f, which are supported by the parts g e and g f,
cannot get deformed or be broken without the whole link
giving way. As the matter composing g e and g f cannot
be shortened, or that which composes d e and d f be lengthened, these four sides will
remain necessarily in their relative positions, by virtue of the large-ended stay h, whose
profile is shown in fig. 220.
We have examined the strength of a link in every direction,
except that perpendicular to its plane. Fig. 221.
represents the assemblage of three links in the above
predicament; but we ought to observe, that the obstacle
C, placed between the links A B, must be necessarily
very small, and could not therefore resist the
pressure or impact of the two lateral links.
Process of manufacturing iron cables.—The implements
and operations are arranged in the following order:—
1. A reverberatory furnace (see Iron), in which a number of rods or round bars of
the best possible wrought-iron, and of proper dimensions, are heated to bright ignition.
2. The cutting by a machine of these bars, in equal lengths, but with opposite
bevels, to allow of the requisite crossing and splicing of the ends in the act of welding.
3. The bending of each of these pieces by a machine, so as to form the links; the last
two operations are done rapidly while the iron is red-hot.
4. The welding of the links at small forge fires, fitted with tools for this express
purpose, and the immediate introduction of the stay, by means of a compound lever
press.
5. Proving the strength of the cables by an hydraulic press, worked by two men turning
a winch furnished with a fly wheel.
The furnace is like those used in the sheet-iron works, but somewhat larger, and needs
no particular description here.
Figs. 222. and 223. are a plan and elevation of the shears with which the rods are cut into
equal pieces, for forming each a link. It is moved at Mr. Brunton’s factory by a small
steam engine, but, for the sake of simplicity, it is here represented worked by four or
more labourers, as it may be in any establishment. These must be relieved however[205]
frequently by others, for I believe each shears’ machine is calculated to require nearly
one horse in steam power. It is portable and must be placed in the neighbourhood of
both the furnace and bending machine.
A and B are the two cast-iron limbs of the shears. The first is fixed and the second
is movable by means of a crank shaft C, driven by a heavy fly-wheel weighing 7 or 8 cwt.
The cutting jaws G are mounted with pieces of steel which are made fast by bolts, and
may be changed at pleasure.
E, the bar of iron to be cut. It is subjected, immediately upon being taken out of the
fire, to the shears, under a determinate uniform angle, care being taken not to let it
turn round upon its axis, lest the planes of the successive incisions should become unequal.
F is a stop which serves to determine, for the same kind of chain, the equality of
length in the link pieces.
Figs. 224, 225, 226. plan and elevations of the machine for bending the links into an
elliptic form. It is represented at the moment when a link is getting bent upon it.
A is an elliptic mandrel of cast-iron; it is fixed upon the top of a wooden pillar
B, solidly supported in the ground. C is the jaw of the vice, pressed by a square-headed
screw against the mandrel A.
D part of the mandrel comprehended between X and Y, formed as an inclined plane, so
as to preserve an interval equal to the diameter of the rod between the two surfaces that
are to be welded together.
E rectangular slots (shears) passing through the centre of the nut of the mandrel, in
which each of the pins F may be freely slidden.
G horizontal lever of wrought-iron six feet long. It carries at H a pulley or friction-roller
of steel, whose position may be altered according to the diameter of the links. It
is obvious that as many mandrels are required as there are sizes and shapes of links.
[206]
The piece of iron intended to form a link being cut, is carried, while red-hot, to the
bending machine, where it is seized with the jaw of the vice C, by one of its ends, the
slant of the cut being turned upwards; this piece of iron has now the horizontal direction
m n; on pushing the lever G in the line of the arrow, the roller H will force m n to
be applied successively in the elliptic groove of the mandrel; thus finally the two faces
that are to be welded together will be placed right opposite each other.
The length of the small diameter of the ellipse ought to exceed by a little the length
of the stay-piece, to allow of this being readily introduced. The difference between
the points F, E is equal to the difference of the radii vectores of the ellipse. Hence
it will be always easy to find the eccentricity of the ellipse.
Fig. 227. is a lever press for squeezing the links upon their stays, after the links are
welded. This machine consists of a strong cast-iron piece A, in the form of a square, of
which one of the branches is laid horizontally, and fixed to a solid bed by means of
bolts; the other branch, composed of two cheeks, leaving between them a space of two
inches, stands upright. These two cheeks are united at top, and on the back of their
plane by a cross piece B. C, a rectangular staple, placed to the right and left of the
cheeks through which is passed the mandrel D, which represents and keeps the place of
the following link. E, is a press lever, 6 feet long. F, clamp and counterclamp, between
which the link is pressed at the moment when the stay is properly placed. There are
other clamps, as well as staples C, for changing with each changed dimension of links.
The links bent, as we have seen, are carried to the forge hearth to be welded, and to
receive their stay; two operations performed at one heating. Whenever the welding is
finished, while the iron is still red-hot, the link is placed upright between the clamps
F; then a workman introduces into the staple the mandrel D, and now applies
the stay with a pair of tongs or pincers, while another workman strikes down the
lever E forcibly upon it. This mechanical compression first of all joins perfectly the
sides of the link against the concave ends of the stay, and afterwards the retraction of
the iron on cooling increases still more this compression.
If each link be made with the same care, the cable must be sound throughout. It
is not delivered for use however till it be proved by the hydraulic press, at a draw-bench
made on purpose. The press is an horizontal one, having the axis of its ram in the
middle line of the draw-bench, which is about 60 feet long, and is secured to the body
of the press by strong bolts.
The portion of chain under trial, being attached at the one end to the end of the ram
of the press, and at the other to a cross-bar at the extremity of the draw-bench, two
men put the press in action, by turning the winch which works by a triple crank three
forcing pumps alternately; the action being equalized by means of a heavy fly-wheel.
As long as the resistance does not exceed the force of two men, the whole three pumps
are kept in play. After a while one pump is thrown out of geer and next another,
only one being worked towards the conclusion. The velocity of the ram being retarded
first one third and next two thirds, gives the men a proportional increase of mechanical
power.
The strength of two average men thus applied being computed, enables us to know at
every instant the resistance opposed by the chain to the pressure of the ram. The strain
usually applied to the stronger cables is about 500 tons.
The side beams of the draw-bench are of cast-iron, 6 inches in diameter; the different
pieces composing it are adjusted to each other end-wise by turned joints. Props
also of cast-iron support the beams two feet asunder, and at the height of 30 inches
above the ground. The space between them is filled with an oak plank on which the
trial chain is laid.
[207]
Strength of iron-cables compared to hemp cables:—
Iron Cables.
Diameter of Iron Rod. |
Hemp Cables.
Circumference of Rope. |
Resistance. |
| Inches. |
Inches. |
Tons. |
| 0 |
7⁄8 |
9 |
|
12 |
| 1 |
|
10 |
|
18 |
| 1 |
1⁄8 |
11 |
|
26 |
| 1 |
1⁄4 |
12 |
|
32 |
| 1 |
5⁄16 |
13 |
|
35 |
| 1 |
3⁄8 |
14 |
to 15 |
38 |
| 1 |
1⁄2 |
16 |
|
44 |
| 1 |
5⁄8 |
17 |
|
52 |
| 1 |
3⁄4 |
18 |
|
60 |
| 1 |
7⁄8 |
20 |
|
70 |
| 2 |
|
22 |
to 24 |
80 |
It would be imprudent to put hemp cables to severer strains than those indicated in
the preceding table, drawn up from Brunton’s experiments; but the iron cables of the
above sizes will support a double strain without breaking. They ought never in common
cases however to be exposed to a greater stress. A cable destined for ships of a
certain tonnage, should not be employed in those of greater burden. Thus treated it
may be always trusted to do its duty, and will last longer than the ship to which it belongs.
A considerable part of this decided superiority which iron cables have over hemp
ones, is undoubtedly due to the admirable form contrived by Brunton. Repeated
experiments have proved that his cables possess double the strength of the iron rods
with which they are made—a fact which demonstrates that no stronger form can be
devised or is in fact possible.
One of the most valuable qualities of iron cables is their resisting lateral as well as
longitudinal strains as explained under figs. 219. and 221.
Vessels furnished with such cables have been saved by them from the most imminent
peril. The Henry, sent out with army stores during the peninsular war, was caught
on the northern coast of Spain in a furious storm. She run for shelter into the Bay of
Biscay among the rocks, where she was exposed for three days to the hurricane. She
possessed fortunately one of Brunton’s 70 fathom chain cables, which held good all the
time, but it was found afterwards to have had the links of its lower portion polished
bright by attrition against the rocky bottom. A hemp cable would have been speedily
torn to pieces in such a predicament.
In the contracts of the Admiralty for chain cables for the British navy, it is stipulated
that “the iron shall have been manufactured in the best manner from pig iron, smelted
from iron-stone only, and selected of the best quality for the purpose, and shall not have
received in any process whatever subsequent to the smelting, the admixture of either the
cinder or oxides produced in the manufacture of iron; and shall also have been puddled
in the best manner upon iron bottoms, and at least three times sufficiently drawn out at
three distinct welding heats, and at least twice properly fagotted.”
The following is a table of the breaking proof of chain cables, and of the iron for the
purpose of making them, also of the proofs required by her majesty’s navy for chains.
| Size of Bolt. |
Proof of Bolt. |
Proof of Chain. |
Navy
Proof of Chain. |
| Inches. |
Tons. |
Cwt. |
Tons. |
Cwt. |
Tons. |
| |
1⁄2 |
5 |
7 |
8 |
11 |
4 |
1⁄2 |
| |
5⁄8 |
8 |
7 |
13 |
4 |
5 |
1⁄2 |
| |
3⁄4 |
12 |
1 |
19 |
5 |
10 |
7⁄8 |
| |
7⁄8 |
16 |
4 |
26 |
5 |
13 |
3⁄4 |
| 1 |
|
21 |
8 |
34 |
5 |
18 |
|
| 1 |
1⁄8 |
27 |
2 |
48 |
15 |
22 |
3⁄4 |
| 1 |
1⁄4 |
33 |
10 |
53 |
11 |
28 |
1⁄2 |
| 1 |
3⁄8 |
40 |
10 |
65 |
0 |
34 |
|
| 1 |
1⁄2 |
48 |
4 |
77 |
0 |
40 |
1⁄2 |
| 1 |
5⁄8 |
56 |
11 |
90 |
10 |
47 |
1⁄2 |
| 1 |
3⁄4 |
65 |
12 |
105 |
0 |
55 |
1⁄8 |
| 1 |
7⁄8 |
75 |
6 |
120 |
10 |
63 |
1⁄4 |
| 2 |
|
85 |
14 |
137 |
0 |
72 |
|
| 2 |
1⁄8 |
96 |
15 |
155 |
0 |
81 |
1⁄4 |
In Brunton’s cable the matter in the link is thrown very much into one plane; the[208]
link being of an oval form, and provided with a stay. As there are emergencies in which
the cable must be severed, this is accomplished in those of iron by means of a bolt and
sheckle (shackle), at every fathom or two fathoms; so that by striking out this bolt or
pin, this cable is parted with more ease than a hempen one can be cut.
CACAO, BUTTER OF. See Cocoa, and Oils, Unctuous.
CADMIUM, is a metal discovered about the beginning of the year 1818. It occurs
chiefly in Silesia in several ores of zinc; and may be readily recognized by means of
the blowpipe; for at the first impression of the reducing or smoky part of the flame, the
ores containing cadmium stain the charcoal all round them with a reddish yellow circle
of oxide of cadmium. The Silesian native oxide of zinc contains from 11⁄2 to 11 per
cent. of cadmium.
The cadmium may be extracted by dissolving the ore in sulphuric acid, leaving
the solution acidulous, and diluting it with water, then transmitting through it a
stream of sulphuretted hydrogen, till the yellow precipitate ceases to fall. This
powder which is sulphuret of cadmium, is to be dissolved in concentrated muriatic
acid, the excess of which is to be expelled by evaporation; and the muriatic
salt being dissolved in water, carbonate of ammonia is to be added in excess, whereby
the cadmium separates as a carbonate, while the small portion of adhering copper
or zinc is retained in solution by the ammonia. Herapath has shown that, in distilling
zinc per descensum (see Zinc), the first portions of gaseous metal which are disengaged
burn with a brown flame and deposit the brown oxide of cadmium.
Cadmium has the colour and lustre of tin; and is susceptible of a fine polish. Its
fracture is fibrous; it crystallizes readily in regular octahedrons, and when it suddenly
solidifies, its surface gets covered with fine mossy vegetations. It is soft, easily bent,
filed, and cut, soils like lead any surface rubbed with it. It is harder and more tenacious
than tin, and emits a creaking sound when bent, like that metal. It is very ductile,
and may be drawn out into fine wire, and hammered into thin leaves without
cracking at the edges. Its specific gravity, after being merely melted, is 8·604; and
8·6944 after it has been hammered. It is very fusible, melting at a heat much under
redness; indeed at a temperature little exceeding that of boiling mercury, it boils and
distils over in drops. Its vapours have no smell. It is but slightly altered by exposure
to air. When heated in the atmosphere, it readily takes fire, and burns with a brownish
yellow smoke which is destitute of smell. In strong acids it dissolves with disengagement
of hydrogen, and forms colourless solutions. Chromate of potash causes no precipitate
in them, unless zinc or lead be present.
There is only one oxide of cadmium, the brown above-mentioned. Its specific gravity
is 8·183. It is neither fusible nor volatile at a very high temperature. When in
the state of a hydrate it is white. The oxide of cadmium consists of 87·45 parts of
metal, and 12·55 oxygen in 100 parts. Berzelius states its atomic weight to be 55·833
to hydrogen 1·000. Its sulphuret has a fine orange yellow colour, and would form a
beautiful pigment, could the metal be found in sufficient quantity for the purposes of
art. The sulphate is applied to the eyes by surgeons for removing specks of the cornea.
CAFEINE. A chemical principle discovered in coffee, remarkable for containing
much azote. See Coffee.
CAJEPUT OIL is obtained from the leaves of the tree called Melaleuca Leucadendron
by Linnæus, which grows upon the mountains of Amboyna, and in other of
the Molucca islands. It is procured by distillation of the dried leaves along with
water, is prepared in great quantities in the island of Banda, and sent to Holland
in copper flasks. Hence as it comes to us, it has a green colour. It is very limpid,
lighter than water, of a strong smell resembling camphor, and pungent taste like
cardamoms. When rectified the copper remains in the retort, and the oil comes over
colourless. It is used in medicine as a stimulant. See Oils Ethereous.
CALAMANCO. A sort of woollen stuff of a shining appearance, chequered in
the warp, so that the checks are seen only upon one side.
CALAMINE. A native carbonate of zinc. See Zinc.
CALCAREOUS EARTH. (Terre calcaire, Fr.; Kalkerde, Germ.) Commonly
denotes lime, in any form; but, properly speaking, it is pure lime.
CALCAREOUS SPAR. Crystallized native carbonate of lime.
CALCEDONY. A hard mineral of the siliceous family, often cut into seals.
Under it may be grouped common calcedony, heliotrope, chrysoprase, plasma, onyx,
sardonyx, and sard.
CALCHANTUM. The ancient name of native copperas or sulphate of iron.
CALCINATION, is the chemical process of subjecting metallic bodies to heat
with access of air, whereby they are converted into a pulverulent matter, somewhat
like lime in appearance, called calx in Latin. The term calcination, however, is now
used when any substance whatever is exposed to a roasting heat.
CALCIUM. The metallic basis of lime. See Lime.
[209]
CALC-SINTER. The incrustations of carbonate of lime upon the ground, or the
pendulous conical pieces called stalactites, attached to the roofs of caverns, are so called.
CALC-TUFF. A semi-hard irregular deposit of carbonate of lime, formed from
the waters of calcareous springs.
CALCULUS. The stony-looking morbid concretion, occasionally formed in the
bladder of urine, gall-bladder, cystic duct, kidneys, and other parts of living animals.
Its examination belongs to medical chemistry.
CALENDER, (Calandre, Fr.; Kalander, Germ.) a word derived from the Greek
kalindros (cylinder), is the name of a machine, consisting of two or more cylinders, revolving
so nearly in contact with each other that cloth passed through between them is smoothed,
and even glazed, by their powerful pressure. It is employed either to finish goods for the
market, or to prepare cotton and linen webs for the calico-printer, by rendering their surfaces
level, compact, and uniform. This condensation and polish, or satinage, as the French
call it, differ in degree according to the object in view, and may be arranged into three
distinct series. 1. For goods which are to receive the first impression by the block,
a very strong pressure is required; for, upon the uniformity of the polish, the neatness
and regularity of the printing, and the correspondence of its members, depend. In
many establishments the calico is passed twice through the calender before being sent
to the tables. 2. The pieces already dyed up at the madder bath, or otherwise, and
which remain to be filled in with other colours, or grounded-in, as it is technically styled,
must receive a much less considerable gloss. This is a principle every where admitted
and acted upon, because the outline of the figured design being deranged by the
washing, and sometimes in consequence of the peculiar texture of the cloth, the printer,
in order to apply his grounding blocks properly, and to fit them to the contours of the
figures already impressed, is obliged to stretch the piece sometimes in the direction of
the warp, and sometimes of the weft, which would be impossible if they had been hard
glazed by the calender. 3. The degree of glazing given to finished goods depends upon
the taste of purchasers, and the nature of the article; but it is, in general, much less
than for the first course of block-printing.
The most complete calender probably in existence is that used by some of the
eminent calico-printers of Alsace, as contrived by M. Charles Dollfus, and constructed
by MM. Witz, Blech, and Co. 1. It passes two pieces at once, and thus does double the
work of any ordinary machine. 2. It supersedes the necessity of having a workman to
fold up the goods, as they emerge from the calender, with the aid of a self-acting folder.
3. It receives, at pleasure, the finished pieces upon a roller, instead of laying them in
folds; and, by a very simple arrangement, it hinders the hands of the workmen from
being caught by the rollers.
Calenders, in consequence of the irregular demand for foreign orders and shipments,
are worked very irregularly, being sometimes overloaded with duty, and at others
altogether unemployed. A machine which can, when required, turn out a double
quantity of goods must, therefore, be a desirable possession. For the first course of
the printers, where high calendering is necessary, the goods are usually passed twice
through between two paper cylinders, to give that equality of surface which could not
be obtained by one passage, however strong the pressure; and therefore the simplification
of this calender will prove no economy. Besides, in order to increase the pressure to
the requisite degree, the cylinders would need to be made bulging at their middle part,
and with such cylinders common smoothing could not be given; for the pieces would
be glazed in the central line, and rough towards the edges. For pieces already printed
in part, and requiring only to be grounded-in for other colours, the system of double
effect has fewer objections, as a single passage through the excellent calender described
under Bleaching, page 134., is found to answer very well.
The most remarkable feature of M. Dollfus’s machine is its being managed by a single
workman. Six or eight pieces are coiled upon the feed-roller, and they are neither pasted
nor stitched together, but the ends are merely overlapped half a yard or so. The
workman is careful not to enter the second piece till one third or one half of the first
one has passed through on the other side, to prevent his being engrossed with two ends
at a time. He must, no doubt, go sometimes to the one side and sometimes to the
other of the machine to see that no folds or creases occur, and to be ready for supplying
a fresh piece as the preceding one has gone through. The mechanism of the folder in
the Alsace machine is truly ingenious: it performs extremely well, really saves the
attendance of an extra workman, and is worthy the attention of manufacturers intent
upon economising hand labour. The lapping-roller works by friction, and does its duty
fully better than similar machines guided by the hand.
The numerous accidents which have happened to the hands of workmen engaged in
calenders should direct the attention towards its effective contrivance for preventing
such misfortunes. These various improvements in the Alsace machine may be easily
adapted to the ordinary calenders of almost every construction.
[210]
The folder is a kind of cage, in the shape of an inverted pyramid, shut on the four
sides, and open at top and bottom: the top orifice is about five inches, the bottom one
an inch and a half: the front and the back, which are about four feet broad, are made
of tin-plate or smooth pasteboard, and the two sides are made of strong sheet-iron; the
whole being bolted together by small bars of iron. Upon the sheet-iron of the sides,
iron uprights are fixed, perforated with holes, through which the whole cage is supported
freely by means of studs that enter into them. One of the uprights is longer than the
other, and bears a slot with a small knob, which, by means of the iron piece, joins the
guide to the crank of the cylinder, and thereby communicates to the cage a seesaw
movement: at the bottom extremity of the great upright, there is a piece of iron in
the shape of an anchor, which may be raised, or lowered, or made fast, by screws.
At the ends of this anchor are friction-rollers, which may be drawn out or pushed back
and fixed by screws: these rollers lift alternately two levers made of wood, and fixed to
a wooden shaft.
The paws are also made of wood: they serve to lay down alternately the plies of the
cloth which passes upon the cage, and is folded zigzag upon the floor, or upon a board
set below the cage: a motion imparted by the seesaw motion of the cage itself. See
Stretching Machine.
To protect the fingers of the workmen, above the small plate of the spreading-board
or bar, there is another bar, which forms with the former an angle of about 75°: they
come sufficiently near together for the opening at the summit of the angle to allow the
cloth to pass through, but not the fingers. See Bulletin de la Société Industrielle de
Mulhausen, No. 18.
I shall now describe, more minutely, the structure of the powerful but less complicated
calender mechanisms employed in the British manufactories.
A front elevation of a four-rollered calender (five rollers are often introduced) for glazing
goods is given in fig. 228. d l are two pasteboard or paper cylinders, each 20 inches in diameter,
whose structure will be presently described: f is a cast-iron cylinder turned perfectly
smooth (its fellow is often placed between e and d): it is eight inches in diameter outside,
four inches inside, with two inches thickness of metal. e is another pasteboard cylinder,
fourteen inches in diameter: the strong cast-iron frame contains the bushes in which
the journals of the rollers turn. o p, is one of the pair of levers for communicating a
graduated pressure according to the quality of the goods. Fig. 229, 230. are end views of
the same machine to show the working geer. The wheel s, on the end of the upper iron
cylinder, is ten inches in diameter; that on the end of the fellow iron cylinder below
(when it is present) is thirteen inches; both are connected by the larger carrier wheel
t. The lower wheel u is one third larger than the upper wheel, and therefore receives
from the carrier wheel t, a proportionally slower motion, which it imparts to the central
pasteboard roller e, lying upon it, causing it to move one third more slowly than the
upper pasteboard roller. Thus a sort of sliding motion is produced, which, by rubbing
their surfaces, glazes the goods.
The iron rollers are made hollow for the purpose of admitting either a hot roller of[211]
iron, or steam when hot calendering is required. The other cylinders used formerly to
be made of wood, but it was liable to many defects. The advantage of the paper roller
consists in its being devoid of any tendency to split, crack, or warp, especially when
exposed to a considerable heat from the contact and pressure of the hot iron rollers.
The paper, moreover, takes a vastly finer polish, and, being of an elastic nature, presses
into every pore of the cloth, and smooths its surface more effectually than any wooden
cylinder, however truly turned, could possibly do.
The paper cylinder is constructed as follows:—The axis of the cylinder is a strong
square bar of the best wrought iron, cut to the proper length. Upon this bar a strong
round plate of cast iron is first put, somewhat less in diameter than the cylinder when
finished. A quantity of thick stout pasteboard is then procured, and cut into round
pieces an inch larger in diameter than the iron plate. In the centre of the plates, and
of every piece of the pasteboard, a square hole must be cut to receive the axis; and, the
circle being divided into six equal parts, a hole must also be cut at each of the divisions,
an inch or two within the rim. These pieces of pasteboard being successively put
upon the axis, a long bolt of malleable iron, with a head at one end, and screwed at the
other, is also introduced through each of the holes near the rim; and this is continued
until a sufficient number of pasteboards are thus placed to form a cylinder of the
length required, proper allowance being made for the compression which the pasteboard
is afterwards to undergo. Another round plate is then applied, and, nuts being put
upon the screws, the whole are screwed tight, and a cylinder formed. This cylinder is
now to be placed in a stove, exposed to a strong heat, and must be kept there for at least
several days; and, as the pasteboard shrinks by exposure to the heat, the screws must
be frequently tightened until the whole mass has been compressed as much as possible.
When the cylinder is thus brought to a sufficient degree of density it is removed from
the stove; and, when allowed to cool, the pasteboard forms a substance almost inconceivably
dense and hard. Nothing now remains but to turn the cylinder; and this is an
operation of no slight labour and patience. The motion in turning must be slow, not
exceeding about forty revolutions in a minute; the substance being now so hard and
tough that tools of a very small size must be used to cut, or rather scrape it, until it
is true. Three men are generally employed for the turning, even when the motion of
the cylinder is effected by mechanical power, two being necessary to sharpen tools, for
the third who turns, as quickly as he blunts them.
Let us suppose it to be a five-rollered machine: when a person stands in front of
the calender, the cloth coming from behind above the uppermost cylinder 1, passes between
1 and 2: proceeding behind 2, it again comes to the front between 2 and 3:
between 3 and 4 it is once more carried behind, and, lastly, brought in front between
4 and 5, where it is received, and smoothly folded on a clean board, or in a box, by a
person placed there for the purpose. In folding the cloth at this time, care must be
taken that it may be loosely done, so that no mark may appear until it be again folded
in the precise length and form into which the piece is to be made up. The folding may
be done either by two persons or by one, with the aid of two sharp polished spikes
placed at a proper distance, to ascertain the length of the fold, and to make the whole
equal. When folded into lengths, it is again folded across upon a smooth clean table,
according to the shape intended, which varies with the different kinds of goods, or the
particular market for which the goods are designed.
When the pieces have received the proper fold, the last operation previous to packing
them is the pressing. This is commonly performed by placing a certain number of pieces,
divided by thin smooth boards of wood, in a common screw press, similar to those used
by printers for taking out the impression left by the types in the printing-press. Besides
the wooden boards, a piece of glazed pasteboard is placed above and below every
piece of cloth, that the outer folds may be as smooth and glossy as possible. The
operation of the common screw press being found tedious and laborious, the hydraulic
press is now in all well mounted establishments had recourse to. See Hydraulic
Press.
No improvements that have taken place in calendering can exceed the power and facility
of the water press: one of these presses may be worked by two men, who can
with great ease produce a pressure of 400 tons; but, in considerable establishments, the
presses are worked by power. See Bandanna.
The appearance and finish of the goods, in consequence of such an immense weight
acting on them, are materially improved.
The press is also used for the purpose of packing; whereby the bale is rendered
much more compact than formerly. It is commonly roped, &c., while in this compressed
state; the dimensions, are therefore, greatly diminished from what they would
otherwise be by any other method. For instance, the same quantity of goods packed
in a bale are from one third to one half less bulky than if they were packed in a box
with the utmost force of the hands.
[212]
For lawns and muslins of a light texture, the operation of smoothing requires a different
process in some respects than close heavy fabrics. They only require to be slightly
smoothed to remove any marks which they may have received at the bleaching; and, as
their beauty depends rather on their transparency than their closeness, the more the cylindrical
form of the yarn is preserved the better. They are therefore put through a
small machine, consisting of three rollers or cylinders; and, as the power required to
move this is small, the person who attends it generally drives it by a small winch; or
the same effect may be produced by passing the muslins between only two or three rollers
of the above calender, lightly loaded.
In the thick fabrics of cloth, including those kinds which are used for many parts of
household furniture, as also those for female dress, the operation of glazing is used both
to add to the original beauty of the cloth, and to render it more impervious to dust or
smoke. The glazing operation is performed entirely by the friction of any smooth
substance upon the cloth; and, to render the gloss brighter, a small quantity of bleached
wax is previously rubbed over the surface. The operation of glazing by the common
plan is very laborious, but the apparatus is of the most simple kind. A table is mounted
with a thick stout cover of level and well-smoothed wood, forming an inclined plane;
that side where the operator stands at work being the lowest. The table is generally
placed near a wall, both for convenience in suspending the glazing apparatus, and for the
sake of light. A long piece of wood is suspended in a groove formed between two longitudinal
beams, placed parallel to the wall, and fixed to it. The groove resembles
exactly the aperture between the shears of a common turning lathe. The lever, of which
the groove may be supposed to be the centre or fulcrum, is faced at the bottom with a
semi-cylindrical piece of finely polished flint, which gives the friction to the cloth stretched
upon the table below. Above the flint are two cross handles, of which the operator
lays hold, and moves them backward and forward with his hands, keeping the flint pressing
slightly upon the cloth. When he has glazed a portion equal to the breadth of the
flint, he moves his lever between the shears sidewise, and glazes a fresh part: thus he
proceeds from one side or selvage of the cloth to the other: and when all which is upon
the table is sufficiently glazed, he draws it over, and exposes a new portion to the same
operation. To preserve the cloth at a proper tension, it may be wound smoothly upon
a roller or beam, which being set so as to revolve upon its own axis behind the table,
another roller to receive the cloth may be placed before, both being secured by a catch,
acting in a ratchet wheel. Of late years, however, a great part of the labour employed
in glazing cloth has been saved, as the common four or five bowl calender has been altered
to fit this purpose by direct pressure.
As a matter of accommodation, the different processes of packing, cording of boxes,
sheeting of trunks, and, in general, all the arrangements preparatory to shipments, and
also the intimations and surveys necessary for obtaining drawbacks, debentures, or
bounties, according to the excise laws, are generally conducted at the calender houses
where goods are finished. These operations sufficiently account for the general meaning
attached to the word.
CALICO-PRINTING (Impression d’Indiennes, Fr.; Zeugdruckerei, Germ.) is the
art of impressing cotton cloth with topical dyes of more or less permanence. Of late
years, silk and woollen fabrics have been made the subjects of a similar style of
dyeing. Linens were formerly stained with various coloured designs, but since the
modern improvements in the manufacture of cotton cloth they are seldom printed, as
they are both dearer, and produce less beautiful work, because flax possesses less affinity
than cotton for colouring matters.
This art is of very ancient date in India, and takes its English name from Calicut, a
district where it has been practised with great success from time immemorial. The
Egyptians, also, appear from Pliny’s testimony to have practised at a remote era some of
the most refined processes of topical dyeing. “Robes and white veils,” says he, “are
painted in Egypt in a wonderful way. They are first imbued, not with dyes, but with
dye-absorbing drugs, by which, though they seem to be unaltered, yet, when immersed
for a little while in a cauldron of the boiling dye-liquor, they are found to become
painted. Yet, as there is only one colour in the cauldron, it is marvellous to see many
colours imparted to the robe, in consequence of the influence of the excipient drug. Nor
can the dye be washed out. A cauldron, which would of itself merely confuse the colours
of cloths previously dyed, is thus made to impart several pigments from a single dye-stuff,
painting as it boils.” The last expression pingitque dum coquit, is perfectly graphic
and descriptive of calico-printing.
The cotton chintz counterpanes of great size, called pallampoors, which have been
manufactured in Madras from the earliest ages, have in like manner peculiar dye-absorbing
drugs applied to them with the pencil, as also wax, to protect certain parts of the
surface from the action of the dye, and are afterwards immersed in a staining liquor, which,
when wax is applied, is usually the cold indigo-vat, but without the wax is a hot liquor
similar to the Egyptian. M. Koechlin Roder, of Mulhouse, brought home lately from[213]
India a rich collection of cloths in this state of preparation, which I saw in the
cabinet of the Société Industrielle of that interesting emporium of calico-printing.
The native implements for applying the wax and colouring bases are placed alongside
of the cloths, and form a curious picture of primeval art. There is among
other samples an ancient pallampoor, five French yards long, and two and a half broad,
said to be the labour of Hindoo princesses, which must have taken a lifetime to execute.
The printing machinery of great Britain has begun to supersede, for these
styles of work, the cheapest hand labour of India.
Calico-printing has been for several hundred years practised by the oriental methods
in Asia Minor and the Levant, but it was unknown as an English art till 1696,
when a small print-ground was formed upon the banks of the Thames, near Richmond,
by a Frenchman; probably a refugee from his own country, in consequence of the
revocation of the edict of Nantes. Some time afterwards, a considerable printing
work was established at Bromley Hall, in Essex, and several others sprung up successively
in Surrey, to supply the London shops with chintzes, their import from India
having been prohibited by act of parliament in 1700. The silk and woollen weavers,
indeed, had all along manifested the keenest hostility to the use of printed calicoes,
whether brought from the East or made at home. In the year 1680 they mobbed the
India House in revenge for some large importations then made of the chintzes of
Malabar. They next induced the government, by incessant clamours, to exclude altogether
the beautiful robes of Calicut from the British market. But the printed goods,
imported by the English and Dutch East India companies, found their way into this
country, in spite of the excessive penalties annexed to smuggling, and raised a new alarm
among the manufacturing population of Spitalfields. The sapient legislators of that day,
intimidated, as would appear, by the East London mobs, enacted in 1720 an absurd sumptuary
law, prohibiting the wearing of all printed calicoes whatsoever, either of foreign or
domestic origin. This disgraceful enactment, worthy of the meridian of Cairo or
Algiers, proved not only a death blow to rising industry in this ingenious department
of the arts, but prevented the British ladies from attiring themselves in the becoming
drapery of Hindostan. After an oppressive operation of ten years, this act was repealed
by a partially enlightened set of senators, who were then pleased to permit what they
called British calicoes, if made of linen warp, with merely weft of the hated cotton, to be
printed and worn, upon paying a duty of no less than sixpence the square yard. Under
this burden, English calico-printing could not be expected to make a rapid progress.
Accordingly, even so lately as the year 1750, no more than 50,000 pieces of mixed
stuff were printed in Great Britain, and that chiefly in the neighbourhood of London;
whereas a single manufacturer, Mr. Coates of Manchester, now-a-days will turn off
nearly twenty times that quantity, and there are very many others who manufacture
several hundred thousand pieces per annum. It was not till about 1766 that this art
migrated into Lancashire, where it has since taken such extraordinary development;
but it was only after 1774 that it began to be founded upon right principles, in consequence
of the repeal of that part of the act of 1730 which required the warp to be made
of linen yarn. Henceforth the printer, though still saddled with a heavy duty of 3d.
the square yard, was allowed to apply his colours to a homogeneous web, instead of the
mixed fabric of linen and cotton substances, which differ in their affinities for dyes.
France pursued for some time a similar false policy with regard to calico-printing, but
she emerged sooner from the mists of manufacturing monopoly than England. Her
avowed motive was to cherish the manufacture of flax, a native product, instead of that
of cotton, a raw material, for which prejudice urged that money had to be exported.
Her intelligent statesmen of that day, fully seventy years ago, replied, that the money
expended in the purchase of cotton was the produce of French industry, beneficially
employed, and they therefore took immediate measures to put the cotton fabrics upon a
footing of equality. Meanwhile the popular prejudices became irritated to such a degree,
by the project of permitting the free manufacture and sale of printed cottons, that every
French town possessed of a chamber of commerce made the strongest remonstrances
against it. The Rouen deputies declared to the government, “that the intended measure
would throw its inhabitants into despair, and make a desert of the surrounding
country;” those of Lyons said, “the news had spread terror through all its work-shops:”
Tours “foresaw a commotion likely to convulse the body of the state:” Amiens
said, “that the new law would be the grave of the manufacturing industry of France;”
and Paris declared that “her merchants came forward to bathe the throne with their tears
upon that inauspicious occasion.”
The government persisted in carrying its truly enlightened principles into effect, and
with so manifest advantage to the nation, as to warrant the inspector-general of manufactures
to make, soon afterwards, the following appeal to those prejudiced bodies:—“Will
any of you now deny that the fabrication of printed cottons has occasioned a vast
extension of the industry of France, by giving profitable employment to a great many[214]
hands in spinning, weaving, bleaching, and printing the colours? Look only at the
dyeing department, and say whether it has not done more good to France in a few years
than many of your other manufactures have in a century?”
The despair of Rouen has been replaced by the most signal prosperity in the cotton
trade, and especially in printed calicoes, for the manufacture of which it possesses 70
different establishments, producing upwards of a million of pieces of greater average size
and price than the English. In the district of the Lower Seine, round that town, there
are 500 cotton factories of different kinds, which give employment to 118 thousand
operatives of all orders, and thus procure a comfortable livelihood to probably not less
than half a million of people.
The repeal, in 1831, of the consolidated duty of 31⁄2d. per square yard upon printed
calicoes in Great Britain is one of the most judicious acts of modern legislation. By
the improvements in calico-printing, due to the modern discoveries and inventions in
chemistry and mechanics, the trade had become so vast as to yield in 1830 a revenue of
2,280,000l. levied upon 8,596,000 of pieces, of which, however, about three fourths were
exported, with a drawback of 1,579,000l. 2,281,512 pieces were consumed in that year
at home. When the expenses of collection were deducted, only 350,000l. found their
way into the exchequer, for which pitiful sum thousands of frauds and obstructions were
committed against the honest manufacturer. This reduction of duty enables the consumer
to get this extensive article of clothing from 50 to 80 per cent. cheaper than
before, and thus places a becoming dress within the reach of thousands of handsome
females in the humbler ranks of life. Printed goods, which in 1795 were sold for two
shillings and three-pence the yard, may be bought at present for eight-pence. In fact a
woman may now purchase the materials of a pretty gown for two shillings. The repeal
of the tax has been no less beneficial to the fair dealers, by putting an end to the contraband
trade, formerly pursued to an extent equally injurious to them and the revenue.
It has, moreover, emancipated a manufacture, eminently dependent upon taste, science,
and dexterity, from the venal curiosity of petty excisemen, by whom private improvements,
of great value to the inventor, were in perpetual jeopardy of being pirated and
sold to any sordid rival. The manufacturer has now become a free agent, a master of
his time, his workmen, and his apparatus; and can print at whatever hour he may receive
an order; whereas he was formerly obliged to wait the convenience of the excise
officer, whose province it was to measure and stamp the cloth before it could be packed,—an
operation fraught with no little annoyance and delay. Under the patronage of parliament,
it was easy for needy adventurers to buy printed calicoes, because they could
raise such a sum by drawbacks upon the export of one lot as would go far to pay for
another, and thus carry on a fraudulent system of credit, which sooner or later merged
in a disastrous bankruptcy. Meanwhile the goods thus obtained were pushed off to
some foreign markets, for which they were, possibly, not suited, or where they produced,
by their forced sales a depreciation of all similar merchandize, ruinous to them and who
meant to pay for his wares.
The principles of calico-printing have been very profoundly studied by many of the French
manufacturers, who generally keep a chemist, who has been educated in the Parisian
schools of science, constantly at work, making experiments upon colours in a well-mounted
laboratory. In that belonging to M. Daniel Kœchlin, of Mulhausen, there are upwards
of 3000 labelled phials, filled with chemical reagents, and specimens subservient to dyeing.
The great disadvantage under which the French printers labour is the higher price they
pay for cotton fabrics, above that paid by the English printers. It is this circumstance
alone which prevents them from becoming very formidable rivals to us in the markets of
the world. M. Barbet, deputy and mayor of Rouen, in his replies to the ministerial
commission of inquiry, rates the disadvantage proceeding from that cause at 2 francs per
piece, or about 5 per cent. in value. In the annual report of the Société Industrielle of
Mulhausen, made in December, 1833, the number of pieces printed that year in Alsace
is rated at 720,000, to which if we add 1,000,000 for the produce of the department of
the Lower Seine, and 280,000 for that of St. Quentin, Lille, and the rest of France, we
shall have for the total amount of this manufacture 2,000,000 of pieces, equivalent to
nearly 2,400,000 pieces English; for the French piece usually measures 331⁄2 aunes, = 41
yards nearly; and it is also considerably broader than the English pieces upon
an average. It is therefore probable that the home consumption of France in printed
goods is equal in quantity, and superior in value, to that of England. With regard to
the comparative skill of the workmen in the two countries, M. Nicholas Kœchlin, deputy
of the Upper Rhine, says, that one of his foremen, who worked for a year in a
print-field in Lancashire, found little or no difference between them in that respect. The
English wages are considerably higher than the French. The machines for multiplying
production, which for some time gave us a decided advantage, are now getting into
very general use among our neighbours. In my recent visit to Mulhausen, Rouen, and
their environs, I had an opportunity of seeing many printing establishments mounted
with all the resources of the most refined mechanisms.
[215]
The calico-printing of this country still labours under the burden of considerable taxes
upon madder and gallipoli oil, which have counteracted the prosperity of our Turkey red
styles of work, and caused them to nourish at Elberfeld, and some other places on the
continent, whither a good deal of the English yarns are sent to be dyed, then brought
back, and manufactured into ginghams, checks, &c., or forwarded directly thence to our
Russian customers. This fact places our fiscal laws in the same odious light as the
facility of pirating printer’s patterns with impunity does our chancery laws.
Before cloth can receive good figured impressions its surface must be freed from
fibrous down by Singeing, and be rendered smooth by the Calender. See these articles.
They are next bleached, with the exception of those destined for Turkey red. See
Bleaching and Madder. After they are bleached, dried, singed, and calendered, they
are lapped round in great lengths of several pieces, stitched endwise together, by means
of an apparatus called, in Manchester, a candroy, which bears on its front edge a rounded
iron bar, transversely grooved to the right and left from the centre, so as to spread out
the web as it is drawn over it by the rotation of the lapping roller. See a figure of
this bar subservient to the cylinder printing-machine.
Four different methods are in use for imprinting figures upon calicoes: the first is by
small wooden blocks, on whose face the design is cut, which are worked by hand; the second
is by larger wood-cut blocks, placed in either two or three planes, standing at right angles
to each other, called a Perrotine, from the name of its inventor; the third is by flat copper
plates, a method now almost obsolete; and the fourth is by a system of copper cylinders,
mounted in a frame of great elegance, but no little complexity, by which two, three,
four, or even five colours may be printed on in rapid succession by the mere rotation
of the machine driven by the agency of steam or water. The productive powers of this
printing automaton are very great, amounting for some styles to a piece in the minute,
or a mile of cloth in the hour. The fifth colour is commonly communicated by means
of what is called a surface cylinder, covered with wooden figures in bas-relief, which,
by rotation, are applied to a plane of cloth imbued with the thickened mordants.
The hand blocks are made of sycamore or pear-tree wood, or of deal faced with these
woods, and are from two to three inches thick, nine or ten inches long, and five broad, with
a strong box handle on the back for seizing them by. The face of the block is either
carved in relief into the desired design, like an ordinary wood-cut, or the figure is formed
by the insertion edgewise into the wood of narrow slips of flattened copper wire. These
tiny fillets, being filed level on the one edge, are cut or bent into the proper shape, and
forced into the wood by the taps of a hammer at the traced lines of the configuration.
Their upper surfaces are now filed flat, and polished into one horizontal plane, for the
sake of equality of impression. As the slips are of equal thickness in their whole depth,
from having been made by running the wire through between the steel cylinders of a
flatting mill, the lines of the figure, however much they get worn by use, are always
equally broad as at first; an advantage which does not belong to wood-cutting. The
interstices between the ridges thus formed are filled up with felt-stuff. Sometimes a
delicate part of the design is made by the wood-cutter, and the rest by the insertion of
copper slips.
The colouring matter, properly thickened, is spread with a flat brush, by a child, upon
fine woollen cloth, stretched in a frame over the wax cloth head of a wooden drum or
sieve, which floats inverted in a tubful of old paste, to give it elastic buoyancy. The
inverted sieve drum should fit the paste tub pretty closely. The printer presses
the face of the block on the drum head, so as to take up the requisite quantity of
colour, applies it to the surface of the calico, extended upon a flat table covered
with a blanket, and then strikes the back of the block with a wooden mallet, in
order to transfer the impression fully to the cloth. This is a delicate operation,
requiring equal dexterity and diligence. To print a piece of cloth 28 yards long,
and 30 inches broad, no less than 672 applications of a block, 9 inches long and 5
inches broad, are requisite for each colour; so that if there are 3 colours, or 3 hands, as
the French term it, no less than 2016 applications will be necessary. The blocks
have pin-points fixed into their corners, by means of which they are adjusted to their
positions upon the cloth, so as to join the different parts of the design with precision.
Each printer has a colour-tub placed within reach of his right hand; and
for every different colour he must have a separate sieve. Many manufacturers cause
their blocks to be made of three layers of wood, two of them being deal with the
grain crossed to prevent warping, and the third sycamore for engraving.
The printing shop is an oblong apartment, lighted with numerous windows at each
side, and having a solid table opposite to each window. The table B, fig. 231. is formed
of a strong plank of well-seasoned hard wood, mahogany, or marble, with a surface truly
plane. Its length is about 6 feet, its breadth 2 feet, and its thickness 3, 4, or 5 inches.
It stands on strong feet, with its top about 36 inches above the floor. At one of its
ends there are two brackets C for supporting the axles of the roller E, which carries the[216]
white calico to be printed. The hanging rollers E are laid across joists fixed near the
roof of the apartment above the printing shop, the ceiling and floor between them being
open bar work, at least in the middle of the room. Their use is to facilitate the
exposure, and, consequently, the drying of the printed pieces, and to prevent one figure
being daubed by another. Should they come to be all filled, the remainder of the
goods must be folded lightly upon the stool D.
The printer stretches a length of the piece upon his table A B, taking care to place
the selvage towards himself, and one inch from the edge. He presents the block
towards the end, to determine the width of its impression, and marks this line A B, by
means of his square and tracing point. The spreader now besmears the cloth with the
colour, at the commencement, upon both sides of the sieve head; because, if not uniformly
applied, the block will take it up unequally. The printer seizes the block in his right
hand, and daubs it twice in different directions upon the sieve cloth, then he transfers it
to the calico in the line A B, as indicated by the four points a b c d, corresponding to
the four pins in the corners of the block. Having done so, he takes another daub of
the colour, and makes the points a b fall on c d, so as to have at the second stamp
a′ b′, covering a b and c′ d′; and so on, through the rest, as denoted by the accented
letters. When one table length is finished, he draws the cloth along, so as to bring a
new length in its place.
The grounding in, or re-entering (rentrage), of the other colours is the next process.
The blocks used for this purpose are furnished with pin-points, so adjusted that, when
they are made to coincide with the pin-points of the former block, the design will be
correct; that is to say, the new colour will be applied in its due place upon the flower
or other figure. The points should not be allowed to touch the white cloth, but should
be made to fall upon the stem of a leaf, or some other dark spot. These rentrages are
of four sorts:—1. One for the mordants, as above; 2. one for topical colours; 3. one
for the application of reds; and, 4., one for the application of resist pastes or reserves.
These styles have superseded the old practice of pencilling.
The Perrotine is a machine for executing block-printing by mechanical power; and
it performs as much work, it is said, as 20 expert hands. I have seen its operation, in
many factories in France and Belgium, in a very satisfactory manner; but I have
reason to believe that there are none of them as yet in this country. Three wooden
blocks, from 21⁄2 to 3 feet long, according to the breadth of the cloth, and from 2 to 5
inches broad, faced with pear-tree wood, engraved in relief, are mounted in a powerful
cast-iron frame work, with their planes at right angles to each other, so that each
of them may, in succession, be brought to bear upon the face, top, and back of a
square prism of iron covered with cloth, and fitted to revolve upon an axis between the
said blocks. The calico passes between the prism and the engraved blocks, and
receives successive impressions from them as it is successively drawn through by a
winding cylinder. The blocks are pressed against the calico through the agency of
springs, which imitate the elastic pressure of the workman’s hand. Each block receives
a coat of coloured paste from a woollen surface, smeared after every contact with a
mechanical brush. One man, with one or two children for superintending the colour-giving
surfaces, can turn off about 30 pieces English per day, in three colours, which
is the work of fully 20 men and 20 children in block printing by hand. It executes
some styles of work to which the cylinder machine, without the surface roller, is
inadequate.
[217]
The copper-plate printing of calico is almost exactly the same as that used for
printing engravings on paper from flat plates, and being nearly superseded by the next
machine, need not be described.
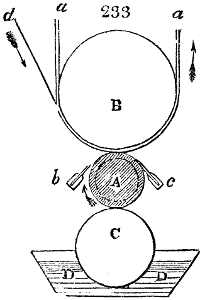
The cylinder printing machine consists, as its name imports, of an engraved copper
cylinder, so mounted as to revolve against another cylinder lapped in woollen cloth, and
imbued with a coloured paste, from which it derives the means of communicating
coloured impressions to pieces of calico passed over it. Fig. 233.
will give the reader a general idea of this elegant and expeditious
plan of printing. The pattern is engraved upon the
surface of a hollow cylinder of copper, or sometimes gun-metal,
and the cylinder is forced by pressure upon a strong
iron mandrel, which serves as its turning shaft. To facilitate
the transfer of the impression from the engraving to the cotton
cloth, the latter is lapped round another large cylinder, rendered
elastic by rolls of woollen cloth, and the engraved cylinder
presses the calico against this elastic cushion, and thereby prints
it as it revolves. Let A be the engraved cylinder mounted upon
its mandrel, which receives rotatory motion by wheels on its
end, connected with the steam or water power of the factory.
B is a large iron drum or roller, turning in bearings of the end
frames of the machine. Against that drum the engraved cylinder
A is pressed by weights or screws; the weights acting steadily, by levers, upon its
brass bearings. Round the drum B the endless web of felt or blanket stuff a a, travels in
the direction of the arrow, being carried round along with the drum B, which again is
turned by the friction of contact with the cylinder A. c represents a clothed wooden roller,
partly plunged into the thickened colour of the trough D D. That roller is also made
to bear, with a moderate force, against A, and thus receives, by friction, in some cases,
a movement of rotation. But it is preferable to drive the roller C from the cylinder A,
by means of a system of toothed wheels attached to their ends, so that the surface speed
of the wooden or paste roller shall be somewhat greater than that of the printing cylinder,
whereby the colour will be rubbed, as it were, into the engraved parts of the latter.
As the cylinder A is pressed upwards against B, it is obvious that the bearers of the
trough and its roller must be attached to the bearings of the cylinder A, in order to
preserve its contact with the colour-roller C. b is a sharp-edged ruler of gun-metal or
steel, called the colour doctor, screwed between two gun-metal stiffening bars; the edge
of which wiper is slightly pressed as a tangent upon the engraved roller A. This ruler
vibrates with a slow motion from side to side, or right to left, so as to exercise a
delicate shaving action upon the engraved surface, as this revolves in the direction of the
arrow. c is another similar sharp-edged ruler, called the lint doctor, whose office it is
to remove any fibres which may have come off the calico in the act of printing, and
which, if left on the engraved cylinder, would be apt to occupy some of the lines, or at
least to prevent the colour from filling them all. This lint doctor is pressed very
slightly upon the cylinder A, and has no traverse motion.
What was stated with regard to the bearers of the colour trough D, namely, that they
are connected, and moved up and down together with the bearings of the cylinder A,
may also be said of the bearers of the two doctors.
The working of this beautiful mechanism may now be easily comprehended. The
web of calico, indicated in the figure by the letter d, is introduced or carried in along
with the blanket stuff a a, in the direction of the arrow, and is moved onward by the
pressure of the revolving cylinder A, so as to receive the impression of the pattern engraved
on that cylinder.
Before proceeding to describe the more complex calico-machine which prints upon
cloth, 3, 4, or 5 colours at one operation, by the rotation of so many cylinders, I shall
explain the modern methods of engraving the cylinder, which I am enabled to do by the
courtesy of Mr. Locket, of Manchester, an artist of great ingenuity in this department,
who politely allowed me to inspect the admirable apparatus and arrangements of his
factory.
To engrave a copper cylinder 3 or 4 inches in diameter, and from 30 to 36 inches long
with the multitude of minute figures which exist in many patterns, would be a very
laborious and expensive operation. The happy invention made by Mr. Jacob Perkins,
in America, for transferring engravings from one surface to another by means of steel
roller dies, was with great judgment applied by Mr. Locket to calico-printing, so long
ago as the year 1808, before the first inventor came to Europe with the plan. The
pattern is first drawn upon a scale of about 3 inches square, so that this size of figure
being repeated a definite number of times, will cover the cylinder. This pattern is next
engraved in intaglio upon a roller of softened steel, about 1 inch in diameter, and 3 inches
long, so that it will exactly occupy its surface. The engraver aids his eye with a[218]
lens, when employed at this delicate work. This roller is hardened by heating it to a
cherry-red in an iron case containing pounded bone-ash, and then plunging it into cold
water; its surface being protected from oxidizement by a chalky paste. This hardened
roller is put into a press of a peculiar construction, where, by a rotatory pressure, it
transfers its design to a similar roller in the soft state; and as the former was in intaglio,
the latter must be in relievo. This second roller being hardened, and placed in an appropriate
volutory press, is employed to engrave by indentation upon the full-sized copper
cylinder, the whole of its intended pattern. The first roller engraved by hand is called
the die; the second, obtained from it by a process like that of a milling tool, is called the
mill. By this indentation and multiplication system, an engraved cylinder may be had
for seven pounds, which engraved by hand would cost fifty or upwards. The restoration
of a worn-out cylinder becomes extremely easy in this way; the mill being preserved, need
merely be properly rolled over the copper surface again.
At other times, the hard roller die is placed in the upper bed of a screw press, not unlike
that for coining, while the horizontal bed below is made to move upon strong rollers
mounted in a rectangular iron frame. In the middle of that bed a smooth cake or flat
disc of very soft iron, about 1 inch thick, and 3 or 4 inches in diameter, is made fast
by four horizontal adjusting screws, that work in studs of the bed frame. The die being
now brought down by a powerful screw, worked by toothed wheel-work, and made to
press with force upon the iron cake, the bed is moved backwards and forwards, causing
the roller to revolve on its axles by friction, and to impart its design to the cake. This
iron disc is now case-hardened by being ignited amidst horn shavings in a box, and then
suddenly quenched in water, when it becomes itself a die in relievo. This disc die is
fixed in the upper part of a screw press with its engraved face downwards, yet so as to be
movable horizontally by traverse screws. Beneath this inverted bed, sustained at its
upper surface by friction-rollers, a copper cylinder 30 inches long, or thereby, is mounted
horizontally upon a strong iron mandrel, furnished with toothed wheels at one of its
ends, to communicate to it a movement upon its axis through any aliquot arcs of the
circle. The disc die being now brought down to bear upon the copper cylinder, this is
turned round through an arc corresponding in length to the length of the die; and thus,
by the steady downward pressure of the screw, combined with the revolution of the cylinder,
the transfer of the engraving is made in intaglio. This is I believe the most convenient
process for engraving, by transfer, the copper of a one-cylinder machine. But
when 2, 3, or 4 cylinders are to be engraved with the same pattern for a two, three, or
four-coloured machine, the die and the mill roller plan of transfer is adopted. In this
case, the hardened roller die is mounted in the upper bed of the transfer press, in such a
way as to be capable of rotation round its axis, and a similar roller of softened steel is
similarly placed in the under bed. The rollers are now made to bear on each other by
the action of the upper screw, and while in hard contact, the lower one is caused to revolve,
which, carrying round the upper by friction, receives from it the figured impression
in relief. When cylinders for a three-coloured machine are wanted, three such mills
are made fac-similes of each other; and the prominent parts of the figure which belong
to the other two copper cylinders are filed off in each one respectively. Thus three differently
figured mills are very readily formed, each adapted to engrave its particular figure
upon a distinct copper cylinder.
Some copper cylinders for peculiar styles are not graved by indentation, as just
described, but etched by a diamond point, which is moved by mechanism in the most
curious variety of configurations, while the cylinder slowly revolves in a horizontal line
beneath it. The result is extremely beautiful, but it would require a very elaborate set
of drawings to represent the machinery by which Mr. Locket produces it. The copper
is covered by a resist varnish while being heated by the transmission of steam through its
axis. After being etched, it is suspended horizontally by the ends, for about five minutes,
in an oblong trough charged with dilute nitric acid.
With regard to the two and three-coloured machines, we must observe, that as the
calico in passing between the cylinders is stretched laterally from the central line of the
web, the figures engraved upon the cylinders must be proportionally shortened, in their
lateral dimensions especially, for the first and second cylinder.
Cylinder printing, though a Scotch invention, has received its wonderful development
in England, and does the greatest honour to this country. The economy
of labour introduced by these machines is truly marvellous; one of them, under
the guidance of a man to regulate the rollers, and the service of a boy, to supply
the colour troughs, being capable of printing as many pieces as nearly 200 men and
boys could do with blocks. The perfection of the engraving is most honourable to
our artisans. The French with all their ingenuity and neat-handedness can produce
nothing approaching in excellence to the engraved cylinders of Manchester,—a painful
admission, universally made to me by every eminent manufacturer in Alsace, whom I
visited in my late tour.
[219]
Another modification of cylinder printing, is that with wooden rollers cut in relief: it
is called surface printing, probably because the thickened colour is applied to a tense
surface of woollen cloth, from which the roller takes it up by revolving in contact with
the cloth. When the copper cylinders, and the wooden ones, are combined in one apparatus,
it has got the appropriate name of the union printing machine.
In mounting three or more cylinders in one frame, many more adjustments become
necessary than those described above. The first and most important is that which
ensures the correspondence between the parts of the figures in the successive printing
rollers, for unless those of the second and subsequent engraved cylinders be accurately
inserted into their respective places, a confused pattern would be produced upon the cloth
as it advances round the pressure cylinder B, figs. 233, 234.
Each cylinder must have a forward adjustment in the direction of rotation round its
axis, so as to bring the patterns into correspondence with each other in the length of the
piece; and also a lateral or traverse adjustment in the line of its axis, to effect the correspondence
of the figures across the piece; and thus, by both together, each cylinder
may be made to work symmetrically with its fellows.
Fig. 234. is a cross section of a four-colour cylinder machine, by which the working parts
are clearly illustrated.
A A A is a part of the two strong iron frames or cheeks, in which the various rollers are
mounted. They are bound together by the rods and bolts a a a a.
B is the large iron pressure cylinder, which rests with its gudgeons in bearings or
bushes, which can be shifted up and down in slots of the side cheeks A A. These bushes
are suspended from powerful screws b, which turn in brass nuts, made fast to the top of
the frame A, as is plainly shown in the figure. These screws serve to counteract the
strong pressure applied beneath that cylinder, by the engraved cylinders D E.
C D E F are the four printing cylinders, named in the order of their operation. They
consist of strong tubes of copper or gun-metal, forcibly thrust by a screw press upon the
iron mandrels, round which as shafts they revolve.
The first and last cylinder C and F are mounted in brass bearings, which may be shifted
in horizontal slots of the frame A. The pressure roller B, against whose surface they
bear with a very little obliquity downwards, may be nicely adjusted to that pressure by
its elevating and depressing screws. By this means C and F can be adjusted to B with
geometrical precision, and made to press it in truly opposite directions.
The bearings of the cylinders D and E are lodged also in slots of the frame A, which
point obliquely upwards, towards the centre of B. The pressure of these two print
cylinders C and F is produced by two screws c and d, which work in brass nuts, made fast
to the frame, and very visible in the figure. The frame-work in which these bearings
and screws are placed, has a curvilinear form, in order to permit the cylinders to be
readily removed and replaced; and also to introduce a certain degree of elasticity.
Hence the pressure applied to the cylinders C and F, partakes of the nature of a spring;
a circumstance essential to their working smoothly, on account of the occasional inequalities
in the thickness of the felt web and the calico.
The pressure upon the other two print cylinders D and E is produced by weights
acting with levers against the bearings. The bearings of D are, at each of their ends,
acted upon by cylindrical rods, which slide in long tubular bosses of the frame, and press
with their nuts g at their under end upon the small arms of two strong levers G, which lie
on each side of the machine, and whose fulcrum is at h (in the lower corner at the left
hand). The long arms of these levers G, are loaded with weights H, whereby they are
made to press up against the bearings of the roller D, with any degree of force, by screwing
up the nut g, and hanging on the requisite weights.
The manner in which the cylinder E is pressed up against B, is by a similar construction
to that just described. With each of its bearings, there is connected by the
link k, a curved lever I, whose fulcrum or centre of motion is at the bolt l. To the
outer end of this lever, a screw, m, is attached, which presses downwards upon the link n,
connected with the small arm of the strong lever k, whose centre of motion is at o. By
turning therefore the screw m, the weight L, laid upon the end of the long arm of the
lever K (of which there is one upon each side of the machine), may be made to act or
not at pleasure upon the bearings of the cylinder E.
In tracing the operation of this exquisite printing machine, we shall begin with the
first engraved cylinder C. Its bearings or bushes shift, as was already stated, in slots of
the frame A. Each of them consists of a round piece of iron, to which the end of the
screw c is joined, in the same way as at d, in the opposite side. In each of these iron
bearings, a concave brass is inserted to support the collar of the shaft, and in a dovetailed
slit of this brass, a sliding piece is fitted, upon which a set or adjusting screw in
the iron bearing acts, and which, being forced against the copper cylinder C, serves to
adjust the line of its axis, and to keep it steady between its bearings, and true in its
rotatory motion. Upon the iron bearing a plate is screwed, provided with two flanges,[220]
which support the colour trough q, and the colour roller M. This trough, as well as the
others to be mentioned presently, is made of sheet copper in the sides and bottom, and
fixed upon a board; but its ends are made of plates of cast copper or gun-metal to serve as
bearings to the colour roller M. The trough and its roller may be shifted both together
into contact with the printing cylinder C, by means of the screw r. Near s, seen above the
roller, C, and t below it, are sections of the two doctors, which keep the engraved cylinders
in sound working condition; the former being the colour doctor, and the latter the
lint doctor. Their ends lie in brasses, which may be adjusted by the screws u and V,
working in the respective brackets, which carry their brasses, and are made fast to the
iron bearings of the cylinder.
The pressure of the colour doctor is produced by two weights w (see high up on the
frame work), which act on a pair of small levers x, (one on each side of the machine,)
and thus, by means of the chains, tend to lift the arms y, attached to the end axles of the
doctor. The pressure of the lint doctor upon the cylinder C, is performed by the screw
z, pressing upon an arm which projects downwards, and is attached to the axle of that
doctor.
The bearings of the second printing cylinder D, consist at each end of a mass of iron
(removed in the drawing to show the mechanism below it), which shifts in the slanting
slot of the frame A. In each of these masses there is another piece of iron, which slides
in the transverse direction, and may be shifted by the adjusting screw a′ fixed to it, and
working in a nut cast upon the principal bearing above described. To the inner bearings,
which carry the brasses in which the shaft lies, are screwed the two curved arms b′ b′ to
which are attached the bearings, &c., for the colour trough, and the doctors. In these
brasses there are also dovetailed pieces, which slide and are pressed by set screws furnished
with square heads in the iron secondary bearings, which serve, as before said, to
adjust the printing cylinder in the line of its axis, while other screws adjust the distance
of the cloth upon which the second colour is printed, and the line of contact with the
cylinder B.
N, is the colour roller of D, and d′ the colour trough, which rests by its board upon the
lever e′; whose centres of motion f′, are made fast to the curved arms b′, fixed at the[221]
bearings of the cylinder, and whose ends are suspended by screws g′; whereby the colour
roller N, may be pressed with greater or less force to the cylinder D. h′ and i′ are the
two doctors of this cylinder; the former being the colour, the latter the lint doctor.
They rest, as was said of the cylinder C, in brasses which are adjustable by means of
screws, that work in the studs or brackets by which the brasses are supported. These
brackets must of course be screwed to the secondary bearing-pieces, in order that they
may keep their position, into whatever direction the bearings may be shifted. k′ and
l′ are these set screws for the colour and lint doctors. The pressure of the former upon
the cylinder D, is produced by weights m′, acting upon levers n′, and pressing by rods
or links o′, upon arms attached to each end of the axis of the doctor. (See the left hand
side of the figure near the bottom). The lint-doctor i′, is pressed in a similar way at
the other side upon the cylinder D, by the weights acting upon levers p′, and by rods q′
upon arms fixed at each end of the axis of the doctor.
The bearings of the third printing cylinder E, are of exactly the same construction as
that above described, and therefore require no particular detail. The lint doctor s, is
here pressed upon the engraved cylinder by screws t′, working in the ends of studs or
arms fixed upon each end of the axis of the doctor, and pressing upon flanges cast upon
the brackets in which the brasses of the doctor’s axis lie, which are made fast to the
bearings of the cylinder E.
The bearings of the fourth copper cylinder F, are also constructed in a similar way.
Each consists of a first bearing, to which is joined the end of the screw d, by which it
is made to slide in a slot of the frame. Another bearing, which contains the brass for
the shaft of the cylinder, can be shifted up and down in a transverse direction by a screw
z′, of the second bearing, working in a nut cast upon the first bearing. To this secondary
bearing, plates are made fast by the screws v′ v′ to the inside, to carry the studs or
brackets of the doctors x′ and y′. In the brasses of the cylinder shaft, dovetailed pieces
are made to slide, being pressed by set screws w′, against the engraved cylinder F, similar
to what has been described for adjusting the cylinders to one another. This cylinder has
no separate colour roller, nor trough, properly speaking, but the colour doctor y′ is made
concave to serve the purpose of a trough in supplying the engraved lines of the cylinder
with colour. With this view the top plate of the doctor is curved to contain the coloured
paste, and it is shut up at the ends by pieces of wood made to fit the curvature of the doctor.
Its pressure against the engraved surface is produced by weights a′′, acting at the ends
of arms b′′, attached to the ends of the axis of the doctor. The pressure of the lint
doctor x′ is given by screws c′′, working in arms attached to the ends of the axis of the
doctor, and pressing upon the flanges d′′, cast upon the brackets which carry the brasses
for the axis of the doctor. These brasses are themselves adjustable, like those of all the
other cylinders, by set screws in the brackets, which work in the nuts formed in the
brasses.
e′′ e′′, is the endless web of felt stuff which goes round the cylinder B, and constitutes
the soft elastic surface upon which the printing cylinders C, D, E, and F exercise their
pressure. This endless felt is passed over a set of rollers at a certain distance from the
machine, to give opportunity for the drying up of any colouring paste which it may
have imbibed from the calico in the course of the impressions. In its return to the machine
in the direction of the arrow, it is led over a guide roller o, which is thereby made
to revolve. Upon the two ends of this, and outside of the bearings which are fixed upon
the tops of the frame A, are two eccentrics, one of which serves to give a vibratory traverse
movement to the colour doctors s′, h′, and r′ of the three cylinders, C, D, and E
whilst the other causes the colour doctor y′ of the cylinder F, to make lateral vibrations.
Q is one of a pair of cast-iron brackets, screwed on at the back of the side-frames or
cheeks A A, to carry the roller filled with white calico R, ready for the printing operations.
Upon the end of the shaft whereon the calico is coiled, a pulley is fixed, over which a
rope passes suspending a weight in order to produce friction, and thereby resistance to
the action which tends to unwind the calico. In winding it upon that and similar
rollers, the calico is smoothed and expanded in breadth by being passed over one or
more grooved rods, or over a wooden bar S, fig. 235. the surface of
which is covered with wire, so as to have the appearance of a
united right and left-handed screw. By this device, the calico,
folded or creased at any part, is stretched laterally from the
centre, and made level. It then passes over the guide-roller
o, where it comes upon the surface of the felt e′′ e′′, and thence proceeds under its
guidance to the series of printing cylinders.
Three and four-colour machines, similar to the above, are now at work in many establishments
in Lancashire, which will turn off a piece of 28 yards per minute, each of
the three or four cylinders applying its peculiar part of the pattern to the cloth as it
passes along, by ceaseless rotation of the unwearied wheels. At this rate, the astonishing[222]
length of one mile of many-coloured web is printed with elegant flowers and other figures
in an hour. When we call to mind how much knowledge and skill are involved
in this process, we may fairly consider it as the greatest achievement of chemical and
mechanical science.
Before entering upon the different styles of work which constitute calico printing, I
shall treat, in the first place, of what is common to them all, namely, the thickening of
the mordants and colours. This is an operation of the greatest importance towards the
successful practice of the art. Several circumstances may require the consistence of the
thickening to be varied; such as the nature of the mordant, its density, and its acidity.
A strong acid mordant cannot be easily thickened with starch; but it may be by roasted
starch, vulgarly called British gum, and by gum arabic or senegal. Some mordants
which seem sufficiently inspissated with starch, liquefy in the course of a few days; and
being apt to run in the printing-on make blotted work. In France, this evil is readily
obviated, by adding one ounce of spirits of wine to half a gallon of colour; a remedy
which the English excise duties render too costly.
The very same mordant, when inspissated to different degrees, produces different tints
in the dye-copper; a difference due to the increased bulk from the thickening substance;
thus, the same mordant, thickened with starch, furnishes a darker shade than when
thickened with gum. Yet there are circumstances in which the latter is preferred, because
it communicates more transparency to the dyes, and because, in spite of the washing,
more or less of the starch always sticks to the mordant. The gum has the inconvenience,
however, of drying too speedily, and of also increasing too much the volume of the
mordants; by both of which causes it obstructs their combination with the stuff, and the
tints become thin or scratchy.
The substances generally employed as thickeners, are the following:—
- Wheat starch.
- Flour.
- Roasted starch.
- Gum senegal.
- Gum tragacanth.
- Salep.
- Pipe-clay, mixed with gum senegal.
- Sulphate of lead.
- Sugar.
- Molasses.
- Glue.
After thickening with gum, we ought to avoid adding metallic solutions in the liquid
state; such as nitrate of iron, of copper, solutions of tin, of subacetate of lead, &c.; as
they possess the property of coagulating gum. I shall take care to specify the nature
and proportion of thickening to be employed for each colour; a most important matter,
hitherto neglected by English writers upon calico printing.
The atmosphere of the printing shops should never be allowed to cool under 65° or
70° F.; and it should be heated by proper stoves in cold weather, but not rendered too
dry. The temperature and moisture should therefore both be regulated with the aid of
thermometers and hygrometers, as they exercise a great influence upon all the printing
processes, and especially upon the combination of the mordant with the cloth. In the
course of the desiccation, a portion of the acetic acid evaporates with the water, and subacetates
are formed, which combine with the stuff in proportion as the solvent principle
escapes; the water as it evaporates carries off acetic acid with it, and thereby aids the fixation
of bases. These remarks are peculiarly appropriate to delicate impressions by the
cylinder machine, where the printing and drying are both rapidly effected. In the lapis
lazuli style, the strong mordants are apt to produce patches, being thickened with pipe-clay
and gum, which obstruct the evaporation of the acids. They are therefore apt to
remain, and to dissolve a portion of the mordants at their immersion in the blue vat, or
at any rate, in the dung bath. In such a case a hot and humid air is indispensable,
after the application of the mordants; and sometimes the stuffs so impregnated, must be
suspended in a damp chamber. To prevent the resist pastes becoming rapidly crusty,
substances apparently useless are mixed with them, but which act beneficially by their
hygrometric qualities, in retarding the desiccation. Oil also is sometimes added with
that view.
It is often observed that goods printed upon the same day, and with the same mordant,
exhibit inequalities in their tints. Sometimes the colour is strong and decided in one
part of the piece, while it is dull and meagre in another. The latter has been printed
in too dry an atmosphere. In such circumstances a neutral mordant answers best,
especially if the goods be dried in a hot flue, through which humid vapours are in constant
circulation.
In padding, where the whole surface of the calico is imbued with mordant, the drying[223]
apartment or flue, in which a great many pieces are exposed at once, should be so constructed
as to afford a ready outlet to the aqueous and acid exhalations. The cloth ought
to be introduced into it in a distended state; because the acetic acid may accumulate in the
foldings, and dissolve out the earthy or metallic base of the mordant, causing white and
gray spots in such parts of the printed goods. Fans may be employed with great
advantage, combined with Hot Flues. (See this article.)
In the colour laboratory, all the decoctions requisite for the print work should be
ready prepared. They are best made by a steam heat, by means of copper boilers of a
cylindric form, rounded at the bottom, and encased within a cast-iron cylinder, the steam
being supplied to the space between the two vessels, and the dye-stuff and water being
introduced into the interior one, which for some delicate purposes may be made of tin,
or copper tinned inside. A range of such steam apparatus should be placed either along
one of the side walls, or in the middle line of the laboratory. Proper tables, drawers,
phials, with chemical reagents, measures, balances, &c., should also be provided. The
most useful dye-extracts are the following:—
Decoction of logwood, of Brazil wood, of Persian berries, of quercitron bark, of nut-galls,
of old fustic, of archil or cutbear, of cochineal, of cochineal with ammonia, of
catechu.
The following mordants should also be kept ready prepared:—
1. Aluminous mordant.
1. Take 50 gallons of boiling water.
1. Take 100 lbs. of alum.
1. Take 10 lbs. of soda crystals.
1. Take 75 lbs. of acetate of lead.
The soda should be added slowly to the solution of the alum in the water, and when
the effervescence is finished, the pulverized acetate of lead is put in and well stirred about
till it be all dissolved and decomposed. During the cooling, the mixture should be
raked up a few times, and then allowed to settle. The supernatant liquor is the mordant;
it has a density of 11° or 111⁄2° Baumé. It serves for reds and pinks, and enters into the
composition of puce and lilac.
2. Aluminous mordant.
2. Take 50 gallons of water.
2. Take 100 lbs. of alum.
2. Take 10 lbs. of soda crystals.
2. Take 100 lbs. of acetate of lead;—operate as above directed.
The supernatant liquor here has a density of 12° Baumé; it is employed for lapis
resists or reserves, and the cylinder printing of madder reds.
3. Aluminous mordant.
3. Take 50 gallons of water.
3. Take 100 lbs. of alum.
3. Take 6 lbs. of soda crystals.
3. Take 50 lbs. of acetate of lead;—operate as above directed.
This mordant is employed for uniform yellow grounds.
4. Aluminous mordant.
This is made by adding potash to a solution of alum, till its earth begins to be
separated, then boiling the mixture to precipitate the subsulphate of alumina, which is to
be strained upon a filter, and dissolved in acetic acid of moderate strength with the aid
of heat. This mordant is very rich in alumina, and marks 20° B.
5. Aluminous mordant.
5. Take 121⁄2 gallons of water.
5. Take 100 lbs. of alum.
5. Take 150 lbs. of liquid pyrolignite of lime at 111⁄2° Baumé.
This mordant is made with heat like the first; after cooling, some alum crystallizes,
and it marks only 121⁄2° B.
A mordant is made by solution of alum in potash, commonly called—
6. Aluminate of potash. The caustic lye is prepared by boiling together for an hour
100 gallons of water, 200 lbs. of potash, and 80 lbs. of quicklime; the mixture is then
allowed to settle, the supernatant liquor is decanted, and evaporated till its density be
35° B. In 30 gallons of that lye at a boiling heat, 100 lbs. of ground alum are to be
dissolved. On cooling, crystals of sulphate of potash separate. The clear liquor is to
be decanted off, and the crystals being washed with a little water, this is to be added to
the lye. About 33 gallons of mordant should be obtained.
Mordant for Black.
The pyrolignite of iron called iron liquor in this country, is the only mordant used in
calico-printing for black, violet, puce, and brown colours. The acetate of alumina, prepared
from pyrolignous acid, is much used by the calico-printers under the name of red
or yellow liquor, being employed for these dyes.
[224]
We may observe that a strong mordant, like No. 2., does not keep so well as one of
mean density, such as No 1. Too much mordant relatively to the demands of the
works should therefore not be made at a time.
There are eight different styles of calico-printing, each requiring different methods of
manipulation, and peculiar processes.
1. The madder style, to which the best chintzes belong, in which the mordants are
applied to the white cloth with many precautions, and the colours are afterwards brought
up in the dye-bath. These constitute permanent prints.
2. The padding or plaquage style, in which the whole surface of the calico is imbued
with a mordant, upon which afterwards different coloured figures may be raised, by the
topical application of other mordants joined to the action of the dye-bath.
3. The reserve style, where the white cloth is impressed with figures in resist paste,
and is afterwards subjected first to a cold dye, as the indigo vat, and then to a hot dye-bath,
with the effect of producing white or coloured spots upon a blue ground.
4. The discharge or rongeant style, in which thickened acidulous matter either pure
or mixed with mordants, is imprinted in certain points upon the cloth, which is afterwards
padded with a dark-coloured mordant, and then dyed, with the effect of showing bright
figures on a darkish ground.
5. China blues; a style resembling blue stone-ware, which requires very peculiar treatment.
6. The decolouring or enlevage style; by the topical application of chlorine or chromic
acid to dyed goods. This is sometimes called a discharge.
7. Steam colours; a style in which a mixture of dye extracts and mordants are
topically applied to calico, while the chemical reaction which fixes the colours to the fibre
is produced by steam.
8. Spirit colours; produced by a mixture of dye extracts, and solution of tin, vulgarly
called spirit by dyers. These colours are brilliant but fugitive.
I. The madder style; called by some dip colours. The true chintz patterns belong to
it; they have from 5 to 7 colours, several of which are grounded-in after the first dye
has been given in the madder bath.
In dyeing with madder; sumach, fustic or quercitron, is sometimes added to the bath,
in order to produce a variety of tints with the various mordants at one operation.
1. Suppose we wish to produce flowers or figures of any kind containing red, purple,
and black colours, we may apply the three mordants at once, by the three-colour cylinder
machine, putting into the first trough acetate of alumina thickened; into the second,
acetate of iron; and into the third, a mixture of the two; then drying in the air for a
few days to fix the iron, dunging, and dyeing up in a bath of madder and sumach. If
we wish to procure the finest madder reds and pinks, besides the purple and black, we
must apply at first only the acetate of alumina of two densities, by two cylinders, dry,
dung, and dye up, in a madder bath. The mordants of iron liquor for the black, and of iron
liquor mixed with the aluminous for purple, must be now grounded-in by blocks, taking
care to insert these mordants into their precise spots: the goods being then dried with
airing for several days, and next dunged, are dyed up in a bath of madder and sumach.
They must be afterwards cleared by branning. See Bran, Dunging, and Madder.
2. Suppose we wish to produce yellow with red, pink, purple and black; in this case
the second dye-bath should contain quercitron or fustic, and the spots intended to be
yellow should receive the acetate of alumina mordant.
3. The mordant for a full red may be acetate of alumina, of spec. grav. 1·055 thickened
with starch, and tinged with Brazil wood; that for a pale red or pink, the same at spec.
gravity 1·014, thickened with gum; that for a middling red, the same at spec. gravity,
1·027, thickened with British gum; and for distinction’s sake, it may be tinged yellow
with Persian berries. The mordant for black is a pyrolignous acetate of iron, of specific
gravity 1·04; for purple the same, diluted with six times its volume of water; for
chocolate, that iron liquor mixed with acetate of alumina, in various proportions according
to the shade wanted. Sumach is mixed with the madder for all these colours except for the
purple. The quantity of madder required varies according to the body of colour to be put
upon the cloth, being from one pound per piece to three or even four. The goods must
be entered when the copper is cool, be gradually heated during two or three hours, up to
ebullition, and sometimes boiled for a quarter of an hour; the pieces being all the while
turned with a wince from the one side of the copper to the other. (See Wince.) They
are then washed and boiled in bran and water for ten or fifteen minutes. When there
is much white ground in the chintz, they must be branned a second or even a third time,
with alternate washing in the dash-wheel. To complete the purification of the white,
they are spread upon the grass for a few days; or what is more expeditious, and equally
good if delicately managed, they are winced for a few minutes in a weak solution of
chloride of lime.
4. In the grounding-in for yellow, after madder reds, the aluminous mordant being[225]
applied, &c., the piece is dyed, for about an hour, with one pound of quercitron bark, the
infusion being gradually heated to 150° or 160°, but not higher.
5. A yellow is sometimes applied in chintz work after the other colours are dyed, by
means of a decoction of Persian berries mixed with the aluminous mordant, thickened
with flour or gum, and printed-on with the block; the piece, when dry, is passed
through a weak carbonated alkaline water, or lime water, then washed and dried for
the market.
6. Black mordant.—Take half a gallon of acetate of iron, of spec. grav. 1·04, 4 ounces
of starch, and 4 ounces of flour. The starch must first be moistened with the acetate,
then the flour must be added, the rest of the acetate well mixed with both, and the
whole made to boil over a brisk fire for five minutes, stirring meanwhile to prevent
adhesion to the bottom of the pot. The colour must be poured into an earthen pipkin,
and well mixed with half an ounce of gallipoli oil. In general, all the mordants,
thickened with starch and flour, must be boiled, for a few minutes. With British gum
or common gum, they must be heated to 160° F., or thereby, for the purpose merely of
dissolving them. The latter should be passed through a sieve to separate the impurities
often present in common gum.
7. Puce mordant.—Take a quart of acetate of alumina and acetate of iron, each of spec.
grav. 1·04, mixed and thickened like the black, No. 6. To give the puce a reddish
tinge, the acetate of alumina should have a specific gravity of 1·048, and the iron
liquor only 1·007.
Red mordants are thickened with British gum, and are sufficiently coloured with the
addition of any tingeing decoction.
8. Violet mordants.—These consist either of a very weak solution of acetate of iron,
of spec. gravity 1·007, for example; or of a little of the stronger acetate of 1·04,
mixed with acetate of alumina, and a little acetate of copper, thickened with starch or
British gum. The shades may be indefinitely varied by varying the proportions of the
acetates.
When black is one of the colours wanted, its mordant is very commonly printed-on
first, and the goods are then hung upon poles in the drying-room, where they are aired
for a few days, in order to fix the iron by its peroxidizement; the mordants for red,
violet, &c., are then grounded in, and the pieces are dyed up, after dunging and
washing, in the madder bath, into which, for certain shades, sumach, galls, or fustic, is
added. The goods are brightened with a boil in soap water; occasionally also in a
bath, containing a small quantity of solution of tin or common salt. The following
mode of brightening is much extolled by the French, who are famous for their reds
and roses.
1. A soap boil of forty minutes, at the rate of 1 pound for every 2 pieces. Rinse in
clear water.
2. Pass through chloride of soda solution of such strength that two parts of it
decolour one part of Gay Lussac’s test liquor. See Chloride of Lime and Indigo.
Wince the pieces through it for 40 minutes. Rinse again.
3. Pass it again through the soap bath, No. 1.
4. Brighten it in a large bath of boiling water, containing 4 pounds of soap, and
1 pound of a cream-consistenced salt of tin, containing nearly half its weight of the
muriate of tin, combined with as much nitric acid of spec. grav. 1·288. This strong
nitro-muriate having been diluted with a little water, is to be slowly poured into
the bath of soap water, and well mixed by stirring. The pieces are now put in,
and winced through it for one half, or three quarters of an hour.
5. Repeat the soap boil, No. 1. Rinse and dry.
9. Grounding in of Indigo blue.
Take half a gallon of water of 120° F., 8 ounces of ground indigo, and 8 ounces
of red sulphuret of arsenic (orpiment), 8 ounces of quicklime, mix together, and heat
the mixture to the boiling point; withdraw from the fire, and add, when it is lukewarm,
6 ounces of carbonate of soda, stir and leave the whole at rest till the next day. Then
decant the clear liquor, and thicken every quart of it with half a pound of gum. This
colour ought to be green, and be preserved in a close vessel. When used it is put
into a pot with a narrow orifice, the pencil is dipped into it, wiped on the edge of the
pot, and immediately applied by hand. This plan is tedious, and is nearly superseded
by the following grounding blue.
Take half a gallon of caustic soda lye of spec. grav. 1·15, heated to 120° F.
12 ounces of hydrate of protoxide of tin, obtained by precipitating it from the muriate
of tin by solution of potash.
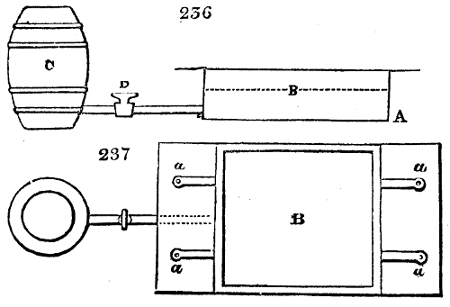
8 ounces of ground indigo; heat these mixed ingredients to the boiling point, then
move the pot off and on the fire two or three times in succession, and finally thicken,
with 3 pounds of raw sugar. In order to apply this by the block, the following
apparatus is employed, called the canvass frame; figs. 236. 237. It is formed of a copper[226]
case or box A, in which is laid a frame B, filled with pretty stout canvass. The box
communicates by a tube with the cistern C, mounted with a stop-cock D. Fig. 237.
represents the apparatus in plan: A, the box; B, the canvass, with its edges a a a a,
fixed by pin points to the sides. The colour is teared (tiré), or spread even, with a
wooden scraper as broad as the canvass. In working with this apparatus, the colour
being contained in the vessel C is drawn off into the case A, by opening the stop-cock D,
till it rises to the level of the canvass. The instant before the printer daubs the block
upon the canvass, the tearer (tireur), boy or girl, runs the scraper across it to renew its
surface; and the printer immediately transfers the colour to the cloth. In this kind
of printing great skill is required to give evenly impressions. As the blue is usually
applied to somewhat large designs, it is very apt to run; an inconvenience counteracted
by dusting fine dry sand upon the cloth as soon as it is blocked. The goods must
be washed within 24 hours after being printed.
10. Topical grounding blue for the cylinder press.
Take 31⁄2 gallons of caustic soda lye of spec. grav. 1·15.
Take 31⁄2 lbs. of ground indigo.
Take 5 lbs. of precipitated protoxide of tin (as above).
Boil the mixed ingredients for ten minutes, take them from the fire, and add, first,
3 lbs. of Venice turpentine; then 11 lbs. of gum.
Put this mixture into the colour trough, print with it, and after two days wash in
the dash-wheel; then pass it through a soap bath, along with a little soda, to brighten
the blue, and to take off its greyish tint.
The use of the turpentine is easily explained; it serves to exclude the atmospherical
oxygen, and prevent the regeneration of the indigo blue, before it is spread upon
the cloth.
After the application to white calico of a similar blue, into which a little acid muriate
of tin has been put, the goods are dipped for ten minutes in thin milk of lime, shaking
the frame all the time. They are then washed, and cleared with a soap boil. The following
colour remains long in the deoxidized state from its containing 8 ounces of indigo, 10
ounces of hydrated protoxide of tin, and 11⁄2 pounds of solution of muriate of tin, to 2
quarts of soda lye of 1·15, thickened with 21⁄2 pounds of gum. This blue may be
applied by either the block or the cylinder.
11. Topical Prussian blue for grounding.
2 quarts of water with 8 ounces of starch, are to be mixed and boiled; add 21⁄4 ounces of a
liquid Prussian blue colour, prepared by triturating three quarters of an ounce of that
pigment with as much muriatic acid, leaving the ingredients to react upon each other for
24 hours, and then adding three quarters of an ounce of water.
Add 4 ounces of liquid perchloride of tin (oxymuriate).
Mix all together, and pass through a searce. This colour is not very fast; cloth
printed with it will bear only rinsing.
12. Prussian blue figures are impressed as follows:—
Dissolve 8 ounces of sulphate of iron, and as much acetate of lead, separately in 2 quarts
of boiling water; mix well, and settle. Take one quart of this clear liquor reduced to
spec. grav. 1·02, one quart of mucilage containing 3 pounds of gum, coloured with a
little prussiate of potash, mix into a mordant, and print it on with the cylinder. Two
days afterwards wash in tepid water containing a little chalk, and then pass the cloth
through a solution of prussiate of potash in water, sharpened with a little muriatic acid,
till it takes the desired hue. Finally rinse.
II. The padding or plaquage style, called foulard also by the French. See Padding.
Any mordant whatever, such as the acetates of alumina, or of iron, or their mixture,
may be applied to the piece by the padding machine, after which it is dried in the HOT-FLUE,
washed, dunged, dyed, washed, and brightened.
Colours from metallic oxides are very elegantly applied by the padding process.
Thus the iron buff, the manganese bronze, and the chrome yellows and greens are given.
1. Iron buff or chamois.
Take 50 gallons of boiling water;
Take 150 pounds of sulphate of iron; dissolve along with
Take 10 pounds of alum; which partly saturate by the gradual addition of
Take 5 pounds of crystals of soda; and in this mixture dissolve[227]
Take 50 pounds of pyrolignous acetate of lead. Allow the whole to settle, and draw off
the clear supernatant liquid.
For furniture prints this bath should have the spec. grav. 1·07.
The calico being padded in it, is to be dried in the hot-flue; and after 48 hours suspension
is to be washed in water at 170° containing some chalk, by the wince apparatus.
It is then washed, by the same apparatus, in hot water, containing a pailful of soda lye
of spec. grav. 1·04.
For light tints the padding liquor should be reduced to the spec. grav. 1·01. The
dye in either case may be brightened by wincing through a weak solution of chloride of
lime.
Nitrate of iron diffused through a body of water may be also used for padding, with
alternate washings in water, and a final wincing in a weak alkaline lye.
With a stronger solution, similar to the first, the boot-top colour is given.
2. The bronze or solitaire.
The goods are to be padded in a solution of the sulphate or muriate of manganese,
of a strength proportional to the shade desired, dried in the hot-flue, and then raised
by wincing them in a boiling-hot caustic lye, of spec. grav. 1·08, and next through a
weak solution of chloride of lime, or soda. They are afterwards rinsed. Instead of
passing them through the chloride, they may be merely exposed to the air till the manganese
attracts oxygen, then rinsed, and dried.
When the manganese solution has the density of 1·027, it gives a light shade; at the
density of 1·06, a shade of moderate depth, and at 1·12 a dark tint.
The texture of the stuff is apt to be injured during the oxidation of the manganese.
3. Carmelite is obtained by padding in a mixture of muriate or sulphate of manganese
and acetate of iron, then proceeding as above.
4. Copper green is given by padding in a mixed solution of sulphate and acetate of
copper with a little glue, drying in the hot-flue, and next day padding in a caustic lye
of spec. grav. 1·05. The goods are then rinsed, and padded through a solution made
with 8 ounces of arsenious acid combined with 4 ounces of potash diluted with 2 gallons
of water. They are finally rinsed and dried.
5. Olive and cinnamon colours are given by padding through mixed solutions of the
acetate of iron and sulphate of copper; drying, and padding in a caustic lye of spec.
grav. 1·05.
6. Green and solitaire form a pleasing umber, or hellebore shade, which may be obtained
by padding through a mixed solution of manganese and aceto-sulphate of copper
and raising the shades, as above prescribed.
7. Chrome yellow.
Pad in a solution of bichromate of potash containing 8 ounces of it to the gallon of water;
then dry with moderate heat, and pad in a solution of acetate or nitrate of lead, containing
6 or 8 ounces in the gallon of water; wash, and dry. Or we may pad first in a
solution of acetate of lead containing a little glue; dry, and pad in solution of bichromate
of potash. Then rinse. The last process is apt to occasion cloudiness. To
obtain a light lemon tint, we must pad in a solution of acetate of lead of double the above
strength, or 16 ounces to the gallon, then wince the pieces through weak milk of lime,
rince, pad through bichromate of potash, rinse, and dry.
8. Chrome orange.
Pad through a mixed solution of the subacetate and acetate of lead, three times in
succession, and dry in the hot-flue; then wince for ten minutes through weak milk of
lime; rinse; wince for a quarter of an hour in a warm solution of bichromate of potash;
and finally raise the colour by wincing the goods through hot lime water.
9. Prussian blue.
Pad in the preceding chamois liquor of the spec. grav. 1·007; dry in the hot-flue;
wince well in chalky water at 160° F., and then dye by wincing in the following
liquor:—
Dissolve 5 ounces of prussiate of potash, in 25 gallons of water heated to 90° or 100°,
adding 2 ounces of sulphuric acid; afterwards rinse, and brighten in a very dilute
sulphuric acid.
10. Green is given by padding goods, previously dyed in the indigo vat, in a solution
of acetate of lead containing a little glue; and then padding them in a warm solution of
bichromate of potash; finally rinsing and drying.
III. Resist pastes or reserves; these are subservient to the cold indigo vat, and they
may be distributed under four heads; 1. fat reserves; 2. reserves with bases of metallic
salts; 3. coloured reserves capable of assuming different tints in the dyeing; 4. reserves
with mordants, for the cloth to be afterwards subjected to a dyeing bath, whereby variously
coloured figures are brought up on a blue ground, so as to resemble the mineral
called lazulite; whence the name lapis or lapis lazuli.
1. The fatty resists are employed in the printing of silk; which see infra.
[228]
2. With regard to reserves the following general observations may be made. After printing-on
the paste, the goods must be hung up in a chamber, rather humid than too dry,
and left there for a certain time, more or less, according to the nature of the reserve. In
dipping them into the blue vat, if the reserve be too dry, it is apt to swell, scale off, and
vitiate the pattern. This accident is liable to happen also when the vat is deficient in
lime, especially with deep blues.
1. Simple white resist paste for a full body of blue.
Take 1 gallon of water, in which are to be dissolved,
1 pound of binacetate of copper (distilled verdigris), and 3 libs. of sulphate of copper.
This solution is to be thickened with
2 libs. of gum senegal, 1 lib. of British gum, and 4 libs. of pipe-clay; adding afterwards, 2 ounces of nitrate of copper—as a deliquescent substance.
2. White reserve for light blues.
Take 1 gallon of water, in which dissolve
4 ounces of binacetate of copper,
1 lib. of sulphate of copper; and thicken this solution with
2 libs. of gum senegal, 1 lib. of British gum, and 4 libs. of pipe-clay.
3. White reserve for the cylinder machine.
Take 11⁄2 gallons of water; in which dissolve
21⁄2 libs. of binacetate of copper,
10 libs. of sulphate of copper; and add to the solution
6 libs. of acetate of lead; then thicken with
10 libs. of gum; adding afterwards 10 libs. of sulphate of lead.
After printing-on this reserve, the goods are to be hung up for two days, then dipped
till the proper blue tint be obtained. Finally they must be winced through dilute sulphuric
acid to clear up the white, by removing the cupreous tinge.
3. Coloured reserves.
1. Chamois reserve.
Take 1 gallon of the chamois bath (No. 1. page 226, at bottom); to which add
8 ounces of nitrate of copper,
24 ditto of muriate of zinc; thicken with
6 pounds of pipe clay, and 3 libs. of gum senegal.
After printing-on this paste, the goods must be hung up for five or six days in a
somewhat damp room. Then after having dipped them in the vat, they are to be steeped
in water for half an hour, and slightly washed. Next wince for half an hour, through
water at 100° F. containing 2 pounds of soda crystals per 30 gallons. Rinse and dry.
2. Chrome yellow reserve.
Take 1 gallon of water; in which dissolve
3 libs. of nitrate of lead,
1 lib. of binacetate of copper; to the solution, add
1⁄2 lib. of subacetate of lead; and thicken the mixed solution with
3 libs. of gum.
6 libs. of pipe clay. Grind all the ingredients together, and pass through a searce.
After treating the goods as in No. 1., they must be winced for half an hour in a solution
containing 5 ounces of bichromate of potash, per piece of calico, and also in a
dilute muriatic bath, till the chrome yellow become sufficiently bright.
A chrome orange reserve may be made by introducing a larger proportion of subacetate
of lead, and passing the reserve printed goods through weak milk of lime, as
already prescribed for producing an orange by chrome.
The basis of the resist pastes used at Manchester is sometimes of more complex composition
than the above; since, according to the private information I received from
an extensive calico printer, they contain “china clay” (instead of pipe-clay which often
contains iron) strong solution of sulphate of copper, oil, tallow, and soap; the whole
incorporated by trituration with heat.
In the Lancashire print-works, a little tartaric acid is added to the nitrate of lead,
which prevents the colour from taking a dingy cast.
4. Reserves with mordants, or the lazulite style.
1. Black upon a blue ground.
At Manchester the black pattern is printed-on with a mixture of iron liquor and extract
of logwood, and the resist paste by the cylinder machine; in France the black is
given by the following recipe:—
Take 1 gallon of decoction of galls of spec. grav. 1·04, mixed and boiled into a paste with
14 ounces of flour; into the paste, when nearly cold, there are added,
8 ounces of an acetated peroxide of iron, made by adding 1 lib. acetate of lead to 3
libs. of nitrate of iron, spec. grav. 1·56.
1⁄8 ounce of gallipoli oil.
[229]
This topical black forms a fast colour, and resists the fine blue vat, weak potash lye,
bichromate of potash, boiling milk of lime, dunging and maddering.
The preceding answers best for the block; the following for the cylinder,—
2. Take 1 gallon decoction of galls of spec. grav. 1·056.
18 ounces of flour, mix, boil into a paste, to which, when cool, add
8 ounces of the aceto-nitrate of iron of the preceding formula, and
1 quart of iron liquor of spec. grav. 1·110.
In Lancashire a little prussiate of potash is sometimes added to nitrate of iron and
decoction of logwood; and the goods are after washing, &c. finished by passing through a
weak solution of bichromate of potash. The chromic acid gives depth and permanence
to the black dye, being supposed to impart oxygen to the iron, while it does not affect
any of the other colours that may happen to be impressed upon the cloth, as solution of
chloride of lime would be apt to do. The solution of the bichromate deepens the spirit
purples into blacks, and therefore with such delicate dyes becomes a very valuable
application. This interesting fact was communicated to me by an eminent calico-printer
in Lancashire.
Having premised the composition of the topical black dye, we are now prepared to
apply it in the lazulite style.
1. Black resist.
Take 1 gallon of the above black without the flour,
2 ounces of sulphate of copper,
1 ounce of muriate of ammonia, dissolve and thicken with
4 pounds of pipe-clay and 2 pounds of gum.
Another good formula is the following:—
Take 1 gallon of iron liquor of 1·056 spec. grav. dissolve in it,
2 ounces of binacetate of copper,
8 ounces of sulphate of copper; and thicken as just described.
2. Puce reserve paste, contains acetate of alumina mixed with the iron liquor.
3. Full red reserve.
Take 1 gallon of acetate of alumina, (made with 50 gallons water, 100 libs. alum, 10 libs.
soda crystals, and 100 libs. acetate of lead; the supernatant liquid being of spec. grav. 1·085); dissolve in it
4 ounces of corrosive sublimate; thicken with
2 pounds of gum senegal,
4 pounds of pipe-clay, and mix in 8 ounces of gallipoli oil.
4. Reserve paste for a light red.
Take 1 gallon of the weaker sulpho-acetate of alumina formerly prescribed; dissolve in it
4 ounces of corrosive sublimate; and thicken with
4 pounds of pipe-clay, and 2 pounds of gum; adding to the mixture
8 ounces of oil.
5. Neutral resist paste.
Take 1 gallon of water; in which dissolve,
31⁄4 libs. of binarseniate of potash, and
12 ounces of corrosive sublimate; thicken with
3 libs. of gum, and 6 libs. of pipe-clay, adding to the paste 16 ounces of oil.
6. Carmelite reserve paste.
Take 1 half gallon of acetate of alumina spec. grav. 1·014; (see second aluminous mordant p. 223).
1 half gallon iron liquor of spec. grav. 1·027; dissolve in them
4 ounces of sulphate of copper, 4 ounces of verdigris, and 1 ounce of nitrate of copper; thicken with
2 libs. of gum,
4 libs. of pipe-clay.
7. Neutral reserve paste.
Take 1 gallon of water; dissolve in it,
44 ounces of binarseniate of potash, and
12 ounces of corrosive sublimate; thicken with
3 libs. of gum,
6 libs. of pipe-clay,
16 oz. of oil.
To explain fully the manipulation of the lazulite style, we shall suppose that the
calicoes are printed with the following reserves, taken in their order:—
- Black reserve, No. 1. above.
- Full red reserve, No. 3.
- Light red reserve, No. 4.
- Neutral reserve, No. 7.
Four days after printing-on these reserves, the goods must be twice dipped in the blue[230]
vat, ten minutes in and ten minutes out each time; but more dips may be given
according to the desired depth of shade. The cloth must be afterwards rinsed in
running water for half an hour. The next process is to remove the paste; which is
done by wincing the goods in a bran bath, lowered to 150°, during twenty minutes.
They are then winced for five minutes in a bath of water slightly sharpened with vinegar.
When well cleansed, they are ready for the madder bath. The lapis goods are finally
cleared in a bran bath, by exposure on the grass, and a soap boil.
The lazulite style is susceptible of many modifications.
8. Deep blue ground, with light blue, carmelite, and white figures.
- Print-on the white reserve, No. 1.
- Dip in the strongest blue vat; rinse and dry.
- Ground-in with the block, the carmelite reserve (containing the mixed acetates of iron and alumina.)
- Ground-in the neutral reserve.
- Dip for the light blue; rinse.
- Dung, dye, and clear, as above.
By varying the proportions of the reserve mordants, and the dye stuffs, as madder,
quercitron, &c. a great variety of effects may be produced.
9. Deep green ground, with buff and white figures.
- Print-on the white reserve.
- Dip in the blue vat; rinse and dry.
- Pad in the buff liquor, as formerly prescribed.
- Ground in upon the buff spots, the discharge, No. 2. presently to be described.
- Wash away the paste in chalky water.
- Wince through a boiling alkaline lye, to raise the buff iron colour.
IV. The Discharge style; first, of simple discharges.
1. Discharge for block printing.
Take 1 gallon of lemon or lime-juice, of spec. grav. 1·09, in which dissolve
1 pound of tartaric acid,
1 pound of oxalic acid, and thicken the solution with
4 pounds of pipe or china clay, and 2 pounds of pulverised gum; as soon as the gum is dissolved, the mixture must be put through a searce.
2. Another discharge is made of half the above acid strength.
3. A third with one half of the solid acids of the second.
4. Take 1 gallon of water, in which dissolve with heat
1 pound of cream of tartar adding, to facilitate the solution,
1 pound of warm sulphuric acid of spec. grav. 1·7674; after 24 hours mix
4 libs. of pipe or China clay, and three libs. of gum with the decanted clear liquor.
In some cases British gum is used alone, as a thickener.
5. Discharge for the cylinder machine.
Take 1 gallon of lime juice, of spec. grav. 1·085; dissolve in it
3 pounds of tartaric acid, and one pound of oxalic acid; thicken with
6 pounds of gum senegal, or 5 pounds of British gum.
6., 7. A stronger and weaker discharge is made of the same materials; and one is made
without the tartaric acid.
Second; combination of discharges with mordants.
1. Black, red, lilac, and white figures upon an olive ground.
The olive being given in a madder bath, and the ground well whitened (see Madder),
the cloth is padded in a weak buff mordant; and upon the parts that are to remain
white, the weakest simple discharge No. 3. is printed-on by the cylinder; (in some works
the discharge paste is applied and made dry before padding through the iron liquor;)
the goods are cleared of the paste in a tepid chalky water, then dyed in a quercitron
bath, containing a little glue, and cleared in a bran bath.
Discharge mordants upon mordants may be regarded as a beautiful modification of the
preceding style. Example.
A violet ground or impression, with red and white.
1. Pad with an acetate of iron of 1·004; or print-on with the cylinder, iron liquor
of 1·027 thickened with British gum.
2. Print-on a red mordant, strongly acidulated with lime juice of 1·226.
3. Ground in the discharge No. 2.; dry.
4. Clear off the paste in chalky water.
5. Dung, madder, and brighten.
6. Ground-in the topical colours at pleasure.
V. China blues.
Take 16 pounds of coarsely ground indigo, and
4 pounds of sulphuret of arsenic; dissolve 22 pounds of sulphate of iron in 6
gallons of water; introduce these three matters into the indigo mill, and grind them for[231]
three days. If it be wished to have a thickened blue, this mixture must have pounded
gum added to it, but if not, 5 gallons of water are added. This colour may be called
blue No. 1.
The following table exhibits the different gradations of China blue:—
| Course. |
Quantity
by measure of
No. 1. |
Quantity
by measure of
water or mucilage. |
| No. |
1 |
1 |
0 |
| |
2 |
11 |
1 |
| |
3 |
10 |
2 |
| |
4 |
8 |
4 |
| |
5 |
6 |
6 |
| |
6 |
4 |
8 |
| |
7 |
2 |
10 |
| |
8 |
2 |
12 |
| |
9 |
2 |
14 |
| |
10 |
2 |
16 |
| |
11 |
2 |
18 |
| |
12 |
2 |
20 |
I shall now give examples of working this style by the block and cylinder:—
Impression of a single blue with small dots.
For the block, blue No. 5. thickened with starch.
For the cylinder, No. 4. thickened with gum.
Impression of two different blues with the block.
First blue, No. 4. with starch.
Second blue, No. 9. with gum.
Impression of three blues with the block.
First blue, No. 5. with starch.
Second blue, No. 7. with starch.
Third blue, No. 10. with gum.
After printing-on the blues, the pieces are hung up for two days in a dry and airy
place, but not too dry; then they are dipped as follows:—Three vats are mounted,
which may be distinguished by the numbers, 1., 2., 3.—
No. 1. 300 pounds of lime to 1,800 gallons of water.
No. 2. Solution of sulphate of iron of spec. grav. 1·048.
No. 3. Solution of caustic soda of spec. grav. 1·055; made from soda crystals, quicklime,
and water, as usual.
The pieces being suspended on the frames, are to be dipped in the first vat, and left
in it ten minutes; then withdrawn, drained for five minutes; next plunged into the
second vat for ten minutes, and drained also for five, &c. These operations will be most
intelligible when put into the form of a table:—
| Dip in the 1st vat. |
During 10 minutes. |
Drain during 5 minutes. |
| 2 |
— |
— |
| 1 |
— |
— |
| 2 |
— |
— |
| 3 |
— |
— |
| 2 |
— |
— |
| 1 |
— |
— |
| 2 |
— |
— |
| 1 |
— |
— |
| 2 |
— |
— |
| 3 |
— |
— |
In the dipping of China blues, care should be taken to swing the frames during the
operation; and when the last dip is given, the piece is to be plunged upon its frame into
a fourth vat, containing dilute sulphuric acid of spec. grav. 1·027. This immersion
is for the purpose of removing the oxide of iron, deposited upon the calico in the alternate
passages through the sulphate of iron and lime vats. They are then rinsed an hour in
running water, and finally brightened in the above dilute sulphuric acid, slightly tepid.
Sometimes they are subjected to a soap bath, at the temperature of 120°. By the
addition of nitrate of lead to the indigo vat, the blue becomes more lively. Some use
the roller dyeing apparatus for running the pieces through the respective baths instead
of the square frames. (See Wincing.) But the frame-dip gives the most evenly dyes,
and preserves the vats in good condition for a much longer time.
[232]
The various phenomena which occur in the dipping of China blues, are not difficult
of explanation with the lights of modern chemistry. We have, on the one hand, indigo
and sulphate of iron alternately applied to the cloth; by dipping it into the lime, the
blue is deoxidized, because a film of the sulphate of iron is decomposed, and protoxide
of iron comes forth to seize the oxygen of the indigo, to make it yellow-green, and
soluble, at the same time, in lime-water. Then, it penetrates into the heart of the
fibres, and, on exposure to air, absorbs oxygen, so as to become insoluble and fixed
within their pores. On dipping the calico into the second vat of sulphate of iron, a
layer of oxide is formed upon its whole surface, which oxide exercises an action only
upon those parts that are covered with indigo, and deoxidizes a portion of it; thus
rendering a second dose soluble by the intervention of the second dip in the lime-bath.
Hence we see that while these alternate transitions go on, the same series of deoxidizement,
solution, and re-oxidizement recurs; causing a progressively increasing fixation
of indigo within the fibres of the cotton. A deposit of sulphate of lime and oxide of
iron necessarily falls upon the cloth, for which reason the frame should be shaken in the
lime water vat, to detach the sulphate; but, on the contrary, it should be held motionless
in the copperas bath, to favour the deposition of as much protoxide upon it as possible.
These circumstances serve to account for the various accidents which sometimes befall
the China blue process. Thus the blues sometimes scale off, which may proceed from
one of two causes:—1. If the goods are too dry before being dipped, the colour swells,
and comes off in the vats, carrying along with it more or less indigo. 2. If the
quantity of sulphate of lime formed upon the cloth be considerable, the crust will fall
off, and take with it more or less of the blue; whence arise inequalities in the impression.
The influence of temperature is important; when it falls too low, the colours take a
gray cast. In this case it should be raised with steam.
VI. The decolouring or enlevage style; not by the removal of the mordant, but the
destruction of the dye. The acid, which is here mixed with the discharge paste, is
intended to combine with the base of the chloride, and set the chlorine free to act upon
the colour. Among the topical colours for this style are the following:—
1. Black.—Take one gallon of iron liquor of spec. grav. 1·086.
1. Black.—One pound of starch; boil together, and while the paste is hot, dissolve in it
1. Black.—One pound of tartaric acid in powder; and when cold, add
1. Black.—Two pounds of Prussian blue, prepared with muriatic acid, see p. 226.
1. Black.—Two ounces of lamp black, with four ounces of oil.
2. White discharge.—Take one gallon of water; in which dissolve
2. White discharge.—One pound and a half of oxalic acid,
2. White discharge.—Three pounds of tartaric acid; add
2. White discharge.—One gallon of lime juice of spec. grav. 1·22; and thicken with
2. White discharge.—Twelve pounds of pipe clay, and six pounds of gum.
3. Chrome-green discharge.—
3. Chrome-green disTake one gallon of water, thicken with 18 ounces of starch; boil
3. Chrome-green disand dissolve in the hot paste
3. Chrome-green disTwo pounds and a half of powdered nitrate of lead,
3. Chrome-green disOne pound and a half of tartaric acid,
3. Chrome-green disTwo pounds of Prussian blue, as above.
4. Blue discharge.—Take one gallon of water, thicken with
4. Blue discharge.18 ounces of gum; while the boiled paste is hot, dissolve in it
4. Blue discharge.Two pounds of tartaric acid, and mix one pound of Prussian blue.
5. Chrome-yellow discharge.—This is the same as the chrome-green given above, but
without the Prussian blue.
6. A white discharge on a blue ground, requires the above white discharge to be
strengthened with 8 ounces of strong sulphuric acid, per gallon.
7. White discharge for Turkey red needs to be very strong.
7. Take one gallon of lime juice of sp. grav. 1·086; dissolve in it
7. Five pounds of tartaric acid; thicken with
7. Eight pounds of pipe-clay, four pounds of gum; then dissolve in the mixture
7. Three pounds of muriate of tin in crystals; and add, finally,
7. Twenty-four ounces of sulphuric acid.
8. Yellow discharge for Turkey red.—
8. Yellow Take one gallon of lime juice of spec. grav. 1·086; in which dissolve
8. Yellow Four pounds of tartaric acid,
8. Yellow Four pounds of nitrate of lead; thicken the solution with
8. Yellow Six pounds of pipe-clay, and three pounds of gum.
9. For green discharge, add to the preceding 24 ounces of Prussian blue, as above.
The decolouring or chlorine bath is usually formed of wood lined with lead, and
has an area of about 5 feet square, with a depth of 6 feet. A square frame, mounted
with a horizontal series of rollers at top and bottom, may be let down by cords, at[233]
pleasure, into the cistern. The pieces are introduced and guided in a serpentine path,
round the upper and lower rollers alternately, by a cord.
This bath is filled with a solution of chloride of lime, of the spec. grav. 1·045, whose
decolouring strength is 65° by Gay Lussac’s indigo chlorometer. It ought to be made
turbid by stirring before putting in the goods, which should occupy three minutes in
their passage. The piece is drawn through by a pair of squeezer cylinders at the end of
the trough, opposite to that at which the piece enters. With black, white, and blue
impressions of all shades, the goods are floated in a stream of water for an hour; then
rinsed and dried. When there is yellow or green, the pieces must be steeped in water,
then merely washed by the wince, and passed through solution of bichromate of potash,
containing from 3 to 5 ounces of the salt per piece. Here the pieces are winced during
15 or 20 minutes, rinsed, and next passed through dilute muriatic acid to clear the
ground; then rinsed and dried.
Discharge by the intervention of the chromic acid.
After having dipped the pieces to the desired shade, they are padded in a solution of
bichromate of potash; dried in the shade without heat; and then printed with the
following mordant:—
- Take 1 gallon of water; dissolve in it
- 2 pounds of oxalic and 1 pound of tartaric acid; thicken with
- 6 pounds of pipe clay, and 3 pounds of gum; lastly, add
- 8 ounces of muriatic acid.
After the impression, the pieces are winced in chalky water, at 120° F., then washed,
and passed through a dilute sulphuric acid.
M. Daniel Kœchlin, of Mulhausen, the author of this very ingenious process, considers
the action of the bichromate here as being analogous to that of the alkaline
chlorides. At the moment that the block applies the preceding discharge to the bichromate
dye, there is a sudden decoloration, and a production of a peculiar odour.
The pieces padded with the bichromate must be dried at a moderate temperature,
and in the shade. Whenever watery solutions of chromate of potash and tartaric acid
are mixed, an effervescence takes place, during which the mixture possesses the power
of destroying vegetable colours. This property lasts no longer than the effervescence.
VII. Steam colours.—This style combines a degree of brilliancy with solidity of
colour, which can hardly be obtained in any other way, except by the chintz dyes.
The steam apparatus, employed for fixing colours upon goods, may be distributed under
five heads:—1. the column; 2. the lantern; 3. the cask; 4. the steam-chest; and,
5. the chamber.
The column is what is most generally used in this country. It is a hollow
cylinder of copper, from three to five inches in diameter, and about 44 inches long,
perforated over its whole surface with holes of about one sixteenth of an inch,
placed about a quarter of an inch asunder. A circular plate, about 9 inches diameter,
is soldered to the lower end of the column, destined to prevent the coil of cloth from
sliding down off the cylinder. The lower end of the column terminates in a pipe,
mounted with a stop-cock for regulating the admission of steam from the main steam-boiler
of the factory. In some cases, the pipe fixed to the lower surface of the disc is
made tapering, and fits into a conical socket, in a strong iron or copper box, fixed to a
solid pedestal; the steam pipe enters into one side of that box, and is provided, of
course, with a stop-cock. The condensed water of the column falls down into that
chest, and may be let off by a descending tube and a stop-cock. In other forms of the
column, the conical junction pipe is at its top, and fits there into an inverted socket
connected with a steam chest, while the bottom has a very small tubular outlet, so that
the steam may be exposed to a certain pressure in the column, when it is encased
with cloth.
The pieces, after being printed with the topical colours presently to be described, and
dried, are lapped round this column, but not in immediate contact with it; for the copper
cylinder is first enveloped in a few coils of blanket stuff; then with several coils of
white calico; next with the several pieces of the printed goods, stitched endwise; and
lastly, with an outward mantle of white calico. In the course of the lapping and
unlapping of such a length of webs, the cylinder is laid in a horizontal frame, in which
it is made to revolve. In the act of steaming, however, it is fixed upright, by one of
the methods above described. The steaming lasts for 20 or 30 minutes, according to
the nature of the dyes; those which contain much solution of tin admit of less steaming.
Whenever the steam is shut off, the goods must be immediately uncoiled, to prevent the
chance of any aqueous condensation. I was much surprised, at first, on finding the
unrolled pieces to be free from damp, and requiring only to be exposed for a few
minutes in the air, to appear perfectly dry. Were water condensed during the process, it
would be apt to make the colours run.
Steam colours are all topical, though, for many of them, the pieces are previously[234]
padded with mordants of various kinds. Some manufacturers run the goods before
printing them through a weak solution of the perchloride of tin, with the view of brightening
all the colours subsequently applied or raised upon them. I shall now illustrate
steam calico-printing by some examples, kindly furnished me by a practical printer near
Manchester, who conducts a great business with remarkable success.
Steam blue.—Prussiate of potash, tartaric acid, and a little sulphuric acid, are dissolved
in water, and thickened with starch; then applied by the cylinder, dried at a
moderate heat, and steamed for 25 minutes. They are rinsed and dried after the
steaming. The tartaric acid, at a high temperature, decomposes here a portion of the
ferrocyanic acid, and fixes the remaining ferrocyanate of iron (Prussian blue) in the fibre
of the cloth. The ground may have been previously padded and dyed; the acids will
remove the mordant from the points to which the above paste has been applied, and bring
out a bright blue upon them.
Steam purple.—This topical colour is made by digesting acetate of alumina upon
ground logwood with heat; straining, thickening with gum senegal, and applying the
paste by the cylinder machine.
Steam pink.—A decoction of Brazil-wood with a small quantity of the solution
of muriate of tin, called, at Manchester, new tin crystals[15], and a little nitrate of copper to
assist in fixing the colour; properly thickened, dried, and steamed for not more than 20
minutes, on account of the corrosive action of muriate of tin when the heat is too strong.
Cochineal pink.—Acetate of alumina is mixed with decoction of cochineal, a little
tartaric acid and solution of tin; then thickened with starch, dried, and steamed.
Steam brown.—A mixed infusion of logwood, cochineal, and Persian berries, with
cream of tartar, alum (or acetate of alumina), and a little tartaric acid, thickened, dried,
and steamed.
Green, blue, chocolate, with white ground, by steam.—Prussiate of potash and tartaric
acid, thickened, for the blue; the same mixture with berry-liquor and acetate of alumina,
thickened, for the green; extract of logwood with acetate of alumina and cream of
tartar, thickened, for the chocolate. These three topical colours are applied at once by
the three-colour cylinder machine; dried and steamed. Though greens are fixed by the
steam, their colour is much improved by passing the cloth through solution of bichromate
of potash.
In France, solution of tin is much used for steam colours.
VIII. Spirit or Fancy colours.—These all owe their vivacity, as well as the moderate
degree of permanency they possess, to their tin mordant. After printing-on the topical
colour, the goods must be dried at a gentle heat, and passed merely through the rinsing
machine. Purple, brown, or chocolate, red, green, yellow, blue, and white discharge; any
five of these are printed on at once by the five-colour cylinder machine. See Rinsing
Machine.
Chocolate, is given by extract of Brazil-wood, extract of logwood, nitromuriate of tin,
with a little nitrate of copper; all mixed, thickened, and merely printed-on.
Red, by extract of Brazil-wood and tin, with a little nitrate of copper.
Green, by prussiate of potash, with muriate of tin and acetate of lead, dissolved,
thickened, and printed-on.
The goods after rinsing must be passed through solution of bichromate of potash, to
convert the Prussian blue colour into green, by the formation of chrome yellow upon it.
Blue.—Prussian blue ground up with solution (nitromuriate) of tin; thickened, &c.
Yellow.—Nitrate of lead dissolved in solution of tartaric acid, thickened, tenderly
dried, passed through the bichromate vat or padding machine, washed and dried.
This yellow is pretty fast; though topical, it can hardly, therefore, be called a fancy
colour.
When purple is to be inserted instead of the above blue, extract of logwood with tin is
used in the place of the Prussian blue. Tartaric acid is a useful addition to tin in
brightening fancy colours.
Chocolate.—A good topical chocolate is made by digesting logwood with liquid acetate
of alumina, adding a little cream of tartar to the infusion; thickening, applying by the
cylinder, drying, washing, then passing through solution of bichromate of potash, which
serves to darken and fix the colour.
I shall conclude my account of the printing of cotton goods with some miscellaneous
formulæ, which were given me by skilful calico-printers in Lancashire.
Prussian blue is prepared for topical printing by grinding it in a handmill, like that
for grinding pepper or coffee, and triturating the powder with solution of muriate of tin.
Green.—The deoxidized indigo vat liquor is mixed with a little pearlash, and
thickened with gum. This is applied by the cylinder or block to goods previously[235]
padded with nitrate of lead; the goods, after being dried, are passed through milky lime-water,
rinsed, and then winced or padded through the bichromate of potash bath.
Another green.—Nitrate of lead, prussiate of potash, and tartaric acid, dissolved, and
mixed with a little sulphate, nitrate, and muriate of iron; this mixture is either thickened
for cylinder printing, or used in its liquid state in the padding trough. The goods subjected
to one of these two processes are dried, padded in weak solution of carbonate of
potash, which serves to precipitate the oxide of lead from the nitrate; they are finally
padded with bichromate of potash, which induces a yellow upon the blue, constituting a
green colour of any desired tint, according to the proportion of the materials.
Chocolate and black, with white discharge; a fast colour.—The cloth is padded with
acetate of alumina, and dried in the hot flue; it is then passed through a two-colour
machine, the one cylinder of which prints-on lime-juice discharge, thickened with gum
senegal; the other a black topical dye (made with logwood extract and iron liquor).
The cloths are now hung up to be aired during a week, after which they are dunged, and
dyed up with madder, fustic, and quercitron bark, heated with steam in the bath.
Blue, white, and olive or chocolate.—1. Pad with the aluminous mordant; 2. Apply
thickened lemon juice for discharge by the cylinder; 3. Dung the goods after they
are thoroughly dried; 4. Pass them through the bath of madder, fustic, and quercitron,
which dye a brown ground, and leave the discharge points white; then print-on a reserve
paste of China clay and gum with sulphate of copper; dry, dip in the blue vat, which
will communicate an olive tint to the brown ground; or a chocolate, if madder alone had
been used.
When a black ground is desired, with white figures, the acid discharge paste should be
printed-on by the cylinder, and dried before the piece is padded in the iron liquor. By
following this plan the whites are much purer than when the iron is first applied.
Green, black, white.—The black is first printed-on by a mixture of iron liquor, and
infusion (not decoction) of logwood; then resist or reserve paste is applied by the block,
and dried; after which the goods are blued in the indigo vat, rinsed, dried, passed
through solution of acetate of lead; next, through milky lime water; lastly, through a
very strong solution of bichromate of potash.
Turkey red, black, yellow.—Upon Turkey red cloth, print with a strong solution of
tartaric acid, mixed with solution of nitrate of lead, thickened with gum; dry. The
cloth is now passed through the chloride of lime bath, washed, and chromed. Lastly,
the black is printed-on by the block as above, with iron liquor and logwood.
Black ground dotted white, with red or pink and black figures.—1. Print-on the
lime juice discharge-paste by the cylinder; dry; 2. Then pad with iron liquor,
containing a little acetate of alumina, and hang up the goods for a few days to fix
the iron; 3. Dye in a logwood bath to which a little madder has been added; clear
with bran. The red or pink is now put in by the block, with a mixture of extract of
Brazil-wood, nitromuriate of tin, and nitrate of copper, as prescribed in a preceding
formula.
Orange or brown; black; white; pink.—The black is topical, as above; it is printed-on,
as also the lemon-juice discharge and red mordant, with muriate of tin (both
thickened), by the three-colour machine. Then, after drying the cloth, a single-cylinder
machine is made to apply in diagonal lines to it a mixture of acetate of iron and alumina.
The cloth, being dried and dunged, is next dyed in a bath of quercitron, madder, and
fustic.
Here the orange is the result of the mordant of tin and alumina; the brown, of the
alumina and iron; white, of the citric acid discharge. The tin mordant, wherever it has
been applied, resists the weaker mordant impressed in the diagonal lines. The pink is
blocked-on at the end.
Orange brown, or aventurine; black and white.—The topical black (as above), and
discharge lemon juice, are printed-on by the two-colour machine; then the cloth is
subjected to the diagonal line cylinder, supplied with the alumino-iron mordant. The
cloth is dried, dunged, and dyed in a bath of bark, madder, and fustic.
The manganese or solitaire ground admits of a great variety of figures being easily
brought upon it, because almost every acidulous mordant will dissolve the oxide of
manganese from the spot to which it is applied, and insert its own base in its place; and
of course, by dyeing such mordanted goods in various baths, any variety of coloured
designs may be produced. Thus, if the paste of nitrate of lead and tartaric acid solution
be applied, and the goods after drying be passed first through lime water, and then
through a chrome bath, bright yellow spots will be made to appear upon the bronze
ground.
Manganese bronze, buff and green; all metallic colours.—Pad-on the manganese
solution, and dry; apply the aceto-sulphate of iron, of spec. grav. 1·02, and Scheele’s
green (both properly thickened), by the two-colour machine. The goods are next to
be dried, and padded through a cold caustic lye of spec. grav. 1·086. They are then[236]
rinsed, and passed through a weak solution of chloride of lime, to raise the bronze,
again rinsed, and passed through a solution of arsenious acid to raise the green.
Scheele’s green for the calico-printer is made as follows:—
Take 1 gallon of water, in which dissolve with heat,
5 pounds of sulphate of copper, and 1 pound of verdigris. When the two salts
are dissolved, remove the kettle from the fire, and put into it 1 quart of solution of
nitrate of copper, and 5 pounds of acetate of lead. Stir the mixture to facilitate the
decomposition, and allow the pigment to subside.
It must be thickened with 21⁄2 libs. of gum per gallon, for pencilling; or 12 oz. of starch
for the block. The goods printed with this paste are to be winced through a caustic lye,
till a fine sky-blue be produced; then washed well and rinsed. They are now to be
passed through water, containing from half an ounce to an ounce of white arsenic per
piece; 4 turns are sufficient; if it be too long immersed, it will take a yellow tint.
Catechu has been considerably employed by calico-printers of late years, as it affords
a fine permanent substantive brown, of the shade called carmelite by the French. The
following formula will exemplify its mode of application:—
Take 1 gallon of water;
1 pound of catechu in fine powder; reduce by boiling to half a gallon, pass the
decoction through a fine sieve, and dissolve in it 4 ounces of verdigris; allow it then
to cool, and thicken the solution with 5 ounces of starch; while the paste is hot,
dissolve in it 5 ounces of pulverized muriate of ammonia.
Print-on this paste, dry, and wash. It is a fast colour.
I shall subjoin the prescriptions for two fancy cochineal printing colours.
Amaranth by cochineal.—Pad the pieces in the aluminous mordant of spec. grav. 1·027,
page 224.
Dry in the hot flue; and after hanging up the goods during 3 days, wince well through
chalky water, and then dye, as follows:—
For each piece of 28 or 30 yards, 8 ounces of cochineal are to be made into a decoction
of 2 gallons in bulk, which is to be poured into a kettle with a decoction of 3 ounces
of galls, and with 2 ounces of bran. The pieces are to be entered, and winced as in the
madder bath, during two hours and a half; then washed in the dash wheel. On mixing
with the amaranth bath a certain quantity of logwood, very beautiful lilacs and violets
may be obtained.
Mixture of quercitron and cochineal.—Pad in the aluminous mordant, and dye with 2
libs. of quercitron, and 4 ounces of cochineal, when a capuchin colour will be obtained. If
we pad with the following mordant; viz. 1 gallon of acetate of alumina of 1·056 spec.
grav., and 1 of iron liquor of 1·02 spec. grav., and dye with 1 pound of quercitron,
and 1 ounce of cochineal, we shall obtain a shade like boot-tops, of extreme vivacity.
Two ounces of cochineal will print a long piece of calico with rich pink figures, having
acetate of alumina for a mordant. As the ground is hardly tinged by the dye, it neither
needs nor admits of much clearing.
I have already mentioned that goods are sometimes padded with solution of perchloride
of tin before printing-on them the steam colours, whereby they acquire both permanence
and vivacity. I have also stated that the salts of tin at a high temperature are apt to
corrode the fibre of the stuff, and therefore must be used with discretion. This danger
is greatly lessened by adding to the perchloride of tin a sufficient quantity of caustic
potash lye to form a stannate of potash. The goods are padded through this substance,
diluted with water, dried with a moderate heat, and then immersed in very dilute sulphuric
acid, which saturates the potash, and precipitates the tin oxide within the pores of
the cloth. Calico thus prepared affords brilliant and permanent colours by the steam
process, above described.
Printing of silks or woollen stuffs, such as merinoes and mousselin de laine, as also of mixed
stuffs of silk and wool, such as chalys.—All these prints are applied, not by the cylinder
but the block, and are fixed by the application of steam in one of four ways; 1. By the
lanthorn; 2. By the cask; 3. By the chest; or 4. By the chamber.
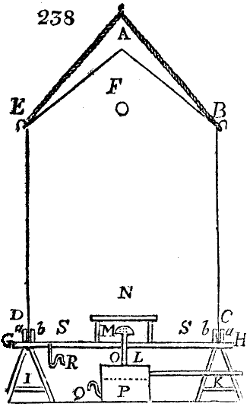
1. By the lanthorn.—In this mode of exposure to steam, the goods are stretched upon
a frame; and therefore the apparatus may be described under two heads; the lanthorn and
the frame. The former is made of copper, in the shape of a box A B C D E, fig. 238., open
below, and with a sloping roof above, to facilitate the trickling down of the water condensed
upon the walls. The sides B C D E are 41⁄2 feet high, 6 feet long, and 4 feet wide.
The distance of the point A from the line E B is 2 feet. At F is a brass socket, which
may be stopped with a cork; and there is a similar one at the other side. This kind
of penthouse may be raised by means of a pully with cords fixed to the four angles
of the roof E B; and it rests upon the table G H, a little larger than the area of the box,
which stands upon the four feet I K. Round the borders of the table there is a
triangular groove a b, for receiving the lower edges of the box, and it is stuffed steam-tight
with lists of cloth. Through the centre of the table, the two-inch steam pipe M[237]
passes; it is surmounted with an hemispherical rose pierced with numerous holes for
the equal distribution of the steam. Right above it, a disc N is placed upon four feet.
The tube L communicates with a box P, which has a syphon Q
to let off the condensed water. At the upper part of this box
the tube L terminates which brings the steam. The little table
G H slopes towards the part G, where the syphon R is placed
for drawing off the water.
The frame has such dimensions, that it may stand in the four
corners of the table at S S, as pointed out by the dotted lines.
The second part embraces an open square frame, which is
formed by spars of wood 2 inches square, mortised together;
and is 3 feet 8 inches wide, 5 feet 8 inches long, and 4 feet 3
inches high; it is strengthened with cross bars. Upon the two
sides of its breadth, two rows of round brass hooks are placed,
about half an inch apart; they are soldered to a copper plate
fixed to uprights by means of screws.
Before hanging up the goods, a piece of cloth 3 feet 8 inches
long, and 4 feet wide, is placed upon the row of hooks; and 3
feet of it are left hanging out.
One foot within, the hooks pass through the cloth. A similar one is fitted to the other
side. This cloth is intended to cover the goods hung upon the hooks; and it is kept
straight by resting upon strings. The pieces are attached zig-zag from one hook to
another. When the frame is filled, the bag is put within the cloths; it has the same
rectangular shape as the frame. The pieces are in this way all encased in the cloth; a
bit of it being also put beneath to prevent moisture affecting that part.
When shawls are framed, they are attached with pins; and if they be too large, they
are doubled back to back, with the fringes at top.
These arrangements being made, the frame is set upon the table, the penthouse is placed
over it, and the steam is admitted during from 35 to 45 minutes, according to circumstances.
The orifice F is opened at first to let the air escape, and when it begins to discharge
steam it is stopped. The frame is taken out at the proper time, the bag is
removed, the cloths are lifted off, and the goods are spread out for airing. Three frames
and six bags are required for a constant succession of work. The above apparatus is particularly
suitable for silks.
2. The drum.—This is the most simple mode of steaming. The apparatus is a drum
of white wood, 2 inches thick, fig. 239.; the bottom is pierced with a hole which admits
the steam-pipe F, terminating in a perforated rose. Four inches from the bottom there
is a canvass partition E, intended to stop any drops of water projected from the tube F,
and also to separate the condensed water from the body of the apparatus. The drum is
covered in by a wooden head H, under which the goods are placed. It is made fast either
by bolts, or by hooks, G G, thus  , to which weighted cords are
hung. The frame 1, fig. 240. rests upon a hoop, a a, a few inches
from the edge. The goods are hung upon the frame in the ordinary
way, and then wrapped round with flannel. The frame is
studded with pin points, like that of the indigo vat, fixed about
5 inches asunder. From 20 to 30 minutes suffice for one steaming
operation. The upper part of the frame must be covered
also with flannels to prevent the deposition of moisture upon it.
At the bottom of the drum there is a stopcock to let off the condensed
water. According to the size of the figure, which is 3
feet 2 inches, 50 yards may be hung up single; but they may be
doubled on occasion.
, to which weighted cords are
hung. The frame 1, fig. 240. rests upon a hoop, a a, a few inches
from the edge. The goods are hung upon the frame in the ordinary
way, and then wrapped round with flannel. The frame is
studded with pin points, like that of the indigo vat, fixed about
5 inches asunder. From 20 to 30 minutes suffice for one steaming
operation. The upper part of the frame must be covered
also with flannels to prevent the deposition of moisture upon it.
At the bottom of the drum there is a stopcock to let off the condensed
water. According to the size of the figure, which is 3
feet 2 inches, 50 yards may be hung up single; but they may be
doubled on occasion.
3. The box.—This steaming
apparatus is convenient from the
large quantity of goods admissible
at a time: it answers best
for woollen stuffs. From 12 to
16 pieces, of 36 yards each, may
be operated upon at once; and
from 240 to 260 shawls. It is
formed of a deal box, A B C D, fig.
241., 4 feet wide, 6 long, and 3
high; the wood being 4 inches thick. It is closed by a cover of the same substance, I,
which is made steam-tight at the edges by a list of felt. The lid is fastened down by 5
cross bars of iron, a a a a a, which are secured by screws, c c c c c, fig. 242. The ends of
these cross bars are let into the notches, b b b b b, on the edge of the box. The safety
valve M, fig. 241., is placed upon the lid. For taking off the lid, there are rings at the four[238]
corners, d d d d, bearing cords, F F F F. These join at the centre into one, which passes
over a pulley. Eight inches from the bottom of the box there is a horizontal canvass partition,
beneath which the steam is discharged from the pipe L, fig. 243. There are two
ledges, E F G H, at the sides for receiving the bobbins. The tube L runs round the box, as
shown by the letters d a e b: the end d is shut;
but the side and top are perforated with many
holes in the direction towards the centre of the box.
Fig. 244. shows the arrangement of the lower set
of bobbins: that of the upper set is shown by the
dotted lines: it is seen to be in an alternate position,
one lying between two others. They are
formed of pieces of deal 4 inches broad, 1 inch
thick, and of a length equal to the width of the
box. They are first wrapped round with 5 or 6
turns of doubled flannel or calico: the piece of
goods is laid over it upon a table, and then
wrapped round. At the end of the piece, several
folds of the covering must be put, as, also, a roll
of flannel. The two ends must be slightly tied
with packthread. When these flat bobbins are
arranged in the box, the steam is let on them, and
continued about 45 minutes: it is then shut off,
the lid is removed, and the pieces are unrolled.
4. The chamber.—The interior height of the
chamber, A B C D, fig. 245., is 9 feet, the length 12
feet, and the breadth 9 feet. The steam is introduced
into it by two pipes, a b c, d e f. Their two ends,
d c, are shut; but their sides are all along perforated
with small holes. The frames E F G H, E F G H, are
moveable, and run upon rollers: they are taken out
by front doors, which are made of strong planks,
shut by sliding in slots, and are secured by strong
iron bars and pressure screws. The cross rods,
E F G H, are provided with hooks for hanging up the pieces. There is a safety-valve in the
top of this large chamber. The dimensions of the frame are 10 feet long, 3 feet wide,
and 7 high. Three feet and a half from the upper part of the frame, a row of hooks
is fixed for hanging on a double row of pieces, as shown in the figure. Over the frame,
woollen blankets are laid to protect it from drops of water that might fall from the
roof of the chamber. When the hooks are two thirds of an inch apart, 24 pieces, of 28
yards each, may be suspended at once. The period of steaming is from 45 to 60
minutes.
Muslins and silks do not require so high a temperature as woollen goods. When the
stuffs are padded with colour, like merinos and chalys, they must not be folded together,
for fear of stains, which are sometimes occasioned by the column in steam calico-printing,
where the end which receives the first impression of the steam is seldom of the same
shade as the rest of the roll of goods. The duration of the steaming depends upon
the quantity of acid in the mordant, and of saline solution in the topical colour; the
more of which are present, the shorter should be the steaming period. A dry vapour is
requisite in all cases; for when it becomes moist, from a feeble supply or external condensation,
the goods become streaky or stained by the spreading of the colours.
1. Black figures are given by decoction of logwood thickened with starch, to which
a little oxalic acid is added while hot, and, after it is cold, neutralised solution of nitrate
of iron.
2. Dark blue for a ground.—Decoction of logwood, and archil thickened with starch;
to which, while the paste is hot, a little soluble Prussian blue is added; and, when it is
cold, neutralised nitrate of iron; see supra.
[239]
3. Deep poppy or ponceau colour.—Cochineal boiled in starch water, with oxalic acid
(or tartaric), and perchloride of tin.
4. Rose.—Cochineal infusion; oxalic acid; perchloride of tin; thickened with gum.
5. Dark amaranth.—Decoctions of archil and cochineal, thickened with starch: to
the paste, alum and perchloride of tin are added.
6. Capuchin colour.—Quercitron and cochineal thickened with starch; to the paste
add oxalic acid, and perchloride of tin.
7. Annotto orange.—Dissolve the annotto in soda lye, of spec. grav. 1·07, at a boiling
heat; add aluminate of soda, and thicken with gum.
8. Golden yellow.—Decoction of Persian berries thickened with starch; to which some
alum and muriate of tin are added, with a little perchloride of tin and oxalic acid.
9. Lemon yellow.—Persian berries; starch; alum.
10. An ammoniacal solution of cochineal is used for making many violet and mallow
colours. It is prepared by infusing cochineal in water of ammonia for 24 hours; then
diluting with water, heating to ebullition, and straining.
11. Fine violet is given by ammoniacal cochineal, with alum and oxalic acid; to which
a little aceto-sulphate of indigo is added, and gum for thickening. The following blue
may be used instead of the solution of indigo. The mallow tint is given by adding a
little perchloride of tin to the above formula, and leaving out the blue.
12. Dark blue.—Soluble Prussian blue; tartaric acid; alum; thicken with gum.
13. Emerald green.—One quart of decoction, equivalent to 1 pound of Persian
berries; 1 quart of infusion of quercitron, of spec. grav. 1·027; in which dissolve 12
ounces of alum in powder; and add 6 ounces of the following blue bath for greens;
thicken with 20 ounces of gum.
14. Blue bath for greens. Half a gallon of water at 140° F., 1 pound of soluble
Prussian blue, 3 ounces of tartaric acid, and 2 ounces of alum.
I. Printing of Silks.—1. Of the madder style. This is one of the most difficult to execute,
requiring both much skill and experience. The first step is the removal of the gum. A copper
being nearly filled with water, the pieces, tied up in a linen bag, are put into it, with a
quarter of a pound of soap for every pound of silk, and are boiled for 3 hours. If the silk
be Indian, half an ounce of soda crystals must be added. When the goods are taken
out, they are rinsed in the river, then passed through water at 140° F., holding 8
ounces of crystallised soda in solution, as a scourer. They are next rinsed in cold
water, and steeped in water very faintly acidulated with sulphuric acid, during 4
hours, then rinsed, and dried.
Preparation of Mordants.—1 gallon of boiling water; 2 pounds of alum; dissolve:
1 pound of acetate of lead; 4 ounces of sal-ammoniac; 1 of chalk; mix well together;
after decomposition and subsidence, draw off clear.
1. Red.—1 gallon of the above mordant, thickened with 14 ounces of starch, and tinged
with decoction of Brazil wood. If dark red be wanted, dissolve, in a gallon of the
above red, 4 ounces of sulphate of copper.
2. Black.—1 gallon of iron liquor, of 1·056 spec. grav.; thicken with 14 ounces of
starch; and dissolve in the hot paste 2 ounces of sulphate of copper.
3. Violet. Take 1 gallon of iron liquor of 1·04 spec. grav.;
3. Violet. 2 ounces of cream of tartar; 2 ounces of nitre; 2 ounces of copperas;
3. Violet. 1 ounce of alum: dissolve, and mix the solution with
3. Violet. 1 gallon of gum water, containing 6 libs. of gum.
4. Puce. Half a gallon of red mordant; half a gallon of iron liquor of 1·07;
4. Puce. 7 ounces of starch for thickening colour with logwood.
Manipulation of the above colours.—Print-on the black, then the puce, next the violet,
and, lastly the red. Dry in the hot flue, and, 48 hours after the impression, wash away
the paste. The copper employed for dyeing is of a square form: a boil is given with
bran, at the rate of 4 libs. per piece of the foulards: cold water is added to lower the
temperature to 130° F. The pieces must be entered with the printed surface undermost,
and winced for half an hour, taking care to keep them expanded and well covered
with the liquor: they are then taken out and rinsed. When grounds are to be made
on the foulards, 2 ounces of sumach must be added per piece.
Maddering.—Suppose 48 pieces are to be grounded with madder. 12 pounds of
madder must be put into the copper, 1 pound of sumach, and 6 pounds of bran; the
bath must be tepid when the pieces are entered: it must be heated to 104° F. in 20 minutes,
and to the boiling point in an hour and a half. The goods must be briskly winced all
the time, and finally turned out into cold water.
When they come out of the madder bath they are much loaded with colour. They are
cleared by a boil of half an hour in bran, then turned out into cold water, and rinsed. A
copper must be now mounted with 3 pounds of soap, 1 ounce of solution of tin, and 2
pailsful of bran, in which the goods are to be boiled for half an hour, then rinsed, and
passed through a very dilute sulphuric acid bath. Then rinse, and dry. By following
this process a light salmon ground is obtained.
[240]
II. Steam colours upon silk.—The same plan of operations may be adopted here as is
described for calico-printing; the main difference being in the method of mordanting
the stuffs. After boiling in soap water, in the proportion of 4 ounces per pound of silk,
the goods are washed in cold water, and then in hot water at 140°; they are next rinsed,
passed through weak sulphuric acid, rinsed, squeezed between rollers, and afterwards steeped
in a bath containing 8 ounces of alum per gallon, where they remain for four hours, with
occasionally wincing. They are now rinsed, and dried. The subsequent treatment resembles
that of steam-colour printed cottons.
Black.—Take a gallon of decoction, made with 4 libs. of logwood, with which
Black.—14 ounces of starch are to be combined: mix in
Black.—2 ounces of powdered nut-galls: boil, and pour the colour into a pipkin containing
Black.—2 ounces of tartaric acid; 2 ounces of oxalic, both in powder, and
Black.—2 ounces of olive oil. Stir the colour till it is cold, and add
Black.—8 ounces of nitrate of iron, and 4 ounces of nitrate of copper.
The red, violet, lilac, yellow colours, &c. are the same as for steam colours upon cotton.
Topical colours are also applied without mordanting the silk beforehand. In this case
a little muriate of tin is introduced. Thus, for
Yellow.—Take 1 gallon of a decoction, made with 4 libs. of Persian berries: dissolve
in it 8 ounces of salt of tin (muriate), and 4 ounces of the nitro-muriatic
solution of tin. Thicken with 2 pounds of gum.
Printing of foulard pieces. The tables which serve for the impression of silk goods are so
constructed as to receive them in their full breadth. Towards the part between the colour
or sieve tub and the table, the roller is mounted upon which the piece is wound. This roller,
A B, fig. 246., has a groove, C, cut out parallel to its axis.
Into this a bar is pressed, which fixes the end of the
piece. The head, B, of the roller is pierced with several
holes, in which an iron pin passes for stopping its rotation
at any point, as is shown at B. At the other end
of the table there is placed a comb, fig. 247., which
is supported by pivots A B at its ends. The teeth of the comb are on a level with the
cloth.
The piece is arranged for printing as follows:—It is unwound, and its end is brought
upon the teeth of the comb, and made to pass into them by slight taps with a brush.
It is now stretched, by turning round the roller, and fixing it by the pin-handle.
After tracing the outline, the printing blocks are applied. Care should be taken, in
the course of printing, always to fix the teeth of the comb in the middle line between
two handkerchiefs. The operation of grounding-in is much facilitated by this plan of
extension.
The pieces are washed in running water, and must be rapidly dried. The subsequent
dressing is given by gum tragacanth: they are dried upon a stretching frame, and then
folded up for the market.
III. Mandarining of silk stuffs and chalys.—This style of printing depends upon the
property which nitric acid possesses of giving to silk and woollen stuffs a yellow colour.
The first step is the scouring with a soap boil, as already described.
The designs are printed-on as also above described.
The swimming or colour-tub is usually double, and serves for two tables; instead of
being placed, therefore, at the end of the table, it is put between two, and, consequently,
behind the printer. It is formed of a
copper chest, fig. 248., A B C D, in which steam
may circulate, introduced by the pipe I; the
excess being allowed to escape by the tube J,
as also the water of condensation. The frame
is placed in the hollow box K K. Between two
such frames there is a plate of copper, L, which
closes the box; it serves for laying the plates in
order to keep them hot. At E and H are prolongations of the box, in which are set
the vessels F G for holding the reserve paste.
Preparation of the reserve or resist paste.—Melt in a kettle 21⁄2 libs. of rosin; 1
lib. of suet: mix well, and put it into the basins F G. By means of steam the
reserve is kept melted, as well as the false colour upon which the sieve floats.
The piece of silk being laid upon the table, and the reserve spread upon the frame,
the printer heats his block, which should be mounted with lead, if the pattern
will permit, upon the little table L. He takes up the colour from the frame, and transfers
it instantly to the piece. He must strike the block lightly, and then lift it, lest, by
its cooling, it might stick to the silk. When the table pattern is completed, he dusts it
over with sand, and proceeds to another portion of the silk. The piece must not be[241]
taken out of the stretch till it is quite dry, which requires usually 6 hours. Let us
consider first the most common case, that of a white upon an orange ground. We shall
afterwards describe the other styles, which may be obtained by this process. The piece,
being printed and dry, must next be subjected to the mandarining operation.
The apparatus here employed consists of a sandstone trough
A B C D, fig. 249. Upon the two sides, A C, B D, of this trough
are fixed two wooden planks, pierced with a hole an inch from
the bottom to receive the roller E, under which the piece passes.
In this trough the acid mixture is put. That trough is put into
a wooden or copper trough, F G H I. Into the latter, water
is put, which is heated by means of steam, or a convenient
furnace. Before and behind are placed two winces, or reels,
K L: one serves to guide the piece in entering into the trough,
and the other in its leaving it. The piece falls immediately
into a stream of cold water, or, failing that, into a large back,
containing a mixture of chalk and water. The two winces are
moved by handles: the velocity is proportioned to the action of
the acid. The wince L ought to be higher than K, to allow the
acid to drain off. Fig. 250. shows a section of the apparatus.
The temperature of the acid mixture ought to be maintained
between 95° and 100° F.; for if it be raised higher, the resist
would run the risk of melting, and the impression would
become irregular and blotty.
The proportions of the acid mixture are the following:—1
gallon of water; and 1 gallon of nitric acid, of spec. grav. 1·288, which may be
increased with the strength of the silk. It should be a little weaker for chalys. For
the strong greens it may be 2 measures of acid of 1·288 to 1 measure of water. The
duration of the passage through the acid should be 1 minute at most.
Mixture of orange colour, and clearing away of the resist.—The goods, on coming
out of the mandarining apparatus, are rinsed in running water; then boiled in
soap water, quickened with a little soda, at the rate of 2 libs. of the former and
4 oz. of the latter for a piece of 30 yards. They must be worked by the wince for half
an hour. They are now rinsed in cold water, then passed through hot, again rinsed, and
dried. I shall give some examples of the mode of manufacture, which is undoubtedly
one of the most curious applications of chemical ingenuity.
1. Orange ground with white figures.
(1.) Print-on the fat reserve; (2.) mandarine; (3.) brighten the orange, and clear.
2. Orange ground, with blue figures.
(1.) Dip in the indigo vat as for calico; (2.) print-on the fat resist to preserve the
blue; (3.) mandarine; 4. clear, and brighten the orange by the boil.
3. Orange ground, with blue and white figures.
(1.) Print-on the resist to preserve the white; (2.) dip in the vat, rinse, and dry; (3.)
ground-in the fat resist to preserve the blue; (4.) mandarine; (5.) cleanse, and brighten.
4. Full green ground, and white figures.
(1.) Print-on the resist; (2.) mandarine, and rinse without drying; (3.) dip in
the blue vat; (4.) cleanse, and brighten.
5. Full green ground, and blue figures.
(1.) Dip a pale blue, rinse, and dry; (2.) print-on the fat resist; (3.) mandarine,
wash and dry; (4.) dip full blue; (5.) clean, and brighten.
6. Full green ground, with white and blue figures.
(1.) Print-on the resist; (2.) dip a pale blue, and dry; (3.) ground-in the fat
resist; (4.) mandarine and rinse; (5.) dip a full blue; (6.) clean, and brighten.
7. Full green ground, with white, blue, and orange figures.
(1.) Print-on the fat reserve; (2.) dip a pale blue, and dry; (3.) ground-in the
reserve; (4.) mandarine, rinse, and dry; (5.) ground-in the reserve; (6.) dip a full
blue; (7.) clean, and brighten.
If blue grounds with white figures be wanted, the resist must be applied, and then
the goods must be dipped in the blue vat: the resist is afterwards removed by a boil in
soap-water.
The above processes are applicable to chalys.
The property which nitric acid possesses of staining animal matters yellow, such as
the skin, wool, and silk, is here applied to a very elegant purpose.
Of the bronze or solitaire style by mandarining.—The mandarining mixture, is
1 gallon of nitric acid, of 1·17 spec. grav.; mixed with 3 pints of solution of
nitrate of iron, of spec. grav. 1·65. If the quantity of nitrate of iron be increased, a
darker tint will be obtained. The temperature of the mixture should be 94° F. The
pieces, after mandarining, are let fall into water, and steeped for an hour.
[242]
In order to raise the bronze, and clear away the fat resist, the goods must be boiled
in a bath of soap and soda, as described for orange.
1. Bronze ground, with white figures.
(1.) Print on the fat resist; (2.) dip in the blue vat, and dry; (3.) pad in a decoction
of logwood, of 4 libs. per gallon; dry, taking care to turn over the selvages; (4.)
mandarine, and steep in water for an hour; (5.) cleanse, and pass through soap.
2. Bronze ground, with blue figures.
(1.) Dip in the blue vat, and dry; (2.) print-on the fat resist; (3.) pad in the
above decoction of logwood, and dry; (4.) mandarine, and steep an hour; (5.)
cleanse, and brighten.
3. Bronze ground, with white and blue.
(1.) Print-on the fat resist; (2.) dip in the blue vat, and dry; (3.) ground-in
the fat resist; (4.) pad in the logwood liquor, and dry; (5.) mandarine, and steep for
an hour; (6.) cleanse, and give the brightening boil with soap.
This style of manufacture may be executed on chalys; and is capable of producing
beautiful effects, which will in vain be sought for by other means.
With silks, advantage may be derived from various metallic solutions which possess
the property of staining animal substances; among which are nitrate of silver, nitrate of
mercury, and muriate of iron. The solutions of these salts may be thickened with gum,
and printed-on.
An orange upon an indigo vat ground.—After the blue ground has been dyed, orange
figures may be produced by printing-on the following discharge paste:—
1 gallon of water, made into a paste with 1 pound of starch: when cold, add to
it from 16 to 24 ounces of nitric acid, of spec. grav. 1·288. After fixing the colour by
steam, the orange is brightened with a soap boil.
An orange upon a Prussian-blue ground.—The dye is first given by Prussian blue in
the ordinary way, and then the following discharge is printed-on:—
A caustic lye being prepared, of 1·086 specific gravity, dissolve in a gallon of it 2
pounds of annotto, and thicken with 3 pounds and a quarter of gum. Two days
after the impression of this paste, pass the goods through steam, and wash them in
running water. With these two designs, the logwood and gall black, formerly described,
may be associated, to produce a rich effect.
To the preceding practical instructions for printing calicoes, silks, woollens, and
mixed fabrics, made of the two latter, a few annotations may be added.
When an uniform colour is to be applied to both sides of the cloth, the padding process
is employed; but, when only one side is to be thus coloured, diagonal lines are cut
very closely to each other upon the cylinder, which transfer so much colour from the trough
to the cloth passed under it as to make the surface appear uniformly stained. This
process is called mattage by the French. Mordants or topical dyes, to be applied in this
way, should not be much thickened.
The doubler is the piece of felt or blanket stuff placed between the cloth to be printed,
and the block printing table, or the cylinders. It should be kept very clean; because,
were it soiled with acetate of iron, it would spoil all the light shades made with acetate
of alumina.
Filters for the colour shop of a print house are best made of wool, formed into a substantial
conical cap by felting. A filter ought to be set apart for each different dye
stuff.
When the goods after dyeing are washed, by being held by the selvage, dipped, and
shaken in a stream of water, the process is called giving a list by the French (donner
une lisière). The piece is transferred alternately from one hand to another.
Stains. When we observe stains produced by mordants, upon spots where no colour
is to come, we must, before dunging the goods, apply a little of the lime juice, or tartaro-oxalic
acid discharge paste, to the place. If, on the contrary, the stains are not perceived till
after the maddering, we must then apply to it first a strong solution of chloride of lime
with a pencil, next a solution of oxalic acid mixed with a little muriatic with another
pencil, and immediately afterwards wash with water. Every madder stain will be
effaced by this means.
Rust stains are removable by a mixture of oxalic and muriatic acids.
Indigo stains by the combined action of chloride of lime and muriatic acid.
Topical yellow stains, or yellow dyes, by the same combination.
Metallic greens and Scheele’s green by the acid alone.
Chrome green, and Prussian blue. The blue may be taken out by a caustic alkali;
after which the goods must be washed: the residuary rust stain may be removed by the
mixture of oxalic and muriatic acids. The above methods refer to cotton and linen.
The stains on silk and woollen stuffs should be removed before fixing the colours by the
soap boil; which may generally be done by scratching with the finger, with the aid of a
little water.
For a direct calico green, see oxide of Chrome.
[243]
Mr. Hudson, of Gale, near Rochdale, obtained a patent, in December, 1834, for a
mechanism which furnishes a continual and regular supply of colour to the sieve or tear
(tiré, Fr.) into which the printer has to dip his block, for the purpose of receiving the
colour about to be transferred to the fabric in the operations of printing calicoes or paper
hangings. The contrivance consists in a travelling endless web, moved by power, which,
by passing progressively from the colour vat over the diaphragm, brings forward continuously
an equable supply of the coloured paste for the workman’s block.
Fig. 251. represents the
construction of this ingenious
apparatus, shown
partly in section. a a is a
vessel of iron, supported
upon wooden standards b b,
over the upper surface of
which vessel a sheet or
diaphragm, c c, of oiled
cloth, or other suitable
elastic material, is distended,
and made fast at
its edges by being bent
over a flange, and packed
or cemented to render the joints water-tight. A vertical pipe d is intended to conduct
water to the interior of the vessel a, and, by a small elevation of the column, to create
such upward pressure as shall give to the diaphragm a slight bulge like the swimming
tub.
An endless web, e e e, passing over the surface of the diaphragm, is distended over three
rollers, f g h, the lower of which, f, is in contact with the colour-roller i in the colour-trough
K. On the axle of the roller i a pulley wheel is fixed, which allows the roller to
be turned by a band from any first mover; or the roller may receive rotatory motion by a
winch fixed on its axle. On this said axle there is also a toothed wheel, taking into
another toothed wheel on the axle of the roller f; hence, the rotation of the colour-roller
i in the one direction will cause the roller f to revolve in the opposite, and to carry
forward the endless web e e e, over the elastic diaphragm, the web taking with it a stratum
of colour received from the roller i, evenly distributed over its surface, and ready
for the printer to dip his block into.
The axles of the rollers f and g turn in stationary bearings; but the axle of h is
mounted in sliding nuts, which may be moved by turning the screws m, for the purpose
of tightening the endless web. The axle of the colour-roller i turns in mortises, and
may be raised by screws n, in order to bring its surface into contact with the endless
web. To prevent too great a quantity of colour being taken up, the endless web passes
through a long slit, or parallel aperture, in a frame o, which acts as a scraper or doctor,
and is adjustable by a screw p, to regulate the quantity of colour carried up. The contents
of the vessel a, and of the colour-trough K, may be discharged when required by a
cock in the bottom of each. See Paper Hangings, for the Fondu style.
CALOMEL. (Chlorure de Mercure, Fr.; Versüsstes Quecksilber, Germ.) The
mild protochloride of mercury. The manufacture of this substance upon the great scale
may be performed in two ways. The cheapest and most direct consists in mixing
11⁄8 part of pure quicksilver with 1 part of pure nitric acid, of sp. grav. from
1·2 to 1·25; and in digesting the mixture till no more metal can be dissolved, or
till the liquid has assumed a yellow colour. At the same time, a solution of 1 part
of common salt is made in 32 parts of distilled water, to which a little muriatic acid is
added; and, when heated to nearly the boiling point, it is mixed with the mercurial solution.
The two salts exchange bases, and a protochloride of mercury precipitates in a
white powder, which, after being digested for some time in the acidulous supernatant
liquor, is to be washed with the greatest care in boiling water. The circumstances which
may injure the process are the following:—1. When less mercury is employed than the
acid can dissolve, there is formed a deuto-nitrate of mercury, which forms some corrosive
sublimate with the common salt, and causes a proportional defalcation of calomel. 2. If
the liquors are perfectly neutral at the moment of mixing them, some subnitrate of mercury
is thrown down, which cannot be removed by washing, and which gives a noxious
contamination to the bland calomel. The acid prescribed in the above formula obviates
this danger.
The second manner of manufacturing calomel is to grind very carefully 4 parts of
corrosive sublimate (bi-chloride of mercury) with 3 parts of quicksilver, adding a little water
or spirits to repress the noxious dust during the trituration. The mass is then introduced
into a glass globe, and sublimed at a temperature gradually raised. The quicksilver
combines with the deutochloride, and converts it into the protochloride, or calomel. The[244]
following formula, upon the same principle, was recommended to the chemical manufacturer
in Brande’s Journal, for July, 1818:—
“Prepare an oxysulphate of mercury, by boiling 25 pounds of mercury with 35 pounds
of sulphuric acid to dryness. Triturate 31 pounds of this dry salt with 20 pounds 4
ounces of mercury, until the globules disappear, and then add 17 pounds of common
salt. The whole is to be thoroughly mixed, and sublimed in earthen vessels. Between
46 and 48 pounds of pure calomel are thus produced: it is to be washed and levigated
in the usual way.” The above is the process used at Apothecaries’ Hall, London. The
oxysulphate is made in an iron pot; and the sublimation is performed in earthen vessels.
The crystalline crust or cake of calomel should be separated from the accompanying
gray powder, which is nearest the glass, and consists of mercury mixed with corrosive
sublimate.
An ingenious modification of the latter process, for which a patent, now expired, was
obtained by Mr. Jewell, consists in conducting the sublimed vapours over an extensive
surface of water contained in a covered cistern. The calomel thus obtained is a superior
article, in an impalpable powder, propitious to its medical efficacy.
The presence of corrosive sublimate in calomel is easily detected by digesting alcohol
upon it, and testing the decanted alcohol with a drop of caustic potash, when the characteristic
brick-coloured precipitate will fall, if any of the poisonous salt be present.
To detect subnitrate of mercury in calomel, digest dilute nitric acid on it, and test the
acid with potash, when a precipitate will fall in case of that contamination. As it is
a medicine so extensively administered to children at a very tender age, its purity ought
to be scrupulously watched.
118 parts of calomel contain 100 of quicksilver.
CALORIC. The chemical name of the power or matter of heat.
CALORIFÈRE OF WATER. (Calorifère d’eau, Fr.; Wasser-Heitzung, Germ.)
In the Dictionnaire Technologique, vol. iv., published in 1823, we find the following
description of this apparatus, of late years so much employed in Great Britain for heating
conservatories, &c. by hot water circulating in pipes:—
“This mode of heating is analogous to that by stove pipes: it is effected by the circulation
of water, which, like air, is a bad conductor, but may serve as a carrier of caloric
by its mobility. We may readily form an idea of the apparatus which has been employed
for this purpose. We adapt to the upper part of either a close kettle, or of an ordinary
cylindric boiler A, fig. 252, a tube B, which rises to a certain
height, then descends, making several sinuosities with a gentle
slope till it reaches the level of the bottom of the boiler, to
whose lowest part, as that which is least heated, it is fitted at C.
At the highest point of the tube F we adapt a vertical pipe, destined
to serve as an outlet to the steam which may be formed if
the temperature be too much raised: it serves also for the
escape of the air expelled from the water by the heat; and it
permits the boiler to be replenished from time to time as the
water is dissipated by evaporation; lastly, it is a tube of safety.
“The apparatus being thus arranged, and all the tubes as
well as the boiler filled with water, if we kindle fire in the grate
D, the first portions of water heated, having become specifically
lighter, will tend to rise: they will actually mount into the
upper part of the boiler, and, of course, enter the tube B F: at
the same time an equivalent quantity of water will re-enter the
boiler by the other extremity C of the tube. We perceive that
these simultaneous movements will determine a circulation in
the whole mass of the liquid, which will continue as long as heat is generated in the
fire-place; and if we suppose that the tubes, throughout their different windings, are
applied against the walls of a chamber, or a stove-room, the air will get warmed by
contact with the hot surfaces; and we may accelerate the warming by multiplying these
contacts in the mode indicated.
“This calorifère cannot be employed so usefully as those with heated air, when
it is wished to heat large apartments. In fact, the passage of heat through metallic
plates is in the ratio of the difference of temperature and quantity of the heating surfaces.
In the present case, the temperature of the water, without pressure, in the tubes, must be
always under 100° C. (212° F.), even in those points where it is most heated, and less
still in all the other points, while the temperature of the flues in air stoves, heated directly
by the products of combustion, may be greatly higher. In these stoves, also, the pipes
may without inconvenience have a large diameter, and present, consequently, a large heating
surface; whereas, with the water calorifère, the pressure exercised by the liquid upon
the sides of the tubes being in the ratio of the surfaces, we are obliged, in order to avoid
too great pressure, to employ a multitude of small tubes, which is expensive. Lastly, if[245]
the hot-water circulation is to be carried high, as may be often necessary in lofty buildings,
the pressure resulting from the great elevation would call for proportional thickness
in the tubes and the boiler: for these reasons, and others which we shall state in treating
of heating by steam, it appears that water cannot be advantageously substituted for air
or steam in the applications above stated; yet this mode of heating presents very decided
advantages where it is useful to raise the temperature a small number of degrees in a
uniform manner.” See Incubation, artificial.
“M. Bonnemain applied, with much success, these ingenious processes of heating
by the circulation of water, to maintain a very equal temperature in hot-houses (serres-chaudes),
in stoves adapted to artificial incubation, and in preserving or quickening vegetation
within hot-houses, or outside of their walls, during seasons unpropitious to horticulture.
“Since the capacity of water for heat is very great, if the mass of it in a circulation-apparatus
be very considerable, and the circulation be accelerated by proper arrangements,
as by cooling the descending tube exterior to the stove-room, we may easily obtain by
such means a moderately high and uniform temperature, provided the heat generated in
the fire-place be tolerably regular. We may easily secure this essential point by the aid
of the fire-regulator, an instrument invented by M. Bonnemain, and which is described
under the article Incubation, because there its use seems to be indispensable.”
From the above quotation, and, more especially, from the evidence adduced in the
article Incubation, we see how little claim the Marquis de Chabannes, or any of his
followers, can have to invention in their arrangements for heating apartments by the
calorific motions of the particles of water, enclosed in pipes of any kind.
CAMBRIC. (Batiste, Fr.; Kammertuch, Germ.) A sort of very fine and rather
thin linen fabric, first made at Cambray. An excellent imitation of this fabric is made
in Lancashire, woven from fine cotton yarn hard twisted. Linen cambric of a good
quality is also now manufactured in the United Kingdom from power-spun flax.
CAMLET OR CAMBLET. A light stuff, much used for female apparel. It is
made of long wool hard spun, sometimes mixed in the loom with cotton or linen yarn.
CAMPHOR, or CAMPHIRE. This immediate product of vegetation was known
to the Arabs under the names of kamphur and kaphur, whence the Greek and Latin
name camphora. It is found in a great many plants, and is secreted, in purity, by
several laurels: it occurs combined with the essential oils of many of the labiatæ; but
it is extracted, for manufacturing purposes only, from the Laurus camphora, which
abounds in China and Japan, as well as from a tree which grows in Sumatra and Borneo,
called, in the country, Kapour barros, from the name of the place where it is most
common. The camphor exists, ready formed, in these vegetables, between the wood
and the bark; but it does not exude spontaneously. On cleaving the tree Laurus
sumatrensis, masses of pure camphor are found in the pith.
The wood of the laurus is cut into small pieces, and put, with plenty of water, into
large iron boilers, which are covered with an earthen capital or dome, lined within with
rice straw. As the water boils, the camphor rises with the steam, and attaches itself as
a sublimate to the stalks, under the form of granulations of a grey colour. In this state
it is picked off the straw, and packed up for exportation to Europe.
Formerly Venice held the monopoly of refining camphor, but now France, England,
Holland, and Germany refine it for their own markets. All the purifying processes
proceed on the principle that camphor is volatile at the temperature of 400° F. The
substance is mixed, as intimately as possible, with 2 per cent. of quicklime, and the
mixture is introduced into a large bottle made of thin uniform glass, sunk in a sand
bath. The fire is slowly raised till the whole vessel becomes heated, and then its upper
part is gradually laid bare in proportion as the sublimation goes on. Much attention
and experience are required to make this operation succeed. If the temperature be
raised too slowly, the neck of the bottle might be filled with camphor before the heat
had acquired the proper subliming pitch; and, if too quickly, the whole contents might
be exploded. If the operation be carried on languidly, and the heat of the upper part
of the bottle be somewhat under the melting point of camphor, that is to say, a little
under 350° F., the condensed camphor would be snowy, and not sufficiently compact
and transparent to be saleable. Occasionally, sudden alternations of temperature cause
little jets to be thrown up out of the liquid camphor at the bottom upon the cake
formed above, which soil it, and render its re-sublimation necessary.
If, to the mixture of 100 parts of crude camphor and 2 of quicklime, 2 parts of
bone-black, in fine powder, be added, the small quantity of colouring matter in the
camphor will be retained at the bottom, and whiter cakes will be produced. A spiral
slip of platina foil immersed in the liquid may tend to equalise its ebullition.
By exposing some volatile oils to spontaneous evaporation, at the heat of about 70° F.,
Proust obtained a residuum of camphor; from oil of lavender, 25 per cent. of its weight;
from oil of sage, 121⁄2; from oil of marjoram, 10.
[246]
Refined camphor is a white translucid solid, possessing a peculiar taste and smell.
It may be obtained, from the slow cooling of its alcoholic solution, in octahedral
crystals. It may be scratched by the nail, is very flexible, and can be reduced into powder
merely by mixing it with a few drops of alcohol. Its specific gravity varies from 0·985
to 0·996. Mixed and distilled with six times its weight of clay, it is decomposed, and
yields a golden yellow aromatic oil, which has a flavour analogous to that of a mixture
of thyme and rosemary; along with a small quantity of acidulous water tinged with
that oil, charcoal remains in the retort. In the air, camphor takes fire on contact of an
ignited body, and burns all away with a bright fuliginous flame.
Camphor is little soluble in water; one part being capable of communicating smell
and taste to 1000 of the fluid. 100 parts of alcohol, spec. grav. 0·806, dissolve 120
parts of camphor, at ordinary temperatures. It is separated, in a pulverulent state, by
water. Ether and oils, both expressed and volatile, also dissolve it.
When distilled with eight parts of aquafortis, camphor is converted into camphoric
acid. Camphor absorbs 144 times its volume of muriatic acid gas, and is transformed
into a colourless transparent liquid, which becomes solid in the air, because the acid
attracts humidity, which precipitates the camphor. One part of strong acetic acid
dissolves two parts of camphor. By my analysis, camphor consists of 77·38 carbon,
11·14 hydrogen, and 11·48 oxygen. Berzelius’s numbers are certainly erroneous.
CAMWOOD. An article imported from Sierra Leone, which seems to possess similar
dyeing powers with Brazil or Nicaragua wood.
CANDLE. (Chandelle, Fr.; Kerze, Licht, Germ.) I shall first briefly describe the
ordinary manufacture of candles. They are either dipped or moulded. But the first
part of the process is the sorting of the tallow. Mutton suet with a proportion of
ox-tallow is selected for mould candles, because it gives them gloss and consistence.
Coarser tallow is reserved for the dipped candles. After being sorted, it is cut into
small pieces, preparatory to being melted or rendered; and the sooner this is done after
the fat is taken from the carcase the better, because the fibrous and fleshy matters mixed
with it promote its putrefaction. Tallow is too commonly melted by a naked fire
applied to the bottom of the vessel, whereas it should be done either in a cold set pan,
where the flame plays only round the sides a little way above the bottom, or in a steam-cased
pan. After being fused a considerable time, the membranous matters collect at
the surface, constituting the cracklings used sometimes for feeding dogs, after the fat
has been squeezed out of it by a press. The liquid tallow is strained through a sieve into
another copper, where it is treated with water at a boiling temperature in order to wash
it. After a while, when the foul water has settled to the bottom, the purified tallow is
lifted out, by means of tinned iron buckets, into tubs of a moderate size, where it concretes,
and is ready for use.
It is a remarkable circumstance, that the wicks for the best candles are still cotton
rovings imported from Turkey, notwithstanding the vast extension and perfection of
cotton-spinning in this country. Four or more of these Turkey skeins, according to the
intended thickness of the wick, are wound off at once into bottoms or clues, and afterwards
cut by a simple machine into lengths corresponding to those of the candles to be
made. Mr. Colebank obtained a patent, in June, 1822, for a machine for cutting, twisting,
and spreading wicks, which, though convenient, does not seem to have come into
general use. The operations are performed upon a series of threads at once. The
apparatus is placed in a box, in front of which the operator sits. A reel extends across
the box, at the hinder part, upon which the cotton threads have been previously wound:
from this reel they are drawn off in proper lengths, doubled, and cut by an ingenious
mechanism. By dipping the wicks into the melted tallow, rubbing them between the palms
of the hands, and allowing the tallow which adheres to harden, they may be arranged
with facility upon the broaches for the purpose of dipping. The dipping room is furnished
with a boiler for melting the tallow, the dipping mould, or cistern, and a large
wheel for supporting the broaches. From the ceiling of the workshop a long balance-shaped
beam is suspended, to one end of which a wooden frame is attached for holding
the broaches with the wicks arranged at proper distances. The opposite arm is loaded
with a weight to counterbalance the wooden frame, and to enable the workman to
ascertain the proper size of the candles. The end of the lever which supports the frame
is placed immediately above the dipping cistern; and the whole machine is so balanced
that, by a gentle pressure of the hand, the wicks are let down into the melted tallow as
often as may be required.
The following convenient apparatus for dipping candles has been long in use at Edinburgh.
In the centre of the dipping-room a strong upright post A A, fig. 253., is erected,
with turning iron pivots at its two ends. Near its middle, six mortises are cut at small
distances from one another, into each of which is inserted a long bar of wood B B, which
moves vertically upon an iron pin, also passing through the middle of the shaft. The
whole presents the appearance of a large horizontal wheel with twelve arms. A complete[247]
view of two of them only is given in the figure. From the extremity of each arm is
suspended a frame, or port, as the workmen call it, containing 6 rods, on each of which
are hung 18 wicks, making the whole
number of wicks upon the wheel 1296.
The machine, though apparently
heavy, turns round by the smallest effort
of the workman; and each port,
as it comes in succession over the dipping-mould,
is gently pressed downwards,
by which means the wicks are
regularly immersed in melted tallow.
As the arms of the lever are all of the
same length, and as each is loaded
with nearly the same weight, it is
obvious that they will all naturally
assume a horizontal position. In
order, however, to prevent any oscillation
of the machine in turning
round, the levers are kept in a horizontal
position by means of small
chains a a, one end of which is fixed
to the top of the upright shaft, and
the other terminates in a small square
piece of wood b, which exactly fills
the notch c in the lever. As one end of the levers must be depressed at each dip,
the square piece of wood is thrown out of the notch by the workman pressing down
the handle D, which communicates with the small lever e, inserted into a groove in
the bar B. In order that the square piece of wood, fixed in one extremity of the chain,
may recover its position upon the workman’s raising the port, a small cord is attached
to it, which passes over a pulley inserted in a groove near c, and communicates with
another pulley and weight, which draws it forward to the notch. In this way the
operation of dipping may be conducted by a single workman with perfect ease and
regularity, and even dispatch. No time is lost, and no unnecessary labour expended, in
removing the ports after each dip; and, besides, the process of cooling is much accelerated
by the candles being kept in constant motion through the air. The number of
revolutions which the wheel must make, in order to complete one operation, must
obviously depend upon the state of the weather and the size of the candles; but it is
said that, in moderately cold weather, not more than two hours are necessary for a single
person to finish one wheel of candles of a common size. Upon the supposition, therefore,
that six wheels are completed in one day, no less a number than 7776 candles will be
manufactured in that space of time by one workman.
I shall next describe the process of moulding, which, if possible, is even less complicated
in its details than that of dipping. The moulds are made of some metallic
substance, usually pewter, and consist of two parts. The shaft or great body of the mould
is a hollow cylinder, finely polished in the inside, and open at both extremities. The top
of the mould is a small metallic cup, having a moulding within-side, and a hole to admit
the wick. The two parts are soldered together, and when united, as will readily be
imagined, have the shape of a moulded candle. A third piece, called the foot, is sometimes
added; it is a kind of small funnel, through which the liquid tallow runs into the
mould, and, being screwed to the opposite extremity of the shaft, is removable at pleasure.
This additional piece may certainly be useful in very mild weather; since, by removing
it, the candles may be drawn more easily from the moulds; but, in general, it may be
dispensed with.
Eight or twelve of these moulds, according to their size, are fixed in a frame, which
bears a great resemblance to a wooden stool, the upper surface of which forms a kind of
trough. The top of the moulds points downwards, and the other extremity, which is
open, is inserted into the bottom trough or top of the stool, and made quite level with its
upper surface. In order to introduce the wicks into the mould, the workman lays the
frame upon its side on an adjoining table, and holding in his left hand a quantity of wicks,
previously cut to the proper length, he introduces into the mould a long wire with a
hooked point. As soon as the hook of the wire appears through the hole in the top of
the mould, he attaches to it the looped end of the wick, and, immediately drawing back
the wire, carries the wick along with it. In this manner each mould in succession is
furnished with a wick. Another workman now follows, and passes a small wire through
the loop of each wick. This wire is obviously intended to keep the wick stretched, and
to prevent it from falling back into the mould upon the frame being placed in the proper
position for filling. The frame is then handed to the person that fills the moulds, who[248]
previously arranges the small wires in such a manner that each wick may be exactly in
the middle of the mould.
The moulds are filled by running tallow into each of them, or into the trough, from a
cistern furnished with a cock, and which is regularly supplied with tallow of the proper
temperature from an adjoining boiler. When the workman observes that the moulds are
nearly half filled he turns the cock, and, laying hold of that portion of the wick which hangs
out of the mould, pulls it tight, and thus prevents any curling of the wick, which might
injure the candles: he then opens the cock, and completes the process of filling. The frame
is now set aside to cool; and when the tallow has acquired a proper consistence, which the
workman easily discovers by a snapping noise emitted by the candles upon pressing his
thumb against the bottom of the moulds, he first withdraws the small wires which kept the
wicks tense, and then, scraping off the loose tallow from the top of the frame with a small
wooden spade, he introduces a bodkin into the loop of the wick, and thus draws each
candle in succession from its mould. The candles are now laid upon a table for the
inspection of the exciseman, and afterwards removed to the storehouse. Previous to
storing them up, some candle-makers bleach their candles, by exposing them to the air
and dews for several days. This additional labour can be necessary only when the dealer
is obliged to have early sales; for if the candles are kept for some months, as they
ought to be, before they are brought to market, they become sufficiently whitened by
age.
Wax candles.—Next to tallow, the substance most employed in the manufacture
of candles is wax. Wax candles are made either by the hand or with a ladle. In the
former case, the wax, being kept soft in hot water, is applied bit by bit to the wick, which
is hung from a hook in the wall; in the latter, the wicks are hung round an iron circle,
placed immediately over a large copper-tinned basin full of melted wax, which is poured
upon their tops, one after another, by means of a large ladle. When the candles have by
either process acquired the proper size, they are taken from the hooks, and rolled upon
a table, usually of walnut-tree, with a long square instrument of box, smooth at the
bottom.
A few years ago I made a set of experiments upon the relative intensities of light, and
duration of different candles, the results of which are contained in the following
table.
Number in
a pound. |
Duration
of a
candle. |
Weight
in
grains. |
Consump-
tion per
hour
in grains. |
Propor-
tion of
light. |
Economy
of light. |
Candles
equal one
Argand. |
| |
h. |
m. |
|
|
|
|
|
| 10 |
mould |
5 |
9 |
|
682 |
132 |
12 |
1⁄4 |
68 |
|
5 |
·7 |
| 10 |
dipped |
4 |
36 |
|
672 |
150 |
13 |
|
65 |
1⁄2 |
5 |
·25 |
| 8 |
mould |
6 |
31 |
|
856 |
132 |
10 |
1⁄2 |
59 |
1⁄2 |
6 |
·6 |
| 6 |
ditto |
7 |
2 |
1⁄2 |
1160 |
163 |
14 |
2⁄3 |
66 |
|
5 |
·0 |
| 4 |
ditto |
9 |
3 |
·6 |
1707 |
186 |
20 |
1⁄4 |
80 |
|
3 |
·5 |
| Argand oil flame |
— |
— |
512 |
69 |
·4 |
100 |
|
|
A Scotch mutchkin, or 1⁄8 of a gallon of good seal oil, weighs 6010 gr., or 131⁄10 oz.,
avoirdupois, and lasts in a bright Argand lamp 11 hours 44 minutes. The weight of
oil it consumes per hour is equal to 4 times the weight of tallow in candles 8 to the
pound, and 1⁄7 the weight of tallow in candles 6 to the pound. But, its light being
equal to that of 5 of the latter candles, it appears from the above table that 2 pounds
weight of oil, value 9d. in an Argand, are equivalent in illuminating power to 3 pounds
of tallow candles, which cost about two shillings. The larger the flame in the above
candles the greater the economy of light.
In June, 1825, M. Gay Lussac obtained a patent in England for making candles from
margaric and stearic acids, improperly called stearine, by converting tallow into the above
fat acids by the following process:—Tallow consists, by Chevreul’s researches, of stearine,
a solid fat, and elaine, a liquid fat; the former being in much the larger proportion.
When tallow is treated with an alkaline body, such as potash, soda, or lime, it is saponified;
that is, its stearine and elaine become respectively stearic and elaic acids, and, as such,
form compounds with these bases. When by the action of an acid, such as the sulphuric
or muriatic, these combinations are decomposed, the fats reappear in the altered form of
stearic and elaic acids; the former body being harder than tallow, and of a texture,
somewhat like spermaceti, the latter body being fluid, like oil. “The decomposition of the
soap should be made,” says the patentee, “in a large quantity of water, kept well stirred
during the operation, and warmed by steam introduced in any convenient way. When
the mixture has been allowed to stand, the acid of the tallow or fat will rise to the
surface, and the water being drawn off will carry the alkaline or saline matters with it;
but, if the acids of the tallow should retain any portion of the salts, fresh water may be[249]
thrown upon it, and the whole well agitated, until the acids have become perfectly free
from the alkaline matters; and, when allowed to cool, the acids will be formed into a
solid mass. This mass is now to be submitted to considerable pressure in such an apparatus
as is employed in expressing oil from seeds; when the liquid acid will run off in the
form of a substance resembling oil, leaving a solid matter, similar, in every respect, to
spermaceti, which is fit for making candles.”
The wick to be used in the manufacture of these improved candles, and which forms
one of the features of this invention, is to be made of cotton yarn, twisted rather hard,
and laid in the same manner as wire is sometimes coiled round the bass strings of
musical instruments. For this purpose, straight rods or wires are to be procured, of
suitable lengths and diameters, according to the intended size of the candles about to be
made; and these wires, having been covered with cotton coiled round them, as described,
are to be inserted in the candle moulds as the common wicks are; and when the candle
is made, and perfectly hard, the wire is to be withdrawn, leaving a hollow cylindrical
aperture entirely through the middle of the candle. See Stearine.
CANE-MILL. See Mill and Sugar.
CANNON. For the composition of these implements of destruction, see Bronze.
CANVASS (Canevas, Fr.; Segeltuch, Germ.) It has been found that sails of ships
made with the selvages and seams of the canvass running down parallel to their edges, are
very apt to bag, and become torn in the middle, from the strain to which they are subjected
by the pressure of the wind. To obviate this inconvenience, a mode of making sails, with
the seams and selvages running diagonally, was proposed by Admiral Brooking, and a
patent granted to him for the same on 4th of September, 1828. The invention of
Messrs. Ramsay and Orr, which we are about to describe, has a similar object, viz., that
of giving additional strength to sails by a peculiar manner of weaving the canvass of which
they are made.
The improvement proposed under their patent of March, 1830, consists in weaving
the canvass with diagonal threads; that is, placing the weft yarn, or shoot, in weaving, at
an oblique angle to the warp yarns, instead of making the decussation of the warp, or
weft threads, or yarns, at right angles to each other, as in the ordinary mode of weaving.
To accomplish this object the loom must be peculiarly constructed; that is, its
warp and work beams must stand at an oblique angle with the sides of the loom, and the
batten and slay must be hung in a peculiar manner, in order to beat up the weft, or
shoot, in lines ranging diagonally with the warp. No drawing is shown of the method
by which this arrangement of the loom is to be made, but it is presumed that any weaver
would know how to accomplish it: the invention consisting solely in producing sail
cloth with the threads, or yarns, of the weft ranging diagonally at any desired angle with
the direction of the warp thread.
CAOUTCHOUC, GUM-ELASTIC, OR INDIAN-RUBBER, (Federharz, Germ.)
occurs as a milky juice in several plants, such as the siphonia cahuca, called also hevea
guianensis, cautschuc, jatropha elastica, castilleja elastica, cecropia pellata, ficus religiosa and
indica, urceolaria elastica, &c. It is, however, extracted chiefly from the first plant, which
grows in South America and Java. The tree has incisions made into it through the bark
in many places, and it discharges the milky juice, which is spread upon clay moulds, and
dried in the sun, or with the smoke of a fire, which blackens it.
The juice itself has been of late years imported. It is of a pale yellow colour, and
has the consistence of cream. It becomes covered in the bottles containing it with a
pellicle of concrete caoutchouc. Its spec. grav. is 1·012. When it is dried it loses 55
per cent. of its weight: the residuary 45 is elastic gum. When the juice is heated it
immediately coagulates, in virtue of its albumen, and the elastic gum rises to the
surface. It mixes with water in any proportion; and, when thus diluted, it coagulates
with heat and alcohol as before.
The specific gravity of caoutchouc is 0·925, and it is not permanently increased by
any degree of pressure. By cold or long quiescence it becomes hard and stiff. When
the milky juice has become once coherent, no means hitherto known can restore it to
the emulsive state. By long boiling in water it softens, swells, and becomes more
readily soluble in its peculiar menstrua; but when exposed to the air it speedily resumes
its pristine consistence and volume. It is quite insoluble in alcohol; but in ether, deprived
of alcohol by washing with water, it readily dissolves, and affords a colourless
solution. When the ether is evaporated, the caoutchouc becomes again solid, but is
somewhat clammy for a while. When treated with hot naphtha, distilled from native
petroleum, or from coal tar, it swells to 30 times its former bulk; and if then triturated
with a pestle, and pressed through a sieve, it affords a homogeneous varnish, which being
applied by a flat edge of metal or wood to cloth, prepares it for forming the patent
water-proof cloth of Mackintosh. Two surfaces of cloth, to which several coats of the
above varnish have been applied, are, when partially dried, brought evenly in contact, and
then passed between rollers, in order to condense and smooth them together. This double[250]
cloth is afterwards suspended in a stove-room to dry, and to discharge the disagreeable
odour of the naphtha.
Caoutchouc dissolves in the fixed oils, such as linseed oil, but the varnish has not the
property of becoming concrete upon exposure to air.
It has been lately asserted that caoutchouc is soluble in the oils of lavender and sassafras.
It melts at 248° F., and stands afterwards a much higher heat without undergoing
any further change. When the melted caoutchouc is exposed to the air, it becomes
hard on the surface in the course of a year. When kindled it burns with a bright flame
and a great deal of smoke.
Neither chlorine, sulphurous acid gas, muriatic acid gas, ammonia, nor fluosilicic acid
gas, affect it, whence it forms very valuable flexible tubes for pneumatic chemistry.
Cold sulphuric acid does not readily decompose it, nor does nitric acid, unless it be somewhat
strong. The strongest caustic potash lye does not dissolve it even at a boiling heat.
Caoutchouc, according to my experiments, which have been confirmed by those of Mr.
Faraday, contains no oxygen, as almost all other solid vegetable products do, but is a mere
compound of carbon and hydrogen, in the proportion, by my results, of 90 carbon to 10
hydrogen, being three atoms of the former to two of the latter. Mr. Faraday obtained
only 87·2 carbon, from which I would infer that some of the carbon, which in this substance
is difficult to acidify by peroxide of copper, had escaped its action. It is obvious
that too little carbonic acid gas may be obtained, but certainly not more than corresponds
to the carbon in the body. No carbon can be created in the process of ultimate
analysis by pure peroxide of copper such as I employed; and I repeated the ignition
after attrition of the mixture used in the experiment. Melted caoutchouc forms a very
excellent chemical lute, as it adheres very readily to glass vessels, and withstands the corrosive
action of acid vapours. This substance is much used for effacing the traces of plumbago
pencils, whence it derived the name of Indian-rubber. It has been lately employed
very extensively for making elastic bands or braces. The caoutchouc bottles are skilfully
cut into long spiral slips, which are stretched, and kept extended till nearly deprived of
their elasticity, and till they form a thread of moderate fineness. This thread is put into a
braid machine, and covered with a sheath of cotton, silk, linen, or worsted. The clothed
caoutchouc is then laid as warp in a loom, and woven into an elegant riband. When
woven, it is exposed, upon a table to the action of a hot smoothing iron, which restoring
to the caoutchouc all its primitive elasticity, the riband retracts considerably in length,
and the braiding corrugates equally upon the caoutchouc cores. Such bands possess a
remarkable elasticity, combined with any desired degree of softness. Sometimes cloth
is made of these braided strands of caoutchouc used both as warp and as weft, which is
therefore elastic in all directions. When a light fabric is required, the strands of caoutchouc,
either naked or braided, are alternated with common warp yarns. For this mixed
fabric a patent has been obtained. The original manufacturer of these elastic webs is
a major in the Austrian service, who has erected a great factory for them at St. Denys,
near Paris. See Elastic Bands.
Mr. William Henry Barnard, in the course of some experiments upon the impregnation
of ropes with caoutchouc, at the factory of Messrs. Enderby at Greenwich, discovered that
when this substance was exposed to
a heat of about 600° F. it resolved
itself into a vapour, which, by
proper refrigeratory methods, was
condensable into a liquid possessing
very remarkable properties, to
which the name caoutchoucine has
been given. For this invention
“of a solvent not hitherto used in
the arts” Mr. Barnard obtained a
patent, in August, 1833. His
process for preparing it is described
in his specification as follows:—I
take a mass of the said caoutchouc,
or Indian rubber, as imported, and
having cut it into small lumps,
containing about two cubic inches
each (which I prefer), I throw these
lumps into a cast-iron still (which
I find adapted for the purpose, and
a diagram of which is annexed to,
and forms part of, this my specification),
with a worm attached; fig. 254., A is the still, B the cover ground to a metallic[251]
fit, to admit of a thermometer to take the temperature; C the fire-place, D the ash-pit,
E the worm-tub and worm, F the brick-work of the still, G a roller and carriage, in conjunction
with a crane, or other means, to raise the cover to take out the residue, and to
charge the same; H the chain.
I then apply heat to the still in the usual manner, which heat is increased until the
thermometer ranges at 600 degrees of Fahrenheit, or thereabouts. And, as the thermometer
ranges progressively upwards to 600 degrees of Fahrenheit, a dark-coloured oil
or liquid is distilled over, which I claim as my said invention, such liquid being a
solvent of caoutchouc, and other resinous and oleaginous substances. When the thermometer
reaches 600 degrees, or thereabouts, nothing is left in the still but dirt and
charcoal.
I have found the operation of distillation to be facilitated by the addition of a portion
of this oil, either previous or subsequent to rectification, as hereinafter mentioned, in the
proportion of one third of oil to two thirds of caoutchouc.
I afterwards subject the dark-coloured liquid thus distilled to the ordinary process
of rectification, and thereby obtain fluids varying in specific gravity, of which the
lightest hitherto has not been under 670, taking distilled water at 1000, which fluids I
also claim as my said invention.
At each rectification the colour of the liquid becomes more bright and transparent,
until at the specific gravity of 680, or thereabouts, it is colourless and highly volatile.
In the process of rectification (for the purpose of obtaining a larger product of the oil
colourless) I put about one third of water into the still. In each and every state the
liquid is a solvent of caoutchouc, and several resinous and oleaginous substances, and
also of other substances (such as copal), in combination with very strong alcohol.
Having experienced much difficulty in removing the dirt which adheres to the bottom
of the still, I throw into the still, lead and tin in a state of alloy (commonly called solder),
to the depth of about half an inch, and, as this becomes fused, the dirt which lies on the
surface of it is more easily removed.
Objections have been made to the smell of this liquid:—I have found such smell removed
by mixing and shaking up the liquid with nitro-muriatic acid, or chlorine, in the
proportion of a quarter of a pint of the acid (of the usual commercial strength) to a
gallon of the liquid.
The discovery of the chemical solvent, which forms the subject of the patent above
described, has excited considerable interest in the philosophic world, not only from its
probable usefulness as a new article of commerce, but also from two very extraordinary
characteristics which it is found to possess, viz., that, in a liquid state, it has less specific
gravity than any other liquid known to chemists, being considerably lighter than
sulphuric ether, and, in a state of vapour, is heavier than the most ponderous of the
gases.
Its elementary constituents are,
| Carbon |
6·812 |
8 proportions. |
| Hydrogen |
1·000 |
7 ditto. |
This new material (when mixed with alcohol) is a solvent of all the resins and particularly
of copal, which it dissolves, without artificial heat, at the ordinary temperature of the atmosphere;
a property possessed by no other solvent known; and hence it is peculiarly useful
for making varnishes in general. It also mixes readily with oils, and will be found to be a
valuable and cheap menstruum for liquefying oil-paints; and without in the slightest
degree affecting the most delicate colours, will, from its ready evaporation, cause the
paint to dry almost instantly.
Cocoa-nut oil, at the common temperature of the atmosphere, always assumes a concrete
form; but a portion of this caoutchoucine mixed with it will cause the oil to become
fluid, and to retain sufficient fluidity to burn in a common lamp with extraordinary
brilliancy.
Caoutchoucine is extremely volatile; and yet its vapour is so exceedingly heavy, that
it may be poured, without the liquor, from one vessel into another like water.
CAPERS. The caper is a small prickly shrub, cultivated in Spain, Italy, and the
southern provinces of France. The flowers are large roses of a pretty appearance, but
the flower buds alone are the objects of this cultivation.
They are plucked before they open, and thrown into strong vinegar slightly salted,
where they are pickled. The crop of each day is added to the same vinegar tub, so
that in the course of the six months during which the caper shrub flowers, the vessel gets
filled, and is sold to persons who sort the capers, (the smallest being most valued) by
means of copper sieves. This metal is attacked by the acid, wherefrom the fruit acquires
a green colour, much admired by ignorant connoisseurs.
The capers, as found in the French market, are distinguished into five sorts; the
non-pareille, the capucine, the capote, the second, and the third; this being the decreasing
order of their quality, which depends upon the strength of the vinegar used in
pickling them, as also the size and colour of the buds.
[252]
The caper shrub grows in the driest situations, even upon walls, and does not disdain
any soil; but it loves a hot and sheltered exposure. It is multiplied by grafts made in
autumn, as also by slips of the roots taken off in spring.
CAPSTAN. (Cabestan, Fr.; Spille, Germ.) A machine whereon the cable is wound
successively in weighing the anchor of a vessel. It is a species of wheel and axle; the
axle being vertical, and pierced with holes near its top for the insertion of the ends of
horizontal levers, called handspikes, which represent the wheel. These are turned by
the force of men moving in a circle. The power applied to the lever is to the resistance
to be overcome, (the weight of the anchor, for example,) when the forces are in
equilibrio, as the radius of the cylinder round which the cable is coiled is to the circumference
described by the power.
It is manifest that the radius of the axle must be augmented in this computation by
half the diameter of the cable, which is supposed to lie always one coil thick upon it.
The force of a man, thus applied, has been commonly estimated as equal to the traction
of 27 pounds hanging over a pulley.
Friction being so variable a quantity in capstans, renders the exact calculation of its
mechanical effect somewhat uncertain.
A stout man, stationed near the bottom of the axle, holds fast the loose part of the
cable, which has already made two or three turns; and, being aided by its friction upon
the wood, he both prevents it from slipping backwards, and uncoils each turn as it is
progressively made.
Mr. Hindmarsh, master mariner of Newcastle, obtained a patent, in February, 1827,
for a contrivance to enable a capstan or windlass to be occasionally worked with increased
mechanical advantage. With this view, he placed toothed wheel-work, partly in the
drum-head of the capstan, and partly in the upper part of the barrel, upon which the
cable is coiled and uncoiled in successive portions.
The drum-head, and also the barrel, turn loosely upon a central spindle, independent
of each other, and are connected together either by the toothed geer, or by bolts. On
raising or withdrawing the connecting pinion from the toothed wheels, and then locking
the drum-head and barrel together, the capstan works with a power equal only to that
exerted by the men at the capstan-bars, as an ordinary capstan; but on lowering the
pinion into geer with the wheel-work, and withdrawing the bolts which locked the
drum-head to the barrel, the power exerted by the men becomes increased in proportion
to the diameter and numbers of teeth in the wheels and pinions.
Fig. 255. is the external appearance of this capstan. Fig. 256. a horizontal view of the
toothed geer at the top of the barrel. The barrel, with the whelps a a, turns loosely upon
a verticle spindle fixed into
the deck of the vessel. The
drum-head b also turns loosely
upon the same spindle. The
circular frame c c, in fig. 256.,
in which the axes of the
toothed wheels d d d are
mounted, is fixed to the central
spindle. The rim e e e,
with internal teeth, is made
fast to the top of the barrel;
and the pinion f, which slides
upon the spindle, is connected
to the drum-head.
When it is intended to work
the capstan with ordinary
power, the pinion f is raised
up into the recess of the drum-head,
by means of a screw g,
fig. 255., which throws it out
of geer with the toothed wheels,
and it is then locked up by a
pin z: the bolts h h are now
introduced, for the purpose of fastening the drum-head and barrel together, when it
becomes an ordinary capstan.
But when it is required that the same number of men shall exert a greater power,
the bolts h are withdrawn, and the pinion f lowered into geer, with the toothed wheels.
The rotation of the drum-head, then carrying the pinion round, causes it to drive the
toothed wheels d d d; and these working into the toothed rim e e, attached to the barrel,
cause the barrel to revolve with an increased power.
Thus, under particular circumstances, a smaller number of men at the capstan or[253]
windlass (which is to be constructed upon the same principle) will be enabled to haul
in the cable and anchor, or warp off the vessel, which is an important object to be
effected.
In 1819, Captain Phillips obtained a patent for certain improvements in capstans, a
part of which invention is precisely the same as this in principle, though slightly varied
in its adaptation.
James Brown, ship-rigger, in his capstan, patented in 1833, instead of applying the
moving power by handspikes, having fixed two rims of teeth round the top of the capstan,
acts upon them by a rotatory worm, or pinions turned by a winch.
Fig. 257. is an elevation of this capstan, and fig. 258. is a horizontal top view. a is an
upright shaft, fixed firmly to the deck, serving as an axle round which the body of the
capstan revolves. A frame c, fixed to the top of a stationary shaft a, above the body of
the capstan, carries the driving apparatus.
The upper part of the body of the capstan has a ring of oblique teeth d formed round
its edge; and above this, on the top of the capstan, is a ring of bevel teeth e. A horizontal
shaft f, mounted in the top frame c, has a worm or endless screw, which takes into
the teeth of the ring d; and a short axle g, having its bearings in the central shaft a,
and in the frame c, carries a bevel pinion, which takes into the bevel teeth of the ring c.
The bearings of the shaft f, in the top frame, are in long slots, with angular returns,
something like the fastening of a bayonet, which is for the purpose of enabling the shaft
to be readily lifted in and out of geer with the teeth of the ring d: the outer bearing of
the axle g of the bevel pinion is also supported in the frame c, in a similar way, in
order to put it in and out of geer with the teeth of the bevel ring e. A mode of shifting
these is essential; because the two toothed rings, and their driving worm and pinion,
give different speeds, and, of course, cannot be both in operation at the same time.
The worm of the shaft f, being placed in geer with the teeth of the ring d, on applying
rotatory power thereto, by means of winches attached to the ends of the shaft, the
barrel or body of the capstan will be made to revolve with a slow motion, but with
great power; and thus two men at the winches will do the same work as many men
with capstan bars in the ordinary way.
If a quicker movement than that of the endless screw is desired, then the driving power
may be applied by a winch to the axle g of the bevel pinion, that pinion being put into
geer with the bevel ring e, and the endless screw withdrawn. It should, however, be
here remarked, that the patentee proposes to employ two short axles g, placed opposite to
each other, with bevel pinions acting in the bevel-toothed ring, though only one is
shown in the figure to avoid confusion. He also contemplates a modification of the
same contrivance, in which four short axles g, placed at right angles, with pinions taking
into a bevel ring, may be employed, and made effective in giving rotatory motion to the
barrel of a capstan by means of winches applied to the outer ends of the axle, and turned
by the labour of four men.
CARAT or CARACT is a weight used by goldsmiths and jewellers. See Assay
and Diamond.
CARBON, (Carbone, Fr.; Kohlenstoff, Germ.) in a perfectly pure state, constitutes
diamond. Carbonaceous substances are usually more or less compound, containing hydrogen,
or sometimes oxygen, and azote, along with earthy and metallic matters. Carbon,
tolerably pure, abounds in the mineral kingdom; and, in a combined state, it forms a
main constituent of vegetable and animal bodies. Anthracite is a mineral charcoal,
differing from common pit-coal in containing no bitumen, and, therefore, burning without
flame or smoke. Coke is the carbonaceous mass which remains after pit-coal has
been exposed to ignition for some time out of contact of air; its volatile parts having
been dissipated by the heat. It is a spongy substance, of an iron-black colour, a somewhat
metallic lustre, and does not easily burn unless several pieces are kindled together.
With a good draught, however, it produces a most intense heat. Wood charcoal is obtained
by the calcination of wood in close vessels, as described under the article Acetic
Acid, or in piles of various shapes, covered with loam, to screen it from the free action
of the atmosphere, which would otherwise consume it entirely. See Charcoal. Such
carbon is a solid, without smell or taste, and bears the strongest heats of our furnaces
without suffering any change, provided air be excluded: it is a bad conductor of heat,
but conducts electricity very well. When burned, it unites with oxygen, and forms carbonic
acid, the fixed air of Dr. Black, the choke-damp of the miner. When this carbonic
acid is made to traverse red hot charcoal it dissolves a portion of it, and becomes
carbonic oxide, which contains only one half of its volume of oxygen; whereas carbonic
acid consists of one volume of oxygen combined with one volume of the vapour
of carbon, the two being condensed into one volume. If the specific gravity of oxygen,
= 1·1025, be deducted from that of carbonic acid, = 1·5245, the difference, = 0·422,
will be the specific gravity of the vapour of carbon; as well as the proportion present in
that weight of the acid.
[254]
Charcoal obtained by the action of a rapid fire in close vessels is not so solid and
so good a fuel as that which is made in the ancient way by the slow calcination of pyramidal
piles covered with earth. One of the most economical ovens for making wood
charcoal is that invented by M. Foucauld, which he calls a shroud, or abri. To construct
one of these, 30 feet in diameter at the base, 10 feet at its summit, and from 8 to 9 feet
high, he forms, with wood 2 inches square, a frame 12 feet long, 3 feet broad at one end,
and one foot at the other. The figure will explain the construction. The uprights,
A B and C D, of this frame are furnished with three wooden handles a a a, and a′ a′ a′,
by means of which they can be joined together, by passing through two contiguous
handles a wooden fork, the frame being previously provided with props, as shewn in
fig. 259, and covered with loam mixed with grass. A flat cover of 10 feet diameter, made
of planks well joined, and secured by four cross bars, is mounted with two trap doors,
M N, fig. 261., for giving egress to the smoke at the commencement of the operation;
a triangular hole P, cut out in the cover, receives the end of a conduit Q R S, (figs. 262.
and 261.) of wood formed of three deals, destined to convey the gases and condensed
liquids into the casks F G H. Lastly, a door T, which may be opened and shut at pleasure,
permits the operator to inspect the state of the fire. The charcoal calcined by this
abri, has been found to be of superior quality.
When it is wished to change the place where the abri is erected, and to transport it to
a store of new-felled timber, the frame is taken down, after beating off the clay which
covers it, the joints are then cut by a saw, as well as the ends of the forks which fixed
the frames to one another. This process is economical in use, simple and cheap in
construction; since all the pieces of the apparatus are easily moved about, and may be
readily mounted in the forests. For obtaining a compact charcoal, for the use of artisans,
this mixed process of Foucauld is said to be preferable to either the close iron cylinder
or the pile.
For making gunpowder-charcoal the lighter woods, such as the willow, dogwood, and
alder answer best; and in their carbonization care should be taken to let the vapours freely
escape, especially towards the end of the operation, for when they are re-absorbed, they
greatly impair the combustibility of the charcoal.
By the common process of the forests, about 18 per cent. of the weight of the wood is
obtained; by the process of Foucauld about 24 per cent. are obtained, with 20 of crude
pyrolignous acid of 10 degrees Baumé. By the process described under Acetic Acid,
27 of charcoal, and 18 of acid at 6 degrees, are procured from 100 parts of wood, besides
the tar. These quantities were the results of careful experimenting, and are greater than
can be reckoned upon in ordinary hands.
Charcoal for chemical purposes may be extemporaneously prepared by calcining pieces
of wood covered with sand in a crucible, till no more volatile matter exhales.
The charcoal of some woods contains silica, and is therefore useful for polishing metals.
Being a bad conductor of heat, charcoal is employed sometimes in powder to encase
small furnaces and steam-pipes. It is not affected by water; and hence, the extremities
of stakes driven into moist ground are not liable to decomposition. In like manner
casks when charred inside preserve water much better than common casks, because they
furnish no soluble matter for fermentation or for food to animalcules.
Lowitz discovered that wood charcoal removes offensive smells from animal and vegetable
substances, and counteracts their putrefaction. He found the odour of succinic[255]
and benzoic acids, of bugs, of empyreumatic oils, of infusions of valerian, essence of
wormwood, spirits distilled from bad grain, and sulphureous substances were all absorbable
by freshly calcined charcoal properly applied. A very ingenious filter has been constructed
for purifying water, by passing it through strata of charcoal of different fineness.
When charcoal is burned, one third of the heat is discharged by radiation, and two
thirds by conduction.
The following table of the quantity of charcoal yielded by different woods was published
by Mr. Mushet, as the result of experiments carefully made upon the small scale.
He says, the woods before being charred were thoroughly dried, and pieces of each kind
were selected as nearly alike in every respect as possible. One hundred parts of each
sort were taken, and they produced as under:—
| Lignum Vitæ |
afforded |
26·0 of charcoal of a greyish colour, resembling coke. |
| Mahogany |
25·4 tinged with brown, spongy and porous. |
| Laburnam |
24·5 velvet black, compact, very hard. |
| Chesnut |
23·2 glossy black, compact, firm. |
| Oak |
22·6 black, close, very firm. |
| Walnut |
20·6 dull black, close, firm. |
| Holly |
19·9 dull black, loose and bulky. |
| Beech |
19·9 dull black, spongy, firm. |
| Sycamore |
19·7 fine black, bulky, moderately firm. |
| Elm |
19·5 fine black, moderately firm. |
| Norway Pine |
19·2 shining black, bulky, very soft. |
| Sallow |
18·4 velvet black, bulky, loose and soft. |
| Ash |
17·9 shining black, spongy, firm. |
| Birch |
17·4 velvet black, bulky, firm. |
| Scottish Pine |
16·4 tinged with brown, moderately firm. |
Messrs. Allen and Pepys, from 100 parts of the following woods, obtained the quantities
of charcoal as under:—
| Beech |
15·00 |
| Mahogany |
15·75 |
| Lignum Vitæ |
17·25 |
| Oak |
17·40 |
| Fir |
18·17 |
| Box |
20·25 |
It is observable that the quantities obtained by Messrs. Allen and Pepys are in general
less than those given by Mr. Mushet, which may be owing to Mr. Mushet not having
applied sufficient heat, or operated long enough, to dissipate the aqueous matter of the
gaseous products.
To those persons who buy charcoal by weight, it is important to purchase it as soon
after it is made as possible, as it quickly absorbs a considerable portion of water from
the atmosphere. Different woods, however, differ in this respect. Messrs. Allen and
Pepys found that by a week’s exposure to the air, the charcoal of
| Lignum Vitæ |
gained |
9·6 |
per cent. |
| Fir |
13·0 |
ditto. |
| Box |
14·0 |
ditto. |
| Beech |
16·3 |
ditto. |
| Oak |
16·5 |
ditto. |
| Mahogany |
18·0 |
ditto. |
The following is a tabular view of the volumes of the different gases which were absorbed
in the course of 24 hours, by one volume of charcoal, in the experiments of
M. Theodore de Saussure, which were conducted in a way likely to produce correct
results. Each portion of charcoal was heated afresh to a red heat, and allowed to cool
under mercury. When taken from the mercury, it was instantly plunged into the
vessel of gas.
| Ammoniacal gas |
90 |
|
| Muriatic acid gas |
85 |
|
| Sulphurous acid |
65 |
|
| Sulphuretted hydrogen |
55 |
|
| Nitrous oxide |
40 |
|
| Carbonic acid gas |
35 |
|
| Bicarburetted hydrogen |
35 |
·00 |
| Carbonic oxide |
9 |
·42 |
| Oxygen gas |
9 |
·25 |
| Nitrogen |
7 |
·50 |
| Carburetted hydrogen |
5 |
·00 |
| Hydrogen gas |
1 |
·75 |
Neumann, who made many experiments on charcoal, informs us that for the reduction
of the metallic oxides, the charcoal of the heavier woods, as that of the oak and the
beech, is preferable, and that, for common fuel, such charcoal gives the greatest heat, and
requires the most plentiful supply of air to keep it burning; while those of the lighter
woods preserve a glowing heat with a much less draught of air; and that for purposes
where it is desirable to have a steady and a still fire, charcoal should be employed which[256]
has been made from wood previously divested of its bark, since it is the cortical part
which crackles and flies off in sparks during combustion, while the coal of the wood itself
seldom does.
For making crayons of charcoal, the willow is the best wood that can be employed,
as the softness is uniform in all its parts. Its durability may be seen in several of our old
churchyards, where the letters made with lamp-black are still perfect, though the white
lead with which the body of the stones was painted is entirely destroyed.
This property of carbon is shewn, however, in a more striking manner by the writings
that were found in the ruins of Herculaneum, which have retained their original blackness
for two thousand years. The ancients wrote with ink made from ground charcoal.
If it be required to purify any carbonaceous matter, to render it fitter for delicate
pigments, this may be done by first calcining it in a close vessel, and then lixiviating it
in water slightly acidulated by nitric acid.
The incorruptibility of charcoal was well known to the ancients, and they availed
themselves of this property upon all important occasions.
About sixty years ago a quantity of oak stakes were found in the bed of the Thames,
in the very spot where Tacitus says that the Britons fixed a vast number of such stakes,
to prevent the passage of Julius Cæsar and his army. These stakes were charred to a
considerable depth, had retained their form completely, and were firm at the heart.
Most of the houses in Venice stand upon piles of wood, which have all been previously
charred for their preservation. In this country, estates were formerly marked out by
charred stakes driven to a considerable depth into the ground. See Bone-black,
Charcoal, and Graphite.
CARBONATED WATER, is water either pure, or holding various saline matters
in solution, impregnated with carbonic acid gas. For general sale in this country, the
water usually contains a little soda, which being charged with the gas, is called Soda
water; see this article for a description of an excellent machine for the manufacture of this
fashionable beverage.
CARBONATES. Saline compounds in definite proportions, of carbonic acid, with
alkalis, earths, and the ordinary metallic oxides.
The carbonates principally used in the arts and manufactures are those of ammonia,
copper, iron, lead, lime, magnesia, potash, soda. Native carbonate of copper is the beautiful
green mineral called Malachite.
Carbonates are easily analyzed by estimating either by weight or measure the quantity
of carbonic acid which they evolve under the decomposing action of somewhat dilute
sulphuric, nitric, or muriatic acid; for as they are all compounds of acid, and base in
equivalent proportions, the quantity of acid will indicate the quantity of base. Thus,
as pure limestone consists of 56 of lime and 44 of acid, in 100 parts, if upon examining a
sample of limestone we find it to give out only 22 per cent. of carbonic acid gas, during
its slow solution in muriatic acid, we are sure that there are only 28 parts of lime present.
I have described, in the Annals of Philosophy, for October, 1817, a simple form
of apparatus for analyzing the carbonates with equal readiness and precision. The
simple rule by measure to which I was led, may be thus stated: From the bulk of evolved
gas, expressed in cubic inches and tenths, deduct 1⁄20, the remainder will express the proportion
of real limestone present in the grains employed. Pure magnesian limestone yields
very nearly a cubic inch of the gas for every grain in weight.
CARBONATE OF AMMONIA. A salt called in modern chemistry sesquicarbonate,
to denote its being composed of one and a half equivalent primes of carbonic
acid, and one of ammonia. It consists by my analysis of 55·89 carbonic acid, 28·86
ammonia, and 15·25 water, in 100 parts. It is generally prepared by mixing from 11⁄4 to
11⁄2 parts of well-washed dry chalk, with 1 of sal-ammoniac, introducing the mixture into
an earthen or cast-iron retort, or subliming pot, and exposing it to a heat gradually
raised to redness. By double decomposition, the ammonia is volatilized in combination
with the carbonic acid of the chalk, and the vapours are received in a condensing
receiver made either of glass, stone ware, or lead. The chlorine of the sal-ammoniac
remains in the retort, associated with the basis of the chalk in the state of chloride of
calcium. Some ammonia gas escapes during the process.
The saline mass thus sublimed is purified by a second sublimation in glass, or salt-glazed
earthen vessels. The salt may be obtained, by the above method carefully conducted,
in rhomboidal octahedrons, but it is generally made for the market in a compact
semi-crystalline white cake. It has a pungent ammoniacal smell, a hot, pungent, alkaline
taste, a strong alkaline reaction, and dissolves in two parts of cold water. It must
be kept in well-closed vessels, as by exposure to the air a portion of its ammonia exhales,
and it passes into the state of the scentless bi-carbonate. It is employed much in medicine,
chemical analysis, and by the pastry-cooks to give sponginess to their cakes in
consequence of its volatilization from their dough in the oven. See Sal-Ammoniac.
For the other carbonates used in the arts, see their respective bases; copper, lead,
lime, &c.
[257]
CARBONIC ACID (Acide carbonique, Fr.; Kohlensäure, Germ.), consists of
1 prime equivalent of carbon = 6·125 + 2 of oxygen = 16·026, whose joint sum =
22·151, represents the atomic weight or combining ratio of this acid, in the neutral or
protocarbonate salts. Its composition by volume is stated under Carbon. Its natural
form is a gas, whose specific gravity is 1·5245, compared to atmospheric air 1·000; and
being so dense, it may be poured out of one vessel into another. Hence it was called
at first aërial acid. From its existing copiously, in a solid state, in limestones and the
mild alkalies, it was styled fixed air by its proper discoverer, Dr. Black. About one
volume of it exists in 1000 volumes of common atmospheric air, which may be made
manifest by the crust of carbonate it occasions upon the surface of lime water. Carbonic
acid gas is found accumulated in many caverns of volcanic districts, and particularly
in the grotto dei cani at Pausilippo, near Puzzuoli; being disengaged in such
circumstances by the action of subterranean fire, and, possibly, of certain acids, upon
the limestone strata. It often issues from fountains in copious currents, as at Franzensbrunn,
near Eger, in Polterbrunnen; near Trier; and Byrreshorn. This acid gas
occurs also frequently in mines and wells, being called choke damp, from its suffocating
quality. Its presence may, at all times, be detected, by letting down a lighted candle,
suspended from a string, into the places suspected of containing this mephitic air. It
exists, in considerable quantities, in the water of every pump well, and gives it a fresh
and pleasant taste. Water, exposed some time to the air, loses these aerial particles, and
becomes vapid. Many springs are highly impregnated with carbonic acid gas, and
form a sparkling beverage; such as the Selterswasser, from Selters upon the Lahn, in
the grand duchy of Nassau; of which no less than two millions and a half of bottles
are sold every year. A prodigious quantity of a similar water is also artificially
prepared in Great Britain, and many other countries, under the name of aërated or
soda water.
Carbonic acid occurs in nature, combined with many salifiable bases; as in the
carbonates of soda, baryta, strontia, magnesia; the oxides of iron, manganese, zinc,
copper, lead, &c. From these substances it may be separated, generally speaking, by
strong ignition, or, more readily, by the superior affinity of muriatic, sulphuric, or
nitric acid, for the earth or metallic oxide. It is formed whenever vegetable or animal
substances are burned with free access of air, from the union of their carbonaceous
principle with atmospheric oxygen. It is also formed in all cases of the spontaneous
decomposition of organic substances, particularly in the process of fermentation; and
constitutes the pungent, noxious, heavy gas thrown off, in vast volumes, from beer vats.
See Distillation and Fermentation. Carbonic acid is also generated in the breathing
of animals; from 4 to 5 per cent., in volume, of the inhaled oxygen being converted,
at each expiration, into this gas, which contaminates the air of crowded apartments,
and renders ventilation essential to health, and even to life: witness the horrible
catastrophe of the Black-hole at Calcutta.
Carbonic acid gas is destitute of colour, has a sourish, suffocating smell, an acidulous
pungent taste, imparts to moist, but not dry, litmus paper, a transient reddish tint, and
weighs per 100 cubic inches, 461⁄2 grains; and per cubic foot, 8031⁄2 grains; a little more
than 33⁄4 oz. avoirdupoid. A cubic foot of air weighs about two thirds of that quantity,
or 527 grains. It may be condensed into the liquid state by a pressure of 40 atmospheres,
and this liquid may be then solidified by its own sudden spontaneous evaporation.
If air contain more than 15 per cent. in bulk of this gas, it becomes unfit
for respiration and combustion, animal life and candles being speedily extinguished
by it.
Before a person ventures into a deep well, or vault containing fermenting materials,
he should introduce a lighted candle into the space, and observe how it burns. Carbonic
acid, being so much denser than common air, may be drawn out of cellars or
fermenting tubs, by a pump furnished with a leather hose, which reaches to the bottom.
Quicklime, mixed with water, may be used also to purify the air of a sunk apartment
by its affinity for, or power of, absorbing this aërial acid. See Mineral Waters and
Soda Water.
CARBONIC OXIDE. See the article Carbon.
CARBUNCLE. A gem highly prized by the ancients; most probably a variety of
the noble garnet of modern mineralogists.
CARBURET OF SULPHUR, called also sulphuret of carbon, and alcohol of
sulphur, is a limpid volatile liquid, possessing a penetrating fetid smell, and an acrid
burning taste. Its specific gravity is 1·265; and its boiling point is about 112° Fahr. It
evaporates so readily, and absorbs so much heat in the vaporous state, that if a tube
containing quicksilver, surrounded with lint dipped in this liquid, be suspended in the
receiver of an air-pump, on making the vacuum, the quicksilver will be congealed. It
consists of 15·8 carbon and 84·2 sulphur, in 100 parts; being two equivalent primes of
the latter to one of the former.
[258]
CARBURETTED HYDROGEN. A compound of carbon and hydrogen, of
which there are several species—such as oil-gas, coal-gas, olefiant gas, oil of lemons,
otto of roses, oil of turpentine, petroleum, naphta, naphthaline, oil of wine, caoutchoucine
and caoutchouc.
CARDS, PLAYING. (Cartes à jouer, Fr.; Karten, Germ.) Mr. de la Rue obtained, in
February, 1832, a patent for certain improvements in the manufacture of playing cards,
which he distributed under three heads: first, printing the pips, and also the picture or
court-cards, in oil colours by means of types or blocks; secondly, effecting the same in
oil colours by means of lithography; and thirdly, gilding or silvering borders, and other
parts of the characters, by the printing process, either by types, blocks, or lithography.
In the ordinary mode of manufacturing playing cards, their devices are partly produced
by copperplate printing, and they are filled up with water colours by the means called
stencilling.
The patentee does not propose any material alteration in the devices or forms upon
the cards, but only to produce them with oil colours; and, to effect this, he follows
precisely the same mode as that practised by calico printers.
A set of blocks or types properly devised, are produced for printing the different pips
of hearts, diamonds, spades, and clubs, or they are drawn, as other subjects, in the usual
way upon stone. The ink or colour, whether black or red, is to be prepared from the
best French lamp-black, or the best Chinese vermillion ground in oil, and laid on the
types and blocks, or on the stone, in the same way as printers’ ink, and the impressions
taken-on to thick drawing paper by means of a suitable press in the ordinary manner of
printing.
The picture or court-cards are to be produced by a series of impressions in different
colours, fitting into each other exactly in the same way as in printing paper hangings,
or silks and calicoes, observing that all the colours are to be prepared with oil.
For this purpose a series of blocks or types are to be provided for each subject, and
which, when put together, will form the whole device. These blocks are to be used
separately, that is, all the yellow parts of the picture, for instance, are to be printed at
one impression, then all the red parts, next all the flesh colour, then the blue portions,
and so on, finishing with the black outlines, which complete the picture.
If the same is to be done by lithography, there must be as many stones as there are
to be colours, each to print its portion only; and the impression, or part of the picture
given by one stone, must be exactly fitted into by the impression given from the next
stone, and so on until the whole subject is complete.
A superior kind of card is proposed to be made, with gold or silver devices in parts
of the pictures, or gold or silver borders round the pips. This is to be effected by
printing the lines which are to appear as gold or silver, with gilders’ size, in place of
ink or colour; and immediately after the impression has been given, the face of the card
is to be powdered over with gold dust, silver, or bronze, by means of a soft cotton or
wool dabber, by which the gold, silver, or bronze will be made to adhere to the picture,
and the superfluous portions of the metal will wipe off by a very slight rubbing. When
the prints are perfectly dry, the face of the card may be polished by means of a soft
brush.
If it should be desirable to make these improved cards to resemble ivory, that may be
done by preparing the face of the paper in the first instance with a composition of size and
fine French white, and a drying oil, mixed together to about the consistence of cream;
this is to be washed over the paper, and dried before printing, and when the cards are
finished, they will exactly resemble ivory.
The only thing remaining to be described, is the means by which the successive impressions
of the types, blocks, or stones forming the parts of the pictures, are to be
brought exactly to join each other, so as to form a perfect whole design when complete;
this is by printers called registering, and is to be effected much in the usual way, by
points in the tympan of the press, or by marks upon the stones.
The parts of the subject having been all accurately cut or drawn to fit, small holes are
to be made with a fine awl through a quire or more of the paper at once, by placing
upon the paper a gauge-plate, having marks or guide-holes, and by observing these, the
same sheet laid on several times, and always made to correspond with the points or
marks, the several parts of the picture must inevitably register, and produce a perfect
subject.
CARD CUTTING. Mr. Dickinson’s patent machine for cutting cards, consists of
a pair of rollers with circular revolving cutters, the edges of which are intended to act
against each other as circular shears, and the pasteboards in passing between these
rollers are cut by the circular shears into cards of the desired dimensions. These rollers
are mounted in suitable standards, with proper adjustments, and are made to revolve by[259]
a band and pulley connected to the axle of a crank, or by any other convenient
means.
Fig. 263. is a front view of
this machine; a a and b b
are the two rollers, the
upper one turning upon
an extended axle, bearing
in the standards, the lower
one upon pivots. These
rollers are formed by a
series of circular blocks,
between a series of circular
steel cutters, which
are slidden on to iron
shafts, and held together
upon their axle by nuts
screwed up at their ends.
The accurate adjustment
of the cutters is of the
first importance to their
correct performance; it is
therefore found necessary
to introduce spiral springs
within the blocks, in order
to press the cutters up to
their proper bearings. A section of one of the blocks is shewn at fig. 265, and an end
view of the same at fig. 266, with the spiral springs inserted.
At the outer extremity of the axle of the roller a, a rigger c, is attached, whence
a band passes to a pulley d, on the crank shaft e, to which a flywheel f, is affixed, for the
purpose of rendering the action uniform. Rotatory motion being given to the crank
shaft, the upper roller is turned, the lower roller moving at the same time by the friction
against the edges of the cutters.
Fig. 264 is an end view of the rollers, showing the manner in which the pasteboards
are guided and conducted between the cutters. In the front of the machine a movable
frame g, is to be placed, for the purpose of receiving the pasteboards, preparatory to
cutting them into cards, and a stop is screwed to this frame for the edge of the pasteboard
to bear against, which stop is adjustable to suit different sizes. From the back
part of this frame an arm h, extends, the extremity of which acts against the periphery
of a ratchet wheel i, fixed at the end of the roller b, and hence, as the roller goes round,
the frame is made to rise and fall upon its pivots, for the purpose of guiding the pasteboard
up to the cutters; at the same time a rod k, hanging in arms from the sides of the
standards (shewn by dots in fig. 263), falling upon the pasteboard, confines it, while the
cutters take hold, and racks, corresponding with the indentations of the rollers, are
placed as at l l, by means of which the cards, when cut, are pushed out of the grooves.
As various widths of cards will require to be cut by this machine, the patentee proposes
to have several pairs of rollers ready adjusted to act together, when mounted in
the standards, in preference to shifting the circular cutters, and introducing blocks of
greater or less width.
The second part of the invention is a machine for pasting the papers, and pressing
the sheets together to make pasteboard. This machine consists of several reels (we
suppose rollers are intended) on which the paper is to be wound, along with a paste
trough, and rotatory brushes. The several parts of this machine, and their operations
in making pasteboard, are described in the specification, but the patentee having
omitted the letters of reference in the drawing which he has enrolled, it becomes
difficult to explain it.
As far as we are enabled to understand the machine, it appears, that damped paper is
to be wound upon two rollers, and conducted from thence over two other rollers; that
two fluted rollers revolving in the paste trough are to supply paste to two circular brushes,
and that by those brushes the papers are to be pasted upon one side, and then pressed
together, to make the pasteboard; after this, the pasteboard is to be drawn on to a
table, and to remain there until sufficiently dry to be wound upon other rollers. By
comparing this description with the figure, perhaps the intended operations of the
machine may be discovered, it is the best explanation we are enabled to give.
CARDS, (Cardes, Fr.; Karden, Ger.) are instruments which serve to disentangle
the fibres of wool, cotton, or other analogous bodies, to arrange them in an orderly lap
or fleece, and thereby prepare them for being spun into uniform threads. The fineness
and the levelness of the yarn, as well as the beauty of the cloth into which it enters,[260]
depend as much upon the regularity and perfection of the carding, as upon any subsequent
operations of the factory. The quality of the carding depends more upon that of
the cards than upon any attention or skill in the operative; since it is now nearly an
automatic process, conducted by young women called card-tenters.
Cards are formed of a sheet or fillet of leather pierced with a multitude of small holes,
in which are implanted small staples of wire with bent projecting ends called teeth.
Thus every piece of wire is double toothed. The leather is afterwards applied to a flat
or cylindrical surface of wood or metal, and the co-operation of two or more such surfaces
constitutes a card. The teeth of cards are made thicker or slenderer, according as
the filaments to be carded are coarser or finer, stiffer or more pliant, more valuable or
cheaper. It is obviously of great importance that the teeth should be all alike, equably
distributed, and equally inclined over the surface of the leather, a degree of precision
which is scarcely possible with handwork. To judge of the difficulty of this manipulation
we need only inspect the annexed figures. The wire must first be bent at right
angles in c and d, fig. 268, then each branch must receive a second bend in a and b at a
determinate obtuse angle, invariable for each system of cards. It is indispensable that
the two angles c a e and d b f be mathematically equal, not only as to the twin teeth of one
staple, but through the whole series; for it is easy to see that if one of the teeth be more
or less sloped than its fellow, it will lay hold of more or less wool than it, and render
the carding irregular. But though the perfect regularity of the teeth be important, it
is not the sole condition towards making a good card. It must be always kept in view
that these teeth are to be implanted by pairs in a piece of leather, and kept in it by the
cross part c d. The leather must therefore be pierced with twin holes at the distance
c d; and pierced in such a manner, that the slope of the holes, in reference to the
plane of the leather, be invariably the same; for otherwise the length of the teeth would
vary with this angle of inclination, and the card would be irregular.
A third condition essential towards producing perfect regularity, is that the leather
ought to be of the same thickness throughout its whole surface, otherwise the teeth,
though of the same length and fixed at the same angle, would be rendered unequal by
the different thicknesses of the leather, and the operation of carding would be in consequence
extremely defective. Fig. 267. shows the card-teeth acting against each other, as
indicated by the arrows in two opposite directions; in fig. 269. they work one way.
Of late years very complex but complete and well-acting machines have been constructed
for splitting the leather or equalizing it by shaving, for bending and cutting the
wires, and implanting them in the leather, into holes pierced with perfect regularity.
Card machines which fashion the teeth with great precision and rapidity, and pierce the
leather, have been for a considerable time in use at Halifax, in Yorkshire, a town famous
for the excellence of its card-cloth, as also at Leeds, Glasgow, and several other places.
The wires and the leather thus prepared are given out by the manufacturer to women
and children, who put them together.
1. The simplest machine for equalizing the leather which can be employed, is that
which I saw operating in MM. Scrive’s automatic card factory at Lille, the most magnificent
I believe in the world, where the leather was drawn forwards by a roller over a
solid horizontal table, or bed, and passed under a nicely adjusted vertical blade, which
shaved it by a scraping motion to a perfectly uniform thickness. About one half the
weight of the leather is lost in this process, and in the subsequent squaring and trimming.
The machine for making cards, invented I believe by a Mr. Ellis of the United States,
for which a first patent was obtained in this country by Joseph Cheeseborough Dyer, Esq.
of Manchester, in 1811, and a second and third with further improvements in 1814,
and 1824, is one of the most elegant automatons ever applied to productive industry.
It is however necessarily so complicated with different mechanisms as to render its
representation impracticable in such engravings as are compatible with the scope of
this dictionary. I must therefore content myself with the following general description
of its constituent parts.
The first thing to be done after having, as above, prepared the long sheets or fillets of
leather, of suitable length, breadth, and thickness, for making the cards, is to stretch the
leather, and hold it firmly; which is accomplished by winding the fillet of leather upon
the roller or drum, like the warp roller of a loom, and then conducting it upwards between
guide rollers, to a receiving or work roller at top of the machine, where the
fillet is held fast by a cramp, by which means the leather is kept stretched.
Secondly, the holes are pierced in the leather to receive the wire staples or teeth of the[261]
card, by means of a sliding fork, the points of which are presented to the face of the leather;
while the fork is made to advance and recede continually, by the agency of levers
worked by rotatory cams upon a revolving main shaft.
The points of the fork being thus made to penetrate into the leather, the holes for receiving
the staples are pierced, at regular distances, and in correct order, by shifting the
leather fillet so as to bring different parts of its surface opposite to the points of the sliding
fork. This is done by cams, or indented wheels and gear, which shift the guide rollers
and confining drums laterally, as they revolve, and consequently move the fillet of leather
at intervals a short distance, so as to present to the points of the fork or piercer at every
movement, a different part of the surface of the leather.
Thirdly, the wire of which the teeth or points of the card are to be made, is supplied
from a coil on the side of the machine, and is brought forward at intervals, by a pair
of sliding pincers, which are slidden to and fro through the agency of levers actuated
by rotatory cams upon the main shaft. The pincers having advanced a distance equal to
the length of wire intended to form one staple, or two points, this length of wire is
pressed upon exactly in the middle by a square piece of steel, and being there confined,
a cutter is brought forward, which cuts it off from that part of the wire held in the pincers.
The length of wire thus separated and confined, is now, by a movement of the machine,
bent up along the sides of the square steel holder, and shaped to three edges of the square,
that is, formed as a staple; and in the same way, by the continued movements of the
machine, a succession of pieces of wire are cut off, and bent into staples for making the
teeth of the card as long as the mechanism is kept in action.
Fourthly, the wire staple thus formed is held with its points or ends outwards, closely
contiguous to the forked piercer described above, and by another movement of the mechanism,
the staple is protruded forward, its end entering into the two holes made
previously in the leather by the sliding of the fork.
While the wire staple is being thus introduced into the leather, its legs or points are
to be bent, that is, formed with a knee or angle, which is the fifth object to be effected.
This is done by means of a small apparatus consisting of a bar or bed, which bears up
against the under side of the wire staple when it has been passed half-way into the
holes in the leather, and another bar above it, which being brought down behind the
staple, bends it over the resisting bar to the angle required; that is, forms the knee in each
leg. A pusher now acts behind the staple, and drives it home into the leather, which
completes the operation.
The leather being thus conducted, and its position shifted before the piercer progressively,
a succession of the above described operations of cutting the wire, forming the
staple, passing it into the leather, and bending its legs to the angular form, produces
a sheet of card of the kind usually employed for carding or combing wool, cotton, and
other fibrous materials. It may be necessary to add, that as these wire staples are required
to be set in the leathers sometimes in lines crossing the sheet, which is called
ribbed, and at other times in oblique lines, called twilled, these variations are produced
by the positions of the notches or steps upon the edge or periphery of the cam or
indented wheel, which shifts the guide rollers that hold the fillet or sheet of leather as
already described.
CARMINE, (Eng. and Fr.; Karminstoff, Ger.), is, according to Pelletier and Caventou,
a triple compound of the colouring substance, and an animal matter contained in
cochineal, combined with an acid added to effect the precipitation. The preparation of this
article is still a mystery, because upon the one hand, its consumption being very limited,
few persons are engaged in its manufacture, and upon the other, the raw material being
costly, extensive experiments on it cannot be conveniently made. Success in this business
is said to depend not a little upon dexterity of manipulation, and upon knowing
the instant for arresting the further action of heat upon the materials.
There is sold at the shops different kinds of carmine, distinguished by numbers, and
possessed of a corresponding value. This difference depends upon two causes, either
upon the proportion of alumina added in the precipitation, or of a certain quantity of
vermillion put in to dilute the colour. In the first case the shade is paler, in the second,
it has not the same lustre. It is always easy to discover the proportion of the adulteration.
By availing ourselves of the property of pure carmine to dissolve in water of ammonia,
the whole foreign matter remains untouched, and we may estimate its amount by drying
the residuum.
To make Ordinary Carmine.
Take 1 pound of cochineal in powder;
Take 3 drachms and a half of carbonate of potash;
Take 8 drachms of alum in powder;
Take 3 drachms and a half of fish-glue.
The cochineal must be boiled along with the potash in a copper containing five pailfuls
of water (60 pints); the ebullition being allayed with cold water. After boiling a[262]
few minutes the copper must be taken from the fire, and placed on a table at such an
angle as that the liquor may be conveniently transvased. The pounded alum is then
thrown in, and the decoction is stirred; it changes colour immediately, and inclines to a
more brilliant tint. At the end of fifteen minutes the cochineal is deposited at the
bottom, and the bath becomes as clear as if it had been filtered. It contains the colouring
matter, and probably a little alum in suspension. We decant it then into a copper of
equal capacity, and place it over the fire, adding the fish-glue dissolved in a great deal of
water, and passed through a searce. At the moment of ebullition, the carmine is perceived
to rise up to the surface of the bath, and a coagulum is formed, like what takes
place in clarifications with white of egg. The copper must be immediately taken from
the fire, and its contents be stirred with a spatula. In the course of fifteen or twenty
minutes the carmine is deposited. The supernatant liquor is decanted, and the deposit
must be drained upon a filter of fine canvas or linen. If the operation has been well
conducted, the carmine when dry crushes readily under the fingers. What remains after
the precipitation of the carmine is still much loaded with colour, and may be employed
very advantageously for carminated lakes. See Lake.
By the old German process carmine is prepared by means of alum without any other
addition. As soon as the water boils the powdered cochineal is thrown into it, stirred
well, and then boiled for six minutes; a little ground alum is added, and the boiling is
continued for three minutes more; the vessel is removed from the fire, the liquor is filtered
and left for three days in porcelain vessels, in the course of which time a red matter falls
down, which must be separated and dried in the shade. This is carmine, which is sometimes
previously purified by washing. The liquor after three days more lets fall an
inferior kind of carmine, but the residuary colouring matter may also be separated by
the muriate of tin.
The proportions for the above process are 580 parts of clear river water, 16 parts of
cochineal, and 1 part of alum; there is obtained from 11⁄2 to 2 parts of carmine.
Another carmine with tartar.—To the boiling water the cochineal is added, and after
some time a little cream of tartar; in eight minutes more we add a little alum, and continue
the boiling for a minute or two longer. Then take it from the fire and pour it
into glass or porcelain vessels, filter and let it repose quietly till the carmine falls down.
We then decant and dry in the shade. The proportions are 8 pounds of water, 8 oz.
of cochineal, 1⁄2 oz. of cream of tartar, 3⁄4 oz. of alum, and the product is an ounce of
carmine.
The process of Alxon or Langlois.—Boil two pails and a half of river water (30 pints),
throw into it, a little afterwards, a pound of cochineal, add a filtered solution of six
drachms of carbonate of soda and a pound of water, and let the mixture boil for half an
hour; remove the copper from the fire, and let it cool, inclining it to one side. Add
six drachms of pulverized alum, stir with a brush to quicken the solution of the salt, and
let the whole rest 20 minutes. The liquor, which has a fine scarlet colour, is to be carefully
decanted into another vessel, and there is to be put into it the whites of two eggs
well beat up with half a pound of water. Stir again with a brush. The copper is replaced
on the fire, the alumina becomes concrete, and carries down the colouring matter
with it. The copper is to be taken from the fire, and left at rest for 25 or 30 minutes to
allow the carmine to fall down. When the supernatant liquor is drawn off, the
deposit is placed upon filter cloth stretched upon a frame to drain. When the carmine has
the consistence of cream cheese, it is taken from the filter with a silver or ivory knife
and set to dry upon plates covered with paper, to screen it from dust. A pound of
cochineal gives in this way an ounce and a half of carmine.
Process of Madame Cenette of Amsterdam, with salt of sorrel.—Into six pails of river
water boiling hot throw two pounds of the finest cochineal in powder, continue the
ebullition for two hours and then add 3 oz. of refined saltpetre, and after a few minutes
4 oz. of salt of sorrel. In ten minutes more take the copper from the fire and let it
settle for four hours; then draw off the liquor with a syphon into flat plates and leave it
there for three weeks. Afterwards there is formed upon the surface a pretty thick mouldiness,
which is to be removed dexterously in one pellicle by a slip of whalebone. Should
the film tear and fragments of it fall down, they must be removed with the utmost care.
Decant the supernatant water with a syphon, the end of which may touch the bottom of
the vessel, because the layer of carmine is very firm. Whatever water remains must be
sucked away by a pipette. The carmine is dried in the shade, and has an extraordinary
lustre.
Carmine by the salt of tin, or the Carmine of China.—Boil the cochineal in river water,
adding some Roman alum, then pass through a fine cloth to remove the cochineal, and
set the liquor aside. It becomes brighter on keeping. After having heated this liquor,
pour into it drop by drop solution of tin till the carmine be precipitated. The proportions
are one pailful of water, 20 oz. of cochineal and 60 grains of alum, with a solution
of tin containing 4 oz. of the metal.
[263]
To revive or brighten carmine.—We may brighten ordinary carmine, and obtain a very
fine and clear pigment, by dissolving it in water of ammonia. For this purpose we leave
ammonia upon carmine in the heat of the sun, till all its colour be extracted, and the
liquor has got a fine red tinge. It must be then drawn off and precipitated, by acetic
acid and alcohol, next washed with alcohol, and dried. Carmine dissolved in ammonia
has been long employed by painters, under the name of liquid carmine.
Carmine is the finest red colour which the painter possesses. It is principally
employed in miniature painting, water colours, and to tint artificial flowers, because it is
more transparent than the other colours. For Carminium, see Cochineal.
CARPET. (Tapis, Fr.; Teppich, Germ.) A thick woollen fabric of variegated
colours, for covering the floors of the better sort of apartments. This luxurious manufacture
took its origin in Persia and Turkey, whence the most beautiful patterns were wont
to come into Europe; but they have been for some time surpassed by the workmanship
of France, Great Britain, and Belgium. To form a just conception of the elegant
and ingenious processes by which carpets are made, we should visit the royal establishment
of the Gobelins at Paris, where we would see the celebrated carpet manufactory
of the Savonnerie, which has been transported thither. A detailed set of engravings
of this art is given by Roland de la Platière in the first and second volumes of the
Encyclopédie Méthodique, to which I must refer my readers, as a due exposition of its
machines and operations would far exceed the scope of the present volume.
The warp, says M. Roland, being the foundation of the fabric, ought to be of fine
wool, equally but firmly spun, and consist of three yarns twisted into one thread. The
yarns that are to form the velvety surface of the carpet, ought also to be of the best
quality, but soft and downy in their texture, so that the dye may penetrate every
filament. Hemp, or linen yarns, are likewise employed in this manufacture, as a woof,
to bind the warp firmly together after each shoot of the velvety threads. Thus we see
that good carpeting consists essentially of two distinct webs woven at the same time,
and firmly decussated together by the woof threads. Hence the form of the pattern is
the same upon the two sides of the cloth, only the colours are reversed, so that what was
green upon one side becomes red or black upon the other, and vice versâ. The smaller
the figures the more frequent the decussations of the two planes, and the firmer and more
durable the fabric.
The carpet manufacture, as now generally practised, may be distributed into two
systems—that of double fabrics, and that cut in imitation of velvet. Of late years the
Jacquard loom has been much used in weaving carpets, the nature of which will be
found fully explained under that title.
For the sake of illustration, if we suppose the double carpets to be composed of only
two colours, the principle of weaving will be easily understood; for it is only necessary
to raise the warp of each web alternately for the passage of the shuttle, the upper web
being entirely above when the under web is being woven, or decussated, and vice versâ.
In a Brussels carpet the worsted yarn raised to form the pile, and make the figure, is
not cut; in the Wilton the pile is cut to give it a velvety aspect and softness. In the
imperial Brussels carpet the figure is raised above the ground, and its pile is cut,
but the ground is uncut; and in the royal Wilton, the pile is both raised higher
than in the common Wilton, and it is cut, whereby it has a rich cushion-like
appearance. The cloth of all these superior carpets consists of woollen and linen,
or hemp; the latter being put upon a beam, and brought, of course, through heddles
and a reed; but as its only purpose is to bind together the worsted fabric, it should
not be visible upon the upper face of the carpet. The worsted yarn is wound upon
small bobbins or pirns, with a weight affixed to each, for giving proper tension to
the threads. Their number varies, for one web, from 1300 to 1800, according as the
carpet is to be 27 or 36 inches wide; and, they are placed, in frames, behind the loom,
filled with differently coloured yarn, to correspond with the figure. This worsted warp
is then drawn through the harness, heddles, and reed, to be associated with the linen
yarn in the compound fabric.
In Kidderminster carpeting, both warp and weft appear upon the face of the cloth,
whereas, in the Brussels style, only the warp is seen, its binding weft being fine hempen
or linen threads. The three-ply imperial carpet, called the Scotch, is coming very
much into vogue, and is reckoned by many to be little inferior in texture, look, and
wear to the Brussels. Kilmarnock has acquired merited distinction by this ingenious
industry. In this fabric, as well as in the two-ply Kidderminster, the weft predominates,
and displays the design; but, in the French carpets, the worsted warp of the web shows
the figure. Plain Venetian carpets, as used for stairs and passages, are woven in simple
looms, provided merely with the common heddles and reed. The warp should be a
substance of worsted yarn, so heavy as to cover in the weft completely from the view.
Figured Venetian carpets are woven in the two-ply Kidderminster looms, and are[264]
provided with a mechanism to raise the pattern upon the worsted warp. The weft is
an alternate shoot of worsted and linen yarn, and must be concealed.
The following figure and description will explain the construction of the three-ply
imperial Scotch and two-ply Kidderminster carpet loom, which is merely a modification
of the Jacquard métier. The Brussels carpet-loom, on the contrary, is a draw-boy loom
on the damask plan, and requires the
weaver to have an assistant. Fig. 270.
A A A, is the frame of the loom, consisting
of four upright posts, with caps
and cross rails to bind them together.
The posts are about six feet high. C C,
the cloth-beam, is a wooden cylinder,
six inches or thereby in diameter, of
sufficient length to traverse the loom,
with iron gudgeons in the two ends,
which work in bushes in the side frame.
On one end of this beam is a ratchet
wheel, with a tooth to keep it from
turning round backwards by the tension
of the web. D, the lay, with its reed,
its under and upper shell, its two lateral
rulers or swords, and rocking-tree above.
There are grooves in the upper and
under shell, into which the reed is fitted.
E, the heddles, or harness, with a double
neck attached to each of the tower or
card mechanisms F F, of the Jacquard
loom. The heddles are connected and
work with the treddles B B, by means of cords, as shown in the figure. G G are wooden
boxes for the cards. H, the yarn, or warp beam.
In draw-looms of every kind, there is no sinking of any portion of the warp, as in
plain cloth-weaving; but the plane of the warp is placed low, and the threads under
which the shuttle is to pass are raised, while all the rest remains stationary. The
harness part of this carpet loom is moved by an assistant boy or girl, who thus allows
the weft to be properly decussated, while the weaver attends to working the front
mounting or heddles. Fig. 271., A represents
the frame of a carpet draw-loom;
B is a box or frame of pulleys, over
which the cords of the harness pass, and
are then made fast to a piece of wood,
seen at E, which the weavers call a table.
From the tail of the harness the simples
descend, and to the end of each is attached
a small handle G, called a bob.
These handles being disposed in pairs,
and their regularity preserved by means
of a perforated board C, it is merely
necessary to pull every handle in succession;
the weaver, at the same time,
working his treddles with his feet, as
in any other loom. The treddles are
four in number, the fabric being that of plain or alternate cloth, and two treddles
allotted for each web. The harness part of the carpet draw-loom is furnished
with mails, or metallic eyes, to save friction; two threads being drawn through
each eye. The design or pattern of a carpet is drawn upon cross-rule paper, exactly
in the same way as every other kind of fancy-loom work, and is transferred
from the paper to the mounting by the rules for damask weaving. Suppose that
a double web is so mounted that every alternate thread of the one may be raised, so as
to form a sufficient shed-way for the shuttle, without depressing the other in the least.
Then suppose another web placed above the former, at such a distance that it will
exactly touch the convexity of those threads of the former, which are raised. Then, if
the threads of the latter web are sunk while the others are raised, the two would be
entirely incorporated. But if this be only partially done, that is, at particular places,
only those parts immediately operated upon will be affected by the action of the apparatus.
If the carpet is a two-coloured pattern, as black and red, and if upon the upper
surface, as extended in the loom, red flowers are to be represented upon a black ground,
then all those species of design paper which are coloured may be supposed to represent[265]
the red, and those which are vacant the black. Then counting the spaces upon the
paper, omit those which are vacant, and cord those which are coloured, and the effect
will be produced. But as the two webs are to be raised alternately, whatever is corded
for the first handle must be passed by for the second, and vice versâ; so that the one will
form the flower, and the other the ground.
The board by which the simples are regulated appears at F. D shows the weights.
CARTHAMUS, or safflower (carthamus tinctorius), (Carthame, Fr.; Färber distel,
Germ.), the flower of which alone is used in dyeing, is an annual plant cultivated in
Spain, Egypt, and the Levant. There are two varieties of it—one which has large
leaves, and the other smaller ones. It is the last which is cultivated in Egypt, where it
forms a considerable article of commerce.
Carthamus contains two colouring matters, one yellow and the other red. The first
alone is soluble in water; its solution is always turbid: with re-agents it exhibits the
characters usually remarked in yellow colouring matters. The acids render it lighter,
the alkalies deepen it, giving it more of an orange hue: both produce a small dun precipitate,
in consequence of which it becomes clearer. Alum forms a precipitate of a deep
yellow, in small quantity. The solution of tin and the other metallic solutions cause
precipitates which have nothing remarkable in them.
The yellow matter of carthamus is not employed; but in order to extract this portion,
the carthamus is put into a bag, which is trodden under water, till no more colour can
be pressed out. The flowers, which were yellow, become reddish, and lose in this operation
nearly one half of their weight. In this state they are used.
For extracting the red part of carthamus, and thereafter applying it to stuff, the
property which alkalies possess of dissolving it is had recourse to, and it is afterwards
precipitated by an acid.
The process of dyeing consists, therefore, in extracting the colouring matter by means
of an alkali, and precipitating it on the stuff by means of an acid. It is this fecula which
serves for making the rouge employed by ladies.
As to this rouge, the solution of carthamus is prepared with crystallised carbonate of
soda, and it is precipitated by lemon juice. It has been remarked that lemons, beginning
to spoil, were fitter for this operation than those which were less ripe, whose juice retained
much mucilage. After squeezing out the lemon juice, it is left to settle for some
days. The precipitate of carthamus is dried at a gentle heat upon plates of stone-ware;
from which it is detached and very carefully ground with talc, which has been reduced
to a very subtile powder, by means of the leaves of shave-grass (presle), and successively
passed through sieves of increasing fineness. It is the fineness of the talc, and the greater
or less proportion which it bears to the carthamus precipitate, which constitute the difference
between the high and low priced rouges.
Carthamus is used for dyeing silk, poppy, nacarat (a bright orange-red), cherry, rose
colour, and flesh colour. The process differs according to the intensity of the colour, and
the greater or less tendency to flame colour that is wanted. But the carthamus bath,
whose application may be varied, is prepared as follows:
The carthamus, from which the yellow matter has been extracted, and whose lumps
have been broken down, is put into a trough. It is repeatedly sprinkled with cendres
gravelées (crude pearl ashes), or soda (barilla) well powdered and sifted at the rate of
6 pounds for 120 lbs. of carthamus; but soda is preferred, mixing carefully as the alkali
is introduced. This operation is called amestrer. The amestred carthamus is put into
a small trough with a grated bottom, first lining this trough with a closely woven cloth.
When it is about half filled, it is placed over the large trough, and cold water is poured
into the upper one, till the lower becomes full. The carthamus is then set over another
trough, till the water comes from it almost colourless. A little more alkali is now
mixed with it, and fresh water is passed through it. These operations are repeated
till the carthamus be exhausted, when it turns yellow.
After distributing the silk in hanks upon the rods, lemon juice, brought in casks from
Provence, is poured into the bath till it becomes of a fine cherry colour; this is called
turning the bath (virer le bain). It is well stirred, and the silk is immersed and turned
round the skein-sticks in the bath, as long as it is perceived to take up the colour. For
ponceau (poppy colour), it is withdrawn, the liquor is run out of it upon the peg, and it
is turned through a new bath, where it is treated as in the first. After this it is dried and
passed through fresh baths, continuing to wash and dry it between each operation, till it
has acquired the depth of colour that is desired. When it has reached the proper point,
a brightening is given it by turning it round the sticks seven or eight times in a bath of
hot water, to which about half a pint of lemon juice for each pailful of water has been
added.
When silk is to be dyed ponceau or flame colour, it must be previously boiled as for
white; it must then receive a slight foundation of annotto, as explained in treating
of this substance. The silk should not be alumed.
[266]
The nacarats, and the deep cherry colours, are given precisely like the ponceaux, only
they receive no annotto ground; and baths may be employed which have served for the
ponceau, so as to complete their exhaustion. Fresh baths are not made for the latter
colours, unless there be no occasion for the poppy.
With regard to the lighter cherry-reds, rose colour of all shades and flesh colours, they are
made with the second and last runnings of the carthamus, which are weaker. The deepest
shades are passed through first.
The lightest of all these shades, which is an extremely delicate flesh colour, requires
a little soap to be put into the bath. This soap lightens the colour, and prevents it from
taking too speedily, and becoming unevenly. The silk is then washed, and a little
brightening is given it, in a bath which has served for the deeper colours.
All these baths are employed the moment they are made, or as speedily as possible,
because they lose much of their colour upon keeping, by which they are even entirely
destroyed at the end of a certain time. They are, moreover, used cold, to prevent the
colour from being injured. It must have been remarked in the experiments just described,
that the caustic alkalies attack the extremely delicate colour of carthamus, making
it pass to yellow. This is the reason why crystals of soda are preferred to the other alkaline
matters.
In order to diminish the expense of the carthamus, it is the practice in preparing the
deeper shades to mingle with the first and the second bath about one fifth of the
bath of archil.
Dobereiner regards the red colouring matter of carthamus as an acid, and the yellow
as a base. His carthamic acid forms, with the alkalies, colourless salts, decomposed by the
tartaric and acetic acids, which precipitate the acid of a bright rose-red. Heat has a remarkable
influence upon carthamus, rendering its red colour yellow and dull. Hence, the
colder the water is by which it is extracted, the finer is the colour. Light destroys
the colour very rapidly, and hitherto no means have been found of counteracting this
effect. For this reason this brilliant colour must be dried in the shade, its dye must be
given in a shady place, and the silk stuffs dyed with it must be preserved as much as
possible from the light. Age is nearly as injurious as light, especially upon the dye
in a damp state. The colour is very dear, because a thousand parts of carthamus contain
only five of it.
In preparing the finest rouge, the yellow colouring matter being separated by washing
with water, the red is then dissolved by the aid of alkali, and is thrown down on linen
or cotton rags by saturating the solution with vegetable acid. The colour is rinsed out
of these rags, dissolved anew in alkalis, and once more precipitated by lemon juice. The
best and freshest carthamus must be selected. It is put into linen bags, which are placed
in a stream of water, and kneaded till the water runs off colourless. The bags are then
put into water soured with a little vinegar, kneaded till the colour is all expelled, and
finally rinsed in running water. By this treatment the carthamus loses nearly half its
weight. 6633 cwts. of safflower were imported into the United Kingdom in 1835, of
which 2930 cwts. were retained for internal consumption.
CASE-HARDENING, is the name of the process by which iron tools, keys, &c.,
have their surfaces converted into steel.
Steel when very hard is brittle, and iron alone is for many purposes, as for fine keys,
far too soft. It is therefore an important desideratum to combine the hardness of a
steely surface with the toughness of an iron body. These requisites are united by the
process of case-hardening, which does not differ from the making of steel, except in the
shorter duration of the process. Tools, utensils, or ornaments, intended to be polished,
are first manufactured in iron and nearly finished, after which they are put into an iron
box, together with vegetable or animal charcoal in powder, and cemented for a certain
time. This treatment converts the external part into a coating of steel, which is usually
very thin, because the time allowed for the cementation is much shorter than when the
whole substance is intended to be converted. Immersion of the heated pieces into water
hardens the surface, which is afterwards polished by the usual methods. Moxon in his
Mechanic Exercises, p. 56., gives the following receipt for case-hardening:—“Cow’s horn
or hoof is to be baked or thoroughly dried and pulverised. To this add an equal
quantity of bay salt; mix them with stale chamber-lye or white wine vinegar: cover
the iron with this mixture, and bed it with the same in loam, or enclose it in an iron box:
lay it on the hearth of the forge to dry and harden: then put it into the fire, and blow
till the lump have a blood-red heat, and no higher, lest the mixture be burnt too much.
Take the iron out, and immerse it in water to harden.” I consider the vinegar to be
quite superfluous.
I shall now describe the recent application of prussiate (ferrocyanate) of potash to this
purpose. The piece of iron, after being polished, is to be made brightly red-hot, and
then rubbed or sprinkled over with the above salt in fine powder, upon the part intended
to be hardened. The prussiate being decomposed, and apparently dissipated, the iron is[267]
to be quenched in cold water. If the process has been well managed, the surface of the
metal will have become so hard as to resist the file. Others propose to smear over the
surface of the iron with loam made into a thin paste with a strong solution of the prussiate,
to dry it slowly, then expose the whole to a nearly white heat, and finally
plunge the iron into cold water, when the heat has fallen to dull redness. See
Steel.
CASHMERE or CACHEMERE, a peculiar textile fabric first imported from the
kingdom of Cashmere, and now well imitated in France and Great Britain. The
material of the Cashmere shawls is the downy wool found about the roots of the hair of
the Thibet goat. The year 1819 is remarkable in the history of French husbandry for
the acquisition of this breed of goats, imported from the East under the auspices of their
government, by the indefatigable courage and zeal of M. Jaubert, who encountered every
fatigue and danger to enrich his country with these valuable animals, aided by the
patriotism of M. Ternaux, who first planned this importation, and furnished funds for
executing it at his own expence and responsibility. He placed a portion of the flock
brought by M. Jaubert, at his villa of Saint Ouen, near Paris, where the climate seemed
to be very favourable to them, since for several successive years after their introduction
M. Ternaux was enabled to sell a great number of both male and female goats. The
quantity of fine fleece or down afforded by each animal annually, is from a pound and a
half to two pounds.
The wool imported into Europe comes by the way of Casan, the capital of a government
of the Russian empire upon the eastern bank of the Wolga; it has naturally a
grayish colour, but is easily bleached. Its price a few years back at Paris was 17 francs
per kilogramme; that is, about 6 shillings the pound avoirdupois. The waste in picking,
carding, and spinning, amounts to about one third of its weight.
The mills for spinning Cachemere wool have multiplied very much of late years in
France, as appears from the premiums distributed at the exposition of 1834, and the
prices of the yarn have fallen from 25 to 30 per cent. notwithstanding their improved
fineness and quality. There is a fabric made with a mixture of Cachemere down and
spun silk, which is becoming very general. One of the manufacturers, M. Hindenlang,
exhibited samples of Cachemere cloth woven with yarn so fine as No. 130 for warp, and
No. 228 for weft.
Messrs. Pollino, brothers, of Paris, produced an assortment of Cachemere pieces from 22
to 100 francs the yard, dyed of every fancy shade. Their establishment at Ferté-Bernard
occupies 700 operatives, with an hydraulic wheel of 60 horse power.
The oriental Cashmere shawls are woven by processes extremely slow and consequently
costly; whence their prices are very high. They are still sold in Paris at from 4,000 to
10,000 francs a piece; and from 100 to 400 pounds sterling in London. It became
necessary therefore either to rest satisfied with work which should have merely a surface
appearance, or contrive economical methods of weaving, to produce the real Cachemere
style with much less labour. By the aid of the draw-loom and still better of the Jacquard
loom, M. Ternaux first succeeded in weaving Cachemere shawls perfectly similar to the
oriental in external aspect, which became fashionable under the name of French Cachemere.
But to construct shawls altogether identical on both sides with the eastern, was a
more difficult task, which was accomplished only at a later period by M. Bauson of
Paris.
In both modes of manufacture, the piece is mounted by reading-in the warp for the
different leaves of the heddles, as is commonly practised for warps in the Jacquard looms.
The weaving of imitation shawls is executed, as usual, by as many shuttles as there are
colours in the design, and which are thrown across the warp in the order established by
the reader. The greater number of these weft yarns being introduced only at intervals
into the web, when the composition of the pattern requires it, they remain floating loose
at the back of the piece, and are cut afterwards, without affecting in the least the quality
of the texture; but there is a considerable waste of stuff in the weaving, which is worked
up into carpets.
The weaving of the imitation of real Cachemere shawls is different from the above.
The yarns intended to form the weft are not only equal in number to that of the colours
of the pattern to be imitated, but besides this, as many little shuttles or pirns (like those
used by embroiderers) are filled with these yarns, as there are to be colours repeated in
the breadth of the piece; which renders their number considerable when the pattern is
somewhat complicated and loaded with colours. Each of these small bobbins or shuttles
passes through only that portion of the flower in which the colour of its yarn is to appear,
and stops at the one side and the other of the cloth exactly at its limit; it then returns
upon itself after having crossed the thread of the adjoining shuttle. From this reciprocal
intertexture of all the yarns of the shuttles, it results, that although the weft is
composed of a great many different threads, they no less constitute a continuous line in
the whole breadth of the web, upon which the lay or batten acts in the ordinary way[268]
We see therefore that the whole art of manufacturing this Cachemere cloth consists in
avoiding the confusion of the shuttles, and in not striking up the lay till all have fulfilled
their function. The labour does not exceed the strength of a woman, even though
she has to direct the loom and work the treddles. Seated on her bench at the end
opposite to the middle of the beam, she has for aids in weaving shawls from 45 to 52
inches wide, two girl apprentices, whom she directs and instructs in their tasks. About
four hundred days of work are required for a Cachemere shawl of that breadth. For
the construction of the loom, see Jacquard.
In the oriental process all the figures in relief are made simply with a slender pirn
without the shuttle used in European weaving. By the Indians the flower and its
ground are made with the pirn, by means of an intertwisting, which renders them in some
measure independent of the warp. In the Lyons imitation of this style, the leaves of
the heddles lift the yarns of the warp, the needles embroider as in lappett weaving, and
the flower is united to the warp by the weft thrown across the piece. Thus a great deal
of labour is saved, the eye is pleased with an illusion of the loom, and the shawls cost
little more than those made by the common fly shuttle.
Considered in reference to their materials, the French shawls present three distinct
classes, which characterise the three fabrics of Paris, Lyons, and Nimes.
Paris manufactures the French Cachemere, properly so called, of which both the warp
and the weft are the yarn of pure Cachemere down. This web represents with fidelity
the figures and the shades of colour of the Indian shawl, which it copies; the deception
would be complete if the reverse of the piece did not show the cut ends. The Hindoo
shawl, also woven at Paris, has its warp in spun silk, which reduces its price without
impairing its beauty much.
Lyons however has made the greatest progress in the manufacture of shawls. It excels
particularly in the texture of its Thibet shawls, the weft of which is yarn spun with a
mixture of wool and spun silk.
Nimes is remarkable for the low price of its shawls, in which spun silk, Thibet down,
and cotton, are all worked up together.
The value of shawls exported from France in the following years was:—
| |
1831. |
1832. |
1833. |
| |
Francs. |
Francs. |
Francs. |
| Woollen |
1,863,147 |
2,070,926 |
4,319,601 |
| Cachemere down |
433,410 |
655,200 |
609,900 |
| Spun silk |
401,856 |
351,152 |
408,824 |
It appears that M. J. Girard at Sèvres, near Paris, has succeeded best in producing
Cachemere shawls equal in stuff and style of work to the oriental, and at a lower price.
They have this advantage over the Indian shawls, that they are woven without seams, in
a single piece, and exhibit all the variety and the raised effect of the eastern colours.
Women and children alone are employed in his factory.
CASK, (Tonneau, Fr.; Fass, Germ.) manufacture of by mechanical power.
Mr. Samuel Brown obtained a patent in Nov., 1825, for certain improvements in
machinery for making casks, which seems to be ingenious and worthy of record. His
mechanism consists in the first place of a circular saw attached to a bench, with a sliding
rest, upon which rest each piece of wood intended to form a stave of a cask is fixed;
and the rest being then slidden forward in a curved direction, by the assistance of an
adjustable guide, brings the piece of wood against the edge of the rotatory saw, and causes
it to be cut into the curved shape required for the edge of the stave. The second feature
is an apparatus with cutters attached to a standard, and traversing round with their
carrier upon a centre, by means of which the upper and lower edges of the cask are cut
round and grooved, called chining, for the purpose of receiving the heads. Thirdly, an
apparatus not very dissimilar to the last, by which the straight pieces of wood designed
for the heads of the cask are held together, and cut to the circular figure required, and
also the bevelled edges produced. And fourthly, a machine in which the cask is made
to revolve upon an axis, and a cutting tool to traverse for the purpose of shaving the
external part of the cask, and bringing it to a smooth surface.
The pieces of wood intended to form the staves of the cask, having been cut to their
required length and breadth, are placed upon the slide-rest of the first mentioned machine,
and confined by cramps; and the guide, which is a flexible bar, having been previously
bent to the intended curve of the stave and fixed in that form, the rest is then slidden
forward upon the bench by the hand of the workman, which as it advances (moving in a
curved direction) brings the piece of wood against the edge of the revolving circular
saw, by which it is cut to the curved shape desired.
The guide is a long bar held by a series of movable blocks fitted to the bench by[269]
screws, and is bent to any desired curve by shifting the screws: the edge of the slide-rests
which holds the piece of wood about to be cut, runs against the long guide bar, and of
consequence is conducted in a corresponding curved course. The circular saw receives
a rapid rotatory motion by means of a band or rigger from any first mover; and the piece
of wood may be shifted laterally by means of racks and pinions on the side-rest, by the
workman turning a handle, which is occasionally necessary in order to bring the piece
of wood up to, or away from, the saw.
The necessary number of staves being provided, they are then set round within a
confining hoop at bottom, and brought into the form of a cask in the usual way, and
braced by temporary hoops. The barrel part of the cask being thus prepared, in order
to effect the chining, it is placed in a frame upon a platform, which is raised up by a
treddle lever, that the end of the barrel may meet the cutters in a sort of lathe above: the
cutters are then made to traverse round within the head of the barrel, and, as they proceed,
occasionally to expand, by which means the bevels and grooves are cut on the
upper edge of the barrel, which is called chining. The barrel being now reversed, the
same apparatus is brought to act against the other end, which becomes chined in like
manner.
The pieces of wood intended to form the heads of the cask are now to be cut straight
by a circular saw in a machine, similar to the first described; but in the present instance
the slide-rest is to move forward in a straight course. After their straight edges are
thus produced, they are to be placed side by side, and confined, when a scribing cutter
is made to traverse round, and cut the pieces collectively into the circular form desired
for heading the cask.
The cask having now been made up, and headed by hand as usual, it is placed between
centres, or upon an axle in a machine, and turned round by a rigger or band with a
shaving cutter, sliding along a bar above it, which cutter being made to advance, and
recede as it slides along, shaves the outer part of the cask to a smooth surface.
CASSAVA. Cassava bread, conaque, &c., are different names given to the starch
of the root of the Manioc (Jatropha Manihot, Linn.), prepared in the following manner
in the West Indies, the tropical regions of America, and upon the African coast. The
tree belongs to the natural family of the euphorbiaceæ.
The roots are washed, and reduced to a pulp by means of a rasp or grater. The pulp
is put into coarse strong canvas bags, and thus submitted to the action of a powerful
press, by which it parts with most of its noxious juice (used by the Indians for poisoning
the barbs of their arrows). As the active principle of this juice is volatile, it is easily
dissipated by baking the squeezed cakes of pulp upon a plate of hot iron. Fifty pounds
of the fresh juice, when distilled, afford, at first, three ounces of a poisonous water, possessing
an intolerably offensive smell; of which, 35 drops being administered to a slave
convicted of the crime of poisoning, caused his death in the course of six minutes, amid
horrible convulsions.[16]
The pulp dried in the manner above described concretes into lumps, which become
hard and friable as they cool. They are then broken into pieces, and laid out in the sun
to dry. In this state they afford a wholesome nutriment, and are habitually used as such
by the negroes, as also by many white people. These cakes constitute the only provisions
laid in by the natives, in their voyages upon the Amazons. Boiled in water with
a little beef or mutton they form a kind of soup similar to that of rice.
The Cassava cakes sent to Europe (which I have eaten with pleasure) are composed
almost entirely of starch, along with a few fibres of the ligneous matter. It may be
purified by diffusion through warm water, passing the milky mixture through a linen
cloth, evaporating the strained liquid over the fire, with constant agitation. The starch
dissolved by the heat, thickens as the water evaporates, but on being stirred, it becomes
granulated, and must be finally dried in a proper stove. Its specific gravity is 1·530—that
of the other species of starch.
The product obtained by this treatment is known in commerce under the name of tapioca;
and being starch very nearly pure, is often prescribed by physicians as an aliment
of easy digestion. A tolerably good imitation of it is made by heating, stirring, and
drying potato starch in a similar way.
The expressed juice of the root of manioc contains in suspension a very fine fecula, which
it deposits slowly upon the bottom of the vessels. When freed by decantation from the supernatant
liquor, washed several times and dried, it forms a beautiful starch, which
creaks on pressure with the fingers. It is called cipipa, in French Guyana; it is
employed for many delicate articles of cookery, especially pastry, as also for hair powder,
starching linen, &c.
Cassava flour, as imported, may be distinguished from arrow-root and other kinds[270]
of starch, by the appearance of its particles viewed in a microscope. They are
spherical, all about 1-1000th of an inch in diameter, and associated in groups; those of
potato starch are irregular ellipsoids, varying in size from 1-300th to 1-3000th of an
inch; those of arrow-root have the same shape nearly, but vary in size from 1-500th to
1-800th of an inch; those of wheat are separate spheres 1-1000th of an inch.
CASSIS, the black currant (ribes nigra, Linn.), which was formerly celebrated
for its medicinal properties with very little reason.
The only technical use to which it is now applied is in preparing the agreeable liqueur
called ratafia, by the following French recipe:—Stone, and crush three pounds of black
currants, adding to the magma one drachm of cloves, two of cinnamon, four quarts of spirit
of wine, at 18° Baumé (see Aréomètre of Baumé), and 21⁄2 pounds of sugar. Put the
mixture into a bottle which is to be well corked; let it digest for a fortnight, shaking
the bottle once daily during the first eight days; then strain through a linen cloth, and
finally pass through filtering paper.
CASTING OF METALS. (See Founding.) Casts from elastic moulds.—Being
much engaged in taking casts from anatomical preparations, Mr. Douglas Fox, Surgeon,
Derby, found great difficulty, principally with hard bodies, which, when undercut, or
having considerable overlaps, did not admit of the removal of moulds of the ordinary
kind, except with injury. These difficulties suggested to him the use of elastic moulds,
which, giving way as they were withdrawn from complicated parts, would return to
their proper shape; and he ultimately succeeded in making such moulds of glue, which
not only relieved him from all his difficulties, but were attended with great advantages,
in consequence of the small number of pieces into which it was necessary to divide
the mould.
The body to be moulded, previously oiled, must be secured one inch above the surface
of a board, and then surrounded by a wall of clay, about an inch distant from its sides.
The clay must also extend rather higher than the contained body: into this, warm melted
glue, as thick as possible so that it will run, is to be poured, so as to completely cover
the body to be moulded; the glue is to remain till cold, when it will have set into an
elastic mass, just such as is required.
Having removed the clay, the glue is to be cut into as many pieces as may be necessary
for its removal, either by a sharp-pointed knife, or by having placed threads in
the requisite situations of the body to be moulded, which may be drawn away when the
glue is set, so as to cut it out in any direction.
The portions of the glue mould having been removed from the original, are to be placed
together and bound round by tape.
In some instances it is well to run small wooden pegs through the portions of glue, so
as to keep them exactly in their proper positions. If the mould be of considerable size, it is
better to let it be bound with moderate tightness upon a board to prevent it bending
whilst in use; having done as above described, the plaster of Paris, as in common casting,
is to be poured into the mould, and left to set.
In many instances wax may also be cast in glue, if it is not poured in whilst too hot;
as the wax cools so rapidly when applied to the cold glue, that the sharpness of the impression
is not injured.
Glue has been described as succeeding well where an elastic mould is alone applicable;
but many modifications are admissible. When the moulds are not used soon
after being made, treacle should be previously mixed with the glue (as employed by
printers) to prevent it becoming hard.
The description thus given is with reference to moulding those bodies which cannot
be so done by any other than an elastic mould; but glue moulds will be found greatly
to facilitate casting in many departments, as a mould may be frequently taken by this
method in two or three pieces, which would, on any other principle, require many.
CASTOR. (Eng. and Fr.; Biber, Germ.) The castor is an amphibious quadruped,
inhabiting North America; also found in small numbers in the islands of the
Rhone. In the arts, the skin of this animal is employed either as a fur or as affording
the silky hair called beaver, with which the best hats are covered. Beaver skins, which
form a very considerable article of trade, are divided into 3 sorts: 1. The fresh beaver
skins from castors, killed in winter before shedding their hair; these are most in request
among the furriers, as being the most beautiful. 2. The dry or lean beavers are
the skins of the animals killed during the moulting season; they are not much esteemed,
as the skin is rather bare. 3. The fat castors: these are the skins of the first sort, which
have been worn for some time upon the persons of the savages and have got imbued with
their sweat. The last are principally used in the hat manufacture. In France, the
marine otter has been for many years substituted in the place of the castor or
beaver.
CASTOR or CASTOREUM. This name is given to a secretion of the castors,[271]
contained in pear-shaped cellular organic sacs, placed near the genital organs of both the
male and female animals. It is a substance analogous to civet and musk, of a consistence
similar to thick honey. It has a bitter acrid taste; a powerful, penetrating, fetid, and very
volatile smell; but, when dried, it becomes inodorous. Several chemists, and in particular
Bouillon Lagrange, Laugier, and Hildebrandt have examined castor; and found
it to be composed of a resin, a fatty substance, a volatile oil, an extractive matter, benzoic
acid, and some salts.
The mode of preparing it is very simple. The sacs are cut off from the castors when they
are killed, and are dried to prevent the skin being affected by the weather. In this state,
the interior substance is solid, of a dark colour, and a faint smell; it softens with heat,
and becomes brittle by cold. Its fracture betrays fragments of membranes, indicating its
organic structure. When chewed, it adheres to the teeth somewhat like wax; it has a
bitter, slightly acrid, and nauseous taste.
The castor bags, as imported, are often joined in pairs by a kind of ligature. Sometimes
the substance which constitutes their value is sophisticated; a portion of the castoreum
being extracted, and replaced by lead, clay, gums, or some other foreign matters.
This fraud may be easily detected, even when it exists in a small degree, by the absence
of the membranous partitions in the interior of the bags, as well as by the altered smell
and taste.
The use of castoreum in medicine is considerable, especially in nervous and spasmodic
diseases, and it is often advantageously combined with opium.
CASTORINE. A chemical principle lately discovered to the amount of a few parts
per cent. in Castoreum.
CASTOR OIL. The expressed oil of the seeds of the Palma Christi, or Ricinus
communis, a native tree of the West Indies and South America; but which has been cultivated
in France, Italy, and Spain. Bussy and Lecanu discovered in it 3 species of
fatty matters, obtained partly by saponification, and partly by dry distillation—the margaritic,
ricinic, and elaiodic acids. None of these has been separately applied to any
use in the arts.
The quantity of castor oil imported in 1835 into the United Kingdom, was
1,109,307 libs.; retained for home consumption, 670,205 libs. See Oils.
CATECHU, absurdly called Terra Japonica, is an extract made from the wood of
the tree mimosa catechu, which grows in Bombay, Bengal, and other parts of India. It
is prepared by boiling the chips of the interior of the trunk in water, evaporating the
solution to the consistence of syrup over the fire, and then exposing it in the sun to
harden. It occurs in flat rough cakes, and under two forms. The first, or the Bombay,
is of uniform texture, of a dark red colour, and of specific gravity 1·39. The second
is more friable and less solid. It has a chocolate colour, and is marked inside with red
streaks. Its specific gravity is 1·28.
According to Sir H. Davy, these two species are composed as follows:—
| |
Bombay. |
Bengal. |
| Tannin |
54 |
·5 |
48 |
·5 |
| Extractive |
34 |
·0 |
36 |
·5 |
| Mucilage |
6 |
·5 |
8 |
|
| Insoluble matters, sand and lime |
5 |
|
7 |
|
| |
100 |
·0 |
100 |
·0 |
Areka nuts are also found to yield catechu; for which purpose they are cut into
pieces watered in an earthen pot with solution of nitre, and have a little of the bark of
a species of mimosa added to them. The liquor is then boiled with the nuts, and affords
an inspissated decoction.
Good catechu is a brittle, compact solid, of a dull fracture. It has no smell, but a
very astringent taste. Water dissolves the whole of it, except the earthy matter, which
is probably added during its preparation. Alcohol dissolves its tannin and extractive.
The latter may be oxidized, and thus rendered insoluble in alcohol, by dissolving the
catechu in water, exposing it for some time to a boiling heat, and evaporating to
dryness.
The tannin of catechu differs from that of galls, in being soluble in alcohol, and more
soluble in water. It precipitates iron of an olive colour, and gelatine in a mass which
gradually becomes brown.
It has been long employed in India for tanning skins, where it is said to effect this object
in five days. I have seen a piece of sole leather completely tanned by it in this country in
ten days, the ox-hide having been made into a bag, with the hair outside, and kept filled
with the solution of catechu. In India it has also been used to give a brown dye to[272]
cotton goods, and of late years it has been extensively introduced into the calico print-works
of Europe. The salts of copper with sal ammoniac cause it to give a bronze
colour, which is very fast; the proto-muriate of tin, a brownish yellow; the per-chloride
of tin, with the addition of nitrate of copper, a deep bronze hue; acetate of alumina
alone, a reddish brown, and, with nitrate of copper, a reddish olive gray; nitrate of iron,
a dark brown gray. For dyeing a golden coffee brown, it has entirely superseded
madder; one pound of it being equivalent to six pounds of this root.
A solution of one part of catechu in ten parts of water, which is reddish brown,
exhibits the following results with—
| Acids |
A brightened shade. |
| Alkalis |
A darkened shade. |
| Proto-sulphate of iron |
Olive brown precipitate. |
| Per-sulphate of iron |
Olive green do. |
| Sulphate of copper |
Yellowish brown. |
| Alum |
A brightening of the liquor. |
| Per-nitrate of iron |
Olive green precipitate. |
| Nitrate of copper |
Yellowish brown do. |
| Nitrate of lead |
Salmondo. |
| Proto-nitrate of mercury |
Milk-coffee do. |
| Muriate of alumina |
Brown yellow. |
| Muriate of tin |
Do. do. |
| Per-chloride of tin |
Do.darker. |
| Corrosive sublimate |
Light chocolate do. |
| Acetate of alumina |
Brightening of the liquor. |
| Acetate of copper |
Copious brown precipitate. |
| Acetate of lead |
Salmon coloureddo. |
| Bichromate of potash |
Copious browndo. |
Pure tannin may be obtained from catechu, by treating it with sulphuric acid and carbonate
of lead; but this process has no manufacturing application.
CATGUT, (Corde à boyau, Fr.; Darmsaite, Germ.) the name absurdly enough
given to cords made of the twisted intestines of the sheep. The guts being taken while
warm out of the body of the animal, are to be cleared of feculent matter, freed from any
adhering fat, and washed in a tub of water. The small ends of all the intestines are
next to be tied together, and laid on the edge of the tub, while the body of them is left
to steep in some water, frequently changed, during two days, in order to loosen the
peritoneal and mucous membranes. The bundle of intestines is then laid upon a
sloping table which overhangs the tub, and their surface is scraped with the back of a
knife, to try if the external membrane will come away freely in breadths of about half
the circumference. This substance is called by the French manufacturers filandre, and
the process filer. If we attempt to remove it by beginning at the large end of the
intestine, we shall not succeed. This filandre is employed as thread to sew intestines,
and to make the cords of rackets and battledores. The flayed guts are put again into fresh
water, and after steeping a night, are taken out and scraped clean next day, on the
wooden bench with the rounded back of a knife. This is called curing the gut. The
large ends are now cut off, and sold to the pork-butchers. The intestines are again
steeped for a night in fresh water, and the following day in an alkaline lixivium made
by adding 4 ounces of potash, and as much pearlash, to a pail of water containing about
3 or 4 imperial gallons. This lye is poured in successive quantities upon the intestines,
and poured off again, after 2 or 3 hours, till they be purified. They are now drawn
several times through an open brass thimble, and pressed against it with the nail, in order
to smooth and equalize their surface. They are lastly sorted, according to their sizes, to
suit different purposes.
Whip-cord is made from the above intestines, which are sewed together endwise by the
filandre, each junction being cut aslant, so as to make it strong and smooth. The cord
is put into the frame, and each end is twisted separately; for whip-cord is seldom made
out of two guts twisted together. When twisted it is to be sulphured (see Sulphuring)
once or twice. It may also be dyed black with common ink, pink with red ink,
which the sulphurous acid changes to pink, and green with a green dye which the
colour dealers sell for the purpose. The guts take the dyes readily. After being well
smoothed, the cord is to be dried, and coiled up for sale.
Hatter’s cords for bowstrings.—The longest and largest intestines of sheep, after being
properly treated with the potash, are to be twisted 4, 6, 8, 10, or 12 together, according
to the intended size of the cord, which is usually made from 15 to 25 feet long. This cord
must be free from seams and knots. When half dry, it must be exposed twice to the
fumes of burning sulphur; and, after each operation, it is to be well stretched and
smoothed; it should be finally dried in a state of tension.
Clockmaker’s cord.—This cord should be extremely thin, and be therefore made from[273]
very small intestines, or from intestines slit up in their length by a knife fitted for the
purpose; being a kind of lancet surmounted with a ball of lead or wood. The wet gut
is strained over the ball which guides the knife, and the two sections fall down into a
vessel placed beneath. Each hand pulls a section. Clockmakers also make use of
stronger cords made of 2 or more guts twisted together.
Fiddle and harp strings.—These require the greatest care and dexterity on the part
of the workmen. The treble strings are peculiarly difficult to make, and are best made
at Naples, probably because their sheep, from their small size and leanness, afford the
best raw material.
The first scraping of the guts intended for fiddle-strings must be very carefully performed;
and the alkaline lyes being clarified with a little alum, are added, in a progressively stronger
state from day to day, during 4 or 5 days, till the guts be well bleached and swollen.
They must then be passed through the thimble, and again cleansed with the lixivium;
after which they are washed, spun, or twisted and sulphured during two hours. They are
finally polished by friction, and dried. Sometimes they are sulphured twice or thrice
before being dried, and are polished between horse-hair cords.
It has been long a subject of complaint, as well as a serious inconvenience to musicians,
that catgut strings cannot be made in England of the same goodness and strength
as those imported from Italy. These are made of the peritoneal covering of the intestines
of the sheep; and, in this country, they are manufactured at Whitechapel, and
probably elsewhere in considerable quantity; the consumption of them for harps, as
well as for the instruments of the violin family, being very great. Their chief fault is
weakness; whence it is difficult to bring the smaller ones, required for the higher notes,
to concert pitch; maintaining at the same time, in their form and construction, that
tenuity or smallness of diameter, which is required to produce a brilliant and clear tone.
The inconvenience arising from their breaking when in use, and the expense in the
case of harps, where so many are required, are such as to render it highly desirable to improve
a manufacture which, to many individuals may, however, appear sufficiently contemptible.
It is well known to physiologists, that the membranes of lean animals are far more
tough than of those animals which are fat or in high condition; and there is no reason to
doubt that the superiority of the Italian strings arises from the state of the sheep in that
country. In London, where no lean animals are slaughtered, and where, indeed, an
extravagant and useless degree of fattening, at least for the purpose of food, is given to
sheep in particular, it is easy to comprehend why their membranes can never afford a
material of the requisite tenacity. It is less easy to suggest an adequate remedy; but a
knowledge of the general principle, should this notice meet the eyes of those interested in
the subject, may at least serve the purpose of diminishing the evil and improving the manufacture,
by inducing them to choose in the market the offal of such carcases as appear
least overburthened with fat. It is probable that such a manufacture might be advantageously
established in those parts of the country where the fashion has not, as in
London, led to the use of meat so much overfed; and it is equally likely, that in the
choice of sheep for this purpose, advantage would arise from using the Welch, the Highland,
or the Southdown breeds, in preference to those which, like the Lincoln, are prone
to excessive accumulations of fat. It is equally probable, that sheep dying of some
of the diseases accompanied by emaciation, would be peculiarly adapted to this
purpose.
That these suggestions are not merely speculative is proved by comparing the strength
of the membranes in question, or that of the other membranous parts, in the unfattened
Highland sheep, with that of those found in the London markets.
CATHARTINE. The name proposed by MM. Feneulle and Lassaigne for a chemical
principle, which they suppose to be the active constituent of senna.
CAUSTIC. Any chemical substance corrosive of the skin and flesh; as potash, called
common caustic, and nitrate of silver, called lunar caustic, by surgeons.
CAVIAR. The salted roe of certain species of fish, especially the sturgeon. This
product forms a considerable article of trade, being exported annually from the town of
Astrachan alone, upon the shores of the Caspian sea, to the amount of several hundred
tons. The Italians first introduced it into Eastern Europe from Constantinople, under
the name of caviale. Russia has now monopolized this branch of commerce. It is prepared
in the following manner:—
The female sturgeon is gutted; the roe is separated from the other parts, and cleaned
by passing it through a very fine searce, by rubbing it into a pulp between the hands:
this is afterwards thrown into tubs, with the addition of a considerable quantity of salt;
the whole is then well stirred, and set aside in a warm apartment. There is another
sort of caviar, the compressed, in which the roe, after having been cured in strong brine, is
dried in the sun, then put into a cask, and subjected to strong pressure.
CAWK. The English miner’s name for sulphate of baryta, or heavy spar.
[274]
CEDRA, (Cedrat, Fr.) is the fruit of a species of orange, citron, or lemon, a tree which
bears the same name. Its peel is very thick, and covered with an epidermis which encloses
a very fragrant and highly prized essential oil. The preserves flavoured with it
are very agreeable. The citrons are cut into quarters for the dry comfits, but are put whole
into the liquid ones. The liquorist-perfumer makes with the peel of the cedra an excellent
liqueur; for which purpose, he plucks them before they are quite ripe; grates
down the peel into a little brandy, or cuts them into slices, and infuses these in the
spirits. This infusion is distilled for making perfume; but the flavour is better when
the infusion itself is used. See Essences, Liquorist, Perfumery.
CELESTINE. Native sulphate of strontia, found abundantly near Bristol, in the
red marl formation. It is decomposed, by ignition with charcoal, into sulphuret of
strontia, which is converted into nitrate by saturation with nitric acid, evaporation, and
crystallization. This nitrate is employed for the production of the red light in theatrical
fire-works.
CEMENTATION. A chemical process, which consists in imbedding a solid
body, in a pulverulent matter, and exposing both to ignition in an earthen or metallic
case. In this way, iron is cemented with charcoal to form steel, and bottle glass with
gypsum powder, or sand, to form Reaumur’s porcelain.
CEMENTS. (Ciments, Fr.; Cämente, Kitte, Germ.) Substances capable of taking the
liquid form, and of being in that state applied between the surfaces of two bodies, so as to
unite them by solidifying. They may be divided into two classes, those which are applied
through the agency of a liquid menstruum, such as water, alcohol, or oil, and those which
are applied by fusion with heat.
The diamond cement for uniting broken pieces of china, glass, &c. which is sold as a
secret at an absurdly dear price, is composed of isinglass soaked in water till it becomes
soft, and then dissolved in proof spirit, to which a little gum resin, ammoniac, or galbanum,
and resin mastic are added, each previously dissolved in a minimum of alcohol.
When to be applied, it must be gently heated to liquefy it; and it should be kept for
use in a well-corked phial. A glass stopper would be apt to fix so as not to be removable.
This is the cement employed by the Armenian jewellers in Turkey for glueing
the ornamental stones to trinkets of various kinds. When well made it resists
moisture.
Shell-lac dissolved in alcohol, or in a solution of borax, forms a pretty good cement.
White of egg alone, or mixed with finely sifted quick lime, will answer for uniting
objects which are not exposed to moisture. The latter combination is very strong, and
is much employed for joining pieces of spar and marble ornaments. A similar composition
is used by copper-smiths to secure the edges and rivets of boilers; only bullock’s
blood is the albuminous matter used instead of white of egg. Another cement in which
an analogous substance, the curd or caseum of milk is employed, is made by boiling
slices of skim-milk cheeses into a gluey consistence in a great quantity of water, and
then incorporating it with quicklime on a slab with a muller, or in a marble mortar.
When this compound is applied warm to broken edges of stoneware, it unites them very
firmly after it is cold.
A cement which gradually indurates to a stony consistence may be made by mixing
20 parts of clean river sand, two of litharge, and one of quicklime, into a thin putty
with linseed oil. The quicklime may be replaced with litharge. When this cement is
applied to mend broken pieces of stone, as steps of stairs, it acquires after some time a
stony hardness. A similar composition has been applied to coat over brick walls, under
the name of mastic.
The iron-rust cement is made of from 50 to 100 parts of iron borings, pounded and
sifted, mixed with one part of sal-ammoniac, and when it is to be applied moistened with
as much water as will give it a pasty consistency. Formerly flowers of sulphur were used,
and much more sal-ammoniac in making this cement, but with decided disadvantage, as
the union is effected by the oxidizement, consequent expansion and solidification of the
iron powder, and any heterogeneous matter obstructs the effect. The best proportion of
sal-ammoniac is, I believe, one per cent. of the iron borings. Another composition of the
same kind is made by mixing 4 parts of fine borings or filings of iron, 2 parts of potter’s
clay, and 1 part of pounded potsherds, and making them into a paste with salt and
water. When this cement is allowed to concrete slowly on iron joints, it becomes very
hard.
For making architectural ornaments in relief, a moulding composition is formed of
chalk, glue, and paper paste. Even statues have been made with it, the paper aiding the
cohesion of the mass.
Mastics of a resinous or bituminous nature which must be softened or fused by heat are
the following:—
Mr. S. Varley’s consists of sixteen parts of whiting sifted and thoroughly dried by a
red heat, adding when cold a melted mixture of 16 parts of black rosin and 1 of bees’-wax,
and stirring well during the cooling.
[275]
Mr. Singer’s electrical and chemical apparatus cement consists of 5 lbs. of rosin, 1 of
bees’-wax, 1 of red ochre, and two table-spoonsful of Paris-plaster, all melted together.
A cheaper one for cementing voltaic plates into wooden troughs is made with 6 pounds
of rosin, 1 pound of red ochre, 1⁄2 of a pound of plaster of Paris, and 1⁄4 of a pound of linseed
oil. The ochre and the plaster of Paris should be calcined beforehand, and added
to the other ingredients in their melted state. The thinner the stratum of cement that
is interposed, the stronger generally speaking is the junction.
Boiled linseed oil and red lead mixed together into a putty are often used by coppersmiths
and engineers, to secure joints. The washers of leather or cloth are smeared with
this mixture in a pasty state.
The resin mastic alone is sometimes used by jewellers to cement by heat cameos of
white enamel or coloured glass to a real stone, as a ground to produce the appearance of
an onyx. Mastic is likewise used to cement false backs or doublets to stones to alter
their hue.
Melted brimstone either alone, or mixed with rosin and brick dust, forms a tolerably
good and very cheap cement.
Plumber’s cement consists of black rosin one part, brick dust two parts, well incorporated
by a melting heat.
The cement of dihl for coating the fronts of buildings consists of linseed oil, rendered
dry by boiling with litharge, and mixed with porcelain clay in fine powder, to give it the
consistence of stiff mortar. Pipe-clay would answer equally well if well dried, and any
colour might be given with ground bricks, or pottery. A little oil of turpentine to thin
this cement aids its cohesion upon stone, brick, or wood. It has been applied to sheets of
wire cloth, and in this state laid upon terraces, in order to make them water tight; but
it is little less expensive than lead.
The bituminous or black cement for bottle corks consists of pitch hardened by the addition
of rosin and brick-dust.
In certain localities where a limestone impregnated with bitumen occurs, it is dried,
ground, sifted, and then mixed with about its own weight of melted pitch, either mineral,
vegetable, or that of coal tar. When this mixture is getting semifluid, it may be moulded
into large slabs or tiles in wooden frames lined with sheet iron, previously smeared over
with common lime mortar, in order to prevent adhesion to the moulds, which, being in
movable pieces, are easily dismounted so as to turn out the cake of artificial bituminous
stone. This cement is manufactured upon a great scale in many places, and used for
making Italian terraces, covering the floors of balconies, flat roofs, water reservoirs, water
conduits, &c. When laid down, the joints must be well run together with hot irons. The
floor of the terrace should be previously covered with a layer of Paris plaster or common
mortar, nearly an inch thick, with a regular slope of one inch to the yard. Such bituminous
cement weighs 144 pounds the cubic foot; or a foot of square surface, one inch
thick, weighs 12 pounds. Sometimes a second layer of these slabs or tiles is applied
over the first, with the precaution of making the seams or joints of the upper correspond
with the middle of the under ones. Occasionally a bottom bed, of coarse cloth or gray
paper, is applied. The larger the slabs are made, as far as they can be conveniently
transported and laid down, so much the better. For hydraulic cements, see Mortar.
CERASIN. The name given by Dr. John to those gums which swell, but do not
dissolve in water; such as gum tragacanth. It is synonymous with Bassorine,
which see.
CERATE from cera, wax. An unguent, of rather a stiff consistence, made of oil, or
lard and wax, thickened occasionally with pulverulent matters.
CERINE. A substance which forms from 70 to 80 per cent. of bees’-wax. It may
be obtained by digesting wax, for some time, in spirit of wine, at a boiling temperature.
The myricine separates, while the cerine remains dissolved, and may be obtained from
the decanted liquor by evaporation. Cerine is white, analogous to wax, fusible at
134° F., hardly acted upon by hot nitric acid, but is readily carbonized by hot sulphuric
acid. When treated with caustic alkaline lye, it is converted into margaric acid
and ceraïne.
CERIUM. A peculiar metal discovered in the rare mineral, called cerite, found
only in the copper mine of Bastnaes, near Riddarhytta, in Sweden. Cerium, extracted
from its chloride by potassium, appears as a dark red or chocolate powder, which
assumes a metallic lustre by friction. It does not conduct electricity well, like other
metals; it is infusible; its specific gravity is unknown. It has been applied to no use
in the arts.
CERUSE. A name of white lead. See Lead.
CETINE. The name given by Chevreul to spermaceti.
CHAINWORK is a peculiar style of textile fabric, to which hosiery and tambouring
belong. See Hosiery.
CHALK. (Craie, Fr.; Kreide, Germ.) A friable carbonate of lime, white, opaque,[276]
soft, dull, or without any appearance of polish in its fracture. Its specific gravity varies
from 2·4 to 2·6. It usually contains a little silica, alumina, and oxide of iron. It
may be purified by trituration, and elutriation. The siliceous and ferruginous
matters subside first, and the finer chalky particles floating in the supernatant liquid,
may be decanted with it, and obtained by subsidence. When thus purified, it is called
whitening and Spanish white, in England; schlemmkreide, in Germany; blanc de
Troyes, and blanc de Meudon, in France. Pure chalk should dissolve readily in dilute
muriatic acid, and the solution should afford no precipitate with water of ammonia.
CHALK—Black. A mineral, called also drawing-slate.
CHALK—French. Steatite, or soap stone; a soft magnesian mineral.
CHALK—Red. A clay coloured with the peroxide of iron, of which it contains
about 17 per cent.
CHARCOAL. The fixed residuum of vegetables exposed to ignition out of
contact of air. In the article Carbon, I have described the general properties of
charcoal and the simplest mode of making it. I shall here detail the best systems
of manufacturing this product upon the continent of Europe.
To carbonize wood under a movable covering, the plan of meiler, or heaps, is employed
very much in Germany. The wood is arranged either in horizontal layers, or in nearly
vertical ones, with a slight slope, so as to form conical rounded heaps of different sizes.
The former are called lying meiler, fig. 272.; the latter standing meiler, figs. 273. and 274.
Both are distributed in much the same way.
In districts where the wood can be transported into one place by means of rivers, or
mountain slides, a dry flat space must be pitched upon, screened from storms and floods,
which may be walled round, having a slight declivity made in the ground, towards the
centre. See fig. 275. Into this space the tarry acid will partially fall, and may be
conducted outwards, through a covered gutter beneath, into a covered tank. The
mouth of the tank must be shut, during the coaking, with an iron or stone slab, luted
with clay. A square iron plate is placed over the inner orifice of the gutter, to prevent
it being choked with coal ashes.
Fig. 275. represents a walled meiler
station; a, the station; b, the
gutter; c, the tank, which is covered
with the slab d; e, a slab
which serves to keep the gutter
clear of coals. The cover of the heaps is formed of earth, sand, ashes, or such other
matter as may be most readily found in the woods. They should be kindled in the
centre. From 6 days to 4 weeks may be required for charring a heap, according to its
size; hard wood requiring most time; and the slower the process, the better and
greater is the product, generally speaking.
Charring of wood in mounds
(Haufe or liegende werke) figs. 276.
and 277. differs from that in the
meiler, because the wood in the
haufe is successively charred, and
the charcoal is raked out by little
and little. The product is said
to be greater in this way, and also
better. Uncleft billets, 6 or 8
feet long, being laid over each
other, are covered with ashes, and
then carbonized. The station is
sometimes horizontal, and sometimes
made to slope. The length
may be 24 feet, the breadth 8
feet; and the wood is laid crosswise.[277]
Piles are set perpendicularly to support the roof, made of boughs and leaves,
covered with ashes. Pipes are occasionally laid within the upper part of the mounds,
which serve to catch and carry off some of the liquid products into proper tanks.
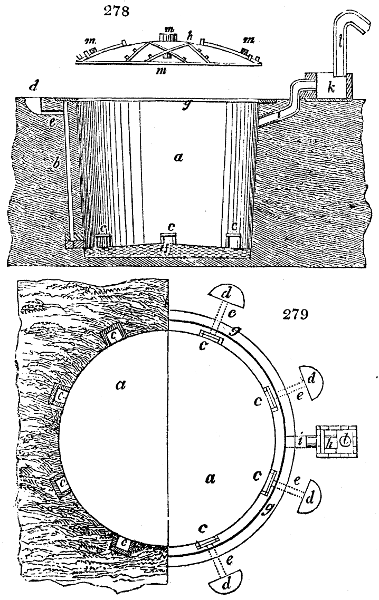
Fig. 278. is a vertical section,
and fig. 279. a half bird’s-eye view,
and half cross section, at the height
of the pit-bottom, of Chabeaussière’s
kiln for making wood charcoal.
a is the oven; b, vertical
air-pipes; c c, horizontal flues for
admitting air to the kiln; d d,
small pits which communicate by
short horizontal pipes e e, with the
vertical ones; f, the sole of the
kiln, a circle of brickwork, upon
which the cover or hood h reposes;
i, a pipe which leads to the cistern
k; l, the pipe destined for carrying
off the gaseous matter; m m, holes
in the iron cover or lid.
The distribution of the wood is
like that in the horizontal meilers,
or heaps; it is kindled in the central
vertical canal with burning
fuel, and the lid is covered with
a few inches of earth. At the beginning
of the operation all the
draught flues are left open, but
they are progressively closed, as
occasion requires. In eight kilns
of this kind, 500 decasters of oak
wood are carbonized, from which
16,000 hectolitres of charcoal are
obtained, equal to 64,000 pounds
French, being about 25 per cent.;
besides tar and 3000 velts of
wood vinegar, of from 2° to 3°.
Baumé.
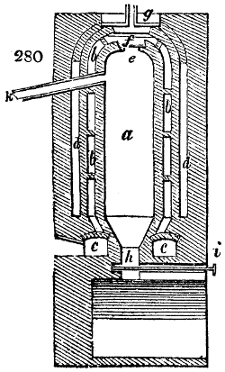
At Crouy upon the Ourcq, near Meaux, there is a well constructed kiln for making
turf-charcoal. It resembles most nearly a tar-kiln. In fig. 280. a is the cylindrical
coaking place, whose surrounding walls are heated by
the flame which passes through the intermediate space
b. The place itself is divided by partitions of fire tiles
into three stages, through the apertures in which the
flames of the fire c c, rise, and heat the exterior of the
coaking apartment. In order to confine the heat, there
is in the enclosing walls of the outer kiln a cylindrical
hollow space d, where the air is kept stagnant. Through
the apertures left in the upper end at e, the turf is introduced;
they are then shut with an iron plate f,
which is covered with ashes or sand. The fire-place
opens above this aperture, and its outlet is provided
with a moveable iron cover g, in which there is a small
hole for the issue of the gases. The sole of the kiln
consists of a cast iron slab h, which may be raised by
means of a hook i upon it. This is drawn back after
the carbonization is completed, whereby the charcoal
falls from the coaking space into a subjacent vault. The
volatile products are carried off by the pipe k, and led
into the condensing cistern; the gases escaping to the
fire-place where they are burned. The iron slab is protected from the corrosion of the
acid vapours by a layer of coal ashes.
CHICA is a red colouring principle made use of in America by some Indian tribes
to stain their skins. It is extracted from the bignonia chica by boiling its leaves in
water, decanting the decoction, and allowing it to settle and cool, when a red matter
falls down, which is formed into cakes and dried. This substance is not fusible, and,[278]
when burned, diffuses the same odour as animal bodies do. It is insoluble in cold water,
very soluble in alcohol and ether, but, after the evaporation of these liquids, it is recovered
unchanged. Fats and unctuous oils both dissolve it. It is soluble in carbonated
and caustic alkaline lyes, from which it is precipitated by the acids without alteration.
An excess of alkali, however, speedily decomposes it. Nitric acid transforms it into
oxalic acid, and a bitter matter. Chlorine makes it white.
The savages mix this pigment with the fat of the cayman or alligator, and rub their
skins with the mixture. It may probably be turned to account in the arts of civilized
nations.
CHIMNEY. (Cheminée, Fr.; Schornstein, Germ.) Chimney is a modern invention
for promoting the draught of fires and carrying off the smoke, introduced into
England so late as the age of Elizabeth, though it seems to have been employed in
Italy 100 years before. The Romans, with all their luxurious refinements, must have
had their epicurean cookery placed in perpetual jeopardy from their kitchen fires, which,
having no vent by a vertical tunnel in the walls, discharged their smoke and frequently
their flames at the windows, to the no small alarm of their neighbours, and annoyance
of even the street passengers.
Chimneys in dwelling houses serve also the valuable purpose of promoting salubrious
circulation of air in the apartments, when not foolishly sealed with anti-ventilating stove-chests.
The first person who sought to investigate the general principles of chimney draughts,
in subserviency to manufacturing establishments, was the celebrated Montgolfier. As the
ascent of heated air in a conduit depends upon the diminution of its specific gravity, or,
in other words, upon the increase of its volume by the heat, the ascensional force may
be deduced from the difference between the density of the elastic fluid in the interior of
the chimney, and of the external air; that is, between the different heights of the internal
and external columns of elastic fluid supposed to be reduced to the same density. In
the latter case, the velocity of the gaseous products of combustion in the interior of
the chimney is equal to that of a heavy body let fall from a height equal to the difference
in height of the two aerial columns.
To illustrate this position by an example, let us consider the simple case of a chimney
of ventilation for carrying off foul air from a factory of any kind; and suppose that
the tunnel of iron be incased throughout with steam at 212 degrees Fahr. Suppose
this tunnel to be 100 yards high, then the weight of the column of air in it will be to
that of a column of external air 100 yards high, assumed at 32° F. inversely as its expansion
by 180°; that is, as 1000 is to 1·375; or as 72·727 is to 100. The column of
external air at 32° being 100 yards, the internal column will be represented by 72·727;
and the difference = 27·27, will be the amount of unbalanced weight or pressure, which
is the effective cause of the ventilation. Calculating the velocity of current due to this difference
of weight by the well-known formula for the fall of heavy bodies, that is to say,
multiplying the above difference, which is 27·27, by the constant factor 19·62, and extracting
the square root of the product; thus, √19·62 × 27·27 = 23·13 will be the velocity
in yards per second, which, multiplied by 3, gives 69·39 feet. The quantity of air which
passes in a second is obtained of course by multiplying the area or cross section of the
tunnel by this velocity. If that section is half a yard, that is = a quadrangle 21⁄4 feet
by 2, we shall have 23·13 × 0·5 = 11·565 cubic yards, = 3121⁄4 cubic feet.
The problem becomes a little more complicated in calculating the velocity of air which
has served for combustion, because it has changed its nature, a variable proportion of its
oxygen gas of specific gravity 1·111, being converted into carbonic acid gas of specific
gravity 1·524. The quantity of air passed through well-constructed furnaces may, in
general, be regarded as double of what is rigorously necessary for combustion, and the
proportion of carbonic acid generated, therefore, not one half of what it would be were
all the oxygen so combined. The increase of weight in such burned air of the temperature
of 212°, over that of pure air equally heated, being taken into account in the
preceding calculation, will give us about 19 yards or 57 feet per second for the velocity
in a chimney 100 yards high incased in steam.
Such are the deductions of theory; but they differ considerably from practical results,
in consequence of the friction of the air upon the sides of the chimneys, which varies likewise
with its form, length, and quality. The direction and force of the winds also exercise
a variable influence upon chimney furnaces differently situated. In chimnies made of
wrought iron, like those of steam boats, the refrigeration is considerable, and causes a
diminution of velocity far greater than what occurs in a factory stalk of well-built brick
work. In comparing the numbers resulting from the trials made on chimneys of different
materials and of different forms, it has been concluded that the obstruction to the
draught of the air, or the deduction to be made from the theoretical velocity of efflux,
is directly proportional to the length of the chimneys and to the square of the velocity,
and inversely to their diameter. With an ordinary wrought-iron pipe, of from 4 inches[279]
to 5 inches diameter, attached to an ordinary stove, burning good charcoal, the difference
is prodigious between the velocity calculated by the above theoretical rule, and that observed
by means of a stop-watch, and the ascent of a puff of smoke from a little tow,
dipped in oil of turpentine thrust quickly into the fire. The chimney being 45 feet
high, the temperature of the atmosphere 68° Fahr., the velocity per second was,—
| Trials. |
By
theory. |
By
experiment. |
Mean temperature
of chimney. |
| 1 |
26·4 |
feet |
5 |
|
feet |
190 |
° Fahr. |
| 2 |
29·4 |
|
5 |
·76 |
|
214 |
|
| 3 |
34·5 |
|
6 |
·3 |
|
270 |
|
To obtain congruity between calculation and experiment, several circumstances must
be introduced into our formulæ. In the first place, the theoretical velocity must be
multiplied by a factor, which is different according as the chimney is made of bricks,
pottery, sheet iron, or cast iron. This factor must be multiplied by the square root of
the diameter of the chimney (supposed to be round), divided by its length, increased by
four times its diameter. Thus, for pottery, its expression is 2·06
√DL + D; D being the diameter,
and L the length of the chimney.
A pottery chimney, 33 feet high, and 7 inches in diameter, when the excess of its mean
temperature above that of the atmosphere was 205° Fahr., had a pressure of hot air
equal to 11·7 feet, and a velocity of 7·2 feet per second. By calculating from the last
formula, the same number very nearly is obtained. In none of the experiments did the
velocity exceed 12 feet per second, when the difference of temperature was more than
410° Fahr.
Every different form of chimney would require a special set of experiments to be
made for determining the proper factor to be used.
This troublesome operation may be saved by the judicious application of a delicate
differential barometer, such as that invented by Dr. Wollaston; though this instrument
does not seem to have been applied by its very ingenious author in measuring the
draughts or ventilating powers of furnaces.
If into one leg of this differential syphon, water be put, and fine spermaceti oil into
the other, we shall have two liquids, which are to each other in density as the numbers
8 and 7. If proof spirit be employed instead of water, we shall then have the relation
of very nearly 20 to 19. I have made experiments on furnace draughts with the instrument
in each of these states, and find the water and oil syphon to be sufficiently sensible: for
the weaker draughts of common fire-places the spirits and oil will be preferable
barometric fluids.
To the lateral projecting tube of the instrument, as described by Dr. Wollaston, I
found it necessary to attach a stop-cock, in order to cut off the action of the chimney,
while placing the syphon, to allow of its being fixed in a proper state of adjustment,
with its junction line of the oil and water at the zero of the scale. Since a slight deviation
of the legs of the syphon from the perpendicular, changes very considerably the
line of the level, this adjustment should be made secure by fixing the horizontal pipe
tightly into a round hole, bored into the chimney stalk, or drilled through the furnace
door. On gently turning the stop-cock, the difference of atmospherical pressure corresponding
to the chimney draught, will be immediately indicated by the ascent of the
junction-line of the liquids in the syphon. This modification of apparatus permits the
experiment to be readily rectified by again shutting off the draught, when the air will
slowly re-enter the syphon; because the projecting tube of the barometer is thrust into
the stop-cock, but not hermetically joined; whereby its junction line is allowed to
return to the zero of the scale in the course of a few seconds.
Out of many experiments made with this instrument, I shall content myself with
describing a few, very carefully performed at the breweries of Messrs. Trueman, Hanbury,
and Buxton, and of Sir H. Meux, Bart., and at the machine factory of Messrs.
Braithwaite; in the latter of which I was assisted by Captain Ericsson. In the first
trials at the breweries, the end of the stop-cock attached to the differential barometer
was lapped round with hemp, and made fast into the circular peep-hole of the furnace
door of a wort copper, communicating with two upright parallel chimneys, each 18 inches
square, and 50 feet high. The fire was burning with fully its average intensity at the
time. The adjustment of the level being perfect, the stop-cock orifice was opened, and
the junction level of the oil and water rose steadily, and stood at 11⁄4 inches, corresponding
to 1·258 = 0·156 of 1 inch of water, or a column of air 10·7 feet high. This difference
of pressure indicates a velocity of 26 feet per second. In a second set of experiments,
the extremity of the stop-cock was inserted into a hole, bored through the chimney
stalk of the boiler of a Boulton and Watt steam-engine of twenty-horse power. The
area of this chimney was exactly 18 inches square at the level of the bored hole, and its
summit rose 50 feet above it. The fire-grate was about 10 feet below that level. On[280]
opening the stop-cock, the junction line rose 21⁄4 inches. This experiment was verified
by repetition upon different days, with fires burning at their average intensity, and consuming
fully 12 lbs. of the best coals hourly for each horse’s power, or nearly one ton
and a third in twelve hours. If we divide the number 21⁄4 by 8, the quotient 0·28 will
represent the fractional part of 1 inch of water, supported in the syphon by the unbalanced
pressure of the atmosphere in the said chimney; which corresponds to 191⁄4 feet
of air, and indicates a velocity in the chimney current of 35 feet per second. The
consumption of fuel was much more considerable in the immense grate under the wort
copper, than it was under the steam-engine boiler.
In my experiments at Messrs. Braithwaite’s factory, the maximum displacement of
the junction line was 1 inch, when the differential oil and water barometer was placed
in direct communication with a chimney 15 inches square, belonging to a steam boiler,
and when the fire was made to burn so fiercely, that, on opening the safety-valve of the
boiler, the excess of steam beyond the consumption of the engine, rushed out with such
violence as to fill the whole premises. The pressure of one-eighth of an inch of water
denotes a velocity of draught of 23·4 feet per second.
In building chimneys, we should be careful to make their area rather too large than
too small; because we can readily reduce it to any desired size, by means of a sliding
register plate near its bottom, or a damper plate applied to its top, adjustable by wires
or chains, passing over pulleys. Wide chimneys are not so liable as narrow ones to
have their draught affected by strong winds. In a factory, many furnace flues are
often conducted into one vertical chimney stalk, with great economy in the first
erection, and increased power of draught in the several fires.
Vast improvements have been made in this country, of late years, in building stalks
for steam boilers and chemical furnaces. Instead of constructing an expensive, lofty
scaffolding of timber round the chimney, for the bricklayers to stand upon, and to
place their materials, pigeon-holes, or recesses, are left at regular intervals, a few feet
apart, within the chimney, for receiving the ends of stout wooden bars, which are
laid across, so as to form a species of temporary ladder in the interior of the tunnel.
By means of these bars, with the aid of ropes and pulleys, every thing may be progressively
hoisted, for the building of the highest engine or other stalks. An expert
bricklayer, with a handy labourer, can in this way raise, in a few weeks, a considerable
chimney, 40 feet high, 5 feet 8 inches square outside, 2 feet 8 inches inside at the base,
28 inches outside, and 20 inches inside at the top. To facilitate the erection, and at
the same time increase the solidity of an insulated stalk of this kind, it is built
with three or more successive plinths, or recedures, as shown in fig. 281. It is necessary
to make such chimneys thick and substantial near the base, in order that they
may sustain the first violence of the fire, and prevent the sudden dissipation of the
heat. When many flues are conducted into one chimney stalk, the area of the latter
should be nearly equal to the sum of the areas of the former, or at least of as many of
them as shall be going simultaneously. When the products of combustion from any
furnace must be conducted downwards, in order to enter near the bottom of the main
stalk, they will not flow off until the lowest part of the channel be heated by burning
some wood shavings or straw in it, whereby the air syphon is set agoing. Immediately
after kindling this transient fire at that spot, the orifice must be shut by which
it was introduced; otherwise the draught of the furnace would be seriously impeded.
But this precaution is seldom necessary in great factories, where a certain degree of
heat is always maintained in the flues, or, at least, should be preserved, by shutting
the damper plate of each separate flue, whenever its own furnace ceases to act.
Such chimneys are finished at top with a coping of stone-slabs, to secure their brickwork
against the infiltration of rains, and they should be furnished with metallic
conducting rods, to protect them from explosions of lightning.
When small domestic stoves are used, with very slow combustion, as has been
recently proposed, upon the score of a misjudged economy, there is great danger of the
inmates being suffocated or asphyxied, by the regurgitation of the noxious burned air.
The smoke doctors who recommend such a vicious plan, from their ignorance of
chemical science, are not aware that the carbonic acid gas, of coke or coal, must be heated
250° F. above the atmospheric air, to acquire the same low specific gravity with it. In
other words, unless so rarefied by heat, that gaseous poison will descend through the orifice
of the ash-pit, and be replaced by the lighter air of the apartment. Drs. Priestley and
Dalton have long ago shown the co-existence of these two-fold crossing currents of air,
even through the substance of stone-ware tubes. True economy of heat, and salubrity,
alike require vivid combustion of the fuel, with a somewhat brisk draught inside of the
chimney, and a corresponding abstraction of air from the apartment. Wholesome
continuous ventilation, under the ordinary circumstances of dwelling houses, cannot
be secured in any other way. Were these mephitic stoves, which have been of late
so ridiculously puffed in the public prints, generally introduced, the faculty would[281]
need to be immediately quadrupled to supply the demand for medical advice; for
headaches, sickness, nervous ailments, and apoplexy, would become the constant inmates
of every inhabited mansion. The phenomena of the grotto of Pausilippo might then
be daily realised at home, among those who ventured to recline upon sofas in such carbonated
apartments; only instead of a puppy being suffocated pro tempore, human beings
would be sacrificed, to save two-penny worth of fuel per diem.
[282]
The figures upon the preceding page represent one of the two chimneys, recently
erected at the Camden Town station, for the steam boilers of the two engines of 60
horse-power each, belonging to the London and Birmingham Railway Company.
These engines draw their train of carriages up the inclined plane of Hampstead Hill.
The chimneys were designed by Robert Stephenson, Esq., engineer to the Company,
executed by William Cubitt, Esq., of Gray’s Inn Road,—and do equal honour to
both gentlemen, being probably the most elegant and substantial specimens of this style
of architecture in the world. In the section, fig. 281.,
A represents a bed of concrete, 6 feet thick, and 24 feet square.
B, brick footings set in cement; the lower course 19 feet square.
C, Bramley-fall stone base, with a chain of wrought iron let into it.
D, a portion, 15 feet high, curved to a radius of 113 feet, built entirely of Malm
paviours, (a peculiarly good kind of bricks.)
E, shaft built of Malm paviours in mortar.
F, ditto, built from the inside, without exterior scaffolding.
G, the cap ornamented, (as shown in the plan alongside,) with Portland stone, the
dressings being tied together with copper cramps and an iron bond.
Fig. 282. represents the mouldings of the top, upon an enlarged scale.
Fig. 283., a plan of the foundation, ditto.
Fig. 284., ditto, at the level of the entrance of the flue, as seen in
Fig. 285., the elevation of the chimney.
Fig. 286., plan at the ground level I, in fig. 281. and 285.
K, fig. 281., the lightning conducting rod.
CHINTZ is a peculiar style of fast-printed calico, in which figures of at least five
different colours are impressed upon a white or light coloured ground.
CHLORATE OF POTASH, commonly called oxymuriate of potash. This interesting
saline compound has become the object of a pretty extensive manufacture, in
consequence of its application to make matches for procuring instantaneous light, and a
detonating powder for fire-arms. It may be prepared both in the humid and dry way.
Having made a strong solution of purified potash, or carbonate of potash, with from
two to three parts of water, we pass through it in a Woulfe’s apparatus a current of chlorine
gas, till it ceases to absorb any more. Chloride of potash and chloride of potassium
alone are formed as long as there is an excess of alkali in the solution; but afterwards in
the further reaction of the materials, the chloride passes into the state of a chlorate, and,
as such, precipitates from the solution. During the first half of the operation, that is, till
the potash be about one half saturated with chlorine, as indicated by litmus paper ceasing
to be darkened and beginning to be blanched, only the chloride of potassium or muriate
of potash falls. The process should be interrupted at this point in order to remove the
salt, to wash it, to add the washings to the liquor, and then to transmit the gas freely through
the solution. As the operation advances, less muriate of potash is formed, and at length
nothing but the pure chlorate is separated in crystals. When finally the bubbles of
gas pass through without being sensibly absorbed, the process is known to be completed;
the liquid may then be allowed to settle, and be poured off from the crystals of chlorate
of potash, which are purified from the muriate by dissolving them in three times their
weight of boiling water, and filtering the solution while hot. On its cooling, the chlorate
will separate in pearly-looking crystalline plates. It may be rendered quite pure
by a second crystallization, in which state it does not affect solution of nitrate of silver.
The above potash lye usually gets a reddish tint in the course of the process in consequence
of a little manganesic acid coming over with the chlorine, but it gradually loses
this colour as the saturation becomes complete, when the solution turns yellow. The
tubes for conveying the gas should be of large diameter, if they be plunged into the
saline solution, because the crystallization which takes place in it is apt to choke them
up. This inconvenience may however be obviated by attaching to the end of the glass
tube, a tube of caoutchouc terminated in a small glass funnel, or simply the neck of a
caoutchouc bottle with a part of its body, whose width will not be readily closed with a
saline crust. The residuary lixivium may be used against another operation, or it may
be evaporated down to half its bulk and set aside to crystallize, whereby some more
chlorate will be obtained, mixed indeed with muriate and carbonate, from which however
it may be separated by a second crystallization. In general the pure chlorate obtained
does not exceed one tenth the weight of the potash employed; because in thus treating
potash with chlorine, five-sixths of it are converted into muriate of potash and only one
sixth into chlorate, and a part of the latter adheres to the muriate, or is lost in the mother
waters of the crystallizations.
The chlorate of potash may be more conveniently manufactured, like that of lime, in
the dry way. St. Romer patented at Vienna the following method for that purpose in
1821:—Ten pounds of crystallised peroxide of manganese are to be finely pulverised,
mixed with ten pounds of plumbago, and thirty pounds of common salt, and put into the[283]
leaden retort represented in fig. 287. p. 287. From the middle of the helmet-shaped
lid of this vessel, a lead tube, two feet long and two inches wide, conducts to the receiver,
which is a square earthen pan, hard glazed both within and without, of the same capacity
with the retort. The end of the tube must be made fast to a frame at the height of
six inches above the bottom of the receiver. Upon its inner sides four inches apart,
brackets are to be fixed for supporting a series of laths or shelves of white wood, on which
a number of little paper or paste-board boxes are to be laid. In these boxes ten pounds
of the purest carbonate of potash, prepared from tartar, are to be spread. The receiver
must now be covered with a lid made tight by a water lute. Twenty pounds of
concentrated sulphuric acid previously diluted with sixteen pounds of water, and then
cooled, are to be poured upon the mixed materials in the retort, the lid immediately
secured, with the tube adjusted in the receiver. The whole must be allowed to operate
spontaneously without heat for twelve hours. At the end of this time the retort is to be
surrounded with a water bath and steadily heated during twelve hours, and then left to
cool for six hours. The apparatus must now be opened, the cakes of chlorate of potash
removed, and freed from muriate by solution and crystallization.
M. Liebig proposes the following process for obtaining chlorate of potash:—
Heat chloride of lime in water till it ceases to destroy vegetable colours. In this case a
mixture of chloride of calcium and chlorate of potash is obtained. This is to be dissolved
in hot water, and to the solution concentrated by evaporation, chloride of potassium is
to be added, and then suffered to cool. After cooling, a quantity of crystals of chlorate
of potash is obtained, which are to be redissolved and crystallized again to purify them.
M. Liebig considers that this will be a cheap process for obtaining chlorate of potash.
From 12 ounces of chloride of lime, of so bad a quality that it left 65 per cent. of insoluble
matter, he obtained an ounce of chlorate of potash.
The only difficulty to overcome in this process is, from the chloride of lime not being
so easily decomposed by heat as is generally supposed; a solution of it may be kept boiling
for an hour without losing its bleaching power. The best method is to form a thin
paste with chloride of lime and water, and then to evaporate it to dryness. If it be required
to prepare it by passing chlorine into cream of lime, it is advantageous to keep
it very hot.
The chlorate of potash which separates from the solution by crystallization, has not
the form of scales which it usually possesses, but is prismatic: whether this is occasioned
by some admixture has not been ascertained; but on re-crystallizing, it is obtained in
the usual form.
The solution ought not merely to be left to cool, in order to procure crystals, for
the crystallization is far from being terminated even after complete cooling; crystals
continue to be deposited for 3 or 4 days.
The following modification of the process for making chlorate of potash is that of M.
Vée. A solution of chloride of lime marking 18° or 20° Baumé, is to be set upon the
fire in a lead or cast iron pot, and when it begins to get hot, there is to be dissolved in
it, a quantity of chloride of potassium sufficient to raise the hydrometer 3 or 4 degrees.
It must be then concentrated as quickly as possible till it marks 30° or 31°, taking care
that it does not boil over by the sudden extrication of oxygen. The concentrated liquor
is set aside to crystallize in a cool place; where a deposit of chlorate of potash forms,
mixed with chloride of potassium. The mother waters being evaporated to the density
of 36°, afford another crop of crystals, after which they may be thrown away.
The salts obtained at the first crystallization are to be re-dissolved, and the solution
being brought to 15° or 16° is to be filtered, when it will afford upon cooling pure chlorate
of potash.
Chlorate or oxymuriate of potash has a cooling, somewhat unpleasant and nitrous
taste. It does not bleach. At 60° F. 100 parts of water dissolve six parts of it, and at
its boiling point or 220°, sixty parts. When heated to dull ignition in a glass retort it
gives out 39·15 per cent. of its weight of oxygen, and becomes thereby chloride of
potassium. When strongly triturated in a mortar it crackles, throws out sparks, and becomes
luminous. It deflagrates upon red-hot cinders like nitre: when triturated along with
sulphur, or phosphorus, it detonates with great violence, not without danger to the hands
of the operator, if they be not protected by a thick glove. Similar detonations may be
produced with cinnabar or vermillion, sulphuret of potassium, sugar, volatile oils, &c.;
but they can be effected only by the smart blow of a heated hammer and anvil. A
mixture of sugar or starch with chlorate of potash is readily inflamed by a drop of sulphuric
acid, and this experiment is the basis of the preparation of the oxygenated matches,
as they have been commonly called. The following formula forms a good paste for
tipping the said matches, made of narrow slips of either wood or card. Thirty parts of
the chlorate in fine powder are to be mixed gently with a spatula upon paper with ten
parts of flowers of sulphur well levigated, eight of sugar, five of gum arabic, and enough
of vermillion to give the whole a rose tint. We begin by mixing tenderly together[284]
the sugar, the gum, and the salt previously pulverised; we then add as much water as shall
reduce the mixture to a thin paste, and lastly introduce the sulphur; after which all must
be well incorporated. The points of the matches, either previously tipped with sulphur
or not, are to be dipped in that paste, so as to get coated with a little of it, and are lastly
laid in a warm place till they become thoroughly dry. To kindle one of them, it must
be touched with strong sulphuric acid, which for this purpose is usually kept in a small
well-stoppered phial, and thickened with amianthus. Aspen is reckoned the best wood
for matches.
Of late years a detonating priming for fire-arms has been much used with the percussion
locks. The simplest formula for making it is to take ten parts of gunpowder, to
lixiviate it with water, and to mix the residuum, while moist, with five parts and a
quarter of chlorate of potash, reduced to an extremely fine powder. The paste may be
made pretty thin, for the salt is sparingly soluble in the cold water, and it mixes best
when tolerably fluid. This powder when dry is dangerous to handle, being very apt to
explode. But this danger is guarded against by letting fall a drop of the paste into each
copper percussion cap, and leaving it to dry there. In the detonation of this powder, besides
muriate of potash, there are generated a little sulphate of potash and chlorine gas,
which rust the metal very fast. For which reason fulminate of mercury is now preferred by
many sportsmen as a detonating powder. See Fulminate.
CHLORATES, compounds of chloric acid with the salifiable bases. The only acid
belonging to this class of any manufacturing importance is the following:
CHLORIC ACID; the acid constituent of the preceding salt; it consists of one
equivalent prime of chlorine = 35·476, + 5 of oxygen, = 40·065; of which the sum
75·535 is the prime equivalent of the acid.
CHLORINE; the most energetic of the undecompounded bodies, or chemical
elements as they are usually called, exists, under ordinary circumstances, as a greenish
yellow gas, but, when exposed to a pressure of 4 atmospheres, it becomes a yellow
transparent liquid. In the first state, its density compared to air, reckoned 1·000, is
2·47; in the second, its density compared to water, 1·000, is 1·33. No degree of cold,
hitherto tried, has liquefied the gas when dry. It is obtained by putting into a glass
retort a mixture of 3 parts of common salt, with 2 parts of peroxide of manganese, and
pouring upon it 2 parts of sulphuric acid diluted with its own weight of water; or, more
conveniently, by pouring moderately strong muriatic acid upon peroxide of manganese in
a retort; and in either case applying the gentle heat of a spirit lamp or a water bath, while
the beak of the retort is plunged under brine upon the shelf of the pneumatic trough.
The gas issues, and may be received in the usual way into inverted glass jars, or phials;
but the first which comes over being mixed with the air of the retort, must be rejected.
It has a peculiar smell, and irritates the nostrils most violently when inhaled, as also the
windpipe and lungs. It is eminently noxious to animal life, and, if breathed in its undiluted
state, would prove instantly fatal. It supports the combustion of many bodies,
and indeed spontaneously burns several without their being previously kindled. The
resulting combinations are called chlorides, and act most important parts in many manufacturing
processes.
Water absorbs, at the ordinary temperature of the atmosphere, about double its volume
of chlorine, and acquires the colour, smell, and taste of the gas, as well as its power
of destroying or bleaching vegetable colours. When this aqueous chlorine is cooled to
36° F. dark yellow crystalline plates appear in it of the hydrate of chlorine, which are
composed in 100 parts of 27·7 chlorine, and 72·3 water. If these crystals be heated
to about 45° they liquefy, and the gas flies off.
Chlorine has a powerful affinity for hydrogen, not only combining with it rapidly in
the gaseous, but seizing it in many of its liquid and solid combinations, as in volatile
oils, which it inflames, and in yellow wax, cotton, and flax, which it whitens. The compound
of chlorine and hydrogen gases is muriatic acid gas. Manganese, when mixed
with liquid muriatic acid, as in the above process, abstracts the hydrogen, and lets the
chlorine gas go free. When chlorine is passed into water, it decomposes some of it, seizes
its hydrogen to form a little muriatic acid, and enables its oxygen to unite either with the
chlorine, into chlorous acid, or with the remaining water, and to constitute oxygenated
water. Hence, aqueous chlorine, exposed to the sunbeam, continually evolves oxygen,
and, ere long, becomes muriatic acid.
This watery compound acts in a powerful way upon coloured vegetable fibres, extracting
their hydrogen or colouring element by the twofold affinities of the chlorine and oxygen
for it.
Hence chlorine, as a bleaching agent, requires to be tempered by the quiescent affinity
of some alkaline base, potash or lime. Malaria, or morbific and putrescent miasmata,
consist chiefly of hydrogenous matter as their basis, and are best counteracted by chlorine,
where it can be conveniently applied.
Chlorides of Potash, Soda, and Lime.—These are the most important preparations[285]
through which chlorine exercises its peculiar powers upon the objects of manufactures.
When a weak solution of caustic potash or soda is saturated with chlorine, it affords a
bleaching liquor which is still used by some bleachers and calico-printers for their most
delicate processes; but the price of the alkalis has led to the disuse of these chlorides as a general
means, and has occasioned an extensive employment of chloride of lime. Upon the
manufacture of this interesting compound I made an elaborate series of experiments
several years ago, and published the results in the 13th volume of Brande’s Journal, for
April 1822. I have no reason to suppose, from any thing that has been published since,
that the processes there described have been essentially improved, or that any errors,
either theoretical or practical, of any moment, exist in that memoir. I shall therefore
first present my readers with a brief abstract of it, and then make such observations as
subsequent inquiries suggest.
In the researches which I made, at many different times, upon the nature of the chloride
of lime, I generally sought to combine the information flowing from both synthesis and
analysis; that is, I first converted a known portion of hydrate of lime into bleaching-powder,
and then subjected this chloride to analysis.
Two hundred grains of the atomic proto-hydrate of pure lime were put into a glass
globe, which was kept cold by immersion in a body of water at 50°. A stream of
chlorine, after being washed in water of the same temperature in another glass globe,
connected to the former by a long narrow glass tube, was passed over the calcareous
hydrate. The globe with the lime was detached from the rest of the apparatus from
time to time, that the process might be suspended as soon as the augmentation of weight
ceased. This happened when the 200 grains of hydrate, containing 151·9 of lime, had
absorbed 130 grains of chlorine. By one analytical experiment it was found, that dilute
muriatic acid expelled from 50 grains of the chloride, 20 grains of chlorine, or 40
per cent.; and by another, from 40 grains, 16·25 of gas, which is 40·6 per cent. From
the residuum of the first 39·7 grains of carbonate of lime were obtained by carbonate
of ammonia; from that of the second, 36·6 of ignited muriate of lime. The whole
results are therefore as follows:—
| |
Synthesis. |
1st
Analysis. |
2d
Analysis. |
Mean. |
|---|
| Chlorine |
39·39 |
40·00 |
40·62 |
40·31 |
| Lime |
46·00 |
44·74 |
46·07 |
45·50 |
| Water |
14·60 |
15·26 |
13·31 |
14·28 |
| |
100·00 |
100·00 |
100·00 |
100·00 |
Though the heat generated by the action of the dilute acid had carried off in the analytical
experiments a small portion of moisture with the chlorine, yet their accordance
with the synthetic experiment is sufficiently good to confirm the general results. The
above powder appears to have been a pure chloride, without any mixture of muriate.
But it exhibits no atomic constitution in its proportions.
To 200 grains of that hydrate of lime 30 grains of water being added, the powder
was subjected to a stream of chlorine in the above way, till saturation took place. Its
increase of weight was 150 grains.
It ought to be remarked, that in this and the preceding experiment, there was no appreciable
pneumatic pressure employed to aid the condensation of the chlorine. In the
last case, we see that the addition of 30 grains of water has enabled the lime to absorb
20 grains more of chlorine, being altogether a quantity of gas nearly equal to that of
the dry lime. Thus, an atom of lime seems associated with 7⁄9 of an atom of chlorine.
Analysis by muriatic acid confirmed this composition. It gave
| Chlorine |
39·5 |
= 51·8 cubic inches. |
| Lime |
39·9 |
| Water |
20·6 |
| |
100·0 |
A great variety of apparatus has been at different times contrived for favouring the
combination of chlorine with the slacked lime for the purposes of commerce. One of
the most ingenious forms, is that of a cylinder, or barrel, furnished with narrow wooden
shelves within, and suspended on a hollow axis by which the chlorine was admitted, and
round which the barrel was made to revolve. By this mode of agitation, the lime-dust
being exposed on the most extensive surface, was speedily impregnated with the gas to
the requisite degree. Such a mechanism I saw at MM. Oberkampf and Widmer’s celebrated
fabrique de toiles peintes, at Jouy, in 1816. But this is a costly refinement, inadmissible
on the largest scale of British manufacture. The simplest, and, in my
opinion, the best construction for subjecting lime-powder to chlorine, is a large chamber[286]
8 or 9 feet high, built of siliceous sandstone, having the joints of the masonry secured
with a cement composed of pitch, resin, and dry gypsum in equal parts. A door is
fitted into it at one end, which can be made air-tight by strips of cloth and clay lute.
A window on each side enables the operator to judge how the impregnation goes on
by the colour of the air, and also gives light for making the arrangements within at the
commencement of the process. As water lutes are incomparably superior to all others
where the pneumatic pressure is small, I would recommend a large valve or door on this
principle to be made in the roof, and two tunnels of considerable width at the bottom of
each side wall. The three covers could be simultaneously lifted off by cords passing
over a pulley, without the necessity of the workman approaching the deleterious gas,
when the apartment is to be opened. A great number of wooden shelves, or rather
trays, 8 or 10 feet long, 2 feet broad, and 1 inch deep, are provided to receive the riddled
slacked lime, containing generally about 2 atoms of lime to 3 of water. These shelves
are piled one over another in the chamber, to the height of 5 or 6 feet, cross bars below
each keeping them about an inch asunder, that the gas may have free room to circulate
over the surface of the calcareous hydrate.
The alembics for generating the chlorine, which are usually nearly spherical, are in
some cases made entirely of lead, in others of two hemispheres, joined together in the
middle, the upper hemisphere being lead, the under one cast-iron. The first kind of
alembic is enclosed for two-thirds from its bottom, in a leaden or iron case, the interval
of two inches between the two being destined to receive steam from an adjoining boiler.
Those which consist below of cast-iron, have their bottom directly exposed to a very
gentle fire; round the outer edge of the iron hemisphere a groove is cast, into which the
under edge of the leaden hemisphere fits, the joint being rendered air-tight by Roman
or patent cement. In this leaden dome there are four apertures, each secured by a
water-lute. The first opening is about 10 or 12 inches square, and is shut with a
leaden valve, with incurvated edges, that fit into the water channel at the margin of the
hole. It is destined for the admission of a workman to rectify any derangement in the
apparatus of rotation, or to detach hard concretions of salt from the bottom.
The second aperture is in the centre of the top. Here a tube of lead is fixed, which
descends nearly to the bottom, and down through which the vertical axis passes. To
its lower end the cross bars of iron, or of wood, sheathed with lead, are attached, by
whose revolution the materials receive the proper agitation for mixing the dense manganese
with the sulphuric acid and salt. The motion is communicated either by the
hand of a workman applied from time to time to a winch at top, or it is given by connecting
the axis with wheel work, impelled by a stream of water or a steam-engine.
The third opening admits the syphon-formed funnel, through which the sulphuric acid
is introduced; and the fourth is the orifice of the eduction-pipe.
Manufacturers differ much from each other in the proportion of their materials for
generating chlorine. In general, 10 cwt. of salt are mixed with from 10 to 14 cwt. of
manganese, to which mixture, after its introduction into the alembic, from 12 to 14 cwt.
of sulphuric acid are added in successive portions. That quantity of oil of vitriol must,
however, be previously diluted with water, till its specific gravity becomes about 1·6.
But, indeed, this dilution is seldom actually made, for the manufacturer of bleaching-powder
almost always prepares his own sulphuric acid for the purpose, and therefore
carries its concentration no higher in the leaden boilers than the density of 1·65, which
from my table of sulphuric acid, indicates 1⁄4th of its weight of water, and therefore 1⁄3d
more of such acid must be used.
The fourth aperture, I have said, admits the eduction pipe. This pipe is afterwards
conveyed into a leaden chest or cylinder, in which all the other eduction pipes also
terminate. They are connected with it simply by water-lutes, having a hydrostatic
pressure of 2 or 3 inches. In this general diversorium the chlorine is washed from
adhering muriatic acid, by passing through a little water, in which each tube is immersed,
and from this the gas is let off by a pretty large leaden tube, into the combination
room. It usually enters in the top of the ceiling, whence it diffuses its heavy
gas equally round.
Four days are required, at the ordinary rate of working, for making good marketable
bleaching-powder. A more rapid formation would merely endanger an elevation of
temperature, productive of muriate of lime, at the expense of the bleaching quality. But
skilful manufacturers use here an alternating process. They pile up, first of all, the
wooden trays only in alternate shelves in each column. At the end of two days the
distillation is intermitted, and the chamber is laid open. After two hours the workman
enters, to introduce the alternate trays covered with fresh hydrate of lime, and at the
same time rakes up thoroughly the half-formed chloride in the others. The door is then
secured, and the chamber, after being filled for two days more with chlorine, is again
opened, to allow the first set of trays to be removed, and to be replaced by others, containing
fresh hydrate, as before. Thus the process is conducted in regular alternation;[287]
thus, to my knowledge, very superior bleaching-powder is manufactured, and thus the
chlorine may be suffered to enter in a pretty uniform stream. But for this judicious
plan, as the hydrate advances in impregnation, its faculty of absorption becoming
diminished, it would be requisite to diminish proportionately the evolution of chlorine,
or to allow the excess to escape to the great loss of the proprietor, and, what is of more
consequence, to the great detriment of the health of the workmen.
The manufacturer generally reckons on obtaining from one ton of rock-salt, employed
as above, a ton and a half of good bleaching-powder. But the following analysis of the
operation will show that he ought to obtain two tons.
When a mixture of sulphuric acid, common salt, and black oxide of manganese are
the ingredients used, as by the manufacturer of bleaching-powder, the absolute proportions
are, upon the oxygen scale of equivalents:—
| 1 |
atom muriate of soda |
7 |
·5 |
29·70 |
100·0 |
| 1 |
atom peroxide of manganese |
5 |
·5 |
21·78 |
73·3 |
| 2 |
atoms oil of vitriol 1·846 |
12 |
·25 |
48·52 |
163·3 |
| |
25 |
·25 |
100·00 |
|
And the products ought to be:—
| Chlorine disengaged |
1 |
atom. |
4 |
·5 |
17·82 |
| Sulphate of soda |
1 |
— |
9 |
·0 |
35·64 |
| Proto-sulphate of manganese |
1 |
— |
9 |
·5 |
37·62 |
| Water |
2 |
— |
2 |
·25 |
8·92 |
| |
25 |
·25 |
100·00 |
These proportions are, however, very different from those employed, by many, nay
I believe by all manufacturers; and they ought to be so, on account of the impurity
of their oxide of manganese. Yet making allowance for this, I am afraid that many
of them commit great errors in the relative quantities of their materials.
From the preceding computation, it is evident that 1 ton of salt with 1 ton of the
above native oxide of manganese properly treated, would yield 0·59 of a ton of chlorine,
which would impregnate 1·41 tons of slaked lime, producing 2 tons of bleaching-powder,
stronger than the average of the commercial specimens; or allowing for a little loss,
which is unavoidable, would afford 2 tons of ordinary powder, with a little more slaked
lime.
Fig. 287. represents a retort of lead, well adapted to the evolution of chlorine from the
mixture of salt, manganese, and sulphuric acid, or from manganese and muriatic acid.
The interior vessel is cast in lead, and it has round its bottom part a cast-iron steam
case. The salt and manganese are introduced by the aperture C, and the sulphuric
acid by the syphon funnel F. The contact of these three substances is continually
renewed by the agitator or stirrer B, which consists of wrought or cast iron sheathed
with lead. e is the gas discharge pipe. The residuums are drawn off by the bottom
discharge pipe G. The heating case receives its steam by the pipe h.
The chlorine gas fig. 288. is conveyed from the retort B into the chamber I, by the tube
E E E. This chamber is divided into four compartments, to receive the gas disengaged
from four retorts, like the above. The bottom of it is covered with a stratum three
or four inches thick of quicklime, newly slaked and sifted, which is stirred about from[288]
time to time, by the rakes L L L L. When the saturation is sufficient, the chloride of
lime is taken out by the doors K K K K. The size of this apparatus allows 2 cwt. of
manganese, and its equivalent quantity of salt and sulphuric acid, or of muriatic acid,
to be introduced at once into the retort. D is the handle of the agitator.
The same form of retort will suit perfectly well to prepare chlorine for making
liquid chloride of lime, which is preferred by many bleachers and calico-printers who
have conveniences for preparing it themselves. The most concentrated solutions of the
dry chloride of lime do not mark more than 6° B. (sp. grav. 1·04), and discolour only
50 volumes of Gay Lussac’s solution of indigo, whilst the chloride made in the humid
way marks from 8° to 9° B. (about 1·060), and discolours 80 volumes of the same
solution.
In the chloride of lime apparatus, most generally used by the skilful calico-printers
of Mulhausen, the mixture of muriatic acid and manganese is put into glass globes,
with long necks, heated upon a sand-bath. The chlorine is conveyed by glass tubes
into a cylindrical stone cistern, containing milk of lime. The furnace of the sand
baths is made of cast iron, and has brick partitions, to give each retort its own fire.
The smoke of all these fires goes off by a flue into sheet iron pipes. The cistern is
made of siliceous sandstone. Its cover is of wood, coated with a resinous cement; and
it fits at its edges into grooves cut in the stone. A wheel serves to agitate the liquid
continually; its paddles being kept at two inches distance from the sides of the cistern.
The milk of lime is introduced by a funnel, and the chloride is drawn off by a
discharge pipe. I think the lead retort and agitator used in this country greatly
preferable to the experimental laboratory plan described above. In all such apparatus
we should avoid giving any pressure to the tubes or vessels, and should not
therefore dip the extremities of the gas pipes beneath the surface of the liquid, but
rather facilitate the combination of the chlorine and the lime, by enlarging the surfaces
of contact and by agitating. Intermediate vessels containing water, or the chemical
cascade of M. Clement, are very useful for absorbing any muriatic acid which may
be disengaged along with the chlorine, and thereby preventing the needless formation of
muriate of lime in the chambers or cisterns of impregnation.
When the solution of the chloride of lime is mixed with hydrate of lime, it bears,
without decomposing, a pretty high temperature, provided it be not too long continued;
it may even, in certain cases, be raised to near the boiling point without suffering a
marked loss of its discolouring power; but when the chloride is deprived of that
excess of lime, it is decomposed in a short time, even at a heat of 110° F.
When chlorine is admitted to milk of lime, it infallibly produces some muriate of
lime; but the quantity is kept at a minimum by constantly presenting an excess of
lime to the gas with the agitator, and by keeping the temperature as low as possible.
Hence the influx of gas should not be so rapid as to generate much heat. An automatic
agitator, moved by steam or water power, is therefore much better than one driven by the
hand of the operator, who is apt to intermit his labours. If the liquor becomes hot
at the end of the process, it should be immediately drawn off into large stone bottles,
and cooled. The rose-colour, which sometimes supervenes, is due to a minute quantity
of manganese. The strongest liquid chloride of lime that can be prepared will not
discolour more than 80 times its volume of Gay Lussac’s indigo test.
On acting upon cotton cloth with a concentrated solution of chloride of lime, at from
110° to 120° F., pure carbonic acid gas is disengaged, and the texture of the cloth is
injured. Here the hydrogen of the water and the cotton being seized by the chlorine,
the liberated oxygen combines with the carbon to form carbonic acid. In the discharge
troughs where printed calicoes are passed through strong solutions of chloride of lime,
stalactitic crusts of carbonate of lime come to be formed in this way.
The chlorometre of Gay Lussac consists of a test solution of indigo and a graduated
tube. One part of the best indigo, passed through a silk sieve, is to be dissolved in
nine parts of concentrated sulphuric acid, by the aid of a water-bath heat applied for
six hours. The sulphate of indigo is now to be diffused through such a body of water
that one volume of chlorine gas shall discolour exactly ten times its volume of this dilute
solution. The test liquor should be protected from the agency of light.
Mr. Crum, of Thorniebank, near Glasgow, has lately modified Dr. Dalton’s copperas
test for chloride of lime, and made it convenient to the practical man. The Doctor justly
considered that the more chlorine any bleaching powder contains, the more of the green
sulphate of iron will it convert into the red sulphate, so that we have only to add successive
portions of the chloride to a given weight of the dissolved copperas, and note
the point at which all the iron gets peroxidized. See Bleaching.
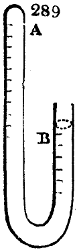
Besides the method of analysis already quoted from my memoir on the manufacture
of the chloride of lime, another occurred to me long ago, which I often practised as an
easy and expeditious test. Chlorine decomposes ammonia. If therefore water of
ammonia, faintly tinged with litmus, be added slowly to a solution of a given weight of[289]
chloride of lime, the colour will continue to disappear till the chlorine be all neutralized
by the reaction of the hydrogen of the ammonia. The quantity of liquid ammonia
of a certain strength requisite to neutralize in this way, a certain volume, say, one cubic
inch, or a thousand grain measures of chlorine gas, may be assumed as the standard of
such a chlorometer. As chlorine or chloride of lime, when mixed with water of
ammonia, causes the disengagement of azote, the quantity of this gas evolved may
also be made the foundation of an accurate and convenient chlorometer. The two substances
should be mixed over mercury, in a graduated syphon tube. The shut
end A and the open end B are both graduated to one scale; for example, to hundredths
of a cubic inch, or to grain or 10 grain measures. The tube is to be
filled with mercury, and then 10 measures of it are to be displaced at the open
end, by inserting a wooden plug. This space, being filled with the solution
of chloride of lime, is to be turned up into the shut end by covering the open
end with the finger, and inverting the tube; a few drops of water may be sent
through to wash the mercury. The ammonia being now let up, will cause
a reaction, and evolve a quantity of azote, equivalent to the chlorine present.
The action may be quickened by holding the sealed end of the tube obliquely
over a lamp heat. The mercury is protected from the chlorine by the ammonia;
and should any notion be entertained of such an action, the ammonia
may be let up first. I have made innumerable researches over mercury with
a detached apparatus of that kind, which combines precision with rapidity of
result. It was by a similar mercurial syphon that I analyzed the carbonates, as
described in the first edition of my Dictionary of Chemistry, twenty-one years ago.
M. Gay Lussac takes, as the basis of his indigo chlorometer, the fact, that one pound
of pure crystallized peroxide of manganese is capable of affording, with muriatic acid,
0·7964 parts of a pound of chlorine; or one kilogramme yields 2511⁄4 litres; that is,
one pound yields 2511⁄4 pound measures. Hence 3·98 grammes of that manganese are
capable of affording 1000 gramme measures, or 1 litre of chlorine; or, in round
numbers, 4 grains will yield 1000 grain measures. This quantity of gas, being received
into that volume of milk of lime, constitutes therefore Gay Lussac’s primary standard.
The small retort in which the manganese and muriatic acid are put, ought to be heated
to ebullition, to discharge every particle of chlorine. To prevent the manganese, in
this experiment, from sticking to the bottom in a cake, it has been proposed to mix it
previously with a little plumbago. See Chlorometry.
For preparing the chlorides of potash and soda, the same apparatus may be
employed as for the liquid chloride of lime. The alkaline solutions should be weak,
containing not more than a pound to the gallon of water. Potash liquor saturated
with chlorine, is much employed at Paris for whitening linen, under the name of the
water of Javelle, the place where it was first made as a manufacture. One hundred
parts of chlorine are said to saturate 133 parts of pure potash, and 195 of the carbonate;
but the latter should not be used for preparing the bleaching fluid, as the carbonic
acid resists the combination of the chlorine. A chloride of carbonate of soda has
been lately recommended as a disinfecting substance against contagious miasmata or
fomites. One hundred parts of chlorine will saturate 150 of the dry carbonate,
and 405 of the crystallized. M. Payen prepares this medicinal chloride by adding 138
parts of carbonate of soda to a liquid, consisting of water 1800, chloride of lime 100,
at 98° of strength, by Gay Lussac’s standard. The chloride of lime is to be dissolved,
and the sediment well washed; the carbonate of soda, dissolved by heat, is to be poured
into the solution, the precipitate allowed to subside, the clear fluid decanted, and the
solid matter washed upon a filter. The collected solutions are neutral chloride of soda.
Sixty-two parts of the carbonate of soda are then to be dissolved in the remainder of the
water, and added to the preparation; the whole being thus filtered, a limpid liquor is
obtained, indicating 5° by the hydrometer of Baumé.
The chloride of magnesia was long ago proposed by Sir H. Davy for bleaching
linen, as being preferable to chloride of lime, because the resulting muriate of
magnesia was not injurious to the fibre of cloth, as muriate of lime may be, under
certain circumstances. I prepared a quantity of chloride of magnesia, by exposing a
hydrate of that earth in the chlorine chamber of a large manufactory of chloride of
lime at Glasgow, and obtained a compound possessed of considerable discolouring
powers; but I found that the chlorine was so feebly saturated by the base, that it
destroyed the colours of fast-dyed calicoes as readily as chlorine gas or chlorine water did,
and was therefore dangerous for common bleaching, and destructive in clearing the
grounds of printed goods, which is one of the most valuable applications of the
calcareous and alkaline chlorides. The occasion of my making these experiments
was the importation of a considerable quantity of magnesite, or native atomic carbonate
of magnesia, from the district of Madras, by an enterprising friend of mine. Encouraged
by the encomiums bestowed on the chloride of magnesia by many chemical writers, he[290]
expected to have benefited both the country and himself, by bringing home the earthy
base of that compound, at a moderate price; but was disappointed to his cost.
Dr. Thomson is of opinion that the bleaching compound of lime and chlorine is not
a chloride of lime, but a combination of chlorous acid with lime and of chlorine with
calcium; consisting in its most concentrated state of
| 3 atoms of chloride of calcium |
= |
21 |
| 1 atom of chlorite of lime |
= |
11 |
| |
32 |
So that about one third of the weight is chlorite of lime, to which alone the bleaching
powers of the substance are owing. He admits a fact, rather inconsistent with this opinion,
that bleaching powder does not attract moisture from the atmosphere with nearly so
much rapidity as might be expected from a mixture containing two thirds of its weight
of so deliquescent a salt as muriate of lime; unless this indeed be prevented by the
chloride and chlorite being united into a double salt, which is a mere conjecture without
either proof or analogy. And further, when dilute sulphuric or muriatic acid is poured
upon bleaching powder, a profusion of chlorine is given out immediately, which he also
admits to be inconsistent with the notion of its being a mixture of chloride of calcium
and chlorite of lime, for no such evolution takes place when the above acids are mixed
with solutions of chloride of calcium and chlorate of potash. Though I am of opinion that
bleaching powder is simply a chloride of lime, in which the lime corresponds to the water in
the aqueous chlorine, yet I cannot see the truth or appositeness of his last reason, because
chlorine is certainly given out when chlorate of potash is acted upon by dilute muriatic
acid, as any man may prove by adding to a mixture of these two substances a vegetable
colour; for it will be speedily blanched. Dr. Thomson considers the chloride which is
at present made in Mr. Tennant’s great factory, as containing one atom of chlorine associated
with one atom of lime, or, taking his numbers, as consisting of
| Hydrate of lime |
4 |
·625 |
| Chlorine |
4 |
·5 |
Or nearly equal weights of the chlorine and the base; indicating a surprising degree
of excellence in the preparation. The average commercial samples of bleaching
powder from different factories which I examined some years ago, did not possess nearly
that strength; but varied in their quantity of chlorine from 20 to 28 per cent. In my
synthetic experiments related above, the greatest quantity of chlorine that would combine
with the atomic hydrate of lime, was in the proportion of 130 to 200; but there is no
doubt that if the lime contains additional water, it will condense more gas. I have never
seen a chloride of lime of the strength mentioned by Dr. Thomson, and I should think
there must be some fallacy in his statements. I have recorded in the paper above quoted
an experiment which proves that with additional moisture, a chloride of lime may be obtained
of the following composition:—
| Chlorine |
39·5 |
| Lime |
39·9 |
| Water |
20·6 |
| |
100·0 |
In the article Bleaching, of the Encyclopædia Britannica, Dr. Thomson deduces
from a test trial of Mr. Crum, that the best bleaching powder is a compound of 1 atom
chlorite of lime = 11, 3 atoms chloride of calcium = 21, and 8 atoms of water = 9. “But,”
adds he, “in general the whole lime is not accurately saturated with chlorine. Accordingly,
when the bleaching powder is dissolved in water a small residue almost always remains
undissolved. Unless the powder be fresh made, a portion of chlorite is always
converted into chloride of calcium. It is probable therefore that the best bleaching
powder, as it comes into the hands of the bleachers, consists of
| 1 |
atom chlorite of lime |
11 |
|
| 3 |
atoms chloride of calcium |
21 |
|
| 6 |
atoms water |
6 |
·75 |
| |
Impurity |
2 |
·25 |
| |
41 |
·00 |
“If we consider the bleaching powder as a compound of chlorine and lime, our mode of
calculating will not be altered. Instead of 1 atom chlorite of lime, and 3 atoms chloride
of calcium, we shall have 4 atoms chloride of lime, 6 atoms water, and 2·25 of impurity
as before.” In such ambiguity does this able chemist place this interesting compound,
for theoretical reasons, of which I cannot see the value. Surely there is no difficulty in
conceiving chlorine to exercise a direct attractive force towards the hydrate of lime, as it
is known to do towards each of its elementary constituents, the oxygen and the calcium.
Such refinements as the preceding tend merely to mystify a plain matter. Even the[291]
chlorous acid here brought into play to form the ideal chlorite, is by his own admission
a hypothetical being. “When chlorate of potash” says Dr. Thomson, “is mixed with
sulphuric acid, and made into small balls the size of a pea, if we expose these balls to a
heat somewhat lower than that of boiling water, a bright yellowish green gas separates,
which may be received over mercury. Its smell is peculiar and aromatic. Water absorbs
at least seven times its volume of it. It destroys vegetable blues. Its constituents are,
| 1 volume chlorine |
2 |
·5 |
or |
4 |
·5 |
| 2 volumes oxygen |
2 |
·222 |
or |
4 |
. |
Thus this compound consists in weight of chlorine 4·5, oxygen 4 = 8·5. It has been called
quarteroxide of chlorine, but it is more probably a teroxide. It has been supposed by some
to possess acid properties, and has therefore been called chlorous acid. But this is only
as yet a hypothesis.”
Surely this by the Doctor’s own showing is very slender authority for renouncing our
long-received doctrines concerning the constitution of bleaching powder. I shall
conclude by remarking that the ultra-atomists are now in a dilemma about this substance;
M. Welter, and many French chemists calling it a sub-chloride, of 1 atom
of chlorine to 2 atoms of lime, and Dr. Thomson showing that Mr. Tennant, the greatest
and best manufacturer of it, has produced it in the state of a chloride, or 1 atom of each.
The fact is, in chloride of lime, as in water of ammonia, alcohol, and muriatic acid,
there is no sufficient reason for definite proportion in any term short of saturation, and
therefore we shall find that chloride in every gradation of strength from 1 per cent. of
chlorine up to 40 per cent.—the strongest which I succeeded in preparing, though
I passed a constant stream of chlorine in great excess over a pure hydrate of lime
for upwards of 24 hours, with frequent renewal of the surface; indeed, till it refused
to absorb any more gas, as indicated by its remaining stationary in weight.
CHLOROMETRY; Chlorometrie, is the name given by the French to the process for
testing the decolouring power of any combination of chlorine, but especially of the
commercial articles, the chlorides of lime, potash, and soda. M. Gay Lussac proposed
many years ago the following graduated method of applying indigo to this purpose. As
indigo varies much in its dyeing quality, and of consequence in the proportion of chlorine
required for its decoloration, he assumes as the unity of blanching power, one litre of
chlorine gas, measured at the mean pressure of 29·6 inches, and at the temperature of
melting ice. This volume of gas, when combined with a determinate quantity of water,
is employed to test the standard solution of indigo. For this purpose a solution in sulphuric
acid of any sample of indigo is taken, and diluted with water to such a degree
that 10 measures of it, in a graduated tube, are decoloured by that one measure of combined
chlorine gas. Each measure of indigo solution so destroyed is called a degree,
and this measure being divided into five parts, the real test of chlorine is given to fiftieths,
which is sufficiently nice. For the standard of the assays, a chloride of lime as pure
and fully saturated as possible is taken, and dissolved in such a quantity of water, that
the solution shall contain, or be equivalent to, one volume of chlorine gas. Calculation
proves that this condition is exactly fulfilled by dissolving 4938 grammes of the said
chloride in half a litre of water; or in English measures, 5 gr. very nearly in 500 grain
measures of water. This solution, which serves for a type, indicates 10° in the assay,
or proof; that is to say, each single volume destroys the colour of 10 volumes of the
dilute indigo solution. It may be remarked, that a greater degree of precision is in
general attainable with a weak solution of chlorine or a chloride, for example at 4° or 5°,
than with one much stronger; consequently if, after a preliminary trial, the standard
considerably exceeds 10°, a given volume of water must be added to the solution,
and then the above proof must be taken. If the volume of water added was double,
the number of degrees afterwards found must be tripled, to obtain the true title
of the chloride. It is, however, to be observed that the degree of decoloration
varies with the time taken in making the mixture; the more slowly the chlorine
is added to the indigo, the less of it escapes into the atmosphere, and the more
effective it becomes in destroying the colour. The best mode of obtaining comparable
results, is to pour suddenly into the test quantity of chlorine the whole volume of the
indigo solution likely to be decoloured; but it is requisite to find approximately beforehand,
what quantity of indigo-blue will probably be destroyed. When it comes to the
verge of destruction, it is green; but yellowish-brown when entirely decomposed.
I have tried the indigo test in many ways, but never could confide in it. The sulphuric
solution of indigo is very liable to change by keeping, and thus to lead to erroneous
results. The method of testing the chlorides by green sulphate of iron, described
under bleaching, is in my opinion preferable to the above.
M. Gay Lussac has recently proposed another proof of chlorine, founded on the same
principle as that by green vitriol, namely, the quantity of it requisite to raise a metallic
substance from a lower to a higher stage of oxidizement. He now prescribes as the
preferable plan of chlorometry, to pour very slowly from a graduated glass tube, a[292]
standard solution of the chloride, to be tested upon a determinate quantity of arsenious acid
dissolved in muriatic acid, till the whole arsenious be converted into the arsenic acid. The
value of the chloride is greater the less of it is required to produce this effect. It is easy
to recognize, by a few drops of solution of indigo, the instant when all the arsenious
acid has disappeared; for then the blue tint is immediately effaced, and cannot be restored
by the addition of a fresh drop of the indigo solution.
In graduating the arsenical chlorometer, M. Gay Lussac takes for his unity the decolouring
power of one volume of chlorine at 32° Fahr., and divides it into 100 parts.
Suppose that we prepare a solution of chlorine containing its own volume of the gas, and an
arsenious solution, such, that under a like volume, the two solutions shall reciprocally
destroy each other. Let us call the first, the normal solution of chlorine, and the second,
the normal arsenious solution. We shall fix at 10 grammes the weight of chloride of lime
subjected to trial; and dissolve it in water, so that the total volume of the solution shall
be a litre (1000 grammes measure), including the sediment. If we take a constant
volume of this solution, 10 centimetres cube (10 gramme measures), for example, divided
into 100 equal parts, and pour into it gradually the arsenious solution (measured
by like portions), till the chlorine be destroyed, the bleaching power will be
proportional to the number of portions of the arsenious solution, which the chloride
shall have required. If the chloride has destroyed 100 portions of the arsenious solution,
its title will be 100; if it has destroyed 80 portions, its title will be 80, &c. and so forth.
On pouring the acidulous arsenious solution into the chloride of lime, this will become
very acid; the chlorine will be emitted abundantly, and the proof will be quite incorrect.
If, on the contrary, we pour the solution of the chloride of lime into the arsenious solution,
this evil will not occur, since the chlorine will always find plenty of arsenious acid
to act upon, whatever be the dilution of the one or the other; but in this case, the standard
of the chlorine is not given directly, as it is in the inverse ratio of the number of
portions which are required to destroy the measures of the arsenious solution. If 50
portions of the chloride have been required, the proof will be 100 × 10050 = 200°; if 200
have been required, the proof will be 100 × 100200 = 50°, &c. This evil is not, however,
very serious, since we have merely to consult a table, in which we can find the proof
corresponding to each volume of the chloride employed for destroying the constant
measure of the arsenious solution. The arsenious solution should be slightly tinged with
sulphate of indigo, so as to show, by the disappearance of the colour, the precise point or
instant of its saturation with chlorine, that is, its conversion into arsenic acid. If the
arsenious acid be pure, the normal solution may be made directly by dissolving 4·439
grammes of it in muriatic acid (free from sulphurous acid), and diluting the solution
till it occupies one litre, or 1000 grammes measure. Annales de Chimie et Physique,
LX. 225.
CHOCOLATE. Is an alimentary preparation of very ancient use in Mexico, from
which country it was introduced into Europe by the Spaniards in the year 1520, and
by them long kept a secret from the rest of the world. Linnæus was so fond of it, that
he gave the specific name, theobroma, food of the gods, to the cacao tree which produced
it. The cacao-beans lie in a fruit somewhat like a cucumber, about 5 inches long and
31⁄2 thick, which contains from 20 to 30 beans, arranged in 5 regular rows with partitions
between, and which are surrounded with a rose-coloured spongy substance, like
that of water-melons. There are fruits, however, so large as to contain from 40 to 50
beans. Those grown in the West India islands, Berbice and Demerara, are much
smaller, and have only from 6 to 15; their development being less perfect than in
South America. After the maturation of the fruit, when their green colour has changed
to a dark yellow, they are plucked, opened, their beans cleared of the marrowy substance,
and spread out to dry in the air. Like almonds, they are covered with a thin
skin or husk. In the West Indies they are immediately packed up for the market
when they are dried; but in the Caraccas they are subjected to a species of slight fermentation,
by putting them into tubs or chests, covering them with boards or stones,
and turning them over every morning, to equalize the operation. They emit a good
deal of moisture, lose the natural bitterness and acrimony of their taste by this process,
as well as some of their weight. Instead of wooden tubs, pits or trenches dug in the
ground are sometimes had recourse to for curing the beans; an operation called earthing
(terrer). They are lastly exposed to the sun, and dried. The latter kind are reckoned
the best; being larger, rougher, of a darker brown colour, and, when roasted, throw off
their husk readily, and split into several irregular fragments; they have an agreeable
mild bitterish taste, without acrimony. The Guiana and West India sorts are smaller,
flatter, smoother-skinned, lighter coloured, more sharp and bitter to the taste. They
answer best for the extraction of the butter of cacao, but afford a less aromatic and
agreeable chocolate. According to Lampadius, the kernels of the West India cacao
beans contain, in 100 parts, besides water, 53·1 of fat or oil, 16·7 of an albuminous
brown matter, which contains all the aroma of the bean, 10·91 of starch, 73⁄4 of gum or[293]
mucilage, 0·9 of lignine, and 2·01 of a reddish dye stuff somewhat akin to the pigment of
cochineal. The husks form 12 per cent. of the weight of the beans; they contain no fat,
but, besides lignine, or woody fibre, which constitutes half their weight, they yield a
light brown mucilaginous extract by boiling in water. The fatty matter is of the consistence
of tallow, white, of a mild agreeable taste, called butter of cacao, and not apt to
turn rancid by keeping. It melts only at 122° Fahr., and should, therefore, make tolerable
candles. It is soluble in boiling alcohol, but precipitates in the cold. It is
obtained by exposing the beans to strong pressure in canvass bags, after they have been
steamed or soaked in boiling water for some time. From 5 to 6 ounces of butter may
be thus obtained from a pound of cacao. It has a reddish tinge when first expressed,
but it becomes white by boiling with water.
The beans, being freed from all spoiled and mouldy portions, are to be gently roasted
over a fire in an iron cylinder, with holes in its ends for allowing the vapours to escape;
the apparatus being similar to a coffee-roaster. When the aroma begins to be well
developed, the roasting is known to be finished; and the beans must be turned out,
cooled, and freed by fanning and sifting from their husks. The kernels are then
to be converted into a paste, either by trituration in a mortar heated to 130° F., or
by the following ingenious and powerful machine. The chocolate paste has usually
in France a little vanilla incorporated with it, and a considerable quantity of sugar,
which varies from one third of its weight to equal parts. For a pound and a half of
cacao, one pod of vanilla is sufficient. Chocolate paste improves in its flavour by keeping,
and should therefore be made in large quantities at a time. But the roasted beans
soon lose their aroma, if exposed to the air.
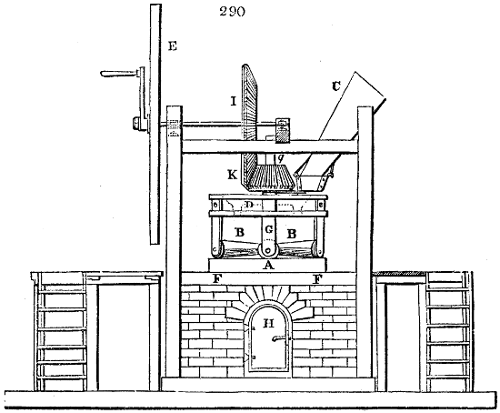
Fig. 290. represents the chocolate mill. Upon the sole A, made of marble, six conical
rollers B B, are made to run by the revolution of the upright axis or shaft q, driven by
the agency of the fly wheel E and bevel wheels I K. The sole A rests upon a strong
iron plate, which is heated by a small stove, introduced at the door H. The wooden
frame work F, forms a ledge, a few inches high, round the marble slab, to confine
the cocoa in the act of trituration. C is the hopper of the mill through which the
roasted beans are introduced to the action of the rollers, passing first into the flat
vessel D to be thence evenly distributed. After the cacao has received the first trituration,
the paste is returned upon the slab, in order to be mixed with the proper quantity
of sugar, and vanilla, previously sliced and ground up with a little hard sugar. When
the chocolate is sufficiently worked, and while it is thin with the heat and trituration,
it must be put carefully into the proper moulds. If introduced too warm, it will be
apt to become damp and dull on the surface; and, if too cold, it will not take the
proper form. It must be previously well kneaded with the hands to ensure the expulsion
of every air bubble.
In Barcelona, chocolate mills on this construction are very common, but they are
turned by a horse-gin set to work in the under story, corresponding to H in the above
figure. The shaft G is, in this case, extended down through the marble slab, and is[294]
surrounded at its centre with a hoop to prevent the paste coming into contact with it.
Each of these horse-mills turns out about ten pounds of fine chocolate in the hour, from
a slab two feet seven inches in diameter.
Chocolate is flavoured with cinnamon and cloves, in several countries, instead of the
more expensive vanilla. In roasting the beans the heat should be at first very slow, to
give time to the humidity to escape; a quick fire hardens the surface, and injures the
process. In putting the paste into the tin plate, or other moulds, it must be well
shaken down to insure its filling up all the cavities, and giving the sharp and polished
impression so much admired by connoisseurs. Chocolate is sometimes adulterated
with starch; in which case it will form a pasty consistenced mass when treated with
boiling water. The harder the slab upon which the beans are triturated, the better;
and hence porphyry is far preferable to marble. The grinding rollers of the mill should
be made of iron, and kept very clean.
CHROMATES, saline compounds of chromic acid with the bases. See Chromium.
CHROMIC ACID; see Chromium.
CHROMIUM. The only ore of this metal, which occurs in sufficient abundance
for the purposes of art, is the octohedral chrome-ore, commonly called chromate of iron,
though it is rather a compound of the oxides of chromium and iron. The fracture of
this mineral is uneven; its lustre imperfect metallic; its colour between iron-black and
brownish-black, and its streak brown. Its specific gravity, in the purest state, rises
to 4·5; but the usual chrome-ore found in the market varies from 3 to 4. According
to Klaproth, this ore consists of oxide of chromium, 43; protoxide of iron, 34·7;
alumina, 20·3; and silica, 2; but Vauquelin’s analysis of another specimen gave as
above, respectively, 55·5, 33, 6, and 2. It is infusible before the blowpipe; but it acts
upon the magnetic needle, after having been exposed to the reducing smoky flame. It
is entirely soluble in borax, at a high blowpipe heat, and imparts to it a beautiful
green colour.
Chrome-ore is found at the Bare Hills, near Baltimore, in Maryland; in the Shetland
isles, Unst and Fetlar; the department of Var, in France, in small quantity; and
near Portsoy, in Banffshire; as also in Silesia and Bohemia.
The chief application of this ore is to the production of chromate of potash, from
which salt the various other preparations of this metal used in the arts are obtained.
The ore, freed, as well as possible, from its gangue, is reduced to a fine powder, by
being ground in a mill under ponderous edge-wheels, and sifted. It is then mixed
with one third or one half its weight of coarsely bruised nitre, and exposed to a
powerful heat, for several hours, on a reverberatory hearth, where it is stirred about
occasionally. In the large manufactories of this country, the ignition of the above
mixture in pots is laid aside, as too operose and expensive. The calcined matter is raked
out, and lixiviated with water. The bright yellow solution is then evaporated briskly,
and the chromate of potash falls down in the form of a granular salt, which is lifted
out from time to time from the bottom with a large ladle, perforated with small holes,
and thrown into a draining-box. This saline powder may be formed into regular crystals
of neutral chromate of potash, by solution in water and slow evaporation; or it may be
converted into a more beautiful crystalline body, the bichromate of potash, by treating
its concentrated solution with nitric, muriatic, sulphuric, or acetic acid, or, indeed, any
acid exercising a stronger affinity for the second atom of the potash than the chromic
acid does.
Bichromate of potash, by evaporation of the above solution, and slow cooling, may be
obtained in the form of square tables, with bevelled edges, or flat four-sided prisms.
They are permanent in the air, have a metallic and bitter taste, and dissolve in about one
tenth of their weight of water, at 60° F.; but in one half of their weight of boiling water.
They consist of chromic acid 13, potash 6; or, in 100 parts, 68·4 + 31·6. This salt is
much employed in calico-printing and in dyeing; which see.
Chromate of lead, the chrome-yellow of the painter, is a rich pigment of various
shades, from deep orange to the palest canary yellow. It is made by adding a limpid
solution of the neutral chromate (the above granular salt), to a solution, equally limpid,
of acetate or nitrate of lead. A precipitate falls, which must be well washed, and carefully
dried out of the reach of any sulphuretted vapours. A lighter shade of yellow is
obtained by mixing some solution of alum, or sulphuric acid, with the chromate, before
pouring it into the solution of lead; and an orange tint is to be procured by the addition
of subacetate of lead, in any desired proportion.
For the production of chromate of potash from chrome ore, various other processes
have been recommended. The following formulæ, which have been verified in practice,
will prove useful to the manufacturers of this important article:—
| I. |
Two parts of chrome ore, containing about 50 per cent. of protoxide of chromium: |
| |
One part of saltpetre.[295] |
| II. |
Four parts of chrome ore, containing 34 per cent. of protoxide of chromium. |
| |
Two parts of potashes. |
| |
One part of saltpetre. |
| III. |
Four parts of chrome ore,cont—ining34per cent. of pro— |
| |
Two of potashes. |
| |
Four tenths of a part of peroxide of manganese. |
| IV. |
Three parts of chrome ore. |
| |
Four parts of saltpetre. |
| |
Two parts of argal. |
Some manufacturers have contrived to effect the conversion of the oxide into an
acid, and of course to form the chromate of potash, by the agency of potash alone, in a
calcining furnace, or in earthen pots fired in a pottery kiln.
After lixiviating the calcined mixtures with water, if the solution be a tolerably pure
chromate of potash, its value may be inferred, from its specific gravity, by the following
table:—
| At specific gravity |
1·28 |
it contains about |
50 |
per cent. of the salt. |
| |
1·21 |
|
33 |
|
| |
1·18 |
|
25 |
|
| |
1·15 |
|
20 |
|
| |
1·12 |
|
16 |
|
| |
1·11 |
|
14 |
|
| |
1·10 |
|
12 |
|
In making the red bichromate of potash from these solutions of the yellow salt, nitric
acid was at first chiefly used; but, in consequence of its relatively high price, sulphuric,
muriatic or acetic acid has been frequently substituted upon the great scale.
There is another application of chrome which merits some notice here; that of its green
oxide to dyeing and painting on porcelain. This oxide may be prepared by decomposing,
with heat, the chromate of mercury, a salt made by adding to nitrate of protoxide of mercury,
chromate of potash, in equivalent proportions. This chromate has a fine cinnabar
red, when pure; and, at a dull red heat, parts with a portion of its oxygen and its
mercurial oxide. From M. Dulong’s experiments it would appear, that the purest
chromate of mercury is not the best adapted for preparing the oxide of chrome to be
used in porcelain painting. He thinks it ought to contain a little oxide of manganese
and chromate of potash, to afford a green colour of a fine tint, especially for pieces that
are to receive a powerful heat. Pure oxide of chrome preserves its colour well enough
in a muffle furnace; but, under a stronger fire, it takes a dead-leaf colour.
The green oxide of chrome has come so extensively into use as an enamel colour for
porcelain, that a fuller account of the best modes of manufacturing it must prove acceptable
to many of my readers.
That oxide, in combination with water, called the hydrate, may be economically
prepared by boiling chromate of potash, dissolved in water, with half its weight of
flowers of sulphur, till the resulting green precipitate ceases to increase, which may be
easily ascertained by filtering a little of the mixture. The addition of some potash
accelerates the operation. This consists in combining the sulphur with the oxygen of
the chromic acid, so as to form sulphuric acid, which unites with the potash of the
chromate into sulphate of potash, while the chrome oxide becomes a hydrate. An
extra quantity of potash facilitates the deoxidizement of the chromic acid by the formation
of hyposulphite and sulphuret of potash, both of which have a strong attraction
for oxygen. For this purpose the clear lixivium of the chromate of potash is sufficiently
pure, though it should hold some alumina and silica in solution, as it generally does.
The hydrate may be freed from particles of sulphur by heating dilute sulphuric acid
upon it, which dissolves it; after which it may be precipitated, in the state of a
carbonate, by carbonate of potash, not added in excess.
By calcining a mixture of bichromate of potash and sulphur in a crucible, chromic
acid is also decomposed, and a hydrated oxide may be obtained; the sulphur being
partly converted into sulphuret of potassium, and partly into sulphuric acid (at the
expense of the chromic acid), which combines with the rest of the potash into a
sulphate. By careful lixiviation, these two new compounds may be washed away, and
the chrome green may be freed from the remaining sulphur, by a slight heat.
Liebig and Wöhler have lately contrived a process for producing a subchromate of
lead of a beautiful vermillion hue. Into saltpetre, brought to fusion in a crucible at
a gentle heat, pure chrome yellow is to be thrown by small portions at a time. A
strong ebullition takes place at each addition, and the mass becomes black, and continues
so while it is hot. The chrome yellow is to be added till little of the saltpetre
remains undecomposed, care being taken not to overheat the crucible, lest the colour
of the mixture should become brown. Having allowed it to settle for a few minutes,
during which the dense basic salt falls to the bottom, the fluid part, consisting of[296]
chromate of potash and saltpetre, is to be poured off, and it can be employed again in
preparing chrome yellow. The mass remaining in the crucible is to be washed with
water, and the chrome red being separated from the other matters, is to be dried after
proper edulcoration. It is essential for the beauty of the colour, that the saline solution
should not stand long over the red powder, because the colour is thus apt to become of
a dull orange hue. The fine crystalline powder subsides so quickly to the bottom after
every ablution, that the above precaution may be easily observed.
As Chromic Acid will probably ere long become an object of interest to the calico
printer, I shall describe here the best method of preparing it. To 100 parts of yellow
chromate of potash, add 136 of nitrate of barytes, each in solution. A precipitate of the
yellow chromate of barytes falls, which being washed and dried would amount to 130
parts. But while still moist it is to be dissolved in water by the intervention of a little
nitric acid, and then decomposed by the addition of the requisite quantity of sulphuric
acid, whereby the barytes is separated, and the chromic acid remains associated with the
nitric acid, from which it can be freed by evaporation to dryness. On re-dissolving the
chromic acid residuum in water, filtering and evaporating to a proper degree, 50 parts
of chromic acid may be obtained in crystals.
This acid may also be obtained from chromate of lime, formed by mixing chromate of
potash and muriate of lime; washing the insoluble chromate of lime which precipitates,
and decomposing it by the equivalent quantity of oxalic acid, or for ordinary purposes
even sulphuric acid may be employed.
Chromic acid is obtained in quadrangular crystals, of a deep red colour; it has a very
acrid and styptic taste. It reddens powerfully litmus paper. It is deliquescent in the air.
When heated to redness, it emits oxygen and passes into the deutoxide. When a little of
it is fused along with vitreous borax, the compound assumes an emerald green colour.
As chromic acid parts with its last dose of oxygen very easily, it is capable in certain
styles of calico printing of becoming a valuable substitute for chlorine where this more
powerful substance would not from peculiar circumstances be admissible. For this ingenious
application, the arts are indebted to that truly scientific manufacturer, M. Daniel
Kœchlin, of Mulhouse. He discovered that whenever chromate of potash has its acid
set free by its being mixed with tartaric or oxalic acid, or a neutral vegetable substance,
(starch or sugar for example), and a mineral acid, a very lively action is produced, with
disengagement of heat, and of several gases. The result of this decomposition is the active
reagent, chromic acid, possessing valuable properties to the printer. Watery solutions
of chromate of potash and tartaric acid being mixed, an effervescence is produced which
has the power of destroying vegetable colours. But this power lasts no longer than the
effervescence. The mineral acids react upon the chromate of potash only when
vegetable colouring matter, gum, starch, or a vegetable acid are present, to determine the
disengagement of gas. During this curious change carbonic acid is evolved; and when it
takes place in a retort, there is condensed in the receiver a colourless liquid, slightly
acid, exhaling somewhat of the smell of vinegar, and containing a little empyreumatic
oil. This liquid heated with the nitrates of mercury or silver reduces these metals.
On these principles M. Kœchlin discharged indigo blue by passing the cloth through a
solution of chromate of potash, and printing nitric acid thickened with gum upon certain
spots. It is probable that the employment of chromic acid would supersede the
necessity of having recourse in many cases to the more corrosive chlorine.
The following directions have been given for the preparation of a blue oxide of chrome.
The concentrated alkaline solution of chromate of potash is to be saturated with weak
sulphuric acid, and then to every 8 lbs. is to be added 1 lb. of common salt, and half-a-pound
of concentrated sulphuric acid; the liquid will now acquire a green colour. To be
certain that the yellow colour is totally destroyed, a small quantity of the liquor is to
have potash added to it, and filtered; if the fluid is still yellow, a fresh portion of salt
and of sulphuric acid is to be added: the fluid is then to be evaporated to dryness, redissolved,
and filtered; the oxide of chrome is finally to be precipitated by caustic potash.
It will be of a greenish-blue colour, and being washed, must be collected upon a filter.
Chromate of Potash, adulteration of, to detect. The chromate of potash has the
power of combining with other salts up to a certain extent without any very sensible
change in its form and appearance; and hence it has been sent into the market falsified
by very considerable quantities of sulphate and muriate of potash, the presence of
which has often escaped observation, to the great loss of the dyers who use it so extensively.
The following test process has been devised by M. Zuber, of Mulhouse.
Add a large excess of tartaric acid to the chromate in question, which will decompose
it, and produce in a few minutes a deep amethyst colour. The supernatant liquor
will, if the chromate be pure, afford now no precipitate with the nitrates of barytes or
silver; whence the absence of the sulphates and muriates may be inferred. We must,
however, use dilute solutions of the chromate and acid, lest bitartrate of potash be precipitated,
which will take place if less than 60 parts of water be employed. Nor must[297]
we test the liquid till the decomposition be complete, and till the colour verge rather
towards the green than the yellow. Eight parts of tartaric acid should be added to one
of chromate to obtain a sure and rapid result. If nitrate of potash (saltpetre) is the
adulterating ingredient, it may be detected by throwing it on burning coals, when
deflagration will ensue. The green colour is a certain mark of the transformation of the
chromic acid partially into the chrome oxide; which is effected equally by the sulphurous
acid and sulphuretted hydrogen. Here this metallic acid is disoxygenated by the
tartaric, as has been long known. The tests which I should prefer, are the nitrates of
silver and baryta, having previously added so much nitric acid to the solution of the suspected
chromate, as to prevent the precipitation of the chromate of silver or baryta.
The smallest adulteration by sulphates or muriates will thus be detected.
CINNABAR; the native red sulphuret of mercury. It occurs sometimes crystallized
in rhomboids; has a specific gravity varying from 6·7 to 8·2; a flat conchoidal
fracture; is fine grained; opaque; has an adamantine lustre, and a colour passing from
cochineal to ruby red. The fibrous and earthy cinnabar has a scarlet hue. It is met
with disseminated in smaller or larger lumps in veins, which are surrounded by a black
clay, and is associated with native quicksilver, amalgam with iron-ore, lead-glance,
blende, copper-ore, gold, &c. Its principal localities are Almaden in Spain, Idria in
the Schiefergebirge, Kremnitz and Schemnitz in Hungary; in Saxony, Bavaria,
Bohemia, Nassau, China, Japan, Mexico, Columbia, Peru. It consists of two primes
of sulphur, = 32·240, combined with one of mercury, = 202,863; or in 100 parts of
12·7 sulphur + 87·3 mercury. It is the most prolific ore of this metal; and is easily
smelted by exposing a mixture of it with iron or lime to a red heat in retorts. Factitious
cinnabar is called in commerce Vermillion, which see, as also Mercury.
CINNAMON. (Cannelle, Fr.; Zimmt, Germ.) Is the inner bark of the laurus cinnamomum,
a handsome-looking tree, which grows naturally to the height of 18 or 20 feet,
in Java, Sumatra, Ceylon, and other islands in the East Indian seas. It has been transplanted
to the Antilles, particularly Guadaloupe and Martinique, as well as Cayenne,
but there it produces a bark of very inferior value to the Oriental.
Cinnamon is gathered twice a year, but not till after the tree has attained to a certain
age and maturity. The young twigs yield a bark of better quality than the larger
branches. The first and chief harvest takes place from April to August; the second,
from November to January. After having selected the proper trees, all the branches
more than three years old are cut off; the epidermis is first removed with a two-edged
pruning knife, then a longitudinal incision is made through the whole extent of the
bark, and lastly, with the bluntest part of the knife, the true bark is carefully stripped
off in one piece. All these pieces of bark are collected, the smaller ones are laid within
the larger, and in this state they are exposed to the sun, whereby in the progress of
drying, they become rolled into the shape of a quill. These convoluted pieces are
formed into oblong bundles of 20 or 30 lbs. weight, which are placed in warehouses,
sorted and covered with mats. Good cinnamon should be as thin as paper, have its
peculiar aromatic taste, without burning the tongue, and leave a sweetish flavour in the
mouth. The broken bits of cinnamon are used in Ceylon for procuring the essential
oil by distillation. 445,367 lbs. of cinnamon were imported into this kingdom in 1835,
of which 16,604 only were retained for internal consumption.
CITRIC ACID. (Acide citrique, Fr.; Citronensäure, Germ.) Scheele first procured
this acid in its pure state from lemon juice, by the following process. The juice put into a
large tub, is to be saturated with dry chalk in fine powder, noting carefully the quantity
employed. The citrate of lime which precipitates being freed from the supernatant foul
liquor, is to be well washed with repeated affusion and decantation of water. For every 10
pounds of chalk employed, nine and a half pounds of sulphuric acid, diluted with six
times its weight of water, are to be poured while warm upon the citrate of lime, and
well mixed with it. At the end of twelve hours, or even sooner, the citrate will be all
decomposed, dilute citric acid will float above, and sulphate of lime will be found at
the bottom. The acid being drawn off, the calcareous sulphate must be thrown on a
canvass filter, drained, and then washed with water to abstract the whole acid.
The citric acid thus obtained may be evaporated in leaden pans, over a naked fire till
it acquires the specific gravity 1·13; after which it must be transferred into another vessel,
evaporated by a steam or water bath till it assumes a syrupy aspect, when a pellicle
appears first in patches, and then over the whole surface. This point must be watched
with great circumspection, for if it be passed, the whole acid runs a risk of being spoiled
by carbonization. The steam or hot water must be instantly withdrawn, and the concentrated
acid put into a crystallizing vessel in a dry, but not very cold apartment. At
the end of four days, the crystallization will be complete. The crystals must be drained,
re-dissolved in a small portion of water, the solution set aside to settle its impurities,
then decanted, re-evaporated, and re-crystallized. A third or fourth crystallization may
be necessary to obtain a colourless acid.
[298]
If any citrate of lime be left undecomposed by the sulphuric acid, it will dissolve in
the citric acid, and obstruct its crystallization, and hence it will be safer to use the
slightest excess of sulphuric acid, than to leave any citrate undecomposed. There should
not however be any great excess of sulphuric acid. If there be, it is easily detected by
nitrate of barytes, but not by the acetate of lead as prescribed by some chemical authors;
because the citrate of lead is not very soluble in the nitric acid, and might thus be confounded
with the sulphate, whereas citrate of barytes is perfectly soluble in that test
acid. Sometimes a little nitric acid is added with advantage to the solution of the
coloured crystals, with the effect of whitening them.
Twenty gallons of good lemon juice will afford fully ten pounds of white crystals of
citric acid.
Attempts were made both in the West Indies and Sicily, to convert the lime and
lemon juice into citrate of lime, but they seem to have failed through the difficulty of
drying the citrate for shipment.
The crystals of citric acid are oblique prisms with four faces, terminated by dihedral
summits, inclined at acute angles. Their specific gravity is 1·617. They are unalterable
in the air. When heated, they melt in their water of crystallization; and at a
higher heat, they are decomposed. They contain 18 per cent. of water, of which one
half may be separated in a dry atmosphere, at about 100° F., when the crystals fall into
a white powder.
Citric acid in crystals is composed by my analysis of carbon, 35·8, oxygen 59·7, and
hydrogen 45; results which differ very little from those of Dr. Prout, subsequently
obtained. I found its atomic weight to be 8·375, compared to oxygen 1,000. I cannot
account for Berzelius’s statements relative to the composition of this acid.
Citric acid in somewhat crude crystals is employed with much advantage in calico-printing.
If adulterated with tartaric acid, the fraud may be detected by adding potash
to the solution of the acid, which will occasion a precipitate of cream of tartar.
CIVET. (Civette, Fr.; Zibeth, Germ.) This substance approaches in smell to
musk and ambergris; it has a pale yellow colour, a somewhat acrid taste, a consistence
like that of honey, and a very strong aromatic odour. It is the product of two small
quadrupeds of the genus viverra (v. zibetha and v. civetta), of which the one inhabits
Africa, the other Asia. They are reared with tenderness, especially in Abyssinia.
The civet is contained in a sac, situated between the anus and the parts of generation,
in either sex. The animal frees itself from an excess of this secretion by a contractile
movement which it exercises upon the sac, when the civet issues in a vermicular
form, and is carefully collected. The negroes are accustomed to increase the secretion
by irritating the animal; and likewise introduce a little butter, or other grease, by the
natural slit in the bag, which mixes with the odoriferous substance, and increases its
weight. It is employed only in perfumery.
According to M. Boutron-Chalard, it contains a volatile oil, to which it owes its
smell, some free ammonia, resin, fat, an extractiform matter, and mucus. It affords, by
calcination, an ash, in which there are some carbonate and sulphate of potash, phosphate
of lime, and oxide of iron.
CLAY (Argile, Fr.; Thon, Germ.) is a mixture of the two simple earths, alumina
and silica, generally tinged with iron. Lime, magnesia, with some other colouring metallic
oxides, are occasionally present in small quantities in certain natural clays.
The different varieties of clay possess the following common characters:—
1. They are readily diffusible through water, and are capable of forming with it a
plastic ductile mass, which may be kneaded by hand into any shape. This plasticity
exists, however, in very different degrees in the different clays.
2. They concrete into a hard mass upon being dried, and assume, upon exposure to
the heat of ignition, a degree of hardness sometimes so great as to give sparks by collision
with hardened steel. In this state they are no longer plastic with water, even
when pulverised. Tolerably pure clays, though infusible in the furnace, become readily
so by the admixture of lime, iron, manganese, &c.
3. All clays, even when previously freed from moisture, shrink in the fire in virtue
of the reciprocal affinity of their particles; they are very absorbent of water in their
dry state, and adhere strongly to the tongue.
4. Ochrey, impure clays emit a disagreeable earthy smell when breathed upon.
Brongniart distributes the clays into:—
1. Fire-clays, (argiles apyres, Fr.; feuerfeste, Germ.)
2. Fusible, (schmelzbare, Germ.)
3. Effervescing (brausende, Germ.), from the presence of chalk.
4. Ochrey (ocreuses, Fr.; ockrige, Germ.)
Fire-clay is found in the greatest abundance and perfection for manufacturing purposes
in,
1. Slate-clay. (Thon-schiefer, Germ.) Its colour is gray or grayish-yellow. Massive,[299]
dull, or glimmering from admixture of particles of mica. Fracture slaty, approaching
sometimes to earthy. Fragments tabular. Soft, sectile, and easily broken. Sp. gr. = 2·6.
Adheres to the tongue, and breaks down in water. It occurs along with pit coal; which
see. Slate-clay is ground, and reduced into a paste with water, for making fire-bricks;
for which purpose it should be as free as possible from lime and iron.
2. Common clay or loam.—This is an impure coarse pottery clay, mixed with iron
ochre, and occasionally with mica. It has many of the external characters of plastic
clay. It is soft to the touch, and forms, with water, a somewhat tenacious paste;
but is in general less compact, more friable, than the plastic clays, which are more
readily diffusible in water. It does not possess the property of acquiring in water
that commencement of translucency which the purer clays exhibit. Although soft
to the touch, the common clay wants unctuosity, properly so called. The best example
of this argillaceous substance is afforded in the London clay formation, which
consists chiefly of bluish or blackish clay, mostly very tough. Those of its strata which
effervesce with acids partake of the nature of marl. This clay is fusible at a strong heat,
in consequence of the iron and lime which it contains. It is employed in the manufacture
of bricks, tiles, and coarse pottery ware.
3. Potter’s clay, or Plastic clay.—This species is compact, soft, or even unctuous to
the touch, and polishes with the pressure of the finger; it forms, with water, a tenacious,
very ductile, and somewhat translucent paste. It is infusible in a porcelain kiln,
but assumes in it a great degree of hardness. Werner calls it pipe-clay. Good plastic
clay remains white, or if gray before, becomes white in the porcelain kiln.
The geological position of the plastic clay is beneath the London clay, and above the
sand which covers the chalk formation. The plastic clay of the Paris basin is described as
consisting of two beds separated by a bed of sand. The lower bed is the proper plastic
clay. The plastic clay of Abondant, near the forest of Dreux, analysed by Vauquelin,
gave—
Silica, 43·5; alumina, 33·2; lime, 0·35; iron, 1; water, 18.
This clay is employed as a fire clay for making the bungs or seggars, or coarse earthenware
cases, in which china ware is fired.
The plastic clay of Dorsetshire and Devonshire supplies the great Staffordshire potteries.
It is gray coloured, less unctuous than that of Dreux, and consequently more
friable. It becomes white in the pottery kiln, and is infusible at that heat. It causes
no effervescence with nitric acid, but falls down quickly in it, and becomes higher coloured.
Its refractoriness allows of a harder glaze being applied to the ware formed
from it without risk of the heat requisite for making the glaze flow, affecting the biscuit
either in shape or colour. “Most of the plastic clays of France,” says M. Brongniart,
“employed for the same ware, have the disadvantage of reddening a little in a somewhat
strong heat; and hence it becomes necessary to coat them with a soft glaze, fusible by
means of excess of lead at a low heat, in order to preserve the white appearance of the
biscuit. Such a glaze has a dull aspect, and cracks readily into innumerable fissures by
alternations of hot and cold water.” Hence one reason of the vast inferiority of the
French stone-ware to the English.
4. Porcelain clay or Kaolin earth.—The Kaolins possess very characteristic properties.
They are friable in the hand, meagre to the touch, and difficultly form a paste
with water. When freed from the coarse and evidently foreign particles interspersed
through them, they are absolutely infusible in the porcelain kiln, and retain their white
colour unaltered. They harden with heat like other clays, and perhaps in a greater degree;
but they do not acquire an equal condensation or solidity, at least when they are
perfectly pure. The Kaolins in general appear to consist of alumina and silica in nearly
equal proportions. Most of the Kaolin clays contain some spangles of mica which betray
their origin from disintegrated granite.
This origin may be regarded as one of their most distinctive features. Almost all
the porcelain clays are evidently derived from the decomposition of the felspars, granites,
and principally those rocks of felspar and quartz, called graphic granite. Hence, they
are to be found only in primitive mountain districts, among banks or blocks of granite,
forming thin seams or partings between them. In the same partings, quartz and mica
occur, being relics of the granite; while some seams of Kaolin retain the external form
of felspar.
The most valuable Kaolins have been found:—
In China and Japan. The specimens imported from these countries appear pretty
white; but are more unctuous to the touch, and more micaceous than the porcelain clays
of France.
In Saxony. The Kaolin employed in the porcelain manufactories of that country
has a slight yellow or flesh colour, which disappears in the kiln, proving as Wallerius
observed, that this tint is not owing to any metallic matter.
In France, at Saint-Yriex-la-Perche, about 10 leagues from Limoges. The Kaolin[300]
occurs there in a bed, or perhaps a vein of beds of granite, or rather of that felspar rock
called Pe-tun-tse, which exists here in every stage of decomposition. This Kaolin is
generally white, but sometimes a little yellowish with hardly any mica. It is meagre
to the touch, and some beds include large grains of quartz, called pebbly by the China
manufacturers. This variety, when ground, affords, without the addition of any fusible
ingredient, a very transparent porcelain.
Near Bayonne. A Kaolin possessing the lamellated structure of felspar, in many
places. The rock containing it is a graphic granite in every stage of decomposition.
In England, in the county of Cornwall. This Kaolin or China clay is very white,
and more unctuous to the touch than those upon the continent of Europe mentioned
above. Like them it results from the decomposition of the felspars and granites, occurring
in the middle of these rocks. Mr. Wedgewood found it to contain 60 of alumina
or pure clay, and 40 of silica, in 100 parts.
Pure clay, the alumina of the chemist, is absolutely infusible; but when subjected to
the fire of a porcelain kiln, it contracts into about one half of its total bulk. It must,
however, be heated very cautiously, otherwise it will decrepitate and fly in pieces, owing
to the sudden expansion into steam of the water combined with its particles, which is
retained with a considerable attractive force. It possesses little plasticity, and consequently
affords a very short paste, which is apt to crack when kneaded into a
cake.
It is not only infusible by itself, but it will not dissolve in the fusible glasses;
making them merely opaque. If either lime or silica be added separately to pure clay,
in any proportion, the mixture will not melt in the most violent furnace; but if alumina,
lime, and silica be mixed together, the whole melts, and the more readily, the nearer the
mixture approaches to the following proportions:—1 of alumina, 1 of lime, and 3 of
sand. If the sand be increased to five parts, the compound becomes infusible. These
interesting facts show the reciprocal action of those earths which are mixed most commonly
in nature with alumina.
Iron in small quantity, but in a state not precisely determined, though probably of
protoxide, does not colour the clays till they are subjected to a powerful heat. There
are very white clays, such as those of Montereau, which do not become red till calcined
in the porcelain kiln; the oxide of iron contained in them, which colours them in that
case, was previously imperceptible. It appears from this circumstance, that the clays
fit for making fine white stone ware, as also the Kaolins adapted to the manufacture of
porcelain, are very rare.
Iron, in larger proportion, usually colours the clays green or slate-blue, before they
have been heated. Such clays, exposed to the action of fire, become yellow or red according
to the quantity of iron which they contain. When the iron is very abundant,
it renders the clays fusible; but a little lime and silica must also be present for this effect.
The earthenware made with these ferruginous clays, can bear but a moderate
baking heat; it is thick, porous, and possesses the advantage merely of cheapness, and
of bearing considerable alternations of temperature without breaking.
Alumina and the very aluminous natural clays which possess most plasticity, are apt
to crack in drying, or to lose their shape. This very serious defect for the purposes of
pottery is rectified, in some measure, by adding to that earth a certain quantity of sand
or silica. Thus, a compound is formed which possesses less attraction for water, and
dries more equably from the openness of its body. The principal causes of the distortion
of earthenware vessels, are the unequal thickness of their parts, and quicker desiccation
upon one side than another. Hard burnt stone-ware ground to powder, and incorporated
with clay, answers still better than sand for counteracting the great and irregular
contraction which natural pottery paste is apt to experience. Such ground biscuit is
called cement; and its grains interspersed through the ware, may be regarded as so many
solutions of continuity, which arrest the fissures.
The preceding observations point out the principles of those arts which employ clay
for moulding by the wheel, and baking in a kiln. See Porcelain and Pottery.
CLOTH, MANUFACTURE OF. See Textile Fabrics, Weaving, Wool.
CLOTH-BINDING. Nothing places in so striking a point of view the superior
taste, judgment, and resources of London tradesmen over those of the rest of the world,
than the extensive substitution which they have recently made of embossed silks and
calicoes for leather in the binding of books. In old libraries, cloth-covered boards
indeed may occasionally be seen, but they have the meanest aspect, and are no more
to be compared with our modern cloth-binding, than the jupon of a trull, with the ballet
dress of Taglioni. The silk or calico may be dyed of any shade which use or fancy may
require, impressed with gold or silver foil in every form, and variegated by ornaments
in relief, copied from the most beautiful productions in nature. This new style of
binding is distinguished not more for its durability, elegance, and variety, than for the
economy and dispatch with which it ushers the offspring of intellect into the world.[301]
For example, should a house eminent in this line, such as that of Westleys, Friar-street,
Doctors’-commons, receive 5000 volumes from Messrs. Longman & Co. upon
Monday morning, they can have them all ready for publication, within the incredibly
short period of two days; being far sooner than they could have rudely boarded them
upon the former plan. The reduction of price is not the least advantage incident to
the new method, amounting to fully 50 per cent. upon that with leather.
The dyed cloth being cut by a pattern to the size suited to the volume, is passed
rapidly through a roller press, between engraved cylinders of hard steel, whereby it receives
at once the impress characteristic of the back, and the sides, along with embossed
designs over the surface in sharp relief. The cover thus rapidly fashioned, is as rapidly
applied by paste to the stitched and pressed volume; no time being lost in mutual adjustments;
since the steel rollers turn off the former, of a shape precisely adapted to the
latter. Hard glazed and varnished calico is moreover much less an object of depredation
to moths, and other insects, than ordinary leather has been found to be.
COBALT. This metal being difficult to reduce from its ores, is therefore very little
known, and has not hitherto been employed in its simple state in any of the arts; but its
oxide has been extensively used on account of the rich blue colour which it imparts to
glass, and the glaze of porcelain and stone-ware. The principal ores of cobalt are
those designated by mineralogists under the names of arsenical cobalt and gray cobalt.
The first contains, in addition to cobalt, some arsenic, iron, nickel, and occasionally silver,
&c. The other is a compound of cobalt with iron, arsenic, sulphur, and nickel.
Among the gray cobalts, the ore most esteemed for its purity is that of Tunaberg in
Sweden. It is often in regular crystals which possess the lustre and colour of polished
steel. The specific gravity of cobalt pyrites is 6·36 to 4·66. The Tunaberg variety
afforded to Klaproth, cobalt, 44; arsenic, 55·5; sulphur, 0·5: so that it is an arseniuret.
Others, however, contain much sulphur as well as iron. It imparts at the blowpipe
a blue colour to borax and other fluxes, and gives out arsenical fumes.
The ore being picked to separate its concomitant stony matters, is pounded fine
and passed through a sieve; and is also occasionally washed. The powder is then
spread on the sole of a reverberatory furnace, the flue of which leads into a long horizontal
chimney. Here it is exposed to calcination for several hours, to expel the sulphur
and arsenic that may be present; the former burning away in sulphurous acid gas,
the latter being condensed into the white oxide or arsenious acid, whence chiefly the
market is supplied with this article. This calcining process can never disengage the
whole of these volatile ingredients, and there is therefore a point beyond which it is
useless to push it; but the small quantities that remain are not injurious to the subsequent
operations. The roasted ore is sifted anew; reduced to a very fine powder, and
then mixed with 2 or 3 parts of very pure siliceous sand, to be converted into what is
called zaffre. With this product glasses are generally coloured blue, as well as enamels
and pottery glaze. In the works where cobalt ores are treated, a blue glass is prepared
with the zaffre, which is well known under the name of smalt or azure blue.
This azure is made by adding to the zaffre 2 or 3 parts of potash, according to its richness
in cobalt, and melting the mixture in earthen crucibles. The fused mass is
thrown out while hot into water; and is afterwards triturated and levigated in mills
mounted for the purpose. There remains at the bottom of the earthen pot a metallic
lump, which contains a little cobalt, much nickel, arsenic, iron, &c. This is called
speiss.
As it is the oxide of cobalt which has the colouring quality, the calcination serves the
purpose of oxidizement, as well as of expelling the foreign matters.
A finer cobalt-oxide is procured for painting upon hard porcelain, by boiling the cobalt
ore in nitric acid, which converts the arsenic into an acid, and combines it with the different
metals present in the mineral. These arseniates being unequally soluble in nitric
acid, may be separated in succession by a cautious addition of carbonate of soda or potash;
and the arseniate of cobalt as the most soluble remains unaffected. It has a
rose colour; and is easily distinguishable, whence the precipitation may be stopped at
the proper point. The above solution should be much diluted, and the alkali should
be cautiously added with frequent agitation.
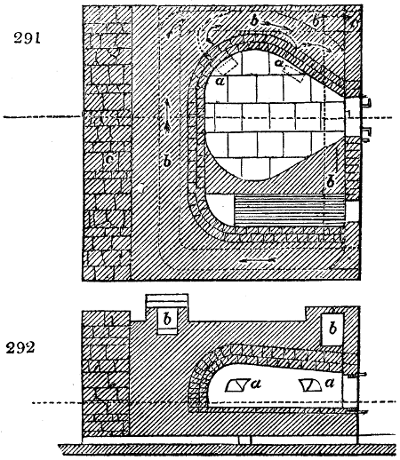
The cobalt ores, rich in nickel, are exposed to slow oxidizement in the air, whereby
the iron, cobalt, arsenic, and sulphur get oxygenated by the atmospheric moisture, but
the nickel continues in the metallic state. This action of the weather must not be
extended beyond a year, otherwise the nickel becomes affected, and injures the cobalt
blue. The ore hereby increases in weight, from 8 to 10 per cent. Fig. 291. is a
longitudinal section of the furnace: fig. 292., a horizontal section upon a level with the
sole of the hearth. It is constructed for wood fuel, and the hearth is composed of
fire-bricks or tiles. The vapours and gases disengaged in the roasting, pass off
through the flues a a, into the channels b b, and thence by c into the common vent, or
poison chamber. See the representation of the poison tower of Altenberg, under the[302]
article Arsenic. The flues are cleared out by means of openings left at suitable
situations in the brick-work of the chimneys.
The azure manufacture is carried
on chiefly in winter, in order that
the external cold may favour the
more complete condensation of the
acids of arsenic. From 3 to 5 cwt.
of Schlich (pasty ore), are roasted
at one operation, and its bed is laid
from 5 to 6 inches thick. After two
hours, it must be turned over; and
the stirring must be repeated every
half hour, till no more arsenic is
observed to exhale. The process
being then finished, the ore must be
raked out of the furnace, and another
charge introduced.
The duration of the roasting is
regulated partly by the proportion
of sulphur and arsenic present, and
partly by the amount of nickel;
which must not be suffered to become
oxidized, lest it should spoil
the colour of the smalt. The latter
ores should be but slightly roasted,
so as to convert the nickel into speiss.
The roasted ore must be sifted in a
safety apparatus. The loss of weight
in the roasting amounts, upon the
average, to 36 per cent. The roasted
ore has a brownish gray hue, and is
called safflor in German, and is distributed
into different sorts. F F S
is the finest safre; F S, fine; O S,
ordinary; and M S, middling. These
varieties proceed from various mixtures
of the calcined ores. The
roasted ore is ground up along with
sand, elatriated, and, when dry, is
called zaffre. It is then mixed with
a sufficient quantity of potash for
converting the mixture into a glass.
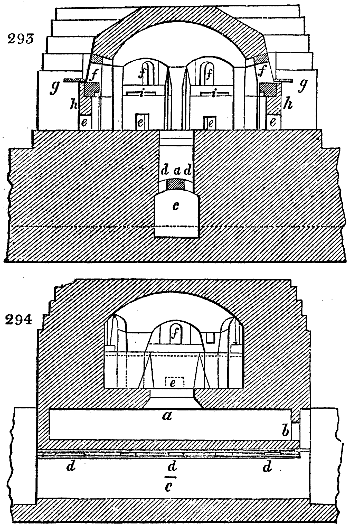
Figs. 293. and 294. represent a
round smalt furnace, in two vertical
sections, at right angles to each
other. The fire-place is vaulted or
arched; the flame orifice a, is in the
middle of the furnace; b is the feed
hole; c, a tunnel which serves as an
ash-pit, and to supply air; d, openings
through which the air arrives
at the fuel, the wood being placed
upon the vault; e, knee holes for
taking out the scoriæ from the pot
bottoms; f, working orifices, with
cast-iron plates g, in front of them.
Under these are the additional outlets
h. The smoke and flame pass
off through the orifices i, which terminate
in expanded flues, where the
sand may be calcined or the wood
may be baked. Eight hours are
sufficient for one vitrifying operation,
during which the glass is stirred
about several times in the earthen melting pots.
The preparation of the different shades of blue glass are considered as secrets in the
smelting works; and marked with the following letters:—F F F C, the finest; F C,[303]
fine; M C, middling; O C, ordinary. A melting furnace, containing 8 pots of glass;
produces in 24 hours, from 24 cwts. of the mixture, 19 cwts. of blue glass; and from
1⁄2 to 3⁄4 cwt. of scoriæ or speiss (speise). The composition speise, according to Berthier,
is,—nickel, 49·0; arsenic, 37·8; sulphur, 7·8; copper, 1·6; cobalt, 3·2 in 100.
Nickel, arsenic, and sulphur, are its essential constituents; the rest are accidental, and
often absent. The freer the cobalt ore is from foreign metals, the finer is the colour,
and the deeper is the shade; paler tints are easily obtained by dilution with more
glass. The presence of nickel gives a violet tone.
The production of smalt in the Prussian states amounted, in 1830, to 74521⁄2 cwts.;
and, in Saxony, to 9697 cwts.; in 1825, to 12,310 cwts.
One process for making fine smalt has been given under the title Azure; I shall introduce
another somewhat different here.
The ore of cobalt is to be reduced to very fine powder, and then roasted with much
care. One part, by weight, is next to be introduced, in successive small portions, into
an iron vessel, in which three parts of acid sulphate of potassa has been previously
fused, at a moderate temperature. The mixture, at first fluid, soon becomes thick
and firm, when the fire is to be increased, until the mass is in perfect fusion, and all
white vapours have ceased. It is then to be taken out of the crucible with an iron
ladle, the crucible is to be recharged with acid sulphate of potash, and the operation
continued as before, until the vessel is useless. The fused mass contains sulphate of
cobalt, neutral sulphate of potassa, and arseniate of iron, with a little cobalt. It is to
be pulverized, and boiled in an iron vessel, with water, as long as the powder continues
rough to the touch. The white, or yellowish white residue, may be allowed to separate
from the solution, either by deposition or filtration. Carbonate of potassa, free from
silica, is then to be added to the solution, and the carbonate of cobalt thrown down is
to be separated and well washed, if possible, with warm water; the same water may be
used to wash other portions of the fused mass. The filtered liquid which first passes,
is a saturated solution of sulphate of potassa: being evaporated to dryness in an iron
vessel, it may be reconverted into acid sulphate by fusing it with one half its weight of
sulphuric acid: this salt is then as useful as at first.
The oxide of cobalt thus obtained, contains no nickel; so little oxide of iron is
present, that infusion of galls does not show its presence; it may contain a little
copper, if that metal exists in the ore, but it is easily separated by the known
methods. Sometimes sulphuretted hydrogen will produce a yellow brown precipitate
in the solution of the fused mass; this, however, contains no arsenic, but is either
sulphuret of antimony or bismuth, or a mixture of both.
It has been found advantageous to add to the fused mass, sulphate of iron, calcined
to redness, and one tenth of nitre when the residue is arseniate of iron, and contains
no arseniate of cobalt. There is then no occasion to act upon the residue a second
time for the cobalt in it.
This process is founded on the circumstances that the sulphate of cobalt is not
decomposed by a red heat, and that the arseniates of iron and cobalt are insoluble
in all neutral liquids. It is quite evident that to obtain a perfect result, the excess
of acid in the bisulphate of potassa must be completely driven off by the red heat
applied.
110,646 lbs. of smalts were imported into the United Kingdom in 1835, and 96,949
were retained for home consumption. In 1834, only 16,223 lbs. were retained.
In 1835, 322,562 lbs. of zaffres were imported, and 336,824 are stated to have been
retained, which is obviously an error. 284,000 lbs. were retained in 1834.
COCCULUS INDICUS, or Indian berry, is the fruit of the Menispermum Cocculus,
a large tree, which grows upon the coasts of Malabar, Ceylon, &c. The fruit is
blackish, and of the size of a large pea. It owes its narcotic and poisonous qualities to
the vegeto-alkaline chemical principle called picrotoxia, of which it contains about one-fiftieth
part of its weight. It is sometimes thrown into waters to intoxicate or kill
fishes; and it is said to have been employed to increase the inebriating qualities of ale
or beer. Its use for this purpose is prohibited by act of parliament, under a penalty of
200l. upon the brewer, and 500l. upon the seller of the drug.
COCHINEAL was taken in Europe at first for a seed, but was proved by the observations
of Lewenhoeck to be an insect, being the female of that species of shield-louse,
or coccus, discovered in Mexico, so long ago as 1518. It is brought to us from
Mexico, where the animal lives upon the cactus opuntia or nopal. Two sorts of cochineal
are gathered—the wild, from the woods, called by the Spanish name grana silvestra;
and the cultivated, or the grana fina, termed also mesteque, from the name of a Mexican
province. The first is smaller, and covered with a cottony down, which increases its
bulk with a matter useless in dyeing; it yields, therefore, in equal weight, much less
colour, and is of inferior price to that of the fine cochineal. But these disadvantages
are compensated in some measure to the growers by its being reared more easily, and[304]
less expensively; partly by the effect of its down, which enables it better to resist rains
and storms.
The wild cochineal, when it is bred upon the field nopal, loses in part the tenacity
and quantity of its cotton, and acquires a size double of what it has on the wild opuntias.
It may therefore be hoped, that it will be improved by persevering care in the rearing
of it, when it will approach more and more to fine cochineal.
The fine cochineal, when well dried and well preserved, should have a gray colour,
bordering on purple. The gray is owing to the powder, which naturally covers it, and
of which a little adheres; as also to a waxy fat. The purple shade arises from the
colour extracted by the water in which they were killed. It is wrinkled with parallel
furrows across its back, which are intersected in the middle by a longitudinal one;
hence, when viewed by a magnifier, or even a sharp naked eye, especially after being
swollen by soaking for a little in water, it is easily distinguished from the factitious,
smooth, glistening, black grains, of no value, called East India cochineal, with which it
is often shamefully adulterated by certain London merchants. The genuine cochineal
has the shape of an egg, bisected through its long axis, or of a tortoise, being rounded
like a shield upon the back, flat upon the belly, and without wings.
These female insects are gathered off the leaves of the nopal plant, after it has ripened
its fruit, a few only being left for brood, and are killed, either by a momentary immersion
in boiling water, by drying upon heated plates, or in ovens: the last become of an
ash-gray colour, constituting the silver cochineal, or jaspeada; the second are blackish,
called negra, and are most esteemed, being probably driest; the first are reddish brown,
and reckoned inferior to the other two. The dry cochineal being sifted, the dust, with
the imperfect insects and fragments which pass through, are sold under the name of
granillo.
Cochineal keeps for a long time in a dry place. Hellot says that he has tried some
130 years old, which produced the same effect as new cochineal.
We are indebted to MM. Pelletier and Caventou for a chemical investigation of
cochineal, in which its colouring matter was skilfully eliminated.
Purified sulphuric ether acquired by digestion with it a golden yellow colour, amounting
by Dr. John to one tenth of the weight of the insect. This infusion left, on evaporation,
a fatty wax of the same colour.
Cochineal, exhausted by ether, was treated with alcohol at 40° B. After 30 infusions
in the digester of M. Chevreul, the cochineal continued to retain colour, although the
alcohol had ceased to have any effect on it. The first alcoholic liquors were of a red
verging on yellow. On cooling, they let fall a granular matter. By spontaneous
evaporation, this matter, of a fine red colour, separated, assuming more of the crystalline
appearance. These species of crystals dissolved entirely in water, which they tinged of
a yellowish-red.
This matter has a very brilliant purple-red colour; it adheres strongly to the sides of
the vessels; it has a granular and somewhat crystalline aspect, very different, however,
from those compound crystals alluded to above; it is not altered by the air, nor does it
sensibly attract moisture. Exposed to the action of heat, it melts at about the fiftieth
degree centigrade (122° Fahr.). At a higher temperature it swells up, and is decomposed
with the production of carburetted hydrogen, much oil, and a small quantity of
water, very slightly acidulous. No trace of ammonia was found in these products.
The colouring principle of cochineal is very soluble in water. By evaporation, the
liquid assumes the appearance of syrup, but never yields crystals. It requires of this
matter a portion almost imponderable to give a perceptible tinge of bright purplish red to
a large body of water. Alcohol dissolves this colouring substance, but, as we have already
stated, the more highly it is rectified the less of it does it dissolve. Sulphuric ether
does not dissolve the colouring principle of cochineal; but weak acids do, possibly
owing to their water of dilution. No acid precipitates it in its pure state. This
colouring principle, however, appears to be precipitable by all the acids, when it is
accompanied by the animal matter of the cochineal.
The affinity of alumina for the colouring matter is very remarkable. When that
earth, newly precipitated, is put into a watery solution of the colouring principle, this is
immediately seized by the alumina. The water becomes colourless, and a fine red lake
is obtained, if we operate at the temperature of the atmosphere; but if the liquor has
been hot, the colour passes to crimson, and the shade becomes more and more violet,
according to the elevation of the temperature, and the continuance of the ebullition.
The salts of tin exercise upon the colouring matter of cochineal a remarkable action.
The muriatic protoxide of tin forms a very abundant violet precipitate in the liquid.
This precipitate verges on crimson, if the salt contains an excess of acid. The muriatic
deutoxide of tin produces no precipitate, but changes the colour to scarlet-red. If
gelatinous alumina be now added, we obtain a fine red precipitate, which does not pass
to crimson by boiling.
[305]
To this colouring principle the name carminium has been given, because it forms the
basis of the pigment called carmine.
The process followed in Germany for making carmine, which consists in pouring a
certain quantity of solution of alum into a decoction of cochineal, is the most simple of
all, and affords an explanation of the formation of carmine, which is merely the carminium
and the animal matter precipitated by the excess of acid in the salt, which has
taken down with it a small quantity of alumina; though it appears that alumina ought
not to be regarded as essential to the formation of carmine. In fact, by another process,
called by the name of Madame Cenette of Amsterdam, the carmine is thrown down, by
pouring into the decoction of cochineal a certain quantity of the binoxalate of potash.
When carbonate of soda is added, then carminated lake also falls down. That carmine
is a triple compound of animal matter, carminium, and an acid appears from the circumstance,
that liquors which have afforded their carmine, when a somewhat strong
acid is poured into them, yield a new formation of carmine by the precipitation of the
last portions of the animal matter. But whenever the whole animal matter is thrown
down, the decoctions, although still much charged with the colouring principle, can
afford no more carmine. Such decoctions may be usefully employed to make carminated
lakes, saturating the acid with a slight excess of alkali, and adding gelatinous
alumina. The precipitates obtained, on adding acids to the alkaline decoctions of
cochineal, are therefore true carmines, since they do not contain alumina; but the small
quantity of alumina which is thrown down by alum in the manufacture of carmine,
augments its bulk and weight. It gives, besides, a greater lustre to the colour, even
though diluting and weakening it a little.
The carmines found in the shops of Paris were analysed, and yielded the same products.
They are decomposed by the action of heat, with the diffusion at first of a very
strong smell of burning animal matter, and then of sulphur. A white powder remained,
amounting to about one-tenth of the matter employed, and which was found to be
alumina. Other quantities of carmine were treated with a solution of caustic potash,
which completely dissolved them, with the exception of a beautiful red powder, not
acted on by potash and concentrated acids, and which was recognized to be red sulphuret
of mercury or vermillion. This matter, evidently foreign to the carmine, appears to
have been added, in order to increase its weight.
The preceding observations and experiments seem calculated to throw some light on
the art of dyeing scarlet and crimson. The former is effected by employing a cochineal
bath, to which there have been added, in determinate proportions, acidulous tartrate of
potash, and nitro-muriatic deutoxide of tin. The effect of these two salts is now well
known. The former, in consequence of its excess of acid, tends to redden the colour,
and to precipitate it along with the animal matter: the latter acts in the same manner,
at first by its excess of acid, then by the oxide of tin which falls down also with the
carmine and animal matter, and is fixed on the wool, with which it has of itself a strong
tendency to combine. MM. Pelletier and Caventou remark, that “to obtain a beautiful
shade, the muriate of tin ought to be entirely at the maximum of oxidizement; and it
is in reality in this state that it must exist in the solution of tin prepared according to
the proportions prescribed in M. Berthollet’s treatise on dyeing.”
We hence see why, in dyeing scarlet, the employment of alum is carefully avoided,
as this salt tends to convert the shade to a crimson. The presence of an alkali would
seem less to be feared. The alkali would occasion, no doubt, a crimson-coloured bath;
but it would be easy in this case to restore the colour, by using a large quantity of tartar.
We should, therefore, procure the advantage of having a bath better charged with
colouring matter and animal substance. It is for experience on the large scale to determine
this point. As to the earthy salts, they must be carefully avoided; and if the
waters be selenitish, it would be a reason for adding a little alkali.
To obtain crimson, it is sufficient, as we know, to add alum to the cochineal bath, or
to boil the scarlet cloth in alum water. It is also proper to diminish the dose of the
salt of tin, since it is found to counteract the action of the alum.
The alkalies ought to be rejected as a means of changing scarlet to crimson. In fact,
crimsons by this process cannot be permanent colours, as they pass into reds by the
action of acids.
According to M. Von Grotthuss, carmine may be deprived of its golden shade by
ammonia, and subsequent treatment with acetic acid and alcohol. Since this fact was
made known, M. Herschel, colour maker at Halle, has prepared a most beautiful
carmine.
The officers of Her Majesty’s Customs have lately detected a system of adulterating
cochineal, which has been practised for many years upon a prodigious scale by a mercantile
house in London. I have analyzed about 100 samples of such cochineal, from which
it appears that the genuine article is moistened with gum-water, agitated in a box or[306]
leather bag, first, with sulphate of baryta in fine powder, afterwards with bone or ivory
black, to give it the appearance of negra cochineal, and then dried. By this means about
12 per cent. of the worthless heavy spar is sold at the price of cochineal, to the enrichment
of the sophisticators, and the disgrace and injury of British trade and manufactures.
The specific gravity of genuine cochineal is 1·25; that of the cochineal loaded with
the barytic sulphate 1·35. It was taken in oil of turpentine and reduced to water as
unity, because the waxy fat of the insects prevents the intimate contact of the latter
liquid with them, and the ready expulsion of air from their wrinkled surface. They are
not at all acted upon by the oil, but are rapidly altered by water, especially when they
have been gummed and barytified.
The quantities of cochineal imported into the United Kingdom in the following years,
were:—
| |
1827. |
1828. |
1829. |
1830. |
1831. |
1832. |
1833. |
1834. |
1835. |
| Libs. |
320,722 |
258,032 |
288,456 |
316,589 |
244,371 |
388,478 |
359,381 |
410,387 |
418,320 |
The quantities re-exported were:—
| Libs. |
145,756 |
158,109 |
153,738 |
100,059 |
168,329 |
138,270 |
130,732 |
265,490 |
352,023 |
Humboldt states that so long ago as the year 1736, there was imported into Europe
from South America cochineal to the value of 15 millions of francs. Its high price had
for a long time induced dyers to look out for cheaper substitutes in dyeing red, and
since science has introduced so many improvements in tinctorial processes, both madder
and lac have been made to supersede cochineal to a very great extent. Its price has, in
consequence of this substitution, as well as from more successful modes of cultivation,
fallen very greatly of late years. At present it is only 7s. per lib. in London. See
Scarlet Dyeing.
COCOA, STEARINE, AND ELAINE. Mr. Soames obtained a patent in September
1829, for making these useful articles, by the following process:
He takes the substance called cocoa-nut oil, in the state of lard, in which it is imported
into this country, and submits it to a strong hydraulic pressure, having made it up in
small packages, 3 or 4 inches wide, 2 feet long, and 1 or 11⁄2 inches thick. These packages
are formed by first wrapping up the said substance in a strong linen cloth, of close texture,
and then in an outward wrapper of strong sail cloth. The packages are to be
placed side by side, in single rows, between the plates of the press, allowing a small
space between the packages for the escape of the elaine.
The temperature at which the pressure is begun, should be from about 50 to 55 degrees,
or in summer as nearly at this pitch as can be obtained, and the packages of the said substance
intended for pressure, should be exposed for several hours previously to about the
same temperature. When the packages will no longer yield their oil or elaine freely
at this temperature, it is to be gradually raised; but it must at no time exceed 65 degrees,
and the lower the temperature at which the separation can be effected, the better
will be the quality of the oil expressed.
When the packages are sufficiently pressed, that is, when they will give out no more
oil, or yield it only in drops at long intervals, the residuum in them is to be taken out and
cleansed and purified, which is done by melting it in a well-tinned copper vessel, which
is fixed in an outer vessel, having a vacant space between, closed at the top, into which
steam is admitted, and the heat is kept up moderately for a sufficient time to allow the
impurities to subside; but if a still higher degree of purity is required, it is necessary
to pass it through filters of thick flannel lined with blotting paper.
Having been thus cleansed or purified, it is fit for the manufacture of candles, which
are made by the ordinary process used in making mould tallow candles. Having thus
disposed of the stearine, or what is called the first product, he proceeds with the elaine
or oil expressed from it, and which he calls the second product, as follows: that is to
say, he purifies it by an admixture, according to the degree of its apparent foulness, of
from 1 to 2 per cent. by weight of the sulphuric acid of commerce, of about 1·80 specific
gravity, diluted with six times its weight of water. The whole is then to be violently
agitated by mechanical means, and he prefers for this purpose the use of a vessel
constructed on the principle of a common barrel churn. When sufficiently agitated, it
will have a dirty whitish appearance, and is then to be drawn off into another vessel, in
which it is to be allowed to settle, and any scum that rises is to be carefully taken off.
In a day or two the impurities will be deposited at the bottom of the oil, which will
then become clear, or nearly so, and it is to be filtered through a thick woollen cloth,
after which it will be fit for burning in ordinary lamps and for other uses.
The process of separating the elaine from the stearine, by pressure, in manner aforesaid,
had never before been applied to the substance called cocoa-nut oil, and consequently
no product had heretofore been obtained thereby from that substance, fit for being manufactured
into candles in the ordinary way, or for being refined by any of the usual[307]
modes, so as to burn in ordinary lamps, both which objects are obtained by this method
of preparing or manufacturing the said substance.
Candles well made from the above material are a very superior article. The light
produced is more brilliant than from the same sized candle made of tallow; the flame is
perfectly colourless, and the wick remains free from cinder, or any degree of foulness
during combustion.
COFFEE. The coffee is the seed of a tree of the family rubiaceæ, and belongs to
the Pentandria monogynia of Linnæus. There are several species of the genus, but the
only one cultivated is the Coffæa Arabica, a native of Upper Ethiopia and Arabia Felix.
It rises to the height of 15 or 20 feet; its trunk sends forth opposite branches in pairs
above and at right angles to each other; the leaves resemble those of the common laurel,
although not so dry and thick. From the angle of the leaf-stalks small groups of white
flowers issue, which are like those of the Spanish jasmine. These flowers fade very
soon, and are replaced by a kind of fruit not unlike a cherry, which contains a yellow
glairy fluid, enveloping two small seeds or berries convex upon one side, flat and furrowed
upon the other in the direction of the long axis. These seeds are of a horny or
cartilaginous nature; they are glued together, each being surrounded with a peculiar
coriaceous membrane. They constitute the coffee of commerce.
It was not till towards the end of the 15th century that the coffee tree began to be cultivated
in Arabia. Historians usually ascribe the discovery of the use of coffee as a beverage
to the superior of a monastery there, who, desirous of preventing the monks from
sleeping at their nocturnal services, made them drink the infusion of coffee upon the report
of shepherds, who pretended that their flocks were more lively after browsing on the
fruit of that plant. The use of coffee was soon rapidly spread, but it encountered much
opposition on the part of the Turkish government, and became the occasion of public
assemblies. Under the reign of Amurath III. the mufti procured a law to shut all the
coffee-houses, and this act of suppression was renewed under the minority of Mahomet
IV. It was not till 1554 under Solyman the Great that the drinking of coffee was accredited
in Constantinople; and a century elapsed before it was known in London and
Paris. Solyman Aga introduced its use into the latter city in 1669, and in 1672 an
Armenian established the first café at the fair of Saint Germain.
When coffee became somewhat of a necessary of life from the influence of habit
among the people, all the European powers who had colonies between the tropics, projected
to form plantations of coffee trees in them. The Dutch were the first who transported
the coffee plant from Moka to Batavia, and from Batavia to Amsterdam. In
1714 the magistrates of that city sent a root to Louis XIV. which he caused to be
planted in the Jardin du Roi. This became the parent stock of all the French coffee
plantations in Martinique.
The most extensive culture of coffee is still in Arabia Felix, and principally in the
kingdom of Yemen, towards the cantons of Aden and Moka. Although these countries
are very hot in the plains, they possess mountains where the air is mild. The
coffee is generally grown halfway up on their slopes. When cultivated on the lower
grounds it is always surrounded by large trees which shelter it from the torrid sun, and
prevent its fruit from withering before their maturity. The harvest is gathered at three
periods, the most considerable occurs in May, when the reapers begin by spreading
cloths under the trees, then shaking the branches strongly, so as to make the fruit drop,
which they collect, and expose upon mats to dry. They then pass over the dried berries
a very heavy roller, to break the envelopes, which are afterwards winnowed away
with a fan. The interior bean is again dried before being laid up in store.
In Demerara, Berbice, and some of our West India islands, where much good coffee
is now raised, a different mode of treating the pulpy fruit and curing the beans is
adopted. When the cherry-looking berry has assumed a deep-red colour it is gathered,
and immediately subjected to the operations of a mill composed of two wooden rollers,
furnished with iron plates, which revolve near a third fixed roller called the chops.
The berries are fed into a hopper above the rollers, and falling down between them and
the chops, they are stripped of their outer skins and pulp, while the twin beans are separated
from each other. These beans then fall upon a sieve, which allows the skin and
the pulp to pass through, while the hard beans accumulate and are progressively slid
over the edge into baskets. They are next steeped for a night in water, thoroughly
washed in the morning, and afterwards dried in the sun. They are now ready for the
peeling mill, a wooden edge wheel turned vertically by a horse yoked to the extremity
of its horizontal axis. In travelling over the coffee, it bursts and detaches the coriaceous
or parchment-like skin which surrounds each hemispherical bean. It is then freed
from the membranes by a winnowing machine, in which four pieces of tin made fast to
an axle are caused to revolve with great velocity. Corn fanners would answer better
than this rude instrument of negro invention. The coffee is finally spread upon mats
or tables, picked clean, and packed up for shipment.
[308]
The most highly esteemed coffee is that of Moka. It has a smaller and a rounder
bean; a more agreeable taste and smell than any other. Its colour is yellow. Next
to it in European reputation is the Martinique and Bourbon coffees; the former is
larger than the Arabian and more oblong; it is rounded at the ends; its colour is greenish,
and it preserves almost always a silver gray pellicle, which comes off in the roasting.
The Bourbon coffee approaches nearest to the Moka from which it originally sprung.
The Saint Domingo coffee has its two extremities pointed, and is much less esteemed
than the preceding.
The coffee tree flourishes in hilly districts where its root can be kept dry, while its
leaves are refreshed with frequent showers. Rocky ground, with rich decomposed
mould in the fissures, agrees best with it. Though it would grow, as we have said, to
the height of 15 or 20 feet, yet it is usually kept down by pruning to that of five feet
for increasing the production of the fruit, as well as for the convenience of cropping.
It begins to yield fruit the third year, but is not in full bearing till the fifth,
does not thrive beyond the twenty-fifth, and is useless in general at the thirtieth.
In the coffee husbandry, the plants should be placed eight feet apart, as the trees throw
out extensive horizontal branches, and in holes ten or twelve feet deep to secure a constant
supply of moisture.
Coffee has been analysed by a great many chemists, with considerable diversity of
results. The best analysis perhaps is that of Schrader. He found that the raw beans
distilled with water in a retort communicated to it their flavour and rendered it turbid,
whence they seem to contain some volatile oil. On reboiling the beans, filtering, and
evaporating the liquor to a syrup, adding a little alcohol till no more matter was precipitated,
and then evaporating to dryness, he obtained 17·58 per cent. of a yellowish-brown
transparent extract, which constitutes the characteristic part of coffee, though it
is not in that state the pure proximate principle, called cafeine. Its most remarkable
reaction is its producing, with both the protoxide and the peroxide salts of iron, a fine
grass green colour, while a dark green precipitate falls, which re-dissolves when an acid
is poured into the liquor. It produces on the solution of the salts of copper scarcely
any effect, till an alkali be added, when a very beautiful green colour is produced which
may be employed in painting. Coffee beans contain also a resin, and a fatty substance
somewhat like suet. According to Robiquet, ether extracts from coffee beans nearly 10
per cent. of resin and fat, but he probably exaggerates the amount. The peculiar substance
cafeine contained in the above extract is crystallizable. It is remarkable in
regard to composition, that after urea and the uric acid, it is among organic products
the richest in azote. It was discovered and described in 1820 by Runge. It does not
possess alkaline properties. Pfaff obtained only 90 grains of cafeine from six pounds
of coffee beans. There is also an acid in raw coffee to which the name of cafeic acid
has been given. When distilled to dryness and decomposed, it has the smell of roasted
coffee.
Coffee undergoes important changes in the process of roasting. When it is roasted
to a yellowish brown it loses, according to Cadet, 121⁄2 per cent. of its weight, and is in
this state difficult to grind. When roasted to a chestnut brown it loses 18 per cent.,
and when it becomes entirely black, though not at all carbonised, it has lost 23 per cent.
Schrader has analyzed roasted coffee comparatively with raw coffee, and he found in
the first 121⁄2 per cent. of an extract of coffee, soluble in water and alcohol, which possesses
nearly the properties of the extract of the raw coffee, although it has a deeper
brown colour, and softens more readily in the air. He found also 10·4 of a blackish
brown gum; 5·7 of an oxygenated extract or rather apothème soluble in alcohol, insoluble
in water; 2 of a fatty substance and resin; 69 of burnt vegetable fibre, insoluble.
On distilling roasted coffee with water, Schrader obtained a product which contained
the aromatic principle of coffee; it reddened litmus paper, and exhaled a strong and
agreeable odour of roasted coffee. If we roast coffee in a retort, the first portions of
the aromatic principle of coffee condense into a yellow liquid in the receiver; and these
may be added to the coffee roasted in the common way, from which this matter has
been expelled and dissipated in the air.
Chenevix affirmed that by the roasting of coffee a certain quantity of tannin possessing
the property of precipitating gelatine is generated. Cadet made the same observation,
and found, moreover, that the tannin was most abundant in the lightly roasted
coffee, and that there was nearly none of it in coffee highly roasted. Payssé and
Schrader, on the contrary, state that solution of gelatine does not precipitate either the
decoction of roasted coffee or the alcoholic extract of this coffee. Runge likewise asserts
that he could obtain no precipitate with gelatine; but he says that albumen precipitates
from the decoction of roasted coffee the same kind of tannin as is precipitated
from raw coffee by the acetate of lead, and set free from the lead by sulphuretted hydrogen.
With these results my own experiments agree. Gelatine certainly does not
disturb clear infusion of roasted coffee, but the salts of iron blacken it.
[309]
Schrader endeavoured to roast separately the different principles of coffee, but none
of them exhaled the aromatic odour of roasted coffee except the horny fibrous matter.
He therefore concludes that this substance contributes mainly to the characteristic taste
of roasted coffee, which cannot be imitated by any other vegetable matter, and which,
as we have seen, should be ascribed chiefly to the altered cafeic acid. According to
Garot we may extract the cafeine without alteration from roasted coffee by precipitating
its decoction by subacetate of lead, treating the washed precipitate with sulphuretted
hydrogen, and evaporating the liquid product to dryness.
Of late years, much ingenuity has been expended in contriving various forms of
apparatus for making infusions of coffee for the table. I have tried most of them, and
find, after all, none so good as a cafetière à la Belloy, the coffee biggin, with the perforated
tin plate strainer, especially when the filtered liquor is kept simmering in a close
vessel, set over a lamp or steam pan. The useful and agreeable matter in coffee is
very soluble: it comes off with the first waters of infusion, and needs no boiling.
To roast coffee rightly we should keep in view the proper objects of this process,
which are to develop its aroma, and destroy its toughness, so that it may be readily
ground to powder. Too much heat destroys those principles which we should wish to
preserve, and substitutes new ones which have nothing in common with the first, but
add a disagreeable empyreumatic taste and smell. If, on the other hand, the rawness
or greenness is not removed by an adequate heat, it masks the flavour of the bean, and
injures the beverage made with it. When well roasted in the sheet iron cylinders set
to revolve over a fire, it should have a uniform chocolate colour, a point readily hit by
experienced roasters, who now manage the business very well for the principal coffee
dealers both of London and Paris, so far as my judgment can determine. The development
of the proper aroma is a criterion by which coffee roasters frequently regulate
their operations. When it loses more than 20 per cent. of its weight, coffee is sure
to be injured. It should never be ground till immediately before infusion.
COKE, is carbonized pitcoal. See Charcoal; and Pitcoal at the end.
COLCOTHAR OF VITRIOL, (Rouge d’Angleterre, Fr.; Rothes Eisenoxyd,
Germ.) is the brown-red peroxide of iron, produced by calcining sulphate of iron with
a strong heat, levigating the resulting mass, and elutriating it into an impalpable powder.
A better way of making it so as to complete the separation of the acid, is to mix
100 parts of the green sulphate of iron with 42 of common salt, to calcine the mixture,
wash away the resulting sulphate of soda, and levigate the residuum. The sulphuric
acid in this case expels the chlorine of the salt in the form of muriatic acid gas, and saturates
its alkaline base produced by the chemical reaction; whence an oxide will be
obtained free from acid, much superior to what is commonly found in the shops. The
best sort of polishing powder called jewellers’ red rouge or plate powder is the precipitated
oxide of iron prepared by adding solution of soda to solution of copperas, washing,
drying, and calcining the powder in shallow vessels with a gentle heat, till it assumes
a deep brown red colour. See Iron.
COLOPHANY, black rosin, the solid residuum of the distillation of turpentine,
when all the oil has been worked off.
COLOURING MATTER. (Matière colorante, Fr.; Farbstoff, Germ.) See
Dyeing, the several dye-stuffs and pigments.
COLUMBIUM, a peculiar metal extracted from a rare mineral brought from Haddam
in Connecticut. It is also called Tantalium from the mineral tantalite and yttrotantalite,
found in Sweden. It has hitherto no application to the arts. It combines
with two successive doses of oxygen; by the second it becomes an acid.
COLZA, is a variety of cabbage, the brassica oleracea, whose seeds afford, by pressure,
an oil much employed in France and Belgium for burning in lamps, and for many
other purposes. This plant requires a rich but light soil; it does not succeed upon
either sandy or clayey lands. The ground for it must be deeply ploughed and well
dunged. It should be sown in July, and be afterwards replanted in a richly manured
field. In October it is to be planted out in beds, 15 or 18 inches apart. Colza may
also be sowed in furrows 8 or 10 inches asunder.
Land which has been just cropped for wheat is that usually destined to colza; it may
be fresh dunged with advantage. The harvest takes place in July, with the sickle, a
little before the seeds are completely ripe, lest they should drop off. As the seed is
productive of oil, however, only in proportion to its ripeness, the cut plants are allowed
to complete their maturation, by laying them in heaps under airy sheds, or placing
them in a stack, and thatching it with straw.
The cabbage stalks are thrashed with flails, the seeds are winnowed, sifted, spread
out in the air to dry; then packed away in sacks, in order to be subjected to the oil
mill at the beginning of winter. The oil-cake is a very agreeable food to cattle, and
serves to fatten them. It is reckoned to defray the cost of the mill.
Colza impoverishes the soil very much, as do, indeed, all the plants cultivated for the[310]
sake of their oleaginous seeds. It must not, therefore, be come back upon again for six
years, if fine crops be desired. The double ploughing which it requires, effectually
cleans the ground. See Oils, Unctuous.
COMB, the name of an instrument made of a thin plate either plane or curved of
wood, horn, tortoise-shell, ivory, bone, or metal, cut out upon one or both of its sides
or edges, into a series of somewhat long teeth, not far apart; which is employed for
disentangling, laying parallel and smooth the hairs of man, horses, or other animals.
A thin steel saw bow, mounted in an iron or wooden handle, is the implement used
by the comb-maker to cut the bone, ivory, and wood into slices of from a twelfth to a
quarter of an inch thick, and of a size suitable to that of the comb. The pieces of
tortoise-shell as found in commerce are never flat, or, indeed, of any regular curvature,
such as the comb must have. They are therefore steeped in boiling water sufficiently
long to soften them, and set to cool in a press between iron or brass moulds, which impart
to them the desired form which they preserve after cooling. After receiving their
outline shape, and curvature, by proper flat files or fine rasps, the place of the teeth is
marked with a triangular file, and then the teeth themselves are cut out with a double
saw, composed of two thin slips of tempered steel, such as the main-spring of a watch,
notched with very fine sharp teeth. These slips are mounted in a wooden or iron stock
or handle, in which they may be placed at different distances to suit the width of the
comb teeth. A comb-maker, however, well provided in tools, has an assortment of
double saws set at every ordinary width. The two slips of this saw have their teeth in
different planes, so that when it begins to cut, the most prominent slip alone acts, and
when the teeth of this one have fairly entered into the comb, the other parallel blade
begins to saw. The workman, meanwhile, has fixed the plate of tortoise-shell or ivory
between the flat jaws of two pieces of wood, like a vice made fast to a bench, so that
the comb intended to be cut is placed at an angle of 45° with the horizon. He now
saws perpendicularly, forming two teeth at a time, proceeding truly in the direction of
the first tracing.
A much better mode of making combs is to fix upon a shaft or arbour in a lathe
a series of circular saws, with intervening brass washers or discs to keep them at suitable
distances; to set in a frame like a vice, in front of these saws, the piece of ivory or horn
to be cut; and to press it forward upon the saws at an angle of 45 degrees, by means
of a regulated screw motion. When the teeth are thus cut, they are smoothed and polished
with files, and by rubbing with pumice stone and tripoli.
Mr. Bundy, of Camden Town, obtained a patent so long ago as 1796, for an apparatus
of that kind, which had an additional arbour fitted with a series of circular saws, or
rather files, for sharpening the points of the comb teeth.
More recently, Mr. Lyne has invented a machine in which, by means of pressure, two
combs are cut out at once with chisels from any tough material, such as horn or tortoise-shell,
somewhat softened at the moment by the application of a heated iron to it. The
piece of horn is made fast to a carriage, which is moved forwards by means of a screw
until it comes under the action of a ratchet-wheel, toothed upon a part of its circumference.
The teeth of this wheel bring a lever into action, furnished with a chisel or
knife, which cuts out a double comb from the flat piece, the teeth of which combs are
opposite to each other. By this means no part of the substance is lost, as in sawing out
combs. The same carriage may be used, also, to bear a piece of ivory in the hard state
towards a circular saw, on the principles above explained, with such precision, that from
80 to 100 teeth can be formed in the space of one inch by a proper disposition of the
tool.
Bullocks’ horns, after the tips are sawed off, are roasted in the flame of a wood
fire, till they are sufficiently softened; when they are slit up, pressed in a machine between
two iron plates, and then plunged into a trough of cold water, whereby they are
hardened. A paste of quicklime, litharge, and water is used to stain the horn to resemble
tortoise-shell. See Horn.
COMBINATION (Combinaison, Fr.; Verbindung, Germ.); a chemical term
which denotes the intimate union of dissimilar particles of matter, into a homogeneous
looking compound, possessed of properties generally different from those of the separate
constituents.
COMBUSTIBLE (Eng. and Fr.; Brennstoff, Germ.); any substance which exposed
in the air to a certain temperature, consumes spontaneously with the emission
of heat and light. All such combustibles as are cheap enough for common use go under
the name of Fuel; which see. Every combustible requires a peculiar pitch of temperature
to be kindled, called its accendible point. Thus phosphorus, sulphur, hydrogen,
carburetted hydrogen, carbon, each takes fire at successively higher heats.
COMBUSTION (Eng. and Fr.; Verbrennung, Germ.) results in common cases
from the mutual chemical reaction of the combustible, and the oxygen of the atmosphere,[311]
whereby a new compound is formed; the heat and light evolved being most probably
produced by the rapid motions of the particles during the progress of this combination.
COMPOUND COLOURS. If the effects of the colouring particles did not vary
according to the combinations which they form, and the actions exercised upon them by
the different substances present in a dyeing bath, we might determine with precision
the shade which ought to result from the mixture of any two colours, or of the ingredients
affording these colours separately. Though the chemical action of the mordants,
and of the liquor in the dye-bath often changes the results, yet theory may always predict
them within a certain degree. It is not the colour appropriate to the dye-stuffs
which is to be considered as the constituent part of compound colours, but that which
they must assume with a certain mordant and dye-bath. Our attention ought therefore
to be directed principally to the operation of the chemical agents employed.
1. The mixture of blue and yellow dyes produces green. D’Ambourney, indeed,
says that he has extracted a fast green from the fermented juice of the berries of the
buckthorn (rhamnus frangula), but no dyer would trust to such a colour.
2. The mixture of red and blue produces violet, purple, columbine (dove-colour),
pansy, amaranth, lilac, mallow, and a great many other shades, determined by the nature
and tone of the red and blue dye-stuffs, as well as their relative proportions in the
bath.
3. The mixture of red and yellow produces orange, mordoré, cinnamon, coquelicot,
brick, capuchin; with the addition of blue, olives of various shades; and with duns instead
of yellows, chestnut, snuff, musk, and other tints.
4. Blacks of the lighter kinds constitute grays; and, mixed with other colours, produce
marrone (marroons), coffees, damascenes. For further details upon this subject,
see Calico Printing, Dyeing, as also the individual colours in their alphabetical
places.
CONCRETE. The name given by architects to a compact mass of pebbles, sand
and lime cemented together, in order to form the foundations of buildings. Semple
says that the best proportions are 80 parts of pebbles, each about 7 or 8 ounces in
weight, 40 parts sharp river sand, and 10 of good lime; the last is to be mixed with
water to a thinnish consistence, and grouted in. It has been found that Thames ballast,
as taken from the bed of the river, consists nearly of 2 parts of pebbles to 1 of sand, and
therefore answers exceedingly well for making concrete; with from one-seventh to one-eighth
part of lime. The best mode of making concrete, according to Mr. Godwin,
is to mix the lime, previously ground, with the ballast in a dry state; sufficient water
is now thrown over it to effect a perfect mixture, after which it should be turned over at
least twice with shovels, or oftener; then put into barrows, and wheeled away for use
instantly. It is generally found advisable to employ two sets of men to perform this
operation, with three in each set; one man to fetch the water, &c., while the other two
turn over the mixture to the second set, and they, repeating the process, turn over the
concrete to the barrow-men. After being put into the barrows, it should at once be
wheeled up planks, so raised as to give it a fall of some yards, and thrown into the
foundation, by which means the particles are driven closer together, and greater solidity
is given to the whole mass. Soon after being thrown in, the mixture is observed
usually to be in commotion, and much heat is evolved with a copious emission of
vapour. The barrow-load of concrete in the fall spreading over the ground, will form
generally a stratum of from 7 to 9 inches thick, which should be allowed to set before
throwing in a second.
Another method of making concrete, is first to cover the foundation with a certain
quantity of water, and then to throw in the dry mixture of ballast and lime. It is next
turned and levelled with shovels; after which more water is pumped in, and the
operation is repeated. The former method is undoubtedly preferable.
In some cases it has been found necessary to mix the ingredients in a pug-mill,
as in mixing clay, &c. for bricks. For the preparation of a concrete foundation, as the
hardening should be rapid, no more water should be used than is absolutely necessary to
effect a perfect mixture of the ingredients. Hot water accelerates the induration. There
is about one-fifth of contraction in volume in the concrete, in reference to the bulk of
its ingredients. To form a cubical yard of concrete, about 30 feet cube of ballast and
31⁄2 feet cube of ground lime must be employed, with a sufficient quantity of water.
CONGELATION (Eng. and Fr.; Gefrierung, Germ.); the act of freezing
liquids. Many means are supplied by chemistry of effecting or promoting this process,
but they do not constitute any peculiar art or manufacture. See Ice-House.
COOLING OF FLUIDS. In Mr. Derosnes’s method, the cooling agents employed
are a current of atmospheric air, and warm water of the same or nearly the same
temperature as that of the vapours which are to be operated upon.
Fig. 295. represents merely a diagram of the general features of an apparatus constructed[312]
upon the principles proposed to be employed, which will serve to explain the
nature of this improvement.
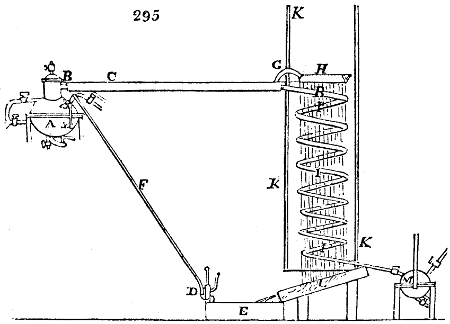
Let A be the source of
the vapours, or the vessel,
boiler, alembic, or closed
pan that contains the
liquid or syrup to be evaporated
or concentrated.
The pipe B, through which
the vapour passes as it
rises in the boiler, is surrounded
by another tube
C, of larger diameter, closed
at both ends. A pump D,
draws from the reservoir E,
warm water, which water
has been heated by its previous
and continual passage
through the apparatus
in contact with the
surface of the vapour pipes.
This pump forces the water by the pipe F, into the annular space or chamber between the
pipes B and C, in which chamber, by its immediate contact with the pipe B, it acquires the
temperature of the vapours intended to be refrigerated. The pipe G conveys the water from
the pipe C, into the annular colander or sieve H, which has a multitude of small holes pierced
through its under part, and from whence the warm water descends in the form of a
continued shower of rain. To the end of the pipe B, a distiller’s worm I I, is connected,
which is placed beneath the colander H. The entire length of the worm-pipe should
be bound round with linen or cotton cloth, as a conductor of the heat, which cloth will
be continually moistened by the rain in its descent from the colander. As this water
has been heated in passing along the tube C, the shower of rain descending from the
colander will be at a higher temperature than that of the atmosphere, and, consequently,
by heating the surrounding air as it descends, a considerable upward draft will
be produced through the coils of the worm-pipe.
If the colander and the worm-pipe are enclosed within a chimney or upright tube, as
K K, open at top and bottom, a current of ascending air will be produced within it by
the descending shower of hot water, similar in effect to that which would be produced
in a chimney communicating with a furnace, or to that of the burner of an argand
lamp. Consequently, it will be perceived that in opposition to the descending rain, a
strong upward current of air will blow through that part of the cylinder K K, which is
beneath the colander. When the air first enters the lower aperture of the chimney or
tube K, it is of the same temperature and moisture as the external atmosphere; but in
its passage up the tube it meets with a warmer and damper atmosphere, caused by the
heat given out from the hot fluid continually passing through the pipes, and by the hot
shower of rain, and also by the steam evolved from the surfaces of the coils of the worm,
which are continually wetted by the descending rain, the evaporation being considerably
augmented by the cloth bound round the worm-pipe, retaining the water as it descends
in drops from coil to coil.
The atmosphere within the tube being of a higher temperature than without, a
current of air constantly ascends and escapes at the upper aperture K, and its place is
supplied by fresh air from the surrounding atmosphere, entering the tube below. The
fresh air thus admitted at the bottom of the tube being cold and dry, will be suited to
take up the heat and moisture within, because the water within the tube being in a
state of dispersion as rain, presents to the air many points, or a very extended surface,
and also because it is of a higher temperature than the air; and, besides, cold dry air is
continually renewed, and a source of warmth is furnished by the latent caloric to the
steam, as fast as it is evolved. Thus a portion of the descending rain, or water, is
evaporated, and the effect of this evaporation is to subtract caloric not only from the
water held in contact with the coils of the worm-pipe by the cloth enveloping it, but also
from the hot vapours which pass through the worm. This process of evaporation has,
therefore, a cooling power, which is but slight in the lower part of the chimney or tube
K; because the temperature of the water, or rain, and of the worm, at this part, are of
a lower temperature; but its refrigerating power increases as it rises towards the
colander, and there it acquires its maximum of intensity, so that at any point between
the lower aperture of the cylinder and the colander, the current of air is always a little
cooler than the atmosphere of the region through which it passes (that is, at its maximum);
and in passing this region of higher temperature, it is not only put in equilibrium[313]
of temperature, but also made to take up an additional quantity of aqueous
vapours, which equalises the new temperature it acquires with its capacity of saturation.
The cooling caused by the evaporation acts in an incessant and progressive manner from
the lower aperture of the cylinder to the under side of the colander; and this cooling
not only acts as an agent of the evaporation which the current of air cools, but it refrigerates
also, because it becomes warmed in abstracting caloric from the vapours or
liquids passing through the worm; and this refrigeration acts also incessantly and progressively
from the lower part of the tube or chimney to the colander.
The patentee states, in conclusion, that “the velocity or force of the current of air
that passes through the chimney or tube K, can be accelerated by artificial means, either
by conducting the air and vapour passing from the upper aperture of the cylinder into
the chimney or flues of a furnace, or by means of a revolving, forcing, or exhausting
fan, or ventilator, or any other contrivance which will produce an increased current of
air, but which it is not necessary to be particularly described, as I only wish to explain
the principles of a simple apparatus, constructed in any convenient form; and I would
remark, that the area of the lower aperture through which the air is introduced into the
chimney or tube K, and also the area of the upper aperture, or that through which it
passes to the atmosphere, should be in accordance with the effect intended to be obtained.
“It is further to be remarked, that in order to obtain from this apparatus the best
effect, the velocity of the current of air must be itself a maximum; and as the speed or
velocity of the current of air is owing to and determined by the excess of the temperature
of the descending water, or rain, and of the coils of the worm to that of the
exterior atmosphere, it ensues that the temperature of the water, or rain, must be a
maximum. But this excess of temperature is a maximum only when the source of the
rain is at the same temperature as the vapours to be condensed: if less warm, it would
attract less air; or, if warmer, it would augment the temperature of the vapours intended
to be condensed. Consequently, the shower of water employed in the tube K, as the
agent for cooling, bestows its maximum of effect when it is as warm as the vapours to
be condensed; therefore, I may express this proposition, viz., ‘That in refrigerating
with water, less of it may be expended when it is warm than when it is cold, and that
the least quantity of water will be evaporated when it is as warm as the aqueous or
spirituous vapours upon which it is to operate.’
“This proposition may appear strange, nevertheless it is conformable to the laws of
nature; and appears only strange, because until now warm water has not been employed
with currents of air for refrigerating.
“Hence it is necessary to raise the temperature of the water in the colander to the temperature
of the vapours to be condensed: therefore, I cause the lukewarm water, pumped
from the reservoir E, to circulate in the chamber C. In this circulation it also begins to
act as a refrigerating medium, taking up a portion of heat from the vapours that pass
through the pipe B, and afterwards it acts as a further condenser in the cylinder, in the
way described. Finally, the portion of this water that is still in the fluid state, after
having fallen down from coil to coil, arrives lukewarm to the inclined surface L, which
conducts it into the reservoir E, from whence it is pumped up into the chamber C, as
before described.
“The tube or chimney K, may have more or less altitude; the higher it is the greater
is the current produced. The force or velocity of the current of air can be governed by
the areas of the introduction and exit apertures. If the cylinder rises only to the height
of the sieve, the effect is much less than when it is prolonged beyond this height. I
would further remark, that if the cylinder was removed, a slight effect might be produced,
provided that a current of air be preserved in the cylindrical space limited by the
coils of the worm, and also if the current was produced between the coils; or a central
passage might be formed in an apparatus of another shape than that above described.
“I have only shown the application of the worm, because intending only to explain
the principles of this method of condensing and refrigerating.
“The small quantity of water wasted in this manner of condensation, (that is, that
portion passed off to the atmosphere in the form of vapours, at the upper aperture of the
cylinder K,) may be replaced by a small stream of cold water, which may be brought to
the apparatus, and perhaps most conveniently introduced into the reservoir E, or into
the chamber between the pipes B and C. When operating upon aqueous vapours, the
waste of water is always less in weight than that of the vapours liquefied. When this
apparatus is applied to the purposes of distillation, the end of the worm should terminate
in a vessel M, which is to receive the produce of the condensation. It will be
seen that this improved process is applicable to various purposes, where condensation or
refrigeration is required; for instance, in the boiling or concentration of sugar; to condensing
and refrigerating distilled vapours, or steam, or saline liquids, either in vacuum
or not; to cooling brewers’ worts; and to the refrigeration of other liquors, or any
other processes, when it may be required.”
I have inserted the specification of this patent verbatim. M. Derosne has busied[314]
himself during a long life with a prodigious number of ingenious little contrivances for
clarifying and boiling syrups, distillation, &c., but he has in this invention taken a
bolder flight, having secured the exclusive privilege of condensing vapours, and cooling
liquors, with hot water, in preference to cold. No man at all versant in the scientific
doctrines, or the practical applications of caloric, will ever seek to meddle with his monopoly
of such a scheme. He may find, perhaps, some needy coppersmith ready to espouse
that or any other equally foolish project, provided a productive job can be made of it,
against credulous customers.
For some rational methods of cooling liquors, and condensing vapours, see Refrigeration,
Still, and Sugar.
COPAL, a resin which exudes spontaneously from two trees, the Rhus copallinum,
and the Elæocarpus copalifer, the first of which grows in America, and the second in
the East Indies. A third species of copal tree grows on the coasts of Guinea, especially
on the banks of some rivers, among whose sands the resin is found. It occurs in
lumps of various sizes and of various shades of colour, from the palest greenish yellow to
darkish brown. I found its specific gravity to vary in different specimens from 1·059 to
1·071, being intermediate in density between its two kindred resins, animé and amber.
Some rate its specific gravity so high as 1·139, which I should think one of the errors with
which chemical compilations teem. Copal is too hard to be scratched by the nail,
whence the excellence of its varnish. It has a conchoidal fracture, and is without smell
or taste. When exposed to heat in a glass retort over a spirit lamp it readily melts
into a liquid, which being further heated boils with explosive jets. A viscid oily-looking
matter then distils over. After continuing the process for some time, no succinic acid
is found in the receiver, but the copal blackens in the retort. Anhydrous alcohol
boiled upon it, causes it to swell, and transforms it by degrees into an elastic viscid
substance. It is not soluble in alcohol of 0·825 at the boiling point, as I have ascertained.
Copal dissolves in ether, and this ethereous solution may be mixed with alcohol
without decomposition. Caoutchoucine acts very slightly upon it by my experiments,
even at the boiling temperature of this very volatile fluid; but a mixture of it
with alcohol of 0·825 in equal parts dissolves it very rapidly in the cold into a perfectly
liquid varnish. Alcohol holding camphor in solution also dissolves it, but not nearly so
well as the last solvent. According to Unverdorben, copal may be completely dissolved
by digesting one part of it for 24 hours with one part and a half of alcohol (probably anhydrous),
because that portion of copal which is insoluble in alcohol, dissolves in a very
concentrated solution of the soluble portion. Oil of petroleum and turpentine dissolve
only 1 or 2 per cent. of raw copal. By particular management, indeed, oil of turpentine
may be combined with copal, as we shall describe under the article Varnish.
Fused copal possesses different properties from the substance in its solid state; for
it then may be made to combine both with alcohol and oil of turpentine.
Unverdorben has extracted from the copal of Africa, five different kinds of resin, none
of which has however been applied to any use in the arts.
The ultimate constituents of copal by my analysis are, carbon 79·87, hydrogen 9·00,
oxygen 11·1; being of hydrogen 7·6 in excess above the quantity necessary to form
water with the oxygen. Of copal and animé, 551,166 libs. were imported in 1835.
COPPER is one of the metals most anciently known. It was named from the
island of Cyprus, where it was extensively mined and smelted by the Greeks. It has a
reddish brown colour inclining to yellow; a faint but nauseous and rather disagreeable
taste; and when rubbed between the fingers it imparts a smell somewhat analogous
to its taste. Its specific gravity is from 8·8 to 8·9. It is much more malleable
than it is ductile; so that far finer leaves may be obtained from it than wire. It melts
at the 27th degree of Wedgewood’s pyrometer, and at a higher temperature it evaporates
in fumes which tinge the fire of a bluish green. By exposure to heat with access of air,
it is rapidly converted into black scales of peroxide. In tenacity it yields to iron; but
surpasses gold, silver, and platinum, considerably in this respect.
In mineralogy, the genus copper includes about 13 different species, and each
of these contains a great many varieties. These ores do not possess any one general
exterior character by which they can be recognized; but they are readily distinguished by
chemical re-agents. Water of ammonia digested upon any of the cupreous ores in a pulverized
state, after they have been calcined either alone or with nitre, assumes an
intense blue colour, indicative of copper. The richest of the ordinary ores appear
under two aspects; the first class has a metallic lustre, a copper red, brass yellow, iron
gray, or blackish gray colour, sometimes inclining to blue; the second is without metallic
appearance, has a red colour, verging upon purple, blue, or green, the last tint
being the most usual. Few copper ores are to be met with, indeed, which do not betray
the presence of this metal by more or less of a greenish film.
1. Native copper, occurs in crystals, branches and filaments, its most common locality
being in primitive rocks. It is found abundantly in Siberia, at the mines
of Tourinski, in those of Hungary, of Fundo-Moldavi in Gallicia, of Fahlun in Sweden,[315]
of Cornwall, &c. The gangues of native copper are granite, gneiss, mica-slate, clay-slate,
quartz, carbonate or fluate of lime, sulphate of barytes, &c. The most remarkable
masses of native copper hitherto observed were; first, one in Brazil, 14 leagues
from Basa, which weighed 2616 pounds; and secondly, another which Dr. Francis-le-Baron
discovered in America to the south of Lake Superior. It was nearly 15 feet
in circumference.
2. Sulphuret of Copper, the vitreous ore of Brochant. The texture of this ore is
compact: its fracture, conchoidal, surface sometimes dull; colour, iron black or lead
gray, often bluish, iridescent, or reddish from a mixture of protoxide. It is easily
melted even by the heat of a candle; but more difficult of reduction than protoxide.
This ore yields to the knife, assuming a metallic lustre when cut. Its density varies
from 4·8 to 5·34. Its composition according to Klaproth is 78·5 copper, 18·5 sulphur,
with a little iron and silica. Its equivalent constitution by theory is 80 copper + 20
sulphur = 100; whence 78·5 of metal should be associated with 19·6 of sulphur. This
ore is therefore one of the richest ores, and forms very powerful veins, which likewise contain
some orange protoxide. It is to be found in all considerable copper districts; in
Siberia, Saxony, Sweden, and especially Cornwall, where the finest crystals occur.
3. Copper Pyrites, resembles in its metallic yellow hue, sulphuret of iron; but the
latter is less pale, harder, and strikes fire more easily with steel. It presents the most
lively rainbow colours. Its specific gravity is 4·3. It contains generally a good deal
of iron; as the following analysis will show; copper 30, sulphur 37, iron 33, in 100
parts. According to Hisinger, the Swedish pyrites contains 63 of copper, 12 of iron,
and 25 of sulphur. These ores occur in primitive and transition districts in vast masses
and powerful veins; and are commonly accompanied with gray copper, sulphuret of
iron, sparry iron, sulphurets of lead, and zinc.
4. Gray Copper, has a steel gray colour, more or less deep, either shining or dull;
fracture uneven; a distinct metallic lustre; difficult of fusion at the blowpipe; it communicates
to glass of borax a yellowish-red colour. Its density in crystals is 4·86. Its
composition is very variable; consisting essentially of copper, iron, antimony, and sulphur.
The exploration of this ore is profitable, in consequence of the silver which it
frequently contains. It occurs in primitive mountains; and is often accompanied
with red silver ore, copper pyrites, and crystallized quartz.
5. Protoxide of Copper, or red oxide of Copper: its colour is a deep red, sometimes
very lively, especially when bruised. It is friable, difficult of fusion at the blowpipe,
reducible on burning charcoal, soluble with effervescence in nitric acid, forming a green
liquid. Its constitution when pure, is 88·9 copper + 11·1 oxygen = 100.
6. Black oxide of Copper, is of a velvet black, inclining sometimes to brown or blue;
and it acquires the metallic lustre on being rubbed. It is infusible at the blowpipe.
Its composition is, copper 80 + oxygen 20; being a true peroxide.
7. Hydrosilicate of Copper, consists essentially of oxide of copper, silica, and water.
Its colour is green; and its fracture is conchoidal with a resinous lustre, like most minerals
which contain water. Its specific gravity is 2·73. It is infusible at the blowpipe
alone, but it melts easily with borax.
8. Dioptase Copper, or Emerald Malachite; a beautiful but rare cupreous mineral,
consisting of oxide of copper, carbonate of lime, silica, and water in varying proportions.
9. Carbonate of Copper, Malachite; is of a blue or green colour. It occurs often
in beautiful crystals.
10. Sulphate of Copper, Blue Vitriol, similar to the artificial salt of the laboratory.
The blue water which flows from certain copper mines, is a solution of this salt. The
copper is easily procured in the metallic state by plunging pieces of iron into it.
11. Phosphate of Copper, is of an emerald green, or verdigris colour with some spots
of black. It presents fibrous or tuberculous masses with a silky lustre in the fracture.
It dissolves in nitric acid without effervescence, forming a blue liquid; melts at the
blowpipe, and is reducible upon charcoal, with the aid of a little grease, into a metallic
globule. Its powder does not colour flame green, like the powder of muriate of copper.
12. Muriate of Copper, is green of various shades; its powder imparts to flame a
remarkable blue and green colour. It dissolves in nitric acid without effervescence;
and is easily reduced before the blowpipe. Its density is 3·5. By Klaproth’s analysis
it consists of oxide of copper 73, muriatic acid 10, water 17.
13. Arseniate of Copper. It occurs in beautiful blue crystals. Before the blowpipe
it melts exhaling fumes of a garlic odour, and it affords metallic globules when in
contact with charcoal. See more upon the ores at the end of this article.
In the article Metallurgy, I have described the mode of working certain copper
mines; and shall content myself here with giving a brief account of two cupreous formations,
interesting in a geological point of view; that of the copper slate of Mansfeldt,
and of the copper veins of Cornwall.
The curious strata of bituminous schist in the first of these localities, are among the[316]
most ancient of any which contain the exuviæ of organised bodies not testaceous. From
among their tabular slabs the vast multitudes of fossil fish were extracted, which have
rendered the cantons of Mansfeldt, Eisleben, Ilmenau, and other places in Thuringia
and Voigtland so celebrated. Many of the fish are transformed into copper pyrites.
Here, also, have been found the fossil remains of the lizard family, called Monitors.
Such is the influence of a wise administration upon the prosperity of mines, that the
thin layer of slate in this formation, of which 100 pounds commonly contain but one
pound and a half of copper, occasionally argentiferous, has been for several centuries the
object of smelting works of the greatest importance to the territory of Mansfeldt and
the adjoining country.
The frequent derangements which this metallic deposit experiences, led skilful directors
of the under-ground operations at an early period to study the order of superposition
of the accompanying rocks. From their observations, there resulted a system
of facts which have served to guide miners, not only in the country of Mansfeldt, but
over a great portion of Germany, and in several other countries where the same series
of rocks, forming the immediate envelope of the cupreous schists, were found to occur
in the same order of superposition.
Of the English copper works.—The deposits of copper in Cornwall occur always as
veins in granite, or in the schistose rocks which surround and cover it; and hence, the
Cornish miners work mostly in the granite or greenish clay slate; the former of which
they call growan, the latter killas. But tin is sometimes disseminated in small veins in
porphyry or elvan, which itself forms great veins in the above rocks. No stratification
has been observed in Cornwall.
The copper veins are abundant in the killas and rare in the granite; but most numerous
near the line of junction of the two rocks. The different kinds of mineral veins
in Cornwall may be classed as follows:—
1. Veins of elvan; elvan courses, or elvan channels.
2. Tin veins, or tin lodes; the latter word being used by the Cornish miners to signify
a vein rich in ore, and the word course, to signify a barren vein.
3. Copper veins running east and west; east and west copper lodes.
4. Second system of copper veins, or contra copper lodes.
5. Crossing veins; cross courses.
6. Modern copper veins; more recent copper lodes.
7. Clay veins; of which there are two sets, the more ancient, called Cross-Fluckans;
and the more modern, called Slides.
There are therefore three systems of copper veins in Cornwall; of which the first is
considered to be the most ancient, because it is always traversed by the two others, and
because, on the contrary, it never cuts them off. The width of these veins does not exceed
6 feet, though occasional enlargements to the extent of 12 feet sometimes take
place. Their length is unknown, but the one explored in the United Mines has been
traced over an extent of seven miles. The gangue of these veins is generally quartz,
either pure, or mixed with green particles analogous to chlorite. They contain
iron pyrites, blende, sulphuret, and several other compounds of copper, such as the carbonate,
phosphate, arseniate, muriate, &c. The most part of the copper veins are
accompanied with small argillaceous veins, called by the miners fluckan of the lode. These
are often found upon both sides of the vein, so as to form cheeks or salebandes.
When two veins intersect each other, the direction of the one thrown out becomes an
object of interest to the miner and geologist. In Saxony it is regarded as a general fact
that the rejected portion is always to the side of the obtuse angle; this also holds generally
in Cornwall, and the more obtuse the angle of incidence, the more considerable the
out-throw.
The great copper vein of Carharack, in the parish of Gwenap, is a most instructive
example of intersection. The power of this vein is 8 feet; it runs nearly from east to
west, and dips towards the north at an inclination of 2 feet in a fathom. Its upper part
is in the killas, its lower part in the granite. The vein has suffered two intersections;
the first results from encountering the vein called Steven’s fluckan, which runs from
north-east to south-west, throwing it out several fathoms. The second has been caused
by another vein, almost at right angles to the first, and which has driven it 20 fathoms out
to the right side. The fall of the vein occurs, therefore, in one case to the right, and in
the other to the left; but in both instances, it is to the side of the obtuse angle. This
disposition is very singular; for one portion of the vein appears to have ascended, while
another has sunk.
The mining works in the copper veins are carried on by reverse steps; see Mines.
The grand shafts for drainage and extraction are vertical, and open upon the roof side
of the vein, traversing it to a certain depth. These pits are sunk to the lowest point of
the exploration; and, in proportion as the workings descend, by means of excavations
in the vein, the pits are deepened and put into communication towards their bottom with[317]
each new gallery of elongation, by means of transverse galleries. At present, the main
shafts are fully 160 fathoms deep. Their horizontal section is oblong, and is divided into two
compartments; the one destined for extraction, the other for the pumps. Their timbering
has nothing remarkable, but is executed with every attention to economy, the whole
wood employed in these mines being brought from Norway.
The descent of the workmen is effected by inclined shafts scooped out of the vein;
the ladders are slightly inclined; they are interrupted every 10 fathoms by floors; the
steps are made of iron, and, to prevent them from turning under the foot, the form of a
miner’s punch or jumper has been given them, the one end being round, and the other
being wedge-shaped.
The ore is raised either by means of horse-gins, or by steam-engine power most frequently
of high pressure. I shall take the Consolidated Mines as an example.
The draining, which is one of the most considerable sources of expense, both from
the quantity of water, and from the depth of the mine, is executed by means of sucking
and forcing pumps, the whole piston-rods of which, 120 feet long, are attached to a
main-rod suspended at the extremity of the working beam of a steam-engine.
On this mine three steam-engines are erected of very great power, for the purpose of
drainage; the one called the Maria engine is of the first-rate force, and most improved
construction. The cylinder is 90 inches in internal diameter, and the length of the
stroke is 9 feet 11 inches. It works single stroke, and is encased in a coating of bricks
to prevent dissipation of the heat. The vapour is admitted at the upper end of the
cylinder during the commencement of the fall of the piston, at a pressure capable of
forming an equilibrium with a column of 60 inches of mercury. The introduction of
the steam ceases whenever the piston has descended through a certain space, which may
be increased or diminished at pleasure. During the remainder of the descent the piston
is pressed merely by this vapour in its progressive expansion, while the under side of
the piston communicates with the condenser. It ascends by the counterweight at the
pump end of the working beam. Hence, it is only during the descent of the piston,
that the effective stroke is exerted. Frequently the steam is admitted only during the
sixth part of the course of the piston, or 18 inches. In this way the power of the engine
is proportioned to the work to be done; that is, to the body of water to be raised.
The maximum force of the above engine is about 310 horses; though it is often made
to act with only one third of this power.
The copper mines of the isle of Anglesey, those of North Wales, of Westmoreland,
the adjacent parts of Lancashire and Cumberland, of the south west of Scotland, of
the Isle of Man, and of the south east of Ireland, occur also in primitive or transition
rocks. The ores lie sometimes in masses, but more frequently in veins. The mine of
Ecton in Staffordshire, and that of Cross-gill-burn, near Alston-moor in Cumberland,
occur in transition or metalliferous limestone.
The copper ores extracted both from the granitic and schistose localities, as well as
from the calcareous, are uniformly copper pyrites more or less mixed with iron pyrites;
the red oxide, carbonate, arseniate, phosphate, and muriate of copper, are very rare in
these districts.
The working of copper in the isle of Anglesey may be traced to a very remote era. It
appears that the Romans were acquainted with the Hamlet mine near Holyhead; but
it was worked with little activity till about 70 years ago. This metalliferous deposit
lies in a greenish clay slate, passing into talc slate; a rock associated with serpentine and
euphotide (gabbro of Von Buch). The veins of copper are from one to two yards
thick; and they converge towards a point where their union forms a considerable mass
of ore. On this mass the mine was first pierced by an open excavation, which is now
upwards of 300 feet deep, and appears from above like a vast funnel. Galleries are
formed at different levels upon the flank of the excavation to follow the several small
veins, which run in all directions, and diverge from a common centre like so many radii.
The ore receives in these galleries a kind of sorting, and is raised by means of hand
windlasses, to the summit of a hill, where it is cleaned by breaking and riddling.
The water is so scanty in this mine that it is pumped up by a six-horse steam-engine.
A great proportion of it is charged with sulphate of copper. It is conveyed into reservoirs
containing pieces of old iron; the sulphate is thus decomposed into copper of
cementation. The Anglesea ore is poor, yielding only from 2 to 3 per cent. of copper:
a portion of its sulphur is collected in roasting the ore.
Mechanical preparation of the copper ores in Cornwall.—The ore receives a first sorting,
either within the mine itself, or at its mouth, the object of which is to separate all
the pieces larger than a walnut. These are then reduced by the hammer to a smaller
size; after which the whole are sorted into four lots, according to their relative richness.
The fragments of poor ore are pounded in the stamps so that the metallic portion
may be separated by washing.
The rich ore is broken into small bits, of the size of a nut, with a flat beater, formed[318]
of a piece of iron 6 inches square and 1 inch thick, adapted to a wooden handle. The
ore to be broken is placed upon plates of cast-iron; each about 16 inches square and 11⁄2
inch thick. These iron plates are set towards the edge of a small mound about a yard
high, constructed with dry stones rammed with earth. The upper surface of this
mound is a little inclined from behind forwards. The work is performed by women,
each furnished with a beater; the ore is placed in front of them beyond the plates; they
break it, and strew it at their feet, whence it is lifted and disposed of to the smelting-houses.
Inferior ores, containing a notable proportion of stony matters, are also broken with
the beater, and the rich parts are separated by riddling and washing from the useless
matters.
The smaller ore is washed on a sieve by shaking it in a stream of water, which carries
away the lighter stony pieces, and leaves the denser metalliferous. They are then
sorted by hand. Thus by beating, stamping, and riddling in water, the stony substances
are in a great measure separated. The finer ground matter is washed on a plane table,
over which a current of water is made to flow. Finally, the ore nearly fine is put into a large
tub with water, and briskly stirred about with a shovel, after which it settles in the order
of richness, the pure metallic ore being nearest the bottom. The stamps used for copper
ore in Cornwall are the same as those used for tin ores, of which we shall speak in treating
of the latter metal, as well as of the boxes for washing the fine powder or slime.
These in fact do not differ essentially from the stamping mills and washing apparatus
described in the article Metallurgy. Crushing rolls are of late years much employed.
See Lead and Tin.
Cornwall being destitute of coal, the whole copper ore which this county produces
is sent for smelting to South Wales. Here are 15 copper works upon the Swansea and
Neath, which pursue a nearly uniform and much improved process, consisting in a series
of calcinations, fusions, and roastings, executed upon the ores and the matters resulting
from them.
The furnaces are of the reverberatory construction; they vary in their dimensions and
in the number of their openings, according to the operations for which they were intended.
There are 5 of them:—1. The calcining furnace or calciner; 2. The melting
furnace; 3. The roasting furnace or roaster; 4. The refining furnace; 5. The heating
or igniting furnace.
1. The calcining furnace rests upon a vault, C, into which the ore is raked down after
being calcined; it is built of bricks, and bound with iron bars, as shown in the elevation,
fig. 296. The hearth, B B, figs. 296. and 298. is placed upon a level with the lower horizontal
binding bar, and has nearly the form of an ellipse, truncated at the two extremities of
its great axis. It is horizontal, bedded with fire-bricks set on edge, so that it may be removed
and repaired without disturbing the arch upon which it reposes. Holes, not visible
in the figure, are left in the shelves before each door, c c, through which the roasted ore is
let fall into the subjacent vault. The dimensions of the hearth B B are immense, being
from 17 to 19 feet in length, and from 14 to 16 in breadth. The fire-place, A, fig. 298., is
from 41⁄2 to 5 feet long, and 3 feet wide. The bridge or low wall, b, fig. 302., which separates
the fire-place from the hearth, is 2 feet thick; and in Mr. Vivian’s smelting-works is
hollow, as shown in the figure, and communicates at its two ends with the atmosphere,
in order to conduct a supply of fresh air to the hearth of the furnace. This judicious
contrivance will be described in explaining the roasting operation. The arched roof of
the furnace slopes down from the bridge to the beginning of the chimney, f, fig. 296,
298., its height above the hearth being at the first point about 26 inches, and from 8 to
12 at the second.
Such great calcining furnaces have 4 or 5 doors, c c c c, fig. 298., one for the fire-place,
as shown at the right hand in fig. 297., and 3 or 4 others for working the ore upon the[319]
reverberatory hearth. If there be 3, 2 of them are placed between the vertical binding
bars upon one side, and a third upon the opposite side of the furnace; if there be 4, 2
are placed upon each side, facing one another. These openings are 12 inches square,
and are bound with iron frames. The chimney is about 22 feet high, and is placed at
one angle of the hearth, as at f, fig. 298., being joined by an inclined flue to the furnace.
For charging it with ore there is usually placed above the upper part of the vault 2
hoppers, E E, in a line with the doors; they are formed of 4 plates of iron, supported in
an iron frame. Beneath each of them there is an orifice for letting the ore down into
the hearth.
These furnaces serve for calcining the ore, and the matts or crude coppers: for the latter
purpose, indeed, furnaces of two stories are sometimes employed, as represented in fig. 301.
The dimensions of each floor in this case are a little less than the preceding. Two doors,
c c, correspond to each hearth, and the workmen, while employed at the upper story,
stand upon a raised movable platform.
2. Melting furnace, figs. 299 and 300.—The form of the
hearth is also elliptical, but the dimensions are smaller than
in the calcining furnace. The length does not exceed 11 or 111⁄2
feet, and the breadth varies from 7 to 8. The fire-place is
however larger in proportion, its length being from 31⁄2 feet
to 4, and its breadth from 3 to 31⁄2; this size being requisite
to produce the higher temperature of this furnace. It has
fewer openings, there being commonly three; one to the
fire-place at D, a second one, O, in the side, kept generally
shut, and used only when incrustations need to be scraped
off the hearth, or when the furnace is to be entered for
repairs; and the third or working-door, G, placed on the
front of the furnace beneath the chimney. Through it the
scoriæ are raked out, and the melted matters are stirred and
puddled, &c.
The hearth is bedded with infusible sand, and slopes
slightly towards the side door, to facilitate the discharge of
the metal. Above this door there is a hole in the wall of the
chimney (fig. 300.) for letting the metal escape. An iron gutter, O, leads it into a pit, K,
bottomed with an iron receiving-pot, which may be lifted out by a crane. The pit M is
filled with water, and the metal becomes granulated as it falls into the receiver. The
melting furnaces are surmounted by a hopper, L, as shown in fig. 299.
Melting furnaces are sometimes used also for calcination.
There are some such near Swansea, which serve this double
purpose; they are composed of 3 floors (fig. 301.) The floor
A is destined for melting the calcined ore; the other two,
B C, serve for calcination. The heat being less powerful,
upon the upper sole C, the ore gets dried upon it, and begins
to be calcined—a process completed on the next floor.
Square holes, d, left in the hearths B and C, put them in
communication with each other, and with the lower one A;
these perforations are shut during the operation by a sheet
of iron, removable at pleasure.
The hearths b and c are made of bricks; they are horizontal at top and slightly vaulted
beneath; they are 2 bricks thick, and their dimensions are larger than those of the inferior
hearths, as they extend above the fire-place. On the floors destined for calcination
the furnace has two doors on one of its sides: on the lower story there are also two; but
they are differently collocated. The first, being in the front of the furnace, serves for
drawing off the scoriæ, for working the metal, &c.; and the second, upon the side, admits
workmen to make necessary repairs. Below this door the discharge or tap-hole A is
placed, which communicates by a cast-iron gutter with a pit filled with water. The
dimensions of this furnace in length and breadth are nearly the same as those of the
melting furnace above described; the total height is nearly 12 feet. It is charged by
means of one or two hoppers.
3. Roasting furnace.—The furnaces employed for this purpose are in general analogous
to the calcining ones; but in the smelting works of Hafod, the property of Messrs.
Vivian, these furnaces, alluded to above, present a peculiar construction, for the purpose
of introducing a continuous current of air upon the metal, in order to facilitate its oxidizement.
This process was originally invented by Mr. Sheffield, who disposed of his
patent right to Messrs. Vivian.
The air is admitted by a channel, c c, through the middle of the fire-bridge, fig. 302, and
extending all its length; it communicates with the atmosphere at its two ends c c;
square holes, b b, left at right angles to this channel, conduct the air into the furnace.[320]
This very simple construction produces a powerful effect in the roasting operation.
It not only promotes the oxidizement of the metals, but burns the smoke, and
assists in the vaporisation of the sulphur; while by keeping the bridge cool it preserves
it from wasting, and secures uniformity of temperature to the hearth.
4. Refining furnace.—In this, as in the melting furnace, the sole slopes towards
the door in front, instead of towards the side doors, because in the refining furnace the
copper collects into a cavity formed in the hearth towards the front door, from which it
is lifted out by ladles; whereas, in the melting furnaces, the metal is run out by a tap-hole
in the side. The hearth sole is laid with sand; but the roof is higher than in the
melting furnace, being from 32 to 36 inches. If the top arch were too much depressed,
there might be produced upon the surface of the metal a layer of oxide very prejudicial
to the quality of the copper. When the metal in that case is run out, its surface solidifies
and cracks, while the melted copper beneath breaks through and spreads irregularly
over the cake. This accident, called the rising of the copper, hinders it from being
laminated, and requires it to be exposed to a fresh refining process, when lead must be
added to dissolve the oxide of copper. This is the only occasion upon which the addition
of lead is proper in refining copper. When the metal to be refined is mixed with
others, particularly with tin, as in extracting copper from old bells, then very wide furnaces
must be employed, to expose the metallic bath upon a great surface, and in a thin
stratum, to the oxidizing action of the air.
The door G, fig. 300., upon the side of the refining furnace, is very large, and is shut
with a framed brick door, balanced by a counter-weight. This door being open during
the refining process, the heat is stronger at B than at A (figs. 299, 300.)
5. Heating furnaces, being destined to heat the pigs or bars of copper to be laminated,
as well as the copper sheets themselves, are made much longer in proportion to their
breadth. Their hearth is horizontal, the vault not much depressed; they have only one
door, placed upon the side, but which extends nearly the whole length of the furnace:
this door may be raised by means of a counter-weight, in the same way as in the furnaces
for the fabrication of sheet-iron and brass.
Series of operations to which the ore is subjected.—The ores which are smelted in the
Swansea works are cupreous pyrites, more or less mingled with gangue (vein-stone).
The pyrites is composed of nearly equal proportions of sulphuret of copper and sulphuret
of iron.
The earthy matters which accompany the pyrites are usually siliceous, though in some
mines the metalliferous deposit is mixed with clay or fluate of lime. Along with these
substances, pretty uniformly distributed, tin and arsenical pyrites occur occasionally
with the copper; and though these two metals are not chemically combined, yet they
cannot be separated entirely in the mechanical preparations. The constituent parts of
the ore prepared for smelting are, therefore, copper, iron, sulphur, with tin, arsenic,
and earthy matters in some cases. The different ores are mixed in such proportions
that the average metallic contents may amount to 81⁄2 per cent. The smelting process
consists in alternate roastings and fusions. The following description of it is chiefly
taken from an excellent paper, published by John Vivian, esq., in the Annals of Philosophy
for 1823.
In the roasting operation the volatile substances are disengaged mostly in the gaseous
state, while the metals that possess a strong affinity for oxygen become oxidized. In
the fusion the earthy substances combine with these oxides, and form glassy scoriæ or
slags, which float upon the surface of the melted metal.
These calcinations and fusions take place in the following order:—
1. Calcination of the ore. 2. Melting of the calcined ore. 3. Calcination of the
coarse metal. 4. Melting of the calcined coarse metal. 5. Calcination of the fine
metal (second matt). 6. Melting of the calcined fine metal. 7. Roasting of the
coarse copper. In some smelting works, this roasting is repeated four times; in
which case a calcination and a melting are omitted. In the Havod works, however,
the same saving is made without increasing the number of roastings. 8. Refining or
toughening the copper.
Besides these operations, which constitute the treatment of copper properly speaking,
two others are sometimes performed, in which only the scoriæ are melted. These may
be designated by the letters a and b. a is the re-melting of the portion of the scoriæ
of the second process, which contain some metallic granulations. b is a particular melting
of the scoriæ of the fourth operation. This fusion is intended to concentrate the
particles of copper in the scoriæ, and is not practised in all smelting works.
First operation. Calcination of the ore.—The different ores, on arriving from Cornwall
and other districts where they are mined, are discharged in continuous cargoes at
the smelting works, in such a way, that by taking out a portion from several heaps at
a time, a tolerably uniform mixture of ores is obtained; which is very essential in
a foundry, because, the ores being different in qualities and contents, they act as[321]
fluxes upon each other. The ore thus mixed is transported to the works in wooden
measures that hold a hundred-weight. The workmen entrusted with the calcination
convey the ore into the hoppers of the calcining furnace, whence it falls into the hearth;
other workmen spread it uniformly on the surface by iron rakes. The charge of a furnace
is from three tons to three tons and a half. Fire is applied and gradually increased,
till, towards the end of the operation, the temperature be as high as the ore can support
without melting or agglutinating. To prevent this running together, and to aid the
extrication of the sulphur, the surfaces are renewed, by stirring up the ore at the end of
every hour. The calcination is usually completed at the end of 12 hours, when the
ore is tumbled into the arch under the sole of the furnace. Whenever the ore is cold
enough to be moved, it is taken out of the arch, and conveyed to the calcined heap.
The ore in this process hardly changes weight, having gained in oxidizement nearly
as much as it has lost in sulphur and arsenic; and if the roasting has been rightly managed,
the ore is in a black powder, owing to the oxide of iron present.
Second operation. Fusion of the calcined ore.—The calcined ore is likewise given to
the melters in measures containing a hundred-weight. They toss it into hoppers, and
after it has fallen on the hearth, they spread it uniformly. They then let down the
door, and lute it tightly. In this fusion there are added about 2 cwt. of scoriæ proceeding
from the melting of the calcined matt, to be afterwards described. The object
of this addition is not only to extract the copper that these scoriæ may contain, but especially
to increase the fusibility of the mixture. Sometimes also, when the composition
of the ore requires it, lime, sand, or fluor spar is added; and particularly the last
fluxing article.
The furnace being charged, fire is applied, and the sole care of the founder is to keep
up the heat so as to have a perfect fusion; the workman then opens the door, and
stirs about the liquid mass to complete the separation of the metal (or rather of the
matt) from the scoriæ, as well as to hinder the melted matter from sticking to the sole.
The furnace being ready, that is, the fusion being perfect, the founder takes out the
scoriæ by the front door, by means of a rake. When the matt is thus freed from the
scoriæ, a second charge of calcined ore is then introduced to increase the metallic bath;
which second fusion is executed like the first. In this way, new charges of roasted ore
are put in till the matt collected on the hearth rises to a level with the door-way, which
happens commonly after the third charge. The tap hole is now opened; the matt
flows out into the pit filled with water, where it is granulated during its immersion;
and it collects in the pan placed at the bottom. The granulated matt is next conveyed
into the matt warehouse. The oxidation with which the grains get covered by
the action of the water, does not allow the proper colour of the matt or coarse metal to
be distinguished; but in the bits which stick in the gutter, it is seen to be of a steel
gray. Its fracture is compact, and its lustre metallic. The scoriæ often contain
metallic grains; they are broken and picked with care. All the portions which include
some metallic particles are re-melted in an accessory process. The rejected scoriæ
have been found to be composed of siliceous matter 59, oxide of copper 1, oxide
of tin 0·7.
In this operation, the copper is concentrated by the separation of a great part of the
matters with which it was mixed or combined. The granulated matt produced, contains
in general 33 per cent. of copper; it is therefore four times richer than the ore;
and its mass is consequently diminished in that proportion. The constituent parts are
principally copper, iron, and sulphur.
The most important point to hit in the fusion just described, is to make a fusible
mixture of the earths and the oxides, so that the matt of copper may, in virtue of its
greater specific gravity, fall to the under-part, and separate exactly from the slag. This
point is attained by means of the metallic oxides contained in the scoriæ of the fourth
operation, of which 2 cwt. were added to the charge. These consist almost entirely of
black oxide of iron. When the ores are very difficult to melt, a measure of about half
a hundred-weight of fluor spar is added; but this must be done with precaution, for
fear of increasing the scoriæ too much.
The business goes on day and night. Five charges are commonly put through hands
in the course of 24 hours; but when all circumstances are favourable, that is to say,
when the ore is fusible, when the fuel is of the first quality, and when the furnace is in
good condition, even six charges a day have been despatched.
The charge is a ton and a half of calcined ore, so that a melting furnace corresponds
nearly to a calcining furnace; the latter turning out nearly 7 tons of calcined ore in 24
hours.
The workmen are paid by the ton.
Third operation. Calcination of the coarse metal, or the matt.—The object of this
operation is principally to oxidize the iron, an oxidation easier to execute, than in the first[322]
calcining, because the metal is now disengaged from the earthy substances, which screened
it from the action of the air.
This calcination is executed in the furnace already represented, fig. 296, 297, 298.
page 318. exactly in the same way as the ore was calcined. The metal must be perpetually
stirred about, to expose all its surfaces to the action of the hot air, and to hinder the
clotting together. The operation lasts 24 hours; during the first six, the fire should be very
moderate, and thereafter gradually increased to the end of the calcination. The charge
is, like that of the first, 3 tons and a half.
Fourth operation. Melting of the calcined coarse metal, or calcined matt.—In the
fusion of this first calcined matt, some scoriæ of the latter operations must be added,
which are very rich in oxide of copper, and some crusts from the hearth, which are likewise
impregnated with it. The proportion of these substances varies according to the
quality of the calcined matt.
In this second fusion, the oxide of copper contained in the scoriæ, is reduced by the
affinity of the sulphur, one portion of which passes to the state of acid, while the other
forms a subsulphuret with the copper become free. The matt commonly contains a sufficient
quantity of sulphur to reduce the oxide of copper completely; but if not, which
may happen if the calcination of the matt has been pushed too far, a small quantity of
uncalcined matt must be introduced, which, by furnishing sulphur, diminishes the
richness of the scoriæ, and facilitates the fusion.
The scoriæ are taken out by the front door, by drawing them forward with a rake.
They have a great specific gravity; are brilliant with metallic lustre, very crystalline,
and present, in the cavities, crystals like those of pyroxene; they break easily into very
sharp-edged fragments. They contain no granulated metal in the interior; but it
sometimes occurs, on account of the small thicknesses of the stratum of scoriæ, that
these carry off with them, when they are withdrawn, some metallic particles.
These scoriæ, as we have already stated, under the fusion of the roasted ore, are in
general melted with it. In some cases, however, a special melting is assigned to them.
The matt obtained in this second fusion is either run out into water like the first, or
moulded into pigs (ingots), according to the mode of treatment which it is to undergo.
This matt, called by the smelters fine metal when it is granulated, and blue metal when it
is in pigs, is of a light grey colour, compact, and bluish at the surface. It is collected
in the first form when it is to be calcined anew; and in the second, when it must
immediately undergo the operation of roasting. Its contents in copper are 60 per cent.
This operation, which is but sometimes had recourse to, lasts 5 or 6 hours. The charge
is 1 ton.
(b) Particular fusion of the scoriæ of the fourth operation.—In re-melting these scoriæ,
the object is to procure the copper which they contain. To effect this fusion, the scoriæ
are mixed with pulverized coal, or other carbonaceous matters. The copper and several
other metals are deoxidized, and furnish a white and brittle alloy. The scoriæ resulting
from this melting are in part employed in the first melting, and in part thrown away.
They are crystalline, and present crystals often in the cavities, which appear to belong
to bisilicate of iron. They have a metallic lustre, and break into very sharp-edged
fragments. The white metal is melted again, and then united to the product of the
second fusion.
Fifth operation. Calcination of the second matt, or fine metal of the smelter.—This is
executed in precisely the same way as that of the first matt. It lasts 24 hours; and the
charge is usually 3 tons.
Sixth operation. Melting of the calcined fine metal.—This fusion is conducted like
that of the first matt. The black copper, or coarse copper, which it produces, contains
from 70 to 80 per cent. of pure metal; it is run into ingots, in order to undergo the
operation of roasting.
The scoriæ are rich in copper; they are added to the fusion of the calcined coarse
metal of the fourth operation.
In the smelting houses of Messrs. Vivian, at Hafod, near Swansea, the fifth and sixth
operations have been omitted of late years. The second matt is run into pigs, under the
name of blue metal, to be immediately exposed to the roasting.
The disposition of the canal a a′, fig. 302., which introduces a continuous current of air
to the hearth of the furnace, accelerates and facilitates the calcination of the matt; an
advantage which has simplified the treatment, by diminishing the number of calculations.
Seventh operation. Roasting of the coarse copper, the product of the sixth operation.
The chief object of this operation is oxidizement; it is performed either in an ordinary
roasting furnace, or in the one belonging to fig. 302., which admits a constant current of
air. The pigs of metal derived from the preceding melting are exposed, on the hearth
of the furnace, to the action of the air, which oxidizes the iron and other foreign metals
with which the copper is still contaminated. The duration of the roasting varies from[323]
12 to 24 hours, according to the degree of purity of the crude copper. The temperature
should be graduated, in order that the oxidizement may have time to complete, and
that the volatile substances which the copper still retains may escape in the gaseous form.
The fusion must take place only towards the end of the operation.
The charge varies from a ton and a quarter to a ton and a half. The metal obtained
is run out into moulds of sand. It is covered with black blisters, like steel of cementation;
whence it has got the name of blistered copper. In the interior of these pigs, the copper
presents a porous texture, occasioned by the ebullition produced by the escape of the
gases during the moulding. The copper being now almost entirely purged from the
sulphur, iron, and the other substances with which it was combined, is in a fit state to be
refined. This operation affords some scoriæ; they are very heavy, and contain a great
deal of oxide of copper, sometimes even metallic copper.
These scoriæ, as well as those of the third melting and of the refining, are added to
the second fusion, as we have already stated, in describing the fourth operation.
In some works, the roasting is repeated several times upon the blue metal, in order to
bring it to a state fit for refining. We shall afterwards notice this modification of the
treatment.
Eighth operation. Refining or toughening.—The pigs of copper intended for refining
are put upon the sole of the refining furnace through the door in the side. A slight heat
is first given, to finish the roasting or oxidation, in case this operation has not already
been pushed far enough. The fire is to be increased by slow degrees, so that, by the end
of 6 hours, the copper may begin to flow. When all the metal is melted, and when the
heat is considerable, the workman lifts up the door in the front, and withdraws with a
rake the few scoriæ which may cover the copper bath. They are red, lamellated, very
heavy, and closely resemble protoxide of copper.
The refiner takes then an assay with a small ladle, and when it cools, breaks it in a
vice, to see the state of the copper. From the appearance of the assay, the aspect of the
bath, the state of the fire, &c., he judges if he may proceed to the toughening, and what
quantity of wooden spars and wood charcoal he must add to render the metal malleable,
or, in the language of the smelters, bring it to the proper pitch. When the operation of
refining begins, the copper is brittle or dry, and of a deep red colour approaching to
purple. Its grain is coarse, open, and somewhat crystalline.
To execute the refining, the surface of the metal is covered over with wood charcoal,
and stirred about with a spar or rod of birch wood. The gases which escape from the
wood, occasion a brisk effervescence. More wood charcoal is added from time to time,
so that the surface of the metal may be always covered with it, and the stirring is continued
with the rods, till the operation of refining be finished; a circumstance indicated
by the assays taken in succession. The grain of the copper becomes finer and finer,
and its colour gradually brightens. When the grain is extremely fine, or closed, when
the trial pieces half cut through and then broken, present a silky fracture, and when the
copper is of a fine light red, the refiner considers the operation to be completed; but he
verifies still further the purity of the copper, by trying its malleability. For this purpose,
he takes out a sample in his small ladle, and pours it into a mould. When the
copper is solidified, but still red-hot, he forges it. If it is soft under the hammer, if it
does not crack on the edges, the refiner is satisfied with its ductility, and he pronounces
it to be in its proper state. He orders the workmen to mould it; who then lift the
copper out of the furnace in large iron ladles lined with clay, and pour it into moulds
of the size suitable to the demands of commerce. The ordinary dimensions of the ingots
or pigs are 12 inches broad, 18 long, and from 2 to 21⁄2 thick.
The period of the refining process is 20 hours. In the first six, the metal heats, and
suffers a kind of roasting; at the end of this time it melts. It takes four hours to reach
the point at which the refining, properly speaking, begins; and this last part of the
process lasts about 4 hours. Finally, 6 hours are required to arrange the moulds,
cast the ingots, and let the furnace cool.
The charge of copper in the refining process depends upon the dimensions of the furnace.
In the Hafod works, one of the most important in England, the charge varies from
3 to 5 tons; and the quantity of pure copper manufactured in a week is from 40 to 50 tons.
The consumption of fuel is from 15 to 18 parts of coal, for one part of refined copper
in pigs.
When the copper offers difficulties in the refining, a few pounds of lead are added to
it. This metal, by the facility with which it scorifies, acts as a purifier, aiding the
oxidation of the iron and other metals that may be present in the copper. The lead
ought to be added immediately after removing the door to skim the surface. The
copper should be constantly stirred up, to expose the greatest possible surface to the
action of the air, and to produce the complete oxidation of the lead; for the smallest
quantity of this metal alloyed in copper, is difficult to clear up in the lamination; that
is to say, the scale of oxide does not come cleanly from the surface of the sheets.
[324]
The operation of refining copper is delicate, and requires, upon the part of the workmen,
great skill and attention to give the metal its due ductility. Its surface ought to
be entirely covered with wood charcoal; without this precaution, the refining of the
metal would go back, as the workmen say, during the long interval which elapses in the
moulding; whenever this accident happens, the metal must be stirred up anew with the
wooden pole.
Too long employment of the wooden rod gives birth to another remarkable accident,
for the copper becomes more brittle than it was prior to the commencement of the refining;
that is, when it was dry. Its colour is now of a very brilliant yellowish red,
and its fracture is fibrous. When this circumstance occurs, when the refining, as the
workmen say, has gone too far, the refiner removes the charcoal from the top of the
melted metal; he opens the side door, to expose the copper to the action of the air, and
it then resumes its malleable condition.
Mr. Vivian, to whom we owe the above very graphic account of the processes, has
explained, in a very happy manner, the theory of refining. He conceives, we may conclude,
that the copper in the dry state, before the refining, is combined with a small portion
of oxygen, or, in other words, that a small portion of oxide of copper is diffused
through the mass, or combined with it; and that this proportion of oxygen is expelled
by the deoxidizing action of the wood and charcoal, whereby the metal becomes malleable.
2. That when the refining process is carried too far, the copper gets combined with
a little charcoal. Thus copper, like iron, is brittle when combined with oxygen and
charcoal; and becomes malleable only when freed entirely from these two substances.
It is remarkable, that copper, in the dry state, has a very strong action upon iron; and
that the tools employed in stirring the liquid metal become very glistening, like those
used in a farrier’s forge. The iron of the tools consumes more rapidly at that time,
than when the copper has acquired its malleable state. The metal requires, also, when
dry, more time to become solid, or to cool, than when it is refined; a circumstance depending,
probably, upon the difference in fusibility of the copper in the two states, and
which seems to indicate, as in the case of iron, the presence of oxygen.
When the proper refining point has been passed, another very remarkable circumstance
has been observed; namely, that the surface of the copper oxidizes more difficultly,
and that it is uncommonly brilliant; reflecting clearly the bricks of the furnace
vault. This fact is favourable to the idea suggested above, that the metal is in that case
combined with a small quantity of carbon; which absorbs the oxygen of the air, and
thus protects the metal from its action.
Copper is brought into the market in different forms, according to the purposes which
it is to serve. What is to be employed in the manufacture of brass is granulated. In
this condition it presents more surface to the action of zinc or calamine, and combines
with it more readily. To produce this granulation, the metal is poured into a large
ladle, pierced with holes, and placed above a cistern filled with water, which must be
hot or cold, according to the form wished in the grains. When it is hot, round grains
are obtained analogous to lead shot; and the copper in this state is called bean shot.
When the melted copper falls into cold water perpetually renewed, the granulations are
irregular, thin, and ramified; constituting feathered shot. The bean shot is the form employed
in brass making.
Copper is also made into small ingots, about 6 ounces in weight. These are intended
for exportation to the East Indies, and are known in commerce by the name of Japan
copper. Whenever these little pieces are solidified, they are thrown, while hot, into cold
water. This immersion slightly oxidizes the surface of the copper, and gives it a fine red
colour.
Lastly, the copper is often reduced into sheets, for the sheathing of ships, and many
other purposes. The Hafod works possess a powerful rolling mill, composed of four
pairs of cylinders. It is moved by a steam engine, whose cylinder has 40 inches
diameter. See the representation of the rolling mill of the Royal Mint, under
Gold.
The cylinders for rolling copper into sheets are usually 3 feet long, and 15 inches in
diameter. They are uniform. The upper roller may be approached to the under one,
by a screw, so that the cylinders are brought closer, as the sheet is to be made thinner.
The ingots of copper are laid upon the sole of a reverberatory furnace to be heated;
they are placed alongside each other, and they are formed into piles in a cross-like arrangement,
so that the hot air may pass freely round them all. The door of the furnace
is shut, and the workman looks in through a peep-hole from time to time, to see if they
have taken the requisite temperature; namely, a dull red. The copper is now passed
between the cylinders; but although this metal be very malleable, the ingots cannot be
reduced to sheets without being several times heated; because the copper cools, and acquires,
by compression, a texture which stops the progress of the lamination.
These successive heatings are given in the furnace indicated above; though, when the[325]
sheets are to have a very great size, furnaces somewhat different are had recourse to.
They are from 12 to 15 feet long, and 5 wide. See Brass.
The copper, by successive heating and lamination, gets covered with a coat of oxide,
which is removed by steeping the sheets for a few days in a pit filled with urine; they
are then put upon the sole of the heating furnace. Ammonia is formed, which acts on the
copper oxide, and lays bare the metallic surface. The sheets are next rubbed with a piece
of wood, then plunged, while still hot, into water, to make the oxide scale off; and lastly,
they are passed cold through the rolling press to smooth them. They are now cut
square, and packed up for home sale or exportation.
The following estimate has been given by MM. Dufrénoy and Elie de Beaumont of
the expense of manufacturing a ton of copper in South Wales.
| |
£ |
s. |
d. |
| 121⁄2 tons of ore, yielding 81⁄2 per cent. of copper |
55 |
0 |
0 |
| 20 tons of coals |
8 |
0 |
0 |
| Workmen’s wages, rent, repairs, &c. |
13 |
0 |
0 |
| |
76 |
0 |
0 |
The exhalations from the copper smelting works are very detrimental to both vegetable
and animal life. They consist of sulphurous acid, sulphuric acid, arsenic and arsenious acids,
various gases and fluoric vapours, with solid particles mechanically swept away into the air,
besides the coal smoke. Mr. Vivian has invented a very ingenious method of passing the
exhalations from the calcining ores and matts along horizontal flues or rather galleries of
great dimensions, with many crossings and windings of the current, and exposure during
the greater part of the circuit to copious showers of cold water. By this simple and
powerful system of condensation, the arsenic is deposited in the bottoms of the flues,
the sulphurous acid is in a great measure absorbed, and the nuisance is remarkably
abated.
The following figures represent certain modifications of the copper calcining and
smelting copper furnaces of Swansea.
Fig. 304. is the section of the roasting furnace lengthwise; fig. 303. the ground plan;
in which a is the fire-door; b the grate; c the fore-bridge; d the chimney; e e working
apertures on each of the
long sides of the furnace,
through which the ore is
introduced, spread, and
turned over; f f cast-iron
hoppers; g g openings in
the vaulted roof; h the
hearth-sole; i i holes in
this; k a vaulted space
under the hearth. The
hearth has a suitable oval
shape, and is covered with
a flat arch. Its length is
16 feet, breadth 131⁄2, mean
height 2 feet.
Fig. 305. is a longitudinal section of the melting furnace; fig. 306. the ground plan
in which a is the fire door; b the grate; c the fire bridge; d the chimney; e the side
openings; f the working doors; g the raking-out hole; h iron spouts, which conduct
the melted metal into pits filled with water.
The melting furnace is altogether smaller; but its firing hearth is considerably larger[326]
than in the roasting furnace. The long axis of the oval hearth is 14 feet; its short
axis 10 feet; its mean height 2 feet.
The principal ore smelted at Chessy
is the azure copper, which was discovered
by accident in 1812. Red copper ore,
also, has come into operation there since
1825. The average metallic contents of
the richest azure ore are from 33 to 36
per cent.; of the poorer, from 20 to 24.
The red ore contains from 40 to 67 parts
in 100. The ore is sorted, so that the
mean contents of metal may be 27 per
cent., to which 20 per cent. of limestone
are added; whence the cinder will
amount to 50 per cent. of the ore. A
few per cents. of red copper slag, with
some quicklime and gahrslag, are added
to each charge, which consists of 200
pounds of the above mixture, and 150
pounds of coke. When the furnace (fourneau à manche, see the Scotch smelting hearth,
under Lead), is in good action, from 10 to 14 such charges are worked in 12 hours.
When the crucible is full of metal at the end of this period, during which the cinder
has been frequently raked off, the blast is stopped, and the matt floating over the
metal being sprinkled with water and taken off, leaves the black copper to be treated
in a similar way, and converted into rosettes. The refining of this black copper is
performed in a kind of reverberatory furnace.
The cinders produced in this reduction process are either vitreous and light blue,
which are most abundant; cellular, black, imperfectly fused from excess of lime; or,
lastly, red, dense, blistery, from defect of lime, from too much heat, and the passage of
protoxide into the cinders. They consist of silicate of alumina, of lime, protoxide of
iron; the red contain some silicate of copper.
The copper-refining
furnace at Chessy, near
Lyons, is of the kind
called Spleiss-ofen (split
hearths) by the Germans.
Fig. 307. is a section
lengthwise in the dotted
line A B of fig. 308., which
is the ground plan.
The foundation-walls
are made of gneiss; the
arch, the fire-bridge, and
the chimney, of fire-bricks.
The hearth, a, is
formed of a dense mixture
of coal-dust, upon a
bottom of well-beat clay
b, which reposes upon a
bed of brickwork c. Beneath
this there is a slag
bottom d; e is the upper,
and f the under discharge
hole. The hearth is egg-shaped;
the longer axis
being 8 feet, the shorter
61⁄2 feet: in the middle it is 10 inches deep, and furnished with the outlets g g, which
lead to each of the Spleiss-hearths h h, fig. 308. These outlets are contracted with fire-bricks
i i, till the proper period of the discharge. The two hearths are placed in communication
by a canal h; they are 31⁄2 feet in diameter, 16 inches deep; are floored
with well-beat coal ashes, and receive about 27 cwt. for a charge.
l is the grate; m, the fire-bridge; n, the boshes in which the tuyères lie; o, the
chimney; p, the working door through which the slags may be drawn off. Above
this is a small chimney, to carry off the flame and smoke whenever the door is
opened.
The smelting post or charge, to be purified at once, consists of 60 cwt. of black
copper, to which a little granular copper and copper of cementation is added; the[327]
consumption of pit-coal amounts to 36 cwt. As soon as the copper is melted, the
bellows are set a-going, and the surface of the metal gets soon covered with a
moderately thick layer of cinder, which is drawn off. This is the first skimming or
decrassage. By and by, a second layer of cinder forms, which is in like manner
removed; and this skimming is repeated, to allow the blast to act upon fresh metallic
surfaces. After 4 or 5 hours, no more slag appears, and then the fire is increased.
The melted mass now begins to boil or work (travailler), and continues so to do, for
about 3⁄4 of an hour, or an hour, after which the motion ceases, though the fire be kept
up. The gahrproof is now taken; but the metal is seldom fine in less than 3⁄4 of an
hour after the boil is over. Whenever the metal is run off by the tap-hole into the
two basins i i, called SPLIT-HEARTHS, a reddish vapour or mist rises from its surface,
composed of an infinite number of minute globules, which revolve with astonishing
velocity upon their axes, constituting what the Germans called spratzen (crackling) of
the copper. They are composed of a nucleus of metal, covered with a film of protoxide,
and are used as sand for strewing upon manuscript. The copper is separated, as usual, by
sprinkling water upon the surface of the melted metal, in the state of rosettes, which are
immediately immersed in a stream of water. This refining process lasts about 16 or 17
hours; the skimmings weigh about 50 cwt.; the refuse is from 15 to 17 per cent.; the
loss from 2 to 3 per cent. The gahrslag amounts to 11 cwt.
The refining of the eliquated copper (called darrlinge) from which the silver has been
sweated out by the intervention of lead, can be performed only in small hearths. The
following is the representation of such a furnace, called, in German, Kupfergahrheerd.
Fig. 309. is the section lengthwise; fig. 310. is the section across; and fig. 311. is the
ground plan, in which a is the hearth-hollow; b, a massive wall; c, the mass out of
which the hearth is formed; d, cast-iron plates covering the hearth; e, opening for[328]
running off the liquid slag; f, a small wall; g, iron curb for keeping the coals
together.
The hearth being heated with a bed of charcoal, 3⁄4 cwt. of darrlinge are laid over it,
and covered with more fuel: whenever this charge is melted, another layer of the coal
and darrlinge is introduced, and thus in succession till the hearth become full, or
contain from 21⁄4 to 21⁄2 cwt. In Neustadt 71⁄2 cwt. of darrlinge have been refined in one
furnace, from which 5 cwt. of gahrcopper has been obtained. The blast oxidizes the
foreign metals, namely, the lead, nickel, cobalt, and iron, with a little copper, forming
the gahrslag; which is, at first, rich in lead oxide, and poor in copper oxide; but, at the
end, this order is reversed. The slag, at first blackish, assumes progressively a copper
red tint. The slag flows off spontaneously along the channel e, from the surface of the
hearth. The gahre is tested by means of a proof rod of iron, called gahr-eisen, thrust
through the tuyère into the melted copper, then drawn out and plunged in cold water.
As soon as the gahrspan (scale of copper) appears brownish red on the outside, and
copper red within, so thin that it seems like a net-work, and so deficient in tenacity
that it cannot be bent without breaking, the refining is finished. The blast is then
stopped; the coals covering the surface, as also the cinders must be raked off the copper,
after being left to cool a little; the surface is now cooled by sprinkling water upon it,
and the thick cake of congealed metal (rondelle) is lifted off with tongs, a process called
schleissen (slicing), or sheibenreissen (shaving), which is continued till the last convex cake
at the bottom of the furnace, styled the kingspiece, is withdrawn. These rondelles are
immediately immersed in cold water, to prevent the oxidation of the copper; whereupon
the metal becomes of a cochineal red colour, and gets covered with a thin film of
protoxide. Its under surface is studded over with points and hooks, the result of
tearing the congealed disc from the liquid metal. Such cakes are called rosette copper.
When the metal is very pure and free from protoxide, these cakes may be obtained very
thin, one 24th of an inch for example.
The refining of two cwts. and a half of darrlinge takes three quarters of an hour, and
yields one cwt. and a half of gahr copper in 36 rosettes, as also some gahrslag. Gahr
copper generally contains from 11⁄2 to 21⁄2 per cent. of lead, along with a little nickel,
silver, iron, and aluminum.
Smelting of the Mansfeldt copper schist, or bituminous Mergelschiefer.—The cupreous
ore is first roasted in large heaps, of 2000 cwts., interstratified with brush-wood, and
with some slates rich in bituminous matter, mixed with the others. These heaps are
3 ells high, and go on burning 15 weeks in fair and 20 in rainy weather. The bitumen
is decomposed; the sulphur is dissipated chiefly in the form of sulphurous acid; the metal
gets partially oxidized, particularly the iron, which is a very desirable circumstance
towards the production of a good smelting slag. The calcined ore is diminished one-tenth
in bulk, and one-eighth in weight; becoming of a friable texture and a dirty yellow
gray colour. The smelting furnaces are cupolas (schachtofen), 14 to 18 feet high; the
fuel is partly wood charcoal, partly coke from the Berlin gas-works, and Silesia. The
blast is given by cylinder bellows, recently substituted for the old barbarous Blasebälgen,
or wooden bellows of the household form.
The cupreous slate is sorted, according to its composition, into slate of lime, clay, iron,
&c., by a mixture of which the smelting is facilitated. For example, 1 post or charge may
consist of 20 cwt. of the ferruginous slate, 14 of the calcareous, 6 of the argillaceous, with
3 of fluor spar, 3 of rich copper slags, and other refuse matters. The nozzle at the
tuyère is lengthened 6 or 8 inches, to place the melting heat near the centre of the
furnace. In 15 hours 1 fodder of 48 cwts. of the above mixture may be smelted,
whereby 4 to 5 cwts. of matte (crude copper, called Kupferstein in Germany) and a
large body of slags are obtained. The matte contains from 30 to 40 per cent. of copper,
and from 2 to 4 loths (1 to 2 oz.) of silver. The slags contain at times one-tenth their
weight of copper.
The matte is composed of the sulphurets of copper, iron, silver, zinc, along with some
arsenical cobalt and nickel. The slaty slag is raked off the surface of the melted matte
from time to time. The former is either after being roasted six successive times,
smelted into black copper; or it is subjected to the following concentration process. It
is broken to pieces, roasted by brushwood and coals three several times in brick-walled
kilns, containing 60 cwts., and turned over after every calcination; a process of four
weeks’ duration. The thrice roasted mass, called spurrost, being melted in the cupola
fig. 313. with ore-cinder, yields the spurstein, or concentrated matte. From 30 to 40 cwts.
of spurrost are smelted in 24 hours; and from 48 to 60 per cent. of spurstein are obtained,
the slag from the slate smelting being employed as a flux. The spurstein contains from
50 to 60 per cent. of copper, combined with the sulphurets of copper, of iron, and
silver.
The spurstein is now mixed with dünnstein (a sulphuret of copper and iron produced
in the original smeltings) roasted six successive times, in a quantity of 60 cwts., with[329]
brushwood and charcoal; a process which requires from 7 to 8 weeks. The product of
this six-fold calcination is the Gahrrost of the Germans (done and purified); it has a
colour like red copper ore, varying from blue gray into cochineal red; a granular fracture;
it contains a little of the metal, and may be immediately reduced into metallic
copper, called kupfermachen. But before smelting the mass, it is lixiviated with water,
to extract from it the soluble sulphate, which is concentrated in lead pans, and
crystallized.
The lixiviated gahröste mixed with from 1⁄4 to 1⁄5 of the lixiviated dünnsteinrost, and 1⁄6 to 1⁄10
of the copper slate slag, are smelted with charcoal or coke fuel in the course of 24 hours,
in a mass of 60 or 80 cwts. The product is black copper, to the amount of about 1⁄4 the
weight, and 1⁄6 of dünnstein, or thin matte. This black copper contains in the cwt. from
12 to 20 loths (6 to 10 oz.) of silver. The dünnstein consists of from 60 to 70 per
cent. of copper combined with sulphur, sulphuret of iron and arsenic; and when thrice
roasted, yields a portion of metal. The black copper lies undermost in the crucible of
the furnace, above it is the dünnstein, covered with the stone slag, or copper cinder,
resulting from the slate-smelting. The slags being raked off, and the crucible sufficiently
full, the eye or nozzle hole is shut, the dünnstein removed by cooling the
surface, and breaking the crust, which is about 1⁄4 to 1⁄2 inch thick. The same method is
adopted for taking out the black copper in successive layers. For the de-silvering of
this, and similar black coppers, see Silver.
Fig. 312. is a vertical section
through the form or tuyère in
the dotted line A B of fig. 314.
Fig. 313. is a vertical section in
the dotted line C D of fig. 315. a
is the shaft of the furnace, b the
rest, c c the forms; d the sole or
hearth-stone, which has a slope
of 3 inches towards the front
wall; e e, &c. casing walls of fire
bricks; f f, &c. filling up walls
built of rubbish stones; g g a
mass through which the heat is
slowly conducted; h h the two
holes through one or other of
which alternately the product of
the smelting process is run off
into the fore-hearth. Beneath
the hearth-sole there is a solid
body of loam; and the fore-hearth
is formed with a mixture
of coal-dust and clay; k is the
discharge outlet. Fig. 314. is a
horizontal section of the furnace
through the hole or eye in the
dotted line E F of fig. 312.; fig.
315. a horizontal section of the
shaft of the furnace through the
form in the dotted line G H of figs. 312 and 313. The height of the shaft, from the line
E F to the top, is 14 feet; from E to G, 25 inches; from c to the line below b, 2 feet;
from that line to the line opposite g g, 2 feet. The width at the line g g is 3 feet 3 inches,
and at c 26 inches. The basins i i, fig. 314., are 3 feet diameter, and 20 inches deep.
The refining of copper is said to be well executed at Seville, in Spain; and, therefore,
some account of the mode of operating there may be acceptable to the reader.
The first object is to evaporate in a reverberatory furnace all the volatile substances,
such as sulphur, arsenic, antimony, &c., which may be associated with the sulphur;
and the second, to oxidize and to convert into scoriæ the fixed substances, such as iron,
lead, &c., with the least possible expense and waste. The minute quantities of gold
and silver which resist oxidation cannot be in any way injurious to the copper. The hearth
is usually made of a refractory sand and clay with ground charcoal, each mixed in equal
volumes, and worked up into a doughy consistence with water. This composition is
beat firmly into the furnace bottom. But a quartzose hearth is found to answer better,
and to be far more durable; such as a bed of fire-sandstone.
Before kindling the furnace, its inner surface is smeared over with a cream-consistenced
mixture of fire-clay and water.
The cast pigs, or blocks of black or crude copper, are piled upon the hearth, each successive
layer crossing at right angles the layer beneath it, in order that the flame may[330]
have access to play upon the surface of the hearth, and to heat it to a proper pitch
for making the metal flow.
The weight of the charge should be proportional to the capacity of the furnace, and
such that the level of the metallic bath may be about an inch above the nozzle of the
bellows; for, were it higher, it would obstruct its operation, and were it too low, the
stream of air would strike but imperfectly the surface of the metal, and would fail to
effect, or would retard at least, the refining process, by leaving the oxidation and volatilization
of the foreign metals incomplete.
As the scoriæ form upon the surface, they are drawn off with an iron rabble fixed to
the end of a wooden rod.
Soon after the copper is melted, charcoal is to be kindled in three iron basins lined
with loam, placed alongside the furnace, to prepare them for receiving their charge of
copper, which is to be converted in them, into rosettes.
The bellows are not long in action before the evaporation of the mineral substances
is so copious, as to give the bath a boiling appearance; some drops rise up to the roof
of the reverberatory, others escape by the door, and fall in a shower of minute spherical
globules. This phenomenon proves that the process is going on well; and, when it
ceases, the operation is nearly completed. A small proof of copper, of the form of a
watch-case, and therefore called montre, is taken out from time to time, upon the round
end of a polished iron rod, previously heated. This rod is dipped two or three inches
into the bath, then withdrawn and immersed in cold water. The copper cap is detached
from the iron rod, by a few blows of a hammer; and a judgment is formed from its
thickness, colour, and polish, as to the degree of purity which the copper has acquired.
But these watches need not be drawn till the small rain, above spoken of, has ceased to
fall. At the end of about 11 hours of firing, the numerous small holes observable in
the first watch samples begin to disappear; the outer surface passes from a bright red
to a darker hue, the inner one becomes of a more uniform colour, and always less and
less marked with yellowish spots. It will have acquired the greatest pitch of purity
that the process can bestow, when the watches become of a dark crimson colour.
Care must be taken to stop this refining process at the proper time; for, by prolonging
it unduly, a small quantity of cupreous oxide would be formed, which, finding
no oxygen to reduce it, would render the whole body of copper hard, brittle, and incapable
of lamination.
The basins must now be emptied of their burning charcoal, the opening of the tuyère
must be closed, and the melted copper allowed to flow into them through the tap-hole,
which is then closed with loam. Whenever the surface is covered with a solid crust, it
is bedewed with water; and as soon as the crust is about 11⁄2 inch thick it is raised upon
hooks above the basin, to drain off any drops, and then carried away from the furnace.
If these cakes, or rosettes, be suddenly cooled by plunging them immediately in water,
they will assume a fine red colour, from the formation of a film of oxide.
Each refining operation produces, in about 12 hours, 17⁄10 tons of copper, with the
consumption of about 4⁄5 of a ton of dry wood.
Care should be taken that the copper cake or rosette be all solidified before plunging
it into water, otherwise a very dangerous explosion might ensue, in consequence of the
sudden extrication of oxygen from the liquid metal, in the act of condensation. On
the other hand, the cake should not be allowed to cool too long in the air, lest it get
peroxidized upon the surface, and lose those fine red, purple, and yellow shades, due to
a film of the protoxide, which many dealers admire.
When a little oxide of antimony and oxide of copper are combined with copper, they
occasion the appearance of micaceous scales in the fractured faces. Such metal is hard,
brittle, yellowish within, and can be neither laminated nor wire-drawn. These defects
are not owing to arsenic, as was formerly imagined; but, most probably, to antimony
in the lead, which is sometimes used in refining copper. They are more easily prevented
than remedied.
According to M. Frèrejean, proprietor of the great copper works of Vienne, in
Dauphiny, too low a temperature or too much charcoal, gives to the metal a cubical
structure, or that of divergent rays; in either of which states it wants tenacity. Too
high a temperature, or too rapid a supply of oxygen, gives it a brick red colour, a
radiated crystallization without lustre, or a very fine grain of indeterminate form; the
last structure being unsuitable for copper that is to be worked under the hammer or in
the rolling-press. The form which indicates most tenacity is radiated with minute
fibres glistening in mass. Melted copper will sometimes pass successively through
these three states in the space of ten minutes.
Fig. 316. represents a roasting mound of copper pyrites in the Lower Hartz, near Goslar,
where a portion of the sulphur is collected. It is a vertical section of a truncated quadrangular
pyramid. A layer of wooden billets is arranged at the base of the pyramid in
the line a a.
[331]
C, a wooden chimney which stands in the centre of the mound with a small pile of
charcoal at its bottom, c; d d are large lumps of ore surrounded by smaller pieces; f f,
are rubbish and earth to form a covering.
A current of air is admitted under
the billets by an opening, in the middle
of each of the four sides of the base
a a, so that two principal currents of
air cross under the vertical axis C of
the truncated pyramid, as indicated in
the figure.
The fire is applied through the chimney C; the charcoal at its bottom c, and the pile
a a are kindled. The sulphureous ores d, f, are raised to such a high temperature as to
expel the sulphur in the state of vapour.
In the Lower Hartz a roasting mound continues burning during four months. Some
days after it is kindled the sulphur begins to exhale, and is condensed by the air at the
upper surface of the pyramid. When this seems impregnated with it, small basins l l
are excavated, in which some liquid sulphur collects; it is removed from time to time
with iron ladles, and thrown into water, where it solidifies. It is then refined and cast
into roll brimstone.
A similar roasting mound contains, in the Lower Hartz, from 100 to 110 tons of ore
and 730 cubic feet of wood. It yields in four months about one ton and a half of
sulphur from copper pyrites. Lead ore is treated in the same way, but it furnishes less
sulphur.
There are usually from 12 to 15 roasting heaps in action at once for three smelting
works of the Lower Hartz. After the first roasting two heaps are united to form a
third, which is calcined anew, but under a shed; the ores are then stirred up and roasted
for the third time, whence a crude mixture is procured for the smelting-house.
The most favourable seasons for roasting in the open air are spring and autumn; the
best weather is a light wind accompanied with gentle rain. When the wind or rain
obstruct the operation, this inconvenience is remedied by planks distributed round the
upper surface of the truncated pyramid over the sulphur basins.
Manufacturing assays of copper.—The first thing is to make such a sample as will
represent the whole mass to be valued; with which view, fragments must be taken from
different spots, mixed, weighed, and ground together. A portion of this mixture being
tried by the blow-pipe, will show, by the garlic or sulphurous smell of its fumes, whether
arsenic, sulphur, or both, be the mineralizers. In the latter case, which often occurs,
100 gr. or 1000 gr. of the ore are to be mixed with one half its weight of saw-dust,
then imbued with oil, and heated moderately in a crucible till all the arsenical fumes be
dissipated. The residuum being cooled and triturated, is to be exposed in a shallow
earthen cup to a slow roasting heat, till the sulphur and charcoal be burned away. What
remains being ground and mixed with half its weight of calcined borax, one-twelfth its
weight of lamp black, next made into a dough with a few drops of oil, is to be pressed
down into a crucible, which is to be covered with a luted lid, and to be subjected, in a
powerful air furnace, first to a dull red heat, and then to vivid ignition for 20 minutes.
On cooling and breaking the crucible, a button of metallic copper will be obtained. Its
colour and malleability indicate pretty well the quality, as does its weight, the relative
value of the ore. It should be cupelled with lead, to ascertain if it contains silver or
gold. See Assay, and Silver.
If the blow-pipe trial showed no arsenic, the first calcination may be omitted; and if
neither sulphur nor arsenic, a portion of the ground ore should be dried, and treated
directly with borax, lamp black and oil. It is very common to make a dry assay of
copper ores, by one roasting and one fusion along with 3 parts of black flux; from the
weight of the metallic button the richness of the ore is inferred.
The humid assay is more exact, but it requires more skill and time.
The sulphur and the silica are easily got rid of, by the acids which do not dissolve
them, but only the metallic oxides and the other earths. These oxides may then be
thrown down by their appropriate reagents, the copper being precipitated in the state of
either the black oxide, or pure metal. 105 parts of black oxide represent 100 of copper.
Before entering upon the complete analysis of an ore, preliminary trials should be made,
to ascertain what are its chief constituents. If it be sulphuret of copper, or copper
pyrites, without silver or lead, 100 grains exactly of its average powder may be weighed
out, treated in a matras with boiling muriatic acid for some time, gradually adding a
few drops of nitric acid, till all action ceases, or till the ore be all dissolved. The insoluble
matter found floating in the liquid contains most of the sulphur; it may be separated
upon a filter, washed, dried, and weighed; then verified by burning away. The incombustible
residuum, treated by muriatic acid, may leave an insoluble deposit, which is
to be added to the former. To the whole of the filtered solutions carbonate of potash is[332]
to be added; and the resulting precipitate, being washed, and digested repeatedly in
water of ammonia, all its cupric oxide will have been dissolved, whenever the ammonia
is no longer rendered blue.
Caustic potash, boiled with the ammoniacal solution, will separate the copper in the
state of black oxide; which is to be thrown upon a filter, washed, dried, and weighed.
The matter left undissolved by the ammonia, consists of oxide of iron, with probably a
little alumina. The latter being separated by caustic potash, the iron oxide may be
also washed, dried, and weighed. The powder which originally resisted the muriatic
acid, is silica.
Assay of copper ores, which contain iron, sulphur, silver, lead, and antimony.
100 grains of these ores, previously sampled, and pulverized, are to be boiled with
nitric acid, adding fresh portions of it from time to time, till no more of the matter be
dissolved. The whole liquors which have been successively digested and decanted off,
are to be filtered and treated with common salt, to precipitate the silver in the state of
a chloride.
The nitric acid, by its reaction upon the sulphur, having generated sulphuric acid,
this will combine with the lead oxidized at the same time, constituting insoluble sulphate
of lead, which will remain mixed with the gangue. Should a little nitrate of lead
remain in the liquid, it may be thrown down by sulphate of soda, after the silver has
been separated. The dilute liquid being concentrated by evaporation, is to be mixed
with ammonia in such excess as to dissolve all the cupric oxide, while it throws down
all the oxide of iron and alumina; which two may be separated, as usual, by a little
caustic potash. The portion of ore insoluble in the nitric acid, being digested in
muriatic acid, every thing will be dissolved except the sulphur and silica. These
being collected upon a filter, and dried, the sulphur may be burned away, whereby the
proportion of each is determined.
Ores of the oxide of copper, are easily analyzed by solution in nitric acid, the addition
of ammonia, to separate the other metals, and precipitation by potash. The native carbonate
is analyzed by calcining 100 grains; when the loss of weight will shew the
amount of water and carbonic acid; then that of the latter may be found, by expelling
it from another 100 grains, by digestion in a given weight of sulphuric acid. The
copper is, finally, obtained in a metallic state by plunging bars of zinc into the solution
of the sulphate.
The native arseniates of copper are analyzed by drying them first at a moderate heat;
after which they are to be dissolved in nitric acid. To this solution, one of nitrate of
lead is to be added, as long as it occasions a precipitate; the deposit is to be drained upon
a filter, and the clear liquid which passes through, being evaporated nearly to dryness, is
to be digested in hot alcohol, which will dissolve every thing except a little arseniate
of lead. This being added to the arseniate first obtained, from the weight of the whole,
the arsenic acid, constituting 35 per cent., is directly inferred. The alcoholic solution
being now evaporated to dryness, the residue is to be digested in water of ammonia, when
the cupric oxide will be dissolved, and the oxide of iron will remain. The copper is
procured, in the state of black oxide, by boiling the filtered ammoniacal solution with
the proper quantity of potash.
The analysis of muriate of copper—atacamite—is an easy process. The ore being
dissolved in nitric acid, a solution of nitrate silver is added, and from the weight of the
chloride precipitated, the equivalent amount of muriate or chloride of copper is given;
for 100 of chloride of silver represent 93 of chloride of copper, and 43·8 of its metallic
basis. This calculation may be verified by precipitating the copper of the muriate
from its solution in dilute sulphuric acid, by plates of zinc.
The phosphate of copper may be analyzed either by solution in nitric acid, and precipitation
by potash; or by precipitating the phosphoric acid present, by means of
acetate of lead. The phosphate of lead thus obtained, after being washed, is to be
decomposed by dilute sulphuric acid. The insoluble sulphate of lead being washed,
dried, and weighed, indicates by its equivalent the proportion of phosphate of lead, as
also of phosphate of copper; for 100 of sulphate of lead correspond to 92·25 phosphate
of lead, and 89·5 phosphate of copper; and this again to 52·7 of the black oxide.
Copper forms the basis of a greater number of important ALLOYS than any other metal.
With zinc it forms Brass in all its varieties; which see.
Bronze and Bell Metal are alloys of copper and tin. This compound is prepared
in crucibles when only small quantities are required; but in reverberatory hearths, when
statues, bells, or cannons are to be cast. The metals must be protected as much as possible
during their combination from contact of air by a layer of pounded charcoal, otherwise
two evils would result, waste of the copper by combustion, and a rapid oxidizement
of the tin, so as to change the proportions and alter the properties of the alloy. The
fused materials ought to be well mixed by stirring, to give uniformity to the compound.
See Bronze.
[333]
An alloy of 100 of copper and 4·17 of tin has been proposed by M. Chaudet for the
ready manufacture of medals. After melting this alloy he casts it in moulds made of
such bone-ash as is used for cupels. The medals are afterwards subjected to the action
of the coining press, not for striking them, for the mould furnishes perfect impressions,
but for finishing and polishing them.
By a recent analysis of M. Berthier, the bells of the pendules, or ornamental clocks,
made in Paris, are found to be composed, of copper 72·00, tin 26·56, iron 1·44, in 100
parts.
An alloy of 100 of copper and 14 of tin is said by M. Dussaussy to furnish tools,
which hardened and sharpened in the manner of the ancients, afford an edge nearly equal
to that of steel.
Cymbals, gongs, and the tamtam of the Chinese are made of an alloy of 100 of copper
with about 25 of tin. To give this compound the sonorous property in the highest
degree it must be subjected to sudden refrigeration. M. D’Arcet, to whom this discovery
is due, recommends to ignite the piece after it is cast, and to plunge it immediately
into cold water. The sudden cooling gives the particles of the alloy such a disposition
that, with a regulated pressure by skilful hammering, they may be made to slide
over each other, and remain permanently in their new position. When by this means
the instrument has received its intended form, it is to be heated and allowed to cool
slowly in the air. The particles now take a different arrangement from what they
would have done by sudden refrigeration; for instead of being ductile they possess such
an elasticity, that on being displaced by a slight compression, they return to their primary
position after a series of extremely rapid vibrations; whence a very powerful
sound is emitted. Bronze, bell-metal, and probably all the other alloys of tin with copper
present the same peculiarities.
The alloy of 100 of copper with from 60 to 33 of tin forms common bell-metal. It is
yellowish or whitish gray, brittle, and sonorous, but not so much so as the preceding.
The metal of house-clock bells contain a little more tin than that of church-bells, and
the bell of a repeater contains a little zinc in addition to the other ingredients.
The bronze-founder should study to obtain a rapid fusion, in order to avoid the causes
of waste indicated above. Reverberatory furnaces have been long adopted for this operation;
and among these, the elliptical are the best. The furnaces with spheroidal domes
are used by the bell-founders, because their alloy being more fusible, a more moderate
melting heat is required; however, as the rapidity of the process is always a matter of
consequence, they also would find advantage in employing the elliptical hearths (see the
form of the melting furnace, as figured under Smelting of copper ores.) Coal is now universally
preferred for fuel.
The alloy of 100 of copper with 50 of tin, or more exactly of 32 of the former with
141⁄2 of the latter, constitutes speculum metal, for making mirrors of reflecting telescopes.
This compound is nearly white, very brittle, and susceptible of a fine polish with a
brilliant surface. The following compound is much esteemed in France for making
specula. Melt 2 parts of pure copper and 1 of grain-tin in separate crucibles, incorporate
thoroughly with a wooden spatula, and then run the metal into moulds. The
lower surface is the one that should be worked into a mirror.
Mr. Edwards, in the Nautical Almanack for 1787, gave the following instructions for
making speculum metal.
The quality of the copper is to be tried by making a series of alloys with tin, in the
proportion of 100 of the former to 47, to 48, to 49, and to 50 of the latter metal;
whence the proportions of the whitest compound may be ascertained. Beyond the last
proportion, the alloy begins to lose in brilliancy of fracture, and to take a bluish tint.
Having determined this point, take 32 parts of the copper, melt, and add one part of brass
and as much silver, covering the surface of the mixture with a little black flux; when
the whole is melted, stir with a wooden rod, and pour in from 15 to 16 parts of
melted tin (as indicated by the preparatory trials), stir the mixture again, and immediately
pour it out into cold water. Then melt again at the lowest heat, adding for
every 16 parts of the compound 1 part of white arsenic, wrapped in paper, so that it
may be thrust down to the bottom of the crucible. Stir with a wooden rod as long as
arsenical fumes rise, and then pour it into a sand mould. While still red hot, lay the
metal in a pot full of very hot embers, that it may cool very slowly, whereby the danger
of its cracking or flying into splinters is prevented.
Having described the different alloys of copper and tin, I shall now treat of the method
of separating these metals from each other as they exist in old cannons, damaged
bells, &c. The process employed on a very great scale in France during the Revolution,
for obtaining copper from bells, was contrived by Fourcroy; founded upon the chemical
fact that tin is more fusible and oxidizable than copper.
1. A certain quantity of bell metal was completely oxidized by calcination in a
reverberatory furnace; the oxide was raked out, and reduced to a fine powder.
2. Into the same furnace a fresh quantity of the same metal was introduced; it was[334]
melted, and there was added to it one half of its weight of the oxide formed in the first
operation. The temperature was increased, and the mixture well incorporated; at the
end of a few hours, there was obtained on the one hand copper almost pure, which subsided
in a liquid state, and spread itself upon the sole of the hearth, while a compound of
oxide of tin, oxide of copper, with some of the earthy matters of the furnace collected
on the surface of the metallic bath in a pasty form. These scoriæ were removed with
a rake, and as soon as the surface of the melted copper was laid bare, it was run out.
The scoriæ were levigated, and the particles of metallic copper were obtained after
elutriation. By this process, from 100 pounds of bell metal, about 50 pounds of copper
were extracted, containing only one per cent. of foreign matters.
3. The washed scoriæ were mixed with 1⁄8 their weight of pulverised charcoal; the
mixture was triturated to effect a more intimate distribution of the charcoal; and it was
then put into a reverberatory hearth, in which, by aid of a high heat, a second reduction
was effected, yielding a fluid alloy consisting of about 60 parts of copper and 20 of tin;
while the surface of the bath got covered with new scoriæ containing a larger proportion
of tin than the first.
4. The alloy of 60 of copper with 40 of tin was next calcined in the same reverberatory
furnace, but with stirring of the mass. The air in sweeping across the surface of the bath,
oxidized the tin more rapidly than the copper; whence proceeded crusts of oxide that
were skimmed off from time to time. This process was continued till the metallic alloy
was brought to the same standard as bell metal, when it was run out to be subjected to
the same operations as the metal of No. 1.
The layers of oxide successively removed in this way were mixed with charcoal, and
reduced in a fourneau à manche, or Scotch lead smelting furnace.
I shall not prosecute any further the details of this complicated process of Fourcroy;
because it has been superseded by a much better one contrived by M. Bréant. He
employed a much larger quantity of charcoal to reduce the scoriæ rich in tin; and increased
the fusibility by adding crushed oyster-shells, bottle glass, or even vitrified scoriæ,
according to the nature of the substance to be reduced; and he treated them directly
in a reverberatory furnace.
The metal, thus procured, was very rich in tin. He exposed it in masses on a sloping
hearth of a reverberatory furnace, where, by a heat regulated according to the proportions
of the two metals in the alloy, he occasioned an eliquation or sweating out of the tin.
Metallic drops were seen to transpire round the alloyed blocks or pigs, and, falling like
rain, flowed down the sloping floor of the furnace; on whose concave bottom the metal
collected, and was ladled out into moulds. When the alloy, thus treated, contained lead,
this metal was found in the first portions that sweated out. The purest tin next came
forth, while the last portions held more or less copper in solution. By fractioning the
products, therefore, there was procured:
- 1. Tin with lead.
- 2. Tin nearly pure.
- 3. Tin alloyed with a little copper.
A spongy mass remained, exhibiting sometimes beautiful crystallizations; this mass,
commonly too rich in copper to afford tin by liquation, was treated by oxidizement. In
this manner, M. Bréant diminished greatly the reductions and oxidations; and therefore
incurred in a far less degree the enormous waste of tin, which flies off with the draught
of air in high and long continued heats. He also consumed less fuel as well as labour,
and obtained purer products of known composition, ready to be applied directly in
many arts.
He treated advantageously in this manner more than a million of kilogrammes
(1000 tons) of scoriæ, for every 2 cwts. of which he paid 40 centimes (four-pence), while
several million kilogrammes of much richer scoriæ had been previously sold to other
refiners at 5 centimes or one sous.
I have said that the ancients made their tools and military weapons of bronze.
Several of these have been analyzed, and the results are interesting.
An antique sword found in 1799, in the peat moss of the Somme, consisted of copper
87·47; tin 12·53, in 100 parts.
The bronze springs for the balistæ, according to Philo of Byzantium, were made of
copper 97, tin 3.
Hard and brittle nails afforded by analysis, 92 of copper, and 8 of tin.
Of three antique swords found in the environs of Abbeville, one was found to consist
of 85 of copper to 15 of tin. The nails of the handle of this sword were flexible; they
were composed of copper 95, tin 5.
Another of the swords consisted of 90 of copper and 10 of tin; and the third, of 96
copper, with 4 tin.
A fragment of an ancient scythe afforded to analysis 92·6 copper, and 7·4 tin.
The process of coating copper with tin, exemplifies the strong affinity between the
two metals. The copper surface to be tinned is first cleared up with a smooth sandstone;[335]
then it is heated and rubbed over with a little sal ammoniac, till it be perfectly
clean and bright: the tin, along with some pounded rosin, is now placed on the copper,
which is made so hot as to melt the tin, and allow of its being spread over the surface with
a dossil or pad of tow. The layer thus fixed on the copper is exceedingly thin; Bayen
found that a copper pan, 9 inches in diameter and 31⁄4 inches deep, being weighed
immediately before and after tinning, became only 21 grains heavier. Now as the
area tinned, including the bottom, amounted to 155 square inches, 1 grain of tin had
been spread over nearly 71⁄2 square inches; or only 20 grains over every square foot.
Copper and Arsenic form a white-coloured alloy, sometimes used for the scales of
thermometers and barometers; for dials, candlesticks, &c. To form this compound,
successive layers of copper clippings and white arsenic are put into an earthen crucible;
which is then covered with sea salt, closed with a lid, and gradually heated to redness.
If 2 parts of arsenic have been used with 5 of copper, the resulting compound commonly
contains one tenth of its weight of metallic arsenic. It is white, slightly
ductile, denser, and more fusible than copper, and without action on oxygen at ordinary
temperatures; but, at higher heats, it is decomposed with the exhalation of arsenious
acid. The white copper of the Chinese consists of 40·4 copper; 31·6 nickel; 25·4
zinc; and 2·6 iron. This alloy is nearly silver white; it is very sonorous, well
polished, malleable at common temperatures, and even at a cherry red, but very brittle
at a red-white heat. When heated with contact of air, it oxidizes, burning with a
white flame. Its specific gravity was 8·432. When worked with great care, it may
be reduced to thin leaves, and to wires as small as a needle. See German Silver,
infra.
Tutenag, formerly confounded with white copper, is a different composition from
the above. Keir says it is composed of copper, zinc, and iron; and Dick describes it
as a short metal, of a grayish colour, and scarcely sonorous. The Chinese export it, in
large quantities, to India.
Copper, White, or German silver. M. Gersdorf, of Vienna, states, that the proportions
of the metals in this alloy should vary according to the uses for which it is
destined. When intended as a substitute for silver, it should be composed of 25 parts
of nickel, 25 of zinc, and 50 of copper. An alloy better adapted for rolling, consists of
25 of nickel, 20 of zinc, and 60 of copper. Castings, such as candlesticks, bells, &c.,
may be made of an alloy, consisting of 20 of nickel, 20 of zinc, and 60 of copper; to
which 3 of lead are added. The addition of 2 or 21⁄2 of iron (in the shape of tin plate?)
renders the packfong much whiter but, at the same time, harder and more brittle.
Keferstein has given the following analysis of the genuine German silver, as made
from the original ore found in Hildburghausen, near Suhl, in Henneberg:—
| Copper |
40·4 |
| Nickel |
31·6 |
| Zinc |
25·4 |
| Iron |
2·6 |
| |
100·0 |
Chinese packfong, according to the same authority, consists of 5 parts of copper,
alloyed with 7 parts of nickel, and 7 parts of zinc.
The best alloy for making plummer blocks, bushes, and steps for the steel or iron
gudgeons, and pivots of machinery to run in, is said to consist of 90 parts of copper,
5 of zinc, and 5 of antimony.
A factitious protoxide of copper, of a fine red colour, may be made by melting
together, with a gentle heat, 100 parts of sulphate of copper, and 59 of carbonate of
soda in crystals, and continuing the heat till the mass become solid. This being
pulverized, and mixed exactly with 15 parts of copper filings, the mixture is to be
heated to whiteness, in a crucible, during the space of 20 minutes. The mass, when
cold, is to be reduced to powder, and washed. A beautiful metallic pigment may be
thus prepared, at the cost of 2s. a pound.
All the oxides and salts of copper are poisonous; they are best counteracted by administering
a large quantity of sugar, and sulphuretted hydrogen water.
The following scientific summary of copper ores in alphabetical order may prove
acceptable to many readers, amid the present perplexing distribution of the native metallic
compounds in mineralogical systems.
1. Arseniate of Copper.
A. Erinite, rhomboidal arseniate of copper, micaceous copper, kupferglimmer.
Emerald green; specific gravity 4·043; scratches calc-spar; yields water by heat;
fusible at the blowpipe, and reducible into a white metallic globule. Soluble in nitric
acid; the solution throws down copper by iron. It consists of arsenic acid 33·78;
oxide of copper 59·24; water 5; alumina 1·77. It is found in Cornwall, Ireland,
Hungary.
B. Liroconite; octahedral arseniate of copper; lens ore, so called from the flatness[336]
of the crystal. Blue; specific gravity 2·88; scratches calc-spar. It consists of arsenic
acid 14; oxide of copper 49; water 35. It is found in Huel-Mutrel, Huel-Gorland,
Huel-Unity, mines in Cornwall.
C. Olivenite; right prismatic arseniate of copper; olive-ore. Dull green; specific
gravity 4·28; scratches fluor; yields no water by heat; fusible at the blowpipe into
a glassy bead, enclosing a white metallic grain. It consists of arsenic acid 45, oxide
of copper 50·62. It affords indications of phosphoric acid, which the analysts seem to
have overlooked. It occurs in the above and many other mines in Cornwall.
D. Aphanese. Trihedral arseniate of copper. Bluish green, becoming gray upon
the surface; specific gravity 4·28; scarcely scratches calc-spar; yields water with heat;
and traces of phosphoric acid.
The fibrous varieties called wood copper, contain water, and resemble the last species
in composition.
2. Carbonate of Copper.
A. Azurite; kupferlazur. Blue. Crystallizes in oblique rhomboidal prisms; specific
gravity 3 to 3·83; scratches calc-spar, is scratched by fluor; yields water with heat,
and blackens. Its constituents are, carbonic acid 25·5; oxide of copper 69·1; water
5·4. The Chessy and Banat azurite is most profitably employed to make sulphate of
copper.
B. Malachite; green carbonate or mountain green. Crystallizes in right rhomboidal
prisms; specific gravity 3·5; affords water with heat, and blackens. It consists of carbonic
acid 18·5; oxide of copper 72·2; water 9·3.
C. Mysorine; anhydrous carbonate of copper. Dark brown generally stained green
or red; conchoidal fracture; soft, sectile; specific gravity 2·62. It consists of carbonic
acid 16·7; oxide of copper 60·75; peroxide of iron 19·5; silica 2·10. This is a rare
mineral found in the Mysore.
3. Chromate of Copper and Lead; vauquelinite. Green of various shades; specific
gravity 6·8 to 7·2; brittle; scratched by fluor; fusible at the blowpipe with froth and
the production of a leaden bead. It consists of chromic acid 28·33; oxide of lead 60·87;
oxide of copper 10·8. It occurs at Berezof in Siberia along with chromate of lead.
4. Dioptase; silicate of copper; emerald copper. Specific gravity 3·3; scratches
glass with difficulty; affords water with heat, and blackens; infusible at the blowpipe.
It consists of silica 43·18; oxide of copper 45·46; water 11·36. This rare substance
comes from the government of Kirgis.
The silicate of Dillenberg is similar in composition.
5. Gray copper ore called Panabase, from the number of metallic bases which it
contains; and Fahlerz. Steel gray; specific gravity 4·79 to 5·10; crystallizes in regular
tetrahedrons; fusible at the blowpipe, with disengagement of fumes of antimony and
occasionally of arsenic; swells up and scorifies, affording copper with soda flux. Is acted
upon by nitric acid with precipitation of antimony; becomes blue with ammonia;
yields a blue precipitate with ferrocyanide of potassium; as also indications frequently
of zinc, mercury, silver, &c. Its composition which is very complex is as follows:
sulphur 26·83; antimony 12·46; arsenic 10·19; copper 40·60; iron 4·66; zinc 3·69;
silver 0·60. Some specimens contain from 5 to 31 per cent. of silver. The gray copper
ores are very common; in Saxony; the Hartz; Cornwall; at Dillenberg; in Mexico;
Peru, &c. They are important on account both of their copper and silver. Tennantite
is a variety of Fahlerz. It occurs in Cornwall. Its constituents are, sulphur 28·74;
arsenic 11·84; copper 45·32; iron 9·26.
6. Hydrated silicate of Copper; or Chrysocolla. Green or bluish green; specific
gravity 2·03 to 2·16; scratched by steel; very brittle; affords water with heat, and
blackens; is acted upon by acids, and leaves a siliceous residuum. Solution becomes
blue with ammonia. Its constituents are silica 26; oxide of copper 50; water 17;
carbonic acid 7.
7. Muriate of Copper. Atakamite; green; crystallizes in prisms; specific gravity
4·43. Its constituents are, chlorine 15·90; copper 14·22; oxide of copper 54·22;
water 14·16; oxide of iron 1·50. The green sand of Peru, collected by the inhabitants
of Atakama, is this substance in a decomposed state.
8. Oxide of Copper.
A. Black, or Melaconise; a black earthy looking substance found at Chessy and
other places. It is deutoxide of copper.
B. Protoxide or red oxide of copper; ziegelerz. Crystallizes in the regular octahedron;
specific gravity 5·69; scratches calc-spar; fusible at the blowpipe into the black
oxide; and reducible in the smoke of the flame to copper; acted upon by nitric acid
with disengagement of nitrous gas; solution is rendered blue by ammonia. Its constituents
are oxygen 11·22; copper 88·78. It occurs near Chessy, and upon the eastern
slope of the Altai mountains.
9. Phosphate of Copper. Dark green; crystallizes in octahedrons; specific gravity
3·6 to 3·8; scratches calc-spar; yields water with heat; and affords metallic copper[337]
with soda flux; acted on by nitric acid. Its constituents are, phosphoric acid 28·7;
oxide of copper 63·9; water 7·4. It occurs at the mines of Libethen in Hungary.
10. Pyritous Copper; Kupferkies; a metallic looking substance, of a bronze-yellow
colour, crystallizing in octahedrons which pass into tetrahedrons; specific gravity 4·16;
fusible at the blowpipe into beads attractable by the magnet, and which afterwards
afford copper with a soda flux; soluble in nitric acid; solution is rendered blue by
ammonia, and affords an abundant precipitate of iron. Its composition is, sulphur 36;
copper 34·5; iron 30·5; being a combined sulphuret of these two metals. This is the
most important metallurgic species of copper ores. It occurs chiefly in primitive formations,
as among gneiss and mica slate, in veins or more frequently masses in very
many parts of the world—Cornwall, Anglesea, Wicklow, &c. It is found among the
early secondary rocks, in Shetland, Yorkshire, Mansfeldt, &c. The finest crystallized
specimens come from Cornwall, Derbyshire, Freyberg, and Saint Marie-aux-Mines in
France.
11. Seleniate of Copper; Berzeline. Is of metallic aspect; silver white; ductile;
fusible at the blowpipe into a gray bead, somewhat malleable; is acted upon by nitric
acid; consists of selenium 40; copper 64.
12. Sulphate of Copper; Cyanose. Blue; soluble, &c. like the artificial sulphates,
which see.
Brochantite is a subsulphate of copper observed in small crystals at Ekaterinenbourg
in Siberia.
13. Sulphuret of Copper; Kupferglanz. Of a steel gray metallic aspect; crystallizes
in rhomboids; specific gravity 5·69; somewhat sectile, yet brittle; fusible with intumescence
at the blowpipe, and yields a copper bead with soda; soluble in nitric acid;
becomes blue with ammonia, but lets fall scarcely any oxide of iron. Its constituents
are, sulphur 19; copper 79·5; iron 0·75; silica 1·00. It occurs in small quantities in
Cornwall, &c.
The chemical preparations of copper which constitute distinct manufactures are,
Blue or Roman vitriol; for which see Sulphate of Copper; Scheele’s green and Schweinurth
green, Verditer, and Verdigris. See these articles in their alphabetical places.
COPPER, Statistics of.—Copper ores may be imported into Great Britain for
smelting, from any country, and under any flag. On arrival of the cargo at Swansea
or elsewhere, a bond is given at the Custom-house, which binds the party to return the
quantity of copper which the lot of ores shall be ascertained to contain, into bond within
a limited period, or pay thereon the duty as foreign copper, which is 27l. per ton.
The cargo of ore is then weighed out by the custom-house officer, and samples are
taken which are sent to two assay-masters in Cornwall, the highest produce of the
two being entered as that of the cargo. This fixes the quantity of copper that must be
exported under the bond.
The copper produced from foreign ores must then find a market, as cake or pig copper,
in France, Holland, Germany, Italy, the United States of America, &c. At Calcutta, it
is subject to a duty of 6 per cent.; and at Bombay, to a duty of 10 per cent. ad valorem.
The export of British unwrought copper to the continent of Europe, and to the
United States of America, was formerly inconsiderable. These countries drew the bulk
of their supplies either from the north of Europe, or direct from South America in pig
copper. In point of fact, the copper derived from the import of foreign ores for smelting,
has produced for itself a new market, as the following table, taken from the official
returns will show.
Export of unwrought copper from Great Britain to all parts, except Asia:—
| Years ending Jan. 5th. |
1830 |
881 |
tons. |
| — |
1831 |
857 |
— |
| — |
1832 |
1326 |
— |
| — |
1833 |
2471 |
— |
| — |
1834 |
2523 |
— |
| — |
1835 |
3267 |
— |
| — |
1836 |
4083 |
— |
| — |
1837 |
2546 |
— |
In the last year, that ended with 5th January, 1838, the export of unwrought copper
was about 5000 tons.
Let any candid and practical man consider attentively this table, and compare it with
the import of foreign ores for the same period, and with the gradual advance in the
value of copper; and then let him, if he can, avoid the conclusion that the admission of
foreign ores for smelting was a great boon conferred upon the British copper mines, for
it made this country what it now is, the regulator and distributor of the copper produce
of the world—the country to which all others consuming and not producing copper,
must look for a regular, certain, and economical supply. We want the admission merely
under proper and safe regulations, of foreign copper for refining, to draw to this country[338]
the whole supply of copper for the world, by which prices would be regulated and maintained,
and our copper-mining interests put beyond the reach of successful rivalry.
This country did not furnish any supply of unwrought copper to the continent of
Europe, or to the United States of America, which was worthy of notice, before the year
1830; in fact, previous to that time, we imported considerable quantities of foreign
copper for re-exportation to India. It is easy to explain how the produce of foreign
ores, being prohibited from export in any other shape, has, in fact, opened for itself a
new debouché, and this is illustrated by the table, showing the growth of the export of
unwrought copper from 1830. To prove that this is not merely a simultaneous advance
in the export of all sorts of copper, a corrected table is subjoined from the official returns,
comprising the whole export, and divided so as to illustrate the operation of the copper
produce of foreign ores upon our foreign copper trade.
Copper exported:—
| Years ending |
Wrought. |
Unwrought. |
Total. |
| To all parts. |
To India. |
To all parts. |
To all parts. |
| |
Tons. |
Tons. |
Tons. |
Tons. |
| 5th January, |
1825 |
- |
- |
- |
- |
960 |
|
|
| 1826 |
- |
- |
- |
- |
|
1⁄2 |
|
| 1827 |
- |
- |
- |
- |
130 |
|
|
| 1828 |
- |
- |
- |
- |
1329 |
|
|
| 1829 |
- |
- |
- |
- |
1079 |
|
|
| 1830 |
5327 |
1801 |
2682 |
|
8,009 |
|
| 1831 |
6172 |
2317 |
3150 |
|
9,322 |
|
| 1832 |
5171 |
2423 |
3714 |
|
8,885 |
|
| 1833 |
5855 |
2312 |
4569 |
|
10,424 |
|
| 1834 |
5417 |
1769 |
4019 |
|
9,436 |
|
| 1835 |
4787 |
2104 |
5283 |
|
10,072 |
|
| 1836 |
5948 |
1993 |
5935 |
|
11,883 |
|
| 1837 |
6105 |
1588 |
3909 |
|
10,014 |
[17] |
Production of Copper in Great Britain:—
| Years. |
Ores. |
Metal. |
| |
Tons. |
Tons. |
| 1771-1781 |
28,185 |
3380 |
|
| 1781-1791 |
32,854 |
4123 |
|
| 1791-1801 |
48,034 |
4083 |
|
| 1801-1811 |
67,533 |
6060 |
|
| 1811-1816 |
78,237 |
7181 |
|
| 1816 |
83,058 |
7045 |
|
| 1817 |
75,016 |
6608 |
|
| 1818 |
80,525 |
6714 |
|
| 1819 |
92,234 |
7214 |
|
| 1820 |
92,672 |
7364 |
|
| 1821 |
98,803 |
8163 |
|
| 1822 |
106,723 |
9331 |
|
| 1826 |
128,459 |
— |
| 1827 |
— |
12,381 |
|
| 1828 |
153,600 |
12,169 |
|
| 1829 |
— |
11,994 |
|
| 1830 |
— |
13,097 |
|
| 1831 |
— |
14,480 |
|
| 1832 |
— |
14,463 |
[18] |
[339]
Quantity of Copper produced in the several districts of Great Britain and Ireland:—
| With Ores from— |
1828. |
1829. |
1830. |
1831. |
1832. |
| |
Tons. |
Tons. |
Tons. |
Tons. |
Tons. |
| Cornwall |
1966 |
9763 |
10,890 |
12,218 |
12,099 |
| Devonshire |
434 |
318 |
368 |
312 |
249 |
| Other parts of England |
71 |
36 |
10 |
31 |
42 |
| Island of Anglesea |
738 |
901 |
815 |
809 |
852 |
| Other parts of Wales |
259 |
172 |
237 |
123 |
237 |
| Ireland |
706 |
790 |
768 |
972 |
974 |
| Isle of Man |
— |
4 |
9 |
15 |
12 |
Total copper from the
ores of the United Kingdom |
12,169 |
11,994 |
13,097 |
14,480 |
14,465 |
Copper smelted from
Foreign ores |
— |
30 |
124 |
100 |
56 |
| General total |
12,169 |
12,024 |
13,221 |
14,580 |
14,521 |
Statistics of Copper for Cornwall in 1837.—The total quantity of ore sold was 142,089
tons (of 21 cwts.), yielding an average produce of eight per cent.; the quantity of fine
copper being 11,209 tons 1 cwt.; and the average price of the ore 5l. 15s. 6d.; the
total amount of the sales for the twelve months being 822,516l. The standard upon
the 5th of January was 127l. 16s.; this was the highest for the year. Upon the 22d of
June it was at the lowest, being only 93l. 18s. It went up again to 120l. 10s. upon the
5th of October; but declined with some slight fluctuation to 107l. 18s. upon the 28th
of December. The largest quantity sold at any one ticketing, was 4670 tons, upon the
4th of May: and the smallest 1088, upon the 17th of August. The highest produce
was nine and five-eighths per cent. upon the 13th of July; and the lowest, seven, upon
the 26th of January. The greatest weekly total was 25,887l., upon the 2nd of November,
and the least 5694l. upon the 17th of August. The average sum per week was
15,817l.[19]
Table of the produce of Copper Ores and fine Metal in Cornwall, from 1800 to 1830.
| Years. |
Ores. |
Metal. |
Value of Ore. |
Metal. |
Average
Standard. |
| |
Tons of
21 Cwts |
Tons. |
Cwt. |
|
Per Cent.
of Ore. |
Price
per Ton. |
| |
|
|
£ |
s. |
d. |
|
£ |
s. |
d. |
| 1800 |
55,981 |
5187 |
0 |
550,925 |
0 |
0 |
9 |
1⁄4 |
133 |
3 |
6 |
| 1801 |
56,611 |
5268 |
0 |
476,313 |
0 |
0 |
9 |
1⁄4 |
117 |
8 |
0 |
| 1802 |
53,937 |
5228 |
15 |
445,094 |
0 |
0 |
9 |
5⁄8 |
110 |
18 |
0 |
| 1804 |
64,637 |
5374 |
18 |
507,840 |
11 |
0 |
8 |
3⁄8 |
136 |
5 |
0 |
| 1806 |
79,269 |
6863 |
10 |
730,845 |
6 |
0 |
8 |
5⁄8 |
138 |
5 |
0 |
| 1808 |
67,867 |
6795 |
13 |
495,303 |
10 |
0 |
10 |
|
100 |
7 |
0 |
| 1810 |
66,048 |
5682 |
19 |
570,035 |
8 |
0 |
8 |
1⁄2 |
132 |
5 |
0 |
| 1812 |
71,547 |
6720 |
7 |
549,665 |
6 |
0 |
9 |
3⁄8 |
111 |
0 |
0 |
| 1814 |
74,322 |
6369 |
13 |
627,501 |
10 |
0 |
8 |
1⁄2 |
130 |
12 |
0 |
| 1816 |
77,334 |
6697 |
4 |
447,959 |
17 |
0 |
8 |
5⁄8 |
98 |
13 |
0 |
| 1818 |
86,174 |
6849 |
7 |
686,005 |
4 |
0 |
7 |
7⁄8 |
134 |
15 |
0 |
| 1820 |
91,473 |
7508 |
0 |
602,441 |
12 |
0 |
8 |
1⁄8 |
113 |
15 |
0 |
| 1822 |
104,523 |
9140 |
8 |
663,085 |
13 |
0 |
8 |
3⁄4 |
104 |
0 |
0 |
| 1824 |
99,700 |
7823 |
15 |
587,178 |
0 |
0 |
7 |
7⁄8 |
110 |
0 |
0 |
| 1826 |
117,308 |
9026 |
12 |
788,971 |
15 |
0 |
7 |
5⁄8 |
123 |
3 |
0 |
| 1828 |
130,366 |
9921 |
1 |
756,174 |
16 |
0 |
7 |
5⁄8 |
112 |
7 |
0 |
| 1829 |
124,502 |
9656 |
10 |
717,334 |
0 |
0 |
7 |
3⁄4 |
109 |
14 |
0 |
| 1830 |
143,296 |
11,224 |
19 |
887,900 |
0 |
0 |
7 |
3⁄4 |
114 |
4 |
0 |
| 1834 |
150,617 |
12,271 |
14 |
893,402 |
15 |
0 |
8 |
1⁄8 |
106 |
11 |
0 |
| 1835 |
[340]
Produce of Copper Mines in Cornwall, (on the authority of John Taylor, Esq. F.R.S.)
| Years. |
Ore. |
Metal. |
Value. |
Produce. |
Standard. |
| |
Tons. |
Tons. |
£. |
s. |
d. |
Per Cwt. |
|
| 1831 |
144,402 |
12,044 |
806,090 |
15 |
6 |
8 |
1⁄4 |
100 |
| 1832 |
137,357 |
11,948 |
825,612 |
6 |
0 |
8 |
5⁄8 |
100 |
| 1833 |
138,300 |
11,191 |
858,708 |
10 |
0 |
8 |
1⁄8 |
111 |
| 1834 |
143,296 |
11,226 |
887,902 |
0 |
0 |
7 |
3⁄4 |
114 |
| 1835 |
150,617 |
12,270 |
893,402 |
14 |
0 |
8 |
1⁄8 |
106 |
| 1836 |
140,981 |
11,647 |
957,752 |
8 |
6 |
8 |
1⁄4 |
115 |
| 1837 |
140,753 |
10,832 |
908,613 |
15 |
0 |
7 |
5⁄8 |
120 |
An account of the quantities of Foreign wrought and unwrought Copper, and Copper
Ore imported and exported, and of British wrought and unwrought Copper exported
from the United Kingdom; together with the quantities and value of Copper Ore smelted
in Cornwall and Swansea, and the quantity of Copper produced in those places; and in
the county of Devon; together with the market prices of sheet and cake Copper, in the
year ending 5th January, 1835.
| |
Quan-
tity. |
Value. |
| Foreign Copper imported:— |
|
|
£ |
s. |
d. |
| Unwrought in bricks or pigs, rose and cast copper |
Cwts. |
|
5,389 |
|
|
| Part wrought, viz., bars, rods, or ingots, hammered or raised |
|
|
1,968 |
|
|
| Wrought plates and coin |
|
|
2 |
|
|
| Wr — htold for re-manufacture |
|
|
493 |
|
|
| Copper ore Foreign |
|
|
278,900 |
|
|
| Manufactures of copper, entered by weight |
|
|
650 |
|
|
| Manufactur — f copper, entered at value |
|
— |
5,353 |
0 |
0 |
| Foreign Copper exported, viz.:— |
|
|
|
| Unwrought, in bricks and pigs, rose and cast copper |
Cwts. |
|
6,898 |
|
|
| Part wrought, viz., bars, rods, or ingots, hammered or raised |
|
|
2,013 |
|
|
| Old, fit only for re-manufacture |
|
|
265 |
|
|
| Smelted in the United Kingdom from foreign ore |
|
|
55,456 |
|
|
| Manufactures of copper, entered by weight |
|
|
650 |
|
|
| Manufactur — f copper, entered at value |
|
— |
112 |
0 |
0 |
| |
|
|
| BRITISH COPPER. |
|
|
| Exported, unwrought, in bricks and pigs |
Cwts. |
|
63,252 |
|
|
| E—rtedwrought sheets, nails, &c. |
|
|
103,433 |
|
|
| Exported, —ughtwire |
|
|
56 |
|
|
| Exported, —ughtof other sorts |
|
|
15,197 |
|
|
| E—rtedTotal of British copper exported |
|
|
182,225 |
|
|
| Ores sold in Cornwall:— |
|
|
|
| Quantity of ore |
Tons |
|
150,617 |
|
|
| Value of ditto |
|
— |
893,403 |
0 |
0 |
| Quantity of metal |
Tons |
|
12,270 |
|
|
| Standard |
|
— |
106 |
11 |
0 |
| Produce per cent. |
|
|
8 |
1⁄2 |
|
| Ores sold, &c. in Swansea:— |
|
|
|
| Quantity of ore |
Tons |
|
28,746 |
|
|
| Value of ditto |
|
— |
223,958 |
0 |
0 |
| Quantity of metal |
Tons |
|
2,832 |
|
|
| Standard |
|
— |
101 |
18 |
0 |
| Produce per cent. |
|
|
9 |
7⁄8 |
|
| Copper sold in Devonshire {oremetal} |
Tons |
{ |
5,114455 |
|
|
| Total quantity of copper raised in the United Kingdom, exclusive of Anglesea and Staffordshire, and deducting 1083 tons of metal, value 88,207l., the produce of 4985 tons of foreign ore sold at Swansea, included above. |
14,474 |
|
|
[341]
COPPERAS. (Couperose verte, Fr.; Eisenvitriol, Germ.) Sulphate of iron.
CORAL, (Corail, Fr.; Koralle, Germ.) is a calcareous substance, formed by a
species of sea polypus, which constructs in concert immense ramified habitations, consisting
of an assemblage of small cells, each the abode of an animal. The coral is therefore
a real polypary, which resembles a tree stripped of its leaves. It has no roots, but a foot not
unlike a hemispherical skull-cap, which applies closely to every point of the surface upon
which it stands, and is therefore difficult to detach. It merely serves as a basis or support
to the coral, but contributes in no manner to its growth, like the root of an ordinary
tree; for detached pieces have been often found at the bottom of the sea in a state of
increase and reproduction. From the above base a stem usually single proceeds, which
seldom surpasses an inch in diameter, and from it a small number of branches ramify in
very irregular directions, which are studded over with cells, each containing an insect.
The polypi, when they extend their arms, feelers, or tentacula, resemble flowers, whence,
as well as from the form of the coral, they were classed among vegetable productions.
They are now styled zoophytes by the writers upon Natural History.
The finest coral is found in the Mediterranean. It is fished for upon the coasts of
Provence, and constitutes a considerable branch of trade at Marseilles. The coral is attached
to the submarine rocks, as a tree is by its roots, but the branches, instead of
growing upwards, shoot downwards towards the bottom of the sea; a conformation
favourable to breaking them off and bringing them up. For this kind of fishing, eight
men, who are excellent divers, equip a felucca or small boat, called commonly a coralline.
They carry with them a large wooden cross, with strong, equal, and long arms,
each bearing a stout bag-net. They attach a strong rope to the middle of the cross,
and let it down horizontally into the sea, having loaded its centre with a weight sufficient
to sink it. The diver follows the cross, pushes one arm of it after another into the hollows
of the rocks, so as to entangle the coral in the nets. Then his comrades in the boat
pull up the cross and its accompaniments.
Coral fishing is nearly as dangerous as pearl fishing, on account of the number of sharks
which frequent the seas where it is carried on. One would think the diving-bell in its
now very practicable state might be employed with great advantage for both purposes.
Coral is mostly of a fine red colour, but occasionally it is flesh-coloured, yellow, or
white. The red is preferred for making necklaces, crosses, and other female ornaments.
It is worked up like precious stones. See Lapidary.
CORK, (Liége, Fr.; Kork, Germ.) is the bark of the quercus liber, Linn., a species of
oak-tree, which grows abundantly in the southern provinces of France, Italy, and Spain.
The bark is taken off by making coronal incisions above and below the portions to be
removed; vertical incisions are then made from one of these circles to another, whereby
the bark may be easily detached. It is steeped in water to soften it, in order to be flattened
by pressure under heavy stones, and next dried at a fire which blackens its surface.
The cakes are bound up in bales and sent into the market.
There are two sorts of cork, the white and the black; the former grows in France and
the latter in Spain. The cakes of the white are usually more beautiful, more smooth,
lighter, freer from knots and cracks, of a finer grain, of a yellowish gray colour on both
sides, and cut more smoothly than the black. When this cork is burned in close vessels
it forms the pigment called Spanish black.
This substance is employed to fabricate not only bottle corks, but small architectural
and geognostic models, which are very convenient from their lightness and solidity.
The cork-cutters divide the boards of cork first into narrow fillets, which they afterwards
subdivide into short parallelopipeds, and then round these into the proper conical
or cylindrical shape. The bench before which they work is a square table, where 4
workmen are seated, one at every side, the table being furnished with a ledge to prevent
the corks from falling over. The cork-cutter’s knife is a broad blade, very thin, and fine
edged. It is whetted from time to time upon a fine-grained dry whetstone. The workman
ought not to draw his knife edge over the cork, for he would thus make misses, and
might cut himself, but rather the cork over the knife edge. He should seize the knife
with his left hand, rest the back of it upon the edge of the table; into one of the notches
made to prevent it from slipping, and merely turns its edge sometimes upright and sometimes
to one side. Then holding the squared piece of cork by its two ends, between his
finger and his thumb, he presents it in the direction of its length to the edge; the cork is
now smoothly cut into a rounded form by being dexterously turned in the hand. He next
cuts off the two ends, when the cork is finished and thrown into the proper basket alongside,
to be afterwards sorted by women or boys.
Of late years a much thicker kind of cork boards have been imported from Catalonia,
from which longer and better corks may be made. In the art of cork-cutting the French
surpass the English, as any one may convince himself by comparing the corks of their
champagne bottles with those made in this country.
Cork, on account of its buoyancy in water, is extensively employed for making floats[342]
to fishermen’s nets, and in the construction of life-boats. Its impermeability to water has
led to its employment for inner soles to shoes.
When cork is rasped into powder, and subjected to chemical solvents, such as alcohol,
&c., it leaves 70 per cent. of an insoluble substance, called suberine. When it is treated
with nitric acid, it yields the following remarkable products:—White fibrous matter
0·18, resin 14·72, oxalic acid 16·00, suberic acid (peculiar acid of cork) 14·4 in 100
parts.
Machine cork-cutting.—A patent was obtained some years ago by Sarah Thomson for
this purpose. The cutting of the cork into slips is effected by fixing it upon the sliding
bed of an engine, and bringing it, by a progressive motion, under the action of a circular
knife, by which it is cut into slips of equal widths. The nature or construction of a
machine to be used for this purpose may be easily conceived, as it possesses no new mechanical
feature, except in its application to cutting cork. The motion communicated
to the knife by hand, steam, horse, or other power, moves at the same time the bed also,
which carries the cork to be cut.
The second part of the invention, viz. that for separating the cork into square pieces,
after it has been cut in slips as above, is effected by a moving bed as before, upon which
the slips are to be placed and submitted to the action of a cutting lever, which may be
regulated to chop the cork into pieces of any given length.
The third part of the invention, viz., that for rounding or finishing the corks, consists
of an engine to which is attached a circular knife that turns vertically, and a carriage or
frame upon its side that revolves upon an axle horizontally.
This carriage or frame contains several pairs of clamps, intended respectively to hold
a piece of the square cut cork by pressing it at the ends, and carrying it lengthways perpendicularly;
which clamps are contrived to have a spindle motion, by means of a pinion
at the lower end of their axles, working into a spur-wheel.
The machinery, thus arranged, is put in motion by means of bands and drum-wheels,
or any other contrivance which may be found most eligible; and at the same time that
the circular knife revolves vertically, the frame containing the clamps with the pieces of
cork, turns horizontally, bringing the corks, one by one, up to the edge of the knife, when,
to render each piece of cork cylindrical, the clamps, as above described, revolve upon their
axes, independently of their carriage, by which means the whole circumference of the
cork is brought under the action of the knife, the superfluous parts are uniformly pared
off, and the cork finished smooth and cylindrical.
CORROSIVE SUBLIMATE; bichloride of mercury.
CORUNDUM; or Telesie; a very hard genus of aluminous minerals, to which the
gems, sapphire, ruby, salamstein, and adamantine spar belong.
COTTON DYEING. (Teinture de Coton, Fr.; Baumwollenfärberei, Germ.)
Cotton and linen yarns and cloths have nearly the same affinity for dyes, and
may therefore with propriety be treated, in this respect, together. After they have
acquired the proper degree of whiteness (see Bleaching) they are still unfit to
receive and retain the dyes in a permanent manner. It is necessary, before dipping
them into the dye-bath, to give them a tendency to condense the colouring particles
within their cavities or pores, and to communicate such chemical properties as will
fix these particles so that they will not separate, to whatever ordinary trial they may
be subjected. All the colours which it would be desirable to transfer to these stuffs unfortunately
do not possess this permanence. Men of science engaged in this important art have
constantly aimed at the discovery of some new processes which may transfer into the class
of fast colours those dyes which are at present more or less fugitive. Almost all the
goods manufactured of cotton, flax, or hemp, are intended to be washed, and ought,
therefore, to be so dyed as to resist the alkaline and soapy solutions commonly used in
the laundry. Vitalis distinguished dyed cottons into three classes; 1. the fugitive, or
fancy-coloured (petit teint), which change their hue or are destroyed by one or two
boils with soap; 2. those which resist five or six careful washings with soap, are good dyes,
(bon teint); and those which were still more durable, such as Turkey reds, may be
called fast colours (grand teint). The colours of Brazil wood, logwood, annotto, safflower,
&c., are fugitive; those made with madder without an oily base, are good; and
those of madder with an oily mordant, are fast. It is, however, possible to point out
certain processes for giving these different orders of dyes a greater degree of fixity.
I shall describe, in the five following paragraphs, the operations conducive to the
fixation of colours upon cotton and linen.
1. Galling. Either gall nuts alone, or sumach alone, or these two substances united,
are employed to give to cotton the fast dye preparation. 2 or 3 ounces of galls for every
pound of cotton, being coarsely pounded, are to be put into a copper containing about
30 gallons of water for every 100 pounds of cotton, and the bath is to be boiled till the bits
of galls feel pasty between the fingers. The fire being withdrawn, when the bath becomes
moderately cool, it is passed through a hair-cloth sieve. If during this operation the[343]
liquor should become cold, it must be made once more as hot as the hand can bear. A
portion of it is now transferred into another vessel, called a back, in which the cotton
is worked till it be well penetrated with the decoction. It is then taken out, wrung at
the peg or squeezed in a press, and straightway hung up in the drying house. Some
more of the fresh decoction being added to the partially exhausted liquor in the back,
the process is resumed upon fresh goods.
The manipulation is the same with sumach, but the bath is somewhat differently
made; because the quantity of sumach must be double that of galls, and must be
merely infused in very hot water, without boiling. When galls and sumach are both
prescribed, their baths should be separately made and mixed together.
2. Aluming. Alum is a salt which serves to prepare cotton for receiving an indefinite
variety of dyes. Its bath is made as follows: For 100 pounds of scoured cotton, about
30 gallons of water being put into the copper, are heated to about 122° F., when 4
ounces of alum, coarsely pounded, are thrown in for every pound of cotton, and instantly
dissolved. Whenever the heat of the bath has fallen to about 98° F., the cotton is well
worked in it, in order that the solution may thoroughly penetrate all its pores. It is
then taken out, wrung at the peg or squeezed in the press, and dried in the shade. The
solution of alum is of such constant employment in this kind of dyeing, that it should
be made in large quantities at a time, kept in the alum tun, where it can suffer no
deterioration, and drawn off by a spigot or stop-cock as wanted.
There are certain colours which require alum to be deprived of a portion of its acid
excess, as a supersalt; which may be done by putting 1 ounce of crystals of soda into
the tun for every pound of alum. But so much soda should never be used as to cause
any permanent precipitation of alumina. When thus prepared, it is called saturated
alum, though it is by no means neutral to litmus paper; but it crystallizes differently
from ordinary alum.
Cotton does not take up at the first aluming a sufficient quantity of alum; but it
must receive a second, or even a third immersion. In every case the stuff should be
thoroughly dried, with an interval of one or two days between each application; and it
may even be left for 10 or 12 hours moist with the alum bath before being hung in the
air. When the cotton is finally dry, it must be washed before being plunged into the dye
bath; otherwise, the portion of alum, not intimately combined with the cotton, but
adhering externally to its filaments, would come off by the heat, mix with the bath,
alter the colour by dissolving in it, and throw it down to the bottom of the copper, in
the form of a lake, to the great loss of the dyer. Madder reds, weld yellows, and some
other colours, are more brilliant and faster when acetate of alumina, prepared with
acetate of lead, alum, and a little potash, is used, than even saturated alum. This
mordant is employed cold, and at 4° Baumé.
3. Mordants. See this article in its alphabetical place.
4. Dye baths, are distinguished into two classes; the colouring bath, and the dyeing
bath. The former serves to extract the colouring matters of the different substances,
with the exception of madder, which is always used in substance, and never as an
extract, infusion, or decoction. In all these cases, when the colour is extracted, that is,
when the dye bath is completed by the degree of heat suited to each substance, it is then
allowed to cool down a certain way, and the cotton is worked or winced through it, to
get the wished-for tint. This is what is called the dye bath. Several colouring baths
are made in the cold; and they serve to dye also in the cold; but the greater part
require a heat of 90° or 100° to facilitate the penetration of the stuffs by the colouring
particles. The description of the several dye baths is given under the individual dyes.
5. Of the washing after the dyeing.—The washing of the cottons after they have
received the dyes, is one of the most important operations in the business. If it is not
carefully performed, the excess of colour not combined with the fibres, is apt to stain
whatever it touches. This inconvenience would be of little consequence, if the friction
carried off the colour equally from all the points; but it does not do so, and hence the
surface appears mottled. A well-planned dye house should be an oblong gallery, with
a stream of water flowing along in an open conduit in the middle line, a series of dash
wheels arranged against the wall, at one side, and of dyeing coppers, furnished with self-acting
winces or reels, against the other. In such a gallery, the washing may be done
either by hand, by the rinsing machine, or by the dash wheel, according to the quality
of the dye, and the texture of the stuffs. And they may be stripped of the water either
by the jack and pin, by the squeezing roller, or by the press. Wooden pins are placed in
some dye-houses on each side of the wash cistern or pool. They are somewhat conical,
11⁄2 foot high, 31⁄2 inches in diameter at the base, 11⁄2 at the top, are fixed firmly upright,
and at a level of about 3 feet above the bottom of the cistern, so as to be handy
for the workmen. See Brazil wood, Fustic, Madder, Black Dye, Brown Dye,
&c., as also Bleaching, Bran, Calico Printing, Dunging, Dyeing, &c.
COTTON MANUFACTURE. (Filature de Coton, Fr.; Baumwollespinnerei,[344]
Germ.) Cotton is a filamentous down, which invests the seeds of the plant called
gossypium by Linnæus, and placed by him in the class monadelphia and order
monandria, but belonging to the natural family of malvaceæ. It has a cup-shaped
calyx, obtusely five-toothed, inclosed in a three-cleft exterior calyx; the leaflets
are united at their base, of a heart shape and toothed; stigmas three to five; capsule
three to five celled and many-seeded; seeds bearing a downy wool. Thirteen
species are described by Decandolle, but their characters are very uncertain, and no
botanist can assign to a definite species of the plant, the very dissimilar staples of the
cotton filaments found in commerce. The leaves are generally palmate and hairy; and
the blossoms are large, and of a beautiful yellow. The gossypium religiosum of
Tranquebar has white blossoms in some of its varieties, to which, probably, the white
cotton of Rome, cultivated in the Jardin des Plantes at Paris, belongs. The filaments
differ in length, flexibility, tenacity, and thickness, in different cottons, whence the
great differences of their value to the cotton-spinner, as the prices current in the market
show. Thus, at Liverpool, on the 1st of December, 1835, the following values were
assigned to the following cottons:—
| |
s. |
d. |
|
s. |
d. |
| Sea-island |
1 |
6 |
|
to |
2 |
6 |
|
| Demerara and Berbice |
0 |
9 |
|
|
1 |
0 |
|
| Pernambuco |
0 |
10 |
3⁄4 |
|
1 |
1 |
1⁄2 |
| Egyptian |
0 |
11 |
1⁄2 |
|
1 |
2 |
1⁄2 |
| New Orleans |
0 |
7 |
1⁄8 |
|
1 |
0 |
|
| Bahia |
0 |
8 |
1⁄4 |
|
0 |
10 |
|
| Upland Georgia |
0 |
7 |
1⁄8 |
|
0 |
11 |
1⁄2 |
| West Indian |
0 |
7 |
3⁄4 |
|
0 |
9 |
|
| Surat |
0 |
6 |
1⁄8 |
|
0 |
8 |
|
| Madras |
0 |
6 |
1⁄2 |
|
0 |
8 |
|
| Bengal |
0 |
5 |
1⁄4 |
|
0 |
6 |
1⁄2 |
But it is to be observed, that there are varieties of the Sea-island Georgian cotton,
so highly prized by the spinner of fine yarn, as to fetch 3s., 4s., or even 5s. per pound.
The filaments of cotton, when examined with a good microscope, are seen to be more
or less ribbon-like, and twisted; having a breadth varying from 1⁄800 of an inch in the
strongest Smyrna or candle-wick cotton of the Levant, to 1⁄2500 of an inch in the finest
Sea-island.
The main distinction between cottons in the pod, is that of the black seeded, and the
green seeded; for the former part with their downy wool very readily to a pair of
simple rollers, made to revolve nearly in contact, by the power of the human arm;
while the latter retain the wool with much force, and require to be ginned, as the
operation is called, by a powerful revolving circular saw-mechanism, usually driven by
horse or water power. After the cotton wool is thus separated from the seeds, it is
packed in large canvas bags, commonly with the aid of a screw or hydraulic press, into a
very dense bale, for the convenience of transport. Each of the American bags contains
about 340 lbs. of cotton wool. When this cotton is delivered to the manufacturer, it is
so foul and flocky, that he must clean and disentangle it with the utmost care, before
he can subject it to the carding operation.
Fig. 317. A B, is a roller, about 9 inches in diameter,
which revolves in the direction of the arrow. This
cylinder consists of a parallel series of oblique pointed
circular saws made fast to one axis, and parted from
each other by wooden rings nearly one inch and a half
in thickness. Above the cylinder is a kind of hopper
E F, into which ginner throws the seed cotton, which
falls upon a grating, up though which small segments
of the saw-teeth project, so as to lay hold of the fibres in their revolution, and pull them
through, while the seeds being thus separated, roll down the slope of the grid, to be
discharged from the spout I K. M is a cylindrical brush placed below the grating,
which revolves against the saw teeth, so as to clear them of the adhering cotton
filaments.
The willow, which was originally a cylindrical willow basket, whence its name, but is
now a box made of wood, with revolving iron spikes, is the first apparatus to which
cotton wool is exposed, after it has been opened up, picked, and sorted by hand or a
rake, in what is called a bing. The willow exercises a winnowing action, loosens the
large flocks, and shakes out much of the dirt contained in them. The frame of the
willow is about 2 feet wide, and turns with its spikes at the rapid rate of 600 revolutions
per minute, whereby it tosses the cotton about with great violence. The heavy impurities
fall down through the grid bottom. It is exposed, however, for only a few
minutes to the action of this machine. For factories which work up chiefly the coarser
and fouler cottons of India, and Upland Georgia, the conical self-acting willow, as[345]
constructed by Mr. Lillie at Manchester, is much employed. In it, the cotton is put
in at the narrow end of the truncated cone, which, being spiked, and revolving rapidly
within a nearly concentric case upon a horizontal axis, wafts it on towards the wide end,
while its impurities are partly shaken out through the grid or perforated bottom, and
partly sucked up through revolving squirrel wire cages, by the centrifugal action of a
fan. This is a powerful automatic engine, deserving the study of the curious, and is
as safe as it is powerful. The cone of this huge machine makes from 400 to 600 turns
per minute, and will clean 7200 pounds, or 24 bags, in a day.
After shaking out the grosser impurities by the willow, the cotton spinner proceeds
to separate each individual filament of cotton wool from its fellow, so as to prepare it
for carding, and to free it from every particle of foreign matter, whether lighter or
heavier than itself. This second operation is performed by what are called batting
(beating), scutching, and blowing machines, which are all now much the same, whatever
difference of signification the name may have. Indeed, each machine not only
beats, scutches, but blows. Fig. 318. exhibits
a longitudinal section of a good
blowing engine of modern construction.
The machine is about 18 or 19 feet long,
and three feet across within the case. The
whole frame is made of cast-iron, lined
with boards, forming a close box, which has
merely openings for introducing the raw
cotton wool, for taking out the cleansed
wool, and removing the dust as it collects
at the bottom. These doors are shut during
the operation of the machine, but may be
opened at pleasure, to allow the interior to
be inspected and repaired.
The introduction of the cotton is effected
by means of an endless cloth or double
apron, which moves in the direction of
the arrow a a, at the left end of the figure,
by passing round the continually revolving
rollers at b and c. The two rollers at e,
being the ones which immediately introduce
the cotton into the jaws, as it were,
of the machine, are called the feed rollers.
The batting arm, or revolving diameter,
f e, turns in the direction of the arrow, and
strikes the flocks violently as they enter, so
as to throw down any heavy particles upon
the iron grating or grid at n, while the
light cotton filaments are wafted onwards
with the wind, from the rotation of the
scutcher in the direction of arrow a′, along
the second travelling apron, upon which the
squirrel cage cylinder presses, and applies
the cotton in the form of a lap. Above the
cylindric cage h, which turns in the direction
of its arrow, there is a pipe k, the
continuation of the case i. This pipe,
though broken off in the figure, communicates
by a branch pipe with an air-sucking
fan ventilator, not seen in this
figure, but explained under Foundry. The
cage h, by its rotation, presses down, as we
have said, the half-cleaned cotton upon the
cloth a′, which carries it forward to the
second scutcher f′, by the second set of
feed rollers e′. The second scutcher throws
down the heavy dust upon the second
grid n′, through which it falls upon the
bottom of the case. The first scutcher
makes about 1280 strokes of each of its
two arms in a minute; the second 1300.
The feed rollers for each are fluted. The feed cloth is either sustained by a board, or
is made of parallel spars of wood, to secure it against bagging, which would render the[346]
delivery of the cotton irregular. The feed rollers make 8 turns in the minute, and as
their diameter is 11⁄2 inches, they will introduce 8 times their circumference, or 37·7
inches of the cotton spread upon the apron in that time. Upon every 12th part of an
inch of the cotton, therefore, nearly 3 blows of the scutcher arm will be applied. The
second feed rollers move relatively with more slowness, so that for every 2·4 blows of
the scutcher, only one twelfth of an inch of cotton wool is presented.
The fan is inclosed in a cylindrical case. The wings or vanes revolve from 120 to
150 times in the minute; and while they throw the air out with nearly this velocity
at their excentric outlet in the circumference, they cause it to enter, with equal
velocity, at the centre. With this centre the squirrel cage is connected by a pipe, as
above stated. The sound filaments
of the cotton are arrested by
the sieve surface of the cylindric
cage, and nothing but the broken
fragments and the light dust can
pass through.
The cotton wool in the blowing
machine is wafted by the second
scutcher into the space x, w w,
provided with a fine grid bottom;
or it is sometimes wound up
there by rollers into a lap.
In fig. 318. an additional ventilator
is introduced beneath at
m, o o, to aid the action of the
scutchers in blowing the cotton
onwards into the oblong trough
a. The outlet of that fan is at
t; and it draws in the air at its
axis q. u and v, are two doors
or lids for removing the cleaned
cotton wool. This last fan is
suppressed in many blowing machines,
as the scutching arms
supply a sufficient stream of air.
The dotted lines show how the
motion is transmitted from the
first mover at s, to the various
parts of the machine. 6′ 6′ represent
the bands leading to the
main shafting of the mill. A
machine of this kind can clean
fully 600 pounds of short-stapled
cotton wool in a day, with the
superintendence of one operative,
usually a young woman, to distribute
the cotton upon the first
feed cloth.
The second Blowing machine
is usually called a lap machine,
because, after blowing and scutching
the cotton, as above described,
it eventually coils the fleece upon
a wooden roller at the delivering
end of the apparatus. It is
sometimes also called a spreading
machine. A section of it is
shown in fig. 319. The breadth
of this machine is about 3 feet as
the lap formed is prepared for
the usual breadth of the breaker
cards, namely 3 feet. Where the
cards are only 18 inches broad,
the lap machine is also made of
the same breadth. In the figure we see the feed-cloth, the scutching barrel, the
squirrel suction, and spreading cage, and the rollers for coiling up the lap. The
lever shown below is for removing the pressure weight from the axis of the lap[347]
rollers, when a full one is to be removed, and replaced by an empty one. m, at the top,
is the commencement of the pipe which leads to the suction fan, or ventilator. The
thickness of the lap in this machine must be nicely regulated, as it determines, in a
great measure, the grist of the card ends, and even the rovings. In 12 hours such a
lap machine will prepare 650 pounds of cotton.
Fig. 320. is the first scutching machine, now never seen except in the oldest factories.
A B is the feed cloth; G H and M N are the two scutcher frames.
Carding is the next operation in a cotton factory. Cards are destined to disentangle
the individual filaments from each other, and to lay them lengthwise, instead of being
doubled up and convoluted, as they usually are in leaving the blowing and lap
machines. Carding consists in the mutual action of two opposite surfaces, which are
studded thick with oblique angled hooks. The wires of which these hooks are made must
be very hard drawn in order to render them stiff and elastic. The middle part of the
figures shows one of the staples or double teeth, the structure of which has been partly
explained under Card. Suppose a, fig. 321. to be a piece of a card fillet, and b to be
another piece, each being made fast with pins to a board; the teeth of these two cards
are set in opposite directions, but are very near together, and parallel. Now suppose
a flock or tuft of cotton placed between two such bristling surfaces. Let a be moved
in the direction of its arrow, and let b be moved in the opposite direction, or even let it
remain at rest. Every filament of the cotton will be laid hold of by each set of teeth,
when their surfaces are thus drawn over each other; the teeth of a will pull them in a
forward direction, while those of b will tend to retain them, or to pull them backwards.
The loops or doublings will, by both movements, be opened or drawn out, so that the
flocks will be converted into rows of parallel filaments, lying alongside or before each
other. Each tooth will secure to itself one or more of them, and by the friction of its
sides, as well as the hooks of its points, will draw them to their utmost elongation.
Though one stroke of the opposite cards be inadequate to produce this equable arrangement,
yet many repeated strokes must infallibly accomplish the end in view, of laying
the fibres parallel.
Let us suppose this end effected, and that all the fibres have been transferred to the
card a, a transverse stroke of b will draw over to it a certain number of them, and indeed
at each stroke there will be a new partition between the two cards, with increased
parallelism, but still each card will retain a great deal of the cotton. To make one
card strip another, the teeth of one of them must be placed in a reverse position, as
shown in fig. 322.
If a be now drawn in the direction of its arrow along the face of b, it will inevitably
comb out all, or almost all, the filaments from it, since the hooks of b have, in this position,
no power of retaining them. Even the doubled fibres or loops will slip over the
sloping point of b, in obedience to the traction of a. By considering these two relative
positions of the cards, which take place in hand cards, simply by reversing one of them,
any person will be able to understand the play of a cylinder card against its flat top, or
against another cylinder card, the respective teeth being in what we may call the teazing
position of fig. 321.; and also the play of a cylinder card against the doffer cylinder,
in what may be called the stripping position of fig. 322.
Cylinder cards, so essential to the continuity and dispatch of cotton factory labour,
were the ingenious invention of Lewis Paul of Northampton, but were greatly improved
and brought into nearly their present operative state by Sir Richard Arkwright. A[348]
carding engine consists of one or more cylinders, covered with card-leather (sometimes
called card cloth), and a set of plane surfaces similarly covered, made to work against
each other, but so that their points do not come into absolute contact. Some cards
consist entirely of cylinders, the central main cylinder being surrounded by a series of
smaller ones called urchins or squirrels. These are used solely for preparing the coarser
stapled cotton, and sheep’s wool for the wool spinner.
Fig. 323. represents a card of excellent construction, which may be called a breaker
and finisher, as it is capable of working up the fleece roll of the lapping machine directly
into a card-end or riband fit for the drawing machine. In fine spinning mills
there are always, however, two cards; one coarser, called a breaker, which turns off
the cotton in a broad fleece of extreme thinness, which is lapped round a cylinder;
and constitutes the material presented to the finisher card, which has teeth of a finer
construction.
a is one of the two upright slots, which are fixed at each side of the engine for receiving
the iron gudgeons of the wooden cylinders round which the fleece of the lapping
machine is rolled. The circumference of this coil rests upon a roller b, which is made to
turn slowly in such a direction as to aid the unfolding of the lap by the fluted cylinders
e. The lap proceeds along the table seen beneath the letter c, in its progress to the fluted
rollers, which are an inch and one-sixth in diameter, and have 28 flutings in their circumference.
g is a weight which hangs upon the axis of the upper roller, and causes
it to press upon the under one: f is the main card drum; g g g, the arch formed by
the flat top cards; h, the small card cylinder for stripping off the cotton, and therefore
called the doffer, as we have said; i, the doffer-knife or comb for stripping the fleecy
web from the doffer; k l q m, the lever mechanism for moving these parts. At d there is
a door for permitting the tenter to have access to the interior of the engine, and to remove
whatever dirt, &c. may happen to fall into it. In fig. 324. we see the manner of fixing
the flat tops g g over the drum; and for making the matter clearer, three of the tops
are removed. Upon the arched cast-iron side of the frame, a row of strong iron pins k
are made fast in the middle line; and each top piece has, at each of its ends, a hole,
which fits down upon two such opposite pins. l l are screws whose heads serve as
supports to the tops, by coming into contact with the bottom of the holes, which are not
of course bored through the wood of the tops. By turning the heads of these screws a
little the one way or the other, the pins may be lengthened or shortened in any degree,
so as to set the tops very truly in adjustment with the drum teeth revolving beneath
them, h′ is the small runner or urchin, and i′ the large runner; both of which are
spirally covered from end to end with narrow card fillets, in the same manner as the
doffer. The main drum is on the contrary covered with card cloth, in strips laid on
parallel to its axis, with interjacent parallel smooth leather borders. The teeth of these
several cards are set as represented in the figure, and their cylinders revolve as the arrows
indicate. The runners as well as the doffer cylinder may be set nearer to or farther
from the drum f; but the screws intended for this adjustment are omitted in the drawings,
to avoid confusion of the lines.
The card-end or fleece taken off the doffer h by the crank and comb mechanism i k m,
passes through the tin plate or brass funnel n, fig. 323., whereby it is hemmed in and
contracted into a riband, which is then passed through between a pair of drawing rollers
o. It is next received by the rollers u v, which carry it off with equable velocity,
and let it fall into the tin cans placed below, or conduct it over a friction pulley, to be
wound along with many other card-ends upon a lap roller or large bobbin. The latter
mechanism is not shown in this figure. A sloping curved tin or brass plate, channelled or[349]
ridged along its surface, conducts the card ribands separately; there are two smooth
iron rollers for condensing the several ribands, and a wooden pin round which the
ribands are lapped, resting between two leather-covered rollers, one of which receives
motion from mill geering, and imparts it by friction to the lap roller over it. The iron
ends of the lap roller lie in upright slots, which allow them freedom to rise as the roller
gets filled with fleece.
The two pairs of rollers at o, effect the extension of the card-end, and reduce its size.
The under rollers are made of iron and fluted; the upper ones are also made of iron,
but they are covered with a coat of leather, nicely glued on over a coat of flannel, which
two coats render them both smooth and elastic. Two weights, w, press the upper cylinders
steadily down upon the under ones. Between the first and second pair there is a
certain interval, which should be proportioned to the length of the cotton staple. The
second, or that furthest from the funnel, revolves with greater velocity than the first, and
therefore turns out a greater length of riband than it receives from its fellow; the consequence
is a corresponding extension of the riband in the interval between the two
pairs of rollers.
The motions of the several parts of the engine are effected in the following way. The
band, p p, fig. 324., which comes down from the pulley upon the main shaft near the ceiling
of the work-room, drives, by means of the pulley q, the drum f, fig. 323., with a velocity of
from 120 to 140 revolutions in a minute. From another pulley r, on the axis of the drum,
the axis of t is driven by the band s working round the pulley t on its end. This shaft
drives the crank and lever mechanism of the stripper knife i. A third pulley of the same
size as r is fixed just within the frame to the other end of the drum, and from it a crossed
or close band r′ goes to a pulley upon the small runner h′, to give this its rapid rotation.
Upon the opposite end of the engine in fig. 323., these wheels and pulleys are marked
with dotted lines. Here we may observe, first, a pulley y upon the drum, and a pulley
a′, which receives motion from it by means of the band z. The axis of a′, carries
in front a pinion m′, which sets in motion the wheel n′. The latter imparts motion,
by means of a pinion and intermediate wheel o′, to the wheel h on the doffer
cylinder, and consequently to that cylinder on the one hand; and it turns, by the carrier
wheel p′, a wheel x, whose axis is marked also with x in fig. 323., upon the other
hand. The axis of x′, fig. 323., carries, towards the middle of the engine, a very broad
wheel, which is represented by a small dotted circle. The toothed wheel v of the smooth
roller v′, fig. 323., and the two toothed wheels o o, fig. 324., of the under rollers o o, fig.
323., work into that broad wheel. The wheel of the second or delivery fluted roller is
seen to be smaller than that of the first, by which means the difference of their velocities
is obtained. The large runner i is driven from the main drum pulley, by means of the
band s′, and the pulley u′, fig. 323. The said band is crossed twice, and is kept in
tension by the pulley t′, round which it passes. The motion of the fluted rollers e, which
feed in the cotton fleece, is effected by means of a bevel wheel b′ on the end of the
doffer, which works into a similar wheel c′ on the oblique axis d′ (dotted lines across the
drum), of the pinion e′ upon the lower end of the same axis which turns the wheel f′,
upon the under feed roller.
Each of the feed rollers, fig. 324., bears a pinion e e at one end, so that the upper roller
turns round with the under one. The roller b, fig. 323., is set in motion by means of[350]
its wheel x′; which is driven by a wheel v′ on the other end of the under feed roller,
through the intervention of the large carrier wheel w′. The original or first motion of
b must be as quick as that of the fluted feed rollers e, in order that the former may uncoil
as much lap as the latter can pass on.
The annexed table exhibits the proper velocities of the different cylinders and rollers
of the carding engine, which, however, are not invariable, but may be modified according
to circumstances, by changing the pinions e′, fig. 323., and w′, according to the quality
or length of the cotton staple. The velocities stated in the table will be obtained
when the pulley a′, fig. 323., is made greater than y in the proportion of 3 to 2, and the
wheels and pinions have the following number of teeth: m′, 18; n′, 50; its pinion, 18;
h, 128; x, 24; the broad wheel upon the shaft of x, 37 teeth; the wheel o of the first
fluted roller, 35; that of the second, 21; v, 44; b′ and e′, 54; e′, 10;
f′, 63.
| Names of the parts. |
Diameter
in inches. |
Circum-
ference
in inches. |
Revolutions
in one
minute. |
Velocity. |
| Drum f |
35 |
|
109 |
·9 |
130 |
|
142 |
·87 |
| Doffer h |
14 |
|
43 |
·96 |
4 |
·38 |
192 |
·5 |
| Runner or urchin i′ |
6 |
·25 |
19 |
·62 |
5 |
· |
98 |
·1 |
| Ditto h′ |
3 |
·5 |
11 |
· |
470 |
· |
5170 |
· |
| Fluted feed roller e |
1 |
·167 |
3 |
·664 |
0 |
·696 |
2 |
·55 |
| First drawing roller o |
1 |
· |
3 |
·14 |
68 |
·71 |
215 |
·75 |
| Second ditto |
1 |
·167 |
3 |
·664 |
114 |
·52 |
419 |
·6 |
| Smooth delivery roller v |
2 |
·5 |
7 |
·85 |
54 |
·66 |
429 |
·08 |
The operation of the runners, h′ and i′, becomes very plain on comparing their speed
with one another and with that of the main-drum, and taking into account the direction
of the card teeth. The cotton wool, taken off from the feed-rollers by the drum, is
caught by the opposite teeth of the large runner i′, which, on account of its slower surface
rotation (98 inches per minute) may be considered to be at rest with reference to the
drum, and therefore, by holding the cotton in its teeth, will commence its carding. The
small runner h′, in consequence of its greater surface velocity (5170 inches per minute)
will comb the cotton-wool back out of the teeth of the large runner, but it will
give it up in its turn to the swifter teeth of the drum, which, in carrying it forwards,
encounters the teeth of the top cards, and delivers up the filaments to their keeping for
some time. We thus see how essential the runners are to the perfection as well as to
the acceleration of the carding process for ordinary cotton wool, though for the slenderer
and longer filaments of the sea-island kind they are not so well adapted. In cleaning
the carding-engines the little runner must be looked to every time that the drum is examined.
The large runner and the doffer require to be cleaned together. The quantity
of cotton spread upon the feed-cloth, the velocity of it, and of the drawing-rollers, must
all be carefully adjusted to the grist of the yarn intended to be spun.
Suppose the sizes and velocities to be as represented in the preceding table, that the
engine is a double card 36 inches broad, and that it is furnished with a lap from the lap-machine
of which 30 feet in length weigh 5 lbs. In one minute the surface of the feed-rollers,
e, passes 2·55 inches of that lap onwards; in the same time the main drum f will
work it off. To card the whole 30 feet, therefore, 141 minutes, or 2 hours and 21 minutes
will be required. In this time the circumference of the rollers, u v, moves through a
space of 141 × 42,908 in. = 5042 ft., and delivers a card-end of that length, weighing
5 lbs., minus 6 per cent. for waste, that is 4 lbs. 111⁄2 oz. One pound will form a riband
1072 feet long, being, according to the English mode of counting, about number 1⁄3, or
0·357. The extension of the cotton-fleece to this degree proceeds as follows:—In the
141 minutes which the feed-rollers take to introduce the 30 feet of lap, the doffer, h,
makes 617·58 revolutions, and the comb, or doffer knife, i, detaches from the doffer
teeth, a thin fleecy web of 2262 feet in length. The first drawing pair of fluted rollers,
by its quick motion, with the aid of the funnel,
m, converts this fleece into a riband 2535 feet
long. The second pair of the fluted rollers extends
this riband to 4390 feet, since their surface
velocity is greater than the first pair in that
proportion. The slight elongation (of only 112
feet, or about 1⁄44) which takes place between
the delivery fluted rollers and the smooth cylinders,
v, u, serves merely to keep the card-end
steadily upon the stretch without folding. Fig.
325. is a plan of the card and the fleece, where
h is the cylinder, n is the funnel, u the pressing
rollers, and h′ the card-ends in the can.
[351]
Figs. 326, 327. represent skeletons of the old cards to facilitate the comprehension of
these complex machines. Fig. 326. is a plan; F is the main drum; M M is the doffer
knife or comb; G, the carded fleece hemmed in by the funnel a, pressed between the
rollers b, and then falling in narrow fillets into its can. Fig. 327. K L are the feed
rollers; A B, the card drum; C D, the tops; E F, the doffer card; M N, the doffer knife;
d, b, c, the card-end passing between compressing rollers into the can a.
The drawing and doubling are the next operation. The ends, as they come from the
cards, are exceedingly tender and loose, but the filaments of the cotton are not as yet laid
so parallel with each other as they need to be for machine spinning. Before any degree
of torsion therefore be communicated, a previous process is required to give the filaments
a level arrangement in the ribands. The drawing out and doubling accomplish this purpose,
and in a manner equally simple and certain. The means employed are drawing-rollers,
whose construction must here be fully explained, as it is employed in all the following
machines; one example of their use occurred, indeed, in treating of the cards.
Let a and b, fig. 328., represent the section of two rollers lying
over each other, which touch with a regulated pressure, and turn in
contact upon their axes, in the direction shown by the arrows.
These rollers will lay hold of the fleecy riband presented to them at
a, draw it through between them, and deliver it quite unchanged.
The length of the piece passed through in a given time will be equal
to the space which a point upon the circumference of the roller
would have percured in the same time; that is, equal to the periphery
of one of the rollers multiplied by the number of its entire
revolutions. The same thing holds with regard to the transmission
of the riband through between a second pair of rollers,
c, d, and a third, e, f. Thus the said riband issues from the third pair exactly the
same as it entered at a, provided the surface speed of all the rollers be the same.
But if the surface speed of c and d be greater than that of a and b, then the first-named
pair will deliver a greater length of riband than the last receives and transmits to
it. The consequence can be nothing else in these circumstances than a regulated drawing
or elongation of the riband in the interval betwixt a, b, and c, d, and a condensation
of the filaments as they glide over each other, to assume a straight parallel direction. In
like manner the drawing may be repeated by giving the rollers, e, f, a greater surface
speed than that of the rollers, c and d. This increase of velocity may be produced,
either by enlarging the diameter, or by increasing the number of turns in the same time,
or finally by both methods conjoined. In general the drawing-machine is so adjusted,
that the chief elongation takes place between the second and third pairs of rollers, while
that between the first and second is but slight and preparatory. It is obvious, besides,
that the speed of the middle pair of rollers can have no influence upon the amount of
the extension, provided the speed of the first and third pair remains unchanged. The
rollers, a, b, and c, d, maintain towards each other continually the same position, but they
may be removed with their frame-work, more or less, from the third pair, e, f, according
as the length of the cotton staple may require. The distance of the middle point from
b and d, or its line of contact with the upper roller, is, once for all, so calculated, that it
shall exceed the length of the cotton filaments, and thereby that these filaments are never
in danger of being torn asunder by the second pair pulling them while the first holds
them fast. Between d and f, where the greatest extension takes place, the distance must
be as small as it can be without risk of tearing them in that way; for thus will the uniformity
of the drawing be promoted. If the distance between d and f be very great, a
riband passing through will become thinner, or perhaps break in the middle; whence we
see that the drawing is more equable, the shorter is the portion submitted to extension at
a time, and the nearer the rollers are to each other, supposing them always distant
enough not to tear the staple.
[352]
The under rollers b d f are made of iron, and, to enable them to lay firmer hold of the
filaments, their surfaces are fluted with triangular channels parallel to their axes. The
upper rollers, a c e, are also made of iron, but they are smooth, and covered with a double
coating, which gives them a certain degree of softness and elasticity. A coat of
flannel is first applied by sewing or gluing the ends, and then a coat of leather in the
same way. The junction edges of the leather are cut slanting, so that when joined by
the glue (made of isinglass dissolved in ale) the surface of the roller may be smoothly
cylindrical. The top rollers are sometimes called the pressers, because they press by
means of weights upon the under ones. These weights are suspended to the slight rods
k k′; of which the former operates on the roller e alone, the latter on the two rollers
a and e together. For this purpose the former is hung to a C shaped curve i, whose
upper hook embraces the roller e; the latter to a brass saddle h, which rests upon a and
c. A bar of hard wood, g, whose under surface is covered with flannel, rests, with merely
its own weight, upon the top rollers, and strips off all the loose hanging filaments. Similar
bars with the same view are made to bear up under the fluted rollers b d f, and
press against them by a weight acting through a cord passing over a pulley. Instead of
the upper dust-covers, light wooden rollers covered with flannel are occasionally
applied.
Were the drawing of a riband continued till all its fibres acquired the desired degree
of parallelism, it would be apt, from excessive attenuation, to tear across, and thereby
to defeat the purpose of the spinner. This dilemma is got rid of in a very simple way,
namely, by laying several ribands together at every repetition of the process, and incorporating
them by the pressure of the rollers. This practice is called doubling. It is an exact
imitation of what takes place when we draw a tuft of cotton wool between our fingers and
thumb in order to ascertain the length of the staple, and replace the drawn filaments
over each other, and thus draw them forth again and again, till they are all parallel
and of nearly equal length. The doubling has another advantage, that of causing the
inequalities of thickness in the ribands to disappear, by applying their thicker to their
thinner portions, and thereby producing uniformity of substance.
The drawing frame, as shown in section in figs. 328. 330., and in a back view in fig.
329., will require, after the above details, little further explanation. l l are the weights which
press down the top rollers upon the under ones, by means of the rods k k′ and hook i. Each
fluted roller is, as shown at f, fig. 329., provided in the middle of its length with a thinner
smooth part called the neck, whereby it is really divided into two fluted portions, represented
by e e in the figure. Upon this middle neck in the pressure rollers, the hook i and the
saddle h immediately bear, as shown in the former fig. 328. The card-ends, to the number
probably of six, are introduced to the drawing frame either from tin cans, placed at e e, fig.
330., and at A, fig. 329., or from lap-bobbins; and, after passing through it, the ribands or slivers
are received either into similar tin cans, as g, or upon other lap-bobbins upon the other
side. These appendages may be readily conceived, and are therefore not exhibited in all
the drawings. Three of the slivers being laid together, are again introduced to the one
fluted portion a b, fig. 328., and three other slivers to the other portion. The sloping
curved tin or brass plate s, fig. 329., with its guide pins t, serves to conduct the slivers
to the rollers. When the two threefold slivers have passed through between the three
pairs of rollers, and been thereby properly drawn, they run towards each other in an
oblique direction, behind the last roller pair e f, fig. 328., and unite, on issuing through the[353]
conical funnel m, fig. 329., into a single riband or spongy sliver; which is immediately
carried off with equable velocity by two smooth cast-iron rollers, n o, fig. 329. and 330.
and either dropped into a can, or wound upon a large bobbin. The surface speed of
these rollers is made a trifle greater than that of the delivery drawing rollers, in order to
keep the portion of sliver between them always in an extended state. Four fluted drawing
portions are usually mounted in one drawing frame, which are set a-going or at rest together.
To save all unnecessary carrying of the cans from the back to the front of
the frame, the drawing heads are so placed, that the first and third, discharge their slivers
at the one side, and the second and fourth at the other. By this arrangement, the cans
filled behind one head, are directly pushed aside in front of the next drawing head;
by which alternate distribution the work goes on without interruption.
The fast pulley u, fig. 330., by which the whole machine is driven, derives its motion
from the main shaft of the mill by means of the band w. The similar pulley x, which
sits loose upon the axis, and turns independently of it, is called the loose pulley; both
together being technically styled riggers. When the operative desires to stop the machine,
he transfers the band from the fast to the loose pulley by means of a lever, bearing a fork
at its end, which embraces the band. Upon y, four pulleys such as x are fixed, each of
which sets in motion a drawing head, by means of a band like w going round the pulleys
x and u. On account of the inverted position of the heads, which requires the
motion of u to be inverted, the bands of the first and third heads are open, but those of
the second and fourth are crossed. Every head is provided with a loose pulley v, as well
as the fast pulley u, in order to make the one stop or move without affecting the others.
The shaft of the pulley u is the prolonged shaft of the backmost fluted roller f. It carries
besides a small pulley q, which, by means of the band r, and the pulley p, fig. 329., sets
in motion the undermost condensing roller o. The upper roller n, presses with its whole
weight upon it, and therefore turns by friction. The toothed wheel-work, by which the
motions are communicated from the backmost fluted roller to the middle and front ones,
are seen in fig. 330.
The wheel f, fig. 328., of 20 teeth, works in a 44-toothed carrier-wheel, on whose
axis there are two smaller wheels; 2 with 26 teeth, and 1 with 22 teeth. The wheel d, fig.
330., of the middle roller, and the wheel b of the front roller, are set in motion by other
carrier wheels; the first has 27 teeth, and the last 40. For every revolution of b, the
roller d makes nearly 13⁄4 turns, and the roller f, 4 revolutions. The top rollers revolve,
as we have stated, simply by the friction of contact with the lower ones. Now suppose
the diameter of the rollers b and d to be 1 inch or 12 lines, that of f, 11⁄4 inches or 15
lines, the surface velocities of the three pairs of rollers in the series will be as 1, 13⁄4, and 5.
Every inch of the cotton sliver will be therefore extended between the first and second
pair of rollers into 13⁄4 inches, and between the second and third or delivery pair into
5 inches; and after the sliver has passed through all the four drawing heads, its length
will be increased 625 times = 5 × 5 × 5 × 5.
The further the drawing process is pushed, the more perfectly will its object be accomplished;
namely the parallelism of the filaments. The fineness of the appearance
of the sliver after the last draught depends upon the number of doublings conjointly
with the original fineness and number of drawings. The degree of extension may be
increased or diminished, by changing the wheels in fig. 330., for others with a different
number of teeth. Thus the grist or fineness of the sliver may be modified in any desired
degree; for, when the subsequent processes of the mill remain the same, the finer
the drawings the finer will be the yarn. For spinning coarse numbers or low counts, for
example, six card-ends are usually transmitted through the first drawing head, and converted
into one riband. Six such ribands again form one in the second draught; six
of these again go together into the third sliver; and this sliver passes five-fold through the
last draught. By this combination 1080 of the original card-ends are united in the
finished drawn sliver = 6 × 6 × 6 × 5. The fineness of the sliver is, however, in consequence
of these doublings not increased but rather diminished. For, by the drawing, the card-end
has been made 625 times longer, and so much smaller; by the doubling alone it
would have become 1080 times thicker; therefore the original grist is to the present as
1, to the fraction 625⁄1080; that is, supposing 1072 feet of the riband delivered by the card
to weigh one pound, 625 feet, the sliver of the last drawing, will also weigh a pound,
which corresponds in fineness to number 0·24, or nearly 1⁄4.
The rearmost or last drawing roller has a circumference of nearly 4 inches, and
makes about 150 revolutions per minute; hence, each of these drawing heads may
turn off 35,000 feet of sliver in 12 hours.
Some manufacturers have lately introduced a double roller beam, and a double
draught at the same doubling, into their drawing frames. I have seen this contrivance
working satisfactorily in mills where low counts were spun, and where the tube roving
frame was employed; but I was informed by competent judges, that it was not advisable
where a level yarn was required for good printing calicoes.
[354]
The loss which the cotton suffers in the drawing frame is quite inconsiderable. It
consists of those filaments which remain upon the drawing rollers, and collect, in a great
measure, upon the flannel facing of the top and bottom cleaner bars. It is thrown
among the top cleanings of the carding engine. When from some defect in the rollers,
or negligence in piecing the running slivers, remarkably irregular portions occur
in the ribands, these must be torn off, and returned to the lap machine to be carded
anew.
The fifth operation may be called the first spinning process, as in it, the cotton sliver
receives a twist; whether the twist be permanent as in the bobbin and fly frame, or be
undone immediately, as in the tube-roving machine. In fact, the elongated slivers of
parallel filaments could bear little further extension without breaking asunder, unless
the precaution were taken to condense the filaments by a slight convolution, and at the
same time to entwine them together. The twisting should positively go no further
than to fulfil the purpose of giving cohesion, otherwise it would place an obstacle in the
way of the future attenuation into level thread. The combination of drawing and
twisting is what mainly characterizes the spinning processes, and with this fifth operation
therefore commences the formation of yarn. As however a sudden extension to
the wished-for fineness is not practicable, the draught is thrice repeated in machine
spinning, and after each draught a new portion of torsion is given to the yarn, till at
last it possesses the degree of fineness and twist proportioned to its use.
The preliminary spinning process is called roving. At first the torsion is slight in
proportion to the extension, since the solidity of the still coarse sliver needs that cohesive
aid only in a small degree, and looseness
of texture must be maintained to facilitate
to the utmost the further elongation.
Fig. 331. is a section of the can roving
frame, the ingenious invention of Arkwright,
which till within these 14 years
was the principal machine for communicating
the incipient torsion to the spongy
cord furnished by the drawing heads. It
differs from that frame in nothing but
the twisting mechanism; and consists of
two pairs of drawing rollers, a and b, between
which the sliver is extended in the
usual way; c are brushes for cleaning the
rollers; and d is the weight which presses
the upper set upon the lower. The wiping
covers (not shown here) rest upon a b.
The surface speed of the posterior or second
pair of rollers is 3, 4, or 5 times greater
than that of the front or receiving pair,
according to the desired degree of attenuation.
Two drawn slivers were generally united into one by this machine, as is shown
in the figure, where they are seen coming from the two cans e e, to be brought together
by the pressure rollers, before they reach the drawing rollers a b. The sliver, as it escapes
from these rollers, is conducted into the revolving conical lantern g, through the funnel
f at its top. This lantern-can receives its motion by means of a cord passing over a
pulley k, placed a little way above the step on which it turns. The motion is steadied
by the collet of the funnel f, being embraced by a brass busk. Such a machine generally
contained four drawing heads, each mounted with two lanterns; in whose side
there was a door for taking out the conical coil of roving.
The motion imparted to the back roller by the band pulley or rigger m, was conveyed
to the front one by toothed wheel work.
The vertical guide pulley at bottom n, served to lead the driving band descending
from the top of the frame round the horizontal whorl or pulley upon the under end of
the lantern. The operation of this can-frame was pleasing to behold; as the centrifugal
force served both to distribute the soft cord in a regular coil, and also to condense a
great deal of it most gently within a moderate space. Whenever the lantern was filled,
the tenter carried the roving to a simple machine, where it was wound upon bobbins by
hand. Notwithstanding every care in this transfer, the delicate texture was very apt to
be seriously injured, so as to cause corresponding injuries in every subsequent operation,
and in the finished yarn. Messrs. Cocker and Higgins, of Salford, had the singular
merit, as I have said, of superseding that beautiful but defective mechanism, which
had held a prominent place in all cotton mills from almost the infancy of the factory
system, by the following apparatus.
The Bobbin and Fly frame is now the great roving machine of the cotton manufacture;[355]
to which may be added, for coarse spinning, the tube roving frame. Of such a
complicated machine as the bobbin and fly frame, it is not possible to give an adequately
detailed description in the
space due to the subject in this
Dictionary. Its mechanical combinations
are however so admirable
as to require such an account as
will make its functions intelligible
by the general reader.
Fig. 332. exhibits a back view
of this machine; and fig. 333. a
section of some of the parts not
very visible in the former figure.
The back of the machine is the
side at which the cotton is introduced
between the drawing rollers.
The cans, or lap-bobbins filled
with slivers at the drawing frame,
are placed in the situation marked
B, fig. 333., in rows parallel with
the length of the machine. The
sliver of each can or the united
slivers of two contiguous cans are
conducted upwards along the surface
of a sloping board f, and
through an iron staple or guide e,
betwixt the usual triple pair of
drawing rollers, the first of which
is indicated by a, b. In fig. 332.,
for the purpose of simplifying the
figure, the greater part of these
rollers and their subordinate parts
are omitted. After the slivers
have been sufficiently extended
and attenuated between the rollers,
they proceed forwards, towards the
spindles i i i, where they receive the
twist, and are wound upon the
bobbins h. The machine delineated
contains thirty spindles, but
many bobbin and fly frames contain
double or even four times that
number. Only a few of the spindles
are shown in fig. 332., for
fear of confusing the drawing.
With regard to the drawing functions of this machine, I have already given abundant[356]
explanation, so far as the properties and operation of the rollers are concerned. The
frame-work of this part of the machine, called the roller-beam, is a cast iron bench, upon
which nine bearers c, are mounted for carrying the rollers. The fluted rollers a a a, fig.
334., are constructed in four pieces for the whole length, which are parted from each
other by thinner smooth cylindric portions z, called necks. Seven such partings for
four rollers, and one parting for two rollers, constitute together the 30 fluted rollers of
which the whole series consists. The coupling of these roller subdivisions into one
cylinder, is secured by the square holes x, and square pins y, fig. 334., which fit into the
holes of the adjoining subdivision.
The top or pressure rollers b, are
two-fold over the whole set; and the
weighted saddle presses upon the
neck w, which connects every pair,
as was already explained under fig. 329. These weights g, g, fig. 333., are applied in
this as in the drawing frame; d, are the bars faced with flannel for cleaning the top
rollers. A similar bar is applied beneath the rollers, to keep the flutings clean.
The structure and operation of the spindles i, may be best understood by examining
the section fig. 335. They are made of iron, are cylindrical from
the top down to a2, but from this part down to the steel tipt rounded
points they are conical. Upon this conical portion there is a pulley
k, furnished with two grooves in its circumference, in which the cord
runs that causes the spindle to revolve. The wooden bobbin h, is
slid upon the cylindrical part, which must move freely upon it, as
will be presently explained. To the bobbin another two-grooved
pulley or whorl q is made fast by means of a pin r, which passes
through it; by removing this pin, the bobbin can be instantly taken
off the spindle. The upper end of the spindle bears a fork s t,
which may be taken off at pleasure by means of its left-handed
screw; this fork or flyer, has a funnel-formed hole at v. One arm
of the fork is a tube s, u, open at top and bottom; the leg t, is
added merely as a counterpoise to the other. In fig. 333., for the
sake of clearness, the forks or flyers of the two spindles here represented
are left out; and in fig. 332. only one is portrayed for the
same reason. It is likewise manifest from a comparison of these
two figures that the spindles are alternately placed in two rows, so
that each spindle of the back range stands opposite the interval
between two in the front range. The object of this distribution
is economy of space, as the machine would need to be greatly longer
if the spindles stood all in one line. If we suppose the spindles and
the bobbins (both of which have independent motions) to revolve
simultaneously and in the same direction, their operation will be
as follows: The sliver properly drawn by the fluted rollers, enters
the opening of the funnel v, proceeds thence downwards through
the hole in the arm of the fork, runs along its tube u, s, and then
winds round the bobbin. This path is marked in fig. 335. by a
dotted line.
The revolution of the spindles in the above circumstances effects the twisting of the
sliver into a soft cord; and the flyer s, t, or particularly its tubular arm s, lays this cord
upon the bobbin. Were the speed of the bobbins equal to that of the spindles, that is,
did the bobbin and spindle make the same number of turns in the same time, the
process would be limited to mere twisting. But the bobbin anticipates the flyers a
little, that is, it makes in a given time a somewhat greater number of revolutions than
the spindle, and thereby effects the continuous winding of the cord upon itself.
Suppose the bobbin to make 40 revolutions, while the spindle completes only 30; 30 of
these revolutions of the bobbin will be inoperative towards the winding-on, because the
flyers follow at that rate, so that the cord or twisted sliver will only be coiled 10 times
round the bobbin, and the result as to the winding-on will be the same as if the spindle
had stood still, and the bobbin had made 40 - 30 = 10 turns. The 30 turns of the
spindles serve, therefore, merely the purpose of communicating twist.
The mounting and operation of the spindles are obviously the same as they are upon
the household flax wheel. In the bobbin and fly frame there are some circumstances
which render the construction and the winding-on somewhat difficult, and the mechanism
not a little complicated. It may be remarked in the first place, that as the cord is wound
on, the diameter of the bobbin increases very rapidly, and therefore every turn made
round it causes a greater length of roving to be taken up in succession. Were the
motions of the bobbins to continue unchanged in this predicament, the increased
velocity of the winding-on would require an increased degree of extension, or it would[357]
occasion the rupture of the cord, because the front fluted rollers move with uniform
speed, and therefore deliver always the same length of sliver in the same time. It
is therefore necessary to diminish the velocity of the bobbins, or the number of their
turns, in the same proportion as their diameter increases, in order that the primary
velocity may remain unchanged. Moreover, it is requisite for the proper distribution
of the cord upon the bobbin, and the regular increase of its diameter, that two of its
successive convolutions should not be applied over each other, but that they should be
laid close side by side. This object is attained by the up and down sliding motion of the
bobbin upon the spindle, to the same extent as the length of the bobbin barrel. This
up and down motion must become progressively slower, since it increases the diameter
of the bobbin at each range, by a quantity equal to the diameter of the sliver. What
has now been stated generally, will become more intelligible by an example.
Let it be assumed that the drawing rollers deliver, in 10 seconds, 45 inches of
roving, and that this length receives 30 twists. The spindles must, in consequence,
make 30 revolutions in 10 seconds, and the bobbins must turn with such speed, that
they wind up the 45 inches in 10 seconds. The diameter of the bobbin barrels being
11⁄2 inches, their circumference of course 41⁄2 inches, they must make 10 revolutions more
in the same time than the spindles. The effective speed of the bobbins will be thus
30 + 10 = 40 turns in 10 seconds. Should the bobbins increase to 3 inches diameter,
by the winding-on of the sliver, they will take up 9 inches at each turn, and consequently
45 inches in 5 turns. Their speed should therefore be reduced to 30 + 5 = 35
turns in 10 seconds. In general, the excess in number of revolutions, which the
bobbins must make over the spindles, is inversely as the diameter of the bobbins.
The speed of the bobbins must remain uniform during the period of one ascent or
descent upon the spindle, and must diminish at the instant of changing the direction
of their up and down motion; because a fresh range of convolutions then begins with
a greater diameter. When, for example, 30 coils of the sliver or roove are laid in one
length of the bobbin barrel, the bobbin must complete its vertical movement up or
down, within 30 seconds in the first case above mentioned, and within 60 seconds in
the second case.
The motions of the drawing rollers, the spindles, and bobbins, are produced in the
following manner:—A shaft c′, fig. 332. and 333., extending the whole length of the
machine, and mounted with a fly wheel d′, is set in motion by a band from the running
pulley upon the shaft of the mill, which actuates the pulley a′. b′ is the loose pulley
upon which the band is shifted when the machine is set at rest. Within the pulley a′,
but on the outside of the frame, the shaft c′ carries a toothed wheel b2 with 50 teeth,
which by means of the intermediate wheel c2 turns the wheel d2 upon the prolonged
shaft of the backmost fluted roller (m2, fig. 333.) This wheel d2 has usually 54 teeth;
but it may be changed when the roove is to receive more or less twist; for as the
spindles revolve with uniform velocity, they communicate the more torsion the less
length of sliver is delivered by the rollers in a given time. Upon the same shaft with
d2, a pinion e2 of 32 teeth is fixed, which works in a wheel f2 of 72 teeth. Within
the frame a change pinion g2 is made fast to the shaft of f2. This pinion, which has
usually from 24 to 28 teeth, regulates the drawing, and thereby the fineness or number
of the roving. It works in a 48-toothed wheel h2 upon the end of the backmost fluted
roller a, fig. 333. The other extremity of the same roller, or, properly speaking, line of
rollers, carries a pinion l2, furnished with 26 teeth, which, by means of the broad
intermediate wheel k2, sets in motion the pinion i′2 of 22 teeth upon the middle roller.
When the diameter of all the drawing rollers is the same, suppose 1 inch, their proportional
velocities will be, with the above number of teeth in the wheel work, if g2 have
24 teeth, as 1 : 1·18 : 4·5; and the drawn sliver will have 41⁄2 times its original length.
The front or delivery roller of the drawing frame is of late years usually made 11⁄4 or
13⁄8 inches in diameter. If 625 feet of the sliver from the drawing frame weighed one
pound, 2790 feet of the roving will now go to this weight, and the number will be
1·12; that is, 1 hank and 12 hundredths to the pound. The front pair of fluted
rollers makes about 90 revolutions, and delivers 282·6 inches of roving in the minute,
when of one inch diameter.
The spindles i, (fig. 332. and 333.), rest, with their lower ends, in steps l, which are
fixed in an immoveable beam or bar m. To protect it from dust and cotton filaments,
this beam is furnished with a wooden cover n, in which there are small holes for the
passage of the spindles right over the steps. In fig. 332., two of the eight covers n, which
compose the whole range m, are removed to let the steps be seen. The cylindrical part
of each spindle passes through a brass ring o; and all these 30 rings, whose centres
must be vertically over the steps l, are made fast to the copping beam p. This beam
is so called, because it is destined not merely to keep the spindles upright by the rings
attached to it, but, at the same time, to raise and lower along the spindles the bobbins[358]
which rest on these rings; for which purpose the two racks, or toothed bars m2 m2,
made fast to it, are designed, as will be presently explained. To effect the revolution
of the spindles, there are attached to the main shaft c′ two whorls or pulleys e′ f′, each
bearing four grooves of equal diameter. Each of these pulleys puts one half of the
spindles in motion, by means of a cord, which, after going round the whorls k, turns
four times about the pulleys of the shaft c′. Two guide pulleys h′, each four-grooved,
and two others i′, with a single groove, which turn independently of the others, upon
the above shaft, serve to give the whorl cords the proper direction, as well as to keep
them tight. The spindles revolve 200 times or thereby in the minute; and therefore
impart two turns or twists to every three inches of the roving.
The revolution of the bobbins is independent of that of the spindles, although it likewise
proceeds from the shaft c′, and differs from it in being a continually retarded
motion. The simplest method of effecting this motion, is by means of the wooden or
tin plate cone k′′, which revolves equally with the shaft c′, and at the same time slides
along it.
The manner in which this operates is shown in section in fig. 336. Here, we perceive
the rod q2, which extends from the base towards the narrow end of the truncated
cone, and p2 a forked bearer or carrier made fast to the shaft c′ by a screw, which
compels the cone by means of that rod, to obey the movements of c′. In the large end
of the cone there is an aperture, through which the bearer can be got at. The smaller
end carries outside a projection o2, provided with a groove, which is embraced by the
forked end of a rod q′, fig. 337., that serves to shove the cone along upon the shaft c′.
Directly under the cone, there is an upright round pillar p′, upon which the holder o′
of the two guide pulleys l′ is adjustable. A bar r2 placed along-side of the holder,
prevents its turning round, but allows it to slide along p′ by friction. The weight of the
holder and the pulley is sufficient to distend the endless band n′, which runs from the
cone k′, through under the pulley l′, and round the small drum m′ on the shaft s2. A
pulley or whorl t2 with four grooves, is made fast by means of a tube to this shaft, and
slides along it backwards and forwards, without ever ceasing to follow its revolutions.
The shaft possesses for this purpose a long fork, and the interior of the tube a corresponding
tongue or catch. There is besides upon the tube beneath the pulley, at u2, a
groove that goes round it, in which the staple or forked end of an arm like v2, fig. 333.,
made fast to the copping beam p, catches. By the up and down movement of that
beam, the pulley t2 takes along with it the arm that embraces the tube, which therefore
rises and falls equally with the bobbins h′, and their pulleys or whorls q. This is
requisite, since the bobbins are made to revolve by the pulleys t2, by means of 2 endless
cords or bands.
The most intricate part of the mechanism is the adjustment, by which the revolution
of the bobbins is continually retarded, and their up and down, or copping motion, along
the spindles, is also retarded in like proportion. The vertical pulley f′, (towards the
left end of the shaft c′) has at its right side a somewhat larger disc or sheave g′,
with a perfectly uniform, but not a very smooth surface. Upon this sheave, a smaller
horizontal pulley x′ rubs, whose upper face is covered with leather to increase the friction.
The under end of the shaft y2 of the pulley x′ turns in a step, which is so connected with
the arm v′ of the large bent lever t′ v′, that it always stands horizontally, whatever
[359]direction the arms of that lever may assume. The shaft y2 is steadied at top by an
annular holder or bush, which embraces the fast arm x2 with its forked end. Upon its
opposite side, this arm carries a pulley y2, upon which a cord goes, that is made fast to
the holder of the shaft y2, and loaded with the weight z′. The weight presses the
pulley x′ against the surface of g′, in such wise as to effect the degree of friction necessary
in order that the revolution of g′ may produce an uninterrupted revolution in x′. A
pinion w′, whose length must be equal at least to the semi-diameter of the sheave g′, is
placed upon the under end of the shaft y2. It has 22 teeth, and takes into a 62-toothed
horizontal wheel z2. Upon the upper end of this wheel the conical pinion a3 is made
fast, which may be changed for changing the speed, but usually has from 28 to 30 teeth.
By this pinion the conical wheel b3 is turned, which has 30 teeth, and whose shaft is c3.
This shaft carries upon its opposite end a six-leaved pinion, d3, which takes into the
calender wheel f3, formed with cogs like a trundle, upon the long shaft e3. In fig. 338.
the wheel f3 is exhibited with its pinion d3. Here we may remark that in the circumference
of the wheel there is a vacant place, g3, void of teeth. When by the motion of
the wheel, the pinion comes opposite to this opening, it turns round about the last tooth
of the wheel, falls into the inside of the toothed circle marked by the dotted lines, and
thus gives now an inverse movement to the wheel f3, while itself revolves always in the
same direction. This reversed motion continues till the opening g3 comes once more
opposite to the pinion, when this turns round about the last tooth of that side, and begins
again to work in the exterior teeth. Thus, by the uniform motion of d3 and its
dependent parts, the wheel f3, with its shaft e3, revolves alternately to the right hand
and the left. That this result may ensue, the shaft c3 of the pinion must be able to
slide endwise, without losing its hold of a3 and b3. This adjustment is effected by
placing the end of the said shaft, nearest b3, in a box or holder i3, in which it can turn,
and which forms a vertical tube to this box, as a downward prolongation which is fixed
to the tail of the conical pinion a3. Fig. 339. shows this construction in section upon
an enlarged scale. The second bearer of the shaft nearest d3, must possess likewise the
means of lateral motion. When therefore the pinion d3 shifts through the opening
of the wheel f3 outwards or inwards, its shaft c3, makes a corresponding small angular
motion upon the pivot of a3, by means of the tube i3; a3 and b3 remain thereby completely
in geer with one another.
The above-described alternate revolutions of the wheel f3 serve to produce the up
and down motions of the bobbins. The shaft e3 has for this purpose two pinions n2 n2,
which work in the rack teeth m2 m2 of the copping rail p, and thus alternately raise and
sink it with the bobbins which rest upon it. The weight of the copping beam and all
its dependent parts, is poised by two counterweights m4, whose cords run over the
pulleys o4 o4 o4, fig. 332., and have their ends made fast to the frame, so as to make the
upwards motion as easy as the downwards. The two upper pulleys out of the three of
each weight, are fixed to the frame; the under one, round which the cord first runs, is
attached to the copping beam, rising and falling along with it.
As long as the friction disc x′ remains at the same height, the pulley g′ derives its
motion from the same circle of the said disc, and the up and down motion of the copping
beam is also uniform. But when that disc ascends so as to describe with its edge a small
circle upon the face of g′, its motion must become proportionally more slow. This is the
method, or principle of retarding the copping motions of the bobbins. It has been shown,
however, that the rotation of the bobbins should be also retarded in a progressive manner.
This object is effected by means of the cone k′, which, as the band n′ progressively
approaches towards its smaller diameter, drives the pulleys or whorls q of the bobbins
with decreasing speed, though itself moves uniformly quick with the shaft c′. To effect
this variation, the cone is shifted lengthwise along its shaft, while the band running
upon it remains continually in the same vertical plane, and is kept distended by the
weight of the pulley o′. The following mechanism serves to shift the cone, which may
[360]be best understood by the aid of the figures 340., 341., and 337. A long cast iron bar m3,
which bears two horizontal projecting puppets, o3 o3, is made fast to the front upright
face of the copping beam A. Through the above puppets a cylindrical rod n3 passes freely,
which is left out in fig. 337., that the parts lying behind it may be better seen. Upon
this rod there is a kind of fork, p3 p3, to which the alternating rack bars q3 are made
fast. The teeth of these racks are at unequal distances from each other, and are so
arranged, that each tooth of the under side corresponds to the space between two teeth
in the upper side. Their number depends upon the number of coils of roving that may
be required to fill a bobbin; and consists in the usual machines of from 20 to 22. The
rod n3 may be shifted in the puppet o3, like the fork p3 of the rack-rod, upon the rod
n3, and along the surface of m3, where two wings u3 u3 are placed, to keep the fork in
a straight direction. Upon the bar m3, there are the pivots or fulcra of two stop catches
w3 x3, of which the uppermost presses merely by its own weight, but the undermost by
means of a counterweight y3, against the rack, and causes them thus to fall in between
the teeth. In fig. 341., v3 shows the pivot of the catch or detent w3 by itself, the
detent itself being omitted, to render the construction plainer. A pushing rod l3, upon
which there is a pin above at s3, that passes behind the rack rod, between this and
the bar m3, has for its object to remove at pleasure the one or the other of the two
catches; the upper, when the upper end of the rod pushes against it; the under, by
means of the above mentioned pin s3. Both the catches are never raised at once, but
either the under or the upper holds the rack bar fast, by pressing against one of the
teeth. The vertical motion up or down, which the rod l3 must take to effect the lifting
of the catches, is given to it from the copping beam p; since upon it a horizontal arm
v2, fig. 341., is fixed, that lays hold of that rod. Upon the pushing rod are two rings,
h3 and k3, each made fast by a screw. When the copping beam is in the act of going
up, the arm v3 at the end of this movement, pushes against the ring h3, raises up the
rod l3, and thus removes the catch w3, fig. 337., from the teeth of the rod q3, before
which it lies flat. At the descent of the copping rail, v2 meets the ring k3, when the
motion in this direction is nearly completed, draws down the rod l3 a little, by means of
the same, and thereby effects the removal of the catch x3, fig. 337., from the rod q3.
Every time that one of the catches is lifted, the rack recovers its freedom to advance a
little bit in the direction of the arrow; so far, namely, till the other catch lays hold upon
the tooth that next meets it. The reason is thus manifest why the teeth of the upper
and under sides of the bar q3 are not right opposite to each other, but in an alternate
position.
From the rack-bar, the sliding of the cone k′, and the raising of the shaft y2, each by
minute steps at a time, is produced as follows:—
A large rectangular lever t1, v1, whose centre of motion is at p4, has at the upper end
of its long arm t1, a long slot through which a stud r3 upon the rack q3 goes (fig. 340.,
341., 337.,) so that the lever must follow the motions of the rack bar. The end of the
short arm of the lever bears, as already mentioned, the step of the shaft y2; hence the
friction disc x1 will be raised in proportion as the rack bar advances, and will come
nearer to the middle point of g1; consequently, its revolution and the shifting of the
bobbins will become slower. Upon the cylindrical rod n3, the piece s1 s1 furnished
with a long slot is made fast, by means of a tube z3, (fig. 337.) and a screw. A fork
u u, which by means of the screw nut a4 is made fast in the slot, embraces the arm t1 of
the bent lever; and a tube r1 rivetted to the surface of s1, is destined to take up the draw
rod q1 of the cone k1, fig. 337. A weight f4, whose cord b4 is made fast to the cylindrical
rod n3, endeavours to draw this rod continually in the direction of the arrow. In
consequence of this arrangement, every time that the pushing bar l3 lifts up one of the
[361]catches, the cone k1, the lever t1 v1, and by it the rack bar q3, are set in motion. It is
obvious, that the motion of the cone may be made greater or less, according as the
fork u u is fixed further up or down in the slot of s1.
The number of the teeth upon the bar q3 is so ordered, that the bobbins are quite full
when the last tooth has reached the catch and is released by it. The rack bar, being restrained
by nothing, immediately slides onwards, in consequence of the traction of the
weight f4 and brings the machine to repose by this very movement, for which purpose
the following construction is employed. A rectangular lever which has its centre of
motion in g4 is attached to the side face of the beam A, and has at the end of its horizontal
arm a pulley d4, over which the cord b4 of the counterweight f4 is passed. The
end of the perpendicular arm is forked and embraces the long and thin rod k4, to whose
opposite end the fork l4 is made fast. Through this fork the band which puts the
machine in motion passes down to the pulley a1. With the bent lever another rod c4 is
connected at h4, which lies upon the puppet e3 with a slot at e4, and hereby keeps the
lever g4 in its upright position notwithstanding the weight f4. In the moment when,
as above stated, the rack bar q3 becomes free, the arm p3 of its fork pushes in its rapid
advance against the under oblique side of e4, raises this rod, and thereby sets the lever g4
free, whose upright arm bends down by the traction of the weight, drives the rod k4
before it into the ring i4 fastened to it, and thus by means of the fork l4 shifts the band
upon the loose pulley b1. But the machine may be brought to repose or put out of geer
at any time merely by shifting the rod k4 with the hand.
The operation of the bobbin and fly frame may be fully understood from the preceding
description. A few observations remain to be made upon the cone k1, the rack-bar
q3, and the speed of the work.
When we know the diameter of the empty bobbins, and how many turns they should
make in a given time in order to wind-on the sliver delivered by the fluted rollers and the
spindles; when we consider the diameters of the spindle pullies q, and t2, as also the drum.
m1, fig. 332., we may easily find the diameter which the cone must have for producing
that number of turns. This is the diameter for the greatest periphery of the base. The
diameter of the smaller is obtained in the same way, when the diameter of the bobbins
before the last winding-on, as well as the number of turns necessary in a given time, are
known.
A bobbin and fly frame of the construction just described delivers from each spindle
in a day of twelve hours, from 6 to 8 lbs of roving of the fineness of 11⁄2 English counts.
One person can superintend two frames, piece the broken slivers, and replace the full
bobbins by empty ones. The loss of cotton wool in this machine consists in the
portions carried off from the torn slivers, and must be returned to the lapping machine.
The fine bobbin and fly frame does not differ essentially from the preceding machine.
The rovings from the coarse bobbin and fly frame are placed in their bobbins in a frame
called the creel, behind and above the roller beam, two bobbins being allowed for one
fluted portion of the rollers. These rovings are united into one, so as to increase the
uniformity of the slivers.
The invention of the beautiful machine above described is due to Messrs. Cocker and
Higgins of Manchester, and as lately improved by Henry Houldsworth, junr. Esq., it
may be considered the most ingeniously combined apparatus in the whole range of productive
industry.
In the fine roving frame the sliver is twisted in the contrary direction to that of the
coarse roving frame. For this reason the position of the cone is reversed, so as to present
in succession to the band or strap, diameters continually greater, in order that the
rotation of the bobbins may be accelerated in proportion as their size is increased,
because here the flyer and the bobbin turn in the same direction, and the winding-on
is effected by the precession of the bobbin; but if the winding-on took place by its falling
behind, as in the coarse bobbin and fly frame, that is, if the flyer turned less quickly
than the bobbin, the rotatory speed of the bobbin would be uniformly retarded; in
which case the cone would be disposed as in the coarse frame.
When by any means whatever an uniform length of thread is delivered by the rollers
in a given time, the bobbin must wind it up as it is given out, and must therefore turn
with a speed decreasing with the increase of its diameter by successive layers of thread.
Hence proceeds the proposition, that the velocity of the bobbin must be in the inverse
ratio of its diameter, as already explained.
With respect to the bobbin and fly frame, the twist is given to the sliver by
means of a spindle or flyer which turns in the same direction with the bobbin, but
quicker or slower than it, which establishes two predicaments. The first case is where
the flyer turns faster than the bobbin. Here the winding-on goes in advance, as in
the coarse roving frame, or as in throstle spinning, where the yarn is wound on
merely in consequence of the friction of the lower disc or washer of the bobbin upon the
copping rail, and of the drag of the yarn. The second case is where the flyer revolves
more slowly than the bobbin. Here the winding goes on in arrear, and as the bobbin[362]
turns faster, it must receive a peculiar motion, which is uniformly retarded in the ratio
of its increase of diameter. This is the case with the fine bobbin and fly frame. When
the cone is placed as in fig. 332, the winding-on, in either the coarse or fine frame, results
from the difference, whether greater or less, between the rotatory speed of the flyer and
bobbin.
The motion of the bobbin and spindle is simultaneous, and takes place in the same
direction, with a difference varying more or less with the varying diameters of the
bobbins. To render the matter still clearer, suppose for a moment the spindle to be
motionless, then the bobbin must revolve with such a speed, as to lap-on the roving as
fast as the rollers deliver it. The sliver comes forward uniformly; but the bobbin, by
its increase of diameter, must revolve with a speed progressively slower. Now, suppose
the spindle set a-whirling, it is obvious that the bobbin must add to the movement
requisite for winding-on the sliver, that of the spindle in the case of winding-on in
arrear, or when it follows the flyers, and subtract its own motion from the twisting
motion of the spindles, in the case of winding-on in advance, that is, when the bobbin
precedes or turns faster than the flyers; for the diameter of the bobbin being 11⁄2
inch, 10 turns will take up 45 inches. Deducting these 10 turns from the 30 made
by the spindle in the same time, there will remain for the effective movement of the
bobbin only 20 turns; or when the diameter of the bobbin becomes 3 inches, 5 turns
will take up the 45 inches, if the spindle be at rest; but if it makes 30 turns in the
time, the effective velocity of the bobbin will be 25 turns, = 30 - 5. Hence in the
fine bobbin and fly frame, the number of turns of the spindle, minus the number of
turns made by the bobbin in equal times, is in the inverse ratio of the diameter of the
bobbin. We thus perceive, that in the coarse frame the bobbin should move faster
than the spindle, and that its speed should always diminish; whilst in the fine frame
the bobbin should move slower than the spindle, but its speed should always increase.
It is easy to conceive, therefore, why the cones are placed in reverse directions in the
two machines. Not that this inversion is indispensably necessary; the cone of the fine
roving frame might, in fact, be placed like that of the coarse roving frame; but as the
torsion of the roving becomes now considerable, and as on that account the bobbin
would need to move still faster, which would consume a greater quantity of the moving
power, it has been deemed more economical to give its movement an opposite direction.
We mentioned that the twist of the sliver in the fine roving frame was the reverse of
that in the coarse; this is a habit of the spinners, for which no good reason has been
given.
The divisions of the rack-bar, and the successive diameters of the cone, must be
nicely adjusted to each other. The first thing to determine is how much the rack
should advance for every layer or range of roving applied to the bobbin, in order that
the cone may occupy such a place that the strap which regulates the pulley barrel may
be at the proper diameter, and thus fulfil every condition. The extent of this progressive
movement of the rack depends upon the greater or less taper of the cone, and
the increase which the diameter of the bobbin receives with every traverse, that is,
every layer of roving laid on. But care should be taken not to taper the cone too
rapidly, especially in the fine roving frame, because in its progress towards the smaller
end, the strap would not slide with certainty and ease. We have already shown that
the number of effective turns of the bobbin is inversely, as the diameter of the bobbin,
or directly, as the successive diameters of the different points of the cone.
H. Houldsworth, jun. Esq. has introduced a capital improvement into the bobbin and
fly frame, by his differential or equation-box mechanism, and by his spring fingers,
which, by pressing the soft sliver upon the bobbin, cause at least a double quantity to
be wound upon its barrel. With the description of his patent equation-box, I shall
conclude the description of the bobbin and fly frame.
Fig. 342. represents a portion of a fly frame with Mr. Houldsworth’s invention.
a a a are the front drawing rollers, turning upon bearings in the top of the machine,
and worked by a train of toothed wheels, in the way that drawing rollers are usually
actuated.
From the drawing rollers, the filaments of cotton or other material, b b, are brought
down to, and passed through the arms of the flyers c c, mounted on the tops of the
spindles d d, which spindles also carry the loose bobbins e e. In the ordinary mode of
constructing such machines, the spindles are turned by cords or bands passing from a
rotatory drum round their respective pulleys or whirls f, and the loose bobbins e, turn
with them by the friction of their slight contact to the spindle, as before said; in the
improved machine, however, the movements of the spindles and the bobbins are independent
and distinct from each other, being actuated from different sources.
The main shaft of the engine g, turned by a band and rigger A as usual, communicates
motion by a train of wheels h, through the shaft i, to the drawing rollers at the
reverse end of the machine, and causes them to deliver the filaments to be twisted.[363]
Upon the main shaft g, is mounted a cylindrical hollow box or drum-pulley, whence
one cord passes to drive the whirls and spindles f and d, and another to drive the
bobbins e.
This cylindrical box pulley is made in two parts, k and l, and slipped upon the axle with
a toothed wheel m, intervening between them. The box and wheel are shewn detached
in fig. 343., and partly in section at fig. 344. That portion of the box with its pulley
marked l, is fixed to the shaft g; but the other part of the box and its pulley k, and the
toothed wheel m, slide loosely round upon the shaft g, and when brought in contact and
confined by a fixed collar n, as in the machine shewn at fig. 342., they constitute two
distinct pullies, one being intended to actuate the spindles, and the other the bobbins.
In the web of the wheel m, a small bevel pinion o, is mounted upon an axle standing
at right angles to the shaft g, which pinion is intended to take into the two bevel pinions
p and q, respectively fixed upon bosses, embracing the shaft in the interior of the boxes k
and l. Now it being remembered that the pinion q, and its box l, are fixed to the shaft
g, and turn with it, if the loose wheel m be independently turned upon the shaft, with a
different velocity, its pinion o, taking into q, will be made to revolve upon its axle, and
to drive the pinion p, and pulley box k, in the same direction as the wheel m; and this
rotatory movement of the box k and wheel m, may be faster or slower than the shaft g,
and box l, according to the velocity with which the wheel m is turned.
Having explained the construction of the box pullies k and l, which are the peculiar
features of novelty claimed under this patent, their office and advantage will be seen by
describing the general movements of the machine.
The main shaft g, being turned by the band and rigger A, as above said, the train of
wheels h, connected with it, drives the shaft i, which at its reverse end has a pinion (not
seen in the figure,) that actuates the whole series of drawing rollers a. Upon the shaft
i there is a sliding pulley r, carrying a band s, which passes down to a tension pulley t,
and is kept distended by a weight. This band s, in its descent, comes in contact with
the surface of the cone u, and causes the cone to revolve by the friction of the band
running against it. The pulley r is progressively slidden along the shaft i, by means of
a rack and weight not shewn, but well understood as common in these kind of machines,
and which movement of the pulley is for the purpose of progressively shifting the band
s from the smaller to the larger diameter of the cone, in order that the speed of its
rotation may gradually diminish as the bobbins fill by the winding-on of the yarns.
At the end of the axle of the cone u a small pinion v is fixed, which takes into the
teeth of the loose wheel m, and, as the cone turns, drives the wheel m round upon the
shaft g, with a speed dependent always upon the rapidity of the rotation of the cone.
Now the box pulley l, being fixed to the main shaft g, turns with one uniform speed,
and by cords passing from it over guides to the whorls f, drives all the spindles and
flyers, which twist the yarns with one continued uniform velocity; but the box pulley k,[364]
being loose upon the shaft, and actuated by the bevel pinions within, as described, is
made to revolve by the rotation of the wheel m, independent of the shaft, and with a
different speed from the pulley box l; cords passing from this pulley box k, over guides
to small pullies under the bobbins, communicate the motion, whatever it may be, of the
pulley box k, to the bobbins, and cause them to turn, and to take up or wind the yarn
with a speed derived from this source, independent of, and different from, the speed of
the spindle and flyer which twist the yarn.
It will now be perceived, that these parts being all adjusted to accommodate the
taking up movements to the twisting or spinning of any particular quality of yarn
intended to be produced, any variations between the velocities of the spinning and
taking up, which another quality of yarn may require, can easily be effected, by merely
changing the pinion v, for one with a different number of teeth, which will cause the
wheel m, and the pulley box k, to drive the bobbins faster or slower, as would be required
in winding-on fine or coarse yarn, the speed of the twisting or spinning being the
same.
The rovings or spongy cords, of greater or less tenuity, made on the bobbin and fly,
or tube roving frame, are either spun immediately into firm cohesive yarn, or receive a
further preparation process in the stretching frame, which is, in fact, merely a mule-jenny,
without the second draught and second speed, and therefore need not be described
at present, as it will be in its place afterwards.
The finishing machines of a cotton mill, which spin the cohesive yarn, are of two
classes; 1. the water-twist or throstle, in which the twisting and winding are performed
simultaneously upon progressive portions of the roving; and, 2. the mule, in which the
thread is drawn out and stretched, with little twist, till a certain length of about 5 feet
is extended, then the torsion is completed, and the finished thread is immediately
wound upon the spindles into double conical coils called cops.
The water-twist frame, so called by its inventor, Sir R. Arkwright, because it was first
driven by water, is now generally superseded by the throstle frame, in which the mechanical
spinning fingers, so to speak, are essentially the same, but the mode of communicating
the motion of the mill-geering to them is somewhat different. Fig. 345. exhibits a
vertical section of the throstle. This machine is double, possessing upon each side of
its frame, a row of spindles with all their subsidiary parts. The bobbins, filled with
rovings from the bobbin and fly, or the tube frame, are set up in the creel a a, in two
ranges, b, c, d, are the three usual pairs of drawing rollers, through which the yarn
is attenuated to the proper degree of fineness, upon the principles already explained.
At its escape from the front rollers, every thread runs through a guide eyelet e of wire,
which gives it the vertical direction down towards the spindles f, g. The spindles which
perform at once and uninterruptedly the twisting and winding-on of the thread delivered
by the rollers, are usually made of steel, and tempered at their lower ends. They
stand at g in steps, pass at v through a brass bush or collet which keeps them upright,
and revolve with remarkable speed upon their axes. The bobbins h, destined to take
up the yarn as it is spun, are stuck loosely upon the spindles, and rest independently of
the rotation of the spindles upon the copping beam l, with a leather washer between.
Upon the top of the spindles an iron-wire fork, called a fly or flyer, i, k, is made
fast by a left-hand screw, and has one of its forks turned round at the end into a little
ring. The branch of the flyer at f is tubular, to allow the thread to pass through, and to
escape by a little hole at its side, in order to reach the eyelet at the end of that fork.
From this eyelet i, it proceeds directly to the bobbin. By the twirling of the spindle,
the twisting of the portion of thread between the front roller d, and the nozzle f, is
effected. The winding-on takes place in the following way:—Since the bobbin has no
other connection with the spindle than that of the thread, it would but for it remain
entirely motionless, relatively to the spindle. But the bobbin is pulled after it by the
thread, so that it must follow the rotation of the spindle and fly. When we consider
that the thread is pinched by the front roller d, and is thereby kept fully upon the
stretch, we perceive that the rotation of the bobbin must be the result. Suppose now
the tension to be suspended for an instant, while the rollers d, deliver, for example, one
inch of yarn. The inertia or weight of the bobbin, and its friction upon the copping
beam l, by means of the leather washer, will, under this circumstance, cause the bobbin
to hang back in a state of rest, till the said inch of yarn be wound on by the whirling
of the fly i, and the former tension be restored. The delivery of the yarn by the drawing
rollers, however, does not take place inch after inch, by starts, but at a certain continuous
rate; whence results a continuous retardation or loitering, so to speak, of the
bobbins behind the spindles, just to such an amount that the delivered yarn is wound
up at the same time during the rotation.
This process in spinning is essentially the same as what occurs in the fine bobbin
and fly frame, but is here simplified, as the retardation regulates itself according to
the diameter of the bobbin by the drag of the thread. In the fly frame the employment[365]
of this tension is impossible, because the roving has too little cohesion to bear the
strain; and hence it is necessary to give the bobbins that independent movement of
rotation which so complicates this machine.
The up and down motion of the bobbins along the spindles, which is required for
the equal distribution of the yarn, and must have the same range as the length of the
bobbin barrels, is performed by the following mechanism. Every copping rail l,
is made fast to a bar m, and this, which slides in a vertical groove or slot at the end of
the frame, is connected by a rod n, with an equal-armed, moveable lever o. The rod p
carries a weight r, suspended from this lever; another rod q, connects the great lever o
with a smaller one s, t, upon which a heart-shaped disc or pulley u, works from below at t.
By the rotation of the disc u, the arm t, being pressed constantly down upon it by the
reaction, the weight r must alternately rise and fall; and thus the copping rail l must
obviously move with the bobbins h up and down; the bobbins upon one side of the frame
rising, as those upon the other sink. Strictly considered, this copping motion should
become slower as the winding-on proceeds, as in the fly roving frame; but, on account
of the smallness of the finished thread, this construction, which would render the
machine complicated, is without inconvenience neglected, with the result merely that
the coils of the yarn are successively more sparsely laid on, as the diameter of the
bobbin increases.
The movement of the whole machine proceeds from the shaft of a horizontal drum,
which drives the spindles by means of the endless bands x x. Each spindle is mounted
with a small pulley or wharf w, at its lower part, and a particular band, which goes
round that wharf or whorl, and the drum y. The bands are not drawn tense, but hang
down in a somewhat slanting direction, being kept distended only by their own weight.
Thus every spindle, when its thread breaks, can readily be stopt alone, by applying a slight
pressure with the hand or knee, the band meanwhile gliding loosely round the whorl.
The velocities of rotation of the three drawing rollers are, according to this arrangement,
in the proportion of 1 : 11⁄2 : 8; and as their diameters are the same, namely, one
inch, the elongation of the yarn in spinning is eight-fold. If, for example, the roving
was of the number 41⁄2, the yarn would become No. 36. The extension of the thread
may be changed by changing the wheels of the drawing rollers. To perceive the
power of this change, let us put, for example, in the place of the 18-toothed wheel of
the back rollers, a wheel with 16 teeth; we shall find that the elongation will amount,
in that case, only to 71⁄2 times, whence the number of the yarn would come out 32 = 71⁄2 ×
41⁄2. The extension by the throstle is extremely various; it amounts, in some cases, to
only 4 times; at others to 10, 12, or even 15.
The copping motion of the bobbins is produced in consequence of a bevel pinion
working in a small bevel wheel upon an upright shaft; while this wheel gives a slow
motion by means of a worm screw to the wheel of the heart-shaped pulley u, fig. 345.
The driving pulley makes about 600
turns in a minute; and as the diameter of
the drum y, fig. 345., is six times the diameter
of the spindle wharves w, it will
give 3600 turns to the spindle in that time.
If the pulley be driven faster, for example
700 times in a minute, it will increase the
revolutions of the spindles to 4200. The
degree of twist which will be thereby imparted
to the yarn, depends, with like speed
of spindles, upon the rate at which the soft
yarn is delivered by the drawing rollers;
for the quicker this delivery, the quicker is
the winding-on, and the less twist goes into
a given length of yarn. If, for example,
the front rollers d, turn 24 times in a
minute, giving out of course 72 inches of
yarn in this time, upon which the 3600
revolutions of the spindle are expended,
there will be 50 twists to every inch of
yarn. By changing the wheel-work of
fig. 345., or by sticking greater or smaller wharves upon the spindles, the proportion
between their velocity and that of the drawing rollers, and thence the degree of twist
can be modified at pleasure.
The number of spindles in a throstle frame 12 feet long, is about 60 on each side.
The drawing rollers are coupled together as in the bobbin and fly frame, so that each
row forms one continuous cylinder. There is a complete roller beam on each side;
each of the rollers of the front row is pressed by its top rollers with a weight of ten or[366]
twelve pounds; but those of the middle and back rows bear weights of only one pound.
In the throstles, there is a guide bar which traverses a small way horizontally to the left
and right, in front of the roller beam, to lead the thread along different points of the
rollers, and thus prevent the leather of the top ones from being grooved by its constant
pressure in one line.
For the service of 240 spindles, in two double frames, one young woman, and an assistant
piecer are sufficient. They mend the broken ends, and replace the empty bobbins
in the creel with full ones, and the full bobbins of the throstle by empty ones. The
average quantity of yarn turned off in a week of 69 hours is about 24 hanks per spindle
of 30′s twist. Throstle yarn is of a firm wiry quality, adapted to the warps of fustians
and other strong stuffs, as well as to the manufacture of stockings and sewing thread.
There are many modifications of the throstle system besides the one above described;
the most celebrated of which are Danforth’s, called the American throstle, Montgomery’s,
and Gore’s. I must refer for an account of them to my work entitled “The
Cotton Manufacture of Great Britain,” where they are minutely described and illustrated
with accurate figures.
Mule-spinning.—The general principles of the mule have been already stated. This
machine is so named because it is the offspring, so to speak, of two older machines, the
jenny and the water-frame. A mule is mounted with from 240 to 1000 spindles, and
spins of course as many threads.
Fig. 346. represents the
original jenny of Hargreaves,
by which one
person was enabled to
spin from 16 to 40
threads at once. The
soft cords of rovings
wound in double conical
cops upon skewers were
placed in the inclined
frame at C; the spindles
for first twisting and
then winding-on the
spun yarn were set upright
in steps and bushes
at A, being furnished
near their lower ends with whorls, and endless cords, which were driven by passing round
the long-revolving drum of tin plate E. D is the clasp or clove, having a handle for
lifting its upper jaw a little way, in order to allow a few inches of the soft roving to be
introduced. The compound
clove D being now
pushed forward upon its
friction wheels to A, was
next gradually drawn
backward, while the spindles
were made to revolve
with proper speed by
the right hand of the
operative turning the flywheel
B. Whenever one
stretch was thereby spun,
the clove frame was slid
home towards A; the
spindles being simultaneously
whirled slowly
to take up the yarn,
which was laid on in a
conical cop by the due
depression of the faller
wire at A with the spinner’s
left hand.
Fig. 347. is a diagram
of Arkwright’s original
water-frame spinning
machine, called afterwards
the water-twist[367]
frame. The rovings mounted upon bobbins
in the creel A A, have their ends led
through between the three sets of twin
rollers below B B, thence down through
the eyelet hooks upon the end of the flyers
of the spindles C, and finally attached to
their bobbins. The spindles being driven
by the band D D upon their lower part, continuously
twist and wind the finished yarn
upon the bobbins; constituting the first
unremitting automatic machine for spinning
which the world ever saw.
Contrast with the above admirable system,
the primitive cotton wheel of India, as
represented in the annexed figure 348. By
the aid of mechanical fingers, one Englishman
at his mule can turn off daily more
yarn and of far finer quality than 200 of
the most diligent spinsters of Hindostan.
Fig. 349., is a transverse section of the mule, in which its principal parts are shown.
The machine consists of two main parts; a fixed one corresponding in some measure
to the water-frame or throstle, and a moveable one corresponding to the jenny.
The first contains in a suitable frame the drawing roller-beam and the chief moving
machinery: the second, is called the carriage, in which the remainder of the moving
mechanism and the spindles are mounted.
The frame of the fixed part consists of two upright sides, and two or more intermediate
parallel bearings, upon which the horizontal roller beam a, the basis of the drawing rollers
is supported, b, c, d, are the three ranges of fluted iron rollers; e, f, g, are the upper iron
rollers covered with leather; h, the wooden wiper-rollers covered with flannel, which
being occasionally rubbed with chalk, imparts some of it to the pressure rollers beneath,
so as to prevent the cotton filaments adhering to them. The rollers are made throughout[368]
the whole length of the mule in portions containing six flutings, which are coupled
together by squared ends fitted into square holes.
The skewers upon which the bobbins containing the rovings from the bobbin and fly
or stretching frame, are set up, are seen at a1, a1, a1, arranged in three rows in the
creel z. The soft threads unwound from these bobbins, in their way to the drawing
rollers, pass first through eyelets in the ends of the wire arms b1, then through the
rings or eyes of the guide bar w, and enter between the back pair of rollers. The
number of these bobbins is equal to the number of spindles in the mule, and twice as
great as the number of fluted portions of the rollers; for two threads are assigned to
each portion.
The carriage consists of two cast-iron side pieces, and several cast-iron intermediate
similar pieces, such as f2, which all together are made fast to the planks b2, c2, d2. The
top is covered in with the plank k2. The carriage runs by means of its cast-iron grooved
wheels, upon the cast-iron railway l2, which is fixed level on the floor.
The spindles stand upon the carriage in a frame, which consists of two slant rails x2,
x2, connected by two slender rods y2, and which frame may be set more or less obliquely.
The lower rail carries the brass steps for the points of the spindles b3; upon the upper
rail brass slips are fixed pierced with holes through which the tops of the spindles play.
The spindles are as usual made of steel, perfectly straight, turned truly round, and are
all arranged in one plane. To each of them a small wooden or cast-iron whorl g2 is
made fast. They are distributed into groups of 24, and the whorls are arranged at
such different heights, that only two of them in each group are upon a level with each
other. A small brass head h2, which every spindle has beneath the upper slant rail of
the frame x2, prevents their sitting down into the step, during their rotation, or
sliding off their cop of yarn.
c3 are drums, mounted in the carriage in a plane at right angles to the plane in
which the spindles are placed. At top they have a double groove for a cord to run
in, and the motion which they receive from the great fly wheel, or rim of the mule (not
visible in this view) they impart to the spindles. Such a drum is assigned to every 24
spindles; and therefore a mule of 480 spindles contains 20 drums. In the middle of
the carriage is seen the horizontal pulley k3, furnished with three grooves, which stands
in a line with the drums c3.
The motion is given to the drums c3, upon the right hand half of the carriage by a
single endless band or cord which proceeds from the middle groove of the pulley k3.
The rotation of the spindles is produced by a slender cord, of which there are 12 upon
each drum c3; because every such cord goes round the drum, and also every two wharves
which stand at the same level upon the spindles. It is obvious that the drums, and
consequently the spindles, must continue to revolve as long as the main rim of the mule
is turned, whether the carriage be at rest or in motion upon its railway.
If we suppose the carriage to be run in to its standing point, or to be pushed home
to the spot from which it starts in spinning, its back plank d2 will strike the post q3
upon the fixed frame, and the points of the spindles will be close in front of the roller
beam. The rollers now begin to turn and to deliver threads, which receive immediately
a portion of their twist from the spindles; the carriage retires from the roller beam
with somewhat greater speed than the surface speed of the front rollers, whereby the
threads receive a certain degree of stretching, which affects most their thicker and less
twisted portions, and thereby contributes greatly to the levelness of the yarn. When
the carriage has run out to the end of its course, or has completed a stretch, the fluted
rollers suddenly cease to revolve (and sometimes even beforehand, when a second
stretch is to be made), but the spindles continue to whirl till the fully extended threads
have received the proper seconder after-twist. Then the carriage must be put up, or run
back towards the rollers, and the threads must be wound upon the spindles.
This is the order of movements which belong to the mule. It has been shown how
the rotation of the spindles is produced.
For winding-on the yarn the carriage has a peculiar apparatus, which we shall now
describe. In front of it, through the whole extent to the right hand as well as the left,
a slender iron rod, d5, runs horizontally along, in a line somewhat higher than the middle
of the copping portion of the spindles, and is supported by several props, such as
e5. Upon each end of the two rods, d5, there is an arm, g5; and betwixt these arms an
iron wire, called the copping wire, f5, is stretched, parallel with the rod d5. For the
support of this wire, there are several slender bent arms h5 extended from the rod d5
at several points betwixt the straight arms g5. The rod d5 has, besides a wooden
handle at the place opposite to where the spinner stands, by which it can be readily
grasped. This movement is applied at the left division of the machine, and it is communicated
to the right by an apparatus which resembles a crane’s bill. The two arms,
g5, in the middle of the machine, project over the rods d5, and are connected by hinges
with two vertical rods j5, which hang together downwards in like manner with two arms
[369]i5, proceeding from a horizontal axis k5.
By means of that apparatus the yarn is wound upon the spindles in the following
manner. As long as the stretching and twisting go on, the threads form an obtuse angle
with the spindles, and thereby slide continually over their smooth rounded tips
during their revolution, without the possibility of coiling upon them. When, however,
the spinning process is completed, the spinner seizes the carriage with his left hand and
pushes it back towards the roller beam, while with his right hand he turns round the
handle of the rim or fly wheel, and consequently the spindles. At the same time, by
means of the handle upon the rod d5, he moves the copping-wire, f5, so that it presses
down all the threads at once, and places them in a direction nearly perpendicular to
the spindles; as shown by the dotted line y5. That this movement of the copping wire,
however, may take place without injury to the yarn, it is necessary to turn the rim beforehand
a little in the opposite direction, so that the threads may get uncoiled from the
upper part of the spindles, and become slack; an operation called in technical language,
the backing off. The range upon which the threads should be wound, in order to form
a conical cop upon the spindle, is hit by depressing the copping wire to various angles,
nicely graduated by an experienced eye. This faller wire alone is not, however, sufficient
for the purpose of winding-on a seemly cop, as there are always some loose threads
which it cannot reach without breaking others.
Another wire called the counter-faller, l5, must be applied under the threads. It may
be raised to an elevation limited by the angular piece p5; and is counterpoised by a very
light weight m5, applied through the bent lever n5, which turns upon the fulcrum o5. This
wire, which applies but a gentle pressure, gives tension to all the threads, and brings them
regularly into the height and range of the faller f5. This wire must be raised once
more, whenever the carriage approaches the roller beam. At this instant a new stretch
commences; the rollers begin again to revolve, and the carriage resumes its former
course. These motions are performed by the automatic machinery.
There is a little eccentric pulley mechanism for moving the guide beam to and fro
with the soft yarns, as they enter between the back rollers. On the right hand
end of the back roller shaft, a worm screw is formed which works into the oblique teeth
of a pinion attached to the end of the guide beam, in which there is a series of holes for
the passage of the threads, two threads being assigned to each fluted roller. In the
flat disc of the pinion, an eccentric pin stands up which takes into the jointed lever
upon the end of the guide beam, and as it revolves, pushes that beam alternately to the
left and the right by a space equal to its eccentricity. This motion is exceedingly slow,
since for each revolution of the back roller, the pinion advances only by one tooth out
of the 33 which are cut in its circumference.
After counting the number of teeth in the different wheels and pinions of the mule, or
measuring their relative diameters, it is easy to compute the extension and twist of the
yarns; and when the last fineness is given to ascertain their marketable value. Let the ratio
of speed between the three drawing rollers be 1 : 13⁄22 : 71⁄2; and the diameter of the back and
middle roller three quarters of an inch: that of the front roller one inch; in which case
the drawing is thereby increased 11⁄3 times, and 71⁄2 × 11⁄3 = 10. If the rovings in the creel
bobbins have been No. 4. the yarn, after passing through the rollers, will be No. 40. By
altering the change pinion (not visible in this view) the fineness may be changed within
certain limits, by altering the relative speed of the rollers. For one revolution of the
great rim or fly wheel of the mule, the front roller makes about 6-tenths of a turn, and
delivers therefore 22·6 lines or 12ths of an inch of yarn, which, in consequence of
the tenfold draught through the rollers, corresponds to 2·26 lines of roving fed in at the
back rollers. The spindles or their whorls make about 66 revolutions for one turn of
the rim. The pulleys or grooved wheels on which the carriage runs, perform 0·107
part of a turn while the rim makes one revolution, and move the carriage 24·1 lines
upon its rails, the wheels being 6 inches in diameter.
The 22·6 lines of soft yarn delivered by the front rollers, will be stretched 11⁄2 lines
by the carriage advancing 24·1 lines in the same time. Let the length of the railway,
or of each stretch be 5 feet, the carriage will complete its course after 30 revolutions of
the rim wheel, and the 5 feet length of yarn (of which 561⁄2 inches issue from the drawing
rollers, and 31⁄2 inches proceed from the stretching) is, by the simultaneous whirling of
the spindles, twisted 1980 times, being at the rate of 33 twists for every inch. The second
twist, which the threads receive after the carriage has come to repose, is regulated according
to the quality of the cotton wool, and the purpose for which the yarn is spun.
For warp yarn of No. 40 or 50, for example, 6 or 8 turns of the rim wheel, that is, from
396 to 528 whirls of the spindles for the whole stretch, therefore from 7 to 9 twists
per inch will be sufficient. The finished yarn thus receives from 40 to 42 twists per
inch.
One spinner attends to two mules, which face each other, so that he needs merely
turn round in the spot where he stands, to find himself in the proper position for the
other mule. For this reason the rim wheel and handle, by which he operates, are not[370]
placed in the middle of the length of the machine, but about two fifths of the spindles
are to the right hand and three fifths to the left; the rim wheel being towards his right
hand. The carriage of the one mule is in the act of going out and spinning, while that
of the other is finishing its twist, and being put up by the spinner.
The quantity of yarn manufactured by a mule in a given time, depends directly
upon the number of the spindles, and upon the time taken to complete every stretch of
the carriage. Many circumstances have an indirect influence upon that quantity, and
particularly the degree of skill possessed by the spinner. The better the machine, the
steadier and softer all its parts revolve, the better and more abundant is its production.
When the toothed wheels do not work truly into their pinions, when the spindles shake
in their bushes, or are not accurately made, many threads break, and the work is
much injured and retarded. The better the staple of the cotton wool, and the more
careful has been its preparation in the carding, drawing, and roving processes, the more
easy and excellent the spinning will become: warmth, dryness, cold, and moisture
have great influence on the ductility, so to speak, of cotton. A temperature of 65° F.,
with an atmosphere not too arid, is found most suitable to the operations of a spinning
mill. The finer the yarn, the slower is the spinning. For numbers from 20 to 36,
from 2 to 3 stretches of warp may be made in a minute, and nearly 3 stretches of weft;
for numbers above 50 up to 100, about 2 stretches; and for numbers from 100 to 150,
one stretch in the minute. Still finer yarns are spun more slowly, which is not
wonderful, since in the fine spinning mills of England, the mules usually contain
upwards of 500 spindles each, in order that one operative may manage a great number
of them, and thereby earn such high wages as shall fully remunerate his assiduity
and skill.
In spinning fine numbers, the second speed is given before the carriage is run out to
the end of its railway; during which course of about six inches, it is made to move very
slowly. This is called the second stretch, and is of use in making the yarn level by
drawing down the thicker parts of it, which take on the twist less readily than the
thinner, and therefore remain softer and more extensible. The stretch may therefore
be divided into three stages. The carriage first moves steadily out for about 4 feet,
while the drawing rollers and spindles are in full play; now the rollers stop, but the
spindles go on whirling with accelerated speed, and the carriage advances slowly, about
6 inches more; then it also comes to rest, while the spindles continue to revolve for a
little longer, to give the final degree of twist. The acceleration of the spindles in the
second and third stages, which has no other object but to save time, is effected by
a mechanism called the counter, which shifts the driving band, at the proper time, upon
the loose pulley, and, moreover, a second band, which had, till now, lain upon its loose
pulley, upon a small driving pulley of the rim-shaft. At length, both bands are
shifted upon their loose pulleys, and the mule comes to a state of quiescence.
The SELF-ACTOR MULE, or the IRON MAN, as it has been called in Lancashire, is an
invention to which the combinations among the operative spinners obliged the masters
to have recourse. It now spins good yarn up to 40 s with great uniformity and
promptitude, and requires only juvenile hands to conduct it, to piece the broken yarns,
to replace the bobbins of rovings in the creel, and to remove the finished cops from the
spindles.
The self-acting mules were first constructed, I believe, by Messrs. Eaton, formerly of
Manchester, who mounted ten or twelve of them in that town, four at Wiln, in Derbyshire,
and a few in France. From their great complexity and small productiveness,
the whole were soon relinquished, except those at Wiln. M. de Jong obtained
two patents for self-acting mules, and put twelve of them in operation in a mill at Warrington,
of which he was part proprietor; but with an unsuccessful result. I saw the
débris of one of M. de Jong’s self-actors in the factory of M. Nicolas Schlumberger, at
Guebwiller, in Alsace, where the machine had been worked for three months, without
advantage, under the care of the inventor, who is a native of that valley.
The first approximation to a successful accomplishment of the objects in view, was an
invention of a self-acting mule, by Mr. Roberts, of Manchester; one of the principal
points of which was the mode of governing the winding-on of the yarn into the form of
a cop; the entire novelty and great ingenuity of which invention was universally
admitted, and proved the main step to the final accomplishment of what had so long
been a desideratum. For that invention a patent was obtained in 1825, and several
headstocks upon the principle were made, which are still working successfully.
In 1830, Mr. Roberts obtained a patent for the invention of certain improvements;
and by a combination of both his inventions, he produced a self-acting mule, which is
generally admitted to have exceeded the most sanguine expectations, and which has
been extensively adopted. There are, probably, at present, upwards of half a million
of spindles of Messrs. Sharp, Roberts, and Co.’s construction, at work in the United
Kingdom, and giving great satisfaction to their possessors. The advantages of these
self-actors are the following:—
[371]
The saving of a spinner’s wages to each pair of mules, piecers only being required,
as one overlooker is sufficient to manage six or eight pairs of mules. The production
of a greater quantity of yarn, in the ratio of from 15 to 20 per cent. The yarn
possesses a more uniform degree of twist, and is not liable to be strained during the
spinning, or in winding-on, to form the cop; consequently fewer threads are broken in
these processes, and the yarn, from having fewer piecings is more regular.
The cops are made firmer, of better shape, and with undeviating uniformity; and,
from being more regularly and firmly wound, contain from one third to one half more
yarn than cops of equal bulk wound by hand; they are consequently less liable to
injury in packing or in carriage, and the expense of packages and freight (when
charged by measurement) is considerably reduced.
From the cops being more regularly and firmly wound, combined with their superior
formation, the yarn intended for warps less frequently breaks in winding or reeling,
consequently there is a considerable saving of waste in those processes.
Secondly, the advantages connected with weaving.
The cops being more regularly and firmly wound, the yarn, when used as weft,
seldom breaks in weaving; and as the cops also contain a greater quantity of weft,
there are fewer bottoms, consequently there is a very material saving of waste in the
process of weaving.
From those combined circumstances, the quality of the cloth is improved, by being
more free from defects caused by the breakage of the warp or weft, as well as the
selvages being more regular.
The looms can also be worked at greater speed; and, from there being fewer stoppages,
a greater quantity of cloth may be produced.
That the advantages thus enumerated, as derivable from the use of self-acting mules,
have not been overrated, but, in many instances, have been considerably exceeded, I
have, by extensive personal inquiry and observation, had ample opportunity of ascertaining.
Statement of the quantity of yarn produced on Messrs. Sharp, Roberts, and Co.’s
self-acting mules, in twelve working hours, including the usual stoppages connected
with spinning, estimated on the average of upwards of twenty mills:—
| No. of Yarn. |
No. of Twist. |
No. of Weft. |
| 16 |
4 |
1⁄2 |
hanks |
4 |
7⁄8 |
hanks per spindle. |
| 24 |
4 |
1⁄4 |
— |
4 |
5⁄8 |
— |
| 32 |
4 |
|
— |
4 |
3⁄8 |
— |
| 40 |
3 |
3⁄4 |
— |
4 |
1⁄8 |
— |
Of the intermediate numbers the quantities are proportionate.
Results of trials made by Messrs. Sharp, Roberts, and Co., at various mills, to
ascertain the comparative power required to work self-acting mules, in reference to
hand-mules, during the spinning, up to the period of backing off.
Particulars of the trials referred to, and their results:—
At what Mill, and the
Description of Mule. |
No.
and kind
of Yarn. |
Diameter
of
Pulley
or
Rim
Wheel. |
Revo-
lutions
of
Pulley
or
Rim
Wheel. |
Re-
quired
Force
for
Motion. |
Total
Force
Employed
in
Spinning. |
| Messrs. Birley and Kirk. |
Weft. |
Ins. |
|
lbs. |
lbs. |
| Self-acting mule, 360 sps. |
30 to 34 |
12 |
58 |
30 |
|
|
5463 |
|
| [20]Hand mule, 180 sps. |
ditto |
15 |
36 |
26 |
|
|
3669 |
|
- |
| |
|
|
|
|
× 2 = |
7338 |
| |
|
|
|
|
|
| Messrs. Leech and Vandrey. |
Twist. |
|
|
|
|
| [21]Self-acting mule, 324 sps. |
36 |
12 |
70 |
36 |
|
|
7912 |
|
| Hand mules, 324 sps. |
36 |
29 |
58 |
16 |
1⁄2 |
|
7273 |
|
| |
|
|
|
|
|
| Messrs. Duckworth & Co. |
Twist. |
|
|
|
|
| Self-acting mule, 324 sps. |
40 |
12 |
62 |
33 |
|
|
6421 |
|
| Hand mule, 324 sps. |
40 |
47 |
36 |
15 |
1⁄2 |
|
6646 |
|
The mode adopted to make the trials was as follows, viz.:
A force, indicated by weight in pounds, was applied to the strap working upon the[372]
driving-pulley of the respective mules, sufficient to maintain the motion of the mule
whilst spinning, which weight, being multiplied by the length of strap delivered by
each revolution of the pulley, and again by the number of revolutions made by the
pulley whilst spinning, gave the total force in pounds, applied to the respective mules
whilst spinning; for instance, suppose a mule to be driven by a pulley 12 inches
diameter (3·14 ft. in circumference), such pulley making 58 revolutions during the spinning
as above, and that it required a force equal to 30 lbs. weight to maintain the motion
of the mule, then 30 lbs. × 3·14 feet circumference of pulley × 58 revolutions in spinning
= 5463 lbs. of force employed during the spinning, to the period of backing off.
Mr. James Smith, of Deanstone cotton works in Scotland, obtained a patent for the
invention of a self-actor, in February, 1834. He does not perform the backing-off by
reversing the rotation of the spindle, as in common mules, or as in Mr. Roberts’, but by
elevating the counterfaller wire, which, being below the ends of the yarn or thread,
along the whole extent of the carriage, thereby pulls off or strips the spiral coils at the
point of the spindle, instead of unwinding them, as of old. This movement he considers
to be of great importance towards simplifying the machinery for rendering the
mule self-acting; and the particular way in which he brings the stripper into action is
no doubt ingenious, but it has been supposed by many to strain the yarn. He claims
as his invention the application and adaptation of a mangle wheel or mangle rack to the
mule, for effecting certain successive movements, either separately or in conjunction; he
claims that arrangement of the carriages of a pair of mules, by which the stretch is
caused to take place over part of the same ground by both carriages, and thereby the
space required for the working of a pair of mules is greatly diminished; and he
claims the application of a weight, spring, or friction, for balancing the tension of the
ends of the threads.
A patent was granted, in April, 1835, to Mr. Joseph Whitworth, engineer in Manchester,
for some ingenious modifications of the mechanism of the mule, subservient to
automatic purposes. His machinery is designed, first, to traverse the carriage in and
out, by means of screws or worm-shafts, which are placed so as to keep the carriage
parallel to the drawing rollers, and prevent the necessity of squaring bands, hitherto
universally employed; secondly, his invention consists in an improved manner of working
the drums of a self-acting mule by geer; thirdly, in the means of effecting the
backing off; fourthly, in the mechanism for working the faller-wire in building
the cops; and fifthly, in the apparatus for effecting the winding of the yarns upon
the spindles. As regards the throstles and doubling frames, his improvements apply,
first, to the peculiar method of constructing and adapting the flyers and spindles, and
producing the drag; and, secondly, to the arrangement of the other parts of the doubling
machinery.
See Lace-Making, Singeing, Textile Fabric, Thread Manufacture, and
Weaving.
The Imports of Cotton Wool for home consumption into the United Kingdom were
in the year ending 5th January,
| |
1836. |
1837. |
| |
lbs. |
lbs. |
| From the British possessions in America |
1,346,220 |
1,041,434 |
| Fro—the Bdo.h possedo.ns in>East Indies |
43,404,058 |
34,060,055 |
| Fro—the United States of America |
287,346,721 |
309,027,306 |
| Fro—the Brazil |
26,879,779 |
20,822,509 |
| Fro—the Egypt |
5,184,743 |
7,465,774 |
| Otherwise imported |
6,789,603 |
5,602,602 |
| Total |
370,951,124 |
378,019,680 |
| |
£ |
£ |
| The Exports of Cotton Manufactures |
18,511,692 |
13,625,464 |
| The export—of CottonYarn |
6,120,366 |
6,953,467 |
COURT PLASTER, is a considerable object of manufacture. It is made as
follows:
Black silk is strained and brushed over ten or twelve times with the following preparation:—Dissolve
1⁄2 an ounce of balsam of benzoin in 6 ounces of rectified spirits of
wine; and in a separate vessel dissolve 1 ounce of isinglass in as little water as may be.
Strain each solution, mix them, and let the mixture rest, so that any undissolved parts
may subside; when the clear liquid is cold it will form a jelly, which must be warmed
before it is applied to the silk. When the silk coated with it is quite dry, it must be[373]
finished off with a coat of a solution of 4 ounces of China turpentine in 6 ounces of
tincture of benzoin, to prevent its cracking.[22]
CRAPE. (Crêpe, Fr.; Krepp, Germ.) A transparent textile fabric, somewhat
like gauze, made of raw silk, gummed and twisted at the mill. It is woven with any
crossing or tweel. When dyed black, it is much worn by ladies as a mourning dress.
Crapes are crisped (crepés) or smooth; the former being double, are used in close
mourning, the latter in less deep. White crape is appropriate to young unmarried
females, and to virgins on taking the veil in nunneries. The silk destined for the
first is spun harder than for the second; since the degree of twist, particularly of the
warp, determines the degree of crisping which it assumes after being taken from the
loom. It is for this purpose steeped in clear water, and rubbed with prepared wax.
Crapes are all woven and dyed with the silk in the raw state. They are finished with
a stiffening of gum water.
Crape is a Bolognese invention, but has been long manufactured with superior
excellence at Lyons in France, and Norwich in England. There is now a magnificent
fabric of it at Yarmouth, by power-loom machinery.
There is another kind of stuff, called crepon, made either of fine wool, or of wool and
silk, of which the warp is twisted much harder than the weft. The crepons of Naples
consist altogether of silk.
CRAYONS. (Eng. and Fr.; Pastelstifte, Germ.) Slender, soft, and somewhat
friable cylinders, variously coloured for delineating figures upon paper, usually called
chalk drawings. Red, green, brown, and other coloured crayons, are made with fine
pipe or china clay paste, intimately mixed with earthy or metallic pigments, or in
general with body or surface colours, then moulded and dried. The brothers Joel, in
Paris, employ as crayon cement the following composition: 6 parts of shell-lac, 4 parts
of spirit of wine, 2 parts of turpentine, 12 parts of a colouring powder, such as Prussian-blue,
orpiment, whitelead, vermillion, &c., and 12 parts of blue clay. The clay being
elutriated, passed through a hair sieve, and dried, is to be well incorporated by trituration
with the solution of the shell-lac in the spirit of wine, the turpentine, and the
pigment; and the doughy mass is to be pressed in proper moulds, so as to acquire the
desired shape. They are then dried by a stove heat.
In order to make cylindrical crayons, a copper cylinder is employed, about 2 inches
in diameter, and 11⁄2 inches long, open at one end, and closed at the other with a perforated
plate, containing holes corresponding to the sizes of the crayons. The paste is
introduced into the open end, and forced through the holes of the bottom by a piston
moved by a strong press. The vermicular pieces that pass through are cut to the
proper lengths, and dried. As the quality of the crayons depends entirely upon the
fineness of the paste, mechanical means must be resorted to for effecting this object in
the best manner. The following machine has been found to answer the purpose exceedingly
well.
[374]
Fig. 350. is a vertical section through the centre of the crayon mill. Fig. 351. is a
view of the mill from above. A, the mill tub, whose bottom B must be a hard flat plate
of cast iron; the sides A being of wood or iron at pleasure. In the centre of the bottom
there is a pivot C, screwed into a socket cast upon the bottom, and which may be
strengthened by two cross bars D, made fast to the frame E. F, the millstone of cast-iron,
concave, whose diameter is considerably smaller than that of the vessel A; it is
furnished within with a circular basin of wood G, which receives the materials to be
ground, and directs them to the holes H, which allow them to pass down between the
under part of the muller, and the bottom of the tub, to undergo trituration.
By the centrifugal motion, the paste is driven towards the sides of the vessel, rises
over the sides of the muller, and comes again through the holes H, so as to be repeatedly
subjected to the grinding operation. This millstone is mounted upon an upright shaft
I, which receives rotatory motion from the bevel wheel work K, driven by the winch L.
The furnace in which some kinds of crayons, and especially the factitious blacklead
pencils are baked, is represented in fig. 352. in a front elevation; and in fig. 353., which
is a vertical section through the middle of the chimney.
A A, six tubes of greater or less size, according as the substance of the crayons is a
better or worse conductor of heat. These tubes, into which the crayons intended for
baking are to be put, traverse horizontally the laboratory B of the furnace, and are supported
by two plates C, pierced with six square holes for covering the axes of the tubes
A. These two plates are hung upon a common axis D; one of them, with a ledge, shuts
the cylindrical part of the furnace, as is shown in the figure. At the extremity of the
bottom, the axis D is supported by an iron fork fixed in the brickwork; at the front it
crosses the plate C, and lets through an end about 4 inches square to receive a key, by
means of which the axis D may be turned round at pleasure, and thereby the two plates
C, and the six tubes A, are thus exposed in succession to the action of the fire in
an equal manner upon each of their sides. At the two extremities of the furnace are
two chimnies E, for the purpose of diffusing the heat more equably over the body of the
crayons. F, fig. 352., is the door of the fire-place, by which the fuel is introduced; G,
fig. 353., the ash-pit; H, the fire-place; I, holes of the grate which separate the fire-place
from the ash-pit; K, brickwork exterior to the furnace.
General Lomet proposes the following composition for red crayons. He takes
the softest hematite, grinds it upon a porphyry slab; and then carefully elutriates it.
He makes it into a plastic paste with gum arabic and a little white soap, which he
forms by moulding, as above, through a syringe, and drying, into crayons. The proportions
of the ingredients require to be carefully studied.
CRAYONS, lithographic. Various formulæ have been given for the formation of
these crayons. One of these prescribes, white wax, 4 parts; hard tallow-soap, shell-lac,
of each 2 parts; lamp black, 1 part. Another is, dried tallow soap and white wax, each
6 parts; lamp black, 1 part. This mixture being fused with a gentle heat, is to be cast
into moulds for forming crayons of a proper size.
CREOSOTE, or the flesh-preserver, from κρεας and σωζω, is the most important of the
five new chemical products obtained from wood tar by Dr. Reichenbach. The other four,
paraffine, eupione, picamar, and pittacal, have hitherto been applied to no use in the arts,
and may be regarded at present as mere analytical curiosities.
Creosote may be prepared either from tar or from crude pyrolignous acid. The tar
must be distilled till it acquires the consistence of pitch, and at the utmost till it begins
to exhale the white vapours of paraffine. The liquor which passes into the receiver
divides itself into 3 strata, a watery one in the middle, placed between a heavy and a light
oil. The lower stratum alone is adapted to the preparation of creosote.
1. The liquor being saturated with carbonate of potash, is to be allowed to settle, and
the oily matter which floats at top is to be decanted off. When this oil is distilled, it
affords at first, products lighter than water, which are to be rejected, but the heavier oil
which follows is to be separated, washed repeatedly by agitation, with fresh portions of
dilute phosphoric acid, to free it from ammonia, then left some time at rest, after which
it must be washed by water from all traces of acidity, and finally distilled along with
a new portion of dilute phosphoric acid, taking care to cohobate, or pour back the distilled
product repeatedly into the retort.
2. The oily liquid thus rectified is colourless; it contains much creosote, but at the
same time some eupione, &c. It must therefore be mixed with potash lye at 1·12 sp.
grav., which dissolves the creosote. The eupione floats upon the surface of that solution,
and may be decanted off. The alkaline solution is to be exposed to the air, till it
blackens by decomposition of some foreign matter. The potash being then saturated
with dilute sulphuric acid, the creosote becomes free, when it may be decanted or
syphoned off and distilled.
3. The treatment by potash, sulphuric acid, &c., is to be repeated upon the brownish[375]
creosote till it remains colourless, or nearly so, even upon exposure to air. It must
be now dissolved in the strongest potash lye, subjected to distillation anew, and lastly,
re-distilled with the rejection of the first products which contain much water, retaining
only the following, but taking care not to push the process too far.
In operating upon pyrolignous acid, if we dissolve effloresced sulphate of soda in it to
saturation, at the temperature of 167° F., the creosote oil will separate, and float upon
the surface. It is to be decanted, left in repose for some days, during which it will part
with a fresh portion of the vinegar and salt. Being now saturated while hot, with carbonate
of potash and distilled with water, an oily liquor is obtained, of a pale yellow
colour. This is to be rectified by phosphoric acid, &c., like the crude product of
creosote from tar.
Creosote is apparently composed of 76·2 carbon, 7·8 hydrogen, and 16·0 oxygen, in
100 parts. It is an oily looking liquid, slightly greasy to the touch, void of colour,
having an acrid burning taste, and capable of corroding the epidermis in a short time.
It possesses a penetrating disagreeable smell, like that of highly smoked hams, and when
inhaled up the nostrils, causes a flow of tears. Its specific gravity is 1·037, at 58° F.
Its consistence is similar to that of oil of almonds. It has no action upon the colours
of litmus or turmeric, but communicates to white paper a stain which disappears spontaneously
in a few hours, and rapidly by the application of heat.
It boils without decomposition at 398° F., under the average barometric pressure,
remains fluid at 16° F., is a non-conductor of electricity, refracts light powerfully, and
burns in a lamp with a ruddy smoky flame.
When mixed with water at 58° F. it forms two different combinations, the first being
a solution of 1 part of creosote in 400 of water; the second, a combination of 1 part of
water with 10 parts of creosote. It unites in all proportions with alcohol, hydric ether,
acetic ether, naphtha, eupione, carburet of sulphur, &c.
Creosote dissolves a large quantity of iodine and phosphorus, as also of sulphur with
the aid of heat, but it deposits the greater part of them in crystals, on cooling. It combines
with potash, soda, ammonia, lime, baryta, and oxide of copper. Oxide of mercury
converts creosote into a resinous matter, while itself is reduced to the metallic state.
Strong sulphuric and nitric acids decompose it.
Creosote dissolves several salts, particularly the acetates, and the chlorides of calcium
and tin; it reduces the nitrate and acetate of silver. It also dissolves indigo blue; a
remarkable circumstance. Its action upon animal matters is very interesting. It
coagulates albumen, and prevents the putrefaction of butcher’s meat and fish. For this
purpose these substances must be steeped a quarter of an hour in a weak watery solution
of creosote, then drained and hung up in the air to dry. Hence Reichenbach has inferred
that it is owing to the presence of creosote, that meat is cured by smoking; but he is not
correct in ascribing the effect to the mere coagulation of the albumen, since fibrine alone,
without creosote, will putrefy in the course of 24 hours, during the heats of summer. It
kills plants and small animals. It preserves flour paste unchanged for a long time.
Creosote exists in the tar of beech-wood, to the amount of from 20 to 25 per cent.,
and in crude pyrolignous acid, to that of 11⁄2.
It ought to be kept in well-stoppered bottles, because when left open, it becomes
progressively yellow, brown, and thick.
Creosote has considerable power upon the nervous system, and has been applied to
the teeth with advantage in odontalgia, as well as to the skin in recent scalds. But its
medicinal and surgical virtues have been much exaggerated. Its flesh-preserving quality
is rendered of little use, from the difficulty of removing the rank flavour which it
imparts.
CRUCIBLES; (Creusets, Fr.; Schmelztiegel, Germ.) are small conical vessels,
narrower at the bottom than the mouth, for reducing ores in docimasy by the dry
analysis, for fusing mixtures of earthy and other substances, for melting metals, and
compounding metallic alloys. They ought to be refractory in the strongest heats, not
readily acted upon by the substances ignited in them, not porous to liquids, and capable
of bearing considerable alternations of temperature without cracking; on which account
they should not be made too thick. The best crucibles are formed from a pure fire clay,
mixed with finely ground cement of old crucibles, and a portion of black-lead or graphite.
Some pounded coak may be mixed, with the plumbago. The clay should be
prepared in a similar way as for making pottery ware; the vessels after being formed
must be slowly dried, and then properly baked in the kiln. Crucibles formed of a
mixture of 8 parts in bulk of Stourbridge clay and cement, 5 of coak, and 4 of graphite,
have been found to stand 23 meltings of 76 pounds of iron each, in the Royal Berlin
foundry. Such crucibles resisted the greatest possible heat that could be produced, in
which even wrought iron was melted, equal to 150° or 155° Wedgewood; and bore
sudden cooling without cracking. Another composition for brass-founding crucibles is
the following:—1⁄2 Stourbridge clay; 1⁄4 burned clay cement; 1⁄8 coak powder; 1⁄8 pipe[376]
clay. The pasty mass must be compressed in moulds. The Hessian crucibles from
Great Almerode and Epterode are made from a fire clay which contains a little iron,
but no lime; it is incorporated with siliceous sand. The dough is compressed in a
mould, dried, and strongly kilned. They stand saline and leaden fluxes in docimastic
operations very well; are rather porous on account of the coarseness of the sand, but are
thereby less apt to crack from sudden heating or cooling. They melt under the fusing
point of bar iron. Beaufay in Paris has lately succeeded in making a tolerable imitation
of the Hessian crucibles with a fire clay found near Namur in the Ardennes.
Berthier has published the following elaborate analyses of several kinds of crucibles:—
| |
Hes-
sian. |
Beau-
fay. |
English
for
Cast
Steel. |
St.
Etienne
for
Cast
Steel. |
Glass
Pots
at
Ne-
mours. |
Bohe-
mian
Glass
Pots. |
Glass
Pots,
of
Creu-
sot. |
| Silica |
70 |
·9 |
64 |
·6 |
63 |
·7 |
|
65 |
·2 |
67 |
·4 |
68 |
·0 |
68 |
·0 |
| Alumina |
24 |
·8 |
34 |
·4 |
20 |
·7 |
|
25 |
·0 |
32 |
·0 |
29 |
·0 |
28 |
·0 |
| Oxide of Iron |
3 |
·8 |
1 |
·0 |
4 |
·0 |
|
7 |
·2 |
0 |
·8 |
2 |
·2 |
2 |
·0 |
| Magnesia |
trace |
- |
- |
- |
- |
trace |
trace |
0 |
·5 |
trace |
| Water |
- |
- |
- |
- |
10 |
·3 |
[23] |
- |
- |
- |
- |
- |
- |
1 |
·0 |
Wurzer states the composition of the sand and clay in the Hessian crucibles as
follows:—
| Clay; |
silica |
10·1; |
alumina |
65·4; |
oxides of iron and manganese |
1·2; |
lime |
0·3; |
water |
23 |
| Sand; |
|
95·6; |
|
2·1; |
|
1·5; |
|
0·8 |
Black lead crucibles are made of two parts of graphite and one of fire clay; mixed
with water into a paste, pressed in moulds, and well dried; but not baked hard in the
kiln. They bear a higher heat than the Hessian crucibles, as well as sudden changes of
temperature; have a smooth surface, and are therefore preferred by the melters of gold
and silver. This compound forms excellent small or portable furnaces.
Mr. Anstey describes his patent process for making crucibles, as follows: Take two
parts of fine ground raw Stourbridge clay, and one part of the hardest gas coak, previously
pulverized, and sifted through a sieve of one-eighth of an inch mesh (if the coak
is ground too fine, the pots are very apt to crack). Mix the ingredients together with
the proper quantity of water, and tread the mass well. The pot is moulded by hand upon
a wooden block, supported on a spindle which turns in a hole in the bench; there is a
gauge to regulate the thickness of the melting pot, and a cap of linen or cotton placed
wet upon the core before the clay is applied, to prevent the clay from sticking partially to
the core, in the taking off; the cap adheres to the pot only while wet, and may be
removed without trouble or hazard when dry. He employs a wooden bat to assist in
moulding the pot; when moulded it is carefully dried at a gentle heat. A pot dried as
above, when wanted for use, is first warmed by the fire-side, and is then laid in the furnace
with the mouth downwards (the red coaks being previously damped with cold
ones in order to lessen the heat); more coak is then thrown in till the pot is covered,
and it is now brought up gradually to a red heat. The pot is next turned and fixed
in a proper position in the furnace, without being allowed to cool, and is then charged
with cold iron, so that the metal, when melted, shall have its surface a little below the
mouth of the pot. The iron is melted in about an hour and a half, and no flux or addition
of any kind is made use of. A pot will last for fourteen or even eighteen successive
meltings, provided it is not allowed to cool in the intervals; but if it cool, it will
probably crack. These pots it is said can bear a greater heat than others without
softening, and will, consequently, deliver the metal in a more fluid state than the best
Birmingham pots will. See a figure of the crucible mould under Steel.
CRYSTAL, is the geometrical form possessed by a vast number of mineral and saline
substances; as also by many vegetable and animal products. The integrant particles of
matter have undoubtedly determinate forms, and combine with one another, by the
attraction of cohesion, according to certain laws, and points of polarity, whereby they
assume a vast variety of secondary crystalline forms. The investigation of these laws
belongs to crystallography, and is foreign to the practical purpose of this volume.
Instructions are given under each object of manufacture which requires crystallization,
how to conduct this process; see Borax, Salt, &c.
CUDBEAR was first made an article of trade in this country, by Dr. Cuthbert
Gordon, from whom it derived its name, and was originally manufactured on a great
scale by Mr. G. Mackintosh at Glasgow, nearly 60 years ago. Cudbear or persio is a[377]
powder of a violet red colour, difficult to moisten with water, and of a peculiar but not
disagreeable odour. It is partially soluble in boiling water, becomes red with acids, and
violet blue with alkalis. It is prepared in the same way as archil, only toward the
end the substance is dried in the air, and is then ground to a fine powder, taking care to
avoid decomposition, which renders it glutinous. In Scotland they use the lichen tartareus,
more rarely the lichen calcareus, and omphalodes; most of which lichens are imported
from Sweden and Norway, under the name of rock moss. The lichen is suffered
to ferment for a month, and is then stirred about to allow any stones which may be present
to fall to the bottom. The red mass is next poured into a flat vessel, and left to
evaporate till its urinous smell has disappeared, and till it has assumed an agreeable
colour verging upon violet. It is then ground to fine powder. During the fermentation
of the lichen, it is watered with stale urine, or with an equivalent ammoniacal liquor of
any kind, as in making archil.
CUPELLATION; is a mode of analyzing gold, silver, palladium, and platinum, by
adding to small portions of alloys, containing these metals, a bit of lead, fusing the
mixture in a little cup of bone earth called a cupel, then by the joint action of heat and
air, oxidizing the copper, tin, &c., present in the precious metals. The oxides thus produced,
are dissolved and carried down into the porous cupel in a liquid state, by the
vitrified oxide of lead. See Assay, Gold, and Silver.
CURRYING OF LEATHER, (Corroyer, Fr.; Zurichten, Germ.) is the art of
dressing skins after they are tanned, for the purposes of the shoe-maker, coach and harness
maker, &c., or of giving them the necessary smoothness, lustre, colour, and suppleness.
The currier’s shop has no resemblance to the tanner’s premises, having a quite different
set of tools and manipulations.
The currier employs a strong hurdle about a yard square, made either of basket twigs,
or of wooden spars, fixed rectangularly like trellis work, with holes 3 inches square,
upon which he treads the leather, or beats it with a mallet or hammer, in order to soften
it, and render it flexible.
The head knife, called in French couteau a revers, on account of the form of its edge,
which is much turned over, is a tool 5 or 6 inches broad, and 15 or 16 long; with
two handles, one in the direction of the blade, and the other perpendicular to it, for the
purpose of guiding the
edge more truly upon
the skin. The pommel
(paumelle) is so called
because it clothes the
palm of the hand, and
performs its functions.
It is made of hard wood,
and of a rectangular
shape, 1 foot long, 5
inches broad, flat above
and rounded below. It is
furrowed over the rounded
surface with transverse
parallel straight grooves.
These grooves are in
section sharp-edged isosceles
triangles. Fig.
354. and 355., represent
the pommel in an
upper and under view. The flat surface is provided with a leather strap for securing
it to the hand of the workman. Pommels are made of different sizes, and
with grooves of various degrees of fineness. Cork pommels are also used, but they
are not grooved. Pommels serve to give grain and pliancy to the skins.
The stretching iron, fig. 356., is a flat plate of iron or copper, fully a fourth of an
inch thick at top, and thinning off at bottom in a blunt edge, shaped like the arc of a
circle of large diameter, having the angles a and b rounded, lest in working they should
penetrate the leather. The top c is mounted with leather to prevent it from hurting the
hands. A copper stretching knife is used for delicate skins. The workman holds this
tool nearly perpendicular, and scrapes the thick places powerfully with his two hands,
especially those where some tan or flesh remains. He thus equalizes the thickness of
the skin, and renders it at the same time more dense and uniform in texture. This tool
is of very general use in currying.
The round knife, fig. 357. and 358. (lunette in French), is a circular knife from 10 to
12 inches in diameter, with a round 4 or 5 inch hole in its centre, for introducing the
hands and working it. It is concave, as shown in the section fig. 358., presenting the[378]
form of a spherical zone. The concave part is that applied to the skin. Its edge is not
perfectly straight; but is a little turned over on the side opposite to the skin, to prevent
it from entering too far into the leather.
The currier first slopes off with
the head knife from the edges, a portion
equal to what he afterwards removes
with the round one. By this
division the work is done sooner and
more exactly. All the oiled or greased
skins are dressed with the round knife.
The cleaner is a straight two-handled
knife two inches broad, of which
there are two kinds, a sharp-edged and a blunt one. Fig. 359.
The mace is made of wood, having a handle 30 inches long, with a cubical head or
mallet; upon the two faces of which, parallel to the line of the handle, there are 4 pegs
of hard wood turned of an egg-shape, and well polished, so as not to tear the moistened
leather when it is strongly beat and softened with the mace.
The horse or trestle, fig. 360., consists of a strong wooden frame, A B C D, which serves as
a leg or foot. Upon the middle of this frame there are two uprights, E F, and a strong
cross beam, G, for supporting the thick plank H, upon which the skins are worked. This
plank may be set at a greater or less slope, according as its lower end is engaged in one
or other of the cross bars, I I I I, of the frame. In the figure, a skin K is represented upon
the plank with the head knife upon it, in the act of being pared.
A cylindrical bar fixed horizontally at its ends to two buttresses projecting from the
wall, serves by means of a parallel stretched cord, to fix a skin by a coil or two in order
to dress it. This is accordingly called the dresser. The tallow cloth is merely a mop
made of stout rags, without the long handle; of which there are several, one for wax,
another for oil, &c. Strong-toothed pincers with hook-end handles, drawn together
by an endless cord, are employed to stretch the leather in any direction, while it is being
dressed. The currier uses clamps like the letter U, to fix the edges of the leather to his
table. His polisher is a round piece of hard wood, slightly convex below, with a handle
standing upright in its upper surface, for seizing it firmly. He first rubs with sour beer,
and finishes with barberry juice.
Every kind of tanned leather not intended for soles or such coarse purposes, is
generally curried before being delivered to the workmen who fashion it, such as shoemakers,
coachmakers, saddlers, &c. The chief operations of the currier are four:—
1. Dipping the leather, which consists in moistening it with water, and beating it
with the mace, or a mallet upon the hurdle. He next applies the cleaners, both blunt
and sharp, as well as the head knife, to remove or thin down all inequalities. After the
leather is shaved, it is thrown once more into water, and well scoured by rubbing the
grain side with pumice stone, or a piece of slaty grit, whereby it parts with the bloom, a
whitish matter, derived from the oak bark in the tan pit.
2. Applying the pommel to give the leather a granular appearance, and correspondent
flexibility. The leather is first folded with its grain side in contact, and rubbed strongly
with the pommel, then rubbed simply upon its grain side; whereby it becomes extremely
flexible.
3. Scraping the leather. This makes it of uniform thickness. The workman holds
the tool nearly perpendicular upon the leather, and forcibly scrapes the thick places with
both his hands.
4. Dressing it by the round knife. For this purpose he stretches the leather upon
the wooden cylinder, lays hold of the pendent under edge with the pincers attached to
his girdle, and then with both hands applies the edge of the knife to the surface of the
leather, slantingly from above downwards, and thus pares off the coarser fleshy parts of the
skin. This operation requires great experience and dexterity; and when well performed
improves greatly the look of the leather.
The hide or skin being rendered flexible and uniform, is conveyed to the shed or drying
house, where the greasy substances are applied, which is called dubbing (daubing), or
stuffing. The oil used for this purpose is prepared by boiling sheep-skins or doe-skins,
in cod oil. This application of grease is often made before the graining board or pommel
is employed.
Before waxing, the leather is commonly coloured by rubbing it with a brush dipped
into a composition of oil and lamp black on the flesh side, till it be thoroughly black; it is
then black-sized with a brush or sponge, dried, tallowed with the proper cloth, and
slicked upon the flesh with a broad smooth lump of glass; sized again with a sponge;
and when dry, again curried as above described.
Currying leather on the hair or grain side, termed black on the grain, is the same in
the first operation with that drest on the flesh, till it is scoured. Then the first black[379]
is applied to it while wet, by a solution of copperas put upon the grain, after this has
been rubbed with a stone; a brush dipped in stale urine is next rubbed on, then an iron
slicker is used to make the grain come out as fine as possible. It is now stuffed with
oil. When dry, it is seasoned; that is, rubbed over with a brush dipped in copperas
water, on the grain, till it be perfectly black. It is next slicked with a good grit-stone,
to take out the wrinkles, and smooth the coarse grain. The grain is finally raised with
the pommel or graining board, by applying it to the leather in different directions.
When thoroughly dry, it is grained again in two or three ways.
Hides intended for covering coaches are shaved nearly as thin as shoe hides, and
blacked upon the grain.
CUTLERY. (Coutellerie, Fr.; Messerschmidwaare, Germ.) Three kinds of steel
are made use of in the manufacture of different articles of cutlery, viz. common steel,
shear steel, and cast steel. Shear steel is exceedingly plastic and tough. All the edge
tools which require great tenacity without great hardness are made of it, such as table
knives, scythes, plane-irons, &c.
Cast steel is formed by melting blistered steel in covered crucibles, with bottle glass,
and pouring it into cast-iron moulds, so as to form it into ingots: these ingots are then
taken to the tilt, and drawn into rods of suitable dimensions. No other than cast
steel can assume a very fine polish, and hence all the finer articles of cutlery are made
of it, such as the best scissors, penknives, razors, &c.
Formerly cast steel could be worked only at a very low heat; it can now be made so
as to be welded to iron with the greatest ease. Its use is consequently extended to
making very superior kinds of chisels, plane-irons, &c.
Forging of table knives.—Two men are generally employed in the forging of table
knives; one called the foreman or maker, and the other the striker.
The steel called common steel is employed in making the very common articles; but
for the greatest part of table knives which require a surface free from flaws, shear steel
is generally preferred. That part of the knife termed the blade, is first rudely formed
and cut off. It is next welded to a rod of iron about 1⁄2 inch square, in such a manner
as to leave as little of the iron part of the blade exposed as possible. A sufficient
quantity of the iron now attached to the blade, is taken off from the rod to form the
bolster or shoulder, and the tang.
In order to make the bolster of a given size, and to give it at the same time shape
and neatness, it is introduced into a die, and a swage placed upon it; the swage has a
few smart blows given it by the striker. This die and swage are, by the workman,
called prints.
After the tangs and bolster are finished, the blade is heated a second time, and the
foreman gives it its proper anvil finish; this operation is termed smithing. The blade
is now heated red-hot, and plunged perpendicularly into cold water. By this means it
becomes hardened. It requires to be tempered regularly down to a blue colour: in
which state it is ready for the grinder.
Mr. Brownill’s method of securing the handles upon table-knives and forks, is, by
lengthening the tangs, so as to pass them completely through the handle, the ends of
which are to be tinned after the ordinary mode of tinning iron; and, when passed
through the handle, the end of the tang is to be spread by beating, or a small hole
drilled through it, and a pin passed to hold it upon the handle. After this, caps of
metal, either copper plated, or silver, are to be soldered on to the projecting end of the
tang, and while the solder is in a fluid state, the cap is to be pressed upon the end of
the handle and held there until the solder is fixed, when the whole is to be cooled by
being immersed in cold water.
Mr. Thomason’s patent improvements consist in the adaptation of steel edges to the
blades of gold and silver knives. These steel edges are to be attached to the other
metal of whatever quality it may be, of which the knife, &c. is made, by means of
solder, in the ordinary mode of effecting that process. After the edge of steel is thus
attached to the gold, silver, &c., it is to be ground, polished, and tempered by immersion
in cold water, or oil, after being heated. This process being finished, the other parts
of the knife are then wrought and ornamented by the engraver or chaser, as usual.
A patent was obtained in 1827, by Mr. Smith of Sheffield, for rolling out knives at
one operation.
In the ordinary mode of making knives, a sheet of steel being provided, the blades
are cut out of the sheet, and the backs, shoulders, and tangs, of wrought iron, are
attached to the steel blades, by welding at the forge. The knife is then ground to the
proper shape, and the blade polished and hardened.
Instead of this welding process, the patentee proposes to make the knives entirely of
steel, and to form them by rolling in a heated state between massive rollers; the shoulders
or bolsters, and the tangs for the handles being produced by suitable recesses in
the peripheries of the rollers; just as rail-way rails are formed. When the knife is to[380]
be made with what is called a scale tang, that is a broad flat tang, to which the handle
is to be attached in two pieces, riveted on the sides of the tang, the rollers are then only
to have recesses cut in them, in a direction parallel to the axis for forming the bolster.
The plate of steel having been heated, is to be pressed between the two rollers, by
which the blades and the parts for the scale tangs will be pressed out flat and thin, and
those parts which pass between the grooves or recess will be left thick or protuberant,
forming the bolster for the shoulder of the blade. But if the tangs are to be round in
order to be fixed into single handles, then it will be necessary also to form transverse
grooves in the rollers, that is, at right angles to those which give shape to the bolsters,
the transverse grooves corresponding in length to the length of the intended tang.
When the plates of steel have been thus rolled, forming three or more knives in a
breadth, the several knives are to be cut out by the ordinary mode of what is called
slitting, and the blades and shoulders ground, hardened, and polished in the usual way.
Forks are generally a distinct branch of manufacture from that of knives, and are
purchased of the fork makers by the manufacturers of table knives, in a state fit for receiving
the handles.
The rods of steel from which the forks are made, are about 3⁄8ths of an inch square.
The tang and shank of the fork are first roughly formed. The fork is then cut off,
leaving at one end about 1 inch of the square part of the steel. This part is afterwards
drawn out flat to about the length of the prongs. The shank and tang are now
heated, and a proper form given to them by means of a die and swage. The prongs are
afterwards formed at one blow by means of the stamp; this machine is very similar to
that used in driving piles, but it is worked by one man. It consists of a large anvil
fixed in a block of stone nearly on a level with the ground. To this anvil are attached
two rods of iron of considerable thickness, fixed 12 inches asunder, perpendicularly to
the anvil, and diagonally to each other. These are fastened to the ceiling. The hammer
or stamp, about 100 lbs. in weight, having a groove upon either side corresponding to
the angles of the upright rods, is made to slide freely through its limited range, being
conducted by its two iron supporters. A rope is attached to the hammer, which goes
over a pulley on the floor of the room above, and comes down to the person who works
the stamp: two corresponding dies are attached, one to the hammer, and the other to
the anvil. That part of the fork intended to form the prongs, is heated to a pretty white
heat and placed in the lower die, and the hammer containing the other die is made to
fall upon it from a height of about 7 or 8 feet. This forms the prongs and the middle
part of the fork, leaving a very thin substance of steel between each prong, which is
afterwards cut out with an appropriate instrument called a flie-press. The forks are now
annealed by surrounding a large mass of them with hot coals, so that the whole shall
become red hot. The fire is suffered gradually to die out, and the forks to cool without
being disturbed. This process is intended to soften, and by that means to prepare them
for filing. The inside of the prongs are then filed, after which they are bent into their
proper form and hardened. When hardened, which is effected by heating them red-hot
and plunging them into cold water, they are tempered by exposing them to the degree
of heat at which grease inflames. See Stamps.
Penknives are generally forged by a single hand, with the hammer and the anvil simply.
The hammer in this trade is generally light, not exceeding 31⁄2 lbs. The breadth
of the face, or the striking part, is about one inch; if broader, it would not be convenient
for striking so small an object. The principal anvil is about 5 inches, and 10
upon the face, and is provided with a groove into which a smaller anvil is wedged. The
smaller anvil is about 2 inches square upon the face. The blade of the knife is first
drawn out at the end of the rod of steel, and as much more is cut off along with it as
is thought necessary to form the joint. The blade is then taken in a pair of tongs, and
heated a second time to finish the joint part, and at the same time to form a temporary
tang for the purpose of driving into a small haft used by the grinder. Another heat
is taken to give the blade a proper finish. The small recess called the nail hole, used
in opening the knife, is made while it is still hot by means of a chisel, which is round
on one side, and flat upon the other.
Penknives are hardened by heating the blade red hot, and dipping them into water up
to the shoulder. They are tempered by setting them side by side, with the back downwards
upon a flat iron plate laid upon the fire, where they are allowed to remain till
they are of a brown or purple colour.
The blades of pocket knives, and all that come under the denomination of spring
knives, are made in the same way.
The forging of razors is performed by a foreman and striker, as in making table
knives.
They are generally made of cast steel. The rods, as they come from the tilt, are about
1⁄2 inch broad, and of a thickness sufficient for the back of the razor.
There is nothing peculiar in the tools made use of in forging razors: the anvil is a[381]
little rounded at the sides, which affords the opportunity of making the edge thinner,
and saves an immense labour to the grinder.
Razors are hardened and tempered in a similar manner to penknives. They are,
however, left harder, being only let down to yellow or brown colour.
The forging of scissors is wholly performed by the hammer, and all the sizes are made
by a single hand. The anvil of the scissor-maker weighs about 11⁄2 cwt.; it measures,
on the face, about 4 by 11 inches. It is provided with two gates or grooves for the reception
of various little indented tools termed by the workman bosses; one of these
bosses is employed to give proper figure to the shank of the scissors; another for
forming that part which has to make the joint; and a third is made use of for giving
a proper figure to the upper side of the blade. There is also another anvil placed on
the same block, containing two or three tools called beak-irons, each consisting of an
upright stem about 6 inches high, at the top of which a horizontal beak projects; one
of these beaks is conical, and is used for extending the bow of the scissors; the other is
a segment of a cylinder with the round side upwards, containing a recess for giving a
proper shape and smoothness to the inside of the bow.
The shank of the scissors is first formed by means of one of the bosses, above described,
leaving as much steel at the end as will form the blade. A hole is then punched
about 1⁄4 inch in width, a little above the shank. The blade is drawn out and finished,
and the scissors separated from the rod a little above the hole. It is heated a third
time, and the small hole above mentioned is extended upon the beak-irons so as to form
the bow. This finishes the forging of scissors. They are promiscuously made in this
way, without any other guide than the eye, having no regard to their being in pairs.
They are next annealed for the purpose of filing such parts of them as cannot be
ground, and afterwards paired.
The very large scissors are made partly of iron, the blades being of steel.
After the forging, the bow and joints, and such shanks as cannot be ground, are
filed. The rivet hole is then bored, through which they are to be screwed or riveted
together. This common kind of scissors is only hardened up to the joint. They
are tempered down to a purple or blue colour. In this state they are taken to the
grinder.
Grinding and polishing of cutlery.—The various processes which come under this denomination
are performed by machinery, moving in general by the power of the steam-engine
or water-wheel.
Grinding wheels or grinding mills are divided into a number of separate rooms;
every room contains six places called troughs; each trough consists of a convenience for
running a grindstone and a polisher at the same time, which is generally occupied by
a man and a boy.
The business of the grinder is generally divided into three stages, viz. grinding,
glazing, and polishing.
The grinding is performed upon stones of various qualities and sizes, depending on
the articles to be ground. Those exposing much flat surface, such as saws, fenders, &c.
require stones of great diameter, while razors, whose surface is concave, require to be
ground upon stones of very small dimensions. Those articles which require a certain
temper, which is the case with most cutting instruments, are mostly ground on a wet
stone; for which purpose the stone hangs within the iron trough, filled with water to
such a height that its surface may just touch the face of the stone.
Glazing is a process following that of grinding: it consists in giving that degree of
lustre and smoothness to an article which can be effected by means of emery of the
various degrees of fineness. The tool on which the glazing is performed, is termed a
glazer. It consists of a circular piece of wood, formed of a number of pieces in such
a manner that its edge or face may always present the endway of the wood. Were it
made otherwise, the contraction of the parts would destroy its circular figure. It is
fixed upon an iron axis similar to that of the stone. Some glazers are covered on the
face with leather, others with metal, consisting of an alloy of lead and tin; the latter
are termed caps. In others, the wooden surface above is made use of. Some of the
leather-faced glazers, such as are used for forks, table knives, edge tools, and all the
coarser polished articles, are first coated with a solution of glue, and then covered with
emery. The surfaces of the others are prepared for use by first turning the face very
true, then filling it with small notches by means of a sharp-ended hammer, and lastly
filling up the interstices with a compound of tallow and emery.
The pulley of the glazer is so much less than that of the stone, that its velocity is
more than double, having in general a surface speed of 1500 feet in a second.
The process of polishing consists in giving the most perfect polish to the different
articles. Nothing is subjected to this operation but what is made of cast steel, and has
been previously hardened and tempered.
The polisher consists of a circular piece of wood covered with buff leather, the surface[382]
of which is covered from time to time, while in use, with the crocus of iron, called also
colcothar of vitriol.
The polisher requires to run at a speed much short of that of the stone, or the glazer.
Whatever may be its diameter, the surface must not move at a rate exceeding 70 or 80
feet in a second.
CYANATES; saline compounds of cyanic acid with the bases potash, soda, ammonia,
baryta, &c. The first is prepared by calcining at a dull red heat, a mixture of
ferro-cyanide of potassium (prussiate of potash) and black oxide of manganese. The
cyanates have not hitherto been applied to any use in the arts.
CYANHYDRIC Acid; another name for the hydrocyanic or prussic acid. See
Prussian Blue and Prussic Acid.
CYANIDES; compounds of cyanogen with the metals; as cyanide of potassium,
sodium, barium, calcium, iron, mercury. The last is the only one of importance in a
manufacturing point of view, since from it prussic acid is made.
CYANIDES, FERRO. Double compounds of cyanogen with iron, and of cyanogen
with another metal, such as potassium, sodium, barium, &c. The ordinary yellow
prussiate of potash has this constitution, and is called the ferro-cyanide.
CYANOGEN. A gaseous compound of two prime equivalents of charcoal = 12,
and one of azote = 14 = 26; hydrogen being the radix or, 1. It consists of two volumes
of vapour of carbon, and one volume of azote, condensed into one volume; and has
therefore a density equal to the sum of the weights of these 3 gaseous volumes = 1·815.
Cyanogen is readily procured by exposing the cyanide of mercury to a dull red heat in
a retort; the gas is evolved and may be collected over mercury. Its smell is very
sharp and penetrating; it perceptibly reddens tincture of litmus; it is condensable by
pressure at a low temperature into a liquid; and by a still greater degree of cold, it is
solidified. When a lighted taper is applied to a mixture of cyanogen and oxygen, an
explosion takes place; carbonic acid is formed, and the azote is set at liberty.
For a connected view of the various compounds of cyanogen employed in the arts,
see Prussian Blue.
CYDER; (Cidre, Fr.; Apfelwein, Germ.) the vinous fermented juice of the apple.
The ancients were acquainted with cyder and perry, as we learn from the following passage
of Pliny the naturalist: “Wine is made from the Syrian pod, from pears and apples of every
kind.” Book xiv. chap. 19. The term cyder or cidre in French, at first written sidre, is
derived from the latin word sicera, which denoted all other fermented liquors except grape
wine. Cyder seems to have been brought into Normandy by the Moors of Biscay, who had
preserved the use of it after coming into that country from Africa. It was afterwards spread
through some other provinces of France, whence it was introduced into England, Germany,
and Russia. It is supposed that the first growths of Normandy afford still the
best specimens of cyder. Devonshire and Herefordshire are the counties of England
most famous for this beverage.
Strong and somewhat elevated ground, rather dry, and not exposed to the air of the
sea, or to high winds, are the best situations for the growth of the cyder apple. The
fruit should be gathered in dry weather. The juice of apples is composed of a great
deal of water; a little sugar analogous to that of the grape; a matter capable of causing
fermentation with contact of air; a pretty large proportion of mucilage, with malic acid,
acetic acid, and an azotized matter in a very small quantity. The seeds contain a bitter
substance and a little essential oil; the pure parenchyma or cellular membrane constitutes
not more than two per cent. of the whole. After the apples are gathered, they are left
in the barn-loft for fifteen days or upwards to mellow; some of them in this case, however,
become soft and brown. This degree of maturation diminishes their mucilage,
and developes alcohol and carbonic acid; in consequence of which the cyder suffers no
injury. There is always however a little loss; and if this ripening goes a little further
it is very apt to do harm, notwithstanding the vulgar prejudice of the country people to
the contrary. Too much care, indeed, cannot be taken to separate the sound from the
spoiled apples; for the latter merely furnish an acid leaven, give a disagreeable taste to
the juice, and hinder the cyder from fining, by leaving in it a certain portion of the
parenchyma, which the gelatinous matter or the fermentation has diffused through it.
Unripe apples should be separated from the ripe also, for they possess too little saccharum
to be properly susceptible of the vinous fermentation.
In France, where cyder making is most scientifically practised, it is prepared by
crushing the apples in a mill with revolving edge-stones, turned in a circular stone
cistern by one or two horses. When the fruit is half mashed, about one fifth of its
weight of river water is added, or the water of lakes. The latter have been found by
experience to be preferable to other water.
In some places a mill composed of two cast-iron fluted cylinders placed parallel to
each other under the bottom of a hopper, is employed for crushing the apples. One of
the cylinders is turned by a winch, and communicates its motion in the opposite direction[383]
by means of the flutings working into each other. Each portion of the fruit must
be passed thrice through this rude mill in order to be sufficiently mashed; and the same
quantity of water must be added as in the edge stone mill.
After the apples are crushed they are usually put into a large tub or tun for 12 or 24
hours. This steeping aids the separation of the juice, because the fermentative motion
which takes place in the mass breaks down the cellular membranes; but there is
always a loss of alcohol carried off by the carbonic acid disengaged, while the skins
and seeds develope a disagreeable taste in the liquid. The vatting might be suppressed
if the apples were so comminuted as to give out their juice more readily. With slight
modifications, the process employed in rasping and squeezing the beet-roots might in
my opinion be applied with great advantage to the cyder manufacture. See Sugar.
After the vatting, the mashed fruit is carried to the press and put upon a square wicker
frame or into a hair bag, sometimes between layers of straw, and exposed stratum super
stratum to strong pressure till what is called a cheese or cake is formed. The mass
is to be allowed to drain for some time before applying pressure, which ought to
be very gradually increased. The juice which exudes with the least pressure
affords the best cyder; that which flows towards the end acquires a disagreeable taste
from the seeds and the skins. The must is put into casks with large bungholes, where
it soon begins to exhibit a tumultuous fermentation. The cask must be completely
filled, in order that all the light bodies suspended in the liquid when floated to the top
by the carbonic acid may flow over with the froth; this means of clearing cyder is
particularly necessary with the weak kinds, because it cannot be expected that these
matters in suspension will fall to the bottom of the casks after the motion has ceased.
In almost every circumstance besides, when no saccharine matter has been added to the
must, that kind of yeast which rises to the top must be separated, lest by precipitation
it may excite an acid fermentation in the cyder. The casks are raised upon gawntrees
or stillions, in order to place flat tubs below them to receive the liquor which flows over
with the froth. At the end of two or three days, for weak cyders which are to be
drunk somewhat sweet, of 6 or 10 days or more for stronger cyders, with variations for
the state of the weather, the fermentation will be sufficiently advanced, and the cyder may
be racked off into other casks. Spirit puncheons preserve cyder better than any other,
but in all cases the casks should be well seasoned and washed. Sometimes a sulphur
match is burned in them before introducing the cyder, a precaution to be generally
recommended, as it suspends the activity of the fermentation, and prevents the formation
of vinegar.
The cyder procured by the first expression is called cyder without water. The cake
remaining in the press is taken out, divided into small pieces, and mashed anew, adding
about half the weight of water, when the whole is carried back to the press and treated
as above described. The liquor thus obtained furnishes a weaker cyder which will not
keep, and therefore must be drunk soon.
The cake is once more mashed up with water, and squeezed, when it yields a liquor
which may be used instead of water for moistening fresh ground apples.
The processes above described, although they have been long practised, and have
therefore the stamp of ancestral wisdom, are extremely defective. Were the apples
ground with a proper rotatory rasp which would tear all their cells asunder, and the
mash put through the hydraulic press in bags between hurdles of wicker-work, the
juice would be obtained in a state of perfection fit to make a cyder superior to many
wines. An experimental process of this kind has been actually executed in France
upon a considerable scale, with the best results. The juice had the fine flavour of the
apple, was fermented by itself without any previous fermentation in the mash, and
afforded an excellent strong cyder which kept well.
When the must of the apples is weak or sour, good cyder cannot be made from it
without the addition of some saccharine matter. The syrup into which potato farina is
convertible by diastase (saccharine ferment), see Starch and Sugar, would answer well
for enriching poor apple juice.
DAHLINE, the same as Inuline, the fecula obtained from elecampane, analogous
in many respects to starch. It is not employed in the arts.
DAMASCUS BLADES, are swords or scymitars, presenting upon their surface
a variegated appearance of watering, as white, silvery, or black veins, in fine lines,
or fillets; fibrous, crossed, interlaced or parallel, &c. They are brought from the East,
being fabricated chiefly at Damascus, whence their name. Their excellent quality has
become proverbial; for which reason these blades are much sought after by military[384]
men, and are high priced. The oriental processes have never been satisfactorily described;
but of late years methods have been devised in Europe to imitate the fabric
very well.
Clouet and Hachette pointed out the three following processes for producing Damascus
blades: 1, that of parallel fillets; 2, that by torsion; 3, the mosaic. The first,
which is still pursued by some French cutlers, consists in scooping out with a graving
tool the faces of a piece of stuff composed of thin plates of different kinds of steel.
These hollows are by a subsequent operation filled up, and brought to a level with the
external faces, upon which they subsequently form tress-like figures. 2. The method of
torsion, which is more generally employed at present, consists in forming a bundle of
rods or slips of steel, which are welded together into a well-wrought bar, twisted
several times round its axis. It is repeatedly forged, and twisted alternately; after
which it is slit in the line of its axis, and the two halves are welded with their outsides
in contact; by which means their faces will exhibit very various configurations. 3. The
mosaic method consists in preparing a bar, as by the torsion plan, and cutting this bar
into short pieces of nearly equal length, with which a faggot is formed and welded
together; taking care to preserve the sections of each piece at the surface of the blade.
In this way, all the variety of the design is displayed, corresponding to each fragment
of the cut bar.
The blades of Clouet, independently of their excellent quality, their flexibility, and
extreme elasticity, have this advantage over the oriental blades, that they exhibit in the
very substance of the metal, designs, letters, inscriptions, and, generally speaking, all
kinds of figures which had been delineated beforehand.
Notwithstanding these successful results of Clouet, it was pretty clear that the watered
designs of the true Damascus scymitar were essentially different. M. Bréant has
at last completely solved this problem. He has demonstrated that the substance
of the oriental blades is a cast-steel more highly charged with carbon than our
European steels, and in which, by means of a cooling suitably conducted, a crystallization
takes place of two distinct combinations of carbon and iron. This separation is
the essential condition; for if the melted steel be suddenly cooled in a small crucible
or ingot, there is no damascene appearance.
If an excess of carbon be mixed with iron, the whole of the metal will be converted
into steel; and the residuary carbon will combine in a new proportion with a portion of
the steel so formed. There will be two distinct compounds; namely, pure steel, and
carburetted steel or cast-iron. These at first being imperfectly mixed will tend to
separate, if while still fluid they be left in a state of repose; and form a crystallization
in which the particles of the two compounds will place themselves in the crucible in an
order determined by their affinity and density conjoined. If a blade forged out of steel
so prepared be immersed in acidulous water, it will display a very distinct damascus
appearance; the portions of pure steel becoming black, and those of carburetted steel
remaining white, because the acids with difficulty disengage its carbon. The slower
such a compound is cooled, the larger the damascus veins will be. Travernier relates
that the steel crucible ingots, like those of wootz, for making the true oriental damascus,
come from Golconda, that they are of the size of a halfpenny roll, and when cut in
two, form two swords.
Steel combined with manganese forges easily, but it is brittle when cold; it displays
however the damascus appearance very strongly.
A mixture of 100 parts of soft iron, and 2 of lamp black, melts as readily as ordinary
steel. Several of the best blades which M. Bréant presented to the Société d’Encouragement
are the product of this combination. This is an easy way of making cast-steel
without previous cementation of the iron. 100 parts of filings of very gray cast-iron,
and 100 parts of like filings previously oxidized, produced, by their fusion together,
a beautiful damascene steel, fit for forging into white arms, sabres, swords, &c. This
compound is remarkable for its elasticity, an essential quality, not possessed by the
old Indian steel. The greater the proportion of the oxidized cast iron, the tougher is the
steel. Care should be taken to stir the materials during their fusion, before it is allowed
to cool; otherwise they will not afford a homogeneous damasc. If the steel contains
much carbon it is difficult to forge, and cannot be drawn out except within a
narrow range of temperature. When heated to a red-white it crumbles under the
hammer; at a cherry-red it becomes hard and brittle; and as it progressively cools it
becomes still more unmalleable. It resembles completely Indian steel, which European
blacksmiths cannot forge, because they are ignorant of the suitable temperature for
working it. M. Bréant, by studying this point, succeeded in forging fine blades.
Experience has proved that the orbicular veins, called by the workmen knots or
thorns (ronces), which are seen upon the finest Eastern scymitars, are the result of the
manner of forging them, as well as the method of twisting the Damascus bars. If
these be drawn in length, the veins will be longitudinal; if they be spread equally in all[385]
directions, the stuff will have a crystalline aspect; if they be made wavy in the two
directions, undulated veins will be produced like those in the oriental damascus.
DAMASK is a variegated textile fabric, richly ornamented with figures of flowers,
fruits, landscapes, animals, &c., woven in the loom, and is by far the most rich, elegant,
and expensive species of ornamental weaving, tapestry alone excepted. The name is
said to be derived from Damascus, where it was anciently made.
Damask belongs to that species of texture which is distinguished by practical men
by the name of tweeling, of which it is the richest pattern. The tweel of damask is
usually half that of full satin, and consequently consists of eight leaves moved either in
regular succession or by regular intervals, eight leaves being the smallest number which
will admit of alternate tweeling at equal intervals.
In the article Carpet, two representations have been given of the damask draw-loom.
The generic difference of tweeling, when compared with common cloth, consists in
the intersections, although uniform and equidistant, being at determinate intervals, and
not between the alternate threads. Hence we have specimens of tweeled cloth, where
the intersections take place at the third, fourth, fifth, sixth, seventh, eighth, or sixteenth
interval only. The threads thus deflecting only from a straight line at intervals,
preserve more of their original direction, and a much greater quantity of materials can
be combined in an equal space, than in the alternate intersection, where the tortuous
deflection, at every interval, keeps them more asunder. On this principle tweeled
cloths of three and four leaves are woven for facility of combination alone. The
coarser species of ornamented cloths, known by the names of dornock and diaper,
usually intersect at the fifth, or half satin interval. The sixth and seventh are rarely
used, and the intersection at the eighth is distinguished by the name of satin in
common, and of damask in ornamental tweeling. It will further be very obvious, that
where the warp and woof cross only at every eighth interval, the two sides of the cloth
will present a diversity of appearance; for on one side the longitudinal or warp threads
will run parallel from one end of a web to the other, and, on the other, the threads of
woof will run also parallel, but in a transverse direction across the cloth, or at right
angles to the former. The points of intersection being only at every eighth interval,
appear only like points; and in regular tweeling these form the appearance of diagonal
lines, inclined at an angle of 45° (or nearly so) to each of the former.
The appearance, therefore, of a piece of common tweeled cloth is very similar to that
of two thin boards glued together, with the grain of the upper piece at right angles to
that of the under one. That of an ornamental piece of damask may, in the same
manner, be very properly assimilated to a piece of veneering, where all the wood is of
the same substance and colour, and where the figures assume a diversity of appearance
from the ground, merely by the grain of the one being disposed perpendicularly to that
of the other. See Textile Fabric.
From this statement of the principle, it results that the most unlimited variety of
figures will be produced, by constructing a loom by which every individual thread of
warp may be placed either above or below the woof at every intersection; and to effect
this, in boundless variety, is the object of the Jacquard mounting; which see.
The chief seat of this manufacture is probably the town and neighbourhood of
Dunfermline, in Fifeshire, and Lisburn and Ardoyne, near Belfast, where it is considered
as the staple, having proved a very profitable branch of traffic to the manufacturer,
and given employment to many industrious people.
The material used there is chiefly linen; but many have been recently woven of
cotton, since the introduction of that article into the manufacture of cloth has become
so prevalent. The cotton damasks are considerably cheaper than those of linen; but
are not considered either so elegant or durable. The cotton, also, unless frequently
bleached, does not preserve the purity of the white colour nearly so well as the linen.
DAMASKEENING; the art of ornamenting iron, steel, &c., by making incisions
upon its surface, and filling them up with gold or silver wire; chiefly used in enriching
sword blades, guards, and gripes, locks of pistols, &c.
Its name shows the place of its origin, or, at least, the place where it has been
practised in the greatest perfection; viz. the city of Damascus, in Syria; though
M. Felibien attributes the perfection of the art to his countryman, Cursinet, who
wrought under the reign of Henry IV.
Damaskeening is partly mosaic work, partly engraving, and partly carving. As
mosaic work, it consists of pieces inlaid; as engraving, the metal is indented, or cut in
intaglio; and as carving, gold and silver are wrought into it in relievo.
There are two ways of damaskeening; in the first, which is the most beautiful, the
artists cut into the metal with a graver, and other tools proper for engraving upon
steel, and afterwards fill up the incisions, or notches, with a pretty thick silver or gold
wire. In the other, which is only superficial, they content themselves to make hatches,[386]
or strokes across the iron, &c. with a cutting knife, such as is used in making of small
files. As to the first, it is necessary for the gravings or incisions to be made in the
dove-tail form; that the gold or silver wire, which is thrust forcibly into them, may
adhere the more strongly. As to the second, which is the more usual, the method is
this:—Having heated the steel till it changes to a violet, or blue colour, they hatch it
over and across with the knife; then draw the ensign or ornament intended, upon this
hatching, with a fine brass point or bodkin. This done, they take fine gold wire, and
conducting or chasing it according to the figures already designed, they sink it carefully
into the hatches of the metal with a copper tool.
DAMASSIN is a kind of damask, with gold and silver flowers, woven in the warp
and woof; or occasionally with silk organzine.
DAMPS, in mining, are noxious exhalations, or rather gases, so called from the
German dampf, vapour. There are two principal kinds of mine gases, the fire-damp, or
carburetted hydrogen, and the choke-damp, or carbonic acid gas. See Mines.
DAPHNINE; the bitter principle of the Daphne Alpina.
DATOLITE. A mineral composed of silica, lime, and boracic acid.
DECANTATION, (Eng. and Fr.; Abgiessen, Germ.) is the act of pouring off the clear
supernatant fluid from any sediment or deposit. It is much employed in the chemical
arts; and is most conveniently effected by a syphon.
DECOCTION, (Eng. and Fr.; Abkochung, Germ.) means either the act of boiling
a liquid along with some organic substance, or the liquid compound resulting from that
act.
DECOMPOSITION, (Eng. and Fr.; Zersetzung, Germ.) is the separation of the
constituent principles of any compound body. The following table, the result of important
researches recently made by M. Persoz, Professor of Chemistry at Strasburgh, shows
the order in which decompositions take place among the successive substances.
| Nitric Acid. |
Muriatic Acid. |
| Oxide of Magnesium |
Oxide of Magnesium |
| Ox— of Silver |
Ox— of Cobalt |
| Ox— of Cobalt |
Ox— of Nickel |
| Ox— of Nickel |
Protox. of Mercury |
| Protox. of Cerium |
Pro—. of Cerium |
| Oxide of Zinc |
Oxide of Zinc |
| Protox. of Manganese |
Protox. of Manganese |
| Oxide of Lead |
Pro—. of Iron |
| Ox— of Cadmium |
Pro—. of Uranium |
| Ox— of Copper |
Pro—. of Copper |
| Ox— of Glucinum |
Pro—. of Tin |
| Ox— of Alumium |
Oxide of Glucinum |
| Ox— of Uranium |
Ox— of Alumium |
| Ox— of Chromium |
Ox— of Uranium |
| Protox. of Mercury |
Ox— of Chromium |
| Oxide of Mercury |
Ox— of Iron |
| Ox— of Iron |
Ox— of Tin |
| Ox— of Bismuth |
Ox— of Bismuth |
| |
Ox— of Antimony |
By means of the cupric oxide we may separate, 1, the ferric oxide from the manganous
oxide; 2, the cobaltic, nickelic, zincic and cerous oxides from the uranic, ferric,
chromic, and aluminic oxides; 3, the ferrous oxide from the chromic oxide, when dissolved
in the muriatic acid.
In boiling a muriatic solution of the cobaltic, nickelic, and manganous oxides, with
the mercuric oxide, the first two oxides alone are precipitated. Alumina separates the
cadmic oxide from the bismuthic oxide, the stannous oxide from the stannic oxide, and
the stannous oxide from the antimonic acid. The cupric oxide separates also by precipitation,
the aluminic, uranic, chromic, titanic, and vanadic oxides from all the oxides
which are precipitable in the state of sulphuret, by hydrosulphuret of ammonia.
As an example of this mode of analysis—
Dissolve pech-blende in aqua regia, precipitate its copper by sulphuretted hydrogen,
boil the liquid along with nitric acid, in order to transform all the uranium into uranic
acid. Next boil it along with cupric oxide, which precipitates only the uranic and
ferric oxides. Redissolve the precipitate in nitric acid, and boil the solution with mercuric
oxide, which does not precipitate the ferric oxide. Finally, separate the copper
and the mercury from the uranium, by means of sulphuretted hydrogen. In this process
we may substitute plumbic oxide for the cupric oxide, and succeed equally well.
Knowledge, like the above, of the elective affinities and habitudes of chemical bodies,
simple and compound, imparts to its possessor an irresistible power over the unions and[387]
disunions of the elements, which he can exercise with certainty in effecting innumerable
transformations in the arts.
DECREPITATION, (Eng. and Fr.; Verknistern, Germ.) is the crackling noise,
attended with the flying asunder of their parts, made by several salts and minerals,
when heated. It is caused by the unequal sudden expansion of their substance by the
heat. Sulphate of baryta, chloride of sodium, calcareous spar, nitrate of baryta, and many
more bodies which contain no water, decrepitate most violently, separating at the natural
joints of their crystalline structure. Some chemists have preposterously enough ascribed
the phenomenon to the expansion of the combined water into steam. What a specimen
of inductive philosophy!
DEFECATION, (Eng. and Fr.; Klaren, Germ.) the freeing from dregs or impurities.
DEFLAGRATION, (Eng. and Fr.; Verpuffung, Germ.) the sudden blazing up
of a combustible; as of a charcoal or sulphur when thrown into melted nitre.
DELPHINIA. The vegeto-alkaline principle of the Delphinium staphysagria, or
stavesacre. It is poisonous.
DELIQUESCENT, (Zerfliessen, Germ.) is said of a solid which attracts so much
moisture from the air as to become spontaneously soft or liquid; such as potash and
muriate of lime.
DEPHLEGMATION is the process by which liquids are deprived of their watery
particles. It is applied chiefly to spirituous liquors, and is now nearly obsolete, as
involving the alchemistical notion of a peculiar principle called phlegm.
DEPHLOGISTICATED; deprived of phlogiston,—formerly supposed to be the
common combustible principle. It is nearly synonymous with oxygenated. The idea
originally attached to the word having proceeded from false logic, the word itself
should never be used either in science or manufactures.
DEPILATORY. (Depilatoire, Fr.; Enthaarensmittel, Germ.) is the name of
any substance capable of removing hairs from the human skin without injuring its
texture. They act either mechanically or chemically. The first are commonly glutinous
plasters formed of pitch and rosin, which stick so closely to the part of the skin
where they are applied, that when removed, they tear away the hairs with them. This
method is more painful, but less dangerous than the other, which consists in the solvent
action of a menstruum, so energetic as to penetrate the pores of the skin, and
destroy the bulbous roots of the hairs. This is composed either of caustic alkalis,
sulphuret of baryta, or arsenical preparations. Certain vegetable juices have also been
recommended for the same purpose; as spurge and acacia. The bruised eggs of ants
have likewise been prescribed. But the oriental rusma yields to nothing in depilatory
power. Gadet de Gassincourt has published in the Dictionnaire des Sciences Medicales,
the following recipe for preparing it.
Mix two ounces of quicklime, with half an ounce of orpiment or realgar, (sulphuret
of arsenic;) boil that mixture in one pound of strong alkaline lye, then try its strength
by dipping a feather into it, and when the flue falls off, the rusma is quite strong enough.
It is applied to the human skin by a momentary friction, followed by washing with
warm water. Such a caustic liquid should be used with the greatest circumspection,
beginning with it somewhat diluted. A soap is sometimes made with lard and
the above ingredients; or soft soap is combined with them; in either case to form a
depilatory pommade. Occasionally one ounce of orpiment is taken to eight ounces of
quicklime, or two to twelve, or three to fifteen; the last mixture being of course the most
active. Its causticity may be tempered by the addition of one eighth of starch or rye
flour, so as to form a soft paste, which being laid upon the hairy spot for a few minutes,
usually carries away the hairs with it.
The rusma should never be applied but to a small surface at a time, for independently
of the risk of corroding the skin, dangerous consequences might ensue from
absorption of the arsenic.
DETONATION. See Fulminating, for the mode of preparing detonating powder
for the percussion caps of fire-arms.
DEUTOXIDE literally means the second oxide, but is usually employed to denote
a compound containing two atoms or two prime equivalents of oxygen to one or more
of a metal. Thus we say deutoxide of copper, and deutoxide of mercury. Berzelius
has abbreviated this expression by adopting the principles of the French nomenclature
of 1787; according to which the higher stage of oxidizement is characterized by the
termination ic, and the lower by ous, and he writes accordingly cupric and mercuric,
to designate the deutoxides of these two metals; cuprous and mercurous to designate
their protoxides. I have adopted this nomenclature in the article Decomposition, and
in some other parts of this Dictionary, as being short and sufficiently precise.
DEXTRINE, is a matter of a gummy appearance into which the interior substance
of the molecules of starch are converted, through the influence of diastase or acids. It
derives its name from the circumstance that it turns, more than any other body, the[388]
plane of polarization to the right hand. It is white, insipid, without smell, transparent
in thin plates, friable, with a glassy fracture when well dried. It is not altered by the
heat of boiling water, but at 280° F. it becomes brown, and acquires the flavour of
toasted bread. It is not coloured by iodine, like starch, it does not form muric acid
with the nitric, as common gum does, and it is transformed into grape sugar, when
heated along with dilute sulphuric acid or diastase.
Dextrine is much employed by the French pastrycooks and confectioners; it is a good
substitute for gum arabic in medicine. For the conversion of potato or other starch
into dextrine, by the action of diastase, see this article.
DIAMOND. Since this body is merely a condensed form of carbon, it cannot in a
chemical classification be ranked among stones; but as it forms in commerce the most
precious of the gems, it claims our first attention in a practical treatise on the arts.
Diamonds are distinguishable by a great many peculiar properties, very remarkable and
easily recognized, both in their rough state, and when cut and polished. Their most
absolute and constant character is a degree of hardness superior to that of every mineral,
whence diamonds scratch all other bodies, and are scratched by none. Their peculiar
adamantine lustre, not easy to define, but readily distinguishable by the eye from that of
every other gem, is their most obvious feature. Their specific gravity is 3·55. Whether
rough or polished, diamonds acquire by friction, positive electricity, but do not retain it
for more than half an hour. The natural form of diamonds is derivable from an octahedron,
and they never present crystals having one axis longer than the other. Their
structure is very perceptibly lamellar, and therefore, notwithstanding their great hardness,
they are brittle and give way in the line of their cleavage, affording a direct means of
arriving at their primitive form, the regular octahedron.
The diamond possesses either single or double refraction, according to its different crystalline
forms; its refractive power on light is far greater than it ought to be in the ratio of
its density; the index of refraction being 2·44, whence Newton long ago supposed it to
consist of inflammable matter. Its various forms in nature present a circumstance
peculiar to this body; its faces are rarely terminated by planes, like most other native
crystals, but they are often rounded off, and the edges between them are curved.
When these secondary faces are attentively examined with a lens, we remark that they
are marked with striæ, sometimes very fine and almost imperceptible, but at others well
defined; and that these striæ are parallel to the edges of the octahedron, and consequently
to those of the plates that are applied on the primitive faces of this figure.
Diamonds are usually colourless and transparent; when coloured, their ordinary
tint verges upon yellow, or smoke-yellow, approaching sometimes to blackish-brown.
Green diamonds are next to yellow the most common; the blue possess rarely a lively
hue, but they are much esteemed in Scotland. The rose or pink diamonds are the most
valued of the coloured kind, and exceed sometimes in price the most limpid; though
generally speaking the latter are the most highly prized.
The geological locality of the diamond seems to be in diluvial gravel, and among
conglomerate rocks; consisting principally of fragments of quartz, or rolled pebbles of
quartz mixed with ferruginous sand, which compose sometimes hard aggregated masses.
This kind of formation is called cascalho in Brazil. Its accompanying minerals are few
in number, being merely black oxide of iron, micaceous iron ore, pisiform iron ore, fragments
of slaty jasper, several varieties of quartz, principally amethyst. In Mr. Heuland’s
splendid collection there was a Brazilian diamond imbedded in brown iron ore; another
in the same, belonging to M. Schuch, librarian to the Crown Princess of Portugal;
and in the cabinet of M. Eschwege there is a mass of brown iron ore, containing a
diamond in the drusy cavity of a green mineral, conjectured to be arseniate of iron.
From these facts it may be inferred with much probability that the matrix or original
repository of the diamond of Brazil is brown iron ore, which occurs in beds of slaty
quartzose micaceous iron ore, or in beds composed of iron-glance and magnetic iron
ore, both of which are apparently subordinate in that country to primitive clay slate.
The loose earth containing diamonds lies always a little way beneath the surface of
the soil, towards the lower outlet of broad valleys, rather than upon the ridges of the
adjoining hills.
Only two places on the earth can be adduced with certainty, as diamond mines, or
rather districts; a portion of the Indian peninsula, and of Brazil.
India has been celebrated from the most remote antiquity as the country of diamonds.
Its principal mines are in the kingdoms of Golconda and Visapour, extending from
Cape Comorin to Bengal, at the foot of a chain of mountains called the Orixa, which
appear to belong to the trap rock formation. In all the Indian diamond soils, these
gems are so dispersed, that they are rarely found directly, even in searching the richest
spots, because they are enveloped in an earthy crust, which must be removed before they
can be seen. The stony matter is therefore broken into pieces, and is then, as well as
the looser earth, washed in basins scooped out on purpose. The gravel thus washed is[389]
collected, spread out on a smooth piece of ground, and left to dry. The diamonds are
now recognized by their sparkling in the sun, and are picked out from the stones.
The diamond mines of Brazil were discovered in 1728, in the district of Serro-do-Frio.
The ground in which they are imbedded has the most perfect resemblance to that
of the East Indies, where the diamonds occur. It is a solid or friable conglomerate,
consisting chiefly of a ferruginous sand, which encloses fragments of various magnitude
of yellow and bluish quartz, of schistose jasper, and grains of gold disseminated with
oligist iron ore; all mineral matters different from those that constitute the neighbouring
mountains; this conglomerate, or species of pudding-stone, almost always superficial,
occurs sometimes at a considerable height on the mountainous table-land. The most
celebrated diamond mine is that of Mandarga, on the Jigitonhonha, in the district of
Serro-do-Frio to the north of Rio-Janeiro. The river Jigitonhonha, three times broader
than the Seine at Paris, and from 3 to 9 feet deep, is made nearly dry, by drawing the
waters off with sluices at a certain season; and the cascalho or diamond-gravel is removed
from the channel by various mechanical means, to be washed elsewhere at leisure. This
cascalho, the same as the matrix of the gold mines, is collected in the dry season, to be
searched into during the rainy; for which purpose it is formed into little mounds of 15
or 16 tons weight each. The washing is carried on beneath an oblong shed, by means
of a stream of water admitted in determinate quantities into boxes containing the
cascalho. A negro washer is attached to each box; inspectors are placed at regular
distances on elevated stools, and whenever a negro has found a diamond, he rises up and
exhibits it. If it weighs 171⁄2 carats, he receives his liberty. Many precautions are taken
to prevent the negroes from secreting the diamonds. Each squad of workmen consists
of 200 negroes, with a surgeon and an almoner or priest.
The flat lands on either side of the river are equally rich in diamonds over their whole
surface, so that it becomes very easy to estimate what a piece of ground not yet washed
may produce.
It is said that the diamonds surrounded with a greenish crust, are of the first water,
or are the most limpid when cut. The diamonds received in the different mines of the
district, are deposited once a month in the treasury of Tejuco; and the amount of what
was thus delivered from 1801 to 1806, may be estimated at about 18 or 19 thousand
carats per annum.
On the banks of the torrent called Rio-Pardo, there is another mine of diamonds.
The ground presents a great many friable rocks of pudding-stone, distributed in irregular
strata. It is chiefly in the bed of this stream, that masses of cascalho occur, peculiarly
rich in diamonds. They are much esteemed, particularly those of a greenish-blue colour.
The ores that accompany the diamond at Rio-Pardo differ somewhat from those of the
washing grounds of Mandanga, for they contain no pisiform iron ore; but a great many
pebbles of slaty jasper. This table land seems to be very high, probably not less than
5500 feet above the level of the sea.
Tocaya, a principal village of Minas-Novas, is 34 leagues to the north-east of Tejuco,
in an acute angle of the confluence of the Jigitonhonha, and the Rio-Grande. In the
bed of the streamlets which fall westward into the Jigitonhonha, those rolled white
topazes are found which are known under the name of minas-novas with blue topazes, and
aquamarine beryls. In the same country are found the beautiful cymophanes or chrysoberyls
so much prized in Brazil. And it is from the cantons of Indaia and Abaite
that the largest diamonds of Brazil come; yet they have not so pure a water as those of
the district of Serro-do-Frio, but incline a little to the lemon yellow.
Diamonds are said to come also from the interior of the island of Borneo, on the banks
of the river Succadan, and from the peninsula of Malacca.
It is known that many minerals become phosphorescent by heat, or exposure to the
sun’s light. Diamonds possess this property, but all not in equal degree, and certain
precautions must be observed to make it manifest. Diamonds need to be exposed to
the sunbeam for a certain time, in order to become self-luminous; or to the blue rays of
the prismatic spectrum, which augment still more the faculty of shining in the dark.
Diamonds susceptible of phosphorescence exhibit it either after a heat not raised to
redness, or the electric discharge. They possess not only a great refractive power in
the mean ray of light, but a high dispersive agency, which enables them to throw out
the most varied and vivid colours in multiplied directions.
Louis de Berquem discovered in 1476, the art of cutting diamonds by rubbing them
against one another, and of polishing them with their own powder. These operations may
be abridged by two methods: 1. by availing ourselves of the direction of the laminæ of
the diamond to split them in that direction, and thus to produce several facets. This
process is called cleaving the diamond. Some, which appear to be macle crystals, resist
this mechanical division, and are called diamonds of nature. 2. by sawing the diamonds
by means of a very delicate wire, coated with diamond powder.
Diamonds take precedence of every gem for the purposes of dress and decoration; and[390]
hence the price attached to those of a pure water, increases in so rapid a proportion, that
beyond a certain term, there is no rule of commercial valuation. The largest diamond
that is known seems to be that of the Rajah of Mattan, in the East Indies. It is of the
purest water, and weighs 367 carats, or at the rate of 4 grains to a carat, upwards of 3
ounces troy. It is shaped like an egg, with an indented hollow near the smaller end;
it was discovered at Landak about 100 years ago; and though the possession of it has
cost several wars, it has remained in the Mattan family for 90 years. A governor of
Batavia, after ascertaining the qualities of the gem, wished to be the purchaser, and
offered 150,000 dollars for it, besides two war brigs with their guns and ammunition,
together with a certain number of great guns, and a quantity of powder and shot. But
this diamond possessed such celebrity in India, and was regarded as a talisman involving
the fortunes of the Rajah and his family, that he refused to part with it at any price.
The diamond possessed in the time of the traveller Tavernier, by the emperor of
Mogul, a kingdom now no more, weighed 279 carats, and was reckoned worth upwards
of 400,000l. sterling. It was said to have lost the half of its original weight in
the cutting. After these prodigious gems, the next are:—1. That of the emperor of
Russia, bought by the late empress Catherine, which weighs 193 carats. It is said to
be of the size of a pigeon’s egg, and to have been bought for 90,000l., besides
an annuity to the Greek merchant of 4000l. It is reported that the above diamond
formed one of the eyes of the famous statue of Sheringan, in the temple of Brama, and
that a French grenadier, who had deserted into the Malabar service, found the means of
robbing the pagoda of this precious gem; and escaped with it to Madras, where he
disposed of it to a ship captain for 2,000l., who resold it to a Jew for 12,000l. From
him it was transferred for a large sum to the Greek merchant. 2. That of the emperor
of Austria, which weighs 139 carats, and has a slightly yellowish hue. It has, however,
been valued at 100,000l. 3. That of the king of France, called the Regent or Pitt
diamond, remarkable for its form and its perfect limpidity. Although it weighs only
136 carats, its fine qualities have caused it to be valued at 160,000l. though it cost only
100,000l.
The largest diamond furnished by Brazil, now in possession of the crown of Portugal,
weighs, according to the highest estimates, 120 carats. It was found in the streamlet
of Abaïte, in a clay-slate district.
The diamonds possessed of no extraordinary magnitude, but of a good form and a
pure water, may be valued by a certain standard rule. In a brilliant, or rose-diamond
of regular proportions, so much is cut away that the weight of the polished gem does
not exceed one half the weight of the diamond in the rough state; whence the value of
a cut diamond is esteemed equal to that of a similar rough diamond of double weight,
exclusive of the cost of workmanship. The weight and value of diamonds is reckoned
by carats of 4 grains each; and the comparative value of two diamonds of equal
quality but different weights, is as the squares of these weights respectively. The average
price of rough diamonds that are worth working, is about 2l. for one of a single
carat; but as a polished diamond of one carat must have taken one of 2 carats, its price
in the rough state is double the square of 2l., or 8l. Therefore, to estimate the value
of a wrought diamond, ascertain its weight in carats, double that weight, and multiply
the square of this product by 2l.
| Hence, a wrought diamond of |
1 |
carat is worth |
£8 |
|
| 2 |
— |
32 |
| 3 |
— |
72 |
| 4 |
— |
128 |
| 5 |
— |
200 |
| 6 |
— |
288 |
| 7 |
— |
392 |
| 8 |
— |
512 |
| 9 |
— |
612 |
| 10 |
— |
800 |
| 20 |
— |
3200 |
, |
beyond which weight the prices
can no longer rise in this geometrical progression, from the small number of purchasers
of such expensive toys. A very trifling spot or flaw of any kind, lowers exceedingly
the commercial value of a diamond.
Diamonds are used not only as decorative gems, but for more useful purposes, as
for cutting glass by the glazier, and all kinds of hard stones by the lapidary.
On the structure of the glazier’s diamond, we possess some very interesting observations
and reflections by Dr. Wollaston. He remarks, that the hardest substances
brought to a sharp point scratch glass, indeed, but do not cut it, and that diamond alone
possessed that property; which he ascribes to the peculiarity of its crystallization in
rounded faces, and curvilinear edges. For glass-cutting, those rough diamonds are always
selected which are sharply crystallized, hence called diamond sparks; but cut[391]
diamonds are never used. The inclination to be given to a set diamond in cutting glass
is comprised within very narrow limits; and it ought, moreover, to be moved in the
direction of one of its angles. The curvilinear edge adjoining the curved faces, entering
as a wedge into the furrow opened up by itself, thus tends to separate the parts of the
glass; and in order that the crack which causes the separation of the vitreous particles
may take place, the diamond must be held almost perpendicular to the surface of the
glass. The Doctor proved this theory by an experiment. If, by suitable cutting with the
wheel, we make the edges of a spinel ruby, or corundum-telesie (sapphire) curvilinear,
and the adjacent faces curved, these stones will cut glass as well as a glazier’s diamond,
but being less hard than it, they will not preserve this property so long. He found that
upon giving the surface of even a fragment of flint the same shape as that of the cutting
diamond, it acquired the same property; but, from its relative softness, was of little duration.
The depth to which the fissure caused by the glazier’s diamond penetrates, does not
seem to exceed the two-hundredth of an inch.
I shall here introduce Mr. Milburn’s valuable observations on the choice of rough
diamonds, as published in his work on Oriental Commerce.
The colour should be perfectly crystalline, resembling a drop of clear spring water, in the
middle of which you will perceive a strong light, playing with a great deal of spirit. If
the coat be smooth and bright, with a little tincture of green in it, it is not the worse,
and seldom proves bad, but if there is a mixture of yellow with green, then beware of
it; it is a soft greasy stone, and will prove bad.
If the stone has a rough coat, so that you can hardly see through it, and the coat be
white and look as if it were rough by art, and clear of flaws or veins, and no blemish
cast in the body of the stone, (which may be discovered by holding it against the light)
the stone will prove good.
It often happens that a stone will appear of a reddish hue on the outward coat, not
unlike the colour of rusty iron, yet by looking through it against the light, you may
observe the heart of the stone to be white (and if there be any black spots, or flaws, or
veins in it, they may be discovered by a true eye, although the coat of the stone be the
same), and such stones are generally good and clear.
If a diamond appears of a greenish bright coat, resembling a piece of green glass,
inclining to black, it generally proves hard, and seldom bad; such stones have been
known to have been of the first water, and seldom worse than the second; but if any
tincture of yellow seems to be mixed with it, you may depend on its being a very bad
stone.
All stones of a milky cast, whether the coat be bright or dull, if ever so little inclining
to a bluish cast, are naturally soft, and in danger of being flawed in the cutting; and
though they should have the good fortune to escape, yet they will prove dead and
milky, and turn to no account.
All diamonds of cinnamon colour are dubious; but if of a bright coat mixed with
a little green, then they are certainly bad, and are accounted among the worst of colours.
You will meet with a great many diamonds of a rough cinnamon-coloured coat, opaque;
this sort is generally very hard, and, when cut, contain a great deal of life and spirit;
but the colour is very uncertain; it is sometimes white, sometimes brown, and sometimes
of a fine yellow. Rough diamonds are frequently beamy, that is look fair to the
eye, yet are so full of veins to the centre, that no art or labour can polish them. A good
diamond should never contain small spots of a white or gray colour of a nebulous
form; it should be free from small reddish and brownish grains, that sometimes occur on
their surface, or in their interior. A good diamond should split readily in the direction
of the cleavage; it sometimes happens, however, that the folia are curved, as is the case
in twin crystals. When this happens, the stone does not readily cut and polish, and is
therefore of inferior value.
In the cut and polished gem, the thickness must always bear a certain proportion to
the breadth. It must not be too thin nor thick; for, when too thin, it loses much of
its fire, and appears not unlike glass.
The term carat is said to be derived from the name of a bean, the produce of a species
of erythina, a native of the district of Shangallas, in Africa; a famous mart of gold-dust.
The tree is called kuara, a word signifying sun in the language of the country; because
it bears flowers and fruit of a flame colour. As the dry seeds of this pod are always of
nearly uniform weight, the savages have used them from time immemorial to weigh
gold. The beans were transported into India, at an ancient period, and have been
long employed there for weighing diamonds. The carat of the civilized world is, in
fact, an imaginary weight, consisting of 4 nominal grains, a little lighter than 4 grains
troy (poids de marc); it requires 74 carat grains and 1⁄16 to equipoise 72 of the other.
In valuing a cut diamond, we must reckon that one half of its weight has been lost in
the lapidary’s hands; whence its weight in this state should be doubled before we
calculate its price by the general rule for estimating diamonds. The French multiply[392]
by 48 the square of this weight, and they call the product in francs the value of the
diamond. Thus, for example, a cut diamond of 10 carats would be worth (10 × 2)2
× 48 = 19,200 francs, or 768l., allowing only 25 francs to the pound sterling.
The diamond mines of Brazil have brought to its government, from the year 173~
till 1814, 3,023,000 carats; being at the average rate annually of 36,000 carats, or a
little more than 16 libs., weight. They have not been so productive in the later
years of that period; for, according to Mr. Mawe, between 1801 and 1806, only
115,675 carats were obtained, being 19,279 a year. The actual expenses incurred by
the government, during this interval, was 4,419,700 francs; and, deducting the production
in gold from the washings of the diamond gravel, or cascalho, it is found that
the rough diamonds cost in exploration, per carat, 38 francs 20 c., or nearly 31s.
British money. The contraband is supposed to amount to one third of the above
legitimate trade. Brazil is almost the only country where diamonds are mined at the
present day; it sends annually to Europe from 25 to 30 thousand carats, or from 10 to
161⁄2 libs.
DIAMONDS, cutting of. Although the diamond is the hardest of all known substances,
yet it may be split by a steel tool, provided a blow be applied; but this requires
a perfect knowledge of the structure, because it will only yield to such means in certain
directions. This circumstance prevents the workman from forming facettes or planes
generally, by the process of splitting; he is therefore obliged to resort to the process
of abrasion, which is technically called cutting. The process of cutting is effected by
fixing the diamond to be cut on the end of a stick, or handle, in a small ball of
cement, that part which is to be reduced being left to project. Another diamond is
also fixed in a similar manner; and the two stones being rubbed against each other
with considerable force, they are mutually abraded, flat surfaces, or facettes, being
thereby produced. Other facettes are formed by shifting the diamonds into fresh
positions in the cement, and when a sufficient number are produced, they are fit for
polishing. The stones, when cut, are fixed for this purpose, by imbedding them in soft
solder, contained in a small copper cup, the part, or facette, to be polished, being left
to protrude.
A flat circular plate of cast-iron is then charged with the powder produced during
the abrasion of the diamonds; and by this means a tool is formed which is capable of
producing the exquisite lustre so much admired on a finely-polished gem. Those
diamonds that are unfit for working, on account of the imperfection of their lustre or
colour, are sold, for various purposes, under the technical name of Bort. Stones of
this kind are frequently broken in a steel mortar, by repeated blows, until they are
reduced to a fine powder, which is used to charge metal plates, of various kinds, for
the use of jewellers, lapidaries, and others. Bort, in this state of preparation, is
incapable of polishing any gems; but it is used to produce flat surfaces on rubies and
other precious stones.
Fine drills are made of small splinters of bort, which are used for drilling small holes
in rubies, and other hard stones, for the use of watch-jewellers, gold and silver wire-drawers,
and others, who require very fine holes drilled in such substances. These
drills are also used to pierce holes in china, where rivets are to be inserted; also for
piercing holes in artificial enamel teeth, or any vitreous substances, however hard.
DIAMOND MICROSCOPES, were first suggested by Dr. Goring, and have
been well executed by Mr. Pritchard. Previous to grinding a diamond into a spherical
figure, it should be ground flat and parallel upon both sides, that by looking
through it, as opticians try flint glass, we may see whether it has a double or triple refractive
power, as many have, which would render it useless as a lens. Among the
14 different crystalline forms of the diamond, probably the octahedron and the cube are
the only ones that will give single vision. It will, in many cases, be advisable to
grind diamond lenses, plano-convex, both because this figure gives a low spherical
aberration, and because it saves the trouble of grinding one side of the gem. A concave
tool of cast iron, paved with diamond powder, hammered into it by a hardened
steel punch, was employed by Mr. Pritchard. This ingenious artist succeeded in completing
a double convex of equal radii, of about 1⁄25 of an inch focus, bearing an aperture
of 1⁄30 of an inch with distinctness upon opaque objects, and its entire diameter
upon transparent ones. This lens gives vision with a trifling chromatic aberration; in
other respects, like Dr. Goring’s Amician reflector, but without its darkness, its light is
said to be superior to that of any compound microscope whatever, acting with the same
power, and the same angle of aperture. The advantage of seeing an object without
aberration by the interposition of only a single magnifier, instead of looking at a picture
of it with an eye-glass, is evident. We thus have a simple direct view, whereby
we shall see more accurately and minutely the real texture of objects.
DIAPER, is the name of a kind of cloth, used chiefly for table linen. It is known
among the French by the name of toile fourré, and is ornamented with the most extensive[393]
figures of any kind of tweeled cloth, excepting damask. The mounting of a loom for
working diaper is, in principle, much the same as a draw-loom, but the figures being
less extensive, the mounting is more simple, and is wrought entirely by the weaver,
without the aid of any other person. As tweeled cloths, of any number of leaves, are
only interwoven at those intervals when one of the leaves is raised, the woof above, and
the warp below, is kept floating or flushed, until the intersection takes place. Of consequence,
the floating yarn above, appears across the fabric, and that below longitudinally.
This property of tweeled cloths is applied to form the ornamental figures of all
kinds of tweeled goods, merely by reversing the floating yarn when necessary. In the
simpler patterns, this is effected by a few additional leaves of treddles; but when the
range of pattern becomes too great to render this convenient, an apparatus called a
back harness is employed, and the cloth woven with this mounting is called diaper.
Diapers are generally five-leaf tweels, that is to say, every warp floats under four
threads of woof, and is raised, and of course interwoven with the fifth. This is done
either successively, forming diagonals at 45° upon the cloth, or by intervals of two
threads, which is called the broken tweel. The latter is generally, if not universally
adopted in the manufacture of diaper. The reason of preferring the broken to the
regular tweel, where ornaments are to be formed, is very obvious. The whole depending
upon reversed flushing to give the appearance of oblique or diagonal lines, through
either, would destroy much of the effect, and materially injure the beauty of the fabric.
The broken tweel, on the contrary, restores to the tweeled cloth a great similarity
of appearance to plain, or alternately interwoven fabrics, and, at the same time, preserves
the facility of producing ornaments by reversing the flushing. The simplest kinds
of reversed tweels will be found described under Textile Fabrics.
DIASTASE. This curious substance, extracted by water from crushed malt, and
precipitated from that infusion by alcohol, as is described under Fermentation, has
been made the subject of new researches by M. Guerin Varry. The conclusions deducible
from his interesting experiments are the following:—
1. One part of diastase, dissolved in 30 parts of cold water, put with 408 parts of
potato starch out of contact of air, did not exercise the slightest action upon this substance
in the course of 63 days, under a temperature varying from 68° to 79° Fahr.
2. Two parts of diastase do not in the course of an hour, cause the globules of three
parts of starch to burst, at a temperature approaching very nearly to that of the hot water
which bursts them into a paste. It follows that diastase acts no part in the process
of germination, towards eliminating the teguments of the starch, or transforming
its interior portion into sugar, and a gummy matter assimilated by plants.
3. Diastase liquefies and saccharifies the paste of starch without absorption or disengagement
of gas; a reaction which takes place equally in vacuo, as in the open air.
4. 100 parts of starch made into a paste with 39 times their weight of water, mixed
with 6·13 parts of diastase dissolved in 40 parts of water, and kept for an hour between
140° and 149° Fahr., afforded 86·91 parts of sugar.
5. A paste containing 100 parts of starch, and 1393 parts of water, put in contact
with 12·25 parts of diastase dissolved in 367 parts of cold water, having been maintained
at 68° Fahr. during 24 hours, produced 77·64 parts of sugar.
6. The preceding experiment, repeated at the temperature of melting ice, afforded at
the end of 2 hours, 11·82 parts of sugar.
7. The most favourable proportions and circumstances for the production of a great
quantity of sugar, are a slight excess of diastase or barley malt, (at least 25 per cent.
of the latter), about 50 parts of water to one of starch, and a temperature between 140°
and 149° Fahr. It is of the greatest consequence for the saccharification to take
place as speedily as possible, so that the sugar produced may not be left in contact with
much gummy matter (dextrine), in which case, the diastase will not convert the latter
into sugar. In fact, the liquefaction and saccharification should proceed simultaneously.
8. The sugar of starch, prepared either with diastase, or sulphuric acid, crystallizes
in cauliflowers, or in prisms with rhomboidal facets. It has the same composition as
sugar of grapes.
9. Diastase even in excess does not saccharify the gummy matter dissolved in the
water along with the starch sugar, but when the gum is insulated, it is convertible almost
entirely into sugar.
10. Gum arabic, cane sugar, and beer yeast, suffer no change from diastase.
11. A watery solution of diastase readily decomposes on keeping, either in contact
or out of contact of air.
12. When starch-sugar, whether obtained by means of diastase or sulphuric acid,
is submitted to the spirituous fermentation, the sum of the weights of the alcohol, carbonic
acid, and water of crystallization of the sugar, is less than the weight of the
sugar by about 31⁄2 per cent. This difference proceeds in a great measure from the formation[394]
of some acetic acid, lactic acid, volatile oil, and probably some other unknown
products in the act of fermentation.
DIMITY, is a kind of cotton cloth originally imported from India, and now manufactured
in great quantities in various parts of Britain, especially in Lancashire. Dr.
Johnson calls it dimmity, and describes it as a kind of fustian. The distinction between
fustian and dimity seems to be, that the former designates a common tweeled cotton
cloth of a stout fabric, which receives no ornament in the loom, but is most frequently
dyed after being woven. Dimity is also a stout cotton cloth, but not usually of so thick
a texture; and is ornamented in the loom, either with raised stripes or fancy figures,
is seldom dyed, but usually worn white, as for bed and bed-room furniture. The
striped dimities are the most common, they require less labour in weaving than the
others; and the mounting of the loom being more simple, and consequently less expensive,
they can be sold at much lower rates. See Textile Fabrics, for particular details
of the plan of mounting them.
DIES FOR STAMPING, (Coins, Fr.; Münzstempeln, Germ.) The first circumstance
that claims particular attention in the manufacture of dies, is the selection
of the best kind of steel for the purpose, and this must in some measure be left to the
experience of the die-forger, who, if well skilled in his art, will be able to form a tolerably
correct judgment of the fitness of the metal for the purpose, by the manner in
which it works upon the anvil. It should be rather fine-grained than otherwise, and
above all things perfectly even and uniform in its texture, and free from spots and
patches finer or coarser than the general mass. But the very fine and uniform steel
with a silky fracture, which is so much esteemed for some of the purposes of cutlery,
is unfit for our present purpose, from the extreme facility with which it acquires great
hardness by pressure, and its liability to cracks and flaws. The very coarse-grained, or
highly crystalline steel, is also equally objectionable; it acquires fissures under the die-press,
and seldom admits of being equally and properly hardened. The object, therefore,
is to select a steel of a medium quality as to fineness of texture, not easily acted
upon by dilute sulphuric acid, and exhibiting an uniform texture when its surface is
washed over with a little aqua-fortis, by which its freedom from pins of iron, and other
irregularities of composition, is sufficiently indicated.
The best kind of steel being thus selected, and properly forged at a high heat into
the rough die, it is softened by very careful annealing, and in that state, having been
smoothed externally, and brought to a table in the turning lathe, it is delivered to the
engraver.
The process of annealing the die consists in heating it to a bright cherry red, and
suffering it to cool gradually, which is best effected by bedding it in a crucible or iron
pot of coarsely-powdered charcoal, that of animal substances being generally preferred.
In this operation it is sometimes supposed that the die, or at least its superficial
parts, becomes super-carbonized, or highly-converted steel, as it is sometimes
called; but experience does not justify such an opinion, and I believe the composition
of the die is scarcely, certainly not materially, affected by the process, for it does not
remain long enough in the fire for the purpose.
The engraver usually commences his labours by working out the device with small
steel tools, in intaglio; he rarely begins in relief (though this is sometimes done); and
having ultimately completed his design, and satisfied himself of its general effect and
correctness, by impressions in clay, and dabs, or casts in type metal, the die is ready for
the important operation of hardening, which, from various causes, a few of which I
shall enumerate, is a process of much risk and difficulty; for should any accident now
occur, the labour of many months may be seriously injured, or even rendered quite
useless.
The process of hardening soft steel is in itself very simple, though not very easily
explained upon mechanical or chemical principles. We know by experience, that it
is a property of this highly valuable substance, to become excessively hard, if heated
and suddenly cooled; if, therefore, we heat a bar of soft malleable and ductile steel red
hot, and then suddenly quench it in a large quantity of cold water, it not only becomes
hard, but fragile and brittle. But as a die is a mass of steel of considerable dimensions,
this hardening is an operation attended by many and peculiar difficulties, more
especially as we have at the same time to attend to the careful preservation of the
engraving. This is effected by covering the engraved face of the die with a protecting
face, composed of fixed oil of any kind, thickened with powdered charcoal: some
persons add pipe-clay, others use a pulp of garlic, but pure lamp-black and linseed oil
answer the purpose perfectly. This is thinly spread upon the work of the die, which,
if requisite, may be further defended by an iron ring; the die is then placed with its
face downwards in a crucible, and completely surrounded by powdered charcoal. It is
heated to a suitable temperature, that is, about cherry red, and in that state is taken out
with proper tongs, and plunged into a body of cold water, of such magnitude as not[395]
to become materially increased in temperature; here it is rapidly moved about, until all
noise ceases, and then left in the water till quite cool. In this process it should produce
a bubbling and hissing noise; if it pipes and sings, we may generally apprehend a
crack or fissure.
No process has been found to answer better than the above simple and common mode
of hardening dies, though others have had repeated and fair trials. It has been proposed
to keep up currents and eddies of cold water in the hardening cistern, by means of
delivery-pipes, coming from a height; and to subject the hot die, with its face uppermost,
to a sudden and copious current of water, let upon it from a large pipe, supplied
from a high reservoir; but these means have not in any way proved more successful,
either in saving the die, or in giving it any good qualities. It will be recollected, from
the form of the die, that it is necessarily only, as it were, case-hardened, the hardest
strata being outside, and the softer ones within, which envelope a core, something in
the manner of the successive coats of an onion; an arrangement which we sometimes
have an opportunity of seeing displayed in dies which have been smashed by a violent
blow.
The hardening having been effected, and the die being for the time safe, some further
steps may be taken for its protection; one of these consists in a very mild kind of
tempering, produced by putting it into water, gradually raised to the boiling point,
till heated throughout, and then suffering it gradually to cool. This operation renders
the die less apt to crack in very cold weather. A great safeguard is also obtained by
thrusting the cold die into a red-hot iron ring, which just fits it in that state, and which,
by contracting as it cools, keeps its parts together under considerable pressure, preventing
the spreading of external cracks and fissures, and often enabling us to employ
a split or die for obtaining punches, which would break to pieces without the protecting
ring.
If the die has been successfully hardened, and the protecting paste has done its duty,
by preserving the face from all injury and oxidizement, or burning, as it is usually
called, it is now to be cleaned and polished, and in this state constitutes what is
technically called a MATRIX: it may of course be used as a multiplier of medals, coins,
or impressions, but it is not generally thus employed, for fear of accidents happening
to it in the coining press, and because the artist has seldom perfected his work
upon it in this state. It is, therefore, resorted to for the purpose of finishing a
PUNCH, or steel impression for relief. For this purpose a proper block of steel is selected,
of the same quality, and with the same precautions as before, and being carefully
annealed, or softened, is turned like the matrix, perfectly true and flat at the
bottom, and obtusely conical at top. In this state, its conical surface is carefully compressed
by powerful and proper machinery upon the matrix, which being very hard,
soon allows it to receive the commencement of an impression; but in thus receiving
the impression, it becomes itself so hard by condensation of texture as to require during
the operation to be repeatedly annealed, or softened, otherwise it would split into small
superficial fissures, or would injure the matrix; much practical skill is therefore required
in taking this impression, and the punch, at each annealing, must be carefully protected,
so that the work may not be injured.
Thus, after repeated blows in the die-press, and frequent annealing, the impression
from the matrix is at length perfected, or brought completely up, and having been retouched
by the engraver, is turned, hardened, and collared, like the matrix, of which it
is now a complete impression in relief, and, as we have before said, is called a punch.
This punch becomes an inexhaustible parent of dies, without further reference to the
original matrix; for now by impressing upon it plugs of soft steel, and by pursuing
with them an exactly similar operation to that by which the punch itself was obtained,
we procure impressions from it to any amount, which of course are fac-similes
of the matrix, and these dies being turned, hardened, polished, and, if necessary, tempered,
are employed for the purposes of coinage.
The distinction between striking medals, and common coin, is very essential, and the
work upon the dies is accordingly adjusted to each. Medals are usually in very high
relief, and the effect is produced by a succession of blows; and as the metal in which
they are struck, be it gold, silver, or copper, acquires considerable hardness at each
stroke of the press, they are repeatedly annealed during the process of bringing them up.
In a beautiful medal, which Mr. Wyon some time since completed for the Royal Navy
College, the obverse represents a head of the King, in very bold relief; it required
thirty blows of a very powerful press to complete the impression, and it was necessary
to anneal each medal after every third blow, so that they went ten times into the fire
for that purpose. In striking a coin or medal, the lateral spread of the metal, which
otherwise would ooze out as it were from between the dies, is prevented by the application
of a steel collar, accurately turned to the dimensions of the dies, and which,
when left plain, gives to the edge of the piece a finished and polished appearance; it is[396]
sometimes grooved, or milled, or otherwise ornamented, and occasionally lettered, in
which case it is made in three separate and moveable pieces, confined by a ring, into
which they are most accurately fitted, and so adjusted that the metal may be forced
into the letters by its lateral spread, at the same time that the coin receives the blow
of the screw-press.
Coins are generally completed by one blow of the coining-press. These presses are
worked in the Royal Mint by machinery, so contrived that they shall strike, upon an
average, sixty blows in a minute; the blank piece, previously properly prepared and
annealed, being placed between the dies by part of the same mechanism.
The number of pieces which may be struck by a single die of good steel, properly
hardened and duly tempered, not unfrequently amounts at the Mint to between three
and four hundred thousand, but the average consumption of dies is of course much
greater, owing to the variable qualities of steel, and to the casualties to which the
dies are liable: thus, the upper and lower die are often violently struck together, owing
to an error in the layer-on, or in that part of the machinery which ought to put the
blank into its place, but which now and then fails so to do. This accident very commonly
arises from the boy who superintends the press neglecting to feed the hopper of the
layer-on with blank pieces. If a die is too hard, it is apt to break or split, and is especially
subject to fissures, which run from letter to letter upon the edge. If too soft, it swells,
and the collar will not rise and fall upon it, or it sinks in the centre, and the work becomes
distorted and faulty. He, therefore, who supplies the dies for an extensive coinage, has
many accidents and difficulties to encounter. There are eight presses at the Mint,
frequently at work for ten hours each day, and the destruction of eight pair of dies per
day (one pair for each press) may be considered a fair average result, though they much
more frequently fall short of, than exceed this proportion. It must be remembered,
that each press produces 3600 pieces per hour, but, making allowance for occasional
stoppages, we may reckon the daily produce of each press at 30,000 pieces; the eight
presses therefore will furnish a diurnal average of 240,000 pieces.
DIGESTER, is the name of a strong kettle or pot of small dimensions, made very
strong, and mounted with a safety valve in its top. Papin, the contriver of this apparatus,
used it for subjecting bones, cartilages, &c. to the solvent action of high-pressure
steam, or highly heated water, whereby he proposed to facilitate their digestion in
the stomach. This contrivance is the origin of the French cookery pans, called
autoclaves, because the lid is self-keyed, or becomes steam-tight by turning it round
under clamps or ears at the sides, having been previously ground with emery to fit the
edge of the pot exactly. In some autoclaves the lid is merely laid on with a fillet of
linen as a lute, and then secured in its place by means of a screw bearing down upon
its centre from an arched bar above. The safety valve is loaded either by a weight
placed vertically upon it, or by of a lever of the second kind pressing near its
fulcrum, and acted upon by a weight which may be made to bear upon any point of
its graduated arm.
Chevreul has made a useful application of the digester to vegetable analysis. His
instrument consists of a strong copper cylinder, into which enters a tight cylinder of
silver, having its edge turned over at right angles to the axis of the cylinder, so as to
form the rim of the digester. A segment of a copper sphere, also lined with silver
stops the aperture of the silver cylinder, being applied closely to its rim. It has a
conical valve pressed with a spiral spring, of any desired force, estimated by a steelyard.
This spring is enclosed within a brass box perforated with four holes; which may be
screwed into a tapped orifice in the top of the digester. A tube screwed into another hole
serves to conduct away the condensable vapours at pleasure into a Woulfe’s apparatus.
DISTILLATION, (Eng. and Fr.; Branntweinbrennerei, Germ.) means, in the
commercial language of this country, the manufacture of intoxicating spirits; under
which are comprehended the four processes, of mashing the vegetable materials, cooling
the worts, exciting the vinous fermentation, and separating by a peculiar vessel called
a still, the alcohol combined with more or less water. This art of evoking the fiery
demon of drunkenness from his attempered state in wine and beer, was unknown to the
ancient Greeks and Romans. It seems to have been invented by the barbarians of the
north of Europe, as a solace to their cold and humid clime; and was first made known
to the southern nations in the writings of Arnoldus de Villa Nova, and his pupil,
Raymond Lully of Majorca, who declares this admirable essence of wine to be an
emanation of the Divinity, an element newly revealed to man, but hid from antiquity,
because the human race were then too young to need this beverage, destined to revive
the energies of modern decrepitude. He further imagined that the discovery of this
aqua vitæ, as it was called, indicated the approaching consummation of all things—the
end of this world. However much he erred as to the value of this remarkable essence,
he truly predicted its vast influence upon humanity, since to both civilized and savage
nations it has realized greater ills than were threatened in the fabled box of Pandora.
[397]
I shall consider in this place the first three of these subjects, reserving for the
article Still an account of the construction and use of that apparatus.
Whiskey, from the Irish word Usquebaugh, is the British name of the spirituous
liquor manufactured by our distillers, and corresponds to the Eau de vie of the French,
and the Branntwein of the Germans. It is generated by that intestine change which
grape juice and other glutino-saccharine liquids spontaneously undergo when exposed
to the atmosphere at common temperatures; the theory of which will be expounded
under the article Fermentation. The production of whiskey depends upon the simple
fact, that when any vinous fluid is boiled, the alcohol being very volatile, evaporates first,
and may thereby be separated from the aqueous vegetable infusion in which it took its
birth. Sugar is the only substance which can be transformed into alcohol. Whatsoever
fruits, seeds, or roots afford juices or extracts capable of conversion into vinous liquor,
either contain sugar ready formed, or starch susceptible of acquiring the saccharine state
by proper treatment. In common language, the intoxicating liquor obtained from the
sweet juices of fruits is called wine; and that from the infusions of farinaceous seeds,
beer; though there is no real difference between them in chemical constitution. A
similar beverage, though probably less palatable, is procurable from the juices and infusions
of many roots, by the process of fermentation. Wine, cyder, beer, and fermented
wash of every kind, when distilled, yields an identical intoxicating spirit, which differs
in these different cases merely in flavour, in consequence of the presence of a minute
quantity of volatile oils of different odours.
I. The juices of sweet fruits contain a glutinous ingredient which acts as a ferment in
causing their spontaneous change into a vinous condition; but the infusions of seeds,
even in their germinated or malted state, require the addition of a glutinous substance
called yeast, to excite the best fermentation. In the fabrication of wine or beer for drinking,
the fermentative action should be arrested before all the fruity saccharum is
decomposed; nor should it on any account be suffered to pass into the acetous stage;
whereas for making distillery wash, that action should be promoted as long as the
proportion of alcohol is increased, because the formation of a little acetic acid is not
injurious to the quality of the distilled spirit, but rather improves its flavour by the
addition of acetic ether, while all the undecomposed sugar is lost. Distillers operate
upon the saccharine matter from corn of various kinds in two methods; in the first they
draw off a pure watery extract from the grain, and subject this species of wort to fermentation;
in the second they ferment and distil the infused mass of grains. The
former is the practice of the distillers in the United Kingdom, and is preferable on
many accounts; the latter, which is adopted in Germany, Holland, and the north
of Europe, is less economical, more uncertain in the product, and affords a cruder
spirit, in consequence of the fetid volatile oil evolved from the husks in the
still. The substances employed by the distillers may be distributed into the following
classes:—
1. Saccharine juices. At the head of these stands cane-juice, which fresh from the
mill contains from 12 to 16 per cent. of raw sugar, and like the must of the grape
enters into the vinous fermentation without the addition of yeast, affording the species
of spirit called Rum, which is possessed of a peculiar aroma derived from an essential
oil in the cane. An inferior sort of rum is fabricated from molasses, mixed with the
skimmings and washings of the sugar pans. When molasses or treacle is diluted with
twenty times its weight of warm water, and when the mixture has cooled to 78° F., if
one twelfth of its weight of yeast be added, fermentation will speedily ensue, and an
ardent spirit will be generated, which when distilled has none of the aroma of rum;
proving this to reside in the immediate juice or substance of the cane, and to be dissipated
at the high temperature employed in the production of molasses. Though the
cane juice will spontaneously undergo the vinous fermentation, it does so more slowly
and irregularly than the routine of business requires, and therefore is quickened by the
addition of the lees of a preceding distillation. So sensible are the rum distillers of
the advantage of such a plan, that they soak woollen cloths in the yeast of the fermenting
vats, in order to preserve a ferment from one sugar season to another. In Jamaica
and some other of our colonies, 50 gallons of spent wash or lees are mixed with 6 gallons
of molasses, 36 gallons of sugar-pan skimmings (a substance rich in aroma), and
8 gallons of water; in which mixture there is about one twelfth part of solid saccharum.
Those who attend more to the quality than the quantity of their rum, will use
a smaller proportion of the spent wash, which is always empyreumatic, and imparts more
or less of its odour to the spirit distilled from it. The fermentation is seldom complete
in less than 9 days, and most commonly it requires from 12 to 15; the period being
dependent upon the capacity of the fermenting tun, and the quality of its contents.
The liquid now becomes clear, the froth having fallen to the bottom, and few bubbles
of gas are extricated from it, while its specific gravity is reduced from 1·050 down to
0·992. The sooner it is subjected to distillation after this period the better, to prevent[398]
the loss of alcohol by the supervention of the acetous stage of fermentation, an accident
very liable to happen in the sugar colonies. The crude spirit obtained from the large
single still at the first operation, is rectified in a smaller still. About 114 gallons of
rum, proof strength, specific gravity 0·920, are obtained from 1200 gallons of wash.
Now these 1200 gallons weigh 12,600 libs., and contain nearly one eighth of their weight
of sugar = 1575 libs.; which should yield nearly its own weight of proof spirit, whose
bulk is = 15750·92 = 1712 pound measures = 171·2 gallons; whereas only 114 are obtained;
proving the processes to be conducted in a manner far from economical, even with
every reasonable allowance.
Mr Edwards gives the following estimate: “The total amount of sweets from an
estate in Jamaica which makes 200 hogsheads of sugar, is 16,666 gallons. The wash set
at the rate of 12 per cent. sweets, should return 34,720 gallons of low wines, which should
give 14,412 gallons of rum, or 131 puncheons of 110 gallons each.”
By my own experiments on the quantity of proof spirit obtainable from molasses by fermentation
(afterwards to be detailed), one gallon of sweets should yield one gallon of spirit;
and hence the above 16,666 gallons should have afforded the same bulk of rum. But here
we are left somewhat in the dark, by not knowing the specific gravity of the rum spoken
of by Mr. Edwards. The only light let in upon us is when he mentions rum oil-proof,
that is, a spirit in which olive oil will sink; indicating a density nearly the same with
our actual excise proof, for olive oil at 60° F. has the specific gravity 0·919. When a
solution of sugar of the proper strength is mixed with wine lees, and fermented, it
affords a spirit by distillation not of the rum, but of the brandy flavour.
The sweet juices of palm trees and cocoa nuts, as also of the maple, and ash, birch,
&c., when treated like cane juice, afford vinous liquors from which ardent spirits, under
various names, are obtained; as arrack, &c.; the quantity being about 50 pounds of
alcohol of 0·825 for every 100 pounds of solid saccharine extract present. Honey
similarly treated affords the metheglin so much prized by our ancestors. Good whey,
freed from curd by boiling, will yield 4 per cent. of spirit of wine, when fermented with
the addition of a little yeast.
2. The juices of apples, pears, currants, and such fruits, afford by fermentation
quantities of alcohol proportional to the sugar they contain. But the quality of the
spirit is much better when it is distilled from vinous liquids of a certain age, than from
recently fermented must. Cherries are employed in Germany, and other parts of the
Continent, for making a high-flavoured spirit called Kirsch-wasser, or cherry water.
The fully ripe fruit is crushed by a roller press, or an edge-stone mill, along with the
kernels; the pulp is fermented in a mass, the liquid part is then drawn off, and distilled.
More or less prussic acid enters from the kernels into this spirit, which renders it
very injurious, as a liquor, to many constitutions. I was once nearly poisoned by
swallowing a wine glass of it in the valley of Chamouni. The ripened red fruit of the
mountain ash constitutes a good material for vinous fermentation. The juice being
mixed with some water and a little yeast, affords when well fermented, according to
Hermstaedt, 12 pounds, or 11⁄2 gallons, of alcohol from 2 bushels of the ripe berries.
3. Many roots contain sugar, particularly beet, from which no less than 7 per
cent. of it may be extracted by judicious means. Hermstaedt recommends to mash the
steam boiled clean roots, and add to the paste two-thirds of its weight of boiling water,
and a thirtieth of its weight of ground malt, mixing the materials well, and then leaving
them three hours in a covered vessel. The mixture must now be passed through a wire
sieve, with meshes of one-third of an inch square each; the residuum is washed with a little
cold water, and, when the temperature has fallen to 77° F., the proper quantity of yeast
must be added, and the fermentation suffered to proceed in a covered tun. In 5 or 6
days it will be complete, and will afford by distillation, from 100 pounds of beet root,
about 10 or 12 pounds of proof spirits. Carrots and parsnips, when similarly treated,
yield a considerable quantity of alcohol.
II. Ardent spirits or whiskey from fecula or starchy materials.
I have already pointed out, in the article Beer, how the starch is transformed into a
saccharine condition, by malting and mashing; and how a fermentable wort may be
obtained from starchy meal. By like operations may all vegetable substances, which
consist chiefly of starch, become materials for a whiskey distillery. To this class belong
all the farinaceous grains, potatos, and the pods of shell fruits, as beans, vetches, horse-chesnuts,
acorns, &c.
1. Whiskey from corn. All those species of corn which are employed in breweries
answer for distilleries; as wheat, rye, barley, and oats; as well as buckwheat, and maize or
Indian corn. The product of spirits which these different grains afford, depends upon
the proportion of starch they contain, including the small quantity of uncrystallizable
sugar present in them. Hermstaedt, who has made exact experiments upon the subject,
reckons a quart (Prussian or British) spirits, containing 30 per cent. of the absolute
alcohol of Richter, for 2 pounds of starch. Hence 100 pounds of starch should yield[399]
35 pounds of alcohol; or 4·375 gallons imperial, equal to 7·8 gallons of spirits, excise
proof.
100 pounds of the following grains afford in spirits of specific gravity 0·9427, containing
45 per cent. of absolute alcohol (= 9⁄11 of British proof,) the following quantities:—
Wheat, 40 to 45 pounds of spirits; rye, 36 to 42; barley, 40; oats, 36; buckwheat,
40; maize, 40. The mean of the whole may be taken at 40 pounds, equal to 41⁄4
gallons imperial, of 0·9427 specific gravity = 3·47 gallons, at excise proof. The chief
difference in these several kinds of corn consists in their different bulks under the same
weight; a matter of considerable importance; for since a bushel of oats weighs little
more than the half of a bushel of wheat, the former becomes for some purposes less convenient
in use than the latter, though it affords a good spirit.
Barley and rye are the species of grain most commonly employed in the European
distilleries for making whiskey. Barley is mostly taken either partly or altogether in
the malted state; while the other corns are not malted, but merely mixed with a certain
proportion of barley malt to favour the saccharine fermentation in the mashing. It is
deemed preferable to use a mixture of several sorts of grain, instead of a single one; for
example, wheat with barley and oats; or barley with rye and wheat; for the husks of
the oats diffused through the wheat flour and rye meal keep it open or porous when
mashed, and thus favour the abstraction of the wort; while the gluten of the wheat
tends to convert the starch of the barley and oats into sugar. When the whole of the
grain, however, is malted, a much more limpid wort is obtained than from a mixture of malt
with raw grain; hence the pure malt is preferable for the ale and porter brewer, while
the mixture affords a larger product, at the same cost of materials, to the distiller.
When barley is the only grain employed, from one-third to one-sixth of malt is usually
mixed with it; but when wheat and rye are also taken, the addition of from one-eighth
to one-sixteenth of barley malt is sufficient. Oats are peculiarly proper to
be mixed with wheat, to keep the meal open in the mashing.
The following are the proportions used by some experienced Scotch distillers.
| 250 |
bolls, containing 6 bushels each, being used for a mashing, consist of, |
| 25 |
bolls of |
oats, |
weighing |
284 |
lbs. per boll, or |
47 |
1⁄3 |
lbs. per bushel; |
| 42 |
|
malt |
|
240 |
|
40 |
|
| 25 |
|
rye |
|
320 |
|
53 |
1⁄3 |
|
| 158 |
|
barley |
|
320 |
|
53 |
1⁄3 |
|
| 250 |
mean |
48 |
1⁄2 |
|
From each boll, weighing 291 lbs., 14 imperial gallons of proof whiskey are obtained
on an average; equivalent to 11·2 gallons at 25 over proof.
The malting for the distilleries is to be conducted on the same principles as for the
breweries, but the malt ought to be lightly kiln-dried, and that preferably at a steam
heat, instead of a fire, which is apt to give an empyreumatic smell to the grain that
passes into the spirits. For such persons, indeed, as relish the smell of burned turf,
called peat-reek in Scotland, the malt should be dried by a turf fire, whereby the
whiskey will acquire that peculiar odour.
But this smell, which was originally prized as a criterion of whiskey made from pure
malt, moderately fermented and distilled with peculiar care, has of late years lost its
value, since the artifice of impregnating bad raw grain whiskey with peat-smoke has
been extensively practised.
Dr. Kolle, in his treatise on making spirits, describes a malting kiln with a copper
plate heated with steam, 18 feet long, and 12 feet broad, on which a quantity of malt
being spread thin, is changed every 3 or 4 hours, so that in 24 hours he turns out
upwards of 28 cwt. of an excellent and well-kilned article. The malt of the distiller
should be as pale as possible, because with the deepening of the colour an empyreumatic
principle is generated.
When Indian corn is the subject of distillation, it must be malted in the same way as
described in the article Beer. According to Hermstaedt, its flour may be advantageously
mixed with the crushed malt in the mash tun. But its more complete dissolution
may be accomplished by Siemen’s mode of operating upon potatos, presently to
be described.
1. Mashing. Barley and raw grain are ground to meal by millstones, but malt is
merely crushed between rollers. If only one-tenth or one-eighth of malt be used with
nine-tenths or seven-eighths of barley, some husks of oats are added, to render the mash
mixture more drainable.
When 40 bushels of barley and 20 of malt form one mashing, from 600 to 700
gallons of water, heated to 150° F., are mixed with these 60 bushels in the mash tun,[400]
and carefully incorporated by much manual labour with wooden oars, or in great concerns
by the mechanical apparatus used in the breweries. This agitation must be continued
for 2 or 3 hours, with the admission from time to time of about 400 additional
gallons of water, at a temperature of 190°, to counteract the cooling of the materials.
But since the discovery of diastase, as the best heat for saccharifying starch is shewn to
be not higher than 160° F., it would be far better to mash in a tun, partially, at least,
steam encased, whereby we could preserve the temperature at the appropriate degree
for generating the greatest quantity of sugar.
If the wort be examined every half-hour of the mashing period, it will be found
to become progressively sweeter to the taste, thinner in appearance, but denser in
reality.
The wort must be drawn off from the grains whenever it has attained its maximum
density, which seldom exceeds 150 lbs. per barrel; that is, 360 + 150360 = 1·42, or 42
per cent. As the corn of the distiller of raw grain has not the same porosity as the
brewer’s, the wort cannot be drawn off from the bottom of the tun, but through a series
of holes at the level of the liquor, bored in a pipe stuck in at the corner of the vessel.
About one-third only of the water of infusion can thus be drawn off from the pasty
mass. More water is therefore poured on at the temperature of 190°, well mixed by
agitation for half an hour, then quietly infused for an hour and a half, and finally drawn
off as before. Fully 400 gallons of water are used upon this occasion, and nearly as
much liquor may be drawn off. Lastly, to extract from the grains every thing soluble,
about 700 gallons of boiling hot water are turned in upon them, thoroughly incorporated,
then left quietly to infuse, and drawn off as above. This weak wort is commonly
reserved for the first liquor of the next mashing operation upon a fresh quantity of meal
and malt.
The English distiller is bound by law to make his mixed worts to be let down into
the fermenting tun of a specific gravity not less than 1·050, nor more than 1·090; the
Scotch and Irish distillers not less than 1·030, nor more than 1·080; which numbers
are called, gravity 50, 90, 30, and 80, respectively.
With the proportion of malt, raw grain, and water, above prescribed, the infusion first
drawn off may have a strength = 20 per cent. = spec. grav. 1·082, or 73 lbs. per
barrel; the second of 50 lbs. per barrel, or 14 per cent.; and the two together would
have a strength of 61·2 lbs. per barrel = 17 per cent., or spec. grav. 1·070. From experiments
carefully made upon a considerable scale, it appears that no more than four-fifths
of the soluble saccharo-starchy matter of the worts is decomposed in the best regulated
fermentations of the distiller from raw grain. For every 2 lbs. so decomposed, 1 lb. of
alcohol, spec. grav. 0·825 is generated; and as every gallon of spirits of the spec. grav.
0·909 contains 4·6 lbs. of such alcohol, it will take twice 4·6 or 9·2 lbs. of saccharine
matter to produce the said gallon. To these 9·2 lbs., truly transmuted in the process,
we must add one-fifth, or 1·84 lbs., which will raise to 11·04 the amount of solid matter
employed in producing a gallon of the above spirits.
Some distillers mash a fourth time; and always use the feeble wort so obtained in
mashing fresh grain.
2. As the imperfect saccharine infusion obtained from raw grain is much more acescent
than the rich sugary solution got from malt in the breweries, the distiller must
use every precaution to cool his worts as quietly as possible, and to keep them clear from
any acetous taint. The different schemes of cooling worts are considered under Beer
and Refrigeration. As the worts cool, a quantity of starchy matter is precipitated, but
it is all carefully swept along into the fermenting tun, and undoubtedly contributes to increase
the production of alcohol. During the winter and temperate months, when the
distilleries are most actively at work, the temperature at which the worts are set is
usually about 70° F. When much farinaceous deposit is present, the heat may be only
65°, because, in this case, a slow fermentation seems to favour the conversion of that
starch into sugar. In some German distilleries a little chalk is mixed with the worts, to
check acidity.
3. The fermentation.
The yeast added to the worts as a ferment, ought to be the best top barm of the
London porter breweries. About 1 gallon of it is requisite for every 2 bushels of meal
and malt worked up in the mashing process; and of this quantity only a certain proportion
is introduced at the beginning; the remainder being added by degrees, on the
second and third day.
Should the fermentation flag, a little more may be added on the fourth or fifth day,
and the contents of the tun may be roused by an agitator. About 8 or 9 gallons may
be introduced four days in succession to the quantity of worts extracted from 60 bushels
of the farinaceous materials; or the third day’s dose may be intermitted, and joined to
the fourth on the subsequent day.
[401]
Great diversity, and no little caprice prevail among distillers in respect of the periods of
administering the yeast; but they should be governed very much by the appearance of
the fermentation. This process continues from nine to twelve or even fourteen days,
according to circumstances; the tuns being left quite open during the first five days, but
being covered moderately close afterwards to favour the full impregnation of the liquor
with carbonic acid, as a fermenting agent. In consequence of the great attenuation of
the wort by the generation of so much alcohol, no good body of yeast continues to float
on the surface, and what is formed is beat down into the liquor on purpose to promote
the fermentation. The temperature of the wash gradually increases till towards the end
of the fourth day, when it attains its maximum height of about 25° above the pitch of
55° or 60° at which it may have been set. The time of the greatest elevation of temperature,
as well as its amount, depends conjointly upon the quality of the yeast, the
nature of the saccharo-starchy matter, and the state of the weather. It is highly probable
that the electrical condition of the atmosphere exercises a considerable influence
upon fermentation. We know the power of a thunderstorm to sour vinous fluids. An
experimental inquiry into the relation between electricity and fermentation, could not
fail to prove both curious and profitable.
The diminution of the density of the wort is carefully watched by the distiller, as the
true criterion of the success of his process. This attenuation, as he calls it, is owing
partly to the decomposition of the sugar, which communicated its gravity to the solution,
and partly to the introduction of the lighter alcoholic particles. Were all the saccharo-starchy
matter resolved into gaseous compounds, the wort would become water; but
since a part of it remains undecomposed, and a portion of alcohol is produced at the
expense of the decomposed part, the degree of attenuation becomes a somewhat complicated
problem in a theoretical point of view; the density due to the residuary sugar
being masked and counteracted by the spirit evolved. Could the alcohol be drawn off
as it is formed, the attenuation would probably become greater, because the alcohol
checks the fermentative action, and eventually stops it, before all the saccharum is
decomposed. After the wash has taken its highest degree of temperature, not much
more spirit is found to be generated; were this therefore removed by proper means,
the remaining vegetable matter would undoubtedly yield a further product of alcohol.
In the attenuation of raw-grain wash, the specific gravity seldom arrives at 1·000;
but most commonly stops short at 1·002 or 1·004. When the vinous fermentation
comes to an end, the acetous is apt to commence, and to convert a portion of the alcohol
into vinegar; a result which is easily ascertained by the increasing specific gravity, sour
smell, and acidulous reaction of the wash upon litmus paper, which remains after the
paper is heated, showing that the red colour is not caused by carbonic acid.
Fermentation proceeds with more uniformity and success in the large tuns of the distiller,
than in the experimental apparatus of the chemist; because the body of heat
generated in the former case maintains the action. But I have succeeded in obviating
this inconvenience in operating upon 80 or 90 gallons, by keeping up the temperature,
when it begins to flag, by transmitting hot water through a recurved pipe plunged into
the tun.
We have already mentioned that one gallon of spirits, one in ten over-proof, is upon
the average generated from 11·04 libs. of starch sugar; hence we conclude that one
pound water-measure of spirits at proof (= 1⁄10 imperial gallon) is produced from one
pound of the saccharum.
Malt whiskey.—The treatment and produce of malt distilleries are in some respects
different from those of raw grain. Having been professionally employed by the
proprietors of both, I am prepared to state the peculiarities of the latter, by an
example. 500 bushels of ground malt are first mashed with 9000 gallons of water,
heated to the temperature of 160° F.: 6000 gallons of worts are drawn off into the
coolers, and let down into the fermenting tun at 68°. From 3 to 4 per cent. of a
mixture of London porter yeast with quick Scotch barm, are added, and well stirred
through the mass. At the end of two or three days, in general, the fermentation is
finished. On the residuary grains of the malt, from 4500 to 5000 gallons of water at
180° are run, which after proper mashing as before, are drawn off; then 4500 more are
poured on, the drainage of which is added to the second. Both of these together, constituting
9000 gallons, are heated next day, and employed for the mashing of 500 bushels
of fresh malt. During the fermentation, the wash which was set at the spec. grav. 1·065,
comes down to water = 1·000.
The wash is distilled in two stills, appropriated to it, of about 800 gallons capacity
each, provided with a rotatory chain apparatus for preventing the lees from adhering to
the bottom of the still. Into about 800 gallons of wash 8 lbs. of soap are put. The
liquor obtained at this first distillation is called low-wines. These low-wines are redistilled
in the spirit stills; the first and last portions of liquid being more or less blue or
milky in colour, and rank in flavour, are run into a separate receiver called the faints-back;[402]
while the middle portion, constituting in a well-managed distillery, from three-fourths to
four-fifths of the whole, are received into the spirit-back. The faints are mixed with a large
quantity of water, and redistilled, in order to free them from the fetid oil derived from
the husks of the grain. The interception of this noxious oil may be best effected by a
self-regulating bath, between the capital of the still and the refrigeratory, as will be
explained in treating of Stills. The capitals of the common Scotch stills are made from
15 to 20 feet high, in order to prevent the chance of the wash boiling over into the
worm; and they are, towards the beginning of the process, struck from time to time
with a rod, and by the sound emitted it is known whether they be empty, partially
filled, or in danger of an overflow; in which case the fire is damped, by a spout
near the furnace door, connected by a leather pipe with an elevated reservoir of water.
When very pure spirits are wished for, a third or even a fourth distillation is had recourse
to; there being a quantity of water mixed each time with the spirit in the still, to
prevent its acquiring a harsh alcoholic flavour.
According to some experienced distillers from raw grain, the mashing temperature of
the first liquor should not exceed 140° F.; whereas with malt it may be safely and
beneficially 165° or 170°. When rye is used instead of malt, 90 bushels of it are mixed
with 190 bushels of raw grain, constituting 280 bushels in whole, for the mashing of
which 5200 gallons of water are required. An hour and a half more time is necessary
for settling the mashing of the above mixture, than of grain alone. Gin is made in this
way.
The distiller of malt whiskey calculates on obtaining two gallons of proof spirits from
one bushel of malt, in average years. The highest yield is 20 gallons per quarter of 8
bushels; and the lowest is 16, when the malt and fermentation are indifferent. The best
temperature to set the fermenting tuns with malt wash is about 70° or 72° F.
When malt is 5s. the bushel, 6 bushels at 30s. will yield 12 gallons of proof spirits.
These cost therefore 2s. 6d. per gallon for the malt; to which must be added 3d. per
bushel for the amount of malt duty not returned, or 11⁄2d. on the gallon; this added to the
Scotch duty of 3s. 4d. the gallon, makes the price altogether 5s. 111⁄2d.; besides the expenses
in fuel, yeast, labour, and rent, which may be estimated at 81⁄2d. per gallon. But 3d.
may be deducted for what is paid by the dairymen for the spent wash and grains. The
total cost, therefore, exclusive of use of capital, is 6s. 5d. per gallon in Scotland.
The following is the work of a Scotch distillery, where good malt whiskey was
made.
One bushel of the malt weighed 35 libs., or the boll, = 6 bushels, 210 libs. In
mashing each boll of malt, 110 gallons of water were run on it at 160° F. As soon
as the fermenting tun of 3000 gallons capacity was charged with the wash at from 64°
to 74° F., 2 gallons per cent. of barm were added. When the wash had become attenuated
from 1·060 to 1·040, another gallon of barm was introduced.
The temperature of the fermenting wash sometimes rises to 96°, which is, however,
an extreme case, and not desirable. When the bubbles of carbonic acid mount in rapid
succession, it is reckoned an excellent sign. If the tun be small, and stand in a cool
apartment, it should be started at a higher temperature than in the reverse predicament.
Should the fermentation be suffered to flag, it is in general a hopeless task to restore
vigorous action. Some try the addition of bubs, that is of some wort brought into a state
of rapid fermentation in a tub, by a large proportion of yeast, but seldom with much
success. Indeed the law prohibits the addition of any wort to the tun at a later period
than 24 hours after it is set; so that if bubs are used afterwards, the distiller is apt to
incur a penalty.
The maximum quantity of proof spirits obtained on the great scale at any time from
raw grain mixed with from one-fourth to one-eighth of malt, seems to be 22 gallons per
quarter.
By the British laws a distiller is not allowed to brew and distil at the same time
but he must work alternately, one week, for instance, at fermentation, and next week at
distillation.
In fermenting solutions of sugar mixed with good yeast, the attenuation has been
carried down to 0·984, and even 0·982, that is, in the language of the excise, 16 and 18 degrees
below water, from 1·060, the density at which it was originally set in the tun. This
was excellent work done on the scale of a great distillery nearly 30 years ago, when
distillation from sugar was encouraged, in consequence of bad corn harvests.
In an experiment which I made in 1831 for the information of a committee of the
House of Commons, on the use of molasses in the breweries and distilleries, I dissolved
1 cwt. of raw sugar in water; so as to form 741⁄2 gallons, inclusive of 2 gallons of yeast.
The specific gravity of the mixture was 1·0593 on the 31st of March. By the 6th of
April, that is in 6 days, the gravity had sunk to 0·992, or 8 degrees under water, which
was reckoned a good attenuation, considering the circumstances and the small quantity
operated upon. By distillation it afforded at the rate of 14·875 gallons of proof spirits
for 100 gallons of the wash.
[403]
When the distillers first worked from sugar, they only obtained upon an average
from 1 cwt. 10·09 gallons imp. of proof spirit; but they afterwards got no less than
11·92 imp. gallons.
The following experiment, which I made upon the fermentation of West India molasses
into spirits, for the information of the said committee, may prove not uninteresting to
my readers. 150 libs. were dissolved in water and mixed with 2 gallons of yeast,
weighing exactly 20 libs. The wash measured 70 gallons, and had a spec. gravity of
1·0647 at 60° F. In two days the gravity had fallen to 1·0055; in three days to
1·0022; and in five days to 1·001. The temperature was kept up at from 80° to 90° F.,
during the two last days, by means of a steam pipe, to favour the fermentation. The
product of spirits was 11 gallons, and 35⁄100 of a gallon. Now 150 libs. of the above
molasses were found to contain of solid matter, chiefly uncrystallizable, 112 libs. And as
112 libs. of sugar are estimated by the revenue laws to afford by fermentation 111⁄2 gallons
imp. of proof spirit, the result of that experiment upon molasses must be considered
satisfactory, bearing in mind that the saccharine substance in molasses has been not only
partially decomposed by heat, but is mixed with some of the glutinous or extractive
matter of the cane.
Since the alteration of the excise laws relative to distillation in 1825 and 1826, when
permission was given to set the wort at lower gravities, the quantity of spirits produced
from 1 quarter of corn has been much increased, even up to fully 20 gallons; and the
proportion of malt has been much diminished. The latter was soon reduced from three-sevenths
malt, and four-sevenths barley, or two-fifths malt and three-fifths barley, to
one-fifth of malt, and now to one-tenth or even one-sixteenth.
A discussion having lately taken place in Ireland between certain persons connected
with the distilleries and the officers of the excise, whether, and to what extent, raw
grain worts would pass spontaneously into the vinous fermentation, the Board in
London requested me to superintend a series of researches in a laboratory fitted up at
their office, to settle this important point. I shall content myself here with giving the
result of one experiment, out of several, which seems to me quite decisive. Three
bushels of mixed grains were taken, consisting of two of barley, one half of oats, and one
half of malt, which, being coarsely ground by a hand-mill, were mashed in a new tun
with 24 gallons of water at 155°. The mash liquor drawn off amounted to 18
gallons, at the density of 1·0465; and temperature of 82° F. Being set in a new tun,
it began to ferment in the course of 12 hours, and in 4 days it was attenuated down to
gravity 1·012. This yielded, upon distillation in low wines, 3·22 gallons, and by rectification,
in spirits, 3·05; while the quantity equivalent to the attenuation by the
tables was 3·31, being an excellent accordance in such circumstances.
The inquisitorial regime imposed by law upon our distilleries, might lead a stranger
to imagine that our legislators were desirous of repressing by every species of annoyance
the fabrication of the fiery liquid which infuriates and demoralizes the lower population
of these islands. But alas! credit can be given them for no such moral or philanthropic
motive. The necessity of the exchequer to raise a great revenue, created by the wasteful
expenditure of the state, on the one hand, and the efforts of fraudulent ingenuity on the
other, to evade the payment of the high duties imposed, are the true origin of that regime.
Examinations in distilleries are constantly made by the officers of excise. There is a
survey at 6 o’clock in the morning, when the officers take their accounts and gauges,
and make calculations which occupy several hours. At 10 o’clock they again survey,
going over the whole premises, where they continue a considerable time, frequently till
the succeeding officer comes on duty; at 2 in the afternoon another survey takes place,
but not by the same people; at 6 in the evening the survey is repeated; at 10 there
comes another survey by an officer who had not been engaged in any of the previous
surveys of that day. He is not relieved till 6 o’clock next morning. In addition to
these regular inspections, the distilleries are subject to frequent and uncertain visits of
the surveyor and general surveyor. “We are never,” says Mr. Smith, the eminent
distiller of Whitechapel, “out of their hands.”[24]
Before the fermented wort goes into the still, a calculation is made of the quantity
of wash drawn from the wash back, and which is first pumped into what is called the
wash charger. If the quantity in the wash charger exceeds the quantity in the wash
back, the distiller is charged upon the higher quantity; if it contains less, he must pay
according to the wash back, as being the larger quantity. When the quantity of wash is
all transferred to the charger, the discharge cock of the wash charger is unlocked, and
the wash is allowed to be drawn off from the charger into the still, the charging and
discharging cock of the still being locked by the officer. There can be no transfer of
wash but through the pumps, which are locked also. The first distillation from the
wash is worked into the low-wine receiver, which is also a locked-up vessel; then of[404]
those low wines, the strength and quantity are ascertained by the excise. The account
of them affords a comparison with the quantity which the contents of the wash-back had
been estimated to produce; they are then pumped from the low-wine receiver, through
pumps previously locked into the low-wine charger, which is also a locked-up vessel;
from the locked-up charger, after the officer has done his duty regarding it, they are allowed
to be drawn off into the low-wine still, which is a distillation of the second extraction;
then that low wine still works into another locked-up cask, called the spirit
receiver, for the receiving of raw spirits; when that distillation is finished, the officer,
attending again on regular notice for that purpose, takes the quantity and strength of
the spirits therein, and upon the quantity so ascertained he charges the duty. In distilling
low wines, one portion of them goes into the spirit receiver, and a portion into
what is called the faint receiver, which is another locked-up vessel. These faints are
in the next distillation united with the low wines, from the succeeding wash-back on
their second distillation, and are worked together; the united produce of these goes
partly into the spirit cask, and partly back again into the faint cask. The operation is
thus continued till all the backs in the house are emptied.[25]
There is a kind of ardent spirits manufactured in Holland, vulgarly called Dutch gin,
Hollands, and sometimes geneva, from genievre, the French for juniper, a plant with the
essential oil of whose berries it is flavoured. One cwt. of ground malt mixed with two
cwt. of rye meal are mashed for two hours, with about 450 gallons of water at the temperature
of 160° F. The mash drawn off is reduced with cold water till the liquid part
has the density of 45 libs. per barrel, = specific gravity 1·047; and is then put altogether
into the fermenting back at the temperature of 80° F. One or two gallons of yeast are
added. The fermentation soon becomes so vigorous as to raise the heat to 90° and upwards,
but it is not pushed far, being generally over in two days, when the gravity of the wash, still
indicates 12 pounds of saccharum per barrel. By this moderate attenuation, like that
practised by the contraband distillers of the Highlands of Scotland, it is supposed that
the fetid oil of the husks is not evolved, or at least in very small quantity. The grains
are put into the alembic along with the liquid wash, and distilled into low wines, which are
rectified twice over, some juniper berries and hops being added at the last distillation.
But the junipers are sometimes bruised and put into the mash. The produce of worts so
imperfectly fermented, is probably little more than one half of what the British distiller
draws from the same quantity of grain. But the cheapness of labour and of grain, as
well as the superior flavour of the Schiedam spirits, enables the Dutch distiller to carry on
his business with a respectable profit. In opposition to the above facts, Dubrunfaut says
that about one third more spirits is obtained in Holland from grain than in France, because
a very calcareous spring water is employed in the mashing operation. Were this
account well founded, all that the distillers of other countries would have to do would
be merely to introduce a portion of chalk into their mash tuns, in order to be on a par
with the Dutch. But the statement is altogether a mistake.
In the vine countries, the inferior wines or those damaged by keeping, as also a fermented
mash of the pressed grapes, mixed with water, are distilled to form the eau de
vie de Cognac of the French, called Brandy in this country. It contains less essential
oil, and that of a more agreeable flavour, than corn spirits. See Brandy.
Berzelius says that there are distillers who are guilty of putting a little arsenious acid
into the still; that the spirits contain pretty frequently traces of arsenic, which may
be detected by adding to them a little muriatic acid, then evaporating off the alcohol,
and passing a current of sulphuretted hydrogen gas through the residuary liquid, which
will give it the characteristic orpiment yellow tinge, arsenic being present. Copper, which
is sometimes introduced into distilled grain, or even malt spirits, in consequence of the
soap employed in the process of distillation, may be detected best by the brown precipitate
which it occasions with ferroprussiate of potash. No arsenic is ever used in this country.
When damaged grain has been mashed in making whiskey, a peculiar oily substance
makes its appearance in it. On approaching the nostrils to such whiskey slightly heated,
this volatile matter irritates the pituitary membrane and the eyes very powerfully. These
spirits have exactly the smell of an alcoholic solution of cyanogene; they intoxicate more
powerfully than pure alcohol of equal strength, and produce even temporary frenzy,
with subsequent sickness and disordered functions. This volatile body is not cyanogene,
though it be so like it, for it forms no such combinations as cyanogene does. It
may be extracted from diluted alcohol by agitating it with an unctuous oil, and then
distilling the oil along with water. At the end of 3 or 4 months, this volatile matter
disappears in a great measure, even when the spirits impregnated with it are inclosed
in well-corked bottles; obviously from its undergoing a spontaneous decomposition.
It may be preserved much longer in the state of a watery solution.
When acetic ether is added to well purified or clean spirits, such as the distillers call[405]
silent whiskey, it gives it somewhat of the flavour of brandy. For this purpose, also,
the spirits are rectified from bruised prunes, or the lees of the cognac distilleries,
whereby they acquire additional flavour. The astringent taste of old brandy is imitated
by the introduction of a little catechu into the British spirits. Burned sugar is employed
as a colouring in these imitations.
IV. Of making whiskey from potatos.—This root in certain localities where it
abounds at a moderate price, is an excellent material for fermenting into alcohol. When
sound, it possesses from 20 to 25 per cent. of solid substance, of which starch constitutes
at least three-fourths; hence 100 pounds contain from 16 to 22 pounds of starch susceptible
of being saccharified. In the expressed juice there is a small quantity of tartaric
acid.
Previously to mashing, potatos must be first well washed in a horizontal cylindrical
cage revolving partially in a trough of water, as will be described in treating of
the manufacture of sugar from beet root. They must be then boiled in a close
vessel with steam, provided with a perforated bottom a few inches above the real
one. The top has an opening with a cover fitted tightly to it; through that the potatos
are introduced; and immediately above the false bottom there is a similar aperture
through which the boiled potatos are taken out. The steam-pipe enters at the top,
runs down the side a little way; and terminates in a widened mouth. The large lids
are secured by cross bars, the small hole by folds of linen. In the lower valve there are
two small holes closed with pins, for inserting a wire to feel whether the potatos be sufficiently
boiled. If so, the steam is immediately stopped off, the lower lid is removed, and
the potatos pulled out with a
hook into a tub. They must
be immediately made into a
homogeneous paste before they
get cold. Fig. 361. represents,
in plan, or horizontal section,
the apparatus used in France
for this purpose. A B are two
cylinders covered with wire
cloth, but open at the ends;
C C and D D are two pieces of
wood fixed on the two axes,
in the form of two cones, with
the adjoining surfaces truncated;
upon which, as also
upon iron rings E F, of the
same diameter, made fast to the axes, the wire cylinder rests. Of the two wheels G, H,
the smaller has 18, the greater has 21 teeth. The diameter of each cylinder is 14
inches, the length 18. Above and between the two cylinders, there is a hopper for the
reception of the boiled potatos. This machine triturates 1200 pounds of potatos per
hour. Their paste must be forthwith mashed with some ground wheat or barley, and
a proportion of malt; then be set a fermenting.
As in the above mode of trituration, the potatos are apt to cool to such a degree as
to obstruct their ready admixture with water, it is better to make them into a paste
in the vessel in which they are steamed. The apparatus contrived by Siemens fully answers
this end. It consists essentially of a tub A, represented in fig. 362. in section. It
is cylindrical, and made of planks from 3 to 4 inches thick, joined firmly and steam-tight;
the upper and under ends being well secured with iron hoops. The lower part is about
2 inches more in diameter than the upper. About a foot from the bottom, in a circular
groove, a cast iron partition W or disc full of holes is made fast, which serves the purpose
of a scarce, the apertures being an inch asunder; above, from 1⁄8 to 1⁄10 of an inch in diameter,
and below, scooped out to half an inch. This disc is half an inch thick in the
edges, and five fourths of an inch in the middle.
Through the female screw a in the top of the cylinder, there passes the screwed
rod b, one and a half inches thick, provided at top with a strong cross bar C C, for
turning it round. The under end of this rod has a square piece terminating in a
short screw, upon which a wrought iron cross is secured by means of a screw
nut, so as to stand at right angles to the rod. This cross is composed of two distinct
arms; of which one of them is mounted on the upper side with little knives an
inch and a half long; the other, upon the under side, with a wire brush, that may
be made to rub against the perforated cast iron disc. On the side of the cylinder at E,
fig. 362., there is a narrow aperture provided with a bung secured by a cross bar, and
near the bottom at H there is another like it. Both openings serve for taking out the residuary
matter. Through the opening E, the above two arms are introduced; and
secured to the square of the rod by the screw nut. In the top there is an opening, D,[406]
for putting in the potatos which may be shut in the same way. From the lid there
likewise issues a lateral tube F, which terminates in a tubful of water, for condensing
the waste steam. G is the tube connected with the steam boiler, for conducting the
steam into the space under the iron disc W.
With this apparatus the potatos are prepared as follows: when the screw rod is so
fixed that the cross touches the disc, the cylinder is to be filled with washed potatos to
within one foot of the top, leaving them some space to expand. The orifice D is to be
then closed, and the steam admitted. When the potatos are boiled enough, two labourers
lay hold of the lever handles C C, of the screw rod b, and turn it round with the effect of
screwing up the spiked cross, and of triturating the potatos; an operation which may be
still more effectually done by screwing it down again. The potato paste is now let off by
the plug hole H, into the tub L, where it is mixed with about 30 per cent. of boiling
water, and one thousandth part of potash, made caustic with quicklime, in order to
dissolve the albuminous matter coagulated by the heat, and give complete fluidity to the
mass. The alkali also neutralises the tartaric acid present. The mashed matter must now
be mixed with the crushed malt diffused through 40 or 50 pounds of cold water for
every 100 pounds of potatos, which lowers the temperature to 167°. The wort must
be then diligently stirred during two hours; mixed with 40 or 50 pounds of cold
water for 100 pounds of potatos, and when reduced to the temperature of 77° put into
the fermenting tun along with the proper quantity (3 or 4 per cent.) of yeast. As potatos
readily pass into the acetous fermentation, the admixture of the malt, the mashing,
and the cooling should be rapidly performed, while the utmost cleanliness must be observed.
The fermentation is brisk, probably from the agency of the albumen, and furnishes a
good head of barm, which answers well for the bakers; 100 pounds of potatos yield
from 18 to 20 pounds measure of spirits, nine elevenths of our excise proof; or about
16 pounds measure of proof, = about 12⁄3 gallons.
It has been observed that after the month of December potatos begin to yield a smaller
product of fermented spirits; and when they have once sprouted or germinated, they
afford very little indeed. From the difficulty of keeping and transporting potatos, distillation
from them, even though our laws now permit it, can never become general till some
plan be adopted for overcoming these disadvantages. A scheme of this kind, however, has
been successfully practised in Vienna, which consists in subjecting the washed potatos
to strong pressure in a perforated chest by a hydraulic or screw press, whereby they lose
about three fourths of their weight, and may then be readily dried into a white flour,
that may be kept for several years without injury, and transported to considerable distances
with comparative ease. This flour, mixed with a moderate quantity of ground
malt, and saccharified by mashing with water, at the temperature of 167° F., becomes
capable of affording a sweet wort convertible by fermentation either into beer or
whiskey.
Horse-chestnuts, according to Hermstaedt, are an eligible material for producing
alcohol, as 128 pounds of them afford 100 pounds of meal; which 100 pounds yield, by
proper treatment, 34 pounds of spirits, containing 36 per cent. of absolute alcohol, by
Richter’s tables. Barley to the extent of 10 pounds per 100 should be ground up with
them, after they have been boiled in a steam apparatus, not only for the purpose of
softening them, but freeing them from their bitter astringent matter. Acorns are
productive of alcohol by similar treatment.
The best means hitherto discovered for depriving bad whiskey of its nauseous smell
and taste, is to pass it through well-burned and coarsely pulverised charcoal, distributed
as follows in a series of cylindrical casks. Each vessel must have a double bottom,
the false one being perforated with conical holes, and placed a few inches above the
true. Upon this perforated board a layer of chopped clean straw one inch thick is laid;
and over the straw, a stratum of small river gravel, the size of large peas. This is to be
covered with a pretty thick stratum of the charcoal, previously freed from dirt and dust
by washing; upon which a piece of close canvass is to be spread, and pressed down by
a thin bed of river sand. The cylinder or cask should be filled with these successive
layers to within two inches of its top, and it is then to be closed air-tight. Immediately
below the head, a round orifice is pierced in the side, for receiving an overflow tube,
which is either screwed rectangularly to another elbow pipe, or is bent (when of block
tin) so as to enter tight into an orifice beneath the false bottom of the second cylinder or
cask. In this way, the series may be continued to any desired number of vessels; the
last discharging the purified spirit into the store-back. The foul spirit must be made to
flow into the bottom space of the first cylinder down through a pipe in communication
with a charging-back placed upon such an elevated level as to give sufficient pressure to
force the spirits up through the series of filters; the supply-pipe being provided with a regulating
stop-cock. The spirit may be filtered downwards through sand and cloth in[407]
its final passage to the receiver. It has been found, with very crude spirits, that eight
successive cylinders were required to deprive them entirely of the rank flavour.
In the year 1831, 23,000,000 gallons of spirits were made in the United Kingdom,
equivalent to the consumption of 1,500,000 quarters of grain, and for that year and the
four preceding years, there were imported annually 2,000,000 of quarters of foreign
barley.
| In |
1832, |
20,778,521. |
gallons paid excise duty. |
| |
1834, |
23,397,806. |
|
| |
1836, |
27,137,000; |
of which 14,000,000 were Irish. |
We may add to the last quantity, 3 millions of gallons at least on the score of smuggling,
in licensed and illicit distilleries; making 30 millions to be the frightful amount
of whiskey consumed by the British people, independent of other intoxicating liquors.
DOCIMACY, from the Greek Δοκιμαζω, I prove; (Docimasie, Fr.; Probierkunst,
Germ.;) is the art by which the nature and proportions of an ore are determined.
This analytical examination was originally conducted in the dry way, the metal being
extracted from its mineralizers, by means of heat and certain fluxes. But this method
was eventually found to be insufficient and even fallacious, especially when volatile
metals were in question, or when the fluxes could absorb them. The latter circumstance
became a very serious evil, whenever the object was to appreciate an ore that was
to be worked at great expense. Bergmann first demonstrated, in an elaborate dissertation,
that the humid analysis was much to be preferred; and since his time the dry
way has been consecrated chiefly to the direction of metallurgic operations, or, at
least, it has been employed merely in concert with the humid, in trials upon the
small scale.
After discovering an ore of some valuable metal, it is essential to ascertain if its
quantity and state of combination will justify an adventurer in working the mine, and
smelting its products. The metal is rarely found in a condition approaching to purity;
it is often disseminated in a mineralizing gangue far more bulky than itself; and more
frequently still it is combined with simple non-metallic substances, such as sulphur,
carbon, chlorine, oxygen, and acids, more or less difficult to get rid of. In these
compound states its distinctive characters are so altered, that it is not an easy task
either to recognize its nature, or to decide if it can be smelted with advantage. The
assayer, without neglecting any of the external characters of the ore, seeks to penetrate,
so to speak, into its interior; he triturates it to an impalpable powder, and then subjects
it to the decomposing action of powerful chemical reagents; sometimes with the aid of
alkalies or salts appropriate to its nature, he employs the dry way by fire alone; at
others, he calls in the solvent power of acids with a digesting heat; happy, if after a
series of labours, long, varied, and intricate, he shall finally succeed in separating a
notable proportion of one or more metals either in a pure state, or in a form of combination
such, that from the amount of this known compound, he can infer, with
precision, the quantity of fine metal, and thereby the probable value of the mine. The
blow-pipe, skilfully applied, affords ready indications of the nature of the metallic
constituents, and is therefore usually the preliminary test. The separation of the
several constituents of the ore can be effected, however, only by a chemist, who joins
to the most extensive knowledge of the habitudes of mineral substances, much experience,
sagacity, and precision, in the conduct of analytical operations. Under the
individual metals, as also in the articles Metallurgy, Mines, and Ores, I have endeavoured
to present such a copious and correct detail of docimastic processes, as will
serve to guide the intelligent student through this most mysterious labyrinth of nature
and art.
DORNOCK, is a species of figured linen of stout fabric, which derives its name
from a town in Scotland, where it was first manufactured for table-cloths. It is the
most simple in pattern of all the varieties of the diaper or damask style, and therefore
the goods are usually of coarse quality for common household wear. It receives the
figure by reversing the flushing of the warp and woof at certain intervals, so as to form
squares, or oblong rectangles upon the cloth. The most simple of these is a succession
of alternate squares, forming an imitation of a checker board or mosaic work. The
coarsest kinds are generally woven as tweels of three leaves, where every thread floats
over two, and is intersected by the third in succession. Some of the finer are tweels
of four or five leaves, but few of more; for the six and seven leaf tweels are seldom or
never used, and the eight leaf tweel is confined almost exclusively to damask. See
Textile Fabric.
DRAGON’S BLOOD; (Sang dracon, Fr.; Drachenblut, Germ.) is a resinous
substance, which comes to us sometimes in small balls of the size of a pigeon’s egg,
sometimes in rods, like the finger, and sometimes in irregular cakes. Its colour, in
lump, is dark brown red; in powder, bright red; friable; of a shining fracture,
sp. grav. 1·196. It contains a little benzoic acid, is insoluble in water, but dissolves[408]
readily in alcohol, ether, and oils. It is brought from the East Indies, Africa, South
America, as the produce of several trees, the Dracæna Draco, the Pterocarpus santalinus,
the Pterocarpus Draco, and the Calamus Rotang.
Dragon’s blood is used chiefly for tingeing spirit and turpentine varnishes, for
preparing gold lacquer, for tooth tinctures and powders, for staining marble, &c.
According to Herbenger, it consists of 9·07 parts of red resin, 2 of fat oil, 3 of benzoic
acid, 1·6 of oxalate, and 3·7 of phosphate of lime.
DRUGGET, is a coarse, but rather slight, woollen fabric, used for covering carpets,
and as an article of clothing by females of the poorer classes. It is now-a-days nearly
superseded by coarse cotton goods.
DRYING HOUSE. An apartment fitted up in a peculiar manner for drying
calicoes, and other textile fabrics. Mr. Southworth, of Sharples, a Lancashire bleacher,
obtained a patent, in 1823, for the following ingenious arrangement, which has been
since generally adopted, with certain modifications, in most of our extensive bleaching
and printing works. Fig. 363. is a section of the drying-house, where a is a furnace and
boiler for the purpose of generating steam; it is furnished with a safety valve in the
tube b, at top, and from this tube the steam main c passes down to the floor of the
basement story. From this main, a series of steam-pipes, as d d, extend over the surface
of the floor, and from them heat is intended to be diffused for the purpose of warming
the drying-house.
Along the middle of the building a strong beam of timber e e, extends, and is
supported by cast-iron pillars; from this beam, to bearings on the side walls, a series
of rails are carried in a cross direction, over which rails the wet cloth is to be hung in
folds, and the steam or evaporation emitted in drying is allowed to escape through
apertures or ventilators in the roof.
The mode in which the cloth is delivered on to the rails, on either side of the beam,
will be best understood by reference to the delivering carriage, which is shown, with its
rollers partly in section.
The wet cloth is first to be coiled upon a roller, and then placed in the carriage, as
at f, with its pivots bearing upon inclined planes. The carriage is to be placed at the
commencement of the rails, running upon the middle beam, and also upon the side-bearings
or railways extending along the side walls of the building, parallel to and
upon a level with the same beam. It is made to travel by means of an endless band
passing over two riggers, g and h, in fig. 363., and over pulleys and a band-wheel
attached to the carriage, as will be explained. The rigger g, which moves this endless
band, is actuated by bevel geer, seen at i, which is put in motion by a pinion at the end
of a revolving shaft leading from a steam engine.
In the same fig., k k is the endless band passing over a pulley under the band-wheel,
and over the pulley n, by which it will be perceived that the traversing of the band, as
described, would cause these pulleys and wheels to revolve. On the axle of the band-wheel
m, there is a drum against which the roll of wet cloth f presses, and as this
drum revolves, the roll of wet cloth is, by its friction, made to turn in a contrary direction,
and to deliver off the cloth on to the periphery of the drum, whence it
passes over a roller and descends to the tails. Upon the end of the axle of the[409]
band-wheel m, there is a pinion which takes into the teeth of the large wheel, and
upon the axle of this large wheel there is a pinion that actuates the intermediate wheel,
which turns another toothed wheel. This last-mentioned toothed wheel takes into
cogs upon the side railway, and hence, as the train of wheels moves round, the carriage
to which the wheels are attached is slowly impelled forward.
As soon as the wheels begin to move, and the carriage to advance, the wet cloth
begins to uncoil, and to pass down over the first roller; a small roller attached to the
carriage, as it passes over the rails in succession, holds the cloth against each rail for a
short space of time, and prevents it from slipping, by which means the cloth descends
in folds or loops between the rails, and is thereby made to hang in a series of folds or
loops, as shown in the figure.
It will be perceived that as the pivots of the cloth roller f bear upon inclined planes,
the roller will continually slide down as the cloth diminishes in bulk, keeping in contact
with the drum, and delivering the cloth from the roller on to the several rails,
as described.
In order to stop the carriage in any part of its course, or to adjust any of the folds of
the cloth, a man is usually placed upon the platform travelling with the carriage,
over which he has perfect command. This apparatus may be also employed for taking
the cloth when dried off the rails; in which case the carriage must be made to travel
backwards, and by first guiding the end of the cloth on to the roller f, and then putting
the wheels in a retrograde motion, the cloth will be progressively coiled upon the roller
f, in a similar way to that by which it was uncoiled.
DUCTILITY, (Streckbarkeit, Germ.) is the property of being drawn out in length
without breaking, possessed in a pre-eminent degree by gold and silver, as also by many
other metals, by glass in the liquid state, and by many semifluid resinous and gummy
substances. The spider and the silk-worm exhibit the finest natural exercise of ductility
upon the peculiar viscid secretions from which they spin their threads. When
a body can be readily extended in all directions under the hammer, it is said to be
malleable, and when into fillets under the rolling press, it is said to be laminable.
Table of the ductility and malleability of Metals.
Metals ductile
and malleable
in alphabetical
order. |
Brittle metals
in
alphabetical
order. |
Metals in the
order of their
wire-drawing
ductility. |
Metals in the
order of their
laminable
ductility. |
| Cadmium. |
Antimony. |
Gold. |
Gold. |
| Copper. |
Arsenic. |
Silver. |
Silver. |
| Gold. |
Bismuth. |
Platinum. |
Copper. |
| Iron. |
Cerium. ? |
Iron. |
Tin. |
| Iridium. |
Chromium. |
Copper. |
Platinum. |
| Lead. |
Cobalt. |
Zinc. |
Lead. |
| Magnesium. |
Columbium. ? |
Tin. |
Zinc. |
| Mercury. |
Iridium. |
Lead. |
Iron. |
| Nickel. |
Manganese. |
Nickel. |
Nickel. |
| Osmium. |
Molybdenum. |
Palladium. ? |
Palladium. ? |
| Palladium. |
Osmium. |
Cadmium. ? |
Cadmium. ? |
| Platinum. |
Rhodium. |
|
|
| Potassium. |
Tellurium. |
|
|
| Silver. |
Titanium. |
|
|
| Sodium. |
Tungsten. |
|
|
| Tin. |
Uranium. |
|
|
| Zinc. |
|
|
|
There appears to be therefore a real difference between ductility and malleability;
for the metals which draw into the finest wire are not those which afford the thinnest
leaves under the hammer or in the rolling press. Of this fact iron affords a good
illustration. Among the metals permanent in the air, 17 are ductile and 16 are brittle.
But the most ductile cannot be wire-drawn or laminated to any considerable extent
without being annealed from time to time during the progress of the extension, or
rather, the sliding of the particles alongside of each other, so as to loosen their lateral
cohesion.
DUNGING, in calico-printing, is the application of a bath of cowdung, diffused
through hot water, to cotton goods in a particular stage of the manufacture. Dunging
and scouring are commonly alternated, and are two of the most important steps in the
process. The operation of dunging has for its objects:—
1. To determine the entire combination of the aluminous sub-salts with the stuffs, by[410]
separating almost all the acetic acid which was not volatilized in the stove-drying of
the mordant.
2. To dissolve and carry off from the cloth a portion of the thickening matters.
3. To separate from the cloth the part of the mordant that is uncombined, and merely
mixed mechanically with the gum or starch.
4. To prevent, by the peculiar action of the dung, the uncombined mordant, as well
as the acetic acid with which the bath is apt to get loaded, from affecting the blank parts
of the cloth, or being injurious to the mordant.
The aluminous base or mordant on the cloth, more or less neutralized by the dunging,
is next subjected to the dash-wheel or fulling mill, where by the stream of water the
remainder of the thickening and other impurities are washed away.
No very exact analysis has been made of cowdung. Morin’s, which is the most
recent and elaborate, is as follows:—
| Water |
70·00 |
| Vegetable fibre |
24·08 |
| Green resin and fat acids |
1·52 |
| Undecomposed biliary matter |
0·60 |
| Peculiar extractive matter (bubuline) |
1·60 |
| Albumen |
0·40 |
| Biliary resin |
1·80 |
According to M. Kœchlin’s practical knowledge on the great scale, it consists of a
moist fibrous vegetable substance, which is animalized, and forms about one-tenth of its
weight; 2. of albumen; 3. of animal mucus; 4. of a substance similar to bile; 5. of
muriate of soda, muriate and acetate of ammonia, phosphate of lime and other salts;
6. of benzoin or musk.
Probably the hot water in which the calico-printer diffuses the dung, exerts a powerful
solvent action, and in proportion as the uncombined mordant floats in the bath it is
precipitated by the albumen, the animal mucus, and the ammoniacal salts; but there is
reason to think that the fibrous matter in part animalized or covered with animal
matter, plays here the principal part; for the great affinity of this substance for the
aluminous salts is well known.
All practical men are aware that the affinity of cotton for alumina is increased by
its combination with oil or animal substances, to such a degree as to take it from the
dung bath; which would not be possible without this combination. It would therefore
appear that the principal function of dunging is to hinder the uncombined mordant,
diffused in the dung bath, from attaching itself to the unmordanted portion of the
cloth, as already observed; for if we merely wished to abstract the thickening stuffs, or
to complete by the removal of acetic acid the combination of the aluminous base with
the goods, dung would not be required, for hot water would suffice. In fact, we may
observe, that in such cases the first pieces passed through the boiler are fit for dyeing;
but when a certain number have been passed through, the mordant now dissolved in
the water is attracted to the white portions of the cloth, while the free acid impoverishes
the mordanted parts, so that they cannot afford good dyes, and the blank
spaces are tarnished.
The cow dung may be in some measure replaced by bran, but not with perfect success.
The former both answers the purpose better and is cheaper. The bran is only
preferred for the most delicate yellows, for cochineal pinks and lilacs, to which the
dung may sometimes impart a greenish cast. It is to be presumed that the action of
the bran in this process has much analogy with that of the dung, and that the ligneous
fibre is the most active constituent; with which the gluten and mucilage co-operate, no
doubt, in seizing the aluminous salts.
It seems to be ascertained that the mordant applied to the cloth does not combine
entirely with it during the drying; that this combination is more or less perfect according
to the strength of the mordants, and the circumstances of the drying; that the
operation of dunging, or passing through hot water, completes the combination of the
cloth with the aluminous base now insoluble in water; that this base may still contain
a very minute quantity of acetic acid or sulphate of alumina; that a long ebullition
in water impoverishes the mordant but a little; and that even then the liquid does
not contain any perceptible quantity of acetate or subsulphate of alumina.
The manner of immersing the goods, or passing them through the dung bath, is an
important circumstance. They should be properly extended and free from folds, which
is secured by a series of cylinders.
The cistern is from 10 to 12 feet long, 41⁄2 feet wide, and 6 or 8 feet deep. The
piece passes alternately over the upper rollers and under rollers near the bottom.
There are two main squeezing rollers at one end, which draw the cloth through between
them. Whenever the goods come out of the bath they are put into the dash-wheel.[411]
The immersion should take place as fast as possible, for the moment the hot water
penetrates the mordanted cloth, the acetic acid quits it; and, therefore, if the immersion
was made slowly or one ply after another, the acid as well as the uncombined
mordant become free, would spread their influence, and would have time to dissolve
the aluminous subsalts now combined with the cloth; whence inequalities and impoverishment
of the colours would ensue.
It is difficult to determine the number of pieces which may be passed through a given
quantity of dung and water. This depends upon the state of the mordants, whether
they are strong or acid, and on the quantity of the surface covered with the figures.
The number varies usually from 20 to 60 pieces, for from 240 to 300 gallons of water
and 6 gallons of dung. The time of the immersion varies with the concentration of the
mordants, and the nature of their thickening. The temperature must be regulated by
the same circumstances; for starch or flour paste a much warmer bath is needed than
for gum. The heat varies usually from 130° to 212° F. When the printing is heavy
and the thickening is starch or flour, the goods are usually twice dunged, with two
washings between the two dungs. A strong acid mordant is more difficult to dung and
to wash than a neutral mordant, especially when it is to receive the madder dye. Sometimes
a little chalk is added to the bath, when the goods have been padded in an acid
mordant. Too much dung is injurious to weak mordants, as well as to pinks. It has
also been remarked that a mordant when neutralized does not produce as brilliant tints,
especially yellows. The latter are obtained of a finer shade when, instead of dunging,
they are exposed for an hour in a stream of water, provided its temperature is not too
low. In winter they are passed through a slightly chalky water, then washed at the
wheel, and dyed in quercitron or weld.
A very able and learned memoir upon this subject, by M. Penot, Professor of
Chemistry, appeared in the Bulletin of the Society of Mulhausen, in October, 1834,
with an ingenious commentary upon it, under the title of a Report by M. Camille
Kœchlin, in March, 1835.
Experience has proved that dunging is one of the most important steps in the process
of calico printing, and that if it be not well performed the dyeing is good for nothing.
Before we can assign its peculiar function to the dung in this case, we must know its
composition. Fresh cow’s dung is commonly neutral when tested by litmus paper; but
sometimes it is slightly alkaline, owing, probably, to some peculiarity in the food of
the animal.
The total constituents of 100 parts of cow dung are as follows: Water, 69·58;
bitter matter, 0·74; sweet substance, 0·93; chlorophylle, 0·28; albumine, 0·63;
muriate of soda, 0·08; sulphate of potash, 0·05; sulphate of lime, 0·25; carbonate
of lime, 0·24; phosphate of lime, 0·46; carbonate of iron, 0·09; woody fibre, 26·39;
silica, 0·14; loss, 0·14.
In dunging calicoes the excess of uncombined mordant is in part attracted by the
soluble matters of the cow’s dung, and forms an insoluble precipitate, which has no
affinity for the cloth, especially in presence of the insoluble part of the dung, which
strongly attracts alumina. The most important part which that insoluble matter
plays, is to seize the excess of the mordants, in proportion as they are dissolved by the
water of the bath, and thus to render their reaction upon the cloth impossible. It
is only in the deposit, therefore, that the matters carried off from the cloth by the
dung are to be found.
M. Camille Kœchlin ascribes the action of cow dung chiefly to its albuminous constituent,
combining with the alumina and iron, of the acetates of these bases dissolved
by the hot water of the bath. The acids consequently set free, soon become
evident by the test of litmus paper, after a few pieces are passed through, and require
to be got rid of either by a fresh bath or by adding chalk to the old one. The dung
thus serves also to fix the bases on the cloth, when used in moderation. It exercises
likewise a disoxidating power on the iron mordant, and restores it to a state more fit
to combine with colouring matter.
DYEING, (Teinture, Fr.; Färberei, Germ.) is the art of impregnating wool, silk,
cotton, linen, hair, and skins, with colours not removable by washing, or the ordinary
usage to which these fibrous bodies are exposed when worked up into articles of furniture
or raiment. I shall here consider the general principles of the art, referring for the
particular dyes, and peculiar treatment of the stuffs to be dyed, to the different tinctorial
substances in their alphabetical places; such as cochineal, indigo, madder, &c.
Dyeing is altogether a chemical process, and requires for its due explanation and
practice an acquaintance with the properties of the elementary bodies, and the laws
which regulate their combinations. It is true that many operations of this, as of other
chemical arts, have been practised from the most antient times, long before any just
views were entertained of the nature of the changes that took place. Mankind, equally
in the rudest and most refined state, have always sought to gratify the love of distinction[412]
by staining their dress sometimes even their skin, with gaudy colours. Moses speaks
of raiment dyed blue, and purple, and scarlet, and of sheep-skins dyed red; circumstances
which indicate no small degree of tinctorial skill. He enjoins purple stuffs for
the works of the tabernacle and the vestments of the high priest.
In the article Calico Printing, I have shown from Pliny that the antient Egyptians
cultivated that art with some degree of scientific precision, since they knew the use of
mordants, or those substances which, though they may impart no colour themselves, yet
enable white robes (candida vela) to absorb colouring drugs (colorem sorbendibus medicamentis).
Tyre, however, was the nation of antiquity which made dyeing its chief
occupation and the staple of its commerce. There is little doubt that purple, the sacred
symbol of royal and sacerdotal dignity, was a colour discovered in that city, and that it
contributed to its opulence and grandeur. Homer marks no less the value than the
antiquity of this dye, by describing his heroes as arrayed in purple robes. Purple habits
are mentioned among the presents made to Gideon by the Israelites from the spoils of
the kings of Midian.
The juice employed for communicating this dye was obtained from two different
kinds of shell-fish, described by Pliny under the names of purpura and buccinum; and
was extracted from a small vessel, or sac, in their throats, to the amount of only one
drop from each animal. A darker and inferior colour was also procured by crushing
the whole substance of the buccinum. A certain quantity of the juice collected from
a vast number of shells being treated with sea-salt, was allowed to ripen for three days;
after which it was diluted with five times its bulk of water, kept at a moderate heat for
six days more, occasionally skimmed to separate the animal membranes, and when thus
clarified was applied directly as a dye to white wool, previously prepared for this purpose
by the action of lime-water, or of a species of lichen called fucus. Two operations were
requisite to communicate the finest Tyrian purple; the first consisted in plunging the
wool into the juice of the purpura; the second, into that of the buccinum. Fifty drachms
of wool required one hundred of the former liquor, and two hundred of the latter. Sometimes
a preliminary tint was given with coccus, the kermes of the present day, and the
cloth received merely a finish from the precious animal juice. The colours, though probably
not nearly so brilliant as those producible by our cochineal, seem to have been very
durable, for Plutarch says, in his Life of Alexander, (chap. 36.), that the Greeks found
in the treasury of the king of Persia a large quantity of purple cloth, which was as
beautiful as at first, though it was 190 years old.[26]
The difficulty of collecting the purple juice, and the tedious complication of the dyeing
process, made the purple wool of Tyre so expensive at Rome that in the time of
Augustus a pound of it cost nearly 30l. of our money.[27] Notwithstanding this enormous
price, such was the wealth accumulated in that capital, that many of its leading
citizens decorated themselves in purple attire, till the emperors arrogated to themselves
the privilege of wearing purple, and prohibited its use to every other person. This
prohibition operated so much to discourage this curious art as eventually to occasion its
extinction, first in the western and then in the eastern empire, where, however, it existed
in certain imperial manufactories till the eleventh century.
Dyeing was little cultivated in antient Greece; the people of Athens wore generally
woollen dresses of the natural colour. But the Romans must have bestowed some
pains upon this art. In the games of the circus parties were distinguished by
colours. Four of these are described by Pliny, the green, the orange, the grey, and the
white. The following ingredients were used by their dyers. A crude native alum mixed
with copperas, copperas itself, blue vitriol, alkanet, lichen rocellus, or archil, broom,
madder, woad, nut-galls, the seeds of pomegranate, and of an Egyptian acacia.
Gage, Cole, Plumier, Reaumur, and Duhamel have severally made researches concerning
the colouring juices of shell-fish caught on various shores of the ocean, and have
succeeded in forming a purple dye, but they found it much inferior to that furnished by
other means. The juice of the buccinum is at first white; it becomes by exposure to
air of a yellowish green bordering on blue; it afterwards reddens, and finally changes
to a deep purple of considerable vivacity. These circumstances coincide with the minute
description of the manner of catching the purple-dye shell-fish which we possess in
the work of an eye-witness, Eudocia Macrembolitissa, daughter of the Emperor Constantine
VIII., who lived in the eleventh century.
The moderns have obtained from the New World several dye-drugs unknown to the
antients; such as cochineal, quercitron, Brazil wood, logwood, annatto; and they have[413]
discovered the art of using indigo as a dye, which the Romans knew only as a pigment.
But the vast superiority of our dyes over those of former times must be ascribed principally
to the employment of pure alum and solution of tin as mordants, either alone or
mixed with other bases; substances which give to our common dye-stuffs remarkable
depth, durability, and lustre. Another improvement in dyeing of more recent date is the
application to textile substances of metallic compounds, such as Prussian blue, chrome
yellow, manganese brown, &c.
Indigo, the innoxious and beautiful product of an interesting tribe of tropical plants,
which is adapted to form the most useful and substantial of all dyes, was actually denounced
as a dangerous drug, and forbidden to be used, by our parliament in the reign
of Queen Elizabeth. An act was passed authorizing searchers to burn both it and logwood
in every dye-house where they could be found. This act remained in full force
till the time of Charles II.; that is, for a great part of a century. A foreigner might
have supposed that the legislators of England entertained such an affection for their
native woad, with which their naked sires used to dye their skins in the old times, that
they would allow no outlandish drug to come in competition with it. A most instructive
book might be written illustrative of the evils inflicted upon arts, manufactures, and
commerce, in consequence of the ignorance of the legislature.[28]
Colours are not, properly speaking, material; they are impressions which we receive
from the rays of light reflected, in a decomposed state, by the surfaces of bodies. It is
well known that a white sunbeam consists of an indeterminate number of differently
coloured rays, which being separated by the refractive force of a glass prism, form the
solar spectrum, an image distinguishable into seven sorts of rays; the red, orange,
yellow, green, blue, indigo, and violet. Hence, when an opaque body appears coloured,
for example, red, we say that it reflects the red rays only, or in greatest abundance,
mixed with more or less of the white beam, which has escaped decomposition. According
to this manner of viewing the colouring principle, the art of dyeing consists in
fixing upon stuffs, by means of corpuscular attraction, substances which act upon light
in a different manner from the surfaces of the stuffs themselves. The dyer ought, therefore,
to be familiar with two principles of optics; the first relative to the mixture of
colours, and the second to their simultaneous contrast.
Whenever the different coloured rays, which have been separated by the prism, are
totally reunited, they reproduce white light. It is evident, that in this composition
of light, if some rays were left out, or if the coloured rays be not in a certain proportion,
we should not have white light, but light of a certain colour. For example; if we
separate the red rays from the light decomposed by a prism, the remaining coloured
rays will form by their combination a peculiar bluish green. If we separate in like
manner the orange rays, the remaining coloured rays will form by their combination
a blue colour. If we separate from the decomposed prismatic light the rays of greenish
yellow, the remaining coloured rays will form a violet. And if we separate the rays of
yellow bordering on orange, the remaining coloured rays will form by their union an
indigo colour.
Thus we see that every coloured light has such a relation with another coloured light
that, by uniting the first with the second, we reproduce white light; a relation which we
express by saying that the one is the complement of the other. In this sense, red is the
complementary colour of bluish green; orange, of blue; greenish yellow, of violet; and
orange yellow, of indigo. If we mix the yellow ray with the red, we produce orange;
the blue ray with the yellow, we produce green; and the blue with the red, we produce
violet or indigo, according as there is more or less red relatively to the blue. But
these tints are distinguishable from the orange, green, indigo, and violet of the solar
spectrum, because when viewed through the prism they are reduced to their elementary
component colours.
If the dyer tries to realize the preceding results by the mixture of dyes, he will
succeed only with a certain number of them. Thus, with red and yellow he can make
orange; with blue and yellow, green; with blue and red, indigo or violet. These
facts, the results of practice, have led him to conclude that there are only three
primitive colours; the red, yellow, and blue. If he attempts to make a white, by
applying red, yellow, and blue dyes in certain quantities to a white stuff, in imitation of
the philosopher’s experiment on the synthesis of the sunbeam, far from succeeding, he
will deviate still further from his purpose, since the stuff will by these dyes become so
dark coloured, as to appear black.
This fact must not, however, lead us to suppose that in every case where red, yellow,
and blue are applied to white cloth, black is produced. In reality, when a little ultramarine,
cobalt blue, Prussian blue, or indigo, is applied to goods with the view of giving
them the best possible white, if only a certain proportion be used, the goods will appear
whiter after this addition than before it. What happens in this case? The violet blue[414]
forms, with the brown yellow of the goods, a mixture tending to white, or less coloured
than the yellow of the goods and the blue together were. For the same reason, a
mixture of prussian blue and cochineal pink has been of late years used in the whitening
or the azuring of silks, in preference to a pure blue; for on examining closely the
colour of the silk to be neutralized, it was found by the relations of the complementary
colours, that the violet was more suitable than the indigo blue formerly used. The dyer
should know, that when he applies several different colouring matters to stuffs, as
yellow and blue, for example, if they appear green, it is because the eye cannot distinguish
the points which reflect the yellow from those which reflect the blue; and that,
consequently, it is only where the distinction is not possible, that a mixture or combination
appears. When we examine certain gray substances, such as hairs, feathers, &c.,
with the microscope, we see that the gray colour results from black points, disseminated
over a colourless or slightly coloured surface. In reference to compound colours, this
instrument might be used with advantage by the dyer.
The dyer should be acquainted also with the law of the simultaneous contrast of
colours. When the eye views two colours close alongside of each other, it sees them
differing most in their optical composition, and in the height of their tone, when the
two are not equally pale or full-bodied. They appear most different as to their optical
composition, when the complementary of the one of them is added to the colour of the
other. Thus, put a green zone alongside of an orange zone; the red colour complementary
of green, being added to the orange, will make it appear redder; and in like
manner the blue, complementary of orange, being added to the green, will make it appear
more intensely blue. In order to appreciate these differences, let us take two green
stripes and two orange stripes, placing one of the green stripes near one of the orange;
then place the two others so that the green stripe may be at a distance from the other
green stripe, but on the same side, and the orange at a distance from the other orange,
also on the same side.
As to the contrast in the height of the tone, we may satisfy ourselves by taking the
tones No. 1. No. 2. No. 15. and No. 16. from a graduated pallet of reds: for example,
by placing No. 2. and No. 15. close alongside, putting No. 1. at a distance from No. 2.
on the same side, and No. 16. at a distance from No. 15. on the same side,—we shall
see (if the pallet is sufficiently lowered in tone) No. 2. equal to No. 1., and No. 15.
equal to No. 16.; whence it follows that No. 2., by the vicinity of No. 15., will appear
to have lost some of its colour; while No. 15. will appear to have acquired colour.
When black or gray figures are printed upon coloured grounds, these figures are of
the colour complementary of the ground. Consequently, in order to judge of their
colour, we must cut out spaces in a piece of gray or white paper, so as to allow the
eye to see nothing but the figures; and if we wish to compare figures of the same
colour, applied upon grounds of different colours, we can judge rightly of the figures
only by insulating them from the grounds.
The relations of dyeing with the principles of chemistry, constitute the theory of the
art, properly speaking; this theory has for its basis, the knowledge—1. of the species
of bodies which dyeing processes bring into contact; 2. of the circumstances in which
these species act; 3. of the phenomena which appear during their action; and 4. of
the properties of the coloured combinations which are produced. These generalities
may be specified under the ten following heads:—
1. The preparation of the stuffs to be dyed, whether fibres, yarn, or cloth; under
the heads of ligneous matter, cotton, hemp, flax; and of the animal matters, silk and
wool.
2. The mutual action of these stuffs, and simple bodies.
3. The mutual action of these stuffs, and acids.
4. The mutual action of these stuffs, and salifiable bases, as alumina, &c.
5. The mutual action of these stuffs, and salts.
6. The mutual action of these stuffs, and neutral compounds not saline.
7. The mutual action of these stuffs, and of one or more definite compounds.
8. Of dyed stuffs considered in reference to the fastness of their colour, under the
influence of heat, light, water, oxygen, air, boilings with soap, and reagents.
9. Of dyeing, considered in its connections with chemistry.
10. Of dyeing, considered in its relations with caloric, mechanics, hydraulics, and
optics.
1. The preparation of stuffs.
The operations to which stuffs are subjected before dyeing, are intended—1. to separate
from them any foreign matters; 2. to render them more apt to unite with the
colouring tinctures which the dyer proposes to fix upon them, in order to give them a
more agreeable, or more brilliant aspect, or to lessen their tendency to assume a soiled
appearance by use, which white surfaces so readily do. The foreign matters are either
naturally inherent in the stuffs, or added to them in the spinning, weaving, or other[415]
manipulation of manufacture. The ligneous fibres must be freed from the coloured
azotized varnish on their surface, from a yellow colouring matter in their substance,
from some lime and iron, from chlorophylle or leaf-green, and from pectic acid; all
natural combinations. Some of these principles require to be oxygenized, before alkaline
lyes can cleanse them, as I have stated in the article Bleaching, which may be
consulted in reference to this subject. See also Silk and Wool. A weak bath of soda
has the property of preparing wool for taking on a uniform dye, but it must be well
rinsed and aired before being put into the dye-vat.
2. Mutual action of stuffs, and simple bodies.
Stuffs chemically considered being composed of three or four elements, already in a
state of reciprocal saturation, have but a feeble attraction for simple substances. We
know in fact, that the latter combine only with each other, or with binary compounds,
and that in the greater number of cases where they exert an action upon more complete
compounds, it is by disturbing the arrangement of their elements, and not by a
resulting affinity with the whole together.
3, 4. Although stuffs may in a general point of view be considered as neutral in
relation to colouring reagents, yet experience shows that they are more disposed to
combine with acid than with alkaline compounds; and that consequently their nature
seems to be more alkaline than acid. By steeping dry wool or other stuff in a clean
state in an alkaline or acid solution of known strength, and by testing the liquor after
the stuff is taken out, we shall ascertain whether there be any real affinity between
them, by the solution being rendered more dilute in consequence of the abstraction of
alkaline or acid particles from it. Wool and silk thus immersed, abstract a portion of
both sulphuric and muriatic acids; but cotton and flax imbibe the water, with the
rejection of a portion of the acid. The acid may be again taken from the stuffs by
washing them with a sufficient quantity of water.
5. The affinity between saline bodies and stuffs may be ascertained in the same way
as that of acids, by plunging the dry stuffs into solutions of the salts, and determining
the density of the solution before the immersion, and after withdrawing the stuffs.
Wool abstracts alum from its solution, but it gives it all out again to boiling water.
The sulphates of protoxide of iron, of copper and zinc resemble alum in this respect.
When silk is steeped for some time in solution of protosulphate of iron, it abstracts
the oxide, gets thereby dyed, and leaves the solution acidulous. Wool put in contact
with cream of tartar decomposes a portion of it; it absorbs the acid into its pores,
and leaves a neutral salt in the liquor. The study of the action of salts upon stuffs
is at the present day the foundation of the theory of dyeing; and some of them are
employed immediately as dye-drugs.
6. Mutual action of stuffs, and neutral compounds not saline.
Several sulphurets, such as those of arsenic, lead, copper, antimony, tin, are susceptible
of being applied to stuffs, and of dyeing them in a more or less fast manner. Indigo,
hematine, breziline, carmine, and the peculiar colouring principles of many dyes belong
to this division.
7. Mutual action of goods with one or more definite compounds, and dye-stuffs.
I shall consider here in a theoretical point of view, the most general results which
a certain number of organic colouring matters present, when applied upon stuffs by
the dyer.
Indigo. This dye-drug, when tolerably good, contains half its weight of indigotine.
The cold vat is prepared commonly with water, copperas, indigo, lime, or sometimes
carbonate of soda, and is used almost exclusively for cotton and linen; immersion in
acidulated water is occasionally had recourse to for removing a little oxide of iron which
attaches itself to the cloth dyed in this vat.
The indigo vat for wool and silk is mounted exclusively with indigo, good potashes
of commerce, madder and bran. In this vat, the immediate principles with base of
carbon and hydrogen, such as the extracts of madder and bran, perform the disoxidizing
function of the copperas in the cold vat. The pastel vats require most skill and experience,
in consequence of their complexity. The greatest difficulty occurs in keeping
them in a good condition, because they vary progressively as the dyeing goes on, by the
abstraction of the indigotine, and the modification of the fermentable matter employed
to disoxygenate the indigo. The alkaline matter also changes by the action of the air.
By the successive additions of indigo, alkali, &c., this vat becomes very difficult to
manage with profit and success. The great affair of the dyer is the proper addition of
lime; too much or too little being equally injurious.
Sulphate of indigo or Saxon blue is used also to dye silk and wool. If the wools be
ill sorted it will show their differences by the inequalities of the dye. Wool dyed in
this bath put into water saturated with sulphuretted hydrogen, becomes soon colourless,
owing to the disoxygenation of the indigo. The woollen cloth when exposed to the air
for some time, resumes its blue colour, but not so intensely as before.
[416]
The properties of hematine explain the mode of using logwood. When stuffs are
dyed in the infusion or decoction of this wood, under the influence of a base which acts
upon the hematine in the manner of an alkali, a blue dye bordering upon violet is
obtained. Such is the process for dyeing cotton and wool a logwood blue by means of
verdigris, crystallized acetate of copper, and acetate of alumina.
When we dye a stuff yellow, red, or orange, we have always bright tints; with blue
we may have a very dark shade, but somewhat violet; the proper black can be obtained
only by using the three colours, blue, red, and yellow, in proper proportions. Hence
we can explain how the tints of yellow, red, orange, blue, green, and violet, may be
browned, by applying to them one or two colours which along with themselves would
produce black; and also we may explain the nature of that variety of blacks and grays
which seems to be indefinite. Nutgalls and sulphate of iron, so frequently employed
for the black dye, give only a violet or bluish gray. The pyrolignite of iron, which
contains a brown empyreumatic matter, gives to stuffs a brown tint, bordering upon
greenish yellow in the pale hues, and to chestnut brown in the dark ones. By galling
cotton and silk, and giving them a bath of pyrolignite of iron, we may after some
alternations dye them black. Galls, logwood, and a salt of iron, produce merely a
very deep violet blue; but by boiling and exposure to air, the hematate of iron is
changed, becoming red-brown, and favours the production of black. Galls and salts of
copper dye stuffs an olive drab, logwood and salts of copper a violet blue; hence their
combination should produce a black. In using sumach as a substitute for galls, we
should take into account the proportion of yellow matter it contains. When the best
possible black is wanted upon wool, we must give the stuff a foundation of indigo, then
pass it into a bath of logwood, sumach, and proto-sulphate of iron. The sumach may
be replaced by one third of its weight of nutgalls.
8. Of dyed stuffs considered in reference to the fastness of their colours, when
exposed to water, light, heat, air, oxygen, boiling and reagents.
Pure water without air has no action upon any properly dyed stuff.
Heat favours the action of certain oxygenized bodies upon the carbonaceous and
hydrogenous constituents of the stuff; as is seen with regard to chromic acid, and peroxide
of manganese upon cotton goods. It promotes the solvent action of water, and
it even affects some colours. Thus Prussian blue applied to silk, is reduced to peroxide
of iron by long boiling.
Light without contact of air affects very few dyes.
Oxygen, especially in the nascent state, is very powerful upon dyes. See Bleaching.
The atmosphere in a somewhat moist state affects many dyes, at an elevated temperature.
Silk dyed pink, with safflower, when heated to 400° F. becomes of a dirty
white hue in the course of an hour. The violet of logwood upon alumed wool becomes
of a dull brown at the same temperature in the same time. But both stand a heat of
300° F. Brazil red dye, turmeric, and weld yellow dyes display the same phenomena.
These facts shew the great fixity of colours commonly deemed tender. The stuffs
become affected to a certain degree, under the same circumstances as the dyes. The
alterability even of indigo in the air is shewn in the wearing of pale blue clothes; in
the dark blue cloth there is such a body of colour, that it resists proportionally longer;
but the seams of coats exhibit the effect very distinctly. In silk window curtains,
which have been long exposed to the air and light, the stuff is found to be decomposed
as well as the colour.
Boiling was formerly prescribed in France as a test of fast dyes. It consisted in
putting a sample of the dyed goods in boiling water, holding in solution a determinate
quantity of alum, tartar, soap, and vinegar, &c. Dufay improved that barbarous test.
He considered that fast-dyed cloth could be recognized by resisting an exposure of
twelve hours to the sunshine of summer, and to the midnight dews; or of sixteen days
in winter.
In trying the stability of dyes, we may offer the following rules:—
That every stuff should be exposed to the light and air; if it be intended to be
worn abroad, it should be exposed also to the wind and rain; that carpets moreover
should be subjected to friction and pulling, to prove their tenacity; and that cloths
to be washed should be exposed to the action of hot water and soap.
In examining a piece of dyed cotton goods, we may proceed as follows:—
Suppose its colour to be orange-brown. We find first that it imparts no colour to
boiling water; that protochloride of tin takes out its colour; that plunged into a solution
of ferroprussiate of potash it becomes blue; and that a piece of it being burned,
leaves a residuum of peroxide of iron; we may thence conclude that the dyeing matter
is peroxide of iron.
Suppose we have a blue stuff which may have been dyed either with indigo or with
Prussian blue, and we wish to know what it will become in use. We inquire first into
the nature of the blue. Hot water slightly alkaline will be coloured blue by it, if[417]
it has been dyed with sulphate of indigo; it will not be coloured if it was dyed in
the indigo vat, but it will become yellow by nitric acid. Boiling water, without becoming
coloured itself, will destroy the Prussian blue dye; an alkaline water will
convert its colour into an iron rust tint; nitric acid, which makes the indigo dye yellow,
makes that of Prussian blue green. The liquor resulting from boiling alkaline water
on the Prussian blue cloth, will convert sulphate of iron into Prussian blue.
9. Division. Of dyeing viewed in its relation to chemistry.
The phenomena of dyeing have been ascribed to very different causes; by some they
were supposed to depend upon mechanical causes, and by others upon the forces from
which chemical effects flow. Hellot, in conformity with the first mode of explanation,
thought that the art of dyeing consisted essentially in opening the pores in order to
admit colouring matters into them, and to fix them there by cooling, or by means of a
mordant imagined to act like a cement.
Dufay in 1737, Bergmann in 1776, Macquer in 1778, and Berthollet in 1790, had recourse
to chemical affinities, to explain the fixation of the colouring principles upon stuffs,
either without an intermedium, like indigo, walnut peels, annotto; or by the intervention
of an acid, a salifiable base, or a salt, which were called mordants. When bodies
present phenomena which we refer to an attraction uniting particles of the same
nature, whether simple or compound, to form an aggregate, or to an affinity which
unites the particles of different natures to form them into a chemical compound, these
bodies are in apparent contact. This happens precisely in all the cases of the mutual
action of bodies in an operation of dyeing; if their particles were not in apparent contact,
there would be absolutely no change in their respective condition. When we see
stuffs and metallic oxides in apparent contact, form a mutual union of greater or less
force, we cannot therefore help referring it to affinity. We do not know how many
dyes may be fixed upon the same piece of cloth; but in the operations of the dye-house
sufficiently complex compounds are formed, since they are always stuffs, composed of
three or four elements, which are combined with at least binary acid or basic compounds;
with simple salts compounded themselves of two immediate principles at least
binary; with double salts composed of two simple salts; and finally with organic dye-stuffs
containing three or four elements. We may add that different species belonging
to one of these classes, and different species belonging to different classes, may unite
simultaneously with one stuff. The union of stuffs with colouring matters appears, in
general, not to take place in definite proportions; though there are probably some
exceptions.
We may conclude this head by remarking, that, besides the stuff and the colouring
matter, it is not necessary, in dyeing, to distinguish a third body, under the name of
mordant; for the idea of mordant does not rest upon any definite fact; the body to
which this name has been given being essentially only one of the immediate principles
of the coloured combination which we wish to fix upon the stuff.
10. Division. Of dyeing in its relation with caloric, mechanics, hydraulics, pneumatics,
and optics.
Dyeing baths, or coppers, are heated directly by a furnace, or by means of steam
conducted in a pipe from a boiler at a certain distance from the bath. In the first
case, the vessels are almost always made of copper; only, in special cases, for the
scarlet and some delicate silk dyes, of tin; in the second case, they are of copper, iron,
or wood. A direct fire is more economical than heating steam pipes, where there is
only one or two baths to heat, or where the labours are often suspended. Madder and
indigo vats, when heated by steam, have it either admitted directly into the liquor, or
made to circulate through pipes plunged into it, or between the copper and an exterior
iron or wood case. See the end of this article.
Every thing else being equal, dyeing with heat presents fewer difficulties towards
obtaining an evenly colour, than dyeing in the cold; the reason of which may be found
in the following facts:—The air adhering to the surface of stuffs, and that interposed
between the fibres of their constituent yarns, is more easily extricated in a hot bath
than a cold one, and thus allows the dye liquor to penetrate more easily into their
interior: in the second place, the currents which take place in a hot bath, and which
tend incessantly to render its contents uniform, by renewing continually the strata of
liquid in contact with the stuff, contribute mainly to render the dyeing evenly. In
cold dyeing, it is necessary to stir up the bath from time to time; and when goods are
first put in, they must be carefully dipped, then taken out, pressed, and wrung, several
times in succession till they be uniformly moistened.
The mechanical relations are to be found in the apparatus employed for wincing,
siring, and pressing the goods, as we have described under Calico Printing and
Bandanna. The hydraulic relations refer to the wash-wheels and other similar apparatus,
of which an account is given under the same articles. The optical relations[418]
have been already considered. In the sequel of this article an automatic dyeing vat
will be described.
The extracts of solutions of native dye-stuffs may be divided into two classes, in
reference to their habitudes with the oxygen of the atmosphere; such as continue
essentially unaltered in the air, and such as suffer oxidation, and thereby precipitate a
determinate colouring matter. The dyes contained in the watery infusions of the
different vegetable and animal substances which do not belong to the second class, are
feebly attached to their solvents, and quit them readily for any other bodies that possess
an attraction for them. On this principle, a decoction of cochineal, logwood, brazil
wood, or a solution of sulphate of indigo, by digestion with powdered bone black, lose
their colour, in consequence of the colouring particles combining by a kind of capillary
attraction with the porous carbon, without undergoing any change. The same thing
happens when well-scoured wool is steeped in such coloured liquids; and the colours
which the wool assumes by its attraction for the dye, is, with regard to most of the
above coloured solutions, but feeble and fugitive, since the dye may be again abstracted
by copious washing with simple water, whose attractive force therefore overcomes that
of the wool. The aid of a high temperature, indeed, is requisite for the abstraction
of the colour from the wool and the bone-black, probably by enlarging the size of the
pores, and increasing the solvent power of the water.
Those dye-baths, on the contrary, whose colouring matter is of the nature of extractive
or apothème, form a faster combination with stuffs. Thus the yellow, fawn, and brown
dyes, which contain tannin and extractive, become oxygenated by contact of air, and
insoluble in water; by which means they can impart a durable dye. When wool is
impregnated with decoctions of that kind, its pores get charged by capillarity, and
when the liquid becomes oxygenated, they remain filled with a colour now become
insoluble in water. A similar change to insolubility ensues when the yellow liquor of the
indigo vat gets oxidized in the pores of cotton and wool, into which it had been introduced
in a fluid state. The same change occurs when protosulphate of iron is converted
into persulphate, with the deposition of an insoluble peroxide in the substance of the
stuff. The change here effected by oxidation can, in other circumstances, be produced
by acids which have the power of precipitating the dye-stuff in an insoluble state, as
happens with decoction of fustic.
Hence we perceive that the dyeing of fast colours rests upon the principle, that the
colours dissolved in the vat, during their union with the stuff, should suffer such a
change as to become insoluble in their former menstruum. The more this dye, as
altered in its union with the stuff, can resist other menstrua or agents, the faster it will
be. This is the essential difference between dyeing and painting; or applying a coat of
pigment devoid of any true affinity for the surface.
If we mix a clear infusion of a dye with a small quantity of a solution of an earthy
or metallic salt, both in water, the limpid liquids soon become turbid, and there gradually
subsides sooner or later, according to the nature of the mixture, a coloured
precipitate, consisting of the altered dye united with a basic or subsalt. In this compound
the colouring matter seems to act the part of an acid, which is saturated by a
small quantity of the basis, or in its acid relationship is feeble, so that it can also
combine with acids, being in reference to them a base. The decomposition of a salt, as
alum, by dyes, is effected principally through the formation of an insoluble subsalt,
with which the colour combines, while a supersalt remains in the bath, and modifies, by
its solvent reaction, the shade of the dyed stuff. Dyed stuffs may be considered as
composed of the fibrous body intimately associated with the colouring matter, the oxide,
and acid, all three constituting a compound salt. Many persons have erroneously
imagined, that dyed goods contained none of the acid employed in the dye bath; but
they forget that even potash added to alum does not throw down the pure earthy basis,
but a subsalt; and they should not ascribe to colouring matter a power of decomposition
at all approaching to that of an alkali. Salts, containing strong acids, saturate a very
large quantity of colouring matter, in proportion to their place in the scale of chemical
equivalents. Mere bases, such as pure alumina, and pure oxide of tin, have no power
of precipitating colouring matter; when they seem to do so, they always contain some
acid.
Such salts, therefore, as have a tendency to pass readily into the basic state, are
peculiarly adapted to act as mordants in dyeing, and to form coloured lakes. Magnesia
affords as fine a white powder as alumina, and answers equally well to dilute lakes,
but its soluble salts cannot be employed to form lakes, because they do not pass into
the basic state. This illustration is calculated to throw much light upon dyeing
processes in general.
The colour of the lake depends very much upon the nature of the acid, and the
basis of the precipitating salt. If it be white, like alumina and oxide of tin, the lake
will have, more or less, the colour of the dye, but brightened by the reflection of white[419]
light from the basis; while the difference of the acid occasions a difference in the hue.
The coloured bases impart more or less of their colour to the lakes, not merely in virtue
of their own tints, but of their chemical action upon the dye.
Upon these principles a crimson precipitate is obtained from infusions of cochineal by
alum and salt of tin, which becomes scarlet by the addition of tartar; by acetate of
lead, a violet blue precipitate is obtained, which is durable in the air; by muriate of
lime, a pink brown precipitate falls, which soon becomes black, and at last dirty green;
by the solution of a ferruginous salt, the precipitates are dark violet, and black; and,
in like manner, all other salts with earthy or metallic bases, afford diversities of shade
with cochineal. If this dye stuff be dissolved in weak water of ammonia, and be
precipitated with acetate of lead, a green lake is obtained, which, after some time, will
become green on the surface by contact of air, but violet and blue beneath. Hence it
appears, that the shade of colour of a lake depends upon the degree of oxidation or
change of the colour caused by the acid of the precipitating salt, upon the degree of
oxidation or colour of the oxide which enters into union with the dye, and upon its
quantity in reference to that of the colouring principle.
Such lakes are the difficultly soluble salts which constitute the dyeing materials of
stuffs. Their particles, however, for the purposes of dyeing, must exist in a state of
extremely fine division in the bath liquor, in order that they may penetrate along with
it into the minute pores of textile fibres, and fill the cavities observed by means of the
microscope in the filaments of wool, silk, cotton, and flax. I have examined these stuffs
with an achromatic microscope, and find that when they are properly dyed with fast
colours, the interior of their tubular texture is filled, or lined at least, with colouring
matter. When the bath contains the colouring particles, so finely divided that they can
pass through filtering paper, it is capable of dyeing; but if the infusion mixed with its
mordant be flocculent and ready to subside, it is unfit for the purpose. In the latter case,
the ingredients of the dye have already become aggregated into compounds too coherent
and too gross for entering into combination with fibrous stuffs. Extractive matter and tannin
are particularly liable to a change of this kind, by the prolonged action of heat in the
bath. Hence also an alkaline solution of a colouring matter, affords no useful dye
bath, when mixed with the solution of a salt having an earthy or metallic basis.
These circumstances, which are of frequent occurrence in the dye-house, render it
necessary always to have the laky matter in a somewhat soluble condition, and to effect its
precipitation within the pores of the stuffs, by previously impregnating them with the
saline solutions by the aid of heat, which facilitates their introduction.
When a mordant is applied to any stuff, the portion of it remaining upon the surface
of the fibres should be removed; since, by its combination with the colouring matter,
it would be apt to form an external crust of mere pigment, which would block up the
pores, obstruct the entrance of the dye into the interior, and also exhaust to no purpose
the dyeing power of the bath. For this reason the stuffs, after the application of the
mordant, are drained, squeezed, washed, and sometimes (particularly with cotton and
linen, in calico printing), even hard dried in a hot stove.
The saline mordants, moreover, should not in general possess the crystallizing property
in any considerable degree, as this opposes their affinity of composition for the cloth.
On this account the deliquescent acetates of iron and alumina are more ready to aid the
dyeing of cotton than copperas and alum.
Alum is the great mordant employed in wool dyeing. It is frequently dissolved in
water, holding tartar equal to one fourth the weight of the alum in solution; by which
addition its tendency to crystallize is diminished, and the resulting colour is brightened.
The alum and tartar combine with the stuff without suffering any change, and are decomposed
only by the action of the colouring matters in the dye bath. The alum operates
solely in virtue of its sulphuric acid, and earthy basis; the sulphate of potash
present in that salt being rather injurious. Hence, if a sulphate of alumina free from
iron could be readily obtained, it would prove a preferable mordant to alum. It is also
probable, for the reason above assigned, that soda alum, a salt much less apt to crystallize
than potash or ammonia alum, would suit the dyer very well. In order to counteract
the tendency of common alum to crystallize, and to promote its tendency to pass
into a basic salt, one eighth part of its weight of potash is added to its solution, or the
equivalent in chalk or soda.
We shall conclude this account of the general principles of dyeing, with Mr. Delaval’s
observations on the nature of dyes, and a list of the different substances used in dyeing,
in reference to the colours produced by them.
Sir Isaac Newton supposed coloured matters to reflect the rays of light; some bodies
reflecting the more, others the less, refrangible rays most copiously; and this he conceived
to be the true, and the only reason of their colours. Mr. Delaval, however, proved in
the 2d vol. of the “Memoirs of the Philosophical and Literary Society of Manchester,”
that, “in transparent coloured substances, the colouring substance does not reflect any[420]
light; and that when, by intercepting the light which was transmitted, it is hindered
from passing through substances, they do not vary from their former colour to any
other colour, but become entirely black;” and he instances a considerable number of coloured
liquors, none of them endued with reflective powers, which, when seen by transmitted
light, appeared severally in their true colours; but all of them, when seen by
incident light, appeared black; which is also the case of black cherries, black currants,
black berries, &c., the juices of which appeared red when spread on a white ground,
or otherwise viewed by transmitted instead of incident light; and he concludes, that
bleached linen, &c. “when dyed or painted with vegetable colours, do not differ in
their manner of acting on the rays of light, from natural vegetable bodies; both yielding
their colours by transmitting through the transparent coloured matter, the light which is
reflected from the white ground:” it being apparent, from different experiments, “that
no reflecting power resides in any of their components, except in their white matter
only,” and that “transparent coloured substances, placed in situations by which transmission
of light through them is intercepted, exhibit no colour, but become entirely
black.”
The art of dyeing, therefore, (according to Mr. Delaval) “consists principally in covering
white substances, from which light is strongly reflected, with transparent coloured
media, which, according to their several colours, transmit more or less copiously the
rays reflected from the white,” since “the transparent media themselves reflect no light;
and it is evident that if they yielded their colours by reflecting, instead of transmitting
the rays, the whiteness or colour of the ground on which they are applied, would not
in anywise alter or affect the colours which they exhibit.”
But when any opaque basis is interposed, the reflection is doubtless made by it, rather
than by the substance of the dyed wool, silk, &c., and more especially when such basis
consists of the white earth of alum, or the white oxide of tin; which, by their strong
reflective powers, greatly augment the lustre of colours. There are, moreover, some
opaque colouring matters, particularly the acetous, and other solutions of iron, used to
stain linen, cotton, &c., which must necessarily themselves reflect, instead of transmitting
the light by which their colours are made perceptible.
The compound or mixed colours, are such as result from the combination of two
differently coloured dye stuffs, or from dyeing stuffs with one colour, and then with
another. The simple colours of the dyer, are red, yellow, blue, and black, with which,
when skilfully blended, he can produce every variety of tint. Perhaps the dun or fawn
colour might be added to the above, as it is directly obtained from a great many
vegetable substances.
1. Red with yellow, produces orange; a colour, which upon wool, is given usually
with the spent scarlet bath. To this shade may be referred flame colour, pomegranate,
capuchin, prawn, jonquil, cassis, chamois, café au lait, aurora, marigold, orange peel,
mordorés, cinnamon, gold, &c. Snuff, chesnut, musk, and other shades are produced by
substituting walnut peels or sumach for bright yellow. If a little blue be added to
orange, an olive is obtained. The only direct orange dyes are annotto, and subchromate
of lead; see Silk and Wool Dyeing.
2. Red with blue produces purple, violet, lilac, pigeon’s neck, mallow, peach-blossom,
bleu de roi, lint-blossom, amaranth.
3. Red with black; brown, chocolate, marone, &c.
4. Yellow with blue; green of a great variety of shades; such as nascent green,
gay green, grass green, spring green, laurel green, sea green, celadon green, parrot
green, cabbage green, apple green, duck green.
5. Mixtures of colours, three and three, and four and four, produce an indefinite diversity
of tints; thus red, yellow and blue, form brown olives, and greenish grays; in
which the blue dye ought always to be first given, lest the indigo vat should be soiled
by other colours. Red, yellow, and gray, (which is a gradation of black), give the
dead-leaf tint, as well as dark orange, snuff colour, &c. Red, blue and gray give a
vast variety of shades; as lead gray, slate gray, wood-pigeon gray, and other colours,
too numerous to specify. See Brown Dye.
The following list of dyes, and the colouring substances which produce them, may
prove useful.
Red. Cochineal, kermes, lac, madder, archil, carthamus or safflower, brazil wood,
logwood, periodide of mercury, alkanet.
Yellow. Quercitron, weld, fustic (yellow wood), annotto, sawwort, dyer’s broom,
turmeric, fustet (rhus cotinus), Persian and Avignon berries (rhamnus infectorius), willow,
peroxide of iron; chromate of lead (chrome yellow), sulphuret of arsenic, hydrosulphuret
of antimony; nitric acid on silk.
Blue. Indigo, woad or pastel, Prussian blue, turnsole or litmus, logwood with a
salt of copper.
[421]
Black. Galls, sumach, logwood, walnut peels, and other vegetables which contain
tannin and gallic acid, along with ferruginous mordants. The anacardium of India.
Green. These are produced by the blue and yellow dyes skilfully combined; with
the exception of the chrome green, and perhaps the copper green of Schweinfurt.
Orange. Annotto, and mixtures of red and yellow dyes; subchromate of lead.
Brown. See the remarks at the beginning of this article; Brown in its alphabetical
place; Calico Printing, Catechu, and Manganese.
Fawn, Dun or Root. Walnut peels, sumach, birch tree, henna, sandal wood. See
Calico Printing, for a great variety of these dyes.
Fig. 364. and 365. represent in a cross and longitudinal section the automatic dyeing
steam copper, so generally employed in the well-appointed factories of Lancashire.
A is the long reel, composed at each end of six
radial iron arms or spokes, bound at their outer extremities
with a six-sided wooden frame; these two
terminal hexagons are connected by long wooden
laths, seen above and below A in fig. 365. F shows
the sloping border or ledge of the copper. B and C
are rollers laid horizontally, for facilitating the continuous
motion of the series of pieces of goods
stitched together into an endless web, which are
made to travel by the incessant rotations of the reel.
Immediately above the roller B in fig. 364., all the
spare foldings of the web are seen resting upon the
sloping wooden grating, which guides them onwards
in the direction indicated by the arrow. The dye
stuffs are put within the middle grating, like a hen-coop,
marked G. Each copper is 6 feet long, 31⁄2 feet
wide, 31⁄2 feet deep, exclusive of the top ledge, 9
inches high. Such steam coppers are usually erected
in pairs, and moved by a common horizontal bevel
wheel seen at D in fig. 365., fixed upon a vertical shaft,
shifted into geer by a wheel at its top, with one of the driving shafts of the factory. Upon
each side of D, the two steam pipes for supplying the right and left hand coppers are
seen; each provided with a stop cock for admitting, regulating, or cutting off the steam.
These steam pipes descend at E E, the horizontal branch having several orifices in its
upper surface. The horizontal shaft in a line with the axes of the reels, and which
turns them, is furnished upon each side with a clutch for putting either of the reels
into or out of geer, that is to say, setting it a going, or at rest, in a moment by the
touch of a forked lever.
The steam pipe of distribution E lies horizontally near the bottom of the middle coop,
as shown under G in fig. 364., and sends up the steam through its numerous orifices,
among the dye-stuffs and water by which it is covered. Thus the infusion or decoction[422]
is continually advancing in the copper, during the incessant loco-motion of the
endless web. The horizontal pipe traverses the copper from end to end, and is not
stopped short in the middle. Each of these coppers can receive two, three or more
parallel pieces of goods at a time, the reel and copper being divided into so many
compartments by transverse wooden spars.
EARTHS. (Terres, Fr.; Erden, Germ.) Modern science has demonstrated that the
substances called primitive earths, and which prior to the great electro-chemical career
of Sir H. Davy, were deemed to be elementary matter, are all compounds of certain
metallic bases and oxygen, with the exception of silica, whose base, silicon, being analogous
to boron, has led that compound to be regarded as an acid; a title characteristic
of the part it extensively performs in neutralizing alkaline bodies, in mineral nature,
and in the processes of art. Four of the earths, when pure, possess decided alkaline
properties, being more or less soluble in water, having (at least 3 of them) an acrid
alkaline taste, changing the purple infusion of red cabbage to green, most readily saturating
the acids, and affording thereby neutro-saline crystals. These four are baryta,
strontia, lime (calcia), and magnesia. The earths proper are five in number; alumina,
glucina, yttria, zirconia, and thorina. These do not change the colour of infusion of cabbage
or tincture of litmus, do not readily neutralize acidity, and are quite insoluble in
water. The alkalies are soluble in water, even when carbonated; a property which distinguishes
them from the alkaline earths. Lithia must for this reason be considered to
be an alkali. See the above substances in their alphabetical places.
EAU DE COLOGNE. This preparation has long possessed great celebrity, in
consequence chiefly of the numerous virtues ascribed to it by its venders; and is resorted
to by many votaries of fashion as a panacea against ailments of every kind. It
is however nothing more than aromatized alcohol, and as such, an agreeable companion
of the toilet. Numerous fictitious recipes have been offered for preparing eau de
Cologne; the following may be reckoned authentic, having been imparted by Farina
himself to a friend.
Take 60 gallons of silent brandy; sage, and thyme, each 6 drachms; balm-mint and
spearmint, each 12 ounces; calamus aromaticus, 4 drachms; root of angelica, 2 drachms;
camphor, 1 drachm; petals of roses and violets, each 4 ounces; flowers of lavender, 2
ounces; flowers of orange, 4 drachms; wormwood, 1 ounce; nutmegs, cloves, cassia
lignea, mace, each 4 drachms. Two oranges and two lemons, cut in pieces. Allow
the whole to macerate in the spirit during 24 hours, then distil off 40 gallons by the
heat of a water bath. Add to the product:
Essence of lemons, of cedrat, of balm-mint, of lavender, each 1 ounce 4 drachms;
neroli and essence of the seed of anthos, each 4 drachms; essence of jasmin, 1 ounce;
of bergamot, 12 ounces. Filter and preserve for use.
Cadet Gassincourt has proposed to prepare eau de Cologne by the following recipe:
Take alcohol at 32° B., 2 quarts; neroli, essence of cedrat, of orange, of lemon, of bergamot,
of rosemary, each 24 drops; add 2 drachms of the seeds of lesser cardamoms,
distil by the heat of a water bath a pint and a half. When prepared as thus by simple
mixture of essences without distillation, it is never so good.
EAU DE LUCE, is a compound formed of the distilled oil of amber and water of
ammonia.
ELEMI, is a resin which exudes from incisions made during dry weather through
the bark of the amyris elemifera, a tree which grows in South America and Brazil. It
comes to us in yellow, tender, transparent lumps, which readily soften by the heat of
the hand. They have a strong aromatic odour, a hot spicy taste, and contain 121⁄2 per
cent. of ethereous oil. The crystalline resin of elemi has been called Elémine. It is
used in making lacquer, to give toughness to the varnish.
EBULLITION. (Eng. and Fr.; Kochen, Germ.) When the bottom of an open
vessel containing water is exposed to heat, the lowest stratum of fluid immediately
expands, becomes therefore specifically lighter, and is forced upwards by the superior gravity
of the superincumbent colder and heavier particles. The heat is in this way diffused
through the whole liquid mass, not by simple communication of that power from particle
to particle as in solids, called the conduction of caloric, but by a translation of the
several particles from the bottom to the top, and the top to the bottom, in alternate
succession. This is denominated the carrying power of fluids, being common to both
liquid and gaseous bodies. These internal movements may be rendered very conspicuous
and instructive, by mingling a little powdered amber with water, contained in a[423]
tall glass cylinder, standing upon a sand-bath. A column of the heated and lighter
particles will be seen ascending near the axis of the cylinder, surrounded by a hollow
column of the cooler ones descending near the sides. That this molecular translation
or loco-motion is almost the sole mode in which fluids get heated, may be demonstrated
by placing the middle of a pretty long glass tube, nearly filled with water, obliquely
over an argand flame. The upper half of the liquid will soon boil, but the portion
under the middle will continue cool, so that a lump of ice may remain for a considerable
time at the bottom. When the heat is rapidly applied, the liquid is thrown into
agitation, in consequence of elastic vapour being suddenly generated at the bottom of
the vessel, and being as suddenly condensed at a little distance above it by the surrounding
cold columns. These alternate expansions and contractions of volume become
more manifest as the liquid becomes hotter, and constitute the simmering vibratory
sound which is the prelude of ebullition. The whole mass being now heated to
a pitch compatible with its permanent elasticity, becomes turbulent and explosive
under the continued influence of fire, and emitting more or less copious volumes of vapour
is said to boil. The further elevation of temperature, by the influence of caloric,
becomes impossible in these circumstances with almost all liquids, because the vapour
carries off from them as much heat in a latent state as they are capable of receiving
from the fire.
The temperature at which liquids boil in the open air varies with the degree of
atmospheric pressure, being higher as that is increased, and lower as it is diminished.
Hence boiling water is colder by some degrees in bad weather, or in an elevated situation,
with a depressed barometer, than in fine weather, or at the bottom of a coal-pit,
when the barometer is elevated. A high column of liquid also by resisting the discharge
of the steam raises the boiling point. In vacuo, all liquids boil at a temperature
about 124° F. lower than under the average atmospheric pressure. For a table of elasticities,
see Vapour. Gay Lussac has shown that liquids are converted into vapours
more readily or with less turbulence, when they are in contact with angular or irregular,
than with smooth surfaces; that they therefore boil at a heat 2° F. lower in metallic than
in glass vessels, probably owing to the greater polish of the latter. For example, if into
water about to boil in a glass matras, iron filings, ground glass, or any other insoluble
powder be thrown, such a brisk ebullition will be instantly determined, as will sometimes
throw the water out of the vessel; the temperature at the same time sinking two
degrees F. It would thence appear that the power of caloric, like that of electricity,
becomes concentrated by points.
The following table exhibits the boiling heats, by Fahrenheit’s scale, of the most
important liquids:—
| Ether, specific gravity 0·7365 at 48° |
|
100 |
° |
| Carburet of sulphur, |
|
113 |
|
| Alcohol, sp. grav. 0·813 |
Ure, |
173 |
·5 |
| Nitric acid, . grav.1·500 |
Dalton, |
210 |
|
| Water, |
|
212 |
|
| Saturated solution of Glauber salt, |
Biot, |
213 |
1⁄3 |
| Satdo.ted soludo.n ofAcetate of lead |
do. |
215 |
2⁄3 |
| Satdo.ted soludo.n ofSea salt |
do. |
224 |
1⁄3 |
| Satdo.ted soludo.n ofMuriate of lime, |
Ure, |
285 |
|
| Satdo.ted soludo.n of Muriado.of lime, 3 1 + water 2, |
do. |
230 |
|
| Satdo.ted soludo.n of Muriado.of lime35·5 + wdo.r 64·5, |
do. |
235 |
|
| Satdo.ted soludo.n of Muriado.of lime40·5 + wdo.r 59·5, |
do. |
240 |
|
| Muriatic acid, sp. grav. 1·094 |
Dalton, |
232 |
|
| Muriado.c acid, sdo.av.1·127 |
do. |
222 |
|
| Nitric acid,id, sp.do.av.1·420 |
do. |
248 |
|
| Nitrdo. acid, sp. do.av.1·30 |
do. |
236 |
|
| Rectified petroleum |
Ure, |
306 |
|
| Oil of turpentine |
do. |
316 |
|
| Sulphuric acid, sp. grav. 1·848 |
Dalton, |
600 |
|
| Sulphdo.c acid, spdo.av.1·810 |
do. |
473 |
|
| Sulphdo.c acid, spdo.av.1·780 |
do. |
435 |
|
| Sulphdo.c acid, spdo.av.1·700 |
do. |
374 |
|
| Sulphdo.c acid, spdo.av.1·650 |
do. |
350 |
|
| Sulphdo.c acid, spdo.av.1·520 |
do. |
290 |
|
| Sulphdo.c acid, spdo.av.1·408 |
do. |
260 |
|
| Sulphdo.c acid, spdo.av.1·300+ |
do. |
240 |
|
| Phosphorus |
do. |
554 |
|
| Sulphur |
do. |
570 |
|
| Linseed oil[424] |
do. |
640 |
|
| Mercury |
Dulong, |
662 |
|
| do. |
Crighton, |
656 |
|
| Saturated solution of |
acetate of soda, containing |
60 |
|
per cent. |
Griffiths, |
256 |
|
| Saturatedo. |
Nitrate of soda, |
60 |
|
|
do. |
246 |
|
| Saturatedo. |
Rochelle salt, |
90 |
|
|
do. |
240 |
|
| Saturatedo. |
Nitre, |
74 |
|
|
do. |
238 |
|
| Saturatedo. |
Muriate of ammonia, |
50 |
|
|
do. |
236 |
|
| Saturatedo. |
Tartrate of potash, |
68 |
|
|
do. |
234 |
|
| Saturatedo. |
Muriate of soda, |
30 |
|
|
do. |
224 |
|
| Saturatedo. |
Sulphate of magnesia, |
57 |
·5 |
|
do. |
222 |
|
| Saturatedo. |
Borax, |
52 |
·5 |
|
do. |
222 |
|
| Saturatedo. |
Phosphate of soda, |
? |
|
do. |
222 |
|
| Saturatedo. |
Carbonate of soda, |
? |
|
do. |
220 |
|
| Saturatedo. |
Alum, |
52 |
|
|
do. |
220 |
|
| Saturatedo. |
Chlorate of potash, |
40 |
|
|
do. |
218 |
|
| Saturatedo. |
Sulphate of copper, |
45 |
|
|
do. |
216 |
|
EDULCORATE, (Edulcorer, Fr.; Aussüssen, Germ.) is a word introduced by the
alchemists to signify the sweetening, or rather rendering insipid, of acrimonious pulverulent
substances, by copious ablutions with water. It means, in modern language, the
washing away of all particles soluble in water, by agitation or trituration with this
fluid, and subsequent decantation or filtration.
EFFERVESCENCE. (Eng. and Fr.; Aufbrausen, Germ.) When gaseous
matter is suddenly extricated with a hissing sound during a chemical mixture, or by the
application of a chemical solvent to a solid, the phenomenon, from its resemblance to
that of simmering or boiling water, is called effervescence. The most familiar example
is afforded in the solution of sodaic powders; in which the carbonic acid gas of sesquicarbonate
of soda, is extricated by the action of citric, or tartaric acid.
EFFLORESCENCE, (Eng. and Fr.; Verwittern, Germ.) is the spontaneous conversion
of a solid, usually crystalline, into a powder, in consequence either of the
abstraction of the combined water by the air, as happens to the crystals of sulphate and
carbonate of soda; or by the absorption of oxygen and the formation of a saline compound,
as in the case of alum schist, and iron pyrites. Saltpetre appears as an efflorescence
upon the ground and walls in many situations.
EDGE-TOOLS. See Cutlery and Steel.
EIDER-DOWN, is a kind of precious down, so called because it is obtained from
the Eider-duck. These birds build their nests among precipitous rocks, and the female
lines them with fine feathers plucked from her breast, among which she lays her five
eggs. The natives of the districts frequented by the eider-ducks let themselves down
by cords among the dangerous cliffs, to collect the down from the nests. It is used to
fill coverlets, pillows, cushions, &c.
ELAINE is the name given by Chevreul to the thin oil, which may be expelled
from tallow, and other fats, solid or fluid, by pressure either in their natural state, or
after being saponified, so as to harden the stearine. It may be extracted also by digesting
the fat in 7 or 8 times its weight of boiling alcohol, spec. grav. 0·798, till it dissolves
the whole. Upon cooling the solution, the stearine falls to the bottom, while the elaine
collects in a layer like olive oil, upon the surface of the supernatant solution, reduced by
evaporation to one eighth of its bulk. If this elaine be now exposed to a cold temperature,
it will deposit its remaining stearine, and become pure. See Fat, Oils, and
Stearine.
ELASTIC BANDS. (Tissus Elastiques, Fr.; Federharz-zeige, Germ.) The
manufacture of braces and garters, with threads of caoutchouc, either naked or covered,
seems to have originated, some time ago, in Vienna, whence it was a few years since
imported into Paris, and thence into this country. At first the pear-shaped bottle of
Indian rubber was cut into long narrow strips by the scissors; a single operative
turning off only about 100 yards in a day, by cutting the pear in a spiral direction. He
succeeded next in separating with a pair of pincers the several layers of which the bottle
was composed. Another mode of obtaining fine threads was to cut them out of a bottle
which had been rendered thin by inflation with a forcing pump. All these operations
are facilitated by previously steeping the caoutchouc in boiling water, in its moderately
inflated state. More recently, machines have been successfully employed for cutting
out these filaments, but for this purpose the bottle of caoutchouc is transformed into
a disc of equal thickness in all its parts, and perfectly circular. This preliminary
operation is executed as follows: 1. the bottle, softened in hot water, is squeezed between
the two plates of a press, the neck having been removed beforehand, as useless in this
point of view; 2. the bottle is then cut into two equal parts, and is allowed to consolidate[425]
by cooling before subjecting it to the cutting instrument. When the bottle is
strong enough, and of variable thickness in its different points, each half is submitted to
powerful pressure in a very strong cylindrical mould of metal, into which a metallic
plunger descends, which forces the caoutchouc to take the form of a flat cylinder with a
circular base. The mould is plunged into hot water during the compression. A stem
or rod of iron, which goes across the hollow mould and piston, retains the latter in its
place, notwithstanding the resilience of the caoutchouc, when the mould is taken from
the press. The mould being then cooled in water, the caoutchouc is withdrawn.
The transformation of the disc of caoutchouc into fine threads is performed by two
machines; the first of which cuts it into a riband of equal thickness in its whole extent,
running in a spiral direction from the circumference to the centre; the second subdivides
this riband lengthwise into several parallel filaments much narrower but equally
thick.
The following figs. 366, 367, 368. represent the machine for cutting the spiral riband.
The disc D, placed horizontally, turns round its vertical axis, so as to present its
periphery to the edge of a knife C, formed like a circular blade, whose plane is perpendicular
to that of the bases of the disc. This knife turns round its centre, which is
fixed. The rotatory motion of the disc forces the knife to penetrate further and further
into its mass, and the motion of the knife itself makes it cut the riband more easily. It
is obvious, that if the disc alone revolved, the motionless knife could act only by pressure,
and would meet with an enormous resistance. A third movement becomes necessary.
In proportion as the disc is diminished by the removal of the spiral band, the centre of
this disc must advance upon the knife, in order that the riband may have always the
same breadth. The inspection of fig. 368. will make the accordance of the three motions
intelligible.
The knife C is placed upon a shaft or axis A, which carries a pulley, round which a
belt or cord runs which drives the whole machine. This knife is six inches in diameter.
In order that by being kept cool it may cut the caoutchouc better, it is plunged at its
lower part into a trough B, full of water; a stopcock R, serves to empty this trough.
The shaft A bears a pinion p, which takes into a wheel R, placed upon the shaft A′;
upon which there is cut a worm or endless screw, V, V. This worm bears a nut E,
which advances as the screw turns, and carries with it a tie L, which in its turn pushes
the disc D, carried upon a shoulder constantly towards the knife. This shoulder is
guided by two ears which slide in two grooves cut in the thickness of the table. The
diameter of the pinion p is about one fifth of that of the wheel R; so that the arbour
A turns five times less quickly than the arbour A; and the fineness of the screw V contributes
further to slacken the movement of translation of the disc.
When the disc is all cut down, the shoulder, the tie, and the nut, are brought back
to their original position by lifting the nut, which is hinged on. The disc is fixed
upon the shoulder by means of sharp points, and an upper washer. The shoulder
and the washer have a very small diameter, in order that the knife may, in cutting
down the disc, advance as near as possible to the centre.
The rotatory movement of the disc and its shoulder, is given by an endless screw
W, W, which governs a pinion p′, provided with 10 teeth, and carried by the shaft A,
upon which the shoulder is mounted. The arbour A′ of this endless screw receives
its motion from the first shaft A, by means of the wheels S and S′ mounted upon these
shafts, and of an intermediate wheel S′′. This wheel, of a diameter equal to that of
the shaft A′′, is intended merely to allow this shaft to recede from the shaft A. The
diameter of the wheel of this last shaft is to that of the two others in the ratio of 10
to 8.
[426]
Second machine for sub-dividing the ribands. Fig. 369.—The riband is engaged
between the circular knives, C, C, which are mounted upon the rollers R, R; thin brass
washers keep these knives apart at a distance which may be varied, and two extreme
washers mounted with screws on each roller maintain the whole system. The axes of
these rollers traverse two uprights M, M, furnished with brasses, and with adjusting
screws to approximate them at pleasure. The axis of the lower roller carries a wheel
r, which takes into another smaller wheel r′, placed upon the same shaft as the pulley P,
which is driven by a cord. The diameter of the wheel r is three times greater than the
wheel r′. The pulley P is twice the size of the wheel r′; and its cord passes round a
drum B, which drives the rest of the machine.
The threads when brought to this state of slenderness, are put successively into tubs
filled with cold water; they are next softened in hot water, and elongated as much as
possible in the following manner:—They are wound upon a reel turned quickly, while
the operative stretches the caoutchouc thread with his hand. In this way it is rendered
8 or 10 times longer. The reels when thus filled are placed during some days in a cold
apartment, where the threads become firm, and seem to change their nature.
This state of stiffness is essential for the success of the subsequent operations. The
threads are commonly covered with a sheath of silk, cotton, or linen, by a braiding machine,
and are then placed as warp in a loom, in order to form a narrow web for braces,
garters, &c. If the gum were to exercise its elasticity during this operation, the different
threads would be lengthened and shortened in an irregular manner, so as to form
a puckered tissue. It is requisite therefore to weave the threads in their rigid and inextensible,
or at least incontractile condition, and after the fabric is woven to restore
to the threads of caoutchouc their appropriate elasticity. This restoration is easily
effected by passing a hot smoothing iron over the tissue laid smoothly upon a table
covered with blanket stuff. See Braiding Machine.
ELECTIVE AFFINITY, (Wahlverwandtschaft, Germ.) denotes the order of preference,
so to speak, in which the several chemical substances choose to combine; or
really, the gradation of attractive force infused by Almighty Wisdom among the different
objects of nature, which determines perfect uniformity and identity in their compounds
amidst indefinite variety of combination. The discussion of this interesting subject
belongs to pure chemistry. See Decomposition.
ELEMENTS (Eng. and Fr.; Grundstoffe, Germ.) The ancients considered fire,
air, water, and earth, as simple substances, essential to the constitution of all terrestrial
beings. This hypothesis, evidently incompatible with modern chemical discovery,
may be supposed to correspond, however, to the four states in which matter seems to
exist; namely, 1. the unconfinable powers or fluids,—caloric, light, electricity; 2. ponderable
gases, or elastic fluids; 3. liquids; 4. solids. The three elements of the
alchemists, salt, earth, mercury, were, in their sense of the word, mere phantasms.
In modern science, the term Element signifies merely a substance which has not yet
been resolved by analysis into any simpler form of matter; and it is therefore synonymous
with undecompounded. This class comprehends 54 different bodies, of which no less
than 41 are metallic. Five may be styled Archæal, from the intensity and universality
of their affinities for the other bodies, which they penetrate, corrode, and apparently
consume, with the phenomena of light and heat. These 5 are chlorine, oxygen, iodine,
bromine, fluorine. Eight elements are eminently inflammable when acted upon by any
of the preceding five, and are thereby converted into incombustible compounds. The
simple non-metallic inflammables are hydrogen, azote, sulphur, phosphorus, selenium,
carbon, boron, silicon.
The following table exhibits all the undecompounded bodies in alphabetical order,
with their prime equivalent numbers, atomic weights, or reciprocal combining and
saturating proportions, as given by Berzelius, in reference to oxygen, reckoned
100,000.:—
[427]
Table of undecompounded Bodies, or modern Chemical Elements.
A signifies Archæal; I, Inflammable; M, Metal.
| Aluminium |
M. |
171,167 |
| Antimony |
— |
806,542 |
| Arsenic |
— |
470,042 |
| Azote |
I. |
88,518 |
| Barium |
M. |
856,880 |
| Bismuth |
— |
886,000 |
| Boron |
I. |
135,983 |
| Bromine |
A. |
489,150 |
| Cadmium |
M. |
696,970 |
| Calcium |
— |
256,019 |
| Carbon |
I. |
76,437 |
| Cerium |
M. |
574,718 |
| Chlorine |
A. |
221,325 |
| Chromium |
M. |
351,819 |
| Cobalt |
— |
369,991 |
| Copper |
— |
395,695 |
| Fluorine |
I. |
116,900 |
| Gold |
M. |
1243,013 |
| Hydrogen |
I. |
62,398 |
| Iodine |
A. |
789,145 |
| Iridium |
M. |
1233,260 |
| Iron |
— |
339,213 |
| Lead |
— |
1294,489 |
| Lithium |
— |
81,320 |
| Magnesium |
— |
158,353 |
| Manganesium |
— |
345,900 |
| Mercury |
M. |
1265,822 |
| Molybdenum |
— |
598,525 |
| Nickel |
— |
369,675 |
| Osmium |
— |
1244,210 |
| Oxygen |
A. |
100,000 |
| Palladium |
M. |
665,840 |
| Phosphorus |
I. |
196,155 |
| Platinum |
M. |
1233,260 |
| Rhodium |
— |
651,400 |
| Selenium |
I. |
494,582 |
| Silicon |
— |
277,478 |
| Silver |
M. |
675,804 |
| Strontium |
— |
547,285 |
| Sulphur |
I. |
201,165 |
| Tantalum |
M. |
1153,715 |
| Tellurium |
— |
801,760 |
| Thorinum |
— |
744,900 |
| Tin |
— |
735,294 |
| Titanium |
— |
303,686 |
| Tungsten |
— |
1183,000 |
| Uranium |
— |
2711,360 |
| Vanadium |
— |
855,840 |
| Yttrium |
— |
401,840 |
| Zinc |
— |
403,226 |
| Zirconium |
— |
420,238 |
ELUTRIATE. (Soutirer, Fr.; Schlemmen, Germ.) When an insoluble pulverulent
matter, like whitening or ground flints, is diffused through a large body of
water, and the mixture is allowed to settle for a little, the larger particles will subside.
If the supernatant liquid be now carefully decanted, or run off, with a syphon, it will
contain an impalpable powder, which on repose will collect at the bottom, and may be
taken out to dry. This process is called elutriation.
EMBALMING. (Embaument, Fr.; Einbalsamen, Germ.) Is an operation in
which balsams (baumes, Fr.) were employed to preserve human corpses from putrefaction;
whence the name.
The ancient Egyptians had recourse to this process for preserving the bodies of
numerous families, and even of the animals which they loved or worshipped. An excellent
account of their methods is given in Mr. Pettigrew’s work upon Mummies.
Modern chemistry has made us acquainted with many means of counteracting putrefaction
more simple and efficacious than the Egyptian system of salting, smoking,
spicing, and bituminizing. See Putrefaction.
EMBOSSING WOOD. (Bossage, Fr.; Erhabenes Arbeit, Germ.) Raised figures
upon wood, such as are employed in picture frames and other articles of ornamental
cabinet work, are usually produced by means of carving, or by casting the pattern in
plaster of Paris, or other composition, and cementing, or otherwise fixing it on the
surface of the wood. The former mode is expensive; the latter is inapplicable on
many occasions. The invention of Mr. Streaker may be used either by itself, or in aid
of carving; and depends on the fact, that if a depression be made by a blunt instrument
on the surface of the wood, such depressed part will again rise to its original level by
subsequent immersion in the water.
The wood to be ornamented having been first worked out to its proposed shape, is in
a state to receive the drawing of the pattern; this being put on, a blunt steel tool, or
burnisher, or die, is to be applied successively to all those parts of the pattern intended
to be in relief, and, at the same time, is to be driven very cautiously, without breaking
the grain of the wood, till the depth of the depression is equal to the intended prominence
of the figures. The ground is then to be reduced by planing or filing to the
level of the depressed part; after which, the piece of wood being placed in water, either
hot or cold, the part previously depressed will rise to its former height, and will then
form an embossed pattern, which may be finished by the usual operations of carving.
For this invention the Society of Arts voted to Mr. Streaker their silver Isis medal,
and ten guineas.
EMBOSSING CLOTH. Mr. Thomas Greig, of Rose Bank, near Bury, patented
an invention, in November 1835, which consists in an ingenious construction of machinery[428]
for both embossing and printing silk, cotton, woollen cloth, paper, and other
fabrics, in one or more colours, at one operation.
Figs. 370, 370* represent three distinct printing cylinders of copper, or other suitable
material, A, B, C, with
their necessary appendages
for printing three
different colours upon
the fabric as it passes
through the machine:
either of these cylinders
A, B, or C, may be employed
as an embossing
cylinder, without performing
the printing
process, or may be made
to effect both operations
at the same time.
The fabric or goods
to be operated upon being
first wound tightly
upon a roller, that roller
is to be mounted upon
an axle or pivot, bearing
in arms or brackets at
the back of the machine,
as shown at D. From
this roller the fabric
a a a a is conducted between
tension rails, and
passed under the bed
cylinder or paper bowl
E, and from thence proceeds
over a carrier
roller F, and over steam
boxes not shown in the
drawing, or it may be
conducted into a hot
room, for the purpose of
drying the colours.
The cylinders A, B,
and C, having either engraved
or raised surfaces,
are connected to feeding
rollers b b b, revolving
in the ink or coloured
troughs c c c; or endless felts, called sieves, may be employed, as in ordinary printing
machines, for supplying the colour, when the device on the surface of the cylinders is
raised: these cylinders may be furnished with doctors or scrapers when required, or the
same may be applied to the endless felts.
The blocks have adjustable screws g g, for the purpose of bringing the cylinders up
against the paper bowl, with any required degree of pressure: the cylinder B is
supported by its gudgeons running in blocks, which blocks slide in the lower parts
of the side frames, and are connected to perpendicular rods i, having adjustable
screw nuts.
The lower parts of these rods bear upon weighted levers k k, extending in front of
the machine; and by increasing the weights l l, any degree of upward pressure may be
given to the cylinder B.
The colour boxes or troughs c c c, carrying the feeding rollers b b b, are fixed on
boards which slide in grooves in the side frames, and the rollers are adjusted and brought
into contact with the surface of the printing cylinders by screws.
If a back cloth should be required to be introduced between the cylindrical bed or
paper bowl E, and the fabric a a a, as the ordinary felt or blanket, it may, for printing
and embossing cotton, silk, or paper, be of linen or cotton; but if woollen goods are to
be operated upon, a cap of felt, or some such material, must be bound round the paper
bowl, and the felt or blanket must be used for the back cloth, which is to be conducted
over the rollers H and I.
For the purpose of embossing the fabric, either of the rollers A, B, or C, may be[429]
employed, observing that the surface of the roller must be cut, so as to leave the pattern
or device elevated for embossing velvets, plain cloths, and papers; but for woollens the
device must be excavated, that is, cut in recess.
The pattern of the embossing cylinder will, by the operation, be partially marked
through the fabric on to the surface of the paper bowl E; to obliterate which marks
from the surface of the bowl, as it revolves, the iron cylinder roller G is employed; but
as in the embossing of the same patterns on paper, a counter roller is required to
produce the pattern perfectly, the iron roller is in that case dispensed with, the impression
given to the paper bowl being required to be retained on its surface until the
operation is finished.
In this case the relative circumferences of the embossing cylinder, and of the paper
bowl, must be exactly proportioned to each other; that is, the circumference of the
bowl must be equal, exactly, to a given number of circumferences of the embossing
cylinder, very accurately measured, in order to preserve a perfect register or coincidence,
as they continue revolving between the pattern on the surface of the embossing cylinder,
and that indented into the surface of the paper bowl.
The axle of the paper bowl E, turns in brasses fitted into slots in the side frames, and
it may be raised by hand from its bearings when required, by a lever k, extending in
front. This lever is affixed to the end of a horizontal shaft L, L, crossing the machine
seen in the figures, at the back of which shaft there are two segment levers P, P, to
which bent rods Q, Q, are attached, having hooks at their lower ends, passed under the
axle of the bowl. At the reverse end of the shaft L, a ratchet-wheel r, is affixed, and a
pall or click mounted on the side of the frame takes into the teeth of the wheel r, and
thereby holds up the paper bowl when required.
When the iron roller G, is to be brought into operation, the vertical screws t, t,
mounted in the upper parts of the side frames, are turned, in order to bring down the
brasses N, which carry the axle of that roller and slide in slots in the side frames.
The cylinders A, B, and C, are represented hollow, and may be kept at any desired
temperature during the operation of printing, by introducing steam into them; and
under the colour boxes c, c, c, hollow chambers are also made for the same purpose.
The degree of temperature required to be given to these must depend upon the nature
of the colouring material, and of the goods operated upon. For the purpose of conducting
steam to these hollow cylinders and colour boxes, pipes, as shown at v, v, v, are
attached, which lead from a steam boiler. But when either of these cylinders is
employed for embossing alone, or for embossing and printing at the same time, and
particularly for some kinds of goods where a higher temperature may be required, a red-hot
heater is then introduced into the hollow cylinder in place of steam.
If the cylinder B, is employed as the embossing cylinder, and it is not intended to
print the fabric by that cylinder simultaneously with the operation of embossing, the
feeding rolling b, must be removed, and also the colour box c, belonging to that cylinder;
and the cylinders A, and C, are to be employed for printing the fabric, the one
applying the colour before the embossing is effected, the other after it. It is however
to be remarked, that if A, and C, are to print colours on the fabric, and B, to emboss it,
in that case it is preferred, where the pattern would allow it. A and C, are wooden
rollers having the pattern upon their surfaces, and not metal, as the embossing cylinders
must of necessity be.
It will be perceived that this machine will print one, two, or three colours at the
same time, and that the operation of embossing may be performed simultaneously with
the printing, by either of the cylinders A, B, or C, or the operation may be performed
consecutively by the cylinders, either preceding or succeeding each other.
The situations of the doctors, when required to be used for removing any superfluous
colour from the surface of the printing cylinder, are shown at d, d, d; those for removing
any lint which may attach itself, at e, e, e. They are kept in their bearings by weighted levers
and screws, and receive a slight lateral movement to and fro, by means of the vertical
rod m, which is connected at top to an eccentric, on the end of the axle of the roller H,
and at its lower end to a horizontal rod mounted at the side of the frame; to this horizontal
rod, arms are attached, which are connected to the respective doctors; and thus
by the rotation of the eccentric, the doctors are made to slide laterally.
When the cylinders A, B, or C, are employed for embossing only, those doctors will not
be required. The driving power is communicated to the machine from any first mover
through the agency of the toothed geer, which gives rotatory motion to the cylinder B,
and from thence to the other cylinders A, and C, by toothed geer shown in fig. 370.
EMBROIDERING MACHINE. (Machine à broder, Fr.; Steckmaschine, Germ.)
This art has been till of late merely a handicraft employment, cultivated on account of its
elegance by ladies of rank. But a few years ago M. Heilmann of Mulhausen invented a machine
of a most ingenious kind, which enables a female to embroider any design with 80
or 140 needles as accurately and expeditiously as she formerly could do with one. A brief[430]
account of this remarkable invention will therefore be acceptable to many readers. It
was displayed at the national exposition of the products of industry in Paris for 1834,
and was unquestionably the object which stood highest in public esteem; for whether at
rest or in motion, it was always surrounded with a crowd of curious visiters, admiring
the figures which it had formed, or inspecting its movements and investigating its
mechanism. 130 needles were occupied in copying the same pattern with perfect regularity,
all set in motion by one person.
Several of these machines are now mounted in France, Germany, and Switzerland.
I have seen one factory in Manchester, where a great many of them are doing beautiful
work.
The price of a machine having 130 needles, and of consequence 260 pincers or fingers
and thumbs to lay hold of them, is 5000 francs, or 200l. sterling; and it is estimated to
do daily the work of 15 expert hand embroiderers, employed upon the ordinary frame. It
requires merely the labour of one grown-up person, and two assistant children. The
operative must be well taught to use the machine, for he has many things to attend to:
with the one hand he traces out, or rather follows the design with the point of the pantograph;
with the other he turns a handle to plant and pull all the needles, which are
seized by pincers and moved along by carriages, approaching to and receding from the
web, rolling all the time along an iron railway; lastly, by means of two pedals, upon
which he presses alternately with the one foot and the other, he opens the 130 pincers
of the first carriage, which ought to give up the needles after planting them in the stuff,
and he shuts with the same pressure the 130 pincers of the second carriage, which is to
receive the needles, to draw them from the other side, and to bring them back again.
The children have nothing else to do than to change the needles when all their threads
are used, and to see that no needle misses its pincers.
This machine deserves particular attention, because it is no less remarkable for the
happy arrangement of its parts, than for the effects which it produces. It may be
described under four heads: 1. the structure of the frame; 2. the disposition of the
web; 3. the arrangement of the carriages; and 4. the construction of the pincers.
1. The structure of the frame. It is composed of cast-iron, and is very massive.
Fig. 371. exhibits a front elevation of it. The length of the machine depends
upon the number of pincers to be worked. The model at the exposition had 260
pincers, and was 2 metres and a half (about 100 inches or 8 feet 4 inches English)
long. The figure here given has been shortened considerably, but the other proportions
are not disturbed. The breadth of the frame ought to be the same for every
machine, whether it be long or short, for it is the breadth which determines the length of
the thread to be put into the needles, and there is an advantage in giving it the full
breadth of the model machine, fully 100 inches, so that the needles may carry a thread
at least 40 inches long.
Disposition of the piece to be embroidered.—We have already stated that the pincers
which hold the needles always present themselves opposite to the same point, and that
in consequence they would continually pass backwards and forwards through the same
hole, but the piece is displaced with sufficient precision to bring opposite the tips progressively
of the needles, every point upon which they are to work a design, such as a flower.
The piece is strained perpendicularly upon a large rectangular frame, whose four
sides are visible in fig. 371.; namely the two vertical sides at F F, and the two horizontal
sides, the upper and lower at F′ F′′. We see also in the figure two long wooden rollers
G and G, whose ends, mounted with iron studs, are supported upon the sides F of the
frame, so as to turn freely. These form a system of beams upon which the piece destined
to receive the embroidery, is wound and kept vertically stretched to a proper degree,
for each of these beams bears upon its end a small ratchet wheel g, g; the teeth
of one of them being inclined in the opposite direction to those of the other. Besides
this system of lower beams, there is another of two upper beams, which is however but
imperfectly seen in the figure, on account of the interference of other parts in this view
of the machine. One of these systems presents the web to the inferior needles, and
the other to the upper needles. As the two beams are not in the same vertical plane,
the plane of the web would be presented obliquely to the needles were it not for a
straight bar of iron, round whose edge the cloth passes, and which renders it
vertical. The piece is kept in tension crosswise by small brass templets, to which the
strings g′′ are attached, and by which it is pulled towards the sides of the frame F. It
remains to shew by what ingenious means this frame may be shifted in every possible
direction. M. Heilmann has employed for this purpose the pantograph which
draughtsmen use for reducing or enlarging their plans in determinate proportions.
b b′ f b′′ (fig. 371.) represents a parallelogram of which the four angles b, b′, f, b′′, are
jointed in such a way that they may become very acute or very obtuse at pleasure,
while the sides of course continue of the same length; the sides b, b′ and b, b′′ are prolonged,
the one to the point d, and the other to the point c, and these points c and d,[431]
are chosen under the condition that in one of the positions of the parallelogram, the
line c d which joins them passes through the point f; this condition may be fulfilled in
an infinite number of manners, since the position of the parallelogram remaining the
same, we see that if we wished to shift the point d further from the point b′, it would
be sufficient to bring the point c near enough to b′′, or vice versa; but when we
have once fixed upon the distance b′ d, it is evident that the distance b′′ c is its necessary
consequence. Now the principle upon which the construction of the pantograph rests
is this; it is sufficient that the three points d, f, and c be in a straight line, in one only
of the positions of the parallelogram, in order that they shall remain always in a
straight line in every position which can possibly be given to it.
We see in the figure that the side b c, has a handle B′′ with which the workman
puts the machine in action. To obtain more precision and solidity in work, the sides
of the pantograph are joined, so that the middle of their thickness lies exactly in the
vertical plane of the piece of goods, and that the axes of the joints are truly perpendicular
to this plane, in which consequently all the displacements are effected. We
arrive at this result by making fast to the superior great cross bar D′′ an elbow piece d2,
having a suitable projection, and to which is adapted in its turn the piece d′, which[432]
receives in a socket the extremity of the side b, d; this piece d′ is made fast to d′′ by
a bolt, but it carries an oblong hole, and before screwing up the nut, we make the
piece advance or recede, till the fulcrum point comes exactly into the plane of the web.
This condition being fulfilled, we have merely to attach the frame to the angle f of the
parallelogram, which is done by means of the piece F′′.
It is now obvious that if the embroiderer takes the handle B′′ in his hand and makes
the pantograph move in any direction whatever, the point f will describe a figure similar
to the figure described by the point c, and six times smaller, but the point f cannot
move without the frame, and whatever is upon it moving also. Thus, in the movement
of the pantograph, every point of the web describes a figure equal to that described by
the point f, and consequently similar to that described by the point c, but six times
smaller; the embroidered object being produced upon the cloth in the position of that
of the pattern. It is sufficient therefore to give the embroidering operative who holds
the handle B′′, a design six times greater than that to be executed by the machine, and to
afford him at the same time a sure and easy means of tracing over with the point c, all the
outlines of the pattern. For this purpose he adapts to c, perpendicularly to the plane
of the parallelogram, a small style terminated by a point C′, and he fixes the pattern
upon a vertical tablet E, parallel to the plane of the stuff and the parallelogram, and
distant from it only by the length of the style c C′′; this tablet is carried by the iron
rod c′, which is secured to a cast iron foot E′, serving also for other purposes, as we shall
presently see. The frame loaded with its beams and its cloth forms a pretty heavy
mass, and as it must not swerve from its plane, it needs to be lightened in order that the
operative may cause the point of the pantograph to pass along the tablet without straining
or uncertainty in its movements. M. Heilmann has accomplished these objects in the
following way. A cord e attached to the side b c of the pantograph passes over a return
pulley, and carries at its extremity, a weight which may be graduated at pleasure; this
weight equipoises the pantograph, and tends slightly to raise the frame. The lower
side of the frame carries two rods H and H, each attached by two arms h h, a little bent
to the left; both of these are engaged in the grooves of a pulley. Through this mechanism
a pressure can be exercised upon the frame from below upwards, which may be
regulated at pleasure, and without preventing the frame from moving in all directions,
it hinders it from deviating from the primitive plane to which the pantograph
was adjusted. The length of the rods H ought to be equal to the amount of the lateral
movement of the frame. Two guides i i carried by two legs of cast iron, present vertical
slits in which the lower part of the frame F′ is engaged.
Disposition of the carriages.—The two carriages, which are similar, are placed the
one to the right, and the other to the left of the frame. The carriage itself is composed
merely of a long hollow cylinder of cast iron L, carrying at either end a system
of two grooved castors or pulleys L′, which roll upon the horizontal rails K; the pulleys
are mounted upon a forked piece l′, with two ends to receive the axes of the pulleys,
and the piece l′ is itself bolted to a projecting ear l cast upon the cylinder.
This assemblage constitutes properly speaking the carriage, resting in a perfectly
stable equilibrium upon the rails K, upon which it may be most easily moved backwards
and forwards, carrying its train of needles to be passed or drawn through the cloth.
M. Heilmann has contrived a mechanism by which the operative without budging
from his place may conduct the carriages, and regulate as he pleases the extent of their
course, as well as the rapidity of their movements. By turning the axes M′′ in the one
direction or the other, the carriage may be made to approach to, or recede from the
web.
When one of the carriages has advanced to prick the needles into the stuff, the other
is there to receive them; it lays hold of them with its pincers, pulls them through,
performs its course by withdrawing to stretch the thread, and close the stitch, then it
goes back with the needles to make its pricks in return. During these movements the
first carriage remains at its post waiting the return of the second. Thus the two chariots
make in succession an advance and a return, but they never move together.
To effect these movements M. Heilmann has attached to the piece O′ made fast to
the two uprights A C and A D of the frame, a bent lever n o n′ n′′ movable round the
point o; the bend n′ carries a toothed wheel O′, and the extremity n′′ a toothed wheel
O′′; the four wheels M M′ O′ and O′′ have the same number of teeth and the same
diameter; the two wheels O′ and O′′ are fixed in reference to each other, so that it is
sufficient to turn the handle N to make the wheel O′′ revolve, and consequently the
wheel O′; when the lever n o is vertical, the wheel O′ touches neither the wheel M nor the
wheel M′; but if it be inclined to the one side or the other, it brings the wheel O′ alternately
into geer with the wheel M or the wheel M′. As the operative has his two hands
occupied, the one with the pantograph and the other with the handle of impulsion, he
has merely his feet for acting upon the lever n o, and as he has many other things to
do, M. Heilmann has adapted before him a system of two pedals, by which he executes[433]
with his feet a series of operations no less delicate than those which he executes with
his hands.
The pedals P are moveable round the axis p, and carry cords p′ wound in an opposite
direction upon the pulleys P′; these pulleys are fixed upon a moveable shaft P′′, supported
upon one side by the prop E′, and on the other in a piece K′ attached to the two great
uprights of the frame. In depressing the pedal P (now raised in the figure), the upper
part of the shaft P′′ will turn from the left to the right, and the lever n o will become
inclined so as to carry the wheel O′ upon the wheel M′, but at the same time the pedal
which is now depressed will be raised, because its cord will be forced to wind itself upon
its pulley, as much as the other cord has unwound itself; and thus the apparatus will
be ready to act in the opposite direction, when wanted.
Disposition of the pincers.—The shaft L′ carries, at regular intervals of a semi-diameter,
the appendages q q cast upon it, upon which are fixed, by two bolts, the curved
branches Q destined to bear the whole mechanism of the pincers. When the pincers are
opened by their appropriate leverage, and the half of the needle, which is pointed at
each end, with the eye in the middle, enters the opening of its plate, it gets lodged in an
angular groove, which is less deep than the needle is thick, so that when the pincers are
closed, the upper jaw presses it into the groove. In this way the needle is firmly held,
although touched in only three points of its circumference.
Suppose, now, that all the pincers are mounted and adjusted at their proper distances
upon their prismatic bar, forming the upper range of the right carriage. For opening
all the pincers there is a long plate of iron, U, capable of turning upon its axis, and
which extends from the one end of the carriage to the other. This axis is carried by a
kind of forks which are bolted to the extremity of the branches Q. By turning that
axis the workman can open the pincers at pleasure, and they are again closed by springs.
This movement is performed by his feet acting upon the pedals.
The threads get stretched in proportion as the carriage is run out, but as this tension
has no elastic play, inconveniences might ensue which are prevented by adapting to the
carriage a mechanism by means of which all the threads are pressed at the same time
by a weight susceptible of graduation. A little beneath the prismatic bar, which carries
the pincers, we see in the figure, a shaft Y, going from one end of the carriage to the
other, and even a little beyond it; this shaft is carried by pieces y which are fixed to the
arms Q, and in which it can turn. At its left end it carries two small bars y′ and w′,
and at its right a single bar y′, and a counterweight (not visible in this view); the ends
of the two bars y′ are joined by an iron wire somewhat stout and perfectly straight.
When the carriage approaches the web, and before the iron wire can touch it, the little
bar w presses against a pin w′, which rests upon it, and tends to raise it more and more.
In what has preceded we have kept in view only the upper range of pincers and needles,
but there is an inferior range quite similar, as the figure shows, at the lower ends of the
arms Q. In conclusion, it should be stated, that the operative does not follow slidingly
with the pantograph the trace of the design which is upon the tablet or the picture, but he
must stop the point of the style upon the point of the pattern into which the needle should
enter, then remove it, and put it down again upon the point by which the needle ought
to re-enter in coming from the other side of the piece, and so on in succession. To
facilitate this kind of reading off, the pattern upon the tablet is composed of right lines
terminated by the points for the entrance and return of the needle, so that the operative
(usually a child) has continually under her eyes the series of broken lines which must be
followed by the pantograph; if she happens to quit this path an instant, without having
left a mark of the point at which she had arrived, she is under the necessity of looking
at the piece to see what has been already embroidered, and to find by this comparison
the point at which she must resume her work, so as not to leave a blank, or to repeat
the same stitch.
Explanation of figure:
A, lower cross bars, which unite the legs of the two ends of the frame.
a, the six feet of the front end of the frame.
a′, the six feet of the posterior end of the frame.
a′′, curved pieces which unite the cross bars A′′ to the uprights.
B′′, handle of the pantograph.
b b′ b′′, three of the angles of the pantograph.
c, point of the side b b′′ on which the point is fixed.
C′′, point of the pantograph.
D′′, cross bar in form of a gutter, which unites the upper parts of the frame.
d, fixed point, round which the pantograph turns.
E, tablet upon which the pattern to be embroidered is put.
E′, support of that tablet.
e, cord attached at one end to the side b c of the pantograph passing over a guide pulley,
[434]and carrying a weight at the other end.
e′, iron rod by which the tablet E is joined to its support E′.
F F, uprights of the cloth-carrying frame.
F′ F′, horizontal sides of the same frame.
G, four roll beams.
G′′, the piece of cloth.
g′′, the strings, which serve to stretch the cloth laterally.
EMERALD. (Emeraude, Fr.; Smaragd, Germ.) Is a precious stone of a beautiful
green colour; valued next to diamond, and in the same rank as oriental ruby
and sapphire. It occurs in prisms with a regular hexagonal base; sp. grav. 2·7;
scratches quartz with difficulty; is scratched by topaz; fusible at the blowpipe into a
frothy bead; the precipitate afforded by ammonia, from its solution, is soluble, in a
great measure, in carbonate of ammonia. Its analysis is given very variously by different
chemists. It contains about 14 per cent. of glucina, which is its characteristic
constituent; along with 68 of silica, 16 of alumina, a very little lime and iron. The
beautiful emerald of Peru is found in a clay schist mixed with some calcareous matter.
A stone of 4 grains weight is said to be worth from 4l. to 5l.; one of 8 grains, 10l.;
one of 15 grains, being fine, is worth 60l.; one of 24 grains fetched, at the sale of
M. de Drée’s cabinet, 2400 francs, or nearly 100l.
The beryl is analogous in composition to the emerald, and is employed (when of the
common opaque kind, found near Limoges,) by chemists, for procuring the earth
glucina.
EMERY. This mineral was long regarded as an ore of iron; and was called by
Haüy fer oxidé quartzifère. It is very abundant in the island of Naxos, at cape Emeri,
whence it is imported in large quantities. It occurs also in the islands of Jersey and
Guernsey, at Almaden, in Poland, Saxony, Sweden, Persia, &c. Its colour varies from
red brown to dark brown; its specific gravity is about 4·000; it is so hard as to scratch
quartz and many precious stones. By Mr. Tennant’s analysis, it consists of alumina,
80; silica, 3; iron, 4. Another inferior kind yielded 32 of iron, and only 50 of
alumina.
The alumina of emery is believed to be aggregated to the same degree of hardness
as in corundum or adamantine spar; which is one of the hardest minerals known.
Emery is extensively employed for grinding metals, glass, &c.; for which purpose it is
reduced to powders of different degrees of fineness by grinding and elutriation. When
so treated, it is sold under the name of flour of emery, or washed emery.
EMPYREUMA, means the offensive smell produced by fire applied to organic
matters, chiefly vegetable, in close vessels. Thus, empyreumatic vinegar is obtained
by distilling wood at a red heat, and empyreumatic oil from many animal substances in
the same way.
ENAMELS, (Emaux, Fr.; Schmelzglas, Germ.) are varieties of glass, generally
opaque and coloured, always formed by the combination of different metallic oxides, to
which certain fixed fusible salts are added, such as the borates, fluates, and phosphates.
The simplest enamel, and the one which serves as a basis to most of the others, is
obtained by calcining first of all a mixture of lead and tin, in proportions varying from
15 to 50 parts of tin for 100 of lead. The middle term appears to be the most suitable
for the greater number of enamels; and this alloy has such an affinity for oxygen, that
it may be calcined with the greatest ease in a flat cast-iron pot, and at a temperature
not above a cherry red, provided the dose of tin is not too great. The oxide is drawn
off to the sides of the melted metal according as it is generated, new pieces of the alloy
being thrown in from time to time, till enough of the powder be obtained. Great care
ought to be taken that no metallic particles be left in the oxide, and that the calcining
heat be as low as is barely sufficient; for a strong fire frits the powder, and obstructs its
subsequent comminution. The powder when cold is ground in a proper mill, levigated
with water, and elutriated, as will be described under Red lead. In this state of fineness
and purity, it is called calcine, or flux, and it is mixed with siliceous sand and
some alkaline matter or sea-salt. The most ordinary proportions are, 4 of sand, 1 of
sea-salt, and 4 of calcine. Chaptal states, that he has obtained a very fine product from
100 parts of calcine, made by calcining equal parts of lead and tin, 100 parts of ground
flint, and 200 parts of pure subcarbonate of potash. In either case, the mixture is put
into a crucible, or laid simply on a stratum of sand, quicklime spontaneously slacked, or
wood-ashes, placed under a pottery or porcelain kiln. This mass undergoes a semi-vitrification;
or even a complete fusion on its surface. It is this kind of frit which
serves as a radical to almost every enamel; and by varying the proportions of the ingredient,
more fusible, more opaque, or whiter enamels are obtained. The first of these
qualities depends on the quantity of sand or flux, and the other two on that of the tin.
The sea-salt employed as a flux may be replaced either by salt of tartar, by pure
potash, or by soda; but each of these fluxes gives peculiar qualities to the enamel.
Most authors who have written on the preparation of enamels, insist a great deal on[435]
the necessity of selecting carefully the particular sand that should enter into the composition
of the frit, and they even affirm that the purest is not the most suitable.
Clouet states, in the 34th volume of the Annales de Chimie, that the sand ought to contain
at least 1 part of talc for 3 of siliceous matter, otherwise the enamel obtained is
never very glassy, and that some wrinkled spots from imperfect fusion are seen on its
surface; and yet we find prescribed in some old treatises, to make use of ground flints,
fritted by means of salt of tartar or some other flux. It would thence appear that the
presence of talc is of no use towards the fusibility of the silica, and that its absence may
be supplied by increasing the dose of the flux. In all cases, however, we ought to beware
of metallic oxides in the sand, particularly those of iron and manganese, which most
frequently occur, and always injure the whiteness of the frit.
The ancients carried the art of enamelling to a very high perfection, and we occasionally
find beautiful specimens of their work, of which we know neither the composition, nor
the manner of applying it. Then, as at present, each artist made a mystery of the
means that succeeded best with him, and thus a multitude of curious processes have
been buried with their authors. Another cause contributes powerfully to this sort of
declension in the arts. Among the vast number of recipes which have been published for
the formation of enamels, there are several in which substances are mentioned that can
no longer be procured, whether owing to a change of denomination, or because the
substances cannot now be found in commerce, or because they are not of the same
nature as of old. Hence, in many cases, we find it impossible to obtain satisfactory
results. What we have now said renders it desirable that the operations should be
resumed anew, or upon new bases, and availing ourselves of all the known chemical
facts, we should employ in the production of enamels, raw materials of the purest kind.
The Venetians are still in possession of the best enamel processes, and they supply
the French and other nations with the best kinds of enamel, of every coloured shade.
Enamels are distinguished into transparent and opaque; in the former all the
elements have experienced an equal degree of liquefaction, and are thus run into crystal
glass, whilst in the others, some of their elements have resisted the action of heat more,
so that their particles retain sufficient aggregation to prevent the transmission of light.
This effect is produced, particularly by the oxide of tin, as we shall perceive in treating
of white enamel.
The frits for enamels that are to be applied to metallic surfaces require greater fusibility,
and should therefore contain more flux; and the sand used for these should be
calcined beforehand with one-fourth its weight of sea-salt; sometimes, indeed, metallic
fluxes are added, as minium or litharge. For some metallic colours, the oxides of lead
are very injurious, and in this case recourse must be had to other fluxes. Clouet states
that he has derived advantage from the following mixtures, as bases for purples, blues,
and some other delicate colours:—
Three parts of siliceous sand, one of chalk, and three of calcined borax; or, three of
glass (of broken crystal goblets), one of calcined borax, one-fourth of a part of nitre,
and one part of well washed diaphoretic antimony. These compositions afford a very
white enamel, which accords perfectly well with blue.
It is obvious that the composition of this primary matter may be greatly varied; but
we should never lose sight of the essential quality of a good enamel; which is, to
acquire, at a moderate heat, sufficient fluidity, to take a shining surface, without running
too thin. It is not complete fusion which is wanted; but a pasty state, of such a
degree as may give it, after cooling, the aspect of having suffered complete liquefaction.
Dead-white Enamel.—This requires greater nicety in the choice of its materials than
any other enamel, as it must be free from every species of tint, and be perfectly white;
hence the frit employed in this case should be itself composed of perfectly pure ingredients.
But a frit should not be rejected hastily because it may be somewhat discoloured,
since this may depend on two causes; either on some metallic oxides, or on
fuliginous particles proceeding from vegetable or animal substances. Now the latter
impurities may be easily removed by means of a small quantity of peroxide of manganese,
which has the property of readily parting with a portion of its oxygen, and of
thus facilitating the combustion, that is to say, the destruction of the colouring carbonaceous
matter. Manganese indeed possesses a colouring power itself on glass, but only
in its highest state of oxidizement, and when reduced to the lower state, as is done by
incombustible matters, it no longer communicates colour to the enamel combinations.
Hence the proportion of manganese should never exceed what is just; for the surplus
would cause colour. Sometimes, indeed, it becomes necessary to give a little
manganese-colour, in order to obtain a more agreeable shade of white; as a little azure
blue is added to linens, to brighten or counteract the dulness of their yellow tint.
A white enamel may be conveniently prepared also with a calcine composed of two parts
of tin and one of lead calcined together; of this combined oxide, one part is melted
with two parts of fine crystal and a very little manganese, all previously ground together.[436]
When the fusion is complete, the vitreous matter is to be poured into clear
water, and the frit is then dried, and melted anew. The pouring into water and fusion
are sometimes repeated 4 times, in order to secure a very uniform combination. The
crucible must be carefully screened from smoke and flame. The smallest portions of
oxide of iron or copper admitted into this enamel will destroy its value.
Some practitioners recommend the use of washed diaphoretic antimony (antimoniate
of potash, from metallic antimony and nitre deflagrated together) for white enamel; but
this product cannot be added to any preparation of lead or other metallic oxides; for it
would tend rather to tarnish the colour than to clear it up; and it can be used therefore
only with ordinary glass, or with saline fluxes. For three parts of white glass (without
lead) one part of washed diaphoretic antimony is to be taken; the substances are well
ground together, and fused in the common way.
Blue Enamel.—This fine colour is almost always obtained from the oxide of cobalt
or some of its combinations, and it produces it with such intensity that only a very
little can be used, lest the shade should pass into black. The cobalt blue is so rich
and lively that it predominates in some measure over every other colour, and masks
many so that they can hardly be perceived; it is also most easily obtained. To bring
it out, however, in all its beauty, the other colours must be removed as much as possible,
and the cobalt itself should be tolerably pure. This metal is associated in the
best known ores with a considerable number of foreign substances, as iron, arsenic, copper,
nickel, and sulphur, and it is difficult to separate them completely; but for enamel
blues, the oxide of cobalt does not require to be perfectly free from all foreign metals;
the iron, nickel, and copper being most prejudicial, should be carefully eliminated.
This object may be most easily attained by dissolving the ore in nitric acid, evaporating
the solution to a syrupy consistence, to expel the excess of acid, and separate a portion
of arsenic. It is now diluted with water, and solution of carbonate of soda is dropped
slowly into it with brisk agitation, till the precipitate, which is at first of a whitish
gray, begins to turn of a rose-red. Whenever this colour appears, the whole must be
thrown on a filter, and the liquid which passes through must be treated with more of
the carbonate of soda, in order to obtain the arseniate of cobalt, which is nearly pure.
Since arsenic acid and its derivatives are not capable of communicating colour themselves,
and as they moreover are volatile, they cannot impair the beauty of the blue, and
hence this preparation affords it in great perfection.
Metallic fluxes are not the most suitable for this colour; because they always communicate
a tint of greater or less force, which never fails to injure the purity of the blue.
Nitre is a useful addition, as it keeps the oxide at the maximum of oxidation, in which
state it produces the richest colour.
Yellow Enamel.—There are many processes for making this colour in enamel; but it
is somewhat difficult to fix, and it is rarely obtained of an uniform and fine tint. It
may be produced directly with some preparations of silver, as the phosphate or sulphate;
but this method does not always succeed, for too strong a heat or powerful fluxes readily
destroy it, and nitre is particularly prejudicial. This uncertainty of success with the
salts of silver causes them to be seldom employed; and oxides of lead and antimony are
therefore preferred, which afford a fine yellow when combined with some oxides that are
refractory enough to prevent their complete vitrification. One part of white oxide of
antimony may be taken with from one to three parts of white lead, one of alum, and
one of sal-ammoniac. Each of these substances is to be pulverized, and then all are to
be exactly mixed, and exposed to a heat adequate to decompose the sal-ammoniac. This
operation is judged to be finished when the yellow colour is well brought out. There
is produced here a combination quite analogous to that known under the name of
Naples yellow.
Other shades of yellow may be procured either with the oxide of lead alone, or by
adding to it a little red oxide of iron; the tints varying with the proportion of the
latter.
Clouet says, in his memoir on enamels, that a fine yellow is obtained with pure oxide
of silver, and that it is merely necessary to spread a thin coat of it on the spot to be coloured.
The piece is then exposed to a moderate heat, and withdrawn as soon as this
has reached the proper point. The thin film of metallic silver revived on the surface
being removed, the place under it will be found tinged of a fine yellow, of hardly any
thickness. As the pellicle of silver has to be removed which covers the colour, it is
requisite to avoid fixing this film with fluxes; and it ought therefore to be applied
after the fusion of the rest. The yellows require in general little flux, and they answer
better with one of a metallic nature.
Green Enamel.—It is known that a green colour may be produced by a mixture of
yellow and blue; but recourse is seldom had to this practice for enamels, as they can be
obtained almost always directly with the oxide of copper; or still better with the oxide
of chrome, which has the advantage of resisting a strong heat.
[437]
Chemists describe two oxides of copper, the protoxide, of an orange red colour, which
communicates its colour to enamels, but it is difficult to fix; the deutoxide is blue in
the state of hydrate, but blackish-brown when dry, and it colours green all the vitreous
combinations into which it enters. This oxide requires, at most, one or two proportions
of flux, either saline or metallic, to enter into complete fusion; but a much smaller
dose is commonly taken, and a little oxide of iron is introduced. To four pounds of frit,
for instance, two ounces of oxide of copper and 48 grains of red oxide of iron are used;
and the ordinary measures are pursued for making very homogeneous enamel.
The green produced by the oxide of chrome is much more solid; it is not affected
by a powerful fire, but it is not always of a fine shade. It generally inclines too
much to the dead-leaf yellow, which depends on the degree of oxygenation of the chrome.
Red Enamel.—We have just stated, that protoxide of copper afforded a fine colour
when it could be fixed, a result difficult to obtain on account of the fugitive nature of
this oxide; slight variations of temperature enabling it to absorb more oxygen. The
proper point of fusion must be seized, for taking it from the fire whenever the desired
colour is brought out. Indeed, when a high temperature has produced peroxidizement,
this may be corrected by adding some combustible matter, as charcoal, tallow, tartar,
&c. The copper then returns to its minimum of oxidizement, and the red colour which
had vanished, reappears. It is possible, in this way, and by pushing the heat a little,
to accomplish the complete reduction of a part of the oxide; and the particles of metallic
copper thereby disseminated in a reddish ground, give this enamel the aspect of the
stone called avanturine. The surest and easiest method of procuring protoxide of
copper is to boil a solution of equal parts of sugar, and sulphate or rather acetate of
copper, in four parts of water. The sugar takes possession of a portion of the oxygen
of the cupreous oxide, and reduces it to the protoxide; when it may be precipitated in
the form of a granular powder of a brilliant red. After about two hours of moderate
ebullition, the liquid is set aside to settle, decanted off the precipitate, which is washed
and dried.
This pure oxide, properly employed by itself, furnishes a red which vies with the
finest carmine, and by its means every tint may be obtained from red to orange, by
adding a greater or smaller quantity of peroxide of iron.
The preparations of gold, and particularly the oxide and purple of Cassius, are likewise
employed, with advantage, to colour enamel red, and this composition resists a
powerful fire tolerably well. For some time back, solutions of gold, silver, and platinum
have been used with success instead of their oxides; and, in this way, a more intimate
mixture may be procured, and, consequently, more homogeneous tints.
Black Enamel.—Black enamels are made with peroxide of manganese or protoxide
of iron; to which more depth of colour is given with a little cobalt. Clay alone,
melted with about a third of its weight of protoxide of iron, gives, according to Clouet,
a fine black enamel.
Violet Enamel.—The peroxide of manganese in small quantity by itself furnishes,
with saline or alkaline fluxes, an enamel of a very fine violet hue; and variations of
shade are easily had by modifying the proportions of the elements of the coloured
frit. The great point is to maintain the manganese in a state of peroxidation, and consequently
to beware of placing the enamel in contact with any substance attractive
of oxygen.
Such are the principal coloured enamels hitherto obtained by means of metallic
oxides; but since the number of these oxides is increasing every day, it is to be wished
that new trials be made with such as have not yet been employed. From such researches
some interesting results would unquestionably be derived.
Of painting on Enamel.—Enamelling is only done on gold and copper; for silver
swells up, and causes blisters and holes in the coat of enamel. All enamel paintings
are, in fact, done on copper or gold.
The goldsmith prepares the plate that is to be painted upon. The gold should be
22 carats fine: if purer, it would not be sufficiently stiff; if coarser, it would be subject
to melt; and its alloy should be half white and half red, that is, half silver and half
copper; whereby the enamel with which it is covered will be less disposed to turn
green, than if the alloy were entirely copper.
The workman must reserve for the edge of the plate a small fillet, which he calls the
border. This ledge serves to retain the enamel, and hinders it from falling off when
applied and pressed on with a spatula. When the plate is not to be counter-enamelled,
it should be charged with less enamel, as, when exposed to heat, the enamel draws up
the gold to itself, and makes the piece convex. When the enamel is not to cover the
whole plate, it becomes necessary to prepare a lodgement for it. With this view, all
the outlines of the figure are traced on the plate with a black-lead pencil, after which
recourse is had to the graver.
The whole space enclosed by the outlines must be hollowed out in bas-relief, of a[438]
depth equal to the height of the fillet, had the plate been entirely enamelled. This
sinking of the surface must be done with a flat graver as equally as possible; for if
there be an eminence, the enamel would be weaker at that point, and the green would
appear. Some artists hatch the bottom of the hollow with close lines, which cross each
other in all directions; and others make lines or scratches with the end of a file broken
off square. The hatchings or scratches lay hold of the enamel, which might otherwise
separate from the plate. After this operation, the plate is cleansed by boiling it in
an alkaline ley, and it is washed first with a little weak vinegar, and then with clear
water.
The plate thus prepared is to be covered with a coat of white enamel, which is done
by bruising a piece of enamel in an agate or porcelain mortar to a coarse powder like
sand, washing it well with water, and applying it in the hollow part in its moist state.
The plate may meanwhile be held in an ordinary forceps. The enamel powder is
spread with a spatula. For condensing the enamel powder, the edges of the plate are
struck upon with this spatula.
Whenever the piece is dry, it is placed on a slip of sheet iron perforated with several
small holes, see fig. 375., which is laid on hot cinders; and it is left there until it
ceases to steam. It must be kept hot till it goes to the fire; for were it allowed to cool
it would become necessary to heat it again very gradually at the mouth of the furnace
of fusion, to prevent the enamel from decrepitating and flying off.
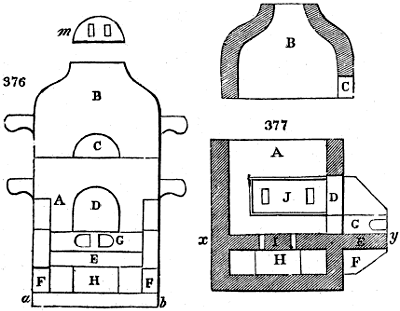
Before describing the manner of exposing the piece to the fire, we must explain the
construction of the furnace. It is square, and is shewn in front elevation in fig. 376.
It consists of two pieces, the lower part A, or the body of the furnace, and the upper
part B, or the capital, which is laid on the lower part as is shewn in fig. 377., where these
two parts are separately represented. The furnace is made of good fire-clay, moderately
baked, and resembles very closely the assay or cupellation furnace. Its inside dimensions
are 9 inches in width; 13 inches in height in the body, and 9 in the capital. Its
general thickness is 2 inches.
The capital has an aperture or door C, fig. 376., which is closed by a fire-brick stopper
m, when the fire is to be made active. By this door fuel is supplied.
[439]
The body of the furnace has likewise a door D, which reaches down to the projecting
shelf E, called the bib (mentonnière), whose prominence is seen at E, fig. 376. This
shelf is supported and secured by the two brackets F, F; the whole being earthenware.
The height of the door D, is abridged by a peculiar fire-brick G, which not only covers
the whole projection of the shelf E, but enters within the opening of the door D, filling
its breadth, and advancing into the same plane with the inner surface of the furnace.
This plate is called the hearth; its purpose will appear presently; it may be taken out
and replaced at pleasure, by laying hold of the handle in its front.
Below the shelf E, a square hole, H, is seen, which serves for admitting air, and for
extracting the ashes. Similar holes are left upon each side of the furnace, as is shown
in the ground plan of the base, fig. 377., at H H H.
On a level with the shelf, in the interior of the furnace, a thin fire-tile I rests, perforated
with numerous small holes. This is the grate represented in a ground view in
fig. 375. Fig. 378, 379, 380. represent, under different aspects, the muffle. Fig. 377. shows
the elevation of its further end; fig. 379. its sides; and fig. 380. its front part. At J, fig. 377.
the muffle is seen in its place in the furnace, resting on two bars of iron, or, still better,
on ledges of fire-clay, supported on brackets attached to the lateral sides of the furnace.
The muffle is made of earthenware, and as thin as possible. The fuel consists of dry
beech-wood, or oaken branches, about an inch in diameter, cut to the length of 9 inches,
in order to be laid in horizontal strata within the furnace, one row only being placed
above the muffle. When the muffle has attained to a white-red heat, the sheet iron
tray, bearing its enamel plate, is to be introduced with a pair of pincers into the front
of the muffle, and gradually advanced towards its further end. The mouth of the
muffle is to be then closed with two pieces of charcoal only, between which the artist
may see the progress of the operation. Whenever the enamel begins to flow, the tray
must be turned round on its base to ensure equality of temperature; and as soon as the
whole surface is melted, the tray must be withdrawn with its plate, but slowly, lest the
vitreous matter be cracked by sudden refrigeration.
The enamel plate, when cold, is to be washed in very dilute nitric acid, and afterwards
in cold water, and a second coat of granular enamel paste is to be applied,
with the requisite precautions. This, being passed through the fire, is to be treated in
the same way a third time, when the process will be found complete. Should any
chinks happen to the enamel coat, they must be widened with a graver, and the space
being filled with ground enamel, is to be repaired in the muffle. The plate, covered
with a pure white enamel, requires always to be polished and smoothed with sandstone
and water, particularly if the article have a plane surface; and it is then finally glazed
at the fire.
The painting operation now follows. The artist prepares his enamel colours by
pounding them in an agate mortar, with a pestle of agate, and grinding them on an
agate slab, with oil of lavender, rendered viscid by exposure to the sun in a shallow
vessel, loosely covered with gauze or glass. The grinding of two drachms of enamel
pigment into an impalpable powder, will occupy a labourer a whole day. The painter
should have alongside of him a stove in which a moderate fire is kept up, for drying his
work whenever the figures are finished. It is then passed through the muffle.
Enamelling at the Lamp.—The art of the lamp enameller is one of the most agreeable
and amusing that we know. There is hardly a subject in enamel which may not be
executed by the lamp-flame in very little time, and more or less perfectly, according to
the dexterity of the artist, and his acquaintance with the principles of modelling.
In working at the lamp, tubes and rods of glass and enamel must be provided, of all
sizes and colours.
The enamelling table is represented in fig. 373., round which several workmen, with
their lamps, may be placed, while the large double bellows D below is set a-blowing by
a treadle moved with the foot. The flame of the lamp, when thus impelled by a powerful
jet of air, acquires surprising intensity. The bent nozzles or tubes A A A A, are made
of glass, and are drawn to points modified to the purpose of the enameller.
Fig. 374. shows, in perspective, the lamp A of the enameller standing in its cistern B;
the blowpipe C is seen projecting its flame obliquely upwards. The blowpipe is adjustable
in an elastic cork D, which fills up exactly the hole of the table into which it enters.
When only one person is to work at a table provided with several lamps, he sits down at
the same side with the pedal of the bellows; he takes out the other blowpipes, and plugs
the holes in the table with solid corks.
The lamp is made of copper or tin-plate, the wick of cotton threads, and either
tallow or oil may be used. Between the lamp and the workman a small board or
sheet of white iron B, called the screen, is interposed to protect his eyes from the glare
of light. The screen is fastened to the table by a wooden stem, and it throws its
shadow on his face.
[440]
The enamelling workshop ought to admit little or no daylight, otherwise the artist,
not perceiving his flame distinctly, would be apt to commit mistakes.
It is impossible to describe all the manipulations of this ingenious art, over which
taste and dexterity so entirely preside. But we may give an example. Suppose the
enameller wishes to make a swan. He takes a tube of white enamel, seals one of
its ends hermetically at his lamp, and while the matter is sufficiently hot, he blows on
it a minikin flask, resembling the body of the bird; he draws out, and gracefully bends
the neck; he shapes the head, the beak, and the tail; then, with slender enamel rods
of a proper colour, he makes the eyes; he next opens up the beak with pointed
scissors; he forms the wings and the legs; finally attaching the toes, the bird stands
complete.
The enameller also makes artificial eyes for human beings, imitating so perfectly the
colours of the sound eye of any individual, as to render it difficult to discover that he
has a blind and a seeing one.
It is difficult to make large articles at the blowpipe; those which surpass 5 or 6 inches
become nearly unmanageable by the most expert workmen.
EQUIVALENTS, CHEMICAL. (Stöchiometrie, Germ.) This expression was
first employed by Dr. Wollaston, to denote the primary proportions in which the
various chemical bodies reciprocally combine; the numbers representing these proportions
being referred to one standard substance of general interest, such as oxygen or
hydrogen reckoned unity, or 1,000. Dr. Dalton, who is the true author of the grand
discovery of definite, and multiple chemical ratios, calls these equivalent numbers atomic
weights, when reduced to their lowest terms, either hydrogen or oxygen being the
radix of the scale. Though it belongs to a chemical work, to discuss the principles
and develope the applications of the Atomic Theory, I shall be careful, upon all proper
occasions, to point out the vast advantages which the chemical manufacturer may derive
from it, and to show how much he may economize and improve his actual processes
by its means. See Element.
ESSENCES, are either ethereous oils, in which all the fragrance of vegetable products
reside; or the same combined and diluted with alcohol. See Oils, Ethereous.
ESSENCE D’ORIENT, the name of a pearly looking matter procured from the
blay or bleak, a fish of the genus cyprinus. This substance, which is found principally
at the base of the scales, is used in the manufacture of artificial pearls. A large quantity
of the scales being scraped into water in a tub, are there rubbed between the hands
to separate the shining stuff, which subsides on repose. The first water being decanted,
more is added with agitation till the essence is thoroughly washed from all impurities;
when the whole is thrown upon a sieve; the substance passes through, but the scales
are retained. The water being decanted off, the essence is procured in a viscid state, of
a bluish white colour, and a pearly aspect. The intestines of the same fish are also
covered with this beautiful glistening matter. Several other fish yield it, but in smaller
proportion. When well prepared, it presents exactly the appearance and reflections of the
real pearls, or the finest mother of pearl; properties which are probably owing to the
interposition of some portions of this same substance, between the laminæ of these shelly
concretions. Its chemical nature has not been investigated; it putrefies readily when
kept moist, an accident which may however be counteracted by water of ammonia.
See Pearls.
ETCHING Varnish. (Aetzgrund-Deckfirniss, Germ.) Though the practice of this
elegant art does not come within the scope of our Dictionary, the preparation of the
varnishes, and of the biting menstrua which it employs, legitimately does.
The varnish of Mr. Lawrence, an English artist resident in Paris, is made as follows:
Take of virgin wax and asphaltum, each two ounces, of black pitch and burgundy-pitch
each half an ounce. Melt the wax and pitch in a new earthenware glazed pot, and
add to them, by degrees, the asphaltum, finely powdered. Let the whole boil till such
time as that, taking a drop upon a plate, it will break when it is cold, on bending it
double two or three times betwixt the fingers. The varnish, being then enough boiled,
must be taken off the fire, and after it cools a little, must be poured into warm water
that it may work the more easily with the hands, so as to be formed into balls, which
must be kneaded, and put into a piece of taffety for use.
Care must be taken, first, that the fire be not too violent, for fear of burning the ingredients,
a slight simmering being sufficient; secondly, that whilst the asphaltum is
putting in, and even after it is mixed with the ingredients, they should be stirred continually
with the spatula; and, thirdly, that the water into which this composition is
thrown should be nearly of the same degree of warmth with it, in order to prevent a
kind of cracking that happens when the water is too cold.
The varnish ought always to be made harder in summer than in winter, and it will
become so if it be suffered to boil longer, or if a greater proportion of the asphaltum or[441]
brown rosin be used. The experiment above mentioned, of the drop suffered to cool,
will determine the degree of hardness or softness that may be suitable to the season
when it is used.
Preparation of the hard varnish used by Callot, commonly called the Florence Varnish:—Take
four ounces of fat oil very clear, and made of good linseed oil, like that
used by painters; heat it in a clean pot of glazed earthenware, and afterwards put to it
four ounces of mastick well powdered, and stir the mixture briskly till the whole be well
melted, then pass the mass through a piece of fine linen into a glass bottle with a long
neck, that can be stopped very securely; and keep it for the use that will be explained
below.
Method of applying the soft varnish to the plate, and of blackening it.—The plate
being well polished and burnished, as also cleansed from all greasiness by chalk or Spanish
white, fix a hand-vice on the edge of the plate where no work is intended to be, to
serve as a handle for managing it when warm; then put it upon a chafing dish, in which
there is a moderate fire, and cover the whole plate equally with a thin coat of the varnish;
and whilst the plate is warm, and the varnish upon it in a fluid state, beat every part
of the varnish gently with a small ball or dauber made of cotton tied up in taffety,
which operation smooths and distributes the varnish equally over the plate.
When the plate is thus uniformly and thinly covered with the varnish, it must be
blackened by a piece of flambeau, or of a large candle which affords a copious smoke;
sometimes two or even four such candles are used together for the sake of dispatch,
that the varnish may not grow cold, which if it does during the operation, the plate
must be heated again, that it may be in a melted state when that operation is performed;
but great care must be taken not to burn it, which when it happens may be easily perceived
by the varnish appearing burnt and losing its gloss.
The menstruum used and recommended by Turrell, an eminent London artist, for
etching upon steel, was prepared as follows:—
| Take |
Pyrolignous acid |
4 |
parts by measure, |
| |
Alcohol |
1 |
part, mix, and add |
| |
Nitric acid |
1 |
part. |
This mixed liquor is to be applied from 11⁄2 to 15 minutes, according to the depth
desired. The nitric acid was employed of the strength of 1·28—the double aquafortis
of the shops.
The eau forte or menstruum for copper, used by Callot, as also by Piranesi, with a slight
modification, is prepared, with
| 8 |
parts of strong French vinegar, |
| 4 |
parts of verdigris, |
| 4 |
ditto sea salt, |
| 4 |
ditto sal ammoniac, |
| 1 |
ditto alum, |
| 16 |
ditto water. |
The solid substances are to be well ground, dissolved in the vinegar, and diluted with
the water; the mixture is now to be boiled for a moment, and then set aside to cool.
This menstruum is applied to the washed, dried, and varnished plate, after it has
suffered the ordinary action of aquafortis, in order to deepen and finish the delicate
touches. It is at present called the eau forte à passer.
ETHER, is the name of a class of very light, volatile, inflammable, and fragrant
spirituous liquids, obtained by distilling in a glass retort, a mixture of alcohol with
almost any strong acid. Every acid modifies the result, in a certain degree, whence
several varieties of ether are produced. The only one of commercial importance is
sulphuric ether, which was first made known under the name of sweet oil of vitriol,
in 1540, by the receipt of Walterus Cordus. Froberus, 190 years after that date,
directed the attention of chemists afresh to this substance, under the new denomination
of ether.
There are two methods of preparing it; by the first, the whole quantity of acid and
alcohol are mixed at once, and directly subjected to distillation; by the second, the
alcohol is admitted, in a slender streamlet, into a body of acid previously mixed with a
little alcohol, and heated to 220° Fahr.
1. Mix equal weights of alcohol at spec. grav. 0·830, and sulphuric acid at 1·842, by
introducing the former into a large tubulated retort, giving it a whirling motion, so
that the alcohol may revolve round a central conical cavity. Into this species of whirlpool
the acid is to be slowly poured. The mixture, which becomes warm, is to be
forthwith distilled by attaching a spacious receiver to the retort, and applying the heat
of a sand-bath. The formation of ether takes place only at a certain temperature. If
the contents of the retort be allowed to cool, and be then slowly heated in a water bath,
alcohol alone will come over for some time without ether, till the mixture acquires the[442]
proper degree of heat. The first receiver should be a globe, with a tube proceeding
from its bottom, into a second receiver, of a cylindric shape, surrounded with ice-cold
water. The joints must be well secured by lutes, after the expanded air has been
allowed to escape. The liquid in the retort should be kept in a steady state of bullition.
The ether, as long as it is produced, condenses in the balloon and neck of the
receiver in striæ; when these disappear the process is completed. The retort must
now be removed from the sand; otherwise it would become filled with white fumes
containing sulphurous acid, and denser striæ would flow over, which would contaminate
the light product with a liquid called sweet oil of wine.
The theory of etherification demonstrates that when strong sulphuric acid is mixed
with alcohol, there is formed, on the one hand, a more aqueous sulphuric acid, and, on
the other, sulphovinic acid. When this mixture is made to boil, the sulphovinic acid is
decomposed, its dihydrate of carbon combines with the alcohol, and constitutes ether;
while the proportion of sulphovinic acid progressively diminishes. Mr. Hennell, of the
Apothecaries’ Hall, first explained these phenomena, and he was confirmed in his views
by the interesting researches of Serullas. The acid left in the retort is usually of a
black colour, and may be employed to convert into ether half as much alcohol again;
an experiment which may be repeated several times in succession.
The most profitable way of manufacturing ether has been pointed out by Boullay.
It consists in letting the alcohol drop in a slender stream into the acid, previously heated
to the etherifying temperature. If the acid in this case were concentrated to 1·846,
the reaction would be too violent, and the ether would be transformed into bicarburetted
hydrogen (dihydrate of carbon.) It is therefore necessary to dilute the acid down to
the density of 1·780; but this dilution may be preferably effected with alcohol instead
of water, by mixing three parts of the strongest acid with 2 of alcohol, specific gravity
0·830, and distilling off a portion of the ether thereby generated; after which the
stream of alcohol is to be introduced into the tubulure of the retort through a small
glass tube plunged into the mixture; this tube being the prolongation of a metallic
syphon, whose shorter leg dips into a bottle filled with the alcohol. The longer leg is
furnished with a stop-cock, for regulating at pleasure the alcoholic streamlet. The distilled
vapours should be transmitted through a worm of pure tin surrounded by cold
water, and the condensed fluid received in a glass bottle. The quantity of alcohol
which can be thus converted into ether by a given weight of sulphuric acid, has not
hitherto been accurately determined; but it is at least double. In operating in this way,
neither sulphurous acid, nor sweet oil of wine is generated, while the residuary liquid in
the retort continues limpid and of a merely brownish yellow colour. No sulphovinic acid
is formed, and according to the experiments of Geiger, the proportion of ether approaches
to what theory shows to be the maximum amount. In fact 57 parts of alcohol
of 0·83 sp. grav. being equivalent to 46·8 parts of anhydrous alcohol, yield according
to Geiger, 331⁄2 parts of ether; and by calculation, they should yield 371⁄4.
The ether of the first distillation is never pure, but always contains a certain quantity
of alcohol. The density of that product is usually 0·78, and if prepared by the first of
the above methods, contains besides alcohol, pretty frequently sulphurous acid, and
sweet oil of wine, impurities from which it must be freed. Being agitated with its
bulk of milk of lime, both the acid and the alcohol are removed at the same time; and
if it be then decanted and agitated, first with its bulk of water, next decanted into a retort
containing chloride of calcium in coarse powder and distilled, one third of perfectly
pure ether may be drawn over. Gay Lussac recommends to agitate the ether,
first with twice its volume of water, to mix it, and leave it in contact with powdered unslaked
lime for 12 or 14 hours, and then to distil off one third of pure ether. The
remaining two thirds consist of ether containing a little alcohol. If in preparing ether
by Boullay’s method, the alcohol be too rapidly introduced, much of this liquid will
come over unchanged. If in this state the ether be shaken with water, a notable quantity
of it will be absorbed, because weak alcohol dissolves it very copiously. The above
product should therefore be re-distilled, and the first half that comes over may be considered
as ether, and treated with water and lime. The other half must be exposed
afresh to the action of sulphuric acid.
Pure ether possesses the following properties. It is limpid, of spec. grav. 0·713, or
0·715 at 60°; has a peculiar penetrating strong smell; a taste at first acrid, burning,
sweetish, and finally cooling. It has neither an acid nor alkaline reaction; is a non-conductor
of electricity, and refracts light strongly. It is very volatile, boiling at
96° or 97° F., and produces by its evaporation a great degree of cold. At the temperature
of 62·4, the vapour of ether balances a column of mercury 15 inches high,
or half the weight of the atmosphere. When ether is cooled to -24° F. it begins to
crystallize in brilliant white plates, and at -47° it becomes a white crystalline solid.
When vapour of ether is made to traverse a red hot porcelain tube, it deposits within it
one half per cent. of charcoal, and there are condensed in the receiver one and two thirds[443]
per cent. of a brown oil, partly in crystalline scales, and partly viscid. The crystalline
portion is soluble in alcohol, but the viscid only in ether. The remainder of the decomposed
ether consists of bi-carburetted hydrogen gas, tetrahydric carburet, carbonic
oxide gas, and one per cent. at most of gaseous carbonic acid.
Ether takes fire readily, even at some distance from a flame, and it should not therefore
be poured from one vessel to another in the neighbourhood of a lighted candle.
It may be likewise set on fire by the electric spark. It burns all away with a bright
fuliginous flame. When the vapour of ether is mixed with 10 times its volume of
oxygen, it burns with a violent explosion, absorbs 6 times its bulk of oxygen, and produces
4 times its volume of carbonic acid gas.
Ether alters gradually with contact of air; absorbing oxygen, and progressively changing
into acetic acid and water. This conversion takes place very rapidly when the ether
is boiled in an open vessel, while the acid enters into a new combination forming acetic
ether. Ether should be preserved in bottles perfectly full and well corked, and kept in
a cool place, otherwise it becomes sour, and is destroyed. It contains in this state 15 per
cent. of its bulk of azote, but no oxygen gas, as this has combined with its elements.
Ether is composed of oxygen 21·24; hydrogen 13·85; carbon 65·05. This composition
may be represented by 1 prime equivalent of water, and 4 primes of bi-carburetted
hydrogen gas; in other words, ether contains for 1 prime of water, once as much
olefiant gas as alcohol, and its prime equivalent is therefore 468·15 to oxygen 100.
By my analysis, as published in the Phil. Trans. for 1822, ether is composed of oxygen
27·10; hydrogen 13·3; and carbon 59·6 in 100 parts. The density of my ether
was 0·700. One volume of vapour of ether consists of one volume of aqueous vapour
and two volumes of olefiant gas (bi-carburetted hydrogen,) while alcohol consists of
two volumes of each.
ETHER, ACETIC, is used to flavour silent corn spirits in making imitation brandy.
It may be prepared by mixing 20 parts of acetate of lead, 10 parts of alcohol, and
111⁄2 of concentrated sulphuric acid; or 16 of the anhydrous acetate, 5 of the acid, and
41⁄2 of absolute alcohol; distilling the mixture in a glass retort into a very cold receiver,
agitating along with weak potash lye the liquor which comes over, decanting the supernatant
ether, and rectifying it by re-distillation over magnesia and ground charcoal.
Acetic ether is a colourless liquid of a fragrant smell and pungent taste, of spec.
grav. 0·866 at 45° F., boiling at 166° F, burning with a yellowish flame, and disengaging
fumes of acetic acid. It is soluble in 8 parts of water.
Acetic ether may be economically made with 3 parts of acetate of potash, 3 of very
strong alcohol, and 2 of the strongest sulphuric acid, distilled together. The first product
must be re-distilled along with one fifth of its weight of sulphuric acid; as much
ether will be obtained as there was alcohol employed.
ETHIOPS, is the absurd name given by the alchemists to certain black metallic
preparations. Martial ethiops was the black oxide of iron; mineral ethiops, the black
sulphuret of mercury; and ethiops per se, the black oxide of mercury.
EVAPORATION, (Eng. and Fr.; Abdampfen; Abdunsten, Germ.) is the process by
which any substance is converted into, and carried off in, vapour. Though ice, camphor,
and many other solids evaporate readily in dry air, I shall consider, at present, merely
the vaporization of water by heat artificially applied.
The vapour of water is an elastic fluid, whose tension and density depend upon the
temperature of the water with which it is in contact. Thus the vapour rising from
water heated to 165° F. possesses an elastic force capable of supporting a column of mercury
10·8 high; and its density is such that 80 cubic feet of such vapour contain one
pound weight of water; whereas 321⁄2 cubic feet of steam of the density corresponding
to a temperature of 212° and a pressure of 30 inches of mercury, weigh one pound.
When the temperature of the water is given, the elasticity and specific gravity of the
vapour emitted by it, may be found.
Since the vapour rises from the water only in virtue of the elasticity due to its gaseous
nature, it is obvious that no more can be produced, unless what is already incumbent
upon the liquid have its tension abated, or be withdrawn by some means. Suppose the
temperature of the water to be midway between freezing and boiling, viz. 122° Fahr., as
also that of the air in contact with it, to be the same but replete with moisture, so that
its interstitial spaces are filled with vapour of corresponding elasticity and specific gravity
with that given off by the water, it is certain that no fresh formation of vapour can
take place in these circumstances. But the moment a portion of vapour is allowed to
escape, or is drawn off by condensation to another vessel, an equivalent portion of vapour
will be immediately exhaled from the water.
The pressure of the air and of other vapours upon the surface of water in an open vessel,
does not prevent evaporation of the liquid; it merely retards its progress. Experience
shows that the space filled with an elastic fluid, as air or other gaseous body, is capable
of receiving as much aqueous vapour as if it were vacuous, only the repletion of that[444]
space with the vapour proceeds more slowly in the former predicament than in the latter,
but in both cases it arrives eventually at the same pitch. Dr. Dalton has very ingeniously
proved, that the particles of aeriform bodies present no permanent obstacle to
the introduction of a gaseous atmosphere of another kind among them, but merely
obstruct its diffusion momentarily, as if by a species of friction. Hence, exhalation at
atmospheric temperatures is promoted by the mechanical diffusion of the vapours
through the air with ventilating fans or chimney draughts; though under brisk ebullition,
the force of the steam readily overcomes that mechanical obstruction.
The quantities of water evaporated under different temperatures in like times, are
proportional to the elasticities of the steam corresponding to these temperatures. A
vessel of boiling water exposing a square foot of surface to the fire, evaporates 725
grains in the minute; the elasticity of the vapour is equivalent to 30 inches of mercury.
To find the quantity that would be evaporated from the same surface per minute at a
heat of 88° F. At this temperature the steam incumbent upon water is capable of supporting
1·28 inch of mercury; whence the rule of proportion is 30 : 1·28 ∷ 725 : 30·93;
showing that about 31 grains of water would be evaporated in the minute. If the air
contains already some aqueous vapour, as it commonly does, then the quantity of evaporation
will be proportional to the difference between the elastic force of that vapour, and
what rises from the water.
Suppose the air to be in the hygrometric state denoted by 0·38 of an inch of mercury,
then the above formula will become: 30 : 1·28 - 0·38 ∷ 725 : 21·41; showing that
not more than 211⁄2 grains would be evaporated per minute under these circumstances.
The elastic tension of the atmospheric vapour is readily ascertained by the old experiment
of Le Roi, which consists in filling a glass cylinder (a narrow tumbler for
example) with cool spring water, and noting its temperature at the instant it becomes
so warm that dew ceases to be deposited upon it. This temperature is that which
corresponds to the elastic tension of the atmospheric vapour. See Vapour, Table of.
Whenever the elasticity of the vapour, corresponding to the temperature of the water,
is greater than the atmospheric pressure, the evaporation will take place not only from
its surface, but from every point in its interior; the liquid particles throughout the
mass assuming the gaseous form, as rapidly as they are actuated by the caloric, which
subverts the hydrostatic equilibrium among them, to constitute the phenomena of ebullition.
This turbulent vaporization takes place at any temperature, even down to the
freezing point, provided the pneumatic pressure be removed from the liquid by the
air pump, or any other means. Ebullition always accelerates evaporation, as it serves
to carry off the aqueous particles not simply from the surface, but from the whole body
of the water.
The vapours, exhaled from a liquid at any temperature, contain more heat than the
fluid from which they spring; and they cease to form whenever the supply of heat into
the liquid is stopped. Any volume of water requires for its conversion into vapour five
and a half times as much heat as is sufficient to heat it from the freezing to the boiling
temperature. The heat, in the former case, seems to be absorbed, being inappreciable by
the thermometer; for steam is no hotter than the boiling water from which it rises. It
has been therefore called latent heat; in contradistinction to that perceived by the touch
and measured by the thermometer, which is called sensible heat. The quantity of heat
absorbed by one volume of water in its conversion into steam, is about 1000° Fahr.;
it would be adequate to heat 1000 volumes of water, one degree of the same scale; or
to raise one volume of boiling water, confined in a non-conducting vessel, to 1180°.
Were the vessel charged with water so heated, opened, it would be instantaneously emptied
by vaporization, since the whole caloric equivalent to its constitution as steam, is
present. When, upon the other hand, steam is condensed by contact with cold substances,
so much heat is set free as is capable of heating five and a half times its weight
of water, from 32° to 212° F. If the supply of heat to a copper be uniform, five hours
and a half will be required to drive off its water in steam, provided one hour was
taken in heating the water, from the freezing to the boiling pitch, under the atmospherical
pressure.
Equal weights of vapour of any temperature contain equal quantities of heat; for
example, the vapour exhaled from one pound of water, at 77° F., absorbs during its
formation, and will give out in its condensation, as much heat as the steam produced by
one pound of water, at 212° F. The first portion of vapour with a tension = 30 inches,
occupies a space of 27·31 cubic feet; the second, with a tension of 0·92 inch, occupies a
space of 890 cubic feet.[29] Suppose that these 890 volumes were to be compressed into
27·31 in a cylinder capable of confining the heat, the temperature of the vapour would
rise from 77° to 212°, in virtue of the condensation, as air becomes so hot by compression[445]
in a syringe, as to ignite amadou. The latent heat of steam at 212° F. is
1180° - 180 = 1000; that of vapour, at 77°, is 1180 - 45 = 1135°; so that, in fact,
the lower the temperature at which the vapour is exhaled, the greater is its latent heat,
as Joseph Black and James Watt long ago proved by experiments upon distillation
and the steam engine.
From the preceding researches it follows, that evaporation may be effected upon two
different plans:—
1. Under the ordinary pressure of the atmosphere; and that either,
A, by external application of heat to boilers, with a, an open fire; b, steam; c, hot
liquid media.
B, by evaporation with air; a, at the ordinary temperature of the atmosphere; b,
by currents of warm air.
2. Under progressively lower degrees of pressure than the atmospheric, down to
evaporation in as perfect a vacuum as can be made.
It is generally affirmed, that a thick metallic boiler obstructs the passage of the heat
through it so much more than a thin one, as to make a considerable difference in their
relative powers of evaporating liquids. Many years ago, I made a series of experiments
upon this subject. Two cylindrical copper pans, of equal dimensions, were provided;
but the metal of the one was twelve times thicker than that of the other. Each being
charged with an equal volume of water, and placed either upon the same hot plate of
iron, or immersed, to a certain depth, in a hot solution of muriate of lime, I found that
the ebullition was greatly more vigorous in the thick than in the thin vessel, which I
ascribed to the conducting substance up the sides, above the contact of the source of
heat, being 12 times greater in the former case than in the latter.
If the bottom of a pan, and the portions of the sides, immersed in a hot fluid medium,
solution of caustic potash or muriate of lime, for example, be corrugated, so as to contain
a double expanse of metallic surface, that pan will evaporate exactly double the quantity
of water, in a given time, which a like pan, with smooth bottom and sides, will do
immersed equally deep in the same bath. If the corrugations contain three times the
quantity of metallic surface, the evaporation will be threefold in the above circumstances.
But if the pan, with the same corrugated bottom and sides, be set over a fire,
or in an oblong flue, so that the current of flame may sweep along the corrugations, it
will evaporate no more water from its interior than a smooth pan of like shape and
dimensions placed alongside in the same flue, or over the same fire. This curious fact
I have verified upon models constructed with many modifications. Among others, I
caused a cylindrical pan, 10 inches diameter, and 6 inches deep, to be made of tin-plate,
with a vertical plate soldered across its diameter; dividing it into two equal
semi-cylindrical compartments. One of these was smooth at the bottom, the other
corrugated; the former afforded as rapid an evaporation over the naked fire as the
latter, but it was far outstripped by its neighbour when plunged into the heated liquid
medium.
If a shallow pan of extensive surface be heated by a subjacent fire, by a liquid medium,
or a series of steam pipes upon its bottom; it will give off less vapour in the same time
when it is left open, than when partially covered. In the former case, the cool incumbent
air precipitates by condensation a portion of the steam, and also opposes
considerable mechanical resistance to the diffusion of the vaporous particles. In the
latter case, as the steam issues with concentrated force and velocity from the contracted
orifice, the air must offer less proportional resistance, upon the known hydrostatic
principle of the pressure being as the areas of the respective bases, in communicating
vessels.
In evaporating by surfaces heated with ordinary steam, it must be borne in mind
that a surface of 10 square feet will evaporate fully one pound of water per minute, or
725 × 10 = 7250 gr., the same as over a naked fire; consequently the condensing surface
must be equally extensive. Suppose that the vessel is to receive of water 2500
libs, which corresponds to a boiler 5 feet long, 4 broad, and 2 deep, being 40 cubic
feet by measure, and let there be laid over the bottom of this vessel 8 connected tubes,
each 5 inches in diameter and 5 feet long, possessing therefore a surface of 5 feet square.
If charged with steam, they will cause the evaporation of half a pound of water per
minute. The boiler to supply the steam for this purpose must expose a surface of 5
square feet to the fire. It has been proved experimentally that 10 square feet surface
of thin copper can condense 3 libs of steam per minute, with a difference of temperature
of 90 degrees Fahr. In the above example, 10 square feet evaporate 1 lib. of water
per minute; the temperature of the evaporating fluid being 212° F., consequently
3 : 1 ∷ 90 : 903. During this evaporation the difference of the temperature is therefore = 30°.
Consequently the heat of the steam placed in connection with the interior
of the boiler, to produce the calculated evaporation should be, 212 + 30 = 242°,
corresponding to an elastic force of 53·6 inches of mercury. Were the temperature of[446]
the steam only 224, the same boiler in the same time would produce a diminished quantity
of steam, in the proportion of 12 to 30; or to produce the same quantity the boiler
or tubular surface should be enlarged in the proportion of 30 to 12. In general, however,
steam boilers employed for this mode of evaporation are of such capacity as to
give an unfailing supply of steam.
I shall now illustrate by some peculiar forms of apparatus, different systems of evaporation.
Fig. 381. explains the principles of evaporating in vacuo. A B represents
a pan or kettle charged with the liquor to be evaporated. The somewhat wide orifice
c, secured with a screw-plug, serves to admit the hand for the purpose of cleaning it
thoroughly out when the operation is finished; h is the pipe of communication with the
steam boiler; b is a tube prolonged and then bent down with its end plunged into the
liquor to be evaporated, contained in the charging back, (not shown in the figure). H is
a glass tube communicating with the vacuum pan at the top and bottom, to shew by the
height of the column the quantity of liquid within. The eduction evaporating pipe
c is provided with a stop-cock to cut off the communication when required. i is a tube
for the discharge of the air and the water from the steam-case or jacket; the refrigerator
E is best formed of thin copper tubes about 1 inch in diameter, arranged zig-zag or spirally
like the worm of a still in a cylinder. The small air-tight condenser F, connected
with the efflux pipe f of the refrigerator, is furnished below with a discharge cock g,
and surrounded by a cooling case, for the collection of the water condensed by the
refrigerator. In its upper part there is a tube k, also furnished with a cock, which
communicates with the steam boiler, and through which the pan A B is heated.
The operation of this apparatus is as follows: after opening the cocks C, f, g,
and before admitting the cold water into the condenser E, the cock of the pipe k is
opened, in order that by injecting steam it may expel the included air; after which the
cocks k and g are to be shut. The water must now be introduced into the condenser,
and the cock b opened, whereon the liquid to be evaporated rises from the charging back,
through the tube b, and replenishes the vacuum pan to the proper height, as shown by
the register glass tube H. Whenever the desired evaporation or concentration is effected,
the cock C must be closed, the pipe k opened, so as to fill the pan with steam, and
then the efflux cock a is opened to discharge the residuary liquor. By shutting the
cocks a and k, and opening the cock b, the pan will charge itself afresh with liquor, and
the operation will be begun anew, after b has been shut and C opened.
The contents of the close water cistern F, may be drawn off during each operation.
For this purpose, the cock f must first be shut, the cold water is to be then run out of
the condenser G, and k and g are to be opened. The steam entering by k makes the
water flow, but whenever the steam itself issues from the cock g, this orifice must be
immediately shut, the cock f opened, and the cold water again introduced, whereupon
the condensed water that had meanwhile collected in the under part of the refrigerator,
flows off into the condenser vessel F. Since some air always enters with the liquor[447]
sucked into the pan, it must be removed at the time of drawing off the water from the
two condensers, by driving steam through the apparatus. This necessity will be less
urgent if the liquor be made to boil before being introduced into the vacuum pan.
Such an apparatus may be modified in size and arrangement to suit the peculiar
object in view, when it will be perfectly adapted for the concentration of extracts of
every kind, as well as saline solutions containing vegetable acids or alkalis. The interior
vessel of A B should be made of tinned or plated copper. For an account of Howard’s
vacuum pan, made upon the same principle, see Sugar.
When a boiler is set over a fire, its bottom should not be placed too near the grate,
lest it refrigerate the flame, and prevent that vivid combustion of the fuel essential to
the maximum production of heat by its means. The evil influence of leaving too little
room between the grate and the copper may be illustrated by a very simple experiment.
If a small copper or porcelain capsule containing water be held over the flame of a candle
a little way above its apex, the flame will suffer no abatement of brightness or size,
but will continue to keep the water briskly boiling. If the capsule be now lowered into
the middle of the flame, this will immediately lose its brightness, becoming dull and smoky
covering the bottom of the capsule with soot; and, owing to the imperfect combustion,
though the water is now surrounded by the flame, its ebullition will cease.
Fig. 382. is a section of two evaporating coppers en suite, so mounted as to favour
the full combustion of the fuel. A is the hearth, in which wood or coal may be burned.
For coal, the grate should be set higher and be somewhat smaller, a is the door for feeding
the fire; d, an arch of fire-bricks over the hearth; c, a grate through which the
ashes fall into the pit beneath, capable of being closed in front to any extent by a sliding
door b. B and C are two coppers encased in brickwork; f the flue. At the end of the
hearth near m, where the fire plays first upon the copper, the sole is made somewhat
lower and wider, to promote the spreading of the flame under the vessel. The second
copper, C, receives the benefit of the waste heat; it may be placed upon a higher level,
so as to discharge its concentrated liquor by a stop-cock or syphon into the first. When
coals are burned for heating such boilers, the grate should be constructed as shown in
the figure of the brewing copper, page 116.
Fig. 383. represents a pan for evaporating liquids, which are apt, during concentration,
to let fall crystals or other sediment.
These would be injured either by the fire playing
upon the bottom of the pan, or, by adhesion
to it, they would allow the metal to get red
hot, and in that state run every risk of being
burnt or rent on the sudden intrusion of a little
liquor through the incrustation. When large
coppers have their bottoms planted in loam, so
that the flame circulates in flues round their
sides, they are said to be cold-set.
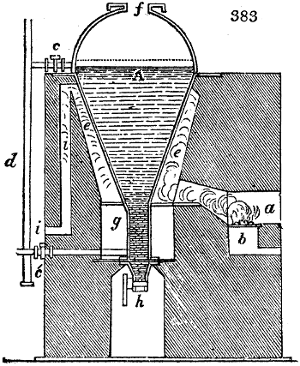
A is a pear-shaped pan, charged with the liquid
to be evaporated; it is furnished with a
dome cover, in which there is an opening with
a flange f, for attaching a tube, to conduct the
steam wherever it may be required. a is the
fire-place; b, the ash-pit. The conical part terminates
below in the tube g, furnished with a
stop-cock at its nozzle h. Through the tube
c d c′, furnished above and below with the stop-cocks
c and c′, the liquid is run from the[448]
charging back or reservoir. During the operation, the upper cock c is kept partially
open, to replace the fluid as it evaporates; but the under cock c′ is shut. The flame
from the fire-place plays round the kettle in the space e, and the smoke escapes downwards
through the flue i into the chimney. The lower cylindrical part g, remains thus
comparatively cool, and collects the crystalline or other solid matter. After some time,
the under stop-cock c′, upon the supply-pipe, is to be opened to admit some of the cold
liquor into the cylindrical neck. That cock being again shut, the sediment settled, and
the large stop-cock (a horizontal slide-valve would be preferable) h opened, the crystals
are suffered to descend into the subjacent receiver; after which the stop-cock h is shut,
and the operation is continued. A construction upon this principle is well adapted for
heating dyeing coppers, in which the sediment should not be disturbed, or exposed
to the action of the fire. The fire-place should be built as for the brewing copper.
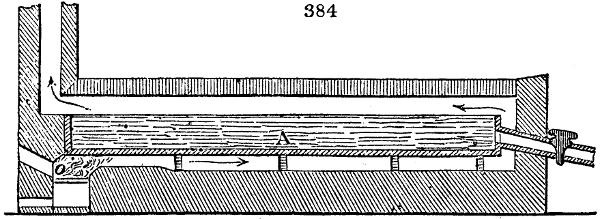
Fig. 384. represents
an oblong evaporating
pan, in which the flame,
after beating along its
bottom, turns up at its
further end, plays back
along its surface, and
passes off into the chimney.
A is a rectangular
vessel, from 10 to 15
feet long, 4 to 6 feet broad, and 1 or 11⁄2 feet deep. The fire-bricks, upon which the pan
rests, are so arranged as to distribute the flame equably along its bottom.
EUDIOMETER, is the name of any apparatus subservient to the chemical examination
of the atmospheric air. It means a measure of purity, but it is employed merely
to determine the proportion of oxygen which it may contain. The explosive eudiometer,
in which about two measures of hydrogen are introduced into a graduated glass
tube, containing five measures of atmospheric air, and an electric spark is passed across
the mixture, is the best of all eudiometers; and of these the syphon form, proposed
by me in a paper published by the Royal Society of Edinburgh in 1819, is probably the
surest and most convenient. Volta’s explosive eudiometer as made in Paris, costs 3
guineas; mine may be had nicely graduated for 6 or 8 shillings.
EXPANSION (Eng. and Fr.; Ausdehnung, Germ.), is the increase of bulk experienced
by all bodies when heated, unless a change of chemical texture takes place,
as in the case of clays in the potter’s kiln. Table I. exhibits the linear expansion of
several solids by an increase of temperature from 32° to 212° Fahr.; Table II. exhibits
the expansion in bulk of certain liquids.
TABLE I.—Linear Dilatation of Solids by Heat.
Dimensions which a bar takes at 212°, whose length at 32° is 1·000000.
| Substances. |
Authority. |
Dilatation
in
Decimals. |
Dilatation
in Vulgar
Fractions. |
| Glass tube, |
Smeaton, |
1·00083333 |
|
| Glasdo. |
Roy, |
1·00077615 |
|
| Glasdo. |
Deluc’s mean, |
1·00082800 |
1⁄1116 |
| Glasdo. |
Dulong and Petit, |
1·00086130 |
1⁄1148 |
| Glasdo. |
Lavoisier and Laplace, |
1·00081166 |
1⁄1122 |
| Plate glass, |
do. do. |
1·000890890 |
1⁄1142 |
| Pldo. crown glass, |
do. do. |
1·00087572 |
1⁄1114 |
| Pldo.crowdo. |
do. do. |
1·00089760 |
1⁄1090 |
| Pldo.crowdo. |
do. do. |
1·00091751 |
|
| Pldo. rod, |
Roy, |
1·00080787 |
|
| Deal, |
Roy, as glass, |
— |
|
| Platina, |
Borda, |
1·00085655 |
|
| Pldo. |
Dulong and Petit, |
1·00088420 |
1⁄1131 |
| Pldo. |
Troughton, |
1·00099180 |
|
| Pldo.na and glass, |
Berthoud, |
1·00110000 |
|
| Palladium, |
Wollaston, |
1·00100000 |
|
| Antimony, |
Smeaton, |
1·00108300 |
|
| Cast-iron prism, |
Roy, |
1·00110940 |
|
| Cast-iron, |
Lavoisier, by Dr Young |
1·00111111 |
|
| Steel, |
Troughton, |
1·00118990 |
|
| Steel rod,[449] |
Roy, |
1·00114470 |
|
| Blistered Steel, |
Phil. Trans. 1795, 428, |
1·00112500 |
|
| Blistedo. |
Smeaton, |
1·00115000 |
|
| Steel not tempered, |
Lavoisier and Laplace, |
1·00107875 |
1⁄927 |
| Stdo. do.tedo. |
do. do. |
1·00107956 |
1⁄926 |
| Stdo. tempered yellow, |
do. do. |
1·00136900 |
|
| Stdo.temdo.ed yedo. |
do. do. |
1·00138600 |
|
| Stdo.temdo. ed at a higher heat, |
do. do. |
1·00123956 |
1⁄807 |
| Steel, |
Troughton, |
1·00118980 |
|
| Hard Steel, |
Smeaton, |
1·00122500 |
|
| Annealed steel, |
Muschenbroek, |
1·00122000 |
|
| Tempered steel, |
do. |
1·00137000 |
|
| Iron, |
Borda, |
1·00115600 |
|
| Ido. |
Smeaton, |
1·00125800 |
|
| Soft iron, forged, |
Lavoisier and Laplace, |
1·00122045 |
|
| Round iron, wire drawn, |
do. do. |
1·00123504 |
|
| Iron wire, |
Troughton, |
1·00144010 |
|
| Iron, |
Dulong and Petit, |
1·00118203 |
1⁄846 |
| Bismuth, |
Smeaton, |
1·00139200 |
|
| Annealed gold, |
Muschenbroek, |
1·00146000 |
|
| Gold, |
Ellicot, by comparison, |
1·00150000 |
|
| Gdo. procured by parting, |
Lavoisier and Laplace, |
1·00146606 |
1⁄682 |
| Gdo. Paris standard, unannealed, |
do. do. |
1·00155155 |
1⁄645 |
| Gdo.Paris s do. dardannealed, |
do. do. |
1·00151361 |
1⁄661 |
| Copper, |
Muschenbroek, |
1·0019100 |
|
| Codo. |
Lavoisier and Laplace, |
1·00172244 |
1⁄581 |
| Codo. |
do. do. |
1·00171222 |
1⁄584 |
| Codo. |
Troughton, |
1·00191880 |
|
| Codo. |
Dulong and Petit, |
1·00171821 |
1⁄582 |
| Brass, |
Borda, |
1·00178300 |
|
| Bdo. |
Lavoisier and Laplace, |
1·00186671 |
|
| Bdo. |
do. do. |
1·00188971 |
|
| Brass scale, supposed from Hamburg, |
Roy, |
1·00185540 |
|
| Cast brass, |
Smeaton, |
1·00187500 |
|
| English plate-brass, in rod, |
Roy, |
1·00189280 |
|
| Endo.h plado.rass, in a trough form, |
do. |
1·00189490 |
|
| Brass, |
Troughton, |
1·00191880 |
|
| Brass wire, |
Smeaton, |
1·00193000 |
|
| Brass, |
Muschenbroek, |
1·00216000 |
|
| Copper 8, tin 1, |
Smeaton, |
1·00181700 |
|
| Silver, |
Herbert, |
1·00189000 |
|
| Sido. |
Ellicot, by comparison, |
1·0021000 |
|
| Sido. |
Muschenbroek, |
1·00212000 |
|
| Sido.r, of cupel, |
Lavoisier and Laplace, |
1·00190974 |
1⁄524 |
| Sido.r, Paris standard, |
do. do. |
1·00190868 |
1⁄524 |
| Silver, |
Troughton, |
1·0020826 |
|
| Brass 16, tin 1, |
Smeaton, |
1·00190800 |
|
| Speculum metal, |
do. |
1·00193300 |
|
| Spelter solder; brass 2, zinc 1, |
do. |
1·00205800 |
|
| Malacca tin, |
Lavoisier and Laplace, |
1·00193765 |
1⁄516 |
| Tin from Falmouth, |
do. do. |
1·00217298 |
1⁄462 |
| Fine pewter, |
Smeaton, |
1·00228300 |
|
| Grain tin, |
do. |
1·00248300 |
|
| Tin, |
Muschenbroek, |
1·00284000 |
|
| Soft solder; lead 2, tin 1, |
Smeaton, |
1·00250800 |
|
| Zinc 8, tin 1, a little hammered, |
do. |
1·00269200 |
|
| Lead. |
Lavoisier and Laplace, |
1·00284836 |
1⁄351 |
| Ldo. |
Smeaton, |
1·00286700 |
|
| Zinc, |
do. |
1·00294200 |
|
| Zinc, hammered out 1⁄2 inch per foot, |
do. |
1·00301100 |
|
| Glass, from 32°, to 212°, |
Dulong and Petit, |
1·00086130 |
1⁄1161 |
| G do. from 212°, to 392°, |
do. do. |
1·00091827 |
1⁄1089 |
| G do. from 392°, to 572°, |
do. do. |
1·00101114 |
1⁄987 |
The last two measurements by an air thermometer.
[450]
TABLE II.
Expansion of certain Liquids by being Heated from 32° to 212°.
| Substances. |
Authority. |
Expansion
in
Decimals. |
Expansion
in Vulgar
Fractions. |
| Mercury, |
Dulong and Petit. |
0·01801800 |
1⁄55·5 |
| Medo.ry, in glass, |
do. do. |
0·01543200 |
1⁄65 |
| Water, from its maximum density, |
Kirwan. |
0·04332 |
1⁄23 |
| Muriatic acid (sp. gr. 1·137), |
Dalton. |
0·0600 |
1⁄17 |
| Nitric acid (sp. gr. 1·40), |
do. |
0·1100 |
1⁄9 |
| Sulphuric acid (sp. gr. 1·85), |
do. |
0·0600 |
1⁄17 |
| Alcohol (to its boiling point)? |
do. |
0·1100 |
1⁄9 |
| Water, |
do. |
0·0460 |
1⁄22 |
| Water, saturated with common salt, |
do. |
0·0500 |
1⁄20 |
| Sulphuric ether (to its boiling point)? |
do. |
0·0700 |
1⁄14 |
| Fixed oils, |
do. |
0·0800 |
1⁄12·5 |
| Oil of turpentine, |
do. |
0·0700 |
1⁄14 |
| If the density of water at 39° be called |
1·00000, |
| If the density at 212° it becomes |
0·9548, |
| If the density and its volume has increased to |
1·04734; |
| If the density at 77° it becomes |
0·9973587, |
| If the density and its volume has increased to only |
1·00265, |
| which, though one fourth of the whole range of temperature, is only 1⁄18 of the total expansion. |
| If the density Water at 60° F. has a specific gravity of |
0·9991953, |
| If the density and has increased in volume from 39° to |
1·00008, |
| which is only about 1⁄58 of the total expansion to 212°, with 1⁄64 of the total range of temperature. |
All gases expand the same quantity by the same increase of temperature, which from
32° to 212° Fahr. = 180°480 = 3⁄8, or 100 volumes become 137·5. For each degree of Fahr.
the expansion is 1⁄480.
When dry air is saturated with moisture, its bulk increases, and its specific gravity
diminishes, because aqueous vapour is less dense than air, at like temperatures.
The following Table gives the multipliers to be employed for converting one volume
of moist gas at the several temperatures, into a volume of dry gas.
| Temperature. |
Multiplier. |
| 53 |
° F. |
0·9870 |
| 54 |
|
0·9864 |
| 55 |
|
0·9858 |
| 56 |
|
0·9852 |
| 57 |
|
0·9846 |
| 58 |
|
0·9839 |
| 59 |
|
0·9833 |
| 60 |
|
0·9827 |
| 61 |
|
0·9820 |
| 62 |
|
0·9813 |
| 63 |
|
0·9806 |
| 64 |
|
0·9799 |
| 65 |
|
0·9793 |
| 66 |
|
0·9786 |
| 67 |
|
0·9779 |
| 68 |
|
0·9772 |
| 69 |
|
0·9765 |
| 70 |
|
0·9758 |
| 71 |
|
0·9751 |
| 72 |
|
0·9743 |
| 73 |
|
0·9735 |
EXTRACTS. (Extraits, Fr.; Extracten, Germ.) The older apothecaries used
this term to designate the product of the evaporation of any vegetable juice, infusion,
or decoction; whether the latter two were made with water, alcohol, or ether; whence
arose the distinction of aqueous, alcoholic, and ethereous extracts.
Fourcroy made many researches upon these preparations, and supposed that they
had all a common basis, which he called the extractive principle. But Chevreul and
other chemists have since proved that this pretended principle is a heterogeneous and
very variable compound. By the term extract therefore is now meant merely the
whole of the soluble matters obtained from vegetables, reduced by careful evaporation
to either a pasty or solid consistence. The watery extracts, which are those most commonly
made, are as various as the vegetables which yield them; some containing
chiefly sugar or gum in great abundance, and are therefore innocent or inert; while
others contain very energetic impregnations. The conduct of the evaporating heat is
the capital point in the preparation of extracts. They should be always prepared if
possible from the juice of the fresh plant, by subjecting its leaves or other succulent[451]
part, to the action of a powerful screw or hydraulic press; and the evaporation should
be effected by the warmth of a water bath, heated not beyond 100° or 120° F. Steam
heat may perhaps be applied advantageously in some cases, where it is not likely to decompose
any of the principles of the plant. But by far the best process for making extracts
is in vacuo, upon the principles explained in the article Evaporation. It is much
easier to fit up a proper apparatus of this kind, than most practical men imagine. The
vacuum may either be made through the agency of steam, as there pointed out, or by
means of an air-pump. One powerful air-pump may form and maintain a good vacuum
under several receivers, placed upon the flat-ground flanges of so many basins, each
provided with a stop-cock at its side for exhaustion. The air-less basin containing the
juice being set on the shelf of a water-bath, and exposed to a proper temperature, will
furnish in a short time, a large quantity of medicinal extract, possessing the properties
of the plant unimpaired.
For exceedingly delicate purposes, the concentration may be performed in the cold,
by placing saucers filled with the expressed juice over a basin containing sulphuric
acid, putting a glass receiver over them, and exhausting its air.
FAHLERZ. Gray copper-ore, called also Panabase, from the many oxides it contains.
FAINTS, is the name of the impure spirit, which comes over first and last in the
distillation of whiskey; the former being called the strong, and the latter, which is
much more abundant, the weak faints. This crude spirit is much impregnated with
fetid essential oil, is therefore very unwholesome, and must be purified by rectification.
FAN (Eventail, Fr.; Fächer, Germ.); is usually a semi-circular piece of silk or
paper, pasted double, enclosing slender slips of wood, ivory, tortoise-shell, whale-bone,
&c., arranged like the tail of a peacock in a radiating form, and susceptible of being
folded together, and expanded at pleasure. This well-known hand ornament is used
by ladies to cool their faces by agitating the air. Fans made of feathers, like the wing
of a bird, have been employed from time immemorial by the natives of tropical countries.
Fan is also the name of the apparatus for winnowing corn. For an account of the
powerful blowing and ventilating fan machine, see Foundry and Ventilator.
FARINA (Farine, Fr.; Mehl, Germ.); is the flour of any species of corn, or
starchy root, such as potato, arrow root, &c. See Bread and Starch.
FATS, (Graisses, Fr.; Fette, Germ.) occur in a great number of the animal
tissues, being abundant under the skin in what is called the cellular membrane, round
the kidneys, in the folds of the omentum, at the base of the heart, in the mediastinum,
the mesenteric web, as well as upon the surface of the intestines, and among many of
the muscles. They vary in consistence, colour, and smell, according to the animals
from which they are obtained; thus, they are generally fluid in the cetaceous tribes,
soft and rank-flavoured in the carnivorous, solid and nearly scentless in the ruminants,
usually white and copious in well-fed young animals; yellowish and more scanty in the
old. Their consistence varies also according to the organ of their production; being firmer
under the skin, and in the neighbourhood of the kidneys, than among the movable
viscera. Fat forms about one twentieth of the weight of a healthy animal. But as
taken out by the butcher it is not pure, for being of a vesicular structure it is always
enclosed in membranes, mixed with blood, blood-vessels, lymphatics, &c. These foreign
matters must first be separated in some measure mechanically, after the fat is minced
small, and then more completely by melting it along with hot water, passing it through
a sieve, and letting the whole cool very slowly. By this means a cake of cleansed fat
will be obtained. Many plans of purifying fats have been proposed; one of the best is
to mix two per cent. of strong sulphuric acid with a quantity of water, in which the
tallow is heated for some time with much stirring; to allow the materials to cool, to
take off the supernatant fat, and re-melt it with abundance of hot water. More tallow
will thus be obtained, and that considerably whiter and harder than is usually procured
by the melters.
I have found that chlorine, and chloride of lime do not improve, but rather deteriorate
the appearance of oils and other fatty bodies. According to Appert, minced suet
subjected to the action of high-pressure steam in a digester, at 250° or 260° F., becomes
so hard as to be sonorous when struck, whiter, and capable when made into candles, of
giving a superior light. A convenient mode of rendering minced tallow, or melting it, is to
put it in a tub, and drive steam through it from numerous orifices in ramifying pipes
placed near the bottom. Mr. Watt assures me that his plan of purifying fats, patented
in March 1836, has been quite successful. He employs dilute sulphuric acid, to which
he adds a little nitric acid, with a very small quantity of bichromate of potash, “to supply[452]
oxygen;” and some oxalic acid. These are mixed with the fat in the steaming tub.
When the lumps of it are nearly dissolved, he takes for every ton of fat, one pound of
strong nitric acid, diluted with one quart of water; to which he adds two ounces of
alcohol, naphta, sulphuric ether, or spirits of turpentine; and after introducing this
mixture, he continues the boiling for half an hour. The fat is finally washed.—As I
do not comprehend the modus operandi of these ingredients, I shall abstain from any
comment upon the recipe.
Others have proposed to use vegetable or animal charcoal first, especially for rancid
oils, then to heat them with a solution of sulphate of copper and common salt, which is
supposed to precipitate the fetid albuminous matter. Milk of lime has been also prescribed;
but it is I believe always detrimental.
Davidson treats whale oil with infusion of tan, in order to separate the gelatine
and albumine in flocks; next with water and chloride of lime, to destroy the
smell; and lastly, with dilute sulphuric acid, to precipitate all the lime in the state of
a sulphate. This is certainly one of the cheapest and most effective methods of purifying
that substance.
Braconnot and Raspail have shown that solid animal fats are composed of very small,
microscopic, partly polygonal, partly reniform particles, which are connected together
by very thin membranes. These may be ruptured by mechanical means, then separated
by triturating the fresh fats with cold water, and passing the unctuous matter through
a sieve. The particles float in the water, but eventually collect in a white granular
crystalline appearance, like starch. Each of them consists of a vesicular integument, of
the nature of stearine, and an interior fluid like elaine, which afterwards exudes. The
granules float in the water, but subside in spirits of wine. When digested in strong
alcohol, the liquid part dissolves, but the solid remains. These particles differ in shape
and size, as obtained from different animals; those of the calf, ox, sheep, are polygonal,
from 1⁄50 to 1⁄350 of an inch in diameter; those of the sow are kidney-shaped, and from 1⁄50 to
1⁄100; those of man are polygonal, and from 1⁄50 to 1⁄600; those of insects are spherical, and
at most 1⁄500 of an inch.
Fats all melt at a temperature much under 212° F. When strongly heated with contact
of air, they diffuse white pungent fumes, then blacken, and take fire. When subjected
to distillation, they afford a changed fluid oil, carburetted hydrogen, and the other products
of oily bodies. Exposed for a certain time to the atmosphere, they become
rancid, and generate the same fat acids as they do by saponification. In their fresh
state they are all composed principally of stearine, margarine, and oleine, with a little
colouring and odorous matter; and, in some species, hircine, from the goat; phocenine,
from the dolphin; and butyrine, from butter. By subjecting them to a great degree of
cold, and compressing them between folds of blotting paper, a residuum is obtained,
consisting chiefly of stearine and margarine; the latter of which may be dissolved out
by oil of turpentine.
Beef and Mutton Suet.—When fresh, this is an insipid, nearly inodorous fat, of a firm
consistence, almost insoluble in alcohol, entirely so if taken from the kidneys and
mesenteric web of the ox, the sheep, the goat, and the stag. It varies in its whiteness,
consistence, and combustibility, with the species and health of the animals. That of
the sheep is very white, and very solid. They may all be purified in the manner above
described. Strong sulphuric acid develops readily the acid fats by stirring it through
melted suet. Alkalis, by saponification, give rise at once to the three acids,—the
stearic, margaric, and oleic. Beef suet consists of stearine, margarine, and oleine;
mutton and goat suet contain a little hircine. The specific gravity of the tallow, of
which common candles are made is, by my experiments, 0·936. The melting point of
suet is from 98° to 104° F. The proportion of solid and fluid fat in it is somewhat
variable, but the former is in much larger proportion. Mutton suet is soluble in
44 parts of boiling alcohol, of 0·820; beef suet in 44 parts. Marrow fat consists of
76 of stearine, and 24 of oleine; it melts at 115° F.
Hog’s-lard is soft, fusible at 81° F., convertible, by an alkaline solution, into a stearate,
margarate, oleate, and glycerine. Its sp. grav. is 0·938, at 50° F; It consists of 62 of
oleine, and 38 of stearine, in 100 parts.
Goose-fat, consists of 68 oleine and 32 stearine.
Butter, in summer, consists of 60 of oleine and 40 of stearine; in winter, of 35 of
oleine, and 65 of stearine; the former substance being yellow and the latter white.
It differs, however, as produced from the milk of different cows, and also according to
their pasture.
The ultimate constituents of stearine, according to Chevreul are, 79 carbon; 11·7
hydrogen; and 9·3 oxygen, in 100 parts.
1,294,009 cwts. of the tallow imported in 1837, were retained for internal consumption.
See Margarine, Oleine, Soap, Stearine.
FAULTS (Failles, Fr.); in mining, are disturbances of the strata which interrupt[453]
the miner’s operations, and put him at fault, to discover where the vein of ore or bed of
coal has been thrown by the convulsions of nature. Many examples of faults are exhibited
under Pitcoal.
FEATHERS (Plumes, Fr.; Federn, Germ.), constitute the subject of the
manufacture of the Plumassier, a name given by the French (and also the English) to
the artisan who prepares the feathers of certain birds for ornaments to the toilette
of ladies and for military men, and to him also who combines the feathers in various
forms. We shall content ourselves with describing the method of preparing ostrich
feathers, as most others are prepared in the same way.
Several qualities are distinguished in the feathers of the ostrich; those of the male,
in particular, are whiter and more beautiful. Those upon the back and above the wings
are preferred; next, those of the wings, and lastly, of the tail. The down is merely
the feathers of the other parts of the body, which vary in length from 4 to 14 inches.
This down is black in the males, and gray in the females. The finest white feathers of
the female have always their ends a little grayish, which lessens their lustre, and lowers
their price. These feathers are imported from Algiers, Tunis, Alexandria, Madagascar,
and Senegal; this being the order of their value.
The scouring process is thus performed:—4 ounces of white soap, cut small, are dissolved
in 4 pounds of water, moderately hot, in a large basin; and the solution is made
into a lather by beating with rods. Two bundles of the feathers, tied with packthread,
are then introduced, and are rubbed well with the hands for five or six minutes. After
this soaping they are washed in clear water, as hot as the hand can bear.
The whitening or bleaching is performed by three successive operations.
1. They are immersed in hot water mixed with Spanish white, and well agitated in
it; after which they are washed in three waters in succession.
2. The feathers are azured in cold water containing a little indigo tied up in a fine
cloth. They should be passed quickly through this bath.
3. They are sulphured in the same way as straw hats are (see Sulphuring); they
are then dried by hanging upon cords, when they must be well shaken from time to
time to open the fibres.
The ribs are scraped with a bit of glass cut circularly, in order to render them very
pliant. By drawing the edge of a blunt knife over the filaments they assume the curly
form so much admired. The hairs of a dingy colour are dyed black. For 20 pounds
of feathers, a strong decoction is made of 25 pounds of logwood in a proper quantity of
water. After boiling it for 6 hours, the wood is taken out, 3 pounds of copperas are
thrown in; and, after continuing the ebullition for 15 or 20 minutes, the copper is
taken from the fire. The feathers are then immersed by handfuls, thoroughly soaked,
and worked about; and left in for two or three days. They are next cleansed in a very
weak alkaline lye, and soaped three several times. When they feel very soft to the
touch, they must be rinsed in cold water, and afterwards dried. White feathers are
very difficult to dye a beautiful black. The acetate of iron is said to answer better than
the sulphate, as a mordant.
For dyeing other colours, the feathers should be previously well bleached by the
action of the sun and the dew; the end of the tube being cut sharp like a toothpick,
and the feathers being planted singly in the grass. After fifteen days’ exposure, they are
cleared with soap as above described.
Rose colour or pink, is given with safflower and lemon juice.
Deep red, by a boiling hot bath of Brazil wood, after aluming.
Crimson. The above deep red feathers are passed through a bath of cudbear.
Prune de Monsieur. The deep red is passed through an alkaline bath.
Blues of every shade, are dyed with the indigo vat.
Yellow; after aluming, with a bath of turmeric or weld.
Other tints may be obtained by a mixture of the above dyes.
Feathers have some more useful employments than the decoration of the heads of
women and soldiers. In one case, they supply us with a soft elastic down on which
we can repose our wearied frames, and enjoy sweet slumbers. Such are called bed
feathers. Others are employed for writing, and these are called quills.
Goose feathers are most esteemed for beds, and they are best when plucked from the
living bird, which is done thrice a year, in spring, midsummer, and the beginning of
harvest. The qualities sought for in bed feathers, are softness, elasticity, lightness, and
warmth. Their only preparation when cleanly gathered are a slight beating to clear
away the loose matter, but for this purpose they must be first well dried either by the
sun or a stove. Bleaching with lime water is a bad thing, as they can never be freed
from white dust afterwards.
The feathers of the eider duck, anas mollissima, called eider down, possess in a
superior degree all the good qualities of goose down. It is used only as a covering to
beds, and never should be slept upon, as it thereby loses its elasticity.
[454]
Quills for writing. These consist usually of the feathers plucked out of the wings of
geese. Dutch quills have been highly esteemed, as the Dutch were the first who hit
upon the art of preparing them well, by clearing them both inside and outside from a
fatty humour with which they are naturally impregnated, and which prevents the ink
from flowing freely along the pens made with them. The Dutch for a long time
employed hot cinders or ashes to attain this end; and their secret was preserved very
carefully, but it at length transpired, and the process was then improved. A bath of very
fine sand must be kept constantly at a suitable temperature, which is about 140° F.;
into this, the quill end of the feather must be plunged, and left in it a few instants. On
taking them out they must be strongly rubbed with a piece of flannel, after which they
are found to be white and transparent. Both carbonate of potash in solution and dilute
sulphuric acid have been tried to effect the same end, but without success. The yellow
tint which gives quills the air of age, is produced by dipping them for a little in dilute
muriatic acid, and then making them perfectly dry. But this process must be preceded
by the sand-bath operation. The above is the French process.
Quills are dressed by the London dealers in two ways; by the one, they remain of
their natural colour; by the other, they acquire a yellow tint. The former is called the
Dutch method, and the principal workman is called a Dutcher. He sits before a small
stove fire, into which he thrusts the barrel of the quill for about a second, then lays its
root quickly below his blunt-edged knife called a hook, and, pressing this firmly with
the left hand, draws the quill briskly through with his right. The bed on which the
quill is laid to receive this pressure is called the plate. It is a rectangular smooth
lump of iron, about 3 inches long, 11⁄2 broad, and 21⁄2 thick, which is heated on his stove
to about the 350th degree Fahr. The hook is a ruler of about 15 inches in length,
somewhat like the patten-makers’ knife, its fulcrum being formed at the one end by a
hook and staple, and the power of pressure being applied by the hand at the other end.
The quill, rendered soft and elastic by the heat, endures the strong scraping action of
the tool, and thus gets stripped of its opaque outer membrane, without hazard of being
split. A skilful workman can pass 2000 quills through his hands in a day of 10 hours.
They are next cleaned by being scrubbed by a woman with a piece of rough dog-fish
skin, and finally tied up by a man in one quarter of hundred bundles.
In another mode of dressing quills, they are steeped a night in decoction of turmeric,
to stain them yellow; taken out and dried in warm sand contained in a pot, then
scraped by the Dutcher as above described. The first are reckoned to make the best
pens, though the second may appear more beautiful.
Crow quills for draughtsmen, as well as swan quills, are prepared in the same way.
The quills plucked from well-fed living birds have most elasticity, and are least subject
to be moth-eaten. The best are those plucked, or which are spontaneously cast in the
month of May or June, because they are then fully ripe. In the goose’s wing the five
exterior feathers only are valuable for writing. The first is the hardest and roundest
of all, but the shortest. The next two are the best of the five. They are sorted into
those of the right and the left wing, which are differently bent. The heaviest quills
are, generally speaking, the best. Lately, steaming for four hours has been proposed
as a good preparation.
FECULA (Fecule, Fr.; Stärkemehl, Germ.); sometimes signifies corn flour, sometimes
starch from whatever source obtained.
FELSPAR (Orthose, Fr.; Feldspath, Germ.) is a mineral crystallizing in oblique
rhomboidal prisms, susceptible of two cleavages; lustre more pearly than vitreous;
spec. grav. 2·39 to 2·58; scratches glass; yields no water when calcined; fusible at
the blowpipe into a white enamel; not affected by acids. The liquid left from its
analytical treatment with nitrate of baryta, nitric acid, and carbonate of ammonia,
affords on evaporation an alkaline residuum which precipitates platina from its chloride,
and appears from this, as well as other tests, to be potash. Felspar consists of—silica,
66·75; alumina, 17·50; potash, 12; lime, 1·25; oxide of iron, 0·75. Rose. This
mineral is a leading constituent of granite; and in its decomposed state furnishes the
petuntse or Cornish stone, so much used in the porcelain and best pottery manufactures.
FELTING; (Feutrage, Fr.; Filzen, Germ.) is the process by which loose flocks
of wool, and hairs of various animals, as the beaver, rabbit, hare, &c., are mutually interlaced
into a compact textile fabric. The first step towards making felt is to mix, in
the proper proportions, the different kinds of fibres intended to form the stuff; and then,
by the vibratory strokes of the bowstring, to toss them up in the air, and to cause them
to fall as irregularly as possible, upon the table, opened, spread, and scattered. The
workman covers this layer of loose flocks with a piece of thick blanket stuff slightly
moistened; he presses it with his hands, moving the hairs backwards and forwards in
all directions. Thus the different fibres get interlaced, by their ends pursuing ever tortuous
paths; their vermicular motion being always, however, root foremost. As the[455]
matting gets denser, the hand pressure should be increased in order to overcome the increasing
resistance to the decussation.
A first thin sheet of soft spongy felt being now formed, a second is condensed upon it in
like manner, and then a third, till the requisite strength and thickness be obtained. These
different pieces are successively brought together, disposed in a way suitable to the
wished-for article, and united by continued dexterous pressure. The stuff must be next
subjected to the fulling mill. See Hat Manufacture.
FERMENT (Eng. and Fr.; Hefe, Germ.) is the substance which, when
added in a small quantity to vegetable or animal fluids, tends to excite those intestine
motions and changes which accompany fermentation. It seems to be the result of an
alteration which vegetable albumen and gluten undergo with contact of air amidst a fermenting
mass. The precipitate or lees which fall down when fermentation is finished
consist of a mixture of the fermenting principle with the insoluble matters contained
in the fermented liquor, some of which, like hordeine, existed in the worts, and others
are probably generated at the time.
To prepare a pure ferment, or at least a compound rich in that principle, the precipitate
separated during the fermentation of a clear infusion of malt, commonly called
yeast or barm, is made use of. This pasty matter must be washed in cold distilled water,
drained and squeezed between the folds of blotting paper. By this treatment it becomes
a pulverulent mass, composed of small transparent grains, yellowish gray when viewed
in the compound microscope. It contains much water, and is therefore soft, like moist
gluten and albumen. When dried, it becomes like these bodies, translucid, yellowish
brown, horny, hard, and brittle. In the soft humid state it is insipid, inodorous, insoluble
in water and alcohol. If, in this state, the ferment be left to itself at a temperature
of from 60° to 70° F., but not in too dry a situation, it putrefies with the same
phenomena as vegetable gluten and albumen, and leaves, like them, a residuum resembling
old cheese.
At the beginning of this change, particularly if the ferment be enclosed in a limited
portion of air, there is an absorption of oxygen gas with a fivefold disengagement of carbonic
acid gas; while acetic acid makes its appearance in the substance. When distilled
by itself it affords the same products as gluten. Dilute acids dissolve it very readily;
and so does potash with the production of ammonia, a peculiar circumstance, for in
dissolving gluten the alkali causes no such evolution.
The property possessed by yeast of determining the fermentation of a properly
diluted solution of sugar is very fleeting, and is lost by very trifling alterations. It is
destroyed by complete desiccation, and cannot be restored by moistening it again. The
attempts made in London to squeeze out the liquid part of yeast in bags placed in a
powerful press, and to obtain a solid cake, in order to transport ferment to India, have
had but a very partial success; for its virtue is so impaired that it will rarely excite a
perfect fermentation in the best prepared worts. The same method is adopted in Germany,
to send yeast to only moderate distances; and therefore with more advantage.
If yeast be boiled for ten minutes, it loses the greater part of its fermenting power,
and by longer boiling it becomes inert.
When alcohol is poured upon yeast, it immediately destroys its fermenting faculties,
though, on filtering it off, it seems to carry no remarkable principle with it. One thousandth
part of sulphuric acid equally deprives yeast of its peculiar property, and so does a
little strong acetic acid. All the acids and the salts, especially those which part readily
with their oxygen, produce the same effect. A very small quantity of sulphurous acid,
or sulphites, mustard powder, particularly the volatile oil of mustard, and in general
the volatile oils that contain sulphur, as well as the vegetables which yield them, such
as horse-radish and garlick, all kill the fermenting agent. Lastly, fermentation is completely
stopped by a moderate depression of temperature.
During fermentation the yeast undergoes a change; it loses the property of causing
another wort to ferment. This change probably depends upon the chemical reaction
between the ferment and the sugar that is decomposed; for a certain quantity of yeast
can effect the fermentation of only a certain quantity of sugar, and all the sugar
exceeding this quantity remains unaltered in the liquor. It has been concluded from
some rather loose experiments, that one part and a half of yeast (supposed to be in the
dry state), is adequate to the fermentation of a solution of 100 parts of pure sugar.
When such a solution is fermented by the precise proportion of yeast, the fermenting
principle is exhausted, for no new yeast is formed in it. There is a deposit indeed to
about half the weight of the yeast employed, of a white matter insoluble in water,
which affords no ammonia by dry distillation, and is incapable of acting as a ferment
upon a fresh saccharine solution.
Of all the bodies convertible into yeast during fermentation, vegetable gluten
and albumen possess the most rapid and energetic powers. But ordinary glue, isinglass,
animal fibrine, curd or caseum, albumine, urine and other azotized substances, all[456]
enjoy the property of causing a solution of sugar to ferment; with this difference,
that whilst yeast can establish a complete fermentation in less than an hour, at a temperature
of about 68°, the above substances require several days, with a heat of from
77° to 87° F., for becoming ferments, and for occasioning fermentation. Substances
devoid of nitrogen do not produce a ferment.
FERMENTATION. (Eng. and Fr.; Gährung, Germ.) When organic substances,
under the influence of water, air, and warmth, are abandoned to the reciprocal operation
of their proximate principles, (sugar, starch, gluten, &c.), they are entirely changed and
decomposed, so that their ultimate principles (oxygen, hydrogen, carbon, and in some cases
azote,) combine in new proportions, and thus give birth to various new compounds. To
this process, the general name of fermentation has been given. These operations and their
products differ according to the differences of the substances, and of the circumstances
in which they are placed. The following may be enumerated as sufficiently distinct
species of fermentation. 1. The saccharine fermentation, in which starch and gum
are changed into sugar. 2. The vinous fermentation, in which sugar is converted into
alcohol. 3. The mucilaginous fermentation, in which sugar is converted into slime,
instead of alcohol. 4. The acetous fermentation, in which alcohol and other substances
are converted into vinegar. 5. The putrid fermentation or putrefaction, which
characterizes particularly the decomposition of azotized organic substances.
1. The saccharine fermentation. When a paste made by boiling one part of starch
with twelve parts of water is left entirely to itself, water merely being stirred in as it
evaporates, at the end of a month or two in summer weather it is changed into sugar,
equal in weight to from one third to one half of the starch, and into gum, equal to from
one fifth to one tenth, with a residuum of starch paste somewhat altered. This saccharifying
process advances much quicker through the co-operation of vegetable albumine
or gluten, acting as a ferment. If we boil two parts of potato starch into a
paste with twenty parts of water, mix this paste with one part of the gluten of wheat
flour, and set the mixture for 8 hours in a temperature of from 122° to 167° F., the
mixture soon loses its pasty character, and becomes by degrees limpid, transparent and
sweet, passing at the same time first into gum, and then into sugar. The remainder
consists of the unchanged starch with the altered gluten, which has become sour, and
has lost the faculty of acting upon fresh portions of starch. It is probable, however,
that the sugar formation in the first case, when the starch undergoes a spontaneous
change, may be due to the action of a small portion of gluten and albumine left in
the starch, since a putrefactive smell is eventually evolved indicative of that azotized
matter. The gum into which during this process the starch is first converted, and which
becomes afterwards sugar, is of the same nature as British gum, formed by the roasting
of starch.
This production of sugar takes place in the germination and kiln-drying of malt; and
the mashing of the brewer as well as the sweetening of bread in baking, rests upon the
same principles. In many cases the vinous fermentation precedes the saccharification,
or accompanies it; the starchy parts of the fermenting mass changing into sugar,
while the previously formed sugar becomes wine or beer. In the sweetening of fruits
by keeping, a similar process occurs; the gummy and starchy fibres become sugar from
the action of the glutinous ferment which they contain; as happens also to the juices
of many fruits which sweeten for a little while after they have been expressed.
The nature of this sugar formation through the influence of gluten upon starch, is
undoubtedly the same as the conversion of starch into sugar, by boiling it with sulphuric
acid; though the whole theory of this change is not entirely developed.
The most energetic substance for the conversion of starch into sugar, is the malt of
barley. According to the researches of Payen and Persoz, the gum which by this
process is first formed, may be prevented from going into sugar, by merely exposing it
to a boiling heat, and hence we have it in our power either to make sugar or gum at
pleasure. Of finely ground malt from 10 to 25 parts must be taken for 100 parts of starch.
Into a pan placed in a water bath, 400 parts of water being warmed to from 77° to 86° F.,
the ground malt must be stirred in, and the temperature must be raised to 140°. The 100
parts of starch must now be added, and well mixed. The heat is then to be increased
to 158°F.; and be so regulated that it shall not fall below 149°, nor rise above 167°.
In the course of 20 or 30 minutes the originally milky and pasty liquid will become
gradually more attenuated, and eventually it will turn as fluid nearly as water. This
is the point of time in which the starch has passed into gum, or into the substance
lately denominated dextrine by the chemists. Should this mucilaginous matter, which
appears to be a mixture of gum and a little starchy sugar, be wished for in that state,
the temperature of the liquid must be suddenly raised to the boiling point, whereby the
further action of the malt upon it is stopped. But on the other hand if sugar be
desired, then the temperature must be steadily maintained at from 158° to 167° for
three quarters of an hour, in which time the greater part of the starch will have become[457]
sugar, and from the evaporation of the fluid a starchy syrup will be obtained, entirely
similar to that procurable by the action of very dilute sulphuric acid upon starch.
The substance which operates this saccharine change, or the appropriate yeast of the
sugar fermentation, which had been previously imagined to be a residuum of gluten or
vegetable albumen in the germinated grain, has been traced by Payen and Persoz to a
peculiar proximate vegetable principle called by them diastase. This substance is
generated during the germination of barley, oats, and wheat, and may be obtained separately
by infusing the ground malt in a small quantity of cold water, straining off the
liquor, then filtering it, and heating the clear solution in a water bath to the temperature
of 158° F. The greater part of the vegetable albumen is thus coagulated,
and must be separated by a fresh filtration; the liquid is afterwards treated with
alcohol as long as the flocculent precipitate of diastase falls. In order to purify it still
more completely from the azotized matter, it may be once more dissolved in water, and again
precipitated by alcohol. When dried at a low temperature, it appears as a white solid,
which contains no azote, is insoluble in strong alcohol, but dissolves in weak alcohol and
water. Its solution is neutral and tasteless; and if left to itself, it changes spontaneously
sooner or later according to the degree of warmth, and becomes sour. At the temperature
of from 149° to 168°, it has the property of converting starch into gum or
dextrine, and sugar; and, when sufficiently pure, it does this with such energy, that one
part of it is capable of saccharifying 2000 parts of dry starch. It acts the more rapidly the
larger its proportion. Whenever the solution of diastase with starch or dextrine, has
been heated to the boiling point, it loses the property of transforming these substances.
One hundred parts of well malted barley appear to contain about one part of this new body.
2. The Vinous Fermentation.—In this fermentation the sugar existing in watery solution
is, by the operation of the ferment or yeast, converted into alcohol, with disengagement
of carbonic acid gas. If we dissolve one part of pure sugar in ten parts of water, and
leave the solution in a temperature of from 68° to 77° F., which is that most favourable
to fermentation, it will remain unaltered. But if we stir into that solution some beer
yeast, the phenomena of fermentation soon appear in the above circumstances; for carbonic
acid gas is evolved, with intestine movements of the liquid, and an increase of its temperature.
A body of yeast rises to the surface, and exhibits a continual formation and
rupture of air bubbles. At length the sugar being in a great measure decomposed, the
motions cease, the liquor becomes clear, and instead of being a syrup, it is now a dilute
alcohol. The yeast has by this time fallen to the bottom in a somewhat compact form,
and of a whitish colour, deprived of the property of exciting fermentation in fresh syrup,
provided no undue excess of it was added at first, for that alone would remain effective.
Experience shows that for the conversion of a determinate quantity of sugar by fermentation,
a determinate quantity of yeast is necessary, which has been estimated at about
11⁄2 per cent. in the dry state. When the yeast has been decomposed by fermenting its
definite proportion of sugar, it loses its fermentable property, and leaves the excess of
sugar unaffected, forming a sweet vinous solution. The same thing happens if the
yeast be separated from the wort by a filter in the progress of the fermentation, for then
all intestine motion speedily stops, although much saccharine matter remains.
In the juices of sweet fruits, of grapes, for example, the ferment is intimately associated
with the sugar. It is at first soluble and inactive, till it absorbs oxygen from the
atmosphere, whereby it becomes an operative ferment, but, at the same time, insoluble,
so as to precipitate at the end of the process. When the expressed juice of the grape,
or must, is inclosed in a vessel out of contact of air, and there subjected to the heat of
boiling water, the small portion of oxygen present is rendered inactive, and the liquor experiences
no fermentative change. If the grapes be squeezed in an atmosphere deprived of
oxygen, and confined in the same, the juice will also remain unaltered. Recently expressed
grape juice is limpid, and manifests the commencement of fermentation by the separation
of the yeasty substance, which can take place only with access of air. The solution
becomes turbid after a certain time, gas begins to be evolved, and the separated ferment
decomposes the sugar. At the end of the process the yeast collects at the bottom of the
vessel, usually in larger quantity than was sufficient to complete the fermentation; and
hence a considerable portion of it possesses still the fermentative faculty. The fermentation
itself, when once begun, that is, whenever the yeasty particles are evolved, and
float in the liquid, for which evolution a very minute quantity of oxygen is sufficient, is
thenceforth independent of the contact of air, and goes on as well in close as in open
vessels; so that the production of alcohol and carbonic acid depends solely upon the
mutual reaction of the ferment and the sugar.
The yeast, which may be obtained tolerably pure from a fine infusion of malt in a
state of fermentation, after being washed with cold water to separate the soluble, gummy,
and saccharine matter, and after being pressed between folds of blotting paper, constitutes a
pulverulent, grayish yellow, granular substance, destitute of both taste and smell, insoluble
both in water and alcohol. Cold water dissolves, indeed, only 1⁄400, and boiling water
very little more.
[458]
The essentially operative constituent of yeast is a peculiar azotized matter, which in
the wine vat is mixed with some tartar and other salts, and in the beer tun with gum,
starch, &c. This animalized substance may be obtained in a separate state, according
to Braconnot, by acting upon the washed yeast powder with a weak lye of carbonate of
potash, and by decomposing the solution with vinegar, whereby the matter is thrown
down in a gelatinous form. The substance thus obtained is insoluble in cold water
and alcohol, but dissolves readily in very dilute alkaline lyes, and even in lime water.
When diffused through water, it assumes a homogeneous aspect, as if it were really
dissolved; but when this mixture is heated, the animalized matter coagulates, and
separates in thick flocks. In this state it has lost its former properties, being no longer
soluble in alkaline lyes, even when concentrated. Acids exercise no solvent power over
this peculiar matter; they precipitate it from its solutions, as do also the earthy and
metallic salts, which, moreover, combine with it. This is also the case with tannin.
The combination of the ferment stuff with acids increases the stability of its constitution,
and counteracts its tendency to influence solutions of sugar. These properties of
the operative principle of yeast explain many of the phenomena of fermentation, as we
shall presently see.
The animalized matter of yeast resembles gluten, albumen, caseum, and other azotized
substances; if any one of these be put into a saccharine solution ready for fermentation, it
will begin to operate a change, when aided by warmth and time, if it be previously decomposed
in some measure to facilitate its influence; or if these substances be brought
into a slightly putrescent state beforehand, they will cause more speedy fermentation.
Thus white of egg, when added to saccharine liquors, requires a period of three weeks,
with a temperature of 96° F., before it will excite fermentation; afterwards the excess
of the albumen forms a precipitate which may be used instead of yeast upon other sweet
worts. The rapidity with which such azotized substances are capable of being converted
into ferments of more or less purity and power is very variable; vegetable
gluten and albumen being best fitted for this purpose. This conversion is accelerated
when the sweet liquor in which the substance is diffused or dissolved has already begun
to ferment; whence it appears that the presence of carbonic acid gas, combined with the
liquor, is here of singular influence. Upon it, in fact, the formation and elimination
of the yeast in fermenting liquors depend.
A solution of pure sugar, which has been made to ferment by the addition of yeast, furnishes
no new yeast; but there remains after the process a portion of the yeast originally
mixed, in an altered inoperative condition, should its quantity have been exactly adequate
to the decomposition of the sugar, or in an operative state, should the quantity
have been originally excessive.
But if the fermentable liquor contains vegetable albumen and gluten, as is commonly
the case with the sweet juices of fruits and beer worts, these substances become changed
into ferments in the course of the fermentation induced by the yeast, and, being superfluous,
so to speak, for that particular process, they remain entire at the end, and
may be collected for use in other operations.
Upon this principle is founded the increased production of yeast, and the manufacture
of what has been called artificial barm, in which the fermentation is conducted chiefly with
a view to the formation of yeast. To the fermenting mass, those kinds of meal are
added which abound in albumen and gluten, as barley, beans, or wheat, for instance;
and the process is similar to the production of a great lump of leaven, from the action
of a small piece of it upon dough. The following prescription will illustrate this subject.
Take three ounces of bean flour, add to it five quarts of boiling water, and
boil the mixture for half an hour. Pour the decoction into a vessel, and stir into it,
while hot, 56 ounces of wheaten flour. After the mixture cools to the temperature of
54° F., add to it about two quarts of beer barm, stirring the whole well together. About
24 hours after the commencement of the fermentation, incorporate with the mixture
112 ounces of barley or bean flour, till it becomes a uniform dough, which must be
thoroughly kneaded, rolled out into cakes about an inch thick, and cut into pieces of
the size of a dollar. These cakelets must be dried upon laths in the sun in favourable
weather, and then put up in a dry situation. For use, one of these discs is to be broken
into pieces, laid in warm water, and set in a warm place during 12 hours. The soft
mass will then serve the purpose of beer yeast.
Or we may mix equal parts of barley malt, wheat malt, and crushed rye, pour water
at the temperature of 122° F. over them into a tub till it stand a span above their surface;
then stir well together, and allow the whole to remain at rest for a few hours,
till it cools to about 65° F. We must now add for each pound of the mingled meals,
a quarter of an ounce of beer barm. The tub must be then covered, and preserved at
a temperature of 63° F. The husks, as they begin to rise to the surface, in consequence
of the fermentation, must be taken off, and squeezed through a cloth over the vessel.
When the meal comes afterwards to subside to the bottom, the whole must be strained[459]
through a canvas bag, and freed from the superfluous moisture by squeezing. The bag
with its doughy mass must next be surrounded with dry ashes, to remove the remaining
humidity, and to arrest any further fermentation. This consistent ferment may be used
instead of beer yeast.
It is difficult to prepare an artificial yeast without barm. The best process for this
purpose is the following. Take five parts of honey, one part of powdered tartar, and
sixteen parts of wheat or barley malt, stir the whole in water of the temperature of
122° F., and place in a fermenting heat; when the yeast will, as usual, be eliminated.
The change which gluten or vegetable albumen undergoes in the different kinds of
meal, when it becomes a ferment, consists apparently in an oxidation, since analysis
shows that this ferment contains more oxygen than gluten does.
It has been already stated that yeast in its liquid condition readily putrefies, and
becomes altogether useless for the process of fermentation. In order to preserve it for
some time, it must be dried to such a degree as to resist spontaneous decomposition
without losing its fermentative faculty; but completely dried yeast loses that property,
and does not recover it by being again moistened. Beer barm may be dried after being
washed several times with cold water, till the last quantity comes off clear; but the
insoluble portion must be allowed to settle fully before the water is poured away from
it. The residuum being freed as much as possible from water, by drainage and
pressure between flannel cloths, is to be dried in the shade by a current of warm
air as quickly as possible, with the aid of frequent turning over. It must be afterwards
kept in dry earthen vessels. Yeast may also be preserved a short time in activity by
being kneaded with as much barley or wheat flour as it can take up without losing the
doughy consistence. Dried yeast has, however, always an impaired activity. The easiest
and most certain method of preserving yeast in its primitive power, is by mixing it,
after pressure in flannel, with as much pulverized sugar as will render it dry, and
putting up the mixture in air-tight vessels. The fermentative power of yeast is
destroyed by the following means: 1. as already stated, by making it completely dry
either by the evaporation of the water, or its abstraction by alcohol; 2. by boiling,
which if continued for ten minutes renders yeast quite inoperative; 3. by the action of
such substances as dissolve out its essential constituents; by alkalis, for instance, since the
particles of yeast seem to be operative only in their insoluble granular state; 4. by such
substances as form combinations with it, and thereby either alter its nature, or at least
increase the cohesion of its constituent parts, so that they can no longer operate upon
sweet liquors by the decomposing affinity of its ultimate particles. Such bodies are the
acids, especially the mineral ones, tannin and most salts, particularly the metallic, which
unite with the yeast into new compounds. The volatile oils which contain sulphur
exercise the same paralyzing influence upon yeast.
The circumstances which promote, and are necessary to, the vinous fermentation are,
conformably to the above views, the following:—1. The presence of the proper quantity
of active yeast, and its proper distribution through the worts. If in the course of
a slack fermentation the yeast subsides to the bottom, the intestine motions cease
entirely, but they may be excited anew by stirring up the ingredients, or rousing the
tun, as the brewers say. 2. A certain degree of warmth, which should never be less
than 51° F., nor more than 86°; the temperature of from 68° to 77° being the most propitious
for the commencement and progress of fermentation. When other circumstances
are the same, the rapidity of the fermentation is proportional to the temperature
within certain limits, so that by lowering it, the action may be moderated at pleasure.
3. The fermentation proceeds the better and more equably the greater the mass of
fermenting liquor, probably on account of the uniformly high temperature, as well as
the uniform distribution of the active particles of the yeast by the greater energy of the
intestine movements. 4. The saccharine solution must be sufficiently diluted with water;
when too much concentrated it will not ferment. Hence very sweet musts furnish
wines containing much undecomposed sugar. For a complete fermentative action, one
part of sugar should be dissolved in ten parts of water.
Fermentation maybe tempered or stopped: 1. by those means which render the
yeast inoperative, particularly by the oils that contain sulphur, as oil of mustard; as
also by the sulphurous and sulphuric acids. The operation of the sulphurous acid in
obstructing the fermentation of must consists partly, no doubt, in its absorbing oxygen,
whereby the elimination of the yeasty particles is prevented. The sulphurous acid,
moreover, acts more powerfully upon fermenting liquors that contain tartar, as grape
juice, than sulphuric acid. This acid decomposes the tartaric salts, and, combining with
their bases, sets the vegetable acid free, which does not interfere with the fermentation;
but the sulphurous acid operates directly upon the yeast: 2. by the separation of the
yeast, either with the filter or by subsidence: 3. by lowering the temperature to
45° F. If the fermenting mass become clear at this temperature, and be drawn off
from the subsided yeast, it will not ferment again, though it should be heated to the
proper pitch.
[460]
The products of vinous fermentation are carbonic acid gas, and alcohol; of which the
former escapes during the process, except in the case of the sparkling wines, like champaign,
that are partially fermented in close vessels. The alcohol remains in the fermented
liquor. 100 parts of sugar afford by complete decomposition nearly 50 parts of
alcohol. According to Thenard, 100 parts of sugar are converted into 46·8 parts of
carbonic acid, and 49·38 of alcohol; besides 3·82 parts of carbon otherwise employed,
which the sugar contained, above what is present in the former two products. This chemist
found in the fermented liquor 4 per cent. of an extractive matter, soluble in water, and
having an acidulous reaction, to whose formation, probably, that excess of carbon
may be necessary. In what way the action of the yeasty particles upon the saccharine
substance is carried on in the vinous fermentation, or what may be the interior working of
this process, is not accurately understood. The quantitative relation of the carbonic acid
and alcohol to the sugar is pretty well made out; but the determination of the ultimate
principles of the ferment itself, before and after the vinous change, and of the residuum
dissolved in the fermented liquor, has not been well ascertained. It is probable that
the yeast undergoes in the process a similar decomposition to that of the putrefactive,
and that its elementary constituents enter into new combinations, and abstract so much carbon
and hydrogen from the sugar, that the remainder, amounting to 96 per cent.
of the whole, may constitute one atom of alcohol and one of carbonic acid.
3. The slimy or glutinous fermentation.—This process takes place in weak solutions
of sugar, at ordinary fermenting temperatures, where, from defect of good yeast, the
vinous fermentation cannot proceed. In such circumstances from one part of sugar, one
third part of gum is formed. According to Desfosses however, 100 parts of sugar afford
109·48 of gum or slime. This is formed when one part of sugar is dissolved in twenty
parts of water, which had been previously boiled with washed barm or gluten, and then
filtered. The process proceeds slowly and quietly, equally well in close vessels, as with
contact of air, and continues at ordinary temperatures about 12 days; but it goes on more
rapidly and completely at the heat of from 77° to 86° F. A small quantity of hydrogen
and carbonic acid gas is disengaged, in the proportion of two to one by volume. The
fermented liquor becomes turbid, and assumes a tough thready appearance, like a decoction
of linseed. A small addition of sulphuric or sulphurous acid, of muriatic acid and
alum, or of tannin, impedes this species of fermentation; because these substances combine,
as in the vinous fermentation, with the ferment into an insoluble precipitate, unsusceptible
of further change. In many wines, especially when bottled, this slimy fermentation
occurs, and occasions their ropiness, which may be best remedied or prevented
by the addition of as much tannin as will precipitate the dissolved mucous matter. This
species of fermentation attacks very rapidly the rinsing waters of the sugar refiner,
which always contain some fermentative gluten. A little alum is the best preventative
in this case, because it precipitates the dissolved ferment.
4. The acetous or sour fermentation.—In this process, alcohol, more or less dilute, is
resolved into water and vinegar, in consequence of the operation of the ferment; oxidizement
of the alcohol being effected by the oxygen of the atmospherical air. The
requisites of this process have been already detailed under the article Acetic Acid.
They are the presence of atmospherical air; alcohol diluted to a certain degree with
water ferment or yeast, and a temperature above 66° F. The most active ferments are
such substances as have already passed into the acetous state; hence vinegar, especially
when it contains some yeasty particles, or is combined with porous and spongy bodies,
so as to multiply its points of contact with the vinous liquor, is particularly powerful.
Common yeast may also be employed for vinegar ferments, if it be imbued with a little
vinegar, with leaven, crusts of bread soaked in vinegar, the stalks and husks of grapes,
sawdust and shavings of beech or oak impregnated with vinegar, or the slimy sediment
of vinegar casks called mother; all of which operate as ferments chiefly in consequence
of the vinegar which they contain. The inside shavings of the staves of vinegar tuns
act on the same principle.
The acetous fermentation may, moreover, go on along with the vinous in the same
liquor, when this contains sugar as well as alcohol. Whilst the acidification of the alcohol
is effected by the absorption of oxygen from the atmosphere, the sugar becomes alcohol
with disengagement of carbonic acid, and then passes into vinegar. Since most liquors
intended for making vinegar, such as wine, juices of fruits, ales, &c., contain still a little
sugar, they disengage always a little carbonic acid. Besides spirits, some other substances,
such as gum, the mucilage of plants, and starch paste, directly ferment into vinegar.
Sugar also seems to be convertible into vinegar without any vinous change. The albuminous
matter of potato juice, precipitated by vinegar, serves as a proper ferment for
that purpose, when added in its moist state to weak syrup. 5. See Putrefaction.
Mr. William Black, in his treatise on Brewing, has, with much ingenuity and apparent
truth, endeavoured to show that the process of fermentation is strongly influenced by
electricity, not only that of the atmosphere, as has been long known from the circumstance[461]
of beer and wine becoming speedily sour after thunderstorms, but the voltaic, produced
by electric combinations of metals in the fermenting tuns. He therefore recommends
these tuns to be made with as little metallic work as possible, and to be insulated
from the floor of the brewhouse. For the propriety of this advice he adduces some
striking examples. Wort which had become stationary in its fermentation, on being
pumped out of square gyles imbedded in the floor, into casks placed upon wooden
stillions, began immediately to work very well, and gained about 6 degrees of attenuation
while throwing off its yeast. From the stagnation of the process in the gyles, he had
in the morning predicted an approaching thunderstorm, which accordingly supervened
in the course of the evening. In further support of his views he instances the fact, that, in
dairies where the milk is put into porcelain vessels, and placed upon wooden shelves,
it is seldom injured by lightning; but when contained in wooden or leaden vessels, and
placed upon the ground, it almost invariably turns sour in thundery weather. His
general conclusion is “that the preservation or destruction of beer depends upon electricity;
and the most certain mode of preservation is to insulate as much as possible,
both the squares and all other utensils or vessels connected with the brewing or storing
of beer.”
Mr. Black further considers that unsoundness of worts is often the result of electricity
excited between the mash tun and the copper.
Why is beer liable to get spoiled in thunder storms, though apparently well insulated
in glass bottles?
I shall conclude this article with Mr. Black’s description of the phenomena of beer
fermentation. In every regular process there are five distinct stages. In the first
we see a substance like cream forming all round the edges of the gyle tun; which extends
towards the centre until the whole is creamed over, constituting the first change.
Next a fine curl appears like cauliflower, which also spreads over the square surface,
and according to the strength and appearance of this curl, the quality of the fermentation
may be predicated. This he calls the second stage. What is technically called
the stomach or vinous vapour now begins to be smelt, and continues to gain strength
till the process is concluded. From the vinous energy of this odour, and the progressive
attenuation of the wort, the vigour of the fermentation may be inferred. The experienced
brewer is much guided in his operations by the peculiarity of this effluvium. The
third change is when the cauliflower or curling top rises to a fine rocky or light yeasty
head; and when this falls down, the fourth stage has arrived. Finally the head should
rise to what is called close yeasty, having the appearance of yeast all over. About this
period the gas becomes so powerful as to puff up occasionally in little bells or bladders
about the size of a walnut, which immediately break. The bells should appear bright
and clear. If they be opaque or whey coloured, there is some unsoundness in the wort.
The great point is to add just so much yeast as to carry the fermentation completely
through these five changes at the regular periods.
FERROCYANATE, or, more correctly, FERROCYANIDE. (Ferrocyanure,
Fr.; Eisencyanid, Germ.) Several compounds of cyanogen and metals possess the
property of uniting together into double cyanides; of which there are none so remarkable
in this respect, as the protocyanide of iron. This appears to be capable of combining
with several simple cyanides, such as that of potassium, sodium, barium,
strontium, calcium, and ammonium. The only one of these double cyanides of any
importance in manufactures is the first, which is described under its commercial name,
Prussiate of Potash.
FERROPRUSSIATES; another name for Ferrocyanides.
FIBRE, VEGETABLE, called also Lignine; (Ligneux, Fr.; Pflanzen-faserstoff,
Germ.) is the most abundant and general ingredient of plants, existing in all their
parts, the root, the leaves, the stem, the flowers, and the fruit; amounting in the
compact wood to 97 or 98 per cent. It is obtained in a pure state by treating saw-dust
successively with hot alcohol, water, dilute muriatic acid, and weak potash lye, which
dissolve, first, the resinous; second, the extractive, and saline matters; third, the carbonate
and phosphate of lime; and, lastly, any residuary substances. Ligneous fibres,
such as saw-dust, powdered barks, straw, hemp, flax, linen, and cotton cloth, are convertible
by the action of strong sulphuric acid into a gummy substance analogous to
dextrine, and a sugar resembling that of the grape.
If we put into a glass mortar 24 parts, by weight, of dry old cordage, chopped small,
and sprinkle over it 34 parts of sulphuric acid, by degrees, so as to avoid heating the
mixture, while we constantly stir it; and if, in a quarter of an hour, we triturate the
mass with a glass pestle, the fibres will disappear without the disengagement of gas.
A tenacious mucilage will be produced, almost entirely soluble in water. The gum
being thus formed, may be separated from the acid by dilution with water, and addition
of the requisite quantity of chalk; then straining the saturated liquid through linen
cloth, concentrating it by evaporation, throwing down any remaining lime by oxalic[462]
acid, filtering anew, and mixing the mucilage with alcohol in great excess, which will
take up the free acid, and throw down the gum. From 24 parts of hemp fibres thus
treated, fully 24 parts of a gummy mass may be obtained, containing, however, probably
some water.
When, instead of saturating the diluted acid paste with chalk, we boil it for 10 hours,
the gummy matter disappears, and is replaced by sugar, which may be purified without
any difficulty, by saturation with chalk, filtration, and evaporation to the consistence of
syrup. In 24 hours crystallization begins, and, in 2 or 3 days, a concrete mass of grape
sugar is formed; which needs merely to be pressed strongly between old linen cloths
doubled, and then crystallized a second time. If this syrup be treated with bone black,
a brilliant white sugar will be procured. 20 parts of linen rags yield 23 of good sugar.
Braconnot. Guerin got 871⁄2 of dry sugar from 100 parts of rags, treated with 250 of
sulphuric acid. See Wood.
FIBRINE, (Eng. and Fr.; Thierischer Faserstoff, Germ.) constitutes the principal
part of animal muscle; it exists in the chyle, the blood, and may be regarded as the
most abundant constituent of animal bodies. It may be obtained in a pure state by
agitating or beating new drawn blood with a bundle of twigs, when it will attach itself
to them in long reddish filaments, which may be deprived of colour by working them
with the hands under a streamlet of cold water, and afterwards freed from any adhering
grease by digestion in alcohol or ether.
Fibrine, thus obtained, is solid, white, flexible, slightly elastic, insipid, inodorous,
denser than water, but containing 4 fifths of its weight of it, and without action on
litmus. When dried, it becomes semi-transparent, yellowish, stiff, and brittle: water
restores its softness and flexibility. 100 parts of fibrine consist of 53·36 carbon, 19·68
oxygen, 7·02 hydrogen, and 19·31 azote. As the basis of flesh, it is a very nutritious
substance, and is essential to the sustenance of carnivorous animals.
FILE (Lime, Fr.; Feile, Germ.), is a well known steel instrument, having teeth
upon the surface for cutting and abrading metal, ivory, wood, &c.
When the teeth of these instruments are formed by a straight sharp-edged chisel, extending
across the surface, they are properly called files; but when by a sharp-pointed tool, in
the form of a triangular pyramid, they are termed rasps. The former are used for all
the metals, as well as ivory, bone, horn, and wood; the latter for wood and horn.
Files are divided into two varieties, from the form of their teeth. When the teeth
are a series of sharp edges, raised by the flat chisel, appearing like parallel furrows,
either at right angles to the length of the file, or in an oblique direction, they are termed
single cut. But when these teeth are crossed by a second series of similar teeth, they
are said to be double cut. The first are fitted for brass and copper, and are found to
answer better when the teeth run in an oblique direction. The latter are suited for the
harder metals, such as cast and wrought iron and steel. Such teeth present sharp
angles to the substance, which penetrate it, while single cut files would slip over the
surface of these metals. The double cut file is less fit for filing brass and copper,
because its teeth would be very liable to become clogged with the filings.
Files are also called by different names according to their various degrees of fineness.
Those of extreme roughness are called rough; the next to this is the bastard cut; the
third is the second cut; the fourth, the smooth; and the finest of all, the dead
smooth. The very heavy square files used for heavy smith-work, are sometimes a little
coarser than the rough; they are known by the name of rubbers.
Files are also distinguished from their shape, as flat, half-round, three-square, four-square,
and round. The first are sometimes of uniform breadth and thickness throughout,
and sometimes tapering. The cross section is a parallelogram. The half-round is
generally tapering, one side being flat, and the other rounded. The cross section is a
segment of a circle, varying a little for different purposes, but seldom equal to a semi-circle.
The three-square generally consists of three equal sides, being equilateral
prisms, mostly tapering; those which are not tapering are used for sharpening the
teeth of saws. The four-square has four equal sides, the section being a square. These
files are generally thickest in the middle, as is the case with the smith’s rubber. In the
round file, the section is a circle, and the file generally conical.
The heavier and coarser kinds of files are made from the inferior marks of blistered
steel. Those made from the Russian iron, known by the name of old sable, called from
its mark CCND, are excellent. The steel made from the best Swedish iron, called
hoop L or Dannemora, makes the finest Lancashire files, for watch and clock makers;
a manufacture for which the house of Stubbs in Warrington is celebrated.
The steel intended for files is more highly converted than for other purposes, to give
them proper hardness. It should however be recollected, that if the hardness be not
accompanied with a certain degree of tenacity, the teeth of the file break, and do but
little service.
Small files are mostly made of cast steel, which would be the best for all others, if[463]
it were not for its higher price. It is much harder than the blistered steel, and from
having been in the fluid state, is entirely free from those seams and loose parts so common
to blistered steel, which is no sounder than as it comes from the iron forge
before conversion.
The smith’s rubbers are generally forged in the common smith’s forge, from the converted
bars, which are, for convenience, made square in the iron before they come into
this country. The files of lesser size are made from bars or rods, drawn down from the
blistered bars, and the cast ingots, and known by the name of tilted steel.
The file-maker’s forge consists of large bellows, with coak as fuel. The anvil-block,
particularly at Sheffield, is one large mass of mill-stone girt. The anvil is of considerable
size, set into and wedged fast into the stone; and has a projection at one end, with
a hole to contain a sharp-edged tool for cutting the files from the rods. It also
contains a deep groove for containing dies or bosses, for giving particular forms to
the files.
The flat and square files are formed entirely by the hammer. One man holds the
hot bar, and strikes with a small hammer. Another stands before the anvil with a two-handed
hammer. The latter is generally very heavy, with a broad face for the large
files. They both strike with such truth as to make the surface smooth and flat, without
what is called hand-hammering. This arises from their great experience in the same
kind of work. The expedition arising from the same cause is not less remarkable.
The half-round files are made in a boss fastened into the groove above mentioned.
The steel being drawn out, is laid upon the rounded recess, and hammered till it fills
the die.
The three-sided files are formed similarly in a boss, the recess of which consists of
two sides, with the angle downwards. The steel is first drawn out square, and then
placed in a boss with an angle downwards, so that the hammer forms one side, and the
boss two. The round files are formed by a swage similar to those used by common
smiths, but a little conical.
The file-cutter requires an anvil of a size greater or less, proportioned to the size of his
files, with a face as even and flat as possible. The hammers weigh from one to five or six
pounds. The chisels are a little broader than the file, sharpened to an angle of about
20 degrees. The length is just sufficient for them to be held fast between the finger
and thumb, and so strong as not to bend with the strokes of the hammer, the intensity
of which may be best conceived by the depth of the impression. The anvil is placed in
the face of a strong wooden post, to which a wooden seat is attached, at a small distance
below the level of the anvil’s face. The file is first laid upon the bare anvil, one end
projecting over the front, and the other over the back edge of the same. A leather
strap now goes over each end of the file, and passes down upon each side of the block to
the workman’s feet, which, being put into the strap on each side, like a stirrup, holds
the file firmly upon the anvil as it is cut. While the point of the file is cutting, the
strap passes over one part of the file only, the point resting upon the anvil, and the tang
upon a prop on the other side of the strap. When one side of the file is single cut, a
fine file is run slightly over the teeth, to take away the roughness; when they are to be
double cut, another set of teeth is cut, crossing the former nearly at right angles. The
file is now finished upon one side, and it is evident that the cut side cannot be laid
upon the bare anvil to cut the other. A flat piece of an alloy of lead and tin is interposed
between the toothed surface and the anvil, while the other side is cut, which
completely preserves the side already formed. Similar pieces of lead and tin, with
angular and rounded grooves, are used for cutting triangular and half-round files.
Rasps are cut precisely in the same way, by using a triangular punch instead of a flat
chisel. The great art in cutting a rasp is to place every new tooth as much as possible
opposite to a vacancy.
Many abortive attempts have been made to cut the teeth of files by machinery. The
following plan, for which a patent was obtained by Mr. William Shilton, of Birmingham,
in April 1833, is replete with ingenious mechanical resources, and deserves to
succeed.
The blanks of steel for making the files and rasps, are held in a pair of clamps in
connexion with a slide, and are moved forward at intervals under the head of the tilt
hammer which carries the tool; the distance which the blank is to be advanced at
every movement being dependent upon the required fineness or coarseness of the cut of
the file, which movement is effected and regulated by a rack and pinion, actuated by a
pall and ratchet wheel, or the movement may be produced by any other convenient
means.
When the machine is employed for cutting or indenting the teeth of rasps, the cutting
tool being pointed and only producing one tooth at a blow, the tilt hammer carrying the
tool must be made to traverse at intervals across the width of the blank piece of steel[464]
from one edge to the other and back again; the blank being advanced in length only
when the hammer has produced the last cut or tooth toward either edge of the rasp.
In order to render this invention better understood, two views of the apparatus for
producing the cross-cut or teeth of the files, are given.
Fig. 384*. is an elevation of the
upper part of the file-cutting machine,
as seen on one side; fig. 385.
is a plan or horizontal view, as the
machine appears on the top.
a, is the head of the tilt hammer
placed in the end of the lever b,
which is mounted on an axle c,
turning in proper bearings in the
frame work of the machine; d, is the
tilt wheel mounted on another axle
s, also turning in bearings on the
frame work of the machine, and
having any required number of projections
or tappets upon it for depressing
the tail or shorter end of the
hammer or tilt lever b.
The tilt wheel d, receives its rotatory
motion from the toothed wheel
f, mounted upon the same axle, and
it takes into geer with a pinion g,
upon the main shaft h, which is actuated
by a band passed from any
first mover to the rigger on its end,
or in any other convenient manner.
The bed upon which the blank piece
of steel bears is marked i. This bed
is firmly supported upon masonry
placed upon proper sleepers: j, is
one of the blank pieces of steel under
operation, and is shown secured in
the pair of jaws or holding clamps k,
mounted on centre pins in the slide
l, fig. 385.; which slide is held down
by a spring and slide beneath, and is moved backwards and forwards in the machine
upon the (v) edges m, m, of the frame, by means of the rack n, and its pinion; the
latter being mounted upon the axle of the ratchet wheel p, and which ratchet wheel is
made to turn at intervals by means of the pall q, upon the end of the lever r, fig. 385.
This lever is depressed, after every cut has been effected upon the blank by means of
the teeth or tappets of the wheel s, coming in contact with the inclined plane t, upon
the lever r. The tappet wheel s, is mounted upon the end of the axle e, of the tilt
wheel, and consequently revolves with it, and by depressing the lever r, every time that
a tooth passes the inclined plane t, the click q, is made to drive the ratchet wheel p,
and thereby the advancing movement of the blank is effected after each blow of the tilt
hammer.
There is a strong spring u, attached to the upper side of the tilt hammer, its end
being confined under an adjustable inclined plane v, mounted in the frame w, which
inclined plane can be raised or lowered by its adjusting screws as required, to produce
more or less tension of the spring.
A similar spring is placed on the under side of the tilt hammer, to raise and sustain
the cutter or tool clear of the bed after every blow, and in conjunction with safety
holders or catchers, to counteract any vibration or tendency the spring u, may have to
cause the hammer to reiterate the blow.
The end of the lower spring acts on an inclined plane, mounted in the frame w, which
has an adjusting screw similar to v, to regulate the tension of the spring.
In case the under spring should raise, that is, return the hammer, with sufficient
force or velocity to cause the top spring u, to reiterate the blow, the ends of the safety
holders or catchers are made to move under and catch the tail of the lever b, immediately
on its being raised by the under springs, which is effected by the following means:—The
holders are mounted upon a plate or carriage 1, fig. 384., which turns upon a small pin or
axle mounted in the ears of a cross bar; the upper ends of the holders are kept inclined
towards the tail of the tilt hammer by means of a spring fixed to the cross bar, and
which acts upon one end of the plate or carriage 1.
[465]
In order that the holders may be removed out of the way of the tail of the hammer
b, when the tilt wheel is about to effect a blow, the tooth of the tilt wheel which
last acted upon the hammer comes in contact with an inclined plane fixed on
the plate or carriage 1, and by depressing that end of the plate, causes the upper
ends of the holders to be withdrawn from under the tail of the hammer b. The
tilt wheel continuing to revolve, the next tooth advances, and depresses the tail of the
hammer, but before it leaves the tail of the hammer, the tooth last in operation will
have quitted the inclined plane and allowed the spring to return the holders into their
former position. After the tooth has escaped from the tail of b, the hammer will immediately
descend and effect the blow or cut on the blank, and as the tail of the
hammer rises, it will come in contact with the inclined planes at the upper ends of the
holders, and force them backwards; and as soon as the tail of the hammer has passed
the top of the holders, the spring will immediately force the holders forward under the
tail of the hammer, and prevent the hammer rising again until the next tooth of the
tilt wheel is about to depress the end of the hammer, when the same movements of the
parts will be repeated, and the machine will continue in operation until a sufficient
length of the blank of steel (progressively advanced under the hammer) has been
operated upon, when it will be thrown out of geer by the following means:—
Upon the sliding bar 6, there is placed an adjustable stop, against which the foremost
end of the slide l l, fig. 385. comes in contact, as it is moved forward by the rack n, and its
pinion. The sliding bar 6, is connected at its left end to the bent lever 8, the other end
of this lever being formed into a forked arm, which embraces a clutch upon the main
shaft, and as the slide l continues to advance, it will come in contact with a stop; and
when it has brought a sufficient length of the blank pieces of steel under the operation
of the cutting tool, the slide l, in its progress, will have moved that stop and the bar 6
forward, and that bar, by means of the bent lever 8, will withdraw the clutch on the
main shaft, from locking into the boss of the fly-wheel, and consequently stop the further
progress of the machine; the rigger and fly-wheel turning loosely upon the main shaft.
The cut file can now be removed from out of the clamps, and reversed to cut the other
side, or another blank piece put in its place; and after throwing back the pall q of the
ratchet wheel p, the slide l, and with it the fresh blank may be moved back into the
machine by turning the winch handle, on the axle of the ratchet wheel p, the reverse
way, which will turn the pinion backwards, and draw back the rack n, without affecting
any other parts of the machine; and on moving back the bar 6, by the handle 11, placed
on the stop, the clutches will be thrown into geer again, and the machine proceed to cut
the next blank.
When the blanks have been thus cut on one side, and are reversed in the machine to
form the teeth upon the other side, there should be a piece of lead placed between the
blank and the bed to protect the fresh cut teeth.
It will be seen that the position of the stop upon the bar 6, will determine the length
or extent of the blank piece of steel which shall be cut or operated upon; and in order
that the progressive movement of the blanks under the cutting tool may be made to suit
different degrees of fineness or coarseness of the teeth (that is the distance between the
cuts), there is an adjusting screw upon the lever r, the head of which screw stops against
the under side of an ear projecting from the frame-work, and thereby determines the
extent of the motion of the lever r, when depressed by the tappets of the wheel s, acting
upon the inclined plane t, consequently determining the number of teeth the ratchet
wheel p shall be moved round by the pall q; and hence the extent of motion communicated
by the rack and pinion to the slide l, and the blank j, which regulates the
distance that the teeth of the file are apart, and the lever r is forced upwards by a spring
pressing against its under side.
It will be perceived that the velocity of the descent of the hammer, and consequently
the force of the blow, may be regulated by raising or lowering the inclined plane v of
the spring u; and in order to accommodate the bed upon which the blanks rest to the
different inclinations they may be placed at, that part of the bed is formed of a semi-globular
piece of hardened steel, which fits loosely into a similar concavity in the bed r,
and is therefore capable of adjusting itself, so that the blanks shall be properly presented
to the cutting tool, and receive the blow or cut in an equal and even manner; or the
piece of steel may be of a conical shape, and fit loosely in a similar shaped concavity.
There are guides 16, placed on the top of the bed i, for the purpose of keeping the
blanks in their proper position towards the cutting tool, and these can be regulated to
suit blanks of any width, by turning the right and left handed screw 17. There is also
another adjustable stop on the jaws or clamps k which serves as a guide when placing
the blanks within the jaws: and 19 is a handle or lever for raising the clamps when
required, which has a weight suspended from it for the purpose of keeping down the
blanks with sufficient pressure upon the bed.
The cutting tool in the face of the hammer, can be placed at any required angle or[466]
inclination with the blank, it being secured in the head of the hammer by clamps and
screws. In cutting fine files a screw is employed in preference to the rack and
pinion, for advancing the slide l, and the blank piece of steel in the machine.
Hardening of files.—This is the last and most important part of file making.
Whatever may be the quality of the steel, or however excellent the workmanship, if it is
not well hardened all the labour is lost.
Three things are strictly to be observed in hardening; first, to prepare the file on the
surface, so as to prevent it from being oxidated by the atmosphere when the file is red
hot, which effect would not only take off the sharpness of the tooth, but render the
whole surface so rough that the file would, in a little time, become clogged with the
substance it had to work. Secondly, the heat ought to be very uniformly red throughout,
and the water in which it is quenched, fresh and cold, for the purpose of giving it the
proper degree of hardness. Lastly, the manner of immersion is of great importance, to
prevent the files from warping, which in long thin files is very difficult.
The first object is accomplished by laying a substance upon the file, which when
it fuses, forms as it were, a varnish upon the surface, defending the metal from the
action of the oxygen of the air. Formerly the process consisted in first coating the
surface of the file with ale grounds, and then covering it over with pulverized common
salt, (muriate of soda.) After this coating became dry, the files were heated red hot,
and hardened; after this, the surface was lightly brushed over with the dust of cokes,
when it appeared white and metallic, as if it had not been heated. This process has
lately been improved, at least so far as relates to the economy of the salt, which from
the quantity used, and the increased thickness, had become a serious object. Those who
use the improved method are now consuming about one fourth the quantity of salt used
in the old method. The process consists in dissolving the salt in water to saturation,
which is about three pounds to the gallon, and stiffening it with ale grounds, or with
the cheapest kind of flour, such as that of beans, to about the consistence of thick cream.
The files require to be dipped only into this substance, and immediately heated and
hardened. The grounds or the flour are of no other use, than to give the mass consistence,
and by that means to allow a larger quantity of salt to be laid upon the surface.
In this method, the salt forms immediately a firm coating. As soon as the water is
evaporated, the whole of it becomes fused upon the file. In the old method the dry
salt was so loosely attached to the file, that the greatest part of it was rubbed off into
the fire, and was sublimed up the chimney, without producing any effect.
The carbonaceous matter of the ale grounds is supposed to have some effect in giving
hardness to the file, by combining with the steel, and rendering it more highly carbonated.
It will be found, however, upon experiment, that vegetable carbon does not
combine with iron, with sufficient facility to produce any effect, in the short space of
time a file is heating, for the purpose of hardening. Some file makers are in the habit
of using the coal of burnt leather, which doubtless produces some effect; but the carbon
is generally so ill prepared for the purpose, and the time of its operation so short, as to
render the result inconsiderable. Animal carbon, when properly prepared and mixed,
with the above hardening composition, is capable of giving hardness to the surface even
of an iron file.
This carbonaceous matter may be readily obtained from any of the soft parts of
animals, or from blood. For this purpose, however, the refuse of shoemakers and
curriers is the most convenient. After the volatile parts have been distilled over, from
an iron still, a bright shining coal is left behind, which, when reduced to powder, is fit
to mix with the salt. Let about equal parts, by bulk, of this powder, and muriate of
soda be ground together, and brought to the consistence of cream, by the addition of
water. Or mix the powdered carbon with a saturated solution of the salt, till it become
of the above consistence. Files which are intended to be very hard, should be covered
with this composition, previous to hardening. All files intended to file iron or steel,
particularly saw files, should be hardened with the aid of this mixture, in preference
to that with the flour or grounds. Indeed, it is probable, that the carbonaceous powder
might be used by itself, in point of economy, since the ammonia or hartshorn, obtained
by distillation, would be of such value as to render the coal of no expense. By means
of this method the files made of iron, which, in itself, is unsusceptible of hardening,
acquire a superficial hardness sufficient for any file whatever. Such files may, at the
same time, be bent into any form; and, in consequence, are particularly useful for
sculptors and die-sinkers.
The next point to be considered is the best method of heating the file for hardening.
For this purpose a fire, similar to the common smiths’ fire, is generally employed.
The file is held in a pair of tongs by the tang, and introduced into the fire, consisting
of very small cokes, pushing it more or less into the fire for the purpose of heating it
regularly. It must frequently be withdrawn with the view of observing that it is not
too hot in any part. When it is uniformly heated, from the tang to the point, of a[467]
cherry red colour, it is fit to quench in the water. At present an oven, formed of fire-bricks,
is used for the larger files, into which the blast of the bellows is directed, being
open at one end, for the purpose of introducing the files and the fuel. Near to the top
of the oven are placed two cross bars, on which a few files are placed, to be partially
heating. In the hardening of heavy files, this contrivance affords a considerable
saving, in point of time, while it permits them also to be more uniformly and thoroughly
heated.
After the file is properly heated for the purpose of hardening, in order to produce the
greatest possible hardness, it should be cooled as soon as possible. The most common
method of effecting this is by quenching it in the coldest water. Some file-makers
have been in the habit of putting different substances in their water, with a view to
increase its hardening property. The addition of sulphuric acid to the water was
long held a great secret in the hardening of saw files. After all, however, it will be
found, that clear spring water, free from animal and vegetable matter, and as cold as
possible, is the best calculated for hardening files of every description.
In quenching the files in water, some caution must be observed. All files, except the
half-round, should be immersed perpendicularly, as quickly as possible, so that the upper
part shall not cool. This management prevents the file from warping. The half-round
file must be quenched in the same steady manner; but, at the same time that it is kept
perpendicular to the surface of the water, it must be moved a little horizontally, in the
direction of the round side, otherwise it will become crooked backwards.
After the files are hardened, they are brushed over with water, and powdered cokes,
when the surface becomes perfectly clean and metallic. They ought also to be washed
well in two or three clean waters, for the purpose of carrying off all the salt, which, if
allowed to remain, will be liable to rust the file. They should moreover be dipped
into lime-water, and rapidly dried before the fire, after being oiled with olive oil,
containing a little oil of turpentine, while still warm. They are then finished.
FILLIGREE (Filigrane, Fr.; Filigran, or Feine Drahtgeflecht, Germ.); is, as the
last term justly expresses it, intertwisted fine wire, used for ornamenting gold and silver
trinkets. The wire is seldom drawn round, but generally flat or angular; and soldered
by gold or silver solder with borax and the blowpipe. The Italian word,
filigrana, is compounded of filum and granum, or granular net-work; because the
Italians, who first introduced this style of work, placed small beads upon it.
FILTRATION (Eng. and Fr.; Filtriren, Germ.), is a process purely mechanical, for
separating a liquid from the undissolved particles floating in it, which liquid may be either
the useful part, as in vegetable infusions, or of no use, as the washings of mineral precipitates.
The filtering substance may consist of any porous matter in a solid, foliated, or
pulverulent form; as porous earthen ware, unsized paper, cloth of many kinds, or sand.
The white blotting paper sold by the stationers answers extremely well for filters in chemical
experiments, provided it be previously washed with dilute muriatic acid, to remove
some lime and iron that are generally present in it. Filter papers are first cut square,
and then folded twice diagonally into the shape of a cornet, having the angular parts
rounded off. Or the piece of paper being cut into a circle, may be folded fan-like from
the centre, with the folds placed exteriorly, and turned out sharp by the pressure of the
finger and thumb, to keep intervals between the paper and the funnel into which it
is fitted, to favour the percolation. The diameter of the funnel should be about three-fourths
of its height, measured from the neck to the edge. If it be more divergent,
the slope will be too small for the ready efflux of the fluid. A filter covered with the
sediment is most conveniently washed by spouting water upon it with a little syringe.
A small camel’s-hair paint brush is much employed for collecting and turning over
the contents in their soft state. Agitation or vibration is of singular efficacy in quickening
percolation, as it displaces the particles of the moistened powders, and opens up
the pores which had become closed. Instead of a funnel, a cylindrical vessel may be
employed, having its perforated bottom covered with a disc of filtering powder folded
up at the edges, and made tight there by a wire ring. Linen or calico is used for weak
alkaline liquors; and flannels, twilled woollen cloth, or felt-stuff for weak acid ones.
These filter bags are often made conical like a fool’s cap, and have their mouths supported
by a wooden or metallic hoop. Cotton wool put loose into the neck of a funnel
answers well for filtering oils upon the small scale. In the large way, oil is filtered
in conical woollen bags, or in a cask with many conical tubes in its bottom, filled with
tow or cotton wool. Stronger acid and alkaline liquors must be filtered through a layer
of pounded glass, quartz, clean sand, or bruised charcoal. The alcarrhazas are a porous
biscuit of stone ware made in Spain, which are convenient for filtering water, as also the
porous filtering stone of Teneriffe, largely imported into England at one time, but now
superseded in a great measure by the artificial filters patented under many forms,
consisting essentially of strata of gravel, sand, and charcoal powder.
It is convenient to render the filter self-acting, by accommodating the supply of liquid[468]
to the rate of percolation, so that the pressure upon the porous surface may be always
equally great. Upon the small scale, the lamp-fountain or bird’s-glass form so generally
used for lamps, will be found to answer.
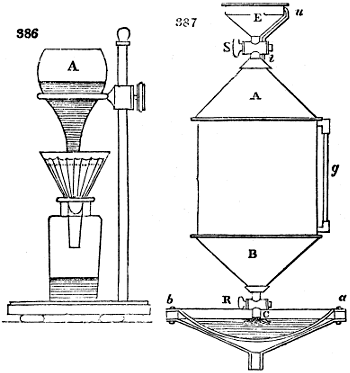
Fig. 386. represents a glass bottle A, partly filled with the fluid to be filtered, supported
in the ring of a chemical stand, and having its mouth inverted into the same liquor in
the filter funnel. It is obvious, that whenever this liquor by filtration falls below the
lip of the bottle, air will enter into it, let down a fresh supply to feed the filter, and
keep the funnel regularly charged. If larger quantities are to be operated upon, the
following apparatus may
be employed. Fig. 387.
A B is a metallic vessel
which may be made air-tight;
C is the under pipe
provided with a stopcock
R, for letting down the
liquor into the filter a b.
The upper pipe t, through
which the fluid is poured
by means of the funnel E,
has also a stopcock which
opens or shuts, at the same
time, the small side tube u t,
through which, during the
entrance of the fluid, the
air is let off from the receiver.
A glass tube g,
shows the level of the
liquor in the body of the
apparatus. In using it,
the cock R must be first
closed, and the cock S
must be opened to fill the
receiver. Then the filter
is set a going, by re-opening
the cock R, so as to keep the fluid in the filter upon a level with the opening of the
tube C. Both these pieces of apparatus are essentially the same.
In many manufactures, self-acting filters are fed by the plumber’s common contrivance
of a ball-cock in which the sinking and rising of the ball, within certain
limits, serves to open or
shut off the supply of
liquor, as it may be required
or not. Dumont
has adopted this expedient
for his system of filtering
syrup through a stratum of
granularly ground animal
charcoal or bone-black.
Fig. 388. is a front view
of this apparatus with 4
filters C; and fig. 389. is a
cross section. The framework
B supports the cistern
A, in which the syrup is contained. From it the liquor flows through the stop-cock[469]
b, and the connection-tube a, into the common pipe c, which communicates, by the
short branch tubes e, with each of the four filters. The end of the branch tube, which
is inside of the filter tub, is provided with a stopcock d f, whose opening, and thereby
the efflux of the liquor from the cistern through the tube a, is regulated by means of
the float-ball g. Upon the brickwork D the filter tub stands, furnished at h with a false
bottom of zinc or copper pierced with fine holes; besides which, higher up at i there is
another such plate of metal furnished with a strong handle k, by which it may be removed,
when the bone-black needs to be changed. In the intervening space l, the
granular coal is placed. o is the cover of the filter tub, with a handle also for lifting it.
One portion of it may be raised by a hinge, when it is desired to inspect the progress
of the filtration within. m m is a slender vertical tube, forming a communication between
the bottom part h, and the upper portion of the filter, to admit of the easy escape of the
air from that space, and from among the bone-black as the syrup descends; otherwise
the filtration could not go on. p is the stopcock through which the fluid collected in
the space under h is let off from time to time into the common pipe q, fig. 388. r is a
trickling channel or groove lying parallel to the tube q, and in which, by means of a
tube s, inserted at pleasure, the syrup is drawn off in case of its flowing in a turbid state,
when it must be returned over the surface of the charcoal.
The celerity with which any fluid passes through the filter depends, 1. upon the
porosity of the filtering substance; 2. upon the pressure exercised upon it; and 3. upon
the extent of the filtering surface. Fine powders in a liquor somewhat glutinous, or
closely compacted, admit of much slower filtration than those which are coarse and
free; and the former ought, therefore, to be spread in a thinner stratum and over a
more extensive surface than the latter, for equal effect; a principle well exemplified in
the working of Dumont’s apparatus, just described.
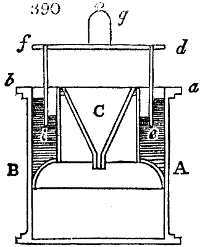
In many cases filtration may be accelerated by the increase of hydrostatic or pneumatic
pressure. This happens when we close the top of a filtering cylinder, and connect
it by a pipe with a cistern of fluid placed upon a higher level. The pressure of the
air may be rendered operative also either by withdrawing it partially from a close
vessel, into which the bottom of the filter enters, or by increasing its density over the
top of the liquor to be filtered. Either the air pump or steam may be employed to
create a partial void in the receiver beneath the filter. In like manner, a forcing pump
or steam may be employed to exert pressure upon the surface of the filtering liquor. A
common syphon may, on the same principle, be made a good pressure filter, by making
its upper leg trumpet-shaped, covering the orifice with filter paper or cloth, and filling
the whole with liquor, the lower leg being of such length so as to create considerable
pressure by the difference of hydrostatic level. This apparatus is very convenient either
on the small or great scale, for filtering off a clear fluid from a light muddy sediment.
The pressure of the atmosphere may be elegantly applied to common filters, by the
apparatus represented in fig. 390., which is merely a funnel inclosed within a gasometer.
The case A B bears an annular hollow vessel a b, filled with water, in which
receiver the cylindrical gasometer d, e, f, i, is immersed. The filter funnel C is secured
at its upper edge to the inner surface of the annular vessel a b. In consequence of the
pressure of the gasometer regulated by the weight g, upon the air inclosed within it, the
liquid is equally pressed, and the water in the annular space rises to a corresponding
height on the outer surface of the gasometer, as shown in the figure. Were the
apparatus made of sheet iron, the annular space might be charged with mercury.
In general, relatively to the application of pressure to filters, it may be remarked,
that it cannot be pushed very far, without the chance of deranging the apparatus, or
rendering the filtered liquor muddy. The enlargement of the surface is, generally
speaking, the safest and most efficacious plan of increasing the rapidity of filtration,
especially for liquids of a glutinous nature. This expedient is well illustrated in the
creased bag filter now in use in most of the sugar refineries of London. See Sugar.
In many cases it is convenient so to construct the filtering apparatus, as that the
liquid shall not descend, but mount by hydrostatic pressure. This method has two
advantages: 1. that without much expensive apparatus, any desired degree of hydrostatic
pressure may be given, as also that the liquid may be forced up through several filtering
surfaces placed alongside of each other; 2. that the object of filtering, which is to
separate the particles floating in the fluid without disturbing the sediment, may be perfectly
attained, and thus very foul liquids be cleared without greatly soiling the filtering
surface.
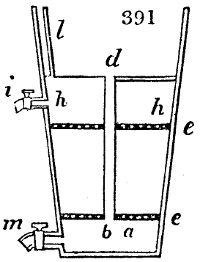
Such a construction is peculiarly applicable to the purification of water, either alone,
or combined with the downwards plan of filtration. Of the former variety an example
is shown in fig. 391. The wooden or zinc conical vessel is provided with two perforated
bottoms or sieves e e, betwixt which the filtering substance is packed. Over
this, for the formation of the space h h, there is a third shelf, with a hole in its middle,
through which the tube d b is passed, so as to be water tight. This places the upper[470]
open part of the apparatus in communication with the lowest space a. From the compartment
h h a small air tube l runs upwards. The filtering substance consists at bottom
of pebbles, in the middle of gravel, and at the top of fine sand, which may be mixed
with coarsely ground bone-black, or covered with a layer of the same. The water to
be filtered being poured into the cistern at top, fills through the tube b d the inferior
compartment a, from which the hydrostatic pressure forces the water upward through
the perforated shelf, and the filtering materials. The pure water collects in the space
h h, while the air escapes by the small tube l, as the liquid enters. The stopcock i serves to
draw off the filtered water. As the motion of the fluid in the filter is slow, the particles
suspended in it have time to subside by their own gravity; hence there collects
over the upper shelf at d, as well as over the under one at a, a precipitate or deposit
which may be washed out of the latter cavity by means of the stopcock m.
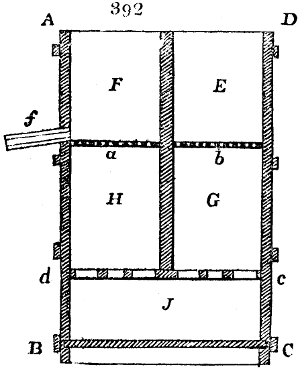
As an example of an upwards and downwards filter, fig. 392. may be exhibited. A B C D
is a wooden or metallic cistern
furnished with the perforated
shelf c d near its under part, upon
which a vertical partition is fixed
through the axis of the vessel.
A semicircular perforated shelf
is placed at a, and a second
similar one at b. These horizontal
shelves rest upon brackets in
the sides of the cisterns, so that
they may be readily lifted out.
The space G is filled with coarse
sand, J with moderately fine, and
H with very fine. The foul water
is poured into the chamber E,
and presses through G J H and
into the space F; whence it may
be drawn by the stopcock f.
Fig. 393. represents in section a filtering apparatus consisting of two concentric
chambers; the interior being destined for downwards filtration, and the exterior for
upwards. Within the larger cistern A, a smaller one B is placed concentrically, with its
under part, and is left open from
distance to distance, to make a
communication between the interior
cavity and the exterior
annular space. These cavities
are filled to the marked height
with sand and gravel. The
inner cylindrical space has fine
sand below, then sharper sand
with granular charcoal, next
coarse sand, and lastly gravel.
The annular space has in like
manner fine sand below. The
foul water is introduced by the
pipe E, the orifice at whose end
is acted upon by a ball-cock
with its lever a; whereby the
water is kept always at the same
level in the inner vessel. The water sinks through the sand strata of the middle vessel,
passes outwards at its bottom into the annular space, thence up through the sand in it,
and collecting above it, is let off by the stopcock on the pipe b. When a muddy deposit
forms after some time, it may be easily cleared out. The cord e, running over the
pulleys f f, being drawn tight, the ball lever will shut up the valve. The stopcock d made
fast to the conducting tube E must then be opened, so that the water now overflows into
the annular space at A; the tube c, in communication with the inner space B, being opened
by taking out the stopper h. The water thereby percolates through the sand strata in the
reverse direction of its usual course, so as to clear away the impurities in the space B,
and to discharge them by the pipe c h. An apparatus of this kind of moderate size is
capable of filtering a great body of water. It should be constructed for that purpose of
masonry; but upon a small scale it may be made of stone-ware.
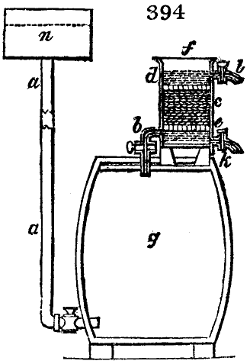
A convenient apparatus for filtering oil upwards is represented in fig. 394. g is an oil
cask, in which the impure parts of the oil have accumulated over the bottom. Immediately
above this, a pipe a is let in, which communicates with an elevated water cistern[471]
n. f is the filter, (placed on the lid of the cask) furnished with two perforated shelves,
one at e and another at d; which divide the interior of the filter into three compartments.
Into the lower space immediately over the shelf e,
the tube b, furnished with a stopcock enters, to establish a
communication with the cask; the middle cavity e is filled with
coarsely ground charcoal or other filtering materials; and the
upper one has an eduction pipe l. When the stopcocks of the
tubes a and b are opened, the water passes from the cistern into
the oil cask, occupies from its density always the lowest place,
and presses the oil upwards, without mixing the two liquids;
whereby first the upper and purer portion of the oil is forced
through tube b into the filter, and thence out through the pipe l.
When the fouler oil follows, it deposits its impurities in the
space under the partition c, which may from time to time be
drawn off through the stopcock k, while the purer oil is pressed
upwards through the filter. In this way the different strata
of oil in the cask may be filtered off in succession, and kept
separate, if found necessary for sale or use, without running any risk of mixing up
the muddy matter with what is clear. According to the height of the water cistern n,
will be the pressure, and of course the filtering force. When the filter gets choked
with dirt, it may be easily re-charged with fresh materials.
In filtering caustic alkaline lyes through linen or quartz, it is proper to exclude the
free contact of air; which is done by inclosing the upper vessel, and attaching a pipe of
communication between its cover, and the shoulder of the lower vessel, or recipient of
the lyes. In proportion as these flow down, they will displace their bulk of air, and
drive it into the top of the upper vessel above the foul lyes.
Many modifications of the above described apparatus are now on sale in this country;
but certainly the neatest, most economical, and effective means of transforming the
water of a stagnant muddy pool, into that of a crystalline fountain, is afforded by the
Royal Patent Filters of George Robins.
FIRE ARMS, Manufacture of. This art is divided into two branches, that of
the metallic and of the wooden work. The first includes the barrel, the lock, and the
mounting, as also the bayonet and ramrod, with military arms. The second comprises
the stock, and in fowling pieces, likewise the ramrod.
1. The Barrel. Its interior is called the bore; its diameter, the calibre; the back
end, the breech; the front end, the muzzle; and the closing of the back end, the breech
pin or plug. The barrel is generally made of iron. Most military musquets and
low-priced guns are fashioned out of a long slip of sheet-iron folded together edge-wise
round a skewer into a cylinder, are then lapped over at the seam, and welded at a
white heat. The most ductile and tenacious soft iron, free from all blemishes, must be
selected for this slip. It is frequently welded at the common forge, but a proper air-furnace
answers better, not being so apt to burn it. It should be covered with ashes
or cinders. The shape of the bore is given by hammering the cylinder upon a steel
mandril, in a groove of the anvil. Six inches of the barrel at either end are left open
for forming the breech and the muzzle by a subsequent welding operation; the extremity
put into the fire being stopped with clay, to prevent the introduction of cinders.
For every length of two inches, there are from two to three welding operations, divided
into alternating high and low heats; the latter being intended to correct the defects of
the former. The breech and muzzle are not welded upon the mandril, but upon the
horn of the anvil; the breech being thicker in the metal, is more highly heated, and is
made somewhat wider to save labour to the borer. The barrel is finally hammered in
the groove of the anvil without the mandril, during which process it receives a heat
every two minutes. In welding, the barrel extends about one-third in length; and
for musquets, is eventually left from 3 to 31⁄2 feet long; but for cavalry pistols, only 9
inches.
The best iron plates for gun-barrels are those made of stub iron, that is of old
horse-shoe nails welded together, and forged into thin bars, or rather narrow ribands.
At one time damascus barrels were much in vogue; they were fashioned either as above
described, from plates made of bars of iron and steel laid parallel, and welded together,
or from ribands of the same damascus stuff coiled into a cylinder at a red heat, and then
welded together at the seams. The best modern barrels for fowling pieces are constructed
of stub-nail iron in this manner. The slip or fillet is only half an inch broad
or sometimes less, and is left thicker at the end which is to form the breech, and thinner
at the end which is to form the muzzle, than in the intermediate portion. This fillet
being moderately heated to increase its pliancy, is then lapped round the mandril in a
spiral direction till a proper length of cylinder is formed; the edges being made to
overlap a little in order to give them a better hold in the welding process. The coil[472]
being taken off the mandril and again heated, is struck down vertically with its muzzle
end upon the anvil, whereby the spiral junctions are made closer and more uniform. It
is now welded at several successive heats, hammered by horizontal strokes, called jumping,
and brought into proper shape on the mandril. The finer barrels are made of still
narrower, stub-iron slips, whence they get the name of wire twist. On the Continent,
barrels are made of steel wire, welded together lengthwise, then coiled spirally into a
cylinder. Barrels that are to be rifled, require to be made of thicker iron, and that of
the very best quality, for they would be spoiled by the least portion of scale upon
their inside. Soldiers’ musquets are thickened a little at the muzzle, to give a stout
holding to the bayonet.

The barrels thus made are annealed with a gentle heat in a proper furnace, and
slowly cooled. They are now ready for the borer, which is an oblong square bit of
steel, pressed in its rotation against the barrel, by a slip of wood applied to one of its
flat sides, and held in its place by a ring of metal. The boring bench works horizontally,
and has a very shaky appearance, in respect at least of the bit. In some cases,
however, it has been attempted to work the barrels and bits at an inclination to the
horizon of 30°, in order to facilitate the discharge of the borings. The barrel is held in
a slot by only one point, to allow it to humour the movements of the borer, which
would otherwise be infallibly
broken. The bit, as represented
in fig. 395., has merely its
square head inserted into a
clamp-chuck of the lathe, and
plays freely through the rest of
its length.
Fig. 396. represents in plan
the boring bench for musquet
barrels; f f is the sledge or
carriage frame in which the barrel is supported; a is the revolving chuck of the lathe,
into which the square end of the bit, fig.. 395., is inserted; b is the barrel, clamped at
its middle to the carriage, and capable of being pressed onwards against the tapering
bit of the borer, by the bent lever c, worked by the left hand of the operative against
fulcrum knobs at d, which stand about two inches asunder. Whenever the barrel has
been thereby advanced a certain space to the right, the bent end of the lever is shifted
against another knob or pin. The borer appears to a stranger to be a very awkward
and unsteady mechanism, but its perpetual vibrations do not affect the accuracy of the
bore. The opening broach may be of a square or pentagonal form; and either gradually
tapered from its thickest part, or of uniform diameter till within two inches of
the end, whence it is suddenly tapered to a point.
A series of bits may be used for boring a barrel, beginning with the smallest and
ending with the largest. But this multiplication of tools becomes unnecessary, by
laying against the cutting part of the bit, slips of wood, called spales, of gradually
increasing thickness, so that the edge is pressed by them progressively further from the
axis. The bore is next polished. This is done by a bit with a very smooth edge,
which is mounted as above, with a wedge of wood besmeared with a mixture of oil and
emery. The inside is finished by working a cylindrical steel file quickly backwards and
forwards within it, while it is revolving slowly.
In boring, the bit must be well oiled or greased, and the barrel must be kept cool by
letting water trickle on it; for the bit, revolving at the rate of 120 or 140 times a
minute, generates a great deal of heat. If a flaw be detected in the barrel during the
boring, that part is hammered in, and then the bit is employed to turn it out.
Many sportsmen are of opinion that a barrel with a bore somewhat narrowed towards
the muzzle serves to keep shot better together; and that roughening its inside with
pounded glass has a good effect, with the same view. For this purpose, also, fine
spiral lines have been made in their interior surface. The justness of its calibre is tried
by means of a truly turned cylinder of steel, 3 or 4 inches long, which ought to move
without friction, but with uniform contact from end to end of the barrel. Whatever irregularities
appear must be immediately removed.
The outer surface of the barrel is commonly polished upon a dry grindstone, but it
is better finished, and less dangerously to the workman, at a turning lathe with a slide
rest. If a stone be used, it should be made to revolve at the mouth of a tunnel of some
kind, into which there is a good draught to carry off the ferruginous particles. A
piece of moist cloth or leather should be suspended before the orifice.
Rifle barrels have parallel grooves of a square or angular form cut within them, each
groove being drawn in succession. These grooves run spirally, and form each an
aliquot part of a revolution from the chamber to the muzzle. Rifles should not be too
deeply indented; only so much as to prevent the ball turning round within the barrel.[473]
and the spires should be truly parallel, that the ball may glide along with a regular
pace. See infra.
The Parisian gun-makers, who are reckoned very expert, draw out the iron for the
barrels at hand forges, in fillets only one-ninth of an inch thick, one inch and a half
broad, and four feet long. Twenty-five of these ribands are laid upon each other, between
two similar ones of double thickness, and the bundle, weighing 60 pounds, bound with
wire at two places, serves to make two barrels. The thicker plates are intended to
protect the thinner from the violence of the fire in the numerous successive heats necessary
to complete the welding, and to form the bundle into a bar two-thirds of an inch
broad, by half an inch thick; the direction of the individual plates relatively to the
breadth being preserved. This bar folded flat upon itself, is again wrought at the
forge, till it is only half an inch broad, and a quarter of an inch thick, while the plates of
the primitive ribands are now set perpendicular to the breadth of the narrow fillet; the
length of which must be 15 or 16 feet French (16 or 17 English), to form a fowling
piece from 28 to 30 inches long. This fillet, heated to a cherry red in successive
portions, is coiled into as close a spiral as possible, upon a mandril about two-fifths
of an inch in diameter. The mandril has at one end a stout head for drawing it out,
by means of the hammer and the grooves of the anvil, previous to every heating. The
welding is performed upon a mandril introduced after each heat; the middle of the
barrel being first worked, while the fillets are forced back against each other, along the
surface of the mandril, to secure their perfect union. The original plates having in the
formation of the ultimate long riband become very thin, appear upon the surface of the
barrel like threads of a fine screw, with blackish tints to mark the junctions. In making
a double-barrelled gun, the two are formed from the same bundle of slips, the coils of
the one finished fillet being turned to the right hand, and those of the other to the left.
The Damascus barrels forged as above described, from a bundle of steel and iron
plates laid alternately together, are twisted at the forge several times, then coiled and
welded as usual. Fifteen Parisian workmen concur in one operation: six at the forge;
two at the boring mill; seven at filing, turning, and adjusting; yet all together make
only six pairs of barrels per week, which are sold at from 100 to 300 francs the pair,
ready for putting into the stock.
The breeching is of three kinds: the common; the chamber, plug, or mortar, fig. 397.;
and the patent, fig. 398. The common
was formerly used for soldiers’ musquets
and inferior pieces. The second is a
trifling improvement upon it. In the
patent breeching, the screws do not interfere
with the touch-hole, and the ignition
is quicker in the main chamber.
The only locks which it is worth while
to describe are those upon the percussion
principle, as flint locks will certainly soon
cease to be employed even in military
musquets. Forsyth’s lock (fig. 399.) was
an ingenious contrivance. It has a magazine
a, for containing the detonating powder,
which revolves round a roller b, whose
end is screwed into the breech of the
barrel. The priming powder passes
through a small hole in the roller, which
leads to a channel in communication
with the chamber of the gun.
The pan for holding the priming is placed immediately over the little hole in the roller.
There is a steel punch c, in the magazine, whose under end stands above the pan, ready[474]
to ignite the priming when struck upon the top by the cock d, whenever the trigger is
drawn. The punch immediately after being driven down into the pan is raised by the
action of a spiral spring. For each explosion, the magazine must be turned so far
round as to let fall a portion of the percussion powder into the pan; after which it is
turned back, and the steel punch recovers its proper position for striking another blow
into the pan.
The invention of the copper percussion cap was another great improvement upon the
detonating plan. Fig. 400. represents the ordinary percussion lock, which is happily
divested of three awkward projections upon the flint lock, namely, the hammer, hammer
spring, and the pan. Nothing now appears upon the plate of the lock, but the cock
or striking hammer, which inflicts the proper blow upon the percussion cap. It is
concave, with a small metallic ring or border, called a shield or fence, for the purpose
of enclosing the cap, as it were, and preventing its splinters doing injury to the sportsman,
as also protecting against the line of flame which may issue from the touch-hole in the
cap nipple. This is screwed into the patent breech, and is perforated with a small
hole.
The safety lock of Dr. Somerville is a truly humane invention. Its essential feature
is a slide stop or catch, placed under the trigger A, fig. 401. It is pulled forward into
a notch in the trigger, by means of a spring B, upon the front of the guard, which is
worked by a key C, pressing upon the spring when the piece is discharged. In another
safety plan there is a small movable curved piece of iron, A, which rises through an opening
B, in the lock-plate C, and prevents the cock from reaching the nipple, as represented in
the figure, until it is drawn back within the plate of the lock when the piece is fired.
To fire this gun, two different points must be pressed at the same time. If by
accident the key which works the safety be touched, nothing happens, because the trigger
is not drawn; and the trigger touched alone can produce no effect, because it is locked.
The pressure must be applied to the trigger and the key at the same instant, otherwise
the lock will not work.
The French musquet is longer than the British, in the proportion of 44·72 inches
to 42; but the French bayonet is 15 inches, whereas the British is 17.
[475]
| |
Eng.
Dimensions. |
Fr.
Dimensions. |
| Diameter of the bore |
0 |
·75 in. |
0 |
·69. in. |
| Diameter of the ball |
0 |
·676 |
0 |
·65 |
| Weight of the ball in oz. |
1 |
·06 |
0 |
·958 |
| Weight of the firelock and bayonet in libs. |
12 |
·25 |
10 |
·980 |
| Length of the barrel and bayonet |
59 |
·00 |
59 |
·72 |
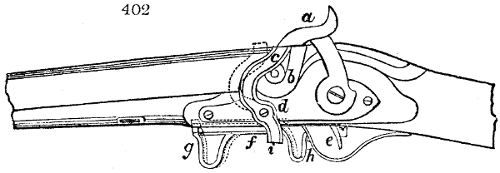
Within these few years a great many contrivances have been brought forward, and
several have been patented for fire arms. The first I shall notice is that of Charles
Random, Baron de Berenger. Fig. 402. shows the lock and breech of a fowling piece,
with a sliding protector on one of the improved plans; a is the hammer, b the nipple of
the touch-hole, c a bent lever, turning upon a pin, fixed into the lock-plate at d. The
upper end of this bent lever stands partly under the nose of the hammer, and while in
that situation stops it from striking the nipple. A slider g f h, connected with the under
part of the gun-stock, is attached to the tail of the bent lever at i; and when the piece
is brought to the shoulder for firing, the hand of the sportsman pressing against the bent
part of the slider at g, forces this back, and thereby moves the end of the lever c forwards
from under the nose of the cock or hammer, as shown by the dotted lines. The trigger
being now drawn, the piece will be discharged; and on removing the hand from the end
g, of the slider f, the spring at h acting against the guard, will force the slider forward,
and the lever into the position first described.
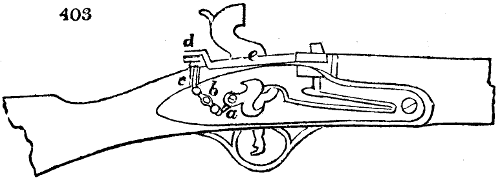
Mr. Redford, gun-maker of Birmingham, proposes a modification of the lock for
small fire-arms, in which the application of pressure to the sear spring for discharging
the piece is made by means of a plug, depressed by the thumb, instead of the force of
the finger exerted against the trigger. Fig. 403. represents a fowling piece partly in
section. The sear spring is shown at a. It is not here connected with the trigger as in
other locks; but is attached by a double-jointed piece to a lever b, which turns upon a
fulcrum pin in its centre. At the reverse end of this lever an arm extends forwards,
like that of an ordinary sear spring, upon which arm the lower end of the plug c is
intended to bear; and when this plug is depressed by the thumb bearing upon it, that
end of the lever b will be forced downwards, and the reverse end will be raised, so as to
draw up the end of the sear spring, and set off the piece. For the sake of protection,
the head of the plug c is covered by a movable cap d, forming part of a slider e, which
moves to and fro in a groove in the stock, behind the breech end of the barrel; this
slider e is acted upon by the trigger through levers, which might be attached to the other
side of the lock-plate; but are not shown in this figure to avoid confusion. When the
piece is brought to the shoulder for firing, the fore-finger must be applied as usual to
the trigger, but merely for the purpose of drawing back the slider e, and uncovering the
head of the plug; when this is done, the thumb is to be pressed upon the head of the
plug, and will thus discharge the piece. A spring bearing against the lever of the slider
e, will, when the finger is withdrawn from the trigger, send the slider forward again, and
cover the head of the plug, as shown.
It is with pleasure I again advert to the humane ingenuity of the Rev. John Somerville,
of Currie. In April, 1835, he obtained a patent for a further invention to prevent the
accidental discharge of fire arms. It consists in hindering the hammer from reaching the
nipple of a percussion lock, or the flint reaching the steel of an ordinary one, by the
interposition of movable safety studs or pins, which protrude from under the false
breech before the hammers of the locks, and prevent them from descending to strike.[476]
These safety studs or pins are moved out of the way by the pressure of the right hand of
the person using the gun only when in the act of firing, that is, when the force of the right
hand and arm is exerted to press the butt end of the stock of the gun against the shoulder
while the aim is taken and the trigger pulled. In carrying the gun at rest, the proper
parts of the thumb or hand do not come over Mr. Somerville’s movable buttons or
studs.
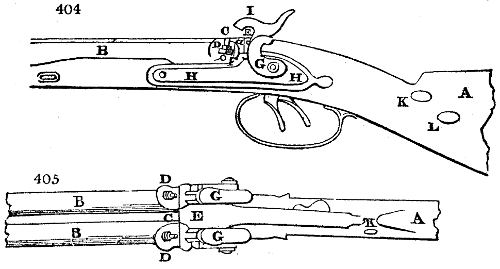
Fig. 404. is a side view of part of a double percussion gun; and fig. 405. is a top or
plan view, which will serve to explain these improvements, and show one, out of many,
methods of carrying them into effect. A is the stock of the gun; B the barrels; C the
breech; D the nipples; E the false breech, on the under side of which the levers which
work the safety studs or pins are placed; F is the shield of the false breech; G, triggers;
H the lock-plate; and I the hammers: all of which are constructed as usual: a a are
the safety studs or pins, which protrude before the shield F, and work through guide
pieces on the under side of the false breech. The button piece is placed in the
position for the thumb of the right hand to act upon it; but when the pressure of the
ball of the right thumb is to produce the movement of the safety studs, it must be
placed in or near the position K; and when the heel of the right hand is to effect the
movements of the safety studs, the button piece must be placed at L, or nearly so.
In these last two positions, the lever (which is acted upon by the button piece
to work the safety studs through a slide) would require to be of a different shape
and differently mounted. When the hammers are down upon the nipples after discharging
the gun, the ends of the safety pins press against the inner sides of the
hammers. When this invention is adapted to single-barrelled guns, only one pin, a, one
lever and button piece will be required.
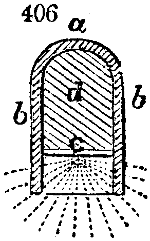
Mr. Richards, gun-maker, Birmingham, patented, in March, 1836, a modification of
the copper cap for holding the percussion powder, as represented fig. 406.; in which
the powder is removed from the top of the cap, and brought nearer the mouth; a being
the top, b the sides, and c the position of the priming. The dotted lines show the direction
of the explosion, whereby it is seen that the metal case is opened or distended only in
a small degree, and not likely to burst to pieces, as in the common caps, the space
between a and c being occupied by a piece of any kind of hard metal d, soldered or
otherwise fastened in the cap.
George Lovell, Esq., director of the Royal Manufactory of Arms at Enfield, has recently
made a great improvement upon the priming chamber. He forms it into a vertical
double cone, joined in the middle by the common apex; the base of the upper cone being
in contact with the percussion cap, presents the most extensive surface to the fulminate
upon the one hand, while the base of the under one being in a line with the interior
surface of the barrel, presents the largest surface to the gunpowder charge, upon the
other. In the old nipple the apex of the cone being at its top, afforded very injudiciously
the minimum surface to the exploding force.
Guns, Rifling of the Barrels.—The outside of rifle barrels is, in general, octagonal.
After the barrel is bored, and rendered truly cylindrical, it is fixed upon the rifling
machine. This instrument is formed upon a square plank of wood 7 feet long, to which
is fitted a tube about an inch in diameter, with spiral grooves deeply cut internally
through its whole length; and to this a circular plate is attached, about 5 inches
diameter, accurately divided in concentric circles, into from 5 to 16 equal parts, and
supported by two rings made fast to the plank, in which rings it revolves. An arm
connected with the dividing graduated plate, and pierced with holes, through which a[477]
pin is passed, regulates the change of the tube in giving the desired number of grooves
to the barrel. An iron rod, with a movable handle at the one end, and a steel cutter
in the other, passes through the above rifling tube. This rod is covered with a core of
lead one foot long. The barrel is firmly fixed by two rings on the plank, standing in
a straight line on the tube. The rod is now drawn repeatedly through the barrel, from
end to end, until the cutter has formed one groove of the proper depth. The pin is
then shifted to another hole in the dividing plate, and the operation of grooving is
repeated till the whole number of riflings is completed. The barrel is next taken out
of the machine, and finished. This is done by casting upon the end of a small iron
rod a core of lead, which, when besmeared with a mixture of fine emery and oil, is
drawn, for a considerable time, by the workmen, from the one end of the barrel to the
other, till the inner surface has become finely polished. The best degree of spirality is
found to be from a quarter to half a revolution in a length of three feet.
Military Rifles.—An essential improvement in this destructive arm has lately been
introduced into the British service, at the suggestion of Mr. Lovell:
The intention in all rifles is to impart to the ball a rotatory or spinning motion
round its axis, as it passes out through the barrel. This object was attained, to a
certain degree, in the rifles of the old pattern, by cutting seven spiral grooves into the
inside of the barrel, in the manner shewn by fig. 407., the spherical ball, fig. 408., being
a little larger than the bore, was driven down with a mallet, by which the projecting
ribs were forced into the surface of the ball, so as to keep it in contact with their
curvatures, during its expulsion. Instead of this laborious and insecure process, the
barrel being now cut with only two opposite grooves, fig. 409., and the ball being formed
with a projecting belt, or zone, round its equator, of the same form as the two grooves,
fig. 410., it enters so readily into these hollows, that little or no force is required to
press it down upon the powder. So much more hold of the barrel is at the same time
obtained, that instead of one quarter of a turn, which was the utmost that could be
safely given in the old way, without danger of stripping the ball, a whole turn round
the barrel, in its length, can be given to the two grooved rifles; whereby a far more
certain and complete rotatory motion is imparted to the ball. The grand practical
result is, that better practice has been performed by several companies of the Rifle
Corps, at 300 yards, than could be produced with the best old military rifles at 150
yards; the soldier being meanwhile enabled to load with much greater ease and
despatch. The belt is bevelled to its middle line, and not so flat as shown in the figure.
This mode of rifling is not, however, new in England. In fact, it is one of the
oldest upon record; and appears to have fallen into disuse from faults in the execution.
The idea was revived within the last few years in Brunswick, and it was tried in
Hanover also, but with a lens-shaped (Linsenförmig) ball. The judicious modifications
and improvements it has finally received in Mr. Lovell’s hands, have brought out all its[478]
advantages, and rendered it, when skilfully used, a weapon of unerring aim, even at the
prodigious distance of 700 yards.
Mr. Lovell’s Lock.
The locks, also, for the military service generally, are now receiving an important improvement
by means of his labours, having been simplified in a remarkable manner. The
action of the main spring is reversed, as shown by fig. 411.; thus rendering the whole
mechanism more solid, compact, and convenient; while the ignition of the charge
being effected by percussion powders in a copper cap, the fire of the British line will,
in future, be more murderous than ever, as a mis-fire is hardly ever experienced with
the fire-arms made at the Royal manufactory, under Mr. Lovell’s skilful superintendence.
FIRE-DAMP; the explosive carburetted hydrogen of coal mines. See Pitcoal.
FIRE-WORKS. (Feux d’artifice, Fr.; Feuerwerke, Germ.) The composition of
luminous devices with explosive combustibles, is a modern art resulting from the discovery
of gunpowder. The finest inventions of this kind are due to the celebrated
Ruggieri, father and son, who executed in Rome and Paris, and the principal capitals of
Europe, the most brilliant and beautiful fireworks that were ever seen. The following
description of their processes will probably prove interesting to many of my readers.
The three prime materials of this art are, nitre, sulphur, and charcoal, along with
filings of iron, steel, copper, zinc, and resin, camphor, lycopodium, &c. Gunpowder
is used either in grain, half crushed, or finely ground, for different purposes. The
longer the iron filings, the brighter red and white sparks they give; those being
preferred which are made with a very coarse file, and quite free from rust. Steel
filings and cast-iron borings contain carbon, and afford a more brilliant fire, with
wavy radiations. Copper filings give a greenish tint to flame; those of zinc, a
fine blue colour; the sulphuret of antimony gives a less greenish blue than zinc, but
with much smoke; amber affords a yellow fire, as well as colophony, and common salt;
but the last must be very dry. Lampblack produces a very red colour with gunpowder,
and a pink with nitre in excess. It serves for making golden showers. The yellow
sand or glistening mica, communicates to fire-works golden radiations. Verdigris
imparts a pale green; sulphate of copper and sal-ammoniac, a palm-tree green. Camphor
yields a very white flame and aromatic fumes, which mask the bad smell of other
substances. Benzoin and storax are used also on account of their agreeable odour.
Lycopodium burns with a rose colour and a magnificent flame; but it is principally
employed in theatres to represent lightning, or to charge the torch of a fury.
Fire-works are divided into three classes: 1. those to be set off upon the ground; 2.
those which are shot up into the air; and 3. those which act upon or under water.
Composition for jets of fire; the common preparation for rockets not more than 3⁄4 of an
inch in diameter, is: gunpowder, 16 parts; charcoal, 3 parts. For those of larger diameter:
gunpowder, 16; steel filings, 4.
Brilliant revolving wheel; for a tube less than 3⁄4 of an inch: gunpowder, 16; steel
filings, 3. When more than 3⁄4: gunpowder, 16; filings, 4.
Chinese or Jasmine fire; when less than 3⁄4 of an inch: gunpowder, 16; nitre, 8;
charcoal (fine), 3; sulphur, 3; pounded cast-iron borings (small), 10. When wider
than 3⁄4: gunpowder, 16; nitre, 12; charcoal, 3; sulphur, 3; coarse borings, 12.
A fixed brilliant; less than 3⁄4 in diameter: gunpowder, 16; steel filings, 4; or, gunpowder,
16; and finely pounded borings, 6.
Fixed suns are composed of a certain number of jets of fire distributed circularly, like
the spokes of a wheel. All the fusees take fire at once through channels charged with
quick matches. Glories are large suns with several rows of fusees. Fans are portions
of a sun, being sectors of a circle. The Patte d’oie is a fan with only three jets.
The mosaic represents a surface covered with diamond shaped compartments, formed
by two series of parallel lines crossing each other. This effect is produced by placing
at each point of intersection, four jets of fire, which run into the adjoining ones. The
intervals between the jets must be associated with the discharge of others, so as to keep
up a succession of fires in the spaces.
Palm trees. Ruggieri contrived a new kind of fire, adapted to represent all sorts of
trees, and especially the palm. The following is the composition of this magnificent
green fire-work: crystallized verdigris, 4 parts; sulphate of copper, 2; sal-ammoniac,
1. These ingredients are to be ground and moistened with alcohol. An artificial tree
of any kind being erected, coarse cotton rovings about 2 inches in diameter, impregnated
with that composition, are to be festooned round the trunk, branches, and among
the leaves; and immediately kindled before the spirits have had time to evaporate.
Cascades, imitate sheets or jets of water. The Chinese fire is best adapted to such
decorations.
Fixed stars. The bottom of a rocket is to be stuffed with clay, and one diameter in
height of the first preparation being introduced, the vacant space is to be filled with the
following composition, and the mouth tied up. The pasteboard must be pierced into
the preparation, with five holes, for the escape of the luminous rays, which represent
a star.
[479]
Composition of fixed stars:—
| |
Ordinary. |
Brighter. |
Coloured. |
| Nitre, |
16 |
12 |
0 |
| Sulphur, |
4 |
6 |
6 |
| Gunpowder meal, |
4 |
12 |
16 |
| Antimony, |
2 |
1 |
2 |
Lances, are long rockets of small diameter, made with cartridge paper. Those which
burn quickest should be the longest. They are charged by hand without any mould,
with rods of different lengths, and are not strangled at the mouth, but merely stuffed
with a quick match of tow. These lances form the figures of great decorations; they
are fixed with sprigs upon large wooden frame works, representing temples, palaces,
pagodas, &c. The whole are placed in communication by conduits, or small paper
cartridges like the lances, but somewhat conical, that they may fit endwise into one
another to any extent that may be desired. Each is furnished with a match thread
fully 11⁄2 inches long, at its two ends.
Composition for the white lances: nitre, 16; sulphur, 8; gunpowder, 4 or 3. For a
bluish-white: nitre, 16; sulphur, 8; antimony, 4. For blue lances: nitre, 16; antimony,
8. For yellow: nitre, 16; gunpowder, 16; sulphur, 8; amber, 8. For yellower
ones: nitre, 16; gunpowder, 16; sulphur, 4; colophony, 3; amber, 4. For
greenish ones: nitre, 16; sulphur, 6; antimony, 6; verdigris, 6. For pink lances;
nitre, 16; gunpowder, 3; lampblack, 1. Others less vivid are made with: nitre, 16;
colophony, 3; amber, 3; lycopodium, 3.
Cordage is represented in fire-works, by imbuing soft ropes with a mixture of, nitre,
2; sulphur, 16; antimony, 1; resin of juniper, 1.
The Bengal flames rival the light of day. They consist of, nitre, 7; sulphur, 2;
antimony, 1. This mixture is pressed strongly into earthen porringers, with some bits
of quick match strewed over the surface. These flames have a fine theatrical effect for
conflagrations.
Revolving suns, are wheels upon whose circumference rockets of different styles are
fixed, and which communicate by conduits, so that one is lighted up in succession after
another. The composition of their common fire is, for sizes below 3⁄4 of an inch: gunpowder
meal, 16; charcoal, not too fine, 3. For larger sizes: gunpowder, 20; charcoal,
not too fine, 4. For fiery radiations: gunpowder, 16; yellow micaceous sand, 2 or 3.
For mixed radiations: gunpowder, 16; pitcoal, 1; yellow sand, 1 or 2.
The waving or double Catherine wheels, are two suns turning about the same axis in
opposite directions. The fusees are fixed obliquely and not tangentially to their peripheries.
The wheel spokes are charged with a great number of fusees; two of the four
wings revolve in the one direction, and the other two in the opposite; but always in a
vertical plane.
The girandoles, caprices, spirals, and some others have on the contrary a horizontal
rotation. The fire-worker may diversify their effects greatly by the arrangement and
colour of the jets of flame. Let us take for an example the globe of light. Imagine a
large sphere turning freely upon its axis, along with a hollow hemisphere, which revolves
also upon a vertical axis passing through its under pole. If the two pieces be covered
with coloured lances or cordage, a fixed luminous globe will be formed, but if horizontal
fusees be added upon the hemisphere, and vertical fusees upon the sphere, the
first will have a relative horizontal movement, the second a vertical movement, which
being combined with the first, will cause it to describe a species of curve, whose effect
will be an agreeable contrast with the regular movement of the hemisphere. Upon the
surface of a revolving sun, smaller suns might be placed, to revolve like satellites round
their primaries.
Ruggieri exhibited a luminous serpent pursuing with a rapid winding pace, a butterfly
which flew continually before it. This extraordinary effect was produced in the
following way. Upon the summits of an octagon he fixed eight equal wheels turning
freely upon their axles, in the vertical plane of the octagon. An endless chain passed round
their circumference, going from the interior to the exterior, covering the outside semi-circumference
of the first, the inside of the second, and so in succession; whence arose
the appearance of a great festooned circular line. The chain, like that of a watch,
carried upon a portion of its length a sort of scales pierced with holes for receiving
coloured lances, in order to represent a fiery serpent. At a little distance there was a
butterfly constructed with white lances. The piece was kindled commonly by other
fireworks, which seemed to end their play, by projecting the serpent from the bosom of
the flames. The motion was communicated to the chain by one of the wheels, which
received it like a clock from the action of a weight. This remarkably curious mechanism
was called by the artists a salamander.
The rockets which rise into the air with a prodigious velocity, are among the most[480]
common, but not least interesting fire-works. When employed profusely they form those
rich volleys of fire which are the crowning ornaments of a public fête. The cartridge
is similar to that of the other jets, except in regard to its length, and the necessity of
pasting it strongly, and planing it well; but it is charged in a different manner. As
the sky-rockets must fly off with rapidity, their composition should be such as to kindle
instantly throughout their length, and extricate a vast volume of elastic fluids. To effect
this purpose, a small cylindric space is left vacant round the axis; that is, the central
line is tubular. The fire-workers call this space the soul of the rocket (ame de la fusée).
On account of its somewhat conical form, hollow rods, adjustable to different sizes of
broaches or skewers, are required in packing the charge; which must be done while the
cartridge is sustained by its outside mould, or copper cylinder. The composition of sky-rockets
is as follows:—
| When the bore is |
3⁄4 of
an inch; |
3⁄4 to 11⁄4; |
12⁄3; |
| Nitre |
16 |
|
16 |
|
16 |
| Charcoal |
7 |
|
8 |
|
9 |
| Sulphur |
4 |
|
4 |
|
4 |
| Brilliant Fire. |
|
|
|
| Nitre |
16 |
|
16 |
|
16 |
| Charcoal |
6 |
|
7 |
|
8 |
| Sulphur |
4 |
|
4 |
|
4 |
| Fine steel filings |
3 |
|
4 |
|
5 |
| Chinese Fire. |
|
|
|
| Nitre |
16 |
|
16 |
|
16 |
| Charcoal |
4 |
|
5 |
|
6 |
| Sulphur |
3 |
|
3 |
|
4 |
| Fine borings of cast iron |
3 |
coarser |
4 |
mixed |
5 |
The cartridge being charged as above described, the pot must be adjusted to it, with
the garniture; that is, the serpents, the crackers, the stars, the showers of fire, &c. The
pot is a tube of pasteboard wider than the body of the rocket, and about one third of its
length. After being strangled at the bottom like the mouth of a phial, it is attached
to the end of the fusee by means of twine and paste. These are afterwards covered with
paper. The garniture is introduced by the neck, and a paper plug is laid over it. The
whole is inclosed within a tube of pasteboard terminating in a cone, which is firmly
pasted to the pot. The quick-match is now finally inserted into the soul of the rocket.
The rod attached to the end of the sky-rockets to direct their flight, is made of willow
or any other light wood. M. Ruggieri replaced the rod by conical wings containing
explosive materials, and thereby made them fly further and straighter.
The garnitures of the sky-rocket pots are the following:—
1. Stars are small, round, or cubic solids, made with one of the following compositions,
and soaked in spirits. White stars, nitre, 16; sulphur, 8; gunpowder, 3. Others
more vivid consist of nitre, 16; sulphur, 7; gunpowder, 4.
Stars for golden showers, nitre, 16; sulphur, 10; charcoal, 4; gunpowder, 16; lamp-black,
2. Others yellower are made with nitre, 16; sulphur, 8; charcoal, 2; lamp-black,
2; gunpowder, 8.
The serpents are small fusees made with one or two playing cards; their bore being
less than half an inch. The lardons are a little larger, and have three cards; the vetilles
are smaller. Their composition is, nitre, 16; charcoal, not too fine, 2; gunpowder, 4;
sulphur, 4; fine steel filings, 6.
The petards are cartridges filled with gunpowder and strangled.
The saxons are cartridges clayed at each end, charged with the brilliant turning fire,
and perforated with one or two holes at the extremity of the same diameter.
The cracker is a round or square box of pasteboard, filled with granulated gunpowder,
and hooped all round with twine.
Roman candles are fusees which throw out very bright stars in succession. With the
composition (as under) imbued with spirits and gum-water, small cylindric masses are
made, pierced with a hole in their centre. These bodies, when kindled and projected
into the air, form the stars. There is first put into the cartridge a charge of fine gunpowder
of the size of the star; above this charge a star is placed; then a charge of
composition for the Roman candles.
The stars, when less than 3⁄4 of an inch, consist of nitre, 16; sulphur, 7; gunpowder, 5.
When larger, of nitre, 16; sulphur, 8; gunpowder, 8.
Roman candles, nitre, 16; charcoal, 6; sulphur, 3. When above 3⁄4 of an inch
nitre, 16; charcoal, 8; sulphur, 6.
The girandes, or bouquets, are those beautiful pieces which usually conclude a fire-work[481]
exhibition; when a multitude of jets seem to emblazon the sky in every direction,
and then fall in golden showers. This effect is produced by distributing a number of
cases open at top, each containing 140 sky-rockets, communicating with one another by
quick-match strings planted among them. The several cases communicate with each
other by conduits, whereby they take fire simultaneously, and produce a volcanic display.
The water fire-works are prepared like the rest; but they must be floated either by
wooden bowls, or by discs and hollow cartridges fitted to them.
Blue fire for lances may be made with nitre, 16; antimony, 8; very fine zinc filings, 4.
Chinese paste for the stars of Roman candles, bombs, &c.:—Sulphur, 16; nitre, 4;
gunpowder meal, 12; camphor, 1; linseed oil, 1; the mixture being moistened with
spirits.
The feu grégois of Ruggieri, the son:—Nitre, 4; sulphur, 2; naphtha, 1. See
Pyrotechny and Rockets.
The red fire composition is made by mixing 40 parts of nitrate of strontia, 13 of
flowers of sulphur, 5 of chlorate of potash, and 4 of sulphuret of antimony.
White fire is produced by igniting a mixture of 48 parts nitre; 131⁄4 sulphur; 71⁄4 sulphuret
of antimony; or, 24 nitre, 7 sulphur, 2 realgar; or, 75 nitre, 24 sulphur,
1 charcoal; or, finally, 100 of gunpowder meal, and 25 of cast-iron fine borings.
The blue fire composition is, 4 parts of gunpowder meal; 2 of nitre; sulphur and
zinc, each 3 parts.
FISH-HOOKS (Hameçons, Fr.; Fischangeln, Germ.); are constructed with simple
tools, but require great manual dexterity in the workmen. The iron wire of which
they are made should be of the best quality, smooth, and sound. A bundle of such
wire is cut in lengths, either by shears or by laying it down upon an angular wedge of
hard steel fixed horizontally in a block or anvil, and striking off the proper lengths by
the blows of a hammer. In fashioning the barbs of the hooks, the straight piece of wire
is laid down in the groove of an iron block made on purpose, and is dexterously struck
by the chisel in a slanting direction, across so much of the wire as may be deemed
necessary. A sharp-pointed little wedge is thus formed, whose base graduates into the
substance of the metal.
The end of the wire where the line is to be attached is now flattened or screw-tapped;
the other end is sharp pointed, and the proper twisted curvature is given. The soft iron
hooks are next case-hardened, to give them the steely stiffness and elasticity, by imbedding
them in animal charcoal contained in an earthen or iron box; see Case-Hardening;
after which they are brightened by heating and agitating them with bran, and
finally tempered by exposure to a regulated temperature upon a hot iron plate. Hooks
for salt-water fishing are frequently tinned, to prevent them wearing rapidly away in
rust. See Tin Plate.
FLAKE WHITE; is the name sometimes given to pure white-lead.
FLAME (Flamme, Fr. and Germ.); is the combustion of an explosive mixture of an
inflammable gas or vapour with air. That it is not, as many suppose, combustion merely
at the exterior surface, is proved by plunging a fragment of burning phosphorus or sulphur
into the centre of a large flame of alcohol. Either of these bodies will continue to burn
there with its peculiar light; thus proving that oxygen is mixed with the whole of the
burning vapour. If we mix good coal gas with as much atmospheric air as can convert
all its carbon into carbonic acid, the mixture will explode with a feeble blue light; but
if we mix the same gas with a small quantity of air, it will burn with a rich white
flame. In the latter case, the carbonaceous particles are precipitated, as Sir H. Davy
first showed, in the interior of the flame, become incandescent, and constitute white
light: for from the ignition of solid matter alone can the prismatic rays be emitted in that
concentrated union. Towards the interior of the flame of a candle, a lamp, or a gas jet,
where the air is scanty, there is a deposition of solid charcoal, which first by its ignition,
and afterwards by its combustion, increases in a high degree the intensity of the light.
If we hold a piece of fine wire gauze over a jet of coal gas close to the orifice, and if we
then kindle the gas, it will burn above the wire with its natural brilliancy; but if we
elevate the gauze progressively higher, so as to mix more and more air with it before it
reaches the burning point, its flame will become fainter and less white. At a certain
distance it becomes blue, like that of the above explosive mixture. Since the combustion
of all the constituents is in this case direct and complete, the heat becomes greatest
in proportion nearly as the light is diminished. If a few platina wires be held in that
dim flame they will grow instantly white hot, and illuminate the apartment. On
reversing the order of this experiment, by lowering progressively a flat piece of wire
gauze from the summit towards the base of a gas flame, we shall find no charcoal
deposited at its top, because plenty of air has been introduced there to convert all the
carbon of the gas into carbonic acid, and therefore the apex is blue; but as we descend,
more and more charcoal will appear upon the meshes. At the very bottom, indeed,[482]
where the atmospheric air impinges upon the gauze, the flame is again blue, and no
charcoal can therefore be deposited.
The fact of the increase of the brilliancy and whiteness of flame by the development
and ignition of solid matter in its bosom, illustrates many curious phenomena. We
can thus explain why olefiant gas affords the most vivid illumination of all the gases;
because, being surcharged with charcoal, its hydrogen lets it go in the middle of the
flame, as it does in an ignited porcelain tube, whereby its solid particles first get ignited
to whiteness, and then burn away. When phosphorus is inflamed, it always yields a
pure white light, from the ignition of the solid particles of the snowy acid thus produced.
In the blowpipe, the inner blue flame has the greatest heat, because there the combustion
of the whole fatty vapour is complete. The feeble light of burning hydrogen,
carbonic oxide, and sulphur, may, upon the principles now expounded, be increased by
simply placing in them a few particles of oxide of zinc, slender filaments of amianthus,
or fine platina wire. Upwards of twenty years ago, I demonstrated in my public
lectures in Glasgow, that by narrowing the top of a long glass chimney over an argand
flame either from oil or coal gas, the light could be doubled, at the same cost of
material. The very tall chimneys used by the Parisian lampists are very wasteful. I
find that with a narrow chimney of half the length of theirs, I can have as good a light, and
save 30 per cent. of the oil. Thus the light of a flame may be increased by diminishing
its heat, or the intensity of its combustion; and conversely the heat of a flame may be
increased by diminishing its light.
FLANNEL; a plain woollen stuff of a rather open and slight fabric.
FLAX. By this term we understand the bast or inner bark of the Linum usitatissimum,
which is spun into yarn for weaving linen webs. This plant blossoms in June
or July, and commonly ripens its seeds in September. As varieties, we distinguish the
spring flax, with short knotty stems, whose seed capsules at the period of maturity,
spring open with a perceptible sound; and the close flax, with longer smoother stems,
whose capsules give out their seeds only when threshed. The Germans, who have
bestowed much attention upon the culture of flax, call the former Klanglein or Springlein,
and the latter Dreschlein. This is the kind most commonly grown, but from the
difference of climate, soil, and culture, it affords flax of very different qualities. The
best ground for this plant is an open, somewhat friable clay, mingled with sand and
mould. The early flax is usually sown in the end of April or beginning of May, the
late, in June. The seeds ought to be sown thick, whereby the stalks are forced to grow
more slender, and the fibres of the bast or harl are not only smoother and finer, but more
uniform in length. If the raising of seed be the principal object, the flax must be more
thinly sown, whereby it will produce stronger stalks, but more knotty, with shorter
fibres, and more productive of tow.
Whenever the flax is ripe, which is shown by the bottom of the stalk becoming yellow,
and the leaves beginning to drop off, it must be immediately reaped by pulling it up by the
roots. The seeds are still immature, fit merely for the oil press, and not for sowing.
When the seed crop is the object, the plant must be suffered to acquire its full maturity;
in which case the fibres are less fine and soft.
The flax is carried off the field in bundles to be rippled, or stripped of its seeds, which
is done by drawing it by handfuls, through an iron comb with teeth eight inches long,
fixed upright in a horizontal beam. When the seeds are more fully ripened, they may
be separated by the threshing mill.
The operations next performed upon the flax, will be understood by attending to the
structure of the stem. In it, two principal parts are to be distinguished; the woody
heart or boon, and the harl (covered outwardly with a fine cuticle), which encloses the
former like a tube, consisting of parallel lines. In the natural state, the fibres of the
harl are attached firmly not only to the boon, but to each other by means of a green or
yellowish substance. The rough stems of the flax after being stripped of their seeds,
lose in moisture by drying in warm air, from 55 to 65 per cent. of their weight; but
somewhat less when they are quite ripe and woody. In this dry state, they consist in
100 parts of from 20 to 23 per cent. of harl, and from 80 to 77 per cent. of boon. The
latter is composed upon the average of 69 per cent. of a peculiar woody substance, 12 per
cent. of a matter soluble in water, and 19 per cent. of a body not soluble in water, but
in alkaline lyes. The harl contains at a mean 58 per cent. of pure flaxen fibres, 25 parts
soluble in water (apparently extractive and albumen), and 17 parts insoluble in water,
being chiefly gluten. By treating the harl with either cold or hot water, the latter
substance is dyed brown by the soluble matter, while the fibres retain their coherence to
one another. Alkaline lyes, and also, though less readily, soap water, dissolve the
gluten, which seems to be the cement of the textile fibres, and thus set them free.
The cohesion of the fibres in the rough harl is so considerable that by mechanical
means, as by beating, rubbing, &c., a complete separation of them cannot be effected,
unless with great loss of time, and rupture of the filaments. This circumstance[483]
shows the necessity of having recourse to some chemical method of decomposing the
gluten. The process employed with this view is a species of fermentation, to which the
flax stalks are exposed; it is called retting, a corruption of rotting, since a certain degree
of putrefaction takes place. The German term is rusting. This is the first important
step in the preparation of flax. After the retting is completed, the boon of the stalks
must be removed by the second operation called breaking, and other subordinate
processes. The harl freed from the woody parts contains still a multitude of fibres, more
or less coherent, or entangled, and of variable lengths, so as to be ill adapted for spinning.
These are removed by the heckle, which separates the connected fibres into their finest
filaments, removes those that are too short, and disentangles the longer ones.
I. Of retting.—The fermentation of this process may be either rendered rapid by
steeping the flax in water, or slow by using merely the ordinary influence of the atmospheric
damp, dews, and rain. Hence the distinction of water-retting and dew-retting.
Both may also be combined.
Prior to being retted, the flax should be sorted according to the length and thickness
of its stalks, and its state of maturity; the riper the plant, the longer must the retting
last. The due length of the process is a point too little studied.
Water-retting.—When flax stalks are macerated in water, at a temperature not too
low, fermentation soon begins, evinced in the dingy infusion, by disengagement of
carbonic acid gas, and the production of vinegar. If the flax be taken out at the end of
a few days, dried, and rubbed, the textile filaments are found to be easily separable from
each other. By longer continuance of the steep, the water ceases to be acid, it becomes
to a certain degree alkaline, from the production of ammonia, diffuses a fetid odour,
from the disengagement of sulphuretted hydrogen gas, along with the carbonic acid; the
acetous fermentation being in fact now changed into the putrid. The filaments become
yellowish brown, afterwards dark brown and lose much of their tenacity, if the process
be carried further.
When the operation is conducted with discernment, the water-retting may be completed
by the acetous fermentation alone, as the putrefaction should never be suffered to
proceed to any length; because when over-retted, flax is partially rotten, gets a bad
colour, and yields a large proportion of tow.
For water-retting, the flax must be bound up in sheaves, placed in layers over each
other in the water, or sometimes upright, with the roots undermost. Straw may be put
below to keep it from touching the ground, and boards may be laid upon the top, with
weights to hold it immersed about a foot beneath the surface, especially when the fermentative
gases make it buoyant. As soon as it sinks at the end of the fermentation, it
must be inspected at least twice a day, and samples must be taken out to see that no
over-retting ensues. A single day too long often injures the flax not a little. We may
judge that the retting is sufficient when the harl separates easily from the boon by the
fingers, when the boon breaks across without bending, and when several stalks knotted
together sink to the bottom upon being thrown into the water. For this completion, a
shorter or longer time is required according to the quality of the flax, the temperature,
&c., so that the term may vary from five to fourteen days. It may be done either in
running or in stagnant water. For the latter purpose, tanks five feet deep are dug in the
ground. In stagnant water, the process is sooner finished, but it is more hazardous, and
gives a deeper stain to the fibres, than in a stream, which carries off much of the colour.
The best place for steeping flax is a pond with springs of water at its bottom; or a tank
into which a rivulet of water can be occasionally admitted, while the foul water is let off.
For every fresh quantity of flax, the pond should be emptied, and supplied with clear
water. Water impregnated with iron, stains flax a permanent colour, and should
therefore never be used. After retting, the flax should be taken out without delay,
rinsed in clean water, and exposed in an airy situation to dry by the sun.
Rough rippled flax stalks, well seasoned before being retted, and dried afterwards,
show a loss of weight, amounting to 20 or 30 per cent., affecting both the boon and the
harl. This loss is greater the finer the stems, and the longer the retting. The harl
contains, beside the textile filaments, a certain portion of a glutinous cement; but
nothing soluble in water. The destruction of the gluten cannot be pushed to the last
point by steeping, without doing an essential injury to the filaments.
Dew-retting.—The fetid and noxious exhalations which the water-retting diffuses
over an extensive district of country, and the danger of over-retting in that way,
especially with stagnant water, are far from recommending that process to general
adoption. Dew-retting accomplishes the same purpose, by the agency of the air, dews,
and rain, in a much more convenient, though far slower manner. The flax, with this
view, should be spread out thin upon meadow or grass lands, but never upon the bare
ground, and turned over, from time to time, till the stems, on being rubbed between
the fingers, show that the harl and the boon are ready to part. The duration of dew-retting
is, of course, very various, from 2 to 6, or 8 weeks, as it depends upon the state[484]
of the weather; a moist air being favourable, and dry sunshine the reverse. The loss of
weight by dew-retting is somewhat less than by water-retting; and the textile fibres are
of a brighter colour, softer and more delicate to the touch.
Mixed retting.—This may be fairly regarded as the preferable plan, the retting being
begun in the water, and finished in the air. The flax should be taken out of the steep
whenever the acetous fermentation is complete, before the putrid begins, and exposed,
for 2 or 3 weeks, on the grass.
II. The breaking is performed by an instrument called a brake. In order to give the
wood or boon such a degree of brittleness as to make it part readily from the harl,
whereby the execution of this process is rendered easy, the flax should be well dried in
the sun, or what is more suitable to the late period of the year, in a stove. Such is
often attached to the bakers’ ovens in Germany, and other flax-growing countries.
The drying temperature should never exceed 120° F., for a higher heat makes it brittle,
easy to tear, and apt to run into tow. Before subjecting the flax to the brake, the
stems should be equalized and laid parallel by the hand, and the entangled portions
should be straightened with a coarse heckle. The brake has one general construction,
and consists of two principal parts, the frame or case, and the sword or beater. In the
simplest brakes, the frame e, fig. 412., is a piece of wood cleft lengthwise in the middle,
supported by the legs a and c. The sword f, also of hard wood, is formed with an edge
beneath, and turns round the centre of motion at q, when seized by the handle h, and
moved up and down. As it descends, the sword enters the cleft of the frame, and breaks
the flax stalks laid transversely upon it, scattering the boon in fragments.
But those hand brakes are more convenient which are provided with a double cleft,
or triple row of oblong teeth; with a double sword. This construction will be understood
by inspecting figs. 412, 413, 414. Fig. 412. is the section of that side at which
the operative sits; fig. 413. is a section in the line A, B, of fig. 412; and fig. 414., the
ground plan. The whole machine is made of hard wood, commonly red beech. Two
planks, a and c, form the legs of the implement. a is mortised in a heavy block, to give
the brake a solid bearing; two stretchers d, bind a and c, firmly together. The frame e
consists of three thin boards, which are placed edgewise, and have their ends secured in
a and c. The sword f is a piece of wood, so chamfered from i to k, that it appears
forklike, and embraces the middle piece of the frame; its centre of motion is the wooden
pin q; in front is the handle h, which the operative seizes with the right hand. Both
the lathes of the frame, and those of the sword are sharpened, from l to the front end, as
is best shown in fig. 413.; but the edges must not be too sharp, for fear of injuring the
flax; and, for the same reason, the sword should not sink too far between the lathes of
the frame. Such hand-brakes are laborious in use, and often tear the harl into tow.
The operative, usually a female, in working the brake, seizes with her left hand a bundle
of flax, lays it transversely across the frame, and strikes it smartly with repeated blows
of the sword, pushing forwards continually new portions of the flax into the machine.
She begins with the roots, turns next round the tips, then goes on through the length
of the stalks. Flax is frequently exposed twice to the brake, with a stove drying
between the two applications.
The brake machines afford a far preferable means of cleaning flax than the above
hand tools. The essential part of such a machine, consists in several deeply fluted rollers
of wood or iron, whose teeth work into each other, and while they stretch out the
flaxen stalks betwixt them, they break the wood or boon, without doing that violence
to the harl which hand mechanisms are apt to do. The following may be regarded as[485]
one of the best constructions hitherto contrived for breaking flax. Fig. 415. is a view
of the right side of this machine. Fig. 416., the view from behind, where the broken
flax issues from between the rollers. The frame is formed by the two side pillars or
walls a, a, which are mortised into the bottom b, b; and are firmly fixed to it by
braces. Two transverse rods d, d, secure the base, two others d′ d′′, the sides. In
each of these a lateral arm e, is mortised in an oblique direction; a cross bar f,
unites both arms. Fig. 417. shows the
inside of the left side of the frame,
with the subsidiary parts. The three
rollers g, i, k, may be made of red
beech, with iron gudgeons, and fluted
in their length, each of the flutes being
5⁄12 of an inch broad, and 4⁄12ths deep.
The large roller g, bears upon the
right side, a handle h, which on being
turned, sets the whole train in motion.
The side partitions a, a, are furnished
with brasses in whose round holes l, g,
fig. 417., the gudgeons g work. For
the extremities of the two smaller
rollers, there are at a and e, slots in
brasses, as may be seen in fig. 415.
Within the partition a, there are movable
brasses l, for the pivots of i and
k, shewn in fig. 417. Each brass slides
in a groove, between two ledges. A
strong cord made fast at m to the partition
a, runs over the brass of i, next
over that of k, then descends perpendicularly,
and passes over the cross bar
n, fig. 415. and 416. This construction
being repeated at both ends of the
rollers, the rod n, binds both cords.
Against the cross bar d′ of the frame, a lever o is sustained, which lies upon the rod
n, and carries a weight p. The farther or nearer this weight hangs towards the end of
the lever, it stretches the cord more or less, and presses by means of the brasses l, the
rollers i, k, towards the main roller g. A table q, serves for spreading out the flax to
be broken, and a second one r, for the reception of the stalks at their issuing from
between the rollers. Both tables hang by means of iron hooks to rings of the frame s,
t, fig. 415. and 417., and are supported by the movable legs u, u, u, fig. 415. and 416.[486]
In using the machine the operative lays an evenly spread handful of flax upon the table
q, introduces their root ends with his left hand between the rollers g and i, and turns
round the handle h, with the right. The stems are first broken betwixt g and i, then
between g and k, and come out upon the table r. The handle is moved alternately
forwards and backwards, in order that the flax may be rolled alternately in the same
directions, and be more perfectly broken. The boon falls down in very small pieces,
and the harl remains expanded in parallel bands. This should be drawn over the
points of a heckle, then laid for a couple of days in a cellar to absorb some moisture,
and afterwards worked once more through the machine, whereby the flax acquires a
peculiar softness.
The advantages of this brake machine are chiefly the following:—
It takes up little room, and from its simplicity is easily and cheaply constructed; it
requires no more power to work, than the ordinary hand-brake; it tears none of the
filaments, and grinds nothing except the boon, in consequence of the flutings of the
rollers going much less deep into each other, than the sword of the hand-brake; it prevents
all entanglements of the flax, whence in the subsequent heckling the quantity of
short fibres or tow is diminished; and it accomplishes the cleaning of even the shortest
flax, which cannot be well done by hand machines.
The comminution of the boon of the stems, which is the object of the breaking
process, can however be performed by threshing or beating, although in this way the
separation of the woody matter from the textile fibres is much less completely effected.
It is the practice in Great Britain, instead of
breaking, to employ a water-driven wooden
mallet, between which and a smooth stone the
flax is laid. In that part of Belgium where
the preparation of flax has been studied, the
brake is not used, but beating by means of the
Bott-hammer, to the great improvement, it is
said, of the flax. The Bott-hammer, fig. 418., is
a wooden block, having on its under face, channels
or flutings, 5 or 6 lines deep, and it is fixed
to a long bent helve or handle. In using it, a
bundle of the dried flax stalks is spread evenly
upon the floor, then powerfully beaten with the
hammer, first at the roots, next at the points,
and lastly in the middle. When the upper
surface has been well beat in this way, it is
turned over, that the under surface may get its
turn. The flax is then removed, and well shaken
to free it from the boon.
By the brake or the hammer the whole wood is never separated from the textile
fibres, but a certain quantity of chaffy stuff adheres to them, which is removed by another
operation. This consists either in rubbing or shaking. The rubbing is much practised
in Westphalia, and the neighbouring districts. In this process, the operative lays the
rubbing apron on a piece of dressed leather, one foot square, upon her knee; then seizes
a bundle of flax in the middle with her left hand, and scrapes it strongly with the Ribbe-knife
held in her right, fig. 419. This tool, which consists of a wooden handle s, and a
thin iron blade r, with a blunt and somewhat bent edge, acts admirably in cleaning and
also in parting the filaments, without causing needless waste in flax previously well
broken.
The winnowing, which has the very same object as the rubbing, is, however, much
more generally adopted than the latter. Two distinct pieces of apparatus belong to it,
namely, the swing-stock and the swing-knife. The first consists of an upright board with
a groove in its side, into which a handful of flax is so placed that it hangs down over
half the surface of the board. While the left hand holds the flax fast above, the right
carries the swing-knife, a sabre-shaped piece of wood from 11⁄2 to 2 feet long, planed to
an edge on the convex side, and provided with a handle. With this knife the flax is
struck parallel to the board, with perpendicular blows, so as to scrape off its woody
asperities. The breadth of the swing-knife is an important circumstance; when too
narrow it easily causes the flax to twist round it, and thereby tears away a portion of
the fibres. When 8 or 10 inches broad, it is found to act best. Knives made of iron
will not answer, for they injure the filaments.
Figs. 420, 421. show the best construction of the swing-stock. The board a has
for its base a heavy block of wood b, upon which two upright pins e e, are fixed. The
band f, which is stretched between the pins, serves to guide the swing-knife in its
movements, and prevent the operative from wounding his feet. The under edge of the
groove c, upon which the flax comes to be laid, is cut obliquely and rounded off (see d[487]
in fig. 420.); thus we perceive that the swing-knife can never strike against that edge,
so as to injure the flax.
Fig. 422. exhibits the form of a very convenient implement which is employed in
Belgium instead of the swing-knife. It is a sort of
wooden hatchet, which is not above two lines thick,
and at the edge g h is reduced to the thickness of the
back of a knife. The fly k gives force to the blow,
and preserves the tool in an upright position. The
short flat-pressed helve i is glued to that side of the
leaf which in working is turned from the swing-stock;
and is, moreover, fastened with a wooden pin.
The rubbing and swinging throw off the coarsest
sort of tow, by separating and shaking out the shortest
fibres and those that happen to get torn. That
tow is used for the inferior qualities of sacking, being
mixed with many woody fibres.
We may in general estimate that 100 pounds of
the stalks of retted flax, taken in the dry state, afford
from 45 to 48 pounds of broken flax, of which, in the
swinging or scutching, about 24 pounds of flax, with 9 or 10 pounds of scutch tow are
obtained. The rest is boon-waste. The breaking of 100 pounds of stalks requires, in the
ordinary routine of a double process by hand, about 20 hours; and with the above described
machine, from 17 to 18 hours. To scutch 100 pounds of broken flax clean, 130
hours of labour are required by the German swinging method.
Mr. Bundy obtained a patent in 1819, for certain machinery for breaking and preparing
flax, which merits description here. Fig. 423. A A A A, is the frame made either of
wood or metal, which supports the two conical rollers B and C. These revolve independently
of each other in proper brass bearings. A third conical roller D is similarly
supported under the top piece E of the machine. All these rollers are frusta of cones,
made of cast iron. Whatever form of tooth be adopted, they must be so shaped and
disposed with regard to each other as to have considerable play between them, in order
to admit the quantity of flax stem which is intended to be broken and prepared. The
upper piece E of the machine which carries the upper conical roller D, is fixed or attached
to the main frame A A A A by strong hinges or any other moveable joint at G, and
rods of iron or other sufficiently strong material; H H is attached at its upper end by a
joint to the top piece E, through a hole near I, and is fixed at its lower end by another
joint K to the treadle or lever K L, which turns upon the joint or hinges M. A spring or
weight (but the former is preferable for many reasons) is applied to the machine in such
manner, that its action will always keep the upper piece E, and consequently the upper
roller D, in an elevated or raised position above the rollers B and C, when the machine is
not in action; and of course the end L of the treadle will also be raised, which admits of
the flax to be worked being introduced between the rollers, viz. over the two lower
rollers B, C, and under the upper roller D; such a spring may be applied in a variety of
ways, as between the top piece E, and the top or platform of the machine at N; or it[488]
may be a strong spiral wire spring, having its upper end fastened to the platform while
its lower extremity is fixed to the rod H H, round which it coils as shown at O, or it may
be placed under the end L of the treadle; but in every case its strength must be no
more than will be just sufficient to raise the upper roller D about two inches from the
lower rollers, otherwise it will occasion unnecessary fatigue to the person working the
machine.
The manner of using it is as follows: the upper and lower rollers being separated as
aforesaid, a small handful of dried flax or hemp stems is to be introduced between them,
and held extended by the two hands, while the rollers are brought together by the pressure
of the foot upon the treadle L. This pressure being continued, the flax or hemp is
to be drawn backwards and forwards by the hands between the rollers, in a direction at
right angles to their axes, and eventually withdrawn by pulling with one hand only.
The foot is now to be removed until the flax or hemp is again replaced, and each end is
this way to be drawn several times through the machine, until such ends are respectively
finished.
By a succession of these operations, using the pressure of the foot upon L, each time
that the flax or hemp is introduced between the rollers, and regulating such pressure according
to the progress of the work, the flax or hemp will soon be sufficiently worked,
and the fibre brought into a clean and divided state fit for bleaching; or if it be required
to spin it in the yellow state, it may be made sufficiently fine by a longer continuation
of the same process, particularly if worked between the smaller ends of the
rollers.
Indeed, the operation may be commenced and continued for some time, with the
larger part of the rollers, and finished with their smaller ends; and, in this point of
view, the invention of conical rollers will be found both convenient and useful; for as
the flutes, grooves, or teeth, vary in their distance from each other at all points between
the large and small ends, so it becomes almost impossible for the workman to draw the
flax or hemp through such rollers in the same track; and thus the breaking of the boon
must be much more irregular, and the fibre will be much more effectually cleansed than
it can be by the flutes, grooves, or teeth of cylinders, or other such contrivances formerly
employed; because they would probably fall frequently upon the same points of the
fibres. If it is intended that the flax shall be bleached before it is spun, then the second
part of Mr. Bundy’s invention may be had recourse to, which consists in moving certain
trays or cradles in the water, or other fluid used for bleaching the flax or hemp, in the
manner following, viz.: The flax or hemp, after having been broken and worked in the
machine, should be divided into small quantities of about one ounce each, and these should
be tied loosely in the middle with a string, and in this state laid in the trays or cradles, and
then be soaked in cold soft water for a day or two, when each parcel should be worked
separately, while wet, through a machine, precisely similar to that already described, except
only that the rollers should be cylindrical, and made entirely of wood with metal axles,
and the teeth, which will be parallel, should be similar in form to those shown in
section at Q, fig. 423*. Such operation will loosen the gluten and colouring matter, for
the rinsing and wringing which must follow. The flax must then be again disposed in
a flat and smooth manner, in such trays or cradles, and once more set to soak in sufficient
soft water to cover it, in which a small quantity of soap, in the proportion of about
seven pounds of soap to each hundred weight of flax, has been previously dissolved, and
in this state it should remain for two or three days longer, and then be finally worked
through the machine, rinsed with clear water, and wrung; which will render it sufficiently
white for most purposes.
III. The Heckling.—We have already stated that, by the operation of heckling, a
three-fold object is proposed: 1. the parting of the filaments into their finest fibrils;
2. the separation of the short fibres which are unfit for spinning; 3. the equable and
parallel arrangements of the long filaments. The instrument of accomplishing these objects
is a comb-fashioned tool, called the heckle or hackle; a surface studded more or less
thickly with metal points, called
heckle teeth; over which the flax is
drawn in such a way that the above
three required operations may be properly
accomplished.
The common construction of the
heckle is the following: (see fig.
424.) Fig. 424. is the ground plan,
and fig. 425. is the section. Upon
an oblong plank a b, two circular or
square blocks of wood c and d are
fixed, in which the heckle teeth stand
upright. To give these a firmer hold they are stuck into holes in a brass or iron plate,[489]
with which the upper surface of c and d is covered. Both heckles may be either associated
upon one board or separated; and of different finenesses; that is, the teeth of the one
may be thinner, and stand closer together; because the complete preparation of the
flax requires for its proper treatment, a two-fold heckling; one upon the coarse, and
one upon the fine heckle; nay, sometimes 3 or 4 heckles are employed of progressive
fineness. The heckle teeth are usually made of iron, occasionally of steel, and from
1 to 2 inches long. Their points must be very sharp and smooth, all at an equal level,
and must all graduate very evenly into a cylindrical stem, like that of a sewing needle,
without any irregularity. The face of the heckle block must be uniformly beset with
teeth, which is done by different arrangements, some persons setting them in a
circle, and others in parallel rows; the former being practised in Germany, the latter in
England. The coarse heckle is furnished with teeth about one tenth of an inch thick,
one and a quarter of an inch long, and tapering from the middle into a very fine point. In
the centre of the circular heckle is a tooth planted; the rest are regularly set in 12 similar
concentric circles, of which the outermost is 53⁄4 inches in diameter. The fine heckles
contain no fewer than 1109 teeth. Instead of making the points of the teeth round, it
is better to make them quadrangular, in a rhombus form, in which case the edges serve
to separate or dissect the fibres.
The operation of heckling is simple in principle, although it requires much experience
to acquire dexterity. The operative seizes a flock of flax by the middle with the right
hand, throws it upon the points of the coarse heckle, and draws it towards him, while
he holds the left hand upon the other side of the heckle, in order to spread the flax, and
to prevent it from sinking too deeply among the teeth. From time to time the short
fibres or tow sticking to the teeth are removed. Whenever one half of the length of the
strake of flax is heckled, it is turned round to heckle the other half. This process is
repeated upon the fine heckle. From 100 pounds of well-cleaned flax, about 45 or 50
pounds of heckled flax may be obtained by the hand labour of 50 hours; the rest being
tow, with a small waste in boony particles and dust. The process is continued, till by
careful handling little more tow is formed.
Many contrivances have been made to heckle by machinery, but it may be doubted
whether any of them as yet make such good work with so little loss as hand labour.
In heckling by the hand, the operative feels at once the degree of resistance, and can
accommodate the traction to it, or throw the flax more or less deep among the teeth,
according to circumstances, and draw it with suitable force and velocity. To aid the
heckle in splitting the filaments, three methods have been had recourse to; beating,
brushing, and boiling with soap-water, or an alkaline lye.
Beating flax either after it is completely heckled, or between the first and second
heckling, is practised in Bohemia and Silesia. Each heckled tress of flax is folded in
the middle, twisted once round, its ends being wound about with flaxen threads; and
this head, as it is called, is then beat by a wooden mallet upon a block, and repeatedly
turned round till it has become hot. It is next loosened out, and rubbed well between the
hands. The brushing is no less a very proper operation for parting the flax into fine
filaments, softening and strengthening it without risk of tearing the fibres. This
process requires in tools, merely a stiff brush made of swines’ bristles, and a smooth
board, 3 feet long and one foot broad, in which a wooden pin is made fast. The end
of the flax is twisted two or three times round this pin to hold it, and then brushed
through its whole length. Well heckled flax suffers no loss in this operation; unheckled,
only a little tow; which is of no consequence, as the waste is thereby diminished
in the following process. A cylindrical brush turned by machinery might be
employed here to advantage.
The boiling of flax with potash lye alone, or with lye and soap, dissolves that portion
of the glutinous cement which had resisted the retting, completes the separation of the
fibres, and is therefore a good practical means of improving flax. When it is performed
upon the heckled fibres, a supplementary brushing is requisite to free it from the dust,
soapy particles, &c.
Can flax be prepared without retting?—The waste of time and labour in the steeping
of flax; the dyeing of the fibres consequent thereon, which must be undone by bleaching;
the danger of injuring the staple by the action of putrescent water; and, lastly,
the diminished value of flax which is much water-retted, are all circumstances which
have of late years suggested the propriety of superseding that process entirely by
mechanical operations. It was long hoped, that by the employment of breaking
machines, the flax merely dried could be freed from its woody particles, while the
textile filaments might be sufficiently separated by a subsequent heckling. Experience
has, however, proved the contrary. The machines, which consisted for the most part of
fluted rollers of iron or wood, though expensive, might have been expected to separate
the ligneous matter from the fibres; but, in the further working of the flax no advantage
was gained over the water-retting process.
[490]
1. Unretted flax requires a considerably longer time for breaking than retted, under
the employment of the same manipulations.
2. Unretted stalks deliver in the breaking and heckling a somewhat greater product
than the same weight of flax which has been retted; but there is no real advantage in
this, as the greater weight of the unretted flax consists in the remainder of ligneous or
glutinous matter, which being foreign to the real fibre, must be eventually removed.
In the bleaching process, the water and the alkaline lyes take away that matter, so that
the weight of the bleached fibre is not greater from the unretted than the retted flax.
3. The parting of the fibres in the unretted stalks is imperfectly effected by the
heckling, the flax either remains coarser as compared with the retted article, and affords
a coarser thread, or if it be made to receive greater attenuation by a long continued
heckling, it yields incomparably more torn filaments and tow.
4. The yarn of unretted flax feels harder, less glossy, and rougher; and, on
account of these qualities, turns out worse in the weaving than the retted flax. Nor is
the yarn of unretted flax, whether unbleached or bleached, in any degree stouter than
the yarn of the retted flax.
5. Fabrics of unretted flax require for complete bleaching about a sixth less time
and materials than those of the retted. This is the sole advantage, but it is more than
counterbalanced by the other drawbacks above specified.
In Mr. Wordsworth’s improved apparatus for heckling flax and hemp, a succession of
stricks is subjected to the operation of several series of revolving heckles of different degrees
of fineness, for the purpose of gradually separating or combing the long fibres, and
dressing them smooth; while at the same time, the tow or entangled refuse portions of
the material taken off from the stricks by the heckle points are removed from the heckles
by rotatory brushes and rollers covered with wire cards, and discharged into suitable
receivers, whence it may be taken to a carding engine, to be worked in the ordinary
way.
The accompanying figures represent in plan and section, the heckling machine which
is made double, for the purpose of allowing two series of stricks of flax to be acted upon
at one time. Fig. 426. is a horizontal view of the machine; fig. 427. is an end view, the
whole being represented in working order, and the respective letters of reference pointing
out corresponding parts of the machine.
A A are two large barrels or drums, upon the surfaces of which are fixed longitudinally
several series of brass ribs a, b, c, d, e, f, g, h, i, holding heckle points. These ribs
are placed at small distances apart round the barrels, all the heckle points standing radially
from the axes, and the barrels are mounted upon axles supported by pedestals,
with plummer blocks bearing on the rails of the end frames. B B, are two horizontal
wheels or pulleys turning upon vertical shafts, which pulleys conduct an endless chain
C C C C, carrying the holders, whereon the stricks of flax or other material intended to be
heckled are suspended.
At one end of the axle of each of the barrels a toothed wheel D D, is made fast, and these[491]
are connected by a similar wheel E, and a pinion F, fig. 427., the latter being fixed upon
the axle of the driving rigger G.
The power of a steam engine, or any other first mover, being applied by a band and
rigger, or otherwise to the axle of G, the pinion F, is driven round, which, being in geering
with the toothed wheels E and D D, causes the heckle barrels A A to revolve simultaneously
in opposite directions, as shown by the arrows in fig. 427.
The stricks of flax intended to be operated upon are severally confined between pairs
of clamps k, fastened together, which clamps, with the stricks, are then suspended in
their respective holders H H, attached to the endless chain C: the lower portion of the
flax hanging down for the purpose of being acted upon by the rotatory heckles, while
the upper portions are turned up in loops and confined by spring levers attached to each
carrier.
The respective holders of the clamps consist of a forked frame, with hooks at the
lower parts of their arms, which receive the ends of the clamps k, that confine the strick
of flax. From the upper part of each forked frame, a perpendicular pin extends, which
pins when inserted into the sockets l l l, in front of the chain, form axles for the frames to
turn upon at certain periods of the operation.
On the upper end of each pin, a small arm or tappet piece m, fig. 427., is fixed,
standing at right angles to the face of the forked frame of the holder H. Those tappets
as the endless chain conducts the holders along at certain periods, come in contact with
stationary pins or wipers n n, fixed to the guide rails o, on which the chain C slides; and
these wipers acting against the tappets as they pass, cause the holders to be turned round
at those periods for the purpose of bringing the reverse side of the strick of flax on to
the heckle points.
Let it now be supposed, that all the holders connected to the endless chain have been
furnished with stricks of flax, or other material to be heckled, and that the barrels A A,
are put in motion in the way described, revolving in the direction of the arrows shown
in fig. 427. A pinion on the end of the axles of one of the barrels A, will drive a train
of toothed geer J K L M and N, on the axle of the latter, of which there is a bevelled
pinion taking into a bevelled wheel, turning horizontally at the lower end of the perpendicular
shaft of one of the chain pulleys. It will hence be perceived that as the
barrels go round, such rotatory motion will be communicated to the pulley B, as will
cause it to drive the chain C forward, and by that means conduct the several stricks of
flax progressively along the barrel.
When each successive holder, with its strick of flax or other material, is brought to
the part z, fig. 426., the fibres come in contact with the rotatory barrel, and first strike
upon the series of coarse heckles a a, placed upon an inclined or conical surface of the
barrel, by which means the lower ends of the flax in each strick are first acted upon; and
as it advances, the upper part, and ultimately the whole length of the long fibres of the
suspended strick are gradually brought on to the heckles, which progressive operation
prevents the long fibres from being broken, and causes a smaller quantity of tow to be
produced than is usually taken off in any of the ordinary modes of heckling.
After the strick of flax or other material has been carried by the travelling chain past
the first inclined or conical surface a, of the heckling barrel, it then comes upon the
cylindrical part b, of the barrel, which is also furnished with coarse heckles that penetrate
and comb down the whole pendant lengths of the fibres. But in order that both sides
of the strick of flax may be equally operated upon, the holder is now to be turned round
upon its pin or pivot, which movement is effected by one arm of the lever or tappet m,
(as the carrying chain moves onward), coming against the stationary pin or wiper n,
which changes the position of the holder, as shown at p, in the horizontal view fig. 426.
The under part of the guide rail o, upon which the chain slides, is at this part cut
away, for the purpose of allowing the holder to turn round horizontally; and a pin or
projection at the under side of the guide rail, as the chain continues moving, acts against[492]
the side of the carrier frame, and forces it into a position parallel with the chain. The
other side of the strick of flax is by these means brought on to the heckles of the second
inclined or conical surface of the barrel at c; and the travelling chain proceeding onward,
the fibres of the material are in succession passed over and combed by the heckles
of increasing fineness, d, e, and f, on the cylindrical part of the revolving barrel, until
the strick having arrived at the second wiper n, the frame or holder is at q, turned round
as before, and the reverse side of the strick, or that first operated upon by the heckles a
and b, is brought progressively on to the heckles of increasing fineness, g, h, and i; and
having passed the last series of rotatory heckles, the holders are in succession to be removed
from the machine, the material having been sufficiently dressed.
The clamps of the holders are now opened by the attendant, and the stricks of flax
or other material are taken out, and again placed between the clamps in reversed positions,
in order that the other ends of the fibres may be operated upon. The clamps,
with the stricks, are then suspended again in the holders, the uncombed ends of the
fibres hanging down upon the heckle barrel.
In order to avoid interrupting the continual operation of the machine, it is proposed
that the strick, on its second introduction, shall be placed in the holders on the opposite
side at y, which is one of the reasons for constructing a double machine, and the strick
being thence carried along by the travelling endless chain in the way already described,
the fibres will be first brought under the operation of the coarse heckles on the inclined
or conical surface of the second revolving barrel, and then of the other heckles increasing
in fineness on the cylindrical part of the barrel, until having reached the end,
as in the former instance, the fibres of the flax may be considered to be sufficiently
dressed, and may then be withdrawn.
It may be necessary here to remark, that as different kinds and qualities of material
will require different degrees of working by the heckles, this can be effected by varying
the comparative speeds of the travelling holders and the heckle barrels. These comparative
speeds, it will be perceived, depend upon the diameters of the wheels and
pinions by which the pulley B is driven from the rotation of the heckle barrel. These
wheels and pinions are therefore intended to be removed and changed for others of different
diameters, as circumstances may require. It will be perceived that the faster the
stricks travel through the machine compared to the rotatory speed of the heckle barrels,
so much the less will the material be acted upon by the rotatory heckles; but as different
qualities of material must be differently operated upon, according to circumstances,
it is impossible to set out any definite speeds or proportions of speed: that will, however,
be readily perceived by competent workmen when working at the machine.
In the process of opening the fibres of the material by the rotatory heckles, a quantity
of short or loose fibres, as tow, will be taken off the stricks by the heckle points, and
will remain adhering to the barrel between the points of the heckles: in order, therefore,
to remove this tow, or other loose entangled materials from the heckles, several
series of brushes, or blocks, with bristles, are affixed longitudinally to rotatory barrels
Q Q.
These brush barrels are mounted parallel to the heckle barrels upon axles, supported
in plummer blocks affixed to brackets extending from the end frames of the machine.
Those parts of the brush barrels which are opposite to the cylindrical portions of the
heckle barrel are cylindrical, and those parts which are opposite to the bevels are contra-bevelled,
or made as frustums of cones reversed, or in an opposite angle, as r, s, so as to
run parallel to the inclined surfaces of the heckle barrels a and c.
Upon the periphery of these barrels Q Q, ribs or blocks, with bristles or brushes, are
fixed longitudinally, at suitable distances apart, the bristles all standing radially from the
axle, and taking into the points of the heckles.
Rotatory motions are given to the brush barrels Q Q, by bands passing from the riggers
at G, over pulleys R R, fixed at the end of each of the axles of the brush barrels. Hence,
it will be perceived, that the barrels Q Q will revolve in opposite directions to the heckle
barrels, and with sufficient speed to enable the brushes to pass through between the
points of the heckles, and in so doing, to remove the tow or other loose matter
therefrom.
The tow or other loose fibrous material collected upon the brushes is transferred
thence on to wire cards placed round the periphery of the barrels S S, which barrels are
mounted upon axles parallel to the brush rollers, and turn in plummer blocks upon
brackets, extending from the end frames of the machine.
These barrels are cylindrical, and covered with sheets of wire cards at those parts
which are opposite to the cylindrical portions of the brush barrels, but those portions of
the barrel S, which are opposite to the bevelled points r and s, of the brush barrels, are
bevelled or made conical at t u, to fit or correspond with the inclined surfaces r and s; these
are covered with sheets of wire card also.
Rotatory motions are communicated to the card barrels S S, by bands from the pulley[493]
T, fixed on to the side of the toothed wheel M, (see fig. 427.) which band drives similar
pulleys V V, mounted upon studs fixed in the end frame. Upon the side of each of these
pulleys V V, a pinion t is fixed, which pinion takes into the teeth of the wheel W, on the
end of the axle of each of the card barrels S S; by which means such slow motions are
given to the barrels S, as will allow the brushes of the barrels Q to comb off, and deposit
the tow or other fibrous material upon the wire cards as they revolve, and from whence
it is to be removed by a doffing comb, and let fall into any convenient receptacle below,
in the same way as in ordinary carding engines.
The doffing combs, X X X, are formed to the shape of the card barrels, and are attached
to straight bars extending along the machine on both sides, which are supported at their
extremities by levers Y Y, vibrating upon fulcrum pivots at w w. To these levers perpendicular
rods Z Z are connected by joints, and the lower end of each of these rods is
attached to an eccentric disk, roller or crank x x, on the axle of the brush barrel; whence
it will be perceived that by the rotation of the eccentrics x, the levers Y will be made to
vibrate and strike off, or doff the tow or other material from the card barrels, in a
similar manner to the operations of the doffing comb of an ordinary carding engine.
Mr. Evans’ patent improvements in machinery for preparing and dressing flax and
hemp apply, first, to the operation of scutching, swingling, or beating away the boom
or woody particles of the rind which covers the flax, or hemp, in its rough state; and,
secondly, to the subsequent operation of heckling, combing, or opening of the fibres of
the material preparatory to spinning it into yarns.
Fig. 428. represents the scutching or swingling machine, in different positions.
Fig. 428. is an end view of the machine in operation; fig. 429. is a front view of the
same. The essential parts of the machine, and those in which the invention especially
consists, are two pairs of revolving beaters or scutchers, each formed by long ribs or
blades mounted upon arms. The blades of the beaters a a, may be made of ribs of hard
wood, or other suitable material, broad but thin, and slightly rounded on their edges, to
prevent their cutting the fibres of the flax or hemp when they strike it. The two blades
are placed parallel to each other, and mounted upon a hexagonal frame, the arms b b
inclining or forming obtuse angles with the blades, and from the middle of the arms
short axles c c, extend, upon which the beaters revolve.
The axles of both pairs of beaters are mounted in plummer boxes, bearing upon
horizontal rails at the ends of the machine, as shown in fig. 428., and are at such distance
apart as will allow of the arms and the beaters of each pair passing alternately
within those of the other pair as they revolve in opposite directions, which they are
enabled to do without coming in contact, in consequence of the inclination of the arms.
On the axle at one end of each pair of beaters a toothed wheel d, is affixed, and these
wheels being of similar diameters, and taking into each other, cause the beaters to
revolve with similar speed in opposite directions, rotatory motion being given to them
by a band and rigger fixed upon one of the axles; and in order that the beaters in
revolving may not come in contact as they pass, the positions of the two pairs are so
arranged that the blades of one shall be in a perpendicular situation, while those of the
other are horizontal.
The rind of the flax or hemp having been previously broken by any of the ordinary
modes of performing that operation, small bunches or stricks of the material are spread
out, and their ends confined between the jaws of clamps or holders.
[494]
These clamps or holders differ considerably from the clamps which are commonly
used. I shall therefore particularly describe their construction, before showing them in
operation. Fig. 430. and 431. are views of the clamp in two different positions; a and b
are two boards united together by a hinge c, at top,
which of course allows them to shut and open. The
lower parts, forming the jaws of the clamps, are
made with teeth or indentations, between which
parts the ends of the flax or hemp are securely held
when the clamps are brought together; d d, are two
pieces projecting from the board b, at the end of each
of which is an eye shown by dots, and at the back of
the board a, (see fig. 430.,) there is a double armed
lever e, turning upon a fixed pin f, which lever carries
two circular wedges g g. These wedges pass into the eyes of the pieces d d, when
the clamps are closed, and hold them fast. There is a segment ratchet h, at the upper
part of the board a, which turns upon a stud i, and is pressed downward by a spring k.
This ratchet receives the end of the lever e, and consequently keeps the circular wedges
firm in the eyes, which hold the clamps securely together, and prevents their opening by
the shaking of the machine.
When it is required to open the clamps, the ratchet h must be raised, and the lever e
pushed aside by its handle l, which draws the circular wedges f from the eyes of the
pieces d d, and the boards of the clamps immediately separate. For the convenience of
suspending the holders in the machines, a piece of sheet iron m, is bent at right angles,
and fastened to the back of the board b, as seen in fig. 431., forming a groove by means
of which the holders are enabled to slide into the machine and hang there.
These clamps or holders are, when charged with the material, placed in the scutching
machine, as shown at e e e in figs. 428. and 429., bearing upon the edge-rail or bar f.
The beaters are now made to revolve in the manner already described, by which the
edges of the blades will strike against the pendent stricks of flax or hemp alternately on
each side, and beat off, scutch or swingle the boom from the material, and render it fit
for the operation of heckling which is to follow.
The whole machine is encased with boards, to prevent the inconvenience arising from
dust, and an apparatus might be adapted with a blower to conduct away the dust
created by the machine, and to discharge it out of the building.
In introducing these stricks of flax or hemp into the machine, the holder is placed
upon the projecting end of the bar or edge-rail f, and is thence slidden into the machine;
and after the material has been sufficiently scutched or swingled, the holders with the
stricks are removed through the top of the machine, and others successively introduced
at the end, and pushed along the rail.
If, however, it should be thought
desirable, the stricks may be
progressively carried through the
scutching machine, and delivered
into a similar edge-rail in the
heckling machine, there to be
operated upon in the way about to
be described, by which means the
whole process of scutching and
heckling may go on without interruption.
Fig. 432. represents the heckling
or combing machine by which
the fibres of the material are to be
opened, and the tow removed.
It is a transverse section, taken
nearly through the middle, in a
vertical direction. Perpendicular
standards form the ends of the
machine, which are connected together
by longitudinal rods or
bars secured by nuts. The heckle
points intended to act upon the
flax are mounted in the frames
a, b, c, and d, and the stricks of
flax held in the clamps e, e, e, as
described, are suspended from the
bar or edge-rail extending through
the machine.
[495]
In order to render the principles of this machine and its mode of working evident, it
may be desirable to show in an abstract form the manner in which the heckles are
brought into operation upon the flax, and for this purpose two diagrams are delineated
in figs. 433, 434.
Suppose two sets of combs or heckle points be mounted upon frames a and b, as in
these figures, each frame being moveable by means of cranks c, c, and d, d, connected in
such manner that they both turn with the same speed in opposite directions, it is evident
that every part of the frames and combs will move in circles corresponding to those
described by the cranks; the points of the combs travelling in the directions of the
arrows, and in circles represented by dots.
During this movement, whilst performing the first descending quarter of the circle,
the cranks bring the frames together as in fig. 433. They begin after this to separate in
describing the second descending quarter, and come to the position fig. 434., when, continuing
to revolve, they move further from each other in describing the first ascending
quarter of the circle, and arrive at the position where the distance is the greatest;
lastly, they describe the second ascending quarter returning to the third position. If,
therefore, a strick of flax be suspended between the two sets of combs as in fig. 433., and
the rotatory motion be continued for a sufficient length of time, the flax will be combed
in the whole length which is submitted to the actions of the combs, although the points
severally have only operated in very small space.
Such a system of combs or heckles would make a very good and simple heckling
engine, if it were not for the inconvenience experienced by the points dragging some of
the fibres with them when withdrawing from the flax, which would produce a great
waste of material; and to obviate this it would be necessary to introduce some contrivance
for clearing the points, which must be attended with considerable complication. The
plan, however, of the present improved engine, affords the means of producing the same
effect by more simple and efficient means.
There are two series of combs, see fig. 435., attached to two movable frames represented
at a and b. Each frame is formed
by vertical bars a b, with lateral branches
or arms, which carry the heckle points.
The branches or arms are parallel, and at
equal distances apart, but fixed in such
positions in each frame that they may occupy
the intervening space when the frames
are brought together as fig. 436. The
frames are put in motion by means of revolving
cranks to which they are attached,
as shown in fig. 436., and when the cranks
turn upon their axes, the branches of one
frame pass between those of the other
without touching. This forms what may
be called a set of combs; but one of the improved machines contains two such sets,
the points of the combs of one set being opposed to the points of the combs in the
other set.
The way in which the series of combs that compose one set act upon the flax, is shown
in the side view, fig. 435. When the cranks are nearly vertical, the points of both frames
are away from the flax, but as the cranks move round in the direction of the arrows, the
frames come into another position, and it is then that the points or heckles of one of the
frames a, begin to penetrate the flax, and descending they comb or divide its fibres. The
rotation of the cranks continuing, the two frames a and b come into the position shown
at fig. 435., the points of the frame a, withdrawing from the flax, and those of the frame
b, approaching and pushing the fibres off from the former, which are now combed by the
descending stroke of the points.
It will hence be perceived that as the combs of the frame a and b, respectively
advance, they will push forward the whole of the strick of flax, and render it impossible
for the fibres to be raised and entangled, as each frame in advancing clears the fibres
from the points which preceded it.
A single set, however, of such combs or heckles acting only on one side of the flax,
would but imperfectly perform the operation of opening its fibres; it is therefore
necessary, in order to accomplish the desired object in the most effectual way, that two
such sets of combs or heckles should be brought to act on opposite sides of the strick of
flax, which may be done in the manner shown in the figures. The cranks of the two
opposite sets of comb-frames or heckles a, b, and c, d, are connected by a pair of toothed
wheels e, f, as fig. 437., or by four toothed wheels, by which the heckles are actuated at
once, the two sets moving in opposite directions, but with similar speeds, and the
combing or heckling of the material will go on in the way shown in the figure last
indicated.
[496]
Thus far I have considered only two frames of combs or heckles constituting a set, as
acting upon each side of the strick of flax; but in order to perform a greater quantity of
work, several sets may be mounted in one machine, working alongside of each other,
extending over the breadth of the machine. The combs may then be supported upon
three frames, of which the middle one may have branches or arms extending upon both
sides, and the other two frames branches extending inwards only. To drive the frames
so arranged they must be connected to treble cranks.
Such is the principle of the improved machine for combing or heckling, exhibited in
the several figures of which I now proceed to describe the particular construction. The
machine or engine, fig. 432., has four sets of combs, acting both at the back and front
of the flax; a b are the front set of combs, and c d, the back set of combs; e e e, are the
clamps holding the stricks of flax previously scutched, which clamps hang upon the edge-rail.
The comb frames are attached at top and bottom to the cranks g g, which are all
connected by toothed geer, and driven by a band and rigger.
The combs or heckles being put in motion in the way described, act upon the suspended
stricks of flax, and upon their fibres, as explained; which stricks are progressively
conducted through the machine by their clamps sliding upon the edge-rail through the
agency of the endless chain, to which the clamps are severally attached, by a hook falling
into one of the links. The chain is driven by a spur wheel upon the axle of a bevel
wheel, which receives a slow rotatory motion through a bevel pinion on the axis of a
similar wheel, actuated by another pinion on the end of the upper crank axle. By these
means, clamps, with the stricks of flax placed on the edge-rail, are slowly carried through
the machine, when the flax will be gradually acted upon first by heckle points of a coarse
kind, set wide apart, and ultimately by finer points set near together; after which, the
clamp with the strick of flax is discharged from the machine, at the reverse end of the edge-rail.
But should the workman neglect to remove the holder or clamp, when it arrives
at the end of the rail, the machine would be stopped by means of a jointed lever, having
a fork at its end, which pushes the band from the fast rigger on to the loose one, and
throws off the driving power.
As the combs or heckles, in acting upon the flax to divide its fibres, tear parts of the
fibres, and reduce them into tow, the downward motion of the heckles brings the tow
with them out of the flax, which is deposited between two fluted rollers p p, fig. 432.,
and is by them conducted down to the large drum q, where it becomes lapped in two
endless sheets round the periphery of the drum; the one of coarse tow, the other of fine,
the adhesion being assisted by a pressing roller r; and when a quantity of the tow has
been thus accumulated round the periphery of the drum, it may be removed thence by
cutting it off in sheets. The fluted rollers, and also the large drum, are driven by geer
bands.
After the strick of flax has been thus carried through the scutching machine or the
heckling machine, the jaws of the clamps are to be opened, the ends of the flax reversed,
and the strick again confined in the clamps, so that the other end of the strick may be
operated upon in a similar way. In order to prevent any part of the flax from attaching
itself to the branches of the movable frames, each frame is furnished with a shield or
guard of polished iron or brass plate, which covers a part of the combs and the heads of
the screws by which they are fixed to the branches. When the plate metal is bent into
the form of a shield, it is slipped on to the branches of the heckle frames, and is sufficiently
elastic to hold fast.
But it is to be observed, that the edges of the shields are to vary in the extent of
their projection according to the situation in which they are to be placed; those which
are to shield the upper branches of heckles are to project but little, so as to leave the
points uncovered and free to enter the strick of flax; but the shields of the lower
heckles are to project considerably over the points, to prevent them from penetrating too
far into the fibres, which is so contrived for the purpose of facilitating the falling of the
tow, which would otherwise be with difficulty removed from the lower combs, were it
thrust upon the whole length of the points.
It being advantageous that each strick of flax should be combed near the lower
extremities before the middle is acted upon, it is necessary, in order to obtain this effect,
to remove some of the points of the combs in the upper branches. By these means, the
operation of the heckles upon the flax begins and proceeds gradually, and ceases at the
opposite extremity of the machine in the same gradual way, which is very advantageous
in clearing completely the flax from the tow.
IV. Flax spinning.—If we compare flax with other spinning materials, such as wool
and cotton, we shall find it to possess several characteristic properties. While cotton
and wool are presented by nature in the form of insulated fibres, the former requiring
merely to be separated from its seeds, and the latter to be purified from dirt and grease
before being delivered to the spinner, flax must have its filaments separated from each[497]
other by tedious and painful treatment. In reference to the spinning and the subsequent
operations, the following properties of flax are influential and important:—
1. The considerable length of the fibres, which renders it difficult, on the one hand,
to form a fine, level, regular thread, on the other, gives the yarn a considerably greater
tenacity, so that it cannot be broken by pulling out the threads from each other, but
by tearing them across.
2. The smooth and slim structure of the filaments, which gives to linen its peculiar
polished aspect, and feel so different from cotton, and especially from woollen stuffs, unless
when disguised by dressing. The fibres of flax have no mutual entanglement, whereby one
can draw out another as with wool, and they must therefore be made adhesive by moisture.
This wetting of the fibres renders them more pliant and easier to twist together.
3. The small degree of elasticity, by which the simple fibres can be stretched only one
twenty-fifth of their natural length before they break, while sheep’s wool will stretch from
one-fourth to one half before it gives way.
Good flax should have a bright silver gray or yellowish colour (inclining neither to
green nor black); it should be long, fine, soft, and glistening, somewhat like silk, and
contain no broad tape-like portions, from undissevered filaments. Tow differs from
flax in having shorter fibres, of very unequal length, and more or less entangled.
Hemp agrees in its properties essentially with flax, and must be similarly treated in the
spinning processes.
The manufacture of linen and hemp yarn, and the tow of either, may be effected by
different processes; by the distaff, the hand-wheel, and spinning machinery. It will be
unnecessary to occupy the pages of this volume with a description of the first two well
known domestic employments. I shall therefore proceed directly to describe the last
method, or
Spinning of Flax by Machinery.—This branch of manufacture has been much more
recently brought to a practical state than the spinning of cotton and wool by machines,
of which the cause must be sought for in the nature of flax as above described. The
first attempts at the machine spinning of flax, went upon the principle of cutting the
filaments into short fragments before beginning the operation. But in this way the most
valuable property of linen yarn, its cohesive force, was greatly impaired; or these attempts
were restricted to the spinning of tow, which on account of its short and somewhat
tortuous fibres, could be treated like cotton, especially after it had been further torn by
the carding engine. The first tolerably good results with machinery seem to have been
obtained by the brothers Girard at Paris, about the year 1810. But the French have
never carried the apparatus to any great practical perfection. The towns of Leeds in
Yorkshire, of Dundee in Scotland, and Belfast in Ireland, have the merit of bringing
the spinning of flax by machines into a state of perfection little short of that for which
the cotton trade has been so long celebrated.
For machine spinning, the flax is sometimes heckled by hand, and sometimes by machinery.
The series of operations is the following:—
1. The heckling.
2. The conversion of the flax into a band of parallel rectilinear filaments, which
forms the foundation of the future yarn.
3. The formation of a sliver from the riband, by drawing it out into a narrower
range of filaments.
4. The coarse spinning, by twisting the sliver into a coarse and loose thread.
5. The fine spinning, by the simultaneous extension and twisting of that coarse thread.
The spinning of tow requires a different treatment: we shall first treat of the
heckling of flax by machines; and secondly, of the mechanical spinning of flax. The
mechanical carding and spinning of tow are very similar to those of cotton; which see.
Though machine heckling be far from perfect, yet the tow it throws off can be spun
into very good yarn by machines, while it would afford very indifferent yarn to the
hand spinner.
All heckle machines have this common property, that the flax is not drawn through
them, as in working by hand, but on the contrary, the system of heckles is moved through
the flax properly suspended or laid. Differences exist in the shape, arrangement, and
movements of the heckles, as also in regard to the means by which the adhering tow is
removed from them. The simplest and most common construction is to place the
heckles upon the surface of a horizontal cylinder, while the flax is held either by mechanical
means or by the hand during its exposure to the heckle points. Many
machines have been made upon this principle. It is proper in this case to set the heckle
teeth obliquely in the direction in which the cylinder turns, whereby they penetrate
the fibres in a more parallel line, effect their separation more easily, and cause less waste
in torn filaments. To conduct the flax upon the cylinders, two horizontal fluted rollers
of iron are employed, which can be so modified in a moment by a lever as to present
the flax more or less to the heckling mechanism. The operator seizes a tress lock of[498]
flax with her hand and introduces it between the fluted rollers, so that the tips on which
the operation must begin, reach the heckles first, and by degrees the advancing flax gets
heckled through two-thirds or three-fourths of its length, after which the tress or strick is
turned, and its other end is subjected to the same process. By its somewhat rapid revolution
the heckle cylinder creates a current of air which not only carries away the boomy
particles, but also spreads out the flax like a sheaf of corn upon the spikes, effecting the
same object as is done by the dexterous swing of the hand. The tow collects betwixt the
teeth of the heckle, and may, when its quantity has become considerable, be removed in
the form of a flock of parallel layers.
The essential parts of such a construction will be understood from fig. 438., though the
fluted rollers are absent. The flax a, b, is held by the hand, or in a kind of clamp.
The cylinder is partly covered with a curvilinear plate of iron c, d, which serves to sustain
the flax, and to guide it in circular tresses round the periphery of the heckle. At the
beginning it is placed near b, when the tips of the flax are only presented to the heckles;
during the working the shield is continually drawn back in the direction from d to c,
and thus lets the operation be performed upon the remaining part of the flax.
First operation; the conversion of flax into ribands or slivers.—This is effected by subjecting
the flax to a series of advancing gills or heckle-teeth, and at the same time drawing
out its fibres by means of rollers. Figs. 439, 440, 441, show the outline of the construction[499]
of a machine for this purpose. Here two rows of heckles are placed alongside
of each other, though only one of them be shown in the ground plan, fig. 440., in order to
allow the parts beneath the other to be seen. The flax is placed in the sheet iron
channels a a, by laying down one handful after another, so that the points of the second
strick reach to only the middle of the first, and thus preserve a uniformity of thickness
in the feeding. This process is necessary, since, as every one knows, the heckled stricks
are always thick in the middle, and thin at the ends. The flax being introduced between
the rollers b and c, is drawn out by their agency, and at the same time subdivided by the
heckles d, between whose teeth the pins of the roller e press it down. At the rollers
f3 it is loosened from the heckles by the transverse bars which rise from the springs g,
after which it is seized by the rollers h i, and drawn again. A little beyond these rollers,
it runs through a funnel l, in order to gather the fibres together; in front of these rollers
the slivers from both rows of heckles are united, and proceed in one riband through that
polished brass funnel; the rollers m n extend this riband, pressing it gently together,
and then let it fall into a tin can. The union of the two slivers contributes to the
uniformity, since the irregular thicknesses are thereby compensated. The diameter of
the roller c, is equal to that of each of the cylinders f, f1, f2, f3; and the whole five
move with equal velocity. The same correspondence exists between the rollers n and i.
Thus the sliver of flax is not stretched either by its passage from e, upon the heckles,
nor between i and n, but solely in passing from the heckles to the rollers i h. The
heckle teeth of this machine do not stand perpendicularly, but are bent somewhat
backwards; so as to retain the flax more firmly. The revolving cylindrical brush o,
is placed over and a little in front of the pressing roller h, in order to take off all the
filaments of flax adhering to their circumference, and to toss them onwards where they
may again unite with the slivers. For the sake of perspicuity, the rollers h, and those
brushes are left out in fig. 440., but the latter are particularly shown in fig. 442., while a
portion of their axis q, is however shown in fig. 440. The pressure of the cylinder h,
upon the cylinder i, is produced by the weight r, fig. 439., which hangs upon the lever
s; the lever pulls down at t, a vertical rod, whose upper hook-shaped end embraces the
axis of h in the middle of its length.
Second principal operation; the formation of rovings.—Mr. Wordsworth’s improvements
in machinery for preparing, drawing, and roving flax, hemp, wool, and other
fibrous substances, consists in a novel contrivance or mechanism to be adapted to the
machine commonly called the gill, employed for preparing, drawing, and roving flax and
hemp, and for combing and spinning long wool; which improvements allow the points
of the travelling heckles to continue longer in operation than in the ordinary construction
of gill, and cause the heckle points to be withdrawn from the fibres at the end of the
stroke without the possibility of their drawing the fibres down with them.
The manner of effecting this object will be seen by reference to the several figures
which exhibit a gill on this
improved plan in different
views. Fig. 443. is a plan or
horizontal view, exhibiting the
upper surface of the machine;
and fig. 444. is a longitudinal
section taken through the
middle of the machine: fig.
445. is a representation of the
front of the machine, but in
which several parts have been
removed to show the action
of the heckles more perfectly.
The several heckles a a a
are formed by a series of
needles or heckle points set
into a metal bar, as represented
on an enlarged scale in
figs. 446. and 447. These
bars are each of them suspended
in a frame or carriage
b b b (shown in two views at figs. 448. and 449.), by means of double jointed levers c c,
seen in two positions, at figs. 450. and 451.; the heckle bar, its levers and carriage or
frame, being shown put together in figs. 452. and 453.
When the heckles are in operation, the points are raised, as in fig. 452.; but when
they are withdrawn from the fibres, then the points are sunk down into the carrying
frames, as fig. 453.
These two positions of the heckles are produced by the knobs or parts d, that project[500]
from the jointed levers c, acting against the edges of guide bars, which will be explained
in describing the operations of the machine.
The several heckles are
adapted and made to work
in the machine by attaching
the ends of the respective
frames or carriages b, to travelling
endless chains e e,
seen in figs. 443., 444., and
445. These endless chains
pass over fluted guide rollers
f f, seen best in figs. 444.
and 445., and over horizontal
bars g g, seen best in figs.
443. and 444. The chains
with the heckles are driven
through the machine by rotatory
spur wheels h h; see
figs. 443. and 444., the teeth
of which take into the spaces
between the cylindrical parts
of the several heckle carriages
b b, and consequently drive
the heckles forward; and
these spur wheels are actuated
by a train of toothed
geer from the first driving
shaft i, which gives motion
to all the operative parts of
the machine.
If flax, hemp, long wool,
or other fibrous material, be
passed into the machine at the
back part by a feeding cloth
or creeper through a guide
k, best seen in figs. 443. and
444., and be conducted under
and over the feeding rollers
l, m, and n, and over the
heckles a a a to the drawing
rollers o and p, and thence to
the flyer and bobbin, or to a
receiving can, the fibres will
be opened in their progress,
and combed by the points of
the heckles entering into and
separating the fibres, the material
being drawn by a different
speed to that with
which the heckles travel.
This operation of preparing,
drawing, and roving flax
and hemp, and the general
construction of a machine of
this kind being well understood,
it is not necessary to
explain its details, excepting
as respects those parts which
constitute the present improvements.
It will be perceived, by
reference to figs. 443. and
444., that the knobs d, which
project from the jointed levers
c, as they travel along the machine,
bear against the outer
edges of the two fixed guide bars q q that extend along the top of the machine above[501]
the heckles, which keep the heckle points raised, as in fig. 451. This will also be very
evidently seen in the front view of the machine, fig. 445., where the upper heckle bar a
is raised in its carriage b, by the knobs d d bearing against the outer edges of these
guide bars q q. But when the endless chains e e, which support and conduct the frames
or carriages of the heckles, have advanced the heckle points to within a very little distance
of the drawing rollers (see fig. 444.) then the knob d of the jointed levers at each
end of the heckle bar passes the ends of the guide bars q q, and they immediately come
in contact with two inclined planes r r, seen in figs. 443. and 444., which instantly
depress the levers c, and consequently cause the heckle bar a, with its points to descend
in the frame or carriage b, withdrawing the points from the fibres of the material almost
in a perpendicular direction.
The heckles that have become thus depressed pass with their carriages by the traversing
of the endless chains along the under part of the machine, and when they arrive at
the back, and begin to rise, the guide bars q q, being at their commencement slightly bent,
conduct the knobs b of the levers c until they are forced back into the positions first
described, whereby the heckle points are raised, as they come to the upper part of the
machine, into effective operation. The fibres of material operated upon, after passing
through the drawing process between the rollers, may be roved, twisted, or spun, by the
employment of a bobbin and flyer, as shown in fig. 444., or may be delivered into a
can, to be roved, twisted, or spun, by other machinery, by substituting a pair of conducting
rollers instead of the bobbin and flyer, which shall conduct the sliver of material
into a tin can below.
The descent of the heckles a, into their frames b, by the falling of the levers c, c, precludes
the possibility of the fibres of the material operated upon being carried down
under the machine by the points, as frequently happens in gill machines of the ordinary
construction; and this mode of mounting the heckles and traversing them with the
assistance of the guide bars q, q, and inclined planes r, r, allows the heckle points to be
brought much nearer to the drawing rollers o, p, by means of the metal bars in which
the heckle points or needles are set, falling below the centre of the endless chain e, e,
as shown in figs. 443. and 444., and thereby affords the means of preparing, drawing, and
roving various qualities of flax, hemp, wool and other fibrous materials, particularly
such as have a much shorter staple than any fibrous materials hitherto operated upon in
gill machinery.
Another most ingenious and effective improvement made of late years in the flax
spinning machinery, is that patented by Messrs. Westley and Lawson, in August 1833,
and since then introduced into practice with great advantage. It applies to the gill or
mechanism employed for opening, straightening, and separating the fibres of flax,
hemp, and long wool in the operation of slivering. The peculiar feature here is a
method of driving the heckle bars through the gill machine by means of perpetual
screws or worm shafts, instead of by chains and spur wheels, as in the former constructions.
The heckle bars which lie across the machine, are, by the present patentees, supported
at their ends by fixed horizontal guide rails, on which they slide, while the extremities
of the heckle bars are inserted in the helical grooves of the worm shafts, which are
placed in horizontal positions at the sides of the machine; and hence the rotatory motions
given to these screw shafts, cause the heckle bars to be driven along the guide rails
with an uniform simultaneous movement.
The heckle bars having performed their usual office, that is, having combed and separated
the fibres of the material as they move onward, are at the front part of the
machine depressed and put out of operation by means of rotatory cams; and by the
assistance of guide levers, each heckle bar, when it arrives at the end of the upper horizontal
guide rail, is conducted down to the lower horizontal guide rails, where the
extremities of the comb-bars falling into the helical grooves of a lower pair of worm
shafts, revolving in an opposite direction to the former, thereby give the heckle bars a
retrograde movement. When they arrive at the back end of their horizontal guide rails,
they are, by similar rotatory cams, raised again to the upper horizontal guide rails,
which coming into geer with the upper worm shafts, are moved onwards as at first.
By this means a succession of heckles is continually advancing upon the upper guide
rails, having their points in constant operation between the fibres of the textile materials,
while their vertical position is secured during their whole course.
Fig. 454. is a horizontal representation of a gill machine, shewing the present improvements;
but some of the upper portions of the machine are removed, to let the working
parts be seen more clearly. Fig. 455. is a side view of the gill; and fig. 456. a vertical
section taken longitudinally. The driving rigger or pulley a, is fixed upon the front
roller b, commonly called the drawing roller, because when pressed upon by the upper
wooden roller c, it draws out the fibres between them. The rollers d, e, f, are the ordinary
back or holding rollers, for retaining the fibres, while they suffer powerful traction[502]
by the rollers b, c, over the needles or points of the heckle bars. The upper guide rail
above mentioned, upon which the heckle bars slide, is shown at g, in fig. 456., and the
lower guide rail at h; the series of heckle bars with their needles are represented at
i, i, i, i, i, i; the upper worm shafts k, k, are mounted in brackets made fast to the sides
of the frame; a similar pair of worm
shafts l, being mounted in like manner
below. These worm shafts k and l, on
each side are connected together by
toothed wheels m, and upon the axles
of the lower worm shafts, bevelled pinions
n are fixed, which take into corresponding
bevel pinions on the transverse
shaft or axle o. This shaft o,
being connected by a train of toothed
wheel work with the axle of the drawing
roller b, as shown in figs. 454. and
455., the rotation of the roller b, causes
the shaft o to turn also, and the bevel
geer n and o, produce the rotatory
motion of the worm shafts k and l,
which turn in contrary directions.
It will be seen, from fig. 454., that
the ends of the heckle bars i, have nibs
or projections which fall into the
grooves of the screw or worm shaft,
and that being supported below, upon
their guide rails, as the worm-shafts k k
revolve, the upper range of heckle bars
will be progressively advanced towards
the front part of the machine. By referring
to fig. 456. it will be perceived,
that as each heckle bar arrives at the
front end of the guide rail g, a finger p,
called a tappet or cam, on the shaft k,
strikes it down to the lower guide rails
h; and, in order that its descent may
be truly vertical, weighted levers q q, in
front, are made to press against the face
of the heckle bar as it descends. This
bar having now arrived at the lower guide rails h, lets fall its nibs into the grooves of
the lower worm shafts l, by whose rotation the heckle bar is made to retrograde, or return
towards the back of the machine. When the heckle bar has reached the hinder end
of the guide rail h, a finger or tappet, r, on the lower worm shaft, comes under it and
raises the heckle bar, guided by the back-weighted levers s, as shown in fig. 456., till it
is elevated to the level of the upper guide rail g; when the threads of the upper worm-shafts
take hold of its nibs as before, and conduct it forward upon the guide rail in the[503]
way already described. Thus the continued rotation of the worm shafts k k, and l l,
causes the whole series of heckle bars to travel along the guide rails, and the tappets
p and r, by alternately depressing and raising them at the ends of the said rails, cause
them to move in a regular circuit, yet so as to preserve their verticality.
The claim made under this patent is, for every mode in which screw or worm
shafts may be adapted to conduct the bars carrying the needles or heckle-teeth through
a machine for preparing, drawing, or roving textile fibres.
In December 1835, Messrs. Hope and Dewhurst obtained a patent for improvements
in the manufacture of flax, which deserve notice. These are of both a chemical and
mechanical nature. The first consists in steeping the flax in dilute sulphuric acid, of a
certain strength, and for a certain time, proportioned to the quality of the fibres, the
coarser requiring the stronger application. By this means the gummy matter and the
outer shell will be loosened and easily detached. It is then to be passed between
squeezing rollers, afterwards well washed, boiled in a solution of soap and water for a
few hours, and finally passed again through the rollers. These processes may be repeated
till the flax acquires the desired glossiness and separation of fibres. It is next to be
beaten, and passed once or twice over an ordinary heckle or stiff brush.
The second part, or the mechanical,
is represented by the
figures 457., 458., 459., 460., and
461. Fig. 457. is a sectional
elevation in part of the construction
of the spindle, bobbin and
flyer proposed for spinning all
kinds of flax or hemp. Fig. 458.
answers for spinning coarser
yarns; fig. 459. shows how yarns
are to be spun for weft, and
wound upon what is called a
“pin cop bobbin.”
a a a is the stationary or fixed
spindle of the ordinary throstle
frame, which is surrounded by the
tube b b, and connected to the
wharve or pulley c, by which the
flyer d is driven. The flyer is
furnished with guides or conductors
e e, which lead the yarn
immediately to the bobbin; this
flyer is also provided with a small central shaft which supports it, and runs in the small
cup or recess at the top of the stationary spindle a, and is fixed with the flyer to the
tube b b, which is altogether carried round or driven by the wharve c.
It will be seen by fig. 460., that the wharve c, and tube b, are connected at bottom
by a half-lap coupling joint or clutch; this is for the purpose of allowing the tube b
to be slidden up the spindle, and more readily removing the bobbin when it is full of
yarn, without stopping the frame, or removing the band from the wharve c, the tube of
which runs in the step or cup h, fixed upon the bolster rail near the bottom of the
throstle frame. The traversing of the bobbin or the copping motion is effected exactly
in the same manner as in ordinary throstles, that is, by the lifting and lowering of the
copping rail i, which in this instance supports the bobbin. In fig. 458. the flyer is
constructed of twice the length of the bobbin, to allow this to rise and fall freely within
it, and is connected at top by a slight cross piece, for the purpose of preventing the
arms of the flyer from expanding by the centrifugal force, when turning with great
velocity. The flyer for spinning coarse numbers requires to have an inner tube k,
to support the spindle. The bobbins are supported upon a washer l, l. The spindle
is allowed to revolve in a slight degree by the friction of the drag-weight m, m. This
weight has a hole formed in it with a flat side, as shown in fig. 461.
Flax has been for a long period spun wet in the mills; a method no doubt copied
from the practice of housewives moistening their yarn with their saliva at the domestic
wheel. Within a few years the important improvement has been introduced, of substituting
hot for cold water, in the troughs through which the fibres in the act of
spinning pass. By this means a much finer, smoother, and more uniform thread can
be spun than in the old way. The flax formerly spun to twelve pounds a bundle, is,
with hot water, spun to six. The inconvenience of the spray thrown from the yarn on
the flyers remains; aggravated by increased heat and dampness of the room, where this
hot process goes on. Being a new expedient, it receives daily changes and ameliorations.
When first employed, the troughs of hot water were quite open; they are now[504]
usually covered in, so as almost entirely to obviate the objections to which they were
previously liable. With the covers has been also introduced a new method of piecening
or joining on any end, which may have been run down, namely, by splicing it to
the adjoining roving, whereby it is carried through the water without imposing a
necessity on the spinner to put her hand into the water at all. In some places she
uses a wire, for the purpose of drawing through the end of the roving to mend a broken
yarn.
This may be considered the inherent evil of flax-spinning,—the spray thrown off by
the wet yarn, as it whirls about with the flyer of the spindles. A working dress,
indeed, is generally worn by the spinners; but, unless it be made of stuff impermeable
to water, like Macintosh’s cloth, it will soon become uncomfortable, and cause injury
to health by keeping the body continually in a hot bath. In some mills, water-proof
cloth and leather aprons have actually been introduced, which are the only practicable
remedy; for the free space which must be left round the spindles for the spinner to see
them play, is incompatible with any kind of fixed guard or parapluie.
There was before the late Factory Bill passed, a class of very young children
employed in the flax mills, under the name of little doffers, forming generally a troop
of from four to ten in each spinning-room, who, the moment they perceived the bobbins
of any frame or side of a frame exhausted of roving, ran together, and furnished it with
full ones as quickly as possible. They were not numerous in all, but they had an
occupation requiring a great activity and attention. It was practised also in the fine
spinning-rooms, which are perfectly free from dust; and, as it involved a kneeling and
stooping position, seemed peculiarly appropriate to children, and is still done by them
at a somewhat more advanced age.
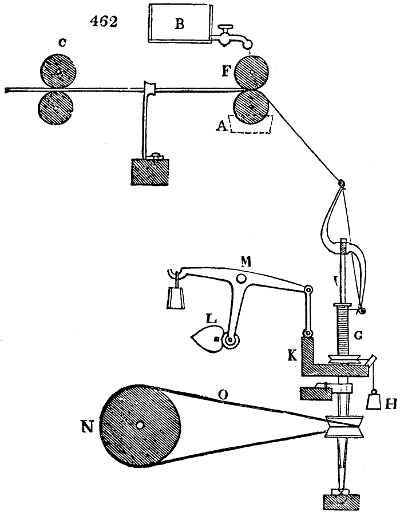
The adjoining fig. 462. will serve to explain the mechanism by which the fine
spinning of flax is performed.
The front pair of drawing rollers
represented at F, was at one time
moistened by letting water trickle
upon it, from a vessel B, furnished
with a stopcock placed a little
above, or by immersing one half
of the under-roller in the water-trough
as at A. The roller pair
C, which receives the fine rovings
from bobbins placed on skewers
or upright pins in the creel behind,
is so mounted as to be fixed at any
desired distance from the front
rollers F. This distance should be
always a little more than the average
length of the filaments of the
line; for if it were equal to it,
they would be seized at both ends
by the two pairs of rollers, which
move with different velocities, and
would be torn asunder, instead of
being drawn out alongside of each
other. The front rollers indeed
move in many such machines four
times faster than the back pair.
The rest of this flax-spinning apparatus
resembles in every respect
the throstle frame of the cotton-spinner.
The thread, as it escapes from the front rollers, gets twisted by the spindle
and flyer, and wound up in constant progression on the bobbin, the motion of the latter
being retarded either by a washer of leather beneath its lower end, or sometimes, as
shown in the figure, by a weighted lever H, suspended from a cord, which embraces the
pulley-groove turned on the lower end of the bobbin. This friction of this cord on the
pulley, which may be varied by changing the length of leverage at which the weight
acts, gives the bobbin the requisite retardation for winding up the yarn.
The bobbin G, at the same time that it has this retarded movement of revolution on
its axis, has another motion up and down on the spindle I, to present itself at different
points to the thread, and to cause the equal distribution of this over the surface of the
bobbin-barrel. This latter motion is given by a double eccentric L, which by turning
slowly on its axis, makes the balance-lever M oscillate, and thereby raises or depresses
the bobbin-rail with its row of spindles. N is a section of the long tin drum, which[505]
extends the whole breadth of the frame, and communicates its rotatory motion, derived
from the steam-pulley, to the spindles, by the intervention of the endless cotton cords O,
as also to the fluted rollers C, F, and to the axis of the heart-shaped or eccentric wheel L,
working in an endless screw.
The ratio of the velocity of the rollers of supply C, with the front or delivering
rollers F, and with the spindles, is proportional to the fineness of the yarn. For low
numbers, the draught is usually fourfold. The speed of the spindles also varies with
the quality of the yarn, according as it is intended for warp or weft; the former
requiring more twist than the latter; but never so much as to cause it to snarl into a
knot, when left free to turn on itself.
One of the most important improvements hitherto made in the spinning of flax is that
for which James Kay, of Preston, obtained a patent in July, 1825. Its peculiar feature
is the maceration in warm water of the slivers or rovings, previously to spinning them,
by conducting them into tin cans, with open bottoms, fitted into circular boxes having
holes like a cullender, and immersed into a trough of warm water. The slivers as they
pass from the rollers are let fall through the cans into these boxes, when they are to be
repeatedly pressed and beaten down by a plunger, or the action of rollers, as may be most
convenient. The material must be thoroughly freed from air, and macerated. After
five or six hours it is to be removed from the water, and placed in its compressed state
at the back part of a drawing and spinning machine. The cake being now turned over,
the end of the roving first deposited in the can is drawn out with care, then raised up,
and passed over a tension roller to the drawing apparatus. The first pair of rollers for
the drawing process merely retains the filaments; while at a distance of two inches and
a half the drawing rollers are placed. Both are fluted for the purpose of taking firm
hold of the material; and the drawing pair is made to move eight times quicker than
the retaining. As the flax fibres have in this state little or no elasticity, and as they
adhere loosely in their macerated condition, the drawing rollers must be placed thus
close to the retaining rollers, and being made to move at a proper speed, produce an
extremely attenuated thread.
The adjoining table represents, in three compartments, the most important rooms in a
flax-mill, viz.:—
I. The tow preparing room.
II. The line preparing room for the long flax.
III. One room of spinning machines as a pattern for the rest.
TOW PREPARING ROOM.
A, lap machine; B, 4-feet breaker card; C, 3 feet 6 inches ditto; D, 3-feet finisher
card, 3 workers; E, cut tow, second drawing, 5 heads; F, cut tow, first drawing, 4 heads;
G, cut tow, reg. roving, 32 spindles; H, 4-feet breaker card; I, 4-feet finisher ditto; K,
long tow, first drawing, 3 heads; L, long tow, second drawing, 4 heads; M, long tow,
roving 4 spindles.
[506]
LINE PREPARING ROOM.
A, cut line, first drawing; B, cut line, second drawing, 4 heads; C, cut line, third
drawing, 5 heads; D, cut line, reg. roving 32 spindles each; E, long line, first drawing;
F, long line, second drawing, 3 heads each; G, long line, third drawing, 4 heads each;
H, long line, roving 16 spindles.
SPINNING ROOM.
I. The line preparing room comprehends:—
- 1. Heckling machines with heckles.
- 2. Line spreaders, or first drawing slivers.
- 3. Frames for the second drawing, of 3 heads each.
- 4. Frames for the third drawing, of 4 heads each.
- 5. Roving frames of 16 spindles each.
- 6. Spare fallers for first drawing with gills.
- 7. Ditto ditto for second and third drawing with ditto.
- 8. Ditto ditto for roving.
II. The cut flax line preparing room:—
- 1. Sets of heckling frames (excentric.)
- 2. Cutting or breaking machine.
- 3. Line spreaders or drawing ditto.
- 4. Frames for second drawing, 4 heads each.
- 5. Ditto es for third ditto,awing,5 ditto.
- 6. Ditto, regulator roving, 32 spindles each.
- 7. Spare fallers with gills for first drawing.
- 8. Ditto, dittors with gills for second and third ditto.
- 9. Ditto, ditto, with gills for roving.
III. Long or uncut flax tow preparation:—
- 1. Lap machine.
- 2. Breaker cards, 4 feet diameter.
- 3. Finisher ditto, ditto.
- 4. Frames for first drawing, 3 heads each.
- 5. Ditto for second drawing, 3 heads each.
- 6. Ditto for roving, 16 spindles each.
- 7. Spare fallers, with gills for first and second drawing.
- 8. Ditto, ditto, ditto,h gillsfor roving.
IV. Cut flax tow preparation:—
- 1. Lap machine.
- 2. First breaker cards, 4 feet diameter.
- 3. Second ditto, ditto, 3 feet 6 inches ditto.
- 4. Finisher cards with 8 workers.
- 5. First drawing frames, of 4 heads each.
- 6. Second ditto, ditto, of 5 ditto.
- 7. Frames for regulator roving, 32 spindles each frame.
- 8. Spare fallers with gills for first and second drawing.
- 9. Ditto, ditto,s with gills for roving.
V. Spinning rooms for both lines and tows:—spindles in frames in a number proportional
to the number of the above preparation machines; and consequently to
the quantity and quality of the flax yarn intended to be spun.
VI. Utensils and tools; such as cards clothing with needle pointed filleting.
Observations upon the above statement of the series of machinery requisite in a
modern flax mill of the most improved construction:—
The long or uncut flax to be spun into yarns averaging 30 leas per lb.
Each heckling machine will produce about 41⁄2 cwts. per day, which would be distributed
into 200 lbs. of line, and 2662⁄3 of tow.
The total with 3 machines would be therefore 600 lbs. of line, and 800 lbs. of tow.
The preceding statement contains three systems of line preparing, each system being
composed of—[507]
- 1 line spreader, or first drawing;
- 1st frame of 3 heads; 2d ditto, 2 slivers each;
- 1 ditto of of 4 ditto;s;3d ditto, ditto ditto;
- 2 ditto rovings of 32 spindles, which are capable of supplying about 640 spinning ditto;
- 1 line spreader being allowed for contingencies.
The above statement contains 3 systems of tow (uncut) preparation, each system being
composed of—
- 1 breaker card;
- 2 finisher ditto,
- 1 frame of first drawing, 3 heads of 4 slivers each;
- 1 dittoe of second ditto, 4 ditto,s of 4 ditto ditto;
- 21⁄3 ditto rovings or 37 spindles, which are capable of supplying about 660 spinning ditto;
- 1 lap machine being sufficient for 2 or 3 systems;
- 1 extra finisher is deemed desirable.
The statement contains 2 systems of heckling machines for cut flax, a system consisting
of either 8 or 10 machines; for the coarser work, 8 machines in succession finer
and finer, are sufficient; but for the finest 10 or 12 are required. Each system will
produce between 2 and 300 lbs. per diem, of raw flax, heckled, divided on the average
into 170 lbs. line, 280 lbs. tow, which will about equal the supply of the 5th system
contained in the statement, each consisting of—
- 1 line spreader or 1st drawing;
- 1 frame 2d drawing; 4 heads 4 slivers each;
- 1 dittoe 3d ditto,ing; 5 dittods4 ditto ditto;
- 1 ditto roving 32 spindles;
and are capable of supplying about 480 ditto, of spinning.
The statement contains 2 systems of tow (cut flax) preparings, each system being
composed of—
- 2 second breaker card;
- 4 finishers ditto;
- 4 frames 1st drawing, 4 heads each 4 slivers;
- 4 dittoes 2d ditto,ing, 5 ditto ditto,h 4 ditto;
- 4 regulator rovings 128 spindles,
and are capable to supply about 1800 spinning ditto.
- 1 first-breaker card and lap frame are sufficient to 2 or 3 systems.
Summary view:—
| Long |
or uncut |
line |
3 |
systems of |
640 |
spindles |
= |
1920 |
|
| Ditto |
— |
tow |
3 |
ditto |
660 |
ditto |
1980 |
3900 |
| Cut |
— |
line |
5 |
ditto |
480 |
ditto |
2400 |
|
| Ditto |
— |
tow |
2 |
ditto |
1800 |
ditto |
3600 |
6000 |
| Total of spinning spindles |
9900 |
3900 spindles, at an average of 30 leas yarn per lb., would turn off 9 leas per spindle
per diem with waste circa 1400 lbs.
6000 spindles, at an average of 100 leas yarn per lb., would turn off 6 leas per spindle
per diem with waste circa 450 lbs.
| Yarns produced: |
£. |
s. |
d. |
| Of average |
30 |
leas |
per lb. |
per week |
circa |
1050 |
boles at 9s. |
|
472 |
10 |
0 |
| Of ditto |
100 |
ditto |
— |
— |
|
1080 |
— |
|
486 |
0 |
0 |
| Total weekly produce |
2130 |
|
958 |
10 |
0 |
| |
£. |
s. |
d. |
|
| Weekly charges, wages, &c. |
150 |
0 |
0 |
|
| Flax |
400 |
0 |
0 |
|
| Weekly expenses |
40 |
0 |
0 |
|
| Interest on 60,000l. 10 per annum |
120 |
0 |
0 |
710 |
0 |
0 |
| Weekly profit |
248 |
10 |
0 |
Measures of flax yarn; and statistics of the linen trade for the United Kingdom.
| One lea of flax yarn at Leeds is |
= |
300 |
yards. |
| One spindle Scotch |
= |
38 |
leas |
= |
11400 |
yards. |
| One rand |
= |
6 |
ditto |
= |
1800 |
ditto. |
| One dozen is 12 rands |
= |
72 |
ditto |
= |
21600 |
ditto. |
When yarn is estimated in Nos. it implies the number of leas in one pound weight;
as in cotton, it means the number of hanks of 840 yards each in one pound.
[508]
Imports of flax and tow, or codilla of hemp and flax, at a duty of 1d. per cwt., in
| |
1834. |
1835. |
1837. |
1838. |
| |
lbs. |
lbs. |
lbs. |
lbs. |
| |
811,722 |
740,814 |
1,529,116 |
1,002,256 |
| Retained for consumption. |
| |
794,272 |
728,143 |
1,532,059 |
1,002,408 |
| Linen yarn exported |
|
2,611,215 |
|
|
Linen manufactures exported, in-
cluding flax yarn, declared value |
£3,208,139 |
£3,645,097 |
£2,613,293 |
FLINT. (Pierre à fusil, Fr; Feuerstein, Germ.) The fracture of this fossil is
perfectly conchoidal, sometimes glossy, and sometimes dull on the surface. It is very
hard, but breaks easily, and affords very sharp-edged splintery fragments; whence it is
a stone which strikes most copious sparks with steel. It is feebly translucid, has so fine
and homogeneous a texture as to bear polishing, but possesses little lustre. Its colours
are very various, but never vivid. The blackish-brown flint is that usually found in
the white chalk. It is nearly black and opaque, loses its colour in the fire, and becomes
grayish-white, and perfectly opaque. Flints occur almost always in nodules or tubercular
concretions of various and very irregular forms. These nodules, distributed in
strata among the chalk, alongside of one another and almost in contact, form extensive
beds; interrupted, indeed, by a multitude of void spaces, so as to present, if freed from
the earthy matter in which they are imbedded, a species of network with meshes, very
irregular both in form and dimension.
The nodules of silex, especially those found in the chalk, are not always homogeneous
and solid. Sometimes there is remarked an organic form towards their centre, as a
madrepore or a shell, which seems to have served as their nucleus; occasionally the
centre is hollow, and its sides are studded over with crystals of quartz, carbonate of
iron, pyrites, concretionary silex or calcedony, filled with pulverulent silica nearly pure,
or silex mixed with sulphur; a very singular circumstance.
Flints are observed to be generally humid when broken immediately after being dug
out of the ground; a property which disappears after a short exposure to the air.
When dried they become more brittle and more splintery, and sometimes their surfaces
get covered at old fractures with a thin film or crust of opaque silex.
Flints calcined and ground to a powder enter into the composition of all sorts of fine
pottery ware.
The next important application of this siliceous substance is in the formation of gun-flints,
for which purpose it must be cut in a peculiar manner. The following characters
distinguish good flint nodules from such as are less fit for being manufactured. The
best are somewhat convex, approaching to globular; those which are very irregular,
knobbed, branched and tuberose, are generally full of imperfections. Good nodules
seldom weigh more than 20 pounds; when less than 2, they are not worth the working.
They should have a greasy lustre, and be particularly smooth and fine grained. The
colour may vary from honey-yellow to blackish-brown, but it should be uniform
throughout the lump, and the translucency should be so great as to render letters legible
through a slice about one-fiftieth of an inch thick, laid down upon the paper. The
fracture should be perfectly smooth, uniform, and slightly conchoidal; the last property
being essential to the cutting out of perfect gun-flints.
Four tools are employed by the gun-flint makers.
First, a hammer or mace of iron with a square head, from 1 to 2 pounds weight, with
a handle 7 or 8 inches long. This tool is not made of steel, because so hard a metal
would render the strokes too harsh, or dry as the workmen say, and would shatter the
nodules irregularly, instead of cutting them with a clean conchoidal fracture.
Second, a hammer with 2 points, made of good steel well hardened, and weighing
from 10 to 16 ounces, with a handle 7 inches long passing through it in such a way
that the points of the hammer are nearer the hand of the workman than the centre of
gravity of the mass.
Third, the disc hammer or roller, a small solid wheel, or flat segment of a cylinder,
parallel to its base, only two inches and a third in diameter, and not more than 12
ounces in weight. It is formed of steel not hardened, and is fixed upon a handle 6
inches long, which passes through a square hole in its centre.
Fourth, a chisel tapering and bevelled at both extremities, 7 or 8 inches long, and 2
inches broad, made of steel not hardened; this is set on a block of wood, which serves
also for a bench to the workmen. To these 4 tools a file must be added, for the purpose
of restoring the edge of the chisel from time to time.
After selecting a good mass of flint, the workman executes the following four operations
on it.
1. He breaks the block. Being seated upon the ground, he places the nodule of flint on[509]
his left thigh, and applies slight strokes with the square hammer to divide it into smaller
pieces of about a pound and a half each, with broad surfaces and almost even fractures.
The blows should be moderate, lest the lump crack and split in the wrong direction.
2. He cleaves or chips the flint. The principal point is to split the flint well, or to
chip off scales of the length, thickness, and shape adapted for the subsequent formation
of gun flints. Here the greatest dexterity and steadiness of manipulation are necessary;
but the fracture of the flint is not restricted to any particular direction, for it may be
chipped in all parts with equal facility.
The workman holds the lump of flint in his left hand, and strikes with the pointed
hammer upon the edges of the great planes produced by the first breaking, whereby the
white coating of the flint is removed in small scales, and the interior body of the flint
is laid bare; after which he continues to detach similar scaly portions from the clean mass.
These scaly portions are nearly an inch and a half broad, two inches and a half
long, and about one-sixth of an inch thick in the middle. They are slightly convex
below, and consequently leave in the part of the lump from which they were separated
a space slightly concave, longitudinally bordered by two somewhat projecting straight
lines or ridges. The ridges produced by the separation of the first scales must naturally
constitute nearly the middle of the subsequent pieces; and such scales alone as have
their ridges thus placed in the middle are fit to be made into gun-flints. In this manner
the workman continues to split or chip the mass of flint in various directions, until
the defects usually found in the interior render it impossible to make the requisite fractures,
or until the piece is too-much reduced to sustain the smart blows by which the
flint is divided.
3. He fashions the gun-flints. Five different parts may be distinguished in a gun-flint.
1. The sloping facet or bevel part, which is impelled against the hammer of the
lock. Its thickness should be from two to three twelfths of an inch; for if it were
thicker it would be too liable to break; and if more obtuse, the scintillations would be
less vivid. 2. The sides, or lateral edges, which are always somewhat irregular. 3.
The back or thick part opposite the tapering edge. 4. The under surface, which is
smooth and rather concave. And 5. The upper face, which has a small square plane
between the tapering edge and the back, for entering into the upper claw of the cock.
In order to fashion the flint, those scales are selected which have at least one of the
above mentioned longitudinal ridges; the workman fixes on one of the two tapering
borders to form the striking edge, after which the two sides of the stone that are to form
the lateral edges, as well as the part that is to form the back, are successively placed on
the edge of the chisel in such a manner that the convex surface of the flint, which rests
on the forefinger of the left hand, is turned towards that tool. Then with the disc hammer
he applies some slight strokes to the flint just opposite the edge of the chisel underneath,
and thereby breaks it exactly along the edge of the chisel.
4. The finishing operation is the trimming, or the process of giving the flint a smooth
and equal edge; this is done by turning up the stone and placing the edge of its tapering
end upon the chisel, in which position it is completed by 5 or 6 slight strokes of
the disc hammer. The whole operation of making a gun-flint, which I have used so
many words to describe, is performed in less than one minute. A good workman is able
to manufacture 1000 good chips or scales in a day (if the flint-balls be of good quality),
or 500 gun-flints. Hence, in the space of 3 days, he can easily cleave and finish 1000
gun-flints without any assistance.
A great quantity of refuse matter is left, for scarcely more than half the scales are
good, and nearly half the mass in the best flints is incapable of being chipped out; so
that it seldom happens that the largest nodules furnish more than 50 gun-flints.
Flints form excellent building materials; because they give a firm hold to the mortar
by their irregularly rough surfaces, and resist, by their nature, every vicissitude of
weather. The counties of Kent, Essex, Suffolk, and Norfolk contain many substantial
specimens of flint-masonry.
FLOSS, of the puddling furnace, is the fluid glass floating upon the iron produced
by the vitrification of the oxides and earths which are present.
FLOSS-SILK (Filoselle, Bourre de soie, or fleuret, Fr.); is the name given to the
portions of ravelled silk broken off in the filature of the cocoons, which is carded like
cotton or wool, and spun into a soft coarse yarn or thread, for making bands, shawls,
socks, and other common silk fabrics. The floss or fleuret, as first obtained, must be
steeped in water, and then subjected to pressure, in order to extract the gummy matter,
which renders it too harsh and short for the spinning wheel. After being dried it is
made still more pliant by working a little oil into it with the hands. It is now ready
to be submitted to the carding engine. See Cotton Manufacture. It is spun upon
the flax wheel.
The female peasants of Lombardy generally wear clothes of homespun floss silk. Of
late years, by improved processes, pretty fine fabrics of this material have been produced[510]
both in England and France. M. Ajac, of Lyons, presented at one of the
French national exhibitions of the objects of industry, a great variety of scarfs and
square shawls, of bourre de sole, closely resembling those of cachemere.
FLOUR; the finely ground meal of wheat, and of any other corns or cerealia. See
Bread.
FLOUR OF WHEAT, Adulterations of, to detect.
The first method is by specific gravity. If potato flour be added, which is frequently
done in France, since a vessel which contains one pound of wheat flour will contain one
pound and a half of the fecula, the proportion of this adulteration may be easily estimated.
If gypsum or ground bones be mixed with the flour, they will not only increase
its density still more; but they will remain after burning away the meal.
The second method is by ascertaining the quantity of gluten which the suspected
sample will afford, by the process prescribed under the article Bread. The two following
chemical criteria may also be employed.
1st. Nitric acid has the property of colouring wheat flour of a fine orange yellow,
whereas it affects the colour neither of fecula nor starch.
2nd. Pure muriatic acid colours good wheat flour of a deep violet, but dissolves fecula
or starch, and forms with it a light, colourless, viscous fluid, decomposable by alkalis.
It may also be observed, that as fecula absorbs less water than flour, this affords a ready
means of detection.
The adulteration with bean or pea flour may be detected by pouring boiling water
upon it, which developes the peculiar smell of these two substances.
FLOWERS (Fleurs, Fr.; Blumen, Germ.) of benzoin, of sulphur, of zinc, &c., is
the appellation given by the older chemists to such substances as were obtained in a
pulverulent or rather minutely crystalline form by the process of sublimation.
FLOWERS, ARTIFICIAL, MANUFACTURE OF. The art of representing
by flowers, leaves, plants, &c., vegetable nature in her ornamental productions, constitutes
the business of the artificial florist. The Italians appear to have been the first
people in Europe who excelled in the art of making artificial flowers; but of late years
the French have been most ingenious in this branch of industry.
Ribbons folded in different forms and of different colours were originally employed for
imitating flowers, by being attached to wire stems. This imitation soon gave way to
that by feathers, which are more delicate in texture, and more capable of assuming a
variety of flower-like figures. But a great difficulty was encountered in dyeing them
with due vivacity. The savages of South America manufacture perfect feather flowers,
derived from the brilliant plumage of their birds, which closely resemble the products of
vegetation. The blossoms and leaves are admirable, while the colours never fade.
The Italians employ frequently the cocoons of the silkworm for this purpose; these
take a brilliant dye, preserve their colour, and possess a transparent velvety appearance,
suitable for petals. Of late years, the French have adopted the finest cambric for
making petals, and the taffeta of Florence for the leaves. M. de Bernardière employs
whalebone in very thin leaves for artificial flowers; and by bleaching and dyeing them of
various hues, he has succeeded in making his imitations of nature to be very remarkable.
The colouring matters used in flower dyeing are the following:—
For red; carmine dissolved in a solution of salt of tartar.
For blue; indigo dissolved in sulphuric acid, diluted and neutralized in part by
Spanish whitening.
For bright yellow; a solution of turmeric in spirit of wine. Cream of tartar
brightens all these colours.
For violet; archil, and a blue bath.
For lilac; archil.
Some petals are made of velvet, and are coloured merely by the application of the
finger dipped in the dye.
FLUATES, more properly fluorides (Eng. and Fr.; Flusssäure, Germ.); compounds
of fluorine and the metals; as fluor spar, for example, which consists of fluorine
and calcium.
FLUOR SPAR. (Chaux fluatée, Fr.; Spath fluor, Germ.) This mineral often
exhibits a variety of vivid colours. It crystallizes in the cubic system; with regular
octahedral and tetrahedral cleavages; spec. grav. 3·1 to 3·2; scratches calc spar, but is
scratched by a steel point; usually phosphorescent with heat; fusible at the blowpipe
into an opaque bead; acted on by the acids, with disengagement of a vapour which
corrodes glass; its solution affords precipitates with the oxalates, but not with ammonia.
Its constituents are, fluorine, 48·13; calcium, 51·87 in 100.
Fluor spar occurs subordinate to metallic veins; as to those of lead, in Derbyshire;
of tin, in Saxony and Bohemia; but it is found also in masses or veins, either in crystalline
rocks, associated with quartz, heavy spar, &c., as in Auvergne, Forez, Vosges,
Norberg in Sweden; Norway; Petersburg; near Hall; Gourock, in Scotland, &c.; or[511]
among secondary limestones, slates, and sandstones, in Derbyshire, Cumberland, Cornwall,
and New Jersey. It exists also in the amygdaloids of Scotland, and in the volcanic
products of Monte Somma at Vesuvius. The variously coloured specimens, called
Derbyshire spar, are worked upon the turning lathe into vases and other ornamental
objects.
FLUX, (Eng. and Fr.; Fluss, Germ.) signifies any substance capable of promoting
the fusion of earths or metallic ores by heat. White flux is the residuum of the deflagration
in a red hot crucible, of a mixture of two parts of nitre, and one of cream of
tartar. It is in fact merely a carbonate of potash. Black flux is obtained when equal
parts of nitre and tartar are deflagrated. It owes its colour to the carbonaceous matter
of the tartaric acid, which remains unconsumed; the quantity of nitre being too
small for that purpose. The presence of the charcoal renders this preparation a convenient
flux for reducing calcined or oxidized ores to the metallic state. Limestone, fluor-spar,
borax, and several earthy or metallic oxides are employed as fluxes in metallurgy.
FLY POWDER; the black coloured powder obtained by the spontaneous oxidizement
of metallic arsenic in the air.
FODDER; is the name of a weight by which lead and some other metals are sold
in this country. It varies in its amount in different parts of the kingdom; being in
Northumberland estimated at 21 cwts., and in other counties 22, 23 or even more cwts.
FONDUS; is the name given by the French to a particular style of calico printing
resembling the rainbow, in which the colours are graduated or melted (fondus) into one
another, as in the prismatic spectrum. See Paper hangings, for a description of the
process.
FORGE; (Eng. and Fr.; Feuer, Germ.) is the name either of the furnace, where
wrought iron is hammered and fashioned with the aid of heat, or the great workshop
where iron is made malleable. The former is called a smith’s forge, the latter a
shingling mill. See Iron.
Fig. 466. represents a portable
truck forge of a very commodious
construction. A is the cylindric
leather bellows, pressed down by a
helical spring, and worked by
means of the handle at B, which
moves the horizontal shaft C, with
its two attached semicircular levers
and chains. D, is the pipe which
conducts the blast to the nozzle at
E. The hearth may be covered
with a thin fire-tile or with cinders.
F is a vice fixed to the strong
rectangular frame. This apparatus
answers all the ordinary purposes
of a smith’s forge; and is
peculiarly adapted to ships, and to
the execution of engineering jobs
upon railways, or in the country.
The height is 2 feet 6 inches; the
length is 2 feet 9 inches; the
width 2 feet. Weight about 2
cwt.
FORMIATES; are compounds of formic acid, with the salifiable bases. Many of
them are susceptible of crystallization.
FORMIC ACID; (Acide Formique, Fr.; Ameisensäure, Germ.) exists in the
bodies of wood ants, associated with the malic or acid of apples. The artificial formation
of this animal secretion, is one of the most remarkable triumphs of modern
chemistry. If 10 parts of tartaric acid, 14 of black oxide of manganese, 15 of concentrated
sulphuric acid, and from 20 to 30 of water be mixed and distilled in a retort, formic acid
will be the liquid product; while carbonic acid will be disengaged. It may also be generated
from other mixtures. This acid is transparent and colourless, of a pungent sour
smell, a strongly acid taste, of specific gravity 1·1168 at 60° F., and may be re-distilled
without suffering any change. It contains in its most concentrated form 193⁄4 per cent.
of water. The dry acid, as it exists in the formiates, is composed of 32·54 carbon, 2·68
hydrogen, and 64·78 oxygen; or of two volumes carbonic oxide gas, and one volume
of vapour of water. It reduces the oxides of mercury and silver to the metallic state.
It has not hitherto been applied to any use in the arts.
FORMULÆ, CHEMICAL, are symbols representing the different substances,
simple and compound.
[512]
| Name. |
Formula. |
Oxygen
= 100. |
Hydrogen
= 1. |
| Oxygen |
O |
100·000 |
16·026 |
| Hydrogen |
H |
6·2398 |
1·000 |
| 2H |
12·4796 |
2·000 |
| Nitrogen |
N |
88·518 |
14·186 |
| 2N |
177·086 |
28·372 |
| Phosphorus |
P |
196·155 |
31·436 |
| 2P |
392·310 |
68·872 |
| Chlorine |
Cl |
221·325 |
35·470 |
| 2Cl |
442·650 |
70·940 |
| Iodine |
I |
768·781 |
123·206 |
| 2I |
1537·562 |
246·412 |
| Carbon |
C |
76·437 |
12·250 |
| 2C |
152·875 |
24·500 |
| Boron |
B |
135·983 |
21·793 |
| 2B |
271·966 |
43·586 |
| Silicon |
Si |
277·478 |
44·469 |
| Selenium |
Se |
494·582 |
79·263 |
| Arsenic |
As |
470·042 |
75·329 |
| 2As |
940·084 |
150·659 |
| Chromium |
Cr |
351·819 |
56·383 |
| 2Cr |
703·638 |
112·766 |
| Molybdenum |
Mo |
598·525 |
95·920 |
| Tungstenium |
Tu or W |
1183·200 |
189·621 |
| Antimony |
Sb |
806·452 |
129·243 |
| 2Sb |
1612·904 |
258·486 |
| Tellurium |
Te |
806·452 |
129·243 |
| Tantalum |
Ta |
1153·715 |
184·896 |
| 2Ta |
2307·430 |
369·792 |
| Titanium |
Ti |
389·092 |
62·356 |
| Gold (aurum) |
Au |
1243·013 |
199·207 |
| 2Au |
2486·026 |
398·415 |
| Platina |
Pt |
1215·220 |
194·753 |
| Rhodium |
R |
750·680 |
120·305 |
| 2R |
1501·360 |
240·610 |
| Palladium |
Pd |
714·618 |
114·526 |
| Silver (argentum) |
Ag |
1351·607 |
216·611 |
| Mercury (hydrargyrus) |
Hg |
1265·822 |
202·863 |
| 2Hg |
2531·645 |
405·725 |
| Copper (cuprum) |
Cu |
395·695 |
63·415 |
| 2Cu |
791·390 |
126·829 |
| Uranium |
U |
2711·360 |
434·527 |
| 2U |
5422·720 |
869·154 |
| Bismuth |
Bi |
1330·376 |
213·208 |
| 2Bi |
2660·752 |
426·416 |
| Tin (stannum) |
Sn |
735·294 |
117·839 |
| Lead (plumbum) |
Pb |
1294·498 |
207·458 |
| 2Pb |
2588·996 |
414·917 |
| Cadmium |
Cd |
696·767 |
111·665 |
| Zinc |
Zn |
403·226 |
64·621 |
| Nickel |
Ni |
369·675 |
59·245 |
| Cobalt |
Co |
368·991 |
59·135 |
| 2Co |
737·982 |
118·270 |
| Iron (ferrum) |
Fe |
339·213 |
54·363 |
| 2Fe |
678·426 |
108·725 |
| Manganese |
Mn |
355·787 |
57·019 |
| 2Mn |
711·575 |
114·038 |
| Cerium |
Ce |
574·718 |
92·105 |
| 2Ce |
1149·436 |
184·210 |
| Zirconium |
Zr |
420·238 |
67·348 |
| 2Zr |
840·476 |
134·696 |
| Yttrium |
Y |
401·840 |
64·395 |
| Beryllium (glucinum)[513] |
Be |
331·479 |
53·123 |
| 2Be |
662·958 |
106·247 |
| Aluminum |
Al |
171·167 |
27·431 |
| 2Al |
342·234 |
54·863 |
| Magnesium |
Mg |
158·353 |
25·378 |
| Calcium |
Ca |
256·019 |
41·030 |
| Strontium |
Sr |
547·285 |
87·709 |
| Baryum |
Ba |
856·88 |
137·325 |
| Lithium |
L |
127·757 |
20·474 |
| Natrium (sodium) |
Na |
290·897 |
46·620 |
| 2Na |
581·794 |
93·239 |
| Kalium (potassium) |
K |
489·916 |
78·515 |
| Ammonia |
2N 2H3 |
214·474 |
34·372 |
| Cyanogen |
2NC |
329·911 |
52·872 |
| Sulphuretted hydrogen |
2HS |
213·644 |
34·239 |
| Hydrochloric acid |
2HCl |
455·129 |
72·940 |
| Hydrocyanic acid |
2HNC |
342·390 |
54·872 |
| Water |
2. |
112·479 |
18·026 |
| 2H |
| Protoxide of nitrogen |
2. |
277·036 |
44·398 |
| 2N |
| Deutoxide of nitrogen |
. |
188·518 |
30·212 |
| N |
| Nitrous acid |
2... |
477·036 |
76·449 |
| 2N |
| Nitric acid |
.·.·. |
677·036 |
108·503 |
| 2N |
| Hyposulphurous acid |
. |
301·165 |
48·265 |
| S |
| Sulphurous acid |
.. |
401·165 |
64·291 |
| S |
| Hyposulphuric acid |
.·.·. |
902·330 |
144·609 |
| 2S |
| Sulphuric acid |
... |
501·165 |
80·317 |
| S |
| Phosphoric acid |
.·.·. |
892·310 |
143·003 |
| 2P |
| Chloric acid |
.·.·. |
942·650 |
151·071 |
| 2Cl |
| Perchloric acid |
::: |
1042·650 |
167·097 |
| 2Cl |
| Iodic acid |
.·.·. |
2037·562 |
326·543 |
| 2I |
| Carbonic acid |
.. |
276·437 |
44·302 |
| C |
| Oxalic acid |
2... |
452·875 |
72·578 |
| 2C |
| Boracic acid |
2::: |
871·966 |
139·743 |
| 2B |
| Silicic acid |
... |
577·478 |
92·548 |
| Si |
| Selenic acid |
.. |
694·582 |
111·315 |
| Se |
| Arsenic acid |
.·.·. |
1440·084 |
230·790 |
| 2As |
| Protoxide of chrome |
2... |
1003·638 |
160·840 |
| 2Cr |
| Chromic acid |
... |
651·819 |
104·462 |
| Cr |
| Molybdic acid |
... |
898·525 |
143·999 |
| Mo |
| Tungstic, or wolfram acid |
... |
1483·200 |
237·700 |
| W |
| Oxide of antimony |
2... |
1912·904 |
306·565 |
| 2Sb |
| Antimonious acid |
.. |
1006·452 |
161·296 |
| Sb |
| .... |
2012·904 |
322·591 |
| 2Sb |
| Antimonic acid[514] |
2.·.·. |
2112·904 |
338·617 |
| 2Sb |
| Oxide of tellurium |
.. |
1006·452 |
161·296 |
| Te |
| Tantalic acid |
... |
2607·430 |
417·871 |
| 2Ta |
| Titanic acid |
.. |
589·092 |
94·409 |
| Ti |
| Protoxide of gold |
. |
2586·026 |
414·441 |
| 2Au |
| Peroxide of gold |
... |
2786·026 |
446·493 |
| 2Au |
| Oxide of platina |
.. |
1415·220 |
226·086 |
| Pt |
| Oxide of rhodium |
2... |
1801·360 |
228·689 |
| 2R |
| Oxide of palladium |
. |
814·618 |
130·552 |
| Pd |
| Oxide of silver |
. |
1451·607 |
232·637 |
| Ag |
| Protoxide of mercury |
. |
2631·645 |
421·752 |
| 2Hg |
| Peroxide of mercury |
. |
1365·822 |
218·889 |
| Hg |
| Protoxide of copper |
. |
801·390 |
142·856 |
| 2Cu |
| Peroxide of copper |
. |
495·695 |
79·441 |
| Cu |
| Protoxide of uranium |
. |
2811·360 |
450·553 |
| U |
| Peroxide of uranium |
2... |
5722·720 |
917·132 |
| 2U |
| Oxide of bismuth |
2... |
2960·752 |
474·49 |
| 2Bi |
| Protoxide of tin |
. |
835·294 |
133·866 |
| Sn |
| Peroxide of tin |
.. |
935·294 |
149·892 |
| Sn |
| Oxide of lead |
. |
1394·498 |
223·484 |
| Pb |
| Minium |
... |
2888·996 |
462·995 |
| 2Pb |
| Brown oxide of lead |
.. |
1494·498 |
239·511 |
| Pb |
| Oxide of cadmium |
. |
796·767 |
127·691 |
| Cd |
| Oxide of zinc |
. |
503·226 |
80·649 |
| Zn |
| Oxide of nickel |
. |
469·675 |
75·271 |
| Ni |
| Oxide of cobalt |
. |
468·991 |
75·161 |
| Co |
| Peroxide of cobalt |
... |
1037·982 |
166·349 |
| 2Co |
| Protoxide of iron |
. |
439·213 |
70·389 |
| Fe |
| Peroxide of iron |
... |
978·426 |
156·804 |
| 2Fe |
| Protoxide of manganese |
. |
455·787 |
73·045 |
| Mn |
| Oxide of manganese |
... |
1011·575 |
162·117 |
| 2Mn |
| Peroxide of manganese |
.. |
555·787 |
89·071 |
| Mn |
| Manganesic acid |
.·.·. |
1211·575 |
194·169 |
| 2Mn |
| Protoxide of cerium |
. |
674·718 |
108·132 |
| Ce |
| Oxide of cerium |
... |
1449·436 |
232·289 |
| 2Ce |
| Zirconia |
... |
1140·476 |
182·775 |
| 2Zr |
| Yttria |
. |
501·840 |
80·425 |
| Y |
| Glucina, or berryllia[515] |
... |
962·958 |
154·325 |
| 2Be |
| Alumina |
... |
642·334 |
109·942 |
| 2Al |
| Magnesia |
. |
258·353 |
41·404 |
| Mg |
| Lime |
. |
356·019 |
57·056 |
| Ca |
| Strontia |
. |
647·285 |
103·735 |
| Sr |
| Baryta |
. |
956·880 |
153·351 |
| Ba |
| Lithia |
. |
227·757 |
36·501 |
| L |
| Natron, or soda |
. |
390·897 |
62·646 |
| Na |
| Peroxide of sodium |
... |
881·794 |
141·318 |
| 2Na |
| Kali, or potassa |
. |
589·916 |
94·541 |
| K |
| Peroxide of potassium |
... |
789·916 |
126·593 |
| K |
| Sulphate of potassa |
. ... |
1091·081 |
174·859 |
| K S |
| Protosulphate of iron |
. ... |
940·378 |
150·706 |
| Fe S |
| Persulphate of iron |
... ... |
2481·906 |
397·754 |
| 2Fe S3 |
| Protochloride of iron |
Fe 2Cl |
781·863 |
125·303 |
| Perchloride of iron |
2Fe 2Cl3 |
2006·376 |
321·545 |
| Protochloride of mercury |
2Hg 2Cl |
2974·295 |
476·666 |
| Perchloride of mercury |
Hg 2Cl |
1708·472 |
273·803 |
| Ferrocyanide of iron |
Fe2NC + 2K2NC |
2308·778 |
370·008 |
| Alum |
. ... + 2... ... + 24 2 . |
5936·406 |
951·378 |
| K S + 2Al S3 + 24 2H |
| Felspar |
. ... + 2 ... ... |
3542·162 |
567·673 |
| K Si + 2Al Si3 |
FOUNDING of metals, chiefly of Iron. The operations of an iron foundry consist
in re-melting the pig-iron of the blast furnaces, and giving it an endless variety of forms,
by casting it in moulds of different kinds, prepared in appropriate manners. Coke is the
only kind of fuel employed to effect the fusion of the cast iron.
The essential parts of a well-mounted iron foundry, are,
1. Magazines for pig irons of different qualities, which are to be mixed in certain
proportions, for producing castings of peculiar qualities; as also for coal, coke, sands,
clay, powdered charcoal, and cow-hair for giving tenacity to the loam mouldings.
2. One or more coke ovens.
3. A workshop for preparing the patterns and materials of the moulds. It should
contain small edge millstones for grinding and mixing the loam, and another mill
for grinding coal and charcoal.
4. A vast area, called properly the foundry, in which the moulds are made and filled
with the melted metal. These moulds are in general very heavy, consisting of two parts
at least, which must be separated, turned upside down several times, and replaced very
exactly upon one another. The casting is generally effected by means of large ladles
or pots, in which the melted iron is transported from the cupola, where it is fused.
Hence the foundry ought to be provided with cranes, having jibs movable in every
direction.
5. A stove in which such moulds may be readily introduced, as require to be entirely
deprived of humidity, and where a strong heat may be uniformly maintained.
6. Both blast and air furnaces, capable of melting speedily the quantity of cast-iron
to be employed each day.
7. A blowing machine to urge the fusion in the furnaces.
Fig. 467. represents the general plan of a well-mounted foundry.
a, is a cupola furnace of which the section and view will be afterwards given; it is
capable of containing 5 tons of cast-iron.
a′, is a similar furnace, but of smaller dimensions, for bringing down 13⁄4 tons.
a′′, is a furnace like the first, in reserve for great castings.
[516]
b, b, b, b, a vast foundry apartment, whose floor to a yard in depth, is formed of sand
and charcoal powder, which have already been used for castings, and are ready for heaping
up into a substratum, or to be scooped out
when depth is wanted for the moulds. There are
besides several cylindrical pits, from five to seven
yards in depth, placed near the furnaces. They
are lined with brick work, and are usually left full
of moulding sand. They are emptied in order to
receive large moulds, care being had that their top
is always below the orifice from which the melted
metal is tapped.
These moulds, and the ladles full of melted
metal are lifted and transported by the arm of one
or more men, when their weight is moderate; but
if it be considerable, they are moved about by
cranes whose vertical shafts are placed at c, d, e,
in correspondence, so that they may upon occasion
transfer the load from one to another. Each crane
is composed principally of an upright shaft, embraced at top by a collet, and turning
below upon a pivot in a step; next of a horizontal beam, stretched out from nearly the
top of the former, with an oblique stay running downwards, like that of a gallows. The
horizontal beam supports a movable carriage, to which the tackle is suspended for raising
the weights. This carriage is made to glide backwards or forwards along the beam by
means of a simple rack and pinion mechanism, whose long handle descends within reach
of the workman’s hand.
By these arrangements in the play of the three cranes, masses weighing five tons may
be transported and laid down with the greatest precision upon any point whatever in the
interior of the three circles traced upon fig. 467. with the points c, d, e, as centres.
c, d, e, are the steps, upon which the upright shafts of the three cranes rest and turn.
Each shaft is 16 feet high.
f, f, is the drying stove, having its floor upon a level with that of the foundry.
f′, f′, is a supplementary stove for small articles.
g, g, g, are the coaking ovens.
h, is the blowing machine or fan.
i, is the steam-engine, for driving the fan, the loam-edge stones,
k, and the charcoal mill.
i′′, are the boiler and the furnace of the engine.
k′, workshop for preparing the loam and other materials of moulding.
l, is the apartment for the patterns.
The pig-iron, coals, &c. are placed either under sheds or in the open air, round the
above buildings; where are also a smith’s forge, a carpenter’s shop, and an apartment
mounted with vices for chipping and rough cleaning the castings by chisels and files.
Such a foundry may be erected upon a square surface of about 80 yards in each side,
and will be capable, by casting in the afternoon and evening of each day, partly in large
and partly in small pieces, of turning out from 700 to 800 tons per annum, with an establishment
of 100 operatives, including some moulding boys.
Of making the Moulds.—1. Each mould ought to present the exact form of its object.
2. It should have such solidity that the melted metal may be poured into it, and fill
it entirely without altering its shape in any point.
3. The air which occupies the vacant spaces in it, as well as the carburetted gases
generated by the heat, must have a ready vent; for if they are but partially confined,
they expand by the heat, and may crack, even blow up the moulds, or at any rate
become dispersed through the metal, making it vesicular and unsound.
There are three distinct methods of making the moulds:—
1. In green sand; 2. In baked sand; 3. In loam.
To enumerate the different means employed to make every sort of mould exceeds the
limits prescribed to this work. I shall merely indicate for each species of moulding,
what is common to all the operations; and I shall then describe the fabrication of a few
such moulds as appear most proper to give general views of this peculiar art.
Moulding in green sand.—The name green is given to a mixture of the sand as it
comes from its native bed, with about one twelfth its bulk of coal reduced to powder,
and damped in such a manner as to form a porous compound, capable of preserving the
forms of the objects impressed upon it. This sand ought to be slightly argillaceous, with
particles not exceeding a pin’s head in size. When this mixture has once served for a
mould, and been filled with metal, it cannot be employed again except for the coarsest
castings, and is generally used for filling up the bottoms of fresh moulds.
For moulding any piece in green sand, an exact pattern of the object must be prepared[517]
in wood or metal; the latter being preferable, as not liable to warping,
swelling, or shrinkage.
A couple of iron frames form a case or box, which serves as an envelope to the mould.
Such boxes constitute an essential and very expensive part of the furniture of a foundry.
It is a rectangular frame, without bottom or lid, whose two largest sides are united by
a series of cross bars, parallel to each other, and placed from 6 to 8 inches apart.
The two halves of the box carry ears corresponding exactly with one another; of
which one set is pierced with holes, but the other has points which enter truly into
these holes, and may be made fast in them by cross pins or wedges, so that the pair
becomes one solid body. Within this frame there is abundance of room for containing
the pattern of the piece to be moulded with its encasing sand, which being rammed into
the frame, is retained by friction against the lateral faces and cross bars of the mould.
When a mould is to be formed, a box of suitable dimensions is taken asunder, and
each half, No. 1. and No. 2., is laid upon the floor of the foundry. Green sand is thrown
with a shovel into No. 1. so as to fill it; when it is gently pressed in with a rammer.
The object of this operation is to form a plane surface upon which to lay in the pattern
with a slight degree of pressure, varying with its shape. No. 1. being covered with sand,
the frame No. 2. is laid upon it, so as to form the box. No. 2. being now filled carefully
with the green sand, the box is inverted, so as to place No. 1. uppermost, which is
then detached and lifted off in a truly vertical position; carrying with it the body of
sand formed at the commencement of the operation. The pattern remains imbedded in
the sand of No. 2., which has been exactly moulded upon a great portion of its surface.
The moulder condenses the sand in the parts nearest to the pattern, by sprinkling a little
water upon it, and trimming the ill-shaped parts with small iron trowels of different
kinds. He then dusts a little well-dried finely-sifted sand over all the visible surface of
the pattern, and of the sand surrounding it; this is done to prevent adhesion when he
replaces the frame No. 1.
He next destroys the preparatory smooth bed or area formed in this frame, covers the
pattern with green sand, replaces the frame 1. upon 2. to reproduce the box, and proceeds
to fill and ram No. 1., as he had previously done No. 2. The object of this operation
is to obtain very exactly a concavity in the frame No. 1., having the shape of the part
of the model impressed coarsely upon the surface formed at the beginning, and which
was meant merely to support the pattern and the sand sprinkled over it, till it got
imbedded in No. 2.
The two frames in their last position, along with their sand, may be compared to a box
of which No. 1. is the lid, and whose interior is adjusted exactly upon the enclosed
pattern.
If we open this box, and after taking out the pattern, close its two halves again, then
pour in melted metal till it fill every void space, and become solid, we shall obviously
attain the wished-for end, and produce a piece of cast iron similar to the pattern. But
many precautions must still be taken before we can hit this point. We must first lead
through the mass of sand in the frame No. 1., one or more channels for the introduction
of the melted metal; and though one may suffice for this purpose, another must be made
for letting the air escape. The metal is run in by several orifices at once, when the
piece has considerable surface, but little thickness, so that it may reach the remotest
points sufficiently hot and liquid.
The parts of the mould near the pattern must likewise be pierced with small holes, by
means of wires traversing the whole body of the sand, in order to render the mould more
porous, and to facilitate the escape of the air and the gases. Then, before lifting off
the frame No. 1., we must tap the pattern slightly, otherwise the sand enclosing it would
stick to it in several points, and the operation would not succeed. These gentle jolts
are given by means of one or more pieces of iron wire which have been screwed vertically
into the pattern before finally ramming the sand into the frame No. 1., or which enter
merely into holes in the pattern. These pieces are sufficiently long to pass out through
the sand when the box is filled; and it is upon their upper ends that the horizontal
blows of the hammer are given; their force being regulated by the weight and magnitude
of the pattern. These rods are then removed by drawing them straight out; after
which the frame No. 1. may be lifted off smoothly from the pattern.
The pattern itself is taken out, by lifting it in all its parts at once, by means of screw
pins adjusted at the moment. This manœuvre is executed, for large pieces, almost
always by several men, who while they lift the pattern with one hand, strike it with the
other with small repeated blows to detach the sand entirely, in which it is generally more
engaged than it was in that of the frame No. 1. But in spite of all these precautions,
there are always some degradations in one or other of the two parts of the mould; which
are immediately repaired by the workman with damp sand, which he applies and presses
gently with his trowel, so as to restore the injured forms.
Hitherto I have supposed all the sand rammed into the box to be of one kind; but[518]
from economy, the green sand is used only to form the portion of the mould next the
pattern, in a stratum of about an inch thick; the rest of the surrounding space is filled
with the sand of the floor which has been used in former castings. The interior layer
round the pattern is called in this case, new sand.
It may happen that the pattern is too complex to be taken out without damaging the
mould, by two frames alone; then 3 or more are mutually adjusted to form the box.
When the mould, taken asunder into two or more parts, has been properly repaired,
its interior surface must be dusted over with wood charcoal reduced to a very fine
powder, and tied up in a small linen bag, which is shaken by hand. The charcoal is
thus sifted at the moment of application, and sticks to the whole surface which has been
previously damped a little. It is afterwards polished with a fine trowel. Sometimes,
in order to avoid using too much charcoal, the surfaces are finally dusted over with sand,
very finely pulverized, from a bag like the charcoal. The two frames are now replaced with
great exactness, made fast together by the ears, with wedged bolts laid truly level, or at
the requisite slope, and loaded with considerable weights. When the casting is large,
the charcoal dusting as well as that of fine sand, is suppressed. Every thing is now
ready for the introduction of the fused metal.
Moulding in baked or used sand.—The mechanical part of this process is the same as
of the preceding. But when the castings are large, and especially if they are tall, the
hydrostatic pressure of the melted metal upon the sides of the mould cannot be counteracted
by the force of cohesion which the sand acquires by ramming. We must in that
case adapt to each of these frames a solid side, pierced with numerous small holes to give
issue to the gases. This does not form one body with the rest of the frame, but is attached
extemporaneously to it by bars and wedged bolts. In general no ground coal is mixed
with this sand. Whenever the mould is finished, it is transferred to the drying stove,
where it may remain from 12 to 24 hours at most, till it be deprived of all its humidity.
The sand is then said to be baked, or annealed. The experienced moulder knows how
to mix the different sands placed at his disposal, so that the mass of the mould as it
comes out of the stove, may preserve its form, and be sufficiently porous. Such moulds
allow the gases to pass through them much more readily than those made of green
sand; and in general the castings they turn out are less vesicular, and smoother upon
the surface. Sometimes in a large piece, the three kinds of moulding, that in green
sand, in baked sand, and in loam, are combined to produce the best result.
Moulding in loam.—This kind of work is executed from drawings of the pieces to be
moulded, without being at the expense of making patterns. The mould is formed of a
pasty mixture of clay, water, sand, and cow’s hair, or other cheap filamentous matter,
kneaded together in what is called the loam mill. The proportions of the ingredients
are varied to suit the nature of the casting. When the paste requires to be made very
light, horse dung or chopped straw is added to it.
I shall illustrate the mode of fabricating loam moulds, by a simple case, such as that
of a sugar pan. Fig. 468. is the pan. There is laid upon the floor of the foundry, an
annular platform of cast-iron a b, fig. 469.; and upon its centre c, rests the lower extremity
of a vertical shaft, adjusted so as to turn freely upon itself, while it makes a
wooden pattern e f, fig. 470., describe a surface of revolution identical with the internal
surface reversed of the boiler intended to be made. The outline e g, of the pattern is
fashioned so as to describe the surface of the edge of the vessel. Upon the part a d b d,
fig. 469., of the flat cast-iron ring, there must next be constructed, with bricks laid either
flat or on their edge, and clay, a kind of dome, h i k, fig. 470., from two to four inches
thick, according to the size and weight of the piece to be moulded. The external surface
of the brick dome ought to be everywhere two inches distant at least, from the surface
described by the arc e, f. Before building up the dome to the point i, coals are to be
placed in its inside upon the floor, which may be afterwards kindled for drying the
mould. The top is then formed, leaving at i, round the upright shaft of revolution,
only a very small outlet. This aperture, as also some others left under the edges of the
iron ring, enable the moulder to light the fire when it becomes necessary, and to graduate
it so as to make it last long enough without needing more fuel, till the mould
be quite finished and dry. The combustion should be always extremely slow.
Over the brick dome a pasty layer of loam is applied, and rounded with the mould[519]
g e f; this surface is then coated with a much smoother loam, by means of the concave
edge of the same mould. Upon the latter surface, the inside of the sugar pan is cast;
the line e g having traced, in its revolution, a ledge m. The fire is now kindled, and as
the surface of the mould becomes dry, it is painted over by a brush, with a mixture of
water, charcoal powder, and a little clay, in order to prevent adhesion between the surface
already dried and the coats of clay about to be applied to it. The board g e f is
now removed, and replaced by another, g′ e′ f′, fig. 471., whose edge e′ f′ describes the
outer surface of the pan. Over the surface e, f, a layer of loam is applied, which is
turned and polished so as to produce the surface of revolution e′ f′, as was done for the
surface e f; only in the latter case, the line e′ g′ of the board does not form a new
shoulder, but rubs lightly against m.
The layer of loam included between the two surfaces e f, e′ f′, is an exact representation
of the sugar pan. When this layer is well dried by the heat of the interior fire, it
must be painted like the former. The upright shaft is now removed, leaving the small
vent hole through which it passed to promote the complete combustion of the coal.
There must be now laid horizontally upon the ears of the platform d d, fig. 469., another
annular platform p q, like the former, but a little larger, and without any cross-bar.
The relative position of these two platforms is shown in fig. 473. Upon the surface
e′ f′, fig. 472., a new layer of loam is laid, two inches thick, of which the surface is
smoothed by hand. Then upon the platform p q, fig. 473., a brick vault is constructed,
whose inner surface is applied to the layer of loam. This contracts a strong adherence with
the bricks which absorb a part of its moisture, while the coat of paint spread over the
surface e′ f′, prevents it from sticking to the preceding layers of loam. The brick
dome ought to be built solidly.
The whole mass is now to be thoroughly dried by the continuance of the fire, the
draught of which is supported by a small vent left in the upper part of the new dome; and
when all is properly dry, the two iron platforms are adjusted to each other by pin points,
and p q is lifted off, taking care to keep it in a horizontal position. Upon this platform
are removed the last brick dome, and the layer of loam which had been applied next to
it; the latter of which represents exactly by its inside the mould of the surface e′ f′, that
is of the outside of the pan. The crust contained between e f and e′ f′ is broken away,
an operation easily done without injury to the surface e f, which represents exactly the
inner surface of the pan; or only to the shoulder m, corresponding to the edge of the
vessel. The top aperture through which the upright shaft passed must be now closed;
only the one is kept open in the portion of the mould lifted off upon p q; because through
this opening the melted metal is to be poured in the process of casting. The two platforms
being replaced above each other very exactly, by means of the adjusting pin-points,
the mould is completely formed, and ready for the reception of the metal.
When the object to be moulded presents more complicated forms than the one now
chosen for the sake of illustration, it is always by analogous processes that the workman
constructs his loam moulds, but his sagacity must hit upon modes of executing many
things which at first sight appear to be scarcely possible. Thus, when the forms of the
interior and exterior do not permit the mould to be separated in two pieces, it is divided
into several, which are nicely fitted with adjusting pins. More than two cast-iron
rings or platforms are sometimes necessary. When ovals or angular surfaces must be
traced instead of those of revolution, no upright shaft is used, but wooden or cast-iron
guides made on purpose, along which the pattern cut-out board is slid according to the
drawing of the piece. Iron wires and claws are often interspersed through the brick
work to give it cohesion. The core, kernel, or inner mould of a hollow casting is frequently
fitted in when the outer shell is moulded. I shall illustrate this matter in the
case of a gas-light retort, fig. 474. The core of the retort ought to have the form e e e e,
and be very solid, since it cannot be fixed in the outer mould, for the casting, except in
the part standing out of the retort towards m m. It must be modelled in loam, upon
a piece of cast-iron called a lantern, made expressly for this purpose. The lantern is a
cylinder or a truncated hollow cone of cast iron, about half an inch thick; and differently
shaped for every different core. The surface is perforated with holes of about
half an inch in diameter. It is mounted by means of iron cross bars, upon an iron axis,[520]
which traverses it in the direction of its length. Fig. 475. represents a horizontal
section through the axis of the core; g h is the axis of the lantern, figured itself at i k
k i; o i i o is a kind of disc or dish, perpendicular to the axis, open at i i, forming one
piece with the lantern, whose circumference o o presents a curve similar to the section
of the core, made at right angles to its axis. We shall see presently the two uses for
which this dish is intended. The axis g h is laid upon two gudgeons, and handles are
placed at each of its extremities, to facilitate the operation in making the core. Upon
the whole surface of the lantern, from the point h to the collet formed by the dish, a hay
cord as thick as the finger is wound. Even two or more coils may be applied, as
occasion requires, over which loam is spread to the exact form of the core, by applying
with the hand a board, against the dish o o, with its edge cut out to the desired shape;
as also against another dish, adjusted at the time towards h; while by means of the
handles a rotatory movement is given to the whole apparatus.
The hay interposed between the lantern and the loam, which represents the crust of
the core, aids the adhesion of the clay with the cast iron of the lantern, and gives passage
to the holes in its surface, for the air to escape through in the casting.
When the core is finished, and has been put into the drying stove, the axis g h is
taken out, then the small opening which it leaves at the point h, is plugged with clay.
This is done by supporting the core by the edges of the dish, in a vertical position. It
is now ready to be introduced into the hollow mould of the piece.
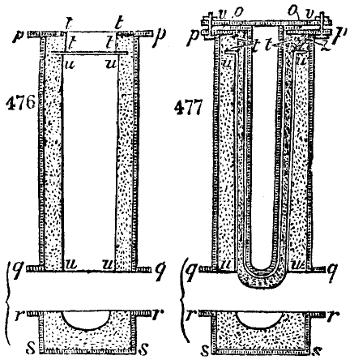
This mould executed in baked sand consists of three pieces, two of which absolutely
similar, are represented, fig. 476., at p q, the third is
shown at r s. The two similar parts p q, present each
the longitudinal half of the nearly cylindrical portion
of the outer surface of the gas retort; so that when
they are brought together, the cylinder is formed; r s
contains in its cavity the kind of hemisphere which
forms the bottom of the retort. Hence, by adding this
part of the mould to the end of the two others, the
resulting apparatus presents in its interior, the exact
mould of the outside of the retort; an empty cylindrical
portion t t, whose axis is the same as that of the
cylinder u u, and whose surface, if prolonged, would be
every where distant from the surface u u, by a quantity
equal to the desired thickness of the retort. The
diameter of the cylinder t t is precisely equal to that of the core, which is slightly conical,
in order that it may enter easily into this aperture t t, and close it very exactly
when it is introduced to the collet or neck.
The three parts of the mould and the core being prepared, the two pieces p q, must
first be united, and supported in an upright position; then the core must be let down
into the opening t t, fig. 477. When the plate or disc o o of the core is supported upon
the mould, we must see that the end of the core is every where equally distant from the
edge of the external surface u u, and that it does not go too far beyond the line q q.
Should there be an inaccuracy, we must correct it by slender iron slips placed under the
edge of the disc o o; then by means of a cast iron cross, and screw bolts v v, we fix the
core immovably. The whole apparatus is now set down upon r s, and we fix with screw
bolts the plane surface q q upon r r; then introduce the melted metal by an aperture z,
which has been left at the upper part of the mould.
When, instead of the example now selected, the core of the piece to be cast must go
beyond the mould of the external surface, as is the case with a pipe open at each end,
the thing is more simple, because we may easily adjust and fix the core by its two ends.
In casting a retort, the metal is poured into the mould set upright. It is important to
maintain this position in the two last examples of casting; for all the foreign matters
which may soil the metal during its flow, as the sand, the charcoal, gases, scoriæ, being
less dense than it, rise constantly to the surface. The hydrostatic pressure produced by
a high gate, or filling-in aperture, contributes much to secure the soundness and solidity
of the casting. This gate piece being superfluous, is knocked off almost immediately[521]
after, or even before the casting cools. Very long, and somewhat slender pieces, are
usually cast in moulds set up obliquely to the horizon. As the metal shrinks in cooling,
the mould should always be somewhat larger than the object intended to be cast. The
iron founder reckons in general upon a linear shrinkage of a ninety-sixth part; that is
one-eighth of an inch per foot.
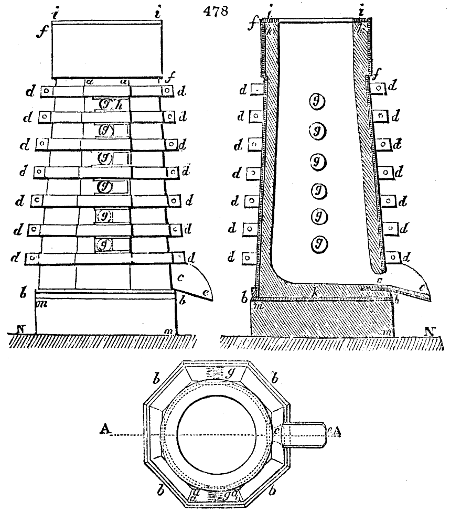
Melting of the cast-iron.—The metal is usually melted in a cupola furnace, of which
the dimensions are very various. Fig. 478. represents in plan, section, and elevation,
one of these furnaces of the largest size; being capable of founding 5 tons of cast-iron at
a time. It is kindled by laying a few chips of wood upon its bottom, leaving the orifice
c open, and it is then filled up to the throat with coke. The fire is lit at c, and in a
quarter or half an hour, when the body of fuel is sufficiently kindled, the tuyère blast is
set in action. The flame issues then by the mouth as well as the orifice c, which has
been left open on purpose to consolidate it by the heat. Without this precaution, the
sides which are made up in argillaceous sand after each day’s work, would not present
the necessary resistance. A quarter of an hour afterwards, the orifice c is closed with a
lump of moist clay, and sometimes, when the furnace is to contain a great body of melted
metal, the clay is supported by means of a small plate of cast-iron fixed against the
furnace. Before the blowing machine is set a going, the openings g g g had been kept
shut. Those of them wanted for the tuyères are opened in succession, beginning at the
lowest, the tuyères being raised according as the level of the fused iron stands higher in
the furnace. The same cupola may receive at a time from one to six tuyères, through
which the wind is propelled by the centrifugal action of an excentric fan or ventilator.
It does not appear to be ascertained whether there be any advantage in placing more
than two tuyères facing each other upon opposite sides of the furnace. Their diameter
at the nozzle varies from 3 to 5 inches. They are either cylindrical or slightly conical.
A few minutes after the tuyères have begun to blow, when the coke sinks in the furnace,
alternate charges of coke and pig iron must be thrown in. The metal begins to melt in
about 20 minutes after its introduction; and successive charges are then made every
10 minutes nearly; each charge containing from 2 cwt. to 5 cwt. of iron, and a quantity
proportional to the estimate given below. The amount of the charges varies of course
with the size of the furnace, and the speed required for the operation. The pigs must
be previously broken into pieces weighing at most 14 or 16 pounds. The vanes of the
blowing fan make from 625 to 650 turns per minute. The two cupolas represented
fig. 478., and another alongside in the plan, may easily melt 61⁄2 tons of metal in 23⁄4 hours;
that is 21⁄3 tons per hour. This result is three or four times greater than what was[522]
formerly obtained in similar cupolas, when the blast was thrown in from small nozzles
with cylinder bellows, moved by a steam engine of 10 horses power.
In the course of a year, a considerable foundry like that represented in the plan,
fig. 467., will consume about 300 tons of coke in melting 1240 tons of cast iron; consisting
of 940 tons of pigs of different qualities, and 300 tons of broken castings, gate-pieces,
&c. Thus, it appears that 48 pounds of coke are consumed for melting every 2 cwt.
of metal.
Somewhat less coke is consumed when the fusion is pushed more rapidly to collect a
great body of melted metal, for casting heavy articles; and more is consumed when, as in
making many small castings, the progress of the founding has to be slackened from time
to time; otherwise, the metal would remain too long in a state of fusion, and probably
become too cold to afford sharp impressions of the moulds.
It sometimes happens that in the same day, with the same furnace, pieces are to be
cast containing several proportions of different kinds of iron; in which case, to prevent
an intermixture with the preceding or following charges, a considerable bed of coke is
interposed. Though there be thus a little waste of fuel, it is compensated by the
improved adaptation of the castings to their specific objects. The founding generally
begins at about 3 o’clock, P. M., and goes on till 6 or 8 o’clock. One founder, aided by
four labourers for charging, &c., can manage two furnaces.
The following is the work of a well-managed foundry in Derby.
200 lbs. of coke are requisite to melt, or bring down (in the language of the founders),
1 ton of cast-iron, after the cupola has been brought to its proper heat, by the combustion
in it of 9 baskets of coak, weighing by my trials, 40 pounds each, = 360 lbs.
The chief talent of the founder consists in discovering the most economical mixtures,
and so compounding them as to produce the desired properties in the castings. One
piece, for example, may be required to have great strength and tenacity to bear heavy
weights or strains; another must yield readily to the chisel or the file; a third must
resist sudden alternations of temperature; and a fourth must be pretty hard.
The filling in of the melted metal is managed in two ways. For strong pieces, whose
moulds can be buried in the ground at 7 or 8 yards distance from the furnace, the metal
may be run in gutters, formed in the sand of the floor, sustained by plates or stones.
The clay plug is pierced with an iron rod, when all is ready.
When from the smaller size, or greater distance of the moulds, the melted metal
cannot be run along the floor from the furnace, it is received in cast-iron pots or ladles,
lined with a coat of loam. These are either carried by the hands of two or more men,
or transported by the crane. Between the successive castings, the discharge hole of the
furnace is closed with a lump of clay, applied by means of a stick, having a small disc of
iron fixed at its end.
After the metal is somewhat cooled, the moulds are taken asunder, and the excrescences
upon the edges of the castings are broken off with a hammer. They are afterwards
more carefully trimmed or chipped by a chisel when quite cold. The loss of weight in
founding is about 61⁄2 per cent. upon the pig iron employed. Each casting always
requires the melting of considerably more than its own weight of iron. This excess
forms the gates, false seams, &c.; the whole of which being deducted, shows that 1 cwt.
of coke is consumed for every 3 cwt. of iron put into the furnace; for every 138 cwt. of
crude metal, there will be 100 cwt. of castings, 32 of refuse pieces, and 6 of waste.
Explanation of the plates.
Manner of constructing the Mould of a Sugar-pan.
Fig. 468. View of the pan.
—g.469. Flat ring of cast-iron for supporting the inner mould.
—g.470. Construction of the inner mould.
—g.471. Formation of the outer surface of the pan.
—g.472. Finished mould.
—g.473. Position of the two flat cast-iron rings, destined to sustain the moulds of
the inner and the outer surface.
Gas-retort Moulding.
—g.474. Vertical projection, perpendicular to the axis of the retort; and two sections,
the one upright, the other horizontal.
—g.475. Construction of the core of the retort.
—g.476. Disposition of the outer mould.
—g.477. Adjustment of the core in the mould.
—g.478. Cupola furnace. It is 3 feet wide within, and 131⁄2 high.
m m, solid body of masonry, as a basis to the furnace.
[523]
b b, octagonal platform of cast iron, with a ledge in which the plates a a a a are engaged.
a a, eight plates of cast iron, 1 inch thick, absolutely similar; only one of them is
notched at its lower part in c, to allow the melted metal to run out, and two of the
others have six apertures g g g, &c. to admit the tuyères.
c, orifice for letting the metal flow out. A kind of cast iron gutter, e, lined with loam
is fitted to the orifice.
d, hoops of hammered iron, 41⁄4 inches broad; one half of an inch thick for the bottom
ones; and a quarter of an inch for the upper ones. The intermediate hoops decrease
in thickness from below upwards between these limits.
e, cast iron gutter or spout, lined with loam, for running off the metal.
f f, cylindrical piece of cast iron, for increasing the height and draught of the
furnace.
g, side openings for receiving the tuyères, of which there are six upon each side of the
furnace. Each of them may be shut at pleasure, by means of a small cast iron plate h,
made to slide horizontally in grooves sunk in the main plate, pierced with the holes
g g.
k k, interior lining of the surface, made of sand, somewhat argillaceous, in the following
way. After having laid at
the bottom of the furnace a
bed of sand a few inches thick,
slightly sloped towards the
orifice of discharge, there is
set upright, in the axis of the
cupola, a wooden cylinder of
its whole height, and of a
diameter a little less than that
of the vacant space belonging
to the top of the furnace.
Sand is to be then rammed in
so as to fill the whole of the
furnace; after which the
wooden cylinder is withdrawn,
and the lining of sand
is cut or shaved away, till it
has received the proper form.
This lining lasts generally
5 or 6 weeks, when there are
6 meltings weekly.
i i, cast iron circular plate,
through which the mouth of
the furnace passes, for protecting
the lining in k during
the introduction of the charges.
N N, level of the floor of
the foundry. The portion of
it below the running out orifice
consists of sand, so that it
may be readily sunk when it
is wished to receive the
melted metal in ladles or pots
of large dimensions.
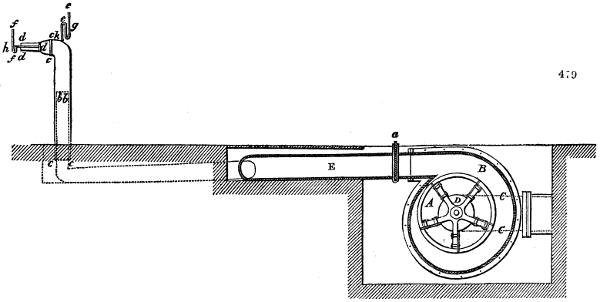
The fan distributes the blast
from the main pipe to three
principal points, by three
branch tubes of distribution.
A register, consisting of a
cast-iron plate sliding with
friction in a frame, serves to
intercept the blast at any moment,
when it is not desirable
to stop the moving power.
A large main pipe of zinc or
sheet iron is fitted to the orifice
of the slide valve. It is
square at the beginning, or
only rounded at the angles;[524]
but at a little distance it becomes cylindrical, and conducts the blast to the divaricating
points. There, each of the branches turns up vertically, and terminates at b b, fig. 479.,
where it presents a circular orifice of 71⁄2 inches. Upon each of the upright pipes b, the
one end of an elbow-tube of zinc c c c c, fig. 479., is adjusted rather loosely, and the
other end receives a tuyère of wrought iron d d, through the intervention of a shifting
hose or collar of leather c c d, hooped with iron wire to both the tube and the tuyère.
The portion c c c c may be raised or lowered, by sliding upon the pipe b, in order to
bring the nozzle of the tuyère d d, to the requisite point of the furnace. The portion
c c c c may be made also of wrought iron. A power of 4 horses is adequate to drive
this fan, for supplying blast to 3 furnaces.
The founders have observed the efflux of air was not the same when blown into the
atmosphere, as it was when blown into the furnaces; the velocity of the fan, with the
same impulsive power, being considerably increased in the latter case. They imagine
that this circumstance arises from the blast being sucked in, so to speak, by the draught
of the furnace, and that the fan then supplied a greater quantity of air.
The following experimental researches show the fallacy of this opinion. Two water
syphons, e e e, f f f, made of glass tubes, one-fifth of an inch in the bore, were inserted
into the tuyère, containing water in the portions g g g, h h h. The one of these manometers
for measuring the pressure of the air was inserted at k, the other in the centre of
the nozzle. The size of this glass tube was too small to obstruct in any sensible degree
the outlet of the air. It was found that when the tuyères of the fan discharged into the
open air, the expenditure by a nozzle of a constant diameter was proportional to the
number of the revolutions of the vanes. It was further found, that when the speed of
the vanes was constant, the expenditure by one or by two nozzles was proportional to
the total area of these nozzles. The following formulæ give the volume of air furnished
by the fan, when the number of turns and the area of the nozzles are known.
Volume = 25·32 S n 1,000,000n(1)
Volume = 0·86′6′7 S n1,000,000(2)
The volume is measured at 32° Fahr., under a pressure of 29·6 inches barom.
S = is the total area of the orifices of the tuyères in square inches.
n = the number of turns of the vanes in a minute.
After measuring the speed of the vanes blowing into the atmosphere, if we introduce
the nozzle of discharge into the orifice of the furnace, we shall find that their speed immediately
augments in a notable degree. We might, therefore, naturally suppose that
the fan furnishes more air in the second case than in the first; but a little reflection
will show that it is not so. In fact, the air which issues in a cold state from the tuyère
encounters instantly in the furnace a very high temperature, which expands it, and
contributes, along with the solid matters with which the furnace is filled, to diminish
the facility of the discharge, and consequently to retard the efflux by the nozzles. The
oxygen gas consumed is replaced by a like volume of carbonic acid gas, equally expansible
by heat. Reason leads us to conclude that less air flows from the nozzles into the
furnace than into the open atmosphere.
The increase in the velocity of the vanes takes place precisely in the same manner,
when after having made the nozzles blow into the atmosphere, we substitute for these
nozzles others of a smaller diameter, instead of directing the larger ones into the furnace.
Hence we may conceive that the proximity of the charged furnace acts upon the blast
like the contraction of the nozzles. When the moving power is uniform, and the
velocity of the vanes remains the same, the quantity of air discharged must also be the
same in the two cases.
Two tuyères, one 5 inches in diameter, the other 41⁄2, and which, consequently, presented
a total area of 351⁄2 square inches, discharged air into one of the furnaces, from a
fan whose vanes performed 654 turns in the minute. These two nozzles being briskly
withdrawn from the furnace, and turned round to the free air, while a truncated pasteboard
cone of 31⁄2 inches diameter was substituted for the nozzle of 41⁄2 inches, whereby
the area of efflux was reduced to 29·3 square inches, the velocity of the vanes continued
exactly the same. The inverse operation having been performed, that is to say, the two
original nozzles having been smartly replaced in the furnace, to discover whether or not
the moving power had changed in the interval of the experiment, they betrayed no perceptible
alteration of speed. From the measures taken to count the speed, the error
could not exceed 3 revolutions per minute, which is altogether unimportant upon the
number 654.
[525]
It follows, therefore, that when the vanes of the fan have the velocity of 654 turns
per minute, the expenditure by two nozzles, whose joint area is 351⁄2 square inches,
both blowing into a furnace, is to the expenditure which takes place, when the same
nozzles blow into the air, as 35·5 is to 29·3; that is, a little more than 4-fifths.
If this be, as is probable, a general rule for areas and speeds considerably different
from the above, to find the quantity of air blown into one or more furnaces by the fan,
we should calculate the volume by one of the above formulæ (1) or (2), and take 4-fifths
of the result, as the true quantity.
The fan A C here represented is of the best excentric form, as constructed by Messrs.
Braithwaite and Ericsson. D is the circular orifice round the axis by which the air is
admitted; and C C B is the excentric channel through which the air is wafted towards
the main discharge pipe E.
FOUNTAIN; a stream of water rising up through the superficial strata of the
earth. See Artesian Wells.
FOXING, is a term employed by brewers to characterize the souring of beer, in the
process of its fermentation or ripening.
FRANKFORT BLACK; is made by calcining vine branches, and the other refuse
lees of the vinegar vats in Germany. They must be previously washed.
FREEZING. (Congelation, Fr.; Gefrierung, Germ.) The three general forms,
solid, liquid, and gaseous, under one or other of which all kinds of matter exist, seem
to be immediately referrible to the influence of heat; modifying, balancing, or subduing
the attraction of cohesion. Every solid may be liquefied, and every liquid may be vaporized,
by a certain infusion of caloric, whether this be regarded as a moving power, or
an elastic essence. The converse of this proposition is equally true; for many gases,
till lately styled permanent, may be liquefied, nay, even solidified, by diminution of their
temperature, either alone, or aided by a condensing force, to bring their particles within
the sphere of aggregative attraction. When a solid is transformed into a liquid, and a
liquid into a gas or vapour, a quantity more or less considerable of heat is absorbed, or
becomes latent, to use the term of Dr. Black, the celebrated discoverer of this great law
of nature. When the opposite transformation takes place, the heat absorbed is again
emitted, or what was latent becomes sensible caloric. Upon the first principle, or the
absorption of heat, are founded the various artificial methods of producing cold and
congelation.
Tables, exhibiting a collective view of all the Frigorific Mixtures contained in
Mr. Walker’s publication, 1808.
I.—Table consisting of Frigorific Mixtures, composed of ice, with chemical salts
and acids.
Frigorific Mixtures with Ice.
| MIXTURES. |
Thermometer sinks. |
Deg. of
cold
produced. |
| |
|
|
| Snow, or pounded ice |
2 |
parts |
From
any
tem-
pera-
ture |
- |
|
to -5° |
* |
| Muriate of soda |
1 |
|
| Snow, or pounded ice |
5 |
parts |
to -12° |
* |
| Muriate of soda |
2 |
|
| Muriate of ammonia |
1 |
|
| Snow, or pounded ice |
24 |
parts |
to -18° |
* |
| Muriate of soda |
10 |
|
| Muriate of ammonia |
5 |
|
| Nitrate of potash |
5 |
|
| Snow, or pounded ice |
12 |
parts |
to -25° |
* |
| Muriate of soda |
5 |
|
| Nitrate of ammonia |
5 |
|
| |
|
|
| Snow |
3 |
parts |
From +32° to -23° |
55 |
| Diluted sulphuric acid |
2 |
|
| Snow |
8 |
parts |
From +32° to -27° |
59 |
| Muriatic acid |
5 |
|
| Snow |
7 |
parts |
From +32° to -30° |
62 |
| Diluted nitric acid |
4 |
|
| Snow |
4 |
parts |
From +32° to -40° |
72 |
| Muriate of lime |
5 |
|
| Snow |
2 |
parts |
From +32° to -50° |
82 |
| Cryst. muriate of lime |
3 |
|
| Snow |
3 |
parts |
From +32° to -51° |
83 |
| Potash |
4 |
|
[526]
N. B.—The reason for the omissions in the last column of the preceding table is,
the thermometer sinking in these mixtures to the degree mentioned in the preceding
column, and never lower, whatever may be the temperature of the materials at mixing.
II.—Table, consisting of Frigorific Mixtures, having the power of generating or
creating cold, without the aid of ice, sufficient for all useful and philosophical purposes,
in any part of the world at any season.
Frigorific Mixtures without Ice.
| MIXTURES. |
Thermometer sinks. |
Deg. of
cold
produced. |
| Muriate of ammonia |
5 |
parts |
From +50° to +10° |
40 |
° |
| Nitrate of potash |
5 |
|
| Water |
16 |
|
| Muriate of ammonia |
5 |
parts |
From +50° to +4° |
46 |
|
| Nitrate of potash |
5 |
|
| Sulphate of soda |
8 |
|
| Water |
16 |
|
| Nitrate of ammonia |
1 |
part |
From +50° to +4° |
46 |
|
| Water |
1 |
|
| Nitrate of ammonia |
1 |
part |
From +50° to -7° |
57 |
|
| Carbonate of soda |
1 |
|
| Water |
1 |
|
| Sulphate of soda |
3 |
parts |
From +50° to -3° |
53 |
|
| Diluted nitric acid |
2 |
|
| Sulphate of soda |
6 |
parts |
From +50° to -10° |
60 |
|
| Muriate of ammonia |
4 |
|
| Nitrate of potash |
2 |
|
| Diluted nitric acid |
4 |
|
| Sulphate of soda |
6 |
parts |
From +50° to -14° |
64 |
|
| Nitrate of ammonia |
5 |
|
| Diluted nitric acid |
4 |
|
| Phosphate of soda |
9 |
parts |
From +50° to -12° |
62 |
|
| Diluted nitric acid |
4 |
|
| Phosphate of soda |
9 |
parts |
From +50° to -21° |
71 |
|
| Nitrate of ammonia |
6 |
|
| Diluted nitric acid |
4 |
|
| Sulphate of soda |
8 |
parts |
From +50° to 0° |
50 |
|
| Muriatic acid |
5 |
|
| Sulphate of soda |
5 |
parts |
From +50° to +3° |
47 |
|
| Diluted sulphuric acid |
4 |
|
N. B.—If the materials are mixed at a warmer temperature than that expressed in
the table, the effect will be proportionably greater; thus, if the most powerful of these
mixtures be made when the air is +85°, it will sink the thermometer to +2°.
III.—Table consisting of Frigorific Mixtures selected from the foregoing Tables,
and combined so as to increase or extend cold to the extremest degrees.
Combinations of Frigorific Mixtures.
| MIXTURES. |
Thermometer sinks. |
Deg. of
cold
produced. |
| Phosphate of soda |
5 |
parts |
From 0° to -34° |
34 |
| Nitrate of ammonia |
3 |
|
| Diluted nitric acid |
4 |
|
| Phosphate of soda |
3 |
parts |
From -34° to -50° |
16 |
| Nitrate of ammonia |
2 |
|
| Diluted mixed acids |
4 |
|
| Snow |
3 |
parts |
From 0° to -46° |
46 |
| Diluted nitric acid |
2 |
|
| Snow[527] |
8 |
parts |
From -10° to -56° |
46 |
| Diluted sulphuric acid |
3 |
|
| Diluted nitric acid |
3 |
|
| Snow |
1 |
part |
From -20° to -60° |
40 |
| Diluted sulphuric acid |
1 |
|
| Snow |
3 |
parts |
From +20° to -48° |
68 |
| Muriate of lime |
4 |
|
| Snow |
3 |
parts |
From +10° to -54° |
64 |
| Muriate of lime |
4 |
|
| Snow |
2 |
parts |
From -15° to -68° |
53 |
| Muriate of lime |
3 |
|
| Snow |
1 |
part |
From 0° to -66° |
66 |
| Cryst. muriate of lime |
2 |
|
| Snow |
1 |
part |
From -40° to -73° |
33 |
| Cryst. muriate of lime |
3 |
|
| Snow |
8 |
parts |
From -68° to -91° |
23 |
| Diluted sulphuric acid |
10 |
|
N. B.—The materials in the first column are to be cooled, previously to mixing, to
the temperature required, by mixtures taken from either of the preceding tables.
Water absorbs 1000 degrees of heat in becoming vapour; whence, if placed in a
saucer within an exhausted receiver, over a basin containing strong sulphuric acid, it
will freeze by the rapid absorption of its heat into the vapour so copiously formed under
these circumstances.
But the most powerful means of artificial refrigeration is afforded by the evaporation
of liquefied carbonic acid gas; for the frozen carbonic acid thus obtained, has probably
a temperature 100° under zero; so that when a piece of it is laid upon quicksilver,
it instantly congeals this metal. The more copious discussion of this subject belongs to
chemical science.
FRICTION, counteraction of; see Lubrication.
FRIT; see Enamel and Glass.
FUEL; (Combustible, Fr; Brennstoff, Germ.).
Such combustibles as are used for fires or furnaces are called fuel, as wood, turf, pitcoal.
These differ in their nature, and in their power of giving heat.
I. Wood, which is divided into hard and soft. To the former belong the oak,
the beech, the alder, the birch, and the elm; to the latter, the fir, the pine of different
sorts, the larch, the linden, the willow, and the poplar.
Under like dryness and weight, different woods are found to afford equal degrees of
heat in combustion. Moisture diminishes the heating power in three ways; by diminishing
the relative weight of the ligneous matter, by wasting heat in its evaporation,
and by causing slow and imperfect combustion. If a piece of wood contain, for example,
25 per cent. of water, then it contains only 75 per cent. of fuel, and the evaporation
of that water will require 1⁄28 part of the weight of the wood. Hence the damp
wood is of less value in combustion by 8⁄28 or 2⁄7 than the dry. The quantity of moisture
in newly felled wood amounts to from 20 to 50 per cent.; birch contains 30, oak 35,
beech and pine 39, alder 41, fir 45. According to their different natures, woods which
have been felled and cleft for 12 months contain still from 20 to 25 per cent. of water.
There is never less than 10 per cent. present, even when it has been kept long in a dry
place, and though it be dried in a strong heat, it will afterwards absorb 10 or 12 per
cent. of water. If it be too strongly kiln dried, its heating powers are impaired by the
commencement of carbonization, as if some of its hydrogen were destroyed. It may
be assumed as a mean of many experimental results, that 1 pound of artificially dried
wood will heat 35 pounds of water from the freezing to the boiling point; and that a
pound of such wood as contains from 20 to 25 per cent. of water will heat 26 pounds
of ice-cold water to the same degree. It is better to buy wood by measure than by
weight, as the bulk is very little increased by moisture. The value of different woods
for fuel is inversely as their moisture, and this may easily be ascertained by taking their
shavings, drying them in a heat of 140° F., and seeing how much weight they lose.
From every combustible the heat is diffused either by radiation or by direct communication
to bodies in contact with the flame. In a wood fire the quantity of radiating heat
is to that diffused by the air, as 1 to 3; or it is one fourth of the whole heating power.
[528]
II. Charcoal. The different charcoals afford, under equal weights, equal quantities
of heat. We may reckon, upon an average, that a pound of dry charcoal is capable of
heating 73 pounds of water from the freezing to the boiling point; but when it has been
for some time exposed to the air, it contains at least 10 per cent. of water, which is partially
decomposed in the combustion into carburetted hydrogen, which causes flame,
whereas pure dry charcoal emits none.
A cubic foot of charcoal from soft wood weighs upon an average from 8 to 9 pounds,
and from hard wood 12 to 13 pounds; and hence the latter are best adapted to maintain
a high heat in a small compass. The radiating heat from charcoal fires constitutes one
third of the whole emitted.
III. Pitcoal. The varieties of this coal are almost indefinite, and give out very
various quantities of heat in their combustion. The carbon is the heat-giving constituent,
and it amounts, in different coals, to from 75 to 95 per cent. One pound of
good pitcoal will, upon an average, heat 60 pounds of water from the freezing to the
boiling point. Small coal gives out three-fourths of the heat of the larger lumps. The
radiating heat emitted by burning pitcoal is greater than that by charcoal.
IV. The coke of pitcoal.—The heating power of good coke is to that of pitcoal as
75 to 69. One pound of the former will heat 65 pounds of water from 32° to 212°; so
that its power is equal to nine-tenths of that of wood charcoal.
V. Turf or peat.—One pound of this fuel will heat from 25 to 30 pounds of water
from freezing to boiling. Its value depends upon its compactness and freedom from earthy
particles; and its radiating power is to the whole heat it emits in burning, as 1 to 3.
VI. Carburetted hydrogen or coal gas.—One pound of this gas, equal to about 24
cubic feet, disengages in burning, as much heat as will raise 76 pounds of water from
the freezing to the boiling temperature.
In the following table the fourth column contains the weight of atmospherical air,
whose oxygen is required for the complete combustion of a pound of each particular
substance.
| Species of combustible. |
Pounds of
water which
a pound can
heat from
0° to 212°. |
Pounds of
boiling water
evaporated
by 1 pound. |
Weight of
atmospheric
air at 32°,
to burn
1 pound. |
| Perfectly dry wood |
35·00 |
6·36 |
5·96 |
| Wood in its ordinary state |
26·00 |
4·72 |
4·47 |
| Wood charcoal |
73·00 |
13·27 |
11·46 |
| Pitcoal |
60·00 |
10·90 |
9·26 |
| Coke |
65·00 |
11·81 |
11·46 |
| Turf |
30·00 |
5·45 |
4·60 |
| Turf charcoal |
64·00 |
11·63 |
9·86 |
| Carburetted hydrogen gas |
76·00 |
13·81 |
14·58 |
| Oil |
|
- |
78·00 |
14·18 |
15·00 |
| Wax |
| Tallow |
| Alcohol of the shops |
52·60 |
9·56 |
11·60 |
The quantity of air stated in the fourth column, is the smallest possible required to
burn the combustible, and is greatly less than would be necessary in practice, where
much of the air never comes into contact with the burning body, and where it consequently
never has its whole oxygen consumed. The heating power stated in the
second column is also the maximum effect, and can seldom be realized with ordinary
boilers. The draught of air usually carries off at least 1⁄7 of the heat, and more if its
temperature be very high when it leaves the vessel. In this case it may amount to one
half of the whole heat or more; without reckoning the loss by radiation and conduction,
which however may be rendered very small by enclosing the fire and flues within proper
non-conducting and non-radiating materials.
It appears that in practice, the quantity of heat which may be obtained from any
combustible in a properly mounted apparatus, must vary with the nature of the object to
be heated. In heating chambers by stoves, and water boilers by furnaces, the effluent heat
in the chimney which constitutes the principal waste, may be reduced to a very moderate
quantity, in comparison of that which escapes from the best constructed reverberatory
hearth. In heating the boilers of steam engines, one pound of coal is reckoned adequate
to convert 71⁄2 pounds of boiling water into vapour; or to heat 411⁄4 pounds of water from
the freezing to the boiling point. One pound of fir of the usual dryness will evaporate
4 pounds of water, or heat 22 pounds to the boiling temperature; which is about two-thirds
of the maximum effect of this combustible. According to Watt’s experiments
upon the great scale, one pound of coal can boil off with the best built boiler, 9 pounds
of water; the deficiency from the maximum effect being here 10⁄57, or nearly one-sixth.
In many cases the hot air which passes into the flues or chimneys may be beneficially[529]
applied to the heating, drying, or roasting of objects; but care ought to be taken
that the draught of the fire be not thereby impaired, and an imperfect combustion of the
fuel produced. For at a low smothering temperature both carbonic oxide and carburetted
hydrogen may be generated from coal, without the production of much heat in the
fire-place.
To determine exactly the quantity of heat disengaged by any combustible in the act
of burning, three different systems of apparatus have been employed; 1. the calorimeter
of Lavoisier and Laplace, in which the substance is burned in the centre of a vessel,
whose walls are lined with ice; and the amount of ice melted, measures the heat
evolved; 2. the calorimeter of Watt and Rumford, in which the degree of heat communicated
to a given body of water affords the measure of temperature; and 3. by the
quantity of water evaporated by different kinds of fuel in similar circumstances.
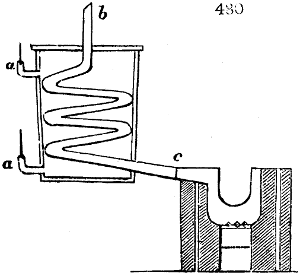
If our object be to ascertain the relative heating powers of different kinds of fuel, we
need not care so much about the total waste of heat in the experiments, provided it be
the same in all; and therefore they should be burned in the same furnace, and in the
same way. But the more economically the heat is applied, the greater certainty will
there be in the results. The apparatus, fig. 480.,
is simple and well adapted to make such comparative
trials of fuel. The little furnace is
covered at top, and transmits its burned air by c,
through a spiral tube immersed in a cistern of
water, having a thermometer inserted near its
top, and another near its bottom, into little side
orifices a a, while the effluent air escapes from the
upright end of the tube b. Here also a thermometer
bulb may be placed. The average indication
of the two thermometers gives the mean
temperature of the water. As the water evaporates
from the cistern, it is supplied from a vessel
placed alongside of it. The experiment should be
begun when the furnace has acquired an equability
of temperature. A throttle valve at c serves to regulate the draught, and to
equalize it in the different experiments by means of the temperature of the effluent
air. When the water has been heated the given number of degrees, which should be
the same in the different experiments, the fire may be extinguished, the remaining
fuel weighed, and compared with the original quantity. Care should be taken to make
the combustion as vivid and free from smoke as possible.
FULGURATION; designates the sudden brightening of the melted gold and silver
in the cupel of the assayer, when the last film of vitreous lead and copper leaves their
surface.
FULLER’S EARTH, (Terre à foulon, Argile Smectique, Fr.; Walkererde, Germ.)
is a soft, friable, coarse or fine grained mass of lithomarge clay. Its colour is greenish,
or yellowish gray; it is dull, but assumes a fatty lustre upon pressure with the
fingers, feels unctuous, does not adhere to the tongue, and has a specific gravity varying
from 1·82 to 2·19. It falls down readily in water, into a fine powder, with extrication
of air bubbles, and forms a non-plastic paste. It melts at a high heat into a brown
slag. Its constituents are 53·0 silica; 10·0 alumina; 9·75 red oxide of iron; 1·25
magnesia; 0·5 lime; 24 water, with a trace of potash. Its cleansing action upon
woollen stuffs depends upon its power of absorbing greasy matters. It should be
neither tenacious nor sandy; for in the first case, it would not diffuse itself well
through water, and in the second it would abrade the cloth too much. The finely divided
silica is one of its useful ingredients.
Fuller’s earth is found in several counties of England; but in greatest abundance in
Bedfordshire, Berkshire, Hampshire, and Surry.
In the county of Surry there are great quantities of fuller’s earth found about Nutfield,
Ryegate, and Blechingley, to the south of the Downs, and some, but of inferior
quality, near Sutton and Croydon, to the north of them. The most considerable pits
are near Nutfield, between which place and Ryegate, particularly on Redhill, about a
mile to the east of Ryegate, it lies so near the surface as frequently to be turned up by
the wheels of the waggons. The fuller’s earth to the north of the road between Redhill
and Nutfield, and about a quarter of a mile from the latter place, is very thin; the
seam in general is thickest on the swell of the hill to the south of the road. It is not
known how long this earth has been dug in Surry; the oldest pit now wrought is said
to have lasted between 50 and 60 years, but it is fast wearing out. The seam of fuller’s
earth dips in different directions. In one, if not in more cases, it inclines to the west
with a considerable angle. There are two kinds of it, the blue and the yellow: the
former, on the eastern side of the pit, is frequently within a yard of the surface, being[530]
covered merely with the soil—a tough, wet, clayey loam. A few yards to the west,
the blue kind appears with an irony sand-stone, of nearly two yards in thickness, between
it and the soil. The blue earth in this pit is nearly 16 feet deep. In some
places the yellow kind is found lying upon the blue; there seems, indeed, to be no
regularity either in the position or inclination of the strata where the fuller’s earth is
found, nor any mark by which its presence could be detected. It seems rather thrown
in patches than laid in any continued or regular vein. In the midst of the fuller’s
earth are often found large pieces of stone of a yellow colour, translucent and remarkably
heavy, which have been found to be sulphate of barytes, encrusted with quartzose
crystals. These are carefully removed from the fuller’s earth, as the workmen say they
often spoil many tons of it which lie about them. There is also found with the yellow
fuller’s earth a dark brown crust, which the workmen consider as injurious also. In
Surry the price of fuller’s earth seems to have varied very little, at least for these last 80
years. In 1730, the price at the pit was 6d. a sack, and 6s. per load or ton. In 1744,
it was nearly the same. It is carried in waggons, each drawing from three to four tons,
to the beginning of the iron railway near Westham, along which it is taken to the
banks of the Thames, where it is sold at the different wharfs for about 25s. or 26s. per
ton. It is then shipped off either to the north or west of England.
The next characteristic stratum, owing to its forming a ridge of conspicuous hills
through the country, is the Woburn land, a thick ferruginous stratum, which below its
middle contains a stratum of fuller’s earth. This is thicker and more pure in Aspley
and Hogstye-end, two miles north-west of Woburn, than in any known place.
Fuller’s earth is found at Tillington, and consumed in the neighbouring fulling mills.
Mode of preparing fuller’s earth:—
After baking it is thrown into cold water, where it falls into powder, and the separation
of the coarse from the fine is effectually accomplished, by a simple method used in
the dry colour manufactories, called washing over. It is done in the following manner:
Three or four tubs are connected on a line by spouts from their tops; in the first the
earth is beat and stirred, and the water, which is continually running from the first to
the last through intermediate ones, carries with it and deposits the fine, whilst the
coarse settles in the first. The advantages to be derived from this operation are, that
the two kinds will be much fitter for their respective purposes of cleansing coarse or
fine cloth; for without baking the earth they would be unfit, as before noticed, to incorporate
so minutely with the water in its native state; it would neither so readily fall
down, nor so easily be divided into different qualities, without the process of washing
over. When fuel is scarce for baking the earth, it is broken into pieces of the same
size, as mentioned above, and then exposed to the heat of the sun.
The various uses of fuller’s earth may be shortly explained. According to the above
method, the coarse and fine of one pit being separated, the first is used for cloths or
an inferior, and the second for those of a superior quality. The yellow and the blue
earths of Surry are of different qualities naturally, and are like the above, obtained artificially,
and used for different purposes. The former, which is deemed the best, is
employed in fulling the kerseymeres and finer cloths of Wiltshire and Gloucestershire,
whilst the blue is principally sent into Yorkshire for the coarser cloths. Its effects on
these cloths is owing to the affinity which alumine has for greasy substances; it unites
readily with them, and forms combinations which easily attach themselves to different
stuffs, and thereby serve the purpose of mordants in some measure. The fullers generally
apply it before they use the soap.
FULLING; for the theory of the process, see Felting, and Wool.
FULLING MILL. Willan and Ogle obtained a patent in 1825 for improved fulling
machinery, designed to act in a similar
way to the ordinary stocks, in which
cloths are beaten, for the purpose of washing
and thickening them; but the standard
and the bed of the stocks are made of iron
instead of wood as heretofore; and a steam
vessel is placed under the bed, for heating
the cloths during the operation of fulling;
whereby their appearance is said to be
greatly improved.
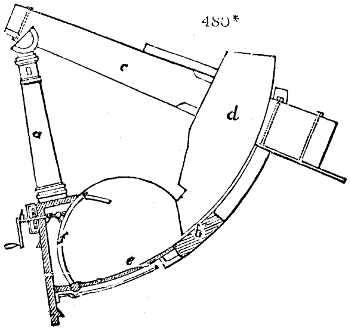
Fig. 480*. is a section of the fulling
machine or stocks; a, is a cast-iron pillar,
made hollow for the sake of lightness; b,
is the bed of the stocks, made also of iron,
and polished smooth, the side of the stock
being removed to shew the interior; c, is
the lever that carries the beater d. The
cloths are to be placed on the bed b, at[531]
bottom, and water allowed to pass through the stock, when by the repeated blows of the
beater d, which is raised and let fall in the usual way, the cloths are beaten, and
become cleansed and fulled.
A part of the bed at e, is made hollow, for the purpose of forming a steam box, into
which steam from a boiler is introduced by a pipe with a stop-cock. This steam heats
the bed of the stock, and greatly facilitates, as well as improves the process of cleansing
and fulling the cloths.
The smoothness of the surface of the polished metal, of which the bed of the stock is
constituted, is said to be very much preferable to the roughness of the surface of wood
of which ordinary fulling stocks are made, as by these iron stocks less of the nap or felt
of the cloth is removed, and its appearance when finished is very much superior to
cloths fulled in ordinary stocks.
In the operation of fulling, the cloths are turned over on the bed, by the falling of
the beaters, but this turning over of the cloths will depend in a great measure upon the
form of the front or breast of the stock. In these improved stocks therefore, there is a
contrivance by which the form of the front may be varied at pleasure, in order to suit
cloths of different qualities; f, is a movable curved plate, constituting the front of the
stock; its lower part is a cylindrical rod, extending along the entire width of the bed,
and being fitted into a recess, forms a hinge joint upon which the curved plate moves;
g, is a rod attached to the back of the curved plate f, with a screw thread upon it; this
rod passes through a nut h, and by turning this nut, the rod is moved backward or forward,
and consequently, the position of the curved plate altered.
The nut h, is a wheel with teeth, taking into two other similar toothed wheels, one
on each side of it, which are likewise the nuts of similar rods jointed to the back of the
curved plate f; by turning the central wheel, therefore, which may be done by a winch,
the other two wheels are turned also, and the curved plate moved backward or forward.
At the upper part of the plate there are pins passing through curved slots, which act as
guides when the plate is moved.
The patentees state in conclusion, that steam has been employed before for heating
cloths while fulling them, they therefore do not exclusively claim its use, except in the
particular way described; the advantages arising from the construction of iron stocks,
with polished surfaces in place of wooden ones, together with the movable curved plates
described, are in their opinion “sufficiently important to constitute a patent right.”
FULMINATES, or fulminating powders. Of these explosive compounds, there are
several species; such as fulminating gold, mercury, platinum, silver; besides the old
fusible mixture of nitre, sulphur, and potash. The only kind at all interesting in a
manufacturing point of view is the fulminate of mercury, now so extensively used as a
priming to the caps of percussion locks. Having published a paper in the Journal of
the Royal Institution for 1831, upon gunpowder (see Gunpowder), the result of an
elaborate suite of experiments, I was soon afterwards requested by the Hon. the Board
of Ordnance to make such researches as would enable me to answer, in a satisfactory
practical manner, a series of questions upon fulminating powders, subservient to the
future introduction of percussion musquets into the British army. The following is a
verbatim copy of my report upon the subject:—
To the Secretary of the Board of Ordnance.
“Sir,—I have the honour of informing you, for the instruction of the Honourable
the Master General and the Board of Ordnance, that the researches on fulminating
mercury, which I undertook by their desire, have been brought to a satisfactory conclusion,
after a numerous, diversified, and somewhat hazardous series of experiments. The
following are the questions submitted to me, with their respective answers:—
Question 1. What proportions of mercury, with nitric acid and alcohol of certain
strengths, will yield the greatest quantity of pure fulminate of mercury?
Answer. One hundred parts, by weight, of mercury, must be dissolved with a gentle
heat, in 1000 parts (also by weight) of nitric acid, spec. gr. 1·4; and this solution, at
the temperature of about 130° Fahr. must be poured into 830 parts by weight of
alcohol, spec. gr. 0·830.—Note. 830 parts of such alcohol, by weight, constitute 1000
by measure; and 1000 parts of such nitric acid, by weight, constitute 740 by measure.
Hence, in round numbers, one ounce weight of quicksilver must be dissolved in 71⁄2 oz.
measures of the above designated nitric acid, and the resulting solution must be poured
into 10 oz. measures of the said alcohol.
Question 2. What is the most economical and safe process for conducting the manipulation,
either as regards the loss of nitrous gas and residuum, or as respects danger to
the operator; also, what is the readiest and safest mode of mixing the fulminate intimately
with its due proportions of common gunpowder.
Answer. The mercury should be dissolved in the acid in a glass retort, the beak of
which is loosely inserted into a large balloon or bottle of glass or earthenware, whereby[532]
the offensive fumes of the nitrous gas disengaged during the solution, are, in a considerable
measure, condensed into liquid acid, which should be returned into the retort. As
soon as the mercury is all dissolved, and the solution has acquired the prescribed temperature
of about 130°, it should be slowly poured, through a glass or porcelain funnel,
into the alcohol contained in a glass matrass or bottle capable of holding fully 6 times
the bulk of the mixed liquids. In a few minutes bubbles of gas will proceed from the
bottom of the liquid; these will gradually increase in number and magnitude till a
general fermentative commotion, of a very active kind, is generated, and the mixture
assumes a somewhat frothy appearance. A white voluminous gas now issues from the
orifice of the matrass, which is very combustible, and must be suffered to escape freely
into the air, at a distance from any flame. These fumes consist of an ethereous gas,
holding mercury in suspension or combination. I have made many experiments with
the view of condensing this gas, or, at least, the mercury, but with manifest disadvantage
to the perfection of the process of producing fulminate. When the said gas is transmitted,
through a glass tube, into a watery solution of carbonate of soda, a little oxide
of mercury is, no doubt, recovered; but the pressure on the fermentative mixture,
though slight, necessary to the displacement of the soda solution, seems to obstruct or
impair the generation of the fulminate; this effect is chiefly injurious towards the end
of the operation when the gaseous fumes are strongly impregnated with nitrous gas.
When this is not allowed freely to come off, a portion of subnitrate or nitrate of mercury
is apt to be formed, to the injury of the general process and the product.
As soon as the effervescence and concomitant emission of gas are observed to cease,
the contents of the matrass should be turned out upon a paper double filter, fitted into a
glass or porcelain funnel, and washed by the affusion of cold water till the drainings no
longer redden litmus paper. The powder adhering to the matrass should be washed out
and thrown on the filter by the help of a little water. Whenever the filter is thoroughly
drained, it is to be lifted out of the funnel, and opened out on plated copper or stone
ware, heated to 212° Fahr. by steam or hot water. The fulminate being thus dried, is to
be put up in paper parcels of about 100 grains each; the whole of which may be afterwards
packed away in a tight box, or a bottle with a cork stopper. The excellence of
the fulminate may be ascertained, by the following characters. It consists of brownish-gray
small crystals which sparkle in the sun, are transparent when applied to a
slip of glass with a drop of water, and viewed by transmitted light. These minute
spangles are entirely soluble in 130 times their weight of boiling water; that is to say,
an imperial pint of boiling water will dissolve 67 grs. of pure fulminate. Whatever
remains indicates impurity. From that solution beautiful pearly spangles of fulminate
fall down as the liquid cools.
It may now be proper to show within what nice and narrow limits the best proportions
of the ingredients used in making the fulminate of mercury lie. The following
are selected from among many experiments instituted to determine that point, as well
as the most economical process.
1. According to the formula given by the celebrated chemist Berzelius, in the 4th
vol. of his “Traité de Chimie,” recently published (p. 383.), the mercury should be
dissolved in 12 times its weight of nitric acid sp. gr. 1·375; and alcohol of sp. gr.
0·850, amounting to 16·3 times the weight of the mercury, should be poured at intervals
into the nitric solution. The mixture is then to be heated till effervescence with the
characteristic cloud of gas appears. On the action becoming violent, alcohol is to be
poured in from time to time to repress it, till additional 16·3 parts have been employed.
On this process I may remark, that it is expensive, troublesome, dangerous, and unproductive
of genuine pure fulminate. One fifth more nitric acid is expended very
nearly than what is necessary, and almost four times the weight of alcohol which is
beneficial. Of alcohol at 0·83, 8·3 parts by weight are sufficient; whereas Berzelius
prescribes nearly 4 times this quantity in weight, though the alcohol is somewhat
weaker, being of sp. gr. 0·850. By using such an excess of alcohol, much of the fulminate
is apt to be revived into globules of quicksilver at the end of the process,
as I showed in my paper on this subject published in the Journal of the Royal Institution
two years ago. There is no little hazard in pouring the alcohol into the nitric solution;
for at each effusion an explosive blast takes place, whereas by pouring the
solution into the alcohol, as originally enjoined by the Hon. Mr. Howard, the inventor
of the process, no danger whatever is incurred. 100 parts of mercury treated in the
way recommended by Berzelius afforded me only 112 parts of fulminate, instead of the
130 obtained by my much more economical and safe proportions and process from the
same weight of quicksilver.
2. If 10 parts of nitric acid of sp. gr. 1·375 be used for dissolving 1 of quicksilver,
and if 14 parts of alcohol of sp. gr. 0·85 be thereafter mixed with the solution, the product
of such proportions will either be not granular, and therefore not fulminating, or[533]
it will be partially granular and partially pulverulent, being a mixture of fulminate and
subnitrate of mercury ill adapted for priming detonating caps. Instead of 130 parts
of genuine fulminate, as I do obtain, probably not more than 10 parts of powder will be
produced, and that of indifferent quality. In fact, whenever the ethereous fermentation
is defective, or not vigorous, little true fulminate is generated; but much of the mercury
remains in the acidulated alcoholic liquid.
3. If the alcohol be poured in successive portions, and of proper strength (sp. gr. 0·83)
into a proper nitric solution of mercury, the explosive action which accompanies each
effusion dissipates much of the alcohol, and probably impairs the acid, so that the subsequent
ethereous fermentation is defective, and little good fulminate is formed. From
100 parts of mercury submitted to this treatment, I obtained in one experiment carefully
made, only 51 parts of a powder, which was impalpable, had a cream colour, and
was not explosive either by heat or percussion.
4. When, with 100 parts of mercury, 800 of nitric acid of sp. gr. 1·375 are employed
with 650 of alcohol of sp. gr. 846, no fulminate whatever is generated.
5. When with the proper proportions of mercury, acid, and alcohol, the process is
advanced into a proper energy of fermentative commotion, if the matrass be immersed
in cold water so as materially to repress that action, the process will be impaired, and
will turn out ultimately defective both as to the quantity and quality of the fulminate.
It is therefore evident that a certain energy or vivacity of etherization is essential to the
full success of this curious process, and that any thing which checks it, or obstructs its
taking place, is injurious and to be avoided.
When my proportions are observed in making fulminating mercury, somewhat less
than one fourth of the nitric acid used in making the solution remains in the alcoholic
mixture along with the fulminate. When other proportions are taken, much more acid
remains. This acid is not recoverable to any useful or economical purpose, nor is the
alcohol that is associated with it. Many distillations with various reagents have led me
to this practical conclusion. In fact, when the process is most complete, as described
in the first paragraph, the alcohol is entirely and profitably employed in etherization,
and generating fulminic acid.
I have made a series of analytical experiments on the pure fulminate of mercury,
with the view of determining its composition, the quantity of quicksilver present in it,
and consequently the loss of mercury in the operation. I have stated that my maximum
product of fulminate from 100 grs. of quicksilver is 130 grs. Occasionally from
slight differences in the temperature of the mixture, or the ambient atmosphere, 2 grs.
less may be obtained.
A. I dissolved 130 grs. with a gentle heat in muriatic acid contained in a small matrass,
adding a few drops of the nitric to quicken the solution. On evaporating it to dryness,
with much care to avoid volatilization of the salt, I obtained 125 grs. of corrosive sublimate
or bi-chloride of mercury. But 125 grs. of this bi-chloride contain only 91·1
grs. of quicksilver. Therefore, by this experiment, 130 grs. of fulminate contain no
more than 91·1 of mercury, indicating an exhalation of 8·9 parts in the form of fumes,
or a retention in the residuary liquid of some of these 8·9 parts, out of the 100 originally
employed.
B. In another experiment for analysis, 130 grs. dissolved as above, were thrown
down by carbonate of soda. 95 grs. of black oxide of mercury were obtained, which
are equivalent to 91·2 grs. of quicksilver; affording a confirmation of the preceding
result.
C. 130 grs. of fulminate were dissolved in strong muriatic acid, and the solution was
decomposed by crystals of proto-muriate of tin at a boiling temperature. The mercury
was precipitated in globules to such amount as to verify the two preceding experiments.
Regarding fulminate of mercury as a bi-cyanate, that is, as a compound of one atom
or one equivalent prime of deutoxide of mercury, and two primes of cyanic acid, we
shall find its theoretical composition to be as follows, hydrogen being the radix, or 1.
| 2 |
Primes of |
Cyanic or fulminic Acid = |
34 × 2 = |
68 |
24 |
| 1 |
|
Deutoxide of Mercury = |
|
216 |
76 |
| |
284 |
100 |
As these 284 parts of fulminate contain 200 of quicksilver, so 142 parts of fulminate
will contain 100 of quicksilver. Whence it appears, that when only 130 parts of fulminate
can be obtained in practice from 100 of quicksilver, 81⁄2 parts of quicksilver out
of the 100 are unproductive, that is, are expended in the etherized gas, or left in the
residuary acidulous liquid. By the above experimental and theoretical analysis, 91·5
parts of quicksilver enter into the composition of 130 parts of true crystalline fulminate.
The complete accordance here exhibited between theory and practice removes every[534]
shadow of doubt as to the accuracy of the statements. 100 parts of fulminate
consist of—
| Mercury |
|
- |
70·4 |
|
- |
Peroxide |
76 |
·0 |
| Oxygen |
5·6 |
| Fulminic acid |
24 |
|
| 100 |
·0 |
Question 3. May the gas or vapour produced by the inflammation of the fulminate
of mercury, when combined with a portion of gunpowder, be considered in its nature
corrosive of iron or brass?
Answer. I have suggested to Mr. Lovell, of Waltham Abbey works, that the fulminate
may be probably diluted most advantageously with spirit varnish made of a
proper consistence by dissolving sandarach in alcohol. When well mixed with this
varnish, a small drop of the mixture will suffice for priming each copper cap or disc;
and as the spirit evaporates immediately, the fulminate will be fixed to the copper
beyond the risk of shaking or washing away. On the Continent, tincture of benjamin
is used for the same purpose; but as that balsamic resin leaves in combustion a voluminous
coal, which sandarach does not, the latter, which is the main constituent of
spirit varnish, seems better adapted for this purpose. It is sufficiently combustible, and
may be yet made by a due proportion, to soften the violence of the explosive mercury
on the nipple of the touch-hole. Fulminate prepared by my formula has no corrosive
influence whatsoever on iron or steel; and, therefore, if such a medium of applying it, as
I have now taken leave to suggest, should be found to answer, all fears on the score of
corrosion may for ever be set at rest.
Question 4. How far is the mixture (of fulminate and gunpowder) liable to be
affected by the moisture of the atmosphere, or by the intrusion of water; and will such
an accident affect its inflammability when dried again?
Answer. Well made fulminate, mixed with gunpowder and moistened, undergoes no
change, nor is it apt to get deteriorated by keeping any length of time in a damp
climate or a hazy atmosphere. Immersion in water would be apt to wash the nitre out
of the pulverine; but this result would be prevented if the match or priming mixture
were liquefied or brought to the pasty consistence not with water, but spirit varnish.
Such detonating caps would be indestructible, and might be alternately moistened
and dried without injury.
Question 5. Is it at all probable that the composition would be rendered more inflammable
or dangerous of use, by the heat of tropical climates?
Answer. No elevation of temperature of an atmospheric kind, compatible with human
existence, could cause spontaneous combustion of the fulminating mercury, or the
detonating matches made with it. In fact, its explosive temperature is so high as 367°
of Fahrenheit’s scale, and no inferior heat will cause its detonation.
Question 6. Is the mercurial vapour or gas arising from the ignition of a great
number of primers, and combined with the smoke of gunpowder in a confined space (as
in the case of troops in close bodies, squares, casemates, &c.) likely in its nature to be
found prejudicial to human health?
Answer. I have exploded in rapid succession of portions, 100 grains of fulminate of
mercury (equivalent to 300 or 400 primers), in a close chamber of small dimensions,
without experiencing the slightest inconvenience at the period, or afterwards, though my
head was surrounded by the vapours all the time of the operation. These vapours are,
in fact, so heavy that they subside almost immediately. When the fulminate mixed
with pulverine is exploded in the primers by condensed masses of troops, the mercury
will cause no injury to their health, nor one 100th part of the deleterious impression on
weak lungs which the gases of exploded gunpowder might by possibility inflict. These
gases are all, theoretically speaking, noxious to respiration; such as carbonic acid gas,
azote, carburetted hydrogen, and sulphuretted hydrogen, a deadly gas. Yet the soldier
who should betray any fear of gunpowder smoke would be an object of just ridicule.”
In the following September, I executed for the Board of Ordnance a set of experiments
complementary to those of the memoir, with the view of ascertaining the best
manner of protecting the fulminate when applied to the copper caps, from being
detached by carriage, or altered by keeping. The following were my results and conclusions.
1. Fulminate of mercury moistened upon copper is speedily decomposed by the superior
affinity of the copper over mercury, for oxygen and fulminic acid. Dryness
is, therefore, essential to the preservation of the fulminate; and hence charcoal, which is
apt to become moist, should not be introduced into percussion caps destined for distant
service.
2. An alcoholic solution of sandarach, commonly called spirit varnish, acts powerfully
on copper, with the production of a green efflorescence, which decomposes fulminate[535]
of mercury. Indeed, sandarach can decompose the salts of copper. It is therefore
ill adapted for attaching the fulminate to copper caps.
3. An alcoholic solution of shell-lac acts on copper, though more feebly than the
sandarach.
4. A solution of mastic in spirits of turpentine, whether alone or mixed with fulminate,
has no action whatever on bright copper, but protects it from being tarnished.
Such a varnish is very cheap, dries readily, adheres strongly, screens the fulminate from
damp, and does not impair or counteract its detonating powers. This, therefore, is
in my opinion the fittest medium for attaching the fulminate, and for softening the force
of its impulsion in any degree proportional to the thickness of the varnish.”
Fulminate of mercury is obtained in white grains, or short needles, of a silky lustre,
which become gray upon exposure to light, and detonate either by a blow or at a heat
under 370° F.; with the disengagement of azote, carbonic acid, as also of aqueous and
mercurial vapours; to the sudden formation of which gaseous products the report is
due. It detonates even in a moist condition; and when dry it explodes readily when
struck between two pieces of iron, less so between iron and bronze, with more difficulty
between marble and glass, or between two surfaces of marble or glass. It is hardly
possible to explode it by a blow with iron upon lead; and impossible by striking it with
iron upon wood. It fulminates easily when rubbed between two wooden surfaces; less
so between two of marble, two of iron, or one of iron against one of wood or marble.
The larger its crystals, the more apt they are to explode. By damping it with 5 per
cent. of water, it becomes less fulminating; the part of it struck still explodes with a
proper blow, but will not kindle the adjoining portion. Though moistened with 30
per cent. of water, it will occasionally explode by trituration between a wooden muller
and a marble slab, but only to a small extent, and never with any danger to the operator.
When an ounce of it, laid upon the bottom of a cask, is kindled, it strikes a round hole
down through it, as if it had been exposed to a four-pound shot, without splintering the
wood. If a train of fulminate of mercury be spread upon a piece of paper, covered with
some loose gunpowder, in exploding the former the latter will not be kindled, but
merely scattered. When gunpowder, however, is packed in a cartridge, or otherwise, it
may be certainly kindled by a percussion cap of the fulminate, and more completely
than by a priming of gunpowder. 81⁄2 parts of gunpowder exploded by a percussion cap,
have an equal projectile force as 10 exploded by a flint lock. If we add to this economy
in the charge of the barrel, the saving of the powder for priming, the advantage in
military service of the percussion system will become conspicuous.
The French calculate that 1 kilogramme of mercury will furnish 11⁄4 kil. (21⁄2 lbs.
nearly) of fulminate, which will be sufficient to charge 40,000 percussion caps. For
this purpose they grind the crystalline salt along with 30 per cent. of water upon a
marble table with a wooden muller; mixing with every 10 parts of the fulminate 6 of
gunpowder. A consistent dough is thus obtained, which, being dried in the air, is ready
for introducing into the bottoms of the copper caps. One quarter of a grain of the fulminate
is said to be fully sufficient for one priming.
Mr. Lovell, of the Royal Manufactory of Arms, has lately executed a series of experiments
upon priming powders. His trials, which occupied nearly 18 months, were made
for the purpose of ascertaining what is the advantage in point of force obtained by using
percussion primes. He had anticipated some extra energy would be imparted to the
charge of powder in the barrel, because he had repeatedly proved that a good strong
cap, exploded by itself on the nipple of the musquet, (without any charge of gunpowder),
will exert sufficient force upon the air within the barrel to blow a candle out
at a distance of 12 feet from the muzzle. He concluded also that stopping the escape
of fluid from the vent, as is done by the cap, would have some effect, but he attributed
most to the quickness and energy with which the powder of the charge is ignited by
the vivid stream of flame, generated by the percussion prime. The trials were made
from one and the same barrel, having a percussion lock on one side and a flint lock on
the other. The balls were fired against Austen’s recoiling target, a very delicate plegometer,
beginning with a charge of 150 grains (the present musquet charge), and
descending by 10 grains at a time (firing 30 rounds with each weight), down to 50
grains. The machine marked the decrease of force at each reduction in the charge very
satisfactorily, and the result of the whole average was that 8·84 parts of gunpowder
fired by percussion are equal to 10 parts fired by the flint.
To find out what sort of liberties might be taken with fulminate of mercury in
handling it, he placed 3 grains on an anvil, putting the end of a steel punch gently
on the top of it, and while so placed he covered the fulminate over with a drachm of dry
gunpowder. He then ignited the fulminate by a blow on the punch with the hammer,
but not a grain of the gunpowder was lighted, though it was blown about in all
directions. He then placed a train of fulminate as thick as a quill, and about 3 feet long,
on a table, and covered it over entirely with gunpowder except about an inch at one[536]
end; this he lighted with a hot iron, when the whole train went off without blazing a
grain of the gunpowder, which he swept together and blew up afterwards with a
match. He then took a tin box containing 500 copper caps, made a hole in the top of
the box, and through this hole ignited one of the caps in the middle, by means of the
punch and hammer on the outside; only two other caps besides the one struck exploded;
no injury was sustained by the remainder, except being discoloured. This he tried
repeatedly, and always with the same kind of result, never more than 3 or 4 caps exploding.
He then made a steel rammer red hot, and passed it through the hole in the box right
in amongst the caps, but it only ignited them where the hot iron came in actual contact
with the priming composition; when, however, he placed a few grains of gunpowder
loose among the caps, the hot iron lighted this, and produced a flame that blew off the
whole of them.
The same thing has been tried at Woolwich, where large packages of percussion caps
(some thousands) have been fired at with musquet balls, and only a few of the caps actually
hit by the ball exploded; but when any cartridges were connected with the packages, the
whole, caps and all were blown up. The flame of the fulminate is therefore hazardous, but
being so very ethereal, it requires for making primes, an admixture of some combustible
matter, as a little gunpowder, to condense or modify the flame.
FULMINIC ACID; (Acide fulminique, Fr.; Knallsäure, Germ.) is the explosive
constituent of the fulminating mercury of Howard, and the fulminating silver of Brugnatelli,
being generated by the reaction of alcohol and the acid nitrates of these
metals. It is a remarkable chemical fact, that fulminic acid has exactly the same
composition as cyanic acid; though the salts of the latter possess no detonating property,
and afford, in their decomposition by an oxygen acid, ammonia with carbonic
acid; while those of the former afford ammonia and prussic acid. All attempts to insulate
fulminic acid have proved unsuccessful, as it explodes with the slightest decomposing
force. It consists, by weight, of 2 primes of carbon, 1 of azote, and 1 of oxygen; or of
two volumes of carbonic acid, and one of azote. When two different bodies, like the
above, have the same composition, they are said to be isomeric.
FUMIGATION, is the employment of
fumes or vapours to purify articles of apparel,
and goods or apartments supposed
to be imbued with some infectious or contagious
poison or fumes. The vapours of
vinegar, the fumes of burning sulphur, explosion
of gunpowder, have been long prescribed
and practised, but they have in all
probability little or no efficacy. The diffusion
of such powerful agents as chlorine
gas, muriatic acid gas, or nitric acid vapour,
should alone be trusted to for the
destruction of morbific effluvia.
FURNACE OF ASSAY. Under
Assay, I have referred to a furnace constructed
by Messrs. Anfrye and d’Arcet,
which gives some peculiar facilities and
economy to the ancient process by fire. It
had originally a small pair of bellows attached
to it, for raising the heat rapidly to
the proper vitrifying pitch. The furnace,
171⁄2 inches high, and 71⁄2 inches wide, made
of pottery or fine clay, is represented fig.
481., supported upon a table, having a pair
of bellows beneath it. The laboratory is
at b, the blow-pipe of the bellows at d,
with a stop-cock, and the dome is surmounted
by a chimney a, c, in whose
lower part there is an opening with a sliding
door, for the introduction of the charcoal
fuel. The furnace is formed in three
pieces; a dome, a body, and an ash-pit.
A pair of tongs, a stoking hook, and cupel,
are seen to the right hand, and the plan of
the stone-ware grate, pierced with conical
holes, and a poker, are seen to the left. This
grate suits the furnace represented under
Assay. The following are comparative experiments made by means of this furnace:
[537]
| Numbers. |
Silver
employed. |
Lead
employed. |
Time of
Assay. |
Standards. |
Charcoal
used. |
| 1 |
1 Grain. |
4 Grains. |
12 |
minutes. |
947 |
millièmes. |
173 |
Grains. |
| 2 |
— |
— |
11 |
|
950 |
|
86 |
|
| 3 |
— |
— |
13 |
|
949 |
|
93 |
|
| 4 |
— |
— |
10 |
|
949 |
|
60 |
|
Each assay was therefore performed at an average in 111⁄2 minutes, and not much
more than a quarter of a pound of charcoal was used. An experiment of verification
in the ordinary assay furnace showed the standard to be 949 thousandths.
This furnace becomes a very convenient one for melting small quantities of metals in
analyses, by removing the muffle, and closing the several apertures with their appropriate
stoppers. A small pedestal may be then set in the middle of the grate, to support a
crucible, which may be introduced through the opening h. Coak may also be used as
fuel, either by itself or mixed with charcoal. For descriptions of various furnaces, see
Assay; Beer; Copper; Evaporation; Iron; Metallurgy; Ores; Silver;
Tin; &c.
FUSIBILITY. That property by which solids assume the fluid state.
Some chemists have asserted that fusion is simply a solution in caloric; but this
opinion includes too many yet undecided questions, to be hastily adopted.
Fusibility of Metals, as given by M. Thenard.
| |
Centigr. |
|
| 1. |
Fusible below
a red heat. |
Mercury |
|
-39 |
° |
|
| Potassium |
+58 |
° |
|
- |
Gay Lussac and Thenard. |
| Sodium |
90 |
| |
| Tin |
210 |
|
|
- |
Newton. |
| Bismuth |
256 |
|
| Lead |
260 |
|
Biot. |
| Tellurium |
A little less fusible than lead.—Klaproth. |
| Arsenic |
Undetermined. |
| Zinc |
370 |
° |
|
Brongniart. |
| Antimony |
A little below a red heat. |
| Cadmium |
|
Stromeyer. |
| |
Pyrometer of
Wedgewood. |
|
| 2. |
Infusible below
a red heat. |
Silver |
|
20 |
° |
|
Kennedy. |
| Copper |
|
27 |
|
|
- |
Wedgewood. |
| Gold |
|
32 |
| Cobalt |
|
A little less difficult to melt than iron. |
| Iron |
- |
|
130 |
|
Wedgewood. |
| 158 |
Sir G. M’Kenzie. |
| Manganese |
|
|
160 |
|
Guyton. |
| Nickel |
|
As manganese.—Richter. |
| Palladium |
|
- |
Nearly infusible; and to be obtained at a
forge heat only in small buttons. |
| Molybdenum |
| Uranium |
| Tungsten |
| Chromium |
| |
| Titanium |
|
- |
Infusible at the forge furnace. Fusible at the
oxyhydrogen blowpipe. See Blowpipe. |
| Cerium |
| Osmium |
| Iridium |
| Rhodium |
| Platinum |
| Columbium |
FUSIBLE METAL. See Alloy.
FUSTET. (Fustec, Fr.) The wood of the rhus cotinus, a fugitive yellow dye.
FUSTIAN, is a species of coarse thick tweelled cotton, and is generally dyed of an
olive, leaden, or other dark colour. Besides the common fustian, which is known by the
name of pillow (probably pilaw), the cotton stuffs called corduroy, velverett, velveteen,
thicksett, used for men’s wearing apparel, belong to the same fabric. The commonest
kind is merely a tweel of four, or sometimes five leaves, of a very close stout texture, and
very narrow, seldom exceeding 17 or 18 inches in breadth. It is cut from the loom in
half pieces, or ends as they are usually termed, about 35 yards long, and after undergoing
the subsequent operations of dyeing, dressing, and folding, is ready for the
market.
[538]
The draught and cording of common fustian is very simple, being generally a regular
or unbroken tweel of four or five leaves. Below are specimens of a few different kinds,
selected from those most general in Lancashire.
The number of leaves of heddles are represented by the lines across the paper, and the
cording by the cyphers in the little squares, those which raise every leaf being distinguished
by these marks, and those which sink them left blank, as more particularly
explained in the article Textile Fabric.
Of velvet, there are properly only two kinds, that with a plain, and that with a
tweeled, or, as it is here called, a Genoa ground, or back. When the material is silk, it
is called velvet, when cotton, velveteen; and this is the sole difference. In the same way
a common tweeled cloth, when composed of silk is called satin; when of cotton, fustian
or jean; of woollen, plaiding, serge, or kerseymere; and in the linen trade is distinguished
by a variety of names according to the quality or fineness, or the place where the article
is manufactured.
| |
No. 1.—Pillow Fustian. |
|
No. 2.—Plain Velveret. |
|
| |
0 |
|
|
|
|
4 |
|
5 |
|
1 |
|
§ |
|
0 |
|
|
|
|
3 |
|
1 |
|
|
| |
|
0 |
|
|
|
3 |
|
6 |
|
2 |
|
§ |
0 |
|
|
|
|
5 |
|
|
| |
|
|
0 |
|
6 |
|
2 |
|
3 |
|
§ |
0 |
|
|
0 |
0 |
|
0 |
|
2 |
|
|
| |
|
|
|
0 |
|
5 |
|
1 |
|
4 |
|
§ |
|
|
|
0 |
|
|
6 |
|
4 |
|
|
| |
2 |
4 |
3 |
1 |
|
4 |
6 |
2 |
3 |
1 |
|
| |
5 |
|
Of the above, each contains four leaves of heddles or healds; that represented by
No. 1. is wrought by four treddles, and that which is distinguished by No. 2. by five;
the succession of inserting the threads of warp into the heddles will be discovered by
the figures between the lines, and the order in which the treddles are to be successively
pressed down by the figures below.
| |
No. 3.—Double Jean. |
|
No. 4.—Plain Thicksett. |
|
| |
0 |
|
|
0 |
|
1 |
|
§ |
|
0 |
|
|
|
8 |
|
|
| |
0 |
|
0 |
|
|
2 |
|
§ |
|
0 |
0 |
0 |
|
|
6 |
|
4 |
|
|
| |
|
0 |
0 |
|
|
3 |
|
§ |
|
|
|
0 |
|
|
5 |
|
2 |
|
|
| |
|
0 |
|
0 |
4 |
|
§ |
0 |
|
|
0 |
0 |
|
7 |
|
3 |
|
1 |
|
|
| |
4 |
2 |
3 |
1 |
|
4 |
6 |
2 |
3 |
1 |
|
| |
5 |
|
7 |
|
These, like the former, are wrought with leaves. No. 3. requires four, and No. 4.
five treddles. The succession of inserting the threads of warp, and of working the
treddles, are marked by the respective numbers between and under the lines, as in the
former example. Both are fabrics of cloth in very general use and estimation as low
priced articles.
| |
No. 5.—Best Thicksett. |
|
No. 6.—Velvet Tuft. |
|
| |
0 |
|
|
0 |
0 |
|
3 |
|
1 |
|
§ |
|
0 |
|
|
|
5 |
|
3 |
|
1 |
|
|
| |
|
|
|
|
0 |
|
5 |
|
§ |
|
0 |
0 |
|
|
|
4 |
|
2 |
|
|
| |
|
0 |
|
|
|
|
2 |
|
§ |
0 |
|
|
0 |
0 |
|
4 |
|
2 |
|
|
| |
|
0 |
0 |
|
|
6 |
|
4 |
|
§ |
|
|
|
0 |
|
|
5 |
|
3 |
|
1 |
|
| |
6 |
4 |
2 |
3 |
1 |
|
6 |
4 |
2 |
3 |
1 |
|
| |
5 |
|
These are further specimens of what may be, and is, executed with four leaves, and
in both examples five treddles are used. With two other specimens we shall conclude
our examples of this description of work, and shall then add a very few specimens of
the more extensive kinds.
| |
No. 7.—Cord and Velveret. |
|
No. 8.—Thicksett Cord. |
|
| |
|
0 |
|
|
|
|
3 |
|
1 |
|
3 |
|
1 |
§ |
0 |
|
|
0 |
0 |
|
5 |
|
3 |
|
1 |
|
|
| |
|
0 |
0 |
|
|
|
5 |
|
7 |
|
5 |
|
§ |
|
0 |
|
|
|
|
4 |
|
2 |
|
|
| |
0 |
|
|
0 |
0 |
6 |
|
8 |
|
2 |
|
§ |
|
|
|
|
|
|
9 |
|
7 |
|
|
| |
|
|
|
0 |
|
|
4 |
|
2 |
|
6 |
|
4 |
|
§ |
|
0 |
0 |
|
|
10 |
|
8 |
|
6 |
|
|
| |
4 |
|
2 |
3 |
1 |
|
5 |
4 |
3 |
2 |
1 |
|
| |
6 |
5 |
|
In these the succession of drawing and working are marked like the former. The
next are examples of patterns wrought with six leaves. No. 9. has eight, and No. 10.
five heddles.
[539]
| |
No. 9.—Double Corduroy. |
|
No. 10.—Genoa Thicksett. |
|
| |
|
|
|
0 |
|
0 |
|
0 |
|
1 |
|
§ |
|
|
|
0 |
0 |
|
1 |
|
|
| |
|
0 |
|
|
|
0 |
|
|
|
2 |
|
§ |
|
|
0 |
|
0 |
|
2 |
|
|
| |
0 |
0 |
0 |
0 |
0 |
|
|
|
|
3 |
|
§ |
0 |
|
0 |
0 |
|
|
3 |
|
|
| |
|
|
|
0 |
|
0 |
|
|
|
4 |
|
§ |
|
0 |
|
0 |
0 |
|
4 |
|
|
| |
|
0 |
|
|
|
|
0 |
|
|
5 |
|
§ |
0 |
|
0 |
|
0 |
|
5 |
|
|
| |
|
0 |
|
0 |
|
|
|
|
6 |
|
§ |
|
0 |
|
0 |
|
6 |
|
|
| |
2 |
4 |
6 |
8 |
10 |
12 |
3 |
1 |
|
4 |
2 |
5 |
3 |
1 |
|
| |
7 |
5 |
|
8 |
6 |
11 |
9 |
7 |
|
| |
11 |
9 |
|
1 |
2 |
10 |
|
In both these the warp is inserted into the heddles the same way. The difference
is entirely in the application of the cords, and in the succession of pressing down the
treddles. We now give four specimens of the flushed and cut work, known by the name
of velveteen. They are also upon six leaves, and the difference is solely in the cording
and in the treading.
| |
No. 11.Queen’s Velveteens.No. 12. |
|
| |
|
0 |
|
0 |
0 |
|
|
1 |
|
§ |
|
|
|
0 |
|
0 |
|
1 |
|
|
| |
|
|
0 |
|
0 |
|
|
2 |
|
§ |
|
|
0 |
|
0 |
0 |
|
2 |
|
|
| |
|
|
0 |
0 |
|
|
|
3 |
|
§ |
|
|
|
0 |
0 |
|
|
3 |
|
|
| |
|
|
|
0 |
0 |
0 |
|
4 |
|
§ |
|
0 |
|
0 |
|
0 |
|
4 |
|
|
| |
|
|
0 |
|
0 |
|
|
5 |
|
§ |
|
|
|
|
0 |
0 |
|
5 |
|
|
| |
0 |
|
0 |
0 |
|
|
6 |
|
§ |
0 |
|
|
0 |
0 |
|
6 |
|
|
| |
1 |
2 |
12 |
8 |
4 |
2 |
|
2 |
4 |
3 |
|
1 |
|
| |
5 |
7 |
|
6 |
|
6 |
8 |
7 |
|
5 |
|
| |
9 |
11 |
|
10 |
|
10 |
12 |
11 |
9 |
|
| |
No. 13.—Plain Velveteen. |
|
No. 14.—Genoa Velveteen. |
|
| |
|
|
|
|
0 |
|
1 |
|
§ |
|
|
0 |
0 |
|
0 |
|
1 |
|
|
| |
0 |
|
|
0 |
|
|
2 |
|
§ |
|
0 |
0 |
|
|
|
|
2 |
|
|
| |
|
|
|
|
0 |
|
3 |
|
§ |
0 |
0 |
|
0 |
|
|
|
3 |
|
|
| |
|
|
0 |
0 |
|
|
4 |
|
§ |
|
|
0 |
0 |
|
|
|
4 |
|
|
| |
|
|
|
|
0 |
|
5 |
|
§ |
|
0 |
0 |
|
0 |
|
|
5 |
|
|
| |
|
0 |
|
0 |
|
6 |
|
§ |
|
0 |
|
0 |
|
|
6 |
|
|
| |
1 |
3 |
2 |
4 |
8 |
|
2 |
4 |
8 |
12 |
3 |
1 |
|
| |
5 |
7 |
6 |
|
6 |
|
7 |
5 |
|
| |
10 |
|
11 |
9 |
|
The additional varieties of figure which might be given are almost endless, but the
limits of this article will not admit a further detail. Those already given are the articles
in most general use. The varieties of fancy may be indulged to great extent, but
it is universally found, that the most simple patterns in every department of ornamental
weaving, are those which attract attention and command purchasers. We shall therefore
only add two examples of king’s cord or corduroy, two of Genoa and common
velvet, and two more of jean. These will be found below.
| |
No. 15.—King’s Cord. |
|
No. 16.—Dutch Cord. |
|
| |
|
|
|
|
0 |
0 |
|
1 |
|
§ |
|
|
0 |
|
|
|
4 |
|
1 |
|
|
| |
|
|
0 |
|
|
0 |
|
2 |
|
§ |
|
0 |
|
|
0 |
|
5 |
|
2 |
|
|
| |
|
|
0 |
0 |
|
|
|
7 |
|
3 |
|
§ |
0 |
|
|
0 |
|
|
6 |
|
3 |
|
|
| |
|
|
|
0 |
0 |
|
8 |
|
4 |
|
§ |
|
0 |
0 |
|
|
|
7 |
|
|
| |
|
0 |
|
|
0 |
0 |
|
5 |
|
§ |
0 |
|
0 |
|
0 |
|
8 |
|
|
| |
0 |
|
0 |
|
|
|
|
6 |
|
§ |
0 |
0 |
|
0 |
0 |
9 |
|
|
| |
1 |
3 |
8 |
6 |
4 |
2 |
|
6 |
4 |
2 |
3 |
1 |
|
| |
5 |
7 |
|
5 |
|
| |
No. 17.—Genoa Velvet. |
|
No. 18.—Plain Velvet. |
|
| |
|
|
0 |
|
|
0 |
|
1 |
|
§ |
|
|
|
|
|
|
1 |
|
|
| |
|
0 |
0 |
0 |
|
|
|
2 |
|
§ |
|
|
|
|
|
|
2 |
|
|
| |
0 |
0 |
|
|
|
|
|
3 |
|
§ |
|
|
|
|
|
|
3 |
|
|
| |
|
|
0 |
0 |
|
|
|
4 |
|
§ |
|
|
|
|
|
|
4 |
|
|
| |
|
0 |
|
0 |
|
|
|
5 |
|
§ |
|
|
|
|
|
|
5 |
|
|
| |
|
0 |
|
0 |
|
|
6 |
|
§ |
|
|
|
|
|
6 |
|
|
| |
2 |
4 |
8 |
12 |
3 |
1 |
|
1 |
3 |
4 |
2 |
8 |
|
| |
6 |
|
7 |
5 |
|
7 |
5 |
|
| |
10 |
|
11 |
9 |
|
After the fustian cloth is taken from the loom-beam, it is carried to the cutter, who
rips up the surface-threads of weft, and produces thereby a hairy-looking stuff.
[540]
Preparatory to its being cut, the cloth is spread evenly upon a table about six feet
long, upon each end of which a roller mounted with a ratchet-wheel is fixed; the one
to give off, and the other to wind up the piece, in the above six-feet lengths.
The knife is a steel rod about two feet long, and three-eighths of an inch square,
having a square handle at the one end; the other end is tapered away to a blade, as thin
as paper. To prevent this point from turning downwards and injuring the cloth, its
under side is covered by a guide which serves to stiffen it, as well as to prevent its
lower edge from cutting the fustian.
The operative (male or female) grasps the handle in the right hand, and insinuating
the projecting point of the guide under the weft, pushes the knife smartly forward
through the whole length of six feet, with a certain dexterous movement of the shoulder
and right side, balancing the body meanwhile, like a fencer, upon the left foot. This
process is repeated upon every adhesive line of the weft.
The next process to which fustians are exposed is steeping in hot water, to take out
the dressing paste. They are then dried, reeled, and brushed by a machine, &c.
From twenty to thirty pieces, each eighty yards long, may be brushed in an hour. The
breadth of the cloth is twenty inches. The maceration is performed by immersing the
bundled pieces in tanks of water, heated by waste steam; and the washing by means of
a reel or winch, kept revolving rapidly under the action of a stream of cold water, for
an hour or longer.
After being thus ripped up, it is taken to the brushing or teazling machine, to make
it shaggy.
This consists of a series of wooden rollers, turning freely upon iron axles, and covered
with tin-plate, rough with the burs of punched holes; and blocks of wood, whose concave
under surfaces are covered with card-cloth or card-brushes, and which are made to
traverse backwards and forwards in the direction of the axes of the revolving rollers,
during the passage of the cloth over them.
After they are brushed in the machine, the goods are singed by passing their cut
surface over a cylinder of iron, laid in a horizontal direction, and kept red hot by a flue.
See Singeing. They are now brushed again by the machine, and once more passed over
the singeing surface. The brushing and singeing are repeated a third or even occasionally
a fourth time, till the cord acquires a smooth polished appearance.
The goods are next steeped, washed, and bleached, by immersion in solution of chloride
of lime. They are then dyed by appropriate chemical means. After which they
are padded (imbued by the padding machine of the calico printers) with a solution of
glue, and passed over steam cylinders to stiffen them.
Smooth fustians, when cropped or shorn before dyeing, are called moleskins; but
when shorn after being dyed, are called beaverteen, they are both tweeled fabrics. Cantoon
is a fustian with a fine cord visible upon the one side, and a satiny surface of yarns
running at right angles to the cords upon the other side. The satiny side is sometimes
smoothed by singeing. The stuff is strong, and has a very fine aspect. Its price is
one shilling and sixpence a yard.
Common plain fustian, of a brown or drab colour, with satin top, is sold as low as
sevenpence a yard.
A fustian, with a small cord running in an oblique direction, has a very agreeable
appearance. It is called diagonal. Moleskin shorn, of a very strong texture, and a
drab dyed tint, is sold at 20d. per yard.
The weight of 90 yards of the narrow velveteen, in the green or undressed state, is
about 24 pounds. The goods made for the German, Italian, and Russian markets are
lighter, on account of the peculiarity in the mode of levying the import duty in these
countries.
Velveteens as they come from the loom, are sold wholesale by weight, and average a
price of 20d. per pound. They are usually woven with yarns of Upland and Brazil
cotton wool, spun together for the warp; or, sometimes, New Orleans alone. The
weft is usually Uplands, sometimes mixed with East India cotton wools.
Trowser velveteens are woven 19 inches wide, if they are to be cut up; if not, they
are woven 30 inches, and called beaverteen.
Cutting or cropping fustians by hand is a very laborious and delicate operation.
The invention of an improved apparatus for effecting the same end with automatic precision
and despatch, was therefore an object of no little interest to this peculiar manufacture
of Manchester. An ingenious machine, apparently well calculated for this purpose,
was made the subject of a patent by Messrs. William Wells and George Scholefield,
of Salford in November, 1834.
FUSTIC. (Bois jaune, Fr.; Gelbholz, Germ.) The old fustic of the English dyer,
as the article fustet is their yellow fustic. It is the wood of the Morus tinctoria. It
is light, not hard, and pale yellow with orange veins; it contains two colouring matters,
one resinous, and another soluble in water. The latter resembles weld, but it has more
of an orange cast, and is not so lively.
[541]
Its decoctions in water are brightened by the addition of a little glue, and more by
curdled milk. This wood is rich in colour, and imparts permanent dyes to woollen
stuffs, when aided by proper mordants. It unites well with the blue of the indigo vat,
and Saxon blue, in producing green of various shades. Alum, tartar, and solution of
tin, render its colour more vivid; sea salt and sulphate of iron deepen its hue. From 5
to 6 parts of old fustic are sufficient to give a lemon colour to 16 parts of cloth. The
colour of weld is however purer and less inclining to orange; but that of fustic is less
affected by acids than any other yellow dye. This wood is often employed with sulphate
of iron in producing olive and brownish tints, which agree well with its dull yellow. For
the same reason it is much used for dark greens.
GABRONITE, is a yellowish stony substance, of a greasy lustre and spec. gr.
= 2·74; affording no water by calcination; fusible at the blowpipe into an opaque
glass; soluble in muriatic acid; solution affords hardly any precipitate by oxalate of
ammonia. This mineral is distinguished by the large quantity of soda which it contains;
its constituents being,—silica, 54; alumina, 24; soda, 17·25; magnesia, 1·5; oxide of
iron, 1·25; water, 2. It belongs to the species Nepheline.
GADOLINITE; called also Yttrite and Ytterbite; is a mineral of a black, brownish,
or yellowish colour, granular, or compactly vitreous, and conchoidal fracture; of
spec. grav. 4·23? readily scratching glass; fusible at the blowpipe into an opaque glass,
sometimes with intumescence. It affords, with acids, a solution that lets fall, with caustic
soda, a precipitate partly re-soluble in carbonate of ammonia. It is remarkable for
containing from 45 to 55 per cent. of the earth Yttria; its remaining constituents being
silica, 25·8; oxide of cerium, 17·92; oxide of iron, 11·43. This mineral is very rare,
having been hitherto found only in the neighbourhood of Fahlun and Ytterby, in
Sweden; its peculiar constituent was discovered by Professor Gadolin.
GALACTOMETER, or LACTOMETER, is an instrument to ascertain the quality
of milk; an article often sophisticated in various ways. Fresh milk, rich in cream, has
a less specific gravity, than the same milk after it has been skimmed; and milk diluted
with water becomes proportionably lighter. Hence, when our purpose is to determine
the quantity of cream, the galactometer may consist merely of a long graduated glass
tube standing upright upon a sole. Having filled 100 measures with the recent milk,
we shall see, by the measures of cream thrown up, its value in this respect. A delicate
long-ranged glass hydrometer, graduated from 1·000 up to 1·060, affords the most
convenient means of detecting the degree of watery dilution, provided the absence of
thickening materials has been previously ascertained by filtration. Good fresh milk
indicates from 1·030 to 1·032; when the cream is removed, 1·035 to 1·037. When
its density is less than 1·028, we may infer it has been thinned with water.
GALBANUM, is a gum-resin, which occurs sometimes in yellow, shining tears,
easily agglutinated; of a strong durable smell; an acrid and bitter taste; at other
times in lumps. It exudes either spontaneously or from incisions made into the stem
of the bubon galbanum, a plant of the family of umbelliferæ, which grows in Africa,
particularly in Ethiopia. It contains 67 of resin; 19·3 of gum; 6·4 of volatile oil
and water; 7·5 of woody fibres and other impurities; with traces of acid malate of lime.
GALENA; (Plomb sulfuré, Fr.; Bleiglanz, Germ.;) is a metallic looking substance
of a lead-gray colour, which crystallizes in the cubical system, and is susceptible
of cleavages parallel to the faces of the cube; spec. gr. 7·7592; cannot be cut; fusible
at the blowpipe with exhalation of sulphureous vapours; is easily reduced to metallic
lead. Nitric acid first dissolves it, and then throws down sulphate of lead in a white
precipitate; the solution affording with plates of zinc, brilliant laminæ of lead (arbor
Saturni.) It consists of sulphur, 13; lead, 85; with a little iron, and sometimes a
minute quantity of silver. This is the richest ore of lead, and it occurs in almost every
geological formation, in veins, in masses, or in beds. It is almost always accompanied
by sulphuret of zinc, different salts of lead, heavy spar, fluor spar, &c. Galena in
powder, called Alquifoux, is employed as a glaze for coarse stoneware.
GALIPOT, is a name of a white semi-solid viscid rosin found on fir-trees; or an
inferior sort of turpentine, poor in oil.
GALLATES; salts consisting of gallic acid combined with bases; the most important
being that with oxide of iron, constituting a principal part of the black dye.
GALLIC ACID, is the peculiar acid extracted from gall-nuts; which see.
GALLIPOLI OIL, is a coarse olive oil, containing more or less mucilage; imported
from a sea port so named, of the province of Otranto, in the kingdom of Naples.
GALL-NUTS, or GALLS; (Noix de Galle, Fr.; Galläpfel, Germ.;) are excrescences
found upon the loaves and leaf-stalks of a species of oak, called Quercus infectoria,
which grows in the Levant. They are produced in consequence of the puncture
of the female of the gall wasp, (Cynips folii quercus), made in order to deposit her[542]
eggs; round which the juice of the tree exudes, and dries in concentric portions.
When the insect gets fully formed, it eats through the nut, and flies off.
The Levant galls are of two different appearances and qualities; the first are heavy,
compact, imperforated, the insect having not been sufficiently advanced to eat its way
through the shell; prickly on the surface; of a blackish or bluish green hue; about
the size of a musket ball. These are called black, blue, or Aleppo galls. The second
are light, spongy, pierced with one or more holes; smooth upon the surface, of a pale
grayish or reddish yellow colour, generally larger than the first, and are called white
galls. Besides the galls of the Levant, others come from Dalmatia, Illyria, Calabria,
&c.; but they are of inferior quality, being found upon the Quercus Cerris; they are
smaller, of a brownish colour, and of inferior value. The further south the galls are
grown, they are reckoned the better.
Galls consist principally of three substances; tannin or tannic acid; yellow extractive;
and gallic acid. Their decoction has a very astringent and unpleasant bitter taste.
The following are their habitudes with various reagents:—
Litmus paper is powerfully reddened.
Stannous chloride (protomuriate of tin), produces an isabel yellow precipitate.
Alum; a yellowish gray precipitate.
Acetate of lead; a thick yellowish white precipitate.
Acetate of copper; a chocolate brown precipitate.
Ferric sulphate (red sulphate of iron); a blue precipitate.
Sulphuric acid; a dirty yellowish precipitate.
Acetic acid brightens the muddy decoction.
The galls of the Quercus Cerris and common oak (Galles à l’épine, Fr.; Knoppern,
Germ.) are of a dark-brown colour, prickly on the surface, and irregular in shape and
size. They are used chiefly for tanning in Hungary, Dalmatia, and the southern provinces
of the Austrian states, where they abound.
Tannin or tannic acid is prepared as follows: Into a long narrow glass adopter tube
shut at its lower orifice with a cotton wick, a quantity of pounded galls are put, and
slightly pressed down. The tapering end of the tube being inserted into a matrass or
bottle, the vacant upper half of the tube is filled with sulphuric ether, and then closed with
a ground-glass stopper. Next day there will be found in the bottle a liquid in two distinct
strata; of which the more limpid occupies the upper part, and the other, of a syrupy
consistence and amber colour, the lower. More ether must be filtered through the
galls, till the thicker liquid ceases to augment. Both are now poured into a funnel,
closed with the finger, and after the dense liquor is settled at the bottom, it is steadily
run off into a capsule. This, after being washed repeatedly with ether, is to be transferred
into a stove chamber, or placed under the receiver of an air pump to be evaporated.
The residuary matter swells up in a spongy crystalline form of considerable brilliancy,
sometimes colourless, but more frequently of a faintly yellowish hue.
This is pure tannin, which exists in galls to the amount of from 40 to 45 per cent.
It is indispensable that the ether employed in the preceding process be previously agitated
with water, or that it contain some water, because by using anhydrous ether, not
a particle of tannin will be obtained.
Tannic acid is a white or yellowish solid, inodorous, extremely astringent, very soluble
in water and alcohol, much less so in sulphuric ether, and uncrystallizable. Its
watery solution, out of contact of air, undergoes no change; but if, in a very dilute
state, it be left exposed to the atmosphere, it loses gradually its transparency, and lets
fall a slightly grayish crystalline matter, consisting almost entirely of gallic acid. For
procuring this acid in a perfectly pure state, it is merely necessary to treat that solution
thus changed with animal charcoal, and to filter it in a boiling state, through
paper previously washed with dilute muriatic acid. The gallic acid will fall down in
crystals as the liquid cools.
If the preceding experiment be made in a graduated glass tube containing oxygen
over mercury, this gas will be absorbed, and a corresponding volume of carbonic acid
gas will be disengaged. In this case the liquor will appear in the course of a few weeks
as if traversed with numerous crystalline colourless needles of gallic acid.
Tannin or tannic acid consists of carbon 51·56; hydrogen 4·20; oxygen 44·24.
From the above facts it is obvious that gallic acid does not exist ready formed in
gall nuts, but that it is produced by the reaction of atmospheric oxygen upon the tannin
of these concretions.
Gallic acid is a solid, feebly acidulous and styptic to the taste, inodorous, crystallizing
in silky needles of the greatest whiteness; soluble in about 100 times its weight of
cold, and in a much smaller quantity of boiling water; more soluble in alcohol than in
water, but little so in sulphuric ether.
Gallic acid does not decompose the salts of protoxide of iron, but it forms, with the
sulphate of the peroxide, a dark blue precipitate, much less insoluble than the tannate
of iron. Gallic acid takes the oxide from the acetate and nitrate of lead, and throws[543]
down a white gallate unchangeable in the air, when it is mixed with that acetate and
nitrate. It occasions no precipitate in solutions of gelatine (isinglass or glue), by
which criterion its freedom from tannin is verified.
Gallic acid occurs but seldom in nature; and always united to brucine, veratrine, or
lime. Its constituents are, carbon 49·89; hydrogen 3·49; oxygen 46·62. In the
crystalline state it contains one atom of water, which it loses by drying.
Scheele obtained gallic acid by infusing pounded galls for 3 or 4 days in 8 times their
weight of water, and exposing the infusion to the air, in a vessel covered loosely with
paper. At the end of two months, the liquor had almost all evaporated, leaving some
mouldiness mixed with a crystalline precipitate. The former being removed, the deposit
was squeezed in a linen cloth, and then treated with boiling water. The solution
being gradually evaporated, yielded crystals of gallic acid, granular or star-like, of a
grayish colour. These crystals might be whitened by boiling their solution along with
a little animal charcoal. About one fifth of gallic acid may be obtained by Scheele’s
process from good gall-nuts.
From a decoction of 500 parts of galls, Sir H. Davy obtained 185 parts of solid extract;
which consisted of 130 parts of tannin; 31 parts of gallic acid with extractive;
13 parts of mucilage; 12 parts of lime and salts. Hence gall-nuts would seem to contain,
by this statement, more than two-thirds of their weight of tannin. This result is
now seen, from the above experiments of Pelouze, to have been incorrect, in consequence
of the admixture of yellow extractive in Davy’s tannin.
The uses of galls in many processes of dyeing, and in making black ink, are detailed
under their respective heads.
GALL OF ANIMALS, or OX-GALL, purification of. Painters in water colours,
scourers of clothes, and many others employ ox-gall or bile, but when it is not purified,
it is apt to do harm from the greenness of its own tint. It becomes therefore an important
object to clarify it, and to make it limpid and transparent like water. The
following process has been given for that purpose. Take the gall of newly killed oxen,
and after having allowed it to settle for 12 or 15 hours in a basin, pour the supernatant
liquor off the sediment into an evaporating dish of stone ware, and expose it to a boiling
heat in a water bath, till it is somewhat thick. Then spread it upon a dish, and
place it before a fire till it becomes nearly dry. In this state it may be kept for years
in jelly pots covered with paper, without undergoing any alteration. When it is to be
used, a piece of it of the size of a pea is to be dissolved in a table spoonful of water.
Another and probably a better mode of purifying ox-gall is the following. To a pint
of the gall boiled and skimmed, add one ounce of fine alum in powder, and leave the
mixture on the fire till the alum be dissolved. When cooled, pour into a bottle, which
is to be loosely corked. Now take a like quantity of gall also boiled and skimmed, add
an ounce of common salt to it, and dissolve with heat; put it when cold into a bottle,
which is likewise to be loosely corked. Either of these preparations may be kept for
several years without their emitting a bad smell. After remaining three months, at a
moderate temperature, they deposit a thick sediment, and become clearer, and fit for
ordinary uses, but not for artists in water colours and miniatures, on account of their
yellowish-green colour. To obviate this inconvenience, each of the above liquors is to
be decanted apart, after they have become perfectly settled, and the clear portion of both
mixed together in equal parts. The yellow colouring matter still retained by the
mixture coagulates immediately and precipitates, leaving the ox-gall perfectly purified
and colourless. If wished to be still finer, it may be passed through filtering paper;
but it becomes clearer with age, and never acquires a disagreeable smell, nor loses any
of its good qualities.
Clarified ox-gall combines readily with colouring matters or pigments, and gives them
solidity either by being mixed with or passed over them upon paper. It increases the
brilliancy and the durability of ultramarine, carmine, green, and in general of all delicate
colours, whilst it contributes to make them spread more evenly upon the paper, ivory,
&c. When mixed with gum Arabic, it thickens the colours without communicating to
them a disagreeable glistering appearance; it prevents the gum from cracking, and
fixes the colours so well that others may be applied over them without degradation.
Along with lamp black and gum, it forms a good imitation of China ink. When a coat
of ox-gall is put upon drawings made with black lead or crayons, the lines can no longer
be effaced, but may be painted over safely with a variety of colours previously mixed up
with the same ox-gall.
Miniature painters find a great advantage in employing it; by passing it over ivory,
it removes completely the unctuous matter from its surface; and when ground with the
colours, it makes them spread with the greatest ease, and renders them fast.
It serves also for transparencies. It is first passed over the varnished or oiled paper,
and is allowed to dry. The colours mixed with the gall are then applied, and cannot
afterwards be removed by any means.
It is adapted finally for taking out spots of grease and oil.
[544]
GALL OF GLASS, called also sandiver, is the neutral salt skimmed off the surface
of melted crown glass; which, if allowed to remain too long, is apt to be reabsorbed
in part, and to injure the quality of the metal, as the workmen call it.
GALVANIZED IRON, is the somewhat fantastic name newly given in France
to iron tinned by a peculiar patent process, whereby it resists the rusting influence of
damp air, and even moisture, much longer than ordinary tin plate. The following is
the prescribed process. Clean the surface of the iron perfectly by the joint action of
dilute acid and friction, plunge it into a bath of melted zinc, and stir it about till it be
alloyed superficially with this metal; then take it out, and immerse it in a bath of tin,
such as is used for making tin plate. The tin forms an exterior coat of alloy. When
the metal thus prepared is exposed to humidity, the zinc is said to oxidize slowly by a
galvanic action, and to protect the iron from rusting within it, whereby the outer tinned
surface remains for a long period perfectly white, in circumstances under which iron
tinned in the usual way would have been superficially browned and corroded with rust.
GAMBOGE; (Gomme Gutte, Fr.; Gutti, Germ.) is a gum resin, concreted in
the air, from the milky juice which exudes from several trees. The gambogia gutta, a tree
which grows wild upon the coasts of Ceylon and Malabar, produces the coarsest kind of
gamboge; the guttaefera vera (Stalagmites cambogioides) of Ceylon and Siam affords the
best. It comes to us in cylindrical lumps, which are outwardly brown yellow, but
reddish yellow within, as also in cakes; it is opaque, easily reducible to powder, of
specific gravity 1·207, scentless, and nearly devoid of taste, but leaves an acrid feeling in
the throat. Its powder and watery emulsion are yellow. It consists of 80 parts of a
hyacinth red resin, soluble in alcohol; and 20 parts of gum; but by another analysis, of
89 of resin, and 10·5 of gum. Gamboge is used as a pigment, and in miniature painting,
to tinge gold varnish; in medicine as a powerful purge. It should never be employed
by confectioners to colour their liqueurs, as they sometimes do.
GANGUE. A word derived from the German gang, a vein or channel. It signifies
the mineral substance which either encloses or usually accompanies any metallic ore in
the vein. Quartz, lamellar carbonate of lime, sulphate of baryta, sulphate and fluate of
lime, generally form the gangues; but a great many other substances become such
when they predominate in a vein. In metallurgic works the first thing is to break the
mixed ore into small pieces, in order to separate the valuable from the useless parts, by
processes called stamping, picking, sorting. See Metallurgy and Mines.
GARNET (Grenat, Fr.; Granat, Germ.); is a vitreous mineral of the cubic
system, of which the predominating forms are the rhomboidal dodecahedron and the
trapœzohedron; specific gravity varying from 3·35 to 4·24; fusible at the blowpipe.
Its constituents are, silica, 42; alumina, 20·0; lime, 34·0; protoxide of iron, 4. Garnets
are usually disseminated, and occur in all the primitive strata from gneiss to clay
slate. The finer varieties, noble garnet or Almandine, and the reddish varieties of
Grossulaire (Essonite), are employed in jewellery; the first are called the Syrian or
oriental; the others, hyacinth. In some parts of Germany garnets are so abundant as
to be used as fluxes to some iron ores; in others, the garnet gravel is washed, pounded,
and employed as a substitute for emery. The garnets of Pegu are most highly valued.
Factitious garnets may be made by the following composition:—Purest white glass, 2
ounces; glass of antimony, 1 ounce; powder of cassius, 1 grain; manganese, 1 grain.
GAS (Eng. and Fr.; Gaz, Germ.); is the generic name of all those elastic fluids
which are permanent under a considerable pressure, and at the temperature of zero of
Fahrenheit. In many of them, however, by the joint influence of excessive cold and
pressure, the repulsive state of the particles may be balanced or subverted, so as to
transform the elastic gas into a liquid or a solid. For this most interesting discovery,
we are indebted to the fine genius of Mr. Faraday.
The following table exhibits the temperatures and pressures at which certain gases
are liquefied.
| Name of the gas. |
Becomes liquid |
Calculated
boiling point;
Barom. =
30 inches. |
| At |
Under a pressure of |
| Sulphurous acid |
59 |
° |
F. |
3 |
atmospheres. |
- |
4 |
° |
Fahr. |
| Chlorine |
60 |
|
4 |
|
- |
22 |
|
| Ammonia |
50 |
|
6 |
·5 |
- |
64 |
|
| Sulphuretted hydrogen |
50 |
|
17 |
|
- |
142 |
|
| Carbonic acid |
32 |
|
36 |
|
- |
229 |
|
| Hydrochloric or muriatic acid |
50 |
|
50 |
|
- |
249 |
|
| Deutoxide of azote |
45 |
|
50 |
|
- |
254 |
|
[545]
Liquid carbonic acid becomes solidified, into a snowy-looking substance, by its own
rapid evaporation. Oxygen, hydrogen, and azote, have hitherto resisted all attempts to
divest them of their elastic form. For this purpose, it is probable that a condensing
force equal to that of 650 atmospheres, will be required.
The volume of any gas is, generally speaking, inversely as the pressure to which it is
exposed; thus, under a double pressure its bulk becomes one-half; under a triple
pressure, one-third; and so on. For the change of volume in gaseous bodies by heat,
see Expansion.
Ammonia, carbonic acid, carburetted hydrogen, chlorine, muriatic acid, sulphurous
acid, sulphuretted hydrogen, are the gases of most direct interest in the arts and manufactures.
Their detailed examination belongs to a work on chemistry.
GAS-LIGHT. (Eclairage par gas, Fr.; Gaslicht, Germ.) Dr. Clayton demonstrated,
by numerous experiments in 1737 and 1738, that bituminous pit-coal,
subjected to a red heat in close vessels, afforded a great deal of an air similar
to the fire-damp of mines, but which burned with a brighter flame. It does
not appear that this species of factitious air was ever produced from pit-coal for
the purpose of artificial illumination till 1792, when Mr. William Murdoch, engineer to
Messrs. Bolton and Watt, employed coal gas for lighting his house and offices, at Redruth
in Cornwall. The gas was generated in an iron retort, whence it was received in
a gasometer, distributed in different situations by pipes, and finally burned at small
apertures which could be opened and stopped at pleasure. He moreover made this
light movable, by confining the gas in portable tin-plate vessels, and burning it wherever
he pleased. Between this period and 1802, Mr. Murdoch continued at intervals
to make similar experiments; and upon occasion of the national illumination in the
spring of the latter year, at the peace of Amiens, he lighted up part of the Soho manufactory
with a public display of gas lights.
The earliest application of this artificial light, on a large systematic scale, was made
at Manchester; where an apparatus for lighting the great cotton mills of Messrs.
Philips and Lee, was fitted up in 1804 and 1805, under the direction of Mr. Murdoch.
A quantity of light, nearly equal to 3000 candles, was produced and distributed in this
building. This splendid pattern has been since followed very generally in Great
Britain, and more or less in many parts of the continents of Europe and America. By
the year 1822, gas-lighting in London had become the business of many public companies.
At the Peter-street station, for example, 300 retorts had been erected, supplying
15 gasometers, having each an average capacity of 20·626 cubic feet, but, being never
quite filled, their total contents in gas might be estimated at 309,385 cubic feet. The
extent of main pipes of distribution belonging to this station was then about 57 miles,
with two separate mains in some of the streets. The product of gas was from 10,000
to 12,000 cubic feet from a chaldron of coals. The annual consumption of coals was
therefore altogether 9282 chaldrons, affording 11,384,000 cubic feet of gas, allowing
153 retorts to be in constant daily action, upon an average of the year; and illuminating
10,660 private lamps, 2248 street lamps, and 3894 theatre lamps.
At the Brick-lane works, 371 retorts were fixed in 1822, 133 being worked on an
average of summer and winter. There were 12 gasometers, charged with an average
quantity of gas amounting to 197,214 cubic feet. Of coals, 8060 chaldrons were
annually consumed; 96,720,000 cubic feet of gas were generated; for the supply of
1978 public lamps, and 7366 private ones, connected with main pipes 40 miles long.
At the Curtain-road gas establishment, there were 240 retorts; but the greatest number
worked in 1821 was only 80, and the lowest 21. The six gasometers had an average
contents of 90,467 cubic feet. Of coals, 3336 chaldrons were annually consumed,
yielding 40,040,000 cubic feet of gas, that supplied 3860 private lamps, and 629 public
ones, by means of mains 25 miles long. The above three stations belonged to the
London Gas Light and Coke Company.
The City of London Gas Light Company, Dorset-street, had built up 230 retorts,
and 6 gasometers, while two were preparing; having a total capacity of 181,282 cubic
feet. Of private lamps 5423 were lighted, and 2413 public ones, from mains extending
50 miles. The quantity of coals carbonized amounted to 8840 chaldrons; producing
106,080,000 cubic feet of gas.
The South London Gas Light and Coke Company had mounted at Bankside 143
retorts, with 3 gasometers; the contents of the whole being 41,110 cubic feet, connected
with mains from 30 to 40 miles long. At their other station, in Wellington-street, 3
large gasometers were then erecting, with a capacity of 73,565 cubic feet, which were
to be supplied with gas from Bankside, till retorts were mounted for them.
The Imperial Gas Light and Coke Company had at that time 6 gasometers in progress
at their Hackney station.
In 1822 there were thus four great companies, having in all 47 gasometers at work,
capable of containing 917,940 cubic feet of gas, supplied by 1315 retorts, which generated[546]
per annum upwards of 397,000,000 cubic feet of gas, by which 61,203 private
lamps, and 7268 public or street lamps, were lighted in the metropolis. Besides these
public companies, there were likewise several private ones.
1. Of the generation of illuminating gases.—Pure hydrogen gas burns with too feeble
a flame to be employed for illumination. But carburetted hydrogen having the property
of precipitating its carbon in the act of burning, its solid particles become incandescent,
and diffuse a vivid light. The more carbon it contains, the more brightly does it burn.
This gas exists in two distinct states of combination. In the first, two measures of
hydrogen gas are combined with one measure of the vapour of carbon, forming
together one measure whose specific gravity is of course the sum of the weights of the
constituents, or 0·559; atmospherical air being 1·000. This is the gas which is found
in mines, and is also evolved in ditches from decomposing vegetable matter. In the
second, two measures of hydrogen gas are combined with two of gaseous carbon, forming
also one volume or measure whose weight or specific gravity is 0·985. This was at one
time called the olefiant gas, because when mixed with chlorine an oily looking compound
was produced. It may be called as well oil gas, because it is generated in
considerable quantities by the igneous decomposition of oil. Thus the olefiant gas
contains in the same volume double the quantity of carbon of common carburetted
hydrogen, and it burns with a proportionably brighter flame. The gaseous oxide of
carbon, as well as sulphuretted hydrogen gas, burns with a feeble blue light, but
the latter produces in combustion sulphurous acid, an offensive and noxious gas.
By dry distillation or carbonization in close vessels, all bodies of vegetable and animal
origin disengage carburetted hydrogen gas; even charcoal when placed in ignition in
contact with steam, by decomposing the water, produces abundance of carbonic acid,
carburetted hydrogen, hydrogen, and carbonic oxide. After separating the carbonic
acid with lime water, that mixed gas contains in 100 measures, 20 of carburetted hydrogen;
the rest being hydrogen and carbonic oxide, so that the gaseous mixture
cannot be used for illumination. The best substances for furnishing a gas rich in
luminifereous materials are, pitcoal, especially the cannel coal, resin, oil, fats of all kinds,
tar, wax, &c. In some cases the gases evolved during the igneous decomposition of
bones and other animal matters for the production of ammonia, may be employed for
procuring light, but they are apt to emit a fetid odour.
When coals are heated in a cast-iron retort to ignition, the progress of decomposition
is as follows. First, and before the retort becomes red hot, steam issues along with the
atmospheric air. When the retort begins to redden, tar distils in considerable quantity
with some combustible gas, of which hydrogen mixed with ammoniacal gas forms a part.
The evolution of gas increases as the retort becomes hotter, with a continual production
of tar and ammoniacal liquor as well as sulphurous acid from the pyrites of the coal, which
unites with the ammonia. When the retort has come to a bright cherry red heat, the
disengagement of gas is most active. By and bye the gaseous production diminishes,
and eventually ceases entirely, although the heat be increased. In the retort a quantity
of carbonized coal or coke remains, while tar is found at the bottom of the receiver,
covered with the ammoniacal liquor, and combined with carbonic and sulphurous acids,
and sulphuretted hydrogen.
If during this distillation, the combustible gas be collected and examined at the
several stages of the process, it is found to differ extremely in its luminiferous powers.
That which comes off before the retort has acquired its proper temperature, gives a
feeble light, and resembles the gas obtained by the ignition of moist charcoal, consisting
chiefly of hydrogen. That evolved when the retort has just acquired throughout a
vivid red heat, is the best of all, consisting chiefly of bicarburetted hydrogen or olefiant
gas. From good coal, it consists, for example in 100 measures, of 13 of olefiant gas,
82·5 of carburetted hydrogen, 3·2 carbonic oxide, 1·3 azote; the mixture having a
specific gravity of 0·650. At a later period, as after 5 hours, it contains 7 measures of
olefiant gas, 56 of carburetted hydrogen, 11 of carbonic oxide, 21·3 of hydrogen, 4·7 of
azote; the specific gravity of the whole being 0·500. Towards the end of the operation,
as after 10 hours, it contains twenty measures of carburetted hydrogen, 10 of carbonic
oxide, 60 of hydrogen, 10 of azote, with a specific gravity of only 0·345. The hydrogen
becomes sulphuretted hydrogen, if there be much pyritous matter in the coal. The
larger proportion of the gas is disengaged during the first hour, amounting to about
one fifth of the whole; in the three following hours the disengagement is tolerably
uniform, constituting in all fifty-four hundredths; in the sixth hour, it is one tenth; in
the seventh and eighth hours, sixteen hundredths.
From these observations are derived the rules for the production of a good light gas
from coals. They show that the distillation should commence with a retort previously
heated to a cherry red, since thereby good gas is immediately produced, and a portion
of the tar is also converted into gas, instead of being simply distilled over into the condenser
pit; that this heat should be steadily continued during the whole operation,
from 5 to 8 hours; that it should not be increased, especially towards the end, for fear[547]
of generating carbonic oxide and hydrogen gases, as well as of injuring the retort when
the cooling agency of gasefication has become feeble; and that the operation should be
stopped some time before gas ceases to come over, lest gases with feeble illuminating power
should impoverish the contents of the gasometer. Upon the average, a pound of good coal
affords four cubic feet of gas, or a chaldron = 26 cwt. London measure, affords from 12,000
to 15,000 cubic feet, according to the form of the retort, and the manner of firing it.
When oil, fats, rosin, tar, &c. are employed for the production of a light gas, it is not
sufficient to introduce these substances into the retorts, and to heat them, as is done
with coals. In this case, the greater part of them would distil over in the state of volatile
oils, and very little gas be generated, only as much as corresponded to the quantity
of fat, &c. in immediate contact with the retort. It becomes therefore necessary to
fill the retorts with pieces of brick or coke; and to keep them in ignition, while the
oil, &c. is slowly introduced into their interior. The fats instantly assume the vaporous
state, and thus coming into contact upon an extensive surface with the ignited bricks,
are decomposed into combustible gases. A small portion of carbonaceous matter remains
in the retort, while much olefiant gas is formed, possessing a superior illuminating
power to common coal gas, and entirely free from sulphureous impregnation.
The best oil gas is generated at a dull red, a heat much below what is requisite for the
decomposition of coal. A more intense heat would indeed produce a greater volume
of gas, but of a poorer quality, because the olefiant gas thereby deposits one half of
its carbon, and is converted into common carburetted hydrogen. Oil affords at a lively
red heat, gases which contain in 100 measures, 19 of olefiant gas, 32·4 of carburetted
hydrogen, 12·2 of carbonic oxide gas, 32·4 of hydrogen, and 4 of azote; the mean specific
gravity being only 0·590. At a more moderate temperature it yields 22·5 of the
olefiant, 50·3 carburetted hydrogen, 15·5 carbonic oxide, 7·7 hydrogen, and 4 azote,
with a specific gravity of 0·758. It contains when generated by dull ignition, as is
usual in works on the manufacturing scale, in 100 parts, from 38 to 40 of olefiant gas,
and besides the carburetted hydrogen, a few per cents. of carbonic oxide and azote,
with a specific gravity of 0·900, and even upwards. One pound of oil or fluid fat affords
15 cubic feet of gas; of tar affords about 12 cubic feet; of rosin or pitch, 10 cubic feet.
When the oil gas is compressed by a force of from 15 to 20 atmospheres, as was the
practice of the Portable Gas Company, about one fifth of the volume of the gas becomes
liquefied into an oily, very volatile fluid, having the specific gravity 0·821. It is a
mixture of three fluids (consisting of carburetted hydrogen), of different degrees of
volatility. The most volatile of these boils even under 32° F. Some of the vapour of
this gas-oil is mixed with the olefiant gas in the general products of decomposition; in
consequence of which they are sometimes richer in carbon than even olefiant gas, and
have a higher illuminating power. Oil gas contains about 22 per cent. and coal gas
about 31⁄4 per cent. of this oily vapour. In the estimations of the composition of the
gases given above, this vapour is included under olefiant gas. This vapour combines
readily with sulphuric acid, and is thus precipitated from the gaseous mixture. The
amount of olefiant gas is shown, by adding to the gas, contained over water, one half of
its volume of chlorine, which, in the course of an hour or two, condenses the olefiant gas
into an oily looking liquid (chloride of hydrocarbon.) After the mixture, the gases
must be screened from the light, otherwise the common carburetted hydrogen would also
combine with the chlorine, while water and carbonic acid would make their appearance.
The oil employed for affording gas is the crudest and cheapest that can be bought;
even the blubber and sediment of whale oil are employed with advantage. After all,
however, coal is so much cheaper, and the gas produced from it is now so well purified,
that oil and rosin are very little used in gas apparatus.
Apparatus for Coal Gas.—Coal gas, as it issues from the retort, cannot be directly
employed for illumination; for it contains vapours of tar and coal oil, as also steam
impregnated with the carbonate, sulphite, and hydrosulphuret of ammonia. These
vapours would readily condense in the pipes through which the gas must be distributed,
and would produce obstructions; they must therefore be so far removed by
previous cooling, as to be liable to occasion no troublesome condensation at ordinary
temperatures. The crude coal gas contains moreover sulphuretted hydrogen, whose combustion
for light would exhale an offensive sulphureous odour, that ought to be got
rid of as much as possible. Carbonic acid and carbonic oxide gases, generated at first
from the decomposition of the steam by the ignited coal, enfeeble the illuminating power
of the gas, and should be removed. The disengagement of gas in the retorts is never
uniform, but varies with the degree of heat to which they are exposed; for which
reason the gas must be received in a gasometer, where it may experience uniform
pressure, and be discharged uniformly into the pipes of distribution, in order to
ensure a steady discharge of gas, and uniform intensity of light in the burners. A coal
gas apparatus ought therefore to be so constructed as not only to generate the gas itself,
but to fulfil the above conditions.
[548]
In fig. 482., such an apparatus is represented, where the various parts are shown
connected with each other, in section.
A is the furnace with its set of cylindrical or elliptical retorts, five in number. From
each of these retorts, a tube b proceeds perpendicularly upwards, and then by a curve
or saddle-tube, it turns downwards,
where it enters a long
horizontal cylinder under B, shut
at each end with a screw cap, and
descends to beneath its middle,
so as to dip about an inch into
the water contained in it. From
one end of this cylinder the tube
d passes downwards, to connect
itself with a horizontal tube which
enters into the tar pit or cistern
C, by means of the vertical branch
f. This branch reaches to near
the bottom of the cylindrical vessel,
which sits on the sole of the
tar cistern. From the other side
of the vertical branch f, the main
pipe proceeds to the condenser D,
and thence by the pipe l, into the
purifier E; from which the gas
is immediately transmitted by the
pipe p into the gasometer F.
The operation proceeds in the
following way:—As soon as gas
begins to be disengaged from the
ignited retort, tar and ammoniacal
liquor are deposited in the
cylindrical receiver B, and fill it
up till the superfluity runs over
by the pipe d, the level being
constantly preserved at the line
shown in the figure. By the
same tarry liquid, the orifices of
the several pipes b, issuing from
the retorts, are closed; whereby
the gas in the pipe d has its communication
cut off with the gas
in the retorts. Hence if one of
the retorts be opened and emptied,
it remains shut off from the
rest of the apparatus. This insulation
of the several retorts is
the function of the pipe under B,
and therefore the recurved tube b
must be dipped as far under the
surface of the tarry liquid, as to
be in equilibrio with the pressure
of the gas upon the water in
the purifier. The tube b is
closed at top with a screw cap,
which can be taken off at pleasure,
to permit the interior to be
cleansed.
Both by the overflow from the
receiver-pipe B, and by subsequent
condensation in the tube d, tar and ammoniacal liquor collect progressively in the
cistern or pit under C, by which mingled liquids the lower orifice of the vertical tube f is
closed, so that the gas cannot escape into the empty space of this cistern. These liquids
flow over the edges of the inner vessel when it is full, and may, from time to time,
be drawn off by the stopcock at the bottom of the cistern.
Though the gas has, in its progress hitherto, deposited a good deal of its tarry and
ammoniacal vapours, yet, in consequence of its high temperature, it still retains a considerable
portion of them, which must be immediately abstracted, otherwise the tar[549]
would pollute the lime in the vessel E, and interfere with its purification. On this account
the gas should, at this period of the process, be cooled as much as possible, in
order to condense these vapours, and to favour the action of the lime in the purifier E,
upon the sulphuretted hydrogen, which is more energetic the lower the temperature of
the gas. The coal gas passes, therefore, from the tube f into the tube h of the condenser
D, which is placed in an iron chest g filled with water, and it deposits more tar
and ammoniacal liquor in the under part of the cistern at t, t. When these liquids have
risen to a certain level, they overflow into the tar-pit, as shown in the figure, to be
drawn off by the stopcock as occasion may require.
The refrigerated gas is now conducted into the purifier E, which is filled with milk
of lime, made by mixing one part of slaked lime with 25 parts of water. The gas, as it
enters by the pipe l, depresses the water in the wide cylinder n, thence passes under the perforated
disc in the under part of that cylinder, and rising up through innumerable small
holes is distributed throughout the lime liquid in the vessel m. By contact with the
lime on this extended surface, the gas is stripped of its sulphuretted hydrogen and carbonic
acid, which are condensed into the hydro-sulphuret and carbonate of lime; it now
enters the gasometer F in a purified state, through the pipe p t, and occupies the space
q. The gasometer, pressing with a small unbalanced force over the counterweight s,
expels it through the main u u, in communication with the pipes of distribution through
the buildings or streets to be illuminated.
The parts A B C D E and F, of which this apparatus consists, are essential constituents
of every good coal-gas work. Their construction rests upon peculiar principles, is susceptible
of certain modifications, and therefore deserves to be considered in detail.
The Retorts.—These are generally made of cast iron, though they have occasionally
been made of baked clay, like common earthenware retorts. The original form was a
cylinder, which was changed to an ellipse, with the long axis in a horizontal direction,
then into the shape of the letter D with the straight line undermost, and lastly into a
semi-cylinder, with its horizontal diameter 22 inches, and its vertical varying from 9 to
12. The kidney form was at one time preferred, but it has been little used of late.
The form of retort represented in fig. 483. has been found to yield the largest quantity
of good gas in the shortest time, and
with the least quantity of firing. The
length is 71⁄2, and the transverse area, from
one foot to a foot and a half square.
The arrows show the direction of the
flame and draught in this excellent bench
of retorts, as mounted by Messrs. Barlow.
The charge of coals is most conveniently
introduced in a tray of sheet iron,
made somewhat like a grocer’s scoop,
adapted to the size of the retort, which is
pushed home to its further end, inverted
so as to turn out the contents, and then
immediately withdrawn.
The duration of the process, or the
time of completing a distillation, depends
upon the nature of the coal and the form
of the retort. With cylindrical retorts it
cannot be finished in less than 6 hours,
but with elliptical and semi-cylindrical
retorts, it may be completed in 4 or 5
hours. If the distillation be continued
in the former for 8 hours, and in the latter for 6, gas will continue to be obtained, but
during the latter period of the operation, of indifferent quality.
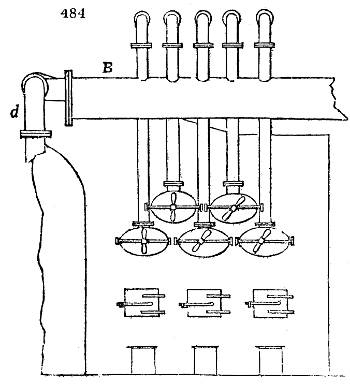
The Receiver.—If the furnace contains only 2 or 3 retorts, a simple cylindrical vessel
standing on the ground half filled with water, may serve as a receiver; into which the
tube from the retort may be plunged. It should be provided with an overflow pipe for
the tar and ammoniacal liquor. For a range of several retorts, a long horizontal cylinder
is preferable, like that represented at B in fig. 484. Its diameter is from 10 to 15
inches. This cylinder may be so constructed as to separate the tar from the ammoniacal
liquor, by means of a syphon attached to one of its ends.
The Condenser.—The condenser, represented in fig. 482., consists of a square chest,
g, made of wrought iron plates open at top, but having its bottom pierced with a row
of holes, to receive a series of tubes. To these holes the upright four-inch tubes h h are
secured by flanges and screws, and they are connected in pairs at top by the curved or
saddle tubes. The said bottom forms the cover of the chest t, t, which is divided by vertical
iron partitions, into half as many compartments as there are tubes.
[550]
These partition plates are left open at bottom, so as to place the liquids of each compartment
in communication. Thereby the gas passes up and down the series of tubes,
in proceeding from one compartment to another. The condensed liquids descend into
the box t, t, and flow over into the
tar cistern, when they rise above the
level t, t. The tar may be drawn off
from time to time by the stopcock.
Through the tube k, cold water flows
into the condenser chest, and the
warm water passes away by a pipe
at its upper edge.
The extent of surface which the
gas requires for its refrigeration before
it is admitted into the washing-lime
apparatus, depends upon the
temperature of the milk of lime, and
the quantity of gas generated in a
certain time.
It may be assumed as a determination
sufficiently exact, that 10
square feet of surface of the condenser
can cool a cubic foot of gas
per minute to the temperature of the
cooling water. For example, suppose
a furnace or arch with 5 retorts
of 150 pounds of coal each, to produce
in 5 hours 3000 cubic feet of gas, or 10 cubic feet per minute, there would be
required, for the cooling surface of the condenser, 100 square feet = 10 × 10. Suppose
100,000 cubic feet of gas to be produced in 24 hours, for which 8 or 9 such arches
must be employed, the condensing surface must contain from 800 to 900 square feet.
The Purifier.—The apparatus represented in the preceding figure is composed of a
cylindrical iron vessel, with an air-tight cover screwed upon it, through which the
cylinder n is also fixed air-tight. The bottom of this cylinder spreads out like the brim
of a hat, forming a horizontal circular partition, which is pierced with holes. Through
a stuffing box, in the cover of this interior cylinder, the vertical axis of the agitator
passes, which is turned by wheel and pinion work, in order to stir up the lime from the
bottom of the water in the purifier. The vessel o serves for introducing fresh milk of
lime, as also for letting it off by a stopcock when it has become too foul for further use.
The quantity of lime should be proportioned to the quantity of sulphuretted hydrogen
and carbonic acid contained in the gas. Supposing that in good coal gas there is 5 per
cent. of these gases, about one pound and a half of lime will be requisite for every hundred
cubic feet of coal gas generated, which amounts to nearly one-sixteenth of the
weight of coal subjected to decomposition. This quantity of lime mixed with the
proper quantity of water will form about a cubic foot of milk of lime. Consequently,
the capacity of the purifier, that is, of the interior space filled with liquid, may be taken
at four-sevenths of a cubic foot for every hundred cubic feet of gas passing through it in
one operation; or for 175 cubic feet of gas, one cubic foot of liquor. After every
operation, that is, after every five or six hours, the purifier must be filled afresh. Suppose
that in the course of one operation 20,000 cubic feet of gas pass through the
machine, this should be able to contain 20,000175 = 114 cubic feet of milk of lime; whence
its diameter should be seven feet, and the height of the liquid three feet. If the capacity
of the vessel be less, the lime milk must be more frequently changed.
In some of the large gas works of London the purifier has the following construction,
whereby an uninterrupted influx and efflux of milk of lime takes place. Three single
purifiers are so connected together, that the second vessel stands higher than the first,
and the third than the second; so that the discharge tube of the superior vessel, placed
somewhat below its cover, enters into the upper part of the next lower vessel; consequently,
should the milk of lime in the third and uppermost vessel rise above its ordinary
level, it will flow over into the second, and thence in the same way into the first;
from which it is let off by the eduction pipe. A tube introduces the gas from the condenser
into the first vessel, another tube does the same thing for the second vessel, &c.,
and the tube of the third vessel conducts the gas into the gasometer. Into the third
vessel, milk of lime is constantly made to flow from a cistern upon a higher level.
By this arrangement, the gas passing through the several vessels in proportion as it
is purified, comes progressively into contact with purer milk of lime, whereby its purification
becomes more complete. The agitator c, provided with two stirring paddles, is[551]
kept in continual rotation. The pressure which the gas has here to overcome is
naturally three times as great as with a single purifier of like depth.
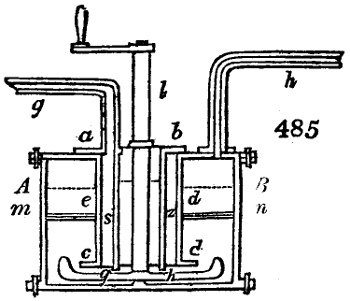
Fig. 485. is a simple form of purifier, which has been found to answer well in practice.
Through the cover of the vessel A B, the wide cylinder e d is inserted, having its
lower end pierced with numerous holes. Concentric with that cylinder is the narrower
one s z, bound above with the flange a b, but open at top and bottom. The under edge
g h of this cylinder descends a few inches below the end c d of the outer one. About
the middle of the vessel the perforated shelf m n is placed. The shaft of the agitator l,
passes through a stuffing box upon the top of the vessel. The gas-pipe g, proceeding
from the condenser, enters through the flange a b in the outer cylinder, while the gas-pipe
h goes from the cover to the gasometer. A stopcock upon the side, whose orifice
of discharge is somewhat higher than the under edge of the outer cylinder, serves to
draw off the milk of lime. As the gas enters through the pipe g into the space between
the two cylinders, it displaces the liquor till it arrives at the holes in the under edge of
the outer cylinder, through which, as well as under the edge, it flows, and then passes
up through the apertures of the shelf m n into the milk of lime chamber; the level of
which is shown by the dotted line. The stirrer, l, should be turned by wheel work,
though it is here shown as put in motion by a winch handle.
In order to judge of the degree of purity of the gas after its transmission through
the lime machine, a slender syphon tube provided with a stopcock may have the one
end inserted in its cover, and the other dipped into a vessel containing a solution of
acetate of lead. Whenever the solution has been rendered turbid by the precipitation
of sulphuret of lead, it should be renewed. The saturated and fetid milk of lime is
evaporated in oblong cast-iron troughs placed in the ash-pit of the furnaces, and the
dried lime is partly employed for luting the apparatus, and partly disposed of for a
mortar or manure.
By this purifier, and others of similar construction, the gas in the preceding parts of
the apparatus, as in the retorts and the condenser, suffers a pressure equal to a column
of water about two feet high; and in the last described purifier even a greater pressure.
This pressure is not disadvantageous, but is of use in two respects; 1. it shows by
a brisk jet of gas when the apparatus is not air-tight, and it prevents common air from
entering into the retorts; 2. this compression of the gas favours the condensation of the
tar and ammoniacal liquor. The effect of such a degree of pressure in expanding the
metal of the ignited retorts is quite inconsiderable, and may be neglected. Two contrivances
have, however, been proposed for taking off this pressure in the purifier.
In fig. 486., m m are two similar vessels of a round or rectangular form, furnished at
their upper border with a groove filled with water, into which the under edge of the
cover fits, so as to make the vessel air-tight. The cover is suspended by a cord or chain,
which goes over a pulley, and may be raised or lowered at pleasure. The vessels themselves
have perforated bottoms, r r′, covered with wetted moss or hay sprinkled over with
slaked and sifted quicklime. The gas passes through the loosely compacted matter of
the first vessel, by entering between its two bottoms, rises into the upper space t, thence
it proceeds to the second vessel, and, lastly, through the pipe u into the gasometer.
This method, however, requires
twice as much lime as the former,
without increasing the purity of
the gas.
The second method consists in
compressing the gas by the action
of an Archimedes screw, to
such a degree, before it is admitted
into the purifier, as that it
may overcome the pressure of
the column of water in that
vessel. Fig. 487. exhibits this
apparatus in section. D D is the
Archimedes worm, the axis of
which revolves at bottom upon
the gudgeon e; it possesses a
three-fold spiral, and is turned
in the opposite direction to that
in which it scoops the water.
The cistern which contains it has
an air-tight cover. The gas to
be purified passes through the
pipe C into the space D, over the
water level d; the upper cells of the worm, scoop in the gas at this point, and[552]
carry it downwards, where it enters at g into the cavity E of a second cistern. In order
that the gas, after it escapes from the bottom of the worm, may not partially return
through g into the cavity D, an annular plate g h is attached to its under edge, so as to
turn over it. The compressed gas is conducted from the cavity E through the pipe G
into the purifying machine; a is a manometer, to indicate the elastic tension of the gas
in D. On the top of the worm a mechanism is fitted for keeping it in constant
rotation.
A perfect purification of light-gas from sulphuretted hydrogen, either by milk of
lime or a solution of the green sulphate of iron, is attended with some difficulty, when
carried so far as to cause no precipitation of sulphuret in acetate of lead, because such
a degree of washing is required as is apt to diminish its illuminating power, by abstracting
the vapour of the rich oily hydrocarburet which it contains. Moreover, the coal
gas obtained towards the end of the distillation contains some sulphuret of carbon,
which affords sulphurous acid on being burned, and can be removed by no easy method
hitherto known. The lime in the purifier disengages from the carbonate and hydrosulphuret
of ammonia carried over with the gas, especially when it has been imperfectly
cooled in the condenser, a portion of ammoniacal gas, which, however, is not injurious
to its illuminating power. The best agent for purifying gas would be the
pyrolignite of lead, were it not rather expensive, because it would save the trouble of
stirring, and require a smaller and simpler apparatus.
The Gasometer.—The gasometer serves not merely as a magazine for receiving the
gas when it is purified, and keeping it in store for use, but also for communicating to
the gas in the act of burning such an uniform pressure as may secure a steady unflickering
flame. It consists of two essential parts; 1. of an under cistern, open at top and
filled with water; and 2. of the upper floating cylinder or chest, which is a similar
cistern inverted, and of somewhat smaller dimensions, called the gas-holder: see F,
fig. 482. The best form of this vessel is the round or cylindrical; both because under
equal capacity it requires least surface of metal, and it is least liable to be warped by its
own weight or accidents. Since a cylindrical body has the greatest capacity with a
given surface when its height is equal to its semi-diameter, its dimensions ought to be
such that when elevated to the highest point in the water, the height may be equal to
the radius of the base. For example, let the capacity of the gas-holder in cubic
feet be k, the semi-diameter of its base be x, the height out of the water be h;
h is = x = ∛k3·14. This height may be increased by one or two feet, according to
its magnitude, to prevent the chance of any gas escaping beneath its under edge, when
it is raised to its highest elevation in the water.
The size of the gasometer should be proportional to the quantity of gas to be consumed
in a certain time. If 120,000 cubic feet be required, for instance, in 10 hours
for street illumination, and if the gas retorts be charged four times in 24 hours, 30,000
cubic feet of gas will be generated in 6 hours. Hence the gasometer should have a capacity
of at least 70,000 cubic feet, supposing the remaining 50,000 cubic feet to be produced
during the period of consumption. If the gasometer has a smaller capacity, it must be
supplied from a greater number of retorts during the lighting period, which is not
advantageous, as the first heating of the supernumerary retorts is wasteful of fuel.
Some engineers consider that a capacity of 30,000 cubic feet is the largest which can
with propriety be given to a gasometer; in which case, they make its diameter 42 feet,
and its height 23. When the dimensions are greater, the sheet iron must be thicker
and more expensive; and the hollow cylinder must be fortified by strong internal cross
braces.
The water cistern is usually constructed in this country with cast-iron plates bolted
together, and made tight with rust-cement.
In cases where the weight of water required to fill such a cistern might be inconvenient
to sustain, it may be made in the form represented in fig. 488.; which, however, will cost
nearly twice as much. Parallel with the side of the cistern, a second cylinder C, of the
same shape but somewhat smaller, is fixed in an inverted position to the bottom of the
first, so as to leave an annular space B B between them, which is filled with water, and
in which the floating gasometer A plays up and down. The water must stand above the
cover of the inverted cylinder. a and b are the pipes for leading the gas in and out.
Through an opening in the masonry upon which the gasometer apparatus rests, the
space C may be entered, in order to make any requisite repairs.
The water cistern may also be sunk in the ground, and the sides made tight with
hydraulic mortar, as is shown in fig. 489., and to make it answer with less water, a
concentric cylindrical mass of masonry may be built at a distance of 2 or 3 inches within it.
Every large gasometer must be strengthened interiorly with cross iron rods, to stiffen
both its top and bottom. The top is supported by rods stretching obliquely down to[553]
the sides, and to the under edge an iron ring is attached, consisting of curved cast-iron
bars bolted together; with which the oblique rods are connected by perpendicular ones.
Other vertical rods stretch directly from the top to the bottom edge. Upon the
periphery of the top, at the end of the rods, several rings are made fast, to which the
gas-holder is suspended, by means of a common chain which runs over a pulley at the
centre. Upon the other end of the chain there is a counterpoise, which takes off the
greater part of the weight of the gas-holder, leaving only so much as is requisite for the
expulsion of the gas. The inner and outer surfaces of the gas-holder should be a few
times rubbed over with hot tar, at a few days’ interval between each application. The
pulley must be made fast to a strong frame.
If the water cistern be formed with masonry, the suspension of the gas-holder may be
made in the following way. A A, fig. 489., is a hollow cylinder of cast iron, standing up
through the middle of the gasometer, and which is provided at either end with another
small hollow cylinder G, open at both ends and passing through the top, with its axis
placed in the axis of the gas-holder. In the hollow cylinder G, the counterweight
moves up and down, with its chain passing over the three pulleys B, B, B, as shown in
fig. 489. E F are the gas pipes made fast to a vertical iron rod. Should the gasometer
be made to work without a counterweight, as we shall presently see, the central
cylinder A A, serves as a vertical guide.
In proportion as the gas-holder sinks in the water of the cistern, it loses so much of
its weight, as is equal to the weight of the water displaced by the sides of the sinking
vessel; so that the gas-holder when entirely immersed, exercises the least pressure upon
the gas, and when entirely out of the water, it exercises the greatest pressure. In order
to counteract this inequality of pressure, which would occasion an unequal velocity in
the efflux of the gas, and of course an unequal intensity of light in its flame, the weight of
the chain upon which the gas-holder hangs is so adjusted as to be equal, throughout
the length of its motion, to one half of the weight which the gas-holder loses by
immersion. In this case, the weight which it loses by sinking into the water, is replaced
by the portion of the chain which passing the pulley, and hanging over, balances so
much of the chain upon the side of the counterweight; and the weight which it gains by
rising out of the water, is counterpoised by the links of the chain which passing over the
pulley, add to the amount of the counterweight. The pressure which the gas-holder
exercises upon the gas, or that with which it forces it through the first main pipe, is
usually so regulated as to sustain a column of from one to two inches of water; so that
the water will stand in the cistern from one to two inches higher within, than without
the gas-holder. The following computation will place these particulars in a clear light.
Let the semi-diameter of the gas-holder, equal to the vertical extent of its motion into
and out of the water, = x; let the weight of a foot square of the side of the gas-holder,
including that of the strengthening bars and ring, which remain plunged under the
water, be = p; then
[554]
1. the weight of the gas-holder in its highest position = 3 p π x2;
2. the weight of the sides of the gas-holder which play in the water = 2 p π x2;
3. the cubic contents of the immersed portion of the gas-holder = 2 p π x2400;
4. its loss of weight in water = 112400 p π x2;
5. the weight of the gas-holder in its lowest position =
p π x2 (3 - 112400) = 2·72 p π x2;
6. the weight of n inches, height of water = 5612 n π x2;
7. the amount of the counterweight = π x2 (3 p - 56 n12);
8. the weight of the chain for the length x = 112800 p π x.
If we reduce the weight of the gas-holder in its highest and lowest positions to the
height of a stratum of water equal to the surface of its top, this height is that of the
column of water which would press the gas within the gasometer, were no counterweight
employed; it consists as follows;—
9. for the highest position = 3 p56;
10. for the lowest = 2·72 p50;
For the case, when the height of the gas-holder is different from its semi-diameter,
let this height = m x; then the height of the water level is
11. for the highest position = p (1 + 2 m56);
12. for the lowest = p (1 + 1·72 m6);
13. the counterweight = π x2 (p (1 + 2m) - 56 n12);
14. the weight of the equalizing chain = 112800 p π m x2.
For example, let the diameter of the gas-holder be 30 feet, the height 15 (the
contents in cubic feet will be 10,597), p = 4 pounds; then the counterweight for a
height of an inch and a half of water pressure = 3532 pounds; the weight of the chain
for a length of 15 feet = 395 pounds. Were no counterweight employed, so that the
gas-holder pressed with its whole weight upon the gas, then the height of the equivalent
column of water in its highest position = 2·56 inches; and in its lowest, 2·33. The
counterweight may hence be lessened at pleasure, if the height of the pressing water-column
n be increased. The weight of the equalising or compensating portion of the
chain remains the same. When n = 2 inches, for instance, the counterweight = 1886
pounds.
The velocity with which the gas passes along the mains for supplying the various jets
of light, may be further regulated by opening the main-cock or slide-valve in a greater
or less degree.
Gasometers whose height is greater than their semi-diameter, are not only more
costly in the construction, but require heavier counterweights and equilibration chains.
The above estimate is made on the supposition of the gas in the gas-holder being of
the same specific gravity as the atmospherical air, which would be nearly true with
regard to oil gas under the ordinary pressure. But coal gas, whose specific gravity
may be taken on an average at about 0·5, exercises a buoyancy upon the top of the gas-holder,
which of course diminishes its absolute weight. Supposing the cubic foot of gas
to be = 0·0364 pounds, the buoyancy will be = 0·0364 π x3 pounds; a quantity which
deserves to be taken into account for large gasometers. Hence,
15. the weight of the gas-holder in its highest position = 3 p π x2 - 0·1143 x3;
16. the counterweight = π x2 (3 p - 56 n12) - 0·1143 x2;
17. The weight of the chain for the length x, = 112800 p π x2 0·1143 x32;
18. The height of the water pressure for the highest position, without the counterweight
= 3 p π - 0·1143 x56 π;
19. the same for the lowest position = 2·72 p56 in feet.
[555]
The preceding values of p and x, are,
(16) = 3147; (17) = 203; (18) = 2·44 inches; (19) = 2·33 inches.
The water columns in the highest and lowest situations of the gas-holder here differ
about 0·1 of an inch, and this difference becomes still less when p has a smaller value,
for example, 3 pounds, or when the diameter of the gas-holder is still greater.
It would thus appear that for coal-gas gasometers, in which the height of the gas-holder
does not exceed its semi-diameter, and especially when it has a considerable size, neither
a compensation chain nor a counterweight is necessary. The only thing requisite, is to
preserve the vertical motion of the gas-holder by a sufficient number of guide rods or
pillars, placed either within the water cistern, or round about it. Should the pressure
of the gas in the pipe proceeding from the gasometer, be less than in the gasometer
itself, this may be regulated by the main valve, or by water valves of various kinds. Or
a small intermediate regulating gasometer may be introduced between the great gas-holder,
and the main pipe of distribution. With a diameter of 61 feet in the gas-holder,
the pressure in the highest and lowest positions is the same.
The gasometers employed in storing up gas until required for use, occupy, upon the
old plan, much space, and are attended with considerable expense in erecting. The
water tank, whether sunk in the ground, or raised, must be of equal dimensions with
the gasometer, both in breadth and depth. The improved construction which we are
about to describe, affords a means of reducing the depth of the tank, dispensing with the
bridge of suspension, and of increasing at pleasure the capacity of the gasometer, upon a
given base; thus rendering a small apparatus capable, if required, of holding a large
quantity of gas, the first cost of which will be considerably less than even a small gasometer
constructed upon the ordinary plan.
Mr. Tait, of Mile-End Road, the inventor, has, we believe, been for some years
connected with gas establishments, and is therefore fully aware of the practical defects
or advantages of the different constructions of
gasometers now in use. Fig. 490. is a section
of Mr. Tait’s improved contrivance; a a is the
tank, occupied with water, b b two iron columns,
with pulley-wheels on the top, c c,
chains attached to a ring of iron, d d, extending
round the gasometer, which chains pass
over the pulley-wheels, and are loaded at their
extremities, for the purpose of balancing the
weight of the materials of which the gasometer
is composed.
The gasometer is formed by 2 or 3 cylinders,
sliding one within the other, like the tubes
of a telescope; e, e, e, is the first or outer cylinder,
closed at the top, and having the ring
of iron d, passing round it, by which the whole
is suspended; f f, is the second cylinder,
sliding freely within the first, and there may
be a third and fourth within these if necessary.
When there is no gas in the apparatus, all the cylinders are slidden down, and remain
one within the other immersed in the tank of water; but when the gas rises through the
water pressing against the top of the gasometer, its buoyancy causes the cylinder e to ascend.
Round the lower edge of this cylinder a groove is formed by the turning in of the plate
of iron, and as it rises, the edge takes hold of the top rim of the cylinder f, which is
overlapped for that purpose. The groove at the bottom of the cylinder fills itself with
water as it ascends, and by the rim of the second cylinder falling into it, an air-tight
hydraulic joint is produced.
Thus, several cylinders may be adapted to act in a small tank of water, by sliding
one within the other, with lapped edges forming hydraulic joints, and by supporting the
apparatus in the way shown, the centre of gravity will always be below the points of
suspension. A gasometer may be made upon this plan of any diameter, as there will
be no need of frame work, or a bridge to support it; and the increasing weight of the
apparatus, as the cylinders are raised one after the other, may be counterpoised by loading
the ends of the chains c c.
The water in the gasometer need not be renewed; but merely so much of it as
evaporates or leaks out, is to be replaced. Indeed the surface of the water in the cistern
gets covered with a stratum of coal oil, a few inches deep, which prevents its evaporation,
and allows the gas to be saturated with this volatile substance, so as to increase its
illuminating powers.
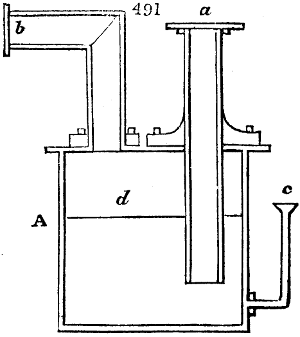
The gasometer may be separated from the purifier by an intermediate vessel, such
as is represented fig. 491., with which the two gas pipes are connected. A is the[556]
cylindrical vessel of cast iron, a, the end of the gas pipe which comes from the
purifier, immersed a few inches deep into the
liquid with which the vessel is about two-thirds
filled; b is the gas-pipe which leads into the gasometer,
c is a perpendicular tube, placed over the
bottom of the vessel, and reaching to within one-third
of the top, through which the liquid is introduced
into the vessel, and through which it escapes
when it overflows the level d. In this tube the
liquid stands towards the inner level higher, in proportion
to the pressure of the gas in the gasometer.
The fluid which is condensed in the gas pipe, b,
and in its prolongation from the gasometer, runs
off into the vessel A; and therefore the latter must
be laid so low that the said tube may have the requisite
declivity. A straight stop-cock may also be
attached to the side over the bottom, to draw off
any sediment.
II. Application of Light-Gas.
1. Distribution of the pipes.—The pressure by which the motion of the gas is maintained
in the pipes, corresponds to a certain height of water in the cistern of the gasometer.
From the magnitude of this pressure, and the quantity of gas which in a given time, as
an hour, must be transmitted through a certain length of pipes, depends the width or the
diameter that they should have, in order that the motion may not be retarded by the
friction which the gas, like all other fluids, experiences in tubes, and thereby the gas
might be prevented from issuing with the velocity required for the jets of flame. The
velocity of the gas in the main pipe increases in the ratio of the square root of the
pressing column of water upon the gasometer, and therefore by increasing this pressure,
the gas may be forced more rapidly along the remoter and smaller ramifications of the
pipes. Thus it happens, however, that the gas will be discharged from the orifices near
the gasometer, with superfluous velocity. It is therefore advisable to lay the pipes in
such a manner, that in every point of their length, the velocity of discharge may be
nearly equal. This may be nearly effected as follows;—
From experiment it appears that the magnitude of the friction, or the resistance which
the air suffers in moving along the pipes, under a like primary pressure, that is for equal
initial velocity, varies with the square root of the length. The volume of gas discharged
from the end of a pipe, is directly proportional to the square of its diameter, and inversely
as the square root of its length; or, calling the length L, the diameter D, the cubic
feet of gas discharged in an hour k; then k = D2√L. Experience likewise shows, that
for a pipe 250 feet long, which transmits in an hour 200 cubic feet of gas, one inch is
a sufficient diameter.
Consequently, 200 : k ∷ 1144 √250 : D2√L; and D = √k √L455,000
From this formula the following table of proportions is calculated.
Number of
cubic feet
per hour. |
Length of
pipe,
in feet. |
Diameter,
in inches. |
| 50 |
100 |
0 |
·40 |
| 250 |
200 |
1 |
·00 |
| 500 |
600 |
1 |
·97 |
| 700 |
1000 |
2 |
·65 |
| 1000 |
1000 |
3 |
·16 |
| 1500 |
1000 |
3 |
·87 |
| 2000 |
1000 |
4 |
·47 |
| 2000 |
2000 |
5 |
·32 |
| 2000 |
4000 |
6 |
·33 |
| 2000 |
6000 |
7 |
·00 |
| 6000 |
1000 |
7 |
·75 |
| 6000 |
2000 |
9 |
·21 |
| 8000 |
1000 |
8 |
·95 |
| 8000 |
2000 |
16 |
·65 |
These dimensions are applicable to the case where the body of gas is transmitted through[557]
pipes without being let off in its way by burners, that is, to the mains which conduct
the gas to the places where it is to be used. If the main sends off branches for burners,
then for the same length the diameter may be reduced, or for like diameter the length
may be greater. For example, if a pipe of 5·32 inches, which transmits 2000 cubic
feet through a length of 2000 feet, gives off, in this space, 1000 cubic feet of gas; then the
remainder of the pipe, having the same diameter, can continue to transmit the gas through
a length of 2450 feet = (450,000k)2, with undiminished pressure for the purposes of
lighting. Inversely, the diameter should be progressively reduced in proportion to the
number of jets sent off in the length of the pipe.
Suppose for instance, the gasometer to discharge 2000 cubic feet per hour, and the last
point of the jets to be at a distance of 4000 feet. Suppose also that from the gasometer
to the first point of lighting, the gas proceeds through 1000 feet of close pipe, the
diameter of the pipe will be here 4·47 inches; in the second 1000 feet of length, suppose
the pipe to give off, at equal distances, 1000 cubic feet of gas, the diameter in
this length (calculated at 1500 cubic feet for 1000 feet long) = 3·87 inches; in the
third extent of 1000 feet, 600 cubic feet of gas will be given off, and the diameter
(reckoning 700 cubic feet for 1000 feet long) will be 2·65 inches; in the fourth and
last space (for 200 cubic feet in 1000 feet long) the pipe has a diameter of only an
inch and a half, for which, in practice, a two-inch cast iron pipe is substituted; this
being the smallest used in mains, into which branch pipes can be conveniently inserted.
The same relations hold with regard to branch pipes through which the gas is transmitted
into buildings and other places to be illuminated. If such pipes make frequent
angular turnings, whereby they retard the motion of the gas, they must be a third or a
half larger in diameter. The smallest tubes of distribution are never less than one
fourth of an inch in the bore.
Where, from one central gas work, a very great quantity of light is required in particular
localities, there ought to be placed near these spots gasometers of distribution,
which, being filled during the slack hours of the day, are ready to supply the burners
at night, without making any considerable demand upon the original main pipe. Suppose
the first main be required to supply 8000 cubic feet in the hour, for an illumination
of 8 hours, at the distance of 2000 feet, a pipe 102⁄3 inches in diameter would be
necessary; but if two or three gasometers of distribution, or station gasometers be had
recourse to, into which the gas during the course of 24 hours would flow through the
same distance continuously from the central gas works, the quantity required per hour
from them would be only one third of 8000, = 2666·6 cubic feet; consequently the
diameter for such a pipe is only 6·15 inches.
All the principal as well as branch pipes, whose interior diameter exceeds an inch
and a half, are made of cast iron from 6 to 8 feet long, with elbow pipes cast in them
where it is necessary. These pipe lengths are shown in fig. 492., having at one end
a wide socket a, and at the other a nozzle b, which fits the former. After inserting the
one in the other in their proper horizontal position, a coil of hemp soaked with tar is
driven home at the junction; then a luting of clay is applied at the mouth, within
which a ring of lead is cast into the socket, which is driven tight home with a mallet
and blunt chisel.
The pipes should be proved by a force pump before being received into the gas
works; two or three lengths of them should be joined before laying them down, and
they should be placed at least two feet below the surface, to prevent their being affected
by changes of temperature, which would loosen the joints. The tubes for internal
distribution, when of small size are made of lead, copper, wrought iron, or tin.
[558]
Instead of a stopcock for letting off the gas in regulated quantities from the gasometer,
a peculiarly formed water or mercurial valve is usually employed. Fig. 493.
shows the mode of construction for a water trap or lute, and is, in fact, merely a gasometer
in miniature. C D E F is a square cast iron vessel, in the one side of which a pipe A
is placed in communication with the gasometer, and in the other, one with the main B.
The movable cover or lid H G I K has a partition, L M, in its middle. If this cover be
raised by its counterweight, the gas can pass without impediment from A to B; but if the
counterweight be diminished so as to let the partition plate L M sink into the water, the
communication of the two pipes is thereby interrupted. In this case the water-level
stands in the compartment A so much lower than outside of it, and in the compartment
B, as is equivalent to the pressure in the gasometer; therefore the pipes A and B must
project thus far above the water. In order to keep the water always at the same height,
and to prevent it from flowing into the mouths of these pipes, the rim C D of the outer
vessel stands somewhat lower than the orifices A B; and thence the vessel may be kept
always full of water.
If a quicksilver valve be preferred, it may be constructed as shown in fig. 494. A B are
the terminations of the two gas pipes,
which are made fast in the rectangular
iron vessel M. E is an iron vessel of the
same form, which is filled with quicksilver
up to the level a, and which, by means of
the screw G, which presses against its
bottom, and works in the fixed female screw
C C, may be moved up or down, so that the
vessel M may be immersed more or less into
the quicksilver. The vessel M is furnished
with a vertical partition m; the passage of
the gas from A to B is therefore obstructed
when this partition dips into the quicksilver,
and from the gradual depression of
the vessel E by its screw, the interval between
the quicksilver and the lower edge
of the partition, through which the gas
must enter, may be enlarged at pleasure,
whereby the pressure of the gas in B may
be regulated to any degree. The transverse
section of that interval is equal to
the area of the pipe or rather greater; the
breadth of the vessel M from A to B
amounts to the double of that space, and
its length to the mere diameter of A or B.
The greatest height to which the partition
m can rise out of the quicksilver, is also
equal to the above diameter, and in this
case the line a comes to the place of b.
The vertical movement of the outer vessel
E, is secured by a rectangular rim or hoop
which surrounds it, and is made fast to the
upper part of the vessel M, within which
guide it moves up and down. Instead of
the lever D D, an index with a graduated
plate may be employed to turn the screw,
and to indicate exactly the magnitude in
the opening of the valve.
In order to measure the quantity of gas
which passes through a pipe for lighting a
factory, theatre, &c., the gas-meter is employed,
of whose construction a sufficiently
precise idea may be formed from the consideration
of fig. 495., which shows the instrument
in a section perpendicular to its
axis.
Within the cylindrical case a, there is
a shorter cylinder b b, shut at both ends,
and movable round an axis, which is
divided into four compartments, that communicate by the opening d, with the
interval between this cylinder and the outer case. The mode in which this[559]
cylinder turns round its axis is as follows:—The end of the tube c, which is
made fast to the side of the case, and by which the gas enters, carries a pivot or
gudgeon, upon which the centre of its prop turns; the other end of the axis
runs in the cover, which here forms the side of a superior open vessel, in which,
upon the same axis, there is a toothed wheel. The vessel is so far filled with
water, that the tube c just rises above it, which position is secured by the level of
the side vessel. When the gas enters through the tube c, by its pressure upon the
partition e, (fig. 495.) it turns the cylinder from right to left upon its axis, till the exterior
opening d rises above the water, and the gas expands itself in the exterior space,
whence it passes off through a tube at top. At every revolution a certain volume of gas
thus goes through the cylinder, proportional to its known capacity. The wheel on the
axis works in other toothed wheels, whence, by means of an index upon a graduated
disc or dial, placed at top or in front of the gas-meter, the number of cubic feet of gas,
which pass through this apparatus in a given time, is registered.
B. Employment of the gas for lighting.—The illuminating power of different gases
burned in the same circumstances, is proportional, generally speaking, to their specific
gravity, as this is to the quantity of carbon they hold in combination. The following
table exhibits the different qualities of gases in respect to illumination.
| Density or specific gravity. |
Proportion of light
afforded by
coal gas to oil gas. |
| Coal gas. |
Oil gas. |
| 0 |
·659 |
0 |
·818 |
100 |
: |
140 |
| 0 |
·578 |
0 |
·910 |
100 |
: |
225 |
| 0 |
·605 |
1 |
·110 |
100 |
: |
250 |
| 0 |
·407 |
0 |
·940 |
100 |
: |
354 |
| 0 |
·429 |
0 |
·965 |
100 |
: |
356 |
| 0 |
·508 |
1 |
·175 |
100 |
: |
310 |
| Mean 0 |
·529 |
0 |
·96 |
100 |
: |
272 |
In the last three proportions, the coal gas was produced from coals of middle
quality; in the first three proportions from coals of good quality; and therefore the
middle proportion of 100 to 270 may be taken to represent the fair average upon the
great scale. On comparing the gas from bad coals, with good oil gas, the proportion
may become 100 to 300. Nay, coal gas of specific gravity 0·4, compared to oil gas of
1·1, gives the proportion of 1 to 4. A mould tallow candle, of 6 in the pound, burning
for an hour, is equivalent to half a cubic foot of ordinary coal gas, and to four tenths of a
foot of good gas. The flame of the best argand lamp of Carcel, in which a steady supply of
oil is maintained by pump-work, consuming 42 grammes = 649 grains English in an hour,
and equal in light to 9·38 such candles, is equivalent to 3·75 cubic feet of coal gas per
hour. The sinumbra lamp, which consumes 50 grammes = 772 grains English, of
oil per hour, and gives the light of 8 of the above candles, is equivalent to the light
emitted by 3·2 cubic feet of coal gas burning for an hour. A common argand lamp,
equal to 4 candles, which consumes 30 grammes = 463 grains English per hour, is
represented by 1·6 cubic feet of gas burning during the same time. A common lamp,
with a flat wick and glass chimney, whose light is equal to 1·13 tallow candles, and
which consumes 11 grammes = 169·8 grains English per hour, is represented by 0·452
of a cubic foot of gas burning for the same time.
Construction of the Burners.—The mode of burning the gas as it issues from the jets
has a great influence upon the quantity and quality of its light. When carburetted
hydrogen gas is transmitted through ignited porcelain tubes, it is partially decomposed
with a precipitation of some of its carbon, while the resulting gas burns with a feebler
flame. Coal gas, when kindled at a small orifice in a tube, undergoes a like decomposition
and precipitation. Its hydrogen, with a little of its carbon, burns whenever it
comes into contact with the atmospherical air, with a bluish coloured flame; but the
carbonaceous part not being so accendible, takes fire only when mixed with more air;
therefore at a greater distance from the beak, and with a white light from the vivid
ignition of its solid particles. Upon this principle pure hydrogen gas may be made to
burn with a white instead of its usual blue flame, by dusting into it particles of lamp
black; or by kindling it at the extremity of a tube containing finely pulverized zinc.
The metallic particles become ignited, and impart their bright light to the pale blue
flame. Even platinum wire and asbestos, when placed in the flame of hydrogen gas,
serve to whiten it. Hence it has been concluded, that the intensity of light which a
gas is capable of affording is proportional to the quantity of solid particles which it[560]
contains, and can precipitate in the act of burning. Carbonic oxide gas burns with the
feeblest light next to hydrogen, because it deposits no carbon in the act of burning.
Phosphuretted hydrogen gives a brilliant light, because the phosphoric acid, into which
its base is converted during the combustion, is a solid substance, capable of being
ignited in the flame. Olefiant gas, as also the vapour of hydro-carbon oil, emits a
more vivid light than common coal gas; for the first is composed of two measures of
hydrogen and two measures of the vapour of carbon condensed into one volume; while
the last contains only one measure of the vapour of carbon in the same bulk, and
combined with the same proportion of hydrogen. Olefiant gas may therefore be
expected to evolve a double quantity of carbon in its flame, which should emit a
double light.
The illuminating power of the flame of coal gas is, on the contrary, impaired, when,
by admixture with other species of gas which precipitate no carbon, its own ignited
particles are diffused over a greater surface. This happens when it is mixed with
hydrogen, carbonic oxide, carbonic acid, and nitrogen gases, and the diminution of the
light is proportional to the dilution of the coal gas.
In like manner the illuminating power of coal gas is impaired, when it is consumed
too rapidly to allow time for the separation and ignition of its carbonaceous matter; it
burns, in this case, without decomposition, and with a feeble blue flame. 1. This
occurs when the light-gas is previously mixed with atmospherical air, because the
combustion is thereby accelerated throughout the interior of the flame, so as to prevent
the due separation of carbon. A large admixture of atmospherical air makes the flame
entirely blue. 2. When it issues, with considerable velocity, from a minute orifice,
whereby the gas, by expansion, gets intimately mixed with a large proportion of
atmospherical air. If the jet be vertical, the bottom part of the flame is blue, and the
more so the less carbon is contained in the gas. The same thing may be observed in
the flame of tallow, wax, or oil lights. The burning wick acts the part of a retort, in
decomposing the fatty matter. From the lower part of the wick the gases and vapours
of the fat issue with the greatest velocity, and are most freely mixed with the air; while
the gases disengaged from the upper part of the wick compose the interior of the flame,
and being momentarily protected from the action of the atmosphere, acquire the proper
high temperature for the deposition of carbon, which is then diffused on the outer
surface in an ignited state, and causes its characteristic white light. Hence with coal
gas, the light increases in a certain ratio with the size of the flame as it issues from a
larger orifice, because the intermixture of air becomes proportionately less. 3. If by
any means too great a draught be given to the flame, its light becomes feebler by the
rapidity and completeness with which the gas is burned, as when too tall a chimney is
placed over an argand burner, see fig. 496. Fig. 497. c, is a view of the upper plate,
upon which the glass chimney b rests. The gas issues through the smaller openings of
the inner ring, and forms a hollow cylindrical flame, upon the outside as well as the
inside of which the atmospherical air acts. The illuminating power of this flame may be
diminished at pleasure, according as more or less air is allowed to enter through the orifices
beneath. With a very full draught the light almost vanishes, leaving only a dull blue flame
of great heating power, like that of the blowpipe, corresponding to the perfect combustion
of the gas without precipitation of its carbon. 4. On the other hand, too small a
draught of air is equally prejudicial; not merely because a portion of the carbon thus
escapes unconsumed in smoke, but also because the highest illuminating power of the
flame is obtained only when the precipitated charcoal is heated to whiteness, a circumstance
which requires a considerable draught of air. Hence the flame of dense oil gas,
or of oil in a wick, burns with a yellow light without a chimney; but when it is increased
in intensity by a chimney draught, it burns with a brilliant white flame.
From the consideration of the preceding facts, it is possible to give to coal gas its
highest illuminating power. The burners are either simple beaks perforated with a
small round hole, or circles with a series of holes to form an argand flame, as
shown in fig. 497, or two holes drilled obliquely, to make the flame cross, like a swallow’s
tail, or with a slit constituting the sheet of flame called a bat’s wing, like most of
the lamps in the streets of London. These burners are mounted with a stop-cock for
regulating the quantity of gas.
The height of the flame, which with like pressure depends upon the size of the orifice,
and with like orifice upon the amount of pressure, the latter being modified by the stop-cock,
is for simple jets in the open air, as follows:—
| Length of the flame |
2 |
3 |
4 |
5 |
6 inches |
| Intensity of the light |
55·6 |
100 |
150 |
197·8 |
247·4 |
|
| Volume of gas consumed |
60·5 |
101·4 |
126·3 |
143·7 |
182·2 |
|
| Light with equal consumption |
100 |
109 |
131 |
150 |
150 |
|
When the length exceeds five inches, nothing is gained in respect to light. For oil[561]
gas the same statements will serve, only on account of its superior richness in carbon, it
does not bear so long a flame without smoke. Thus:—
| Length of the flame |
1 |
2 |
3 |
4 |
5 inches |
| Intensity of the light |
22 |
63·7 |
96·5 |
141 |
178 |
|
| Gas consumed |
33·1 |
78·5 |
90 |
118 |
153 |
|
| Light with equal consumption |
100 |
122 |
159 |
181 |
174 |
|
The diameter of the orifice for single jets, or for several jets from the same beak, is
one twenty-eighth of an inch for coal gas, and one forty-fifth for oil gas.
When several jets issue from the same burner, the light is improved by making all
the flames unite into one. In this case the heat becomes greater, for the combined
flame presents a smaller surface to be cooled, than the sum of the smaller flames. The
advantage gained in this way, may be in the ratio of 3 to 2, or 50 per cent. In an argand
burner, the distances of the orifices for coal gas should be from 16⁄100 to 18⁄100 of an inch, and
for oil gas 12⁄100. If the argand ring has ten orifices, the diameter of the central opening
should be = 4⁄10 of an inch; if 25 orifices, it should be one inch for coal gas; but for oil
gas with 10 orifices, the central opening should have a diameter of half an inch, and for
20 orifices, one inch. The pin holes should be of equal size, otherwise the larger ones
will cause smoke, as in an argand flame with an uneven wick. The glass chimney is
not necessary to promote the combustion of an argand coal gas flame, but only to prevent
it from flickering with the wind, and therefore it should be made so wide as to
exercise little or no influence upon the draught. A narrow chimney is necessary
merely to prevent smoke, when a very strong light, with a profusion of gas is desired.
Oil gas burned in an argand beak requires a draught chimney, like a common argand
lamp, on account of the large quantity of carbon to be consumed. The most suitable
mode of regulating the degree of draught can be determined only by experiment, and
the best construction hitherto ascertained is that represented in fig. 498. Fig. 499. exhibits
the view from above, of the rim or ring c, upon
which the chimney b stands, and which surrounds the
perforated beak. The ring is made of open fretwork,
to permit the free passage of air upwards to strike
the outside of the flame. The thin annular disc d,
which is laid over its fellow disc c, in the bottom
of the chimney-holder, being turned a little one way
or other, will allow more or less air to pass through
for promoting more or less, the draught or ventilation.
The draught in the central tube of the
burner may be regulated by the small disc e, whose
diameter is somewhat smaller than that of the ring
of the burner, and which by turning the milled
head f, of the screw, may be adjusted with the greatest nicety, so as to admit a greater
or smaller body of air into the centre of the cylindrical flame.
In mounting gas-lights, and in estimating beforehand their illuminating effects, we
must keep in mind the optical proposition, that the quantity of light is inversely as the
square of the distance from the luminous body, and we must distribute the burners
accordingly. When for example a gas-light placed at a distance of ten feet, is required
for reading or writing to afford the same light as a candle placed at a distance of two
feet; squaring each distance, we have 100 and 4; therefore 1004 = 25, shows us that 25
such lights will be necessary at the distance of 10 feet.
Concerning portable gas-light, with the means of condensing it, and carrying it from
the gas works to the places where it is to be consumed, we need say nothing, as by the
improvements lately made in the purification and distribution of coal-gas, the former
system has been superseded.
It is well known that light gas deteriorates very considerably by keeping, especially
when exposed to water over an extensive surface; but even to a certain degree over oil,
or in close vessels. An oil-gas which when newly prepared has the specific gravity of
1·054, will give the light of a candle for an hour, by consuming 200 cubic inches; will,
after two days, give the same light by consuming 215 cubic inches per hour; and after four
days, by consuming 240 cubic inches in the like time. With coal-gas the deterioration
appears to be more rapid. When newly prepared, if it affords the light of a candle with
a consumption of 400 cubic inches per hour; it will not give the same light after being
kept two days, except with a consumption of 430 inches; and after four days, of 460.
Oil-gas three weeks old has become so much impaired in quality that 600 inches of it
were required per hour to furnish the light of a candle. All light gas should be used
therefore as soon as possible after it is properly purified.
Economical considerations.—The cost of gas-light depends upon so many local circumstances,
that no estimate of it can be made of general application; only a few[562]
leading points may be stated. The coals required for heating the retorts used to constitute
one half of the quantity required for charging the retorts themselves. When five retorts
are heated by one fire, the expenditure for fuel is only one third of that when each retort
has a fire. The coak which remains in the retorts constitutes about 60 per cent. of the
weight of the original coal; but the volume is increased by the coaking in the
proportion of 100 to 75. When the coak is used for heating the retorts, about one
half of the whole is required. If we estimate the coak by its comparative heating
power, it represents 65 per cent. of the coals consumed. One hundred pounds of good
coal yield in distillation 10 pounds of ammoniacal liquor, from which sulphate or muriate
of ammonia may be made, by saturation with sulphuric or muriatic acid, and evaporation.
The liquor contains likewise some cyanide of ammonia, which may be converted
into prussian blue by the addition of sulphate of iron, after saturation with muriatic acid.
Two hundred pounds of coal afford about 17 pounds of tar. This contains in 100 pounds
26 pounds of coal oil, and 48 pounds of pitch. The tar is sometimes employed as a
paint to preserve wood and walls from the influence of moisture, but its disagreeable
smell limits its use. The coal oil when rectified by distillation, is extensively employed
for dissolving caoutchouc in making the varnish of waterproof cloth, and also for burning
in a peculiar kind of lamps under the name of naphtha. Oil of turpentine however
is often sold and used for this purpose, by the same name. If the coal oil be mixed
with its volume of water, and the mixture be made to boil in a kettle, the mingled vapours
when passed through a perforated nozzle may be kindled, and employed as a powerful
means of artificial heat. The water is not decomposed, but it serves by its vapour
to expand the bulk of the volatile oil, and to make it thereby come into contact with a
larger volume of atmospherical air, so as to burn without smoke, under a boiler or any
other vessel. The pitch may be decomposed into a light-gas.
The relative cost of light from coal gas and oil gas may be estimated as one to six, at
least. Rosin gas is cheaper than oil gas. See Rosin.
I shall conclude this article with a summary of the comparative expense of different
modes of illumination, and some statistical tables.
One pound of tallow will last 40 hours in six mould candles burned in succession,
and costs 8d.; a gallon of oil, capable of affording the light of 15 candles, for 40
hours costs 5s., being therefore 1⁄2 of the price of mould candles, and 6⁄15 of the price of
dips. The cost of wax is about 31⁄2 times that of tallow; and coal gas, as sold at the rate
of 9s. for 1000 cubic feet, will be one sixth the price of mould candles; for 500 cubic
inches of coal gas give a light equal to the above candle for an hour; therefore 40 × 500
= 20,000 cubic inches = 11·57 cubic feet, worth 11⁄4d., which multiplied by 6 gives 71⁄2d.
the average price of mould candles per pound.
The author of the article Gas-light in the Encyclopædia Britannica, observes, in reference
to the economy of this mode of illumination, that while the price of coal, in consequence
of the abundant and regular supply of that article, is liable to little fluctuation,
the cost of wax, tallow, and oil, on account of the more precarious nature of the sources
from which they are obtained, varies exceedingly in different seasons. “Assuming that
a pound of tallow candles, which last when burned in succession forty hours, costs nine-pence,”
(seven-pence halfpenny is the average price), “that a gallon of oil, yielding the
light of 600 candles for an hour, costs two shillings,” (five shillings is the lowest price of
a gallon of such oil as a gentleman would choose to burn in his lamp), “that the expense
of the light from wax is three times as great as from tallow, and that a thousand
cubic feet of coal gas cost nine shillings;” he concludes the relative cost to be for the
same quantity of light,—from wax, 100; tallow, 25; oil, 5; and coal-gas, 3. I conceive
the estimate given above to be much nearer the truth; when referred to wax called 100,
it becomes, for tallow, 28·6; oil, 14·3; coal gas, 4·76.
Gas-lighting has received a marvellous development in London. In the year 1834,
the number of gas lamps in this city was 168,000, which consumed daily about 4,200,000
cubic feet of gas. For the purpose of generating this gas, more than 200,000 chaldrons,
or 10,800,000 cubic feet of coals were required.
For the following valuable statistical details upon gas-light, my readers are
indebted to Joseph Hedley, Esq., engineer, of the Alliance Gas Works, Dublin; a
gentleman who to a sound knowledge of chemistry, joins such mechanical talent and
indefatigable diligence, as qualify him to conduct with success, any great undertaking
committed to his care. He has long endeavoured to induce the directors of the London
gas-works to employ a better coal, and generate a more richly carburetted gas, which in
much smaller quantity would give as brilliant a light, without heating the apartments
unpleasantly, as their highly hydrogenated gas now does. Were his judicious views
adopted, coal gas would soon supersede oil, and even wax candles, for illuminating private
mansions.
[563]
Copy of a paper laid before a Committee of the House of Commons, showing not
only the relative values of the Gases produced at the undermentioned places, but
showing in like manner the relative economy of Gas as produced at the different places,
over candles. By Joseph Hedley, Esq.
Names of the Places
where Experiments
were made. |
Illuminating
power of a
single Jet of
Gas-flame
four inches
high, taken
by a
comparison
of Shadows. |
The Jet of
Gas burnt,
four inches
high,
consumed
per hour
and was
equal to the
Candles
in the last
column. |
Gas required
to be equal
to 100 lbs.
of mould
Candles,
6 to the lb.,
9 inches
long each.
[A] |
Selling
price
of Gas
per meter
per 1000
cubic feet. |
Cost of
Gas equal
in illumi-
nating
power to
100 lbs. of
candles.[B] |
Average
discount
allowed
off the
charge
for Gas. |
Net cost of
Gas equal
to 100 lbs.
of Candles. |
Specific
gravity
of the
Gas. |
| |
Equal to
Candles. |
Cubic
Feet. |
Cubic
Feet. |
s. |
d. |
L. |
s. |
d. |
Per
Cent. |
L. |
s. |
d. |
|
| Birmingham; |
|
- |
2·572 |
1 |
·22 |
2704 |
10 |
0 |
1 |
7 |
0 |
9 |
|
1 |
4 |
7 |
·541 |
Birmingham and
Staffordshire;
two Companies |
| Stockport |
3·254 |
|
·85 |
1489 |
10 |
0 |
0 |
14 |
11 |
12 |
1⁄2 |
0 |
13 |
0 |
·539 |
| Manchester |
3·060 |
|
·825 |
1536 |
8 |
0 |
0 |
12 |
3 |
11 |
1⁄4 |
0 |
10 |
10 |
·534 |
Liverpool Old
Company[C] |
2·369 |
1 |
·1 |
2646 |
10 |
0 |
1 |
6 |
5 |
6 |
1⁄4 |
1 |
4 |
9 |
·462 |
Liverpool New
Gas Company |
4·408 |
|
·9 |
1164 |
10 |
0 |
0 |
11 |
8 |
6 |
1⁄4 |
0 |
9 |
10 |
·580 |
| Bradford |
2·190 |
1 |
·2 |
3123 |
9 |
0 |
1 |
8 |
1 |
12 |
1⁄2 |
1 |
4 |
6 |
·420 |
| Leeds |
2·970 |
|
·855 |
1644 |
8 |
0 |
0 |
13 |
2 |
6 |
1⁄4 |
0 |
12 |
4 |
·530 |
| Sheffield |
2·434 |
1 |
·04 |
2440 |
8 |
0 |
0 |
19 |
6 |
6 |
1⁄4 |
0 |
18 |
3 |
·466 |
| Leicester |
2·435 |
1 |
·1 |
2575 |
7 |
6 |
0 |
19 |
3 |
15 |
|
0 |
16 |
5 |
·528 |
| Nottingham |
1·645 |
1 |
·3 |
4200 |
9 |
0 |
1 |
17 |
9 |
15 |
|
1 |
11 |
3 |
·424 |
| Derby |
1·937 |
1 |
·2 |
3521 |
10 |
0 |
1 |
15 |
4 |
15 |
|
1 |
10 |
0 |
·448 |
| Preston |
2·136 |
1 |
·15 |
3069 |
10 |
0 |
1 |
10 |
8 |
15 |
|
1 |
6 |
2 |
·419 |
| London |
2·083 |
1 |
·13 |
3092 |
10 |
0 |
1 |
10 |
11 |
none
allowed. |
1 |
10 |
11 |
·412 |
| [A] 100 lbs. of candles are estimated to burn 5700 hours. |
| [B] The candles cost 3l. 2s. 6d. |
| [C] The Liverpool Old Company have since resorted to the use of Cannel coal, and consequently very nearly assimilate to the Liverpool New Company in illuminating power. |
Names of the Places
where Experiments
were made. |
Illuminating
power of a
single Jet of
Gas-flame
four inches
high, taken
by a
comparison
of Shadows. |
The Jet of
Gas burnt,
four inches
high,
consumed
per hour
and was
equal to the
Candles
in the last
column. |
Gas required
to be equal
to 100 lbs.
of mould
Candles,
6 to the lb.,
9 inches
long each.
[A] |
Selling
price
of Gas
per meter
per 1000
cubic feet. |
| |
Equal to
Candles. |
Cubic
Feet. |
Cubic
Feet. |
s. |
d. |
| Birmingham; |
|
- |
2·572 |
1 |
·22 |
2704 |
10 |
0 |
Birmingham and
Staffordshire;
two Companies |
| Stockport |
3·254 |
|
·85 |
1489 |
10 |
0 |
| Manchester |
3·060 |
|
·825 |
1536 |
8 |
0 |
Liverpool Old
Company[C] |
2·369 |
1 |
·1 |
2646 |
10 |
0 |
Liverpool New
Gas Company |
4·408 |
|
·9 |
1164 |
10 |
0 |
| Bradford |
2·190 |
1 |
·2 |
3123 |
9 |
0 |
| Leeds |
2·970 |
|
·855 |
1644 |
8 |
0 |
| Sheffield |
2·434 |
1 |
·04 |
2440 |
8 |
0 |
| Leicester |
2·435 |
1 |
·1 |
2575 |
7 |
6 |
| Nottingham |
1·645 |
1 |
·3 |
4200 |
9 |
0 |
| Derby |
1·937 |
1 |
·2 |
3521 |
10 |
0 |
| Preston |
2·136 |
1 |
·15 |
3069 |
10 |
0 |
| London |
2·083 |
1 |
·13 |
3092 |
10 |
0 |
| [A] 100 lbs. of candles are estimated to burn 5700 hours. |
| [C] The Liverpool Old Company have since resorted to the use of Cannel coal, and consequently very nearly assimilate to the Liverpool New Company in illuminating power. |
Names of the Places
where Experiments
were made. |
Cost of
Gas equal
in illumi-
nating
power to
100 lbs. of
candles.[B] |
Average
discount
allowed
off the
charge
for Gas. |
Net cost of
Gas equal
to 100 lbs.
of Candles. |
Specific
gravity
of the
Gas. |
| |
L. |
s. |
d. |
Per
Cent. |
L. |
s. |
d. |
|
| Birmingham; |
|
- |
1 |
7 |
0 |
9 |
|
1 |
4 |
7 |
·541 |
Birmingham and
Staffordshire;
two Companies |
| Stockport |
0 |
14 |
11 |
12 |
1⁄2 |
0 |
13 |
0 |
·539 |
| Manchester |
0 |
12 |
3 |
11 |
1⁄4 |
0 |
10 |
10 |
·534 |
Liverpool Old
Company[C] |
1 |
6 |
5 |
6 |
1⁄4 |
1 |
4 |
9 |
·462 |
Liverpool New
Gas Company |
0 |
11 |
8 |
6 |
1⁄4 |
0 |
9 |
10 |
·580 |
| Bradford |
1 |
8 |
1 |
12 |
1⁄2 |
1 |
4 |
6 |
·420 |
| Leeds |
0 |
13 |
2 |
6 |
1⁄4 |
0 |
12 |
4 |
·530 |
| Sheffield |
0 |
19 |
6 |
6 |
1⁄4 |
0 |
18 |
3 |
·466 |
| Leicester |
0 |
19 |
3 |
15 |
|
0 |
16 |
5 |
·528 |
| Nottingham |
1 |
17 |
9 |
15 |
|
1 |
11 |
3 |
·424 |
| Derby |
1 |
15 |
4 |
15 |
|
1 |
10 |
0 |
·448 |
| Preston |
1 |
10 |
8 |
15 |
|
1 |
6 |
2 |
·419 |
| London |
1 |
10 |
11 |
none
allowed. |
1 |
10 |
11 |
·412 |
| [B] The candles cost 3l. 2s. 6d. |
| [C] The Liverpool Old Company have since resorted to the use of Cannel coal, and consequently very nearly assimilate to the Liverpool New Company in illuminating power. |
Memorandum.—It will not fail to be observed that in deducing the comparative value
between candles and gas by these experiments, the single jet (and in every instance,
of course, it was the same), has been the medium. This however, though decidedly
the most correct way of making the comparative estimate of the illuminating power
of the several gases, is highly disadvantageous in the economical comparison, inasmuch
as gas burnt in a properly regulated argand burner, with its proper sized glass, air
aperture, and sufficient number of holes, gives an advantage in favour of gas consumed
in an argand, over a jet burner, of from 30 to 40 per cent. At the same time it
must not be overlooked that in many situations where great light is not required, it
will be found far more economical to adopt the use of single jets, which by means of
swing brackets and light elegant shades, becomes splendid substitutes for candles, in
banking establishments, offices, libraries, &c. &c.
Note.—In Glasgow, Edinburgh, Dundee, Perth, and the Scotch towns generally
the Parrot or Scotch Cannel coal is used; in illuminating power and specific gravity
the gas produced is equal to that from the best description of Cannel coal in England.
The price per 1000 cubic feet ranges about 9s., with from 5 to 30 per cent. off for
discounts, leaving the net price about 9s. to be equal in the above table to 100 lbs. of
candles.
Epitome of Experiments made in Gas produced from different qualities of Coal, and
consumed in different kinds of Burners:
Tried at the Sheffield Gas Light Company’s Works, and laid before a Committee of the
House of Commons. By Joseph Hedley, Esq.
Date
1835. |
Description
of Burner. |
Species of
Coal. |
Specific
Gravity
of Gas. |
Distance
of Candle
from
Shadow. |
Gas
consumed
per Hour. |
Height
of Gas
Flame. |
Equal to
Mould Tallow
Candles, 6 to
the pound,
9 inches
long each. |
Gas equal
to 100 lbs.
of Mould
Candles. |
Cost of Gas
at 8s. per
1000 cubic feet. |
Cost of 100
lbs. of Mould
Candles at 7s.
6d. per dozen
lbs. |
| May. |
|
|
|
Inches. |
Cubic
Feet. |
Inches. |
Candles. |
Cubic
Feet. |
L. |
s. |
d. |
|
L. |
s. |
d. |
| 8 |
|
Single Jet |
|
Deep Pit |
·410 |
75 |
|
1 |
· |
4 |
|
2 |
·36 |
2415 |
0 |
19 |
3 |
1⁄2 |
|
- |
3 |
2 |
6 |
| 9 |
|
Ditto |
|
Mortormley |
·450 |
74 |
|
|
·95 |
4 |
|
2 |
·434 |
2224 |
0 |
17 |
9 |
1⁄2 |
| 9 |
|
Ditto |
|
Cannel |
·660 |
61 |
1⁄4 |
|
·7 |
4 |
|
3 |
·54 |
1127 |
0 |
9 |
0 |
|
| 8 |
- |
|
Argand
14 holes |
|
- |
Deep Pit |
·410 |
34 |
|
3 |
·3 |
3 |
1⁄2 |
11 |
·53 |
1631 |
0 |
13 |
0 |
1⁄2 |
| 9 |
|
Ditto |
|
Mortormley |
·450 |
33 |
|
3 |
·1 |
3 |
1⁄2 |
12 |
·24 |
1443 |
0 |
11 |
6 |
1⁄2 |
| 9 |
|
Ditto |
|
Cannel |
·660 |
29 |
|
2 |
·6 |
3 |
1⁄3 |
15 |
·85 |
935 |
0 |
7 |
5 |
3⁄4 |
| |
|
|
|
|
|
|
|
|
|
|
Date
1835. |
Description
of Burner. |
Species of
Coal. |
Specific
Gravity
of Gas. |
Distance
of Candle
from
Shadow. |
Gas
consumed
per Hour. |
Height
of Gas
Flame. |
| May. |
|
|
|
Inches. |
Cubic
Feet. |
Inches. |
| 8 |
|
Single Jet |
|
Deep Pit |
·410 |
75 |
|
1 |
· |
4 |
|
| 9 |
|
Ditto |
|
Mortormley |
·450 |
74 |
|
|
·95 |
4 |
|
| 9 |
|
Ditto |
|
Cannel |
·660 |
61 |
1⁄4 |
|
·7 |
4 |
|
| 8 |
- |
|
Argand
14 holes |
|
- |
Deep Pit |
·410 |
34 |
|
3 |
·3 |
3 |
1⁄2 |
| 9 |
|
Ditto |
|
Mortormley |
·450 |
33 |
|
3 |
·1 |
3 |
1⁄2 |
| 9 |
|
Ditto |
|
Cannel |
·660 |
29 |
|
2 |
·6 |
3 |
1⁄3 |
Date
1835. |
Description
of Burner. |
Species of
Coal. |
Equal to
Mould Tallow
Candles, 6 to
the pound,
9 inches
long each. |
Gas equal
to 100 lbs.
of Mould
Candles. |
Cost of Gas
at 8s. per
1000 cubic feet. |
Cost of 100
lbs. of Mould
Candles at 7s.
6d. per dozen
lbs. |
| May. |
|
|
Candles. |
Cubic
Feet. |
L. |
s. |
d. |
|
L. |
s. |
d. |
| 8 |
|
Single Jet |
|
Deep Pit |
2 |
·36 |
2415 |
0 |
19 |
3 |
1⁄2 |
|
- |
3 |
2 |
6 |
| 9 |
|
Ditto |
|
Mortormley |
2 |
·434 |
2224 |
0 |
17 |
9 |
1⁄2 |
| 9 |
|
Ditto |
|
Cannel |
3 |
·54 |
1127 |
0 |
9 |
0 |
|
| 8 |
- |
|
Argand
14 holes |
|
- |
Deep Pit |
11 |
·53 |
1631 |
0 |
13 |
0 |
1⁄2 |
| 9 |
|
Ditto |
|
Mortormley |
12 |
·24 |
1443 |
0 |
11 |
6 |
1⁄2 |
| 9 |
|
Ditto |
|
Cannel |
15 |
·85 |
935 |
0 |
7 |
5 |
3⁄4 |
| |
|
|
|
|
|
|
[564]
Copy of Experiments made at the Alliance Gas Company’s Works in Dublin, during
the past year 1837. By Joseph Hedley, Esq.
Results of experiments on the qualities of various coals for the production of gas;
its value in illuminating power; produce of coke, and quality; and other particulars
important in gas-making:—
1st Experiment, Saturday, May 27th, 1837.—Deane coal, (Cumberland). 2 cwt. of
112 lbs. each (or 224 lbs.) produced 970 cubic feet of gas; 4 bushels of coke of middling
quality; specific gravity of the gas, 475. Consumed in a single-jet burner, flame 4
inches high, 14⁄10ths cubic feet per hour; distance from shadow 76 inches or 2·3 mould
candles. Average quantity of gas made from the charge (6 hours) 4·33 cubic feet per
lb., or 9,700 cubic feet per ton of 20 cwt. Increase of coke over coal in measure, not
quite 30 per cent. Loss in weight between coal, coke and breize 56 lbs., converted
into gas, tar, ammonia, &c.
2nd Experiment, May 28th.—Carlisle coal, (Blenkinsopp). 224 lbs. produced 1010
cubic feet of gas, 4 bushels of coke of good quality though small; increase of coke
over coal in measure not quite 30 per cent. Loss in weight, same as foregoing experiment.
Average quantity of gas made from the charge (6 hours) 4·5 cubic feet per lb.
or 10,080 per ton.
Illuminating power of the Gas.
| |
Consumed
per hour,
single jet. |
Distance
from
candle. |
Equal
to
candles. |
Specific
gravity. |
| |
feet. |
inches. |
|
|
| At the end of the 1st hour |
1 |
1⁄10 |
70 |
2 |
·72 |
·475 |
Dittodittowith 20-hole
argand burner |
|
- |
5 |
|
25 |
21 |
·33 |
·475 |
| When charge nearly off |
1 |
4⁄10 |
85 |
1 |
·84 |
·442 |
When charge quite off, with
20-hole argand burner |
|
- |
9 |
|
100 |
not 1 |
|
·256 |
| |
|
|
|
|
3rd Experiment, May 29th.—Carlisle coal (Blenkinsopp). 112 lbs. produced 556 cubic
feet of gas. Other products, loss of weight, &c., same proportion as foregoing experiment.
Average quantity of gas made from the charge (6 hours) 4·96 cubic feet per lb.,
or 11,120 per ton.
In this experiment the quantity of gas generated every hour was ascertained; the
illuminating power, the specific gravity, and the quantity of gas consumed by the single
jet with a flame 4 inches high, was tried at the end of each hour, with the respective gases
generated at each hour; and the following is a table of results.
RESULTS.
| Hour. |
Gas
produced. |
Consumed
per hour
per single jet,
4 inches high. |
Specific
gravity. |
Distance
of candle
from
shadow. |
Illuminating
power
equal to
mould candles. |
| |
cubic
feet. |
cubic
feet. |
|
inches. |
|
| 1st. |
150 |
- |
|
|
111⁄2-10ths.
or 1·15 |
|
- |
·534 |
70 |
2·72 |
| 2nd. |
120 |
|
11 |
|
·495 |
75 |
2·36 |
| 3rd. |
95 |
|
12 |
|
·344 |
75 |
2·36 |
| 4th. |
95 |
|
15 |
|
·311 |
80 |
2·08 |
| 5th. |
80 |
|
17 |
|
·270 |
85 |
1·81 |
| 6th. |
16 |
|
29 |
|
·200 |
100 |
not one |
| Total |
556 |
or |
921⁄3 or 2 feet 9 inches. |
| Average of the above gas, 6-hour charge. |
| |
921⁄3 |
|
16-10ths. nearly |
·359 |
81 |
2·03 |
| Average of the above gas at 4-hour charge. |
| |
115 |
|
121⁄3-10ths. |
·421 |
75 |
2·36 |
Production of gas in 6 hours 556 feet, or at the rate of 11,120 cubic feet per ton.
Produc Dittof gas in 4 hours 460 feet, or at the rate of 19,200cubic ditto.
[565]
The relative value of these productions of gas is as follows, viz.:
11,120 at 16-10ths per hour nearly, (or 1·5916 accurately) and equal to 203 candles;
the 11,120 feet would be equal to and last as long as 1597 candles, or 2661⁄6 lbs. of
candles.
9200 at 121⁄3-10ths. per hour, (or 1·2375 accurately,) and equal to 236 candles; the
9200 feet would be equal to 1949 candles, or 3245⁄6 lbs. candles.
Now 2661⁄6 lbs. of mould candles, at 7s. 6d. per dozen lbs. will cost 18l. 6s. 41⁄2d., whilst
Now 3245⁄6 lbs. of mdo.d cado.es,at 7s. 6d. perdozedo.bs. wdo.ost 10l. 3s.
Shewing the value of 4-hour charges, over 6-hour charges; and of 9,200 cubic feet
over 11,120 cubic feet.
Note.—9500 cubic feet of Wigan cannel coal gas are equal in illuminating power to 859 1-6th lbs. of
candles, which at 7s. 6d. per dozen lbs. will cost 25l. 10s. 51⁄2d. It is also found that any burner with
superior gas, will consume only about half the quantity it would do with common gas.
4th Experiment, May 30th.—Cannel and Cardiff coal mixed 1⁄2 and 1⁄2, together 112 lbs.,
produced 460 feet of gas; 2 bushels of coke of good quality; increase of coke over coal
in measure about 30 per cent.; loss in weight, 41 lbs.; coke weighed 71 lbs., no breize.
Average quantity of gas made from the charge, (4 hours) 4·1 cubic feet, per lb., or
9·200, per ton.
Illuminating power.—At end of first hour.
| |
Candles. |
|
Cubic feet. |
Distance of candle from
shadow |
|
- |
73 or 2·49 |
- |
|
Consumed per hour, single
jet, 4 inches high |
|
- |
12-10ths |
| At end of 2nd hour, do. |
70 or 2·72 |
|
Do.umeddo.er hodo. |
|
111⁄2-10ths |
| At end of 3d hour. |
This gas very indifferent. |
| Average of the three |
70 or 2·72 |
|
Do.umeddo.er hodo. |
|
111⁄2-10ths |
Specific gravity 3·44; 5 feet per hour, with a 20-hole argand burner, equal to 14·66
candles.
5th Experiment, May 31st.—Carlisle coal, 112 lbs. produced 410 feet of gas; other
products, same as in former experiments with this coal, but heat very low.
Illuminating power and produce of gas.
| 410 ft |
- |
|
1st |
hour |
120 |
cubic feet |
|
- |
Average of this gas: specific gravity, 540; distance of candle from shadow, 55 inches, or 4·4 candles consumed per single jet, 9-10ths of a cubic foot per hour. 20-hole argand burner, 4 feet per hour, equal to 21·33 candles. |
| 2nd |
100 |
| 3d |
90 |
| 4th |
100 |
It is possible, from the superior quality of this gas, that a little of the cannel gas
made for a particular purpose, may have have got intermixed with it in the experimental
gasholder and apparatus.
A variety of other experiments were tried on different qualities of coal, and mixtures
of ditto, too tedious to insert here, though extremely valuable, and all tending to shew
the superior value of gas produced at short over long charges; and also showing the
importance and value of coal producing gas of the highest illuminating power; among
which the cannel coal procured in Lancashire, Yorkshire, and some other counties of
England and Wales, and the Parrot or splent coal of Scotland, stand pre-eminent.
Note.—In all the foregoing experiments the same single-jet burner was used; its flame in all instances
exactly 4 inches high.
The coal when drawn from the retort was slaked with water, and after allowing some short time
for drying, was weighed.
A Table of the number of hours Gas is burnt in each month, quarter and year.
| Time of Burning. |
July. |
Aug. |
Sep. |
Oct. |
Nov. |
Dec. |
Jan. |
Feb. |
Mar. |
Apl. |
May. |
June. |
Mid.
quar. |
Mic.
quar. |
Xms.
quar. |
Lady
day
quar. |
Totl.
of
Year. |
|
| o’clock. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| From Dusk: |
to 6 |
— |
— |
2 |
31 |
62 |
80 |
65 |
33 |
4 |
— |
— |
— |
— |
2 |
173 |
102 |
277 |
For
Sun-
days
off,
de-
duct
one
sev-
enth. |
| — |
7 |
— |
14 |
22 |
62 |
92 |
111 |
96 |
61 |
31 |
4 |
— |
— |
4 |
36 |
265 |
188 |
493 |
| — |
8 |
— |
40 |
52 |
93 |
122 |
142 |
127 |
89 |
62 |
28 |
4 |
— |
32 |
92 |
357 |
278 |
759 |
| — |
9 |
13 |
71 |
82 |
124 |
152 |
173 |
158 |
117 |
93 |
58 |
29 |
8 |
95 |
166 |
449 |
368 |
1078 |
| — |
10 |
44 |
102 |
112 |
155 |
182 |
204 |
189 |
145 |
124 |
88 |
60 |
38 |
186 |
258 |
541 |
458 |
1443 |
| — |
11 |
75 |
133 |
142 |
186 |
212 |
235 |
220 |
173 |
155 |
118 |
91 |
68 |
277 |
350 |
633 |
548 |
1808 |
| — |
12 |
106 |
164 |
172 |
217 |
242 |
266 |
251 |
201 |
186 |
148 |
122 |
98 |
368 |
442 |
725 |
638 |
2173 |
| All night |
- |
217 |
307 |
345 |
421 |
473 |
527 |
512 |
411 |
382 |
295 |
242 |
195 |
732 |
869 |
1421 |
1305 |
4327 |
| Morning from |
4 |
— |
16 |
48 |
80 |
110 |
137 |
137 |
98 |
71 |
28 |
2 |
— |
30 |
64 |
327 |
306 |
727 |
| — |
5 |
— |
— |
18 |
49 |
80 |
106 |
106 |
70 |
40 |
3 |
— |
— |
3 |
18 |
235 |
216 |
472 |
| — |
6 |
— |
— |
— |
18 |
50 |
75 |
75 |
42 |
9 |
— |
— |
— |
— |
— |
143 |
126 |
269 |
| — |
7 |
— |
— |
— |
— |
20 |
44 |
44 |
14 |
— |
— |
— |
— |
— |
— |
64 |
58 |
122 |
| Time of Burning. |
July. |
Aug. |
Sep. |
Oct. |
Nov. |
Dec. |
|
| o’clock. |
|
|
|
|
|
|
|
| From Dusk: |
to 6 |
— |
— |
2 |
31 |
62 |
80 |
For
Sun-
days
off,
de-
duct
one
sev-
enth. |
| — |
7 |
— |
14 |
22 |
62 |
92 |
111 |
| — |
8 |
— |
40 |
52 |
93 |
122 |
142 |
| — |
9 |
13 |
71 |
82 |
124 |
152 |
173 |
| — |
10 |
44 |
102 |
112 |
155 |
182 |
204 |
| — |
11 |
75 |
133 |
142 |
186 |
212 |
235 |
| — |
12 |
106 |
164 |
172 |
217 |
242 |
266 |
| All night |
- |
217 |
307 |
345 |
421 |
473 |
527 |
| Morning from |
4 |
— |
16 |
48 |
80 |
110 |
137 |
| — |
5 |
— |
— |
18 |
49 |
80 |
106 |
| — |
6 |
— |
— |
— |
18 |
50 |
75 |
| — |
7 |
— |
— |
— |
— |
20 |
44 |
| Time of Burning. |
Jan. |
Feb. |
Mar. |
Apl. |
May. |
June. |
|
| o’clock. |
|
|
|
|
|
|
|
| From Dusk: |
to 6 |
65 |
33 |
4 |
— |
— |
— |
For
Sun-
days
off,
de-
duct
one
sev-
enth. |
| — |
7 |
96 |
61 |
31 |
4 |
— |
— |
| — |
8 |
127 |
89 |
62 |
28 |
4 |
— |
| — |
9 |
158 |
117 |
93 |
58 |
29 |
8 |
| — |
10 |
189 |
145 |
124 |
88 |
60 |
38 |
| — |
11 |
220 |
173 |
155 |
118 |
91 |
68 |
| — |
12 |
251 |
201 |
186 |
148 |
122 |
98 |
| All night |
- |
512 |
411 |
382 |
295 |
242 |
195 |
| Morning from |
4 |
137 |
98 |
71 |
28 |
2 |
— |
| — |
5 |
106 |
70 |
40 |
3 |
— |
— |
| — |
6 |
75 |
42 |
9 |
— |
— |
— |
| — |
7 |
44 |
14 |
— |
— |
— |
— |
| Time of Burning. |
Mid.
quar. |
Mic.
quar. |
Xms.
quar. |
Lady
day
quar. |
Totl.
of
Year. |
|
| o’clock. |
|
|
|
|
|
|
| From Dusk: |
to 6 |
— |
2 |
173 |
102 |
277 |
For
Sun-
days
off,
de-
duct
one
sev-
enth. |
| — |
7 |
4 |
36 |
265 |
188 |
493 |
| — |
8 |
32 |
92 |
357 |
278 |
759 |
| — |
9 |
95 |
166 |
449 |
368 |
1078 |
| — |
10 |
186 |
258 |
541 |
458 |
1443 |
| — |
11 |
277 |
350 |
633 |
548 |
1808 |
| — |
12 |
368 |
442 |
725 |
638 |
2173 |
| All night |
- |
732 |
869 |
1421 |
1305 |
4327 |
| Morning from |
4 |
30 |
64 |
327 |
306 |
727 |
| — |
5 |
3 |
18 |
235 |
216 |
472 |
| — |
6 |
— |
— |
143 |
126 |
269 |
| — |
7 |
— |
— |
64 |
58 |
122 |
[566
567]
Copy of a Paper submitted to a Committee of the House of Commons in the Session of 1837,
being a Synopsis of the proceedings of the undermentioned principal Gas-Light Establishments
of England; and procured by actual Survey and
Experiments between the Years 1834 and 1837. By Joseph Hedley, Esq.
| Name of the Place where Gas Works are situated. |
Price of Gas per Meter, and Discounts allowed. |
Price of Coal, and Description; delivered per Ton. |
Average Quantity of Gas made per Ton of Coals. |
Coke made from a Ton of Coal. |
Selling Price of Coke. |
Material used to heat Retorts. |
Quantity used per Ton of Coal. |
No. of Public or Street Lamps supplied. |
Description.
—
Size or Sort. |
Price paid per Annum for Ditto. |
Who lights, cleans, puts out, and repairs. |
No. of Hours, or Time burnt in the Year. |
Gas consumed in each Lamp per Hour. |
Rate per Meter Cubic Feet received for Ditto. |
Amount deducted for cleaning, lighting, extinguishing, providing Lamp Posts; &c. |
Per Centage of Loss of Gas made. |
Greatest Quantity of Gas delivered in One Night. |
Duration of Charges. |
Method of Purification. |
Number of Gas Holders. |
Specific Gravity of the Gas. |
Distance of Candle from Shadow. |
Gas equal to Candles. Gas burnt in a single Jet Four Inches high. |
Gas consumed per Hour with a Four-Inch Flame. |
Gas Flame reduced to Candle burnt per Hour. |
Height of Gas Flame equal to Light from Candle. |
| |
|
|
Cu. ft. |
|
|
|
|
|
|
L. |
s. |
d. |
|
|
|
s. |
d. |
s. |
d. |
|
Cubic Feet. |
|
|
|
|
Inch. |
Candles |
Cu. ft. |
Cu. ft. |
Inch. |
| Birmingham Gas Company. |
| 10s. per meter cub. feet. |
| Discounts |
| 10l. |
to |
30l. |
per
an. |
2 |
1⁄2 |
per
cent. |
| 30l. |
to |
50l. |
5 |
|
| 50l. |
to |
75l. |
7 |
1⁄2 |
| 75l. |
to |
100l. |
10 |
|
| 100l. |
& upwards |
15 |
|
|
Lump coal from West Bromwich pits risen much of late. 1837, 11s. 10d. |
6,500 |
32 bushels. |
2s. 1d. per quarter delivered, or about 3d. per bushel. |
Slack. |
About 5 cwt. of slack, at 6s. per ton, 25 per cent. |
490 |
Batswings, 460 30 |
1
2 |
10
0 |
0
0 |
Company, and provides posts, services, &c. |
226 nights, or 2938 hours, 9 months, omitting 5 nights for moons. |
5 feet per hour. |
30
40 |
10
18 |
|
18 |
0 |
Receives net about 6s. 8d. per meter cubic feet. |
48 millions in the year. |
6 hours. |
Dry lime. |
4, and 2 in the town, and large new gas station. |
·453 |
72 |
1,929 |
1 |
·22 |
|
·8 |
2 |
1⁄2 |
| Birmingham and Staffordshire. |
10s. per meter cub. feet. Discounts as above. |
From West Bromwich pits, 1837, 9s. 3d. |
6,500 |
24 bush. but larger measure than Birmingham. |
2s. 10d. per sack of 8 bushels. |
Slack and Tar. |
5 cwt. of slack, at 4s. 25 per cent. |
1,500 |
Batswings. |
average |
Ditto. |
234 nights, or 3042 hours. |
Ditto. |
1 |
3 |
1⁄2 |
18 |
0 |
Receives net about 5s. 6d. per meter cubic feet. |
85 millions in the year. |
Ditto. |
Ditto. |
6, and 6 in the town 7 miles off. |
·455 |
72 |
1,929 |
1 |
·22 |
|
·8 |
2 |
1⁄2 |
| 1 |
18 |
0 |
| Macclesfield. |
| 10s. per meter cub. feet. |
| Discounts |
A-
bove |
50l. |
and
not
ex-
ceed-
ing |
75l. |
5 |
|
per
cent. |
| 75l. |
100l. |
7 |
1⁄2 |
| 100l. |
125l. |
10 |
|
| 125l. |
150l. |
12 |
1⁄2 |
| 150l. |
175l. |
15 |
|
| 175l. |
200l. |
17 |
1⁄2 |
| |
200l. |
& upwards |
20 |
. |
|
Common, 8s. average 1831 |
6,720 |
12 cwt. |
10s. per ton. |
Coke. |
No account kept. |
220 |
Ditto. |
2 |
10 |
0 |
Company. |
8 months, omitting 5 nights for moons. |
4 feet per hour. |
3 |
0 |
|
12 |
0 |
Could not say. |
80,000. Total for year about 15 millions. |
8 hours. |
Ditto. |
3 gas holders. |
Not taken. |
70 |
204 |
Not taken. |
|
·8 |
2 |
3⁄4 |
| Stockport. |
10s. per meter cub. feet. Discounts same as Macclesfield. Macclesfield discounts taken from Stockport card. |
Coal 10s. 6d. cannel 19s. 6d. about half and half used. Average 15s. 1834. |
7,800 |
7 cwt. |
6s. 8d. per ton. |
Coal, coke, and tar. |
Ditto. |
230 |
Ditto. |
2 |
10 |
0 |
Comrs. provide lamps and posts. Company's service light, repair, clean, and extinguish. |
8 months. 4 nights omitted for moons. 237 nights—2800 hours. |
Ditto. |
2 |
6 |
|
12 |
6 |
Ditto. |
65,000. Total for year about 12 millions. |
Ditto. |
Ditto. |
4 gas holders. |
·539 |
64 |
2,441 |
|
·85 |
|
·55 |
2 |
5⁄8 |
| 1834. |
| 2 |
0 |
0 |
| 1837. |
| Manchester. |
| 10s. per m. cub. ft. 1834. |
| 9s. and 8s. cu—ft. 1837. |
| Discounts |
| 50l. |
and
un-
der |
100l. |
2 |
1⁄2 |
per
cent. |
| 100l. |
150l. |
5 |
|
| 150l. |
200l. |
7 |
1⁄2 |
| 200l. |
225l. |
10 |
|
| 225l. |
250l. |
12 |
1⁄2 |
| 250l. |
300l. |
15 |
|
| 300l. |
400l. |
17 |
1⁄2 |
| 400l. |
and upwards |
20 |
|
|
| 15s. 2d. average. |
| Oldham |
|
- |
cannel. |
| Watergate |
| Wigan |
| Mixed, 1834. |
|
9,500 |
14 cwt. |
Ditto. |
Coke. |
4, 2-3ds cwt. |
2,375 |
Single-jets and flat flames, about half and half. |
1
2 |
2
0 |
0
0 |
Commissioners of police. |
3390 hours. |
1 foot, 2 feet, per hour. |
6
5 |
6
6 |
|
nothing. |
About 15 to 171⁄2 per cent. receive about 7s. 4d. per meter cubic feet, public and private. Nearly all by meter. |
500,000. Total for year 100 millions. |
6 hours. |
Wet lime. |
10 gas holders, and 2 in the town. |
·534 |
66 |
2,295 |
|
·825 |
|
·475 |
2 |
1⁄4 |
| Liverpool Old Company, 1834. |
| 10s. per meter cub. feet. |
| Discounts |
| 10l. |
& under |
50l. |
2 |
1⁄2 |
per
ct. |
| 50l. |
to |
100l. |
5 |
|
| 100l. |
to |
200l. |
7 |
1⁄2 |
| 300l. |
& upwards |
10 |
|
|
7s. 3d. per ton of 112 lbs. per cwt. Ormskirk or Wigan slack. |
8,200 |
113⁄4 cwt. |
8s. 4d. per ton of 112 lb. per cwt. |
Slack 7s. 3d. per ton. |
61⁄2 cwt. |
1,700
30 |
Batswings,
1 jet,
2 —
3 —
4 — |
4
2
2
2
3 |
10
5
13
2
13 |
0
0
0
9
11 |
Company light, clean, put out, and repair. |
3600 hours. |
5 feet per hour. |
4 |
4 |
|
12 |
0 |
Could not learn in the absence of the manager. |
360,000. Total for year 72 millions. |
8 hours, large, retorts holding 6 cwt. each. |
Wet and dry lime, principally dry. |
8 gas holders in all, 4 in the town, 1000 yards off the works. |
·462 |
75 |
1,777 |
1 |
·1 |
|
·75 |
2 |
5⁄8 |
| Ditto ditto. |
In 1835 this Company resorted to the use of cannel coal similar to the Liverpool New Gas and Coal Company, producing nearly similar results, which see. |
| Liverpool New Gas and Coke, 1835. |
10s. per meter cub. feet. Discounts same as Liverpool Old Company. |
18s. all cannel Wigan. |
9,500 |
13 cwt. |
7s. 6d. per ton. |
Coke and slack. |
51⁄2 cwt. |
Only a few. |
Argands. |
4 |
0 |
0 |
Commissioners. |
3000 hours. |
31⁄2 feet per hour. |
5 |
6 |
|
nothing |
Nearly all by meter. |
Not sufficiently long at work. |
4 hours. |
Wet lime. |
2 large gas holders. |
·580 |
55 |
3,306 |
|
·9 |
|
·45 |
2 |
|
| Bradford, 1834. |
| 9s. per meter cubic feet to large consumers. |
| Discounts |
| 20l. |
to |
30l. |
5 |
|
per
cent. |
| 30l. |
to |
40l. |
7 |
1⁄2 |
| 40l. |
to |
60l. |
10 |
|
| 60l. |
to |
80l. |
12 |
1⁄2 |
| 80l. |
to |
100l. |
15 |
|
| 100l. |
& upwards |
20 |
|
| Small consumers, 10s. per meter cub. feet, and 5 per cent. off from 10l. to 20l. |
|
8s. 6d. per ton. 3 sorts used average. Slack 5s. 6d. Low moor 8s. 10d. Catherine slack 8s. |
8,000 |
13 cwt. |
12s. per ton. |
Coke. |
81⁄2 cwt. |
220 |
Batswings. |
2 |
12 |
6 |
Company light, repair, &c. |
8 months, omitting 7 nights, 2600 hours to 4 o’clock in the morning. |
5 feet per hour. |
3 |
1 |
|
12 |
6 |
Receive 8s. per meter cubic feet, less 51⁄2 per cent. |
42,500. Total for year 8,619,000. |
8 hours. |
Dry lime. |
4 gas holders. |
·420 |
78 |
1,643 |
|
·12 |
|
·9 |
3 |
|
| Leeds, 1834. |
| 8s. per meter cubic feet. |
| Discounts |
| 2 |
1⁄2 |
|
- |
per cent.
on half-
yearly
payments |
- |
|
15l. |
| 5 |
|
30l. |
| 7 |
1⁄2 |
50l. |
| 10 |
|
100l. |
|
8s. per ton average. 2-3ds common 7s. 1-3d cannel, 10s. |
6,500 |
12 cwt. |
7s. 6d. per ton. |
Ditto. |
51⁄4 cwt. |
517 |
Ditto. |
2 |
12 |
6 |
Commissioners, except extinguishing, for which Company pay 3s. 10d. per lamp. |
2330 hours. |
4 feet per hour. |
5 |
2 |
|
3 |
10 |
Receive for public and private 6s. 8d. per meter cubic feet. Public 5s., private 7s. ; meters used to 5 to 1 for private rental. |
176,000. Total for year 31 millions. |
6 hours. |
Ditto. |
5 gas holders. |
·530 |
67 |
2,228 |
|
·855 |
|
·51 |
2 |
1⁄4 |
| Sheffield, 1835. |
8s. per meter cubic feet. Discounts same as Leeds. |
7s. 9d. per ton average. 3 sorts used 1, 2-10ths. cannel, at 16s. 8, 2-10ths. deep pit, 7s. 1-10th silk stone, 10s. |
8,000 |
10 cwt. of saleable coke. |
10s. per ton. |
Ditto. |
31⁄2 cwt. |
600 |
Ditto. |
2 |
10 |
0 |
Company provide lamps, clean, repair, put out, &c. |
2200 hours. |
Ditto. |
3 |
2 |
1⁄2 |
18 |
0 |
Receive for public and private lts. 5s. per meter cubic feet. Public 3s. 21⁄2d., private 5s. 91⁄2d. Few meters used. |
220,000. Total for year 40 millions. |
Ditto. |
Ditto. |
4 gas holders, and 2 more erecting. |
·466 |
74 |
1,826 |
1 |
·04 |
|
·735 |
2 |
3⁄4 |
| Leicester, 1837. |
| 7s. 6d. per meter cub. ft. |
Discounts on half-yearly rental not
exceeding 10l., 5 per cent. |
A-
bove |
10l. |
and
not
ex-
ceed-
ing |
20l. |
7 |
1⁄2 |
per
cent. |
| 20l. |
30l. |
10 |
|
| 30l. |
40l. |
12 |
1⁄2 |
| 40l. |
50l. |
15 |
|
| 50l. |
60l. |
20 |
|
| 60l. |
& upwards |
25 |
|
|
13s. 6d. average. Derbyshire soft coal. |
7,500 |
4 quarters. |
10s. 8d. or 2s. 8d. per qr. |
Coke, tar, &c. |
About 1-3d of coke. |
414 |
Ditto. |
2 |
18 |
6 |
Company light, put out, and clean. |
From August 14th to September 1st, omitting 3 nights for moons, 3000 hours. |
5 feet per hour. |
3 |
4 |
3⁄4 |
7 |
0 |
Not sufficiently long, at 7s. 6d. |
Total for year 18 millions. |
Ditto. |
Ditto. |
3 gas holders, and 1 erecting. |
·528 |
74 |
1,826 |
1 |
·1 |
|
·75 |
2 |
3⁄4 |
| Derby, 1834. |
10s. per meter cub. feet. |
Same coal used as at Leicester. |
7,000 |
Ditto. |
Ditto. |
Coke. |
Ditto. |
219 |
Ditto. |
2 |
2 |
0 |
Commissioners light, put out, &c. |
2173 hours, from August to May. |
Ditto. |
4 |
0 |
|
— |
Lose about 171⁄2 per cent. |
Ditto. |
Ditto. |
Wet lime. |
4 gas holders. |
·448 |
83 |
1,453 |
1 |
·2 |
|
·925 |
3 |
|
| Discounts 5 to 35 per cent. |
2 |
7 |
0 |
nearly. |
| Nottingham, 1834. |
9s. per meter cubic feet. Discounts as above. |
Ditto. |
7,000 |
Ditto. |
Ditto. |
Ditto. |
Ditto. |
300 |
Ditto. |
3 |
3 |
0 |
Commissioners light, clean, repair, &c. |
All the year, 4327 hours. |
Ditto. |
3 |
0 |
|
— |
Could not learn. |
Ditto. |
Ditto. |
Ditto. |
— |
·424 |
90 |
1,234 |
1 |
·3 |
1 |
·175 |
3 |
|
| nearly. |
| London, 1834. |
10s. per meter cub. feet. No discounts. |
17s. average. Newcastle. |
8,500 |
36 bush. |
12s. per chaldron. |
Ditto. |
13 bush. |
26,280 |
Ditto. |
4 |
0 |
0 |
Company light, clean, put out, but not repair. |
4327 hours, all the year. |
4 feet per hour. |
4 |
0 |
|
12 |
0 |
Receive for public and private lights 7s. public, 4s. private, 8s. few meters used. |
Total for year 1000 millions. Longest night 4,910,000. |
Ditto. |
Ditto. |
130 gas holders. |
·412 |
80 |
1,562 |
1 |
·13 |
|
·84 |
2 |
3⁄4 |
| Ditto, 1837. |
Ditto. |
Ditto. |
8,500 |
Ditto. |
Ditto. |
Ditto. |
Ditto. |
30,400 |
Ditto. |
4 |
0 |
0 |
Ditto. |
Ditto. |
Ditto. |
4 |
0 |
|
12 |
0 |
Ditto. |
Total for year 1460 millions. Longest night 7,120,000. |
Ditto. |
Ditto. |
176 gas holders. |
·412 |
80 |
1,562 |
1 |
·13 |
|
·84 |
2 |
3⁄4 |
| Name of the Place where Gas Works are situated. |
Price of Gas per Meter, and Discounts allowed. |
Price of Coal, and Description; delivered per Ton. |
Average Quantity of Gas made per Ton of Coals. |
Coke made from a Ton of Coal. |
| |
|
|
Cu. ft. |
|
| Birmingham Gas Company. |
| 10s. per meter cub. feet. |
| Discounts |
| 10l. |
to |
30l. |
per
an. |
2 |
1⁄2 |
per
cent. |
| 30l. |
to |
50l. |
5 |
|
| 50l. |
to |
75l. |
7 |
1⁄2 |
| 75l. |
to |
100l. |
10 |
|
| 100l. |
& upwards |
15 |
|
|
Lump coal from West Bromwich pits risen much of late. 1837, 11s. 10d. |
6,500 |
32 bushels. |
| Birmingham and Staffordshire. |
10s. per meter cub. feet. Discounts as above. |
From West Bromwich pits, 1837, 9s. 3d. |
6,500 |
24 bush. but larger measure than Birmingham. |
| Macclesfield. |
| 10s. per meter cub. feet. |
| Discounts |
A-
bove |
50l. |
and
not
ex-
ceed-
ing |
75l. |
5 |
|
per
cent. |
| 75l. |
100l. |
7 |
1⁄2 |
| 100l. |
125l. |
10 |
|
| 125l. |
150l. |
12 |
1⁄2 |
| 150l. |
175l. |
15 |
|
| 175l. |
200l. |
17 |
1⁄2 |
| |
200l. |
& upwards |
20 |
. |
|
Common, 8s. average 1831 |
6,720 |
12 cwt. |
| Stockport. |
10s. per meter cub. feet. Discounts same as Macclesfield. Macclesfield discounts taken from Stockport card. |
Coal 10s. 6d. cannel 19s. 6d. about half and half used. Average 15s. 1834. |
7,800 |
7 cwt. |
| Manchester. |
| 10s. per m. cub. ft. 1834. |
| 9s. and 8s. cu—ft. 1837. |
| Discounts |
| 50l. |
and
un-
der |
100l. |
2 |
1⁄2 |
per
cent. |
| 100l. |
150l. |
5 |
|
| 150l. |
200l. |
7 |
1⁄2 |
| 200l. |
225l. |
10 |
|
| 225l. |
250l. |
12 |
1⁄2 |
| 250l. |
300l. |
15 |
|
| 300l. |
400l. |
17 |
1⁄2 |
| 400l. |
and upwards |
20 |
|
|
| 15s. 2d. average. |
| Oldham |
|
- |
cannel. |
| Watergate |
| Wigan |
| Mixed, 1834. |
|
9,500 |
14 cwt. |
| Liverpool Old Company, 1834. |
| 10s. per meter cub. feet. |
| Discounts |
| 10l. |
& under |
50l. |
2 |
1⁄2 |
per
ct. |
| 50l. |
to |
100l. |
5 |
|
| 100l. |
to |
200l. |
7 |
1⁄2 |
| 300l. |
& upwards |
10 |
|
|
7s. 3d. per ton of 112 lbs. per cwt. Ormskirk or Wigan slack. |
8,200 |
113⁄4 cwt. |
| Ditto ditto. |
In 1835 this Company resorted to the use of cannel coal similar to the Liverpool New Gas and Coal Company, producing nearly similar results, which see. |
| Liverpool New Gas and Coke, 1835. |
10s. per meter cub. feet. Discounts same as Liverpool Old Company. |
18s. all cannel Wigan. |
9,500 |
13 cwt. |
| Bradford, 1834. |
| 9s. per meter cubic feet to large consumers. |
| Discounts |
| 20l. |
to |
30l. |
5 |
|
per
cent. |
| 30l. |
to |
40l. |
7 |
1⁄2 |
| 40l. |
to |
60l. |
10 |
|
| 60l. |
to |
80l. |
12 |
1⁄2 |
| 80l. |
to |
100l. |
15 |
|
| 100l. |
& upwards |
20 |
|
| Small consumers, 10s. per meter cub. feet, and 5 per cent. off from 10l. to 20l. |
|
8s. 6d. per ton. 3 sorts used average. Slack 5s. 6d. Low moor 8s. 10d. Catherine slack 8s. |
8,000 |
13 cwt. |
| Leeds, 1834. |
| 8s. per meter cubic feet. |
| Discounts |
| 2 |
1⁄2 |
|
- |
per cent.
on half-
yearly
payments |
- |
|
15l. |
| 5 |
|
30l. |
| 7 |
1⁄2 |
50l. |
| 10 |
|
100l. |
|
8s. per ton average. 2-3ds common 7s. 1-3d cannel, 10s. |
6,500 |
12 cwt. |
| Sheffield, 1835. |
8s. per meter cubic feet. Discounts same as Leeds. |
7s. 9d. per ton average. 3 sorts used 1, 2-10ths. cannel, at 16s. 8, 2-10ths. deep pit, 7s. 1-10th silk stone, 10s. |
8,000 |
10 cwt. of saleable coke. |
| Leicester, 1837. |
| 7s. 6d. per meter cub. ft. |
Discounts on half-yearly rental not
exceeding 10l., 5 per cent. |
A-
bove |
10l. |
and
not
ex-
ceed-
ing |
20l. |
7 |
1⁄2 |
per
cent. |
| 20l. |
30l. |
10 |
|
| 30l. |
40l. |
12 |
1⁄2 |
| 40l. |
50l. |
15 |
|
| 50l. |
60l. |
20 |
|
| 60l. |
& upwards |
25 |
|
|
13s. 6d. average. Derbyshire soft coal. |
7,500 |
4 quarters. |
| Derby, 1834. |
10s. per meter cub. feet. |
Same coal used as at Leicester. |
7,000 |
Ditto. |
| Discounts 5 to 35 per cent. |
| Nottingham, 1834. |
9s. per meter cubic feet. Discounts as above. |
Ditto. |
7,000 |
Ditto. |
| London, 1834. |
10s. per meter cub. feet. No discounts. |
17s. average. Newcastle. |
8,500 |
36 bush. |
| Ditto, 1837. |
Ditto. |
Ditto. |
8,500 |
Ditto. |
| Name of the Place where Gas Works are situated. |
Selling Price of Coke. |
Material used to heat Retorts. |
Quantity used per Ton of Coal. |
No. of Public or Street Lamps supplied. |
Description.
—
Size or Sort. |
Price paid per Annum for Ditto. |
Who lights, cleans, puts out, and repairs. |
| |
|
|
|
|
|
L. |
s. |
d. |
|
| Birmingham Gas Company. |
2s. 1d. per quarter delivered, or about 3d. per bushel. |
Slack. |
About 5 cwt. of slack, at 6s. per ton, 25 per cent. |
490 |
Batswings, 460 30 |
1
2 |
10
0 |
0
0 |
Company, and provides posts, services, &c. |
| Birmingham and Staffordshire. |
2s. 10d. per sack of 8 bushels. |
Slack and Tar. |
5 cwt. of slack, at 4s. 25 per cent. |
1,500 |
Batswings. |
average |
Ditto. |
| 1 |
18 |
0 |
| Macclesfield. |
10s. per ton. |
Coke. |
No account kept. |
220 |
Ditto. |
2 |
10 |
0 |
Company. |
| Stockport. |
6s. 8d. per ton. |
Coal, coke, and tar. |
Ditto. |
230 |
Ditto. |
2 |
10 |
0 |
Comrs. provide lamps and posts. Company's service light, repair, clean, and extinguish. |
| 1834. |
| 2 |
0 |
0 |
| 1837. |
| Manchester. |
Ditto. |
Coke. |
4, 2-3ds cwt. |
2,375 |
Single-jets and flat flames, about half and half. |
1
2 |
2
0 |
0
0 |
Commissioners of police. |
| Liverpool Old Company, 1834. |
8s. 4d. per ton of 112 lb. per cwt. |
Slack 7s. 3d. per ton. |
61⁄2 cwt. |
1,700
30 |
Batswings,
1 jet,
2 —
3 —
4 — |
4
2
2
2
3 |
10
5
13
2
13 |
0
0
0
9
11 |
Company light, clean, put out, and repair. |
| Ditto ditto. |
In 1835 this Company resorted to the use of cannel coal similar to the Liverpool New Gas and Coal Company, producing nearly similar results, which see. |
| Liverpool New Gas and Coke, 1835. |
7s. 6d. per ton. |
Coke and slack. |
51⁄2 cwt. |
Only a few. |
Argands. |
4 |
0 |
0 |
Commissioners. |
| Bradford, 1834. |
12s. per ton. |
Coke. |
81⁄2 cwt. |
220 |
Batswings. |
2 |
12 |
6 |
Company light, repair, &c. |
| Leeds, 1834. |
7s. 6d. per ton. |
Ditto. |
51⁄4 cwt. |
517 |
Ditto. |
2 |
12 |
6 |
Commissioners, except extinguishing, for which Company pay 3s. 10d. per lamp. |
| Sheffield, 1835. |
10s. per ton. |
Ditto. |
31⁄2 cwt. |
600 |
Ditto. |
2 |
10 |
0 |
Company provide lamps, clean, repair, put out, &c. |
| Leicester, 1837. |
10s. 8d. or 2s. 8d. per qr. |
Coke, tar, &c. |
About 1-3d of coke. |
414 |
Ditto. |
2 |
18 |
6 |
Company light, put out, and clean. |
| Derby, 1834. |
Ditto. |
Coke. |
Ditto. |
219 |
Ditto. |
2 |
2 |
0 |
Commissioners light, put out, &c. |
| 2 |
7 |
0 |
| Nottingham, 1834. |
Ditto. |
Ditto. |
Ditto. |
300 |
Ditto. |
3 |
3 |
0 |
Commissioners light, clean, repair, &c. |
| London, 1834. |
12s. per chaldron. |
Ditto. |
13 bush. |
26,280 |
Ditto. |
4 |
0 |
0 |
Company light, clean, put out, but not repair. |
| Ditto, 1837. |
Ditto. |
Ditto. |
Ditto. |
30,400 |
Ditto. |
4 |
0 |
0 |
Ditto. |
| Name of the Place where Gas Works are situated. |
No. of Hours, or Time burnt in the Year. |
Gas consumed in each Lamp per Hour. |
Rate per Meter Cubic Feet received for Ditto. |
Amount deducted for cleaning, lighting, extinguishing, providing Lamp Posts; &c. |
Per Centage of Loss of Gas made. |
Greatest Quantity of Gas delivered in One Night. |
| |
|
|
s. |
d. |
s. |
d. |
|
Cubic Feet. |
| Birmingham Gas Company. |
226 nights, or 2938 hours, 9 months, omitting 5 nights for moons. |
5 feet per hour. |
30
40 |
10
18 |
|
18 |
0 |
Receives net about 6s. 8d. per meter cubic feet. |
48 millions in the year. |
| Birmingham and Staffordshire. |
234 nights, or 3042 hours. |
Ditto. |
1 |
3 |
1⁄2 |
18 |
0 |
Receives net about 5s. 6d. per meter cubic feet. |
85 millions in the year. |
| Macclesfield. |
8 months, omitting 5 nights for moons. |
4 feet per hour. |
3 |
0 |
|
12 |
0 |
Could not say. |
80,000. Total for year about 15 millions. |
| Stockport. |
8 months. 4 nights omitted for moons. 237 nights—2800 hours. |
Ditto. |
2 |
6 |
|
12 |
6 |
Ditto. |
65,000. Total for year about 12 millions. |
| Manchester. |
3390 hours. |
1 foot, 2 feet, per hour. |
6
5 |
6
6 |
|
nothing. |
About 15 to 171⁄2 per cent. receive about 7s. 4d. per meter cubic feet, public and private. Nearly all by meter. |
500,000. Total for year 100 millions. |
| Liverpool Old Company, 1834. |
3600 hours. |
5 feet per hour. |
4 |
4 |
|
12 |
0 |
Could not learn in the absence of the manager. |
360,000. Total for year 72 millions. |
| Ditto ditto. |
In 1835 this Company resorted to the use of cannel coal similar to the Liverpool New Gas and Coal Company, producing nearly similar results, which see. |
| Liverpool New Gas and Coke, 1835. |
3000 hours. |
31⁄2 feet per hour. |
5 |
6 |
|
nothing |
Nearly all by meter. |
Not sufficiently long at work. |
| Bradford, 1834. |
8 months, omitting 7 nights, 2600 hours to 4 o’clock in the morning. |
5 feet per hour. |
3 |
1 |
|
12 |
6 |
Receive 8s. per meter cubic feet, less 51⁄2 per cent. |
42,500. Total for year 8,619,000. |
| Leeds, 1834. |
2330 hours. |
4 feet per hour. |
5 |
2 |
|
3 |
10 |
Receive for public and private 6s. 8d. per meter cubic feet. Public 5s., private 7s. ; meters used to 5 to 1 for private rental. |
176,000. Total for year 31 millions. |
| Sheffield, 1835. |
2200 hours. |
Ditto. |
3 |
2 |
1⁄2 |
18 |
0 |
Receive for public and private lts. 5s. per meter cubic feet. Public 3s. 21⁄2d., private 5s. 91⁄2d. Few meters used. |
220,000. Total for year 40 millions. |
| Leicester, 1837. |
From August 14th to September 1st, omitting 3 nights for moons, 3000 hours. |
5 feet per hour. |
3 |
4 |
3⁄4 |
7 |
0 |
Not sufficiently long, at 7s. 6d. |
Total for year 18 millions. |
| Derby, 1834. |
2173 hours, from August to May. |
Ditto. |
4 |
0 |
|
— |
Lose about 171⁄2 per cent. |
Ditto. |
| nearly. |
| Nottingham, 1834. |
All the year, 4327 hours. |
Ditto. |
3 |
0 |
|
— |
Could not learn. |
Ditto. |
| nearly. |
| London, 1834. |
4327 hours, all the year. |
4 feet per hour. |
4 |
0 |
|
12 |
0 |
Receive for public and private lights 7s. public, 4s. private, 8s. few meters used. |
Total for year 1000 millions. Longest night 4,910,000. |
| Ditto, 1837. |
Ditto. |
Ditto. |
4 |
0 |
|
12 |
0 |
Ditto. |
Total for year 1460 millions. Longest night 7,120,000. |
| Name of the Place where Gas Works are situated. |
Duration of Charges. |
Method of Purification. |
Number of Gas Holders. |
Specific Gravity of the Gas. |
Distance of Candle from Shadow. |
| |
|
|
|
|
Inch. |
| Birmingham Gas Company. |
6 hours. |
Dry lime. |
4, and 2 in the town, and large new gas station. |
·453 |
72 |
| Birmingham and Staffordshire. |
Ditto. |
Ditto. |
6, and 6 in the town 7 miles off. |
·455 |
72 |
| Macclesfield. |
8 hours. |
Ditto. |
3 gas holders. |
Not taken. |
70 |
| Stockport. |
Ditto. |
Ditto. |
4 gas holders. |
·539 |
64 |
| Manchester. |
6 hours. |
Wet lime. |
10 gas holders, and 2 in the town. |
·534 |
66 |
| Liverpool Old Company, 1834. |
8 hours, large, retorts holding 6 cwt. each. |
Wet and dry lime, principally dry. |
8 gas holders in all, 4 in the town, 1000 yards off the works. |
·462 |
75 |
| Ditto ditto. |
In 1835 this Company resorted to the use of cannel coal similar to the Liverpool New Gas and Coal Company, producing nearly similar results, which see. |
| Liverpool New Gas and Coke, 1835. |
4 hours. |
Wet lime. |
2 large gas holders. |
·580 |
55 |
| Bradford, 1834. |
8 hours. |
Dry lime. |
4 gas holders. |
·420 |
78 |
| Leeds, 1834. |
6 hours. |
Ditto. |
5 gas holders. |
·530 |
67 |
| Sheffield, 1835. |
Ditto. |
Ditto. |
4 gas holders, and 2 more erecting. |
·466 |
74 |
| Leicester, 1837. |
Ditto. |
Ditto. |
3 gas holders, and 1 erecting. |
·528 |
74 |
| Derby, 1834. |
Ditto. |
Wet lime. |
4 gas holders. |
·448 |
83 |
| Nottingham, 1834. |
Ditto. |
Ditto. |
— |
·424 |
90 |
| London, 1834. |
Ditto. |
Ditto. |
130 gas holders. |
·412 |
80 |
| Ditto, 1837. |
Ditto. |
Ditto. |
176 gas holders. |
·412 |
80 |
| Name of the Place where Gas Works are situated. |
Gas equal to Candles. Gas burnt in a single Jet Four Inches high. |
Gas consumed per Hour with a Four-Inch Flame. |
Gas Flame reduced to Candle burnt per Hour. |
Height of Gas Flame equal to Light from Candle. |
| |
Candles |
Cu. ft. |
Cu. ft. |
Inch. |
| Birmingham Gas Company. |
1,929 |
1 |
·22 |
|
·8 |
2 |
1⁄2 |
| Birmingham and Staffordshire. |
1,929 |
1 |
·22 |
|
·8 |
2 |
1⁄2 |
| Macclesfield. |
204 |
Not taken. |
|
·8 |
2 |
3⁄4 |
| Stockport. |
2,441 |
|
·85 |
|
·55 |
2 |
5⁄8 |
| Manchester. |
2,295 |
|
·825 |
|
·475 |
2 |
1⁄4 |
| Liverpool Old Company, 1834. |
1,777 |
1 |
·1 |
|
·75 |
2 |
5⁄8 |
| Ditto ditto. |
In 1835 this Company resorted to the use of cannel coal similar to the Liverpool New Gas and Coal Company, producing nearly similar results, which see. |
| Liverpool New Gas and Coke, 1835. |
3,306 |
|
·9 |
|
·45 |
2 |
|
| Bradford, 1834. |
1,643 |
|
·12 |
|
·9 |
3 |
|
| Leeds, 1834. |
2,228 |
|
·855 |
|
·51 |
2 |
1⁄4 |
| Sheffield, 1835. |
1,826 |
1 |
·04 |
|
·735 |
2 |
3⁄4 |
| Leicester, 1837. |
1,826 |
1 |
·1 |
|
·75 |
2 |
3⁄4 |
| Derby, 1834. |
1,453 |
1 |
·2 |
|
·925 |
3 |
|
| Nottingham, 1834. |
1,234 |
1 |
·3 |
1 |
·175 |
3 |
|
| London, 1834. |
1,562 |
1 |
·13 |
|
·84 |
2 |
3⁄4 |
| Ditto, 1837. |
1,562 |
1 |
·13 |
|
·84 |
2 |
3⁄4 |
[568]
A Table shewing the Rate per Thousand Cubic feet received for any Burner consuming from 1⁄2 a Cubic foot to 10 Cubic feet per hour, at any given
price per annum, and to the times below stated. By Joseph Hedley, Esq.
| Time of Burning per annum. |
No.
of
Hours.
[D] |
Single Jets. |
2 Jets. |
3 Jets. |
Small Argand. |
Large Argand. |
Fancy and extravagant
Burners. |
Cub.
ft.
1⁄2 |
Cub.
ft.
3⁄4 |
Cub.
ft.
1 |
Cub.
ft.
11⁄4 |
Cub.
ft.
11⁄2 |
Cub.
ft.
13⁄4 |
Cub.
ft.
2 |
Cub.
ft.
21⁄2 |
Cub.
ft.
3 |
Cub.
ft.
31⁄2 |
Cub.
ft.
4 |
Cub.
ft.
41⁄2 |
Cub.
ft.
5 |
Cub.
ft.
6 |
Cub.
ft.
7 |
Cub.
ft.
8 |
Cub.
ft.
9 |
Cub.
ft.
10 |
| From Dusk to |
8 |
o’clock |
781 |
2 |
·56 |
1 |
·706 |
1 |
·28 |
1 |
·026 |
|
·853 |
|
·731 |
|
·64 |
|
·5132 |
|
·4268 |
|
·3658 |
|
·3201 |
|
·2846 |
|
·2561 |
|
·2134 |
|
·1829 |
|
·16 |
|
·1423 |
|
·128 |
| ditto and Sundays |
902 |
2 |
·216 |
1 |
·478 |
1 |
·108 |
|
·887 |
|
·739 |
|
·633 |
|
·554 |
|
·4434 |
|
·3695 |
|
·3168 |
|
·2771 |
|
·2464 |
|
·2217 |
|
·1848 |
|
·1584 |
|
·1383 |
|
·1232 |
|
·1108 |
| ditto and from 6 o’clock mornings |
1050 |
1 |
·904 |
1 |
·27 |
|
·952 |
|
·762 |
|
·635 |
|
·544 |
|
·476 |
|
·381 |
|
·3174 |
|
·272 |
|
·2381 |
|
·2116 |
|
·1905 |
|
·1587 |
|
·136 |
|
·119 |
|
·1058 |
|
·0952 |
| ditto and Sundays and from ditto |
1172 |
1 |
·706 |
1 |
·138 |
|
·853 |
|
·682 |
|
·569 |
|
·487 |
|
·426 |
|
·3412 |
|
·2844 |
|
·2438 |
|
·2133 |
|
·1896 |
|
·1706 |
|
·1422 |
|
·1219 |
|
·1067 |
|
·0948 |
|
·0853 |
| 9 |
o’clock |
1054 |
1 |
·896 |
1 |
·264 |
|
·948 |
|
·759 |
|
·632 |
|
·542 |
|
·474 |
|
·3794 |
|
·3162 |
|
·271 |
|
·2371 |
|
·2108 |
|
·1897 |
|
·1581 |
|
·1355 |
|
·1185 |
|
·1054 |
|
·0948 |
| ditto and Sundays |
1221 |
1 |
·638 |
1 |
·092 |
|
·819 |
|
·675 |
|
·546 |
|
·463 |
|
·409 |
|
·3376 |
|
·273 |
|
·234 |
|
·2047 |
|
·182 |
|
·1638 |
|
·1365 |
|
·117 |
|
·1024 |
|
·091 |
|
·0819 |
| ditto and from 6 o’clock mornings |
1323 |
1 |
·510 |
1 |
·066 |
|
·755 |
|
·604 |
|
·503 |
|
·431 |
|
·378 |
|
·3022 |
|
·2519 |
|
·2158 |
|
·1889 |
|
·1678 |
|
·1511 |
|
·1259 |
|
·1079 |
|
·0945 |
|
·0839 |
|
·0755 |
| ditto and Sundays and from ditto |
1490 |
1 |
·342 |
|
·894 |
|
·671 |
|
·536 |
|
·447 |
|
·383 |
|
·335 |
|
·2684 |
|
·2236 |
|
·1918 |
|
·1675 |
|
·1492 |
|
·1312 |
|
·1118 |
|
·0959 |
|
·0839 |
|
·0746 |
|
·0671 |
| 10 |
o’clock |
1367 |
1 |
·462 |
|
·974 |
|
·731 |
|
·585 |
|
·487 |
|
·418 |
|
·366 |
|
·2926 |
|
·2438 |
|
·209 |
|
·1829 |
|
·1626 |
|
·1463 |
|
·1219 |
|
·1045 |
|
·0914 |
|
·0813 |
|
·0731 |
| ditto and Sundays |
1586 |
1 |
·26 |
|
·84 |
|
·63 |
|
·504 |
|
·42 |
|
·36 |
|
·315 |
|
·2522 |
|
·2101 |
|
·1802 |
|
·1576 |
|
·14 |
|
·1261 |
|
·1051 |
|
·0901 |
|
·0789 |
|
·07 |
|
·0630 |
| ditto and from 6 o’clock mornings |
1636 |
1 |
·222 |
|
·814 |
|
·611 |
|
·489 |
|
·407 |
|
·349 |
|
·305 |
|
·2444 |
|
·2037 |
|
·1746 |
|
·1528 |
|
·1358 |
|
·1222 |
|
·1019 |
|
·0873 |
|
·0764 |
|
·0679 |
|
·0611 |
| ditto and Sundays and from ditto |
1855 |
1 |
·078 |
|
·718 |
|
·539 |
|
·431 |
|
·359 |
|
·308 |
|
·269 |
|
·2156 |
|
·1796 |
|
·154 |
|
·1347 |
|
·1198 |
|
·1078 |
|
·0898 |
|
·077 |
|
·0674 |
|
·0599 |
|
·0539 |
| 11 |
o’clock |
1680 |
1 |
·19 |
|
·794 |
|
·595 |
|
·476 |
|
·397 |
|
·34 |
|
·297 |
|
·238 |
|
·1984 |
|
·17 |
|
·1488 |
|
·133 |
|
·119 |
|
·0992 |
|
·085 |
|
·0744 |
|
·0665 |
|
·0595 |
| ditto and Sundays |
1951 |
1 |
·024 |
|
·682 |
|
·512 |
|
·409 |
|
·341 |
|
·293 |
|
·256 |
|
·2048 |
|
·1707 |
|
·1466 |
|
·1281 |
|
·1138 |
|
·1024 |
|
·0854 |
|
·0733 |
|
·064 |
|
·0569 |
|
·0512 |
| ditto and from 6 o’clock mornings |
1949 |
1 |
·026 |
|
·684 |
|
·513 |
|
·41 |
|
·342 |
|
·294 |
|
·256 |
|
·2052 |
|
·171 |
|
·1466 |
|
·1282 |
|
·114 |
|
·1026 |
|
·0855 |
|
·0733 |
|
·0641 |
|
·057 |
|
·0513 |
| ditto and Sundays and from ditto |
2220 |
|
·9 |
|
·6 |
|
·45 |
|
·36 |
|
·3 |
|
·257 |
|
·225 |
|
·1802 |
|
·1501 |
|
·1286 |
|
·1126 |
|
·1 |
|
·0981 |
|
·0751 |
|
·0643 |
|
·0563 |
|
·05 |
|
·045 |
| 12 |
o’clock |
1993 |
1 |
· |
|
·668 |
|
·502 |
|
·4 |
|
·334 |
|
·287 |
|
·251 |
|
·2006 |
|
·1672 |
|
·1434 |
|
·1254 |
|
·1114 |
|
·1003 |
|
·0836 |
|
·0717 |
|
·0627 |
|
·0557 |
|
·0502 |
| ditto and Sundays |
2316 |
|
·862 |
|
·574 |
|
·432 |
|
·345 |
|
·287 |
|
·247 |
|
·215 |
|
·1726 |
|
·1439 |
|
·1236 |
|
·1079 |
|
·0958 |
|
·0863 |
|
·0719 |
|
·0618 |
|
·0539 |
|
·0479 |
|
·0432 |
| ditto and from 6 o’clock mornings |
2262 |
|
·884 |
|
·59 |
|
·442 |
|
·353 |
|
·295 |
|
·255 |
|
·221 |
|
·1768 |
|
·1476 |
|
·1274 |
|
·1105 |
|
·0982 |
|
·0884 |
|
·0737 |
|
·0637 |
|
·0552 |
|
·0491 |
|
·0442 |
| ditto and Sundays and from ditto |
2585 |
|
·772 |
|
·514 |
|
·387 |
|
·309 |
|
·257 |
|
·221 |
|
·193 |
|
·1546 |
|
·1289 |
|
·1104 |
|
·0967 |
|
·0858 |
|
·0773 |
|
·0645 |
|
·0552 |
|
·0483 |
|
·0429 |
|
·0387 |
| 1 |
o’clock |
2306 |
|
·866 |
|
·578 |
|
·434 |
|
·347 |
|
·289 |
|
·247 |
|
·217 |
|
·1734 |
|
·1445 |
|
·1238 |
|
·1080 |
|
·0962 |
|
·0867 |
|
·0723 |
|
·0619 |
|
·0542 |
|
·0481 |
|
·0434 |
| ditto and Sundays |
2681 |
|
·746 |
|
·498 |
|
·373 |
|
·298 |
|
·249 |
|
·213 |
|
·186 |
|
·1492 |
|
·1243 |
|
·1066 |
|
·0932 |
|
·0828 |
|
·0746 |
|
·0621 |
|
·0533 |
|
·0466 |
|
·0414 |
|
·0373 |
| ditto and from 6 o’clock mornings |
2575 |
|
·776 |
|
·518 |
|
·388 |
|
·31 |
|
·259 |
|
·222 |
|
·194 |
|
·1552 |
|
·1294 |
|
·111 |
|
·0971 |
|
·0862 |
|
·0776 |
|
·0647 |
|
·0555 |
|
·0485 |
|
·0431 |
|
·0388 |
| ditto and Sundays and from ditto |
2950 |
|
·678 |
|
·452 |
|
·339 |
|
·271 |
|
·226 |
|
·193 |
|
·169 |
|
·1356 |
|
·113 |
|
·0968 |
|
·0847 |
|
·0754 |
|
·0678 |
|
·0565 |
|
·0484 |
|
·0424 |
|
·0377 |
|
·0339 |
| All night |
4327 |
|
·462 |
|
·308 |
|
·231 |
|
·185 |
|
·154 |
|
·132 |
|
·115 |
|
·6924 |
|
·077 |
|
·066 |
|
·0578 |
|
·0515 |
|
·0462 |
|
·0385 |
|
·033 |
|
·0289 |
|
·0257 |
|
·0231 |
| To use the Table.—Select the hour to which it is agreed the gas is to burn,—9, 10, 11 o’clock, Sundays, &c., as the case may be, and the description of the burner.—Multiply the decimal number opposite to it by the amount in shillings agreed to be paid per annum, and the product will be the sum received per m. cubic feet for the gas. |
| Example.—Suppose a small argand which should burn 31⁄2 feet per hour, is agreed for till 9 o’clock at 2l. per annum. Look along the line of 9 o’clock till you arrive at the column of 31⁄2 feet per hour, and you find the number, ·271. Multiply this number by 40s. and the result gives 10s. 10d. per m. cubic feet. But suppose instead of keeping to 9 o’clock the party burns till 1 o’clock, Sundays and mornings, and by enlarging the holes or height of flame consumes 8 cubic feet of gas per hour; then you have the number, ·0424, which multiplied by 40s., still the price paid, gives 1s. 8d. per m. cubic feet only, and so on for any greater or lesser variation of the agreement. |
| [D] The “number of hours” includes 1⁄4 of an hour allowed for shutting shops, and 1 hour’s extra burning on Saturday nights. |
| Time of Burning per annum. |
No.
of
Hours.
[D] |
Single Jets. |
2 Jets. |
Cub.
ft.
1⁄2 |
Cub.
ft.
3⁄4 |
Cub.
ft.
1 |
Cub.
ft.
11⁄4 |
Cub.
ft.
11⁄2 |
Cub.
ft.
13⁄4 |
| From Dusk to |
8 |
o’clock |
781 |
2 |
·56 |
1 |
·706 |
1 |
·28 |
1 |
·026 |
|
·853 |
|
·731 |
| ditto and Sundays |
902 |
2 |
·216 |
1 |
·478 |
1 |
·108 |
|
·887 |
|
·739 |
|
·633 |
| ditto and from 6 o’clock mornings |
1050 |
1 |
·904 |
1 |
·27 |
|
·952 |
|
·762 |
|
·635 |
|
·544 |
| ditto and Sundays and from ditto |
1172 |
1 |
·706 |
1 |
·138 |
|
·853 |
|
·682 |
|
·569 |
|
·487 |
| 9 |
o’clock |
1054 |
1 |
·896 |
1 |
·264 |
|
·948 |
|
·759 |
|
·632 |
|
·542 |
| ditto and Sundays |
1221 |
1 |
·638 |
1 |
·092 |
|
·819 |
|
·675 |
|
·546 |
|
·463 |
| ditto and from 6 o’clock mornings |
1323 |
1 |
·510 |
1 |
·066 |
|
·755 |
|
·604 |
|
·503 |
|
·431 |
| ditto and Sundays and from ditto |
1490 |
1 |
·342 |
|
·894 |
|
·671 |
|
·536 |
|
·447 |
|
·383 |
| 10 |
o’clock |
1367 |
1 |
·462 |
|
·974 |
|
·731 |
|
·585 |
|
·487 |
|
·418 |
| ditto and Sundays |
1586 |
1 |
·26 |
|
·84 |
|
·63 |
|
·504 |
|
·42 |
|
·36 |
| ditto and from 6 o’clock mornings |
1636 |
1 |
·222 |
|
·814 |
|
·611 |
|
·489 |
|
·407 |
|
·349 |
| ditto and Sundays and from ditto |
1855 |
1 |
·078 |
|
·718 |
|
·539 |
|
·431 |
|
·359 |
|
·308 |
| 11 |
o’clock |
1680 |
1 |
·19 |
|
·794 |
|
·595 |
|
·476 |
|
·397 |
|
·34 |
| ditto and Sundays |
1951 |
1 |
·024 |
|
·682 |
|
·512 |
|
·409 |
|
·341 |
|
·293 |
| ditto and from 6 o’clock mornings |
1949 |
1 |
·026 |
|
·684 |
|
·513 |
|
·41 |
|
·342 |
|
·294 |
| ditto and Sundays and from ditto |
2220 |
|
·9 |
|
·6 |
|
·45 |
|
·36 |
|
·3 |
|
·257 |
| 12 |
o’clock |
1993 |
1 |
· |
|
·668 |
|
·502 |
|
·4 |
|
·334 |
|
·287 |
| ditto and Sundays |
2316 |
|
·862 |
|
·574 |
|
·432 |
|
·345 |
|
·287 |
|
·247 |
| ditto and from 6 o’clock mornings |
2262 |
|
·884 |
|
·59 |
|
·442 |
|
·353 |
|
·295 |
|
·255 |
| ditto and Sundays and from ditto |
2585 |
|
·772 |
|
·514 |
|
·387 |
|
·309 |
|
·257 |
|
·221 |
| 1 |
o’clock |
2306 |
|
·866 |
|
·578 |
|
·434 |
|
·347 |
|
·289 |
|
·247 |
| ditto and Sundays |
2681 |
|
·746 |
|
·498 |
|
·373 |
|
·298 |
|
·249 |
|
·213 |
| ditto and from 6 o’clock mornings |
2575 |
|
·776 |
|
·518 |
|
·388 |
|
·31 |
|
·259 |
|
·222 |
| ditto and Sundays and from ditto |
2950 |
|
·678 |
|
·452 |
|
·339 |
|
·271 |
|
·226 |
|
·193 |
| All night |
4327 |
|
·462 |
|
·308 |
|
·231 |
|
·185 |
|
·154 |
|
·132 |
| To use the Table.—Select the hour to which it is agreed the gas is to burn,—9, 10, 11 o’clock, Sundays, &c., as the case may be, and the description of the burner.—Multiply the decimal number opposite to it by the amount in shillings agreed to be paid per annum, and the product will be the sum received per m. cubic feet for the gas. |
| Example.—Suppose a small argand which should burn 31⁄2 feet per hour, is agreed for till 9 o’clock at 2l. per annum. Look along the line of 9 o’clock till you arrive at the column of 31⁄2 feet per hour, and you find the number, ·271. Multiply this number by 40s. and the result gives 10s. 10d. per m. cubic feet. But suppose instead of keeping to 9 o’clock the party burns till 1 o’clock, Sundays and mornings, and by enlarging the holes or height of flame consumes 8 cubic feet of gas per hour; then you have the number, ·0424, which multiplied by 40s., still the price paid, gives 1s. 8d. per m. cubic feet only, and so on for any greater or lesser variation of the agreement. |
| [D] The “number of hours” includes 1⁄4 of an hour allowed for shutting shops, and 1 hour’s extra burning on Saturday nights. |
| Time of Burning per annum. |
No.
of
Hours.
[D] |
3 Jets. |
Small Argand. |
Cub.
ft.
2 |
Cub.
ft.
21⁄2 |
Cub.
ft.
3 |
Cub.
ft.
31⁄2 |
Cub.
ft.
4 |
Cub.
ft.
41⁄2 |
| From Dusk to |
8 |
o’clock |
781 |
|
·64 |
|
·5132 |
|
·4268 |
|
·3658 |
|
·3201 |
|
·2846 |
| ditto and Sundays |
902 |
|
·554 |
|
·4434 |
|
·3695 |
|
·3168 |
|
·2771 |
|
·2464 |
| ditto and from 6 o’clock mornings |
1050 |
|
·476 |
|
·381 |
|
·3174 |
|
·272 |
|
·2381 |
|
·2116 |
| ditto and Sundays and from ditto |
1172 |
|
·426 |
|
·3412 |
|
·2844 |
|
·2438 |
|
·2133 |
|
·1896 |
| 9 |
o’clock |
1054 |
|
·474 |
|
·3794 |
|
·3162 |
|
·271 |
|
·2371 |
|
·2108 |
| ditto and Sundays |
1221 |
|
·409 |
|
·3376 |
|
·273 |
|
·234 |
|
·2047 |
|
·182 |
| ditto and from 6 o’clock mornings |
1323 |
|
·378 |
|
·3022 |
|
·2519 |
|
·2158 |
|
·1889 |
|
·1678 |
| ditto and Sundays and from ditto |
1490 |
|
·335 |
|
·2684 |
|
·2236 |
|
·1918 |
|
·1675 |
|
·1492 |
| 10 |
o’clock |
1367 |
|
·366 |
|
·2926 |
|
·2438 |
|
·209 |
|
·1829 |
|
·1626 |
| ditto and Sundays |
1586 |
|
·315 |
|
·2522 |
|
·2101 |
|
·1802 |
|
·1576 |
|
·14 |
| ditto and from 6 o’clock mornings |
1636 |
|
·305 |
|
·2444 |
|
·2037 |
|
·1746 |
|
·1528 |
|
·1358 |
| ditto and Sundays and from ditto |
1855 |
|
·269 |
|
·2156 |
|
·1796 |
|
·154 |
|
·1347 |
|
·1198 |
| 11 |
o’clock |
1680 |
|
·297 |
|
·238 |
|
·1984 |
|
·17 |
|
·1488 |
|
·133 |
| ditto and Sundays |
1951 |
|
·256 |
|
·2048 |
|
·1707 |
|
·1466 |
|
·1281 |
|
·1138 |
| ditto and from 6 o’clock mornings |
1949 |
|
·256 |
|
·2052 |
|
·171 |
|
·1466 |
|
·1282 |
|
·114 |
| ditto and Sundays and from ditto |
2220 |
|
·225 |
|
·1802 |
|
·1501 |
|
·1286 |
|
·1126 |
|
·1 |
| 12 |
o’clock |
1993 |
|
·251 |
|
·2006 |
|
·1672 |
|
·1434 |
|
·1254 |
|
·1114 |
| ditto and Sundays |
2316 |
|
·215 |
|
·1726 |
|
·1439 |
|
·1236 |
|
·1079 |
|
·0958 |
| ditto and from 6 o’clock mornings |
2262 |
|
·221 |
|
·1768 |
|
·1476 |
|
·1274 |
|
·1105 |
|
·0982 |
| ditto and Sundays and from ditto |
2585 |
|
·193 |
|
·1546 |
|
·1289 |
|
·1104 |
|
·0967 |
|
·0858 |
| 1 |
o’clock |
2306 |
|
·217 |
|
·1734 |
|
·1445 |
|
·1238 |
|
·1080 |
|
·0962 |
| ditto and Sundays |
2681 |
|
·186 |
|
·1492 |
|
·1243 |
|
·1066 |
|
·0932 |
|
·0828 |
| ditto and from 6 o’clock mornings |
2575 |
|
·194 |
|
·1552 |
|
·1294 |
|
·111 |
|
·0971 |
|
·0862 |
| ditto and Sundays and from ditto |
2950 |
|
·169 |
|
·1356 |
|
·113 |
|
·0968 |
|
·0847 |
|
·0754 |
| All night |
4327 |
|
·115 |
|
·6924 |
|
·077 |
|
·066 |
|
·0578 |
|
·0515 |
| To use the Table.—Select the hour to which it is agreed the gas is to burn,—9, 10, 11 o’clock, Sundays, &c., as the case may be, and the description of the burner.—Multiply the decimal number opposite to it by the amount in shillings agreed to be paid per annum, and the product will be the sum received per m. cubic feet for the gas. |
| Example.—Suppose a small argand which should burn 31⁄2 feet per hour, is agreed for till 9 o’clock at 2l. per annum. Look along the line of 9 o’clock till you arrive at the column of 31⁄2 feet per hour, and you find the number, ·271. Multiply this number by 40s. and the result gives 10s. 10d. per m. cubic feet. But suppose instead of keeping to 9 o’clock the party burns till 1 o’clock, Sundays and mornings, and by enlarging the holes or height of flame consumes 8 cubic feet of gas per hour; then you have the number, ·0424, which multiplied by 40s., still the price paid, gives 1s. 8d. per m. cubic feet only, and so on for any greater or lesser variation of the agreement. |
| [D] The “number of hours” includes 1⁄4 of an hour allowed for shutting shops, and 1 hour’s extra burning on Saturday nights. |
| Time of Burning per annum. |
No.
of
Hours.
[D] |
Large Argand. |
Fancy and extravagant
Burners. |
Cub.
ft.
5 |
Cub.
ft.
6 |
Cub.
ft.
7 |
Cub.
ft.
8 |
Cub.
ft.
9 |
Cub.
ft.
10 |
| From Dusk to |
8 |
o’clock |
781 |
|
·2561 |
|
·2134 |
|
·1829 |
|
·16 |
|
·1423 |
|
·128 |
| ditto and Sundays |
902 |
|
·2217 |
|
·1848 |
|
·1584 |
|
·1383 |
|
·1232 |
|
·1108 |
| ditto and from 6 o’clock mornings |
1050 |
|
·1905 |
|
·1587 |
|
·136 |
|
·119 |
|
·1058 |
|
·0952 |
| ditto and Sundays and from ditto |
1172 |
|
·1706 |
|
·1422 |
|
·1219 |
|
·1067 |
|
·0948 |
|
·0853 |
| 9 |
o’clock |
1054 |
|
·1897 |
|
·1581 |
|
·1355 |
|
·1185 |
|
·1054 |
|
·0948 |
| ditto and Sundays |
1221 |
|
·1638 |
|
·1365 |
|
·117 |
|
·1024 |
|
·091 |
|
·0819 |
| ditto and from 6 o’clock mornings |
1323 |
|
·1511 |
|
·1259 |
|
·1079 |
|
·0945 |
|
·0839 |
|
·0755 |
| ditto and Sundays and from ditto |
1490 |
|
·1312 |
|
·1118 |
|
·0959 |
|
·0839 |
|
·0746 |
|
·0671 |
| 10 |
o’clock |
1367 |
|
·1463 |
|
·1219 |
|
·1045 |
|
·0914 |
|
·0813 |
|
·0731 |
| ditto and Sundays |
1586 |
|
·1261 |
|
·1051 |
|
·0901 |
|
·0789 |
|
·07 |
|
·0630 |
| ditto and from 6 o’clock mornings |
1636 |
|
·1222 |
|
·1019 |
|
·0873 |
|
·0764 |
|
·0679 |
|
·0611 |
| ditto and Sundays and from ditto |
1855 |
|
·1078 |
|
·0898 |
|
·077 |
|
·0674 |
|
·0599 |
|
·0539 |
| 11 |
o’clock |
1680 |
|
·119 |
|
·0992 |
|
·085 |
|
·0744 |
|
·0665 |
|
·0595 |
| ditto and Sundays |
1951 |
|
·1024 |
|
·0854 |
|
·0733 |
|
·064 |
|
·0569 |
|
·0512 |
| ditto and from 6 o’clock mornings |
1949 |
|
·1026 |
|
·0855 |
|
·0733 |
|
·0641 |
|
·057 |
|
·0513 |
| ditto and Sundays and from ditto |
2220 |
|
·0981 |
|
·0751 |
|
·0643 |
|
·0563 |
|
·05 |
|
·045 |
| 12 |
o’clock |
1993 |
|
·1003 |
|
·0836 |
|
·0717 |
|
·0627 |
|
·0557 |
|
·0502 |
| ditto and Sundays |
2316 |
|
·0863 |
|
·0719 |
|
·0618 |
|
·0539 |
|
·0479 |
|
·0432 |
| ditto and from 6 o’clock mornings |
2262 |
|
·0884 |
|
·0737 |
|
·0637 |
|
·0552 |
|
·0491 |
|
·0442 |
| ditto and Sundays and from ditto |
2585 |
|
·0773 |
|
·0645 |
|
·0552 |
|
·0483 |
|
·0429 |
|
·0387 |
| 1 |
o’clock |
2306 |
|
·0867 |
|
·0723 |
|
·0619 |
|
·0542 |
|
·0481 |
|
·0434 |
| ditto and Sundays |
2681 |
|
·0746 |
|
·0621 |
|
·0533 |
|
·0466 |
|
·0414 |
|
·0373 |
| ditto and from 6 o’clock mornings |
2575 |
|
·0776 |
|
·0647 |
|
·0555 |
|
·0485 |
|
·0431 |
|
·0388 |
| ditto and Sundays and from ditto |
2950 |
|
·0678 |
|
·0565 |
|
·0484 |
|
·0424 |
|
·0377 |
|
·0339 |
| All night |
4327 |
|
·0462 |
|
·0385 |
|
·033 |
|
·0289 |
|
·0257 |
|
·0231 |
| To use the Table.—Select the hour to which it is agreed the gas is to burn,—9, 10, 11 o’clock, Sundays, &c., as the case may be, and the description of the burner.—Multiply the decimal number opposite to it by the amount in shillings agreed to be paid per annum, and the product will be the sum received per m. cubic feet for the gas. |
| Example.—Suppose a small argand which should burn 31⁄2 feet per hour, is agreed for till 9 o’clock at 2l. per annum. Look along the line of 9 o’clock till you arrive at the column of 31⁄2 feet per hour, and you find the number, ·271. Multiply this number by 40s. and the result gives 10s. 10d. per m. cubic feet. But suppose instead of keeping to 9 o’clock the party burns till 1 o’clock, Sundays and mornings, and by enlarging the holes or height of flame consumes 8 cubic feet of gas per hour; then you have the number, ·0424, which multiplied by 40s., still the price paid, gives 1s. 8d. per m. cubic feet only, and so on for any greater or lesser variation of the agreement. |
| [D] The “number of hours” includes 1⁄4 of an hour allowed for shutting shops, and 1 hour’s extra burning on Saturday nights. |
[569]
GENERAL SUMMARY.
For lighting London and its suburbs with gas, there are—
18 public gas works.
12publdo.gascompanies.
2,800,000l. capital employed in works, pipes, tanks, gas-holders, apparatus.
450,000l. yearly revenue derived.
180,000 tons of coals used in the year for making gas.
1,460,000,000 cubic feet of gas made in the year.
134,300 private burners supplied to about 40,000 consumers.
30,400 public or street do. N. B. about 2650 of these are in the city of London.
380 lamplighters employed.
176 gas-holders; several of them double ones, capable of storing 5,500,000 cubic feet.
890 tons of coals used in the retorts on the shortest day, in 24 hours.
7,120,000 cubic feet of gas used in longest night, say 24th December.
About 2500 persons are employed in the metropolis alone, in this branch of manufacture.
Between 1822 and 1827 the quantity nearly doubled itself, and that in 5 years.
Between 1827 and 1837 it doubled itself again.
Mr. Kirkham, engineer, obtained a patent, in June, 1837, for an improved mode of
removing the carbonaceous incrustation from the internal surfaces of gas retorts. He
employs a jet or jets of heated atmospheric air, or other gases containing oxygen, which
he impels with force into the interior of such retorts as have become incrusted in consequence
of the decomposition of the coal. The retort is to be kept thoroughly red hot
during the application of the proposed jets. An iron pipe, constructed with several
flexible joints, leading from a blowing machine, is bent in such a way as to allow its
nozzle end to be introduced within the retort, and directed to any point of its surface.
I should suppose that air, even at common temperatures, applied to a retort ignited to
the pitch of making gas, would burn away the incrustations; but hot air will, no doubt,
be more powerful.
GAS-HOLDER; a vessel for containing and preserving gas, of which various forms
are described by chemical writers.
GASOMETER, means properly a measurer of gas, though it is employed often to
denote a recipient of gas of any kind. See the article Gas-Light.
GAUZE WIRE CLOTH; is a textile fabric, either plane or tweelled, made of
brass, iron, or copper wire, of very various degrees of fineness and openness of texture.
Its chief uses are for sieves, and safety lamps.
GAY-LUSSITE, is a white mineral of a vitreous fracture, which crystallizes in
oblique rhomboidal prisms; specific gravity from 1·93 to 1·95; scratches gypsum, but
is scratched by calcspar; affords water by calcination; it consists of carbonic acid
28·66; soda, 20·44; lime, 17·70; water, 32·20; clay, 1·00. It is in fact, by my analysis,
a hydrated soda-carbonate of lime in atomic proportions. This mineral occurs
abundantly in insulated crystals, disseminated through the bed of clay which covers the
urao, or native sesquicarbonate of soda, at Lagunilla in Colombia.
GELATINE; (Eng. and Fr.; Gallert, Leim, Germ.) is an animal product which is
never found in the humours, but it may be obtained by boiling with water the soft and solid
parts; as the muscles, the skin, the cartilages, bones, ligaments, tendons, and membranes.
Isinglass consists almost entirely of gelatine. This substance is very soluble in boiling
water; the solution forms a tremulous mass of jelly when it cools. Cold water has little
action upon gelatine. Alcohol and tannin (tannic acid, see Gall-nuts) precipitate gelatine
from its solution; the former by abstracting the water, the latter by combining with the
substance itself into an insoluble compound; of the nature of leather. No other acid,
except the tannic, and no alkali possesses the property of precipitating gelatine. But
chlorine and certain salts render its solution more or less turbid; as the nitrate and
bi-chloride of mercury, the proto-chloride of tin, and a few others. Sulphuric acid
converts a solution of gelatine at a boiling heat into sugar. See Ligneous Fibre.
Gelatine consists of carbon, 47·88; hydrogen, 7·91; oxygen, 27·21. See Glue and
Isinglass.
GEMS, are precious stones, which, by their colour, limpidity, lustre, brilliant polish,
purity, and rarity, are sought after as objects of dress and decoration. They form the
principal part of the crown jewels of kings, not only from their beauty, but because
they are supposed to comprize the greatest value in the smallest bulk; for a diamond,
no larger than a nut or an acorn, may be the representative sign of the territorial value
of a whole country, the equivalent in commercial exchange of a hundred fortunes,
acquired by severe toils and privations.
Among these beautiful minerals mankind have agreed in forming a select class, to which
the title of gems or jewels has been appropriated; while the term precious stone is more
particularly given to substances which often occur under a more considerable volume
than fine stones ever do.
[570]
Diamonds, sapphires, emeralds, rubies, topazes, hyacinths, and chrysoberyls, are
reckoned the most valuable gems.
Crystalline quartz, pellucid opalescent or of various hues, amethyst, lapis lazuli,
malachite, jasper, agate, &c., are ranked in the much more numerous and inferior class
of ornamental stones. These distinctions are not founded upon any strict philosophical
principle, but are regulated by a conventional agreement, not very well defined; for it
is impossible to subject these creatures of fashion and taste to the rigid subdivisions of
science. We have only to consider the value currently attached to them, and take care
not to confound two stones of the same colour, but which may be very differently
prized by the virtuoso.
Since it usually happens that the true gems are in a cut and polished state, or even set
in gold or silver, we are thereby unable to apply to them the criteria of mineralogical and
chemical science. The cutting of the stone has removed or masked its crystalline
character, and circumstances rarely permit the phenomena of double or single refraction
to be observed; while the test by the blowpipe is inadmissible. Hence the only
scientific resources that remain are the trial by electricity, which is often inconclusive;
the degree of hardness, a criterion requiring great experience in the person who employs
it; and, lastly, the proof by specific gravity, unquestionably one of the surest means of
distinguishing the really fine gems from ornamental stones of similar colour. This
proof can be applied only to a stone that is not set; but the richer gems are usually
dismounted, when offered for sale.
This character of specific gravity may be applied by any person of common intelligence,
with the aid of a small hydrostatic balance. If, for example, a stone of a fine
crimson-red colour, be offered for sale, as an oriental ruby; the purchaser must ascertain
if it be not a Siberian tourmaline, or ruby spinel. Supposing its weight in air to be 100
grains, if he finds it reduced to 69 grains, when weighed in water, he concludes that its
bulk is equal to that of 31 grains of water, which is its loss of weight. Now, a real
sapphire which weighs 100 grains in air, would have weighed 76·6 in water; a spinel
ruby of 100 grains would have weighed 72·2 in water, and a Siberian tourmaline of
100 grains would have weighed only 69 grains in water. The quality of the stone in
question is, therefore, determined beyond all dispute, and the purchaser may be thus
protected from fraud.
The sard of the English jewellers (Sardoine, French) is a stone of the nature of
agate, having an orange colour more or less deep, and passing by insensible shades
into yellow, reddish, and brown; whence it has been agreed to unite under this denomination
all the agates whose colour verges upon brown. It should be remarked,
however, that the sard presents, in its interior and in the middle of its ground, concentric
zones, or small nebulosities, which are not to be seen in the red cornelian, properly
so called. The ancients certainly knew our sard, since they have left us a great
many of them engraved, but they seem to have associated under the title sarda both the
sardoine of the French, and our cornelians and calcedonies. Pliny says that the sarda
came from the neighbourhood of a city of that name in Lydia, and from the environs of
Babylon. Among the engraved sards which exist in the collection of antiques in the
Bibliothèque Royale of Paris, there is an Apollo remarkable for its fine colour and great
size. When the stone forms a part of the agate-onyx, it is called sardonyx. For further
details upon Gems, and the art of cutting and engraving them, see Lapidary.
GEOGNOSY, means a knowledge of the structure of the earth; Geology, a description
of the same. The discussion of this subject does not come within the province
of this Dictionary.
GERMAN SILVER. See the latter end of the article Copper.
GERMINATION; (Eng. and Fr.; Das Keimen, Germ.) is the first sprouting of a
seed after it is sown, or when, after steeping, it is spread upon the malt floor. See Beer.
GIG MACHINES, are rotatory drums, mounted with thistles or wire teeth for
teazling cloth. See Woollen Manufacture.
GILDING (Dorure, Fr.; Vergoldung, Germ.); is the art of coating surfaces with
a thin film of gold. For a full discussion of this subject, see Gold. Mr. Elkington,
gilt toy maker, obtained a patent, in June, 1836, for gilding copper, brass, &c., by
means of potash or soda combined with carbonic acid, and with a solution of gold.
Dissolve, says he, 5 oz. troy of fine gold in 52 oz. avoirdupois of nitro-muriatic acid of
the following proportions: viz. 21 oz. of pure nitric acid, of spec. grav. 1·45, 17 oz.
of pure muriatic acid, of spec. grav. 1·15; with 14 oz. of distilled water.
The gold being put into the mixture of acids and water, they are to be heated in
a glass or other convenient vessel till the gold is dissolved; and it is usual to continue
the application of heat after this is effected, until a reddish or yellowish vapour ceases
to rise.
The clear liquid is to be carefully poured off from any sediment which generally
appears and results from a small portion of silver, which is generally found in alloy with
gold. The clear liquid is to be placed in a suitable vessel of stone, pottery ware is preferred.[571]
Add to the solution of gold 4 gallons of distilled water, and 20 pounds of bicarbonate
of potash of the best quality; let the whole boil moderately for 2 hours, the
mixture will then be ready for use.
The articles to be gilded having been first perfectly cleaned from scale or grease, they
are to be suspended on wires, conveniently for a workman to dip them in the liquid,
which is kept boiling. The time required for gilding any particular article will depend
on circumstances, partly on the quantity of gold remaining in the liquid, and partly on
the size and weight of the article; but a little practice will readily give sufficient
guidance to the workman.
Supposing the articles desired to be gilded be brass or copper buttons, or small
articles for gilt toys, or ornaments of dress, such as earrings or bracelets, a considerable
number of which may be strung on a hoop, or bended piece of copper or brass wire, and
dipped into the vessel containing the boiling liquid above described, and moved therein,
the requisite gilding will be generally obtained in from a few seconds to a minute; this
is when the liquid is in the condition above described, and depending on the quality of
the gilding desired; but if the liquid has been used some time, the quantity of gold will
be lessened, which will vary the time of operating to produce a given effect, or the
colour required, all which will quickly be observed by the workman; and by noting
the appearance of the articles from time to time, he will know when the desired object
is obtained, though it is desirable to avoid as much as possible taking the articles out of
the liquid.
When the operation is completed, the workman perfectly washes the articles so gilded
with clean water; they may then be submitted to the usual process of colouring.
If the articles be cast figures of animals, or otherwise of considerable weight, compared
with the articles above mentioned, the time required to perform the process
will be greater.
In case it is desired to produce what is called a dead appearance, it may be performed
by several processes: the one usually employed is to dead the articles in the process of
cleaning, as practised by brass-founders and other trades; it is produced by an acid,
prepared for that purpose, sold by the makers under the term “deading aquafortis,”
which is well understood.
It may also be produced by a weak solution of nitrate of mercury, applied to the
articles previous to the gilding process, as is practised in the process of gilding with
mercury, previous to spreading the amalgam, but generally a much weaker solution; or
the articles having been gilded may be dipped in a solution of nitrate of mercury, and
submitted to heat to expel the same, as is practised in the usual process of gilding.
It is desirable to remark, that much of the beauty of the result depends on the well
cleaning of the articles, and it is better to clean them by the ordinary processes, and at
once pass them into the liquid to be gilded. See Gold, towards the end.
GIN, or Geneva, from Genievre (juniper), is a kind of ardent spirits manufactured in
Holland, and hence called Hollands gin in this country, to distinguish it from British
gin. The materials employed in the distilleries of Schiedam, are two parts of unmalted
rye from Riga, weighing about 54 lbs. per bushel, and one part of malted bigg,
weighing about 37 lbs. per bushel. The mash tun, which serves also as the fermenting
tun, has a capacity of nearly 700 gallons, being about five feet in diameter at the mouth,
rather narrower at the bottom, and 41⁄2 feet deep; the stirring apparatus is an oblong
rectangular iron grid, made fast to the end of a wooden pole. About a barrel, = 36
gallons of water, at a temperature of from 162° to 168° (the former heat being best for
the most highly dried rye), are put into the mash tun for every 11⁄2 cwt. of meal, after
which the malt is introduced and stirred, and lastly the rye is added. Powerful agitation
is given to the magma till it becomes quite uniform; a process which a vigorous workman
piques himself upon executing in the course of a few minutes. The mouth of the
tun is immediately covered over with canvas, and further secured by a close wooden lid, to
confine the heat; it is left in this state for two hours. The contents being then stirred
up once more, the transparent spent wash of a preceding mashing is first added, and next
as much cold water as will reduce the temperature of the whole to about 85° F. The
best Flanders yeast, which had been brought, for the sake of carriage, to a doughy consistence
by pressure, is now introduced to the amount of one pound for every 100 gallons
of the mashed materials.
The gravity of the fresh wort is usually from 33 to 38 lbs. per Dicas’ hydrometer;
and the fermentation is carried on from 48 to 60 hours, at the end of which time the
attenuation is from 7 to 4 lbs., that is, the specific gravity of the supernatant wash is
from 1·007 to 1·004.
The distillers are induced by the scarcity of beer-barm in Holland, to skim off a
quantity of the yeast from the fermenting tuns, and to sell it to the bakers, whereby they
obstruct materially the production of spirit, though they probably improve its quality,
by preventing its impregnation with yeasty particles; an unpleasant result which seldom
fails to take place in the whiskey distilleries of the United Kingdom.
[572]
On the third day after the fermenting tun is set, the wash containing the grains is transferred
to the still, and converted into low wines. To every 100 gallons of this liquor, two
pounds of juniper berries, from 3 to 5 years old, being added along with about one quarter
of a pound of salt, the whole are put into the low wine still, and the fine Hollands spirit
is drawn off by a gentle and well-regulated heat, till the magma becomes exhausted;
the first and the last products being mixed together; whereby a spirit, 2 to 3 per cent.
above our hydrometer proof, is obtained, possessing the peculiar fine aroma of gin. The
quantity of spirit varies from 18 to 21 gallons per quarter of grain; this large product
being partly due to the employment of the spent wash of the preceding fermentation;
an addition which contributes at the same time to improve the flavour.
For the above instructive details of the manufacture of genuine Hollands, I am
indebted to Robert More, Esq., formerly of Underwood, distiller, who after studying
the art at Schiedam, tried to introduce that spirit into general consumption in this
country, but found the palates of our gin-drinkers too much corrupted to relish so pure
a beverage.
GINNING, is the name of the operation by which the filaments of cotton are separated
from the seeds. See Cotton Manufacture.
GLANCE COAL, or anthracite, of which there are two varieties, the slaty and the
conchoidal. See Anthracite.
GLASS (Verre, Fr.; Glas, Germ.); is a transparent solid formed by the fusion of
siliceous and alkaline matter. It was known to the Phenicians, and constituted for a
long time an exclusive manufacture of that people, in consequence of its ingredients,
natron, sand, and fuel, abounding upon their coasts. It is probable that the more
ancient Egyptians were unacquainted with glass, for we find no mention of it in the
writings of Moses. But according to Pliny and Strabo, the glass works of Sidon and
Alexandria were famous in their times, and produced beautiful articles; which were
cut, engraved, gilt, and stained of the most brilliant colours, in imitation of precious
stones. The Romans employed glass for various purposes; and have left specimens in
Herculaneum of window-glass, which must have been blown by methods analogous to
the modern. The Phenician processes seem to have been learned by the Crusaders, and
transferred to Venice in the 13th century, where they were long held secret, and formed
a lucrative commercial monopoly. Soon after the middle of the 17th century, Colbert
enriched France with the blown mirror glass manufacture.
Chance undoubtedly had a principal share in the invention of this curious fabrication,
but there were circumstances in the most ancient arts likely to lead to it; such as the
fusing and vitrifying heats required for the formation of pottery, and for the extraction of
metals from their ores. Pliny ascribes the origin of glass to the following accident.
A merchant-ship laden with natron being driven upon the coast at the mouth of the
river Belus, in tempestuous weather, the crew were compelled to cook their victuals
ashore, and having placed lumps of the natron upon the sand, as supports to the kettles,
found to their surprise masses of transparent stone among the cinders. The sand of
this small stream of Galilee, which runs from the foot of Mount Carmel, was in consequence
supposed to possess a peculiar virtue for making glass, and continued for ages to
be sought after and exported to distant countries for this purpose.
Agricola, the oldest author who has written technically upon glass, describes furnaces
and processes closely resembling those employed at the present day. Neri, Kunckel,
Henckel, Pott, Achard, and some other chemists, have since then composed treatises
upon the subject; but Neri, Bosc, Antic, Loysel, and Allut, in the Encyclopédie
Méthodique, are the best of the elder authorities.
The window-glass manufacture was first begun in England in 1557, in Crutched
Friars, London; and fine articles of flint-glass were soon afterwards made in the Savoy
House, Strand. In 1635 the art received a great improvement from Sir Robert Mansell,
by the use of coal fuel instead of wood. The first sheets of blown glass for looking
glasses and coach windows were made in 1673 at Lambeth, by Venetian artisans employed
under the patronage of the Duke of Buckingham.
The casting of mirror-plates was commenced in France about the year 1688, by Abraham
Thevart; an invention which gave rise soon afterwards to the establishment of the
celebrated works of St. Gobin, which continued for nearly a century the sole place
where this highly prized object of luxury was well made. In excellence and cheapness,
the French mirror-plate has been, however, for some time rivalled by the English.
The analysis of modern chemists, which will be detailed in the course of this article,
and the light thrown upon the manufacture of glass in general by the accurate means
now possessed of purifying its several ingredients, would have brought the art to the
highest state of perfection in this country, but for the vexatious interference and obstructions
of our excise laws.
The researches of Berzelius having removed all doubts concerning the acid character
of silica, the general composition of glass presents now no difficulty of conception.
This substance consists of one or more salts; which are silicates with bases of potash,[573]
soda, lime, oxide of iron, alumina, or oxide of lead; in any of which compounds we
can substitute one of these bases for another, provided that one alkaline base be left.
Silica in its turn may be replaced by the boracic acid, without causing the glass to lose
its principal characters.
Under the title glass are therefore comprehended various substances fusible at a high
temperature, solid at ordinary temperatures, brilliant, generally more or less transparent,
and always brittle. The following chemical distribution of glasses has been proposed.
1. Soluble glass; a simple silicate of potash or soda; or of both these alkalis.
2. Bohemian or crown glass; silicate of potash and lime.
3. Common window and mirror glass; silicate of soda and lime; sometimes also of
potash.
4. Bottle glass; silicate of soda, lime, alumina and iron.
5. Ordinary crystal glass; silicate of potash and lead.
6. Flint glass; silicate of potash and lead; richer in lead than the preceding.
7. Strass; silicate of potash and lead; still richer in lead.
8. Enamel; silicate and stannate or antimoniate of potash or soda, and lead.
The glasses which contain several bases are liable to suffer different changes when
they are melted or cooled slowly. The silica is divided among these bases, forming
new compounds in definite proportions, which by crystallizing, separate from each other,
so that the general mixture of the ingredients which constituted glass is destroyed.
It becomes then very hard, fibrous, opaque, much less fusible, a better conductor of
electricity and of heat; forming what Reaumur styled devitrified glass; and what is
called after him, Reaumur’s porcelain.
This altered glass can always be produced in a more or less perfect state, by melting
the glass and allowing it to cool very slowly; or merely by heating it to the softening
pitch, and keeping it at this heat for some time. The process succeeds best with the
most complex vitreous compounds, such as bottle glass; next with ordinary window
glass; and lastly with glass of potash and lead.
This property ought to be kept constantly in view in manufacturing glass. It shows
why in making bottles we should fashion them as quickly as possible with the aid of a
mould, and reheat them as seldom as may be absolutely necessary. If it be often heated
and cooled, the glass loses its ductility, becomes refractory, and exhibits a multitude
of stony granulations throughout its substance. When coarse glass is worked at the
enameller’s lamp, it is apt to change its nature in the same way, if the workman be not
quick and expert at his business.
From these facts we perceive the importance of making a careful choice of the glass
intended to be worked in considerable masses, such as the large object glasses of telescopes;
as their annealing requires a very slow process of refrigeration, which is apt to
cause devitrified specks and clouds. For such purposes, therefore, no other species of
glass is well adapted except that with basis of potash and lead; or that with basis
of potash and lime. These two form the best flint glass, and crown glass; and they
should be exclusively employed for the construction of the object glasses of achromatic
telescopes.
GLASS-MAKING, general principles of. Glass may be defined in technical phraseology,
to be a transparent homogeneous compound formed by the fusion of silica with
oxides of the alkaline, earthy, or common metals. It is usually colourless, and then resembles
rock crystal, but is occasionally stained by accident or design with coloured metallic
oxides. At common temperatures it is hard and brittle, in thick pieces; in thin
plates or threads, flexible and elastic; sonorous when struck; fracture conchoidal, and
of that peculiar lustre called vitreous; at a red heat, becoming soft, ductile and plastic.
Besides glass properly so called, other bodies are capable of entering into vitreous
fusion, as phosphoric acid, boracic acid, arsenic acid, as also certain metallic oxides, as of
lead, and antimony, and several chlorides; some of which are denominated glasses.
Impure and opaque vitriform masses are called slags; such are the productions of blast
iron furnaces and many metallurgic operations.
Silica, formerly styled the earth of flints, which constitutes the basis of all commercial
glass, is infusible by itself in the strongest fire of our furnaces; but its vitreous fusion is
easily effected by a competent addition of potash or soda, either alone or mixed with
lime or litharge. The silica, which may be regarded as belonging to the class of acids,
combines at the heat of fusion with these bases, into saline compounds; and hence
glass may be viewed as a silicate of certain oxides, in which the acid and the bases exist
in equivalent proportions. Were these proportions, or the quantities of the bases which
silica requires for its saturation at the melting point, exactly ascertained, we might
readily determine beforehand the best proportions of materials for the glass manufacture.
But as this is far from being the case, and as it is, moreover, not improbable that the
capacity of saturation of the silica varies with the temperature, and that the properties
of glass also vary with the bases, we must, in the present state of our knowledge, regulate
the proportions rather by practice than by theory, though the latter may throw an[574]
indirect light upon the subject. For example, a good colourless glass has been found by
analysis to consist of 72 parts of silica, 13 parts of potash, and 10 parts of lime, in 95
parts. If we reduce these numbers to the equivalent ratios, we shall have the following
results; taking the atomic weights as given by Berzelius.
| 1 |
atom |
potash |
= |
590 |
14·67 |
|
| 1 |
lime |
356 |
8·84 |
| 3 |
silica |
1722 |
42·79 |
|
- |
71·49 |
| 2 |
silica |
1155 |
28·70 |
| |
3823 |
95·00 |
|
This glass would therefore have been probably better compounded with the just
atomic proportions, to which it nearly approaches, viz. 71·49 silica, 14·67 potash, and
8·84 lime, instead of those given above as its actual constituents.
The proportions in which silica unites with the alkaline and other oxides are modified
by the temperature as above stated; the lower the heat, the less silica will enter
into the glass, and the more of the base will in general be required. If a glass which
contains an excess of alkali be exposed to a much higher temperature than that of its
formation, a portion of the base will be set free to act upon the materials of the earthen
pot, or to be dissipated in fumes, until such a silicate remains as to constitute a permanent
glass corresponding to that temperature. Hence the same mixture of vitrifiable
materials will yield very different results, according to the heats in which it is fused
and worked in the glass-house; and therefore the composition should always be referrible
to the going of the furnace. When a species of glass which at a high temperature
formed a transparent combination with a considerable quantity of lime, is kept for
some time in fusion at a lower temperature, a portion of the lime unites with the silica
into another combination of a semi-vitreous or even of a stony aspect, so as to spoil
the transparency of the glass altogether. There is probably a supersilicate, and a subsilicate
formed in such cases; the latter being much the more fusible of the two compounds.
The Reaumur’s porcelain produced by exposing bottle glass to a red heat
for 24 hours, is an example of this species of vitreous change in which new affinities
are exercised at a lower temperature. An excess of silica, caused by the volatilization
of alkaline matter with too strong firing, will bring on similar appearances.
The specific gravity of glass varies from 2·3 to 3·6. That of least specific gravity
consists of merely silica and potash fused together; that with lime is somewhat denser,
and with oxide of lead denser still. Plate glass made from silica, soda, and lime, has
a specific gravity which varies from 2·50 to 2·6; crystal or flint glass from 3·0 to 3·6.
The power of glass to resist the action of water, alkalis, acids, air, and light, is in
general the greater, the higher the temperature employed in its manufacture, the smaller
the proportion of its fluxes, and the more exact the equivalent ratios of its constituents.
When glass contains too much alkali, it is partially soluble in water. Most crystal glass
is affected by having water boiled in it for a considerable time; but crown glass being
poorer in alkali, and containing no lead, resists that action much longer, and is therefore
better adapted to chemical operations. The affinity of glass for water, or its hygrometric
attraction, is also proportional to the quantity of alkali which it contains. In general
also potash glass is more apt to become damp than soda glass, agreeably to the respective
hygrometric properties of these two alkalis, and also to the smaller proportion of soda
than of potash requisite to form glass.
Air and light operate upon glass probably by their oxidizing property. Bluish or
greenish coloured glasses become by exposure colourless, in consequence undoubtedly
of the peroxidizement of the iron, to whose protoxide they owe their tint;
other glasses become purple red from the peroxidizement of the manganese. The glasses
which contain lead, suffer another kind of change in the air, if sulphuretted hydrogen
be present; the oxide of lead is converted into a sulphuret, with the effect of rendering
the surface of the glass opaque and iridescent. The more lead is in the glass, the
quicker does this iridescence supervene. By boiling concentrated sulphuric acid in a
glass vessel, or upon glass, we can ascertain its power of resisting ordinary menstrua.
Good glass will remain smooth and transparent; bad glass will become rough
and dim.
The brittleness of unannealed glass by change of temperature is sometimes very
great. I have known a thick vessel to fly by vicissitudes of the atmosphere alone. This
defect may be corrected by slowly heating the vessel in salt-water or oil to the highest
pitch consistent with the nature of these liquids, and letting it cool very slowly.
Within the limits of that range of heat, it will, in consequence of this treatment,
bear alternations of temperature without cracking as before.
It has been said that glass made from silica and alkalis alone, will not resist the action
of water, but that the addition of a little lime is necessary for this effect. In general[575]
100 parts of quartzose sand require 33 parts of dry carbonate of soda for their vitrification,
and 45 parts of dry carbonate of potash. But to make unchangeable alkaline
glass, especially with potash, a smaller quantity of this than the above should be used,
with a very violent heat. A small proportion of lime increases the density, hardness,
and lustre of glass; and it aids in decomposing the alkaline sulphates and muriates always
present in the pearl ash of commerce. From 7 to 20 parts of dry slaked lime
have been added for 100 of silica, with advantage, it is said, in some German glass
manufactories, where the alkaline matter is soda; for potass does not assimilate well
with the calcareous earth.
In many glass works on the Continent, sulphate of soda is the form under which alkaline
matter is introduced into glass. This salt requires the addition of 8 per cent. of
charcoal to decompose and dissipate its acid; a result which takes place at a high heat,
without the addition of any lime. 88 pounds of quartz-sand, 44 pounds of dry glauber
salt, and 3 pounds of charcoal, properly mixed and fused, afford a limpid, fluent, and
workable glass; with the addition of 17 pounds of lime, these materials fuse more readily
into a plastic mass. If less carbon be added, the fusion becomes more tedious. The two
following formulæ afford good glauber salt glass.
| |
1. |
2. |
| Sand |
100 |
60·3 |
| Calcined sulphate of soda |
50 |
26·8 |
| Lime |
20 |
10·8 |
| Charcoal |
2·65 |
2·1 |
The first mixture has been proved in the looking-glass manufactory of Neuhaus near
Vienna, and the second by the experiments of Kirn. The fusion of the first requires
18, of the second 21 hours. The bluish-green tinge which these otherwise beautiful
and brilliant glasses possess, is not removable by the ordinary means, such as manganese
or arsenic, which decolour alkaline glass. When the sulphate of soda and charcoal are
used in smaller proportions, the glass becomes more colourless. The tinge is no doubt
owing to the sulphur combining with the oxide of sodium, in some such way as in the
pigment ultramarine.
By a proper addition of galena (the native sulphuret of lead), to glauber salt and
quartz sand, without charcoal, it is said a tolerably good crystal glass may be formed.
The sulphuric acid of the salt is probably converted by the reaction of the sulphuret of
lead into sulphurous acid gas, which is disengaged.
One atom of sulphuret of lead = 1495·67, is requisite to decompose 3 atoms of sulphate
of soda = 2676. It is stated, on good authority, that a good colourless glass
may be obtained by using glauber salt without charcoal, as by the following formula.
| Quartz-sand |
100 |
pounds |
| Calcined glauber salt |
24 |
| Lime |
20 |
| Cullet of soda glass |
12 |
The melting heat must be continued for 261⁄2 hours. A small quantity of the sand is
reserved to be thrown in towards the conclusion of the process, in order to facilitate the
expulsion of air bubbles. The above mixture will bear to be blanched by the addition
of manganese and arsenic. The decomposition of the salt is in this case effected by the
lime, with which the sulphuric acid first combines, is then converted into sulphurous
acid, and dissipated. Glass made in this way was found by analysis to consist of 79
parts of silica, 12 lime, and 9·6 soda, without any trace of gypsum or sulphuric acid.
Glauber salt is partially volatilized by the heat of the furnace, and acts upon the arch
of the oven and the tops of the pots. This is best prevented by introducing at first
into the pots the whole of the salt mixed with the charcoal, the lime, and one fourth
part of the sand; fusing this mixture at a moderate heat, and adding gradually afterwards
the remainder of the sand, increasing the temperature at the same time. If we
put in the whole ingredients together, as is done with potash glass, the sand and lime
soon fall to the bottom, while the salt rises to the surface, and the combination becomes
difficult and unequal.
Sulphate of potash acts in the same way as sulphate of soda.
Muriate of soda also, according to Kirn, may be used as a glass flux with advantage.
The most suitable proportions are 4 parts of potash, 2 of common salt, and 3 of lime,
agreeably to the following compositions:—
| |
1. |
2. |
| Quartz-sand |
60·0 |
75·1 |
| Calcined carbonate of potash |
17·8 |
19·1 |
| Common salt |
8·9 |
9·5 |
| Lime |
13·3 |
14·3 |
[576]
For No. 1., the melting heat must be 10 hours, which turns out a very pure, solid,
good glass; for No. 2., 23 hours of the furnace are required. Instead of the potash,
glauber salt may be substituted; the proportions being then 19·1 glauber salt, 9·5 muriate
of soda, 14·3 lime, 75·1 sand, and 1·3 charcoal.
The oxide of lead is an essential constituent of the denser glasses, and may be regarded
as replacing the lime, so as to form with the quartz-sand a silicate of lead. It
assimilates best with purified pearl ash, on account of the freedom of this alkali from
iron, which is present in most sodas.
Its atomic constitution may be represented as follows:—
| |
|
Computation. |
Analysis. |
| Silicic acid |
5 |
atoms |
= |
2877· |
|
59·19 |
59·20 |
| Oxide of lead |
1 |
= |
1394· |
5 |
28·68 |
28·20 |
| Potash |
1 |
= |
590· |
0 |
12·13 |
9·00 |
| Oxides of iron and manganese |
|
— |
— |
1·40 |
| |
|
4861· |
5 |
100·00 |
100·00 |
The above analysis by Berthier relates to a specimen of the best English crystal
glass, perfectly colourless and free from air-bubbles. This kind of glass may however
take several different proportions of potash and silica to the oxide of lead.
The composition of mirror plate, as made on the Continent, is as follows:—
| White quartz-sand |
300 |
pounds |
| Dry carbonate of soda |
100 |
| Lime slaked in the air |
43 |
| Cullet, or old glass |
300 |
The manganese should not exceed one half per cent. of the weight of the soda.
Optical glass requires to be made with very peculiar care. It is of two different kinds;
namely, crown glass and flint glass. The latter contains a considerable proportion of
lead, in order to give it an increased dispersive power upon the rays of light, in proportion
to its mean refractive power.
Optical crown glass should be perfectly limpid, and have so little colour, that a pretty
thick piece of it may give no appreciable tinge to the rays of light. It should be exempt
from striæ or veins as well as air-bubbles, and have not the slightest degree of milkiness.
It should moreover preserve these qualities when worked in considerable quantities.
Potash is preferable to soda for making optical crown glass, because the latter alkali is
apt to make a glass which devitrifies and becomes opalescent, by long exposure to heat
in the annealing process. A simple potash silicate would be free from this defect, but
it would be too attractive of moisture, and apt to decompose eventually by the humidity
of the atmosphere. It should therefore contain a small quantity of lime, and as little
potash as suffices for making a perfect glass at a pretty high temperature. It is probably
owing to the high heats used in the English crown glass works, and the moderate
quantity of alkali (soda) which is employed, that our crown glass has been found to
answer so well for optical purposes.
Practical details of the Manufacture of Glass.
The Venetians were the first in modern times who attained to any degree of excellence
in the art of working glass, but the French became eventually so zealous of
rivalling them, particularly in the construction of mirrors, that a decree was issued by
the court of France, declaring not only that the manufacture of glass should not
derogate from the dignity of a nobleman, but that nobles alone should be masters of
glass-works. Within the last 30 or 40 years, Great Britain has made rapid advances
in this important art, and at the present day her pre-eminence in every department
hardly admits of dispute.
There are five different species of glass, each requiring a peculiar mode of fabrication,
and peculiar materials: 1. The coarsest and simplest form of this manufacture is
bottle glass. 2. Next to it in cheapness of material maybe ranked broad or spread
window glass. An improved article of this kind is now made near Birmingham, under the
name of British or German plate. 3. Crown glass comes next, or window glass, formed
in large circular plates or discs. This glass is peculiar to Great Britain. 4. Flint glass,
crystal glass, or glass of lead. 5. Plate or fine mirror glass.
The materials of every kind of glass are vitrified in pots made of a pure refractory
clay; the best kind of which is a species of shale or slate clay dug out of the coal-formation
near Stourbridge. It contains hardly any lime or iron, and consists of silica
and alumina in nearly equal proportions. The masses are carefully picked, brushed,
and ground under edge iron wheels of considerable weight, and sifted through sieves[577]
having 20 meshes in the square inch. This powder is moistened with water (best hot),
and kneaded by the feet or a loam-mill into an uniform smooth paste. A large body of
this dough should be made up at a time, and laid by in a damp cellar to ripen. Previously
to working it into shapes, it should be mixed with about a fourth of its weight of
cement of old pots, ground to powder. This mixture is sufficiently plastic, and being
less contractile by heat, forms more solid and durable vessels. Glass-house pots have
the figure of a truncated cone, with the narrow end undermost; those for bottle and
window-glass, being open at top, about 30 inches diameter at bottom, 40 inches at the
mouth, and 40 inches deep; but the flint-glass pots are covered in at top with a
dome-cap, having a mouth at the side, by which the materials are introduced, and the
glass is extracted. Bottle and crown-house pots are from 3 to 4 inches thick; those
for flint-houses are an inch thinner, and of proportionally smaller capacity.
The well-mixed and kneaded dough is first worked upon a board into a cake for
the bottom; over this the sides are raised, by laying on its edges rolls of clay above
each other with much manual labour, and careful condensation. The clay is made
into lumps, is equalized, and slapped much in the same way as for making
Pottery. The pots thus fashioned must be dried very prudently, first in the
atmospheric temperature, and finally in a stove floor, which usually borrows its heat
directly from the glass-house. Before setting the pots in the furnace, they are annealed
during 4 or 5 days, at a red heat in a small reverberatory vault, made on purpose.
When completely annealed, they are transferred with the utmost expedition into their
seat in the fire, by means of powerful tongs supported on the axle of an iron-wheel
carriage frame, and terminating in a long lever for raising them and swinging them
round. The pot-setting is a desperate service, and when unskilfully conducted without
due mechanical aids, is the forlorn hope of the glass-founder.—Quæque ipse miserrima
vidi. The celebrated chemist, Dr. Irvine, caught his last illness by assisting imprudently
at this formidable operation. The working breast of the hot furnace must be laid bare
so as to open a breach for the extraction of the faulty pot, and the insertion of the fresh
one, both in a state of bright incandescence. It is frightful to witness the eyes and
fuming visages of the workmen, with the blackening and smoking of their scorched
woollen clothes, exposed so long to the direct radiations of the flame. A light mask
and sack dress coated with tinfoil, would protect both their faces and persons from any
annoyance, at a very cheap rate.
The glass-houses are usually built in the form of a cone, from 60 to 100 feet high,
and from 50 to 80 feet in diameter at the base. The furnace is constructed in the centre
of the area, above an arched or groined gallery which extends across the whole space, and
terminates without the walls, in large folding doors. This cavern must be sufficiently high
to allow labourers to wheel out the cinders in their barrows. The middle of the vaulted
top is left open in the building, and is covered over with the grate-bars of the furnace.
1. Bottle glass.—The bottle-house and its furnace resemble nearly fig. 505. The
furnace is usually an oblong square chamber, built of large fire-bricks, and arched over
with fire-stone, a siliceous grit of excellent quality extracted from the coal measures of
Newcastle. This furnace stands in the middle of the area; and has its base divided into
three compartments. The central space is occupied by the grate-bars; and on either
side is the platform or fire-brick siege, (seat,) raised about 12 inches above the level of
the ribs upon which the pots rest. Each siege is about 3 feet broad.
In the sides of the furnace, semi-circular holes of about a foot diameter are left
opposite to, and a little above the top of, each pot, called working holes, by which
the workmen shovel in the materials, and take out the plastic glass. At each angle
of the furnace there is likewise a hole of about the same size, which communicates
with the calcining furnace of a cylindrical form, dome-shaped at top. The flame
that escapes from the founding or pot-furnace is thus economically brought to reverberate
on the raw materials of the bottle glass, so as to dissipate their carbonaceous or volatile
impurities, and convert them into a frit. A bottle-house has generally eight other
furnaces or fire-arches; of which six are used for annealing the bottles after they are
blown, and two for annealing the pots, before setting them in the furnace.
The laws of this country till lately prohibited the use for making common bottles of
any fine materials. Nothing but the common river sand, and soap-boilers’ waste, was
allowed. About 3 parts of waste, consisting of the insoluble residuum of kelp, mixed
with lime and a little saline substance, were used for 1 part of sand. This waste was first
of all calcined in two of the fire arches or reverberatories reserved for that purpose, called
the coarse arches, where it was kept at a red heat, with occasional stirring, from 24 to 30
hours, being the period of a journey or journée, in which the materials could be melted
and worked into bottles. The roasted soap-waste was then withdrawn, under the name
of ashes, from its arch, coarsely ground, and mixed with its proper proportion of sand.
This mixture was now put into the fine arch, and calcined during the working journey,
which extended to 10 or 12 hours. Whenever the pots were worked out, that frit[578]
was immediately transferred into them in its ignited state, and the founding process
proceeded with such despatch that this first charge of materials was completely melted
down in 6 hours, so that the pots might admit to be filled up again with the second charge
of frit, which was founded in 4 hours more. The heat was briskly continued, and in the
course of from 12 to 18 hours, according to the size of the pots, the quality of the
fuel, and the draught of the furnace, the vitrification was complete. Before blowing the
bottles, however, the glass must be left to settle, and to cool down to the blowing consistency,
by shutting the cave doors and feeding holes, so as to exclude the air from the
fire-grate and the bottom of the hearth. The glass or metal becomes more dense, and
by its subsidence throws up the foreign lighter earthy and saline matters in the form of
a scum on the surface, which is removed with skimming irons. The furnace is now
charged with coal, to enable it to afford a working heat for 4 or 5 hours, at the end of
which time more fuel is cautiously added, to preserve adequate heat for finishing the
journey.
It is hardly possible to convey in words alone a correct idea of the manipulations
necessary to the formation of a wine bottle; but as the manufacturers make no mystery
of this matter, any person may have an opportunity of inspecting the operation. Six
people are employed at this task; one, called a gatherer, dips the end of an iron tube,
about five feet long, previously made red-hot, into the pot of melted metal, turns the rod
round so as to surround it with glass, lifts it out to cool a little, and then dips and
turns it round again; and so in succession till a ball is formed on its end sufficient to
make the required bottle. He then hands it to the blower, who rolls the plastic lump
of glass on a smooth stone or cast-iron plate, till he brings it to the very end of the
tube; he next introduces the pear-shaped ball into an open brass or cast-iron mould,
shuts this together by pressing a pedal with his foot, and holding his tube vertically,
blows through it, so as to expand the cooling glass into the form of the mould. Whenever
he takes his foot from the pedal-lever, the mould spontaneously opens out into two
halves, and falls asunder by its bottom hinge. He then lifts the bottle up at the end
of the rod, and transfers it to the finisher, who, touching the glass-tube at the end of the
pipe with a cold iron, cracks off the bottle smoothly at its mouth-ring. The finished
bottles are immediately piled up in the hot annealing arch, where they are afterwards
allowed to cool slowly for 24 hours at least. See Bottle Mould.
2. Broad or spread window glass.—This kind of glass is called inferior window glass,
in this country, because coarse in texture, of a wavy wrinkled surface, and very cheap,
but on the Continent spread window glass, being made with more care, is much
better than ours, though still far inferior in transparency and polish to crown glass,
which has, therefore, nearly superseded its use among us. But Messrs. Chance and
Hartley, of West Bromwich near Birmingham, have of late years mounted a spread-glass
work, where they make British sheet glass, upon the best principles, and turn out an article
quite equal, if not superior to any thing of the kind made either in France or Belgium.
Their materials are those used in the crown-glass manufacture. The vitrifying
mixture is fritted for 20 or 30 hours in a reverberatory arch, with considerable stirring
and puddling with long-handled shovels and rakes; and the frit is then transferred by
shovels while red hot, to the melting pots to be founded. When the glass is rightly
vitrified, settled, and brought to a working heat, it is lifted out by iron tubes, as will be
described under the article Crown Glass, blown into pears, which being elongated
into cylinders, are cracked up along one side, parallel to the axis, by touching them with
a cold iron dipped in water, and are then opened out into sheets. Glass cylinders
are spread in France, and at West Bromwich, on a bed of smooth stone Paris-plaster,
or laid on the bottom of a reverberatory arch; the cylinder being placed on its side
horizontally, with the cracked line uppermost, gradually opens out, and flattens on the
hearth. At one time, thick plates were thus prepared for subsequent polishing into
mirrors; but the glass was never of very good quality; and this mode of making
mirror-plate has accordingly been generally abandoned.
The spreading furnace or oven is that in which cylinders are expanded into tables or
plates. It ought to be maintained at a brisk red heat, to facilitate the softening of the
glass. The oven is placed in immediate connection with the annealing arch, so that the
tables may be readily and safely transferred from the former to the latter. Sometimes
the cylinders are spread in a large muffle furnace, in order to protect them from being
tarnished by sulphureous and carbonaceous fumes.
Fig. 500. represents a ground plan of both the spreading and annealing furnace;
fig. 501. is an oblong profile in the direction of the dotted line X X, fig. 500.
a is the fire-place; b b the canals or flues through which the flame rises into both
furnaces; c the spreading furnace, upon whose sole is the spreading slab. d is the cooling
and annealing oven; e e iron bars which extend obliquely across the annealing arch,
and serve for resting the glass tables against, during the cooling. f f the channel
along which the previously cracked cylinders are slid, so as to be gradually warmed;[579]
g the opening in the spreading furnace, for enabling the workmen to regulate the process;
h a door in the annealing arch, for introducing the tools requisite for raising up
and removing the tables.
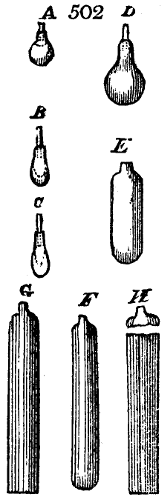
In forming glass-plates by the extension of a cylinder into a plane, the workman first
blows the lump of glass into the shape of an oblong pear, the length of which must be
nearly equal to the length of the intended plate, and its diameter such, that the circumference
when developed, will be equal to the breadth of the plate. He now rests the
blowing-iron on a stool or iron bar, while an assistant with a pointed iron, pierces a hole
into the extreme end of the pear, in the line of the blowing-pipe. This opening is then
enlarged, by introducing the blade of a pair of spring-tongs, while the glass is turned
round; and by skilful management, the end of the pear is eventually opened out into a
cylindrical mouth. The workman next mounts upon a stool, and holds the blowing-iron
perpendicularly. The blown cylinder is now cracked off, a punto rod of iron having been
previously stuck to its one end, to form a spindle for working the other by. This rod has
a flat disc on its end, or three prongs, which being dipped in melted glass, are applied to the
mouth of the cylinder. By this as a handle, the glass cone is carried to the fire, and the
narrow end being heated, is next opened by spring tongs, and formed into a cylinder of the
same size as the other end. The cylinder thus equalized, is next cracked or slit down in its
side with a pair of shears, laid on a smooth copper plate, detached from the iron rod, spread
out by heat into a plane surface, and finally annealed. This series of transformations, is
represented in fig. 502., at A, B, C, D, E, F, G, H.
Fig. 503. and 504. represent a Bohemian furnace in which excellent white window
glass is founded. Fig. 503. is a longitudinal section of the glass and annealing furnace.
Fig. 504. is the ground plan. a is the ash pit vaulted under the sole of the furnace; the fireplace
itself is divided into three compartments; with a middle slab at d, which is hollowed
in the centre, for collecting any spilt glass, and two hearth tiles or slabs b b. c c are the
draught or air holes; e e are
arches upon which the bearing
slabs f f partly rest. In the
middle between these arches,
the flame strikes upwards upon
the pots g g, placed as closely
together as possible, for economy
of room. h is the breast
wall of the furnace; i, fig.
504., the opening through which
the pots are introduced; it is
bricked up as soon as they are
set. k k, is the base of the
cone or dome of the furnace;
l l l, the working orifices, which
are made larger or smaller according
to the size of the glass
articles to be made. m is the
flue which leads to the annealing
stove n, with an arched door.
Exterior to this, there is usually
a drying kiln not shown in the figure; and there are adjoining stoves called arches,
for drying and annealing the new pots before they are set.
The cooling or annealing arch, or leer, is often built independent of the glass-house
furnace, is then heated by a separate fire-place, and constructed like a very long reverberatory
furnace. See Copper.
The leer pans or trays of sheet iron, are laid upon its bottom in an oblong series, and
hooked to each other.
3. Crown-glass.—The crown-glass house with its furnace is represented in fig. 505.,
where the blowing operation is shewn on the one side of the figure, and the flashing on
the other. The furnace is usually constructed to receive 4 or 6 pots, of such dimensions
as to make about a ton of glass each at a time. There are, however, several subsidiary[580]
furnaces to a crown-house. 1. A reverberatory furnace or calcar, for calcining or
fritting the materials; 2. a blowing
furnace, for blowing the pear-shaped
balls made at the pot-holes,
into large globes; 3. a
flashing furnace, and bottoming
hole for communicating a softening
heat, in expanding the globe
into a circular plate; 4. the
annealing arch for the finished
tables; 5. the reverberatory oven
for annealing the pots prior to
their being set upon the founding
siege.
The materials of crown glass
used to be, fine sand, by measure
5 parts, or by weight 10; ground
kelp by measure 11 parts, or by
weight 161⁄2; but instead of kelp,
soda ash is now generally employed.
From 6 to 8 cwt. of sand, lime,
and soda-ash, mixed together in
wooden boxes with a shovel, are thrown on the sole of a large reverberatory,
such as is represented in the article Copper. Here the mixture is well worked
together, with iron paddles, flat shovels, and rakes with long handles; the area of this
furnace being about 6 feet square, and the height 2 feet. The heat soon brings the
materials to a pasty consistence, when they must be diligently turned over, to favour the
dissipation of the carbon, sulphur, and other volatile matters of the kelp or soda ash, and
to incorporate the fixed ingredients uniformly with the sand. Towards the end of 3 hours,
the fire is considerably raised, and when the fourth hour has expired, the fritting operation
is finished. The mass is now shovelled or raked out into shallow cast-iron square cases,
smoothed down, and divided before it hardens by cooling, into square lumps, by cross
sections with the spade. These frit-bricks are afterwards piled up in a large apartment
for use; and have been supposed to improve with age, by the efflorescence of their
saline constituents into carbonate of soda on their surface.
The founding-pots are filled up with these blocks of frit, and the furnace is powerfully
urged by opening all the subterranean passages to its grate, and closing all the doors
and windows of the glass-house itself. After 8 or 10 hours the vitrification has made
such progress, and the blocks first introduced are so far melted down, that another
charge of frit can be thrown in, and thus the pot is fed with frit till the proper quantity
is used. In about 16 hours the vitrification of the frit has taken place, and a considerable
quantity, amounting often to the cwt. of liquid saline matter floats over the glass.
This salt is carefully skimmed off into iron pots with long ladles. It is called Sandiver
or Glass-gall, and consists usually of muriate of soda, with a little sulphate. The pot
is now ready for receiving the topping of cullet, which is broken pieces of window glass,
to the amount of 3 or 4 cwt. This is shovelled in at short intervals; and as its pressure
forces up the residuary saline matter, this is removed; for were it allowed to remain,
the body of the glass would be materially deteriorated.
The heat is still continued for several hours till the glass is perfect, and the extrication
of gas called the boil, which accompanies the fusion of crown glass, has nearly
terminated, when the fire is abated, by shutting up the lower vault doors and every
avenue to the grate, in order that the glass may settle fine. At the end of about 40
hours altogether, the fire being slightly raised by adding some coals, and opening the
doors, the glass is carefully skimmed, and the working of the pots commences.
Before describing it, however, we may state that the marginal figure 506. shews the
base of the crown-house cone, with the
four open pots in two ranges on opposite
sides of the furnace, sitting on their raised
sieges, at each side of the grate. At one
side of the base the door of the vault is
shewn, and its course is marked by the
dotted lines.
Detailed description of the crown-glass furnace, figs. 507. 508.—It is an oblong square,
built in the centre of a brick cone, large enough to contain within it, two or three pots
at each side of the grate room, which is either divided as shown in the plan, or runs the
whole length of the furnace, as the manufacturer chooses. Fig. 507. is a ground plan, and
fig. 508. a front elevation, of a six-pot furnace. 1, 2, 3, fig. 507., are the working holes[581]
for the purposes of ventilation, of putting in the materials, and of taking out the metal
to be wrought. 4, 5, 6, 7, are pipe holes for warming the pipes before beginning to
work with them. 8, 9, 10, are foot holes for mending the pots and sieges. 11 is a bar
of iron for binding the furnace, and keeping it from swelling.
The arch is of an elliptic form; though a barrel arch, that is, an arch shaped like the
half of a barrel cut longwise through the centre, is sometimes used. But this soon gives
way when used in the manufacture of crown glass, although it does very well in the
clay-furnace used for bottle houses.
The best stone for building furnaces is fire-stone, from Coxgreen in the neighbourhood
of Newcastle. Its quality is a close grit, and it contains a greater quantity of
talc than the common fire-stone, which seems to be the chief reason of its resisting the
fire better. The great danger in building furnaces is, lest the cement at the top should
give way with the excessive heat, and by dropping into the pots, spoil the metal. The top
should therefore be built with stones only, as loose as they can hold together after the centres
are removed, and without any cement whatever. The stones expand and come quite
close together when annealing; an operation which takes from eight to fourteen days
at most. There is thus less risk of any thing dropping from the roof of the furnace.
The inside of the square of the furnace is built either of Stourbridge fire-clay annealed,
or the Newcastle fire-stone, to the thickness of sixteen inches. The outside is built of
common brick about nine inches in thickness.
The furnace is thrown over an ash-pit, or cave as it is called, which admits the atmospheric
air, and promotes the combustion of the furnace. This cave is built of stone
until it comes beneath the grate room, when it is formed of fire-brick. The abutments
are useful for binding and keeping the furnace together, and are built of masonry. The
furnaces are stoutly clasped with iron all round, to keep them tight. In four-pot furnaces
this is unnecessary, provided there be four good abutments.
Fig. 509. is an elevation of the flashing furnace. The outside is built of common
brick, the inside of fire-brick, and the mouth or nose of Stourbridge fire-clay.
Fig. 510. is the annealing kiln. It is built of common brick, except round the grate
room, where fire-brick is used.
Few tools are needed for blowing and flashing crown-glass. The requisite ball of
plastic glass is gathered, in successive layers as for bottles, on the end of an iron tube, and
rolled into a pear-shape, on a cast-iron plate; the workman taking care that the air
blown into its cavity is surrounded with an equal body of glass, and if he perceives any
side to be thicker than another, he corrects the inequality by rolling it on the sloping
iron table called marver, (marbre). He now heats the bulb in the fire, and rolls it so as[582]
to form the glass upon the end of the tube, and by a dexterous swing or two he
lengthens it, as shewn in I, fig. 511. To extend the neck of that pear, he next rolls it
over a smooth iron rod, turned round in a horizontal direction, into the shape K, fig. 511.
By further expansion at the blowing-furnace, he now brings it to the shape L, represented
in fig. 511.
This spheroid having become cool and somewhat stiff, is next carried to the bottoming
hole (like fig. 509.), to be exposed to the action of flame. A slight wall erected before one
half of this hole, screens the workman from the heat, but leaves room for the globe to pass
between it and the posterior wall. The blowing-pipe is made to rest a little way from the
neck of the globe, on a hook fixed in the front wall; and thus may be made easily to revolve
on its axis, and by giving centrifugal force to the globe, while the bottom of it, or
part opposite to the pipe, is softened by the heat, it soon assumes the form exhibited in
M, fig. 511.
In this state the flattened globe is removed from the fire, and its rod being rested on
the casher box covered with coal cinders, another workman now applies the end of a solid
iron rod tipped with melted glass, called a punto, to the nipple or prominence in the
middle; and thus attaches it to the centre of the globe, while the first workman cracks
off the globe by touching its tubular neck with an iron chisel dipped in cold water. The
workman having thereby taken possession of the globe by its bottom or knobbed pole
attached to his punty rod, he now carries it to another circular opening, where he
exposes it to the action of moderate flame with regular rotation, and thus slowly
heats the thick projecting remains of the former neck, and opens it slightly out, as
shewn at N, in fig. 511. He next hands it to the flasher, who resting the iron rod in a
hook placed near the side of the orifice A, fig. 509., wheels it rapidly round opposite to
a powerful flame, till it assumes first the figure O, and finally that of a flat circular table.
The flasher then walks off with the table, keeping up a slight rotation as he moves
along, and when it is sufficiently cool, he turns down his rod into a vertical position,
and lays the table flat on a dry block of fire-clay, or bed of sand, when an assistant
nips it off from the punto with a pair of long iron shears, or cracks it off with a touch
of cold iron. The loose table or plate is lastly lifted up horizontally on a double pronged
iron fork, introduced into the annealing arch fig. 510. and raised on edge; an assistant with
a long-kneed fork preventing it from falling too rapidly backwards. In this arch a great
many tables of glass are piled up in iron frames, and slowly cooled from a heat of about
600° to 100° F., which takes about 24 hours; when they are removed. A circular
plate or table of about 5 feet diameter weighs on an average 9 pounds.
4. Flint glass.—This kind of glass is so called because originally made with calcined
flints, as the siliceous ingredient. The materials at present employed in this country
for the finest flint glass or crystal, are first, Lynn sand, calcined, sifted, and washed;
second, an oxide of lead, either red lead or litharge; and third, pearl ash. The pearl
ash of commerce must however be purified by digesting it in a very little hot water,
which dissolves the carbonate of potash, and leaves the foreign salts, chiefly sulphate of
potash, muriate of potash, and muriate of soda. The solution of the carbonate being
allowed to cool and become clear in lead pans, is then run off into a shallow iron boiler,
and evaporated to dryness. Nitre is generally added as a fourth ingredient of the body
of the glass; and it serves to correct any imperfections which might arise from
accidental combustible particles, or from the lead being not duly oxidized. The above
four substances constitute the main articles; to which we may add arsenic and manganese,
introduced in very small quantities, to purify the colour and clear up the
transparency of the glass. The black oxide of manganese, when used in such quantity
only as to peroxidize the iron of the sand, simply removes the green tinge caused by the
iron; but if more manganese be added than accomplishes that purpose, it will give a
purple tinge to the glass; and in fact, most manufacturers prefer to have an excess
rather than a defect of manganese, since cut glass has its brilliancy increased by a faint
lilac hue. The arsenic is supposed to counteract the injury arising from excess of manganese,
but is itself very apt on the other hand to communicate some degree of opalescence,[583]
or at least, to impair the lustre of the glass. When too much manganese has been
added, the purple tinge may indeed be removed by any carbonaceous matter, as by
thrusting a wooden rod down into the liquid glass; but this cannot be done with good
effect in practice, since the final purple tinge is not decided till the glass is perfectly
formed, and then the introduction of charcoal would destroy the uniformity of the whole
contents of the pot.
The raw materials of flint glass, are always mixed with about a third or a fourth of
their weight of broken crystal of like quality; this mixture is thrown into the pot
with a shovel; and more is added whenever the preceding portions by melting subside; the
object being to obtain a pot full of glass, to facilitate the skimming off the impurities, and
sandiver. The mouth of the pot is now
shut, by applying clay-lute round the
stopper, with the exception of a small
orifice below, for the escape of the liquid
saline matter. Flint glass requires
about 48 hours for its complete vitrification,
though the materials be more
fusible than those of crown glass; in
consequence of the contents of the pot
being partially screened by its cover
from the action of the fire, as also from
the lower intensity of the heat.
Fig. 512. represents a flint glass
house for 6 pots, with the arch or leer
on one side for annealing the crystal
ware. In fig. 513., the base of the
cone is seen, and the glass pots in situ
on their platform ranged round the central
fire grate. The dotted line denotes
the contour of the furnace, fig. 512.
Whenever the glass appears fine, and
is freed from its air bubbles, which it
usually is in about 36 hours, the heat is
suffered to fall a little by closing the
bottom valves, &c., that the pot may
settle; but prior to working the metal,
the heat is somewhat raised again.
It would be useless to describe the
manual operations of fashioning the
various articles of the flint-glass manufacture, because they are indefinitely varied to suit
the conveniences and caprices of human society.
Every different flint-house has a peculiar proportion of glass materials. The following
have been offered as good practical mixtures.
| 1. |
Fine white sand |
300 |
parts. |
| Red lead or litharge |
200 |
| Refined pearl ashes |
80 |
| Nitre |
20 |
| Arsenic and manganese, a minute quantity. |
In my opinion, the proportion of lead is too great in the above recipe, which is given
on the authority of Mr. James Geddes, of Leith. The glass made with it would be
probably yellowish, and dull.
| 2. |
Fine sand |
50·5 |
| Litharge |
27·2 |
| Refined pearl ashes (carbonate of potash, with 5 per cent. of water) |
17·5 |
| Nitre |
4·8 |
| 100·0 |
To these quantities from 30 to 50 parts of broken glass or cullet are added; with
about a two-thousandth part of manganese, and a three-thousandth part of arsenic. But
manganese varies so extremely in its purity, and contains often so much oxide of iron,
that nothing can be predicated as to its quantity previously to trial.
M. Payen, an eminent manufacturing chemist in France, says that the composition
of crystal does not deviate much from the following proportions:—
| |
Wood
fire. |
Coal
fire. |
| Siliceous sand |
3 |
|
3 |
|
| Minium |
2 |
|
2 |
1⁄4 |
| Carbonate of potash |
1 |
1⁄2 |
1 |
2⁄3 |
[584]
I conceive that this glass contains too much lead and potash. Such a mixture will
produce a dull metal, very attractive of moisture: defects to which the French crown-glass
also is subject.
The flint-glass leer for annealing glass, is an arched gallery or large flue, about 36
feet long, 3 feet high, 4 wide; having its floor raised above 2 feet above the ground of
the glass-house. The hot air and smoke of a fire-place at one end pass along this gallery,
and are discharged by a chimney 8 or 10 feet short of the other end. On the floor
of the vault, large iron trays are laid and hooked to each other in a series, which are drawn
from the fire end towards the other by a chain, wound about a cylinder by a winch-handle
projecting through the side. The flint-glass articles are placed in their hot state into the
tray next the fire, which is moved onwards to a cooler station whenever it is filled, and
an empty tray is set in its place. Thus, in the course of about 20 hours, the glass
advances to the cool end thoroughly annealed.
Besides colourless transparent glass, which forms the most important part of this
manufacture, various coloured glasses are made to suit the taste of the public. The
taste at Paris was lately for opaline crystal; which may be prepared by adding to the
above composition (No. 2.) phosphate of lime, or well burnt bone-ash in fine powder,
washed, and dried. The article must be as uniform in thickness as possible, and
speedily worked into shape, with a moderate heat. Oxide of tin, putty, was formerly
used for making opalescent glass, but the lustre of the body was always impaired by its
means.
Crystal vessels have been made recently of which the inner surface is colourless, and all
the external facets coloured. Such works are easily executed. The end of the blowing-rod
must be dipped first in the pot containing colourless glass, to form a bulb of a
certain size, which being cooled a little is then dipped for an instant into the pot of
coloured glass. The two layers are associated without intermixture; and when the
article is finished in its form, it is white within and coloured without. Fluted lines
somewhat deeply cut, pass through the coloured coat, and enter the colourless one; so
that when they cross, their ends alone are coloured.
For some time past, likewise, various crystal articles have been exhibited in the
market with coloured enamel-figures on their surface, or with white incrustations of a
silvery lustre in their interior. The former are prepared by placing the enamel object
in the brass mould, at the place where it is sought to be attached. The bulb of glass
being put into the mould, and blown while very hot, the small plate of enamel gets
cemented to the surface. For making the white argentine incrustations, small figures
are prepared with an impalpable powder of dry porcelain paste, cemented into a solid
by means of a little gypsum plaster. When these pieces are thoroughly dried, they are
laid on the glass while it is red hot, and a large patch of very liquid glass is placed above
it, so as to encase it and form one body with the whole. In this way the incrustation
is completely enclosed; and the polished surface of the crystal which scarcely touches
it, gives a brilliant aspect, pleasing to the eye.
An uniform flint-glass, free from striæ, or wreath, is much in demand for the optician.
It would appear that such an article was much more commonly made by the English
manufacturers many years ago, than at present; and that in improving the brilliancy of
crystal-glass they have injured its fitness for constructing optical lenses, which depends
not so much on its whiteness and lustre as on the layers of different densities being
parallel to each other. The oxide of lead existing in certain parts of a potful of glass
in greater proportion than in other parts, increases the density unequally in the same
mass, so that the adjoining strata are often very different in this respect. Even a potful
of pretty uniform glass, when it stands some time liquid, becomes eventually unequable
by the subsidence of the denser portions; so that striæ and gelatinous appearances
begin to manifest themselves, and the glass becomes of little value. Glass allowed
to cool slowly in mass in the pot is particularly full of wreath; and if quickly refrigerated,
that is in two or three hours, it is apt to split into a multitude of minute splinters,
of which no use can be made. For optical purposes, the glass must be taken out in its
liquid state, being gathered on the end of the iron rod from the central portion of a
recently skimmed pot, after the upper layers have been worked off in general articles.
M. Guinand, of Brennets near Geneva, appears to have hit upon processes that furnished
almost certainly pieces of flint-glass capable of forming good lenses of remarkable
dimensions, even of 11 inches diameter; of adequate density and transparency, and
nearly free from striæ. M. Cauchoix, the eminent French optician, says, that out of
ten object glasses, 4 inches in diameter, made with M. Guinand’s flint-glass, eight or
nine turned out very good, while out of an equal number of object glasses made of the
flint-glass of the English and French manufactories, only one, or two at most, were found
serviceable. The means by which M. Guinand arrived at these results have not been
published. He has lately died, and it is not known whether his son be in possession of
his secret.
[585]
An achromatic object glass for telescopes and microscopes consists of at least two
lenses; the one made with glass of lead, or flint glass, and the other with crown glass;
the former possessing a power of dispersing the coloured rays relatively to its mean
refractive power, much greater than the latter; upon which principle, the achromatism
of the image is produced, by re-uniting the different coloured rays into one focus. Flint
glass to be fit for this delicate purpose must be perfectly homogeneous, or of uniform
density throughout its substance, and free from wavy veins or wreathes; for every such
inequality would occasion a corresponding inequality in the refraction and dispersion of
the light; like what is perceived in looking through a thick and thin solution of gum
Arabic imperfectly mixed. Three plans have been prescribed for obtaining homogeneous
pieces of optical glass: 1. to lift a mass of it in large ladles, and let it cool in
them; 2. to pour it out from the pots into moulds; 3. to allow it to cool in the
pots, and afterwards to cut it off in horizontal strata. The last method, which is the
most plausible, seldom affords pieces of uniform density, unless peculiar precautions have
been adopted to settle the flint glass in uniform strata; because its materials are of such
unequal density, the oxide of lead having a specific gravity of 8, and silica of 2·7, that
they are apt to stand at irregular heights in the pots.
One main cause of these inequalities lies in the construction of the furnace, whereby
the bottom of the pot is usually much less heated than the upper part. In a plate glass
furnace the temperature of the top of the pot has been found to be 130° Wedgew., while
that of the bottom was only 110°, constituting a difference of no less than 2610° F.
The necessary consequence is that the denser particles which subside to the bottom,
during the fusion of the materials, and after the first extrication of the gases, must
remain there, not being duly agitated by the expansive force of caloric, acting from below
upwards.
The preparation of the best optical glass is now made a great mystery by one or two
proficients. The following suggestions, deduced from a consideration of principles, may
probably lead to some improvements, if judiciously applied. The great object is to
counteract the tendency of the glass of lead to distribute itself into strata of different
densities; which may be effected either by mechanical agitation or by applying the
greatest heat to the bottom of the pot. But however homogeneous the glass may be
thereby made, its subsequent separation into strata of different densities must be prevented
by rapid cooling and solidification. As the deeper the pots, the greater is the
chance of unequal specific gravity in their contents, it would be advisable to make them
wider and shallower than those in use for making ordinary glass. The intermixture
may be effected either by lading the glass out of one pot into another in the furnace, and
back again, with copper ladles, or by stirring it up with a rouser, then allowing it to
settle for a short time, till it becomes clear and free from air bubbles. The pot may
now be removed from the furnace, in order to solidify its contents in their homogeneous
state; after which the glass may be broken in pieces, and be perfected by subjecting it
to a second fusion; or what is easier and quicker, we may form suitable discs of glass
without breaking down the potful, by lifting it out in flat copper ladles with iron shanks,
and transferring the lumps after a little while into the annealing leer.
To render a potful of glass homogeneous by agitation, is a
more difficult task, as an iron rod would discolour it, and a
copper rod would be apt to melt. An iron rod sheathed in
laminated platinum would answer well, but for its expense.
A stone-ware tube supported within by a rod of iron, might
also be employed for the purpose in careful hands; the stirring
being repeated several times, till at last the glass is
suffered to stiffen a little by decrease of temperature. It
must be then allowed to settle and cool, after which the pot,
being of small dimensions, may be drawn out of the fire.
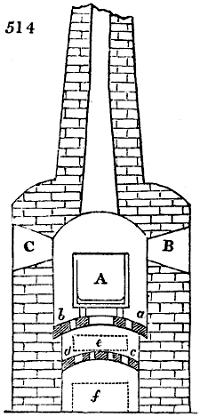
2. The second method of producing the desired uniformity
of mixture, consists in applying a greater heat to the bottom
than to the upper part of the melting pot. Fig. 514. represents
in section a furnace contrived to effect this object. It is
cylindrical, and of a diameter no greater than to allow the
flames to play round the pot, containing from three to four
cwts. of vitreous materials. A is the pot, resting upon the
arched grid b a, built of fire-bricks, whose apertures are wide
enough to let the flames rise freely, and strike the bottom and
sides of the vessel. From 11⁄2 to 2 feet under that arch, the
fuel grate c d is placed. B C are the two working openings
for introducing the materials, and inspecting the progress of
the fusion; they must be closed with fire-tiles and luted with
fire-clay at the beginning of the process. At the back of the furnace, opposite the mouth[586]
of the fire-place, there is a door-way, which is bricked up, except upon occasion of
putting in and taking out the pot. The draught is regulated by means of a slide-plate
upon the mouth of the ash-pit f. The pot being heated to the proper pitch, some
purified pearl ash, mixed with fully twice its weight of colourless quartz sand, is to be
thrown into it, and after the complete fusion of this mixture, the remaining part of the
sand along with the oxide of lead (fine litharge) is to be strown upon the surface. These
siliceous particles in their descent serve to extricate the air from the mass. Whenever
the whole is fused, the heat must be strongly urged to ensure a complete uniformity of
combination by the internal motions of the particles. As soon as the glass has been
found by making test phials to be perfectly fine, the fire must be withdrawn, the two
working holes must be opened, as well as the mouths of the fire-place and ash-pit, to
admit free ingress to cooling currents of air, so as to congeal the liquid mass as quickly
as possible; a condition essential to the uniformity of the glass. It may be worth while
to stir it a little with the pottery rod at the commencement of the cooling process. The
solidified glass may be afterwards detached by a hammer in conchoidal discs, which
after chipping off their edges, are to be placed in proper porcelain or stone-ware dishes,
and exposed to a softening heat, in order to give them a lenticular shape. Great care
must be taken that the heat thus applied by the muffle furnace be very equable, for
otherwise wreathes might be very readily re-produced in the discs. A small oven upon
the plan of a baker’s, is best fitted for this purpose, which being heated to dull redness,
and then extinguished, is ready to soften and afterwards anneal the conchoidal pieces.
Guinand’s dense optical flint glass, of specific gravity 3·616, consists by analysis, of
oxide of lead 43·05; silica 44·3; and potash 11·75; but requires for its formation the
following ingredients: 100 pounds of ground quartz; 100 pounds of fine red lead; 35
pounds of purified potash; and from 2 to 4 pounds of saltpetre. As this species of glass
is injured by an excess of potash, it should be compounded with rather a defect of it,
and melted by a proportionally higher or longer heat. A good optical glass has been
made in Germany with 7 parts of pure red lead, 3 parts of finely ground quartz, and 2
parts of calcined borax.
5. Plate glass.
This, like English crown-glass, has a soda flux, whereas flint-glass requires potash,
and is never of good quality when made with soda. We shall distribute our account
of this manufacture under two heads.
1. The different furnaces and principal machines, without whose knowledge it would
be impossible to understand the several processes of a plate-glass factory.
2. The materials which enter into the composition of this kind of glass, and the
series of operations which they undergo; devoting our chief attention to the changes
and improvements which long experience, enlightened by modern chemistry, has introduced
into the great manufactory of Saint-Gobin in France, under the direction of
M. Tassaert. It may however be remarked that the English plate-glass manufacture
derives peculiar advantages from the excellence of its grinding and polishing machinery.
The clay for making the bricks and pots should be free from lime and iron, and very
refractory. It is mixed with the powder of old pots passed through a silk sieve. If
the clay be very plastic it will bear its own weight of the powder, but if shorter in
quality, it will take only three-fifths. But before mingling it with the cement of old
pots, it must be dried, bruised, then picked, ground, and finally elutriated by agitation
with water, decantation through a hair sieve, and subsidence. The clay fluid after
passing the sieve is called slip (coulis.)
The furnace is built of dry bricks, cemented with slip, and has at each of its four
angles a peculiar annealing arch, which communicates with the furnace interiorly, and
thence derives sufficient heat to effect in part, if not wholly, the annealing of the pots,
which are always deposited there a long time before they are used. Three of these
arches exclusively appropriated to this purpose, are called pot-arches. The fourth is
called the arch of the materials, because it serves for drying them before they are founded.
Each arch has, moreover, a principal opening called the throat, another called bonnard,
by the French workmen, through which fire may be kindled in the arch itself, when it
was thought to be necessary for the annealing of the pots; a practice now abandoned.
The duration of a furnace is commonly a year, or at most 14 months; that of the
arches is 30 years or upwards, as they are not exposed to so strong a heat.
In the manufacture of plate-glass two sorts of crucibles are employed, called the
pots and the basins, (cuvettes). The first serve for containing the materials to be
founded, and for keeping them a long time in the melted state. The cuvettes receive the
melted glass after it is refined, and decant it out on the table to be rolled into a plate.
Three pots hold liquid glass for six small basins, or for three large ones, the latter being
employed for making mirrors of great dimensions, that is, 100 inches long and upwards.[587]
Furnaces have been lately constructed with 6 pots, and 12 cuvettes, 8 of which
are small, and 4 large; and cuvettes of three sizes are made, called small, middling, and
large. The small are perfect cubes, the middling and the large ones are oblong parallelopipeds.
Towards the middle of their height, a notch or groove, two or three inches
broad, and an inch deep, is left, called the girdle of the cuvette, by which part they are
grasped with the tongs, or rather are clamped in the iron frame. This frame goes round
the four sides of the small cuvettes, and may be placed indifferently upon all their
sides; in the other cuvettes, the girdle extends only over the two large sides, because
they cannot be turned up. See m T, fig. 515., p. 590.
The pot is an inverted truncated cone, like a crown glass pot. It is about 30 inches
high, and from 30 to 32 inches wide, including its thickness. There is only a few
inches of difference between the diameter of the top and that of the bottom. The
bottom is 3 inches thick, and the body turns gradually thinner till it is an inch at the
mouth of the pot.
The large building or factory, of which the melting furnace occupies the middle
space, is called the halle in French. At Ravenhead in Lancashire it is called the
foundry, and is of magnificent dimensions, being probably the largest apartment under
one roof in Great Britain, since its length is 339 feet, and its breadth 155. The famous
halle of St. Gobin is 174 feet by 120. Along the two side walls of the halle, which
are solidly constructed of hewn stone, there are openings like those of common ovens.
These ovens, destined for the annealing of the newly cast plates, bear the name of carquaises.
Their soles are raised two feet and a half above the level of the ground, in order to
bring them into the same horizontal plane with the casting tables. Their length, amounting
sometimes to 30 feet, and their breadth to 20, are required in order to accommodate
6, 8, or even 10 plates of glass, alongside of each other. The front aperture is called
the throat, and the back door the little throat (gueulette). The carquaise is heated by
means of a fire-place of a square form called a tisar, which extends along its side.
The founding or melting furnace is a square brick building laid on solid foundations,
being from 8 to 10 feet in each of its fronts, and rising inside into a vault or crown about
10 feet high. At each angle of this square, a small oven or arch is constructed, likewise
vaulted within, and communicating with the melting furnace by square flues, called
lunettes, through which it receives a powerful heat, though much inferior to that round
the pots. The arches are so distributed as that two of the exterior sides of the furnace
stand wholly free, while the two other sides, on which the arches encroach, offer a free
space of only 3 feet. In this interjacent space, two principal openings of the furnace, of
equal size in each side, are left in the building. These are called tunnels. They are
destined for the introduction of the pots and the fuel.
On looking through the tunnels into the inside of the furnace, we perceive to the
right hand and the left, along the two free sides, two low platforms or sieges, at least 30
inches in height and breadth. See figs. 506. 508.
These sieges (seats) being intended to support the pots and the cuvettes filled with
heavy materials, are terminated by a slope, which ensures the solidity of the fire-clay
mound. The slopes of the two sieges extend towards the middle of the furnace so
near as to leave a space of only from 6 to 10 inches between them for the hearth. The
end of this is perforated with a hole sufficiently large to give passage to the liquid glass
of a broken pot, while the rest is preserved by lading it from the mouth into the adjoining
cuvette.
In the two large parallel sides of the furnace, other apertures are left much smaller
than the tunnels, which are called ouvreaux (peep holes). The lower ones, or the ouvreaux
en bas, called cuvette openings, because being allotted to the admission of these vessels,
they are exactly on a level with the surface of the sieges, and with the floor of the halle.
Plates of cast iron form the thresholds of these openings, and facilitate the ingress and
egress of the cuvettes. The apertures are arched at top, with hewn stone like the tunnels,
and are 18 inches wide when the cuvettes are 16 inches broad.
The upper and smaller apertures, or the higher ouvreaux called the lading holes, because
they serve for transvasing the liquid glass, are three in number, and are placed 31
or 32 inches above the surface of the sieges. As the pots are only 30 inches high, it
becomes easy to work through these openings either in the pots or the cuvettes. The
pots stand opposite to the two pillars which separate the openings, so that a space is left
between them for one or more cuvettes according to the size of the latter. It is obvious
that if the tunnels and ouvreaux were left open, the furnace would not draw or take the
requisite founding heat. Hence the openings are shut by means of fire-tiles. These are
put in their places, and removed by means of two holes left in them, in correspondence
with the two prongs of a large iron fork supported by an axle and two iron wheels, and
terminated by two handles which the workmen lay hold of when they wish to move
the tile.
The closing of the tunnel is more complex. When it is shut or ready for the firing,[588]
the aperture appears built up with bricks and mortar from the top of the arch to the
middle of the tunnel. The remainder of the door-way is closed; 1. on the two sides
down to the bottom, by a small upright wall, likewise of bricks, and 8 inches broad,
called walls of the glaye; 2. by an assemblage of pieces called pieces of the glaye, because
the whole of the closure of the tunnel bears the name of glaye. The upper hole,
4 inches square, is called the tisar, through which billets of wood are tossed into the
fire. Fuel is also introduced into the posterior openings. The fire is always kept up on
the hearth of the tunnel, which is, on this account, 4 inches higher than the furnace-hearth,
in order that the glass which may accidentally fall down on it, and which does
not flow off by the bottom hole, may not impede the combustion. Should a body of
glass, however, at any time obstruct the grate, it must be removed with rakes, by opening
the tunnel and dismounting the fire-tile stoppers of the glaye.
Formerly wood fuel alone was employed for heating the melting-furnaces of the
mirror-plate manufactory of Saint-Gobin; but within these few years, the Director of
the works makes use with nearly equal advantage of pit-coal. In the same establishment,
two melting furnaces may be seen, one of which is fixed with wood, and the other with
coals, without any difference being perceptible in the quality of the glass furnished by
either. It is not true, as has been stated, that the introduction of pit-coal has made it
necessary to work with covered pots in order to avoid the discoloration of the materials,
or that more alkali was required to compensate for the diminished heat in the covered
pots. They are not now covered when pit-coal is used, and the same success is obtained
as heretofore by leaving the materials two or three hours longer in the pots and the cuvettes.
The construction of the furnaces in which coal is burned, is the same as that
with wood, with slight modifications. Instead of the close bottomed hearth of the wood
furnace, there is an iron grate in the coal-hearth through which the air enters, and the
waste ashes descend.
When billets of wood were used as fuel, they were well dried beforehand, by being
placed a few days on a frame work of wood called the wheel, placed two feet above the
furnace and its arches, and supported on four pillars at some distance from the angles of
the building.
Composition of plate-glass.—This is not made now, as formerly, by random trials.
The progress of chemistry, the discovery of a good process for the manufacture of soda
from sea salt, which furnishes a pure alkali of uniform power, and the certain methods
of ascertaining its purity, have rendered this department of glass-making almost entirely
new, in France. At Saint-Gobin no alkali is employed at present except artificial
crystals of soda, prepared at the manufactory of Chauny, subsidiary to that establishment.
Leaden chambers are also erected there for the production of sulphuric acid
from sulphur. The first crop of soda crystals is reserved for the plate-glass manufacture,
the other crystals and the mother-water salts are sold to the makers of inferior
glass.
At the mirror-plate works of Ravenhead, near St. Helen’s in Lancashire, soda crystals,
from the decomposition of the sulphate of soda by chalk and coal, have been also
tried, but without equal success as at Saint-Gobin; the failure being unquestionably
due to the impurity of the alkali. Hence, in the English establishment the soda is obtained
by treating sea-salt with pearl-ash, whence carbonate of soda and muriate of
potash result. The latter salt is crystallized out of the mingled solution, by evaporation
at a moderate heat, for the carbonate of soda does not readily crystallize till the temperature
of the solution fall below 60° Fahr. When the muriate of potash is thus removed,
the alkaline carbonate is evaporated to dryness.
Long experience at Saint-Gobin has proved that one part of dry carbonate of soda is
adequate to vitrify perfectly three parts of fine siliceous sand, as that of the mound of
Aumont near Senlis, of Alum Bay in the Isle of Wight, or of Lynn in Norfolk. It
is also known that the degree of heat has a great influence upon the vitrification, and
that increase of temperature will compensate for a certain deficiency of alkali; for it is
certain that a very strong fire always dissipates a good deal of the soda, and yet the glass
is not less beautiful. The most perfect mirror-plate has constantly afforded to M. Vauquelin
in analysis, a portion of soda inferior to what had been employed in its formation.
Hence, it has become the practice to add for every 100 parts of cullet or broken plate
that is mixed with the glass composition, one part of alkali, to make up for the loss that
the old glass must have experienced.
To the above mentioned proportions of sand and alkali independently of the cullet
which may be used, dry slaked lime carefully sifted is to be added to the amount of one
seventh of the sand; or the proportion will be, sand 7 cwt.; quicklime 1 cwt.; dry
carbonate of soda 2 cwt. and 37 lbs.; besides cullet. The lime improves the quality of
the glass, rendering it less brittle and less liable to change. The preceding quantities
of materials suitably blended, have been uniformly found to afford most advantageous
results. The practice formerly was to dry that mixture, as soon as it was made, in the[589]
arch for the materials, but it has been ascertained that this step may be dispensed with,
and the small portion of humidity present is dissipated almost instantly after they are
thrown into the furnace. The coat of glaze previously applied to the inside of the pot,
prevents the moisture from doing them any harm. For this reason, when the demand
for glass at Saint-Gobin is very great, the materials are neither fritted nor even dried,
but shovelled directly into the pot; this is called founding raw. Six workmen are
employed in shovelling-in the materials either fritted or otherwise, for the sake of
expedition, and to prevent the furnace getting cooled. One-third of the mixture is
introduced at first; whenever this is melted, the second third is thrown in, and then the
last. These three stages are called the first, second, and third fusion or founding.
According to the ancient practice, the founding and refining were both executed in
the pots, and it was not till the glass was refined, that it was laded into the cuvettes,
where it remained only 3 hours, the time necessary for the disengagement of the air
bubbles introduced by the transvasion, and for giving the metal the proper consistence
for casting. At present, the period requisite for founding and refining, is equally
divided between the pots and the cuvettes. The materials are left 16 hours in the pots,
and as many in the cuvettes; so that in 32 hours, the glass is ready to be cast. During the
last two or three hours, the fireman or tiseur ceases to add fuel; all the openings are
shut, and the glass is allowed to assume the requisite fluidity; an operation called
stopping the glass, or performing the ceremony.
The transfer of the glass into the cuvettes, is called lading, (tréjetage). Before this is
done, the cuvettes are cleared out, that is, the glass remaining on their bottom, is removed,
and the ashes of the firing. They are lifted red hot out of the furnace by the
method presently to be described, and placed on an iron plate, near a tub filled
with water. The workmen, by means of iron paddles 6 feet long, flattened at one end
and hammered to an edge, scoop out the fluid glass expeditiously, and throw it into
water; the cuvettes are now returned to the furnace, and a few minutes afterwards the
lading begins.
In this operation, ladles of wrought iron are employed, furnished with long handles,
which are plunged into the pots through the upper openings or lading holes, and
immediately transfer their charge of glass into the buckets. Each workman dips his
ladle only three times, and empties its contents into the cuvette. By these three
immersions (whence the term tréjeter is derived), the large iron spoon is heated so much
that when plunged into a tub full of water, it makes a noise like the roaring of a lion,
which may be heard to a very great distance.
The founding, refining, and ceremony, being finished, they next try whether the glass
be ready for casting. With this view, the end of a rod is dipped into the bucket, which
is called drawing the glass, the portion taken up being allowed to run off, naturally
assumes a pear-shape, from the appearance of which, they can judge if the consistence be
proper, and if any air bubbles remain. If all be right, the cuvettes are taken out of the
furnace, and conveyed to the part of the halle where their contents are to be poured out.
This process requires peculiar instruments and manipulations.
Casting.—While the glass is refining, that is, coming to its highest point of perfection,
preparation is made for the most important process, the casting of the plate, whose
success crowns all the preliminary labours and cares. The oven or carquaise destined to
receive and anneal the plate, is now heated by its small fire or tisar, to such a pitch that
its sole may have the same temperature as that of the plates, being nearly red-hot at the
moment of their being introduced. An unequal degree of heat in the carquaise would
cause breakage of the glass. The casting table is then rolled towards the front door or
throat, by means of levers, and its surface is brought exactly to the level of the sole of
the oven.
The table T, fig. 515., is a mass of bronze, or now preferably cast-iron, about 10 feet
long, 5 feet broad, and from 6 to 7 inches thick, supported by a frame of carpentry,
which rests on three cast-iron wheels. At the end of the table opposite to that next to the
front of the oven, is a very strong frame of timber-work, called the puppet or standard, upon
which the bronze roller which spreads the glass is laid, before and after the casting. This
is 5 feet long by 1 foot in diameter; it is thick in the metal but hollow in the axis.
The same roller can serve only for two plates at one casting, when another is put in its
place, and the first is laid aside to cool; for otherwise the hot roller would at a third
casting, make the plate expand unequally, and cause it to crack. When the rollers are
not in action, they are laid aside in strong wooden trestles, like those employed by sawyers.
On the two sides of the table in the line of its length, are two parallel bars of bronze, t, t,
destined to support the roller during its passage from end to end; the thickness of these
bars determines that of the plate. The table being thus arranged, a crane is had recourse
to for lifting the cuvette, and keeping it suspended, till it be emptied upon the table.
This raising and suspension are effected by means of an iron gib, furnished with
pullies, held horizontally, and which turns with them.
[590]
The tongs T, fig. 515., are made of four iron bars, bent into a square frame in their
middle, for embracing the bucket. Four chains proceeding from the corners of the frame
V, are united at their other ends into a ring which fits into the hook of the crane.
Things being thus arranged, all the workmen of the foundry co-operate in the manipulations
of the casting. Two of them fetch, and place quickly in front of one of the lower
openings, the small cuvette-carriage, which bears a forked bar of iron, having two prongs
corresponding to the two holes left in the fire-tile door. This fork mounted on the axle
of two cast-iron wheels, extends at its other end into two branches terminated by
handles, by which the workmen move the fork, lift out the tile stopper, and set it
down against the outer wall of the furnace.
The instant these men retire, two others push forward into the opening the extremity
of the tongs-carriage, so as to seize the bucket by the girdle, or rather to clamp it.
At the same time, a third workman is busy with an iron pinch or long chisel, detaching
the bucket from its seat, to which it often adheres by some spilt glass; whenever it is
free, he withdraws it from the furnace. Two powerful branches of iron united by a
bolt, like two scissor blades, which open, come together, and join by a quadrant near
the other end, form the tongs-carriage, which is mounted upon two wheels like a truck.
The same description will apply almost wholly to the iron-plate carriage, on which
the bucket is laid the moment it is taken out of the furnace; the only difference in its
construction is, that on the bent iron bars which form the tail or lower steps of this carriage
(in place of the tongs) is permanently fastened an iron plate, on which the bucket
is placed and carried for the casting.
Whenever the cuvette is set upon its carriage, it must be rapidly wheeled to its station
near the crane. The tongs T above described are now applied to the girdle, and are then
hooked upon the crane by the suspension chains. In this position the bucket is skimmed
by means of a copper tool called a sabre, because it has nearly the shape of that
weapon. Every portion of the matter removed by the sabre is thrown into a copper
ladle (poche de gamin), which is emptied from time to time into a cistern of water. After
being skimmed, the bucket is lifted up, and brushed very clean on its sides and bottom;
then by the double handles of the suspension-tongs it is swung round to the table, where
it is seized by the workmen appointed to turn it over; the roller having been previously
laid on its ruler-bars, near the end of the table which is in contact with the annealing
oven. The cuvette-men begin to pour out towards the right extremity E of the roller, and
terminate when it has arrived at the left extremity D. While preparing to do so, and
at the instant of casting, two men place within the ruler-bar on each side, that is
between the bar and the liquid glass, two iron instruments called hands, m, m, m, m,
which prevent the glass from spreading beyond the rulers, whilst another draws along
the table the wiping bar c, c, wrapped in linen, to remove dust, or any small objects
which may interpose between the table and the liquid glass.
Whenever the melted glass is poured out, two men spread it over the table, guiding
the roller slowly and steadily along, beyond the limits of the glass, and then run it
smartly into the wooden standard prepared for its reception, in place of the trestles V, V.
The empty bucket, while still red-hot, is hung again upon the crane, set on its plate-iron
carriage, freed from its tongs, and replaced in the furnace, to be speedily cleared
out anew, and charged with fresh fluid from the pots. If while the roller glides along,
the two workmen who stand by with picking tools, perceive tears in the matter in advance
of the roller, and can dexterously snatch them out, they are suitably rewarded,
according to the spot where the blemish lay, whether in the centre, where it would
have proved most detrimental, or near the edge. These tears proceed usually from[591]
small portions of semi-vitrified matter which fall from the vault of the furnace, and from
their density occupy the bottom of the cuvettes.
While the plate is still red-hot and ductile, about 2 inches of its end opposite to the
carquaise door is turned up with a tool; this portion is called the head of the mirror;
against the outside of this head, the shovel, in the shape of a rake without teeth, is applied,
with which the plate is eventually pushed into the oven, while two other workmen
press upon the upper part of the head with a wooden pole, eight feet long, to preserve
the plate in its horizontal position, and prevent its being warped. The plate is now left
for a few moments near the throat of the carquaise, to give it solidity; after which it is
pushed further in by means of a very long iron tool, whose extremity is forked like the
letter y, and hence bears that name; and is thereby arranged in the most suitable spot
for allowing other plates to be introduced.
However numerous the manipulations executed from the moment of withdrawing the
cuvette from the furnace, till the cast-plate is pushed into the annealing oven, I have seen
them all performed in less than five minutes; such silence, order, regularity, and despatch
prevail in the establishment of Saint-Gobin.
When all the plates of the same casting have been placed in the carquaise, it is
sealed up, that is to say, all its orifices are closed with sheets of iron, surrounded and
made tight with plastic loam. With this precaution, the cooling goes on slowly and
equably in every part, for no cooling current can have access to the interior of the oven.
After they are perfectly cooled, the plates are carefully withdrawn one after another,
keeping them all the while in a horizontal position, till they are entirely out of the carquaise.
As soon as each plate is taken out, one set of workmen lower quickly and
steadily the edge which they hold, while another set raise the opposite edge, till the glass
be placed upright on two cushions stuffed with straw, and covered with canvas. In
this vertical position they pass through, beneath the lower edge of the plate, three girths
or straps each four feet long, thickened with leather in their middle, and ending in
wooden handles; so that one embraces the middle of the plate, and the other two, the
ends. The workmen, six in number, now seize the handles of the straps, lift up the
glass closely to their bodies, and convey it with a regular step to the warehouse. Here
the head of the plate is first cut off with a diamond square, and then the whole is
attentively examined, in reference to its defects and imperfections, to determine the
sections which must be made of it, and the eventual size of the pieces. The pairings
and small cuttings detached are set aside, in order to be ground and mixed with the
raw materials of another glass-pot.
The apartment in which the roughing-down and smoothing of the plates is performed,
is furnished with a considerable number of stone tables, truly hewn and placed
apart like billiard tables, in a horizontal position, about 2 feet above the ground. They
are rectangular, and of different sizes proportional to the dimensions of the plates, which
they ought always to exceed a little. These tables are supported either on stone pillars
or wooden frames, and are surrounded with a wooden board whose upper edge stands
somewhat below their level, and leaves in the space between it and the stone all round
an interval of 3 or 4 inches, of which we shall presently see the use.
A cast plate, unless formed on a table quite new, has always one of its faces, the one
next the table, rougher than the other; and with this face the roughing-down begins.
With this view, the smoother face is cemented on the stone table with Paris-plaster. But
often instead of one plate, several are cemented alongside of each other, those of the same
thickness being carefully selected. They then take one or more crude plates of about
one-third or one-fourth the surface of the plate fixed to the table, and fix it on them
with liquid gypsum to the large base of a quadrangular truncated pyramid of stone; of
a weight proportioned to its extent, or about a pound to the square inch. This pyramidal
muller, if small sized, bears at each of its angles of the upper face a peg or ball,
which the grinders lay hold of in working it; but when of greater dimension, there is
adapted to it horizontally a wheel of slight construction, 8 or 10 feet in diameter, whose
circumference is made of wood rounded so as to be seized with the hand. The upper
plate is now rubbed over the lower ones, with moistened sand applied between.
This operation is however performed by machinery. The under plate being fixed or
imbedded in stucco, on a solid table, the upper one likewise imbedded by the same
cement in a cast-iron frame, has a motion of circum-rotation given to it closely resembling
that communicated by the human hand and arm, moist sand being supplied
between them. While an excentric mechanism imparts this double rotatory movement
to the upper plate round its own centre, and of that centre round a point in the lower
plate, this plate placed on a moveable platform changes its position by a slow horizontal
motion, both in the direction of its length and its breadth. By this ingenious contrivance,
which pervades the whole of the grinding and polishing machinery, a remarkable
regularity of friction and truth of surface is produced. When the plates are sufficiently
worked on one face, they are reversed in the frames, and worked together on[592]
the other. The Paris plaster is usually coloured red, in order to shew any defects in
the glass.
The smoothing of the plates is effected on the same principles by the use of moist
emery washed to successive degrees of fineness, for the successive stages of the operation;
and the polishing process is performed by rubbers of hat-felt and a thin paste of colcothar
and water. The colcothar, called also crocus, is red oxide of iron prepared by
the ignition of copperas, with grinding and elutriation of the residuum.
The last part of the polishing process is performed by hand. This is managed by
females, who slide one plate over another, while a little moistened putty of tin finely
levigated is thrown between.
Large mirror-plates are now the indispensable ornaments of every large and sumptuous
apartment; they diffuse lustre and gaiety round them, by reflecting the rays of
light in a thousand lines, and by multiplying indefinitely the images of objects placed
between opposite parallel planes.
The silvering of plane mirrors consists in applying a layer of tin-foil alloyed with mercury
to their posterior surface. The workshop for executing this operation is provided with
a great many smooth tables of fine freestone or marble, truly levelled, having round
their contour a rising ledge, within which there is a gutter or groove which terminates
by a slight slope in a spout at one of the corners. These tables rest upon an axis of wood
or iron which runs along the middle of their length; so that they may be inclined
easily into an angle with the horizon of 12 or 13 degrees, by means of a hand-screw
fixed below. They are also furnished with brushes, with glass rules, with rolls of woollen
stuff, several pieces of flannel, and a great many weights of stone or cast-iron.
The glass-tinner, standing towards one angle of his table, sweeps and wipes its surface
with the greatest care, along the whole surface to be occupied by the mirror-plate; then
taking a sheet of tin-foil adapted to his purpose, he spreads it on the table, and applies
it closely with a brush, which removes any folds or wrinkles. The table being horizontal,
he pours over the tin a small quantity of quicksilver, and spreads it with a roll
of woollen stuff; so that the tin-foil is penetrated and apparently dissolved by the mercury.
Placing now two rules, to the right and to the left, on the borders of the sheet, he
pours on the middle a quantity of mercury sufficient to form every where a layer about the
thickness of a crown piece; then removing with a linen rag the oxide or other impurities,
he applies to it the edge of a sheet of paper, and advances it about half an inch. Meanwhile
another workman is occupied in drying very nicely the surface of the glass that
is to be silvered, and then hands it to the master workman, who, laying it flat, places its
anterior edge first on the table, and then on the slip of paper; now pushing the glass
forwards, he takes care to slide it along so that neither air nor any coat of oxide on the
mercury can remain beneath the plate. When this has reached its position, he fixes it
there by a weight applied on its side, and gives the table a gentle slope, to run off all
the loose quicksilver by the gutter and spout. At the end of five minutes he covers
the mirror with a piece of flannel, and loads it with a great many weights, which are
left upon it for 24 hours, under a gradually increased inclination of the table. By this
time the plate is ready to be taken off the marble table, and laid on a wooden one sloped
like a reading desk, with its under edge resting on the ground, while the upper is raised
successively to different elevations by means of a cord passing over a pulley in the
ceiling of the room. Thus the mirror has its slope graduated from day to day, till
it finally arrives at a vertical position. About a month is required for draining out
the superfluous mercury from large mirrors; and from 18 to 20 days from those of
moderate size. The sheets of tin-foil being always somewhat larger than the glass-plate,
their edges must be pared smooth off, before the plate is lifted off the marble
table.
Process for silvering concave mirrors.—Having prepared some very fine Paris plaster
by passing it through a silk sieve, and some a little coarser passed through hair-cloth,
the first is to be made into a creamy liquor with water, and after smearing the concave
surface of the glass with a film of olive oil, the fine plaster is to be poured into it, and
spread by turning about, till a layer of plaster be formed about a tenth of an inch thick.
The second or coarse plaster, being now made into a thin paste, poured over the first,
and moved about, readily incorporates with it, in its imperfectly hardened state. Thus
an exact mould is obtained of the concave surface of the glass, which lies about three-quarters
of an inch thick upon it, but is not allowed to rise above its outer edge.
The mould being perfectly dried, must be marked with a point of coincidence on the
glass, in order to permit of its being exactly replaced in the same position, after it has
been lifted out. The mould is now removed, and a round sheet of tin-foil is applied to it, so
large that an inch of its edge may project beyond the plaster all round; this border
being necessary for fixing the tin to the contour of the mould by pellets of white wax
softened a little with some Venice turpentine. Before fixing the tin-foil, however, it
must be properly spread over the mould, so as to remove every wrinkle; which the[593]
pliancy of the foil easily admits of, by uniform and well-directed pressure with the
fingers.
The glass being placed in the hollow bed of a tight sack filled with fine sand, set in a
well-jointed box capable of retaining quicksilver, its concave surface must be dusted
with sifted wood-ashes, or Spanish white contained in a small cotton bag, and then well
wiped with clean linen rags, to free it from all adhering impurity, and particularly the
moisture of the breath. The concavity must be now filled with quicksilver to the very
lip, and the mould being dipped a little way into it, is withdrawn, and the adhering
mercury is spread over the tin with a soft flannel roll, so as to amalgamate and brighten
its whole surface, taking every precaution against breathing on it. Whenever this
brightening seems complete, the mould is to be immersed, not vertically, but one edge
at first, and thus obliquely downwards till the centres coincide; the mercury meanwhile
being slowly displaced, and the mark on the mould being brought finally into coincidence
with the mark on the glass. The mould is now left to operate by its own weight,
in expelling the superfluous mercury, which runs out upon the sand-bag and thence
into a groove in the bottom of the box, whence it overflows by a spout into a leather bag
of reception. After half an hour’s repose, the whole is cautiously inverted, to drain off
the quicksilver more completely. For this purpose, a box like the first is provided
with a central support rising an inch above its edges; the upper surface of the support
being nearly equal in diameter to that of the mould. Two workmen are required to
execute the following operation. Each steadies the mould with the one hand, and raises
the box with the other, taking care not to let the mould be deranged, which they rest
on the (convex) support of the second box. Before inverting the first apparatus, however,
the reception bag must be removed, for fear of spilling its mercury. The redundant
quicksilver now drains off; and if the weight of the sand-bag is not thought sufficient,
supplementary weights are added at pleasure. The whole is left in this position
for two or three days. Before separating the mirror from its mould, the border of tin-foil,
fixed to it with wax, must be pared off with a knife. Then the weight and
sand-bag being removed, the glass is lifted up with its interior coating of tin-amalgam.
For silvering a convex surface.—A concave plaster mould is made on the convex
glass, and their points of coincidence are defined by marks. This mould is to be lined
with tin-foil, with the precautions above described; and the tin surface being first
brightened with a little mercury, the mould is then filled with the liquid metal. The
glass is to be well cleaned, and immersed in the quicksilver bath, which will expel the
greater part of the metal. A sand-bag is now to be laid on the glass, and the whole is
to be inverted as in the former case on a support; when weights are to be applied to
the mould, and the mercury is left to drain off for several days.
If the glass be of large dimensions, 30 or 40 inches, for example, another method
is adopted. A circular frame or hollow ring of wood or iron is prepared, of twice the
diameter of the mirror, supported on three feet. A circular piece of new linen cloth
of close texture is cut out, of equal diameter to the ring, which is hemmed stoutly at
the border, and furnished round the edge with a row of small holes, for lacing the cloth
to the ring, so as to leave no folds in it, but without bracing it so tightly as to deprive
it of the elasticity necessary for making it into a mould. This apparatus being set
horizontally, a leaf of tin-foil is spread over it, of sufficient size to cover the surface of
the glass; the tin is first brightened with mercury, and then as much of the liquid
metal is poured on as a plane mirror requires. The convex glass, well cleaned, is now
set down on the cloth, and its own weight, joined to some additional weights, gradually
presses down the cloth, and causes it to assume the form of the glass which thus comes
into close contact with the tin submersed under the quicksilver. The redundant quicksilver
is afterwards drained off by inversion, as in common cases.
The following recipe has been given for silvering the inside of glass globes. Melt in an
iron ladle or a crucible, equal parts of tin and lead, adding to the fused alloy one part of
bruised bismuth. Stir the mixture well and pour into it as it cools two parts of dry mercury;
agitating anew and skimming off the drossy film from the surface of the amalgam.
The inside of the glass globe being freed from all adhering dust and humidity, is to
be gently heated, while a little of the semi-fluid amalgam is introduced. The liquidity
being increased by the slight degree of heat, the metallic coating is applied to all the
points of the glass, by turning round the globe in every direction, but so slowly as to
favour the adhesion of the alloy. This silvering is not so substantial as that of plane
mirrors: but the form of the vessel, whether a globe, an ovoid, or a cylinder, conceals or
palliates the defects by counter reflection from the opposite surfaces.
Coloured Glasses and Artificial Gems.—The general vitreous body preferred by Fontanieu
in his treatise on this subject, which he calls the Mayence base, is prepared in
the following manner. Eight ounces of pure rock-crystal or flint in powder, mixed
with 24 ounces of salt of tartar, are baked and left to cool. This is afterwards poured[594]
into a basin of hot water, and treated with dilute nitric acid till it ceases to effervesce;
when the frit is to be washed till the water comes off tasteless. The frit is now dried
and mixed with 12 ounces of fine white lead, and the mixture is to be levigated
and elutriated with a little distilled water. An ounce of calcined borax is to be added
to about 12 ounces of the preceding mixture in a dry state, the whole rubbed
together in a porcelain mortar, then melted in a clean crucible, and poured out into cold
water. This vitreous matter must be dried, and melted a second and a third time, always
in a new crucible, and after each melting poured into cold water as at first, taking
care to separate the lead that may be revived. To the last glass ground to powder, five
drachms of nitre are to be added, and the mixture being melted for the last time, a
mass of crystal will be found in the crucible with a beautiful lustre. The diamond is
well imitated by this Mayence base. Another very fine white crystal may be obtained,
according to M. Fontanieu, from eight ounces of white lead, two ounces of powdered
borax, half a grain of manganese, and three ounces of rock-crystal treated as above.
The colours of artificial gems are obtained from metallic oxides. The oriental topaz
is prepared by adding oxide of antimony to the base; the amethyst from manganese
with a little purple precipitate of Cassius; the beryl from antimony and a very little
cobalt; yellow artificial diamond and opal, from horn-silver (chloride of silver); blue
stone from cobalt. See Pastes and Pigments Vitrifiable.
The following are recipes for making the different kinds of glass.
1. Bottle glass.—11 pounds of dry glauber salts; 12 pounds of soaper salts; a half
bushel of waste soap ashes; 56 pounds of sand; 22 pounds of glass skimmings; 1
cwt. of green broken glass; 25 pounds of basalt. This mixture affords a dark green
glass.
2. Yellow or white sand 100 parts; kelp 30 to 40; lixiviated wood ashes from 160
to 170 parts; fresh wood ashes 30 to 40 parts; potter’s clay 80 to 100 parts; cullet
or broken glass 100. If basalt be used, the proportion of kelp may be diminished.
In two bottle-glass houses in the neighbourhood of Valenciennes, an unknown ingredient,
sold by a Belgian, was employed, which he called spar. This was discovered
by chemical analysis to be sulphate of baryta. The glass-makers observed that the
bottles which contained some of this substance were denser, more homogeneous, more
fusible, and worked more kindly, than those formed of the common materials. When
one prime equivalent of the silicate of baryta = 123, is mixed with three primes of the
silicate of soda = (3 × 77·6) = 232·8, and exposed in a proper furnace, vitrification
readily ensues, and the glass may be worked a little under a cherry-red heat, with as
much ease as a glass of lead, and has nearly the same lustre. Since the ordinary run
of glass-makers are not familiar with atomic proportions, they should have recourse to
a scientific chemist, to guide them in using such a proportion of sulphate of baryta as
may suit their other vitreous ingredients; for an excess or defect of any of them will
injure the quality of the glass.
3. Green window glass, or broad glass.—11 pounds of dry glauber salt; 10 pounds
of soaper salts; half a bushel of lixiviated soap waste; 50 pounds of sand; 22 pounds
of glass pot skimmings; 1 cwt. of broken green glass.
4. Crown glass.—300 parts of fine sand; 200 of good soda ash; 33 of lime; from
250 to 300 of broken glass; 60 of white sand; 30 of purified potash; 15 of saltpetre
(1 of borax), 1⁄2 of arsenious acid.
5. Nearly white table glass.—20 pounds of potashes; 11 pounds of dry glauber salts;
16 of soaper salt; 55 of sand; 140 of cullet of the same kind. Another.—100
of sand; 235 of kelp; 60 of wood ashes; 11⁄3 of manganese; 100 of broken glass.
6. White table glass.—40 pounds of potashes; 11 of chalk; 76 of sand; 1⁄2 of manganese;
95 of white cullet.
Another.—50 of purified potashes; 100 of sand; 20 of chalk; and 2 of saltpetre.
Bohemian table or plate glass is made with 63 parts of quartz; 26 of purified potashes;
11 of sifted slaked lime, and some cullet.
7. Crystal glass.—60 parts of purified potashes; 120 of sand; 24 of chalk; 2 of
saltpetre; 2 of arsenious acid; 1⁄16 of manganese.
Another.—70 of purified pearl ashes; 120 of white sand; 10 of saltpetre; 1⁄2 of arsenious
acid; 1⁄3 of manganese.
A third.—67 of sand; 23 of purified pearl ashes; 10 of sifted slaked lime; 1⁄4 of
manganese; (5 to 8 of red lead).
A fourth.—120 of white sand; 50 of red lead; 40 of purified pearl ash; 20 of saltpetre;
1⁄3 of manganese.
A fifth.—120 of white sand; 40 of pearl ash purified; 35 of red lead; 13 of saltpetre;
1⁄12 of manganese.
A sixth.—30 of the finest sand; 20 of red lead; 8 of pearl ash purified; 2 of saltpetre;
a little arsenious acid and manganese.
[595]
A seventh.—100 of sand; 45 of red lead; 35 of purified pearl ashes; 1⁄7 of manganese;
1⁄3 of arsenious acid.
8. Plate glass.—Very white sand 300 parts; dry purified soda 100 parts; carbonate
of lime 43 parts; manganese 1; cullet 300.
Another.—Finest sand 720; purified soda 450; quicklime 80 parts; saltpetre 25
parts; cullet 425.
A little borax has also been prescribed; much of it communicates an exfoliating property
to glass.
Tabular view of the composition of several kinds of Glass.
| |
No. 1. |
No. 2. |
No. 3. |
No. 4. |
No. 5. |
No. 6. |
No. 7. |
No. 8. |
No. 9. |
| Silica |
71·7 |
69·2 |
62·8 |
69·2 |
60·4 |
53·55 |
59·2 |
51·93 |
42·5 |
| Potash |
12·7 |
15·8 |
22·1 |
8·0 |
3·2 |
5·48 |
9·0 |
13·77 |
11·7 |
| Soda |
2·5 |
3·0 |
|
3·0 |
S. pot. |
|
|
|
|
| Lime |
10·3 |
7·6 |
12·5 |
13·0 |
20·7 |
29·22 |
|
|
0·5 |
| Alumina |
0·4 |
1·2 |
|
3·6 |
10·4 |
6·01 |
|
|
1·8 |
| Magnesia |
|
2·0 |
|
|
- |
2·6 |
0·6 |
0·6 |
|
|
|
|
| Oxide of iron |
0·3 |
0·5 |
1·6 |
3·8 |
5·74 |
0·4 |
|
|
| — manganese |
0·2 |
|
|
|
|
1·0 |
|
|
| — lead |
|
|
|
|
|
|
28·2 |
33·28 |
43·5 |
| Baryta |
|
|
|
|
0·9 |
|
|
|
|
| |
No. 1. |
No. 2. |
No. 3. |
No. 4. |
No. 5. |
No. 6. |
No. 7. |
No. 8. |
No. 9. |
| Silica |
71·7 |
69·2 |
62·8 |
69·2 |
60·4 |
53·55 |
59·2 |
51·93 |
42·5 |
| Potash |
12·7 |
15·8 |
22·1 |
8·0 |
3·2 |
5·48 |
9·0 |
13·77 |
11·7 |
| Soda |
2·5 |
3·0 |
|
3·0 |
S. pot. |
|
|
|
|
| Lime |
10·3 |
7·6 |
12·5 |
13·0 |
20·7 |
29·22 |
|
|
0·5 |
| Alumina |
0·4 |
1·2 |
|
3·6 |
10·4 |
6·01 |
|
|
1·8 |
| Magnesia |
|
2·0 |
|
|
- |
2·6 |
0·6 |
0·6 |
|
|
|
|
| Oxide of iron |
0·3 |
0·5 |
1·6 |
3·8 |
5·74 |
0·4 |
|
|
| — manganese |
0·2 |
|
|
|
|
1·0 |
|
|
| — lead |
|
|
|
|
|
|
28·2 |
33·28 |
43·5 |
| Baryta |
|
|
|
|
0·9 |
|
|
|
|
No. 1. is a very beautiful white wine glass of Neuwelt in Bohemia.
No. 2. Glass tubes, much more fusible than common wine glasses.
No. 3. Crown glass of Bohemia.
No. 4. Green glass, for medicinal phials and retorts.
No. 5. Flask glass of St. Etienne, for which some heavy spar is used.
No. 6. Glass of Sèvres.
No. 7. London glass employed for chemical and physical purposes.
No. 8. English flint glass.
No. 9. Guinand’s flint glass.
The manufacture of Glass beads at Murano near Venice, is most ingeniously simple.
Tubes of glass of every colour, are drawn out to great lengths in a gallery adjoining
the glass-house pots, in the same way as the more moderate lengths of thermometer and
barometer tubes are drawn in our glass-houses. These tubes are chopped into very small
pieces of nearly uniform length on the upright edge of a fixed chisel. These elementary
cylinders being then put in a heap into a mixture of fine sand and wood ashes, are
stirred about with an iron spatula till their cavities get filled. This curious mixture
is now transferred to an iron pan suspended over a moderate fire, and continually stirred
about as before, whereby the cylindrical bits assume a smooth rounded form; so that
when removed from the fire and cleared out in the bore, they constitute beads, which
are packed in casks, and exported in prodigious quantities to almost every country,
especially to Africa and Spain.
GLASS CUTTING AND GRINDING, for common and optical purposes. By
this mechanical process the surface of glass may be modified into almost any ornamental
or useful form.
1. The grinding of crystal ware. This kind of glass is best adapted to receive
polished facets, both on account of its relative softness, and its higher refractive power,
which gives lustre to its surface. The cutting shop should be a spacious long apartment,
furnished with numerous sky-lights, having the grinding and polishing lathes
arranged right under them, which are set in motion by a steam-engine or water-wheel at
one end of the building. A shaft is fixed as usual
in gallowses along the ceiling; and from the pulleys
of the shaft, bands descend to turn the different
lathes, by passing round the driving pulleys near
their ends.
The turning lathe is of the simplest construction.
Fig. 516. D is an iron spindle with two well-turned
prolongations, running in the iron puppets a a,
between two concave bushes of tin or type metal,
which may be pressed more or less together by the
thumb-screws shown in the figure. These two
puppets are made fast to the wooden support B,
which is attached by a strong screw and bolt to the
longitudinal beam of the workshop A. E is the fast and loose pulley for putting the[596]
lathe into and out of geer with the driving shaft. The projecting end of the spindle
is furnished with a hollow head-piece, into which the rod c is pushed tight. This
rod carries the cutting or grinding disc plate. For heavy work, this rod is fixed into
the head by a screw. When a conical fit is preferred, the cone is covered with lead to
increase the friction.
Upon projecting rods or spindles of that kind the different discs for cutting the glass
are made fast. Some of these are made of fine sandstone or polishing slate, from 8 to
10 inches in diameter, and from 3⁄4 to 1⁄2 inch thick. They must be carefully turned and
polished at the lathe, not only upon their rounded but upon their flat face, in order to
grind and polish in their turn the flat and curved surfaces of glass vessels. Other discs
of the same diameter, but only 3⁄4 of an inch thick, are made of cast tin truly turned, and
serve for polishing the vessels previously ground; a third set consist of sheet iron from
1⁄6 to 1⁄2 an inch thick, and 12 inches in diameter, and are destined to cut grooves in glass
by the aid of sand and water. Small discs of well-hammered copper from 1⁄2 to 3 inches
in diameter, whose circumference is sometimes flat, and sometimes concave or convex,
serve to make all sorts of delineations upon glass by means of emery and oil. Lastly,
there are rods of copper or brass furnished with small hemispheres from 1⁄24 to 1⁄4 of an inch
in diameter, to excavate round hollows in glass. Wooden discs are also employed for polishing,
made of white wood cut across the grain, as also of cork.
The cutting of deep indentations, and of grooves, is usually performed by the iron
disc, with sand and water, which are allowed constantly to trickle down from a wooden
hopper placed right over it, and furnished with a wooden stopple or plug at the apex, to
regulate by its greater or less looseness the flow of the grinding materials. The same
effect may be produced by using buckets as shown in fig. 517. The
sand which is contained in the bucket F, above the lathe, has a spigot
and faucet inserted near its bottom, and is supplied with a stream of
water from the stopcock in the vessel G, which, together running down
the inclined board, are conducted to the periphery of the disc as
shown in the figure, to whose lowest point the glass vessel is applied
with pressure by the hand. The sand and water are afterwards collected
in the tub H. Finer markings which are to remain without
lustre, are made with the small copper discs, emery, and oil. The polishing
is effected by the edge of the tin disc, which is from time
to time moistened with putty (white oxide of tin) and water. The
wooden disc is also employed for this purpose with putty, colcothar, or
washed tripoli. For fine delineations, the glass is first traced over with
some coloured varnish, to guide the hand of the cutter.
In grinding and facetting crystal glass, the deep grooves are first
cut, for example the cross lines, with the iron disc and rounded edge,
by means of sand and water. That disc is one sixth of an inch thick
and 12 inches in diameter. With another iron disc about half an inch thick, and more
or less in diameter, according to the curvature of the surface, the grooves may be
widened. These roughly cut parts must be next smoothed down with the sandstone
disc and water, and then polished with the wooden disc about half an inch thick, to
whose edge the workman applies, from time to time, a bag of fine linen containing
some ground pumice moistened with water. When the cork or wooden disc edged with
hat felt is used for polishing, putty or colcothar is applied to it. The above several
processes in a large manufactory, are usually committed to several workmen on the
principle of the division of labour, so that each may become expert in his department.
2. The grinding of optical glasses.—The glasses intended for optical purposes being
spherically ground, are called lenses; and are used either as simple magnifiers and spectacles,
or for telescopes and microscopes. The curvature is always a portion of a sphere,
and either convex or concave. This form ensures the convergence or divergence of the
rays of light that pass through them, as the polishing does the brightness of the
image.
The grinding of the lenses is performed in brass moulds, either concave or convex, formed
to the same curvature as that desired in the lenses; and may be worked either by hand or
by machinery. A gauge is first cut out out of brass or copper plate to suit the curvature
of the lens, the circular arc being traced by a pair of compasses. In this way both
a convex and concave circular gauge are obtained. To these gauges the brass moulds are
turned. Sometimes, also, lead moulds are used. After the two moulds are made, they
are ground face to face with fine emery.
The piece of glass is now roughed into a circular form by a pair of pincers, leaving
it a little larger than the finished lens ought to be, and then smoothed round upon the
stone disc, or in an old mould with emery and water, and is next made fast to a holdfast.
This consists of a round brass plate having a screw in its back; and is somewhat
smaller in diameter than the lens, and two thirds as thick. This as turned concave upon[597]
the lathe, and then attached to the piece of glass by drops of pitch applied to several
points of its surface, taking care while the pitch is warm, that the centre of the glass
coincides with the centre of the brass plate. This serves not merely as a holdfast, by
enabling a person to seize its edge with the fingers, but it prevents the glass from bending
by the necessary pressure in grinding.
The glass must now be ground with coarse emery upon its appropriate mould,
whether convex or concave, the emery being all the time kept moist with water. To prevent
the heat of the hand from affecting the glass, a rod for holding the brass plate is
screwed to its back. For every six turns of circular motion, it must receive two or
three rubs across the diameter in different directions, and so on alternately. The middle
point of the glass must never pass beyond the edge of the mould; nor should strong
pressure be at any time applied. Whenever the glass has assumed the shape of the
mould, and touches it in every point, the coarse emery must be washed away, finer be
substituted in its place, and the grinding be continued as before, till all the scratches
disappear, and a uniform dead surface be produced. A commencement of polishing is
now to be given with pumice-stone powder. During all this time the convex mould
should be occasionally worked in the concave, in order that both may preserve their correspondence
of shape between them. After the one surface has been thus finished, the
glass must be turned over, and treated in the same way upon the other side.
Both surfaces are now to be polished. With this view equal parts of pitch and rosin
must be melted together, and strained through a cloth to separate all impurities. The
concave mould is next to be heated, and covered with that mixture in a fluid state to the
thickness uniformly of one quarter of an inch. The cold convex mould is now to be
pressed down into the yielding pitch, its surface being quite clean and dry, in order to
give the pitch the exact form of the ground lens; and both are to be plunged into cold
water till they be chilled. This pitch impression is now the mould upon which the
glass is to be polished, according to the methods above described with finely washed
colcothar and water, till the surface become perfectly clear and brilliant. To prevent the
pitch from changing its figure by the friction, cross lines must be cut in it about 1⁄2 an inch
asunder, and 1-12th of an inch broad and deep. These grooves remove all the superfluous
parts of the polishing powder, and tend to preserve the polishing surface of the pitch clean
and unaltered. No additional colcothar after the first is required in this part of the process;
but only a drop of water from time to time. The pitch gets warm as the polishing advances,
and renders the friction more laborious from the adhesion between the surfaces. No interruption
must now be suffered in the work, nor must either water or colcothar be added;
but should the pitch become too adhesive, it must be merely breathed upon, till the
polish be complete. The nearer the lens is brought to a true and fine surface in the first
grinding, the better and more easy does the polishing become. It should never be
submitted to this process with any scratches perceptible in it, even when examined by a
magnifier.
As to small lenses and spectacle eyes, several are ground and polished together in a
mould about 6 inches in diameter, made fast to a stiffening plate of brass or iron of a
shape corresponding with the mould. The pieces of glass are affixed by means of drops
of pitch as above described, to the mould, close to each other, and are then all treated as
if they formed but one large lens. Plane glasses are ground upon a surface of pitch
rendered plane by the pressure of a piece of plate glass upon it in its softened state.
Lenses are also ground and polished by means of machinery, into the details of which
the limits of this work will not allow me to enter.
A Return to an Order of the Honourable the House of Commons, dated 1st March,
1838, of the Amount of Duty charged on Glass; distinguishing the Amount charged
on Flint, Plate, Broad, Crown, Bottle and German Sheet, in the Year ending the
5th day of January, 1838; together with the Amount of Drawback on each
description of Glass; the produce of the Duties in England, Scotland, and Ireland
stated separately.
| Amount of Duty charged on |
Total. |
| — |
Flint Glass. |
Plate. |
Broad. |
Crown. |
Bottle. |
German Sheet. |
| |
£. |
s. |
d. |
£. |
s. |
£. |
s. |
£. |
s. |
d. |
£. |
s. |
d. |
£. |
s. |
£. |
s. |
d. |
| England |
176,052 |
1 |
0 |
68,902 |
10 |
10,789 |
10 |
533,404 |
6 |
7 |
122,617 |
10 |
2 |
25,511 |
17 |
837,277 |
14 |
9 |
| Scotland |
7,530 |
9 |
4 |
|
|
16,423 |
11 |
6 |
32,246 |
4 |
1 |
- |
- |
56,200 |
4 |
11 |
| Ireland |
6,736 |
12 |
11 |
|
|
- |
- |
|
3,642 |
0 |
3 |
- |
- |
10,378 |
13 |
2 |
| Total |
90,319 |
3 |
3 |
68,902 |
10 |
10,789 |
10 |
549,827 |
18 |
1 |
158,505 |
14 |
6 |
25,511 |
17 |
903,856 |
12 |
10 |
| Amount of Duty charged on |
| — |
Flint Glass. |
Plate. |
Broad. |
Crown. |
| |
£. |
s. |
d. |
£. |
s. |
£. |
s. |
£. |
s. |
d. |
| England |
176,052 |
1 |
0 |
68,902 |
10 |
10,789 |
10 |
533,404 |
6 |
7 |
| Scotland |
7,530 |
9 |
4 |
|
|
16,423 |
11 |
6 |
| Ireland |
6,736 |
12 |
11 |
|
|
- |
- |
|
| Total |
90,319 |
3 |
3 |
68,902 |
10 |
10,789 |
10 |
549,827 |
18 |
1 |
| Amount of Duty charged on |
Total. |
| — |
Bottle. |
German Sheet. |
| |
£. |
s. |
d. |
£. |
s. |
£. |
s. |
d. |
| England |
122,617 |
10 |
2 |
25,511 |
17 |
837,277 |
14 |
9 |
| Scotland |
32,246 |
4 |
1 |
- |
- |
56,200 |
4 |
11 |
| Ireland |
3,642 |
0 |
3 |
- |
- |
10,378 |
13 |
2 |
| Total |
158,505 |
14 |
6 |
25,511 |
17 |
903,856 |
12 |
10 |
[598]
| Amount of Drawback on Exportation. |
| — |
Flint Glass. |
Plate. |
Broad. |
Crown. |
Bottle. |
German Sheet. |
Total. |
| |
£. |
s. |
d. |
£. |
s. |
d. |
£. |
s. |
£. |
s. |
d. |
£. |
s. |
d. |
£. |
s. |
d. |
£. |
s. |
d. |
| England |
15,597 |
2 |
7 |
3,983 |
17 |
9 |
4 |
10 |
168,892 |
10 |
2 |
56,770 |
10 |
5 |
22,889 |
17 |
9 |
268,138 |
8 |
8 |
| Scotland |
1,726 |
15 |
5 |
- |
- |
|
- |
- |
8,626 |
9 |
0 |
14,819 |
8 |
1 |
32 |
15 |
6 |
25,205 |
8 |
0 |
| Ireland |
107 |
14 |
8 |
- |
- |
|
- |
- |
10 |
9 |
1 |
274 |
10 |
5 |
- |
- |
|
392 |
14 |
2 |
| Total |
17,431 |
12 |
8 |
3,983 |
17 |
9 |
4 |
10 |
177,529 |
8 |
3 |
71,864 |
8 |
11 |
22,922 |
13 |
3 |
293,736 |
10 |
10 |
| Amount of Drawback on Exportation. |
| — |
Flint Glass. |
Plate. |
Broad. |
Crown. |
| |
£. |
s. |
d. |
£. |
s. |
d. |
£. |
s. |
£. |
s. |
d. |
| England |
15,597 |
2 |
7 |
3,983 |
17 |
9 |
4 |
10 |
168,892 |
10 |
2 |
| Scotland |
1,726 |
15 |
5 |
- |
- |
|
- |
- |
8,626 |
9 |
0 |
| Ireland |
107 |
14 |
8 |
- |
- |
|
- |
- |
10 |
9 |
1 |
| Total |
17,431 |
12 |
8 |
3,983 |
17 |
9 |
4 |
10 |
177,529 |
8 |
3 |
| Amount of Drawback on Exportation. |
| — |
Bottle. |
German Sheet. |
Total. |
| |
£. |
s. |
d. |
£. |
s. |
d. |
£. |
s. |
d. |
| England |
56,770 |
10 |
5 |
22,889 |
17 |
9 |
268,138 |
8 |
8 |
| Scotland |
14,819 |
8 |
1 |
32 |
15 |
6 |
25,205 |
8 |
0 |
| Ireland |
274 |
10 |
5 |
- |
- |
|
392 |
14 |
2 |
| Total |
71,864 |
8 |
11 |
22,922 |
13 |
3 |
293,736 |
10 |
10 |
The duties payable in the United Kingdom, upon the different descriptions of glass
are, for—
| |
£. |
s. |
d. |
| Flint glass, the finished article |
0 |
0 |
2 |
per lb. |
| British plate or German sheet, and crown glass, |
ditto |
3 |
13 |
6 |
per cwt. |
| Broad glass, |
ditto |
1 |
10 |
0 |
— |
| Bottles, |
ditto |
0 |
7 |
0 |
— |
| Plate glass, the fused material in pot |
3 |
0 |
0 |
— |
GLAZIER, is the workman who cuts plates, or panes of glass, with the diamond, and
fastens them by means of putty in frames or window casements. See Diamond, for an
explanation of its glass-cutting property.
GLAUBER SALT; is the old name of sulphate of soda.
GLOVE MANUFACTURE. In February, 1822, Mr. James Winter of Stoke-under-Hambdon,
in the county of Somerset, obtained a patent for an improvement upon
a former patent machine of his for sewing and
pointing leather gloves. Fig. 518. represents
a pedestal, upon which the instrument called
the jaws is to be placed. Fig. 519. shows the
jaws, which instead of opening and closing by
a circular movement upon a joint, as described
in the former specification, are now made to
open and shut by a parallel horizontal movement,
effected by a slide and screw; a a is the
fixed jaw, made of one piece, on the under
side of which is a tenon, to be inserted into
the top of the pedestal. By means of this
tenon the jaws may be readily removed, and
another similar pair of jaws placed in their
stead, which affords the advantage of expediting
the operation by enabling one person to prepare
the work whilst another is sewing; b b is
the movable jaw, made of one piece. The
two jaws being placed together in the manner
shown at fig. 519., the movable jaw traverses
backwards and forwards upon two guide-bars,
c, which are made to pass through holes exactly
fitted to them, in the lower parts of the
jaws. At the upper parts of the jaws are,
what are called the indexes, d d, which are
pressed tightly together by a spring, shown at
fig. 520., and intended to be introduced between
the perpendicular ribs of the jaws at
e. At f, is a thumb-screw, passing through
the ribs for the purpose of tightening the jaws, and holding the leather fast between the
indexes while being sewn; this screw, however, will seldom, if ever, be necessary if the
spring is sufficiently strong; g is an eye or ring fixed to the movable jaw, through which
the end of a lever h, in fig. 518., passes; this lever is connected by a spring to a treadle
i, at the base of the pedestal, and by the pressure of the right foot upon this treadle, the
movable jaw is withdrawn; so that the person employed in sewing may shift the
leather, and place another part of the glove between the jaws. The pieces called indexes,
are connected to the upper part of the jaws, by screws passing through elongated holes
which render them capable of adjustment.
The patentee states, that in addition to the index described in his former patent, which
is applicable to what is called round-seam sewing only, and which permits the leather to
expand but in one direction, when the needle is passed through it, namely, upwards; he[599]
now makes two indexes of different construction, one of which he calls the receding
index, and the other the longitudinally grooved index. Fig. 521. represents an end view,
and fig. 522. a top view of the receding index, which is particularly adapted for what are
called “drawn sewing, and prick-seam sewing;” this index, instead of biting to the top,
is so rounded off in the inside from the bottom of the cross grooves, as to permit the
needles, by being passed backwards and forwards, to carry the silk thread on each side of
the leather without passing over it. Fig. 523. represents an end view of the longitudinally
grooved index, partly open, to show the section of the grooves more distinctly; and
fig. 524. represents an inside view of one side of the same index, in which the longitudinal
groove is shown passing from k to l. This index is more particularly adapted to round-seam
sewing, and permits the leather to expand in every direction when the needle is
passed through it, by which the leather is less strained, and the sewing consequently
rendered much stronger.
It is obvious that the parallel horizontal movement may be effected by other mechanical
means besides those adopted here, and the chief novelty claimed with respect to that
movement, is its application to the purpose of carrying the index used in sewing and
pointing leather gloves.
Importation of leather gloves for home consumption; and amount of duty in
| 1836. |
1837. |
1836. |
1837. |
| 1,461,769 |
1,221,350 |
£27,558 |
£22,923 |
GLOVE-SEWING. The following simple and ingenious apparatus, invented by
an Englishman, has been employed extensively in Paris, and has enabled its proprietors
to realize a handsome fortune. The French complain that “it has inundated the world
with gloves, made of excellent quality, at 30 per cent. under their former wholesale
prices.” The instrument is
shown in profile ready for action
in fig. 525. It resembles an
iron vice, having the upper portion
of each jaw made of brass,
and tipped with a kind of comb
of the same metal. The teeth
of this comb, only one twelfth
of an inch long, are perfectly
regular and equal. Change
combs are provided for different
styles of work. The vice A A
is made fast to the edge of the
bench or table B, of the proper
height, by a thumb-screw C,
armed with a cramp which lays
hold of the wood. Of the two
jaws composing the machine, the
one D is made fast to the foot
A A, but the other E is movable upon the solid base of the machine, by means of a
hinge at the point F. At I I is shown how the upper brass portion is adjusted to the
lower part made of iron; the two being secured to each other by two stout screws.
The comb, seen separately in fig. 527., is made fast to the upper end of each jaw, by the
three screws n n n. Fig. 526. is a front view of the jaw mounted with its comb, to illustrate
its construction.
The lever K corresponds by the stout iron wire L, with a pedal pressed by the needlewoman’s
foot, whenever she wishes to separate the two jaws, in order to insert between
them the parallel edges of leather to be sewed. The instant she lifts her foot, the two jaws
join by the force of the spring G, which pushes the movable jaw E against the stationary
one D. The spring is made fast to the frame of the vice by the screw H.
After putting the double edge to be sewed in its place, the woman passes her needle
successively through all the teeth of the comb, and is sure of making a regular seam in
every direction, provided she is careful to make the needle graze along the bottom of the
notches. As soon as this piece is sewed, she presses down the pedal with her toes,
whereby the jaws start asunder, allowing her to introduce a new seam; and so in quick
succession.
The comb may have any desired shape, straight or curved; and the teeth may be
larger or smaller, according to the kind of work to be done. With this view, the
combs might be changed as occasion requires; but it is more economical to have sets of
vices ready mounted with combs of every requisite size and form.
GLUCINA (Glucine, Fr.; Berryllerde, Germ.), is one of the primitive earths,[600]
originally discovered by Vauquelin, in the beryl and emerald. It may be extracted
from either of these minerals, by treating their powder successively with potash, with water,
and with muriatic acid. The solution by the latter, being evaporated to dryness, is to be
digested with water, and filtered. On pouring carbonate of ammonia in excess into the
liquid, we form soluble muriate of ammonia, with insoluble carbonates of lime, chrome,
and iron, as also carbonate of glucina, which may be dissolved out from the rest by an
excess of carbonate of ammonia. When the liquid is filtered anew, the glucina passes
through, and may be precipitated in the state of a carbonate by boiling the liquid, which
expels the excess of ammonia. By washing, drying, and calcining the carbonate, pure
glucina is obtained. It is a white insipid powder, infusible in the heat of a smith’s forge,
insoluble in water, but soluble in caustic potash and soda; as also, especially when it is a
hydrate, in carbonate of ammonia. It has a metallic base called glucinum, of which 100
parts combine with 45·252 of oxygen to form the earth. It is too rare to be susceptible
of application in manufactures.
GLUE; (Colle forte, Fr.; Leim, Tischlerleim, Germ.) is the chemical substance
gelatine in a dry state. The preparation and preservation of the skin and other
animal matters employed in the manufacture of glue, constitute a peculiar branch
of industry. Those who exercise it should study to prevent the fermentation of
the substances, and to diminish the cost of carriage by depriving them of as much
water as can conveniently be done. They may then be put in preparation by macerating
them in milk of lime, renewed three or four times in the course of a fortnight
or three weeks. This process is performed in large tanks of masonry. They are
next taken out with all the adhering lime, and laid in a layer, 2 or 3 inches thick,
to drain and dry, upon a sloping pavement, where they are turned over by prongs,
two or three times a day. The action of the lime dissolves the blood and certain
soft parts, attacks the epidermis, and disposes the gelatinous matter to dissolve
more readily. When the cleansed matters are dried, they may be packed in sacks
or hogsheads, and transported to the glue manufactory at any distance. The principal
substances of which glue is made are the parings of ox and other thick hides,
which form the strongest article; the refuse of the leather dresser; both afford from
45 to 55 per cent. of glue. The tendons, and many other offals of slaughter houses,
also afford materials, though of an inferior quality, for the purpose. The refuse of tanneries,
such as the ears of oxen, calves, sheep, &c., are better articles; but parings of
parchment, old gloves, and, in fact, animal skin, in every form, uncombined with tannin,
may be made into glue.
The manufacturer who receives these materials, is generally careful to ensure their
purification by subjecting them to a weak lime steep, and rinsing them by exposure
in baskets to a stream of water. They are lastly drained upon a sloping surface, as above
described, and well turned over till the quicklime gets mild by absorption of carbonic
acid; for, in its caustic state, it would damage the glue at the heat of boiling water. It
is not necessary, however, to dry them before they are put into the boiler, because they
dissolve faster in their soft and tumefied state.
The boiler is made of copper, rather shallow in proportion to its area, with a uniform
flat bottom, equably exposed all over to the flame of the fire. Above the true bottom
there is a false one of copper or iron, pierced with holes, and standing upon feet 3 or 4
inches high; which serves to sustain the animal matters, and prevent them from being
injured by the fire. The copper being filled to two thirds of its height with soft water,
is then heaped up with the bulky animal substances, so high as to surmount its brim.
But soon after the ebullition begins they sink down, and, in a few hours, get entirely
immersed in the liquid. They should be stirred about from time to time, and well
pressed down towards the false bottom, while a steady but gentle boil is maintained.
The solution must be drawn off in successive portions; a method which fractions the
products, or subdivides them into articles of various value, gradually decreasing from
the first portion drawn off to the last. It has been ascertained by careful experiments
that gelatine gets altered over the fire very soon after it is dissolved, and it ought therefore
to be drawn off whenever it is sufficiently fluid and strong for forming a clear
gelatinous mass on cooling, capable of being cut into moderately firm slices by the wire.
This point is commonly determined by filling half an egg-shell with the liquor, and
exposing it to the air to cool. The jelly ought to get very consistent in the course of a
few minutes; if not so, the boiling must be persisted in a little longer. When this term
is attained, the fire is smothered up, and the contents of the boiler are left to settle for a
quarter of an hour. The stop-cock being partially turned, all the thin gelatinous liquor
is run off into a deep boiler, immersed in a warm-water bath, so that it may continue
hot and fluid for several hours. At the end of this time, the supernatant clear liquid is
to be drawn off into congealing boxes, as will be presently explained.
The grounds, or undissolved matters in the boiler, are to be again supplied with a
quantity of boiling water from an adjoining copper, and are to be once more subjected to[601]
the action of the fire, till the contents assume the appearance of dissolved jelly, and afford
a fresh quantity of strong glue liquor, by the stop-cock. The grounds should be subjected
a third time to this operation, after which they may be put into a bag, and squeezed
in a press to leave nothing unextracted. The latter solutions are usually too weak to
form glue directly, but they may be strengthened by boiling with a portion of fresh
skin-parings.
Fig. 528. represents a convenient apparatus for the boiling of skins into glue, in which
there are three coppers upon three different levels; the uppermost being acted upon by
the waste heat of the chimney, provides warm water in the most economical way; the
second contains the crude materials, with water for dissolving them; and the third
receives the solution to be settled. The last vessel is double, with water contained
between the outer and inner one; and discharges its contents by a stop-cock into buckets
for filling the gelatinizing wooden boxes. The last made solution has about one
five hundredth part of alum in powder usually added to it, with proper agitation, after
which it is left to settle for several hours.
The three successive boils furnish three different qualities of glue.
Flanders or Dutch glue, long much esteemed on the Continent, was made in the
manner above described, but at two boils, from animal offals well washed and soaked, so
as to need less boiling. The liquor being drawn off thinner, was therefore less coloured,
and being made into thinner plates was very transparent. The above two boils gave
two qualities of glue.
By the English practice, the whole of the animal matter is brought into solution at
once, and the liquor being drawn off, hot water is poured on the residuum, and made to
boil on it for some time, when the liquor thus obtained is merely used instead of water
upon a fresh quantity of glue materials. The first drawn off liquor is kept hot in a
settling copper for five hours, and then the clear solution is drawn off into the boxes.
These boxes are made of deal, of a square form, but a little narrower at bottom than
at top. When very regular cakes of glue are wished for, cross grooves of the desired
square form are cut in the bottom of the box. The liquid glue is poured into the boxes
placed very level, through funnels furnished with filter cloths, till it stands at the brim
of each. The apartment in which this is done ought to be as cool and dry as possible,
to favour the solidification of the glue, and should be floored with stone flags kept very
clean, so that if any glue run through the seams, it may be recovered. At the end of
12 or 18 hours, or usually in the morning if the boxes have been filled overnight,
the glue is sufficiently firm for the nets, and they are at this time removed to
an upper story, mounted with ventilating windows to admit the air from all quarters.
Here the boxes are inverted upon a moistened table, so that the gelatinous cake thus
turned out will not adhere to its surface; usually the moist blade of a long knife is
insinuated round the sides of the boxes beforehand, to loosen the glue. The mass is first
divided into horizontal layers by a brass wire stretched in a frame, like that of a bow-saw,
and guided by rulers which are placed at distances corresponding to the desired
thickness of the cake of glue. The lines formed by the grooves in the bottom of the
box define the superficial area of each cake, where it is to be cut with a moist knife.
The gelatinous layers thus formed, must be dexterously lifted, and immediately laid
upon nets stretched in wooden frames, till each frame be filled. These frames are set
over each other at distances of about three inches, being supported by small wooden[602]
pegs, stuck into mortise holes in an upright, fixed round the room; so that the air may
have perfectly free access on every side. The cakes must moreover be turned upside
down upon the nets twice or thrice every day, which is readily managed, as each frame
may be slid out like a drawer, upon the pegs at its two sides.
The drying of the glue is the most precarious part of the manufacture. The least
disturbance of the weather may injure the glue during the two or three first days of its
exposure; should the temperature of the air rise considerably, the gelatine may turn so
soft as to become unshapely, and even to run through the meshes upon the pieces below,
or it may get attached to the strings and surround them, so as not to be separable
without plunging the net into boiling water. If frost supervene, the water may freeze
and form numerous cracks in the cakes. Such pieces must be immediately re-melted
and re-formed. A slight fog even produces upon glue newly exposed a serious deterioration;
the damp condensed upon its surface occasioning a general mouldiness. A
thunderstorm sometimes destroys the coagulating power in the whole laminæ at once;
or causes the glue to turn on the nets, in the language of the manufacturer. A wind
too dry or too hot may cause it to dry so quickly, as to prevent it from contracting to
its proper size without numerous cracks and fissures. In this predicament, the closing
of all the flaps of the windows is the only means of abating the mischief. On these
accounts it is of importance to select the most temperate season of the year, such as
spring and autumn, for the glue manufacture.
After the glue is dried upon the nets it may still preserve too much flexibility, or
softness at least, to be saleable; in which case it must be dried in a stove by artificial
heat. This aid is peculiarly requisite in a humid climate, like that of Great Britain.
When sufficiently dry it next receives a gloss, by being dipped cake by cake in hot
water, and then rubbed with a brush also moistened in hot water; after which the glue
is arranged upon a hurdle, and transferred to the stove room, if the weather be not
sufficiently hot. One day of proper drought will make it ready for being packed up
in casks.
The pale-coloured, hard and solid, article, possessing a brilliant fracture, which is
made from the parings of ox-hides by the first process, is the best and most cohesive,
and is most suitable for joiners, cabinet-makers, painters, &c. But many workmen are
influenced by such ignorant prejudices, that they still prefer a dark-coloured article,
with somewhat of a fetid odour, indicative of its impurity and bad preparation, the
result of bad materials and too long exposure to the boiling heat.
There is a good deal of glue made in France from bones, freed from the phosphate of
lime by muriatic acid. This is a poor article, possessing little cohesive force. It dissolves
almost entirely in cold water, which is the best criterion of its imperfection. Glue
should merely soften in cold water, and the more considerably it swells, the better
generally speaking, it is.
Some manufacturers prefer a brass to a copper pan for boiling glue, and insist much
on skimming it as it boils; but the apparatus I have represented renders skimming of
little consequence. For use, glue should be broken into small pieces, put along with some
water into a vessel, allowed to soak for some hours, and subjected to the heat of a boiling-water
bath, but not boiled itself. The surrounding hot water keeps it long in a
fit state for joiners, cabinet-makers, &c.
Water containing only one hundredth part of good glue, forms a tremulous solid.
When the solution, however, is heated and cooled several times, it loses the property of
gelatinizing, even though it be enclosed in a vessel hermetically sealed. Isinglass or fish-glue
undergoes the same change. Common glue is not soluble in alcohol, but is precipitated
in a white, coherent, elastic mass, when its watery solution is treated with that
fluid. By transmitting chlorine gas through a warm solution of glue, a combination
is very readily effected, and a viscid mass is obtained like that thrown down by alcohol.
A little chlorine suffices to precipitate the whole of the glue. Concentrated sulphuric
acid makes glue undergo remarkable changes; during which are produced, sugar of gelatine,
leucine, an animal matter, &c. Nitric acid, with the aid of heat, converts glue into
malic acid, oxalic acid, a fat analogous to suet, and into tannin; so that, in this way,
one piece of skin may be made to tan another. When the mixture of glue and nitric
acid is much evaporated, a detonation at last takes place. Strong acetic acid renders
glue first soft and transparent, and then dissolves it. Though the solution does not
gelatinize, it preserves the property of gluing surfaces together when it dries. Liquid
glue dissolves a considerable quantity of lime, and also of the phosphate of lime recently
precipitated. Accordingly glue is sometimes contaminated with that salt. Tannin
both natural and artificial combines with glue; and with such effect, that one part of
glue dissolved in 5000 parts of water affords a sensible precipitate with the infusion of
nutgalls. Tannin unites with glue in several proportions, which are to each other as
the numbers 1, 11⁄2, and 2; one compound consists of 100 glue and 89 tannin; another
of 100 glue and 60 tannin; and a third of 100 glue and 120 tannin. These two substances[603]
cannot be afterwards separated from each other by any known chemical
process.
Glue may be freed from the foreign animal matters generally present in it, by softening
it in cold water, washing it with the same several times till it no longer gives out
any colour, then bruising it with the hand, and suspending it in a linen bag beneath the
surface of a large quantity of water at 60° F. In this case, the water loaded with the
soluble impurities of the glue gradually sinks to the bottom of the vessel, while the pure
glue remains in the bag surrounded with water. If this softened glue be heated to 92°
without adding water, it will liquefy; and if we heat it to 122°, and filter it, some
albuminous and other impurities will remain on the filter, while a colourless solution of
glue will pass through.
Experiments have not yet explained how gelatine is formed from skin by ebullition.
It is a change somewhat analogous to that of starch into gum and sugar, and takes place
without any appreciable disengagement of gas, and even in close vessels. Gelatine, says
Berzelius, does not exist in the living body, but several animal tissues, such as skin,
cartilages, hartshorn, tendons, the serous membranes, and bones, are susceptible of being
converted into it.
GLUTEN; (Colle Vegetale, Fr.; Kleber, Germ.) was first extracted by Beccaria
from wheat flour, and was long regarded as a proximate principle of plants, till Einhof,
Taddei, and Berzelius, succeeded in showing that it may be resolved by means of alcohol
into three different substances, one of which resembles closely animal albumine, and has
been called Zymome, or vegetable albumine; another has been called Gliadine; and a third
Mucine. The mode of separating gluten from the other constituents of wheat flour, has
been described towards the end of the article Bread.
Gluten when dried in the air or a stove, diminishes greatly in size, becomes hard,
brittle, glistening, and of a deep yellow colour. It is insoluble in ether, in fat and
essential oils, and nearly so in water. Alcohol and acetic acid cause gluten to swell and
make a sort of milky solution. Dilute acids and alkaline lyes dissolve gluten. Its
ultimate constituents are not determined, but azote is one of them, and accordingly
when moist gluten is left to ferment, it exhales the smell of old cheese.
GLYCERINE, is a sweet substance which may be extracted from fatty substances.
If we take equal parts of olive oil, and finely-ground litharge, put them into a basin
with a little water, set this on a sand bath moderately heated, and stir the mixture constantly,
with the occasional addition of hot water to replace what is lost by evaporation,
we shall obtain in a short time, a soap or plaster of lead. After having added more
water to this, we remove the vessel from the fire, decant the liquor, filter it, pass sulphuretted
hydrogen through it to separate the lead, then filter afresh, and concentrate the
liquor as much as is possible without burning upon the sand bath. What remains must
be finally evaporated within the receiver of the air-pump. Glycerine thus prepared is a
transparent liquid, without colour or smell, and of a syrupy consistence. It has a very
sweet taste. Its specific gravity is 1·27 at the temperature of 60°. When thrown upon
burning coals, it takes fire and burns like an oil. Water combines with it in almost all
proportions; alcohol dissolves it readily; nitric acid converts it into oxalic acid; and
according to Vogel, sulphuric acid transforms it into sugar, in the same way as it does
starch. Ferment or yeast does not affect it in any degree.
Its constituents are, carbon 40; hydrogen 9; oxygen 51; in 100.
GNEISS, is the name of one of the great mountain formations, being reckoned the
oldest of the stratified rocks. It is composed of the same substances as granite, viz.
quartz, mica, and felspar. In gneiss however they are not in granular crystals, but in
scales, so as to give the mass a slaty structure. It abounds in metallic treasures.
GOLD. (Eng. and Germ.; Or, Fr.) This metal is distinguished by its splendid
yellow colour; its great density = 19·3, compared to water 1·0; its fusibility at the
32d degree of Wedgewood’s pyrometer; its pre-eminent ductility and malleability,
whence it can be beat into leaves only one 282,000th of an inch thick; and its insolubility
in any acid menstruum, except the mixture of muriatic and nitric acids, styled
by the alchemists aqua regia, because gold was deemed by them to be the king of
metals.
Gold is found only in the metallic state, sometimes crystallized in the cube, and its
derivative forms. It occurs also in threads of various size, twisted and interlaced into
a chain of minute octahedral crystals; as also in spangles or roundish grains, which
when of a certain magnitude are called pepitas. The small grains are not fragments
broken from a greater mass; but they shew by their flattened ovoid shape, and their
rounded outline, that this is their original state. The spec. grav. of native gold varies
from 13·3 to 17·7. Humboldt states that the largest pepita known was one found in
Peru, weighing about 12 kilogrammes (261⁄2 lbs. avoird.); but masses have been quoted
in the province of Quito which weighed nearly four times as much.
Another ore of gold is the alloy with silver, or argental gold, the electrum of Pliny,[604]
so called from its amber shade. It seems to be a definite compound, containing in 100
parts, 64 of gold, and 36 of silver.
The mineral formations in which this metal occurs, are the crystalline primitive
rocks, the compact transition rocks, the trachytic and trap rocks, and alluvial grounds.
It never predominates to such a degree as to constitute veins by itself. It is either
disseminated, and as it were impasted in stony masses, or spread out in thin plates or
grains on their surface, or, lastly, implanted in their cavities, under the shape of filaments
or crystallized twigs. The minerals composing the veins are either quartz, calc-spar,
or sulphate of baryta. The ores that accompany the gold in these veins are chiefly
iron pyrites, copper pyrites, galena, blende, and mispickel (arsenical pyrites.)
In the ores called auriferous pyrites, this metal occurs either in a visible or invisible
form, and though invisible in the fresh pyrites, becomes visible by its decomposition; as
the hydrated oxide of iron allows the native gold particles to shine forth on their reddish-brown
ground, even when the precious metal may constitute only the five millionth
part of its weight, as at Rammelsberg in the Hartz. In that state it has been extracted
with profit; most frequently by amalgamation with mercury, proving that the gold was
in the native state, and not in that of a sulphuret.
Gold exists among the primitive strata, disseminated in small grains, spangles, and
crystals. Brazil affords a remarkable example of this species of gold mine. Beds of
granular quartz, or micaceous specular iron, in the Sierra of Cocäes, 12 leagues beyond
Villa Rica, which form a portion of a mica-slate district, include a great quantity of
native gold in spangles, which in this ferruginous rock replace mica.
Gold has never been observed in any secondary formation, but pretty abundantly in
its true and primary locality, among the trap rocks of igneous origin; implanted on the
sides of the fissures, or disseminated in the veins.
The auriferous ores of Hungary and Transylvania, composed of tellurium, silver
pyrites or sulphuret of silver, and native gold, lie in masses or powerful veins in a rock
of trachyte or in a decomposed felspar subordinate to it. Such is the locality of the
gold ore of Königsberg, of Telkebanya, between Eperies and Tokay in Hungary, and
probably that of the gold ores of Kapnick, Felsobanya, &c., in Transylvania; an
arrangement nearly the same with what occurs in Equatorial America. The auriferous
veins of Guanaxuato, of Real del Monte, of Villalpando, are similar to those of Schemnitz
in Hungary, as to magnitude, relative position, the nature of the ores they include,
and of the rocks they traverse. These districts have impressed all mineralogists with
the evidences of the action of volcanic fire. Breislak and Hacquet have described the
gold mines of Transylvania as situated in the crater of an ancient volcano. It is certain
that the trachytes which form the principal portions of the rocks including gold, are
now almost universally regarded as of igneous or volcanic origin.
It would seem, however, that the primary source of the gold is not in these rocks,
but rather in the sienites and greenstone prophyries below them, which in Hungary
and Transylvania are rich in great auriferous deposits; for gold has never been found
in the trachyte of the Euganean mountains, of the mountains of the Vicentin, of those
of Auvergne; all of which are superposed upon granite rocks, barren in metal.
Finally, if it be true that the ancients worked mines of gold in the island of Ischia, it
would be another example, and a very remarkable one, of the presence of this metal in
trachytes of an origin evidently volcanic.
Gold is, however, much more common in the alluvial grounds than among the
primitive and pyrogenous rocks just described. It is found disseminated under the
form of spangles, in the siliceous, argillaceous, and ferruginous sands of certain plains
and rivers, especially in their re-entering angles, at the season of low water, and after
storms and temporary floods.
It has been supposed that the gold found in the beds of rivers had been torn out
by the waters from the veins and primitive rocks, which they traverse. Some have
even searched, but in vain, at the source of auriferous streams for the native bed of this
precious metal. The gold in them belongs, however, to the grounds washed by the waters
as they glide along. This opinion, suggested at first by Delius, and supported by Deborn,
Guettard, Robitant, Balbo, &c., is founded upon just observations. 1. The soil of these
plains contains frequently, at a certain depth, and in several spots, spangles of gold,
separable by washing. 2. The beds of the auriferous rivers and streamlets contain
more gold after storms of rain upon the plains than in any other circumstances. 3. It
happens almost always that gold is found among the sands of rivers only in a very circumscribed
space; on ascending these rivers their sands cease to afford gold; though
did this metal come from the rocks above, it should be found more abundantly near the
source of the rivers. Thus it is known that the Orco contains no gold except from
Pont to its junction with the Po. The Ticino affords gold only below the Lago Maggiore,
and consequently far from the primitive mountains, after traversing a lake, where
its course is slackened, and into which whatsoever it carried down from these mountains[605]
must have been deposited. The Rhine gives more gold near Strasburg than near
Basle, though the latter be much closer to the mountains. The sands of the Danube
do not contain a grain of gold, while this river runs in a mountainous region; that is,
from the frontiers of the bishoprick of Passau to Efferding; but its sands become auriferous
in the plains below. The same thing is true of the Ems; the sands of the upper
portion of this river, as it flows among the mountains of Styria, include no gold; but
from its entrance into the plain at Steyer, till its embouchure in the Danube, its sands
become auriferous, and are even rich enough to be washed with profit.
The greater part of the auriferous sands, in Europe, Asia, Africa, and America,
are black or red, and consequently ferruginous; a remarkable circumstance in the
geological position of alluvial gold. M. Napione supposes that the gold of these ferruginous
grounds is due to the decomposition of auriferous pyrites. The auriferous
sand occurring in Hungary almost always in the neighbourhood of the beds of lignites,
and the petrified wood covered with gold grains, found buried at a depth of 55 yards in
clay, in the mine of Vorospatak near Abrabanya in Transylvania, might lead us to presume
that the epoch of the formation of the auriferous alluvia is not remote from that
of the lignites. The same association of gold ore and fossil wood occurs in South
America, at Moco. Near the village of Lloro, have been discovered at a depth of 20
feet, large trunks of petrified trees, surrounded with fragments of trap rocks interspersed
with spangles of gold and platinum. But the alluvial soil affords likewise all the characters
of the basaltic rocks; thus in France, the Cèze and the Gardon, auriferous
rivers, where they afford most gold, flow over ground apparently derived from the
destruction of the trap rocks, which occur in situ higher up the country. This fact had
struck Reaumur, and this celebrated observer had remarked that the sand which more
immediately accompanies the gold spangles in most rivers, and particularly in the Rhone
and the Rhine, is composed, like that of Ceylon and Expailly, of black protoxide of
iron and small grains of rubies, corindon, hyacinth, &c. Titanium has been observed
more recently. It has, lastly, been remarked that the gold of alluvial formations is
purer than that extracted from rocks.
Principal Gold Mines.
Spain anciently possessed mines of gold in regular veins, especially in the province of
Asturias; but the richness of the American mines has made them be neglected.
The Tagus, and some other streams of that country, were said to roll over golden sands.
France contains no workable gold mines; but it presents in several of its rivers auriferous
sands. There are some gold mines in Piedmont; particularly the veins of
auriferous pyrites of Macugnagna, at the foot of Monte Rosa, lying in a mountain of
gneiss; and although they do not contain 10 or 11 grains of gold in a hundred weight,
they have long defrayed the expense of working them. On the southern slope of the
Pennine Alps, from the Simplon and Monte Rosa to the valley of Aoste, several auriferous
districts and rivers occur. Such are the torrent Evenson, which has afforded
much gold by washing; the Orco, in its passage from the Pont to the Po; the reddish
grounds over which this little river runs for several miles, and the hills in the neighbourhood
of Chivasso, contain gold spangles in considerable quantity.
In the county of Wicklow, in Ireland, a quartzose and ferruginous sand was discovered
not long ago, containing many particles of gold, with pepitas or solid pieces,
one of which weighed 22 ounces. No less than 1000 ounces of gold were collected.
There are auriferous sands in some rivers of Switzerland, as the Reuss and the Aar.
In Germany no mine of gold is worked, except in the territory of Salzburg, amid the
chain of mountains which separates the Tyrol and Carinthia.
The mines of Hungary and Transylvania are the only gold mines of any importance
in Europe; they are remarkable for their position, the peculiar metals that accompany
them, and their product, estimated at about 1430 pounds avoird. annually. The principal
ones are in Hungary. 1. Those of Konigsberg. The native gold is disseminated
in ores of sulphuret of silver, which occur in small masses and in veins in a decomposing
felspar rock, amid a conglomerate of pumice, constituting a portion of the trachytic
formation. 2. Those of Borson, Schemnitz. And, 3 of Felsobanya; ores also of
auriferous sulphuret of silver, occur in veins of sienite and greenstone porphyry. 4.
Those of Telkebanya, to the south of Kaschau, are in a deposit of auriferous pyrites
amid trap rocks of the most recent formation.
In Transylvania the gold mines occur in veins often of great magnitude, 6, 8, and sometimes
40 yards thick. These veins have no side plates or wall stones, but abut without
intermediate gangues at the primitive rock. They consist of carious quartz, ferriferous
limestone, heavy spar, fluor spar, and sulphuret of silver. The mine of Kapnik deserves
notice, where the gold is associated with orpiment, and that of Vorospatak in granite
rocks; those of Offenbanya, Zalatna, and Nagy-Ag, where it is associated with Tellurium.
The last is in a sienitic rock on the limits of the trachyte.
[606]
In Sweden, the mine of Edelfors in Smoland may be mentioned, where the gold
occurs native and in auriferous pyrites; the veins are a brown quartz, in a mountain of
foliated hornstone.
In Siberia, native gold occurs in a hornstone at Schlangenberg or Zmeof, and at
Zmeino-garsk in the Altai mountains, accompanied with many other ores.
The gold mine of Berezof in the Oural mountains, has been long known, consisting of
partially decomposed auriferous pyrites, disseminated in a vein of greasy quartz. About
1820, a very rich deposit of native gold was discovered upon the eastern side of the Oural
mountains, disseminated at some yards depth, in an argillaceous loam, and accompanied
with the débris of rocks which usually compose the auriferous alluvial soils, as greenstone,
serpentine, protoxide of iron, corundum, &c. The rivers of this district possess auriferous
sands. The annual product of the gold mines of Siberia is 3740 pounds avoirdupois.
In Asia, and especially in its southern districts, there are many mines, streams, rivers,
and wastes, which contain this metal. The Pactolus, a small river of Lydia, rolled over
such golden sands, that it was supposed to constitute the origin of the wealth of Crœsus.
But these deposits are now poor and forgotten. Japan, Formosa, Ceylon, Java, Sumatra,
Borneo, the Philippines, and some other islands of the Indian Archipelago, are supposed
to be very rich in gold mines. Those of Borneo are worked by the Chinese in an
alluvial soil on the western coast, at the foot of a chain of volcanic mountains.
Little or no gold comes into Europe from Asia, because its servile inhabitants place
their fortune in treasure, and love to hoard up that precious metal.
Numerous gold mines occur on the two slopes of the chain of the Cailas mountains
in the Oundès, a province of Little Thibet. The gold lies in quartz veins which traverse
a very crumbling reddish granite.
Africa was, with Spain, the source of the greater portion of the gold possessed by the
antients. The gold which Africa still brings into the market in abundance is always in
dust, showing that the metal is obtained by washing the alluvial soils. None of it is
collected in the north of that continent; three or four districts only are remarkable for
the quantity of gold they produce.
The first mines are those of Kordofan, between Darfour and Abyssinia. The negroes
transport the gold in quills of the ostrich or vulture. These mines seem to have been
known to the antients, who considered Ethiopia to abound in gold. Herodotus relates
that the king of that country exhibited to the ambassadors of Cambyses, all their
prisoners bound with golden chains.
The second and chief exploitation of gold dust is to the south of the great desert of
Zaara, in the western part of Africa, from the mouth of the Senegal to the Cape of
Palms. The gold occurs in spangles, chiefly near the surface of the earth, in the bed of
rivulets, and always in a ferruginous earth. In some places the negroes dig wells in
the soil to a depth of about 40 feet, unsupported by any props. They do not follow any
vein; nor do they construct a gallery. By repeated washings they separate the gold
from the earthy matters.
The same district furnishes also the greater part of what is carried to Morocco, Fez,
and Algiers, by the caravans which go from Timbuctoo on the Niger, across the great
desert of Zaara. The gold which arrives by Sennaar at Cairo and Alexandria, comes from
the same quarter. From Mungo Park’s description, it appears that the gold spangles are
found usually in a ferruginous small gravel, buried under rolled pebbles.
The third spot in Africa where gold is collected, is on the south-east coast, between the
twenty-fifth and the twenty-second degree of south latitude, opposite to Madagascar, in the
country of Sofala. Some persons think that this was the kingdom of Ophir, whence
Solomon obtained his gold.
In modern times, the richest gold mines are found in America, from which there is
exported annually, 3700 or 4000 pounds avoirdupois of this metal. It occurs there
principally in spangles among the alluvial earths, and in the beds of rivers; more rarely
in veins.
There is little gold in the northern part of America. The United States have hitherto
produced but a slight quantity of alluvial gold, collected in the gravel-pits of the creeks
of Rockhole, district of Lebanon, in North Carolina. In 1810, a mass was found there,
weighing 28 pounds. This district has furnished the mint of the United States with
about 100 lbs. avoirdupois of gold.
South America, especially Brazil, Choco, and Chili, are the regions which furnish
most gold.
The gold of Mexico is in a great measure contained in the argentiferous veins, so
numerous in that country, whose principal localities are mentioned under the article
Silver. The silver of the argentiferous ores of Guanaxato, contains one 360th of its
weight of gold; the annual product of the mines being valued at from 2640 to 3300
pounds avoirdupois.
[607]
Oaxaco contains the only auriferous veins exploited as gold mines in Mexico; they
traverse rocks of gneiss and mica slate.
All the rivers of the province of Caracas, to ten degrees north of the line, flow over
golden sands.
Peru is not rich in gold ores. In the provinces of Huailas and Pataz, this metal is
mined in veins of greasy quartz, variegated with red ferruginous spots, which traverse
primitive rocks. The mines called pacos de oro, consist of ores of iron and copper oxides,
containing a great quantity of gold.
All the gold furnished by New Grenada (New Colombia), is the product of washings,
established in alluvial grounds. The gold exists in spangles and in grains, disseminated
among fragments of greenstone and porphyry. At Choco, along with the gold and
platinum, hyacinths, zircons, and titanium occur. There has been found, as already
stated, in the auriferous localities, large trunks of petrified trees. The gold of Antioquia
is 20 carats fine, that of Choco 21, and the largest lump or pepita of gold weighed about
271⁄2 pounds avoirdupois. The gold of Chili also occurs in alluvial formations.
Brazil furnishes the greatest part of the gold now brought into the market. Yet
there is not in this country any gold mine properly so called; for the veins containing
the metal are seldom worked.
It is in the sands of the Mandi, a branch of the Rio-Dolce, at Catapreta, that the auriferous
ferruginous sands were first discovered in 1682. Since then, they have been
found almost everywhere at the foot of the immense chain of mountains, which runs
nearly parallel with the coast, from the 5th degree south to the 30th. It is particularly
near Villa Rica, in the environs of the village Cocäes, that the numerous washings for
gold are established. The pepitas occur in different forms, often adhering to micaceous
specular iron. But in the province of Minas Geräes, the gold occurs also in veins, in
beds, and in grains, disseminated among the alluvial loams. It has been estimated in
annual product, by several authors, at about 2800 pounds avoirdupois of fine metal;
worth nearly a million sterling.
We thus see that almost all the gold brought into the market, comes from alluvial
lands, and is extracted by washing.
The gold coin of the ancients was made chiefly out of alluvial gold, for in these early
times the metallurgic arts were not sufficiently advanced to enable them to purify it. The
gold dust from Bambouk in Africa, is of 221⁄4 carats fine, and some from Morocco is even
23.
The gold of Giron, in New Grenada, is of 233⁄4 carats; being the purest from America.
“For those who traffick in gold,” says Humboldt, “it is sufficient to learn the place
where the metal has been collected, to know its title.”
Metallurgic treatment of gold.—The gold found in the sands of rivers, or in auriferous
soils, needs not be subjected to any metallurgic process, properly speaking. The
Orpaillers, separate it from the sands, by washing them first upon inclined tables,
sometimes covered with a cloth, and then by hand in wooden bowls of a particular form.
Amalgamation is employed to carry off from the sand, the minuter particles of gold they
may contain. The people called Bohemians, Cigans, or Tehinganes, who wash the
auriferous sands in Hungary, employ a plank with 24 transverse grooves cut in its
surface. They hold this plank in an inclined position, and put the sand to be washed
in the first groove; they then throw water on it, when the gold mixed with a little sand
collects usually towards the lowest furrow. They remove this mixture into a flat wooden
basin, and by a peculiar sleight of hand, separate the gold entirely from the sand. The
richest of the auriferous ores consist of the native gold quite visible, disseminated in a
gangue, but the veins are seldom continuous for any length. The other ores are auriferous
metallic sulphurets, such as sulphurets of copper, silver, arsenic, &c., and, particularly
iron.
The stony ores are first ground in the stamping mill, and then washed in hand-basins,
or on wooden tables.
The auriferous sulphurets are much more common, but much poorer than the former
ores; some contain only one 200,000th part of gold, and yet they may be worked with
advantage, when treated with skill and economy.
The gold of these ores is separated by two different processes; namely, by fusion and
amalgamation.
The auriferous metallic sulphurets are first roasted; then melted into mattes, which
are roasted anew; next fused with lead, whence an auriferous lead is obtained, which
may be refined by the process of cupellation.
When the gold ores are very rich, they are melted directly with lead, without preliminary
calcination or fusion. These processes are however little practised, because they
are less economical and certain than amalgamation, especially when the gold ores are
very poor.
If these ores consist of copper pyrites, and if their treatment has been pushed to the point[608]
of obtaining auriferous rose copper, or even black copper including gold, the precious
metal cannot be separated by the process of liquation, because the gold having more
affinity for copper than for lead, can be but partially run off by the latter metal. For
these reasons the process of amalgamation is far preferable.
This process being the same for silver, I shall reserve its description for this metal.
The rich ores in which the native gold is apparent, and merely disseminated in a stony
gangue, are directly triturated with quicksilver, without any preparatory operation. As
to the poor ores, in which the gold seems lost amid a great mass of iron, sulphuret of
copper, &c., they are subjected to a roasting before being amalgamated. This process
seems requisite to lay bare the gold enveloped in the sulphurets. The quicksilver with
which the ore is now ground, seizes the whole of its gold, in however small quantity this
metal may be present.
The gold procured by the refining process with lead, is free from copper and lead, but
it may contain iron, tin, or silver. It cannot be separated from iron and tin without
great difficulty, and expense, if the proportion of gold be too small to admit of the employment
of muriatic acid.
By cupellation with lead, gold may be deprived of any antimony united with it.
Tin gives gold a remarkable hardness and brittleness; a piece of gold, exposed for
some time over a bath of red hot tin, becomes brittle. The same thing happens more
readily over antimony, from the volatility of this metal. A two thousandth part of
antimony, bismuth, or lead, destroys the ductility of gold. The tin may be got rid of
by throwing some corrosive sublimate or nitre into a crucible, containing the melted
alloy. By the first agent, perchloride of tin is volatilized; by the second, stannate of
potash forms, which is carried off in the resulting alkaline scoriæ.
Gold treated by the process of amalgamation, contains commonly nothing but a little
silver. The silver is dissolved out by nitric acid, which leaves the gold untouched;
but to make this parting with success and economy on the great scale, several precautions
must be observed.
If the gold do not contain fully two thirds of its weight of silver, this metal being
thoroughly enveloped by the gold, is partially screened from the action of the acid.
Whenever, therefore, it is known by a trial on a small scale, that the silver is much
below this proportion, we must bring the alloy of gold and silver to that standard by
adding the requisite quantity of the latter metal. This process is called quartation.
This alloy is then granulated or laminated; and from twice to thrice its weight
of sulphuric or nitric acid is to be boiled upon it; and when it is judged that the
solution has been pushed as far as possible by this first acid, it is decanted, and new
acid is poured on. Lastly, after having washed the gold, some sulphuric acid is to be
boiled over it, which carries off a two or three thousandth part of silver, which nitric
acid alone could not dissolve. Thus perfectly pure gold is obtained.
The silver held in solution by the sulphuric or nitric acid is precipitated in the
metallic state by copper, or in the state of chloride by sea-salt. See Parting.
Not only has the ratio between the value of gold and silver varied much in different
ages of the world; but the ratio between these metals and the commodities they represent,
has undergone variations, owing to the circumstances in which their mines have
been successively placed; since they have always poured a greater quantity of the metals
into the market than has been absorbed by use. This quantity has greatly increased
since the discovery of America, a period of little more than 300 years. The mines of
that continent, rich, numerous, and easily worked, by augmenting the mass of gold and
silver, necessarily lessened the value of these metals compared with that of the objects
of commerce represented by them, so that every thing else being equal, there is now
required for purchasing the same quantity of commodities, much more gold or silver
than was necessary in the reign of Henry VII., before the discovery of America. This
productiveness of the American mines has had an influence on those of the ancient
continent; many of whose silver and gold mines have been abandoned, not because the
veins or auriferous sands are less rich than they were; but because their product no
longer represents the value of human labour, and of the goods to be furnished in return
for their exploitation.
In the 3d. vol. of the Mining Journal, p. 331., we have the following statement as to
the produce of the precious metals.—“In 40 years, from 1790 to 1830, Mexico produced
6,436,453l. worth of gold, and 139,818,032l. of silver. Chile, 2,768,488l. of gold, and
1,822,924l. of silver. Buenos Ayres, 4,024,895l. of gold, and 27,182,673l. of silver.
Russia, 3,703,743l. of gold, and 1,502,981l. of silver. Total, 1880 millions sterling, or
47 millions per annum.”
The following table shews what proportion the product of the mines of America
bears to that of the mines of the ancient continent.
[609]
Table of the Quantities of Gold which may be considered as having been brought into
the European Market, every Year on an Average, from 1790 to 1802.
| Continents. |
Gold. |
| Ancient Continent. |
lbs. Avoir. |
| Asia: |
|
| Siberia |
3740 |
| Africa |
3300 |
| Europe: |
|
| Hungary |
1430 |
| Salzbourg |
165 |
| Austrian States |
|
- |
165 |
| Hartz and Hessia |
| Saxony |
| Norway |
| Sweden |
| France |
| Spain, &c. |
| Total of the Ancient Continent |
8800 |
| New Continent. |
|
| North America |
2860 |
| South America: |
|
| Spanish dominions |
22,000 |
| Brazil |
15,400 |
| Total of the New Continent |
40,260 |
The mines of America have sent into Europe three and a half times more gold, and
twelve times more silver, than those of the ancient continent. The total quantity of
silver was to that of gold in the ratio of 55 to 1; a very different ratio from that which
holds really in the value of these two metals, which is in Europe as 1 to 15. This
difference depends upon several causes, which cannot be investigated here at length;
but it may be stated that gold, by its rarity and price, being much less employed in the
arts than silver, the demand for it is also much less; and this cause is sufficient to
lower its price much beneath what it would have been, if it had followed the ratio of its
quantity compared to that of silver. Thus also bismuth, tin, &c., though much rarer
than silver, are, nevertheless, very inferior in price to it. Before the discovery of
America, the value of gold was not so distant from that of silver, because since that era
silver has been distributed in Europe in a far greater proportion than gold. In Asia
the proportion is now actually only 1 to 11 or 12; the product of the gold mines in that
quarter, being not so much below that of the silver mines as in the rest of the
world.
The total annual production of Gold at present has been estimated as follows.
| From |
the ancient Spanish colonies of America |
10,400 |
kilogrammes |
| Brazil |
600 |
|
| Europe and Asiatic Russia |
6,200 |
|
| The Indian Archipelago |
4,700 |
|
| Africa |
14,000 |
? |
| |
35,900 |
= 36 tons nearly |
without taking into account the quantity of gold now extracted from silver.
Gold has less affinity for oxygen than any other metal. When alone, it cannot be
oxidized by any degree of heat with contact of air, although in combination with other
oxidized bodies, it may pass into the state of an oxide, and be even vitrified. The purple
smoke into which gold leaf is converted by an electric discharge is not an oxide,
for it is equally formed when the discharge is made through it in hydrogen gas. There
are two oxides of gold; the first or protoxide is a green powder, which may be obtained
by pouring, in the cold, a solution of potash into a solution of the metallic
chloride. It is not durable, but soon changes in the menstruum into metallic gold,[610]
and peroxide. Its constituents are 96·13 metal, and 3·87 oxygen. The peroxide is
best prepared by adding magnesia to a solution of the metallic chloride; washing the
precipitate with water till this no longer takes a yellow tint from muriatic acid; then
digesting strong nitric acid upon the residuum, which removes the magnesia, and leaves
the peroxide in the form of a black or dark brown powder, which seems to partake more
of the properties of a metallic acid than a base. It contains 10·77 per cent. of
oxygen. For the curious combination of gold and tin, called the Purple Precipitate
of Cassius, see this article, and Pigments Vitrifiable.
Gold beating.—This is the art of reducing gold to extremely thin leaves, by beating
with a hammer. The processes employed for this purpose may be applied to other
metals, as silver, platinum, and copper. Under tin, zinc, &c., we shall mention such
modifications of the processes as these metals require to reduce them to thin leaves.
The Romans used to gild the ceilings and walls of their apartments; and Pliny tells
us, that from an ounce of gold forming a plate of 4 fingers square, about 600 leaves of
the same area were hammered. At the present day, a piece of gold is extended so as
to cover a space 651,590 times greater than its primary surface when cast.
The gold employed in this art ought to be of the finest standard. Alloy hardens
gold, and renders it less malleable; so that the fraudulent tradesman who should attempt
to debase the gold, would expose himself to much greater loss in the operations, than
he could derive of profit from the alloy.
Four principal operations constitute the art of gold beating. 1. The casting of the
gold ingots. 2. The hammering. 3. The lamination; and 4., the beating.
1. The gold is melted in a crucible along with a little borax. When it has become
liquid enough, it is poured out into the ingot-moulds previously heated, and greased on
the inside. The ingot is taken out and annealed in hot ashes, which both soften it and
free it from grease. The moulds are made of cast iron, with a somewhat concave internal
surface, to compensate for the greater contraction of the central parts of the metal
in cooling than the edges. The ingots weigh about 2 ounces each, and are 3⁄4 of an
inch broad.
2. The forging.—When the ingot is cold, the French gold-beaters hammer it out on
a mass of steel 4 inches long and 3 broad. The hammer for this purpose is called the
forging hammer. It weighs about 3 pounds, with a head at one end and a wedge at the
other, the head presenting a square face of 11⁄2 inches. Its handle is 6 inches long. The
workman reduces the ingot to the thickness of 1⁄6 of an inch at most; and during this
Operation he anneals it whenever its substance becomes hard and apt to crack. The
English gold-beaters omit this process of hammering.
3. The lamination.—The rollers employed for this purpose should be of a most perfectly
cylindrical figure, a polished surface, and so powerful as not to bend or yield in
the operation. The ultimate excellence of the gold leaf depends very much on the precision
with which the riband is extended in the rolling press. The laminating machine
represented under the article Mint, is an excellent pattern for this purpose. The gold-beater
desires to have a riband of such thinness that a square inch of it will weigh 61⁄2
grains. Frequent annealings are requisite during the lamination.
4. Beating.—The riband of gold being thus prepared uniform, the gold-beater cuts
it with shears into small squares of an inch each, having previously divided it with compasses,
so that the pieces may be of as equal weight as possible. These squares are piled
over each other in parcels of 150, with a piece of fine calf-skin vellum interposed between
each, and about 20 extra vellums at the top and bottom. These vellum leaves are about 4
inches square, on whose centre lie the gold laminæ of an inch square. This packet is
kept together by being thrust into a case of strong parchment open at the ends, so as to
form a belt or band, whose open sides are covered in by a second case drawn over the
packet at right angles to the first. Thus the packet becomes sufficiently compact to
bear beating with a hammer of 15 or 16 pounds weight, having a circular face nearly 4
inches diameter, and somewhat convex, whereby it strikes the centre of the packet most
forcibly, and thus squeezes out the plates laterally.
The beating is performed on a very strong bench or stool framed to receive a heavy
block of marble, about 9 inches square on the surface, enclosed upon every side by woodwork,
except the front where a leather apron is attached, which the workman lays before
him to preserve any fragments of gold that may fall out of the packet. The hammer
is short-handled, and is managed by the workman with one hand; who strikes fairly on
the middle of the packet, frequently turning it over to beat both sides alike; a feat dexterously
done in the interval of two strokes, so as not to lose a blow. The packet is occasionally
bent or rolled between the hands, to loosen the leaves and secure the ready
extension of the gold; or it is taken to pieces to examine the gold, and to shift the central
leaves to the outside, and vice versa, that every thing may be equalized. Whenever
the gold plates have extended under this treatment, to nearly the size of the vellum,
they are removed from the packet, and cut into four equal squares by a knife. They[611]
are thus reduced to nearly the same size as at first, and are again made up into packets
and enclosed as before, with this difference, that skins prepared from ox-gut are now interposed
between each gold leaf, instead of vellum. The second course of beating is
performed with a smaller hammer, about 10 pounds in weight, and is continued till the
leaves are extended to the size of the skins. During this period, the packet must be often
folded, to render the gold as loose as possible between the membranes; otherwise the leaves
are easily chafed and broken. They are once more spread on a cushion, and subdivided
into four square pieces by means of two pieces of cane cut to very sharp edges, and fixed
down transversely on a board. This rectangular cross being applied on each leaf, with
slight pressure, divides it into four equal portions. These are next made up into a third
packet of convenient thickness, and finally hammered out to the area of fine gold leaf,
whose average size is from 3 to 31⁄2 inches square. The leaves will now have obtained an
area 192 times greater than the plates before the hammering begun. As these were
originally an inch square, and 75 of them weighed an ounce (= 61⁄2 × 75 = 4871⁄2), the
surface of the finished leaves will be 192 × 75 = 14,400 square inches, or 100 square
feet per ounce troy. This is by no means the ultimate degree of attenuation, for an
ounce may be hammered so as to cover 160 square feet; but the waste incident in this
case, from the number of broken leaves, and the increase and nicety of the labour, make
this an unprofitable refinement; while the gilder finds such thin leaves to make less
durable and satisfactory work.
The finished leaves of gold are put up in small books made of single leaves of soft
paper, rubbed over with red chalk to prevent adhesion between them. Before putting
the leaves in these books, however, they are lifted one by one with a delicate pair of pincers
out of the finishing packet, and spread out on a leather cushion by blowing them
flat down. They are then cut to one size, by a sharp-edge square moulding of cane,
glued on a flat board. When this square-framed edge is pressed upon the gold, it cuts
it to the desired size and shape. Each book commonly contains 25 gold leaves.
I shall now describe some peculiarities of the French practice of gold beating. The
workman cuts the laminated ribands of an inch broad into portions an inch and a half
long. These are called quartiers. He takes 24 of them, which he places exactly over
each other, so as to form a thickness of about an inch, the riband being 1⁄2 of a line, or
1⁄24 of an inch thick; and he beats them together on the steel slab with the round face
(panne) of the hammer, so as to stretch them truly out into the square form. He begins
by extending the substance towards the edges, thereafter advancing towards the
middle; he then does as much on the other side, and finally hammers the centre. By
repeating this mode of beating as often as necessary, he reduces at once all the quartiers
(squares) of the same packet, till none of them is thicker than a leaf of gray paper, and
of the size of a square of 2 inches each side.
When the quartiers are brought to this state, the workman takes 56 of them, which
he piles over each other, and with which he forms the first packet (caucher) in the manner
already described; only two leaves of vellum are interposed between each gold leaf.
The empty leaves of vellum at the top and bottom of the packet are called emplures.
They are 4 inches square, as well as the parchment pieces.
The packet thus prepared forms a rectangular parallelopiped; it is enclosed in two
sheathes, composed each of several leaves of parchment applied to each, and glued at
the two sides, forming a bag open at either end.
The block of black marble is a foot square at top, and 18 inches deep, and is framed
as above described. The hammer used for beating the first packet is called the flat,
or the enlarging hammer; its head is round, about 5 inches in diameter, and very slightly
convex. It is 6 inches high, and tapers gradually from its head to the other extremity,
which gives it the form of a hexagonal truncated pyramid. It weighs 14 or 15
pounds.
The French gold-beaters employ besides this hammer, three others of the same form;
namely, 1. The commencing hammer, which weighs 6 or 7 pounds, has a head 4 inches in
diameter, and is more convex than the former. 2. The spreading hammer, (marteau a
chasser); its head is two inches diameter, more convex than the last, and weighs only
4 or 5 pounds. 3. The finishing hammer; it weighs 12 or 13 pounds, has a head four
inches diameter, and is the most convex of all.
The beating processes do not differ essentially from the English described above. The
vellum is rubbed over with fine calcined Paris plaster, with a hare’s foot. The skin of
the gold-beater is a pellicle separated from the outer surface of ox-gut; but before
being employed for this purpose, it must undergo two preparations. 1. It is sweated, in
order to expel any grease it may contain. With this view, each piece of membrane is
placed between two leaves of white paper; several of these pairs are piled over each
other, and struck strongly with a hammer, which drives the grease from the gut into the
paper.
2. A body is given to the pieces of gut; that is, they are moistened with an infusion[612]
of cinnamon, nutmeg, and other warm and aromatic ingredients, in order to preserve
them; an operation repeated after they have been dried in the air. When the leaves of skin
are dry, they are put in a press, and are now ready for use. After the parchment, vellum,
and gut membrane have been a good deal hammered, they become unfit for work, till
they are restored to proper flexibility, by being placed leaf by leaf, between leaves of
white paper, moistened sometimes with vinegar, at others with white wine. They are
left in this predicament for 3 or 4 hours, under compression of a plank loaded with
weights. When they have imbibed the proper humidity, they are put between leaves of
parchment 12 inches square, and beat in that situation for a whole day. They are then
rubbed over with fine calcined gypsum, as the vellum was originally. The gut-skin is
apt to contract damp in standing, and is therefore dried before being used.
The average thickness of common gold leaf is 1⁄282000 of an inch.
The art of Gilding.—This art consists in covering bodies with a thin coat of gold;
which may be done either by mechanical or chemical means. The mechanical mode is
the application of gold leaf or gold powder to various surfaces, and their fixation by
various means. Thus gold may be applied to wood, plaster, pasteboard, leather; and
to metals, such as silver, copper, iron, tin, and bronze; so that gilding generally speaking
includes several arts, exercised by very different classes of tradesmen.
I. Mechanical Gilding.—Oil gilding is the first method under this head, as oil is the
fluid most generally used in the operation of this mechanical art. The following process
has been much extolled at Paris.
1. A coat of impression is to be given first of all, namely, a coat of white lead paint,
made with drying linseed oil, containing very little oil of turpentine.
2. Calcined ceruse is to be ground very well with unboiled linseed oil, and tempered
with essence of turpentine, in proportion as it is laid on. Three or four coats of this
hard tint are to be applied evenly and drily on the ornaments, and the parts which are
to be most carefully gilded.
3. The Gold colour is then to be smoothly applied. This is merely the dregs of the
colours, ground and tempered with oil, which remain in the little dish in which painters
clean their brushes. This substance is extremely rich and gluey; after being ground up,
and passed through fine linen cloth, it forms the ground for gold leaf.
4. When the gold colour is dry enough to catch hold of the leaf gold, this is spread
on the cushion, cut into pieces and carefully applied with the pallet knife, pressed down
with cotton, and in the small ornaments with a fine brush.
5. If the gildings be for outside exposure, as balconies, gratings, statues, &c., they
must not be varnished, as simple oil gilding stands better; for when it is varnished, a
bright sun-beam acting after heavy rain, gives the gilding a jagged appearance. When
the objects are inside ones, a coat of spirit varnish may be passed over the gold leaf, then
a glow from the gilder’s chafing dish may be given, and finally a coat of oil varnish.
The workman who causes the chafing dish to glide in front of the varnished surface,
must avoid stopping for an instant opposite any point, otherwise he would cause the
varnish to boil and blister. This heat brings out the whole transparency of the varnish,
and lustre of the gold.
Oil Gilding is employed with varnish polish, upon equipages, mirror-frames, and other
furniture. The following method is employed by eminent gilders at Paris.
1. White lead, with half its weight of yellow ochre, and a little litharge, are separately
ground very fine; and the whole is then tempered with linseed oil, thinned with essence
of turpentine, and applied in an evenly coat, called impression.
2. When this coat is quite dry, several coats of the hard tint are given, even so many
as 10 or 12, should the surface require it for smoothing and filling up the pores. These
coats are given daily, leaving them to dry in the interval in a warm sunny exposure.
3. When the work is perfectly dry, it is first softened down with pumice stone and
water, afterwards with worsted cloth and very finely powdered pumice, till the hard tint
give no reflection, and be smooth as glass.
4. With a camel’s hair brush, there must be given lightly and with a gentle heat,
from 4 to 5 coats at least, and even sometimes double that number, of fine lac varnish.
5. When these are dry, the grounds of the pannels and the sculptures must be first
polished with shave-grass (de la prèle); and next with putty of tin and tripoli, tempered
with water, applied with woollen cloth; by which the varnish is polished till it shines
like a mirror.
6. The work thus polished is carried into a hot place, free from dust, where it receives
very lightly and smoothly, a thin coat of gold colour, much softened down. This coat is
passed over it with a clean soft brush, and the thinner it is the better.
7. Whenever the gold colour is dry enough to take the gold, which is known by laying
the back of the hand on a corner of the frame work, the gilding is begun and finished
as usual.
8. The gold is smoothed off with a very soft brush, one of camel’s hair for example,
of three fingers’ breadth; after which it is left to dry for several days.
[613]
9. It is then varnished with a spirit of wine varnish; which is treated with the chafing
dish as above described.
10. When this varnish is dry, two or three coats of copal, or oil varnish are applied,
at intervals of two days.
11. Finally, the pannels are polished with a worsted cloth, imbued with tripoli and
water, and lustre is given by friction with the palm of the hand, previously softened
with a little olive oil, taking care not to rub off the gold.
In this country, Burnished gilding is practised by first giving a ground of size whiting,
in several successive coats; next applying gilding size; and then the gold leaf, which is
burnished down with agate, or a dog’s tooth.
Gilding in distemper of the French, is the same as our burnished gilding. Their process
seems to be very elaborate, and the best consists of 17 operations; each of them
said to be essential.
1. Encollage, or the Glue coat. To a decoction of wormwood and garlic in water,
strained through a cloth, a little common salt, and some vinegar are added. This composition,
as being destructive of worms in wood, is mixed with as much good glue; and the
mixture is spread in a hot state, with a brush of boar’s hair. When plaster or marble is to
be gilded, the salt must be left out of the above composition, as it is apt to attract humidity
in damp places, and to come out as a white powder on the gilding. But the salt
is indispensible for wood. The first glue coating is made thinner than the second.
2. White preparation. This consists in covering the above surface, with 8, 10, or 12
coats of Spanish white, mixed up with strong size, each well worked on with the brush,
and in some measure incorporated with the preceding coat, to prevent their peeling off
in scales.
3. Stopping up the pores, with thick whiting and glue, and smoothing the surface
with dog-skin.
4. Polishing the surface with pumice-stone and very cold water.
5. Reparation; in which a skilful artist retouches the whole.
6. Cleansing; with a damp linen rag, and then a soft sponge.
7. Préler. This is rubbing with horse’s tail (shave-grass) the parts to be yellowed,
in order to make them softer.
8. Yellowing. With this view yellow ochre is carefully ground in water, and mixed
with transparent colourless size. The thinner part of this mixture is applied hot over
the white surface with a fine brush, which gives it a fine yellow hue.
9. Ungraining; consists in rubbing the whole work with shave-grass, to remove any
granular appearance.
10. Coat of assiette; trencher coat. This is the composition on which the gold is to
be laid. It is composed of Armenian bole, 1 pound; bloodstone (hematite), 2 ounces;
and as much galena; each separately ground in water. The whole are then mixed together,
and ground up with about a spoonful of olive oil. The assiette well made and applied
gives beauty to the gilding. The assiette is tempered with a white sheepskin glue,
very clear and well strained. This mixture is heated and applied in three successive
coats, with a very fine long-haired brush.
11. Rubbing, with a piece of dry, clean linen cloth; except the parts to be burnished,
which are to receive other two coats of assiette tempered with glue.
12. Gilding. The surface being damped with cold water, (iced in summer) has then
the gold leaf applied to it. The hollow grounds must always be gilded before the prominent
parts. Water is dexterously applied by a soft brush, immediately behind the
gold leaf, before laying it down, which makes it lie smoother. Any excess of water is
then removed with a dry brush.
13. Burnishing, with bloodstone.
14. Deadening. This consists in passing a thin coat of glue slightly warmed, over
the parts that are not to be burnished.
15. Mending; that is moistening any broken points with a brush, and applying bits
of gold leaf to them.
16. The vermeil coat. Vermeil is a liquid which gives lustre and fire to the gold;
and makes it resemble or moulu. It is composed as follows: 2 ounces of annotto, 1
ounce of gamboge, 1 ounce of vermillion, half an ounce of dragon’s blood, 2 ounces of
salt of tartar, and 18 grains of saffron, are boiled in a litre (2 pints English) of water,
over a slow fire, till the liquid be reduced to a fourth. The whole is then passed
through a silk or muslin sieve. A little of this is made to glide lightly over the gold,
with a very soft brush.
17. Repassage; is passing over the dead surfaces a second coat of deadening glue,
which must be hotter than the first. This finishes the work, and gives it strength.
Leaf gilding, on paper or vellum, is done by giving them a coat of gum water or fine
size, applying the gold leaf ere the surfaces be hard dry, and burnishing with agate.
[614]
Gold lettering, on bound books, is given without size, by laying the gold leaf on the
leather, and imprinting it with hot brass types.
The edges of the leaves of books are gilded, while they are in the press, where they have
been cut smooth, by applying a solution of isinglass in spirits, and laying-on the gold
when the edges are in a proper state of dryness. The French workmen employ a ground
of Armenian bole, mixed with powdered sugar-candy, by means of white of egg. This
ground is laid very thin upon the edges, after fine size or gum water has been applied;
and when the ground is dry it is rubbed smooth with a wet rag, which moistens it
sufficiently to take the gold.
Japanners’ gilding is done by sprinkling or daubing with wash leather, some gold
powder, over an oil sized surface, mixed with oil of turpentine. This gives the appearance
of frosted gold. The gold powder may be obtained, either by precipitating gold
from its solution in aqua regia by a solution of pure sulphate of iron, or by evaporating
away the mercury from some gold amalgam.
II. Chemical Gilding, or the application of gold by chemical affinity to metallic surfaces.
A compound of copper with one seventh of brass is the best metal for gilding on;
copper by itself being too soft and dark coloured. Ordinary brass, however, answers very
well. We shall describe the process of wash gilding, with M. D’Arcet’s late improvements,
now generally adopted in Paris.
Wash gilding, consists in applying evenly an amalgam of gold to the surface of a copper
alloy, and dissipating the mercury with heat, so as to leave the gold film fixed. The
surface is afterwards burnished or deadened at pleasure. The gold ought to be quite
pure, and laminated to facilitate its combination with the mercury; which should also
be pure.
Preparation of the amalgam. After weighing the fine gold, the workman puts it in a
crucible, and as soon as this becomes faintly red, he pours in the requisite quantity
of mercury; which is about 8 to 1 of gold. He stirs up the mixture with an iron rod,
bent hookwise at the end, leaving the crucible on the fire till he perceives that all the
gold is dissolved. He then pours the amalgam into a small earthen dish containing water,
washes it with care, and squeezes out of it with his fingers all the running mercury that
he can. The amalgam that now remains on the sloping sides of the vessel is so pasty as
to preserve the impression of the fingers. When this is squeezed in a shamoy leather
bag, it gives up much mercury; and remains an amalgam, consisting of about 33 of mercury,
and 57 of gold, in 100 parts. The mercury which passes through the bag, under
the pressure of the fingers, holds a good deal of gold in solution; and is employed
in making fresh amalgam.
Preparation of the mercurial solution. The amalgam of gold is applied to brass,
through the intervention of pure nitric acid, holding in solution a little mercury.
100 parts of mercury, and 110 parts by weight of pure nitric acid, specific gravity
1·33, are to be put into a glass matrass. On the application of a gentle heat the mercury
dissolves with the disengagement of fumes of nitrous gas, which must be allowed to
escape into the chimney. This solution is to be diluted with about 25 times its weight
of pure water, and bottled up for use.
1. Annealing.—The workman anneals the piece of bronze after it has come out of the
bands of the turner and engraver. He sets it among burning charcoal, or rather peats,
which have a more equal and lively flame; covering it quite up, so that it may be oxidized
as little as possible, and taking care that the thin parts of the piece do not become
hotter than the thicker. This operation is done in a dark room, and when he sees the
piece of a cherry red colour, he removes the fuel from about it, lifts it out with long
tongs, and sets it to cool slowly in the air.
2. The decapage.—The object of this process is to clear the surface from the coat of
oxide which may have formed upon it. The piece is plunged into a bucket filled with
extremely dilute sulphuric acid; it is left there long enough to allow the coat of oxide to
be dissolved, or at least loosened; and it is then rubbed with a hard brush. When the
piece becomes perfectly bright, it is washed and dried. Its surface may however be still
a little variegated; and the piece is therefore dipped in nitric acid, specific gravity 1·33,
and afterwards rubbed with a long-haired brush. The addition of a little common salt
to the dilute sulphuric acid would probably save the use of nitric acid, which is so apt to
produce a new coat of oxide. It is finally made quite dry, (after washing in pure water)
by being rubbed well with tanners’ dry bark, saw-dust, or bran. The surface should
now appear somewhat de-polished; for when it is very smooth, the gold does not adhere
so well.
3. Application of the amalgam.—The gilder’s scratch-brush or pencil, made with
fine brass wire is to be dipped into the solution of nitrate of mercury, and is
then to be drawn over a lump of gold amalgam, laid on the sloping side of an earthen
vessel, after which it is to be applied to the surface of the brass. This process is to be[615]
repeated, dipping the brush into the solution, and drawing it over the amalgam, till the
whole surface to be gilded is coated with its just proportion of gold. The piece is then
washed in a body of water, dried, and put to the fire to volatilize the mercury. If
one coat of gilding be insufficient, the piece is washed over anew with amalgam, and
the operation recommenced till the work prove satisfactory.
4. Volatilization of the mercury.—Whenever the piece is well coated with amalgam,
the gilder exposes it to glowing charcoal, turning it about, and heating it by degrees
to the proper point; he then withdraws it from the fire, lifts it with long pincers, and,
seizing it in his left hand, protected by a stuffed glove, he turns it over in every direction,
rubbing and striking it all the while with a long-haired brush, in order to
equalize the amalgam. He now restores the piece to the fire, and treats it in the same
way till the mercury be entirely volatilized, which he recognises by the hissing sound of
a drop of water let fall on it. During this time he repairs the defective spots, taking
care to volatilize the mercury very slowly. The piece, when thoroughly coated with
gold, is washed, and scrubbed well with a brush in water acidulated with vinegar.
If the piece is to have some parts burnished, and others dead, the parts to be burnished
are covered with a mixture of Spanish white, bruised sugar-candy, and gum dissolved
in water. This operation is called in French epargner (protecting). When the
gilder has protected the burnished points, he dries the piece, and carries the heat high
enough to expel the little mercury which might still remain on it. He then plunges
it, while still a little hot, in water acidulated with sulphuric acid, washes it, dries it,
and gives it the burnish.
5. The burnish is given by rubbing the piece with burnishers of hematite (bloodstone).
The workman dips his burnisher in water sharpened with vinegar, and rubs
the piece always in the same direction backwards and forwards, till it exhibits a fine
polish, and a complete metallic lustre. He then washes it in cold water, dries it with
fine linen cloth, and concludes the operation by drying it slowly on a grating placed
above a chafing dish of burning charcoal.
6. The deadening is given as follows. The piece, covered with the protection on those
parts that are to be burnished, is attached with an iron wire to the end of an iron rod,
and is heated strongly so as to give a brown hue to the epargne by its partial carbonization.
The gilded piece assumes thus a fine tint of gold; and is next coated over with
a mixture of sea salt, nitre, and alum, fused in the water of crystallization of the latter
salt. The piece is now restored to the fire, and heated till the saline crust which covers
it becomes homogeneous, nearly transparent, and enters into true fusion. It is then taken
from the fire and suddenly plunged into cold water, which separates the saline crust,
carrying away even the coat of epargne. The piece is lastly passed through very weak
nitric acid, washed in a great body of water, and dried by exposure either to the air,
over a drying stove, or with clean linen cloths.
7. Of or-moulu colour.—When it is desired to put a piece of gilded bronze into or-moulu
colour, it must be less scrubbed with the scratch-brush than usual, and made to
come back again by heating it more strongly than if it were to be deadened, and allowing
it then to cool a little. The or-moulu colouring is a mixture of hematite, alum, and
sea salt. This mixture is to be thinned with vinegar, and applied with a brush so as to
cover the gilded brass, with reserve of the burnished parts. The piece is then put on
glowing coals, urged a little by the bellows, and allowed to heat till the colour begins
to blacken. The piece ought to be so hot that water sprinkled on it may cause a hissing
noise. It is then taken from the fire, plunged into cold water, washed, and next rubbed
with a brush dipped in vinegar, if the piece be smooth, but if it be chased, weak nitric
acid must be used. In either case, it must be finally washed in a body of pure water,
and dried over a gentle fire.
8. Of red gold colour.—To give this hue, the piece after being coated with amalgam,
and heated, is in this hot state to be suspended by an iron wire, and tempered with the
composition known under the name of gilder’s wax; made with yellow wax, red ochre,
verdigris, and alum. In this state it is presented to the flame of a wood fire, is heated
strongly, and the combustion of its coating is favoured by throwing some drops of the wax
mixture into the burning fuel. It is now turned round and round over the fire, so that
the flame may act equally. When all the wax of the colouring is burned away, and when
the flame is extinguished, the piece is to be plunged in water, washed, and scrubbed
with the scratch-brush and pure vinegar. If the colour is not beautiful, and quite equal
in shade, the piece is coated with verdigris dissolved in vinegar, dried over a gentle fire,
plunged in water, and scrubbed with pure vinegar, or even with a little weak nitric
acid if the piece exhibit too dark a hue. It is now washed, burnished, washed anew,
wiped with linen cloth, and finally dried over a gentle fire.
The following is the outline of a complete, gilding factory, as now fitted up at
Paris.
Fig. 529. Front elevation and plan of a complete gilding workshop.
[616]
P. Furnace of appel, or draught, serving at the same time to heat the deadening pan
(poêlon au mat).
F. Ashpit of this furnace.
N. Chimney of this furnace constructed of bricks, as far as the contraction of the
great chimney S of the forge, and which is terminated by a summit pipe rising 2 or 3
yards above this contraction.
B. Forge for annealing the pieces of bronze; for drying the gilded pieces, &c.
C. Chimney of communication between the annealing forge B, and the space D below
the forge. This chimney serves to carry the noxious fumes into the great vent of the
factory.
U. Bucket for the brightening operation.
A. Forge for passing the amalgam over the piece.
R. Shelf for the brushing operations.
E E. Coal cellarets.
O. Forge for the deadening process.
G. Furnace for the same.
M. An opening into the furnace of appel, by which vapours may be let off from any
operation by taking out the plug at M.
I. Cask in which the pieces of gilded brass are plunged for the deadening process.
The vapours rising thence are carried up the general chimney.
J J. Casement with glass panes, which serves to contract the opening of the hearths,
without obstructing the view. The casement may be rendered movable to admit larger
objects.
H H. Curtains of coarse cotton cloth, for closing at pleasure, in whole or part, one or
several of the forges or hearths, and for quickening the current of air in the places
where the curtains are not drawn.
Q. Opening above the draught furnace, which serves for the heating of the poêlon au
mat (deadening pan).
Gilding on polished iron and steel.—If a nearly neutral solution of gold in muriatic
acid, be mixed with sulphuric ether, and agitated, the ether will take up the gold, and
float above the denser acid. When this auriferous ether is applied by a hair pencil to
brightly polished iron or steel, the ether flies off, and the gold adheres. It must be fixed
by polishing with the burnisher. This gilding is not very rich or durable. In fact the
affinity between gold and iron is feeble, compared to that between gold and copper or
silver. But polished iron, steel, and copper, may be gilded with heat, by gold leaf.
They are first heated till the iron takes a bluish tint, and till the copper has attained to
a like temperature; a first coat of gold leaf is now applied, which is pressed gently[617]
down with a burnisher, and then exposed to a gentle heat. Several leaves either single
or double are thus applied in succession, and the last is burnished down cold.
Cold gilding.—Sixty grains of fine gold and 12 of rose copper are to be dissolved in
two ounces of aqua regia. When the solution is completed, it is to be dropped on clean
linen rags, of such bulk as to absorb all the liquid. They are then dried, and burned
into ashes. These ashes contain the gold in powder.
When a piece is to be gilded, after subjecting it to the preliminary operations of
softening or annealing and brightening, it is rubbed with a moistened cork, dipped in
the above powder, till the surface seems to be sufficiently gilded. Large works are
thereafter burnished with pieces of hematite, and small ones with steel burnishers, along
with soap water.
In gilding small articles, as buttons, with amalgam, a portion of this is taken equivalent
to the work to be done, and some nitrate of mercury solution is added to it in a wooden
trough; the whole articles are now put in, and well worked about with a hard brush,
till their surfaces are equably coated. They are then washed, dried, and put altogether
into an iron frying-pan, and heated till the mercury begins to fly off, when they are turned
out into a cap, in which they are tossed and well stirred about with a painter’s brush.
The operation must be repeated several times for a strong gilding. The surfaces are
finally brightened by brushing them along with small beer or ale grounds.
Gold wire, is formed by drawing a cylindrical rod of the metal as pure as may be,
through a series of holes punched in an iron plate, diminishing progressively in size.
The gold as it is drawn through, becomes hardened by the operation, and requires frequent
annealing.
Gold thread, or spun gold, is a flatted silver-gilt wire, wrapped or laid over a thread of
yellow silk, by twisting with a wheel and iron bobbins. By the aid of a mechanism like
the Braiding Machine, a number of threads may thus be twisted at once by one master
wheel. The principal nicety consists in so regulating the movements that the successive
volutions of the flatted wire on each thread may just touch one another, and form a
continuous covering. The French silver for gilding is said to be alloyed with 5 or 6
pennyweights, and ours with 12 pennyweights of copper in the pound troy. The gold
is applied in leaves of greater or less thickness, according to the quality of the gilt wire.
The smallest proportion formerly allowed in this country by act of parliament, was 100
grains of gold to one pound, or 5760 grains of silver; but more or less may now be used.
The silver rod is encased in the gold leaf, and the compound cylinder is then drawn into
round wire down to a certain size, which is afterwards flatted in a rolling mill such
as is described under Mint.
The liquor employed by goldsmiths to bring out a rich colour upon the surface of their
trinkets, is made by dissolving 1 part of sea-salt, 1 part of alum, 2 parts of nitre, in 3 or
4 of water. This pickle or sauce, as it is called, takes up not only the copper alloy, but
a notable quantity of gold; the total amount of which in the Austrian empire, has been
estimated annually at 47,000 francs. To recover this gold, the liquor is diluted with at
least twice its bulk of boiling water; and a solution of very pure green sulphate of iron
is poured into it. The precipitate of gold is washed upon a filter, dried, and purified by
melting in a crucible along with a mixture of equal parts of nitre and borax.
GONG-GONG; or tam-tam of the Chinese; a kind of cymbal made of a copper
alloy, described towards the end of the article Copper.
GONIOMETER, is the name of a little instrument made either on mechanical or
optical principles, for measuring the angles of crystals. It is indispensable to the mineralogist.
GRADUATOR, called by its contriver M. Wagenmann, Essigbilder, which means
in German, vinegar-maker, is represented fig. 530. It is
an oaken tub, 51⁄2 feet high, 31⁄2 feet wide at top, and 3 at
bottom, set upon wooden beams, which raise its bottom
about 14 inches from the floor. At a distance of 15 inches
above the bottom, the tub is pierced with a horizontal row
of 8 equidistant round holes, of an inch in diameter. At 5
inches beneath the mouth of the tub, a thick beech-wood
hoop is made fast to the inner surface, which supports a
circular oaken shelf, leaving a space round its edge of 11⁄4
inches, which is stuffed water tight with hemp or tow. In
this shelf, 400 holes at least must be bored, about 1⁄8 of an
inch in diameter, and 11⁄2 inches apart; and each of these
must be loosely filled with a piece of packthread, or cotton
wick, which serves to filter the liquid slowly downwards.
In the same shelf there are likewise four larger holes of 11⁄2 inches diameter, and 18
inches apart, each of which receives air-tight a glass tube 3 or 4 inches long, having its
ends projecting above and below the shelf. These tubes serve to allow the air that[618]
enters by the 8 circumferential holes, to circulate freely through the graduator. The
mouth of the tube is covered with a wooden lid, in whose middle is a hole for the insertion
of a funnel, when the liquor of acetification requires to be introduced. One inch
above the bottom, a hole is bored for receiving a syphon-formed discharge pipe, whose
upper curvature stands one inch below the level of the holes in the side of the tub, to
prevent the liquor from rising so high as to overflow through them. The syphon is
so bent as to retain a body of liquor 12 inches deep above the bottom of the tub, and
to allow the excess only to escape into the subjacent receiver. In the upper part
of the graduator, but under the shelf, the bulb of a thermometer is inserted through
the side, some way into the interior, having a scale exteriorly. The whole capacity of
the cask from the bottom up to within one inch of the perforated shelf, is to be filled
with thin shavings of beech wood, grape stalks or birch twigs, previously imbued with
vinegar. The manner of using this simple apparatus, is described under Acetic Acid.
GRANITE, is a compound rock, essentially composed of quartz, felspar, and mica,
each in granular crystals. It constitutes the lowest of the geological formations, and
therefore has been supposed to serve as a base to all the rest. It is the most durable
material for building, as many of the ancient Egyptian monuments testify.
The obelisk in the place of Saint Jean de Lateran at Rome, which was quarried
at Syene, under the reign of Zetus, king of Thebes, 1300 years before the Christian
era; and the one in the place of Saint Pierre, also at Rome, consecrated to the
Sun by a son of Sesostris, have resisted the weather for fully 3000 years. On the
other hand there are many granites, especially those in which felspar predominates,
which crack and crumble down in the course of a few years. In the same mountain, or
even in the same quarry, granites of very different qualities as to soundness and durability
occur. Some of the granites of Cornwall and Limousin readily resolve themselves
into a white kaolin or argillaceous matter, from which pottery and porcelain are made.
Granite when some time dug out of the quarry, becomes refractory, and difficult to
cut. When this rock is intended to be worked it should be kept under water; and that
variety ought to be selected which contains least felspar, and in which the quartz or
gray crystals predominate.
GRANULATION, is the process by which metals are reduced to minute grains.
It is effected by pouring them in a melted state, through an iron cullender pierced
with small holes, into a body of water; or directly upon a bundle of twigs immersed in
water. In this way copper is granulated into bean shot, and silver alloys are granulated
preparatory to Parting; which see.
GRAPHITE; (Plombagine, Fr.; Reissblei, Germ.) is a mineral substance of a lead
or iron gray colour, a metallic lustre, soft to the touch, and staining the fingers with a
lead gray hue. Spec. grav. 2·08 to 2·45. It is easily scratched, or cut with a steel edge,
and displays the metallic lustre in its interior. Burns with great difficulty in the outward
flame of the blow-pipe. It consists of carbon in a peculiar state of aggregation,
with an extremely minute and apparently accidental impregnation of iron. Graphite,
called also plumbago and black lead, occurs in gneiss, mica slate, and their subordinate
clay slates and lime stones; in the form of masses, veins, and kidney-shaped disseminated
pieces; as also in the transition slate, as at Borrodale in Cumberland, where the
most precious deposit exists, both in reference to extent and quality for making pencils.
It has been found also among the coal strata, as near Cumnock in Ayrshire. This
substance is employed for counteracting friction between rubbing surfaces of wood or
metal, for making crucibles and portable furnaces, for giving a gloss to the surface of
cast iron, &c. See Plumbago, for some remarks concerning the Cumberland mine.
GRAUWACKE or GREYWACKE, is a rock formation, composed of pieces of
quartz, flinty slate, felspar and clay slate, cemented by a clay-slate basis; the pieces
varying in size from small grains to a hen’s egg.
GRAY DYE. (Teinture grise, Fr.; Graufarbe, Germ.) The gray dyes in their
numerous shades, are merely various tints of black, in a more or less diluted state, from
the deepest to the lightest hue.
The dyeing materials are essentially the tannic and gallic acid of galls or other
astringents, along with the sulphate or acetate of iron, and occasionally wine stone.
Ash gray is given for 30 pounds of woollen stuff, by one pound of gall-nuts, 1⁄2 lib. of wine
stone (crude tartar), and 21⁄2 libs. of sulphate of iron. The galls and the wine stone being
boiled with from 70 to 80 pounds of water, the stuff is to be turned through the decoction
at a boiling heat for half an hour, then taken out, when the bath being refreshed
with cold water, the copperas is to be added, and, as soon as it is dissolved, the
stuff is to be put in and fully dyed. Or, for 36 pounds of wool; 2 pounds of tartar,
1⁄2 pound of galls, 3 pounds of sumach, and 2 pounds of sulphate of iron are to be
taken. The tartar being dissolved in 80 pounds of boiling water, the wool is to be
turned through the solution for half an hour, and then taken out. The copper being
filled up to its former level with fresh water, the decoction of the galls and sumach is to[619]
be poured in, and the wool boiled for half an hour in the bath. The wool is then taken
out, while the copperas is being added and dissolved; after which it is replaced in the
bath, and dyed gray with a gentle heat.
If the gray is to have a yellow cast, instead of the tartar, its own weight of alum is to be
taken; instead of the galls, one pound of old fustic; instead of the copperas, 3⁄4 of a
pound of Saltzburg vitriol, which consists, in 223⁄8 parts, of 17 of sulphate of iron, and
53⁄8 of sulphate of copper; then proceed as above directed. Or the stuff may be first
stained in a bath of fustic, next in a weak bath of galls with a little alum; then the
wool being taken out, a little vitriol, (common or Saltzburg) is to be put in, previously
dissolved in a decoction of logwood; and in this bath the dye is completed.
Pearl-gray is produced by passing the stuff first through a decoction of sumach and
logwood (2 libs. of the former to one of the latter), afterwards through a dilute solution
of sulphate or acetate of iron; and finishing it in a weak bath of weld containing a
little alum. Mouse-gray is obtained, when with the same proportions as for ash-gray,
a small quantity of alum is introduced.
For several other shades, as tawny-gray, iron-gray, and slate-gray, the stuff must receive
a previous blue ground by dipping it in the indigo vat; then it is passed first
through a boiling bath of sumach with galls, and lastly through the same bath at a
lower temperature after it has received the proper quantity of solution of iron.
For dyeing silk gray, fustet, logwood, sumach, and elder-tree bark, are employed
instead of galls. Archil and annotto are frequently used to soften and beautify the
tint.
The mode of producing gray dyes upon cotton has been sufficiently explained in the
articles Calico Printing and Dyeing.
GREEN DYE is produced by the mixture of a blue and yellow dye, the blue
being first applied. See Dyeing; as also Blue and Yellow Dyes, and Calico
Printing.
GREEN PAINTS. (Couleurs vertes, Fr.; Grüne pigmente, Germ.) Green, which is
so common a colour in the vegetable kingdom, is very rare in the mineral. There is only
one metal, copper, which affords in its combinations the various shades of green in
general use. The other metals capable of producing this colour are, chromium in its
protoxide, nickel in its hydrated oxide, as well as its salts, the seleniate, arseniate, and
sulphate; and titanium in its prussiate.
Green pigments are prepared also by the mixture of yellows and blues; as, for example,
the green of Rinman and of Gellert, obtained by the mixture of cobalt blue, and
flowers of zinc; that of Barth made with yellow lake, prussian blue, and clay; but
these paints seldom appear in the market, because the greens are generally extemporaneous
preparations of the artists.
Mountain green consists of the hydrate, oxide, or carbonate of copper, either factitious,
or as found in nature.
Bremen or Brunswick green is a mixture of carbonate of copper with chalk or lime,
and sometimes a little magnesia or ammonia. It is improved by an admixture of white
lead. It may be prepared by adding ammonia to a mixed solution of sulphate of copper
and alum.
Frise green is prepared with sulphate of copper and sal ammoniac.
Mittis green is an arseniate of copper; made by mixing a solution of acetate or sulphate
of copper with arsenite of potash. It is in fact Scheele’s green.
Sap green is the inspissated juice of buckthorn berries. These are allowed to ferment
for 8 days in a tub, then put in a press, adding a little alum to the juice, and concentrated
by gentle evaporation. It is lastly put up in pigs’ bladders, where it becomes
dry and hard.
Schweinfurt green; see Schweinfurt.
Verona green is merely a variety of the mineral called green earth.
GREEN VITRIOL is sulphate of iron in green crystals.
GUAIAC; (Gaiac, Fr.; Guajaharz, Germ.) is a resin which exudes from the
trunk of the Guaiacum officinale, a tree which grows in the West India islands. It
comes to us in large greenish-brown, semi-transparent lumps, having a conchoidal or
splintery fracture, brittle and easy to pulverize. It has an aromatic smell, a bitterish,
acrid taste, melts with heat, and has a spec. grav. of from 1·20 to 1·22. It consists of
67·88 carbon; 7·05 hydrogen; and 25·07 oxygen; and contains two different resins,
the one of which is soluble in all proportions in ammonia, and the other forms, with
water of ammonia, a tarry consistenced mixture. It is soluble in alkaline lyes, in alcohol,
incompletely in ether, still less so in oil of turpentine, and not at all in fat oils.
Its chief use is in medicine.
GUANO; is a substance of a dark yellow colour; of a strong ambrosial smell;
which blackens in the fire, with the exhalation of an ammoniacal odour; soluble with
effervescence in hot nitric acid. When this solution is evaporated to dryness, it assumes[620]
a fine red colour, evincing the presence of uric acid. Guano is found upon the coasts of
Peru, in the islands of Chinche, near Pisco, and several other places more to the south.
It forms a deposit 50 or 60 feet thick, and of considerable extent; and appears to be
the accumulation of the excrements of innumerable flocks of birds, especially herons
and flamands, which inhabit these islands. It is an excellent manure, and forms the
object of a most extensive and profitable trade.
GUM; (Gomme, Fr.; Gummi, Pflanzenschleim, Germ.) is the name of a proximate
vegetable product, which forms with water a slimy solution, but is insoluble in alcohol,
ether, and oils; it is converted by strong sulphuric acid into oxalic and mucic acids.
There are six varieties of gum: 1. gum arabic; 2. gum senegal; 3. gum of the
cherry and other stone fruit trees; 4. gum tragacanth; 5. gum of Bassora; 6. the gum
of seeds and roots. The first five spontaneously flow from the branches and trunks of
their trees, and sometimes from the fruits, in the form of a mucilage which dries and
hardens in the air. The sixth kind is extracted by boiling water.
Gum arabic and gum senegal consist almost wholly of the purest gum called arabine
by the French chemists; our native fruit trees contain some cerasine, along with arabine;
the gum of Bassora and gum tragacanth consist of arabine and bassorine.
Gum arabic, flows from the acacia arabica, and the acacia vera, which grow upon the
banks of the Nile and in Arabia. It occurs in commerce in the form of small pieces,
rounded upon one side and hollow upon the other. It is transparent, without smell,
brittle, easy to pulverize, sometimes colourless, sometimes with a yellow or brownish
tint. It may be bleached by exposure to the air and the sun-beams, at the temperature of
boiling water. Its specific gravity is 1·355, Moistened gum arabic reddens litmus
paper, owing to the presence of a little supermalate of lime, which may be removed by
boiling alcohol; it shows also traces of the chlorides of potassium and calcium, and the
acetate of potash. 100 parts of good gum, contain 70·40 of arabine, 17·60 of water,
with a few per cents. of saline and earthy matters. Gum arabic is used in medicine, as
also to give lustre to crapes and other silk stuffs.
Gum senegal, is collected by the negroes during the month of November, from the
acacia senegal, a tree 18 or 20 feet high. It comes to us in pieces about the size of a
partridge egg, but sometimes larger, with a hollow centre. Its specific gravity is 1·436.
It consists of 81·10 arabine; 16·10 water; and from 2 to 3 of saline matters. The
chemical properties and uses of this gum are the same as those of gum arabic. It is
much employed in calico-printing.
Cherry-tree gum, consists of 52·10 arabine; 54·90 cerasine; 12 water; and 1 saline
matter.
Gum tragacanth, is gathered about the end of June, from the astragalus tragacantha
of Crete and the surrounding islands. It has the appearance of twisted ribands; is
white or reddish; nearly opaque, and a little ductile. It is difficult to pulverize, without
heating the mortar. Its specific gravity is 1·384. When plunged in water, it
dissolves in part, swells considerably, and forms a very thick mucilage. 100 parts of it
consist of 53·30 arabine; 33·30 bassorine and starch; 11·0 water; and from 2 to 3
parts of saline matters. It is employed in calico printing, and by shoemakers.
Gum of Bassora; see Bassorine.
Gum of seeds, as linseed, consists of 52·70 arabine; 28·9 of an insoluble matter;
10·3 water; and 7·11 saline matter. Neither bassorine nor cerasine seems to be present
in seeds and roots. For British Gum, see Starch.
GUM RESINS. (Gomme-résines, Fr.; Schleimharze, Germ.) When incisions are
made in the stems, branches and roots of certain plants, a milky juice exudes, which
gradually hardens in the air; and appears to be formed of resin and essential oil, held
suspended in water charged with gum, and sometimes with other vegetable matters,
such as caoutchouc, bassorine, starch, wax, and several saline matters. The said concrete
juice is called a gum-resin; an improper name, as it gives a false idea of the nature
of the substance. They are all solid; heavier than water; in general opaque and
brittle; many have an acrid taste, and a strong smell; their colour is very variable.
They are partially soluble in water, and also in alcohol; and the solution in the former
liquid seldom becomes transparent. Almost all the gum resins are medicinal substances,
and little employed in the arts and manufactures. The following is a list of
them: Asa-fœtida; gum ammoniac; bdellium; euphorbium; galbanum; gamboge;
myrrh; olibanum or frankincense; opoponax; and scammony. Some of these are
described in this work under their peculiar names.
GUNPOWDER. The following memoir upon this subject was published by me in
the Journal of the Royal Institution for October, 1830. It contains the results of several
careful analytical experiments, as also of observations made at the Royal Gunpowder
Works at Waltham Abbey, and at some similar establishments in the neighbourhood of
London.
Gunpowder is a mechanical combination of nitre, sulphur, and charcoal; deriving[621]
the intensity of its explosiveness from the purity of its constituents, the proportion in
which they are mixed, and the intimacy of the admixture.
1. On the nitre.—Nitre may be readily purified, by solution in water and crystallization,
from the muddy particles and foreign salts with which it is usually contaminated.
In a saturated aqueous solution of nitre, boiling hot, the temperature is 240° F.; and
the relation of the salt to its solvent is in weight as three to one, by my experiments: not
five to one, as MM. Bottée and Riffault have stated. We must not, however, adopt
the general language of chemists, and say that three parts of nitre are soluble in one of
boiling water, since the liquid has a much higher heat and greater solvent power than
this expression implies.
Water at 60° dissolves only one-fourth of its weight of nitre; or, more exactly, this
saturated solution contains 21 per cent. of salt. Its specific gravity is 1·1415; 100 parts
in volume of the two constituents occupy now 97·91 parts. From these data we may
perceive that little advantage could be gained in refining crude nitre, by making a boiling-hot
saturated solution of it; since on cooling, the whole would concrete into a moist
saline mass, consisting by weight of 23⁄4 parts of salts, mixed with 1 part of water,
holding 1⁄4 of salt in solution, and in bulk of 17⁄8 of salt, with about 1 of liquid; for the
specific gravity of nitre is 2·005, or very nearly the double of water. It is better,
therefore, to use equal weights of saltpetre and water in making the boiling-hot solution.
When the filtered liquid is allowed to cool slowly, somewhat less than three-fourths of
the nitre will separate in regular crystals; while the foreign salts that were present will
remain with fully one-fourth of nitre in the mother liquor. On redissolving these
crystals with heat, in about two-thirds of their weight of water, a solution will result,
from which crystalline nitre, fit for every purpose, will concrete on cooling.
As the principal saline impurity of saltpetre is muriate of soda (a substance scarcely
more soluble in hot than in cold water), a ready mode thence arises of separating that
salt from the nitre in mother waters that contain them in nearly equal proportion.
Place an iron ladle or basin, perforated with small holes, on the bottom of the boiler in
which the solution is concentrating. The muriate, as it separates by the evaporation of
the water, will fall down and fill the basin, and may be removed from time to time.
When small needles of nitre begin to appear, the solution must be run off into the crystallizing
cooler, in which moderately pure nitre will be obtained, to be refined by another
similar operation.
At the Waltham Abbey gunpowder works the nitre is rendered so pure by successive
solutions and crystallizations, that it causes no opalescence in a solution of nitrate of
silver. Such crystals are dried, fused in an iron pot at a temperature of from 500° to
600° F., and cast into moulds. The cakes are preserved in casks.
About the period of 1794 and 1795, under the pressure of the first wars of their
revolution, the French chemists employed by the government contrived an expeditious,
economical, and sufficiently effective mode of purifying their nitre. It must be observed
that this salt, as brought to the gunpowder-works in France, is in general a much cruder
article than that imported into this country from India. It is extracted from the
nitrous salts contained in the mortar-rubbish of old buildings, especially those of the
lowest and filthiest descriptions. By their former methods, the French could not refine
their nitre in less time than eight or ten days; and the salt was obtained in great lumps,
very difficult to dry and divide; whereas the new process was so easy and so quick, that
in less than twenty-four hours, at one period of pressure, the crude saltpetre was converted
into a pure salt, brought to perfect dryness, and in such a state of extreme division
as to supersede the operations of grinding and sifting, whence also considerable waste
was avoided.
The following is a brief outline of this method, with certain improvements, as now
practised in the establishment of the Administration des poudres et salpêtres, in France.
The refining boiler is charged over night with 600 kilogrammes of water, and 1200
kilogrammes of saltpetre, as delivered by the salpêtriers. No more fire is applied than
is adequate to effect the solution of this first charge of saltpetre. It may here be observed,
that such an article contains several deliquescent salts, and is much more soluble than
pure nitre. On the morrow morning the fire is increased, and the boiler is charged at
different intervals with fresh doses of saltpetre, till the whole amounts to 3000 kilogrammes.
During these additions, care is taken to stir the liquid very diligently, and
to skim off the froth as it rises. When it has been for some time in ebullition, and
when it may be presumed that the solution of the nitrous salts is effected, the muriate of
soda is scooped out from the bottom of the boiler, and certain affusions or inspersions of
cold water are made into the pot, to quicken the precipitation of that portion which the
boiling motion may have kept afloat. When no more is found to fall, one kilogramme
of Flanders glue, dissolved in a sufficient quantity of hot water, is poured into the boiler;
the mixture is thoroughly worked together, the froth being skimmed off, with several
successive inspersions of cold water, till 400 additional kilogrammes have been introduced,
constituting altogether 1000 kilogrammes.
[622]
When the refining liquor affords no more froth, and is grown perfectly clear, all
manipulation must cease. The fire is withdrawn, with the exception of a mere kindling,
so as to maintain the temperature till the next morning at about 88° C. = 190·4 F.
This liquor is now transferred by hand-basins into the crystallizing reservoirs, taking
care to disturb the solution as little as possible, and to leave untouched the impure
matter at the bottom. The contents of the long crystallizing cisterns are stirred backwards
and forwards with wooden paddles, in order to quicken the cooling, and the
consequent precipitation of the nitre in minute crystals. These are raked as soon as they
fall, to the upper end of the doubly-inclined bottom of the crystallizer, and thence removed
to the washing chests or boxes. By the incessant agitation of the liquor, no large
crystals of nitre can possibly form. When the temperature has fallen to within 7° or 8°
F., of the apartment, that is, after seven or eight hours, all the saltpetre that it can yield
will have been obtained. By means of the double inward slope given to the crystallizer,
the supernatant liquid is collected in the middle of the breadth, and may be easily laded
out.
The saltpetre is shovelled out of the crystallizer into the washing chests, and heaped
up in them so as to stand about six or seven inches above their upper edges, in order to
allow for the subsidence which it must experience in the washing process. Each
of these chests being thus filled, and their bottom holes being closed with plugs, the salt
is besprinkled from the rose of a watering-can, with successive quantities of water
saturated with saltpetre, and also with pure water, till the liquor, when allowed to run
off, indicates by the hydrometer, a saturated solution. The water of each sprinkling
ought to remain on the salt for two or three hours; and then it may be suffered to drain
off through the plug-holes below, for about an hour.
All the liquor of drainage from the first watering, as well as a portion of the second,
is set aside, as being considerably loaded with the foreign salts of the nitre, in order to
be evaporated in the sequel with the mother waters. The last portions are preserved,
because they contain almost nothing but nitre, and may therefore serve to wash another
dose of that salt. It has been proved by experience, that the quantity of water employed
in washing need never exceed thirty-six sprinklings in the whole, composed of three
waterings, of which the first two consist of fifteen, and the last of six pots = 3 gallons E.;
or in other words, of fifteen sprinklings of water saturated with saltpetre, and twenty-one
of pure water.
The saltpetre, after remaining five or six days in the washing chests, is transported into
the drying reservoirs, heated by the flue of the nearest boiler; here it is stirred up from
time to time with wooden shovels, to prevent its adhering to the bottom, or running
into lumps, as well as to quicken the drying process. In the course of about four hours,
it gets completely dry, in which state it no longer sticks to the shovel, but falls down
into a soft powder by pressure in the hand, and is perfectly white and pulverulent. It
is now passed through a brass sieve, to separate any small lumps or foreign particles
accidentally present, and is then packed up in bags or barrels. Even in the shortest
winter days, the drying basin may be twice charged, so as to dry 700 or 800 kilogrammes.
By this operation, the nett produce of 3000 kilogrammes (3 tons) thus
refined, amounts to from 1750 to 1800 kilogrammes of very pure nitre, quite ready
for the manufacture of gunpowder.
The mother waters are next concentrated; but into their management it is needless
to enter in this memoir.
On reviewing the above process as practised at present, it is obvious that, to meet the
revolutionary crisis, its conductors must have shortened it greatly, and have been content
with a brief period of drainage.
2. On the sulphur.—The sulphur now imported into this country, from the volcanic
districts of Sicily and Italy, for our manufactories of sulphuric acid, is much purer than
the sulphur obtained by artificial heat from any varieties of pyrites, and may, therefore,
by simple processes, be rendered a fit constituent of the best gunpowder. As it not my
purpose here to repeat what may be found in common chemical compilations, I shall say
nothing of the sublimation of sulphur; a process, moreover, much too wasteful for the
gunpowder-maker.
Sulphur may be most easily analyzed, even by the manufacturer himself; for I find it
to be soluble in one tenth of its weight of boiling oil of turpentine, at 316° Fahrenheit,
forming a solution which remains clear at 180°. As it cools to the atmospheric temperature,
beautiful crystalline needles form, which may be washed sufficiently with cold
alcohol, or even tepid water. The usual impurities of the sulphur, which are carbonate
and sulphate of zinc, oxide and sulphuret of iron, sulphuret of arsenic and silica, will
remain unaffected by the volatile oil, and may be separately eliminated by the curious,
though such separation is of little practical importance.
Two modes of refining sulphur for the gunpowder works have been employed; the
first is by fusion, the second by distillation. Since the combustible solid becomes as[623]
limpid as water, at the temperature of about 230° Fahrenheit, a ready mode offers of
removing at once its denser and lighter impurities, by subsidence and skimming. But
I may take the liberty of observing, that the French melting pot, as described in the
elaborate work of MM. Bottée and Riffault, is singularly ill-contrived, for the fire is
kindled right under it, and plays on its bottom. Now a pot for subsidence ought to be
cold set; that is, should have its bottom part imbedded in clay or mortar for four or six
inches up the side, and be exposed to the circulating flame of the fire only round its
middle zone. This arrangement is adopted in many of our great chemical works, and
is found to be very advantageous. With such a boiler, judiciously heated, I believe
that crude sulphur might be made remarkably pure; whereas by directing the heat
against the bottom of the vessel, the crudities are tossed up, and incorporated with
the mass. See Evaporation.
The sulphur of commerce occurs in three prevailing colours; lemon yellow verging
on green, dark yellow, and brown yellow. As these different shades result from the
different degrees of heat to which it has been exposed in its original extraction on the
great scale, we may thereby judge to what point it may still be heated anew in the
refinery melting. Whatever be the actual shade of the crude article, the art of the
refiner consists in regulating the heat, so that after the operation it may possess a
brilliant yellow hue, inclining somewhat to green.
In seeking to accomplish this purpose, the sulphur should first be sorted according to
its shades; and if a greenish variety is to be purified, since this kind has been but little
heated in its extraction, the fusion may be urged pretty smartly, or the fire may be
kept up till every thing is melted but the uppermost layer.
Sulphur of a strong yellow tinge cannot bear so great a heat, and therefore the fire
must be withdrawn whenever three fourths of the whole mass have been melted.
Brown-coloured brimstone, having been already somewhat scorched, should be heated
as little as possible, and the fire may be removed as soon as one half of the mass
is fused.
Instead of melting, separately, sulphurs of different shades, we shall obtain a better result
by first filling up the pot to half its capacity, with the greenish-coloured article, putting
over this layer one quarter volume of the deep yellow, and filling it to the brim with
the brown-coloured. The fire must be extinguished as soon as the yellow is fused.
The pot must then be closely covered for some time; after which the lighter impurities
will be found on the surface in a black froth, which is skimmed off, and the heavier ones
sink to the bottom. The sulphur itself must be left in the pot for ten or twelve hours,
after which it is laded out into the crystallizing boxes or casks.
Distillation affords a more complete and very economical means of purifying sulphur,
which was first introduced into the French gunpowder establishments, when their
importation of the best Italian and Sicilian sulphur was obstructed by the British
navy. Here the sulphur need not come over slowly in a rare vapour, and be deposited
in a pulverulent form called flowers; for the only object of the refiner is to bring over
the whole of the pure sulphur into his condensing chamber, and to leave all its crudities
in the body of the still. Hence a strong fire is applied to elevate a denser mass of
vapours, of a yellowish colour, which passing over into the condenser, are deposited in
a liquid state on its bottom, whilst only a few lighter particles attach themselves to the
upper and lateral surfaces. The refiner must therefore give to the heat in this operation
very considerable intensity; and, at some height above the edge of the boiler, he
should provide an inclined plane, which may let the first ebullition of the sulphur overflow
into a safety recipient. The condensing chamber should be hot enough to maintain
the distilled sulphur in a fluid state,—an object most readily procured by leading
the pipes of several distilling pots into it; while the continuity of the operations is
secured, by charging each of the stills alternately, or in succession. The heat of the
receiver must be never so high as to bring the sulphur to a syrupy consistence, whereby
its colour is darkened.
In the sublimation of sulphur, a pot containing about four cwt. can be worked off
only once in twenty-four hours, from the requisite moderation of its temperature, and
the precaution of an inclined plane, which restores to it the accidental ebullitions.
But, by distillation, a pot containing fully ten cwt. may complete one process in nine
hours at most, with a very considerable saving of fuel. In the former plan of procedure,
an interval must elapse between the successive charges; but in the latter, the
operation must be continuous to prevent the apparatus from getting cooled: in sublimation,
moreover, where communication of atmospheric air to the condensing chamber
is indispensable, explosive combustions of the sulphurous vapours frequently occur,
with a copious production of sulphurous acid, and correspondent waste of the sulphur;
disadvantages from which the distillatory process is in a great measure exempt.
I shall here describe briefly the form and dimensions of the distilling apparatus
employed at Marseilles in purifying sulphur for the national gunpowder works, which[624]
was found adequate to supply the wants of Napoleon’s great empire. This apparatus
consists of only two still-pots of cast iron, formed like the large end of an egg, each
about three feet in diameter, two feet deep, and nearly half an inch thick at the bottom,
but much thinner above, with a horizontal ledge four inches broad. A pot of good
cast iron is capable of distilling 1000 tons of sulphur before it is rendered unserviceable,
by the action of the brimstone on its substance, aided by a strong red heat. The pot is
covered in with a sloping roof of masonry, the upper end of which abuts on the brickwork
of the vaulted dome of condensation. A large door is formed in the masonry in front
of the mouth of the pot, through which it is charged and cleared out; and between the
roof-space over the pot, and the cavity of the vault, a large passage is opened. At the
back of the pot a stone-step is raised to prevent the sulphur boiling over into the
condenser. The vault is about ten feet wide within, and fourteen feet from the bottom
up to the middle of the dome, which is perforated, and carries a chimney about twelve
feet high, and twelve feet diameter inside.
As the dome is exposed to the expansive force of a strong heat, and to a very considerable
pressure of gases and vapours, it must possess great solidity, and be therefore
bound with iron straps. Between the still and the contiguous wall of the condensing
chamber, a space must be left for the circulation of air; a precaution found by experience
indispensable; for the contact of the furnaces would produce on the wall of the chamber
such a heat as to make it crack and form crevices for the liquid sulphur to escape.
The sides of the chamber are constructed of solid masonry, forty inches thick, surmounted
by a brick dome, covered with a layer of stones. The floor is paved with
tiles, and the walls are lined with them up to the springing of the dome; a square
hole being left in one side, furnished with a strong iron door, at which the liquid
sulphur is drawn off at proper intervals. In the roof of the vault are two valve-holes
covered with light plates of sheet-iron, which turn freely on hinges at one end, so as
to give way readily to any sudden expansion from within, and thus prevent dangerous
explosions.
As the chamber of condensation is an oblong square, terminating upwards in an oblong
vault, it consists of a parallelopiped below, and semi-cylinder above, having the following
dimensions:—
| Length of the parallelopiped |
161⁄2 |
feet. |
| Width |
104⁄5 |
| Height |
71⁄4 |
| Radius of the cylinder |
52⁄5 |
| Height or length of semi-cylinder |
161⁄2 |
Whenever the workman has introduced into each pot its charge of ten or twelve
hundred weight of crude sulphur, he closes the charging doors carefully with their iron
plates and cross-bars, and lutes them tight with loam. He then kindles his fire, and
makes the sulphur boil. One of his first duties (and the least neglect in its discharge
may occasion serious accidents) is to inspect the roof-valves and to clean them, so that
they may play freely and give way to any expulsive force from within. By means of a cord
and chain, connected with a crank attached to the valves, he can, from time to time, ascertain
their state, without mounting on the roof. It is found proper to work one of the
pots a certain time before fire is applied to the other. The more steadily vapours of
sulphur are seen to issue from the valves, the less atmospherical air can exist in the
chamber, and therefore the less danger there is of combustion. But if the air be cold,
with a sharp north wind, and if no vapours be escaping, the operator should stand on
his guard, for in such circumstances a serious explosion may ensue.
As soon as both the boilers are in full work the air is expelled, the fumes cease, and
every hazard is at an end. He should bend his whole attention to the cutting off all communication
with the atmosphere, securing simply the mobility of the valves, and a steady
vigour of distillation. The conclusion of the process is ascertained by introducing his
sounding-rod into the pot, through a small orifice made for its passage in the wall. A
new charge must then be given.
By the above process, well conducted, sulphurs are brought to the most perfect state
of purity that the arts can require; while not above four parts in the hundred of the sulphur
itself are consumed; the crude, incombustible residuum varying from five to eight
parts, according to the nature of the raw material. But in the sublimation of sulphur, the
frequent combustions inseparable from this operation carry the loss of weight in flowers to
about twenty per cent. See Sulphur, for a figure of the subliming apparatus.
The process by fusion, performed at some of the public works in this country, does
not afford a return at all comparable with that of the above French process, though a
much better article is operated upon in England. After two meltings of rough sulphur
(as imported from Sicily or Italy), eighty-four per cent. is the maximum amount[625]
obtained, the average being probably under eighty; while the product is certainly inferior
in quality to that by distillation.
3. On the charcoal.—Tender and light woods, capable of affording a friable and
porous charcoal, which burns rapidly away, leaving the smallest residuum of ashes, and
containing therefore the largest proportion of carbon, ought to be preferred for charring
in gunpowder-works.
After many trials made long ago, black dogwood came to be preferred to every plant
for this purpose; but modern experiments have proved that many other woods afford an
equally suitable charcoal. The woods of black alder, poplar, lime-tree, horse-chesnut
and chesnut-tree, were carbonized in exactly similar circumstances, and a similar gunpowder
was made with each, which was proved by the same proof-mortar. The following
results were obtained:—
| |
Toises. |
Feet. |
| Poplar—mean range |
113 |
2 |
| Black alder |
110 |
4 |
| Lime |
110 |
3 |
| Horse-chesnut |
110 |
3 |
| Chesnut-tree |
109 |
|
By subsequent experiments confirmatory of the above, it has been further found that
the willow presents the same advantages as the poplar, and that several shrubs, such as
the hazel-nut, the spindle-tree, the dogberry, the elder-tree, the common sallow, and
some others, may be as advantageously employed. But whichever wood be used, we should
always cut it when full of sap, and never after it is dead; we should choose branches not
more than five or six years old, and strip them carefully, because the old branches and
the bark contain a larger proportion of earthy constituents. The branches ought not
to exceed three-quarters of an inch in thickness, and the larger ones should be divided
lengthwise into four, so that their pith may be readily burned away.
Wood is commonly carbonized in this country into gunpowder-charcoal in cast-iron
cylinders, with their axes laid horizontally, and built in brick-work, so that the flame of
a furnace may circulate round them. One end of the cylinder is furnished with a door,
for the introduction of the wood and the removal of the charcoal; the other end terminates
in a pipe, connected with a worm-tub for condensing the pyrolignous acid, and
giving vent to the carburetted hydrogen gases that are disengaged. Towards the end
of the operation, the connexion of the cylinder with the pyrolignous acid cistern ought
to be cut off, and a very free egress opened for the volatile matter, otherwise the
charcoal is apt to get coated with a fuliginous varnish, and to be even penetrated with
condensable matter, which materially injure its qualities.
In France, the wood is carbonized for the gunpowder works either in oblong vaulted
ovens, or in pits, lined with brick-work or cylinders of strong sheet-iron. In either
case, the heat is derived from the imperfect combustion of the wood itself to be charred.
In general, the product in charcoal by the latter method is from 16 to 17 parts by weight
from 100 of wood. The pit-process is supposed to afford a more productive return, and
a better article; since the body of wood is much greater, and the fuliginous vapours are
allowed a freer escape. The surface of a good charcoal should be smooth, but not
glistening. See Charcoal.
The charcoal is considered by the scientific manufacturers to be the ingredient most
influential, by its fluctuating qualities, upon the composition of gunpowder; and,
therefore, it ought always to be prepared under the vigilant and skilful eye of the
director of the powder establishment. If it has been kept for some time, or quenched
at first with water, it is unsuitable for the present purpose. Charcoal extinguished in a
close vessel by exclusion of air, and afterwards exposed to the atmosphere, absorbs only
from three to four per cent. of moisture, while red-hot charcoal quenched with water
may lose by drying twenty-nine per cent. When the latter sort of charcoal is used for
gunpowder, a deduction of weight must be made for the water present. But charcoal
which has remained long impregnated with moisture, constitutes a most detrimental
ingredient of gunpowder.
4. On Mixing the Constituents and forming the Powder.
The three ingredients thus prepared are ready for manufacturing into gunpowder.
They are, 1. Separately ground to a fine powder, which is passed through sorted silk
sieves or bolting machines; 2. They are mixed together in the proper proportions,
which we shall afterwards discuss; 3. The composition is then sent to the gunpowder[626]
mill, which consists of two edge-stones of a calcareous kind, turning by means of a
horizontal shaft, on a bed-stone of the same nature; incapable of affording sparks by
collision with steel, as sand-stones would do. On this bed-stone the composition is
spread, and moistened with as small a quantity of water as will, in conjunction with the
weight of the revolving stones, bring it into a proper body of cake, but by no means into
a pasty state. The line of contact of the rolling edge-stone is constantly preceded by a
hard copper scraper, which goes round with the wheel, regularly collecting the caking
mass, and bringing it into the track of the stone. From 50 to 60 pounds of cake are
usually worked at one operation, under each millstone. When the mass has been
thoroughly kneaded and incorporated, it is sent to the corning-house, where a separate
mill is employed to form the cake into grains or corns. Here it is first pressed into a hard
firm mass, then broken into small lumps; after which the corning process is performed,
by placing these lumps in sieves, on each of which is laid a disc or flat cake of lignum vitæ.
The sieves are made of parchment skins, or of copper, perforated with a multitude of
round holes. Several such sieves are fixed in a frame, which, by proper machinery, has
such a motion given to it as to make the lignum vitæ runner in each sieve move about
with considerable velocity, so as to break down the lumps of the cake, and force its substance
through the holes, in grains of certain sizes. These granular particles are afterwards
separated from the finer dust by proper sieves and reels.
The corned powder must now be hardened, and its rougher angles removed, by
causing it to revolve in a close reel or cask turning rapidly round its axis. This vessel
resembles somewhat a barrel-churn, and is frequently furnished inside with square bars
parallel to its axis, to aid the polish by attrition.
The gunpowder is finally dried, which is now done generally with a steam heat, or in
some places by transmitting a current of air, previously heated in another chamber, over
canvas shelves, covered with the damp grains.
5. On the proportion of the Constituents.
A very extensive suite of experiments, to determine the proportions of the constituents
for producing the best gunpowder, was made at the Essonne works, by a commission of
French chemists and artillerists, in 1794.
Powders in the five following proportions were prepared:—
| |
Nitre. |
Charcoal. |
Sulphur. |
|
| 1 |
76 |
|
14 |
|
10 |
|
Gunpowder of Bâle. |
| 2 |
76 |
|
12 |
|
12 |
|
Gunpowder works of Grenelle. |
| 3 |
76 |
|
15 |
|
9 |
|
M. Guyton de Morveau. |
| 4 |
77 |
·32 |
13 |
·44 |
9 |
·24 |
Idem. |
| 5 |
77 |
·5 |
15 |
|
7 |
·5 |
M. Riffault. |
The result of more than two hundred discharges with the proof-mortar shewed that
the first and third gunpowders were the strongest; and the commissioners in consequence
recommended the adoption of the third proportions. But a few years thereafter
it was thought proper to substitute the first set of proportions, which had been found
equal in force to the other, as they would have a better keeping quality, from containing
a little more sulphur and less charcoal. More recently still, so strongly impressed have
the French government been with the high value of durability in gunpowders, that they
have returned to their ancient dosage of 75 nitre, 121⁄2 charcoal, and 121⁄2 sulphur. In
this mixture, the proportion of the substance powerfully absorbent of moisture, viz. the
charcoal, is still further reduced, and replaced by the sulphur, or the conservative ingredient.
If we inquire how the maximum gaseous volume is to be produced from the chemical
reaction of the elements of nitre on charcoal and sulphur, we shall find it to be by the
generation of carbonic oxide and sulphurous acid, with the disengagement of nitrogen.
This will lead us to the following proportions of these constituents:—
| |
Hydrogen = 1. |
Per cent. |
| 1 |
prime equivalent of |
nitre |
102 |
75·00 |
| 1 |
... |
sulphur |
16 |
11·77 |
| 3 |
... |
charcoal |
18 |
13·23 |
| |
136 |
100·00 |
[627]
The nitre contains five primes of oxygen, of which three, combining with the three of
charcoal, will furnish three of carbonic oxide gas, while the remaining two will convert
the one prime of sulphur into sulphurous acid gas. The single prime of nitrogen is,
therefore, in this view, disengaged alone.
The gaseous volume, on this supposition, evolved from 136 grains of gunpowder,
equivalent in bulk to 751⁄2 grains of water, or to three-tenths of a cubic inch, will be, at
the atmospheric temperature, as follows:—
| |
Grains. |
|
Cubic Inches. |
| Carbonic oxide |
42 |
= |
141·6 |
| Sulphurous acid |
32 |
= |
47·2 |
| Nitrogen |
14 |
= |
47·4 |
| |
|
236·2 |
being an expansion of one volume into 787·3. But as the temperature of the gases at
the instant of their combustive formation must be incandescent, this volume may be
safely estimated at three times the above amount, or considerably upwards of two
thousand times the bulk of the explosive solid.
But this theoretical account of the gases developed does not well accord with the
experimental products usually assigned, though these are probably not altogether exact.
Much carbonic acid is said to be disengaged, a large quantity of nitrogen, a little
oxide of carbon, steam of water, with carburetted and sulphuretted hydrogen. From
experiments to be presently detailed, I am convinced that the amount of these latter
products printed in italics must be very inconsiderable indeed, and unworthy of ranking
in the calculation; for, in fact, fresh gunpowder does not contain above one per cent. of
water, and can therefore yield little hydrogenated matter. Nor is the hydrogen in the
carbon of any consequence.
It is obvious that the more sulphur is present, the more of the dense sulphurous acid
will be generated, and the less forcibly explosive will be the gunpowder. This is sufficiently
confirmed by the trials at Essonne, where the gunpowder that contained 12 of
sulphur and 12 of charcoal in 100 parts, did not throw the proof-shell so far as that
which contained only 9 of sulphur and 15 of charcoal. The conservative property is,
however, so capital, especially for the supply of our remote colonies and for humid
climates, that it justifies a slight sacrifice of strength, which at any rate may be compensated
by a small addition of charge.
Table of Composition of different Gunpowders.
| |
Nitre. |
Charcoal. |
Sulphur. |
| Royal Mills at Waltham Abbey |
75 |
|
15 |
|
10 |
|
| France, national establishment |
75 |
|
12 |
·5 |
12 |
·5 |
| French, for sportsmen |
78 |
|
12 |
|
10 |
|
| French, for mining |
65 |
|
15 |
|
20 |
|
| United States of America |
75 |
|
12 |
·5 |
12 |
·5 |
| Prussia |
75 |
|
13 |
·5 |
11 |
·5 |
| Russia |
73 |
·78 |
13 |
·59 |
12 |
·63 |
| Austria (musquet) |
72 |
|
17 |
|
16 |
|
| Spain |
76 |
·47 |
10 |
·78 |
12 |
·75 |
| Sweden |
76 |
|
15 |
|
9 |
|
| Switzerland (a round powder) |
76 |
|
14 |
|
10 |
|
| Chinese |
75 |
|
14 |
·4 |
9 |
·9 |
| Theoretical proportions (as above) |
75 |
|
13 |
·23 |
11 |
·77 |
6. On the Chemical Examination of Gunpowders.
I have treated five different samples: 1. The government powder made at Waltham
Abbey; 2. Glass gunpowder made by John Hall, Dartford; 3. The treble strong gunpowder
of Charles Lawrence and Son; 4. The Dartford gunpowder of Pigou and
Wilks; 5. Superfine treble strong sporting gunpowder of Curtis and Harvey. The
first is coarse-grained, the others are all of considerable fineness. The specific gravity
of each was taken in oil of turpentine: that of the first and last three was exactly the
same, being 1·80; that of the second was 1·793, all being reduced to water as unity.
[628]
The above density for specimen first, may be calculated thus:—
| 75 parts of nitre, specific gravity |
= |
2·000 |
| 15 parts of charcoal, specific gr. |
= |
1·154 |
| 10 parts of sulphur, specific gr. |
= |
2·000 |
The volume of these constituents is 55·5, (the volume of their weight of water being
100;) by which if their weight 100 be divided, the quotient is 1·80.
The specific gravity of the first and second of the above powders, including the interstices
of their grains, after being well shaken down in a phial, is 1·02. This is a
curious result, as the size of the grains is extremely different. That of Pigou and
Wilks similarly tried is only 0·99; that of the Battle powder is 1·03; and that of Curtis
and Harvey is nearly 1·05. Gunpowders thus appear to have nearly the same weight
as water, under an equal bulk; so that an imperial gallon will hold from 10 pounds to
10 pounds and a half, as above shown.
The quantities of water which 100 grains of each part with on a steam bath, and absorb
when placed for 24 hours under a moistened receiver standing in water, are as follows:
| 100 grains |
of |
Waltham Abbey, lose |
1·1 |
by steam heat, gain |
0·8 |
over water. |
| of |
Hall |
0·5 |
2·2 |
| Lawrence |
1·0 |
1·1 |
| Pigou and Wilks |
0·6 |
2·2 |
| Curtis and Harvey |
0·9 |
1·7 |
Thus we perceive that the large-grained government powder resists the hygrometric
influence better than the others; among which, however, Lawrence’s ranks nearly as
high. These two are therefore relatively the best keeping gunpowders of the series.
The process most commonly practised in the analysis of gunpowder seems to be tolerably
exact. The nitre is first separated by hot distilled water, evaporated and weighed.
A minute loss of salt may be counted on, from its known volatility with boiling water.
I have evaporated always on a steam bath. It is probable that a small portion of the
lighter and looser constituent of gunpowder, the carbon, flies off in the operations of
corning and dusting. Hence, analysis may show a small deficit of charcoal below the
synthetic proportions originally mixed. The residuum of charcoal and sulphur left on
the double filter-paper, being well dried by the heat of ordinary steam, was estimated, as
usual, by the difference of weight of the inner and outer papers. This residuum was
cleared off into a platina capsule with a tooth-brush, and digested in a dilute solution
of potash at a boiling temperature. Three parts of potash are fully sufficient to dissolve
out one of sulphur. When the above solution is thrown on a filter, and washed first
with a very dilute solution of potash boiling hot, then with boiling water, and afterwards
dried, the carbon will remain; the weight of which deducted from that of the mixed
powder, will show the amount of sulphur.
I have tried many other modes of estimating the sulphur in gunpowder more directly,
but with little satisfaction in the results. When a platina capsule, containing gunpowder
spread on its bottom, is floated in oil heated to 400° Fahrenheit, a brisk exhalation of
sulphur fumes rises, but, at the end of several hours, the loss does not amount to more
than one half of the sulphur present.
The mixed residuum of charcoal and sulphur digested in hot oil of turpentine gives
up the sulphur readily; but to separate again the last portions of the oil from the charcoal
or sulphur, requires the aid of alcohol.
When gunpowder is digested with chlorate of potash and dilute muriatic acid, at a
moderate heat in a retort, the sulphur is acidified; but this process is disagreeable and
slow, and consumes much chlorate. The resulting sulphuric acid being tested by nitrate
of baryta, indicates of course the quantity of sulphur in the gunpowder. A curious
fact occurred to me in this experiment. After the sulphur and charcoal of the gunpowder
had been quite acidified, I poured some solution of the baryta salt into the
mixture, but no cloud of sulphate ensued. On evaporating to dryness, however, and
redissolving, the nitrate of baryta became effective, and enabled me to estimate the sulphuric
acid generated; which was of course 10 for every 4 of the sulphur.
The acidification of the sulphur by nitric or nitro-muriatic acid is likewise a slow and
unpleasant operation.
By digesting gunpowder with potash water, so as to convert its sulphur into a sulphuret,
mixing this with nitre in great excess, drying and igniting, I had hoped to convert
the sulphur readily into sulphuric acid. But on treating the fused mass with dilute
nitric acid, more or less sulphurous acid was exhaled. This occurred even though
chlorate of potash had been mixed with the nitre to aid the oxygenation.
[629]
The following are the results of my analyses, conducted by the first described
method:
| 100 grains afford, of |
Nitre. |
Charcoal. |
Sulphur. |
Water. |
| Waltham Abbey |
74·5 |
14·4 |
10·0 |
1·1 |
|
| Hall, Dartford |
76·2 |
14·0 |
9·0 |
0·5 |
loss 0·3 |
| Pigou and Wilks |
77·4 |
13·5 |
8·5 |
0·6 |
|
| Curtis and Harvey |
76·7 |
12·5 |
9·0 |
1·1 |
loss 0·7 |
| Battle Gunpowder |
77·0 |
13·5 |
8·0 |
0·8 |
loss 0·7 |
It is probable, for reasons already assigned, that the proportions mixed by the manufacturers
may differ slightly from the above.
The English sporting gunpowders have long been an object of desire and emulation in
France. Their great superiority for fowling pieces over the product of the French national
manufactories, is indisputable. Unwilling to ascribe this superiority to any genuine cause,
M. Vergnaud, captain of French artillery, in a little work on fulminating powders lately
published, asserts positively, that the English manufacturers of ‘poudre de chasse’ are
guilty of the ‘charlatanisme’ of mixing fulminating mercury with it. To determine
what truth was in this allegation, with regard at least to the above five celebrated gunpowders,
I made the following experiments:
One grain of fulminating mercury, in crystalline particles, was mixed in water with
200 grains of the Waltham Abbey gunpowder, and the mixture was digested over a
lamp with a very little muriatic acid. The filtered liquid gave manifest indications of
the corrosive sublimate, into which fulminating mercury is instantly convertible by muriatic
acid; for copper was quicksilvered by it; potash caused a white cloud in it that
became yellow, and sulphuretted hydrogen gas separated a dirty yellow white precipitate
of bisulphuret of mercury. When the Waltham Abbey powder was treated alone
with dilute muriatic acid, no effect whatever was produced upon the filtered liquid by
the sulphuretted hydrogen gas.
200 grains of each of the above sporting gunpowders were treated precisely in the
same way, but no trace of mercury was obtained by the severest tests. Since by this
process there is no doubt but one 10,000th part of fulminating mercury could be detected,
we may conclude that Captain Vergnaud’s charge is groundless. The superiority
of our sporting gunpowders is due to the same cause as the superiority of our cotton
fabrics—the care of our manufacturers in selecting the best materials, and their skill
in combining them.
I shall subjoin here some miscellaneous observations upon gunpowder.
In Bengal, mixing is performed by shutting up the ingredients in barrels, which
are turned either by hand or machinery; each containing 50 lbs. weight, or more, of
small brass balls. They have ledges on the inside, which occasion the balls and composition
to tumble about and mingle together, so that the intermixture of the ingredients,
after the process has been gone through, cannot fail to be complete. The
operation is continued two or three hours; and I think it would be an improvement in
Her Majesty’s system of manufacture if this method of mixing were adopted.
In England two or three pints of water are used for a 42 lb. charge: but the quantity
is variable; both the temperature and the humidity of the atmosphere influence it.
Bramah’s hydrostatic press, or a very strong wooden press working with a powerful
screw, lever, and windlass, constitutes the description of mechanism by which density is
imparted to gunpowder. The incorporated or mill-cake powder is laid on the bed or
follower of the press, and separated, at equal distances, by sheets of copper, so that when
the operation is over, it comes out in large thin solid cakes, or strata, distinguished by
the term press-cake. The mill-cake powder at Waltham Abbey, is submitted to a mean
theoretic pressure of 70 to 75 tons per superficial foot.
Gunpowder should be thoroughly dried, but not by too high a degree of heat; that
of 140° or 150° of Fahrenheit’s thermometer is sufficient. It appears to be of no consequence
whether it be dried by solar heat; by radiation from red-hot iron, as in the
gloom stove; or by a temperature raised by means of steam. Her Majesty’s gunpowder
is dried by the last two methods. The grain should not be suddenly exposed to the
highest degree of heat, but gradually.
The method of trial best adapted to shew the real inherent strength and goodness of
gunpowder, appears to be an eight or ten-inch iron or brass mortar, with a truly spherical
solid shot, having not more than one-tenth of an inch windage, and fired with a low
charge. The eight-inch mortar, fired with two ounces of powder, is one of the established
methods of proof at Her Majesty’s works. Gunpowders that range equally in
this mode of trial, may be depended on as being equally strong.
Another proof is by four drachms of powder laid in a small neat heap, on a clean, polished,[630]
copper plate; which heap is fired at the apex, by a red-hot iron. The explosion should
be sharp and quick; not tardy, nor lingering; it should produce a sudden concussion in
the air, and the force and power of that concussion ought to be judged of by comparison
with that produced by powder of known good quality. No sparks should fly off,
nor should beads, or globules of alkaline residuum, be left on the copper. If the copper
be left clean, i. e. without gross foulness, and no lights, i. e. sparks, be seen, the
ingredients may be considered to have been carefully prepared, and the powder to have
been well manipulated, particularly if pressed and glazed; but if the contrary be the
result, there has been a want of skill or of carefulness manifested in the manufacture.
“Gunpowder,” says Captain Bishop “explodes exactly at the 600° of heat by Fahrenheit’s
thermometer; when gunpowder is exposed to 500° it alters its nature altogether;
not only the whole of the moisture is driven off, but the saltpetre and sulphur are
actually reduced to fusion, both of which liquefy under the above degree. The powder
on cooling, is found to have changed its colour from a gray to a deep black; the grain
has become extremely indurated, and by exposure even to very moist air, it then suffers
no alteration by imbibing moisture.”
The mill for grinding the gunpowder cake may be understood from the following
representation: (fig. 531.) p, is the water wheel, which may drive several pairs of stones;
q, q, two vertical bevel wheels, fixed upon the axis of the great wheel; r, r, two horizontal
bevel wheels working in q, q, and turning the shafts s, s; t, t, two horizontal spur wheels
fixed to the upper part of the vertical shafts, and driving the large wheels u, u. To
the shafts of these latter wheels are fixed the runners v, v, which traverse upon the bed
stone w, w; x, x, are the curbs surrounding the bed stone to prevent the powder from
falling off; o is the scraper. Mill A represents a view, and mill B a section of the bed
stone and curb.
GYPSUM, Sulphate of Lime, Alabaster, or Paris Plaster. This substance is found
in three geological positions in the crust of the earth; among transition rocks; in the
red marl formation; and above the chalk, in the tertiary beds.
1. The alpine gypsums are ranged by M. Brochant among the transition class, and
are characterized by the presence of anthracite or stone coal; some of them are white
and pure, others gray or yellowish, and mixed with mica, talc, steatite, black oxide of
iron, pyrites, compact carbonate of lime, sulphur, and common salt. Examples of such
localities are found in the gypsum of Val-Canaria at the foot of Saint Gothard, that of
Brigg in the upper Valais; of the Grilla in the valley of Chamouni, and of Saint Gervais-les-Bains,
near Sallenches in Savoy.
2. The secondary gypsum, or that of the salt mine districts, belongs to the red ground,
immediately beneath the lias in the order of stratification, and therefore a rock relatively
antient. Near Northwick, the red marl beds above the great deposit of rock salt, are
irregularly intersected with gypsum, in numerous laminæ or plates. At Newbiggin in
Cumberland, the gypsum lies in red argillaceous marl, between two strata of sandstone;
and a mile south of Whitehaven, the subterraneous workings for the alabaster extend
30 yards in a direct line; with two or three lateral branches extending about 10 yards,
at whose extremities are large spaces where the gypsum is blasted with gunpowder. It
is generally compact, forming a regular and conformable bed, with crystals of selenite
(crystallized gypsum) in drusy cavities. Gypsum occurs in the red marl in the isle of
Axholme, and various other places in Nottinghamshire. In Derbyshire some considerable
deposits have been found in the same red sandstone, several of which are mined,
as at Chellaston hill, which would exhibit a naked and water-worn rock of gypsum,[631]
were it not for a covering of alluvial clay. It appears in general to present itself
chiefly in particular patches, occasioning a sudden rise, or an insulated hill, by the
additional thickness which it gives to the stratum of the red ground in these places.
The principal demand for the pure white gypsum, or that faintly streaked with red, is by
the potters in Staffordshire, who form their moulds with the calcined powder which it
affords; only particularly fine blocks are selected for making alabaster ornaments on the
turning lathe. In one of the salt pits near Droitwich, the strata sunk through, were,
vegetable mould, 3 feet; red marl, 35 feet; gypsum, 40 feet; a river of brine, 22 inches;
gypsum, 75 feet; a rock of salt, bored into only 5 feet, but probably extending much
deeper. On the Welsh side of the Bristol channel, gypsum occurs in the red marl cliffs
of Glamorganshire, from Pennarth to Lavernock. No organic remains or metallic
minerals have hitherto been found in the gypsum of this formation.
3. The most interesting gypsums in a general point of view, are certainly the tertiary, or
those of the plains, or hills of comparatively modern formation. They are characterized,
by the presence of fossil bones of extinct animals, both mammifera and birds, by shells,
and a large proportion of carbonate of lime, which gives them the property of effervescing
with acids, and the title of limestone gypsums. Such are the gypsums of the
environs of Paris, as at the heights of Montmartre, which contain crystallized sulphate of
lime in many forms, but most commonly the lenticular and lance-shaped.
Sulphate of lime occurs either as a dense compound without water, and is called
anhydrite from that circumstance; or with combined water, which is its most ordinary
state. Of the latter there are 6 sub-species; sparry gypsum or selenite in a variety of
crystalline forms; the foliated granular; the compact; the fibrous; the scaly foliated;
the earthy.
The prevailing colour is white, with various shades of gray, blue, red, and yellow.
More or less translucent. Soft, sectile, yielding to the nail. Specific gravity 2·2.
Water dissolves about one five-hundredth part of its weight of gypsum, and acquires
the quality of hardness, with the characteristic selenitic taste. When exposed on red
hot coals, it decrepitates, becomes white, and splits into a great many brittle plates. At
the heat of a baker’s oven, or about 400° Fahr., the combined water of gypsum escapes
with a species of ebullition. At a higher temperature the particles get indurated.
When rightly calcined and pulverized, gypsum is mixed with water to the consistence
of cream, and poured into moulds by the manufacturers of stucco ornaments and
statues. A species of rapid crystallization ensues, and the thin paste soon acquires a
solid consistence, which is increased by drying the figure in proper stoves. During
the consolidation of the plaster, its volume expands into the finest lines of the mould,
so as to give a sharp and faithful impression.
The plaster stone of the Paris basin contains about 12 per cent. of carbonate of lime.
This body, ground and mixed with water, forms an adhesive mortar much used in
building, as it fixes very speedily. Works executed with pure gypsum never become so
hard as those made with the calcareous kind; and hence it might be proper to add a
certain portion of white slaked lime to our calcined gypsum, in order to give the stucco
this valuable property. Coloured stuccos of great solidity are made by adding to a
clear solution of glue, any desired colouring tincture, and mixing-in the proper quantity
of the calcined calcareous gypsum.
The compact, fine-grained gypseous alabaster is often cut into various ornamental
figures, such as vases, statuary groups, &c., which take a high polish and look beautiful,
but from their softness are easily injured, and require to be kept enclosed within a glass
shade.
In America and France, the virtues of gypsum in fertilizing land have been highly
extolled, but they have not been realized in the trials made in this kingdom.
Pure gypsum consists of lime 28; sulphuric acid 40; water 18; which are the respective
weights of its prime equivalent parts.
M. Gay Lussac, in a short notice, in the Annales de Chimie for April 1829, on the
setting of gypsum, says that the purest plasters are those that harden least, and that the
addition of lime is of no use towards promoting their solidity, nor can the heat proper
for boiling gypsum ever expel the carbonic acid gas from the calcareous carbonate
present in the gypsum of Montmartre. He conceives that a hard plaster-stone having
lost its water, will resume more solidity in returning to its first state, than a plaster-stone
naturally tender or soft; and that it is the primitive molecular arrangement which
is regenerated. See Alabaster.
[632]
HADE; signifies among English miners, the inclination, or deviation from the
vertical, of any mineral vein.
HAIR; (Cheveu, Crin, Fr.; Haar, Germ.) is of all animal products, the one least
liable to spontaneous change. It can be dissolved in water only at a temperature somewhat
above 230° F., in a Papin’s digester, but it appears to be partially decomposed by
this heat, since some sulphuretted hydrogen is disengaged. By dry distillation, hair
gives off several sulphuretted gases, while the residuum contains sulphate of lime,
common salt, much silica, with some oxide of iron and manganese. It is a remarkable
fact that fair hair affords magnesia, instead of these latter two oxides. Horse-hair yields
about 12 per cent. of phosphate of lime.
Hairs are tubular, their cavities being filled with a fat oil, having the same colour
with themselves. Hair plunged in chlorine gas, is immediately decomposed and converted
into a viscid mass; but when immersed in weak aqueous chlorine, it undergoes no change,
except a little bleaching. The application of nitrate of mercury to hairy skins in the
process of secrétage, is explained under Peltry.
For the dyeing of horse-hair, see the next article.
Living hairs are rendered black by applying to them for a short time, a paste made by
mixing litharge, slaked lime, and bicarbonate of potash, in various proportions, according
to the shade of colour desired.
We have no recent analysis of hair. Vauquelin found nine different substances in
black hair; in red hair, a red oil instead of a greenish-black one.
The salts of mercury, lead, bismuth, as well as their oxides, blacken hair, or make it
of a dark violet, by the formation, most probably, of metallic sulphurets.
Hair as an object of manufactures is of two kinds, the curly and the straight. The
former, which is short, is spun into a cord, and boiled in this state, to give it the tortuous
springy form. The long straight hair is woven into cloth for sieves, and also for ornamental
purposes, as in the damask-hair cloth of chair bottoms. For this purpose the
hair may be dyed in the following way.
Forty pounds of tail hair about 26 inches long are steeped in lime water during twelve
hours. Then a bath is made with a decoction of 20 pounds of logwood, kept boiling for
three hours, after which time the fire is withdrawn from the boiler, and ten ounces of
copperas are introduced, stirred about, and the hair is immersed, having been washed from
the lime in river water. The hair should remain in this cooling bath for 24 hours, when
the operation will be finished. For other colours, see the respective dyes.
The looms for weaving hair differ from the common ones, only in the templet and the
shuttle. Two templets of iron must be used to keep the stuff equably, but lightly
stretched. These templets, of which one is represented in fig. 532., are constructed in
the shape of flat pincers; the jaws C C being furnished with teeth inside. A screw D,
binds the jaws together, and hinders the selvage
from going inwards. Upon the side cross
beam of the loom, seen in section at I, a bolt
is fixed which carries a nut F at its end, into
which a screwed iron rod E enters, on one of
whose ends is the handle B. The other extremity
of the screw E is adapted by a washer
and pin to the back of the pincers at the
point H, so that by turning the handle to the
right or the left, we draw onwards or push
backwards the pincers and the stuff at pleasure. The warp of the web is made of
black linen yarn. The weft is of hair, and it is thrown with a long hooked shuttle; or
a long rod, having a catch hook at its end. The length of this shuttle is about 3 feet;
its breadth half an inch, and its thickness one sixth. It is made of box-wood. The
reed is of polished steel; the thread warps are conducted through it in the usual way.
The workman passes this shuttle between the hairs of the warp with one hand, when
the shed or shuttle way is opened by the treddles; a child placed on one side of the loom
presents a hair to the weaver near the selvage, who catches it with the hook of his
shuttle, and by drawing it out passes it through the warp. The hairs are placed in a
bundle on the side where the child stands, in a chest filled with water to keep them moist,
for otherwise they would not have the suppleness requisite to form a web. Each time that
a hair is thrown across, the batten is driven home twice. The warp is dressed with[633]
paste in the usual way. The hair cloth after it is woven, is hot calendered to give it
lustre.
HAIR PENCILS OR BRUSHES for painting. Two sorts are made; those with
coarse hair, as that of the swine, the wild boar, the dog, &c., which are attached usually
to short wooden rods as handles; these are commonly called brushes; and hair pencils
properly so called, which are composed of very fine hairs, as of the minever, the marten,
the badger, the polecat, &c. These are mounted in a quill when they are small or of
moderate size, but when larger than a quill, they are mounted in white-iron tubes.
The most essential quality of a good pencil is to form a fine point, so that all the
hairs without exception may be united when they are moistened by laying them upon
the tongue, or drawing them through the lips. When hairs present the form of an
elongated cone in a pencil, their point only can be used. The whole difficulty consists
after the hairs are cleansed, in arranging them together so that all their points may lie in
the same horizontal plane. We must wash the tails of the animals whose hairs are to be
used, by scouring them in a solution of alum till they be quite free from grease, and then
steeping them for 24 hours in luke-warm water. We next squeeze out the water by pressing
them strongly from the root to the tip, in order to lay the hairs as smooth as possible.
They are to be dried with pressure in linen cloths, combed in the longitudinal direction,
with a very fine-toothed comb, finally wrapped up in fine linen, and dried.
When perfectly dry, the hairs are seized with pincers, cut across close to the skin, and
arranged in separate heaps, according to their respective lengths.
Each of these little heaps is placed separately, one after the other, in small tin pans
with flat bottoms, with the tips of the hair upwards. On striking the bottom of the pan
slightly upon a table, the hairs get arranged parallel to each other, and their delicate
points rise more or less according to their lengths. The longer ones are to be picked out
and made into so many separate parcels, whereby each parcel may be composed of
equally long hairs. The perfection of the pencil depends upon this equality; the
tapering point being produced simply by the attenuation of the tips.
A pinch of one of these parcels is then taken, of a thickness corresponding to the
intended size of the pencil; it is set in a little tin pan, with its tips undermost, and
is shaken by striking the pan on the table as before. The root end of the hairs being tied
by the fisherman’s or seaman’s knot, with a fine thread, it is taken out of the pan, and
then hooped with stronger thread or twine; the knots being drawn very tight by means
of two little sticks. The distance from the tips at which these ligatures are placed, is
of course relative to the nature of the hair, and the desired length of the pencil. The
base of the pencil must be trimmed flat with a pair of scissors.
Nothing now remains to be done but to mount the pencils in quill or tin-plate tubes
as above described. The quills are those of swans, geese, ducks, lapwings, pigeons, or
larks, according to the size of the pencil. They are steeped during 24 hours in water,
to swell and soften them, and to prevent the chance of their splitting when the hair brush
is pressed into them. The brush of hair is introduced by its tips into the large end of
the cut quill, having previously drawn them to a point with the lips, when it is pushed forwards
with a wire of the same diameter, till it comes out at the other and narrower end
of the quill.
The smaller the pencils, the finer ought the hairs to be. In this respect, the manufacture
requires much delicacy of tact and experience. It is said, that there are only
four first-rate hands among all the dexterous pencil-makers of Paris, and that these are
principally women.
HALOGENE; is a term employed by Berzelius to designate those substances
which form compounds of a saline nature, by their union with metals; such are Bromine,
Chlorine, Cyanogene, Fluorine, Iodine. Haloid is his name of the salt thereby formed.
HANDSPIKE, is a strong wooden bar, used as a lever to move the windlass
and capstan in heaving up the anchor, or raising any heavy weights on board a ship.
The handle is smooth, round, and somewhat taper; the other end is squared to fit the
holes in the head of the capstan or barrel of the windlass.
HARDNESS (Dureté, Fr.; Härte, Festigkeit, Germ.); is that modification of
cohesive attraction which enables bodies to resist any effort made to abrade their
surfaces. Its relative intensity is measured by the power they possess of cutting or
scratching other substances. The following table exhibits pretty nearly the successive
hardnesses of the several bodies in the list:—
[634]
| Substances. |
Hardness. |
Sp. Grav. |
| Diamond from Ormus |
20 |
3·7 |
| Pink diamond |
19 |
3·4 |
| Bluish diamond |
19 |
3·3 |
| Yellowish diamond |
19 |
3·3 |
| Cubic diamond |
18 |
3·2 |
| Ruby |
17 |
4·2 |
| Pale ruby from Brazil |
16 |
3·5 |
| Deep blue sapphire |
16 |
3·8 |
| Ditto, paler |
17 |
3·8 |
| Topaz |
15 |
4·2 |
| Whitish topaz |
14 |
3·5 |
| Ruby spinell |
13 |
3·4 |
| Bohemian topaz |
11 |
2·8 |
| Emerald |
12 |
2·8 |
| Garnet |
12 |
4·4 |
| Agate |
12 |
2·6 |
| Onyx |
12 |
2·6 |
| Sardonyx |
12 |
2·6 |
| Occidental amethyst |
11 |
2·7 |
| Crystal |
11 |
2·6 |
| Cornelian |
11 |
2·7 |
| Green jasper |
11 |
2·7 |
| Reddish yellow do. |
9 |
2·6 |
| Schoerl |
10 |
3·6 |
| Tourmaline |
10 |
3·0 |
| Quartz |
10 |
2·7 |
| Opal |
10 |
2·6 |
| Chrysolite |
10 |
3·7 |
| Zeolite |
8 |
2·1 |
| Fluor |
7 |
3·5 |
| Calcareous spar |
6 |
2·7 |
| Gypsum |
5 |
2·3 |
| Chalk |
3 |
2·7 |
HARTSHORN, SPIRIT OF; is the old name for water of ammonia.
HATCHING OF CHICKENS; see Incubation, Artificial.
HAT MANUFACTURE. (L’art de Chapelier, Fr.; Hutmacherkunst, Germ.) Hat
is the name of a piece of dress worn upon the head by both sexes, but principally by the
men, and seems to have been first introduced as a distinction among the ecclesiastics in
the 12th century, though it was not till the year 1400 that it was generally adopted
by respectable laymen.
As the art of making common hats does not involve the description of any curious
machinery, or any interesting processes, we shall not enter into very minute details upon
the subject. It will be sufficient to convey to the reader a general idea of the methods
employed in this manufacture.
The materials used in making stuff hats are the furs of hares and rabbits freed from
the long hair, together with wool and beaver. The beaver is reserved for the finer
hats. The fur is first laid upon a hurdle made of wood or wire, with longitudinal
openings; and the operator, by means of an instrument called the bow, (which is a piece
of elastic ash, six or seven feet long, with a catgut stretched between its two extremities,
and made to vibrate by a bowstick,) causes the vibrating string to strike and play upon the
fur, so as to scatter the fibres in all directions, while the dust and filth descend through
the grids of the hurdle.
After the fur is thus driven by the bow from the one end of the hurdle to the other, it
forms a mass called a bat, which is only half the quantity sufficient for a hat. The
bat or capade thus formed is rendered compact by pressing it down with the hardening
skin, (a piece of half-tanned leather,) and the union of the fibres is increased by covering
them with a cloth, while the workman presses them together repeatedly with his hands.
The cloth being taken off, a piece of paper, with its corners doubled in, so as to give it
a triangular outline, is laid above the bat. The opposite edges of the bat are then folded
over the paper, and being brought together and pressed again with the hands, they form
a conical cap. This cap is next laid upon another bat, ready hardened, so that the joined[635]
edges of the first bat rest upon the new one. This new bat is folded over the other, and
its edges joined by pressure as before; so that the joining of the first conical cap is
opposite to that of the second. This compound bat is now wrought with the hands for a
considerable time upon the hurdle between folds of linen cloth, being occasionally
sprinkled with clear water, till the hat is basoned or rendered tolerably firm.
The cap is now taken to a wooden receiver, like a very flat mill-hopper, consisting of
eight wooden planes, sloping gently to the centre, which contains a kettle filled with
water acidulated with sulphuric acid. The
technical name of this vessel is the battery. It
consists of a kettle A; and of the planks, B C,
which are sloping planes, usually eight in number,
one being allotted to each workman. The
half of each plank next the kettle is made of
lead, the upper half of mahogany. In this liquor
the hat is occasionally dipped, and wrought
by the hands, or sometimes with a roller, upon
the sloping planks. It is thus fulled or thickened
during four or five hours; the knots or
hard substances are picked out by the workman,
and fresh felt is added by means of a wet brush
to those parts that require it. The beaver is
applied at the end of this operation. In the
manufacture of beaver hats, the grounds of beer
are added to the liquor in the kettle.
Stopping, or thickening the thin spots, seen by looking through the body, is performed
by daubing on additional stuff with successive applications of the hot acidulous liquor
from a brush dipped into the kettle, until the body be sufficiently shrunk and made
uniform. After drying, it is stiffened with varnish composition rubbed in with a brush;
the inside surface being more copiously imbued with it than the outer; while the brim
is peculiarly charged with the stiffening.
When once more dried, the body is ready to be covered, which is done at the battery.
The first cover of beaver or napping, which has been previously bowed, is strewed
equably over the body, and patted on with a brush moistened with the hot liquor, until
it gets incorporated; the cut ends towards the root, being the points which spontaneously
intrude. The body is now put into a coarse hair cloth, then dipped and rolled in the
hot liquor, until the root ends of the beaver are thoroughly worked in. This is technically
called rolling off, or roughing. A strip for the brim, round the edge of the inside,
is treated in the same way; whereby every thing is ready for the second cover (of
beaver), which is incorporated in like manner; the rolling, &c. being continued, till a
uniform, close, and well-felted hood is formed.
The hat is now ready to receive its proper shape. For this purpose the workman turns
up the edge or brim to the depth of about 11⁄2 inch, and then returns the point of the cone
back again through the axis of the cap, so as to produce another inner fold of the same
depth. A third fold is produced by returning the point of the cone, and so on till the
point resembles a flat circular piece having a number of concentric folds. In this state
it is laid upon the plank, and wetted with the liquor. The workman pulls out the
point with his fingers, and presses it down with his hand, turning it at the same time
round on its centre upon the plank, till a flat portion, equal to the crown of the hat, is
rubbed out. This flat crown is now placed upon a block, and, by pressing a string
called a commander, down the sides of the block, he forces the parts adjacent to the
crown, to assume a cylindrical figure. The brim now appears like a puckered appendage
round the cylindrical cone; but the proper figure is next given to it, by working and
rubbing it. The body is rendered waterproof and stiff by being imbued with a varnish
composed of shellac, sandarach, mastic, and other resins dissolved in alcohol or naphtha.
The hat being dried, its nap is raised or loosened with a wire brush or card, and
sometimes it is previously pounced or rubbed with pumice, to take off the coarser parts,
and afterwards rubbed over with seal-skin. The hat is now tied with pack-thread upon
its block, and is afterwards dyed. See Hat-dyeing, infra.
The dyed hats are now removed to the stiffening shop. Beer grounds are next
applied on the inside of the crown, for the purpose of preventing the glue from coming
through; and when the beer grounds are dried, glue, (gum Senegal is sometimes used,)
a little thinner than that used by carpenters, is laid with a brush on the inside of the
crown, and the lower surface of the brim.
The hat is then softened by exposure to steam, on the steaming basin, and is brushed
and ironed till it receives the proper gloss. It is lastly cut round at the brim by a knife
fixed at the end of a gauge, which rests against the crown. The brim, however, is not[636]
cut entirely through, but is torn off so as to leave an edging of beaver round the external
rim of the hat. The crown being tied up in a gauze paper, which is neatly ironed
down, is then ready for the last operations of lining and binding.
The furs and wools of which hats are manufactured contain in their early stage of
preparation, hemps and hairs, which must be removed in order to produce a material for
the better description of hats. This separation is effected by a sort of winnowing
machine, which wafts away the finer and lighter parts of the furs and wools from the
coarser. Messrs. Parker and Harris obtained a patent in 1822 for the invention and
use of such an apparatus, whose structure and functions may be perfectly understood,
from its analogy to the blowing and scutching machine of the cotton manufacture; to
which I therefore refer my readers.
I shall now proceed to describe some of the recent improvements proposed in the
manufacture of hats, but their introduction is scarcely possible, on account of the perfectly
organized combination which exists among journeymen hatters throughout the
kingdom, by which the masters are held in a state of complete servitude, having no
power to take a single apprentice into their works beyond the number specified by the
Union, nor any sort of machine which is likely to supersede hand labour in any remarkable
degree. Hence the hat trade is, generally speaking, unproductive to the capitalist,
and incapable of receiving any considerable development. The public of a free country
like this, ought to counteract this disgraceful state of things, by renouncing the wear of
stuff hats, a branch of the business entirely under the controul of this despotic Union, and
betake themselves to the use of silk hats, which, from recent improvements in their
fabric and dyeing, are not a whit inferior to the beaver hats, in comfort, appearance, or
durability, while they may be had of the best quality for one-fourth part of their price.
The annexed figures represent Mr. Ollerenshaw’s machine, now generally employed
for ironing hats. Fig. 534. is the frame-work or standard upon which three of these
lathes are mounted, as A, B, C. The lathe A is intended to be employed when the
crown of the hat is to be ironed. The lathe B, when the flat top, and the upper side
of the brim is ironed, and lathe C, when its under side is ironed; motion being given to
the whole by means of a band passing from any first mover (as a steam-engine, water-wheel,
&c.) to the drum on the main shaft a a. From this drum a strap passes over
the rigger b, which actuates the axle of the lathe A. On to this lathe a sort of chuck
is screwed, and to the chuck the block c is made fast by screws, bolts, or pins. This
block is represented in section, in order to shew the manner in which it is made, of
several pieces held fast by the centre wedge-piece, as seen at fig. 535.
The hat-block being made to turn round with the chuck, at the rate of about twenty
turns per minute, but in the opposite direction to the revolution of an ordinary turning
lathe, the workman applies his hot iron to the surface of the hat, and thereby smooths
it, giving a beautiful glossy appearance to the beaver; he then applies a plush cushion,
and rubs round the surface of the hat while it is still revolving. The hat, with its
block, is now removed to the lath B, where it is placed upon the chuck d, and made to
turn in a horizontal direction, at the rate of about twenty revolutions per minute, for
the purpose of ironing the flat-top of the crown. This lathe B moves upon an upright
shaft e, and is actuated by a twisted band passing from the main shaft, round the[637]
rigger f. In order to iron the upper surface of the brim, the block c is removed from
the lathe, and taken out of the hat, when the block fig. 536. is mounted upon the chuck
d, and made to turn under the hand of the workman, as before.
The hat is now to be removed to the lathe C, where it is introduced in an inverted
position, between the arms g g supporting the rim h h, the top surface of which is
shewn at fig. 537. The spindle i of the lathe turns by similar means to the last, but
slower; only ten turns per minute will be sufficient. The workman now smooths the
under side of the brim, by drawing the iron across it, that is from the centre outwards.
The hat is then carefully examined, and all the burs and coarse hairs picked out, after
which the smoothing process is performed as before, and the dressing of the hat is
complete.
Messrs. Gillman and Wilson, of Manchester, obtained a patent, in 1823, for a
peculiar kind of fabric to be made of cotton, or a mixture of cotton and silk, for the
covering of hats and bonnets, in imitation of beaver. The foundation of the hat may
be of felt, hemp, wool, which is to be covered, by the patent fabric. This debased article
does not seem to have got into use; cotton, from its want of the felting property and
inelasticity, being very ill-adapted for making hat-stuff.
A more ingenious invention of John Gibson, hatter, in Glasgow, consisting of an
elastic fabric of whalebone, was made the subject of a patent, in June, 1824. The
whalebone, being separated into threads no larger than hay stalks, is to be boiled in
some alkaline liquid for removing the oil from it, and rendering it more elastic. The
longest threads are to be employed for warp, the shorter for weft; and are to be woven
in a hair-cloth loom. This fabric is to be passed between rollers, after which it is fit to
be cut out into forms for making hats and bonnets, to be sewed together at the joints,
and stiffened with a preparation of resinous varnishes, to prevent its being acted upon
by perspiration or rain. A very considerable improvement in the lightness and elasticity
of silk hats has been the result of this invention.
The foundation of men’s hats, upon whose outside the beaver, down, or other fine fur
is laid to produce a nap, is, as I have described, usually made of wool felted together
by hand, and formed first into conical caps, which are afterwards stretched and moulded
upon blocks to the desired shape. Mr. Borradaile, of Bucklersbury, obtained a patent
in November 1825, for a machine, invented by a foreigner, for setting up hat bodies,
which seems to be ingeniously contrived; but I shall decline describing it, as it has
probably not been suffered by the Union to come into practical operation, and as I shall
presently give the details of another later invention for the same purpose.
Silk hats, for several years after they were manufactured, were liable to two objections;
first, the body or shell over which the silk covering is laid, was, from its hardness,
apt to hurt the head; second, the edge of the crown being much exposed to blows, the
silk nap soon got abraded, so as to lay bare the cotton foundation, which is not capable
of taking so fine a black die as the silk; whence the hat assumed a shabby appearance.
Messrs. Mayhew and White, of London, hat-manufacturers, proposed in their patent of
February, 1826, to remedy these defects, by making the hat body of stuff or wool, and
relieving the stiffness of the inner part round the brim, by attaching a coating of beaver
upon the under side of the brim, so as to render the hat pliable. Round the edge of
the tip or crown, a quantity of what is called stop wool is to be attached by the ordinary
operation of bowing, which will render the edge soft and elastic. The hat is to be
afterwards dyed of a good black colour, both outside and inside; and being then properly
stiffened and blocked, is ready for the covering of silk.
The plush employed for covering silk hats, is a raised nap or pile woven usually upon
a cotton foundation; and the cotton, being incapable of receiving the same brilliant
black dye as the silk, renders the hat apt to turn brown whenever the silk nap is partially
worn off. The patentees proposed to counteract this evil, by making the foundation of
the plush entirely of silk. To these two improvements, now pretty generally introduced,
the present excellence of the silk hats, may be, in a good measure, ascribed.
The apparatus above alluded to, for making the foundations of hats by the aid of
mechanism, was rendered the subject of a patent, by Mr. Williams, in September, 1826;
but I fear it has never obtained a footing, nor even a fair trial in our manufactures, on
account of the hostility of the operatives to all labour-saving machines.
Fig. 538. is a side view of the carding engine, with a horizontal or plan view of the
lower part of the carding machine, shewing the operative parts of the winding apparatus,
as connected to the carding engine. The doffer cylinder is covered with fillets of
wire cards, such as are usually employed in carding engines, and these fillets are
divided into two, three, or more spaces extending round the periphery of the cylinder,
the object of which division is to separate the sliver into two, three, or more breadths,
which are to be conducted to, and wound upon distinct blocks, for making so many
separate hats or caps.
[638]
The principal cylinder of the carding engine, is made to revolve by a rigger upon its
axle, actuated by a band from any first mover as usual, and the subordinate rollers or
cylinders belonging to the carding engine, are all turned by pullies, and bands, and
geer, as in the ordinary construction.
The wool or other material is supplied to the feeding cloth, and carried through
the engine to the doffer cylinder, as in other carding engines; the doffer comb is
actuated by a revolving crank in the common way, and by means of it the slivers are
taken from the doffer cylinder, and thence received on to the surfaces of the blocks e e.
These blocks, of which two only are shewn to prevent confusion, are mounted upon
axles, supported by suitable bearings in a carriage f f, and are made to revolve by means
of a band g g, leading from a pulley on the axle of a conical drum beneath. The band g
passes over a pulley h, affixed to the axle of one of the blocks, while another pulley i,
upon the same axle, gives motion, by means of a band, to as many other blocks as are
adapted to the machine.
As it is necessary in winding the slivers on to the blocks, to cross them in different
directions, and also to pass the sliver over the hemispherical ends of the blocks, in
order that the wool or other material may be uniformly spread over the surface in
forming the cap or hood for the shell or foundation of the intended hat, the carriage f,
with the blocks, is made to traverse to and fro in lateral directions upon rollers at each
end.
This alternating motion of the carriage is caused by a horizontal lever l l, (seen in
the horizontal view fig. 538.) moving upon a fulcrum pin at m, which lever is attached
to the carriage at one extremity n, and at the other end has a weighted cord which
draws the side of this lever against a cam wheel o. This cam is made to revolve by
means of a band and pulley, which turns the shaft and endless screw q, and this
endless screw taking into a toothed wheel r, on the axle of the cam o, causes the cam
to revolve, the periphery of which cam running against a friction roller on the side of
the lever l, causes the lever to vibrate, and the carriage f f, attached to it, to traverse to
and fro upon the supporting rollers, as described. By these means the slivers are
laid in oblique directions, (varying as the carriage traverses,) over the surface of the
blocks.
The blocks being conically formed, or of other irregular figures, it is necessary, in
order to wind the slivers with uniform tension, to vary their speed according to the
diameter of that part of the block which is receiving the sliver. This is effected by
giving different velocities to the pulley on the axle of the conical drum s, corresponding
with e. There is a similar conical drum t, placed in a reverse position in the lower[639]
part of the frame, which is actuated by a band from any convenient part of the machine
passing over a pulley u, upon the axle of t. From the drum t, to the drum s, there is
a band v, which is made to slide along the drums by the guidance of two rollers at the
end of the lever l.
It will now be seen that when the larger diameter of the cam wheel o forces the
lever outwards, the band v will be guided on to the smaller part of the conical drum t,
and the larger part of s, consequently the drum s will at this time receive its slowest
motion, and the band g will turn the blocks slower also; the reverse end of the lever l,
having by the same movement, slidden the carriage into that position which causes the
slivers to wind upon the larger diameter of the blocks.
When the smaller diameter of the cam is acting against the side of the lever, the
weighted cord draws the end of the lever to the opposite side, and the band v will be
guided on to the larger part of the cord t, and the smaller part of the cone s; consequently,
the quicker movement of the band g will now cause the blocks e e to revolve
with a corresponding speed. The carriage f will also be moved upon its rollers, to
the reverse side, and the sliver of wool or other material be now wound upon the
smaller parts and ends of the blocks, at which time the quicker rotation of the blocks is
required. It may be here observed, that the cam wheel o should be differently formed
according to the different shaped blocks employed, so as to produce the requisite movements
of the lever and carriage suited thereto.
It only remains to state, that there are two heavy conical rollers w w, bearing upon
the peripheries of the blocks e e, which turn loosely upon their axles by the friction
of contact, for the purpose of pressing the slivers of wool or other material on the
blocks as it comes from the doffer cylinder of the carding engine, and when the blocks
have been coated with a sufficient quantity of the sliver, the smaller end of the pressing
rollers is to be raised, while the cap is withdrawn from the block. The process being
continued as before, the formations of other bodies or caps is effected in the manner
above described.
After the caps or bodies of hats, &c. are formed in the above described machine, they
are folded in wet cloths, and placed upon heated plates, where they are rolled under
pressure, for the purpose of being hardened. Fig. 539. represents the front of three
furnaces a a a, the tops of which are covered with iron plates b b b. Upon these plates,
which are heated by the furnace below, or by steam, the bodies wrapped in the wet
cloths c c c, are placed, and pressed upon by the flaps or covers d d d, sliding upon
guide rods, to which flaps a traversing motion is given, by means of chains attached to
an alternating bar e e. This bar is moved by a rotatory crank f, which has its motion
by pulleys from any actuating power. When any one of the flaps is turned up to
remove the bodies from beneath, the chains hang loosely, and the flap remains
stationary.
These caps or hat bodies, after having been hardened in the manner above described,
may be felted in the usual way by hand, or they are felted in a fulling mill, by the
usual process employed for milling cloths, except that the hat bodies are occasionally
taken out of the fulling mill, and passed between rollers, for the purpose of rendering
the felt more perfect.
Mr. Carey, of Basford, obtained
a patent in October, 1834,
for an invention of certain machinery
to be employed in the
manufacture of hats, which is
ingenious and seems to be worthy
of notice in this place. It consists
in the adaptation of a system
of rollers, forming a machine,
by means of which the
operation of roughing or plaiting
of hats may be performed; that
is, the beaver or other fur may
be made to attach itself, and
work into the felt or hat body,
without the necessity of the ordinary
manual operations.
The accompanying drawings represent the machine in several views, for the purpose of
showing the construction of all its parts. Fig. 540. is a front elevation of the machine;
fig. 541. is a side elevation of the same; fig. 542. is a longitudinal section of the machine;
and fig. 543. is a transverse section; the similar letters indicating the same parts
in all the figures.
[640]
Upon a brick or other suitable base, a furnace or fire-place a, is made, having a descending
flue b, for the purpose of carrying
away the smoke. A pan
or shallow vessel c c, formed of
lead, is placed over the furnace;
which vessel is intended to contain
a sour liquor, as a solution
of vitriolic acid and water. On
the edge of this pan is erected a
wooden casing d d d, which encloses
three sides, leaving the
fourth open for the purpose of
obtaining access to the working
apparatus within. A series of
what may be termed lantern
rollers, e e e, is mounted on
axles turning in the side casings;
and another series of similar
lantern rollers, f f f, is
in like manner mounted above.
These lantern rollers are made
to revolve by means of bevel
pinions, fixed on the ends of
their axles, which are turned
by similar bevel wheels on the
lateral shafts g and h, driven by
a winch i, and geer, as shown
in figs. 540. and 541.
Having prepared the bodies
of the hats, and laid upon their
surfaces the usual coatings of
beaver, or other fur, when so prepared they are to be placed between hair cloths, and
these hair cloths folded within a canvass or other suitable wrapper. Three or more hats
being thus enclosed in each wrapper, the packages are severally put into bags or pockets
in an endless band of sackcloth, or other suitable material; which endless band is
extended over the lantern rollers in the machine.
In the first instance, for the purpose of merely attaching the furs to the felts (which is
called slicking, when performed by hand), Mr. Carey prefers to pass the endless band
k k k, with the covered hat bodies, over the upper series f f f, of the lantern rollers, in order
to avoid the inconvenience of disturbing the fur, which might occur from subjecting
them to immersion in the solution contained in the pan, before the fur had become attached
to the bodies.
After this operation of slicking has been effected, he distends the endless band k k k, over
the lower series of lantern rollers e e e, and round a carrier roller l, as shown in fig. 542.;
and having withdrawn the hat bodies for the purpose of examining them, and changing
their folds, he packs them again in a similar way in flannel, or other suitable cloths, and
introduces them into the pockets or bags of the endless bands, as before.
On putting the machinery in rotatory motion in the way described, the hats will be
carried along through the apparatus, and subjected to the scalding solution in the pan,
as also to the pressure, and to a tortuous action between the ribs of the lantern rollers,
as they revolve, which will cause the ends of the fur to work into the felted bodies of the
hats, and by that means permanently to attach the nap to the body; an operation which
when performed by hand, is called rolling off.
The improved stiffening for hat bodies proposed by Mr. Blades, under his patent of
January, 1828, consists in making his solution of shellac in an alkaline lye, instead of
spirits of wine, or pyroxylic spirit, vulgarly called naphtha.
He prepares his water-proof stiffening by mixing 18 pounds of shellac with 11⁄2 pounds
of salt of tartar (carbonate of potash), and 51⁄2 gallons of water. These materials are to
be put into a kettle, and made to boil gradually until the lac is dissolved; when the
liquor will become as clear as water, without any scum upon the top, and if left to
cool, will have a thin crust upon its surface of a whitish cast, mixed with the light
impurities of the gum. When this skin is taken off, the hat body is to be dipped into
the mixture in a cold state, so as to absorb as much as possible of it; or it may be
applied with a brush or sponge. The hat body being thus stiffened, may stand till it
become dry, or nearly so; and after it has been brushed, it must be immersed in very
dilute sulphuric or acetic acid, in order to neutralize the potash, and cause the shellac to[641]
set. If the hats are not to be napped immediately, they may be thrown into a cistern
of pure water, and taken out as wanted.
Should the hat bodies have been worked at first with sulphuric acid (as usual), they
must be soaked in hot water to extract the acid, and dried before the stiffening is applied;
care being taken that no water falls upon the stiffened body, before it has been immersed
in the acid.
This ingenious chemical process has not been, to the best of my knowledge, introduced
into the hat manufacture. A varnish made by dissolving shellac, mastic, sandarach,
and other resins in alcohol, or the naphtha of wood vinegar, is generally employed
as the stiffening and water-proof ingredient of hat bodies. A solution of caoutchouc is
often applied to whalebone and horse-hair hat bodies.
The following recipe has been prescribed as a good composition for stiffening hats:
four parts of shellac, one part of mastic, one half of a part of turpentine, dissolved in
five parts of alcohol, by agitation and subsequent repose, without the aid of heat. This
stiffening varnish should be applied quickly to the body or foundation with a soft oblong
brush, in a dry and rather warm workshop; the hat being previously fitted with
its inside turned outwards upon a block. The body must be immediately afterwards
taken off, to prevent adhesion.
Hat-Dyeing.—The ordinary bath for dyeing hats, employed by the London manufacturers,
consists for 12 dozen, of—
| 144 |
|
pounds of logwood; |
| 12 |
|
pounds of green sulphate of iron, or copperas; |
| 7 |
1⁄2 |
pounds of verdigris. |
The copper is usually made of a semi-cylindrical shape, and should be surrounded with
an iron jacket or case, into which steam may be admitted, so as to raise the temperature
of the interior bath to 190° F., but no higher, otherwise the heat is apt to affect the
stiffening varnish, called the gum, with which the body of the hat has been imbued.
The logwood having been introduced and digested for some time, the copperas and
verdigris are added in successive quantities, and in the above proportions, along with
every successive two or three dozens of hats, suspended upon the dipping machine.
Each set of hats, after being exposed to the bath with occasional airings during 40
minutes, is taken off the pegs, and laid out upon the ground to be more completely
blackened by the peroxidizement of the iron with the atmospheric oxygen. In 3 or 4
hours the dyeing is completed. When fully dyed, the hats are well washed in running
water.
Mr. Buffum states that there are four principal objects accomplished by his patent
invention for dyeing hats.
1. in the operation;
2. the production of a better colour;
3. the prevention of any of the damages to which hats are liable in the dyeing;
4. the accomplishment of the dyeing process in a much shorter time than by the usual
methods, and consequently lessening the injurious effects of the dye-bath upon the
texture of the hat.
Fig. 544. shows one method of constructing the apparatus. a a is a semi-cylindrical
shaped copper vessel, with flat ends, in which the dyeing process is carried on. b b b is
a wheel with several circular rims mounted upon arms, which revolve upon an axle c.
In the face of these rims a number of pegs or blocks are set at nearly equal distances
apart, upon each of which pegs or blocks it is intended to place a hat, and as the wheel[642]
revolves, to pass it into and out of the dyeing liquor in the vat or copper. This wheel
may be kept revolving with a very slow motion, either by geer connecting its axle, c,
with any moving power, or it may be turned round by hand, at intervals of ten minutes;
whereby the hats hung upon the pegs, will be alternately immersed for the space of ten
minutes in the dyeing liquor, and then for the same space exposed to the atmospheric
air. In this way, the process of dyeing, it is supposed, may be greatly facilitated, and
improved, as the occasional transition from the dye vat into the air, and from the air
again into the bath, will enable the oxygen of the atmosphere to strike the dye more
perfectly and expeditiously into the materials of which the hat is composed, than by a
continued immersion in the bath for a much longer time.
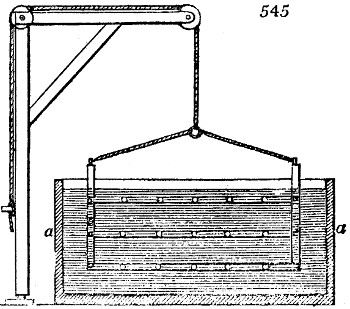
A variation in the mode of performing this process is suggested, and the apparatus
fig. 545. is proposed to be employed, a a is a square vat or vessel containing the dyeing
liquor; b b is a frame or rack having a number of pegs placed in it for hanging the hats
upon, which are about to be dyed, in a manner similar to the wheel above described.
This frame or rack is suspended by cords from a crane, and may in that way be lowered
down with the hats into the vat, or drawn up and exposed in the air; changes which
may be made every 10 or 20 minutes.
I have seen apparatus of this kind doing good work in the hat-dyeing manufactories
of London, that being a department of the business with which the Union has not
thought it worth their while to interfere.
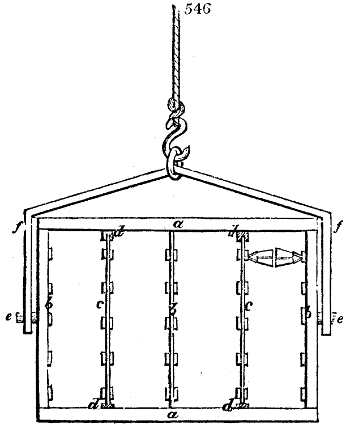
Mr. William Hodge’s patent improvements in hat dyeing, partly founded upon an
invention of Mr. Bowler, consist, first in causing every alternate frame to which the
suspenders or blocks are to be attached, to slide in and out of grooves, for the purpose
of more easily removing the said
suspenders when required. Fig.
546. represents the improved dyeing
frame, consisting of two circular
rims, a a, which are connected
together at top and bottom,
by three fixed perpendicular bars
or the frame-work b b b. Two
other perpendicular frames c c,
similar to the former, slide in
grooves, d d d d, fixed to the upper
and lower rims. These grooves
have anti-friction rollers in them,
for the purpose of making the
frames c c, to slide in and out
more freely. The suspenders or
substitutes for blocks, by these
means, may be more easily got at
by drawing out the frames c c,
about half way, when the suspenders,
which are attached to the
frames with the hats upon them,
may be easily reached, and either
removed or altered in position;
and when it is done on one side, the sliding frame may be brought out on the other,
and the remaining quantity of “suspenders” undergo the same operation.
The patentee remarks, that it is well known to all hat dyers, that after the hats have
been in the dyeing liquor some time, they ought to be taken out and exposed to the
action of the atmospheric air, when they are again immersed in the copper, that part of
the hat which was uppermost in the first immersion, being placed downwards in the
second. This is done for the purpose of obtaining an uniform and regular dye. The
patentee’s mode of carrying this operation into effect, is shown in the figure: e e are
pivots for the dyeing-frame to turn upon, which is supported by the arms f, from a crane
above. The whole apparatus may be raised up or lowered into the copper by means of
the crane or other mechanism. When the dyeing-frame is raised out of the copper, the
whole of the suspenders or blocks are reversed, by turning the apparatus over upon the
pivots e e, and thus the whole surfaces of the hats are equally acted upon by the dyeing
material.
It should be observed, that when the dyeing-frame is raised up out of the copper, it
should be tilted on one side, so as to make all the liquor run out of the hats, as also to
cause the rims of the hats to hang down, and not stick to the body of the hat, or leave
a bad place or uneven dye upon it. The second improvement described by the patentee,
is the construction of “suspenders,” to be substituted instead of the ordinary blocks.
[643]
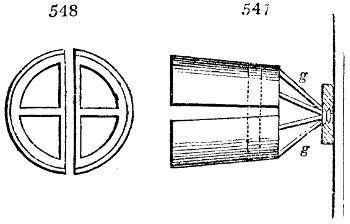
These “suspenders” are composed of thin plates of copper, bent into the required
form, that is, nearly resembling that of a hat block, and made in such a manner as to be
capable of contraction and expansion to suit different sized hats, and keep them
distended, which may be altered by the workman at pleasure, when it is required to
place the hats upon them, or remove them therefrom. The dyeing-frame at fig. 546. is
shown with only two of these “suspenders,” in order to prevent confusion. One of these
suspenders is represented detached at fig. 547., which exhibits a side view; and fig. 548.
a front view of the same. It will be seen by reference to the figure, that the suspenders
consist of two distinct parts, which may be enlarged or collapsed by a variety of means,
and which means may be suggested by any competent mechanic. The two parts of the
suspenders are proposed to be connected together by arms g g, and at the junction of
these arms a key is connected for turning them round when required. It will be seen
on reference to the front view, fig. 548., that the “suspenders” or substitutes for blocks,
are open at the top or crown part of the hat; this is for the purpose of allowing the
dyeing liquor to penetrate.
From the mixture of copperas and verdigris employed in the hat-dye, a vast quantity
of an ochreous muddy precipitate results, amounting to no less than 25 per cent. of the
weight of the copperas. This iron mud forms a deposit upon the hats, which not only
corrodes the fine filaments of the beaver, but causes both them and the felt stuff to turn
speedily of a rusty brown. There is no process in the whole circle of our manufactures,
so barbarous as that of dyeing stuff hats. No ray of chemical science seems hitherto to
have penetrated the dark recesses of their dye shops. Some hatters have tried to remove
this corrosive brown ochre by a bath of dilute sulphuric acid, and then counteract the
evil effect of the acid upon the black dye by an alkaline bath; but with a most unhappy
effect. Hats so treated are most deceptious and unprofitable; as they turn of a dirty
brown hue, when exposed for a few weeks to sunshine and air.
HEALDS, is the harness for guiding the warp threads in a loom; that is, for lifting
a certain number of them alternately to open the shed, and afford passage to the decussating
weft threads of the shuttle. See Weaving.
HEARTH; (Foyer, Fr.; Heerde, Germ.) is the flat or hollow space in a smelting
furnace upon which the ore and fluxes are subjected to the influence of flame. See Copper,
Iron, Metallurgy, &c.
HEAT, is that power or essence called caloric, the discussion of whose habitudes
with the different kinds of matter belongs to the science of chemistry.
HEAT-REGULATOR. The name given by M. Bonnemain to an ingenious apparatus
for regulating the temperature of his incubating stove rooms. See Incubation,
Artificial, for the manner of applying the Heat-Regulator.
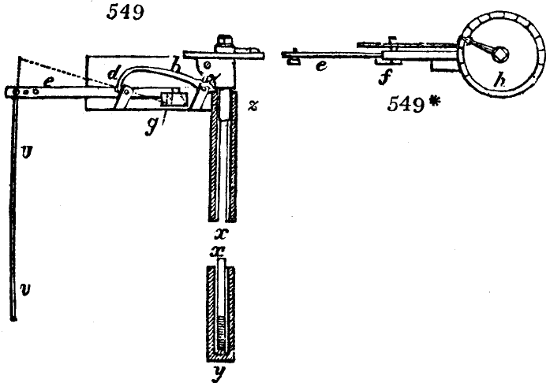
The construction of the regulator is founded upon the unequal dilatation of different
metals by the same degree of heat. A rod of iron x, fig. 549., is tapped at its lower
end into a brass nut y, enclosed in
a leaden box or tube, terminated
above by a brass collet z. This
tube is plunged into the water of
the boiler, alongside of the smoke-pipe.
(Fig. 549*. is a bird’s-eye
view of the dial, &c.) The expansion
of the lead being more
than the iron for a like degree of
temperature, and the rod enclosed
within the tube being less easily
warmed, whenever the heat rises to
the desired pitch, the elongation
of the tube puts the collet z in
contact with the heel a, of the
bent lever a, b, d; thence the
slightest increase of heat lengthens the tube anew, and the collet lifting the heel of
the lever, depresses its other end d through a much greater space, on account of the
relative lengths of its legs. This movement operates near the axis of a balance-bar e,
sinks one end of this, and thereby increases the extent of the movement which is transmitted
directly to the iron skewer v. This pushing down a swing register diminishes
or cuts off the access of air to the fire-place. The combustion is thereby obstructed, and
the temperature falling by degrees, the tube shrinks and disengages the heel of the lever.
The counterpoise g, fixed to the balance-beam e, raises the other extremity of this beam,
by raising the end d of the lever as much as is necessary to make the heel bear upon
the collet of the tube. The swing register acted upon by this means, presents a greater
section to the passage of the air; whence the combustion is increased. To counterbalance[644]
the effect of atmospheric changes, the iron stem which supports the regulator
is terminated by a dial disc, round the shaft of the needle above h, fig. 549*.; on turning
this needle, the stem below it turns, as well as a screw at its under end, which raises or
lowers the leaden tube. In the first case, the heel falls, and opens the swing register,
whence a higher temperature is required to shut it, by the expansion of the tube. We
may thus obtain a regularly higher temperature. If, on the contrary, we raise the tube
by turning the needle in the other direction, the register presents a smaller opening, and
shuts at a lower temperature; in this case, we obtain a regularly lower temperature.
It is therefore easy, says M. Bonnemain, to determine à priori the degree of temperature
to be given to the water circulating in the stove pipes. In order to facilitate the regulation
of the apparatus, he graduated the disc dial, and inscribed upon its top and bottom, the
words, Strong and Weak heat. See Thermostat, for another Heat-Regulator.
HEAVY SPAR, sulphate of Baryta, or Cawk; (Spath pesant, Fr.; Schwerspath,
Germ.) is an abundant mineral, which accompanies veins of lead, silver, mercury, &c.
but is often found, also, in large masses. Its colour is usually white, or flesh-coloured.
It occurs in many crystalline forms, of which the cleavage is a right rhomboidal prism.
It is met with also of a fibrous, radiated, and granular structure. Its spec. grav. varies
from 4·1 to 4·6. It has a strong lustre, between the fatty and the vitreous. It melts at
35° Wedgew. into a white opaque enamel. Its constituents are 65·63 baryta, and
34·37 sulphuric acid. It is decomposed by calcination in contact with charcoal at a
white heat, into sulphuret of baryta; from which all the baryta salts may be readily
formed. Its chief employment in commerce is for adulterating white lead; a purpose
which it readily serves on account of its density. Its presence here is easily detected by
dilute nitric acid, which dissolves the carbonate of lead, and leaves the heavy spar. It
is also a useful ingredient in some kinds of pottery, and glass.
HECKLE; (Seran, Fr.; Hechel, Germ.) is an implement for dissevering the filaments
of flax, and laying them in parallel stricks or tresses. See Flax.
HELIOTROPE; is a variety of jasper, mixed with chlorite, green earth, and diallage;
occasionally marked with blood-red points; whence its vulgar name of bloodstone.
HEMATINE; is the name given by its discoverer Chevreul to a crystalline substance,
of a pale pink colour, and brilliant lustre when viewed in a lens, which he
extracted from logwood, the hæmatoxylon Campechianum of botanists. It is, in fact, the
characteristic principle of this dye-wood. To procure hematine, digest during a few
hours ground logwood in water heated to a temperature of about 130° F.; filter the
liquor, evaporate it to dryness by a steam bath, and put the extract in alcohol of 0·835
for a day. Then filter anew, and after having inspissated the alcoholic solution by evaporation,
pour into it a little water, evaporate gently again, and then leave it to itself in
a cool place. In this way a considerable quantity of crystals of hematine will be
obtained, which may be readily purified by washing with alcohol and drying.
When subjected to dry distillation in a retort, hematine affords all the usual products
of vegetable bodies, along with a little ammonia; which proves the presence of azote.
Boiling water dissolves it abundantly, and assumes an orange-red colour, which passes
into yellow by cooling, but becomes red again with heat. Sulphurous acid destroys the
colour of solution of hematine. Potash and ammonia convert into a dark purple-red
tint, the pale solution of hematine; when these alkalis are added in large quantity, they
make the colour, violet blue, then brown-red, and lastly brown-yellow. By this time,
the hematine has become decomposed, and cannot be restored to its pristine state by
neutralizing the alkalis with acids.
The waters of baryta, strontia, and lime exercise an analogous power of decomposition;
but they eventually precipitate the changed colouring matter.
A red solution of hematine subjected to a current of sulphuretted hydrogen becomes
yellow; but it resumes its original hue when the sulphuretted hydrogen is removed by
a little potash.
The protoxide of lead, the protoxide of tin, the hydrate of peroxide of iron, the
hydrate of oxides of copper and nickel, oxide of bismuth, combine with hematine, and
colour it blue with more or less of a violet cast.
Hematine precipitates glue from its solution in reddish flocks. This substance has
not hitherto been employed in its pure state; but as it constitutes the active principle
of logwood, it enters as an ingredient into all the colours made with that dye stuff.
These colours are principally violet and black. Chevreul has proposed hematine as
an excellent test of acidity.
HEMATITE; (Fer Oligiste, Fr.; Rotheisenstein, Germ.) is a native reddish-brown
peroxide of iron, consisting of oxygen 30·66; iron 60·34. It is the kidney ore of Cumberland,
which is smelted at Ulverstone with charcoal, into excellent steel iron.
HEMP; (Chanvre, Fr.; Hanf, Germ.) is the fibrous rind of the bark of the cannabis[645]
sativa, which is spun into strands or yarn for making ropes, sail-cloth, &c. It is
prepared for spinning in the same way as flax, which see. Hemp-seed contains an oil
which is employed for making soft soap, for painting, and for burning in lamps. See
Oils.
Importation of undressed hemp for home consumption; and amount of duty, in
| |
1837. |
1838. |
1837. |
1838. |
| Cwts. |
596,994·3 |
667,017 |
£2487 |
£2780 |
HEPAR; which signifies liver in Latin, was a name given by the older chemists to
some of those compounds of sulphur with the metals which had a liver-brown colour.
Thus the sulphuret of potassium was called liver of sulphur.
HERMETICAL SEAL, is an expression derived from Hermes, the fabulous
parent of Egyptian chemistry, to designate the perfect stoppage of a hollow vessel, by
the cementing or melting of the lips of its orifice; as in the case of a glass thermometer,
or matrass.
HIDE; (Peau, Fr.; Haut, Germ.) the strong skin of an ox, horse, or other large
animal. See Leather.
Importation of untanned hides for home consumption; and amount of duty, in
| 1837. |
1838. |
1837. |
1838. |
| 332,877 |
301,890 |
£46,190 |
£36,647 |
HIRCINE; from hircus, a ram; is the name given by Chevreul to a liquid fatty
substance, which is mixed with the oleine of mutton suet, and gives it its peculiar rank
smell. Hircine is much more soluble in alcohol than oleine. It produces hircic acid
by saponification.
HONEY; (Mel, Fr.; Honig, Germ.) is a sweet viscid liquor, elaborated by bees
from the sweet juices of the nectaries of flowers, and deposited by them in the waxen cells
of their combs. Virgin honey is that which spontaneously flows with a very gentle heat
from the comb, and common honey is that which is procured by the joint agency of
pressure and heat. The former is whitish or pale yellow, of a granular texture, a fragrant
smell, and a sweet slightly pungent taste; the latter is darker coloured, thicker,
and not so agreeable either in taste or smell. Honey would seem to be merely collected
by the bees, for it consists of merely the vegetable products; such as the sugars of grape,
gum, and manna; along with mucilage, extractive matter, a little wax, and acid.
HONEY-STONE; (Mellite, Fr.; Honigstein, Germ.) is a mineral of a yellowish
or reddish colour, and a resinous aspect, crystallizing in octahedrons with a square
base; specific gravity 1·58. It is harder than gypsum, but not so hard as calc-spar; it
is deeply scratched by a steel point; very brittle; affords water by calcination; blackens,
then burns at the flame of the blowpipe, and leaves a white residuum which becomes
blue, when it is calcined after having been moistened with a drop of nitrate of cobalt.
It is a mellate of alumina, and consists of:
| |
Klaproth. |
Wöhler. |
| Mellitic acid |
46 |
44·4 |
| Alumina |
16 |
14·5 |
| Water |
38 |
41·1 |
| |
100 |
100·0 |
The honey-stone, like amber, belongs to the geological formation of lignites. It has
been hitherto found clearly in only one locality, at Artern in Thuringia.
HOP; (Houblon, Fr.; Hopfen, Germ.) is the name of a well-known plant of the
natural family of Urticeæ, and of the dioecia pentandria of Linnæus. The female flowers,
placed upon different plants from the male, grow in ovoid cones formed of oval leafy
scales, concave, imbricated, containing each at the base an ovary furnished with two
tubular open styles, and sharp pointed stigmata. The fruit of the hop is a small
rounded seed, slightly compressed, brownish coloured, enveloped in a scaly calyx, thin
but solid, which contains, spread at its base, a granular yellow substance, appearing to
the eye like a fine dust, but in the microscope seen to be round, yellow, transparent
grains; deeper coloured, the older the fruit. This secretion, which constitutes the useful
portion of the hop, has been examined in succession by Ives, Planche, Payen, and
Chevallier. I have given a pretty full account of the results of their researches in
treating of the hop, under the article Beer.
[646]
HORDEINE, is the name given by Proust to the peculiar starchy matter of barley.
It seems to be a mixture of the starch, lignine, and husks, which constitutes barley
meal. See Beer.
HORN; (Eng. and Germ.; Corne, Fr.) particularly of oxen, cows, goats, and sheep,
is a substance soft, tough semi-transparent, and susceptible of being cut and pressed
into a variety of forms; it is this property that distinguishes it from bone. Turtle or
tortoise shell seems to be of a nature similar to horn, but instead of being of a uniform
colour, it is variegated with spots.
These valuable properties render horn susceptible of being employed in a variety
of works fit for the turner, snuff-box, and comb maker. The means of softening
the horn need not be described, as it is well known to be by heat; but those of cutting,
polishing, and soldering it, so as to make plates of large dimensions, suitable to
form a variety of articles, may be detailed. The kind of horn to be preferred is
that of goats and sheep, from its being whiter and more transparent than the horn
of any other animals. When horn is wanted in sheets or plates, it must be steeped
in water, in order to separate the pith from the kernel, for about fifteen days in summer,
and a month in winter; and after it is soaked, it must be taken out by one end,
well shaken and rubbed in order to get off the pith; after which it must be put for
half an hour into boiling water, then taken out, and the surface sawed even lengthways;
it must again be put into the boiling water to soften it, so as to render it capable
of separating; then, with the help of a small iron chisel, it can be divided into sheets or
leaves. The thick pieces will form three leaves, those which are thin will form only
two, whilst young horn, which is only one quarter of an inch thick, will form only one.
These plates or leaves must again be put into boiling water, and when they are sufficiently
soft, they must be scraped with a sharp cutting instrument, to render those
parts that are thick even and uniform; they must be put once more into the boiling
water, and finally carried to the press.
At the bottom of the press employed, there must be a strong block, in which is
formed a cavity, of nine inches square, and of a proportionate depth; the sheets of horn
are to be laid within this cavity, in the following manner: at the bottom, first a sheet of
hot iron, upon this a sheet of horn, next again a sheet of hot iron, and so on, taking care
to place at the top a plate of iron even with the last. The press must then be screwed
down tight.
There is a more expeditious process, at least in part, for reducing the horn into
sheets, when it is wanted very even. After having sawed it with a very fine and sharp saw,
the pieces must be put into a copper made on purpose, and there boiled, until sufficiently
soft, so as to be able to be split with pincers; the sheets of horn must then be put in
the press, where they are to be placed in a strong vice, the chaps of which are of iron
and larger than the sheets of horn, and the vice must be screwed as quick and tight as
possible; let them cool in the press or vice, or it is as well to plunge the whole into cold
water. The last mode is preferable, because the horn does not shrink in cooling. Now
draw out the leaves of horn, and introduce other horn to undergo the same process.
The horn so enlarged in pressing, is to be submitted to the action of the saw, which
ought to be set in an iron frame, if the horn is wanted to be cut with advantage, in
sheets of any desired thickness, which cannot be done without adopting this mode. The
thin sheets thus produced must be kept constantly very warm between plates of hot
iron to preserve their softness; every leaf being loaded with a weight heavy enough to
prevent its warping. To join the edges of these pieces of horn together, it is necessary
to provide strong iron moulds suited to the shape of the article wanted, and to place the
pieces in contact with copper-plates or with polished metal surfaces against them; when
this is done, the whole is to be put into a vice and screwed up tight, then plunged into
boiling water, and after some time it is to be removed from thence and immersed in cold
water. The edges of the horn will be thus made to cement together and become perfectly
united.
To complete the polish of the horn, the surface must be rubbed with the subnitrate
of bismuth by the palm of the hand. The process is short, and has this advantage, that
it makes the horn dry promptly.
When it is wished to spot the horn in imitation of tortoise-shell, metallic solutions
must be employed as follows:—To spot it red, a solution of gold in aqua regia must be
employed; to spot it black, a solution of silver in nitric acid must be used; and for
brown, a hot solution of mercury in nitric acid. The right side of the horn must be
impregnated with these solutions, and they will assume the colours intended. The
brown spots can be produced on the horn by means of a paste made of red lead, with a
solution of potash, which must be put in patches on the horn, and subjected some time to
the action of heat. The deepness of the brown shades depends upon the quantity of
potash used in the paste, and the length of time the mixture lies on the horn. A
decoction of Brazil wood, or a solution of indigo, in sulphuric acid, or a decoction of saffron,[647]
and Berbary wood may also be used. After having employed these materials, the horn
may be left for half a day in a strong solution of vinegar and alum.
In France, Holland, and Austria, the comb-makers and horn-turners use the clippings
of horn, which are of a whitish yellow, and tortoise-shell skins, out of which they make
snuff-boxes, powder-horns, and many curious and handsome things. They first soften
the horn and shell in boiling water, so as to be able to submit them to the press in iron
moulds, and by means of heat form them into one mass. The degree of heat necessary
to join the horn clippings must be stronger than that for shell skins, and it can
only be found out by experience. The heat must not however be too great, for fear of
scorching the horn or shell. Considerable care is required in these operations, not to
touch the horn with the fingers, nor with any greasy body, because the grease will prevent
the perfect joining. Wooden instruments should be used to move them, while they are
at the fire, and for carrying them to the moulds.
In making a ring of horn for bell-pulls, &c., the required piece is to be first cut out
in the flat of its proper dimensions, and nearly in the shape of a horse-shoe; it is then
pressed in a pair of dies to give its surface the desired pattern; but previous to the pressure,
both the piece of horn and the dies are to be heated; the piece of horn is to be introduced
between the dies, squeezed in a vice, and when cold, the impression or pattern
will be fixed upon the horn. One particular condition, however, is to be observed in the
construction of the dies, for forming a ring. They are to be so made, that the open
ends of the horse-shoe piece of horn, after being pressed, shall have at one end a nib,
and at the other a recess of a dovetailed form, corresponding to each other; and the
second operation in forming this ring of horn is to heat it, and place it in another pair
of dies, which shall bring its open ends together, and cause the dovetailed joints to be
locked fast into each other, which completes the ring, and leaves no appearance of the
junction.
In forming the handles of table knives and forks, or other things which require to be
made of two pieces, each of the two pieces or sides of the handle is formed in a separate
pair of dies; the one piece is made with a counter-sunk groove along each side, and the
other piece with corresponding leaves or projecting edges. When these two pieces are
formed, by first being cut out of the flat horn, then pressed in the dies in a heated state,
for the purpose of giving the pattern, the two pieces are again heated and put together,
the leaves or edges of the one piece dropping into the counter-sunk grooves of the other
piece, and being introduced between another pair of heated dies, the joints are pressed
together and the two pieces formed into one handle.
In making the knobs for drawers which have metal stems or pins to fasten them into
the furniture, the face of the knob is to be first made in a die, as above described, and
then the back part of the knob with a hole in it; a metal disc of plate-iron is next provided,
in which the metal stem or screw pin is fixed, and the stem being passed through
the aperture in the back piece, and the two, that is, the back and front pieces of horn
put together, they are then heated and pressed in dies as above described; the edge of
the back piece falling into the counter-sunk groove of the front piece, while by the heat
they are perfectly cemented together.
HORNSILVER; (Argent Corné, or Kerargyre, Fr; Hornsilber, Germ.) is a white
or brownish mineral, sectile like wax or horn; and crystallizing in the cubic system.
Its specific gravity varies from 4·75 to 5·55. Insoluble in water; not volatile; fusible
at the blowpipe, but difficult of reduction by it. It deposits metallic silver when
rubbed with water upon a piece of clean copper or iron. It consists of 24·67 chlorine,
and 75·32 silver.
Hornsilver is rare in the European mines, but it occurs in great quantity in the districts
of Zacatecas, Fresnillo, and Catarce, in Mexico; and in Huantajaya, Yauricocha,
&c., in Peru; where it is abundantly mixed with the ores of hydrate of iron, called
Pacos and Colorados, interspersed with veins of metallic silver, which form considerable
deposits in the penæan limestones. There it is profitably mined as an ore of silver.
HORNSTONE; is a variety of rhomboidal quartz. Being both hard and tough,
it is well adapted to form the stones of pottery mills for grinding flints; it is called chert
in Derbyshire, where it abounds.
Hornstone occurs under three modifications; splintery hornstone, conchoidal hornstone,
and woodstone. The colours of the first two are gray, white, and red; they are
all massive; dull, or of a glimmering lustre. Translucent only on the thin edges. Difficult
to break. Hornstone is less brittle than flint; and by its infusibility before the blowpipe it
may be distinguished from petrosilex, which it resembles in external appearance. The geological
locality of hornstone is remarkable; for it occurs in both ancient and recent formations.
It is found frequently in the veins that traverse primitive crystalline rocks,
filling up the interstices, and enveloping their metallic ores. In the lead mine of Huelgoët
in Brittany it is whitish; but its prevailing colour is gray. It occurs likewise in
the middle beds of the coarse limestone (calcaire grossier) in the Paris basin, which is a[648]
comparatively modern formation, as well as in the sand beds of the upper parts of this
district, near Saint Cloud, Neuilly, &c. The hornstone which occurs in secondary
limestone is called chert by the English miners. It is valuable for forming the grinding
blocks of flint mills in the pottery manufacture.
HORSE POWER, in steam engines, is estimated by Mr. Watt at 32,000 pounds
avoirdupois lifted one foot high per minute, for one horse. M. D’Aubuisson, from an
examination of the work done by horses in the whims, or gigs (machines à molettes) for
raising ore from the mines at Freyberg, the horses being of average size and strength,
has concluded that the useful effect of a horse yoked during eight hours, by two relays
of four hours each, in a manege or mill course, may be estimated at 40 kilogrammes
raised 1 mètre per second; which is nearly 16,440 pounds raised one foot per minute;
being very nearly one half of Mr. Watt’s liberal estimate for the work of his steam
engines.
HOSIERY; (Bonnèterie, Fr.; Strumpfweberei, Germ.) The stocking frame, which is the
great implement of this business, though it appears at first sight to be a complicated machine,
consists merely of a repetition of parts easily understood, with a moderate degree
of attention, provided an accurate conception is first formed of the nature of the hosiery
fabric. This texture is totally different from the rectangular decussation which constitutes
cloth, as the slightest inspection of a stocking will show; for this, instead of
having two distinct systems of thread, like the warp and the weft, which are woven
together, by crossing each other at right angles, the whole piece is composed of a single
thread united or looped together in a peculiar manner, which is called stocking-stitch,
and sometimes chain-work.
This is best explained by the view in fig. 550. A single thread is formed into
a number of loops or waves, by arranging it
over a number of parallel needles, as shewn at
R: these are retained or kept in the form of
loops or waves, by being drawn or looped
through similar loops or waves formed by the
thread of the preceding course of the work, S.
The fabric thus formed by the union of a number
of loops is easily unravelled, because the
stability of the whole piece depends upon the
ultimate fastening of the first end of the thread;
and if this is undone, the loops formed by that
end will open, and release the subsequent loops
one at a time, until the whole is unravelled, and drawn out into the single thread from
which it was made. In the same manner, if a thread in a stocking piece fails, or
breaks at any part, or drops a stitch, as it is called, it immediately produces a hole,
and the extension of the rest can only be prevented by fastening the end. It should
be observed that there are many different fabrics of stocking-stitch for various kinds of
ornamental hosiery, and as each requires a different kind of frame or machine to produce
it, we should greatly exceed our limits to enter into a detailed description of them
all. That species which we have represented in fig. 550. is the common stocking-stitch
used for plain hosiery, and is formed by the machine called the common stocking-frame,
which is the groundwork of all the others. The operation, as we see, consists in drawing
the loop of a thread successively through a series of other loops, so long as the work
is continued, as is very plainly shown for one stitch in fig. 551.
There is a great variety of different frames in use for producing various ornamental
kinds of hosiery. The first, which forms the foundation of the whole, is that for knitting
plain hosiery, or the common stocking-frame.
Of this valuable machine, the invention of Mr. Lee of Cambridge, a side elevation is
given in fig. 552., with the essential parts. The framing is supported by four upright
posts, generally of oak, ash, or other hard wood. Two of these posts appear at A A, and
the connecting cross rails are at C C. At B is a small additional piece of framing, which
supports the hosier’s seat. The iron-work of the machine is bolted or screwed to the
upper rails of the frame-work, and consists of two parts. The first rests upon a sole of
polished iron, which appears at D, and to which a great part of the machinery is
attached. The other part, which is generally called the carriage, runs upon the iron
sole at D, and is supported by four small wheels, or trucks, as they are called by the
workmen. At the upper part of the back standard of iron are joints, one of which
appears at Q; and to these is fitted a frame, one side of which is seen extending to H.
By means of these joints, the end at H may be depressed by the hosier’s hand, and it
returns, when relieved, by the operation of a strong spring of tempered steel, acting
between a cross bar in the frame, and another below. The action of this spring is
very apparent in fig. 553. In the front of the frame, immediately opposite to where
the hosier sits, are placed the needles which form the loops. These needles, or rather[649]
hooks, are more or less numerous, according to the coarseness or fineness of the
stocking; and this, although unavoidable, proves a very considerable abatement of
the value of a stocking-frame. In
almost every other machine (for
example those employed in spinning
or weaving), it is easy to
adapt any one either to work
coarser or finer work, as it may
be wanted. But in the manufacture
of hosiery, a frame once
finished, is limited for ever in its
operation to the same quality of
work, with this exception, that
by changing the stuff, the work
may be made a little more dense
or flimsy; but no alteration in
the size or quantity of loops can
take place. Hence where the
manufacture is extensively prosecuted,
many frames may be
thrown idle by every vicissitude
of demand; and where a poor
mechanic does purchase his own
frame, he is for ever limited to
the same kind of work. The
gauge, as it is called, of a stocking-frame
is regulated by the
number of loops contained in
three inches of breadth, and varies very much; the coarsest frames in common use being
about what are termed Fourteens, and the finest employed in great extent about
Forties. The needles are of iron wire, the manufacture of which is very simple; but
long practice in the art is found necessary before a needle-maker acquires the dexterity
which will enable him both to execute his work well, and in sufficient quantity to
render his labour productive.
The process of making the needles is as follows:—Good sound iron wire, of a proper
fineness, is to be selected; that which is liable to split or splinter, either in filing,
punching, or bending, being totally unfit for the purpose. The wire is first to be cut
into proper lengths, according to the fineness of the frame for which the needles are
designed, coarse needles being considerably longer than fine ones. When a sufficient
number (generally some thousands) have been cut, the wire must be softened as much
as possible. This is done by laying them in rows in a flat iron box, about an inch
deep, with a close cover; the box being filled with charcoal between the strata of wires.
This box, being placed upon a moderate fire, is gradually heated until both the wires
and charcoal have received a moderate red heat, because, were the heat increased to what
smiths term the white heat, the wire would be rendered totally unfit for the subsequent
processes which it has to undergo, both in finishing and working. When the box has
been sufficiently heated, it may be taken from the fire, and placed among hot ashes,
until both ashes and box have gradually cooled; for the slower the wires cool, the
softer and easier wrought they will be. When perfectly cool, the next process is to
punch a longitudinal groove in the stem of every needle, which receives the point or
barb, when depressed. This is done by means of a small engine worked by the power
of a screw and lever. The construction of these engines is various; but a profile
elevation of one of the most simple and commonly
used will be found in fig. 553. It
consists of two very strong pieces of malleable
iron, represented at A and C, and these
two pieces are connected by a strong well-fitted
joint at B. The lower piece, or sole of
the engine at C, is screwed down by bolts to
a strong board or table, and the upper piece
A will then rise or sink at pleasure, upon the
joint B. In order that A may be very steady
in rising and sinking, which is indispensable
to its correct operation, a strong bridle of
iron, which is shewn in section E, is added to confine it, and direct its motion. In
the upper part of this bridle is a female screw, through which the forcing screw passes,
which is turned by the handle or lever D. To the sole of the engine C is fixed a bolster[650]
of tempered steel, with a small groove to receive the wire, which is to be punched; and
in the upper or moving part A, is a sharp chisel, which descends exactly into the groove,
when A is depressed by the screw. These are represented at F, and above H. At G is a
strong spring, which forces up the chisel when the pressure of the screw is removed. The
appearance of the groove, when the punching is finished, will be rendered familiar by
inspecting fig. 554., p. 651. When the punching is finished, the wires are to be brought
to a fine smooth point by filing and burnishing, the latter of which should be very completely
done, as, besides polishing the wire, it tends greatly to restore that spring and
elasticity which had been removed by the previous operation of softening. The wire is
next to be bent, in order to form the hook or barb; and this is done with a small piece
of tin plate bent double, which receives the point of the wire, and by its breadth regulates
the length of the barb. The stem of the needle is now flattened with a small
hammer, to prevent it from turning in the tin socket in which it is afterwards to be cast;
and the point of the barb being a little curved by a pair of small plyers, the needle is
completed.
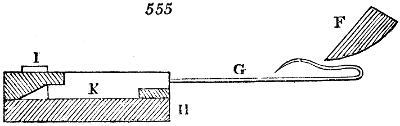
In order to fit the needles for the frame, they are now cast into the tin sockets, or leads
as they are called by the workmen; and this is done by placing the needles in an iron
mould, which opens and shuts by means of a joint, and pouring in the tin while in a
state of fusion. In common operations, two needles are cast into the same socket. The
form of the needle, when complete and fitted to its place in the frame, will be seen in
fig. 555., which is a profile section
of the needle-bar exhibiting one
needle. In this figure a section of
the presser is represented at F;
the needle appears at G, and the
socket or level at K. At H, is a
section of the needle-bar, on the
fore part of which is a small plate
of iron called a verge, to regulate the position of the needles. When placed upon
the bar resting against the verge, another plate of iron, generally lined with soft leather,
is screwed down upon the sockets or leads, in order to keep them all fast. This plate
and the screw appear at I. When the presser at F, is forced down upon the barb, this
sinks into the groove of the stem, and the needle is shut; when the presser rises, the
barb opens again by its own elasticity.
The needles or hooks being all properly fitted, the next part of the stocking-frame to
which attention ought to be paid, is the machinery for forming the loops; and this consists
of two parts. The first of these, which sinks between every second or alternate
needle, is represented at O, fig. 552., and is one of the most important parts of the whole
machine. It consists of two moving parts; the first being a succession of horizontal
levers moving upon a common centre, and called jacks, a term applied to vibrating levers
in various kinds of machinery as well as the stocking-frame. One only of these jacks
can be represented in the profile fig. 552.; but the whole are distinctly shown in a horizontal
position in fig. 556.; and a profile upon a very enlarged scale is given in fig. 557.[651]
The jack shewn in fig. 552., extends horizontally from O to I, and the centre of motion
is at R. On the front, or right hand part of the jack at O, is a joint suspending a very
thin plate of polished iron, which is termed a sinker. One of these jacks and sinkers is
allotted for every second or alternate needle. The form of the sinker will appear at S,
fig. 557.; and in order that all may be exactly uniform in shape, they are cut out and
finished between two stout pieces of iron, which serve as moulds or gauges to direct the
frame-smith. The other end of the jack at I, is tapered to a point; and when the jacks
are in their horizontal position, they are secured by small iron springs, one of which is
represented at I, fig. 552., each spring having a small obtuse angled notch to receive the
point of the jack, against which it presses by its own elasticity. In fig. 557. the centre is
at R; the pointed tail is omitted for want of room, the joint is at O, and the throat of the
sinker, which forms the loop, is at S. The standards at R, upon which the jack moves,
are called combs, and consist of pieces of flat smooth brass, parallel to, and equidistant
from each other. The cross-bar R, which contains the whole, is of iron, with a perpendicular
edge or rim on each side, leaving a vacancy between them, or a space to receive
the bottom part or tails of the combs. The combs are then placed in the bar, with a
flat piece of brass called a countercomb, between each, to ascertain and preserve their
distances from each other. These countercombs are exactly of the same shape as the
combs, but have no tails. When both combs and countercombs are placed in the bar,
it is luted with clay so as to form a mould, into which is poured a sufficient quantity of
melted tin. When the tin has had time to cool, the countercombs having no tails are
easily taken out, and the combs remain well fastened and secured by the tin, which has
been fused entirely round them. Thus they form a succession of standards for the
jacks; and a hole being drilled through each jack and each comb, one polished wire
put through, serves as a common centre for the whole.
The jack sinkers being only used for every alternate or second needle, in order to
complete this part of the apparatus, a second set of sinkers is employed. These are, in
form and shape, every way the same as the jack sinkers, but they are jointed at the top
into pieces of tin, all of which are screwed to the sinker bar H, fig. 552.; and thus a
sinker of each kind descends between the needles alternately. By these sinkers the
loops are formed upon all the needles, and the reason of two sets different in operation
being employed, will be assigned in describing the mode of working the frame. The
presser of the operation, of which something has already been said, appears at F; and
of the two arms which support and give motion to it, one appears very plainly at E, its
centre of motion being at C. The circular bend given to these arms, besides having an
ornamental effect, is very useful,
in order to prevent any part from
interfering with the other parts
which are behind, by elevating
them entirely above them. The
extremity of these arms at the
termination of the bends behind,
are connected by a cross bar,
which has also a circular bend
in the middle, projecting downwards,
for a reason similar to that
already assigned. This bend is
concealed in fig. 552., but visible
in the front elevation, fig. 558.
From the middle of the bend,
the presser is connected with the
middle treadle by a depending
wire appearing at M, fig. 552., and
thus, by the pressure of that
treadle, the presser is forced down
to close the barbs of the needle.
The re-ascent of the presser is
sometimes effected by means of
a counterpoising weight passing
over a pulley behind; and sometimes
by the reaction of a wooden
spring, formed of a strong hoop
like that represented at K. The
latter of these is preferred, especially
by the Nottingham hosiers,
because, as they assert, it makes
the presser spring up with greater[652]
rapidity, and consequently saves time in working. How far this may be practically the
case, it would be superfluous here to investigate; but it is obvious that the wooden
spring, if very stiff, must add much to the hosier’s exertion of his foot, already exercised
against the united spring of all his barbs; and this inconvenience is much complained
of by those who have been accustomed to work with the counterpoise.
At L are two pulleys or wheels, of different diameters, moving upon a common centre,
by which the jack sinkers are relieved from the back springs, and thrown downwards to
form the loops upon the needles. About the larger wheel is a band of whipcord, passing
twice round, the extremities of which are attached to what is called the slur, which
disengages the jacks from the back springs. The smaller pulley, by another band,
communicates with the right and left treadle; so that these treadles, when pressed
alternately, turn the pulleys about in an inverted order. The directions of these bands
also appear more plainly in the front elevation, fig. 558. The construction of the slur,
and its effect upon the jacks, will also be rendered apparent by fig. 559. In this
figure, eight jacks are represented in section, the tail part of three of which, 1, 2, 3, are
thrown up by the slur in its progress from left to right; the fourth is in the act of
rising, and the remaining four, 5, 6, 7, and 8, are still unacted upon, the slur not yet
having reached them. As the slur acts in the direction of the dotted line X, X, fig. 556.,
behind the centres of the jacks, it is hardly necessary to remark, that this forcing up of the
tails must of course depress the joints by which the sinkers in front are suspended. The
jack sinkers falling successively from the loops on every alternate needle, in the way represented
at fig. 560., where both kinds
of sinkers appear in section, the
light part expressing what is above
the point at which the throat of the
sinker operates upon the thread, and
the dark part what is below. The
second set, or, as they are called, the
lead sinkers, from the manner of
jointing them, and suspending them from the bar above, appear still elevated; the
position of the bar being represented by the line A, B. But when these are pulled down
to the level of the former by the operator’s hands, the whole looping will be completed,
and the thread C, D, which is still slack, will be brought to its full and proper degree of
tension, which is regulated by stop screws, so as to be tempered or altered at pleasure.
The sinking of this second set of
sinkers, may be easily explained by
fig. 561. The direction of the sinkers
is expressed by the line E; the
bar from which they are suspended
will be at A; the top frame is in the
direction from A to B; the back
standards at D, and the joint at B, is
the centre of motion. If E is pulled
perpendicularly downwards, the spring C, will be contracted, and its upper extreme point
G, will be brought nearer to its lower extreme point F, which is fixed. Again, when the
force which has depressed E is removed, the spring C will revert to its former state, and
the sinkers will rise. The raising of the jack sinkers and jacks takes place at the
same time, by the hosier raising his hands; and for the cause of this we must revert
to fig. 556. The lead sinkers in rising, lay hold of notches, which raise the extreme
parts of the set of jacks Z, Z, which are called half-jacks. Between the extremities of
these at Z, Z, is a cross bar, which, in descending, presses all the intermediate jacks
behind the common centre, and restores them to their original posture, where they are
secured by the back springs, until they are again relieved by the operation of the slur
recrossing at the next course.
Working of the frame.—In order to work a frame, the whole apparatus being previously
put into complete order, the hosier places himself on the seat B in front, and provides
himself with a bobbin of yarn or stuff. This bobbin he places loosely on a vertical
pin of wire, driven into one side of the frame contiguous to the needles, so that it may
turn freely as the stuff is unwound from it. Taking the thread in his hand, he draws it
loosely along the needles, behind the barbs, and under the throats of the sinkers. He
then presses down one of the treadles to pass the slur along, and unlock the jacks from
the back springs, that they may fall in succession. When this is done, the number of
loops thus formed is doubled by bringing down the lead sinkers, and the new formed
loops are lodged under the barbs of the needles by bringing forward the sinkers. The
preceding course, and former fabric, being then again pushed back, the barbs are shut
by depressing the middle treadle, and forcing down the presser upon the needles. The
former work is now easily brought over the shut needles, after which, by raising the[653]
hands, both sets of sinkers are raised; the jacks are locked by the back springs, and the
hosier goes on to another course.
From this it will be apparent, that the remark made in the outset is well founded,
that there are in reality, no complicated or difficult movements in the stocking-frame.
Almost the whole are merely those of levers moving upon their respective fulcra, excepting
that of the carriage which gives the horizontal motion to the sinkers, and that is merely
an alternate motion on four wheels. Yet the frame is a machine which requires considerable
experience and care, both to work it to advantage, and also to keep it in good
order. This circumstance arises greatly from the small compass in which a number of
moving parts must be included. Owing to this, the needles, unless cautiously and delicately
handled, are easily bent or injured. The same circumstance applies with equal
or greater force to the sinkers, which must be so very thin as to be easily injured. But
as these must work freely, both in a perpendicular and horizontal direction between the
needles, in a very confined and limited space, the slightest variation in either, from being
truly and squarely placed, unavoidably injures the others. When a hosier, either ignorant
of the mechanical laws, of their relation to each other, or too impatient to wait for
the assistance of another, attempts to rectify defects, he in most cases increases them tenfold,
and renders the machine incapable of working at all, until repaired by some more
experienced person. This circumstance has given rise to a set of men employed in this
trade, and distinguished by the name of upsetters; and these people, beside setting new
frames to work, have frequently more employment in repairing old ones injured by
want of care or skill, than many country apothecaries, who live in unhealthy parishes,
find in tampering with the disorders of mankind.
It seems unnecessary to go further into detail respecting a machine so well known,
and which requires practical attention even more than most others. It may, therefore,
be sufficient to describe shortly some of its varieties, the most simple and common of
which is the rib stocking-frame.
Rib stocking-frame.—This frame, which, next to the common frame, is most extensively
in use, is employed for working those striped or ribbed stockings, which are very
common in all the different materials of which hosiery is formed. In principle it does
not differ from the common frame, and not greatly in construction. The preceding general
description will nearly apply to this machine with equal propriety as to the former:
that part, however, by which the ribs or stripes are formed, is entirely an addition, and
to the application of this additional machinery it may be proper to pay the chief attention,
referring chiefly to fig. 558., which is a front elevation.
This figure has been already referred to for the illustration of those parts of
the machinery which are common to both, and those parts therefore require no recapitulation.
The principle of weaving ribbed hosiery possesses considerable affinity to
that which subsists in the weaving of that kind of cloth which is distinguished by the
name of tweeling, for the formation of stripes, with some variation arising merely from
the different nature of the fabric. In cloth weaving, two different kinds of yarn intersecting
each other at right angles, are employed; in hosiery only one is used. In the
tweeling of cloth, striped as dimity, in the cotton or kerseymere, and in the woollen manufacture,
the stripes are produced by reversing these yarns. In hosiery, where only one
kind of yarn is used, a similar effect is produced by reversing the loops. To effect this
reversing of the loops, a second set of needles is placed upon a vertical frame, so that
the bends of the hooks may be nearly under those of the common needles. These
needles are cast into tin moulds, pretty similar to the former, but more
oblique or bevelled towards the point, so as to prevent obstructions in
working them. They are also screwed to a bar of iron, generally lighter
than the other, and secured by means of plates: this bar is not fixed,
but has a pivot in each end, by means of which the bar may have a kind
of oscillatory motion on these pivots. Two frames of iron support this
bar; that in which it oscillates being nearly vertical, but inclined a little
towards the other needles. Fig. 562., which is a profile elevation, will
serve to illustrate the relative position of each bar to the other. The
lower or horizontal frame, the ends only of which can be seen in fig.
558. under a a, appears in profile in fig. 562., where it is distinguished
by d. The vertical frame at a is attached to this by two centre screws,
which serve as joints for it to move in. On the top of this frame is the
rib-needle bar at f, in figs. 552. and 562., and one needle is represented
in fig. 562. at f. At g is a small presser, to shut the barbs of the rib-needles,
in the same manner as the large one does those of the frame.
At h is one of the frame needles, to show the relative position of the one
set to the other. The whole of the rib-bar is not fitted with needles
like the other; for here needles are only placed where ribs or stripes
are to be formed, the intervals being filled up with blank leads, that is[654]
to say, with sockets of the same shape as the others, but without needles; being merely
designed to fill the bar and preserve the intervals. Two small handles depend from the
needle bar, by which the oscillatory motion upon the upper centres is given. The rising
and sinking motion is communicated to this machine by chains which are attached to
iron sliders below, and which are wrought by the hosier’s heel when necessary. The
pressure takes place partly by the action of the small presser, and partly by the motion
of the needles in descending, A small iron slider is placed behind the rib-needles,
which rises as they descend, and serves to free the loops perfectly from each other.
In the weaving of ribbed hosiery, the plain and rib courses are wrought alternately.
When the plain are finished, the rib-needles are raised between the others, but no additional
stuff is supplied. The rib-needles intersecting the plain ones, merely lay hold
of the last thread, and, by again bringing it through that which was on the rib-needle
before, give it an additional looping, which reverses the line of chaining, and raises the
rib above the plain intervals, which have only received a single knitting.
HOT-FLUE, is the name given in England to an apartment heated by stoves or
steam pipes, in which padded and printed calicoes are dried hard. Fig. 563. represents
the simplest form of such a flue, heated by the vertical round iron stove C, from whose
top a wide square pipe proceeds upwards in a slightly inclined direction, which receives
the current of air heated by the body and capital of the stove. In this wide channel
there are pullies, with cords or bands which, suspend by hooks, and conduct the web of[655]
calico, from the entrance at B, where the operative sits, to near the point A, and back
again. This circuit may be repeated once or oftener till the goods are perfectly dried.
At D the driving pulley connected with the main shaft is shown. Near the feet of the
operative is the candroy or reel upon which the moist goods are rolled in an endless
web; so that their circulation in the hot-air channel can be continued without interruption,
as long as may be necessary.
Fig. 564. is a cross section of the apparatus of the regular hot-flue, as it is mounted
in the most scientific calico works of
England, those of James Thomson, Esq.,
of Primrose, near Clitheroe, Lancashire.
a a a a, is an arched apartment, nearly 30
yards long, by 13 feet high, and 10 feet
wide. Through about one half of this
gallery there is a horizontal floor supported
on arches, above which is the driest
space, through which the goods are finally
passed before they escape from the hot-flue,
after they have been previously exposed to
the hot but somewhat moist air of the
lower compartment. A large square flue
covered with cast-iron plates runs along
the whole bottom of the gallery. It is
divided into two long parallel vaults,
whose sections are seen at u, u, fig. 564.,
covered with the cast-iron plates v v,
grooved at their ends into one another. The
thickness of these plates is increased progressively
as they come nearer to the fireplace
or furnace. There are dampers which regulate the draught, and of course the heat
of the stove. h h are the air-passages or vent-holes, left in the side walls, and which by
means of a long iron rod, mounted with iron plates, may be opened or closed together
to any degree. k k are the cast-iron supports of the tinned brass rollers which guide
the goods along, and which are fixed to the cross pieces represented by r r, fig. 564.
l l are iron bars for supporting the ventilators or fans (see the fan under Foundry).
These fans are here enclosed within a wire grating. They make about 300 turns per
minute, and expel the moist air with perfect effect. s indicates the position of the
windows, which extend throughout the length of the building. t is a gas-light jet,
placed at the side of each window to supply illumination for night work.
The piece is stretched along the whole extent of the gallery, and runs through it in
the course of one minute and a half; being exposed during its passage to the heat of
212° Fahr.
In fig. 565., A is the iron door of entrance to the hot-flue gallery; at b is the padding
machine, where the goods are imbued with the general mordant. The speed of
this machine may be varied by means of the two conical drums c c, which drive it; since
when the band c c, is brought by its forks, and adjusting screws, nearer to the narrow
end of the lower drum, the cylinder upon the same shaft with the latter is driven
quicker; and vice versa. Over D D the cords are shown for drawing the drum mechanism
into geer with the main shaft band F, F, E; or for throwing it out of geer. The
pullies F F carry the bands which transmit the motion to the padding machine. A
cylindrical drum exterior to the hot-flue, covered with flannel, serves to receive the end
of the series of pieces, and to draw them through the apartment. This mode of drying[656]
the padded calicoes requires for each piece of 28 yards, 3 pounds of coals for the furnace
when a fan is employed, and 4 pounds without it.
HYDRATES; are compounds of the oxides, salts, &c. with water in definite or
equivalent proportions. Thus slaked lime consists of one atom of quick-lime = 28, +
one atom of water = 9, of which the sum is 37 on the hydrogen scale.
HYDRAULIC PRESS. See Oil, Press, and Stearine.
HYDRIODIC ACID; (Acide Hydriodique, Fr.; Hydriodsäure, Germ.) is an acid
formed by the combination of 99·21 parts of iodine, and 0·79 hydrogen. When pure,
it occurs in the gaseous state, but it combines with water like the hydrochloric or muriatic
acid gas into a liquid acid.
HYDROCHLORIC ACID; the new chemical name of muriatic acid, which see.
HYDROGEN; (Eng. and Fr.; Wasserstoff, Germ.) an undecompounded gaseous
body; the lightest of all ponderable matter, whose examination belongs to chemistry.
HYDROMETER; an instrument for ascertaining the specific gravities of liquids.
Baumé’s hydrometer, which is much used in France, and other countries of the continent
of Europe, when plunged in pure water, at the temperature of 58° Fahr., marks 0 upon
its scale; in a solution containing 15 per cent. of common salt, (chloride of sodium)
and 85 of water by weight, it marks 15°; so that each degree is meant to indicate a
density corresponding to one per cent. of that salt. See Areometer, for comparative
tables of hydrometers.
HYDROSULPHURETS; chemical compounds of bases with sulphuretted hydrogen.
HYMENŒA COURBARIL; a tree growing in South America, from which the
resin animé exudes.
HYOSCIAMUS NIGER. Henbane is a plant used in medicine, from which
modern chemistry has extracted a new crystalline vegetable principle called hyosciamine,
which is very poisonous, and when applied in solution to the eye, determines a remarkable
dilatation of the pupil; as belladonna also does.
HYPOSULPHATES; Hyposulphites; saline compounds of the hyposulphuric
or hyposulphurous acid with bases.
HYPEROXYMURIATES; the old and incorrect name of Chlorates.
JACK, called also jack in a box, and hand-jack, is a portable, mechanical instrument,
consisting of a rack and pinion, or a pair of claws and ratchet bar, moved by a winch
handle, for raising heavy weights a little way off the ground.
JACK and JACK-SINKERS, are parts of a stocking frame; see Hosiery.
JACK-BACK, is the largest jack of the brewer.
JACQUARD. A peculiar and most ingenious mechanism, invented by M. Jacquart
of Lyons, to be adapted to a silk or muslin loom for superseding the employment
of draw-boys, in weaving figured goods. Independently of the ordinary play of the
warp threads for the formation of the ground of such a web, all those threads which
should rise simultaneously to produce the figure, have their appropriate healds, which a
child formerly raised by means of cords, that grouped them together into a system, in
the order, and at the time desired by the weaver. This plan evidently occasioned no
little complication in the machine, when the design was richly figured; but the apparatus
of Jacquart, which subjects this manœuvre to a regular mechanical operation, and
derives its motion from a simple pedal put in action by the weaver’s feet, was generally
adopted soon after its invention in 1800. Every common loom is susceptible of receiving
this beautiful appendage. It costs in France, 200 francs, or 8l. sterling; and a little
more in this country.
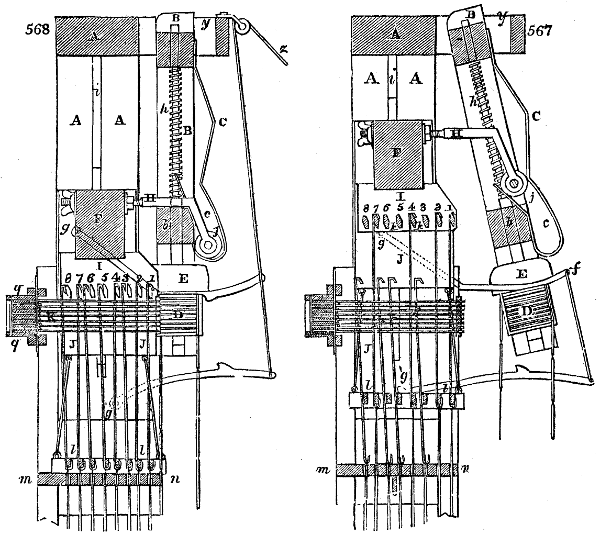
Fig. 566. is a front elevation of this mechanism, supposed to be let down. Fig. 567.
is a cross section, shown in its highest position. Fig. 568. the same section as the preceding,
but seen in its lower position.
A, is the fixed part of the frame, supposed to form a part of the ordinary loom; there
are two uprights of wood, with two cross-bars uniting them at their upper ends, and
leaving an interval x y, between them, to place and work the movable frame B, vibrating
round two fixed points a a, placed laterally opposite each other, in the middle of the
space x y, fig. 566.
C, is a piece of iron with a peculiar curvature, seen in front, fig. 566., and in profile,
figs. 567. and 568. It is fixed on one side upon the upper cross-bar of the frame B, and
on the other, to the intermediate cross-bar b of the same frame, where it shows an
inclined curvilinear space c, terminated below by a semi-circle.
D, is a square wooden axis, movable upon itself round two iron pivots, fixed into its two[657]
ends; which axis occupies the bottom of the movable frame B. The four faces of this
square axis are pierced with three round, equal, truly-bored holes, arranged in a quincunx.
The teeth a, fig. 570., are stuck into each face, and correspond to holes a,
fig. 573., made in the cards which constitute the endless chain for the healds; so that
in the successive application of the cards to each face of the square axis, the holes pierced
in one card may always fall opposite to those pierced in the other.[658]
The right-hand end of the square axis, of which a section is shown in double size,
fig. 569., carries two square plates of sheet iron d, kept parallel to each other and
a little apart, by four spindles e, passed opposite to the corners. This is a kind of
lantern, in whose spindles, the hooks of the levers f f′, turning round fixed points g g′
beyond the right hand upright A, catch hold, either above or below at the pleasure of the
weaver, according as he merely pulls or lets go the cord z, during the vibratory movement
of the frame B.
E is a piece of wood shaped like a T, the stem of which prolonged upwards, passes
freely through the cross-bar b, and through the upper cross-bar of the frame B, which serve
as guides to it. The head of the T piece being applied successively against the two
spindles e, placed above in a horizontal position, first by its weight, and then by the
spiral spring h, acting from above downwards, keeps the square axis in its position, while
it permits it to turn upon itself in the two directions. The name press is given to the
assemblage of all the pieces which compose the movable frame B B.
F is a cross-bar made to move in a vertical direction by means of the lever G, in the
notches or grooves i, formed within the fixed uprights A.
H, is a piece of bent iron, fixed by one of its ends with a nut and screw, upon the cross-bar
F, out of the vertical plane of the piece C. Its other end carries a friction roller J,
which working in the curvilinear space c of the piece C, forces this, and consequently the
frame B to recede from the perpendicular, or to return to it, according as the cross-bar
F is in the top or bottom of its course, as shown in figs. 567. and 568.
I, cheeks of sheet iron attached on either side to the cross-bar F, which serve as a safe
to a kind of claw K, composed here of eight small metallic bars, seen in section fig. 567.
and 568., and on a greater scale in fig. 570.
J, upright skewers of iron wire, whose tops bent down hook-wise, naturally place
themselves over the little bars K. The bottom of these spindles likewise hooked in the
same direction as the upper ones, embraces small wooden bars l, whose office is to keep
them in their respective places, and to prevent them from twirling round, so that the
uppermost hooks may be always directed towards the small metallic bars upon which
they impend. To these hooks from below are attached strings, which after having
crossed a fixed board m n, pierced with corresponding holes for this purpose, proceed
next to be attached to the threads of the loops destined to lift the warp threads. K K,
horizontal spindles or needles, arranged here in eight several rows, so that each spindle
corresponds both horizontally and vertically to each of the holes pierced in the four faces
of the square axis D. There are therefore as many of these spindles as there are holes
in one of the faces of the square.

Fig. 571. represents one of these horizontal spindles. n is an eyelet through which the
corresponding vertical skewer passes. o another elongated eyelet, through which a small
fixed spindle passes to serve as a guide, but which does not hinder it from moving length-wise,
within the limits of the length of the eyelet. p, small spiral springs placed in each
hole of the case q q, fig. 570. They serve the purpose of bringing back to its primitive
position, every corresponding needle, as soon as it ceases to press upon it.
Fig. 572. represents the plan of the upper row of horizontal needles. Fig. 573. is a
fragment of the endless chain, formed with perforated cards, which are made to circulate[659]
or travel by the rotation of the shaft D. In this movement, each of the perforated cards,
whose position, form, and number, are determined by the operation of tying-up of the
warp, comes to be applied in succession against the four faces of the square axis or drum,
leaving open the corresponding holes, and covering those upon the face of the axis, which
have no corresponding holes upon the card.
Now let us suppose that the press B is let down into the vertical position shown in
fig. 568.; then the card applied against the left face of the axis, leaves at rest or
untouched the whole of the horizontal spindles (skewers), whose ends correspond to these
holes, but pushes back those which are opposite to the unpierced part of the card; thereby
the corresponding upright skewers, 3. 5. 6. and 8. for example, pushed out of the perpendicular,
unhook themselves from above the bars of the claw, and remain in their place, when
this claw comes to be raised by means of the lever G; and the skewers 1. 2. 4. and 7.,
which have remained hooked on, are raised along with the warp threads attached to them.
Then by the passage across of a shot of the colour, as well as a shot of the common weft,
and a stroke of the lay after shedding the warp and lowering the press B, an element or
point in the pattern is completed.
The following card, brought round by a quarter revolution of the axis, finds all the
needles in their first position, and as it is necessarily perforated differently from the preceding
card, it will lift another series of warp threads; and thus in succession for all the
other cards, which compose a complete system of a figured pattern.
This machine, complicated in appearance, and which requires some pains to be understood,
acts however in a very simple manner. Its whole play is dependent upon the
movement of the lever G, which the weaver himself causes to rise and fall, by means of
a peculiar pedal; so that without the aid of any person, after the piece is properly read in
and mounted, he can execute the most complex patterns, as easily as he could weave
plain goods; only attending to the order of his weft yarns, when these happen to be of
different colours.
If some warp yarns should happen to break without the weaver observing them, or
should he mistake his coloured shuttle yarns, which would so far disfigure the pattern,
he must undo his work. For this purpose, he makes use of the lower hooked lever f′,
whose purpose is to make the chain of the card go backwards, while working the loom
as usual, withdrawing at each stroke the shot both of the ground and of the figure.
The weaver is the more subject to make mistakes, as the figured side of the web is
downwards, and it is only with the aid of a bit of looking-glass that he takes a peep of
his work from time to time. The upper surface exhibits merely loose threads in different
points, according as the pattern requires them to lie upon the one side or the other.
Thus it must be evident, that such a number of paste-boards are to be provided and
mounted as equal the number of throws of the shuttle between the beginning and end of
any figure or design which is to be woven; the piercing of each paste-board individually,
will depend upon the arrangement of the lifting rods, and their connection with
the warp, which is according to the design and option of the workman; great care must
be taken that the holes come exactly opposite to the ends of the needles; for this purpose
two large holes are made at the ends of the paste-boards, which fall upon conical points,
by which means they are made to register correctly.
It will be hence seen, that, according to the length of the figure, so must be the
number of paste-boards, which may be readily displaced so as to remount and produce
the figure in a few minutes, or remove it, or replace it, or preserve the figure for future
use. The machine, of course, will be understood to consist of many sets of the lifting
rods and needles, shown in the diagram, as will be perceived by observing the disposition
of the holes in the paste-board; those holes, in order that they may be accurately
distributed, are to be pierced from a gauge, so that not the slightest variation shall take
place.
To form these card-slips, an ingenious apparatus is employed, by which the proper
steel punches required for the piercing of each distinct card, are placed in their relative
situations preparatory to the operation of piercing, and also by its means a card may be
punched with any number of holes at one operation. This disposition of the punches is
effected by means of rods connected to cords disposed in a frame, in the nature of a false
simple, on which the pattern of the work to be performed is first read in.
These improved pierced cards, slips, or paste-boards, apply to a weaving apparatus,
which is so arranged that a figure to be wrought can be extended to any distance along
the loom, and by that means the loom is rendered capable of producing broad figured
works; having the long lever G placed in such a situation that it affords power to the foot
of the weaver, and by this means enables him to draw the heaviest morintures and
figured works, without the assistance of a draw-boy.
The machinery for arranging the punches, consists of a frame with four upright
standards and cross-pieces, which contains a series of endless cords passing under a
wooden roller at bottom, and over pulleys at the top. These pulleys are mounted on[660]
axles in two frames, placed obliquely over the top of the standard frame, which pulley-frames
constitute the table commonly used by weavers.
In order better to explain these endless cords, fig. 574. represents a single endless
cord 1, 1, which is here shown in operation, and part of another endless cord 2, 2, shown
stationary. There must be as many endless cords
in this frame as needles in the weaving-loom. a is
the wooden cylinder, revolving upon its axis at the
lower part of the standards; b b, the two pulleys of the
pulley-frames above, over which the individual endless
cord passes; c is a small traverse ring. To each of
these rings a weight is suspended by a single thread,
for the purpose of giving tension to the endless cord.
d is a board resembling a common comber-bar, which
is supported by the cross-bars of the standard frame,
and is pierced with holes, in situation and number,
corresponding with the perpendicular threads that pass
through them; which board keeps the threads distinct
from each other.
At e, the endless cord passes through the eyes of
wires resembling needles, which are contained in a
wooden box placed in front of the machine, and shown
in this figure in section only. These wires are called
the punch-projectors; they are guided and supported by
horizontal rods and vertical pins, the latter of which
pass through loops formed at the hinder part of the
respective wires. At f are two horizontal rods extending
the whole width of the machine, for the purpose
of producing the cross in the cords; g is a thick
brass plate, extending along in front of the machine,
and lying close to the box which holds the punch-projectors;
this plate g, shown also in section, is called the punch-holder; it contains the
same number of apertures as there are punch-projectors, and disposed so as to correspond
with each other. In each of these apertures, there is a punch for the purpose of
piercing the cards, slips, or pasteboards with holes; h is a thick steel plate of the same
size as g, and shown likewise in section, corresponding also in its number of apertures,
and their disposition, with the punch-projectors and the punch-holder. This plate h, is
called the punch-receiver.
The object of this machine is to transfer such of the punches as may be required for
piercing any individual card from the punch-holder g, into the punch-receiver h; when
they will be properly situated, and ready for piercing the individual card or slip, with
such holes as have been read in upon the machine, and are required for permitting the
warp threads to be withdrawn in the loom, when this card is brought against the ends
of the needles. The process of transferring the patterns to the punches will be effected
in the following manner.
The pattern is to be read in, according to the ordinary mode, as in a false simple,
upon the endless cords below the rods f, and passed under the revolving wooden cylinder
a, to a sufficient height for a person in front of the machine to reach conveniently. He
there takes the upper threads of the pattern, called the beard, and draws them forward
so as to introduce a stick behind the cords thus advanced, as shown by dots, for the purpose
of keeping them separate from the cords which are not intended to be operated
upon. All the punch-projectors which are connected with the cords brought forward,
will be thus made to pass through the corresponding apertures of the punch-holder g,
and by this means will project the punches out of these apertures, into corresponding
apertures of the punch-receiver h. The punches will now be properly arranged for
piercing the required holes on a card or slip, which is to be effected in the following
manner.
Remove the punch-receivers from the front of the machine; and having placed one
of the slips of card or pasteboard between the two folding plates of metal, completely
pierced with holes corresponding to the needles of the loom, lay the punch-receiver upon
those perforated plates; to which it must be made to fit by mortises and blocks, the
cutting parts of the punches being downwards. Upon the back of the punch-receiver
is then to be placed a plate or block, studded with perpendicular pins corresponding to
the above described holes, into which the pins will fall. The plates and the blocks thus
laid together, are to be placed under a press, by which means the pins of the block will
be made to pass through the apertures of the punch-receiver; and wherever the punch
has been deposited in the receiver by the above process, the said punches will be forced
through the slip of pasteboard, and pierced with such holes as are required for producing
the figured design in the loom.
[661]
Each card being thus pierced, the punch-receiver is returned to its place in front of
the machine, and all the punches forced back again into the apertures of the punch-holder
as at first. The next set of cords is now drawn forward by the next beard,
as above described, which sends out the punch-projectors as before, and disposes the
punches in the punch-receiver, ready for the operation of piercing the next card. The
process being thus repeated, the whole pattern is, by a number of operations, transferred
to the punches, and afterwards to the cards or slips, as above described.
JADE; axe-stone; (Nephrite, Ceraunite, Fr.) is a mineral commonly of a greenish
colour, compact, and of a fatty lustre. Spec. grav. 2·95; scratches glass, is very tough;
fuses into a white enamel. Its constituents are, silica 50·5; alumina 10; magnesia 31;
oxide of iron 5·50; oxide of chrome 0·05; water 2·75. It comes from China, is used
among rude nations for making hatchets; and is susceptible of being cut into any
form.
JAPANNING, is a kind of varnishing or lacquering, practised with excellence by
the Japanese, whence the name. See Varnish.
JASPER; (Jaspe calcedoine, Fr.; Jaspis, Germ.) is a sub species of calcedony
quartz, of which there are five varieties. 1. The Egyptian red and brown, with ring
or tendril-shaped delineations. 2. Striped jasper. 3. Porcelain jasper. 4. Common
jasper. 5. Agate jasper. The prettiest specimens are cut for seals, and for the inferior
kinds of jewellery ornaments. See Lapidary.
ICEHOUSE; (Glacière, Fr.; Eishaus, Germ.) Under the article Freezing, I
have enumerated the different artificial methods of producing cold. But for the uses
of common life, in these climates, the most economical and convenient means of refrigeration
in hot weather may be procured by laying up a store of ice in winter, in
such circumstances as will preserve it solid during summer.
An icehouse should not be regarded as an object of mere luxury, for pleasing the palates
of gourmands with iced creams and orgeats. In the southern countries of Europe
it is considered among people in easy circumstances as an indispensable appendage to a
country mansion. During the Dog-days, especially at those periods, and in those districts
where the sirocco blows, a lassitude and torpor of mind and body supervene, with
indigestion or total loss of appetite, and sometimes dysenteries, which are obviously occasioned
by the excessive heat, and are to be prevented or counteracted chiefly by the use
of cold beverages. By giving tone to the stomach, iced drinks immediately restore the
functions of the nervous and muscular systems when they are languid; while they enable
persons in health to endure without much inconvenience an atmosphere so close and
sultry as would be intolerable without this remedy. Icehouses, moreover, afford to
country gentlemen, a great advantage in enabling them to preserve their fish, butcher
meat, dead poultry, and game, which would otherwise, in particular states of the weather,
immediately spoil. Considering at how little expense and trouble an icehouse can be
constructed, it is surprising that any respectable habitation in the country should not have
one attached to it. The simplest and most scientific form is a double cone, that is, two
cones joined base to base; the one being of stones or brickwork, sunk under ground
with its apex at the bottom, into which the ice is rammed; the other being a conical
roof of carpentry covered with thatch, and pointed at top. The entrance should be placed
always on the north side; it should consist of a corridor or porch with double doors, and
be screened from the sunbeams by a small shrubbery. Such are, in general, the principles
upon which an icehouse should be formed; but they will be better understood by
the following explanation and figure.
A dry sandy soil should be selected, and, if possible, a spot sheltered by a cliff or other
natural barrier from the direct rays of the sun. Here a cavity is to be dug about 16 feet
in diameter, terminating below like the point of a sugar loaf. Its ordinary depth, for
a moderate family, may be about 24 feet; but the larger its dimensions are, the longer
will it preserve the ice, provided it be filled. In digging, the workman should slope the
ground progressively towards the axis of the cone, to prevent the earth falling in. This
conical slope should be faced with brick or stone work about one foot thick, and jointed
with Roman cement so as to be air and water tight. A well is to be excavated at
the bottom two feet wide and four deep, covered at top with an iron grating for supporting
the ice, and letting the water drain away.
The upper cone may likewise be built of brickwork, and covered with thatch; such a
roof would prove the most durable. This is the construction shown in fig. 575. Whatever
kind of roof be preferred, there must be left in it an oblong passage into the interior.
This porch should face the north, and be at least 8 feet long by 21⁄2 feet wide; and perfectly
closed by a well-fitted door at each end. All round the bottom of this conical
cover, a gutter should be placed to carry off the rain to a distance from the icehouse,
and prevent the circumjacent ground from getting soaked with moisture.
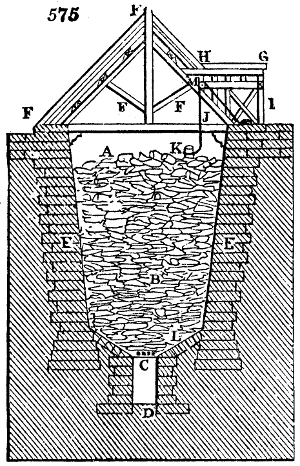
Fig. 575. shows the section of a well-constructed icehouse. Under the ice-chamber
A the ice is rammed into the space B. C is the grate of the drain-sink D. The[662]
portion E E is built in brick or stone; the base L of the ice-chamber slopes inwards towards
the centre at C. The upper part of the brickwork E E is a little way below the
level of the ground. The wooden frame work
F F F F forms the roof, and is covered with thick
thatch. G H is the wooden work of the door I. At
K the bucket is seen for lifting up a charge of ice,
by means of the cord J passing over the pulley M,
which enables the servant to raise it easily.
The icehouse should have no window to admit
light; but be, so to speak, hermetically sealed in
every point, except at its cess-pool, which may
terminate in a water trap to prevent circulation of
air.
A clear day should be selected for charging the
icehouse; but before beginning to fill, a quantity
of long dry straw should be laid on the bottom
crosswise; and as the ice is progressively introduced,
straw is to be spread against the conical sides, to prevent
the ice from coming into contact with the brick
or stone work. The more firmly compacted the ice
is, the better does it keep; with which view it should
be broken into pieces with mallets before being
thrown in. No layers of straw should be stratified
among the ice, for they would make its body porous.
Some persons recommend to pour in a little water
with the successive layers of ice, in order to fill up its small crevices, and convert the
whole into one mass.
Over the top layer a thick bed of straw should be spread, which is to be covered
with boards surmounted with heavy stones, to close up the interstices in the straw. The
inner and outer doors should never be opened at once; but the one should always be
shut before the other is opened.
Dry snow well rammed keeps equally well with hard ice, if care be taken to leave no
cavities in the mass, and to secure its compactness by sprinkling a little water upon the
successive charges.
To facilitate the extraction of the ice, a ladder is set up against its sloping wall at
one side of the door, and left there during the season.
JELLY, VEGETABLE, of ripe currants and other berries, is a compound of
mucilage and acid, which loses its power of gelatinizing by prolonged ebullition.
JELLY, ANIMAL; see Gelatine, Glue, and Isinglass.
JET; (Jaiet or jais, Fr.) a species of pitch-coal or glance-coal, which, being found
abundantly in a beautiful compact form, in the valley of Hers, arrondissement of Pamiers,
department of the Arriège, has been worked up extensively there from time immemorial,
into a multitude of ornamental articles. With this black lignite, buttons,
crosses, rosaries, necklaces, ear-drops, bracelets, waist-buckles, &c. are made, which
were at one time much worn by ladies for mourning dresses. The greater number of these
ornaments are fashioned upon grindstones which turn in a horizontal direction, and
are kept continually wet; others are turned at the lathe, or shaped by files.
About 40 years ago this manufacture employed from 1000 to 1200 operatives; at
present it gives bread to only 60. This falling off may be ascribed to the successful
imitation of the jet articles by those of black glass, which are equally beautiful, and not
nearly so apt to lose their polish by use.
IMPERMEABLE, is the epithet given to any kind of textile fabric, rendered
water-proof by one or other of the following substances:—
1. Linseed oil to which a drying quality has been communicated by boiling with
litharge or sugar of lead, &c.
2. The same oil holding in solution a little caoutchouc.
3. A varnish made by dissolving caoutchouc in rectified petroleum or naphtha, applied
between two surfaces of cloth, as described under Macintosh’s patent. See Caoutchouc.
4. Vegetable or mineral pitch, applied hot with a brush, as in making tarpauling for
covering goods in ships.
5. A solution of soap worked into cloth, and decomposed in it by the action of a
solution of alum; whence results a mixture of acid fats and alumina, which insinuates
itself among all the woolly filaments, fills their interstices, and prevents the passage of
water.
6. A solution of glue or isinglass, introduced into a stuff, and then acted upon by a[663]
clear infusion of galls, whereby the fibres get impregnated with an insoluble, impermeable,
pulverulent leather.
7. Plaster work is rendered impermeable by mixing artificial or natural asphaltum
with it.
JEWELLERY, Art of. See Gem and Lapidary.
INCOMBUSTIBLE CLOTH; is a tissue of the fibrous mineral called amianthus
or asbestos. This is too rare to form the object of any considerable manufacture.
Cotton and linen cloth may be best rendered incapable of taking fire, or burning with
flame, by being imbued with a solution of sal ammoniac.
INCUBATION, ARTIFICIAL. The Egyptians have from time immemorial
been accustomed to hatch eggs by artificial warmth, without the aid of hens, in peculiar
stoves, called Mammals. The inhabitants of the village Bermé, still travel through the most
distant provinces of Egypt at certain seasons of the year, with a portable furnace, heated
by a lamp, and either hatch chickens for sale, or undertake to hatch the eggs belonging
to the natives at a certain rate per dozen. M. de Reaumur published in France about
a century ago, some ingenious observations upon this subject; but M. Bonnemain was
the first person who studied with due attention all the circumstances of artificial incubation,
and mounted the process successfully upon the commercial scale. So far back as
1777 he communicated to the Academy of Sciences an interesting fact, which he had
noticed, upon the mechanism employed by chicks to break their shells; and for some
time prior to the French revolution he furnished the Parisian market with excellent
poultry at a period of the year when the farmers had ceased to supply it. His establishment
was ruined at that disastrous era, and no other has ever since been constructed
or conducted with similar care. As there can be no doubt however of the practicability
and profitableness of the scheme, when judiciously managed, I shall insert a brief
account of his ingenious arrangements. I had the pleasure of making the acquaintance
of this amiable old man at my first visit to Paris, many years ago, and believe all his
statements to be worthy of credit. Some imitations of his plans have been made in this
country, but how far they have succeeded in an economical point of view, it is difficult
to determine. His apparatus derives peculiar interest from the fact, that it was founded
upon the principle of the circulation of hot water, by the intestine motions of its particles,
in a returning series of connected pipes; a subject afterwards illustrated in the experimental
researches of Count Rumford. It has of late years been introduced as a novelty into
this country, and applied to warm the apartments of many public and private buildings.
The following details will prove that the theory and practice of hot-water circulation
were as perfectly understood by M. Bonnemain fifty years ago, as they are by any of
our stove-doctors at the present day. They were then publicly exhibited at his residence
in Paris, and were afterwards communicated to the world at large in the interesting
article of the Dictionnaire Technologique, intitled Incubation Artificielle.
The apparatus of M. Bonnemain consisted: 1. of a boiler and pipes for the circulation
of water; 2. of a regulator calculated to maintain an equable temperature; 3.
of a stove-apartment, heated constantly to the degree best fitted for incubation, which
he called the hatching pitch. He attached to one side a poussinière or chick-room, for
cherishing the chickens during a few days after incubation.
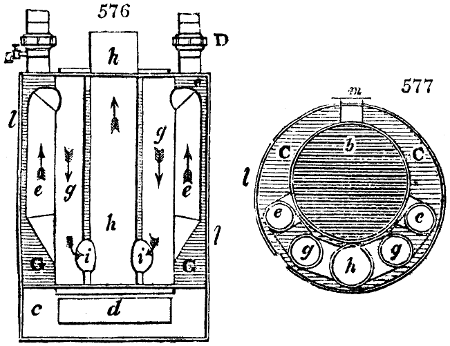
The boiler is represented in vertical section and ground plan, in figs. 576. and 577.
It is composed of a double cylinder of copper or cast-iron l, l, having a grate b (see
plan), an ashpit at d (section). The water occupies the shaded
space C, C. h, g, g, e, e, are five vertical flues, for conducting
the burnt air and smoke, which first rise in the two exterior
flues e, e, then descend in the two adjoining flues g, g, and
finally re-mount through the passages i, i, in the central flue
h. During this upwards and downwards circulation, as shown
by the arrows in the section, the products of combustion are
made to impart nearly the whole of their heat to the water by
which they are surrounded. At the commencement, some
burning paper or wood shavings are inserted at the orifice m,
to establish a draught in this circuitous chimney. The air is
admitted into the ash-pit at the side, in regulated quantities,
through a small square door, movable round a rod which runs
horizontally along its middle line. This swing valve is acted
upon by an expanding bar (see Heat-Regulator), which
opens it more or less, according to the temperature of the
stove apartment in which the eggs are placed.
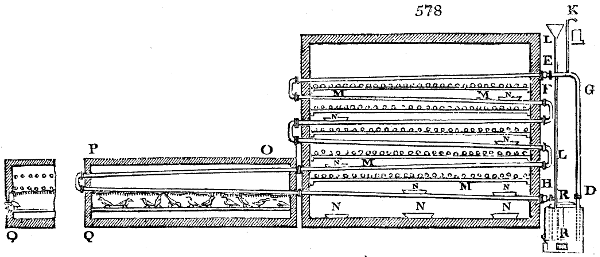
D is the upper orifice of the boiler, by which the hotter and consequently lighter particles
of the water continually ascend, and are replaced by the cooled particles, which
enter the boiler near its bottom, as shown in fig. 578. at R. Into further details relative
to the boiler it is needless to enter; for though its form, as designed by M. Bonnemain,[664]
is excellent and most economical of heat for a charcoal fire, it would not suit one of pit-coal,
on account of the obstruction to the pipes which would soon be occasioned by its soot.
In fig. 578. the boiler is shown at R, with the rod which regulates the air door of the
ash-pit. D is a stopcock for modifying the opening by which the hotter particles of
water ascend; G is the water-pipe of communication, having the heating pipe of distribution
attached between E F, which thence passes backwards and forwards with a very
slight slope from the horizontal direction, till it reaches the poussinière O P Q. It traverses
this apartment, and returns by N N to the orifice of the boiler H, where it turns vertically
downwards, and descends to nearly the bottom of the boiler, discharging at that point
the cooled and therefore denser particles of water to replace those which continually issue
upwards at D. L R is a tube surmounted with a funnel for keeping the range of pipes
always full of water; and K is a syphon orifice for permitting the escape of the disengaged
air, which would otherwise be apt to occupy partially the pipes and obstruct the aqueous
circulation.
The faster the water gets cooled in the serpentine tubes, the quicker its circulation
will be, because the difference of density between the water at the top and bottom of the
boiler, which is the sole cause of its movement, will be greater. N represents small
saucers filled with water, to supply the requisite moisture to the heated air, and to place
the eggs, arranged along the trays M M, in an atmosphere analogous to that under the
body of the hen.
When we wish to hatch eggs with this apparatus, the fire is to be kindled in the
boiler, and as soon as the temperature has risen to about 100° F., the eggs are introduced;
but only one-twentieth of the whole number intended, upon the first day; next
day, a like number is laid upon the trays, and thus in succession for twenty days, so that
upon the twenty-first day the eggs first placed may be hatched for the most part, and
we may obtain daily afterwards an equal number of chicks. In this way, regularity of
care is established in the rearing of them.
During the first days of incubation, natural as well as artificial, a small portion of the
water contained in the egg evaporates by the heat, through the shell, and is replaced by
a like quantity of air, which is afterwards useful for the respiration of the animal. If the
warm atmosphere surrounding the eggs were very dry, such a portion of the aqueous
part of the eggs would evaporate through the pores of the shells, as would endanger the
future life of the chick in ovo. The transpiration from the body of the hen, as she sits
upon her eggs, counteracts this desiccation in general; yet in very dry weather, many
hatching eggs fail from that cause, unless they be placed in moist decomposing straw.
The water saucers N N are therefore essential to success in artificial incubation.
After the chickens are hatched they are transferred into the nursery, O Q, on the front
side of which there is a small grated trough filled with millet seed. Small divisions are
made between the broods of successive days, to enable the superintendent to vary their
feeding to their age.
In order to supply an establishment of the common kind, where 100 eggs are to be
hatched daily, a dozen of hens would be needed, and 150 eggs must be placed under
them, as only two-thirds in general succeed. At this rate, 4300 mothers would be
required to sit. Now supposing we should collect ten times as many hens, or 43,000,
we should not be able to command the above number of chickens, as there is seldom a
tenth part of hens in a brooding state. Besides, there would be in this case no fewer
than 720 hens every day coming out with a fresh brood of chickens, which would require
a regiment of superintendents.
Artificial Incubation, by means of Hot Mineral Waters.—This curious process is[665]
described very briefly in a letter by M. D’Arcet. The following are extracts from this
letter:—
“In June, 1825, I obtained chickens and pigeons at Vichy, by artificial incubation,
effected through the means of the thermal waters of that place. In 1827 I went to the
baths of Chaudes-Aigues, principally for the purpose of doing the same thing there.
Finding the proprietor a zealous man, I succeeded in making a useful application of this
source of heat to the production of poultry.
“The advantage of this process may be comprehended, when it is known that the
invalids who arrive at Vichy, for instance in the month of May, find chickens only the
size of quails; whereas, by this means, they may be readily supplied six months old.
“The good which may be done by establishing artificial incubation in places where
hot springs exist, is incalculable; it may be introduced into these establishments without
at all interfering with the medical treatment of patients, since the hatching would go
on in winter, at a time when the baths for other purposes are out of use.
“There is no other trouble required in breeding chickens, by means of hot baths, than
to break the eggs at the proper time; for, when the apartments are closed, the whole of
the interior will readily acquire a sufficiently elevated and very constant temperature.”
In addition to these details by M. D’Arcet, a letter was received from M. Felgeris, the
proprietor of the baths at Chaudes-Aigues (Cantal), in which he describes the success
he had in following M. D’Arcet’s process. This consists in putting the eggs into a small
basket, suspending it in one of the stove-rooms heated by the hot mineral water, and
turning round the eggs every day. The very first trial was attended with success, and
no failure was experienced in four repetitions of it.
INDIGO. This invaluable blue dye-stuff, for which no tolerable substitute has
been found, was known to the ancients as a pigment under the name of indicum, whence
its present denomination. In modern Europe, it first came into extensive use in Italy,
but, about the middle of the 16th century, the Dutch began to import and employ it in
considerable quantities. Its general introduction into the dye-houses of both England
and France was kept back by absurd laws, founded upon an opinion that it was a
fugitive substance, and even prejudicial to the fibre of wool. See Dyeing, p. 413.
The plants which afford this dye-drug grow in the East and West Indies, in the
middle regions of America, in Africa, and Europe. They are all species of the genera
Indigofera, Isatis, and Nerium.
The following are cultivated:—Indigofera tinctoria affords in Bengal, Malabar,
Madagascar, the Isle of France, and St. Domingo, an article of middling quality,
but in large quantity. The indigofera disperma, a plant cultivated in the East Indies
and America, grows higher than the preceding, is woody, and furnishes a superior
dye-stuff. The Guatimala indigo comes from this species. Indigofera Anil grows in
the same countries, and also in the West Indies. The Indigofera Argentea, which grows
also in Africa; it yields little indigo, but of an excellent quality. Indigofera Pseudotinctoria,
which is cultivated in the East Indies, furnishes the best of all: the Indigofera
Glauca is the Egyptian and Arabian species. There are also the cærulea, cinerea erecta,
hirsuta, glabra, and several others. The Nerium tinctorium of the East Indies affords
some indigo; as does the Isatis tinctoria, or Woad, in Europe; and the Polygonum
tinctorium.
The districts of Kishenagar, Jessore, and Moorshedabad, in Bengal, ranging from 88°
to 90° E.L. and 221⁄2° to 24° N.L., produce the finest indigo. That from the districts
about Burdwan and Benares is of a coarser or harsher grain. Tyroot, in lat. 26°, yields
a tolerably good article. The portion of Bengal most propitious to the cultivation of
indigo lies between the river Hoogly and the main stream of the Ganges.
In the East Indies, after having ploughed the ground in October, November, and
the beginning of December, they sow the seed of the indigo plant in the last half of
March and the beginning of April, while the soil being neither too hot nor too dry,
is most propitious to its germination. A light mould answers best; and sunshine,
with occasional light showers, are most favourable to its growth. Twelve pounds of
seeds are sufficient for sowing an acre of land. The plants grow rapidly, and will bear
to be cut for the first time at the beginning of July, nay, in some districts, so early as
the middle of June. The indications of maturity are the bursting forth of the flower
buds, and the expansion of the blossoms; at which period the plant abounds most in
the dyeing principle. Another indication is taken from the leaves; which, if they
break across, when doubled flat, denote a state of maturity. But this character
is somewhat fallacious, and depends upon the poverty or richness of the soil. When
much rain falls, the plants grow too rapidly, and do not sufficiently elaborate the blue
pigment. Bright sunshine is most advantageous to its production.
The first cropping of the plants is the best; after two months a second is made; after
another interval, a third, and even a fourth; but each of these is of diminished value.
There are only two croppings in America.
[666]
Two methods are pursued to extract the indigo from the plant; the first effects it by
fermentation of the fresh leaves and stems; the second, by maceration of the dried
leaves; the latter process being most advantageous.
1. From the recent leaves.—In the indigo factories of Bengal, there are two large
stone-built cisterns, the bottom of the first being nearly upon a level with the top of
the second, in order to allow the liquid contents to be run out of the one into the other.
The uppermost is called the fermenting vat, or the steeper; its area is 20 feet square,
and its depth 3 feet; the lowermost, called the beater or beating vat, is as broad as the
other, but one third longer. The cuttings of the plant, as they come from the field,
are stratified in the steeper, till this be filled within 5 or 6 inches of its brim. In order
that the plant, during its fermentation, may not swell and rise out of the vat, beams of
wood and twigs of bamboo are braced tight over the surface of the plants, after which
water is pumped upon them till it stands within three or four inches of the edge of the
vessel. An active fermentation speedily commences, which is completed within 14 or 15
hours; a little longer or shorter, according to the temperature of the air, the prevailing
winds, the quality of the water, and the ripeness of the plants. Nine or ten hours after
the immersion of the plant, the condition of the vat must be examined; frothy bubbles
appear, which rise like little pyramids, are at first of a white colour, but soon
become gray-blue; and then deep purple-red. The fermentation is at this time
violent, the fluid is in constant commotion, apparently boiling, innumerable bubbles
mount to the surface, and a copper-coloured dense scum covers the whole. As long as
the liquor is agitated, the fermentation must not be disturbed; but when it becomes
more tranquil, the liquor is to be drawn off into the lower cistern. It is of the
utmost consequence not to push the fermentation too far, because the quality of the
whole indigo is deteriorated; but rather to cut it short, in which case there is, indeed,
a loss of weight, but the article is better. The liquor possesses now a glistening
yellow colour, which, when the indigo precipitates, changes to green. The average
temperature of the liquor is commonly 85° Fahr.; its specific gravity at the surface is
1·0015; and at the bottom 1·003.
As soon as the liquor has been run into the lower cistern, ten men are set to work to
beat it with oars, or shovels 4 feet long, called busquets. Paddle wheels have also been
employed for the same purpose. Meanwhile two other labourers clear away the compressing
beams and bamboos from the surface of the upper vat, remove the exhausted
plant, set it to dry for fuel, clean out the vessel, and stratify fresh plants in it. The
fermented plant appears still green, but it has lost three fourths of its bulk in the
process, or from 12 to 14 per cent. of its weight, chiefly water and extractive matter.
The liquor in the lower vat must be strongly beaten for an hour and a half, when the
indigo begins to agglomerate in flocks, and to precipitate. This is the moment for
judging whether there has been any error committed in the fermentation; which must
be corrected by the operation of beating. If the fermentation has been defective, much
froth rises in the beating, which must be allayed with a little oil, and then a reddish
tinge appears. If large round granulations are formed, the beating is continued, in
order to see if they will grow smaller. If they become as small as fine sand, and if the
water clears up, the indigo is allowed quietly to subside. Should the vat have been over
fermented, a thick fat-looking crust covers the liquor, which does not disappear by the
introduction of a flask of oil. In such a case the beating must be moderated. Whenever
the granulations become round, and begin to subside, and the liquor clears up,
the beating must be discontinued. The froth or scum diffuses itself spontaneously into
separate minute particles, that move about the surface of the liquor; which are marks
of an excessive fermentation. On the other hand, a rightly fermented vat is easy to
work; the froth, though abundant, vanishes whenever the granulations make their
appearance. The colour of the liquor, when drawn out of the steeper into the beater,
is bright green; but as soon as the agglomerations of the indigo commence, it assumes
the colour of Madeira wine; and speedily afterwards, in the course of beating, a small
round grain is formed, which, on separating, makes the water transparent, and falls
down, when all the turbidity and froth vanish.
The object of the beating is threefold: first, it tends to disengage a great quantity
of carbonic acid present in the fermented liquor; secondly, to give the newly developed
indigo its requisite dose of oxygen by the most extensive exposure of its particles to
the atmosphere; thirdly, to agglomerate the indigo in distinct flocks or granulations.
In order to hasten the precipitation, lime-water is occasionally added to the fermented
liquor in the progress of beating, but it is not indispensable, and has been supposed
capable of deteriorating the indigo. In the front of the beater a beam is fixed upright,
in which three or more holes are pierced a few inches in diameter. These are closed
with plugs during the beating, but, two or three hours after it, as the indigo subsides,
the upper plug is withdrawn to run off the supernatant liquor, and then the lower
plugs in succession. The state of this liquor being examined, affords an indication of[667]
the success of both the processes. When the whole liquor is run off, a labourer enters
the vat, sweeps all the precipitate into one corner, and empties the thinner part into a
spout which leads into a cistern, alongside of a boiler, 20 feet long, 3 feet wide and 3 deep.
When all this liquor is once collected, it is pumped through a bag for retaining the
impurities, into the boiler, and heated to ebullition. The froth soon subsides, and shows
an oily looking film upon the liquor. The indigo is by this process not only freed from
the yellow extractive matter, but is enriched in the intensity of its colour, and increased in
weight. From the boiler the mixture is run, after two or three hours, into a general
receiver called the dripping vat, or table, which, for a factory of twelve pairs of preparation
vats, is 20 feet long, 10 feet wide, and 3 feet deep; having a false bottom, 2 feet
under the top edge. This cistern stands in a basin of masonry (made water tight with
Chunam hydraulic cement), the bottom of which slopes to one end, in order to facilitate
the drainage. A thick woollen web is stretched along the bottom of the inner vessel,
to act as a filter. As long as the liquor passes through turbid, it is pumped back into
the receiver. Whenever it runs clear, the receiver is covered with another piece of
cloth to exclude the dust, and allowed to drain at its leisure. Next morning the
drained magma is put into a strong bag, and squeezed in a press. The indigo is then
carefully taken out of the bag, and cut with a brass wire into bits, about 3 inches cube,
which are dried, in an airy house, upon shelves of wicker work. During the drying, a
whitish efflorescence comes upon the pieces, which must be carefully removed with a
brush. In some places, particularly on the coast of Coromandel, the dried indigo
lumps are allowed to effloresce in a cask for some time, and when they become hard they
are wiped and packed for exportation.
From some experiments it would appear that the gas disengaged during the middle
period of the fermentation is composed in 100 parts of 27·5 carbonic acid, 5·8 oxygen,
and 66·7 azote; and towards its end, of 40·5 carbonic acid, 4·5 oxygen, and 55·0 azote.
The fermenting leaves apparently convert the oxygen of the atmosphere into carbonic
acid gas, and leave its azote; besides the quantity of carbonic acid which they spontaneously
evolve. Carburetted hydrogen does not seem to be disengaged. That the
liquor in the beating vat absorbs oxygen from the air in proportion as the indigo becomes
flocculent and granular, has been ascertained by experiment, as well as that sunshine
accelerates the separation of the indigo blue. Out of 1000 parts of the fermented
liquor of specific gravity 1·003, the blue precipitate may constitute 0·75 of a part.
Such a proportion upon the great scale is however above the average, which is not
more than 0·5. When lime water is added, an extractive matter is thrown down, which
amounts to from 20 to 47 parts in 1000 of the liquor. It has a dark brown tint, a viscid
appearance, an unpleasant smell, and a bitter taste. It becomes moist in damp air, and
dissolves in water without decomposition. It is precipitated by lime, alkalis, infusion of
galls, and acetate of lead. All indigo contains a little lime derived from the plant, even
though none has been used in its preparation.
2. Indigo from dried leaves.—The ripe plant being cropped, is to be dried in sunshine
from 9 o’clock in the morning till 4 in the afternoon, during two days, and threshed to
separate the stems from the leaves, which are then stored up in magazines till a sufficient
quantity be collected for manufacturing operations. The newly dried leaves must be
free from spots, and friable between the fingers. When kept dry, the leaves undergo in
the course of 4 weeks, a material change, their beautiful green tint turning into a pale
blue-gray, previous to which the leaves afford no indigo by maceration in water, but
subsequently a large quantity. Afterwards the product becomes less considerable.
The following process is pursued to extract indigo from the dried leaves. They are
infused in the steeping vat with six times their bulk of water, and allowed to macerate for
two hours with continual stirring till all the floating leaves sink. The fine green liquor
is then drawn off into the beater vat, for if it stood longer in the steeper, some of the indigo
would settle among the leaves and be lost. Hot water, as employed by some manufacturers,
is not necessary. The process with dry leaves possesses this advantage, that
a provision of the plant may be made at the most suitable times, independently of the
vicissitudes of the weather, and the indigo may be uniformly made; and moreover, that
the fermentation of the fresh leaves, often capricious in its course, is superseded by a
much shorter period of simple maceration.
The process for obtaining indigo from the Nerium is altogether the same, but hot
water has been generally applied to the dried leaves. For woad, hot water must be employed,
and also lime water as a precipitant, on account of the small proportion of indigo
in the plant. Dilute muriatic acid is digested upon the woad indigo to remove the
lime, without which no dye could be precipitated. According to the warmth of the summer
and the ripeness of the plant, from 2 to 5 ounces of indigo may be obtained from
100 pounds of the dried woad, or upon an average 4 ounces to the hundred weight.
The indigo found in European commerce is imported from Bengal, Coromandel,
Madras, the Mauritius, Manilla, and Java in the Eastern hemisphere; from Senegal,[668]
Caraccas, Guatimala, Brazil, (South Carolina and Louisiana in small quantity), and
formerly from the West India islands, especially St. Domingo. Its quality depends
upon the species of the plant, its ripeness, the soil and climate of its growth, and mode
of manufacture. The East Indian and Brazilian indigo comes packed in chests, the
Guatimala in ox-hides, called surons.
The organ which affords the indigo is confined entirely to the pellicle of the leaves,
and exists in largest quantity at the commencement of maturation while the plant is in
flower. The indigofera is remarkable for giving a blue tinge to the urine of cows that
feed upon its leaves.
According to some manufacturers, the plants should be cut down in dry weather, an
hour or two before sunset, carried off the field in bundles, and immediately spread upon
a dry floor. Next morning the reaping is resumed for an hour and a half, before the
sun acts too powerfully upon vegetation; and the plants are treated in the same way.
Both cuttings become sufficiently dry by three o’clock in the afternoon, so as to permit
the leaves to be separated from the stems by threshing. They are now thoroughly
dried in the sunshine, then coarsely bruised, or sometimes ground to powder in a
mill, and packed up for the operations of manufacture.
In the spring of 1830 I subjected a variety of specimens of indigo to comparative
analyses, by dissolving a few grains of each in strong sulphuric acid, diluting the solutions
with an equal volume of water, and determining the resulting shade of colour in
a hollow prism of plate glass, furnished with a graduated scale. The following are the
results, compared to the shade produced by a like weight of absolute indigo.
I. East India Indigos; prices as at the last October sales.
| No. |
Price. |
Real
indigo
in 100
parts. |
Characters by the Brokers. |
| |
s. |
d. |
|
|
| 1 |
3 |
9 |
42 |
|
Broken, middling violet, and coppery violet spotted. |
| 2 |
3 |
6 |
56 |
·5 |
Ditto, a little being coppery violet and copper. |
| 3 |
3 |
3 |
46 |
·0 |
Ditto, middling red violet, dull violet and lean. |
| 4 |
4 |
3 |
54 |
·5 |
Large broken, and square, even middling red violet. |
| 5 |
4 |
2 |
75 |
·0 |
Much broken and very small, very crumbly and limy, soft, good violet. |
| 6 |
4 |
9 |
60 |
·0 |
Square and large broken, 1⁄2 middling violet, and 1⁄2 good coppery violet. |
| 7 |
5 |
3 |
70 |
·0 |
Large broken, very good; paste a little limy, good violet. |
| 8 |
6 |
6 |
60 |
·0 |
Square and large broken, soft, fine paste, fine violet. |
| 9 |
6 |
0 |
66 |
2⁄3 |
Square, ditto, good red violet. |
| 10 |
7 |
0 |
75 |
|
Square, ditto, fine purple and blue. |
| 11 |
2 |
3 |
37 |
·5 |
Middling ordinary Madras. |
| 12 |
3 |
6 |
60 |
·0 |
Good Madras. |
| 13 |
4 |
3 |
58 |
·0 |
Very fine ditto. |
| 14 |
2 |
0 |
—— |
Low, pale Oude. |
| 15 |
2 |
4 |
27 |
3⁄4 |
Middling, ordinary Oude. |
| 16 |
3 |
3 |
54 |
|
Good Oude. |
| 17 |
1 |
9 |
29 |
|
Lundy, very low quality. |
II. American Indigos; wholesale prices at present. (March 1830.)
| Indigo. |
No. |
Price. |
Parts
in 100. |
| |
|
s. |
d. |
|
| Caraca flor. |
1 |
6 |
6 |
54 |
1⁄2 |
| Guatimala |
2 |
5 |
5 |
33 |
1⁄2 |
| —— |
3 |
3 |
3 |
19 |
|
| —— |
4 |
4 |
4 |
32 |
1⁄2 |
| —— |
5 |
5 |
5 |
50 |
|
| —— |
6 |
5 |
5 |
50 |
|
| —— |
7 |
5 |
5 |
35 |
|
| —— |
8 |
4 |
4 |
46 |
|
| —— |
9 |
4 |
4 |
33 |
1⁄2 |
| —— |
10 |
5 |
5 |
50 |
|
Properties of Indigo.—It possesses a dark blue colour, passing into violet-purple, is
void of taste and smell, dull, but by rubbing with a smooth hard body, it assumes the lustre
and hue of copper. It occurs sometimes less and sometimes more dense apparently than
water, which circumstance depends upon its freedom from foreign impurities, as well
as upon the treatment of its paste in the boiling, pressing, and drying operations. It
is insoluble in water, cold alcohol, ether, muriatic acid, dilute sulphuric acid, cold[669]
ethereous and fat oils; but boiling alcohol and oils dissolve a little of it, which they
deposit on cooling. Creosote has the property of dissolving indigo.
Indigo is a mixture of several dye-stuffs, and other substances. Berzelius found in
it a matter resembling vegetable gluten or gliadine, a brown, red, and blue pigment,
besides oxide of iron, clay, lime, magnesia, and silica.
1. Indigo gluten or gliadine is dissolved along with the calcareous and magnesian
salts by acids. If the powder be treated with dilute sulphuric acid, if the solution be
saturated with carbonate of lime, evaporated to dryness, and its residuum treated with
alcohol; the solution thus formed leaves, after being evaporated, a yellow transparent
extract, easily soluble in water, more difficultly in acid liquids; showing that acids
extract only a portion of the gliadine from the indigo. It yields, by dry distillation,
much ammonia, a fetid oil, and comports itself in other respects like vegetable gluten.
2. Indigo-brown, occurs in combination with lime, as also with vegetable acid in considerable
quantity, and more abundantly in the coarser sorts of indigo than in the finer.
Indigo purified by acids is to be treated with hot strong caustic lye, which dissolves the
indigo-brown; the liquid part of the mixture passes with difficulty through the filter,
is black-brown, opaque, and holds some indigo-blue in solution, or diffused in fine powder.
The alkali being neutralized with acetic acid, the liquor is to be evaporated, and alcohol
poured on the residuum, whereby the alkaline acetate is dissolved out from the brown.
This pigment is a dark brown, almost black, but is not yet entirely deprived of
the other constituents of indigo. It is nearly tasteless, is combustible, affords, by dry
distillation, ammonia and fetid oil, forms with acids combinations hardly soluble in water,
with alkalis soluble ones, but with earths hardly soluble. Lime possesses the property
of precipitating the indigo-brown completely from its alkaline solution. Chlorine occasions
a pale yellow brownish precipitate, which consists of indigo brown and muriatic
acid, but causes no further change. By drying, it becomes again dark coloured. Indigo-brown
seems to exist also in woad.
3. Indigo-red, or more properly red resin of indigo. This may be obtained by boiling
alcohol of sp. grav. 0·830 upon some indigo which has been previously treated with acids
and alkalis; for the red substance is hardly soluble in cold alcohol. The solution is dark
red, opaque, and leaves, by distillation, the indigo-red in the form of a black-brown powder,
or a glistening varnish, slightly soluble in alcohol and ether. Alkalis do not dissolve
it, but concentrated sulphuric acid forms with it a dark yellow dye, from which water
causes no precipitation; wool extracts the colour from the acid solution, and becomes
of a dirty brown hue. Chlorine does not seem capable of destroying the colour
for though it makes it yellow, it becomes as dark as ever on being dried. Indigo-red
melts with heat, burns with a bright flame, affords, when heated in vacuo, first a white
crystalline sublimate, and then unchanged indigo-red. That white matter is changed by
nitric acid into indigo-red.
4. Indigo-blue, or pure indigo remains, after treating the indigo of commerce with
dilute acid, alkalis, and alcohol; it retains, however, still traces of the matters thereby
extracted, along with some earthy substances. In order to procure indigo-blue in its
utmost purity, we must deoxidize the above blue residuum, thus form colourless indigo,
which again acquires a blue colour from the air, and constitutes the pure pigment. For
this purpose the above moist indigo is to be mixed with slaked lime, green sulphate
of iron, and hot water in an air-tight matrass. The indigo when deoxidized by protoxide
of iron being soluble in lime-water, the clear yellow solution is to be poured off, and
exposed to the air. The indigo absorbs oxygen, and becomes again blue. By digestion
with dilute muriatic acid the foreign matters are dissolved, and may then be washed
away with distilled water, from the absolute indigo.
The indigo-blue obtained in this manner has a cast of purple red, displaying the
characteristic copper lustre in a high degree, but in powder, it is blue. It is void
of taste and smell, is by my experiments of specific gravity 1·50, affords at 554°
Fahr. a purple vapour, and sublimes in shining purple scales, or slender needles in
an apparatus open to the air, whereby, however, much of it is destroyed. Some
carbon remains after the sublimation. A quick heat produces most sublimate.
These needles contain a brown oily matter, which may be dissolved out by
means of hot alcohol. Their specific gravity is 1·35, according to Mr. Crum. The
sublimate from common indigo does not contain any oil, but some indigo-red and the
above white crystalline matter. According to Mr. Crum, indigo-blue consists of carbon,
73·22; oxygen, 12·60; azote, 11·26; hydrogen, 2·92; while according to Dumas,
crystallized indigo consists of carbon, 73·26; oxygen, 10·43; azote, 13·81; and hydrogen,
2·50: precipitated indigo consists of carbon, 74·81; oxygen, 7·88; azote, 13·98;
and hydrogen, 3·33: sublimed indigo, of carbon, 71·71; oxygen, 12·18; azote, 13·45;
hydrogen, 2·66. My own analysis afforded—carbon, 71·37; oxygen, 14·25; azote, 10·00;
hydrogen, 4·33. In another analysis of Dumas, 3·93 parts of hydrogen were obtained.
Hence we must infer that considerable differences exist in the composition of indigo in[670]
its purest state. Reagents act upon it much as upon common indigo. Chlorine,
iodine, and bromine convert it into a reddish brown soluble substance. Concentrated
sulphuric acid, especially the smoking or anhydrous of Nordhausen, dissolves indigo-blue
with the disengagement of heat, but it makes it suffer some modification; for though it
retains an intense dark blue colour, it has become soluble in water, and may be
blanched by light, which does not happen with indigo itself. Nitric acid destroys indigo-blue,
forms indigotic (carbazotic) acid, carbonic acid, artificial resin, and bitter principle.
Indigo-blue may be reduced by substances oxidized, with the co-operation of alkalis
or alkaline earths; for example, by such substances as have a strong affinity for oxygen,
and are imperfectly saturated with this principle, as the sulphurous and phosphorous
acids and their salts, the protoxides of iron and manganese, the protoxide salts of tin,
and the corresponding compounds of chlorine, as the proto-chlorides of tin and iron; and
the solution of the former in potash. When in these circumstances, in the presence of
alkali, a deoxidation or reduction of the indigo-blue takes place, the other bodies get
oxidized by absorption of the oxygen of the indigo-blue; the protoxides become peroxides,
and the acids in ous become acids in ic, &c. Several metallic sulphurets also
reduce the indigo-blue in the same predicament, as the sulphurets of potassium, of calcium,
of antimony, and of arsenic (orpiment). A similar influence is exercised by
fermenting vegetable substances, such as woad, madder, bran, raw sugar (molasses), starch,
syrup, in consequence of the formation of carbonic and acetic acids, by absorption of the
oxygen of the indigo-blue, for acetic acid and acetic salts are found in the liquor of the
warm blue vat, in which indigo has been reduced by means of woad, madder, and bran.
Formation of colourless reduced indigo-blue, or indigotine.—Purified indigo-blue is to
be treated with copperas and slaked lime, as above described; or the clear wine-yellow
supernatant liquor of the cold blue-vat mixture is to be taken, run by a syphon into
a matrass, a few drops of concentrated acetic or sulphuric acid, deprived of air, are to be
poured into it, and the vessel being made quite full, is to be well corked. The reduced
indigo soon falls in white flocks, or crystalline scales. They must be edulcorated upon
a filter with water deprived of its air by boiling, then pressed between folds of blotting-paper,
and dried under the receiver in vacuo. Indigo-blue may likewise be reduced and
dissolved by solution of hydro-sulphuret of ammonia; and the colourless indigotine may
be precipitated by muriatic acid.
The reduced indigo is sometimes white at the instant of its elimination, sometimes
grayish, of a silky lustre, but becomes very readily greenish, blue green, and blue, in the
air; in which case it absorbs, according to Berzelius, 4·2 per cent. of oxygen; but according
to Liebig, 11·5 per cent. It is void of taste and smell, is insoluble in water;
well boiled water free from air is not affected by it, but is turned blue by common water.
It dissolves in alcohol and ether into a yellow dye; not in dilute acids, but in concentrated
sulphuric acid, whereby probably a portion of this is decomposed, and some hyposulphurous
acid formed; the colour of this solution is blue. Solutions of the caustic and
carbonated alkalis, even the alkaline earths, readily dissolve reduced indigo into a wine-yellow
liquid; but in contact with air, oxygen is absorbed, and indigo-blue falls, while
a purple-coloured froth, passing into copper-red, appears upon the surface, just as in the
indigo vats of the dyer.
The reduced indigo may be combined, by means of complex affinity, with other bases,
with the exception of the oxides of copper, zinc, and mercury, which oxidize it. These
combinations are white, in part crystallizable, become speedily blue in the air, and
afford by sublimation indigo-blue. Berzelius formed with lime a two-fold combination;
one easily soluble in water, and another difficultly soluble, of a lemon colour, which
contained an excess of lime; this is formed both in the hot and the cold blue vat; in the
latter it is occasioned by an overdose of lime.
When pure indigo-blue is treated with concentrated sulphuric acid, and particularly with
six times its weight of the smoking dry acid, it dissolves completely, and several different
compounds are produced in the solution. There is first a blue sulphate of indigo; secondly,
a similar compound with the resulting hyposulphurous acid; thirdly, a combination of
sulphuric acid with the purple of indigo (called Phænicin by Crum), a peculiar substance,
generated from indigo-blue. These three compounds are here dissolved in an excess
of sulphuric acid. The more concentrated the sulphuric acid is, the more blue hyposulphite
is formed. The solution in smoking acid, when diluted with water and filtered,
affords a considerable precipitate of indigo purple, which that in oil of vitriol does not.
The vapour of anhydrous sulphuric acid combines with indigo-blue into a purple fluid.
In order to obtain from the dark blue solution each of these blue acids in a pure state,
we must dilute it with forty times its weight of water, and immerse in the filtered
liquor, well washed wool or flannel, with which the blue acids combine, while most of the
sulphuric acid and some other foreign substances remain free in the liquor. The wool
must be then scoured with water containing about half a per cent. of carbonate of ammonia,
or potash, which neutralizes both of the blue acids, and produces a blue compound.[671]
This being evaporated to dryness at the temperature of 140° F., alcohol of 0·833 is to be
poured upon the residuum, which dissolves the blue hyposulphite, but leaves the blue
sulphate undissolved. From either salt, by precipitating with acetate of lead, by acting
upon the precipitate with sulphuretted hydrogen water, and evaporation, either of the
two blue acids may be obtained. They may be both evaporated to dryness, especially
the blue sulphate of indigo; they both become somewhat moist in the air, they are very
soluble in water, and the blue sulphate also in alcohol; they have a not unpleasant smell,
and an acid astringent taste.
From these habitudes, particularly in reference to the bases, it appears that indigo-blue
does not comport itself like a saline base towards the acids, but rather like an acid, since
it enters into the salts, just as the empyreumatic oil of vinegar and oil of turpentine do
into resin soaps. The blue pigment of both acids is reduced by zinc or iron without the
disengagement of hydrogen gas; as also by sulphuretted hydrogen, tepid protochloride
of tin, while the liquor becomes yellow.
Indigo-blue sulphate of potash, or ceruleo-sulphate of potash, may be obtained by
extracting the blue colour from the wool by water containing 1 per cent. of carbonate
of potash, evaporating nearly to dryness, treating the extract with alcohol
to remove the indigo-blue hyposulphite, then with acetic acid and alcohol to
remove any excess of carbonate of potash. It is found in commerce under the
name of precipitated indigo, indigo paste, blue carmine, and soluble indigo. To
prepare it economically, indigo is to be dissolved in ten times its weight of concentrated
sulphuric acid; the solution after twenty-four hours is to be diluted with ten
times its weight of water, filtered, and imperfectly saturated with carbonate of potash;
whereby a blue powder falls down; for the resulting sulphate of potash throws down the
ceruleo-sulphate, while the hyposulphite of potash remains dissolved. It is a dark
blue copper shining powder, soluble in 140 parts of cold water, and in much less of
boiling water. It is made use of as a dye, and to give starch a blue tint. When mixed
with starch into cakes, it is sold under the name of blue for washerwomen.
Ceruleo-sulphate of ammonia may be formed in the same way. It is much more
soluble in water. Ceruleo-sulphate of lime is obtained by saturating the above dilute
acid with chalk, filtering to separate the undyed gypsum, and washing with water till
the purple colour be extracted. This liquor evaporated and decomposed by alcohol,
affords a bluish flocky precipitate, which is more soluble in water than common gypsum,
and dries up in a purple-blue film. Ceruleo-sulphate of alumina may be obtained
by double affinity; it is dark blue while moist, but becomes black-blue by drying, and
is soluble in water.
The blue present in all these salts of ceruline is destroyed by sunshine, becomes greenish-gray
by caustic alkalis; and turns immediately yellow-brown by alkaline earths. But
when the solution is very dilute, the colour becomes first green, then yellow. The carbonates
of alkalis do not produce these changes. Nitric acid decomposes the colour
quickly. Mr. Crum considers ceruline to be a combination of indigo-blue with water.
Phenicine, or indigo-purple combined with sulphuric acid, is obtained when the solution
of indigo-blue in concentrated sulphuric acid, has been diluted for a few hours with
water, and then filtered. It seems to be an intermediate body into which the indigo-blue
passes, before it becomes soluble ceruline. Hence it occurs in greater quantity soon
after digesting the indigo with the acid, than afterwards. It is dark blue, dissolves gradually
in water, affords after evaporation a blue residuum, of the same appearance as the
above blue acids. When a salt is added to it a purple precipitate ensues, which is a compound
of indigo-purple, sulphuric acid, and the base of the salt. Indigo-purple is
reduced by bodies having a strong attraction for oxygen, if a free alkali or alkaline earth
be present, and its solution is yellow, but it becomes blue in the atmosphere. According
to Mr. Crum, Phenicine contains half as much combined water as ceruline.
The table which I published in 1830 (as given above) shows very clearly how much
the real quality and value of indigo differ from its reputed value and price, as estimated
from external characters by the brokers. Various test or proof processes of this drug
have been proposed. That with chlorine water is performed as follows. It is known
that chlorine destroys the blue of indigo, but not the indigo-red or indigo-brown, which
by the resulting muriatic acid is thrown down from the sulphuric solution in flocks, and
the chlorine acts in the same way on the gliadine or gluten of the indigo. Pure indigo-blue
is to be dissolved in 10 or 12 parts of concentrated sulphuric acid, and the solution is to
be diluted with a given weight of water, as, for example, 1000 parts for 1 of indigo-blue.
If we then put that volume of liquor into a graduated glass tube, and add to it chlorine
water of a certain strength till its blue colour be destroyed by becoming first green and
then red-brown, we can infer the quantity of colour from the quantity of chlorine water
expended to produce the effect. The quantity of real indigo-blue cannot, however, be
estimated with any accuracy in this way, because the other colouring matters in the drug
act also upon the chlorine; and, indeed, the indigo itself soon changes, when dissolved in[672]
sulphuric acid, even out of access of light, while the chlorine water itself is very susceptible
of alteration. Perhaps a better appreciation might be made by avoiding the sulphuric
acid altogether, and adding the finely-powdered indigo to a definite volume of the
chlorine water till its colour ceased to be destroyed, just as prussian-blue is decoloured
by solution of potash in making the ferro-cyanide.
Another mode, and one susceptible of great precision, is to convert 10 or 100 grains
of indigo finely powdered into its deoxidized state, as in the blue vat by the proper
quantity of slaked lime and solution of green sulphate; then to precipitate the indigo,
collect and weigh it. The indigo should be ground upon a muller along with the quicklime,
the levigated mixture should be diluted with water, and added to the solution of
the copperas. This exact analytical process requires much nicety in the operator, and
can hardly be practised by the broker, merchant, or manufacturer.
Employment of indigo in dyeing.—As indigo is insoluble in water, and as it can penetrate
the fibres of wool, cotton, silk, and flax, only when in a state of solution, the dyer
must study to bring it into this condition in the most complete and economical manner.
This is effected either by exposing it to the action of bodies which have an affinity for
oxygen superior to its own, such as certain metals and metallic oxides, or by mixing
it with fermenting matters, or, finally, by dissolving it in a strong acid, such as the sulphuric.
The second of the above methods is called the warm blue, or pastel vat; and
being the most intricate, we shall begin with it.
Before the substance indigo was known in Europe, woad having been used for dyeing
blue, gave the name of woad vats to the apparatus. The vats are sometimes made of
copper, at other times of iron or wood, the last alone being well adapted for the employment
of steam. The dimensions are very variable; but the following may be considered
as the average size: depth, 71⁄2 feet; width below, 4 feet, above, 5 feet. The vats are
built in such a way that the fire does not affect their bottom, but merely their sides half
way up; and they are sunk so much under the floor of the dyehouse, that their upper
half only is above it, and is surrounded with a mass of masonry to prevent the dissipation
of the heat. About 3 or 31⁄2 feet under the top edge an iron ring is fixed, called
the champagne by the French, to which a net is attached in order to suspend the stuffs
out of contact of the sediment near the bottom.
In mounting the vat the following articles are required: 1. woad prepared by fermentation,
or woad merely dried, which is better, because it may be made to ferment
in the vat, without the risk of becoming putrid, as the former is apt to do; 2. indigo,
previously ground in a proper mill; 3. madder; 4. potash; 5. slaked quicklime;
6. bran. In France, weld is commonly used instead of potash.
The indigo mill is represented in figs. 579. and 580. a is a four-sided iron cistern,
cylindrical or rounded in the bottom, which rests upon gudgeons in a wooden frame; it
has an iron lid b, consisting of two leaves, between which the rod c moves to and fro,
receiving a vibratory motion from the crank d. By this construction, a frame e, which
is made fast in the cistern by two points e′ e′, is caused to vibrate, and to impart its swing
movement to six iron rollers f f f, three being on each side of the frame, which
triturate the indigo mixed with water into a fine paste. Whenever the paste is uniformly
ground, it is drawn off by the stopcock g, which had been previously filled up by a
screwed plug, to prevent any of the indigo from lodging in the orifice of the cock, and
thereby escaping the action of the rollers. The cistern is nearly three feet long.
The vat being filled with clear river water, the fire is to be kindled, the ingredients
introduced, and if fermented woad be employed, less lime is needed than with the merely
dried plant. Meanwhile the water is to be heated to the temperature of 160° Fahr.,
and maintained at this pitch till the deoxidizement and solution of the indigo begin
to shew themselves, which, according to the state of the constituents, may happen in 12
hours, or not till after several days. The first characters of incipient solution are blue[673]
bubbles, called the flowers, which rise upon the surface, and remain like a head of soap-suds
for a considerable time before they fall; then blue coppery shining veins appear with
a like coloured froth. The hue of the liquor now passes from blue to green, and an
ammoniacal odour begins to be exhaled. Whenever the indigo is completely dissolved,
an acetic smelling acid may be recognized in the vat, which neutralizes all the alkali,
and may occasion even an acid excess, which should be saturated with quicklime. The
time for doing this cannot be in general very exactly defined. When quicklime has been
added at the beginning in sufficient quantity, the liquor appears of a pale wine-yellow
colour, but if not, it acquires this tint on the subsequent introduction of the lime.
Experience has not hitherto decided in favour of the one practice or the other.
As soon as this yellow colour is formed in the liquor, and its surface becomes blue,
the vat is ready for the dyer, and the more lime it takes up without being alkaline, the
better is its condition. The dyeing power of the vat may be kept up during six months,
or more, according to the fermentable property of the woad. From time to time, madder
and bran must be added to it, to revive the fermentation of the sediment, along with some
indigo and potash, to replace what may have been abstracted in the progress of dyeing.
The quantity of indigo must be proportional, of course, to the depth or lightness of
the tints required.
During the operation of this blue vat two accidents are apt to occur; the first, which
is the more common one, is called the throwing back, in French the cuve rebuté, and in
German, the Scharf or Schwartzwerden (the becoming sharp or black); the second is
the putrefaction of the ingredients. Each is discoverable by its peculiar smell, which it
is impossible to describe. The first is occasioned by the employment of too much quicklime,
whereby the liquor becomes neutral or even alkaline. This fault may be recognized
by the fading of the green, or by the dark green, or nearly black appearance of the
liquor; and by a dull blue froth, owing to a film of lime. The remedy for a slight degree
of this vicious condition, is to suspend in the liquor a quantity of bran tied up in a bag,
and to leave it there till the healthy state be restored. Should the evil be more inveterate,
a decoction of woad, madder, and bran must be introduced. Strong acids are
rather detrimental. Sulphate of iron has been recommended, because its acid precipitates
the lime, while its oxide reduces the indigo to the soluble state.
The decomposition or putrefaction of the blue vat is an accident the reverse of the
preceding, arising from the transition of the acetous into the putrid fermentation,
whereby the dyeing faculty is destroyed. Such a misfortune can happen only towards
the commencement of working the vat, whilst the woad is still powerful, and very little
indigo has been dissolved. Whenever the vat is well charged with indigo, that accident
cannot easily supervene. In both of these distemperatures the elevation of the temperature
of the vat aggravates the evil.
Dyeing in the blue vat is performed as follows:—
Wool is put into a net, and pressed down into the liquor with rods; but cloth is smoothly
stretched and suspended by hooks upon frames, which are steadily dipped into the vat,
with slight motions through the liquor; yarn-hanks must be dipped and turned about
by hand. All unnecessary stirring of the liquor must however be avoided, lest the oxygen
of the atmosphere be brought too extensively into contact with the reduced indigo, for
which reason mechanical agitation with rollers in the vat is inadmissible. The stuffs
to be dyed, take at the first dip only a feeble colour, though the vat be strong, but they
must be deepened to the desired shade by successive immersions of fifteen minutes or
more each time, with intervals of exposure to the air, for absorption of its oxygen.
After the lapse of a certain time, if the fermentative power be impaired, which is recognized
by the dye stuffs losing more colour in a weak alkaline test lye than they ought,
the vat should be used up as far as it will go, and then the liquor should be poured
away, for the indigo present is not in a reduced state, but merely mixed mechanically,
and therefore incapable of forming a chemical combination with textile fibres. If cotton
goods previously treated with an alkaline lye are to be dyed blue, the vat should contain
very little lime.
Theory of the Indigo vat.—The large quantity of extractive matter in woad and
madder; as also the sugar, starch and gluten in the bran and woad, when dissolved
in warm water, soon occasion a fermentation, with an absorption of oxygen, from the
air, but especially from the indigo of the woad, and from that introduced in a finely
ground state. When thus disoxygenated, it becomes soluble in alkaline menstrua; the
red-brown of the indigo being dissolved at the same time. When lime is added, the
indigo-blue dissolves, and still more readily if a little potash is conjoined with it; but
whatever indigo-brown may have been dissolved by the potash, is thrown down by the lime.
Lime in too large a quantity, however, forms an insoluble combination with the reduced
indigo, and thus makes a portion of the dye ineffective; at the same time it combines
with the extractive. In consequence of the fermentative action, carbonic acid, acetic acid,
and ammonia are disengaged; the first two of which neutralize a portion of the lime,[674]
and require small quantities of this earth to be added in succession; hence also
a considerable quantity of the carbonate of lime is found as a deposit on the sides and
bottom of the vat. In the sound condition of the indigo vat, no free lime should be
perceived, but on the contrary a free acid. Yet when the disengaged carbonic and
acetic acids saturate the lime completely, no indigo can remain at solution; therefore a
sufficient supply of lime must always be left to dissolve the dye, otherwise the indigo
would fall down and mix with the extractive matter at the bottom. Goods dyed in the
blue vat are occasionally brightened by a boil in a logwood bath, with a mordant of
sulpho-muriate of tin, or in a bath of cudbear.
Another mode of mounting the indigo vat without woad and lime, is by means of
madder, bran, and potash. The water of the vat is to be heated to the temperature of
122° F.; and for 120 cubic feet of it, 12 pounds of indigo, 8 pounds of madder, and as
much bran are to be added, with 24 pounds of good potashes; at the end of 36 hours,
12 pounds more of potash are introduced, and a third 12 pounds in other 12 hours. In
the course of 72 hours, all the characters of the reduction and solution of the indigo
become apparent; at which time the fermentation must be checked by the addition of
quick-lime. The liquor has a bright full colour, with a beautiful rich froth. In feeding
the vat with indigo, an equal weight of madder, and a double weight of potash should
be added. The odour of this vat in its mild but active state is necessarily different from
that of the woad vat, as no ammonia is exhaled in the present case, and the sediment is
much smaller. The reduced indigo is held in solution by the carbonated potash, while
the small addition of quicklime merely serves to precipitate the indigo-brown.
A potash vat dyes in about half the time of the ordinary warm vat, and penetrates
fine cloth much better; while the goods thus dyed lose less colour in alkaline and soap
solutions. This vat may moreover be kept with ease in good condition for several
months; is more readily mounted; and from the minute proportion of lime present, it
cannot impair the softness of the woollen fibres. It is merely a little more expensive.
It is said that cloth dyed in the potash indigo vat, requires one third less soap in the
washing at the fulling mill, and does not soil the hands after being dressed. At Elbœuf
and Louviers in France, such vats are much employed. Wool, silk, cotton, and linen
may all be dyed in them.
Cold vats.—The copperas or common blue vat of this country is so named because the
indigo is reduced by means of the protoxide of iron. This salt should therefore be as
free as possible from the red oxide, and especially from any sulphate of copper, which
would re-oxidize the indigo. The necessary ingredients are: copperas (green sulphate
of iron), newly slaked quicklime, finely ground indigo, and water; to which sometimes
a little potash or soda is added, with a proportional diminution of the lime. The operation
is conducted in the following way: the indigo well triturated with water or an
alkaline lye, must be mixed with hot water in the preparation vat, then the requisite
quantity of lime is added, after which the solution of copperas must be poured in with
stirring. Of this preparation vat, such a portion as may be wanted is laded into
the dyeing vat. For one pound of indigo three pounds of copperas are taken, and four
pounds of lime (or 1 of indigo, 21⁄2 of copperas, and 3 of lime). If the copperas be partially
peroxidized, somewhat more of it must be used.
A vat containing a considerable excess of lime is called a sharp vat, and is not well
adapted for dyeing. A soft vat, on the contrary, is that which contains too much copperas.
In this case the precipitate is apt to rise, and to prevent uniformity of tint in
the dyed goods. The sediment of the copperas vat consists of sulphate of lime, oxide
of iron, lime with indigo brown, and lime with indigo blue, when too much quicklime
has been employed. The clear, dark wine yellow fluid contains indigo blue in a
reduced state, and indigo red, both combined with lime and with the gluten of indigo
dissolved. After using it for some time the vat should be refreshed or fed with copperas
and lime, upon which occasion, the sediment must first be stirred up, and then allowed
time to settle again, and become clear. For obtaining a series of blue tints, a series of
vats of different strengths is required.
Linen and cotton yarn, before being dyed should be boiled with a weak alkaline lye,
then put upon frames or tied up in hanks, and after removing the froth from the vat,
plunged into, and moved gently through it. For pale blues, an old, nearly exhausted
vat, is used; but for deep ones, a fresh nearly saturated vat. Cloth is stretched upon a
proper square dipping frame made of wood, or preferably of iron, furnished with sharp
hooks or points of attachment. These frames are suspended by cords over a pulley, and
thus immersed and lifted out alternately at proper intervals. In the course of 8 or 10
minutes, the cloth is sufficiently saturated with the solution of indigo, after which it is
raised and suspended so as to drain into the vat. The number of dippings determines
the depth of the shade; after the last the goods are allowed to dry, taken off the frame,
plunged into a sour bath of very dilute sulphuric or muriatic acid, to remove the adhering
lime, and then well rinsed in running water. Instead of the dipping frames some[675]
dyers use a peculiar roller apparatus, called gallopers, similar to what has been described
under Calico Printing; particularly for pale blues. This cold vat is applicable to
cotton, linen and silk goods.
When white spots are to appear upon a blue ground, resist pastes are to be used, as
described under Calico Printing.
The urine vat is prepared by digestion of the ground indigo in warmed stale urine,
which first disoxygenates the indigo, and then dissolves it by means of its ammonia.
Madder and alum are likewise added, the latter being of use to moderate the fermentation.
This vat was employed more commonly of old than at present, for the purpose of
dyeing woollen and linen goods.
The mode of making the china blue dye has been described under Calico Printing;
as well as the pencil blue, or blue of application.
A blue dye may likewise be given by a solution of indigo in sulphuric acid. This
process was discovered by Barth, at Grossenhayn in Saxony, about the year 1740, and
is hence called the Saxon blue dye. The chemical nature of this process has been
already fully explained. If the smoking sulphuric acid be employed, from 4 to 5 parts
are sufficient for 1 of indigo; but if oil of vitriol, from 7 to 8 parts. The acid is to be
poured into an earthen-ware pan, which in summer must be placed in a tub of cold
water, to prevent it getting hot, and the indigo in fine powder, is to be added with careful
stirring, in small successive portions. If it become heated, a part of the indigo is decomposed,
with the disengagement of sulphurous acid gas, and indigo green is produced.
Whenever all the indigo has been dissolved, the vessel must be covered up, allowed to
stand for 48 hours, and then diluted with twice its weight of clear river water.
The undiluted mass has a black blue colour, is opaque, thick, attracts water from
the air, and is called indigo composition or chemic blue. It must be prepared beforehand,
and kept in store. In this solution, besides the cerulin, there are also indigo-red, indigo-brown,
and gluten, by which admixture the pure blue of the dye is rendered foul,
assuming a brown or a green cast. To remove these contaminations, wool is had recourse
to. This is plunged into the indigo previously diffused through a considerable body of
water, brought to a boiling heat in a copper kettle, and then allowed to macerate
as it cools for 24 hours. The wool takes a dark blue dye by absorbing the indigo-blue
sulphate and hyposulphite, while at the same time the liquor becomes greenish blue;
and if the wool be left longer immersed, it becomes of a dirty yellow. It must therefore
be taken out, drained, washed in running water till this runs off colourless, and
without an acid taste. It must next be put into a copper full of water, containing
one or two per cent. of carbonate of potash, soda, or ammonia (to about one third the
weight of the indigo), and subjected to a boiling heat for a quarter of an hour. The blue
salts forsake the wool, leaving it of a dirty red brown, and dye the water blue. The
wool is in fact dyed with the indigo red, which is hardly soluble in alkali. The blue
liquor may now be employed as a fine dye, possessed of superior tone and lustre. It
is called distilled blue and soluble blue. Sulphuric acid throws down from it the small
quantity of indigo red, which had been held in solution by the alkali.
When wool is to be dyed with this sulphate of indigo blue, it must be first boiled in
alum, then treated with the blue liquor, and thus several times alternately, in order to
produce an uniform blue colour. Too long continuance of boiling is injurious to the
beauty of the dye. In this operation the woollen fibres get impregnated with the
indigo-blue sulphate of alumina.
With sulphate of indigo, not only blues of every shade are dyed, but also green, olive,
gray, as also a fast ground to logwood blues; for the latter purpose the preparatory boil
is given with alum, tartar, sulphates of copper and iron, and the blue solution; after
which the goods are dyed up with a logwood bath containing a little potash.
Statistical Tables of Indigo; per favour of James Wilkinson, Esq.,
of Leadenhall-Street.
East India Indigo.
| Years. |
Produce
in
India. |
Con-
sumption
of World;
average,
4 years. |
Stock in
England
31st De-
cember. |
Highest
Price. |
Good
middling
Violet. |
| |
|
|
|
|
Per lb. |
|
| |
Chests. |
Chests. |
Chests. |
|
s. |
d. |
s. |
d. |
s. |
d. |
| 1811 |
21,000 |
22,200 |
26,900 |
|
10 |
6 |
5 |
6 |
6 |
0 |
| 1812 |
23,500 |
22,500 |
29,500 |
|
11 |
6 |
6 |
9 |
7 |
3 |
| 1813 |
22,800 |
22,800 |
24,500 |
|
15 |
5 |
9 |
0 |
9 |
6 |
| 1814 |
28,500 |
23,000 |
24,900 |
|
13 |
0 |
7 |
9 |
8 |
3 |
| 1815 |
30,500 |
23,200 |
30,400 |
|
11 |
0 |
6 |
9 |
7 |
6 |
| 1816 |
25,000 |
26,900 |
25,700 |
|
10 |
0 |
5 |
0 |
5 |
6 |
| 1817[676] |
20,500 |
27,000 |
23,500 |
|
10 |
0 |
7 |
3 |
7 |
9 |
| 1818 |
19,100 |
26,500 |
24,000 |
|
9 |
3 |
6 |
9 |
7 |
3 |
| 1819 |
20,700 |
26,400 |
19,700 |
|
8 |
6 |
5 |
6 |
6 |
0 |
| 1820 |
27,200 |
24,200 |
14,500 |
|
9 |
0 |
6 |
3 |
6 |
9 |
| 1821 |
21,100 |
25,300 |
9,800 |
|
11 |
6 |
8 |
6 |
9 |
0 |
| 1822 |
25,700 |
26,000 |
8,200 |
|
12 |
0 |
9 |
0 |
9 |
6 |
| 1823 |
29,800 |
25,300 |
13,100 |
|
10 |
0 |
7 |
3 |
7 |
9 |
| 1824 |
24,100 |
26,500 |
12,200 |
|
15 |
0 |
12 |
0 |
12 |
6 |
| 1825 |
43,500 |
23,500 |
16,400 |
|
15 |
6 |
12 |
0 |
12 |
6 |
| 1826 |
28,000 |
27,300 |
22,300 |
|
11 |
3 |
7 |
6 |
7 |
9 |
| 1827 |
45,300 |
28,900 |
22,800 |
|
12 |
6 |
8 |
0 |
8 |
6 |
| 1828 |
30,000 |
31,000 |
31,100 |
|
10 |
0 |
6 |
3 |
6 |
6 |
| 1829 |
43,200 |
33,000 |
31,200 |
|
8 |
9 |
5 |
3 |
5 |
9 |
| |
|
|
|
Years. |
|
|
| 1830-1 |
32,100 |
32,800 |
37,600 |
1831 |
7 |
9 |
4 |
3 |
4 |
9 |
| 1831-2 |
32,500 |
34,500 |
35,700 |
1832 |
6 |
3 |
4 |
3 |
4 |
6 |
| 1832-3 |
35,200 |
35,500 |
32,500 |
1833 |
6 |
0 |
4 |
2 |
4 |
4 |
| 1833-4 |
27,100 |
34,600 |
35,800 |
1834 |
8 |
0 |
6 |
3 |
6 |
6 |
| 1834-5 |
30,500 |
33,800 |
29,319 |
1835 |
7 |
0 |
5 |
3 |
5 |
6 |
| 1835-6 |
32,600 |
34,700 |
21,449 |
1836 |
6 |
3 |
4 |
9 |
5 |
0 |
| 1836-7 |
- - |
32,600 |
26,219 |
1837 |
8 |
9 |
6 |
9 |
7 |
0 |
East India and Spanish, &c. Indigo.
| Years. |
Importations. |
Exported. |
Home
Consump-
tion. |
East
India. |
Spanish,
&c. |
| |
lbs. |
lbs. |
lbs. |
lbs. |
| 1785 |
154,291 |
1,539,218 |
584,885 |
|
| 1786 |
253,345 |
1,724,945 |
466,696 |
|
| 1787 |
364,046 |
1,514,784 |
502,800 |
|
| 1788 |
622,691 |
1,473,920 |
445,857 |
|
| 1789 |
371,469 |
1,594,618 |
673,630 |
|
| 1790 |
531,619 |
1,307,088 |
821,131 |
|
| 1791 |
465,198 |
1,141,589 |
870,185 |
|
| 1792 |
581,827 |
1,274,538 |
880,951 |
|
| 1793 |
890,766 |
1,066,817 |
929,707 |
|
| 1794 |
1,403,650 |
1,487,642 |
1,623,908 |
|
| 1795 |
2,862,684 |
1,424,941 |
1,387,171 |
|
| 1796 |
3,897,120 |
680,915 |
1,883,320 |
|
| 1797 |
1,754,233 |
535,845 |
3,105,610 |
|
| 1798 |
3,862,188 |
192,060 |
1,718,624 |
|
| 1799 |
2,529,377 |
512,459 |
2,585,755 |
|
| 1800 |
2,674,317 |
1,076,417 |
2,586,833 |
|
| 1801 |
2,123,637 |
827,696 |
2,281,812 |
|
| 1802 |
2,264,199 |
669,679 |
1,961,346 |
|
| 1803 |
2,632,110 |
522,825 |
1,130,194 |
|
| 1804 |
2,765,871 |
395,258 |
1,523,095 |
|
| 1805 |
4,666,292 |
687,319 |
1,845,035 |
|
| 1806 |
2,612,181 |
319,394 |
2,904,614 |
|
| 1807 |
5,326,032 |
715,809 |
2,006,463 |
|
| 1808 |
5,314,860 |
477,625 |
1,568,351 |
|
| 1809 |
2,179,083 |
674,048 |
3,179,861 |
|
| 1810 |
5,243,613 |
883,061 |
2,485,679 |
|
| 1811 |
4,453,932 |
658,577 |
1,566,056 |
|
| 1812 |
4,461,793 |
354,171 |
1,853,916 |
|
| 1813 |
Accounts destroyed by Fire
at Custom House. |
|
| 1814 |
6,803,064 |
328,881 |
5,501,851 |
3,406,282 |
| 1815 |
5,543,852 |
79,253 |
4,278,674 |
2,774,091 |
| 1816 |
7,247,227 |
39,275 |
4,214,454 |
1,899,819 |
| 1817 |
5,001,280 |
134,313 |
2,427,443 |
2,377,659 |
| 1818 |
5,497,768 |
187,257 |
2,963,462 |
2,302,163 |
| 1819[677] |
3,689,052 |
129,682 |
3,126,739 |
2,033,601 |
| 1820 |
4,924,222 |
161,164 |
4,378,857 |
2,288,196 |
| 1821 |
3,943,592 |
119,517 |
2,985,364 |
1,959,509 |
| 1822 |
2,549,284 |
374,230 |
2,378,948 |
2,004,062 |
| 1823 |
6,557,296 |
664,408 |
2,783,504 |
2,322,221 |
| 1824 |
4,595,707 |
485,110 |
2,795,740 |
2,493,350 |
| 1825 |
6,233,335 |
560,296 |
3,870,929 |
2,381,233 |
| 1826 |
7,699,439 |
386,312 |
4,365,163 |
1,901,047 |
| 1827 |
5,404,811 |
662,936 |
3,315,675 |
2,399,365 |
| 1828 |
9,683,626 |
229,384 |
4,588,658 |
3,064,915 |
| 1829 |
5,978,527 |
769,757 |
4,286,605 |
2,113,830 |
| 1830 |
7,920,924 |
295,516 |
4,686,784 |
2,676,945 |
| 1831 |
7,004,510 |
290,089 |
4,374,241 |
2,490,134 |
| 1832 |
6,221,725 |
131,340 |
5,346,725 |
2,395,653 |
| 1833 |
6,304,016 |
331,016 |
3,664,814 |
2,323,300 |
| 1834 |
3,798,144 |
357,152 |
3,928,226 |
2,447,827 |
| 1835 |
3,986,233 |
183,480 |
4,074,598 |
2,606,772 |
| 1836 |
6,753,898 |
418,800 |
3,691,951 |
2,864,274 |
| 1837 |
5,872,601 |
673,270 |
3,587,561 |
2,240,451 |
INDIAN RUBBER, is the vulgar name of caoutchouc in this country.
INK; (Encre, Fr.; Tinte, Germ.) is a coloured liquid for writing on paper, parchment,
linen, &c. with a pen.
Black ink.—Nut-galls, sulphate of iron, and gum, are the only substances truly
useful in the preparation of ordinary ink; the other things often added merely modify
the shade, and considerably diminish the cost to the manufacturer upon the great scale.
Many of these inks contain little gallic acid, or tannin, and are therefore of inferior
quality. To make 12 gallons of ink we may take,—
| 12 |
pounds of nutgalls, |
| 5 |
pounds of green sulphate of iron, |
| 5 |
pounds of gum senegal, |
| 12 |
gallons of water. |
The bruised nutgalls are to be put into a cylindrical copper, of a depth equal to its
diameter, and boiled, during three hours, with three fourths of the above quantity of
water, taking care to add fresh water to replace what is lost by evaporation. The
decoction is to be emptied into a tub, allowed to settle, and the clear liquor being
drawn off, the lees are to be drained. Some recommend the addition of a little bullock’s
blood or white of egg, to remove a part of the tannin. But this abstraction tends to lessen
the product, and will seldom be practised by the manufacturer intent upon a large return
for his capital. The gum is to be dissolved in a small quantity of hot water, and the
mucilage, thus formed, being filtered, is added to the clear decoction. The sulphate of
iron must likewise be separately dissolved, and well mixed with the above. The colour
darkens by degrees, in consequence of the peroxidizement of the iron, on exposing the
ink to the action of the air. But ink affords a more durable writing when used
in the pale state, because its particles are then finer, and penetrate the paper more
intimately. When ink consists chiefly of tannate of peroxide of iron, however black,
it is merely superficial, and is easily erased or effaced. Therefore whenever the
liquid made by the above prescription has acquired a moderately deep tint, it should be
drawn off clear into bottles, and well corked up. Some ink-makers allow it to mould
a little in the casks before bottling, and suppose that it will thereby be not so liable to
become mouldy in the bottles. A few bruised cloves, or other aromatic perfume, added
to ink, is said to prevent the formation of mouldiness, which is produced by the ova
of infusoria animalcules. I prefer digesting the galls, to boiling them.
The operation may be abridged, by peroxidizing the copperas beforehand, by moderate
calcination in an open vessel; but, for the reasons above assigned, ink made with such
a sulphate of iron, however agreeable to the ignorant, when made to shine with gum
and sugar, under the name of japan ink, is neither the most durable nor the most
pleasant to write with.
From the comparatively high price of gall-nuts, sumach, logwood, and even[678]
oak bark, are too frequently substituted, to a considerable degree, in the manufacture
of ink.
The ink made by the prescription given above, is much more rich and powerful
than many of the inks commonly sold. To bring it to their standard, a half more water
may safely be added, or even 20 gallons of tolerable ink may be made from that weight
of materials, as I have ascertained.
Sumach and logwood admit of only about one half of the copperas that galls will take
to bring out the maximum amount of black dye.
Chaptal gives a prescription in his Chimie appliquée aux arts, which, like many other
things in that book, are published with very little knowledge and discrimination. He
uses logwood and sulphate of copper, in addition to the galls and sulphate of iron;
a pernicious combination productive of a spurious fugitive black, and a liquor corrosive
of pens. It is, in fact, a modification of the vile dye of the hatters.
Lewis, who made exact experiments on inks, assigned the proportion of 3 parts
of galls to 1 of sulphate of iron, which, with average galls, will answer very well; but
good galls will admit of more copperas.
Gold ink is made by grinding upon a porphyry slab, with a muller, gold leaves along
with white honey, till they be reduced to the finest possible division. The paste is
then collected upon the edge of a knife or spatula, put into a large glass, and diffused
through water. The gold by gravity soon falls to the bottom, while the honey dissolves
in the water, which must be decanted off. The sediment is to be repeatedly
washed till entirely freed from the honey. The powder, when dried, is very brilliant,
and when to be used as an ink, may be mixed up with a little gum water. After the
writing becomes dry, it should be burnished with a wolf’s tooth.
Silver ink is prepared in the same manner.
Indelible ink.—A very good ink, capable of resisting chlorine, oxalic acid, and ablution
with a hair pencil or sponge, may be made by mixing some of the ink made by the
preceding prescription, with a little genuine China ink. It writes well. Many other
formulæ have been given for indelible inks, but they are all inferior in simplicity and
usefulness to the one now prescribed. Solution of nitrate of silver thickened with gum,
and written with upon linen or cotton cloth, previously imbued with a solution of soda,
and dried, is the ordinary permanent ink of the shops. Before the cloths are washed,
the writing should be exposed to the sun-beam, or to bright daylight, which blackens
and fixes the oxide of silver. It is easily discharged by chlorine and ammonia.
Red ink.—This ink may be made by infusing, for 3 or 4 days in weak vinegar,
Brazil wood chipped into small pieces; the infusion may be then boiled upon the wood
for an hour, strained, and thickened slightly with gum arabic and sugar. A little
alum improves the colour. A decoction of cochineal with a little water of ammonia,
forms a more beautiful red ink, but it is fugitive. An extemporaneous red ink of the
same kind may be made by dissolving carmine in weak water of ammonia, and adding a
little mucilage.
Green ink.—According to Klaproth, a fine ink of this colour may be prepared by
boiling a mixture of two parts of verdigris in eight parts of water, with one of cream of
tartar, till the total bulk be reduced one half. The solution must be then passed
through a cloth, cooled, and bottled for use.
Yellow ink is made by dissolving 3 parts of alum in 100 of water, adding 25 parts of
Persian or Avignon berries bruised, boiling the mixture for an hour, straining the
liquor, and dissolving in it 4 parts of gum arabic. A solution of gamboge in water
forms a convenient yellow ink.
By examining the different dye-stuffs, and considering the processes used in dyeing
with them, a variety of coloured inks may be made.
China ink.—Proust says, that lamp-black purified by potash lye, when mixed with
a solution of glue, and dried, formed an ink which was preferred by artists to that of
China. M. Merimée, in his interesting treatise, entitled, De la peinture à l’huile, says,
that the Chinese do not use glue in the fabrication of their ink, but that they add
vegetable juices, which render it more brilliant and more indelible upon paper.
When the best lamp-black is levigated with the purest gelatine or solution of glue, it
forms, no doubt, an ink of a good colour, but wants the shining fracture, and is not so
permanent on paper as good China ink; and it stiffens in cold weather into a tremulous
jelly. Glue may be deprived of the gelatinizing property by boiling it for a long time,
or subjecting it to a high heat in a Papin’s digester; but as ammonia is apt to be generated
in this way, M. Merimée recommends starch gum made by sulphuric acid (British
gum) to be used in preference to glue. He gives, however, the following directions for
preparing this ink with glue. Into a solution of glue he pours a concentrated solution
of gall-nuts, which occasions an elastic resinous-looking precipitate. He washes this
matter with hot water, and dissolves it in a spare solution of clarified glue. He filters[679]
anew, and concentrates it to the proper degree for being incorporated with the purified
lamp-black. The astringent principle in vegetables does not precipitate gelatine when
its acid is saturated, as is done by boiling the nutgalls with limewater or magnesia.
The first mode of making the ink is to be preferred. The lamp-black is said to be
made in China, by collecting the smoke of the oil of sesame. A little camphor (about
2 per cent.) has been detected in the ink of China, and is supposed to improve it.
infusion of galls renders the ink permanent on paper.
Sympathetic ink. The best is a solution of muriate of cobalt.
Printer’s ink. See this article.
By decomposing vanadate of ammonia with infusion of galls, a liquid is obtained
of a perfectly black hue, which flows freely from the pen, is rendered blue by acids, is
insoluble in dilute alkalis, and resists the action of chlorine. Whenever the metal vanadium
shall become more abundant, as it probably may ere long, we shall possess the
means of making an ink, at a moderate price, much superior to the tannate and gallate
of iron.
To prepare the above vanadic salt cheaply, the cinder or hammerschlag obtained
from the iron made at Ekersholm, in Sweden, or other iron which contains vanadium,
being reduced to a fine powder, is to be mixed with two thirds of its weight
of nitre, and one third of effloresced soda. The mixture is to be ignited in a crucible;
cooled and lixiviated, whereby solutions of the vanadates of potash and soda are obtained,
not pure, indeed, but sufficiently so for being decomposed, by means of sal ammoniac,
into a vanadate of ammonia. This being rendered nearly neutral with any acid, constitutes
an excellent indelible ink.
INULINE; (Eng. and Fr.) is a substance first extracted from the root of the Inula-Hellenium,
or Elecampane. It is white and pulverulent like starch; and differs from
this substance chiefly because its solution, when it cools, lets fall the inuline unchanged
in powder, whereas starch remains dissolved in the cold, as a jelly or paste.
Inuline is obtained by boiling the root sliced in 3 or 4 times its weight of water, and
setting the strained decoction aside till it cools, when the pulverulent inuline precipitates.
It exists also in the roots of colchicum, and pellitory.
IODINE; (Iode, Fr.; Iod, Germ.) is one of the archæal undecompounded chemical
bodies, which was discovered accidentally in 1812 by M. Courtois, a manufacturer of
saltpetre, in the mother-waters of that salt. Its affinities for other substances are so
powerful as to prevent it from existing in an insulated state. It occurs combined with
potassium and sodium in many mineral waters, such as the brine spring of Ashby-de-la-Zouche,
and other strongly saline springs. This combination exists sparingly in sea-water,
abundantly in many species of fucus or sea-weed, and in the kelp made from
them; in sponges; in several marine molluscæ, such as the doris, the venus, oysters, &c.;
in several polyparies, and sea plants, as the gorgonia, the zostera marina, &c.; particularly
in the mother-waters of the salt works upon the Mediterranean sea; and it has been
found in combination with silver, in some ores brought from the neighbourhood of
Mexico.
Iodine is most economically procured from the mother-water of kelp, as furnished by
those manufacturers of soap in Scotland and elsewhere who employ this crude alkaline
matter. By pouring an excess of sulphuric acid upon that liquid, and exposing the
mixture to heat in a retort, iodine rises in violet vapours (whence its name), and condenses
in the receiver into black, brilliant, soft, scaly crystals, resembling graphite or plumbago.
An addition of the peroxide of manganese to the above mixture, favours the production
of iodine. Soubeiran has proposed, as a means of extracting it in greater abundance
from a given quantity of the said mother-waters, to transform the iodide of potash or
soda, present, into an insoluble iodide of copper, by pouring into them solution of
sulphate of copper, which precipitates first of all one half of the iodine. He then
decants the supernatant liquor, and adds to it a fresh quantity of the sulphate along
with some iron filings. The latter metal seizes the oxygen and sulphuric acid of
the cupreous salt, sets the copper free, which then seizes the other half of the
iodine. To separate this iodide from the remaining iron filings, he agitates the
whole with water, and decants the liquor. The filings immediately subside, but the
iodide of copper remains for some time in a state of suspension. This compound,
separated by a filter cloth, is to be mixed with twice its weight of the black peroxide
of manganese, and as much sulphuric acid as will make the mixture into a paste; which
mixture being introduced into a retort, and distilled, the iodine comes over in its characteristic
violet vapours, which are condensed into the glistening black substance in the
receiver.
Iodine is always solid at atmospheric temperatures, though it slowly flies off with a
peculiar offensive penetrating odour somewhat like chlorine. Its specific gravity is
4·946 at the temperature of 58° Fahr. Its prime equivalent, according to Berzelius, is[680]
63·283, one volume of hydrogen being 1·000; but 126·566, if two volumes of hydrogen
be reckoned unity, as most British chemists estimate it, from the composition of water.
It possesses in a high degree electro-negative properties, like oxygen and chlorine; and
therefore makes its appearance at the positive pole, when its compounds are placed in
the voltaic circuit. It stains the skin yellow; and if applied for some time to it, is apt
to produce painful ulcerations.
Iodine melts only at about 390° Fahr.; but with the vapour of water it volatilizes at
212°. It has a great affinity for hydrogen, and constitutes by that union hydriodic acid; a
compound resembling in some respects muriatic or hydrochloric acid. It also can be
combined with oxygen, and forms thereby iodic acid. Its compounds with carbon,
phosphorus, sulphur, chlorine, azote, and many metals have not been applied to any
manufacturing purpose, and therefore need not be described here.
The chief application of iodine in the arts, is for the detection of starch, which its
watery solution, though containing only one part in 5000, does readily, by the production
of a deep purple colour; this vanishes by exposing the starch to the air for
some time, or more quickly by heating it.
As a medicine, iodine and its compounds, such as the iodides of potassium and iron,
are supposed to possess great powers in resolving glandular swellings. The periodide
of mercury is a brilliant red pigment, but somewhat evanescent.
Chlorine, bromine, and iodine are frequently associated; and it has hitherto been
reckoned a difficult problem to separate them from one another. The following plan is
proposed by M. Lövig.
Heat the mixture of the dried chloride and bromide (or chloride and iodide) while
a current of chlorine is made to pass over it, till no more bromine is carried off by the
chlorine. Receive the gases in a solution of potash; saturate this fluid mixture of the
chloride of potassium, and the chlorate and bromate of potash with nitric acid, adding
afterwards nitrate of silver. A mixture of bromate and chloride of silver will precipitate.
Dry the precipitate, calcine it, and calculate the proportion of bromine from the
volume of oxygen gas now disengaged. It would be preferable to digest in a phial, the
precipitate while moist, along with water of baryta, which decomposes the bromate of
silver without acting upon the chloride. The excess of baryta being thrown down by
carbonic acid, and the liquid being evaporated, a bromate of baryta is obtained, which
may be washed with alcohol of 0·840. The solution of bromate of baryta may also be
neutralized by nitric acid, and the bromic acid may be precipitated by nitrate of silver.
The same method is applicable to the separation of iodine from chlorine.
After throwing down the solution of the mixed salts by nitrate of silver, Berzelius
digests the washed precipitate in a closed bottle of water of baryta; whence results
bromate of baryta without any chloride of barium. On evaporating the liquor we obtain
crystallized bromate of baryta, which may be freed from a small accidental quantity
of chloride, by washing with alcohol at 0·840. By calcination we then obtain bromide
of barium, which being distilled with sulphuric acid and peroxide of manganese, affords
bromine.
IRIDIUM, is a metal discovered by Descotils in 1803, as also by Tennant in 1804;
and is so called because its different solutions exhibit all the colours of the rainbow. It
occurs only in the ore of platinum, being found there in two states; 1. united to that
metal, and 2., as alloy of osmium and iridium, in the form of small, insulated, hard
grains. Iridium is the most refractory of all the metals; and appears as a gray metallic
powder. It is not fused by the flame of the hydroxygen lamp.
IRON; (Fer, Fr.; Eisen, Germ.) is a metal of a bluish-gray colour, and a dull fibrous
fracture, but it is capable of acquiring a brilliant surface by polishing. Its specific gravity
is 7·78. It is the most tenacious of metals, and the hardest of all those which are malleable
and ductile. It is singularly susceptible of the magnetic virtue, but in its pure state soon
loses it. When rubbed it has a slight smell, and it imparts to the tongue a peculiar
astringent taste, called chalybeate. In a moist atmosphere, iron speedily oxidizes, and
becomes covered with a brown coating, called rust.
Every person knows the manifold uses of this truly precious metal; it is capable of
being cast in moulds of any form; of being drawn out into wires of any desired strength
or fineness; of being extended into plates or sheets; of being bent in every direction;
of being sharpened, hardened, and softened at pleasure. Iron accommodates itself to all
our wants, our desires, and even our caprices; it is equally serviceable to the arts, the
sciences, to agriculture, and war; the same ore furnishes the sword, the ploughshare, the
scythe, the pruning hook, the needle, the graver, the spring of a watch or of a carriage,
the chisel, the chain, the anchor, the compass, the cannon, and the bomb. It is a
medicine of much virtue, and the only metal friendly to the human frame.
The ores of iron are scattered over the crust of the globe with a beneficent profusion,
proportioned to the utility of the metal; they are found under every latitude, and every[681]
zone; in every mineral formation, and are disseminated in every soil. Considered in
a purely mineralogical point of view, without reference to their importance for reduction,
they may be reckoned to be 19 in number; namely, 1. native iron of three kinds: pure,
nickeliferous, and steely; 2. arsenical iron; 3. yellow sulphuret of iron; 4. white
sulphuret of iron; 5. magnetic sulphuret of iron; 6. black oxide of iron, either the
loadstone, or susceptible of magnetism, and titaniferous; 7. compact fer oligiste, specular
iron ore, as of Elba, and scaly fer oligiste; 8. hematite, affording a red powder; 9. hematite
or hydrate of iron, affording a yellow powder, of which there are several varieties;
10. pitchy iron ore; 11. siliceo-calcareous iron, or yenite; 12. sparry carbonate of
iron, and the compact clay iron-stone of the coal formation; 13. phosphate of iron;
14. sulphate of iron, native copperas; 15. chromate of iron; 16. arseniate of iron;
17. muriate of iron; 18. oxalate of iron; 19. titanate of iron.
Among all these different species, ten are worked by the miner, either for the sake of
the iron which they contain; for use in their native state; or for extracting some principles
from them advantageous to the arts and manufactures; such are arsenical iron,
sulphate of iron, sulphuret of iron, and chromate of iron.
1. Native iron A. Pure.—This species is very rare, and its existence was long matter of
dispute; though it has been undoubtedly found not only in volcanic formations, but in
veins properly so called. It is not entirely like our malleable iron; but is whiter, more
ductile, more permanent or less oxidizable in the air, and somewhat less dense. Among
the best attested examples of pure native iron is that observed by M. Schreber, in the
mountain of Oulle near Grenoble. The metal was entangled in a vein running
through gneiss, and appeared in ramifying stalactites, enveloped in fibrous brown-oxide
of iron mixed with quartz and clay.
B. The native nickeliferous or meteoric iron is very malleable, often cellular, but sometimes
compact, and in parallel plates, which pass into rhomboids or octahedrons. It
is naturally magnetic, and by its nickel is distinguishable from terrestrial native iron.
Macquart, in describing the famous mass found at mount Kemir in Siberia, says that
the iron is perfectly flexible, and fit for making small instruments at a moderate heat;
but in too strong a fire, the metal becomes short, brittle, and falls into grains under the
hammer. Meteoric iron is covered with a sort of varnish which preserves its surface
from the rusting action of the air; but this preservative property does not extend to
the interior. Chladni has given a list of masses of meteoric iron, which have been
known to fall at different times from the atmosphere, and of many specimens which
indicate their atmospheric origin, by their aspect and composition. A portion of the
mass of meteoric iron found at Santa-Rosa near Santa-Fe-de-Bogota, was made into a
sword, and presented to Bolivar.
C. Native steel-iron.—This substance has all the characters of cast-steel; it occurs in a
kind of small button ingots, with a finely striated surface, and a fracture exceedingly
fine grained. It is hardly to be touched by the file, and will scarcely flatten under the
hammer. M. Mossier found this native steel at the village of Bouiche, near Nery,
department of the Allier, in a spot where there had existed a seam of burning coal. A
mass of 16 pounds and 6 ounces of native steel was discovered in that place, besides a
great many small globules.
2. Arsenical iron, Arsenikkies or Mispickel, is a tin-white mineral, which emits a
garlic smell at the blowpipe, or even when sparks are struck from it by steel, accompanied
with a small train of white smoke. It contains generally more or less sulphur
and sometimes a little silver, associated with metallic arsenic and iron.
3. Yellow sulphuret of iron, commonly called Marcasite, or Martial pyrites. The bronze
or brass-yellow colour enables us to recognize this mineral. At the blowpipe it gives
off its sulphur, and is converted into a globule attractable by the blowpipe. It is a
bisulphuret of iron containing 32 of sulphur and 28 of metal.
Copper pyrites may be distinguished from it by its golden yellow colour, which is
frequently iridescent, and by its inferior hardness; for it does not strike fire with steel,
like the preceding persulphuret. There is no vein, stratum, or mass of metallic ore
which does not contain some iron pyrites; and it is often the sole mineral that fills the
veins in quartz. It sometimes contains gold, and at other times silver.
4. White sulphuret of iron.—This is distinguishable from the preceding species only
by its colour and form of crystallization, and was hence till lately confounded with it by
mineralogists. Its surface is often radiated.
5. Magnetic sulphuret of iron, the Magnetkies of the Germans.—This ore is attractable
by the magnet like common iron. Its colour is reddish-yellow, passing into
brown; its fracture is rough. It consists of 16 of sulphur and 28 of iron.
6. Black oxide of iron, magnet ore, or native loadstone.—One variety of this species
has two poles in each specimen, which manifest a repulsive action against the corresponding
poles of a magnetic needle. All the varieties furnish a black powder. Its
external colour is a gray approaching to that of metallic iron, but somewhat duller;[682]
with occasional iridescence of surface. Neither nitric acid nor the blowpipe has any
action upon it. Its specific gravity varies from 4·24 to 5·4; and its constituents are
71·86 peroxide, and 28·14 protoxide, according to Berzelius; or in 100 parts, 71·74 of
metallic iron, and 28·26 of oxygen. Assuming the prime equivalent of iron to be 28,
with the British chemists, then an ore consisting, like the above, of two prime proportions
of peroxide, and one of protoxide, would be represented by the number 116 = 80
+ 36; and would consist in 100 parts, of iron 72·4, oxygen 27·6.
Magnetic iron-ore belongs to primitive rock formations, and occurs abundantly in
Sweden, Dalecarlia, Norway, Siberia, China, Siam, and the Philippine Isles; but it is
rare in England and France. It is worked extensively in Sweden, and furnishes an
excellent iron.
The titaniferous oxide of iron, or iron sand, is also attractable by the magnet. Its
colour is a deep black, with some metallic lustre; it is perfectly opaque: its fracture is
conchoidal; it is hard and difficult to grind under the pestle into a dull black powder,
which stains the fingers when it is very fine; it melts at a high heat into a black enamel
without lustre. All volcanic rocks contain a greater or less quantity of titanic iron-ore,
disseminated through them, which may be recognised by its brilliant metallic lustre, and
its perfect conchoidal fracture.
7. Fer oligiste, iron-glance, specular iron and red iron-ore.—This ore has the colour of
polished steel; and the light transmitted through the thin edges of its crystals appears
of a beautiful red. Its powder is always of a well marked brown-red hue, passing into
cherry-red, which distinguishes it from the black-oxide ore. Its fracture is rough, or
vitreous in certain varieties; it breaks easily; but it is hard enough to scratch glass. It
usually contains from 60 to 70 of metallic iron in 100 parts; the equivalent proportion
of oxygen in the pure red oxide of iron being 30 parts combined with 70 of metal. It
is a mistake to suppose any specular iron ore capable of yielding 85 per cent. of iron,
for 100 parts of even protoxide of iron contain only 77·77 parts of metal.
The compact variety comprises the crystals of the island of Elba, and of Framont in
the Vosges, which have a rough-grained fracture. It exists in very great masses, constituting
even entire mountains; in the cavities and fissures of these masses, the beautiful
crystals so much prized by collectors of minerals, occur.
The island of Elba is equally celebrated for its inexhaustible abundance of rich
specular iron-ore, and for the immemorial antiquity of its mining operations. Fig. 581.
is a vertical section passing through the three workings, called Pietamonte (D), Sanguinaccio
(E), Antenna (F), through an antient excavation a, through the coast o, and
the mole p, ending at the canal of Piombino. The total height of the metalliferous
mountain above the level of the sea, is no more than 180 metres, or 600 feet.
The rock which constitutes the body of this little mountain d l, is called bianchetta
by the workmen. It is a white slaty talc, slightly ochreous, or yellowish, consisting
chiefly of silica and alumina, with some magnesia.
The ore of Antenna (F) is a very hard compact fer oligiste, of a brilliant metallic
aspect. The workable bed has a height of 66 feet, and consists of metalliferous blocks
mixed confusedly with sterile masses of the rock; the whole covered with a rocky
detritus, under a brownish mould. From its metallic appearance and toughness, this
bed is called vena ferrata, the iron vein. In Pietamonte the workable bed is composed
entirely of micaceous specular iron ore (fer oligiste), with its fissures filled with yellow
ochre. This bed rests upon the rock called bianchetta; the brilliant aspect of ore in
this place has gained for it the name of vena lucciola.
The metalliferous hill d l, extends to the north-east, about a mile beyond the workings
D E F. The ore contains about 65 per cent. of iron, and is smelted in Catalan
forges.
The following description of the figure will make the structure of this extraordinary
mine well understood. a, is a great excavation, the result of antient workings.
1, 1; 2, 2; 3, 3, 4, 4, 5, 6, and 7, are roads for carrying off the rubbish, in correspondence
with the several working levels.
[683]
b, b, b, masses of old rubbish (deblais).
c, c, ditto, from the present workings D, E, F.
d, the rocky mass called bianchetta, against which the ore extracted from a, abuts.
e, the surface of a bed of ore, near the streamlet g.
f, f, indication of beds of iron pyrites and fer oligiste.
g, a small rivulet preceding from the infiltration of rains, and which is impregnated
with acidulous sulphate of iron.
h, h, ravine which separates the metalliferous hill d l, from the barren hill i.
k, masses of slags from ancient smelting operations; such are very common in this
island. None of any consequence now exists; nearly the whole of the ore being exported
to Tuscany, the Romagna, the Genoese territories, Piedmont, Naples, and
Corsica.
l, a considerable body of rubbish from ancient workings, towards the summit of the
metalliferous hill d, l.
m, m, part of this hill covered with rubbish, the result of old workings.
n, the site called Vigneria.
o, houses upon the shore called Marine de Rio, where the workpeople live, and the
mineral is kept in store.
p, wooden pier (mole) whence the ore is shipped; terminated by a small tower q.
Compact fer oligiste occurs also in the Vosges, in Corsica, at Altenberg and Freyburg
in Saxony, Presnitz in Bohemia, Norberg and Bisberg in Sweden, &c.
The varieties called specular fer oligiste, and scaly fer oligiste, or iron-glance, do not
differ essentially from the compact. None of them affects the magnetic needle, and
their powder is a red of greater or less vivacity.
8. Red oxide of iron.—The varieties included under this species afford a red powder,
do not affect the magnetic needle, and are destitute of metallic lustre. At the blowpipe
they all become black, or deep brown; and then they act on the needle. The crystallized
variety consists of 70 iron and 30 oxygen in 100 parts. The concretionary kind,
or hematite, has a brown-red colour; is solid, compact, and sometimes very hard; its
surface may be filed and polished so as to acquire a lustre almost metallic; its internal
structure is fibrous, and it exhibits sometimes a resemblance to splinters of wood. Its
outer surface is constantly concretionary, mammelated, and presents occasionally sections
of a sphere, or cylinders attached to each other. This is the blood-stone of the burnisher
of metals. It is a very common mineral. The ochry variety or red-iron-ochre is distinguished
from the solid hematite by the brightness of its colour. It is used as a
pigment.
9. Brown oxide of iron, brown iron-stone.—This affords always a yellow powder,
without any shade of red, which passes sometimes into the bistre brown, or velvet black.
At the blowpipe this oxide becomes brown, and very attractable by the magnet; but after
calcination and cooling, the ore yields a red powder, which stains paper nearly as red
as hematite does; and which is much employed in polishing metals. All the yellow or
brown oxides contain a large proportion of water, in chemical combination; and hence
this species has been called hydrate of iron. There are several varieties which assume
globular, reniform, stalactitic, and fruticose shapes. As impure varieties of the species we
must consider some of the clay-iron-ores, such as the granular, the common, the pisiform,
and the reniform clay-iron-ore. According to D’Aubuisson, the present species consists
of peroxide of iron, from 82 to 84 per cent.; water, 14 to 11; oxide of manganese, 2;
silica, 1 to 2. It is therefore a hydrated peroxide of iron; and ought by theory, to
consist, in its absolute state, of 81·63 peroxide, and 18·37 water. It occurs both in beds
and veins. The œtites or eagle-stones form a particular variety of this ore. On breaking
the balls so named, they are observed to be composed of concentric coats, the outside
ones being very hard, but the interior becoming progressively softer towards the centre,
which is usually earthy and of a bright yellow colour; sometimes however the centre is
quite empty, or contains only a few drops of water. Œtites occur in abundance, often
even in continuous beds in secondary mountains, and in certain argillaceous strata.
These stones are still considered by the French shepherds as amulets or talismans, and
may be found in the small bags which they suspend to the necks of their favourite rams;
and they are in such general use that a large quantity is annually imported into France
from the frontiers of Germany, for this superstitious purpose. When smelted, they
yield a good iron.
The variety called granular brown oxide, or bone ore, is merely a modification of
the preceding. It occurs in grains nearly round, varying in size from a millet seed to a
pea, each being composed of concentric coats, hard outside and soft within. They are
generally agglutinated by a calcareous or argillaceous paste; but are occasionally quite
loose. This ore occurs in calcareous formations, and is sometimes accompanied with
shells, such as terebratulæ. The brittle quality of the iron afforded by it, has been
ascribed to the phosphorus derived from the large quantity of organic bodies, with[684]
which the ore is frequently mixed. The bog-iron-ore, and swamp iron ore belong to
this species.
10. Pitchy hydrate of iron.—This is a rare mineral of a resinous aspect, found in a
vein in the mine of Braunsdorf, two leagues from Freyberg, and seems to consist of red
oxide of iron and water.
11. Yenite, is a mineral species rather rare, composed of red oxide of iron, silica, and
lime.
12. Carbonate of iron, sparry iron, or brown-spar.—This important species has been
divided into two varieties; spathose iron, and the compact carbonate. The first has a
sparry and lamellar fracture; with a colour varying from yellowish-gray to isabella
yellow, or even to brownish-red. It turns brown without melting at the blowpipe, and
becomes attractable by the magnet after being slightly roasted in the flame of a candle.
Even by a short exposure to the air, after its extraction from the mine, it also assumes
the same brown tint, but without acquiring the magnetic quality. It affords but a
slight effervescence with nitric acid, changing merely to a red-brown colour. Its specific
gravity varies from 3·00 to 3·67. Its primitive form is like that of carbonate of lime,
an obtuse rhomboid. Without changing this form, its crystals are susceptible of containing
variable quantities of carbonate of lime, till it passes wholly into this mineral.
Manganese and magnesia enter also occasionally into its composition.
Sparry carbonate of iron belongs to primitive formations; forming powerful veins in
mountains of gneiss, and is associated in these veins with quartz, copper pyrites, gray
copper, fibrous brown oxide of iron, and a variety of ramose carbonate of lime, vulgarly
called flos ferri. Thus it is found at Allevard and Vizille, near Grenoble, at Saint-George
d’Huretière, in the Alps of Savoy; at Baigorry, in the Lower Pyrenees; at
Eisenerz, in Styria; at Hüttenberg, in Carinthia; at Schwartz, in the Tyrol; in
Saxony, Hungary, other places in Germany, as also in Spain, Sweden, Norway,
and Siberia. It also occurs along with galena, and other ores of lead, in the mines of
Lead-Hills, and Wanlockhead, in Scotland; and in the mines of Cumberland, Northumberland,
and Derbyshire; likewise with tin-ore, at Wheal Maudlin, Saint-Just, and
other places in Cornwall.
This ore viewed as a metallurgic object, is one of the most interesting and valuable
that is known; it affords natural steel with the greatest facility, and accommodates itself
best to the Catalan smelting forge. It was owing in a great measure to the peculiar
quality of the iron which it produces, that the excellence long remarked in the cutlery
of the Tyrol, Styria, and Carinthia was due. It was called by the older mineralogists
steel ore.
The carbonate of iron of the coal formation, is the principal ore from which iron is
smelted in England and Scotland, and it yields usually from 30 to 33 per cent. of cast
metal. We are indebted to Dr. Colquhoun for several elaborate analyses of the sparry-irons
of the Glasgow coal field; ores which afford the best qualities of iron made in that
district. The richest specimen out of the nine which he tried, came from the neighbourhood
of Airdrie; it had a specific gravity of 3·0533, and afforded in 100 parts;
carbonic acid, 35·17; protoxide of iron, 53·03; lime, 3·33; magnesia, 1·77; silica,
1·4; alumina, 0·63; peroxide of iron, 0·23; carbonaceous or bituminous matter, 3·03;
moisture and loss, 1·41. Its contents in metallic iron are 41·25.
The compact carbonate of iron has no relation externally with the sparry variety. It
comprehends most of the clay-iron-stones, and particularly that which occurs in flattened
spheroidal masses of various size, among the coal measures. The colour of this ore is
often a yellowish-brown, reddish-gray, or a dirty brick-red. Its fracture is close grained;
it is easily scratched, and gives a yellowish-brown powder. It adheres to the tongue,
has an odour slightly argillaceous when breathed upon, makes no effervescence with any
acid, blackens at the blowpipe without melting, and becomes attractable by the magnet
with the slightest calcination.
This ore affords from 30 to 40 per cent. of iron of excellent quality; and it is the
object of most extensive workings in Great Britain. It occurs in the slaty clay which
serves as a roof or floor to the strata of coal; and also in continuous beds, from 2 to 18
inches thick, among the coal measures, as in Staffordshire, Shropshire, and Wales. It is
remarkable, that the coal-basin of Newcastle contains little clay iron-stone, while the
coal-basin of Dudley is replete with it.
13. Phosphate of iron.—A dull blue colour is the most remarkable external character
of this species, which occurs in small masses composed of aggregated plates, sometimes
in an excessively fine powder, or giving other bodies a blue tinge. It assumes at the
blowpipe a rusty hue, and is then reduced to a button of a metallic aspect. It dissolves
completely in dilute nitric acid, as well as in ammonia, but it does not communicate its
colour to them, and oil turns it black; characters which distinguish it readily from blue
carbonate of copper, whose colour is not altered by ammonia. It is of no use as a
smelting ore.
[685]
14. Sulphate of iron, native green vitriol.—This is formed by the oxygenation of sulphuret
of iron, and is unimportant in a metallurgic point of view.
15. Chromate of iron.—For the treatment and use of this ore, see Chrome.
16. Arseniate of iron, Wurfelerz.
17. Muriate of iron.
18. Oxalate of iron; Humboldtite, found by M. Breithaupt in the lignite of Kolaw.
It consists of protoxide of iron, 53·86; oxalic acid, 46·14; in 100.
19. Titanate of iron, consists of protoxide and peroxide of iron, 86; titanic acid, 8;
oxide of manganese, 2; gangue, 1 = 97. See Black Oxide of iron.
Of the assay of iron-ores by fusion.—In the assays by the dry way, the object is to
separate exactly all the iron which the ore may contain, with the view of comparing the
result with the product of smelting on the great scale. In order to succeed in this operation,
we must deoxidize the iron, and produce at the same time such a temperature as will
melt the metal and the earths associated with it in the ore, and obtain the former in a
dense button at the bottom of a crucible, and the latter in a lighter glass or slag, above
it. Sometimes the gangue of the ores, consisting mostly of a single earth, as quartz,
alumina, or lime, is of itself very refractory, and hence some flux must be added to bring
about the fusion. The substance most commonly employed for this purpose is borax;
but ordinary flint glass may be substituted for it. Sometimes, also, instead of adding
borax, which always succeeds, lime or clay may be added to the ore, according to the
nature of its mineralizer; that is, lime for a clay iron-stone, and clay for a calcareous carbonate
of iron; and both, when the gangue is siliceous, as occurs with the black oxide.
The ore, pulverized and passed through a silk sieve, is to be well mixed with the flux,
and the mixture introduced into the smooth concavity made in the centre of a crucible
lined with hard rammed damp charcoal dust. Were the mixture diffused through the
charcoal, the reduced iron would be apt to remain scattered in little globules through
the crucible, and no metallic button would be formed at its bottom. The mingled ore
and flux must be covered with charcoal. The crucible thus filled must be shut with
an earthen lid luted on with fire-clay; and it is then set on its base, either in an air
furnace, or on the hearth of a forge urged with a smith’s bellows. The heat should be
very slowly raised, not employing the bellows till three quarters of an hour have
expired. In this way, the water of the damp charcoal (brasque) is allowed to exhale
slowly, and the deoxidation is completed before the fusion begins; for by acting otherwise,
the slags formed would dissolve some oxide of iron, and the assay would not
indicate the whole of the iron to be obtained from the ore. At the end of the above
period, the fire must be raised progressively to a white heat, at which pitch it must be
maintained for a quarter of an hour, after which the crucible should be withdrawn.
Whenever it has cooled, it is to be opened, the brasque must be carefully removed or put
aside, and the button of cast-iron taken out and weighed. The brasque may sometimes
contain a few globules, which must be collected by washing in water, or the application
of a magnetic bar. The quantity of iron denotes, of course, the richness of the ore.
These assays furnish always a gray cast-iron; and, therefore, the quality of the products
can hardly be judged of, except by an experiment on the large scale. The temperature
necessary for the success of an assay is about 150° of Wedgewood.
In the assays by the humid way, we may expect to find manganese, silica, alumina,
lime, magnesia, and sometimes carbonic acid, associated with the iron. 100 grains of
the ore in fine powder are to be digested with nitro-muriatic acid; which will leave only
the silica with perhaps a very little alumina. If an effervescence takes place in the
cold with a dilute acid, the loss of weight will indicate the amount of carbonic acid gas
expelled. The muriatic solution contains the iron, the manganese, the lime, magnesia,
and most of the alumina, with a little silica. On evaporating to dryness, and digesting
in water, all the silica will remain in an insoluble state. If the solution somewhat
acidulated be treated with oxalate of ammonia, the lime will fall down in the form of
an oxalate; ammonia will now precipitate the alumina and the oxide of iron together,
while the manganese and magnesia will continue dissolved in the state of triple salts
(ammonia-muriates). The alumina may be separated from the ferric oxide by potash-lye.
The manganese may be thrown down by hydrosulphuret of potash; and, finally,
the magnesia may be precipitated by carbonate of soda. 100 parts of the red oxide of
iron contain 69·34 of metal, and 30·66 of oxygen.
If phosphorus be present in the ore, the nitro-muriatic solution being rendered nearly
neutral, will afford with muriate of lime a precipitate of phosphate of lime, soluble in
an excess of muriatic acid.
When the sole object is to learn readily the per-centage of iron, the ore may be treated
with hot nitro-muriatic, the acid solution filtered, and supersaturated with ammonia,
which will throw down only the iron oxide and alumina; because the lime is not precipitable
by that alkali, nor is magnesia and manganese, when in the state of ammonia-muriates.[686]
The red precipitate being digested with some potash-lye, will lose its alumina,
and will leave the ferric oxide nearly pure. The presence of sulphur, phosphorus, or
arsenic, in iron ores, may always be detected by the blowpipe, or ustulation in the assay
muffle, as described under Furnace.
Of the smelting of iron-ores.—We shall describe, in the first place, the methods practised
in Great Britain, and shall afterwards consider those pursued in other countries, in
the treatment of their peculiar ores.
Iron is divided into three kinds, according to the different metallic states in which it
may be obtained; and these are called crude or cast iron; steel; and bar or malleable
iron. These states are determined essentially by the different proportions of charcoal or
carbon held in chemical combination; cast iron containing more than steel, and steel
more than malleable iron; which last, indeed, ought to be the pure metal, a point of
perfection, however, rarely if ever attained. It is impossible to assign the limits between
these three forms of iron, or their relative proportions of carbon, with ultimate precision;
for bar iron passes into steel by insensible gradations, and steel and cast iron make such
mutual transitions as to render it difficult to define where the former commences, and
the latter ceases, to exist. In fact, some steels may be called crude iron, and some cast
irons may be reckoned among steels.
Towards the conclusion of the last century the manufacture of iron underwent a very
important revolution in Great Britain, by the substitution of pitcoal for charcoal of
wood, the only combustible previously used in smelting the ores of this metal. This
improvement served not merely to diminish the cost of reduction, but it furnished a
softer cast iron, fit for many new purposes in the arts. From this era, iron works have
assumed an immense importance in our national industry, and have given birth to many
ingenious and powerful machines for fashioning the metal into bars of every form, with
almost incredible economy and expedition.
The profusion of excellent coal, and its association in many localities with iron-stone,
have procured hitherto for our country a marked superiority over all others in the iron
trade; though now every possible effort is making by foreign policy to rival or to limit
our future operations. In 1802, M. de Bonnard, now divisionary inspector in the royal
corps of mines of France, and secretary of the general council, made a tour in England,
in order to study our new processes of manufacturing iron, and published on his return,
in the Journal des Mines, tom. 17., a memoir descriptive of them. Since the peace,
many French engineers and iron-masters have exerted themselves in naturalizing in
France this species of industry; and M. de Gallois, in particular, after a long residence
in Great Britain, where he was admitted to see deliberately and minutely every department
of the iron trade, returned with ample details, and erected at Saint-Etienne a
large establishment entirely on the English model. More recently, MM. Dufrénoy and
Elie de Beaumont, and MM. Coste and Perdonnet, have published two very copious
accounts of their respective metallurgic tours in Great Britain, illustrated with plans
and sections of our furnaces, for the instruction of the French nation.
The argillaceous carbonate of iron, or clay ironstone of the coal measures, is the chief
ore smelted in England. Some red hematite is used as an auxiliary in certain works in
Cumberland and Lancashire; but nowhere is the iron-sand, or other ferruginous
matters of the secondary strata, employed at present for procuring the metal.
Among the numerous coal-basins of England there are two, in particular, which furnish
more than three-fourths of the whole cast iron produced in the kingdom; namely,
the coal field of Dudley, in the south of Staffordshire; and the coal fields of Monmouthshire,
in South Wales, along with those of Gloucestershire and Somersetshire.
Dudley is peculiarly favoured by nature. There are found associated the coal, the
iron ore, the limestone for flux, and the refractory fire-clay for constructing the interior
brick-work of the furnaces. This famous clay is mined at Stourbridge, and exported
to every part of the kingdom for making cast-steel crucibles and glass-house melting
pots.
At Merthyr-Tydvil, the centre of the iron works of Wales, the iron-stone is extremely
plentiful, forming 16 beds, or rather constituting an integrant portion of 16 beds of
slate-clay. Sometimes it occurs in pretty long tables adjoining each other, so as to
resemble a continuous stratum; but more frequently it forms nodules of various size
and abundance, placed in planes both above and below the coal seam. Eight varieties
of ore, belonging to different beds, have been distinguished by the following barbarous
names: black balls, black pins, six-inch-wide vein, six-inch jack, blue vein, blue pins,
gray pins, seven pins. The bed containing the first quality of iron-stone is analogous
to the black ore of Staffordshire called gubbin; it is often cleft within like septaria, and
its cavities are sometimes besprinkled with crystals of carbonate of lime or quartz. In
the superior beds there are nodules decomposing into concentric coats, of which the
middle is clay. Crystals of oxide of titanium are occasionally found in the middle of[687]
the balls of clay iron-stone; to which the metallic titanium observed in the inside of the
dome of blast furnaces, may be traced. Both at Dudley and South Wales, casts of shells
belonging to the genus unio, are observed on the iron-stone.
The average richness of the iron-stones of South Wales is somewhat greater than those
of Staffordshire. The former is estimated at 33 parts of cast iron, while the latter
rarely exceeds 30 parts in 100 of ore; and this richness, joined to the superior quality
or cheapness of the coals, and the proximity of the sea, gives South Wales a decided
advantage as a manufacturing district.
The number of blast furnaces in the parish of Merthyr-Tydvil amounts to upwards of
30. The cast iron produced is, however, seldom brought into the market, but is almost
entirely converted into bar iron, of which, at Mr. Crawshay’s works, 600 tons are manufactured
in a week. Numerous iron railways, extending through a length of 220 miles,
facilitate the transport of the materials and the exportation of the products. That
concurrence of favourable circumstances, which we have noticed as occurring at Dudley,
prevails in an equal degree in South Wales.
The same economy which the use of coal has introduced into the smelting of cast iron
from the ore, also extends to its refinery into bars. And this process would supersede
in every iron work the use of wood charcoal, were not the iron produced by the latter
combustible, better for many purposes, particularly the manufacture of steel. In some
English smelting works, indeed, where sheet iron is prepared for making tin plate, a
mixed refining process is employed, where the cast iron is made into bar iron by wood
charcoal, and laminated by the aid of a coal fire.
Till 1740, the smelting of iron ores in England was executed entirely with wood
charcoal; and the ores employed were principally brown and red hematites. Earthy
iron ores were also smelted; but it does not appear that the clay iron-stones of the coal-basins
were then used, though they constitute almost the sole smelting material at
the present day. At that era, there were 59 blast furnaces, whose annual product was
17,350 tons of cast iron; that is, for each furnace, 294 tons per annum, and 51⁄8 tons per
week. By the year 1788, several attempts had been made to reduce iron ore with coaked
coal; and there remained only 24 charcoal blast furnaces, which produced altogether
13,000 tons of cast iron in the year; being at the rate of 546 tons for each per annum,
or nearly 11 tons per week. This remarkable increase of 11 tons for 51⁄8, was due chiefly
to the substitution of cylinder blowing machines worked with pistons, for the common
wooden bellows. Already 53 blast furnaces fired with coke were in activity; which
furnished in toto 48,800 tons of iron in a year; which raises the annual product of each
furnace to 907 tons, and the weekly product to about 171⁄2 tons. The quantity of
cast iron produced that year (1788)
| by means of coal, was |
48,800 |
tons, |
| and that by wood charcoal, was |
13,100 |
|
| constituting a total quantity of |
61,900 |
tons. |
In 1796, the wood charcoal process was almost entirely given up; when the returns
of the iron trade made by desire of Mr. Pitt, for establishing taxes on the manufacture,
afforded the following results:—
121 blast furnaces, furnishing in whole per annum 124,879 tons, constituting an
average amount for each furnace of 1032 tons.
In 1802, Great Britain possessed 168 blast furnaces, yielding a product of about
170,000 tons; and this product amounted, in 1806, to 250,000 tons, derived from 227
coke furnaces, of which only 159 were in activity at once. These blast furnaces were
distributed as follows:—
| In the principality of Wales |
52 |
| In Staffordshire |
42 |
| In Shropshire |
42 |
| In Derbyshire |
17 |
| In Yorkshire |
28 |
| In the counties of Gloucester, Monmouth, Leicester, Lancaster, Cumberland, and Northumberland |
18 |
| In Scotland |
28 |
| |
227 |
In 1820, the iron trade had risen to the amount shewn in the following table:—
| |
Tons. |
| Wales manufactured, per annum |
150,000 |
| Shropshire and Staffordshire |
180,000 |
| Yorkshire and Derbyshire |
50,000 |
| Scotland, with some places in England |
20,000 |
| Total |
400,000 |
[688]
In a statistical view given by M. de Villefosse, of the French and English iron works,
he assigns to the latter, in 1826, 305 blast furnaces, distributed as follows:—
| In the principality of Wales |
87 |
| In Staffordshire |
78 |
| In Shropshire, Derbyshire, Yorkshire, &c. |
84 |
| In Scotland |
56 |
| |
305 |
Out of these, 280 were in activity at the same time; and if we suppose their mean product
to have been 50 tons a week, the total product would have been, in 1826, 728,000
tons. But this estimate seems to be somewhat above the truth; for, from the information
communicated by Mr. Philip Taylor to M. Achille Chaper, a considerable French iron-master,
who, in the summer of 1826, inspected two-thirds of the blast furnaces of Great
Britain, their product during this year was about 600,000 tons.
The preceding details shew the successive increments which the manufacture of cast
iron has received; and a similar progression has taken place in its refinery into wrought
iron. This operation was formerly effected by the agency of wood charcoal in refineries
analogous to those still made use of in France. But when that kind of fuel began to be
scarce in this island, it came to be mixed with coke in various proportions. The bar iron
thus produced was usually hard, and required much time to convert, so that an establishment
which could produce 20 tons of bar iron in a week, was deemed considerable. At
that time, England imported annually from Sweden and Russia the enormous quantity
of 70,000 tons of iron.
Mr. Cort, to whom Great Britain is indebted for the methods now pursued in this
country, succeeded about that time, after many unsuccessful experiments, in converting
cast iron into bar iron, by exposing it on the hearth of a reverberatory furnace to the
flame of pitcoal. This method, which possessed the advantage of employing this species of
combustible alone, likewise simplified the treatment, because it required no blast apparatus.
But this mode of refinery, consisting in the use of a reverberatory furnace alone,
did not produce altogether the desired result. It was irregular; sometimes the loss of
iron was small, but at others it was very considerable; and there were great variations
in the quality of the iron, as well as in the quantity of fuel consumed. Mr. Cort succeeded
in removing this uncertainty of result, by causing the puddling in the reverberatory
furnace to be preceded by a kind of refinery with coke. The intent of this operation
was to decarburate the iron, and to prepare it for becoming malleable. The metal
took in that case the name of finery metal, called, for sake of brevity, fine-metal.
He also substituted the drawing cylinders for the extension under the hammer, an improvement
which accelerated greatly the manufacture of bar iron. The iron then yielded
by the operation of puddling, was of a very inferior quality, and could not be directly employed
in the arts. In order to give it more consistence, it was subjected to a second heating
in a reverberatory furnace; and whenever this method had arrived at a high enough
degree of perfection to afford products fit for the market, it became exclusively employed
in Great Britain. This new method of transforming cast-iron into malleable iron,
speedily gained such an extension, that of late years, a single iron-work, Cyfartha in
Wales, manufactured annually more than twice as much as was made annually from
1740 to 1750, in the whole kingdom.
In surveying the improvements which the iron manufacture has received in England
in the space of the last 60 years, they are seen to be resolvable into two; the first set
relating to the smelting of the ores; the other, to the conversion of the pigs into bar
iron; hence naturally arise two heads under which the subject of iron must be treated.
1. Manufacture of cast-iron by coke and coal.—The cast-iron produced by the
English and Scotch blast furnaces is in general black and very soft; but yet may be
distinguished into several qualities, of which three are particularly noticed.
No. 1. Very black cast-iron, in large rounded grains, obtained commonly near the
commencement of the casting, when an excess of carbon is present; in flowing, it
appears pasty, and throws out blue scintillations. It exhibits a surface where crystalline
vegetations develope themselves rapidly in very fine branches; it congeals or fixes
very slowly; its surface when cold is smooth, concave, and often charged with plumbago;
it has but a moderate tenacity, is tender under the file, and susceptible of a dull polish.
When melted over again, it passes into No. 2., and forms the best castings.
No. 2. Black cast-iron, has a somewhat lighter shade than the preceding, and may
therefore on comparison be called blackish-gray. It presents less large granulations
than No. 1.; is tenacious, easily turned, filed, and polished; excellent for casting when
it approaches to No. 1., and for the manufacture of bar iron when it has on the contrary
a shade somewhat lighter. If repeatedly melted, it passes into the next quality, or
No. 3. White cast-iron; this is brittle, and indicates always some derangement in the[689]
working of the furnace; it flows imperfectly, and darts out in casting, abundance of
brilliant white scintillations; it fixes very quickly; and on cooling, exhibits on its surface
irregular asperities, which make it extremely rough. It is easily broken, and presents a
lamellar and radiated fracture; and is so hard that tempered steel cannot act upon it.
It is cast only into weights, bullets or bombs, but never into pieces of machinery. When
exposed to the refinery processes, it affords a bad bar iron. It is owing probably to the
different nature of the cast-iron obtained in different counties in England, that Staffordshire
and Shropshire furnish the greater part of the great iron castings, while Wales
manufactures almost exclusively malleable iron. The lower price of coals in Wales
is perhaps the cause to a certain extent of this difference in the results of these two iron
districts. It will be interesting at any rate, to describe separately the processes employed
in Staffordshire and Wales.
The blast furnaces of Staffordshire, in the neighbourhood of Dudley, Bilston, and
Wednesbury, are constructed almost wholly of bricks. Their outer form is frequently
a cone, often also a pyramid with a square base. They are bound about with a great
many iron hoops, or with iron bars placed at different heights. This powerful armour
allows the furnaces to be built much less massively than they formerly were; and admits
of lighter and more elegant external forms. They are seldom insulated; but are usually
associated to the number of two or three in the same line. A narrow passage is left
between them, which leads to the lateral openings where the tuyères are placed. At the
front of the furnace, a large shed is always raised. The roofs of these sheds present in
general circular profiles, and being made of cast or bar iron, they display a remarkable
lightness of construction. The cast-iron columns likewise, which support the joists
and girders, give additional elegance.
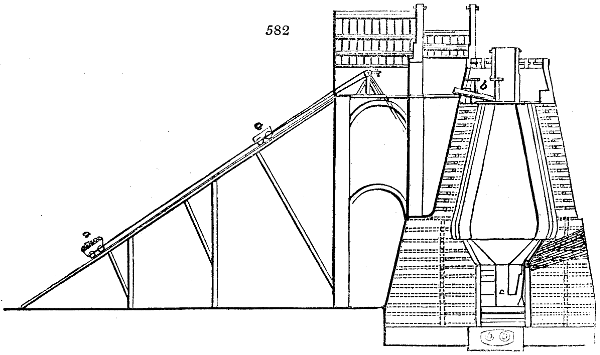
In the Dudley field, the furnaces are almost always in the middle of the plain, and an inclined
rail-way must be formed to reach their platform. These inclined planes, composed
of beams or rails placed alongside of each other, and sustained by props and cross-bars, as
indicated in fig. 582., are set up mostly against the posterior face of the furnace. Two
chains or ropes, passing over the drums of gins, moved by a steam engine (commonly
the same that drives the bellows), draw up the waggons of wood or sheet iron a a, which
contain the various materials for supplying the furnace. To facilitate this service, the
platform round the furnace is sometimes enlarged behind by a floor; while a balustrade,
which opens when the waggons arrive at the platform, prevents accidents. This projection
is occasionally covered by a roof. For a furnace of the largest size, the force
expended by this lifting apparatus, is not more than a two-horse power.
Fig. 582. is a vertical section through the furnace from front to rear, or at right
angles to the line of the lateral tuyères. The erection of a pair of blast furnaces,
of 40 feet high each, costs, in the Dudley district, 1800 pounds sterling; and requires
for building each, 160,000 common bricks for the outside work, 3900 fire-bricks for the
lining or shirt of the furnace, and 825 for the boshes. The dimensions of the fire-bricks
are various; 5 kinds are employed for the lining, and 9 kinds for the boshes. They are
all 6 inches thick, and are curved to suit the voussoirs.
The number of charges given in 12 hours is different in different furnaces; being
sometimes 20, 25, and even so high as 40; but 30 is a fair average. Each charge is[690]
composed of from 5 to 6 cwt. of coak, (or now of 3 to 4 cwt. of coal with the hot
blast); 3, 4, and sometimes 6 cwt. of the roasted mine, according to its richness and the
quality of cast iron wanted; the limestone flux is usually one-third of the weight of
the roasted iron stone. There are 2 casts in 24 hours; one at 6 in the morning, and
another at 6 in the evening.
The height of the blast furnaces is very variable; some being only 36 feet high
including the chimney, whilst others have an elevation of 60 feet. These extreme limits
are very rare: so that the greater part of the furnaces are from 45 to 50 feet high.
They are all terminated by a cylindrical chimney of from 8 to 12 feet long; being about
one-fifth of the total height of the furnace. The inside diameter of this chimney is the
same as that of the throat or mouth; and varies from 4 to 6 feet. The chimney is frequently
formed of a single course of bricks, and acquires solidity from its hoops of iron,
so thickly placed that one half of the surface is often covered with them. At its lower
end, the mouth presents one or two rectangular openings, through which the charge
is given. It is built on a basement circle of cast-iron, which forms the circumference
of the throat; and a sloping plate of cast-iron b is so placed as to make the materials
slide over into the furnace, as shown in the figure.
The inside of the blast furnaces of Staffordshire is most frequently of a circular form,
except the hearth and working area. The inner space is divided into four portions,
different in their forms, and the functions which they fulfil in the smelting of the ore.
The undermost, called the hearth, or crucible, in which the cast-iron collects, is a right
rectangular prism, elongated in a line perpendicular to the axes of the tuyères. The
sides of the hearth consist in general of refractory sandstone (fire-stone), obtained mostly
from the bed of the coal basin, called millstone grit; and the bottom of the hearth is
formed of a large block of the same nature, laid on a cast-iron plate.
The second portion is also made of the same refractory grit stone. It has the form of
a quadrangular pyramidal, approaching considerably to a prism, from the smallness of
the angle included between the sides and the axis.
The third portion or lower body of the furnace is conical, but here the interior space
suddenly expands; the slope outwards at this part seems to have a great influence on
the quality of the cast-iron obtained from the furnace. When No. 2. of the blackest kind
is wanted for castings, the inclination of this cavity of the furnace is in general less
considerable than when No. 2. cast iron for conversion into bar iron is required. The
inclination of this conical chamber, called the boshes, varies from 55 to 60 degrees with
the horizon. The diameter of this part is equal to that of the belly, and is from 11 to
13 feet. The boshes are built of masonry, as shown in figs. 583, 584.
The fourth part, which constitutes about two-thirds of the height of the furnace from
the base of the hearth up to the throat, presents the figure of a surface of revolution,
generated by a curve whose concavity is turned towards the axis of the furnace, and
whose last tangent towards the bottom is almost vertical. This surface is sloped off with
that of the boshes (étalages in French), so that no sharp angle may exist at the belly.
In some furnaces of considerable dimensions, as in that with three tuyères, this portion
of the furnace is cylindrical for a certain height.
[691]
The following measurements represent the interior structure of two well-going
furnaces.
| |
No. 1. |
No. 2. |
| |
Feet. |
Feet. |
| Height from the hearth to the throat or mouth |
45 |
|
49 |
|
| Height of the crucible or hearth |
6 |
1⁄2 |
6 |
|
| H—ht of the boshes |
8 |
|
7 |
|
| H—ht of the cone |
30 |
1⁄2 |
36 |
|
| H—ht of the chimney or mouth |
8 |
|
12 |
3⁄4 |
| Width of the bottom of the hearth |
2 |
1⁄2 |
2 |
|
| Ditto at its upper end |
3 |
|
2 |
2⁄3 |
| Ditto of the boshes |
12 |
2⁄3 |
13 |
1⁄2 |
| Ditto at one-third of the belly |
12 |
|
11 |
1⁄2 |
| Ditto at two-thirds of ditto |
8 |
2⁄3 |
9 |
1⁄2 |
| Ditto at the mouth |
4 |
1⁄2 |
3 |
2⁄3 |
| Inclination of the boshes |
59 |
° |
52 |
° |
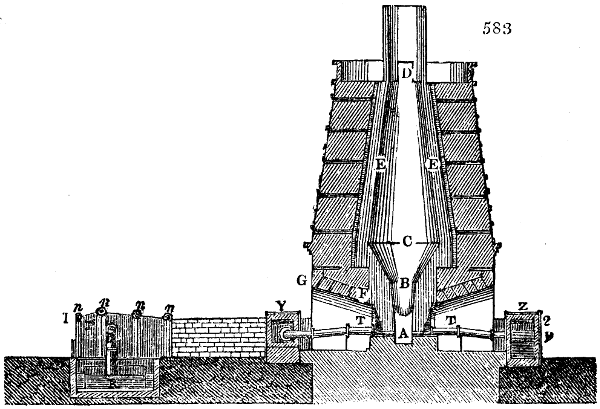
The conical orifice called the tuyère, in which the tapered pipes are placed, for imparting
the blast, is seen near the bottom of the furnace, fig. 583. at A. Nose tubes of various
sizes, from 2 to 4 inches in diameter, are applied to the extremity of the main blast-pipe.
Under A is the bottom of the hearth, which, in large furnaces, may be two feet
square. B is the top of the hearth, about two feet six inches square. A, B, is the height
of the hearth, about six feet six inches. B shows the round bottom of the conical or
funnel part, called in this country, the boshes, standing upon the square area of the
hearth. C is the top of the boshes, which may be about 12 feet in diameter, and 8 feet
in perpendicular height. D is the furnace top or mouth (gueulard in French), at which
the materials are charged. It may be 41⁄2 feet in diameter. The line between C, D, is the
height of the internal cavity of the furnace, from the top of the boshes upwards, supposed
to be 30 feet. A, D, is the total height of the interior of the furnace, reckoned at
441⁄2 feet. E E is the lining, which is built in the nicest manner with the best fire-bricks,
from 12 to 14 inches long, 3 inches thick, and curved to suit the circle of the cone. A
vacancy of 3 inches wide is left all round the outside of the first lining by the builder;
which is sometimes filled with coak dust, but more generally with sand firmly rammed.
This void space in the brick-work is for the purpose of allowing for any expansion
which might occur, either by an increase in the bulk of the building, or by the pressure
and weight of the materials when descending to the bottom of the furnace. Exterior
to E E is a second lining of fire-bricks similar to the first. At F, on either side, is
a cast-iron lintel, 81⁄2 feet long, by 10 inches square, upon which the bottom of the
arches is supported. F, G, is the rise of the tuyère arch, which may be 14 feet high
upon the outside, and 18 feet wide. The extreme size of the bottom or sole of the
hearth, upon each side of A, may be 10 feet square. This part and the boshing stones,
are preferably made from a coarse sandstone grit, containing large rounded grains
of quartz, united by a siliceo-argillaceous cement.
The bottom of the hearth consists, first, of a course of the said gritstone; beneath
which is a layer of bedding sand, having, in its under
part, passages for the escape of the vapours generated
by damps; the whole being supported upon pillars of
brick.
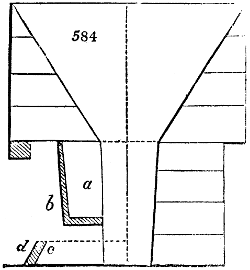
Fig. 584. represents the hearth and boshes, in a
vertical side section. a is the tymp stone, and b the
tymp plate for confining the liquid metal in the
hearth. The latter is wedged firmly into the side-walls
of the hearth; c is the dam-stone, which occupies
the whole breadth at the bottom of the hearth,
excepting about 6 inches, which space, when the furnace
is at work, is filled before every cast, with a
strong binding sand. This stone is faced outside
by a cast-iron plate d, called the dam-plate, of considerable
thickness, and peculiar shape. The top of
the dam-stone, or rather the notch of the dam-plate,
lies from 4 to 8 inches under the level of the tuyère
hole. The space under the tymp plate, for 5 or 6 inches down, is rammed full, for
every cast, with strong loamy earth, or even fine clay; a process called the tymp
stopping. The area of the base of this furnace being 38 feet; its extreme height is 55
feet.
The blast furnaces of Staffordshire have always two tuyères, at least, placed on opposite[692]
sides, but so pointed that the blast may not pursue directly opposite lines. In a furnace
acting well in the neighbourhood of Dudley, the one of the tuyères was 10 inches distant
from the posterior wall of the hearth, and the other only 4 inches. In other furnaces
with 3 tuyères; the side ones are placed, the one 161⁄2 inches, and the other 61⁄2 inches
from the back. Three tuyères are seldom made to blow simultaneously. The third is
brought into action only when the furnace seems to be choaked up, and when it
becomes necessary to clear it up by a powerful concussion. Too much pains cannot be
bestowed on the masonry and brickwork of a blast furnace, and on the solidity of its
foundation. In a soft ground it should rest on piles, so driven that the channel left
beneath for the drainage of the building may be above any water level. Small passages
should likewise be left throughout the body of the work, for the transpiration of moisture.
The blowing machines employed in Staffordshire, are generally cast-iron cylinders, in
which a metallic piston is exactly fitted as for a steam engine, and made in the same
way. Towards the top and bottom of the blowing cylinders orifices are left covered
with valves, which open inside when the vacuum is made with the cylinders, and
afterwards shut by their own weight. Adjutages conduct into the iron globe or chest,
the air expelled by the piston, both in its ascent and descent; because these blowing
machines have always a double stroke.
The pressure of the air is made to vary through a very considerable range, according
to the nature of the fuel and season of the year; for as in summer the atmosphere is
more rarefied, it must be expelled with a compensating force. The limits are from 11⁄2
pounds to 31⁄2 pounds on the inch; but these numbers represent extreme proportions,
the average amount in Staffordshire being 3 pounds. With this pressure a furnace
usually works, which affords 60 tons of cast iron in the week; and the pressure may be
21⁄2 pounds on an average. The orifices, or nose-pipes, through which the air issues,
also vary with the nature of the coke and the ore. In Staffordshire they are generally
from 2 inches and 5 tenths to 2 inches and 8 tenths in diameter.
The blowing machines of Staffordshire are always impelled by steam engines. At
Mr. Bagnall’s works, two blast furnaces, 40 feet high, exclusive of the chimney or top,
and two finery furnaces, are worked by a steam engine of 40 horses power; and therefore
the power of one horse corresponds to the production of 21⁄2 tons of cast iron per
weekly, independently of the finery.
In South Wales, especially at Pontypool, there are slighter blast furnaces, whose upper
portion is composed of a single range of bricks, each of which is 20 inches long,
4 thick, and 9 broad. The interior of the chimney represents an inverted cone. These
furnaces derive solidity, and power to resist the expansions and contractions from
change of temperature, by being cased, as it were, in horizontal hoops, placed 3 feet, or,
even in some cases, only 6 inches asunder. These flat rings consist of four pieces,
which are joined by means of vertical bars, that carry a species of ears or rings, into
which the hoops enter, and are retained by bolts or keys. Instead of these ears, screw
nuts are also employed for the junction. Each hoop is alternately connected to each of
the eight vertical bars. The interior of these furnaces is the same as of the others;
being generally from 12 to 14 feet diameter at the belly, and from 50 to 55 feet high.
Though slight, they last as long as those composed of an outer body of masonry and
a double lining of bricks; and have continued constantly at work for three years.
In Wales also the blast furnaces are generally somewhat larger than in Staffordshire;
because there the object being to refine the cast iron, they wish to procure as large a
smelting product as possible. But in Staffordshire, a fine quality of casting iron is chiefly
sought after, and hence their furnaces have less height, but nearly the same width.
In a blast apparatus employed at the Cyfartha works, moved by a 90-horse steam
power, the piston rod of the blowing cylinder is connected by a parallelogram mechanism
with the opposite end of the working beam of the steam engine. The cylinder is
9 feet 4 inches diameter, and 8 feet 4 inches high. The piston has a stroke 8 feet long,
and it rises 13 times in the minute. By calculating the sum of the spaces percurred
by the piston in a minute, and supposing that the volume of the air expelled is equal to
only 96 per cent. of that sum, which must be admitted to hold with machines executed
with so much precision, we find that 12,588 cubic feet of air are propelled every
minute. Hence a horse power applied to blowing machines of this nature gives, on an
average, 137 cubic feet of air per minute. The pressure on the air as it issues, rarely
exceeds two pounds on the square inch in the Welsh works.
At the establishment of Cyfartha, for blowing seven smelting furnaces, and the seven
corresponding fineries, three steam engines are employed, one of 90 horse-power,
another of 80, and a third of 40; which constitutes in the whole, a force of 210 horses,
or 26 horses and 1⁄5 per furnace, supposing the fineries to consume one-eighth of the blast.
In the whole of the works of Messrs. Crawshay, the proprietors of Cyfartha, the power
of about 350 horses is expended in blowing 12 smelting furnaces, and their subordinate
fineries; which gives from 25 to 26 horses for each, allowing as before one-eighth for the
fineries. As these furnaces produce each about 60 tons of cast iron weekly, we find[693]
that a horse power corresponds to 2 tons and a tenth in that time. Each of the furnaces
consumes about 3567 cubic feet of air per minute. These works have been greatly
increased of late years.
The following analyses of the English coal ironstones have been made by M. Berthier,
at the school of mines in Paris.
| |
Rich Welsh ore. |
Poor Welsh ore. |
Rich ore of Dudley,
or gubbin. |
| Loss by ignition |
30 |
·00 |
27 |
·00 |
31 |
·00 |
| Insoluble residuum |
8 |
·40 |
22 |
·03 |
7 |
·66 |
| Lime |
0 |
·0 |
6 |
·00 |
2 |
·66 |
| Peroxide of iron |
60 |
·00 |
42 |
·66 |
58 |
·33 |
On calculating the quantities of carbonate of iron, and metallic iron, to which the
above peroxide corresponds, we have:— |
| Carbonate of iron |
88 |
·77 |
65 |
·09 |
85 |
·20 |
| Metallic iron |
42 |
·15 |
31 |
·38 |
40 |
·45 |
The mean richness of the ores of carbonate of iron of these coal basins, is not far from
33 per cent. About 28 per cent. is dissipated on an average, in the roasting of the ores.
Every ferruginous clay-stone is regarded as an iron ore, when it contains more than 20
per cent. of metal; and it is paid for according to its quality, being on an average at
12 shillings per ton in Staffordshire. The gubbin however fetches so high a price as 16
or 17 shillings. The ore must be roasted before it is fit for the blast furnace, a process
carried on in the open air. A heap of ore mingled with small coal (if necessary) is
piled up over a stratum of larger pieces of coal; and this heap may be 6 or 7 feet high,
by 15 or 20 broad. The fire is applied at the windward end, and after it has burned a
certain way, the heap is prolonged at the other extremity, as far as the nature of the
ground or convenience of the work requires. The quantity of coal requisite for roasting
the ore varies from one to four hundred weight per ton, according to the proportion
of bituminous matter associated with the iron-stone. The ore loses in this operation
from 25 to 30 per cent. of its weight. Three and a quarter tons of crude ore, or two
and a quarter tons of roasted ore are required to produce a ton of cast iron; that is to
say, the crude material yields on an average 30·7 per cent., and the roasted ore 44·4 of
pig metal. In most smelting works in Staffordshire, about equal weights of the rich
ore in round nodules called gubbin, and the poorer ore in cakes called blue flat, are employed
together in their roasted state; but the proportions are varied, in order to have
an uniform mixture, capable of yielding from 30 to 33 per cent. of metal.
The transition or carboniferous limestone of Dudley is used as the flux; it is compact
and contains little clay. The bulk of the flux is made nearly equal to that of the
ore. To treat two tons and a quarter of roasted ore, which furnish one ton of pig iron,
19 hundred weight of limestone are employed; constituting nearly 1 of limestone for
3 of unroasted ore. The limestone costs 6 shillings the ton.
Carbonized pitcoal or coke was, till within these few years, the sole combustible used
in the blast furnaces of Staffordshire.
The coal is distributed in circular heaps, about 5 feet diameter, by 4 feet high; and
the middle is occupied by a low brick chimney, piled with loose bricks, so open as to
leave interstices between them, especially near the ground. The larger lumps of coal
are arranged round this chimney, and the smaller towards the circumference of the heap.
When every thing is adjusted, a kindling of coals is introduced into the bottom of the
brick chimney; and to render the combustion slow, the whole is covered over with a
coat of coal dross, the chimney being loosely closed with a slab of any kind. Openings
are occasionally made in the crust and afterwards shut up, to quicken and retard the
ignition at pleasure, during its continuance of 24 hours. Whenever the carbonization
has reached the proper point for forming good coke, the covering of coal dross is removed,
and water is thrown on the heap to extinguish the combustion; a circumstance deemed
useful to the quality of the coke. In this operation the Staffordshire coal loses the half
of its weight, or two tons of coal produce one of coke.
As soon as the blast furnace gets into a regular heat, which happens about 15 days or
three weeks after fires have been put in it, the working consists simply in charging it, at
the opening in the throat, whenever there is a sufficient empty space; the only rule
being to keep the furnace always full. The coke is measured in a basket, thirteen of
which go to the ton. The ore and the flux (limestone) are brought forwards in wheelbarrows
of sheet-iron. In 24 hours, there are thrown into a furnace such as fig.
582., 141⁄3 tons of coke, 16 tons of roasted ore, and 63⁄4 tons of limestone; from which
about 7 tons of pig iron are procured. This is run off every 12 hours; in some
works the blast is suspended during the discharge. The metal intended to be converted[694]
into bar iron, or to be cast again into moulds, is run into small pigs 3 feet long, and 4
inches diameter; weighing each about 2 hundred weight and a half.
The disorders to which blast furnaces are liable, have a tendency always to produce
white cast iron. The colour of the slag or scoriæ is the surest test of these derangements,
as it indicates the quality of the products. If the furnace is yielding an iron
proper for casting into moulds, the slag has an uniform vitrification, and is slightly translucid.
When the dose of ore is increased in order to obtain a gray pig iron, fit for
fabrication into bars, the slag is opaque, dull, and of a greenish-yellow tint, with blue
enamelled zones. Lastly, when the furnace is producing a white metal, the slags are
black, glassy, full of bubbles, and emit an odour of sulphuretted hydrogen. The scoriæ
from a coke, are much more loaded with lime than those from a charcoal blast furnace.
This excess of lime appears adapted to absorb and carry off the sulphur, which would
otherwise injure the quality of the iron. The slags, when breathed on, emit an argillaceous
odour.
A blast furnace of 50 or 60 feet in height, gives commonly from 60 to 70 tons of cast
iron per week; one from 50 to 55 feet high, gives 60 tons; two united of 45 feet, produce
together, 100 tons; and one of 36 feet furnishes from 30 to 40. A blast furnace
should go for four or five years without needing restoration. From 31⁄2 to 4 tons of coal,
inclusive of the coal of calcination, are required in Staffordshire to obtain one ton of
cast iron; and the expense in workmen’s wages is about 15 shillings on that quantity.
At the Cyfartha works of Messrs. Crawshay in South Wales, the average price of the
lithoid carbonate of iron, ready for roasting, is only 7s. 6d. a ton, and its richness is about
33 per cent. The furnaces for roasting the ore in that country are made in the form
of cylinders, placed above an inverted cone. The cylindrical part is 6 feet high and
wide, and the cone is about 4 feet high, with a base equal to that of the cylinder; towards
the bottom or narrowest part of the inverted cone, there is an aperture which
terminates in an outlet on a level with the bottom of the terrace in which the furnace
is built. Sometimes, however, all the roasting furnaces are in a manner combined into
one, which resembles a long pit about 6 feet in width and depth, and whose bottom
presents a series of inverted hollow quadrangular pyramids, 6 feet in each side, and 4
deep. The bottom or apex of each of these pyramids, communicates with a mouth or
door-way that opens on a lower terrace, through which the ore falls in proportion as it
is roasted; and whence it is wheeled and tumbled into the throat of an adjoining blast
furnace, on the same level with the terrace; for in Wales the blast furnace is generally
built up against the face of a hill, which makes one of its fronts. The above roasting
furnaces, which closely resemble lime-kilns, after being filled with alternate strata of
small coal and ore, are set on fire; and the roasted ore is progressively withdrawn below,
as already mentioned.
The product of coke from a certain weight of coal is greater in Wales than in Staffordshire,
though the mode of manufacture is the same. At Pen-y-Darran, for example,
5 of coal furnish 31⁄2 of coke; or 100 give 70; at Dowlais 100 of coal afford 71 of coke,
and the product would be still greater if more pains were bestowed upon the process.
At Dowlais, coal costs only 2 shillings a ton; at Cyfartha, it is worth from 2s. 6d. to
5 shillings. About 2 tons of coke are employed in obtaining 1 ton of cast iron.
According to M. Berthier’s analysis, the slag or cinder of Dowlais consists of silica,
40·4; lime, 38·4; magnesia, 5·2; alumina, 11·2; protoxide of iron, 3·8; and a trace of sulphur.
He says that the silica contains as much oxygen as all the other bases united; or is
equivalent to them in saturating power; and to the excess of lime he ascribes the freedom
from sulphur, and the good quality of the iron produced. The specimen examined
was from a furnace at Merthyr-Tydvil. Other slags from the same furnace, and one
from Dudley, furnished upwards of 2 per cent. of manganese. Those which he analysed
from Saint Etienne in France afforded about 1 per cent. of sulphur.
The consumption of coal in the Welsh smelting furnaces may be estimated, on an
average, at 3 tons per ton of cast iron; corresponding to 2·1 of their coke. From this
economy in the quantity of fuel, as well as from its cheapness and that of the iron ore,
the iron of South Wales can be brought into the market at a much lower rate than that
of any other district. These blast furnaces remain in action from 5 to 10 years; at the
end of which time only their interior surface has to be repaired. The lining of the
upper part lasts much longer; for examples are not wanting of its holding good for
nearly 40 years.
One of the greatest improvements ever made by simple means in any manufacture is
the employment of hot air instead of the ordinary cold air of the atmosphere, in supplying
the blast of furnaces for smelting and founding iron. The discovery of the superior
power of a hot over a cold blast in fusing refractory lumps of cast iron, was
accidentally observed by my pupil Mr. James Beaumont Neilson, engineer to the Glasgow
gas works, about the year 1827, at a smith’s forge in that city, and it was made the
subject of a patent in the month of September of the following year. No particular
construction of apparatus was described by the inventor by which the air was to be[695]
heated, and conveyed to the furnace; but it was merely stated that the air may be
heated in a chamber or closed vessel, having a fire under it, or in a vessel connected in
any convenient manner with the forge or furnace. From this vessel the air is to be forced
by means of bellows into the furnace. The quantity of surface which a heating furnace is
required to have for a forge, is about 1260 cubic inches; for a cupola furnace, about 10,000
cubic inches. The vessel may be enclosed in brickwork, or fixed in any other manner
that may be found desirable, the application of heated air in any way to furnaces or
forges, for the purposes of working iron, being the subject claimed as constituting the
invention.
Wherever a forced stream of air is employed for combustion, the resulting temperature
must evidently be impaired by the coldness of the air injected upon the fuel. The heat
developed in combustion is distributed into three portions; one is communicated to the
remaining fuel, another is communicated to the azote of the atmosphere, and to the volatile
products of combustion, and a third to the iron and fluxes, or other surrounding
matter to be afterwards dissipated by wider diffusion. This inevitable distribution takes
place in such a way, that there is a nearly equal temperature over the whole extent of a
fire-place, in which an equal degree of combustion exists.
We thus perceive that if the air and the coal be very cold, the portions of heat absorbed
by them might be very considerable, and sufficient to prevent the resulting temperature
from rising to a proper pitch; but if they were very hot they would absorb
less caloric, and would leave more to elevate the common temperature. Let us suppose
two furnaces charged with burning fuel, into one of which cold air is blown, and into
the other hot air, in the same quantity. In the same time, nearly equal quantities of fuel
will be consumed with a nearly equal production of heat; but notwithstanding of this,
there will not be the same degree of heat in the two furnaces, for the one which receives
the hot air will be hotter by all the excess of heat in its air above that of the
other, since the former air adds to the heat while the latter abstracts from it. Nor are
we to imagine that by injecting a little more cold air into the one furnace, we can raise
its temperature to that of the other. With more air indeed we should burn more coals
in the same time, and we should produce a greater quantity of heat, but this heat being
diffused proportionally among more considerable masses of matter, would not produce
a greater temperature; we should have a larger space heated, but not a greater intensity
of heat in the same space.
Thus, according to the physical principles of the production and distribution of heat,
fires fed with hot air should, with the same fuel, rise to a higher pitch of temperature
than fires fed with common cold air. This consequence is independent of the masses,
being as true for a small stove which burns only an ounce of charcoal in a minute, as
for a furnace which burns a hundred weight; but the excess of temperature produced
by hot air cannot be the same in small fires as in great; because the waste of heat is
usually less the more fuel is burned.
This principle may be rendered still more evident by a numerical illustration. Let
us take, for example, a blast furnace, into which 600 cubic feet of air are blown per
minute; suppose it to contain no ore but merely coal or coke, and that it has been
burning long enough to have arrived at the equilibrium of temperature, and let us see
what excess of temperature it would have if blown with air of 300° C. (572° F.), instead
of being blown with air at 0° C.
600 cubic feet of air under the mean temperature and pressure, weigh a little more
than 45 pounds avoirdupois; they contain 10·4 pounds of oxygen, which would burn
very nearly 4 pounds of carbon, and disengage 16,000 times as much heat as would
raise by one degree cent. the temperature of two pounds of water. These 16,000 portions
of heat, produced every minute, will replace 16,000 other portions of heat, dissipated
by the sides of the furnace, and employed in heating the gases which escape from
its mouth. This must take place in order to establish the assumed equilibrium of caloric.
If the 45 pounds of air be heated beforehand up to 300° C., they will contain about
the eighth part of the heat of the 16,000 disengaged by the combustion, and there will
be therefore in the same space one eighth of heat more, which will be ready to operate
upon any bodies within its range, and to heat them one eighth more. Thus the blast
of 300° C. gives a temperature which is nine-eighths of the blast at zero C., or at
even the ordinary atmospheric temperature; and as we may reckon at from 2200° to
2700° F. (from 1200° to 1500° C.), the temperature of blast furnaces worked in the
common way, we perceive that the hot-air blast produces an increase of temperature
equal to from 270° to 360° F.
Now in order to appreciate the immense effects which this excess of temperature may
produce in metallurgic operations, we must consider that often only a few degrees more
temperature are required to modify the state of a fusible body, or to determine the play
of affinities dormant at lower degrees of heat. Water is solid at 1° under 32° F.; it
is liquid at 1° above. Every fusible body has a determinate melting point, a very few[696]
degrees above which it is quite fluid, though it may be pasty below it. The same observation
applies to ordinary chemical affinities; charcoal, for example, which reduces
the greater part of metallic oxides, begins to do so only at a determinate pitch of temperature,
under which it is inoperative, but a few degrees above, it is in general lively
and complete. It is unnecessary, in this article, to enter into any more details to show
the influence of a few degrees of heat, more or less, in a furnace, upon chemical operations,
or merely upon physical changes of state.
These consequences might have been deduced long ago, and industry might thus have
been enriched with a new application of science; but philosophers have been and still
are too much estranged from the study of the useful arts, and content themselves too
much with the minutiæ of the laboratory or theoretic abstractions. Within the space
of 7 years, the use of the hot blast has been so much extended in Great Britain, as to
have enabled many proprietors of iron works to add 50 per cent. to their weekly production
of metal, to diminish the expenses of smelting by 50 per cent., and, in many
cases, to produce a better sort of cast iron from indifferent materials.
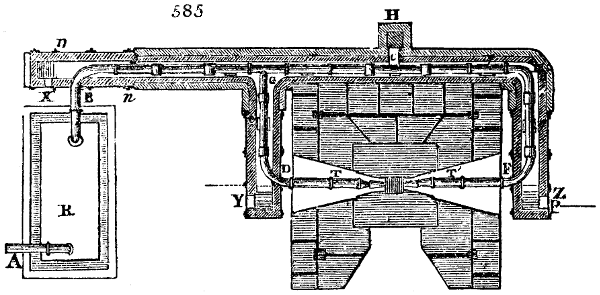
The figures here given represent the blast furnace, and all the details of the air-heating
at one view. Fig. 583. is a vertical section of
the furnace and the apparatus; fig. 585. represents
the plan at the height of the line 1, 2. of
fig. 583. The blowing machine, which is not
shown in this view, injects the air through the
pipe A, into the regulator chamber R, fig. 585.;
the air thence issues by the pipe B, proceeds to
C, where it is subdivided into two portions; the
one passes along the pipe C D to get to the
tuyère T, the other passes behind the furnace,
and arrives at the tuyère T′ by the pipe C E F.
These pipes are distributed in a long furnace
or flue, whose bottom, sides, and top are formed
with fire-brick, where they are exposed to the
action of the flame of the three fires X, Y, Z.
The flame of the fire X plays round the pipe
B at its entrance into the flue, and quits it only
to go into the chimney H; that of the fire Y
acts from the point D to the same chimney,
passing by the elbow C; that of the fire Z acts
equally upon F and H, in passing by the elbow E.
Disposition of the fires and furnace.—Fig.
586. represents, upon a scale three times larger than fig. 585., the section of the[697]
fire X, of which the plan is seen in fig. 585., and the elevation in fig. 583.; as also in the
outside view of the blast furnace, fig. 589.
The grate is at L; the fuel is introduced by the door P, fig. 583.; the flame rises
above the bridge I K, and proceeds along the vaulted flue towards the chimney H. Through
a length of about 13 feet including the grate, the furnace is on each side supported by
oblong plates of cast iron, which are bound together by 4 upright ribbed or feathered
bars, also on each side; these bars n being bound together by iron rods furnished with
screw nuts at their ends (figs. 583, 585, 586.) Beyond this distance, the outside of the
furnace is mere brickwork.
The fires Y and Z have exactly a like disposition with the above.
Fig. 586. indicates the dimensions and the curvature of the arch above the grate,
near the bridge; fig. 587. represents the section of the furnace and of the pipe beyond
the cast-iron casing.
I find that the furnace is only about 3 feet wide at the bottom, and that the elevation
of the arch above the bottom is no more than 30 inches. Perhaps it might be made a
little wider with advantage; the combustion would be more vigorous and effective; and
if the sides also were a little thicker, the heat would be better confined.
| The distance from the fire-place |
X to the chimney H, is |
43 |
1⁄2 |
feet. |
|
| The di—ance from the fire— |
Y to the point C, is |
13 |
|
— |
|
| The di—ance from the fire— |
Z to the chimney, is |
29 |
|
— |
including the turn of the elbow E. |
Distribution of the pipes.—At B, the pipe is 18 inches diameter outside, and one
inch thick of metal, and it tapers to C; from C to D and from D to C the pipes are only
11 inches in external diameter, and three-fourths of an inch thick; they are 5 feet long,
and are united by two kinds of joints; the ordinary ones, and those of compensation,
to give play for the expansion and contraction. One of these is seen between B and C,
one between C and D, one between C and E, and a fourth between E and F. These pipes
and their adjustment are seen more at large in fig. 588.; U V is one of these pipes, its
widened mouth receives the extremity M of the preceding pipe. These pieces are truly
bored and turned to fit each other, and slide out and in like telescope tubes, by the
effect of dilatation and contraction of the pipes with changes of temperature.
At certain distances castors or friction-rollers of cast iron are placed to carry the
pipes, which roll upon oblong plates of cast iron laid upon the floor of the flues.
These castors are shown at a, b, c, d, e, f, g, fig. 585.; one of them is shown separate
upon a larger scale at G, fig. 587., as also the plate or rail S, on which it runs.
The tuyères T T′ are adjusted into the pipe behind them; this is truly bored, so as to
allow the thick end of the tuyère to slide tightly backwards and forwards in it, like a
piston in the barrel of a pump; a diaphragm moreover prevents the tuyère from being
drawn or forced entirely out of its tube. At the side of this tube there is a small
orifice, which may be shut or opened at pleasure with a stopcock or screw-plug: it
serves to try the degree of heat of the air-blast; if a lead wire does not melt when
held at this hole, the temperature is reckoned too low; being under the 612th degree of
Fahrenheit. The nozzles are 2 inches in diameter.
Near the fire-places of the air-heating furnaces the pipes are at a cherry-red heat;
and lest they should be burned, they are there coated with a lute of fire-clay, as shown
near K fig. 586. By this means the air is kept up at the heat of 350° C, or 662° F., a
little above the boiling point of quicksilver.
Quantity of air and pressure.—The blowing-machine belonging to the above blast-furnace
is moved by a water wheel of 22-horse power, the pistons are 4 feet in diameter,
have a 31⁄2-feet stroke, work double, and expel 1200 cubic feet of air in the minute; or
600 cubic feet for each nozzle. The pressure of the air is equivalent to no more than
2 or 21⁄4 inches of mercury; formerly with cold air it amounted to 31⁄2 inches. This furnace
yields, upon an average, 51⁄4 tons of cast iron daily, and consumes 11⁄3 cwt. of coke for
each cwt. of cast iron produced; being 7 tons of coke per diem.
The consumption of the three flue fires is 30 pounds of small coal, for 100 pounds of
cast-iron produced, which may be reckoned equivalent to 15 pounds of coke; hence
altogether each ton of cast iron requires for its production 11⁄2 tons of coke.
The same furnace worked with the cold blast, the same pressure and the same ores,
produced only 31⁄2 tons of cast iron daily, with an expenditure of 2·55 of coke for 1 of
cast iron; in which case the coke amounted to 9 tons daily.
The returns by the hot blast compared with those by the cold, are therefore as the
numbers 3 and 2, which shows an advantage by the former plan of 50 per cent. The
consumption of fuel in the two cases is as 8 to 9, being a saving in this article of about
11 per cent. Coke is used on account of sulphur in the coal.
Hot-blast heated by the flame of the furnace mouth.—This system is mounted in Staffordshire.
The heating apparatus is there set immediately upon the mouth of the furnace;
and is composed of 2 large cast-iron cylinders of the same length, the one within[698]
the other, leaving a space between them. This annular interval amounts to 16 inches,
and it is closed at top and bottom: but the innermost cylinder is open at both ends,
and forms, indeed, the vent of the chimney or furnace. It carries nine rows of pipes,
three in each row, which cross its interior, and open into the annular space.
The flame of the furnace passes between the intervals of the cross pipes, heating
them, and also the two upright cylinders with which they are connected. The air of
the blowing machine arrives by a vertical pipe, which is placed at the back of the
furnace; it enters into the above annular space, and thence circulates, with more or less
velocity, through the 27 cross tubes, upon which the flame is continually playing; lastly,
it is drawn through to the bottom of the annular space; the two tubes which conduct it
to the two tuyères, pass down within the brickwork of the furnace, and thus prevent the
dissipation of its heat.
Below this heating apparatus there is a door for putting the charges into the furnace.
The above arrangement does not seem to be the best for obtaining the greatest possible
heat for the blast, nor for favouring the free action of the furnace; but it illustrates
perfectly well the principle of this application. A serpentine movement in a long bent
hot channel would be much better adapted for communicating heat to so bad a conductor
as air is known to be.
In the month of July, 1836, I paid a visit to Codner Park and Butterly works, in
Derbyshire, belonging to the eminent iron-masters, Messrs. Jessop and Co., where I
was kindly permitted not only to study the various processes of the manufacture of
cast and wrought iron, but to inspect the registers of the products of cast iron in
their blast furnaces for several years back. It appeared that in the year 1829, only
29 tons of cast iron were made weekly in each of the blast furnaces at Codner
Park. They were then worked with coke, and blown with cold air. Each ton of
iron required for its production, at that time, 6·82 tons of coals, made into coke for
smelting; with 2·64 of roasted iron ore (carbonate), called mine; and 0·87 of limestone,
the castine of the French.
In 1835 and 1836, the same furnaces turned out weekly, 49 tons of cast iron each;
and every ton of iron required for its production only 3 tons of coal (not made into
coke); 2·72 tons of mine; and 0·77 of lime.
In 1829, and for many years before, as well as one or two after, each ton of coals is said
to have cost for coking the sum of 6s., whence the 6·82 tons of coals then converted into
coke for smelting one ton of iron, cost fully 40s. in coking alone, in addition to their prime
cost. The saving in this respect, therefore, is 40s. upon each ton of iron, besides the
saving of fully half the coal, and the increased produce of nearly 60 per cent. of metal
per week. The iron-master pays the patentee 1s. upon every ton of iron which he
makes, and at the prices of 1836, he lessened his expenses by, at least, 30s. or 40s. per
ton by the patent improvement.
The following tabular view of the progression in the management and results of the
hot blast, is given by M. Dufrénoy, after visiting the various iron works in this country
where it had been introduced.
“At the Clyde iron works, near Glasgow; in 1829, when the combustion was effected
by the cold air blast,—
| |
Coal. |
| |
Tons. |
cwt. |
lbs. |
| There were consumed, |
for smelting; 3 tons of coke, equivalent to |
6 |
13 |
|
0 |
| — |
for the blowing engine |
1 |
0 |
|
7 |
| |
Total coal per ton of iron |
7 |
13 |
|
7 |
| |
Limestone |
0 |
10 |
1⁄2 |
0 |
| In 1831, with the hot blast at 450° F., coke being still used in smelting,— |
|
| There were consumed, |
for smelting; 1 ton 18 cwt. of coke, equivalent to |
4 |
6 |
|
0 |
| — |
for heating the air, 5 cwt. |
|
- |
0 |
12 |
|
4 |
| — |
for the blowing engine, 7 cwt. 4 lbs. |
| |
Total coal per ton of iron |
4 |
18 |
|
4 |
| |
Limestone |
0 |
9 |
|
0 |
| In July, 1833, with the hot blast at 612° F., raw coal alone being used for smelting,— |
|
| There were consumed, |
for smelting |
2 |
0 |
|
0 |
| — |
for heating the air |
0 |
8 |
|
0 |
| — |
for the blowing engine |
0 |
11 |
|
2 |
| |
Total coal per ton of iron |
2 |
19 |
|
2 |
| |
Limestone |
0 |
7 |
|
0 |
[699]
“At the last period the use of hot air had increased the make of the furnaces by
more than one third, and had consequently produced a great saving of expense in the
article of labour. The quantity of blast necessary for the furnaces was also sensibly diminished;
for a blowing engine of seventy-horse power, which, in 1829, served only for
three blast furnaces, was now sufficient for the supply of four.
“On comparing these several results, we find that the economy of fuel is in proportion
to the temperature to which the air is raised. As for the actual saving, it varies in
every work, according to the nature of the coal, and the care with which the operation
is conducted.
“This process, though it has been four years in use in the works near Glasgow,
(which it has rescued from certain ruin) has scarcely passed the borders of Scotland;
the marvellous advantages, however, which it has produced, are beginning to triumph
over prejudice, and gradually to extend its use into the different English iron districts.
There are one-and-twenty works, containing altogether sixty-seven blast furnaces, in
which hot air is used. The pig iron run out of these furnaces is generally No. 1.,
and is fit for making the most delicate castings. This process is equally applicable
to forge pigs for the manufacture of bar iron; since in order to obtain this quality of
iron, it is only necessary to alter the proportion of fuel and mineral. In the forges
of the Tyne iron-works, near Newcastle, and of Codner Park, near Derby, pigs made in
furnaces blown by hot air, are alone used in the manufacture of bar iron.
“In the side of the tuyère pipe a small hole is made, by means of which the heat of
the air may be ascertained at any moment. This precaution is indispensable, it being
of importance to the beneficial use of hot air, that it be kept at a uniformly high temperature.
With a proper apparatus the air is raised to 612 degrees Fahr., which is a greater
heat, by several degrees, than is necessary for the fusion of lead.”
“At Calder works the consumption of fuel has diminished in the proportion of 7 tons
17 cwt. to 2 tons 2 cwt. There has also been a great diminution of expense in limestone,
of which only 51⁄2 cwt. are now used, instead of 13 cwt., which were used in 1828.
This decrease results, as I have already said, from the high temperature which the
furnace has acquired since the introduction of hot air.
“The quantity of blast has been reduced from 3500 cubic feet per minute, to 2627
cubic feet; the pressure also has been reduced from 31⁄4 to 23⁄4 lbs.”
Of the refinery of cast iron, or its conversion into bar-iron, in England.—This operation
is naturally divisible into three distinct parts. The first, or the finery properly speaking,
is executed in peculiar furnaces called running out fires; the second operation completes
the first, and is called puddling; and the third consists in welding several iron bars
together, and working them under forge hammers, and between rolls.
1. The finery furnaces are composed of a body of brickwork, about 9 feet square; rising
but little above the surface of the ground. The hearth, placed in the middle, is two
feet and a half deep; it is rectangular, being in general, 3 feet by 2, with its greatest
side parallel to the face of the tuyères; and it is made of cast iron in four plates. On the
side of the tuyères there is a single brick wall. On the three other sides, sheet iron doors
are placed, to prevent the external air from cooling the metal, which is almost always
worked under an open shed, or in the open air, but never in a space surrounded by walls.
The chimney, from 15 to 18 feet high, is supported upon four columns of cast iron;
its lintel is four feet above the level of the hearth, in order that the labourers may work
without restraint.
The number of tuyères is from two to three; they are placed at the height of the lip
of the crucible or hearth, and distributed so as to divide its length into equal parts;
their axes being inclined towards the bottom, at an angle of from 25° to 30°, so as to point
upon the bath of melted metal as it flows. The cast-iron nose-pipe is encased, and water
is made to circulate in the hollow space by means of cylindrical tubes; being introduced
by one tube, and let off by another, so as to prevent the tuyères from getting burned in
the process.
Two nozzles are usually placed in each tuyère, to render the blast constant and uniform;
and for the same end, the air impelled by the bellows, is sometimes received at
first in a regulator. The quantity of air blown into the fineries is considerable; being
nearly 400 cubic feet per minute for each finery; or about the eighth part of the consumption
of a blast furnace.
The finery furnace, or running out fire, is represented in figs. 590. and 591. It
is a smelting hearth, in which by first fusing and then cooling gray cast iron in a peculiar
way, it is converted into white cast iron, called fine iron, or fine metal, of the
quality of forge pig, for making malleable iron by the puddling process. The furnace
resembles the forge hearth employed in Germany and France for converting forge pig
into wrought iron; but it differs, particularly in this, that the fused iron is run out into
an oblong iron trough, for sudden congelation.
a is the air-chest, in communication with the blowing cylinder, or bellows; the air[700]
being conducted through at least two blast pipes to the fire, and sometimes through
even 4 or 6 pipes. b is the side of the furnace, corresponding to the tuyère plates, in
which are the openings for the blast pipes. All the sides of the furnace are hollow, and
are kept cool by the circulation of water through the cavity between them. c is the front
wall of the furnace, having a strong cast-iron plate containing the tap holes for running
off the melted metal. d d is the exterior wall of the furnace, which corresponds to the
contre-vent and ash-hearth of the French refining forge. e, is the top plate upon which the
coke is piled up in store. f f, f f, iron props of the chimney, (not shown in this view). g,
cast-iron trough into which the fine iron is run off in fusion; which is sometimes made
in one piece, but more usually in separate plates joined together. Beneath this mould
a stream of water is made to flow. h is the bottom of the hearth, covered with sand.
In the finery process, the hearth or crucible of the furnace is filled with coke; then six
pigs of cast iron are laid horizontally on the hearth, namely, four of them parallel to
the four sides, and two in the middle above; and the whole is covered up in a dome-form,
with a heap of coke. The fire is now lighted, and in a quarter of an hour the
blast is applied. The cast iron flows down gradually, and collects in the crucible; more
coke being added as the first quantity burns away. This operation proceeds by itself;
the melted metal is not stirred about, as in some modes of refinery, and the temperature
is always kept high enough to preserve the metal liquid. During this stage the coals
are observed continually heaving up, a movement due in part to the action of the blast,
and in part to an expansion caused in the metal by the discharge of gaseous oxide of
carbon. When all the pig iron is collected at the bottom of the hearth, which happens
commonly at the end of two hours, or two and a half, the tap hole is opened, and the
fine metal flows out with the slag, into the loam-coated pit, on a plate 10 feet long,
3 broad, and from 2 inches to 21⁄2 thick. A portion of the slag forms a small crust on
the surface of the metal; but most part of it collects in a basin scooped out at the
bottom of the pit, into which the fine metal is run.
A large quantity of water is thrown on the fine metal, with the view of rendering it
brittle, and perhaps of partially oxidizing it. This metal suddenly cooled, is very white,
and possesses in general a fibrous radiated texture; or sometimes a cellular, including a
considerable number of small spherical cavities, like a decomposed amygdaloid rock. If
the cast iron be of bad quality, a little limestone is occasionally used in the above
operation.
Three samples of cinder, analyzed by Berthier, gave,
| Silica |
0·276; |
protox. of iron, |
0·612; |
alumina, |
0·040; |
phosp. acid, 0·072, Dudley. |
| — |
0·368 |
— |
0·610 |
— |
0·015; |
puddling of Dowlais. |
| — |
0·424 |
— |
0·520 |
— |
0·033; |
ditto. |
The remarkable fact of the presence of phosphoric acid, shows how important this
operation is to the purification of the iron. The charge varies from a ton and a quarter
to a ton and a half of pigs; and the loss by the process varies from 12 to 17 per cent.
The fine metal is broken into fragments, and sent to the puddling furnace after the
product of each operation has been weighed. The coal consumed in the fine metal
process is from 4 to 5 hundred weight for the ton of cast iron. About 10 tons may be
refined per diem, a quantity somewhat greater than the supply from a blast furnace; but
the fineries are not worked on the Sundays; and therefore a smelting furnace just keeps
one of them in play. Whatever care be taken in this process, the bar iron finally
resulting is never so good as if wood charcoal had been used in the refinery; and hence
in making sheet iron for the tin plate manufacture, wood charcoal is substituted for
coke in one Welsh establishment. The cast iron treated with charcoal, gets into clots[701]
or lumps in the finery furnace, which are lifted out, set under the hammer, and flattened
into thin cakes.
The main effect of the finery process, is probably the separation of the plumbaginous
part of the charcoal, which is disseminated through the gray cast iron in a state
of imperfect chemical combination. When that is removed the metal becomes more
homogeneous, having no crystalline carbon present to counteract its transition into pure
iron; much of the silica and manganese are also vitrified together, and run off in the
finery cinder.
2. The puddling furnace, is of the reverberatory form. It is bound generally with iron,
as represented in the side view, fig. 592., by means of horizontal and vertical bars, which
are joined together and fixed by wedges, to prevent them from starting asunder. Very
frequently, indeed, the reverberatory furnaces are armed with cast-iron plates over their
whole surface. These are retained by upright bars of cast iron applied to the side walls,
and by horizontal bars of iron, placed across the arch or roof. The furnace itself is
divided interiorly into three parts; the fire-place, the hearth, and flue. The fire-place
varies from 31⁄2 to 41⁄2 feet long, by from 2 feet 8 inches to 3 feet 4 inches wide. The door
way by which the coke is charged, is 8 inches square, and is bevelled off towards the
outside of the furnace. This opening consists entirely of cast iron, and has a quantity
of coal gathered round it. The bars of the fire grate are movable, to admit of more
readily clearing them from ashes.
Fig. 593. is a longitudinal section referring to the elevation, fig. 592., and fig. 594.
is a ground plan. When the furnace is a single one, a square hole is left in the side
of the fire-place opposite to the door, through which the rakes are introduced, in
order to be heated.
a is the fire door; b, the grate; c, the fire bridge; d d, cast-iron hearth plates, resting
upon cast-iron beams e e, which are bolted upon both sides to the cast-iron binding
plates of the furnace. f is the hearth covered with cinders or sand; g, is the main
working door, which may be opened and shut by means of a lever g′, and chain to
move it up and down. In this large door there is a hole 5 inches square, through
which the iron may be worked with the paddles or rakes; it may also be closed air-tight.[702]
There is a second working door h, near the flue, for introducing the cast iron,
so that it may soften slowly, till it be ready for drawing towards the bridge. i, is the
chimney, from 30 to 50 feet high, which receives commonly the flues of two furnaces, each
provided with a damper plate or register. Fig. 595.,
shows the main damper for the top of the common
chimney, which may be opened or shut to any degree
by means of the lever and chain. k, fig. 593., is the tap
or floss hole far running off the slag or cinder.
The sole is sometimes made of bricks, sometimes of
cast iron. In the first case it is composed of fire-bricks
set on edge, forming a species of flat vault. It rests
immediately on a body of brickwork either solid or
arched below. When it is made of cast iron, which is
now beginning to be the general practice, it may be
made either of one piece or of several. It is commonly
in a single piece, which, however, causes the inconvenience of reconstructing the furnace
entirely when the sole is to be changed. In this case it is a little hollow, as is shown in
the preceding vertical section; but if it consists of several pieces, it is usually made flat.
The hearths of cast iron rest upon cast-iron pillars, to the number of four or five;
which are supported on pedestals of cast iron placed on large blocks of stone. Such an
arrangement is shown in the figure, where also the square hole a, fig. 592., for heating the
rake irons, may be observed. The length of the hearth is usually six feet; and its breadth
varies from one part to another. Its greatest breadth, which is opposite the door, is four
feet. In the furnace, whose horizontal plan is given above, and which produces good results,
the sole exhibits, in this part, a species of ear, which enters into the mouth of the door.
At its origin towards the fireplace, it is 2 feet 10 inches wide; from the fire it is
separated, moreover, by a low wall of bricks (the fire-bridge) 10 inches thick, and from
3 inches to 5 high. At the other extremity its breadth is 2 feet. The curvature
presented by the sides of the sole or hearth is not symmetrical; for sometimes it makes
an advancement, as is observable in the plan. At the extremity of the sole furthest from
the fire, there is a low rising in the bricks of 21⁄2 inches, called the altar, for preventing
the metal from running out at the floss-hole when it begins to fuse. Beyond this shelf
the sole terminates in an inclined plane, which leads to the floss, or outlet of the slag
from the furnace. This floss is a little below the level of the sole, and is hollowed out of
the basement of the chimney. The slag is prevented from concreting here, by the flame
being made to pass over it, in its way to the sunk entry of the chimney; and there is
also a plate of cast iron near this opening, on which a moderate fire is kept up to preserve
the fluidity of the scoriæ, and to burn the gases that escape from the furnace, as also to
quicken the draught, and to keep the remote end of the furnace warm. On the top of
this iron plate, and at the bottom of the inclined plane, the cinder accumulates in a
small cavity, whence it afterwards flows away; whenever it tends to congeal, the workman
must clear it out with his rake.
The door is a cast-iron frame filled up inside with fire-bricks; through a small hole
in its bottom the workmen can observe the state of the furnace. This hole is at other
times shut with a stopper. The chimney has an area of from 14 to 16 inches.
The hearth stands 3 feet above the ground. Its arched roof, only one brick thick,
is raised 2 feet above the fire-bridge, and above the level of the sole, taken at the middle
of the furnace. At its extreme point near the chimney, its elevation is only 8 inches;
and the same height is given to the opening of the chimney.
In most iron-works the sole is covered with a layer of refractory sand, from 21⁄2 to 3
inches thick, which is lightly beat down with a shovel. At each operation a portion of
the sand is carried away; and is replaced before another. Within these few years, there
has been substituted for the sand a body of pounded slags; a substitution which has
occasioned, it is said, a great economy of iron and fuel.
The fine metal obtained by the coke is puddled by a continuous operation, which calls
for much care and skill on the part of the workmen. To charge the puddling furnace,
pieces of fine metal are successively introduced with a shovel, and laid one over another
on the sides of the hearth, in the form of piles rising to the roof; the middle being left
open for puddling the metal, as it is successively fused. Indeed, the whole are kept as
far separate as possible, to give free circulation to the air round the piles. The working
door of the furnace is now closed, fuel is laid on the grate, and the mouth of the fire-place
as well as the side opening of the grate, are both filled up with coal, at the same
time that the damper is entirely opened.
The fine metal in about twenty minutes comes to a white-red heat, and its thin-edged
fragments begin to melt and fall in drops on the sole of the furnace. At this period the
workman opens the small hole of the furnace door, detaches with a rake the pieces of
fine metal that begin to melt, tries to expose new surfaces to the action of the heat, and[703]
in order to prevent the metal from running together as it softens, he removes it from the
vicinity of the fire-bridge. When the whole of the fine metal has thus got reduced
to a pasty condition, he must lower the temperature of the furnace, to prevent it from
becoming more fluid. He closes the damper, takes out a portion of the fire, and the
ribs of the grate, and also throws a little water sometimes on the semi-fused mass. He
then works about with his paddle the clotty metal, which swells up, with the discharge
of gaseous oxide of carbon, burning with a blue flame, as if the bath were on fire. The
metal becomes finer by degrees, and less fusible; or in the language of the workmen, it
begins to get dry. The disengagement of the oxide of carbon diminishes, and soon
stops. The workmen continue meanwhile to puddle the metal till the whole charge be
reduced to the state of incoherent sand; and at that time, the ribs of the grate are
replaced, the fire is restored, and the register is progressively opened up. With the
return of the heat, the particles of metal begin to agglutinate, the charge becomes more
difficult to raise, or in the labourers’ language, it works heavy. The refining is now
finished, and nothing remains but to gather the iron into balls. The founder with his
paddle takes now a little lump of metal, as a nucleus, and makes it roll about on the
surface of the furnace, so as to collect more metal, and form a ball of about 60 or 70
pounds weight. With a kind of rake, called in England a dolly, and which he heats
beforehand, the workman sets this ball on that side of the furnace most exposed to the
action of the heat, in order to unite its different particles; which he then squeezes
together to force out the scoriæ. When all the balls are fashioned, (they take about
20 minutes work,) the small opening of the working door is closed with a brick, to cause
the heat to rise, and to facilitate the welding. Each ball is then lifted out, either with
tongs, if roughing rollers are to be used as in Wales, or with an iron rod welded to the
lump as a handle, if the hammer is to be employed, as in Staffordshire. Thus we see
that the operation lasts in whole from 2 hours to 21⁄2; in a quarter of an hour, the fine
metal melts at its edges, when the puddling begins, in order to effect its division; at the
end of an hour or an hour and a half, the metal is entirely reduced to a sand; a state
that is kept up for half an hour by continual stirring; and finally, the balling operation
takes nearly the same time.
The charge for each operation is from 31⁄2 to 4 hundred weight; and sometimes the
cuttings of bar-ends are introduced, which are puddled apart. The loss of iron is here
very variable, according to the degree of skill in the workman, who by negligence may
suffer a considerable body of iron to scorify or to flow into the hearth and raise the
bottom. In good working, the loss is from 8 to 10 per cent. In Wales, the consumption
of coal is estimated at one ton for every ton of fine metal. About five puddling
furnaces are required for the service of one smelting furnace and one finery. The hearth
of the puddling furnace should be exposed to heat for 12 hours before the work begins
on the Mondays; and on the Saturdays, the old sole must be cleared out, by melting it
off; and running it out by the floss-hole.
Mr. Schafthault obtained, in May, 1835, a patent for the conversion of cast into
wrought iron, by adding a mixture of black oxide of manganese, common salt and
potter’s clay, in certain small portions, successively to the melting iron in the puddling
furnace.
The reheating furnaces, balling furnaces, or mill furnaces, are analogous to the puddling
furnaces, but only of larger dimensions.
The wood charcoal forge hearth is employed for working up scrap iron into boiler
plate, &c. Here 22 bushels of charcoal are consumed in making one ton of iron of that
description, from boiler plate parings.
Machines for forging and condensing the iron.—In England there are employed for
the forging and drawing out of the iron, cast-iron hammers of great weight, and cylinders
of different dimensions, for beating out the balls, or extending the iron into bars, as also
powerful shears. These several mechanisms are moved either by a steam engine, as in
Staffordshire, and in almost all the other counties of England, or by water-wheels when
the localities are favourable, as in many establishments in South Wales. We shall here
offer some details concerning these machines.
The main driving shaft usually carries at either end a large toothed wheel, which
communicates motion to the different machines through smaller toothed wheels. Of
these, there are commonly six, four of which drive four different systems of cylinders,
and the two others work the hammer and the shears. The different cylinders of an
iron work should never be placed on the same arbor, because they are not to move
together, and they must have different velocities, according to their diameter. In order
to economise time and facilitate labour, care is taken to associate on one side of the
motive machine the hammer, the shears, and the reducing cylinders; and on the other
side, to place the several systems of cylinders for drawing out the iron into bars. For
the same reason the puddling furnaces ought to be grouped on the side of the hammer;
and the reheating furnaces on the other side of the works.
[704]
The hammers, fig. 596., are made entirely of cast iron; they are nearly 10 feet long,
and consist usually of two parts, the helve c, and the head or pane d. The latter enters
with friction into the former, and is retained in its place by wedges of iron or wood.
The head consists of several faces or planes receding from each other; for the purpose
of giving different forms to the ball lumps. A ring of cast-iron a, called the cam-ring bag,
bearing movable cams b b, drives the hammer d, by lifting it up round its fulcrum f, and
then letting it fall alternately. In one iron work, this ring was found to be 3 feet in
diameter, 18 inches thick, and to weigh 4 tons. The weight of the helve (handle) of
the corresponding hammer was 3 tons and a half, and that of the head of the hammer,
8 hundred weight.
The anvil e consists also of two parts; the one called the pane of the anvil, is the counterpart
of the pane of the hammer; it likewise weighs 8 hundred weight. The second g,
named the stock of the anvil, weighs 4 tons. Its form is a parallelopiped, with the
edges rounded. The bloom, or rough ball, from the puddle furnace, is laid and turned
about upon it, by means of a rod of iron welded to each of them, called a porter. Since
the weight of these pieces is very great, and the shocks very considerable, the utmost
precautions should be taken in setting the hammer and its anvil upon a substantial
mass of masonry, as shown in the figure, over which is laid a double, or even quadruple
flooring of wood, formed of beams placed in transverse layers close to each other. Such
beams possess an elastic force, and thereby partially destroy the injurious reaction of the
shock. In some works, a six-feet cube of cast iron is placed as a pedestal to the anvil.
Forge hammers are very frequently mounted as levers of the first kind, with the
centre of motion about one-third or one-fourth of the length of the helve from the cam
wheel. The principle of this construction will be understood by inspection of fig. 605.
The short end of the lever which is struck down by the tappet c, is driven against the
end of an elastic beam a, and immediately rebounds, causing the long end to strike a
harder blow upon the anvil s.
The shears are composed of two branches, the one fixed and the other movable, each
formed of two pieces. The fixed branch is a cast-iron plate, which forms one mass with
a horizontal base fixed to a piece of wood or cast iron buried in the ground. A sharpened
chisel is fastened to its upper part by screws and nuts. The movable branch is
likewise of cast iron; it bears an axis round which it turns, and this axis passes through
the fixed part. It is also furnished with a cutting chisel, fixed on by nuts and screws.
An excentric or an ellipse, moved directly by a toothed wheel, lifts the movable branch
of the shears, and forces it to cut the iron bars presented to it. The pressure exerted
by these scissors is such, that they can cut without difficulty, iron bars, one-half or two-thirds
of an inch thick.
Cylinders.—The compression between cylinders now effects, in a few seconds, that
condensation and distribution of the fibres, which 40 years ago, could not be accomplished
till after many heats in the furnace, and many blows of the hammer. The
cylinders may be distinguished into two kinds; 1. those which serve to draw out the
ball, called puddling rolls, or roughing rolls, and which are, in fact, reducing cylinders;
2. the cylinders of extension, called rollers, for drawing into bars the massive iron after
it has received a welding, to make it more malleable. This second kind of cylinders is[705]
subdivided into several varieties, according to the patterns of bar iron that are required.
These may vary from 2 inches square to less than one-sixth of an inch.
Beneath the cylinders there is usually formed an oblong fosse, into which the scoriæ
and the scales fall when the iron is compressed. The sides of this fosse, constructed of
stone, are founded on a body of solid masonry, capable of supporting the enormous load
of the cylinders. Beams of wood form in some measure the sides of this pit, to which
cylinders may be made fast, by securing them with screws and bolts. Massive bars of
cast iron are found, however, to answer still better, not only because the uprights and
bearers may be more solidly fixed to them, but because the basement of heavy metal is
more difficult to shatter or displace, an accident which happens frequently to the
wooden beams. A rill of water is supplied by a pipe to each pair of cylinders, to
hinder them from getting hot; as also to prevent the hot iron from adhering to the
cylinder, by cooling its surface, and perhaps producing on it a slight degree of
oxidizement.
The shafts are one foot in diameter for the hammer and the roughing rolls; and six
inches where they communicate motion to the cylinders destined to draw the iron into
bars.
The roughing rolls are employed either to work out the lump or ball immediately
after it leaves the puddling furnace, as in the Welsh forges, or only to draw out the
piece, after it has been shaped under the hammer, as is practised in most of the Staffordshire
establishments. These roughing cylinders are generally 7 feet long, including
the trunnions, or 5 feet between the bearers, and 18 inches diameter; and weigh in the
whole from 4 to 41⁄2 tons. They contain from 5 to 7 grooves, commonly of an elliptical
form, one smaller than another in regular progression, as is seen in fig. 597. The small
axis of each ellipse, as formed by the union of the upper and under grooves, is always
placed in the vertical direction, and is equal to the great axis, or horizontal axis of the
succeeding groove; so that in transferring the bar from one groove to another, it must
receive a quarter of a revolution, whereby the iron gets elongated in every direction.
Sometimes the roughing rolls serve as preparatory cylinders, in which case they bear
towards one extremity rectangular grooves, as the figure exhibits. Several of these
large grooves are bestudded with small asperities analogous to the teeth of files, for
biting the lump of iron, and preventing its sliding. On a level with the under side of
the grooves of the lower cylinder, there is a plate of cast iron with notches in its edge
adapted to the grooves. This piece called the apron, rests on iron rods, and serves to
support the balls and bars exposed to the action of the rollers, and to receive the fragments
of ill-welded metal, which fall off during the drawing. The housing frames in
which the rollers are supported and revolve, are made of great strength. Their height
is 5 feet; their thickness is 1 foot in the side perpendicular to the axis of the cylinders,
and 10 inches in the other. Each pair of bearers is connected at their upper ends by
two iron rods, on which the workmen rest their tongs or pinchers for passing the lump
or bar from one side of the cylinders to the other.
The cods or bushes are each composed of two pieces; the one of hard brass, which
presents a cylindrical notch, is framed into the other which is made of cast iron, as is
clearly seen in fig. 597.
The iron bar delivered from the square grooves, is cut by the shears into short lengths,
which are collected in a bundle in order to be welded together. When this bundle of
bars has become hot enough in the furnace, it is conveyed to the rollers; which differ
in their arrangement according as they are meant to draw iron from a large or small
piece. The first, fig. 597., possess both elliptical and rectangular grooves; are 1 foot
in diameter and 3 feet long between the bearers. The bar is not finished under these
cylinders, but is transferred to another pair, whose grooves have the dimensions proper
for the bar, with a round, triangular, rectangular, or fillet form. The triangular grooves
made use of for square iron, have for their profile, an isosceles triangle slightly obtuse,
so that the space left by the two grooves together may be a rhombus, differing little
from a square, and whose smaller diagonal is vertical. When the bar is to be passed
successively through several grooves of this kind, the larger or horizontal diagonal of
each following groove is made equal to the smaller or upright of the preceding one,
whereby the iron must be turned one fourth round at each successive draught, and thus
receive pressure in opposite directions. Indeed the bar is often turned in succession
through the triangular and rectangular grooves, that its fibres may be more accurately
worked together. The decrement in the capacity of the grooves follows the proportion
of 15 to 11.
When it is intended to reduce the iron to a small rod, the cylinders have such a diameter,
that three may be set in the same housing frame. The lower and middle
cylinders are employed as roughing rollers, while the upper and middle ones are made
to draw out the rod. When a rod or bar is to be drawn with a channel or gutter in
its face, the grooves of the rollers are suitably formed.
[706]
To draw out square rods of a very small size, as nail-rods, a system of small rollers is
employed, called slitters. Their ridges are sharp-edged, and enter into the opposite
grooves 21⁄2 inches deep; so that the flat bar in
passing between such rollers is instantaneously
divided into several slips. For this purpose
the rollers represented in fig. 598. may be
put on and removed from the shaft at pleasure.
The velocity of the cylinders varies with
their dimensions. In one work, cylinders for
drawing out iron of from one-third to two-thirds
of an inch thick, make 140 revolutions
per minute; while those for iron of from two-thirds
of an inch to 3 inches, make only 65.
In another work, the cylinders for two inch
iron, make 95 revolutions per minute; those
for iron from two-thirds of an inch to an inch
and a third, make 128; and those for bars
from one-third to two-thirds of an inch, 150.
The roughing rollers move with only one-third
the velocity of the drawing cylinders.
The shingling and plate-rolling mill is represented
in fig. 597. The shingling mill, for
converting the blooms from the balling furnace
into bars, consists of two sets of grooved
cylinders, the first being called puddling rolls
or roughing rolls; the second are for reducing
or drawing the iron into mill-bars, and are
called simply rolls.
a, a, a, a, are the powerful uprights or standards
called housing frames, of cast iron, in which
the gudgeons of the rolls are set to revolve;
b, b, b, b, are bolt rods for binding these frames
together at top and bottom; c, are the roughing
rolls, having each a series of triangular grooves,
such that between those of the upper and
under cylinder, rectangular concavities are
formed in the circumference with slightly
sloping sides. The end groove to the right
of c, should be channelled like a rough file, in
order to take the better hold of the blooms, or
to bite the metal as the workmen say; and give
it the preparatory elongation for entering into
and passing through the remaining grooves
till it comes to the square ones, where it becomes
a mill-bar. d, d, are the smooth cylinders,
hardened upon the surface, or chilled
as it is called, by being cast in iron moulds,
for rolling iron into plates or hoops. e, e, e, e, are strong screws with rectangular threads,
which work by means of a wrench or key, into the nuts e′ e′ e′ e′, fixed in the standards;
they serve to regulate the height of the plummer blocks or bearers of the gudgeons, and
thereby the distance between the upper and under cylinders. f is a junction shaft; g,
g, g, are solid coupling boxes, which embrace the two separate ends of the shafts, and make
them turn together. h, h, are junction pinions, whereby motion is communicated from the
driving shaft f, through the under pinion to the upper one, and thus to both upper and
under rolls at once. i, i, are the pinion standards in which their shafts run; they are
smaller than the uprights of the rolls. k, k, are screws for fastening the head pieces l to
the top of the pinion standards. All the standards are provided with sole plates m,[707]
whereby they are screwed to the foundation beams, n, of wood or preferably iron, as
shown by dotted lines; o o are the binding screw bolts. Each pair of rolls at work is
kept cool by a small stream of water let down upon it from a pipe and stop-cock.
In the cylinder drawing, the workman who holds the ball in tongs, passes it into the
first of the elliptical grooves; and a second workman on the other side of the cylinders,
receives this lump, and hands it over to the first, who re-passes it between the rollers,
after bringing them somewhat closer to each other, by giving a turn to the adjusting pressure
screws. After the lump has passed five or six times through the same groove, it has
got an elliptical form, and is called in England a bloom. It is next passed through a
second groove of less size, which stretches the iron bar. In this state it is subjected to
a second pair of cylinders, by which the iron is drawn into flat bars, 4 inches broad and
half an inch thick. Fragments of the ball or bloom fall round about the cylinders;
which are afterwards added to the puddling charge. In a minute and a half, the rude
lump is transformed into bars, with a neatness and rapidity which the inexperienced eye
can hardly follow. A steam engine of thirty-horse power can rough down in a week,
200 tons of coarse iron.
This iron called mill-bar iron, is however of too inferior a quality to be employed in
any machinery; and it is subjected to another operation, which consists in welding
several pieces together, and working them into a mass of the desired quality. The iron
bars while still hot, are cut by the shears into a length proportional to the size of iron
bar that is wanted; and four rows of these are usually laid over each other into a heap
or pile, which is placed in the re-heating furnace above described, and exposed to a free
circulation of heat; one pile being set crosswise over another. In a half or three
quarters of an hour, the iron is hot enough, and the pieces now sticking together, are
carried in successive piles to the bar-drawing cylinders, to be converted into strong bars,
which are reckoned of middle quality. When a very tough iron is wanted, as for
anchors, another welding and rolling must be given. In the re-heating ovens, the loss
is from 8 to 10 per cent. on the large bar iron, and from 10 to 12 in smaller work. A
ton of iron consumes in this process, about 150 lbs. of coals.
It is thought by many that a purer iron is obtained by subjecting the balls as they
come out of the puddling furnace, to the action of the hammer at first, than to the
roughing rollers; and that by the latter process vitrified specks remain in the metal,
which the hammer expels. Hence, in some works, the balls are first worked under
the forge-hammer; and these stampings being afterwards heated in the form of pies
or cakes piled over each other, are passed through the roughing rollers.
Having given ample details concerning the manufacturing processes used in England
for making cast iron, it may be proper to subjoin a few observations upon its chemical
constitution. It has been generally believed and taught that the dark gray cast iron,
No. 1. or No. 2., contains more carbon than the white cast iron; and that the superior
quality of the former in tenacity and softness, is to be ascribed to that excess. But
the distinguished German metallurgist, M. Karsten, in his instructive volume, “Handbuch
der Eisenhüttenkunde,” or manual of the art of smelting iron ores, has proved, on
the contrary, that the white cast iron contains most charcoal; that this substance exists in
it in a state of combination with the whole body of the iron; that the foliated or lamellar
white cast iron contains as much carbon as iron can absorb in the liquid state; and that
this constitutes a compound of 4 atoms of iron combined with 1 of charcoal, or 112 + 6;
or 51⁄3 per cent.; whereas the dark gray cast iron contains generally from 3 to 4 per
cent., in the state of plumbago merely dispersed through the metal. He has further
confirmed his opinion, by causing the white variety to pass into the gray, and reciprocally.
Thus, dark gray cast metal melted and suddenly cooled, gives a silvery white
metal, hard and brittle. On the other hand, when the white cast iron is cooled very
slowly after fusion, the condition of the carbon in it changes, and a dark gray cast iron
is obtained. These phenomena shew that the graphite or plumbago, which requires a
high temperature for its formation, cannot be produced but by a slow cooling, which allows
the carbon to agglomerate itself in the iron in the state of graphite; while under a rapid
congelation, the carbon remains dissolved in the mass, and produces a white metal.
Hence we may understand how each successive fusion of dark gray iron hardens and
whitens it, though in contact with coke, by completing that chemical dissolution of the
carbon on which the white state depends.
In the manufacture of the blackest No. 1. cast iron, it sometimes happens that a considerable
quantity of a glistening carburet of iron appears, floating on the top of the
metal as it is run out into the sand-moulds. This substance is called kish by the
English workmen; and it affords a sure test of the good state of the furnace and quality
of the iron.
The most remarkable fact relative to the smelting of cast iron, is the difference of product
between the workings of the summer and the winter season, though all the materials
and machinery be the same. In fact, no cold-blast furnace will carry so great a burden[708]
in summer as in winter, that is, afford so great a product of metal, or bear so great a
charge of ore with the same quantity of coke. This difference is undoubtedly due to
the dilated and humid state of the atmosphere in the warm season. A very competent
judge of this matter, states the diminution in summer at from one-fifth to one-seventh,
independently of deterioration of quality.
Some of the foreign irons, particularly certain Swedish and Russian bars, are imported
into Great Britain in large quantities, and at prices much greater than those of
the English bars, and therefore the modes of manufacturing such excellent metal deserve
examination. All the best English cast steel, indeed, is made from the hoop L, iron
from Dannemora, in Sweden.
The processes pursued in the smelting works of the Continent have frequently in view
to obtain from the ore malleable iron directly, in a pure or nearly pure state. The furnaces
used for this purpose are of two kinds, called in French, 1. Feux de Loupes, or
Forges Catalanes; and 2. Fourneaux à pièce, or Forges Allemandes.
In the Catalan, or French method, the ore previously roasted in a kiln is afterwards
strongly torrefied in the forge before the smelting begins; operations which follow in
immediate succession. Ores treated in this way should be very fusible and very rich;
such as black oxide of iron, hematites, and certain spathose iron ores. From 100
parts of ore, 50 of metallic iron have been procured, but the average product is 35.
The furnaces employed are rectangular hearths, figs. 599. and 600., the water-blowing
machine being employed to give the blast. See Metallurgy. There are three
varieties of this forge; the Catalan, the Navarrese, and the Biscayan. The dimensions
of the first, the one most generally employed, are as follows: 21 inches long, in the
direction p f, fig. 600.; 181⁄2 broad, at the bottom of the hearth or creuset, in the line
A B; and 17 inches deep, fig. 599. The tuyère, q p, is placed 91⁄2 inches above the bottom,
so that its axis is directed towards the opposite side, about 2 inches above the bottom.
But it must be movable, as its inclination needs to be changed, according to the stage
of the operation, or the quantity of the ores. It is often raised or lowered with pellets
of clay; and even with a graduated circle, for the workmen make a great mystery of
this matter. The hearth is lined with a layer of brasque (loam and charcoal dust
worked together), and the ore after being roasted is sifted; the small powder being set
aside to be used in the course of the operation. The ore is piled up on the side opposite
to the blast in a sharp saddle ridge, and it occupies one-third of the furnace. In the
remaining space of two-thirds, the charcoal is put. To solidify the small ore on the
hearth, it is covered with moist cinders mixed with clay.
The fire is urged with moderation during the first two hours, the workman being
continually employed in pressing down more charcoal as the former supply burns
away, so as to keep the space full, and prevent the ore from crumbling down. By a
blast so tempered at the beginning, the ore gets well calcined, and partially reduced in
the way of cementation. But after two hours, the full force of the air is given;
at which period the fusion ought to commence. It is easy to see whether the torrefaction
be sufficiently advanced, by the aspect of the flame, as well as of the ore,
which becomes spongy or cavernous; and the workman now completes the fusion, by
detaching the pieces of ore from the bottom, and placing them in front of the tuyère.
When the fine siftings are afterwards thrown upon the top, they must be watered,
to prevent their being blown away, and to keep them evenly spread over the whole
surface of the light fuel. They increase the quantity of the products, and give a
proper fusibility to the scoriæ. When the scoriæ are viscid, the quantity of siftings
must be diminished; but if thin, they must be increased. The excess of slag is allowed
to run off by the chio or floss hole. The process lasts from five to six hours, after
which the pasty mass is taken out, and placed under a hammer to be cut into lumps,
which are afterwards forged into bars.
Each mass presents a mixed variety of iron and steel; in proportions which may
be modified at pleasure; for by using much of the siftings, and making the tuyère dip
towards the sole of the hearth, iron is the chief product; but if the operation be conducted[709]
slowly, with a small quantity of siftings, and an upraised tuyère, the quantity
of steel is more considerable. This primitive process is favourably spoken of by
M. Brongniart. The weight of the lump of metal varies from 200 to 400 pounds.
As the consumption of charcoal is very great, amounting in the Palatinate or Rheinkreis
to seven times the weight of iron obtained, though in the Pyrenees it is only
thrice, the Catalan forge can be profitably employed only where wood is exceedingly
cheap and abundant.
The Fourneaux à pièce of the French, or Stuck-ofen of the Germans, resembles fig. 313.,
(Copper); the tuyère (not shown there) having a dip towards the bottom of the hearth,
where the smelted matter collects. When the operation is finished, that is at least
once in every 24 hours, one of the sides of the hearth must be demolished, to take
out the pasty mass of iron, more or less pure. This furnace holds a middle place in the
treatment of iron, between the Catalan forge and the cast-iron floss-ofen, or high-blast
furnaces. The stuck-ofen are from 10 to 15 feet high, and about 3 feet in diameter at
the hearth. Most usually there is only one aperture for the tuyère and for working;
with a small one for the escape of the slag; on which account, the bellows are removed
to make way for the lifting out of the lump of metal, which is done through an opening
left on a level with the sole, temporarily closed with bricks and potters’ clay, while the
furnace is in action.
This outlet being closed, and the furnace filled with charcoal, fire is kindled at the
bottom. Whenever the whole is in combustion, the roasted ore is introduced at the
top in alternate charges with charcoal, till the proper quantity has been introduced.
The ore falls down; and whenever it comes opposite to the tuyère the slag begins to
flow, and the iron drops down and collects at the bottom of the hearth into the mass or
stuck; and in proportion as this mass increases, the floss-hole for the slag and the tuyère
is raised higher. When the quantity of iron accumulated in the hearth is judged
to be sufficient, the bellows are stopped, the scoriæ are raked off, the little brick wall is
taken down, and the mass of iron is removed by rakes and tongs. This mass is then
flattened under the hammer, into a cake from 3 to 4 inches thick, and is cut into
two lumps, which are submitted to a new operation; where it is treated in a peculiar
refinery, lined with charcoal brasque, and exposed to a nearly horizontal blast. The
above mass seized in the jaws of powerful tongs, is heated before the tuyère; a portion
of the metal flows down to the bottom of the hearth, loses its carbon in a bath
of rich slags or fused oxides, and forms thereby a mass of iron thoroughly refined.
The portion that remains in the tongs furnishes steel, which is drawn out into bars.
This process is employed in Carniola for smelting a granular oxide of iron. The mass
or stuck amounts to from 15 to 20 hundred weight, after each operation of 24 hours.
Eight strong men are required to lift it out, and to carry it under a large hammer,
where it is cut into pieces of about 1 cwt. each. These are afterwards refined, and drawn
into bars as above described. These furnaces are now almost generally abandoned on
the Continent, in favour of charcoal high or blast furnaces.
Fig. 313. represents a schachtofen, (but without the tuyère, which may be supposed to
be in the usual place), and is, like all the continental Hauts Fourneaux, remarkable for
the excessive thickness of its masonry. The charge is put in at the throat, near the
summit of the octagonal or square concavity, for they are made of both forms. At
the bottom of the hearth there is a dam-stone with its plate, for permitting the overflow
of the slag, while it confines the subjacent fluid metal; as well as a tymp-stone
with its plate, which forms the key to the front of the hearth; the boshes are a wide
funnel, almost flat, to obstruct the easy descent of the charges, whereby the smelting
with charcoal would proceed too rapidly. The bottom of the hearth is constructed of
two large stones, and the hinder part of one great stone, called in German rückstein
(back stone), which the French have corrupted into rustine. In other countries of the
Continent, the boshes are frequently a good deal more tapered downwards, and the
hearth is larger than here represented. The refractory nature of the Hartz iron ores is
the reason assigned for this peculiarity.
In Sweden there are blast-furnaces, schachtofen, 35 feet in height, measured from the
boshes above the line of the hearth, or creuset. Their cavity has the form of an
elongated ellipse, whose small diameter is 8 feet across, at a height of 14 feet above the
bottom of the hearth; hence, at this part, the interior space constitutes a belly corresponding
with the upper part of the boshes. In other respects the details of the construction
of the Swedish furnaces resemble the one figured above. Marcher relates
that a furnace of that kind whose height was only 30 feet, in which brown hydrate of
iron (hematite) was smelted, yielded 47 per cent. in cast iron, at the rate of 5 hundred
weight a day, or 36 hundred weight one week after another; and that in the production
of 100 pounds of cast iron, 130 pounds of charcoal were consumed. That furnace was
worked with forge bellows, mounted with leather.
[710]
The decarburation of cast iron is merely a restoration of the carbon to the surface, in
tracing inversely the same progressive steps as had carried it into the interior during the
smelting of the ore. The oxygen of the air, acting first at the surface of the cast metal,
upon the carbon which it finds there, burns it: fresh charcoal, oozing from the interior,
comes then to occupy the place of what had been dissipated; till, finally, the whole carbon
is transferred from the centre to the surface, and is there converted into either carbonic
acid gas or oxide of carbon; for no direct experiment has hitherto proved which
of these is the precise product of this combustion.
This diffusibility of carbon through the whole mass of iron constitutes a movement
by means of which cast iron may be refined even without undergoing fusion, as is proved
by a multitude of phenomena. Every workman has observed that steel loses a portion
of its steely properties every time it is heated in contact with air.
On the above principle, cast iron may be refined at one operation. Three kinds of
iron are susceptible of this continuous process:—1. The speckled cast-iron, which contains
such a proportion of oxygen and carbon as with the oxygen of the air and the
carbon of the fuel may produce sufficient and complete saturation, but nothing in excess.
2. The dark gray cast-iron. 3. The white cast-iron. The nature of the crude metal
requires variations both in the form of the furnaces, and in the manipulations.
Indeed malleable iron may be obtained directly from the ores by one fusion.
This mode of working is practised in the Pyrenees to a considerable extent. All
the ores of iron are not adapted for this operation. Those in which the metallic
oxide is mixed with much earthy matter, do not answer well; but those composed
of the pure black oxide, red oxide, and carbonate, succeed much better. To extract
the metal from such ores, it is sufficient to expose them to a high temperature, in
contact either with charcoal, or with carbonaceous gases; the metallic oxide is
speedily reduced. But when several earths are present, these tend continually, during
the vitrification which they suffer, to retain in their vitreous mass the unreduced oxide
of iron. Were such earthy ores, as our ironstones, to be put into the low furnaces
called Catalan, through which the charges pass with great rapidity, and in which the contact
with the fuel is merely momentary, there would be found in the crucible or hearth
merely a rich metallic glass, instead of a lump of metal.
In smelting and refining by a continuous operation, three different stages may be distinguished:—1.
The roasting of the ore to expel the sulphur, which would be less
easily separated afterwards. The roasting dissipates likewise the water, the carbonic acid,
and any other volatile substances which the minerals may contain. 2. The deoxidizement
and reduction to metal by exposure to charcoal or carburetted vapours. 3. The
melting, agglutination, and refining of the metal to fit it for the heavy hammers where
it gets nerve. There are several forges in which these three operations seem to be confounded
into a single one, because, although still successive, they are practised at one
single heating without interruption. In other forges, the processes are performed separately,
or an interval elapses between each stage of the work. Three systems of this
kind are known to exist:—1. The Corsican method; 2. The Catalan with wood
charcoal; and 3. The Catalan with coke.
The furnaces of Corsica are a kind of semicircular basins, 18 inches in diameter, and
6 inches deep. These are excavated in an area, or a small elevation of masonry, 8 or
10 feet long by 5 or 6 broad, and covered in with a chimney. This area is quite similar
to that of the ordinary hearths of our blast-furnaces.
The tuyère stands 5 or 6 inches above the basin, and has a slight inclination downwards.
In Corsica, and the whole portion of Italy adjoining the Mediterranean shores,
the iron ore is an oxide similar to the specular ore of the Isle of Elba. This ore contains
a little water, some carbonic acid, occasionally pyrites, but in small quantity. Before
deoxidizing the ore, it is requisite to expel the water and carbonic acid combined
with the oxide, as well as the sulphur of the pyrites.
The operations of roasting, reduction, fusion, and agglutination are executed in the
same furnace. These are indeed divided into two stages, but the one is a continuation
of the other. In the first, the two primary operations are performed at once;—the
reduction of a portion of the roasted ore is begun at the same time that a portion of
the raw ore is roasted: these two substances are afterwards separated. In the second
stage, the deoxidizement of the metal is continued, which had begun in the preceding
stage; it is then melted and agglutinated, so as to form a ball to be submitted to the
forge-hammer.
The roasted pieces are broken down to the size of nuts, to make the reduction of the
metal easier. In executing the first step, the basin and area of the furnace must be
lined with a brasque of charcoal dust, 3, 4, or even 5 inches thick: over this brasque a
mound is raised with lumps of charcoal, very hard, and 4 or 5 inches high. A semi-circle
is formed round the tuyère, the inner radius of which is 5 or 6 inches. This mass
of charcoal is next surrounded with another pile of the roasted and broken ores, which[711]
must be covered with charcoal dust. The whole is sustained with large blocks of the
raw ore, which form externally a third wall.
These three piles of charcoal, with roasted and unroasted ore, are raised in three successive
beds, each 7 inches thick: they are separated from each other by a layer of charcoal
dust of about an inch, which makes the whole 24 inches high. This is afterwards
covered over with a thick coat of pounded charcoal.
The blocks of raw ore which compose the outward wall form a slope; the larger and
stronger pieces are at the bottom, and the smaller in the upper part. The large blocks
are sunk very firmly into the charcoal dust, to enable them better to resist the pressure
from within.
On the bottom of the semicircular well formed within the charcoal lumps, kindled pieces
are thrown, and over these, pieces of black charcoal; after which the blast of a water-blowing
machine (trompe) is given. The fire is kept up by constantly throwing charcoal
into the central well. At the beginning of the operation it is thrust down with
wooden rods, lest it should affect the building; but when the heat becomes too intense
for the workmen to come so near the hearth, a long iron rake is employed for the purpose.
At the end of about 3 hours, the two processes of roasting and reduction are
commonly finished: then the raw ore no longer exhales any fumes, and the roasted
ore, being softened, unites into lumps more or less coherent.
The workman now removes the blocks of roasted ore which form the outer casing,
rolls them to the spot where they are to be broken into small pieces, and pulls down
the brasque (small charcoal) which surrounds the mass of reduced ore.
The second operation is executed by cleaning the basin, removing the slags, covering
the basin anew with 2 or 3 brasques, (coats of pounded charcoal), and piling up to the
right and the left, two heaps of charcoal dust. Into the interval between these conical
piles two or three baskets of charcoal are cast, and on its top some cakes of the reduced
crude metal being laid, the blast is resumed. The cakes, as they heat, undergo a sort
of liquation, or sweating, by the action of the earthy glasses on the unreduced black
oxide present. Very fusible slags flow down through the mass; and the iron, reduced
and melted, passes finally through the coals, and falls into the slag basin below. To the
first parcel of cakes, others are added in succession. In proportion as the slags proceeding
from these run down, and the melted iron falls to the bottom, the thin slag is run
off by an upper overflow or chio hole, and the reduced iron kept by the heat in the
pasty condition, remains in the basin: all its parts get agglutinated, forming a soft
mass, which is removed by means of a hooked pole in order to be forged. Each lump
or bloom of malleable iron requires 3 hours and a half for its production.
The iron obtained by this process is in general soft, very malleable, and but little
steely. In Corsica four workmen are employed at one forge. The produce of their
labour is only about 4 cwt. of iron from 10 cwt. of ore and 20 of charcoal, mingled
with wood of beech and chestnut. Though their ore contains on an average 65 per
cent. of iron, only about 40 parts are extracted; evincing a prodigious waste, which
remains in the slags.
The difference between the Corsican and the Catalonian methods consists in the latter
roasting the ore at a distinct operation, and employing a second one in the reduction,
agglutination, and refining of the metal. In the Catalonian forges, 100 pounds of iron
are obtained from 300 pounds of ore and 310 pounds of charcoal; being a produce of
only 33 per cent. It may be concluded that there is a notable loss, since the sparry
iron ores, which are those principally smelted, contain on an average from 54 to 56 per
cent. of iron. The same ores smelted in the ordinary blast furnace produce about 45
per cent. of cast iron.
On the Continent, iron is frequently refined from the cast metal of the blast furnaces
by three operations, in three different ways. In one, the pig being melted,
with aspersion of water, a cake is obtained, which is again melted in order to form
a second cake. This being treated in the refinery fire, is then worked into a bloom. In
another system, the pig iron is melted and cast into plates: these are melted anew in
order to obtain crude balls, which are finally worked into blooms. In a third mode of
manufacture, the pig-iron is melted and cast into plates, which are roasted, and then
strongly heated, to form a bloom.
The French fusible ores, such as the silicates of iron, are very apt to smelt into white
cast iron. An excess of fluxes, light charcoals, too strong a blast, produce the same results.
A surcharge of ores which deranges the furnace and affords impure slags mixed with
much iron, too rapid a slope in the boshes, too low a degree of heat, and too great condensation
of the materials in the upper part of the furnace; all tend also to produce a
white cast iron. In its state of perfection, white cast iron has a silver colour, and a
bright metallic lustre. It is employed frequently in Germany for the manufacture of
steel, and is then called steel floss, or lamellar floss, a title which it still retains, though it
be hardly silver white, and have ceased to be foliated. When its colour takes a bluish-gray[712]
tinge, and its fracture appears striated or splintery, or when it exhibits gray spots,
it is then styled flower floss. In a third species of white cast iron we observe still
much lustre, but its colour verges upon gray, and its texture is variable. Its fracture
has been sometimes compared to that of a broken cheese. This variety occurs very
frequently. It is a white cast iron, made by a surcharge of ore in the furnace. If the
white colour becomes less clear and turns bluish, if its fracture be contorted, and contains
a great many empty spaces or air-cells, the metal takes the name of cavernous-floss,
or tender-floss. The whitest metal cannot be employed for casting. When the
white is mixed with the gray cast iron, it becomes riband or trout cast iron.
The German refining forge.—Figs. 601, 602. represent one of the numerous refinery
furnaces so common in the Hartz. The example is taken from the Mandelholz works,
in the neighbourhood of Elbingerode. Fig. 602. is an elevation of this forge. D is the
refinery hearth, provided with two pairs of bellows. Fig. 601. is a vertical section,
showing particularly the construction of the crucible or hearth in the refinery forge D.
C is an overshot water-wheel, which gives an alternate impulsion to the two bellows a b
by means of the revolving shaft c, and the cams or tappets d f e g.
D, the hearth, is lined with cast-iron plates. Through the pipe l, cold water may be
introduced, under the bottom plate m, in order to keep down, when necessary, the temperature
of the crucible, and facilitate the solidification of the loupe or bloom. An orifice
n, figs. 601, 602., called the chio (floss hole), allows the melted slag or cinder to flow
off from the surface of the melted metal. The copper pipe or nose piece p, fig. 600.,
conducts the blast of both bellows into the hearth, as shown at b x, fig. 602., and D g p
fig. 600.
The substance subjected to this mode of refinery, is a gray carbonaceous cast iron,
from the works of Rothehütte. The hearth D, being filled and heaped over with live
charcoal, upon the side opposite to the tuyère x, figs. 601, 602, long pigs of cast iron are
laid with their ends sloping downwards, and are drawn forwards successively into the hearth
by a hooked poker, so that the extremity of each may be plunged into the middle of the
fire, at a distance of 6 or 8 inches from the mouth of the tuyère. The workman proceeds
in this way, till he has melted enough of metal to form a loupe. The cast iron, on
melting, falls down in drops to the bottom of the hearth; being covered by the fused
slags, or vitreous matters more or less loaded with oxide of iron. After running them off
by the orifice n, he then works the cast iron by powerful stirring with an iron rake
(ringard), till it is converted into a mass of a pasty consistence.
During this operation, a portion of the carbon contained in the cast iron combines
with the atmospherical oxygen supplied by the bellows, and passes off in the form of
carbonic oxide and carbonic acid. When the lump is coagulated sufficiently, the workman
turns it over in the hearth, then increases the heat so as to melt it afresh, meanwhile
exposing it all round to the blast, in order to consume the remainder of the carbon, that
is, till the iron has become ductile, or refined. If one fusion should prove inadequate to
this effect, two are given. Before the conclusion, the workman runs off a second
stratum of vitreous slag, but at a higher level, so that some of it may remain upon the
metal.
The weight of such a loupe or bloom is about 2 cwts., being the product of 2 cwts. and
7⁄10 of pig iron; the loss of weight is therefore about 26 per cent. 149 pounds of charcoal
are consumed for every 100 pounds of bar iron obtained. The whole operation lasts
about 5 hours. The bellows are stopped as soon as the bloom is ready; this is immediately
transferred to a forge hammer, such as is represented fig. 605.; the cast-iron head
of which weighs 8 or 9 cwts. The bloom is greatly condensed thereby, and discharges
a considerable quantity of semi-fluid cinder. The lump is then divided by the hammer[713]
and a chisel into 4 or 6 pieces, which are re-heated, one after another, in the same refinery
fire, in order to be forged into bars, whilst another pig of cast iron is laid in its place, to
prepare for the formation of a new bloom. The above process is called by the Germans
klump-frischen, or lump-refining. It differs from the durch-brech-frischen, because in
the latter, the lump is not turned over in mass, but is broken, and exposed in separate
pieces successively to the refining power of the blast near the tuyère. The French call
this affinage par portions; it is much lighter work than the other.
The quality of the iron is tried in various ways; as first, by raising a bar by one end,
with the two hands over one’s head, and bringing it forcibly down to strike across a narrow
anvil at its centre of percussion, or one-third from the other extremity of the bar; after
which it may be bent backwards and forwards at the place of percussion several times;
2. a heavy bar may be laid obliquely over props near its end, and struck strongly with a
hammer with a narrow pane, so as to curve it in opposite directions; or while heated
to redness, they may be kneed backwards and forwards at the same spot, on the edge of
the anvil. This is a severe trial, which the hoop L, Swedish iron, bears surprisingly,
emitting as it is hammered, a phosphoric odour, peculiar to it and to the bar iron of
Ulverstone, which also resembles it, in furnishing a good steel. The forging of a horseshoe
is reckoned a good criterion of the quality of iron. Its freedom from flaws is
detected by the above modes; and its linear strength may be determined by suspending
a scale to the lower end of a hard-drawn wire, of a given size, and adding weights till
the wire breaks. The treatises of Barlow and Tredgold may be consulted with advantage
on the methods of proving the strength of different kinds of iron, in a great variety
of circumstances.
Steel of cementation, or blistered steel and cast steel, are treated under the article
Steel. But since in the conversion of cast iron into wrought iron, by a very slight
difference in the manipulations, a species of steel may be produced called natural steel, I
shall describe this process here.
Fig. 603. is a view of the celebrated steel iron works, called Königshütte (king’s-forge),
in Upper Silesia, being one of the best arranged in Germany, for smelting iron ore by
means of coke. The front shown here is about 400 English feet long. a a are two blast
furnaces. A third blast furnace, all like the English, is situated to the left of one of the
towers b. b b are the charging towers, into which the ore is raised by machinery from
the level of the store-houses l l, up to the mouth of the furnaces a a; c c point to the
positions of the boilers of the two steam engines, which drive two cylinder bellows at f.
n n n n are arched cellars placed below the store-houses l l, for containing materials and
tools necessary for the establishment.
Figs. 599., 604., are vertical sections of the forge of Königshütte, for making natural
steel; fig. 599. being drawn in the line A B of
the plan, fig. 600. a is the bottom of the hearth,
consisting of a fire-proof gritstone; b is a space
filled with small charcoal, damped with water,
under which, at n, in fig. 604., is a bed of well
rammed clay; d is a plate of cast iron, which lines
the side of the hearth called rückstein (backstone)
in German, and corrupted by the French into
rustine; f is the plate of the counter-blast; g the
plate of the side of the tuyère: behind, upon the
face d, the fire-place or hearth is only 51⁄2 inches deep; in front as well as upon the
lateral faces, it is 18 inches deep. By means of a mound made of dry charcoal, the
posterior face d, is raised to the height of the face f. i, fig. 600., is the floss-hole, by
which the slags are run off from the hearth during the working, and through which,
by removing some bricks, the lump of steel is taken out when finished.
k l m are pieces of cast iron, for confining the fire in front, that is towards the side where
the workman stands; o is the level of the floor of the works; p a copper tuyère; it is
situated 41⁄2 inches above the bottom a, slopes 5 degrees towards it, and advances 4 inches
into the hearth or fire-place, where it presents an orifice, one half inch in horizontal
length, and one inch up and down; q the nose pipes of two bellows, like those represented[714]
in fig. 602., and under Silver; the round orifice of each of them within the tuyère being
one inch in diameter. r is the lintel or top arch of the tuyère, beneath which is seen
the cross section of the pig of cast iron under operation.
For the production of natural steel, a white cast iron is preferred, which contains
little carbon, which does not flow thin, and which being cemented over or above the
wind, falls down at once through the blast to the bottom of the hearth in the state of
steel. With this view, a very flat fire is used; and should the metal run too fluid, some
malleable lumps are introduced to give the mass a thicker pasty consistence.
If the natural steel be supposed to contain too little carbon, which is a very rare case,
the metal bath covered with its cinder slag, is diligently stirred with a wooden pole, or
it may receive a little of the more highly carburetted iron. If it contains the right dose
of carbon, the earthy and other foreign matters are made progressively to sweat out,
into the supernatant slag. When the mass is found by the trial of a sample to be completely
converted, and has acquired the requisite stiffness, it is lifted out of the furnace,
by the opening in front, subjected to the forge hammer, and drawn into bars. In Sweden,
the cast-iron pigs are heated to a cherry-red, and in this state broken to pieces under the
hammer, before they are exposed in the steel furnace. These natural steels are much
employed on the Continent in making agricultural implements, on account of their
cheapness. The natural steel of Styria is regarded as a very good article.
Wootz is a natural steel prepared from a black ore of iron in Hindostan, by a process
analogous to that of the Catalan hearth, but still simpler. It seems to contain a minute
portion of the combustible bases of alumina and silica, to which its peculiar hardness
when tempered, may possibly be ascribed. It is remarkable for the property of assuming
a damask surface, by the action of dilute sulphuric acid, after it has been forged and
polished. See Damascus and Steel.
Fig. 605. is the German forge-hammer; to the left of 1, is the axis of the rotatory
cam, 2, 3, consisting of 8 sides,
each formed of a strong broad bar
of cast iron, which are joined together
to make the octagon wheel.
4, 5, 6, are cast-iron binding rings
or hoops; made fast by wooden
wedges. b, b, are standards of the
frame work e, l, m, in which the
helve of the forge hammer has its
fulcrum near u. h, the sole part
of the frame. Another cast-iron
base or sole is seen at m. n is a
strong stay, to strengthen the
frame-work. At r two parallel
hammers are placed, with cast-iron
heads and wooden helves. s is the anvil, a very massive piece of cast iron. t is
the end of a vibrating beam, for throwing back the hammer from it forcibly by recoil.
x y is the outline of the water-wheel which drives the whole. The cams or tappets are
shown mounted upon the wheel 6, g, 6.
Analysis of Irons.—Oxidized substances cannot exist in metallic iron, and the foreign
substances it does contain are present in such small quantities, that it is somewhat
difficult to determine their amount. The most intricate point is, the proportion of
carbon. The free carbon, which is present only in gray cast iron, may, indeed, be
determined nearly, for most of it remains after solution of the metal in acids. The
combined charcoal, however, changes by the action of muriatic acid into gas and oil;
sulphuric acid also occasions a great loss of carbon, and nitric acid dissipates it almost
entirely. Either nitre or chloride of silver may be employed to ascertain the amount
of carbon; but when the iron contains chromium and much phosphorus, the determination
of the carbon is attended with many difficulties.
The quantity of sulphur is always so small, that it can scarcely be ascertained by the
weight of the precipitate of sulphate of barytes from the solution of the iron in nitro-muriatic
acid. The iron should be dissolved in muriatic acid; and the hydrogen, as it
escapes charged with the sulphur, should be passed through an acidulous solution of
acetate of lead. The weight of the precipitated sulphuret shows the amount of sulphur,
allowing 13·45 of the latter for 100 of the former. In this experiment the metal
should be slowly acted upon by the acid. Cast iron takes from 10 to 15 days to dissolve,
steel from 8 to 10, and malleable iron 4 days. The residuum of a black colour
does not contain a trace of sulphur.
Phosphorus and chromium are determined in the following way. The iron must be
dissolved in nitro-muriatic acid, to oxygenate those two bodies. The solution must be
evaporated cautiously to dryness in porcelain capsules, and the saline residuum heated[715]
to redness. A little chloride of iron is volatilized, and the remainder resembles the
red-brown oxide. This must be mixed with thrice its weight of carbonate of potash,
and fused in a platinum crucible; the quantity of iron being from 40 to 50 grains
at most.
The mixture after being acted upon by boiling water, is to be left to settle, to allow
the oxide to be deposited, for it is so fine as to pass through a filter. If the iron contained
manganese, this would be found at first in the alkaline solution; but manganese
spontaneously separates by exposure to the air. The alkaline liquor must be
supersaturated with muriatic acid, and evaporated to dryness. The liquor acidulated,
and deprived of its silica by filtration, is to be supersaturated with ammonia; when
the alumina will precipitate in the state of a subphosphate. When the liquor is now
supersaturated with acetic acid, and then treated with acetate of lead, a precipitate
of phosphate of lead almost always falls. There is hardly a bit of iron to be found
which does not contain phosphorus. The slightest trace of chrome is detected by
the yellow colour of the lead precipitate; if this be white there is none of the colouring
metal present.
100 parts of the precipitated phosphate of lead contain, after calcination, 19·4 parts
of phosphoric acid. The precipitate should be previously washed with acetic acid, and
then with water. These 19·4 parts contain 8·525 parts of phosphorus.
Cast iron sometimes contains calcium and barium, which may be detected by their
well-known reagents, oxalate of ammonia, and sulphuric acid. In malleable iron they
are seldom or never present.
The charcoal found in the residuum of the nitro-muriatic solution is to be burned away
under a muffle. The solution itself contains along with the oxide of iron, protoxide of
manganese, and other oxides, as well as the earths, and the phosphoric and arsenic acids.
Tartaric acid is to be added to it, till no precipitate be formed by supersaturation with
caustic ammonia. The ammoniacal liquor must be treated with hydrosulphuret of
ammonia as long as it is clouded, then thrown upon a filter. The precipitate is usually
very voluminous, and must be well washed. The liquor which passes through is to be
saturated with muriatic acid, to decompose all the sulphurets.
The solution still contains all the earths and the oxide of titanium, besides the phosphoric
acid. It is to be evaporated to dryness, whereby the ammonia is expelled, and
the carbonaceous residuum must be burned under a muffle. If the iron contains much
phosphorus, the ashes are strongly agglutinated. They are to be fused as already
described along with carbonate of potash, and the mass is to be treated with boiling
water. The residuum may be examined for silica, lime, barytes, and oxide of titanium.
Muriatic acid being digested on it, then evaporated to dryness, and the residuum treated
with water; will leave the silica. Caustic ammonia, poured into the solution, will
separate the alumina, if any be present, and the oxide of titanium; but the former
almost never occurs.
Manganese is best sought for by a distinct operation. The iron must be dissolved
at the heat of boiling water, in nitro-muriatic acid; and the solution, when very
cold, is to be treated with small successive doses of solution of carbonate of ammonia.
If the iron has been oxidized to a maximum, and if the liquor has been
sufficiently acid, and diluted with water, it will retain the whole of the manganese.
This process is as good as that by succinate of ammonia, which requires many
precautions.
The liquor is often tinged yellow by carbon, after it has ceased to contain a single
trace of iron oxide. As soon as litmus paper begins to be blued by carbonate of
ammonia, we should stop adding it; immediately throw the whole upon a filter, and
wash continuously with cold water. What passes through is to be neutralized with
muriatic acid, and concentrated by evaporation. It may contain besides manganese, some
lime, or barytes. It should therefore be precipitated with hydrosulphuret of ammonia,
the hydrosulphuret of manganese should be collected, dissolved in strong muriatic acid,
filtered, and treated, at a boiling heat, with carbonate of potash. The precipitate, well
washed and calcined, contains, in 100 parts, 72·75 parts of metallic manganese.
The copper, arsenic, lead, tin, bismuth, antimony, or silver, are best separated by a
stream of sulphuretted hydrogen gas passed through the solution in nitro-muriatic acid,
after it is largely diluted with water. The precipitate must be cautiously roasted in a
porcelain test, to burn away the large quantity of sulphur which is deposited in consequence
of the conversion of the peroxide of iron into the protoxide. If nothing
remains upon the test, none of these metals is present. If a residuum be obtained, it
must be dissolved in nitro-muriatic acid, and subjected to examination. But, in fact,
carbon, sulphur, phosphorus, silicon, and manganese, are the chief contaminators of
iron.
Chloride of silver affords the means of determining the proportion of carbon contained
in iron, and of ascertaining the state in which that substance exists in the metal. Fused[716]
chloride of a pale yellow colour must be employed. The operation is to be performed
in close vessels, with the addition of a great deal of water, and a few drops of muriatic
acid. The carbonaceous residuum is occasionally slightly acted upon. We may
judge of this circumstance by the gases disengaged, as well as by the appearance of the
charcoal.
Ductile iron and soft steel, as well as white cast-iron which has been rendered gray
by roasting, when decomposed by chloride of silver, afford a blackish-brown unmagnetic
charcoal, and a plumbaginous substance perfectly similar to what is extracted from the
same kinds of iron, by solution in acids. A portion of this plumbago is also converted
into charcoal of a blackish brown colour, by the action of the chloride. Hence this
agent does not afford the means of obtaining what has been called the poly-carburet, till
it has produced a previous decomposition. But we obtain it, in this manner, purer and in
greater quantity than we could by dissolving the metal in the acids. The only subject
of regret is, that we possess no good criterion for judging of the progress of this
analytical operation.
Gray cast iron leaves, besides the polycarburet, a residuum of plumbago, and carbon
which was not chemically combined with the iron; while tempered steel and white cast
iron afford merely a blackish brown charcoal; but the operation is extremely slow with
the latter two bodies, because a layer of charcoal forms upon the surface, which obstructs
their oxidizement. For this reason the white cast iron ought to be previously
changed into gray by fusion in a crucible lined with charcoal, before being subjected to the
chloride of silver; if this process be employed for tempered steel, the combined carbon
becomes merely a polycarburet. It would not be possible to operate upon more than
15 grains, which require from 60 to 80 times that quantity of the chloride, and a period
of 15 days for the experiment.
The residuum, which is separable from the silver only by mechanical means, should
be dried a long time at the heat of boiling water. It contains almost always iron and
silica. After its weight is ascertained, it is to be burned in a crucible of platinum till the
ashes no longer change their colour, and are not attractable by the magnet. The difference
between the weights of the dried and calcined residuum is the weight of the
charcoal. The oxide of iron is afterwards separated from the silica by muriatic acid.
In operating upon gray cast iron, we should ascertain separately the proportion of
graphite or plumbago, and that of the combined charcoal. To determine the former,
we dissolve a second quantity of the cast iron in nitric acid, with a little muriatic; the
residuum, which is graphite, is separated from the silica and the combined carbon by
the action of caustic potash. After being washed and dried, it must be weighed. The
weight of the graphite obtained being deducted from the quantity of carbon resulting
from the decomposition effected by the chloride of silver, the remainder is the amount
of the chemically combined carbon.
By employing muriatic acid, we could dissipate at once the combined carbon; but
this method would be inexact, because the hydrogen disengaged would carry off a
portion of the graphite.
According to Karsten, Mushet’s table of the quantities of carbon contained in different
steels and cast irons is altogether erroneous. It gives no explanation why, with
equal proportions of charcoal, cast iron constitutes at one time a gray, soft, granular
metal, and at another, a white, hard, brittle metal in lamellar facets. The incorrectness
of Mushet’s statement becomes most manifest when we see the white lamellar cast iron
melted in a crucible lined with charcoal, take no increase of weight, while the gray cast
iron treated in the same way becomes considerably heavier.
Analysis has never detected a trace of carbon unaltered or of graphite in white cast iron,
if it did not proceed from small quantities of the gray mixed with it; while perfect
gray cast iron affords always a much smaller quantity of carbon altered by combination,
and a much greater quantity of graphite. Neither kind of cast iron, however, betrays
the presence of any oxygen. Steel affords merely altered carbon, without graphite;
the same thing holds true of malleable iron; while the iron obtained by fusion with
25 per cent. of scales of iron contains no carbon at all.
The graphite of cast iron is obtained in scales of a metallic aspect, whereas the combined
carbon is obtained in a fine powder. When the white cast iron has been roasted,
and become gray, and is as malleable as the softest gray cast iron, it still affords no
graphite as the latter does, though in appearance both are alike. Yet in their properties
they are still essentially dissimilar.
With 41⁄4 per cent. of carbon, the white cast iron preserves its lamellar texture; but
with less carbon, it becomes granular and of a gray colour, growing paler as the dose
of carbon is diminished, while the metal after passing through an indefinite number
of gradations, becomes steely cast iron, very hard steel, soft steel, and steely wrought
iron.
The steels of the forge and the cast steels examined by Karsten, afforded him from[717]
2·3 to 11⁄4 per cent. of carbon; in the steel of cementation, (blistered steel) he never found
above 13⁄4 of carbon. Some wrought irons which ought to contain no charcoal, hold as
much as 1⁄2 per cent. and they then approach to steel in nature. The softest and purest
irons contain still 0·2 per cent. of carbon.
The quantity of graphite which gray cast iron contains, varies, according to Karsten’s
experiments, from 2·57 to 3·75 per cent.; but it contains besides, some carbon in
a state of alteration. The total contents in carbon varied from 3·15 to 4·65 per cent.
When the congelation of melted iron is very slow, the carbon separates, probably in
consequence of its crystallizing force, so as to form a gray cast iron replete with plumbago.
If the gray do not contain more charcoal than the white from which it has
been formed, and if it contain the charcoal in the state of mechanical mixture, then it
can have little or none in a state of combination, even much less than what some steels
contain. Hence we can account for some of its peculiarities in reference to white
cast iron; such as its granular texture, its moderate hardness, the length of time it
requires to receive annealing colours, the modifications it experiences by contact of air
at elevated temperatures, the high degree of heat requisite to fuse it, its liquidity, and
finally its tendency to rust by porosity, much faster than the white cast iron.
We thus see that carbon may combine with iron in several manners; that the gray
cast iron is a mixture of steely iron and plumbago; that the white, rendered gray and
soft by roasting, is a compound of steely iron and a carburet of iron, in which the carbon
predominates; and that untempered steel is in the same predicament.
For the following analyses of cast irons, we are indebted to MM. Gay Lussac and
Wilson.
Table.—In 100 parts.
| Cast iron. |
Iron. |
Carbon. |
Silica. |
Phos-
phorus. |
Man
ganese. |
Remarks. |
| White cast from |
Siegen |
94·338 |
2·690 |
0·230 |
0·162 |
2·590 |
By wood
charcoal |
| Do. |
Coblentz |
94·654 |
2·441 |
0·230 |
0·185 |
2·490 |
do. |
| Do. |
a. d. Champ |
96·133 |
2·324 |
0·840 |
0·703 |
a trace |
do. |
| Do. |
Isère |
94·687 |
2·636 |
0·260 |
0·280 |
2·137 |
do. |
| Gray |
Nivernais |
95·673 |
2·254 |
1·030 |
1·043 |
a trace |
do. |
| Do. |
Berry |
95·573 |
2·319 |
1·920 |
0·188 |
do |
Mix. of
coke & do. |
| Do. |
a. d. Champ |
95·971 |
2·100 |
1·060 |
0·869 |
do. |
Charcoal |
| Do. |
Creusot |
93·385 |
2·021 |
3·490 |
0·604 |
do. |
Coke |
| Do. |
a. d. Franche
Comté |
95·689 |
2·800 |
1·160 |
0·351 |
do. |
do. |
| Do. |
Wales |
94·842 |
1·666 |
3·000 |
0·492 |
do. |
do. |
| Do. |
Do. |
95·310 |
2·550 |
1·200 |
0·440 |
do. |
do. |
| Do. |
Do. |
95·150 |
2·450 |
1·620 |
0·780 |
do. |
do. |
Karsten has given the following results as to carbon, in 100 parts of gray cast
iron.
| Gray cast iron. |
Combined
carbon. |
Free
carbon. |
Total
carbon. |
Remarks. |
| Siegen, from brown iron-stone |
0·89 |
3·71 |
4·60 |
By wood charcoal |
Siegen (Widderstein), from
brown and sparry iron |
1·03 |
3·62 |
4·65 |
do. |
| Malapane, from spherosiderite |
0·75 |
3·15 |
3·90 |
do. |
| Königshütte, from brown ore |
0·58 |
2·57 |
3·15 |
coke |
| Do. at a lower smelting heat |
0·95 |
2·70 |
3·65 |
do. |
Fig. 607. represents in section, and fig. 606. in plan, the famous cupola furnace for casting
iron employed at the Royal Foundry in Berlin. It rests upon a foundation a, from 18
to 24 inches high, which supports the basement plate of cast iron, furnished with ledges,
for binding the lower ends of the upright side plates or cylinder, e. Near the mouth
there is a top-plate d, made in several pieces, which serves to bind the sides at their
upper end, as also to cover in the walls of the shaft. These plates are most readily secured
in their places by screws and bolts. Within this iron case, at a little distance
from it, the proper furnace-shaft e, is built with fire-bricks, and the space between this
and the iron is filled up with ashes. The sole of the hearth f, over the basement-plate,
is composed of a mixture of fire-clay and quartz-sand firmly beat down to the thickness
of 6 or 8 inches, with a slight slope towards the discharge-hole for running off the[718]
metal. g is the form or the tuyère (there are sometimes one on each side); h the
nose pipe; the discharge aperture i is 12 inches wide and 15 inches high; across
which the sole of the hearth is rammed down. During the melting operation, this
opening is filled up with fire-clay; when it is completed, a small hole merely is pierced
through it at the lowest point, for running off the liquid metal. The hollow shaft should
be somewhat wider at bottom than at top. Its dimensions vary with the magnitude of
the foundry. When 5 feet high, its width at the level of the tuyère or blast-hole may
be from 20 to 22 inches. From 250 to 300 cubic feet of air per minute are required
for the working of such a cupola. For running down 100 pounds of iron, after the
furnace has been brought to its heat, 48 pounds of ordinary coke are used; but with the
hot blast much less will suffice. The furnace requires feeding with alternate charges
of coke and iron every 8 or 10 minutes. The waste of iron, by oxidization and slag,
amounts in most foundries to fully 5 per cent. For carrying off the burnt air, a chimney-hood
is commonly erected over the cupola. See Foundry.
The double-arched air or wind-furnace used in the foundries of Staffordshire for
melting cast iron, has been found advantageous in saving fuel, and preventing waste by
slag. It requires fire-bricks of great size and the best composition.
The main central key-stone is constructed of large fire-bricks made on purpose; against
that key-stone the two arches press, having their abutments at the sides against the walls.
The highest point of the roof is only 8 inches above the melted metal. The sole of the
hearth is composed of a layer of sand 8 inches thick, resting upon a bed of iron or of
brickwork. The edge of the fire-bridge is only 3 inches above the fluid iron.
In from 2 to 4 hours from 1 to 3 tons of metal may be founded in such a furnace,
according to its size; but it ought always to be heated to whiteness before the iron is
introduced. 100 pounds of cast iron require from 1 to 11⁄2 cubic foot of coal to melt them.
The waste varies from 5 to 9 per cent.
I shall conclude the subject of iron with a few miscellaneous observations and statistical
tables. Previously to the discovery by Mr. Cort, in 1785, of the methods of puddling
and rolling or shingling iron, this country imported 70,000 tons of this metal from
Russia and Sweden; an enormous quantity for the time, if we consider that the cotton
and other automatic manufactures, which now consume so vast a quantity of iron, were
then in their infancy; and that two years ago, the whole of our importation from
these countries did not exceed 40,000 tons. From the following table of the prices of
bar iron in successive years, we may infer the successive rates of improvement and economy,
with slight vicissitudes.
| Years. |
Per Ton. |
| |
£ |
s. |
|
£ |
s. |
| 1824 |
9 |
0 |
to |
10 |
0 |
| 1825 |
10 |
0 |
— |
14 |
0 |
| 1826 |
8 |
10 |
— |
10 |
0 |
| 1827 |
8 |
0 |
— |
9 |
0 |
| 1828 |
7 |
10 |
— |
8 |
0 |
| 1829 |
5 |
10 |
— |
7 |
0 |
| 1830 |
5 |
5 |
— |
6 |
0 |
| 1831 |
5 |
5 |
— |
5 |
10 |
| 1832 |
5 |
0 |
— |
5 |
10 |
| 1833 |
5 |
10 |
— |
6 |
0 |
| 1834 |
6 |
0 |
— |
6 |
10 |
| 1835 |
5 |
10 |
— |
7 |
0 |
I have been informed upon good authority that the total production of iron in Great
Britain, in the year 1836, was almost exactly ONE MILLION OF TONS!
[719]
The export of iron that year, in bars, rods, pigs, castings, wire, anchors, hoops, nails,
and old iron, amounted to 189,390 tons; in unwrought steel to 3,014, and in cutlery,
to 21,072; in whole to 213,478: leaving apparently for internal consumption 776,522
tons, from which however one tenth probably should be deducted for waste, in the conversion
of the bar iron. Hence 700,000 tons may be taken as the approximate quantity
of iron made use of in the United Kingdom, in the year 1836.
The years 1835 and 1836 being those of the railway mania over the world, produced
a considerable temporary rise in the price of bar iron; but as this increased demand
caused the construction of a great many more smelting and refining furnaces, it has tended
eventually to lower the prices; an effect also to be ascribed to the more general use of
the hot blast.
The relative cost of making cast iron at Merthyr Tydvil in South Wales, and at
Glasgow, was as follows, eight or nine years ago.
| At Merthyr. |
| |
s. |
|
Tons. |
Cwts. |
Qrs. |
£ |
s. |
d. |
| Raw mine |
at |
10 |
per ton, |
3 |
7 |
0 |
1 |
13 |
6 |
| Coal |
at |
6 |
|
2 |
16 |
0 |
0 |
16 |
6 |
| Limestone |
1 |
5 |
2 |
0 |
1 |
4 |
| Other charges |
0 |
9 |
1 |
| Total Cost |
3 |
0 |
5 |
| At Glasgow. |
| |
s. |
d. |
|
Tons. |
Cwts. |
|
£ |
s. |
d. |
| Raw mine at |
4 |
6 |
|
3 |
10 |
|
0 |
16 |
3 |
| Splint Coal at |
2 |
5 |
|
5 |
15 |
|
0 |
14 |
0 |
| Limestone at |
0 |
3 |
|
0 |
14 |
|
0 |
3 |
6 |
| Coals for the engine |
1 |
10 |
|
0 |
3 |
0 |
| Other charges |
1 |
1 |
0 |
| Total cost |
2 |
17 |
9 |
The cost is still nearly the same at Merthyr, but it has been greatly decreased at
Glasgow.
The saving of fuel by the hot-blast is said to be in fact so great, that blowing
cylinders, which were adequate merely to work three furnaces at the first period, were
competent to work four furnaces at the last period. The saving of materials has moreover
been accompanied by an increase of one-fourth in the quantity of iron, in the same
time; as a furnace which turned out only 60 tons a week with the cold blast, now turns
out no less than 80 tons. That the iron so made is no worse, but probably better, when
judiciously smelted, would appear from the following statement. A considerable order
was not long since given to four iron-work companies in England, to supply pipes to
one of the London water companies. Three of these supplied pipes made from the
cold-blast iron; the fourth, it is said, supplied pipes made with the hot-blast iron. On
subjecting these several sets of pipes to the requisite trials by hydraulic pressure, the
last lot was found to stand the proof far better than any of the former three.—That
iron was made with raw coal.
I have been since told by eminent iron-masters of Merthyr, that this statement stands
in need of confirmation, or is probably altogether apocryphal, and that as they find the
hot blast weakens the iron, they will not adopt it.
Between the cast irons made in different parts of Great Britain, there are characteristic
differences. The Staffordshire metal runs remarkably fluid, and makes fine sharp
castings. The Welsh is strong, less fluent, but produces bar iron of superior quality.
The Derbyshire iron also forms excellent castings, and may be worked with care into
very good bar iron. The Scotch iron is very valuable for casting into hollow wares, as
it affords a beautiful smooth skin from the moulds, so remarkable in the castings of the
Carron company, in Stirlingshire, and of the Phœnix foundry, at Glasgow. The Shropshire
iron resembles the Staffordshire in its good qualities.
The average quantity of fine metal obtainable from the forge-pigs at Merthyr Tydvil,
from the finery furnace, is one ton for 221⁄2 cwt. of cast iron, with a consumption of about
91⁄2 cwt. of coal per ton.
[720]
Estimate of the average cost of erecting three blast furnaces.
| BUILDING EXPENSES. |
| Foundations |
£480 |
| Masonry of hewn grit-stones |
600 |
| Common bricklayers’ work |
1200 |
| Lining of the furnace, hearth, &c., in fire-bricks |
1140 |
| Fire-clay for building |
80 |
| Lime and sand |
800 |
| CAST IRON. |
| Cast-iron pieces, such as dam-plates, tymp-plates, beams, tuyère-plates, &c., weighing about 24 tons for each furnace;—in whole |
1140 |
| WROUGHT IRON. |
| For the binding-hoops, keys, &c.; 5 tons for each |
300 |
| COST OF LABOUR. |
| Bricklayers, masons, and labourers in building |
1080 |
| VARIOUS EXPENSES. |
| Scaffolding |
48 |
| Tools |
160 |
| Shed in front of each furnace |
480 |
| Terracing, cost of ground, &c. |
2400 |
| Total cost of erecting the furnaces |
9908 |
| INCIDENTAL CHARGES. |
| Blowing machinery, and steam engine of 80-horse power |
6400 |
| Inclined railway for mounting the charges |
120 |
| Gallery for charging |
160 |
| Steam engine house |
400 |
| Chimneys, boilers, &c. |
480 |
| Roasting kilns |
480 |
| Coke kilns |
800 |
| Dwelling-houses for workmen |
800 |
| Total cost of 3 furnaces complete |
£19,548 |
Estimate from the Neath-Abbey Works in S. Wales, of the cost of machines requisite for
a forge and shingling-mill, capable of turning out 120 tons of bar iron per week.
| 1. |
Steam-engine upon Bolton and Watt’s construction; of 40 inches diameter in the cylinder, and 8-feet stroke; with boilers, pipes, grate, bars, fire-doors, &c. &c., complete |
£1600 |
| 2. |
System of great-geering for transmitting the crank-motion of the engine to the mill-work, with fly-wheel, &c. |
1090 |
| 3. |
A system of roughing rolls, with pinions, uprights, and every thing else necessary |
525 |
| 4. |
Two pairs of finisher-rolls, with all their accessories |
525 |
| 5. |
Two pairs of shear-machines, at 170l. apiece |
340 |
| 6. |
One pair of rolls of 10 inches diameter, for making small bar iron, with all their accessories |
230 |
| 7. |
Forge hammer, including the anvil, the cam-shafts, and all the other requisites |
185 |
| 8. |
A complete turning lathe |
200 |
| |
£4695 |
| 9. |
To the above must be added, spare cylinders weighing about 60 tons |
960 |
| 10. |
Duplicate articles for the steam-engine |
? |
| 11. |
150 tons of cast-iron plates, to cover the floor of the mill |
900 |
| 12. |
Eight tons of cast-iron pieces for a reverberatory furnace |
52 |
| 13. |
Tools of malleable iron; rakes, oars, &c. |
28 |
| 14. |
Castings for mounting a cupola furnace |
50 |
| 15. |
Blowing-machine for the cupola |
80 |
| 16. |
Pieces of iron for a small forge, with two fires, two bellows, two anvils, iron tools faced with steel, and common iron tools, &c.[721] |
100 |
| 17. |
Eight tons of cast-iron pieces, and wrought-iron pieces for 14 puddling furnaces |
983 |
| 18. |
Seven tons of cast-iron pieces, and wrought iron for 4 re-heating furnaces |
252 |
| 19. |
Tools for the puddlers and other workmen |
15 |
| 20. |
Iron mountings for two cranes, partly made of wood |
50 |
| |
Total cost of machines, and pieces of iron |
£8165 |
| To the above, the cost of the steam engine house is to be added, that of another forge hammer, and incidental expenses. |
In Staffordshire the following estimate has been given:
| A steam-engine of 60-horse power |
2016 |
| Rolls, with the iron work of the furnaces, &c., to make 120 tons of bar iron weekly |
2572 |
| |
£4588 |
The Neath-Abbey estimate is greater, but that company has a high character for
making substantial well-finished machinery.
Bar iron made entirely from ore without admixture of cinder, or vitrified oxide, is
always reckoned worth 10s. a ton more than the average iron in the market, which is
frequently made by smelting 25 per cent. of cinder with 75 of ore or mine, as it is called.
Importation of iron in bars or unwrought, for home consumption; and amount of duty, in
| 1836. |
1837. |
1836. |
1837. |
| 18,978 tons 18 cwt. |
13,470 tons 4 cwt. |
£28,450 |
£20,065 |
M. Virlet’s Statistical Table of the produce of Iron in Europe.
| |
Quintals. |
| England (1827) |
7,098,000 |
| France (1834) |
2,200,000 |
| Russia (1834) |
1,150,000 |
| Austria (1829) |
850,000 |
| Sweden (1825) |
850,000 |
| Prussia |
800,000 |
| The Hartz Mountains |
600,000 |
| Holland and Belgium |
600,000 |
| Elba and Italy |
280,000 |
| Piedmont |
200,000 |
| Spain |
180,000 |
| Norway |
150,000 |
| Denmark |
135,000 |
| Bavaria |
130,000 |
| Saxony |
80,000 |
| Poland |
75,000 |
| Switzerland |
30,000 |
| Savoy |
25,000 |
| Total |
13,433,000 |
| (equal to about 672,000 tons.) |
For additional statistics of iron, see Pitcoal, at the end.
Bronzing of polished iron.—The barrels of fowling-pieces and rifles are occasionally
bronzed and varnished, to relieve the eye of the sportsman from the glare of a polished
metal, and to protect the surface from rusting. The liquid used for browning the barrels
is made by mixing nitric acid of specific gravity 1·2, with its own weight of spirit of nitric
ether, of alcohol, and tincture of muriate of iron; and adding to that mixture, a quantity
of sulphate of copper equal in weight to the nitric acid and ethereous spirit taken together.
The sulphate must be dissolved in water before being added; and the whole being
diluted with about 10 times its weight of water, is to be bottled up for use. This liquid
must be applied by friction with a rag to the clear barrel, which must then be rubbed
with a hard brush; processes to be alternated two or three times. The barrel should be
afterwards dipped in boiling water, rendered feebly alkaline with carbonate of potash or
soda, well dried, burnished, and heated slightly for receiving several coats of tin-smith’s
lacquer, consisting of a solution of shellac in alcohol, coloured with dragon’s blood.
ISINGLASS, or Fish-glue, called in Latin ichthyocolla, is a whitish, dry, tough, semi-transparent[722]
substance, twisted into different shapes, often in the form of a lyre, and
consisting of membranes rolled together. Good isinglass is unchangeable in the air, has
a leathery aspect, and a mawkish taste nearly insipid; when steeped in cold water it
swells, softens, and separates in membranous laminæ. At the boiling heat it dissolves
in water, and the solution, on cooling, forms a white jelly, which is semi-transparent,
soluble in weak acids, but is precipitated from them by alkalies. It is gelatine nearly
pure; and if not brittle, like other glue, this depends on its fibrous and elastic texture.
The whitest and finest is preferred in commerce. Isinglass is prepared from the air-bladders
of sturgeons, and especially the great sturgeon, the accipenser huso; which is
fished on the shores of the Caspian sea, and in the rivers flowing into it, for the sake
chiefly of its swim bladder.
The preparation of isinglass in this part of Russia, and particularly at Astracan,
consists in steeping these bladders in water, removing carefully their external coat, and
the blood which often covers them, putting them in a hempen bag, squeezing them,
softening them between the hands, and twisting them into small cylinders, which are
afterwards bent into the shape of a lyre. They are ready for the market immediately
after being dried in the sun, and whitened with the fumes of burning sulphur.
In some districts of Moldavia, another process is followed. The skin, the stomach,
the intestines, and the swim bladder of the sturgeon are cut in small pieces, steeped in
cold water, and then gently boiled. The jelly thus obtained is spread in thin layers to
dry, when it assumes the appearance of parchment. This being softened in a little
water, then rolled into cylinders, or extended into plates, constitutes an inferior article.
The swim bladder of the cod and many other fishes, also furnishes a species of isinglass,
but it is much more membranous, and less soluble than that of the sturgeon.
The properties of isinglass are the same as those of gelatine or pure glue; and its uses
are very numerous. It is employed in considerable quantities to clarify ale, wine, liqueurs,
and coffee. As an article of food to the luxurious in the preparation of creams and jellies,
it is in great request. Four parts of it convert 100 of water into a tremulous jelly,
which is employed to enrich many soups and sauces. It is used along with gum as a
dressing to give lustre to ribbons and other silk articles. The makers of artificial pearls
employ it to fix the essence d’Orient on the glass globules which form these pearls, and
the Turks set their precious stones or jewellery by means of isinglass dissolved in alcohol
along with gum ammoniac; a combination which is also employed in this country
to join broken pieces of china and glass, under the name of diamond cement. That
setting preserves its transparency after it solidifies, if it be well made.
It is by covering taffety or thin silk with a coat of isinglass that court plaster is
made. A solution of isinglass coloured with carmine forms an excellent injection liquor
to the anatomist. M. Rochen has made another pretty application of isinglass. He
plunges into a limpid solution of it, made by means of a water bath, sheets of wire gauze
set in window or lamp frames, which, when cold, have the appearance of glass, and answer
instead of it for shades and other purposes. If one dip be not sufficient to make
a proper transparent plate of isinglass, several may be given in succession, allowing each
film to harden in the interval between the dips. The outer surface should be varnished
to protect it from damp air. These panes of gelatine are now generally used for lamps
instead of horn, in the maritime arsenals of France.
Isinglass imported for home consumption; and duties paid in
| 1835. |
1836. |
1835. |
1836. |
| 1,814 cwts. |
1,735 cwts. |
£4,290 |
£4,125 |
ISLAND MOSS (Lichen d’Islande, Fr.; Flechte Isl., Germ.); is a lichen, the
Cetraria islandica, which contains a substance soluble in hot water, but forming a
jelly when it cools, styled lichenine by M. Guerin. Lichenine has a yellowish tint in
the dry state, is transparent in thin plates, insipid, inodorous, and difficult to pulverize.
Cold water makes it swell, but does not dissolve it. It is precipitated in white flocks
by alcohol and ether. Iodine tinges it of a brownish green. Sulphuric acid converts
it into sugar; and the nitric into oxalic acid. Lichenine is prepared by extracting
first of all from the plant a bitter colouring matter, by digesting 1 pound of it in 16
pounds of cold water containing 1 ounce of pearl-ash; then draining the lichen,
edulcorating with cold water, and boiling it in 9 pounds of boiling water, till 3
pounds be evaporated. The jelly which forms, upon cooling the filtered solution, is
dark coloured, but, being dried and redissolved in hot water, it becomes clear and
colourless. Lichenine consists of 39·33 carbon, 7·24 hydrogen, and 55·43 oxygen.
With potash, lime, oxide of lead, and tincture of galls, the habitudes of lichenine and
starch are the same. The mucilage of island moss is preferred in Germany to common
paste for dressing the warp of webs in the loom, because it remains soft, from its
hygrometric quality. It is also mixed with the pulp for sizing paper in the vat.
IVORY (Ivoire, Fr.; Elfenbein, Germ.); is the osseous matter of the tusks[723]
teeth of the elephant, the hippopotamus, or morse, wild boar, several species of phocæ,
as well as the horn or tooth of the narwhal. Ivory is a white, fine-grained, dense
substance, of considerable elasticity, in thin plates, and more transparent than paper
of equal thickness. The outside of the tusk is covered by the cortical part, which is
softer and less compact than the interior substance, with the exception of the brown
plate that sometimes lines the interior cavity. The hardest, toughest, whitest, and
most translucent ivory, has the preference in the market; and the tusks of the sea-horse
are considered to afford the best. In these, a rough glassy enamel covers the
cortical part, of such hardness, as to strike sparks with steel. The horn of the narwhal
is sometimes ten feet long, and consists of an ivory of the finest description, as hard
as that of the elephant, and susceptible of a better polish; but it is not in general so
much esteemed as the latter.
Ivory has the same constituents as the teeth of animals, three-fourths being phosphate,
with a little carbonate of lime; one-fourth cartilage. See Bones.
It is extensively employed by miniature painters for their tablets; by turners, in
making numberless useful and ornamental objects; by cutlers, for the handles of knives
and forks; by comb-makers; as also by philosophical instrument makers, for constructing
the scales of thermometers, &c. The ivory of the sea-horse is preferred by dentists for
making artificial teeth; that of the East India elephant is better than of the African.
When it shows cracks or fissures in its substance, and when a splinter broken off has
a dull aspect, it is reckoned of inferior value. Ivory is distinguishable from bone by
its peculiar semi-transparent rhombohedral net-work, which may be readily seen in
slips of ivory cut transversely.
Ivory is very apt to take a yellow-brown tint by exposure to air. It may be whitened
or bleached, by rubbing it first with pounded pumice-stone and water, then
placing it moist under a glass shade luted to the sole at the bottom, and exposing it
to sunshine. The sunbeams without the shade would be apt to occasion fissures in
the ivory. The moist rubbing and exposure may be repeated several times.
For etching ivory, a ground made by the following recipe is to be applied to the
polished surface:—Take of pure white wax, and transparent tears of mastick, each
one ounce; asphalt, half an ounce. The mastick and asphalt having been separately
reduced to fine powder, and the wax being melted in an earthenware vessel over the
fire, the mastick is to be first slowly strewed in and dissolved by stirring; and then the
asphalt in like manner. This compound is to be poured out into lukewarm water, well
kneaded, as it cools, by the hand, into rolls or balls about one inch in diameter. These
should be kept wrapped round with taffety. If white rosin be substituted for the
mastick, a cheaper composition will be obtained, which answers nearly as well; 2 oz.
asphalt, 1 oz. rosin, 1⁄2 oz. white wax; being good proportions. Callot’s etching ground
for copper plates, is made by dissolving with heat 4 oz. of mastick in 4 oz. of very fine
linseed oil; filtering the varnish through a rag, and bottling it for use.
Either of the two first grounds being applied to the ivory, the figured design is
to be traced through it in the usual way, a ledge of wax is to be applied, and the
surface is to be then covered with strong sulphuric acid. The effect comes better out
with the aid of a little heat; and by replacing the acid, as it becomes dilute by absorption
of moisture, with concentrated oil of vitriol. Simple wax may be employed instead
of the copperplate engravers’ ground; and strong muriatic acid instead of
sulphuric. If an acid solution of silver or gold be used for etching, the design will
become purple or black, on exposure to sunshine. The wax may be washed away with
oil of turpentine. Acid nitrate of silver affords the easiest means of tracing permanent
black lines upon ivory.
Ivory may be dyed by using the following prescriptions:—
1. Black dye.—If the ivory be laid for several hours in a dilute solution of neutral
nitrate of pure silver, with access of light, it will assume a black colour, having a
slightly green cast. A still finer and deeper black may be obtained by boiling the
ivory for some time in a strained decoction of logwood, and then steeping it in a solution
of red sulphate or red acetate of iron.
2. Blue dye.—When ivory is kept immersed for a longer or shorter time in a dilute
solution of sulphate of indigo (partly saturated with potash), it assumes a blue tint of
greater or less intensity.
3. Green dye.—This is given by dipping blued ivory for a little while in solution of
nitromuriate of tin, and then in a hot decoction of fustic.
4. Yellow dye—is given by impregnating the ivory first with the above tin mordant,
and then digesting it with heat in a strained decoction of fustic. The colour passes
into orange, if some brazil wood has been mixed with the fustic. A very fine unchangeable
yellow may be communicated to ivory by steeping it 18 or 24 hours in a
strong solution of the neutral chromate of potash, and then plunging it for some time in
a boiling hot solution of acetate of lead.
[724]
5. Red dye—may be given by imbuing the ivory first with the tin mordant, then
plunging it in a bath of brazil wood, cochineal, or a mixture of the two. Lac-dye may
be used with still more advantage, to produce a scarlet tint. If the scarlet ivory be
plunged for a little in a solution of potash, it will become cherry red.
6. Violet dye—is given in the logwood bath, to ivory previously mordanted for a
short time with solution of tin. When the bath becomes exhausted, it imparts a lilac
hue. Violet ivory is changed to purple-red by steeping it a little while in water containing
a few drops of nitro-muriatic acid.
With regard to dyeing ivory, it may in general be observed, that the colours penetrate
better before the surface is polished than afterwards. Should any dark spots appear,
they may be cleared up by rubbing them with chalk; after which the ivory should be
dyed once more to produce perfect uniformity of shade. On taking it out of the boiling
hot dye bath, it ought to be immediately plunged into cold water, to prevent the chance
of fissures being caused by the heat.
If the borings and chips of the ivory-turner, called ivory dust, be boiled in water,
a kind of fine size is obtained.
The importation of elephants’ teeth for home consumption was, in 1834, 4,282 cwts.;
in 1835, 3,698, and in 1836, 4,584 cwts.; duty, 1l. per cwt.
IVORY BLACK (Noir d’ivoire, Fr.; Kohle von Elfenbein, Germ.); is prepared
from ivory dust, by calcination in the very same way as is described under Bone
Black.
The calcined matter being ground and levigated on a porphyry slab, affords a beautiful
velvety black, much used in copperplate printing. Ivory black may be prepared
upon the small scale, by a well regulated ignition of the ivory dust in a covered
crucible.
KALI. The Arabs gave this name to an annual plant which grows near the sea-shore;
now known under the name of salsola soda, and from whose ashes they
extracted a substance, which they called alkali, for making soap. The term kali is used
by German chemists to denote caustic potash; and kalium, its metallic basis; instead
of our potassa and potassium, of preposterous pedigree, being derived from the words
pot ashes, that is ashes prepared in a pot.
KAOLIN, (Terre à porcelaine, Fr.; Porzellanerde, Germ.), is the name given by the
Chinese to the fine white clay with which they fabricate the biscuit of their porcelains.
See Clay. Berthier’s analyses of two porcelain earths are as follows:—
| Analyses. |
From Passau. |
From Saint Yriex. |
| Silica |
45 |
·06 |
46 |
·8 |
| Alumina |
32 |
·00 |
37 |
·3 |
| Lime |
0 |
·74 |
— |
| Oxide of iron |
0 |
·90 |
— |
| Potass |
— |
2 |
·5 |
| Water |
18 |
·0 |
13 |
·0 |
| |
96 |
·7 |
99 |
·6 |
KARABÉ, a name of amber, of Arabic origin, in use upon the Continent.
KELP; (Varec, Fr.; Wareck, Germ.), is the crude alkaline matter produced by
incinerating various species of fuci, or sea-weed. They are cut with sickles from the
rocks in the summer season, dried and then burned, with much stirring of the pasty
ash. I have analyzed many specimens of kelp, and found the quantity of soluble
matter in 100 parts of the best to be from 53 to 62, while the insoluble was from 47 to
38. The soluble consisted of—
| Sulphate of Soda |
8·0 |
19·0 |
| Soda in carbonate and sulphuret |
8·5 |
5·5 |
| Muriate of soda and potash |
36·5 |
37·5 |
| |
53·0 |
62·0 |
[725]
The insoluble matter consisted of—
| Carbonate of lime |
24·0 |
10·0 |
| Silica |
8·0 |
0·0 |
| Alumina tinged with iron oxide |
9·0 |
10·0 |
| Sulphate of lime |
0·0 |
9·5 |
| Sulphur and loss |
6·0 |
8·5 |
| |
100·0 |
100·0 |
The first of these specimens was from Heisker, the second from Rona, both in the
isle of Skye, upon the property of Lord Macdonald. From these, and many other
analyses which I have made, it appears that kelp is a substance of very variable composition,
and hence it was very apt to produce anomalous results, when employed as
the chief alkaline flux of crown glass, which it was for a very long period. The fucus
vesiculosus and fucus nodosus are reckoned to afford the best kelp by incineration; but
all the species yield a better product when they are of two or three years growth, than
when cut younger. The varec, made on the shores of Normandy, contains almost no
carbonate of soda, but much sulphate of soda and potash, some hyposulphate of potash,
chloride of sodium, iodide of potassium, and chloride of potassium; the average composition
of the soluble salts being, according to M. Gay Lussac, 56 of chloride of sodium,
25 of chloride of potassium, and a little sulphate of potash. The very low price at which
soda ash, the dry crude carbonate from the decomposition of sea salt, is now sold, has
nearly superseded the use of kelp, and rendered its manufacture utterly unprofitable—a
great misfortune to the Highlands and Islands of Scotland.
KERMES. There are two substances so called, of totally different natures. Kermes
mineral is merely a factitious sulphuret of antimony in a state of impalpable comminution,
prepared in the moist way. Its minute examination belongs to pharmaceutical chemistry.
It may be obtained perfectly pure, by diluting the proto-chloride of antimony
with solution of tartaric acid, and precipitating the metal with sulphuretted hydrogen;
or by exposing the finely levigated native sulphuret to a boiling solution of carbonate of
potash for some time, and filtering the liquor while boiling hot. The kermes falls
down in a brown-red powder, as the liquor cools.
Kermes-grains, alkermes, are the dried bodies of the female insects of the species coccus
ilicis, which lives upon the leaves of the quercus ilex (prickly oak). The word kermes
is Arabic, signifies little worm. In the middle ages, this dye stuff was therefore
called vermiculus in Latin, and vermillion in French. It is curious to consider how the
name vermillion has been since transferred to red sulphuret of mercury.
Kermes has been known in the East since the days of Moses; it has been employed
from time immemorial in India to dye silk; and was used also by the ancient Greek
and Roman dyers. Pliny speaks of it under the name of coccigranum, and says that
there grew upon the oak in Africa, Sicily, &c. a small excrescence like a bud, called
cusculium; that the Spaniards paid with these grains, half of their tribute to the
Romans; that those produced in Sicily were the worst; that they served to dye
purple; and that those from the neighbourhood of Emerita in Lusitania (Portugal)
were the best.
In Germany, during the ninth, twelfth, thirteenth, and fourteenth centuries, the rural
serfs were bound to deliver annually to the convents, a certain quantity of kermes,
the coccus polonicus, among the other products of husbandry. It was collected from the
trees upon Saint John’s day, between eleven o’clock and noon, with religious ceremonies,
and was therefore called Johannisblut, (Saint John’s blood), as also German cochineal.
At the above period, a great deal of the German kermes was consumed in Venice, for
dyeing the scarlet to which that city gives its name. After the discovery of America,
cochineal having been introduced, began to supersede kermes for all brilliant red dyes.
The principal varieties of kermes are the coccus quercus, the coccus polonicus, the
coccus fragariæ, and the coccus uva ursi.
The coccus quercus insect lives in the south of Europe upon the kermes oak. The
female has no wings, is of the size of a small pea, of a brownish-red colour, and is
covered with a whitish dust. From the middle of May to the middle of June the eggs
are collected, and exposed to the vapour of vinegar, to prevent their incubation. A
portion of eggs is left upon the tree for the maintenance of the brood. In the
department of the Bouches-du-Rhone, one half of the kermes crop is dried. It amounts
annually to about 60 quintals or cwts., and is warehoused at Avignon.
The kermes of Poland, or coccus polonicus, is found upon the roots of the scleranthus
perennis and the scleranthus annuus, in sandy soils of that country and the Ukraine. This
species has the same properties as the preceding; one pound of it, according to Wolfe,
being capable of dyeing 10 pounds of wool; but Hermstaedt could not obtain a fine
colour, although he employed 5 times as much of it as of cochineal. The Turks, Armenians,[726]
and Cossacks, dye with kermes, their morocco leather, cloth, silk, as well as the
manes and tails of their horses.
The kermes called coccus fragariæ, is found principally in Siberia, upon the root of
the common strawberry.
The coccus uva ursi is twice the size of the Polish kermes, and dyes with alum a fine
red. It occurs in Russia.
Kermes is found not only upon the lycopodium complanatum in the Ukraine, but upon
a great many other plants.
Good kermes is plump, of a deep red colour, of an agreeable smell, and a rough and
pungent taste. Its colouring matter is soluble in water and alcohol; it becomes yellowish
or brownish with acids, and violet or crimson with alkalis. Sulphate of iron
blackens it. With alum it dyes a blood-red; with copperas an agate gray; with copperas
and tartar, a lively gray; with sulphate of copper and tartar, an olive green; with
tartar and salt of tin, a lively cinnamon yellow; with more alum and tartar, a lilac; with
sulphate of zinc and tartar, a violet. Scarlet and crimson dyed with kermes, were called
grain colours; and they are reckoned to be more durable than those of cochineal, as is
proved by the brilliancy of the old Brussels tapestry.
Hellot says that previous to dyeing in the kermes bath, he threw a handful of wool
into it, in order to extract a blackish matter, which would have tarnished the colour.
The red caps for the Levant are dyed at Orleans with equal parts of kermes and madder;
and occasionally with the addition of some Brazil wood.
Cochineal and lac-dye have now nearly superseded the use of kermes as a tinctorial
substance, in England.
KILLAS, is the name by which clay-slate is known among the Cornish miners.
KILN; (Four, Fr.; Ofen, Germ.) is the name given to various forms of furnaces
and stoves, by which an attempered heat may be applied to bodies; thus there are brick-kilns,
hop-kilns, lime-kilns, malt-kilns, pottery-kilns. Hop and malt kilns, being
designed merely to expel the moisture of the vegetable matter, may be constructed in
the same way. See Brick, Limestone, Malt, Pottery, for a description of their
respective kilns.
KINIC ACID; a peculiar acid extracted by Vauquelin from cinchona.
KINO, is an extractive matter obtained from the nauclea gambir, a shrub which
grows at Bancoul and Sumatra, but principally in Prince of Wales’ Island. It is of a
reddish-brown colour, has a bitter styptic taste, and consists chiefly of tannin. It is
used only as an astringent in medicine. Kino is often called a gum, but most improperly.
KIRSCHWASSER, is an alcoholic liquor obtained by fermenting and distilling
bruised cherries, called kirschen in German. The cherry usually employed in Switzerland
and Germany is a kind of morello, which on maturation becomes black, and has a
kernel very large in proportion to its pulp. When ripe, the fruit being made to fall by
switching the trees, is gathered by children, thrown promiscuously, unripe, ripe, and
rotten into tubs, and crushed either by hand, or with a wooden beater. The mashed
materials are set to ferment, and whenever this process is complete, the whole is transferred
to an old still covered with verdigris, and the spirit is run off in the rudest manner
possible, by placing the pot over the common fire-place.
The fermented mash is usually mouldy before it is put into the alembic, the capital
of which is luted on with a mixture of mud and dung. The liquor has accordingly,
for the most part, a rank smell, and is most dangerous to health, not only from its own
crude essential oil, but from the prussic acid, derived from the distillation of the cherry-stones.
There is a superior kind of kirschwasser made in the Black Forest, prepared with
fewer kernels, from choice fruit, properly pressed, fermented, and distilled.
KNOPPERN, are excrescences produced by the puncture of an insect upon the
flower-cups of several species of oak. They are compressed or flat, irregularly pointed,
generally prickly and hard; brown when ripe. They abound in Styria, Croatia, Sclavonia,
and Natolia; those from the latter country being the best. They contain a great deal
of tannin, are much employed in Austria for tanning, and in Germany for dyeing fawn,
gray, and black. Wool, with a mordant of sulphate of zinc, takes a grayish nankeen
colour. See Galls.
KOUMISS, is the name of a liquor which the Calmucs make by fermenting mare’s
milk, and from which they distil a favourite intoxicating spirit, called rack or racky.
Cow’s milk is said to produce only one third as much spirit, from its containing probably
less saccharine matter.
The milk is kept in bottles made of hides, till it becomes sour, is shaken till it casts
up its cream, and is then set aside in earthen vessels in a warm place to ferment, no
yeast being required, though sometimes a little old koumiss is added. 21 pounds of
milk put into the still afford 14 ounces of low wines, from which 6 ounces of pretty
strong alcohol, of an unpleasant flavour, are obtained by rectification.
[727]
LABDANUM or Ladanum, is an unctuous resin, of an agreeable odour, found besmearing
the leaves and twigs of the cystus creticus, a plant which grows in the island of
Candia, and in Syria. It is naturally a dark-brown soft substance, but it hardens on
keeping. Its specific gravity is 1·186. It has a bitter taste. Its chief use is in surgery
for making plasters.
LABRADORITE; opaline or Labradore felspar, is a beautiful mineral, with brilliant
changing colours, blue, red, and green, &c. Spec. grav. 2·70 to 2·75. Scratches
glass; affords no water by calcination; fusible at the blow-pipe into a frothy bead;
soluble in muriatic acid; solution affords a copious precipitate with oxalate of ammonia.
Cleavages of 931⁄2° and 861⁄2°; one of which is brilliant and pearly. Its constituents are,
silica, 55·75; alumina, 26·5; lime, 11; soda, 4; oxide of iron, 1·25; water, 0·5.
LABYRINTH, in metallurgy, means a series of canals distributed in the sequel
of a stamping-mill; through which canals a stream of water is transmitted for suspending,
carrying off, and depositing, at different distances, the ground ores. See
Metallurgy.
LAC, LAC-DYE. (Laque, Fr.; Lack, Lackfarben, Germ.) Stick-lac is produced
by the puncture of a peculiar female insect, called coccus lacca or ficus, upon the
branches of several plants; as the ficus religiosa, the ficus indica, the rhamnus jujuba, the
croton lacciferum, and the butea frondosa, which grow in Siam, Assam, Pegu, Bengal,
and Malabar. The twig becomes thereby encrusted with a reddish mammelated resin,
having a crystalline-looking fracture.
The female lac insect is of the size of a louse; red, round, flat, with 12 abdominal
circles, a bifurcated tail, antennæ, and 6 claws, half the length of the body. The male is
twice the above size, and has 4 wings; there is one of them to 5000 females. In November
or December the young brood makes its escape from the eggs, lying beneath the dead
body of the mother; they crawl about a little way, and fasten themselves to the bark of
the shrubs. About this period the branches often swarm to such a degree with this vermin,
that they seem covered with a red dust; in this case, they are apt to dry up,
by being exhausted of their juices. Many of these insects, however, become the
prey of others, or are carried off by the feet of birds, to which they attach themselves,
and are transplanted to other trees. They soon produce small nipple-like incrustations
upon the twigs, their bodies being apparently glued, by means of a transparent
liquor, which goes on increasing to the end of March, so as to form a cellular texture.
At this time, the animal resembles a small oval bag, without life, of the size of cochineal.
At the commencement, a beautiful red liquor only is perceived, afterwards eggs make
their appearance; and in October or November, when the red liquor gets exhausted, 20
or 30 young ones bore a hole through the back of their mother, and come forth. The
empty cells remain upon the branches. These are composed of the milky juice of the
plant, which serves as nourishment to the insects, and which is afterwards transformed
or elaborated into the red colouring matter that is found mixed with the resin, but
in greater quantity in the bodies of the insects, in their eggs, and still more copiously
in the red liquor secreted for feeding the young. After the brood escapes, the cells
contain much less colouring matter. On this account, the branches should be broken
off before this happens, and dried in the sun. In the East Indies this operation is
performed twice in the year; the first time in March, the second in October. The
twigs encrusted with the radiated cellular substance, constitute the stick-lac of commerce.
It is of a red colour more or less deep, nearly transparent, and hard, with a brilliant
conchoidal fracture. The stick-lac of Siam is the best; a piece of it presented to me by
Mr. Rennie, of Fenchurch-street, having an incrustation fully one quarter of an inch
thick all round the twig. The stick-lac of Assam ranks next; and, last, that of Bengal,
in which the resinous coat is scanty, thin, and irregular. According to the analysis of
Dr. John, stick-lac consists, in 120 parts, of
| An odorous common resin |
80·00 |
| A resin insoluble in ether |
20·00 |
| Colouring matter analogous to that of cochineal |
4·50 |
| Bitter balsamic matter |
3·00 |
| Dun yellow extract |
0·50 |
| Acid of the stick-lac (laccic acid) |
0·75 |
| Fatty matter, like wax |
3·00 |
| Skins of the insects, and colouring matter |
2·50 |
| Salts[728] |
1·25 |
| Earths |
0·75 |
| Loss |
4·75 |
| |
120·00 |
According to Franke, the constituents of stick-lac are, resin, 65·7; substance of the
lac, 28·3; colouring matter, 0·6.
Seed-lac.—When the resinous concretion is taken off the twigs, coarsely pounded,
and triturated with water in a mortar, the greater part of the colouring matter is dissolved,
and the granular portion which remains being dried in the sun, constitutes
seed-lac. It contains of course less colouring matter than the stick-lac, and is much less
soluble. John found in 100 parts of it, resin, 66·7; wax, 1·7; matter of the lac, 16·7;
bitter balsamic matter, 2·5; colouring matter, 3·9; dun yellow extract, 0·4; envelopes
of insects, 2·1; laccic acid, 0·0; salts of potash and lime, 1·0; earths, 6·6; loss, 4·2.
In India the seed-lac is put into oblong bags of cotton cloth, which are held over a
charcoal fire by a man at each end, and, as soon as it begins to melt, the bag is twisted so
as to strain the liquefied resin through its substance, and to make it drop upon smooth
stems of the banyan tree (musa paradisa). In this way, the resin spreads into thin
plates, and constitutes the substance known in commerce by the name of shell-lac.
The Pegu stick-lac, being very dark coloured, furnishes a shell-lac of a corresponding
deep hue, and therefore of inferior value. The palest and finest shell-lac is brought
from the northern Circar. It contains very little colouring matter. A stick-lac of an
intermediate kind comes from the Mysore country, which yields a brilliant lac-dye and
a good shell-lac.
Lac-dye is the watery infusion of the ground stick-lac, evaporated to dryness, and
formed into cakes about two inches square and half an inch thick. Dr. John found it
to consist of, colouring matter, 50; resin, 25; and solid matter, composed of alumina,
plaster, chalk, and sand, 22.
Dr. Macleod, of Madras, informs me that he prepared a very superior lac-dye from
stick-lac, by digesting it in the cold in a slightly alkaline decoction of the dried leaves
of the Memecylon tinctorium (perhaps the M. capitellatum, from which the natives of
Malabar and Ceylon obtain a saffron-yellow dye). This solution being used along with
a mordant consisting of a saturated solution of tin in muriatic acid, was found to dye
woollen cloth of a very brilliant scarlet hue.
The cakes of lac-dye imported from India, stamped with peculiar marks to designate
their different manufacturers, are now employed exclusively in England for dyeing
scarlet cloth, and are found to yield an equally brilliant colour, and one less easily
affected by perspiration than that produced by cochineal. When the lac-dye was first
introduced, sulphuric acid was the solvent applied to the pulverized cakes, but as muriatic
acid has been found to answer so much better, it has entirely supplanted it. A
good solvent (No. 1.) for this dye-stuff may be prepared by dissolving 3 pounds of tin
in 60 pounds of muriatic acid, of specific gravity 1·19. The proper mordant for the
cloth is made by mixing 27 pounds of muriatic acid of sp. grav. 1·17, with 11⁄2 pounds
of nitric acid of 1·19; putting this mixture into a salt-glazed stone bottle, and adding
to it in small bits at a time, grain tin, till 4 pounds be dissolved. This solution (No. 2.)
may be used within twelve hours after it is made, provided it has become cold and clear.
For dyeing; three quarters of a pint of the solvent No. 1. is to be poured upon each
pound of the pulverized lac-dye, and allowed to digest upon it for six hours. The cloth
before being subjected to the dye bath, must be scoured in the mill with fullers’ earth.
To dye 100 pounds of pelisse cloth, a tin boiler of 300 gallons capacity should be filled
nearly brimful with water, and a fire kindled under it. Whenever the temperature
rises to 150° Fahr., a handful of bran, and half a pint of the solution of tin (No. 2.)
are to be introduced. The froth, which rises as it approaches ebullition, must be
skimmed off; and when the liquor boils, 101⁄2 pounds of lac-dye, previously mixed with 7
pints of the solvent No. 1., and 31⁄2 pounds of solution of tin No. 2., must be poured in.
An instant afterwards, 101⁄2 pounds of tartar, and 4 pounds of ground sumach, both tied up
in a linen bag, are to be suspended in the boiling bath for five minutes. The fire being
now withdrawn, 20 gallons of cold water, with 101⁄2 pints of solution of tin being poured
into the bath, the cloth is to be immersed in it, moved about rapidly during ten minutes;
the fire is to be then re-kindled, and the cloth winced more slowly through the bath,
which must be made to boil as quickly as possible, and maintained at that pitch for an
hour. The cloth is to be next washed in the river; and lastly with water only, in the
fulling mill. The above proportions of the ingredients produce a brilliant scarlet
tint, with a slightly purple cast. If a more orange hue be wanted, white Florence argal
may be used, instead of tartar, and some more sumach. Lac-dye may be substituted for
cochineal in the orange-scarlets; but for the more delicate pink shades, it does not[729]
answer so well, as the lustre is apt to be impaired by the large quantity of acid necessary
to dissolve the colouring matter of the lac.
Shell-lac, by Mr. Hatchett’s analysis, consists of resin, 90·5; colouring matter, 0·5;
wax, 4·0; gluten, 2·8; loss, 1·8; in 100 parts.
The resin may be obtained pure by treating shell-lac with cold alcohol, and filtering
the solution in order to separate a yellow gray pulverulent matter. When the alcohol
is again distilled off, a brown, translucent, hard, and brittle resin, of specific gravity
1·139, remains. It melts into a viscid mass with heat, and diffuses an aromatic
odour. Anhydrous alcohol dissolves it in all proportions. According to John, it consists
of two resins, one of which dissolves readily in alcohol, ether, the volatile and
fat oils; while the other is little soluble in cold alcohol, and is insoluble in ether and
the volatile oils. Unverdorben, however, has detected no less than four different resins,
and some other substances in shell-lac. Shell-lac dissolves with ease in dilute muriatic
and acetic acids; but not in concentrated sulphuric acid. The resin of shell-lac
has a great tendency to combine with salifiable bases; as with caustic potash, which it
deprives of its alkaline taste.
This solution, which is of a dark red colour, dries into a brilliant, transparent, reddish
brown mass; which may be re-dissolved in both water and alcohol. By passing
chlorine in excess through the dark-coloured alkaline solution, the lac-resin is precipitated
in a colourless state. When this precipitate is washed and dried, it forms, with alcohol,
an excellent pale-yellow varnish, especially with the addition of a little turpentine and
mastic.
With the aid of heat, shell-lac dissolves readily in a solution of borax.
The substances which Unverdorben found in shell-lac are the following:
1. A resin, soluble in alcohol and ether;
2. A resin, soluble in alcohol, insoluble in ether;
3. A resinous body, little soluble in cold alcohol;
4. A crystallizable resin;
5. A resin, soluble in alcohol and ether, but insoluble in petroleum, and uncrystallizable.
6. The unsaponified fat of the coccus insect, as well as oleic and margaric acids.
7. Wax.
8. The laccine of Dr. John.
9. An extractive colouring matter.
Statistical Table of Lac-dye and Lac-lake, per favour of James Wilkinson, Esq.,
of Leadenhall-street.
| |
Import. |
Export. |
Home
Consump-
tion. |
Prices. |
Stocks. |
| |
lbs. |
lbs. |
lbs. |
s. |
d. |
s. |
d. |
Chests. |
| 1802 |
253 |
none |
none |
|
|
| 1803 |
1,735 |
|
accot.
burned |
|
|
| 1804 |
531 |
|
|
|
|
| 1805 |
1,987 |
|
|
|
|
| 1806 |
none |
|
|
|
|
| 1807 |
25,350 |
|
|
|
|
| 1808 |
5,731 |
|
|
|
|
| 1809 |
40,632 |
|
|
|
|
| 1810 |
235,154 |
|
|
|
|
| 1811 |
378,325 |
|
|
|
|
| 1812 |
198,250 |
|
|
|
|
| 1813 |
289,654 |
|
|
|
|
| 1814 |
278,899 |
5,071 |
133,935 |
|
|
| 1815 |
598,592 |
8,441 |
137,915 |
|
|
| 1816 |
269,373 |
27,412 |
162,894 |
|
|
| 1817 |
384,909 |
23,091 |
234,763 |
|
|
| 1818 |
242,572 |
32,079 |
323,169 |
|
|
| 1819 |
179,511 |
21,707 |
207,063 |
|
|
| 1820 |
441,486 |
49,519 |
912,514 |
|
|
| 1821 |
641,755 |
91,925 |
322,837 |
|
|
| 1822 |
872,967 |
29,578 |
349,351 |
|
|
| 1823 |
534,220 |
13,050 |
414,714 |
|
|
| 1824 |
604,269 |
53,843 |
483,339 |
|
|
| 1825 |
541,443 |
61,908 |
385,734 |
|
|
| 1826 |
760,729 |
68,603 |
395,609 |
|
|
| 1827[730] |
756,315 |
76,875 |
448,270 |
1 |
9 |
4 |
0 |
11,538 |
| 1828 |
512,874 |
54,999 |
397,867 |
1 |
3 |
3 |
9 |
11,085 |
| 1829 |
475,632 |
39,344 |
433,851 |
1 |
3 |
3 |
6 |
11,976 |
| 1830 |
534,341 |
78,099 |
548,865 |
0 |
9 |
3 |
3 |
11,834 |
| 1831 |
913,562 |
175,717 |
597,568 |
0 |
4 |
2 |
6 |
12,559 |
| 1832 |
378,843 |
69,842 |
594,155 |
0 |
4 |
2 |
3 |
11,420 |
| 1833 |
326,894 |
66,447 |
426,460 |
0 |
9 |
2 |
4 |
11,457 |
| 1834 |
708,959 |
89,229 |
398,832 |
0 |
11 |
2 |
4 |
11,928 |
| 1835 |
528,564 |
203,840 |
573,288 |
0 |
11 |
3 |
0 |
10,454 |
| 1836 |
642,436 |
200,975 |
642,615 |
1 |
0 |
4 |
0 |
9,492 |
| 1837 |
1,011,674 |
133,959 |
427,890 |
1 |
0 |
3 |
9 |
8,780 |
| The Stock includes 2,200 chests of Lac-lake. |
LACCIC ACID crystallizes, has a wine-yellow colour, a sour taste, is soluble in
water, alcohol, and ether. It was extracted from stick-lac by Dr. John.
LACCINE is the portion of shell-lac which is insoluble in boiling alcohol. It is
brown, brittle, translucid, consisting of agglomerated pellicles, more like a resin than any
thing else. It is insoluble in ether and oils. It has not been applied to any use.
LACE MANUFACTURE. The pillow-made, or bone-lace, which formerly gave
occupation to multitudes of women in their own houses, has, in the progress of mechanical
invention, been nearly superseded by the bobbin-net lace, manufactured at first
by hand-machines, as stockings are knit upon frames, but recently by the power of
water or steam. This elegant texture possesses all the strength and regularity of the
old Buckingham lace, and is far superior in these respects to the point-net and warp
lace, which had preceded, and in some measure paved the way for it. Bobbin-net
may be said to surpass every other branch of human industry in the complex
ingenuity of its machinery; one of Fisher’s spotting frames being as much beyond the
most curious chronometer in multiplicity of mechanical device, as that is beyond a
common roasting-jack.
The threads in bobbin-net lace form, by their intertwisting and decussation, regular
hexagonal holes or meshes, of which the two opposite sides, the upper and under, are
directed along the breadth of the piece, or at right angles to the selvage or border.
Fig. 608. shows how, by the crossing and
twisting of the threads, the regular six-sided
mesh is produced, and that the texture
results from the union of three separate
sets of threads, of which one set proceed
downwards in serpentine lines, a second
set proceeds from the left to the
right, and a third from the right to the
left, both in slanting directions. These
oblique threads twist themselves round the
vertical ones, and also cross each other betwixt
them, in a peculiar manner, which
may be readily understood by examining
the representation. In comparing bobbin-net
with a common web, the perpendicular
threads in the figure, which are parallel to
the border, may be regarded as the warp,
and the two sets of slanting threads, as the
weft.
These warp threads are extended up and down, in the original mounting of the piece
between a top and bottom horizontal roller or beam, of which one is called the warp
beam, and the other the lace beam, because the warp and finished lace are wound upon
them respectively. These straight warp threads receive their contortion from the tension
of the weft threads twisted obliquely round them alternately to the right and the left
hand. Were the warp threads so tightly drawn that they became inflexible, like
fiddle-strings, then the lace would assume the appearance shown in fig. 609.; and
although this condition does not really exist, it may serve to illustrate the structure of[731]
the web. The warp threads stand in the positions a a, a′ a′, and a′′ a′′; the one half
of the weft proceeds in the direction b b, b′ b′ and b′′ b′′; and the second crosses the
first by running in the direction c c, or
c′ c′, towards the opposite side of the fabric.
If we pursue the path of a weft
thread, we find it goes on till it reaches
the outermost or last warp thread, which it
twists about; not once, as with the others,
but twice; and then returning towards the
other border, proceeds in a reverse direction.
It is by this double twist, and
by the return of the weft threads, that the
selvage is made.
The ordinary material of bobbin-net is
two cotton yarns, of from No. 180. to No.
250., twisted into one thread; but sometimes
strongly twisted single yarn has been
used. The beauty of the fabric depends
upon the quality of the material, as well as
the regularity and smallness of the meshes.
The number of warp threads in a yard in
breadth is from 600 to 900; which is equivalent to from 20 to 30 in an inch. The size
of the holes cannot be exactly inferred from that circumstance, as it depends partly
upon the oblique traction of the threads. The breadth of the pieces of bobbin-net
varies from edgings of a quarter of an inch, to webs 12, or even 20 quarters, that is, 5
yards wide.
Bobbin-net lace is manufactured by means of very costly and complicated machines,
called frames. The limits of this Dictionary will admit of an explanation of no more
than the general principles of the manufacture. The threads for crossing and twisting
round the warp, being previously gassed, that is, freed from loose fibres by singeing with
gas, are wound round small pulleys, called bobbins, which are, with this view, deeply
grooved in their periphery. Figs. 610, 611. exhibit the bobbin alone, and with its carriage.
In the section of the bobbin a, fig. 610., the deep groove is shown in which the thread is
wound. The bobbin consists of two thin discs of brass, cut out in a stamp-press, in the
middle of each of which there is a hollow space c. These discs are riveted together, leaving
an interval between their edge all round, in which the thread is coiled. The round hole
in the centre, with the little notch at top, serves for spitting them upon a feathered rod,
in order to be filled with thread by the rotation of that rod in a species of reel, called
the bobbin-filling machine. Each of these bobbins (about double the size of the
figure), is inserted into the vacant space G of the carriage, fig. 611. This is a small
iron frame (also double the size of the figure), which, at e e, embraces the grooved
border of the bobbin, and by the pressure of the spring at f, prevents it from falling out.
This spring serves likewise to apply sufficient friction to the bobbin, so as to prevent it
from giving off its thread at g by its rotation, unless a certain small force of traction be
employed upon the thread. The curvilinear groove h h, sunk in each face or side of
the carriage, has the depth shown in the section at h. This groove corresponds to the
interval between the teeth of the comb, or bars of the bolt, in which each carriage is
placed, and has its movement. A portion of that bolt or comb is shown at a, fig. 612.
in plan, and one bar of a circular bolt machine at b, in section. If we suppose two
such combs or bolts placed with the ends of the teeth opposite each other, but a little
apart, to let the warp threads be stretched, in one vertical plane, between their ends or
tips, we shall have an idea of the skeleton of a bobbin-net machine. One of these two[732]
combs, in the double bolt machine, has an occasional lateral movement called shogging,
equal to the interval of one tooth or bolt, by which, after it has received the bobbins,
with their carriages, into its teeth, it
can shift that interval to the one
side, and thereby get into a position
to return the bobbins, with their
carriages, into the next series of interstices
or gates, in the other bolt.
By this means the whole series of
carriages receives successive side
steps to the right in one bolt, and to
the left in the other, so as to perform
a species of countermarch, in
the course of which they are made
to cross and twist round about the
vertical warp threads, and thus to
form the meshes of the net.
The number of movements required
to form a row of meshes in
the double tier machine, that is, in a
frame with two combs or bars, and
2 rows of bobbins, is six; that is,
the whole of the carriages (with their bobbins) pass from one bar or comb to the other
six times, during which passages the different divisions of bobbin and warp threads
change their relative positions 12 times.
This interchange or traversing of the carriages with their bobbins, which is the most
difficult thing to explain, but at the same time the most essential principle of the lace-machine,
may be tolerably well understood by a careful study of fig. 613., in which the
simple line  represents the bolts or teeth, the sign
represents the bolts or teeth, the sign  the back line of carriages, and the
sign
the back line of carriages, and the
sign 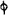 the front line of carriages. H is the front comb or bolt bar, and I the back bolt
bar. The former remain is always fixed or stationary, to receive the carriages as they
may be presented to it by the shogging of the latter. There must be always one odd
carriage at the end; the rest being in pairs.
the front line of carriages. H is the front comb or bolt bar, and I the back bolt
bar. The former remain is always fixed or stationary, to receive the carriages as they
may be presented to it by the shogging of the latter. There must be always one odd
carriage at the end; the rest being in pairs.
No. 1. represents the carriages in the front comb or bar, the odd carriage being at the
left end. The back line of carriages is first moved on to the back bar I, the odd
carriage, as seen in No. 1., having been left behind, there being no carriage opposite to
drive it over to the other comb or bar. The carriages then stand as in No. 2. The bar
I now shifts to the left, as shown in No. 3.; the front carriages then go over into the
back bar or comb, as is represented by No. 4. The bar I now shifts to the right, and
gives the position No. 5. The front carriages are then driven over to the front bar, and
leave the odd carriage on the back bar at the right end, for the same reason as before
described, and the carriages stand as shown in No. 6. The bar I next shifts to the
left, and the carriages stand as in No. 7. (the odd carriage being thereby on the back
bar to the left.) The back carriages now come over to the front bar, and stand as in
No. 8. The back bar or comb I shifts to the right as seen in No. 9., which completes
the traverse. The whole carriages with their bobbins have now changed their
position, as will be seen by comparing No. 9. with No. 1. The odd carriage, No. 1.
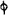 has advanced one step to the right, and has become one of the front tier; one of
the back tier or line
has advanced one step to the right, and has become one of the front tier; one of
the back tier or line 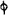 has advanced one step to the left, and has become the odd
carriage; and one of the front ones
has advanced one step to the left, and has become the odd
carriage; and one of the front ones 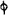 has gone over to the back line. The bobbins
and carriages throughout the whole width of the machine have thus crossed each other’s
course, and completed the mesh of net.
has gone over to the back line. The bobbins
and carriages throughout the whole width of the machine have thus crossed each other’s
course, and completed the mesh of net.
The carriages with their bobbins are driven a certain way from the one comb to
the other, by the pressure of two long bars (one for each) placed above the level of
the comb, until they come into such a position that their projecting heels or catches
i i, fig. 611., are moved off by two other long flat bars below, called the locker plates,
and thereby carried completely over the interval between the two combs.
There are six different systems of bobbin-net machines. 1. Heathcoate’s patent[733]
machine. 2. Brown’s traverse warp. 3. Morley’s straight bolt. 4. Clarke’s pusher
principle, single tier. 5. Leaver’s machine, single tier. 6. Morley’s circular bolt. All
the others are mere variations in the construction of some of their parts. It is a
remarkable fact, highly honourable to the mechanical judgment of Mr. Morley of Derby,
that no machines except those upon his circular bolt principle, have been found capable
of working successfully by mechanical power.
The circular bolt machine (comb with curved teeth) was used by Mr. Morley, for
making narrow breadths or edgings of lace immediately after its first invention, and
it has been regularly used by the trade for that purpose ever since, in consequence of
the inventor having declined to secure the monopoly of it to himself by patent. At
that time the locker bars for driving across the carriages had only one plate or blade.
A machine so mounted is now called “the single locker circular bolt.” In the year
1824, Mr. Morley added another plate to each of the locker bars, which was a great
improvement on the machines for making plain net, but an obstruction to the making
of narrow breadths upon them. This machine is now distinguished from the former by
the term “double locker.”[31]
A rack of lace, is a certain length of work counted perpendicularly, and contains
240 meshes or holes. Well-made lace has the meshes a little elongated in the direction
of the selvage.
The term gauge, in the lace manufacture, means the number of gates, slits, or interstices,
in one inch of the bolt-bar or comb; and corresponds therefore to the number
of bobbins in an inch length of the double tier. Thus, when we say “gauge
nine points,” we mean that there are nine gates with nine bobbins in one inch of the
comb or bolt-bar. Each of such bobbins with its carriage is therefore no more than
one ninth of an inch thick. The common proportion or gauge up and down the machine
is 16 holes in the inch for ten bobbins transversely. Circular bolt double tier
machines can turn off by steam power fully 360 racks each day of 18 hours, with a
relay of superintendents.
The number of new mechanical contrivances to which this branch of manufacture has
given rise, is altogether unparalleled in any other department of the arts. Since
Mr. Heathcoate’s first successful patent, in 1809, a great many other patents have
been granted for making lace. In the year 1811, Mr. Morley, then of Nottingham,
invented his straight bolt frame, more simple in construction, better combined, and
more easy in its movements, than the preceding machines; but the modest inventor
did not secure it, as he might have done, by patent. The pusher machine was invented
in the same year, by Samuel Mart and James Clark, also of Nottingham. The
following year is remarkable in the history of the lace trade, for the invention of the
circular bolt machine, by Mr. Morley—a mechanism possessing all the advantages of
his straight bolt machine, without its disadvantages.
Nearly at the same time Mr. John Leaver brought forward the lever machine, conjointly
with one Turton, both of New Radford, near Nottingham. About the year
1817, or 1818, Mr. Heathcoate applied the rotatory movement to the circular bolt machine,
and mounted a manufactory on that plan, by mechanical power, at Tiverton, after
he and his partner, Mr. Boden, had been driven from Loughborough, in 1816, by the
atrocious violence of the frame-destroying Luddites.
Such has been the progress of improvement and economy in this manufacture, that
the cost of labour in making a rack, which was, twenty years ago, 3s. 6d., or 42 pence,
is now not more than one penny. The prices of this beautiful fabric have fallen in an
equally remarkable manner. At the former period, a 24 rack piece, five quarters broad,
fetched 17l. sterling, in the wholesale market; the same is now sold for 7s.! The
consequence is, that in lace decoration, the maid servant may now be more sumptuously
arrayed than her mistress could afford to be twenty years ago.
LACQUER, is a varnish, consisting chiefly of a solution of pale shell-lac in alcohol,
tinged with saffron, annotto, or other colouring matters. See Varnish.
LACTIC ACID. (Acide Lactique, Fr.; Milchsäure, Germ.) This acid was discovered
by Scheele in buttermilk, where it exists most abundantly; but it is present
also in fresh milk in small quantity, and communicates to it the property of reddening
litmus. Lactic acid may be detected in all the fluids of the animal body; either free
or saturated with alkaline matter.
Scheele obtained this acid by evaporating the sour whey of clotted milk to an eighth
part of its bulk, saturating this remainder with slaked lime, in order to throw down the
subphosphate of lime held in solution, filtering the liquor, diluting it with thrice its weight
of water, and precipitating the lime circumspectly, by the gradual addition of oxalic acid.[734]
He next filtered, evaporated to dryness on a water bath, and digested the residuum in
strong alcohol, which dissolved the lactic acid, and left the sugar of milk. On evaporating
off the alcohol, the acid was obtained. As thus procured, it requires to be purified
by saturation with carbonate of lead (pure white lead), and precipitating the solution
of this lactate with sulphate of zinc, not added in excess. Sulphate of lead falls, and the
supernatant lactate of zinc being evaporated affords crystals, at first brown, but which
become colourless on being dissolved and re-crystallized twice or thrice. If the sulphuric
acid of the dissolved salt be thrown down by water of baryta, the liquid when filtered and
evaporated yields a pure lactic acid, of a syrupy consistence, colourless and void of smell.
It has a pungent acid taste, which it loses almost entirely when moderately diluted with
water. It does not crystallize. Its salts, with the exception of those of magnesia and
zinc, have a gummy appearance, and are very soluble in alcohol, unless they hold an
excess of base. Lactic acid consists of 44·92 carbon; 6·55 hydrogen; 48·53 oxygen.
It contains 9·92 per cent. of water. It has not hitherto been applied to any use in the
arts, except by the Dutch in their old process of bleaching linen with sour milk.
LACTOMETER is the name of an instrument for estimating the quality of milk,
called also a Galactometer, which see. The most convenient form of apparatus would be
a series of glass tubes each about 1 inch in diameter, and 12 inches long, graduated
through a space of 10 inches, to tenths of an inch, having a stop-cock at the bottom, and
suspended upright in a frame. The average milk of the cow being poured in to the
height of 10 inches, as soon as the cream has all separated at top, the thickness of its
body may be measured by the scale; and then the skim-milk may be run off below
into a hydrometer glass, in order to determine its density, or relative richness in caseous
matter.
LAKES. Under this title are comprised all those colours which consist of a vegetable
dye, combined by precipitation with a white earthy basis, which is usually alumina.
The general method of preparation is to add to the coloured infusion a solution of common
alum, or rather a solution of alum saturated with potash, especially when the infusion
has been made with the aid of acids. At first only a slight precipitate falls, consisting
of alumina and the colouring matter; but on adding potash, a copious precipitation
ensues, of the alumina associated with the dye. When the dyes are not injured, but are
rather brightened by alkalis, the above process is reversed; a decoction of the dye-stuff
is made with an alkaline liquor, and when it is filtered, a solution of alum is poured
into it. The third method is practicable only with substances having a great affinity
for subsulphate of alumina; it consists in agitating recently precipitated alumina with
the decoction of the dye.
Yellow lakes are made with a decoction of Persian or French berries, to which
some potash or soda is added; into the mixture a solution of alum is to be poured as
long as any precipitate falls. The precipitate must be filtered, washed, and formed into
cakes, and dried. A lake may be made in the same way with quercitron, taking the
precaution to purify the decoction of the dye-stuff with buttermilk or glue. After filtering
the lake it may be brightened with a solution of tin. Annotto lake is formed by
dissolving the dye-stuff in a weak alkaline lye, and adding alum water to the solution.
Solution of tin gives this lake a lemon yellow cast; acids a reddish tint.
Red lakes.—The finest of these is carmine.
This beautiful pigment was accidentally discovered by a Franciscan monk at Pisa.
He formed an extract of cochineal with salt of tartar, in order to employ it as a medicine,
and obtained, on the addition of an acid to it, a fine red precipitate. Homberg
published a process for preparing it, in 1656. Carmine is the colouring matter of
cochineal, prepared by precipitation from a decoction of the drug. Its composition
varies according to the mode of making it. The ordinary carmine is prepared with
alum, and consists of carminium (see Cochineal), a little animal matter, alumina, and
sulphuric acid. See Carmine.
Carminated lake, called lake of Florence, Paris, or Vienna. For making this pigment,
the liquor is usually employed which is decanted from the carmine process.
Into this, newly precipitated alumina is put; the mixture is stirred, and heated a little,
but not too much. Whenever the alumina has absorbed the colour, the mixture is
allowed to settle, and the liquor is drawn off.
Sometimes alum is dissolved in the decoction of cochineal, and potash is then added,
to throw down the alumina in combination with the colouring matter; but in this way
an indifferent pigment is obtained. Occasionally, solution of tin is added, to brighten
the dye.
A lake may be obtained from kermes, in the same way as from cochineal; but now
it is seldom had recourse to.
Brazil-wood lakes.—Brazil wood is to be boiled in a proper quantity of water for 15
minutes; then, alum and solution of tin being added, the liquor is to be filtered, and a
solution of potash poured in as long as it occasions a precipitate. This is separated by[735]
the filter, washed in pure water, mixed with a little gum water, and made into cakes.
Or, the Brazil wood may be boiled along with a little vinegar, the decoction filtered,
alum and salt of tin added, and then potash-lye poured in to precipitate the lake. For
1 pound of Brazil wood, 30 to 40 pounds of water, and from 11⁄2 to 2 pounds of alum,
may be taken, in producing a deep red lake; or, the same proportions with half a
pound of solution of tin. If the potash be added in excess, the tint will become violet.
Cream of tartar occasions a brownish cast.
Madder lake.—A fine lake may be obtained from madder, by washing it in cold
water as long as it gives out colour; then sprinkling some solution of tin over it, and
setting it aside for some days. A gentle heat may also be applied. The red liquor
must be then separated by the filter, and decomposed by the addition of carbonate of
soda, when a fine red precipitate will be obtained. Or, the reddish brown colouring
matter of a decoction of madder may be first separated by acetate of lead, and then the
rose-red colour with alum. Or, madder tied up in a bag is boiled in water; to the
decoction, alum is added, and then potash. The precipitate should be washed with
boiling water, till it ceases to tinge it yellow; and it is then to be dried.
The following process merits a preference.
Diffuse 2 pounds of ground madder in 4 quarts of water, and after a maceration of 10
minutes, strain and squeeze the grounds in a press. Repeat this maceration, &c. twice
upon the same portion of madder. It will now have a fine rose colour. It must then
be mixed with 5 or 6 pounds of water and half a pound of bruised alum, and heated upon
a water bath for 3 or 4 hours, with the addition of water, as it evaporates, after which
the whole must be thrown upon a filter cloth. The liquor which passes is to be filtered
through paper, and then precipitated by carbonate of potash. If the potash be added
in three successive doses, three different lakes will be obtained, of successively diminishing
beauty. The precipitates must be washed till the water comes off colourless.
Blue lakes are hardly ever prepared, as indigo, prussian blue, cobalt blue, and ultramarine,
answer every purpose of blue pigments.
Green lakes are made by a mixture of yellow lakes with blue pigments; but chrome
yellows mixed with blues produce almost all the requisite shades of green.
LAMINABLE is said of a metal which may be extended by passing between
steel or hardened (chilled) cast-iron rollers.
For a description of metal rolling presses, see Iron and Mint; and
For a table of the relative laminability of metals, see Ductility.
LAMIUM ALBUM, or the dead nettle, is said by Leuchs to afford in its leaves a
greenish-yellow dye. The L. purpureum dyes a reddish-grey with salt of tin, and a
greenish tint with iron liquor.
LAMPS differ so much in principle, form, and construction, as to render their
description impossible, as a general subject of manufacture. In fact, the operations of
the lampist, like those of the blacksmith, cabinet-maker, cooper, coppersmith, tinman,
turner, &c., belong to a treatise upon handicraft trades. I shall here, however, introduce
a tabular view of the relative light and economy of the lamps most generally
known.
| Kind of Lamps. |
Intensity of light during |
Mean
of 7
hours. |
Consump-
tion per
hour in
grammes. |
Light
from
100
parts
of oil. |
1
hour |
2
hours |
3
hours |
4
hours |
5
hours |
6
hours |
| |
|
|
|
|
|
|
|
|
|
1. Mechanical lamp
of Carcel |
|
- |
|
|
|
|
|
|
100 |
|
42 |
|
238 |
| |
|
|
|
|
|
|
|
|
|
2. Fountain lamp,
and a chimney with
flat wick |
|
- |
100 |
98 |
98 |
97 |
96 |
96 |
125 |
|
11 |
|
113 |
| 3. Dome argand |
103 |
90 |
72 |
61 |
42 |
34 |
31 |
|
26 |
·714 |
116 |
| 4. Sinumbra lamp |
102 |
95 |
83 |
81 |
78 |
66 |
56 |
|
37 |
·145 |
150 |
5. Do. with fountain
above |
|
- |
100 |
90 |
70 |
52 |
41 |
32 |
85 |
|
43 |
|
197 |
| |
|
|
|
|
|
|
|
|
|
6. Do. with another
beak |
|
- |
100 |
97 |
95 |
92 |
89 |
86 |
41 |
|
18 |
|
227 |
| |
|
|
|
|
|
|
|
|
|
7. Girard’s hydrostatic
lamp |
|
- |
101 |
96 |
84 |
81 |
76 |
70 |
63 |
·66 |
34 |
·714 |
182 |
| |
|
|
|
|
|
|
|
|
|
8. Thilorier’s or
Parker’s do. lamp |
|
- |
106 |
103 |
100 |
94 |
92 |
90 |
107 |
·66 |
51 |
·143 |
215 |
| |
|
|
|
|
|
|
|
|
|
[736]
In the above table, for the purpose of comparing the successive degrees of intensity,
100 represents the mean intensity of light during the first hour. The quantity of
oil consumed per hour is given in grammes, of 151⁄2 grains each. The last column
expresses the quantity of light produced with a like consumption of oil, which was in
all cases 100 grammes. See Candles.
The following table of M. Peclet is perhaps more instructive:—
| Nature of the light. |
Inten-
sity. |
Consump-
tion per
hour in
grammes. |
Cost |
Fat pro-
ducing
the same
light. |
Cost
per
hour. |
per
kilogr. |
of
light
per
hour. |
| |
|
|
francs. |
cents. |
gram-
mes. |
cents. |
| 1. |
Mechanical lamp |
100 |
|
42 |
|
1 |
·40 |
5 |
·8 |
42 |
|
5 |
·8 |
| 2. |
Flat-wick mechan. do. |
12 |
·05 |
11 |
|
1 |
·40 |
1 |
·5 |
88 |
|
12 |
·3 |
| 3. |
Hemispherical dome lamp |
31 |
·0 |
26 |
·714 |
1 |
·40 |
3 |
·7 |
86 |
·16 |
12 |
·0 |
| 4. |
Sinumbra lamp |
85 |
|
43 |
|
1 |
·40 |
6 |
·0 |
50 |
·58 |
7 |
·0 |
| 5. |
Do. with a lateral fountain or vase |
41 |
|
18 |
|
1 |
·40 |
2 |
·5 |
43 |
·90 |
6 |
·1 |
| 6. |
Do. with a fountain above |
90 |
|
43 |
|
1 |
·40 |
6 |
·0 |
47 |
·77 |
6 |
·6 |
| 7. |
Girard’s hydrostatic lamp |
63 |
·66 |
34 |
·71 |
1 |
·40 |
4 |
·8 |
54 |
·52 |
7 |
·6 |
| 8. |
Thilorier’s or Parker’s do. |
107 |
·66 |
51 |
·143 |
1 |
·40 |
7 |
·1 |
47 |
·5 |
6 |
·6 |
| 9. |
Candle, 6 in lb. |
10 |
·66 |
8 |
·51 |
1 |
·40 |
1 |
·2 |
70 |
·35 |
9 |
·8 |
| 10. |
Do. 8 in do. |
8 |
·74 |
7 |
·51 |
1 |
·40 |
1 |
·0 |
85 |
·92 |
12 |
·0 |
| 11. |
Do. 6 with smaller wick |
7 |
·50 |
7 |
·42 |
2 |
·40 |
1 |
·7 |
98 |
·93 |
23 |
·7 |
| 12. |
Wax candle, 5 in lb. |
13 |
·61 |
8 |
·71 |
7 |
·60 |
5 |
·7 |
64 |
·04 |
48 |
·6 |
| 13. |
Sperm candle, do. |
14 |
·40 |
8 |
·92 |
7 |
·60 |
5 |
·8 |
61 |
·94 |
47 |
·8 |
| 14. |
Stearine candle, do. |
14 |
·30 |
9 |
·35 |
6 |
·00 |
5 |
·5 |
65 |
·24 |
37 |
·1 |
| 15. |
Coal gas |
127 |
|
136
litres |
|
5 |
·0 |
107
litres |
3 |
·9 |
| 16. |
Oil gas |
127 |
|
136 do. |
|
5 |
·0 |
30 |
|
3 |
·9 |
The light of the mechanical lamp is greatly over-rated relatively to that of gas. The
cost of the former is at least 5 times greater than of the latter, in London.
LAMP OF DAVY consists of a common oil lamp, surmounted with a covered
cylinder of wire gauze, for transmitting light to the miner without
endangering the kindling of the atmosphere of fire-damp which
may surround him; because carburetted hydrogen, in passing
through the meshes of the cylindric cover, gets cooled by the conducting
power of the metallic gauze, below the point of its accension.
The apertures in the gauze should not be more than 1-20th of
an inch square. Since the fire-damp is not inflamed by ignited
wire, the thickness of the wire is not of importance, but wire from
1-40th to 1-60th of an inch in diameter is the most convenient.
The cage or cylinder should be made by double joinings, the
gauze being folded over in such a manner as to leave no apertures.
When it is cylindrical, it should not be more than two inches in
diameter; because in larger cylinders, the combustion of the fire-damp
renders the top inconveniently hot; a double top is always
a proper precaution, fixed 1⁄2 or 3⁄4 of
an inch above the first top. See fig.
614.
The gauze cylinder should be fastened
to the lamp by a screw b, fig.
615., of four or five turns, and fitted
to the screw by a tight ring. All
joinings in the lamp should be made
with hard solder; as the security depends
upon the circumstance, that
no aperture exists in the apparatus,
larger than in the wire-gauze.
[737]
The parts of the lamp are,
1. The brass cistern a, d, fig. 615., which contains the oil. It is pierced at one side of
the centre with a vertical narrow tube, nearly filled with a wire which is recurved above,
at the level of the burner, to trim the wick, by acting on the lower end of the wire e
with the fingers. It is called the safety-trimmer.
2. The rim b is the screw neck for fixing on the gauze cylinder, in which the wire-gauze
cover is fixed, and which is fastened to the cistern by a screw fitted to b.
3. An aperture c for supplying oil. It is fitted with a screw or a cork, and communicates
with the bottom of the cistern by a tube at f. A central aperture for the wick.
4. The wire-gauze cylinder, fig. 614., which should not have less than 625 apertures
to the square inch.
5. The second top, 3⁄4 of an inch above the first, surmounted by a brass or copper plate,
to which the ring of suspension may be fixed. It is covered with a wire cap in the
figure.
6. Four or six thick vertical wires, g′ g′ g′ g′, joining the cistern below with the top
plate, and serving as protecting pillars round the cage. g is a screw-pin to fix the cover,
so that it shall not become loosened by accident or carelessness. The oil-cistern fig. 615.
is drawn upon a larger scale than fig. 614., to show its minuter parts.
When the wire-gauze safe-lamp is lighted and introduced into an atmosphere gradually
mixed with fire-damp, the first effect of the fire-damp is to increase the length and size
of the flame. When the inflammable gas forms so much as 1-12th of the volume of the
air, the cylinder becomes filled with a feeble blue flame, while the flame of the wick
appears burning brightly within the blue flame. The light of the wick augments till
the fire-damp increases to 1-6th or 1-5th, when it is lost in the flame of the fire-damp,
which in this case fills the cylinder with a pretty strong light. As long as any explosive
mixture of gas exists in contact with the lamp, so long it will give light; and when it is
extinguished, which happens whenever the foul air constitutes so much as 1-3d of the
volume of the atmosphere, the air is no longer proper for respiration; for though animal
life will continue where flame is extinguished, yet it is always with suffering. By
fixing a coil of platinum wire above the wick, ignition may be maintained in the metal
when the lamp itself is extinguished; and from this ignited wire the wick may be
again rekindled, on carrying it into a less inflammable atmosphere.
“We have frequently used the lamps where the explosive mixture was so high as
to heat the wire-gauze red-hot; but on examining a lamp which has been in constant
use for three months, and occasionally subjected to this degree of heat, I cannot perceive
that the gauze cylinder of iron wire is at all impaired. I have not, however, thought
it prudent, in our present state of experience, to persist in using the lamps under such
circumstances, because I have observed, that in such situations the particles of coal
dust floating in the air, fire at the gas burning within the cylinder, and fly off in
small luminous sparks. This appearance, I must confess, alarmed me in the first instance,
but experience soon proved that it was not dangerous.
“Besides the facilities afforded by this invention to the working of coal-mines
abounding in fire-damp, it has enabled the directors and superintendents to ascertain,
with the utmost precision and expedition, both the presence, the quantity, and correct
situation of the gas. Instead of creeping inch by inch with a candle, as is usual, along
the galleries of a mine suspected to contain fire-damp, in order to ascertain its presence,
we walk firmly on with the safe-lamps, and, with the utmost confidence, prove the actual
state of the mine. By observing attentively the several appearances upon the flame of
the lamp, in an examination of this kind, the cause of accidents which happened to
the most experienced and cautious miners is completely developed; and this has hitherto
been in a great measure matter of mere conjecture.
“It is not necessary that I should enlarge upon the national advantages which must
necessarily result from an invention calculated to prolong our supply of mineral coal,
because I think them obvious to every reflecting mind; but I cannot conclude without
expressing my highest sentiments of admiration for those talents which have developed
the properties, and controlled the power, of one of the most dangerous elements which
human enterprise has hitherto had to encounter.”—See Letter to Sir H. Davy, in
Journal of Science, vol. i. p. 302., by John Buddle, Esq., generally and justly esteemed
one of the most scientific coal-miners in the kingdom.
Mr. Buddle, in a letter dated 21st August, 1835, which is published in Dr. Davy’s
life of his brother Sir Humphrey, says;—
“In the evidence given in my last examination before a committee of the House of
Commons, I stated that after nearly twenty years’ experience of ‘the Davy’ with from
1000 to 1500 lamps in daily use, in all the variety of circumstances incidental to
coal mining, without a single accident having happened which could be attributed to[738]
a defect in its principle, or even in the rules for its practical application, as laid down
by Sir Humphrey—I maintained that ‘the Davy’ approximated perfection, as nearly
as any instrument of human invention could be expected to do. We have ascertained
distinctly that the late explosion did not happen in that part of the mine where the
Davys were used. They were all found in a perfect state after the accident—many
of them in the hands of the dead bodies of the sufferers.”
LAMPATES and LAMPIC ACID. When a spirit of wine lamp has its cotton
wick surmounted with a spiral coil of platinum wire, after lighting it for a little, it
may be blown out, without ceasing to burn the alcohol; for the coil continues ignited,
and a current of hot vapour continues to rise, as long as the spirit lasts. This vapour
was first condensed and examined by Professor Daniell, who called it lampic acid. It
has a peculiar, strongly acid, burning taste, and a spec. grav. of 1·015. It possesses
in an eminent degree the property of reducing certain metallic solutions; such as those
of platinum, gold, and silver. The lampates may be prepared by saturating the above
acid with the alkaline and earthy carbonates.
LAPIDARY, Art of. The art of the lapidary, or that of cutting, polishing, and
engraving gems, was known to the ancients, many of whom have left admirable specimens
of their skill. The Greeks were passionate lovers of rings and engraved stones; and the
most parsimonious among the higher classes of the Cyrenians are said to have worn rings
of the value of ten minæ (about 30l. of our money.) By far the greater part of the antique
gems that have reached modern times, may be considered as so many models for forming
the taste of the student of the fine arts, and for inspiring his mind with correct ideas of
what is truly beautiful. With the cutting of the diamond, however, the ancients were
unacquainted, and hence they wore it in its natural state. Even in the middle ages, this
art was still unknown; for the four large diamonds which enrich the clasp of the
imperial mantle of Charlemagne, as now preserved in Paris, are uncut, octahedral
crystals. But the art of working diamonds was probably known in Hindostan and
China, in very remote periods. After Louis de Berghen’s discovery, in 1476, of polishing
two diamonds by their mutual attrition, all the finest diamonds were sent to Holland
to be cut and polished by the Dutch artists, who long retained a superiority, now no
longer admitted by the lapidaries of London and Paris.
The operation of gem cutting is abridged by two methods; 1. by cleavage; 2. by
cutting off slices with a fine wire, coated with diamond powder, and fixed in the stock
of a hand-saw. Diamond is the only precious stone which is cut and polished with
diamond powder, soaked with olive oil, upon a mill plate of very soft steel.
Oriental rubies, sapphires, and topazes, are cut with diamond powder soaked with
olive oil, on a copper wheel. The facets thus formed are afterwards polished on another
copper wheel, with tripoli, tempered with water.
Emeralds, hyacinths, amethysts, garnets, agates, and other softer stones, are cut at a
lead wheel, with emery and water; and are polished on a tin wheel with tripoli and
water, or, still better, on a zinc wheel, with putty of tin and water.
The more tender precious stones, and even the pastes, are cut on a mill-wheel of
hard wood, with emery and water; and are polished with tripoli and water, on another
wheel of hard wood.
Since the lapidary employs always the same tools, whatever be the stone which he cuts
or polishes, and since the wheel discs alone vary, as also the substance he uses with them,
we shall describe, first of all, his apparatus, and then the manipulations for diamond-cutting,
which are applicable to every species of stone.
The lapidary’s mill, or wheel, is shewn in perspective in fig. 616. It consists of
a strong frame made of oak carpentry, with
tenon and mortised joints, bound together
with strong bolts and screw nuts. Its form is
a parallelopiped of from 8 to 9 feet long, by
from 6 to 7 high; and about 2 feet broad.
These dimensions are large enough to contain
two cutting wheels alongside of each
other, as represented in the figure.
Besides the two sole bars B B, we perceive
in the breadth, 5 cross bars, C, D, E, F, G.
The two extreme bars C and G, are a part of
the frame-work, and serve to bind it. The
two cross-bars D and F, carry each in the
middle of their length, a piece of wood as
thick as themselves, but only 41⁄2 inches long
(see fig. 617.), joined solidly by mortises and tenons with that cross bar, as well as[739]
with the one placed opposite on the other parallel face. These two pieces are called
summers (lintels); the one placed at D is the upper; the one at F, the lower.
In fig. 617. this face is shewn inside, in order to explain how the mill wheel is placed
and supported. The same letters point out the same objects, both in the preceding and
the following figures.
In each of these summers a square hole is cut out, exactly opposite to the other;
in which are adjusted by friction, a square piece of oak a a,
fig. 617., whose extremities are perforated with a conical
hole, which receives the two ends of the arbor H of the
wheel I, and forms its socket. This square bar is adjusted
at a convenient height, by a double wooden wedge b b.
The cross bar in the middle E supports the table c c, a
strong plank of oak. It is pierced with two large holes
whose centres coincide with the centre of the conical holes
hollowed out at the end of the square pins. These holes,
of about 6 inches diameter each, are intended to let the
arbor pass freely through, bearing its respective wheel. (See
one of these holes at I, in fig. 621. below.)
Each wheel is composed of an iron arbor H, fig. 618.,
of a grinding-wheel I, which differs in substance according
to circumstances, as already stated, and of the pulley J, furnished
with several grooves (see fig. 619.), which has a
square fit upon the arbor. The arbor carries a collet d, on
which are 4 iron pegs or pins that enter into the wheel to
fasten it.
The wheel plate, of which the ground plan is shown at K,
is hollowed out towards its centre to half its thickness;
when it is in its position on the arbor, as indicated in fig.
619., a washer or ferrule of wrought iron is put over it, and
secured in its place by a double wedge. In fig. 619. the
wheel-plate is represented in section, that the connection of
the whole parts may be seen.
A board g (see fig. 616. and fig. 624.), about 71⁄2 inches
high, is fixed to the part of the frame opposite to the side
at which the lapidary works, and it prevents the substances
made use of in the cutting and polishing, from being thrown
to a distance by the centrifugal force of the wheel-plate.
Behind this apparatus is mounted for each grinding-plate,
a large wheel L (see fig. 616.), similar to a cutler’s, but placed
horizontally. This wheel is grooved round its circumference
to receive an endless cord or band, which passes round one of the grooves of the
pulley J, fixed below the wheel-plate. Hence, on turning the fly-wheel L, the plate
revolves with a velocity relative to the velocity communicated to the wheel L, and to
the difference of diameter of the wheel L and the pulley J. Each wheel L, is mounted
on an iron arbor, with a crank (see M, fig. 620.)
The lower pivot of that arbor h is conical, and turns in a socket fixed in the floor.
The great wheel L rests on the collet i, furnished with its 4 iron pins, for securing the
connection. Above the wheel an iron washer is laid, and the whole is fixed by a double
wedge, which enters into the mortise l, fig. 620.
Fig. 621. exhibits a ground-plan view of all
this assemblage of parts, to explain the structure
of the machine. Every thing that stands above
the upper summer-bar has been suppressed in this
representation. Here we see the table c c; the
upper summer m; the one wheel-plate l, the other
having been removed to shew that the endless cord
does not cross; the two large wheels L L, present
in each machine, the crank bar N, seen separate
in fig. 622, which serves for turning the wheel L.
This bar is formed of 3 iron plates n, o; p, q; and q, r; (fig. 622.) The first is[740]
bent round at the point n, to embrace the stud s; the second p q, is of the same
breadth and thickness as the first; and the third, is adjusted to the latter with a hinge
joint, at the point q, where they are both turned into a circular form, to embrace the
crank M. When all these pieces are connected, they are fixed at the proper lengths by
the buckles or square rings t t t, which embrace these pieces, as is shown in fig. 622.
The stud s, seen in fig. 622., is fixed to the point v by a wedge-key upon the arm P,
represented separately, and in perspective, in fig. 623. The labourer seizing the two upright
pegs or handles x x; by the alternate forward and backward motion of his arm, he
communicates the same motion to the crank rod, which transmits it to the crank of the
arbor M, and impresses on that arbor, and the wheel which it bears, a rotatory movement.
Fig. 624. shows piece-meal and in perspective, a part of the lapidary’s wheel-mill.
There we see the table c c, the grind-plate I, whose axis is kept in a vertical position by
the two square plugs a a, fixed into the two summers by the wedges b b. On the two
sides of the wheel-plate we perceive an important instrument called a dial, which serves
to hold the stone during the cutting and polishing. This instrument has received lately
important ameliorations, to be described in fig. 625. The lapidary holds this instrument
in his hand, he rests it upon the iron pins u u fixed in the table, lest he should be
affected by the velocity of the revolving wheel-plate. He loads it sometimes with
weights e, e, to make it take better hold of the grinding plate.
One of the most expert lapidaries of Geneva works by means of the following improved
mechanism, of his own invention, whereby he cuts and polishes the facets with
extreme regularity, converting it into a true dial.
Fig. 625. shows this improvement. Each of the two jaws bears a
large conchoidal cavity, into which is fitted a brass ball, which carries
on its upper part a tube e, to whose extremity is fixed a dial-plate
f f, engraved with several concentric circles, divided into equal parts,
like the toothed-wheel cutting engine-plate, according to the number
of facets to be placed in each cutting range. The tube receives
with moderate friction the handle of the cement rod, which is fixed
at the proper point by a thumb-screw, not shown in the figure, being
concealed by the vertical limb d, about to be described.
A needle or index g, placed with a square fit on the tail of the cement rod, marks by
its point the divisions on the dial plate f f. On the side m n of the jaw A, there is
fixed by two screws, a limb d, forming a quadrant whose centre is supposed to be at the
centre of the ball. This quadrant is divided as usual into 90 degrees, whose highest
point is marked 0, and the lowest would mark about 70; for the remainder of the arc
down to 90 is concealed by the jaw. The two graduated plates are used as follows:—
When the cement rod conceals zero or 0 of the limb, it is then vertical, and serves to
cut the table of the brilliant; or the point opposite to it, and parallel to the table. On
making it slope a little, 5 degrees for example, all the facets will now lie in the same
zone, provided that the inclination be not allowed to vary. On turning round the
cement rod the index g marks the divisions, so that by operating on the circle with
16 divisions, stopping for some time at each, 16 facets will have been formed, of perfect
equality, and at equal distances, as soon as the revolution is completed.
Diamonds are cut at the present day in only two modes; into a rose diamond, and a
brilliant. We shall therefore confine our attention to these two forms.
The rose diamond is flat beneath, like all weak stones, while the upper face rises into
a dome, and is cut into facets. Most usually six facets are put on the central region,[741]
which are in the form of triangles, and unite at their summits; their bases abut upon
another range of triangles, which being set in an inverse position to the preceding, present
their bases to them, while their summits terminate at the sharp margin of the stone. The
latter triangles leave spaces between them which are likewise cut each into two facets.
By this distribution the rose diamond is cut into 24 facets; the surface of the diamond
being divided into two portions, of which the upper is called the crown, and that forming
the contour, beneath the former, is called dentelle (lace) by the French artists.
According to Mr. Jefferies, in his Treatise on Diamonds, the regular rose diamond is
formed by inscribing a regular octagon in the centre of the table side of the stone, and
bordering it by eight right-angled triangles, the bases of which correspond with the sides
of the octagon; beyond these is a chain of 8 trapeziums, and another of 16 triangles.
The collet side also consists of a minute central octagon, from every angle of which proceeds
a ray to the edge of the girdle, forming the whole surface into 8 trapeziums, each
of which is again subdivided by a salient angle (whose apex touches the girdle) into one
irregular pentagon and two triangles.
To fashion a rough diamond into a brilliant, the first step is to modify the faces of the
original octahedron, so that the plane formed by the junction of the two pyramids shall
be an exact square, and the axis of the crystal precisely twice the length of one of the
sides of the square. The octahedron being thus rectified, a section is to be made parallel
to the common base or girdle, so as to cut off 5 eighteenths of the whole height from the
upper pyramid, and 1 eighteenth from the lower one. The superior and larger plane
thus produced is called the table, and the inferior and smaller one is called the collet; in
this state it is termed a complete square table diamond. To convert it into a brilliant,
two triangular facets are placed on each side of the table, thus changing it from a square
to an octagon; a lozenge-shaped facet is also placed at each of the four corners of the
table, and another lozenge extending lengthwise along the whole of each side of the original
square of the table, which with two triangular facets set on the base of each
lozenge, completes the whole number of facets on the table side of the diamond; viz. 8
lozenges, and 24 triangles. On the collet side are formed 4 irregular pentagons, alternating
with as many irregular lozenges radiating from the collet as a centre, and bordered
by 16 triangular facets adjoining the girdle. The brilliant being thus completed,
is set with the table side uppermost, and the collet side implanted in the cavity made
to receive the diamond. The brilliant is always three times as thick as the rose diamond.
In France, the thickness of the brilliant is set off into two unequal portions;
one third is reserved for the upper part or table of the diamond, and the remaining two
thirds for the lower part or collet (culasse). The table has eight planes, and its circumference
is cut into facets, of which some are triangles, and others lozenges. The collet
is also cut into facets called pavillons. It is of consequence that the pavillons lie in
the same order as the upper facets, and that they correspond to each other, so that the
symmetry be perfect, for otherwise the play of the light would be false.
Although the rose-diamond projects bright beams of light in more extensive proportion
often than the brilliant, yet the latter shows an incomparably greater play, from
the difference of its cutting. In executing this, there are formed 32 faces of different
figures, and inclined at different angles all round the table, on the upper side of the
stone. On the collet (culasse) 24 other faces are made round a small table, which converts
the culasse into a truncated pyramid. These 24 facets, like the 32 above, are
differently inclined and present different figures. It is essential that the faces of the top
and the bottom correspond together in sufficiently exact proportions to multiply the
reflections and refractions, so as to produce the colours of the prismatic spectrum.
The other precious stones, as well as their artificial imitations, called pastes, are cut in
the same fashion as the brilliant; the only difference consists in the matter constituting
the wheel plates, and the grinding and polishing powders,
as already stated.
In cutting the stones, they are mounted on the cement-rod
B, fig. 626., whose stem is set upright in a socket placed
in the middle of a sole piece at A, which receives the stem of
the cement-rod. The head of the rod fills the cup of A. A
melted alloy of tin and lead is poured into the head of the
cement-rod, into the middle of which the stone is immediately
plunged; and whenever the solder has become solid,
a portion of it is pared off from the top of the diamond, to
give the pyramidal form shown in the figure at B.
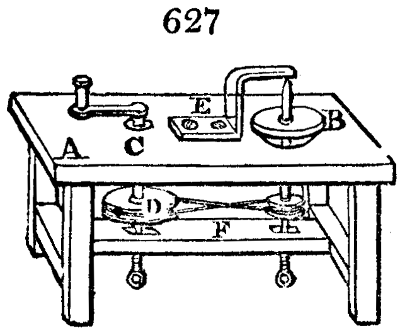
There is an instrument employed by the steel polishers for pieces of clock work, and
by the manufacturers of watch-glasses for polishing their edges. It consists of a
solid oaken table, fig. 627. The top is perforated with two holes, one for passing
through the pulley and the arbor of the wheel-plate B, made either of lead or of hard[742]
wood, according to circumstances; and the other C for receiving the upper part of the
arbor of the large pulley D. The upper pulley of the wheel-plate is supported by an
iron prop E, fixed to the table by two wooden screws. The inferior pivots of the two
pieces are supported by screw-sockets, working in an iron screw-nut sunk into the summer-bar
F. The legs of the table are made longer or shorter, according as the workman
chooses to stand or sit at his employment. Emery with oil is used for grinding
down, and tin-putty or colcothar for polishing. The workman lays the piece on the
flat of the wheel-plate with one hand, and presses it down with a lump of cork, while he
turns round the handle with the other hand.
The Sapphire, Ruby, Oriental Amethyst, Oriental Emerald, and Oriental Topaz, are
gems next in value and hardness to diamond; and they all consist of nearly pure alumina
or clay, with a minute portion of iron as the colouring matter. The following
analyses show the affinity in composition of the most precious bodies with others in little
relative estimation.
| |
Sapphire. |
Corundum
Stone. |
Emery. |
| Alumina or clay |
98 |
·5 |
89 |
·50 |
86 |
·0 |
| Silica |
0 |
·0 |
5 |
·50 |
3 |
·0 |
| Oxide of iron |
1 |
·0 |
1 |
·25 |
4 |
·0 |
| Lime |
0 |
·5 |
0 |
·00 |
0 |
·0 |
| |
100 |
·0 |
96 |
·25 |
93 |
·0 |
Salamstone is a variety which consists of small transparent crystals, generally six-sided
prisms, of pale reddish and bluish colours. The corundum of Battagammana is frequently
found in large six-sided prisms: it is commonly of a brown colour, whence it is called
by the natives curundu gallé, cinnamon stone. The hair-brown and reddish-brown
crystals are called adamantine spar. Sapphire and salamstone are chiefly met with in
secondary repositories, as in the sand of rivers &c., accompanied by crystals and grains
of octahedral iron-ore and of several species of gems. Corundum is found in imbedded
crystals in a rock, consisting of indianite. Adamantine spar occurs in a sort of granite.
The finest varieties of sapphire come from Pegu, where they occur in the Capelan
mountains near Syrian. Some have been found also at Hohenstein in Saxony, Bilin in
Bohemia, Puy in France, and in several other countries. The red variety, the ruby, is
most highly valued. Its colour is between a bright scarlet and crimson. A perfect
ruby above 31⁄2 carats is more valuable than a diamond of the same weight. If it weigh
one carat, it is worth 10 guineas; 2 carats, 40 guineas; 3 carats, 150 guineas; 6 carats,
above 1000 guineas. A deep coloured ruby, exceeding 20 carats in weight, is generally
called a carbuncle; of which 108 were said to be in the throne of the Great Mogul,
weighing from 100 to 200 carats each; but this statement is probably incorrect. The
largest oriental ruby known to be in the world, was brought from China to Prince
Gargarin, governor of Siberia. It came afterwards into the possession of Prince Menzikoff,
and constitutes now a jewel in the imperial crown of Russia.
A good blue sapphire of 10 carats is valued at 50 guineas. If it weighs 20 carats, its
value is 200 guineas; but under 10 carats, the price may be estimated by multiplying
the square of its weight in carats into half a guinea; thus, one of 4 carats would be
worth 42 × 1⁄2 G. = 8 guineas. It has been said that the blue sapphire is superior in
hardness to the red, but this is probably a mistake arising from confounding the corundum
ruby with the spinelle ruby. A sapphire of a barbel blue colour, weighing 6 carats,
was disposed of in Paris by public sale for 70l. sterling; and another of an indigo blue,
weighing 6 carats and 3 grains, brought 60l.; both of which sums much exceed
what the preceding rule assigns, from which we may perceive how far fancy may go in
such matters. The sapphire of Brasil is merely a blue tourmaline, as its specific gravity
and inferior hardness show. White sapphires are sometimes so pure, that when properly
cut and polished they have been passed for diamonds.
The yellow and green sapphires are much prized under the names of Oriental topaz
and emerald. The specimens which exhibit all these colours associated in one stone are
highly valued, as they prove the mineralogical identity of these varieties.
Besides these shades of colour, sapphires often emit a beautiful play of colours, or
chatoiement, when held in different positions relative to the eye or incident light; and
some likewise present star-like radiations, whence they are called star-stones or asterias;
sending forth 6 or even 12 rays, that change their place with the position of the stone.
This property so remarkable in certain blue sapphires, is not however peculiar to these
gems. It seems to belong to transparent minerals which have a rhomboid for their[743]
nucleus, and arises from the combination of certain circumstances in their cutting and
structure. Lapidaries often expose the light-blue variety of sapphire to the action of
fire, in order to render it white and more brilliant; but with regard to those found at
Expailly in France, fire deepens their colour.
3. Chrysoberyl, called by Haüy Cymophane, and by others Prismatic corundum, ranks
next in hardness to sapphire, being 8·5 on the same scale of estimation. Its specific
gravity is 3·754. It usually occurs in rounded pieces about the size of a pea, but it is
also found crystallised in many forms, of which 8-sided prisms with 8-sided summits are
perhaps the most frequent. Lustre vitreous; colour asparagus green, passing into
greenish-white and olive-green. It shows a bluish opalescence, a light undulating as it
were in the stone, when viewed in certain directions; which property constitutes its
chief attraction to the jeweller. When polished, it has been sometimes mistaken for a
yellow diamond; and from its hardness and lustre is considerably valued. Good specimens
of it are very rare. It has been found only in the alluvial deposits of rivers, along
with other species of gems. Thus it occurs in Brasil, along with diamonds and prismatic
topaz; also in Ceylon. Its constituents are, alumina 68·66; glucina 16·00;
silica 6·00; protoxide of iron 4·7; oxide of titanium 2·66; moisture 0·66, according to
Seybert’s analysis of a specimen from Brasil. It is difficultly but perfectly fusible
before the blowpipe, with borax and salt of phosphorus. In composition it differs
entirely from sapphire, or the rhombohedral corundum.
4. Spinelle Ruby, called Dodecahedral corundum by some mineralogists, and Balas
ruby by lapidaries. Its hardness is 8. Specific gravity 3·523. Its fundamental form
is the hexahedron, but it occurs crystallized in many secondary forms: octahedrons, tetrahedrons
and rhombohedrons. Fracture conchoidal; lustre vitreous; colour red, passing
into blue and green, yellow, brown and black; and sometimes it is nearly white. Red
spinelle consists of, alumina 74·5; silica 15·5; magnesia 8·25; oxide of iron 1·5; lime
0·75. Vauquelin discovered 6·18 per cent. of chromic acid in the red spinelle. The
red varieties exposed to heat, become black and opaque; on cooling they appear first
green, then almost colourless, but at last resume their red colour. Pleonaste is a variety
which yields a deep green globule with borax.
Crystals of spinelle from Ceylon have been observed imbedded in limestone, mixed
with mica, or in rocks containing adularia, which seem to have belonged to a primitive
district. Other varieties like the pleonaste occur in the drusy cavities of rocks ejected
by Vesuvius. Crystals of it are often found in diluvial and alluvial sand and gravel,
along with true sapphires, pyramidal zircon, and other gems, as also with octahedral iron
ore, in Ceylon. Blue and pearl-gray varieties occur in Südermannland in Sweden, imbedded
in granular limestone. Pleonaste is met with also in the diluvial sands of
Ceylon. Clear and finely coloured specimens of spinelle are highly prized as ornamental
stones. When the weight of a good spinelle exceeds 4 carats, it is said to be valued at
half the price of a diamond of the same weight. M. Brard has seen one at Paris, which
weighed 215 grains.
5. Zircon or Hyacinth. Its fundamental form is an isosceles 4-sided pyramid; and
the secondary forms have all a pyramidal character. Fracture conchoidal, uneven;
lustre more or less perfectly adamantine; colours, red, brown, yellow, gray, green, white;
which with the exception of some red tints, are not bright. Hardness 7·5. Specific
gravity 4·5. Zircon and hyacinth consist, according to Klaproth, of almost exactly the
same constituents; namely, zirconia 70; silica 25; oxide of iron 5. In the white
zirconia there is less iron and more silica. Before the blowpipe the hyacinth loses its
colour, but does not melt. The brighter zircons are often worked up into a brilliant
form, for ornamenting watch cases. As a gem, hyacinth has no high value. It has
been often confounded with other stones, but its very great specific gravity makes it to
be readily recognized.
6. Topaz. The fundamental form is a scalene 4-sided pyramid; but the secondary
forms have a prismatic character; and are frequently observed in oblique 4-sided
prisms, acuminated by 4 planes. The lateral planes of the prism are longitudinally
striated. Fracture conchoidal, uneven; lustre vitreous; colours, white, yellow, green,
blue, generally of pale shades. Hardness 8; specific gravity 3·5. Prismatic topaz
consists, according to Berzelius, of alumina 57·45; silica 34·24; fluoric acid 7·75. In
a strong heat the faces of crystallization, but not those of cleavage, are covered with
small blisters, which however immediately crack. With borax, it melts slowly into a
transparent glass. Its powder colours the tincture of violets green. Those crystals
which possess different faces of crystallization on opposite ends, acquire the opposite
electricities on being heated. By friction, it acquires positive electricity.
Most perfect crystals of topaz have been found in Siberia, of green, blue, and white
colours, along with beryl, in the Uralian and Altai mountains, as also in Kamschatka;
in Brazil, where they generally occur in loose crystals, and pebble forms of bright yellow[744]
colours; and in Mucla in Asia Minor, in pale straw-yellow regular crystals. They
are also met with in the granitic detritus of Cairngorm in Aberdeenshire. The blue
varieties are absurdly called oriental aquamarine by lapidaries. If exposed to heat, the
Saxon topaz loses its colour and becomes white; the deep yellow Brazilian varieties
assume a pale pink hue; and are then sometimes mistaken for spinelle, to which, however,
they are somewhat inferior in hardness. Topaz is also distinguishable by its double
refractive property. Tavernier mentions a topaz, in the possession of the Great Mogul,
which weighed 157 carats, and cost 20,000l. sterling. There is a specimen in the museum
of natural history at Paris which weighs 4 ounces 2 gros.
Topazes are not scarce enough to be much valued by the lapidary.
7. Emerald and Beryl, are described in their alphabetical places. Emerald loses its
lustre by candle-light; but as it appears to most advantage when in the company of
diamonds, it is frequently surrounded with brilliants, and occasionally with pearls. Beryl
is the aqua-marine of the jewellers, and has very little estimation among lapidaries.
8. Garnet. See this stone in its alphabetical place.
9. Chrysolite, called Peridot by Haüy; probably the topaz of the ancients, as our
topaz was their chrysolite. It is the softest of the precious stones, being scratched by
quartz and the file. It refracts double.
10. Quartz, including, as sub-species, Amethyst, Rock-crystal, Rose-quartz, Prase or
Chrysoprase, and several varieties of calcedony, as Cat’s eye, Plasma, Chrysoprase, Onyx,
Sardonyx, &c. Lustre vitreous, inclining sometimes to resinous; colours, very various;
fracture conchoidal; hardness, 7; specific gravity, 2·69.
11. Opal, or uncleavable quartz. Fracture, conchoidal; lustre, vitreous or resinous;
colours, white, yellow, red, brown, green, gray. Lively play of light; hardness, 5·5 to
6·5; specific gravity, 2·091. It occurs in small kidney-shaped and stalactitic shapes,
and large tuberose concretions. The phenomena of the play of colours in precious
opal has not been satisfactorily explained. It seems to be connected with the regular
structure of the mineral. Hydrophane, or oculis mundi, is a variety of opal without
transparency, but acquiring it when immersed in water, or in any transparent fluid.
Precious opal was found by Klaproth to consist of silica, 90; water, 10; which is a
very curious combination. Hungary has been long the only locality of precious opal,
where it occurs near Caschau, along with common and semi-opal, in a kind of porphyry.
Fine varieties have, however, been lately discovered in the Faroe islands; and most
beautiful ones, sometimes quite transparent, near Gracias a Dios, in the province of
Honduras, America. The red and yellow bright coloured varieties of fire-opal are
found near Zimapan, in Mexico. Precious opal, when fashioned for a gem, is generally
cut with a convex surface; and if large, pure, and exhibiting a bright play of colours,
is of considerable value. In modern times, fine opals of moderate bulk have been
frequently sold at the price of diamonds of equal size; the Turks being particularly
fond of them. The estimation in which opal was held by the ancients is hardly credible.
They called it Paideros, or Child beautiful as love. Nonius, the Roman senator,
preferred banishment to parting with his favourite opal, which was coveted by Mark
Antony. Opal which appears quite red when held against the light, is called girasol
by the French; a name also given to the sapphire or corundum asterias or star-stone.
12. Turquois, or Calaite. Mineral turquois, occurs massive; fine-grained impalpable;
fracture conchoidal; colour, between a blue and a green, soft, and rather
bright; opaque; hardness, 6; spec. grav. 2·83 to 3·0. Its constituents are, alumina,
73; oxide of copper, 4·5; oxide of iron, 4; water, 18; according to Dr. John. But
by Berzelius, it consists of phosphate of alumina and lime, silica, oxides of copper and
iron, with a little water. It has been found only in the neighbourhood of Nichabour
in the Khorassan, in Persia; and is very highly prized as an ornamental stone in that
country. There is a totally different kind of turquois, called bone turquois, which seems
to be phosphate of lime coloured with oxide of copper. When the oriental stone is cut
and polished, it forms a pleasing gem of inferior value. Malachite, or mountain green,
a compact carbonate of copper, has been substituted sometimes for turquois, but their
shades are different. Malachite yields a green streak, and turquois a white one.
13. Lapis lazuli, is of little value, on account of its softness.
LEAD. (Plomb, Fr.; Blei, Germ.) This is one of the metals most antiently known,
being mentioned in the books of Moses. It has a gray blue colour, with a bright
metallic lustre when newly cut, but it becomes soon tarnished and earthy looking in the
air. Its texture is close, without perceptible cleavage or appearance of structure; the
specific gravity of common lead is 11·352; but of the pure metal, from 11·38 to
11·44. It is very malleable and ductile, but soft and destitute of elasticity; fusible
at 612° Fahr., by Crighton, at 634° by Kupfer, and crystallizable on cooling, into
octahedrons implanted into each other so as to form an assemblage of four-sided
pyramids.
[745]
There are four oxides of lead. 1. The suboxide of a grayish blue colour, which
forms a kind of crust upon a plate of lead long exposed to the air. It is procured in a
perfect state by calcining oxalate of lead in a retort; the dark gray powder which remains
is the pure suboxide. 2. The protoxide is obtained by exposing melted lead to the
atmosphere, or, more readily, by expelling the acid from the nitrate of lead by heat in a
platinum crucible. It is yellow, and was at one time prepared as a pigment by calcining
lead; but is now superseded by the chromate of this metal. Litharge is merely
this oxide in the form of small spangles, from having undergone fusion; it is more or
less contaminated with iron, copper, and sometimes a little silver. It contains likewise
some carbonic acid. The above oxide consists of 104 of metal, and 8 of oxygen, its
prime equivalent being 112, upon the hydrogen scale; and it is the base of all the salts
of lead. 3. The plumbeous suroxide of Berzelius, the sesquioxide of some British
chemists, is the well-known pigment called RED LEAD or minium. It consists of 100
parts of metal and 10 of oxygen. 4. The plumbic suroxide of Berzelius, or the
peroxide of the British chemists, is obtained by putting red lead in chlorine water, or
in dilute nitric acid. It is of a dark brown, almost black colour, which gives out
oxygen when heated, and becomes yellow oxide. It kindles sulphur when triturated
with it. This oxide is used by the analytical chemist to separate, by condensation,
the sulphurous acid existing in a gaseous mixture.
Among the ores of lead some have a metallic aspect; are black in substance, as
well as when pulverized; others have a stony appearance, and are variously coloured,
with usually a vitreous or greasy lustre. The specific gravity of the latter ores is
always less than 5. The whole of them, excepting the chloride, become more or less
speedily black, with sulphuretted hydrogen or with hydrosulphurets; and are easily
reduced to the metallic state upon charcoal, with a flux of carbonate of soda, after they
have been properly roasted. They diffuse a whitish or yellowish powder over the
charcoal, which, according to the manner in which the flame of the blowpipe is directed
upon it, becomes yellow or red; thus indicating the two characteristic colours of the
oxides of lead.
We shall not enter here into the controversy concerning the existence of native lead,
which has been handled at length by M. Brongniart in the Dictionnaire des Sciences
Naturelles, article Plomb, Mineralogie.
The lead ores most interesting to the arts are:—
1. Galena, sulphuret of lead. This ore has the metallic lustre of lead with a crystalline
structure derivable from the cube. When heated cautiously at the blowpipe it is
decomposed, the sulphur flies off, and the lead is left alone in fusion; but if the heat be
continued, the coloured surface of the charcoal indicates the conversion of the lead into
its oxides. Galena is a compound of lead and sulphur, in equivalent proportions, and
therefore consists, in 100 parts, of 862⁄3 of metal, and 131⁄3 of sulphur, with which numbers
the analysis of the galena of Clausthal by Westrumb exactly agrees. Its specific
gravity, when pure, is 7·56. Its colour is blackish gray, without any shade of red, and
its powder is black; characters which distinguish it from blende or sulphuret of zinc.
Its structure in mass is lamellar, passing sometimes into the fibrous or granular, and
even compact. It is brittle. The specular galena, so called from its brightly polished
aspect, is remarkable for forming the slickensides of Derbyshire—thin seams, which
explode with a loud noise when accidentally scratched in the mine.
The argentiferous galena has in general all the external characters of pure galena.
The proportions of silver vary from one-fifth part of the whole, as at Tarnowitz, in
Silesia, to three parts in ten thousand, as in the ore called by the German miners
Weisgültigerz; but it must be observed, that whenever this lead ore contains above
5 per cent. of silver, several other metals are associated with it. The mean proportion
of silver in galena, or that which makes it be considered practically as an argentiferous
ore, because the silver may be profitably extracted, is about two parts in the thousand.
See Silver. The above rich silver ores were first observed in the Freyberg mines,
called Himmelsfürst and Beschertglück, combined with sulphuret of antimony; but
they have been noticed since in the Hartz, in Mexico, and several other places.
The antimonial galena (Bournonite) exhales at the blowpipe the odour peculiar to
antimony, and coats the charcoal with a powder partly white and partly red. It usually
contains some arsenic.
2. The Seleniuret of lead, resembles galena, but its tint is bluer. Its chemical characters
are the only ones which can be depended on for distinguishing it. At the
blowpipe, it exhales a very perceptible smell of putrid radishes. Nitric acid liberates
the selenium. When heated in a tube, oxide of selenium of a carmine red rises along
with selenic acid, white and deliquescent. The specific gravity of this ore varies from
6·8 to 7·69.
3. Native minium or red lead, has an earthy aspect, of a lively and nearly pure red
colour, but sometimes inclining to orange. It occurs pulverulent, and also compact,[746]
with a fracture somewhat lamellar. When heated at the blowpipe upon charcoal, it
is readily reduced to metallic lead. Its specific gravity varies from 4·6 to 8·9. This
ore is rare.
4. Plomb-gomme. This lead ore, as singular in appearance as in composition, is of
a dirty brownish or orange-yellow, and occurs under the form of globular, or gum-like
concretions. It has also the lustre and translucency of gum; with somewhat of a
pearly aspect at times. It is harder than fluor spar. It consists of oxide of lead, 40;
alumina, 37; water, 18·8; foreign matters and loss, 4·06; in 100. Hitherto it has
been found only at Huelgoët, near Poullaouen, in Brittany, covering with its tears or
small concretions the ores of white lead and galena which compose the veins of that
lead mine.
5. White lead, carbonate of lead. This ore in its purest state, is colourless and transparent
like glass, with an adamantine lustre. It may be recognized by the following
characters:—
Its specific gravity is from 6 to 6·7; it dissolves with more or less ease, and with
effervescence, in nitric acid; becomes immediately black by the action of sulphuretted
hydrogen, and melts on charcoal before the blowpipe into a button of lead. According
to Klaproth, the carbonate of Leadhills contains 82 parts of oxide of lead, and 16
of carbonic acid, in 98 parts. This mineral is tender, scarcely scratches calc-spar,
and breaks easily with a waved conchoidal fracture. It possesses the double refracting
property in a very high degree; the double image being very visible on looking
through the flat faces of the prismatic crystals. Its crystalline forms are very numerous,
and are referrible to the octahedron, and the pyramidal prism.
6. Vitreous lead, or sulphate of lead. This mineral closely resembles carbonate of
lead; so that the external characters are inadequate to distinguish the two. But the
following are sufficient. When pure, it has the same transparency and lustre. It does
not effervesce with nitric acid; it is but feebly blackened by sulphuretted hydrogen;
it first decrepitates and then melts before the blowpipe into a transparent glass, which
becomes milky as it cools. By the combined action of heat and charcoal, it passes first into
a red pulverulent oxide, and then into metallic lead. It consists, according to Klaproth,
of 71 oxide of lead, 25 sulphuric acid, 2 water, and 1 iron. That specimen was from
Anglesea; the Wanlockhead mineral is free from iron. The prevailing form of crystallization
is the rectangular octahedron, whose angles and edges are variously modified.
The sulphato-carbonate, and sulphato tri-carbonate of lead, now called Leadhillite, are
rare minerals which belong to this head.
7. Phosphate of lead.—This, like all the combinations of lead with an acid, exhibits no
metallic lustre, but a variety of colours. Before the blowpipe, upon charcoal, it melts
into a globule externally crystalline, which, by a continuance of the heat, with the addition
of iron and boracic acid, affords metallic lead. Its constituents are 80 oxide of lead,
18 phosphoric acid, and 1·6 muriatic acid, according to Klaproth’s analysis of the mineral
from Wanlockhead. The constant presence of muriatic acid in the various specimens
examined is a remarkable circumstance. The crystalline forms are derived from an
obtuse rhomboid. Phosphate of lead is a little harder than white lead; it is easily
scratched, and its powder is always gray. Its specific gravity is 6·9. It has a vitreous
lustre, somewhat adamantine. Its lamellar texture is not very distinct; its fracture is
wavy, and it is easily frangible. The phosphoric and arsenic acids being, according to
M. Mitscherlich, isomorphous bodies, may replace each other in chemical combinations
in every proportion, so that the phosphate of lead may include any proportion, from the
smallest fraction of arsenic acid, to the smallest fraction of phosphoric acid, thus graduating
indefinitely into arseniate of lead. The yellowish variety indicates, for the most
part, the presence of arsenic acid.
8. Muriate of lead. Horn-lead, or murio-carbonate.—This ore has a pale yellow colour,
is reducible to metallic lead by the agency of soda, and is not altered by the hydrosulphurets.
At the blowpipe it melts first into a pale yellow transparent globule, with salt
of phosphorus and oxide of copper; and it manifests the presence of muriatic acid by a
bluish flame. It is fragile, tender, softer than carbonate of lead, and is sometimes almost
colourless, with an adamantine lustre. Spec. grav. 606. Its constituents, according to
Berzelius, are, lead, 25·84; oxide of lead, 57·07; carbonate of lead, 6·25; chlorine,
8·84; silica, 1·46; water, 0·54; in 100 parts. The carbonate is an accidental ingredient,
not being in equivalent proportion. Klaproth found chlorine, 13·67; lead, 39·98;
oxide of lead, 22·57; carbonate of lead, 23·78.
9. Arseniate of lead.—Its colour of a pretty pure yellow, bordering slightly on the
greenish, and its property of exhaling by the joint action of fire and charcoal a very
distinct arsenical odour, are the only characters which distinguish this ore from the phosphate
of lead. The form of the arseniate of lead when it is crystallized, is a prism with
six faces, of the same dimensions as that of phosphate of lead. When pure, it is reducible
upon charcoal, before the blowpipe, into metallic lead, with the copious exhalation[747]
of arsenical fumes; but only in part, and leaving a crystalline globule, when it contains
any phosphate of lead. The arseniate of lead is tender, friable, sometimes even pulverulent,
and of specific gravity 5·04. That of Johann-Georgenstadt consists, according to
Rose, of oxide of lead 77·5; arsenic acid 12·5; phosphoric acid 7·5, and muriatic
acid 1·5.
10. Red lead, or Chromate of lead.—This mineral is too rare to require consideration
in the present work.
11. Plomb vauquelinite. Chromate of lead and copper.
12. Yellow lead. Molybdate of lead.
13. Tungstate of lead.
Having thus enumerated the several species of lead ore, we may remark, that galena
is the only one which occurs in sufficiently great masses to become the object of mining
and metallurgy. This mineral is found in small quantity among the crystalline primitive
rocks, as granite. It is however among the oldest talc-schists and clay slates, that
it usually occurs. But galena is much more abundant among the transition rocks,
being its principal locality, where it exists in interrupted beds, masses, and more rarely
in veins. The blackish transition limestone is of all rocks that which contains most
galena; as at Pierreville in Normandy; at Clausthal, Zellerfeldt, and most mines of
the Harz; at Fahlun, in Sweden; in Derbyshire and Northumberland, &c. In the
transition graywacke of the south of Scotland, the galena mines of Leadhills occur. The
galena of the primitive formations contains more silver than that of the calcareous.
The principal lead mines at present worked in the world, are the following: 1.
Poullaouen and Huelgoët near Carhaix in France, department of Finisterre, being veins
of galena, which traverse a clay slate resting upon granite. They have been known for
upwards of three centuries; the workings penetrate to a depth of upwards of 300 yards,
and in 1816 furnished 500 tons of lead per annum, out of which 1034 pounds avoirdupois
of silver were extracted. 2. At Villeforte and Viallaz, department of the Lozère,
are galena mines said to produce 100 tons of lead per annum, with 400 kilogrammes of
silver (880 libs. avoird.). 3. At Pezey and Macot, to the east of Moutiers in Savoy, a
galena mine exists in talc-schist, which has produced annually 200 tons of lead, and
about 600 kilogrammes of silver (1320 libs avoird.). 4. The mine of Vedrin, near
Namur in the Low Countries, is opened upon a vein of galena, traversing compact
limestone of a transition district; it has furnished 200 tons of lead, from which 385
pounds avoird. of silver were extracted. 5. In Saxony the galena mines are so rich in
silver as to make the lead be almost overlooked. They are enumerated under silver ores.
6. The lead mines of the Harz, have been likewise considered as silver ores. 7. Those of
Bleyberg in the Eifel are in the same predicament. 8. The galena mines of Bleyberg and
Villach in Carinthia, in compact limestone. 9. In Bohemia, to the south-west of Prague.
10. The mines of Joachimsthal, and Bleystadt, on the southern slope of the Erzgebirge,
produce argentiferous galena. 11. There are numerous lead mines in Spain, the most
important being in the granite hills of Linarès, upon the southern slope of the Sierra
Morena, and in the district of the small town of Canjagar. Sometimes enormous
masses of galena are extracted from the mines of Linarès. There are also mines of
galena in Catalonia, Grenada, Murcia, and Almeria, the ore of the last locality being
generally poor in silver. 12. The lead mines of Sweden are very argentiferous, and
worked chiefly with a view to the silver. 13. The lead mines of Daouria are numerous
and rich, lying in a transition limestone, which rests on primitive rocks; their
lead is neglected on account of the silver.
14. Of all the countries in the world, Great Britain is that which annually produces
the greatest quantity of lead. According to M. Villefosse, in his Richesse Minerale, published
in 1810, we had furnished every year 12,500 tons of lead, whilst all the rest of
Europe taken together, did not produce so much; but from more recent documents,
that estimate seems to have been too low. Mr. Taylor has rated the total product of the
United Kingdom per annum at 31,900 tons, a quantity fully 21⁄2 times greater than the
estimate of Villefosse (see Conybeare and Phillips’ Geology, p. 354). Mr. Taylor
distributes this product among the different districts as follows:—
| |
Tons. |
| Wales, (Flintshire and Denbighshire) |
7,500 |
| Scotland, (in transition graywacke) |
2,800 |
| Durham, Cumberland, and Yorkshire, (in carboniferous lime) |
19,000 |
| Derbyshire, (probably in carboniferous lime) |
1,000 |
| Shropshire |
800 |
| Devon and Cornwall, (transition and primitive rocks) |
800 |
| Total |
31,900 |
We thus see that Cumberland, and the adjacent parts of the counties of Durham and[748]
York, furnish of themselves nearly three-fifths of the total product. Derbyshire was
formerly much more productive. In Cornwall and Devonshire, the lead ore is found
in veins in killas, a clay-slate passing into greywacke. In North Wales and the adjacent
counties, as well as in Cumberland and Derbyshire, the lead occurs in the carboniferous
limestone.
The English lead-miners distinguish three different kinds of deposits of lead ore;
rake-veins, pipe-veins, and flat-veins. The English word vein corresponds to the French
term filon; but miners make use of it indifferently in England and France, to indicate
all the deposits of this ore, adding an epithet to distinguish the different forms; thus,
rake veins are true veins in the geological acceptation of the word vein; pipe-veins are
masses usually very narrow, and of oblong shape, most frequently parallel to the plane
of the rocky strata; and flat-veins are small beds of ores interposed in the middle of
these strata.
Rake-veins are the most common form in which lead ore occurs in Cumberland.
They are in general narrower in the sandstone which covers the limestone, than in the
calcareous beds. A thickness of less than a foot in the former, becomes suddenly 3 or
4 feet in the latter; in the rich vein of Hudgillburn, the thickness is 17 feet in the
Great limestone, while it does not exceed 3 feet in the overlying Watersill or sandstone.
This influence exercised on the veins by the nature of the enclosing rock, is instructive;
it determines at the same time almost uniformly their richness in lead ore, an observation
similar to what has been made in other countries, especially in the veins of Kongsberg
in Norway. The Cumberland veins are constantly richer, the more powerful they are, in
the portions which traverse the calcareous rocks, than in the beds of sandstone, and more
particularly the schistose rocks. It is rare in the rock called plate (a solid slaty clay)
for the vein to include any ore; it is commonly filled with a species of potter’s earth.
The upper calcareous beds are also in general more productive than the lower ones. In
most of these mines, the veins were not worked till lately below the fifth calcareous bed
(the four-fathom limestone), which is 307 yards beneath the millstone-grit; and as the
first limestone stratum is 108 yards beneath it, it follows that the thickness of the part
of the ground where the veins are rich in lead does not in general exceed 200 yards. It
appears however that veins have been mined in the neighbourhood of Alston Moor,
downwards to the eleventh calcareous stratum, or Tyne bottom limestone, which is 418
yards under the millstone-grit of the coal formation, immediately above the whin-sill;
and that they have been followed above the first limestone stratum, as high as the grindstone
sill, which is only 83 yards below the same stratum of millstone-grit; so that in
the total thickness of the plumbiferous formation there is more than 336 yards. It has
been asserted that lead veins have been traced even further down, into the Memerby
scar limestone; but they have not been mined.
The greatest enrichment of a vein takes place commonly in the points where its two
sides, being not far asunder, belong to the same rock; and its impoverishment occurs
when one side is calcareous and the other a schistose clay. The minerals which most
frequently accompany the galena, are carbonate of lime, fluate of lime, sulphate of
baryta, quartz, and pyrites.
The pipe-veins (amas in French), are seldom of great length; but some have a
considerable width; their composition being somewhat similar to that of the rake-veins.
They meet commonly in the neighbourhood of the two systems, sometimes being in
evident communication together; they are occasionally barren; but when a wide pipe-vein
is metalliferous, it is said to be very productive.
The flat veins, or strata veins, seem to be nothing else than expansions of the matter
of the vein between the planes of the strata; and contain the same ores as the veins in
their vicinity. When they are metalliferous, they are worked along with the adjacent
rake vein; and are productive to only a certain distance from that vein, unless they get
enriched by crossing a rake vein. Some examples have been adduced of advantageous
workings in flat veins in the great limestone of Cumberland, particularly in the mines of
Coalcleugh and Nenthead. The rake veins, however, furnish the greater part of the
lead which Cumberland and the adjacent counties send every year into the market.
Mr. Forster gives a list of 165 lead mines, which have been formerly, or are now, worked
in that district of the kingdom.
The metalliferous limestone occupies, in Derbyshire, a length of about 25 miles from
north-west to south-east, under a very variable breadth, which towards the south,
amounts to 25 miles. Castleton to the north, Buxton to the north-west, and Matlock
to the south-east, lie nearly upon its limits. It is surrounded on almost all sides by the
millstone grit which covers it, and which is, in its turn, covered by the coal strata.
The nature of the rocks beneath the limestone is not known. In Cumberland the
metalliferous limestone includes a bed of trap, designated under the name of whinsill.
In Derbyshire the trap is much more abundant, and it is thrice interposed between the
limestone. These two rocks constitute of themselves the whole mineral mass, through a[749]
thickness of about 550 yards, measuring from the millstone grit; only in the upper
portion, that is near the millstone grit, there is a pretty considerable thickness of argillo-calcareous
schists.
Four great bodies or beds of limestone are distinguishable, which alternate with three
masses of trap, called toadstone. The lead veins exist in the calcareous strata, but
disappear at the limits of the toadstone. It has now been ascertained however that they
recur in the limestone underneath.
Treatment of the Ores of Lead.
The mechanical operations performed upon the lead ores in Great Britain, to bring
them to the degree of purity necessary for their metallurgic treatment, may be divided
into three classes, whose objects are,—
1. The sorting and cleansing of the ores;
2. The grinding;
3. The washing, properly so called.
The apparatus subservient to the first objects are sieves, running buddles, and gratings.
The large sieves employed in Derbyshire for sorting the ore at the mouth of the mine,
into coarse and fine pieces, is a wire gauze of iron; its meshes are square, and an inch
long in each side. There is a lighter sieve of wire gauze, similar to the preceding, for
washing the mud from the ore, by agitating the fragments in a tub filled with water.
But in Derbyshire, instead of using this sieve, the pieces of ore are sometimes merely
stirred about with a shovel, in a trough filled with water. This is called a standing
buddle; a most defective plan.
The running buddle serves at once to sort and cleanse the ore. It consists of a plane
surface made of slabs or planks, very slightly inclined forwards, and provided behind
and on the sides with upright ledges, the back one having a notch to admit a stream of
water. The ore is merely stirred about with a shovel, and exposed on the slope to the
stream. For this apparatus, formerly the only one used at the mines of Alston Moor,
the following has been substituted, called the grate. It is a grid, composed of square
bars of iron, an inch thick, by from 24 to 32 inches long, placed horizontally, and
parallelly to each other, an inch apart. There is a wooden canal above the grate, which
conducts a stream of water over its middle; and an inclined plane is set beneath it,
which leads to a hemispherical basin, about 24 inches inches in diameter, for collecting
the metallic powder washed out of the ore.
The apparatus subservient to grinding the ore are,—
1. The bucker, or beater, formed of a cast-iron plate, 3 inches square, with a socket
in its upper surface, for receiving a wooden handle. In the neighbourhood of Alston
Moor, crushing cylinders have been substituted for the beating bucker; but even now,
in Derbyshire, buckers are generally employed for breaking the pieces of mixed ore,
called knock-stone-stuff.
At the mines of this county, the knocker’s workshop, or striking floor, is provided
either with a strong stool, or a wall 3 feet high, beyond which there is a flat area 4 feet
broad, and a little raised behind. On this area, bounded, except in front, by small
walls, the ore to be bruised is placed. On the stool, or wall, a very hard stone slab, or
cast-iron plate is laid, 7 feet long, 7 inches broad, and 11⁄2 inches thick, called a knock-stone.
The workmen seated before it, break the pieces of mixed ore, called bowse in
Derbyshire, with the bucker.
Crushing machines are in general use at Alston Moor, to break the mingled ores,
which they perform with great economy of time and labour. They have been employed
there for nearly forty years.
This machine is composed of one pair of fluted cylinders, x x, fig. 628., and of two pairs
of smooth cylinders z z, z z, which serve altogether for crushing the ore. The two
cylinders of each of the three pairs turn simultaneously in an inverse direction, by
means of two toothed wheels, as at m, fig. 629., upon the shaft of every cylinder, which
work by pairs in one another. The motion is given by a single water wheel, of which
the circle a a a represents the outer circumference. One of the fluted cylinders is
placed in the prolongation of the shaft of this wheel, which carries besides a cast-iron
toothed wheel, geered with the toothed wheels e e, fixed upon the ends of two
of the smooth cylinders. Above the fluted cylinders, there is a hopper, which discharges
down between them, by means of a particular mechanism, the ore brought
forward by the waggons A. These waggons advance upon a railway, stop above the
hopper, and empty their contents into it through a trap-hole, which opens outwardly in
the middle of their bottom. Below the hopper there is a small bucket called a shoe,
into which the ore is shaken down, and which throws it without ceasing upon the
cylinders, in consequence of the constant jolts given it by a crank-rod i (fig. 629.)
attached to it, and moved by the teeth of the wheel m. The shoe is so regulated, that
too much ore can never fall upon the cylinders, and obstruct their movement. A small[750]
stream of water is likewise led into the shoe, which spreads over the cylinders, and
prevents them from growing hot. The ore, after passing between the fluted rollers,
falls upon the inclined planes N, N, which turn it over to one or other of the pairs of
smooth rolls.
These are the essential parts of this machine; they are made of iron, and the smooth
ones are case-hardened, or chilled, by being cast in iron moulds. The gudgeons of
both kinds move in brass bushes fixed upon iron supports k, made fast by bolts to the
strong wood-work basis of the whole machine. Each of the horizontal bars has an
oblong slot, at one of whose ends is solidly fixed one of the plummer-blocks or bearers
of one of the cylinders f, and in the rest of the slot the plummer-block of the other
cylinder g slides; a construction which permits the two cylinders to come into contact,
or to recede to such a distance from each other, as circumstances may require.
The movable cylinder is approximated to the fixed one by means of the iron levers X X,
which carry at their ends the weights P, and rest upon wedges M, which may be slidden
upon the inclined plane N. These wedges then press the iron bar O, and make it approach
the movable cylinder by advancing the plummer-block which supports its axis.
When matters are so arranged, should a very large or hard piece present itself to one of
the pairs of cylinders, one of the rollers would move away, and let the piece pass without
doing injury to the mechanism.
Besides the three pairs of cylinders which constitute essentially each crushing machine,
there is sometimes a fourth, which serves to crush the ore when not in large fragments,
for example, the chats and cuttings (the moderately rich and poorer pieces), produced
by the first sifting with the brake sieve, to be presently described. The cylinders
composing that accessory piece, which, on account of their ordinary use, are called chats-rollers,
are smooth, and similar to the rollers z z, and z z. The one of them is usually
placed upon the prolongation of the shaft of the water-wheel, of the side opposite to
the principal machine; and the other, which is placed alongside, receives its motion from
the first, by means of toothed wheel-work.
The stamp mill is employed in concurrence with the crushing cylinders. It serves
particularly to pulverize those ores whose gangue is too hard to yield readily to the rollers,
and also those which being already pulverized to a certain degree, require to be
ground still more finely. The stamps employed in the neighbourhood of Alston Moor
are moved by water wheels. They are similar to those described under Tin.
Proper sifting or jigging apparatus.—The hand sieve made of iron wire meshes, of
various sizes, is shaken with the two hands in a tub of water, the ore vat, being held
sometimes horizontally, and at others in an inclined position. This sieve is now in
general use only for the cuttings that have passed through the grating, and which though
not poor enough to require finer grinding, are too poor for the brake sieve. When the
workman has collected a sufficient quantity of these smaller pieces, he puts them in his
round hand sieve, shakes it in the ore vat with much rapidity and a dexterous toss, till
he has separated the very poor portions called cuttings, from the mingled parts called
chats, as well as from the pure ore. He then removes the first two qualities, with a[751]
sheet-iron scraper called a limp, and he finds beneath them, a certain portion of ore
which he reckons to be pure.
The brake sieve is rectangular, as well as the cistern in which it is agitated. The
meshes are made of strong iron wire, three-eighths of an inch square. This sieve is suspended
at the extremity of a forked lever, or brake, turning upon an axis by means of
two upright arms about 5 feet long, which are pierced with holes for connecting them
with bolts or pins, both to the sieve-frame and to the ends of the two branches of the
lever. These two arms are made of wrought iron, but the lever is made of wood; as it
receives the jolt. A child placed near its end, by the action of leaping, jerks it smartly
up and down, so as to shake effectually the sieve suspended at the other extremity.
Each jolt not only makes the fine parts pass through the meshes, but changes the relative
position of those which remain on the wires, bringing the purer and heavier pieces
eventually to the bottom. The mingled fragments of galena, and the stony substances
called chats lie above them; while the poor and light pieces called cuttings, are at
top. These are first scraped off by the limp, next the mixed lumps, or chats, and lastly
the pure ore, which is carried to the bing heap. The cuttings are handed to a particular
class of workmen, who by a new sifting, divide them into mere stones, or second cuttings,
and into mixed ore analogous to chats.
The poor ore, called chats, is carried to a crushing machine, where it is bruised
between two cylinders appropriated to this purpose under the name of chats rollers; after
which it is sifted afresh. During the sifting many parcels of small ore and stony substances
pass through the sieve, and accumulate at the bottom of the cistern. When it
is two-thirds filled, water is run slowly over it, and the sediment called smitham is taken
out, and piled up in heaps. More being put into the tub, a child lifts up the smitham,
and lays it on the sieve, which retains still on its meshes the layer of fine ore. The sifter
now agitates in the water nearly as at first, from time to time removing with the limp
the lighter matters as they come to the surface; which being fit for washing only in
boxes, are called buddler’s offal, and and are thrown into the buddle hole.
Mr. Petherick, the manager of Lanescot and the Fowey Consol mines, has contrived
an ingenious jigging machine, in which a series of 8 sieves are fixed in a stationary circular
frame, connected with a plunger or piston working in a hollow cylinder, whereby
a body of water is alternately forced up through the crushed ore in the sieves, and
then left to descend. In this way of operating, the indiscriminate or premature passage
of the finer pulverulent matter through the meshes is avoided, because a regulated
stream of water is made to traverse the particles up and down. This mode has proved
profitable in washing the copper ores of the above mentioned copper mines.
Proper washing apparatus.—For washing the ore after sifting it, the running buddle
already described is employed, along with several chests or buddles of other kinds.
1. The trunk buddle is a species of German chest (see Metallurgy and Tin) composed
of two parts; of a cistern or box into which a stream of water flows, and of a
large tank with a smooth level bottom. The ore to be trunked being placed in the box,
the workman furnished with a shovel bent up at its sides, agitates it, and removes from
time to time the coarser portions; while the smaller are swept off by the water and
deposited upon the level area.
2. The stirring buddle, or chest for freeing the schlamms or slimy stuff from clay, is
analogous to the German chests, and consists of two parts; namely, 1. a trough which
receives a stream of water through a plug hole, which is tempered at pleasure, to admit a
greater or less current; 2. a settling tank with a horizontal bottom. The metallic slime
being first floated in the water of the trough, then flows out and is deposited in the tank;
the purest parts falling first near the beginning of the run.
3. The nicking buddle is analogous to the tables called dormantes or jumelles by the
French miners. See Metallurgy. They have at their upper end a cross canal or
spout, equal in length to the breadth of the table, with a plug hole in its middle for
admitting the water. Alongside of this channel there is a slightly inclined plank, called
nicking board, corresponding to the head of the twin table, and there is a nearly level
plane below. The operation consists in spreading a thin layer of the slime upon the
nicking board, and in running over its surface a slender sheet of water, which in its progress
is subdivided into rills, which gradually carry off the muddy matters, and strew
them over the lower flat surface of the tank, in the order of their density.
4. The dolly tub or rinsing bucket, fig. 630., has an upright shaft,
which bears the vane or dolly A B, turned by the winch handle. This
apparatus serves to bring into a state of suspension in water, the fine
ore, already nearly pure; the separation of the metallic particles from
the earthy ones by repose, being promoted by the sides of the tub being
struck frequently during the subsidence.
5. Slime pits.—In the several operations of cleansing ores from mud,
in grinding, and washing, where a stream of water is used, it is impossible to prevent[752]
some of the finely attenuated portions of the galena called sludge, floating in the water,
from being carried off with it. Slime pits or labyrinths, called buddle holes in Derbyshire,
are employed to collect that matter, by receiving the water to settle, at a little
distance from the place of agitation.
These basins or reservoirs are about 20 feet in diameter, and from 24 to 40 inches
deep. Here the suspended ore is deposited, and nothing but clear water is allowed to
escape.
The workmen employed in the mechanical preparation of the ores, are paid, in Cumberland,
by the piece, and not by day’s wages. A certain quantity of crude ore is
delivered to them, and their work is valued by the bing, a measure containing 14 cwt.
of ore ready for smelting. The price varies according to the richness of the ore. Certain
qualities are washed at the rate of two and sixpence, or three shillings the bing;
while others are worth at least ten shillings. The richness of the ore varies from 2 to
20 bings of galena per shift of ore; the shift corresponding to 8 waggons load.
1. The cleansing and sorting of the ores are well performed in Cumberland. These
operations seem however to be inferior to the cleansing on the grid steps, grilles à gradin,
of Saxony (see Metallurgy), an apparatus which in cleaning the ores, has the advantage
of grouping them in lots of different qualities and dimensions.
2. The breaking or bruising by means of the crushing machine, is much more expeditious
than the Derbyshire process by buckers; for the machine introduces not only
great economy into the breaking operation, but it likewise diminishes considerably the
loss of galena; for stamped ores may be often subjected to the action of the cylinders
without waste, while a portion of them would have been lost with the water that runs
from the stamp mill. The use of these rollers may therefore be considered as one of
the happiest innovations hitherto made in the mechanical preparation of ores.
3. The brake sieves appear to be preferable to the hand ones.
4. The system of washing used in Cumberland differs essentially from that of Brittany.
The slime pits are constructed with much less care than in France and Germany.
They never present, as in these countries, those long windings backwards and forwards,
whence they have been called labyrinths; probably because the last deposits, which are
washed with profit in France and Germany, could not be so in Cumberland. There is
reason to believe, however, that the introduction of brake tables, (tables à secousses, see
Metallurgy) would enable deposits to be saved, which at present run to waste in
England.
5. From what we have now said about the system of washing, and the basins of
deposit or settling cisterns, it may be inferred that the operation followed in Cumberland
is more expeditious than that used in Brittany, but it furnishes less pure ores, and
occasions more considerable waste; a fact sufficiently obvious, since the refuse stuff at
Poullaouen is often resumed, and profitably subjected to a new preparation. We cannot
however venture to blame this method, because in England, fuel being cheap, and
labour dear, there may possibly be more advantage in smelting an ore somewhat impure,
and in losing a little galena, than in multiplying the number of washing processes.
6. Lastly, the dolly tub ought to be adopted in all the establishments where the galena
is mixed with much blende (sulphuret of zinc); for schlich (metallic slime) which appears
very clean to the eye, gives off a considerable quantity of blende by means of
the dolly tub. While the vane is rapidly whirled, the sludge is gradually let down into
the revolving water, till the quantity is sufficiently great. Whenever the ore is thoroughly
disseminated in the liquid, the dolly is withdrawn. The workmen then strike on the
sides of the tub for a considerable time, with mallets or wooden billets, to make the
slime fall fast to the bottom. The lighter portions, consisting almost entirely of refuse matter,
fall only after the knocking has ceased; the water is now run away; then the
very poor slime upon the top of the deposit is skimmed off; while the pure ore found
at the bottom of the tub is lifted out, and laid on the bingstead. In this way the blende,
which always accompanies galena in a greater or smaller quantity, is well separated.
Smelting of lead ores.—The lead ores of Derbyshire and the north of England were
antiently smelted in very rude furnaces, or boles, urged by the natural force of the wind,
and were therefore placed on the summits or western slopes of the highest hills. More
recently these furnaces were replaced by blast hearths, resembling smith’s forges, but
larger; and were blown by strong bellows, moved by men or water-wheels. The
principal operation of smelting is at present always executed in Derbyshire in reverberatory
furnaces, and at Alston Moor in furnaces similar to those known in France by
the name of Scotch furnaces. Before entering into the detail of the founding processes,
we shall give a description of the furnaces essential for both the smelting and accessory
Operations.
1. The reverberatory furnace called cupola, now exclusively used in Derbyshire for
the smelting of lead ores, was imported thither from Wales, about the year 1747, by a
company of Quakers. The first establishment in this county was built at Kalstedge, in
the district of Ashover.
[753]
In the works where the construction of these furnaces is most improved, they are
interiorly 8 feet long by 6 wide in the middle, and two feet high at the centre. The
fire, placed at one of the extremities, is separated from the body of the furnace by a body
of masonry, called the fire-bridge, which is two feet thick, leaving only from 14 to 18
inches between its upper surface and the vault. From this, the highest point, the vault
gradually sinks towards the further end, where it stands only 6 inches above the sole.
At this extremity of the furnace, there are two openings separated by a triangular prism
of fire-stone, which lead to a flue, a foot and a half wide, and 10 feet long, which is
recurved towards the top, and runs into an upright chimney 55 feet high. The above
flue is covered with stone slabs, carefully jointed with fire-clay, which may be removed
when the deposit formed under them (which is apt to melt), requires to be cleaned out.
One of the sides of the furnace is called the labourers’ side. It has a door for throwing
coal upon the fire-grate, besides three small apertures each about 6 inches square.
These are closed with movable plates of cast iron, which are taken off when the working
requires a freer circulation of air, or for the stirring up of the materials upon the hearth.
On the opposite side, called the working side, there are five apertures; namely, three
equal and opposite to those just described, shutting in like manner with cast iron plates,
and beneath them two other openings, one of which is for running out the lead, and
another for the scoriæ. The ash pit is also on this side, covered with a little water, and
so disposed as that the grate-bars may be easily cleared from the cinder slag.
The hearth of the furnace is composed of the reverberatory furnace slags, to which a
proper shape has been given by beating them with a strong iron rake, before their
entire solidification. On the labourers’ side, this hearth rises nearly to the surface of
the three openings, and falls towards the working side, so as to be 18 inches below the
middle aperture. In this point, the lowest of the furnace, there is a tap-hole, through
which the lead is run off into a large iron boiler (lea-pan), placed in a recess left outside
in the masonry. From that lowest point, the sole gradually rises in all directions,
forming thus an inside basin, into which the lead runs down as it is smelted. At the
usual level of the metal bath, there is on the working side, at the end furthest from
the fire, an aperture for letting off the slag.
In the middle of the arched roof there is a small aperture, called the crown-hole, which
is covered up during the working with a thick cast iron plate. Above this aperture a
large wooden or iron hopper stands, leading beneath into an iron cylinder, through
which the contents of the hopper may fall into the furnace when a trap or valve is
opened.
2. The roasting furnace.—This was introduced about 30 years ago, in the neighbourhood
of Alston Moor, for roasting the ore intended to pass through the Scotch
furnace, a process which greatly facilitates that operation. Since its first establishment
it has successively received considerable improvements.
Figs. 631, 632, 633., represent the cupola furnace at the Marquess of Westminster’s
lead smelting works, two miles from Holywell. The hearth is hollowed out below the[754]
middle door of the furnace; it slopes from the back and ends towards this basin. The
distance from the lowest point of this concavity up to the sill of the door, is usually 24
inches, but it is sometimes a little less, according to the quality of the ores to be smelted.
This furnace has no hole for running off the slag, above the level of the top hole for the
lead i, like the smelting furnace of Lea, near Matlock. A single chimney stalk serves
for all the establishments; and receives all the flues of the various roasting and reducing
furnaces. Fig. 633. gives an idea of the distribution of these flues. a a a, &c. are the
furnaces; b, the flues, 18 inches square; these lead from each furnace to the principal
conduit c, which is 5 feet deep by 21⁄2 wide; d is 6 feet deep by 3 wide; e is a round
chamber 15 feet in diameter; f is a conduit 7 feet high by 5 wide; g another, 6 feet
high by 3 wide. The chimney at h has a diameter at bottom of 30 feet, at top of 12
feet, including the thickness of its sides, forming a truncated cone 100 feet high; whose
base stands upon a hill a little way from the furnaces, and 62 feet above their level.
a, figs. 631, 632., is the grate; b, the door of the fire-place; c, the fire-bridge; d, the
arched roof; e, the hearth; f f f, &c., the working doors; g g, flues running into one
conduit, which leads to the subterranean condensing chamber, e, and thence to the
general chimney; h, a hopper-shaped opening in the top of the furnace, for supplying
it with materials.
This magnificent structure is not destined solely for the reduction of the ores, but for
dissipating all the vapours which might prove noxious to the health of the work-people
and to vegetation.
The ores smelted at Holywell are very refractory galenas, mixed with blende, calamine,
pyrites, carbonate of lime, &c., but without any fluate of lime. They serve mutually as
fluxes to one another. The coal is of inferior quality. The sole of each furnace is
formed of slags obtained in the smelting, and they are all of one kind. In constructing
it, 7 or 8 tons of these slags are first of all thrown upon the brick area of the hearth;
are made to melt by a brisk fire, and in their stiffening state, as they cool, they permit
the bottom to be sloped and hollowed into the desired shape. Four workmen, two
at each side of the furnace, perform this task.
The ordinary charge of ore for one smelting operation is 20 cwt., and it is introduced
through the hopper; see Copper, fig. 304. An assistant placed at the back doors spreads
it equally over the whole hearth with a rake; the furnace being meanwhile heated only
with the declining fire of a preceding operation. No regular fire is made during the first
two hours, but a gentle heat merely is kept up by throwing one or two shovelfuls of
small coal upon the grate from time to time. All the doors are closed, and the register-plate
of the chimney is lowered.
The outer basin in front of the furnace is at this time filled with the lead derived
from a former process, the metal being covered with slags. A rectangular slit above the
tap hole is left open, and remains so during the whole time of the operation, unless the
lead should rise in the interior basin above the level of that orifice; in which case a
little mound must be raised before it.
The two doors in front furthest from the fire being soon opened, the head-smelter
throws in through them, upon the sole of the furnace, the slags swimming upon the
bath of lead, and a little while afterwards he opens the tap-hole, and runs off the metallic
lead reduced from these slags. At the same time his assistant turns over the ore
with his paddle, through the back doors. These being again closed, while the above
two front doors are open, the smelter throws a shovelful of small coal or coak cinder
upon the lead bath, and works the whole together, turning over the ore with the paddle
or iron oar. About three quarters of an hour after the commencement of the operation,
he throws back upon the sole of the hearth the fresh slags which then float upon the
bath of the outer basin, and which are mixed with coaly matter. He next turns over
these slags, as well as the ore with the paddle, and shuts all the doors. At this time the
smelter runs off the lead into the pig-moulds.
The assistant now turns over the ore once more through the back doors. A little
more than an hour after the operation began, a quantity of lead proceeding from the
slag last remelted, is run off by the tap; being usually in such quantity as to fill one
half of the outer basin. Both the workmen then turn over the ore with the paddles, at
the several doors of the furnace. Its interior is at this time of a dull red heat; the
roasting being carried on rather by the combustion of the sulphurous ingredients, than
by the action of the small quantity of coal in the grate. The smelter, after shutting
the front doors, with the exception of that next the fire-bridge, lifts off the fresh slags
lying upon the surface of the outside bath, drains them, and throws them back into
the furnace.
An hour and a half after the commencement, the lead begins to ooze out in small
quantities from the ore; but little should be suffered to flow before two hours have expired.
About this time the two workmen open all the doors, and turn over the ore,
each at his own side of the furnace. An hour and three quarters after the beginning,[755]
there are few vapours in the furnace, its temperature being very moderate. No more
lead is then seen to flow upon the sloping hearth. A little coal being thrown into the
grate to raise the heat slightly, the workmen turn over the ore, and then close all the
doors.
At the end of two hours, the first fire or roasting being completed, and the doors
shut, the register is to be lifted a little, and coal thrown upon the grate to give the
second fire, which lasts during 25 minutes. When the doors are now opened, the inside
of the furnace is of a pretty vivid red, and the lead flows down from every side towards
the inner basin. The smelter with his rake or paddle pushes the slags upon that basin
back towards the upper part of the sole, and his assistant spreads them uniformly over
the surface through the back doors. The smelter next throws in by his middle door, a
few shovelfuls of quicklime upon the lead bath. The assistant meanwhile, for a quarter
of an hour, works the ore and the slags together through the three back doors, and then
spreads them out, while the smelter pushes the slags from the surface of the inner basin
back to the upper parts of the sole. The doors being now left open for a little, while
the interior remains in repose, the metallic lead, which had been pushed back with the
slags, flows down into the basin. This occasional cooling of the furnace is thought to be
necessary for the better separation of the products, especially of the slags from the lead
bath.
In a short time the workmen resume their rakes, and turn over the slags along with
the ore. Three hours after the commencement, a little more fuel is put into the grate,
merely to keep up a moderate heat of the furnace during the paddling. After three
hours and ten minutes, the grate being charged with fuel for the third fire, the register
is completely opened, the doors are all shut, and the furnace is left in this state for three
quarters of an hour. In nearly four hours from the commencement, all the doors being
opened, the assistant levels the surfaces with his rake, in order to favour the descent of
any drops of lead; and then spreads the slags, which are pushed back towards him by
the smelter. The latter now throws in a fresh quantity of lime, with the view not
merely of covering the lead bath and preventing its oxidizement, but of rendering the
slags less fluid.
Ten minutes after the third fire is completed, the smelter puts a new charge of fuel
in the grate, and shuts the doors of the furnace to give it the fourth fire. In four hours
and forty minutes from the commencement, this fire being finished, the doors are
opened, the smelter pierces the tap-hole to discharge the lead into the outer basin, and
throws some quicklime upon the slags in the inner basin. He then pushes the slags
thus dried up towards the upper part of the hearth, and his assistant rakes them out by
the back doors.
The whole operation of a smelting shift takes about four hours and a half, or at most
five hours, in which four periods may be distinguished.
1. The first fire for roasting the ores, requires very moderate firing, and lasts two
hours.
2. The second fire, or the smelting, requires a higher heat, with shut doors; at the
end the slags are dried up with lime, and the furnace is also allowed to cool a little.
3, 4. The last two periods, or the third and fourth fires, are likewise two smeltings or
foundings, and differ from the first only in requiring a higher temperature. The heat
is greatest in the last. The form and dimensions of the furnace are calculated to cause
a uniform distribution of heat over the whole surface of the hearth. Sometimes billets
of green wood are plunged into the metallic lead of the outer basin, causing an ebullition
which favours the separation of the slags, and consequently the production of a
purer lead; but no more metallic metal is obtained.
Ten cwts. of coal are consumed at Holywell in smelting one ton of the lead-ore schlich
or sludge; but at Grassington, near Skipton in Yorkshire, with a similar furnace worked
with a slower heat, the operation taking from seven hours to seven hours and a half,
instead of five, only 71⁄2 cwts. of coal are consumed. But here the ores are less refractory,
have the benefit of fluor spar as a flux, and are more exhausted of their metal, being
smelted upon a less sloping hearth.
Theory of the above operations.—At Holywell, Grassington, and in Cornwall, the
result of the first graduated roasting heat, is a mixture of undecomposed sulphuret of
lead, with sulphate and oxide of lead, in proportions which vary with the degree of care
bestowed upon the process. After the roasting, the heat is raised to convert the sludge
into a pasty mass; in which the oxide and sulphate re-act upon the sulphuret, so as to
produce a sub-sulphuret, which parts with the metal by liquation. The cooling of the
furnace facilitates the liquation every time that the sub-sulphuret is formed, and the ore
has passed by increase of temperature from the pasty into the liquid state. Cooling
brings back the sludge to the pasty condition, and is therefore necessary for the due
separation of the different bodies. The drying up of the thin slags by lime is intended
to liberate the oxide of lead, and allow it to re-act upon any sulphuret which may have[756]
resisted roasting or decomposition. It is also useful as a thickener, in a mechanical point
of view. The iron of the tools, which wear away very fast, is also serviceable in reducing
the sulphuret of lead. The small coal added along with the lime at Grassington,
and also sometimes at Holywell, aids in reducing the oxide of lead, and in transforming
the sulphate into sulphuret.
3. The smelting furnace or ore hearth.—This furnace, called by the French écossais, is
from 22 to 24 inches in height and 1 foot by 11⁄2 in area inside; but its horizontal section,
always rectangular, varies much in its dimensions at different levels, as shown in
fig. 634.
The hearth and the sides are of cast iron; the sole-plate A B is also of cast iron, 21⁄2 inches
thick, having on its back and two sides an upright ledge, A C, 21⁄2 inches thick, and 41⁄4
high. In front of the hearth there is another cast iron plate M N, called the work-stone,
surrounded on every side excepting towards the sole of the furnace, by a ledge
one inch in thickness and height. The plate slopes from behind forwards, and its posterior
ledge, which is about 41⁄2 inches above the surface of the hearth, is separated from
it by a void space q, which is filled with a mixture of bone ash and galena, both in fine
powder, moistened and pressed down together. The melted lead cannot penetrate into
this body, but after filling the basin at the bottom of the furnace, flows naturally out
by the gutter (nearly an inch deep) through a groove in the work-stone; and then
passes into a cauldron of reception P, styled the melting-pot, placed below the front edge
of the work-stone.
The posterior ledge of the sole is surmounted by a piece of cast iron C D, called the
back-stone, 28 inches long, and 61⁄2 high; on which the tuyère or blast-pipe is placed. It
supports another piece of cast iron E, called pipe-stone, scooped out at its under part, in
the middle of its length, for the passage of the tuyère. This piece advances 2 inches into
the interior of the furnace, the back wall of which is finally crowned by another piece
of cast iron E H, called also back-stone.
On the ledges of the two sides of the sole, are placed two pieces of cast iron, called
bearers, each of which is 5 inches in breadth and height, and 26 inches long. They
advance an inch or two above the posterior and highest edge of the work-stone, and contribute
effectually to fix it solidly in its place. These bearers support, through the intervention
of several ranges of fire-bricks, a piece of cast iron called a fore-stone, which
has the same dimensions as the piece called the back-stone, on which the base of the
blowing-machine rests. This piece is in contact, at each of its extremities, with another
mass of cast iron, 6 inches cube, called the key-stone, supported on the masonry. Lastly,
the void spaces left between the two key-stones and the back part of the furnace are filled
up with two masses of cast iron exactly like the key-stones.
The front of the furnace is open for about 12 inches from the lower part of the front
cross-piece called fore-stone, up to the superior part of the work-stone. It is through this
opening that the smelter operates.
The gaseous products of the combustion, on escaping from this ore-hearth, are frequently
made to pass through a long flue, sloped very slightly upwards, in which they
deposit all the particles of ore that they may have swept along; these flues, whose length
is sometimes more than 100 yards, are usually 5 feet high and 3 feet wide in the inside,
and always terminate in a chimney stalk. The matters deposited near the commencement
of the flue require to be washed; but not the other dusty deposits. The whole
may then be carried back to the roasting furnace, to be calcined and re-agglutinated, or
introduced without any preparation into the slag-hearth.
4. Figs. 635, 636. represent a slag-hearth, the fourneau à manche (elbow furnace) of the
French, and the krummofen (crooked furnace) of the Germans; such as is used at Alston
Moor, in Cumberland, for the reduction of the lead-slag. It resembles the Scotch
furnace. The shaft is a parallelopiped, whose base is 26 inches by 22 in area inside, and
whose height is 3 feet; the sole-plate a, of cast iron, slopes slightly down to the basin
of reception, or the fore-hearth b. Upon both of the long sides of the sole-plate there are
cast iron beams, called bearers C C, of great strength, which support the side walls built
of a coarse grained sand-stone, as well as the cast-iron plate d (fore-stone), which forms[757]
the front of the shaft. This stands 7 inches off from the sole-plate, leaving an empty
space between them. The back side is made of cast iron, from the sole-plate to the
horizontal tuyère in its middle; but above this point it is made of sand-stone. The
tuyère is from 11⁄5 to 2 inches in diameter. In front of the fore-hearth b, a cistern e, is
placed, through which water continually flows, so that the slags which spontaneously
overflow the fore-hearth may become inflated and shattered, whereby the lead disseminated
through them may be readily separated by washing. The lead itself flows from
the fore-hearth b, through an orifice, into an iron pot f, which is kept hot over a fire.
The metal obtained from this slag-hearth is much less pure than that extracted directly
from the ore.
The whole bottom of the furnace is filled to a height of 17 inches, that is, to
within 2 or 3 inches of the tuyère, with the rubbish of coke reduced to coarse powder
and beat strongly down. At each smelting shift, this bed must be made anew, and the
interior of the furnace above the tuyère repaired, with the exception of the front, consisting
of cast iron. In advance of the furnace there is a basin of reception, which is
also filled with coke rubbish. Farther off is a pit, full of water, replenished by a cold
stream, which incessantly runs in through a pipe. The scoriæ, in flowing out of the
furnace, pass over the coke bed in the basin of reception, and then fall into the water,
whose coolness makes them fly into small pieces, after which they are easily washed, so
as to separate the lead that may be entangled among them.
These furnaces are urged, in general, by wooden bellows; fig. 637. But at the smelting
works of Lea, near Matlock, the blowing-machine consists of two casks, which move
upon horizontal axes. Each of these casks is divided into two equal parts by a fixed
plane that passes through its axis, and is filled with water to a certain height. The
water of one side communicates with that of the other by an opening in the lower part
of the division. Each cask possesses a movement of oscillation, produced by a rod
attached to a crank of a bucket-wheel. At each demi-oscillation, one of the compartments,
being in communication with the external air, is filled; whilst the other, on the
contrary, communicates with the nozzle, and supplies wind to the furnace.
5. Refining or cupellation furnace. See Silver.
6. Smelting by the reverberatory furnace, is adopted exclusively in Derbyshire, and in
some works at Alston-moor. The charge in the hopper consists commonly of 16 cwt.,
each weighing 120 lbs. avoirdupois, composed of an intimate mixture of 5, 6, 7 or even
8 kinds of ore, derived from different mines, and prepared in different ways. The
proportions of the mixture are determined by experience, and are of great consequence
to the success of the work.
The ore is rather in the form of grains than of a fine schlich; it is sometimes very
pure, and affords 75 per cent.; but usually it is mixed up with a large proportion of carbonate
and fluate of lime; and its product varies from 65 to 23 per cent.
After scraping the slaggy matters out of the furnace, a fresh smelting shift is introduced
at an interval of a few minutes; and thus, by means of two alternate workmen,
who relieve each other every seven or eight hours, the weekly operations continue
without interruption. The average product in lead of the reverberatory furnaces in
Derbyshire, during several years, has been 66 per cent. of the ore. Very fine ore has,
however, afforded 76.
7. Smelting of the drawn slag, on the slag-mill hearth.—The black slag of the reverberatory[758]
furnace is broken by hammers into small pieces, and mixed in proper proportions
with the coal cinders that fall through the grate of the reverberatory fire. The leaden
matts that float on the surface of the bath, and the dust deposited in the chimney,
are added, along with some poor ore containing a gangue of fluor spar and limestone,
which had been put aside during the mechanical preparation. With such a mixture, the
slag-hearth, already described, figs. 635, 636., is charged. By the action of heat and coal,
the lead is revived, the earthy matters flow into very liquid scoriæ, and the whole is
made to pass across the body of fire into a basin of reception placed beneath. The
scoriæ are thickened by throwing quicklime upon them, and they are then raked
away. At the end of the operation the lead is cast into pigs or ingots of a peculiar
form. This is called slag-lead. It is harder, more sonorous than the lead obtained
from the reverberatory furnace, and is preferred for the manufacture of minium,
lead shot, and some other purposes.
8. Treatment of lead ores by the Scotch furnace, or ore-hearth.—This furnace is generally
employed in the counties of Northumberland, Cumberland, and Durham, for the
smelting of lead ores, which were formerly carried to them without any preparation,
but now they are exposed to a preliminary calcination. The roasted ore yields in the
Scotch furnace a more considerable product than the crude ore, because it forms in
the furnace a more porous mass, and at the same time it works drier, to use the
founders’ expression; that is, it allows the stream of air impelled by the bellows to
diffuse itself more completely across the matters contained in the furnace.
The charge of the roasting furnace, figs. 631, 632, 633., is from 9 to 11 cwt. of ore,
put into the furnace without any addition. Three such shifts are usually passed
through in eight hours. The fire should be urged in such a manner as to produce
constantly a dense smoke, without letting any part of the ore melt and form a slag;
an accident which would obstruct the principal end of the process, which is to burn
off the sulphur and antimony, and to expel the carbonic acid of the carbonate of lead.
The ore must be frequently turned over, by moving it from the bridge to the other end
and back again. To prevent the ore from running into masses as it cools, it is made
to fall out of the furnace into a pit full of water, placed below one of the lateral doors.
Smelting of the lead ores in the Scotch furnace.—When a smelting shift has been
finished in the Scotch furnace, a portion of the ore, called browse, remains in a semi-reduced
state, mixed with coke and cinders. It is found of more advantage to preserve
the browse for beginning the following operation, than to take raw or even roasted
ore. To set the furnace in action, the interior of it is filled with peats, cut into the
form of bricks. The peats towards the posterior part are heaped up without order,
but those near the front are piled up with care in the form of a wall. A kindled peat
is now placed before the nozzle of the bellows, which are made to blow, and the blast
spreads the combustion rapidly through the whole mass. To increase the heat, and to
render the fire more steady and durable, a few shovelfuls of coals are thrown over the
turf. A certain quantity of the browse is to be next introduced; and then (or sometimes
before all the browse is put in) the greater part of the matters contained in the
furnace is drawn over on the work-stone, by means of a large rake called a gowelock; the
refuse of the ore called gray slag, which a skilful smelter knows by its shining more
than the browse, is taken off with a shovel, and thrown to the right hand into a corner
outside of the furnace. The browse left on the work-stone is to be now thrown back
into the furnace, with the addition of a little coal, if necessary. If the browse be not
well cleaned from the slag, which is perceived by the whole mass being in a soft state,
and shewing a tendency to fuse, quicklime must be added, which by its affinity for the
argillaceous, siliceous, and ferruginous substances, dries up the materials, as the
smelters say, and gives to the earthy parts the property of concreting into lumps or
balls; but if, on the other hand, the siliceous, argillaceous, or ferruginous parts contained
in the ore be too refractory, lime is also to be added, but in smaller quantity,
which, by rendering them more fusible, communicates the property of concreting into
balls. These lumps, called gray slag, contain from one-tenth to one-fifteenth of the
lead which was present in the ore. They must be smelted afterwards at a higher temperature
in the slag hearth, to extract their lead. After the browse has been thrown
back into the furnace, as has been said, a few shovelfuls of ore are to be strewed over
it; but before doing this, and after removing the scoriæ, there must be always placed
before the tuyère half a peat, a substance which, being extremely porous and combustible,
not only hinders any thing from entering the nozzle of the bellows, but spreads the blast
through all the vacant parts of the furnace. After an interval of from 10 to 15 minutes,
according to circumstances, the materials in the furnace are drawn afresh upon the
work-stone, and the gray slag is removed by the rake. Another peat being placed
before the tuyère, and coal and quicklime being introduced in suitable proportions, the
browse is thrown back into the furnace, a fresh portion of ore is charged above it, and
left in the furnace for the above mentioned time.
[759]
This mode of working, continued for 14 or 15 hours, forms what is called a smelting
shift; in which time from 20 to 40 cwt. of lead, and even more, are produced.
By this process the purest part of the lead, as well as the silver, are sweated out, as it
were, from the materials, with which they are mixed, without any thing entering into
fusion except these two metals in the state of alloy. It is probable that the moderate
temperature employed in the Scotch furnace is the main cause of the purity of the lead
which it yields.
9. Smelting of the scoriæ of the Scotch furnace on the slag hearth.—Before putting fire to
the slag hearth already described, figs. 635, 636., its empty space is to be filled with peats,
and a lighted one being placed before the tuyère, the bellows are made to play. A layer
of coke is to be now thrown upon the burning peats, and as soon as the heat is sufficiently
high, a layer of the gray slag is to be introduced, or of any other scoriæ that are to be
reduced. From time to time, as the fit moment arrives, alternate strata of coke and
slag are to be added. In this operation, though the slag and the lead are brought to a
state of perfect fluidity; the metal gets separated by filtering down through the bed of
peat cinders, which the slag cannot do on account of its viscidity. Whenever that coke
bed becomes covered with fluid slag, the workman makes a hole in it, of about an
inch diameter, by means of a kneed poker; and runs it off by this orifice, as it cannot sink
down into the hard rammed cinders, which fill the basin of reception. The slag flows
over it in a glowing stream into the pit filled with water, where it gets granulated and
ready for washing.
When lead is obtained from galena without the addition of combustible matter, we
have an example on the great scale, of the mutual decomposition of the oxides and sulphates
formed during the roasting heat, by the still undecomposed galena, especially
when this action is facilitated by working up and skilfully mingling the various matters,
as happens in the reverberatory and Scotch furnaces. It is therefore the sulphuret of
lead itself which serves as the agent of reduction in regard to the oxide and sulphate,
when little or no charcoal has been added. Sometimes, however, towards the end of the
operation in the reverberatory hearth, it becomes necessary to throw in some wood or
charcoal, because the oxidizement having become too complete, there does not remain a
sufficient body of sulphuret of lead to effect the decompositions and reductions just
mentioned, and therefore it is requisite to regenerate some galena by means of carbonaceous
matter, which immediately converts the sulphate of lead into the sulphuret. The
sulphur and oxygen are eventually all separated in the form of sulphurous acid.
Roasted galena contains sometimes no less than 77 per cent. of sulphate of lead.
At Viconago in the Valais, the process of smelting lead ore in the reverberatory furnace
with the addition of iron, as practised at Vienne on the Isère, was introduced;
but the difficulty of procuring a sufficient supply of old iron has led to an interesting
modification.
On the hearth of the reverberatory furnace, 10 quintals of moderately rich ore are
spread; these are heated temperately for some time, and stirred about to promote the
sublimation of the sulphur. After three or four hours, when the ore seems to be sufficiently
de-sulphuretted, the heat is raised so as to melt the whole materials, and whenever
they flux into a metallic glass, a few shovelfuls of bruised charcoal or cinders are
thrown in, which soon thicken the liquid, and cause metallic lead to appear. By this
means three-fourths of the lead contained in the ore are usually extracted; but at
length the substance becoming less and less fluid, yields no more metal. Stamped and
washed carbonate of iron (sparry iron ore) is now added, in the proportion of about 10
per cent. of the lead ore primarily introduced.
On stirring and working together this mixture, it assumes the consistence of a stiff
paste, which is raked out of the furnace. When this has become cold, it is broken into
pieces, and thereafter smelted in a slag-hearth, without the addition of flux. By this
operation, almost the whole lead present is obtained. 100 quintals of schlich yield 45
of argentiferous lead; and in the production of 100 quintals (cwts.) of marketable lead,
140 cubic feet of beech-wood, and 3571⁄2 quintals of charcoal are consumed.
This process is remarkable for the use of iron-ore in smelting galena.
10. Reduction in the reverberatory furnace, of the litharge obtained in the refining of
lead.—The litharge of Alston Moor is seldom sold as such, but is usually converted
into lead, in a reverberatory furnace.
In commencing this reduction, a bed of coal about 2 inches thick is first of all laid
on the hearth; which is soon kindled by the flame of the fire-place, and in a little
while is reduced to red hot cinders. Upon these a certain quantity of a mixture of
litharge and small coal is uniformly spread; the heat of the fire-place being meanwhile
so managed as to maintain in the furnace a suitable temperature for enabling the combustible
to deprive the litharge of its oxygen, and to convert it into lead. The metal is
run out by the tap-hole into an iron pot; and being cast into pigs of half a hundred
weight, is sold under the name of refined lead at a superior price.
[760]
The quantity of small coal mixed with the litharge, should be somewhat less than
what may be necessary to effect the reduction, because if in the course of the process,
a deficiency of it is perceived in any part of the furnace, more can always be added;
whereas a redundancy of coal necessarily increases the quantity of slag, which, at the
end of the shift, must be removed from the furnace before a new operation is begun,
whereby lead is lost. In the reverberatory furnace, six fodders of lead may be revived
in nine or ten hours; during the first six of which the mixture of litharge and coal is
added at short intervals. A fodder is from 21 to 24 cwts.
It deserves to be remarked that the work does not go on so well nor so quick when
the coal and litharge are in a pulverulent form; because the reduction in this case
takes place only at the surface, the air not being able to penetrate into the body, and to
keep up its combustion, and the mutual action of the litharge and carbon in the interior.
But on the other hand, when the litharge is in porous pieces as large as a hen’s egg, the
action pervades the whole body, and the sooty fumes of the coal effect the reduction
even in the centre of the fragments of the litharge, penetrating into every fissure and
carrying off the oxygen. The heat ought never to be urged so far as to melt the
litharge.
The grounds of the cupel, and the slag of the reduction furnace, being a mixture of
small coke, coal ash, and oxide of iron, more or less impregnated with lead, are smelted
upon the slag hearth, along with coke, and by way of flux, with a certain quantity of the
black scoriæ obtained from the same furnace, prepared for this purpose, by running it
out in thin plates, and breaking it into small pieces. The lead thus obtained is usually
very white, very hard, and not susceptible of refinement.
MM. Dufrénoy and Beaumont consider the smelting of lead ore by the reverberatory
furnace as practised in Derbyshire, as probably preferable to that with the slag hearth
as carried on in Brittany; a process which seldom gives uniform products, while it
occasions a more considerable waste of lead, and consumption of fuel.
The mixed process employed in Cumberland of roasting the ore, and afterwards
smelting it in a small furnace resembling that called the Scotch, apparently yields a
little less lead than if both operations were executed in the reverberatory furnace; but
according to Mr. Forster, (see his Treatise on a Section of the Strata from Newcastle
upon Tyne, &c.) this slight loss is more than compensated by the smaller consumption
of fuel, the increased rapidity of the operation, and especially by the much greater
purity of the lead obtained from the Scotch furnace. When it comes to be refined, the
loss is only about one-twelfth or one-thirteenth, whereas the lead revived in the reverberatory
furnace, loses frequently a ninth. Moreover, the lead furnished by the first
method admits of being refined with profit, when it yields only 5 ounces of silver per
fodder of 20 quintals, poids de marc, while that produced by the reverberatory furnace
cannot be cupelled unless it gives 10 ounces per fodder; and as in the English cupellation,
lead is constantly added anew without skimming, the litharge obtained in the
second case can never be brought into the market, whereas the litharge of the leads
from the Scotch furnace is of good quality. See the new method of enriching lead for
cupellation, under Silver.
As the smelting of galena, the principal ore of lead, is not a little complex, the following
tabular view of the different processes may prove acceptable to the metallurgist:—
| |
Treatment of |
|
Process of |
|
I. Class.
Treated in reverberatory furnaces. |
- |
|
A
De-sulphuration by roasting. |
- |
|
1. Pure ores |
|
- |
Pesey, Spain, &c. |
|
| 2. Ores mixed with saline gangues. |
|
- |
England, in general. |
| 3. Ores mixed with earthy gangues. |
|
- |
Viconago in Italy, and Redruth in Cornwall. |
| 4. Ores mixed with several sulphurets. |
|
- |
Combined with the above. |
| 5. Ores with earthy saline, and sulphurous gangues. |
|
B
De-sulphuration by iron. |
- |
|
6. Ores with mattes, as at Vienne, in Dauphiny. |
|
- |
Vienne, Poullaouen, and Tarnowitz. |
II. Class.
Treated in the mill-slag-hearth, the fourneau à manche, or Scotch furnace. |
- |
|
A
Founding after roasting in a heap, or in a reverberatory. |
- |
|
7. Ores producing slags of various silicates. |
- |
|
Mattes, with raw lead. |
|
- |
Many places.[761] |
| Workable lead, without mattes. |
|
- |
Villefort. |
| 8. Ores producing compound silicate slags. |
- |
|
Mattes and workable lead. |
|
- |
Several places. |
| Workable lead. |
|
- |
Pont Gibaud and Scotch furnace. |
B
Founding with direct desulphuration by metallic iron. |
- |
|
9. Ores producing slags composed of silicates and subsilicates. |
- |
|
Mattes and workable lead. |
|
- |
Baad-Ems, Hartz, Tarnowitz. |
| - |
|
Poor mattes and workable lead. |
|
- |
Tarnowitz. |
The annual production of lead in Europe may be estimated at about 80,000 tons; of
which four-sevenths are produced in England, two-sevenths in Spain, the remainder in
Germany and Russia. France does not produce more than one five-hundredth part of
the whole; and only one-fiftieth of its consumption.
See Litharge, Minium, or Red Lead, Solder, Sugar or Acetate of Lead, Type
Metal, and White Lead.
LEAD-SHOT; (Plomb de chasse, Fr.; Schrot, Flintenschrot, Germ.) The origin
of most of the imperfections in the manufacture of lead-shot is the too rapid cooling of
the spherules by their being dropped too hot into the water, whereby their surfaces form
a solid crust, while their interior remains fluid, and in its subsequent concretion, shrinks,
so as to produce the irregularities of the shot.
The patent shot towers originally constructed in England obviate this evil by exposing
the fused spherules after they pass through the cullender, to a large body of air during their
descent into the water tub placed on the ground. The greatest erection of this kind is
probably at Villach in Carinthia, being 240 Vienna, or 249 English feet high.
The quantity of arsenic added to the mass of melted lead, varies according to the
quality of this metal; the harder and less ductile the lead is, the more arsenic must
be added. About 3 pounds of either white arsenic or orpiment is enough for one
thousand parts of soft lead, and about 8 for the coarser kinds. The latter are employed
preferably for shot, as they are cheaper and answer sufficiently well. The arsenical alloy
is made either by introducing some of this substance at each melting; or by making
a quantity of the compound considerably stronger at once, and adding a certain portion of
this to each charge of lead. If the particles of the shot appear lens-shaped, it is a proof
that the proportion of arsenic has been too great; but if they are flattened upon one
side, if they are hollowed in their middle, called cupping by the workman, or drag with
a tail behind them, the proportion of arsenic is too small.
The following is the process prescribed by the patentees, Ackerman and Martin.
Melt a ton of soft lead, and sprinkle round its sides in the iron pot, about two shovelfuls
of wood ashes, taking care to leave the centre clear; then put into the middle about
40 pounds of arsenic to form a rich alloy with the lead. Cover the pot with an iron
lid, and lute the joints quickly with loam or mortar to confine the arsenical vapours,
keeping up a moderate fire to maintain the mixture fluid for three or four hours; after
which skim carefully, and run the alloy into moulds to form ingots or pigs. The composition
thus made is to be put in the proportion of one pig or ingot into 1000 pounds
of melted ordinary lead. When the whole is well combined, take a perforated skimmer
and let a few drops of it fall from some height into a tub of water. If they do not
appear globular, some more arsenical alloy must be added.
Lead which contains a good deal of pewter or tin must be rejected, because it tends
to produce elongated drops or tails.
From two to three tons are usually melted at once in the large establishments. The
surface of the lead gets covered with a crust of oxide of a white spongy nature, sometimes
called cream by the workmen, which is of use to coat over the bottom of the cullender,
because without such a bed the heavy melted lead would run too rapidly through
the holes for the granulating process, and would form oblong spheroids. The mounting
of this filter, or lining of the cullender, is reckoned to be a nice operation by the
workmen, and is regarded usually as a valuable secret.
The cullenders are hollow hemispheres of sheet iron about 10 inches in diameter,
perforated with holes, which should be perfectly round and free from burs. These must
be of an uniform size in each cullender; but of course a series of different cullenders
with sorted holes for every different size of lead shot, must be prepared. The holes have
nearly the following diameters for the annexed numbers of shot.
[762]
| No. |
0. |
1⁄50 |
of an inch. |
| |
1. |
1⁄58 |
— |
| |
2. |
1⁄66 |
— |
| |
3. |
1⁄72 |
— |
| |
4. |
1⁄80 |
— |
From No. 5. to No. 9. the diameter decreases by regular gradations, the latter being
only 1⁄360 of an inch.
The operation is always carried on with three cullenders at a time; which are supported
upon projecting grates of a kind of chafing dish made of sheet iron somewhat
like a triangle. This chafing dish should be placed immediately above the fall; while at
its bottom there must be a tub half filled with water for receiving the granulated lead.
The cullenders are not in contact, but must be parted by burning charcoal in order to
keep the lead constantly at the proper temperature, and to prevent its solidifying in the
filter. The temperature of the lead bath should vary with the size of the shot; for the
largest, it should be such that a bit of straw plunged into it will be scarcely browned, but
for all it should be nicely regulated. The height from which the particles should be let
fall varies likewise with the size of the shot; as the congelation is the more rapid, the
smaller they are. With a fall of 33 yards or 100 feet, from No. 4. to No. 9. may be
made; but for larger sizes, 150 feet of height will be required.
Every thing being arranged as above described, the workman puts the filter-stuff into
the cullender, pressing it well against the sides. He next pours lead into it with an
iron ladle, but not in too great quantity at a time, lest it should run through too fast.
The shot thereby formed and found in the tub are not all equal.
The centre of the cullender being less hot affords larger shot than the sides, which are
constantly surrounded with burning charcoal. Occasionally, also, the three cullenders
employed together may have holes of different sizes, in which case the tub may contain
shot of very various magnitudes. These are separated from each other by square
sieves of different fineness, 10 inches broad and 16 inches long, their bottoms being of
sheet iron pierced with holes of the same diameters as those of the cullenders. These
sieves are suspended by means of two bands above boxes for receiving the shot; one
sieve being usually set above another in consecutive numbers, for instance 1 and 2. The
shot being put into the upper sieve, No. O. will remain in it, No. 1. will remain in the
lower sieve, and No. 2. will, with all the others, pass through it into the chest below. It
is obvious that by substituting sieves of successive fineness, shot of any dimension may
be sorted.
In the preceding process the shot has been sorted to size; it must next be sorted to
form, so as to separate all the spheroids which are not truly round, or are defective in
any respect. For this purpose a board is made use of about 27 inches long and 16
broad, furnished partially with upright ledges; upon this tray a handful or two of the
shot to be sorted being laid, it is inclined very slightly, and gently shaken in the horizontal
direction, when the globular particles run down by one edge, into a chest set to
receive them, while those of irregular forms remain on the sides of the tray, and are
reserved to be re-melted.
After being sorted in this way, the shot requires still to be smoothed and polished
bright. This object is effected by putting it into a small octagonal cask, through a
door in its side, turning upon a horizontal iron axis, which rests in plummer boxes at its
ends, and is made to revolve by any mechanical power. A certain quantity of plumbago
or black lead is put in along with the shot.
LAZULITE (Eng. and Fr.; Lazulith, Germ.); is a blue vitreous mineral, crystallizing
in rhomboidal dodecahedrons; spec. grav. 2·76 to 2·94; scratches glass; affords
a little water by calcination; fusible into a white glass; dissolves in acids with loss
of colour; solution leaves an alkaline residuum, after being treated with carbonate of
ammonia, filtered, evaporated, and calcined. It consists of silica, 35·8; alumina, 34·8;
soda, 23·2; sulphur, 3·1; carbonate of lime, 3·1. This beautiful stone affords the
native ultramarine pigment, which was very costly till a mode of making it artificially
was lately discovered. See Ultramarine.
LEATHER, (Cuir, Fr.; Germ., Leder); is the skin of animals, so modified by chemical
means as to have become unalterable by the external agents which tend to decompose
it in its natural state. The preparation in a rude manner of this valuable substance,
has been known from the most antient times, but it was not till the end of the last, and
the beginning of the present century, that it began to be manufactured upon right principles,
in consequence of the researches of Macbride, Deyeux, Seguin, and Davy. There
are several varieties of leather; such as sole leather, boot or upper leather, shamoy leather,
kid or glove leather, &c. Skins may be converted into leather either with or without
their hairy coat.
We shall treat first of sole and upper leathers, being the most important, and most[763]
costly and difficult to prepare in a proper manner. These kinds consist of organized
fibrous gelatine or skin, combined with the proximate vegetable principle, tannin, and
probably also some vegetable extractive. Under the articles Galls and Tannin, will
be found an account of the properties of this substance, and the means of obtaining it in a
state of purity. Calf leather quickly tanned by an infusion of galls, consists of 61 parts
of skin, and 39 of vegetable matter in 100 by weight; by solution of catechu, it consists
of 80 of skin, and 20 of vegetable matter; by infusion of Leicester willow, of 74·5 skin,
and 25·5 vegetable matter; and by infusion of oak bark, of 73·2 skin, and 26·8 vegetable
matter. By the slow process of tanning, continued for three months, the increase of
weight upon the skin in its conversion into leather, is greatly less; the vegetable constituents
being from Leicester willow only 13 per cent. of the leather, and from oak bark
15 per cent. Sole leather, however, generally contains no less than 40 per cent. of vegetable
matter. In every astringent bark, the inner white part next to the alburnum, contains
the largest quantity of tannin, and the middle coloured part contains most extractive
matter. The outer surface or epidermis seldom furnishes either tannin or extractive
matter. Young trees abound most in the white cortical layers, and are hence more productive
of tannin under equal weights, than the barks of old trees. In no case is there
any reason to believe that the gallic acid of astringent vegetables is absorbed in the process
of making leather; hence Seguin’s theory of the agency of that substance in disoxygenating
skin, falls to the ground. The different qualities of leather made with the
same kind of skin, seem to depend very much upon the different quantities of extractive
matter it may have absorbed. The leather made with infusion of galls, is generally
harder and more liable to crack than the leather obtained from infusions of barks; and
it always contains a much larger proportion of tannin, and a smaller proportion of
extractive matter.
When calf skin is slowly tanned in weak solutions of the bark, or of catechu, it combines
with a good deal of extractive matter, and though the increase of the weight of the
skin be comparatively small, yet it has become perfectly insoluble in water, forming a
soft, but at the same time a strong leather. The saturated infusions of astringent barks
contain much less extractive matter in proportion to their tannin, than the weak infusions;
and when skin is quickly tanned in the former, it produces a worse and less
durable leather than when slowly tanned in the latter. In quick tanning, a considerable
quantity of vegetable extractive matter is thus lost to the manufacturer, which might
have been made to enter as a useful constituent into the leather. These observations
show that there is sufficient foundation for the opinion of the common workmen, concerning
what is technically called feeding of leather, in the slow method of tanning;
and though the processes of this art have been unnecessarily protracted by defective
methods of steeping, and want of progressive infiltration of the astringent liquor through
the skins, yet in general they appear to have arrived, in consequence of old experience, at
a degree of perfection in the quality of the leather, which cannot be far exceeded by
means of any theoretical suggestions which have been advanced.
On the first view it may appear surprising, that in those cases of quick tanning,
where extractive matter forms a certain portion of the leather, the increase of weight is
less than when the skin is combined with the pure tannin; but the fact is easily
accounted for, when we consider that the attraction of skin for tannin must be probably
weakened by its union with extractive matter; and whether we suppose that the tannin
and extractive matter enter together into combination with the matter of skin, or unite
with separate portions of it, still, in either case, the primary attraction of skin for tan
must be to a certain extent diminished.
In examining astringent vegetables in relation to their power of making leather, it is
necessary to take into account not only the quantity they may contain of the substance
precipitable by gelatine, but likewise the quantity and the nature of the extractive
matter; and in cases of comparison, it is essential to employ infusions of the same
degree of concentration.
Of all astringent substances hitherto examined, catechu is that which contains the
largest proportion of tannin; and in supposing, according to the usual estimation, that
from four to five pounds of common oak bark are required to produce one pound of
leather, it appears, from the various synthetical experiments, that about half a pound of
catechu would answer the same purpose. Mr. Purkis found, by the results of different
accurate experiments, that 1 pound of catechu was equivalent to 7 or 8 of oak bark.
For the common purposes of the tanner, 1 pound of it would be equivalent also to 21⁄4
pounds of galls, to 71⁄2 of the Leicester willow, to 11 of the bark of the Spanish chesnut,
to 18 of the bark of the common elm, to 21 of the bark of the common willow, and to
3 pounds of sumach.
Various menstrua have been proposed for the purpose of expediting and improving
the process of tanning, among others, lime water, and solution of pearl-ash; but as these
two substances form compounds with tannin which are not decomposable by gelatine, it[764]
follows that their effects must be prejudicial. There is very little reason to suppose
that any bodies will be found which, at the same time that they increase the solubility of
tannin in water, will not likewise diminish its attraction for skin.
In this country all tanned leather is distinguished into two kinds, called hides and
skins; the former term being appropriated to that made from the larger animals, as
bulls, buffaloes, oxen, and cows, into thick strong sole leather; and the latter to that
made from calves, seals, &c., into thinner and more flexible upper leather. Sometimes
the hides are brought into the market merely dried, as from Buenos-Ayres; or dried
and salted, as from Bahia and Pernambuco; but the greater part are fresh from recently
slaughtered animals. The heaviest ox hides are preferred for forming butts or backs,
which are manufactured as follows:—
The washing process must be more or less elaborate, according to the state of the
skins. Those that are salted and dry require to be steeped, beaten, and rubbed several
times alternately, to bring them to the fresh condition.
After removing the horns, the softened or recent hides are laid in a heap for two or
three days, after which they are suspended on poles in a close room called a smoke-house,
heated somewhat above the common temperature by a smouldering fire. In these
circumstances, a slight putrefaction supervenes, which loosens the epidermis, and renders
the hair easily detachable by the fleshing knife; a large two-handled implement, with a
blunt edge, and bent to suit the curvature of the rounded beam of the wooden horse
upon which the hide is scraped. See Currying.
The next step is immersion in a pit containing water impregnated with about
a 1000th part of sulphuric acid. This process is called raising, because it distends the
pores, and makes the fibres swell, so as to render the skins more susceptible of the action
of the tanning infusions. Forty-eight hours in general suffice for this operation, but
more time may be safely taken.
When the hides are found to be sufficiently raised, they are transferred to a pit, in
which they are stratified with oak bark, ground by a proper mill into a coarse powder.
The pit is then filled up with an infusion of oak bark called ooze, and the hides are
allowed to remain in it for about a month or six weeks. By this time the tannin and
extractive matter of the bark having combined intimately with the animal fibre, the
pit is exhausted of its virtue, and must be renewed, by taking out the spent bark, and
subjecting the skins to a fresh dose of oak bark and ooze. The hides which were
placed near the top of the first pit, must be placed near the bottom of the next. In this
mixture they remain, upon the old practice, about three months. The last process
being repeated twice or thrice, perfectly tanned leather is the result. The hides are now
removed from the pit, and hung up in a shed. In the progress of drying, which should
be slow, they are compressed with a steel tool, and beaten smooth, to render them more
firm and dense.
Some manufacturers place on the bottom of the pit 5 or 6 inches of spent bark, over
it 2 inches of fresh bark, then a skin; and so, alternately, a layer of new bark and a skin,
till the pit is nearly full, reserving a small space at top for a thicker layer of bark, over
which weighted boards are laid, to condense the whole down into the tanning infusion.
The operation of tanning sole leather in the above way, lasts a year or a year and a
half, according to the quality wanted, and the nature of the hides.
A perfect leather is recognized by its section, which should have a glistening marbled
appearance, without any white streaks in the middle.
Crop hides are manufactured by immersion, during three or four days, in pits containing
milk of lime; in which they are occasionally moved up and down in order to expose
them equally to the action of this menstruum. They are then removed, and cleared
from hair and impurities, by using the fleshing knife upon the horse; after which they
must be completely freed from the lime by a thorough washing. They are next
plunged in pits containing a weak ooze or infusion of oak bark, from which they are
successively transferred into other pits with stronger ooze; all the while being daily
handled, that is, moved up and down in the infusion. This practice is continued for
about a month or six weeks. They are now ready to be subjected to a mixture of
ground oak bark and stronger ooze in other pits, to a series of which they are progressively
subjected during two or three months.
The hides are next put into large vats, called layers, in which they are smoothly
stratified with more oak bark, and a stronger infusion of it. After six weeks they are
taken out of these vats, and subjected to a new charge of the same materials for two
months. This simple process is repeated twice or thrice, at the option of the manufacturer,
till the hides are thoroughly tanned. They are then slowly dried, and condensed
in the manner above described. These crop hides form the principal part of the sole
leather used for home consumption in England.
The process of tanning skins (as of calves, seals, &c.) is in some respects peculiar.
They are left in the lime pits for about twelve days, when they are stripped of their[765]
hair, washed in water, then immersed in a lixivium of pigeons’ dung, called a grainer, of
an alkaline nature. Here they remain from eight to ten days, according to the state of
the atmosphere, during which time they are frequently handled, and scraped on both
sides upon a convex wooden beam. This scraping or working, as it is termed, joined to
the action of the grainer, serves to separate the lime, oil, and glutinous matter, and to
render the skin pliant, soft, and ready to imbibe the tanning principle. They are with
this view transferred into pits containing a weak solution of bark, in which they undergo
nearly the same treatment as described above for crop hides; but they are not commonly
stratified in the layers. The time occupied in tanning them is usually limited
to three months. They are then dried, and disposed of to the currier, who dresses and
blackens them for the upper leathers of boots and shoes, for harness, and other
purposes. The light and thin sorts of cow and horse hides are often treated like calf
skins.
In all the above processes, as the animal fibres on the surface of the skin absorb most
readily the tanning principles, and thereby obstruct, in a certain degree, their passage
into the interior fibres, especially of thick hides, it becomes an object of importance to
contrive some method of overcoming that obstacle, and promoting the penetration of
the tan. The first manufacturer who appears to have employed efficacious mechanical
means of favouring the chemical action was Francis G. Spilsbury, who in April, 1823,
obtained a patent for the following operation:—After the hides are freed from the
hairs, &c. in the usual way, they are minutely inspected as to their soundness, and if
any holes be found, they are carefully sewed up, so as to be water tight. Three frames
of wood are provided of equal dimensions, fitted to each other, with the edges of
the frames held together by screw bolts. A skin about to be tanned is now laid upon
the frame, and stretched over its edges, then the second frame is to be placed upon
it, so that the edges of the two frames may pinch the skin all round and hold it securely;
another such skin is then stretched over the upper surface of the second frame, in like
manner, and a third frame being set upon this, confines the second skin. The three
frames are then pinched tightly together by a series of screw bolts, passing through
ears set round their outer edges, which fix the skin in a proper manner for being
operated upon by the tanning liquor.
A space has been thus formed between the two skins, into which, when the frames
are set upright, the infusion is introduced by means of a pipe from the cistern above,
while the air is permitted to escape by a stopcock below. This cock must of course
be shut whenever the bag is filled, but the one above is left open to maintain a communication
with the liquor cistern, and to allow the hydrostatic pressure to force the
liquor through the cutaneous pores by a slow infiltration, and thus to bring the tannin
into contact with all the fibres indiscriminately. The action of this pressure is evinced
by a constant perspiration on the outer surfaces of the skins.
When the tanning is completed, the upper stopcock is closed, and the under is
opened to run off the liquor. The frames are now removed, the bolts are unscrewed,
and the pinched edges of the skins pared off; after which they are to be dried and
finished in the usual manner.
A modification of this ingenious and effectual process was made the subject of a
patent, by William Drake, of Bedminster, tanner, in October, 1831. The hides, after
the usual preparatory processes, are immersed in a weak tan liquor, and by frequent
handling or turning over, receive an incipient tanning before being submitted to the
infiltration plan. Two hides, as nearly of the same size and shape as possible, are placed
grain to grain, when their corresponding edges are sewed firmly together all round
by shoemaker’s waxed thread, so as to form a bag sufficiently tight to hold tan liquor.
This bag must then be suspended by means of loops sewed to its shoulder end, upon
pegs, in such a manner that it may hang within a wooden-barred rack, and be confined
laterally into a book form. About an inch of the bag is left unsewed at the upper end,
for the purpose of introducing a funnel through which the cold tan liquor is poured
into the bag till it be full. After a certain interval which varies with the quality of
the hides, the outer surface becomes moist, and drops begin to form at the bottom of
the bag. These are received in a proper vessel, and when they accumulate sufficiently
may be poured back into the funnel; the bag being thus, as well as by a fresh supply
from above, kept constantly distended.
When the hides are observed to feel hard and firm, while every part of them feels
equally damp, the air of the tanning apartment having been always well ventilated, is
now to be heated by proper means to a temperature gradually increasing from 70°
to 150° of Fahrenheit’s scale. This heat is to be maintained till the hides become
firmer and harder in all parts. When they begin to assume a black appearance in some
parts, and when the tan liquor undergoes little diminution, the hides may be considered
to be tanned, and the bag may be emptied by cutting a few stitches at its bottom.
The outer edges being pared off, the hides are to be finished in the usual way. During[766]
their suspension within the racks, the hides should be shifted a little sideways, to
prevent the formation of furrows by the bars, and to facilitate the equable action of
the liquor.
By this process the patentee says, that a hide may be tanned as completely in ten
days as it could be in ten months by the usual method. I have seen a piece of sole
leather thus rapidly tanned, and it seemed to be perfect. How it may wear, compared
with that made in the old way, I cannot pretend to determine.
Messrs. Knowlys and Duesbury obtained a patent in August, 1826, for accelerating
the impregnation of skins with tannin, by suspending them in a close vessel, from which
the air is to be extracted by an air pump, and then the tanning infusion is to be admitted.
In this way, it is supposed to penetrate the hide so effectually as to tan it
uniformly in a short time.
About 32 years ago, a similar vacuum scheme was employed to impregnate with
weaver’s paste or starch, the cops of cotton weft, for the dandy looms of Messrs. Radcliff
and Ross, of Stockport.
Danish leather is made by tanning lamb and kid skins with willow bark, whence it
derives an agreeable smell. It is chiefly worked up into gloves.
Of the tawing or dressing of skins for gloves, and white sheep leather.
The operations of this art are: 1. washing the skins; 2. properly treating them with
lime; 3. taking off the fleece; 4. treatment in the leather steep.
A shed erected upon the side of a stream, with a cistern of water for washing the
skins; wooden horses for cleaning them with the back of the fleshing knife; pincers
for removing the fibres of damaged wool; a plunger for depressing the skins in the
pits; a lime pit; a pole with a bag tied to the end of it; a two-handed fleshing knife;
a rolling pin, from 15 to 18 inches long, thickened in the middle; such are some of the
utensils of a tawing establishment. There must be provided also a table for applying
the oil to the skins; a fulling mill, worked by a water-wheel or other power; a dressing
peg; a press for squeezing out the fatty filth; a stove; planks mounted upon legs, for
stretching the skins, &c.
Fresh skins must be worked immediately after being washed, and then dried, otherwise
they ferment, and contract either indelible spots, or get tender in certain points,
so as to open up and tear under the tools. When received in the dry state they should
be steeped in water for two days, and then treated as fresh skins. They are next
strongly rubbed on the convex horse-beam with a round-edged knife, in order to make
them pliant. The rough parts are removed by the fleshing knife. One workman can
in this way prepare 200 skins in a day.
The flesh side of each being rubbed with a cold cream of lime, the skins are piled
together with the woolly side of each pair outermost, and the flesh sides in contact.
They are left in this state for a few days, till it is found that the wool may be easily
removed by plucking.
They are next washed in running water, to separate the greater part of the lime,
stripped of the wool by small spring tweezers, and then fleeced smooth by means of the
rolling-pin, or sometimes by rubbing with a whetstone. Unless they be fleeced soon
after the treatment with lime, they do not well admit of this operation subsequently, as
they are apt to get hard.
They are now steeped in the milk of lime-pit, in order to swell, soften, and cleanse
them; afterwards in a weak pit of old lime-water, from which they are taken out and
drained. This steeping and draining upon inclined tables, are repeated frequently
during the space of 3 weeks. Only the skins of young animals, or those of inferior
value are tawed. Sometimes the wool is left on, as for housings, &c.
The skins, after having been well softened in the steeps, are rubbed on the outside
with a whetstone set in a wooden case with two handles, in order to smooth them
completely by removing any remaining filaments of wool. Lamb skins are rubbed
with the pin in the direction of their breadth, to give them suppleness; but sheep skins
are fulled with water alone. They are now ready for the branning, which is done by
mixing 40 lbs. of bran with 20 gallons of water, and keeping them in this fermentable
mixture for three weeks—with the addition, if possible, of some old bran water. Here
they must be frequently turned over, and carefully watched, as it is a delicate operation.
In the course of two days in summer, and eight in winter, the skins are said to be
raised, when they sink in the water. On coming out of the bran, they are ready
for the white stuff; which is a bath composed of alum and sea-salt. Twelve, fourteen,
and sometimes eighteen pounds of alum for 100 skins, form the basis of the bath; to
which two and a half pounds of salt are added in winter, and three in summer. These
ingredients are introduced into a copper with twelve gallons of water. The salt aids
in the whitening action. When the solution is about to boil, three gallons of it are[767]
passed through the cullender into a basin; in this 26 skins are worked one after
another, and after draining, they are put together into the bath, and left in it for ten
minutes to imbibe the salts. They are now ready to receive the paste. For 100 skins,
from 13 to 15 pounds of wheat flour are used along with the yolks of 50 eggs. After
having warmed the alum bath through which the skins have been passed, the flower is
dusted into it, with careful stirring. The paste is well kneaded by the gradual addition
of the solution, and passed through the cullender, whereby it becomes as clear as honey.
To this the yolks being added, the whole is incorporated with much manual labour.
The skins are worked one after another in this paste; and afterwards the whole together
are left immersed in it for a day. They are now stretched and dried upon poles,
in a proper apartment, during from 8 to 15 days, according to the season.
The effects of the paste are to whiten the skins, to soften them, and to protect them
from the hardening influence of the atmosphere, which would naturally render them
brittle. They would not bear working upon the softening iron, but for the emulsion
which has been introduced into their substance. With this view they are dipped in a tub
of clear water during five or six minutes, and then spread and worked upon the board.
They are increased by this means in length, in the proportion of 5 to 3. No hard points
must be left in them. The whiteness is also better brought out by this operation,
which is performed upon the flesh side. The softening tool is an iron plate, about one
foot broad, rounded over above, mounted upon an upright beam, 30 inches high, which
is fixed to the end of a strong horizontal plank, 31⁄2 feet long, and 1 broad. This
plank is heavily loaded, to make it immovable upon the floor. Sometimes the skins are
next spread over an undressed clean skin upon the horse, and worked well with the
two-handled knife, for the purpose of removing the first and second epidermis, called
the fleur and arrière-fleur by the French megissiers. They are then dried while
stretched by hooks and strings. When dry they are worked on the stretching iron, or
they are occasionally polished with pumice stone. A delicate yellow tint is given by
a composition made of two parts of whitening, and one of ochre, applied in a moistened
state, and well worked in upon the grain side. After being polished with pumice,
they are smoothed with a hot iron, as the laundresses do linen, whereby they acquire a
degree of lustre, and are ready to be delivered to the glover.
For housings, the best sheepskins are selected, and such as are covered with the longest
and most beautiful fleece. They are steeped in water, in order to be cleaned and
softened; after which they are thinned inside by the fleshing knife. They are now
steeped in an old bran pit for 3 or 4 days, when they are taken out and washed. They
are next subjected to the white or alum bath, the wool being carefully folded within;
about 18 pounds of alum being used for 100 skins. The paste is made as for the
fleeced skins, but it is merely spread upon their flesh side, and left upon them for 18
hours, so as to stiffen. They are then hung up to dry. They are next moistened by
sprinkling cold water upon them, folded up, piled in a heap, and covered with boards
weighted with heavy stones; in which state they remain for two days. They are next
opened with a round iron upon the horse, and subjected to the stretching iron, being
worked broadwise. They are dried with the fleece outermost, in the sun if possible;
and are finished upon the stretcher.
Calf and lamb skins with their hair and wool are worked nearly in the same manner;
only the thicker the skin, the stronger the alum bath ought to be. One pound of alum
and one of salt are required for a single calf skin. It is left four days in this bath, after
which it is worked upon the stretcher, then fulled. When half dry the skins are opened
upon the horse. In eight days of ordinary weather, they may be completely dressed.
Lamb skins are sometimes steeped during eight days in a bath prepared with unbolted
rye flour and cold water, in which they are daily moved about two or three times.
They are then dried, stretched upon the iron, and switched upon the fleecy side.
Chamois or Shamoy leather.—The skins are first washed, limed, fleeced, and branned
as above described. They are next efflowered, that is, deprived of their epidermis by a
concave knife, blunt in its middle part, upon the convex horse-beam. The cutting part
serves to remove all excrescences, and to equalize the thickness, while the blunt part
softens and smooths. The skins of goats, does, and chamois are always treated in this
way. They are next subjected to the fermenting bran steep for one or two days, in
ordinary weather; but in hot weather for a much shorter time, sometimes only moving
them in the sour bran liquor for a few minutes. They are lastly wrung at the peg, and
subjected to the fulling mill.
When the skins have been sufficiently swelled and suppled by the branning, they may
receive the first oil as follows: a dozen skins being stretched upon the table, the fingers
are dipped in the oil, and shaken over the skins in different places, so as to impart
enough of it to imbue the whole surface slightly, by friction with the palms of the
hands. It is to the outside or grain that the oil is applied. The skins are folded four
together, so as to form balls of the size of a hog’s bladder, and thrown into the trough[768]
of the fulling mill, to the number of twelve dozen at once. Here they remain exposed
to the beater for two, three, or four hours, according to their nature and the state of
the weather. They are taken out, aired, oiled, and again fulled. The airing and fulling
are repeated several times, with more or less frequent oilings. Any cheap animal
oil is employed.
After these operations, the skins require to be subjected to a fermenting process, to
dilate their pores, and to facilitate their combination with the oil. This is performed
in a chamber only 6 feet high, and 10 or 12 feet square. Poles are suspended horizontally
a few inches from the ceiling, with hooks fixed in them to which the skins are
attached. A somewhat elevated temperature is maintained, and by a stove if need be.
This operation requires great skill and experience.
The remainder of the epidermis is next removed by a blunt concave knife and the
horse; whereby the surface is not cut, but rather forcibly scraped.
The skins are now scoured to carry off the redundant oil; which is effected by a
potash lye, at two degrees Baumé, heated no hotter than the hand can bear. In this
they are stirred briskly, steeped for an hour, and lastly wrung at the peg. The soapy
liquor thus expelled is used for inferior purposes. The clean skins after being dried,
are finished first on the stretcher-iron, and then on the herse or stretching frame.
Leather of Hungary.—This is manufactured by impregnating strong hides with alum,
common salt, and suet; by a rapid process which is usually completed in the space of
two months. The workshop is divided into two parts; 1. a shed on the side of a
stream, furnished with wooden horses, fleshing knives, and other small tools. In one
corner is a furnace with a boiler for dissolving the alum, a vat for immersing the hides
in the solution, and several subsidiary tubs. 2. A chamber, 6 feet high, by 15 feet
square, capable of being made very tight, for preserving the heat. In one corner is a
copper boiler, of sufficient size to contain 170 pounds of tallow. In the middle of the
stove is a square stone slab, upon which an iron grate is placed about a yard square.
This is covered with charcoal. At each side of the stove are large tables, which occupy
its whole length, and on which the leather is spread to receive the grease. The upper
part below the ceiling is filled with poles for hanging the leather upon to be heated.
The door is made to shut perfectly close.
The first operations are analogous to those of tanning and tawing; the skins being
washed, cut in halves, shaved, and steeped for 24 hours in the river. They are
then cleaned with 5 or 6 pounds of alum, and 31⁄2 pounds of salt, for a piece of hide
which weighs from 70 to 80 pounds. The common salt softens the effect of the alum,
attracts the moisture of the air, and preserves the suppleness of the skin. When the
alum and salt are dissolved, hot water is poured upon the hides placed in a vat, and they
are tramped upon by a workman walking repeatedly from one end of the vat to the
other. They are then transferred into a similar vat containing some hot water, and
similarly tramped upon. They are next steeped for eight days in alum water. The
same round of operations is repeated a second time.
The skins are now dried either in the air, or a stove room; but before being quite
dry, they are doubled together, well stretched to take out the wrinkles, and piled up.
When dry, they are again tramped to open the pores as well as to render the skin pliant,
after which they are whitened by exposure to the sun.
Tallow of inferior quality is employed for greasing the leather. With this view the
hides are hung upon the poles in the close stove room, then laid upon the table, and
besmeared with the tallow melted till it begins to crackle. This piece is laid on another
table, is there covered with a second, similarly greased, and so forth. Three pounds of
fat are commonly employed for one piece of leather.
When the thirty strips, or fifteen hides passed through the grease in one operation
are completed, two workmen take the first piece in their hands, and stretch it over the
burning charcoal on the grate for a minute, with the flesh side to the fire. The rest
are passed over the flame in like manner. After flaming, the pieces are successively
laid on an inclined table exposed to the fire, where they are covered with a cloth. They
are finally hung upon poles in the air to dry; and if the weather be warm, they are
suspended only during the night, so as to favour the hardening of the grease. Instead
of the alum bath, M. Curaudau has employed with advantage a steep of dilute sulphuric
acid.
Morocco leather.—The true morocco leather is goat skin tanned and then dyed on the
side of the grain. Sheep skins are treated in the same way. The skins are steeped first
in a fermenting mixture of bran water for a few days, they are then worked upon the
horse, steeped in fresh water for 12 hours, and rinsed in the same. They are next drained,
steeped in weak lime pits for a proper time, till the hairs can be readily detached. They
are now subjected to the action of a blunt knife upon the horse-beam, in order to strip
off their hair, after which they are cleansed in running water. Any excrescences must
be carefully removed with the fleshing knife, and their edges neatly pared. The next[769]
process is rubbing them strongly with a piece of hard schist, set in a wooden frame, in
order to expel by the pressure any lime which may still adhere, and to soften the
grain. They are now worked upon the horse-beam with the blunt knife, and subjected
to a species of fulling, by being agitated by pegs in a revolving cask along
with water. Many manufacturers prefer a weak alkaline lye, or putrified urine, to the
lime bath.
The skins are immersed for a night and a day, in a bran bath, in a certain state
of fermentation, then worked on the horse, and salted, to preserve them till they are to
be dyed.
Preparatory to being dyed, each skin is sewed together edgewise, with the grain on
the outside, and it is then mordanted either with a solution of tin, or with alum water.
The colour is given by cochineal, of which from 10 to 12 ounces are required for a dozen
of skins. The cochineal being boiled in water along with a little tartar or alum for a few
minutes, forms a red liquor, which is filtered through a linen cloth, and put into a
clean cask. The skins are immersed in this bath, and agitated in it for about half
an hour; they are taken out and beaten, and then subjected to a second immersion in
the cochineal bath. After being thus dyed, they are rinsed and tanned with Sicilian
sumach, at the rate of two pounds for a skin of moderate size. This process is performed
in a large tub made of white wood, in the liquor of which the skins are floated
like so many bladders, and moved about by manual labour during four hours. They
are then taken out, drained, and again subjected to the tanning liquor; the whole process
requiring a space of twenty-four hours. The skins are now unstitched, rinsed,
fulled with beetles, drained, rubbed hard with a copper blade, and lastly hung up
to dry.
Some manufacturers brighten the colour by applying to the surface of the skins, in
a damp state, a solution of carmine in ammonia with a sponge; others apply a decoction
of saffron to enliven the scarlet tint. At Paris the morocco leather is tanned by
agitation with a decoction of sumach in large casks made to revolve upon a horizontal
axis, like a barrel churn. White galls are sometimes substituted for sumach; a pound
being used for a skin. The skins must be finally cleaned with the utmost care.
The black dye is given by applying with the brush a solution of red acetate of iron to
the grain side. Blue is communicated by the common cold indigo vat; violets, with a
light blue followed by cochineal red; green, by Saxon blue followed by a yellow dye,
usually made with the chopped roots of the barberry. This plant serves also for yellows.
To dye olive, the skins are first passed through a weak solution of green vitriol, and
then through the decoction of barberry root, containing a little Saxon blue. Puce
colour is communicated by logwood with a little alum; which may be modified by the
addition of a little Brazil wood. In all these cases, whenever the skins are dyed, they
should be rinsed, wrung or rather drained, stretched upon a table, then besmeared
on the grain side with a film of linseed oil applied by means of a sponge, in order to
promote their glossiness when curried, and to prevent them becoming horny by too
rapid drying.
The last process in preparing morocco leather is the currying, which brings out the
lustre, and restores the original suppleness. This operation is practised in different
manners, according to the purpose the skins are to serve. For pocket-books, portfolios,
and case-making in general, they must be thinned as much as possible upon the flesh
side, moistened slightly, then stretched upon the table, to smooth them; dried again,
moistened, and lastly passed two or three times through the cylinder press in different
directions, to produce the crossing of the grain. The skins intended for the shoemaker,
the saddler, the bookbinder, &c., require more pliancy, and must be differently curried.
After being thinned, they are glazed with a polisher while still moist, and a grain
is formed upon the flesh side with the roughened lead plate or grainer of the curriers,
called in French pommelle; they are glazed anew to remove the roughness produced by
the pommel, and finally grained on the flesh side with a surface of cork applied under a
pommel of white wood.
Russia leather.—The Russians have long been possessed of a method of making a
peculiar leather, called by them jucten, dyed red with the aromatic saunders wood.
This article has been much sought after, on account of not being subject to mould
in damp situations, being proof against insects, and even repelling them from the
vicinity of its odour. The skins are freed from the hair or fleece, by steeping in an
ash-lye too weak to act upon the animal fibres. They are then rinsed, fulled for a
longer or shorter time according to their nature, and fermented in a proper steep, after
having been washed in hot water. They are taken out at the end of a week, but they
may be steeped a second time if deemed necessary, to open their pores. They are now
cleaned by working them at the horse on both the flesh and grain sides.
A paste is next composed, for 200 skins, of 38 pounds of rye flour, which is set to[770]
ferment with leaven. This dough is worked up with a sufficient quantity of water to
form a bath for the skins, in which they are soaked for 48 hours; they are then transferred
into small tubs, where they remain during fifteen days, after which they are
washed at the river. These operations serve to prepare the skins for absorbing the
astringent juices with uniformity. A decoction of willow bark (salix cinerea, and salix
caprea) being made, the skins are immersed in the boiler whenever the temperature of
the liquor is sufficiently lowered not to injure the animal fibres, and handled and pressed
for half an hour. This manipulation is repeated twice daily during the period of a
week. The tanning infusion is then renewed, and applied to the same skins for another
week; after which being exposed to the air to dry, they are ready for being dyed, and
then curried with the empyreumatic oil of the bark of the birch tree. To this substance
the Russia leather owes its peculiarities. Many modes have been prescribed for preparing
it; but the following is the one practised in Russia.
The whitish membranous epidermis of the birch, stripped of all woody parts, is introduced
into an iron boiler, which, when stuffed full, is covered tight with a vaulted iron lid,
having a pipe rising from its centre. A second boiler into which this pipe passes without
reaching its bottom, is set over the first, and is luted to it at the edges, after the two are
bolted together. They are then inverted, so that the upper one contains the birch
bark. The under half of this apparatus is sunk in the earth, the surface of the upper
boiler is coated over with a clay lute, then surrounded with a fire of wood, and exposed
to a red heat, till the distillation be completed. This operation, though rude in appearance,
and wasteful of wood, answers its purpose perfectly well. The iron cylinder
apparatus used in Britain for distilling wood vinegar, would, however, be much more
convenient and productive. When the above boilers are unluted, there is found
in the upper one a very light powder of charcoal, and in the under one which served
as a receiver, there is an oily, brown, empyreumatic fluid, of a very strong smell,
which is mixed with the tar, and which floats over a small quantity of crude vinegar.
The former matter is the oil employed to impregnate the skins, by working it into the
flesh side with the curriers’ tools. It is difficult to make this oil penetrate with uniformity;
and the Russians do not always succeed in this process, for they turn out many
skins in a spotted state. This oil is at present obtained in France by distilling the
birch bark in copper stills, and condensing the products by means of a pipe plunged in
cold water. About 60 per cent. of the weight of the bark is extracted.
The skins imbibe this oil most equally before they are fully dry. Care must be
taken not to apply too much of it, for fear of its passing through and staining the grain-side
of the leather. Chevreul has investigated the chemical nature of this odoriferous
substance, and finding it to be a peculiar compound, has called it betuline.
LEDUM PALUSTRE. This plant is employed in Russia to tan the skins of goats,
calves, and sheep, into a reddish leather of an agreeable smell; as also in the preparation
of the oil of birch, for making what is commonly called Russia leather.
LEGUMINE, is the name of a vegeto-alkali supposed to exist in leguminous
plants.
LEVIGATION, is the mechanical process whereby hard substances are reduced to
a very fine powder.
LEUCITE, is a hard Vesuvian mineral, consisting of silica, 54; alumina, 23; potash,
23.
LEUCINE, is a white crystalline substance produced by acting upon flesh with
sulphuric acid.
LEWIS, is the name of one kind of shears used in cropping woollen cloth.
LIAS, is a fine-grained argillaceous limestone, whose geological position is under the
oolite; it is the proper lithographic stone.
LIBAVIUS, Liquor of, is the bichloride of tin, prepared by dissolving that
metal with the aid of heat, in aqua regia, or by passing chlorine gas through a solution
of muriate of tin till no more gas be absorbed, evaporating the solution, and setting it
aside to crystallize. The anhydrous bichloride is best prepared by mixing four parts
of corrosive sublimate with one part of tin, previously amalgamated with just so much
mercury as to render it pulverizable; and by distilling this mixture with a gentle heat.
A colourless fluid, the dry bichloride of tin, or the proper fuming liquor of Libavius,
comes over. When it is mixed with one-third of its weight of water it becomes solid.
The first bichloride of tin is used in calico-printing.
LIGNEOUS MATTER, is vegetable fibre. See Fibrous Matter.
LIGNITE, is one of the most recent geological formations, being the carbonaceous
remains of forest trees. From this substance, as found in the neighbourhood of Cologne,
the brown colours, called umber and earth of Cologne, are prepared.
[771]
LIMESTONE (Calcaire, Fr.; Kalkstein, Germ.); may be classed under the following
heads:—
1. Calcareous spar occurs in colourless crystals or crystalline masses; dissolves with
effervescence in muriatic acid; is scratched by soft iron, but not by the nail; specific
gravity 2·7; loses 46 per cent. by the expulsion of carbonic acid, and calcines into
quicklime.
2. Calcsinter, or stalactitic carbonate of lime, called also concretionary limestone, because
formed of zones more or less undulated, and nearly parallel. These zones have a fibrous
structure, arising from the successive deposits of the crystalline limestone from its solvent
water. The long conical pieces called stalactites, show fibres converging to the
axis. The tubercular consists of irregular lumps often sprinkled over with small
crystals, and associated so as to exhibit the appearance of cauliflower. The stratiform,
commonly called stalagmite, or alabaster limestone, represents zones not concentric, but
spread out, waving, and parallel; its texture is sometimes lamellar, and sometimes
fibrous. These waving strata are distinguishable from one another by their different
densities, and by their degrees of translucency. This stalagmitic mass bears the name
of oriental alabaster, when it is reddish-yellow with distinct zones, and is susceptible of
a fine polish. Stalactites are formed in the large excavations of calcareous rocks. The
water percolating down through them, and dropping from the roofs of the caverns, is
usually charged with carbonate of lime held in suspension by an excess of carbonic
acid. The exposure to air, the motion, and the consequent diminution of pressure,
cause the precipitation of the carbonate of lime in the solid state. Each drop of water,
on falling through the vault, abandons a small film of limestone, which enlarges by
degrees, and forms either a cylinder or solid mass. This alabaster differs from marble
in its parallel and waving layers, and its faint degree of transparency.
This alabaster serves for the decoration of public buildings, and is occasionally introduced
into certain pieces of furniture. The fine Egyptian alabaster was anciently
brought from the mountains of the Thebaid, between the Nile and the Red Sea, near
a town called Alabastron, whence probably the name. Very fine red alabaster, of great
hardness, was found at one time in the quarries of Montmartre, but the stock was soon
exhausted.
The incrusting concretionary limestone differs little from the preceding except in
the rapidity of its formation, and in being moulded upon some body whose shape it
assumes. These deposits from calcareous springs, form equally on vegetable bodies, on
stones, metals, within pipes of cast iron, wood, or lead. The incrustations on vegetable
and animal substances are vulgarly called petrifactions, as the organic fibres are replaced
by stone. One of the most curious springs of this nature is at the baths of Saint Philip,
in Tuscany, where the water flows in almost a boiling state, over an enormous mass of
alabaster which it has produced. The carbonate of lime seems to be held in solution
here by sulphuretted hydrogen, which flies off when the water issues to the day. Dr.
Vegny has taken advantage of this property of the spring, to obtain basso-relievo figures
of great whiteness and solidity. He makes use of sulphur moulds.
Calcareous tuf consists of similar incrustations made by petrifying rivulets running
over mud, sand, vegetable remains, &c. It is porous, even cellular, somewhat soft,
impure, and of a dirty gray colour. Its surface is wavy, rough, and irregular. These
incrustations or deposits are, however, sometimes so abundant, and the resulting stony
matters so hard that buildings may be constructed with them. The stone with which
the town of Pasti, in Italy, is built has been called pipe-stone by the Italians; and it has
apparently derived its origin from incrustations upon large reeds.
The travertino, which served to construct all the monuments of Rome, appears to
have been formed by the deposits of the Anio and the solfatara of Tivoli. The temples
of Pæstum, which are of extreme antiquity, have been built with a travertino formed
by the sediment of the waters which still flow in this territory. All these stones
acquire great hardness in the air, and M. de Breislak thinks that it is to the happy
union of travertino and pouzzolana in the same spot, that the monuments of Rome owe
their great solidity.
Spongy limestone, usually called Agaric mineral, stone marrow, &c., belongs to this
kind of formation. It has a very white colour, a very fine grain, is soft to the touch,
very tender, and light enough to float for an instant on water. It occurs in rather thin
layers, in the crevices of calcareous rocks, and is so common in Switzerland as to be
employed for whitening houses.
3. Compact limestone, is of a grain more or less fine, does not polish, nor afford large
blocks free from fissures, has a conchoidal, or uneven scaly fracture. Colours very
various. Its varieties are; a, The sub-lamellar, compact, with some appearance of a
foliated texture. b, Compact fine-grained limestone, the zechstein of the Germans, to
which M. Brongniart refers the lithographic stone in his classification of rocks (Dictionnaire[772]
des Sciences Naturelles,) but the English geologists place the locality of the famous
lithographic quarry of Solenhofen much higher in the plane of secondary superposition.
Its fracture is conchoidal; colour from gray to whitish; c, Compact common limestone.
Grain of middle size; earthy aspect; uneven fracture; perfectly opaque; colour,
whitish to pale gray, yellow, or reddish. The limestones of the Jura formation are
referred to this head, as well as most of those interspersed among the coal strata.
d, The coarse compact, or Cornbrash; texture somewhat open, earthy aspect, rough to
the touch, ragged fracture, colour yellow, gray, or dirty red. e, Compact cellular, the
Rauchekalk and Holekalk of the Germans, on account of the numerous holes or
caverns distributed through it.
4. Oolite or roe-stone.—It consists of spherical grains of various size, from a millet
seed, to a pea, or even an egg; texture compact; fracture even; colours, whitish,
yellow, gray, reddish, brownish. The larger balls have almost always a foreign body
for their centre or nucleus.
5. Chalk; texture earthy; grains fine, tender, friable; colours white, grayish, or pale
yellowish.
6. Coarse-grained limestone; an earthy texture, in large particles, often loose; fracture
foliated, uneven; colour pale and dirty yellow. Coarse lias has has been referred to
this head.
7. Marly limestone; lake and fresh-water limestone formation; texture fine-grained,
more or less dense; apt to crumble down in the air; colour white or pale yellow;
fracture rough-grained, sometimes conchoidal; somewhat tenacious. Texture occasionally
cavernous; with cylindrical winding cavities. This true limestone must not be
confounded with the lime-marl, composed of calcareous matter and clay.
8. Siliceous limestone; of a compact texture; scratching steel, and scratched by it;
leaves a siliceous residuum after the action of muriatic acid.
9. Calp; texture compact; fine-grained; schistose structure; hard, as the preceding;
not burning into quicklime, affording to dilute muriatic acid a copious residuum
of clay and silica; colour blackish; found in beds in the transition district near
Dublin.
10. Lucullite or stinkstone; texture compact or sub-lamellar, colour grayish; emits
the smell of sulphuretted hydrogen by friction or a blow. It occurs at Assynt, in
Sutherlandshire; in Derbyshire; counties of Kilkenny, Cork, and Galway.
11. Bituminous limestone; black or blackish colour; diffusing by the action of fire a
bituminous odour, and becoming white.
Of all common limestones the purity may most readily be determined by the quantity
of carbonic acid which is evolved during their solution in dilute nitric or muriatic acid.
Perfect carbonate of lime loses in this way 46 per cent.; and if any particular limestone
loses only 23 per cent., we may infer that it contains only one half its weight of calcareous
carbonate. This method is equally applicable to marls, which are mixtures in
various proportions of carbonate of lime, clay, and sand, and may all be recognized by
their effervescing with acids.
The chief use of calcareous stones is for procuring quicklime by calcination in proper
furnaces; and they are all adapted to this purpose provided they are not mixed with too
large a proportion of sand and ferruginous clay, whereby they acquire a vitrescent
texture in a high heat, and will not burn into lime. Limestone used to be calcined in
a very rude kiln, formed by enclosing a circular space of 10 or 15 feet diameter, by rude
stone walls 4 or 5 feet high, and filling the cylindrical cavity with alternate layers of
turf or coal and limestone broken into moderate pieces. A bed of brushwood was
usually placed at the bottom, to facilitate the kindling of the kiln. Whenever the combustion
was fairly commenced, the top, piled into a conical form, was covered in with
sods, to render the calcination slow and regular. This method being found relatively
inconvenient and ineffectual, was succeeded by a permanent kiln built of stones or brickwork,
in the shape of a truncated cone with the narrow end undermost, and closed at
bottom by an iron grate. Into this kiln, the fuel and limestone were introduced at the
top in alternate layers, beginning of course with the former; and the charge was either
allowed to burn out, when the lime was altogether removed at a door near the bottom, or the
kiln was successively fed with fresh materials, in alternate beds, as the former supply sunk
down by the calcination, while the thoroughly burnt lime at the bottom was successively
raked out by a side door immediately above the grate. The interior of the lime kiln
has been changed of late years from the conical to the elliptical form; and probably the
best is that of an egg placed with its narrow end undermost, and truncated both above
and below; the ground plot or bottom of the kiln being compressed so as to give an
elliptical section, with an eye or draft-hole towards each end of that ellipse. A kiln
thus arched in above gives a reverberatory heat to the upper materials, and also favours
their falling freely down in proportion as the finished lime is raked out below; advantages[773]
which the conical form does not afford. The size of the draft-notes for extracting
the quicklime, should be proportionate to the size of the kiln, in order to admit a sufficient
current of air to ascend with the smoke and flame, which is found to facilitate
the extrication of the carbonic acid. The kilns are called perpetual, because the operation
is carried on continuously as long as the building lasts; and draw-kilns, from the mode of
discharging them by raking out the lime into carts placed against the draft-holes. Three
bushels of calcined limestone, or lime-shells, are produced on an average for every bushel
of coals consumed. Such kilns should be built up against the face of a cliff, so that
easy access may be gained to the mouth for charging, by making a sloping cart road to
the top of the bank.
Figs. 638, 639, 640, 641. represent the lime-kiln of Rüdersdorf near Berlin, upon the
continuous plan, excellently constructed for economizing fuel. It is triple, and yields a
threefold product. Fig. 640. is a view of it as seen from above; fig. 641., the elevation
and general appearance of one side; fig. 638, a vertical section, and fig. 639. the ground
plan in the line A B C D of fig. 638. The inner shaft fig. 638. has the form of two truncated
cones, with their larger circular ends applied to each other; it has the greatest width at
the level of the fire-door b, where it is 8 feet in diameter; it is narrower below at the
discharge door, and at the top orifice, where it is about 6 feet in diameter. The interior
wall d, of the upper shaft is built with hewn stones, to the height of 38 feet, and below
that for 25 feet, with fire-bricks d′ d′, laid stepwise. This inner wall is surrounded with
a mantle e, of limestones, but between the two there is a small vacant space of a few
inches filled with ashes, in order to allow of the expansion of the interior with heat
taking place without shattering the mass of the building.
The fire-grate b, consists of fire-tiles, which at the middle, where the single pieces
press together, lie upon an arched support f. The fire-door is also arched, and is secured
by fire-tiles. g is the iron door in front of that orifice. The tiles which form the grate
have 3 or 4 slits of an inch wide for admitting the air, which enters through the canal h.
The under part of the shaft from the fire to the hearth, is 7 feet, and the outer enclosing
wall is constructed of limestone, the lining being of fire-bricks. Here are the ash-pit i,
the discharge outlet a, and the canal k, in front of the outlet. Each ash-pit is shut with
an iron door, which is opened only when the space i becomes filled with ashes. These[774]
indeed are allowed to remain till they get cool enough to be removed without inconvenience.
The discharge outlets are also furnished with iron doors, which are opened only for
taking out the lime, and are carefully luted with loam during the burning. The outer
walls l m n of the kiln, are not essentially necessary, but convenient, because they afford
room for the lime to lie in the lower floor, and the fuel in the second. The several
stories are formed of groined arches o, and platforms p, covered over with limestone slabs.
In the third and fourth stories the workmen lodge at night. See fig. 641. Some enter
their apartments by the upper door q; others by the lower door s. r is one of the
chimneys for the several fire-places of the workmen. t u v are stairs.
As the limestone is introduced at top, the mouth of the kiln is surrounded with a
strong iron balustrade to prevent the danger of the people tumbling in. The platform is
laid with rails w, for the waggons of limestone, drawn by horses, to run upon. x is
another rail-way, leading to another kiln. Such kilns are named after the number of
their fire-doors, single, twofold, threefold, fourfold, &c.; from three to five being the
most usual. The outer form of the kiln also is determined by the number of the
furnaces; being a truncated pyramid of equal sides; and in the middle of each alternate
side there is a fire-place, and a discharge outlet. A cubic foot of limestone requires for
burning, one and five-twelfths of a cubic foot of wood, and one and a half of turf.
When the kiln is to be set in action, it is filled with rough limestones, to the height
C D, or to the level of the firing; a wood fire is kindled in a, and kept up till the lime is
calcined. Upon this mass of quicklime, a fresh quantity of limestones is introduced, not
thrown in at the mouth, but let down in buckets, till the kiln be quite full; while over
the top a cone of limestones is piled up, about 4 feet high. A turf-fire is now kindled
in the furnaces b. Whenever the upper stones are well calcined, the lime under the
fire-level is taken out, the superior column falls in, a new cone is piled up, and the
process goes on thus without interruption, and without the necessity of once putting a fire
into a; for in the space C B, the lime must be always well calcined. The discharge of
lime takes place every 12 hours, and it amounts at each time in a threefold kiln, to from
20 to 24 Prussian tonnes of 6 imperial bushels each; or to 130 bushels imperial upon
the average. It is found by experience, that fresh-broken limestone which contains a
little moisture, calcines more readily than what has been dried by exposure for some
time to the air; in consequence of the vapour of water promoting the escape of the
carbonic acid gas; a fact well exemplified in distilling essential oils, as oil of turpentine
and naphtha, which come over with the steam of water, at upwards of 100 degrees
F. below their natural term of ebullition. Six bushels of Rüdersdorf quicklime weigh
from 280 to 306 pounds.
When coals are used for fuel in a well-constructed perpetual, or draw kiln, about 1
measure of them should suffice for 4 or 5 of limestone.
The most extensive employment of quicklime is in agriculture, on which subject instructive
details are given in Loudon’s Encyclopædias of Agriculture and Gardening.
Quicklime is employed in a multitude of preparations subservient to the arts; for
clarifying the juice of the sugar-cane and the beet-root; for purifying coal gas; for
rendering the potash and soda of commerce caustic in the soap manufacture, and in the
bleaching of linen and cotton; for purifying animal matters before dissolving out their
gelatine; for clearing hides of their hair in tanneries; for extracting the pure volatile
alkali from muriate or sulphate of ammonia; for rendering confined portions of air
very dry; for stopping the leakage of stone reservoirs, when mixed with clay and thrown
into the water; for making a powerful lute with white of egg or serum of blood; for
preparing a depilatory pommade with sulphuret of arsenic, &c. Lime water is used in
medicine, and quicklime is of general use in chemical researches. Next to agriculture
the most extensive application of quicklime is to Mortar-Cements, which see.
LINSEED (Graine de lin, Fr.; Leinsame, Germ.); contains in its dry state, 11·265
of oil; 0·146 of wax; 2·488 of a soft resin; 0·550 of a colouring resinous matter;
0·926 of a yellowish substance analogous to tannin; 6·154 of gum; 15·12 of vegetable
mucilage; 1·48 of starch; 2·932 of gluten; 2·782 of albumine; 10·884 of saccharine
extractive; 44·382 of envelopes, including some vegetable mucilage. It contains also
free acetic acid; some acetate, sulphate, and muriate of potash, phosphate and sulphate
of lime; phosphate of magnesia; and silica. See Oils, Unctuous.
LIQUATION (Eng. and Fr.; Saigerung, Germ.); is the process of sweating out,
by a regulated heat, from an alloy, an easily fusible metal from the interstices of a metal
difficult of fusion. Lead and antimony are the metals most commonly subjected to
liquation; the former for the purpose of carrying off by a superior affinity the silver
present in any complex alloy, a subject discussed under Silver; the latter will be
considered here, as referred to from the article Antimony.
[775]
Figs. 642, 643, 644. represent the celebrated antimonial liquation furnaces of Malbosc,
in the department of Ardèche, in France. Fig.
642. is a ground plan taken at the level of the
draught holes g g, fig. 643., and of the dotted line
E F; fig. 643. is a vertical section through the
dotted line A B, of fig. 642.; and fig. 644. is a
vertical section through the dotted line C D of
fig. 642. In the three figures, the same letters
denote like objects, a b c are three grates upon
the same level above the floor of the works, 41⁄2
feet long, by 101⁄2 inches broad; between which
are two rectangular galleries, d e, which pass
transversely through the whole furnace, and lie
at a level of 12 inches above the ground. They
are separated by two walls from the three fire places. The walls have three openings,
f g h, alternately placed for the flames to play through. The ends of these
galleries are shut in with iron doors i i, containing peep holes. In each gallery are two
conical cast-iron crucibles k k, into which the eliquating sulphuret of antimony drops.
Their height is from 12 to 14 inches, the width of the mouth is 10 inches, that of the
bottom is 6, and the thickness four-tenths of an inch. They are coated over with fire clay,
to prevent the sulphuret from acting upon them; and they stand upon cast-iron pedestals
with projecting ears, to facilitate their removal from the gallery or platform. Both of
these galleries are lined with tiles of fire-clay l l, which also serve as supports to the
vertical liquation tubes m m, made of the same clay. The tiles are somewhat curved
towards the middle, for the purpose of receiving the lower ends of these tubes, and have
a small hole at n, through which the liquid sulphuret flows down into the crucible.
The liquation tubes are conical, the internal diameter at top being 10 inches, at
bottom 8; the length fully 40 inches, and the thickness six-tenths of an inch. They have
at their lower ends notches or slits o, fig. 644., from 3 to 5 inches long, which look outwards,
to make them accessible from the front and back part of the furnaces through
small conical openings p p, in the walls. These are closed during the operation with
clay stoppers, and are opened only when the gangue, rubbish, and cinders are to be raked
out. The liquation tubes pass across the arch of the furnace q q, the space of the arch
being wider than the tubes; they are shut in at top with fire-covers r r. s s, the middle
part of the arch, immediately under the middle grate, is barrel-shaped, so that both
arches are abutted together. The flames, after playing round about the sides of the
liquation tubes, pass off through three openings and flues into the chimney t, about 13
feet high; u being the one opening, and v the two others, which are provided with
register plates. In front of the furnace is a smoke flue w, to carry off the sulphureous
vapours exhaled during the clearing out of the rubbish and slag; another x, begins
over y y, at the top of the tubes; a wall z, separates the smoke flue into halves, so
that the workmen upon the one side may not be incommoded by the fumes of the other.
This wall connects at the same time the front flue w with the chimney t. a′ a′ and
b′ b′ are iron and wooden bearer beams and rods for strengthening the smoke-flue, c′ c′
are arches upon both sides of the furnace, which become narrower from without inwards,[776]
and are closed with well-fitted plates d′ d′. They serve, in particular circumstances, to
allow the interior to be inspected, and to see if either of the liquation furnaces be out of
order.
Each tube being charged with about 500 lbs. of the antimonial ore, previously
warmed upon the roof of the furnace, in a short time the sulphuret of a blue colour
begins to flow out. Whenever the liquation ceases, the cinders are raked out by the
side openings, and the tubes are charged afresh. The luted iron crucibles are suffered
to become three-fourths full, are then drawn out from the galleries, left to cool, and
emptied. The ingots weigh about 85 pounds. The charging is renewed every three
hours, and, when the process is in good train, 100 lbs. of sulphuret of antimony are
obtained every hour. The average duration of the tubes is 3 weeks, though in some
cases it may be 40 days. The product from the ore is from 40 to 50 per cent. The
above plan of operation is remarkable for the small consumption of fuel, the economy of
labour, and the complete exhaustion of the ore.
LIQUEURS, LIQUORISTE; names given by the French to liquors compounded
of alcohol, water, sugar, and different aromatic substances; and to the person who compounds
them. I shall insert here a few of their most approved recipes.
Infusion of the peels of fruits.—The outer skin pared off with a sharp knife, is to be
dropped into a hard glazed jar, containing alcohol of 34° B., diluted with half its bulk
of water, and the whole is to be transferred into well-corked carboys. After an infusion
of six weeks, with occasional agitation, the aromatized spirit is to be distilled off. In
this way are prepared the liquors of cedrat, lemons, oranges, limettes (a sort of sweet
lemon), poncires (the large citron), bergamots, &c.
Infusion of aromatic seeds.—These must be pounded, put into a carboy, along with
alcohol diluted as above, infused with agitation for six weeks, and then distilled.
Infusions of aromatic woods are made in the same way.
The liquorist should not bring his infusions and tinctures into the market till six
months after their distillation.
Liqueurs have different titles, according to their mode of fabrication.
Thus waters are liquors apparently devoid of viscidity; creams and oils possess it in
a high degree.
Water of cedrat, is made by dissolving six pounds of sugar in seven quarts of water;
adding two quarts of spirit of cedrat, and one of spirit of citron. Boil the whole for a
minute, and filter hot through a proper bag. Set it for a considerable time aside in a
corked carboy, before it be bottled.
Oil or cream of cedrat.—Take eight quarts of river water, two of spirit of cedrat, one
of spirit of citron, and as much rich syrup as is necessary to give the mixture an oily
consistence. Stir it well and set it aside in carboys. Should it be at all clouded, it must
be filtered till it be perfectly pellucid.
Balm of Molucca, is made by infusing for ten days, in a carboy capable of holding
fully four gallons, 10 pounds of spirits of 18° B., 4 pounds of white sugar, 4 pounds of
river water, 4 drachms of pounded cloves, and 48 grains of pounded mace. The mixture
is to be shaken 3 or 4 times daily, coloured with caramel (burnt sugar), filtered at
the end of ten days, and set aside in bottles.
Tears of the widow of Malabar, are compounded with the preceding quantity of spirits,
sugar, and water, adding 4 drachms of ground cinnamon, 48 grains of cloves, and a like
quantity of mace, both in powder. It may be slightly coloured with caramel.
The delight of the Mandarins.—Take spirit, sugar, and water, as above, adding 4
drachms of anisum Chinæ, (Gingi), as much ambrette (seeds of the hibiscus abelmoschus,
Lin.) all in powder; 2 drachms of safflower.
The sighs of love.—Take spirits, water, and sugar, as above. Perfume with essence
(otto) of roses; give a very pale pink hue with tincture of cochineal, filter and bottle up.
Crème de macarons.—Add to the spirit, sugar, and water as above, half a pound of
bitter almonds, blanched and pounded; cloves, cinnamon, and mace in powder, of each
48 grains. A violet tint is given by the tinctures of turnsole and cochineal.
Curaçoa.—Put into a large bottle nearly full of alcohol of trente-six (34° Baumé),
the peels of six smooth Portugal oranges, (Seville?) and let them infuse for 15 days;
then put into a carboy 10 pounds of spirits of 18° B., 4 pounds of white sugar, and 4
pounds of river water. When the sugar is dissolved, add a sufficient quantity of the
orange zestes to give flavour, then spice the whole with 48 grains of cinnamon, and
as much mace, both in powder. Lastly introduce an ounce of ground Brazil wood, and
infuse during 10 days, agitating 3 or 4 times daily. A pretty deep hue ought to be given
with caramel.
Swiss extract of wormwood, is compounded as follows:—
| Tops of the absinthium majus 4 pounds; |
| Ditto, absinthium minus 2 pounds; |
| Roots of angelica,[777] |
|
- |
of each a few grains at pleasure; |
| Calamus aromaticus, |
| Seeds of anisum Chinæ, |
| Leaves of the dittany of Crete, |
| Alcohol of 20° B., four gallons Imp. |
Macerate these substances during eight days, then distil by a gentle fire; draw off two
gallons of spirits, and add to it 2 drachms of essential oil of anise-seed. The two gallons
left in the still serve for preparing the vulnerary spirituous water.
Of colouring the liqueurs.
Yellow is given with the yellow colouring matter of safflower (carthamus,) which is
readily extracted by water.
Fawn is given by caramel, made by heating ground white sugar in an iron spoon over
a charcoal fire, till it assumes the desired tint, and then pouring it into a little cold
water.
Red is given by cochineal alone, or with a little alum.
Violet is given by good litmus (turnsole).
Blue and green.—Sulphate of indigo gives the first. After saturating it nearly with
chalk, alcohol being digested upon it, becomes blue. This tincture mixed with that of
carthamus forms a good green.
LIQUID AMBER, is obtained from the liquidambar styraciflua, a tree which grows
in Mexico, Louisiana, and Virginia. Some specimens are thin, like oil, and others are
thickish, like turpentine. It is transparent, amber coloured, has an agreeable and
powerful smell, and an aromatic taste, which feels pungent in the throat. Boiling
alcohol dissolves it almost entirely. It contains a good deal of benzoic acid, some of
which effloresces whenever the liquid amber hardens with keeping.
LITHARGE (Eng. and Fr.; Glätte, Germ.); is the fused yellow protoxide of lead,
which on cooling passes into a mass consisting of small six-sided plates, of a reddish
yellow colour, and semitransparent. It generally contains more or less red lead, whence
the variations of its colour; and carbonic acid, especially when it has been exposed to
the air for some. time. See Lead, and Silver, for its mode of preparation.
LITHIA, is a simple earthy or alkaline substance, discovered not many years ago, in
the minerals called petalite and triphane. It is white, very caustic, reddens litmus, and
red cabbage, and saturates acids with great facility. When exposed to the air it
attracts humidity and carbonic acid. It is more soluble in water than baryta; and has
such a strong affinity for it, as to be obtained only in the state of a hydrate. It forms
neutral salts with all the acids. It is most remarkable for its power of acting upon, or
corroding platinum.
LITHIUM, is the metallic basis of Lithia; the latter substance consists of 100 of
metal, and 123 of oxygen.
LITHOGRAPHY. Though this subject belongs rather to the arts of taste and
design than to productive manufactures, its chemical principles fall within the province
of this Dictionary.
The term lithography is derived from λιθος, a stone, and γραφη, writing, and designates
the art of throwing off impressions upon paper, of figures and writing previously traced
upon stone. The processes of this art are founded:—
1. Upon the adhesion to a smoothly-polished limestone, of an encaustic fat which
forms the lines or traces.
2. Upon the power acquired by the parts penetrated by this encaustic, of attracting
to themselves, and becoming covered with a printer’s ink, having linseed oil for its basis.
3. Upon the interposition of a film of water, which prevents the adhesion of the
ink in all the parts of the surface of the stone not impregnated with the encaustic.
4. Lastly, upon a pressure applied by the stone, such as to transfer to paper the
greater part of the ink which covers the greasy tracings of the encaustic.
The lithographic stones of the best quality are still procured from the quarry of
Solenhofen, a village at no great distance from Munich, where this mode of printing
had its birth. They resemble in their aspect the yellowish white lias of Bath, but their
geological place is much higher than the lias. Abundant quarries of these fine-grained
limestones occur in the county of Pappenheim, along the banks of the Danube, presenting
slabs of every required degree of thickness, parted by regular seams, and ready
for removal with very little violence. The good quality of a lithographic stone is generally
denoted by the following characters; its hue is of a yellowish gray, and uniform
throughout; it is free from veins, fibres, and spots; a steel point makes an impression
on it with difficulty; and the splinters broken off from it by the hammer, display a conchoidal
fracture.
The Munich stones are retailed on the spot in slabs or layers of equal thickness;
they are quarried with the aid of a saw, so as to sacrifice as little as possible of the irregular[778]
edges of the rectangular tables or plates. One of the broad faces is then dressed,
and coarsely smoothed. The thickness of these stones is nearly proportional to their
other dimensions; and varies from an inch and two-thirds to 3 inches.
In each lithographic establishment, the stones receive their finishing, dressing, and
polishing; which are performed like the grinding and polishing of mirror plate. The
work is done by hand, by rubbing circularly a movable slab over another cemented
in a horizontal position, with fine sifted sand and water interposed between the two.
The style of work that the stone is intended to produce, determines the kind of polish
that it should get. For crayon drawing the stone should be merely grained more or
less fine according to the fancy of the draughtsman. The higher the finish of the surface,
the softer are the drawings; but the printing process becomes sooner pasty, and a
smaller number of impressions can be taken. Works in ink require the stone to be more
softened down, and finally polished with pumice and a little water. The stones thus
prepared are packed for use with white paper interposed between their faces.
Lithographic crayons.—Fine lithographic prints cannot be obtained unless the crayons
possess every requisite quality. The ingredients composing them ought to be of such
a nature as to adhere strongly to the stone, both after the drawing has undergone the
preparation of the acid, and during the press-work. They should be hard enough to
admit of a fine point, and trace delicate lines without risk of breaking. The following
composition has been successfully employed for crayons by MM. Bernard and Delarue,
at Paris:—
| Pure wax, (first quality) |
4 |
| Dry white tallow soap |
2 |
| White tallow |
2 |
| Gum lac |
2 |
| Lamp black, enough to give a dark tint |
1 |
| Occasionally copal varnish |
1 |
The wax is to be melted over a gentle fire, and the lac broken to bits is then to be
added by degrees, stirring all the while with a spatula; the soap is next introduced in
fine shavings; and when the mixture of these substances is very intimately accomplished,
the copal-varnish, incorporated with the lamp black, is poured in. The heat and
agitation are continued till the paste has acquired a suitable consistence; which may
be recognised by taking out a little of it, letting it cool on a plate, and trying its
quality with a penknife. This composition, on being cut, should afford brittle slices.
The boiling may be quickened by setting the rising vapours on fire, which increases the
temperature, and renders the exhalations less offensive. When ready, it is to be poured
into a brass mould, made of two semi-cylinders joined together by clasps or rings,
forming between them a cylindric tube of the crayon size. The mould should be previously
smeared with a greasy cloth.
M. Lasteyrie prescribes a more simple composition, said to be equally fit for the
lithographer’s use:—
| Dried white tallow soap |
6 |
parts. |
| White wax |
6 |
— |
| Lamp black |
1 |
— |
The soap and tallow are to be put into a small goblet and covered up. When the
whole is thoroughly fused by heat, and no clots remain, the black is gradually sprinkled
in with careful stirring.
Lithographic ink is prepared nearly on the same principles:—
| Wax |
16 |
parts. |
| Tallow |
6 |
— |
| Hard tallow soap |
6 |
— |
| Shell-lac |
12 |
— |
| Mastic in tears |
8 |
— |
| Venice turpentine |
1 |
— |
| Lamp black |
4 |
— |
The mastic and lac, previously ground together, are to be heated with care in the
turpentine; the wax and tallow are to be added after they are taken off the fire, and
when their solution is effected, the soap shavings are to be thrown in. Lastly, the lamp
black is to be well intermixed. Whenever the union is accomplished by heat, the
operation is finished; the liquor is left to cool a little, then poured out on tables, and,
when cold, cut into square rods.
Lithographic ink of good quality ought to be susceptible of forming an emulsion so
attenuated, that it may appear to be dissolved when rubbed upon a hard body in distilled[779]
or river water. It should be flowing in the pen, not spreading on the stone; capable
of forming delicate traces, and very black to show its delineations. The most
essential quality of the ink is to sink well into the stone, so as to re-produce the most
delicate outlines of the drawing, and to afford a great many impressions. It must
therefore be able to resist the acid with which the stone is moistened in the preparation,
without letting any of its greasy matter escape.
M. de Lasteyrie states that after having tried a great many combinations, he gives the
preference to the following:—
| Tallow soap, dried |
30 |
parts. |
| Mastic, in tears |
30 |
— |
| White soda of commerce |
30 |
— |
| Shell-lac |
150 |
— |
| Lamp black |
12 |
— |
The soap is first put into the goblet and melted over the fire, to which the lac being
added fuses immediately; the soda is then introduced, and next the mastic, stirring all
the while with a spatula. A brisk fire is applied till all these materials be melted completely,
when the whole is poured out into the mould.
The inks now prescribed may be employed equally with the pen and the hair pencil,
for writings, black-lead drawings, aqua tinta, mixed drawings, those which represent engravings
on wood (wood cuts), &c. When the ink is to be used it is to be rubbed down
with water, in the manner of China ink, till the shade be of the requisite depth. The
temperature of the place ought to be from 84° to 90° Fahr., or the saucer in which the
ink-stick is rubbed should be set in a heated plate. No more ink should be dissolved
than is to be used at the time, for it rarely keeps in the liquid state for 24 hours; and
it should be covered or corked up.
Autographic paper.—Autography, or the operation by which a writing or a drawing
is transferred from paper to stone, presents not merely a means of abridging labour, but
also that of reverting the writings or drawings into the direction in which they were
traced, whilst, if executed directly upon the stone, the impression given by it is
inverted. Hence, a writing upon stone must be inverted from right to left to obtain
direct impressions. But the art of writing thus is tedious and difficult to acquire,
while, by means of the autographic paper and the transfer, proofs are obtained in the
same direction with the writing and drawing.
Autographic ink.—It must be fatter and softer than that applied directly to the stone,
so that though dry upon the paper, it may still preserve sufficient viscidity to stick to
the stone by mere pressure.
To compose this ink, we take—
| White soap |
100 |
parts. |
| White wax of the best quality |
100 |
— |
| Mutton suet |
30 |
— |
| Shell-lac |
50 |
— |
| Mastic |
50 |
— |
| Lamp black |
30 or 35 |
— |
These materials are to be melted as above described for the lithographic ink.
Lithographic ink and paper.—The following recipes have been much commended:
| Virgin or white wax |
8 |
parts |
| White soap |
2 |
— |
| Shell-lac |
2 |
— |
| Lamp black |
3 |
table-spoonsful. |
Preparation.—The wax and soap are to be melted together, and before they become
so hot as to take fire, the lamp black is to be well stirred in with a spatula, and then the
mixture is to be allowed to burn for 30 seconds; the flame being extinguished, the lac is
to be added by degrees, carefully stirring all the time; the vessel is to be put upon the
fire once more in order to complete the combination, and till the materials are either
kindled or nearly so. After the flame is extinguished, the ink must be suffered to cool
a little, and then put into the moulds.
With the ink crayons thus made, lines may be drawn as fine as with the point of the
graver, and as full as can be desired, without risk of its spreading in the carriage. Its
traces will remain unchanged on paper for years before being transferred.
Some may think it strange that there is no suet in the above composition, but it has
been found that ink containing it is only good when used soon after it is made, and when[780]
immediately transferred to the stone, while traces drawn on paper with the suet ink
become defective after 4 or 5 days.
Lithographic paper.—Lay on the paper, 3 successive coats of sheep-feet jelly,
Lithographic paper.—Lay on the paper, 1 layer of white starch,
Lithographic paper.—Lay on the paper, 1 layer of gamboge.
The first layer is applied with a sponge dipped in the solution of the hot jelly, very
equally over the whole surface, but thin; and if the leaf be stretched upon a cord, the
gelatine will be more uniform. The next two coats are to be laid on, until each is dry.
The layer of starch is then to be applied with a sponge, and it will also be very thin
and equal. The coat of gamboge is lastly to be applied in the same way. When the
paper is dry, it must be smoothed by passing it through the lithographic press; and
the more polished it is, the better does it take on the ink in fine lines.
Transfer.—When the paper is moistened, the transfer of the ink from the gamboge
is perfect and infallible. The starch separates from the gelatine, and if, after taking
the paper off the stone, we place it on a white slab of stone, and pour hot water over it,
it will resume its primitive state.
The coat of gamboge ought to be laid on the same day it is dissolved, as by
keeping, it becomes of an oily nature; in this state it does not obstruct the transfer,
but it gives a gloss to the paper which renders the drawing or tracing more difficult,
especially to persons little habituated to lithography.
The starch paste can be employed only when cold, the day after it is made, and after
having the skin removed from its surface.
A leaf of such lithographic paper may be made in two minutes.
In transferring a writing, an ink drawing, or a lithographic crayon, even the impression
of a copper-plate, to the stone, it is necessary, 1. that the impressions be made upon
a thin and slender body like common paper; 2. that they may be detached and fixed totally
on the stone by means of pressure; but as the ink of a drawing sinks to a certain
depth in paper, and adheres pretty strongly, it would be difficult to detach all its parts,
were there not previously put between the paper and the traces, a body capable of being
separated from the paper, and of losing its adhesion to it by means of the water with
which it is damped. In order to produce this effect, the paper gets a certain preparation,
which consists in coating it over with a kind of paste ready to receive every delineation
without suffering it to penetrate into the paper. There are different modes of communicating
this property to paper. Besides the above, the following may be tried.
Take an unsized paper, rather strong, and cover it with a varnish composed of:—
| Starch |
120 |
parts |
| Gum arabic |
40 |
— |
| Alum |
20 |
— |
A paste of moderate consistence must be made with the starch and some water, with
the aid of heat, into which the gum and alum are to be thrown, each previously dissolved
in separate vessels. When the whole is well mixed, it is to be applied, still hot,
on the leaves of paper, with a flat smooth brush. A tint of yellow colour may be given
to the varnish, with a decoction of the berries of Avignon, commonly called French
berries by our dyers. The paper is to be dried, and smoothed by passing under the
scraper of the lithographic press.
Steel pens are employed for writing and drawing with ink on the lithographic
stones.
LITMUS (Tournesol, Fr.; Lackmus, Germ.); is prepared in Holland from the
species of lichen called Lecanora tartarea, Roccella tartarea, by a process which has been
kept secret, but which is undoubtedly analogous to that for making archil and cudbear.
The ground lichens are first treated with urine containing a little potash, and allowed
to ferment, whereby they produce a purple-red; the coloured liquor, treated with quicklime
and some more urine, is set again to ferment during two or three weeks, then it is
mixed with chalk or gypsum into a paste, which is formed into small cubical pieces,
and dried in the shade. Litmus has a violet-blue colour, is easy to pulverize, is partially
soluble in water and dilute alcohol, leaving a residuum consisting of carbonate of
lime, of clay, silica, gypsum, and oxide of iron combined with the dye. The colour of
litmus is not altered by alkalis, but is reddened by acids; and is therefore used in
chemistry as a delicate test of acidity, either in the state of solution or of unsized paper
stained with it. It is employed to dye marble blue.
LIXIVIATION (Lessivage, Fr.; Auslagen, Germ.); signifies the abstraction by
water of the soluble alkaline or saline matters present in any earthy admixture; as from
that of quicklime and potashes to make potash lye, from that of effloresced alum schist
to make aluminous liquors, &c.
LOADSTONE, MAGNETIC IRON-STONE (Fer oxydulé, Fr.; Magneteisenstein,[781]
Germ.); an iron ore consisting of the protoxide and peroxide of iron in a state
of combination.
LOAM (Terre-limoneuse, Fr.; Lehm, Germ.); a native clay mixed with quartz sand
and iron ochre, and occasionally with some carbonate of lime.
LODE, is the name given by the Cornish miners to a vein, whether it be filled with
metallic or earthy matter.
LOGWOOD (Bois de Campèche, Bois bleu, Fr.; Blauholz, Germ.); is the wood of
the Hæmatoxylon Campechianum, a native tree of central America, grown in Jamaica since
1715. It was first introduced into England in the reign of Elizabeth, but as it afforded
to the unskilful dyers of her time a fugitive colour, it was not only prohibited from
being used, under severe penalties, but was ordered to be burned wherever found, by a
law passed in the 23d year of her reign. The same prejudice existed, and the same law
was enacted against indigo. At length, after a century of absurd prohibition, these two
most valuable tinctorial matters, by which all our hats, and the greater part of our
woollen cloths, are dyed, were allowed to be used.
Old wood, with black bark and with little of the white alburnum, is preferred. Logwood
is denser than water, very hard, of a fine compact grain, and almost indestructible
by the atmospheric elements; it has a sweet and astringent taste, and a peculiar not
inoffensive smell.
For its chemical composition, see Hematin.
When chipped logwood is for some time exposed to the air, it loses a portion of its
dyeing power. Its decoction absorbs the oxygen of the atmosphere, and then acquires
the property of precipitating with gelatine, which it had not before. The dry extract
of logwood, made from an old decoction, affords only a fugitive colour.
For its applications in dyeing, see Black Dye; Brown Dye; Calico Printing;
Dyeing; Hat Dyeing, &c.
The imports of logwood for home use, were, in 1836, 12,880 tons, 13 cwts.; in 1837,
14,677 tons, 13 cwts. And the amount of duty received was, in 1836, 2,480l.; in 1837,
2,552l.
LOOM (Metier a tisser, Fr.; Weberstuhl, Germ.); is the ancient and well-known
machine for weaving cloth by the decussation of a series of parallel threads, which run
lengthwise, called the warp or chain, with other threads thrown transversely with the
shuttle, called the woof or weft. See Jacquard Loom and Weaving.
LUBRICATION. The following simple and efficacious plan of lubricating the
joints and bearings of machinery by capillary attraction, has been kindly communicated
to me, by its ingenious inventor, Edward Woolsey, Esq.:—
Fig. 645. represents a tin cup, which has a small tin tube A, which passes through
the bottom, as shown by the dotted lines. It may have a tin cover to keep out the dust.
Fig. 646. is a plan of the same.
Fig. 647. is a section of the same. Oil is poured into the cup, and one end of a
worsted or cotton thread is dipped into the oil, and the other end passed through the
tube. The capillary attraction causes the oil to ascend and pass over the orifice of the
tube, whence it gradually descends, and drops slower or quicker, according to the length
of the thread, or its thickness, until every particle of oil is drawn over by this capillary
syphon. The tube is intended to be put into the bearings of shafts, &c., and is made
of any size that may be wished. If oil, or other liquids, is desired to be dropped upon a
grindstone or other surface, this cup can have a handle to it, or be hung from the
ceiling.
Fig. 648. It is frequently required to stop the capillary action when the machinery is
not going; and this has been effected by means of a tightening screw, which passes
through a screw boss in the cover of the cup, and presses against the internal orifice
of the tube, preventing the oil from passing.
Fig. 649. As I find when these screw cups (fig. 648.) are used upon beams of engines
and moving bearings, that the screw is apt to be tightened by the motion; and also, as
I think the action of the screw is uncertain, from the workman neglecting to screw it
down sufficiently, it answers best to take out the capillary thread when the lubrication
is not required; and to effect this easily, I have a tin top to the cup, with a round
pipe soldered to it: this pipe has a slit in it, like a pencil case, and allows a bolt B
to slide easily in it. In fig. 650. the bolt is down; in fig. 651., the bolt, which is a
piece of brass wire, is drawn up, and there is no capillary action between the thread and
the oil. In fig. 651. it will be observed, that the bolt is kept in its place by its head
C, resting in a lateral slit in the pipe, and it cannot be drawn out on account of the
pin E. One end of the thread is fastened to the eye-hole at the bottom of the bolt,
and the other end is tied to a small wire which crosses the lower orifice of the tube at
D and which is shown in plan fig. 652.
By this simple contrivance the capillary action can be stopped or renewed in a second,
without removing the top of the lubricator.
[782]
The saving by this plan, instead of pouring oil into the bearings, is 2 gallons out
of 3, while the bearings are better oiled.
“I send you the drawings of the lubricators, with a detailed explanation. I have
omitted to state, that the saving in labour is considerable where there are many joints
to keep oiled three or four times a day; and that the workman does not, with this
apparatus, run the risk of being caught by the machinery. Perhaps your friends may
be at a loss how to tie on the cotton or worsted thread. I pass a long thread through
the eye-hole E of the bolt, and then draw the two ends through the tube by a fine wire
with a hook to it, one end on one side of the cross wire D, and the other end on the
other side. I then put the cover on, and the bolt in the position shown in fig. 651.; when
by drawing the two ends of the thread, and tying them across the wire D, you have the
exact length required. When you wish to see the quantity of oil remaining in the
lubricator, the bolt must be dropped as in fig. 650., and you can then lift the cover a little
way off, without breaking the thread, and replenish with oil. The cost of fig. 650. in
tin plate is 9d. The figures in the wood cuts are one third of the full size.
“Believe me to be yours sincerely,
“E. J. Woolsey.”
LUPININE, is a substance of a gummy appearance, so named by M. Cussola,
because it was obtained from Lupines.
LUPULINE, from Humulus Lupulus; is the peculiar bitter aromatic principle of
the hop. See Beer.
LUTE (from lutum, clay; Lut, Fr.; Kitte, Beschläge, Germ.); is a pasty or loamy
matter employed to close the joints of chemical apparatus, or to coat their surfaces, and
protect them from the direct action of flame. Lutes differ according to the nature of
the vapours which they are destined to confine, and the degree of heat which they are
to be exposed to.
1. Lute of linseed meal, made into a soft plastic dough with water, and immediately
applied pretty thick to junctions of glass, or stone-ware, makes them perfectly tight, hardens
speedily, resists acid and ammoniacal vapours, as also a moderate degree of heat. It
becomes stronger when the meal is kneaded with milk, lime-water, or solution of glue.
2. Lute of thick gum-water, kneaded with clay, and iron filings, serves well for permanent
junctions, as it becomes extremely solid.
3. By softening in water a piece of thick brown paper, kneading it first with rye-flour[783]
paste, and then with some potter’s clay, till it acquire the proper consistence, a
lute is formed which does not readily crack or scale off.
4. Lute, consisting of a strong solution of glue kneaded into a dough with new
slaked lime, is a powerful cement, and with the addition of white of egg, forms the
lut d’ane;—a composition adapted to mend broken vessels of porcelain and stone-ware.
5. Skim-milk cheese, boiled for some time in water, and then triturated into paste
with fresh-slaked lime, forms also a good lute.
6. Calcined gypsum, diffused through milk, solution of glue or starch, is a valuable
lute, in many cases.
7. A lute made with linseed, melted caoutchouc, and pipe-clay, incorporated into a
smooth dough, may be kept long soft when covered in a cellar, and serves admirably to
confine acid vapours. As it does not harden, it may therefore be applied and taken off
as often as we please.
8. Caoutchouc itself, after being melted in a spoon, may be advantageously used for
securing joints against chlorine and acid vapours, in emergencies when nothing else
would be effectual. It bears the heat at which sulphuric acid boils.
9. The best lute for joining crucibles inverted into each other, is a dough made with
a mixture of fresh fire-clay, and ground fire-bricks, worked with water. That cement if
made with solution of borax answers still better, upon some occasions, as it becomes a
compact vitreous mass in the fire. See Cements.
LUTEOLINE, is a yellow colouring matter discovered by Chevreul in weld.
When sublimed, it crystallizes in needles.
LYCOPODIUM CLAVATUM. The seeds of the lycopodium ripen in September.
They are employed, on account of their great combustibility, in theatres, to
imitate the sudden flash of lightning, by throwing a quantity of them from a powder
puff, or bellows, across the flame of a candle.
LYDIAN STONE, is flint-slate.
MACARONI, is a dough of fine wheat flour, made into a tubular or pipe form, of
the thickness of goose-quills, which was first prepared in Italy, and introduced into
commerce under the name of Italian or Genoese paste. The wheat for this purpose
must be ground into a coarse flour, called gruau or semoule, by the French, by means of
a pair of light mill-stones, placed at a somewhat greater distance than usual. This
semoule is the substance employed for making the dough. For the mode of manufacturing
it into pipes, see Vermicelli.
MACE, is a somewhat thick, tough, unctuous membrane, reticulated or chapt, of a
yellowish-brown or orange colour. It forms the envelop of the shell of the fruit of the
myristica moschata, which contains the nutmeg. It is dried in the sun, after being
dipped in brine; sometimes it is sprinkled over with a little brine, before packing, to
prevent the risk of moulding. Mace has a more agreeable flavour than nutmeg;
with a warm and pungent taste. It contains two kinds of oil; the one of which is
unctuous, bland, and of the consistence of butter; the other is volatile, aromatic, and
thinner. The membrane is used as a condiment in cookery, and the aromatic oil in
medicine.
MACERATION (Eng. and Fr.; Einweichen, Germ.), is a preparatory steep to
which certain vegetable and animal substances are submitted, with the view of distending
their fibres or pores, and causing them to be penetrated by such menstrua as are best
adapted to extract their soluble parts. Water, alone, or mixed with acids, alkalis, or
salts; alcohol and ether, are the liquids usually employed for that purpose.
MACLE, is the name of certain diagonal black spots in minerals, like the ace of
diamonds in cards, supposed to proceed from some disturbance of the particles in the
act of crystallization.
MADDER (Garance, Fr.; Färberröthe, Germ.), a substance very extensively used
in dyeing, is the root of the Rubia tinctorum, a plant, of which two species are distinguished
by Linnæus.
The best roots are those which have the size of a writing quill, or, at most, of the
little finger. They are semitransparent, and reddish; have a strong odour, and a smooth
bark. They should be of two or three years’ growth.
The madder, taken from the ground and picked, must be dried in order to be
ground and preserved. In warm climates it is dried in the open air; but, elsewhere,
stoves must be employed.
[784]
The stringy filaments and epidermis are to be removed, called mulle; as also the pith,
so as to leave nothing but the ligneous fibres.
The preparation of madders is carried on in the department of the Rhone, in the following
manner.
The roots are dried in a stove, heated by means of a furnace, from which the air is
allowed to issue only at intervals, at the moment when it is judged to be saturated
with moisture. The furnace-flue occupies a great portion of the floor; above are
three close gratings, on which the roots are distributed in layers of about two decimetres
(nearly 8 inches). At the end of 24 hours, those which are on the first grated floor
directly above the stove are dry, when they are taken away and replaced by those of the
superior floors. This operation is repeated whenever the roots over the stove are dry.
The dry roots are thrashed with a flail, passed through fanners similar to those employed
for corn, and then shaken upon a very coarse sieve. What passes through is farther
winnowed and sifted through a finer sieve than the first. These operations are
repeated five times, proceeding successively to sieves still finer and finer, and setting
aside every time what remains on the sieve. What passes through the fifth sieve is
rejected as sand and dust. After these operations, the whole fibrous matters remaining
on the sieve are cleaned with common fanners, and women separate all the foreign matters
which had not been removed before. For dividing the roots, afterwards, into different
qualities, a brass sieve is made use of, whose meshes are from six to three millimetres in
diameter (from 1⁄4th to 1⁄8th inch E.) What passes through the finest is rejected; and
what passes through the coarsest is regarded as of the best quality. These roots thus
separated, are carried into a stove, of a construction somewhat different from the first.
They are spread out in layers of about a decimetre in thickness (nearly 4 inches E.),
on large lattice-work frames, and the drying is known to be complete, when on taking
up a handful and squeezing it, the roots break easily. On quitting the stove, the
madder is carried, still hot, into a machine, where it is minced small, and a sieve
separates the portion of the bark reduced to powder. This operation is repeated three
or four times, and then the boulter is had recourse to. What passes through the sieve,
or the brass meshes of the boulter, is regarded as common madder; and what issues
at the extremity of the boulter is called the flour. Lastly, the madder which passes
through the boulter is ground in a mill with vertical stones, and then passed through
sieves of different sizes. What remains above is always better than what goes through.
The madder of Alsace is reduced to a very fine powder, and its colouring matter is
extracted by a much longer ebullition than is necessary for the lizari of the Levant.
The prepared madders ought to be carefully preserved from humidity, because they
easily imbibe moisture, in which case fermentation spoils their colour.
D’Ambourney and Beckman have asserted, that it is more advantageous to employ
the fresh root of madder than what has been submitted to desiccation, especially by
means of stoves. But in its states of freshness, its volume becomes troublesome in the
dyeing bath, and uniform observation seems to prove that it ameliorates by age.
Besides, it must be rendered susceptible of keeping and carrying easily.
It appears that madder may be considered as composed of two colouring substances,
one of which is dun (tawny), and the other is red. Both of these substances may
combine with the stuff. It is of consequence, however, to fix only the red part. The
dun portion appears to be more soluble, but its fixity on stuffs may possibly be increased
by the affinity which it has for the red portion.
The different additions made to madder, and the multiplied processes to which it is
sometimes exposed, have probably this separation for their chief object.
The red portion of madder is soluble, but in small quantity, in water. Hence but a
limited concentration can be given to its solution. If the portion of this substance be
too much increased, so far from obtaining a greater effect, we merely augment the proportion
of the dun part, which is the more soluble of the two.
In consequence of the Société Industrielle of Mulhausen having offered in the year
1826 large premiums to the authors of the best analytical investigation of madder, eight
memoirs were transmitted to it in the year 1827. They were examined with the
greatest care by a committee consisting of able scientific and practical men. None of
the competitors however fulfilled the conditions of the programme issued by the society;
but four of them received a tribute of esteem and gratitude from it; MM. Robiquet
and Colin at Paris, Kuhlmann at Lille, and Houton-Libillardière. Fresh premiums
were offered for next year, to the amount of 2000 francs.
Every real discovery made concerning this precious root, would be of vast consequence
to dyers and calico-printers. Both M. Kuhlmann, and Robiquet and Colin, conceived
that they had discovered a new principle in madder, to which they gave the name
alizarine. The latter two chemists treated the powdered madder with sulphuric acid,
taking care to let it heat as little as possible. By this action the whole is carbonized,
except perhaps the red matter. The charcoal thus obtained is pulverized, mixed with[785]
water, thrown upon a filter, and well washed in the cold. It is next dried, ground, and
diffused through fifty parts of water, containing six parts of alum. This mixture is then
boiled for one quarter of an hour, and thrown upon a filter cloth while boiling hot. The
residuum is once more treated with a little warm alum water. The two liquors are to
be mixed, and one part of sulphuric acid poured into them; when they are allowed to
cool with occasional agitation. Flocks now make their appearance; the clear liquid is
decanted, and the grounds are thrown upon a filter. The precipitate is to be washed,
first with acidulated water, then with pure water, and dried, when the colouring matter
is obtained in a red or purple state. This purple substance, when heated dry, gives out
alizarine, and an empyreumatic oil, having an odour of animal matter; while a charcoally
matter remains.
M. Dan. Kœchlin, the justly celebrated calico-printer of Mulhausen, has no faith in
alizarine as the dyeing principle of madder; and thinks moreover that, were it of value,
it could not be extracted on the great scale, on account of the destructive heat which
would result from the acid acting upon a considerable body of the ground madder. Their
alizarine is not a uniform substance, as it ought to be, if a proximate principle; for
samples of it obtained in different repetitions of the process have produced very variable
effects in dyeing. The madders of Avignon, though richer in colour than those of Alsace,
afford however little or no alizarine. In fact, purpurine, the crude substance from which
they profess to extract alizarine, is a richer dye than this pure substance itself.
Madder contains so beautiful and so fast a colour, that it has become of almost
universal employment in dyeing; but that colour is accompanied with so many other
substances which mask and degrade it, that it can be brought out and fixed only after a
series of operations more or less difficult and precarious. This dye is besides so little
soluble, that much of it is thrown away in the dye-house; the portion supposed to be
exhausted being often as rich as other fresh madder; hence it would be a most valuable
improvement in this elegant art to insulate this tinctorial body, and make it a new
product of manufacture.
Before the time of Haussmann, an apothecary at Colmar, the madder bath was subject
to many risks, which that skilful chemist taught dyers how to guard against, by introducing
a certain quantity of chalk into the bath. A change of residence led Haussmann
to this fortunate result. After having made very fine reds at Rouen, he encountered
the greatest obstacles in dyeing the same reds at Logelbach near Colmar, where he went
to live. Numerous trials, undertaken with the view of obtaining the same success in
his new establishment, proved that the cause of his favourable results at Rouen existed in
the water, which contained carbonate of lime in solution, whilst the water of Logelbach
was nearly pure. He then tried a factitious calcareous water, by adding chalk to his
dye bath. Having obtained the most satisfactory results, he was not long of producing
here as beautiful and as solid reds as he had done at Rouen. This practice became
soon general among the calico-printers of Alsace, though in many dye-works the chalk
is now replaced by lime, potash, or soda. But when the madder of Avignon is used,
all these antacid correctives become unnecessary, because it contains a sufficient quantity
of carbonate of lime; an important fact first analytically demonstrated by that accurate
chemist M. Henri Schlumberger of Mulhausen. Avignon madder indicates the presence
of carbonate of lime in it, by effervescing with dilute acids, which Alsace madder
does not.
M. Kuhlmann found a free acid resembling the malic, in his analysis of madders.
But his experiments were confined to those of Alsace. The madders of Avignon are on
the contrary alkaline, as may be inferred from the violet tint of the froth of their
infusions; whereas that of the Alsace madders is yellowish, and it strongly reddens
litmus paper. This important difference between the plants of these two districts,
depends entirely upon the soil; for madders grown in a calcareous shelly soil in Alsace,
have been found to be possessed of the properties of the Avignon madder.
The useful action of the carbonate and the phosphate of lime in the madder of
Avignon, explains why madders treated with acids which remove their calcareous salts,
without taking away their colouring matter, lose the property of forming fast dyes.
Many manufacturers are in the habit of mixing together, and with advantage, different
sorts of madder. That of Avignon contains so much calcareous matter that, when
mixed with the madder of Alsace, it can compensate for its deficiency. Some of the latter
is so deficient as to afford colours nearly as fugitive as those of Brazil wood and quercitron.
The Alsace madders by the addition of chalk to their baths, become as fit for dyeing
Turkey reds as those of Avignon. When the water is very pure, one part of chalk ought
to be used to five of Alsace madder, but when the waters are calcareous, the chalk should
be omitted. Lime, the neutral phosphate of lime, the carbonate of magnesia, oxide and
carbonate of zinc, and several other substances have the property of causing madder to
form a fast dye, in like manner as the carbonate of lime.
[786]
The temperature of from 50° to 60° R. (145° to 167° F.), is the best adapted to the
solution of the colouring matter, and to its combination with the mordants; and thus a
boiling heat may be replaced advantageously by the long continuance of a lower temperature.
A large excess of the dye-stuff in the bath is unfavourable in two points of
view; it causes a waste of colouring matter, and renders the tints dull. It is injurious
to allow the bath to cool, and to heat it again.
In a memoir published by the Society of Mulhausen, in September, 1835, some
interesting experiments upon the growth of madders in factitious soils are related by
MM. Kœchlin, Persoz, and Schlumberger. A patch of ground was prepared containing
from 50 to 80 per cent. of chalky matter, and nearly one fifth of its bulk of good
horse-dung. Slips of Alsace and Avignon madders were planted in March, 1834, and
a part of the roots were reaped in November following. These roots, though of only six
months growth, produced tolerably fast dyes, nor was any difference observable between
the Alsace and the Avignon species; whilst similar slips or cuttings, planted in a natural
non-calcareous soil, alongside of the others, yielded roots which gave fugitive dyes.
Others were planted in the soil of Palud, transported from Avignon, which contained
more than 90 per cent. of carbonate of lime, and they produced roots that gave still
faster dyes than the preceding. Three years are requisite to give the full calcareous
impregnation to the indigenous madders of Avignon.
As to the function of the chalk, valuable observations, made long ago by M. Daniel
Kœchlin, have convinced him, that the combination of two different bases with a colouring
matter, gave much more solidity to the dye, in consequence, undoubtedly, of a greater
insolubility in the compound. Experiments recently made by him and his colleagues
above named, prove that in all cases of madder-dyeing under the influence of chalk, a
certain quantity of lime becomes added to the aluminous mordant. In the subsequent
clearing with a soap bath, some of the alumine is removed, and there remains upon the
fibre of the cloth a combination of these two earths in atomic proportions. Thus the
chalk is not for the purpose of saturating the acid, as had been supposed, but of forming
a definite compound with alumina, and probably also with the fatty bodies, and the
colouring matter itself.
The red mordants are prepared commonly in Alsace, as follows:—The crushed
alum and acetate of lead being weighed, the former is put into a deep tub, and dissolved
by adding a proper quantity of hot water, when about one tenth of its weight of soda
crystals is introduced to saturate the excess of acid in the alum. The acetate of lead
is now mixed in; and as this salt dissolves very quickly, the reaction takes place almost
instantly. Care must be taken to stir for an hour. The vessel should not be covered,
lest its contents should cool too slowly.
The different mordants most generally employed for madder, are detailed under
Colours, in Calico-Printing and Mordant.
Much mordant should not be prepared at once, for sooner or later it will deposit
some sub-acetate of alumina. This decomposition takes place even in corked phials in
the cold; and the precipitate does not readily dissolve again in acetic acid. All practical
men know that certain aluminous mordants are decomposed by heating them, and
restored on cooling, as Gay Lussac has pointed out. He observed, that by adding to
pure acetate of alumina, some alum or sulphate of potash, the mixture acquires the
property of forming a precipitate with a heat approaching the boiling point, and of redissolving
on cooling. The precipitate is alumina nearly pure, according to M. Gay
Lussac; but, by M. Kœchlin’s more recent researches, it is shown to be sub-sulphate of
alumina, containing eight times as much base as the neutral sulphate.
Madder dye.—On account of the feeble solubility of its colouring matter in water,
we cannot dye with its decoction; but we must boil the dye-stuff along with the goods
to be dyed; thereby the water dissolves fresh portions of the dye, and imparts it in
succession to the textile fibres. In dyeing with madder, we must endeavour to fix as
little of the dun matter as possible upon the cloth.
Dyeing on wool.—Alumed wool takes, in the madder bath, a red colour, which is not
so bright as cochineal red, but it is faster; and as it is far cheaper, it is much used
in England to dye soldiers cloth. A mordant of alum and tartar is employed; the
bath of madder, at the rate of from 8 to 16 ounces for the pound of cloth, is heated to
such a degree that we can just hold our hand in it, and the goods are then dyed by the
wince, without heating the bath more till the colouring matter be fixed. Vitalis prescribes
as a mordant, one fourth of alum, and one sixteenth of tartar; and for dyeing,
one third of madder, with the addition of a 24th of solution of tin diluted with its weight
of water. He raises the temperature in the space of an hour, to 200°, and afterwards
he boils for 3 or 4 minutes; a circumstance which is believed to contribute to the fixation
of the colour. The bath, after dyeing, appears much loaded with yellow matter,
because this has less affinity for the alum mordant than the red. Sometimes a little
archil is added to the madder, to give the dye a pink tinge; but this is fugitive.
[787]
Silk is seldom dyed with madder, because cochineal affords brighter tints.
Dyeing on cotton and linen.—The most brilliant and fastest madder red is the Turkey
or Adrianople. The common madder reds are given in the following way:—The yarn
or cloth is boiled in a weak alkaline bath, washed, dried and galled, by steeping the
cotton in a decoction of bruised galls or of sumach. After drying, it is twice alumed;
for which purpose, for every 4 parts of the goods, one part of alum is taken, mixed with
1-16th of its weight of chalk. The goods are dipped into a warm solution of the alum,
wrung out, dried, and alumed afresh, with half the quantity. The acetate of alumina
mordant, described above, answers much better than common alum for cotton. After
the goods are dried and rinsed, they are passed through the dye bath, which is formed
of 3⁄4 lb. of good madder for every pound of cotton; and it is raised to the boiling point
by degrees, in the space of 50 or 60 minutes. Whenever the ebullition has continued
a few minutes, the goods must be removed, washed slightly, and dyed a second time in
the same way, with as much madder. They are then washed and passed through a
warm soap bath, which removes the dun colouring matter.
Hölterhoff prescribes for ordinary madder red the following proportions:—20 pounds
of cotton yarn; 14 pounds of Dutch madder; 3 pounds of nut-galls; 5 pounds of
alum; to which 1⁄2 lb. of acetate of lead has been first added, and then a quarter of a
pound of chalk.
In the calico-print works the madder goods are passed through a bran bath first,
immediately after dyeing; next, after several days exposure to the air, when the dun dye
has become oxidized, and is more easily removed. An addition of chalk, on the principles
explained above, is sometimes useful in the madder bath. If bran be added to
the madder bath, the colour becomes much lighter, and of an agreeable shade. Sometimes
bran-water is added to the madder bath, instead of bran.
Adrianople or Turkey red.—This is the most complicated and tedious operation in
the art of dyeing; but it produces the fastest colour which is known. This dye was
discovered in India, and remained long a process peculiar to that country. It was
afterwards practised in other parts of Asia and in Greece. In 1747, Ferquet and
Goudard brought Greek dyers into France, and mounted near Rouen, and in Languedoc,
Turkey-red dye works. In 1765, the French government, convinced of the
importance of this business, caused the processes to be published. In 1808, Reber, at
Mariakirch, furnished the finest yarn of this dye, and M. Köchlin became celebrated
for his Turkey-red cloth.
Process for Turkey-red.—The first step consists in clearing the yarn or cloth in alkaline
baths, and dipping them in oily liquors, to which sheep’s dung was formerly
added. This operation is repeated several times, the goods being dried after each immersion.
There next follows the cleansing with alkaline liquors to remove the excess
of oil, the galling, the aluming, the maddering, the brightening or removing the dun
part of the dye by boiling, at a high temperature, with alkaline liquid, and the rosing
by boiling in a bath of salt of tin. We shall give some details concerning this tedious
manipulation, and the differences which exist in it in the principal dye-works.
At Rouen, where the process was first brought to perfection, two methods are pursued,
called the gray and the yellow course or march. In the gray, the dye is given
immediately after the cotton has received the oily mordant, the gall, and the alum, as it
has then a gray colour. In the yellow course, it is passed through fresh oils, alum,
and galls before the maddering, the cotton having then a yellow tint.
Different views have been taken of the principles of the Turkey red dye, and the object
and utility of the various steps. The most ancient notion is that of animalizing
the cotton by dung and blood, but experience has proved that without any animal matter
the finest colour may be obtained. According to Dingler, the cotton is imbued with
oil by steeping it in combinations of oil and soda; the oil is altered by repeated dryings
at a high temperature; it attracts oxygen from the air, and thereby combines intimately
with the cotton fibre, so as to increase the weight of the stuff. The dung, by
a kind of fermentation, accelerates the oxidizement, and hence crude oil is preferable to
pure. In England, the mucilaginous oils of Gallipoli are preferred, and in Malabar,
oils more or less rancid. The drying oils do not answer. The subsequent treatment
with the alkaline liquors removes the excess of oil, which has not been oxidized and
combined; a hard drying completely changes that which remains in the fibres; the
aluming which follows combines alumina with the cotton; the galling tans the fibres,
producing a triple compound of oil and alum, which fixes the colouring matter. The
object of the other steps is obvious.
According to Wuttich the treatment with oil opens the cotton so as to admit the mordant
and the colouring matter, but the oil and soap do not combine with the fibres.
In the alkaline baths which follow, the oil is transformed into soap and removed;
whence the cotton should not increase in weight in the galling and aluming; the cotton[788]
suffers a kind of tanning, and the saline parts of the blood assist in fixing the madder
dye.
The German process improved, according to Dingler, consists of the following operations:
mordant of an oily soap or a soapy liniment, hard drying; alkaline bath,
drying, steeping, rinsing away of the uncombined mordant, drying; galling, drying;
aluming, drying, steeping in water containing chalk, rinsing; maddering, airing, rinsing;
brightening with an alkaline boil, and afterwards in a bath containing salt of tin;
then washing and drying.
The yarn or the cloth must be first well worked in a bath of sheep’s dung and oil, compounded
as follows:—25 pounds of sheep’s dung are to be bruised in a solution of
pure caustic potash of hydrometer strength 3°, and the mixed liquor is to be passed
through a sieve. Two pounds of fine oil are now to be poured into 16 pounds of this lye,
after which 30 pounds of coarse oil are to be added, with agitation for 1⁄4 of an hour. Other
4 pounds of hot lye are to be well stirred in, till the whole is homogeneous. This proportion
of mordant is sufficient for 100 pounds of cotton yarn, for 90 pounds of unbleached
or 100 pounds of bleached cotton goods. The cotton stuff, after being well wrung out, is
to be laid in a chest and covered with a lid loaded with weights, in which state it should
remain for five days. At the end of 24 hours, the cotton becomes hot with fermentation,
gets imbued with the mordant, and the oil becomes rapidly altered. The goods are
next exposed freely to the air during the day, and in the evening they are dried in a hot
chamber, exposed to a temperature of 158° F., for 6 or 8 hours, which promotes the
oxidizement of the oil.
The goods are now passed the second time through a soapy-oil mordant similar to
the first, then dried in the air by day, and in the hot stove by night. The third and
fourth oil-soap steeps are given in the same way, but without the dung. The fifth
steep is composed of a lye at 2°, after which the goods must also be dried. Indeed
from the first to the fourth steep, the cotton stuff should be put each time into a chamber
heated to 145° F. for 12 or 15 hours, and during 18 hours after the fifth steep.
The uncombined oil must, in the next place, be withdrawn by the degraissage, which
consists in steeping the goods for 6 hours in a very weak alkaline ley. After rinsing
and wringing, they are dried in the air, and then put into the hot stove.
The goods are now galled in a bath formed of 36 pounds of Sicilian sumach, boiled
for 3 hours in 260 pounds of water, and filtered. The residuum is treated with 190
fresh pounds of water. This decoction is heated with 12 pounds of pounded nut-galls
to the boiling point, allowed to cool during the night, and used next morning as hot as
the hand can bear; the goods being well worked through it. They are again dried in
the air, and afterwards placed in a stove moderately heated. They are next passed
through a tepid alum bath, containing a little chalk; left afterwards in a heap during
the night, dried in the air, and next in the stove. The dry goods are finally passed
through hot water containing a little chalk, wrung out, rinsed, and then maddered.
For dyeing, the copper is filled with water, the fire is kindled, and an ounce and a
half of chalk is added for every pound of madder; a pound and a quarter of madder being
taken for every pound of cotton yarn. The goods are now passed through the bath, so
that they penetrate to near its bottom. The fire must be so regulated, that the copper
will begin to boil in the course of from 21⁄2 to 3 hours; and the ebullition must be
continued for an hour; after which the yarn is aired and rinsed. Cloth should be put
into the dye-bath when its temperature is 77°, and winced at a heat of from 100° to
122° during the first hour; at 167° during the second; and at the boiling point when the
third hour begins. It is to be kept boiling for half an hour; so that the maddering
lasts four hours. Dingler does not add sumach or galls to the madder bath, because
their effect is destroyed in the subsequent brightening, and he has no faith in the utility
of blood.
After being dyed, the goods are washed, pressed, and subjected to a soapy alkaline
bath at a high heat, in a close boiler, by which the dun parts of the galls and the
madder are dissolved away, and the red colour remains in all its lustre. This operation
is called brightening. It is repeated in a similar liquor, to which some muriate of tin is
added for the purpose of enlivening the colour and giving it a rosy tint. Last of all,
the goods are rinsed, and dried in the shade.
The Elberfeld process consists for 100 libs. of the following steps:—
1. Cleaning the cotton by boiling it for four hours in a weak alkaline bath, cooling
and rinsing.
2. Working it thoroughly four times over in a steep, consisting of 300 pounds of
water, 15 pounds of potash, 1 pailful of sheep’s dung, and 121⁄2 pounds of olive oil, in
which it should remain during the night. Next day it is drained for an hour, wrung
out and dried. This treatment with the dung steep, and drying, is repeated 3 times.
3. It is now worked in a bath containing 120 quarts of water, 18 pounds of potash,[789]
and 6 quarts of olive oil; then wrung out and dried. This steep is also repeated
4 times.
4. Steeping for a night in the river is the next process; a slight rinsing without
wringing, and drying in the air.
5. Bath made of a warm decoction (100° F.) of sumach and nut-galls, in which the
goods remain during the night; they are then strongly wrung, and dried in the air.
6. Aluming with addition of potash and chalk; wringing; working it well through
this bath, where it is left during the night.
7. Draining, and strong rinsing the following day; piling up in a water cistern.
8. Rinsing repeated next day, and steeping in water to remove any excess of alum
from the fibres; the goods continue in the water till they are taken to the dyeing-bath.
9. The maddering is made with the addition of blood, sumach, and nut-galls; the
bath is brought to the boil in 1 hour and 3⁄4, and kept boiling for half an hour.
10. The yarn is rinsed, dried, boiled from 24 to 36 hours in a covered copper, with
an oily alkaline liquid; then rinsed twice, laid for two days in clear water, and dried.
11. Finally, the greatest brightness is obtained by boiling for three or four hours in
a soap bath, containing muriate of tin; after which the yarn is rinsed twice over, steeped
in water, and dried.
Process of Haussmann.—He treats cotton twice or 4 times in a solution of aluminated
potash, mixed with one thirty-eighth part of linseed oil. The solution is made by
adding caustic potash to alum. He dries and rinses each time, and dries after the last
operation. He then rinses and proceeds to the madder bath. For the rose colour, he
takes one pound of madder for one pound of cotton; for carmine red, he takes from 2
to 3 pounds; and for the deepest red, no less than 4 pounds. It is said that the colour
thus obtained surpasses Turkey red.
The French process, by Vitalis of Rouen.—First operation. Scouring with a soda
lye, of 1° Baumé, to which there is usually added the remainder of the white preparation
bath, which consists of oil and soda with water. It is then washed, wrung
out, and dried.
In the second operation, he states that from 25 to 30 pounds of sheep’s dung are
commonly used for 100 pounds of cotton yarn. The dung is first steeped for some days
in a lye of soda, of 8° to 10° B. This is afterwards diluted with about 500 pints of a
weaker ley, and at the same time bruised with the hand in a copper basin whose bottom
is pierced with small holes. The liquor is then poured into a vat containing 5 or 6
pounds of fat oil (Gallipoli), and the whole are well mixed. The cotton is washed in
this, and the hanks of yarn are then stretched on perches in the open air, and turned from
time to time, so as to make it dry equably. After receiving thus a certain degree of desiccation,
it is carried into the drying house, which is heated to 50° Reaumur (144°
Fahrenheit), where it loses the remainder of its moisture, which would have prevented
it from combining with the other mordants which it is afterwards to receive. What is
left of the bath is called avances, and is added to the following bath. Two, or even three
dung baths are given to the cotton, when it is wished to have very rich colours. When
the cotton has received the dung baths, care must be taken not to leave it lying in heaps
for any length of time, lest it should take fire; an accident which has occasionally
happened.
The white bath is prepared by pouring 6 pounds of fat oil, into 50 pints of soda water,
at 1° or sometimes less, according as, by a preliminary trial, the oil requires. This bath
ought to be repeated two, three, or even a greater number of times, as more or less body
is to be given to the colour.
To what remains of the white bath, and which is also styled avances, about 100 pints
of soda lye of two or three degrees are added. Through this the cotton is passed as
usual. Formerly it was the practice to give two, or three, or even four oils. Now,
two are found to be sufficient.
The cotton is steeped for five or six hours in a tepid solution of soda, of 1° at most;
it is set to drain, is then sprinkled with water, and at the end of an hour is washed,
hank by hank, to purge it entirely from the oil. What remains of the water of degraissage,
serves for the scouring or first operation.
For 100 pounds of cotton, from 20 to 25 pounds of galls in sorts must be taken, which
are bruised and boiled in about 100 pints of water, till they crumble easily between the
fingers. The galling may be done at two operations, dividing the above quantity of
galls between them, which is thought to give a richer and more uniform colour.
The aluming of 100 pounds of cotton requires from twenty-five to thirty pounds
of pure alum, that is, alum entirely free from ferruginous salts. The alum should
be dissolved without boiling, in about 100 pints of river or rain water. When the
alum is dissolved, there is to be poured in a solution of soda, made with the sixteenth
part of the weight of the alum. A second portion of the alkaline solution must not
be poured in till the effervescence caused by the first portion has entirely ceased,—and[790]
so in succession. The bath of saturated alum, being merely tepid, the cotton is
passed through it, as in the gall bath, so as to impregnate it well, and it is dried with
the precautions recommended above. The dyers who gall at two times, alum also
twice, for like reasons.
For 25 pounds of cotton, 25 pints of blood are prescribed, and 400 pints of water.
Whenever the bath begins to warm, 50 pounds of madder are diffused through the
bath; though sometimes the maddering is given at two operations, by dividing the
madder into two portions.
The brightening bath is prepared always for 100 pounds of cotton, with from four
to five pounds of rich oil, six pounds of Marseilles white soap, and 600 litres of soda
water of 2° B.
The rosing is given with solution of tin, mixed with soap water.
The Turkey-red dye of Messrs. Monteith and Co., of Glasgow, is celebrated all over
the world, and merits a brief description here.
The calico is taken as it comes from the loom without bleaching, for the natural colour
of the cotton wool harmonizes well with the dye about to be given; it is subjected
to a fermentative steep for 24 hours, like that preliminary to bleaching, after which
it is washed at the dash wheel. It is then boiled in a lye, containing about 1 pound of
soda crystals for 12 pounds of cloth. The oiling process now begins. A bath is made
with 10 gallons of Gallipoli oil, 15 gallon measures of sheep’s dung not indurated; 40
gallons of solution of soda crystals, of 1·06 specific gravity; 10 gallons of solution of
pearl-ash of spec. grav. 1·04; and 140 gallons of water; constituting a milk-white,
soapy solution of about spec. grav. 1·022. This liquor is put into a large cylindrical
vat, and constantly agitated by the rotation of wooden vanes, which are best constructed
on the plan of the mashing apparatus of a brewery, but far slighter. This saponaceous
compound is let off as wanted by a stopcock into the trough of a padding machine, in
order to imbue every fibre of the cloth in its passage. This impregnation is still more
fully ensured by laying the padded cloth aside in wooden troughs during 16 or 18 days.
The sheep’s dung has been of late years disused by many Turkey-red dyers both in
England and France, but it is found to be advantageous in producing the very superior
colour of the Glasgow establishment. It is supposed, also, to promote the subsequent
bleaching during the exposure on the green; which is the next process in
favourable weather, but in bad weather the goods are dried over a hot-flue.
The cloth is padded again with the saponaceous liquor; and again spread on the
grass, or dried hard in the stove. This alternation is repeated a third time, and occasionally,
even a fourth.
The cloth by this time is varnished as it were with oil, and must be cleansed in a
certain degree by being passed through a weak solution of pearl-ash, at the temperature
of about 122° F. It is then squeezed by the rollers and dried.
A second system of oiling now commences, with the following liquor:—10 gallons
of Gallipoli oil; 30 gallons of soda crystals lye, of sp. grav. 1·06; and 10 gallons of
caustic potash lye, of specific gravity 1·04, thoroughly diffused through 170 gallons of
water. With this saponaceous liquor the cloth is padded as before, and then passed between
squeezing-rollers, which return the superfluous liquor into the padding-trough.
The cloth may be now laid on the grass if convenient; but at any rate it must be
hard dried in the stove.
These saponifying, grassing, and drying processes, are repeated three times; whereby
the cloth becomes once more very oleaginous, and must be cleansed again by steeping
in a compound lye of soda crystals and pearl-ash of the spec. grav. 1·012, at the temperature
of 122°. The cloth is taken out, squeezed between rollers to save the liquor,
and washed. A considerable portion of the mingled alkalis disappear in this operation,
as if they entered into combination with the oil in the interior of the cotton filaments.
The cloth is now hard dried.
Galling is the next great step in the Turkey-red preparation; and for its success
all the oil should have been perfectly saponified.
From 18 to 20 pounds of Aleppo galls (for each 100 libs of cloth) are to be bruised
and boiled for 3 or 4 hours, in 25 gallons of water, till 5 gallons be evaporated; and
the decoction is to be then passed through a searce. Two pounds of sumach may be
substituted for every pound of galls. The goods must be well padded with this decoction,
kept at 90° F., passed through squeezing-rollers, and dried. They are then
passed through a solution of alum of the sp. gr. 1·04, to which a certain portion of
chalk is added to saturate the acid excess of that supersalt; and in this cretaceous mixture,
heated to 110°, the cloth is winced and steeped for 12 hours. It is then passed
between squeezing-rollers, and dried in the stove.
The maddering comes next.
From two to three pounds of madder, ground to powder in a proper mill, are taken
for every pound of cloth. The cloth, as usual in maddering, is entered into the cold[791]
bath, and winced by the automatic reel during one hour that the bath takes to boil, and
during an ebullition of two hours afterwards. One gallon of bullock’s blood is added
to the cold bath for every 25 pounds of cloth; being the quantity operated upon in one
bath. The utility of the blood in improving the colour has been ascribed to its colouring
particles; but it is more probably owing to its albuminous matter combining with the
margarates of soda and potash condensed in the fibres.
As madder contains a dingy brown colouring matter associated with the fine red, the
goods must be subjected to a clearing process to remove the former tinge, which is more
fugitive than the latter. Every hundred pounds of cloth are therefore boiled during 12
hours at least, with water containing 5 pounds of soda crystals, 8 pounds of soap, and
16 gallons of the residual pearl-ash and soda-lye of the last cleansing operation. By
this powerful means the dun matter is well nigh removed; but it is completely so by a
second boil, at a heat of 250° F., in a tight globular copper, along with 5 pounds of
soap, and 1 pound of muriate of tin crystals, dissolved in a sufficient body of water for
100 pounds of cloth. The muriate of tin serves to raise the madder red to a scarlet hue.
A margarate of tin is probably fixed upon the cloth in this operation.
When the weather permits, the goods should be now laid out for a few days on the
grass. Some manufacturers give them a final brightening with a weak bath of a chloride
of lime; but it is apt to impoverish the colour.
According to the latest improvements of the French dyers, each of the four processes
of oiling, mordanting, dyeing, and brightening differs, in some respects, from the
above.
1. Their first step is boiling the cloth for four hours, in water containing one pound
of soap for every four pieces. Their saponaceous bath of a creamy aspect is used at a temperature
of 75° F.; and it is applied by the padding machine 6 times, with the grassing
and drying alternations. In winter, when the goods cannot be exposed on the grass, no
less than 12 alternations of the saponaceous or white bath are employed, and 8 in spring.
They consider the action of the sun-beam to aid greatly in brightening this dye; but
at Midsummer, if it be continued more than 4 hours, the scarlet colour produced begins
to be impaired.
They conceive that the oiling operation impregnates the fibres with super-margarate
of potash or soda, insoluble salts which attract and condense the alumina, and the red
colouring particles of the madder, so firmly that they can resist the clearing boil.
2. Their second step, the mordanting, consists first in padding the pieces through a
decoction of galls mixed with a solution of an equal weight of alum; and after drying
in the hot-flue, &c., again padding them in a solution of an acetate of alumina, made by
decomposing a solution of 16 libs. of alum with 16 libs of acetate of lead, for 6 pieces
of cloth, each 32 aunes long.
3. The maddering is given at two successive operations; with 4 pounds of Avignon
madder per piece at each time.
4. The brightening is performed by a 12 hours’ boil in water with soda crystals, soap,
and salt of tin; and the rosing by a 10 hours’ boil with soap and salt of tin. Occasionally,
the goods are passed through a weak solution of chloride of potash. When the
red has too much of a crimson cast, the pieces are exposed for two days on the grass,
which gives them a bright scarlet tint.
Process of M. Werdet to dye broad cloth and wool by madder:—
“Preparation for 24 pounds of scoured wool:
“Take 41⁄4 pounds of cream of tartar, 41⁄4 pounds of pure alum; boil the wool gently
for 2 hours, transfer it into a cool place, and wash it next day in clear water.
“Dyeing.—12 pounds of Avignon madder, infused half an hour at 30° R. (100° F.)
Put into the bath 1 pound of muriate of tin, let the colour rose for three quarters of an
hour at the same heat, and drain or squeeze the madder through canvas. The whole of
the red dye will remain upon the filter, but the water which has passed through will be
as deep a yellow as a weld bath. The boiler with the dye must now be filled up with
clear river water, and heated to 100° F. Two ounces of the solution of the tartar and
alum must be poured into it, and the wool must be turned over in it for an hour and a
half, while the heat is gradually raised to the boiling point. The wool is then removed
and washed. It must be rosed the following day.
“Rosing.—Dissolve in hot water 1 pound of white Marseilles soap; let the bath cool,
and pass the wool through it till it has acquired the desired shade; 15 or 20 minutes
are sufficient. On coming out of this bath it should be washed.
“Solution of deuto-muriate of tin:—
“2 ounces of pure muriatic acid; 4 drachms of pure nitric acid; 1 ounce of distilled
water. Dissolve in it, by small portions at a time, 2 drachms of grain tin, in a large
bottle of white glass, shutting it after putting in the tin. This solution may be preserved
for years, without losing its virtue.”
I have inserted this process, as recently recommended by the French minister of commerce,[792]
and published by M. Pouillet in vol. i. of his Portefeuille Industriel, to show
what official importance is sometimes given by our neighbours to the most frivolous things.
| Madders imported for home consumption. |
Gross amount of Duty paid in |
| 1836. |
1837. |
1836. |
1837. |
| Cwts. 106,172 |
cwts. 79,228 |
£10,810 |
£8,081 |
MADREPORES, are calcareous incrustations produced by polypi contained in cells
of greater or less depth, placed at the surface of calcareous ramifications, which are fixed
at their base, and perforated with a great many pores. The mode of the increase, reproduction
and death of these animals is still unknown to naturalists. Living madrepores
are now-a-days to be observed only in the South American, the Indian, and the
Red seas; but although their polypi are not found in our climate at present, there
can be no doubt of their having existed in these northern latitudes in former times,
since fossil madrepores occur in both the older and newer secondary strata of Europe.
MAGISTERY, is an old chemical term to designate white pulverulent substances,
spontaneously precipitated in making certain metallic solutions; as magistery of
bismuth.
MAGISTRAL, in the language of the Spanish smelters of Mexico and South
America, is the roasted and pulverized copper pyrites, which is added to the ground
ores of silver in their patio, or amalgamation magma, for the purpose of decomposing
the horn silver present. See Silver, for an account of this curious process of
reduction.
MAGMA, is the generic name of any crude mixture of mineral or organic matters,
in a thin pasty state.
MAGNANIER, is the name given in the southern departments of France to the
proprietor of a nursery in which silk-worms are reared upon the great scale, or to the
manager of the establishment. The word is derived from magnans, which signifies silkworms
in the language of the country people. See Silk.
MAGNESIA (Eng. and Fr.; Bittererde, Talkerde, Germ.), is one of the primitive
earths, first proved by Sir H. Davy to be the oxide of a metal, which he called magnesium.
It is a fine, light, white powder, without taste or smell, which requires 5150
parts of cold water, and no less than 36,000 parts of boiling water, for its solution. Its
specific gravity is 2·3. It is fusible only by the heat of the hydroxygen blowpipe. A
natural hydrate is said to exist which contains 30 per cent. of water. Magnesia changes
the purple infusion of red cabbage to a bright green. It attracts carbonic acid from the
air, but much more slowly than quicklime. It consists of 61·21 parts of metallic
basis, and 38·79 of oxygen; and has, therefore, 20 for its prime equivalent upon the
hydrogen scale. Its only employment in the arts is for the purification of fine oil, in
the preparation of varnish.
Magnesia may be obtained by precipitation with potash or soda, from its sulphate,
commonly called Epsom salt; but it is usually procured by calcining the artificial or
natural carbonate. The former is, properly speaking, a subcarbonate, consisting of
44·69 magnesia, 35·86 carbonic acid, and 19·45 water. It is prepared by adding to
the solution of the sulphate, or the muriate (the bittern of sea-salt evaporation works),
a solution of carbonate of soda, or of carbonate of ammonia distilled from bones
in iron cylinders. The sulphate of magnesia is generally made by acting upon magnesian
limestone with somewhat dilute sulphuric acid. The sulphate of lime precipitates,
while the sulphate of magnesia remains in solution, and may be made to
crystallize in quadrangular prisms, by suitable evaporation and slow cooling. Where
muriatic acid may be had in profusion for the trouble of collecting it, as in the soda
works in which sea salt is decomposed by sulphuric acid, the magnesian limestone
should be first acted upon with as much of the former acid as will dissolve out the lime,
and then, the residuum being treated with the latter acid, will afford a sulphate at the
cheapest possible rate; from which magnesia and all its other preparations may be readily
made. Or, if the equivalent quantity of calcined magnesian limestone be boiled for
some time in bittern, the lime of the former will displace the magnesia from the muriatic
acid of the latter. This is the most economical process for manufacturing magnesia.
The subcarbonate, or magnesia alba of the apothecary, has been proposed by Mr. E.
Davy to be added by the baker to damaged flour, to counteract its acescency.
MAGNESIAN LIMESTONE (Dolomie, Fr.; Bittertalk, Talkspath, Germ.), is
a mineral which crystallizes in the rhombohedral system. Spec. grav. 2·86; scratches
calc-spar; does not fall spontaneously into powder, when calcined, as common limestone
does. It consists of 1 prime equivalent of carbonate of lime = 50, associated with 1 of
carbonate of magnesia = 42.
Massive magnesian limestone, is yellowish-brown, cream-yellow, and yellowish-gray;
brittle. It dissolves slowly and with feeble effervescence in dilute muriatic acid; whence[793]
it is called Calcaire lent dolomie by the French mineralogists. Specific gravity 2·6
to 2·7.
Near Sunderland, it is found in flexible slabs. The principal range of hills composing
this geological formation in England, extends from Sunderland on the northeast
coast to Nottingham, and its beds are described as being about 300 feet thick on
the east of the coal field in Derbyshire, which is near its southern extremity. On the
western side of the Cumberland mountains magnesian limestone overlies the coal
measures near Whitehaven. The stratification of this rock is very distinct, the individual
courses of stone not exceeding in general the thickness of a common brick.
The lime resulting from the calcination of magnesian limestone appears to have an
injurious action on vegetation, unless applied in quantities considerably less than
common lime, when it is found to fertilize the soil. After two years, its hurtful
influence on the ground seems to become exhausted, even when used in undue quantity.
Great quantities of it are annually brought from Sunderland to Scotland by the Fifeshire
farmers, and employed beneficially by them, as a manure, in preference to other
kinds of lime. It has been unfairly denounced by Mr. Tennent and Sir H. Davy, as a
sterilizer.
This rock is used in many places for building; indeed our most splendid monument
of Gothic architecture, York Minster, is constructed of magnesian limestone.
MAGNESIA, NATIVE (Brucite; Guhr magnésien, Fr.; Wassertalk, Germ.), is a
white, lamellar, pearly-looking mineral, soft to the touch. Spec. grav. 2·336; tender;
scratched by calc-spar; affording water by calcination; leaving a white substance
which browns turmeric paper; and, by calcination with nitrate of cobalt, becoming of a
lilac hue. It consists of 69·75 magnesia, and 30·25 water. It occurs in veins in the
serpentine at Hoboken, in New Jersey, as also at Swinaness, in the island of Unst,
Shetland.
MAGNESITE, Giobertite; native carbonate of magnesia, occurs in white, hard,
stony masses, in the presidency of Madras, and in a few other localities. It dissolves
very slowly in muriatic acid, and gives out carbonic acid in the proportion of 22 parts
by weight to 42 of the mineral, according to my experiments, and is therefore an atomic
carbonate. It forms an excellent and beautiful mortar cement for terraces; a purpose
to which it has been beneficially applied in India by Dr. Macleod.
MAGNET, NATIVE, is a mineral consisting of the protoxide and peroxide of
iron combined in equivalent proportions. See Iron.
MAHALEB. The fruit of this shrub affords a violet dye, as well as a fermented
liquor like Kirschwasser. It is a species of cherry cultivated in our gardens.
MALACHITE, or mountain green, is native carbonate of copper of a beautiful green
colour, with variegated radiations and zones; spec. grav. 3·5; it scratches calc-spar, but
not fluor; by calcination it affords water and turns black. Its solution in the acids,
deposits copper upon a plate of iron plunged into it. It consists of carbonic acid 18·5;
deutoxide of copper 72·2; water 9·3.
MALATES, are saline compounds of the bases, with
MALIC ACID. (Acide malique, Fr.; Aepfelsäure, Germ.) This acid exists in the
juices of many fruits and plants, alone, or associated with the citric, tartaric, and oxalic
acids; and occasionally combined with potash or lime. Unripe apples, sloes, barberries,
the berries of the mountain ash, elder berries, currants, gooseberries, strawberries, raspberries,
bilberries, brambleberries, whortleberries, cherries, ananas, afford malic acid;
the house-leek and purslane contain the malate of lime.
The acid may be obtained most conveniently from the juice of the berries of the
mountain ash, or barberries. This must be clarified, by mixing with white of egg, and
heating the mixture to ebullition; then filtering, digesting the clear liquor with carbonate
of lead, till it becomes neutral; and evaporating the saline solution, till crystals,
of malate of lead be obtained. These are to be washed with cold water, and purified by
re-crystallization. On dissolving the white salt in water, and passing a stream of sulphuretted
hydrogen through the solution, the lead will be all separated in the form of a
sulphuret, and the liquor, after filtration and evaporation, will yield yellow granular
crystals, or cauliflower concretions, of malic acid, which may be blanched by re-dissolution
and digestion with bone-black, and re-crystallization.
Malic acid has no smell, but a very sour taste, deliquesces by absorption of moisture
from the air, is soluble in alcohol, fuses at 150° Fahr., is decomposed at a heat of 348°,
and affords by distillation a peculiar acid, the pyromalic. It consists in 100 parts, of
41·47 carbon; 3·51 hydrogen; and 55·02 oxygen; having nearly the same composition
as citric acid. A crude malic acid might be economically extracted from the fruit of
the mountain ash, applicable to many purposes; but it has not hitherto been manufactured
upon the great scale.
MALLEABILITY, is the property belonging to certain metals, of being extended
under the hammer. A table of malleability is given in the article Ductility.
[794]
MALT; (Eng. and Fr.; Malz, Germ.) is barley-corn, which has been subjected to
an artificial process of germination. See Beer.
Table of the Quantity of Malt consumed by the undermentioned Brewers of
London and Vicinity, from October 10th, 1836, to October 10th, 1837.
| Brewers. |
Qrs. |
| Barclay and Co. |
100005 |
| Hanbury and Co. |
82798 |
| Whitbread and Co. |
47012 |
| Reid and Co. |
43945 |
| Combe and Co. |
40366 |
| Hoare and Co. |
32347 |
| Calvert and Co. |
32335 |
| Meux and Co. |
30575 |
| Elliot and Co. |
24154 |
| Taylor and Co. |
23556 |
| Charrington and Co. |
18842 |
| Thorne and Son |
16404 |
| Gardner |
15256 |
| Ramsbottom and Co. |
15227 |
| J. & C. Goding (11 months) |
14023 |
| Bricheno |
9863 |
| Courage and Co. |
9284 |
| Wood and Co. |
7834 |
| Goding, Thos. |
7095 |
| Hazard |
6674 |
| Mann, Jas. |
6588 |
| Harris, Thos. |
6042 |
| More |
6025 |
| M’Leod, B. |
4960 |
| Farren and Till |
4783 |
| Manners and Co. |
4552 |
| Hale, George. |
4547 |
| Halford and Topham |
3786 |
| Stains and Fox |
5783 |
| Lamont and Co. |
3600 |
| Laxton |
3583 |
| Richmond |
3174 |
| Maynard |
3133 |
| M’Leod and Thompson |
2834 |
| Tubb |
2826 |
| Johnson and Wyatt |
2809 |
| Duggan and Co. |
2665 |
| Hodgson |
2400 |
| Sherborn and Co. |
2347 |
| Griffith |
2221 |
| Cox, John |
2151 |
| Masterman |
1914 |
| Hill and Rice |
1853 |
| Gray and Dacre |
1760 |
| Plimmer |
1747 |
| Hayward |
1737 |
| Verey, W. and C. |
1573 |
| Williamson and Co. |
1566 |
| Honeyball |
1512 |
| Satchell and Son |
1441 |
| Clarke, C. |
1330 |
| Colyer |
1299 |
| Filmer and Wall |
1298 |
| Nicholls and Co. |
1240 |
| Hagan |
1143 |
| Hume |
1126 |
| Buckley and Co. |
1025 |
| Verey, J. |
1017 |
| Collins, J. |
966 |
| Jones |
956 |
| Ufford and Oldershaw |
953 |
| Blogg, B. |
943 |
| Ing |
900 |
| Keep |
886 |
| Soulby |
861 |
| Clarke, R. |
834 |
| Jenner |
833 |
| Manvell |
824 |
| M’Leods |
820 |
| Braithwaite |
799 |
| Addison |
768 |
| Turner |
766 |
| Holt |
756 |
| Church |
742 |
| Clarke, S. |
741 |
| Mann, Joel |
733 |
| Turner |
712 |
| Mantell |
693 |
| Lock |
651 |
| Hood |
649 |
| Pink, A. |
636 |
| Collins |
598 |
| Wright |
588 |
| West |
565 |
| Abbott |
560 |
| Hett (6 months) |
552 |
| Wells |
520 |
| Higgs |
475 |
| Harris, Robt. |
470 |
| Woodward |
462 |
| Wicks |
441 |
| Bell |
440 |
| Thompson |
406 |
| Mattam |
400 |
| M’Intosh |
397 |
| Thurlby |
392 |
| Griffiths |
391 |
| Kay |
360 |
| Tidman |
332 |
| Lindsay |
326 |
| Cooper |
315 |
| West |
306 |
| Carpenter |
299 |
| Green |
292 |
| Chapman |
286 |
| Brace |
266 |
| Clark |
248 |
| Allen |
245 |
| Powditch |
238 |
| Garnett |
232 |
| Hill |
222 |
| Olley |
214 |
| Ward |
206 |
| Bye |
201 |
| Newton |
175 |
| Chadwick |
169 |
| Prosser |
166 |
| Smith |
164 |
| Edwards[795] |
156 |
| Pugh |
155 |
| Hainstock |
155 |
| Lloyd |
154 |
| Reynolds |
151 |
| Latham |
142 |
| Meaton |
140 |
| Brewer |
135 |
| Stirling |
133 |
| Ambler |
130 |
| Potter |
122 |
| Champion |
121 |
| Miller |
115 |
| Edwards |
108 |
| Easton |
105 |
| Griffiths |
105 |
| Hopkins |
91 |
| Hudson |
90 |
| Thorpe |
89 |
| Burt |
88 |
| Bowden |
88 |
| Batt |
84 |
| Phillips |
83 |
| Jewit |
82 |
| Tyler |
76 |
| Whittaker |
75 |
| Begbie |
75 |
| Carter |
75 |
| Priddle |
74 |
| Coomber |
73 |
| Stallwood |
71 |
| Jones |
71 |
| Rose |
67 |
| Norris |
67 |
| Remnant |
62 |
| Kearney |
62 |
| Smith |
62 |
| Woodroffe |
60 |
| Knight |
60 |
| Graves |
54 |
| Sheppard |
52 |
| Field |
51 |
| Bradfield |
51 |
| Webb |
50 |
| Chapman |
48 |
| Price |
45 |
| Godfrey |
45 |
| Hobbs |
32 |
| Denman |
31 |
| |
Qrs. |
| Quantity used |
1836, |
754,313 |
| Quantity used |
1837, |
714,488 |
| Decrease |
1837, |
39,825 |
| John Slater, Cask Inspector. |
| Hop-Duty, 1837. (Old) £178,578. 3s. 01⁄2d. |
Table of the Quantity of Malt from Barley, which paid Duty in
| Years. |
England. |
Scotland. |
Ireland. |
| |
Bushels. |
Bushels. |
Bushels. |
| 1834. |
34,949,646 |
3,580,758 |
1,776,883 |
| 1835. |
36,078,855 |
3,604,816 |
1,825,300 |
| 1836. |
37,196,998 |
4,168,854 |
1,872,104 |
| Amount of Duties paid: |
| |
£ |
£ |
£ |
| 1834. |
4,449,745 |
462,514 |
229,514 |
| 1835. |
4,660,185 |
465,622 |
235,767 |
| 1836. |
4,804,612 |
538,477 |
241,813 |
MALT KILN; (Darre, Germ.) The improved malt kiln of Pistorius is represented
fig. 653. in a top view; fig. 654. in a longitudinal view and section; and
fig. 655., in transverse section. a a, are two quadrangular smoke flues, constructed
of fire-tiles, or fire-stones, and covered with iron plates, over which a pent-house roof is
laid; the whole bound by the cross pieces b (figs. 654, 655.) These flues are built above
a grating c c, which commences at c′; in front of c′ there is a bridge of bricks. Instead
of such a brick flue covered with plates, iron pipes may be used, covered with semi-cylindrical
tiles, to prevent the malt that may happen to fall from being burned. d d,
are the breast walls of the kiln, 3 feet high, furnished with two apertures shut with iron
doors, through which the malt that drops down may be removed from time to time.
e is a beam of wood lying on the breast wall, against which the hurdles are laid down[796]
slantingly towards the back wall of the kiln; f f, are two vertical flues left in the
substance of the walls, through which the hot air, discharged by open pipes laid in a
subjacent furnace, rises into the space between the pent-house roof and the iron plates,
and is thence allowed to issue through apertures in the sides. g is the discharge flue
in the back wall of the kiln for the air now saturated with moisture; h is the smoke-pipe,
from which the smoke passes into the anterior flue a, provided with a slide-plate,
for modifying the draught; the smoke thence flows off through a flue fitted also with a
damper-plate into the chimney i. k is the smoke-pipe of a subsidiary fire, in case no
smoke should pass through h. The iron pipes are 11 inches in diameter, the air-flue f,
5 inches, and the smoke-pipe h, 10 inches square; the brick flues 10 inches wide, and
the usual height of bricks.
MALTHA; Bitume Glutineux, or mineral pitch. It is a soft glutinous substance,
with the smell of pitch. It dissolves in alcohol, but leaves a bituminous residuum; as
also in naphtha, and oil of turpentine. It seems to be inspissated petroleum.
MANGANESE, (Eng. and Fr.; Mangan, Braunsteinmetal, Germ.) is a grayish-white
metal, of a fine-grained fracture, very hard, very brittle, with considerable lustre,
of spec. grav. 8·013, and requiring for fusion the extreme heat of 160° Wedgewood.
It should be kept in closely stoppered bottles, under naphtha, like potassium, because
with contact of air it speedily gets oxidized, and falls into powder. It decomposes
water slowly at common temperatures, and rapidly at a red heat. Pure oxide of manganese
can be reduced to the metallic state only in small quantities, by mixing it with
lamp black and oil into a dough, and exposing the mixture to the intense heat of a
smith’s forge, in a luted crucible; which must be shaken occasionally to favour the
agglomeration of the particles into a button. Thus procured, it contains, however,
a little carbon.
Manganese is susceptible of five degrees of oxigenation:—
1. The protoxide may be obtained from a solution of the sulphate by precipitation
with carbonate of potash, and expelling the carbonic acid from the washed and dried
carbonate, by calcination in a close vessel filled with hydrogen gas, taking care that no
air have access during the cooling. It is a pale green powder, which slowly attracts
oxygen from the air, and becomes brown; on which account it should be kept in glass
tubes, containing hydrogen, and hermetically sealed. It consists of 77·57 metal and
22·43 oxygen. It forms with 24 per cent. of water a white hydrate; and with acids,
saline compounds; which are white, pink, or amethyst coloured. They have a bitter,
acerb taste, and afford with hydrogenated sulphuret of ammonia, a flesh-red precipitate,
but with caustic alkalis, one which soon turns brown-red, and eventually black.
2. The deutoxide of manganese exists native in the mineral called Braunite; but it
may be procured either by calcining, at a red heat, the proto-nitrate, or by spontaneous
oxidizement of the protoxide in the air. It is black; when finely pulverized, dark
brown, and is convertible, on being heated in acids, into protoxide, with disengagement
of oxygen gas. It consists of 69·75 metal, and 30·25 oxygen. It forms with 10 per
cent. of water, a liver-brown hydrate, which occurs native under the name of Manganite.
It dissolves readily in tartaric and citric acids, but in few others. This oxide constitutes
a bronze ground in calico-printing.
3. Peroxide of manganese; Braunstein, occurs abundantly in nature. It gives out
oxygen freely when heated, and becomes an oxidulated deutoxide. It consists of 63·36
metal, and 36·64 oxygen.
[797]
4. Manganesic acid, forms green-coloured salts, but has not hitherto been insulated
from the bases. It consists of 53·55 metal, and 46·45 oxygen.
5. Hypermanganesic acid, consists of 49·70 metal, and 50·30 oxygen.
Ores of manganese.—There are two principal ores of this metal which occur in
great masses; the peroxide and the hydrated oxide; the first of which is frequently
found in primitive formations.
1. Metalloide oxide of manganese; pyrolusite, or gray manganese ore; has a metallic
lustre, a steel gray colour, and affords a black powder. Spec. grav. 4·85. Scratches
calc-spar. It effervesces briskly with borax at the blow-pipe, in consequence of the disengagement
of oxygen gas. This is the most common ore of manganese, and a very
valuable one, being the substance mostly employed in the manufacture of chloride of
lime and of flint-glass. It is the peroxide. Great quantities are found near Tavistock,
in Devonshire, and Launceston, in Cornwall.
2. Braunite, is a dark brown substance, of a glassy metallic lustre, affording a brown
powder. Spec. grav. 4·8. It scratches felspar; but is scratched by quartz. Infusible
at the blow-pipe, and effervesces but slightly when fused with glass of borax. It is the
deutoxide. It gives out at a red heat only 3 per cent. of oxygen.
3. Manganite, or hydroxide of manganese; is brownish-black or iron-black, powder
brown, with somewhat of a metallic lustre. Spec. grav. 4·3. Scratches fluor spar;
affords water by calcination in a glass tube; infusible at the blow-pipe; and effervesces
slightly when fused with glass of borax. It consists of about 90 of deutoxide, and 10
of water.
4. Haussmanite, black braunstein; is brownish-black, affords a reddish-brown powder.
Spec. grav. 4·7; scratches fluor spar; infusible at the blow-pipe; does not
effervesce when fused with borax. It is a deutoxide. This is a rare mineral, and of no
value to the arts.
5. Barytic oxide of manganese; fibrous wad. It is a combination of deutoxide and
peroxide, with some baryta.
6. Manganese blende, or sulphuret of manganese; has a metallic aspect; is black, or
dark steel gray; spec. grav. 3·95; has no cleavage; cannot be cut; infusible, but
affords after being roasted distinct evidence of manganese, by giving a violet tinge to
soda at the blow-pipe. Soluble in nitric acid; solution yields a white precipitate with
the ferro-cyanide of potassium. It consists of sulphur 53·65; manganese 66·35.
7. Carbonate of manganese; dialogite. Spec. grav. 3·4; affords a green frit by fusion
with carbonate of soda; is soluble with some effervescence in nitric acid; solution when
freed from iron by succinate of ammonia, gives a white precipitate, with ferrocyanide of
potassium. It consists of 28 carbonic acid, 56 protoxide of manganese, 5·4 of lime,
4·5 protoxide of iron, and 0·8 magnesia.
8. Hydrosilicate of manganese; is a black metallic looking substance, which yields a
yellowish-brown powder, and water by calcination; is acted upon by muriatic acid, but
affords no chlorine. It consists of silica 25; protoxide of manganese 60; water 13.
9. Ferriferous phosphate of manganese, is brown or black. Spec. grav. 3·6; scratches
fluor; affords by calcination a very little of an acid water which corrodes glass; very
fusible at the blow-pipe into a black metalloid magnetic bead; is acted upon by nitric
acid: solution lets fall a blue precipitate with ferrocyanide of potassium; which tested
by soda is shown to be manganese. It consists of phosphoric acid 32·78; protoxide of
iron 31·90; protoxide of manganese 32·60; phosphate of lime 3·2. Another phosphate
called hureaulite, contains 38 of phosphoric acid; 11·10 of protoxide of iron;
32·85 of protoxide of manganese, and 18 of water.
Black wad, is the old English name of the hydrated peroxide of manganese. It
occurs in various imitative shapes, in froth-like coatings upon other minerals, as also
massive. Some varieties possess imperfect metallic lustre. The external colour is
brown of various shades, and similar in the streak, only shining. It is opaque, very
sectile, soils and writes. Its specific gravity is about 3·7. Mixed with linseed oil into
a dough, black wad forms a mass that spontaneously inflames. A variety from the
Hartz, analyzed by Klaproth, afforded peroxide of manganese 68; oxide of iron 6·5;
water 17·5; carbon 1; barytes and silica 9. The localities of black wad are particularly
Cornwall and Devonshire, the Hartz, and Piedmont. I have analyzed many
varieties of the black wad sold to the manufacturers of bleaching salt, and flint glass,
and have found few of them so rich in peroxide of manganese as the above. Very
generally they contained no less than 25 per cent. of oxide of iron, 8 or 9 of silica, about
7 of water, and the remainder amounting to only 60 per cent. of the peroxide.
M. Gay Lussac has proposed to determine the commercial value of manganese ore,
by the quantity of chlorine which it affords when treated with liquid muriatic acid. He
places the manganese powder in a small retort or matras, pours over it the acid, and the
chlorine being disengaged with the aid of a gentle heat, is transmitted into a vessel containing
milk of lime or potash water. This liquor is thereafter poured into a dilute[798]
solution of sulphate of indigo; and the quantity of chlorine is inferred from the quantity
of the blue solution which is decoloured. I pass the chlorine into test solution of indigo.
The manufacturer of flint glass uses a small proportion of the black manganese ore, to
correct the green tinge which his glass is apt to derive from the iron present in the sand
he employs. To him it is of great consequence to get a native manganese containing
as little iron oxide as possible; since in fact the colour or limpidity of his product will
depend altogether upon that circumstance.
Sulphate of manganese has been of late years introduced into calico printing, to give
a chocolate or bronze impression. It is easily formed by heating the black oxide, mixed
with a little ground coal, with sulphuric acid. See Calico Printing.
The peroxide of manganese is used also in the formation of glass pastes, and in
making the black enamel of pottery. See Oxalic Acid.
MANGLE. (Calandre, Fr.; Mangel, Germ.) This is a well known machine for
smoothing table cloths, table napkins, as well as linen and cotton furniture. As
usually made, it consists of an oblong rectangular wooden chest, filled with stones,
which load it to the degree of pressure that it should exercise upon the two cylinders
on which it rests, and which, by rolling backwards and forwards over the linen spread
upon a polished table underneath, render it smooth and level. The moving wheel,
being furnished with teeth upon both surfaces of its periphery, and having a notch cut
out at one part, allows a pinion, uniformly driven in one direction, to act alternately
upon its outside and inside, so as to cause the reciprocating motion of the chest. This
elegant and much admired English invention, called the mangle-wheel, has been introduced
with great advantage into the machinery of the textile manufactures.
Mr. Warcup, of Dartford, obtained a patent several years ago for a mangle, in which
the linen, being rolled round a cylinder revolving in stationary bearings, is pressed
downwards by heavy weights hung upon its axes, against a curved bed, made to slide to
and fro, or traverse from right to left, and left to right, alternately.
Mr. Hubie, of York, patented in June, 1832, another form of mangle, consisting of
three rollers, placed one above another in a vertical frame, the axle of the upper roller
being pressed downwards by a powerful spring. The articles intended to be smoothed
are introduced into the machine by passing them under the middle roller, which is made
to revolve by means of a fly wheel; the pinion upon whose axis works in a large
toothed wheel fixed to the shaft of the same roller. The linen, &c. is lapped as
usual in protecting cloths. This machine is merely a small Calender.
MANIOC, is the Indian name of the nutritious matter of the shrub jatropha manihot,
from which cassava and tapioca are made in the West Indies.
MANNA, is the concrete saccharine juice of the Fraxinus ornus, a tree much cultivated
in Sicily and Calabria. It is now little used, and that only in medicine.
MARBLE. This title embraces such of the primitive, transition, and purer
compact limestones of secondary formation, as may be quarried in solid blocks without
fissures, and are susceptible of a fine polished surface. The finer the white, or more
beautifully variegated the colours of the stone, the more valuable, ceteris paribus, is the
marble. Its general characters are the following:—
Marble effervesces with acids; affords quicklime by calcination; has a conchoidal
scaly fracture; is translucent only on the very edges; is easily scratched by the knife;
has a spec. grav. of 2·7; admits of being sawn into slabs; and receives a brilliant polish.
These qualities occur united in only three principal varieties of limestone; in the saccharoid
limestone, so called from its fine granular texture resembling that of loaf sugar,
and which constitutes modern statuary marble, like that of Carrara; 2. in the foliated
limestone, consisting of a multitude of small facets formed of little plates applied to one
another in every possible direction, constituting the antique statuary marble, like that of
Paros; 3. in many of the transition and carboniferous, or encrinitic limestones, subordinate
to the coal formation.
The saccharoid and lamellar, or statuary marbles, belong entirely to primitive and
transition districts. The greater part of the close-grained coloured marbles belong also
to the same geological localities; and become so rare in the secondary limestone formations,
that immense tracts of these occur without a single bed sufficiently entire
and compact to constitute a workable marble. The limestone lying between the
calcareo-siliceous sands and gritstone of the under oolite, and which is called Forest
marble in England, being susceptible of a tolerable polish, and variegated with imbedded
shells, has sometimes been worked into ornamental slabs in Oxfordshire, where it
occurs in the neighbourhood of Whichwood forest; but this case can hardly be considered
as an exception to the general rule. To constitute a profitable marble-quarry,
there must be a large extent of homogeneous limestone, and a facility of transporting
the blocks after they are dug. On examining these natural advantages of the beds of
Carrara marble, we may readily understand how the statuary marbles discovered in the
Pyrenees, Savoy, Corsica, &c. have never been able to come into competition with it in[799]
the market. In fact, the two sides of the valley of Carrara may be regarded as mountains
of statuary marble of the finest quality.
Gypseous alabaster may be readily distinguished from marbles, because it does not
effervesce with acids, and is soft enough to be scratched by the nail; stalagmitic alabaster
is somewhat harder than marble, translucent, and variegated with regular stripes or
undulations.
Some granular marbles are flexible in thin slabs, or, at least, become so by being
dried at the fire; which shews, as Dolomieu suspected, that this property arises from a
diminution of the attractive force among the particles, by the loss of the moisture.
The various tints of ornamental marbles generally proceed from oxides of iron; but
the blue and green tints are sometimes caused by minute particles of hornblende, as in
the slate-blue variety called Turchino, and in some green marbles of Germany. The
black marbles are coloured by charcoal, mixed occasionally with sulphur and bitumen;
when they constitute stinkstone.
Brard divides marbles, according to their localities, into classes, each of which contains
eight subdivisions:—
1. Uni-coloured marbles; including only the white and the black.
2. Variegated marbles; those with irregular spots or veins.
3. Madreporic marbles, presenting animal remains in the shape of white or gray
spots, with regularly disposed dots and stars in the centre.
4. Shell marbles; with only a few shells interspersed in the calcareous base.
5. Lumachella marbles, entirely composed of shells.
6. Cipolin marbles, containing veins of greenish talc.
7. Breccia marbles, formed of a number of angular fragments of different marbles,
united by a common cement.
8. Puddingstone marbles; a conglomerate of rounded pieces.
Antique marbles.—The most remarkable of these are the following:—Parian marble,
called lychnites by the ancients, because its quarries were worked by lamps; it has a yellowish-white
colour; and a texture composed of fine shining scales, lying in all directions.
The celebrated Arundelian tables at Oxford consist of Parian marble, as well as the
Medicean Venus. Pentelic marble, from Mount Penteles, near Athens, resembles the
Parian, but is somewhat denser and finer grained, with occasional greenish zones, produced
by greenish talc, whence it is called by the Italians Cipolino statuario. The
Parthenon, Propyleum, the Hippodrome, and other principal monuments of Athens,
were of Pentelic marble; of which fine specimens may be seen among the Elgin collection,
in the British Museum. Marmo Greco, or Greek white marble, is of a very
lively snow white colour, rather harder than the preceding, and susceptible of a very fine
polish. It was obtained from several islands of the Archipelago, as Scio, Samos,
Lesbos, &c. Translucent white marble, Marmo statuario of the Italians, is very much
like the Parian, only not so opaque. Columns and altars of this marble exist in Venice,
and several towns of Lombardy; but the quarries are quite unknown. Flexible white
marble, of which five or six tables are preserved in the house of Prince Borghese, at
Rome. The White marble of Luni, on the coast of Tuscany, was preferred by the
Greek sculptors to both the Parian and Pentelic. White marble of Carrara, between
Specia and Lucca, is of a fine white colour, but often traversed by gray veins, so that
it is difficult to procure moderately large pieces free from them. It is not so apt to
turn yellow as the Parian marble. This quarry was worked by the ancients, having
been opened in the time of Julius Cæsar. Many antique statues remain of this marble.
Its two principal quarries at the present day are those of Pianello and Polvazzo. In
the centre of its blocks very limpid rock-crystals are sometimes found, which are called
Carrara diamonds. As the finest qualities are becoming excessively rare, it has risen
in price to about 3 guineas the cubic foot. The White marble of Mount Hymettus, in
Greece, was not of a very pure white, but inclined a little to gray. The statue of
Meleager, in the French Museum, is of this marble.
Black antique marble, the Nero antico of the Italians. This is more intensely black
than any of our modern marbles; it is extremely scarce, occurring only in sculptured
pieces. The red antique marble, Egyptum of the ancients, and Rosso antico of the Italians,
is a beautiful marble of a deep blood-red colour, interspersed with white veins and with
very minute white dots, as if strewed over with grains of sand. There is in the Grimani
palace at Venice, a colossal statue of Marcus Agrippa in rosso antico, which was
formerly preserved in the Pantheon at Rome. Green antique marble, verde antico, is a
kind of breccia, whose paste is a mixture of talc and limestone, while the dark green
fragments consist of serpentine. Very beautiful specimens of it are preserved at Parma.
The best quality has a grass-green paste, with black spots of noble serpentine, but is
never mingled with red spots. Red spotted green antique marble, has a dark green ground
marked with small red and black spots, with fragments of entrochi changed into white
marble. It is known only in small tablets. Leek marble; a rare variety of that colour,[800]
of which there is a table in the Mint at Paris. Marmo verde pagliocco is of a yellowish
green colour, and is found only in the ruins of ancient Rome. Cervelas marble of a
deep red, with numerous gray and white veins, is said to be found in Africa, and highly
esteemed in commerce. Yellow antique marble, giallo antico of the Italians; colour of
the yolk of an egg, either uniform or marked with black or deep yellow rings. It is
rare, but may be replaced by Sienna marble. Red and white antique marbles, found only
among the ruins of ancient Rome. Grand antique, a breccia marble, containing shells,
consists of large fragments of a black marble, traversed by veins or lines of a shining
white. There are four columns of it in the Museum at Paris. Antique Cipolino marble.
Cipolin is a name given to all such marbles as have greenish zones produced by green
talc; their fracture is granular and shining, and displays here and there plates of talc.
Purple antique breccia marble, is very variable in the colour and size of its spots.
Antique African breccia, has a black ground, variegated with large fragments of a grayish-white,
deep red, or purplish wine colour; and is one of the most beautiful marbles.
Rose-coloured antique breccia marble is very scarce, occurring only in small tablets.
There are various other kinds of ancient breccias, which it would be tedious to particularize.
Modern marbles.—1. British. Black marble is found at Ashford, Matlock, and
Monsaldale in Derbyshire; black and white in the north part of Devonshire; the variegated
marbles of Devonshire are generally reddish, brownish, and grayish, variously
veined with white and yellow, or the colours are often intimately blended; the marbles
from Torbay and Babbacombe, display a great variety in the mixture of their colours;
the Plymouth marble is either ash-coloured with black veins, or blackish-gray and white,
shaded with black veins; the cliffs near Marychurch exhibit marble quarries not only
of great extent, but of superior beauty to any other in Devonshire, being either of a
dove-coloured ground with reddish-purple and yellow veins, or of a black ground mottled
with purplish globules. The green marble of Anglesea is not unlike the verde antico;
its colours being greenish-black, leek-green, and sometimes dull purplish, irregularly
blended with white. The white part is limestone, the green shades proceed from
serpentine and asbestos. There are several fine varieties of marble in Derbyshire; the
mottled-gray in the neighbourhood of Moneyash, the light gray being rendered
extremely beautiful by the number of purple veins which spread upon its polished surface
in elegant irregular branches; but its chief ornament is the multitude of entrochi, with
which this transition limestone-marble abounds. Much of the transition and carboniferous
limestone of Wales and Westmoreland is capable of being worked up into agreeable
dark marbles.
In Scotland, a particularly fine variety of white marble is found in immense beds, at
Assynt in Sutherlandshire. A beautiful ash-gray marble of a very uniform grain, and
susceptible of a fine polish, occurs on the north side of the ferry of Ballachulish in
Invernesshire. One of the most beautiful varieties is that from the hill of Belephetrich
in Tiree, one of the Hebrides. Its colours are pale blood-red, light flesh-red, and
reddish-white, with dark green particles of hornblende, or rather sahlite, diffused through
the general base. The compact marble of Iona is of a fine grain, a dull white colour,
somewhat resembling pure compact felspar. It is said by Bournon, to consist of an
intimate mixture of tremolite and carbonate of lime, sometimes with yellowish or
greenish-yellow spots. The carboniferous limestone of many of the coal basins in the
lowlands of Scotland may be worked into a tolerably good marble for chimney-pieces.
In Ireland, the Kilkenny marble is the one best known, having a black ground more
or less varied with white marks produced by petrifactions. The spar which occupies
the place of the shells, sometimes assumes a greenish-yellow colour. An exceedingly
fine black marble has also been raised at Crayleath in the county of Down. At Louthlougher,
in the county of Tipperary, a fine purple marble is found, which when polished
looks very beautiful. The county of Kerry affords several variegated marbles, not
unlike the Kilkenny.
France possesses a great many marble quarries which have been described by Brard,
and of which a copious abstract is given under the article marble,—Rees’ Cyclopedia.
The territory of Genoa furnishes several beautiful varieties of marble, the most
remarkable of which is the polzevera di Genoa, called in French the vert d’Egypte and
vert de mer. It is a mixture of granular limestone with a talcose and serpentine substance
disposed in veins; and it is sometimes mixed with a reddish body. This marble
was formerly much employed in Italy, France, and England, for chimney-pieces, but its
sombre appearance has put it out of fashion.
Corsica possesses a good statuary marble of a fine close grain, and pure milky
whiteness, quarried at Ornofrio; it will bear comparison with that of Carrara; also a
gray marble (bardiglio), a cipolin, and some other varieties. The island of Elba has
immense quarries of a white marble with blackish-green veins.
[801]
Among the innumerable varieties of Italian marbles, the following deserve especial
notice.
The rovigio, a white marble found at Padua; the white marble of St. Julien, at Pisa,
of which the cathedral and celebrated slanting tower are built; the Biancone marble,
white with a tinge of gray, quarried at Magurega for altars and tombs. Near Mergozza
the white saline marble with gray veins is found, with which the cathedral of Milan is
built. The black marble of Bergamo is called paragone, from its black colour, like
touchstone; it has a pure intense tint, and is susceptible of a fine polish. The pure
black marble of Como is also much esteemed. The polveroso of Pistoya, is a black
marble sprinkled with dots; and the beautiful white marble with black spots, from the
Lago Maggiore, has been employed for decorating the interior of many churches in the
Milanese. The Margorre marble found in several parts of the Milanese, is bluish veined
with brown, and composes part of the dome of the cathedral of Milan. The green
marble of Florence owes its colour to a copious admixture of steatite. Another green
marble, called verde di Prado, occurs in Tuscany, near the little town of Prado. It is
marked with spots of a deeper green than the rest, passing even into blackish-blue.
The beautiful Sienna marble, or brocatello di Siena, has a yellow colour like the yolk of
an egg, which is disposed in large irregular spots, surrounded with veins of bluish-red,
passing sometimes into purple. At Montarenti, two leagues from Sienna, another
yellow marble is met with, which is traversed by black and purplish-black veins. The
Brema marble is yellow with white spots. The mandelato of the Italians is a light
red marble with yellowish-white spots, found at Luggezzana, in the Veronese. The red
marble of Verona is of a red rather inclining to yellow or hyacinth; a second variety of
a dark red, composes the vast amphitheatre of Verona. Another marble is found near
Verona, with large white spots in a reddish and greenish paste. Very fine columns
have been made of it. The occhio di pavone is an Italian shell marble, in which the
shells form large orbicular spots, red, white, and bluish. A madreporic marble known
under the name of pietra stellaria, much employed in Italy, is entirely composed of star
madrepores, converted into a gray and white substance, and is susceptible of an excellent
polish. The village of Bretonico, in the Veronese, furnishes a splendid breccia marble,
composed of yellow, steel-gray, and rose-coloured spots. That of Bergamo consists of
black and gray fragments in a greenish cement. Florence marble, called also ruin and
landscape marble, is an indurated calcareous marl.
Sicily abounds in marbles, the most valuable of which is that called by the English
stone-cutters, Sicilian jasper; it is red with large stripes like ribands, white, red, and
sometimes green, which run zigzag with pretty acute angles.
Among the Genoese marbles we may notice the highly esteemed variety called portor,
on account of the brilliant yellow veins in a deep black ground. The most beautiful
kind comes from Porto-Venese, and Louis XIV. caused a great deal of it to be worked
up for the decoration of Versailles. It costs now two pounds per cubic foot.
Of cutting and polishing marble.—The marble saw is a thin plate of soft iron, continually
supplied during its sawing motion, with water and the sharpest sand. The
sawing of moderate pieces is performed by hand, but that of large slabs is most economically
done by a proper mill.
The first substance used in the polishing process is the sharpest sand, which must be
worked with till the surface becomes perfectly flat. Then a second, and even a third
sand of increasing fineness is to be applied. The next substance is emery of progressive
degrees of fineness, after which tripoli is employed; and the last polish is given with
tin-putty. The body with which the sand is rubbed upon the marble, is usually a plate
of iron; but for the subsequent process, a plate of lead is used with fine sand and emery.
The polishing rubbers are coarse linen cloths, or bagging, wedged tight into an iron
planing tool. In every step of the operation, a constant trickling supply of water is
required.
Visiters of Derby may have an opportunity of inspecting Brown’s extensive machinery
for cutting marble into many ornamental forms, which has been well described
in Rees’ Cyclopedia.
Sir James Jelf patented, in 1822, a combination of machinery for cutting any description
of parallel mouldings upon marble slabs, for ornamental purposes; in which,
tools, supplied with sand and water, are made to traverse to and fro.
Mr. Tullock obtained a patent, in 1824, for improvements in machinery for sawing
and grooving marble; the power being applied by means of toothed wheels bearing
cranks, which gave the see-saw motion to the cutting iron plates.
In November, 1829, Mr. Gibbs secured, by patent, an invention for working ornamental
devices in marble, by means of a travelling drill, guided by a mould of wood,
&c., in counter relief; and in April, 1833, Mr. G. W. Wilds obtained a patent for
machinery, which consists of a series of circular cutters, for separating slabs from a
block of marble; the block being advanced slowly to meet the cutters, by the progressive[802]
movement of a platform upon wheels, driven by the agency of a rack and pinion, as in
the cylinder boring machine of the steam-engine manufacturer. Sand and water must
be supplied, of course, from a hopper, to these smooth cutting discs of iron or copper.
See Glass-Cutting. He proposes also to mould and polish marble, by applying a
rotatory wheel or cylinder of any shape to it, in its carrying frame.
MARCASITE, is a variety of iron pyrites, containing generally a little arsenic.
MARGARATES, are saline compounds of margaric acid with the bases.
MARGARIC ACID, is one of the acid fats, produced by saponifying tallow with
alkaline matter, and decomposing the soap with dilute acid. The term Margaric signifies
Pearly-looking.
The physical properties of the margaric and stearic acids are very similar; the chief
difference is that the former is more fusible, melting at 140° F. The readiest mode
of obtaining pure margaric acid, is to dissolve olive oil soap in water, to pour into the
solution, a solution of neutral acetate of lead, to wash and dry the precipitate, and then
to remove its oleate of lead by ether, which does not affect its margarate of lead. The
residuum being decomposed by boiling hot muriatic acid, affords margaric acid. When
heated in a retort this acid boils. It is insoluble in water, very soluble in alcohol and
ether; it reddens litmus paper, and decomposes with the aid of heat, the carbonates of
soda and potash.
MARINE ACID. See Muriatic Acid.
MARL (Marne, Fr.; Mergel, Germ.), is a mixed earthy substance, consisting of
carbonate of lime, clay, and siliceous sand, in very variable proportions; it is sometimes
compact, sometimes pulverulent. According to the predominance of one or other of
these three main ingredients, marls may be distributed into calcareous, clayey, and sandy.
See Limestone.
MARQUETRY, is a peculiar kind of cabinet work, in which the surface of wood is
ornamented with inlaid pieces of various colours and forms. The marqueteur puts gold,
silver, copper, tortoise-shell, mother-of-pearl, ivory, horn, &c. under contribution.
These substances being reduced to laminæ of proper thinness, are cut out into the
desired forms by punches, which produce at once the full pattern or mould, and the
empty one, which enclosed it; and both serve their separate purposes in marquetry.
For the methods of dyeing the woods, &c. see Ivory.
MARTIAL, signifies belonging to iron; from Mars, the mythological name of this
metal.
MASSICOT, is the yellow oxide of lead.
MASTIC (Eng. and Fr.; Mastix, Germ.), is a resin produced by making incisions
in the Pistacia Lentiscus, a tree cultivated in the Levant, and chiefly in the island of
Chios. It comes to us in yellow, brittle, transparent, rounded tears; which soften
between the teeth; with bitterish taste and aromatic smell, and a specific gravity of
1·07. Mastic consists of two resins; one soluble in dilute alcohol; but both dissolve
in strong alcohol. Its solution in spirit of wine constitutes a good varnish. It dissolves
also in oil of turpentine. See Varnish.
MATRASS, is a bottle with a thin egg-shaped bottom, much used for digestions in
chemical researches.
MATTE, is a crude black copper reduced, but not refined from sulphur and other
heterogeneous substances.
MEADOW ORE, is conchoidal bog iron ore.
MEDALS. For their composition, see Bronze and Copper.
MEERSCHAUM (Germ.; sea-froth, Eng.; Ecume de Mer, Magnésie carbonatée
silicifère, Fr.), is a white mineral, of a somewhat earthy appearance, always soft, but
dry to the touch, and adhering to the tongue. Specific gravity, 2·6 to 3·4; affords
water by calcination; fuses with difficulty at the blowpipe into a white enamel; and is
acted upon by acids. It consists, according to Klaproth, of silica, 41·5; magnesia,
18·25; water and carbonic acid, 39. Other analysts give, silica 50, magnesia 25,
water 25. It occurs in veins or kidney-shaped nodules, among rocks of serpentine, at
Egribos, in the island of Negropont, Eski-Schehir in Anatolia, Brussa at the foot of
Mount Olympus, at Baldissero in Piedmont, in the serpentine veins of Cornwall, &c.
When first dug up, it is soft, greasy, and lathers like soap; and is on that account
used by the Tartars in washing their linen. The well-known Turkey tobacco-pipes
are made from it, by a process analogous to that for making pottery ware. The bowls
of the pipes, when imported into Germany, are prepared for sale by soaking them first in
tallow, then in wax, and finally by polishing them with shave-grass.
MELLITE. (Eng. and Fr.; Honigstein, Germ.) See Honeystone.
MELLITIC ACID, which is associated with alumina in the preceding mineral,
crystallizes in small colourless needles, is without smell, of a strongly acid taste, permanent
in the air, soluble in water and alcohol, as also in boiling hot concentrated
sulphuric acid, but is decomposed by hot nitric acid, and consists of 50·21 carbon, and[803]
49·79 oxygen. It is carbonized at a red heat, without the production of any inflammable
oil.
MELLON, is a new compound of carbon and azote, discovered by M. Liebig, by
heating bi-sulpho-cyanide of mercury. The mellon remains at the bottom of the retort
under the form of a yellow powder.
MENACHANITE, an ore of titanium, found in the bed of a rivulet which flows
into the valley Menacan, in Cornwall.
MERCURY or QUICKSILVER. This metal is distinguished by its fluidity at
common temperatures; its density = 13·6; its silver blue lustre; and its extreme
mobility. A cold of 39° below zero of Fahrenheit, or -40° cent., is required for its
congelation, in which state its density is increased in the proportion of 10 to 9, or it
becomes of spec. grav. 15·0. At a temperature of 656° F. it boils and distils off in an
elastic vapour; which, being condensed by cold, forms purified mercury.
Mercury combines with great readiness with certain metals, as gold, silver, zinc, tin,
and bismuth, forming, in certain proportions, fluid solutions of these metals. Such
mercurial alloys are called amalgams. This property is extensively employed in many
arts; as in extracting gold and silver from their ores; in gilding, plating, making looking-glasses,
&c. Humboldt estimates at 16,000 quintals, of 100 lbs. each, the quantity
of mercury annually employed at his visit to America, in the treatment of the mines of
New Spain; three-fourths of which came from European mines.
The mercurial ores may be divided into four species:—
1. Native quicksilver.—It occurs in most of the mines of the other mercurial ores, in
the form of small drops attached to the rocks, or lodged in the crevices of other ores.
2. Argental mercury, or native silver amalgam.—It has a silver-white colour, and is
more or less soft, according to the proportion which the mercury bears to the silver.
Its density is sometimes so high as 14. A moderate heat dissipates the mercury, and
leaves the silver. Klaproth states its constituents at silver 36, and mercury 64, in 100;
but Cordier makes them to be, 271⁄2 silver, and 721⁄2 mercury. It occurs crystallized in a
variety of forms. It has been found in the territory of Deux-Ponts, at Rozenau and
Niderstana, in Hungary, in a canton of Tyrol, at Sahlberg in Sweden, at Kolyvan in
Siberia, and at Allémont in Dauphiny; in small quantity at Almaden in Spain, and at
Idria in Carniola. By the chemical union of the mercury with the silver, the amalgam,
which should by calculation have a spec. grav. of only 12·5, acquires that of 14·11,
according to M. Cordier.
3. Sulphuret of mercury, commonly called Cinnabar, is a red mineral of various
shades; burning at the blowpipe with a blue flame, volatilizing entirely with the smell
of burning sulphur, and giving a quicksilver coating to a plate of copper held in the
fumes. Even the powder of cinnabar rubbed on copper whitens it. Its density varies
from 6·9 to 10·2. It becomes negatively electrical by friction. Analysed by Klaproth,
it was found to consist of mercury 84·5, sulphur 14·75. Its composition, viewed as a
bisulphuret of mercury, is, mercury 86·2, sulphur 13·8. The finest crystals of sulphuret
of mercury come from China, and Almaden in Spain. These contain, according to
Klaproth, 85 per cent. of mercury.
A bituminous sulphuret of mercury appears to be the base of the great exploration of
Idria; it is of a dark liver-red hue; and of a slaty texture, with straight or twisted
plates. It exists in large masses in the bituminous schists of Idria. M. Beurard
mentions also the locality of Munster-Appel, in the duchy of Deux-Ponts, where the ore
includes impressions of fishes, curiously spotted with cinnabar.
The compact variety of the Idria ore seems very complex in composition, according
to the following analysis of Klaproth:—Mercury, 81·8; sulphur, 13·75; carbon, 2·3;
silica, 0·65; alumina, 0·55; oxide of iron, 0·20; copper, 0·02; water, 0·73; in 100
parts. M. Beurard mentions another variety from the Palatinate, which yields a large
quantity of bitumen by distillation; and it was present in all the specimens of these ores
analyzed by me for the German Mines Company. At Idria and Almaden the sulphurets
are extremely rich in mercury.
4. Muriated mercury, or the Chloride of mercury, commonly called Horn mercury.
This ore occurs in very small crystals of a pearl-gray or greenish-gray colour, or in
small nipples which stud, like crystals, the cavities, fissures, or geodes among the ferruginous
gangues of the other ores of mercury. It is brittle, and entirely volatile at the
blowpipe, characters which distinguish it from horn silver.
The geological position of the mercurial ores, in all parts of the world, is in the strata
which commence the series of secondary formations. Sometimes they are found in the
red sandstone above the coal, as at Menildot, in the old dutchy of Deux-Ponts, at
Durasno in Mexico, at Cuença in New Granada, at Cerros de Gauzan and Upar in
Peru; in the subordinate porphyries, as at Deux-Ponts, San Juan de la Chica in Peru,
and at Cerro-del-Fraile, near the town of San-Felipe, they occur also among the strata
below, or subordinate to the calcareous formation, called zechstein, in Germany, or[804]
among the accompanying bituminous schists, as at Idria in Carniola; and, lastly, they
form masses in the zechstein itself. Thus, it appears that the mercurial deposits are
confined within very narrow geological limits, between the calcareous beds of zechstein,
and the red sandstone. They occur at times in carbonaceous nodules, derived from
the decomposition of mosses of various kinds; and the whole mercurial deposit is occasionally
covered with beds of charcoal, as at Durasno.
They are even sometimes accompanied with the remains of organic bodies, such as
casts of fishes, fossil shells, silicified wood, and true coal. The last fact has been
observed at Potzberg, in the works of Drey-Koenigszug, by M. Brongniart. These
sandstones, bituminous schists, and indurated clays, contain mercury both in the state of
sulphuret and in the native form. They are more or less penetrated with the ore, forming
sometimes numerous beds of very great thickness; while, in the more antient or
the primitive formations, these ores exist only in very small quantity associated with tin.
Mercury is, generally speaking, a metal sparingly distributed in nature, and its mines
are very rare.
The great exploitations of Idria in Friuli, in the county of Goritz, were discovered in
1497, and the principal ore mined there is the bituminous sulphuret. The workings
of this mine have been pushed to the depth of 280 yards. The product in quicksilver
might easily amount annually to 6000 metric quintals = 600 tons British; but, in
order to uphold the price of the metal, the Austrian government has restricted the production
to 150 tons. The memorable fire of 1803 was most disastrous to these mines. It
was extinguished only by drowning all the underground workings. The sublimed
mercury in this catastrophe occasioned diseases and nervous tremblings to more than
900 persons in the neighbourhood.
Pliny has recorded two interesting facts: 1. that the Greeks imported red cinnabar
from Almaden 700 years before the Christian era; and 2. that Rome, in his time, annually
received 700,000 pounds from the same mines. Since 1827, they have produced
22,000 cwts. of mercury every year, with a corps of 700 miners and 200 smelters; and,
indeed, the veins are so extremely rich, that though they have been worked pretty constantly
during so many centuries, the mines have hardly reached the depth of 330 yards,
or something less than 1000 feet. The lode actually under exploration is from 14 to
16 yards thick, and it becomes thicker still at the crossing of the veins. The totality of
the ore is extracted. It yields in their smelting works only 10 per cent. upon an
average, but there is no doubt, from the analysis of the ores, that nearly one half of the
quicksilver is lost, and dispersed in the air, to the great injury of the workmen’s health,
in consequence of the barbarous apparatus of aludels employed in its sublimation; an
apparatus which has remained without any material change for the better since the days
of the Moorish dominion in Spain. M. Le Play, the eminent Ingenieur des Mines, who published,
in a recent volume of the Annales des Mines, his Itinéraire to Almaden, says,
that the mercurial contents of the ores are notablement plus elevées than the product.
These veins extend all the way from the town of Chillon to Almadenejos. Upon
the borders of the streamlet Balde Azogues, a black slate is also mined which is abundantly
impregnated with metallic mercury. The ores are treated in 13 double furnaces,
which I shall presently describe. “Le mercure,” says M. Le Play, “a sur la
santé des ouvriers la plus funeste influence, et l’on ne peut se défendre d’un sentiment
pénible en voyant l’empressement avec lequel des jeunes gens, pleins de force et de
santé, se disputent la faveur d’aller chercher dans les mines, des maladies cruelles, et
souvent une mort prématurée. La population des mineurs d’Almaden méritent le plus
haut interêt.” These victims of a deplorable mismanagement are described as being a
laborious, simple-minded, virtuous race of beings, who are thus condemned to breathe
an atmosphere impregnated far and near with the fumes of a volatile poison, which
the lessons of science, as I shall presently demonstrate, might readily repress, with the
effect of not only protecting the health of the population, but of vastly augmenting the
revenues of the state.
These celebrated mines, near to which lie those of Las Cuebas and of Almadenejos,
were known to the Romans. After having been the property of the religious knights of
Calatrava, who had assisted in expelling the Moors, they were farmed off to the celebrated
Fugger merchants of Augsbourg; and afterwards explored on account of the government,
from the date of 1645 till the present time. Their produce was, till very lately,
entirely appropriated to the treatment of the gold and silver ores of the new world.
The mines of the Palatinate, situated on the left bank of the Rhine, though they
do not approach in richness and importance to those of Idria and Almaden, merit,
however, all the attention of the government that farms them out. They are numerous,
and varied in geological position. Those of Drey-Koenigszug, at Potzberg, near
Kussel, deserve particular notice. The workings have reached a depth of more than
220 yards; the ore being a sandstone strongly impregnated with sulphuret of mercury.
The produce of these mines is estimated at about 30 tons per annum.
[805]
There are also in Hungary, Bohemia, and several other parts of Germany, some
inconsiderable exploitations of mercury, the total produce of which is valued at about
30 or 40 tons on an average of several years.
The mines of Guancavelica, in Peru, are the more interesting, as their products are
directly employed in treating the ores of gold and silver, which abound in that portion
of America. These quicksilver mines, explored since 1570, produced, up to 1800,
53,700 tons of that metal; but the actual produce of the explorations of these countries
was, according to Helms, about the beginning of this century, from 170 to 180 tons
per annum.
In 1782 recourse was had by the South American miners to the mercury extracted
in the province of Yun-nan, in China.
The metallurgic treatment of the quicksilver ores is tolerably simple. In general,
when the sulphuret of mercury, the most common ore, has been pulverized, and sometimes
washed, it is introduced into retorts of cast iron, sheet iron, or even stoneware, in
mixture with an equal weight of quicklime. These retorts are arranged in various
ways.
Prior to the 17th century, the method called per descensum was the only one in use
for distilling mercury; and it was effected by means of two earthen pots adjusted
over each other. The upper pot, filled with ore, and closed at the top, was covered over
with burning fuel; and the mercurial vapours expelled by the heat, passed down
through small holes in the bottom of the pot, to be condensed in another vessel placed
below. However convenient this apparatus might be, on account of the facility of
transporting it, wherever the ore was found, its inefficiency and the losses it occasioned
were eventually recognized. Hence, before 1635, some smelting works of the Palatinate
had given up the method per descensum, which was, however, still retained in
Idria; and they substituted for it the furnaces called galleries. At first earthenware
retorts were employed in these furnaces; but they were soon succeeded by iron retorts.
In the Palatinate this mode of operating is still in use. At Idria, in the year 1750,
a great distillatory apparatus was established for the treatment of the mercurial ores, in
imitation of those which previously existed at Almaden, in Spain, and called aludel-furnaces.
But, since 1794, these aludels have been suppressed, and new distillatory
apparatus have been constructed at Idria, remarkable only for their magnitude; exceeding,
in this respect, every other metallurgic erection.
There exist, therefore, three kinds of apparatus for the distillation of mercury: 1. the
furnace called a gallery; 2. the furnace with aludels; and 3. the large apparatus of
Idria. I shall describe each of these briefly, in succession.
1. Furnace called Gallery of the Palatinate.—The construction of this furnace is
disposed so as to contain four ranges, a a′, b b′, of large retorts, styled cucurbits, of cast
iron, in which the ore of mercury is subjected to distillation. This arrangement is
shewn in fig. 656., which presents a vertical section in the line a b of the ground plan,
fig. 657. In the ground plan, the roof e e′ of the furnace (fig. 656.) is supposed to be
lifted off, in order to shew the disposition of the four ranges of cucurbits upon the grate
c f, figs. 656, 657., which receives the pit-coal employed as fuel. Under this grate
extends an ash-pit d. Fig. 658., which exhibits an elevation of the furnace, points out
this ash-pit, as well as one of the two doors c, by which the fuel is thrown upon the grate
c f. Openings e e, (fig. 656.) are
left over the top arch of the furnace,
whereby the draught of air
may receive a suitable direction.
The grate of the fire-place extends
over the whole length of
the furnace, fig. 657., from the
door c to the door f, situated at
the opposite extremity. The
furnace called gallery includes
commonly 30 cucurbits, and in
some establishments even 52.
Into each are introduced from
56 to 70 pounds of ore, and 15 to
18 pounds of quicklime, a mixture
which fills no more than
two-thirds of the cucurbit; to
the neck a stoneware receiver is
adapted, containing water to half
its height. The fire, at first moderate, is eventually pushed till the cucurbits are red
hot. The operation being concluded, the contents of the receivers are poured out into
a wooden bowl placed upon a plank above a bucket; the quicksilver falls to the bottom[806]
of the bowl, and the water draws over the black mercury, for so the substance that coats
the inside of the receivers is called. This is considered to be a mixture of sulphuret
and oxide of mercury. The black mercury, taken out of the tub and dried, is distilled
anew with excess of lime; after which the residuum in the retorts is thrown away, as
useless.
Aludel furnaces of Almaden.—Figs. 659. and 660. represent the great furnaces with
aludels in use at Almaden, and anciently in Idria; for between the two establishments
there was in fact little difference before the year 1794. Figs. 659. and 662. present two
vertical sections; figs. 660. and 661. are two plans of two similar furnaces, conjoined in
one body of brickwork. In the four
figures the following objects are to be
remarked; a door a, by which the wood
is introduced into the fire-place b. This
is perforated with holes for the passage
of air; the ash-pit c, is seen beneath.
An upper chamber d, contains the mercurial
ores distributed upon open arches,
which form the perforated sole of this
chamber. Immediately over these
arches, there are piled up in a dome
form, large blocks of a limestone, very
poor in quicksilver ore; above these
are laid blocks of a smaller size, then
ores of rather inferior quality, and
stamped ores mixed with richer minerals.
Lastly, the whole is covered up
with soft bricks, formed of clay kneaded
with schlich, and with small pieces of
sulphuret of mercury. Six ranges of
aludels or stoneware tubes, f f, of a pear
shape, luted together with clay, are
mounted in front of each of the two
furnaces, on a double sloping terrace,
having in its lowest middle line two
gutters t v, a little inclined towards the intermediate wall m. In each range the aludel
placed at the line t m v of fig. 660., that is to say at the lowest point, g, figs. 659. 662., is
pierced with a hole. Thereby the mercury which had been volatilized in d, if it be
already condensed by the cooling in the series of aludels f g, may pass into the corresponding
gutter, next into the hole m, fig. 660., and after that into the wooden pipes
h h′, fig. 659., which conduct it across the masonry of the terrace into cisterns filled with
water; see q, fig. 661., which is the plan of fig. 662.
The portion of mercury not condensed in the range of aludels, f g, which is the most[807]
considerable, goes in the state of vapour, into a chamber k; but in passing under a
partition l l, a certain portion is deposited in a cistern i, filled with water. The greater
part of the vapours diffused in the chamber k′ is thereby condensed, and the mercury
falls down upon the two inclined planes which form its bottom. What may still exist
as vapour passes into an upper chamber k′, by a small chimney n. On one of the sides
of this chamber there is a shutter which may be opened at pleasure from below upwards,
and beneath this shutter, there is a gutter into which a notable quantity of mercury
collects. Much of it is also found condensed in the aludels. These facts prove that
this process has inconveniences, which have been tried to be remedied by the more
extensive but rather unchemical grand apparatus of Idria.
Details of the aludel apparatus: 25 are set in each of the 12 ranges, seen in fig. 661.
constituting 300 pear-shaped stoneware vessels, open at both ends, being merely thrust
into one another, and luted with loam. What a multitude of joints, of which a great
many must be continually giving way by the shrinkage of the luting, whereby the
mercurial fumes will escape with great loss of product, to poison the air!
a, is the door of the fire-place; c, the perforated arches upon which the ore is piled in
the chamber e, through the door d, and an orifice at top; the latter being closed during
the distillation; f f are vents for conducting the mercurial vapours into two chambers i,
separated by a triangular body of masonry m n; h is the smoke chimney of the fire-place;
o o, are the ranges of aludels, in connection with the chamber i, which are laid slantingly
towards the gutter q, upon the double inclined plane terrace, and terminate in the
chamber h q; this being surmounted by two chimneys t. The mercury is collected in
these aludels and in the basins at q and p, fig. 661. r is a thin stone partition set
up between the two principal walls of each of the furnaces. v is the stair of the aludel
terrace, leading to the platform which surmounts the furnace; z is a gutter for conducting
away the rains which may fall upon the buildings.
Great apparatus of Idria.—Before entering into details of this laboratory, it will not
be useless to recapitulate the metallurgic classification of the ores treated in it. 1. The
ores in large blocks, fragments, or shivers, whose size varies from a cubic foot to that of
a nut. 2. The smaller ores, from the size of a nut to that of grains of dust.
The first class of large ores comprises three subdivisions, namely; a, blocks of metalliferous
rocks, which is the most abundant and the poorest species of ore, affording only
one per cent. of mercury; b, the massive sulphuret of mercury, the richest and rarest
ore, yielding 80 per cent. when it is picked; c, the fragments or splinters proceeding
from the breaking and sorting, and which vary in value, from 1 to 40 per cent.
The second class of small ores comprises: d, the fragments or shivers extracted from
the mine in the state of little pieces, affording from 10 to 12 per cent.; e, the kernels of
ore, separated on the sieve, yielding 32 per cent.; f, the sands and paste called schlich,
obtained in the treatment of the poorest ores, by means of the stamps and washing
tables; 100 parts of this schlich give at least 8 of quicksilver.
The general aspect of the apparatus is indicated by figs. 663, 664. and 665. Fig. 665.
represents the exterior, but only one
half, which is enough, as it resembles
exactly the other, which is not shown.
In these three figures the following objects
may be distinguished; figs. 663,
664., a, door of the fire-place; b, the
furnace in which beech-wood is burned
mixed with a little fir-wood; c, door
of the ash-pit, extended beneath; d,
a space in which the ores are deposited upon the seven arches, 1. to 7., as indicated in
figs. 663. and 666.; e e, brick tunnels, by which the smoke of the fuel and the vapours
of mercury pass, on the one side, into successive chambers f k.
f g h i j k l are passages which permit the circulation of the vapours from the furnace
a b c d, to the chimneys l l. Figs. 663. and 664. exhibit clearly the distribution of these[808]
openings on each side of the same furnace, and in each half of the apparatus, which is
double, as fig. 664. shows; the spaces without letters being in every respect similar to
the spaces mentioned below. Fig. 664. is double the scale of fig. 663.
m m′, fig. 664., are basins of reception, distributed before the doors of each of the
chambers f k f′ k′. The condensed mercury which flows out of the chambers is conveyed
thither. n n′ is a trench into which the mercury, after being lifted into the basins m, is
poured, so that it may run towards a common chamber o, in the sloping direction
indicated by the arrows. o leads to the chamber where the mercury is received into a
porphyry trough; out of which it is laded and packed up in portions of 50 or 100 lbs.
in sheep-skins prepared with alum. p p′, fig. 663., are vaulted arches, through which a
circulation may go on round the furnace a b c d, on the ground level, q q′ are the vaults
of the upper stories. r r′, fig. 665., vaults which permit access to the tunnels e′ e′′,
fig. 663.
s s′ and t t′, fig. 665., are the doors of the chambers, f k and f′ k′. These openings are
shut during the distillation by wooden doors faced with iron, and luted with a mortar of
clay and lime. u u′ is the door of the vaults 1. to 7. of the furnace represented in
fig. 663. These openings are hermetically shut, like the preceding. v v′, fig. 663., are
superior openings of the chambers, closed during the operation by luted plugs; they are
opened afterwards to facilitate the cooling of the apparatus, and to collect the mercurial
soot. x y z, fig. 666., are floors which correspond to the doors u u′ of the vaults 1. to 7.,
fig. 665. These floors are reached by stairs set up in the different parts of the building,
which contains the whole apparatus.
On the lower arches the largest blocks of metalliferous rock are laid; over these the
less bulky fragments are arranged, which are covered with the shivers and pieces of less
dimension. On the middle vaults, the small ore is placed, distributed into cylindrical
pipkins of earthenware, of 10 inches diameter and 5 inches depth. The upper vaults
receive likewise pipkins filled with the sands and pastes called schlich.
In 3 hours, by the labour of 40 men, the two double sets of apparatus are charged, and
all the apertures are closed. A quick fire of beech-wood is then kindled; and when the
whole mass has become sufficiently heated, the sulphuret of mercury begins to vapourize;
coming into contact with the portion of oxygen which had not been carbonated,
by combustion, its sulphur burns into sulphurous acid, while the mercury becomes free,
passes with the other vapours into the chambers for condensing it, and precipitates in the
liquid form at a greater or less distance from the fire-place. The walls of the chambers
and the floors, with which their lower portion is covered, are soon coated over with a
black mercurial soot, which, being treated anew, furnishes 50 per cent. of mercury. The
distillation lasts from 10 to 12 hours; during which time the whole furnace is kept at
a cherry-red heat. A complete charge for the two double apparatus, consists of from
1000 to 1300 quintals of ore, which produce from 80 to 90 quintals of running mercury.
The furnace takes from 5 or 6 days to cool, according to the state of the weather; and
if to that period be added the time requisite for withdrawing the residuums, and attending
to such repairs as the furnace may need, it is obvious that only one distillation can
be performed in the course of a week.
In the works of Idria, in 1812, 56,686 quintals and a half of quicksilver ores were
distilled, after undergoing a very careful mechanical preparation. They afforded 4832
quintals of running mercury; a quantity corresponding to about 81⁄2 per cent. of the
ore. These smelting works are about 180 feet long and 30 feet high.
Upon the preceding three systems of smelting mercurial ores, I shall now make some
observations.
It has been long well known, that quicksilver may be most readily extracted from
cinnabar, by heating it in contact with quicklime. The sulphur of the cinnabar combines,[809]
by virtue of a superior affinity with the lime, to the exclusion of the quicksilver,
to form sulphurets of lime and calcium, both of which being fixed hepars, remain in the
retort while the mercury is volatilized by the heat. In a few places, hammerschlag, or
the iron cinder, driven off from the blooms by the tilting hammer, has been used instead
of lime in the reduction of this mercurial ore, whereby sulphurous acid and sulphuret
of iron are formed.
The annual production of the Bavarian Rhine provinces has been estimated at from
400 to 550 quintals; that of Almaden, in the year 1827, was 22,000 quintals; and of
Idria, at present, is not more than 1500 quintals.
All the plans hitherto prescribed for distilling the ore along with quicklime, are
remarkably rude. In that practised at Landsberg by Obermoschel, there is a great
waste of labour, in charging the numerous small cucurbits; there is a great waste of
fuel in the mode of heating them; a great waste of mercury by the imperfect luting
of the retorts to the receivers, as well as the imperfect condensation of the mercurial
vapours; and probably a considerable loss by pilfering.
The modes practised at Almaden and Idria are, in the greatest degree, barbarous;
the ores being heated upon open arches, and the vapours attempted to be condensed by
enclosing them within brick or stone and mortar walls, which can never be rendered
either sufficiently tight or cool.
To obviate all these inconveniences and sources of loss, the proper chemical arrangements
suited to the present improved state of the arts ought to be adopted, by which
labour, fuel, and mercury, might all be economized to the utmost extent. The only
apparatus fit to be employed is a series of cast-iron cylinder retorts, somewhat like
those employed in the coal gas works, but with peculiarities suited to the condensation
of the mercurial vapours. Into each of these retorts, supposed to be at least one foot
square in area, and 7 feet long, 6 or 7 cwt. of a mixture of the ground ore with the
quicklime, may be easily introduced, from a measured heap, by means of a shovel.
The specific gravity of the cinnabar being more than 6 times that of water, a cubic foot
of it will weigh more than 31⁄2 cwt.; but supposing the mixture of it with quicklime
(when the ore does not contain the calcareous matter itself) to be only thrice the
density of water, then four cubic feet might be put into each of the above retorts, and
still leave 11⁄2 cubic feet of empty space for the expansion of volume which may take place
in the decomposition. The ore should certainly be ground to a moderately fine powder,
by stamps, iron cylinders, or an edge wheel, so that when mixed with quicklime, the
cinnabar may be brought into intimate contact with its decomposer, otherwise much
of it will be dissipated unproductively in fumes, for it is extremely volatile.
Figs. 667, 668, 669. represent a cheap and powerful apparatus which I contrived at
the request of the German Mines Company of London, and which is now mounted at
Landsberg, near Obermoschel, in the Bavarian Rhein-Kreis.
Fig. 667. is a section parallel to the front elevation of three arched benches of retorts,
of the size above specified. Each bench contains 3 retorts, of the form represented by
a a a. I, is the single fire-place or furnace, capable of giving adequate ignition by
coal or wood, to the three retorts. The retorts were built up in an excellent manner,
by an English mason perfectly acquainted with the best modes of erecting coal-gas
retorts, who was sent over on purpose. The path of the flame and smoke is precisely
similar to that represented in fig. 483, page 549, whereby the uppermost retort is
immersed in a bath of uniformly ignited air, while the currents reverberated from the
top, play round the two undermost retorts, in their way to the vent-flues beneath[810]
them. The bottom of the uppermost retort is protected from the direct impulse of the
flame by fire-tiles. The dotted lines K K, show the paths of the chimneys which rise at
the back ends of the retorts.
In the section, fig. 668., a is the body of the retort; its mouth at the right hand end
is shut, as usual, by a luted iron lid, secured
with a cross-bar and screw-bolts; its other
end is prolonged by a sloping pipe of cast
iron, 4 inches in diameter, furnished with a
nozzle hole at L, closed with a screw plug.
Through this hole a wire rammer may
be introduced, to ascertain that the tube is
pervious, and to cleanse it from the mercurial
soot, when thought necessary. c, is
a cross section of the main condenser, shown
in a longitudinal section at C C, fig. 669.
This pipe is 18 inches in diameter, and
about 20 feet long. At a a, &c., the back
ends of the retorts are seen, with the
slanting tubes b b, &c., descending through
orifices in the upper surface of the condenser
pipe, and dipping their ends just
below the water-line h i. g, is the cap of
a water valve, which removes all risk from
sudden expansion or condensation. The
condenser is placed within a rectangular trough, made either of wood or stone, through
which a sufficient stream of water passes to keep it perfectly cool, and repress every
trace of mercurial vapour, and it is laid with a slight inclination from i to h, so that the
condensed quicksilver may spontaneously flow along its bottom, and pass through the
vertical tube D into the locked up iron chest, or magazine e. This tube D is from
the beginning closed at bottom, by immersion in a shallow iron cup, always filled with
mercury. k is a graduated gauge rod, to indicate the progressive accumulation of quicksilver
in the chest, without being under the necessity of unlocking it.
This air-tight apparatus was erected about a year ago, and has been found to act
perfectly well; I regret, however, that my professional engagements at home have not
hitherto permitted me to conduct its operations personally for some days. The average
samples of cinnabar ore from Obermoschel are ten times poorer than those of Almaden.
Were such an apparatus as the above, with some slight modifications which have lately
occurred to me, mounted for the Spanish mines, I am confident that their produce in
quicksilver might be nearly doubled, with a vast economy of fuel, labour, and human
life. The whole cost of the 9 large retorts, with their condensing apparatus, iron
magazine, &c., was very little more than two hundred pounds! As the retorts are kept
in a state of nearly uniform ignition, like those of the gas works, neither they, nor
the furnaces are liable to be injured in their joints by the alternate contractions and
expansions, which they would inevitably suffer if allowed to cool; and being always
ready heated to the proper pitch for decomposing the mercurial ores, they are capable
of working off a charge, under skilful management, in the course of 3 hours. Thus,
in 24 hours, with a relay of labourers, 8 charges of at least 5 cwts. of ore each,
might be smelted = 2 tons, with 3 retorts, and 6 tons with 9 retorts; with a daily
product from the rich ores of Almaden, or even Idria, of from 12 cwts. to 20 cwts.
Instead of 3 benches of 3 retorts each, I would recommend 15 benches, containing
45 retorts, to be erected for either the Almaden or Idria mines; which, while they
would smelt all their ores, could be got for a sum not much exceeding 1000l., an outlay
which they would reimburse within a month or two.
Quicksilver is a substance of paramount value to science. Its great density and its
regular rate of expansion and contraction by increase and diminution of temperature,[811]
give it the preference over all liquids for filling barometric and thermometric tubes. In
chemistry it furnishes the only means of collecting and manipulating, in the pneumatic
trough, such gaseous bodies as are condensable over water. To its aid, in this respect,
the modern advancement of chemical discovery is pre-eminently due.
This metal alloyed with tin-foil forms the reflecting surface of looking glasses, and by
its ready solution of gold or silver, and subsequent dissipation by a moderate heat, it
becomes the great instrument of the arts of gilding and silvering copper and brass. The
same property makes it so available in extracting these precious metals from their ores.
The anatomist applies it elegantly, to distend and display the minuter vessels of the
lymphatic system, and secretory systems, by injecting it with a syringe through all their
convolutions. It is the basis of many very powerful medicines, at present probably too
indiscriminately used, to the great detriment of English society; for it is far more
sparingly prescribed by practitioners upon the continent of Europe, not otherwise
superior in skill or science to those of Great Britain.
The nitrate of mercury is employed for the secrétage of rabbit and hare-skins, that is,
for communicating to the fur of these and other quadrupeds the faculty of felting, which
they do not naturally possess. With this view the solution of that salt is applied to them
lightly in one direction with a sponge. A compound amalgam of zinc and tin is probably
the best exciter which can be applied to the cushions of electrical machines. Mercury
imported for home consumption in 1836, 286,808 lbs.; in 1837, 314,036 lbs.
The only mercurial compounds which are extensively used in the arts, are fictitious
cinnabar or Vermillion, and corrosive sublimate.
MERCURY, BICHLORIDE OF; Corrosive sublimate; (Deutochlorure de mercure,
Fr.; Aetzendes quecksilber sublimat, Germ.) is made by subliming a mixture of equal
parts of persulphate of mercury, prepared as above described, and sea-salt, in a stone-ware
cucurbit. The sublimate rises in vapour, and encrusts the globular glass capital
with a white mass of small prismatic needles. Its specific gravity is 5·14. Its taste is
acrid, stypto-metallic, and exceedingly unpleasant. It is soluble in 20 parts of water,
at the ordinary temperature, and in its own weight of boiling water. It dissolves in 21⁄2
times its weight of cold alcohol. It is a very deadly poison. Raw white of egg swallowed
in profusion, is the best antidote. A solution of corrosive sublimate has been
long employed for preserving soft anatomical preparations. By this means the corpse of
Colonel Morland was embalmed, in order to be brought from the seat of war to Paris.
His features remained unaltered, only his skin was brown, and his body was so hard as
to sound like a piece of wood when struck with a hammer.
In the valuable work upon the dry rot, published by Mr. Knowles, secretary of the
committee of inspectors of the navy, in 1821, corrosive sublimate is enumerated among
the chemical substances which had been prescribed for preventing the dry rot in timber;
and it is well known that Sir H. Davy had, several years before that date, used and
recommended to the Admiralty and Navy Board, corrosive sublimate as an anti-dry rot
application. It has been since extensively employed by a joint-stock company for the
same purpose, under the title of Kyan’s patent.
MERCURY, PROTOCHLORIDE OF; Calomel; (Protochlorure de mercure,
Fr., Versüsstes quecksilber, Germ.) This compound, so much used and abused by
medical practitioners, is commonly prepared by triturating four parts of corrosive sublimate
along with three parts of running quicksilver in a marble mortar, till the metallic
globules entirely disappear, with the production of a black powder, which is to be put
into a glass balloon, and exposed to a subliming heat in a sand bath. The calomel,
which rises in vapour, and attaches itself in a crystalline crust to the upper hemisphere of
the balloon, is to be detached, reduced to a fine powder, or levigated and elutriated.
200 lbs. of mercury yield 236 of calomel and 272 of corrosive sublimate.
The following more economical process is that adopted at the Apothecaries’ Hall,
London. 140 pounds of concentrated sulphuric acid are boiled in a cast iron pot upon
100 pounds of mercury, till a dry persulphate is obtained. Of this salt, 124 pounds
are triturated with 81 pounds of mercury, till the globules disappear, and till a protosulphate
be formed. This is to be intimately mixed with 68 pounds of sea-salt, and the
mixture, being put into a large stone-ware cucurbit, is to be submitted to a subliming
heat. See Calomel.
From 190 to 200 pounds of calomel rise in a crystalline cake, as in the former process,
into the capital; while sulphate of soda remains at the bottom of the alembic.
The calomel must be ground to an impalpable powder, and elutriated. The vapours,
instead of being condensed into a cake within the top of the globe or in a capital, may
be allowed to diffuse themselves into a close vessel, containing water in a state of ebullition,
whereby the calomel is obtained at once in the form of a washed impalpable
powder. Calomel is tasteless and insoluble in water. Its specific gravity is 7·176.
For the compound of mercury with fulminic acid, see Fulminate. Periodide of
mercury is a bright but fugitive red pigment. It is easily prepared by dropping a solution[812]
of iodide of potassium into a solution of corrosive sublimate, as long as any precipitation
takes place, decanting off the supernatant muriate of potash, washing and
drying the precipitate.
METALLURGY (Erzkunde, Germ.) is the art of extracting metals from their ores.
This art, which supplies industry with the instruments most essential to its wants,
is alike dependent upon the sciences of chemistry and mechanics; upon the former,
as directing the smelting processes, best adapted to disentangle each metal from its
mineralizer; upon the latter, as furnishing the means of grinding the ores, and separating
the light stony parts from the rich metallic matter.
Notwithstanding the striking analogy which exists between common chemical and
metallurgic operations, since both are employed to insulate certain bodies from others,
there are essential differences which should be carefully noted. In the first place,
the quantity of materials being always very great in metallurgy, requires corresponding
adaptations of apparatus, and often produces peculiar phenomena; in the second place,
the agents to be employed for treating great masses, must be selected with a view to
economy, as well as to chemical action. In analytical chemistry, the main object being
exactness of result, and purity of product, little attention is bestowed upon the value of
the reagents, on account of the small quantity required for any particular process. But
in smelting metals upon the great scale, profit being the sole object, cheap materials and
easy operations alone are admissible.
The metallic ores as presented by nature, are almost always mixed with a considerable
number of foreign substances; and could not therefore be advantageously submitted to
metallurgic operations, till they are purified and concentrated to a certain degree by
various methods.
OF THE PREPARATION OF ORES FOR THE SMELTING HOUSE.
There are two kinds of preparation; the one termed mechanical, from the means
employed, and the results obtained, consists in processes for breaking and grinding the
ores, and for washing them so as to separate the vein-stones, gangues, or other mixed
earthy matters, in order to insulate or concentrate the metallic parts.
Another kind of preparation, called chemical, has for its object to separate, by means
of fire, various volatile substances combined in the ores, and which it is requisite to
clear away, at least in a certain degree, before trying to extract the metals they may
contain.
Lastly, an indispensable operation in several circumstances, is to discover, by simple
and cheap methods, called assays, the quantity of metal contained in the different species
of ores to be treated.
This head of our subject, therefore, falls under three subdivisions:—
§ 1. The mechanical preparation of ores, including picking, stamping, and different
modes of washing.
§ 2. The chemical preparation, consisting especially in the roasting or calcination of
the ores.
§ 3. The assay of ores, comprehending the mechanical part: that is, by washing; the
chemical part, or assays by the dry way; and the assays by the moist way.
Section 1. Of the mechanical preparation or dressing of ores.—I. The first picking
or sorting takes place in the interior, or underground, workings, and consists in separating
the fragments of rocks, that apparently contain no metallic matter, from those
that contain more or less of it. The external aspect guides this separation; as also
the feeling of density in the hand.
The substances when turned out to the day, undergo another sorting, with greater or
less care, according to the value of the included metal. This operation consists in
breaking the lumps of ore with the hammer, into fragments of greater or less size, usually
as large as the fist, whereby all the pieces may be picked out and thrown away that
contain no metal, and even such as contain too little to be smelted with advantage.
There is for the most part, a building erected near the output of the mine, in which the
breaking and picking of the ores are performed. In a covered gallery, or under a shed,
banks of earth are thrown up, and divided into separate beds, on each of which a thick
plate of cast iron is laid. On this plate, elderly workmen, women, and children, break
the ores with hand hammers, then pick and sort them piece by piece. The matters so
treated, are usually separated into three parts; 1. the rock or sterile gangue, which is
thrown away; 2. the ore for the stamping mill, which presents too intimate a mixture
of rock and metallic substance to admit of separation by breaking and picking; and
3. the pure ore, or at least the very rich portion, called the sorted mine or the fat ore.
On the sorting floors there remains much small rubbish, which might form a fourth
subdivision of ore, since it is treated in a peculiar manner, by sifting, as will be presently
mentioned.
The distribution of fragments more or less rich, in one class or another, is relative to the
value of the included metal, taking into account the expenses necessary for its extraction.[813]
Thus in certain lead mines, pieces of the gangues are thrown away, which judged by the
eye may contain 3 per cent. of galena, because it is known that the greater portion of
this would be lost in the washings required for separating the 97 parts of the gangue,
and that the remainder would not pay the expenses.
II. The very simple operations of picking are common to almost all ores; but there
are other operations requiring more skill, care, and expense, which are employed in
their final state of perfection only upon ores of metals possessing a certain value, as those
of lead, silver, &c. We allude to the washing of ores.
The most simple and economical washings are those that certain iron ores, particularly
the alluvial, are subjected to, as they are found near the surface of the ground agglutinated
in great or little pieces. It is often useful to clean these pieces, in order to pick
out the earthy lumps, which would be altogether injurious in the furnaces.
This crude washing is performed sometimes by men stirring in the midst of a stream
of water, with iron rakes or shovels, the lumps of ore placed in large chests, or basins of
wood or iron.
In other situations, this washing is executed more economically by a machine called
a buddle or dolly-tub by our miners. A trough of wood or iron, with a concave bottom,
is filled with the ore to be washed. Within the tub or trough, arms or iron handles
are moved round about, being attached to the arbor of a hydraulic wheel. The trough
is kept always full of water, which as it is renewed carries off the earthy matters,
diffused through it by the motion of the machine, and the friction among the pieces of
the ore. When the washing is finished, a door in one of the sides of the trough is
opened, and the current removes the ore into a more spacious basin, where it is subjected
to a kind of picking. It is frequently indeed passed through sieves in different modes.
See Lead and Tin, for figures of buddles and dollies.
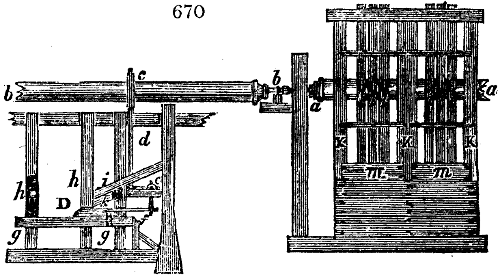
III. Stamping. Before describing the refined methods of washing the more valuable
ores of copper, silver, lead, &c., it is proper to point out the means of reducing them
into a powder of greater or less fineness, by stamping, so called from the name stamps of
the pestles employed for that purpose. Its usefulness is not restricted to preparing the
ores; for it is employed in almost every smelting house for pounding clays, charcoal,
scoriæ, &c. A stamping mill or pounding machine, fig. 670., consists of several
movable pillars of wood l l l,
placed vertically, and supported
in this position between frames
of carpentry K K K. These pieces
are each armed at their under end
with a mass of iron m. An arbor
or axle a a, moved by water, and
turning horizontally, tosses up
these wooden pestles, by means
of wipers or cams, which lay
hold of the shoulders of the pestles
at l l l. These are raised in
succession, and fall into an oblong
trough below m m, scooped out in the ground, having its bottom covered either
with plates of iron or hard stones. In this trough, beneath these pestles, the ore to be
stamped is allowed to fall from a hopper above, which is kept constantly full.
The trough is closed in at the sides by two partitions, and includes three or four
pestles; which the French miners call a battery. They are so disposed that their ascent
and descent take place at equal intervals of time.
Usually a stamping machine is composed of several batteries (two, three, or four), and
the arrangement of the wipers on the arbor of the hydraulic wheel is such that there is
constantly a like number of pestles lifted at a time; a circumstance important for
maintaining the uniform going of the machine.
The matters that are not to be exposed to subsequent washing are stamped dry, that
is without leading water into the trough; and the same thing is sometimes done with
the rich ores, whose lighter parts might otherwise be lost.
Most usually, especially for ores of lead, silver, copper, &c., the trough of the stamper
is placed in the middle of a current of water, of greater or less force; which, sweeping
off the pounded substances, deposits them at a greater or less distance onwards, in the
order of the size and richness of the grain; constituting a first washing, as they escape
from beneath the pestles.
In the dry stamping, the fineness of the powder depends on the weight of the pestles,
the height of their fall, and the period of their action upon the ore; but in the stampers
exposed to a stream of water, the retention of the matters in the trough is longer or
shorter, according to the facility given for their escape. Sometimes these matters flow
out of the chest over its edges, and the height of the line they must surmount has an
influence on the size of the grain; at other times, the water and the pounded matter[814]
which it carries off, are made to pass through a grating, causing a kind of sifting at the
same time. There are, however, some differences in the results of these two methods.
Lastly, the quantity of water that traverses the trough, as well as its velocity, has an
influence on the discharge of the pounded matters, and consequently on the products of
the stampers.
The size of the particles of the pounded ore being different, according to the variable
hardness of the matters which compose them, suggests the means of classing them, and
distributing them nearly in the order of their size and specific gravity, by making the
water, as it escapes from the stamping trough, circulate in a system of canals called a
labyrinth, where it deposits successively, in proportion as it loses its velocity, the earthy
and metallic matters it had floated along. These metalliferous portions, especially when
they have a great specific gravity like galena, would be deposited in the first passages,
were it not that from their hardness being inferior to that of the gangue, they are reduced
to a much finer powder, or into thin plates, which seem to adhere to both the
watery and earthy particles; whence they have to be sought for among the finest portions
of the pulverised gangue, called slime, schlich, or schlamme.
There are several methods of conducting the stamps; in reference to the size of the
grains wished to be obtained, and which is previously determined agreeably to the
nature of the ore, and of the gangue; its richness, &c. The height of the slit that
lets the pounded matters escape, or the diameters of the holes in the grating, their distance,
the quantity of water flowing in, its velocity, &c., modify the result of the
stamping operation.
When it is requisite to obtain powder of an extreme fineness, as for ores that are
to be subjected to the process of amalgamation, they are passed under millstones, as in
common corn mills; and after grinding, they are bolted so as to form a species of flour;
or they are crushed between rolls. See Lead and Tin.
Washing of ores.
IV. The ores pounded under the stamps are next exposed to very delicate operations,
both tedious and costly, which are called the washings. Their purpose is to separate
mechanically the earthy matters from the metallic portion, which must therefore have a
much higher specific gravity; for otherwise, the washing would be impracticable.
The medium employed to diminish the difference of specific gravity, and to move
along the lightest matters, is water; which is made to flow with greater or less velocity
and abundance over the schlich or pasty mud spread on a table of various inclination.
But as this operation always occasions, not only considerable expense, but a certain
loss of metal, it is right to calculate what is the degree of richness below which washing
is unprofitable; and on the other hand, what is the degree of purification of the schlich
at which it is proper to stop, because too much metal would be lost comparatively with
the expense of fusing a small additional quantity of gangue. There cannot, indeed, be
any fixed rule in this respect, since the elements of these calculations vary for every work.
Before describing the different modes of washing, we must treat of the sifting or
riddling, whose purpose, like that of the labyrinth succeeding the stamps, is to distribute
and to separate the ores (which have not passed through the water stamps) in the order
of the coarseness of grain. This operation is practised particularly upon the debris of
the mine, and the rubbish produced in breaking the ores. These substances are put into
a riddle, or species of round or square sieve, whose bottom is formed of a grating instead
of a plate of metal pierced with holes. This riddle is plunged suddenly and repeatedly
into a tub or cistern filled with water. This liquid enters through the bottom, raises up
the mineral particles, separates them and keeps them suspended for an instant, after
which they fall down in nearly the order of their specific gravities, and are thus classed
with a certain degree of regularity. The sieve is sometimes dipped by the immediate
effort of the washer; sometimes it is suspended to a swing which the workman moves;
in order that the riddling may be rightly done, the sieve should receive but a single
movement from below upwards; in this case the ore is separated from the gangue, and if
there be different specific gravities, there is formed in the sieve as many distinct strata,
which the workman can easily take out with a spatula, throwing the upper part away
when it is too poor to be re-sifted. This operation by the hand-sieve, is called riddling
in the tub, or riddling by deposit.
We may observe, that during the sifting, the particles which can pass across the holes of
the bottom, fall into the tub and settle down there; whence they are afterwards gathered
out, and exposed to washing when they are worth the trouble.
Sometimes, as at Poullaouen, the sieves are conical, and held by means of two handles
by a workman; and instead of receiving a single movement, as in the preceding method,
the sifter himself gives them a variety of dexterous movements in succession. His
object is to separate the poor portions of the ore from the richer; in order to subject the
former to the stamp mill.
Among the siftings and washings which ores are made to undergo, we must notice[815]
among the most useful and ingenious, those practised by iron gratings, called on the Continent
grilles anglaises, and the step-washings of Hungary, laveries à gradins. These methods
of freeing the ores from the pulverulent earthy matters, consist in placing them,
at their out-put from the mine, upon gratings, and bringing over them a stream of water,
which merely takes down through the bars the small fragments, but carries off the pulverulent
portions. The latter are received in cisterns, where they are allowed to rest
long enough to settle to the bottom. The washing by steps is an extension of the preceding
plan. To form an idea, let us imagine a series of grates placed successively at different
levels, so that the water, arriving on the highest, where the ore for washing lies, carries
off a portion of it, through this first grate upon a second closer in its bars, thence to a
third, &c., and finally into labyrinths or cisterns of deposition.
The grilles anglaises are similar to the sleeping tables used at Idria. The system of these
en gradins is represented in fig. 671. There are 5 such systems in the works at Idria, for
the sorting of the small morsels of quicksilver ore, intended for the stamping mill. These
fragments are but moderately rich in metal, and are picked up at random, of various
sizes, from that of the fist to a grain of dust.
These ores are placed in the chest a, below the level of which 7 grates are distributed,
so that the fragments which pass through the first b, proceed by an inclined conduit on
to the second grate c, and so in succession. (See the conduits l, o, p). In front, and
on a level with each of the grates b, c, d, &c., a child is stationed on one of the floors,
1, 2, 3, to 7.
A current of water, which falls into the chest a, carries the fragments of ore upon the
grates. The pieces which remain upon the two grates b and c, are thrown on the adjoining
table v, where they undergo a sorting by hand; there the pieces are classified, 1. into
gangue to be thrown away; 2. into ore for the stamp mill; 3. into ore to be sent directly
to the furnace. The pieces which remain on each of the succeeding grates, d, e, f, g, h,
are deposited on those of the floors 3 to 7, in front of each. Before every one of
these shelves a deposit-sieve is established, (see t, u,) and the workmen in charge of it
stand in one of the corresponding boxes, marked 8 to 12. The sieve is represented only
in front of the chest h, for the sake of clearness.
Each of the workmen placed in 8, 9, 10, 11, 12, operates on the heap before him;
the upper layer of the deposit formed in his sieve, is sent to the stamping house, and the
inferior layer directly to the furnace.
As to the grains which, after traversing the five grates, have arrived at the chest x,
they are washed in the two chests y, which are analogous to the German chests to be
presently described. The upper layer of what is deposited in y is sent to the furnace;
the rest is treated anew on three tables of percussion, similar to the English brake-sieves,
also to be presently described.
After several successive manipulations on these tables, an upper stratum of schlich is
obtained fit for the furnace; an intermediate stratum, which is washed anew by the same
process; and an inferior stratum, that is sent to the system of stamps, fig. 672.
This figure represents the general ground plan of a stamping and washing mill. The
stamps F are composed of two batteries similar to fig. 670. The ore passes in succession
under three pestles of cast iron, each of which is heavier the nearer it is to the
sieve through which the sand or pounded matter escapes.
In the upper part of the figure we see issuing from the stamps, two conduits destined
to receive the water and the metalliferous sand with which it is loaded. The first, marked
F, S, w, is used only when a certain quality of ore is stamped, richer in metal than is[816]
usually treated by means of the second conduit, the first being closed. The second conduit,
or that employed for ordinary manipulation when the other is shut, is indicated by
F, 0·7, B; then by 0·58 and 0·29. These numbers express the depth of the corresponding
portions of this conduit. From F to B, the conduit or water-course is divided into three
portions much shallower, called the rich conduit, the middle conduit, and the inferior.
Beyond the basin B, the conduit takes the name of labyrinth. There the muddy sediments
of ore are deposited; being the finer the further they are from the stamps F.
Darts indicate the direction of the stream in the labyrinth. On the German chests, placed
at 3, the sand derived from the rich and middle conduits is treated, in order to obtain
three distinct qualities of schlich, as already mentioned. P is a cloth-covered table, for
treating the deposit of the German chests at 3. M N are two sweep tables (à balai), for
treating the ore collected in the lower conduit, which precedes the midmost of the three
German chests. Upon the three similar tables R T V, are treated in like manner the muddy
deposits of the labyrinth, which forms suite to three parallel German chests situated at 3,
not shown for want of room in the figure, but connected in three rectangular zigzags
with each other, as well as by a transverse branch to the points 0·7 and P. At the upper
part of these five sweep tables, the materials which are to undergo washing are agitated
in two boxes O O, by small paddle-wheels.
We shall now describe the percussion-tables used in the Hartz, for treating the sand
of ore obtained from the conduits represented above.
Figs. 673, 674. and 675. exhibit a plan, a vertical section, and elevation, of one
of these tables, taken in the
direction of its length. The
arbor or great shaft in prolongation
from the stamps mill, is
shown in section perpendicularly
to its axis, at A. The
cams or wipers are shown
round its circumference, one of
them having just acted on n.
These cams, by the revolution
of the arbor, cause the
alternating movements of a
horizontal bar of wood o, u,
which strikes at the point u
against a table d, b, c, u. This
table is suspended by two chains
t, at its superior end, and by
two rods at its lower end.
After having been pushed by
the piece o, u, it rebounds to
strike against a block or
bracket B. A lever p, q, serves
to adjust the inclination of the
movable table, the pivots q
being points of suspension.
The ore-sand to be washed,
is placed in the chest a, into
which a current of water runs.
The ore floated onwards by the
water, is carried through a
sieve on a sloping small table
x, under which is concealed
the higher end of the movable
table d, b, c, u; and it thence
falls on this table, diffusing itself
uniformly over its surface. The
particles deposited on this table
form an oblong talus (slope)
upon it; the successive percussions
that it receives, determine
the weightier matters, and
consequently those richest in
metal, to accumulate towards
its upper end at u. Now the
workman by means of the lever
p, raises the lower end d a little
in order to preserve the same[817]
degree of inclination to the surface on which the deposit is strewed. According as the
substances are swept along by the water, he is careful to remove them from the middle
of the table towards the top, by means of a wooden roller. With this intent, he walks on
the table d b c u, where the sandy sediment has sufficient consistence to bear him. When
the table is abundantly charged with the washed ore, the deposit is divided into three
bands or segments d b, b c, c u. Each of these bands is removed separately and thrown
into the particular heap assigned to it. Every one of the heaps thus formed becomes
afterwards the object of a separate manipulation on a percussion table, but always according
to the same procedure. It is sufficient in general to pass twice over this table the matters
contained in the heap, proceeding from the superior band c u, in order to obtain a pure
schlich; but the heap preceding from the intermediate belt b c, requires always a greater
number of manipulations, and the lower band d b still more. These successive manipulations
are so associated that eventually each heap furnishes pure schlich, which is
obtained from the superior band c u. As to the lightest particles which the water
sweeps away beyond the lower end of the percussion table, they fall into conduits;
whence they are lifted to undergo a new manipulation.
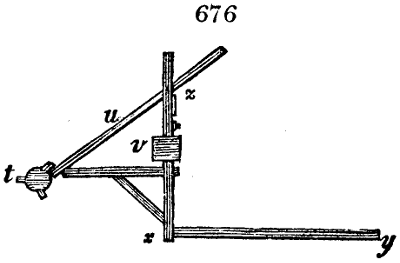
Fig. 676. is a profile of a plan which has been advantageously substituted, in the
Hartz, for that part of the preceding apparatus which causes the jolt of the piece o u
against the table d b c u. By means of this plan, it is easy to vary, according to the
circumstances of a manipulation always delicate, the force of percussion which a bar
x y, ought to communicate by its extremity y. With this view, a slender piece of wood
u is made to slide in an upright piece, v x, adjusted upon an axis at v. To the piece u
a rod of iron is connected, by means of a hinge z; this rod is capable of entering more
or less into a case or sheath in the middle of the piece v x, and of being stopped at the
proper point, by a thumb-screw which presses against this piece. If it be wished to
increase the force of percussion, we must lower the point z; if to diminish it, we must
raise it. In the first case, the extremity of the piece u, advances so much further under
the cam of the driving shaft t; in the second, it goes so much less forwards; whereby
the adjustment is produced.
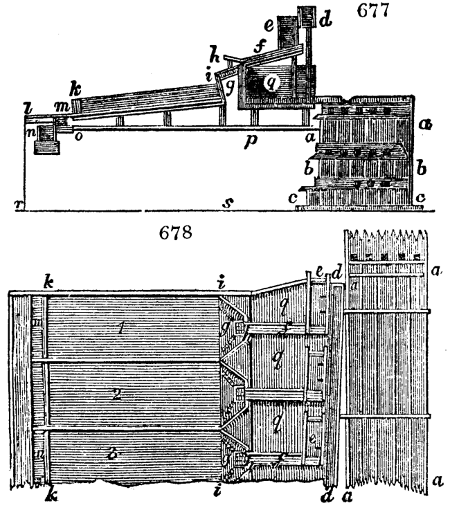
Figs. 677. and 678. represent a complete system of sleeping tables, tables dormantes;
such as are mounted in Idria.
Fig. 678. is the plan, and fig. 677. a
vertical section. The mercurial ores,
reduced to a sand by stamps like
those of fig. 672., pass into a series
of conduits a a, b b, c c, which form
three successive floors below the level
of the floor of the works. The sand
taken out of these conduits is thrown
into the cells q; whence they are
transferred into the trough e, and
water is run upon them by turning
two stopcocks for each trough. The
sand thus diffused upon each table,
runs off with the water by a groove f, comes upon a sieve h, spreads itself upon the
board g, and thence falls into the slanting chest, or sleeping table i k. The under surface
k of this chest is pierced with holes, which may be stopped at pleasure with wooden
plugs. There is a conduit m, at the lower end of each table, to catch the light particles
carried off by the water out of the chest i k, through the holes properly opened,
while the denser parts are deposited upon the bottom of this chest. A general conduit
n passes across at the foot of all the chests i k; it receives the refuse of the washing
operations.
Fig. 679. is a set of stamping and washing works for the ores of argentiferous galena,
as mounted at Bockwiese, in the district of Zellerfeldt, in the Hartz.
A is the stamp mill and its subsidiary parts; among which are a, the driving or[818]
main shaft; b, the overshot water-wheel; c c, six strong rings or hoops of cast iron,
for receiving each a cam or tappet; g, the brake of the machine; k, k, k, the three
standards of the stamps; l l, &c. six pestles of pine
wood, shod with lumps of cast iron. There are
two chests, out of which the ore to be ground falls
spontaneously into the two troughs of the stamps.
Of late years, however, the ore is mostly supplied
by hand; the watercourse terminates a short distance
above the middle of the wheel b. There is
a stream of water for the service of the stamps,
and conduits proceeding from it, to lead the water
into the two stamp troughs; the conduit of discharge
is common to the two batteries or sets of
stamps through which the water carries off the
sand or stamped ore. There is a movable table
of separation, mounted with two sieves. The
sands pass immediately into the conduit placed
upon a level with the floor, and separated into
two compartments, the first of which empties its
water into the second. There are two boards of
separation, or tables, laid upon the ground, with
a very slight slope of only 15 inches from their
top to their bottom. Each of these boards is
divided into four cases with edges; the whole
being arranged so that it is possible, by means of
a flood-gate or sluice, to cause the superfluous
water of the case to pass into the following ones.
Thus the work can go on without interruption,
and alternately upon the two boards. There are
winding canals in the labyrinth, N, N, N, in which are
deposited the particles carried along by the water
which has passed upon the boards. The depth of
these canals gradually increases from 12 to 20
inches, to give a suitable descent for maintaining
the water-flow. At D, two percussion tables are
placed. F G are two German chests. H J are
two percussion tables, which are driven by the
cams z z, fixed upon the main shaft x y. K K′
are two sloping sweep tables (à balai).
The German chests are rectangular, being
about 3 yards long, half a yard broad, with edges
half a yard high; and their inclination is such
that the lower end is about 15 inches beneath
the level of the upper. At their upper end,
usually called the bolster, a kind of trough or
box, without any edge at the side next the chest, is placed, containing the ore to be
washed. The water is allowed to fall upon the bolster in a thin sheet.
The sleeping tables have upright edges; they are from 4 to 5 yards long, nearly 2
yards wide, and have fully a yard of inclination.
The preceding tables are sometimes covered with cloth, particularly in treating ores
that contain gold, on a supposition that the woollen or linen fibres would retain more
surely the metallic particles; but this method appears on trial to merit no confidence,
for it produces a very impure schlich.
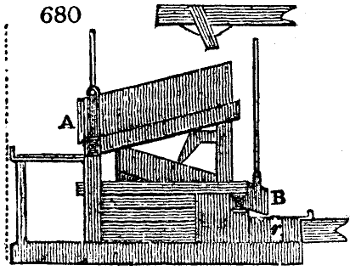
Fig. 680. is a swing-sieve employed in the Hartz, for sifting the small fragments of
the ore of argentiferous lead. Such an apparatus is usually set up in the outside of
a stamp, and washing mill; its place being denoted
by the letter A, fig. 672. The two movable chests or
boxes A B, of the sieve, are connected together, at their
lower ends, with an upright rod, which terminates at
one of the arms of a small balance beam, mounted between
the driving shaft of the stamps and the sieve,
perpendicularly to the length of both. The opposite
arm of this beam carries another upright rod, which
ears (cams or mentonnets), placed on purpose upon the
driving shaft, may push down. During this movement
the two lower ends A, B, are raised; and when the peg-cam of the shaft quits the
rod which it had depressed, the swing chests fall by their own weight. Thus they[819]
are made to vibrate alternately upon their axes. The small ore is put into the upper
part of the chest A, over which a stream of water falls from an adjoining conduit. The
fragments which cannot pass through a cast-iron grid in the bottom of that chest,
are sorted by hand upon a table in front of A, and they are classed by the workman,
either among the ores to be stamped, whether dry or wet, or among the rubbish to be
thrown away, or among the copper ores to be smelted by themselves. As to the small
particles which fall through the grid upon the chest B, supplied also with a stream
of water, they descend successively upon two other brass wire sieves, and also through
the iron wire r, in the bottom of B.
In certain mines of the Hartz, tables called à balais, or sweeping tables, are employed.
The whole of the process consists in letting flow, over the sloping table, in successive
currents, water charged with the ore, which is deposited at a less or greater distance,
as also pure water for the purpose of washing the deposited ore, afterwards carried off
by means of this sweeping operation.
At the upper end of these sweep-tables, the matters for washing are agitated in a
chest, by a small wheel with vanes, or flap-boards. The conduit of the muddy waters
opens above a little table or shelf; the conduit of pure water, which adjoins the
preceding, opens below it. At the lower part of each of these tables, there is a
transverse slit, covered by a small door with hinges, opening outwardly, by falling back
towards the foot of the table. The water spreading over the table, may at pleasure
be let into this slit, by raising a bit of leather which is nailed to the table, so as to
cover the small door when it is in the shut position; but when this is opened, the piece
of leather then hangs down into it. Otherwise the water may be allowed to pass freely
above the leather, when the door is shut. The same thing may be done with a similar
opening placed above the conduit. By means of these two slits, two distinct qualities
of schlich may be obtained, which are deposited into two distinct conduits or canals.
The refuse of the operation is turned into another conduit, and afterwards into ulterior
reservoirs, whence it is lifted out to undergo a new washing.
In the percussion tables, the water for washing the ores is sometimes spread in
slender streamlets, sometimes in a full body, so as to let two cubic feet escape per
minute. The number of shocks communicated per minute, varies from 15 to 36; and
the table may be pushed out of its settled position at one time, three quarters of an
inch, at another nearly 8 inches. The coarse ore-sand requires in general less water,
and less slope of table, than the fine and pasty sand.
The mechanical operations which ores undergo, take place commonly at their out-put
from the mine, and without any intermediate operation. Sometimes, however, the
hardness of certain gangues (vein-stones), and of certain iron-ores, is diminished by
subjecting them to calcination previously to the breaking and stamping processes.
When it is intended to wash certain ores, an operation founded on the difference of
their specific gravities, it may happen that by slightly changing the chemical state of
the substances that compose the ore, the earthy parts may become more easily separable,
as also the other foreign matters. With this view, the ores of tin are subjected to a
roasting, which by separating the arsenic, and oxidizing the copper which are intermixed,
furnishes the means of obtaining, by the subsequent washing, an oxide of tin
much purer than could be otherwise procured. In general, however, these are rare
cases; so that the washing almost always immediately succeeds the picking and stamping;
and the roasting comes next, when it needs to be employed.
The operation of roasting is in general executed by various processes, relatively to the
nature of the ores, the quality of the fuel, and to the object in view. The greatest
economy ought to be studied in the fuel, as well as the labour; two most important
circumstances, on account of the great masses operated upon.
Three principal methods may be distinguished; 1. the roasting in a heap in the open
air, the most simple of the whole; 2. the roasting executed between little walls, and
which may be called case-roasting (rost-stadeln, in German); and 3. roasting in
furnaces.
We may remark, as to the description about to be given of these different processes,
that in the first two, the fuel is always in immediate contact with the ore to be roasted,
whilst in furnaces, this contact may or may not take place.
1. The roasting in the open air, and in heaps more or less considerable, is practised
upon iron ores, and such as are pyritous or bituminous. The operation consists in general
in spreading over a plane area, often bottomed with beaten clay, billets of wood arranged
like the bars of a gridiron, and sometimes laid crosswise over one another, so as to form a
uniform flat bed. Sometimes wood charcoal is scattered in, so as to fill up the interstices,
and to prevent the ore from falling between the other pieces of the fuel. Coal is also
employed in moderately small lumps; and even occasionally, turf. The ore either
simply broken into pieces, or even sometimes under the form of schlich, is piled up over
the fuel; most usually alternate beds of fuel and ore are formed.
[820]
The fire, kindled in general at the lower part, but sometimes, however, at the middle
chimney, spreads from spot to spot, putting the operation in train. The combustion
must be so conducted as to be slow and suffocated, to prolong the ustulation, and let the
whole mass be equably penetrated with heat. The means employed to direct the fire,
are to cover outwardly with earth the portions where too much activity is displayed,
and to pierce with holes or to give air to those where it is imperfectly developed. Rains,
winds, variable seasons, and especially good primary arrangements of a calcination, have
much influence on this process, which requires, besides, an almost incessant inspection at
the beginning.
Nothing in general can be said as to the consumption of fuel, because it varies with
its quality, as well as with the ores and the purpose in view. But it may be laid down
as a good rule, to employ no more fuel than is strictly necessary for the kind of calcination
in hand, and for supporting the combustion; for an excess of fuel would produce,
besides an expense uselessly incurred, the inconvenience, at times very serious, of such
a heat as may melt or vitrify the ores; a result entirely the reverse of a well-conducted
ustulation.
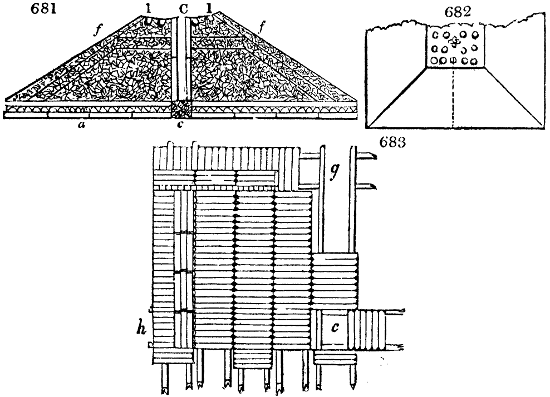
Figs. 681, 682, 683. represent the roasting in mounds, as practised near Goslar in the
Hartz, and at Chessy in the department of the Rhone. Fig. 681. is a vertical section
in the line h c of figs. 682. and 683. In fig. 682. there is shown in plan, only a little
more than one half of the quadrangular truncated pyramid, which constitutes the heap.
Fig. 683. shows a little more than one fourth of a bed of wood, arranged at the bottom
of the pyramid, as shown by a a, fig. 681., and c g h, fig. 683. C is a wooden chimney,
formed within the heap of ore, at whose bottom c
there is a little parcel of charcoal, d d are large
lumps of ore distributed upon the wooden pile
a a; e e are smaller fragments, to cover the larger;
f f is rubbish and clay laid smoothly in a slope
over the whole. g, fig. 683., a passage for air
left under the bed of billets; of which there is
a similar one in each of the four sides of the base
a a, so that two principal currents of air cross
under the upright axis C c, of the truncated pyramid
indicated in fig. 681.
The kindling is thrown in by the chimney C.
The charcoal c, and the wood a a, take fire; the
sulphureous ores d e f are heated to such a high
temperature as to vaporize the sulphur. In the
Lower Hartz, a heap of this kind continues roasting during four months.
2. The second method. The difficulty of managing the fire in the roasting of substances
containing little sulphur, with the greater difficulty of arranging and supporting
in their place the schlichs to be roasted, and last of all, the necessity of giving successive
fires to the same ores, or to inconsiderable quantities at a time, have led to the contrivance
of surrounding the area on which the roasting takes place with three little walls,
or with four, leaving a door in the one in front. This is what is called a walled area,
and sometimes, improperly enough, a roasting furnace. Inside of these little walls,
about 3 feet high, there are often vertical conduits or chimneys made to correspond with
an opening on the ground level, in order to excite a draught of air in the adjacent parts.
When the roasting is once set agoing, these chimneys can be opened or shut at their
upper ends, according to the necessities of the process.
Several such furnaces are usually erected in connexion with each other by their lateral
walls, and all terminated by a common wall, which forms their posterior part; sometimes
they are covered with a shed supported partly by the back wall, built sufficiently
high for this purpose. These dispositions are suitable for the roasting of schlichs, and
in general of all matters which are to have several fires; a circumstance often indispensable
to a due separation of the sulphur, arsenic, &c.
3. The furnaces employed for roasting the ores and the mattes differ much, according[821]
to the nature of the ores, and the size of the lumps. We shall content ourselves with
referring to the principal forms.
When iron ores are to be roasted, which require but a simple calcination to disengage
the combined water and carbonic acid, egg-shaped furnaces, similar to those in which
limestone is burned in contact with fuel, may be conveniently employed; and they present
the advantage of an operation which is continuous with a never-cooling apparatus.
The analogy in the effects to be produced is so perfect, that the same furnace may be
used for either object. Greater dimensions may, however, be given to those destined
for the calcination of iron ores. But it must be remembered that this process is applicable
only to ores broken into lumps, and not to ores in grains or powder.
It has been attempted to employ the same method a little modified, for the roasting of
ores of sulphuret of copper and pyrites, with the view of extracting a part of the sulphur.
More or less success has ensued, but without ever surmounting all the obstacles arising
from the great fusibility of the sulphuret of iron. For sometimes it runs into one mass,
or at least into lumps agglutinated together in certain parts of the furnace, and the
operation is either stopped altogether, or becomes more or less languid; the air not
being able to penetrate into all the parts, the roasting becomes consequently imperfect.
This inconvenience is even more serious than might at first sight appear; for, as the ill-roasted
ores now contain too little sulphur to support their combustion, and as they
sometimes fall into small fragments in the cooling, they cannot be passed again through
the same furnace, and it becomes necessary to finish the roasting in a reverberatory
hearth, which is much more expensive.
In the Pyrenees, the roasting of iron ores is executed in a circular furnace, so disposed
that the fuel is contained and burned in a kind of interior oven, above which lie
the pieces of ore to be calcined. Sometimes the vault of this oven which sustains the
ore, is formed of bricks, leaving between them openings for the passage of the flame and
the smoke, and the apparatus then resembles certain pottery kilns; at other times the
vault is formed of large lumps of ore, carefully arranged as to the intervals requisite to be
left for draught over the arch. The broken ore is then distributed above this arch, care
being taken to place the larger pieces undermost. This process is simple in the construction
of the furnace, and economical, as branches of trees, without value in the forests,
may be employed in the roasting. See Lime-kiln figures.
In some other countries, the ores are roasted in furnaces very like those in which
porcelain is baked; that is to say, the fuel is placed exteriorly to the body of the furnace
in a kind of brick shafts, and the flame traverses the broken ore with which the furnace
is filled. In such an apparatus the calcination is continuous.
When it is proposed to extract the sulphur from the iron pyrites, or from pyritous
minerals, different furnaces may be employed, among which that used in Hungary deserves
notice. It is a rectangular parallelopiped of four walls, each of them being perforated
with holes and vertical conduits which lead into chambers of condensation, where
the sulphur is collected. The ore placed between the four walls on billets of wood
arranged as in figs. 681, 682, 683., for the great roastings in the open air, is calcined
with the disengagement of much sulphur, which finds more facility in escaping by the
lateral conduits in the walls, than up through the whole mass, or across the upper surface
covered over with earth; whence it passes into the chambers of condensation. In
this way upwards of a thousand tons of pyrites may be roasted at once, and a large quantity
of sulphur obtained. See Copper.
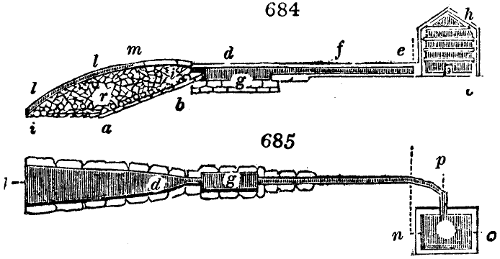
Roasting of Pyrites.—Figs. 684, 685. represent a furnace which has been long employed
at Fahlun in Sweden, and
several other parts of that kingdom,
for roasting iron pyrites in order to
obtain sulphur. This apparatus was
constructed by the celebrated Gahn.
Fig. 684. is a vertical section, in the
line k d n o of fig. 685., which is
a plan of the furnace; the top being
supposed to be taken off. In both
figures the conduit may be imagined
to to be broken off at e; its entire
length in a straight line is 43 feet
beyond the dotted line e n, before the bend, which is an extension of this conduit. Upon
the slope a b of a hillock a b c, lumps r of iron pyrites are piled upon the pieces of wood
i i for roasting. A conduit d f e forms the continuation of the space denoted by r, which
is covered by stone slabs so far as f; and from this point to the chamber h it is constructed
in boards. At the beginning of this conduit, there is a recipient g. The chamber h is
divided into five chambers by horizontal partitions, which permit the circulation of the[822]
vapours from one compartment to another. The ores r being distributed upon the
billets of wood i i, whenever these are fairly kindled, they are covered with small ore,
and then with rammed earth l l. Towards the point m, for a space of a foot square, the
ores are covered with movable stone slabs, by means of which the fire may be regulated,
by the displacement of one or more, as may be deemed necessary. The liquid sulphur
runs into the recipient g, whence it is laded out from time to time. The sublimed sulphur
passes into the conduit f e and the chamber h, from which it is taken out, and
washed with water, to free it from sulphuric acid with which it is somewhat impregnated;
it is afterwards distilled in cast-iron retorts. The residuum of the pyrites is
turned to account in Sweden, for the preparation of a common red colour much used
as a pigment for wooden buildings.
The reverberatory furnace affords one of the best means of ustulation, where it is requisite
to employ the simultaneous action of heat and atmospherical air to destroy certain
combinations, and to decompose the sulphurets, arseniurets, &c. It is likewise
evident that the facility thus offered of stirring the matters spread out on the sole, in
order to renew the surfaces, of observing their appearances, of augmenting or diminishing
the degree of heat, &c., promise a success much surer, a roasting far better
executed, than by any other process. It is known, besides, that flame mingled with
much undecomposed air issuing from the furnace, is highly oxidizing, and is very fit for
burning away the sulphur, and oxidizing the metals. Finally, this is almost the only
method of rightly roasting ores which are in a very fine powder. If it be not employed
constantly and for every kind of ore, it is just because more economy is found in practising
calcination in heaps, or on areas enclosed by walls; besides, in certain mines, a very
great number of these furnaces, and many workmen, would be required to roast the considerable
body of ores that must be daily smelted. Hence there would result from the
construction of such apparatus and its maintenance a very notable outlay, which is
saved in the other processes.
But in every case where it is desired to have a very perfect roasting, as for blende
from which zinc is to be extracted, for sulphuret of antimony, &c., or even for ores reduced
to a very fine powder, and destined for amalgamation, it is proper to perform the
operation in a reverberatory furnace. When very fusible sulphurous ores are treated,
the workman charged with the calcination must employ much care and experience, chiefly
in the management of the fire. It will sometimes, indeed, happen, that the ore partially
fuses; when it becomes necessary to withdraw the materials from the furnace, to
let them cool and grind them anew, in order to recommence the operation. The construction
of these furnaces demands no other attention than to give to the sole or
laboratory the suitable size, and so to proportion to this the grate and the chimney
that the heating may be effected with the greatest economy.
The reverberatory furnace is always employed to roast the ores of precious metals,
and especially those for amalgamation; as the latter often contain arsenic, antimony, and
other volatile substances, they must be disposed of in a peculiar manner.
The sole, usually very spacious, is divided into two parts, of which the one farthest
off from the furnace is a little higher than the other. Above the vault there is a space
or chamber in which the ore is deposited, and which communicates with the laboratory
by a vertical passage; which serves to allow the ore to be pushed down, when it is dried
and a little heated. The flame and the smoke which escape from the sole or laboratory
pass into condensing chambers, before entering into the chimney of draught, so as to
deposit in them the oxide of arsenic and other substances. When the ore on the part
of the sole farthest from the grate has suffered so much heat as to begin to be roasted,
has became less fusible, and when the roasting of that in the nearer part of the sole is
completed, the former is raked towards the fire-bridge, and its ustulation is finished by
stirring it over frequently with a paddle, skilfully worked, through one of the doors left
in the side for this purpose. The operation is considered to be finished when the vapours
and the smell have almost wholly ceased; its duration depending obviously on
the nature of the ores.
When this furnace is employed to roast very arsenical ores, as the tin ores of Schlackenwald
in Bohemia, and at Ehrenfriedersdorf in Saxony, the arsenical pyrites of Geyer
(in Saxony), &c., the chambers of condensation for the arsenious acid are much more
extensive than in the furnaces commonly used for roasting galena, copper, or even silver
ores.
Figs. 686, 687, 688. represent a reverberatory furnace employed in the smelting
works of Lautenthal, in the Hartz, for roasting the schlichs of lead ores, which contain
much blende or sulphuret of zinc. In fig. 686. we see that the two parts A B, B C, are
absolutely like, the two furnaces being built in one body of brickwork. Fig. 687. is
the plan of the furnace B C, taken at the level E F of fig. 686. Fig. 688. is a vertical
section of the similar furnace A B, taken in the prolongation of the line G H in
fig. 687.
[823]
a is the fire-place of the furnace, its grate and ash-pit. b is the conduit of vaporization,
which communicates with the chambers c; c, chambers into which the vaporized
substances are deposited; d, chimney for the escape of the smoke of the fire-place a,
after it has gone through the space b c c; e′, is the charging door, with a hook hanging
in front to rest the long iron rake upon, with which
the materials are turned over; f, chamber containing
a quantity of schlich destined for roasting; this
chamber communicates with the vaulted corridor
(gallery) D, seen in fig. 686.; g, orifice through
which the schlich is thrown into the furnace; h,
area or hearth of the reverberatory furnace, of which
the roof is certainly much too high; i, channels for
the escape of the watery vapours; k l, front arcade,
between which and the furnace, properly speaking,
are the two orifices of the conduits, which terminate
at the channels m, m′. m is the channel for
carrying towards the chimney d, the vapours which
escape by the door e′. n is a walled-up door, which
is opened from time to time, to take out of the
chambers c, c, the substances that may be deposited
in them.
At the smelting works of Lautenthal, in such a
roasting furnace, from 6 to 9 quintals (cwts.) of
schlich are treated at a time, and it is stirred frequently
with an iron rake upon the altar h. The period
of this operation is from 6 to 12 hours, according as
the schlich may be more or less dry, more or less
rich in lead, or more or less charged with blende.
When the latter substance is abundant, the process requires 12 hours, with about
60 cubic feet of cleft billets for fuel.
In such furnaces are roasted the cobalt ores of Schneeberg in Saxony, the tin ores of
Schlackenwald in Bohemia, of Ehrenfriedersdorf in Saxony, and elsewhere; as also the
arsenical pyrites at Geyer in Saxony. But there are poison towers and extensive condensing
chambers attached in the latter case. See Arsenic.
Figs. 689, 690, 691. represent the reverberatory furnace generally employed in the
Hartz, in the district of Mansfeldt, Saxony,
Hungary, &c., for the treatment of black copper,
and for refining rose copper upon the great
scale. An analogous furnace is used at Andreasberg
for the liquefaction or purification of the
mattes, and for workable lead when it is much
loaded with arsenic.
Fig. 689. presents the elevation of the furnace
parallel to the line I K, of the plan fig. 690.;
which plan is taken at the level of the tuyère n,
of fig. 691.; fig. 691. is a vertical section in the line L M, fig. 690. k represents one of
two basins of reception, brasqued with clay and charcoal; n, n, two tuyères, through which
enters the blast of two pairs of bellows, like those shown at Cupellation of Silver; q,
door by which the matter to be melted is laid upon the sole of the furnace; v, v, two
points where the sole is perforated, when necessary to run off the melted matter into
either of the basins h; x, door through which the slags or cinders floating upon the surface[824]
of the melted metal are raked out; y, door of the fire-place. The fuel is laid upon a
grate above an ash-pit, and below the arch of a reverberatory which is contiguous to
the dome or cap of the furnace properly so called. In the section, fig. 691., the following
parts may be noted: 1, 2, 3, mason-work of the foundation; 4, vapour channels
or conduits, for the escape of the humidity; 5, bed of clay; 6, brasque composed of clay
and charcoal, which forms the concavity of the hearth.
Figs. 692, 693, 694., show the
furnace employed for liquation in
one of the principal smelting works
of the Hartz. Fig. 694. exhibits
the working area charged with the
liquation cakes and charcoal, supported
by sheets of wrought iron;
being an image of the process in
action. Fig. 693. is the plan, in
the line F, G, of fig. 692.
A liquation cake is composed
of—
Black copper holding at least 5
or 6 loths (21⁄2 or 3 oz.) of silver per
cwt., and weighing 90 to 96 lbs.
Lead obtained from litharge, 2
cwts. Litharge, 1⁄2 cwt.
From 30 to 32 cakes are successively
worked in one operation,
which lasts about 5 hours; the
furnace is brought into action, as
usual, with the aid of slags; then a
little litharge is added; when the
lead begins to flow, the copper is
introduced, and when the copper
flows, lead is added, so that the
mixture of the metals may be effected
in the best way possible.
From 8 to 16 of these cakes (pains) are usually placed in the liquation furnace, figs.
692, 693, 694. The operation lasts 3 or 4 hours, in which time about 11⁄2 quintals of
charcoal are consumed. The cakes are covered with burning charcoal, supported, as I
have said, by the iron plates. The workable lead obtained flows off towards the basin
in front of the furnace; whence it is laded out into moulds set alongside. See fig. 693.
If the lead thus obtained be not sufficiently rich in silver to be worth cupellation, it is
employed to form new liquation cakes. When it contains from 5 to 6 loths of silver
per cwt., it is submitted to cupellation in the said smelting works. See Silver.
The trompe, or water-blowing engine, figs. 695, 696, 697. Fig. 695. is the elevation;
fig. 696. is a vertical section, made at right angles to the elevation. The machine is formed
of two cylindrical pipes, the bodies of the trompe b b, set upright, called the funnels, which
terminate above in a water cistern a, and below in a close basin under c, called the tub or[825]
drum. The conical part p, of the funnel has been called etranguillon, being strangled, as it
were, in order that the water discharged into the body of the trompe shall not fill the
pipe in falling, but be divided into many streamlets. Below this narrow part, eight holes,
q q, are perforated obliquely through the substance of the trompe, called the vent-holes
or nostrils, for admitting the air, which the water carries with it in its descent. The air
afterwards parts from the water, by dashing upon a cast-iron slab, placed in the drum
upon the pedestal d. An aperture l, at the bottom of the drum, allows the water to
flow away after its fall; but, to prevent the air from escaping along with it, the water
as it issues is received in a chest l m o n, divided into two parts by a vertical slide-plate
between m n. By raising or lowering this plate, the water may be maintained at any
desired level within the drum, so as to give the included air any determinate degree of
pressure. The superfluous water then flows off by the hole o.
The air-pipe e f, fig. 696., is fitted to the upper part of the drum; it is divided, at the
point f, into three tubes, of which the principal one is destined for the furnace of cupellation,
whilst the other two g g, serve for different melting furnaces. Each of these
tubes ends in a leather pocket, and an iron nose-pipe k, adjusted in the tuyère of
the furnace. At Pesey, and in the whole of Savoy, a floodgate is fitted into the upper
cistern a, to regulate the admission of water into the trome; but in Carniola, the funnel p
is closed with a wooden plug, suspended to a cord, which goes round a pulley mounted
upon a horizontal axis, as shewn in fig. 697. By the plug a being raised more or less,
merely the quantity of water required for the operation is admitted. The plug is
pierced lengthwise with an oblique hole c c, in which the small tube c is inserted, with
its top some way above the water level, through which air may be admitted into the heart
of the column descending into the trompe p q.
The ordinary height of the trompe apparatus is about 26 or 27 feet to the upper
level of the water cistern; its total length is 11 mètres (36 feet 6 inches), and its width
2 feet, to give room for the drums. It is situated 10 mètres (331⁄3 feet) from the melting
furnace. This is the case at the smelting works of Jauerberg, in Upper Carniola.
OF THE ASSAY OF ORES.
Assays ought to occupy an important place in metallurgic instructions, and there is[826]
reason to believe that the knowledge of assaying is not sufficiently diffused, since its
practice is so often neglected in smelting houses. Not only ought the assays of the
ores under treatment, to be frequently repeated, because their nature is subject to vary;
but the different products of the furnaces should be subjected to reiterated assays, at the
several periods of the operations. When silver or gold ores are in question, the docimastic
operations, then indispensable, exercise a salutary controul over the metallurgic
processes, and afford a clear indication of the quantities of precious metal which they
ought to produce.
By the title Assays, in a metallurgic point of view, is meant the method of ascertaining
for any substance whatever, not only the presence and the nature of a metal, but its
proportional quantity. Hence the operations which do not lead to a precise determination
of the metal in question, are not to be arranged among the assays now under
consideration. Experiments made with the blow-pipe, although capable of yielding
most useful indications, are like the touchstone in regard to gold, and do not constitute
genuine assays.
Three kinds of assays may be practised in different circumstances, and with more or
less advantage upon different ores. 1. The mechanical assay; 2. the assay by the dry
way; 3. the assay by the humid way.
1. Of mechanical assays.—These kinds of assays consist in the separation of the
substances mechanically mixed in the ores, and are performed by a hand-washing, in a
small trough of an oblong shape, called a sebilla. After pulverizing with more or less
pains the matters to be assayed by this process, a determinate weight of them is put into
this wooden bowl with a little water; and by means of certain movements and some
precautions, to be learned only by practice, the lightest substances may be pretty exactly
separated, namely, the earthy gangues from the denser matter or metallic particles, without
losing any sensible portion of them. Thus a schlich of greater or less purity will be
obtained, which may afford the means of judging by its quality of the richness of the
assayed ores, and which may thereafter be subjected to assays of another kind, whereby
the whole metal may be insulated.
Washing, as an assay, is practised on auriferous sands; on all ores from the stamps, and
even on schlichs already washed upon the great scale, to appreciate more nicely the degree
of purity they have acquired. The ores of tin in which the oxide is often disseminated
in much earthy gangue, are well adapted to this species of assay, because the tin oxide
is very dense. The mechanical assay may also be employed in reference to the ores
whose metallic portion presents an uniform composition, provided it also possesses considerable
specific gravity. Thus the ores of sulphuret of lead (galena) being susceptible
of becoming almost pure sulphurets (within 1 or 2 per cent.) by mere washing skilfully
conducted, the richness of that ore in pure galena, and consequently in lead, may be at
once concluded; since 120 of galena contain 104 of lead, and 16 of sulphur. The sulphuret
of antimony mingled with its gangue may be subjected to the same mode of
assay, and the result will be still more direct, since the crude antimony is brought into
the market after being freed from its gangue by a simple fusion.
The assay by washing is also had recourse to for ascertaining if the scoriæ or other
products of the furnaces contain some metallic grains which might be extracted from
them by stamping and washing on the great scale; a process employed considerably
with the scoriæ of tin and copper works.
Of assays by the dry way.—The assay by the dry way has for its object, to show
the nature and proportion of the metals contained in a mineral substance. To make a
good assay, however, it is indispensably necessary to know what is the metal associated
with it, and even within certain limits, the quantity of the foreign bodies. Only one
metal is commonly looked after; unless in the case of certain argentiferous ores. The
mineralogical examination of the substances under treatment, is most commonly sufficient
to afford data in these respects; but the assays may always be varied with different
views, before stopping at a definite result; and in every instance, only such assays can
be confided in, as have been verified by a double operation.
This mode of assaying requires only a little experience, with a simple apparatus; and
is of such a nature as to be practised currently in the smelting works. The air furnace
and crucibles employed are described in all good elementary chemical books. These
assays are usually performed with the addition of a flux to the ore, or some agent for
separating the earthy from the metallic substances; and they possess a peculiar advantage
relative to the smelting operations, because they offer many analogies between
results on the great scale and experiments on the small. This may even enable us often
to deduce, from the manner in which the assay has succeeded with a certain flux, and
at a certain degree of heat, valuable indications as to the treatment of the ore in the
great way. See Furnace.
In the smelting houses which purchase the ore, as in Germany, it is necessary to
bestow much attention upon the assays, because they serve to regulate the quality and[827]
the price of the schlichs to be delivered. These assays are not by any means free from
difficulties, especially when ores containing several useful metals are treated, and which
are to be dosed or proportioned; ores, for example, including a notable quantity of
lead, copper, and silver, mixed together.
In the central works of the Hartz, as well as in those of Saxony, the schlichs as delivered
are subjected to docimastic assays, which are verified three times, and by three
different persons, one of whom is engaged for the interests of the mining partners,
another for that of the smelting house, and a third as arbiter in case of a difference. If
the first two results of assaying differ by 1⁄2 loth (or 1⁄4 ounce) of silver per cwt. of schlich,
the operations must be resumed; but this rarely happens. When out of the three assays,
the one differs from the two others by no more than 1⁄4 loth of silver per cwt., but by
more in one, and by less in another, the mean result is adopted. As to the contents of the
schlich in lead, the mean results of the assays must be taken. The differences allowed,
are three pounds for the schlich, when it contains from 12 to 30 per cent. of lead, increasing
to six pounds for schlich, when it contains less than 55 per cent. of that metal.
Assaying forms in great establishments, an important object in reference to time and
expense. Thus, in the single work of Franckenscharn, in the Hartz, no less than 300
assays have to be made in a threefold way, every Monday, without taking into account
the several assays of the smelting products which take place every Thursday. Formerly
fluxes more or less compound were employed for these purposes, and every assay cost
about fifteen pence. At present all these assays are made more simply, by much
cheaper methods, and cost a penny farthing each upon an average.
Of the assays by the humid way.—The assays by the humid way, not reducible to
very simple processes, are true chemical analyses, which may in fact be applied with
much advantage, either to ores, or to the products of the furnace; but which cannot be
expected to be practised in smelting-houses, on account of the complication of apparatus
and reagents they require. Moreover, an expert chemist is necessary to obtain
results that can be depended on. The directors of smelting-houses, however, should
never neglect any opportunities that may occur of submitting the materials operated
upon, as well as their products, to a more thorough examination than the dry way
alone can effect. One of the great advantages of similar researches is, to discover
and appreciate the minute quantities of injurious substances which impair the malleability
of the metals, which give them several bad qualities, about whose nature and
cause, more or less error and uncertainty prevail. Chemical analysis, rightly applied
to metallurgy, cannot fail to introduce remarkable improvements into the processes.—See
the different metals, in their alphabetical places.
For assays in the dry way, both of stony and metallic minerals, the process of Dr.
Abich deserves recommendation. In consists in mixing the pulverized mineral with
4 or 6 times its weight of carbonate of baryta in powder, fusing the mixture at a white
heat, and then dissolving it after it cools, in dilute muriatic acid. The most refractory
minerals, even corundum, cyanite, staurolite, zircon, and felspar, yield readily to this
treatment. This process may be employed with advantage upon poor refractory ores.
The platinum crucible, into which the mixed materials are put for fusion, should be
placed in a Hessian crucible, and surrounded with good coak.
The following tabular view of the metallic produce of the British mines, is given
by two very skilful observers, in a work published in 1827, entitled Voyage Metallurgique
en Angleterre, par MM. Dufrénoy et Elie de Beaumont:—
| |
Tons. |
Tons. |
| Tin |
|
Cornwall alone |
|
3,160 |
|
| Copper |
- |
|
Cornwall |
9,331 |
|
- |
11,469 |
| Devonshire |
537 |
| Staffordshire |
38 |
| Anglesey |
738 |
| Wales |
55 |
| Cumberland |
21 |
| Ireland |
738 |
| Scotland |
11 |
| Lead |
- |
|
Wales |
7,500 |
|
- |
31,900 |
| Scotland |
2,800 |
| Cornwall and Devonshire |
800 |
| Shropshire |
800 |
| Derbyshire |
1,000 |
| Cumberland |
19,000 |
| Cast Iron |
|
about |
|
600,000 |
[32] |
[828]
The manganese raised in England exceeds 2000 tons.
M. Heron de Villefosse inserted in the last number of the Annales des Mines for 1827,
the following statistical view of the metallic products of France:—
| |
Tons. |
| Lead in pigs (saumons) |
103 |
|
| Litharge |
513 |
|
| Sulphuret of lead, ground galena (alquifoux) |
112 |
|
| Black copper |
164 |
|
| Antimony |
91 |
|
| Manganese |
765 |
|
| Crude cast iron |
25,606 |
|
| Bar iron |
127,643 |
|
| Steel |
3,500 |
|
| Silver in ingots |
1 |
1⁄6 |
The total value of which is estimated at 80 millions of francs; or about 3,400,000
pounds sterling.
METALS; (Metaux, Fr.; Metalle, Germ.) are by far the most numerous class of
undecompounded bodies in chemical arrangements. They amount to 41; of which 7
form, with oxygen, bodies possessed of alkaline properties; these are, 1. potassium;
2. sodium; 3. lithium; 4. barytium, or barium; 5. strontium; 6. calcium; 7. magnesium;
for even magnesia, the last and feeblest base, tinges turmeric brown, and red
cabbage green. The next 5 metals form, with oxygen, the earths proper; they are,
8. yttrium; 9. glucinum; 10. alumium; 11. zirconium; 12. thorinum. The remaining
29 may be enumerated in alphabetical order, as they hardly admit of being
grouped into subdivisions with any advantage. They are as follows: 13. antimony;
14. arsenic; 15. bismuth; 16. cadmium; 17. cerium; 18. chromium; 19. cobalt;
20. copper; 21. gold; 22. iridium; 23. iron; 24. lead; 25. manganese; 26. mercury;
27. molybdenum; 28. nickel; 29. osmium; 30. palladium; 31. platinum; 32.
rhodium; 33. silver; 34. tantalum; 35. tellurium; 36. tin; 37. titanium; 38.
tungstenium; 39. vanadium; 40. uranium; 41. zinc.
1. They are all, more or less, remarkable for a peculiar lustre, called the metallic.
This property of strongly reflecting light, is connected with a certain state of aggregation
of their particles, but is possessed, superficially at least, by mica, animal charcoal,
selenium, polished indigo;—bodies not at all metallic.
2. The metals are excellent conductors of caloric, and most of them also of electricity,
though probably not all. According to Despretz, they possess the power of conducting
heat according to the following numbers:—Gold, 1000; platinum, 981; silver, 973;
copper, 898; iron, 374; zinc, 363; tin, 304; lead, 179·6.
Becquerel gives the following table of metals, as to electrical conduction:—
Copper, 100; gold, 93·6; silver, 73·6; zinc, 28·5; platina, 16·4; iron, 15·8; tin,
15·5; lead, 8·3; mercury, 3·5; potassium, 1·33.
The metals which hardly, if at all, conduct electricity, are, zirconium; alumium;
tantalum, in powder; and tellurium.
3. Metals are probably opaque; yet gold leaf, as observed by Newton, seems to
transmit the green rays, for objects placed behind it in the sunbeam appear green.
This phenomena has, however, been ascribed to the rays of light passing through an
infinite number of minute fissures in the thinly hammered gold.
4. All metals are capable of combining with oxygen, but with affinities and in
quantities extremely different. Potassium and sodium have the strongest affinity for it;
arsenic and chromium, the feeblest. Many metals become acids by a sufficient dose of
oxygen, while, with a smaller dose, they constitute salifiable bases.
5. Metals combine with each other, forming a class of bodies called alloys, except
when one of them is mercury, in which case the compound is styled an amalgam.
6. They combine with hydrogen into hydrurets; with carbon, into carburets; with
sulphur, into sulphurets; with phosphorus, into phosphurets; with selenium, into
seleniurets; with boron, into borurets (borides?); with chlorine, into chlorides; with
iodine, into iodides; with cyanogen, into cyanides; with silicon, into silicides; and
with fluorine, into fluorides.
7. Metallic salts are definite compounds, mostly crystalline, of the metallic oxides
with the acids. See Haloid.
METEORITES, (Aerolithes, Fr.), are stones of a peculiar aspect and composition,
which have fallen from the air.
METHYLÈNE, a peculiar liquid compound of carbon and hydrogen, extracted
from pyroxilic spirit, which is reckoned to be a bi-hydrate of methylène.
MICA, is a finely foliated mineral, of a pearly metallic lustre. It is harder than
gypsum, but not so hard as calc-spar; flexible and elastic; spec. grav. 2·65. It is[829]
an ingredient of granite and gneiss. The large sheets of mica exposed for sale in
London, are mostly brought from Siberia. They are used, instead of glass, to enclose
the fire, without concealing the flame, in certain stoves.
The mica of Fahlun, analyzed by Rose, afforded, silica, 46·22; alumina, 34·52;
peroxide? of iron, 6·04; potash, 8·22; magnesia, with oxide of manganese, 2·11;
fluoric acid, 1·09; water, 0·98.
MICROCOSMIC SALT; a term given to a salt extracted from human urine,
because man was regarded by the alchemists as a miniature of the world, or the microcosm.
It is a phosphate of soda and ammonia; and is now prepared by mixing,
equivalent proportions of phosphate of soda and phosphate of ammonia, each in solution,
evaporating and crystallizing the mixture. A small excess of ammonia aids the
crystallization.
MILK; (Lait, Fr.; Milche, Germ.) owes its whiteness and opacity to an emulsion
composed of the caseous matter and butter, with sugar of milk, extractive matters,
salts, and free lactic acid; the latter of which causes fresh milk to redden litmus paper.
Milk, in general, contains from 10 to 12 per cent. of solid matter, on being evaporated
to dryness by a steam heat. The mean specific gravity of cows’ milk is 1·030, but it
is less if the milk be rich in cream. The specific gravity of the skimmed milk is 1·035;
and of the cream is 1·0244. 100 parts of creamed milk, contain—
| Caseous matter, containing some butter, |
2 |
·600 |
| Sugar of milk |
3 |
·500 |
| Alcoholic extract, lactic acid, and lactates |
0 |
·600 |
| Salts; muriate and phosphate of potash, and phosphate of lime |
0 |
·420 |
| Water |
92 |
·875 |
| Cream consists of,—Butter separated by churning |
4 |
·5 |
| Caseous matter precipitated by the coagulation of the milk of the butter |
3 |
·5 |
| Butter-milk |
92 |
·0 |
| |
100 |
·0 |
When milk contained in wire-corked bottles, is heated to the boiling point in a water
bath, the oxygen of the included small portion of air under the cork seems to be carbonated,
and the milk will afterwards keep fresh, it is said, for a year or two; as green
gooseberries and peas do by the same treatment.
MILL-STONE, or Buhr-Stone. This interesting form of silica, which occurs
in great masses, has a texture essentially cellular, the cells being irregular in number,
shape, and size, and are often crossed by thin plates, or coarse fibres of silex. The
Buhr-stone has a straight fracture, but it is not so brittle as flint, though its hardness
is nearly the same. It is feebly translucent; its colours are pale and dead, of a whitish,
grayish, or yellowish cast, sometimes with a tinge of blue.
The Buhr-stones usually occur in beds, which are sometimes continuous, and at others
interrupted. These beds are placed amid deposits of sand, or argillaceous and ferruginous
marls, which penetrate between them, filling their fissures and honeycomb cavities.
Buhr-stones constitute a very rare geological formation, being found in abundance
only in the mineral basin of Paris, and a few adjoining districts. Its place of superposition
is well ascertained: it forms a part of the lacustrine, or fresh-water formation,
which, in the locality alluded to, lies above the fossil-bone gypsum, and the stratum of
sand and marine sandstone which cover it. Buhr-stone constitutes, therefore, the uppermost
solid stratum of the crust of the globe; for above it there is nothing but alluvial
soil, or diluvial gravel, sand, and loam.
Buhr-stones sometimes contain no organic forms, at others they seem as if stuffed
full of fresh-water shells, or land shells and vegetables of inland growth. There is no
exception known to this arrangement; but the shells have assumed a siliceous nature,
and their cavities are often bedecked with crystals of quartz. The best Buhr-stones
for grinding corn, have about an equal proportion of solid matter, and of vacant space.
The finest quarry of them is upon the high ground, near La Ferté-sous-Jouarre. The
stones are quarried in the open air, and are cut out in cylinders, from one to two yards in
diameter, by a series of iron and wooden wedges, gradually but equally inserted. The
pieces of buhr-stones are afterwards cut into parallelopipeds, called panes, which are
bound with iron hoops into large millstones. These pieces are exported chiefly to
England and America. Good millstones of a bluish white colour, with a regular
proportion of cells, when six feet and a half in diameter, fetch 1200 francs a-piece, or
48l. sterling. A coarse conglomerate sandstone or breccia is, in some cases, used as
a substitute for buhr-stones; but it is a poor one.
MINERAL WATERS. See Soda Water, and Waters, Mineral.
MINES; (Bergwerke, Germ.) Amidst the variety of bodies apparently infinite, which
compose the crust of the globe, geologists have demonstrated the prevalence of a few[830]
general systems of rocks, to which they have given the name of formations or deposits.
A large proportion of these mineral systems consists of parallel planes, whose length and
breadth greatly exceed their thickness; on which account they are called stratified rocks;
others occur in very thick blocks, without any parallel stratification, or horizontal seams
of considerable extent.
The stratiform deposits are subdivided into two great classes; the primary and the
secondary. The former seem to have been called into existence before the creation of
organic matter, because they contain no exuviæ of vegetable or animal beings; while
the latter are more or less interspersed, and sometimes replete with organic remains.
The primary strata are characterized, moreover, by the nearly vertical or highly inclined
position of their planes; the secondary lie for the most part in a nearly horizontal
position.
Where the primitive mountains graduate down into the plains, rocks of an intermediate
character appear, which, though possessing a nearly vertical position, contain a
few vestiges of animal beings, especially shells. These have been called transition, to
indicate their being the passing links between the first and second systems of ancient
deposits; they are distinguished by the fractured and cemented texture of their planes,
for which reason they are sometimes called conglomerate.
Between these and the truly secondary rocks, another very valuable series is interposed
in certain districts of the globe; namely, the coal-measures, the paramount formation
of Great Britain. The coal strata are disposed in a basin-form, and alternate with
parallel beds of sandstone, slate-clay, iron-stone, and occasionally limestone. Some
geologists have called the coal-measures the medial formation.
In every mineral plane, the inclination and direction are to be noted; the former
being the angle which it forms with the horizon, the latter the point of the azimuth or
horizon, towards which it dips, as west, north-east, south, &c. The direction of the
bed is that of a horizontal line drawn in its plane; and which is also denoted by the
point of the compass. Since the lines of direction and inclination are at right angles to
each other, the first may always be inferred from the second; for when a stratum is said
to dip to the east or west, this implies that its direction is north and south.
The smaller sinuosities of the bed are not taken into account, just as the windings
of a river are neglected in stating the line of its course.
Masses are mineral deposits, not extensively spread in parallel planes, but irregular
heaps, rounded or oval, enveloped in whole or in a great measure by rocks of a different
kind. Lenticular masses being frequently placed between two horizontal or
inclined strata, have been sometimes supposed to be stratiform themselves, and have
been accordingly denominated by the Germans liegende stocke, lying heaps or blocks.
The orbicular masses often occur in the interior of unstratified mountains, or in the
bosom of one bed.
Nests, concretions, nodules, are small masses found in the middle of strata; the first
being commonly in a friable state; the second often kidney-shaped, or tuberous; the
third nearly round, and encrusted, like the kernel of an almond.
Lodes, or large veins, are flattened masses, with their opposite surfaces not parallel,
which consequently terminate like a wedge, at a greater or less distance, and do not run
parallel with the rocky strata in which they lie, but cross them in a direction not far
from the perpendicular; often traversing several different mineral planes. The lodes are
sometimes deranged in their course, so as to pursue for a little way the space between
two contiguous strata; at other times they divide into several branches. The matter
which fills the lodes is for the most part entirely different from the rocks they pass
through, or at least it possesses peculiar features.
This mode of existence, exhibited by several mineral substances, but which has
been long known with regard to metallic ores, suggests the idea of clefts or rents
having been made in the stratum posterior to its consolidation, and of the vacuities
having been filled with foreign matter, either immediately or after a certain interval.
There can be no doubt as to the justness of the first part of the proposition, for there
may be observed round many lodes undeniable proofs of the movement or dislocation of
the rock; for example, upon each side of the rent, the same strata are no longer situated
in the same plane as before, but make greater or smaller angles with it; or the stratum
upon one side of the lode is raised considerably above, or depressed considerably below,
its counterpart upon the other side. With regard to the manner in which the rent has
been filled, different opinions may be entertained. In the lodes which are widest near
the surface of the ground, and graduate into a thin wedge below, the foreign matter
would seem to have been introduced as into a funnel at the top, and to have carried
along with it in its fluid state portions of rounded gravel and organic remains. In
other cases, other conceptions seem to be more probable; since many lodes are largest
at their under part, and become progressively narrower as they approach the surface;
from which circumstance, it has been inferred that the rent has been caused by an[831]
expansive force acting from within the earth, and that the foreign matter, having been
injected in a fluid state, has afterwards slowly crystallized. This hypothesis accounts much
better than the other for most of the phenomena observable in mineral veins, for the
alterations of the rock at their sides, for the crystallization of the different substances
interspersed in them, for the cavities bestudded with little crystals, and for many minute
peculiarities. Thus, the large crystals of certain substances which line the walls of
hollow veins, have sometimes their under surfaces besprinkled with small crystals of
sulphurets, arseniurets, &c., while their upper surfaces are quite smooth; suggesting
the idea of a slow sublimation of these volatile matters from below, by the residual
heat, and their condensation upon the under faces of the crystalline bodies, already
cooled. This phenomenon affords a strong indication of the igneous origin of metalliferous
veins.
In the lodes, the principal matters which fill them are to be distinguished from the
accessory substances; the latter being distributed irregularly, amidst the mass of the
first, in crystals, nodules, grains, seams, &c. The non-metalliferous exterior portion,
which is often the largest, is called gangue, from the German gang, vein. The position
of a vein is denoted, like that of the strata, by the angle of inclination, and the point of
the horizon towards which they dip, whence the direction is deduced.
Veins, are merely small lodes, which sometimes traverse the great ones, ramifying
in various directions, and in different degrees of tenuity.
A metalliferous substance is said to be disseminated, when it is dispersed in crystals,
spangles, scales, globules, &c., through a large mineral mass.
Certain ores which contain the metals most indispensable to human necessities, have
been treasured up by the Creator in very bountiful deposits; constituting either great
masses in rocks of different kinds, or distributed in lodes, veins, nests, concretions, or
beds with stony and earthy admixtures; the whole of which become the objects of
mineral exploration. These precious stores occur in different stages of the geological
formations; but their main portion, after having existed abundantly in the several orders
of the primary strata, suddenly cease to be found towards the middle of the secondary.
Iron ores are the only ones which continue among the more modern deposits, even so
high as the beds immediately beneath the chalk, when they also disappear, or exist
merely as colouring matters of the tertiary earthy beds.
The strata of gneiss and mica-slate constitute in Europe the grand metallic domain.
There is hardly any kind of ore which does not occur there in sufficient abundance to
become the object of mining operations, and many are found no where else. The transition
rocks and the lower part of the secondary ones, are not so rich, neither do they
contain the same variety of ores. But this order of things, which is presented by Great
Britain, Germany, France, Sweden, and Norway, is far from forming a general law;
since in equinoxial America the gneiss is but little metalliferous; while the superior
strata, such as the clay-schists, the sienitic porphyries, the limestones, which complete
the transition series, as also several secondary deposits, include the greater portion of
the immense mineral wealth of that region of the globe.
All the substances of which the ordinary metals form the basis, are not equally
abundant in nature; a great proportion of the numerous mineral species which figure in
our classifications, are mere varieties scattered up and down in the cavities of the great
masses or lodes. The workable ores are few in number, being mostly sulphurets, some
oxides, and carbonates. These occasionally form of themselves very large masses, but
more frequently they are blended with lumps of quartz felspar, and carbonate of lime,
which form the main body of the deposit; as happens always in proper lodes. The
ores in that case are arranged in small layers parallel to the strata of the formation,
or in small veins which traverse the rock in all directions, or in nests or concretions
stationed irregularly, or finally disseminated in hardly visible particles. These deposits
sometimes contain apparently only one species of ore, sometimes several, which must
be mined together, as they seem to be of contemporaneous formation; whilst, in other
cases, they are separable, having been probably formed at different epochs. In treating
of the several metals in their alphabetical order, I have taken care to describe their
peculiar geological positions, and the rocks which accompany or mineralize them.
In mining, as in architecture, the best method of imparting instruction is to display
the master-pieces of the respective arts, which speak clearly to the mind through the
medium of the eye. It is not so easy, however, to represent at once the general effect of
a mine, as it is of an edifice; because there is no point of sight from which the former
can be sketched at once, like the latter. The subterraneous structures certainly afford
some of the finest examples of the useful labours of man, continued for ages, under the
guidance of science and ingenuity; but, however curious, beautiful, and grand in themselves,
they cannot become objects of a panoramic view. It is only by the lights of geometry
and geology that mines can be contemplated and surveyed, either as a whole or in their
details; and, therefore, these marvellous subterranean regions, in which roads are cut[832]
many hundred miles long, are altogether unknown or disregarded by men of the world.
Should any of them, perchance, from curiosity or interest, descend into these dark
recesses of the earth, they are prepared to discover only a few insulated objects, which they
may think strange or possibly hideous; but they cannot recognize either the symmetrical
disposition of mineral bodies, or the laws which govern geological phenomena, and
serve as sure guides to the skilful miner in his adventurous search. It is by exact
plans and sections of subterraneous workings, that a knowledge of the nature, extent,
and distribution of mineral wealth, can be acquired.
698. A general view of mining operations.
As there is no country in the world so truly rich and powerful, by virtue of its mineral
stores, as Great Britain, so there are no people who ought to take a deeper interest in
their scientific illustration. I have endeavoured in the present article to collect from
the most authentic sources the most interesting and instructive examples of mining
operations.
To the magnificent work of Ville-Fosse, Sur la Richesse Minerale, no longer on sale,
I have to acknowledge weighty obligations; many of the figures being copied from his
great Atlas.
Lodes or mineral veins are usually distinguished by English miners into at least four
species. 1. The rake vein. 2. The pipe vein. 3. The flat or dilated vein; and 4. The
interlaced mass (stock-werke), indicating the union of a multitude of small veins mixed
in every possible direction with each other, and with the rock.
1. The rake vein is a perpendicular mineral fissure; and is the form best known
among practical miners. It commonly runs in a straight line, beginning at the superficies
of the strata, and cutting them downwards, generally further than can be reached.
This vein sometimes stands quite perpendicular; but it more usually inclines or hangs
over at a greater or smaller angle, or slope, which is called by the miners the hade or
hading of the vein. The line of direction in which the fissure runs, is called the bearing
of the vein.
2. The pipe vein resembles in many respects a huge irregular cavern, pushing forward
into the body of the earth in a sloping direction, under various inclinations, from an
angle of a few degrees to the horizon, to a dip of 45°, or more. The pipe does not in
general cut the strata across like the rake vein, but insinuates itself between them; so
that if the plane of the strata be nearly horizontal, the bearing of the pipe vein will be
conformable; but if the strata stand up at a high angle, the pipe shoots down nearly
headlong like a shaft. Some pipes are very wide and high, others are very low and
narrow, sometimes not larger than a common mine or drift.
3. The flat or dilated vein, is a space or opening between two strata or beds of stone,
the one of which lies above, and the other below this vein, like a stratum of coal[833]
between its roof and pavement; so that the vein and the strata are placed in the same
plane of inclination. These veins are subject, like coal, to be interrupted, broken, and
thrown up or down by slips, dykes, or other interruptions of the regular strata. In the
case of a metallic vein, a slip often increases the chance of finding more treasure. Such
veins do not preserve the parallelism of their beds, characteristic of coal seams; but
vary excessively in thickness within a moderate space. Flat veins occur frequently in
limestone, either in a horizontal or declining direction. The flat or strata veins open
and close, as the rake veins also do.
4. The interlaced mass has been already defined.
To these may be added the accumulated vein, or irregular mass (butzenwerke), a great
deposit placed without any order in the bosom of the rocks, apparently filling up cavernous
spaces.
The interlaced masses are more frequent in primitive formations, than in the others;
and tin is the ore which most commonly affects this locality. See figure of Tin mine.
The study of the mineral substances, called gangues or vein-stones, which usually
accompany the different ores, is indispensable in the investigation and working of mines.
These gangues, such as quartz, calcareous spar, fluor spar, heavy spar, &c., and a great
number of other substances, although of little or no value in themselves, become of great
consequence to the miner, either by pointing out by their presence that of certain
useful minerals, or by characterising in their several associations, different deposits of
ores of which it may be possible to follow the traces, and to discriminate the relations,
often of a complicated kind, provided we observe assiduously the accompanying gangues.
Mineral veins are subject to derangements in their course, which are called shifts or
faults. Thus, when a transverse vein throws out, or intercepts, a longitudinal one, we
must commonly look for the rejected vein on the side of the obtuse angle which the
direction of the latter makes with that of the former. When a bed of ore is deranged
by a fault, we must observe whether the slip of the strata be upwards or downwards; for
in either circumstance, it is only by pursuing the direction of the fault that we can
recover the ore; in the former case by mounting, in the latter by descending beyond
the dislocation.
When two veins intersect each other, the direction of the offcast is a subject of
interest, both to the miner and the geologist. In Saxony it is considered as a general
fact that the portion thrown out is always upon the side of the obtuse angle, a circumstance
which holds also in Cornwall; and the more obtuse the angle, the out-throw is
the more considerable. A vein may be thrown out on meeting another vein, in a line
which approaches either towards its inclination or its direction. The Cornish miners
use two different terms to denote these two modes of rejection; for the first case, they
say the vein is heaved; for the second, it is started.
The great copper lode of Carharack, d, fig. 699. in the parish of Gwenap, is one of the
most instructive examples of intersection. The power
or thickness of this vein is 8 feet; its direction is
nearly due east and west, and it dips towards the
north at an inclination of two feet per fathom; its
upper part being in the killas (a greenish clay-slate);
its lower part in the granite. The lode has suffered
two intersections; the first produced by meeting the
vein h, called Steven’s fluckan, which runs from north-east
to south-west, and which throws the lode
several fathoms out; the second is produced by
another vein i, almost at right angles with the first, and which occasions another out-throw
of 20 fathoms to the right side. The fall of the vein occurs therefore in the one
case to the right, and in the other to the left; but in both it is towards the side of
the obtuse angle. This distribution is very singular; for one part of the vein appears
to have mounted while the other has descended. N, S denotes North and South. d
is the copper lode running east and west. h, i, are systems of clay-slate veins called
fluckans; the line over S, represents the down-shift, and d′ the up-shift.
General observations on the localities of ores, and on the indications of metallic mines.
1. Tin, exists principally in primitive rocks, appearing either in interlaced masses, in
beds, or as a constituent part of the rock itself, and more rarely in distinct veins. Tin
ore is found indeed sometimes in alluvial land, filling up low situations between lofty
mountains.
2. Gold, occurs either in beds, or in veins, frequently in primitive rocks; though in
other formations, and particularly in alluvial earth, it is also found. When this metal
exists in the bosom of primitive rocks, it is particularly in schists; it is not found in serpentine,
but it is met with in greywacke in Transylvania. The gold of alluvial districts,[834]
called gold of washing or transport, occurs, as well as alluvial tin, among the debris of
the more ancient rocks.
3. Silver, is found particularly in veins and beds, in primitive and transition formations;
though some veins of this metal occur in secondary strata. The rocks richest
in it are, gneiss, mica-slate, clay-slate, greywacke, and old alpine limestone. Localities
of silver-ore itself are not numerous, at least in Europe, among secondary formations;
but it occurs in combination with the ores of copper or of lead.
4. Copper, exists in the three mineral epochas; 1. in primitive rocks, principally in
the state of pyritous copper, in beds, in masses, or in veins; 2. in transition districts,
sometimes in masses, sometimes in veins of copper pyrites; 3. in secondary strata,
especially in beds of cupreous schist.
5. Lead, occurs also in each of the three mineral epochas; abounding particularly in
primitive and transition grounds, where it usually constitutes veins, and occasionally beds
of sulphuretted lead (galena). The same ore is found in strata or in veins among
secondary rocks, associated now and then with ochreous iron-oxide and calamine
(carbonate of zinc); and it is sometimes disseminated in grains through more recent
strata.
6. Iron, is met with in four different mineral eras, but in different ores. Among
primitive rocks, magnetic iron ore and specular iron ore occur chiefly in beds, sometimes
of enormous size; the ores of red or brown oxide of iron (hæmatite) are found generally
in veins, or occasionally in masses with sparry iron, both in primitive and transition
rocks; as also sometimes in secondary strata; but more frequently in the coal-measure
strata, as beds of clay-ironstone, of globular iron oxide, and carbonate of iron. In
alluvial districts we find ores of clay-ironstone, granular iron-ore, bog-ore, swamp-ore,
and meadow-ore. The iron ores which belong to the primitive period have almost
always the metallic aspect, with a richness amounting even to 80 per cent. of iron, while
the ores in the posterior formations become in general more and more earthy, down to
those in alluvial soils, some of which present the appearance of a common stone, and
afford not more than 20 per cent. of metal, though its quality is often excellent.
7. Mercury, occurs principally among secondary strata, in disseminated masses, along
with combustible substances; though the metal is met with occasionally in primitive
countries.
8. Cobalt, belongs to the three mineral epochas; its most abundant deposits are
veins in primitive rocks; small veins containing this metal are found, however, in secondary
strata.
9. Antimony, occurs in veins or beds among primitive and transition rocks.
10, 11. Bismuth and nickel do not appear to constitute the predominating substance
of any mineral deposits; but they often accompany cobalt.
12. Zinc, occurs in the three several formations: namely, as sulphuret or blende,
particularly in primitive and transition rocks; as calamine, in secondary strata, usually
along with oxide of iron, and sometimes with sulphuret of lead.
An acquaintance with the general results collected and classified by geology must be
our first guide in the investigation of mines. This enables the observer to judge whether
any particular district, should from the nature and arrangement of its rocks, be susceptible
of including within its bosom, beds of workable ores; it indicates also, to a certain
degree, what substances may probably be met with in a given series of rocks, and what
locality these substances will preferably affect. For want of a knowledge of these
facts, many persons have gone blindly into researches equally absurd and ruinous.
Formerly indications of mines were taken from very unimportant circumstances;
from thermal waters, the heat of which was gratuitously referred to the decomposition of
pyrites; from mineral waters, whose course is however often from a far distant source;
from vapours incumbent over particular mountain groups; from the snows melting
faster in one mineral district than another; from the different species of forest trees,
and from the greater or less vigour of vegetation, &c. In general, all such indications
are equally fallacious with the divining rod, and the compass made of a lump of pyrites
suspended by a thread.
Geognostic observation has substituted more rational characters of metallic deposits,
some of which may be called negative and others positive.
The negative indications are derived from that peculiar geological constitution, which
from experience or general principles excludes certain metallic matters; for example,
granite, and in general every primitive formation, forbids the hope of finding within them
combustible fossils (pit-coal), unless it be beds of anthracite; there also it would be vain
to seek for sal gem. It is very seldom that granite rocks include silver; or limestones,
ores of tin. Volcanic territories never afford any metallic ores worth the working; nor
do extensive veins usually run into secondary and alluvial formations. The richer ores
of iron do not occur in secondary strata; and the ores of this metal peculiar to these
localities, do not exist among primary rocks.
[835]
Among positive indications, some are proximate and others remote. The proximate
are, an efflorescence, so to speak, of the subjacent metallic masses; magnetic attraction
for iron ores; bituminous stone, or inflammable gas for pit-coal; the frequent occurrence
of fragments of particular ores, &c. The remote indications consist in the geological
epocha, and nature of the rocks. From the examples previously adduced, marks of this
kind acquire new importance when in a district susceptible of including deposits of
workable ores, the gangues or vein-stones are met with which usually accompany any
particular metal. The general aspect of mountains whose flanks present gentle and
continuous slopes, the frequency of sterile veins, the presence of metalliferous sands, the
neighbourhood of some known locality of an ore, for instance that of iron-stone in
reference to coal, lastly the existence of salt springs and mineral waters, may furnish
some indications; but when ferruginous or cupreous waters issue from sands or clays,
such characters merit in general little attention, because the waters may flow from
a great distance. No greater importance can be attached to metalliferous sands and
saline springs.
In speaking of remote indications, we may remark that in several places, and particularly
near Clausthal in the Hartz, a certain ore of red oxide of iron occurs above
the most abundant deposits of the ores of lead and silver; whence it has been named by
the Germans the iron-hat. It appears that the iron ore rich in silver, which is worked
in America under the name of pacos, has some analogy with this substance; but iron
ore is in general so plentifully diffused on the surface of the soil, that its presence can
be regarded as only a remote indication, relative to other mineral substances, except in
the case of clay ironstone with coal.
Of the instruments and operations of subterranean operations.—It is by the aid of geometry
in the first place that the miner studies the situation of the mineral deposits, on
the surface and in the interior of the ground; determines the several relations of the
veins and the rocks; and becomes capable of directing the perforations towards a suitable
end.
The instruments are, 1. the magnetic compass, which is employed to measure the
direction of a metallic ore, wherever the neighbourhood of iron does not interfere with
its functions; 2. the graduated semicircle which serves to measure the inclination, which
is also called the clinometer.
3. The chain or cord for measuring the distance of one point from another.
4. When the neighbourhood of iron renders the use of the magnet uncertain, a
plate or plane table is employed.
The dials of the compasses generally used in the most celebrated mines, are graduated
into hours; most commonly into twice 12 hours. Thus the whole limb is divided into
24 spaces, each of which contains 15° = 1 hour. Each hour is subdivided into 8
parts.
Means of penetrating into the interior of the earth.—In order to penetrate into the
interior of the earth, and to extract from it the objects of his toils, the miner has at his
disposal several means, which may be divided into three classes: 1. manual tools,
2. gunpowder, and 3. fire.
The tools used by the miners of Cornwall and Devonshire are the following:
Fig. 700. The pick. It is a light tool, and somewhat varied in shape according to circumstances.
One side used as a hammer is called the poll, and is employed to drive in
the gads, or to loosen and detach prominences. The point is of steel, carefully tempered,
and drawn under the hammer to the proper form. The French call it pointerolle.
[836]
Fig. 701. The gad. It is a wedge of steel, driven into crevices of rocks, or into small
openings made with the point of the pick.
Fig. 702. The miner’s shovel. It has a pointed form, to enable it to penetrate among
the coarse and hard fragments of the mine rubbish. Its handle being somewhat bent, a
man’s power may be conveniently applied without bending his body.
The blasting or shooting tools are:—
Besides these tools the miner requires a powder-horn, rushes to be filled with gunpowder,
tin cartridges for occasional use in wet ground, and paper rubbed over with
gunpowder or grease, for the smifts or fuses.
The borer, fig. 704., is an iron bar tipped with steel, formed like a thick chisel, and
is used by one man holding it straight in the hole with constant rotation on its axis,
while another strikes the head of it with the iron sledge or mallet, fig. 703. The hole is
cleared out from time to time by the scraper, fig. 707., which is a flat iron rod turned
up at one end. If the ground be very wet, and the hole gets full of mud, it is cleaned
out by a stick bent at the end into a fibrous brush, called a swab-stick.
Fig. 709. represents the plan of blasting the rock, and a section of a hole ready for
firing. The hole must be rendered as
dry as possible, which is effected very
simply by filling it partly with tenacious
clay, and then driving into it a
tapering iron rod, which nearly fills its
calibre, called the claying bar. This
being forced in with great violence,
condenses the clay into all the crevices
of the rock, and secures the dryness of
the hole. Should this plan fail, recourse
is had to tin cartridges furnished
with a stem or tube (see fig. 710.,)
through which the powder may be inflamed.
When the hole is dry, and
the charge of powder introduced, the nail, a small taper rod of copper, is inserted so as
to reach the bottom of the hole, which is now ready for tamping. By this difficult and
dangerous process, the gunpowder is confined, and the disruptive effect produced.
Different substances are employed for tamping, or cramming the hole, the most usual
one being any soft species of rock free from siliceous or flinty particles. Small quantities
of it only are introduced at a time, and rammed very hard by the tamping-bar,
which is held steadily by one man, and struck with a sledge by another. The hole
being thus filled, the nail is withdrawn by putting a bar through its eye, and striking
it upwards. Thus a small perforation or vent is left for the rush which communicates
the fire.
Besides the improved tamping-bar faced with hard copper, other contrivances have
been resorted to for diminishing the risk of those dreadful accidents that frequently
occur in this operation. Dry sand is sometimes used as a tamping material, but there
are many rocks for the blasting of which it is ineffective. Tough clay will answer
better in several situations.
For conveying the fire, the large and long green rushes which grow in marshy
ground are selected. A slit is made in one side of the rush, along which the sharp
end of a bit of stick is drawn, so as to extract the pith, when the skin of the rush closes
again by its own elasticity. This tube is filled up with gunpowder, dropped into the
vent-hole, and made steady with a bit of clay. A paper smift, adjusted to burn a
proper time, is then fixed to the top of the rush-tube, and kindled, when the men of the
mine retire to a safe distance.
In fig. 709. the portion of the rock which would be dislodged by the explosion, is
that included between A and B. The charge of powder is represented by the white part
which fills the hole up to C; from which point to the top, the hole is filled with tamping.
The smift is shewn at D.
Fig. 711. is an iron bucket, or as it is called in Cornwall, a kibble, in which the ore
is raised in the shafts, by machines called whims, worked by horses. The best kibbles[837]
are made of sheet-iron, and hold each about three hundred weight of ore: 120 kibbles
are supposed to clear a cubic fathom of rock.
Fig. 712. represents the wheelbarrow used under ground for conveying ore and
waste to the foot of the shafts. It is made of light deal, except the wheel, which has
a narrow rim of iron.
Fig. 713. represents Mr. Taylor’s ingenious ventilator, or machine for renewing fresh
air in mines. It is so simple in construction, so complete in its operation, requires so
little power to work it, and is so little liable to injury from wear, that nothing further
of the kind can be desired in ordinary metallic mines. The shaft of the mine is represented
at A; at either the top or bottom of which the machine may be placed, as is
found most convenient, but the foul air must be discharged into a floor, furnished with
a valve-door to prevent its return into the mine. B is the air-pipe from the mine, passing
through the bottom of the fixed vessel or cylinder C, which is formed of timber, and
bound with iron hoops. It is filled with water nearly to the top of the pipe B, on which is
fixed a valve opening upwards at D. E, the air, or exhausting cylinder of cast-iron, open
at bottom, and suspended over the air-pipe, but immersed some way in the water. It is
furnished with a wooden top, having an aperture fitted with a valve likewise opening
upwards at F. This exhausting cylinder is moved up and down by the bob G, brought
into connexion with any engine by the horizontal rod H; the weight of the cylinder
being balanced, if necessary, by the counterpoise I. The action is as follows:—When
the cylinder rises, the air from the mine rushes up through the pipe and valve D; and
when it descends, this valve shuts, and prevents the return of the air, which is expelled
through the valve F. With a cylinder two feet in diameter and six feet long, working
from two to three strokes per minute, 200 gallons of air may be discharged in the
same time.
Gunpowder is the most valuable agent of excavation; possessing a power which has
no limit, and which can act every where, even under water. Its introduction, in 1615,
caused a great revolution in the mining art.
It is employed in mines in different manners, and in different quantities, according to
circumstances. In all cases, however, the process resolves itself into boring a hole, and
enclosing a cartridge in it, which is afterwards made to explode. The hole is always
cylindrical, and is usually made by means of the borer, fig. 704., a stem of iron, terminated
by a blunt-edged chisel. It sometimes ends in a cross, formed by two chisels set
transversely. The workman holds the stem in his left hand, and strikes it with an iron
mallet held in his right. He is careful to turn the punch a very little round at every
stroke. Several punches are employed in succession, to bore one hole; the first shorter,
the latter ones longer, and somewhat thinner. The rubbish is withdrawn as it accumulates,
at the bottom of the hole, by means of a picker, which is a small spoon or disc
of iron fixed at the end of a slender iron rod. When holes of a large size are to be[838]
made, several men must be employed; one to hold the punch, and one or more to wield
the iron mallet. The perforations are seldom less than an inch in diameter, and
18 inches deep; but they are sometimes 2 inches wide, with a depth of 50 inches.
The gunpowder, when used, is most commonly put up in paper cartridges. Into
the side of the cartridge, a small cylindrical spindle or piercer is pushed. In this state
the cartridge is forced down to the bottom of the hole, which is then stuffed, by means
of the tamping bar, fig. 708., with bits of dry clay, or friable stones coarsely pounded.[33]
The piercer is now withdrawn, which leaves in its place, a channel through which fire
may be conveyed to the charge. This is executed either by pouring gunpowder into
that passage, or by inserting into it, reeds, straw stems, quills, or tubes of paper filled
with gunpowder. This is exploded by a long match, which the workmen kindle, and
then retire to a place of safety.
As the piercer must not only be slender, but stiff, so as to be easily withdrawn when
the hole is tamped, iron spindles are usually employed, though they occasionally give
rise to sparks, and consequently to dangerous accidents, by their friction against the
sides of the hole. Brass piercers have been sometimes tried; but they twist and break
too readily.
Each hole bored in a mine, should be so placed in reference to the schistose structure
of the rock, and to its natural fissures, as to attack and blow up the least resisting
masses. Sometimes the rock is prepared beforehand for splitting in a certain direction,
by means of a narrow channel excavated with the small hammer.
The quantity of gunpowder should be proportional to the depth of the hole, and the
resistance of the rock; and merely sufficient to split it. Anything additional would
serve no other purpose than to throw the fragments about the mine, without increasing
the useful effect. Into the holes of about an inch and a quarter diameter, and 18 inches
deep, only two ounces of gunpowder are put.
It appears that the effect of the gunpowder may be augmented by leaving an empty
space above, in the middle of, or beneath the cartridge. In the mines of Silesia, the
consumption of gunpowder has been eventually reduced, without diminishing the
product of the blasts, by mixing sawdust with it in certain proportions. The hole has
also been filled up with sand in some cases, according to Mr. Jessop’s plan, instead of
being packed with stones, which has removed the danger of the tamping operation.
The experiments made in this way have given results very advantageous in quarry
blasts with great charges of gunpowder; but less favourable in the small charges
employed in mines.
Water does not oppose an insurmountable obstacle to the employment of gunpowder;
but when the hole cannot be made dry, a cartridge bag impermeable to water must be
had recourse to, provided with a tube also impermeable, in which the piercer is placed.
After the explosion of each mining charge, wedges and levers are employed, to drag
away and break down what has been shattered.
Wherever the rock is tolerably hard, the use of gunpowder is more economical and
more rapid than any tool-work, and is therefore always preferred. A gallery, for
example, a yard and a half high, and a yard wide, the piercing of which by the hammer
formerly cost from five to ten pounds sterling, the running yard, in Germany, is
executed at the present day by gunpowder at from two to three pounds. When, however,
a precious mass of ore is to be detached, when the rock is cavernous, which nearly
nullifies the action of gunpowder, or when there is reason to apprehend that the shock
caused by the explosion may produce an injurious fall of rubbish, hand-tools alone must
be employed.
In certain rocks and ores of extreme hardness, the use both of tools and gunpowder
becomes very tedious and costly. Examples to this effect are seen, in the mass of
quartz mingled with copper pyrites, worked at Rammelsberg, in the Hartz, in the
masses of stanniferous granite of Geyer and Altenberg in the Erzgebirge of Saxony,
&c. In these circumstances, fortunately very rare, the action of fire is used with
advantage to diminish the cohesion of the rocks and the ores. The employment of this
agent is not necessarily restricted to these difficult cases. It was formerly applied very
often to the working of hard substances; but the introduction of gunpowder into the
mining art, and the increase in the price of wood, occasion fire to be little used as an
ordinary means of excavation, except in places where the scantiness of the population has[839]
left a great extent of forest timber, as happens at Kongsberg in Norway, at Dannemora
in Sweden, at Felsobanya in Transylvania, &c.
The action of fire may be applied to the piercing of a gallery, or to the advancement
of a horizontal cut, or to the crumbling down of a mass of ore, by the successive
upraising of the roof of a gallery already pierced. In any of these cases, the process
consists in forming bonfires, the flame of which is made to play upon the parts to be
attacked. All the workmen must be removed from the mine during, and even for
some time after, the combustion. When the excavations have become sufficiently cool
to allow them to enter, they break down with levers and wedges, or even by means of
gunpowder, the masses which have been rent and altered by the fire.
To complete our account of the manner in which man may penetrate into the interior
of the earth, we must point out the form of the excavations that he should make in it.
In mines, three principal species of excavations may be distinguished; viz. shafts,
galleries, and the cavities of greater or less magnitude which remain in the room of the
old workings.
A shaft or pit is a prismatic or cylindrical hollow space, the axis of which is either
vertical or much inclined to the horizon. The dimension of the pit, which is never
less than 32 inches in its narrowest diameter, amounts sometimes to several yards.
Its depth may extend to 1000 feet, and more. Whenever a shaft is opened, means
must be provided to extract the rubbish which continually tends to accumulate at its
bottom, as well as the waters which may percolate down into it; as also to facilitate the
descent and ascent of the workmen. For some time a wheel and axle erected over
the mouth of the opening, which serve to elevate one or two buckets of proper dimensions,
may be sufficient for most of these purposes. But such a machine becomes
ere long inadequate. Horse-whims, or powerful steam-engines, must then be had
recourse to; and effectual methods of support must be employed to prevent the sides
of the shaft from crumbling and falling down.
A Gallery is a prismatic space, the straight or winding axis of which does not usually
deviate much from the horizontal line. Two principal species are distinguished; the
galleries of elongation, which follow the direction of a bed or a vein; and the transverse
galleries, which intersect this direction under an angle not much different from 90°.
The most ordinary dimensions of galleries are a yard wide, and two yards high; but
many still larger may be seen traversing thick deposits of ore. There are few whose
width is less than 24 inches, and height less than 40; such small drifts serve merely as
temporary expedients in workings. Some galleries are several leagues in length. We
shall describe in the sequel the means which are for the most part necessary to support
the roof and the walls. The rubbish is removed by waggons or wheelbarrows of
various kinds. See fig. 712.
It is impossible to advance the boring of a shaft or gallery beyond a certain rate, because
only a limited set of workmen can be made to bear upon it. There are some galleries
which have taken more than 30 years to perforate. The only expedient for accelerating
the advance of a gallery, is to commence, at several points of the line to be pursued,
portions of galleries which may be joined together on their completion.
Whether tools or gunpowder be used in making the excavations, they should be so
applied as to render the labour as easy and quick as possible, by disengaging the mass
out of the rock at two or three of its faces. The effect of gunpowder, wedges, or picks,
is then much more powerful. The greater the excavation, the more important is it to
observe this rule. With this intent, the working is disposed in the form of steps
(gradins), placed like those of a stair; each step being removed in successive portions,
the whole of which, except the last, are disengaged on three sides, at the instant of their
being attacked.
The substances to be mined occur in the bosom of the earth, under the form of
alluvial deposits, beds, pipe-veins, or masses, threads or small veins, and rake-veins.
When the existence of a deposit of ore is merely suspected, without positive proofs,
recourse must be had to labours of research, in order to ascertain the richness, nature,
and disposition of a supposed mine. These are divided into three kinds; open workings,
subterranean workings, and boring operations.
1. The working by an open trench, has for its object to discover the outcropping or
basset edges of strata or veins. It consists in opening a fosse of greater or less width,
which, after removing the vegetable mould, the alluvial deposits, and the matters disintegrated
by the atmosphere, discloses the native rocks, and enables us to distinguish
the beds which are interposed, as well as the veins that traverse them. The trench ought
always to be opened in a direction perpendicular to the line of the supposed deposit.
This mode of investigation costs little, but it seldom gives much insight. It is chiefly
employed for verifying the existence of a supposed bed or vein.
The subterranean workings afford much more satisfactory knowledge. They are
executed by different kinds of perforations; viz. by longitudinal galleries hollowed out[840]
of the mass of the beds or veins themselves, in following their course; by transverse
galleries, pushed at right angles to the direction of the veins; by inclined shafts, which
pursue the slope of the deposits, and are excavated in their mass; or, lastly, by perpendicular
pits.
If a vein or bed unveils itself on the flank of a mountain, it may be explored, according
to the greater or less slope of its inclination, either by a longitudinal gallery opened
in its mass, from the outcropping surface, or by a transverse gallery falling upon it in a
certain point, from which either an oblong gallery or a sloping shaft may be opened.
If our object be to reconnoitre a highly inclined stratum, or a vein in a level country,
we shall obtain it with sufficient precision, by means of shafts, 8 or 10 yards deep, dug
at 30 yards distance from one another; excavated in the mass of ore, in the direction of
its deposit. If the bed is not very much inclined, only 45°, for example, vertical shafts
must be opened in the direction of its roof, or of the superjacent rocky stratum, and
galleries must be driven from the points in which they meet the ore, in the line of its
direction.
When the rocks which cover valuable minerals are not of very great hardness, as
happens generally with the coal formation, with pyritous and aluminous slates, sal gem,
and some other minerals of the secondary strata, the borer is employed with advantage
to ascertain their nature. This mode of investigation is economical, and gives, in such
cases, a tolerably exact insight into the riches of the interior. The method of using the
borer, has been described under Artesian Wells.
OF MINING IN PARTICULAR.
The mode of working mines is two-fold; by open excavations, and subterranean.
Workings in the open air present few difficulties, and occasion little expense, unless
when pushed to a great depth. They are always preferred for working deposits little
distant from the surface; where, in fact, other methods cannot be resorted to, if the
substance to be raised be covered with incoherent matters. The only rules to be
observed are, to arrange the workings in terraces, so as to facilitate the cutting down of
the earth; to transport the ores and the rubbish to their destination at the least possible
expense; and to guard against the crumbling down of the sides. With the latter view,
they ought to have a suitable slope, or to be propped by timbers whenever they are not
quite solid.
Open workings, are employed for valuable clays, sands, as also for the alluvial soils of
diamonds, gold, and oxide of tin, bog iron ores, &c., limestones, gypsums, building stones,
roofing slates, masses of rock salt in some situations, and certain deposits of ores, particularly
the specular iron of the island of Elba; the masses of stanniferous granite of
Geyer, Altenberg, and Seyffen, in the Erzgebirge, a chain of mountains between
Saxony and Bohemia; the thick veins or masses of black oxide of iron of Nordmarch,
Dannemora, &c., in Sweden; the mass of cupreous pyrites of Ræraas, near Drontheim,
in Norway; several mines of iron, copper, and gold in the Ural mountains, &c.
Subterranean workings may be conveniently divided into five classes, viz.:—
1. Veins, or beds, much inclined to the horizon, having a thickness of at least
two yards.
2. Beds of slight inclination, or nearly horizontal, the power or thickness of which
does not exceed two yards.
3. Beds of great thickness, but slightly inclined.
4. Veins, or beds highly inclined, of great thickness.
5. Masses of considerable magnitude in all their dimensions.
Subterranean mining requires two very distinct classes of workings; the preparatory,
and those for extraction.
The preparatory consist in galleries, or in pits and galleries destined to conduct the
miner to the point most proper for attacking the deposit of ore, for tracing it all round
this point, for preparing chambers of excavation, and for concerting measures with a
view to the circulation of air, the discharge of waters, and the transport of the extracted
minerals.
If the vein or bed in question be placed in a mountain, and if its direction forms
a very obtuse angle with the line of the slope, the miner begins by opening in its side, at
the lowest possible level, a gallery of elongation, which serves at once to give issue to
the waters, to explore the deposit through a considerable extent, and then to follow it
in another direction; but to commence the real mining operations, he pierces either
shafts or galleries, according to the slope of the deposit, across the first gallery.
For a stratum little inclined to the horizon, placed beneath a plain, the first thing is
to pierce two vertical shafts, which are usually made to arrive at two points in the same
line of slope, and a gallery is driven to unite them. It is, in the first place, for the sake
of circulation of air that these two pits are sunk; one of them, which is also destined
for the drainage of the waters, should reach the lowest point of the intended workings.[841]
If a vein is intersected by transverse ones, the shafts are placed so as to follow, or, at
least, to cut through the intersections. When the mineral ores lie in nearly vertical
masses, it is right to avoid, as far as possible, sinking pits into their interior. These should
rather be perforated at one side of their floor, even at some considerable distance, to avoid
all risk of crumbling the ores into a heap of rubbish, and overwhelming the workmen.
With a vein of less than two yards thick, as soon as the preparatory labours have
brought the miners to the point of the vein from which the ulterior workings are to
ramify, whenever a circulation of air has been secured, and an outlet to the water and
the matters mined, the first object is to divide the mass of ore into large parallelopipeds,
by means of oblong galleries, pierced 20 or 25 yards below one another, with pits
of communication opened up, 30, 40, or 50 yards asunder, which follow the slope of
the vein. These galleries and shafts are usually of the same breadth as the vein, unless
when it is very narrow, in which case it is requisite to cut out a portion of the roof or
the floor. Such workings serve at once the purposes of mining, by affording a portion
of ore, and the complete investigation of the nature and riches of the vein, a certain
extent of which is thus prepared before removing the cubical masses. It is proper to
advance first of all, in this manner, to the greatest distance from the central point which
can be mined with economy, and afterwards to remove the parallelopiped blocks, in
working back to that point.
This latter operation may be carried on in two different ways; of which one consists
in attacking the ore from above; and another from below. In either case, the excavations
are disposed in steps similar to a stair upon their upper or under side. The first is
styled a working in direct or descending steps; and the second a working in reverse, or
ascending steps.
1. Suppose, for example, that the post N, fig. 714., included between the horizontal
gallery A C, and the shaft A B, is to be excavated by direct steps, a workman stationed
upon a scaffold at the point a, which forms the angle between the shaft and the elongated
drift, attacks the rock in front of him and beneath his feet. Whenever he has cut
out a parallelopiped (a rectangular mass), of from four to six yards broad, and two yards
high, a second miner is set to work upon a scaffold at a′, two yards beneath the first,
who, in like manner, excavates the rock under his feet and before him. As soon as the
second miner has removed a post of four or six yards in width, by two in height, a third
begins upon a scaffold at a′′ to work out a third step. Thus, as many workmen are
employed as there are steps to be made between the two oblong horizontal galleries
which extend above and below the mass to be excavated; and since they all proceed
simultaneously, they continue working in similar positions, in floors, over each other, as
upon a stair with very long wide steps. As they advance, the miners construct before
them wooden floors c c c c, for the purpose of supporting the rubbish which each
workman extracts from his own step. This floor, which should be very solid, serves
also for wheeling out his barrow filled with ore. The round billets which support
the planks sustain the roof or the wall of the mineral vein or bed under
operation. If the rubbish be very considerable, as is commonly the case, the floor
planks are lost. However strongly they may be made, as they cannot be repaired,
they sooner or later give way under the enormous pressure of the rubbish; and as all
the weight is borne by the roof of the oblong gallery underneath, this must be sufficiently
timbered. By this ingenious plan, a great many miners may go to work
together upon a vein without mutual interference; as the portions which they detach
have always two faces at least free, they are consequently more easily separable, either[842]
with gunpowder or with the pick. Should the vein be more than a yard thick, or if its
substance be very refractory, two miners are set upon each step. b b b b indicate the
quadrangular masses that are cut out successively downwards; and 1 1, 2 2, 3 3,
forwards; the lines of small circles are the sections of the ends of the billets which
support the floors.
2. To attack a mass Y, fig. 715., a scaffold m, is erected in one of its terminal pits P P,
at the level of the ceiling of the gallery R R′, where it terminates below. A miner
placed on this scaffold, cuts off at the angle of this mass a parallelopiped 1, from one
to two yards high, by six or eight long. When he has advanced thus far, there is
placed in the same pit, upon another scaffold m′, a second miner, who attacks the vein
above the roof of the first cutting, and hews down, above the parallelopiped 1, a parallelopiped
of the same dimensions 1′, while the first is taking out another 2, in advance
of 1. When the second miner has gone forward 6 or 8 yards, a third is placed also
in the same pit. He commences the third step, while the first two miners are pushing
forwards theirs, and so in succession.
In this mode of working, as well as in the preceding, it is requisite to support the
rubbish and the walls of the vein. For the first object, a single floor n n n, may be
sufficient, constructed above the lower gallery, substantial enough to bear all the
rubbish, as well as the miners. In certain cases, an arched roof may be substituted;
and in others, several floors are laid at different heights. The sides of the vein are
supported by means of pieces of wood fixed between them perpendicularly to their
planes. Sometimes, in the middle of the rubbish, small pits are left at regular distances
apart, through which the workmen throw the ore coarsely picked, down into
the lower gallery. The rubbish occasionally forms a slope f f f, so high that miners
placed upon it can work conveniently. When the rich portions are so abundant as
to leave too little rubbish to make such a sloping platform, the miners plant themselves
upon movable floors, which they carry forward along with the excavations.
These two modes of working in the step-form, have peculiar advantages and disadvantages;
and each is preferred to the other according to circumstances.
In the descending workings or in direct steps, fig. 714., the miner is placed on the very
mass or substance of the vein; he works commodiously before him; he is not exposed
to the splinters which may fly off from the roof; but by this plan he is obliged to
employ a great deal of timber to sustain the rubbish; and the wood is fixed for ever.
In the ascending workings, or in reversed steps, fig. 715., the miner is compelled to
work in the re-entering angle formed between the roof and the front wall of his
excavation, a posture sometimes oppressive; but the weight of the ore conspires with
his efforts to make it fall. He employs less timber than in the workings with direct
steps. The sorting of the ore is more difficult than in the descending working, because
the rich ore is sometimes confounded with the heap of rubbish on which it falls.
When seams of diluvium or gravel-mud, occur on one of the sides of the vein, or
on both, they render the quarrying of the ore more easy, by affording the means of
uncovering the mass to be cut down, upon an additional face.
Should the vein be very narrow, it is necessary to remove a portion of the sterile
rock which encloses it, in order to give the work a sufficient width to enable the
miner to advance. If, in this case, the vein be quite distinct from the rock, the labour
may be facilitated, as well as the separation of the ore, by disengaging the vein, on one
of its faces through a certain extent, the rock being attacked separately. This operation
is called stripping the vein. When it is thus uncovered, a shot of gunpowder is sufficient
to detach a great mass of it, unmixed with sterile stones.
By the methods now described, only those parallelopipeds are cut out, either in
whole or in part, which present indications of richness adequate to yield a prospect of
benefit. In other cases, it is enough to follow out the threads of ore which occur, by
workings made in their direction.
[843]
The miner, in searching within the crust of the earth for the riches which it conceals,
is exposed to many dangers. The rocks amidst which he digs are seldom or never
entire, but are almost always traversed by clefts in various directions, so that impending
fragments threaten to fall and crush him at every instant. He is even obliged at times
to cut through rotten friable rocks or alluvial loams. Fresh atmospheric air follows
him with difficulty in the narrow channels which he lays open before him; and the
waters which circulate in the subterranean seams and fissures filter incessantly into his
excavation, and tend to fill it. Let us now take a view of the means he employs to
escape from these three classes of dangers.
1. Of the timbering of excavations.—The excavations of mines, are divisible into
three principal species; shafts, galleries, and chambers. When the width of these excavations
is inconsiderable, as is commonly the case with shafts and galleries, their sides
can sometimes stand upright of themselves; but more frequently they require to be
propped or stayed by billets of wood, or by walls built with bricks or stones; or even
by stuffing the space with rubbish. These three kinds of support are called timbering,
walling, and filling up.
Timbering is most used. It varies in form for the three species of excavations,
according to the solidity of the walls which it is destined to sustain.
In a gallery, for example, it may be sufficient to support merely the roof, by means
of joists placed across, bearing at their two ends in the rock; or the roof and the two
walls by means of an upper joist S, fig. 716., which is then called a cap or cornice beam,
resting on two lateral upright posts or stanchions, a, b,
to which a slight inclination towards each other is given,
so that they approach a little at the top, and rest entirely
upon the floor. At times, only one of the walls and the
roof need support. This case is of frequent occurrence
in pipe veins. Pillars are then set up only on one side,
and on the other the joists rest in holes of the rock. It
may happen that the floor of the gallery shall not be
sufficiently firm to afford a sure foundation to the standards;
and it may be necessary to make them rest on a
horizontal piece called the sole. This is timbering with
complete frames. The upright posts are usually set
directly on the sole; but the extremities of the cap or
ceiling, and the upper ends of the standards, are mortised
in such a manner that these cannot come nearer, whereby
the cap shall possess its whole force of resistance. In
friable and shivery rocks there is put behind these beams, both upon the ceiling and
the sides, facing boards, which are planks placed horizontally, or spars of cleft wood,
set so close together as to leave no interval. They are called fascines in French.
In ordinary ground, the miner puts up these planks in proportion as he goes forwards;
but in a loose soil, such as sand or gravel, he must mount them a little in advance.
He then drives into the mass behind the wooden frame-work, thick but sharp-pointed
planks or stakes, and which, in fact, form the sides of the cavity, which he proceeds
to excavate. Their one extremity is thus supported by the earth in which it is thrust,
and their other end by the last framing. Whenever the miner gets sufficiently on, he
sustains the walls by a new frame. The size of the timber, as well as the distance
between the frames or stanchions, depends on the degree of pressure to be resisted.
When a gallery is to serve at once for several distinct purposes, a greater height is
given to it; and a flooring is laid on it at a certain level. If, for example, a gallery is
to be employed, both for the transport of the ores and the discharge of the waters, a
floor e e, fig. 715., is constructed above the bottom, over which the carriages are
wheeled, and under which the waters are discharged.
The timbering of shafts varies in form, as well as that of galleries, according to the
nature and the locality of the ground which they traverse, and the purposes which
they are meant to serve. The shafts intended to be stayed with timber are usually
square or rectangular, because this form, in itself more convenient for the miner, renders
the execution of the timbering more easy. The wood-work consists generally of
rectangular frames, the spars of which are about eight inches in diameter, and placed
at a distance asunder of from a yard to a yard and a half. The spars are never placed
in contact, except when the pressure of the earth and the waters is very great. The
pieces composing the frames are commonly united by a half-check, and the longer of
the two pieces extends often beyond the angles, to be rested in the rock. Whether the
shaft is vertical or inclined, the frame-work is always placed so that its plane may be
perpendicular to the axis of the pit. It happens sometimes in inclined shafts that there
are only two sides, or even a single one, which needs to be propped. These are stayed
by means of cross beams, which rest at their two ends in the rock. When the frames[844]
do not touch one another, strong planks or stakes are fastened behind them to sustain
the ground. To these planks the frames are firmly connected, so that they cannot
slide. In this case the whole timbering will be supported, when the lower frame is solidly
fixed, or when the pieces from above pass by its angles to be abutted upon the ground.
In the large rectangular shafts, which serve at once for extracting the ores, for the
discharge of the waters, and the descent of the workmen, the spaces destined for these
several purposes are in general separated by partitions, which also serve to increase the
strength of the timberings, by acting as buttresses to the planks in the long sides of the
frame-work. Occasionally a partition separates the ascending from the descending
basket, to prevent their jostling.—Lastly, particular passages are left for ventilation.
As it is desirable that the wood shall retain its whole force, only those pieces are
squared which absolutely require it. The spars of the frames in shafts and galleries are
deprived merely of their bark, which by holding moisture, would accelerate the decomposition
of the wood. The alburnum of oak is also removed.
Resinous woods, like the pine, last much shorter than the oak, the beech, and the
cherry-tree; though the larch is used with advantage. The oak has been known to
last upwards of 40 years; while the resinous woods decay frequently in 10. The fresher
the air in mines, the more durable is the timbering.
The marginal figs. 717, 718. represent two vertical sections of a shaft, the one at right
angles to the other, with the view of showing the mode of sustaining the walls of the
excavation by timbering. It is copied from an actual mine in the Hartz. There we may
observe the spaces allotted to the descent of the miners by
ladders, to the drainage of the waters by pumps P, and rods t,
and to the extraction of the mineral substances by the baskets
B. a, b, c, f, h, k, various cross timbers; A, C, E, upright do.;
R, pump cistern; V, W, corve-ways. The shafts here shown,
are excavated in the line of the vein itself,—the rock enclosing
it being seen in the second figure.
In a great many mines it is found advantageous to support
the excavations by brick or stone buildings, constructed
either with or without mortar. These constructions are
often more costly than wooden ones, but they last much
longer, and need fewer repairs. They are employed instead
of timberings, to support the walls and roof of galleries, to
line the sides of shafts, and to bear up the roofs of excavations.
Sometimes the two sides of a gallery are lined with vertical
walls, and its roof is supported by an ogee vault, or an
arch. If the sides of the mine are solid, a simple arch is
sufficient to sustain the roof and at other times the whole surface of a gallery is formed of
a single elliptic vault, the great axis of which is vertical; and the bottom is surmounted
by a wooden plank, under which the waters run off; see fig. 719.
Walled shafts also are sometimes constructed in a circular or elliptic form, which is
better adapted to resist the pressure of the earth and waters. Rectangular shafts of all
dimensions, however, are frequently walled.
The sides of an excavation may also be supported by filling it completely with
rubbish. Wherever the sides need to be supported for some time without the necessity
of passing along them, it is often more economical to stuff them up with rubbish,
than to keep up their supports. In the territory of Liege, for example, there have
been shafts thus filled up for several centuries; and which are found to be quite entire
when they are emptied. The rubbish is also useful for forming roads among steep
strata, for closing air-holes, and forming canals of ventilation.
Figs. 719. 720. 721. represent the principal kinds of mason-work employed in the
galleries and shafts of mines. Fig. 722. exhibits the walling in of the cage of an overshot
water-wheel, as mounted within a mine. Before beginning to build, an excavation[845]
large enough must be made in the gallery to leave a space three feet and a half
high for the workmen to stand in, after the brick-work is completed. Between the
two opposite sides, cross beams of wood must be fixed at certain distances, as chords
of the vault, over which the rock must be hollowed out to receive the arch-stones,
and the centring must then be placed, covered with deals to receive the voussoirs,
beginning at the flanks and ending with the key-stone. When the vault is finished
through a certain extent, the interval between the arch and the rock must be
rammed full of rubbish, leaving passages if necessary through it and the arch, for
currents of water.
In walling galleries, attention must be paid to the direction of the pressure, and to
build vertically or with a slope accordingly. Should the pressure be equal in all
directions, a closed vault, like fig. 719., should be formed. For walls not far from the
vertical, salient or buttressed arches are employed, as shown in fig. 720., called in
German überspringende bogen; for other cases, twin-arches are preferred, with an upright
wall between.
Fig. 721. is a transverse section of a walled drain-gallery, from the grand gallery of
the Hartz; see also fig. 722. a is the rock which needs to be supported only at the sides
and top; b, the masonwork, a curve formed of the three circular arcs upon one level;
c, the floor for the watercourse. Fig. 719. is a cross section of a walled gallery, as at
Schneeberg, Rothenburg, Idria, &c.; d, is the rock, which is not solid either at the
flanks, roof, or floor; e, the elliptic masonwork; f, the wooden floor for the waggons,
which is sometimes, however, arched in brick to allow of a watercourse beneath it.
Fig. 720. shows two vertical projections of a portion of a walled shaft with buttresses,
as built at the mine Vater Abraham, near Marienberg. J is a section in the direction of
the vein g h, to show the roof of the shaft. I, a section exhibiting the slope of the vein
g h, into which the shaft is sunk; m is the wall of the vein; k is the roof of the same
vein; n, buttresses resting upon the flanks of the shaft; g, great arcs on which the
buttresses bear; y, vertical masonwork; z, a wall which divides the shaft into two compartments,
of which the larger p is that for extracting the ore, and the smaller for the
draining and descent of the miners.
Fig. 722. C D is the shaft in which the vertical crank-rods c g, e d, move up and
down. F, is a double hydraulic wheel, which can be stopped at pleasure by a brake
mounted upon the machine of extraction. G, is the drum of the gig or whim for raising
the corves or tubs (tonnes); H, is the level of the ground, with the carpentry which supports
the whim and its roof. k, is the key-stone of the ogee arch which covers the
water-wheel; a, is the opening or window, traversed by the extremity of the driving
shaft, upon each side of the water-wheel, through which a workman may enter to adjust
or repair it; c b, line of conduits for the streams of water which fall upon the hydraulic[846]
wheel; c, g, double crank with rods, whose motion is taken off the left side of the wheel;
e, d, the same upon the right side. The distance from H to F is about 22 yards.
Figs. 723. 724. present two vertical sections of the shaft of a mine walled, like the
roof of a cavern, communicating with the galleries of the roof and the wall of the vein,
and well arranged for both the extraction of the ore, and the descent of the miners.
The vertical partition of the shaft for separating the passage for the corves or tubs from
the ladders is omitted in the figure, for the sake of clearness.
In fig. 723., A, B are the side walls supported upon the buttresses C and D; in
fig. 724., E is the masonry of the wall, borne upon the arch F at the entrance to a gallery;
the continuation being at G, which is sustained by a similar arch built lower.
L, is the vault arch of the roof, supported upon another vault M, which presents a
double curvature, at the entrance of a gallery; at H is the continuation of the arch or vault
L, which underneath is supported in like manner at the entrance of a lower gallery.
a b, c d, fig. 723., are small upright guide-bars or rods for one of the corves, or kibbles.
e f, g h, are similar guide-bars for the other corf.
i i, are cross-bars of wood, which support the stays of the ladders of descent.
k k, are also cross-bars by which the guide-rods are secured.
t, a corf, or extraction kibble, furnished with friction rollers; the other corf is supposed
to be drawn up to a higher level, in the other vertical passage.
Figs. 725. 726. represent in a vertical section the mode of timbering the galleries
of the silver and lead mines at Andreasberg in the Hartz. Fig. 725. shows the plan
viewed from above. Upon the roof of the timbering, the workman throws the waste
rubbish, and in the empty space below, which is shaded black, he transports in his
waggons or wheelbarrows the ores towards the mouth of the mine. Fig. 726. is the
cross section of the gallery. In the two figures, a represents the rock, and b the timbering;
round which there is a garniture of small spars or lathes for the purpose of
drainage and ventilation, with the view of promoting the durability of the wood-work.
[847]
The working of minerals by the mass is well exemplified a few leagues to the north
of Siegen, near the village of Müsen, in a mine of iron and other metals, called
Stahlberg, which forms the main wealth of the country. The plan of working is
termed the excavation of a direct or transverse mass. It shows in its upper part the
danger of bad mining, and in its inferior portion, the regular workings, by whose
means art has eventually prevented the destruction of a precious mineral deposit.
Fig. 727. is a vertical section of the bed of ore, which is a direct mass of spathose
iron, contained in transition rock (greywacke). a, a, a, are pillars of the sparry ore,
reserved to support the successive stages or floors, which are numbered 1. 2. 3. &c.;
b, b, b, are excavations worked in the ore; which exhibit at the present day several
floors of arches, of greater or less magnitude, according to the localities. It may be
remarked, that where the metallic deposit forms one entire mass, rich in spathose iron
ore of good quality, there is generally given to the vaults a height of three fathoms;
leaving a thickness over the roof of two fathoms, on account of the numerous fissures
which pervade the mass. But where this mass is divided into three principal branches,
the roof of the vaults has only a fathom and a half of thickness, while the excavation is
three fathoms and a half high. In the actual state of the workings, it may be estimated
that from all this direct mass, there is obtained no more out of every floor than one-third
of the mineral. Two-thirds remain as labours of reserve, which may be resumed
at some future day, in consequence of the regularity and the continuation of the subterranean
workings. e is a shaft for extraction, communicating below with the gallery of
efflux k; h is an upper gallery of drainage, which runs in different directions (one only
being visible in this section) over a length of 400 fathoms. The lower gallery k runs
646 fathoms in a straight line. The mine of Stahlberg has furnished annually on an
average since 1760 about 25,000 cubic feet (French) of an excellent spathose ore
of iron. m m, represents the mass of sparry iron.
Figs. 728, 729, 730. represent the cross system of mining, which consists in forming
galleries through a mineral deposit, from its wall or floor
towards its roof, and not, as usual, in the direction of its
length. This mode was contrived towards the middle of
the 18th century, for working the very thick veins of the
Schemnitz mine in Hungary, and it is now employed with
advantage in many places, particularly at Idria in Carniola.
In the two sections figs. 728., 730., as well as in the ground
plan fig. 729., the wall is denoted by m m, and the roof by t t.
A first gallery of prolongation E F, fig. 730., being formed to the
wall, transverse cuts, a a, are next established at right angles to this gallery, so that between
every two there may be room enough to place three others, b, c, b, fig. 729. From
each of the cuts a, ore is procured by advancing with the help of timbering, till the
roof t be reached. When this is done, these first cuts a, are filled up with rubbish, laid
upon pieces of timber with which the ground is covered, so that if eventually, it should
be wished to mine underneath, no downfall of detritus is to be feared. These heaps
of rubbish rise only to within a few inches of the top of the cuts a, in order that the
working of the upper story may be easier, the bed of ore being there already laid open
upon its lower face.
In proportion as the cuts a, of the first story E F, are thus filled up, the greater part
of the timbering is withdrawn, and made use of elsewhere. The intermediate cuts
b, c, b, are next mined in like manner, either beginning with the cuts c, or the cuts b, according
to the localities. From fig. 729. it appears that the working may be so arranged,
that in case of necessity, there may be always between two cuts in activity the[848]
distance of three cuts, either not made, or filled up with rubbish. Hence, all the portion
of the bed of ore may be removed, which corresponds to a first story E F fig. 730.,
and this portion is replaced by rubbish.
The exploration of the upper stories E′ F′, E2 F2, E3 F3, is now prepared in a similar
manner; with which view shafts h h3, k k3, are formed from below upwards in the wall
m of the deposit, and from these shafts oblong galleries proceed, established successively
on a level with the stories thus raised over one another. See fig. 730. The following
objects may be specified in the figures:—
a a, the first cuts filled up with rubbish, upon the first story E F, fig. 729.
b b, other cuts subsequently filled up, upon the same story.
c, the cut actually working.
d, the front of the cut, or place of actual excavation of the mineral deposit.
e, masses of the barren rock, reserved in the cutting, as pillars of safety.
f, galleries, by means of which the workmen may turn round the mass e, in order to
form, in the roof t, an excavation in the direction of the deposit.
g, rubbish behind the mass e.
k k, two shafts leading from the first story E F, to the upper stories of the workings,
as already stated.
m, the wall, and t the roof of the mineral bed.
In the second story E′ F′, the gallery of prolongation F′, figs. 728. and 730., is not entirely
perforated; but it is further advanced than that of the third story, which, in its turn,
is more than the gallery of the fourth.
From this arrangement there is produced upon fig. 730. the general aspect of a working
by reversed steps.
Whenever the workings of the cuts c in the first story are finished, those of the second,
a′ a′, may be begun in the second; and thus by mounting from story to story,
the whole deposit of ore may be taken out and replaced with rubbish. One great advantage
of this method is, that nothing is lost; but it is not the only one. The facilities
offered by the system of cross workings for disposing of the rubbish, most frequently a
nuisance to the miner, and expensive to get rid of, the solidity which it procures by the
banking up, the consequent economy of timbering, and saving of expense in the excavation
of the rock, reckoning from the second story, are so many important circumstances
which recommend this mode of mining. Sometimes, indeed, rubbish may be
wanted to fill up, but this may always be procured by a few accessory perforations; it
being easy to establish in the vicinity of the workings a vast excavation in the form of
a vault, or kind of subterraneous quarry, which may be allowed to fall in with proper
precautions, and where rubbish will thus accumulate in a short time, at little cost.
Fig. 731. represents a section of the celebrated lead mines of Bleyberg in Carinthia,
not far from Villach.
b, c, is the ridge of the mountains of compact limestone, in whose bosom the
workings are carried on.
e, is the metalliferous valley, running from east to west, between the two parallel[849]
valleys of the Gail and the Drave, but at a level considerably above the waters of these
rivers.
f g, is the direction of a great many vertical beds of metalliferous limestone.
On considering the direction and dip of the marly schist, and metalliferous limestone,
in the space w, w, to the west of
the line 1, s, it would appear that
a great portion of this system of
mountains has suffered a slip between
1, s, and a parallel one towards the
east; whereby, probably, that vertical
position of the strata has been
produced, which exists through a
considerable extent. The metalliferous
limestone is covered to a certain
thickness with a marly schist, and
other more recent rocks. It is in
this schist that the fine marble known
under the name of the lumachella of
Bleyberg is quarried.
The galena occurs in the bosom of this rock in flattened masses, or blocks of a considerable
volume, which are not separated from the rest of the calcareous beds by any
seam. It is accompanied by zinc ore (calamine), especially in the upper parts of the
mountain.
Several of the workable masses are indicated by r, r3; each presents itself as a
solid analogous to a very elongated ellipse, whose axis dips, not according to the inclination
of the surrounding rock, but to an oblique or intermediate line between this
inclination, and the direction of the beds of limestone; as shown by r w, r′ u. Every
thing indicates the contemporaneous formation of the limestone, and the lying beds of
the lead ore.
The accidents or faults called kluft (rent) at Bleyberg are visible on the surface of the
ground. Experienced miners have remarked that the rich masses occur more frequently
in the direction of these accidents than elsewhere.
It is in general by galleries cut horizontally in the body of the mountain, and at different
levels, s, g, s f, that the miner advances towards the masses of ore r, r3.
Many of these galleries are 500 fathoms long before they reach a workable mass. The
several galleries are placed in communication by a few shafts, such as t; but few of these
are sunk deeper than the level of the valley e.
The total length of the mines of Bleyberg is about 10,000 yards, parallel to the
valley e; in which space there are 500 concessions granted by the government to various
individuals or joint stock societies, either by themselves or associated with the government.
The metalliferous valley contains 5000 inhabitants, all deriving subsistence from the
mines; 300 of whom are occupied in the government works.
Each concession has a number and a name; as Antoni, Christoph, Matthæus, Oswaldi,
2, 8, 36, &c.
Fig.. 732 is a section in the quicksilver mine of Idria. 1. is the gray-limestone;
2. is a blackish slate; 5. is a grayish slate. Immediately above these transition rocks
lies the bed containing the ores called corallenerz, which consist of an intimate mixture
of sulphuret of mercury and argillaceous limestone; in which four men can cut out, in
a month, 21⁄2 toises cube of rock.
Fig. 733. represents a section
of part of the copper mine of
Mansfeldt; containing the cellular
limestone, called rauchwacke, always
with the compact marl-limestone
called zechstein; the cupreous
schist, or kupferschiefer;
the wall of grayish-white sandstone,
called the weisse liegende;
and the wall of red sandstone, or
the rothe liegende. The thin dotted
stratum at top is vegetable mould;
the large dotted portion to the
right of the figure is oolite; the
vein at its side is sand; next is rauchwacke; and lastly, the main body of fetid limestone,
or stinkstein.
[850]
Fig. 734. represents one of the Mansfeldt copper schist mines in the district called
Burgoerner, or Preusshoheit.
1. Vegetable mould, with siliceous gravel.
2. Ferruginous clay or loam.
3. Sand, with fragments of quartz.
4. Red clay, a bed of variable thickness as well as the lower strata, according as the
cupreous schist is nearer or farther from the surface.
5. Oolite (roogenstein).
6. Newer variegated sandstone, (bunter sandstein).
7. Newer gypsum; below which, there is
8. A bluish marly clay.
9. Stinkstone, or lucullite.
10. Friable grayish marl.
11. Older gypsum, a rock totally wanting in the other districts of the mines of Rothenberg;
but abounding in Saxon Mansfeldt, where it includes vast caverns known
among the miners by the name of schlotten, as indicated in the figure.
12. The calcareous rock called zechstein. The lower part of this stratum shows
symptoms of the cupriferous schist that lies underneath. It presents three thin bands,
differently modified, which the miner distinguishes as he descends by the names of the
sterile or rotten (faüle) rock; the roof (dachklotz); and the main rock (oberberg.)
13. Is a bed of cupriferous schist (kupferschiefer), also called the bitumino-marly
schist, in which may be noted, in going down, but not marked in the figure:—
- a, the lochberg, a seam 4 inches thick.
- b, the kammschale, 1⁄4 of an inch thick.
- c, the kopfschale, one inch thick.
These seams are not worth smelting; the following, however, are:—
- d, the schiefer kopf, the main copper-schist, 2 inches thick.
- e, a layer called lochen, one inch thick.
14. The wall of sandstone, resting upon a porphyry.
Fig. 735. is a section of the mines of Kiegelsdorf in Hessia, presenting—
1. Vegetable mould.
2. Limestone distinctly stratified, frequently of a yellowish colour, called lagerhafter
kalkstein.
3. Clay, sometimes red, sometimes blue, sometimes a mixture of red, blue, and
yellow.
4. The cellular limestone (rauhkalk). This rock differs both in nature and position
from the rock of the same name at Mansfeldt.
5. Clay, usually red, containing veins of white gypsum, and fine crystals of selenite.
6. Massive gypsum of recent formation.
7. Fetid limestone, compact and blackish gray, or cellular and yellowish gray.
8. Pulverulent limestone, with solid fragments interspersed.
9. Compact marl-limestone, or zechstein, which changes from a brownish colour above
to a blackish schist below, as it comes nearer the cupreous schist, which seems to form a
part of it.
10. Cupreous schist (kuperschiefer), of which the bottom portion, from 4 to 6 inches
thick, is that selected for metallurgic operations. Beneath it, is found the usual wall
or bed of sandstone. A vein of cobalt ore a, which is rich only in the grayish-white
sandstone (weisse liegende), traverses and deranges all the beds wherever it comes.
Of working mines by fire.—The celebrated mine worked since the tenth century in
the mountain called Rammelsberg, in the Hartz, to the south of Goslar, presents a stratified[851]
mass of ores, among the beds of the rock which constitute that mountain. The
mineral deposit is situated in the earth, like an enormous inverted wedge, so that its
thickness (power), inconsiderable near the surface of the ground, increases as it
descends. At about 100 yards from its outcrop, reckoning in the direction of the slope
of the deposit, it is divided into two portions or branches, which are separated from
each other, throughout the whole known depth, by a mass of very hard clay slate,
which passes into flinty slate. The substances composing the workable mass are
copper and iron pyrites with sulphuret of lead (galena), accompanied by quartz, carbonate
of lime, compact sulphate of baryta, and sometimes gray copper ore, sulphuret
of zinc, and arsenical pyrites. The ores of lead and copper contain silver and gold, but
in small proportion, particularly as to the last.
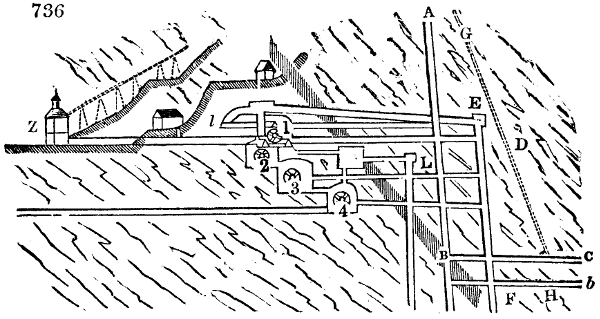
A mine so ancient as that of Rammelsberg, and which was formerly divided among
several adventurous companies, cannot fail to present a great many shafts and excavations;
but out of the 15 pits, only two are employed for the present workings;
namely, those marked A B and E F, in fig. 736., by which the whole extraction and
drainage are executed.—The general system of exploitation by fire, as practised in this
mine, consists of the following operations:—
1. An advance is made towards the deposits of ore, successively at different levels,
by transverse galleries which proceed from the shaft of extraction, and terminate at the
wall of the stratiform mass.
2. There is formed in the level to be worked, large vaults in the heart of the ore, by
means of fire, as we shall presently describe.
3. The floor of these vaults is raised up by means of terraces formed from the rubbish,
in proportion as the roof is scooped out.
4. The ores detached by the fire from their bed, are picked and gathered; sometimes
the larger blocks are blasted with gunpowder.
5. Lastly, the ores thus obtained are wheeled towards the shaft of extraction, and
turned out to the day.
Let us now see how the excavation by fire is practised; and in that view, let us consider
the state of the workings in the mines of Rammelsberg in 1809. We may remark
in fig. 736. the regularity of the vaults previously scooped out above the level B C, and
the other vaults which are in full activity of operation. It is, therefore, towards the
lower levels that the new workings must be directed. For this purpose, the transverse
gallery being already completed, there is prepared on the first of these floors a vault of
exploitation at b, which eventually is to become similar to those of the superior levels.
At the same time, there is commenced at the starting point below it, reached by a
small well dug in the line of the mineral deposit, a transverse gallery in the rock, by
means of blasting with gunpowder. The rock is also attacked at the starting-point
by a similar cut, which advances to meet the first perforation. In this way, whenever
the vaults of the level C are exhausted of ore and terraced up with rubbish, those of the
level beneath it will be in full activity.
Others will then be prepared at a lower level; and the exploitation may afterwards
be driven below this level by pursuing the same plan, by which the actual depth of
excavation has been gained.
In workings by fire we must distinguish, 1. The case where it is necessary to open
a vault immediately from the floor; 2. The case where the vault having already a
certain elevation, it is necessary to heighten its roof. In the former case, the wall or floor
of the mineral deposit is first penetrated by blasting with gunpowder. As soon as this
penetration is effected over a certain length, parallel to the direction of the future
vault, as happens at b, there is arranged on the bottom a horizontal layer of billets of
firwood, over which other billets are piled in nearly a vertical position, which rest upon
the ore, so that the flame in its expansion comes to play against the mineral mass to be[852]
detached. When after some similar operations, the flame of the pile can no longer
reach the ore of the roof on account of its height, a small terrace of rubbish must be
raised on the floor of the deposit; and over this terrace, a new pile of faggots is to be
heaped up as above described. The ancient miners committed the fault of constantly
placing such terraces close to the roof, and consequently arranging the faggots against
this portion of the ore, so that the flame circulated from the roof down to the floor.
The result of such procedure was the weakening of the roof, and the loss of much of the
ore which could not be extracted from so unstable a fabric; and besides, much more
wood was burned than at the present day, because the action of the flame was dissipated
in part against the whole mass of the roof, instead of being concentred on the
portion of the ore which it was desired to dislodge. Now, the flame is usually made to
circulate from the floor to the roof, in commencing a new vault.
When the vault has already a certain height, care is always taken that between the
roof of the vault and the rubbish on which the pile is arranged, no more than two yards
of space should intervene, in order that the flame may embrace equally the whole concavity
of the vault, and produce an uniform effect on all its parts. Here, the pile is
formed of horizontal beds, disposed crosswise above one another, and presents four free
vertical faces, whence it has been called a chest by the miners.
It is usually on Saturday that the fire is applied to all the piles of faggots distributed
through the course of the week. Those in the upper floors of exploitation are first
burned, in order that the inferior piles may not obstruct by their vitiated air, the combustion
of the former. Thus, at 4 o’clock in the morning, the fires are kindled in the
upper ranges; from pile to pile, the fireman and his assistant descend towards the
lower floors, which occupies them till 3 o’clock in the afternoon. Vainly should we
endeavour to describe the majestic and terrific spectacle which the fire presents, as it
unfolds its wings under its metallic vaults, soon filled with vast volumes of smoke and
flame. Let us mark the useful effect which it produces.
When the flame has beat for a few instants on the beds of ore, a strong odour of
sulphur, and sometimes of arsenic is perceived; and soon thereafter loud detonations
are heard in the vaults. Suddenly the flame is seen to assume a blue colour, or even a
white; and at this period, after a slight explosion, flakes of the ore, of greater or less
magnitude, usually fall down on the fire, but the chief portion of the heated mineral
still remains fixed to the vault. The ores pass now into a shattered and divided
condition, which allows them afterwards to be detached by long forks of iron. In this
manner the fire, volatilizing entirely some principles, such as sulphur, zinc, arsenic, and
water, changing the aggregation of the constituent parts of the ore, and causing fissures
by their unequal expansibilities, facilitates the excavation of such materials as resist by
their tenacity the action of gunpowder.
The combustion goes on without any person entering the mine from Saturday evening
till Monday morning, on which day, the fireman and his assistants proceed to
extinguish the remains of the bonfires. On Monday also some piles are constructed in
the parts where the effect of the former ones has been incomplete; and they are kindled
after the workmen have quitted the mine. On Tuesday all hands are employed in
detaching the ores, in sorting them, taking them out, and preparing new piles against
the next Saturday.
The labour of a week consists for every man of five posts during the day, each of 8
hours, and of one post of four hours for Saturday. Moreover, an extra allowance is
made to such workmen as employ themselves some posts during the night.
The labour of one compartment or atelier of the mine consists therefore in arranging
the faggots, in detaching the ore which has already experienced the action of the fire, in
breaking the blocks obtained, in separating the ore from the débris of the pile, and
whenever it may be practicable or useful, in boring holes for blasting with gunpowder.
The heat is so great in this kind of mine, that the men are obliged to work in it without
clothing.
We have already remarked, that besides the working by fire, which is chiefly used
here, recourse is sometimes had to blasting by gunpowder. This is done in order
either to recover the bottom part or ground of the vaults on which the fire can act but
imperfectly, to clear away some projections which would interfere with the effect of the
pile, or lastly to strip the surrounding rock from the mass of the ore, and thence to
obtain schist proper for the construction of the rubbish-terraces.
The blasting process is employed when the foremen of the workshop or mine-chamber
judge that a hole well placed may separate enough of ore to pay the time, the
repair of tools, and the gunpowder expended. But this indemnification is rarely
obtained. The following statement will give an idea of the tenacity which the mineral
deposit often presents.
In 1808, in a portion of the Rammelsberg mine, the ore, consisting of extremely compact
iron and copper pyrites, was attacked by a single man, who bored a mining hole.[853]
After 11 posts of obstinate labour, occupying altogether 88 hours, the workman, being
vigilantly superintended, had been able to advance the hole to a depth of no more than
4 inches; in doing which he had rendered entirely unserviceable 126 punches or borers,
besides 26 others which had been re-tipped with steel, and 201 which had been sharpened;
61⁄4 pounds of oil had been consumed in giving him light; and half a pound of
gunpowder was required for blasting the bore. It was found from a calculation made
upon these facts by the administration of mines, that every inch deep of this hole cost,
at their low price of labour, nearly a florin, value two shillings and sixpence.
It is therefore evident that though the timber, of which the consumption is prodigiously
great, were much less abundant and dearer than it still is at Rammelsberg,
mining by fire would be preferable to every other mode of exploitation. It is even
certain, that on any supposition, the employment of gunpowder would not be practicable
for every part of the mine; and if fuel came to fail, it would be requisite to
renounce the workings at Rammelsberg, although this mountain still contains a large
quantity of metals.
If in all mines the free circulation of air be an object of the highest importance,
we must perceive how indispensable it must be in every part of a mine where the mode
of exploitation maintains the temperature of the air at 112° Fahr., when the workmen
return into it after the combustion of the piles, and in which besides it is necessary that
this combustion be effected with activity in their absence. But in consequence of the
extent and mutual ramifications of the workings, the number of the shafts, galleries,
and their differences of level, the ventilation of the mine is in a manner spontaneously
maintained. The high temperature is peculiarly favourable to it. The aid of art
consists merely in placing some doors judiciously, which may be opened or shut at
pleasure, to carry on the circulation of the air.
In considering the Rammelsberg from its summit, which rises about 400 yards
above the town of Goslar, we observe, first, beds of slaty sandstone, which become the
more horizontal the nearer they approach to the surface. At about 160 yards below
the top level there occurs, in the bosom of the slaty graywacke, a powerful stratum of
shells impasted in a ferruginous sandstone. See D, fig. 730. In descending towards the
face of the ore, the parallel stratification of the clay-slate
which forms its walls and roof grows more and more
manifest. Here the slate is black, compact, and thinly
foliated. The inclination of the different beds of rock
is indicated at B. The substance of the workable mass
is copper and iron pyrites, along with sulphuret of lead,
accompanied by quartz, carbonate of lime, compact sulphate
of baryta, and occasionally gray copper (fahlerz),
sulphuret of zinc, and arsenical pyrites.
The ores are argentiferous and auriferous, but very
slightly so, especially as to the gold. It is the ores of
lead and copper which contain the silver, and in the latter
the gold is found, but without its being well ascertained
in what mineral it is deposited. Sometimes the
copper occurs in the native state, or as copper of cementation.
Beautiful crystals of sulphate of lime are found
in the old workings.
In figs. 736. 737., A B is the shaft of extraction, called
the Kahnenkuhler; N is the ventilation shaft, called Breitlingerwetterschacht;
P is the extraction shaft, called Innier-schacht.
E F, is a new extraction-shaft, called Neuer treibschacht,
by which also the water is pumped up; by A B, and E F,
the whole extraction and draining are carried on. The
ores are raised in these shafts to the level of the waggon-gallery
(galerie de roulage) i, by the whims l, q,
provided with ropes and buckets. 1, 2, 3, 4, fig. 736., represent
the positions of four water-wheels for working the
whims; the first two being employed in extracting the
ores, the last two in draining. The driving stream is
led to the wheel 1, along the drift l; whence it falls in
succession upon the wheels 2, 3, 4. The general system of working consists of the following
operation;—
1. The bed of ore is got at by the transverse galleries, m, n, o, q, r, s, which branch
off from the extraction shaft, and terminate at the wall of the main bed;
2. Great vaults are scooped out at the level of the workings, by means of fire;
[854]
3. The roofs of these vaults are progressively propped with mounds of rubbish;
4. The ores thus detached, or by blasting with gunpowder, are then collected;
5. Lastly, they are wheeled out to the day; and washed near Z.
Comparative Table of celebrated Mines in Europe and America. By F. Burr, Esq.
(Quarterly Mining Review for July, 1835, p. 60.)
| |
Consolidated and United Mines. |
Veta Grande Mines. |
Mine of Valenciana. |
Mine of Himmelsfürst. |
| (At present the richest mines in Cornwall.) |
(At present the richest mines in Mexico.) |
(Richest of the Mexican mines at the beginning of the present century.) |
(Richest of the Saxon mines at the beginning of the present century.) |
| Situation |
Two miles east of Redruth. |
Four miles north of Zacatecas. |
One mile north of Guanaxuato. |
Two miles south-east of Freyberg. |
| Elevation |
Elevation of the surface above the level of the sea, from 200 to 300 ft.; depth of the
bottom of the mine below the level of the sea, about 1,370 feet. |
Elevation of the surface above the level of the sea, supposed to be about 6000 feet.
Elevation of the bottom of the mine above the level of the sea, probably near 5,000 feet. |
Elevation of the surface above the level of the sea, 7,617 feet. Elevation of the bottom
of the mine above the level of the sea, 5,730 feet. |
Elevation of the surface above the level of the sea, 1,346 feet. Elevation of the bottom
of the mine above the level of the sea, 263 feet. |
| Nature of the rock |
The Veta Madre of Guanaxuato, upon which this mine is worked, traverses both clay
slate and porphyry, but it is most productive in the former rock. The clay slate is considered by Humboldt to belong to the transition
class, but situate near the limits of primary formations. This rock in depth, passes into chlorite slate, and talc slate. It contains
subordinate beds of syenite, hornblende slate, and serpentine. The porphyry rests upon the clay slate, and is conformable to it, both in
direction and stratification. |
The rock prevailing in the neighbourhood of Freyberg, in which this and most of the other
mines are situate, is a formation of primary gneiss. |
Primary clay slate resting immediately on granite, a short distance westward of the mines.
The clay slate is intersected by numerous channels of porphyry, which have nearly the same direction as the mineral veins, and are often
of considerable width. The porphyry sometimes appears also to form large irregular masses in the clay slate. Both rocks are traversed by
veins of quartz and clay intersecting the metalliferous veins. |
Transition clay slate, alternating with dolomite, and occasionally with greywacke. This
clay slate is sometimes decomposed; it rests on syenitic rocks, and is in some places covered with porphyry. |
| Nature of the metalliferous deposits |
In the consolidated mines, the eight following lodes are extensively worked:—Wheal
Fortune lode, Cusvea lode, Deeble’s lode, Old lode, Taylor’s lode, Tregonning’s lode, Martin’s lode, and
Glover’s lode. In the united mines, the principal workings are upon the Old lode, and about five or six others are more or less
productive. Numerous smaller lodes or “branches” occur also in both mines. The principal lodes are from 2 or 3, to 7 or 8 feet
wide; the “branches” are generally 12 or 18 inches wide. The direction of the lodes varies from nearly east and west to about
20 degrees north of east and south of west. The underlie of the principal lodes, is from 2 to 3 feet per fathom north, that of the smaller
ones about the same south. |
One principal vein (the Veta Grande) which is generally separated into three
branches, and sometimes into four. When ramified, the width extends to 60 or 70 feet; when united, it varies from 8 or 10 to 20 or 30
feet. The branches are generally about 10 or 12 feet wide, and the upper one is most productive. The direction of the Veta Grande, is from
30 to 40 degrees south of east, and north of west, and its underlie, from two to three feet per fathom south. Other veins of less size,
occur in the neighbourhood of the Veta Grande, which cross it at an acute angle. One of these appears to heave the vein for about 700
feet, being the most remarkable derangement of the kind on record. |
One Veta (the Veta Madre) which is often separated into three branches,
extending from 130 to 160 feet in width. When not ramified, its width varies from 20 or 30 to 60 or 70 feet, but is more commonly from 40
to 50 feet. The direction of the vein, is north-west and south-east; its underlie is south, and about five or six feet per fathom. There
are five veins worked in this mine. |
The principal vein (Teichflache) is from one foot six inches, to three feet in
width, the others are from six to 12 inches wide. The direction of this vein, is nearly north and south, its underlie is west, and about
three feet per fathom. Some of the other veins intersect it. |
| Ores |
Chiefly copper ore, occasionally native copper, blue and green carbonate of copper. Tin, or
oxide of tin, also occurs, but not in very great abundance. |
Chiefly red silver, native silver, sulphuret of silver, and argentiferous pyrites. |
Sulphuret of silver, native silver, prismatic black silver, red silver, native gold,
argentiferous galena. |
Argentiferous sulphuret of lead, native silver, sulphuret of silver, red silver. |
| Produce of the ores |
91⁄4 per cent. of fine copper; average produce in 100 parts of
ore. |
31⁄2 oz. per quintal. |
Four ounces of silver per quintal of 100 lbs., equivalent to
21⁄2
parts of metal in 1,000 of ore, or 1⁄4 per cent. |
Six to seven ounces of silver per quintal of 100 lbs. Equivalent to from
33⁄4 to 41⁄2 parts of metal in 1,000 of ore, or from 3-8ths to nearly
1⁄2 per cent. |
| Veinstone |
Chiefly quartz, of which many varieties occur. |
Chiefly quartz, occasionally amethyst, carbonate of lime, and sulphate of barytes. |
Quartz, amethyst, carbonate of lime, pearlspar, and hornstone. |
Quartz, pearlspar, and calcareous spar. |
| Mineral substances |
The ores are generally accompanied by “gossan”[34] in the backs of the lodes, by blende, and by iron, and
arsenical pyrites in depth. |
The ores are generally accompanied by blende, sulphuret of antimony, and iron pyrites. |
The ores are accompanied by blende, spathose iron, copper and iron pyrites. |
The ores are accompanied by blende, spathose iron, and a little iron and arsenical
pyrites. |
| Depth of the principal shafts |
Woolf’s engine-shaft, 248 fathoms; Pearce’s engine-shaft, 275
fathoms. Some of the other engine shafts are scarcely inferior in depth. |
Tiro General, 182 fathoms; Gallega shaft, 138 fathoms. |
Tiro General, 310 fathoms. |
Frankenschacht, 180 fathoms. |
| [855]Depth of adit
at the principle shafts |
At Woolf’s engine-shaft, 13 fathoms. The average depth of the adit at the other
engine-shafts is about 30 or 40 fathoms. |
There is no adit to this mine. |
There is no adit to this mine. |
The adit at the shaft called Frankenschacht is 47 fathoms in depth. |
| Quantity of water |
Varies from 2,000 to 3,000 gallons per minute. |
About 80 gallons per minute. |
The Valenciana was a dry mine from its commencement in 1760 to 1780, when it first became
troubled with water, in consequence of some of the workings being inadvertently communicated with the adjoining mine of Tepeyac; which,
although upon the same vein, was extremely wet. The quantity of water raised during the late working appears to have been about 110
gallons per minute, but the regular influx was much less. |
50 gallons per minute. |
| Height to which the water is raised |
About 230 fathoms at the consolidated mines, at the united mines, about 110 fathoms. |
On an average about 150 fathoms. |
310 fathoms. |
133 fathoms. |
| Power employed in drainage |
9 steam-engines; 3 of 90-inch cylinder, 3 of 85, 1 of 80, and 2 of 65. A water wheel, 48
feet in diameter. |
Usually 10 malacates.[b] |
A steam-engine of 30-inch cylinder, and 7 malacates. |
Two water-wheels, each 42 feet in diameter. |
| Probable equivalent in actual horsepower |
1,500 constantly at work, or a total number of above 4,500. |
32 horses constantly working, or a total number of about 100 horses.[c] |
65 horses constantly at work, or a total number of about 200. |
16 horses constantly at work or a total number of about 50.[d] |
| Average annual expense in drainage |
12,700l. taking the average of the last ten years.[a] |
20,000l. per annum.[c] |
About 40,000l., per annum.[d] |
Cannot be ascertained, but evidently very small.[d] |
| Quantity of ore annually produced |
16,400 tons of copper ore, a few tons of tin ore.[a] |
21,380 tons of silver ore.[c] |
32,500 tons of silver ore.[d] |
630 tons of silver ore.[d] |
| Produce in metal |
1,517 tons of fine copper, a little tin.[a] |
153,000 lbs. troy of silver.[c] |
221,900 lbs. troy silver.[d] |
6,160 lbs. troy of silver.[d] |
| Total returns, or value of the above |
119,800l.[a] |
423,400l. per annum.[c] |
About 600,000l.[d] |
About 18,000l.[d] |
| Total costs of the mine |
93,500l. exclusive of lord’s dues; 98,600l. including lord’s
dues.[a] |
252,170l. per annum.[c] |
197,900l. per annum.[d] |
9,500l. per annum.[d] |
| Clear profit to the proprietors |
21,000l. per annum.[a] |
171,240l. per annum.[c] |
118,750l. per annum.[d] |
3,560l. per annum.[d] |
| Amount of capital invested |
75,000l.[a] |
130,000l.[c] |
Cannot be ascertained, but known to have been very small.[d] |
Cannot be ascertained, but probably very small.[d] |
| Interest on capital invested |
280 per cent. after paying back the original capital.[a] |
Nearly 700 per cent. after paying back the original capital.[c] |
Not known, but certainly many hundred per cent.[d] |
Not known, but probably very high.[d] |
| Proportion of costs to returns |
Costs exclusive of lord’s dues, 78 per cent.[a] |
About 591⁄2 per cent. |
Costs 60 per cent. In the nine years following, the proportion was 80 per cent., at the
end of that time the working of the mine was stopped by the revolution, in the year 1809.[d] |
Costs 73 per cent.[d] |
| Number of men employed |
About 2,500 persons, of whom about 1,450 are employed under ground. |
About 900, of whom nearly 600 are employed under ground. |
3,100 Indians and Mestizoes, of whom 1,800 are employed under ground. |
700 miners of whom 550 are employed under ground. |
| Wages of the mines per day |
Probably about 3 shillings on an average. |
About 8 or 9 shillings per day. |
From 4 to 5 shillings. |
About 1s. 6d. per day. |
| Quantity and expense of powder |
|
|
1,420 cwt.; value 15,830l. |
240 cwt.; value 1,070l. |
| Manner in which the ores are disposed of |
Sold to the smelting companies, and smelted by them at Swansea, in South Wales. |
Chiefly reduced by the company at the hacienda of Sanceda, by smelting and amalgamation. |
Sold to the Rescatadores, and reduced by smelting and amalgamation at haciendas, in the
neighbourhood of Guanaxuato. |
Delivered to the government reduction works in the neighbourhood of Freyberg, where they
are partly smelted, and partly amalgamated. |
[a] Average of the last Ten Years.
[b] Malacate; a horse whim.
[c] Average of the last Six Years.
[d] Average year at the end of the Eighteenth Century. |
VENTILATION OF MINES.
When men penetrate by narrow passages into the interior of the earth, their respiration,
joined to the combustion of candle and gunpowder, are not long of vitiating
the air. The decomposition of wood contributes to the same effect, as also the
mineral bed itself, especially in coal mines, by the carburetted hydrogen and carbonic
acid evolved, and from the absorption of oxygen by pyrites. In many cases, arsenical
and mercurial vapours are disengaged. Hence the necessity of maintaining in subterranean[856]
cavities a continual circulation of air, which may renew the atmosphere round
the miners. The whole of the means employed to produce this effect, constitutes what
is called the ventilation of mines.
These means are divided into natural and artificial. The natural means are the currents
produced by the difference of density between the air of mines and the external
air; the artificial are air-exhausters or condensers, fires, &c.
The temperature of the air of the subterranean workings surpasses the mean temperature
of the place in which the mine is opened. Hence it is lighter in winter,
but in summer often heavier than the air of the atmosphere. For this reason, when the
mine presents two openings at different levels, the air naturally flows out by the most
elevated in winter, and by the lowest in summer. We may take advantage of this
circumstance, to lead the air into the bottom of even a very long gallery, opening into
the side of the mountain, by piercing a shaft into its roof at some distance from the
entrance, and dividing the gallery by a horizontal floor into two parts, which have no
mutual communication, except at the furthest extremity—the upper part communicating
with the shaft, and the under with the mouth of the gallery. If the two compartments
have different dimensions, the air in the smaller sooner comes into an equilibrium
of temperature with the rock; and the difference of temperature of the two compartments
is sufficient to produce a current. If a streamlet of water flows through this
gallery, it facilitates the flow of the air along the lower compartment. If a mine has
several openings situated on the same level, it rarely happens but some peculiar circumstance
destroys, during the colds of winter and the heats of summer, the equilibrium of
the air. But in spring and autumn, when the external air is nearly of the same temperature
with that of the mines, the above-named causes are almost always too feeble to
excite an issuing current. This effect is, however, frequently obtained by raising over
one of the shafts a chimney 20 or 30 yards high, which alone produces the effect of an
opening at a different level. It has been remarked that stormy weather usually deranges
every system of ventilation. See Pitcoal and Ventilation.
MINIUM. (Eng. and Fr., Red lead; Mennige, Germ.) This pigment is a peculiar
oxide of lead, consisting of two atoms of the protoxide and one of the peroxide; but, as
found in commerce, it always contains a little extra protoxide, or yellow massicot. It
is prepared by calcining lead upon a reverberatory hearth with a slow fire, and frequent
renewal of the surface with a rake, till it becomes an oxide, taking care not to fuse it.
The calcined mass is triturated into a fine powder in a paint mill, where it is elutriated
with a stream of water, to carry off the finely levigated particles, and to deposit them
afterwards in tanks. The powder thus obtained being dried, is called massicot. It is
converted into minium, by being put in quantities of about 50 pounds into iron trays,
1 foot square, and 4 or 5 inches deep. These are piled up upon the reverberatory hearth,
and exposed during the night, for economy of fuel, to the residuary heat of the furnace,
whereby the massicot absorbs more oxygen, and becomes partially red lead. This, after
being stirred about, and subjected to a similar low calcining heat once and again, will
be found to form a marketable red lead.
The best minium, however, called orange mine, is made by the slow calcination of
good white lead (carbonate) in iron trays. If the lead contains either iron or copper,
it affords a minium which cannot be employed with advantage in the manufacture of
flint-glass, for pottery glazes, or for house-painting.
Dumas found several samples of red lead which he examined to consist of the
chemical sesquioxide and the protoxide, in proportions varying from 50 of the former
and 50 of the latter, to 95·3 of the former and 4·7 of the latter. The more oxygen
gas it gives out when heated, the better it is, generally speaking. See Naples
Yellow.
MINT. (Monnaie, Fr.; Münze, Germ.) The chief use of gold and silver is to
serve for the medium of exchange in the sale and purchase of commodities, a function
for which they are pre-eminently fitted by their scarcity, by being unalterable by common
agents, and condensing a great value in a small volume. It would be very inconvenient
in general to barter objects of consumption against each other, because their
carriage would be expensive, and their qualities, in many cases, easily injured by
external agents, &c. Gold is exempt from spontaneous change, and little costly in
conveyance. Mankind at a very early period recognised how much easier it was to
exchange a certain weight of gold or silver for objects of commerce, than to barter these
objects themselves; and thenceforth all agreed to pay for their purchases in bars or ingots
of these precious metals. But as their intrinsic value depends upon their purity, it
became necessary to stamp on these bars their standard quality and their weight.
The inconvenience of using ingots in general trade, on account of the difficulty of
defining fractional values, has determined governments to coin pieces of money, that
is, quantities of metal whose weight and standard were made known and guaranteed by
the effigies of the prince. It is true, indeed, that kings have become frequently coiners[857]
of base money, by altering the weight and purity of the pieces apparently guaranteed
by their impress. By such reductions modern coins represent less of the precious metal
than they did long ago. The ordonnance of 755, for the coining of sous in France, proves
that there was then as much fine silver in a single sous, as there is now in a piece of
5 francs. During the last two centuries, indeed, silver coins have been diminished two
thirds in weight.
But since knowledge has become more generally diffused, it has been shown that these
frauds are equally injurious to the prince and to public faith. A sovereign may, it is
true, declare by a decree that a shilling-piece is to be held worth five; but let us consider
the consequences of this decree. All the individuals who have rents or capital sums to
receive, will be ruined, by getting in metallic value only one-fifth of what is due to
them; for although the nominal value should be the same as what they are entitled to,
the intrinsic value would be but a fifth of the former; so that when they go to purchase
the necessaries or comforts of life, the dealer who sells them will at once raise their price
five-fold. Each article of merchandise would thus acquire a nominal price 5 times
greater; and he who had received payment of a debt in that money, could not with it procure
more than one-fifth of the goods he could have previously commanded. That fraudulent
law would, therefore, favour the debtors at the expense of the creditors; and as the
state is commonly a great debtor, especially when it has recourse to the depreciation of the
currency, it is obvious, that however illicit the gain which it makes, it still does gain;
and this is the reason why princes have so often tampered with the mint. But let us
examine the other consequences of this decree.
If the sovereign is a debtor, he is also a creditor and consumer, and even the most
considerable of any. The taxes which he imposes are paid him in this deteriorated
money, returned to him at its nominal value; and the purveyors of his armies, his
buildings, and his household, sell him their commodities only at the actual market
price. We may infer from this simple development that the coin with which he pays
for any object has the same intrinsic value as the object; and that the name given to
the coin is of no consequence. The prince may call it a crown, a ducat, or a rix-dollar
at his pleasure; and he may assign any value to it that his caprice may suggest, yet this
will not affect its value; for this is fixed beyond his control by the general nature of
things. The prince may, indeed, at the outset, have profited by defrauding his creditors,
and by authorizing each debtor to imitate him, but he will soon lose whatever he
may have gained; and he will thus learn to his cost that it was bad policy to sacrifice
his character by giving an example of a fraud so truly unprofitable in the issue. Moreover,
he will lose still as much in the following years, because his treasury will receive
only one-fifth part of the taxes, unless he has quintupled the imposts. It may be said,
indeed, that he might do the one thing along with the other. But every one knows that
this power is neither generally permitted to princes, nor if it were, could it be safely exercised.
Serious political crises would combine to endanger the stability of the government;
which besides, as the main consumer in the nation, must lose always as much as
it seems to gain.
It is therefore manifest that the alteration of the standard and weight of the coinage
is at once a crime and a ruinous action for the sovereign power to commit; and hence
such disastrous measures have been long abandoned in all well-regulated states. A gold
sovereign is intrinsically worth 20 shillings minus the cost of coinage; for were it worth
more, all our sovereign pieces would be exported or melted down, to obtain the difference
of value, however trifling it might be; and were it worth less, it would be the
source of loss similar to what the state occasions when it depreciates the coin.
To comprehend the true value of a coin, we must regard this piece as an article of
merchandise, whose value depends, as that of every thing else, on its usefulness, the esteem
in which it is held, and the demand for it in the market. Grain increases in value
when there are few sellers and many buyers; gold and silver are in the same predicament.
The value of these metals is much augmented, indeed, by the universal currency
they obtain when struck into money; a value additional to what they possess as
objects of the arts. This value of the precious metals changes with time and place, like
that of every merchandise; their abundance, since the discovery of America, has greatly
lowered their value; that is, with the same weight of metal, we cannot at the present
day purchase the same quantity of corn, land, wool, &c. as formerly. In the countries
where silver abounds, this metal has less value, or, in other terms, commodities are
dearer. Hence the metal tends to resume its equilibrium in flowing into those places
where it is rarer; which means, that the consumer prefers purchasing his commodities
there rather than in another place, if he can easily transport them to where they are
dearer.
It was formerly believed that a country is rich when it has a great deal of gold and
silver; but this popular illusion has passed away. Spain has never been poorer than
since the discovery of America, because its national industry has been ruined, and the[858]
capitals merely passed through its hands to spread over the rest of Europe, from which
it was obliged to import every thing that its want of home manufactures made it necessary
to procure from abroad. We may add to these, the prodigalities of the court,
which, supposing its wealth inexhaustible, tried to corrupt all the ministers of the other
powers, in furtherance of the chimera of universal dominion. The richest state is that
in which there is most industry, whereby the inhabitants may procure every thing indispensable
to the conveniences and comforts of life. Gold as a useful metal, and a medium
of exchange, is undoubtedly very precious, and an adequate quantity for these exchanges
must be had; but as it is good for very little besides, nay, as an excess is even hurtful, it
soon begins to fly of itself towards the places where it is more needed or less common.
With regard to the relative value of gold and silver, several details have already been
given in our view of the mineral wealth of the globe. Three centuries ago, an ounce
of gold was worth at London or Paris 10 ounces of silver; now it may be exchanged for
15 ounces and a half.
The par of two coins results from the comparison of their weight and standard fineness.
Let us take for an example the conversion of English gold sovereigns worth 20
shillings or a pound sterling, in relation to the French louis of 20 francs. The standard
of the sovereign gold is 0·917, fine gold being 1000; its weight is 125·256 gr. English,
or 7·980855 grammes; by multiplying this weight into its standard, we have a product
of 7·318444035; this is, in grammes, the quantity of pure gold contained in the sovereign
piece. The piece of 20 francs has a legal standard of 0·9; and multiplying
this number by the weight of the louis, 6·45161 grammes, we find that it contains
5·806449 of pure metal. We then make this proportion:—
As 5·806449 : 20 francs ∷ 7·31844 : 25·2079 francs; or the value of the English
sovereign is nearly 25·21 francs, in French gold coin. A similar calculation may be
made for silver coins. The French rule for finding the par of a foreign gold coin, or its
intrinsic value in francs, is to multiply its weight by its standard or titre, and that
product by 34⁄9. The par of foreign silver money, or its intrinsic value in francs, is obtained
by multiplying its weight in grammes by its standard in thousand parts, and by 2⁄9.
The French 5-franc piece has its standard or titre at 0·9, and weighs 25 grammes.
The assaying of gold for coin and trinkets requires very delicate management.
The French take half a gramme at most (about 71⁄2 grains) of gold, and fuse it
with thrice its weight of silver, as already described under Assay. The parting is
the next operation. For this purpose the button of gold and silver alloy is first hammered
flat on a piece of steel, and then made feebly red hot in burning charcoal or over
a lamp flame. After being thus annealed, the metal is passed through the rolling press,
till it be converted into a plate about 1⁄70 of an inch thick. After annealing this riband,
it is coiled into a spiral form, introduced immediately into a small matrass of a pear
shape, an assay matrass, and about 500 grains of nitric acid, sp. grav. 1·185, are poured
over it. Heat being now applied to the vessel, the solution of the silver and copper alloys
ensues, and after 22 minutes of constant ebullition, the liquid is poured off and replaced
by an equal quantity of nitric acid, likewise very pure, but of the density 1·28. This
is made to boil for about 10 minutes, and is then poured off, when the matrass is filled up
with distilled water to the brim. In conclusion, a small annealing crucible is inverted
as a cup over the mouth of the matrass, which is now turned upside down with a steady
hand; the slip of metal falls into the crucible through the water; which by sustaining
a part of its weight, softens its descent and prevents its tearing. The matrass is then
dexterously removed, without letting its water overflow the crucible. The water is
gently decanted from the crucible, which is next covered, placed in the middle of
burning charcoal, and withdrawn whenever it becomes red hot. After cooling, the metal
slip is weighed very exactly, whence the weight of fine gold in the alloy is known.
Stronger acid than that prescribed above would be apt to tear the metallic riband to
pieces, and it would be difficult to gather the fine particles of gold together again.
The metallic plate becomes at last merely a golden sieve, with very little cohesion. When
copper is to be separated from gold by cupellation, a higher temperature is requisite
than in cupelling silver coin.
The coining apparatus of the Royal Mint of London is justly esteemed a masterpiece
of mechanical skill and workmanship. It was erected in 1811, under the direction of
the inventor, Mr. Boulton; and has since been kept in almost constant employment.
The melting pots (fig. 738.) are made of cast iron, and hold conveniently 400 pounds
of metal. They are furnished with a spout or lip for pouring out the metal, and with
two ears, on which the tongs of the crane lay hold in lifting them out of the furnace.
The pot rests on pedestals on the grate of the furnace, and has a ring cast on its edge
to prevent the fuel falling into it. Whenever it becomes red hot, the metal properly
prepared and mixed, so as to produce an alloy containing 0·915 parts of gold, is put in,
and during the melting, which occupies some hours, it is occasionally stirred. The moulds
are meanwhile prepared by warming them in a stove, and thereafter by rubbing their[859]
inside surfaces with a cloth dipped in oil, by which means the ingots cast in them get
a better surface. Fig. 739. represents a side view of the carriage, charged with its
moulds. When the proper number of moulds is introduced, the screws at the end, represented
at t T, are screwed fast, to fix them all tight.
The pot of fused metal is lifted out of the furnace by the crane (fig. 740.), then
swung round, and lowered down into the cradle l, m, n, o of the pouring machine,
until the ring on the edge of it rests on the iron hoop n, which, being screwed tight up,
holds it secure, and the crane-tongs are removed. One of the assistants now takes the
winch handle s in one hand, and y in the other. By turning y he moves the carriage
forward, so as to bring the first mould beneath the lip of the melting pot; and by
turning s, he inclines the pot, and pours the metal into the mould. He then fills the
other moulds in succession. The first portion of liquid metal is received in a small iron
spoon, and is reserved for the assay-master; a second sample is taken from the centre
of the pot, and a third from the bottom part. Each of these is examined as to its
quality.
The ingots, which are about 10 inches long, 7 broad, and 6 tenths of an inch thick,
are now carried to the rolling mill.
Fig. 741., where A represents a large spur wheel, fixed on the extremity of a long[860]
horizontal shaft B B, extending beneath the whole mill. This wheel and shaft are driven
by a smaller wheel, fixed on the main or fly-wheel shaft of a steam engine of 36-horse
power. The main shaft B of the rolling mill has wheels C, D, E fixed upon it, to give
motion to the respective rollers, which are mounted at F and G, in strong iron frames,
bolted to the iron sills a a, which extend through the whole length of the mill, and rest
upon the masonry, in which the wheels are concealed. The two large wheels C and E
give motion to the wheels H, I, which are supported on bearings between two standards
b, b, bolted down to the ground sills. On the ends of the axes of these wheels are heads
for the reception of coupling boxes d, d, which unite them to short connecting shafts
K L; and these again, by means of coupling boxes, convey motion to the upper rollers
e, e, of each pair, at F and G. The middle wheel D upon the-main shaft B gives motion to
the lower rollers in a similar manner. Thus both the rollers e, f of each frame receive
their motion from the main shaft with equal velocity, by means of wheels of large
radius, which act with much more certainty than the small pinions usually employed in
rolling mills to connect the upper and lower rollers, and cause them to move together.
The rolling mill contains four pairs of rollers, each driven by its train of wheel work;
the mill, therefore, consists of two such sets of wheels and rollers as are represented in
our figure. The two shafts are situated parallel to each other, and receive their motion
from the same steam engine. This admirable rolling mill was erected by John
Rennie, Esq.
The ingots are heated to redness in a furnace before they are rolled. The two furnaces
for this purpose are situated before two pairs of rollers, which, from being used to
consolidate the metal by rolling whilst hot, are termed breaking-down rollers. Two
men are employed in this operation; one taking the metal from the furnace with a pair
of tongs, introduces it between the rollers; and the other, catching it as it comes
through, lifts it over the top roller, and returns it to his fellow, who puts it through
again, having previously approximated the
rollers a little by their adjusting screws.
After having been rolled in this manner four
or five times, they are reduced to nearly
two-tenths of an inch thick, and increased
lengthwise to about four times the breadth of
the ingot. These plates, while still warm,
are rubbed over with a dilute acid or pickle,
to remove the colour produced by the heat,
and are then cut up into narrow slips across
the breadth of the plate, by means of the circular
shears fig. 742.
This machine is worked by a spur-wheel at the extremity of the main shaft B of the
rolling mill (fig. 741.) It consists of a framing of iron A A, supporting two shafts B B,
which are parallel to each other, and move together by means of two equal spur-wheels
C C, the lower one of which works with the teeth of the great wheel above mentioned,
upon the main shaft of the rolling mill. At the extremities of the two shafts, wheels
or circular cutters are fixed with their edges overlapping each other a little way.
F represents a shelf on which the plate is laid, and advanced forward to present it to the
cutter; and G is a ledge or guide, screwed down on it, to conduct the metal and to regulate
the breadth of the piece to be cut off. Hence the screws which fasten down the
ledge are fitted in oblong holes, which admit of adjustment. The workman holds the
plate flat upon the surface F, and pushing it towards the shears, they will lay hold of it,
and draw it through until they have cut the whole length. The divided parts are also
prevented from curling up into scrolls, as they do when cut by a common pair of shears;
because small shoulders on E and D, behind the cutting edge, keep them straight. Behind
the standard, supporting the back pivots of the shafts B B of the cutter, is a frame l, with
a screw m tapped through it. This is used to draw the axis of the upper cutter D endwise,
and keep its edge in close contact with the edge of the other cutter E. The slips
or ribands of plate are now carried to the other two pairs of rollers in the rolling mill,
which are made of case-hardened iron, and better polished than the breaking-down
rollers. The plates are passed cold between these, to bring them to exactly the same
thickness; whence they are called adjusting or planishing rollers. The workman here
tries every piece by a common gauge, as it comes through. This is a piece of steel having
a notch in it; the inside lines of which are very straight, and inclined to one another at
a very acute angle. They are divided by fine lines, so that the edge of the plate being
pressed into the notch, will have its thickness truly determined by the depth to which
it enters, the divisions showing the thickness in fractions of an inch.
In rolling the plate the second time, all the plates are successively passed through the
rollers; then the rollers being adjusted, they are passed through another time. This
is repeated thrice or even four times; after which they are all tried by the gauge, and[861]
thus sorted into as many parcels as there are different thicknesses. It is a curious circumstance,
that though the rollers are no less than 14 inches in diameter, and their frame
proportionally strong, they will yield in some degree, so as to reduce a thick plate
in a less degree than a thin one; thus the plates which have all passed through the
same rollers, may be of 3 or 4 different degrees of thickness, which being sorted by the
gauge into as many parcels, are next reduced to the exact dimension, by adapting the
rollers to each parcel. The first of the parcel which now comes through is tried, by
cutting out a circular piece with a small hand machine, and weighing it. If it proves
either too light or too heavy, the rollers are adjusted accordingly, till by a few such
trials they are found to be correct, when all the parcel is rolled through. The trial
plates which turn out to be too thin, are returned as waste to the melting-house. By
these numerous precautions, the blanks or circular discs, when cut out by the next
machine, will be very nearly of the same weight; which they would scarcely be, even if
the gauge determined all the plates to the same thickness, because some being more
condensed than others, they would weigh differently under the same volume.
A great improvement has been made on that mode of lamination, by the late Mr.
Barton’s machine for equalizing the thickness of slips of metal for making coin, which
has been for several years introduced into the British mint. A side elevation is shown
in fig. 743., and a plan in fig. 744. It operates in the same way as wire-drawing mechanisms;
namely, pulls the slips of metal forcibly through an oblong opening, left
between two surfaces of hardened steel. The box or case which contains the steel dies,
composed of two hardened cylinders, is represented at C in fig. 743. The pincers employed
to hold the metal, and draw it through, are shown at s r.
The slips of metal to be operated on by the drawing machine, are first rendered
thinner at one end, that they may be introduced between the dies, and also between the
jaws of the pincers. This thinning of the ends is effected by another machine, consisting
of a small pair of rollers, mounted in an iron frame, similar to a rolling-mill.
The upper roller is cylindrical, but the lower is formed with 3 flat sides, leaving merely
portions of the cylinder entire, between these flat sides. The distance between the
centres of the rollers is regulated by screws, furnished with wheels on their upper ends,
similar to what is seen in the drawing dies at C. The two rollers have pinions on their
axes, which make them revolve together; they are set in motion by an endless strap
passing round a drum, upon whose axis is a pinion working into the teeth of a wheel
fixed upon the axis of the lower roller.
The end of a slip of metal is presented between the rollers while they are in motion,
not on that side of the roller which would operate to draw in the slip between them, as
in the rolling-press above described, but on the contrary side, so that when one of the
flat sides of the under roller fronts horizontally the circumference of the upper roller, an
opening is formed, through which the slip of metal is to be inserted until it bears against
a fixed stop at the back of the rollers. As the rollers continue to turn round, the
cylindrical portions come opposite to each other, and press the metal between them,
forcing it outwards, and rendering the part which has been introduced between the
rollers as thin as the space between their cylindrical surfaces. Thus the end of the slip
of metal becomes attenuated enough to pass between the dies of the drawing machine,
and to be seized by the pincers.
In using the drawing machine, a boy takes hold of the handle s of the pincers, their
hook of connexion with the endless chain l, l, not shown in the present figure, being disengaged,
and he moves them upon their wheels towards the die-box C. In this movement
the jaws of the pincers get opened, and they are pushed up so close to the[862]
die-box that their jaws enter a hollow, which brings them near the dies, enabling them
to seize the end of the slip of metal introduced between them by the action of the preparatory
rollers. The boy now holds the handle s on the top of the pincers fast, and with
his other hand draws the handle x backwards. Thus the jaws are closed, and the
metal firmly griped. He now presses down the handle x till a hook on the under side
of the pincers seizes the endless chain as it moves along, when it carries the pincers,
and their slip of metal, onwards with it. Whenever the whole length of the metallic
riband has passed through between the dies, the strain on the pincers is suddenly
relieved, which causes the weight r to raise their hook out of the chain, and stop their
motion. The machine in the mint has two sets of dies, and two endless chains, as
represented in the plan, fig. 744. N N, are toothed wheels in the upper end of the die-box,
furnished with pinions and levers, for turning them round, and adjusting the
distance between the dies. A large spur-wheel G, is fixed upon the axis F, to give motion
to the endless chains; see both figures. This spur-wheel is turned by a pinion H,
fixed upon an axis m, extending across the top of the frame, and working in bearings
at each end. A spur-wheel I, is fixed upon the axis m, and works into the teeth of a
pinion K, upon a second axis across the frame, which carries likewise a drum wheel L,
through which motion is communicated to the whole mechanism by an endless strap.
The cutting-out machine is exhibited in fig. 745. A A is a basement of stone to support
an iron plate B B, on which stand the
columns C C, that bear the upper part
D of the frame. The iron frame of the
machine E, F, E, is fixed down upon
the iron plate B, B. The punch d is fixed
in the lower part of the inner frame,
and is moved up and down by the screw
a, which is worked by wipers turned
by a steam engine, impelling the lever
H, and turning backwards and forwards
the axis G, through a sufficient space
for cutting the thickness of the metallic
lamina. A boy manages this machine.
There are twelve of them mounted on
the same basement frame in a circular
range contained in an elegant room,
lighted from the roof. The whole are
moved by a steam engine of 16-horse
power.
The blanks or planchets thus cut out,
were formerly adjusted by filing the
edges, to bring them to the exact weight; a step which Mr. Barton’s ingenious mechanism
has rendered in a great measure unnecessary. The edge is then milled, by a process
which Mr. Boulton desires to keep secret, and which is therefore not shown in our mint.
But the French mint employs a very elegant machine for the purpose of lettering
or milling the edges, called the cordon des monnaies, invented by M. Gengembre, which
has entirely superseded the older milling machine of M. Castaing, described in the
Encyclopedias. The Napoleon coins of France bear on the
edge, in sunk letters, the legend, Dieu protège la France; and
those of the king, Domine salvum fac regem. This is marked
before striking the blank or flan. One machine imprints
this legend, and its service is so prompt and easy, that a
single man marks in a day 20,000 pieces of 5 francs, or
100,000 francs.
Each of the two arc dies E, D, (fig. 746.) carries one half of
the legend, engraved in relief on the curved face; these arcs
are pieces of steel tempered very hard, and fixed with two
screws, one immoveably at E, on the sill which bears the apparatus;
the other at D, at the extremity of the lever P, D,
which turns round the axis C. The letters of these demi-legends
are exactly parallel, and inscribed in an inverse order
on the dies. An alternating circular motion is communicated
to the handle P. The curvatures of the two dies are
arcs of circles described from the centre C; and the interval
which separates them, or the difference of the radii, is precisely
the diameter of the piece to be milled.
As the centre C sustains the whole strain of the milling, and produces, of consequence,
a hard friction, this axis must possess a considerable size. It is composed of a squat[863]
truncated cone of tempered steel, which enters into an eye of the moveable piece P, D.
This cone is kept on the plate of the metal N N, which bears the whole machine, by a
nut, whose screw, by being tightened or slackened, gives as much freedom as is requisite
for the movement of rotation, or removes the shake which hard service gives to the cone
in its eye. The middle thickness of the hole of the moveable piece P, D, and the axis of
the lever P, which terminates it, are exactly on a level with the engraved letters of the
die, so that no strain can derange the movable piece, or disturb the centre by its
oscillations.
At a is a vertical tube, containing a pile of blanks for milling. It is kept constantly
full; the tube being open at both ends, a little elevated above the circular space a,
K, b, which separates the dies, and fixed by a tail m with a screw to the motionless
piece A, B. The branch I, c, movable with the piece P, D, passes under the tube, and
pushes before it the blank at the bottom of the column, which is received into a small
excavation in the form of a circular step, and carried forwards. Matters are thus so
arranged as to regulate the issue of the blanks, one by one, on the small step, called the
posoir (bed.)
As soon as the blank is pushed forwards into contact with the lower edge of the
engraved grooves, it is seized by them, and carried on by the strain of milling, without
exposing the upper or under surfaces of the blank to any action which may obstruct the
printing on its edge.
The blank is observed to revolve between the two dies according as the lever P
completes its course, and this blank passing from a to K, then to b, meets a circular
aperture b, through which it falls into a drawer placed under the sill.
The range of the movable lever P is regulated by four pieces, F, F, F, F, solidly sunk in
the plate N, N, which bears the whole apparatus. A stud placed on this lever towards D,
makes the arm of the posoir I c retire no farther than is necessary for the little blank to issue
from the column; and a spring fixed to the centre c, and supported on a peg, brings
back the posoir; so that when a screw I comes to strike against the column, the posoir
stops, and the movable die D, which continues its progress, finds the blank in a fit
position for pressing, seizing, and carrying it on, by reaction of the fixed die E. Thus
the edge of the blank is lettered in half a second. A hundred may easily be marked in
about three minutes.
The coining press is the most beautiful part of the whole mechanism in the British
mint; but the limits of this volume will not allow of its being figured upon an adequate
scale. An engraving of it may be seen in the Encyclopedia Britannica.
The only attention which this noble machine requires is that of a little boy, who stands
in a sunk place before the press, and always keeps the tube full of blanks. He has two
strings, one of which, when pulled, will put the press in motion by the concealed mechanism
in the apartment above; and the other string, when snatched, stops the press.
This coining operation goes on at the rate of 60 or 70 strokes per minute; and with
very few interruptions during the whole day. The press-room at the Royal Mint
contains eight machines, all supported on the same stone base; and the iron beams between
the columns serve equally for the presses on each side. The whole has therefore
a magnificent appearance. The eight presses will strike more than 19,000 coins in an
hour, with only a child to supply each. The grand improvement in these presses, consists;
1. in the precision with which they operate to strike every coin with equal force,
which could not be ensured by the old press impelled by manual labour; 2. The rising
collar or steel ring in which they are struck, keeps them all of one size, and makes a fair
edge, which was not the case with the old coins, as they were often rounded and defaced
by the expansion of the metal under the blow; 3. The twisting motion of the upper
die is thought to produce a better surface on the flat parts of the coin; but this is somewhat
doubtful; 4. The feeding mechanism is very complete, and enables the machine
to work much quicker than the old press did, where the workman, being in constant
danger of having his fingers caught, was obliged to proceed cautiously, as well as to place
the coin true on the die, which was seldom perfectly done. The feeding mechanism of
the above press is a French invention; but Mr. Boulton is supposed to have improved
upon it.
MIRRORS. See Copper and Glass.
MISPICKEL, is arsenical pyrites.
MOHAIR, is the hair of a goat which inhabits the mountains in the vicinity of
Angora, in Asia Minor.
MOIRÉE METALLIQUE, called in this country crystallized tin-plate, is a
variegated primrose appearance, produced upon the surface of tin-plate, by applying to it
in a heated state some dilute nitro-muriatic acid for a few seconds, then washing it
with water, drying, and coating it with lacquer. The figures are more or less beautiful
and diversified, according to the degree of heat, and relative dilution of the acid.
This mode of ornamenting tin-plate is much less in vogue now than it was a few
years ago.
[864]
MOLASSE, is a sandstone belonging to the tertiary strata, employed under that
name by the Swiss for building.
MOLASSES, is the brown viscid uncrystallizable liquor, which drains from cane
sugar in the colonies. See Sugar.
MOLYBDENUM (Molybdène, Fr.; Molybdan, Germ.); is a rare metal which
occurs in nature sometimes as a sulphuret, sometimes as molybdic acid, and at others
as molybdate of lead. Its reduction from the acid state by charcoal requires a very
high heat, and affords not very satisfactory results. When reduced by passing hydrogen
over the ignited acid, it appears as an ash-gray powder, susceptible of acquiring
metallic lustre by being rubbed with a steel burnisher; when reduced and fused with
charcoal, it possesses a silver white colour, is very brilliant, hard, brittle, of specific
gravity 8·6; it melts in a powerful air-furnace, oxidizes with heat and air, burns at an
intense heat into molybdic acid, dissolves in neither dilute sulphuric, muriatic, nor
fluoric acids, but in the concentrated sulphuric and nitric.
The protoxide consists of 85·69 of metal, and 14·31 of oxygen; the deutoxide consists
of 75 of metal, and 25 of oxygen; and the peroxide, or molybdic acid, of 66·6 of
metal, and 33·4 of oxygen. These substances are too rare at present to be used in
any manufacture.
MORDANT, in dyeing and calico-printing, denotes a body which, having a twofold
attraction for organic fibres and colouring particles, serves as a bond of union between
them, and thus gives fixity to dyes; or it signifies a substance which, by combining with
colouring particles in the pores of textile filaments, renders them insoluble in hot soapy
and weak alkaline solutions. In order properly to appreciate the utility and the true functions
of mordants, we must bear in mind that colouring matters are peculiar compounds
possessed of certain affinities, their distinctive characters being not to be either acid or
alkaline, and yet to be capable of combining with many bodies, and especially with
salifiable bases, and of receiving from each of them modifications in their colour,
solubility, and alterability. Organic colouring substances, when pure, have a very
energetic attraction for certain bodies, feeble for others, and none at all for some.
Among these immediate products of animal or vegetable life, some are soluble in pure
water, and others become so only through peculiar agents. We may thus readily conceive,
that whenever a dye-stuff possesses a certain affinity for the organic fibre, it will
be able to become fixed on it, or to dye it without the intervention of mordants, if it be
insoluble by itself in water, which, in fact, is the case with the colouring matters of
safflower, annotto, and indigo. The first two are soluble in alkalis; hence, in order to
use them, they need only be dissolved in a weak alkaline lye, be thus applied to the
stuffs, and then have their tinctorial substance precipitated within their pores, by
abstracting their solvent alkali with an acid. The colouring matter, at the instant of
ceasing to be liquid, is in an extremely divided state, and is in contact with the organic
fibres for which it has a certain affinity. It therefore unites with them, and, being
naturally insoluble in water, that is, having no affinity for this vehicle, the subsequent
washings have no effect upon the dye. The same thing may be said of indigo,
although its solubility in the dye-bath does not depend upon a similar cause, but is due
to a modification of its constituent elements, in consequence of which it becomes
soluble in alkalis. Stuffs plunged into this indigo bath get impregnated with the
solution, so that when again exposed to the air, the dyeing substance resumes at once
its primitive colour and insolubility, and washing can carry off only the portions in excess
above the intimate combination, or which are merely deposited upon the surface of
the stuff.
Such is the result with insoluble colouring matters; but for those which are soluble
it should be quite the reverse, since they do not possess an affinity for the organic fibres
which can counterbalance their affinity for water. In such circumstances, the dyer
must have recourse to intermediate bodies, which add their affinity for the colouring
matter to that possessed by the particles of the stuff, and increase by this twofold action
the intimacy and the stability of the combination. These intermediate bodies are the
true mordants.
Mordants are in general found among the metallic bases or oxides; whence they
might be supposed to be very numerous, like the metals; but as they must unite the
twofold condition of possessing a strong affinity for both the colouring matter and the
organic fibre, and as the insoluble bases are almost the only ones fit to form insoluble
combinations, we may thus perceive that their number may be very limited. It is well
known, that although lime and magnesia, for example, have a considerable affinity for
colouring particles, and form insoluble compounds with them, yet they cannot be employed
as mordants, because they possess no affinity for the textile fibres.
Experience has proved, that of all the bases, those which succeed best as mordants are
alumina, tin, and oxide of iron; the first two of which, being naturally white, are the
only ones which can be employed for preserving to the colour its original tint, at least[865]
without much variation. But whenever the mordant is itself coloured, it will cause the
dye to take a compound colour quite different from its own. If, as is usually said, the
mordant enters into a real chemical union with the stuff to be dyed, the application of
the mordant should obviously be made in such circumstances as are known to be most
favourable to the combination taking place; and this is the principle of every day’s practice
in the dyehouse.
In order that a combination may result between two bodies, they must not only be in
contact, but they must be reduced to their ultimate molecules. The mordants that are
to be united with stuffs are, as we have seen, insoluble of themselves, for which reason
their particles must be divided by solution in an appropriate vehicle. Now this solvent
or menstruum will exert in its own favour an affinity for the mordant, which will prove
to that extent an obstacle to its attraction for the stuff. Hence we must select such
solvents as have a weaker affinity for the mordants than the mordants have for the stuffs.
Of all the acids which can be employed to dissolve alumina, for example, vinegar is the
one which will retain it with least energy, for which reason the acetate of alumina is
now generally substituted for alum, because the acetic acid gives up the alumina with
such readiness, that mere elevation of temperature is sufficient to effect the separation of
these two substances. Before this substitution of the acetate, alum alone was employed;
but without knowing the true reason, all the French dyers preferred the alum of Rome,
simply regarding it to be the purest; it is only within these few years that they have
understood the real grounds of this preference. This alum has not, in fact, the same composition
as the alums of France, England, and Germany, but it consists chiefly of cubic
alum having a larger proportion of base. Now this extra portion of base is held by the
sulphuric acid more feebly than the rest, and hence is more readily detached in the form
of a mordant. Nay, when a solution of cubic alum is heated, this redundant alumina
falls down in the state of a subsulphate, long before it reaches the boiling point. This difference
had not, however, been recognised, because Roman alum, being usually soiled with
ochre on the surface, gives a turbid solution, whereby the precipitate of subsulphate of
alumina escaped observation. When the liquid was filtered, and crystallized afresh,
common octahedral alum alone was obtained; whence it was most erroneously concluded,
that the preference given to Roman alum was unjustifiable, and that its only
superiority was in being freer from iron.
Here a remarkable anecdote illustrates the necessity of extreme caution, before we
venture to condemn from theory a practice found to be useful in the arts, or set about
changing it. When the French were masters in Rome, one of their ablest chemists
was sent thither to inspect the different manufactures, and to place them upon a level
with the state of chemical knowledge. One of the fabrics, which seemed to him furthest
behindhand, was precisely that of alum, and he was particularly hostile to the construction
of the furnaces, in which vast boilers received heat merely at their bottoms, and
could not be made to boil. He strenuously advised them to be new modelled upon a
plan of his own; but, notwithstanding his advice, which was no doubt very scientific, the
old routine kept its ground, supported by utility and reputation, and very fortunately,
too, for the manufacture; for had the higher heat been given to the boilers, no more
genuine cubical alum would have been made, since it is decomposed at a temperature
of about 120° F., and common octahedral alum would alone have been produced. The
addition of a little alkali to common alum brings it into the same basic state as the alum
of Rome.
The two principal conditions, namely, extreme tenuity of particles, and liberty of
action, being found in a mordant, its operation is certain. But as the combination to
be effected is merely the result of a play of affinity between the solvent and the stuff to
be dyed, a sort of partition must take place, proportioned to the mass of the solvent, as
well as to its attractive force. Hence the stuff will retain more of the mordant when
its solution is more concentrated, that is, when the base diffused through it is not so
much protected by a large mass of menstruum; a fact applied to very valuable uses by
the practical man. On impregnating in calico printing, for example, different spots of
the same web with the same mordant in different degrees of concentration, there is
obtained in the dye-bath a depth of colour upon these spots intense in proportion to the
strength of their various mordants. Thus, with solution of acetate of alumina in different
grades of density, and with madder, every shade can be produced, from the fullest
red to the lightest pink; and, with acetate of iron and madder, every shade from black
to pale violet.
We hereby perceive that recourse must indispensably be had to mordants at different
stages of concentration; a circumstance readily realized by varying the proportions of
the watery vehicle. See Calico-printing and Madder. When these mordants are to
be topically applied, to produce partial dyes upon cloth, they must be thickened with
starch or gum, to prevent their spreading, and to permit a sufficient body of them to
become attached to the stuff. Starch answers best for the more neutral mordants, and[866]
gum for the acidulous; but so much of them should never be used, as to impede the
attraction of the mordant for the cloth. Nor should the thickened mordants be of too
desiccative a nature, lest they become hard, and imprison the chemical agent before it
has had an opportunity of combining with the cloth, during the slow evaporation of its
water and acid. Hence the mordanted goods, in such a case, should be hung up to
dry in a gradual manner, and when oxygen is necessary to the fixation of the base, they
should be largely exposed to the atmosphere. The foreman of the factory ought, therefore,
to be thoroughly conversant with all the minutiæ of chemical reaction. In cold
and damp weather he must raise the temperature of his drying-house, in order to command
a more decided evaporation; and when the atmosphere is unusually dry and
warm, he should add deliquescent correctives to his thickening, as I have particularized
in treating of some styles of calico-printing. But, supposing the application of the
mordant and its desiccation to have been properly managed, the operation is by no
means complete; nay, what remains to be done is not the least important to success,
nor the least delicate of execution. Let us bear in mind that the mordant is intended
to combine not only with the organic fibre, but afterwards also with the colouring
matter, and that, consequently, it must be laid entirely bare, or scraped clean, so to
speak, that is, completely disengaged from all foreign substances which might invest it,
and obstruct its intimate contact with the colouring matters. This is the principle and
the object of two operations, to which the names of dunging and clearing have been
given.
If the mordant applied to the surface of the cloth were completely decomposed, and
the whole of its base brought into chemical union with it, a mere rinsing or scouring in
water would suffice for removing the viscid substances added to it, but this never
happens, whatsoever precautions may be taken; one portion of the mordant remains
untouched, and besides, one part of the base of the portion decomposed does not enter
into combination with the stuff, but continues loose and superfluous. All these particles,
therefore, must be removed without causing any injury to the dyes. If in this
predicament the stuff were merely immersed in water, the free portion of the mordant
would dissolve, and would combine indiscriminately with all the parts of the cloth not
mordanted, and which should be carefully protected from such combination, as well as
the action of the dye. We must therefore add to the scouring water some substance
that is capable of seizing the mordant as soon as it is separated from the cloth, and of
forming with it an insoluble compound; by which means we shall withdraw it from
the sphere of action, and prevent its affecting the rest of the stuff, or interfering
with the other dyes. This result is obtained by the addition of cow-dung to the scouring
bath; a substance which contains a sufficiently great proportion of soluble animal
matters, and of colouring particles, for absorbing the aluminous and ferruginous salts.
The heat given to the dung-bath accelerates this combination, and determines an insoluble
and perfectly inert coagulum.
Thus the dung-bath produces at once the solution of the thickening paste; a more
intimate union between the alumina or iron and the stuff, in proportion to its elevation
of temperature, which promotes that union; an effectual subtraction of the undecomposed
and superfluous part of the mordant, and perhaps a commencement of mechanical
separation of the particles of alumina, which are merely dispersed among the fibres; a
separation, however, which can be completed only by the proper scouring, which is done
by the dash-wheel with such agitation and pressure (see Bleaching and Dunging) as
vastly facilitate the expulsion of foreign particles. See also Bran.
Before concluding this article, we may say a word or two about astringents, and
especially gall-nuts, which have been ranked by some writers among mordants. It is
rather difficult to account for the part which they play. Of course we do not allude to
their operation in the black dye, where they give the well known purple-black colour
with salts of iron; but to the circumstance of their employment for madder dyes, and
especially the Adrianople red. All that seems to be clearly established is, that the
astringent principle or tannin, whose peculiar nature in this respect is unknown, combines
like mordants with the stuffs and the colouring substance, so as to fix it; but as
this tannin has itself a brown tint, it will not suit for white grounds, though it answers
quite well for pink grounds. When white spots are desired upon a cloth prepared with
oil and galls, they are produced by an oxygenous discharge, effected either through
chlorine or chromic acid.
MORDANT, is also the name sometimes given to the adhesive matter by which
gold-leaf is made to adhere to surfaces of wood and metal in gilding. Paper, vellum,
taffety, &c., are easily gilt by the aid of different mordants, such as the following: 1.
beer in which some honey and gum arabic have been dissolved; 2. gum arabic, sugar,
and water; 3. the viscid juice of onion or hyacinth, strengthened with a little gum
arabic. When too much gum is employed, the silver or gold leaf is apt to crack in
the drying of the mordant. A little carmine should be mixed with the above colourless[867]
liquids, to mark the places where they are applied. The foil is applied by means of a
dossil of cotton wool, and when the mordant has become hard, the foil is polished with
the same.
The best medium for sticking gold and silver leaf to wood, is the following, called
mixtion by the French artists:—1 pound of amber is to be fused, with 4 ounces of
mastic in tears, and 1 ounce of Jewish pitch, and the whole dissolved in 1 pound of
linseed oil rendered drying by litharge.
Painters in distemper sometimes increase the effect of their work, by patches of gold
leaf, which they place in favourable positions; they employ the above mordant. The
manufacturers of paper hangings of the finer kinds attach gold and silver leaf to them
by the same varnish.
MORPHIA (Morphine, Fr.; Morphin, Germ.), is a vegeto-alkali which exists
associated with opian, codeïne, narcotine, meconine, meconic acid, resin, gum, bassorine,
lignine, fat oil, caoutchouc, extractive, &c., in opium. Morphia is prepared as follows:
Opium in powder is to be repeatedly digested with dilute muriatic acid, slightly
heated, and sea-salt is to be added, to precipitate the opian. The filtered liquid is to
be supersaturated with ammonia, which throws down the morphia, along with the meconine,
resin, and extractive. The precipitate is to be washed with water, heated, and
dissolved in dilute muriatic acid; the solution is to be filtered, whereby the foreign
matters are separated from the salt of morphia, which concretes upon cooling, while
the meconine remains in the acid liquid. The muriate of morphia having been
squeezed between folds of blotting paper, is to be sprinkled with water, again squeezed,
next dissolved in water, and decomposed by water of ammonia. The precipitate, when
washed, dried, dissolved in alcohol, and crystallized, is morphia.
These crystals, which contain 6·32 per cent. of combined water, are transparent,
colourless, four-sided prisms, without smell, and nearly void of taste, fusible at a moderate
heat, and then concrete into a radiated translucent mass, but at a higher temperature they
grow purple-red. Morphia consists of 72·34 of carbon; 6·366 of hydrogen; 5 of
azote; and 16·3 of oxygen. It burns with a red and very smoky flame, is stained red
by nitric acid, is soluble in 30 parts of boiling anhydrous alcohol, in 500 parts of boiling
water, but hardly if at all in cold water, and is insoluble in ether and oils. The solutions
have a strong bitter taste, and an alkaline reaction upon litmus paper. The saline
compounds have a bitter taste, are mostly crystallizable, are soluble in water and alcohol
(but not in ether), and give a blue colour to the peroxide salts of iron. It is a very
poisonous substance. Acetate of morphia is sometimes prescribed, instead of opium, in
medicine.
MORTAR, HYDRAULIC, called also Roman Cement, is the kind of mortar used
for building piers, or walls under or exposed to water, such as those of harbours, docks,
&c. The poorer sorts of limestone are best adapted for this purpose, such as contain
from 8 to 25 per cent. of foreign matter, in silica, alumina, magnesia, &c. These,
though calcined, do not slake when moistened; but if pulverized they absorb water without
swelling up or heating, like fat lime, and afford a paste which hardens in a few days
under water, but in the air they never acquire much solidity. Smeaton first discovered
these remarkable facts, and described them in 1759.
The following analyses of different hydraulic limestones, by Berthier, merit confidence:—
| |
No. 1. |
No. 2. |
No. 3. |
No. 4. |
No. 5. |
| A. Analyses of limestones. |
|
|
|
|
|
| Carbonate of lime |
97·0 |
98·5 |
74·5 |
76·5 |
80·0 |
| Carbonate of magnesia |
2·0 |
— |
23·0 |
3·0 |
1·5 |
| Carbonate of protoxide of iron |
— |
— |
— |
3·0 |
— |
| Carbonate of manganese |
— |
— |
— |
1·5 |
— |
| Silica and alumina |
|
|
|
|
|
- |
15·2 |
|
|
- |
18·0 |
| Oxide of iron |
1·0 |
1·5 |
1·2 |
| |
|
|
|
|
|
| |
100·0 |
100·0 |
100·0 |
100·0 |
100·0 |
| B. Analyses of the burnt lime. |
|
|
|
|
|
| Lime |
96·4 |
97·2 |
78·0 |
68·3 |
70·0 |
| Magnesia |
1·8 |
— |
20·0 |
2·0 |
1·0 |
| Alumina |
1·8 |
2·8 |
2·0 |
24·0 |
29·0 |
| Oxide of iron |
— |
— |
— |
5·7 |
— |
| |
100·0 |
100·0 |
100·0 |
100·0 |
100·0 |
[868]
No. 1. is from the fresh-water lime formation of Château-Landon, near Nemours;
No. 2. the large-grained limestone of Paris; both of these afford a fat lime when burnt.
Dolomite affords a pretty fat lime, though it contains 42 per cent. of carbonate of magnesia;
No. 3. is a limestone from the neighbourhood of Paris, which yields a poor lime,
possessing no hydraulic property; No. 4. is the secondary limestone of Metz; No. 5.
is the lime marl of Senonches, near Dreux; both the latter have the property of hardening
under water, particularly the last, which is much used at Paris on this account.
All good hydraulic mortars must contain alumina and silica; the oxides of iron and
manganese, at one time considered essential, are rather prejudicial ingredients. By
adding silica and alumina, or merely the former, in certain circumstances, to fat lime, a
water-cement may be artificially formed; as also by adding to lime any of the following
native productions, which contain silicates; puzzolana, trass or tarras, pumice-stone,
basalt-tuff, slate-clay. Puzzolana is a volcanic product, which forms hills of considerable
extent to the south-west of the Appenines, in the district of Rome, the Pontine
marshes, Viterbo, Bolsena, and in the Neapolitan region of Puzzuoli, whence the name.
A similar volcanic tufa is found in many other parts of the world. According to
Berthier, the Italian puzzolana consists of 44·5 silica; 15·0 alumina; 8·8 lime; 4·7
magnesia; 1·4 potash; 4·1 soda; 12 oxides of iron and titanium; 9·2 water; in 100
parts.
The tufa stone, which when ground forms trass, is composed of 57·0 silica, 16·0 clay,
2·6 lime, 1·0 magnesia, 7·0 potash, 1·0 soda, 5 oxides of iron and titanium, 9·6 water.
This tuff is found abundantly filling up valleys in beds of 10 or 20 feet deep, in the
north of Ireland, among the schistose formations upon the banks of the Rhine, and at
Monheim in Bavaria.
The fatter the lime, the less of it must be added to the ground puzzolana or trass, to
form a hydraulic mortar; the mixture should be made extemporaneously, and must at
any rate be kept dry till about to be applied. Sometimes a proportion of common sand
mortar instead of lime is mixed with the trass. When the hydraulic cement hardens too
soon, as in 12 hours, it is apt to crack; it is better when it takes 8 days to concrete.
Through the agency of the water, silicates of lime, alumina, (magnesia), and oxide of
iron are formed, which assume a stony hardness.
Besides the above two volcanic products, other native earthy compounds are used in
making water cements. To this head belong all limestones which contain from 20 to
30 per cent. of clay and silica. By gentle calcination, a portion of the carbonic acid
is expelled, and a little lime is combined with the clay, while a silicate of clay and lime
results, associated with lime in a subcarbonated state. A lime-marl containing less clay
will bear a stronger calcining heat without prejudice to its qualities as a hydraulic
cement; but much also depends upon the proportion of silica present, and the physical
structure of all the constituents.
The mineral substance most used in England for making such mortar, is vulgarly
called cement-stone. It is a reniform limestone, which occurs distributed in single nodules
or rather lenticular cakes, in beds of clay. They are mostly found in those argillaceous
strata which alternate with the limestone beds of the oolite formation, as also in the
clay strata above the chalk, and sometimes in the London clay. On the coasts of Kent,
in the isles of Sheppey and Thanet, on the coasts of Yorkshire, Somersetshire, and the
Isle of Wight, &c., these nodular concretions are found in considerable quantities, having
been laid bare by the action of the sea and weather. They were called by the older
mineralogists Septaria and Ludus Helmontii (Van Helmont’s coits). When sawn across,
they show veins of calc-spar traversing the siliceous clay, and are then sometimes placed
in the cabinets of virtuosi. They are found also in several places on the Continent, as at
Neustadt-Eberswalde, near Antwerp, near Altdorf in Bavaria; as also at Boulogne-sur-mer,
where they are called Boulogne-pebbles (galets). These nodules vary in size from
that of a fist to a man’s head, they are of a yellow-gray or brown colour, interspersed
with veins of calc-spar, and sometimes contain cavities bestudded with crystals. Their
specific gravity is 2·59.
Analyses of several cement-stones, and of the cement made with them:—
| |
No. 1. |
No. 2. |
No. 3. |
No. 4. |
No. 5. |
| A. Constituents of the cement-stones. |
|
|
|
|
|
| Carbonate of lime |
65·7 |
61·6 |
|
82·9 |
63·8 |
| Carb——e ofmagnesia |
0·5 |
|
|
|
1·5 |
| Carb——e ofprotoxide of iron |
6·0 |
6·0 |
|
|
|
- |
4·3 |
11·6 |
| Carb——e ofmanganese |
1·6 |
|
|
|
| Silica |
18·0 |
15·0 |
|
13·0 |
14·0 |
| Alumina or clay |
6·6 |
4·8 |
|
trace |
5·7 |
| Oxide of iron[869] |
|
3·0 |
|
|
|
| Water |
1·2 |
6·6 |
|
|
3·4 |
| B. Constituents of the cement. |
|
|
|
|
|
| Lime |
55·4 |
54·0 |
55·0 |
|
56·6 |
| Magnesia |
|
|
|
|
1·1 |
| Alumina or clay |
36·0 |
31·0 |
38·0 |
|
21·0 |
| Oxide of iron |
8·6 |
15·0 |
13·0 |
|
13·7 |
No. 1. English cement-stone, analyzed by Berthier; No. 2. Boulogne stone, by
Drapiez; No. 3. English ditto, by Davy; No. 4. reniform limestone nodules from
Arkona, by Hühnefeld; No. 5. cement-stone of Avallon, by Dumas.
In England the stones are calcined in shaft-kilns, or sometimes in mound-kilns,
then ground, sifted, and packed in casks. The colour of the powder is dark-brown-red.
When made into a thick paste with water, it absorbs little of it, evolves hardly any
heat, and soon indurates. It is mixed with sharp sand in various proportions, immediately
before using it; and is employed in all marine and river embankments, for securing the
seams of stone or brick floors or arches from the percolation of moisture, and also for
facing walls to protect them from damp.
The cement of Pouilly is prepared from a Jurassic (secondary) limestone, which contains
39 per cent. of silica, with alumina, magnesia, and iron oxide. Vicat forms a factitious
Roman cement by making bricks with a pasty mixture of 4 parts of chalk, and
1 part of dry clay, drying, burning, and grinding them. River sand must be added
to this powder; and even with this addition, its efficacy is somewhat doubtful; though it
has, for want of a better substitute, been much employed at Paris.
The cement of Dihl consists of porcelain or salt-glaze potsherds ground fine, and
mixed with boiled linseed oil.
Hamelin’s mastic or lithic paint to cover the façades of brick buildings, &c., is composed
of 50 measures of siliceous sand, 50 of lime-marl, and 9 of litharge or red-lead
ground up with linseed oil.
MOSAIC GOLD. For the composition of this peculiar alloy of copper and zinc,
called also Or-molu, Messrs. Parker and Hamilton obtained a patent in November, 1825.
Equal quantities of copper and zinc are to be “melted at the lowest temperature that
copper will fuse,” which being stirred together so as to produce a perfect admixture of
the metals, a further quantity of zinc is added in small portions, until the alloy in the
melting pot becomes of the colour required. If the temperature of the copper be too
high, a portion of the zinc will fly off in vapour, and the result will be merely spelter or
hard solder; but if the operation be carried on at as low a heat as possible, the alloy will
assume first a brassy yellow colour; then, by the introduction of small portions of zinc, it
will take a purple or violet hue, and will ultimately become perfectly white; which is
the appearance of the proper compound in its fused state. This alloy may be poured
into ingots; but as it is difficult to preserve its character when re-melted, it should be
cast directly into the figured moulds. The patentees claim the exclusive right of compounding
a metal consisting of from 52 to 55 parts of zinc out of 100.
Mosaic gold, the aurum musivum of the old chemists, is a sulphuret of tin.
MOSAIC. (Mosaïque, Fr.; Mosaisch, Germ.) There are several kinds of mosaic,
but all of them consist in imbedding fragments of different coloured substances, usually
glass or stones, in a cement, so as to produce the effect of a picture. The beautiful chapel
of Saint Lawrence in Florence, which contains the tombs of the Medici, has been greatly
admired by artists, on account of the vast multitude of precious marbles, jaspers, agates,
avanturines, malachites, &c., applied in mosaic upon its walls. The detailed discussion
of this subject belongs to a treatise upon the fine arts.
MOTHER OF PEARL (Nacre de Perles, Fr.; Perlen mutter, Germ.); is the
hard, silvery, brilliant internal layer of several kinds of shells, particularly oysters, which
is often variegated with changing purple and azure colours. The large oysters of the
Indian seas alone secrete this coat of sufficient thickness to render their shells available
to the purposes of manufactures. The genus of shell fish called pentadinæ furnishes
the finest pearls, as well as mother of pearl; it is found in greatest perfection round the
coasts of Ceylon, near Ormus in the Persian Gulf, at Cape Comorin, and among some
of the Australian seas. The brilliant hues of mother of pearl, do not depend upon the
nature of the substance, but upon its structure. The microscopic wrinkles or furrows
which run across the surface of every slice, act upon the reflected light in such a way as
to produce the chromatic effect; for Sir David Brewster has shown, that if we take, with[870]
very fine black wax, or with the fusible alloy of D’Arcet, an impression of mother of
pearl, it will possess the iridescent appearance. Mother of pearl is very delicate to work,
but it may be fashioned by saws, files, and drills, with the aid sometimes of a corrosive
acid, such as the dilute sulphuric or muriatic; and it is polished by colcothar of vitriol.
MOTHER-WATER, is the name of the liquid which remains after all the salts
that will regularly crystallize have been extracted, by evaporation and cooling, from any
saline solution.
MOUNTAIN SOAP (Savon de montagne, Fr.; Bergseife, Germ.); is a tender
mineral, soft to the touch, which assumes a greasy lustre when rubbed, and falls to pieces
in water. It consists of silica 44, alumina 26·5, water 20·5, oxide of iron 8, lime 0·5. It
occurs in beds, alternating with different sorts of clay, in the Isle of Skye, at Billin in
Bohemia, &c. It has been often, but improperly, confounded with steatite.
MUCIC ACID (Acid mucique, Fr.; Schleimsaüre, Germ.); is the same as the saclactic
acid of Scheele, and may be obtained by digesting one part of gum arabic, sugar
of milk, or pectic acid, with twice or thrice their weight of nitric acid. It forms white
granular crystals, and has not been applied to any use in the arts.
MUCILAGE, is a solution in water of gummy matter of any kind.
MUFFLE, is the earthenware case or box, in the assay furnaces, for receiving the
cupels, and protecting them from being disturbed by the fuel. See Assay and
Furnace.
MUNDIC, is the name of copper pyrites among English miners.
MUNJEET, is a kind of madder grown in several parts of India.
MURIATIC or HYDROCHLORIC ACID; anciently marine acid, and spirit
of salt. (Acide hydrochlorique, and Chlorhydrique, Fr.; Salzsaüre, Germ.) This acid is
now extracted from sea-salt, by the action of sulphuric acid and a moderate heat; but it
was originally obtained from the salt by exposing a mixture of it and of common clay
to ignition in an earthen retort. The acid gas which exhales, is rapidly condensed
by water. 100 cubic inches of water are capable of absorbing no less than 48,000
cubic inches of the acid gas, whereby the liquid acquires a specific gravity of 1·2109;
and a volume of 142 cubic inches. This vast condensation is accompanied with a great
production of heat, whence it becomes necessary to apply artificial refrigeration, especially
if so strong an acid as the above is to be prepared. In general, the muriatic acid of
commerce has a specific gravity varying from 1·15 to 1·20; and contains, for the most
part, considerably less than 40 parts by weight of acid gas in the hundred. The above
stronger acid contains 42·68 per cent. by weight; for since a cubic inch of water, which
weighs 252·5 grains, has absorbed 480 cubic inches = 188 grains of gas; and 252·5
+ 188 = 440·5; then 440·5 : 188 ∷ 100 : 42·68. In general a very good approximation
may be found to the percentage of real muriatic acid, in any liquid sample, by
multiplying the decimal figures of the specific gravity by 200. Thus for example, at
1·162 we shall have by this rule 0·162 × 200 = 32·4, for the quantity of gas in 100
parts of the liquid. Muriatic acid gas consists of chlorine and hydrogen combined,
without condensation, in equal volumes. Its specific gravity is 1·247, air = 1·000.
By sealing up muriate of ammonia and sulphuric acid, apart, in a strong glass tube recurved,
and then causing them to act on each other, Sir H. Davy procured liquid muriatic
acid. He justly observes, that the generation of elastic substances in close vessels, either
with or without heat, offers much more powerful means of approximating their molecules
than those dependent on the application of cold, whether natural or artificial; for as gases
diminish only 1⁄480 in volume for every degree of Fahrenheit’s scale, beginning at ordinary
temperatures, a very slight condensation only can be produced by the most powerful
freezing mixtures, not half as much as would result from the application of a strong
flame to one part of a glass tube, the other part being of ordinary temperature: and
when attempts are made to condense gases into liquids by sudden mechanical compression,
the heat instantly generated presents a formidable obstacle to the success of the
experiment; whereas in the compression resulting from their slow generation in close
vessels, if the process be conducted with common precautions, there is no source of
difficulty or danger; and it may be easily assisted by artificial cold, in cases where
gases approach near to that point of compression and temperature at which they become
vapours.—Phil. Trans. 1823.
The muriatic acid of commerce has usually a yellowish tinge, but when chemically pure
it is colourless. It fumes strongly in the air, emitting a corrosive vapour of a peculiar
smell. The characteristic test of muriatic acid in the most dilute state, is nitrate of silver,
which causes a curdy precipitate of chloride of silver.
The preparation of this acid upon the great scale is frequently effected in this country
by acting upon sea-salt in hemispherical iron pots, or in cast-iron cylinders, with concentrated
sulphuric acid; taking 6 parts of the salt to 5 of the acid. The mouth of the
pot may be covered with a slab of siliceous freestone, perforated with two holes of about
two inches diameter each, into the one of which the acid is poured by a funnel in successive[871]
portions, and into the other, a bent glass, or stone-ware tube, is fixed, for conducting the
disengaged muriatic gas into a series of large globes of bottle glass, one-third filled with
water, and laid on a sloping sand-bed. A week is commonly employed for working off
each pot; no heat being applied to it till the second day.
The decomposition of sea-salt by sulphuric acid, was at one time carried on by some
French manufacturers in large leaden pans, 10 feet long, 5 feet broad, and a foot deep,
covered with sheets of lead, and luted. The disengaged acid gas was made to circulate
in a conduit of glazed bricks, nearly 650 yards long, where it was condensed by a sheet
of water exceedingly thin, which flowed slowly in the opposite direction of the gas down
a slope of 1 in 200. At the end of this canal nearest the apparatus, the muriatic acid
was as strong as possible, and pretty pure; but towards the other end, the water was
hardly acidulous. The condensing part of this apparatus was therefore tolerably complete;
but as the decomposition of the salt could not be finished in the leaden pans, the
acid mixture had to be drawn out of them, in order to be completely decomposed in a
reverberatory furnace; in this way nearly 50 per cent. of the muriatic acid was lost.
And besides, the great quantity of gas given off during the emptying of the lead-chambers
was apt to suffocate the workmen, or seriously injured their lungs, causing
severe hemoptysis. The employment of muriatic acid is so inconsiderable, and the loss of
it incurred in the preceding process is of so little consequence, that subsequently, both in
France and in England, sulphate of soda, for the soda manufacture, has been procured
with the dissipation of the muriatic acid in the air. In the method more lately resorted
to, the gaseous products are discharged into extensive vaults, where currents of water
condense them and carry them off into the river. The surrounding vegetation is thereby
saved in some measure from being burned up, an accident which was previously sure to
happen when fogs precipitated the floating gases upon the ground. At Newcastle,
Liverpool, and Marseilles, where the consumption of muriatic acid bears no proportion
to the manufacture of soda, this process is now practised upon a vast scale.
The apparatus for condensing muriatic acid gas has been modified and changed, of
late years, in many different ways.
The Bastringue apparatus. At the end of a reverberatory furnace, (see Copper,
smelting of, and Soda, manufacture of,) a rectangular lead trough or pan, about 1 foot
deep, of a width equal to that of the interior of the furnace, that is about 5 feet wide,
and 61⁄2 feet long, is encased in masonry, having its upper edges covered with cast-iron
plates or fire tiles, and placed upon a level with the passage of the flame, as it escapes
from the reverberatory. The arch which covers that pan forms a continuation of the
roof of the reverberatory, and is of the same height. The flame which proceeds from
the furnace containing the mixture of salt and sulphuric acid is made to escape between
the vault and the surface of the iron plates or fire tiles, through a passage only 4 inches
in height. When the burned air and vapours reach the extremity of the pan, they are
reflected downwards, and made to return beneath the bottom of the pan, in a flue, which
is afterwards divided so as to lead the smoke into two lateral flues, which terminate in
the chimney. The pan is thus surrounded as it were with the heat and flame discharged
from the reverberatory furnace. See Evaporation. A door is opened near the end of
the pan, for introducing the charge of sea-salt, amounting to 12 bags of 2 cwt. each,
or 24 cwt. This door is then luted on as tightly as possible, and for every 100 parts
of salt, 110 of sulphuric acid are poured in, of specific gravity 1·594, containing 57 per
cent. of dry acid. This acid is introduced through a funnel inserted in the roof of the
furnace. Decomposition ensues, muriatic acid gas mingled with steam is disengaged,
and is conducted through 4 stone-ware tubes into the refrigerators, where it is finally
condensed. These refrigerators consist of large stone-ware carboys, called dame-jeans
in France, to the number of 7 or 8 for each pipe, and arranged so that the neck of the one
communicates with the body of the other; thus the gas must traverse the whole series,
and gets in a good measure condensed by the water in them, before reaching the last.
When the operation is finished, the door opposite the pan is opened, and the residuum
in it, is discharged, in the form of a fluid magma, upon a square bed of bricks, exterior
to the furnace. This paste speedily concretes on cooling, and is then broken into fragments
and carried to the soda manufactory. The immense quantity of gas exhaled in
discharging the pan, renders this part of the operation very painful to the workmen;
and wasteful in reference to the production of muriatic acid. The difficulty of luting
securely the cast-iron plates or fire tiles which cover the pan, the impossibility of completing
the decomposition of the salt, since the residuum must be run off in a liquid
state, finally, the damage sustained by the melting and corrosion of the lead, &c., are
among the causes why no more than 80 or 90 parts of muriatic acid at 1·170 are collected,
equivalent to 25 per cent. of real acid for every 100 of salt employed, instead of much
more than double that quantity, which it may be made to yield by a well conducted
chemical process.
The cylinder apparatus is now much esteemed by many manufacturers. Fig. 747.[872]
represents, in transverse section, a bench of iron cylinder retorts, as built up in a proper
furnace for producing muriatic acid; and fig. 748. a longitudinal section of one retort
with one of its carboys of condensation. a is the grate; b, a fireplace, in which two
iron cylinders, c c, are set alongside of each other. They are 51⁄2 feet long, 20 inches in
diameter, about 1⁄4 of an inch
thick, and take 1·6 cwts. of
salt for a charge; d is the ash-pit;
e, e, are cast-iron lids, for
closing both ends of the cylinders;
f is a tube in the posterior
lid, for pouring in the
sulphuric acid; g is another
tube, in the anterior lid, for
the insertion of the bent pipe
of hard glazed stone-ware h;
i is a three-necked stone-ware
carboy; k is a tube of safety;
l, a tube of communication with
the second carboy; m m, m m,
are the flues leading to the
chimney n.
After the salt has been introduced, and the fire kindled, 831⁄4 per cent. of its
weight of sulphuric acid, of spec. grav. 1·80, should be slowly poured into the cylinder
through a lead funnel, with a syphon-formed pipe. The three-necked carboys may be
either placed in a series for each retort, like a range of Woulfe’s bottles, or all the carboys
of the front range may be placed in communication with one another, while the last carboy
at one end is joined to the first of the second range; and thus in succession. They
must be half filled with cold water; and when convenient, those of the front row at
least, should be plunged in an oblong trough of running water. The acid which condenses
in the carboys of that row is apt to be somewhat contaminated with sulphuric
acid, muriate of iron, or even sulphate of soda; but that in the second and third will be
found to be pure. In this way 100 parts of sea-salt will yield 130 parts of muriatic acid, of
spec. grav. 1·19; while the sulphate of soda in the retort will afford from 208 to 210
of that salt in crystals.
It is proper to heat all the parts of the cylinders equably, to insure the simultaneous
decomposition of the salt, and to protect it from the acid; for the hotter the iron, and
the stronger the acid, the less erosion ensues.
Some manufacturers, with the view of saving fuel by the construction of their furnaces
oppose to the flame as many obstacles as they can, and make it perform numerous
circulations round the cylinders; but this system is bad, and does not even effect the
desired economy, because the passages, being narrow, impair the draught, and become
speedily choked up with the soot, which would be burned profitably in a freer space; the
decomposition also, being unequally performed, is less perfect, and the cylinders are
more injured. It is better to make the flame envelope at once the body of the cylinder;
after which it may circulate beneath the vault, in order to give out a portion of its caloric
before it escapes at the chimney.
The fire should be briskly kindled, but lowered as soon as the distillation commences;
and then continued moderate till the evolution of gas diminishes, when it must be
heated somewhat strongly to finish the decomposition. The iron door is now removed,[873]
to extract the sulphate of soda, and to recommence another operation. This sulphate
ought to be white and uniform, exhibiting in its fracture no undecomposed sea-salt.
Liquid muriatic acid has a very sour corrosive taste, a pungent suffocating smell, and
acts very powerfully upon a vast number of mineral, vegetable, and animal substances.
It is much employed for making many metallic solutions; and in combination with nitric
acid, it forms the aqua regia of the alchemists, so called from its property of dissolving
gold.
Table of Muriatic Acid, by Dr. Ure.
Acid
of 120
in 100. |
Specific
gravity. |
Chlorine. |
Muriatic
Gas. |
| 100 |
1·2000 |
39·675 |
40·777 |
| 99 |
1·1982 |
39·278 |
40·369 |
| 98 |
1·1964 |
38·882 |
39·961 |
| 97 |
1·1946 |
38·485 |
39·554 |
| 96 |
1·1928 |
38·089 |
39·146 |
| 95 |
1·1910 |
37·692 |
38·738 |
| 94 |
1·1893 |
37·296 |
38·330 |
| 93 |
1·1875 |
36·900 |
37·923 |
| 92 |
1·1857 |
36·503 |
37·516 |
| 91 |
1·1846 |
36·107 |
37·108 |
| 90 |
1·1822 |
35·707 |
36·700 |
| 89 |
1·1802 |
35·310 |
36·292 |
| 88 |
1·1782 |
34·913 |
35·884 |
| 87 |
1·1762 |
34·517 |
35·476 |
| 86 |
1·1741 |
34·121 |
35·068 |
| 85 |
1·1721 |
33·724 |
34·660 |
| 84 |
1·1701 |
33·328 |
34·252 |
| 83 |
1·1681 |
32·931 |
33·845 |
| 82 |
1·1661 |
32·535 |
33·437 |
| 81 |
1·1641 |
32·136 |
33·029 |
| 80 |
1·1620 |
31·746 |
32·621 |
| 79 |
1·1599 |
31·343 |
32·213 |
| 78 |
1·1578 |
30·946 |
31·805 |
| 77 |
1·1557 |
30·550 |
31·398 |
| 76 |
1·1536 |
30·153 |
30·990 |
| 75 |
1·1515 |
29·757 |
30·582 |
| 74 |
1·1494 |
29·361 |
30·174 |
| 73 |
1·1473 |
28·964 |
29·767 |
| 72 |
1·1452 |
28·567 |
29·359 |
| 71 |
1·1431 |
28·171 |
28·951 |
| 70 |
1·1410 |
27·772 |
28·544 |
| 69 |
1·1389 |
27·376 |
28·136 |
| 68 |
1·1369 |
26·979 |
27·728 |
| 67 |
1·1349 |
26·583 |
27·321 |
| 66 |
1·1328 |
26·186 |
26·913 |
| 65 |
1·1308 |
25·789 |
26·505 |
| 64 |
1·1287 |
25·392 |
26·098 |
| 63 |
1·1267 |
24·996 |
25·690 |
| 62 |
1·1247 |
24·599 |
25·282 |
| 61 |
1·1226 |
24·202 |
24·874 |
| 60 |
1·1206 |
23·805 |
24·466 |
| 59 |
1·1185 |
23·408 |
24·058 |
| 58 |
1·1164 |
23·012 |
23·050 |
| 57 |
1·1143 |
22·615 |
23·242 |
| 56 |
1·1123 |
22·218 |
22·834 |
| 55 |
1·1102 |
21·822 |
22·426 |
| 54 |
1·1082 |
21·425 |
22·019 |
| 53 |
1·1061 |
21·028 |
21·611 |
| 52 |
1·1041 |
20·632 |
21·203 |
| 51 |
1·1020 |
20·235 |
20·796 |
| 50 |
1·1000 |
19·837 |
20·388 |
| 49 |
1·0980 |
19·440 |
19·980 |
| 48 |
1·0960 |
19·044 |
19·572 |
| 47 |
1·0939 |
18·647 |
19·165 |
| 46 |
1·0919 |
18·250 |
18·757 |
| 45 |
1·0899 |
17·854 |
18·349 |
| 44 |
1·0879 |
17·457 |
17·941 |
| 43 |
1·0859 |
17·060 |
17·534 |
| 42 |
1·0838 |
16·664 |
17·126 |
| 41 |
1·0818 |
16·267 |
16·718 |
| 40 |
1·0798 |
15·870 |
16·310 |
| 39 |
1·0778 |
15·474 |
15·902 |
| 38 |
1·0758 |
15·077 |
15·494 |
| 37 |
1·0738 |
14·680 |
15·087 |
| 36 |
1·0718 |
14·284 |
14·679 |
| 35 |
1·0697 |
13·887 |
14·271 |
| 34 |
1·0677 |
13·490 |
13·863 |
| 33 |
1·0657 |
13·094 |
13·456 |
| 32 |
1·0637 |
12·697 |
13·049 |
| 31 |
1·0617 |
12·300 |
12·641 |
| 30 |
1·0597 |
11·903 |
12·233 |
| 29 |
1·0577 |
11·506 |
11·825 |
| 28 |
1·0557 |
11·109 |
11·418 |
| 27 |
1·0537 |
10·712 |
11·010 |
| 26 |
1·0517 |
10·316 |
10·602 |
| 25 |
1·0497 |
9·919 |
10·194 |
| 24 |
1·0477 |
9·522 |
9·786 |
| 23 |
1·0457 |
9·126 |
9·379 |
| 22 |
1·0437 |
8·729 |
8·971 |
| 21 |
1·0417 |
8·332 |
8·563 |
| 20 |
1·0397 |
7·935 |
8·155 |
| 19 |
1·0377 |
7·538 |
7·747 |
| 18 |
1·0357 |
7·141 |
7·340 |
| 17 |
1·0337 |
6·745 |
6·932 |
| 16 |
1·0318 |
6·348 |
6·524 |
| 15 |
1·0298 |
5·951 |
6·116 |
| 14 |
1·0279 |
5·554 |
5·709 |
| 13 |
1·0259 |
5·158 |
5·301 |
| 12 |
1·0239 |
4·762 |
4·893 |
| 11 |
1·0220 |
4·365 |
4·486 |
| 10 |
1·0200 |
3·968 |
4·078 |
| 9 |
1·0180 |
3·571 |
3·670 |
| 8 |
1·0160 |
3·174 |
3·262 |
| 7 |
1·0140 |
2·778 |
2·854 |
| 6 |
1·0120 |
2·381 |
2·447 |
| 5 |
1·0100 |
1·984 |
2·039 |
| 4 |
1·0080 |
1·588 |
1·631 |
| 3 |
1·0060 |
1·191 |
1·224 |
| 2 |
1·0040 |
0·795 |
0·816 |
| 1 |
1·0020 |
0·397 |
0·408 |
MURIATES were, till the great chemical era of Sir H. Davy’s researches upon
chlorine, considered to be compounds of an undecompounded acid, the muriatic, with the
different bases; but he proved them to be in reality compounds of chlorine with the
metals. They are all, however, still known in commerce by their former appellation.
The only muriates much used in the manufactures are, Muriate of ammonia, or Sal ammoniac;
muriated peroxide of mercury, Mercury, bichloride of; muriate of soda, or chloride
of sodium, see Salt; muriate of tin, see Calico-printing and Tin.
MUSK (Musc, Fr.; Moschus, Germ.), is a peculiar aromatic substance, found in a
sac between the navel and the parts of generation of a small male quadruped of the deer
kind, called by Linnæus, Moschus moschiferus, which inhabits Tonquin and Thibet.
The colour of musk is blackish-brown; it is lumpy or granular, somewhat like dried
blood, with which substance, indeed, it is often adulterated. The intensity of its smell is
almost the only criterion of its genuineness. When thoroughly dried it becomes nearly
scentless; but it recovers its odour when slightly moistened with water of ammonia.
The Tonquin musk is most esteemed. It comes to us in small bags covered with a
reddish-brown hair; the bag of the Thibet musk is covered with a silver-gray hair. All
the analyses of musk hitherto made, teach little or nothing concerning its active or essential
constituent. It is used in medicines, and is an ingredient in a great many perfumes.
[874]
MUSLIN, is a fine cotton fabric, used for ladies’ robes; which is worn either white,
dyed, or printed.
MUST, is the sweet juice of the grape.
MUSTARD (Moutarde, Fr.; Senf, Germ.); is a plant which yields the well-known
seed used as a condiment to food. M. Lenormand gives the following prescription for
preparing mustard for the table.
With 2 pounds of very fine flour of mustard, mix half an ounce of each of the following
fresh plants; parsley, chervil, celery, and tarragon, along with a clove of garlic, and
twelve salt anchovies, all well minced. The whole is to be triturated with the flour of
mustard till the mixture becomes uniform. A little grape-must or sugar is to be added,
to give the requisite sweetness; then one ounce of salt, with sufficient water to form a
thinnish paste by rubbing in a mortar. With this paste the mustard pots being nearly
filled, a redhot poker is to be thrust down into the contents of each, which removes (it
is said) some of the acrimony of the mustard, and evaporates a little water, so as to make
room for pouring a little vinegar upon the surface of the paste. Such table mustard
not only keeps perfectly well, but improves with age.
The mode of preparing table mustard patented by M. Soyés, consisted in steeping
mustard seed in twice its bulk of weak wood vinegar for eight days, then grinding the
whole into paste in a mill, putting it into pots, and thrusting a redhot poker into
each of them.
MUTAGE, is a process used in the south of France to arrest the progress of fermentation
in the must of the grape. It consists either in diffusing sulphurous acid, from
burning sulphur matches in the cask containing the must, or in adding a little sulphite
(not sulphate) of lime to it. The last is the best process. See Fermentation.
MYRICINE, is a vegetable principle which constitutes from 20 to 30 per cent. of
the weight of bees-wax, being the residuum from the solvent action of alcohol upon that
substance. It is a grayish-white solid, which may be vaporized almost without alteration.
MYRRH, is a gum-resin, which occurs in tears of different sizes; they are reddish-brown,
semi-transparent, brittle, of a shining fracture, appear as if greasy under the
pestle, they have a very acrid and bitter taste, and a strong, not disagreeable, smell.
Myrrh flows from the incisions of a tree not well known, which grows in Arabia and
Abyssinia, supposed to be a species of amyris or mimosa. It consists of resin and gum in
proportions stated by Pelletier at 31 of the former and 66 of the latter; but by Braconnot,
at 23 and 77. It is used only in medicine.
NACARAT, is a term derived from the Spanish word nacar, which signifies mother
of pearl; and is applied to a pale red colour, with an orange cast. See Calico-printing.
The nacarat of Portugal or Bezetta is a crape or fine linen fabric, dyed
fugitively of the above tint, which ladies rub upon their countenances to give them a
roseate hue. The Turks of Constantinople manufacture the brightest red crapes of this
kind. See Rouge.
NAILS, MANUFACTURE OF. (Clou, Fr.; Nagel, Germ.)
The forging of nails was till of late years a handicraft operation, and therefore belonged
to a book of trades, rather than to a dictionary of arts. But several combinations of machinery
have been recently employed, under the protection of patents, for making these
useful implements, with little or no aid of the human hand; and these deserve to be
noticed, on account both of their ingenuity and importance.
As nails are objects of prodigious consumption in building their block-houses, the
citizens of the United States very early turned their mechanical genius to good account in
the construction of various machines for making them. So long since as the year 1810, it
appears, from the report of the secretary of their treasury, that they possessed a machine
which performed the cutting and heading at one operation, with such rapidity that it
could turn out upwards of 100 nails per minute. “Twenty years ago,” says the secretary
of the state of Massachusetts, in that report, “some men, then unknown, and then in
obscurity, began by cutting slices out of old hoops, and, by a common vice griping these
pieces, headed them with several strokes of the hammer. By progressive improvements,
slitting-mills were built, and the shears and the heading tools were perfected; yet much
labour and expense were requisite to make nails. In a little time Jacob Perkins, Jonathan
Ellis, and a few others, put into execution the thought of cutting and of heading nails
by water power; but, being more intent upon their machinery than upon their pecuniary
affairs, they were unable to prosecute the business. At different times other men have
spent fortunes in improvements, and it may be said with truth that more than one million[875]
of dollars has been expended; but at length these joint efforts are crowned with
complete success, and we are now able to manufacture, at about one-third of the expense
that wrought nails can be manufactured for, nails which are superior to them for
at least three-fourths of the purposes to which nails are applied, and for most of those
purposes they are full as good. The machines made use of by Odiorne, those invented
by Jonathan Ellis, and a few others, present very fine specimens of American genius.
“To northern carpenters, it is well known that in almost all instances it is unnecessary
to bore a hole before driving a cut nail; all that is requisite is, to place the cutting edge
of the nail across the grain of the wood; it is also true, that cut nails will hold better in
the wood. These qualities are, in some rough building works, worth twenty per cent. of
the value of the article, which is equal to the whole expense of manufacturing. For
sheathing and drawing, cut nails are full as good as wrought nails; only in one respect
are the best wrought nails a little superior to cut nails, and that is where it is necessary
they should be clenched. The manufacture of cut nails was born in our country, and
has advanced, within its bosom, through all the various stages of infancy to manhood;
and no doubt we shall soon be able, by receiving proper encouragement, to render them
superior to wrought nails in every particular.
“The principal business of rolling and slitting-mills, is rolling nail plates; they also
serve to make nail rods, hoops, tires, sheet iron, and sheet copper. In this State we
have not less than twelve.
“These mills could roll and slit 7000 tons of iron a year; they now, it is presumed,
roll and slit each year about 3500 tons, 2400 tons of which, probably, are cut up into
nails and brads, of such a quality that they are good substitutes for hammered nails, and,
in fact, have the preference with most people, for the following reasons; viz., on account
of the sharp corner and true taper with which cut nails are formed; they may be driven
into harder wood without bending or breaking, or hazard of splitting the wood, by
which the labour of boring is saved, the nail one way being of the same breadth or
thickness from head to point.”
Since the year 1820, the following patents have been obtained in England for making
nails; many of them of American origin:—
Alexander Law, September, 1821, for nails and bolts for ships’ fastenings, made in a
twisted form, by hand labour.
Glascott and Mitchell, December, 1823, for ship nails with rounded heads, by hand
labour.
Wilks and Ecroyd, November, 1825, for an engine for cutting wedge-form pieces
from plates.
Ledsom and Jones, December 11, 1827, for machinery for cutting brads and sprigs
from plates; it does not form heads.
The first nail apparatus to which I shall particularly advert, is due to Dr. Church; it
was patented in his absence by his correspondent, Mr. Thomas Tyndall, of Birmingham,
in December, 1827. It consists of two parts; the first is a mode of forming nails, and
the shafts of screws, by pinching or pressing ignited rods of iron between indented rollers;
the second produces the threads on the shafts of the screws previously pressed.
The metallic rods, by being passed between a pair of rollers, are rudely shaped, and then
cut asunder between a pair of shears; after which they are pointed and headed, or otherwise
brought to their finished forms, by the agency of dies placed in a revolving cylinder.
The several parts of the mechanism are worked by toothed wheels, cams, and levers.
The second part of Dr. Church’s invention consists of a mechanism for cutting the
threads of screws to any degree of obliquity or form.[35]
Mr. L. W. Wright’s (American) apparatus should have been mentioned before the preceding,
as the patent for it was sealed in March of the same year; though an amended
patent was obtained in September, 1828. Its object was to form metal screws for wood.
I have seen the machinery, but consider it much too complex to be described in the
present work.
Mr. Edward Hancorne, of Skinner street, London, nail manufacturer, obtained a
patent in October, 1828 for a nail-making machine, of which a brief description may
give my readers a conception of this kind of manufacture. Its principles are similar to
those of Dr. Church’s more elaborate apparatus.
The rods or bars having been prepared in the usual way, either by rolling or hammering,
or by cutting from sheets or plates of iron, called slitting, are then to be made redhot,
and in that state passed through the following machine, whereby they are at once cut
into suitable lengths, pressed into wedge forms for pointing at the one end, and stamped
at the other end to produce the head. A longitudinal view of the machine is shown in
fig. 749. A strong iron frame-work, of which one side is shown at a a, supports the
whole of the mechanism. b is a table capable of sliding to and fro horizontally. Upon[876]
this table are the clamps, which lay hold of the sides of the rod as it advances; as also
the shears which cut the rod into nail lengths.
These clamps or holders consist of a fixed piece and a movable piece; the latter
being brought into action by a lever. The rod or bar of iron shown at c, having been
made redhot, is introduced into the machine by sliding it forward upon the table b,
when the table is in its most advanced position; rotatory motion is then given to the
crank shaft d, by means of a band passing round the rigger pulley e, which causes the
table b to be drawn back by the crank rod f: and as the table recedes, the horizontal
lever is acted upon, which closes the clamps. By these means the clamps take fast hold
of the sides of the heated rod, and draw it forward, when the movable chap of the shears,
also acted upon by a lever, slides laterally, and cuts off the end of the rod held by the
clamps: the piece thus separated is destined to form one nail.
Suppose that the nail placed at g, having been thus brought into the machine and cut
off, is held between clamps, which press it sideways (these clamps are not visible in this
view); in this state it is ready to be headed and pointed.
The header is a steel die h, which is to be pressed up against the end of the nail by a
cam i, upon the crank-shaft; which cam, at this period of the operation, acts against the
end of a rod k, forming a continuation of the die h, and forces up the die, thus compressing
the metal into the shape of a nail-head.
The pointing is performed by two rolling snail pieces or spirals l, l. These pieces are
somewhat broader than the breadth of the nail; they turn upon axles in the side frames.
As the table b advances, the racks m, on the edge of this table, take into the toothed
segments n, n, upon the axles of the spirals, and cause them to turn round.
These spirals pinch the nail at first close under its head with very little force; but as
they turn round, the longer radius of the spiral comes into operation upon the nail, so as
to press its substance very strongly, and squeeze it into a wedge form. Thus the nail
is completed, and is immediately discharged from the clamps or holders. The carriage
is then again by the rotation of the crank-shaft, which brings another portion of the
rod c forward, cuts it off, and then forms it into a nail.
Richard Prosser, July, 1831, for making tacks for ornamental furniture, by soldering
or wedging the spike into the head. This also is the invention of Dr. Church.
Dr. William Church, February, 1832, for improvements in machinery for making
nails. These consist, first, in apparatus for forming rods, bars, or plates of iron, or other
metals; secondly, in apparatus for converting the rods, &c., into nails; thirdly, in improvements
upon Prosser’s patent. The machinery consists in laminating rollers, and
compressing dies.
The method of forming the rods from which the nails are to be made, is very
advantageous. It consists in passing the bar or plate iron through pressing rollers, which
have indentations upon the peripheries of one or both of them, so as to form the bar or
plate into the required shape for the rods, which may be afterwards separated into rods
of any desired breadth, by common slitting rollers.
The principal object of rolling the rods into these wedge forms, is to measure out a
quantity of metal duly proportioned to the required thickness or strength of the nail in
its several parts; which quantity corresponds to the indentations of the rollers.
Thomas John Fuller, February 27, 1834, for an improved apparatus for making square-pointed,
and also flat-pointed nails. He claims as his invention, the application of vertical
and horizontal hammers (mounted in his machine) combined for the purpose of[877]
tapering and forming the points of the nails; which, being made to act alternately, resemble
hand work, and are therefore not so apt to injure the fibrous texture of the iron,
he imagines, as the rolling machinery is. He finishes the points by rollers.
Miles Berry, February 19, 1834, for machinery for forming metal into bolts, rivets,
nails and other articles; being a communication from a foreigner residing abroad. He
employs in his machine holding chaps, heading dies, toggle joints, cams, &c., mechanisms
apparently skilfully contrived, but too complex for admission under the article nail
in this volume.
William Southwood Stocker, July, 1836. This is a machine apparently of American
parentage, as it has the same set of features as the old American mechanisms of Perkins
and Dyer, at the Britannia Nailworks, Birmingham, and all the other American machines
since described, for pressing metal into the forms of nails, pins, screw-shafts,
rivets, &c.; for example, it possesses pressers or hammers for squeezing the rods of metal,
and forming the shanks, which are all worked by a rotatory action; cutters for separating
the appropriate lengths, and dies for forming the heads by compression, also actuated
by revolving cams or cranks.
Mr. Stocker intends, in fact, to effect the same sorts of operations by automatic mechanisms
as are usually performed by the hands of a nail-maker with his hammer and anvil;
viz., the shaping of a nail from a heated rod of iron, cutting it off at the proper length, and
then compressing the end of the metal into the form of the head. His machine may be
said to consist of two parts, connected in the same frame; the one for shaping the shank
of the nail, the other for cutting it off and heading it. The frame consists of a strong
table to bear the machinery. Two pairs of hammers, formed as levers, the one pair
made to approach each other by horizontal movements, the other pair by vertical movements,
are the implements by which a portion at the end of a redhot rod of iron is
beaten or pressed into the wedge-like shape of the shaft of a nail. This having been
done, and the rod being still hot, is withdrawn from the beaters, and placed in the other
part of the machine, consisting of a pair of jaws like those of a vice, which pinch the
shank of the nail and hold it fast. A cutter upon the side of a wheel now comes round,
and, by acting as the moving chap of a pair of shears, cuts the nail off from the rod.
The nail shank being still firmly held in the jaws of the vice, with a portion of its end
projecting outwardly, the heading die is slidden laterally until it comes opposite to the
end of the nail; the dye is then projected forward with great force, for the purpose of
what is termed upsetting the metal at the projecting end of the nail, and thereby blocking
out the head.
A main shaft, driven by a band and rigger as usual, brings, as it revolves, a cam into
operation upon a lever which carries a double inclined plane or wedge in its front or acting
part. This wedge being by the rotatory cam projected forwards between the tails of
one of the pairs of hammers, causes the faces of these hammers to approach each other,
and to beat or press the redhot iron introduced between them, so as to flatten it upon
two opposite sides. The rotatory cam passing round, the wedge lever is relieved, when
springs instantly throw back the hammers; another cam and wedge-lever now brings
the second pair of hammers to act upon the other two sides of the nail in a similar
way. This is repeated several times, until the end of the redhot iron rod, gradually
advanced by the hands of the workman, has assumed the desired form, that is, has
received the bevel and point of the intended nail.
The rod is then withdrawn from between the hammers, and in its heated state is introduced
between the jaws of the holders, for cutting off and finishing the nail. A bevel
pinion upon the end of the main shaft, takes into and drives a wheel upon a transverse
shaft, which carries a cam that works the lever of the holding jaws. The end of the
rod being so held in the jaws or vice, a cutter at the side of a wheel upon the transverse
shaft separates, as it revolves, the nail from the end of the rod, leaving the nail firmly
held by the jaws. By means of a cam, the heading die is now slidden laterally opposite
to the end of the nail in the holding jaws, and by another cam, upon the main shaft, the die
is forced forward, which compresses the end of the nail, and spreads out the nail into the
form of a head. As the main shaft continues to revolve, the cams pass away, and allow
the spring to throw the jaws of the vice open, when the nails fall out; but to guard
against the chance of a nail sticking in the jaws, a picker is provided, which pushes the
nail out as soon as it is finished.
In order to produce round shafts, as for screw blanks, bolts, or rivets, the faces of the
hammers, and the dies for heading, must be made with suitable concavities.
In 1835, 5,180, and in 1836, 5,580 tons of iron nails were exported from the United
Kingdom.
NANKIN, is a peculiarly coloured cotton cloth, originally manufactured in the above
named antient capital of China, from a native cotton of a brown yellow hue. Nankin
cloth has been long imitated in perfection by our own manufacturers; and is now
exported in considerable quantities from England to Canton. The following is the
process for dyeing calico a nankin colour.
[878]
1. Take 300 pounds of cotton yarn in hanks, being the quantity which four workmen
can dye in a day. The yarn for the warp may be about No. 27’s, and that for the weft
23’s or 24’s.
2. For aluming that quantity, take 10 pounds of saturated alum, free from iron (see
Mordant); divide this into two portions; dissolve the first by itself in hot water, so
as to form a solution, of spec. grav. 1° Baumé. The second portion is to be reserved
for the galling bath.
3. Galling, is given with about 80 pounds of oak bark finely ground. This bark may
serve for two quantities, if it be applied a little longer the second time.
4. Take 30 pounds of fresh slaked quicklime, and form with it a large bath of lime-water.
5. Nitro-muriate of tin. For the last bath, 10 or 12 pounds of solution of tin are used,
which is prepared as follows:
Take 10 pounds of strong nitric acid, and dilute with pure water till its specific
gravity be 26° B. Dissolve in it 4633 grains (101⁄2 oz. avoird.) of sal ammoniac, and
3 oz. of nitre. Into this solvent, contained in a bottle set in cold water, introduce successively,
in very small portions, 28 ounces of grain-tin granulated. This solution, when
made, must be kept in a well stoppered bottle.
Three coppers are required, one round, about five feet in diameter, and 32 inches deep,
for scouring the cotton; 2. two rectangular coppers tinned inside, each 5 feet long and 20
inches deep. Two boxes or cisterns of white wood are to be provided, the one for the
lime-water bath, and the other for the solution of tin, each about 7 feet long, 32 inches
wide, and 14 inches deep; they are set upon a platform 28 inches high. In the middle
between these two chests, a plank is fixed, mounted with twenty-two pegs for wringing
the hanks upon, as they are taken out of the bath.
6. Aluming. After the cotton yarn has been scoured with water, in the round copper,
by being boiled in successive portions of 100 pounds, it must be winced in one of the
square tinned coppers, containing two pounds of alum dissolved in 96 gallons of water,
at a temperature of 165° F. It is to be then drained over the copper, exposed for some
time upon the grass, rinsed in clear water, and wrung.
7. The galling. Having filled four-fifths of the second square copper with water, 40
pounds of ground oak bark are to be introduced, tied up in a bag of open canvas, and
boiled for two hours. The bag being withdrawn, the cotton yarn is to be winced through
the boiling tan bath for a quarter of an hour. While the yarn is set to drain above the
bath, 28 ounces of alum are to be dissolved in it, and the yarn being once more winced
through it for a quarter of an hour, is then taken out, drained, wrung, and exposed to
the air. It has now acquired a deep but rather dull yellowish colour, and is ready
without washing for the next process. Bablah may be substituted for oak bark with
advantage.
8. The liming. Into the cistern filled with fresh made lime-water, the hanks of cotton
yarn suspended upon a series of wooden rods, are to be dipped freely three times in rapid
succession; then each hank is to be separately moved by hand through the lime bath, till
the desired carmelite shade appear. A weak soda lye may be used instead of lime water.
9. The brightening, is given by passing the above hanks, after squeezing, rinsing, and
airing them, through a dilute bath of solution of tin. The colour thus produced is said
to resemble perfectly the nankin of China.
Another kind of nankeen colour is given by oxide of iron, precipitated upon the fibre
of the cloth, from a solution of the sulphate, by a solution of soda. See Calico-printing.
NAPLES YELLOW (Jaune minéral, Fr.; Neapelgelb, Germ.); is a fine yellow
pigment, called giallolino, in Italy, where it has been long prepared by a secret process;
for few of the recipes which have been published produce a good colour. It is
employed not only in oil painting, but also for porcelain and enamel. It has a fresh,
brilliant, rich hue, but is apt to be very unequal in different samples.
The following prescription has been confidently recommended. Twelve parts of metallic
antimony are to be calcined in a reverberatory furnace, along with eight parts of
red lead, and four parts of oxide of zinc. These mixed oxides being well rubbed together,
are to be fused; and the fused mass is to be triturated and elutriated into a fine
powder. Chromate of lead has in a great measure superseded Naples yellow.
NAPHTHA, or ROCK-OIL (Huile pétrole, Fr.; Steinöl, Germ.); the Seneca oil of
North America, is an ethereous or volatile oil, which is generated within the crust of the
earth, and issues in many different localities. The colourless kind, called naphtha,
occurs at Baku, near the Caspian Sea, where the vapours which it exhales are kindled,
and the flame is applied to domestic and other economical purposes. Wells are also
dug in that neighbourhood, in which the naphtha is collected. Similar petroleum wells
exist in the territory of the Birmans, at Yananghoung, upon the river Erawaddy, 80
hours’ journey north-east of Pegu, where no less than 520 such springs issue from a
pale blue clay, soaked with oil, which rests upon roofing slate. Under the slate is coal[879]
containing much pyrites. Each spring yields annually 173 casks of 950 pounds each.
Petroleum is also found at Amiano in the duchy of Parma, at Saint Zibio in the grand
duchy of Modena, at Neufchatel in Switzerland, at Clermont in France, upon some
points of the banks of the Iser, at Gabian, a village near Bezières, at Tegernsee in
Bavaria, at Val di Noto in Sicily, in Zante, Gallicia, Wallachia, Trinidad, Barbadoes,
the United States, Rangoon, near Ava, &c. What is found in the market comes mostly
from Trinidad. The city of Parma is lighted with naphtha.
The Persian rock-oil is colourless, limpid, very fluid, of a penetrating odour, a hot taste,
and a specific gravity of 0·753; it is said to boil at 160° F. The common petroleum has
a reddish-yellow colour, which appears blue by reflected light, is transparent, has a spec.
grav. of 0·836, and contains, according to Unverdorben, several oils of different degrees
of volatility, a little oleine and stearine, resin, with a brown indifferent substance held
in solution. By repeated rectifications its density may be reduced to 0·758 at 60° F.
Native naphtha, of specific gravity 0·749, is said by some to boil at 201° F. The condensed
vapour consists of 85·05 carbon, and 14·30 hydrogen.
The naphtha procured by distilling the coal oil of the gas works, is of specific gravity
0·857, boils at 316° F., and consists of, carbon 83·04, hydrogen 12·31, and oxygen
4·65, by my experiments.
Rock-oil is very inflammable; its vapour forms with oxygen gas a mixture which violently
detonates, and produces water and carbonic acid gas. It does not unite with water,
but it imparts a peculiar smell and taste to it; it combines in all proportions with strong
alcohol, with ether and oils, both essential and unctuous; it dissolves sulphur, phosphorus,
iodine, camphor, most of the resins, wax, fats, and softens caoutchouc into a glairy
varnish. When adulterated with oil of turpentine, it becomes thick and reddish brown,
on being agitated in contact with strong sulphuric acid. A very fine black pigment
may be prepared from the soot of petroleum lamps.
NAPHTHALINE, is a peculiar white crystallizable substance, which may be
extracted by distillation from coal tar. It has a pungent aromatic smell and taste, and
a specific gravity of 1·048. It is a solid bicarburet of hydrogen, consisting, by my experiments,
of 92·9 of carbon, and 7·1 of hydrogen. It has not been applied to any use.
NATRON, is the name of the native sesquicarbonate of soda, which occurs in Egypt,
in the west of the Delta; also in the neighbourhood of Fessan, in the province of Sukena
in Northern Africa, where it exists under the name of Trona, crystallized along with
sulphate of soda; near Smyrna, in Tartary, Siberia, Hungary, Hindostan, and Mexico.
In the last country, there are several natron lakes, a little to the north of Zucatecas, as
well as in many other provinces. In Columbia, 48 miles from Merida, native mineral
natron is dug up from the bottom of lakes in large quantities, under the name of Urao.
According to Laugier, the Egyptian natron consists of carbonate of soda 22·44,
sulphate of soda 18·35, muriate of soda 38·64, water 14·0, insoluble matter 6·0.
Trona is composed of carbonate of soda 65·75, sulphate of soda 7·65, muriate of soda
2·63, water 24, insoluble matter 1. The sesquicarbonate may be artificially prepared by
boiling for a short time a solution of the bicarbonate.
NEB-NEB, is the East Indian name of Bablah.
NEEDLE MANUFACTURE. When we consider the simplicity, smallness, and
moderate price of a needle, we would be naturally led to suppose that this little instrument
requires neither much labour nor complicated manipulations in its construction; but
when we learn that every sewing needle, however inconsiderable its size, passes through
the hands of 120 different operatives, before it is ready for sale, we cannot fail to be
surprised.
The best steel, reduced by a wire-drawing machine to the suitable diameter, is
the material of which needles are formed. It is brought in bundles to the needle factory,
and carefully examined. For this purpose, the ends of a few wires in each bundle
are cut off, ignited, and hardened by plunging them into cold water. They are now
snapped between the fingers, in order to judge of their quality; the bundles belonging
to the most brittle wires are set aside, to be employed in making a peculiar kind of
needles.
After the quality of the steel wire has been properly ascertained, it is calibred by means
of a gauge, to see if it be equally thick and round throughout, for which purpose merely
some of the coils of the bundle of wires are tried. Those that are too thick are returned
to the wire-drawer, or set apart for another size of needles.
The first operation, properly speaking, of the needle factory, is unwinding the bundles
of wires. With this view the operative places the coil upon a somewhat conical reel,
fig. 750., whereon he may fix it at a height proportioned to its diameter. The wire is
wound off upon a wheel B, formed of eight equal arms, placed at equal distances round
a nave, which is supported by a polished round axle of iron, made fast to a strong
upright C, fixed to the floor of the workshop. Each of the arms is 54 inches long; and[880]
one of them D, consists of two parts; of an upper part, which bears the cross bar E, to
which the wire is applied; and of an under part, connected with the nave. The part
E slides in a slot in the fixed part F, and is made fast to it by a peg at a proper height
for placing the ends of all the spokes in the circumference of a circle. This arrangement
is necessary, to permit the wire to be readily taken off the reel, after being wound
tight round its eight branches. The peg is then removed, the branch pushed down,
and the coil of wire released. Fig. 751. shows the wheel in profile. It is driven by
the winch-handle G.
The new made coil is cut in two points diametrically opposite, either by hand shears,
of which one of the branches is fixed in a block by a bolt and a nut, as shown in fig. 752.,
or by means of the mechanical shears, represented in fig. 753. The crank A is moved by
a hydraulic wheel, or steam power, and rises and falls alternately. The extremity of
this crank enters into a mortise cut in the arm B of a bent lever B G C, and is made fast
to it by a bolt. An iron rod D F, hinged at one of its extremities to the end of the arm
C, and at the other to the tail of the shears or chisel E, forces it to open and shut alternately.
The operative placed upon the floor under F presents the coil to the action of the
shears, which cut it into two bundles, composed each of 90 or 100 wires, upwards of 8
feet long. The chisel strikes 21 blows in the minute.
These bundles are afterwards cut with the same shears into the desired needle lengths,
these being regulated by the diameter. For this purpose the wires are put into a semi-cylinder
of the proper length, with their ends at the bottom of it, and are all cut across
by this gauge. The wires, thus cut, are deposited into a box placed alongside of the
workman.
Two successive incisions are required to cut 100 wires, the third is lost; hence the
shears, striking 21 blows in a minute, cut in 10 hours fully 400,000 ends of steel
wire, which produce more than 800,000 needles. The wires thus cut are more or less
bent, and require to be straightened. This operation is executed with great promptitude,
by means of an appropriate instrument. In two strong iron rings A B, fig. 754.,
of which one is shown in front view at C, 5000 or 6000 wires, closely packed together, are
put; and the bundle is placed upon a flat smooth bench L M, fig. 757., covered with a
cast-iron plate D E, in which there are two grooves of sufficient depth for receiving the
two ring bundles of wire, or two openings like the rule F, fig. 757., upon which is placed
the open iron rule F, shown in front in fig. 756. upon a greater scale. The two rings
must be carefully set in the intervals of the rule. By making this rule come and go
five or six times with such pressure upon the bundles of wires as causes it to turn upon
its axis, all the wires are straightened almost instantaneously.
The construction of the machine, represented in fig. 757., may require explanation.
It consists of a frame in the form of a table, of which L M is the top; the cast-iron
plate D E is inserted solidly into it. Above the table, seen in fig. 755. in plan,
there are two uprights C H, to support the cross bar A A, which is held in forks cut out
in the top of each of the two uprights. This cross bar A A, enters tightly into a[881]
mortise cut in the swing piece N, at the point N, where it is fixed by a strong pin, so
that the horizontal traverse communicated to
the cross bar A A affects at the same time the swing
piece N. At the bottom of this piece is fixed, as
shown in the figure, the open rule F, seen upon
a greater scale in fig. 756.
When the workman wishes to introduce the
bundle B, he raises, by means of two chains I K,
fig. 757., and the lever G O, the swing piece and
the cross bar. For this purpose he draws down the
chain I; and when he has placed the bundle
properly, so that the two rings enter into the groove
E D, fig. 755., he allows the swing piece to fall back,
so that the same rings enter the open clefts of the
rule F; he then seizes one of the projecting arms
of the cross bar A, alternately pulling and pushing
it in the horizontal direction, whereby he effects, as
already stated, the straightening of the wires.
The wires are now taken to the pointing-tools,
which usually consist of about 30 grindstones
arranged in two rows, driven by a water-wheel.
Each stone is about 18 inches in diameter, and 4
inches thick. As they revolve with great velocity,
and are liable to fly in pieces, they are partially encased by iron plates, having a proper
slit in them to admit of the application of the wires. The workman seated in front
of the grindstone, seizes 50 or 60 wires
between the thumb and forefinger of his
right hand, and directs one end of the
bundle to the stone. By means of a bit of
stout leather called a thumb-piece, of
which A, fig. 758., represents the profile,
and B the plan, the workman presses the
wires, and turns them about with his forefinger,
giving them such a rotatory motion
as to make their points conical. This
operation, which is called roughing down,
is dry grinding; because, if water were
made use of, the points of the needles would be rapidly rusted. It has been observed
long ago, that the siliceous and steel dust thrown off by the stones, was injurious to
the eyes and lungs of the grinders; and many methods have been proposed for preventing
its bad effects. The machine invented for this purpose by Mr. Prior, for which
the Society of Arts voted a premium, deserves to be generally known.
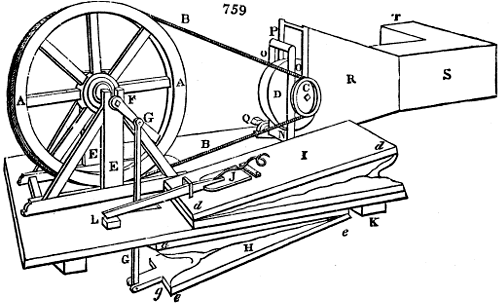
A A, fig. 759., is the fly-wheel of an ordinary lathe, round which the endless cord B B
passes, and embraces the pulley C, mounted upon the axle of the grindstone D. The flywheel
is supported by a strong frame E E, and may be turned by a winch-handle, as usual,
or by mechanical power. In the needle factories, the pointing-shops are in general
very large, and contain several grindstones running on the same long horizontal shaft,
placed near the floor of the apartment, and driven by water or steam power. One of
the extremities of the shaft of the wheel A has a kneed or bent winch F, which by means of
an intermediate crank G G, sets in action a double bellows H I, with a continuous blast,
consisting of the air feeder H below, and the air regulator I above. The first is composed
of two flaps, one of them a a, being fast and attached to the floor, and the other
e e, moving with a hinge-joint; both being joined by strong leather nailed to their
edges. This flap has a tail g, of which the end is forked to receive the end of the
crank G. Both flaps are perforated with openings furnished with valves for the
admission of the air, which is thence driven into a horizontal pipe K, placed beneath
the floor of the workshop, and may be afterwards directed in an uninterrupted blast
upon the grindstone, by means of the tin tubes N O O, which embrace it, and have
longitudinal slits in them. A brass socket is supposed to be fixed upon the ground;
it communicates with the pipe K, by means of a small copper tube, into which one of
the extremities of the pipe N is fitted; the other is supported by the point of a screw Q,
and moves round it as a pivot, so as to allow the two upright branches O O, to be placed
at the same distance from the grindstone. These branches are soldered to the horizontal
pipe N, and connected at their top by the tube P.
The wind which escapes through the slits of these pipes, blows upon the grindstone,
and carries off its dust into a conduit R, fig. 759., which may be extended to S, beyond[882]
the wall of the building, or bent at right angles, as at T, to receive the conduits of the
other grindstones of the factory.
A safety valve J, placed in an orifice formed in the regulator flap I, is kept shut by a
spiral spring of strong iron wire. It opens to allow the superfluous air to escape,
when, by the rising of the bellows, the tail L presses upon a small piece of wood,
and thereby prevents their being injured.
The wires thus pointed at both ends are transferred to the first workshop, and cut in
two, to form two needles, so that all of one quality may be of equal length. For each
sort a small instrument, fig. 760., is employed, being a copper plate nearly square, having
a turned up edge only upon two of its sides; the one of which is intended to receive
all the points, and the other to resist the pressure of the shears. In this small tool a
certain number of wires are put with their points in contact with the border, and they
are cut together flush with the plate by means of the shears, fig. 752., which are moved
by the knee of the workman. The remainder of the wires are then laid upon the same
copper or brass tool, and are cut also even; there being a trifling waste in this operation.
The pieces of wire out of which two needles are formed, are always left a little too
long, as the pointer can never hit exact uniformity in his work.
These pointed wires are laid parallel to each other in little wooden boxes, and transferred
to the head-flattener. This workman, seated at a table with a block of steel
before him, about 3 inches cube, seizes in his left hand 20 or 25 needles, between his
finger and thumb, spreading them out like a fan, with the points under the thumb, and
the heads projecting; he lays these heads upon the steel block, and with a small flat-faced
hammer strikes successive blows upon all the heads, so as to flatten each in an
instant. He then arranges them in a box with the points turned the same way.
The flatted heads have become hardened by the blow of the hammer; when annealed
by heating and slow cooling, they are handed to the piercer. This is commonly a
child, who laying the head upon a block of steel, and applying the point of a small
punch to it, pierces the eye with a smart tap of a hammer, applied first upon the one
side, and then exactly opposite upon the other.
Another child trims the eyes, which he does by laying the needle upon a lump of
lead, and driving a proper punch through its eye; then laying it sidewise upon
a flat piece of steel, with the punch sticking in it, he gives it a tap on each side with
his hammer, and causes the eye to take the shape of the punch. The operation of
piercing and trimming the eyes, is performed by clever children with astonishing rapidity;
who become so dexterous as to pierce with their punch a human hair, and thread
it with another, for the amusement of visitors.
The next operative makes the groove at the eye, and rounds the head. He fixes the
needle in pincers, fig. 761., so that the eye corresponds to their flat side; he then rests the
head of the needle in an angular groove, cut in a piece of hard wood fixed in a vice,
with the eye in an upright position. He now forms the groove with a single stroke
of a small file, dexterously applied, first to the one side of the needle, and then to the
other. He next rounds and smooths the head with a small flat file. Having finished,
he opens the pincers, throws the needle upon the bench, and puts another in its place.
A still more expeditious method of making the grooves and finishing the heads has
been long used in most English factories. A small ram is so mounted as to be made to
rise and fall by a pedal lever, so that the child works the tool with his foot; in the
same way as the heads of pins are fixed. A small die of tempered steel bears the form[883]
of the one channel or groove, another similar die, that of the other, both being in
relief; these being worked by the lever pedal, finish the grooving of the eye at a single
blow, by striking against each other, with the head of the needle between them.
The whole of the needles thus prepared are thrown pell-mell into a sort of drawer or
box, in which they are by a few dexterous jerks of the workman’s hand made to arrange
themselves parallel to each other.
The needles are now ready for the tempering; for which purpose they are weighed
out in quantities of about 30 pounds, which contain from 250,000 to 500,000 needles,
and are carried in boxes to the temperer. He arranges these upon sheet-iron plates,
about 10 inches long, and 5 inches broad, having borders only upon the two longer
sides. These plates are heated in a proper furnace to bright redness for the larger
needles, and to a less intense degree for the smaller; they are taken out, and inverted
smartly over a cistern of water, so that all the needles may be immersed at the same
moment, yet distinct from one another. The water being run off from the cistern,
the needles are removed, and arranged by agitation in a box, as above described.
Instead of heating the needles in a furnace, some manufacturers heat them by means of
a bath of melted lead in a state of ignition.
After being suddenly plunged in the cold water, they are very hard and excessively
brittle. The following mode of tempering them is practised at Neustadt. The needles
are thrown into a sort of frying-pan along with a quantity of grease. The pan being
placed on the fire, the fatty matter soon inflames, and is allowed to burn out; the
needles are now found to be sufficiently well tempered. They must, however, be
re-adjusted upon the steel anvil, because many of them get twisted in the hardening and
tempering.
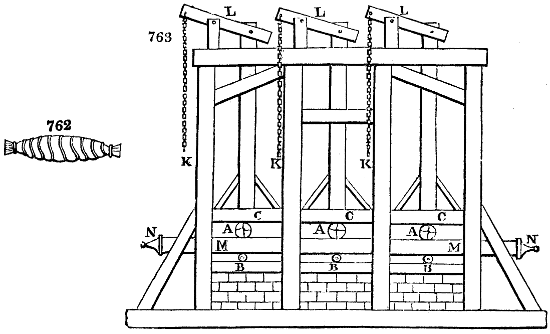
Polishing is the longest, and not the least expensive process in the needle manufacture.
This is done upon bundles containing 500,000 needles; and the same machine under
the guidance of one man, polishes from 20 to 30 bundles at a time; either by water or
steam power. The needles are rolled up in canvas along with some quartzose sand
interstratified between their layers, and the mixture is besmeared with rape-seed oil.
Fig. 762. represents one of the rolls or packets of needles 12 inches long, strongly
bound with cords. These packets are exposed to the to-and-fro pressure of wooden
tables, by which they are rolled about, with the effect of causing every needle in
the bundle to rub against its fellow, and against the siliceous matter, or emery, enclosed
in the bag. Fig. 763. represents an improved table for polishing the needles by attrition-bags.
The lower table M M is movable, whereas in the old constructions it was
fixed; the table C has merely a vertical motion, of pressure upon the bundles, whereas
formerly it had both a vertical and horizontal motion. Several bundles may obviously
be polished at once in the present machine. The table M M may be of any length that
is required, and from 24 to 27 inches broad; resting upon the wooden rollers B, B, B,
placed at suitable distances, it receives a horizontal motion, either by hand or other
convenient power; the packets of needles A, A, A, are laid upon it, and over them the
tables C, C, C, which are lifted by means of the chains K, K, K, and the levers L, L, L, in
order to allow the needles to be introduced or removed. The see-saw motion forces
the rouleaux to turn upon their own axes, and thereby creates such attrition among
their contents as to polish them. The workman has merely to distribute these rolls[884]
upon the table M, in a direction perpendicular to that in which the table moves; and
whenever one of them gets displaced, he sets it right, lifting by the help of the chain
the loaded table. The table makes about 20 horizontal double vibrations in the
minute; whereby each bundle, running over 24 inches each time, passes through 40
feet per minute, or 800 yards in the hour.
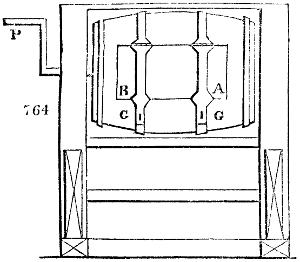
Scouring by the cask. After being worked during 18 or 20 hours under the tables, the
needles are taken out of the packets, and put into wooden bowls, where they are mixed
with sawdust to absorb the black grease upon their surfaces. They are next introduced
into a cask, fig. 764., and a workman seizing the winch P, turns it round a little;
he now puts in some more sawdust at the door, A, B, which is then shut by the clasps
G G, and continues the rotation till the needles be quite clean and clear in their eyes;
which he ascertains by taking out a sample of them from time to time.
Winnowing is the next process, by means of a mechanical ventilator similar to that by
which corn is winnowed. The sawdust is blown away, and the grinding powder is
separated from the needles, which remain apart clean and bright.
The needles are in the next place arranged in order, by being shaken, as above described,
in a small somewhat concave iron tray. After being thus laid parallel to each
other, they are shaken up against the end of the tray, and accumulated in a nearly upright
position, so that they can be seized in a heap and removed in a body upon a pallet
knife, with the help of the forefinger.
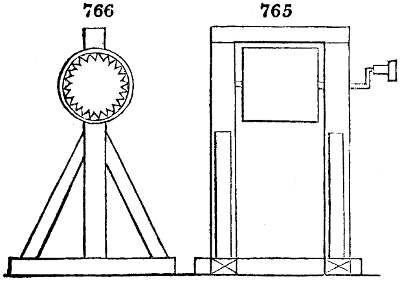
The preceding five operations, of making up the rouleaux, rolling them under the
tables, scouring the needles in the cask, winnowing, and arranging them, are repeated
ten times in succession, in manufacturing the best articles; the only variation being in
the first process. Originally the bundles of needles are formed with alternate layers of
siliceous schistus and needles; but after the seventh time, bran freed from flour by sifting
is substituted for the schistus. The subsequent four processes are, however, repeated
as described. It has been found in England, that emery powder mixed with quartz and
mica or pounded granite, is preferable to every thing else for polishing needles at first by
attrition in the bags; at the second and following operations, emery mixed with olive
oil is used, up to the eighth and ninth, for which putty or oxide of tin with oil is substituted
for the emery; at the tenth the putty is used with very little oil; and lastly
bran is employed to give a finish. In this mode of operating, the needles are scoured in
the copper cask shown in elevation fig. 765., and in section fig. 766. The inner surface
of this cask is studded with points to increase the friction among the needles; and
a quantity of hot soap suds is repeatedly introduced to wash them clean. The cask must
be slowly turned upon its axis, for fear of injuring the mass of needles which it contains.
They are finally dried in the wooden cask by attrition with sawdust; then wiped individually
with a linen rag or soft leather; when the damaged ones are thrown aside.
Sorting of the needles. This operation is performed in a dry upper chamber, kept free
from damp by proper stoves. Here all the points are first laid the same way; and the
needles are then picked out from each other in the order of their polish. The sorting is
effected with surprising facility. The workman places 2000 or 3000 needles in an iron
ring, fig. 767., two inches in diameter, and sets all their heads in one plane; then
on looking carefully at their points, he easily recognises the broken ones; and by means
of a small hook fixed in a wooden handle, fig. 768., he lays hold of the broken needle,
and turns it out. These defective needles pass into the hands of another workman,
who points them anew upon a grindstone, and they form articles of inferior value.
The needles which have got bent in the polishing must now be straightened. The
whole are finally arranged exactly according to their lengths by the tact of the finger and
thumb of the sorter.
The needles are divided into quantities for packing in blue papers, by putting into a[885]
small balance the equivalent weight of 100 needles, and so measuring them out without
the trouble of counting them individually.
The bluer receives these packets, and taking 25 of their needles at a time between the
forefinger and thumb, he presses their points against a very small hone-stone of compact
micaceous schist, mounted in a little lathe, as shown in fig. 769., he turns them briskly
round, giving the points a bluish cast, while he polishes and improves them. This
partial polish is in the direction of the axis; that of the rest of the needle is transverse,
which distinguishes the boundaries of the two. The little hone-stone is not cylindrical,
but quadrangular, so that it strikes successive blows with its corners upon the needles
as it revolves, producing the effect of filing lengthwise. Whenever these angles seem to
be blunted, they are set again by the bluer.
It is easy to distinguish good English needles from spurious imitations; because the
former have their axis coincident with their points, which is readily observed by turning
them round between the finger and thumb.
The construction of a needle requires, as already stated, about 120 operations; but
they are rapidly and uninterruptedly successive. A child can trim the eyes of 4000
needles per hour.
When we survey a manufacture of this kind, we cannot fail to observe, that the diversity
of operations which the needles undergo bears the impress of great mechanical refinement.
In the arts, to divide labour, is to abridge it; to multiply operations, is to
simplify them; and to attach an operative exclusively to one process, is to render him
much more economical and productive.
NEROLI, is the name given by perfumers to the essential oil of orange flowers. It
is procured by distillation with water, in the same way as the other volatile oils. Since
in distilling water from neroli, an aroma is obtained different from that of the orange-flower,
it has been concluded that the distilled water of orange-flowers owes its scent to
some principle different from an essential oil.
NET (Filet, reseau, Fr.; Netz, Germ.); is a textile fabric of knotted meshes, for
catching fish, and other purposes. Each mesh should be so secured as to be incapable of
enlargement or diminution. The French government offered in 1802 a prize of 10,000
francs to the person who should invent a machine for making nets upon automatic
principles, and adjudged it to M. Buron, who presented his mechanical invention to the
Conservatoire des Arts et Métiers. It does not appear, however, that this machine has accomplished
the object in view; for no establishment was ever mounted to carry it into
execution. Nets are usually made by the fishermen and their families during periods of
leisure. The formation of a mesh is too simple a matter to require description in this
Dictionary.
NEUTRALIZATION, is the state produced when acid and alkaline matters are
combined in such proportions that neither predominates, as evinced by the colour of
tincture of litmus and cabbage remaining unaffected by the combination.
NICARAGUA WOOD, is the wood of the Cæsalpinia echinata, a tree which grows
in Nicaraca. It is used with solution of tin as a mordant to dye a bright but fugitive
red. It is an inferior sort of Brazil wood.
NICKEL, is a metal rather sparingly found, and in few localities; being usually associated
with cobalt. Native nickel occurs at Westerwald in the Erzgebirge, in Bohemia,
combined with arsenic, under the significant name of Kupfernickel; with cobalt,
iron, and copper, as Arsenic-nickel, in the Harz; at Riechelsdorf in Hessia; as an oxide,
in Nickelschwärtze; as a sulphuret of nickel in Haarkies; as a sulphuret and arseniate of
nickel in Nickelglanz; and with sulphur and antimony in Nickelspiess glanzerz at Siegen.
Nickel is always present in meteoric stones. Kupfernickel occurs in numerous external
shapes; as reniform, globular, botroidal, arborescent, massive, and disseminated; fracture,
coarse or fine grained, with metallic lustre; colour, copper red, occasionally brown and
gray; in silver and cobalt veins, in gneiss, sienite, mica-slate, kupfer-schiefer, accompanied
by speisse cobalt, native silver, quartz, &c. It is found in Westphalia near Olpe, in
Hessia at Riechelsdorf, and Biber, in Baden; in the Saxon Erzgebirge near Schneeberg,
and Freiberg; in Bohemia, at Joachimsthal; in Thuringia, at Saalfeld; in Steyermark
near Schladming; in Hungary, France, and England.
Since the manufacture of German silver, or Argentane, became an object of commercial
importance, the extraction of nickel has been undertaken upon a considerable scale. The
cobalt ores are its most fruitful sources, and they are now treated by the method of
Wöhler, to effect the separation of the two metals. The arsenic is expelled by roasting
the powdered speise first by itself, next with the addition of charcoal powder, till the garlic
smell be no longer perceived. The residuum is to be mixed with three parts of sulphur
and one of potash, melted in a crucible with a gentle heat, and the product being edulcorated
with water, leaves a powder of metallic lustre, which is a sulphuret of nickel
free from arsenic; while the arsenic associated with the sulphur, and combined with the
resulting sulphuret of potassium, remains dissolved. Should any arsenic still be found[886]
in the sulphuret, as may happen if the first roasting heat was too great, the above process
must be repeated. The sulphuret must be finally washed, dissolved in concentrated
sulphuric acid, with the addition of a little nitric, the metal must be precipitated by a
carbonated alkali, and the carbonate reduced with charcoal.
In operating upon kupfernickel, or speise, in which nickel predominates, after the arsenic,
iron, and copper have been separated, ammonia is to be digested upon the mixed
oxides of cobalt and nickel, which will dissolve them into a blue liquor. This being diluted
with distilled water deprived of its air by boiling, is to be decomposed by caustic
potash, till the blue colour disappears, when the whole is to be put into a bottle tightly
stoppered, and set aside to settle. The green precipitate of oxide of nickel, which slowly
forms, being freed by decantation from the supernatant red solution of oxide of cobalt, is
to be edulcorated and reduced to the metallic state in a crucible containing crown glass.
Pure nickel in the form of a metallic powder is readily obtained by exposing its oxalate
to moderate ignition.
The reduction of the oxide of nickel with charcoal requires the heat of a powerful air
furnace or smith’s forge.
Nickel possesses a fine silver white colour and lustre; it is hard, but malleable, both
hot and cold; may be drawn into wire 1⁄50 of an inch, and rolled into plates 1⁄500 of an
inch thick. A small quantity of arsenic destroys its ductility. When fused it has a
specific gravity of 8·279, and when hammered, of 8·66 or 8·82; it is susceptible of magnetism,
in a somewhat inferior degree to iron, but superior to cobalt. Mariner’s compasses
may be made of it. Its melting point is nearly as high as that of manganese. It
is not oxidized by contact of air, but may be burned in oxygen gas.
There is one oxide and two suroxides of nickel. The oxide is of an ash-gray colour,
and is obtained by precipitation with an alkali from the solution of the muriate or
nitrate. The niccolous suroxide of Berzelius is black, and may be procured by exposing
the nitrate to a heat under redness. The niccolic suroxide has a dirty pale green
colour; but its identity is doubtful.
NICOTIANINE, is the name of an oil recently extracted from the leaves of
tobacco, which possesses the smell of tobacco smoke.
NICOTINE, is a peculiar principle, obtainable from the leaves and seeds of tobacco
(nicotiana tabacum), by infusing them in acidulous water, evaporating the infusion to a
certain point, adding lime to it, distilling and treating the product which comes over
with ether. It is colourless, has an acrimonious taste, a pungent smell, remains liquid
at 20° F., mixes in all proportions with water, but is in a great measure separable from
it by ether, which dissolves it abundantly. It combines with acids, and forms salts acrid
and pungent like itself; the phosphate, oxalate, and tartrate being crystallizable.
Nicotine causes the pupils to contract. A single drop of it is sufficient to kill a dog.
NITRATE OF AMMONIA, is prepared by neutralizing nitric acid with carbonate
of ammonia, and crystallizing the solution.
NITRATE OF LEAD (Nitrate de plomb, Fr.; Salpetersaures bleioxyd, Germ.);
is made by saturating somewhat dilute nitric acid with oxide of lead (litharge), evaporating
the neutral solution till a pellicle appears, and then exposing it in a hot chamber
till it be converted into crystals, which are sometimes transparent, but generally opaque
white octahedrons. Their spec. grav. is 4·068; they have a cooling, sweetish, pungent
taste. They dissolve in 7 parts of cold, and in much less boiling water; they fuse at a
moderate elevation of temperature, emit oxygen gas, and pass into oxide of lead.
Their constituents are 67·3 oxide, and 32·7 acid. Nitrate of lead is much employed
in the chrome yellow style of Calico-printing; which see.
There are three other compounds of nitric acid and lead oxide; viz. the bi-basic,
the tri-basic, and the se-basic; which contain respectively 2, 3, and 6 atoms of base to
1 of acid.
NITRATE OF POTASH, Nitre, Saltpetre. (Nitrate de potasse, Fr.; Salpetersaures
kali, Germ.) This salt occurs native as an efflorescence upon limestones, sandstones,
marls, chalk, and calctuff; it forms a saline crust in caverns, as also upon the
surface of the ground in certain places, especially where animal matters have been
decomposed. Such caverns exist in Germany near Homburg (Burkardush); in Apulia
upon the Adriatic sea (Pulo di Mofetta); in France; in the East Indies; in Ceylon,
where 22 nitriferous caverns are mentioned; in North America, at Crooked river,
Tennessee, Kentucky, and upon the Missouri; in Brazil, Teneriffe, and Africa. Nitre
occurs as an efflorescence upon the ground in Arragon, Hungary, Podolia, Sicily,
Egypt, Persia, Bengal, China, Arabia, North America, and South America. Several
plants contain saltpetre; particularly borage, dill, tobacco, sunflowers, stalks of maize,
beet-root, bugloss, parietaria, &c. It has not hitherto been found in animal substances.
The question has been frequently put; how is nitre annually reproduced upon the
surface of limestones, and the ground, after it has been removed by washing? It has[887]
been said, in reply, that as secondary limestones contain remains of animal matters,
the oxygen of the atmosphere, absorbed in virtue of the porous structure, will combine
with their azote to form nitric acid; whence nitrate of lime will result. Where
potash is present in the ground, a nitrate of that base will be next formed. The generation
of nitre is in all cases limited to a very small distance from the surface of porous stones;
no further, indeed, than where atmospherical air and moisture can penetrate; and none
is ever produced upon the surface of compact stones, such as marble and quartz, or of
argillaceous minerals. Dr. John Davy and M. Longchamp have advanced an opinion,
that the presence of azotized matter is not necessary for the generation of nitric acid or
nitrous salts, but that the oxygen and azote of the atmosphere, when condensed by capillarity,
will combine in such proportions as to form nitric acid, through the agency of
moisture and of neutralizing bases, such as lime, magnesia, potash, or soda. They conceive
that as spongy platina serves to combine oxygen and hydrogen into water, or the vapour
of alcohol and oxygen into acetic acid, and as the peroxide as well as the hydrate of iron,
and argillaceous minerals, serve to generate ammonia from the oxygen of the air and the
hydrogen of water; in like manner, porous limestones, through the agency of water,
operate upon the constituents of the atmosphere to produce nitric acid, without the
presence of animal matter. This opinion may certainly be maintained: for in India,
Spain, and several other countries, at a distance from all habitations, immense quantities
of saltpetre are reproduced in soils which have been washed the year before. But,
on the other hand, it is known that the production of this salt may be greatly facilitated
and increased by the admixture of animal offals with calcareous earths.
The spontaneous generation of nitre in Spain, Egypt, and especially in India, is sufficient
to supply the wants of the whole world. There this salt is observed to form upon
the surface of the ground in silky tufts, or even in slender prismatic crystals, particularly
during the continuance of the hot weather that succeeds copious rains. These saline
efflorescences, after being collected by rude besoms of broom, are lixiviated, allowed to
settle, evaporated, and crystallized. In France, Germany, Sweden, Hungary, &c., vast
quantities of nitrous salts are obtained by artificial arrangements called nitriaries, or
nitre-beds. Very little nitrate of potash, indeed, is obtained in the first place; but
the nitrates of lime and magnesia, which being deliquescent, remain in the nitrous
earths in a semi-liquid state. The operation of converting these salts into good nitre
is often sufficiently complex, in consequence of the presence of several muriates, which
are difficult to eliminate.
The following instructions have been given by the consulting committee of poudres et
salpêtres in France, for the construction of their nitrières artificielles. The permeability
of the materials to the atmospherical air, being found to be as indispensable as is the
presence of a base to fix the nitric acid at the instant of its formation, the first measure
is to select a light friable earth, containing as much carbonate of lime or old mortar-rubbish
as possible; and to interstratify it with beds of dung, five or six inches thick,
till a considerable heap be raised in the shape of a truncated pyramid, which should be
placed under an open shed, and kept moist by watering it from time to time. When
the whole appears to be decomposed into a kind of mould, it is to be spread under sheds
in layers of from two to three feet thick; which are to be watered occasionally with
urine and the drainings of dunghills, taking care not to soak them too much, lest they
should be rendered impermeable to the air, though they should be always damp enough
to favour the absorption and mutual action of the atmospherical gases. Moist garden
mould affords an example of the physical condition most favourable to nitre-beds. The
compost should be turned over, and well mixed with the spade once at least in every
fortnight, and the sides of the shed should be partially closed, for although air be essential,
wind is injurious, by carrying off the acid vapours, instead of allowing them to rest
incumbent upon, and combine with, the bases. The chemical reaction is slow and
successive, and can be made effective only by keeping the agents and materials in a
state of quiescence. The whole process lasts two years; but since organic matters
would yield in the lixiviation several soluble substances detrimental to the extraction of
saltpetre, they must not be added during the operations of the latter six months; nor
must any thing except clear water be used for watering during this period; at the end
of which the whole organic ingredients of the beds will be totally decomposed. Where
dung is not sufficiently abundant for the above stratifications, a nitre-bed should be
formed in a stable with friable earth, covered with a layer of litter; after four months
the litter is to be lifted off, the earth is to be turned over, then another layer of fresh
earth, 8 or 9 inches thick, is to be placed over it, and a layer of the old and fresh litter
over all. At the end of other four months, this operation is to be repeated; and in the
course of a year the whole is ready to be transferred into the regular nitre-beds under a
shed, as above described. Such are the laborious and disagreeable processes practised
by the peasants of Sweden, each of whom is bound by law to have a nitre-bed, and to
furnish a certain quantity of nitre to the state every year. His nitriary commonly consists[888]
of a small hut built of boards, with a bottom of rammed clay, covered by a wooden
floor, upon which is spread a mixture of ordinary earth with calcareous sand or marl,
and lixiviated wood-ashes. This mixture is watered with stable urine, and its surface
is turned over once a week in summer, and once a fortnight in winter. In some
countries, walls 2 or 3 feet thick, and 6 or 7 high, are raised with the nitrifying compost,
interspersed with weeds and branches of trees, in order at once to bind them
together, and to favour the circulation of air. These walls are thatched with straw;
they are placed with one of their faces in the direction of the rains; and must be moistened
with water not rich in animal matter. One side of the walls is upright and
smooth; while the other is sloped or terraced, to favour the admission of humidity into
their interior. The nitre eventually forms a copious efflorescence upon the smooth
side, whence it may be easily scraped off.
M. Longchamp, convinced that organic matters are a useless expense, and not in the
least essential to nitrification, proposes to establish nitre-beds where fuel and labour are
cheapest, as amidst forests, choosing as dry and low a piece of ground as possible, laying
them out upon a square space of about 1000 feet in each side, in the middle of which the
graduation-house may be built, and alongside of it sheds for the evaporation furnaces and
pans. Upon each of the four sides the nitrifying sheds are to be erected, 130 feet long
by 30 feet wide, where the lixiviation would be carried on, and whence the water would
be conducted in gutters to the graduation-house. The sheds are to be closed at the
sides by walls of pisé, and covered with thatch. No substance is so favourable to nitrification
as the natural stony concretion known under the name of lime-tuf. In Touraine,
where it is used as a building stone, the saltpetre makers re-establish the foundations
of old houses at their own expense, provided they are allowed to carry off the old
tuf, which owes its nitrifying properties not only to its chemical nature, but to its
texture, which being of a homogeneous porosity, permits elastic fluids and vapours to
pass through it freely in all directions. With the rough blocks of such tuf, walls about
20 inches thick, and moderately high, are to be raised, upon the principles above prescribed;
in the absence of tuf, porous walls may be raised with a mixture of arable soil,
sand, and mortar-rubbish, chalk or rich marl. The walls ought to be kept moist.
In France, the greater part of the indigenous saltpetre is obtained by lixiviating
the mortar-rubbish of old buildings, especially of those upon the ground-floor, and in
sunk cellars; which are by law reserved for this purpose. The first object of the
manufacturer is then to ascertain the richness of his materials in nitrous salts, to see if
they be worth the trouble of working; and this point he commonly determines merely
by their saline, bitter, and pungent taste, though he might readily have recourse to the
far surer criteria of lixiviation and evaporation. He next pounds them coarsely, and
puts them into large casks open at top, and covered with straw at bottom; which are
placed in three successive levels. Water is poured into the casks till they are full, and
after 12 hours’ digestion it is run off, loaded with the salts, by a spigot near the bottom.
A fresh quantity of water is then added, and drawn off after an interval of four hours;
even a third and fourth lixiviation are had recourse to; but these weak liquors are
reserved for lixiviating fresh rubbish. The contents of the casks upon the second and
third lower levels are lixiviated with the liquors of the upper cask, till the lyes indicate
from 12 to 14 degrees of Baumé’s hydrometer. They are now fit for evaporating to a
greater density, and of then receiving the dose of wood-ashes requisite to convert the
materials of lime and magnesia into nitrate of potash, with the precipitation of the
carbonates of magnesia and lime. The solution of nitre is evaporated in a copper
pan, and as it boils, the scum which rises to the surface must be diligently skimmed off
into a cistern alongside. Muriate of soda being hardly more soluble in boiling than
in cold water, separates during the concentration of the nitre, and is progressively
removed with cullender-shaped ladles. The fire is withdrawn whenever the liquor has
acquired the density of 80° B.; it is allowed to settle for a little while, and is then
drawn off, by a lead syphon adjusted some way above the bottom, into iron vessels, to
cool and crystallize. The crystals thus obtained are set to drain, then re-dissolved and
re-crystallized. The further purification of nitre, is fully described under the article
Gunpowder.
The annual production of saltpetre in France, by the above-described processes,
during the wars of the Revolution, amounted to 2000 tons (2 millions of kilogrammes)
of an article fit for the manufacture of gunpowder; of which seven-twentieths were
furnished by the saltpetre works of Paris alone. Considerably upwards of six times that
quantity of common and cubic nitre were imported into the United Kingdom, for home
consumption, during the year ending January 5, 1838.
Nitrate of potash crystallizes in six-sided prisms, with four narrow and two broad
faces: the last being terminated by a dihedral summit, or two-sided acumination;
they are striated lengthwise, and have fissures in their long axis, which are apt to contain
mother water. The spec. gravity of nitre, varies from 1·93 to 2·00. It possesses[889]
a cooling, bitterish-pungent taste, is void of smell, permanent in the air when pure,
fuses at a heat of about 662, into an oily-looking liquid, and concretes upon cooling
into a solid mass, with a coarsely radiating fracture. This has got the unmeaning
names of sal-prunelle and mineral crystal. At a red heat, nitre gives out at first a
great deal of pretty pure oxygen gas; but afterwards nitrous acid fumes, while potash
remains in the retort. It is soluble in 7 parts of water at 32°; in about 31⁄2 at 60° F.,
in less than half a part at 194°, and in four-tenths at 212°. It is very slightly soluble
in spirit of wine, and not at all in absolute alcohol. It causes a powerful deflagration
when thrown upon burning coals; and when a mixture of it with sulphur is thrown into
a red-hot crucible, a very vivid light is emitted. Its constituents are, 46·55 potash, and
53·45 nitric acid.
Nitre is applied to many purposes:—1, to the manufacture of gunpowder; 2, to that
of sulphuric acid; 3, to that of nitric acid, though nitrate of soda or cubic nitre has lately
superseded this use of it to a considerable extent; 4, to that of flint-glass; 5, it is used
in medicine; 6, for many chemical and pharmaceutical preparations; 7, for procuring
by deflagration with charcoal or cream of tartar, pure carbonate of potash, as also
black and white fluxes; 8, for mixing with salt in curing butcher meat; 9, in some
countries for sprinkling in solution upon grain, to preserve it from insects; 10, for making
fire-works. See Fire-works.
An Account of the quantities of Saltpetre and Cubic Nitre imported into, exported
from, and retained for consumption in the United Kingdom. Duty 6d. per cwt:—
| |
Imported in |
Exported in |
Retained for Consumption in |
| |
1835. |
1836. |
1837. |
1835. |
1836. |
1837. |
1835. |
1836. |
1837. |
| cwts. |
264,338; |
279,902; |
349,993. |
73,379; |
38,414; |
93,024. |
204,580; |
242,131; |
256,969. |
Duty received in 1837, £6,424.
NITRATE OF SILVER (Nitrate d’argent, Fr.; Silbersalpeter, Germ.); is prepared
by saturating pure nitric acid of specific grav. 1·25 with pure silver, evaporating
the solution, and crystallizing the nitrate. When the drained crystals are fused in a
platina capsule, and cast into slender cylinders in silver moulds, they constitute the
lunar caustic of the surgeon. This should be white, and unchangeable by light. It is
deliquescent in moist air. The crystals are colourless transparent 4 and 6 sided tables;
they possess a bitter, acrid, and most disagreeable metallic taste; they dissolve in their
own weight of cold, and in much less of hot water; are soluble in four parts of boiling
alcohol, but not in nitric acid; they deflagrate on redhot coals, like all the nitrates;
and detonate with phosphorus when the two are struck together upon an anvil. They
consist of 68·2 of oxide, and 31·8 of acid. Nitrate of silver, when swallowed, is a very
energetic poison: but it may be readily counteracted, by the administration of a dose
of sea-salt, which converts the corrosive nitrate into the inert chloride of silver.
Animal matter, immersed in a weak solution of neutral nitrate of silver, will keep
unchanged for any length of time; and so will polished iron or steel. Nitrate of
silver is such a delicate reagent of hydrochloric or muriatic acid, as to show by a
sensible cloud, the presence of one 113 millionth part of it, or one 7 millionth part of
sea-salt in distilled water. It is much used under the name of indelible ink, for
writing upon linen with a pen; for which purpose one drachm of the fused salt should be
dissolved in three quarters of an ounce of water, adding to the solution as much water
of ammonia as will re-dissolve the precipitated oxide, with sap-green to colour it, and
gum-water to make the volume amount to one ounce. Traces written with this liquid
should be first heated before a fire to expel the excess of ammonia, and then exposed to
the sun-beam to blacken. Another mode of using nitrate of silver as an indelible ink,
is to imbue the linen first with solution of carbonate of soda, to dry the spot, and write
upon it with a solution of nitrate of silver, thickened with gum, and tinted with sap-green.
NITRATE OF SODA, Cubical Nitre (Nitrate de soude, Fr.; Würfelsalpeter,
Germ.); occurs under the nitre upon the lands in Spain, India, Chile, and remarkably
in Peru, in the districts of Atacama and Taracapa, where it forms a bed several feet
thick. It appears in several places upon the surface, and extends over a space of more
than 40 leagues, approaching near to the frontiers of Chile. It is sometimes efflorescent,
sometimes crystallized, but oftener confusedly mixed with clay and sand.
This immensely valuable deposit is only three days’ journey from the port of Conception
in Chile, and from Iquiqui, another harbour situated in the southern part
of Peru.
Nitrate of soda may be artificially prepared by neutralizing nitric acid with soda, and
crystallizing the solution. It crystallizes in rhomboids, has a cooling, pungent, bitterish
taste, less disagreeable than nitre; it becomes moist in the air; dissolves in 3 parts of
water at 60° F., in less than 1 part of boiling water; deflagrates more slowly than nitre,
and with an orange yellow flame. It consists, in its dry state, of 36·6 soda and 63·4 nitric[890]
acid; but its crystals contain one prime equivalent of water; hence they are composed
of, acid 56·84, base 33·68, water 9·47.
It is susceptible of the same applications as nitre, with the exception of making gunpowder;
for which it is not adapted, on account of its deliquescent property.
NITRATE OF STRONTIA. (Nitrate de strontiane, Fr.; Salpetersaurer strontian,
Germ.) This salt is usually prepared from the sulphuret of strontium, obtained
by decomposing sulphate of strontia with charcoal, by strong ignition of the mixed
powders in a crucible. This sulphuret being treated with water, and the solution
being filtered, is to be neutralized with nitric acid, as indicated by the test of turmeric
paper; care being taken to avoid breathing the noxious sulphuretted hydrogen gas,
which is copiously disengaged. The neutral nitrate being properly evaporated and set
aside, affords colourless, transparent, slender octahedral crystals. It has a cooling, yet
somewhat acrid taste; is soluble in 5 parts of cold, and in one half part of boiling water,
as also in alcohol; is permanent in the air, deflagrates upon burning coals, gives off
oxygen when calcined, and leaves caustic strontia. The salt consists of 48·9 strontia
and 51·1 nitric acid. That salt is anhydrous; but there is another variety of it, which
contains nearly 40 per cent. of water of crystallization, which occurs in large octahedrons.
This is preferred for fire-works, because by efflorescence it is easily obtained in a fine
powder, which mixes more intimately with the chlorate of potash and charcoal, for the
composition of the brilliant red fires, now so much admired in theatrical conflagrations.
NITRIC ACID, Aquafortis (Acide nitrique, Fr.; Salpetersaüre, Germ.); exists,
in combination with the bases, potash, soda, lime, magnesia, in both the mineral and
vegetable kingdoms. This acid is never found insulated. It was distilled from saltpetre
so long ago as the 13th century, by igniting that salt, mixed with copperas or clay, in a
retort. Nitric acid is generated when a mixture of oxygen and nitrogen gases, confined
over water or an alkaline solution, has a series of electrical explosions passed through it.
In this way the salubrious atmosphere may be converted into corrosive aquafortis.
When a little hydrogen is introduced into the mixed gases, standing over water, the
chemical agency of the electricity becomes more intense, and the acid is more rapidly
formed from its elements, with the production of some nitrate of ammonia.
Nitric acid is usually made on the small scale by distilling, with the heat of a sand-bath,
a mixture of 3 parts of pure nitre, and 2 parts of strong sulphuric acid, in a large
glass retort, connected by a long glass tube with a globular receiver surrounded by cold
water. By a well regulated distillation, a pure acid, of specific gravity 1·500, may be
thus obtained, amounting in weight to about two-thirds of the nitre employed. To
obtain easily the whole nitric acid, equal weights of nitre and concentrated sulphuric
acid may be taken; in which case but a moderate heat need be applied to the retort.
The residuum will be bisulphate of potash. When only the single equivalent proportion
of sulphuric acid is used, namely 48 parts for 100 of nitre, a much higher heat is
required to complete the distillation, whereby more or less of the nitric acid is decomposed,
while a compact neutral sulphate of potash is left in the retort, very difficult to
remove by solution in water, and therefore apt to destroy the vessel.
Aquafortis is manufactured upon the great scale in iron pots or cylinders of the same
construction as I have described under muriatic acid. The more concentrated the sulphuric
acid is, the less corrosively will it act upon the metal; and it is commonly used
in the proportion of one part by weight to two of nitre. The salt being introduced into
the cool retort, and the lid being luted tight, the acid is to be slowly poured in through
the aperture f, fig. 748.; while the aperture g is connected by a long glass tube with a
range of balloons inserted into each other, and laid upon a sloping bed of sand. The
bottle i, with 3 tubulures partly filled with water, which is required for condensing
muriatic acid gas, must, for the present purpose, be replaced by a series of empty receivers,
either of glass or salt-glazed stoneware. The cylinders should be only half filled, and
be worked off by a gradually raised heat.
Commercial aquafortis is very generally contaminated with sulphuric and muriatic
acids, as also with alkaline sulphates and muriates. The quantity of these salts may be
readily ascertained by evaporating in a glass capsule a given weight of the aquafortis;
while that of the muriatic acid may be determined by nitrate of silver; and of sulphuric
acid, by nitrate of baryta. Aquafortis may be purified in a great measure, by re-distillation
at a gentle heat; rejecting the first liquid which comes over, as it contains the
chlorine impregnation; receiving the middle portion as genuine nitric acid; and leaving
a residuum in the retort, as being contaminated with sulphuric acid.
Since nitrate of soda has been so abundantly imported into Europe from Peru, it has
been employed by many manufacturers in preference to nitre for the extraction of nitric
acid, because it is cheaper, and because the residuum of the distillation, being sulphate
of soda, is more readily removed by solution from glass retorts, when a range of these
set in a gallery furnace is the apparatus employed. Nitric acid of specific gravity 1·47
may be obtained colourless; but by further concentration a portion of it is decomposed,[891]
whereby some nitrous acid is produced, which gives it a straw-yellow tinge. At this
strength it exhales white or orange fumes, which have a peculiar, though not very disagreeable
smell; and even when largely diluted with water, it tastes extremely sour. The
greatest density at which it can be obtained is 1·51 or perhaps 1·52, at 60° F., in which
state, or even when much weaker, it powerfully corrodes all animal, vegetable, and most
metallic bodies. When slightly diluted it is applied, with many precautions, to silk and
woollen stuffs, to stain them of a bright yellow hue. See Calico-printing; page 240.
In the dry state, as it exists in nitre, this acid consists of 26·15 parts by weight of
azote, and 73·85 of oxygen; or of 2 volumes of the first gas, and 5 volumes of the second.
When of specific gravity 1·5, it boils at about 210° Fahr.; of 1·45, it boils at about
240°; of 1·42, it boils at 253°; and of 1·40, at 246° F. If an acid stronger than 1·420
be distilled in a retort, it gradually becomes weaker; and if weaker than 1·42, it gradually
becomes stronger, till it assumes that standard density. Acid of specific gravity
1·485 has no more action upon tin than water has, though when either stronger or
weaker it oxidizes it rapidly, and evolves fumes of nitrous gas with explosive
violence. In my two papers upon nitric acid published in the fourth and sixth volumes
of the Journal of Science (1818 and 1819), I investigated the chemical relations of
these phenomena. Acid of 1·420 consists of 1 atom of dry acid, and 4 of water; acid
of 1·485, of 1 atom of dry acid, and 2 of water; the latter compound possesses a stable
equilibrium as to chemical agency; the former as to calorific. Acid of specific gravity
1·334, consisting of 7 atoms of water, and 1 of dry acid, resists the decomposing agency
of light. Nitric acid acts with great energy upon most combustible substances, simple
or compound, giving up oxygen to them, and resolving itself into nitrous gas, or even
azote. Such is the result of its action upon hydrogen, phosphorus, sulphur, charcoal,
sugar, gum, starch, silver, mercury, copper, iron, tin, and most other metals.
A Table of Nitric Acid, by Dr. Ure.
Specific
gravity. |
Liq.
Acid
in 100. |
Dry
acid
in 100. |
| 1·5000 |
100 |
79·700 |
| 1·4980 |
99 |
78·903 |
| 1·4960 |
98 |
78·106 |
| 1·4940 |
97 |
77·309 |
| 1·4910 |
96 |
76·512 |
| 1·4880 |
95 |
75·715 |
| 1·4850 |
94 |
74·918 |
| 1·4820 |
93 |
74·121 |
| 1·4790 |
92 |
73·324 |
| 1·4760 |
91 |
72·527 |
| 1·4730 |
90 |
71·730 |
| 1·4700 |
89 |
70·933 |
| 1·4670 |
88 |
70·136 |
| 1·4640 |
87 |
69·339 |
| 1·4600 |
86 |
68·542 |
| 1·4570 |
85 |
67·745 |
| 1·4530 |
84 |
66·948 |
| 1·4500 |
83 |
66·155 |
| 1·4460 |
82 |
65·354 |
| 1·4424 |
81 |
64·557 |
| 1·4385 |
80 |
63·760 |
| 1·4346 |
79 |
62·963 |
| 1·4306 |
78 |
62·166 |
| 1·4269 |
77 |
61·369 |
| 1·4228 |
76 |
60·572 |
| 1·4189 |
75 |
59·775 |
| 1·4147 |
74 |
58·978 |
| 1·4107 |
73 |
58·181 |
| 1·4065 |
72 |
57·384 |
| 1·4023 |
71 |
56·587 |
| 1·3978 |
70 |
55·790 |
| 1·3945 |
69 |
54·993 |
| 1·3882 |
68 |
54·196 |
| 1·3833 |
67 |
53·399 |
| 1·3783 |
66 |
52·602 |
| 1·3732 |
65 |
51·805 |
| 1·3681 |
64 |
51·068 |
| 1·3630 |
63 |
50·211 |
| 1·3579 |
62 |
49·414 |
| 1·3529 |
61 |
48·617 |
| 1·3477 |
60 |
47·820 |
| 1·3427 |
59 |
47·023 |
| 1·3376 |
58 |
46·226 |
| 1·3323 |
57 |
45·429 |
| 1·3270 |
56 |
44·632 |
| 1·3216 |
55 |
43·835 |
| 1·3163 |
54 |
43·038 |
| 1·3110 |
53 |
42·241 |
| 1·3056 |
52 |
41·444 |
| 1·3001 |
51 |
40·647 |
| 1·2947 |
50 |
39·850 |
| 1·2887 |
49 |
39·053 |
| 1·2826 |
48 |
38·256 |
| 1·2765 |
47 |
37·459 |
| 1·2705 |
46 |
36·662 |
| 1·2644 |
45 |
35·865 |
| 1·2583 |
44 |
35·068 |
| 1·2523 |
43 |
34·271 |
| 1·2462 |
42 |
33·474 |
| 1·2402 |
41 |
32·677 |
| 1·2341 |
40 |
31·880 |
| 1·2277 |
39 |
31·083 |
| 1·2212 |
38 |
30·286 |
| 1·2148 |
37 |
29·489 |
| 1·2084 |
36 |
28·692 |
| 1·2019 |
35 |
27·895 |
| 1·1958 |
34 |
27·098 |
| 1·1895 |
33 |
26·301 |
| 1·1833 |
32 |
25·504 |
| 1·1770 |
31 |
24·707 |
| 1·1709 |
30 |
23·900 |
| 1·1648 |
29 |
23·113 |
| 1·1587 |
28 |
22·316 |
| 1·1526 |
27 |
21·519 |
| 1·1465 |
26 |
20·722 |
| 1·1403 |
25 |
19·925 |
| 1·1345 |
24 |
19·128 |
| 1·1286 |
23 |
18·331 |
| 1·1227 |
22 |
17·534 |
| 1·1168 |
21 |
16·737 |
| 1·1109 |
20 |
15·940 |
| 1·1051 |
19 |
15·143 |
| 1·0993 |
18 |
14·346 |
| 1·0935 |
17 |
13·549 |
| 1·0878 |
16 |
12·752 |
| 1·0821 |
15 |
11·955 |
| 1·0764 |
14 |
11·158 |
| 1·0708 |
13 |
10·361 |
| 1·0651 |
12 |
9·564 |
| 1·0595 |
11 |
8·767 |
| 1·0540 |
10 |
7·970 |
| 1·0485 |
9 |
7·173 |
| 1·0430 |
8 |
6·376 |
| 1·0375 |
7 |
5·579 |
| 1·0320 |
6 |
4·782 |
| 1·0267 |
5 |
3·985 |
| 1·0212 |
4 |
3·188 |
| 1·0159 |
3 |
2·391 |
| 1·0106 |
2 |
1·594 |
| 1·0053 |
1 |
0·797 |
NITROGEN, DEUTOXIDE OF; Nitrous gas, Nitric oxide (Deutoxide d’azote,
Fr.; Stickstoffoxyd, Germ.); is a gaseous body which may be obtained by pouring upon
copper or mercury, in a retort, nitric acid of moderate strength. The nitrous gas comes
over in abundance without the aid of heat, and may be received over water freed from
air, or over mercury, in the pneumatic trough. It is elastic and colourless; what taste
and smell it possesses are unknown, because the moment it is exposed to the mouth or
nostrils, it absorbs atmospherical oxygen, and becomes nitrous or nitric acid. Its
specific gravity is 1·0393, or 1·04; whence 100 cubic inches weigh 36·66 gr. Water
condenses not more than 1⁄20 of its volume of this gas. It extinguishes animal life, and
the flame of many combustibles; but of phosphorus well kindled, it brightens the flame in
a most remarkable degree. It consists of 47 parts of nitrogen gas, and 53 of oxygen gas,[892]
by weight; and of equal parts in bulk, without any condensation; so that the specific
gravity of deutoxide of nitrogen is the arithmetical mean of the two constituents. The
constitution of this gas, and the play of affinities which it exercises in the formation of
sulphuric acid, are deeply interesting to the chemical manufacturer.
The Hyponitrous acid (Salpetrigesaüre, Germ.), like the preceding compound, deserves
notice here, on account of the part it plays in the conversion of sulphur into
sulphuric acid, by the agency of nitre. It is formed by mingling four volumes of deutoxide
of nitrogen with one volume of oxygen; and appears as a dark orange vapour
which is condensable into a liquid at a temperature of 4° -zero, Fahr. When distilled,
this liquid leaves a dark yellow fluid. The pure hyponitrous acid consists of 37·12
nitrogen, and 62·88 oxygen; or of two volumes of the first, and three of the second.
Water converts it into nitric acid and deutoxide of nitrogen; the latter of which escapes
with effervescence. This acid oxidizes most combustible bodies with peculiar energy
and though its vapour does not operate upon dry sulphurous acid, yet, through the
agency of steam it converts it into sulphuric acid, itself being simultaneously transformed
into deutoxide of nitrogen; ready to become hyponitrous acid again, and
to perform a circulating series of important metamorphoses. See Sulphuric Acid.
NITROGEN GAS, or AZOTE (Eng. and Fr.; Stickstoffgas, Germ.); constitutes
about 79 hundredths of the bulk of the atmospheric air; it is copiously disengaged from
several mineral springs, as from the natural basins of hot water which supply the baths of
Leuk, near the Gemmi in Switzerland, and from other springs, in the Pyrenees, in Ceylon,
South and North America, &c. It exists also in flesh and most animal substances, as well as
in some vegetable products, being one of their essential constituents. When phosphorus is
burnt within a jar filled with air, standing over water in the pneumatic trough, it consumes
or absorbs the oxygen, and leaves nitrogen, which may be rendered pure by
agitation with water. By exposing nitrite of ammonia to heat in a retort, nitrogen
comes over alone in great abundance; for the hydrogen of the ammonia is sufficient
to saturate the oxygen of the acid, and to convert it into water; while the nitrogen of
both constituents is set at liberty. By transmitting chlorine through water of ammonia,
or digesting lean flesh in warm nitric acid, nitrogen may also be obtained. This permanently
elastic gas is destitute of colour, taste, and smell; it has a specific gravity of
0·976, air being 1·000. Hence 100 cubic inches of it weigh 29·7 gr. It extinguishes all
burning bodies, and when respired without oxygen is fatal to animal life.
NITROGEN, PROTOXIDE OF; Nitrous oxide (Protoxide d’azote, Fr.; Stickstoffoxydul,
Germ.); is a gas which displays remarkable powers when breathed, causing in
many persons unrestrainable feelings of exhilaration, whence it has been called the laughing
or intoxicating gas. It is prepared by exposing crystallized nitrate of ammonia to
a heat of about 350° Fahr., in a glass retort. It is much denser than the air of the
atmosphere, having a spec. grav. of 1·527; whence 100 cubic inches weigh 46·6 grains. It
consists of 63·64 parts of nitrogen, and 36·36 of oxygen, by weight; or of two volumes of
nitrogen and one volume of oxygen, condensed by reciprocal attraction into two volumes.
It is colourless, and possesses all the mechanical properties of the atmosphere. Water
previously freed from air absorbs its own volume of this gas; and thus affords a ready
criterion for estimating its freedom from incondensable gases, as oxygen, nitrogen, and its
deutoxide. Several combustibles burn in this gas with an enlarged blue and very vivid
flame; and it relumes a taper, which has been blown out, provided its tip be redhot.
By powerful pressure it may be liquefied. See Gas.
NITRO-MURIATIC ACID, Aqua regia (Acide nitro-muriatique, Fr.; Salpeter-salzsaüre,
Königswasser, Germ.); is the compound menstruum invented by the
alchemists for dissolving gold. If strong nitric acid, orange-coloured by saturation with
nitrous gas (deutoxide of azote), be mixed with the strongest liquid muriatic acid, no
other effect is produced than might be expected from the action of nitrous acid of
the same strength upon an equal quantity of water; nor has the mixed acid so formed,
any power of acting upon gold or platina. But if colourless aquafortis and ordinary
muriatic acid be mixed together, the mixture immediately becomes yellow, and acquires
the power of dissolving these two noble metals. When gently heated, pure
chlorine gas rises from it, and its colour becomes deeper; when further heated, chlorine
still rises, but now mixed with nitrous acid gas. If the process has been very long
continued, till the colour becomes very dark, no more chlorine can be procured, and the
liquor has lost the power of dissolving gold. It then consists of nitrous and muriatic
acids. It appears, therefore, that aqua regia owes its peculiar properties to the mutual
decomposition of the nitric and muriatic acids; and that water, chlorine, and nitrous
acid gas are the results of that reaction. Aqua regia does not, strictly speaking, oxidize
gold and platinum; it causes merely their combination with chlorine. It may be composed
of very different proportions of the two acids; the nitric being commonly of specific
gravity 1·34; the muriatic, of specific gravity 1·18 or 1·19. Sometimes 3 parts, and
at others 6 parts of the muriatic acid are mixed with 1 of nitric; and occasionally muriate[893]
of ammonia, instead of muriatic acid, is added to nitric acid for particular purposes,
as for making a solution of tin for the dyers. An aqua regia may also be prepared by
dissolving nitre in muriatic acid.
NITROUS ACID (Acide nitreux, Fr.; Salpetrige salpetersaüre, Germ.), may be
procured by distilling, in a coated glass retort, perfectly dry nitrate of lead, into a glass
receiver surrounded with a freezing mixture. The acid passes over in vapour, and condenses
into a liquid; oxygen gas escapes through the safety tube; while oxide of lead
remains in the bottom of the retort. Nitrous acid may also be obtained by distilling
strong fuming nitric acid, at the lowest possible temperature, and rectifying what comes
over. At 4° -zero, Fahr., this acid is colourless; at 32° it is wax yellow; at 60° it has
an orange hue. It possesses a strong smell, has a very pungent, acrid, sour taste, and a
specific gravity of 1·42. It powerfully decomposes organic bodies, staining them yellow.
It boils at 82° Fahr. with the disengagement of red or orange fumes. Its constituents
are, 41·34 of hyponitrous acid, and 58·66 of anhydrous nitric acid; or ultimately, 30·68
nitrogen = 1 volume, and 69·32 oxygen = 2 volumes. In its other habitudes, it is
quite analogous to hyponitrous acid.
A mixture of this double or compound acid with nitric acid, constitutes the orange-brown
fuming nitrous acid of the British apothecaries.
The hyponitrous and nitrous are two acids remarkable for containing no water in
their composition; being therefore dry liquids.
NOPAL, is the Mexican name of the plant cactus opuntia, upon which the cochineal
insect breeds.
NUTMEG (Muscade, Fr.; Muskatennuss, Germ.); is the fruit of the myristica
moschata, a beautiful tree of the family of the laurineæ of Jussieu, which grows in the
Molucca islands. All the parts of this tree are very aromatic; but only those portions
of the fruit called mace and nutmeg are sent into the market. The entire fruit is a
species of drupa, of an ovoid form, of the size of a peach, and furrowed longitudinally.
The nutmeg is the innermost kernel, or seed, contained in a thin shell, which is surrounded
by the mace; and this again is enclosed in a tough fleshy skin, which opening
at the tip, separates into two valves. The nutmeg tree yields three crops annually;
one in April, which is the best; one in August; and one in December.
Good nutmegs should be dense, and feel heavy in the hand. When they have been
perforated by worms, they feel light, and though the holes have been fraudulently
stopped, the unsound ones may be easily detected by this criterion.
Nutmegs afford two oily products. 1. Butter of nutmeg, vulgarly called oil of mace,
is obtained in the Moluccas, by expression, from the fresh nutmegs, to the amount of
50 per cent. of their weight. It is a reddish yellow butter-like substance, interspersed
with light and dark streaks, and possesses the agreeable smell and taste of the nutmeg,
from the presence of a volatile oil. It consists of two fats; one reddish and soft,
soluble in cold alcohol; another white and solid, soluble in hot alcohol. 2. The volatile
oil is solid, or a stereoptène, and has been styled Myristicine.
NUT OIL. See Oils, Unctuous.
NUX VOMICA, a poisonous nut, remarkable for containing the vegeto-alkali
Strychnia.
OATS. (Avoine, Fr.; Hafer, Germ.) The composition of oats is less known than
that of the other Cerealia. Vogel found that 100 parts of oats afforded 66 parts of
flour or meal, and 34 parts of bran; but this proportion would depend upon the quality of
the grain. The flour contains, 2 parts of a greenish-yellow fat oil; 8·25 of bitterish sweet
extractive; 2·5 of gum; 4·30 of a gray substance, more like coagulated albumen than
gluten; 59 of starch; 24 of moisture (inclusive of the loss). Schrader found in the
ashes of oats, silica, carbonate of lime, carbonate of magnesia, alumina, with oxides of
manganese and iron.
OBSIDIAN, is a glassy looking mineral, with a large conchoidal fracture, and of a
blackish colour, which froths much at the blow-pipe before it melts into a white enamel.
OCHRE, yellow and brown (Ocre, Fr.; Ocker, Germ.); is a native earthy mixture
of silica and alumina, coloured by oxide of iron, with occasionally a little calcareous
matter and magnesia. Ochre occurs in beds some feet thick, which lie generally above
the oolite, are covered by sandstone and quartzose sands more or less ferruginous, and
are accompanied by gray plastic clays, of a yellowish or reddish colour; all of them
substances which contribute more or less to its formation. The ochry earths are prepared
for use by grinding under edge millstones, and elutriation. The yellow ochres[894]
may be easily rendered red or reddish brown by calcination in a reverberatory oven,
which oxidizes their iron to a higher degree.
Native red ochre is called red chalk and reddle in England. It is an intimate mixture
of clay and red iron ochre; is massive; of an earthy fracture; is brownish-red, blood-red,
stains and writes red. The oxide of iron is sometimes so considerable, that the
ochre may be reckoned an ore of that metal.
The ochre beds of England are in the iron sand, the lowest of the formations which
intervene between the chalk and oolites. Beds of fuller’s earth alternate with the iron
sand. The following is a section of the ochre pits at Shotover Hill, near Oxford:—
| Beds of highly ferruginous grit, forming the summit of the hill |
6 |
feet. |
| Gray sand |
3 |
do. |
| Ferruginous concretions |
1 |
|
| Yellow sand |
6 |
|
| Cream-coloured loam |
4 |
|
| Ochre |
6 |
inches. |
Beneath this, there is a second bed of ochre, separated by a thin bed of clay.
Bole, or Armenian bole; called also Lemnian earth, and terra sigillata, because when
refined it was stamped with a seal; is massive, with a conchoidal fracture, a feeble lustre,
reddish-yellow or brown, a greasy feel; adheres to the tongue, spec. gray. 1·4 to 2·0.
It occurs in the island Stalimene (the ancient Lesbos), and in several other places,
especially at Sienna; whence the brown pigment called terra di Siena.
OILS (Huiles, Fr.; Oele, Germ.); are divisible into two great classes: the fat or
fixed oils, huiles grasses, Fr.; Fette oele, Germ.; and the essential or volatile oils,
Huiles volatiles, Fr.; Flüchtige, aetherische oele, Germ. The former are usually bland
and mild to the taste; the latter hot and pungent. The term distilled, applied also to
the last class, is not so correct, since some of them are obtained by expression, as the
whole of the first class may be, and commonly are.
All the known fatty substances found in organic bodies, without reference to their
vegetable or animal origin, are, according to their consistence, arranged under the
chemical heads of oils, butters, and tallows. They all possess the same ultimate constituents,
carbon, hydrogen, and generally oxygen, and in nearly the same proportions.
The fat oils are widely distributed through the organs of vegetable and animal nature.
They are found in the seeds of many plants, associated with mucilage, especially in those
of the bicotyledinous class, occasionally in the fleshy pulp surrounding some seeds, as
the olive; also in the kernels of many fruits, as of the nut and almond tree, and finally
in the roots, barks, and other parts of plants. In animal bodies, the oily matter occurs
enclosed in thin membranous cells, between the skin and the flesh, between the muscular
fibres, within the abdominal cavity in the omentum, upon the intestines, and round the
kidneys, and in a bony receptacle of the skull of the spermaceti whale; sometimes in special
organs, as of the beaver; in the gall-bladder, &c., or mixed in a liquid state with other
animal matters, as in the milk.
Braconnot, but particularly Raspail, have shown that animal fats consist of small
microscopic, partly polygonal, and partly reniform particles, associated by means of their
containing sacs. These may be separated from each other by tearing the recent fat asunder,
rinsing it with water, and passing it through a sieve. The membranes being thus
retained, the granular particles are observed to float in the water, and afterwards to
separate, like the globules of starch, in a white pulverulent semi-crystalline form. The
particles consist of a strong membranous skin, enclosing stearine and elaine, or solid and
liquid fat, which may be extracted by trituration and pressure. These are lighter than
water, but sink readily in spirit of wine. When boiled in strong alcohol, the oily
principle dissolves, but the fatty membrane remains. These granules have different
sizes and shapes in different animals; in the calf, the ox, the sheep, they are polygonal,
and from 1⁄70 to 1⁄450 of an inch in diameter; in the hog they are kidney-shaped, and from
1⁄70 to 1⁄140 of an inch; in man, they are polygonal, and from 1⁄70 to 1⁄900 of an inch; in
insects they are usually spherical, and not more than 1⁄600 of an inch.
The following is a list of the Plants which yield the ordinary Unctuous Oils of commerce:
| No. |
Plants. |
Oils. |
Spe-
cific
gravity. |
| 1. |
Linum usitatissum et perenne |
D. |
Linseed oil |
0 |
·9347 |
| 2. |
Coryleus avellana |
|
- |
D. |
Nut oil |
0 |
·9260 |
| 3. |
Juglans regia |
| 4. |
Papaver somniferum |
D. |
Poppy oil |
0 |
·9243 |
| 5. |
Cannabis sativa |
D. |
Hemp oil |
0 |
·9276 |
| 6. |
Sesamum orientale[895] |
G. |
Oil of sesamum |
|
| 7. |
Olea Europea |
G. |
Olive oil |
0 |
·9176 |
| 8. |
Amygdalus communis |
G. |
Almond oil |
0 |
·9180 |
| 9. |
Guilandina mohringa |
G. |
Oil of behen or ben |
|
| 10. |
Cucurbita pepo, and melapepo |
D. |
Cucumber oil |
0 |
·9231 |
| 11. |
Fagus silvatica |
G. |
Beech oil |
0 |
·9225 |
| 12. |
Sinapis nigra et arvensis |
G. |
Oil of mustard |
0 |
·9160 |
| 13. |
Helianthus annuus et perennis |
D. |
Oil of sunflower |
0 |
·9262 |
| 14. |
Brassica napus et campestris |
G. |
Rape seed oil |
0 |
·9136 |
| 15. |
Ricinus communis |
D. |
Castor oil |
0 |
·9611 |
| 16. |
Nicotiana tabacum et rustica |
D. |
Tobacco seed oil |
0 |
·9232 |
| 17. |
Prunus domestica |
G. |
Plum kernel oil |
0 |
·9127 |
| 18. |
Vitis vinifera |
D. |
Grape seed oil |
0 |
·9202 |
| 19. |
Theobroma cacao |
G. |
Butter of cacao |
0 |
·892 |
| 20. |
Cocos nucifera |
G. |
Cocoa nut oil |
|
| 21. |
Cocus butyracea vel avoira elais |
G. |
Palm oil |
0 |
·968 |
| 22. |
Laurus nobilis |
G. |
Laurel oil |
|
| 23. |
Arachis hypogæa |
G. |
Ground-nut oil |
|
| 24. |
Vateria indica |
G. |
Piney tallow |
0 |
·926 |
| 25. |
Hesperis matronalis |
D. |
Oil of Julienne |
0 |
·9281 |
| 26. |
Myagrum sativa |
D. |
Oil of camelina |
0 |
·9252 |
| 27. |
Reseda luteola |
D. |
Oil of weld-seed |
0 |
·9358 |
| 28. |
Lepidium sativum |
D. |
Oil of garden cresses |
0 |
·9240 |
| 29. |
Atropa belladonna |
D. |
Oil of deadly nightshade |
0 |
·9250 |
| 30. |
Gossypium Barbadense |
D. |
Cotton seed oil |
|
| 31. |
Brassica campestris oleifera |
G. |
Colza oil |
0 |
·9136 |
| 32. |
Brassica præcox |
G. |
Summer rapeseed oil |
0 |
·9139 |
| 33. |
Raphanus sativus oleifer |
G. |
Oil of radish seed |
0 |
·9187 |
| 34. |
Prunus cerasus |
G. |
Cherry-stone oil |
0 |
·9239 |
| 35. |
Pyrus malus |
G. |
Apple seed oil |
|
| 36. |
Euonymus Europæus |
G. |
Spindle tree oil |
0 |
·9380 |
| 37. |
Cornus sanguinea |
G. |
Cornil berry tree oil |
|
| 38. |
Cyperus esculenta |
G. |
Oil of the roots of cyper grass |
0 |
·9180 |
| 39. |
Hyosciamus niger |
G. |
Henbane seed oil |
0 |
·9130 |
| 40. |
Æsculus hippocastanum |
G. |
Horse chesnut oil |
0 |
·927 |
| 41. |
Pinus abies |
D. |
Pinetop oil |
028 5 |
The fat oils are contained in that part of the seed which gives birth to the cotyledons;
they are not found in the plumula and radicle. Of all the families of plants, the cruciform
is the richest in oleiferous seeds; and next to that, are the drupaceæ, amentaceæ,
and solaneæ. The seeds of the gramineæ and leguminosæ contain rarely more than a
trace of fat oil. One root alone, that of the cyperus esculenta, contains a fat oil. The
quantity of oil furnished by seeds varies not only with the species, but in the same seed,
with culture and climate. Nuts contain about half their weight of oil; the seeds of the
brassica oleracea and campestris, one third; the variety called colza in France, two fifths;
hempseed, one fourth; and linseed from one fourth to one fifth. Unverdorben states
that a last, or ten quarters, of linseed, yields 40 ahms = 120 gallons English of oil; which
is about 1 cwt. of oil per quarter.
The fat oils, when first expressed without much heat, taste merely unctuous on the
tongue, and exhale the odour of their respective plants. They appear quite neutral by litmus
paper. Their fluidity is very various, some being solid at ordinary temperatures, and
others remaining fluid at the freezing point of water. Linseed oil indeed does not congeal
till cooled from 4° to 18° below 0° F. The same kind of seed usually affords oils
of different degrees of fusibility; so that in the progress of refrigeration one portion
concretes before another. Chevreul, who was the first to observe this fact, considers all
the oils to be composed of two species, one of which resembles suet, and was thence
styled by him stearine; and another which is liquid at ordinary temperatures, and was
called elaine, or oleine. By refrigeration and pressure between the folds of blotting paper,
or in linen bags, the fluid part is separated, and the solid remains. By heating the paper
in water, the liquid oil may be obtained separate. When alcohol is boiled with the
natural oil, the greater part of the stearine remains undissolved.
[896]
Oleine may also be procured by digesting the oil with a quantity of caustic soda
equal to one half of what is requisite to saponify the whole; the stearine is first transformed
into soap, then a portion of the oleine undergoes the same change, but a great
part of it remains in a pure state. This process succeeds only with recently expressed
or very fresh oils. The properties of these two principles of the fat oils vary with the
nature of the respective oils, so that the sole difference does not consist, as many suppose,
in the different proportions of these two bodies, but also in peculiarities of the
several stearines and oleines, which, as extracted from different seeds, solidify at very
different temperatures.
In close vessels, oils may be preserved fresh for a very long time, but with contact of
air they undergo progressive changes. Certain oils thicken and eventually dry into a
transparent, yellowish, flexible substance; which forms a skin upon the surface of the
oil, and retards its further alteration. Such oils are said to be drying or siccative, and
are used on this account in the preparation of varnishes and painters’ colours. Other
oils do not grow dry, though they turn thick, become less combustible, and assume
an offensive smell. They are then called rancid. In this state, they exhibit an acid
reaction, and irritate the fauces when swallowed, in consequence of the presence of a
peculiar acid, which may be removed in a great measure by boiling the oil along with
water and a little common magnesia for a quarter of an hour, or till it has lost the property
of reddening litmus. While oils undergo the above changes, they absorb a
quantity of oxygen equal to several times their volume. Saussure found that a layer of
nut oil, one-quarter of an inch thick, enclosed along with oxygen gas over the surface of
quicksilver in the shade, absorbed only three times its bulk of that gas in the course of
eight months; but when exposed to the sun in August, it absorbed 60 volumes additional
in the course of ten days. This absorption of oxygen diminished progressively, and
stopped altogether at the end of three months, when it had amounted to 145 times the
bulk of the oil. No water was generated, but 21·9 volumes of carbonic acid were disengaged,
while the oil was transformed in an anomalous manner into a gelatinous mass,
which did not stain paper. To a like absorption we may ascribe the elevation of temperature
which happens when wool or hemp, besmeared with olive or rapeseed oil, is
left in a heap; circumstances under which it has frequently taken fire, and caused the
destruction of both cloth-mills and dock-yards.
In illustration of these accidents, if paper, linen, tow, wool, cotton, mats, straw, wood
shavings, moss, or soot, be imbued slightly with linseed or hempseed oil, and placed in
contact with the sun and air, especially when wrapped or piled in a heap, they very soon
become spontaneously hot, emit smoke, and finally burst into flames. If linseed oil and
ground manganese be triturated together, the soft lump so formed will speedily become
firm, and ere long take fire.
The fat oils are completely insoluble in water. When agitated with it, the mixture
becomes turbid, but if it be allowed to settle the oil collects by itself upon the surface.
This method of washing is often employed to purify oils. Oils are little soluble in
alcohol, except at high temperatures. Castor oil is the only one which dissolves in cold
alcohol. Ether, however, is an excellent solvent of oils, and is therefore employed to
extract them from other bodies in analysis; after which it is withdrawn by distillation.
Fat oils may be exposed to a considerably high temperature, without undergoing
much alteration; but when they are raised to nearly their boiling point, they begin to
be decomposed. The vapours that then rise are not the oil itself, but certain products
generated in it by the heat. These changes begin somewhere under 600° of Fahr.,
and require for their continuance temperatures always increasing. The products consist
at first in aqueous vapour, then a very inflammable volatile oil, which causes
boiling oil to take fire spontaneously; and next carburetted hydrogen gas, with carbonic
acid gas. In a lamp, a small portion of oil is raised in the wick by capillarity,
which being heated, boils and burns. See Rosin-gas.
Several fat oils, mixed with one or two per cent. of sulphuric acid, assume instantly
a dark green or brown hue, and, when allowed to stand quietly, deposit a colouring
matter after some time. It consists in a chemical combination of the sulphuric acid,
with a body thus separated from the oil, which becomes in consequence more limpid,
and burns with a brighter flame, especially after it is washed with steam, and clarified by
repose or filtration. Any remaining moisture may be expelled by the heat of a water
bath.
The oils combine with the salifiable bases, and give birth to the substance called
glycerine (the sweet principle), and to the margaric, oleic, and stearic acids. The general
product of their combination with potash or soda, is Soap, which see. Caustic
ammonia changes the oils very difficultly and slowly into a soap; but it readily unites
with them into a milky emulsion called volatile liniment, used as a rubefacient in[897]
medicine. Upon mixing water with this liquor, the oil separates in an unchanged
state. By longer contact, ammonia acts upon oils like the other alkalis. Sea salt dissolves
in small quantity in the oils, and so does verdigris. The latter solution is
green. Oils dissolve also several of the vegetable alkalis, as morphia, cinchonia,
quinia, strychia, and delphia.
| Olive oil consists of |
77 |
·2 |
carbon, |
13 |
·4 |
hydrogen, and |
9 |
·4 |
oxygen, in 100 parts. |
| Spermaceti oil, |
by my analysis, of |
78 |
·9 |
carbon, |
10 |
·97 |
hydrogen, and |
10 |
·13 |
oxygen. |
|
| Castor oil |
do. |
74 |
·0 |
|
10 |
·3 |
|
15 |
·7 |
|
azote, |
| Stearine of olive oil |
82 |
·17 |
|
11 |
·23 |
|
6 |
·30 |
|
0 |
·30 |
Saussure. |
| Oleine of olivdo. |
76 |
·03 |
|
11 |
·54 |
|
12 |
·07 |
|
0 |
·35 |
do. |
| Linseed oil |
76 |
·01 |
|
11 |
·35 |
|
12 |
·64 |
|
do. |
| Nut oil |
79 |
·77 |
|
10 |
·57 |
|
9 |
·12 |
|
0 |
·54 |
do. |
| Oil of almonds |
77 |
·40 |
|
11 |
·48 |
|
10 |
·83 |
|
0 |
·29 |
|
De Saussure concludes that the less fusible fats contain more carbon and less oxygen,
and that oils are more soluble in alcohol, the more oxygen they contain.
I shall now take a short view of the peculiarities of the principal expressed oils.
Oil of almonds, according to Gusseron, contains no stearine; at least he could obtain
none by cooling it and squeezing it successively till it all congealed. Braconnot had,
on the contrary, said, that it contains 24 per cent. of stearine. I believe that Gusseron
is right, and that Braconnot had made fallacious experiments on an impure oil.
Oil of colza, is obtained from the seeds of brassica campestris, to the amount of 39 per
cent. of their weight. It forms an excellent lamp oil, and is much employed in France.
The corylus avellana furnishes in oil 60 per cent. of the weight of the nuts.
Hempseed oil, resembles the preceding, but has a disagreeable smell, and a mawkish
taste. It is used extensively for making both soft soap and varnishes.
Linseed oil, is obtained in greatest purity by cold pressure; but by a steam heat of
about 200° F. a very good oil may be procured in larger quantity. The proportion of
oil usually stated by authors is 22 per cent. of the weight of the seed; but Mr. Blundell
informs me, that, by his plan of hydraulic pressure, he obtains from 26 to 27. In the
Encyclopædia Metropolitana, under Oil Press, a quarter of seed (whose average weight
is 400 lbs.) is said to yield 20 gallons of oil. Now as the gallon of linseed oil weighs
9·3 lbs., the total product will be 186 lbs., which amounts to more than 45 per cent.—an
extravagant statement, about double the ordinary product in oil mills. Even supposing
the gallons not to be imperial, but old English, we should have upwards of 38
per cent. of oil by weight, which is still an impossible quantity. Such are the errors
introduced into respectable books, by adopting without practical knowledge, the puffing
statements of a patentee. It dissolves in 5 parts of boiling alcohol, in 40 parts of cold
alcohol, and in 1·6 parts of ether. When kept long cool in a cask partly open, it
deposits masses of white stearine along with a brownish powder. That stearine is very
difficult of saponification.
Mustard-seed oil. The white or yellow seed affords 36 per cent. of oil, and the black
seed 18 per cent. The oil concretes when cooled a little below 32° F.
Nut oil, is at first greenish coloured, but becomes pale yellow by time. It congeals
at the same low temperature as linseed oil, into a white mass, and has a more drying
quality than it.
Oil of olives, is sometimes of a greenish and at others of a pale yellow colour. A few
degrees above 32° F. it begins to deposit some white granules of stearine, especially if
the oil have been originally expressed with heat. At 22° it deposits 28 per cent. of its
weight in stearine, which is fusible again at 68°, and affords 72 per cent. of oleine.
According to Kerwych, oleine of singular beauty may be obtained by mixing 2 parts of
olive oil with 1 part of caustic soda lye, and macerating the mixture for 24 hours with
frequent agitation. Weak alcohol must then be poured into it, to dissolve the stearine
soap, whereby the oleine, which remains meanwhile unsaponified, is separated, and
floats on the surface of the liquid. This being drawn off, a fresh quantity of spirits is
to be poured in, till the separation of all the oleine be completed. It has a slightly
yellowish tint, which may be removed by means of a little animal charcoal mixed with it
in a warm place for 24 hours. By subsequent nitration, the oleine is obtained limpid
and colourless, of such quality that it does not thicken with the greatest cold, nor
does it affect either iron or copper instruments immersed in it.
There are three kinds of olive oil in the market. The best, called virgin salad oil, is
obtained by a gentle pressure in the cold; the more common sort is procured by
stronger pressure, aided with the heat of boiling water; and thirdly, an inferior kind,
by boiling the olive residuum or marc, with water, whereby a good deal of mucilaginous
oil rises and floats on the surface. The latter serves chiefly for making soaps. A still
worse oil is got by allowing the mass of bruised olives to ferment before subjecting it
to pressure.
[898]
Oil of olives is refined for the watchmakers by the following simple process. Into a
bottle or phial containing it, a slip of sheet lead is immersed, and the bottle is placed
at a window, where it may receive the rays of the sun. The oil by degrees gets
covered with a curdy mass, which after some time settles to the bottom, while itself
becomes limpid and colourless. As soon as the lead ceases to separate any more of
that white substance, the oil is decanted off into another phial for use.
Palm oil melts at 117·5° F., and is said to consist of 31 parts of stearine and 69 of
oleine in 100. It becomes readily rancid by exposure to air, and is whitened at the
same time.
The oil extracted from the plucked tops of the pinus abies, in the Black Forest in
Germany, is limpid, of a golden yellow colour, and resembles in smell and taste the oil
of turpentine. It answers well for the preparation of varnishes.
The oil of plum-stones, is made chiefly in Wurtemberg, and is found to answer very
well for lamps.
Poppy-seed oil, has none of the narcotic properties of the poppy juice. It is soluble
in ether in every proportion.
Rape-seed oil, has a yellow colour, and a peculiar smell. At 25° F. it becomes a
yellow mass, consisting of 46 parts of stearine, which fuses at 50°, and 54 of oleine, in
which the smell resides.
The oils of belladonna seeds, and tobacco seeds, are perfectly bland. The former is
much used for lamps in Swabia and Wurtemberg. The oil-cakes of both are poisonous.
Oil of wine-stones, is extracted to the amount of 10 or 11 per cent. from the seeds of
the grape. Its colour is at first pale yellow, but it darkens with age. It is used as an
article of diet.
FAT OIL MANUFACTURE.
It is the practice of almost all the proprietors in the neighbourhood of Aix, in Provence,
to preserve the olives for 15 days in barns or cellars, till they have undergone
a species of fermentation, in order to facilitate the extraction of their oil. If this practice
were really prejudicial to the product, as some theorists have said, would not the high
reputation and price of the oil of Aix have long ago suffered, and have induced them to
change their system of working? In fact all depends upon the degree of fermentation excited.
They must not be allowed to mould in damp places, to lie in heaps, to soften so as
to stick to each other, and discharge a reddish liquor, or to become so hot as to raise a
thermometer plunged into the mass up to 96° F. In such a case they would afford
an acrid nauseous oil, fit only for the woollen or soap manufactories. A slight fermentative
action, however, is useful, towards separating the oil from the mucilage. The olives
are then crushed under the stones of an edge-mill, and next put into a screw-press, being
enclosed in bullrush-mat bags (cabas), laid over each other to the number of eighteen. The
oil is run off from the channels of the ground-sill, into casks, or into stone cisterns
called pizes, two-thirds filled with water. The pressure applied to the cabas should be
slowly graduated.
What comes over first, without heat, is the virgin oil already mentioned. The cabas
being now removed from the press, their contents are shovelled out, mixed with some
boiling water, again put in the bags, and pressed anew. The hot water helps to carry
off the oil, which is received in other casks or pizes. The oil ere long accumulates at
the surface, and is skimmed off with large flat ladles; a process which is called lever
l’huile. When used fresh, this is a very good article, and quite fit for table use,
but is apt to get rancid when kept. The subjacent water retains a good deal of
oil, by the intervention of the mucilage; but by long repose in a large general cistern,
called l’enfer, it parts with it, and is then drawn off from the bottom by a plug-hole.
The oil which remains after the water is run off, is of an inferior quality, and can be
used only for factory purposes.
The marc being crushed in a mill, boiled with water, and expressed, yields a still
coarser article.
All the oil must be fined by keeping in clean tuns, in an apartment, heated to the 60th
degree Fahr. at least, for twenty days; after which it is run off into strong casks,
which are cooled in a cellar, and then sent into the market.
Oil of almonds, is manufactured by agitating the kernels in bags, so as to separate
their brown skins, grinding them in a mill, then enclosing them in bags, and squeezing
them strongly between a series of cast iron plates, in a hydraulic press; without heat
at first, and then between heated plates. The first oil is the purest, and least apt to
become rancid. It should be refined by filtering through porous paper. Next to olive
oil, this species is the most easy to saponify. Bitter almonds being cheaper than the
sweet, are used in preference for obtaining this oil, and they afford an article equally
bland, wholesome, and inodorous. But a strongly scented oil may be procured, according
to M. Planché, by macerating the almonds in hot water, so as to blanch them,[899]
then drying them in a stove, and afterwards subjecting them to pressure. The volatile
oil of almonds is obtained by distilling the marc or bitter almond cake, along with
water. See Press, Hydraulic, and Stearine.
Linseed, rapeseed, poppyseed, and other oleiferous seeds were formerly treated for
the extraction of their oil, by pounding in hard wooden mortars with pestles shod with
iron, set in motion by cams driven by a shaft turned with horse or water power, then
the triturated seed was put into woollen bags which were wrapped up in hair-cloths,
and squeezed between upright wedges in press-boxes by the impulsion of vertical rams
driven also by a cam mechanism. In the best mills upon the old construction, the
cakes obtained by this first wedge pressure, were thrown upon the bed of an edge-mill,
ground anew, and subjected to a second pressure, aided by heat now, as in the first case.
These mortars and press-boxes constitute what are called Dutch mills. They are still
in very general use both in this country and on the Continent; and are by many
persons supposed to be preferable to the hydraulic presses.
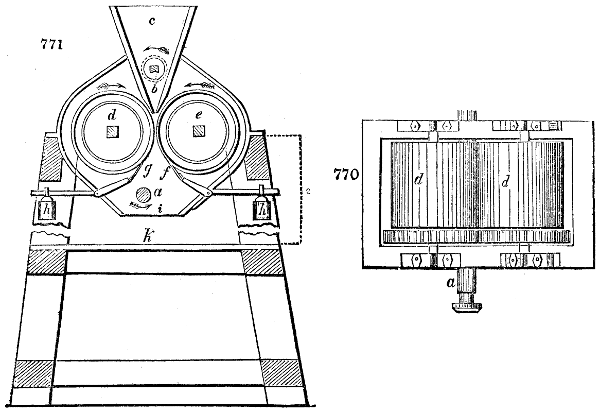
The roller-mill, for merely bruising the linseed, &c., previous to grinding it under edge-stones,
and to heating and crushing it in a Dutch or a hydraulic oil-mill, is represented
in figs. 770. and 771. The iron shaft
a, has a winch at each end, with a
heavy fly-wheel upon the one of them,
when the machine is to be worked by
hand. Upon the opposite end is a pulley,
with an endless cord which passes
round a pulley on the end of the fluted
roller b, and thereby drives it. This fluted
roller b, lies across the hopper c, and by
its agitation causes the seeds to descend
equably through the hopper, between the
crushing rollers d, e. Upon the shaft a,
there is also a pinion which works into
two toothed wheels on the shafts of the
crushing cylinders d and e, thus communicating
to these cylinders motion in
opposite directions. f, g are two scraper-blades,
which by means of the two weights
h, h, hanging upon levers, are pressed
against the surfaces of the cylinders, and
remove any seed-cake from them. The
bruised seeds fall through the slit i of
the case, and are received into a chest
which stands upon the board k.
Machines of this kind are now usually
driven by power. Hydraulic presses have
been of late years introduced into many
seed-oil mills in this country; but it is
still a matter of dispute whether they, or
the old Dutch oil-mill, with bags of seed
compressed between wedges, driven by
cam-stamps, be the preferable; that is,
afford the largest product of oil with the
same expenditure of capital and power.
For figures of hydraulic presses, see Press,
and Stearine.
This bruising of the seed is merely a
preparation for its proper grinding under
a pair of heavy edge-stones, of granite, from 5 to 7 feet in diameter; because unbruised
seed is apt to slide away before the vertical rolling wheel, and thus escape trituration.
The edge-mill, for grinding seeds, is quite analogous to the gunpowder-mill represented
in fig. 531., page 630. Some hoop the stones with an iron rim, but others prefer, and I
think justly, the rough surface of granite, and dress it from time to time with hammers,
as it becomes irregular. These stones make from 30 to 36 revolutions upon their
horizontal bed of masonry or iron in a minute. The centre of the bed, where it is perforated
for the passage of the strong vertical shaft which turns the stones, is enclosed by
a circular box of cast iron, firmly bolted to the bed-stone, and furnished with a cover.
This box serves to prevent any seeds or powder getting into the step or socket, and
obstructing the movement. The circumference of the mill-bed is formed of an upright
rim of oak-plank, bound with iron. There is a rectangular notch left in the edge of the
bed, and corresponding part of the rim, which is usually closed with a slide-plate, and
is opened only at the end of the operation, to let the pasty seed-cake be turned out by[900]
the oblique arm of the bottom scraper. The two parallel stones, which are set near
each other, and travel round their circular path upon the bed, grind the seeds not
merely by their weight, of three tons each, but also by a rubbing motion, or attrition;
because their periphery being not conical, but cylindrical, by its rolling upon a plane
surface, must at every instant turn round with friction upon their resting points. Strong
cast-iron boxes are bolted upon the centres of the stones, which by means of screw
clamps seize firmly the horizontal iron shafts that traverse and drive them, by passing
into a slit-groove in the vertical turning shaft. This groove is lined with strong plates of
steel, which wear rapidly by the friction, and need to be frequently renewed.
The seeds which have been burst between the rolls, or in the mortars of the Dutch
mills, are to be spread as equably as possible by a shovel upon the circular path of the
edge-stones, and in about half an hour the charge will be sufficiently ground into a paste.
This should be put directly into the press, when fine cold-drawn oil is wanted. But in
general the paste is heated before being subjected to the pressure. The pressed cake is
again thrown under the edge-stones, and, after being ground the second time, should be
exposed to a heat of 212° Fahr., in a proper pan, called a steam-kettle, before being
subjected to the second and final pressure in the woollen bags and hair-cloths.
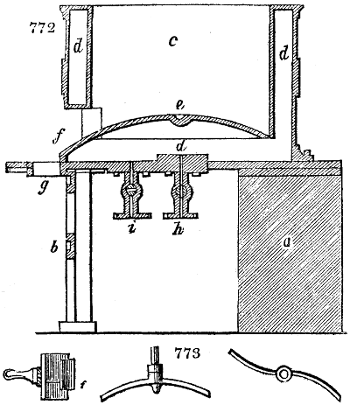
Fig. 772. is a vertical section of the steam-kettle of Hallette, and fig. 773. is a view of
the seed-stirrer. a, is the wall of masonry,
upon which, and the iron pillars
b, the pan is supported. It is enclosed
in a jacket, for admitting steam into the
intermediate space d, d, d, at its sides
and bottom. c, is the middle of the
pan in which the shaft of the stirrer is
planted upright, resting by its lower
end in the step e; f, is an opening, by
which the contents of the pan may be
emptied; g, is an orifice into which
the mouth of the hair or worsted bag
is inserted, in order to receive the
heated seed, when it is turned out by
the rotation of the stirrer and the withdrawal
of the plug f from the discharge
aperture; h, is the steam induction
pipe; and t, the eduction pipe, which
serves also to run off the condensed
water.
The hydraulic oil-press is generally
double; that is, it has two vertical rams
placed parallel to each other, so that
while one side is under pressure, the
other side is being discharged. The bags of heated seed-paste or meal are put into
cast-iron cases, which are piled over each other to the number of 6 or 8, upon the press
sill, and subjected to a force of 300 or 400 tons, by pumps worked with a steam engine.
The first pump has usually 2 or 21⁄2 inches diameter for a ram of 10 inches, and the second
pump one inch. Each side of the press, in a well-going establishment, should work 38
pounds of seed-flour every 5 minutes. Such a press will do 70 quarters of linseed in
the days’ work of one week, with the labour of one man at 20s. and three boys at 5s.
each; and will require a 12-horse power to work it well, along with the rolls and the
edge-stones.
I am indebted to my excellent friend Mr. E. Woolsey, for the following most valuable
notes, taken by him at sundry mills for pressing oil; and remarks upon the subject of
seed-crushing in general.
“The chief point of difference depends upon the quality of seed employed. Heavy
seed will yield most oil, and seed ripened under a hot sun, and where the flax is not
gathered too green, is the best. The weight of linseed varies from 48 to 52 lbs. per
imperial bushel; probably a very fair average is 49 lbs., or 392 lbs. per imperial
quarter. I inspected one of the seed-crusher’s books, and the average of 15 trials of
a quarter each of different seeds in the season averaged 141⁄2 galls. of 71⁄2 lbs. each; say,
109 lbs. of oil per quarter. This crusher, who uses only the hydraulic press, and one
pressing, informed me that
| Archangel seed will yield from |
15 to 16 galls. (of 71⁄2 lbs. each) |
| Best Odessa |
18 and even 19 galls. |
| Good crushing-seed |
151⁄2d even 19 gdo. |
| Low seed, such as weighs 48 lbs. per bushel |
131⁄2d even 19 gdo. |
[901]
“The average of the seed he has worked, which he represents to be of an inferior
quality, for the sake of its cheapness, yields 141⁄2 galls. per quarter. I had some
American seed which weighed 521⁄4 lbs. per imperial bushel, ground and pressed under
my own observation, and it gave me 111 lbs. oil; that is to say, 418 lbs. of seed
gave 111 lbs. oil = 2656⁄100 per cent. A friend of mine, who is a London crusher, told me
the oil varied according to the seed from 14 to 17 galls.; and when you consider the
relative value of seeds, and remember that oil and cake from any kind of seed is of
the same value, it will be apparent that the yield is very different; for example,
25th July, 1836,
prices of seed. |
- |
|
E. India linseed |
worth |
52 |
s. |
per quarter. |
| Petersburg linseed |
|
48 |
to 52 |
do. |
| Odessa |
|
52 |
— |
— |
The difference of 4s. must be paid for in the quantity of oil which at 38s. 6d. per cwt.
(the then price) requires about 111⁄2 lbs. more oil expressed to pay for the difference in
the market value of the seed. Another London crusher informed me that East India
linseed will produce 17 gallons, and he seemed to think that that was the extreme
quantity that could be expressed from any seed. The average of last year’s Russian
seed would be about 14 galls.; Sicilian seed 16 galls.
| Place. |
Engine Power. |
Hydraulic Presses. |
Stampers. |
Rollers. |
Edge-stones. |
Kettles. |
Work done,--reduced to an hour. |
Number of pressings. |
| France |
10 horse power |
1 hydraulic, 200 tons. |
5 light stampers. |
1 pair rolls. |
1 pr. edge-stones. |
5 table kettles small size heated by steam. |
1 English quarter per working hour. |
2 pressings. |
| London |
20 horse power |
1 hydraulic, 800 tons. |
13 light stampers. |
1 pair rolls. |
2 pr. edge-stones. |
8 table kettles small size heated by fire. |
2 English quarters per working hour. |
2 ditto |
| London |
12 horse power, but the engine is used also for other work. |
none |
9 light stampers. |
2 pair rolls, used also for other purposes. |
2 pr. edge-stones, used also for other purposes. |
4 table kettles small size heated by fire. |
7⁄8 English quarter per working hour. |
2 ditto |
| Hull |
18 horse engine, old construction. |
none |
3 very heavy stampers. |
1 pair rolls. |
1 pr. edge-stones. |
3 double case large size steam kettles. |
11⁄4 English quarter per working hour. |
1 ditto |
| Ditto |
22 horse engine |
none |
6 very heavy stampers. |
2 pair rolls. |
2 pr. edge-stones. |
6 double case large size steam kettles. |
Not known. |
1 ditto |
“Rape-seed.—I have not turned my attention to quantity of oil extracted from this
seed; but a French crusher (M. Geremboret), on whom I think one may place considerable
dependence, told me, that
| 31⁄2 |
lbs. of |
best Cambray rape-seed yielded |
1 lb. oil. |
| 33⁄4 |
— |
common rape-seed |
1 lb. oil. |
| 41⁄4 |
— |
com—onpoppy-seed |
1 lb. oil. |
“Rape-seed weighs from 52 to 56 lbs. per imperial bushel.”
The following are the heads of a reference of machinery for a seed oil-mill:—
1. Two pairs of cast-iron rollers, 19 inches long, and 10 inches in diameter, fixed in
a cast-iron frame, with brasses, wheels, shafts, bolts, scrapers, hoppers, shoes, &c.
2. Two pairs of edge-stones, 7 feet diameter each, with two bottom stones, 6 feet
diameter each, cast-iron upright shafts, sweepers, wheels, shafts, chairs, brasses, bolts,
and scrapers, with driving spur-wheels, &c.
3. Five steam kettles, with wheels, shafts, and brasses, bolts, breeches, and steam
pipes, an upright cast-iron shaft, with chairs and brasses at each end; and a large bevel
wheel upon the bottom end of upright shaft, and another, smaller, upon fly-wheel shaft,
for the first motions.
4. Five stamper presses, with press plates of cast iron, cast-iron stamper shaft with
10 arms and 10 rollers, with bosses, brasses, bolts, driving bevel-wheels.
A well made oil-mill, consisting of the above specified parts, will manufacture 200
quarters of seed per week.
I have been assured by practical engineers, conversant in oil-mills, that a double
hydraulic press, with 2 ten-inch rams, will do the work of no more than two of the
stamper presses; that is to say, it will work 22 quarters in 24 hours; while three stamper
presses will work 33 quarters in the same time, and produce one half more oil.
[902]
Castor oil, quantity of,
| |
Im-
ported. |
Retained
for
consumption. |
Ex-
ported. |
| Year. |
Cwts. |
Cwts. |
Cwts. |
| 1835. |
1,109,307 |
670,205 |
61,296 |
| 1836. |
981,585 |
809,559 |
68,515 |
Duty, from British possessions, 2s. 6d. per cwt.; from foreign, 1s. per lb.
Cocoa-nut oil, quantity of,
| |
Im-
ported. |
Retained
for
consumption. |
Ex-
ported. |
| Year. |
Cwts. |
Cwts. |
Cwts. |
| 1835. |
19,838 |
14,015 |
2,238 |
| 1836. |
26,058 |
26,062 |
3,158 |
| 1837. |
41,218 |
28,836 |
|
Olive oil, quantity of,
| |
Im-
ported. |
Retained
for
consumption. |
Ex-
ported. |
| Year. |
Cwts. |
Cwts. |
Cwts. |
| 1835. |
606,166 |
554,196 |
283,734 |
| 1836. |
2,682,016 |
1,844,622 |
150,561 |
| 1837. |
1,720,397 |
1,499,122 |
|
Duties on olive oil, not of Naples and Sicily, 4d.; of Naples and Sicily, 8d.; and,
if in ships of these countries, 10d. per gallon.
Train oil, spermaceti, and blubber, quantity of,
| |
Im-
ported. |
Retained
for
consumption. |
Ex-
ported. |
| Year. |
Cwts. |
Cwts. |
Cwts. |
| 1835. |
24,197 |
16,114 |
8,035 |
| 1836. |
19,489 |
18,722 |
1,365 |
| 1837. |
21,823 |
21,286 |
|
Duties on oil taken by British ships, 1s.; by foreign fishers, £26 18s. per tun.
OILS, VOLATILE OR ESSENTIAL; Manufacture of. The volatile oils occur
in every part of odoriferous plants, whose aroma they diffuse by their exhalation; but in
different organs of different species. Certain plants, such as thyme and the scented
labiatæ, in general contain volatile oil in all their parts; but others contain it only in the
blossoms, the seeds, the leaves, the root, or the bark. It sometimes happens that different
parts of the same plant contain different oils; the orange, for example, furnishes three
different oils, one of which resides in the flowers, another in the leaves, and a third in
the skin or epidermis of the fruit. The quantity of oil varies not only with the species,
but also in the same plant, with the soil, and especially the climate; thus in hot
countries it is generated most profusely. In several plants, the volatile oil is contained
in peculiar orders of vessels, which confine it so closely that it does not escape in the
drying, nor is dissipated by keeping the plants for many years. In other species, and
particularly in flowers, it is formed continually upon their surface, and flies off at the
moment of its formation.
Volatile oils are usually obtained by distillation. For this purpose the plant is introduced
into a still, water is poured upon it, and heat being applied, the oil is volatilized
by the aid of the watery vapour, at the temperature of 212°, though when alone it
would probably not distil over unless the heat were 100° more. This curious fact was
first explained in my New Researches upon Heat, published in the Philosophical Transactions
for 1818. Most of the essential oils employed in medicine and perfumery are
extracted by distillation from dried plants; only a few, such as those of the rose and
orange flower, are obtained, from fresh or succulent salted plants. When the mingled
vapours of the oil and water are condensed into the liquid state, by the refrigerator of the
still, the oil separates, and either floats on the surface or sinks to the bottom of the
water. Some oils of a less volatile nature require a higher heat than 212° to raise them
in vapour, and must be dislodged by adding common salt to the water, whereby the
heat being augmented by 15°, they readily come over. If in such distillations too much[903]
water be added, no oil will be obtained, because it is partially soluble in water; and
thus merely an aromatic water is produced. If on the other hand too little water be
used, the plant may happen to adhere to the bottom of the still, get partially charred, and
thus impart an empyreumatic odour to the product. But as the quality of water distilled
depends less upon the quantity employed, than upon that of the surface exposed to
the heat, it is obvious that by giving a suitable form to the still, we may get rid of every
inconvenience. Hence the narrower and taller the alembic is, within certain limits, the
greater will be the proportion of oil relative to that of the aromatic water, from like
proportions of aqueous and vegetable matter employed. Some place the plants in
baskets, and suspend these immediately over the bottom of the still under the water, or
above its surface in the steam. But the best mode in my opinion is to stuff an upright
cylinder full of the plants, and to drive down through them, steam of any desired force;
its tension and temperature being further regulated by the size of the outlet orifice
leading to the condenser. The cylinder should be made of strong copper tinned inside,
and encased in the worst conducting species of wood, such as soft deal or sycamore.
The distillation is to be continued as long as the water comes over of a milky appearance.
Certain plants yield so little oil by the ordinary processes, notwithstanding
every care, that nothing but a distilled water is obtained. In this case, the same water
must be poured upon a fresh quantity of the plants in the still; which being drawn over,
is again to be poured upon fresh plants; and thus repeatedly, till a certain dose of oil
be separated. This being taken off, the saturated water is reserved for a like distillation.
The refrigeratory vessel is usually a worm or serpentine plunged in a tub of water,
whose temperature should be generally cold; but for distilling the oils of anise-seed,
fennel, &c., which become concrete at low temperatures, the water should not be cooler
than 45° F.
The liquid product is commonly made to run at the worm end, into a vessel called an
Italian or Florentine receiver, which is a conical matrass, standing on its base, with
a pipe rising out of the side close to the bottom, and recurved a little above the middle
of the flask like the spout of a coffee-pot. The water and the oil collected in this
vessel soon separate from each other, according to their respective specific gravities; the
one floating above the other. If the water be the denser, it occupies the under portion
of the vessel, and continually overflows by the spout in communication with the bottom,
while the lighter oil is left. When the oil is the heavier of the two, the receiver should
be a large inverted cone, with a stopcock at its apex to run off the oil from the water
when the separation has been completed by repose. A funnel, having a glass stopcock
attached to its narrow stem, is the most convenient apparatus for freeing the oil finally
from any adhering particles of water. A cotton wick dipped in the oil may also serve
the same purpose by its capillary action. The less the oil is transvased the better, as a
portion of it is lost at every transfer. It may occasionally be useful to cool the distilled
water by surrounding it with ice, because it thus parts with more of the oil with which
it is impregnated.
There are a few essential oils which may be obtained by expression, from the substances
which contain them; such as the oils of lemons and bergamot, found in the
pellicle of the ripe fruits of the citrus aurantium and medica; or the orange and the
citron. The oil comes out in this case with the juice of the peel, and collects upon its
surface.
For collecting the oils of odoriferous flowers which have no peculiar organs for imprisoning
them, and therefore speedily let them exhale, such as violets, jasmine, tuberose, and
hyacinth, another process must be resorted to. Alternate layers are formed of the fresh
flowers, and thin cotton fleece or woollen cloth-wadding, previously soaked in a pure
and inodorous fat oil. Whenever the flowers have given out all their volatile oil to the
fixed oil upon the fibrous matter, they are replaced by fresh flowers in succession, till
the fat oil has become saturated with the odorous particles. The cotton or wool wadding
being next submitted to distillation along with water, gives up the volatile oil.
Perfumers alone use these oils; they employ them either mixed as above, or dissolve
them out by means of alcohol. In order to extract the oils of certain flowers, as for
instance of white lilies, infusion in a fat oil is sufficient.
Essential oils differ much from each other in their physical properties. Most of them
are yellow, others are colourless, red, or brown; some again are green, and a few are blue.
They have a powerful smell, more or less agreeable, which immediately after their
distillation is occasionally a little rank, but becomes less so by keeping. The odour is
seldom as pleasant as that of the recent plant. Their taste is acrid, irritating, and heating,
or merely aromatic when they are largely diluted with water or other substances. They
are not greasy to the touch, like the fat oils, but on the contrary make the skin feel
rough. They are almost all lighter than water, only a very few falling to the bottom of
this liquid; their specific gravity lies between 0·847 and 1·096; the first number
denoting the density of oil of citron, and the second that of oil of sassafras. Although[904]
styled volatile oils, the tension of their vapour, as well as its specific heat, is much less
than that of water. The boiling point differs in different kinds, but it is usually about
316° or 320° Fahr. Their vapours sometimes render reddened litmus paper blue, although
they contain no ammonia. When distilled by themselves, the volatile oils are partially
decomposed; and the gaseous products of the portion decomposed always carry off a
little of the oil. When they are mixed with clay or sand, and exposed to a distilling
heat, they are in a great measure decomposed; or when they are passed in vapour
through a redhot tube, combustible gases are obtained, and a brilliant porous charcoal
is deposited in the tube. On the other hand, they distil readily with water, because the
aqueous vapour formed at the surface of the boiling fluid carries along with it the
vapour of the oil produced in virtue of the tension which it possesses at the 212th deg.
Fahr. In the open air, the volatile oils burn with a shining flame, which deposits a
great deal of soot. The congealing point of the essential oils varies greatly; some do
not solidify till cooled below 32°, others at this point, and some are concrete at the
ordinary temperature of the atmosphere. They comport themselves in this respect like
the fat oils; and they probably consist, like them, of two different oils, a solid and a fluid;
to which the names stearoptène and eleoptène, or stearessence and oleiessence, may be given.
These may be separated from each other by compressing the cooled concrete oil between
the folds of porous paper; the stearessence remains as a solid upon the paper; the
oleiessence penetrates the paper, and may be recovered by distilling it along with water.
When exposed to the air, the volatile oils change their colour, become darker, and
gradually absorb oxygen. This absorption commences whenever they are extracted
from the plant containing them; it is at first considerable, and diminishes in rapidity as
it goes on. Light contributes powerfully to this action, during which the oil disengages
a little carbonic acid, but much less than the oxygen absorbed; no water is formed.
The oil turns gradually thicker, loses its smell, and is transformed into a resin, which
becomes eventually hard. De Saussure found that oil of lavender, recently distilled,
had absorbed in four winter months, and at a temperature below 54° F., 52 times its
volume of oxygen, and had disengaged twice its volume of carbonic acid gases; nor was
it yet completely saturated with oxygen. The stearessence of anise-seed oil absorbed at
its liquefying temperature, in the space of two years, 156 times its volume of oxygen gas,
and disengaged 26 times its volume of carbonic acid gas. An oil which has begun to
experience such an oxidizement is composed of a resin dissolved in the unaltered oil; and
the oil may be separated by distilling the solution along with water. To preserve oils
in an unchanged state, they must be put in phials, filled to the top, closed with ground
glass stopples, and placed in the dark.
Volatile oils are little soluble in water, yet enough so as to impart to it by agitation
their characteristic smell and taste. The water which distils with any oil is in general
a saturated solution of it, and as such is used in medicine under the name of distilled
water. It often contains other volatile substances contained in the plants, and hence
is apt to putrefy and acquire a nauseous smell when kept in perfectly corked bottles;
but in vessels partially open, these parts exhale, and the water remains sweet. The
waters, however, which are made by agitating volatile oil with simple distilled water
are not apt to spoil by keeping in well-corked bottles.
The volatile oils are soluble in alcohol, and the more so the stronger the spirit is.
Some volatile oils, devoid of oxygen, such as the oils of turpentine and citron, are very
sparingly soluble in dilute alcohol; while the oils of lavender, pepper, &c. are considerably
so. De Saussure has inferred from his experiments that the volatile oils are the more
soluble in alcohol, the more oxygen they contain. Such combinations form the odoriferous
spirits which the perfumers incorrectly call waters, as lavender water, eau de Cologne,
eau de jasmin, &c. They become turbid by admixture of water, which seizes the alcohol,
and separates the volatile oils. Ether also dissolves all the essential oils.
These oils combine with several vegetable acids, such as the acetic, the oxalic, the
succinic, the fat acids (stearic, margaric, oleic), the camphoric, and suberic.
With the exception of the oil of cloves, the volatile oils do not combine with the
salifiable bases. They have been partially combined with caustic alkali, as in the case
of Starkey’s soap. This is prepared by triturating recently fused caustic soda in a
mortar, with a little oil of turpentine, added drop by drop, till the mixture has acquired
the consistence of soap. The compound is to be dissolved in spirits of wine, filtered,
and distilled. What remains after the spirit is drawn off, consists of soda combined with
a resin formed in the oil during the act of trituration.
The volatile oils in general absorb six or eight times their bulk of ammoniacal gas;
but that of lavender absorbs 47 times.
The essential oils dissolve all the fat oils, the resins, and the animal fats.
In commerce these oils are often adulterated with fat oils, resins, or balsam of capivi
dissolved in volatile oil. This fraud may be detected by putting a drop of the oil on paper,
and exposing it to heat. A pure essential oil evaporates without leaving any residuum,[905]
whilst an oil mixed with any of the above substances leaves a translucent stain upon the
paper. If fat oil be present, it will remain undissolved, on mixing the adulterated
essential oil with thrice its volume of spirit of wine of specific gravity 0·840. Resinous
matter mixed with volatile oil is easily detected, being left in the alembic after distillation.
Oil diluted with spirit of wine, forms a milky emulsion on the addition of water;
the alcoholic part is absorbed by the water, and the oil afterwards found on the surface,
in a graduated glass tube will show by its quantity the amount of the adulteration.
But it is more difficult to detect the presence of a cheap essential oil in a dear one,
which it resembles. Here the taste and smell are our principal guides. A few drops of
the suspected oil are to be poured upon a bit of cloth, which is to be shaken in the air, and
smelled to from time to time. In this way we may succeed in distinguishing the odour
of the oil which exhales at the beginning, and that which exhales at the end; a method
which serves perfectly to detect oil of turpentine in the finer essential oils. Moreover,
when the debased oil is mixed with spirits of wine at sp. gr. 0·840, the oil of turpentine
remains in a great measure undissolved. If an oil heavier than water, and an oil lighter
than water, be mixed, they may be separated by agitation for some time with that liquid,
and then leaving the mixture at rest. Essential oils may also be distinguished by a
careful examination of their respective densities.
Oil of bitter almonds, is prepared by exposing the bitter almond cake, from which the
bland oil has been expressed, in a sieve to the vapour of water rising within the still.
The steam, as it passes up through the bruised almond parenchyma, carries off its volatile
oil, and condenses along with it in the worm. The oil which first comes over, and
which falls to the bottom of the water, has so pungent and penetrating a smell, that it
is more like cyanogen gas than hydrocyanic or prussic acid. This oil has a golden-yellow
colour, it is heavier than water; when much diluted, it has an agreeable smell, and
a bitter burning taste. When exposed to the air, it absorbs oxygen, and lets fall a heap
of crystals of benzoic acid. This oil consists of a mixture of two oils; one of which is
volatile, contains hydrocyanic acid, and is poisonous; the other is less volatile, is not
poisonous, absorbs oxygen, and becomes benzoic acid. If we dissolve 100 parts of the
oil of bitter almonds in spirit of wine, mix with the solution an alcoholic solution of
potash, and then precipitate the oil with water, we shall obtain a quantity of cyanide of
potash, capable of producing 221⁄2 parts of prussian blue. Oil of bitter almonds combines
with the alkalis. Perfumers employ a great quantity of this oil in scenting their soaps.
One manufacturer in Paris is said to prepare annually 3 cwt. of this oil. A similar
poisonous oil is obtained by distilling the following substances with water:—the leaves
of the peach (amygdalus persica), the leaves of the bay-laurel (prunus lauro-cerasus),
the bark of the plum tree (prunus padus), and the bruised kernels of cherry and plum-stones.
All these oils contain hydrocyanic acid, which renders them poisonous, and they
also generate benzoic acid, by absorbing oxygen on exposure to air.
Oil of anise-seed, is extracted by distillation from the seeds of the pimpinella anisum. It
is either colourless, or has merely a faint yellow colour, with the smell and taste of the
seed. It concretes in lamellar crystals at the temperature of 50°, and does not melt again
till heated to 64° nearly. Its specific gravity at 61° is 0·9958, and at 77°, 0·9857. It
is soluble in all proportions in alcohol of 0·806; but only to the extent of 42 per cent.
in alcohol of 0·84. When it becomes resinous by long exposure to the air, it loses its
congealing property. It consists of two oils; a solid stearessence, and a liquid oleiessence,
which may be separated by compression of the cold concrete oil.
Oil of bergamot, is extracted by pressure from the rind of the ripe fruit of the citrus
bergamium and aurantium. It is a limpid, yellowish fluid, having a smell resembling
that of oranges. Its specific gravity varies from 0·888 to 0·885. It becomes concrete
when cooled a little below 32°.
Oil of cajeput, is prepared in the Moluccas, by distilling the dry leaves of the melaleuca
leucadendron. Cajeput is a native word, signifying merely a white tree. This oil is
green; it has a burning taste, a strong smell of camphor, turpentine, and savine. It is
very fluid, and at 48° has a specific gravity of 0·948. The colour seems to be derived
from the copper vessels in which it is imported, so that it is removed by distillation with
water, which also separates the oil into two sorts; the first which comes over having a
density of 0·897, the last of 0·920. This has a green colour.
The oil of caraway is extracted from the seeds of the carum carui. It has a pale
yellow colour, and the smell and taste of the plant. Its specific gravity is 0·960. The
seeds of the cuminum cyminum (cumin) afford an oil similar to the preceding, but not so
agreeable. Its specific gravity is 0·975.
The oil of cassia, from the laurus cassia, is yellow passing into brown, has a specific
gravity of 1·071, and affords a crystalline stearessence by keeping in a somewhat open
vessel.
The oil of chamomile is extracted by distillation from the flowers of the matricaria
chamomilla. It has a deep blue colour, is almost opaque, and thick; and possesses the[906]
peculiar smell of the plant. In the atmosphere it becomes brown and unctuous. If an
ounce of oil of lemons be added to 3 pounds of this oil, they make it separate more
readily from the adhering water.
Other blue oils, having much analogy with oil of chamomile, are obtained by distilling
the following plants: roman chamomile (anthemis nobilis), the flowers of arnica
montana, and those of milfoil (achillæa millefolia). The last has a spec. grav. of 0·852.
Oil of cinnamon, is extracted by distillation from the bark of the laurus cinnamomum.
It is produced chiefly in Ceylon, from the pieces of bark unfit for exportation. It is
distilled over with difficulty, and the process is promoted by the addition of salt water,
and the use of a low still. It has at first a pale yellow colour, but it becomes brown
with age. It possesses in a high degree both the sweet burning taste, and the agreeable
smell of cinnamon. It is heavier than water; its specific gravity being 1·035. It concretes
below 32° F., and does not fuse again till heated to 41°. It is very sparingly
soluble in water, and when agitated with it readily separates by repose. It dissolves
abundantly in alcohol, and combines with ammonia into a viscid mass, not decomposed
on exposure to air.
When oil of cinnamon is kept for a long time, it deposits a stearessence in large
regular colourless or yellow crystals, which may be pulverized, and which melt at a very
gentle heat into a colourless liquid, which crystallizes on cooling. It has an odour intermediate
between that of cinnamon and vanilla; and a taste at first greasy, but
afterwards burning and aromatic. It crackles between the teeth. It requires a high
temperature for distillation, and becomes then brown and empyreumatic. It is very
soluble in alcohol.
The oil of cloves, is extracted from the dried flower buds of the caryophyllus aromaticus.
It is colourless, or yellowish, has a strong smell of the cloves, and a burning
taste. Its specific gravity is 1·061. It is one of the least volatile oils, and the most
difficult to distil. At the end of a certain time it deposits a crystalline concrete oil. A
similar stearessence is obtained by boiling the bruised cloves in alcohol, and letting the
solution cool. The crystals thus formed are brilliant, white, grouped in globules, without
taste and smell. Oil of cloves has remarkable chemical properties. It dissolves in
alcohol, ether, and acetic acid. It does not solidify at a temperature of 4° under 0° F.,
even when exposed to that cold for several hours. It absorbs chlorine gas, becomes
green, then brown, and turns resinous. Nitric acid makes it red, and if heated upon it,
converts it into oxalic acid. If mixed by slow degrees with one third of its weight of
sulphuric acid, an acid liquor is formed, at whose bottom a resin of a fine purple colour
is found. After being washed, this resin becomes hard and brittle. Alcohol dissolves
it, and takes a red colour; and water precipitates it of a blood red hue. It dissolves
also in ether. When we agitate a mixture of strong caustic soda lye and oil of cloves
in equal parts, the mass thickens very soon, and forms delicate lamellar crystals. If we
then pour water upon it, and distil, there passes along with the water, a small quantity
of an oil which differs from oil of cloves both in taste and chemical properties. During
the cooling, the liquor left in the retort lets fall a quantity of crystalline needles, which
being separated by expression from the alkaline liquid, are almost inodorous, but possess
an alkaline taste, joined to the burning taste of the oil. These crystals require for solution
from 10 to 12 parts of cold water. Potash lye produces similar effects. Ammoniacal
gas transmitted through the oil is absorbed and makes it thick. The concrete
combination thus formed remains solid as long as the phial containing it is corked, but
when opened, the compound becomes liquid; and these phenomena may be reproduced
as many times as we please. Such combinations are decomposed by acids, and the oil
set at liberty has the same taste and smell as at first, but it has a deep red colour. The
alkalis enable us to detect the presence of other oils, as that of turpentine or sassafras,
in that of cloves, because they fix the latter, while the former may be volatilized with
water by distilling the mixture. The oil of cloves found in commerce is not pure, but
contains a mixture of the tincture of pinks or clove-gilly flowers, whose acrid resin is
thereby introduced. It is sometimes sophisticated with other oils.
The oil of elder, is extracted by distillation from the flowers of the sambucus nigra.
It has the consistence of butter. The watery solution is used in medicine.
Oil of fennel, is extracted by distillation from the seeds of the anethum fœniculum. It
is either colourless or of a yellow tint, has the smell of the plant, and a specific gravity
of 0·997. When treated with nitric acid, it affords benzoin. It congeals at the temperature
of 14° F., and then yields by pressure a solid and a liquid oil; the former
appearing in crystalline plates. It is used in this country for scenting soap.
Oils of fermented liquors. The substances usually fermented contain a small quantity
of essential oils, which become volatile along with the alcoholic vapours in distillation,
and progressively increase as the spirits become weaker towards the end of the process.
The vapours then condense into a milky liquor. These oils adhere strongly to the
alcohol, and give it a peculiar acrid taste. They differ according to the vinous wash[907]
from which they are obtained, and combine with greater or less facility with caustic
alkalis.
1. Oil of grain spirits. At the ordinary temperature it is partially a white solid;
when cooled lower it assumes the aspect of suet, and therefore consists chiefly of stearessence.
Its taste and smell are most offensive; it swims upon the surface of water,
and even of spirit containing 30 per cent. of alcohol. It sometimes derives a green
colour from the copper worm of the still. When heated it fuses and turns yellow.
When it has become resinous by the agency of the atmosphere, it gives a greasy stain to
paper. It dissolves in 6 parts of anhydrous alcohol, and in 2 of ether; and is said to
crystallize when the spirit solution has been saturated with it hot, and is allowed to
cool. By exposure to a freezing mixture, the whiskey which contains it lets it fall.
Caustic potash dissolves it very slowly, and forms a soap soluble in 60 parts of water.
It is absorbed by wood charcoal, and still better by bone black; whereby it may be
completely abstracted from bad whiskey. According to Buchner, another oil may also
be obtained from the residuum of the second distillation of whiskey, if saturated with
sea salt, and again distilled. Thus we obtain a pale yellow fluid oil, which does not
concrete with cold, possessed of a disagreeable smell and acrid taste. Its specific gravity
is 0·835. It is soluble in alcohol and ether.
2. The oil from potato spirits, has properties quite different from the preceding. It
is obtained in considerable quantity by continuing the distillation after most of the alcohol
has come over, and it appears in the form of a yellowish oil, mixed with water
and spirits. After being agitated first with water, then with a strong solution of muriate
of lime, and distilled afresh, it possesses the following properties: it is colourless,
limpid, has a peculiar smell, and a bitter hot taste of considerable permanence. It
leaves no greasy stain upon paper, remains liquid at 0° F., but cooled below that point
it crystallizes like oil of anise-seed. When pure it boils at 257° F.; but at a lower
degree, if it contains alcohol. Its specific gravity is 0·821, or 0·823 when it contains a
little water. It burns with a clear flame without smoke, but it easily goes out, if not
burned with a wick. It dissolves in small quantity in water, to which it imparts its
taste and the properties of forming a lather by agitation. It dissolves in all proportions
in alcohol. Chlorine renders it green. Concentrated sulphuric acid converts it into a crimson
solution, from which it is precipitated yellow by water. It dissolves in all proportions
in acetic acid. Concentrated caustic lyes dissolve it, but give it up to water. It
does not appear to be poisonous, like the oil of corn spirits; because, when given by
spoonfuls to dogs, it produced no other effect but vomiting.
3. The oil of brandy or grape spirits, is obtained during the distillation of the fermented
residuum of expressed grapes; being produced immediately after the spirituous liquor
has passed over. It is very fluid, limpid, of a penetrating odour, and an acrid disagreeable
taste. It grows soon yellow in the air. When this oil is distilled, the first
portions of it pass unchanged, but afterwards it is decomposed and becomes empyreumatic.
It dissolves in 1000 parts of water, and communicates to it its peculiar taste
and smell. One drop of it is capable of giving a disagreeable flavour to ten old English
gallons of spirits. It combines with the caustic alkalis, and dissolves sulphur.
Oil of Juniper, is obtained by distilling juniper berries along with water. These should
be bruised, because their oil is contained in small sacs or reservoirs, which must be laid
open before the oil can escape. It is limpid and colourless, or sometimes of a faint
greenish yellow colour. Its specific gravity is 0·911. It has the smell and taste of the
juniper. Water, or even alcohol, dissolves very little of it. Gin contains a very minute
quantity of this oil. Like oil of turpentine, it imparts to the urine of persons who
swallow it, the smell of violets. Oil of juniper is frequently sophisticated with oil of
turpentine introduced into the still with the berries; a fraud easily detected by the
diminished density of the mixture.
The oil of lavender, is extracted from the flowering spike of the lavandula spica. It is
yellow, very fluid, has a strong odour of the lavender, and a burning taste. The
specific gravity of the oil found in commerce is 0·898 at the temperature of 72° F., and
of 0·877 when it has been rectified. It is soluble in all proportions in alcohol of 0·830,
but alcohol of 0·887 dissolves only 42 per cent. of its weight. The fresh oil detonates
slightly when mixed with iodine, with the production of a yellow cloud. There occurs
in commerce a kind of oil of lavender known under the name of oil of aspic or oil of spike,
extracted by distillation from a wild variety of the lavandula spica, which has large
leaves, and is therefore called latifolia. This oil is manufactured in the south of Europe.
Its odour is less characteristic than that of the lavender, resembling somewhat that of
oil of turpentine, with which it is indeed often adulterated. It is also so cheap as
to be sometimes used instead of the latter oil. Oil of lavender deposits, when partially
exposed to the air, a concrete oil, which resembles camphor, to the amount of one fourth
of its weight.
Oil of lemons, is extracted by pressure from the yellow peel of the fruit of the lemon, or[908]
citrus medica. In this state it is a yellowish fluid, having a specific gravity of 0·8517;
but when distilled along with water till three fifths of the oil have come over, it is
obtained in a colourless state, and of a specific gravity of 0·847 at 72° F. This oil does
not become concrete till cooled to 4° below 0° F.
The oil of lemons has a very agreeable smell of the fruit, which is injured by distillation.
It is soluble in all proportions in anhydrous alcohol, but only 14 parts dissolve
in 100 of spirits of wine of specific gravity 0·837. This oil, especially when distilled,
forms with muriatic acid similar camphorated compounds with oil of turpentine,
absorbing no less than 280 volumes of the acid gas.
Oil of lemons kept long, in ill-corked bottles, generates a quantity of stearessence,
which when dissolved in alcohol, precipitated by water, and evaporated, affords brilliant,
colourless, transparent needles. Some acetic acid is also generated in the old oil.
According to Brandes, the specific gravity of oil of lemons is 0·8786.
The oil of mace, lets fall, after a certain time, a concrete oil under the form of a crystalline
crust, called by John myristicine.
The oil of nutmegs, is extracted chiefly from mace, which is the inner epidermis of
these nuts. It is colourless, or yellowish, a little viscid with a strong aromatic odour
of nutmegs, an acrid taste, and a specific gravity of 0·948. It consists of two oils, which
may be easily separated from each other by agitation with water; for one of them, which
is more volatile and aromatic comes to the surface, while the other, which is denser,
white, and of a buttery consistence, falls to the bottom. The latter liquefies by the heat
of the hand.
The oil of orange flowers, called neroli, is extracted from the fresh flowers of the citrus
aurantium. When recently prepared it is yellow; but when exposed for two hours to
the rays of the sun, or for a longer time to diffuse daylight, it becomes of a yellowish-red.
It is very fluid, lighter than water, and has a most agreeable smell. The aqueous
solution known under the name of orange-flower water, is used as a perfume. It is
obtained either by dissolving the oil in water, or by distilling with water the leaves either
fresh or salted; the first being the stronger, but the last being the more fragrant preparation.
Orange-flower water obtained by distillation, contains besides the oil, a
principle which comes over with it, of a nature hitherto unknown; it possesses the property
of imparting to water the faculty of becoming red with a few drops of sulphuric acid.
The water formed from the oil alone, is destitute of this property. The intensity of the
rose-colour is a test in some measure of the richness of the water in oil.
The oil of parsley, is extracted from the apium petroselinum. It is of a pale yellow
colour, having the smell of the plant, and consists of two oils separable by agitation in
water. Its liquid part floats upon the surface in a very fluid form; its stearessence,
which falls to the bottom, is butyraceous and crystallizes at a low temperature. This
concrete oil melts at 86° F.
The oil of pepper, is extracted from the piper nigrum. In the recent state it is limpid
and colourless, but by keeping it becomes yellow. It swims upon the surface of water.
In odour it resembles pepper, but is devoid of its hot taste.
The oil of peppermint is extracted from the mentha piperita. It is yellowish, and endued
with a very acrid burning taste. Its specific gravity is 0·920. At 6° or 7° below
0° F., it deposits small capillary crystals. After long keeping it affords a stearessence
resembling camphor, provided the oil had been obtained from the dry plant gathered in
flower, but not from distillation of the fresh plant. When artificially cooled, it yields
6 per cent. of stearessence, which crystallizes in prisms with three sides, has an acrid
somewhat rank taste, is soluble in ether and alcohol, and is thrown down from the latter
solution by water in the form of a white powder. Peppermint water is characterized
by the sensation of coolness which it diffuses in the mouth.
The oil of pimento, is extracted from the envelopes of the fruits of the myrtus pimenta,
which afford 8 per cent. of it. It is yellowish, almost colourless, of a smell analogous
to that of cloves, an acrid burning taste, and a specific gravity greater than water.
Nitric acid makes it first red, and after the effervescence, of a rusty brown hue. It
combines with the salifiable bases, like oil of cloves.
The oil of rhodium, is extracted from the wood of the convolvolus scoparius. It is very
fluid, and has a yellow colour, which in time becomes red. It has somewhat of the rose
odour, and is used to adulterate the genuine otto. Its taste is bitter and aromatic, which
it imparts to the otto as well as its fluidity.
The oil of roses, called also the attar or otto, is extracted by distillation from the petals
of the rosa centifolia and sempervirens. Our native roses furnish such small quantities
of the oil, that they are not worth distilling for the purpose. The best way of operating
is to return the distilled water repeatedly upon fresh petals, and eventually to cool the
saturated water with ice; whereby a little butyraceous oil is deposited. But the oil
thus obtained has not a very agreeable odour, being injured by the action of the air in
the repeated distillations. In the East Indies, the attar is obtained by stratifying rose[909]
leaves in earthen pans in alternate layers, with the oleiferous seeds of a species of digitalis,
called gengeli, for several days, in a cool situation. The fat oil of the seed absorbs the
essential oil of the rose. By repeating this process with fresh leaves and the same seed,
this becomes eventually swollen, and being then expressed furnishes the oil. The turbid
liquid thus obtained is left at rest, in well-closed vessels, where it gets clarified. The
layer of oil that floats on the top is then drawn off by a capillary cotton wick, and subjected
to distillation along with water, whereby the volatile otto is separated from the
fat seed-oil.
The oil of roses is colourless, and possesses the smell of roses, which is not however
agreeable, unless when diffused, for in its concentrated state it is far from pleasant to
the nostrils, and is apt to occasion headaches. Its taste is bland and sweetish. It is
lighter than water, and at the temperature of 92°, its specific gravity compared to that
of water at 60° is 0·832. At lower temperatures it becomes concrete and butyraceous;
and afterwards fuses at 90°. It is but slightly soluble in alcohol; 1000 parts of this
liquid at 0·806 dissolving only 71⁄2 parts at 58° F. This oil consists of two parts, the
stearessence and oleiessence; the latter being the more volatile odoriferous portion.
The oil of rosemary, is extracted from the rosmarinus officinalis. It is as limpid as water,
has the smell of the plant, and in other respects resembles oil of turpentine. The oil
found in commerce has a specific gravity of 0·911, which becomes 0·8886 by rectification.
It boils at 320° F. (occasionally at 329°). It is soluble in all portions in alcohol of 0·830.
When kept in imperfectly closed vessels, it deposits a stearessence to the amount of one
tenth of its weight, resembling camphor. It is sometimes adulterated with oil of turpentine,
a fraud easily detected by adding anhydrous alcohol, which dissolves only the
oil of rosemary.
The oil of saffron, is extracted from the stigmata of the crocus sativus. It is
yellow, very fluid, falls to the bottom of water, diffuses the penetrating odour of the
plant, and has an acrid and bitter taste. It is narcotic.
The oil of sassafras, is extracted from the woody root of the laurus sassafras. It is
colourless; but at the end of a certain time it becomes yellow or red. It has a peculiar,
sweetish, pretty agreeable, but somewhat burning taste. Its specific gravity is 1·094.
According to Bonastre, this oil separates by agitation with water into an oil lighter and
an oil heavier than this fluid. When long kept, it deposits a stearessence in transparent
and colourless crystals, which have the smell and taste of the liquid oil.
The oil of savine, is extracted from the leaves of the juniperus sabina. It is limpid,
and has the odour and taste of the plant, which is one more productive of volatile
oil than any other.
The oil of tansy has a specific gravity of 0·946, the penetrating odour of the tanacetum
vulgare, with an acrid and bitter taste.
Oil of turpentine, commonly called essence of turpentine. It is extracted from several
species of turpentine, a semi-liquid resinous substance which exudes from certain trees
of the pine tribe, and is obtained by distilling the resin along with water. This oil is
the cheapest of all the volatile species, and, as commonly sold, contains a little resin,
from which it may be freed by re-distillation with water. It is colourless, limpid, very
fluid, and has a very peculiar smell. Its specific gravity at 60° is 0·872; that of the
spirit on sale in the shops is 0·876. This oil always reddens litmus paper, because it
contains a little succinic acid.
100 parts of spirits of wine, of specific gravity 0·84, dissolve only 131⁄2 of oil of turpentine
at 72° F. When agitated with alcohol at 0·830 the oil retains afterwards one fifth
of its bulk of the spirit; hence this proposed method for purifying oil of turpentine is
defective. The oil if left during four months in contact with air is capable of absorbing
20 times its bulk of oxygen gas. One volume of rectified oil of turpentine absorbs at
the temperature of 72°, and under the common atmospheric pressure, 163 times its volume
of muriatic acid gas, provided the vessel be kept cool with ice. This mixture being
allowed to repose for 24 hours, produces out of the oil from 26 to 47 per cent. of a white
crystalline substance, which subsides to the bottom of a brown, smoking, translucent
liquor. Others say that 100 parts of oil of turpentine yield 110 of this crystalline
matter, which was called by Kind, its discoverer, artificial camphor, from its resemblance
in smell and appearance to this substance. Both the solid and the liquid are combinations
of muriatic acid and oil of turpentine; indicating the existence of a stearine and an
oleine in the latter substance. The liquid compound is lighter than water, and is not
decomposed by it, nor does it furnish any more solid matter when more muriatic gas is
passed through it. The solid compound, after being washed first with water containing
a little carbonate of soda, then with pure water, and finally purified by sublimation with
some chalk, lime, ashes, or charcoal, appears as a white, translucent, crystalline body, in
the form of flexible, tenacious needles. It swims upon the surface of water, diffuses a
faint smell of camphor, commonly mixed with that of oil of turpentine, and has rather
an aromatic than a camphorated taste. It does not redden litmus paper. Water[910]
dissolves a very minute quantity; but cold alcohol of 0·806 dissolves fully one third of
its weight, and hot much more, depositing, as it cools, this excess in the form of crystals.
The solution is not precipitated by nitrate of silver, which shows that the nature of the
muriatic acid is perfectly masked by the combination. It is composed, in 100 parts, of
76·4 carbon, 9·6 hydrogen, and 14 muriatic acid. The muriatic acid, or chlorine may
be separated by distilling an alcoholic solution of the artificial camphor 12 or 14 times
in succession with slaked lime.
Oil of turpentine is best preserved in casks enclosed within others, with water between
the two. Its principal use is for making varnishes, and as a remedy for the tape-worm.
The oil of thyme, is extracted from the thymus serpyllum. It is reddish yellow, has an
agreeable smell, and, after being long kept, it lets fall a crystalline stearessence. It is
used merely as a perfume.
The oil of wormwood, is extracted from the artemisia absinthium. It is yellow, or
sometimes green, and possesses the odour of the plant. Its taste resembles that of
wormwood, but without its bitterness. Its specific gravity is 0·9703 according to Brisson
and 0·9725 according to Brandes. It detonates with iodine when it is fresh. Treated
with nitric acid of 1·25 specific gravity, it becomes first blue, and after some time
brown.
OIL OF VITRIOL, is the old name of concentrated Sulphuric Acid.
OLEATES, are saline compounds of oleic acid with the bases.
OLEFIANT GAS, is the name originally given to bi-carburetted hydrogen.
OLEIC ACID, is the acid produced by saponifying olive-oil, and then separating
the base by dilute sulphuric or muriatic acid. See Fats, and Stearine.
OLEINE, is the thin oily part of fats, naturally associated in them with glycerine,
margarine, and stearine.
OLIBANUM, is a gum-resin, used only as incense in Roman-catholic churches.
OLIVE OIL. See Oils, unctuous.
ONYX, an ornamental stone of little value; a subspecies of quartz.
OOLITE, is a species of limestone composed of globules clustered together, commonly
without any visible cement or base. These vary in size from that of small pin-heads
to peas; they sometimes occur in concentric layers, at others they are compact,
or radiated from the centre to the circumference; in which case, the oolite is called
roogenstein by the German mineralogists. In geology the oolitic series includes all the
strata between the iron sand above and the red marl below. It is the great repository
of the best architectural materials which the midland and eastern parts of England
produce; it is divided into three systems:—
1. The upper oolite, including the argillo-calcareous Purbeck strata, which separate the
iron and oolitic series; the oolitic strata of Portland, Tisbury, and Aylesbury; the
calcareous sand and concretions, as of Shotover and Thame; and the argillo-calcareous
formation of Kimmeridge, the oak tree of Smith.
2. The middle oolite; the oolitic strata associated with the coral rag; calcareous sand
and grit; great Oxford clay, between the oolites of this and the following system.
3. The lower oolite; which contains numerous oolitic strata, occasionally subdivided
by thin argillaceous beds; including the cornbrash, forest marble, schistose oolite, and
sand of Stonesfield and Hinton, great oolite and inferior oolite; calcareo-siliceous sand
passing into the inferior oolite; great argillo-calcareous formation of lias, and lias
marl, constituting the base of the whole series.
These formations occupy a zone 30 miles broad in England.
OOST, or OAST; the trivial or provincial name of the stove in which the picked
hops are dried.
OPAL; an ornamental stone of moderate value. See Lapidary.
OPERAMETER, is the name given to an apparatus patented in February, 1829,
by Samuel Walker, cloth manufacturer, in the parish of Leeds. It consists of a train
of toothed wheels and pinions enclosed in a box, having indexes attached to the central
arbor, like the hands of a clock, and a dial plate; whereby the number of rotations of a
shaft projecting from the posterior part of the box is shown. If this shaft be connected
by any convenient means to the working parts of a gig mill, shearing frame, or any other
machinery of that kind for dressing cloths, the number of rotations made by the
operating machine will be exhibited by the indexes upon the dial plate of this apparatus.
In dressing cloths, it is often found that too little or too much work has been expended
upon them, in consequence of the unskilfulness or inattention of the workmen. By the
use of the operameter, that evil will be avoided, as the master may regulate and prescribe
beforehand by the dial the number of turns which the wheels should perform.
A similar clock-work mechanism, called a counter, has been for a great many years
employed in the cotton factories to indicate the number of revolutions of the main shaft
of the mill, and of course the quantity of yarn that might or should be spun, or of cloth
that might be woven in the power looms. A common pendulum or spring clock is[911]
commonly set up alongside of the counter; and sometimes the indexes of both are
regulated to go together, when the mill performs its average work.
OPIUM, is the juice which exudes from incisions made in the heads of ripe poppies,
(papaver somniferum,) rendered concrete by exposure to the air and the sun. The best
opium which is found in the European markets comes from Asia Minor and Egypt;
what is imported from India is reckoned inferior in quality. This is the most valuable
of all the vegetable products of the gum-resin family: and very remarkable for the
complexity of its chemical composition. Though examined by many able analysts, it
still requires further elucidation.
Opium occurs in brown lumps of a rounded form, about the size of the fist, and
often larger; having their surface covered with the seeds and leaves of a species of
rumex, for the purpose of preventing the mutual adhesion of the pieces in their semi-indurated
state. These seeds are sometimes introduced into the interior of the masses
to increase their weight; a fraud easily detected by cutting them across. Good opium
is hard in the cold, but becomes flexible and doughy when it is worked between the hot
hands. It has a characteristic smell, which by heat becomes stronger, and very offensive
to the nostrils of many persons. It has a very bitter taste. Water first softens, and
then reduces it to a pasty magma. Proof spirit digested upon opium forms laudanum,
being a better solution of its active parts than can be obtained by either water or
strong alcohol alone. Water distilled from it acquires its peculiar smell, but carries
over no volatile oil.
Opium was analyzed by Bucholz and Braconnot, but at a period anterior to the
knowledge of the alkaline properties of morphia and opian (narcotine). Bucholz
found in 100 parts of it, 9·0 of resin; 30·4 of gum; 35·6 of extractive matter; 4·8 of
caoutchouc; 11·4 of gluten; 2·0 of ligneous matter, as seeds, leaves, &c.; 6·8 of water
and loss. John, who made his analysis more recently, obtained 2·0 parts of a rancid
nauseous fat; 12·0 of a brown hard resin; 10·0 of a soft resin; 2 of an elastic substance;
12·0 of morphia and opian; 1·0 of a balsamic extract; 25·0 of extractive
matter; 2·5 of the meconates of lime and magnesia; 18·5 of the epidermis of the
heads of the poppy; 15 of water, salts, and odorous matter.
In the Numbers of the Quarterly Journal of Science for January and June, 1830,
I published two papers upon opium and its tests, containing the results of researches
made upon some porter which had been fatally dosed with that drug; for which crime,
a man and his wife had been capitally punished, about a year before, in Scotland.[36]
From the first of these papers the following extract is made:—
“Did the anodyne and soporific virtue of opium reside in one definite principle,
chemical analysis might furnish a certain criterion of its powers. It has been pretty
generally supposed that this desideratum is supplied by Sertürner’s discovery of morphia.
Of this narcotic alkali not more than 7 parts can be extracted by the most rigid
analysis from 100 of the best Turkey opium; a quantity, indeed, somewhat above the
average result of many skilful chemists. Were morphia the real medicinal essence of
the poppy, it should display, when administered in its active saline state of acetate, an
operation on the living system commensurate in energy with the fourteen-fold concentration
which the opium has undergone. But so far as may be judged from the most
authentic recent trials, morphia in the acetate seems to be little, if any, stronger as a
narcotic than the heterogeneous drug from which it has been eliminated. Mr. John
Murray’s experiments would, in fact, prove it to be greatly weaker; for he gave 2
drachms of superacetate of morphia to a cat, without causing any poisonous disorder.
This is perhaps an extreme case, and may seem to indicate either some defect in the
preparation, or an uncommon tenacity of life in the animal. To the same effect
Lassaigne found that a dog lived 12 hours after 36 grains of acetate of morphia in
watery solution had been injected into its jugular vein. The morphia meanwhile was
entirely decomposed by the vital forces, for none of it could be detected in the blood
drawn from the animal at the end of that period. Now, from the effects produced
by 5 grains of watery extract of opium, injected by Orfila into the veins of a dog, we
may conclude that a quantity of it, equivalent to the above dose of the acetate of
morphia, would have proved speedily fatal.
“Neither can we ascribe the energy of opium to the white crystalline substance called
narcotine, or opian, extracted from it by the solvent agency of sulphuric ether; for Orfila
assures us that these crystals may be swallowed in various forms by man, even to the
amount of 2 drachms in the course of 12 hours, with impunity; and that a drachm of it
dissolved in muriatic or nitric acid may be administered in the food of a dog without
producing any inconvenience to the animal. It appears, however, on the same authority,[912]
that 30 grains of it dissolved in acetic or sulphuric acid caused dogs that had swallowed
the dose to die under convulsions in the space of 24 hours, while the head was thrown
backwards on the spine. Oil seems to be the most potent menstruum of narcotine; for
3 grains dissolved in oil readily kill a dog, whether the dose be introduced into the
stomach or into the jugular vein.
“Since a bland oil thus seems to develop the peculiar force of narcotine, and since
opium affords to ether, and also to ammonia, an unctuous or fatty matter, and a resin
(the caoutchouc of Bucholz) to absolute alcohol, we are entitled to infer that the
activity of opium is due to its state of composition, to the union of an oleate or margarate
of narcotine with morphia. The meconic acid associated with this salifiable base
has no narcotic power by itself, but may probably promote the activity of the morphia.”
Opian or narcotine, and morphia, may be well prepared by the following process.
The watery infusion of opium being evaporated to the consistence of an extract, every
3 parts are to be diluted with one and a half parts in bulk of water, and then mixed in
a retort with 20 parts of ether. As soon as 5 parts of the ether have been distilled
over, the narcotic salt contained in the extract will be dissolved. The fluid contents of
the retort are to be poured hot into a vessel apart, and the residuum being washed with
5 other parts of ether, they are to be added to the former. Crystals of narcotine will be
obtained as the solution cools. The remaining extract is to be diluted in the retort
with a little water, and the mixture set aside in a cool place. After some time, some
narcotine will be found crystallized at the bottom. The supernatant liquid thus
freed from narcotine being decanted off, is to be treated with caustic ammonia; and
the precipitate thrown upon a filter. This, when well washed and dried, is to be
boiled with a quantity of spirit of wine at 0·84, equal to thrice the weight of the
opium employed, containing 6 parts of animal charcoal for every hundred parts of the
drug. The alcoholic solution being filtered hot, affords, on cooling, colourless crystals
of morphia.
This alkali may be obtained by a more direct process, without alcohol or ether.
A solution of opium in vinegar, is to be precipitated by ammonia; the washed precipitate
is to be dissolved in dilute muriatic acid, the solution is to be boiled along with
powdered bone black, filtered, and then precipitated by ammonia. This, when washed
upon a filter and dried, is white morphia, which may be dissolved in hot alcohol, if fine
crystals be wanted. See Morphia.
Opium, quantity of,
| |
Imported. |
Retained
for
consumption. |
Exported. |
| Year. |
Libs. |
Libs. |
Libs. |
| 1885. |
85,481 |
31,181 |
74,126 |
| 1836. |
130,794 |
38,943 |
70,824 |
Duty, at present, 1s. per lb.
OPOBALSAM, is the balsam of Peru in a dry state.
OPOPONAX, is a gum-resin resembling gum ammoniac. It is occasionally used
in medicine.
ORANGE DYE, is given by a mixture of red and yellow dyes in various proportions.
Annotto alone dyes orange; but it is a fugitive colour.
ORCINE, is the name of the colouring principle of the lichen dealbatus. The
lichen dried and pulverized is to be exhausted by boiling alcohol. The solution
filtered hot, lets fall in the cooling, crystalline flocks, which do not belong to the
colouring matter. The supernatant alcohol is to be distilled off, the residuum is to
be evaporated to the consistence of an extract, and triturated with water till this liquid
will dissolve no more. The aqueous solution reduced to the consistence of syrup, and
left to itself in a cool place, lets fall, at the end of a few days, long brown brittle
needles, which are to be freed by pressure from the mother water, and dried. That
water being treated with animal charcoal, filtered and evaporated, will yield a second
crop of crystals. These are orcine. Its taste is sweet and nauseous; it melts readily
in a retort into a transparent liquid, and distils without undergoing any change. It is
soluble in water and alcohol. Nitric acid colours it blood-red; which colour afterwards
disappears. Subacetate of lead precipitates it completely. Its conversion into the archil
red is effected by the action of an alkali, in contact with the air. When dissolved,
for example, in ammonia, and exposed to the atmosphere, it takes a dirty brown red
hue; but when the orcine is exposed to air charged with vapours of ammonia, it assumes
by degrees a fine violet colour. To obtain this result, the orcine in powder should be
placed in a capsule, alongside of a saucer containing water of ammonia; and both
should be covered by a large bell glass; whenever the orcine has acquired a dark[913]
brown cast, it must be withdrawn from under the bell, and the excess of ammonia be
allowed to volatilize. As soon as the smell of ammonia is gone, the orcine is to be dissolved
in water; and then a few drops of ammonia being poured into the brownish
liquid, it assumes a magnificent reddish-violet colour. Acetic acid precipitates the
red lake of lichen.
ORES (Mines, Fr.; Erze, Germ.); are the mineral bodies which contain so much
metal as to be worth the smelting, or being reduced by fire to the metallic state.
The substances naturally combined with metals, which mask their metallic characters,
are chiefly oxygen, chlorine, sulphur, phosphorus, selenium, arsenic, water, and several
acids, of which the carbonic is the most common. Some metals, as gold, silver, platinum,
often occur in the metallic state, either alone, or combined with other metals,
constituting what are called native alloys.
I have described in the article Mine, the general structure of the great metallic
repositories within the earth, as well as the most approved methods of bringing them
to the surface; and in the article Metallurgy, the various mechanical and chemical
operations requisite to reduce the ores into pure metals. Under each particular metal,
moreover, in its alphabetical place, will be found a systematic account of its most
important ores.
Relatively to the theory of the smelting of ores, the following observations may
be made. It is probable that the coaly matter employed in that process is not the
immediate agent of their reduction; but the charcoal seems first of all to be transformed
by the atmospherical oxygen into the oxide of carbon; which gaseous product then
surrounds and penetrates the interior substance of the oxides, with the effect of decomposing
them, and carrying off their oxygen. That this is the true mode of action, is
evident from the well-known facts, that bars of iron, stratified with pounded charcoal,
in the steel cementation-chest, most readily absorb the carbonaceous principle to their
innermost centre, while their surfaces get blistered by the expansion of carburetted
gases formed within; and that an intermixture of ores and charcoal is not always
necessary to reduction, but merely an interstratification of the two, without intimate
contact of the particles. In this case, the carbonic acid which is generated at the lower
surfaces of contact of the strata, rising up through the first bed of ignited charcoal, becomes
converted into carbonic oxide; and this gaseous matter, passing up through the
next layer of ore, seizes its oxygen, reduces it to metal, and is itself thereby transformed
once more into carbonic acid; and so on in continual alternation. It may be laid
down, however, as a general rule, that the reduction is the more rapid and complete, the
more intimate the mixture of the charcoal and the metallic oxide has been, because the
formation of both the carbonic acid and carbonic oxide becomes thereby more easy and
direct. Indeed the cementation of iron bars, into steel will not succeed, unless the
charcoal be so porous as to contain, interspersed, enough of air to favour the commencement
of its conversion into the gaseous oxide; thus acting like a ferment in brewing.
Hence also finely pulverized charcoal does not answer well; unless a quantity of
ground iron cinder or oxide of manganese be blended with it, to afford enough of oxygen
to begin the generation of carbonic oxide gas; whereby the successive transformations
into acid, and oxide, are put in train.
ORPIMENT (Eng. and Fr., Yellow sulphuret of arsenic; Operment, Rauschgelb,
Germ.); occurs in indistinct crystalline particles, and sometimes in oblique rhomboidal
prisms; but for the most part, in kidney and other imitative forms; it has a scaly and
granular aspect; texture foliated, or radiated; fracture small granular, passing into conchoidal;
splintery, opaque, shining, with a weak diamond lustre; lemon, orange, or
honey yellow; sometimes green; specific gravity, 3·44 to 3·6. It is found in floetz
rocks, in marl, clay sand-stone, along with realgar, lead-glance, pyrites, and blende,
in many parts of the world. It volatilizes at the blowpipe. It is used as a pigment.
The finest specimens come from Persia, in brilliant yellow masses, of a lamellar
texture, called golden orpiment.
Artificial orpiment is manufactured chiefly in Saxony, by subliming in cast-iron cucurbits,
surmounted by conical cast-iron capitals, a mixture in due proportions of sulphur
and arsenious acid (white arsenic). As thus obtained, it is in yellow compact opaque
masses, of a glassy aspect; affording a powder of a pale yellow colour. Genuine
orpiment is often adulterated with an ill-made compound; which is sold in this
country by the preposterous name of king’s yellow. This fictitious substance is frequently
nothing else than white arsenic combined with a little sulphur; and is quite
soluble in water. It is therefore a deadly poison, and has been administered with
criminal intentions and fatal effects. I had occasion, some years ago, to examine such
a specimen of king’s yellow, with which a woman had killed her child. A proper
insoluble sulphuret of arsenic, like the native or the Saxon, may be prepared by transmitting
sulphuretted hydrogen gas through any arsenical solution. It consists of 38·09
sulphur, and 60·92 of metallic arsenic, and is not remarkably poisonous. The finest[914]
kinds of native orpiment are reserved for artists; the inferior are used for the indigo
vat. They are all soluble in alkaline lyes, and in water of ammonia.
ORYCTNOGNOSY, is the name given by Werner to the knowledge of minerals;
and is therefore synonymous with the English term Mineralogy.
OSTEOCOLLA, is the glue obtained from bones, by removing the earthy phosphates
with muriatic acid, and dissolving the cartilaginous residuum in water at a
temperature considerably above the boiling point, by means of a digester. It is a very
indifferent article.
OSMIUM, is a metal discovered by Mr. Tennant in 1803, among the grains of native
platinum. It occurs also associated with the ore of iridium. As it has not been applied
to any use in the arts, I shall reserve any chemical observations that the subject may
require for the article Platinum.
OXALATES, are saline compounds of the bases with
OXALIC ACID (Acide oxalique, Fr.; Sauerkleesaüre, Germ.); which is the object
of a considerable chemical manufacture. It is usually prepared upon the small scale by
digesting four parts of nitric acid of specific gravity 1·4, upon one part of sugar, in a glass
retort; but on the large scale, in a series of salt-glazed stoneware pipkins, two-thirds
filled, and set in a water bath. The addition of a little sulphuric acid has been found to
increase the product. 15 pounds of sugar yield fully 17 pounds of the crystalline acid.
This acid exists in the juice of wood sorrel, the oxalis acetosella, in the state of a bi-oxalate;
from which the salt is extracted as an object of commerce in Switzerland, and
sold under the name of salt of sorrel, or sometimes, most incorrectly, under that of salt
of lemons.
Some prefer to make oxalic acid by acting upon 4 parts of sugar, with 24 parts of
nitric acid, of specific gravity 1·220, heating the solution in a retort till the acid begins
to decompose, and keeping it at this temperature as long as nitrous gas is disengaged.
The sugar loses a portion of its carbon, which combining with the oxygen of the nitric
acid, becomes carbonic acid, and escapes along with the deutoxide of nitrogen. The remaining
carbon and hydrogen of the sugar being oxidized at the expense of the nitric acid,
generate a mixture of two acids, the oxalic and the malic. Whenever gas ceases to issue,
the retort must be removed from the source of heat, and set aside to cool; the oxalic acid
crystallizes, but the malic remains dissolved. After draining these crystals upon a filter
funnel, if the brownish liquid be further evaporated, it will furnish another crop of them.
The residuary mother water is generally regarded as malic acid, but it also contains both
oxalic and nitric acids; and if heated with 6 parts of the latter acid, it will yield
a good deal more oxalic acid at the expense of the malic. The brown crystals
now formed being, however, penetrated with nitric, as well as malic acid, must be
allowed to dry and effloresce in warm dry air, whereby the nitric acid will be got rid of
without injury to the oxalic. A second crystallization and efflorescence will entirely
dissipate the remainder of the nitric acid, so as to afford pure oxalic acid at the third
crystallization. Sugar affords, with nitric acid, a purer oxalic acid, but in smaller
quantity, than saw-dust, glue, silk, hairs, and several other animal and vegetable
substances.
Oxalic acid occurs in aggregated prisms when it crystallizes rapidly, but in tables of
greater or less thickness when slowly formed. They lose their water of crystallization
in the open air, fall into powder, and weigh 0·28 less than before; but still retain
0·14 parts of water, which the acid does not part with except in favour of another oxide,
as when it is combined with oxide of lead. The effloresced acid contains 20 per cent. of
water, according to Berzelius. By my analysis, the crystals consist of three prime
equivalents, of water = 27, combined, with one of dry oxalic acid = 36; or in 100
parts, of 42·86 of water with 57·14 of acid. The acid itself consists of 2 atoms of carbon
= 12, + 3 of oxygen = 24; of which the sum is, as above stated, 36. This acid has a
sharp sour taste, and sets the teeth on edge; half a pint of water, containing only 1 gr. of
acid, very sensibly reddens litmus paper. Nine parts of water dissolve one part of the
crystals at 60° F. and form a solution, of spec. grav. 1·045, which when swallowed acts
as a deadly poison. Alcohol also dissolves this acid. It differs from all the other acid
products of the vegetable kingdom, in containing no hydrogen, as I demonstrated (in
my paper upon the ultimate analysis of organic bodies, published in the Phil. Trans.
for 1822), by its giving out no muriatic acid gas, when heated in a glass tube with
calomel or corrosive sublimate.
Oxalic acid is employed chiefly for certain styles of discharge in calico-printing,
(which see), and for whitening the leather of boot-tops. Oxalate of ammonia is an
excellent reagent for detecting lime and its salts in any solution. The acid itself, or the
bi-oxalate of potash, is often used for removing ink or iron-mould stains from linen.
A convenient plan of testing the value of peroxide of manganese for bleachers, &c.,
originally proposed by Berthier, has been since simplified by Dr. Thomson, as follows.
In a poised Florence flask weigh 600 grains of water, and 75 grains of crystallized oxalic[915]
acid; add 50 grains of the manganese, and as quickly as possibly afterwards from 150
to 200 grains of concentrated sulphuric acid. Cover the mouth of the flask with paper,
and leave it at rest for 24 hours. The loss of weight it has now suffered, corresponds
exactly to the weight of peroxide of manganese present; because the quantity of
carbonic acid producible by the reaction of the oxalic acid with the peroxide, is precisely
equal to the weight of the peroxide, as the doctrine of chemical equivalents shows.
OXIDES, are neutral compounds, containing oxygen in equivalent proportion.
OXISELS, are salts, consisting of oxygenated acids and oxides, to distinguish them
from the HALOSELS, which are salts consisting of one of the archæal elements; such as
chlorine, iodine, bromine, &c. combined with metals. See Salt.
OXYGEN (Oxigène, Fr.; Sauerstoff, Germ.); is a body which can be examined
only in the gaseous form; for which purpose it is most conveniently obtained in a pure
state by exposing chlorate of potash, or red oxide of mercury, in a glass retort, or
recurved tube, to the heat of a spirit lamp; 100 grains of the salt yield 115 cubic inches
of gas. One pound of nitre, ignited in an iron retort, gives out about 1200 cubic inches
of oxygen, mixed with a little nitrogen. The peroxide of manganese also affords it,
either by ignition alone in an iron or earthen retort, or by a lamp heat in a glass retort,
when mixed with sulphuric acid. Oxygen is void of taste, colour, and smell. It
possesses all the mechanical properties of the atmosphere. Its specific gravity is
1·1026 compared to air 1·0000; whence 100 cubic inches of it weigh 33·85 grains. Combustibles,
even iron and diamonds, once kindled, burn in it most splendidly. It forms
21 parts in 100 by volume of air, being the constituent essential to the atmospheric
functions of supporting animal and vegetable life, as well as flame.
The full development of this subject in its multifarious relations, will be discussed in
my forthcoming new system of chemistry.
Oxygenated-Muriatic, and Oxymuriatic, are the names originally given by the
French chemists, from false theoretical notions, to chlorine, which Sir H. Davy proved
to be an undecompounded substance.
PACKFONG, is the Chinese name of the alloy called white copper, or German
silver.
PACO, or PACOS, is the Peruvian name of an earthy-looking ore, which consists
of brown oxide of iron, with imperceptible particles of native silver disseminated
through it.
PADDING MACHINE (Machine à plaquer, Fr.; Klatsch, or Grundirmaschine,
Germ.); in calico-printing, is the apparatus for imbuing a piece of cotton cloth
uniformly with any mordant. In fig. 774. A B C D represents in section a cast-iron
frame, supporting two opposite
standards above M, in whose vertical
slot the gudgeons a b, of two copper
or bronze cylinders E F, run; the
gudgeons of E turn upon fixed
brasses or plummer blocks; but the
superior cylinder F rests upon the
surface of the under one, and may
be pressed down upon it with
greater or less force by means of
the weighted lever d e f g, whose
centre of motion is at d, and which
bears down upon the axle of F.
K is the roller upon which the pieces
of cotton cloth intended to be padded
are wound; several of them,
being stitched endwise together.
They receive tension from the action
of a weighted belt o, n, which
passes round a pulley n upon the
end of the roller K. The trough G,
which contains the colouring matter
or mordant, rests beneath the
cylinder upon the table L, or other
convenient support. About two inches above the bottom of the trough, there is a
copper dip-roller C, under which the cloth passes, after going round the guide roller m.
Upon escaping from the trough, it is drawn over the half-round stretcher-bar at I, grooved[916]
obliquely right and left, as shown at N, whereby it acquires a diverging extension
from the middle, and enters with a smooth surface between the two cylinders E F.
These are lapped round 6 or 7 times with cotton cloth, to soften and equalize their
pressure. The piece of goods glides obliquely upwards, in contact with one third of
the cylinder F, and is finally wound about the uppermost roller H. The gudgeon of H
revolves in the end of the radius h, k, which is jointed at k, and movable by a mortise at i
along the quadrantal arc towards l, as the roller K becomes enlarged by the convolutions
of the web. The under cylinder E receives motion by a pulley or rigger upon its opposite
end, from a band connected with the driving-shaft of the printshop. To ensure
perfect equability in the application of the mordant, the goods are in some works passed
twice through the trough; the pressure being increased the second time by sliding the
weight g to the end of the lever d f.
A view of a padding machine in connexion with the driving mechanism is given
under Hot Flue; see also Starching Machine.
PAINTS, GRINDING OF. There are many pigments, such as common orpiment,
or king’s yellow, and verdigris, which are strong poisons; others which are very
deleterious, and occasion dreadful maladies, such as white lead, red lead, chrome yellow,
and vermillion; none of which can be safely ground by hand with the slab and muller,
but should always be triturated in a mill. The emanations of white lead cause, first,
that dangerous disease the colica pictonum, afterwards paralysis, or premature decrepitude
and lingering death.
Figs. 775, 776, 777, 778. exhibit the construction of a good colour-mill in three views;
fig. 775. being an elevation shown upon
the side of the handle, or where the
power is applied to the shaft; fig. 776.
a second elevation, taken upon the side
of the line c, d, of the plan or bird’s-eye
view, fig. 777.
The frame-work A A of the mill is
made of wood or cast iron, strongly
mortised or bolted together; and
strengthened by the two cross iron bars
B, B. Fig. 778. is a plan of the millstones.
The lying or nether millstone C,
fig. 776, is of cast iron, and is channelled
on its upper face like corn millstones. It
is fixed upon the two iron bars B, B;
but may be preferably supported upon
the 3 points of adjustable screws, passing
up through bearing-bars. The
millstone C is surrounded by a large
iron hoop D, for preventing the pasty-consistenced
colour from running over
the edge. It can escape only by the sluice hole E, fig. 776., formed in the hoop; and
is then received in the tub X placed beneath.
[917]
The upper or moving millstone F, is also made of cast iron. The dotted lines indicate
its shape. In the centre it has an aperture with ledges G, G; there is also a ledge
upon its outer circumference, sufficiently high to confine the colour which may occasionally
accumulate upon its surface. An upright iron shaft H passes into the turning
stone, and gives motion to it. A horizontal iron bevel wheel K, figs. 776, 777., furnished
with 27 wooden teeth, is fixed upon the upper end of the upright shaft H. A
similar bevel wheel L, having the same number of teeth, is placed vertically upon the
horizontal iron axis M, M, and works into the wheel K. This horizontal axis M, M bears,
at one of its ends, a handle or winch N, by which the workman may turn the millstone
F; and on the other end of the same axis, the fly-wheel O is made fast, which serves
to regulate the movements of the machine. Upon one of the spokes of the fly-wheel
there is fixed, in like manner, a handle P, which may serve upon occasion for turning
the mill. This handle may be attached at any convenient distance from the centre, by
means of the slot and screw-nut J.
The colour to be ground is put into the hopper R, below which the bucket S is suspended,
for supplying the colour uniformly through the orifice in the millstone G. A
cord or chain T, by means of which the bucket S is suspended at a proper height for
pouring out the requisite quantity of colour between the stones, pulls the bucket obliquely,
and makes its beak rest against the square upright shaft H. By this means the
bucket is continually agitated in such a way as to discharge more or less colour, according
to its degree of inclination. The copper cistern X, receives the colour successively
as it is ground; and, when full, it may be carried away by the two handles Z, Z;
it may be emptied by the stopcock Y, without removing the tub.
PAINTS, VITRIFIABLE. See Porcelain, Pottery, and Stained Glass.
PALLADIUM; a rare metal, possessed of valuable properties; was discovered in
1803, by Dr. Wollaston, in native platinum. It constitutes about 1 per cent. of the
Columbian ore, and from 1⁄4 to 1 per cent. of the Uralian ore of this metal; occurring
nearly pure in loose grains, of a steel-gray colour, passing into silver white, and of a
specific gravity of from 11·8 to 12·14; also as an alloy with gold in Brazil, and combined
with selenium in the Harz near Tilkerode. Into the nitro-muriatic solution of
native platinum, if a solution of cyanide of mercury be poured, the pale yellow
cyanide of palladium will be thrown down, which being ignited affords the metal. This
is the ingenious process of Dr. Wollaston. The palladium present in the Brazilian
gold ore may be readily separated as follows: melt the ore along with 2 or 3 parts of
silver, granulate the alloy, and digest it with heat in nitric acid of specific gravity 1·3.
The solution containing the silver and palladium, for the gold does not dissolve, being
treated with common salt or muriatic acid, will part with all its silver in the form of a
chloride. The supernatant liquor being concentrated and neutralized with ammonia,
will yield a rose-coloured salt in long silky crystals, the ammonia-muriate of palladium,
which being washed in ice-cold water, and ignited, will afford 40 per cent. of metal.
The metal obtained by this process is purer than that by the former; and if it be
fused in a crucible along with borax, by the heat of a powerful air-furnace or forge, a
button of malleable and ductile palladium will be produced. When a slip of it is
heated to redness, it takes a bronze-blue shade of greater or less intensity, as the slip is
cooled more or less slowly; but if it be suddenly chilled, as by plunging it into water,
it resumes instantly its white lustre. This curious phenomenon depending upon oxidizement
and de-oxidizement, in different circumstances, serves at once to distinguish
palladium from platinum.
Pure palladium resembles platinum, but has more of a silver hue; when planished by
the hammer into a cup, such as that of M. Bréant, in the museum of the Mint at Paris,
it is a splendid steel-white metal, not liable, like silver, to tarnish in the air. Another cup
made by M. Bréant, weighing 2 lbs. (1 kilogramme), was purchased by Charles X., and
is now in the garde-meuble of the French crown. The specific gravity of this metal, when
laminated, is stated by Dr. Wollaston at 11·8, and by Vauquelin at 12·1. It melts at
from 150° to 160° Wedgewood; and does not oxidize at a white heat. When a drop of
tincture of iodine, is let fall upon the surface of this metal, and dissipated over a lamp
flame, a black spot remains, which does not happen with platinum. A slip of palladium
has been used with advantage to inlay the limbs of astronomical instruments, where
the fine graduated lines are cut, because it is bright, and not liable to alteration, like
silver.
There are a protoxide and peroxide of palladium. The proto-chloride consists of
60 of metal and 40 of chlorine; the cyanide, of 67 of metal, and 33 of cyanogen.
PALM OIL (Huile de palme, Fr.; Palmöl, Germ.); is obtained, in Guinea and
Guyana, by expressing, as also by boiling, the fruit of the avoira elais. It has an
orange colour, a smell of violets, a bland taste, is lighter than water, melts at 84° Fahr.,
becomes rancid and pale by exposure to air, dissolves in boiling alcohol, and consists of
69 parts of oleine, and 31 of stearine, in 100. It is employed chiefly for making yellow[918]
soap. It may be bleached by the action of either chlorine or oxygen gas, as also by
that of light and heat.
Palm oil, quantity of,
| |
Imported. |
Retained for
consumption. |
Exported. |
| Year. |
Cwts. |
Cwts. |
Cwts. |
| 1835. |
260,151 |
242,733 |
30,915 |
| 1836. |
277,017 |
234,357 |
34,379 |
| 1837. |
223,329 |
214,000 |
|
Duty, 1s. 3d. per cwt.
PAPER CUTTING. Mr. T. B. Crompton, of Farnworth, Lancashire, who obtained
a patent in May, 1821, for proposing to conduct the newly formed web of
paper in the Fourdrinier machine over heated cylinders, for the purpose of drying it
expeditiously, in imitation of the mode so long practised in drying calicoes, obtained,
along with Enoch Miller, another, in May, 1828, for cutting the endless web of paper
lengthwise, by revolving circular blades, fixed upon a roller, parallel to a cylinder,
round which the paper is lapped, and progressively unwound.
A patent had been obtained two months before, for certain improvements in cutting
paper, by Mr. Edward Cowper, consisting of a machine, with a reel on which the web
of paper of very considerable length has been previously wound, in the act of being made
in a Fourdrinier’s machine; this web of paper being of sufficient width to produce
two, three, or more sheets, when cut.
The several operative parts of the machine are mounted upon standards, or frame-work,
of any convenient form or dimensions, and consist: of travelling endless tapes to conduct
the paper over and under a series of guide rollers; of circular rotatory cutters for the
purpose of separating the web of paper into strips equal to the widths of the intended
sheets; and of a saw-edged knife, which is made to slide horizontally for the purpose of
separating the strips into such portions or lengths as shall bring them to the dimensions
of a sheet of paper.
The end of the web of paper from the reel a, fig. 779. is first conducted up an inclined
plane b by hand; it
is then taken hold of
by endless tapes extended
upon rollers,
as in Mr. Cowper’s
Printing Machine,
which see. These endless
tapes carry the
web of paper to the
roller c, which is pressed
against the roller d
by weighted levers,
acting upon the plummer
blocks that its
axle is mounted in.
The second roller d
may be either of
wood or metal, having
several grooves formed
round its periphery
for the purpose of receiving
the edges of the
circular cutters e, (see
Card-cutting) mounted
upon an axle turning
upon bearings in the
standards or frame.
In order to allow the web of paper to proceed smoothly between the two rollers c, d, a
narrow rib of leather is placed round the edges of one or both of these rollers, for the
purpose of leaving a free space between them, through which the paper may pass
without wrinkling.
From the first roller c, the endless tapes conduct the paper over the second d, and then
under a pressing roller f, in which progress the edges of the circular knives e, revolving in
the grooves of the second roller d, cut the web of paper longitudinally into strips of such[919]
widths as may be required, according to the number of the circular cutters and distances
between them.
The strips of paper proceed onward from between the knife roller d and pressing roller
f, conducted by tapes, until they reach a fourth roller g, when they are allowed to descend,
and to pass through the apparatus designed to cut them transversely; that is, into
sheet lengths.
The apparatus for cutting the strips into sheets is a sliding knife, placed horizontally
upon a frame at h, which frame, with the knife e, is moved to and fro by a jointed rod i, connected
to a crank on the axle of the pulley k. A flat board or plate l is fixed to the standard
frame in an upright position, across the entire width of the machine; and this board or
plate has a groove or opening cut along it opposite to the edge of the knife. The paper
descending from the fourth roller g passes against the face of this board, and as the carriage
with the knife advances, two small blocks, mounted upon rods with springs m m, come
against the paper, and hold it tight to the board or plate l, while the edge of the knife is
protruded forward into the groove of that board or plate, and its sharp saw-shaped teeth
passing through the paper, cut one row of sheets from the descending strips; which, on
the withdrawing of the blocks, falls down, and is collected on the heap below.
The power for actuating this machine is applied to the reverse end of the axle, on
which the pulley k is fixed, and a band n, n, n, n, passing from this pulley over tension wheels
o, drives the wheel q fixed to the axle of the knife roller d; hence this roller receives the
rotatory motion which causes it to conduct forward the web of paper, but the other
rollers c and f, are impelled solely by the friction of contact.
The rotation of the crank on the axle of k, through the intervention of the crank-rod i,
moves the carriage h, with the knife, to and fro at certain periods, and when the spring
blocks m come against the grooved plate l, they slide their guide rods into them, while the
knife advances to sever the sheets of paper. But as sheets of different dimensions are
occasionally required, the lengths of the slips delivered between each return of the knife
are to be regulated by enlarging or diminishing the diameter of the pulley k, which will of
course retard or facilitate the rotation of the three conducting rollers, c, d, f, and cause a
greater or less length of the paper to descend between each movement of the knife carriage.
The groove of this pulley k, which is susceptible of enlargement, is constructed of
wedge-formed blocks passed through its sides, and meeting each other in opposite
directions, so that on drawing out the wedges a short distance, the diameter of the pulley
becomes diminished; or by pushing the wedges further in, the diameter is increased;
and a tension wheel p being suspended in a weighted frame, keeps the band always tight.
As it is necessary that the paper should not continue descending while it is held by the
blocks m, m to be cut, and yet that it should be led on progressively over the knife roller d,
the fourth roller g, which hangs in a lever j, is made to rise at that time, so as to take up
the length of paper delivered, and to descend again when the paper is withdrawn. This
is effected by a rod r, connected to the crank on the shaft of the aforesaid roller k, and also
to the under part of the lever j, which lever hanging loosely upon the axle of the knife
roller d, as its fulcrum, vibrates with the under roller g, so as to effect the object in the
way described.
The patentee states that several individual parts of this machine are not new, and that
some of them are to be found included in the specifications of other persons, such as the
circular cutters e, which are employed by Mr. Dickinson (Card-cutting), and the horizontal
cutter h, by Mr. Hansard; he therefore claims only the general arrangement of the parts
in the form of a machine for the purpose of cutting paper, as the subject of his invention.
The machine for cutting paper contrived by John Dickinson, Esq. of Nash Mill,
was patented in January, 1829. The paper is wound upon a cylindrical roller a, fig. 780.,
mounted upon an axle, supported in an iron frame or standard. From this roller the
paper in its breadth is extended over a conducting drum b, also mounted upon an axle
turning in the frame or standard, and after passing under a small guide roller, it
proceeds through a pair of drawing or feeding rollers c, which carry it into the cutting
machine.
[920]
Upon a table d, d, firmly fixed to the floor of the building, there is a series of chisel-edged
knives e, e, e, placed at such distances apart as the dimensions of the cut sheets of paper are
intended to be. These knives are made fast to the table, and against them a series of
circular cutters f, f, f, mounted in a swinging frame g, g, are intended to act. The length of
paper being brought along the table over the edges of the knives, up to a stop h, the
cutters are then swung forwards, and by passing over the paper against the stationary
knives, the length of paper becomes cut into three separate sheets.
The frame g, g, which carries the circular cutters f, f, f, hangs upon a very elevated axle,
in order that its pendulous swing may move the cutters as nearly in a horizontal line as
possible; and it is made to vibrate to and fro by an eccentric, or crank, fixed upon a
horizontal rotatory shaft extending over the drum b, considerably above it, which may be
driven by any convenient machinery.
The workmen draw the paper from between the rollers c, and bring it up to the stop h,
in the intervals between the passing to and fro of the swing-cutters.
The following very ingenious apparatus for cutting the paper web transversely into
any desired lengths, was made the subject of a patent by Mr. E. N. Fourdrinier, in
June, 1831, and has since been performing its duty well in many establishments.
Fig. 781. is an elevation, taken upon one side of the machine; and fig. 782. is a longitudinal
section. a, a, a, a,
are four reels, each covered
with one continuous
sheet of paper; which reels
are supported upon bearings
in the frame-work
b, b, b. c, c, c, is an endless
web of felt-cloth passed
over the rollers d, d, d, d,
which is kept in close contact
with the under side of
the drum e, e, seen best in
fig. 782.
The several parallel
layers of paper to be cut,
being passed between the
drum e, and the endless
felt c, will be drawn off
their respective reels, and
fed into the machine, whenever
the driving-band is slid
from the loose to the fast
pulley upon the end of the
main shaft f. But since
the progressive advance of
the paper-webs must be
arrested during the time of
making the cross cut
through it, the following
apparatus becomes necessary.
A disc g, which
carries the pin or stud of a
crank i, is made fast to the
end of the driving shaft f.
This pin is set in an adjustable
sliding piece, which
may be confined by a screw
within the bevelled graduated
groove, upon the
face of the disc g, at variable
distances from the axis, whereby the eccentricity of the stud i, and of course the
throw of the crank, may be considerably varied. The crank stud i is connected by its
rod j, to the swinging curvilinear rack k, which takes into the toothed wheel l, that
turns freely upon the axle of the feed drum e, e. From that wheel the arms m, m,
rise, and bear one or more palls n, which work in the teeth of the great ratchet wheel
o, o, mounted upon the shaft of the drum e.
The crank-plate g being driven round in the direction of its arrow, will communicate
a see-saw movement to the toothed arc k, next to the toothed wheel l in gearing
with it, and an oscillatory motion to the arms m, m, as also to their surmounting pall n.[921]
In its swing to the left hand, the catch of the pall will slide over the slope of the teeth
of the ratchet wheel o; but in its return to the right hand, it will lay hold of these teeth,
and pull them, with their attached drum, round a part of a revolution. The layers of
paper in close contact with the under half of the drum will be thus drawn forward at
intervals, from the reels, by the friction between its surface and the endless felt, and in
lengths corresponding to the arc of vibration of the pall. The knife for cutting these
lengths transversely is brought into action at the time when the swing arc is making its
inactive stroke, viz., when it is sliding to the left over the slopes of the ratchet teeth o.
The extent of this vibration varies according to the distance of the crank stud i, from
the centre f, of the plate g, because that distance regulates the extent of the oscillations
of the curvilinear rack, and that of the rotation of the drum e, by which the paper is fed
forwards to the knife apparatus. The proper length of its several layers being by the
above described mechanism carried forward over the bed r of the cutting knife or shears
r, v, whose under blade r is fixed, the wiper s, in its revolution with the shaft f, lifts the
tail of the lever t, consequently depresses the transverse movable blade v (as shown in
fig. 783.), and slides the slanting blades across each other obliquely, like a pair of scissors,
so as to cause a clean cut across the plies of paper. But just before the shears begin to
operate, the transverse board u descends to press the paper with its edge, and hold it fast
upon the bed r. During the action of the upper blade v, against the under r, the fall
board u, is suspended by a cord passing across pullies from the arm y of the bell-crank
lever t, t. Whenever the lifter cam s, has passed away from the tail of the bell-crank t,
the weight z, hung upon it, will cause the blade v, and the pinching board u, to be
moved up out of the way of the next length of paper, which is regularly brought forward
by the rotation of the drum e, as above described. The upper blade of the shears
is not set parallel to the shaft of the drum, but obliquely to it, and is, moreover, somewhat
curved, so as to close its edge progressively upon that of the fixed blade. The
blade v may also be set between two guide pieces, and have the necessary motion given
to it by levers.
PAPER-HANGINGS, called more properly by the French, papiers peints. The
art of making paper-hangings, papier de tenture, has been copied from the Chinese,
among whom it has been practised from time immemorial. The English first imported
and began to imitate the Chinese paper-hangings; but being exposed till very lately to a
high excise duty upon the manufacture, they have not carried it to that extent and pitch of
refinement which the French genius has been enabled to do, unchecked by taxation. The
first method of making this paper was stencilling; by laying upon it, in an extended state,
a piece of pasteboard having spaces cut out of various figured devices, and applying different
water colours with the brush. Another piece of pasteboard with other patterns cut out
was next applied, when the former figures were dry, and new designs were thus imparted.
By a series of such operations, a tolerable pattern was executed, but with no
little labour and expense. The processes of the calico printer were next resorted to,
in which engraved blocks of the pear or sycamore were employed to impress the
coloured designs.
Paper-hangings may be distinguished into two classes; 1. those which are really
painted, and which are designed in France under the title of papiers peints, with brilliant
flowers and figures; and 2. those in which the designs are formed by foreign matters
applied to the paper, under the name of papier tontisse, or flock paper.
The operations common to paper-hangings, of both kinds, may be stated as follows:—
1. The paper should be well sized.
2. The edges should be evenly cut by an apparatus like the bookbinder’s press.
3. The ends of each of the 24 sheets which form a piece, should be nicely pasted
together; or a Fourdrinier web of paper should be taken.
4. Laying the grounds, is done with earthy colours or coloured lakes thickened with
size, and applied with brushes.
An expert workman, with one or two children, can lay the grounds of 300 pieces in
a day. The pieces are now suspended upon poles near the ceiling, in order to be dried.
They are then rolled up and carried to the apartment where they are polished, by being
laid upon a smooth table, with the painted side undermost, and rubbed with the
polisher. Pieces intended to be satined, are grounded with fine Paris plaster, instead of
Spanish white; and are not smoothed with a brass polisher, but with a hard brush attached
to the lower end of the swing polishing rod. After spreading the piece upon
the table with the grounded side undermost, the paper-stainer dusts the upper surface
with finely powdered chalk of Briançon, commonly called talc, and rubs it strongly with
the brush. In this way the satiny lustre is produced.
THE PRINTING OPERATIONS.
Blocks about two inches thick, formed of three separate boards glued together, of
which two are made of poplar, and one (that which is engraved) of pear-tree or sycamore,[922]
are used for printing paper-hangings, as for calicoes. The grain of the upper
layer of wood should be laid across that of the layer below. As many blocks are required
as there are colours and shades of colour. To make the figure of a rose, for example,
three several reds must be applied in succession, the one deeper than the other,
a white for the clear spaces, two and sometimes three greens for the leaves, and two
wood colours for the stems; altogether from 9 to 12 for a rose. Each block carries
small pin points fixed at its corners to guide the workman in the insertion of the figure
exactly in its place. An expert hand places these guide pins so that their marks are
covered and concealed by the impression of the next block; and the finished piece shows
merely those belonging to the first and last blocks.
In printing, the workman employs the same swimming-tub apparatus which has been
described under block printing (see Calico-printing), takes off the colour upon his
blocks, and impresses them on the paper extended upon a table in the very same way.
The tub in which the drum or frame covered with calf-skin is inverted, contains simply
water thickened with parings of paper from the bookbinder, instead of the pasty mixture
employed by the calico-printers. In impressing the colour by the block upon the paper,
he employs a lever of the second kind, to increase the power of his arm, making it act
upon the block through the intervention of a piece of wood, shaped like the bridge of a
violin. This tool is called tasseau by the French. A child is constantly occupied in
spreading colour with a brush upon the calf-skin head of the drum or sieve, and in
sliding off the paper upon a wooden trestle or horse, in proportion as it is finished.
When the piece has received one set of coloured impressions, the workman, assisted by his
little aid called a tireur (drawer), hooks it upon the drying-poles under the ceiling. A
sufficient number of pieces should be provided to keep the printer occupied during the
whole at least of one day, so that they will be dried and ready to receive another set of
coloured impressions by the following morning.
All the colours are applied in the same manner, every shade being formed by means
of the blocks, which determine all the beauty and regularity of the design. A pattern
drawer of taste may produce a very beautiful effect. The history of Psyche and Cupid,
by M. Dufour, has been considered a masterpiece in this art, rivalling the productions
of the pencil in the gradation, softness, and brilliancy of the tints.
When the piece is completely printed, the workman looks it all over, and if there be
any defects, he corrects them by the brush or pencil, applying first the correction of one
colour, and afterwards of the rest.
A final satining, after the colours are dried, is communicated by the friction of a finely
polished brass roller, attached by its end gudgeons to the lower extremity of a long
swing-frame; and acting along the cylindrical surface of a smooth table, upon which
the paper is spread.

The fondu or rainbow style of paper-hangings, which I have referred to this place in
the article Calico-printing, is produced by means of an assortment of oblong narrow
tin pans, fixed in a frame, close side to side, each being about one inch wide, two inches
deep, and eight inches long; the colours of the prismatic spectrum, red, orange, yellow,
green, &c., are put, in a liquid state, successively in these pans; so that when the
oblong brush A, B, with guide ledges a, b, c, is dipped into them across the whole of the
parallel row at once, it comes out impressed with the different colours
at successive points e, e, e, e, of its length, and is then drawn by
the paper-stainer over the face of the woollen drumhead, or sieve
of the swimming tub, upon which it leaves a corresponding series of stripes in colours,
graduating into one another like those of the prismatic spectrum. By applying his
block to the tear, the workman takes up the colour in rainbow hues, and transfers these
to the paper. f, f, f, f show the separate brushes in tin sheaths, set in one frame.
At M. Zuber’s magnificent establishment in the antient château of Rixheim, near
Mulhouse, where the most beautiful French papiers peints are produced, and where I
was informed that no less than 3000 blocks are required for one pattern, I saw a
two-colour calico machine employed with great advantage, both as to taste and expedition.
Steam-charged cylinders were used to dry the paper immediately after it was
printed, as the colours, not being so rapidly absorbed as they are by calico, would be
very apt to spread.
The operations employed for common paper-hangings, are also used for making flock
paper, only a stronger size is necessary for the ground. The flocks are obtained from
the woollen cloth manufacturers, being cut off by their shearing machines, called lewises
by the English workmen, and are preferred in a white state by the French paper-hanging
makers, who scour them well, and dye them of the proper colours themselves.
When they are thoroughly stove-dried, they are put into a conical fluted mill, like that
for making snuff, and are properly ground. The powder thus obtained is afterwards
sifted by a bolting-machine, like that of a flour mill, whereby flocks of different degrees
of fineness are produced. These are applied to the paper after it has undergone all the[923]
usual printing operations. Upon the workman’s left hand, and in a line with his
printing table, a large chest is placed for receiving the flock powders: it is seven or
eight feet long, two feet wide at the bottom, three feet and a half at top, and from 15 to
18 inches deep. It has a hinged lid. Its bottom is made of tense calf-skin. This chest
is called the drum; it rests upon four strong feet, so as to stand from 24 to 28 inches
above the floor.
The block which serves to apply the adhesive basis of the velvet-powders, bears in
relief only the pattern corresponding to that basis, which is formed with linseed oil,
rendered drying by being boiled with litharge, and afterwards ground up with white
lead. The French workmen call this mordant the encaustic. It is put upon the cloth
which covers the inverted swimming tub, in the same way as the common colours are,
and is spread with a brush by the tireur (corruptly styled tearer by some English writers).
The workman daubs the blocks upon the mordant, spreads the pigment even with a kind
of brush, and then applies it by impression to the paper. Whenever a sufficient surface
of the paper has been thus covered, the child draws it along into the great chest,
sprinkling the flock powder over it with his hands; and when a length of 7 feet is
printed, he covers it up within the drum, and beats upon the calf-skin bottom with a couple
of rods to raise a cloud of flock inside, and to make it cover the prepared portion of
the paper uniformly. He now lifts the lid of the chest, inverts the paper, and beats its
back lightly, in order to detach all the loose particles of the woolly powder.
By the operation just described, the velvet-down being applied every where of the
same colour, would not be agreeable to the eye, if shades could not be introduced to
relieve the pattern. To give the effect of drapery, for example, the appearance of folds
must be introduced. For this purpose, when the piece is perfectly dry, the workman
stretches it upon his table, and by the guidance of the pins in his blocks, he applies to
the flock surface a colour in distemper, of a deep tint, suited to the intended shades, so
that he dyes the wool in its place. Light shades are produced by applying some of his
lighter water-colours.
Gold leaf is applied upon the above mordant, when nearly dry; which then forms a
proper gold size; and the same method of application is resorted to, as for the ordinary
gilding of wood. When the size has become perfectly hard, the superfluous gold leaf
is brushed off with a dossil of cotton wool or fine linen.
The colours used by the paper-hangers are the following:—
1. Whites. These are either white-lead, good whitening, or a mixture of the two.
2. Yellows. These are frequently vegetable extracts; as those of weld, or of Avignon
or Persian berries, and are made by boiling the substances with water. Chrome yellow
is also frequently used, as well as the terra di Sienna and yellow ochre.
3. Reds are almost exclusively decoctions of Brazil wood.
4. Blues are either prussian blue, or blue verditer.
5. Greens, are Scheele’s green, a combination of arsenious acid, and oxide of copper;
the green of Schweinfurth, or green verditer; as also a mixture of blues and yellows.
6. Violets are produced by a mixture of blue and red in various proportions, or they
may be obtained directly by mixing a decoction of logwood with alum.
7. Browns, blacks, and grays. Umber furnishes the brown tints. Blacks are either
common ivory or Frankfort black; and grays are formed by mixtures of prussian blue
and Spanish white.
All the colours are rendered adhesive and consistent, by being worked up with
gelatinous size or a weak solution of glue, liquefied in a kettle. Many of the colours
are previously thickened, however, with starch. Sometimes coloured lakes are employed.
See Lakes.
PAPER, MANUFACTURE OF. (Papeterie, Fr.; Papiermacherkunst, Germ.)
This most useful substance, which has procured for the moderns an incalculable advantage
over the antients, in the means of diffusing and perpetuating knowledge, seems to have
been first invented in China, about the commencement of the Christian era, and was
thence brought to Mecca, along with the article itself, about the beginning of the 8th
century; whence the Arabs carried it, in their rapid career of conquest and colonization,
to the coasts of Barbary, and into Spain, about the end of the 9th or beginning of the
10th century.
By other accounts, this art originated in Greece, where it was first made from
cotton fibres, in the course of the tenth century, and continued there in common use during
the next three hundred years. It was not till the beginning of the 14th century
that paper was made from linen in Europe, by the establishment of a paper-mill in
1390, at Nuremberg in Germany. The first English paper-mill was erected at Dartford
by a German jeweller in the service of Queen Elizabeth, about the year 1588.
But the business was not very successful; in consequence of which, for a long period
afterwards, indeed till within the last 70 years, this country derived its supplies of fine
writing papers from France and Holland. Nothing places in a more striking light the[924]
vast improvement which has taken place in all the mechanical arts of England since the
era of Arkwright, than the condition of our paper-machine factories now, compared
with those on the Continent. Almost every good automatic paper mechanism at present
mounted in France, Germany, Belgium, Italy, Russia, Sweden, and the United States,
has either been made in Great Britain, and exported to these countries, or has been constructed
in them closely upon the English models.
Till within the last 30 years, the linen and hempen rags from which paper was made,
were reduced to the pasty state of comminution requisite for this manufacture by
mashing them with water, and setting the mixture to ferment for many days in close
vessels, whereby they underwent in reality a species of putrefaction. It is easy to see
that the organic structure of the fibres would be thus unnecessarily altered, nay, frequently
destroyed. The next method employed, was to beat the rags into a pulp by stamping
rods, shod with iron, working in strong oak mortars, and moved by water-wheel machinery.
So rude and ineffective was the apparatus, that forty pairs of stamps were
required to operate a night and a day, in preparing one hundred weight of rags. The
pulp or paste was then diffused through water, and made into paper by methods similar
to those still practised in the small hand-mills.
About the middle of the last century, the cylinder or engine mode, as it is called, of
comminuting rags into paper pulp, was invented in Holland; which was soon afterwards
adopted in France, and at a later period in England.
The first step in the paper manufacture, is the sorting of the rags into four or five
qualities. They are imported into this country chiefly from Germany, and the ports of
the Mediterranean. At the mill they are sorted again more carefully, and cut into
shreds by women. For this purpose a table frame is covered at top with wire cloth,
containing about nine meshes to the square inch. To this frame a long steel blade is
attached in a slanting position, against whose sharp edge the rags are cut into squares
or fillets, after having their dust thoroughly shaken out through the wire cloth. Each
piece of rag is thrown into a certain compartment of a box, according to its fineness;
seven or eight sorts being distinguished. An active woman can cut and sort nearly one
cwt. in a day.
The sorted rags are next dusted in a revolving cylinder surrounded with wire cloth,
about six feet long, and four feet in diameter, having spokes about 20 inches long,
attached at right angles to its axis. These prevent the rags from being carried round
with the case, and beat them during its rotation; so that in half an hour, being pretty
clean, they are taken out by the side door of the cylinder, and transferred to the engine,
to be first washed, and next reduced into a pulp. For fine paper, they should be previously
boiled for some time in a caustic lye, to cleanse and separate their filaments.
The construction of the stuff-engine is represented in figs. 785, 786. Fig. 785. is the longitudinal
section, and fig.
786. the plan of the engine.
The large vat is an oblong
cistern rounded at the angles.
It is divided by the partition
b, b, and the whole inside
is lined with lead. The
cylinder c, is made fast to
the spindle d, which extends
across the engine, and is put
in motion by the pinion p,
fixed to its extremity. The
cylinder is made of wood,
and furnished with a number
of blades or cutters,
secured to its circumference,
parallel to the axis,
and projecting about an
inch above its surface.
Immediately beneath the
cylinder a block of wood k
is placed. This is mounted
with cutters like those of
the cylinder, which in their
revolution pass very near to the teeth of the block, but must not touch it. The distance
between these fixed and moving blades is capable of adjustment by elevating or
depressing the bearings upon which the necks e, e, of the shaft are supported. These
bearings rest upon two levers g, g, which have tenons at their ends, fitted into upright
mortises, made in short beams h, h, bolted to the sides of the engine. The one end of[925]
the levers g, g, is movable, while the other end is adapted to rise and fall upon bolts
in the beams h, h, as centres. The front lever, or that nearest to the cylinder c, is
capable of being elevated or depressed, by turning the handle of a screw (not seen in
this view), which acts in a nut fixed to the tenon of g, and comes up through the top of
the beam h, upon which the head of the screw takes its bearing. Two brasses are let
into the middle of the levers g, g, and form the bearings for the shaft of the engine to
turn upon. The above-mentioned vertical screw is used to raise or lower the cylinder,
and cause it to cut coarser or finer, by enlarging or diminishing the space between the
fixed cutters in the block and those in the cylinder.
To the left hand of i, fig. 785., is a circular breasting made of boards, and covered
with sheet lead; it is curved to fit the cylinder very truly, and leaves but very little
space between the teeth and breasting; at its bottom, the block k is fixed. The engine
is supplied with water from a pump, by a pipe, which delivers it into a small cistern,
near to and communicating with the engine. A stopcock cuts off or regulates the
supply of water at pleasure, and a grating covered with hair-cloth is fixed across that small
cistern, to intercept any filth that may be floating in the water; in other cases a flannel
bag is tied round the nose of the stopcock, to act as a filter.
The rags being put into the engine filled with water, are drawn by the rapid rotation
of the cylinder between the two sets of cutters, whereby they are torn into the finest filaments,
and by the impulsion of the cylinder they are floated over the top of the breasting
upon the inclined plane. In a short time more rags and water are raised into that part
of the engine vat. The tendency in the liquid to maintain an equilibrium, puts the
whole contents of the cistern in slow motion down the inclined plane, to the left hand
of i, and round the partition b, b, (see the arrow), whereby the rags come to the cylinder
again in the space of about 20 minutes; so that they are repeatedly drawn out and
separated in all directions till they are reduced to the appearance of a pulp.
This circulation is particularly useful, by turning over the rags in the engine, causing
them to be presented to the cutter at different angles every time; otherwise, as the
blades always act in one direction, the comminution would not be so complete. The
cutting is performed as follows: The teeth of the block are set somewhat obliquely to
the axes of the cylinder, as shown by fig. 787.; but the teeth of the cylinder c itself are set
parallel to its axis; therefore the cutting edges meet at a
small angle, and come in contact, first at the one end, and
then towards the other, by successive degrees, so that any
rags coming between them, are torn as if between the blades
of a pair of forceps. Sometimes the blades k in the block are
bent to an angle in the middle, instead of being straight and
inclined to the cylinder. These are called elbow plates; their
two ends being inclined in opposite directions to the axis of the cylinder. In either case,
the edges of the plates of the block cannot be straight lines, but must be curved, to adapt
themselves to the curve which a line traced on the cylinder will necessarily have. The
plates or blades are united by screwing them together, and fitting them into a cavity
cut into the wooden block k. Their edges are bevelled away upon one side only.
The block is fixed in its place by being made dovetailed, and truly fitted into the
bottom of the cistern, so that the water will not leak through its junction. The end of
it comes through the woodwork of the chest, and projects to a small distance on its
outside, being kept in its place by a wedge. By withdrawing this wedge, the block
becomes loose, and can be removed in order to sharpen the cutters, as occasion may be.
This is done at a grindstone, after detaching the plates from each other.
The cutters of the cylinder, are fixed into grooves, cut in the wood of the cylinder,
at equal distances asunder, round its periphery, in a direction parallel to its axis. The
number of these grooves is twenty, in the machine here represented. For the washer, each
groove has two cutters put into it; then a fillet of wood is driven fast in between them,
to hold them firm; and the fillets are secured by spikes driven into the solid wood of
the cylinder. The beater is made in the same manner, except that each groove contains
three bars and two fillets.
In the operation of the cylinder, it is necessary that it should be enclosed in a case,
or it would throw all the water and rags out of the engine, in consequence of its great
velocity. This case is a wooden box m, m, fig. 785., enclosed on every side except the
bottom; one side of it rests upon the edge of the vat, and the other upon the edge of the
partition b, b, fig. 786. The diagonal lines m, r, represent the edges of wooden frames, which
are covered with hair or wire cloth, and immediately behind these the box is furnished
with a bottom and a ledge towards the cylinder, so as to form a complete trough. The
square figures under n, n, in fig. 785., show the situation of two openings or spouts
through the side of the case, which conduct to flat lead-pipes, one of which is seen
near the upper g in fig. 786., placed by the side of the vat; the beam being cut away
from them. These are waste pipes to discharge the foul water from the engine; because the[926]
cylinder, as it turns, throws a great quantity of water and rags up against the sieves;
the water goes through them, and runs down to the trough under n, n, and thence
into the ends of the flat leaden pipes, through which it is discharged. o, o, fig. 785.,
are grooves for two boards, which, when put down in their places, cover the hair
sieves, and stop the water from going through them, should it be required in the
engine. This is always the case in the beating engines, and therefore they are seldom
provided with these waste pipes, or at most on one side only; the other side of the cover
being curved to conform to the cylinder. Except this, the only difference between
the washing engine and the beater, is that the teeth of the latter are finer, there being
60 instead of 40 blades in the periphery; and it revolves quicker than the washer, so that
it will tear out and comminute those particles which pass through the teeth of the washer.
In small mills, when the supply of water is limited, there is frequently but one engine,
which may be used both for washing and beating, by adjusting the screw so as to let the
cylinder down and make its teeth work finer. But the system in all considerable works,
is to have two engines at least, or four if the supply of water be great. The power
required for a 5 or 6 vat mill, is about 20 horses in a water-wheel or steam engine.
In the above figures only one engine is shown, namely, the finisher; there is another,
quite similar, placed at its end, but on a level with its surface, which is called the
washer, in which the rags are first worked coarsely with a stream of water, running
through them to wash and open their fibres; after this washing they are called half-stuff,
and are then let down into the bleaching engine, and next into the beating engine,
above described.
By the arrangements of the mill gearing, the two cylinders of the washer and
beater engines make from 120 to 150 revolutions per minute, when the water-wheel
moves with due velocity. The beating engine is always made to move, however,
much faster than the washing one, and nearly in the ratio of the above numbers.
The vibratory noise of a washing engine is very great; for when it revolves 120
times per minute, and has 40 teeth, each of which passes by 12 or 14 teeth in the
block at every revolution, it will make nearly 60,000 cuts in a minute, each of them
sufficiently loud to produce a most grating growling sound. As the beater revolves
quicker, having perhaps 60 teeth, instead of 40, and 20 or 24 cutters in the block, it
will make 180,000 cuts in a minute. This astonishing rapidity produces a coarse
musical humming, which may be heard at a great distance from the mill. From this
statement, we may easily understand how a modern engine is able to turn out a vastly
greater quantity of paper pulp in a day than an old mortar machine.
The operation of grinding the rags requires nice management. When first put
into the washing engine they should be worked gently, so as not to be cut, but only
powerfully scrubbed, in order to enable the water to carry off the impurities. This
effect is obtained by raising the cylinder upon its shaft, so that its teeth are separated
considerably from those of the block. When the rags are comminuted too much in the
washer, they would be apt to be carried off in part with the stream, and be lost; for at
this time the water-cock is fully open. After washing in this way for 20 or 30 minutes,
the bearings of the cylinder are lowered, so that its weight rests upon the cutters. Now
the supply of water is reduced, and the rags begin to be torn, at first with considerable
agitation of the mass, and stress upon the machinery. In about three or four hours,
the engine comes to work very smoothly, because it has by this time reduced the rags
to the state of half-stuff. They are then discharged into a large basket, through which
the water drains away.
The bleaching is usually performed upon the half-stuff. At the celebrated manufactory
of Messrs. Montgolfier, at Annonay, near Lyons, chlorine gas is employed
for this purpose with the best effect upon the paper, since no lime or muriate of lime
can be thus left in it; a circumstance which often happens to English paper, bleached
in the washing engine by the introduction of chloride of lime among the rags, after
they have been well washed for three or four hours by the rotation of the engine. The
current of water is stopped whenever the chloride of lime is put in. From 1 to 2
pounds of that chemical compound are sufficient to bleach 1 cwt. of fine rags, but more
roust be employed for the coarser and darker coloured. During the bleaching operation
the two sliders o, o, fig. 785., are put down in the cover of the cylinder, to prevent
the water getting away. The engine must be worked an hour longer with the chloride
of lime, to promote its uniform operation upon the rags. The cylinder is usually
raised a little during this period, as its only purpose is to agitate the mass, but not to
triturate it. The water-cock is then opened, the boards m, m are removed, and the washing
is continued for about an hour, to wash the salt away; a precaution which ought to
be better attended to than it always is by paper manufacturers.
The half-stuff thus bleached, is now transferred to the beating engine, and worked into
a fine pulp. This operation takes from 4 to 5 hours, a little water being admitted from
time to time, but no current being allowed to pass through, as in the washing engine.[927]
The softest and fairest water should be selected for this purpose; and it should be
administered in nicely regulated quantities, so as to produce a proper spissitude of stuff
for making paper.
For printing paper, the sizing is given in the beating engine, towards the end of its
operation. The size is formed of alum in fine powder, ground up with oil; of which
mixture about a pint and a half are thrown into the engine at intervals, during the last
half-hour’s beating. Sometimes a little indigo blue or smalt is also added, when a
peculiar bloom colour is desired. The pulp is now run off into the stuff chest, where
the different kinds are mixed; whence it is taken out as wanted. The chest is usually
a rectangular vessel of stone or wood lined with lead, capable of containing 300 cubic
feet at least, or 3 engines full of stuff. Many paper-makers prefer round chests, as they
admit of rotatory agitators.
When the paper is made in single sheets, by hand labour, as in the older establishments,
a small quantity of the stuff is transferred to the working-vat by means of a pipe,
and there diluted properly with water. This vat is a vessel of stone or wood, about 5
feet square, and 4 deep, with sides somewhat slanting. Along the top of the vat a board
is laid, with copper fillets fastened lengthwise upon it, to make the mould slide more
easily along. This board is called the bridge. The maker stands on one side; and
has to his left hand a smaller board, one end of which is made fast to the bridge, while
the other rests on the side of the vat. In the bridge opposite to this, a nearly upright
piece of wood, called the ass, is fastened. In the vat there is a copper, which communicates
with a steam pipe to keep it hot; there is also an agitator, to maintain the
stuff in a uniform consistence.
The moulds consist of frames of wood, neatly joined at the corners, with wooden bars
running across, about an inch and a half apart. Across these, in the length of the moulds,
the wires run, from fifteen to twenty per inch. A strong raised wire is laid along each
of the cross bars, to which the other wires are fastened; this gives the laid paper its
ribbed appearance.
The water-mark is made by sewing a raised piece of wire in the form of letters, or any
figured device, upon the wires of the mould, which makes the paper thinner in these
places. The frame-work of a wove mould is nearly the same; but instead of sewing on
separate wires, the frame is covered with fine wire cloth, containing from 48 to 64 meshes
per inch square. Upon both moulds a deckel, or movable raised edge-frame, is used;
which must fit very neatly, otherwise the edges of the paper will be rough.
A pair of moulds being laid upon the bridge, the workman puts on the deckel, brings
the mould into a vertical position, dips it about half way down into the stuff before him,
then turning it into a horizontal position, covers the mould with the stuff and shakes it
gently. This is a very delicate operation; for if the mould be not held perfectly level,
one part of the sheet will be thicker than another. The sheet thus formed has, however,
no coherence; so that by turning the mould, and dipping the wire cloth surface in the
vat, it is again reduced to pulp if necessary. He now pushes the mould along the small
board to the left, and removes the deckel. Here another workman called the coucher
receives it, and places it at rest upon the ass, to drain off some of the water. Meanwhile
the vat-man puts the deckel upon the other mould, and makes another sheet. The
coucher stands to the left side of the vat, with his face towards the vat-man or maker,
on his right is the press furnished with felt cloths, or porous flannels; a three-inch-thick
plank lies before him on the ground. On this he lays a cushion of felts, and on this
another felt; he then turns the paper wire mould, and presses it upon the felt, where
the sheet remains. He now returns the mould by pushing it along the bridge.
The maker has by this time another sheet ready for the coucher; which, like the preceding,
is laid upon the ass, and then couched or inverted upon another felt, laid down
for the purpose.
In this way, felts and paper are alternately stratified, till a heap of six or eight quires
is formed, which is from 15 to 18 inches high. This mass is drawn into the press, and
exposed to a force of 100 tons or upwards. After it is sufficiently compressed, the
machine is relaxed, and the elasticity of the flannel makes the rammer descend (if a
hydraulic press be used) with considerable rapidity. The felts are then drawn out on
the other side by an operative called a layer, who places each felt in succession upon one
board, and each sheet of paper upon another. The coucher takes immediate possession
of the felts for his further operations.
Two men at a vat, and a boy as a layer or lifter, can make about 6 or 8 reams in 10
hours. In the evening the whole paper made during the day, is put into another press,
and subjected to moderate compression, in order to get quit of the mark of the felt, and
more of the water. Next day it is all separated, a process called parting, and being
again pressed, is carried into the loft. Fine papers are often twice parted and pressed,
in order to give them a proper surface.
The next operation is the drying, which is performed in the following way. Posts[928]
about 10 or 12 feet high are erected at the distance of ten feet from each other, and
pierced with holes six inches apart; two spars with ropes stretched between them, at
the distance of 5 inches from one another, called a treble or tribble, are placed about 5
feet high between these posts, supported by pins pushed into the holes in the posts.
The workman takes up 4 or 8 sheets of paper, and puts them upon a piece of wood in
the form of a T; passing this T between the ropes, he shifts the sheets upon them, and
proceeds thus till all the ropes are full. He then raises the treble, and puts another in
its place, which he fills and raises in like manner. Nine or ten trebles are placed
in every set of posts. The sides of the drying-room have proper shutters, which can be
opened to any angle at pleasure.
When the paper is dry, it is taken down, and laid neatly in heaps to be sized. Size
is made of pieces of skin, cut off by the curriers before tanning, or sheep’s feet, or any
other matter containing much gelatine. These substances are boiled in a copper to a
jelly; to which, when strained, a small quantity of alum is added. The workman then
takes about 4 quires of paper, spreads them out in the size properly diluted with water,
taking care that they be equally moistened. This is rather a nice operation. The superfluous
size is then pressed out, and the paper is parted into sheets. After being once
more pressed, it is transferred to the drying-room, but must not be dried too quickly.
Three days are required for this purpose. When the paper is thoroughly dry, it is
carried to the finishing-house, and is again pressed pretty hard. It is then picked by
women with small knives, in order to take out the knots, and separate the perfect from
the imperfect sheets. It is again pressed, given to the finisher, to be counted into
reams, and done up. These reams are compressed, tied up, and sent to the warehouse
for sale. A good finisher can count 200 reams, or 96,000 sheets in a day.
Hot pressing is executed by placing a sheet of paper between two smoothed pasteboards,
alternately, and between every 50 pasteboards a heated plate of iron, and
subjecting the pile to the press. This communicates a fine smooth surface to writing-paper.
The grain of the paper is often disfigured by the felts, when they are too much used,
or when the loose fibres do not cover the twisted thread. The two sides of the felt are
differently raised, and that on which the fibres are longest is applied to the sheets which
are laid down. As the felts have to resist the reiterated action of the press, their warp
should be made stout, of long combed wool, and well twisted. The woof, however,
should be of carded wool, and spun into a soft thread, so as to render the fabric spongy,
and capable of imbibing much water.
This operose and delicate process of moulding the sheets of paper by hand, has for
nearly thirty years past been performed, in many manufactories, by a machine which
produces it in a continuous sheet of indefinite length which is afterwards cut into
suitable sizes, by the Paper-cutting Machine.
In 1799, Louis Robert, then employed in the paper works of Essonne in France, contrived
a machine to make paper of a great size, by a continuous motion, and obtained
for it a patent for 15 years, with a sum of 8000 francs from the French government, as
a reward for his ingenuity. The specification of this patent is published in the second
volume of Brevets d’Invention expirés. M. Leger-Didot, then director of the said works,
bought Robert’s machine and patent for 25,000 francs, to be paid by instalments.
Having become proprietor of this machine, which, though imperfect, contained the germ
of a valuable improvement in paper-making, M. Didot came over with it to England,
where he entered into several contracts for constructing and working it.
Meanwhile M. L. Didot having failed to fulfil his obligations to Robert, the latter
instituted a law-suit, and recovered possession of his patent by a decision dated 23d
June, 1810. Didot then sent over to Paris the Repertory of Arts, for Sept. 1808,
which contained the specification of the English patent, with instructions to a friend to
secure the improved machines described in it, by a French patent. The patent was
obtained, but became inoperative in consequence of M. L. Didot failing to return to
France, as he had promised, so as to mount the patent machine within the two years
required by the French patent law. It was not till 1815, that M. Calla, machine-maker
at Paris, constructed the paper apparatus known in England by the name of
Fourdrinier’s, and which, on the authority of the Dictionnaire Technologique, was very
imperfect in comparison of an English-made machine imported about that time into
France. La construction de ces machines, qui n’offre pourtant rien de difficile, est restée
jusqu’à ce jour exclusivement dans les mains des Anglais, is the painful acknowledgment
made in 1829, for his countrymen, by the author of the elaborate article Papeterie in
that national work. If there be nothing difficult in the construction of these machines,
the French mechanicians ought to be ashamed of forcing their countrymen to seek
the sole supply of them in England; for the principal paper works in France, as
those of MM. Canson, Montgolfier, Thomas Varenne, Firmin Didot, Delcambre, De
Maupeon, &c., are mounted with English-made machines.
[929]
The following, for example, are a few of the paper-mills in France which are mounted
with the self-acting machines of Messrs. Bryan Donkin & Co.:—
- Messrs. Canson, at Annonay.
- M. de la Place, at Jean d’Heures, Bar-le-duc.
- Société anonyme, at Sainte Marie, under M. Delatouche.
- Echarcon près Mennecy, (Seine et Oise).
- Firmin Didot, Mesnil sur l’Estrée.
- M. F. M. Montgolfier, à Annonay.
- Muller, Bouchard, Ondin and Co’s., at Gueures, near Dieppe.
- MM. Richard et Comp. à Plainfoing.
- M. Callot-Bellisle; Vieuze et Chantoiseau.
- M. Bechétaile, near St. Etienne, at Bourg Argental.
It deserves particularly to be remarked, to the honour of English mechanism, that the
proprietors of the first five of the above works received gold medals at the last exposition
of their papers at the Louvre, and all the rest received medals either of silver
or bronze.[37]
The following is a true narrative of the rise and progress of the paper automaton.
M. Leger Didot, accompanied by Mr. John Gamble, an Englishman who had resided
for several years in Paris, obtained permission from the French government, in 1800, to
carry over the small working model of Robert’s continuous machine, with the view of getting
the benefit of English capital and mechanical skill to bring it into an operative state
upon the great scale. Fortunately for the vigorous development of this embryo project,
which had proved an abortion in France, they addressed themselves on the one hand, to
a mercantile firm equally opulent and public spirited, and on the other, to engineers
distinguished for persevering energy and mechanical resource. A first patent was
granted to Mr. Gamble on the 20th of April 1801, and a second, for certain improvements
upon the former, on the 7th of June 1803. In January 1804, Mr. Gamble, for
certain considerations, assigned these two patents to Messrs. Henry and Sealy Fourdrinier,
the house above alluded to, who were at that period, and for several years afterwards,
the most considerable stationers and paper-makers in Great Britain. By an
act of parliament passed on the 4th of August 1807, Mr. Gamble’s privilege of 14 years
from April 1801, was prolonged to 15 years after the date of the act, being an extension
of about 7 years upon the original patent.
The proprietors showed good reasons, in the enormous expense of their experiments,
and the national importance of the object, why the patent should have been extended
14 years from the latter date, and would have obtained justice from parliament in this
respect, but for an unworthy artifice of Lord Lauderdale in the House of Lords. “He,
and he only, was the person who took the objection,” and, by introducing a regulation
in a standing order of the House of Lords, that none but the original inventor should
have an extension, though Mr. H. Fourdrinier was the inventor substantially of the
operative machine, he defeated the honourable intentions of his brother peers, whose
committee said, “We will give seven years, and Mr. Fourdrinier may apply again, if it
should turn out that the seven years that we propose to give to Mr. Fourdrinier
should not give sufficient time to afford any chance of his receiving any remuneration
for the expense that he has incurred in introducing this invention.” The bill passed
in the House of Commons for 14 years, but it was limited by this ruse of Lord Lauderdale
to 7, “who put the standing order upon the books (of the upper house) which
prevented Messrs. Fourdrinier from having any benefit from the invention.”[38]
In February 1808, Mr. Gamble, after losing both his time and money savings during
eight years of irksome diligence, assigned over to Messrs. Fourdrinier the whole right
of his share in the patent to which he was entitled under the act of parliament.
Dartford in Kent, which had been long conspicuous as the seat of a good manufactory
of paper and paper moulds, was selected by the proprietors of the patent as the fittest
place for realizing their plans; and happily for them it possessed, in Mr. Hall’s engineering
establishment, every tool requisite for constructing the novel automaton, and
in his assistant Mr. Bryan Donkin, a young and zealous mechanist, who, combining
precision of workmanship with fertility of invention, could turn his local advantages to
the best account. To this gentleman, aided by the generous confidence of Messrs.
Fourdrinier, the glory of rearing to a stately manhood the helpless bantling of M. L.
Didot is entirely due. In 1803, after nearly three years of intense application, he produced
a self-acting machine for making an endless web of paper, which was erected at
St. Neot’s, under the superintendence of Mr. Gamble, and performed in such a manner
as to surprise every beholder.
Since that important era Mr. Donkin has steadily devoted his whole mind and means[930]
to the progressive improvement of this admirable apparatus; and has, by the unfailing
regularity, precision, promptitude, and productiveness of its work, earned for himself a
place along with Watt, Wedgewood, and Arkwright, in the temple of mechanical
fame.
“La France,” says a late official eulogist of her arts, and interpreter of her sentiments,
“ne craint plus la rivalité des autres peuples pour la fabrication des divers
genres de papiers et de cartons.”[39] After this boast, one would not expect to hear him
immediately confess that in 1823 his country possessed only one manufactory of the
papier continu, containing one of the Fourdrinier machines made at London by Mr.
Donkin, for M. Canson, at Vidalon-les-Annonay; that in 1827 there were only 4 of
these machines in France, and that in 1834 there were not many more than a dozen.
He justly observes, that “this mode being more economical, more rapid, and more
powerful, will become henceforth the only one which can be practised without loss.
Then will disappear the antient system of hand-work, which likewise involved the
inconveniences, we may say dangers, resulting from combinations among the operatives.
The machine-made papers possess many advantages: they can receive, so to speak,
unlimited dimensions; they preserve a perfectly uniform thickness throughout all their
length; they may be fabricated in every season of the year; nor do they require to be
sorted, trimmed, and hung up in the drying-house, operations which occasioned great
waste, amounting to no less than one defective sheet out of every five. The continuous
paper at one time retained the impression of the wire-wove web on its under side; a
defect from which it has been freed by a pressure apparatus of Mr. Donkin, recently
imported from England by M. Delatouche.”
It appears from documents presented to a committee of the House of Lords in 1807,
that the Messrs. Fourdrinier had, by that time, withdrawn from their stationery business
the large sum of 60,000l., to further the object of their patent; so many difficulties did
they encounter in bringing the machinery to its then comparatively complete state, and
so little encouragement or support did they receive from the paper manufacturers
throughout the kingdom.
The patentees laid a statement before the public in 1806, containing the following
comparative estimate of the expense attending seven vats, and that attending a machine
employed upon paper sized in the engine, performing the same quantity of work as
seven vats, at the rate of 12 hours daily.
A MACHINE.
| |
Day. |
Week. |
Month. |
Year. |
| |
s. |
d. |
£ |
s. |
d. |
£ |
s. |
d. |
£ |
s. |
d. |
| 2 |
Journeymen |
3 |
6 |
2 |
2 |
0 |
8 |
8 |
0 |
109 |
4 |
0 |
| 2 |
Ditto |
2 |
6 |
1 |
10 |
0 |
6 |
0 |
0 |
78 |
0 |
0 |
| 2 |
Finishers |
3 |
6 |
2 |
2 |
0 |
8 |
8 |
0 |
109 |
4 |
0 |
| 2 |
Dry workers |
3 |
6 |
2 |
2 |
0 |
8 |
8 |
0 |
109 |
4 |
0 |
| |
Parters (none) |
|
|
|
|
| |
Fire (none) |
|
|
|
|
| |
Felting |
|
|
|
24 |
0 |
0 |
| |
Washing, ditto |
|
|
|
5 |
0 |
0 |
| |
Wire |
|
|
|
200 |
0 |
0 |
| 1 |
Man, to keep in repair the mill and machine |
|
|
|
100 |
0 |
0 |
| Total |
9 |
|
|
7 |
16 |
0 |
31 |
4 |
0 |
734 |
12 |
0 |
| |
£ |
s. |
d. |
| Expense of 7 vats per annum (see next page), is |
2,604 |
12 |
0 |
| A machine doing 7 vats’ work, is, per annum |
734 |
12 |
0 |
| Balance saved by the machine per annum |
£1,870 |
0 |
0 |
| N. B.—There are other advantages, to the amount of full 400l. per annum, of which manufacturers are well aware, although not taken into this calculation. |
[931]
SEVEN VATS.
| |
Day. |
Week. |
Month. |
Year. |
| |
s. |
d. |
£ |
s. |
d. |
£ |
s. |
d. |
£ |
s. |
d. |
| 7 |
Vatmen, at |
3 |
3 |
6 |
16 |
6 |
27 |
6 |
0 |
354 |
18 |
0 |
| 7 |
Couchers |
3 |
1 |
6 |
9 |
6 |
25 |
18 |
0 |
336 |
14 |
0 |
| 7 |
Layers |
3 |
1 |
6 |
9 |
6 |
25 |
18 |
0 |
336 |
14 |
0 |
| 3 |
Finishers |
4 |
0 |
3 |
12 |
0 |
14 |
8 |
0 |
187 |
4 |
0 |
| 6 |
Dry-workers |
3 |
1 |
5 |
11 |
0 |
22 |
4 |
0 |
288 |
12 |
0 |
| 3 |
Men to go to press, &c. |
2 |
6 |
2 |
5 |
0 |
9 |
0 |
0 |
117 |
0 |
0 |
| 7 |
Parters (women) |
1 |
4 |
2 |
16 |
0 |
11 |
4 |
0 |
145 |
12 |
0 |
| |
Fire |
|
7 |
0 |
0 |
28 |
0 |
0 |
364 |
0 |
0 |
| |
Felting |
|
|
|
140 |
0 |
0 |
| |
Washing ditto, oil, soap, fire, &c. |
|
1 |
11 |
6 |
6 |
6 |
0 |
81 |
18 |
0 |
| |
Moulds |
|
|
|
140 |
0 |
0 |
| 1 |
Man, and expenses of repairing, in keeping in order 7 vats, vat-presses, &c. |
|
|
|
112 |
0 |
0 |
| Total |
41 |
persons. |
|
42 |
11 |
0 |
170 |
4 |
0 |
2,604 |
0 |
0 |
In the same statement, it was shown that the expense of making paper by hand is
16s. per cwt., whereas by their machine it is only 3s. 9d.; so that upon 432,000 cwts.
the quantity annually made in Great Britain and Ireland (as founded upon the fact that
one vat can make 480 cwts. of paper, and that there were 900 vats in the kingdom), the
annual saving by the machine would be 264,600l., or 345,600l. - 81,000l.
In a second statement laid before the public in 1807, the patentees observe that their
recently improved machine, from its greater simplicity, may be erected at a considerably
reduced expense. “Mr. Donkin, the engineer, will engage to furnish machines of the
dimensions specified below, with all the present improvements, at the prices specified
below.
| |
Inches. |
If driven by straps. |
£ |
| 3 or 4 |
vats |
30 |
between |
the |
deckles |
715 |
| 6 |
ditto |
40 |
ditto |
|
ditto |
845 |
| 8 |
ditto |
44 |
ditto |
|
ditto |
940 |
| 12 |
ditto |
54 |
ditto |
|
ditto |
995 |
| |
|
If driven by wheels. |
|
| 3 or 4 |
vats |
30 |
between |
the |
deckles |
750 |
| 6 |
ditto |
40 |
ditto |
|
ditto |
880 |
| 8 |
ditto |
44 |
ditto |
|
ditto |
980 |
| 12 |
ditto |
54 |
ditto |
|
ditto |
1,040 |
“Instead of 5 men, formerly employed upon 1 machine, 3 are now (in 1813) fully
sufficient, without requiring that degree of attention and skill which were formerly indispensable.
“In 1806 the machine was capable of doing the work of 6 vats in twelve hours; it
is, however, now capable of doing double that quantity, at one-fourth of the expense.
For by the various improvements enumerated above, the consumption of wire is reduced
nearly one-half, and lasts above double the time; the quantity of paper produced is
doubled; and, taking into consideration the work which is now performed by the men
over and above their immediate attendance upon the machine, it may be fairly stated,
that the number of men is reduced to one-half; consequently the expense of wire and
labour is reduced to one-fourth of what it was.
“The other advantages incidental to the nature of the process of making paper by
this machine, may be classed in the following order:—
“1st. That the paper is much superior in strength, firmness, and appearance, to any
which can be made by hand of the same material.
“2d. It requires less drying, less pressing and parting, and consequently comes sooner
to market; for it receives a much harder pressure from the machine than can possibly
be given by any vat press, and is therefore not only drier, but, on account of the closeness
and firmness of texture, even the moisture which remains is far sooner evaporated,
on exposure to the air, than it would be from the more spungy or bibulous paper made
by hand.
[932]
“The superior pressure, and the circumstance of one side of the paper passing under
the polished surface of one of the pressing rollers, contribute to that smoothness which
in hand-made papers can only be obtained by repeated parting and pressing; consequently
a great part of the time necessarily spent in these operations is saved, and the
paper sooner finished and ready for market.
“3dly. The quantity of broken paper and retree is almost nothing compared with
what is made at the vats.
“4th. The machine makes paper with cold water.
“5th. It is durable, and little subject to be out of repair. The machine at Two
Waters, in Hertfordshire, for the last three years, has not cost 10l. a year in repairs.
“6th. As paper mills are almost universally wrought by streams, which vary considerably
in their power from time to time, there will result from this circumstance a
very important advantage in the adoption of the machine. The common paper mill
being limited by its number of vats, no advantage can be taken of the frequent accessions
of power which generally happen in the course of the year, but, on the contrary, as
scarcely any mills are capable of preparing stuff for twelve vats, every accession of power
to the mill, where a machine is employed, will increase its produce without any additional
expense.
“7th. The manufacturer can suspend or resume his work at pleasure; and he is besides
effectually relieved from the perplexing difficulties and loss consequent upon the
perpetual combinations for the increase of wages.”
It is a lamentable fact, that the attention required to mature this valuable invention,
and the large capital which it absorbed, led ultimately to the bankruptcy of this opulent
and public-spirited company; after which disaster no patent dues were collected, though
twelve suits in Chancery were instituted; these being mostly unsuccessful, on account
of some paltry technical objections made to their well-specified patent, by that unscientific
judge Lord Tenterden. The piratical tricks practised by many considerable
paper-makers against the patentees are humiliating to human nature in a civilized and
soi disant Christian community. Many of them have owned, since the bankruptcy of
the house removed the fear of prosecution, that they owed them from 2000l. to 3000l.
apiece.
Nothing can place the advantage of the Fourdrinier machine in a stronger point of
view, than the fact of there being 280 of them now at work in the United Kingdom,
making collectively 1600 miles of paper, of from 4 to 5 feet broad, every day; that they
have lowered the price of paper 50 per cent., and that they have increased the revenue,
directly and indirectly, by a sum of probably 400,000l. per annum. The tissue paper
made by the machine is particularly useful for communicating engraved impressions to
pottery ware; before the introduction of which there was but a miserable substitute.
Messrs. R. and J. Clewes, of Cobridge potteries, in a letter to Messrs. Fourdrinier, state,
“that had not an improvement taken place in the manufacture of paper, the new style
of engraving would have been of no use, as the paper previously used was of too coarse
a nature to draw from the fair engravings any thing like a clear or perfect impression;
and the Staffordshire potteries, in our opinion, as well as the public at large, are deeply
indebted to you for the astonishing improvement that has recently taken place, both as
regards china and earthenware, more particularly the latter.” The following rates of
prices justify the above statement:—
| |
1814. |
1822. |
1833. |
| |
s. |
d. |
s. |
d. |
s. |
d. |
| Demy pottery tissue |
12 |
0 |
9 |
6 |
7 |
0 |
| Royal |
16 |
3 |
12 |
0 |
8 |
9 |
“We have adopted a new mode of printing on china and earthenware, which, but for
your improved system of making tissue paper, must have utterly failed; our patent machine
requiring the paper in such lengths as were impossible to make on the old plan.
On referring to our present stock, we find we have one sheet of your paper more than
1200 yards long. Signed, Machin and Potts; Burslem, February 25th, 1834.”
I have had the pleasure of visiting more than once the mechanical workshops of
Messrs. Bryan Donkin and Co. in Bermondsey, and have never witnessed a more
admirable assortment of exquisite and expensive tools, each adapted to perform its part
with despatch and mathematical exactness, though I have seen probably the best
machine factories of this country and the Continent. The man of science will appreciate
this statement, and may perhaps be surprised to learn that the grand mural circle of
7 feet diameter, made by Troughton, for the Royal Observatory of Greenwich, was turned
with final truth upon a noble lathe in the said establishment. It has supplied no fewer
than 133 complete automatic paper machines, each of a value of from 1200l. to 2000l.,
to different manufactories, not only in the United Kingdom, but in all parts of the
civilized world; as mentioned in the second paragraph of the present article. Each[933]
machine is capable of making, under the impulsion of any
prime mover, all unmatched by a human eye, and unguided by
a human hand, from 20 to 50 feet in length, by 5 feet broad,
of most equable paper in one minute. Of paper of average
thickness, it turns off 30 feet.
Fig. 788. is an upright longitudinal section, representing
the machine in its most complete state, including the drying
steam cylinders, and the compound channelled rollers of
Mr. Wilks, subsequently to be described in detail. The figure
in the upper line shows it all in train, when the paper is to be
wound up wet upon the reels E, E, which being movable round
the centre l of a swing-bar, are presented empty, time about,
to receive the tender web. The figure in the under line contains
the steam or drying cylinders; the points O, O, of whose
frame, replace, at the points P, P, the wet-reel frame, F, F, P.
A is the vat, or receiver of pulp from the stuff-chest.
B is the knot strainer of
Ibotson (p. 936.), to clear the
pulp before passing on to the
wire.
G is the hog, or agitator in
the vat. The arrows show
the course of the currents of
the pulp in the vat.
I is the apron, or receiver
of the water and pulp which
escape through the endless
wire, and which are returned
by a scoop-wheel into the vat.
b is the copper lip of the vat,
over which the pulp flows to
the endless wire, on a leathern
apron extending from this lip
to about 9 inches over the
wire, to support the pulp and
prevent its escaping.
c, c are the bars which bear
up the small tube rollers that
support the wire.
d, d are ruler bars, to support
the copper rollers over which
the wire revolves.
K is the breast roller, round
which the endless wire turns.
N is the point where the
shaking motion is given to the
machine.
M is the guide roller, having
its pivots movable laterally to
adjust the wire and keep it parallel.
L is the pulp roller, or “dandy,” to press out water, and to
set the paper. r, is the place of the second, when it is used.
H is the first or wet press, or couching rollers; the wire
leaves the paper here, which latter is couched upon the
endless felt p; and the endless wire o returns, passing
round the lower couch roller. By Mr. Donkin’s happy invention
of placing these rollers obliquely, the water runs
freely away, which it did not do when their axes were in a
vertical line.
e, e are the deckles, which form the edges of the sheet
of paper, and prevent the pulp passing away laterally.
They regulate the width of the endless sheet.
f, f are the revolving deckle straps.
R is the deckle guide, or driving-pulley.
g, g are tube rollers, over which the wire passes, which do not
partake of the shaking motion; and,
[934]
h, h are movable rollers for stretching the wire, or brass carriages for keeping the
rollers g, g in a proper position.
C is the second press, or dry press, to expel the water in a cold state.
K, K, &c., in the view of the lower line, are the steam cylinders for drying the endless
sheet.
i, i are rollers to convey the paper.
j, j are rollers to conduct the felt; which serves to support the paper, and prevent it
wrinkling or becoming cockled.
D, D are the hexagonal expanding reels for the steam-dried paper web, one only
being used at a time, and made to suit different sizes of sheets. l is their swing fulcrum.
F, F, F, F, is the frame of the machine.
The deckle straps are worthy of particular notice in this beautiful machine. They
are composed of many layers of cotton tape, each one inch broad, and together one
half-inch thick, cemented with caoutchouc, so as to be at once perfectly flexible and
water-tight.
The upper end of each of the two carriages of the roller L is of a forked shape, and
the pivots of the roller are made to turn in the cleft of the forked carriages in such a
manner, that the roller may be prevented from having any lateral motion, while it
possesses a free vibratory motion upwards and downwards; the whole weight of the roller
L being borne by the endless web of woven wire.
The greatest difficulty formerly experienced in the paper manufacture upon the
continuous system of Fourdrinier, was to remove the moisture from the pulp, and condense
it with sufficient rapidity, so as to prevent its becoming what is called water-galled, and
to permit the web to proceed directly to the drying cylinders. Hitherto no invention has
answered so well in practice to remove this difficulty as the channelled and perforated
pulp rollers or dandies of Mr. John Wilks, the ingenious partner of Mr. Donkin; for
which a patent was obtained in 1830. Suppose one of these rollers (see L, in fig. 788.,
and M, M, in fig. 793.,) is required for a machine which is to make paper 54 inches wide,
it must be about 60 inches long, so that its extremities (see figs. 789. and 790.) may extend
over or beyond each edge of the sheet of paper upon which it is laid. Its diameter may
be 7 inches. About 8 grooves, each 1-16th of an inch wide, are made in every inch of
the tube; and they are cut to half the thickness of the copper, with a rectangularly
shaped tool. A succession of ribs and grooves are thus formed throughout the whole
length of the tube. A similar succession is then made across the former, but of 24 in
the inch, and on the opposite surface of the metal, which by a peculiar mode of management
had been prepared for that purpose. As the latter grooves are cut as deep as the
former, those on the inside meet those on the outside, crossing each other at right angles,
and thereby producing so many square holes; leaving a series of straight copper ribs on
the interior surface of the said tube, traversed by another series of ribs coiled round
them on the outside, forming a cylindrical sieve made of one piece of metal. The
rough edges of all the ribs must be rounded off with a smooth file into a semicircular
form. Figs. 789. and 790., A A, are portions
of the ribbed copper tube. Fig. 789.
shows the exterior, and fig. 790. the interior
surface; b, b and b, b show the plain
part at each of the ends, where it is made
fast to the brass rings by rivets or screws;
C, C are the rings with arms, and a centre
piece in each, for fixing the iron pivot or
shaft B; one such pivot is fixed by riveting
it in each of the centre pieces of the
rings, as shown at c, fig. 790.; so that
both the said pieces shall be concentric
with the rings, and have one common
axis with each other, and with the roller.
At a, a, a groove is turned in each of the
pivots, for the purpose of suspending a
weight by a hook, in order to increase
the pressure upon the paper, whenever it
may be found necessary.
Fig. 791. is an end view, showing the
copper tube and its internal ribs A, A; the
brass rings C, C; arm D, D, D; centre piece E,
and pivot B. Fig. 792. is a section of the
said ring, with the arms, &c.
The roller is shown at L, fig. 788., as
lying upon the surface of the wire-web.[935]
The relative position of that perforated roller, and the little roller b, over which
it lies, is such that the axis of L is a little to one side of the axis of b, and not in the
same vertical plane, the latter being about an inch nearer the vat end. Hence, whenever
the wire-web is set in progressive motion, it will cause the roller L to revolve upon
its surface; and as the paper is progressively made, it will pass onwards with the web
under the surface of the roller. Thus the pulpy layer of paper is condensed by
compression under the ribbed roller; while it transmits its moisture through the
perforations, it becomes sufficiently compact to endure the action of the wet press
rollers H, H, and also acquires the appearance of parallel lines, as if made by hand in a
laid mould.
Mr. Wilks occasionally employs a second perforated roller in the same paper machine,
which is then placed at the dotted lines i, i, i.
The patentee has described in the same specification a most ingenious modification of
the said roller, by which he can exhaust the air from a hollowed portion of its periphery,
and cause the paper in its passage over the roller to undergo the sucking operation of
the partial void, so as to be remarkably condensed; but he has not been called upon to
apply this second invention, in consequence of the perfect success which he has experienced
in the working of the first.
The following is a more detailed illustration of Mr. Wilks’ improved roller.
Fig. 793. represents two parts of his double-cased exhausting cylinder.
This consists of two copper tubes,
one nicely lining the other; the inner
being punched full of round holes, as
at K, K, where that tube is shown uncovered:
a portion of the inner surface
of the same tube is shown at
L, L. In this figure also, two portions
of the outer tube are shown at M, M,
and N, N; the former being an external,
and the latter an internal view. Here
we see that the external tube is the
ribbed perforated one already described;
the holes in the inner tube being made
in rows to correspond with the grooves
in the outer. The holes are so distributed
that every hole in one row shall
be opposite to the middle of the space
left between two holes in the next row,
as will appear from inspection of the
figure. The diameter of each of the
punched holes somewhat exceeds the
width of each rib in the inside of the
outer cylinder, and every inside groove
of this tube coincides with a row of
holes in the former, which construction
permits the free transudation or percolation
of the water out of the pulp. At each end of this double-case cylinder, a part
is left at N, N, plain without, and grooved merely in the inside of the outer tube. The
smooth surface allows the brass ends to be securely fixed; the outer edge of the brass
ring fits tight into the inside of the end of the cylinders.
On the inside of each of these rings there are four pieces which project towards the
centre or axis of the cylinder; two of which pieces are shown at a, a, fig. 793. in
section. b, b, is a brass ring with four arms c, c, c, c, and a boss or centre piece d, d.
The outer edge of the last-mentioned ring is also turned cylindrical, and of such a
diameter as to fit the interior of the former ring o, o. The two rings are securely
held together by four screws. e, e is the hollow iron axle or shaft upon which the
cylinder revolves. Its outside is made truly cylindrical, so as to fit the circular holes
in the bosses d, d, of the rings and arms at each end of the cylinder. Hence, if the
hollow shaft be so fixed that it will not turn, the perforated cylinder is capable of
having a rotatory motion given to it round that shaft. This motion is had recourse to,
when the vacuum apparatus is employed. But otherwise the cylinder is made fast to
the hollow axle by means of two screw clamps. To one end of the cylinder, as at p, a
toothed wheel is attached, for communicating a rotatory motion to it, so that its surface
motion shall be the same as that of the paper web; otherwise a rubbing motion might
ensue, which would wear and injure both.
The paper stuff or pulp is allowed to flow from the vat A, fig. 788., on to the surface of
the endless wire-web, as this is moving along. The lines o, o, fig. 788. show the course of[936]
the motion of the web, which operates as a sieve, separating to a certain degree the water
from the pulp, yet leaving the latter in a wet state till it arrives at the first pair of pressing
rollers H, H, between which the web with its sheet of paper is squeezed. Thick
paper, in passing through these rollers, was formerly often injured by becoming water-galled,
from the greater retention of water in certain places than in others. But
Messrs. Donkin’s cylinder, as above described, has facilitated vastly the discharge of the
water, and enabled the manufacturer to turn off a perfectly uniform smooth paper.
In fig. 788., immediately below the perforated cylinder, there is a wooden water-trough.
Along one side of the trough a copper pipe is laid, of the same
length as the cylinder, and parallel to it; the distance between them being about
one fourth of an inch. The side of the pipe facing the cylinder is perforated with a
line of small holes, which transmit a great many jets of water against the surface of
the cylinder, in order to wash it and keep it clean during the whole continuance of the
process.
The principle adopted by John Dickinson, Esq., of Nash Mill, for making paper,
is different from that of Fourdrinier. It consists in causing a polished hollow
brass cylinder, perforated with holes or slits, and covered with wire cloth, to revolve
over and just in contact with the prepared pulp; so that by connecting the cylinder
with a vessel exhausted of its air, the film of pulp, which adheres to the cylinder
during its rotation, becomes gently pressed, whereby the paper is supposed to be rendered
drier, and of more uniform thickness, than upon the horizontal hand moulds,
or travelling wire cloth of Fourdrinier. When subjected merely to agitation, the water
is sucked inwards through the cylindric cage, leaving the textile filaments so completely
interwoven as, if felted among each other, that they will not separate without breaking,
and, when dry, they will form a sheet of paper of a strength and quality relative to the
nature and preparation of the pulp. The roll of paper thus formed upon the hollow
cylinder is turned off continuously upon a second solid one covered with felt, upon
which it is condensed by the pressure of a third revolving cylinder, and is thence delivered
to the drying rollers.
Such is the general plan of Mr. Dickinson’s paper machines, into which he has introduced
numerous improvements since its invention in 1809, many of them secured by
patent right; whereby he has been enabled to make papers of first-rate quality, more
particularly for the printing-press. See infrà.
In July 1830, Mr. Ibotson of Poyle, paper manufacturer, obtained a patent, see B, fig.
788., which has proved very successful, for a peculiar construction of a sieve or strainer. Instead
of wire meshes, he uses a series of bars of gun-metal, laid in the bottom of a box, very
closely together, so that the upper surfaces or the flat sides may be in the same plane,
the edge of each bar being parallel with its neighbour, leaving parallel slits between them
of from about 1-70th to 1-100th of an inch in width, according to the fineness or coarseness
of the paper-stuff to be strained. As this stuff is known to consist of an assemblage of
very fine flexible fibres of hemp, flax, cotton, &c., mixed with water, and as, even in
the pulp of which the best paper is made, the length of the said fibres considerably exceeds
the diameter of the meshes of which common strainers are formed, consequently
the longest and most useful fibres were formerly lost to the paper manufacturer. Mr. Ibotson’s
improved sieve is employed to strain the paper-stuff previously to its being used in
the machine above described. (see its place at B in the vat.) When the strainer is at
work, a quick vertical and lateral jogging motion is given to it, by machinery similar to
the joggling-screens of corn mills.
Since the lateral shaking motion of the wire-web in the Fourdrinier machine, as originally
made, was injurious to the fabric of the paper, by bringing its fibres more closely
together breadthwise than lengthwise, thus tending to produce long ribs, or thick
streaks in its substance, Mr. George Dickinson, of Buckland Mill, near Dover, proposed,
in the specification of a patent obtained in February, 1828, to give a rapid up-and-down
movement to the travelling web of pulp. He does not, however, define with much precision
any proper mechanism for effecting this purpose, but claims every plan which
may answer this end. He proposes generally to mount the rollers, which conduct the
horizontal endless web, upon a vibrating frame. The forepart of this frame is attached,
to the standards of the machine, by hinge joints, and the hinder part, or that upon
which the pulp is first poured out, is supported by vertical rods, connected with a
crank on a shaft below. Rapid rotatory motion being given to this crank-shaft, the
hinder part of the frame necessarily receives a quick up-and-down vibratory movement,
which causes the water to be shaken out from the web of pulp, and thus sets the fibres
of the paper with much greater equality than in the machines formerly constructed. A
plan similar to this was long ago introduced into Mr. Donkin’s machines, in which the
vibrations were actuated in a much more mechanical way.
John Dickinson, Esq., of Nash Mill, obtained a patent in October, 1830, for a method of
uniting face to face two sheets of pulp by means of machinery, in order to produce paper[937]
of extraordinary thickness. Two vats are to be supplied with paper stuff as usual; in
which two hollow barrels or drums are made to revolve upon axles driven by any first
mover; an endless felt is conducted by guide rollers, and brought into contact with the
drums; the first drum gives off the sheet of paper pulp from its periphery to the felt,
which passing over a pressing roller, is conducted by the felt to that part of a second
drum which is in contact with another pressing roller. A similar sheet of paper pulp
is now given off from the second drum, and it is brought into contact with the former
by the pressure of its own roller. The two sheets of paper pulp thus united are carried
forward by the felt over a guide roller, and onward to a pair of pressing rollers, where
by contact the moist surfaces of the pulp are made to adhere, and to constitute one
double thick sheet of paper, which, after passing over the surfaces of hollow drums,
heated by steam, becomes dry and compact. The rotatory movements of the two pulp-lifting
drums must obviously be simultaneous, but that of the pressing rollers should be
a little faster, because the sheets extend by the pressure, and they should be drawn forward
as fast as they are delivered, otherwise creases would be formed. Upon this
invention is founded Mr. Dickinson’s ingenious method of making safety-paper for
Post-office stamps, by introducing silk fibres, &c., between the two laminæ.
The following contrivance of the same inventive manufacturer is a peculiarly elegant
mechanical arrangement, and is likely to conduce to the perfection of machine-made
paper. I have already described Mr. Ibotson’s excellent plan of parallel slits, or gridiron
strainers, which has been found to form paper of superior quality, because it
permits all the elongated tenacious fibres to pass, which give strength to the paper,
while it intercepts the coarser knots and lumps of the paste, that were apt to spoil its
surface. Mr. Turner’s circular wire sieves, presently to be noticed, may do good work,
but they cannot compete with Mr. Dickinson’s present invention, which consists in
causing the diluted paper pulp to pass between longitudinal apertures, about the hundred-and-fifteenth
part of an inch wide, upon the surface of a revolving cylinder.
The pulp being diluted to a consistency suitable for the paper machine, is delivered
into a vat, of which the level is regulated by a waste pipe, so as to keep it nearly full.
From this vat there is no other outlet for the pulp, except through the wire-work periphery
of the revolving cylinder, and thence out of each of its ends into troughs placed
alongside, from which it is conducted to the machine destined to convert it into a
paper web.
The revolving cylinder is constructed somewhat like a squirrel cage, of circular rods,
or an endless spiral wire, strengthened by transverse metallic bars, and so formed that the
spaces between the rings are sufficient to allow the slender fibres of the pulp to pass
through, but are narrow enough to intercept the knots and other coarse impurities,
which must of course remain, and accumulate in the vat. The spaces between the
wires of the squirrel cage may vary from the interval above stated, which is intended
for the finest paper, to double the distance for the coarser kinds.
It has been stated that the pulp enters the revolving cylinders solely through the
intervals of the wires in the circumference of the cylinder; these wires or rods are about
three-eighths of an inch broad without, and two-eighths within, so that the circular slits
diverge internally. The rods are one quarter of an inch thick, and are riveted to the
transverse bars in each quadrant of their revolution, as well as at their ends to the
necks of the cylinder.
During the rotation of the cylinder, its interstices would soon get clogged with the
pulp, were not a contrivance introduced for creating a continual vertical agitation in
the inside of the cylinder. This is effected by the up-and-down motion of an interior
agitator or plunger, nearly long enough to reach from the one end of the cylinder to the
other, made of stout copper, and hollow, but water-tight. A metal bar passes through
it, to whose projecting arm at each end a strong link is fixed; by these two links it is
hung to two levers, in such a way that when the levers move up and down, they raise
and depress the agitator, but they can never make it strike the sides of the cylinder.
Being heavier than its own bulk of water, the agitator, after being lifted by the levers,
sinks suddenly afterwards by its weight alone.
The agitator’s range of up-and-down movement should be about one inch and a
quarter, and the number of its vibrations about 80 or 100 per minute; the flow of the
pulp through the apertures is suddenly checked in its descent, and promoted in its
ascent, with the effect of counteracting obstructions between the ribs of the cylinder.
The sieve cylinder has a toothed wheel fixed upon the tubular part of one of its ends,
which works between two metal flanches made fast to the wooden side of the vat, for
the purpose of keeping the pulp away from the wheel; and it is made to revolve by a
pinion fixed on a spindle, which going across the vat, is secured by two plummer blocks
on the outside of the troughs, and has a rotatory motion given to it by an outside rigger
or pulley, by means of a strap from the driving shaft, at the rate of 40 or 50 revolutions
per minute. This spindle has also two double eccentrics fixed upon it, immediately[938]
under the levers, so that in every revolution it lifts those levers twice, and at the same
time lifts the agitator.
The diameter of the sieve cylinder is not very material, but 14 inches have been
found a convenient size; its length must be regulated according to the magnitude of
the machine which it is destined to supply with pulp. One, four feet long in the cage
part, is sufficient to supply a machine of the largest size in ordinary use, viz., one capable
of making paper 4 feet 6 inches wide. When the cylinder is of this length, it should
have a wheel and pinion at each end.
Metal flanches are firmly fixed to the sides of the vat, with a water-tight joint, and
form the bearings in which the cylinder works.
Mr. Turner of Bermondsey, paper-maker, obtained a patent in March, 1831, for a
peculiar strainer, designed to arrest the lumps mixed with the finer paper pulp, whereby
he can dispense with the usual vat and hog in which the pulp is agitated immediately
before it is floated upon the endless wire-web of the Fourdrinier apparatus. His strainer
may also be applied advantageously to hand paper machines. He constructs his sieves
of a circular form, by combining any desirable number of concentric rings of metal,
with small openings between them, from the 50th to the 100th part of an inch wide. In
order to facilitate the passage of the fine pulp and water, the sieves receive a vibratory
motion up and down, which supersedes the hog employed in other paper-making
machines.
A mechanism to serve the same purpose as the preceding, in which Mr. Ibotson’s
plan of a parallel rod-strainer is modified, was made the subject of a patent by
Mr. Henry Brewer, of Surrey Place, Southwark, in March, 1832. He constructs
square boxes with gridiron bottoms, and gives a powerful up-and-down vibration in the
pulp tub, by levers, rotatory shafts, and cranks.
As the contrivance is not deficient in ingenuity, and may be useful, I shall describe this
mode of adapting his improved strainers to a vat in which paper is to be made by hand
moulds. A hog (or churning rotator) is employed for the purpose of agitating the
pulp at the bottom of the vat, in which the sieve is suspended from a crank-shaft, or
in any other way, so as to receive the up-and-down vibratory motion for the purpose of
straining the pulp. The pulp may be supplied from a chest, and passed through a cock
into a trough, by which it is conveyed to the strainers.
A pipe from the bottom of the vat leads into a lifter-box, which is designed to convey
thin pulp into the sieve, in order to dilute that which is delivered from the chest. This
pipe also allows the small lumps, called rolls, to be re-sifted. The pressure of the pulp
and water in the vat forces the pulp up the pipe into the lifter-box, whence it is taken
by rotatory lifters, and discharged into a trough, where it runs down and mixes with
the thick pulp from the chest, as before mentioned. By these means the contents of
the vat are completely strained or sifted over again in the course of almost every
hour.
A patent was obtained for a paper-pulp strainer by Mr. Joseph Amies, of Loose, in
the county of Kent, paper manufacturer, who makes the bottoms of his improved
strainers with plates of brass or other suitable metal, and forms the apertures for the fine
fibres of pulp to pass through, by cutting short slits through such plates, taking care
that as much metal is left between the ends of each short slit and the next following
as will properly brace or stiffen the ribs of the strainer; and he prefers that the end of
one slit shall be nearly opposite to the middle of the two slits next adjoining it, which
is commonly called blocking the joints. This is for giving rigidity to the bottom of the
strainer, and constitutes the main feature of his improvement. The bottoms of sieves
previously constructed with long metallic rods, he considers to be liable to lateral
vibration in use, and thus to have permitted knots and lumps to pass through their
expanded intervals. This objection is not applicable to Mr. Dickinson’s squirrel-cage
strainer, of which the ribs may be made rigid by a sufficient number of transverse bars;
nor in fact is it applicable to Mr. Ibotson’s original strainer, as it is admirably constructed
by Messrs. Donkin and Co. Each bar which they make being inflexible by a
feathered rib, is rendered perfectly straight in its edge by grinding with emery upon a
flat disc-wheel of block tin, and of invariable length, by a most ingenious method of
turning each set of bars in a lathe. The bars are afterwards adjusted in the metallic
sieve-frame, or chest, at any desired distance apart, from the 120th to the 60th of an
inch, in such a manner as secures them from all risk of derangement by the vibratory
or jogging motion in shaking the pulpy fibres through the lineal intervals between
them.
Mr. James Brown, paper manufacturer, of Esk mills, near Edinburgh, obtained a
patent in May, 1836, for a particular mode of applying suction to the pasty web in
the Fourdrinier’s machine. He places a rectangular box transversely beneath the horizontal
wire cloth, without the interposition of any perforated covering, such as had been[939]
tried in the previously constructed vacuum machines, and which he considers to have
impeded their efficacy in condensing the pulp and extracting the water.
Upon this and all similar contrivances for making a partial vacuum under the pulpy
paper web, it may be justly remarked, that they are more apt to injure than improve
the texture of the article; since when the suction is unequally operative, it draws down not
only the moisture, but many of the vegetable fibres, causing roughnesses, and even
numerous small perforations in the paper.
A modification of Mr. Dickinson’s cylinder-mould continuous paper machine was
made the subject of a patent in Nov. 1830, by Mr. John Hall, jun., of Dartford, as
communicated to him by a foreigner residing abroad. The leading feature of the
invention is a mode of supplying the vat in which the wire cylinder is immersed with a
copious flow of water, for the purpose of creating a considerable pressure upon the
external surface of the cylinder, and thereby causing the fibres of the paper pulp to
adhere to the mould.
There is a semi-cylindrical trough, in which the mould is immersed, and made to
revolve by any convenient means. The pulp is transferred from the vat into that vessel
at its bottom part. On the side of the drum-mould opposite to the vat, there is a cistern
into which a copious flow of water is delivered, which passes thence into the semi-cylindrical
trough. In the interior of the cylindrical mould, a bent or syphon tube is
introduced, on the horizontal part of which tube, inside, the mould revolves. This
tube is connected at the outside to a pump, by which the water is drawn from the interior
of the cylindrical mould. Thus the water in the semi-cylindrical trough, on the
outside of the drum, is kept at a considerably higher level than it is within; and consequently
the pressure of the water, as it passes through the wire gauze, will, it is supposed,
cause the fibres of the paper pulp to adhere to the circumference of the mould.
The water which is withdrawn from the interior of the drum by the recurved tube, is conducted
round into the cistern, where its discharge is impeded by several vertical partitions,
which make the water flow in a gentle stream into the semi-cylindrical mould vat.
In order to keep the pulp properly agitated in the mould vat, a segment frame, having
rails extended across the vat, is moved to and fro; as the drum mould goes round, the
fibres of the pulp are forced against its circumference, and as the water passes through,
the fibres adhere, forming the sheet of paper, which, on arriving at a couching roller
above, is taken up as usual by an endless felt, conducted away to the drying apparatus,
and thence to the reel to be wound up.
The patentee claims merely the application of a pump to draw the water from the
interior of the mould drum, and to throw it upon its external surface.
A rag-cutting and lacerating machine was patented by Mr. Henry Davy, of Camberwell,
in September, 1833, being a communication from a foreigner residing abroad.
The machine consists of an endless feeding-cloth, by which the rough rags supplied by
the attendants are progressively conducted forwards to a pair of feed-rollers (see Cotton,
spinning), and on passing through these rollers, the rags are subjected to the operation
of rotatory cutters, acting against a fixed or ledger blade, which cut and tear them to
pieces. Thence the rags pass down an inclined sieve, upon which they are agitated to
separate the dust. The cleaned fragments are delivered on to a horizontal screen or
sorting table, to suffer examination. When picked here, they are ready for the pulp-engine.
A distinct representation of this machine is given in Newton’s Journal,
conjoined series, vol. iv. pl. IX. fig. 1.
Mr. Jean Jacques Jequier obtained a patent in August, 1831, for a mode of making
paper on the continuous machine with wire-marks. The proposed improvement
consists merely in the introduction of a felted pressing roller, to act upon the paper after
it has been discharged from the mould, and need not therefore be particularly described.
In August, 1830, Mr. Thomas Barratt, paper-maker, of St. Mary Cray, in the county
of Kent, obtained a patent for an apparatus by which paper may be manufactured in a
continuous sheet, with the water-mark and maker’s name, so as to resemble in every
respect paper made by hand, in moulds the size of each separate sheet. On the wire
web, at equal distances apart, repetitions of the maker’s name or other device is placed,
according to the size of the paper when cut up into single sheets. In manufacturing
such paper, the ordinary method of winding upon a reel cannot be employed; and
therefore the patentee has contrived a compensating reel, whose diameter diminishes at
each revolution, equal to the thickness of a sheet of paper. See Newton’s Journal,
C. S. vol. vii. p. 285.
For Mr. Lemuel Wellman Wright’s series of improvements in the manufacture of
paper, specified in his patent of November, 1834, I must refer to the above Journal,
C. S., vol. viii. p. 86.
A committee of the Société d’Encouragement, of Paris, made researches upon the best
composition for sizing paper in the vat, and gave the following recipe:—
[940]
| 100 |
kilogrammes |
of |
dry paper stuff. |
| 12 |
— |
|
starch. |
| 1 |
— |
|
rosin, previously dissolved in 500 grammes of carbonate of soda. |
| 18 |
pails of water. |
M. Braconnot proposed the following formula in the 23d volume of the Annales de
Chimie et de Physique:—To 100 parts of dry stuff, properly diffused through water,
add a boiling uniform solution of 8 parts of flour, with as much caustic potash as will
render the liquor clear. Add to it one part of white soap previously dissolved in hot
water. At the same time heat half a part of rosin with the requisite quantity of weak
potash lye for dissolving the rosin; mix both solutions together, and pour into them
one part of alum dissolved in a little water.
Those who colour prints, size them previously with the following composition:—4
ounces of glue, and 4 ounces of white soap dissolved in 3 English pints of hot water.
When the solution is complete, two ounces of pounded alum must be added, and as soon as
the composition is made homogeneous by stirring, it is ready for use. It is applied cold
with a sponge, or rather with a flat camel’s hair brush. Ackermann’s liquor, as
analyzed by Vauquelin, may be made for sizing paper as follows:—
| 100 |
kilogrammes |
of |
dry stuff. |
| 4 |
— |
|
glue. |
| 8 |
— |
|
resinous soap. |
| 8 |
— |
|
alum. |
The soap is made from 4·8 kilos. of pounded rosin, and 2·22 crystals of carbonate of
soda, dissolved in 100 litres of water. It is then boiled till the mixture becomes quite
uniform; the glue, previously softened by 12 hours’ maceration in cold water, is to be
next added; and when this is totally dissolved, the solution of alum in hot water is
poured in. Three quarts of this size were introduced into the vat with the stuff, and
well mixed with it. The paper manufactured with this paste seemed to be of excellent
quality, and well sized.
The Chinese, in manufacturing paper, sometimes employ linen rags, as we do; at other
times, the fibres of the young bamboo; of the mulberry; the envelope of the silk-worm
cocoon; also a tree, unknown to our botanists, which the natives call chu or ko-chu; cotton
down, and especially the cotton tree. The processes pursued in China to make paper
with the inner bark of their paper-tree (Broussonetia-papyrifera,) or Chinese mulberry,
have been described at great length in the bulletin of the Société d’Encouragement,
for 1826, p. 226; but they will hardly prove serviceable to a European manufacturer.
That tree has been acclimated in France.
Chinese paper is not so well made as the good paper of Europe; it is not so white,
it is thinner, and more brittle, but extremely soft and silky. The longitudinal tenacity
of its filaments, however, renders it fitter for the engraver than our best paper. The Chinese,
after triturating, grinding, and boiling the bamboo, set the paste to ferment in a heap
covered with mats. Chinese paper is readily recognised, because it is smooth on one
side, and bears on the other, the marks of the brush with which it is finished, upon
smooth tables, in order to dry it flat. The kind employed for engravings is in sheets
four feet long, and two broad. It is made of the bamboo; their myrtle-tree paper
would be too strong for this purpose.
Tracing Paper.
The best paper of this kind, sometimes superfluously called vegetable paper, is made
of the refuse of the flax mills, and prepared by the engine without fermentation. It
thus forms a semi-transparent paste, and affords a transparent paper. Bank-note paper
is made of the same materials, but they always undergo a bleaching with chloride of
lime. Great nicety is required in drying this kind of paper. For this purpose, each
sheet must be put between two sheets of gray paper in the press; and this gray paper
must be renewed several times, to prevent the bank-note paper from creasing.
Paper of Safety or Surety; Papier de Sureté.
This subject has occupied the attention of the French Academy for many years, in consequence
of the number of frauds committed upon the stamp revenue in France. One
of the best methods of making a paper which would evince whether any part of a
writing traced upon it had been tampered with or discharged, is to mix in the vat
two kinds of pulp, the one perfectly white, the other dyed of any colour easily affected
by chlorine, acids, and alkalis. The latter stuff being mingled with the former in any
desired proportion, will furnish a material for making a paper which will contain
coloured points distributed throughout all its substance, ready to show, by the changes
they suffer, whether any chemical reaction has been employed.
[941]
Quantity of Paper charged with Duties of Excise, in the United Kingdom, in
| |
1834. |
1835. |
1836. |
| |
lbs. |
lbs. |
lbs. |
| First class |
54,053,721 |
56,179,555 |
66,202,689 |
| Second class |
16,552,168 |
7,863,095 |
15,906,258 |
| Pasteboard, millboard, &c. |
49,392 |
49,772 |
36,340 |
| |
yards. |
yards. |
yards. |
| Stained |
8,749,144 |
8,247,931 |
8,032,577 |
| |
£ |
s. |
d. |
£ |
s. |
d. |
£ |
s. |
d. |
| Amount of duty, |
first class |
675,671 |
10 |
0 |
702,244 |
9 |
0 |
651,699 |
0 |
0 |
| — |
second class |
103,451 |
0 |
0 |
111,644 |
0 |
0 |
99,414 |
0 |
0 |
| — |
pasteboard, &c. |
54,689 |
0 |
0 |
54,548 |
15 |
0 |
39,557 |
0 |
0 |
| — |
stained |
63,795 |
16 |
0 |
60,141 |
0 |
0 |
22,112 |
0 |
0 |
The late reduction of the duty, from 3d. to 11⁄2d. per lb., upon paper of the first class,
viz., on all descriptions of it, except that made out of tarred ropes only, has been already
attended with considerable benefit to the manufacture, and would have acted with much
greater effect, but for the American crisis. The gross amount of the paper duty in the
year ending 5th January, 1836, was 831,057l., and in the year ending 5th January,
1838, it was 554,497l.; instead of being little more than one half, as might have
been the case from the reduction of the duty, which only came into full operation in
the year 1837. At the same time that the tax on common paper was reduced, that
upon stained paper was repealed altogether. The effect of the diminution consequently
made in the price of paper-hangings, has been so great as nearly to double the consumption
of the country, while the manufacture appears to be still rapidly on the
increase.
Declared Value of Stationery and Printed Books exported in
| Years. |
Stationery. |
Printed
Books. |
Total. |
| 1827 |
£195,110 |
£107,199 |
£302,309 |
| 1828 |
208,532 |
102,874 |
311,406 |
| 1829 |
190,652 |
109,878 |
300,530 |
| 1830 |
171,848 |
95,874 |
267,722 |
| 1831 |
179,216 |
101,110 |
280,326 |
| 1832 |
177,718 |
93,038 |
270,756 |
| 1833 |
211,518 |
124,535 |
336,053 |
| 1834 |
211,459 |
122,595 |
334,054 |
| 1835 |
259,105 |
148,318 |
407,423 |
| 1836 |
301,121 |
178,945 |
480,066 |
Till the paper trade shall escape entirely from the clutches of its antient dry-nurse,
the excise, neither it nor the book trade can acquire the same ascendancy in exportation
which all other articles of British manufactures have over the French.
The Value of Stationery exported in France, from 1833, was,—
| Cartons lustrés (polished pasteboards for the cloth manufacture) |
18,992 |
francs |
| Cartons en feuilles (pasteboard in sheets) |
6,352 |
— |
| Cartons moulés (papier-maché) |
215,376 |
— |
| Cartons coupés et assemblés |
54,184 |
— |
| Wrapping paper |
178,544 |
— |
| White paper, and rayé (ruled) pour musique |
2,903,075 |
— |
| Coloured paper in reams |
58,541 |
— |
| Stained paper (paper hangings) in rouleaux, |
1,885,387 |
— |
| Silk paper |
3,240 |
— |
| Total (= £208,000) |
5,323,621 |
francs. |
PARAFFINE. Distil beech-tar to dryness, rectify the heavy oil which collects at the
bottom of the receiver, and when a thick matter begins to rise, set aside what is distilled,
and urge the heat moderately as long as any thing more distils. Pyrélaine passes over,
containing crystalline scales of paraffine. This mixture being digested with its own
volume of alcohol of 0·833, forms a limpid solution, which is to be gradually diluted
with more alcohol, till its bulk becomes 6 or 8 times greater. The alcohol, which at
first dissolves the whole, lets the paraffine gradually fall. The precipitate being washed[942]
with cold alcohol till it becomes nearly colourless, and then dissolved in boiling alcohol,
is deposited on cooling in minute spangles and needles of pure paraffine.
Or the above mixture may be mixed with from 1⁄4 to 1⁄2 its weight of concentrated sulphuric
acid, and subjected for 12 hours to digestion, at a heat of 150° F., till, on cooling, crystals
of paraffine appear upon the surface. These are to be washed with water, dissolved
in hot alcohol, and crystallized. Paraffine is a white substance, void of taste and smell,
feels soft between the fingers, has a specific gravity of 0·87, melts at 112° Fahr., boils
at a higher temperature with the exhalation of white fumes, is not decomposed by dry
distillation, burns with a clear white flame without smoke or residuum, does not stain
paper, and consists of 85·22 carbon, and 14·78 hydrogen; having the same composition
as olefiant gas. It is decomposed neither by chlorine, strong acids, alkalis, nor potassium;
and unites by fusion with sulphur, phosphorus, wax, and rosin. It dissolves
readily in warm fat oils, in cold essential oils, in ether, but sparingly in boiling
absolute alcohol. Paraffine is a singular solid bicarburet of hydrogen; it has not
hitherto been applied to any use, but it would form admirable candles.
PARCHMENT. (Parchemin, Fr.; Pergament, Germ.) This writing material has
been known since the earliest times, but is now made in a very superior manner to what
it was anciently, as we may judge by inspection of the old vellum and parchment
manuscripts. The art of making parchment consists in certain manipulations necessary
to prepare the skins of animals of such thinness, flexibility, and firmness, as may be required
for the different uses to which this substance is applied. Though the skins of all
animals might be converted into writing materials, only those of the sheep or the she-goat
are used for parchment; those of calves, kids, and dead-born lambs for vellum;
those of the he-goat, she-goat, and wolves for drum-heads; and those of the ass for battledores.
All these skins are prepared in the same way, with slight variations, which need
no particular detail.
They are first of all prepared by the leather-dresser. After they are taken out of
the lime-pit, shaved, and well washed, they must be set to dry in such a way as to
prevent their puckering, and to render them easily worked. The small manufacturers
make use of hoops for this purpose, but the greater employ a herse, or stout
wooden frame. This is formed of two uprights and two cross-bars solidly joined together
by tenons and mortises, so as to form a strong piece of carpentry, which is to be fixed up
against a wall. These four bars are perforated all over with a series of holes, of such
dimensions as to receive slightly tapered box-wood pins, truly turned, or even iron
bolts. Each of these pins is transpierced with a hole like the pin of a violin, by means
of which the strings employed in stretching the skin may be tightened. Above the herse,
a shelf is placed, for receiving the tools which the workman needs to have always
at hand. In order to stretch the skin upon the frame, larger or smaller skewers
are employed, according as a greater or smaller piece of it is to be laid hold
of. Six holes are made in a straight line to receive the larger, and four to receive
the smaller skewers or pins. These small slits are made with a tool like a carpenter’s
chisel, and of the exact size to admit the skewer. The string round the skewer is
affixed to one of the bolts in the frame, which are turned round by means of a key,
like that by which pianos and harps are tuned. The skewer is threaded through the
skin in a state of tension.
Every thing being thus prepared, and the skin being well softened, the workman
stretches it powerfully by means of the skewers; he attaches the cords to the skewers,
and fixes their ends to the iron pegs or pins. He then stretches the skin, first
with his hand applied to the pins, and afterwards with the key. Great care must be
taken that no wrinkles are formed. The skin is usually stretched more in length
than in breadth, from the custom of the trade; though extension in breadth would be
preferable, in order to reduce the thickness of the part opposite the backbone.
The workman now takes the fleshing tool represented under Currying. It is a semi-circular
double-edged knife, made fast into a double wooden handle. Other forms of
the fleshing-knife edge are also used. They are sharpened by a steel. The workman
seizes the tool in his two hands, so as to place the edge perpendicularly to the skin, and
pressing it carefully from above downwards, removes the fleshy excrescences, and lays
them aside for making glue. He now turns round the herse upon the wall, in order to
get access to the outside of the skin, and to scrape it with the tool inverted, so as to
run no risk of cutting the epidermis. He thus removes any adhering filth, and squeezes
out some water. The skin must next be ground. For this purpose it is sprinkled
upon the fleshy side with sifted chalk or slaked lime, and then rubbed in all directions
with a piece of pumice-stone, 4 or 5 inches in area, previously flattened upon a sandstone.
The lime gets soon moist from the water contained in the skin. The pumice-stone is
then rubbed over the other side of the skin, but without chalk or lime. This operation
is necessary only for the best parchment or vellum. The skin is now allowed to dry,
upon the frame; being carefully protected from sunshine, and from frost. In the arid[943]
weather of summer a moist cloth needs to be applied to it from time to time, to prevent
its drying too suddenly; immediately after which the skewers require to be tightened.
When it is perfectly dry, the white colour is to be removed by rubbing it with the
woolly side of a lambskin. But great care must be taken not to fray the surface; a circumstance
of which some manufacturers are so much afraid, as not to use either chalk or lime
in the polishing. Should any grease be detected upon it, it must be removed by steeping
it in a lime-pit for 10 days, then stretching it anew upon the herse, after which it is
transferred to the scraper.
This workman employs here an edge tool of the same shape as the fleshing-knife, but
larger and sharper. He mounts the skin upon a frame like the herse above described;
but he extends it merely with cords, without skewers or pins, and supports it generally
upon a piece of raw calfskin, strongly stretched. The tail of the skin being placed towards
the bottom of the frame, the workman first pares off, with a sharp knife, any considerable
roughnesses, and then scrapes the outside surface obliquely downwards with the proper
tools, till it becomes perfectly smooth: the fleshy side needs no such operation; and indeed
were both sides scraped, the skin would be apt to become too thin, the only object of
the scraper being to equalize its thickness. Whatever irregularities remain, may be
removed with a piece of the finest pumice-stone, well flattened beforehand upon a fine
sandstone. This process is performed by laying the rough parchment upon an oblong
plank of wood, in the form of a stool; the plank being covered with a piece of soft parchment
stuffed with wool, to form an elastic cushion for the grinding operation. It is
merely the outside surface that requires to be pumiced. The celebrated Strasburgh
vellum is prepared with remarkably fine pumice-stones.
If any small holes happen to be made in the parchment, they must be neatly
patched, by cutting their edges thin, and pasting on small pieces with gum water.
The skins for drum-heads, sieves, and battledores are prepared in the same way. For
drums, the skins of asses, calves, or wolves are employed; the last being preferred. Ass
skins are used for battledores. For sieves, the skins of calves, she-goats, and, best of all,
he-goats, are employed. Church books are covered with the dressed skins of pigs.
Parchment is coloured only green. The following is the process. In 500 parts of
rain water, boil 8 of cream of tartar, and 30 of crystallized verdigris; when this solution
is cold, pour into it 4 parts of nitric acid. Moisten the parchment with a brush,
and then apply the above liquid evenly over its surface. Lastly, the necessary lustre
may be given with white of eggs, or mucilage of gum arabic.
PARTING (Départ, Fr.; Scheidung, Germ.), is the process by which gold is separated
from silver. See Assay, Gold, Refining, and Silver.
PASTEL, is the French name of coloured crayons.
PASTEL, is a dye-stuff, allied to Indigo, which see.
PASTES, or FACTITIOUS GEMS. (Pierres précieuses artificielles, Fr.; Glaspasten,
Germ.) The general vitreous body called Strass, (from the name of its German
inventor,) preferred by Fontanier in his treatise on this subject, and which he styles the
Mayence base, is prepared in the following manner:—8 ounces of pure rock-crystal or
flint in powder, mixed with 24 ounces of salt of tartar, are to be baked and left to cool.
The mixture is to be afterwards poured into a basin of hot water, and treated with dilute
nitric acid till it ceases to effervesce; and then the frit is to be washed till the water comes
off tasteless. This is to be dried, and mixed with 12 ounces of fine white-lead, and the mixture
is to be levigated and elutriated with a little distilled water. An ounce of calcined
borax being added to about 12 ounces of the preceding mixture in a dry state, the whole is
to be rubbed together in a porcelain mortar, melted in a clean crucible, and poured out
into cold water. This vitreous matter must be dried, and melted a second and a third
time, always in a new crucible, and after each melting poured into cold water, as at
first, taking care to separate the lead that may be revived. To the third frit, ground
to powder, 5 drachms of nitre are to be added; and the mixture being melted for the
last time, a mass of crystal will be found in the crucible, of a beautiful lustre. The
diamond may be well imitated by this Mayence base. Another very fine white crystal may
be obtained, according to M. Fontanier, from 8 ounces of white-lead, 2 ounces of powdered
borax, 1⁄2 grain of manganese, and 3 ounces of rock crystal, treated as above.
The colours of artificial gems are obtained from metallic oxides. The oriental topaz,
is prepared by adding oxide of antimony to the base; the amethyst, by manganese
with a little of the purple of Cassius; the beryl, by antimony and a very little cobalt;
yellow artificial diamond and opal, by horn-silver (chloride of silver); blue-stone
or sapphire, by cobalt. The following proportions have been given:—
For the yellow diamond. To 1 ounce of strass add 24 grains of chloride of silver, or
10 grains of glass of antimony.
For the sapphire. To 24 ounces of strass, add 2 drachms and 26 grains of the oxide
of cobalt.
For the oriental ruby. To 16 ounces of strass, add a mixture of 2 drachms, and 48[944]
grains of the precipitate of Cassius, the same quantity of peroxide of iron prepared by
nitric acid, the same quantity of golden sulphuret of antimony and of manganese calcined
with nitre, and 2 ounces of rock crystal. Manganese alone, combined with the
base in proper quantity, is said to give a ruby colour.
For the emerald. To 15 ounces of strass, add 1 drachm of mountain blue (carbonate
of copper), and 6 grains of glass of antimony; or, to 1 ounce of base, add 20 grains of
glass of antimony, and 3 grains of oxide of cobalt.
For the common opal. To 1 ounce of strass, add 10 grains of horn-silver, 2 grains of
calcined magnetic ore, and 26 grains of an absorbent earth (probably chalk-marl)
Fontanier.
M. Douault-Wiéland, in an experimental memoir on the preparation of artificial
coloured stones, has offered the following instructions, as being more exact than what
were published before.
The base of all artificial stones is a colourless glass, which he calls fondant, or flux;
and he unites it to metallic oxides, in order to produce the imitations. If it be
worked alone on the lapidary’s wheel, it counterfeits brilliants and rose diamonds
remarkably well.
This base or strass is composed of silex, potash, borax, oxide of lead, and sometimes
arsenic. The siliceous matter should be perfectly pure; and if obtained from sand, it
ought to be calcined, and washed, first with dilute muriatic acid, and then with water.
The crystal or flint should be made redhot, quenched in water, and ground, as in the
potteries. The potash should be purified from the best pearlash; and the borax
should be refined by one or two crystallizations. The oxide of lead should be absolutely
free from tin, for the least portion of this latter metal causes milkiness. Good red-lead
is preferable to litharge. The arsenic should also be pure. Hessian crucibles are preferable
to those of porcelain, for they are not so apt to crack and run out. Either a
pottery or porcelain kiln will answer, and the fusion should be continued 24 hours;
for the more tranquil and continuous it is, the denser is the paste, and the greater its
beauty. The following four recipes have afforded good strass:—
| |
Grains. |
| No. I. |
| Or, |
Rock crystal |
4056 |
| |
Minium |
6300 |
| |
Pure potash |
2154 |
| |
Borax |
276 |
| |
Arsenic |
12 |
| No. II. |
| |
Sand |
3600 |
| |
Ceruse of Clichy (pure carbonate of lead) |
8508 |
| |
Potash |
1260 |
| |
Borax |
360 |
| |
Arsenic |
12 |
| No. III. |
| |
Rock crystal |
3456 |
| |
Minium |
5328 |
| |
Potash |
1944 |
| |
Borax |
216 |
| |
Arsenic |
6 |
| No. IV. |
| |
Rock crystal |
3600 |
| |
Ceruse of Clichy |
8508 |
| |
Potash |
1260 |
| |
Borax |
360 |
Topaz.
| |
Grains. |
| |
Very white paste |
1008 |
| |
Glass of antimony |
43 |
| |
Cassius purple |
1 |
| Or, |
|
| |
Paste |
3456 |
| |
Oxide of iron, called saffron of Mars |
36 |
Ruby.
M. Wiéland succeeded in obtaining excellent imitations of rubies, by making use of
the topaz materials. It often happened that the mixture for topazes gave only an opaque
mass, translucent at the edges, and in thin plates of a red colour. 1 part of this substance
being mixed with 8 parts of strass, and fused for 30 hours, gave a fine yellowish crystal-like
paste, and fragments of this fused before the blowpipe, afforded the finest imitation
of rubies. The result was always the same.
The following are other proportions for rubies:—
| |
Grains. |
| Or, |
Paste |
2880 |
| |
Oxide of manganese |
72 |
Emerald.
| Or, |
Paste |
4608 |
| |
Green oxide of pure copper |
42 |
| |
Oxide of chrome |
2 |
[945]
Sapphire.
| |
Grains. |
| Or, |
Paste |
4608 |
| |
Oxide of cobalt |
68 |
This mixture should be carefully fused in a luted Hessian crucible, and be left 30 hours
in the fire.
Amethyst.
| |
Grains. |
| |
Paste |
4608 |
| |
Oxide of manganese |
36 |
| |
Oxide of cobalt |
24 |
| |
Purple of Cassius |
1 |
Syrian Garnet, or Antient Carbuncle.
| |
Grains. |
| |
Paste |
512 |
| |
Glass of Antimony |
256 |
| |
Cassius purple |
2 |
| |
Oxide of manganese |
2 |
Beryl, or Aqua Marina.
| |
Grains. |
| |
Paste |
3456 |
| |
Glass of antimony |
24 |
| |
Oxide of cobalt |
11⁄2 |
In all these mixtures, the substances should be mixed by sifting, fused very carefully,
and cooled very slowly, after having been left in the fire from 24 to 30 hours.
M. Lançon has also made many experiments on the same subject. The following
are a few of his proportions:—
Paste.
| |
Grains. |
| |
Litharge |
100 |
| |
White sand |
75 |
| |
White tartar, or potash |
10 |
Amethyst.
| |
Grains. |
| |
Paste |
9216 |
| |
Oxide of manganese |
from 15 to 24 |
| |
Oxide of cobalt |
1 |
Emerald.
| |
Grains. |
| |
Paste |
9216 |
| |
Acetate of copper |
72 |
| |
Peroxide of iron, or saffron of Mars |
1·5 |
PASTILLE, is the English name of small cones made of gum benzoin, with powder
of cinnamon, and other aromatics, which are burned as incense, to diffuse a grateful
odour, and conceal unpleasant smells in apartments. See Perfumery.
PASTILLE, is the French name of certain aromatic sugared confections; called
also tablettes.
PEARLASH, a commercial form of Potash, which see.
PEARLS (Perles, Fr.; Perlen, Germ.); are the productions of certain shell-fish.
These molluscæ are subject to a kind of disease caused by the introduction of foreign
bodies within their shells. In this case, their pearly secretion, instead of being spread
in layers upon the inside of their habitation, is accumulated round these particles in concentric
layers. Pearl consists of carbonate of lime, interstratified with animal membrane.
The oysters whose shells are richest in mother of pearl, are most productive of
these highly prized spherical concretions. The most valuable pearl fisheries are on the
coast of Ceylon, and at Olmutz in the Persian Gulf, and their finest specimens are more
highly prized in the East than diamonds, but in Europe they are liable to be rated very
differently, according to the caprice of fashion. When the pearls are large, truly
spherical, reflecting and decomposing the light with much vivacity, they are much
admired. But one of the causes which renders their value fluctuating, is the occasional
loss of their peculiar lustre, without our being able to assign a satisfactory reason for it.
Besides, they can be now so well imitated, that the artificial pearls have nearly as rich an
appearance as the real.
PEARLS, ARTIFICIAL. These are small globules or pear-shaped spheroids of
thin glass, perforated with two opposite holes, through which they are strung, and
mounted into necklaces, &c., like real pearl ornaments. They must not only be white
and brilliant, but exhibit the iridescent reflections of mother of pearl. The liquor
employed to imitate the pearly lustre, is called the essence of the east (essence d’orient),
which is prepared by throwing into water of ammonia the brilliant scales, or rather the
lamellæ, separated by washing and friction, of the scales of a small river fish, the blay,
called in French ablette. These scales digested in ammonia, having acquired a degree of
softness and flexibility which allow of their application to the inner surfaces of the glass
globules, they are introduced by suction of the liquor containing them in suspension.
The ammonia is volatilized in the act of drying the globules.
It is said that some manufacturers employ ammonia merely to prevent the alteration
of the scales; that when they wish to make use of them, they suspend them in a
well clarified solution of isinglass, then pour a drop of the mixture into each bead,
and spread it round the inner surface. It is doubtful whether by this method, the same
lustre and play of colours can be obtained as by the former. It seems moreover to be
of importance for the success of the imitation, that the globules be formed of a bluish,
opalescent, very thin glass, containing but little potash and oxide of lead. In every manufactory[946]
of artificial pearls, there must be some workmen possessed of great experience
and dexterity. The French are supposed to excel in this ingenious branch of industry.
PEARLWHITE, is a submuriate of bismuth, obtained by pouring a solution of
the nitrate of that metal into a dilute solution of sea salt, whereby a light and very
white powder is obtained, which is to be well washed and dried. See Bismuth.
PECTIC ACID (Acid pectique, Fr.; Gallertsaüre, Germ.); so named on account
of its jellying property, from πηκτις, coagulum, exists in a vast number of vegetables.
The easiest way of preparing it, is to grate the roots of carrots into a pulp, to express
their juice, to wash the marc with rain or distilled water, and to squeeze it well; 50
parts of the marc are next to be diffused through 300 of rain-water, adding by slow
degrees a solution of one part of pure potash, or two of bicarbonate. This mixture
is to be heated, so as to be made to boil for about a quarter of an hour, and is then to
be thrown boiling-hot upon a filter cloth. It is known to have been well enough
boiled, when a sample of the filtered liquor becomes gelatinous by neutralizing it with
an acid. This liquor contains pectate of potassa, in addition to other matters extricated
from the root. The pectate may be decomposed by a stronger acid, but it is better to
decompose it by muriate of lime; whereby a pectate of lime, in a gelatinous form,
quite insoluble in water, is obtained. This having been washed with cold water upon
a cloth, is to be boiled in water containing as much muriatic acid as will saturate the
lime. The pectic acid thus liberated, remains under the form of a colourless jelly, which
reddens litmus paper, and tastes sour, even after it is entirely deprived of the muriatic
acid. Cold water dissolves very little of it; it is more soluble in boiling water. The
solution is colourless, does not coagulate on cooling, and hardly reddens litmus paper;
but it gelatinizes when alcohol, acids, alkalis, or salts are added to it. Even sugar
transforms it, after some time, into a gelatinous state, a circumstance which serves to
explain the preparation of apple, cherry, raspberry, gooseberry, and other jellies.
PECTINE, or vegetable jelly, is obtained by mixing alcohol with the juice of ripe
currants, or any similar fruit, till a gelatinous precipitate takes place; which is to be
gently squeezed in a cloth, washed with a little weak alcohol, and dried. Thus prepared,
pectine is insipid, without action upon litmus, in small pieces, semi-transparent,
and of a membranous aspect, like isinglass. Its mucilaginous solution in cold water is
not tinged blue with iodine. A very small addition of potash, or its carbonate, converts
pectine into pectic acid; both of which substances are transformed into mucic
and oxalic acids by the nitric.
PELTRY (Pelleterie, Fr.; Pelzwerk, Germ.); is nearly synonymous with fur, and
comprehends the skins of different kinds of wild animals that are found in high
northern latitudes, particularly in the American continent; such as the beaver, bear,
moosedeer, marten, mink, sable, woolverin, wolf, &c. When these skins have received
no preparation but from the hunters, they are most properly called peltry; but when
they have had the inner side tawed or tanned (see Leather) by an aluminous process,
they may then be denominated furs.
The scouring or cleaning of peltry is performed in a large cask, or truncated cone
laid on its side, and traversed by a revolving shaft, which is furnished with a few
rectangular rounded pegs. These are intended to stir round the skins, while they are
dusted over with Paris plaster, whitening, or sometimes sand, made as hot as the
hand can bear. The bottom of the cask should be grated, to allow the impurities to
fall out. The lustrage, which the cleansed skins next undergo, is merely a species of
dyeing, either topical to modify certain disagreeable shades, or general to impart a more
beautiful colour to the fur. Under the articles Dyeing, and the several colours, as also
Hair and Morocco, sufficient instructions will be found for dyeing fur. The mordants
should be applied pretty hot by a brush, on the hair of the skin, stretched upon a solid
table; and after two or three applications, with drying between, the tinctorial infusions
may be rubbed on in the same way. The hair must be freed beforehand from all
greasiness, by lime water, or a weak solution of carbonate of soda; then well washed.
Much nicety, and many successive applications of the dye-stuff, are sometimes requisite
to bring out the desired shade.
Under Hat Manufacture, I referred to this article for a description of the process
of secrétage, whereby the hairs of rabbit and hare skins are rendered fit for felting.
Dissolve 32 parts of quicksilver in 500 of common aquafortis; and dilute the solution
with one half or two thirds of its bulk of water, according to the strength of the acid.
The skin being laid upon a table with the hair side uppermost, a brush, made with the
bristles of the wild boar, is to be slightly moistened with the mercurial solution, and
passed over the smooth surface of the hairs with strong pressure. This application
must be repeated several times in succession, till every part of the fur be equally
touched, and till about two thirds of the length of the hairs be moistened, or a little
more, should they be rigid. In order to complete this impregnation, the skins are laid
together in pairs with the hairy sides in contact, put in this state into the stove-room,[947]
and exposed to a heat higher in proportion to the weakness of the mercurial solution.
The drying should be rapidly effected, otherwise the concentration of the nitrate will not
take due effect in causing the retraction and curling of the hairs.
No other acid, or metallic solution, but the above, has been found to answer the desired
purpose of the hatmaker. After the hairs are properly secreted, they are plucked
off by hand, or shorn off by a machine.
PENCIL MANUFACTURE. (Crayons, fabrique de, Fr.; Bleistifte, verfertigung,
Germ.) The word pencil is used in two senses. It signifies either a small hair brush
employed by painters in oil and water colours, or a slender cylinder of black lead or
plumbago, either naked or enclosed in a wooden case, for drawing black lines upon
paper. The last sort, which is the one to be considered here, corresponds nearly to the
French term crayon, though this includes also pencils made of differently coloured
earthy compositions. See Crayon.
The best black-lead pencils of this country are formed of slender parallelopipeds, cut
out by a saw from sound pieces of plumbago, which have been previously calcined in
close vessels at a bright red heat. These parallelopipeds are generally enclosed in cases
made of cedar wood, though of late years they are also used alone, in peculiar pencil-cases,
under the name of ever-pointed pencils, provided with an iron wire and screw, to
protrude a minute portion of the plumbago beyond the tubular metallic case, in proportion
as it is wanted.
In the year 1795, M. Conté, a French gentleman, well acquainted with the mechanical
arts, invented an ingenious process for making artificial black-lead pencils of superior
quality, by which he and his successor and son-in-law, M. Humblot, have realized
large fortunes.
Pure clay, or clay containing the smallest proportion of calcareous or siliceous matter,
is the substance which he employed to give aggregation and solidity, not only to plumbago
dust, but to all sorts of coloured powders. That earth has the property of diminishing
in bulk, and increasing in hardness, in exact proportion to the degree of heat it is
exposed to, and hence may be made to give every degree of solidity to crayons. The
clay is prepared by diffusing it in large tubs through clear river water, and letting the
thin mixture settle for two minutes. The supernatant milky liquor is drawn off by a
syphon from near the surface, so that only the finest particles of clay are transferred into
the second tub, upon a lower level. The sediment which falls very slowly in this tub, is
extremely soft and plastic. The clear water being run off, the deposit is placed upon a
linen filter, and allowed to dry. It is now ready for use.
The plumbago must be reduced to a fine powder in an iron mortar, then put into a
crucible, and calcined at a heat approaching to whiteness. The action of the fire gives
it a brilliancy and softness which it would not otherwise possess, and prevents it from
being affected by the clay, which it is apt to be in its natural state. The less clay is
mixed with the plumbago, and the less the mixture is calcined, the softer are the pencils
made of it; the more clay is used the harder are the pencils. Some of the best pencils
made by M. Conté, were formed of two parts of plumbago and three parts of clay;
others of equal parts. This composition admits of indefinite variations, both as to the
shade and hardness; advantages not possessed by the native mineral. While the traces
may be made as black as those of pure plumbago, they have not that glistening aspect
which often impairs the beauty of black-lead drawings. The same lustre may, however,
be obtained by increasing the proportion of powdered plumbago relatively to the clay.
The materials having been carefully sifted, a little of the clay is to be mixed with the
plumbago, and the mixture is to be triturated with water into a perfectly uniform paste.
A portion of this paste may be tested by calcination. If on cutting the indurated mass,
particles of plumbago appear, the whole must be further levigated. The remainder of
the clay is now to be introduced, and the paste is to be ground with a muller upon a
porphyry slab, till it be quite homogeneous, and of the consistence of thin dough. It is
now to be made into a ball, put upon a support, and placed under a bell glass inverted
in a basin of water, so as to be exposed merely to the moist air.
Small grooves are to be made in a smooth board, similar to the pencil parallelopipeds,
but a little longer and wider, to allow for the contraction of volume. The wood must be
boiled in grease, to prevent the paste from sticking to it. The above described paste
being pressed with a spatula into these grooves, another board, also boiled in grease, is
to be laid over them very closely, and secured by means of screw-clamps. As the atmospheric
air can get access only to the ends of the grooves, the ends of the pencil-pieces
become dry first, and by their contraction in volume get loose in the grooves, allowing
the air to insinuate further, and to dry the remainder of the paste in succession. When
the whole piece is dried, it becomes loose, and might be turned out of the grooves.
But before this is done, the mould must be put into an oven moderately heated, in
order to render the pencil pieces still drier. The mould should now be taken out, and
emptied upon a table covered with cloth. The greater part of the pieces will be[948]
entire, and only a few will have been broken, if the above precautions have been duly
observed. They are all, however, perfectly straight, which is a matter of the first
importance.
In order to give solidity to these pencils, they must be set upright in a crucible till
it is filled with them, and then surrounded with charcoal powder, fine sand, or sifted
wood ashes. The crucible, after having a luted cover applied, is to be put into a furnace,
and exposed to a degree of heat regulated by the pyrometer of Wedgewood; which
degree is proportional to the intended hardness of the pencils. When they have been
thus baked, the crucible is to be removed from the fire, and allowed to cool with the
pencils in it.
Should the pencils be intended for drawing architectural plans, or for very fine lines, they
must be immersed in melted wax or suet nearly boiling hot, before they are put into
the cedar cases. This immersion is best done by heating the pencils first upon a gridiron,
and then plunging them into the melted wax or tallow. They acquire by this
means a certain degree of softness, are less apt to be abraded by use, and preserve their
points much better.
When these pencils are intended to draw ornamental subjects with much shading,
they should not be dipped as above.
Second process for making artificial pencils, somewhat different from the preceding.—All
the operations are the same, except that some lamp-black is introduced along with the
plumbago powder and the clay. In calcining these pencils in the crucible, the contact
of air must be carefully excluded, to prevent the lamp-black from being burned away
on the surface. An indefinite variety of pencils, of every possible black tint, may thus
be produced, admirably adapted to draw from nature.
Another ingenious form of mould is the following:
Models of the pencil-pieces must be made in iron, and stuck upright upon an iron
tray, having edges raised as high as the intended length of the pencils. A metallic
alloy is made of tin, lead, bismuth, and antimony, which melts at a moderate heat.
This is poured into the sheet-iron tray, and after it is cooled and concreted, it is
inverted, and shaken off from the model bars, so as to form a mass of metal perforated
throughout with tubular cavities, corresponding to the intended pencil-pieces. The
paste is introduced by pressure into these cavities, and set aside to dry slowly. When
nearly dry, the pieces get so much shrunk that they may be readily turned out of the
moulds upon a cloth table. They are then to be completely desiccated in the shade,
afterwards in a stove-room, next in the oven, and lastly ignited in the crucible, with the
precautions above prescribed.
M. Conté recommends the hardest pencils of the architect to be made of lead melted
with some antimony and a little quicksilver.
In their further researches upon this subject, M. Conté and M. Humblot found that
the different degrees of hardness of crayons could not be obtained in a uniform manner
by the mere mixture of plumbago and clay in determinate doses. But they discovered
a remedy for this defect in the use of saline solutions, more or less concentrated, into
which they plunged the pencils, in order to modify their hardness, and increase the
uniformity of their texture. The non-deliquescent sulphates were preferred for this
purpose; such as sulphate of soda, &c. Even syrup was found useful in this way.
PENS, STEEL. The best metal, made from Dannemora or hoop (L) iron, is selected,
and laminated into slips about 3 feet long, and 4 inches broad, of a thickness corresponding
to the desired stiffness and flexibility of the pens. These slips are subjected to the
action of a stamping-press, somewhat similar to that for making buttons. (See Button,
and Plated Ware.) The point destined for the nib is next introduced into an appropriate
gauged hole of a little machine, and pressed into the semi-cylindrical shape; where it is
also pierced with the middle slit, and the lateral ones, provided the latter are to be given.
The pens are now cleaned, by being tossed about among each other, in a tin cylinder,
about 3 feet long, and 9 inches in diameter; which is suspended at each end upon joints,
to two cranks, formed one on each of two shafts. The cylinder, by the rotation of a flywheel,
acting upon the crank-shafts, is made to describe such revolutions as agitate the
pens in all directions, and polish them by mutual attrition. In the course of 4 hours
several thousand pens may be finished upon this machine.
PEPPER. (Poivre, Fr.; Pfeffer, Germ.), Black pepper is composed, according to
M. Pelletier, of the vegetable principle, piperine, of a very acrid concrete oil, a volatile
balsamic oil, a coloured gummy matter, an extractive principle analogous to legumine,
malic and tartaric acids, starch, bassorine, ligneous matter, with earthy and alkaline salts
in small quantity. Cubebs pepper has nearly the same composition.
Pepper imported for home consumption,
| in |
1835, |
1836, |
1837. |
| Lbs. |
2,359,573; |
2,800,980; |
2,626,298. |
| —Duty 6d. per lb. |
[949]
PERFUMERY, ART OF (Parfumerie, Fr.; Wohlriechende-kunst, Germ.); consists
in the preparation of different products, such as fats or pommades, essential oils,
distilled spirits, pastes, pastilles, and essences.
Fats ought to be pounded in a marble mortar, without addition of water, till all the
membranes be completely torn; then subjected to the heat of a water-bath in a proper
vessel. The fat soon melts, and the albumen of the blood coagulating, carries with it
all the foreign substances; the liquid matter should be skimmed, and passed through a
canvas filter.
Of pommades by infusion.—Rose, orange-flower, and cassia. Take 334 pounds of
hog’s lard, and 166 of beef suet. These 500 pounds are put into a pan called bugadier;
and when melted, 150 pounds of rose-leaves nicely plucked are added, taking care to
stir the mixture every hour. The infusion thus prepared is to remain at rest for 24
hours; at the end of this time, the pommade is again melted, and well stirred to
prevent its adherence to the bottom of the melting-pan. The mass is now to be
poured out into canvas, and made into rectangular bricks or loaves, which are subjected
to a press, in order to separate the solid matter from the soft pommade. These brick-shaped
pieces being put into an iron-bound barrel perforated all over its staves, the
pommade is to be allowed to exude on all sides, and flow down into a copper vessel placed
under the trough of the press. This manipulation should be repeated with the same
fat ten or twelve times; or in other words, 3000 pounds of fresh rose-leaves should be
employed to make a good pommade.
The pommade of orange-flowers is made in the same manner, as also the pommade
of cassia.
Of pommades without infusion.—Jasmin, tuberose, jonquil, narcissus, and violet.
A square frame, called tiame, is made of four pieces of wood, well joined together, 2
or 3 inches deep, into which a pane of glass is laid, resting upon inside ledges near the
bottom. Upon the surface of the pane the simple pommade of hog’s lard and suet is spread
with a pallet knife; and into this pommade the sweet-scented flowers are stuck fresh
in different points each successive day, during two or three months, till the pommade
has acquired the desired richness of perfume. The above-described frames are piled
closely over each other. Some establishments at Grasse possess from 3000 to 4000 of
them.
Of oils.—Rose, orange-flower, and cassia oils, are made by infusion, like the pommades
of the same perfumes; taking care to select oils perfectly fresh. As to those of
jasmin, tuberose, jonquil, violet, and generally all delicate flowers, they are made in the
following manner. Upon an iron frame, a piece of cotton cloth is stretched, imbued
with olive oil of the first quality, and covered completely with a thin bed of flowers.
Another frame is similarly treated,—and in this way a pile is made. The flowers
must be renewed till the oil is saturated with their odour. The pieces of cotton cloth
are then carefully pressed to extrude the oil. This last operation requires commonly
7 or 8 days.
Of distillation.—The essential oils or essences, of which the great manufacture is in
the south of France, are of rose, neroli, lavender, lemon thyme, common thyme, and
rosemary. For the mode of distilling the essential oils, see Oils, Essential.
The essence of roses being obtained in a peculiar manner, I shall describe it here.
Put into the body of a still 40 pounds of roses, and 60 quarts of water; distil off one
half of the water. When a considerable quantity of such water of the first distillation
is obtained, it must be used as water upon fresh rose leaves; a process of repetition to
be carried to the fifth time. In the distillation of orange-flower, to obtain the essence of
neroli, the same process is to be followed; but if orange-flower water merely be wanted,
then it is obtained at one distillation, by reserving the first fifth part of water that
comes over. What is called the essence of petit-grain, is obtained by distilling the leaves
of the orange shrub. The essences of lavender, thyme, &c., present nothing peculiar in
their mode of extraction.
OF SCENTED SPIRITS,
From oil of rose, orange, jasmin, tuberose, cassia, violet, and other flowers.
Into each of three digesters, immersed in water-baths, put 25 lbs. of any one of these
oils, and pour into the first digester 25 quarts of spirit of wine; agitate every quarter
of an hour during three days, and at the end of this period, draw off the perfumed
spirit, and pour it into the second digester; then transfer it after 3 days into the third
digester, treating the mixture in the same way; and the spirit thus obtained will be
perfect. The digesters must be carefully covered during the progress of these operations.
On pursuing the same process with the same oil and fresh alcohol, essences of
inferior qualities may be obtained, called Nos. 2, 3, and 4.
Some perfumers state that it is better to use highly scented pommades than oils; but
there is probably little difference in this respect.
[950]
Esprit de suave.
| 7 |
|
Eng. qrts. of spirit of |
jasmin, |
3d operation. |
| 7 |
|
— |
cassia, |
— |
| 3 |
|
— |
wine. |
|
| 2 |
|
— |
tuberose, |
— |
| 1 |
1⁄2 |
ounce essence of cloves. |
| |
1⁄2 |
ounce fine neroli. |
| 1 |
1⁄2 |
ounce essence of bergamote. |
| 8 |
|
ounces essence of musk, 2d infusion. |
| 3 |
|
quarts rose water. |
Spirit of Cytherea.
| 1 |
quart spirit of |
violets. |
| 1 |
— |
jasmin, |
2d operation. |
| 1 |
— |
tuberose, |
— |
| 1 |
— |
clove gilly flower. |
| 1 |
— |
roses, 2d operation. |
| 1 |
— |
Portugal. |
| 2 |
— |
orange-flower water. |
Spirit of flowers of Italy.
| 2 |
|
quarts spirit of |
jasmin, |
2d |
operation. |
| 2 |
|
— |
roses |
— |
| 2 |
|
— |
oranges, |
3d |
— |
| 2 |
|
— |
cassia, |
2d |
operation. |
| 1 |
1⁄2 |
— |
orange-flower water. |
The above spirits mark usually 28 alcometric degrees of Gay Lussac. See Alcohol.
POMMADES.
No less than 20 scented pommades are distinguished by the perfumers of Paris. The
essences commonly employed in the manufacture of pommades, are those of bergamote,
lemons, cédrat, limette (sweet lemon), Portugal, rosemary, thyme, lemon thyme, lavender,
marjoram, and cinnamon.
The following may serve as an example:—
Pommade à la vanille, commonly called Roman.
| 12 |
pounds of |
pommade à la rose. |
| 3 |
— |
oil à la rose. |
| 1 |
— |
vanilla, first quality, pulverized. |
| 6 |
ounces |
bergamote. |
The pommade being melted at the heat of a water-bath, the vanilla is to be introduced
with continual stirring for an hour. The mixture is left to settle during two
hours. The pommade is then to be drawn off, and will be found to have a fine yellow
colour, instead of the brown shade which it commonly has.
In making odoriferous extracts and waters, the spirits of the flowers prepared by
macerating the flowers in alcohol should be preferred to their distillation, as forming the
foundation of good perfumery. The specific gravity of these spirits should be always
under 0·88.
Extract of nosegay (bouquet).
| 2 |
quarts |
spirit of jasmin, |
1st |
operation. |
| 2 |
— |
extract of violets. |
| 1 |
— |
spirit of cassia |
1st |
— |
| 1 |
— |
roses, |
1st |
— |
| 1 |
— |
orange, |
1st |
— |
| 1 |
— |
extract of clove gilly flower. |
| 4 |
drms. of flowers of benzoin (benzoic acid). |
| 8 |
ounces of essence of amber, 1st infusion. |
Extract of peach blossoms.
| 6 |
quarts of spirits of wine. |
| 6 |
pounds of bitter almonds. |
| 2 |
quarts of spirits of orange flower, 2d operation. |
| 4 |
drachms of essence of bitter almonds. |
| 4 |
drachms of balsam of Peru. |
| 4 |
ounces of essence of lemons. |
Eau de Cologne.
Two processes have been adopted for the preparation of this perfume, distillation and
infusion; the first of which, though generally abandoned, is, however, the preferable
one. The only essences which should be employed, and which have given such celebrity
to this water, are the following; bergamote, lemon, rosemary, Portugal, neroli. The
whole of them ought to be of the best quality, but their proportions may be varied
according to the taste of the consumers.
Thirty different odours are enumerated by perfumers; the three following recipes
will form a sufficient specimen of their combinations.
Honey-water.
| 6 |
quarts of spirit of roses, 3d operation. |
| 3 |
quarts do.spirit ofjasmin. |
| 3 |
quarts do.spirit ofspirits of wine. |
| 3 |
ounces essence of Portugal. |
| 4 |
drachms flowers of benzoin. |
| 12 |
ounces of essence of vanilla, 3d infusion. |
| 12 |
ouncesdo.essence of musk,3d indo. |
| 3 |
quarts good orange-flower water. |
Eau de mille fleurs.
| 18 |
quarts of spirits of wine. |
| 4 |
ounces balsam of Peru. |
| 8 |
oudo.esessence of bergamote. |
| 4 |
oudo.es essence ofcloves. |
| 1 |
oudo.esordinary neroli. |
| 1 |
oudo.es essence ofthyme. |
| 8 |
oudo.es essence ofmusk, 3d infusion |
| 4 |
quarts orange-flower water. |
[951]
Eau de mousseline.
| 2 |
quarts spirit of roses, 3d infusion. |
| 2 |
quartsdo.irit ofjasmin, 4thinfdo. |
| 1 |
quartsdo.irit ofclove gilly flower. |
| 2 |
quartsdo.irit oforange flower, 4th do. |
| 2 |
ounces essence of vanilla, 3d infusion. |
| 2 |
ounces do.sence ofmusk,3d infdo. |
| 4 |
drachms of sanders wood. |
| 1 |
quart of orange-flower water. |
Almond pastes.
These are, gray, sweet white, and bitter white.
The first is made either with the kernels of apricots, or with bitter almonds. They
are winnowed, ground, and formed into loaves of 5 or 6 pounds weight, which are put
into the press in order to extract their oil; 300 pounds of almonds affording about 130
of oil. The pressure is increased upon them every two hours during three days; at
the end of which time the loaves or cakes are taken out of the press to be dried,
ground, and sifted.
The second paste is obtained by boiling the almonds in water till their skins are
completely loosened; they are next put into a basket, washed and blanched; then dried,
and pressed as above.
The third paste is prepared like the second, only using bitter almonds.
Liquid almond pastes, such as those of the rose, orange, vanilla, and nosegay.—The
honey paste is most admired. It is prepared as follows:—
| 6 |
pounds of honey. |
| 6 |
poudo.s ofwhite bitter paste. |
| 12 |
pounds oil of bitter almonds. |
| 26 |
yolks of eggs. |
The honey should be heated apart and strained; 6 pounds of almond paste must
then be kneaded with it, adding towards the conclusion, alternately, the quantity of
yolks of eggs and almond oil indicated.
Pastilles à la rose, orange flower, and vanilla.
Pastilles à la rose.
| 12 |
ounces of gum. |
| 12 |
oundo. of olibanum, in tears. |
| 12 |
oundo. of storax,um, indo. |
| 8 |
oundo. of nitre. |
| 16 |
oundo. of powder of pale roses. |
| 3 pounds 14 |
oundo. of charcoal powder. |
| 1 |
oundo. of essence of roses. |
Pastilles of orange flower.
| 12 |
ounces of gum galbanum. |
| 12 |
oundo. of olibanum, in tears. |
| 12 |
oundo. of storax, do. |
| 8 |
oundo. of nitre. |
| 1 |
pound of pure orange powder. |
| 3 |
pdo.ds14 ounces charcoal powder. |
| 1 |
ounce superfine neroli. |
Pastilles à la vanille.
| 12 |
ounces of gum galbanum. |
| 12 |
oudo.s of olibanum, in tears. |
| 12 |
oudo.s of storaxum, in do. |
| 8 |
oudo.s of nitre. |
| 8 |
oudo.s of cloves. |
| 16 |
ounces powder of vanilla. |
| 3 |
pounds 14 ounces charcoal powder. |
| 4 |
drms. essence of cloves. |
| 8 |
ouncessse do. of vanilla, 1st infusion. |
The above mixture in each case is to be thickened with 2 ounces of gum tragacanth
dissolved in 2 pints of rose-water. It is needless to say that the ingredients
of the mixture should be impalpable powders.
Scented cassolettes.
| 8 |
pounds of black amber (ambergris). |
| 4 |
poudo.s ofrose powder. |
| 2 |
ounces of benzoin. |
| 1 |
ounce essence of roses. |
| 1 |
do. gum tragacanth. |
| A few drops of the oil of sanders wood. |
These ingredients are pulverized, and made into a cohesive paste with the gum.
ESSENCES BY INFUSION.
Essence of musk.
| 5 |
ounces of musk from the bladder, cut small. |
| 1 |
oudo.s of civet. |
| 4 |
quarts of spirit of ambrette (purple sweet sultan). |
The whole are put into a matrass, and exposed to the sun for two months during
the hottest season of the year. In winter, the heat of a water-bath must be resorted
to.
Essence of vanilla.
| 3 |
pounds of vanilla in branches, 1st quality, cut small. |
| 4 |
quarts spirit of ambrette. |
| 2 |
drachms of cloves. |
| 1⁄2 |
drado.ms ofmusk from the bladder. |
The same process must be followed as for the essence of musk.
[952]
Essence of ambergris.
| 4 |
ounces of ambergris. |
| 2 |
ounces of bladder musk. |
| 8 |
quarts of spirit of ambrette. |
Treat as above.
Spirit of ambrette (purple sweet sultan).
25 pounds of ambrette are to be distilled with 25 quarts of spirits of wine, adding 12
quarts of water, so as to be able to draw off the 25 quarts.
PERRY, is the fermented juice of pears, prepared in exactly the same way as Cyder.
PERSIAN BERRIES. See Berries, Persian.
PE-TUNT-SE, is the Chinese name of the fusible earthy matter of their porcelain.
It is analogous to our Cornish stone.
PEWTER, PEWTERER. (Potier d’étain, Fr.) Pewter is, generally speaking,
an alloy of tin and lead, sometimes with a little antimony or copper, combined in
several different proportions, according to the purposes which the metal is to serve.
The English tradesmen distinguish three sorts, which they call plate, trifle, and ley
pewter; the first and hardest being used for plates and dishes; the second for beer-pots;
and the third for larger wine measures. The plate pewter has a bright silvery lustre
when polished; the best is composed of 100 parts of tin, 8 parts of antimony, 2 parts of
bismuth, and 2 of copper. The trifle is said by some to consist of 83 of tin, and 17 of
antimony; but it generally contains a good deal of lead. The ley pewter is composed
of 4 of tin, and 1 of lead. As the tendency of the covetous pewterer is always to put in
as much of the cheap metal as is compatible with the appearance of his metal in the
market, and as an excess of lead may cause it to act poisonously upon all vinegars and
many wines, the French government long ago appointed Fourcroy, Vauquelin, and
other chemists, to ascertain by experiment the proper proportions of a safe pewter
alloy. These commissioners found that 18 parts of lead might, without danger of
affecting wines, &c., be alloyed with 82 parts of tin; and the French government in
consequence passed a law, requiring pewterers to use 831⁄2 of tin in 100 parts, with a
tolerance of error amounting to 11⁄2 per cent. This ordonnance, allowing not more than
18 per cent. of lead at a maximum, has been extended to all vessels destined to contain
alimentary substances. A table of specific gravities was also published, on purpose to
test the quality of the alloy; the density of which, at the legal standard, is 7·764.
Any excess of lead is immediately indicated by an increase in the specific gravity above
that number.
The pewterer fashions almost all his articles by casting them in moulds of brass or
bronze, which are made both inside and outside in various pieces, nicely fitted together,
and locked in their positions by ears and catches or pins of various kinds. The moulds
must be moderately heated before the pewter is poured into them, and their surfaces
should be brushed evenly over with pounce powder (sandarach) beaten up with white of
egg. Sometimes a film of oil is preferred. The pieces, after being cast, are turned and
polished; and if any part needs soldering, it must be done with a fusible alloy of tin,
bismuth, and lead.
Britannia metal, the kind of pewter of which English tea-pots are made, is said to be
an alloy of equal parts of brass, tin, antimony, and bismuth; but the proportions differ
in different workshops, and much more tin is commonly introduced. Queen’s metal is
said to consist of 9 parts of tin, 1 of antimony, 1 of bismuth, and 1 of lead; it serves also
for teapots and other domestic utensils.
A much safer and better alloy for these purposes may be compounded by adding to
100 parts of the French pewter, 5 parts of antimony, and 5 of brass to harden it. The
English ley pewter contains often much more than 20 per cent. of lead. Under Tin,
will be found the description of an easy method of analyzing its lead alloys.
PHOSPHORIC ACID, is the acid formed by the vivid combustion of
PHOSPHORUS. This interesting simple combustible, being an object of extensive
consumption, and therefore of a considerable chemical manufacture, I shall describe
the requisite manipulations for preparing it at some detail. Put 1 cwt. of finely ground
bone-ash, such as is used by the assayers, into a stout tub, and let one person work it
into a thin pap with twice its weight of water, and let him continue to stir it constantly
with a wooden bar, while another person pours into it, in a uniform but very slender
stream, 78 pounds of concentrated sulphuric acid.
The heat thus excited in the dilution of the acid, and in its reaction upon the calcareous
base, is favourable to the decomposition of the bone phosphate. Should the resulting
sulphate of lime become lumpy, it must be reduced into a uniform paste, by the addition
of a little water from time to time. This mixture must be made out of doors,
as under an open shed, on account of the carbonic acid and other offensive gases which
are extricated. At the end of 24 hours, the pap maybe thinned with water and, if convenient,[953]
heated, with careful stirring, to complete the chemical change, in a square pan
made of sheet lead, simply folded up at the sides. Whenever the paste has lost its granular
character, it is ready for transfer into a series of tall casks, to be further diluted
and settled, whereby the clear superphosphate of lime may be run off by a syphon from
the deposit of gypsum. More water must then be mixed with the precipitate, after
subsidence of which, the supernatant liquor is again to be drawn off. The skilful operator
employs the weak acid from one cask to wash the deposit in another, and thereby saves
fuel in evaporation.
The collected liquors being put into a leaden, or preferably a copper pan, of proper
dimensions, are to be concentrated by steady ebullition, till the calcareous deposit becomes
considerable; after the whole has been allowed to cool, the clear liquor is to be run
off, the sediment removed, and thrown on a filter. The evaporation of the clear liquor
is to be urged till it acquires the consistence of honey. Being now weighed, it should
amount to 37 pounds. One fourth of its weight of charcoal in fine powder, that is, about
9 pounds, are then to be incorporated with it, and the mixture is to be evaporated to
dryness in a cast-iron pot. A good deal of sulphurous acid is disengaged along with
the steam at first, from the reaction of the sulphuric acid upon the charcoal, and afterwards
some sulphuretted hydrogen. When the mixture has become perfectly dry, as
shown by the redness of the bottom of the pot, it is to be allowed to cool, and packed
tight into stoneware jars fitted with close covers, till it is to be subjected to distillation.
For this purpose, earthen retorts of the best quality, and free from air-holes, must be
taken, and evenly luted over their surface with a compost of fire-clay and horse-dung.
When the coating is dry and sound, the retort is to be two-thirds filled with the powder,
and placed upon proper supports in the laboratory of an air-furnace, having its fire
placed not immediately beneath the retort, but to one side, after the plan of a reverberatory;
whereby the flame may play uniformly round the retort, and the fuel may be
supplied as it is wanted, without admitting cold air to endanger its cracking. The
gallery furnace of the palatinate (under Mercury) will show how several retorts may
be operated upon together, with one fire.
To the beak of the retort properly inclined, the one end of a bent copper tube is to
be tightly luted, while the other end is plunged not more than one quarter of an inch
beneath the surface of water contained in a small copper or tin trough placed beneath,
close to the side of the furnace, or in a wide-mouthed bottle. It is of advantage to let
the water be somewhat warm, in order to prevent the concretion of the phosphorus
in the copper tube, and the consequent obstruction of the passage. Should the beak of
the retort appear to get filled with solid phosphorus, a bent rod of iron may be heated, and
passed up the copper tube, without removing its end from the water. The heat of
the furnace should be most slowly raised at first, but afterwards equably maintained in
a state of bright ignition. After 3 or 4 hours of steady firing, carbonic acid and sulphurous
acid gases are evolved in considerable abundance, provided the materials had
not been well dried in the iron pot; then sulphuretted hydrogen makes its appearance,
and next phosphuretted hydrogen, which last should continue during the whole of
the distillation.
The firing should be regulated by the escape of this remarkable gas, which ought to
be at the rate of about 2 bubbles per second. If the discharge comes to be interrupted,
it is to be ascribed either to the temperature being too low, or to the retort getting
cracked; and if upon raising the heat sufficiently no bubbles appear, it is a proof
that the apparatus has become defective, and that it is needless to continue the operation.
In fact, the great nicety in distilling phosphorus lies in the management of the
fire, which must be incessantly watched, and fed by the successive introduction of fuel,
consisting of coke with a mixture of dry wood and coal.
We may infer that the process approaches its conclusion by the increasing slowness
with which gas is disengaged under a powerful heat; and when it ceases to come over,
we may cease firing, taking care to prevent reflux of water into the retort, from condensation
of its gaseous contents, by admitting air into it through a recurved glass tube,
or through the lute of the copper adopter.
The usual period of the operation upon the great scale is from 24 to 30 hours. Its
theory is very obvious. The charcoal at an elevated temperature disoxygenates the
phosphoric acid with the production of carbonic acid gas at first, and afterwards carbonic
oxide gas, along with sulphuretted, carburetted, and phosphuretted hydrogen, from
the reaction of the water present in the charcoal upon the other ingredients.
The phosphorus falls down in drops, like melted wax, and concretes at the bottom
of the water in the receiver. It requires to be purified by squeezing in a shamoy leather
bag, while immersed under the surface of warm water, contained in an earthen pan.
Each bag must be firmly tied into a ball form, of the size of the fist, and compressed,
under the water heated to 130°, by a pair of flat wooden pincers, like those with which
oranges are squeezed.
[954]
The purified phosphorus is moulded for sale into little cylinders, by melting it at the
bottom of a deep jar filled with water, then plunging the wider end of a slightly tapering
but straight glass tube into the water, sucking this up to the top of the glass, so as to
warm it, next immersing the end in the liquid phosphorus, and sucking it up to any
desired height.
The tube being now shut at bottom by the application of the point of the left index,
may be taken from the mouth and transferred into a pan of cold water to congeal the
phosphorus; which then will commonly fall out of itself, if the tube be nicely tapered,
or may at any rate be pushed out with a stiff wire. Were the glass tube not duly
warmed before sucking up the phosphorus, this would be apt to congeal at the sides,
before the middle be filled, and thus form hollow cylinders, very troublesome and even
dangerous to the makers of phosphoric match-bottles. The moulded sticks of phosphorus
are finally to be cut with scissors under water to the requisite lengths, and put
up in phials of a proper size; which should be filled up with water, closed with ground
stoppers, and kept in a dark place. For carriage to a distance, each phial should be
wrapped in paper, and fitted into a tin-plate case.
Phosphorus has a pale yellow colour, is nearly transparent, brittle when cold, soft and
pliable, like wax, at the temperature of 70° F., crystallizing in rhombo-dodecahedrons
out of its combination with sulphur, and of specific gravity 1·77. It exhales white
fumes in the air, which have a garlic smell, appear luminous in the dark, and spontaneously
condense into liquid phosphorous acid. Phosphorus melts in close vessels, at
95°. F., into an oily-looking colourless fluid, begins to evaporate at 217·5°, boils at
554°, and if poured in the liquid state into ice-cold water, it becomes black, but resumes
its former colour when again melted and slowly cooled. It has an acrid disagreeable
taste, and acts deleteriously in the stomach, though it has been administered as a medicine
by some of the poison-doctors of the present day. It takes fire in the open air at
the temperature of 165°, but at a lower degree if partially oxidized, and burns with great
vehemence and splendour.
Inflammable match-boxes (briquets phosphoriques) are usually prepared by putting
into a small phial of glass or lead a bit of phosphorus, and oxidizing it slightly by
stirring it round with a redhot iron wire. The phial should be unstoppered only at
the instant of plunging into it the tip of the sulphur match which we wish to kindle.
Bendix has given the following recipe for charging such match-phials. Take one part
of fine dry cork raspings, one part of yellow wax, eight parts of petroleum, and four
of phosphorus, incorporate them by fusion, and when the mixture has concreted by
cooling, it is capable of kindling a sulphur match dipped into it. Phosphorus dissolves
in fat oils, forming a solution luminous in the dark at ordinary temperatures. A phial
half filled with this oil, being shaken and suddenly uncorked, will give light enough to
see the dial of a watch by night.
There are five combinations, of phosphorus and oxygen:—1. the white oxide; 2. the
red oxide; 3. hypophosphorous acid; 4. phosphorous acid; 5. phosphoric acid. The
last is the only one of interest in the arts. It may be obtained from the syrupy
superphosphate of lime above described, by diluting it with water, saturating with carbonate
of ammonia; evaporating, crystallizing, and gently igniting the salt in a retort.
The ammonia is volatilized, and may be condensed into water by a Woulfe’s apparatus,
while the phosphoric acid remains in the bottom of the retort. Phosphoric acid may be
more readily produced by burning successive bits of phosphorus in a silver saucer, under
a great bell jar inverted upon a glass plate, so as to admit a little air to carry on the
combustion. The acid is obtained in a fine white snowy deposit; consisting, in this its
dry state, of 44 of phosphorus and 56 of oxygen. That obtained from the syrupy solution
is a hydrate, and contains 9·44 per cent. of water. If the atom of phosphorus
be called 32 upon the hydrogen radix, then 5 atoms of oxygen = 40 will be associated
with it in the dry acid, = 72; and an additional atom of water = 9, in the hydrate, will
make its prime equivalent 81. Phosphorous acid seems to contain no more than 3
atoms of oxygen.
The only salts of this acid much in demand, are the phosphate of soda, and the ammonia
phosphate of soda. The former is prepared by slightly supersaturating superphosphate
of lime with crystals of carbonate of soda; warming the solution, filtering,
evaporating, and crystallizing. It is an excellent purgative, and not unpalatable. The triple
phosphate is used in docimastic operations; and is described under Metallurgy.
PICAMARE, is a thick oil, one of the six new principles detected by M. Reichenbach,
in wood-tar. See Creosote and Paraffine. Picamare constitutes 1-6th of beech-tar.
PICROMEL, is the name given by M. Thenard to a black bitter principle which
he supposed to be peculiar to the bile. MM. Gmelin and Tiedemann have since called
its identity in question.
PICROTOXINE, is an intensely bitter poisonous vegetable principle, extracted from
the seeds of the Menispermum cocculus, (Cocculus Indicus). It crystallizes in small white[955]
needles, or columns; dissolves in water and alcohol. It does not combine with acids, but
with some bases, and is not therefore of an alkaline nature, as had been at first supposed.
PIGMENTS, VITRIFIABLE, belong to five different styles of work: 1. to
enamel painting; 2. to painting on metals; 3. to painting on stoneware; 4. to painting
on porcelain; 5. to stained glass.
PIMENTO; Myrtus pimenta, or Jamaica pepper; consists, according to Bonastre’s
complicated analysis, of:—
| |
Shells
or
Capsules. |
Kernels. |
| Volatile oil |
10·0 |
5·0 |
| Soft green resin |
8·0 |
2·5 |
| Fatty concrete oil |
0·9 |
1·2 |
| Extract containing tannin |
11·4 |
39·8 |
| Gum |
3·0 |
7·2 |
| Brown matter dissolved in potash |
4·0 |
8·0 |
| Resinoid matter |
1·2 |
3·2 |
| Extract containing sugar |
3·0 |
8·0 |
| Gallic and malic acids |
0·6 |
1·6 |
| Vegetable fibre |
50·0 |
16·0 |
| Ashes charged with salts |
2·8 |
1·9 |
| Moisture and loss |
4·1 |
4·8 |
| Pimento imported for home consumption, in |
1835. |
1836. |
| Duty—British possessions, 5d.; foreign, 1s. 3d. |
Lbs. |
344,458. |
400,914. |
PINCHBECK, is a modification of brass; see that article and Copper.
PINE-APPLE YARN and CLOTH. In Mr. Zincke’s process, patented in
December, 1836, for preparing the filaments of this plant, the Bromelia ananas, the leaves
being plucked, and deprived of the prickles round their edges by a cutting instrument,
are then beaten upon a wooden block with a wooden mallet, till a silky-looking
mass of fibres be obtained, which are to be freed by washing from the green fecula.
The fibrous part must next be laid straight, and passed between wooden rollers. The
leaves should be gathered between the time of their full maturity and the ripening of
the fruit. If earlier or latter, the fibres will not be so flexible, and will need to be
cleared by a boil in soapy water for some hours; after being laid straight under the
pressure of a wooden grating, to prevent their becoming entangled. When well washed
and dried, with occasional shaking out, they will now appear of a silky fineness. They
may be then spun into porous rovings, in which state they are most conveniently
bleached by the ordinary methods.
Specimens of cambric, both bleached and unbleached, woven with these fibres, have
been recently exhibited, which excited hopes of their rivalling the finest flax fabrics,
but in my opinion without good reason, on account of their want of strength.
PINEY TALLOW, is a concrete fat obtained by boiling with water the fruit of
the Vateria indica, a tree common upon the Malabar coast. It seems to be a substance
intermediate between tallow and wax; partaking of the nature of stearine. It melts
at 971⁄2° F., is white or yellowish, has a spec. grav. of 0·926; is saponified by alkalies,
and forms excellent candles. Dr. Benjamin Babington, to whom we are indebted for all
our knowledge of piney tallow, found its ultimate constituents to be, 77 of carbon, 12·3
of hydrogen, and 10·7 of oxygen.
PIN MANUFACTURE. (Fabrique d’épingles, Fr.; Nadelfabrik, Germ.) A pin is
a small bit of wire, commonly brass, with a point at one end, and a spherical head at the
other. In making this little article, there are no less than fourteen distinct operations.
1. Straightening the wire. The wire, as obtained from the drawing-frame, is wound
about a bobbin or barrel, about 6 inches diameter, which gives it a curvature that must
be removed. The straightening engine is formed by fixing 6 or 7 nails upright in a
waving line on a board, so that the void space measured in a straight line between the
first three nails may have exactly the thickness of the wire to be trimmed; and that the
other nails may make the wire take a certain curve line, which must vary with its
thickness. The workman pulls the wire with pincers through among these nails, to
the length of about 30 feet, at a running draught; and after he cuts that off, he returns
for as much more; he can thus finish 600 fathoms in the hour. He next cuts these
long pieces into lengths of 3 or 4 pins. A day’s work of one man amounts to 18 or
20 thousand dozen of pin-lengths.
2. Pointing, is executed on two iron or steel grindstones, by two workmen, one of
whom roughens down, and the other finishes. Thirty or forty of the pin wires are
applied to the grindstone at once, arranged in one plane, between the two forefingers
and thumbs of both hands, which dexterously give them a rotatory movement.
[956]
3. Cutting these wires into pin-lengths. This is done by an adjusted chisel. The intermediate
portions are handed over to the pointer.
4. Twisting of the wire for the pin-heads. These are made of a much finer wire, coiled
into a compact spiral, round a wire of the size of the pins, by means of a small lathe
constructed for the purpose.
5. Cutting the heads. Two turns are dexterously cut off for each head, by a regulated
chisel, A skilful workman may turn off 12,000 in the hour.
6. Annealing the heads. They are put into an iron ladle, made redhot over an open
fire, and then thrown into cold water.
7. Stamping or shaping the heads. This is done by the blow of a small ram, raised
by means of a pedal lever and a cord. The pin-heads are also fixed on by the same
operative, who makes about 1500 pins in the hour, or from 12,000 to 15,000 per
diem; exclusive of one-thirteenth, which is always deducted for waste in this department,
as well as in the rest of the manufacture. Cast heads, of an alloy of tin and
antimony, were introduced by patent, but never came into general use.
8. Yellowing or cleaning the pins, is effected by boiling them for half an hour in sour
beer, wine lees, or solution of tartar; after which they are washed.
9. Whitening or tinning. A stratum of about 6 pounds of pins is laid in a copper
pan, then a stratum of about 7 or 8 pounds of grain tin; and so alternately till the
vessel be filled; a pipe being left inserted at one side, to permit the introduction of
water slowly at the bottom, without deranging the contents. When the pipe is withdrawn,
its space is filled up with grain tin. The vessel being now set on the fire,
and the water becoming hot, its surface is sprinkled with 4 ounces of cream of tartar;
after which it is allowed to boil for an hour. The pins and tin grains are, lastly,
separated by a kind of cullender.
10. Washing the pins, in pure water.
11. Drying and polishing them, in a leather sack filled with coarse bran, which is
agitated to and fro by two men.
12. Winnowing, by fanners.
13. Pricking the papers for receiving the pins.
14. Papering, or fixing them in the paper. This is done by children, who acquire
the habit of putting up 36,000 per day.
The pin manufacture is one of the greatest prodigies of the division of labour;
it furnishes 12,000 articles for the sum of three shillings, which have required the united
diligence of fourteen skilful operatives.
The above is an outline of the mode of manufacturing pins by hand labour, but several
beautiful inventions have been employed to make them entirely or in a great measure
by machinery; the consumption for home sale and export amounting to 15 millions
daily, for this country alone. One of the most elaborate and apparently complete, is
that for which Mr. L. W. Wright obtained a patent in May, 1824. A detailed
description of it will be found in the 9th volume of Newton’s London Journal. The following
outline will give my readers an idea of the structure of this ingenious machine:—
The rotation of a principal shaft mounted with several cams, gives motion to various
sliders, levers, and wheels, which work the different parts. A slider pushes pincers
forwards, which draw wire from a reel, at every rotation of the shaft, and advance such a
length of wire as will produce one pin. A dye cuts off the said length of wire by the
descent of its upper chap; the chap then opens a carrier, which takes the pin to the
pointing apparatus. Here it is received by a holder, which turns round, while a bevel-edged
file-wheel rapidly revolves, and tapers the end of the wire to a point. The pin
is now conducted by a second carrier to a finer file-wheel, in order to finish the point
by a second grinding. A third carrier then transfers the pin to the first heading die,
and by the advance of a steel punch, the end of the pin wire is forced into a recess,
whereby the head is partially swelled out. A fourth carrier removes the pin to a second
die, where the heading is perfected. When the heading-bar retires, a forked lever draws
the finished pin from the die, and drops it into a receptacle below.
I believe the chief objection to the raising of the heads by strong mechanical compression
upon the pins, is the necessity of softening the wire previously; whereby the pins
thus made, however beautiful to the eye, are deficient in that stiffness which is so
essential to their employment in many operations of the toilet.
PIPERINE, is a crystalline principle extracted from black pepper, by means of
alcohol. It is colourless, has hardly any taste, fuses at 212° F.; is insoluble in water,
but soluble in acetic acid, ether, and most readily in alcohol.
PITCH, MINERAL, is the same as Bitumen and Asphalt.
PITCH of wood-tar (Poix, Fr.; Pech, Germ.); is obtained by boiling tar in an open
iron pot, or in a still, till the volatile matters be driven off. Pitch contains, pyrolignous
resin, along with colophany (common rosin), but its principal ingredient is the former,
called by Berzelius pyretine. It is brittle in the cold, but softens and becomes ductile[957]
with heat. It melts in boiling water, and dissolves in alcohol and oil of turpentine, as
well as in carbonated or caustic alkaline lyes. For Pyretine, see the mode of preparing
it from birch wood, for the purpose of preparing Russia Leather.
PITCOAL. (Houille, Fr.; Steinkohle, Germ.) This is by far the most valuable of
mineral treasures, and the one which, at least in Great Britain, makes all the others
available to the use and comfort of man. Hence it has been searched after with unremitting
diligence, and worked with all the lights of science, and the resources of art.
The Brora coal-field in Sutherlandshire is the most remarkable example in this, or
in perhaps any country hitherto investigated, of a pseudo coal-basin among the deeper
secondary strata, but above the new sandstone or red marl formation. The Rev.
Dr. Buckland and Mr. C. Lyell, after visiting it in 1824, had expressed an opinion that
the strata there were wholly unconnected with the proper coal formation below the new
red sandstone, and were in fact the equivalent of the oolitic series; an opinion fully
confirmed by the subsequent researches of Mr. Murchison. (Geol. Trans. for 1827,
p. 293.) The Brora coal-field forms a part of those secondary deposits which range
along the south-east coast of Sutherlandshire, occupying a narrow tract of about twenty
miles in length, and three in its greatest breadth.
One stratum of the Brora coal-pit is a coal-shale, composed of a reed-like striated
plant of the natural order Equisetum, which seems to have contributed largely
towards the formation of that variety of coal. From this coal-shale, the next transition
upwards is into a purer bituminous substance approaching to jet, which constitutes
the great bed of coal. This is from 3 feet 3 inches to 3 feet 8 inches thick, and is
divided nearly in the middle by a thin layer of impure indurated shale charged with
pyrites, which, if not carefully excluded from the mass, sometimes occasions spontaneous
combustion upon exposure to the atmosphere; and so much indeed is that
mineral disseminated throughout the district, that the shales might be generally termed
“pyritiferous.” Inattention on the part of the workmen, in 1817, in leaving a large
quantity of this pyritous matter to accumulate in the pit, occasioned a spontaneous
combustion, which was extinguished only by excluding the air; indeed the coal-pit was
closed in and remained unworked for four years. The fires broke out again in the pit
in 1827.
The purer part of the Brora coal resembles common pitcoal; but its powder has the
red ferruginous tinge of pulverized lignites. It may be considered one of the last links
between lignite and true coal, approaching very nearly in character to jet, though less
tenacious than that mineral; and, when burnt, exhaling but slightly the vegetable odour
so peculiar to all imperfectly bituminized substances. The fossil remains of shells and
plants prove the Brora coal to be analogous to that of the eastern moorlands of Yorkshire,
although the extraordinary thickness of the former, compared with any similar
deposit of the latter (which never exceeds from 12 to 17 inches), might have formerly
led to the belief that it was a detached and anomalous deposit of true coal, rather than a
lignite of any of the formations above the new red sandstone: such misconception might
more easily arise in the infancy of geology, when the strata were not identified by their
fossil organic remains.
On the coast of Yorkshire the strata of this pseudo coal formation appear in the
following descending order, from Filey Bay to Whitby. 1. Coral-rag. 2. Calcareous
grit. 3. Shale, with fossils of the Oxford clay. 4. Kelloway rock (swelling out into
an important arenaceous formation). 5. Cornbrash. 6. Coaly grit of Smith. 7. Pier-stone
(according to Mr. Smith, the equivalent of the great oolite). 8. Sandstone and
shale, with peculiar plants and various seams of coal. 9. A bed with fossils of the inferior
oolite. 10. Marl-stone? 11. Alum-shale or lias. All the above strata are identified
by abundant organic remains.
In the oolitic series, therefore, where the several strata are developed in conformity
with the more ordinary type of these formations, we may venture to predict with
certainty, that no carboniferous deposits of any great value will ever be discovered,
at all events in Great Britain. A want of such knowledge has induced many persons
to make trials for coal in beds subordinate to the English oolites, and even superior to
them, in places where the type of formation did not offer the least warrant for such
attempts.
The third great class of terrestrial strata, is the proper coal-measures, called
the carboniferous rocks, our leading object here, and to which we shall presently
return.
The transition rocks which lie beneath the coal-measures, and above the primitive
rocks, or are anterior to the carboniferous order, and posterior to the primitive,
contain a peculiar kind of coal, called anthracite or stone-coal, approaching closely in its
nature to carbon. It is chiefly in the transition clay-slate that the anthracite occurs in
considerable masses. There is one in the transition slate of the little Saint Bernard,
near the village of la Thuile (in the Alps). It is 100 feet long, and 2 or 3 yards thick.[958]
The coal burns with difficulty, and is used only for burning lime. There are several of
the same kind in that country, which extend down the reverse slope of the mountains
looking to Savoy. The slate enclosing them presents vegetable impressions of reeds or
analogous plants. To the transition clay-slate we must likewise refer the beds of
anthracite that M. Hericart de Thury observed at very great heights in the Alps of
Dauphiny, in a formation of schist and grey-wacke with vegetable impressions, which
reposes directly on the primitive rocks.
The great carboniferous formation may be subdivided into four orders of rocks: 1. the
coal-measures, including their manifold alternations of coal-beds, sandstones, and shales;
2. the millstone grit and shale towards the bottom of the coal-measures; 3. the carboniferous
limestone, which projecting to considerable heights above the outcrop of the coal
and grit, acquires the title of mountain limestone; 4. the old red sandstone, or connecting
link with the transition and primary rock basin in which the coal system lies.
The coal-fields of England, from geographical position, naturally fall under the following
arrangement:—1. The great northern district; including all the coal-fields north
of Trent. 2. The central district; including Leicester, Warwick, Stafford, and Shropshire.
3. The western district; subdivided into north-western, including North Wales,
and the south-western, including South Wales, Gloucester, and Somersetshire.
There are three principal coal-basins in Scotland: 1. that of Ayrshire; 2. that of
Clydesdale; and 3. that of the valley of the Forth, which runs into the second in the
line of the Union Canal. If two lines be drawn, one from Saint Andrews on the northeast
coast, to Kilpatrick on the Clyde, and another from Aberlady, in Haddingtonshire, to
a point a few miles south of Kirkoswald in Ayrshire, they will include between them
the whole space where pitcoal has been discovered and worked in Scotland.
The great coal-series consists of a regular alternation of mineral strata deposited in a
great concavity or basin, the sides and bottom of which are composed of transition rocks.
This arrangement will be clearly understood by inspecting fig. 794., which represents
a section of the coal-field south of Malmsbury.
1, 1, old red sandstone; 2, mountain limestone; 3, millstone grit; 4, 4, coal seams;
5, Pennant, or coarse sandstone; 6, new red sandstone, or red marl; 7, 7, lias; 8, 8,
inferior oolite; 9, great oolite; 10, cornbrash and Forest marble.
No. 1., or the old red sandstone, may therefore be regarded as the characteristic lining
of the coal basins; but this sandstone rests on transition limestone, and this limestone
on grey-wacke. This methodical distribution of the carboniferous series is well exemplified
in the coal-basin of the Forest of Dean in the south-west of England, and has
been accurately described by Mr. Mushet.
The grey-wacke consists of highly inclined beds of slaty micaceous sandstone, which
on the one hand alternates with and passes into a coarse breccia, having grains as
large as peas; on the other, into a soft argillaceous slate. The grey-wacke stands
bare on the north-eastern border of the Forest, near the southern extremity of the chain
of transition limestone, which extends from Stoke Edith, near Hereford, to Flaxley on
the Severn. It is traversed by a defile, through which the road from Gloucester to Ross
winds. The abruptness of this pass gives it a wild and mountainous character, and
affords the best opportunity of examining the varieties of the rock.
The Transition limestone consists in its lower beds of fine-grained, tender, extremely
argillaceous slate, known in the district by the name of water-stone, in consequence of the
wet soil that is found wherever it appears at the surface. Calcareous matter is interspersed
in it but sparingly. Its upper beds consist of shale alternating with extensive
beds of stratified limestone. The lowest of the calcareous strata are thin, and alternate with
shale. On these repose thicker strata of more compact limestone, often of a dull blue
colour. The beds are often dolomitic, which is indicated by straw yellow colour, or
dark pink colour, and by the sandy or glimmering aspect of the rock.
The old red sandstone, whose limits are so restricted in other parts of England, here[959]
occupies an extensive area. The space which it covers, its great thickness, its high
inclination, the abrupt character of the surface over which it prevails, and the consequent
display of its strata in many natural sections, present in this strict advantages for
studying the formation, which are not to be met with elsewhere in South Britain. In
the neighbourhood of Mitchel Dean, the total thickness of this formation, interposed
conformably between the transition and mountain limestone, is from 600 to 800 fathoms.
The old red sandstone is characterized in its upper portion by the presence of siliceous
conglomerate, containing siliceous pebbles, which is applied extensively to the fabrication
of millstones near Monmouth, and on the banks of the Wye. This sandstone encircles
the Forest with a ring of very elevated ground, whose long and lofty ridges on the
eastern frontier overhang the valley of the Severn.
The mountain limestone, or carboniferous, is distinguished from transition limestone,
rather by its position than by any very wide difference in its general character or organic
remains. According to the measurements of Mr. Mushet, the total thickness of the
mountain limestone is about 120 fathoms. The zone of limestone belonging to this
coal-basin, is from a furlong to a mile in breadth on the surface of the ground, according
as the dip of the strata is more or less rapid. The angle of dip on the northern and
western border is often no more than 10°, but on the eastern it frequently amounts to
80°. The calcareous zone that defines the outer circle of the basin, suffers only one
short interruption, scarcely three miles in length, where in consequence of a fault the
limestone disappears, and the coal-measures are seen in contact with the old red
sandstone.
Coal measures.—Their aggregate thickness amounts, according to Mr. Mushet, to
about 500 fathoms. 1. The lowest beds, which repose on the mountain limestone, are
about 40 fathoms thick, and consist here, as in the Bristol coal-basin, of a red siliceous
grit, alternating with conglomerate, used for millstones; and with clay, occasionally
used for ochre. 2. These beds are succeeded by a series about 120 fathoms thick, in
which a grey gritstone predominates, alternating in the lower part with shale, and
containing 6 seams of coal. The grits are of a fissile character, and are quarried
extensively for flag-stone, ashlers, and fire-stone. 3. A bed of grit, 25 fathoms thick,
quarried for hearth-stone, separates the preceding series from the following, or the 4th,
which is about 115 fathoms thick, and consists of from 12 to 14 seams of coal alternating
with shale. 5. To this succeeds a straw-coloured sandstone, nearly 100 fathoms thick,
forming a high ridge in the interior of the basin. It contains several thin seams of
coal, from 6 to 16 inches in thickness. 6. On this reposes a series of about 12 fathoms
thick, consisting of 3 seams of coal alternating with shale. 7. This is covered with
alternate beds of grit and shale, whose aggregate thickness is about 100 fathoms, occupying
a tract in the centre of the basin about 4 miles long, and 2 miles broad. The
sandstone No. 5. is probably the equivalent of the Pennant in the preceding figure.
The floor, or pavement, immediately under the coal beds is, almost without exception,
a grayish-slate clay, which, when made into bricks, strongly resists the fire. This fire-clay
varies in thickness from a fraction of an inch to several fathoms. Clay ironstone is
often disseminated through the shale.
The most complete and simplest form of a coal-field is the entire basin-shape, which
we find in some instances without a dislocation. A beautiful example of this is to be
seen at Blairengone, in the county of Perth, immediately adjoining the western boundary
of Clackmannanshire, as represented in fig. 795., where the outer elliptical line, marked
A, B, C, D, represents the crop, outburst, or basset edge of the lower coal, and the inner
elliptical line represents the crop or basset edge of the superior coal. Fig. 796. is the[960]
longitudinal section of the line A B; and fig. 797. the transverse section of the line C D.
All the accompanying coal strata partake of the same form and parallelism. These
basins are generally elliptical, sometimes nearly circular, but are often very eccentric,
being much greater in length than in breadth; and frequently one side of the basin on
the short diameter has a much greater dip than the other, which circumstance throws
the trough or lower part of the basin concavity much nearer to the one side than to the
other. From this view of one entire basin, it is evident that the dip of the coal strata
belonging to it runs in opposite directions, on the opposite sides, and that all the strata
regularly crop out, and meet the alluvial cover in every point of the circumferential space,
like the edges of a nest of common basins. The waving line marks the river Devon.
It is from this basin shape that all the other coal-fields are formed, which are segments
of a basin produced by slips, dikes, or dislocations of the strata. If the coals (fig. 795.)
were dislocated by two slips b c and d e, the slip b c throwing the strata down to the east,
and the slip d e throwing them as much up in the same direction, the outcrops of the
coals would be found in the form represented in fig. 798., of which fig. 799. is the section
in the line A B, and fig. 800. the section in the line C D.
The chief difficulty in exploring a country in search of coal, or one where coal-fields
are known to exist, arises from the great thickness of alluvial and other cover, which
completely hides the outcrop or basset edge of the strata, called by miners the rock-head;
as also the fissures, dikes, and dislocations of the strata, which so entirely change the
structure and bearings of coal-fields, and cause often great loss to the mining adventurer.
The alluvial cover on the other hand is beneficial, by protecting the seams of the strata
from the superficial waters and rains, which would be apt to drown them, if they were
naked. In all these figures of coal-basins, the letter a indicates coal.
The absolute shape of the coal-fields in Great Britain has been ascertained with
surprising precision. To whatever depth a coal-mine is drained of its water, from that
depth it is worked, up to the rise of the water-level line, and each miner continues to
advance his room or working-place, till his seam of coal meets the alluvial cover of the
outcrop, or is cut off by a dislocation of the strata. In this way the miner travels in
succession over every point of his field, and can pourtray its basin-shape most minutely.
Fig. 801. represents a horizontal plan of the Clackmannanshire coal-field, as if the
strata at the outcrop all around were denuded
of the alluvial cover. Only two of the concentric
beds, or of their edges a, a, are represented,
to avoid perplexity. It is to be remembered,
however, that all the series of
attendant strata lie parallel to the above lines.
This plan shows the Ochill mountains, with
the north coal-fields, of an oblong elliptical
shape, the side of the basin next the mountains
being precipitous, as if upheaved by the eruptive
trap-rocks; while the south, the east, and
the west edges of the basin shelve out at a great
distance from the lower part of the concavity
or trough, as miners call it. Thus the alternate
beds of coal, shale, and sandstone, all nearly
concentric in the north coal-field, dip inwards
from all sides towards the central area of the
trough. The middle coal-field of this district,
however, which is formed by the great north
slip, is merely the segment of an elliptical
basin, where the strata dip in every direction to the middle of the axis marked with the
letter X; being the deepest part of the segment. The south coal-field, formed by the great
south slip, is likewise the segment of another elliptical basin, similar in all respects to the
middle coal-field. Beyond the outcrop of the coals and subordinate strata of the south
coal-fields, the counter dip of the strata takes place, producing the mantle-shaped form;
whence the coal strata in the Dunmore field, in Stirlingshire, lie in a direction contrary
to those of the south coal-field of Clackmannanshire. O, are the Ochill mountains.
Fig. 802. is intended to represent an extensive district of country, containing a great
coal-basin, divided into numerous subordinate coal-fields by these dislocations. The
lines marked b are slips, or faults; the broad lines marked c denote dykes: the former
dislocate the strata, and change their level, while dykes disjoin the strata with a wall, but
do not in general affect their elevation. The two parallel lines marked a, represent two
seams of coal, variously heaved up and down by the faults; whereas the dykes are seen
to pass through the strata without altering their relative position. In this manner,
partial coal-fields are distributed over a wide area of country, in every direction.
The only exception to this general form of the coal-fields in Great Britain, is the inverted[961]
basin shape; but this is rare. A few examples occur in some districts of England,
and in the county of Fife; but even in extensive coal-fields, this convex form is
but a partial occurrence, or a deviation by local violence from the ordinary basin.
Fig. 803 is an instance of a convex coal-field exhibited in Staffordshire, at the Castle-hill,
close to the town of Dudley.
1, 1, are limestone strata; 2, 2, are coal.
Through this hill, canals have been
cut, for working the immense beds of
carboniferous limestone. These occur
in the lower series of the strata of
the coal-field, and therefore at a distance
of many miles from the Castle-hill,
beyond the outcrop of all the
workable coals in the proper basin-shaped
part of the field; but by this
apparently inverted basin-form, these
limestone beds are elevated far above
the level of the general surface of the
country, and consequently above the
level of all the coals. We must regard
this seeming inversion as resulting from
the approximation of two coal-basins,
separated by the basset edges of their
mountain limestone repository.

Fig. 804. is a vertical section of the
Dudley coal-basin, the upper coal-bed
of which has the astonishing thickness
of 30 feet; and this mass extends
7 miles in length, and 4 in breadth.
Coal-seams 5 or 6 feet thick, are called
thin in that district.
Fig. 805. is a very interesting section
of the main coal-basin of Clackmannanshire,
as given by Mr. Bald in the
Wernerian Society’s Memoirs, vol. iii.
Here we see it broken into three subordinate
coal-fields, formed by two great faults or dislocations of the strata; but
independently of these fractures across the whole series, the strata continue quite
regular in their respective alternations, and preserve nearly unchanged
their angle of inclination to the horizon. The section shows the south
coal-field dipping northerly, till it is cut across by the great south slip x,
which dislocates the coal and the parallel strata to the enormous extent
of 1230 feet, by which all the coals have been thrown up, not simply to
the day, but are not found again till we advance nearly a mile northward,
on the line of the dip, where the identical seams of coal, shale, &c.
are observed once more with their regular inclination. These coals of
the middle area, dip regularly northward till interrupted by the great
north slip y, which dislocates the strata, and throws them up 700 feet;
that is to say, a line prolonged in the direction of any one well-known
seam, will run 700 feet above the line of the same seam as it emerges
after the middle slip. Immediately adjoining the north slip, the coals
and coal-field resume their course, and dip regularly northward, running
through a longer range than either of the other two members of
the basin, till they arrive at the valley of the Devon, at the foot of the
Ochill mountains, where they form a concave curvature, or trough, a, and
thence rise rapidly in an almost vertical direction at b. Here the coals,
with all their associate strata, assume conformity and parallelism with the face of the
sienitic-greenstone strata of the Ochill mountains c; being raised to the high angle of 73
degrees with the horizon. The coal-seams thus upheaved, are called edge-metals by the
miners.
[962]
In this remarkable coal-field, which has been accurately explored by pitting and
boring to the depth of 703 feet, there are no fewer than 142 beds, or distinct strata of
coal, shale, and sandstone, &c., variously alternating, an idea of which may be had
by inspecting fig. 806. Among these are 24 beds of coal, which would constitute
an aggregate thickness of 59 feet 4 inches; the thinnest seam of coal
being 2 inches, and the thickest 9 feet. The strata of this section contain
numerous varieties of sandstone, slate-clay, bituminous shale, indurated clay, or
fire-clay, and clay ironstone. Neither trap-rock nor limestone is found in connexion
with the workable coals; but an immense bed of greenstone, named
Abbey Craig, occurs in the western boundary of Clackmannanshire, under which
lie regular strata of slate-clay, sandstone, thin beds of limestone, and large spheroidal
masses of clay ironstone, with a mixture of lime.
“With regard to slips in coal fields,” says Mr. Bald, “we find that there is a
general law connected with them as to the position of the dislocated strata,
which is this:—When a slip is met with in the course of working the mines—if
when looking to it, the vertical line of the slip or fissure, it forms an acute angle
with the line of the pavement upon which the observer stands, we are certain
that the strata are dislocated downwards upon the other side of the fissure. On
the contrary, if the angle formed by the two lines above mentioned is obtuse, we
are certain that the strata are dislocated or thrown upwards upon the other side
of the fissure. When the angle is 90°, or a right angle, it is altogether uncertain
whether the dislocation throws up or down on the opposite side of the slip.
When dikes intercept the strata, they generally only separate the strata the
width of the dike, without any dislocation, either up or down; so that if a coal
is intercepted by a dike, it is found again by running a mine directly forward,
corresponding to the angle or inclination of the coal with the horizon.”—Wernerian
Society’s Memoirs, vol. iii. p. 133.[40]
| a. |
Alluvial cover. |
| b. |
Bed of trap or greenstone. |
| c. |
Alternating coal strata. |
| d. |
Coal-seams. |
| e. |
Position of greenstone, not ascertained. |
| f. |
Strata in which no coals have been found. |
| g. |
The overlapped coal. |
| h. |
The double coal. |
The Johnstone coal-field, in Renfrewshire, is both singular and interesting.
The upper stratum of rock is a mass of compact greenstone or trap, above 100
feet in thickness, not at all in a conformable position with the coal strata, but
overlying; next there is a few fathoms of soft sandstone and slate-clay, alternating,
and uncommonly soft. Beneath these beds, there are no fewer than ten
seams of coal, lying on each other, with a few divisions of dark indurated clay.
These coal-seams have an aggregate thickness of no less than 100 feet; a mass of
combustible matter, in the form of coal, unparalleled for its accumulation in so
narrow a space. The greater part of this field contains only 5 beds of coal; but
at the place where the section shown in fig. 807. is taken, these 5 coals seem to
have been overlapped or made to slide over each other by violence. This structure
is represented in fig. 808., which is a section of the Quarrelton coal in the
Johnstone field, showing the overlapped coal and the double coal, with the thick
bed of greenstone, overlying the coal-field.
Before proceeding to examine the modes of working coal, I shall introduce here a
description of the two principal species of this mineral.
1. Cubical coal.—It is black, shining, compact, moderately hard, but easily frangible.
When extracted in the mine, it comes out in rectangular masses, of which the smaller
fragments are cubical. The lamellæ (reed of the coal) are always parallel to the bed or
plane on which the coal rests; a fact which holds generally with this substance. There
are two varieties of cubical coal; the open-burning and the caking. The latter, however
small its fragments may be, is quite available for fuel, in consequence of its agglutinating
into a mass at a moderate heat, by the abundance of its bitumen. This kind is the true
smithy or forge-coal, because it readily forms itself into a vault round the blast of the
bellows, which serves for a cupola in concentrating the heat on objects thrust into the
cavity.
The open-burning cubical coals are known by several local names; the rough coal or[963]
clod coal, from the large masses in which they may be had; and the cherry coal, from
the cheerful blaze with which they spontaneously burn; whereas the caking coals, such
as most of the Newcastle qualities, require to be frequently poked in the grate. Its
specific gravity varies from 1·25 to 1·4.
2. Slate or splint coal.—This is dull-black, very compact, much harder, and more
difficultly frangible than the preceding. It is readily fissile, like slate, but powerfully
resists the cross fracture, which is conchoidal. Specific gravity from 1·26 to 1·40. In
working, it separates in large quadrangular sharp-edged masses. It burns without
caking, produces much flame and smoke, unless judiciously supplied with air, and leaves
frequently a considerable bulk of white ashes. It is the best fuel for distilleries and all
large grates, as it makes an open fire, and does not clog up the bars with glassy scoriæ.
I found good splint coal of the Glasgow field to have a specific gravity of 1·266, and to
consist of—carbon, 70·9; hydrogen, 4·3; oxygen, 24·8.
3. Cannel coal.—Colour between velvet and grayish-black; lustre resinous; fracture
even; fragments trapezoidal; hard as splint coal; spec. grav. 1·23 to 1·28. In working,
it is detached in four-sided columnar masses, often breaks conchoidal, like pitch, kindles
very readily, and burns with a bright white projective flame, like the wick of a candle,
whence its name. It occurs most abundantly in the coal-field of Wigan, in Lancashire,
in a bed 4 feet thick; and there is a good deal of it in the Clydesdale coal-field, of which
it forms the lowest seam that is worked. It produces very little dust in the mine, and
hardly soils the fingers with carbonaceous matter. Cannel coal from Woodhall, near
Glasgow, spec. grav. 1·228, consists by my analysis of—carbon, 72·22; hydrogen, 3·93;
oxygen, 21·05; with a little azote (about 2·8 in 100 parts). This coal has been found to
afford, in the Scotch gas-works, a very rich-burning gas. The azote is there converted
into ammonia, of which a considerable quantity is distilled over into the tar-pit.
4. Glance coal.—This species has an iron-black colour, with an occasional iridescence,
like that of tempered steel; lustre in general splendent, shining, and imperfect metallic;
does not soil; easily frangible; fracture flat conchoidal; fragments sharp-edged. It burns
without flame or smell, except when it is sulphureous; and it leaves a white-coloured
ash. It produces no soot, and seems, indeed, to be merely carbon, or coal deprived of
its volatile matter or bitumen, and converted into coke by subterranean calcination, frequently
from contact with whin-dikes. Glance coal abounds in Ireland, under the name
of Kilkenny coal; in Scotland it is called blind coal, from its burning without flame or
smoke; and in Wales, it is the malting or stone coal. It contains from 90 to 97 per
cent. of carbon. Specific gravity from 1·3 to 1·5; increasing with the proportion of
earthy impurities.
The dislocations and obstructions found in coal-fields, which render the search for coal
so difficult, and their mining so laborious and uncertain, are the following:—
1. Dikes. 2. Slips or Faults. 3. Hitches. 4. Troubles.
The first three infer dislocation of the strata; the fourth changes in the bed of coal itself.
1. A dike is a wall of extraneous matter, which divides all the beds in a coal-field.
Dikes extend not only in one line of bearing through coal-fields for many miles, but
run sometimes in different directions, and have often irregular bendings, but no sharp
angular turns. When from a few feet to a few fathoms in thickness, they occur sometimes
in numbers within a small area of a coal basin, running in various directions, and
even crossing each other. Fig. 809. represents a ground plan of a coal-field, intersected
with greenstone dikes. A B and C D
are two dikes standing parallel to each
other; E F and G H are cross or oblique
dikes, which divide both the coal strata
and the primary dikes A B and C D.
2. Slips or faults run in straight lines
through coal-measures, and at every
angle of incidence to each other.
Fig. 810. represents a ground plan of
a coal-field, with two slips A B and C D
in the line of bearing of the planes of
the strata, which throw them down to
the outcrop. This is the simplest form
of a slip. Fig. 811. exhibits part of a
coal-field intersected with slips, like a
cracked sheet of ice. Here A B is a
dike; while the narrow lines show
faults of every kind, producing dislocations
varying in amount of slip from a few feet to a great many fathoms. The faults
at the points a, a, a vanish; and the lines at c denote four small partial slips called
hitches.
[964]
The effects of slips and dikes on the coal strata appear more prominently when
viewed in a vertical section, than in a ground plan, where they seem to be merely walls,
veins, and lines of demarcation. Fig. 812. is a vertical section of a coal-field, from dip
to rise, showing three strata of coal a, b, c. A B represents a dike at right angles to the
plane of the coal-beds. This rectangular wall merely separates the coal-measures,
affecting their line of rise; but further to the rise, the oblique dike C D interrupts the
coals a, b, c, and not only disjoins them, but throws them and their concomitant strata
greatly lower down; but still, with this depression, the strata retain their parallelism
and general slope. Nearer to the outcrop, another dike E, F, interrupts the coals a, b, c,
not merely breaking the continuity of the planes, but throwing them moderately up, so
as to produce a steeper inclination, as shown in the figure. It sometimes happens that
the coals in the compartment H, betwixt the dikes C and E, may lie nearly horizontal,
and the effect of the dike E, F, is then to throw out the coals altogether, leaving no
vestige of them in the compartment K. “Such,” says Mr. Bald, from whom these
illustrations are borrowed, “are the most prominent changes in the strata, as to their
line of direction, produced by dikes; but of these changes there are various modifications.”
The effect of slips on the strata is also represented in the vertical section, fig. 813., where
a, b, c are coals with their associated strata. A, B, is an intersecting slip, which throws all
the coals of the first compartment
much lower, as is
observable in the second,
No. 2.; and from the amount
of the slip, it brings in other
coal-seams, marked 1, 2, 3,
not in the compartment
No. 1. C, D, is a slip producing
a similar result, but
not of the same magnitude.
E, F, represents a slip across
the strata, reverse in direction
to the former; the effect of which is to throw up the coals, as shown in the area
No. 4. Such a slip occasionally brings into play seams seated under those marked a, b, c,
as seen at 4, 5, 6; and it may happen
that the coal marked 4 lies in the prolongation
of a well-known seam, as c,
in the compartment No. 3., when the
case becomes puzzling to the miner.
In addition to the above varieties, a
number of slips or hitches are often seen
near one another, as in the area marked
No. 5., where the individual displacements
are inconsiderable, but the aggregate
dislocation may be great, in
reference to the seams of the 6th compartment.
The results of dikes and slips on a
horizontal portion of a field are exemplified
in fig. 814. Where the coal-measures
are horizontal, and the faults
run at a greater angle than 45° to the
line of bearing, they are termed dip
and rise faults, as A B, C D, E F.
Coal viewers or engineers regard the
dislocations now described as being subject
in one respect to a general law, which may be thus explained:—Let fig. 815.[965]
be a portion of a coal-measure; A, being the pavement and B the roof of the coal-seam.
If, in pursuing the stratum at C, a dike D occurs, standing at right angles with the
pavement, they conclude that the dike is merely a partition-wall between the beds by
its own thickness, leaving the coal-seam underanged on either side; but if a dike F
forms, as at E, an obtuse angle with the pavement, they conclude that the dike is not a
simple partition between the strata, but has thrown up the several seams into the predicament
shown at G. Finally, should a dike H make at I an acute angle with the
pavement, they conclude that the dike has thrown down the coal-measures into the
position of K.
The same important law holds with slips, as I formerly stated; only when they
form right angles with the pavement, the case is ambiguous; that is, the strata may be
dislocated either upwards or downwards.
Dikes and faults are denominated upthrow or downthrow, according to the position
they are met with in working the mine. Thus, in fig. 812., if the miner is advancing
to the rise, the dike A, B obviously does not change the direction; but C, D is a
downthrow dike of a certain number of fathoms towards the rise of the basin, and
E, F is an upthrow dike likewise towards the rise. On the other hand, when the dikes
are met with by the miner in working from the rise to the dip, the names of the above
dikes would be reversed; for what is an upthrow in the first case, becomes a downthrow
in the second, relative to the mining operations.
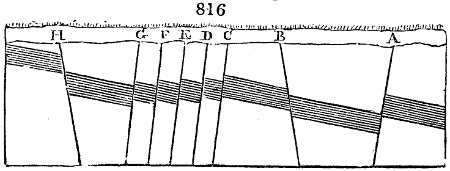
3. We have seen that hitches are small and partial slips, where the dislocation does not
exceed the thickness of the coal-seam; and they are correctly enough called steps by the
miner. Fig. 816. represents the operation of the hitches A, B, C, D, E, F, G, H, on the coal-measures.
Though observed in one
or two seams of a field, they may
not appear in the rest, as is the
case with dikes and faults.
4. Troubles in coal-fields are
of various kinds.
1. Irregular layers of sandstone,
appearing in the middle
of the coal-seam, and gradually
increasing in thickness till they
separate the coal into two distinct
seams, too thin to continue
workable.
2. Nips, occasioned by the
gradual approximation of the
roof and pavement, till not a vestige of coal is left between them; the softer shale
disappearing also at the same time. Figs. 817. and 818. represent this accident, which
is fortunately rare; the first being a vertical, and the second a horizontal view.
3. Shaken coal. It resembles the rubbish of an old waste, being a confused heap of
coal-dust, mixed with small pieces of cubical coal, so soft that it can frequently be dug
with the spade. This shattering is analogous to that observed occasionally in the flint
nodules of the chalk formation; and seems like the effect of some electric tremor of the
strata.
In searching for coal in any country, its concomitant rocks ought to be looked for,
especially the carboniferous or mountain limestone, known by its organic fossils; (see
Ure’s Geology, p. 175, and corresponding plate of fossils;) likewise the outcrop of the
millstone grit, and the newer red sandstone, among some rifts or façades of which,
seams of coal may be discerned. But no assurance of coal can be had without boring
or pitting.
Skill in boring judiciously for coal, distinguishes the genuine miner from the
empirical adventurer, who, ignorant of the general structure of coal-basins, expends
labour, time, and money at random, and usually to no purpose; missing the proper
coal-field, and leading his employer to sink a shaft where no productive seams can be
had. A skilful viewer, therefore, should always direct the boring operations, especially
in an unexplored country.
The boring rods should be made of the best and most tenacious Swedish iron; in
area, about an inch and a quarter square. Each rod is usually 3 feet long, terminating
in a male screw at one end, and a female screw at the other. The boring chisels are
commonly 18 inches long, and from 2 inches and a half to 3 inches and a quarter at
their cutting edge, which must be tipped with good steel. The chisel is screwed to an
intermediate 18-inch rod, called the double box-rod, forming together a rod 3 feet long.
There are, moreover, three short rods, a foot, 18 inches, and 2 feet long each, which
may be screwed, as occasion requires, to the brace-head, to make the height above the[966]
mouth of the bore convenient for the hands of the men in working the rods. Hence
the series of rods becomes a scale of measurement for noting the depth of the bore, and
keeping a journal of the strata that are perforated. The brace-head rod, also 18 inches
long, has two large eyes or rings at its top, set at right angles to each other, through
which arms of wood are fixed for the men to lift and turn the rods by, in the boring
process.
When the bore is intended to penetrate but a few fathoms, the whole work may be
performed directly by the hands; but when the bore is to be of considerable depth, a
lofty triangle of wood is set above the bore-hole, with a pulley depending at its summit
angle, for conducting the rope to the barrel of a windlass or wheel and axle, secured to
the ground with heavy stones. The loose end of the rope is connected to the rods by an
oval iron ring, called a runner; and by this mechanism they may be raised and let fall
in the boring; or the same effect may be more simply produced by substituting for the
wheel and axle, a number of ropes attached to the rod-rope, each of which may be pulled
by a man, as in raising the ram of the pile-engine.
In the Newcastle coal district there are professional master-borers, who undertake to
search for coal, and furnish an accurate register of the strata perforated. The average
price of boring in England or Scotland, where no uncommon difficulties occur, is six
shillings for each of the first five fathoms, twice 6 shillings for each of the second five
fathoms, thrice 6 shillings for each of the third five fathoms, and so on; hence the
series will be—
| 1st five fathoms |
6s. |
each |
£1 |
10 |
| 2nd five fathoms |
12s. |
— |
3 |
0 |
| 3rd five fathoms |
18s. |
— |
4 |
10 |
| 4th five fathoms |
24s. |
— |
6 |
0 |
| 20 fathoms of bore |
£15 |
0 |
Thus the price increases equably with the depth and labour of the bore, and the undertaker
usually upholds his rods. There are peculiar cases, however, in which the expense
greatly exceeds the above rate.
The boring tools are represented in the following figures:—
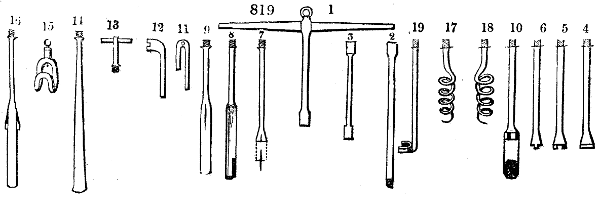
| Fig. 819. |
| 1. |
The brace-head. |
| 2. |
The common rod. |
| 3. |
The double-box rod; intermediate piece. |
| 4. |
The common chisel. |
| 5. |
The indented chisel. |
| 6. |
Another of the same. |
| 7. |
The cross-mouthed chisel. |
| 8. |
The wimble. |
| 9. |
The sludger, for bringing up the mud. |
| 10. |
The rounder. |
| 11. |
The key for supporting the train of rods at the bore-mouth. |
| 12. |
The key for screwing together and asunder the rods. |
| 13. |
The topit, or top-piece. |
| 14. |
The beché, for catching the rod when it breaks in the bore. |
| 15. |
The runner, for taking hold of the topit. |
| 16. |
The tongued chisel. |
| 17. |
The right-handed worm screw. |
| 18. |
The left-handed do. |
| 19. |
The finger-grip or catch. |
We shall now explain the manner of conducting a series of bores in searching ground
for coal.
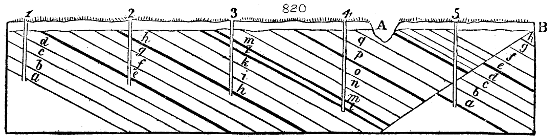
Fig. 820. represents a district of country in which a regular survey has proved
the existence and general distribution of coal strata, with a dip to the south, as
here shown. In this case, a convenient spot should be pitched upon in the north part[967]
of the district, so that the successive bores put down may advance in the line of the
dip. The first bore may therefore be made at No. 1., to the depth of sixty yards.
In the progress of this perforation, many diversities and alternations of strata will be
probably passed through, as we see in the sections of the strata; each of which, as to
quality and thickness, is noted in the journal, and specimens are preserved. This bore
is seen to penetrate the strata d, c, b, a, without encountering any coal. Now, suppose
that the dip of the strata be one yard in ten, the question is, at what distance from bore
No. 1. in a south direction, will a second bore of 60 yards strike the first stratum d, of
the preceding? The rule obviously is, to multiply the depth of the bore by the dip,
that is, 60 by 10, and the product 600 gives the distance required; for, by the rule of
three, if 1 yard of depression corresponds to 10 in horizontal length, 60 yards of
depression will correspond to 600 in length. Hence the bores marked 1, 2, 3, 4, and 5,
are successively distributed as in the figure, the spot where the first is let down being
regarded as the point of level to which the summits of all the succeeding bores are
referred. Should the top of No. 2. bore be 10 yards higher or lower than the top of
No. 1., allowance must be made for this difference in the operation; and hence a surface
level survey is requisite. Sometimes ravines cut down the strata, and advantage should
be taken of them, when they are considerable.
In No. 2. a coal is seen to occur near the surface, and another at the bottom of the
bore; the latter seam resting on the first stratum d, that occurred in bore No. 1.;
and No. 2. perforation must be continued a little farther, till it has certainly descended
to the stratum d. Thus these two bores have, together, proved the beds to the depth
of 120 yards.
No. 3. bore being placed according to the preceding rule, will pass through two
coal-seams near the surface, and after reaching to nearly its depth of 60 yards, it will
touch the stratum h, which is the upper stratum of bore No. 2.; but since a seam of
coal was detected in No. 2., under the stratum h, the proof is confirmed by running the
borer down through that coal. The field has now been probed to the depth of 180
yards. The fourth bore is next proceeded with, till the two coal-seams met in No. 3.
have been penetrated; when a depth of 240 yards has been explored. Hence No. 4.
bore could not reach the lower stratum a, unless it were sunk 240 yards.
The fifth bore (No. 5.) being sunk in like manner, a new coal-seam occurs within a
few yards of the surface; but after sinking to the depth at which the coal at the top of
the fourth bore was found, an entirely different order of strata will occur. In this
dilemma, the bore should be pushed 10 or 20 yards deeper than the 60 yards, to ascertain
the alternations of the new range of superposition. It may happen that no coals
of any value shall be found, as the figure indicates, in consequence of a slip or dislocation
of the strata at B, which has thrown up all the coals registered in the former
borings, to such an extent that the strata b, a, of the first bore present themselves
immediately on perforating the slip, instead of lying at the depth of 300 yards (5 × 60),
as they would have done, had no dislocation intervened. Some coal-fields, indeed, are
so intersected with slips as to bewilder the most experienced miner, which will particularly
happen when a lower coal is thrown upon one side of a slip, directly opposite
to an upper coal situated on the other side of it; so that if the two seams be of the same
thickness, erroneous conclusions are almost inevitable.
When a line of bores is to be conducted from the dip of the strata towards their
outcrop, they should be placed a few yards nearer each other than the rule prescribes,
lest the strata last passed through be overstepped, so that they may disappear from the
register, and a valuable coal-seam may thereby escape notice. In fact, each successive
bore should be so set down, that the first of the strata perforated should be the last
passed through in the preceding bore; as is exemplified by viewing the bores in the
retrograde direction, Nos. 4. 3. and 2. But if the bore No. 2. had gone no deeper than
f, and the bore No. 1. been as represented, then the stratum e, with its immediately
subjacent coal, would have been overstepped, since none of the bores would have
touched it; and they would have remained unnoticed in the journal, and unknown.
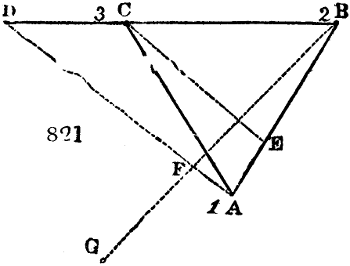
When the line of dip, and consequently the line of bearing which is at right angles
to it, are unknown, they are sought for by making three bores in the following position.—Let
fig. 821. be a horizontal diagram, in which the place of a bore, No. 1., is[968]
shown, which reaches a coal-seam at the depth of 50 yards; bore No. 2. may be made
at B, 300 yards from the former; and bore No. 3. at C, equidistant from Nos. 1. and 2.,
so that the bores are sunk at the three angles of an
equilateral triangle. If the coal occur in No. 2. at the
depth of 30 yards, and in No. 3. of 44 yards, it is
manifest that none of the lines A B, B C, or C A is in the
line of level, which for short distances may be taken
for the line of bearing, with coal-seams of moderate
dip. But since No. 1. is the deepest of the three
bores, and No. 3. next in depth, the line A C joining
them must be nearer the line of level, than either of
the lines A B or B C. The question is, therefore, at
what distance on the prolonged line B C is the point
for sinking a bore which would reach the coal at the same depth as No. 1., namely 50
yards. This problem is solved by the following rule of proportion: as 14 yards (the
difference of depth between bores 2. and 3.) is to 300 yards (the distance between them),
so is 20 (the difference of depth betwixt 1. and 2.) to a fourth proportion, or x = 428 yards,
1 foot, and 8 inches. Now, this distance, measured from No. 2., reaches to the point D on
the prolonged line B C, under which point D the coal will be found at a depth of 50 yards,
the same as under A. Hence the line A D is the true level line of the coal-field; and a
line B F G drawn at right angles to it, is the true dip-line of the plane which leads to the
outcrop. In the present example the dip is 1 yard in 141⁄2; or 1 in 141⁄2, to adopt the judicious
language of the miner; or the sine is 1 to a radius of 141⁄2, measured along the line
from B to F. By this theorem for finding the lines of dip and level, the most eligible
spot in a coal-field for sinking a shaft may be ascertained.
Suppose the distance from B to G in the line of dip to be 455 yards; then, since every
141⁄2 gives a yard of depression, 455 will give 30 yards, which added to 30 yards, the
depth of the bore at B, will make 60 yards for the depth of the same coal-seam at G.
Since any line drawn at right angles to the line of level A D is the line of dip, so any
line drawn parallel to A D is a level line. Hence, if from C the line C E be drawn
parallel to D A, the coal-seam at the points E and C will be found in the same horizontal
plane, or 44 yards beneath the surface level, over these two points. The point E level
with C may also be found by this proportion: as 20 yards (the difference in depth of
the bores under B and A) is to 300 yards (the distance between them), so is 14 yards (the
difference of depth under B and C) to 210 yards, or the distance from B to E.
As boring for coal is necessarily carried on in a line perpendicular to the horizon,
and as coal seams lie at every angle of inclination to it, the thickness of the seam as
given obliquely by the borer, is always greater than the direct thickness of the coal;
and hence the length of that line must be multiplied by the cosine of the angle of dip,
in order to find the true power of the seam.
Of fitting or winning a coal-field.—In sinking a shaft for working coal, the great
obstacle to be encountered, is water, particularly in the first opening of a field, which
proceeds from the surface of the adjacent country; for every coal-stratum, however
deep it may lie in one part of the basin, always rises till it meets the alluvial cover, or
crops out, unless it be met by a slip or dike. When the basset-edge of the strata is
covered with gravel or sand, any body or stream of water will readily percolate downwards
through it, and fill up the porous interstices between the coal-measures, till
arrested by the face of a slip, which acts as a valve or flood-gate, and confines the water
to one compartment of the basin, which may, however, be of considerable area, and
require a great power of drainage.
In reference to water, coal-fields are divided into two kinds; 1., level free coal;
2., coal not level free. In the practice of mining, if a coal-field, or portion of it, is
so situated above the surface of the ocean that a level can be carried from that plane
till it intersects the coal, all the coal above the plane of intersection is said to be level
free; but if a coal-field, though placed above the surface of the ocean, cannot, on
account of the expense, be drained by a level or gallery, but by mechanical power, such
a coal-field is said to be not level free.
Besides these general levels of drainage, there are subsidiary levels, called off-takes or
drifts, which discharge the water of a mine, not at the mouth of the pit, but at some
depth beneath the surface, where, from the form of the country, it may be run off level
free. From 20 to 30 fathoms off-take is an object of considerable economy in
pumping; but even less is often had recourse to; and when judiciously contrived, may
serve to intercept much of the crop water, and prevent it from getting down to the dip
part of the coal, where it would become a heavy load on a hydraulic engine.
Day levels were an object of primary importance with the early miners, who had
not the gigantic pumping power of the steam-engine at their command. Levels ought to
be no less than 4 feet wide, and from 5 feet and a half to 6 feet high: which is large[969]
enough for carrying off water, and admitting workmen to make repairs and clear out
depositions. When a day-level, however, is to serve the double purpose of drainage
and an outlet for coals, it should be nearly 5 feet wide, and have its bottom gutter
covered over. In other instances a level not only carries off the water from the colliery,
but is converted into a canal for bearing boats loaded with coals for the market.
Some subterranean canals are nine feet wide, and twelve feet high, with 5 feet depth of
water.
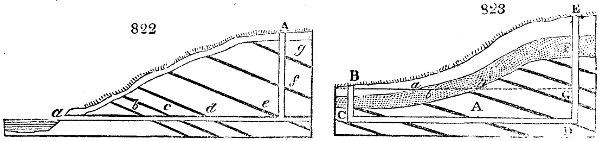
If in the progress of driving a level, workable coals are intersected before reaching
the seam which is the main object of the mining adventure, an air-pit may be sunk, of
such dimension as to serve for raising the coals. These air-pits do not in general
exceed 7 foot in diameter; and they ought to be always cylindrical. Fig. 822.
represents a coal-field where the winning is made by a day-level; a is the mouth
of the gallery on a level with the sea; b, c, d, e, are intersected coal-seams, to be
drained by the gallery. But the coals beneath this level must obviously be drained by
pumping. A represents a coal-pit sunk on the coal e; and if the gallery be pushed
forward, the coal-seams f, g, and any others which lie in that direction, will also be
drained, and then worked by the pit A. The chief obstacle to the execution of day-levels,
is presented by quicksands in the alluvial cover, near the entrance of the gallery.
The best expedient to be adopted amid this difficulty is the following:—Fig. 823.
represents the strata of a coal-field A, with the alluvial earth a, b, containing
the bed of quicksand b. The lower part, from which the gallery is required to be
carried, is shown by the line B d. But the quicksand makes it impossible to push
forward this day-level directly. The pit B C must therefore be sunk through the quicksand
by means of tubbing (to be presently described), and when the pit has descended a
few yards into the rock, the gallery or drift may then be pushed forward to the point D,
when the shaft E D is put down, after it has been ascertained by boring that the rock-head
or bottom of the quicksand at F is a few yards higher than the mouth of the small
pit B. During this operation, all the water and mine-stuff, are drawn off by the pit B;
but whenever the shaft E D is brought into communication with the gallery, the water
is allowed to fill it from C to D, and rise up both shafts till it overflows at the orifice B.
From the surface of the water in the deep shaft at G, a gallery is begun of the common
dimensions, and pushed onwards till the coal sought after is intersected. In this way
no drainage level is lost. This kind of drainage gallery, in the form of an inverted
syphon, is called a drowned or a blind level.
When a coal-basin is so situated that it cannot be rendered level free, the winning
must be made by the aid of machinery. The engines at present employed in the
drainage of coal-mines are:—
- 1. The water-wheel, and water-pressure engine.
- 2. The atmospheric steam-engine of Newcomen.
- 3. The steam-engine, both atmospheric and double stroke, of Watt.
- 4. The expansion steam-engine of Woolf.
- 5. The high-pressure steam-engine, without a condenser.
The depth at which the coal is to be won, or to be drained of moisture, regulates the
power of the engine to be applied, taking into account the probable quantity of water
which may be found, a circumstance which governs the diameter of the working barrels
of the pumps. Experience has proved, that in opening collieries, even in new fields,
the water may generally be drawn off by pumps of from 10 to 15 inches diameter;
excepting where the strata are connected with rivers, sand-beds filled with water, or
marsh-lands. As feeders of water from rivers or sand-beds may be hindered from
descending coal-pits, the growth proceeding from these sources need not be taken into
account; and it is observed, in sinking shafts, that though the influx which cannot be
cut off from the mine, may be at first very great, even beyond the power of the engine
for a little while, yet as this excessive flow of water is frequently derived from the
drainage of fissures, it eventually becomes manageable. An engine working the pumps
for 8 or 10 hours out of the 24, is reckoned adequate to the winning of a new colliery,
which reaps no advantage from neighbouring hydraulic powers. In the course
of years, however, many water-logged fissures come to be cut by the workings, and the
coal seams get excavated towards the outcrop, so that a constant increase of water
ensues, and thus a colliery which has been long in operation, frequently becomes heavily[970]
loaded with water, and requires the action of its hydraulic machinery both night
and day.
Of Engine Pits.—In every winning of coal, the shape of the engine-pit deserves
much consideration. For shafts of moderate depth, many forms are in use; as circular,
oval, square, octagonal, oblong rectangular, and oblong elliptical. In pits of inconsiderable
depth, and where the earthy cover is firm and dry, any shape deemed most
convenient may be preferred; but in all deep shafts, no shape but the circular should
be admitted. Indeed, when a water-run requires to be stopped by tubbing or cribbing,
the circular is the only shape which presents a uniform resistance in every point to the
equable circumambient pressure. The elliptical form is the next best, when it deviates
little from the circle; but even it has almost always given way to a considerable
pressure of water. The circular shape has the advantage, moreover, of strengthening
the shaft walls, and is less likely to suffer injury than other figures, should any failure
of the pillars left in working out the coal cause the shaft to be shaken by subsidence
of the strata. The smallest engine-pit should be ten feet in diameter, to admit of the
pumps being placed in the lesser segment, and the
coals to be raised in the larger one, as shown in
fig. 824., which is called a double pit. If much
work is contemplated in drawing coals, particularly
if their masses be large, it would be advantageous
to make the pit more than 10 feet wide.
When the area of a shaft is to be divided into three compartments, one for the engine
pumps, and two for raising coals, as in fig. 825., which is denominated a triple pit, it
should be 12 feet in diameter. If it is to be divided into four compartments, and made
a quadrant shaft, as in fig. 826., with one space for the pumps, and three for ventilation
and coal-drawing, the total circle should be 15 feet in diameter. These dimensions are,
however, governed by local circumstances, and by the proposed daily discharge of coals.
The shaft, as it passes through the earthy cover, should be securely faced with masonry
of jointed ashler, having its joints accurately bevelled to the centre of the circle. Specific
directions for building the successive masses of masonry, on a series of rings or
cribs of oak or elm, are given by Mr. Bald, article Mine, Brewster’s Encyclopædia,
p. 336.
When the alluvial cover is a soft mud, recourse must be had to the operation of
tubbing. A circular tub, of the requisite diameter, is made of planks from 2 to 3
inches thick, with the joints bevelled by the radius of the shaft, inside of which are cribs
of hard wood, placed from 2 to 4 feet asunder, as circumstances may require. These
cribs are constructed of the best heart of oak, sawn out of the natural curvature of the
wood, adapted to the radius, in segments from 4 to 6 feet long, from 8 to 10 inches in
the bed, and 5 or 6 inches thick. The length of the tub is from 9 to 12 feet, if the
layer of mud have that thickness; but a succession of such tubs must be set on each
other, provided the body of mud be thicker. The first tub must have its lower edge
thinned all round, and shod with sharp iron. If the pit be previously secured to a
certain depth, the tub is made to pass within the cradling, and is lowered down with
tackles till it rests fair among the soft alluvium. It is then loaded with iron weights at
top, to cause it to sink down progressively as the mud is removed from its interior.
Should a single tub not reach the solid rock (sandstone or basalt), then another of like
construction is set on, and the gravitating force is
transferred to the top. Fig. 827. represents a bed
of quicksand resting on a bed of impervious clay,
that immediately covers the rock. A is the finished
shaft; a a, the quicksand; b b, the excavation
necessarily sloping much outwards; c c, the lining
of masonry; d d, the moating or puddle of
clay, hard rammed in behind the stone-work,
to render the latter water-tight. In this case, the quicksand, being thin in body,
has been kept under for a short period, by the hands of many men scooping it rapidly
away as it filled in. But the most effectual method of passing through beds of quicksand,
is by means of cast-iron cylinders; called, therefore, cast-iron tubbing. When
the pit has a small diameter, these tubs are made about 4 feet high, with strong flanges,
and bolt holes inside of the cylinder, and a counterfort ring at the neck of the flange,
with brackets: the first tub, however, has no flange at its lower edge, but is rounded
to facilitate its descent through the mud. Should the pit be of large diameter, then the
cylinders must be cast in segments of 3, 4, or more pieces, joined together with inside
vertical flanges, well jointed with oakum and white lead. When the sand-bed is
thick, eighty feet, for instance, it is customary to divide that length into three sets of
cylinders, each thirty feet long, and so sized as to slide within each other, like the eye
tubes of a telescope. These cylinders are pressed down by heavy weights, taking care to[971]
keep the lower part always further down than the top of the quicksand, where the men
are at work with their shovels, and where the bottom of the pumps hangs for withdrawing
the surface water. This is an improvement adopted of late years in the Newcastle
district with remarkable success.
The engine pit being secured, the process of sinking through the rock is ready to be
commenced, as soon as the divisions of the pit formed of carpentry, called brattices,
are made. In common practice, and where great tightness of jointing is not required,
for ventilating inflammable air, bars of wood, called buntons, about 6 inches thick, and
9 deep, are fixed in a horizontal position across the pit, at distances from each other of
10, 20, or 30 feet, according to circumstances. Being all ranged in the same vertical
plane, deals an inch and a half thick are nailed to them, with their joints perfectly close;
one half of the breadth of a bunton being covered by the ends of the deals. In deep
pits, where the ventilation is to be conducted through the brattice, the side of the
buntons next the pumps is covered with deals in the same way, and the joints are
rendered secure by being caulked with oakum. Fillets of wood are also fixed all the
way down on each side of the brattice, constituting what is called a double pit.
When a shaft is to have 3 compartments, it requires more care to form the brattice,
as none of the buntons stretch across the whole space, but merely meet near the middle,
and join at certain angles with each other. As the buntons must therefore sustain each
other, on the principle of the arch, they are not laid in a horizontal plane, but have a
rise from the sides towards the place of junction of 8 or 9 inches, and are bound
together by a three-tongued iron strap. Fillets of wood are carried down the whole
depth, not merely at the joinings of the brattice with the sides of the pit, but also at
their central place of union; while wooden pillars connect the centre of each set of
buntons with those above and below. Thus the carpentry work acquires sufficient
strength and stiffness.
In quadrant shafts the buntons cross each other towards the middle of the pit, and
are generally let into each other about an inch, instead of being half-checked. Fig. 824.
is a double shaft: A, the pump pit; B, the pit for raising coal. Fig. 825. is a triple
shaft; in which A is the pump compartment; B and C are coal pits. Fig. 826. is a
quadrant shaft: A, the pump pit; B, pit of ventilation or upcast for the smoke; C and D,
pits for raising coals.
A depth of 75 fathoms is fully the average of engine pits in Great Britain. In
practice, it embraces three sets of pumps. Whenever the shaft is sunk so low that the
engine is needed to remove the water, the first set of pumps may be let down by the
method represented in fig. 828.; where A is the pump; a a, strong ears through which
pass the iron rods connected with the spears b b; c c are the lashings;
d, the hoggar pump; e, the hoggar; f f, the tackles; g g, the single
pulleys; h h, the tackle fold leading to the capstans; and i, the pump-spears.
By this mechanical arrangement the pumps are sunk in the most
gradual manner, and of their own accord, so to speak, as the pit descends.
To the arms of the capstans, sledges are fastened with ropes or chains;
these sledges are loaded with weights, as counterpoises to the weight of
the column of pumps, and when additional pumps are joined in, more
weight is laid on the sledges. As the sinking set of pumps is constantly
descending, and the point for the delivery of the water above always varying,
a pipe of equal diameter with the pumps, and about 11 feet long, but
much lighter in the metal, is attached to e, and is terminated by a hose of
leather, of sufficient length to reach the cistern where the water is delivered.
This is called the hoggar-pipe. In sinking, a vast quantity of air
enters with the water, at every stroke of the engine; and therefore the lifting
stroke should be very slow, and a momentary stop should take place
before the returning stroke, to suffer all the air to escape. As the working
barrels are generally 9 or 10 feet long, and the full stroke of the engine
from 7 to 8 feet, when at regular work, it is customary to diminish the
length of stroke, in sinking, to about 6 feet; because, while the pumps
are constantly getting lower, the bucket in the working barrel has its
working range progressively higher.
The usual length for a set of pumps, is from 25 to 30 fathoms. Whenever
this depth is arrived at by the first set, preparations are made for
fixing firmly the upper pit-cistern, into which the upper set of pumps is to
be placed, and the water of the second set is to be thrown. If a strong bed of sandstone
occurs, a scarcement of it is left projecting about 3 feet into the shaft, which is
formed in the course of sinking into a strong chin or bracket, to sustain that part of
the cistern in which the superior set of pumps stands. A few feet beneath this scarcement
the shaft resumes its usual shape.
[972]
But although from 20 to 30 fathoms be the common length of a pump-lift, it sometimes
becomes necessary to make it much longer, when no place can be found in the
shaft for lodging a cistern, on account of the tubbing. Hence a pump-lift
has been occasionally extended to 70 fathoms; which requires extraordinary
strength of materials. The best plan for collaring the pumps in the pit, and
keeping them steady in a perpendicular line, is to fix a strong bunton of timber
under the joints of each pipe; and to attach the pipes firmly to these buntons
by an iron collar, with screws and nuts, as represented in fig. 829.
The water obtained in sinking through the successive strata is, in ordinary cases,
conducted down the walls of the shaft; and if the strata are compact, a spiral groove is
cut down the sides of the shaft, and when it can hold no more, the water is drawn off in
a spout to the nearest pump-cistern; or a perpendicular groove is cut in the side of the
shaft, and a square box-pipe either sunk in it, flush with the sides of the pit, or it is
covered with deal boards well fitted over the cavity. Similar spiral rings are formed
in succession downwards, which collect the trickling streams, and conduct them into the
nearest cistern; or rings, made of wood or cast iron, are inserted flush with the sides of
the pipe; and the water is led from one ring to another, through perpendicular pipes,
until the undermost ring is full, when it delivers its water into the nearest pump-cistern.
Keeping the shaft dry is very important to the comfort of the miners, and
the durability of the work.
When an engine shaft happens to pass through a great many beds of coal, a gallery
a few yards long is driven into each coal-seam, and a bore then put down from one
coal to another, so that the water of each may pass down through these bores to the
pump-cisterns.
While a deep pit is sinking, a register is kept of every part of the excavations, and
each feeder of water is measured daily, to ascertain its rate of discharge, and whether it
increases or abates. The mode of measurement, is by noting the time, with a seconds
watch, in which a cistern of 40 or 50 gallons gets filled. There are three modes of
keeping back or stopping up these feeders; by plank tubbing; iron tubbing; and by
oak cribs. Let fig. 830. represent the sinking of a shaft through a variety of strata,
having a top cover of sand, with much water
resting on the rock summit. Each plane of the
coal-measure rises in a certain direction till it
meets the alluvial cover. Hence, the pressure of
the water at the bottom of the tubbing that rests
on the summit of the rock, is as the depth of
water in the superficial alluvium; and if a stratum
a affords a great body of water, while the superjacent stratum b, and the subjacent c, are
impervious to water; if the porous bed a be 12 feet thick, while no water occurs in the
strata passed through from the rock head, until that depth (supposed to be 50 fathoms
from the surface of the water in the cover); in this case, the tubbing or cribbing must
sustain the sum of the two water pressures, or 62 fathoms; since the stratum a meets the
alluvial cover at d, the fountain head of all the water that occurs in sinking. Thus we
perceive, that though no water-feeder of any magnitude should present itself till the shaft
had been sunk 100 fathoms; if this water required to be stopped up or tubbed off through
the breadth of a stratum only 3 feet thick, the tubbing floodgate would need to have a
strength to resist 100 fathoms of water-pressure. For though the water at first oozes
merely in discontinuous particles through the open pores of the sands and sandstones,
yet it soon fills them up, like a myriad of tubes, which transfer to the bottom the total
weight of the hydrostatic column of 100 fathoms; and experience shows, as we have
already stated, that whatever water occurs in coal-pits or in mines, generally speaking,
proceeds from the surface of the ground. Hence, if the cover be an impervious bed of
clay, very little water will be met with among the strata, in comparison of what would
be found under sand.
When several fathoms of the strata must be tubbed, in order to stop up the water-flow,
the shaft must be widened regularly to admit the kind of tubbing that is to be
inserted; the greatest width being needed for plank-tubbing, and the least for iron-tubbing.
Fig. 831. represents a shaft excavated for plank-tubbing, where a, a, a are the
impervious strata, b, b the porous beds water-logged, and c, c the bottom
of the excavation, made level and perfectly smooth with mason-chisels.
The same precautions are taken in working off the upper part of the
excavation d, d. In this operation, three kinds of cribs are employed;
called wedging, spiking, and main cribs. Besides the stout plank for
making the tub, a quantity of well-seasoned and clean reeded deal is
required for forming the joints; called sheeting deal by the workmen.
This sheeting deal is always applied in pieces laid endwise, with the end of the fibres
towards the area of the pit. Since much of the security from water depends on the[973]
tightness of the tub at its jointing with the rock, several plans have been contrived to
effect this object; the most approved being represented in fig. 832. To make room
for the lower wedging crib, the recess is excavated a few inches wider, as
at c; and from b to c, sheeting deals are laid all round the circle, or a thin stratum
of oakum is introduced. On this the wedging crib d is applied, and neatly
jointed in the radius-line of the pit, each segment being drawn exactly to the
circle: and at each of its segments sheeting deal is inserted. This wedging
crib must be 10 inches in the bed, and 6 inches deep. The vacuity e, at the
back of the crib, about 2 and a half inches wide, is filled with pieces of dry
clean reeded deal, inserted endwise; which is regularly wedged with one set of
wedges all round, and then with a second and a third set of wedges, in the same
regular style, to keep the crib in a truly circular posture. By this process,
well executed, no water can pass downwards by the back of the crib. The next
operation is to fix spiking cribs f, to the rock, about 10 or 12 feet from the lower
crib, according to the length of the planks to be used for the tubs. They must
be set fair to the sweep of the shaft, as on them its true circular figure depends.
The tubbing deals k, must now be fixed. They are 3 inches thick, 6 broad, and planed
on all sides, with the joints accurately worked to the proper bevel for the circle of the
pit. The main cribs g, g, are then to be placed as counterforts, for the support and
strength of the tubbing. The upper ends of the first set of tub-planks being cut square
and level all round, the second spiking crib l, is fixed, and another set of tubbing deals
put round like the former, having sheeting deal inserted betwixt the ends of the two
sets at f. When this is wedged, the cribs h, h, are placed.
Oak cribbing is made with pieces of the best oak, from 3 to 4 feet long, 10 inches in
the bed, and 7 or 8 inches deep.
The third mode of tubbing, by means of iron cylinders cast in segments, is likely
henceforth to supersede the wooden tubbing, from the great reduction in the price of
iron, and its superior strength and durability. Each segment is adjusted piece to piece
in the circular recess of the pit cut out for their reception. The flange for the wedging
joint is best turned inwards. In late improvements of this plan, executed by Mr.
Buddle, where the pressure amounted to several hundred feet, the segments were 6
feet long, 2 feet broad, and an inch thick, counterforted with ribs or raised work on
the back; the lip of the flange was strong, and supported by brackets. These segments
of the iron cylinder are set true to the radius of the pit; and every horizontal and
perpendicular joint is made tight with a layer of sheeting deal. A wedging crib is
fixed at the bottom, and the segments are built up regularly with joints like ashler-work.
This kind of tubbing can be carried to any height, till the water finds an outlet
at the surface, or till strata containing water can be tubbed off, as by the modes of
tubbing already described. A shaft finished in this manner presents a smooth lining-wall
of iron, the flanges being turned towards the outside of the cylinders. In this iron
tubbing, no screw bolts are needed for joining the segments together; as they are
packed hard within the pit, like the staves of a cask. There is a shaft in the
Newcastle district, where 70 fathoms have been executed in this way, under the
direction of Mr. Buddle.

When a porous thin bed or parting betwixt two impervious strata, gives out much
water, or when the fissures of the strata, called cutters, are very leaky, the water can be
completely stopped up by the improved process of wedging. The fissure is
cut open with chisels, to a width of two, and a depth of seven inches, as represented
in fig. 833. The lips being rounded off about an inch and a half,
pieces of clean deal are then driven in, whose face projects no further than the
contour of the lips; when the whole is firmly wedged, till the water is entirely
stopped. By sloping back the edges of the fissures, and wedging back from the
face of the stone, it is not liable to burst or crack off in the operation, as took place
in the old way, of driving in the wedge directly.
Ventilation of Engine Pits.—In ordinary cases, while the sinking of the shaft is going
on, the brattice walls produce a circulation, in consequence of the air being slightly
lighter in one compartment than in another. If this does not occur, the
circulation of air must be produced by artificial means. The most approved
contrivance is, to cover the engine compartment of the shaft with
deals, leaving apertures for the pump-spears and tackling to pass through,
with hatch-doors for the men, and to carry a brick flue at least 3 feet
square, in a horizontal direction, from the mouth of that compartment
to an adjoining high chimney connected with a furnace, as represented
in fig. 834. a, a, are double doors, for the fireman to supply fuel by; b, the
mouth of the horizontal flue; c, the furnace; d, the ash-pit; e, the furnace;
f, the upright chimney for draught, from 50 to 100 feet high,
from 8 to 10 feet square at bottom, and tapering upwards to 3 or 4 feet[974]
square inside. Such a furnace and chimney are also needed for ventilating the coal-mine
through all its underground workings. When a great quantity of gas issues
from one place in a pit, it is proper to carry it up in a square wooden pipe, which
terminating at some distance above the surface in a helmet-shaped funnel, fitted to
turn like a vane, may cause considerable ventilation of itself; or the top of such a
pipe may be connected with a small fireplace, which will cause a rapid current up
through it, from the pit. The stones and rubbish produced in sinking, are drawn up
with horse-gins, when the pit is not deep; but in all shafts of considerable depth, a
steam engine is used, and the workmen have now more confidence in them, as to
personal safety, than in machines impelled by horses.
The great collieries of Newcastle are frequently worked by means of one shaft divided
into compartments, which serves as an engine-pit, and coal-pits, and by these the
whole ventilation is carried on to an extent and through ramifications altogether astonishing.
This system has been adopted on account of the vast expense of a large shaft,
often amounting to 60,000l. or 80,000l., including the machinery. The British
collieries, however, are in general worked by means of an engine-pit, and a series of
other pits, sunk at proper distances for the wants of the colliery.
WORKING OF COAL.
A stratum, bed, or seam of coal, is not a solid mass, of uniform texture, nor always
of homogeneous quality in burning. It is often divided and intersected, with its
concomitant strata, by what are named partings, backs, cutters, reeds, or ends. Besides
the chief partings at the roof and pavement of the coal seam, there are subordinate lines
of parting in the coal mass, parallel to these of variable dimensions. These divisions
are delineated in fig. 835., where A, B, C, D, E F G D, represent a portion of a bed of
coal, the parallelogram A B D C the parting at the
roof, and E F G the parting at the pavement; a b, b c,
d e, and e f, are the subordinate or intermediate partings;
g h, i k, l m, the backs; o p, p q, r s, s t, u v,
and v w, the cutters. It is thus manifest that a bed
of coal, according to the number of these natural divisions,
is subdivided into solid figures of various dimensions,
and of a cubical or rhomboidal shape.
When the engine-pit is sunk, and the lodgement formed, a mine is then run in the
coal to the rise of the field, or a cropping from the engine-pit to the second pit. This
mine may be 6 or 8 feet wide, and carried either in a line directly to the pit bottom, or
at right angles to the backs or web of the coal, until it is on a line with the pit, where
a mine is set off, upon one side, to the pit bottom. This mine or gallery is carried as
nearly parallel to the backs as possible, till the pit is gained. Fig. 836. represents this
mining operation. A is the engine-pit. B, the second or
bye-pit. A C, the gallery driven at right angles to the backs.
C B, the gallery set off to the left hand, parallel to the backs.
The next step is to drive the drip-head or main-levels from
the engine-pit bottom, or from the dip-hand of the backset
immediately contiguous to the engine-pit bottom. In this
business, the best colliers are always employed, as the object
is to drive the gallery in a truly level direction, independently of all sinkings or risings
of the pavement. For coal seams of ordinary thickness, this gallery is usually not more
than 6 feet wide; observing to have on the dip side of the gallery a small quantity of
water, like that of a gutter, so that it shall always be about 4 or 6 inches deep at the
forehead upon the dip-wall. When the level is driven correctly, with the proper depth
of water, it is said to have dead water at the forehead. In this operation, therefore, the
miner pays no regard to the backs or cutters of the coal; but is guided in his line of
direction entirely by the water-level, which he must attend to solely, without regard to
slips or dislocations of the strata throwing the coal up or down. In the last figure, the
coal-field is a portion of a basin; so that if the shape be uniform and unbroken, and if
any point be assumed a dipping from the crop, as D, the level lines from that point will
be parallel to the line of crop, as D E, D F, and the levels from any point whatever a-dipping,
will be also parallel to these; and hence, were the coal-field an entire elliptical
basin, the dip-head levels carried from any point would be elliptical, and parallel to the
crop. If, as is more commonly the case, the coal-field be merely a portion of a basin,
formed by a slip of the strata, as represented in fig. 837.,
where a, a, a, is the crop, and A B, a slip of great magnitude,
forming another coal-field on the side C, then the crop not
only meets the alluvial cover, but is cut off by the slip at A
and at B. Should any point, therefore, be assigned for an engine-pit, the levels from
it will proceed in a line parallel to the crop, as D d, D c, and the level on both sides of[975]
the engine-pit will be also cut off by the slip A B. In this figure, the part included between
the two curve lines, is the breadth or breast of coal-field won by the engine-pit
D; what is not included, is termed the under-dip coal, and can be worked only by one
or more new winnings towards the dip, according to circumstances.
In British practice, there are four different systems of working coal-mines:—
1. Working with pillars and rooms, styled post and stall, where the pillars left, bear
such proportion to the coal excavated, as is just adequate to the support of the incumbent
strata.
2. Working with post and stall, where the pillars are left of an extra size, and
stronger than may be requisite for bearing the superior strata, with the intention of removing
a considerable portion of each massive pillar, whenever the regular working of
post and stall has been finished in the colliery.
3. Working with post and stall, or with comparatively narrow rooms or boards,
whereby an uncommonly large proportion of coal is left, with the view of working back
towards the pits, whenever the colliery is worked in this manner to the extent of the coal-field,
and then taking away every pillar completely, if possible, and allowing the whole
superincumbent strata to crush down, and follow the miners in their retreat.
4. Working the long way, being the Shropshire method; which leaves no pillars, but
takes out all the coal progressively as the workings advance. On this plan, the incumbent
strata crush down, creeping very close to the heads of the miners.
The post and stall system is practised with coals of every thickness. The Shropshire
method is adopted generally with thin coals; for when the thickness exceeds 6 or 7
feet, this mode has been found impracticable.
The following considerations must be had in view in establishing a coal-mine:—
1. The lowest coal of the winning should be worked in such a manner as not to
injure the working or the value of the upper coals of the field; but if this cannot be
done, the upper coals should be worked in the first place.
2. The coals must be examined as to texture, hardness, softness, the number and
openness of the backs and cutters.
3. The nature of the pavement of the coal seam, particularly as to hardness and softness;
and if soft, to what depth it may be so.
4. The nature of the roof of the coal-seam, whether compact, firm, and strong; or
weak and liable to fail; as also the nature of the superincumbent strata.
5. The nature of the alluvial cover of the ground, as to water, quicksands, &c.
6. The situation of rivers, lakes, or marshes, particularly if any be near the outcrop
of the coal strata.
7. The situation of towns, villages, and mansion-houses, upon a coal-field; as to the
chance of their being injured by any particular mode of mining the coal.
Mr. Bald gives the following general rules for determining the best mode of working
coal:—
“1. If the coal, pavement, and roof are of ordinary hardness, the pillars and rooms
may be proportioned to each other, corresponding to the depth of the superincumbent
strata, providing all the coal proposed to be wrought is taken away by the first working,
as in the first system; but if the pillars are to be winged afterwards, they must be left
of an extra strength, as in the second system.
“2. If the pavement is soft, and the coal and roof strong, pillars of an extra size must
be left, to prevent the pillars sinking into the pavement, and producing a creep.
“3. If the coal is very soft, or has numerous open backs and cutters, the pillars must
be left of an extra size, otherwise the pressure of the superincumbent strata will make
the pillars fly or break off at the backs and cutters, the result of which would be a total
destruction of the pillars, termed a crush or sit, in which the roof sinks to the pavement,
and closes up the work.
“4. If the roof is very bad, and of a soft texture, pillars of an extra size are required,
and the rooms or boards comparatively very narrow.
“In short, keeping in view all the circumstances, it may be stated generally, that
when the coal, pavement, and roof are good, any of the systems before mentioned may
be pursued in the working; but if they are soft, the plan is to work with rooms of a
moderate width, and with pillars of great extra strength, by which the greater part of
the coal may be got out at the last of the work, when the miners retreat to the pit bottom,
and there finish the workings of a pit.”
Fig. 838. represents the effects of pillars sinking into the pavement,
and producing a creep; and fig. 839. exhibits large pillars
and a room, with the roof stratum bending down before it falls at
a. Thus the roads will be shut up, the air-courses destroyed, and the
whole economy of the mining operations deranged.
The proportion of coal worked out, to that left in the pillars,
when all the coal intended to be removed is taken out at the first working, varies from[976]
four-fifths to two-thirds; but as the loss of even one-third of the whole area of coal is far
too much, the better mode of working suggested in the third system ought to be
adopted.
The proportion of a winning to be worked maybe thus calculated. Let fig. 840. be a
small portion of the pillars, rooms, and thirlings formed in a coal-field;
a, a, are two rooms; b, the pillars; c, the thirlings (or area worked out).
Suppose the rooms to be 12 feet wide, the thirlings to be the same, and
the pillars 12 feet on each side; adding the face of the pillar to the width
of the room, the sum is 24; and also the end of the pillar to the width
of the thirling, the sum is likewise 24: then 24 × 24 = 576; and the
area of the pillar is 12 × 12 = 144; and as 576 divided by 144, gives 4
for a quotient, the result is, that one fourth of the coal is left in pillars, and three
fourths extracted. Let d, e, f, g, be one winning, and g, e, k, h, another. By inspecting
the figure, we perceive the workings of a coal-field are resolved into quadrangular
areas, having a pillar situated in one of the angles.
In forming the pillars and carrying forwards the boards with regularity, especially
where the backs and cutters are very distinct and numerous, it is of importance to work
the rooms at right angles to the backs, and the thirlings in the direction of the cutters,
however oblique these may be to the backs, as the rooms are by this means conducted
with the greatest regularity with regard to each other, kept equidistant, and the pillars
are strongest under a given area. At the same time, however, it seldom
happens that a back or cutter occurs exactly at the place where a pillar
is formed; but this is of no consequence, as the shearing or cutting
made by the miner ought to be in a line parallel to the backs and cutters.
It frequently happens that the dip-head level intersects the cutters
in its progress at a very oblique angle. In this case, when rooms
and pillars are set off, the face of the pillar and width of the room
must be measured off an extra breadth in proportion to the obliquity,
as in fig. 841. By neglect of this rule, much confusion and irregular
work is often produced. It is, moreover, proper to make the first set of pillars next
the dip-head level much stronger, even where there is no obliquity, in order to protect
that level from being injured by any accidental crush of the strata.
We shall now explain the different systems of working; one of the simplest of which
is shown in fig. 842; where A represents the engine-pit, B the bye-pit, C D the dip-head
levels, always carried in advance of the rooms,
and E the rise or crop gallery, also carried in advance.
These galleries not only open out the
work for the miners in the coal-bed, but, being
in advance, afford sufficient time for any requisite
operation, should the mines be obstructed by
dikes or hitches. In the example before us, the
rooms or boards are worked from the dip to the
crop; the leading rooms, or those most in advance,
are on each side of the crop gallery E; all
the other rooms follow in succession, as shown,
in the figure; consequently, as the rooms advance
to the crop, additional rooms are begun
at the dip-head level, towards C and D. Should the coal work better in a level-course
direction, then the level rooms are next the dip-head level, and the other rooms follow
in succession. Hence the rooms are carried a cropping in the one case, till the coal is
cropped out, or is no longer workable; and in the other, they are extended as far as the
extremity of the dip-head level, which is finally cut off, either by a dike or slip, or by
the boundary of the coal-field.
When the winnings are so very deep as from 100 to 200 fathoms, the first workings
are carried forward with rooms, pillars, and thirlings, but under a different arrangement,
on account of the great depth of the superincumbent strata, the enormous expense
incident to sinking a pit, and the order and severity of discipline indispensable to the due
ventilation of the mines, the preservation of the workmen, and the prosperity of the
whole establishment. To the celebrated Mr. Buddle the British nation is under the
greatest obligations for devising a new system of working coal-mines, whereby nearly
one-third of the coals has been rescued from waste and permanent destruction. This
system is named panel work; because, instead of carrying on the coal-field winning in
one extended area of rooms and pillars, it is divided into quadrangular panels, each panel
containing an area of from 8 to 12 acres; and round each panel is left at first a solid
wall of coal from 40 to 50 yards thick. Through the panel walls roads and air-courses
are driven, in order to work the coal contained within these walls. Thus all the panels
are connected together with the shaft, as to roads and ventilation. Each district or[977]
panel has a particular name; so that any circumstance relative to the details of the
colliery, casualties as to falls and crushes, ventilation, and the safety of the workmen,
can be referred to a specific place.
Fig. 843. represents a part of a colliery laid out in four panels, according to the
improved method. To render it as distinct as possible, the line of the boards is at right
angles with the dip-head level, or level course of the coal. A is the engine-shaft, divided
into three compartments, an engine-pit and two coal-pits, like fig. 825. One of the
coal-pits is the down-cast, by which the atmospheric air is drawn down to ventilate the
works; the other coal-pit is the up-cast shaft, at whose bottom the furnace for rarefying
the air is placed. B C, is the dip-head level; A E, the rise or crop gallery; K, K, the panel
walls; F, G, are two panels completed as to the first work; D, is a panel, with the
rooms a, a, a, in regular progress to the rise; H, is a panel fully worked out, whence
nearly all the coal has been extracted; the loss amounting in general to no more than a
tenth, instead of a third, or even a half, by the old method. By this plan of Mr. Buddle’s,
also, the pillars of a panel may be worked out at any time most suitable for the
economy of the mining operation; whereas formerly, though the size of the pillars and
general arrangement of the mine were made with the view of taking out ultimately a
great proportion of the pillars, yet it frequently happened that, before the workings were
pushed to the proposed extent, some part of the mine gave way, and produced a
crush; but the most common misfortune was the pillars sinking into the pavement, and
deranging the whole economy of the field. Indeed the crush or creep often overran the
whole of the pillars, and was resisted only by the entire body of coal at the wall faces; so
that the ventilation was entirely destroyed, the roads leading from the wall faces to the
pit-bottom shut up and rendered useless, and the recovery of the colliery by means of
new air-courses, new roads, and by opening up the wall faces or rooms, was attended
with prodigious expense and danger. Even when the pillars stood well, the old method
was attended with other very great inconveniences. If water broke out in any particular
spot of the colliery, it was quite impossible to arrest its progress to the engine-pit; and
if the ventilation was thereby obstructed, no idea could be formed where the cause might
be found, there being instances of no less than 30 miles of air-courses in one colliery.
And if from obstructed ventilation an explosion of the fire-damp occurred while many
workmen were occupied along the extended wall faces, it was not possible to determine
where the disaster had taken place; nor could the viewers and managers know where to
bring relief to the forlorn and mutilated survivors.
In Mr. Buddle’s system all these evils are guarded against, as far as human science
and foresight can go. He makes the pillars very large, and the rooms or boards narrow;
the pillars being in general 12 yards broad, and 24 yards long; the boards 4 yards
wide, and the walls or thirlings cut through the pillars from one board to another, only
5 feet wide, for the purpose of ventilation. In the figure, the rooms are represented as
proceeding from the dip to the crop, and the panel walls act as barriers thrown round
the area of the panel, to prevent the weight of the superincumbent strata from overrunning
the adjoining panels. Again, when the pillars of a panel are to be worked, one
range of pillars, as at I (in H), is first attacked; and as the workmen cut away the furthest[978]
pillars, columns of prop-wood are erected betwixt the pavement and the roof, within a
few feet of each other (as shown by the dots), till an area of above 100 square yards is
cleared of pillars, presenting a body of-strata perhaps 130 fathoms thick, suspended
clear and without support, except at the line of the surrounding pillars. This operation
is termed working the goaff. The only use of the prop-wood is to prevent the seam,
which forms the ceiling over the workmen’s heads, from falling down and killing them
by its splintery fragments. Experience has proved, that before proceeding to take away
another set of pillars, it is necessary to allow the last-made goaff to fall. The workmen
then begin to draw out the props, which is a most hazardous employment. They begin
at the more remote props, and knock them down one after another, retreating quickly
under the protection of the remaining props. Meanwhile the roof-stratum begins to
break by the sides of the pillars, and falls down in immense pieces; while the workmen
still persevere, boldly drawing and retreating till every prop is removed. Nay, should
any props be so firmly fixed by the top pressure, that they will not give way to the blows
of heavy mauls, they are cut through with axes; the workmen making a point of honour
to leave not a single prop in the goaff. The miners next proceed to cut away the
pillars nearest to the sides of the goaff, setting prop-wood, then drawing it, and retiring
as before, until every panel is removed, excepting small portions of pillars which require
to be left under dangerous stones to protect the retreat of the workmen. While this
operation is going forward, and the goaff extending, the superincumbent strata being
exposed without support over a large area, break progressively higher up; and when
strong beds of sandstone are thus giving way, the noise of the rending rocks is very
peculiar and terrific; at one time loud and sharp, at another hollow and deep.
As the pillars of the panels are taken away, the panel walls are also worked progressively
backwards to the pit bottom; so that only a very small proportion of coal is
eventually lost. This method is undoubtedly the best for working such coals as those
of Newcastle, considering their great depth beneath the surface, their comparative softness,
and the profusion of inflammable air. It is evident that the larger the pillars and
panel walls are, in the first working, the greater will be the security of the miners, and the
greater the certainty of taking out, in the second stage, the largest proportion of coal.
This system may be applied to many of the British collieries; and it will produce a
vast quantity of coals beyond the post and stall methods, so generally persisted in.
In thus tearing to pieces the massive rocks over his head, the miner displays a determined
and cool intrepidity; but his ingenuity is no less to be admired in contriving
modes of carrying currents of pure atmospheric air through every turning of his gloomy
labyrinth, so as to sweep away the explosive spirit of the mine.
The fourth system of working coal, is called the long way, the long-wall, and the
Shropshire method. The plan must at first have been extremely hazardous; though
now it is so improved as to be reckoned as safe, if not safer, to the workmen, than the
other methods, with rooms and pillars.
The object of the Shropshire system, is to begin at the pit-bottom pillars, and to cut
away at once every inch of coal progressively forward, and to allow the whole superincumbent
strata to crush down behind and over the heads of the workmen. This plan
is pursued chiefly with coals that are thin, and is very seldom adopted when the seam is
7 feet thick; from 4 to 5 feet being reckoned the most favourable thickness for proceeding
with comfort, amidst ordinary circumstances, as to roof, pavement, &c. When
a pit is opened on a coal to be treated by this method, the position of the coals above
the lowest seam sunk to, must first be considered; if the coal beds be contiguous, it
will be proper to work the upper one first, and the rest in succession downwards; but
if they are 8 fathoms or more apart, with strata of strong texture betwixt them, the
working of the lower coals in the first place will do
no injury to that of the upper coals, except breaking
them, perhaps, a little. In many instances, indeed,
by this operation on a lower coal, upper coals are
rendered more easily worked.
When the operation is commenced by working on
the Shropshire plan, the dip-head levels are driven in
the usual manner, and very large bottom pillars are
formed, as represented in fig. 844. Along the rise
side of the dip-head level, chains of wall, or long pillars,
are also made, from 8 to 10 yards in breadth, and
only mined through occasionally, for the sake of ventilation,
or of forming new roads. In other cases no
pillars are left upon the rise side of the level; but,
instead of them, buildings of stone are reared, 4 feet broad at the base, and 9 or 10 feet
from the dip side of the level. Though the roads are made 9 feet wide at first, they
are reduced to half that width after the full pressure of the strata is upon them. Whenever[979]
these points are secured, the operation of cutting away the whole body of the coal
begins. The place where the coal is removed, is named the gobb or waste; and gobbin,
or gobb-stuff, is stones or rubbish taken away from the coal, pavement, or roof, to
fill up that excavation as much as possible, in order to prevent the crush of superincumbent
strata from causing heavy falls, or following the workmen too fast in their
descent. Coals mined in this manner work most easily according to the way in which
the widest backs and cutters are; and therefore, in the Shropshire mode, the walls
stand sometimes in one direction, and sometimes in another; the mine always turning
out the best coals when the open backs and cutters face the workmen. As roads must
be maintained through the crushed strata, the miners in the first place cut away about
15 feet of coal round the pit-bottom pillars, and along the upper sides of the dip-head
chain walls; and then, at the distance of 9 or 10 feet, carry regular buildings of stone
3 feet broad, with props set flush with the faces of these, if necessary. As the miners
advance, they erect small pillars of roof or pavement stone in regular lines with the wall
face, and sometimes with props intermediate.
There are two principal modifications of the Shropshire plan. The first, or the original
system, was to open out the wall round the pit-bottom; and, as the wall face
extended, to set off main roads and branches, very like the branches of a tree. These
roads were so distributed, that between the ends of any two branches there should be a
distance of 30 or 40 yards, as might be most convenient. (see fig. 844.) Each space of
coal betwixt the roads is called a wall; and one half of the coals produced from each
wall is carried to the one road, and the other half to the other road. This is a great
convenience when the roof is bad; and hence a distance of only 20 yards betwixt the
roads is in many instances preferred. In fig. 844. A represents the shaft; B B, the wall-face;
a, the dip-head level; b, the roads, from 20 to 40 yards asunder; c, the gobb or
waste, with buildings along the sides of the roads; and d, the pillars.
The other Shropshire system is represented in fig. 845., where A shows the pit, with
the bottom pillars; b, the dip-head levels; c, the off-break from the level, where no
pillars are left; d, the off-break, where pillars remain
to secure the level. All roads are protected
in the sides by stone buildings, if they can be had,
laid off 9 feet wide. After the crush settles, the
roads generally remain permanently good, and can,
in many cases, be travelled through as easily 50 years
after they have been made, as at the first. Should
stones not be forthcoming, coals must be substituted, which are built about 20 inches
in the base. In this method, the roads are likewise from 20 to 40 yards apart; but
instead of ramifying, they are arranged parallel to each other. The miners secure the
waste by gobbing; and three rows of props are carried forwards next the wall faces a,
with pillars of stone or of coal reared betwixt them. This mode has a more regular
appearance than the other; though it is not so generally practised.
In the post and stall system, each man has his own room, and performs all the labour
of it; but in that of Shropshire, there is a division of labour among the workmen, who
are generally divided into three companies. The first set curves or pools the coal along
the whole line of walls, laying in or pooling at least 3 feet, and frequently 45 inches,
or 5 quarters, as it is called. These men are named holers. As the crush is constantly
following them, and impending over their heads, causing frequent falls of coal, they
plant props of wood for their protection at regular distances in an oblique direction
between the pavement and wall face. Indeed, as a further precaution, staples of coal,
about 10 inches square, are left at every 6 or 8 yards, till the line of holing or curving
is completed. The walls are then marked off into spaces of from 6 to 8 yards in length;
and at each space a shearing or vertical cut is made, as deep as the holing; and when
this is done, the holer’s work is finished. The set who succeed the holers, are called
getters. These commence their operations at the centre of the wall divisions, and drive
out the gibbs and staples. They next set wedges along the roof, and bring down progressively
each division of coal; or, if the roof be hard-bound, the coal is blown down
with gunpowder. When the roof has a good parting, the coals frequently fall down the
moment the gibbs are struck; which makes the work very easy. The getters are relieved
in their turn by the third set, named butty-men, who break down the coals into
pieces of a proper size for sending up the shaft, and take charge of turning out the coal
from the wall face to the ends of the roads. This being done, they build up the stone
pillars, fill up the gobb, set the trees, clear the wall faces of all obstructions, set the
gibbs, and make every thing clear and open for the holers to resume their work. If
the roads are to be heightened by taking down the roof, or removing the pavement,
these butty-men do this work also, building forwards the sides of the roads, and securing
them with the requisite props. When a coal has a following or roof stone, which
regularly separates with the coal, this facilitates the labour, and saves much of the coal;[980]
and should a soft bed of fire-clay occur a foot or two beneath the coal-seam, the holing
is made in it, instead of into the coal, and the stone betwixt the holing and the coal
benched down, which serves for pillars and gobbing. In this way all the vendible coal
becomes available.
Another form of the Shropshire system is, for each miner to have from 6 to 12 feet of
coal before him, with a leading-hand man; and for the several workmen to follow in
succession, like the steps of a stair. When the coal has open backs and cutters, this
work goes on very regularly, as represented in fig. 846., where the leading miner is at a,
next to the outcrop, and b b, &c. are the wall faces of each
workman; A being the shaft, and B the dip-head level.
In this case the roads are carried either progressively
through the gobb, or the gobb is entirely shut up; and
the whole of the coals are brought down the wall-faces,
either to the dip-head level or the road c, c. This method
may be varied by making the walls broad enough to
hold two, three, or four men when each set of miners
performs the whole work of holing, getting, breaking
down, and carrying off the coals.
It is estimated that from one-eighth to one-twelfth part
only of the coals remains underground by the Shropshire plan; nay, in favourable circumstances,
almost every inch of coal may be taken out, as its principle is to leave no
solid pillars nor any coal below, except what may be indispensable for securing the
gobb. Indeed this system might be applied to coal seams of almost any ordinary thickness,
providing stuff to fill up the gobb could be conveniently procured.
In Great Britain, seams of coal are mined when they are only 18 inches thick; but
if thinner, the working of fire-clay or ironstone immediately adjoining must be included.
A few instances may be adduced, indeed, where caking coals of a fine quality
for blacksmiths have been worked, though only in 12-inch seams.
Eighteen-inch seams are best worked by young lads and boys. The coal itself may
be mined without lifting the pavement, or taking down the roof in the rooms; but
roads must be cut either in the pavement or the roof, for removing the coals to the pit-bottom.
All coals less than 2 feet 3 inches thick, are worked with the view of taking
out all the coal, either on the Shropshire system, or with pillar-walls and rooms; with
this peculiarity, that, on account of the thinness of the seam, the rooms are worked as
wide as the roof will bear up; or if a following of the roof-stone, or fall of it, can be
brought on, it proves advantageous, by not only giving head-room, but by filling up
the waste, and rendering the roads easily kept for the working of the pillars. Where
no following takes place, small temporary pillars, about 8 feet square, are left along the
chain-wall side. The walls may vary in thickness from 4 to 16 yards, according to circumstances,
and they are holed through only for ventilation.
Coals from 5 to 8 feet thick are the best suited in every point of view for the effective
work of the miner, and for the general economy of underground operations. When
they exceed that thickness, they require very excellent roofs and pavements, to render
the working either safe or comfortable; or to enable those who superintend the field
to get out a fair proportion of coal from a given area. In such powerful beds the
Shropshire method is impracticable, from want of gobbin; and long props, unless of
prodigious girth, would present an inadequate resistance to the pressure of the massive
ceiling.
When coals do not exceed 20 feet in thickness, and have good roofs, they are sometimes
worked as one bed of coal; but if the coal be tender or free, it is worked as two
beds. One-half of such thick coal, however, is in general lost in pillars; and it is very
seldom that less than one-third can be left. When the coal is free and ready to crumble
by the incumbent pressure, as well as by the action of the air, the upper portion of the
coal is first worked, then a scaffolding of coal is left, 2 or 3 feet thick, according to the
compactness of the coal; and the lower part of the coal is now worked, as shown in
fig. 847. As soon as the workings are completed to the proposed
extent, the coal scaffoldings are worked away, and as much of the
pillars as can be removed with safety. As propwood is of no use in
coal seams of such a height, and as falls from the roof would prove
frequently fatal to the miners, it is customary with tender roofs to leave a ceiling of
coal from 2 to 3 feet thick. This makes an excellent roof; and should it break, gives
warning beforehand, by a peculiar crackling noise, very different from that of roof-stones
crushing down.
One of the thickest coals in Great Britain, worked as one bed from roof to pavement,
is the very remarkable seam near the town of Dudley, known by the name of the ten-yard
coal, about 7 miles long, and 4 broad. No similar coal has been found in the
island; and the mode of working it is quite peculiar, being a species of panel work[981]
totally different from the modern Newcastle system. A compartment, or pannel, formed
in working the coal, is called a side of work; and as the whole operation is exhibited in
one of these compartments, it will be proper to describe the mode of taking the coal from
one of them, before describing the whole extent of the workings of a mine.
Let fig. 848. represent a side of work; A, the ribs or walls of coal left standing round,
constituting the side of work; a, the pillars, 8 yards square; c, the stalls, 11 yards wide;
d, the cross openings, or through puts, also 11 yards
wide; e, the bolt-hole, cut through the rib from the
main road, by which bolt-hole the side of work is
opened up, and all the coals removed. Two, three, or
even four bolt-holes open into a side of work, according
to its extent; they are about 8 feet wide, and 9 feet
high. The working is in a great measure regulated
by the natural fissures and joints of the coal-seam;
and though it is 30 feet thick, the lower band, of
2 feet 3 inches, is worked first; the miners choosing
to confine themselves within this narrow opening, in
order to gain the greater advantage afterwards, in
working the superjacent coal. Whenever the bolt
hole is cut through, the work is opened up by driving
a gallery forward, 4 feet wide, as shown by the
dotted lines. At the sides of this gallery next the
bolt-hole, each miner breaks off in succession a breast
of coal, two yards broad, as at f, f, by means of which the sides of the rib-walls A, are
formed, and the area of the pillars. In this way each collier follows another, as in
one of the systems of the Shropshire plan. When the side of work is laid open along
the rib-walls, and the faces and sides of the pillars have been formed, the upper coals are
then begun to be worked, next the rib-wall. This is done by shearing up to a bed next
the bolt-hole, and on each side, whereby the head coals are brought regularly down in
large cubical masses, of such thickness as suits with the free partings or subordinate
divisions of the coals and bands. Props of wood, or even stone pillars, are placed at
convenient distances for the security of the miners.
In working the ten-yard coal, a very large proportion of it is left underground, not
merely in pillars and rib-walls, but in the state of small coal produced in breaking out
the coal. Hence, from four-tenths to a half of the total amount is lost for ever.

Another method of working coal of uncommon thickness, is by scaffoldings or stages
of coals, as practised in the great coal bed at Johnstone, near Paisley, of which a section
has already been given. In one part of the field the coal is from 50 to 60 feet thick,
and in another it amounts to 90 feet. The seams of stone interspersed through the
coal are generally inconsiderable, and amount in only two cases to 27 inches
in thickness. The roof of the coal is so unsound, and the height so prodigious,
that it could not possibly be worked in one seam, like that of
Staffordshire. About 3 feet of the upper coal is therefore left as a roof,
under which a band of coal, from 6 to 7 feet thick, is worked on the post
and stall plan, with square pillars of extra strength, which are thereafter
penetrated. A platform about 3 feet high is left at the sole; under which
the rooms and pillars are set off and worked in another portion of the coal,
from 5 to 7 feet thick, great care being had to place pillar under pillar, and
partition under partition, to prevent a crush. Where the coal is thickest,
no less than 10 bands of it are worked in this way, as is shown in fig. 849.
When any band of the coal is foul from sulphur or other causes, it is left
for the next platform, so that a large proportion of it is lost, as in the Staffordshire
mines. Much attention must here be paid to the vertical distribution
of the pillars and apartments; the miner’s compass must be continually consulted,
and bore-holes must be put down through the coal scaffoldings, to regulate correctly
the position of the pillars under one another.
Edge coals, which are nearly perpendicular, are worked in a peculiar manner; for the
collier stands upon the coal, having the roof on the one hand, and the floor on the other,
like two vertical walls. The engine-pit is sunk in the most powerful
stratum. In some instances the same stratum is so vertical as
to be sunk through for the whole depth of the shaft.
Whenever the shaft has descended to the required depth, galleries
are driven across the strata from its bottom, till the coals are
intersected, as is shown in fig. 850., where we see the edge coals
at a, a; A, the engine-pit; b, b, the transverse galleries from the
bottom of the shaft; and c, c, upper transverse galleries, for the greater conveniency of
working the coal. The principal edge coal works in Great Britain lie in the neighbourhood[982]
of Edinburgh, and the coals are carried on the backs of women from the
wall-face to the bottom of the engine-pit.
The modes of carrying coals from the point where they are excavated to the pit
bottom, are nearly as diversified as the systems of working.
One method employs hutches, or baskets, having slips or cradle feet shod with iron,
containing from 2 to 3 hundred weight of coals. These baskets are dragged along
the floor by ropes or leather harness attached to the shoulders of the workmen, who are
either the colliers or persons hired on purpose. This method is used in several small
collieries; but it is extremely injudicious, exercising the muscular action of a man in the
most unprofitable manner. Instead of men, horses are sometimes yoked to these
basket-hurdles, which are then made to contain from 4 to 6 hundred weight of coals;
but from the magnitude of the friction, this plan cannot be commended.
An improvement on this system, where men draw the coals, is to place the basket or
corve on a small four-wheeled carriage, called a tram, or to attach wheels to the corve
itself. Thus much more work is performed, provided the floor be hard; but not on a
soft pavement, unless some kind of wooden railway be laid.
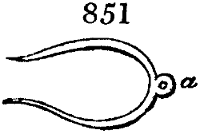
The transport of coals from the wall-face to the bottom of the shaft, was greatly
facilitated by the introduction of cast-iron railways, in place of wooden roads, first
brought into practice by Mr. John Curr of Sheffield. The rails are called tram-rails,
or plate-rails, consisting of a plate from 3 to 4 inches broad, with an edge at right angles
to it about two inches and a half high. Each rail is from 3 to 4 feet long, and is fixed
either to cross bearers of iron, called sleepers, or more usually to wooden bearers. In
some collieries, the miners, after working out the coals, drag them along these railways
to the pit bottom; but in others, two persons called trammers are employed to transport
the coals; the one of whom, in front of the corve, draws with harness; and the other,
called the patter, pushes behind. The instant each corve arrives, from the wall-face, at
a central spot in the system of the railways, it is lifted from the tram by a crane placed
there, and placed on a carriage called a rolley, which generally holds two corves.
Whenever three or four rolleys are loaded, they are hooked together, and the rolley
driver, with his horse, takes them to the bottom of the engine-shaft. The
rolley horses have a peculiar kind of shafts, commonly made of iron,
named limbers, the purpose of which is to prevent the carriage from overrunning
them. One of these shafts is represented in fig. 851. The hole
shown at a, passes over an iron peg or stud in front of the rolley, so that the horse
may be quickly attached or disengaged. By these arrangements the work is carried
on with surprising regularity and despatch.
The power of the engine for drawing the coals up the shaft, is made proportional to
the depth of the pit and the quantity to be raised, the corves ascending at an average
velocity of about 12 feet per second. So admirable is the modern arrangement of this
operation, that the corves are transported from the wall-faces to the pit bottom, and
moved up the shaft, as fast as the onsetters at the bottom, and the banksmen at the top,
can hook the loaded and empty corves on and off the engine ropes. Thus 100 corves of
coals have been raised every hour up a shaft 100 fathoms deep, constituting a lift of 27
tons per hour, or 324 tons in a day, or shift of 12 hours. Coals mined in large cubical
masses cannot, however, be so rapidly raised as the smaller coal of the Newcastle
district.
When coals have so great a rise from the pit bottom to the crop that horses cannot be
used on the rolley ways, the corves descend along the tram-roads, by means of inclined-plane
machines, which are moved either by vertical rope-barrels, or horizontal rope-sheaves.
These inclined planes are frequently divided into successive stages, 200 or 300
yards long, at the end of each of which is an inclined-plane machine, whereby the coals
are lowered from one level to another.
The wheels of the trams and rolleys vary in diameter from 8 to 16 inches, according
to the thickness of the coal. In some, the axles not only revolve on their journals, but
the wheels also revolve on their axles.
Various forms of machines have been employed for raising the coals out of the pits.
The steam engine with fly-wheel and rope-barrels, is, however, now preferred in all considerable
establishments. When of small power, they are usually constructed with a fly
wheel, and short fly-wheel shaft, on which there is a small pinion working into the
teeth of a large wheel, fixed upon the rope barrel. Thus the engine may move with
great rapidity, while it imparts an equable slow motion to the corves ascending in the
shaft. When the engines are of great power, however, they are directly connected with
the rope-barrel; some of these being of such dimensions, that each revolution of the rope-barrel
produces an elevation of 12 yards in the corve. A powerful brake is usually connected
with the circumference of the fly-wheel or rope-barrel, whereby the brakeman,
by applying his foot to the governing lever of the brake, and by shutting at the same
time the steam valves with his hands, can arrest the corve, or pitch its arrival within a[983]
few inches of the required height of every delivery. An endless chain, suspended from
the bottom to the top of the shaft, has, in a few pits of moderate depth, been worked by
a steam engine, for raising corves in constant succession; but the practice has not been
found hitherto applicable on the greater scale.
There is a kind of water engines for raising coals, strictly admissible only in level free
pits, where the ascent of the loaded corve is produced by the descent of a cassoon filled with
water. When the ascent and descent are through equal spaces, the rope barrels for the
cassoon and the corves are of equal diameter; but when the point from which the coals
have to be lifted is deeper than the point of discharge for the water into the dry level,
the cassoon must be larger, and the rope barrel smaller; so that by the time the cassoon
reaches to the half-depth, for example, the corve may have mounted through double the
space. The cassoon is filled with water at the pit mouth, and is emptied by a self-acting
valve whenever it gets to the bottom. The loaded corve is replaced by an empty
one at the pit mouth, and its weight, with that of the descending rope, pull up the
empty cassoon; the motions of the whole mechanism being regulated by a powerful
brake.
Various plans have been devised to prevent collision between the ascending and descending
corves, which sometimes pass each other with a joint velocity of 20 or 30 feet
per second. One method is by dividing the pit from top to bottom, so that each corve
moves in a separate compartment. Another mode was invented by Mr. Curr of
Sheffield, in which wooden guides were attached from top to bottom of the pit; being
spars of deal about 4 inches square, attached perpendicularly to the sides of the shaft,
and to buntons in the middle of the pit. Betwixt these guides, friction-roller sliders are
placed, attached to the gin-ropes, to which sliders the corves are suspended. In this
way, the corves can be raised with great rapidity; but there is a considerable loss of
time in banking the corve at the pit mouth, where shutters or sliding boards must be
used. This plan is highly beneficial where the coals are in large lumps.
Both ropes and chains are used for lifting coals. The round ropes are shroud-laid;
but the preferable rope is the flat band, made of four ropes placed horizontally together,
the ropes being laid alternately right and left. In this way, the ropes counteract one
another in the twist, hanging like a ribbon down the shaft; and are stitched strongly
together by a small cord. Such rope bands are not only very pliable for their strength,
which protects the heart of the rope from breaking, but as they lap upon themselves, a
simple sheave serves as a rope-barrel. They possess the additional advantage, that by so
lapping, they enlarge the diameter of the axle in which they coil, and thus make a compensation
mechanically against the increasing length of rope descending with its corve.
Thus the counterpoise chains, used in deep pits to regulate the descent, have been
superseded. See Rope-spinning.
When chains are preferred to ropes, as in very deep pits, the short pudding-link
chains are mostly used. See Cable.
The corves after being landed or banked at the pit mouth, are drawn to the bin or
coal-hill, either upon slips by horses, or by trammers on a tram-road. But with small
coals, like the Newcastle, the pit head is raised 8 or 9 feet above the common level of
the ground, and the coal-heap slopes downwards from that height. As the bins increase,
tram-roads are laid outwards upon them.
I shall now describe the ventilation of coal mines. Into their furthest recesses, an
adequate supply of fresh air must be carried forwards, for the purposes of respiration,
and the combustion of candles; as also for clearing off the carbonic acid and carburetted
hydrogen gases, so destructive to the miners, who call these noxious airs, from
their most obvious qualities, choke-damp and fire-damp.
Before the steam engine was applied to the drainage of the mines, and the extraction
of the coal, the excavations were of such limited extent, that when inflammable air accumulated
in the foreheads, it was usual in many collieries to fire it every morning.
This was done by fixing a lighted candle to the end of a long pole, which being extended
towards the roof by a person lying flat on the floor, the gas was fired, and the
blast passed safely over him. If the gas was abundant, the explosive miner put on a
wet jacket, to prevent the fire from scorching him. In other situations, where the fire-damp
was still more copious, the candle was drawn forwards into it, by a cord passing
over a catch at the end of the gallery, while the operator stood at a distance. This
very rude and dangerous mode of exploding the inflammable gas, is still practised in a
few mines, under the name of the firing line.
The carbonic acid or choke-damp having a greater specific gravity than atmospheric
air, in the proportion of about 3 to 2, occupies the lower part of the workings, and
gives comparatively little annoyance. Its presence may moreover be always safely
ascertained by the lighted candle. This cannot, however, be said of the fire-damp,
which being lighter and more movable, diffuses readily through the atmospheric air, so
as to form a most dangerous explosive mixture, even at a considerable distance from[984]
the blowers or sources of its extrication from the coal strata. Pure subcarburetted
hydrogen has a specific gravity = 0·555, air being 1; and consists of a volume of vapour
of carbon, and two volumes of hydrogen, condensed by mutual affinity into one volume.
The choke-damp is a mixture of the above, with a little carbonic acid gas, and variable
proportions of atmospheric air. As the pure subcarburetted hydrogen requires twice
its bulk of oxygen to consume it completely, it will take for the same effect about 10
times its bulk of atmospheric air, since this volume of air contains about two volumes
of oxygen. Ten volumes of air, therefore, mixed with one volume of subcarburetted
hydrogen, form the most powerfully explosive mixture. If either less or more air be
intermixed, the explosive force will be impaired; till 3 volumes of air below or above
that ratio, constitute non-explosive mixtures; that is, 1 of the pure fire-damp mixed
with either 7 or 13 of air, or any quantity below the first, or above the second number,
will afford an unexplosive mixture. With the first proportion, a candle will not burn;
with the second, it burns with a very elongated blue flame. The fire-damp should
therefore be still further diluted with common air, considerably beyond the above proportion
of 1 to 13, to render the working of the mine perfectly safe.
These noxious gases are disengaged from the cutters, fissures, and minute pores of the
coal; and if the quantity be considerable, relative to the orifice, a hissing noise is heard.
Though the choke-damp, or carbonic acid gas, be invisible, yet its line of division
from the common air is distinctly observable on approaching a lighted candle to the
lower level, where it accumulates, which becomes extinguished
the instant it comes within its sphere, as if it were plunged in
water. The stratum of carbonic acid sometimes lies 1 or 2 feet
thick on the floor, while the superincumbent air is perfectly good. When the coal has
a considerable dip and rise, the choke-damp will be found occupying the lower parts of
the mine, in a wedge form, as represented in fig. 852., where a shows the place of the
carbonic acid gas, and b that of the common air.
When a gallery is driven in advance of the other workings, and a discharge of this
gas takes place, it soon fills the whole mine, if its direction be in the line of level, and
the mine is rendered unworkable until a supply of fresh air is introduced to dislodge it.
As the flame of a candle indicates correctly the existence of the choke-damp, the miners
may have sufficient warning of its presence, so as to avoid the place which it occupies,
till adequate means be taken to drive it away.
The fire-damp is not an inmate of every mine, and is seldom found, indeed, where
the carbonic acid prevails. It occurs in the greatest quantities in the coal mines of the
counties of Northumberland, Durham, Cumberland, Staffordshire, and Shropshire. It
is more abundant in coals of the caking kind, with a bright steel-grained fracture, than
in cubic coals of an open-burning quality. Splint coals are still less liable to disengage
this gas. In some extensive coal-fields it exists copiously on one range of the line of
bearing, while on the other range, none of it is observed, but abundance of carbonic
acid gas.
In the numerous collieries in the Lothians, south from the city of Edinburgh, the
fire-damp is unknown; while in the coal-fields round the city of Glasgow, and along
the coast of Ayrshire, it frequently appears.
The violent discharge of the gas from a crevice or cutter of the coal, is called a
blower; and if this be ignited, it burns like an immense blowpipe, inflaming the coal
at the opposite side of the gallery. The gas evidently exists in a highly compressed
and elastic state; whence it seems to loosen the texture of the coals replete with it, and
renders them more easily worked. The gas is often peculiarly abundant near a great
dislocation or slip of the strata; so that the fissure of the dislocation will sometimes
emit a copious stream of gas for many years. It has also happened, that from certain
coals, newly worked, and let fall from a height into the hold of a vessel, so much inflammable
gas has been extricated that, after the hatches were secured, and the ship
ready to proceed to sea, the gas has ignited with the flame of a candle, so as to scorch
the seamen, to blow up the decks, and otherwise damage the vessel. In like manner,
when the pillars in a mine are crushed by sudden pressure, a great discharge of gas
ensues. This gas being lighter than common air, always ascends to the roof or to the
rise of the galleries; and, where the dip is considerable, occupies the forehead of the
mine, in a wedge form, as shown in fig. 853., where a represents
the fire-damp, and b the common air. In this case, a candle
will burn without danger near the point c, close to the floor; but if it be advanced a
few feet further towards the roof, an explosion will immediately ensue; since at the line
where the two elastic fluids are in contact, they mix, and form an explosive body.
When this gas is largely diluted with air, the workmen do not seem to feel any
inconvenience from breathing the mixture for a period of many years; but on inhaling
pure carburetted hydrogen, the miner instantly drops down insensible, and, if not
speedily removed into fresh air, he dies.[985]
The production of these noxious gases renders ventilation a primary object in the
system of mining. The most easily managed is the carbonic acid. If an air-pipe
has been carried down the engine pit for the purpose of ventilation in the sinking,
other pipes are connected with it, and laid along the pavement, or are attached to an
angle of the mine next the roof. These pipes are prolonged with the galleries, by
which means the air at the forehead is drawn up the pipes and replaced by atmospheric
air, which descends by the shaft in an equable current, regulated by the draught of the
furnace at the pit mouth. This circulation is continued till the miners cut through
upon the second shaft, when the air-pipes become superfluous; for it is well known
that the instant such communication is made, as is represented in fig. 854., the
air spontaneously descends in the engine pit A, and, passing along the gallery a,
ascends in a steady current in the second pit B. The air, in sinking
through A, has at first the atmospheric temperature, which in
winter may be at or under the freezing point of water; but its
temperature increases in passing down through the relatively
warmer earth, and ascends in the shaft B, warmer than the atmosphere.
When shafts are of unequal depths, as represented in the
figure, the current of air flows pretty uniformly in one direction. If the second shaft has
the same depth with the first, and the bottom and mouth of both be in the same horizontal
plane, the air would sometimes remain at rest, as water would do in an inverted
syphon, and at other times would circulate down one pit and up another, not always
in the same direction, but sometimes up the one, and sometimes up the other, according
to the variations of temperature at the surface, and the barometrical pressures, as modified
by winds. There is in mines a proper heat, proportional to their depth, increasing
about one degree of Fahrenheit’s scale for every 60 feet of descent.
There is a simple mode of conducting air from the pit bottom to the forehead of the
mine, by cutting a ragglin, or trumpeting, as it is termed, in the side of the gallery, as represented
in fig. 855., where A exhibits the gallery in the coal, and B the
ragglin, which is from 15 to 18 inches square. The coal itself forms
three sides of the air-pipe, and the fourth is composed of thin deals applied air-tight,
and nailed to small props of wood fixed between the top and bottom of the lips of the
ragglin. This mode is very generally adopted in running galleries of communication,
and dip-head level galleries, where carbonic acid abounds, or when from the stagnation
of the air the miners’ lights burn dimly.
When the ragglin or air-pipes are not made spontaneously active, the air is sometimes
impelled through them by means of ventilating fanners, having their tube placed at the
pit bottom, while the vanes are driven with great velocity by a wheel and pinion worked
with the hand. In other cases, large bellows like those of the blacksmith, furnished
with a wide nozzle, are made to act in a similar way with the fanners. But these are
merely temporary expedients for small mines. A very slight circulation of air can be
effected by propulsion, in comparison of what may be done by exhaustion; and hence
it is better to attach the air-pipe to the valve of the bellows, than to their nozzle.
Ventilation of collieries has been likewise effected on a small scale, by attaching a
horizontal funnel to the top of air-pipes elevated a considerable height above the pit
mouth. The funnel revolves on a pivot, and by its tail-piece places its mouth so as to
receive the wind. At other times, a circulation of air is produced by placing coal-fires
in iron grates, either at the bottom of an upcast pit, or suspended by a chain a few
fathoms down.
Such are some of the more common methods practised in collieries of moderate
depth, where carbonic acid abounds, or where there is a total stagnation of air. But
in all great coal mines the aërial circulation is regulated and directed by double
doors, called main or bearing doors. These are true air-valves, which intercept a
current of air moving in one direction from mixing with another moving in a different
direction. Such valves are placed on the main roads and passages of the
galleries, and are essential to a just ventilation. Their functions
are represented in the annexed fig. 856., where A shows
the downcast shaft, in which the aerial current is made to
descend; B is the upcast shaft, sunk towards the rise of the
coal; and C, the dip-head level. Were the mine here figured
to be worked without any attention to the circulation, the air
would flow down the pit A, and proceed in a direct line up
the rise mine to the shaft B, in which it would ascend. The consequence would therefore
be, that all the galleries and boards to the dip of the pit A, and those lying on each
side of the pits, would have no circulation of air; or, in the language of the collier, would
be laid dead. To obviate this result, double doors are placed in three of the galleries adjoining
the pit; viz., at a and b, c and d, e and f; all of which open inwards to the shaft A.
By this plan, as the air is not suffered to pass directly from the shaft A to the shaft B, through[986]
the doors a and b, it would have taken the next shortest direction by c d and e f; but the
doors in these galleries prevent this course, and compel it to proceed downwards to
the dip-head level C, where it will spread or divide, one portion pursuing a route to
the right, another to the left. On arriving at the boards g and h, it would have
naturally ascended by them; but this it cannot do, by reason of the building or stopping
placed at g and h. By means of such stoppings placed in the boards next the
dip-head level, the air can be transported to the right hand or to the left for many
miles, if necessary, providing there be a train or circle of aerial communication from
the pit A to the pit B. If the boards i and k are open, the air will ascend in them,
as traced out by the arrows; and after being diffused through the workings, will again
meet in a body at a, and mount the gallery to the pit B, sweeping away with it the
deleterious air which it meets in its path. Without double doors on each main passage,
the regular circulation of the air would be constantly liable to interruptions and
derangements; thus, suppose the door c to be removed, and only d to remain in the
left hand gallery, all the other doors being as represented, it is obvious, that whenever
the door d is opened, the air, finding a more direct passage in that direction, would
mount by the nearest channel l, to the shaft B, and lay dead all the other parts of the
work, stopping all circulation. As the passages on which the doors are placed constitute
the main roads by which the miners go to and from their work, and as the
corves are also constantly wheeling along all the time, were a single door, such as d,
so often opened, the ventilation would be rendered precarious or languid. But the
double doors obviate this inconvenience; for both men and horses, with the corves,
in going to or from the pit bottom A, no sooner enter the door d, than it shuts behind
them, and encloses them in the still air contained between the doors d and c; c having
prevented the air from changing its proper course while d was open. When d is again
shut, the door c may be opened without inconvenience, to allow the men and horses to
pass on to the pit bottom at A; the door d preventing any change in the aerial circulation
while the door c is open. In returning from the pit, the same rule is observed, of
shutting one of the double doors, before the other is opened.
If this mode of disjoining and insulating air-courses from each other be once fairly
conceived, the continuance of the separation through a working of any extent, may be
easily understood.
When carbonic acid gas abounds, or when the fire-damp is in very small quantity,
the air may be conducted from the shaft to the dip-head level, and by placing
stoppings of each room next the level, it may be carried to any distance along the dip-head
levels; and the furthest room on each side being left open, the air is suffered
to diffuse itself through the wastes, along the wall faces, and mount in the upcast
pit, as is represented in fig. 842. But should the air become stagnant along the
wall faces, stoppings are set up throughout the galleries, in such a way as to direct the
main body of fresh air along the wall faces for the workmen, while a partial stream of
air is allowed to pass through the stoppings, to prevent any accumulation of foul air in
the wastes.
In very deep and extensive collieries more elaborate arrangements for ventilation are
introduced. Here the circulation is made active by rarefying the air at the upcast
shaft, by means of a very large furnace placed either at the bottom or top of the
shaft. The former position is generally preferred. Fig. 834. exhibits a furnace
placed at the top of the pit. When it surmounts a single pit, or a single division of
the pit, the compartment intended for the upcast is made air-tight at top, by placing
strong buntons or beams across it, at any suitable distance from the mouth. On these
buntons a close scaffolding of plank is laid, which is well plastered or moated over
with adhesive plastic clay. A little way below the scaffold, a passage is previously
cut, either in a sloping direction, to connect the current of air with the furnace, or it
is laid horizontally, and then communicates with the furnace by a vertical opening.
If any obstacle prevent the scaffold from being erected within the pit, this can be
made air-tight at top, and a brick flue carried thence along the surface to the furnace.
The furnace has a size proportional to the magnitude of the ventilation, and the
chimneys are either round or square, being from 50 to 100 feet high, with an inside
diameter of from 5 to 9 feet at bottom, tapering upwards to a diameter of from 21⁄2 feet
to 5 feet. Such stalks are made 9 inches thick in the body of the building, and a
little thicker at bottom, where they are lined with fire-bricks.
The plan of placing the furnace at the bottom of the pit is, however, more advantageous,
because the shaft through which the air ascends to the furnace at the pit
mouth, is always at the ordinary temperature; so that whenever the top furnace is neglected,
the circulation of air throughout the mine becomes languid, and dangerous to
the workmen; whereas, when the furnace is situated at the bottom of the shaft, its sides
get heated, like those of a chimney, through its total length, so that though the heat of
the furnace be accidentally allowed to decline or become extinct for a little, the circulation[987]
will still go on, the air of the upcast pit being rarefied by the heat remaining in
the sides of the shaft.
To prevent the annoyance to the onsetters at the bottom, from the hot smoke, the following
plan has been adopted, as shown in the wood-cut, fig. 857., where a represents
the lower part of the upcast shaft; b, the furnace, built of
brick, arched at top, with its sides insulated from the solid
mass of coal which surrounds it. Between the furnace
wall and the coal-beds, a current of air constantly passes
towards the shaft, in order to prevent the coal catching
fire. From the end of the furnace a gallery is cut in a
rising direction at c, which communicates with the shaft at
d, about 7 or 8 fathoms from the bottom of the pit. Thus the furnace and furnace-keeper
are completely disjoined from the shaft; and the pit bottom is not only free
from all encumbrances, but remains comfortably cool. To obviate
the inconveniences from the smoke to the banksmen in
landing the coals at the pit mouth, the following plan has been
contrived for the Newcastle collieries. Fig. 858. represents the
mouth of the pit; a is the upcast shaft, provided with a furnace
at bottom; b, the downcast shaft, by which the supply of atmospheric
air descends; and d, the brattice carried above the pit
mouth. A little way below the settle-boards, a gallery c, is pushed,
in communication with the surface from the downcast shaft,
over which a brick tube or chimney is built from 60 to 80 feet
high, 7 or 8 feet diameter at bottom, and 4 or 5 feet diameter at
top. On the top of this chimney a deal funnel is suspended horizontally on a pivot,
like a turn-cap. The vane f, made also of deal, keeps the mouth of the funnel always
in the same direction with the wind. The same mechanism is mounted at the upcast
shaft a, only here the funnel is made to present its mouth in the wind’s eye. It is obvious
from the figure, that a high wind will rather aid than check the ventilation by this plan.
The principle of ventilation being thus established, the next object in opening up a
colliery, and in driving all galleries whatever, is the double mine or double headways
course; on the simple but very ingenious distribution of which, the circulation of air
depends at the commencement of the excavations.
The double headways course is represented in fig. 859., where a is the one
heading or gallery, and b the other; the former being immediately connected with
the upcast side of the pit c, and the latter with
the downcast side of the pit d. The pit itself is
made completely air-tight by its division of deals
from top to bottom, called the brattice wall; so that
no air can pass through the brattice from d to c, and the intercourse betwixt the two
currents of air is completely intercepted by a stopping betwixt the pit bottom and the
end of the first pillar of coal; the pillars or walls of coal, marked e, are called stenting
walls; and the openings betwixt them, walls or thirlings. The arrows show the direction
of the air. The headings a and b are generally made about 9 feet wide, the stenting
walls 6 or 8 yards thick, and are holed or thirled at such a distance as may be most
suitable for the state of the air. The thirlings are 5 feet wide.
When the headings are set off from the pit bottom, an aperture is left in the brattice
at the end of the pillar next the pit, through which the circulation betwixt the upcast
and downcast pits is carried on; but whenever the workmen cut through the first
thirling No. 1., the aperture in the brattice at the pit bottom is shut; in consequence of
which the air is immediately drawn by the power of the upcast shaft through that thirling
as represented by the dotted arrow. Thus a direct stream of fresh air is obviously
brought close to the forehead where the mines are at work. The two headings a and b
are then advanced, and as soon as the thirling No. 2. is cut through, a wall of brick and
mortar, 41⁄2 inches thick, is built across the thirling No. 1. This wall is termed a stopping;
and being air-tight, it forces the whole circulation through the thirling No. 2. In
this manner the air is always led forward, and caused to circulate always by the last-made
thirling next the forehead; care being had, that whenever a new thirling is made,
the last thirling through which the air was circulated, be secured with an air-tight
stopping. In the woodcut, the stoppings are placed in the thirlings numbered 1, 2, 3,
4, 5, 6, and of consequence the whole circulation passes through the thirling No. 7.,
which lies nearest the foreheads of the headings a, b. By inspecting the figure, we
observe, that on this very simple plan, a stream of air may be circulated to any required
distance, and in any direction, however tortuous. Thus, for example, if while the
double headways course a, b, is pushed forward, other double headways courses are required
to be carried on at the same time on both sides of the first headway, the
same general principles have only to be attended to as shown in fig. 860., where[988]
a is the upcast, and b the downcast shaft.
The air advances along the heading c, but
cannot proceed further in that direction than
the pillar d, being obstructed by the double
doors at e. It therefore advances in the direction
of the arrows to the foreheads at f, and
passing through the last thirling made there,
returns to the opposite side of the double doors,
ascends now the heading g to the foreheads at
h, passes through the last-made thirling at that
point, and descends, in the heading i, till it is
interrupted by the double doors at k. The
aerial current now moves along the heading l,
to the foreheads at m, returns by the last-made
thirling there, along the heading n, and finally
goes down the heading o, and mounts by the
upcast shaft a, carrying with it all the noxious
gases which it encountered during its circuitous
journey. This wood-cut is a faithful representation of the system by which collieries
of the greatest extent are worked and ventilated. In some of these, the air
courses are from 30 to 40 miles long. Thus the air conducted by the medium of a
shaft divided by a brattice wall only a few inches thick, after descending in the downcast
in one compartment of the pit at 6 o’clock in the morning, must thence travel through a
circuit of nearly 30 miles, and cannot arrive at its reascending compartment on the other
side of the brattice, or pit partition, till 6 o’clock in the evening, supposing it to move
all the time at the rate of 21⁄2 miles per hour. Hence we see that the primum mobile of
this mighty circulation, the furnace, must be carefully looked after, since its irregularities
may affect the comfort, or even the existence of hundreds of miners spread
over these vast subterraneous labyrinths. On the principles just laid down, it appears,
that if any number of boards be set off from any side of these galleries, either in a level,
dip, or rise direction, the circulation of air may be advanced to each forehead, by an
ingoing and returning current.
Yet while the circulation of fresh air is thus advanced to the last-made thirling next
the foreheads f, h, and m, fig. 860., and moves through the thirling which is nearest
to the face of every board and room, the emission of fire-damp is frequently so abundant
from the coaly strata, that the miners dare not proceed forwards more than a few feet
from that aerial circulation, without hazard of being burned by the combustion of the
gas at their candles. To guard against this accident, temporary shifting brattices are
employed. These are formed of deal, about 3⁄4 of an inch thick, 3 or 4 feet broad,
and 10 feet long; and are furnished with cross-bars for binding the deals together, and
a few finger loops cut through them, for lifting them more expeditiously, in order to place
them in a proper position. Where inflammable air abounds, a store of such brattice
deals should be kept ready for emergencies.
The mode of applying these temporary brattices, or deal partitions, is shown in the
accompanying figure (fig. 861.), which shows how the air circulates freely through the
thirling d, d before the brattices are placed. At b and c, we see two heading
boards or rooms, which are so full of inflammable air as to be unworkable.
Props are now erected near the upper end of the pillar e, betwixt the
roof and pavement, about two feet clear of the sides of the next pillar, leaving
room for the miner to pass along between the pillar side and the brattice.
The brattices are then fastened with nails to the props, the lower
edge of the under brattice resting on the pavement, while the upper edge of
the upper is in contact with the roof. By this means any variation of the
height in the bed of coal is compensated by the overlap of the brattice
boards; and as these are advanced, shifting brattices are laid close to, and
alongside of, the first set. The miner next sets up additional props in the same parallel
line with the former, and slides the brattices forwards, to make the air circulate close
to the forehead where he is working; and he regulates the distance betwixt the brattice
and the forehead by the disengagement of fire-damp and the velocity of the aerial circulation.
The props are shown at d d, and the brattices at f, f. By this arrangement the
air is prevented from passing directly through the thirling a, and is forced along the
right-hand side of the brattice, and, sweeping over the wall face or forehead, returns by
the back of the brattice, and passes through the thirling a. It is prevented, however,
from returning in its former direction by the brattice planted in the forehead c, whereby
it mounts up and accomplishes its return close to that forehead. Thus headways
and boards are ventilated till another thirling is made at the upper part of the pillar.
The thirling a is then closed by a brick stopping, and the brattice boards removed
forward for a similar operation.
[989]
When blowers occur in the roof, and force the strata down, so as to produce a large
vaulted excavation, the accumulated gas must be swept away; because, after filling that
space, it would descend in an unmixed state under the common roof of the coal.
The manner of removing it is represented in fig. 862., where a is the bed of coal,
b the blower, c the excavation left by the downfall of the
roof, d is a passing door, and e a brattice. By this arrangement
the aerial current is carried close to the roof, and
constantly sweeps off or dilutes the inflammable gas of the
blower, as fast as it issues. The arrows show the direction
of the current; but for which, the accumulating gas would be
mixed in explosive proportions with the atmospheric air, and
destroy the miners.
There is another modification of the ventilating system,
where the air-courses are traversed across; that is, when one
air-course is advanced at right angles to another, and must
pass it in order to ventilate the workings on the further side. This is accomplished
on the plan shown in fig. 863., where a is a main road with an air-course, over
which the other air-course b, has to pass. The sides of this air channel are built of
bricks arched over so as to be air-tight, and a gallery is driven in the roof strata as
shown in the figure. If an air-course, as a, be laid over with planks made air-tight,
crossing and recrossing may be effected with facility. The general velocity of the air
in these ventilating channels is from 3 to 4 feet per second, or about 21⁄2 miles per hour,
and their internal dimensions vary from 5 to 6 feet square, affording an area of from 25
to 36 square feet.
Mr. Taylor’s hydraulic air-pump, formerly described, p. 839., deserves to be noticed
among the various ingenious contrivances for ventilating mines, particularly
when they are of moderate extent. a is a large wooden tub, nearly filled with
water, through whose bottom the ventilating pipe b passes down into the recesses
of the mine. Upon the top of b, there is a valve e, opening upwards. Over b,
the gasometer vessel is inverted in a, having a valve also opening outwards at d.
When this vessel is depressed by any moving force, the air contained within it
is expelled through d; and when it is raised, it diminishes the atmospherical
pressure in the pipe b, and thus draws air out of the mine into the gasometer;
which cannot return on account of the valve at e, but is thrown out into the
atmosphere through d at the next descent.
The general plan of distributing the air, in all cases, is to send the first of the
current that descends in the downcast shaft among the horses in the stables, next
among the workmen in the foreheads, after which the air, loaded with whatever
mixtures it may have received, is made to traverse the old wastes. It then passes
through the furnace with all the inflammable gas it has collected, ascends the upcast shaft,
and is dispersed into the atmosphere. This system, styled coursing the air, was invented
by Mr. Spedding of Cumberland. According to the quantity of the fire-damp, the
coursing is conducted either up one room, and returned by the next alternately, through
the whole extent of the works, or it passes along 2 or 3 connected rooms, and returns
by the same number.
This admirable system has received the greatest improvements from the mining
engineers of the Newcastle district, and especially from Mr. Buddle of Wallsend. His
plan being a most complete scale of ventilation, where the aerial current is made
to sweep every corner of the workings, is shown in fig. 865.; in which a
represents the downcast, and b the upcast
shaft. By pursuing the track of the arrows,
we may observe that the air passes first along
the two rooms c, d, having free access to each
through the walls, but is hindered from
entering into the adjoining rooms by the
stoppings which form the air-courses. It
sweeps along the wall faces of the rooms c, d,
and makes a return down the rooms e, f,
but is not allowed to proceed further in that
direction by the stoppings g, h. It then
proceeds to the foreheads i, k, and single
courses all the rooms to the foreheads l, m; from this point it would go directly to the
upcast pit b, were it not prevented by the stopping n, which throws it again into double
coursing the rooms, till it arrives at o, whence it goes directly to the furnace, and
ascends the shaft b. The lines across each other represent the passing doors; and these
may be substituted in any place for a passage where there is a stopping. The stopping
p, near the bottom of the downcast shaft, is termed a main stopping; because if it
were removed, the whole circulation would instantly cease, and the air, instead of[990]
traversing in the direction of the arrows, would go directly from the downcast pit a, to
the upcast pit b, along the gallery q. Hence every gallery and room of the workings
would be laid dead, as it is termed, and be immediately filled with fire-damp, which
might take fire either at the workmen’s candles, or at the furnace next the upcast
shaft b. Thus also a partial stagnation in one district of the colliery, would be produced
by any of the common stoppings being accidentally removed or destroyed, since
the air would thereby always pursue the nearest route to the upcast pit. Main stoppings
are made particularly secure, by strong additional stone buildings, and they are
set up at different places, to maintain the main air courses entire in the event of an
explosion; by which precautions great security is given to human life. This system of
ventilation may be extended to almost any distance from the pit-bottom, provided the
volume of fresh air introduced be adequate to dilute sufficiently the fire-damp, so that
the mixture shall not reach the explosive point. The air, by this management, ventilates
first one panel of work, and then other panels in succession, passing onwards
through the barriers or panel walls, by means of galleries, as in fig. 843., by
the principle either of single, double, or triple coursing, according to the quantity of gas
in the mine.
In ventilating the very thick coal of Staffordshire, though there is much inflammable
air, less care is needed than in the north of England collieries, as the workings are
very roomy, and the air courses of comparatively small extent. The air is conducted
down one shaft, carried along the main roads, and distributed into the sides of work,
as shown in fig. 848. A narrow gallery, termed the air-head, is carried in
the upper part of the coal, in the rib walls, along one or more of the sides. In
the example here figured, it is carried all round, and the air enters at the bolt-hole e.
Lateral openings, named spouts, are led from the air-head gallery into the side of work;
and the circulating stream mixed with the gas in the workings, enters by these spouts,
as represented by the arrows, and returns by the air-head at g, to the upcast pit.
When the fire-damp comes off suddenly in any case, rendering the air foul and explosive
at the foreheads, if no other remedy be found effectual, the working of the coal must
be suspended, and a current of air sent directly from the fresh in-going stream, in order
to dilute the explosive mixture, before it reaches the furnace. This is termed skailing
the air; for otherwise the gas would kindle at the furnace, and flame backwards, like a
train of gunpowder, through all the windings of the work, carrying devastation and
death in its track. By skailing the air, however, time is given for running forward
with water, and drowning the furnace. A cascade of water from the steam engine
pumps is then allowed to fall down the pit, the power of which through a fall of 500 or
600 feet, is so great in carrying down a body of air, that it impels a sufficient current
through every part of the workings. The ventilation is afterwards put into its usual train
at leisure.
In collieries which have been worked for a considerable time, and particularly in such
as have goaves, creeps, or crushed wastes, the disengagement of the fire-damp from these
recesses is much influenced by the state of atmospheric pressure. Should this be suddenly
diminished, as shown by the fall of the barometer, the fire-damp suddenly expands
and comes forth from its retirement, polluting the galleries of the mine with its noxious
presence. But an increase of barometric pressure condenses the gases of the mine, and
restrains them within their sequestered limits. It is therefore requisite that the coal-viewer
should consult the barometer before inspecting the subterraneous workings of an
old mine, on the Monday mornings, in order to know what precautions must be
observed in his personal survey.
The catastrophe of an explosion in an extensive coal-mine is horrible in the extreme.
Let us imagine a mine upwards of 100 fathoms deep, with the workings extended to a
great distance under the surrounding country, with machinery complete in all its parts,
the mining operations under regular discipline, and railways conducted through all its
ramifications; the stoppings, passing doors, brattices, and the entire economy of the
mine, so arranged that every thing moves like a well regulated machine. A mine of
this magnitude at full work is a scene of cheering animation, and happy industry; the
sound of the hammer resounds in every quarter, and the numerous carriages, loaded or
empty, passing swiftly to and fro from the wall faces to the pit bottom, enliven the
gloomiest recesses. At each door a little boy, called a trapper, is stationed, to open, and
shut it. Every person is at his post, displaying an alacrity and happiness pleasingly
contrasted with the surrounding gloom. While things are in this merry train, it has
but too frequently happened that from some unforeseen cause, the ventilation has partially
stagnated, allowing a quantity of the fire-damp to accumulate in one space to the explosive
pitch; or a blower has suddenly sprung forth, and the unsuspecting miner entering
this fatal region with his candle, sets the whole in a blaze of burning air, which immediately
suffocates and scorches to death every living creature within its sphere, while
multitudes beyond the reach of the flame are dashed to pieces by the force of the explosion,
rolling like thunder along the winding galleries. Sometimes the explosive flame[991]
seems to linger in one district for a few moments; then gathering strength for a giant
effort, it rushes forth from its cell with the violence of a hurricane, and the speed of
lightning, destroying every obstacle in its way to the upcast shaft. Its power seems to
be irresistible. The stoppings are burst through, the doors are shivered into a thousand
pieces; while the unfortunate miners, men, women, and boys, are swept along with an
inconceivable velocity, in one body, with the horses, carriages, corves, and coals. Should
a massive pillar obstruct the direct course of the aerial torrent, all these objects are
dashed against it, and there prostrated or heaped up in a mass of common ruin, mutilation,
and death. Others are carried directly to the shaft, and are either buried there
amid the wreck, or are blown up and ejected from the pit mouth. Even at this distance
from the explosive den, the blast is often so powerful, that it frequently tears the brattice
walls of the shaft to pieces, and blows the corves suspended in the shaft as high up into the
open air as the ropes will permit. Not unfrequently, indeed, the ponderous pulley-wheels
are blown from the pit-head frame, and carried to a considerable distance in the bosom
of a thick cloud of coals and coal-dust brought up from the mine by the fire-damp, whose
explosion shakes absolutely the superincumbent solid earth itself with a mimic earthquake.
The dust of the ruins is sometimes thrown to such a height above the pit as to
obscure the light of the sun. The silence which succeeds to this awful turmoil is no less
formidable; for the atmospheric back-draught, rushing down the shaft, denotes the consumption
of vital air in the mine, and the production of the deleterious choke-damp
and azote.
Though many of the miners may have escaped by their distance in the workings from
the destructive blast and the fire, yet their fate may perhaps be more deplorable. They
hear the explosion, and are well aware of its certain consequences. Every one anxious
to secure his personal safety, strains every faculty to reach the pit-bottom. As the
lights are usually extinguished by the explosion, they have to grope their way in utter
darkness. Some have made most marvellous escapes, after clambering over the rubbish
of fallen roofs, under which their companions are entombed; but others wandering into
uncertain alleys, tremble lest they should encounter the pestilential airs. At last they
feel their power, and aware that their fate is sealed, they cease to struggle with their inevitable
doom; they deliberately assume the posture of repose, and fall asleep in death.
Such has been too often the fate of the hardy and intelligent miners who immure themselves
deep beneath the ground, and venture their lives for the comfort of their fellow-men;
and such frequently is the ruinous issue of the best ordered and most prosperous
mining concerns.
In such circumstances the mining engineers or coal viewers have a dangerous and
difficult duty to perform. The pit into which they must descend as soon as possible, is
rendered unsafe by many causes; by the wrecks of loose timber torn away by the
eruption, or by the unrespirable gases; by the ignition perhaps of a portion of the coal
itself, or by the flame of a blower of fire-damp; either of which would produce violent
and repeated explosions whenever the gas may again accumulate to the proper degree.
Such a predicament is not uncommon, and it is one against which no human skill can
guard. Yet even here, the sense of duty, and the hope of saving some workmen from a
lingering death by wounds or suffocation, lead this intrepid class of men to descend
amid the very demons of the mine.
As soon as the ventilation is restored by temporary brattices, the stoppings and doors
are rebuilt in a substantial manner, and the workings are resumed with the wonted
activity. From an inspection of fig. 864., p. 1029, it is obvious that the stability of
the main stopping p, is an important point; for which reason it is counterforted by strong
walls of stone, to resist the explosive force of fire-damp.
When it is known that fire exists in the wastes, either by the burning of the small
coal-dust along the roads, or from the ignition of the solid coal by a blower of gas,
the inspection of the mine is incomparably more hazardous, as safety cannot be insured
for an instant; for if the extrication of gas be great, it rapidly accumulates, and whenever
it reaches the place where the fire exists, a new explosion takes place. There have
been examples of the most furious detonations occurring regularly after the interval of
about an hour, and being thus repeated 36 times in less than two days, each eruption
appearing at the pit mouth like the blast of a volcano. It would be madness for any
one to attempt a descent in such circumstances. The only resource is to moat up the
pit, and check the combustion by exclusion of atmospheric air, or to drown the workings
by letting the water accumulate below ground.
When fire exists in the wastes, with less apparent risk of life, water is driven upon it
by portable fire-extinguishing engines, or small cannon are discharged near the burning
coal, and the concussion thus produced in the air sometimes helps to extinguish the flame.
Since the primary cause of these tremendous catastrophes is the accension of the
explosive gases by the candle of the miner, it has been long a desideratum to procure
light of such a nature as may not possess the power of kindling the fire-damp. The
train of light producible from the friction of flint and steel, by a mechanism called[992]
a steel mill, has been long known, and afforded a tolerable gleam, with which the miners
were obliged to content themselves in hazardous atmospheres.
It consists of a small frame of iron, mounted with a wheel and pinion, which give
rapid rotation to a disk of hard steel placed upright, to whose edge a piece of flint is
applied. The use of this machine entailed on the miner the expense of an attendant,
called the miller, who gave him light. Nor was the light altogether safe, for occasionally the
ignited shower of steel particles attained to a sufficient heat to set fire to the fire-damp.
At length the attention of the scientific world was powerfully attracted to the means
of lighting the miner with safety, by an awful catastrophe which happened at Felling
Colliery, near Newcastle, on the 25th May, 1812. This mine was working with great
vigour, under a well-regulated system of ventilation, set in action by a furnace and air-tube,
placed over a rise pit in elevated ground. The depth of winning was above 100
fathoms; 25 acres of coal had been excavated, and one pit was yielding at the rate of
1700 tons per week. At 11 o’clock in the forenoon the night shift of miners was
relieved by the day shift; 121 persons were in the mine, at their several stations, when,
at half-past 11, the gas fired, with a most awful explosion, which alarmed all the neighbouring
villages. The subterraneous fire broke forth with two heavy discharges from
the dip-pit, and these were instantly followed by one from the rise-pit. A slight
trembling, as from an earthquake, was felt for about half a mile round the colliery, and
the noise of the explosion, though dull, was heard at from 3 to 4 miles’ distance.
Immense quantities of dust and small coal accompanied these blasts, and rose high into
the air, in the form of an inverted cone. The heaviest part of the ejected matter, such
as corves, wood, and small coal, fell near the pits; but the dust borne away by a strong
west wind fell in a continuous shower a mile and a half from the pit. In the adjoining
village of Heworth it caused a darkness like that of early twilight, covering the roads
where it fell so thickly that the footsteps of passengers were imprinted in it. The
heads of both shaft-frames were blown off, their sides set on fire, and their pulleys
shattered to pieces. The coal-dust ejected from the rise-pit into the horizontal part of
the ventilating tube, was about 3 inches thick, and speedily burnt to a cinder; pieces of
burning coal, driven off the solid stratum of the mine, were also blown out of this shaft.
Of the 121 persons in the mine at the time of the explosion, only 32 were drawn up the
pit alive, 3 of whom died a few hours after the accident. Thus no less than 92 valuable
lives were instantaneously destroyed by this pestilential fire-damp. The scene of
distress among the relatives at the pit mouth was indescribably sorrowful.
Dr. W. Reid Clanny, of Sunderland, was the first to contrive a lamp which might
burn among explosive air without communicating flame to the gas in which it was plunged.
This he effected, in 1813, by means of an air-tight lamp, with a glass front, the flame
of which was supported by blowing fresh air from a small pair of bellows through a
stratum of water in the bottom of the lamp, while the heated air passed out through
water by a recurved tube at top. By this means the air within the lamp was completely
insulated from the surrounding atmosphere. This lamp was the first ever taken
into a body of inflammable air in a coal-mine, at the exploding point, without setting
fire to the gas around it. Dr. Clanny made another lamp upon an improved plan, by
introducing into it the steam of water generated in a small vessel at the top of the lamp,
heated by the flame. The chief objection to these lamps is their inconvenience in use.
Various other schemes of safe-lamps were offered to the miner by ingenious mechanicians,
but they have been all superseded by the admirable invention of Sir H. Davy,
founded on his fine researches upon flame. The lamp of Davy was instantly tried
and approved of by Mr. Buddle and the principal mining engineers of the Newcastle
district. A perfect security of accident is therefore afforded to the miner in the use of
a lamp which transmits its light, and is fed with air, through a cylinder of wire gauze;
and this invention has the advantage of requiring no machinery, no philosophical knowledge
to direct its use, and is made at a very cheap rate.
In the course of a long and laborious investigation on the properties of the fire-damp,
and the nature and communication of flame, Sir H. Davy ascertained that the explosions
of inflammable gases were incapable of being passed through long narrow metallic tubes;
and that this principle of security was still obtained by diminishing their length and
diameter at the same time, and likewise diminishing their length, and increasing their
number, so that a great number of small apertures would not pass an explosion, when
their depth was equal to their diameter. This fact led him to trials upon sieves made of
wire-gauze, or metallic plates perforated with numerous small holes; and he found it
was impossible to pass explosions through them.
The apertures in the gauze should never be more than 1-20th of an inch square. In
the working models sent by Sir H. to the mines, there were 748 apertures in the square
inch, and the wire was about the 40th of an inch diameter. The cage or cylinder of
wire gauze should be made by double joinings, the gauze being folded over in such a
manner as to leave no apertures. It should not be more than two inches in diameter;
or in large cylinders the combustion of the fire-damp renders the top inconveniently[993]
hot; and a double top is always a proper precaution, fixed at a distance of about half
an inch above the first top. The gauze cylinder should be fastened to the lamp by a
screw of 4 or 5 turns. All joinings in the lamp should be made with hard solder; and
the security depends upon the condition, that no aperture exists in the apparatus larger
than in the wire gauze.
The forms of the lamp and cage, and the mode of burning the wick, may be greatly
diversified; but the principle which ensures their safety must be strictly attended to.
See Lamp of Davy, Safety Lamp, and Ventilation.
The state of the air in coal mines, from very early periods till the discovery of the
safe-lamp, was judged of by the appearances exhibited by the flame of a candle; and
this test must in many circumstances be still had recourse to. When there is merely a
defect of atmospheric oxygen, the air being also partially vitiated by a little carbonic
acid, either from choke-damp or the lungs and candles of the miners, the lights burn
with a very dull flame, the tallow ceases to melt in the cup formed round the wick, till
the flame flickers and expires. In this case the candle may be kept burning by slanting
it more or less towards a horizontal position, which causes the tallow to melt with the
edge of the flame. The candle is thus rapidly wasted, however; and therefore an oil
lamp is preferable, as it continues to burn where a candle would be extinguished. The
candles of the collier are generally small, with a very small wick; such being found to
produce a more distinct flame than candles of a large size with a thick wick.
In trying the quality of the air by the flame of a candle, the wick must be trimmed
by taking off the snuff, so as to produce a clear, distinct, and steady-burning flame.
When a candle thus trimmed is looked at in common air, a distinct and well-defined
cone of flame is seen, of a fine sky-blue at the bottom next the wick, and thence of a
bright yellow to the apex of the cone. Besides this appearance, there is another,
surrounding the cone, which the brightness of the flame prevents the eye from discerning.
This may be seen by placing one of the hands expanded as a screen betwixt the
eyes and the candle, and at the distance of about an inch, so that the least point of the
apex of the yellow flame may be seen, and no more. By this method, a top, as the
miners term it, will be distinctly observed close to the apex of the yellow flame, from an
eighth to a quarter of an inch in length. This top is of a yellowish-brown colour, and
like a misty haze. This haze is seen not only on the top, but it extends downwards
and surrounds the flame fully half way, about a twentieth of an inch in thickness; here
it assumes a violet colour, which passes into a beautiful blue at the bottom next the
wick. The test of the state of the air in mines, or “trying the candle,” as practised by
miners, depends entirely on the appearance which this haze assumes in shape and
colour at the top of the flame. In fact, this top has distinct appearances when burning
in atmospheric air, carbonated air, azotized air, or fire-damp air; displaying many
modifications, according to the proportions of the various admixtures.
When azote or carbonic acid abounds, the top is frequently an inch or two in length,
of a decided brown colour, and the flame is short and dim. When they are still more
copious, the flame goes out, and the miners immediately retire.
When inflammable air is imagined to exist in considerable quantity, the miner trims
his candle, and advances with cautious step, holding the candle with the left hand, and
screening the flame with the right; and as the fire-damp floats in the upper part of the
gallery next the roof, he holds the candle as low as he can, and keeping his eye fixed on
the tip, he moves forwards. If the gas be small in quantity, he may reach the forehead
without observing any material change in his light. But if in his advance he perceives
the tip to elongate, and take a bluish-gray colour, he is put on his guard, and steps on
with much caution; and if the tip begins to spire, he drops down on one knee, and
holding the candle near the pavement, gradually raises it up, and watches the change it
undergoes as it approaches the roof. If the gas be copious, the flame elongates into a
sharp spire, as well as the top. It is in general reckoned dangerous when the tip
changes from the bluish-gray to a fine blue colour, accompanied with minute luminous
points, which pass rapidly upwards through the flame and top. When the symptoms
are manifestly dangerous, a sudden movement of the hands or body is liable to produce
ignition by agitation of the fire-damp. The experienced miner therefore slowly and
cautiously lowers his candle to the pavement, and then turning round, effects his retreat
slowly, or slips up his right hand and extinguishes the flame with his finger and thumb.
Should he venture too far, and approach the body of gas in an explosive condition, the
tip of the candle rapidly elongates, and the whole rises in a sharp spire several inches in
length; and then the whole surrounding atmosphere is in a blaze, an explosion ensues, and
destructive ravage is the consequence, to an extent proportioned to the quantity of fire-damp.
See Safety Lamp, and Ventilation.
This trying the candle is a delicate operation, requiring much practical sagacity, where
the lives of so many men, and the welfare of the whole establishment, are at stake.
Almost every colliery, after having been worked for some time, gives a peculiar top to[994]
the candle; so that while in one mine liable to fire-damp an explosion will take place
with a top less than an inch long, in another mine the top may be two inches high, and
yet the air be considerably under the point of accension. These differences depend on
several particulars. If the gas has not passed through a long course of ventilation, and
is little mixed with air, it will ignite with a very short top; while on the other hand, a
gas which has run through a ventilation of 20 or 30 miles may cause the production of
a long top without hazard. It is hence obvious, that skilful experience, and thorough
practical knowledge, are the only sure guides in these cases.
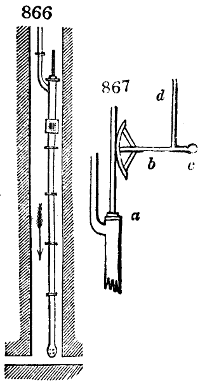
We shall now describe briefly the modern modes of working coals a-dipping of, and
deeper than, the engine-pit bottom. One of these consists in laying a working pump
barrel with a long wind-bore at the bottom of the downset mine, furnished with a smooth
rod working through a collar at the top of the working barrel. At one side of this,
near the top, a kneed pipe is attached, and from it pipes are carried to the point of
delivery, either at the engine-pit bottom or day level, as represented in fig. 866.
The spears are worked sometimes by rods connected with the machinery at the
surface; in which case the spears, if very long, are either suspended
from swing or pendulum rods, or move on friction
rollers. But since the action of the spears, running with great
velocity the total length of the engine stroke, very soon tears
every thing to pieces, the motion of the spears underground has
been reduced from 6 or 8 feet, the length of the engine stroke,
to about 15 inches; and the due speed in the pump is
effected by the centering of a beam, and the attachment of the
spears to it, as represented in fig. 867., where a is the working
barrel, b the beam centered at c, having an arc-head and
martingale sinking-chain. The spears d are fastened by a
strong bolt, which passes through the beam; and there are several holes, by
means of which the stroke in the pumps can be lengthened or shortened at
convenience. The movement of the spears is regulated by a strong iron
quadrant or wheel at the bottom.
In level-free coals, these pumps may be worked by a water-wheel, stationed
near the bottom of the pit, impelled by water falling down the shaft, to be
discharged by the level to the day (day-level).
But the preferable plan of working under-dip coal, is that recently
adopted by the Newcastle engineers; and consists in running a mine a-dipping of the
engine-pit, in such direction of the dip as is most convenient; and both coals and
water are brought up the rise of the coal by means of high-pressure engines, working
with a power of from 30 to 50 pounds on the square inch. These machines are
quite under command, and, producing much power in little space, they are the most
applicable for underground work. An excavation is made for them in the strata above
the coal, and the air used for the furnace under the boiler, is the returned air of the
mine ventilation. In the dip-mine a double tram-road is laid; so that while a number
of loaded corves are ascending, an equal number of empty ones are going down.
Although this improved method has been introduced only a few years back, under-dip
workings have been already executed more than an English mile under-dip of the
engine-pit bottom, by means of three of these high-pressure engines, placed at equal
distances in the under-dip mine. It may hence be inferred, that this mode of working
is susceptible of most extensive application; and in place of sinking pits of excessive
depth upon the dip of the coal, at an almost ruinous expense, much of the under-dip coal
will in future be worked by means of the actual engine-pits. In the Newcastle district,
coals are now working in an engine-pit 115 fathoms deep under-dip of the engine-pit
bottom, above 1600 yards, and fully 80 fathoms of perpendicular depth more than the
bottom of the pit.
If an engine-pit be sunk to a given coal at a certain depth, all the other coals of the
coal-field, both above and below the
coal sunk to, can be drained and
worked to the same depth, by driving
a level cross-cut mine, both to
the dip and rise, till all the coals are
intersected, as represented in fig. 868.,
where A is the engine-pit bottom reaching to the coal a; and b, c, d, e, f, coals lying
above the coal a; the coals which lie below it, g, h, i; k is the forehead of the cross-cut
mine, intersecting all the lower coals; and l, the other forehead of the mine,
intersecting all the upper coals.
| 1. |
First stage of active creep. |
| 2. |
Second do. |
| 3. |
Third do. |
| 4. |
Fourth do. |
| 5. |
The metal ridge closed, and the creep beginning to settle. |
| 6. |
The creep settled, the metal ridges being closely compressed, and supporting the roof. |
In the “Report from the select committee of the House of Lords, appointed to take
into consideration the state of the coal trade in the United Kingdom,” printed in June,
1829, under the head of Mr. Buddle’s evidence we have an excellent description of the[995]
nature and progress of creeps, which we have adverted to in the preceding account.
The annexed fig. 869. exhibits the creep in all its progressive stages, from its commencement
until it has completely closed all the workings, and crushed the pillars of coal.
The section of the figures supposes us standing on the level of the different galleries
which are opened in the seam. The black is the coal pillars between each gallery; when
these are weakened too much, or, in other words, when their bases become too narrow for
the pavement below, by the pressure of the incumbent stratification they sink down into
the pavement, and the first appearance is a little curvature in the bottom of each gallery:
that is the first symptom obvious to sight; but it may generally be heard before it is seen.
The next stage is when the pavement begins to open with a crack longitudinally. The
next stage is when that crack is completed, and it assumes the shape of a metal ridge.
The next is when the metal ridge reaches the roof. The next stage is when the peak
of the metal ridge becomes flattened by pressure, and forced into a horizontal direction,
and becomes quite close; just at this moment the coal pillars begin to sustain part of
the pressure. The next is when the coal pillars take part of the pressure. The last
stage is when it is dead and settled; that is, when the metal or factitious ridge, formed
by the sinking of the pillar into the pavement, bears, in common with the pillars of coal
on each side, the full pressure, and the coal becomes crushed or cracked, and can be no
longer worked, except by a very expensive and dangerous process. Fig. 869.
The quantity of coals, cinders, and culm shipped coastways, and exported from the
several ports of the United Kingdom in the year 1837, was 8,204,301 tons; in 1836,
the quantity was 7,389,272 tons, being an increase of 815,029 tons, or 11·03 per cent.
in favour of 1837.
The following Table shows the separate proportions of this quantity supplied by
England and Wales, Scotland, and Ireland:—
| |
1836. |
1837. |
Increase. |
| |
Tons. |
Tons. |
Tons. |
|
| England and Wales |
6,757,937 |
7,570,254 |
812,317 |
or |
12·02 |
per cent. |
| Scotland |
624,308 |
626,532 |
2,204 |
|
0·36 |
|
| Ireland |
7,027 |
7,515 |
488 |
|
6·94 |
|
| Total |
7,389,272 |
8,204,301 |
815,029 |
or |
11·03 |
per cent. |
PITCOAL, COKING OF. See also Charcoal.
Fig. 870. represents a schachtofen, or pit-kiln, for coking coals in Germany. a is the
lining (chemise), made of fire-bricks; the enclosing
walls are built of the same material; b, b, is a cast-iron
ring covered with a cast-iron plate c. The
floor of the kiln is massive. The coals are introduced,
and the coke taken out, through a hole
in the side d; during the process it is bricked up,
and closed with an iron door. In the surrounding
walls are 4 horizontal rows of flues e, e, e, e, which
are usually iron pipes; the lowest row is upon a
level with the floor of the kiln; and the others are
each respectively one foot and a half higher than
the preceding. Near the top of the shaft there is
an iron pipe f, of from 8 to 10 inches in diameter,
which allows the incoercible vapours generated in
the coking to escape into the condenser, which
consists either of wood or brick chambers. For
kindling the coal, a layer of wood is first placed
on the bottom of the kiln.
The coking of small coal is performed upon vaulted hearths, somewhat like bakers’
ovens, but with still flatter roofs. Of such kilns, several are placed alongside one another[996]
each being an ellipse deviating little from a circle, so that
the mouth may project but a small space. The dimensions
are such, that from 10 to 12 cubic feet of coal-culm may be
spread in a layer 6 inches deep upon the sole of the furnace.
The top of the flat arch of fire brick should be covered with
a stratum of loam and sand.
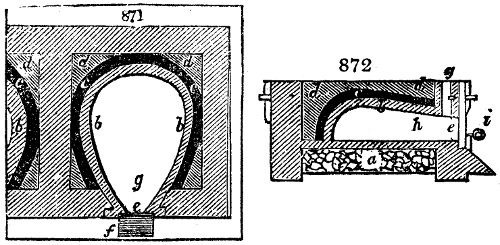
Figs. 871. and 872. represent such a kiln as is mounted
at Zabrze, in Upper Silesia, for coking small coal. Fig. 871.
is the ground plan; fig. 872. the vertical section in the
line of the long axis of fig. 871. a, is the sand-bed of the
hearth, under the brick sole; b, is the roof of large fire-bricks;
c, the covering of loam; d, the top surface of sand;
e, the orifice in the front wall, for admission of the culm,
and removal of the coke, over the sloping stone f. The
flame and vapours pass off above this orifice, through the
chimney marked g, or through the aperture h, into a lateral
chimney. i, is a bar of iron laid across the front of the
door as a fulcrum to work the iron rake upon. A layer of coals is first kindled upon
the hearth, and when this is in brisk ignition, it is covered with the culm in successive
sprinklings. When the coal is sufficiently coked, it is raked out, and quenched with
water.
Fig. 873. represents a simple coking meiler or mound, constructed in a circular form
round a central chimney of loose bricks, towards which small horizontal flues are laid
among the lumps of coals. The sides and top are covered with culm or slack, and
the heap is kindled from certain openings towards the circumference. Fig. 874. represents
an oblong meiler, sometimes made 100 or 150 feet in length, and from 10 to 12 in
breadth. The section in the middle of the figure shows how the lumps are piled up; the
wooden stakes are lifted out when the heap is finished, in order to introduce kindlings
at various points; and the rest of the meiler is then covered with slack and clay, to
protect it from the rains. A jet of smoke and flame is seen issuing from its left end.
An excellent range of furnaces for making a superior article of coke, for the service
of the locomotive engines of the London and Birmingham Railway Company, has been
recently erected at the Camden Town station; consisting of 18 ovens in two lines, the
whole discharging their products of combustion into a horizontal flue, which terminates
in a chimney-stalk, 115 feet high. Fig. 875. is a ground plan of the elliptical ovens, each
being 12 feet by 11 internally, and having 3 feet thickness of walls. a, a, is the mouth, 31⁄2[997]
feet wide outside, and about 23⁄4 feet within. b, b, are the entrances into the flue; they
may be shut more or less completely by horizontal slabs of fire-brick, resting on iron frames,
pushed in from behind, to modify the draught of air. The grooves of these damper-slabs
admit a small stream of air to complete the combustion of the volatilized particles
of soot. By this means the smoke is well consumed. The flue c, c, is 21⁄2 feet high,
by 21 inches wide. The chimney d, at the level of the flue, is
11 feet in diameter inside, and 17 outside; being built from an
elegant design of Robert Stephenson, Esq. (See Chimney.)
d, d, are the keys of the iron hoops, which bind the brickwork of
the oven. Fig. 876. is a vertical section in the line A, B, of fig.
875. showing, at b, b, and e, e, the entrances of the different ovens
into the horizontal flue; the direction of the draught being indicated
by the arrows. f, f, is a bed of concrete, upon which the
whole furnace-range is built, the level of the ground being in the
middle of that bed. g, is a stauncheon on which the crane is
mounted: (see fig. 877.) h is a section of the chimney wall, with
part of the interior to the left of the strong line. Fig. 877. is a
front elevation of two of these elegant coke-ovens; in which the
bracing hoops i, i, i, are shown; k, k, are the cast-iron doors, strengthened outside with
diagonal ridges; each door being 51⁄2 feet high, by 4 feet wide, and lined internally with
fire-bricks. They are raised and lowered by means of chains and counterweights, moved
by the crane l.
Each alternate oven is charged, between 8 and 10 o’clock every morning, with 31⁄2 tons
of good coals. A wisp of straw is thrown in on the top of the heap, which takes fire by
the radiation from the dome (which is in a state of dull ignition from the preceding operation),
and inflames the smoke then rising from the surface, by the re-action of the hot
sides and bottom
upon the body of
the fuel. In this
way the smoke
is consumed at
the very commencement
of the
process, when it
would otherwise
be most abundant.
A neighbour
of the
above coking
ovens, having
lately indicted
them as a nuisance, procured, secundum artem, a parcel of affidavits from sundry
chemical and medical men. Two of the former, who had not entered the premises, but
had espied the outside of the furnaces’ range at some distance, declared that “the coking
process, as performed at the ovens, is a species of distillation of coal”! How rashly do
unpractical theorists affirm what is utterly unfounded, and mislead an unscientific
judge! That the said coking process is in no respect a species of distillation, but a
complete combustion of the volatile principles of the coal, will be manifest from the
following description of its actual progress. The mass of coals is first kindled at the
surface, as above stated, where it is supplied with abundance of atmospheric oxygen;
because the doors of the ovens in front, and the throat-vents behind, are then left open.[998]
The consequence is, that no more smoke is discharged from the top of the chimney, at
this the most sooty period of the process, than is produced by an ordinary kitchen fire.
In these circumstances, the coal gas, or other gas, supposed to be generated in the slightly
heated mass beneath, cannot escape destruction in passing up through the bright open
flame of the oven. As the coking of the coal advances most slowly and regularly
from the top of the heap to the bottom, only one layer is affected at a time, and in succession
downwards, while the surface is always covered with a stratum of redhot cinders,
ready to consume every particle of carburetted or sulphuretted hydrogen gases which
may escape from below. The greatest mass, when calcined in this downward order,
cannot emit into the atmosphere any more of the above-mentioned gases than the
smallest heap; and therefore the argument raised on account of the magnitude of the
operations, is altogether fallacious.
The coke being perfectly freed from all fuliginous and volatile matters by a calcination
of upwards of 40 hours, is cooled down to moderate ignition by sliding in the dampers,
and sliding up the doors, which had been partially closed during the latter part of the
process. It is now observed to form prismatic concretions, somewhat like a columnar
mass of basalt. These are loosened by iron bars, lifted out upon shovels furnished
with long iron shanks, which are poised upon swing chains with hooked ends, and the
lumps are thrown upon the pavement, to be extinguished by sprinkling water upon
them from the rose of a watering-can; or, they might be transferred into a large
chest of sheet-iron set on wheels, and then covered up. Good coals thus treated, yield
80 per cent. of an excellent compact glistening coke; weighing about 14 cwt. per chaldron.
The loss of weight in coking in the ordinary ovens is usually reckoned at 25 per
cent.; and coal, which thus loses one-fourth in weight, gains one-fourth in bulk.
Labourers who have been long employed at rightly-constructed coke ovens, seem to
enjoy remarkably good health.
PITTACALL, is one of the 6 curious principles detected in wood-tar by Reichenbach.
It is a dark-blue solid substance, somewhat like indigo, assumes a metallic
fiery lustre on friction, and varies in tint from copper to golden. It is void of taste
and smell, not volatile; carbonizes at a high heat without emitting an ammoniacal smell;
is soluble or rather very diffusible in water; gives a green solution with a cast of
crimson, in sulphuric acid, with a cast of red blue, in muriatic acid, and with a cast of
aurora red, in acetic acid. It is insoluble in alkalis. It dyes a fast blue upon linen
and cotton goods, with tin and aluminous mordants.
PLASTER OF PARIS; see Gypsum.
PLATED MANUFACTURE. (Fabrique de plaqué, Fr.; Silber plattirung, Germ.)
The silver in this case is not applied to ingots of pure copper, but to an alloy consisting
of copper and brass, which possesses the requisite stiffness for the various articles.
The furnace used for melting that alloy, in blacklead crucibles, is a common air-furnace,
like that for making brass.
The ingot-moulds are made of cast iron, in two pieces, fastened together; the cavity
being of a rectangular shape, 3 inches broad, 11⁄2 thick, and 18 or 20 long. There is an
elevated mouth-piece or gate, to give pressure to the liquid metal, and secure solidity to
the ingot. The mould is heated, till the grease with which its cavity is besmeared, merely
begins to smoke, but does not burn. The proper heat of the melted metal for casting, is
when it assumes a bluish colour, and is quite liquid. Whenever the metal has solidified
in the mould, the wedges that tighten its rings are driven out, lest the shrinkage of the
ingot should cause the mould to crack. See Brass.
The ingot is now dressed carefully with the file on one or two faces, according as it is
to be single or double plated. The thickness of the silver plate is such as to constitute
one fortieth of the thickness of the ingot; or when this is an inch and a quarter thick,
the silver plate applied is one thirty-second of an inch, being by weight a pound troy
of the former, to form 8 to 10 pennyweights of the latter. The silver, which is slightly
less in size than the copper, is tied to it truly with iron wire, and a little of a saturated
solution of borax is then insinuated at the edges. This salt melts at a low heat, and
excludes the atmosphere, which might oxidize the copper, and obstruct the union of the
metals. The ingot thus prepared is brought to the plating furnace.
The furnace has an iron door with a small hole to look through; it is fed with cokes,
laid upon a grate at a level with the bottom of the door. The ingot is placed immediately
upon the cokes, the door is shut, and the plater watches at the peep-hole the instant
when the proper soldering temperature is attained. During the union of the silver and
copper, the surface of the former is seen to be drawn into intimate contact with the
latter, and this species of riveting is the signal for removing the compound bar instantly
from the furnace. Were it to remain a very little longer, the silver would become
alloyed with the copper, and the plating be thus completely spoiled. The adhesion is, in[999]
fact, accomplished here by the formation of a film of true silver-solder at the surfaces
of contact.
The ingot is next cleaned, and rolled to the proper thinness between cylinders as
described under Mint; being in its progress of lamination frequently annealed on a
small reverberatory hearth. After the last annealing, the sheets are immersed in hot
dilute sulphuric acid, and scoured with fine Calais sand; they are then ready to be
fashioned into various articles.
In plating copper wire, the silver is first formed into a tubular shape, with one edge
projecting slightly over the other; through which a redhot copper cylinder being somewhat
loosely run, the silver edges are closely pressed together with a steel burnisher,
whereby they get firmly united. The tube thus completed, is cleaned inside, and put
on the proper copper rod, which it exactly fits. The copper is left a little longer than
its coating tube, and is grooved at the extremities of the latter, so that the silver edges,
being worked into the copper groove, may exclude the air from the surface of the rod.
The compound cylinder is now heated redhot, and rubbed briskly over with the steel
burnisher in a longitudinal direction, whereby the two metals get firmly united, and form
a solid rod, ready to be drawn into wire of any requisite fineness and form; as flat,
half-round, fluted, or with mouldings, according to the figure of the hole in the draw-plate.
Such wire is much used for making bread-baskets, toast-racks, snuffers, and
articles combining elegance with lightness and economy. The wire must be annealed from
time to time during the drawing, and finally cleaned, like the plates, with dilute acid.
Formerly the different shaped vessels of plated metal were all fashioned by the hammer;
but every one of simple form is now made in dies struck with a drop-hammer or
stamp. Some manufacturers employ 8 or 10 drop machines.
Fig. 878. and 879. are two views of the stamp.
A is a large stone, the more massy the better;
b, the anvil on which the die e is secured by four
screws, as shown in the ground plan, fig. 880.
In fig. 878., a a are two upright square prisms,
set diagonally with the angles opposed to each
other; between which the hammer or drop d
slides truly, by means of nicely fitted angular
grooves or recesses in its sides. The hammer
is raised by pulling the rope f, which passes
over the pulley c, and is let fall from different
heights, according to the impulse required.
Vessels which are less in diameter at the top
and bottom than in the middle, must either be
raised by the stamp in two pieces, or raised with
a hand hammer. The die is usually made of
cast steel. When it is placed upon the anvil,
and the plated metal is cut into pieces of proper
size, the top of the die is then surrounded with
a lute made of oil and clay, for an inch or two
above its surface; and the cavity is filled with
melted lead. The under face of the stamp-hammer
has a plate of iron called the licker-up
fitted into it, about the area of the die. Whenever
the lead has become solid, the hammer is raised to a certain
height, and dropped down upon it; and as the under face of the
licker-up is made rough like a rasp, it firmly adheres to the lead,
so as to lift it afterwards with the hammer. The plated metal is
now placed over the die, and the hammer mounted with its lead
is let fall repeatedly upon it, till the impression on the metal is
complete. If the vessel to be struck, be of any considerable depth,
two or three dies may be used, of progressive sizes in succession. But it occasionally
happens that when the vessel has a long conical neck, recourse must be had to an
auxiliary operation, called punching. See the embossing punches, fig. 881. These
are made of cast steel, with their hollows turned out in the lathe.
The pieces a, b are of lead. The punching is performed by a
series of these tools, of different sizes, beginning with the largest,
and ending with the least. By this means a hollow cone, 3 or 4
inches deep, and an inch diameter, may be raised out of a flat
plate. These punches are struck with a hand hammer also, for
small articles, of too great delicacy for the drop. Indeed it frequently
happens that one part of an article is executed by the stamp,
and another by the hand.
[1000]
Cylindrical and conical vessels are mostly formed by bending and soldering. The
bending is performed on blocks of wood, with wooden mallets; but the machine so
much used by the tin-smiths, to form their tubes and cylindric vessels (see the end section,
figs. 882. and 883.), might be employed with advantage.
This consists of 3 iron rollers fixed in an iron frame. A, B, C,
are the three cylinders, and a, b, c, d, the riband or sheet of
metal passed through them to receive the cylindrical or
conical curvature. The upper roller A can be raised or
lowered at pleasure, in order to modify the diameter of
the tube; and when one end of the roller is higher than
the other, the conical curvature is given. The edges of the plated cylinders or cones
are soldered with an alloy composed of silver and brass. An alloy of silver and copper
is somewhat more fusible; but that of brass and silver answers best for plated metal,
the brass being in very small proportion, lest the colour of the plate be affected. Calcined
borax mixed with sandiver (the salt skimmed from the pots of crown glass) is used
along with the alloy, in the act of soldering. The seam of the plated metal being smeared
with that saline mixture made into a pap with water, and the bits of laminated solder,
cut small with scissors, laid on, the seam is exposed to the flame of an oil blowpipe, or
to that of charcoal urged by bellows in a little forge-hearth, till the solder melts and
flows evenly along the junction. The use of the sandiver seems to be, to prevent the
iron wire that binds the plated metal tube from being soldered to it.
Mouldings are sometimes formed upon the edges of vessels, which are not merely
ornamental, but give strength and stiffness. These are fashioned by an instrument
called a swage, represented in figs. 884. and 885. The part A lifts up by a joint, and the
metal to be swaged is placed between the dies, as shown in the figures; the tail b
being held in the jaws of a vice, while the shear-shaped hammer rests upon it. By
striking on the head A, while the metal plate is shifted successively forwards, the beading
is formed. In fig. 884. the tooth a is a guide to regulate the distance between the
bead and the edge. A similar effect is produced of late years in a neater and more expeditious
manner by the rollers, figs. 886. 887. Fig. 888. is a section to show the
form of the bead. The two wheels a, a, fig. 886., are placed upon axes, two of which
are furnished with toothed pinions in their middle; the lower one being turned by the
handle, gives motion to the upper. The groove in the upper wheel corresponds with
the bead in the lower, so that the slip of metal passed through between them assumes
the same figure.
The greatest improvement made in this branch of manufacture, is the introduction of
silver edges, beads and mouldings, instead of the plated ones, which from their prominence
had their silver surface speedily worn off, and thus assumed a brassy look. The
silver destined to form the ornamental edgings is laminated exceedingly thin; a square
inch sometimes weighing no more than 10 or 12 grains. This is too fragile to bear the
action of the opposite steel dies of the swage above described. It is necessary, therefore,
that the sunk part of the die should be steel, and the opposite side lead, as was observed
in the stamping; and this is the method now generally employed to form these silver
ornaments. The inside shell of this silver moulding is filled with soft solder, and then
bent into the requisite form.
The base of candlesticks is generally made in a die by the stamp, as well as the
neck, the dish part of the nozzle or socket, and the tubular stem or pillar. The different
parts are united, some with soft and others with hard solder. The branches of
candlesticks are formed in two semi-cylindrical halves, like the feet of tea-urns. When
an article is to be engraved on, an extra plate of silver is applied at the proper part,[1001]
while the plate is still flat, and fixed by burnishing with great pressure over a hot
anvil. This is a species of welding.
The last finish of plated goods is given by burnishing-tools of bloodstone, fixed in sheet-iron
cases, or hardened steel, finely polished.
The ingots for lamination might probably be plated with advantage by the delicate
pressure process employed for silvering copper wire.
The total value of the plate, plated ware, jewellery, and watches, exported in the
year 1836, was 338,889l.; but the value of the plated goods is not given in the tables
of revenue. M. Parquin, the greatest manufacturer of plated goods in Paris (or
France, for this business is monopolized by the capital), who makes to the value of
700,000 francs per annum, out of the 1,500,000 which, he says, is the whole internal
consumption of the kingdom, states that the internal consumption of the United Kingdom
amounts 30,000,000, or 20 times, that of France! He adds, that our common laminated
copper costs 26 sous the pound, while theirs costs 34. Their plated goods are
fashioned, not in general with stamps, but by the pressure of tools upon wood moulds
in the turning-lathe, which is a great economy of capital to the manufacturer. There
are factories at Birmingham which possess a heavy stock of 300,000 different die-moulds.
See Stamping of Metals.
PLATINA-MOHR. The following easy method of preparing igniferous black platinum,
proposed thirty years ago by Descotils, has been recently recommended by
M. Dobereiner:—
Melt platina ore with double its weight of zinc, reduce the alloy to powder, and treat
it first with dilute sulphuric acid, and next with dilute nitric acid, to oxidize and dissolve
out all the zinc, which, contrary to one’s expectations, is somewhat difficult to do, even
at a boiling heat. The insoluble black-gray powder contains some osmiuret of iridium,
united with the crude platinum. This compound acts like simple platina-black, after it
has been purified by digestion in potash lye, and washing with water. Its oxidizing
power is so great, as to transform not only the formic acid into the carbonic, and alcohol
into vinegar, but even some osmic acid, from the metallic osmium. The above powder
explodes by heat like gunpowder.
When the platina-mohr prepared by means of zinc is moistened with alcohol, it becomes
incandescent, and emits osmic acid; but if it be mixed with alcohol into a paste,
and spread upon a watch-glass, nothing but acetic acid will be disengaged; affording
an elegant means of diffusing the odour of vinegar in an apartment.
PLATINUM, is a metal of a grayish-white colour, resembling in a good measure
polished steel. It is harder than silver, and of about double its density, being of
specific gravity 21. It is so infusible, that no considerable portion of it can be melted
by the strongest heats of our furnaces. It is unchangeable in the air and water; nor
does a white heat impair its polish. The only acid which dissolves it, is the nitro-muriatic;
the muriate or chloride thus formed, affords, with pure ammonia or sal
ammoniac, a triple salt in a yellow powder, convertible into the pure metal by a red
heat. This character distinguishes platinum from every other metal.
Native Platinum.—In the natural state it is never pure, being alloyed with several
other metals. It occurs only under the form of grains, which are usually flattened,
and resemble in shape the gold pepitas. Their size is in general less than linseed,
although in some cases they equal hempseed, and, occasionally, peas. One piece brought
from Choco, in Peru, and presented to the Cabinet of Berlin, by M. Humboldt, weighs
55 grammes = 850 grains, or nearly 2 oz. avoirdupois. The greatest lump of native
platinum known, till of late years, was one in the Royal Museum of Madrid, which was
found in 1814 in the gold mine of Condoto, province of Novita, at Choco. Its size is
greater than a Turkey’s egg, (about 2 inches one diameter, and 4 inches the other,)
and its weight 760 grammes, = 24 oz. or fully 2 lbs. troy. See infrà.
The colour of the grains of native platinum is generally a grayish white, like
tarnished steel. The cavities of the rough grains are often filled with earthy and
ferruginous matters, or sometimes with small grains of black oxide of iron, adhering to
the surface of the platinum grains. Their specific gravity is also much lower than that
of forged pure platinum; varying from 15 in the small particles, to 18·94 in M.
Humboldt’s large specimen. This relative lightness is owing to the presence of iron,
copper, lead, and chrome; besides its other more lately discovered metallic constituents,
palladium, osmium, rhodium, and iridium.
Its main localities in the New Continent, are in the three following districts:—
1. At Choco, in the neighbourhood of Barbacoas, and generally on the coasts of the
South Sea, or on the western slopes of the Cordillera of the Andes, between the 2nd
and the 6th degrees of north latitude. The gold-washings that furnish most platinum,
are those of Condoto, in the province of Novita; those of Santa Rita, or Viroviro, of
Santa Lucia, of the ravine of Iro, and Apoto, between Novita and Taddo. The deposit
of gold and platinum grains is found in alluvial ground, at a depth of about 20[1002]
feet. The gold is separated from the platinum by picking with the hand, and also by
amalgamation; formerly, when it was imagined that platinum might be used to debase
gold, the grains of the former metal were thrown into the rivers, through which mistaken
opinion an immense quantity of it was lost.
2. Platinum grains are found in Brazil, but always in the alluvial lands that contain
gold, particularly in those of Matto-Grosso. The ore of this country is somewhat
different from that of Choco. It is in grains, which seem to be fragments of a spongy
substance. The whole of the particles are nearly globular, exhibiting a surface formed
of small spheroidal protuberances strongly cohering together, whose interstices are
clean, and even brilliant.
This platinum includes many small particles of gold, but none of the magnetic iron-sand
or of the small zircons which accompany the Peruvian ore. It is mixed with
small grains of native palladium, which may be recognised by their fibrous or radiated
structure, and particularly by their chemical characters.
3. Platinum grains are found in Hayti, or Saint Domingo, in the sand of the river
Jacky, near the mountains of Sibao. Like those of Choco, they are in small brilliant
grains, as if polished by friction. The sand containing them is quartzose and
ferruginous. This native platinum contains, like that of Choco, chromium, copper,
osmium, iridium, rhodium, palladium, and probably titanium. Vauquelin could find
no gold among the grains.
Platinum has been discovered lately in the Russian territories, in the auriferous
sands of Kuschwa, 250 wersts from Ekaterinebourg, and consequently in a geological
position which seems to be analogous with that of South America.
These auriferous sands are, indeed, almost all superficial; they cover an argillaceous
soil; and include, along with gold and platinum, debris of dolerite (a kind of greenstone),
protoxide of iron, grains of corundum, &c. The platinum grains are not so flat
as those from Choco, but they are thicker; they have less brilliancy, and more of a
leaden hue. This platinum, by M. Laugier’s analysis, is similar in purity to that of
Choco; but the leaden-gray grains, which were taken for a mixture of osmium and
iridium, are merely an alloy of platinum, containing 25 per cent. of these metals.
The mines of Brazil, Columbia, and Saint Domingo furnish altogether only about
400 kilos. of platinum ore per annum; but those of Russia produce above 1800 kilos.
The latter were discovered in 1822, and were first worked in 1824. They are all situated
in the Ural mountains. The ore is disseminated in an argillaceous sand, of a
greenish-gray colour, resulting from the disintegration of the surrounding rocks, and
constitutes from 1 to 3 parts in 4000 of the sand. Occasionally it has been found in lumps
weighing 8 kilogrammes (16 lbs.!), but it generally occurs in blackish angular grains,
which contain 70 per cent. of platinum, and 3 to 5 of iridium. The ore of Goroblagodat
is in small flattened grains, which contain 88 per cent. of this precious metal. The
osmiure of iridium is found upon a great many points of the Urals, throughout a space of
140 leagues, being a product accessory to the gold washings. 32 kilogrammes of osmiure
are collected there annually, which contain upon an average 2 per cent. of platinum.
M. Vauquelin found nearly ten per cent. of platinum in an ore of argentiferous
copper, which was transmitted to him as coming from Guadalcanal in Spain. This
would be the only example of platinum existing in a rock, and in a vein. As the same
thing has not again been met with, even in other specimens from Guadalcanal, we
must delay drawing geological inferences, till a new example has confirmed the
authenticity of the first.
Platinum has been known in Europe only since 1748, though it was noticed by
Ulloa in 1741. It was compared at first to gold; and was, in fact, brought into the
market under the name of white gold. The term platinum, however, is derived from
the Spanish word plata, silver, on account of its resemblance in colour to that metal.
The whole of the platinum ore from the Urals is sent to St. Petersburg, where it is
treated by the following simple process:—
One part of the ore is put in open platina vessels, capable of containing from 6 to
8 lbs., along with 3 parts of muriatic acid at 25° B. and 1 part of nitric acid at 40°.
Thirty of these vessels are placed upon a sand-bath covered with a glazed dome with
movable panes, which is surmounted by a ventilating chimney to carry the vapours
out of the laboratory. Heat is applied for 8 or 10 hours, till no more red vapours
appear; a proof that the whole nitric acid is decomposed, though some of the muriatic
remains. After settling, the supernatant liquid is decanted off into large cylindrical
glass vessels, the residuum is washed, and the washing is also decanted off. A fresh
quantity of nitro-muriatic acid is now poured upon the residuum. This treatment is
repeated till the whole solid matter has eventually disappeared. The ore requires for
solution from 10 to 15 times its weight of nitro-muriatic acid, according to the size of
its grains.
The solutions thus made are all acid; a circumstance essential to prevent the iridium[1003]
from precipitating with the platinum, by the water of ammonia, which is next added.
The deposit being allowed to form, the mother waters are poured off, the precipitate
is washed with cold water, dried, and calcined in crucibles of platinum.
The mother-waters and the washings are afterwards treated separately. The first
being concentrated to one-twelfth of their bulk in glass retorts, on cooling they let fall
the iridium in the state of an ammoniacal chloride, constituting a dark-purple powder,
occasionally crystallized in regular octahedrons. The washings are evaporated to dryness
in porcelain vessels; the residuum is calcined and treated like fresh ore; but the platinum
it affords needs a second purification.
For agglomerating the platinum, the spongy mass is pounded in bronze mortars; the
powder is passed through a fine sieve, and put into a cylinder of the intended size of the
ingot. The cylinder is fitted with a rammer, which is forced in by a coining press,
till the powder be much condensed. It is then turned out of the mould, and baked 36 hours
in a porcelain kiln, after which it may be readily forged, if it be pure, and may receive
any desired form from the hammer. It contracts in volume from 1-6th to 1-5th during
the calcination. The cost of the manufacture of platinum is fixed by the administration
at 32 francs the Russian pound; but so great a sum is never expended upon it.
For Dr. Wollaston’s process, see Phil. Trans. 1829, Part I.
Platinum furnishes most valuable vessels to both analytical and manufacturing
chemists. It may be beat out into leaves of such thinness as to be blown about with the
breath.
This metal is applied to porcelain by two different processes; sometimes in a
rather coarse powder, applied by the brush, like gold, to form ornamental figures;
sometimes in a state of extreme division, obtained by decomposing its muriatic solution,
by means of an essential oil, such as rosemary or lavender. In this case, it must be
evenly spread over the whole ground. Both modes of application give rise to a steely
lustre.
The properties possessed in common by gold and platinum, have several times given
occasion to fraudulent admixtures, which have deceived the assayers. M. Vauquelin
having executed a series of experiments to elucidate this subject, drew the following
conclusions:—
If the platinum do not exceed 30 or 40 parts in the thousand of the alloy, the gold
does not retain any of it when the parting is made with nitric acid in the usual way;
and when the proportion of platinum is greater, the fraud becomes manifest, 1st by the
higher temperature required to pass it through the cupel, and to form a round button,
2, by the absence of the lightning, fulguration, or coruscation; 3, by the dull white
colour of the button and its crystallized surface; 4, by the straw-yellow colour which
platinum communicates to the aquafortis in the parting; 5, by the straw-yellow colour,
bordering on white, of the cornet, after it is annealed. If the platinum amounts to one
fourth of the gold, we must add to the alloy at least 3 times its weight of fine silver,
laminate it very thin, anneal somewhat strongly, boil it half an hour in the first aquafortis,
and at least a quarter of an hour in the second, in order that the acid may dissolve
the whole of the platinum.
Were it required to determine exactly the proportions of platinum contained in an
alloy of copper, silver, gold, and platinum, the amount of the copper may be found in
the first place by cupellation, then the respective quantities of the three other metals
may be learned by a process founded, 1, upon the property possessed by sulphuric acid
of dissolving silver without affecting gold or platinum; and, 2, upon the property of platinum
being soluble in the nitric acid, when it is alloyed with a certain quantity of gold
and silver.
According to Boussingault, the annual product of Platinum in America does not
exceed 81⁄3 cwts. At Nischne-Tagilsk, in 1824, a lump of native platinum weighing
fully 10 lbs. was found; and in 1830, another lump, of nearly double size, which
weighed 353⁄4 Prussian marcs; fully 18 lbs. avoirdupoise.
PRODUCTION OF PLATINUM IN THE URAL.
| From 1822 to |
1827 |
inclusively, |
52 |
puds[41] and |
22 |
1⁄2 |
pounds. |
| |
1828 |
|
94 |
|
| |
1829 |
|
78 |
|
31 |
1⁄2 |
|
| |
1830 |
|
105 |
|
1 |
|
| |
1831 |
to 1833 |
348 |
|
15 |
|
[1004]
Analyses of the Platinum Ores of the Urals, and of that from Barbacoas on the Pacific,
between the 2nd and 6th degrees of northern latitude.
| |
From Nischne-Tagilsk. |
Goroblagodat. |
Barbacoas. |
| |
Berzelius. |
Osann. |
Berzelius. |
| |
Magnetic. |
Not
Magnetic |
|
|
| Platinum |
73·58 |
78·94 |
83·07 |
86·50 |
84·30 |
| Iridium |
2·35 |
4·97 |
1·91 |
— |
1·46 |
| Rhodium |
1·15 |
0·86 |
0·59 |
1·15 |
3·46 |
| Palladium |
0·30 |
0·28 |
0·26 |
1·10 |
1·06 |
| Iron |
12·98 |
11·04 |
10·79 |
8·32 |
5·31 |
| Copper |
5·20 |
0·70 |
1·30 |
0·45 |
0·74 |
| Undissolved Osmium and Iridium |
2·30 |
1·96 |
1·80 |
1·40 |
— |
| Osmium |
— |
— |
— |
— |
1·03 |
| Quartz |
— |
— |
— |
— |
0·60 |
| Lime |
— |
— |
— |
— |
0·12 |
| |
97·86 |
98·75 |
99·72 |
98·92 |
98·08 |
PLUMBAGO. See Graphite, for its mineralogical and chemical characters.
The mountain at Borrowdale, in which the blacklead is mined, is 2000 feet high, and
the entrance to the mine is 1000 feet below its summit. This valuable mineral became
so common a subject of robbery about a century ago, as to have enriched, it was said,
a great many persons living in the neighbourhood. Even the guard stationed over it
by the proprietors was of little avail against men infuriated with the love of plunder; since
in those days a body of miners broke into the mine by main force, and held possession
of it for a considerable time.
The treasure is now protected by a strong building, consisting of four rooms upon
the ground floor; and immediately under one of them is the opening, secured by a trap-door,
through which alone workmen can enter the interior of the mountain. In this apartment,
called the dressing-room, the miners change their ordinary clothes for their
working dress, as they come in, and after their six hours’ post or journey, they again
change their dress, under the superintendence of the steward, before they are suffered to
go out. In the innermost of the four rooms, two men are seated at a large table, sorting
and dressing the plumbago, who are locked in while at work, and watched by the
steward from an adjoining room, who is armed with two loaded blunderbusses. Such
formidable apparatus of security is deemed requisite to check the pilfering spirit of the
Cumberland mountaineers.
The cleansed blacklead is packed up into strong casks, which hold 1 cwt. each.
These are all despatched to the warehouse of the proprietors in London, where the blacklead
is sold monthly by auction, at a price of from 35s. to 45s. a pound.
In some years, the net produce of the six weeks’ annual working of the mine has, it is
said, amounted to 30,000l. or 40,000l.
PLUSH (Panne, Peluche, Fr.; Wollsammet, Plüsch, Germ.), is a textile fabric, having
a sort of velvet nap or shag upon one side. It is composed regularly of a woof of a
single woollen thread, and a two-fold warp, the one, wool of two threads twisted, the
other, goat’s or camel’s hair. There are also several sorts of plush made entirely of worsted.
It is manufactured, like velvet, in a loom with three treadles; two of which separate
and depress the woollen warp, and the third raises the hair-warp, whereupon the weaver,
throwing the shuttle, passes the woof between the woollen and hair warp; afterwards,
laying a brass broach or needle under that of the hair, he cuts it with a knife (see
Fustian) destined for that use, running its fine slender point along in the hollow of the
guide-broach, to the end of a piece extended upon a table. Thus the surface of the
plush receives its velvety appearance. This stuff is also made of cotton and silk.
POINT NET, is a style of lace formerly much in vogue, but now superseded by
the bobbin-net manufacture.
PORCELAIN, is the finest kind of pottery-ware. It is considered under that
title.
PORPHYRY, is a compound mineral or rock, composed essentially of a base of
hornstone, interspersed with crystals of felspar. It frequently contains also quartz,
mica, and hornblende. That most esteemed is the antient porphyry of Egypt, with
a ground of a fine red colour passing into purple, having snow-white crystals of felspar
imbedded in it. Most beautiful specimens of it are to be seen in the antique colossal
statues in the British Museum.
[1005]
Porphyry occurs in Arran, and in Perthshire between Dalnacardoch and Tummel
bridge. It is much used for making slabs, mullers, and mortars.
PORTER, is a malt liquor, so called from being the favourite beverage of the porters
and workpeople of the metropolis and other large towns of the British empire; it is characterized
by its dark-brown colour, its transparency, its moderately bitter taste, and
peculiar aromatic flavour, which, along with its tonic and intoxicating qualities, make it
be keenly relished by thirsty palates accustomed to its use. At first the essential
distinction of porter arose from its wort being made with highly-kilned brown malt,
while other kinds of beer and ale were brewed from a paler article; but of late years,
the taste of the public having run in favour of sweeter and lighter beverages, the actual
porter is brewed with a less proportion of brown malt, is less strongly hopped, and
not allowed to get hard by long keeping in huge ripening tuns. Some brewers colour
the porter with burnt sugar; but in general the most respectable concentrate a quantity
of their first and best wort to an extract, in an iron pan, and burn this into a colouring
stuff, whereby they can lay claim to the merit of using nothing in their manufacture but
malt and hops. The singular flavour of good London porter seems to proceed, in a
great degree, from that of the old casks and fermenting tuns in which it is prepared.
Though not much addicted to vinous potations of any kind, I feel warranted by long
experience to opine, that the porter brewed by the eminent London houses, when
drunk in moderation, is a far wholesomer beverage for the people than the thin
acidulous wines of France and Germany. See Beer.
PORTLAND STONE, is a fine compact oolite, so named from the island where
it is quarried. It is a convenient but not a durable building-stone.
POTATO (Pomme de terre, Fr.; Kartoffel, Germ.); is the well-known root of the
Solanum tuberosum.
The following Table exhibits several good analyses of the potato:—
| Sort. |
Fibrine. |
Starch. |
Veg.
album. |
Gum. |
Acids
and
Salts. |
Water. |
Analyst. |
| Red potatos |
7 |
·0 |
15 |
·0 |
1 |
·4 |
4 |
·1 |
5 |
·1 |
75 |
·0 |
Einhof. |
| Id. germinated |
6 |
·8 |
15 |
·2 |
1 |
·3 |
3 |
·7 |
— |
73 |
·0 |
— |
| Potato sprouts |
2 |
·8 |
0 |
·4 |
0 |
·4 |
3 |
·3 |
— |
93 |
·0 |
— |
| Kidney potatos |
8 |
·8 |
9 |
·1 |
0 |
·8 |
— |
— |
81 |
·3 |
— |
| Large red do. |
6 |
·0 |
12 |
·9 |
0 |
·7 |
— |
— |
78 |
·0 |
— |
| Sweet do. |
8 |
·2 |
15 |
·1 |
0 |
·8 |
— |
— |
74 |
·3 |
— |
| |
|
|
|
 |
|
|
| Potato of Peru |
5 |
·2 |
15 |
·0 |
1 |
·9 |
1 |
·9 |
76 |
·0 |
Lampad. |
| Potato of England |
6 |
·8 |
12 |
·9 |
1 |
·1 |
1 |
·7 |
77 |
·5 |
— |
| Onion potato |
8 |
·4 |
18 |
·7 |
0 |
·9 |
1 |
·7 |
70 |
·3 |
— |
| Onion Voigtland |
7 |
·1 |
15 |
·4 |
1 |
·2 |
2 |
·0 |
74 |
·3 |
— |
Onion cultivated in
the environs of Paris |
6 |
·79 |
13 |
·3 |
0 |
·92 |
3 |
·3 |
1 |
·4 |
73 |
·12 |
Henry. |
POTASH, or POTASSA. (Potasse, Fr.; Kali, Germ.) This substance was so
named from being prepared for commercial purposes by evaporating in iron pots the
lixivium of the ashes of wood fuel. In the crude state called potashes, it consists, therefore,
of such constituents of burned vegetables as are very soluble in water, and fixed in
the fire. The potash salts of plants which originally contained vegetable acids, will be
converted into carbonates, the sulphates will become sulphites, sulphurets, or even
carbonates, according to the manner of incineration; the nitrates will be changed
into pure carbonates, while the muriates or chlorides will remain unaltered. Should
quicklime be added to the solution of the ashes, a corresponding portion of caustic
potassa will be introduced into the product, with more or less lime, according to the care
taken in decanting off the clear lye for evaporation.
In America, where timber is in many places an incumbrance upon the soil, it is
felled, piled up in pyramids, and burned, solely with a view to the manufacture of
potashes. The ashes are put into wooden cisterns, having a plug at the bottom of
one of the sides under a false bottom; a moderate quantity of water is then poured on
the mass, and some quicklime is stirred in. After standing for a few hours, so as to
take up the soluble matter, the clear liquor is drawn off; evaporated to dryness in iron
pots, and finally fused at a red heat into compact masses, which are gray on the outside,
and pink-coloured within.
Pearlash is prepared by calcining potashes upon a reverberatory hearth, till the whole
carbonaceous matter, and the greater part of the sulphur, be dissipated; then lixiviating
the mass, in a cistern having a false bottom covered with straw, evaporating the clear lye[1006]
to dryness in flat iron pans, and stirring it towards the end into white lumpy granulations.
I find the best pink Canadian potashes, as imported in casks containing about
5 cwts., to contain pretty uniformly 60 per cent. of absolute potassa; and the best pearlashes
to contain 50 per cent.; the alkali in the former being nearly in a caustic state;
in the latter, carbonated.
All kinds of vegetables do not yield the same proportion of potassa. The more
succulent the plant, the more does it afford; for it is only in the juices that the vegetable
salts reside, which are converted by incineration into alkaline matter. Herbaceous
weeds are more productive of potash than the graminiferous species, or shrubs, and
these than trees; and for a like reason, twigs and leaves are more productive than
timber. But plants in all cases are richest in alkaline salts when they have arrived at
maturity. The soil in which they grow also influences the quantity of saline matter.
The following Table exhibits the average product in potassa of several plants, according
to the researches of Vauquelin, Pertuis, Kirwan, and De Saussure:—
| In 1000 parts. |
Potassa. |
| Pine or fir |
0 |
·45 |
| Poplar |
0 |
·75 |
| Trefoil |
0 |
·75 |
| Beechwood |
1 |
·45 |
| Oak |
1 |
·53 |
| Boxwood |
2 |
·26 |
| Willow |
2 |
·85 |
| Elm and maple |
3 |
·90 |
| Wheat straw |
3 |
·90 |
| Barb of oak twigs |
4 |
·20 |
| Thistles |
5 |
·00 |
| Flax stems |
5 |
·00 |
| Small rushes |
5 |
·08 |
| Vine shoots |
5 |
·50 |
| Barley straw |
5 |
·80 |
| Dry beech bark |
6 |
·00 |
| Fern |
6 |
·26 |
| Large rush |
7 |
·22 |
| Stalk of maize |
17 |
·5 |
| Bastard chamomile (Anthemis cotula, L.) |
19 |
·6 |
| Bean stalks |
20 |
·0 |
| Sunflower stalks |
20 |
·0 |
| Common nettle |
25 |
·03 |
| Vetch plant |
27 |
·50 |
| Thistles in full growth |
35 |
·37 |
| Dry straw of wheat before earing |
47 |
·0 |
| Wormwood |
73 |
·0 |
| Fumitory |
79 |
·0 |
Stalks of tobacco, potatos, chesnuts, chesnut husks, broom, heath, furze, tansy, sorrel,
vine leaves, beet leaves, orach, and many other plants, abound in potash salts. In
Burgundy, the well-known cendres gravelées are made by incinerating the lees of wine
pressed into cakes, and dried in the sun; the ashes contain fully 16 per cent. of
potassa.
The purification of pearlash is founded upon the fact of its being more soluble in
water than the neutral salts which debase it. Upon any given quantity of that substance,
in an iron pot, let one and a half times its weight of water be poured, and let a gentle
heat be applied for a short time. When the whole has again cooled, the bottom will be
encrusted with the salts, while a solution of nearly pure carbonate of potash will be found
floating above, which may be drawn off clear by a syphon. The salts may be afterwards
thrown upon a filter of gravel. If this lye be diluted with 6 times its bulk of water
mixed with as much slaked lime as there was pearlash employed, and the mixture be
boiled for an hour, the potash will become caustic, by giving up its carbonic acid to the
lime. If the clear settled lixivium be now siphoned off, and concentrated by boiling
in a covered iron pan, till it assumes the appearance of oil, it will constitute the common
caustic of the surgeon, the potassa fusa of the shops. But to obtain potassa chemically
pure, recourse must be had to the bicarbonate, nitrate, or tartrate of potassa, salts
which, when carefully crystallized, are exempt from any thing to render the potassa
derived from them impure. The bicarbonate having been gently ignited in a silver
basin, is to be dissolved in 6 times its weight of water, and the solution is to be boiled
for an hour, along with one pound of slaked lime for every pound of the bicarbonate
used. The whole must be left to settle without contact of air. The supernatant lye is to
be drawn off by a syphon, and evaporated in an iron or silver vessel provided with a small
orifice in its close cover for the escape of the steam, till it assumes, as above, the
appearance of oil, or till it be nearly redhot. Let the fused potassa be now poured out
upon a bright plate of iron, cut into pieces as soon as it concretes, and put up immediately
in a bottle furnished with a well-ground stopper. It is hydrate of potassa, being
composed of 1 atom of potassa 48, + 1 atom of water 9, = 57.
A pure carbonate of potassa may be also prepared by fusing pure nitre in an earthen
crucible, and projecting charcoal into it by small bits at a time, till it ceases to cause
deflagration. Or a mixture of 10 parts of nitre and 1 of charcoal may be deflagrated in
small successive portions in a redhot deep crucible. When a mixture of 2 parts of
tartrate of potassa, or crystals of tartar, and 1 of nitre, is deflagrated, pure carbonate of[1007]
potassa remains mixed with charcoal, which by lixiviation, and the agency of quicklime,
will afford a pure hydrate. Crystals of tartar calcined alone yield also a pure
carbonate.
Caustic potassa, as I have said, after being fused in a silver crucible at a red heat,
retains 1 prime equivalent of water. Hence its composition in 100 parts is, potassium
70, oxygen 14, water 16. Anhydrous potassa, or the oxide free from water, can be
obtained only by the combustion of potassium in the open air. It is composed of 831⁄3
of metal, and 162⁄3 of oxygen. Berzelius’s numbers are 83·05 and 16·95.
Caustic potassa may be crystallized; but in general it occurs as a white brittle substance
of spec. grav. 1·708, which melts at a red heat, evaporates at a white heat, deliquesces
into a liquid in the air, and attracts carbonic acid; is soluble in water
and alcohol, forms soft soaps with fat oils, and soapy-looking compounds with
resins and wax; dissolves sulphur, some metallic sulphurets, as those of antimony,
arsenic, &c., as also silica, alumina, and certain other bases; and decomposes animal
textures, as hair, wool, silk, horn, skin, &c. It should never be touched with the
tongue or the fingers.
The following Table exhibits the quantity of Fused Potassa in 100 parts of caustic
lye, at the respective densities:—
| Sp. gr. |
Pot.
in 100. |
| 1·58 |
53·06 |
| 1·56 |
51·58 |
| 1·54 |
50·09 |
| 1·52 |
48·46 |
| 1·50 |
46·45 |
| 1·48 |
44·40 |
| 1·46 |
42·31 |
| 1·44 |
40·17 |
| 1·42 |
37·97 |
| 1·40 |
35·99 |
| 1·38 |
34·74 |
| 1·36 |
33·46 |
| 1·34 |
32·14 |
| 1·32 |
30·74 |
| 1·30 |
29·34 |
| 1·28 |
27·86 |
| 1·26 |
26·34 |
| 1·24 |
24·77 |
| 1·22 |
23·14 |
| 1·20 |
21·25 |
| 1·18 |
19·34 |
| 1·16 |
17·40 |
| 1·14 |
15·38 |
| 1·12 |
13·30 |
| 1·10 |
11·28 |
| 1·08 |
9·20 |
| 1·06 |
7·02 |
| 1·04 |
4·77 |
| 1·02 |
2·44 |
| 1·00 |
0·00 |
The only certain way of determining the quantity of free potassa in any solid or liquid,
is from the quantity of a dilute acid of known strength which it can saturate.
The hydrate of potassa, or its lye, often contains a notable quantity of carbonate, the
presence of which may be detected by lime water, and its amount be ascertained by the
loss of weight which it suffers, when a weighed portion of the lye is poured into a
weighed portion of dilute sulphuric acid poised in the scale of a balance.
There are two other oxides of potassium; the suboxide, which consists, according
to Berzelius, of 90·74 of metal, and 9·26 oxygen; and the hyperoxide, an orange-yellow
substance, which gives off oxygen in the act of dissolving in water, and becomes
potassa. It consists of 62 of metal, and 38 of oxygen.
Carbonate of potassa is composed of 48 parts of base, and 22 of acid, according to
most British authorities; or, in 100 parts, of 68·57 and 31·43; but according to Berzelius,
of 68·09 and 31·91.
Carbonate of potassa, as it exists associated with carbon in calcined tartar, passes very
readily into the Bicarbonate, on being moistened with water, and having a current of carbonic
acid gas passed through it. The absorption takes place so rapidly, that the mass
becomes hot, and therefore ought to be surrounded with cold water. The salt should
then be dissolved in the smallest quantity of water at 120° F., filtered, and crystallized.
POTASSIUM (Eng. and Fr.; Kalium, Germ.); is a metal deeply interesting,
not only from its own marvellous properties, but from its having been the first link in
the chain of discovery which conducted Sir H. Davy through many of the formerly
mysterious and untrodden labyrinths of chemistry.
The easiest and best mode of obtaining this elementary substance, is that contrived by
Brunner, which I have often practised upon a considerable scale. Into the orifice of one
of the iron bottles, as A, fig. 889., in which mercury is imported, adapt, by screwing, a piece
of gun-barrel tube, 9 inches long; having brazed into its side, about 3 inches from its outer
end, a similar piece of iron tube. Fill this retort two-thirds with a mixture of 10 parts
of cream of tartar, previously calcined in a covered crucible, and 1 of charcoal, both in
powder; and lay it horizontally in an air-furnace, so that while the screw orifice is at
the inside wall, the extremity of the straight or nozzle tube may project a few inches
beyond the brickwork, and the tube brazed into it at right angles may descend pretty
close to the outside wall, so as to dip its lower end a quarter of an inch beneath the surface
of some rectified naphtha contained in a copper bottle surrounded by ice-cold water.
By bringing the condenser-vessel so near the furnace, the tubes along which the potassium
vapour requires to pass, run less risk of getting obstructed. The horizontal straight
end of the nozzle tube should be shut by screwing a stopcock air-tight into it. By
opening the cock momentarily, and thrusting in a hot wire, this tube may be readily
kept free, without permitting any considerable waste of potassium. The heat should
be slowly applied at first, but eventually urged to whiteness, and continued as long[1008]
as potassuretted hydrogen continues to be disengaged. The retort, and the part of
the nozzle tube exposed to the fire, should be covered with a good refractory lute, as
described under the article Phosphorus. The joints must be perfectly air-tight; and
the vessel freed from every trace of mercury, by ignition, before it is charged with the
tartar-ash.
Tartar skilfully treated in this way will afford 3 per cent. of potassium; and when it is
observed to send forth green fumes, it has commenced the production of the metal. Instead
of the construction above described, the following form of apparatus may be employed.
A. fig. 889., represents the iron bottle,
charged with the incinerated tartar; and
B is a fire-brick support. A piece of fire-tile
should also be placed between the
bottom of the bottle and the back wall
of the furnace, to keep the apparatus
steady during the operation. Whenever
the moisture is expelled, and the mass
faintly ignited, the tube C should be
screwed into the mouth of the bottle,
through a small hole left for this purpose
in the side of the furnace. That
tube should be no longer, and the front
wall of the furnace no thicker, than
what is absolutely necessary. As soon
as the reduction is indicated by the
emission of green vapours, the receiver
must be adapted, d, a, D, E, shown in a
large scale in fig. 890.
This is a condenser, in two pieces, made of thin sheet copper; D, the upper part,
is a rectangular box, open at bottom, about 10 inches high, by 5 or 6 long, and 2 wide;
near to the side a, it is divided inside into two equal compartments, up to two-thirds
of its height, by a partition b, b, in order to make the vapours
that issue from C pursue a downward and circuitous path.
In each of its narrow sides, near the top, a short tube is
soldered, at d and a; the former being fitted air-tight into the
end of the nozzle of the retort, while the latter is closed with
a cork traversed by a stiff iron probe e, which passes through
a small hole in the partition b, b, under c, and is employed to
keep the tube C clear, by its drill-shaped steel point. In one
of the broad sides of the box D, near the top, a bit of pipe is
soldered on at e, for receiving the end of a bent glass tube of
safety, which dips its other and lower end into a glass containing
naphtha. E, the bottom copper box, with naphtha, which
receives pretty closely the upper case D, is to be immersed in
a cistern of cold water containing some lumps of ice.
The chemical action by which potassa is reduced in this
process, seems to be somewhat complicated, and has not been
thoroughly explained. A very small proportion of pure
potassium is obtained; a great deal of it is converted into a
black infusible mass, which passes over with the metal, and
is very apt to block up the tube. Should this resist clearing
out with the probe, the fire must be immediately withdrawn
from the furnace, otherwise the apparatus will probably burst
or blow up. Care must be taken to prevent any moisture
getting into the nozzle, for it would probably produce a violent detonation.
When the operation has proceeded regularly, accompanied to the end with a constant
evolution of gas, the retort becomes nearly empty, or contains merely a little
charcoal, or carbonate of potassa, and the potassium collects in the naphtha at the bottom
of the receiver E, in the form of globules or rounded lumps, of greater or less size, and of
a leaden hue. But the greater part of the metal escapes with the gas, in a state of
combination not well understood. This gaseous compound burns with a white or reddish-white
flame, and deposits potassa. Several ounces of potassium may be produced
in this way at one operation; but, as thus obtained, it always contains some
combined charcoal, which must be separated by distilling it in an iron retort, having its
beak plunged in naphtha.
Pure potassium, as procured in Sir H. Davy’s original method, by acting upon fused
potassa under a film of naphtha, with the negative wire of a powerful voltaic battery,
is very like quicksilver. It is semi-fluid at 60° Fahr., nearly liquid at 92°, and entirely[1009]
so at 120°. At 50° it is malleable, and has the lustre of polished silver; at 32° it is brittle,
with a crystalline fracture; and at a heat approaching to redness, it begins to boil, is
volatilized, and converted into a green-coloured gas, which condenses into globules upon
the surface of a cold body. Its specific gravity in the purest state is 0·865 at 60°.
When heated in the air, it takes fire, and burns very vividly. It has a stronger affinity
for oxygen than any other known substance; and is hence very difficult to preserve
in the metallic state. At a high temperature it reduces almost every oxygenated body.
When thrown upon water, it kindles, and moves about violently upon the surface,
burning with a red flame, till it be consumed; that is to say, converted into potassa.
When thrown upon a cake of ice, it likewise kindles, and burns a hole in it. If a
globule of it be laid upon wet turmeric paper, it takes fire, and runs about, marking
its desultory path with red lines. The flame observed in these cases is owing chiefly
to hydrogen, for it is at the expense of the water that the potassium burns.
Potassa, even in a pretty dilute solution, produces a precipitate with muriate of platinum,
a phenomenon which distinguishes it from soda. It forms, moreover, with sulphuric
and acetic acids, salts which crystallize very differently from the sulphates and acetates of
soda.
POTTERY, PORCELAIN. (Eng. and Fr.; Steingut, Porzellan, Germ.) The
French, who are fond of giving far-fetched names to the most ordinary things, have
dignified the art of pottery with the title of ceramique, from the Greek noun κεραμος, an
earthen pot, compounded of two words which signify, in that language, burned clay. In
reference to chemical constitution, there are only two genera of baked stoneware. The
first consists of a fusible earthy mixture, along with an infusible, which when combined
are susceptible of becoming semi-vitrified and translucent in the kiln. This constitutes
porcelain or china-ware; which is either hard and genuine, or tender and spurious,
according to the quality and quantity of the fusible ingredient. The second kind consists
of an infusible mixture of earths, which is refractory in the kiln, and continues
opaque. This is pottery, properly so called; but it comprehends several sub-species,
which graduate into each other by imperceptible shades of difference. To
this head belong earthenware, stoneware, flintware, fayence, delftware, iron-stone
china, &c.
The earliest attempts to make a compact stoneware, with a painted glaze, seem to
have originated with the Arabians in Spain, about the 9th century, and to have passed
thence into Majorca, in which island they were carried on with no little success. In
the 14th century, these articles, and the art of imitating them, were highly prized by
the Italians, under the name of Majolica, and porcelana, from the Portuguese word for
a cup. The first fabric of stoneware possessed by them, was erected at Fayenza, in the
ecclesiastical state, whence the French term fayence is derived. The body of the ware
was usually a red clay, and the glaze was opaque, being formed of the oxides of lead
and tin, along with potash and sand. Bernhard de Pallissy, about the middle of the
16th century, manufactured the first white fayence, at Saintes, in France; and not
long afterwards the Dutch produced a similar article, of substantial make, under the
name of delftware, and delft porcelain, but destitute of those graceful forms and paintings
for which the ware of Fayenza was distinguished. Common fayence may be, therefore,
regarded as a strong, well-burned, but rather coarse-grained kind of stoneware.
It was in the 17th century that a small work for making earthenware of a coarse
description, coated with a common lead glaze, was formed at Burslem, in Staffordshire,
which may be considered as the germ of the vast potteries now established in that
county. The manufacture was improved about the year 1690, by two Dutchmen, the
brothers Elers, who introduced the mode of glazing ware by the vapour of salt, which they
threw by handfuls at a certain period among the ignited goods in the kiln. But these
were rude, unscientific, and desultory efforts. It is to the late Josiah Wedgewood, Esq.
that this country and the world at large are mainly indebted for the great modern
advancement of the ceramic art. It was he who first erected magnificent factories,
where every resource of mechanical and chemical science was made to co-operate with
the arts of painting, sculpture, and statuary, in perfecting this valuable department of
the industry of nations. So sound were his principles, so judicious his plans of procedure,
and so ably have they been prosecuted by his successors in Staffordshire, that a
population of 60,000 operatives now derives a comfortable subsistence within a district
formerly bleak and barren, of 8 miles long, by 6 broad, which contains 150 kilns,
and is significantly called the Potteries.
OF THE MATERIALS OF POTTERY OR PORCELAIN, AND THEIR PREPARATION.
1. Clay.—The best clay from which the Staffordshire ware is made, comes from
Dorsetshire; and a second quality from Devonshire: but both are well adapted for
working, being refractory in the fire, and becoming very white when burnt. The clay
is cleaned as much as possible by hand, and freed from loosely adhering stones at the[1010]
pits where it is dug. In the factory mounted by Mr. Wedgewood, which may be regarded
as a type of excellence, the clay is cut to pieces, and then kneaded into a pulp
with water, by engines; instead of being broken down with pickaxes, and worked with
water by hand-paddles, in a square pit or water-tank, an old process, called blunging.
The clay is now thrown into a cast-iron cylinder, 20 inches wide, and 4 feet high, or
into a cone 2 feet wide at top, and 6 feet deep, in whose axis an upright shaft revolves,
bearing knives as radii to the shaft. The knives are so arranged, that their flat sides
lie in the plane of a spiral line; so that by the revolution of the shaft, they not only cut
through every thing in their way, but constantly press the soft contents of the cylinder
or cone obliquely downwards, on the principle of a screw. Another set of knives
stands out motionless at right angles from the inner surface of the cylinder, and projects
nearly to the central shaft, having their edges looking opposite to the line of
motion of the revolving blades. Thus the two sets of slicing implements, the one
active, and the other passive, operate like shears in cutting the clay into small pieces,
while the active blades, by their spiral form, force the clay in its comminuted state
out at an aperture at the bottom of the cylinder or cone, whence it is conveyed into a
cylindrical vat, to be worked into a pap with water. This cylinder is tub-shaped,
being about 4 times wider than it is deep. A perpendicular shaft turns also in the
axis of this vat, bearing cross spokes one below another, of which the vertical set on
each side is connected by upright staves, giving the movable arms the appearance of
two or four opposite square paddle-boards revolving with the shaft. This wooden
framework, or large blunger, as it is called, turns round amidst the water and clay
lumps, so as to beat them into a fine pap, from which the stony and coarse sandy particles
separate, and subside to the bottom. Whenever the pap has acquired a cream-consistenced
uniformity, it is run off through a series of wire, lawn, and silk sieves, of
different degrees of fineness, which are kept in continual agitation backwards and
forward by a crank mechanism; and thus all the grosser parts are completely
separated, and hindered from entering into the composition of the ware. This clay
liquor is set aside in proper cisterns, and diluted with water to a standard density.
2. But clay alone cannot form a proper material for stoneware, on account of its great
contractility by heat, and the consequent cracking and splitting in the kiln of the
vessels made of it; for which reason, a siliceous substance incapable of contraction
must enter into the body of pottery. For this purpose, ground flints, called flint-powder
by the potters, is universally preferred. The nodules of flint extracted from
the chalk formation, are washed, heated redhot in a kiln, like that for burning lime,
and thrown in this state into water, by which treatment they lose their translucency,
and become exceeding brittle. They are then reduced to a coarse powder in a stamping-mill,
similar to that for stamping ores; see Metallurgy. The pieces of flint are laid
on a strong grating, and pass through its meshes whenever they are reduced by the
stamps to a certain state of comminution. This granular matter is now transferred
to the proper flint-mill, which consists of a strong cylindrical wooden tub, bottomed
with flat pieces of massive chert, or hornstone, over which are laid large flat blocks of
similar chert, that are moved round over the others by strong iron or wooden arms
projecting from an upright shaft made to revolve in the axis of the mill-tub. Sometimes
the active blocks are fixed to these cross arms, and thus carried round over the
passive blocks at the bottom. See infrà, under Porcelain, figures of the flint and
felspar mill. Into this cylindrical vessel a small stream of water constantly trickles,
which facilitates the grinding motion and action of the stones, and works the flint
powder and water into a species of pap. Near the surface of the water there is a plughole
in the side of the tub, by which the creamy-looking flint liquor is run off from
time to time, to be passed through lawn or silk sieves, similar to those used for the clay
liquor; while the particles that remain on the sieves are returned into the mill. This
pap is also reduced to a standard density by dilution with water; whence the weight
of dry siliceous earth present, may be deduced from the measure of the liquor.
The standard clay and flint liquors are now mixed together, in such proportion by
measure, that the flint powder may bear to the dry clay the ratio of one to five, or occasionally
one to six, according to the richness or plasticity of the clay; and the liquors
are intimately incorporated in a revolving churn, similar to that employed for making
the clay-pap. This mixture is next freed from its excess of water, by evaporation in
oblong stone troughs, called slip-kilns, bottomed with fire-tiles, under which a furnace
flue runs. The breadth of this evaporating trough varies from 2 to 6 feet; its length
from 20 to 50; and its depth from 8 to 12 inches, or more.
By the dissipation of the water, and careful agitation of the pap, an uniform doughy
mass is obtained; which, being taken out of the trough, is cut into cubical lumps.
These are piled in heaps, and left in a damp cellar for a considerable time; that is,
several months, in large manufactories. Here the dough suffers disintegration, promoted
by a kind of fermentative action, due probably to some vegetable matter in the water[1011]
and the clay; for it becomes black, and exhales a fetid odour. The argillaceous and
siliceous particles get disintegrated also by the action of the water, in such a way that
the ware made with old paste is found to be more homogeneous, finer grained, and not
so apt to crack or to get disfigured in the baking, as the ware made with newer paste.
But this chemical comminution must be aided by mechanical operations; the first of
which is called the potter’s sloping or wedging. It consists in seizing a mass of clay in
the hands, and, with a twist of both at once, tearing it into two pieces, or cutting it with
a wire. These are again slapped together with force, but in a different direction from
that in which they adhered before, and then dashed down on a board. The mass is
once more torn or cut asunder at right angles, again slapped together, and so worked repeatedly
for 20 or 30 times, which ensures so complete an incorporation of the different
parts, that if the mass had been at first half black and half white clay, it would now be
of a uniform gray colour. A similar effect is produced in some large establishments by a
slicing machine, like that used for cutting down the clay lumps as they come from the pit.
In the axis of a cast iron cylinder or cone, an upright shaft is made to revolve, from
which the spiral-shaped blades extend, with their edges placed in the direction of rotation.
The pieces of clay subjected to the action of these knives (with the reaction of
fixed ones) are minced to small morsels, which are forced pell-mell by the screw-like
pressure into an opening of the bottom of the cylinder or cone, from which a horizontal
pipe about 6 inches square proceeds. The dough is made to issue through this outlet,
and is then cut into lengths of about 12 inches. These clay pillars or prisms are thrown
back into the cylinder, and subjected to the same operation again and again, till the
lumps have their particles perfectly blended together. This process may advantageously
precede their being set aside to ripen in a damp cellar. In France the stoneware dough
is not worked in such a machine; but after being beat with wooden mallets, a practice
common also in England, it is laid down on a clean floor, and a workman is set to tread
upon it with naked feet for a considerable time, walking in a spiral direction from the
centre to the circumference, and from the circumference to the centre. In Sweden,
and also in China (to judge from the Chinese paintings which represent their manner
of making porcelain), the clay is trodden to a uniform mass by oxen. It is afterwards, in
all cases, kneaded like baker’s dough, by folding back the cake upon itself, and kneading
it out, alternately.
The process of slapping consists in cutting through a large mass with a wire, lifting
up either half in both hands, and casting it down with great violence on the other;
and this violent treatment of the clay is repeated till every appearance of air-bubbles is
removed, for the smallest remaining vesicle expanding in the kiln, would be apt to cause
blisters or warts upon the ware.
Having thus detailed the preparation of the stoneware paste, we have next to describe
the methods of forming it into articles of various forms.
Throwing is performed upon a tool called the potter’s lathe. (See fig., infrà.) This
consists of an upright iron shaft, about the height of a common table, on the top of which
is fixed, by its centre, a horizontal disc or circular piece of wood, of an area sufficiently great
for the largest stoneware vessel to stand upon. The lower end of the shaft is pointed, and
runs in a conical step, and its collar, a little below the top-board, being truly turned, is
embraced in a socket attached to the wooden frame of the lathe. The shaft has a pulley
fixed upon it, with grooves for 3 speeds, over which an endless band passes from a fly-wheel,
by whose revolution any desired rapidity of rotation may be given to the shaft
and its top-board. This wheel, when small, may be placed alongside, as in the turner’s
lathe, and then it is driven by a treadle and crank; or when of larger dimensions, it is
turned by the arms of a labourer. Sometimes, indeed, the wooden plate is replaced by
a large thick disc of Paris plaster, which is whirled round by the hand of the potter,
without the intervention of a pulley and fly-wheel, and affords sufficient centrifugal
power for fashioning small vessels. The mass of dough to be thrown, is weighed out
or gauged by an experienced hand. The thrower dashes down the lump on the
centre of the revolving board, and dipping his hands frequently in an adjoining tub of
water, he works up the clay into a tall irregular cylinder, and then down into a cake,
alternately, till he has secured the final extrication of air-bubbles, and then gives the
proper form to the vessel under a less speed of rotation, regulating its dimensions by
wooden pegs and gauges. He now cuts it off at the base with a piece of fine brass wire,
fastened to a handle at either end. The vessel thus rudely fashioned is placed in a situation
where it may dry gradually to a proper point. At a certain stage of the drying,
called the green state, it possesses a greater tenacity than at any other, till it is baked.
It is then taken to another lathe, called the turning lathe, where it is attached by a
little moisture to the vertical face of a wooden chuck, and turned nicely into its proper
shape with a very sharp tool, which also smooths it. After this it is slightly burnished
with a smooth steel surface.
[1012]
DESCRIPTION OF THE POTTER’S LATHE.
A, fig. 891., is the profile of the English potter’s lathe, for blocking out round
ware; C is the table or tray; a is the head of the lathe, with its horizontal disc; a, b,
is the upright shaft of the head; d, pulleys with several grooves of different
diameters, fixed upon the shaft, for receiving the driving-cord or band; k is a bench
upon which the workman sits astride; e, the treadle foot-board; l, is a ledge-board,
for catching the shavings of clay which fly off from the lathe; h is an instrument,
with a slide-nut i, for measuring the objects in the blocking out; c is the fly-wheel
with its winch-handle r, turned by an assistant; the sole-frame is secured in its place
by the heavy stone p; f is the oblong guide-pulley, having also several grooves
for converting the vertical movement of the fly-wheel into the horizontal movement of
the head of the lathe.
D is one of the intermediate forms given by the potter to the ball of clay, as it revolves
upon the head of the lathe.
In large potteries, the whole of the lathes, both for throwing and turning, are put in
motion by a steam-engine. The vertical spindle of the lathe has a bevel wheel on it,
which works in another bevel toothed wheel fixed to a horizontal shaft. This shaft is
provided with a long conical wooden drum, from which a strap ascends to a similar conical
drum on the main lying shaft. The apex of the one cone corresponds to the base
of the other, which allows the strap to retain the same degree of tension (see the conical
drum apparatus of the Stearine-press), while it is made to traverse horizontally, in order
to vary the speed of the lathe at pleasure. When the belt is at the base of the driving-cone,
it works near the vortex of the driven one, so as to give a maximum velocity to
the lathe, and vice versâ.
During the throwing of any article, a separate mechanism is conducted by a boy,
which makes the strap move parallel to itself along these conical drums, and nicely regulates
the speed of the lathe. When the strap runs at the middle of the cones, the
velocity of each shaft is equal. By this elegant contrivance of parallel cones reversed,
the velocity rises gradually to its maximum, and returns to its minimum or slower motion
when the workman is about finishing the article thrown. The strap is then transferred
to a pair of loose pulleys, and the lathe stops. The vessel is now cut off at the base
with a small wire; is dried, turned on a power lathe, and polished as above described.
The same degree of dryness which admits of the clay being turned on the lathe, also
suits for fixing on the handles and other appendages to the vessels. The parts to be
attached being previously prepared, are joined to the circular work by means of a thin
paste which the workmen call slip, and the seams are then smoothed off with a wet
sponge. They are now taken to a stove-room heated to 80° or 90° F., and fitted up with
a great many shelves. When they are fully dried, they are smoothed over with a small
bundle of hemp, if the articles be fine, and are then ready for the kiln, which is to convert
the tender clay into the hard biscuit.
A great variety of pottery wares, however, cannot be fashioned on the lathe, as they
are not of a circular form. These are made by two different methods, the one called
press-work, and the other casting. The press-work is done in moulds made of Paris
plaster, the one half of the pattern being formed in the one side of the mould, and the
other half on the other side: these moulding-pieces fit accurately together. All vessels
of an oval form, and such as have flat sides, are made in this way. Handles of tea-pots,
and fluted solid rods of various shapes, are formed by pressure also; viz., by
squeezing the dough contained in a pump-barrel through different shaped orifices at its
bottom, by working a screw applied to the piston-rod. The worm-shaped dough, as it
issues, is cut to proper lengths, and bent into the desired form. Tubes may be also
made on the same pressure principle, only a tubular opening must be provided in the
bottom plate of the clay-forcing pump.[1013]
The other method of fashioning earthenware articles is called casting, and is, perhaps,
the most elegant for such as have an irregular shape. This operation consists in pouring
the clay, in the state of pap or slip, into plaster moulds, which are kept in a
desiccated state. These moulds, as well as the pressure ones, are made in halves, which
nicely correspond together. The slip is poured in till the cavity is quite full, and is
left in the mould for a certain time, more or less, according to the intended thickness of
the vessel. The absorbent power of the plaster soon abstracts the water, and makes the
coat of clay in contact with it quite doughy and stiff, so that the part still liquid being
poured out, a hollow shape remains, which when removed from the mould constitutes
the half of the vessel, bearing externally the exact impress of the mould. The thickness
of the clay varies with the time that the paste has stood upon the plaster. These cast
articles are dried to the green state, like the preceding, and then joined accurately with
slip. Imitations of flowers and foliage are elegantly executed in this way. This
operation, which is called furnishing, requires very delicate and dexterous manipulation.
The saggers for the unglazed coloured stoneware should be covered inside with a
glaze composed of 12 parts of common salt and 30 of potash, or 6 parts of potash and
14 of salt; which may be mixed with a little of the common enamel for the glazed
pottery saggers. The bottom of each sagger has some bits of flints sprinkled upon it,
which become so adherent after the first firing as to form a multitude of little prominences
for setting the ware upon, when this does not consist of plates. It is the duty
of the workmen belonging to the glaze kiln to make the saggers during the intervals
of their work; or if there be a relay of hands, the man who is not firing makes the
saggers.
The English kilns differ from those of France and Germany, in their construction,
in the nature of their fuel, and in the high temperature required to produce a surface
sufficiently hard for a perfectly fine glaze.
When the ware is sufficiently dry, and in sufficient quantity to fill a kiln, the next
process is placing the various articles in the baked fire-clay vessels, which may be either
of a cylindrical or oval shape; called gazettes, Fr.; kapseln, Germ. These are from 6
to 8 inches deep, and from 12 to 18 inches in diameter. When packed full of the dry
ware, they are piled over each other in the kiln. The bottom of the upper sagger forms
the lid of its fellow below; and the junction of the two is luted with a ring of soft
clay applied between them. These dishes protect the ware from being suddenly and
unequally heated, and from being soiled by the smoke and vapours of the fuel. Each
pile of saggers is called a bung.
POTTERY KILN OF STAFFORDSHIRE.
Figs. 892, 893, 894, 895, 896., represent the kiln for baking the biscuit, and also for
running the glaze, in the English potteries.
[1014]
a, a, figs. 892, 893, and 894, are the furnaces which heat the kiln; of which b, in
fig. 892., are the upper mouths, and b′ the lower; the former being closed more or less
by the fire-tile z, shown in fig. 896.
f is one fireplace; for the manner of distributing the fuel in it, see fig. 896.
g, y, figs. 892. and 896., are the horizontal and vertical flues and chimneys for conducting
the flame and smoke. l is the laboratory, or body of the kiln; having its floor
k sloping slightly downwards from the centre to the circumference. x, y, is the slit of
the horizontal register, leading to the chimney flue y of the furnace, being the first regulator;
x, u, is the vertical register conduit, leading to the furnace or mouth f, being the
second regulator; v is the register slit above the furnace, and its vertical flue leading
into the body of the kiln; v′, c, slit for regulating flue at the shoulder of the kiln; i is
an arch which supports the walls of the kiln, when the furnace is under repair; c, c,
are small flues in the vault s of the laboratory. h, fig. 893., is the central flue, called
lunette, of the laboratory.
T, T, is the conical tower or howell, strengthened with a series of iron hoops, O′ is the
great chimney or lunette of the tower; p is the door of the laboratory, bound inside
with an iron frame.
A, is the complete kiln and howell, with all its appurtenances.
B, fig. 893., is the plan at the level d, d, of the floor, to
show the arrangement and distribution of all the horizontal
flues, both circular and radiating.
C, fig. 894., is a plan at the level e, e, of the upper mouths
b, of the furnaces, to show the disposition of the fireplaces of
the vertical flues, and of the horizontal registers, or peep-holes.
D, fig. 894., is a bird’s-eye view of the top of the vault or
dome s, to show the disposition of the vent-holes c, c.
E, fig. 895., is a detailed plan at the level c, c, of one furnace
and its dependencies.
F, fig. 896., is a transverse section, in detail, of one furnace
and its dependencies.
The same letters in all the figures indicate the same objects.
Charging of the kiln.—The saggers are piled up first in the
space between each of the upright furnaces, till they rise to the
top of the flues. These contain the smaller articles. Above
this level, large fire tiles are laid, for supporting other saggers, filled with teacups,
sugar-basins, &c. In the bottom part of the pile, within the preceding, the same sorts of
articles are put; but in the upper part all such articles are placed as require a high heat.
Four piles of small saggers, with a middle one 10 inches in height, complete the charge.
As there are 6 piles between each furnace, and as the biscuit kiln has 8 furnaces, a charge
consequently amounts to 48 or 50 bungs, each composed of from 18 to 19 saggers. The
inclination of the bungs ought always to follow the form of the kiln, and should therefore
tend towards the centre, lest the strong draught of the furnaces should make the saggers
fall against the walls of the kiln, an accident apt to happen were these piles perpendicular.
The last sagger of each bung is covered with an unbaked one, three inches deep, in place
of a round lid. The watches are small cups, of the same biscuit as the charge, placed
in saggers, four in number, above the level of the flue-tops. They are taken hastily
out of the saggers, lest they should get smoked, and are thrown into cold water.
When the charging is completed, the firing is commenced, with coal of the best
quality. The management of the furnaces is a matter of great consequence to the
success of the process. No greater heat should be employed for some time than may be
necessary to agglutinate the particles which enter into the composition of the paste, by
evaporating all the humidity; and the heat should never be raised so high as to endanger
the fusion of the ware, which would make it very brittle.
When ever the mouth or door of the kiln is built up, a child prepares several fires in the
neighbourhood of the howell, while a labourer transports in a wheelbarrow a supply of
coals, and introduces into each furnace a number of lumps. These lumps divide the
furnace into two parts; those for the upper flues being placed above, and those for the
ground flues below, which must be kept unobstructed.
The fire-mouths being charged, they are kindled to begin the baking, the regulator
tile z, fig. 896., being now opened; an hour afterwards the bricks at the bottom of the
furnace are stopped up. The fire is usually kindled at 6 o’clock in the evening, and
progressively increased till 10, when it begins to gain force, and the flame rises half-way up
the chimney. The second charge is put in at 8 o’clock, and the mouths of the furnaces are
then covered with tiles; by which time the flame issues through the vent of the tower. An
hour afterwards a fresh charge is made; the tiles z, which cover the furnaces, are slipped[1015]
back; the cinders are drawn to the front, and replaced with small coal. About half-past
11 o’clock the kiln-man examines his furnaces, to see that their draught is properly
regulated. An hour afterwards a new charge of coal is applied; a practice repeated
hourly till 6 o’clock in the morning. At this moment he takes out his first watch, to
see how the baking goes on. It should be at a very pale-red heat; but the watch of 7
o’clock should be a deeper red. He removes the tiles from those furnaces which appear to
have been burning too strongly, or whose flame issues by the orifices made in the shoulder
of the kiln; and puts tiles upon those which are not hot enough. The flames glide
along briskly in a regular manner. At this period he draws out the watches every
quarter of an hour, and compares them with those reserved from a previous standard
kiln; and if he observes a similarity of appearance, he allows the furnaces to burn a
little longer; then opens the mouths carefully and by slow degrees; so as to lower the
heat, and finish the round.
The baking usually lasts from 40 to 42 hours; in which time the biscuit kiln may
consume 14 tons of coals; of which four are put in the first day, seven the next day and
following night, and the four last give the strong finishing heat.
Emptying the kiln.—The kiln is allowed to cool very slowly. On taking the ware
out of the saggers, the biscuit is not subjected to friction, as in the foreign potteries,
because it is smooth enough; but is immediately transported to the place where it is to
be dipped in the glaze or enamel tub. A child makes the pieces ring, by striking with the
handle of the brush, as he dusts them, and then immerses them into the glaze cream;
from which tub they are taken out by the enameller, and shaken in the air. The tub
usually contains no more than 4 or 5 inches depth of the glaze, to enable the workman
to pick out the articles more readily, and to lay them upon a board, whence they are
taken by a child to the glaze kiln.
Glazing.—A good enamel is an essential element of fine stoneware; it should
experience the same dilatation and contraction by heat and cold as the biscuit which it
covers. The English enamels contain nothing prejudicial to health, as many of the foreign
glazes do; no more lead being added to the former than is absolutely necessary to convert
the siliceous and aluminous matters with which it is mixed into a perfectly neutral glass.
Three kinds of glazes are used in Staffordshire; one for the common pipe-clay or
cream-coloured ware; another for the finer pipe-clay ware to receive impressions,
called printing body; a third for the ware which is to be ornamented by painting with
the pencil.
The glaze of the first or common ware is composed of 53 parts of white lead, 16 of
Cornish stone, 36 of ground flints, and 4 of flint glass; or of 40 of white lead, 36 of
Cornish stone, 12 of flints, and 4 of flint or crystal glass. These compositions are not
fritted; but are employed after being simply triturated with water into a thin paste.
The following is the composition of the glaze intended to cover all kinds of figures
printed in metallic colours: 26 parts of white felspar are fritted with 6 parts of soda,
2 of nitre, and 1 of borax; to 20 pounds of this frit, 26 parts of felspar, 20 of white
lead, 6 of ground flints, 4 of chalk, 1 of oxide of tin, and a small quantity of oxide of
cobalt, to take off the brown cast, and give a faint azure tint, are added.
The following recipe may also be used. Frit together 20 parts of flint glass, 6 of
flints, 2 of nitre, and 1 of borax; add to 12 parts of that frit, 40 parts of white lead,
36 of felspar, 8 of flints, and 6 of flint glass; then grind the whole together into an
uniform cream-consistenced paste.
As to the stoneware which is to be painted, it is covered with a glaze composed
of 13 parts of the printing-colour frit, to which are added 50 parts of red lead, 40 of
white lead, and 12 of flint; the whole having been ground together.
The above compositions produce a very hard glaze, which cannot be scratched by the
knife, is not acted upon by vegetable acids, and does no injury to potable or edible
articles kept in the vessels covered with it. It preserves for an indefinite time the glassy
lustre, and is not subject to crack and exfoliate, like most of the Continental stoneware,
made from common pipe-clay.
In order that the saggers in which the articles are baked, after receiving the glaze, may
not absorb some of the vitrifying matter, they are themselves coated, as above mentioned,
with a glaze composed of 13 parts of common salt, and 30 parts of potash, simply dissolved
in water, and brushed over them.
Glaze kiln.—This is usually smaller than the biscuit kiln, and contains no more than
40 or 45 bungs or columns, each composed of 16 or 17 saggers. Those of the first bung
rest upon round tiles, and are well luted together with a finely ground fire-clay of only
moderate cohesion; those of the second bung are supported by an additional tile. The
lower saggers contain the cream-coloured articles, in which the glaze is softer than that
which covers the blue printed ware; this being always placed in the intervals between
the furnaces, and in the uppermost saggers of the columns. The bottom of the kiln,
where the glazed ware is not baked, is occupied by printed biscuit ware.
[1016]
Pyrometric balls of red clay, coated with a very fusible lead enamel, are employed in
the English potteries to ascertain the temperature of the glaze kilns. This enamel is so
rich, and the clay upon which it is spread, is so fine-grained and compact, that even when
exposed for three hours to the briskest flame, it does not lose its lustre. The colour of
the clay alone changes, whereby the workman is enabled to judge of the degree of heat
within the kiln. At first the balls have a pale red appearance; but they become browner
with the increase of the temperature. The balls, when of a slightly dark-red colour,
indicate the degree of baking for the hard glaze of pipeclay ware; but if they become
dark brown, the glaze will be much too hard, being that suited for ironstone ware; lastly,
when they acquire an almost black hue, they show a degree of heat suited to the formation
of a glaze upon porcelain.
The glazer provides himself at each round with a stock of these ball watches, reserved
from the preceding baking, to serve as objects of comparison; and he never slackens the
firing till he has obtained the same depth of shade, or even somewhat more; for it
may be remarked, that the more rounds a glaze kiln has made, the browner the balls
are apt to become. A new kiln bakes a round of enamel-ware sooner than an old one;
as also with less fuel, and at a lower temperature. The watch-balls of these first rounds
have generally not so deep a colour as if they were tried in a furnace three or four
months old. After this period, cracks begin to appear in the furnaces; the horizontal
flues get partially obstructed, the joinings of the brickwork become loose; in consequence
of which there is a loss of heat and waste of fuel; the baking of the glaze takes
a longer time, and the pyrometric balls assume a different shade from what they had on
being taken out of the new kiln, so that the first watches are of no comparable use after
two months. The baking of enamel is commenced at a low temperature, and the heat
is progressively increased; when it reaches the melting point of the glaze, it must be
maintained steadily, and the furnace mouths be carefully looked after, lest the heat
should be suffered to fall. The firing is continued 14 hours, and then gradually
lowered by slight additions of fuel; after which the kiln is allowed from 5 to 6 hours
to cool.
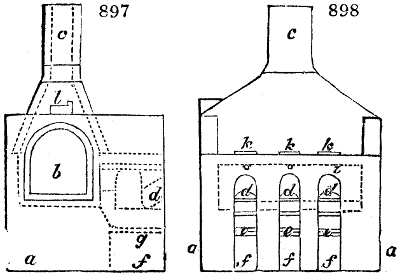
Muffles.—The paintings and the printed figures applied to the glaze of stoneware
and porcelain are baked in muffles
of a peculiar form. Fig. 897. is a lateral
elevation of one of these muffles; fig. 898.
is a front view. The same letters denote
the same parts in the two figures.
a is the furnace; b, the oblong muffle,
made of fire-clay, surmounted with a dome
pierced with three apertures k, k, k, for the
escape of the vaporous matters of the colours
and volatile oils with which they are
ground up; c is the chimney; d, d, feed-holes,
by which the fuel is introduced; e,
the fire-grate; f, the ash-pit; channels are
left in the bottom of the furnace to facilitate
the passage of the flame beneath the muffle; g is a lateral hole, which makes a
communication across the furnace in the muffle, enabling the kiln man to ascertain what
is passing within; k, k, are the lateral chinks for observing the progress of the firing or
flame; l, is an opening scooped out in the front of the chimney to modify its draught.
The articles which are printed or painted upon the glaze are placed in the muffle
without saggers, upon tripods, or movable supports furnished with feet. The muffle
being charged, its mouth is closed with a fire-tile well luted round its edges. The fuel
is then kindled in the fireplaces d, d, and the door of the furnace is closed with bricks,
in which a small opening is left for taking out samples, and for examining the interior of
the muffle. These sample or trial pieces, attached to a strong iron wire, show the progress
of the baking operation. The front of the fireplaces is covered with a sheet-iron plate,
which slides to one side, and may be shut whenever the kiln is charged. Soon after the
fire is lighted, the flame, which communicates laterally from one furnace to another,
envelopes the muffle on all sides, and thence rises up the chimney.
Printing of stoneware.—The printing under the stoneware glaze is generally performed
by means of cobalt, and has different shades of blue according to the quantity
of colouring matter employed. After having subjected this oxide to the processes
requisite for its purification, it is mixed with a certain quantity of ground flints and
sulphate of baryta, proportioned to the dilution of the shade. These materials are
fritted and ground; but before they are used, they must be mixed with a flux consisting
of equal parts by weight of flint glass and ground flints, which serves to fix the colour
upon the biscuit, so that the immersion in the glaze liquor may not displace the lines
printed on, as also to aid in fluxing the cobalt.
[1017]
The following are the processes usually practised in Staffordshire for printing under
the glaze.
The cobalt, or whatever colour is employed, should be ground upon a porphyry slab,
with a varnish prepared as follows:—A pint of linseed oil is to be boiled to the consistence
of thick honey, along with 4 ounces of rosin, half a pound of tar, and half a pint of
oil of amber. This is very tenacious, and can be used only when liquefied by heat;
which the printer effects by spreading it upon a hot cast-iron plate.
The printing plates are made of copper, engraved with pretty deep lines in the
common way. The printer, with a leather muller, spreads upon the engraved plate,
previously heated, his colour, mixed up with the above oil varnish, and removes what is
superfluous with a pallet knife; then cleans the plate with a dossil filled with bran, tapping
and wiping as if he were removing dust from it. This operation being finished, he
takes the paper intended to receive the impression, soaks it with soap-water, and lays it
moist upon the copper-plate. The soap makes the paper part more readily from the
copper, and the thick ink part more readily from the biscuit. The copper-plate is now
passed through the engraver’s cylinder press, the proof leaf is lifted off and handed to
the women, who cut it into detached pieces, which they apply to the surface of the
biscuit. The paper best fitted for this purpose is made entirely of linen rags; it is very
thin, of a yellow colour, and unsized, like tissue blotting-paper.
The stoneware biscuit never receives any preparation before being imprinted, the
oil of the colour being of such a nature as to fix the figures firmly. The printed paper
is pressed and rubbed on with a roll of flannel, about an inch and a half in diameter, and
12 or 15 inches long, bound round with twine, like a roll of tobacco. This is used as a
burnisher, one end of it being rested against the shoulder, and the other end being
rubbed upon the paper; by which means it transfers all the engraved traces to the
biscuit. The piece of biscuit is laid aside for a little, in order that the colour may take
fast hold; it is then plunged into water, and the paper is washed away with a sponge.
When the paper is detached, the piece of ware is dipped into a caustic alkaline lye to
saponify the oil, after which it is immersed in the glaze liquor, with which the printed
figures readily adhere. This process, which is easy to execute, and very economical, is
much preferable to the old plan of passing the biscuit into the muffle after it had been
printed, for the purpose of fixing and volatilizing the oils. When the paper impression
is applied to pieces of porcelain, they are heated before being dipped in the water,
because, being already semi-vitrified, the paper sticks more closely to them than to the
biscuit, and can be removed only by a hard brush.
The impression above the glaze is done by quite a different process, which dispenses
with the use of the press. A quantity of fine clean glue is melted and poured hot upon
a large flat dish, so as to form a layer about a quarter of an inch thick, and of the
consistence of jelly. When cold it is divided into cakes of the size of the copper-plates
it is intended to cover.
The operative (a woman) rubs the engraved copper-plate gently over with linseed oil
boiled thick, immediately after which she applies the cake of glue, which she presses
down with a silk dossil filled with bran. The cake licks up all the oil out of the
engraved lines; it is then cautiously lifted off, and transferred to the surface of the
glazed ware which it is intended to print. The glue cake being removed, the enamel
surface must be rubbed with a little cotton, whereby the metallic colours are attached
only on the lines charged with oil; the piece is then heated under the muffle. The same
cake of glue may serve for several impressions.
Ornaments and colouring.—Common stoneware is coloured by means of two kinds of
apparatus; the one called the blowing-pot, the other the worming-pot. The ornaments
made in relief in France, are made hollow (intaglio) in England, by means of a mould
engraved in relief, which is passed over the article. The impression which it produces
is filled with a thick clay paste, which the workman throws on with the blowing-pot. This
is a vessel like tea-pot, having a spout, but it is hermetically sealed at top with a clay
plug, after being filled with the pasty liquor. The workman, by blowing in at the spout,
causes the liquor to fly out through a quill pipe which goes down through the clay plug
into the liquor. The jet is made to play upon the piece while it is being turned upon
the lathe; so that the hollows previously made in it by the mould or stamp are filled
with a paste of a colour different from that of the body. When the piece has acquired
sufficient firmness to bear working, the excess of the paste is removed by an instrument
called a tournasin, till the ornamental figure produced by the stamp be laid bare;
in which case merely the colour appears at the bottom of the impression. By passing
in this manner several layers of clay liquor of different colours over each other with the
blowing-pot, net-work and decorations of different colours and shades are very rapidly
produced.
The serpentine or snake pots, established on the same principle, are made of tin plate
in three compartments, each containing a different colour. These open at the top of[1018]
the vessel in a common orifice, terminated by small quill tubes. On inclining the vessel,
the three colours flow out at once in the same proportion at the one orifice, and are let
fall upon the piece while it is being slowly turned upon the lathe; whereby curious
serpent-like ornaments may be readily obtained. The clay liquor ought to be in keeping
with the stoneware paste. The blues succeed best when the ornaments are made
with the finer pottery mixtures given above.
Metallic lustres applied to stoneware.—The metallic lustre being applied only to
the outer surface of vessels, can have no bad effect on health, whatever substances be
employed for the purpose; and as the glaze intended to receive it is sufficiently fusible,
from the quantity of lead it contains, there is no need of adding a flux to the metallic
coating. The glaze is in this case composed of 60 parts of litharge, 36 of felspar, and
15 of flints.
The silver and platina lustres are usually laid upon a white ground, while those of
gold and copper, on account of their transparency, succeed only upon a coloured
ground. The dark-coloured stoneware is, however, preferable, as it shows off the
colours to most advantage; and thus the shades may be varied by varying the colours
of the ornamental figures applied by the blowing-pot.
The gold and platina lustre is almost always applied to a paste body made on purpose,
and coated with the above-described lead glaze. This paste is brown, and consists of
4 parts of clay, 4 parts of flints, an equal quantity of kaolin (china clay), and 6 parts
of felspar. To make brown figures in relief upon a body of white paste, a liquor is
mixed up with this paste, which ought to weigh 26 ounces per pint, in order to unite
well with the other paste, and not to exfoliate after it is baked.
Preparation of gold lustre.—Dissolve first in the cold, and then with heat, 48 grains
of fine gold in 288 grains of an aqua regia, composed of 1 ounce of nitric acid and 3
ounces of muriatic acid; add to that solution 41⁄2 grains of grain tin, bit by bit; and then
pour some of that compound solution into 20 grains of balsam of sulphur diluted with
10 grains of oil of turpentine. The balsam of sulphur is prepared by heating a pint of
linseed oil, and 2 ounces of flowers of sulphur, stirring them continually till the mixture
begins to boil; it is then cooled, by setting the vessel in cold water; after which it is
stirred afresh, and strained-through linen. The above ingredients, after being well
mixed, are to be allowed to settle for a few minutes; then the remainder of the solution
of gold is to be poured in, and the whole is to be triturated till the mass has assumed
such a consistence that the pestle will stand upright in it; lastly, there must be added
to the mixture 30 grains of oil of turpentine, which being ground in, the gold lustre is
ready to be applied. If the lustre is too light or pale, more gold must be added, and if
it have not a sufficiently violet or purple tint, more tin must be used.
Platina lustre.—Of this there are two kinds; one similar to polished steel, another
lighter and of a silver-white hue. To give stoneware the steel colour with platina,
this metal must be dissolved in an aqua regia composed of 2 parts of muriatic acid,
and 1 part of nitric. The solution being cooled, and poured into a capsule, there
must be added to it, drop by drop, with continual stirring with a glass rod, a spirit of
tar, composed of equal parts of tar and sulphur boiled in linseed oil and filtered. If
the platina solution be too strong, more spirit of tar must be added to it; but if too
weak, it must be concentrated by boiling. Thus being brought to the proper pitch,
the mixture may be spread ever the piece, which being put into the muffle, will take
the aspect of steel.
The oxide of platina, by means of which the silver lustre is given to stoneware, is
prepared as follows:—After having dissolved to saturation the metal in an aqua regia
composed of equal parts of nitric and muriatic acid, the solution is to be poured into a
quantity of boiling water. At the same time, a capsule, containing solution of sal-ammoniac
is placed upon a sand-bath, and the platina solution being poured into it, the
metal will fall down in the form of the well-known yellow precipitate, which is to be
washed with cold water till it is perfectly edulcorated, then dried, and put up for use.
This metallic lustre is applied very smoothly by means of a flat camel’s hair brush.
It is then to be passed through the muffle kiln; but it requires a second application of
the platinum to have a sufficient body of lustre. The articles sometimes come black
out of the kiln, but they get their proper appearance by being rubbed with cotton.
Platina and gold lustre; by other recipes.
Platina lustre.—Dissolve 1 ounce of platinum in aqua regia formed of 2 parts of
muriatic acid and 1 part of nitric acid, with heat upon a sand-bath, till the liquid is
reduced to two-thirds of its volume; let it cool; decant into a clean vessel, and pour
into it, drop by drop, with constant stirring, some distilled tar, until such a mixture is
produced as will give a good result in a trial upon the ware in the kiln. If the lustre be
too intense, more tar must be added; if it be too weak, the mixture must be concentrated
by further evaporation.
Gold lustre.—Dissolve four shillings’ worth of gold in aqua regia with a gentle heat.[1019]
To the solution, when cool, add 2 grains of grain tin, which will immediately dissolve.
Prepare a mixture of half an ounce of balsam of sulphur with a little essence of turpentine,
beating them together till they assume the appearance of milk. Pour this
mixture into the solution of gold and tin, drop by drop, with continual stirring; and
place the whole in a warm situation for some time.
It is absolutely necessary to apply this lustre only upon an enamel or glaze which has
already passed through the fire, otherwise the sulphur would tarnish the composition.
These lustres are applied with most advantage upon chocolate and other dark
grounds. Much skill is required in their firing, and a perfect acquaintance with the
quality of the glaze on which they are applied.
An iron lustre, is obtained by dissolving a bit of steel or iron in muriatic acid, mixing
this solution with the spirit of tar, and applying it to the surface of the ware.
Aventurine glaze.—Mix a certain quantity of silver leaf with the above-described soft
glaze, grind the mixture along with some honey and boiling water, till the metal assume
the appearance of fine particles of sand. The glaze being naturally of a yellowish hue,
gives a golden tint to the small fragments of silver disseminated through it. Molybdena
may also be applied to produce the aventurine aspect.
The granite-like gold lustre, is produced by throwing lightly with a brush a few drops
of oil of turpentine upon the goods already covered with the preparation for gold lustre.
These cause it to separate and appear in particles resembling the surface of granite.
When marbling is to be given to stoneware, the lustres of gold, platina, and iron are
used at once, which blending in the fusion, form veins like those of marble.
Pottery and stoneware of the Wedgewood colour.—This is a kind of semi-vitrified ware,
called dry bodies, which is not susceptible of receiving a superficial glaze. This pottery
is composed in two ways: the first is with barytic earths, which act as fluxes upon the
clays, and form enamels: thus the Wedgewood jasper ware is made.
The white vitrifying pastes, fit for receiving all sorts of metallic colours, are composed
of 47 parts of sulphate of barytes, 15 of felspar, 26 of Devonshire clay, 6 of
sulphate of lime, 15 of flints, and 10 of sulphate of strontites. This composition is
capable of receiving the tints of the metallic oxides and of the ochrous metallic
earths. Manganese produces the dark purple colour; gold precipitated by tin, a rose
colour; antimony, orange; cobalt, different shades of blue; copper is employed for the
browns and the dead-leaf greens; nickel gives, with potash, greenish colours.
One per cent. of oxide of cobalt is added; but one half, or even one quarter, of a per
cent. would be sufficient, to produce the fine Wedgewood blue, when the nickel and
manganese constitute 3 per cent. as well as the carbonate of iron. For the blacks of
this kind, some English manufacturers mix black oxide of manganese with the black
oxide of iron, or with ochre. Nickel and umber afford a fine brown. Carbonate of
iron, mixed with bole or terra di Sienna, gives a beautiful tint to the paste; as also manganese
with cobalt, or cobalt with nickel. Antimony produces a very fine colour when
combined with the carbonate of iron in the proportion of 2 per cent., along with the
ingredients necessary to form the above-described vitrifying paste.
The following is another vitrifying paste, of a much softer nature than the preceding.
Felspar, 30 parts; sulphate of lime, 23; silex, 17; potter’s clay, 15; kaolin of Cornwall
(china clay), 15; sulphate of baryta, 10.
These vitrifying pastes are very plastic, and may be worked with as much facility as
English pipe-clay. The round ware is usually turned upon the lathe. It may,
however, be moulded, as the oval pieces always are. The more delicate ornaments are
cast in hollow moulds of baked clay, by women and children, and applied with remarkable
dexterity upon the turned and moulded articles. The coloured pastes have such an
affinity for each other, that the detached ornaments may be applied not only with a
little gum water upon the convex and concave forms, but they may be made to adhere
without experiencing the least cracking or chinks. The coloured pastes receive only
one fire, unless the inner surface is to be glazed; but a gloss is given to the outer surface.
The enamel for the interior of the black Wedgewood ware, is composed of 6 parts
of red lead, 1 of silex, and 2 ounces of manganese, when the mixture is made in pounds’
weight.
The operation called smearing, consists in giving an external lustre to the unglazed
semi-vitrified ware. The articles do not in this way receive any immersion,
nor even the aid of the brush or pencil of the artist; but they require a second fire.
The saggers are coated with the salt glaze already described. These cases, or saggers,
communicate by reverberation the lustre so remarkable on the surface of the English
stoneware; which one might suppose to be the result of the glaze tub, or of the brush.
Occasionally also a very fusible composition is thrown upon the inner surface of the
muffle, and 5 or 6 pieces called refractories are set in the middle of it, coated with the
same composition. The intensity of the heat converts the flux into vapour; a part of[1020]
this is condensed upon the surfaces of the contiguous articles, so as to give them the desired
brilliancy.
Mortar body, is a paste composed of 6 parts of clay, 3 of felspar, 2 of silex, and 1 of
china clay.
White and yellow figures upon dark-coloured grounds are a good deal employed. To
produce yellow impressions upon brown stoneware, ochre is ground up with a small
quantity of antimony. The flux consists of flint glass and flints in equal weights.
The composition for white designs is made by grinding silex up with that flux, and
printing it on, as for blue colours, upon brown or other coloured stoneware, which shows
off the light hues.
English porcelain or china.—Most of this belongs to the class called tender or soft
porcelain by the French and German manufacturers. It is not, therefore, composed
simply of kaolin and petuntse. The English china is generally baked at a much lower
heat than that of Sèvres, Dresden, and Berlin; and it is covered with a mere glass.
Being manufactured upon a prodigious scale, with great economy and certainty, and
little expenditure of fuel, it is sold at a very moderate price compared with the foreign
porcelain, and in external appearance is now not much inferior.
Some of the English porcelain has been called ironstone china. This is composed
usually of 60 parts of Cornish stone, 40 of china clay, and 2 of flint glass; or of 42 of
the felspar, the same quantity of clay, 10 parts of flints ground, and 8 of flint glass.
The glaze for the first composition is made with 20 parts of felspar, 15 of flints, 6 of
red lead, and 5 of soda, which are fritted together; with 44 parts of the frit, 22 parts
of flint glass, and 15 parts of white lead, are ground.
The glaze for the second composition is formed of 8 parts of flint glass, 36 of felspar,
40 of white lead, and 20 of silex (ground flints).
The English manufacturers employ three sorts of compositions for the porcelain biscuit;
namely, two compositions not fritted; one of them for the ordinary table service;
another for the dessert service and tea dishes; the third, which is fritted, corresponds to
the paste used in France for sculpture; and with it all delicate kinds of ornaments are
made.
| |
First
composition. |
Second
composition. |
Third
composition. |
| Ground flints |
75 |
|
66 |
Lynn sand |
150 |
| Calcined bones |
180 |
|
100 |
|
300 |
| China clay |
40 |
|
96 |
|
100 |
| Clay |
70 |
Granite |
80 |
Potash |
10 |
The glaze for the first two of the preceding compositions consists of, felspar 45, flints
9, borax 21, flint glass 20, nickel 4. After fritting that mixture, add 12 parts of red
lead. For the third composition, which is the most fusible, the glaze must receive 12
parts of ground flints, instead of 9; and there should be only 15 parts of borax, instead
of 21.
PLAN OF AN ENGLISH POTTERY.
A stoneware manufactory should be placed by the side of a canal or navigable river,
because the articles manufactured do not well bear land carriage.
A Staffordshire pottery is usually built as a quadrangle, each side being about 100
feet long, the walls 10 feet high, and the ridge of the roof 5 feet more. The base of
the edifice consists of a bed of bricks, 18 inches high, and 16 inches thick; upon which
a mud wall in a wooden frame, called pisé, is raised. Cellars are formed in front of the
buildings, as depôts for the pastes prepared in the establishment. The wall of the yard
or court is 9 feet high, and 18 inches thick.
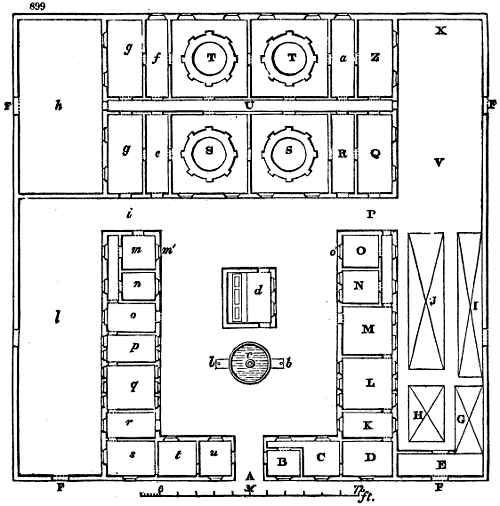
Fig. 899. A, is the entrance door; B, the porter’s lodge; C, a particular warehouse;
D, workshop of the plaster-moulder; E, the clay depôt; F, F, large gates, 6 feet 8 inches
high; G, the winter evaporation stove; H, the shop for sifting the paste liquors; I, sheds
for the paste liquor tubs; J, paste liquor pits; K, workshop for the moulder of hollow
ware; L, ditto of the dish or plate moulder; M, the plate drying-stove; N, workshop of the
biscuit-printers; O, ditto of the biscuit, with o′, a long window; P, passage leading to the
paste liquor pits; Q, biscuit warehouse; R, place where the biscuit is cleaned as it comes
out of the biscuit kilns, S S; T, T, enamel or glaze kilns; U, long passage; V, space left for
supplementary workshops; X, space appointed as a depôt for the sagger fire-clay, as also
for making the saggers; Z, the workshop for applying the glaze liquor to the biscuits;
a, apartment for cleaning the glazed ware; b, b, pumps; c, basin; d, muffles; e, warehouse
for the finished stoneware; f, that of the glazed goods; g, g, another warehouse;
h, a large space for the smith’s forge, carpenter’s shop, packing room, depôt of clays,
saggers, &c. The packing and loading of the goods are performed in front of the[1021]
warehouse, which has two outlets, in order to facilitate the work; i, a passage to the
court or yard; l, a space for the wooden sheds for keeping hay, clay, and other miscellaneous
articles, m, room for putting the biscuit into the saggers; m′, a long window;
n, workshop with lathes and fly-wheels; o, drying-room; p, room for mounting or furnishing
the pieces; q, repairing room; r, drying room of the goods roughly turned; s,
rough turning or blocking-out room; t, room for beating the paste or dough; u,
counting-house.
The declared value of the earthenware exported in 1836, was 837,774l.; in 1837,
558,682l.
There are from 33,000 to 35,000 tons of clay exported annually from Poole, in Dorsetshire,
to the English and Scotch potteries. A good deal of clay is also sent from
Devonshire and Cornwall.
The Spanish alcarazzas, or cooling vessels, are made porous, to favour the exudation
of water through them, and maintain a constantly moist evaporating surface. Lasteyrie
says, that granular sea salt is an ingredient of the paste of the Spanish alcarazzas;
which being expelled partly by the heat of the baking, and partly by the subsequent
watery percolation, leaves the body very open. The biscuit should be charged with a
considerable proportion of sand, and very moderately fired.
OF PORCELAIN.
Porcelain is a kind of pottery ware whose paste is fine grained, compact, very hard,
and faintly translucid; and whose biscuit softens slightly in the kiln. Its ordinary
whiteness cannot form a definite character, since there are porcelain pastes variously
coloured. There are two species of porcelain, very different in their nature, the
essential properties of which it is of consequence to establish; the one is called hard,
and the other tender; important distinctions, the neglect of which has introduced great
confusion into many treatises on this elegant manufacture.
Hard porcelain is essentially composed, first, of a natural clay containing some silica,
infusible, and preserving its whiteness in a strong heat; this is almost always a true
kaolin; secondly, of a flux, consisting of silica and lime, composing a quartzose felspar
rock, called pe-tun-tse. The glaze of this porcelain, likewise earthy, admits of no metallic
substance or alkali.
[1022]
Tender porcelain, styled also vitreous porcelain, has no relation with the preceding
in its composition; it always consists of a vitreous frit, rendered opaque and less fusible
by the addition of a calcareous or marly clay. Its glaze is an artificial glass or crystal,
into which silica, alkalis, and lead enter.
This porcelain has a more vitreous biscuit, more transparent, a little less hard, and
less fragile, but much more fusible than that of the hard porcelain. Its glaze is more
glossy, more transparent, a little less white, much tenderer, and more fusible.
The biscuit of the hard porcelain made at the French national manufactory of Sèvres
is generally composed of a kaolin clay, and of a decomposed felspar rock; analogous to
the china clay of Cornwall, and Cornish stone. Both of the above French materials
come from Saint Yriex-la-perche, near Limoges.
After many experiments, the following composition has been adopted for the service
paste of the royal manufactory of Sèvres; that is, for all the ware which is to be glazed:
silica, 59; alumina, 35·2; potash, 2·2; lime, 3·3. The conditions of such a compound
are pretty nearly fulfilled by taking from 63 to 70 of the washed kaolin or china clay,
22 to 15 of the felspar; nearly 10 of flint powder, and about 5 of chalk. The glaze is
composed solely of solid felspar, calcined, crushed, and then ground fine at the mill.
This rock pretty uniformly consists of silica 73, alumina 16·2, potash 8·4, and
water 0·6.
The kaolin is washed at the pit, and sent in this state to Sèvres, under the name of
decanted earth. At the manufactory it is washed and elutriated with care; and its slip
is passed through fine sieves. This forms the plastic, infusible, and opaque ingredient
to which the substance must be added which gives it a certain degree of fusibility and
semi-transparency. The felspar rock used for this purpose, should contain neither dark
mica nor iron, either as an oxide or sulphuret. It is calcined to make it crushable, under
stamp-pestles driven by machinery, then ground fine in hornstone mills, as represented
in figs. 897, 898, 899, and 900. This pulverulent matter being diffused through
water, is mixed in certain proportions, regulated by its quality, with the argillaceous
slip. The mixture is deprived of the chief part of its water in shallow plaster pans
without heat; and the resulting paste is set aside to ripen, in damp cellars, for many
months.
When wanted for use, it is placed in hemispherical pans of plaster, which absorb the
redundant moisture; after which it is divided into small lumps, and completely dried.
It is next pulverized, moistened a little, and laid on a floor, and trodden upon by a
workman marching over it with bare feet in every direction; the parings and fragments
of soft moulded articles being intermixed, which improve the plasticity of the
whole. When sufficiently tramped, it is made up into masses of the size of a man’s
head, and kept damp till required.
The dough is now in a state fit for the potter’s lathe; but it is much less plastic than
stoneware paste, and is more difficult to fashion into the various articles; and hence
one cause of the higher price of porcelain.
The round plates and dishes are shaped on plaster moulds; but sometimes the paste
is laid on as a crust, and at others it is turned into shape on the lathe. When a crust
is to be made, a moistened sheep-skin is spread on a marble table; and over this the
dough is extended with a rolling pin, supported on two guide-rules. The crust is then
transferred over the plaster mould, by lifting it upon the skin; for it wants tenacity to
bear raising by itself. When the piece is to be fashioned on the lathe, a lump of the
dough is thrown on the centre of the horizontal wooden disc, and turned into form as
directed in treating of stoneware, only it must be left much thicker than in its finished
state. After it dries to a certain degree on the plaster mould, the workman replaces it
on the lathe, by moistening it on its base with a wet sponge, and finishes its form with
an iron tool. A good workman at Sèvres makes no more than from 15 to 20 porcelain
plates in a day; whereas an English potter, with two boys, makes from 1000 to 1200
plates of stoneware in the same time. The pieces which are not round, are shaped in
plaster moulds, and finished by hand. When the articles are very large, as wash-hand
basins, salads, &c., a flat cake is spread above a skin on the marble slab, which is then
applied to the mould with the sponge, as for plates; and they are finished by hand.
The projecting pieces, such as handles, beaks, spouts, and ornaments, are moulded
and adjusted separately; and are cemented to the bodies of china-ware with slip, or
porcelain dough thinned with water. In fact, the mechanical processes with porcelain
and the finer stoneware are substantially the same; only they require more time and
greater nicety. The least defect in the fabrication, the smallest bit added, an unequal
pressure, the cracks of the moulds, although well repaired, and seemingly effaced in the
clay shape, re-appear after it is baked. The articles should be allowed to dry very
slowly; if hurried but a little, they are liable to be spoiled. When quite dry, they are
taken to the kiln.
The kiln for hard porcelain at Sèvres, is a kind of tower in two flats, constructed of[1023]
fire-bricks; and resembles, in other respects, the stoneware kiln already figured and
described. The fuel is young aspin wood, very dry, and cleft very small; it is put into
the apertures of the four outside furnaces or fire-mouths, which discharge their flame
into the inside of the kiln; each floor being closed in above, by a dome pierced with
holes. The whole is covered in by a roof with an open passage, placed at a proper distance
from the uppermost dome. There is, therefore, no chimney proper so called. See
Stone, artificial.
The raw pieces are put into the upper floor of the kiln; where they receive a heat of
about the 60th degree of Wedgewood’s pyrometer, and a commencement of baking,
which, without altering their shape, or causing a perceptible shrinking of their bulk,
makes them completely dry, and gives them sufficient solidity to bear handling. By
this preliminary baking, the clay loses its property of forming a paste with water; and
the pieces become fit for receiving the glazing coat, as they may be dipped in water
without risk of breakage.
The glaze of hard porcelain is a felspar rock: this being ground to a very fine powder,
is worked into a paste with water mingled with a little vinegar. All the articles are
dipped into this milky liquid for an instant; and as they are very porous, they absorb
the water greedily, whereby a layer of the felspar glaze is deposited on their surface, in
a nearly dry state, as soon as they are lifted out. Glaze-pap is afterwards applied with
a hair brush to the projecting edges, or any points where it had not taken; and the
powder is then removed from the part on which the article is to stand, lest it should
get fixed to its support in the fire. After these operations it is replaced in the kiln, to
be completely baked.
The articles are put into saggers, like those of fine stoneware; and this operation is
one of the most delicate and expensive in the manufacture of porcelain. The saggers
are made of the plastic or potter’s clay of Abondant, to which about a third part of
cement of broken saggers has been added.
As the porcelain pieces soften somewhat in the fire, they cannot be set above each
other, even were they free from glaze; for the same reason, they cannot be baked on
tripods, several of them being in one case, as is done with stoneware. Every piece of
porcelain requires a sagger for itself. They must, moreover, be placed on a perfectly
flat surface, because in softening they would be apt to conform to the irregularities of a
rough one. When therefore any piece, a soup plate for example, is to be saggered, there
is laid on the bottom of the case a perfectly true disc or round cake of stoneware,
made of the sagger material, and it is secured in its place on three small props of a clay-lute,
consisting of potter’s clay mixed with a great deal of sand. When the cake is carefully
levelled, it is moistened, and dusted over with sand, or coated with a film of fire-clay
slip, and the porcelain is carefully set on it. The sand or fire-clay hinders it from
sticking to the cake. Several small articles may be set on the same cake, provided they
do not touch one another.
The saggers containing the pieces thus arranged, are piled up in the kiln over each
other, in the columnar form, till the whole space be occupied; leaving very moderate
intervals between the columns to favour the draught of the fires. The whole being
arranged with these precautions, and several others, too minute to be specified here, the
door of the kiln is built up with 3 rows of bricks, leaving merely an opening 8 inches
square, through which there is access to a sagger with the nearest side cut off. In this
sagger are put fragments of porcelain intended to be withdrawn from time to time, in
order to judge of the progress of the baking. These are called time-pieces or watches
(montres). This opening into the watches is closed by a stopper of stoneware.
The firing begins by throwing into the furnace-mouths some pretty large pieces of
white wood, and the heat is maintained for about 15 hours, gradually raising it by the
addition of a larger quantity of the wood, till at the end of that period the kiln has a
cherry-red colour within. The heat is now greatly increased by the operation termed
covering the fire. Instead of throwing billets vertically into the four furnaces, there is
placed horizontally on the openings of these furnaces, aspin wood of a sound texture,
cleft small, laid in a sloping position. The brisk and long flame which it yields dips
into the tunnels, penetrates the kiln, and circulates round the sagger-piles. The heat
augments rapidly, and, at the end of 13 or 15 hours of this firing, the interior of the
kiln is so white, that the watches can hardly be distinguished. The draught, indeed, is
so rapid at this time, that one may place his hand on the slope of the wood without
feeling incommoded by the heat. Every thing is consumed, no small charcoal remains,
smoke is no longer produced, and even the wood-ash is dissipated. It is obvious
that the kiln and the saggers must be composed of a very refractory clay, in order to
resist such a fire. The heat in the Sèvres kilns mounts so high as the 134th degree
of Wedgewood.
At the end of 15 or 20 hours of the great fire; that is, after from 30 to 36 hours’
firing, the porcelain is baked; as is ascertained by taking out and examining the[1024]
watches. The kiln is suffered to cool during 3 or 4 days, and is then opened and discharged.
The sand strewed on the cakes to prevent the adhesion of the articles to
them, gets attached to their sole, and is removed by friction with a hard sandstone; an
operation which one woman can perform for a whole kiln in less than 10 days; and is the
last applied to hard porcelain, unless it needs to be returned into the hot kiln to have
some defects repaired.
The materials of fine porcelain are very rare; and there would be no advantage
in making a gray-white porcelain with coarser and somewhat cheaper materials,
for the other sources of expense above detailed, and which are of most consequence,
would still exist; while the porcelain, losing much of its brightness, would lose the main
part of its value.
Its pap or dough, which requires tedious grinding and manipulation, is also more
difficult to work into shapes, in the ratio of 80 to 1, compared to fine stoneware. Each
porcelain plate requires a separate sagger; so that 12 occupy in the kiln a space sufficient
for at least 38 stoneware plates. The temperature of a hard porcelain kiln being
very high, involves a proportionate consumption of fuel and waste of saggers. With
40 steres (cubic metres) of wood, 12,000 stoneware plates may be completely fired, both
in the biscuit and glaze kilns; while the same quantity of wood would bake at most
only 1000 plates of porcelain.
To these causes of high price, which are constant and essential, we ought to add the
numerous accidents to which porcelain is exposed at every step of its preparation, and
particularly in the kiln; these accidents damage upwards of one-third of the pieces,
and frequently more, when articles of singular form and large dimensions are adventured.
The best English porcelain is made from a mixture of the Cornish kaolin (called
china clay), ground flints, ground Cornish stone, and calcined bones in powder, or bone-ash,
besides some other materials, according to the fancy of the manufacturers. A
liquid pap is made with these materials, compounded in certain proportions, and diluted
with water. The fluid part is then withdrawn by the absorbent action of dry stucco
basins or pans. The dough, brought to a proper stiffness, and perfectly worked and
kneaded on the principles detailed above, is fashioned on the lathe, by the hands of
modellers, or by pressure in moulds. The pieces are then baked to the state of biscuit
in a kiln, being enclosed, of course, in saggers.
This biscuit has the aspect of white sugar, and being very porous, must receive a
vitreous coating. The glaze consists of ground felspar or Cornish stone. Into this,
diffused in water, along with a little flint-powder and potash, the biscuit ware is dipped,
as already described, under stoneware. The pieces are then fired in the glaze-kiln,
care being taken, before putting them into their saggers, to remove the glaze powder from
their bottom parts, to prevent their adhesion to the fire-clay vessel.
TENDER PORCELAIN.
Tender porcelain, or soft china-ware, is made with a vitreous frit, rendered less fusible
and opaque by an addition of white marl or bone-ash. The frit is, therefore, first prepared.
This, at Sèvres, is a composition, made with some nitre, a little sea salt, Alicant
barilla, alum, gypsum, and much siliceous sand or ground flints. That mixture is subjected
to an incipient pasty fusion in a furnace, where it is stirred about to blend the
materials well; and thus a very white spongy frit is obtained. It is pulverized, and
to every three parts of it, one of the white marl of Argenteuil is added; and when the
whole are well ground, and intimately mixed, the paste of tender porcelain is formed.
As this paste has no tenacity, it cannot bear working till a mucilage of gum or black
soap be added, which gives it a kind of plasticity, though even then it will not bear the
lathe. Hence it must be fashioned in the press, between two moulds of plaster. The
pieces are left thicker than they should be; and when dried, are finished on the lathe
with iron tools.
In this state they are baked, without any glaze being applied; but as this porcelain
softens far more during the baking than the hard porcelain, it needs to be supported on
every side. This is done by baking on earthen moulds all such pieces as can be treated
in this way, namely plates, saucers, &c. The pieces are reversed on these moulds,
and undergo their shrinkage without losing their form. Beneath other articles, supports
of a like paste are laid, which suffer in baking the same contraction as the articles, and
of course can serve only once. In this operation saggers are used, in which the pieces
and their supports are fired.
The kiln for the tender porcelain at Sèvres is absolutely similar to that for the
common stoneware; but it has two floors; and while the biscuit is baked in the lower
story, the glaze is fused in the upper one; which causes considerable economy of
fuel. The glaze of soft porcelain is a species of glass or crystal prepared on purpose.
It is composed of flint, siliceous sand, a little potash or soda, and about two-fifth parts[1025]
of lead oxide. This mixture is melted in crucibles or pots beneath the kiln. The
resulting glass is ground fine, and diffused through water mixed with a little vinegar to
the consistence of cream. All the pieces of biscuit are covered with this glazy matter,
by pouring this slip over them, since their substance is not absorbent enough to take it
on by immersion.
The pieces are encased once more each in a separate sagger, but without any supports;
for the heat of the upper floor of the kiln, though adequate to melt the glaze,
is not strong enough to soften the biscuit. But as this first vitreous coat is not very
equal, a second one is applied, and the pieces are returned to the kiln for the third
time. See Stone, artificial, for a view of this kiln.
The manufacture of soft porcelain is longer and more difficult than that of hard; its
biscuit is dearer, although the raw materials may be found every where; and it furnishes
also more refuse. Many of the pieces split asunder, receive fissures, or become
deformed in the biscuit-kiln, in spite of the supports; and this vitreous porcelain,
moreover, is always yellower, more transparent, and incapable of bearing rapid transitions
of temperature, so that even the heat of boiling water frequently cracks it. It
possesses some advantages as to painting, and may be made so gaudy and brilliant in its
decorations, as to captivate the vulgar eye.
DESCRIPTION OF THE PORCELAIN MILL.
1. The following figures of a felspar and flint mill are taken from plans of apparatus
lately constructed by Mr. Hall of Dartford, and erected by him in the royal manufactory
of Sèvres. There are two similar sets of apparatus, fig. 900., which may be employed together
or in succession; composed each of an elevated tub A, and of three successive vats
of reception A′, and two behind it, whose top edges are upon a lower level than the bottom
of the casks A, A, to allow of the liquid running out of them with a sufficient slope. A
proper charge of kaolin is first put into the cask A, then water is gradually run into it
by the gutter adapted to the stopcock a, after which the mixture is agitated powerfully
in every direction by hand with the stirring-bar, which is hung within a hole in the
ceiling, and has at its upper end a small tin-plate funnel to prevent dirt or rust from
dropping down into the clay. The stirrer may be raised or lowered so as to touch any
part of the cask. The semi-fluid mass is left to settle for a few minutes, and then the
finer argillaceous pap is run off by the stopcock a′, placed a little above the gritty
deposit, into the zinc pipe which conveys it into one of the tubs A′; but as this semi-liquid
matter may still contain some granular substances, it must be passed through a
sieve before it is admitted into the tub. There is, therefore, at the spot upon the tub where
the zinc pipe terminates, a wire-cloth sieve, of an extremely close texture, to receive the
liquid paste. This sieve is shaken upon its support, in order to make it discharge
the washed argillaceous kaolin. After the clay has subsided, the water is drawn off
from its surface by a zinc syphon. The vats A′ have covers, to protect their contents
from dust. In the pottery factories of England, the agitation is produced by machinery,
instead of the hand. A vertical shaft, with horizontal or oblique paddles, is
made to revolve in the vats for this purpose.
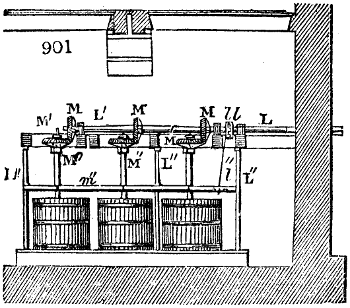
The small triturating mill is represented in fig. 901. There are three similar grinding-tubs
on the same line. The details of the construction are shown in figs. 902, 903,
where it is seen to consist principally of a revolving millstone B (fig. 902.), of a fast
or sleeper millstone B′, and of a vat C, hooped with iron, with its top raised above the
upper millstone. The lower block of hornstone rests upon a very firm basis, b′; it
is surrounded immediately by the strong wooden circle c, which slopes out funnel-wise
above, in order to throw back the earthy matters as they are pushed up by the attrition[1026]
of the stones. That piece is hollowed out, partially to admit the key C, opposite to
which is the faucet and spigot c′, for emptying the tub. When one operation is completed,
the key C is lifted out by means of a
peg put into the holes at its top; the spigot is
then drawn, and the thin paste is run out into vats.
The upper grindstone, B d, like the lower one, is
about two feet in diameter, and must be cut in a
peculiar manner. At first there is scooped out
a hollowing in the form of a sector, denoted by
d e f, fig. 903.; the arc d f is about one-sixth of
the circumference, so that the vacuity of the
turning grindstone is one-sixth of its surface;
moreover, the stone must be channelled, in order
to grind or crush the hard gritty substances. For
this purpose, a wedge-shaped groove d e g, about
an inch and a quarter deep, is made on its under
face, whereby the stone, as it turns in the direction indicated by the arrow, acts with
this inclined plane upon all the particles in its course, crushing them and forcing them
in between the stones, till they be triturated to an impalpable powder. When the
grindstone wears unequally on its lower surface, it is useful to trace upon it little
furrows, proceeding from the centre to the circumference, like those shown by the
dotted lines e′ e′′. It must, moreover, be indented with rough points by the hammer.
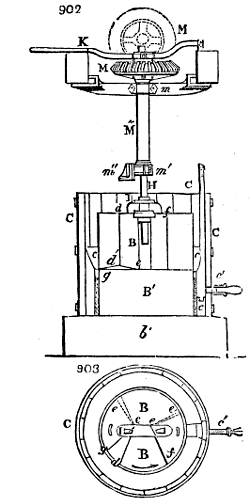
The turning hornstone-block is set in motion by the vertical shaft H, which is fixed
by the clamp-iron cross I to the top of the stone. When the stone is new, its thickness
is about 14 inches, and it is made to answer for grinding till it be reduced to about
8 inches, by lowering the clamp I upon the shaft, so that it may continue to keep its
hold of the stone. The manner in which the grindstones are turned, is obvious from
inspection of fig. 901., where the horizontal axis L, which receives its impulsion from
the great water-wheel, turns the prolonged shaft L′, or leaves it at rest, according as the
clutch l, l′, is locked or opened. This second shaft bears the three bevel wheels M, M, M.
These work in three corresponding bevel wheels M′ M′ M′, made fast respectively to the
three vertical shafts of the millstones, which pass
through the cast-iron guide tubes M′′ M′′. These
are fixed in a truly vertical position by the collar-bar
m′′, m′, fig. 902. In this figure we see at m how
the strong cross-bar of cast iron is made fast to the
wooden beams which support all the upper mechanism
of the mill-work. The bearing m′ is disposed in
an analogous manner; but it is supported against
two cast-iron columns, shown at L′′ L′′, in fig. 901.
The guide tubes M′′ are bored smooth for a small
distance from each of their extremities, and their
interjacent calibre is wider, so that the vertical shafts
touch only at two places. It is obvious, that whenever
the shaft L′ is set a-going, it necessarily turns
the wheels M and M′, and their guide tubes M′′; but
the vertical shaft may remain either at rest, or revolve,
according to the position of the lever click or catch
K, at the top, which is made to slide upon the shaft,
and can let fall a finger into a vertical groove cut
in the surface of that shaft. The clamp-fork of the
click is thus made to catch upon the horizontal bevel-wheel
M′, or to release it, according as the lever K is
lowered or lifted up. Thus each millstone may be
thrown out of or into geer at pleasure.
These stones make upon an average 11 or 12 turns in
a minute, corresponding to 3 revolutions of the water-wheel,
which moves through a space of 3 feet 4 inches in
the second, its outer circumference being 66 feet. The
weight of the upper stone, with its iron mountings, is
about 6 cwt., when new. The charge of each mill in dry
material is 2 cwt.; and the water may be estimated at
from one-half to the whole of this weight; whence the total
load may be reckoned to be at least 3 cwt.; the stone, by
displacement of the magma, loses fully 400 pounds of its weight, and weighs therefore in
reality only 2 cwt. It is charged in successive portions, but it is discharged all at once.
When the grinding of the siliceous or felspar matters is nearly complete, a remarkable[1027]
phenomenon occurs; the substance precipitates to the bottom, and assumes in a few
seconds so strong a degree of cohesion, that it is hardly possible to restore it again to the
pasty or magma state; hence if a millstone turns too slowly, or if it be accidentally
stopped for a few minutes, the upper stone gets so firmly cemented to the under one,
that it is difficult to separate them. It has been discovered, but without knowing why,
that a little vinegar added to the water of the magma almost infallibly prevents that
sudden stiffening of the deposit and stoppage of the stones. If the mills come to be
set fast in this way, the shafts or geering would be certainly broken, were not some
safety provision to be made in the machinery against such accidents. Mr. Hall’s contrivance
to obviate the above danger is highly ingenious. The clutch l, l′, fig. 901., is
not a locking crab, fixed in the common way, upon the shaft L; but it is composed, as
shown in figs. 904, 905, 906, 907, of a hoop u, fixed
upon the shaft by means of a key, of a collar v, and
of a flat ring or washer x, with four projections, which
are fitted to the collar v, by four bolts y. Fig. 905.
represents the collar v seen in front; that is, by the
face which carries the clutch teeth; and fig. 906. represents
its other face, which receives the flat ring x,
fig. 907., in four notches corresponding to the four projections
of the washer-ring. Since the ring u is fixed
upon the shaft L, and necessarily turns with it, it has
the two other pieces at its disposal, namely the collar
v, and the washer x, because they are always connected
with it by the four bolts y, so as to turn with the ring u, when the resistance they encounter
upon the shaft L′ is not too great, and to remain at rest, letting the ring u turn by itself,
when that resistance increases to a certain pitch. To give this degree of friction, we
need only interpose the leather washers z, z′, fig. 904.; and now as the collar coupling-box,
v, slides pretty freely upon the ring u, it is obvious that by tightening more or less
the screw bolts y, these washers will become as it were a lateral brake, to tighten more
or less the bearing of the ring u, to which they are applied: by regulating this pressure,
every thing may be easily adjusted. When the resistance becomes too great, the leather
washers, pressed upon one side by the collar v, of the washer x, and rubbed upon the
other side by the prominence of the ring u, get heated to such a degree, that they are apt
to become carbonized, and require replacement.
This safety clutch may be recommended to the notice of mechanicians, as susceptible
of beneficial application in a variety of circumstances.
GREAT PORCELAIN MILL.
The large felspar and kaolin mill, made by Mr. Hall, for Sèvres, has a flat bed of
hornstone, in one block, laid at the bottom of a great tub, hooped strongly with iron.
In most of the English potteries, however, that bed consists of several flat pieces of
chert or hornstone, laid level with each other. There is, as usual, a spigot and faucet
at the side, for drawing off the liquid paste. The whole system of the mechanism
is very substantial, and is supported by wooden beams.
The following is the manner of turning the upper blocks. In fig. 900. the main
horizontal shaft P bears at one of its extremities a toothed wheel, usually mounted upon
the periphery of the great
water-wheel (fig. 908. shows
this toothed wheel by a dotted
line) at its other end; P carries
the fixed portion p of a
coupling-box, similar to the
one just described as belonging
to the little mill. On the prolongation
of P, there is a second
shaft P′, which bears the movable
portion of that box, and
an upright bevel wheel P′′.
Lastly, in figs. 900. and 908.
there is shown the vertical
shaft Q, which carries at its
upper end a large horizontal
cast-iron wheel Q′, not seen in
this view, because it is sunk within the upper surface of the turning hornstone, like
the clamp d, f, in fig. 902. At the lower end of the shaft Q, there is the bevel wheel
Q′′, which receives motion from the wheel P′′, fig. 900.
The shaft P always revolves with the water-wheel; but transmits its motion to the[1028]
shaft P′ only when the latter is thrown into geer with the coupling-box p′, by means of
its forked lever. Then the bevel wheel P′ turns round with the shaft P′, and communicates
its rotation to the bevel wheel Q′′, which transmits it to the shaft Q, and to the large cast-iron
wheel, which is sunk into the upper surface of the revolving hornstone.
The shaft Q is supported and centred by a simple and solid adjustment; at its lower
part, it rests in a step R, which is supported upon a cast-iron arch Q′, seen in profile in
fig. 900.; its base is solidly fixed by four strong bolts. Four set screws above R, fig. 900.,
serve to set the shaft Q truly perpendicular: thus supported, and held securely at its lower
end, in the step at R, figs. 900. and 908., it is embraced near the upper end by a brass
bush or collar, composed of two pieces, which may be drawn closer together by means
of a screw. This collar is set into the summit of a great truncated cone of cast-iron,
which rises within the tub through two-thirds of the thickness of the hornstone
bed; having its base firmly fixed by bolts to the bottom of the tub, and having a brass
collet to secure its top. The iron cone is cased in wood. When all these pieces are
well adjusted and properly screwed up, the shaft Q revolves without the least vacillation,
and carries round with it the large iron wheel Q′, cast in one piece, and which consists of an
outer rim, three arms or radii, and a strong central nave, made fast by a key to the top of
the shaft Q, and resting upon a shoulder nicely turned to receive it. Upon each of the three
arms, there are adjusted, with bolts, three upright substantial bars of oak, which descend
vertically through the body of the revolving mill to within a small distance of the bed-stone;
and upon each of the three arcs of that wheel-ring, comprised between its three
strong arms, there are adjusted, in like manner, five similar uprights, which fit into hollows
cut in the periphery of the moving stone. They ought to be cut to a level at their
lower part, to suit the slope of the bottom of the tub o, figs. 900. and 908., so as to
glide past it pretty closely, without touching.
The speed of this large mill is eight revolutions in the minute. The turning hornstone
describes a mean circumference of 1411⁄3 inches (its diameter being 45 inches), and
of course moves through about 100 feet per second. The tub O, is 52 inches wide at
bottom, 56 at the surface of the sleeper block (which is 16 inches thick), and 64 at top,
inside measure. It sometimes happens that the millstone throws the pasty mixture out of
the vessel, though its top is 6 inches under the lip of the tub o; an inconvenience which
can be obviated only by making the pap a little thicker; that, is by allowing only from
25 to 30 per cent. of water; then its density becomes nearly equal to 2·00, while that of
the millstones themselves is only 2·7; whence, supposing them to weigh only 2 cwt.,
there would remain an effective weight of less than 1⁄2 cwt. for pressing upon the bottom
and grinding the granular particles. This weight appears to be somewhat too small to
do much work in a short time; and therefore it would be better to increase the quantity
of water, and put covers of some convenient form over the tubs. It is estimated that
this mill will grind nearly 5 cwt. of hard kaolin or felspar gravel, in 24 hours, into a
proper pap.
To the preceding methodical account of the porcelain manufacture, I shall now subjoin
some practical details relative to certain styles of work, with comparisons between
the methods pursued in this country and upon the Continent, but chiefly by our jealous
rivals the French.
The blue printed ware of England has been hitherto a hopeless object of emulation in
France. M. Alexandre Brongniart, membre de l’Institut, and director of the Manufacture
Royal de Sèvres, characterizes the French imitations of the Fayence fine, ou
Anglaise, in the following terms: “Les défauts de cette poterie, qui tiennent à sa
nature, sont de ne pouvoir aller sur le feu pour les usages domestiques, et d’avoir un
vernis tendre, qui se laisse aisément entamer par les instruments d’acier et de fer.
Mais lorsque cette poterie est mal fabriquée, ou fabriquée avec une économie mal
entendue, ses défauts deviennent bien plus graves; son vernis jaunâtre et tendre tressaille
souvent; il se laisse entamer ou user avec la plus grande facilité par les instruments
de fer, ou par l’usage ordinaire. Les fissures que ce tressaillement ou ces
rayures ouvrent dans le vernis permettent aux matières grasses de pénétrer dans le biscuit,
que dans les poteries affectées de ce défaut, a presque toujours une texture lâche; les
pièces se salissent, s’empuantissent, et se brisent même avec la plus grande facilité.”[42]
What a glaze, to be scratched or grooved with soft iron; to fly off in scales, so as to
let grease soak into the biscuit or body of the ware; to become foul, stink, and break with
the utmost ease! The refuse crockery of the coarsest pottery works in the United
Kingdom would hardly deserve such censure.
In the minutes of evidence of the Enquête Ministérielle, published in 1835, MM. de Saint
Cricq and Lebeuf, large manufacturers of pottery-ware at Creil and Montereau, give a very
gratifying account of the English stoneware manufacture. They declare that the English
possess magnificent mines of potter’s clay, many leagues in extent; while those of the[1029]
French are mere patches or pots. Besides, England, they say, having upwards of 200
potteries, can constantly employ a great many public flint-mills, and thereby obtain that
indispensable material of the best quality, and at the lowest rate. “The mill erected by
M. Brongniart, at Sèvres, does its work at twice the price of the English mills. The
fuel costs in England one-fourth of what it does in France. The expense of a kiln-round,
in the latter country, is 200 francs; while in the former it is not more than 60.”
After a two-months tour among the English potteries, these gentlemen made the following
additional observations to their first official statement:—
“The clay, which goes by water carriage from the counties of Devon and Dorset, into
Staffordshire, to supply more than 200 potteries, clustered together, is delivered to them
at a cost of 4 francs (3s. 2d.) the 100 kilogrammes (2 cwt.); at Creil, it costs 4f. 50c.,
and at Montereau, only 2f. 40c. There appears, therefore, to be no essential difference
in the price of the clay; but the quality of the English is much superior, being incontestably
whiter, purer, more homogeneous, and not turning red at a high heat, like the
French.” The grinding of the flints costs the English potter 41⁄2d. per 100 kilos., and the
French 6d.; but as that of the latter is in general ground dry, it is a coarser article. The
kaolin, or china clay, is imported from Cornwall for the use of many French potteries;
but the transport of merchandise is so ill managed in France, that while 2 cwts. cost in
Staffordshire only 8f. 75c. (about 7s. 1d.), they cost 12f. at Creil, and 13f. 50c. at
Montereau. The white lead and massicot, so much employed for glazes, are 62 per cent.
dearer to the French potters than the English. As no French mill has succeeded in
making unsized paper fit for printing upon stoneware, our potters are under the
necessity of fetching it from England; and, under favour of our own custom-house, are
allowed to import it at a duty of 165f. per 100 kilogrammes, or about 8d. per pound
English. No large stock of materials need be kept by the English, because every
article may be had when wanted from its appropriate wholesale dealers; but the case is
quite different with the French, whose stocks, even in small works, can never safely be
less in value than 150,000f. or 200,000f.; constituting a loss to them, in interest upon
their capital, of from 7,500f. to 10,000f. per annum. The capital sunk in buildings is
far less in England than in France, in consequence of the different styles of erecting stoneware
factories in the two countries. M. de Saint Cricq informs us, that Mr. Clewes, of
Shelton, rents his works for 10,000f. (380l.) per annum; while the similar ones of Creil
and Montereau, in France, have cost each a capital outlay of from 500,000f. to 600,000f.,
and in which the products are not more than one half of Mr. Clewes’. “This forms a
balance against us,” says M. St. C., “of about 20,000f. per annum; or nearly 800l.
sterling. Finally, we have the most formidable rival to our potteries in the extreme
dexterity of the English artisans. An enormous fabrication permits the manufacturers
to employ the same workmen during the whole year upon the same piece; thus I have
seen at Shelton a furnisher, for sixpence, turn off 100 pieces, which cost at Creil and
Montereau 30 sous (1s. 21⁄2d.); yet the English workman earns 18f. 75c. a week, while
the French never earns more than 15f. I have likewise seen an English moulder
expert enough to make 25 waterpots a day, which, at the rate of 2d. a piece, bring him
4s. 2d. of daily wages; while the French moulder, at daily wages also of 4s. 2d., turns
out of his hands only 7, or at most 8 pots. In regard to hollow wares, the English may
be fairly allowed to have an advantage over us, in the cost of labour, of 100 per cent.;
which they derive from the circumstance, that there are in Staffordshire 60,000 operatives,
men, women, and children, entirely dedicated to the stoneware manufacture;
concentrating all their energies within a space of 10 square leagues. Hence a most
auspicious choice of good practical potters, which cannot be found in France.”
M. Saint Amans, a French gentleman, who spent some years in Staffordshire, and has
lately erected a large pottery in France, says the English surpass all other nations in
manufacturing a peculiar stoneware, remarkable for its lightness, strength, and elegance;
as also in printing blue figures upon it of every tint, equal to that of the Chinese, by
processes of singular facility and promptitude. After the biscuit is taken out of the
kiln, the fresh impression of the engraving is transferred to it from thin unsized paper,
previously immersed in strong soap water; the ink for this purpose being a compound
of arseniate of cobalt with a flux, ground up with properly boiled linseed oil. The
copper-plates are formed by the graving tool with deeper or shallower lines according to
the variable depth of shades in the design. The cobalt pigment, on melting, spreads so
as to give the soft effect of water-colour drawing. The paper being still moist, is
readily applied to the slightly rough and adhesive surface of the biscuit, and may be
rubbed on more closely by a dossil of flannel. The piece is then dipped in a tub of
water, whereby the paper gets soft, and may be easily removed, leaving upon the pottery
the pigment of the engraved impression. After being gently dried, the piece is dipped
into the glaze mixture, and put into the enamel oven.
[1030]
Composition of the Earthy Mixtures.
The basis of the English stoneware is, as formerly stated, a bluish clay, brought from
Dorsetshire and Devonshire, which lies at the depth of from 25 to 30 feet beneath the
surface. It is composed of about 24 parts of alumina, and 76 of silica, with some other
ingredients in very small proportions. This clay is very refractory in high heats, a
property which, joined to its whiteness when burned, renders it peculiarly valuable for
pottery. It is also the basis of all the yellow biscuit-ware called cream colour, and in
general of what is called the printing body; as also for the semi-vitrified porcelain of
Wedgewood’s invention, and of the tender porcelain.
The constituents of the stoneware are, that clay, the powder of calcined flints, and of the
decomposed felspar called Cornish stone. The proportions are varied by the different
manufacturers. The following are those generally adopted in one of the principal
establishments of Staffordshire:—
| For cream colour, |
Silex or ground flints |
20 |
parts. |
| |
Clay |
100 |
|
| |
Cornish stone |
2 |
|
Composition of the Paste for receiving the Printing Body under the Glaze.
For this purpose the proportions of the flint and the felspar must be increased. The
substances are mixed separately with water into the consistence of a thick cream, which
weighs per pint, for the flints 32 ounces, and for the Cornish stone 28. The china clay
of Cornwall is added to the same mixture of flint and felspar, when a finer pottery or
porcelain is required. That clay cream weighs 24 ounces per pint. These 24 ounces
in weight are reduced to one-third of their bulk by evaporation. The pint of dry
Cornish clay weighs 17 ounces, and in its first pasty state 24, as just stated. The dry
flint powder weighs 141⁄2 ounces per pint; which when made into a cream weighs 32
ounces. To 40 measures of Devonshire clay-cream there are added,
| 13 |
measures of |
flint liquor. |
| 12 |
— |
Cornish clay ditto. |
| 1 |
— |
Cornish stone ditto. |
The whole are well mixed by proper agitation, half dried in the troughs of the slip-kiln,
and then subjected to the machine for cutting up the clay into junks. The above paste,
when baked, is very white, hard, sonorous, and susceptible of receiving all sorts of
impressions from the paper engravings. When the silica is mixed with the alumina in
the above proportions, it forms a compact ware, and the impression remains fixed
between the biscuit and the glaze, without communicating to either any portion of the
tint of the metallic colour employed in the engraver’s press. The felspar gives strength
to the biscuit, and renders it sonorous after being baked; while the china clay has the
double advantage of imparting an agreeable whiteness and great closeness of grain.
PRECIPITATE, is any matter separated in minute particles from the bosom of a
fluid, which subsides to the bottom of the vessel in a pulverulent form.
PRECIPITATION, is the actual subsidence of a precipitate.
PRESS, HYDRAULIC. Though the explanation of the principles of this powerful
machine belongs to a work upon mechanical engineering, rather than to one upon[1031]
manufactures, yet as it is often referred to in this volume, a brief description of it cannot
be unacceptable to many of my readers.
The framing consists of two stout cast-iron plates a, b, which are strengthened by projecting
ribs, not seen in the section, fig. 909. The top or crown plate b, and the base-plate
a, a, are bound most firmly together by 4 cylinders of the best wrought iron, c, c,
which pass up through holes near the ends of the said plates, and are fast wedged in
them. The flat pieces e, e, are screwed to the ends of the crown and base plates, so as
to bind the columns laterally. f, is the hollow cylinder of the press, which, as well as
the ram g, is made of cast iron. The upper part of the cavity of the cylinder is cast
narrow, but is truly and smoothly rounded at the boring-mill, so as to fit pretty closely
round a well-turned ram or piston; the under part of it is left somewhat wider
in the casting. A stout cup of leather, perforated in the middle, is put upon the ram,
and serves as a valve to render the neck of the cylinder perfectly water-tight, by filling
up the space between it and the ram; and since the mouth of the cup is turned downwards,
the greater the pressure of water upwards, the more forcibly are the edges of the
leather valve pressed against the inside of the cylinder, and the tighter does the joint
become. This was Bramah’s beautiful invention.
Upon the top of the ram, the press-plate or table, strengthened with projecting ridges,
rests, which is commonly called the follower, because it follows the ram closely in its
descent. This plate has a half-round hole at each
of its four corners, corresponding to the shape of
the four iron columns along which it glides in its
up-and-down motions of compression and relaxation.
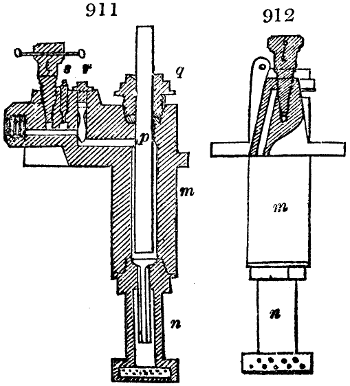
k, k, figs. 909. and 910., is the framing of a
force pump with a narrow barrel; i is the well for
containing water to supply the pump. To spare
room in the engraving, the pump is set close to the
press, but it may be removed to any convenient
distance by lengthening the water-pipe u, which
connects the discharge of the force pump with the
inside of the cylinder of the press. Fig. 911. is
a section of the pump and its valves. The pump
m, is of bronze; the suction-pipe n, has a conical
valve with a long tail; the solid piston or plunger
p, is smaller than the barrel in which it plays, and
passes at its top through a stuffing-box q; r is the pressure-valve, s is the safety-valve,
which, in fig. 910., is seen to be loaded with a weighted lever; t is the discharge-valve,
for letting the water escape, from the cylinder beneath the ram, back
into the well. See the winding passages in fig. 912. u is the tube which conveys
the water from the pump into the press-cylinder. In fig. 910. two centres of motion
for the pump-lever are shown. By shifting the bolt into the centre nearest the pump-rod,
the mechanical advantage of the workman may be doubled. Two pumps are
generally mounted in one frame for one hydraulic press; the larger to give a rapid
motion to the ram at the beginning, when the resistance is small; the smaller to give
a slower but more powerful impulsion, when the resistance is much increased. A pressure
of 500 tons may be obtained from a well-made hydraulic press with a ten-inch
ram, and a two and a one inch set of pumps. See Stearine Press.
PRINCE’S METAL, or Prince Rupert’s metal, is a modification of brass.
PRINTING INK. (Encre d’imprimerie, Fr.; Buchdruckerfarbe, Germ.) After
reviewing the different prescriptions given by Moxon, Breton, Papillon, Lewis, those in
Nicholson’s and the Messrs. Aikins’ Dictionaries, in Rees’ Cyclopædia, and in the French
Printer’s Manual, Mr. Savage[43] says, that the Encyclopædia Britannica is the only
work, to his knowledge, which has given a recipe by which a printing ink might be
made, that could be used, though it would be of inferior quality, as acknowledged by
the editor; for it specifies neither the qualities of the materials, nor their due proportions.
The fine black ink made by Mr. Savage, has, he informs us, been pronounced
by some of our first printers to be unrivalled; and has procured for him
the large medal from the Society for the Encouragement of Arts.
1. Linseed oil.—Mr. S. says, that the linseed oil, however long boiled, unless set
fire to, cannot be brought into a proper state for forming printing ink; and that the
flame may be most readily extinguished by the application of a pretty tight tin cover to
the top of the boiler, which should never be more than half full. The French prefer
nut oil to linseed; but if the latter be old, it is fully as good, and much cheaper, in this
country at least.
2. Black rosin is an important article in the composition of good ink; as by melting[1032]
it in the oil, when that ingredient is sufficiently boiled and burnt, the two combine,
and form a compound approximating to a natural balsam, like that of Canada, which
is itself one of the best varnishes that can be used for printing ink.
3. Soap.—This is a most important ingredient in printer’s ink, which is not even
mentioned in any of the recipes prior to that in the Encyclopædia Britannica. For
want of soap, ink accumulates upon the face of the types, so as completely to clog them
up after comparatively few impressions have been taken; it will not wash off without
alkaline lyes, and it skins over very soon in the pot. Yellow rosin soap is the best for
black inks; for those of light and delicate shades, white curd soap is preferable. Too
much soap is apt to render the impression irregular, and to prevent the ink from drying
quickly. The proper proportion has been hit, when the ink works clean, without
clogging the surface of the types.
4. Lamp black.—The vegetable lamp black, sold in firkins, takes by far the most
varnish, and answers for making the best ink. See Black.
5. Ivory black is too heavy to be used alone as a pigment for printing ink; but it
may be added with advantage by grinding a little of it upon a muller with the lamp
black, for certain purposes; for instance, if an engraving on wood is required to be
printed so as to produce the best possible effect.
6. Indigo alone, or with an equal weight of prussian blue, added in small proportion,
takes off the brown tone of certain lamp-black inks. Mr. Savage recommends a little
Indian red to be ground in with the indigo and prussian blue, to give a rich tone to the
black ink.
7. Balsam of capivi, as sold by Mr. Allen, Plough-court, Lombard-street, mixed,
by a stone and a muller, with a due proportion of soap and pigment, forms an extemporaneous
ink, which the printer may employ very advantageously when he wishes to
execute a job in a peculiarly neat manner. Canada balsam does not answer quite so
well.
After the smoke begins to rise from the boiling oil, a bit of burning paper stuck in
the cleft end of a long stick, should be applied to the surface, to set it on fire, as soon as
the vapour will burn; and the flame should be allowed to continue (the pot being
meanwhile removed from over the fire, or the fire taken from under the pot,) till a
sample of the varnish, cooled upon a pallet-knife, draws out into strings of about half an
inch long between the fingers. To six quarts of linseed oil thus treated, six pounds of
rosin should be gradually added, as soon as the froth of the ebullition has subsided.
Whenever the rosin is dissolved, one pound and three quarters of dry brown soap, of the
best quality, cut into slices, is to be introduced cautiously, for its water of combination
causes a violent intumescence. Both the rosin and soap should be well stirred with the
spatula. The pot is to be now set upon the fire, in order to complete the combination
of all the constituents.
Put next of well ground indigo and prussian blue, each 21⁄2 ounces, into an earthen
pan, sufficiently large to hold all the ink, along with 4 pounds of the best mineral
lamp black, and 31⁄2 pounds of good vegetable lamp black; then add the warm varnish
by slow degrees, carefully stirring, to produce a perfect incorporation of all the ingredients.
This mixture is next to be subjected to a mill, or slab and muller, till it be
levigated into a smooth uniform paste.
One pound of a superfine printing ink may be made by the following recipe of Mr.
Savage:—Balsam of capivi, 9 oz.; lamp black, 3 oz.; indigo and prussian blue,
together, p. æq. 11⁄4 oz.; Indian red, 3⁄4 oz.; turpentine (yellow) soap, dry, 3 oz. This
mixture is to be ground upon a slab, with a muller, to an impalpable smoothness. The
pigments used for coloured printing inks are, carmine, lakes, vermillion, red lead,
Indian red, Venetian red, chrome yellow, chrome red or orange, burnt terra di Sienna,
gall-stone, Roman ochre, yellow ochre, verdigris, blues and yellows mixed for greens,
indigo, prussian blue, Antwerp blue, lustre, umber, sepia, browns mixed with Venetian
red, &c.
PRINTING MACHINE. (Typographie mécanique, Fr.; Druckmaschine, Germ.)
In reviewing those great eras of national industry, when the productive arts, after a long
period of irksome vassalage, have suddenly achieved some new conquest over the inertia
of matter, the contemplative mind cannot fail to be struck with the insignificant part
which the academical philosopher has generally played in such memorable events.
Engrossed with barren syllogisms, or equational theorems, often little better than
truisms in disguise, he nevertheless believes in the perfection of his attainments, and
disdains to soil his hands with those handicraft operations at which all improvements in
the arts must necessarily begin. He does not deem manufacture worthy of his regard,
till it has worked out its own grandeur and independence with patient labour and consummate
skill. In this spirit the men of speculative science neglected for 60 years the
steam engine of Newcomen, till the artisan Watt transformed it into an automatic prodigy;
they have never deigned to illustrate by dynamical investigations the factory mechanisms[1033]
of Arkwright, yet nothing in the whole compass of art deserves it so well; and though
perfectly aware that revolvency is the leading law in the system of the universe, they
have never thought of showing the workman that this was also the true principle of
every automatic machine.
These remarks seem to be peculiarly applicable to book-printing, an art invented for
the honour of learning and the glory of the learned, though they have done nothing for
its advancement; yet by the overruling bounty of Providence it has eventually served as
the great teacher and guardian of the whole family of man.
It has been justly observed by Mr. Cowper, in his ingenious lecture,[44] that no improvement
had been introduced in this important art, from its invention till the year 1798, a
period of nearly 350 years. In Dr. Dibdin’s interesting account of printing, in the
Bibliographical Decameron, may be seen representations of the early printing-presses,
which exactly resemble the wooden presses in use at the present day. A new era has,
however, now arrived, when the demands for prompt circulation of political intelligence
require powers of printing newspapers beyond the reach of the most expeditious
hand presswork.
For the first essential modification of the old press, the world is indebted to the late
Earl Stanhope.[45] His press is formed of iron, without any wood; the table upon which
the form of types is laid, as well as the platen or surface which immediately gives the
impression, is of cast iron, made perfectly level; the platen being large enough to
print a whole sheet at one pull. The compression is applied by a beautiful combination
of levers, which give motion to the screw, cause the platen to descend with progressively
increasing force till it reaches the type, when the power approaches the maximum;
upon the infinite lever principle, the power being applied to straighten an obtuse-angled
jointed lever. This press, however, like all its flat-faced predecessors, does not act by a
continuous, but a reciprocating motion, and can hardly be made automatic; nor does
it much exceed the old presses in productiveness, since it can turn off only 250 impressions
per hour.
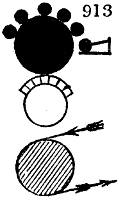
Nicholson’s for
arched type.
Nicholson’s for
common type.

Nicholson’s for
arched type.

Nicholson’s for
common type.
The first person who publicly projected a self-acting printing-press, was Mr.
William Nicholson, the able editor of the Philosophical Journal, who obtained a patent
in 1790-1, for imposing types upon a cylindrical surface; this disposition of types,
plates, and blocks, being a new invention (see fig. 913.); 2, for applying the ink upon
the surface of the types, &c., by causing the surface of a cylinder smeared with the
colouring-matter to roll over them; or else causing the types to apply themselves to
the said cylinder. For the purpose of spreading the ink evenly over this cylinder, he
proposed to apply three or more distributing
rollers longitudinally against the inking cylinder,
so that they might be turned by the
motion of the latter. 3. “I perform,” he
says, “all my impressions by the action of a
cylinder, or cylindrical surface; that is, I
cause the paper to pass between two cylinders,
one of which has the form of types attached
to it, and forming part of its surface; and
the other is faced with cloth, and serves to
press the paper so as to take off an impression
of the colour previously applied; or
otherwise I cause the form of types, previously coloured, to pass in close and successive
contact with the paper wrapped round a cylinder with woollen.” (See figs. 913. and
914.)[46]
In this description Mr. Nicholson indicates pretty plainly the principal parts of
modern printing machines; and had he paid the same attention to any one part of his
invention which he fruitlessly bestowed upon attempts to attach types to a cylinder, or
had he bethought himself of curving stereotype plates, which were then beginning to be
talked of, he would in all probability have realized a working apparatus, instead of
scheming merely ideal plans.
The first operative printing machine was undoubtedly contrived by, and constructed
under the direction of, M. König, a clockmaker from Saxony, who, so early as the year
1804, was occupied in improving printing-presses. Having failed to interest the continental
printers in his views, he came to London soon after that period, and submitted
his plans to Mr. T. Bensley, our celebrated printer, and to Mr. R. Taylor, now one of
the editors of the Philosophical Magazine.
[1034]
These gentlemen afforded Mr. König and his assistant Bauer, a German mechanic,
liberal pecuniary support. In 1811, he obtained a patent for a method of working a common
hand-press by power; but after much expense and labour he was glad to renounce
the scheme. He then turned his mind to the use of a cylinder for communicating the
pressure, instead of a flat plate; and he finally succeeded, sometime before the 28th
November 1814, in completing his printing automaton; for on that day the editors of
the Times informed their readers that they were perusing for the first time a newspaper
printed by steam-impelled machinery; it is a day, therefore, which will be ever memorable
in the annals of typography.
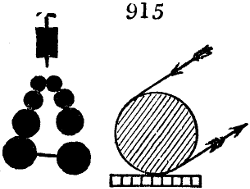
König’s single, for one
side of the sheet.
In that machine the form of type was made to traverse horizontally under the pressure
cylinder, with which the sheet of paper was held in close embrace by means of a series
of endless tapes. The ink was placed in a cylindrical box,
from which it was extruded by means of a powerful screw, depressing
a well-fitted piston; it then fell between two iron
rollers, and was by their rotation transferred to several other
subjacent rollers, which had not only a motion round their
axes, but an alternating traverse motion (endwise). This
system of equalizing rollers terminated in two which applied
the ink to the types. (See fig. 915). This plan of inking evidently
involved a rather complex mechanism, was hence difficult
to manage, and sometimes required two hours to get into good working trim. It has
been superseded by a happy invention of Mr. Cowper, to be presently described.
In order to obtain a great many impressions rapidly from the same form, a paper-conducting
cylinder (one embraced by the paper) was mounted upon each side of
the inking apparatus, the form being made to traverse under both of them. This
double-action machine threw off 1100 impressions per hour when first finished; and
by a subsequent improvement, no less than 1800.
König’s double, for both sides of the sheet.
Mr. König’s next feat was the construction of a machine for printing both sides of
the newspaper at each complete traverse
of the forms. This resembled
two single machines, placed with their
cylinders towards each other, at a distance
of two or three feet; the sheet
was conveyed from one paper cylinder
to another, as before, by means of tapes;
the track of the sheet exactly resembled
the letter S laid horizontally, thus,  ;
and the sheet was turned over or reversed in the course of its passage. At the first
paper cylinder it received the impression from the first form, and at the second it
received it from the second form; whereby the machine could print 750 sheets of book
letter-press on both sides in an hour. This new register apparatus was erected for Mr.
T. Bensley, in the year 1815, being the only machine made by Mr. König for printing
upon both sides. See fig. 916.
;
and the sheet was turned over or reversed in the course of its passage. At the first
paper cylinder it received the impression from the first form, and at the second it
received it from the second form; whereby the machine could print 750 sheets of book
letter-press on both sides in an hour. This new register apparatus was erected for Mr.
T. Bensley, in the year 1815, being the only machine made by Mr. König for printing
upon both sides. See fig. 916.
Donkin and Bacon’s
for type.
Messrs. Donkin and Bacon had for some years previous to this date been busily
engaged with printing machines, and had indeed, in 1813, obtained a patent for an
apparatus, in which the types were placed upon the sides of a revolving
prism; the ink was applied by a roller, which rose and
fell with the eccentricities of the prismatic surface, and the sheet
was wrapped upon another prism fashioned so as to coincide with
the eccentricities of the type prism. One such machine was
erected for the University of Cambridge. (See fig. 917.) It was a
beautiful specimen of ingenious contrivance and good workmanship.
Though it was found to be too complicated for common
operatives, and defective in the mechanism of the inking process;
yet it exhibited for the first time the elastic inking rollers, composed
of glue combined with treacle, which alone constitute one of the finest inventions
of modern typography. In König’s machine the rollers were of metal covered with
leather, and never answered their purpose very well.
Before proceeding further, I may state that the above elastic composition, which
resembles caoutchouc not a little, but is not so firm, is made by dissolving with heat, in
two pounds of ordinary treacle, one pound of good glue, previously soaked during a
night in cold water.
Cowper’s single, for curved
stereotype.
Cowper’s double, for both sides of the
sheet.

Cowper’s single, for curved
stereotype.

Cowper’s double, for both sides of the
sheet.
In the year 1815, Mr. Cowper turned his scientific and inventive mind to the subject
of printing machines, and has since, in co-operation with his partner, Mr. Applegath,
carried them to an unlooked-for degree of perfection. In 1815 Mr. Cowper obtained
a patent for curving stereotype plates, for the purpose of fixing them on a cylinder[1035]
Several machines so mounted, capable of printing 1000 sheets per hour upon both
sides, are at work at the present day; twelve machines on this principle having been
made for the Directors
of the
Bank of England
a short time
previous to their
re-issuing gold.
See figs. 918.
and 919.
It deserves to be remarked here, that the same object seems to have occupied the
attention of Nicholson, Donkin, Bacon, and Cowper; viz., the revolution, of the form
of types. Nicholson sought to effect this by giving to the shank of a type a shape like the
stone of an arch; Donkin and Bacon by attaching types to the sides of a revolving
prism; and Cowper, more successfully, by curving a stereotype plate. (See fig. 918.)
In these machines Mr. Cowper places two paper cylinders side by side, and against
each of them a cylinder for holding the plates; each of these four cylinders is about two
feet in diameter. Upon the surface of the stereotype-plate cylinder, four or five inking
rollers of about three inches in diameter are placed; they are kept in their position by
a frame at each end of the said cylinder, and the axles of the rollers rest in vertical
slots of the frame, whereby having perfect freedom of motion, they act by their gravity
alone, and require no adjustment.
The frame which supports the inking rollers, called the waving-frame, is attached by
hinges to the general framework of the machine; the edge of the stereotype-plate cylinder
is indented, and rubs against the waving-frame, causing it to vibrate to and fro, and
consequently to carry the inking rollers with it, so as to give them an unceasing traverse
movement. These rollers distribute the ink over three-fourths of the surface of the
cylinder, the other quarter being occupied by the curved stereotype plates. The ink is
contained in a trough, which stands parallel to the said cylinder, and is formed by a
metal roller revolving against the edge of a plate of iron; in its revolution it gets
covered with a thin film of ink, which is conveyed to the plate-cylinder by a distributing
roller vibrating between both. The ink is diffused upon the plate cylinder as before
described; the plates in passing under the inking rollers become charged with the
coloured varnish; and as the cylinder continues to revolve, the plates come into contact
with a sheet of paper on the first paper cylinder, which is then carried by means of tapes
to the second paper cylinder, where it receives an impression upon its opposite side from
the plates upon the second cylinder.
Thus the printing of the sheet is completed. Though the above machine be applicable
only to stereotype plates, it has been of general importance, because it formed the
foundation of the future success of Messrs. Cowper and Applegath’s printing machinery,
by showing them the best method of serving out, distributing, and applying the
coloured varnish to the types.
In order to adapt this method of inking to a flat type-form machine, it was merely
requisite to do the same thing upon an extended flat surface or table, which had
been performed upon an extended cylindrical surface. Accordingly, Messrs. Cowper
and Applegath constructed a machine for printing both sides of the sheet from type,
including the inking apparatus, and the mode of conveying the sheet from the one
paper cylinder to the other, by means of drums and tapes. It is highly creditable to
the scientific judgment of these patentees, that in new modelling the printing machine,
they dispensed with forty wheels, which existed in Mr. König’s apparatus, when Mr.
Bensley requested them to apply their improvements to it.
Cowper’s inking
table and roller.
The distinctive advantages of these machines, and which have not hitherto been
equalled, are the uniform distribution of the ink, the equality as well as delicacy
with which it is laid upon the types, the diminution in its expenditure, amounting to
one half upon a given quantity of letter-press, and the facility with
which the whole mechanism is managed. The band inking-roller,
and distributing-table, now so common in every printing-office in Europe
and America, is the invention of Mr. Cowper, and was specified
in his patent. The vast superiority of the inking apparatus in his machines,
over the balls used of old, induced him to apply it forthwith
to the common press, and most successfully for the public; but with
little or no profit to the inventor, as the plan was unceremoniously infringed
throughout the kingdom, by such a multitude of printers,
whether rich or poor, as to render all attempts at reclaiming his rights
by prosecution hopeless. See fig. 920.
Applegath and Cowper’s single.
Applegath and Cowper’s double.

Applegath and Cowper’s single.

Applegath and Cowper’s double.
To construct a printing machine which shall throw off two sides at a
time with exact register, that is, with the second side placed precisely upon the back of the[1036]
first, is a very difficult problem, which was first practically solved by Messrs. Applegath
and Cowper. It is comparatively easy to make a machine which shall print the one side
of a sheet of paper first, and then the other side, by the removal of one form, and the
introduction of another; and thus far did Mr. König advance. A correct register
requires the sheet, after it has received its first impression from one cylinder, to travel
round the peripheries of the cylinders and drums, at such a rate as to meet the types
of the second side at the exact point which will ensure this side falling with geometrical
nicety upon the back of the first. For this purpose, the cylinders and drums
must revolve at the very same speed as the carriage underneath; hence the least incorrectness
in the workmanship will produce such defective typography as will not be
endured in book-printing at the present day, though it may be tolerated in newspapers.
An equable distribution of the ink is of no less importance to beautiful letter-press. See
figs. 921. 922.
The machines represented in figs. 923, 924, 925, are different forms of those which have
been patented by Messrs. Applegath and Cowper. That shown in figs. 923. and 925. prints
both sides of the sheet during its passage, and is capable of throwing off nearly 1000
finished sheets per hour. The moistened quires of blank paper being piled upon a
table A, the boy, who stands on the adjoining platform, takes up one sheet after another,
and lays them upon the feeder B, which has several linen girths passing across its surface,
and round a pulley at each end of the feeder; so that whenever the pulleys begin
to revolve, the motion of the girths carries forward the sheet, and delivers it over the
entering roller E, where it is embraced between two series of endless tapes, that pass
round a series of tension rollers. These tapes are so placed as to fall partly between,
and partly exterior to, the pages of the printing; whereby they remain in close contact
with the sheet of paper on both of its sides during its progress through the machine. The
paper is thus conducted from the first printing cylinder F, to the second cylinder G,
without having the truth of its register impaired, so that the coincidence of the two
pages is perfect. These two great cylinders, or drums, are made of cast iron, turned perfectly
true upon a self-acting lathe;[47] they are clothed in these parts, corresponding to the
typographic impression, with fine woollen cloth, called blankets by the pressmen, and
revolve upon powerful shafts which rest in brass bearings of the strong framing of the
machine. These bearings, or plummer blocks, are susceptible of any degree of adjustment,
by set screws. The drums H and I are made of wood; they serve to conduct the
sheet evenly from the one printing cylinder to the other.
One series of tapes commences at the upper part of the entering drum E, proceeds in
contact with the right-hand side and under surface of the printing cylinder F, passes[1037]
next over the carrier-drum H, and under the carrier-drum I; then encompassing the
left-hand side and under portion of the printing drum G, it passes in contact with the
small tension rollers a, b, c, d, fig. 925., and finally arrives at the roller E, which may
be called the commencement of the one series of endless tapes. The other series may
be supposed to commence at the roller h; it has an equal number of tapes, and corresponds
with the former in being placed upon the cylinders so that the sheets of paper
may be held securely between them. This second series descends from the roller h,
fig. 925., to the entering drum E, where it meets and coincides with the first series in
such a way that both sets of tapes proceed together under the printing cylinder F, over H,
under I, and round G, until they arrive at the roller i, fig. 923., where they separate, after
having continued in contact, except at the places where the sheets of paper are held
between them. The tapes descend from the roller i, to a roller at k, and, after passing
in contact with rollers at l, m, n, they finally arrive at the roller h, where they were
supposed to commence. Hence two series of tapes act invariably in contact, without
the least mutual interference, as may be seen by inspection of the figs. 923, 924, 925.
The various cylinders and drums revolve very truly by means of a system of toothed
wheels and pinions mounted at their ends. Two horizontal forms of types are laid at
a certain distance apart upon the long carriage M, adjoining to each of which there is a
flat metallic plate, or inking table, in the same plane. The common carriage, bearing its
two forms of type and two inking tables, is moved backwards and forwards, from one
end of the printing machine to the other, upon rollers attached to the frame-work, and
in its traverse brings the types into contact with the sheet of paper clasped by the tapes
round the surfaces of the printing cylinders. This alternate movement of the carriage
is produced by a pinion working alternately into the opposite sides of a rack under the
table. The pinion is driven by the bevel wheels K.
The mechanism for supplying the ink, and distributing it over the forms, is one of the
most ingenious and valuable inventions belonging to this incomparable machine, and is
so nicely adjusted, that a single grain of the pigment may suffice for printing one side
of a sheet. Two similar sets of inking apparatus are provided; one at each end of the
machine, adapted to ink its own form of type. The metal roller L, called the ductor
roller, as it draws out the supply of ink, has a slow rotatory motion communicated to it
by a catgut cord, which passes round a small pulley upon the end of the shaft of the
printing cylinder G. A horizontal plate of metal, with a straight-ground edge, is
adjusted by set screws, so as to stand nearly in contact with the ductor roller. This plate
has an upright ledge behind, converting it into a sort of trough or magazine, ready to
impart a coating of ink to the roller, as it revolves over the table. Another roller,
covered with elastic composition (see suprà), called the vibrating roller, is made to travel
between the ductor roller and the inking table; the vibrating roller, as it rises, touches
the ductor roller for an instant, abstracts a film of ink from it, and then descends to
transfer it to the table. There are 3 or 4 small rollers of distribution, placed somewhat
diagonally across the table at M, (inclined only 2 inches from a parallel to the end of the
frame,) furnished with long slender axles, resting in vertical slots, whereby they are
left at liberty to revolve and to traverse at the same time; by which compound movement
they are enabled to efface all inequality in the surface of the varnish, or to effect a perfect[1038]
distribution of the ink along the table. The table thus evenly smeared, being made
to pass under the 3 or 4 proper inking rollers N, fig. 924., imparts to them an uniform[1039]
film of ink, to be immediately transferred by them to the types. Hence each time that
the forms make a complete traverse to and fro, which is requisite for the printing of
every sheet, they are touched no less than eight times by the inking rollers. Both
the distributing and inking rollers turn in slots, which permit them to rise and fall so
as to bear with their whole weight upon the inking table and the form, whereby they
never stand in need of any adjustment by screws, but are always ready for work when
dropped into their respective places.
Motion is given to the whole system of apparatus by a strap from a steam engine
going round a pulley placed at the end of the axle at the back of the frame; one
steam-horse power being adequate to drive two double printing machines; while a
single machine may be driven by the power of two men acting upon a fly-wheel. In
Messrs. Clowes’ establishment, in Stamford-street, two five-horse engines actuate nineteen
of the above described machines.
The operation of printing is performed as follows:—See fig. 926.
The sheets being carefully laid, one by one, upon the linen girths, at the feeder B, the
rollers C and D are made to move, by means of a segment wheel, through a portion of a
revolution. This movement carries on the sheet of paper sufficiently to introduce it between
the two series of endless tapes at the point where they meet each other upon the
entering drum E. As soon as the sheet is fairly embraced between the tapes, the rollers
C and D are drawn back, by the operation of a weight, to their original position, so as
to be ready to introduce another sheet into the machine. The sheet, advancing between
the endless tapes, applies itself to the blanket upon the printing cylinder F, and as it
revolves meets the first form of types, and receives their impression; after being thus
printed on one side, it is carried, over H and under I, to the blanket upon the printing
cylinder G, where it is placed in an inverted position; the printed side being now in
contact with the blanket, and the white side being outwards, meets the second form of
types at the proper instant, so as to receive the second impression, and get completely
printed. The perfect sheet, on arriving at the point i, where the two series of tapes
separate, is tossed out by centrifugal force into the hands of a boy.
The diagram, fig. 926. shows the arrangement of the tapes, agreeably to the preceding
description; the feeder B, with the
rollers C and D, is seen to have an independent
endless girth.
The diagram, fig. 927. explains the
structure of the great machine contrived
by Messrs. Applegath and Cowper for
printing the Times newspaper. Here
there are four places to lay on the sheets,
and four to take them off; consequently,
the assistance of eight lads is required.
P, P, P, P, are the four piles of paper;
F, F, F, F, are the four feeding-boards; E, E, E, E, are the four entering drums, upon which
the sheets are introduced between the tapes t, t, t, t, whence they are conducted to the[1040]
four printing cylinders 1, 2, 3, 4; T is the form of type; I, I, are two inking tables, of
which one is placed at each end of the form. The inking apparatus is similar to that
above described, with the addition of two central inking rollers R, which likewise
receive their ink from the inking tables. The printing cylinders 1, 2, 3, 4, are made to
rise and fall about half an inch; the first and third simultaneously, as also the second
and fourth. The form of type, in passing from A to B, prints sheets at 1 and 3; in
returning from B to A, it prints sheets at 4 and 2; while the cylinder alternately falls
to give the impression, and rises to permit the form to pass untouched.
Each of the lines marked t, consists of two endless tapes, which run in contact at the
parts shown, but separate at the entering drums E, and at the taking off parts o, o, o, o.
The return of the tapes to the entering drum is omitted in the diagram, to avoid confusion
of the lines.
The sheets of paper being laid upon their respective feeding-boards, with the fore
edges just in contact with the entering drum, a small roller, called the drop-down
roller, falls, at proper intervals, down upon the edges of the sheets; the drum and the
roller being then removed, instantly carry on the sheet, between the tapes t, downwards
to the printing cylinder, and thence upwards to o, o, o, o, where the tapes are parted, and
the sheet falls into the hands of the attendant boy. This noble mechanism is so perfectly
equipped, that it is generally in full work within four minutes after the form is
brought into the machine-room. The speed of König’s machine, by which the Times
was formerly printed, was such as to turn out 1800 papers per hour; that of Applegath
and Cowper throws off 4200 per hour, and it has been daily in use during eight
years.
PRUSSIAN BLUE, and PRUSSIATE OF POTASH, are two important
articles of chemical manufacture, which must be considered together. The first is
called by English chemists, Ferrocyanodide of iron, the Cyanure ferroso-ferrique of
Berzelius; Eisenblausaures eisenoxyd, or eisencyanür + eisencyanid, Germ.; the second
is called Ferrocyanodide of potassium, the Cyanure ferroso-potassique of Berzelius;
Eisencyanür-kalium, cyaneisen + cyankalium, or Blausaures eisenoxydul-kali, Germ.
Prussian blue (Berliner-blau, Germ.), is a chemical compound of iron and cyanogen.
When organic matters abounding in nitrogen, as dried blood, horns, hair, skins, or
hoofs of animals, are triturated along with potash in a strongly ignited iron pot, a dark
gray mass is obtained, that affords to water the liquor originally called lixivium sanguinis,
or blood-lye, which, by evaporation, yields lemon-coloured crystals in large rectangular
tables, bevelled at the edges. This salt is called in commerce, prussiate of potash,
and has for its ultimate constituents, potassium, iron, oxygen, and hydrogen, (the latter
two in such proportions as to form water), and the peculiar compound Cyanogen, the
blaustoff of the Germans.
These crystals consist, in 100 parts, of potassium 37·02, iron 12·82, cyanogen 37·40,
water 12·76; or, cyanide of potassium 61·96, cyanide of iron 25·28, and water 12·76.
They may be represented also by the following composition: 44·58 of potassa, 38·82 of
hydrocyanic or prussic acid, and 16·60 of oxide of iron, in 100 parts; but the first appears
to be their true chemical constitution. Dry ferrocyanodide of potassium, is a compound of,
one atom of cyanide of iron, 54 = (28 + 26), and 2 atoms of cyanide of potassium, 132, =
(26 × 2 + 40 × 2); the sum being 186; hydrogen being 1·0 in the scale of equivalents.
The crystals of prussiate of potash are nearly transparent, soft, of a sweetish saline and
somewhat bitterish taste, soluble in 4 parts of water at 52° F., and in 1 part of boiling
water, but insoluble in alcohol. They are permanent in the air at ordinary temperatures,
but in a moderately warm stove-room they part with 123⁄4 per cent. of water, without
losing their form or coherence, and become thereby a white friable anhydrous ferrocyanodide
of potassium, consisting of 42·44 potassium, 42·87 cyanogen, and 14·69 iron,
in 100 parts.
This salt is an excellent reagent for distinguishing metals from each other, as the
following Table of the precipitates which it throws down from their saline solutions
will show:—
| Metallic solutions. |
Colour of precipitate. |
| Antimony |
white. |
| Bismuth |
white. |
| Cadmium |
white, a little yellowish. |
| Cerium (protoxide) |
white, soluble in acids. |
| Cobalt |
green, soon turning reddish-gray. |
| Copper (protoxide) |
white, changing to red. |
| CoDo.r (peroxide) |
brown-red. |
| Iron (protoxide) |
white, rapidly turning blue. |
| IDo. (peroxide) |
dark blue. |
| Lead |
white, with a yellowish cast. |
| Manganese (protoxide) |
white, turning quickly peach or blood-red. |
| Manganese (deutoxide)[1041] |
greenish-gray. |
| Mercury (protoxide) |
white. |
| MeDo.ry(peroxide) |
white, turning blue. |
| Molybdenum |
dark brown. |
| Nickel (oxide) |
white, turning greenish. |
| Palladium (protoxide) |
green (gelatinous). |
| Silver |
white, turning brown in the light. |
| Tantalum |
yellow, dark burned colour. |
| Tin (protoxide) |
white, (gelatinous). |
| Do. (peroxide) |
yellow, perdo. |
| Uranium |
red-brown. |
| Zinc |
white. |
No precipitations ensue with solutions of the alkaline or earthy salts, except that of
yttria, which is white; nor with those of gold, platinum, rhodium, iridium, osmium,
(in concentrated solutions,) tellurium, chromium, tungstenium. All the precipitates
by the ferrocyanodide of iron, are double compounds of cyanide of iron with cyanide of
the metal thrown down, which is produced by the reciprocal decomposition of the
cyanide of potassium and the peculiar metallic oxide present in the solution. The precipitate
from the sulphate of copper has a fine brown colour, and has been used as a
pigment; but it is somewhat transparent, and therefore does not cover well. The precipitate
from the peroxide salts of iron is a very intense prussian blue, called on the
continent, Paris blue. It may be regarded as a compound of prussiate of protoxide and
prussiate of peroxide of iron; or as a double cyanide of the protoxide and peroxide of
iron, as the denomination cyanure ferroso-ferrique denotes. In numbers, its composition
may be therefore stated thus: prussic or hydrocyanic acid, 48·48; protoxide of iron, 20·73;
peroxide of iron, 30·79; or cyanogen, 46·71; iron, 37·36; water, 15·93; which represent
its constitution when it is formed by precipitation with the prussiate of potash or a
salt of iron that contains no protoxide. If the iron be but partially peroxidized in the
salt, it will afford a precipitate, at first pale blue, which turns dark blue in the air, consisting
of a mixture of prussiate of protoxide and prussiate of peroxide. In fact, the
white cyanide of iron (the prussiate of the pure protoxide), when exposed to the air in
a moist condition, becomes, as above stated, dark blue; yet the new combination formed
in this case through absorption of oxygen, is essentially different from that resulting
from the precipitation by the peroxide of iron, since it contains an excess of the peroxide
in addition to the usual two cyanides of iron. It has been therefore called basic prussian
blue, and, from its dissolving in pure water, soluble prussian blue.
Both kinds of prussian blue agree in being void of taste and smell, in attracting
humidity from the air when they are artificially dried, and being decomposed at a heat
above 348° F. The neutral or insoluble prussian blue is not affected by alcohol; the
basic, when dissolved in water, is not precipitated by that liquid. Neither is acted upon
by dilute acids; but they form with concentrated sulphuric acid a white pasty mass,
from which they are again reproduced by the action of cold water. They are decomposed
by strong sulphuric acid at a boiling heat, and by strong nitric acid at common
temperatures; but they are hardly affected by the muriatic. They become green with
chlorine, but resume their blue colour when treated with disoxidizing reagents. When
prussian blue is digested in warm water along with potash, soda, or lime, peroxide of
iron is separated, and a ferroprussiate of potash, soda, or lime remains in solution. If
the prussian blue has been previously purified by boiling in dilute muriatic acid, and
washing with water, it will afford by this treatment a solution of ferrocyanodide of potassium,
from which by evaporation this salt may be obtained in its purest crystalline
state. When the powdered prussian blue is diffused in boiling water, and digested with
red oxide of mercury, it parts with all its oxide of iron, and forms a solution of bi-cyanodide,
improperly called prussiate of mercury; consisting of 79·33 mercury, and 20·67
cyanogen; or, upon the hydrogen equivalent scale, of 200 mercury, and 52 = (26 × 2)
cyanogen. When this salt is gently ignited, it affords gaseous cyanogen. Hydrocyanic
or prussic acid, which consists of 1 atom of cyanogen = 26, + 1 of hydrogen = 1, is
prepared by distilling the mercurial bi-cyanide in a glass retort with the saturating
quantity of dilute muriatic acid. Prussic acid may also be obtained by precipitating the
mercury by sulphuretted hydrogen gas from the solution of its cyanide; as also by
distilling the ferrocyanide of potassium along with dilute sulphuric acid. Prussic acid
is a very volatile light fluid, eminently poisonous, and is spontaneously decomposed
by keeping, especially when somewhat concentrated.
Having expounded the chemical constitution of prussian blue and prussiate of potash,
I shall now treat of their manufacture upon the commercial scale.
1. Of blood-lye, the phlogisticated alkali of Scheele. Among the animal substances used
for the preparation of this lixivium, blood deserves the preference, where it can be had
cheap enough. It must be evaporated to perfect dryness, reduced to powder, and sifted.[1042]
Hoofs, parings of horns, hides, old woollen rags, and other animal offals, are, however,
generally had recourse to, as condensing most azotized matter in the smallest bulk. Dried
funguses have been also prescribed. These animal matters may either be first carbonized in
cast-iron cylinders, as for the manufacture of sal ammoniac (which see), and the residual
charcoal may be then taken for making the ferroprussiate; or the dry animal matters may
be directly employed. The latter process is apt to be exceedingly offensive to the workmen
and neighbourhood, from the nauseous vapours that are exhaled in it. Eight pounds
of horn (hoofs), or ten pounds of dry blood, afford upon an average one pound of charcoal.
This must be mixed well with good pearlash, (freed previously from most of the
sulphate of potassa, with which it is always contaminated,) either in the dry way, or by
soaking the bruised charcoal with a strong solution of the alkali; the proportion being
one part of carbonate of potassa to from 11⁄2 to 2 parts of charcoal, or to about eight
parts of hard animal matter. Gautier has proposed to calcine three parts of dry blood
with one of nitre; with what advantage to the manufacturer, I cannot discover.
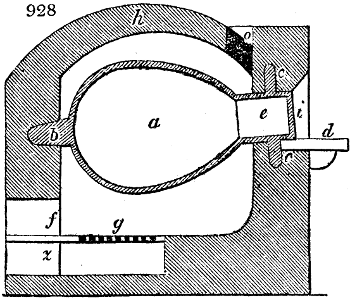
The pot for calcining the mixture of animal and alkaline matter is egg-shaped as
represented at a, fig. 928, and is considerably narrowed at the neck e, to facilitate the
closing of the mouth with a lid i. It is made of cast iron, about two inches thick in the
belly and bottom; this strength being requisite
because the chemical action of the materials
wears the metal fast away. It should
be built into the furnace in a direction sloping
downwards, (more than is shown in the figure,)
and have a strong knob b, projecting from
its bottom to support it upon the back wall,
while its shoulder is embraced at the arms c, c,
by the brickwork in front. The interior of
the furnace is so formed as to leave but a
space of a few inches round the pot, in order
to make the flame play closely over its whole
surface. The fire-door f, and the draught-hole
z, of the ash-pit, are placed in the posterior
part of the furnace, in order that the
workmen may not be incommoded by the heat. The smoke vent o, issues through the
arched top h of the furnace, towards the front, and is thence led backwards by a flue to
the main chimney of the factory. d is an iron or stone shelf, inserted before the mouth
of the pot, to prevent loss in shovelling out the semi-liquid paste. The pot may be
half filled with the materials.
The calcining process is different, according as the animal substances are fresh or
carbonized. In the first case, the pot must remain open, to allow of diligent stirring of
its contents, with a slightly bent flat iron bar or scoop, and of introducing more of the
mixture as the intumescence subsides, during a period of five or six hours, till the nauseous
vapours cease to rise, till the flame becomes smaller and brighter, and till a smell
of ammonia be perceived. At this time, the heat should be increased, the mouth of the pot
should be shut, and opened only once every half hour, for the purpose of working the
mass with the iron paddle. When on opening the mouth of the pot, and stirring the
pasty mixture, no more flame rises, the process is finished.
If the animal ingredients are employed in a carbonized state, the pot must be shut
as soon as its contents are brought to ignition by a briskly urged fire, and opened for a
few seconds only every quarter of an hour, during the action of stirring. At first, a
body of flame bursts forth every time that the lid is removed; but by degrees this
ceases, and the mixture soon agglomerates, and then softens into a paste. Though the
fire be steadily kept up, the flame becomes less and less each time that the pot is opened;
and when it ceases, the process is at an end. The operation, with a mass of 50 pounds of
charcoal and 50 pounds of purified pearlash, lasts about 12 hours, the first time that the
furnace is kindled; but when the pot has been previously brought to a state of ignition,
it takes only 7 or 8 hours. In a well-appointed factory, the fire should be invariably
maintained at the proper pitch, and the pots should be worked with relays of operatives.
The molten mass is now to be scooped out with an appropriate iron shovel, having a long
shank, and caused to cool in small portions, as quickly as possible; but not by throwing
it into water, as has sometimes been prescribed; for in this way a good deal of the cyanogen
is converted into ammonia. If it be heaped up and kept hot in contact with air,
some of the ferrocyanide is also decomposed, with diminution of the product. The
crude mass is to be then put into a pan with cold water, dissolved by the application of
a moderate heat, and filtered through cloths. The charcoal which remains upon the
filter possesses the properties of decolouring syrups, vinegars, &c., and of destroying
smells in a pre-eminent degree. It may also serve, when mixed with fresh animal coal,
for another calcining operation.
[1043]
As the iron requisite for the formation of the ferrocyanide is in general derived from
the sides of the pot, this is apt to wear out into holes, especially at its under side, where
the heat is greatest. In this event, it may be taken out of the furnace, patched up
with iron-rust cement, and re-inserted with the sound side undermost. The erosion of
the pot may be obviated in some measure by mixing iron borings or cinder (hammerschlag)
with the other materials, to the amount of one or two hundredths of the
potash.
The above lixivium is not a solution of pure ferroprussiate; it contains not a little
cyanide of potassium, which in the course of the process had not absorbed the proper
dose of iron to form a ferrocyanide; it contains also more or less carbonate of potash,
with phosphate, sulphate, hydrogenated sulphuret, muriate, and sulpho-cyanide of the same
base, as well as phosphate of lime; substances derived partly from the impure potash,
and partly from the incinerated animal matters. Formerly that very complex impure
solution was employed directly for the precipitation of prussian blue; but now, in all well
regulated works, it is converted by evaporation and cooling into crystallized ferroprussiate
of potash. The mother-water is again evaporated and crystallized, whereby a somewhat
inferior ferroprussiate is obtained. Before evaporating the lye, however, it is advisable
to add as much solution of green sulphate of iron to it, as will re-dissolve the white
precipitate of cyanide of iron which first falls, and thereby convert the cyanide of
potassium, which is present in the liquor, into ferrocyanide of potassium. The commercial
prussiate of potash may be rendered chemically pure by making its crystals
effloresce in a stove, fusing them with a gentle heat in a glass retort, dissolving the mass
in water, neutralizing any carbonate and cyanide of potash that may be present with
acetic acid, then precipitating the ferroprussiate of potash by the addition of a sufficient
quantity of alcohol, and finally crystallizing the precipitated salt twice over in water.
The sulphate of potassa may be decomposed by acetate of baryta, and the resulting
acetate of potassa removed by alcohol.
2. The precipitation of prussian blue.—Green sulphate of iron is always employed
by the manufacturer, on account of its cheapness, for mixing with solution of the ferroprussiate,
in forming prussian blue, though the red sulphate, nitrate, or muriate of iron
would afford a much richer blue pigment. Whatever salt of iron be preferred, should
be carefully freed from any cupreous impregnation, as this would give the pure blue a
dirty brownish cast. The green sulphate of iron is the most advantageous precipitant,
on account of its affording protoxide, to convert into ferrocyanide any cyanide of potassium
that may happen to be present in the uncrystallized lixivium. The carbonate
of potash in that lixivium might be saturated with sulphuric acid before adding the
solution of sulphate of iron; but it is more commonly done by adding a certain portion
of alum; in which case, alumina falls along with the prussian blue; and though it
renders it somewhat paler, yet it proportionally increases its weight; whilst the acid of
the alum saturates the carbonate of potash, and prevents its throwing down iron-oxide,
to degrade by its brown-red tint the tone of the blue. For every pound of pearlash
used in the calcination, from two to three pounds of alum are employed in the precipitation.
When a rich blue is wished for, the free alkali in the prussian lye may be partly
saturated with sulphuric acid, before adding the mingled solutions of copperas and
alum. One part of the sulphate of iron is generally allowed for 15 or 20 parts of dried
blood, and 2 or 3 of horn-shavings or hoofs. But the proportion will depend very
much upon the manipulations; which, if skilfully conducted, will produce more of the
cyanides of iron, and require more copperas to neutralize them. The mixed solutions
of alum and copperas should be progressively added to the lye as long as they produce
any precipitate. This is not at first a fine blue, but a greenish gray, in consequence of
the admixture of some white cyanide of iron; it becomes gradually blue by the absorption
of oxygen from the air, which is favoured by agitation of the liquor. Whenever the
colour seems to be as beautiful as it is likely to become, the liquor is to be run off by a
spigot or cock from the bottom of the precipitation vats, into flat cisterns, to settle.
The clear supernatant fluid, which is chiefly a solution of sulphate of potash, is then
drawn off by a syphon; more water is run on with agitation to wash it, which after
settling is again drawn off; and whenever the washings become tasteless, the sediment is
thrown upon filter sieves, and exposed to dry, first in the air of a stove, but finally upon
slabs of chalk or Paris plaster. But for several purposes, prussian blue may be best
employed in the fresh pasty state, as it then spreads more evenly over paper and other
surfaces.
A good article is known by the following tests: it feels light in the hand, adheres to
the tongue, has a dark lively blue colour, and gives a smooth deep trace; it should not
effervesce with acids, as when adulterated with chalk; nor become pasty with boiling
water, as when adulterated with starch. The Paris blue, prepared without alum, with
a peroxide salt of iron, displays, when rubbed, a copper-red lustre, like indigo. Prussian
blue, degraded in its colour by an admixture of free oxide of iron, may be improved[1044]
by digestion in dilute sulphuric or muriatic acid, washing, and drying. Its
relative richness in the real ferroprussiate of iron may be estimated by the quantity of
potash or soda which a given quantity of it requires to destroy its blue colour.
Sulphuretted hydrogen passed through prussian blue diffused in water, whitens it;
while prussic acid is eliminated, sulphur is thrown down, and the sesquicyanide of iron
is converted into the single cyanide. Iron and tin operate in the same way. When
prussian blue is made with two atoms of ferrocyanide of potassium, instead of one, it
becomes soluble in water.
For the mode of applying this pigment in dyeing, see Calico-printing.
Sesquiferrocyanate of potash, is prepared by passing chlorine gas through a solution
of ferrocyanide of potassium, till it becomes red, and ceases to precipitate the peroxide
salts of iron. The liquor yields, by evaporation, prismatic crystals, of a ruby-red transparency.
They are soluble in 38 parts of water, and consist of 40·42 parts of sesquicyanide
of iron, and 59·58 of cyanide of potassium. The solution of this salt precipitates
the following metals, as stated in the table:—
| Bismuth |
pale yellow. |
| Cadmium |
yellow. |
| Cobalt |
dark brown red. |
| Copper (protoxide) |
red brown. |
| CoDo.r (peroxide) |
yellow green. |
| Iron, protoxide salts of |
blue. |
| Manganese |
brown. |
| Mercury (protoxide) |
red brown. |
| Mercury (peroxide) |
yellow. |
| Molybdenum |
red brown. |
| Nickel |
yellow green. |
| Silver |
red brown. |
| Tin (protoxide) |
white. |
| Uranium |
red brown. |
| Zinc |
orange yellow. |
PUMICE-STONE (Pierre-ponce, Fr.; Bimstein, Germ.); is a spongy, vitreous-looking
mineral, consisting of fibres of a silky lustre, interlaced with each other in all
directions. It floats upon water, is harsh to the touch, having in mass a mean sp. grav.
of 0·914; though brittle, it is hard enough to scratch glass and most metals. Its
colour is usually grayish white; but it is sometimes bluish, greenish, reddish, or
brownish. It fuses without addition at the blowpipe into a white enamel. According
to Klaproth, it is composed of, silica, 77·5; alumina, 17·5; oxide of iron, 2;
potassa and soda, 3; in 100 parts. The acids have hardly any action upon pumice-stone.
It is used for polishing ivory, wood, marble, metals, glass, &c.; as also
skins and parchment. Pumice-stone is usually reckoned to be a volcanic product,
resulting, probably, from the action of fire upon obsidians. The chief localities of
this mineral are, the islands of Lipari, Ponza, Ischia, and Vulcano. It is also found in
the neighbourhood of Andernach, upon the banks of the Rhine, in Teneriffe, Iceland,
Auvergne, &c. It is sometimes so spongy as to be of specific gravity 0·37.
PUOZZOLANA, is a volcanic gravelly product, used in making hydraulic mortar.
See Cements and Mortars.
PURPLE OF CASSIUS, Gold purple (Pourpre de Cassius, Fr.; Gold-purpur,
Germ.); is a vitrifiable pigment, which stains glass and porcelain of a beautiful red or
purple hue. Its preparation has been deemed a process of such nicety, as to be liable
to fail in the most experienced hands. The following observations will, I hope, place
the subject upon a surer footing.
The proper pigment can be obtained only by adding to a neutral muriate of gold a
mixture of the protochloride and perchloride of tin. Every thing depends upon this
intermediate state of the tin; for the protochloride does not afford, even with a concentrated
solution of gold, either a chesnut-brown, a blue, a green, a metallic precipitate,
or one of a purple tone; the perchloride occasions no precipitate whatever,
whether the solution of gold be strong or dilute: but a properly neutral mixture, of
1 part of crystallized protochloride of tin, with 2 parts of crystallized perchloride, produces,
with 1 part of crystallized chloride of gold (all being in solution), a beautiful
purple-coloured precipitate. An excess of the protosalt of tin gives a yellow, blue, or
green cast; an excess of the persalt gives a red and violet cast; an excess in the gold
salt occasions, with heat (but not otherwise), a change from the violet and chesnut-brown
precipitate into red. According to Fuchs, a solution of the sesquioxide of tin
in muriatic acid, or of the sesquichloride in water, serves the same purpose, when dropped
into a very dilute solution of gold.
Buisson prepares gold-purple in the following way. He dissolves, first, 1 gramme
of the best tin in a sufficient quantity of muriatic acid, taking care that the solution
is neutral; next, 2 grammes of tin in aqua regia, composed of 3 parts of nitric
acid, and 1 part of muriatic, so that the solution can contain no protoxide; lastly, 7
grammes of fine gold in a mixture of 1 part of nitric acid, and 6 of muriatic, observing
to make the solution neutral. This solution of gold being diluted with 31⁄2 litres of water
(about 3 quarts), the solution of the perchloride of tin is to be added at once, and
afterwards that of the protochloride, drop by drop, till the precipitate thereby formed[1045]
acquires the wished-for tone; after which it should be edulcorated by washing, as quickly
as possible.
Frick gives the following prescription:—Let tin be set to dissolve in very dilute
aqua regia without heat, till the fluid becomes faintly opalescent, when the metal must be
taken out, and weighed. The liquor is to be diluted largely with water, and a definite
weight of a dilute solution of gold, and dilute sulphuric acid, is to be simultaneously
stirred into the nitro-muriate of tin. The quantity of solution of gold to be poured
into the tin liquor must be such, that the gold in the one is to the tin in the other in
the ratio of 36 to 10.
Gold-purple becomes brighter when it is dry, but appears still as a dirty-brown
powder. Muriatic acid takes the tin out of the fresh-made precipitate, and leaves the
gold either in the state of metal or of a blue powder. At a temperature between 212°
and 300° Fahr., mercury dissolves out all the gold from the ordinary purple of Cassius.
Relative to the constitution of gold-purple, two views are entertained: according to the
first; the gold is associated in the metallic state along with the oxide of tin; according
to the second, the gold exists as a purple oxide along with the sesquioxide or peroxide
of tin. Its composition is differently reported by different chemists. The constituents,
according to—
| |
Gold. |
Tin
oxide. |
| Oberkampf, in the |
purple |
precipitate, |
are |
39 |
·82 |
60 |
·18 |
| |
violet |
ditto |
|
20 |
·58 |
79 |
·42 |
| Berzelius |
30 |
·725 |
69 |
·275 |
| Buisson |
30 |
·19 |
69 |
·81 |
| Gay Lussac |
30 |
·89 |
69 |
·11 |
| Fuchs |
17 |
·87 |
82 |
·13 |
If to a mixture of protochloride of tin, and perchloride of iron, a properly diluted
solution of gold be added, a very beautiful purple precipitate of Cassius will immediately
fall, while the iron will be left in the liquid in the state of a protochloride. The purple
thus prepared keeps in the air for a long time without alteration. Mercury does not
take from it the smallest trace of gold,—Fuchs’ Journal für Chemie, t. xv.
PURPLE OF MOLLUSCA, is a viscid liquor, secreted by certain shell-fish, the
Buccinum lapillus, and others, which dyes wool, &c. of a purple colour, and is supposed
to be the substance of the Tyrian dye, so highly prized in antient Rome for producing
the imperial purple. See Dyeing.
PURPURIC ACID, is an acid obtained by treating uric or lithic acid with dilute
nitric acid. It has a fine purple colour; but has hitherto been applied to no use in
the arts.
PURPURINE, is the name of a colouring principle, supposed by Robiquet and
Colin to exist in madder. Its identity is questionable.
PUTREFACTION, and its Prevention. The decomposition of animal bodies, or of
such plants as contain azote in their composition, which takes place spontaneously when
they are exposed to the air, under the influence of moisture and warmth, is called putrefaction.
During this process, there is a complete transposition of the proximate principles,
the elementary substances combining in new and principally gaseous compounds.
Oxygen is absorbed from the atmosphere, and converted into carbonic acid; one portion
of the hydrogen forms water with the oxygen; another portion forms, with the azote,
the carbon, the phosphorus, and the sulphur respectively, ammonia, carburetted,
phosphuretted, and sulphuretted hydrogen gases, which occasion the nauseous smell
evolved by putrefying bodies. There remains a friable earthy-looking residuum, consisting
of rotten mould and charcoal. Vegetables which contain no azote, like the
ligneous part of plants, suffer their corresponding decomposition much more slowly, and
with different modifications, but they are finally converted into vegetable mould. In
this process, the juices with which the plants are filled first enter into the acetous fermentation
under the action of heat and moisture; the acid thereby generated destroys the
cohesion of the fibrous matter, and thus reduces the solids to a pulpy state. In the
progress of the decomposition, a substance is lastly produced which resembles oxidized
extractive, is soluble in alkalis, and is sometimes called mould. This decomposition of
the plants which contain no azote, goes on without any offensive smell, as none of the
above-named nauseous gases are disengaged. When vegetable matters are mixed with
animal, as in the dung of cattle, this decomposition proceeds more rapidly, because
the animalized portion serves as a ferment to the vegetable. Vegetable acids, resins, fats,
and volatilized oils, are not of themselves subject to putrefaction.
The object of the present article is to detail the principles and processes, according to
which, for various purposes in the arts, the destruction of bodies by putrefaction may be
prevented, and their preservation in a sound state secured for a longer or a shorter
time.
[1046]
I. CONDITIONS OF THE PREVENTION OF PUTREFACTION.
The circumstances by which putrefaction is counteracted, are, 1. the chemical change
of the azotized juices; 2. the abstraction of the water; 3. the lowering of the temperature;
and 4. the exclusion of oxygen.
1. The chemical change of the azotized juices.—The substance which in dead animal
matter is first attacked with putridity, and which serves to communicate it to the solid
fibrous parts, is albumen, as it exists combined with more or less water in all the
animal fluids and soft parts. In those vegetables also which putrefy, it is the albumen
which first suffers decomposition; and hence those plants which contain most of that
proximate principle, are most apt to become putrid, and most resemble, in this respect,
animal substances; of which fact, mushrooms, cabbages, coleworts, &c., afford illustrations.
The albumen, when dissolved in water, very readily putrefies in a moderately
warm air; but when coagulated, it seems as little liable to putridity as fibrin itself.
By this change, it throws off the superfluous water, becomes solid, and may then be
easily dried. Hence, those means which by coagulation make the albumen insoluble,
or form with it a new compound, which does not dissolve in water, but which resists
putrefaction, are powerful antiseptics. Whenever the albumen is coagulated, the uncombined
water may be easily evaporated away, and the residuary solid matter may be
readily dried in the air, so as to be rendered unsusceptible of decomposition.
In this way acids operate, which combine with the albumen, and fix it in a coagulated
state, without separating it from its solution: such is the effect of vinegar, citric
acid, tartaric acid, &c.
Tannin combines with the albuminous and gelatinous parts of animals, and forms insoluble
compounds, which resist putrefaction; on which fact the art of tanning is founded.
Alcohol, oil of turpentine, and some other volatile oils, likewise coagulate albumen,
and thereby protect it from putrescence. The most remarkable operation of this kind is
exhibited by wood vinegar, in consequence of the creosote contained in it, according to
the discovery of Reichenbach. This peculiar volatile oil has so decided a power of
coagulating albumen, that even the minute portion of it present in pyrolignous vinegar
is sufficient to preserve animal parts from putrefaction, when they are simply soaked
in it. Thus, also, flesh is cured by wood smoke. Wood tar likewise protects animal
matter from change, by the creosote it contains. The ordinary pyrolignous acid sometimes
contains 5 per cent. of creosote.
In circumstances where a stronger impregnation with this antiseptic oil may be
necessary, common wood vinegar may be heated to 167° F., and saturated with
effloresced Glauber’s salts, by which expedient the oil is separated and made to float
upon the surface of the warm liquid; whence it should be immediately skimmed off;
because, by cooling and crystallizing, the solution would so diminish in density as to
allow the oil to sink to the bottom; for its specific gravity is considerably greater than
that of water. This oil, which contains, besides creosote, some other volatile constituents,
may be kept dissolved ready for use in strong vinegar or alcohol. Water takes
up of pure creosote only 13⁄4 per cent.; but alcohol dissolves it in every proportion.
The earthy and metallic salts afford likewise powerful means for separating albumen
from its watery solution, their bases having the property of forming insoluble compounds
with it. The more completely they produce this separation, the more effectually
do they counteract putrefaction. The alkaline salts also, as common salt, sal ammoniac,
saltpetre, and tartar, operate against putrescence, though in a smaller degree, because they
do not precipitate the albumen; but, by abstracting a part of its water, they render it
less liable to become putrid. Among the earthy salts, alum is the most energetic, as it
forms a subsalt which combines with albumen; it is three times more antiseptic than
common salt, and from seven to eight times more so than saltpetre. Muriate of soda,
however, may be employed along with alum, as is done in the tawing of sheepskins.
The metallic salts operate still more effectually as antiseptics, because they form with
albumen still more intimate combinations. Under this head we class the green and red
sulphates of iron, the chloride of zinc, the acetate of lead, and corrosive sublimate; the
latter, however, from its poisonous qualities, can be employed only on special occasions.
Nitrate of silver, though equally noxious to life, is so antiseptic, that a solution containing
only 1⁄500 of the salt is capable of preserving animal matters from corruption.
2. Abstraction of water.—Even in those cases where no separation of the albumen
takes place in a coagulated form, or as a solid precipitate, by the operation of a substance
foreign to the animal juices, putrefaction cannot go on, any more than other kinds of
fermentation, in bodies wholly or in a great measure deprived of their water. For the
albumen itself runs so much more slowly into putrefaction, the less water it is dissolved
in; and in the desiccated state, it is as little susceptible of alteration as any other dry
vegetable or animal matter. Hence, the proper drying of an animal substance becomes
a universal preventive of putrescence. In this way fruits, herbs, cabbages, fish, flesh,[1047]
may be preserved from corruption. If the air be not cold and dry enough to cause the
evaporation of the fluids before putrescence may come on, the organic substance must
be dried by artificial means, as by being exposed in thin slices in properly constructed
air-stoves. At temperatures under 140° F., the albumen dries up without coagulation,
and may then be re-dissolved in cold water, with its valuable properties unaltered.
By such artificial desiccation, if flesh is to be preserved for cooking or boiling,
it must not be exposed, however, to so high a degree of heat, which would harden it
permanently, like the baked mummies of Egypt. Mere desiccation, indeed, can hardly
ever be employed upon flesh. Culinary salt is generally had recourse to, either alone
or with the addition of saltpetre or sugar.
These alkaline salts abstract water in their solution, and, consequently, concentrate
the aqueous solution of the albumen; whence, by converting the simple watery fluid
into salt water, which is in general less favourable to the fermentation of animal matter
than pure water, and by expelling the air, they counteract putridity. On this account,
salted meat may be dried in the air much more speedily and safely than fresh meat.
The drying is promoted by heating the meat merely to such a degree as to consolidate
the albumen, and eliminate the superfluous water.
Alcohol operates similarly, in abstracting the water essential to the putrefaction of
animal substances, taking it not only from the liquid albumen, but counteracting its
decomposition, when mixed among the animal solids. Sugar acts in the same way, fixing
in an unchangeable syrup the water which would otherwise be accessory to the fermentation
of the organic bodies. The preserves of fruits and vegetable juices are made upon
this principle. When animal substances are rubbed with charcoal powder or sand,
perfectly dry, and are afterwards freely exposed to the air, they become deprived of
their moisture, and will keep for any length of time.
3. Defect of warmth.—As a certain degree of heat is requisite for the vinous fermentation,
so is it for the putrefactive. In a damp atmosphere, or in one saturated with
moisture, if the temperature stand at from 70° to 80° F., the putrefaction goes on most
rapidly; but it proceeds languidly at a few degrees above freezing, and is suspended
altogether at that point. The elephants preserved in the polar ices are proofs of the
antiseptic influence of low temperature. In temperate climates, ice-houses serve the
purpose of keeping meat fresh and sweet for any length of time.
4. Abstraction of oxygen gas.—As the putrefactive decomposition of a body first
commences with the absorption of oxygen from the atmosphere, so it may be
retarded by the exclusion of this gas. It is not, however, enough to remove the aerial
oxygen from the surface of the body, but we must expel all the oxygen that may be
diffused among the vessels and other solids, as this portion suffices in general to
excite putrefaction, if other circumstances be favourable. The expulsion is most
readily accomplished by a moderate degree of heat, which, by expanding the air, evolves
it in a great measure, and at the same time favours the fixation of the oxygen in the
extractive matter, so as to make it no longer available towards the putrefaction of the
other substances. Milk, soup, solution of gelatine, &c., may be kept long in a fresh
state, if they be subjected in an air-tight vessel every other day to a boiling heat.
Oxygenation may be prevented in several ways: by burning sulphur or phosphorus in
the air of the meat receiver; by filling this with compressed carbonic acid; or with
oils, fats, syrups, &c., and then sealing it hermetically. Charcoal powder recently
calcined is efficacious in preserving meat, as it not only excludes air from the bodies
surrounded by it, but intercepts the oxygen by condensing it. When butcher-meat is
enclosed in a vessel filled with sulphurous acid, it absorbs the gas, and remains for a considerable
time proof against corruption. The same result is obtained if the vessel be
filled with ammoniacal gas. At the end of 76 days such meat has still a fresh look,
and may be safely dried in the atmosphere.
II. PECULIAR ANTISEPTIC PROCESSES.
Upon the preceding principles and experiments depend the several processes employed
for protecting substances from putrescence and corruption. Here we must distinguish
between those bodies which may be preserved by any media suitable to the purpose, as
anatomical preparations or objects of natural history, and those bodies which being
intended for food, can be cured only by wholesome and agreeable means.
A common method for preserving animal substances unchanged in property and
texture, is to immerse them in a spirituous liquor containing about 65 or 70 per cent.
of real alcohol. Camphor may also be dissolved in it, and as much common salt as its
water will take up. A double fold of ox-bladder should be bound over the mouth of
the vessel, in order to impede the evaporation of the watery portion of the liquid, and
its upper surface should be coated with a turpentine varnish. Undoubtedly a little
creosote would be of use to counteract the decomposing influence of the alcohol upon the[1048]
animal substances. With such an addition, a weaker spirit, containing no more than
30 per cent. of alcohol, would answer the purpose.
Instead of alcohol, a much cheaper vehicle is water saturated with sulphurous acid;
and if a few drops of creosote be added, the mixture will become very efficacious. A
solution of red sulphate of iron is powerfully antiseptic; but after some time it gives a
deposit of the oxide, which disguises the preparation in a great degree.
According to Tauffier, animal substances may be preserved more permanently by a
solution of one part of chloride of tin in 20 parts of water, sharpened with a little
muriatic acid, than even by alcohol.
For preserving animal bodies in an embalmed form, mummy-like, a solution of
chloride of mercury and wood vinegar are most efficacious. As there is danger in
manipulating with that mercurial salt, and as in the present state of our knowledge of
creosote we have it in our power to make a suitably strong solution of this substance in
vinegar or spirit of wine, I am led to suppose that it will become the basis of most
antiseptic preparations for the future. From the statements of Pliny, it is plain that
wood vinegar was the essential means employed by the antient Egyptians in preparing
their mummies, and that the odoriferous resins were of inferior consequence.
CURING OF PROVISIONS.
Flesh.—The ordinary means employed for preserving butcher meat are, drying,
smoking, salting, and pickling or souring.
Drying of animal fibre.—The best mode of operating is as follows:—The flesh must
be cut into slices from 2 to 6 ounces in weight, immersed in boiling water for 5 or 6
minutes, and then laid on open trellis-work in a drying-stove, at a temperature kept
steadily about 122° F., with a constant stream of warm dry air. That the boiling
water may not dissipate the soluble animal matters, very little of it should be used,
just enough for the meat to be immersed by portions in succession, whereby it will
speedily become a rich soup, fresh water being added only as evaporation takes place.
It is advantageous to add a little salt, and some spices, especially coriander seeds, to the
water. After the parboiling of the flesh has been completed, the soup should be evaporated
to a gelatinous consistence, in order to fit it for forming a varnish to the meat after
it is dried, which may be completely effected within two days in the oven. By this
process two-thirds of the weight is lost. The perfectly dry flesh must be plunged piece
by piece in the fatty gelatinous matter liquefied by a gentle heat; then placed once more
in the stove, to dry the layer of varnish. This operation may be repeated two or three
times, in order to render the coat sufficiently uniform and thick. Butcher’s meat dried
in this way, keeps for a year, affords, when cooked, a dish similar to that of fresh
meat, and is therefore much preferable to salted provisions. The drying may be
facilitated, so that larger lumps of flesh may be used, if they be imbued with some
common salt immediately after the parboiling process, by stratifying them with salt, and
leaving them in a proper pickling-tub for 12 hours before they are transferred to the
stove. The first method, however, affords the more agreeable article.
Smoking.—This process consists in exposing meat previously salted, or merely
rubbed over with salt, to wood smoke, in an apartment so distant from the fire as not to
be unduly heated by it, and into which the smoke is admitted by flues at the bottom of the
side walls. Here the meat combines with the empyreumatic acid of the smoke, and gets
dried at the same time. The quality of the wood has an influence upon the smell and
taste of the smoke-dried meat; smoke from beech wood and oak being preferable to that
from fir and larch. Smoke from the twigs and berries of juniper, from rosemary,
peppermint, &c., imparts somewhat of the aromatic flavour of these plants. A slow
smoking with a slender fire is preferable to a rapid and powerful one, as it allows the
empyreumatic principles time to penetrate into the interior substance, without drying
the outside too much. To prevent soot from attaching itself to the provisions, they may
be wrapped in cloth, or rubbed over with bran, which may be easily removed at the
end of the operation.
The process of smoking depends upon the action of the wood acid, or the creosote
volatilized with it, which operates upon the flesh. The same change may be produced
in a much shorter time by immersing the meat for a few hours in pyrolignous acid,
then hanging it up in a dry air, which, though moderately warm, makes it fit for keeping,
without any taint of putrescence. After a few days exposure, it loses the empyreumatic
smell, and then resembles thoroughly smoked provisions. The meat dried in
this way is in general somewhat harder than by the application of smoke, and therefore
softens less when cooked, a difference to be ascribed to the more sudden and concentrated
operation of the wood vinegar, which effects in a few hours what would require
smoking for several weeks. By the judicious employment of pyrolignous acid diluted
to successive degrees, we might probably succeed in imitating perfectly the effect of
smoke in curing provisions.
[1049]
Salting.—The meat should be rubbed well with common salt, containing about one
sixteenth of saltpetre, and one thirty-secondth of sugar, till every crevice has been impregnated
with it; then sprinkled over with salt, laid down for 24 or 48 hours, and,
lastly, subjected to pressure. It must next be sprinkled anew with salt, packed into
proper vessels, and covered with the brine obtained in the act of pressing, rendered
stronger by boiling down. For household purposes it is sufficient to rub the meat well
with good salt, to put it into vessels, and load it with heavy weights, in order to squeeze
out as much pickle as will cover its surface. If this cannot be had, a pickle must
be poured on it, composed of 4 pounds of salt, 1 pound of sugar, and 2 oz. of saltpetre,
dissolved in 2 gallons of water.
Pickling with vinegar.—Vinegar dissolves or coagulates the albumen of flesh, and
thereby counteracts its putrescence. The meat should be washed, dried, and then laid in
strong vinegar. Or it may be boiled in the vinegar, allowed to cool in it, and then set
aside with it in a cold cellar, where it will keep sound for several months.
Fresh meat may be kept for some months in water deprived of its air. If we strew on
the bottom of a vessel a mixture of iron filings and flowers of sulphur, and pour over them
some water which has been boiled, so as to expel its air, meat immersed in it will keep
a long time, if the water be covered with a layer of oil, from half an inch to an inch
thick. Meat will also keep fresh for a considerable period when surrounded with oil,
or fat of any kind, so purified as not to turn rancid of itself, especially if the meat be
previously boiled. This process is called potting, and is applied successfully to fish,
fowls, &c.
Prechtl says that living fish may be preserved 14 days without water, by stopping their
mouths with crumbs of bread steeped in brandy, pouring a little brandy into them, and
packing them in this torpid state in straw. When put into fresh water, they come alive
again after a few hours! Prechtl, Encyclop. Technologisches, art. Faülniss Abhaltung.
Eggs.—These ought to be taken new laid. The essential point towards their preservation
is the exclusion of the atmospheric oxygen, as their shells are porous, and
permit the external air to pass inwards, and to excite putrefaction in the albumen.
There is also some oxygen always in the air cell of the eggs, which ought to be expelled
or rendered inoperative, which may be done by plunging them for 5 minutes in water
heated to 140° F. The eggs must be then taken out, wiped dry, besmeared with some
oil (not apt to turn rancid) or other unctuous matter, packed into a vessel with their
narrow ends uppermost, and covered with sawdust, fine sand, or powdered charcoal.
Eggs coated with gum arabic, and packed in charcoal, will keep fresh for a year. Lime
water, or rather milk of lime, is an excellent vehicle for keeping eggs in, as I have
verified by long experience. Some persons coagulate the albumen partially, and also
expel the air by boiling the eggs for two minutes, and find the method successful.
When eggs are intended for hatching, they should be kept in a cool cellar; for example,
in a chamber adjoining an ice-house. Eggs exposed, in the holes of perforated shelves,
to a constant current of air, lose about 3⁄4 of a grain of their weight daily, and become
concentrated in their albuminous part, so as to be little liable to putrefy. For long sea
voyages, the surest means of preserving eggs, is to dry up the albumen and yolk, by
first triturating them into a homogeneous paste, then evaporating this in an air-stove or
a water-bath heated to 125°, and putting up the dried mass in vessels which may be
made air-tight. When used, it should be dissolved in three parts of cold or tepid water.
Grain of all kinds, as wheat, barley, rye, &c., and their flour, may be preserved for an
indefinite length of time, if they be kiln-dried, put up in vessels or chambers free from
damp, and excluded from the air. Well dried grain is not liable to the depredations
of insects.
To preserve fruits in a fresh state, various plans are adopted. Pears, apples,
plums, &c. should be gathered in a sound state, altogether exempt from bruises, and
plucked, in dry weather, before they are fully ripe. One mode of preservation is, to
expose them in an airy place to dry a little for eight or ten days, and then to lay them
in dry sawdust or chopped straw, spread upon shelves in a cool apartment, so as not to
touch each other. Another method consists in surrounding them with fine dry sand in
a vessel which should be made air-tight, and kept in a cool place. Some persons coat
the fruit, including their stalks, with melted wax; others lay the apples, &c., upon
wicker-work shelves in a vaulted chamber, and smoke them daily during 4 or 5 days with
vine branches or juniper wood. Apples thus treated, and afterwards stratified in dry
sawdust, without touching each other, will keep fresh for a whole year.
The drying of garden fruits in the air, or by a kiln, is a well-known method of preservation.
Apples and pears of large size should be cut into thin slices. From 5 to 6
measures of fresh apples, and from 6 to 7 of pears, afford in general one measure of dry
fruit, (biffins). Dried plums, grapes, and currants are a common article of commerce.
Herbs, cabbages, &c., may be kept a long time in a cool cellar, provided they are
covered with dry sand. Such vegetables are in general preserved for the purposes of[1050]
food, by means of drying, salting, pickling with vinegar, or beating up with sugar.
Cabbages should be scalded in hot water previously to drying; and all such plants, when
dried, should be compactly pressed together, and kept in air-tight vessels. Tuberous
and other roots are better kept in an airy place, where they may dry a little without
being exposed to the winter’s frost.
A partial drying is given to various vegetable juices by evaporating them to the consistence
of a syrup, called a rob, in which so much of the water is dissipated as to
prevent them from running into fermentation. The fruits must be crushed, squeezed in
bags to expel the juices, which must then be inspissated either over the naked fire, or
on a water or steam bath, in the air or in vacuo. Sometimes a small proportion of spices is
added, which tends to prevent mouldiness. Such extracts may be conveniently mixed
with sugar into what are called conserves.
Salting is employed for certain fruits, as small cucumbers or gherkins, capers, olives,
&c. Even for peas such a method is had recourse to, for preserving them a certain
time. They must be scalded in hot water, put up in bottles, and covered with saturated
brine, having a film of oil on its surface, to exclude the agency of the atmospheric air.
Before being used, they must be soaked for a short time in warm water, to extract the
salt. The most important article of diet of this class, is the sour kraut of the northern
nations of Europe, (made from white cabbage,) which is prepared simply by salting; a
little vinegar being formed spontaneously by fermentation. The cabbage must be cut
into small pieces, stratified in a cask along with salt, to which juniper berries and carui
seeds are added, and packed as hard as possible by means of a wooden rammer. The
cabbage is then covered with a lid, on which a heavy weight is laid. A fermentation
commences, which causes the cabbage to become more compact, while a quantity of juice
exudes and floats on the surface, and a sour smell is perceived towards the end of the
fermentation. In this condition the cask is transported into a cool cellar, where it is
allowed to stand for a year; and indeed, where, if well made and packed, it may be kept
for several years.
The excellent process for preserving all kinds of butcher meat, fish, and poultry, first
contrived by M. Appert in France, and afterwards successfully practised upon the great
commercial scale by Messrs. Donkin and Gamble, for keeping beef, salmon, soups, &c.
perfectly fresh and sweet for exportation from this country, as also turtle for importation
thither from the West Indies, deserves a brief description.
Let the substance to be preserved be first parboiled, or rather somewhat more, the
bones of the meat being previously removed. Put the meat into a tin cylinder, fill up
the vessel with seasoned rich soup, and then solder on the lid, pierced with a small hole.
When this has been done, let the tin vessel thus prepared be placed in brine and heated
to the boiling point, to complete the remainder of the cooking of the meat. The hole
of the lid is now to be closed perfectly by soldering, whilst the air is rarefied. The vessel
is then allowed to cool, and from the diminution of the volume, in consequence of the
reduction of temperature, both ends of the cylinder are pressed inwards, and become
concave. The tin cases, thus hermetically sealed, are exposed in a test-chamber, for
at least a month, to a temperature above what they are ever likely to encounter; from
90° to 110° of Fahrenheit. If the process has failed, putrefaction takes place, and
gas is evolved, which, in process of time, will cause both ends of the case to bulge, so as
render them convex, instead of concave. But the contents of those cases which stand
the test will infallibly keep perfectly sweet and good in any climate, and for any number
of years. If there be any taint about the meat when put up, it inevitably ferments, and
is detected in the proving process. Mr. Gamble’s turtle is delicious.
This preservative process is founded upon the fact, that the small quantity of oxygen
contained within the vessel gets into a state of combination, in consequence of the high
temperature to which the animal substances are exposed, and upon the chemical
principle, that free oxygen is necessary as a ferment to commence or give birth to the
process of putrefaction.
I shall conclude this article with some observations upon the means of preserving
water fresh on sea voyages. When long kept in wooden casks, it undergoes a kind of
putrefaction, contracts a disagreeable sulphureous smell, and becomes undrinkable. The
influence of the external air is by no means necessary to this change, for it happens in
close vessels even more readily than when freely exposed to the atmospherical oxygen.
The origin of this impurity lies in the animal and vegetable juices which the water originally
contained in the source from which it was drawn, or from the cask, or insects,
&c. These matters easily occasion, with a sufficient warmth, fermentation in the stagnant
water, and thereby cause the evolution of offensive gases. It would appear that
the gypsum of hard waters is decomposed, and gives up its sulphur, which aggravates
the disagreeable odour; for selenitic waters are more apt to take this putrid taint, than
those which contain merely carbonate of lime.
As the corrupted water has become unfit for use merely in consequence of the admixture[1051]
of these foreign matters, for water in itself is not liable to corruption, so it may be
purified again by their separation. This purification may be accomplished most
easily by passing the water through charcoal powder, or through the powder of
rightly calcined bone-black. The carbon takes away not only the finely diffused
corrupt particles, but also the gaseous impurities. By adding to the water a very little
sulphuric acid, about 30 drops to 4 pounds, Lowitz says that two-thirds of the
charcoal may be saved. Undoubtedly the sulphuric acid acts here, as in other
similar cases, by the coagulation and separation of the albuminous matters, combining
with them, and rendering them more apt to be seized by the charcoal. A more effectual
agent for the purification of foul water is to be found in alum. A dram of pounded
alum should be dissolved with agitation in a gallon of the water, and then left to operate
quietly for 24 hours. A sediment falls to the bottom, while the water becomes clear
above, and may be poured off. The alum combines here with the substances dissolved
in the water, as it does with the stuffs in the dyeing copper. In order to decompose
any alum which may remain in solution, the equivalent quantity of crystals of carbonate
of soda may be added to it.
The red sulphate of iron acts in the same way as alum. A few drops of its solution
are sufficient to purge a pound of foul water. The foreign matters dissolved in the water,
which occasion putrefaction, become insoluble, in consequence of oxidizement, like vegetable
extractive, and are precipitated. On this account, also, foul water may be purified,
by driving atmospheric air through it with bellows, or by agitating it in contact with
fresh air, so that all its particles are exposed to oxygen. Thus we can explain the influence
of streams and winds, in counteracting the corruption of water exposed to them.
Chlorine acts still more energetically than the air in purifying water. A little aqueous
chlorine added to foul water, or the transmission of a little gaseous chlorine through
it, cleanses it immediately.
Water-casks ought to be charred inside, whereby no fermentable stuff will be extracted
from the wood. British ships, however, are now commonly provided with iron
tanks for holding their water in long voyages.
PYRITES, is the native bisulphuret of iron. Copper pyrites, called vulgarly
mundick, is a bisulphuret of copper.
PYRO-ACETIC SPIRIT. (Esprit pyro-acétique, Acétone, Fr.; Brennzlicher
Essiggeist, Mesit, Germ.) This liquid was discovered and described by Chenevix long
before pyrolignous spirit was known. It may be obtained by subjecting to dry distillation
the acetates of copper, lead, alkalis, and earths; and as it is formed especially
during the second half of the process, the liquor which comes over then should be set
apart, separated by decantation from the empyreumatic oil, and distilled a second time
by the heat of a water-bath. The fine light fluid which now comes over first, is to be
rectified along with carbonate of potassa, or chloride of calcium. As pyro-acetic spirit
usually retains, even after repeated distillations, a disagreeable empyreumatic smell, like
garlic, a little good bone-black should be employed in its final rectification. According
to Reichenbach, pyro-acetic spirit may be extracted in considerable quantity from beech
tar. (See the next article.) The spirit thus prepared, is a colourless limpid liquid, of
an acrid and burning taste at first, but afterwards cooling; of a penetrating aromatic
smell, different from that of alcohol; of the spec. gravity 0·7921 at 60° F., boiling at
132° F., and remaining fluid at 5°. It consists ultimately of—carbon, 62·148; hydrogen,
10·453; oxygen, 27·329; or, of 1 proportion of carbonic acid + 2 prop. of olefiant
gas + 1 prop. of water; or, 1 prop. of acetic acid—1 prop. of carbonic acid. According
to another view, it is composed of, 51·52 parts of concentrated acetic acid, and
48·488 of oil of wine, being double of the quantity in acetic ether. It is very combustible,
and burns with a brilliant flame, without smoke. When treated by chlorine, it
loses an atom of its hydrogen, and absorbs 2 atoms of chlorine. It is soluble in water,
alcohol, ether, and is not convertible into ether by strong sulphuric acid. It is used
for dissolving the resins commonly called gums, with which the bodies of hats are
stiffened.
PYROLIGNOUS ACID. In addition to what has been said under Acetic Acid,
I shall here describe the process as conducted upon a great scale at an establishment
near Manchester. The retorts are of cast iron, 6 feet long, and 3 feet 8 inches in diameter.
Two of these cylinders are heated by one fire, the flame of which plays round
their sides and upper surface; but the bottom is shielded by fire-tiles from the direct
action of the fire. 2 cwts. of coals are sufficient to complete the distillation of one
charge of wood; 36 imperial gallons of crude vinegar, of specific gravity 1·025, being
obtained from each retort. The process occupies 24 hours. The retort-mouth is then
removed, and the ignited charcoal is raked out for extinction into an iron chest,
having a groove round its edges, into which a lid is fitted.
When this pyrolignous acid is saturated with quicklime, and distilled, it yields one per[1052]
cent. of pyroxilic spirit (sometimes called naphtha); which is rectified by two or
three successive distillations with quicklime.
The tarry deposit of the crude pyrolignous acid, being subjected to distillation by
itself, affords a crude pyro-acetic ether, which may also be purified by re-distillation with
quicklime, and subsequent agitation with water.
The pyrolignite of lime, is made by boiling the pyrolignous acid in a large copper,
which has a sloping spout at its lip, by which the tarry scum freely flows over, as it
froths up with the heat. The fluid compound thus purified, is syphoned off into another
copper, and mixed with a quantity of alum equivalent to its strength, in order to form
the red liquor, or acetate of alumina, of the calico-printer. The acetate of lime, and
sulphate of alumina and potash, mutually decompose each other; with the formation of
sulphate of lime, which falls immediately to the bottom.
M. Kestner, of Thann, in Alsace, obtains, in his manufactory of pyrolignous acid,
5 hectolitres (112 gallons imperial, nearly,) from a cord containing 93 cubic feet of wood.
The acid is very brown, much loaded with tar, and marks 5° Baumé; 220 kilogrammes
of charcoal are left in the cylinders; 500 litres of that brown acid produce, after several
distillations, 375 of the pyrolignous acid of commerce, containing 7 per cent. of acid,
with a residuum of 40 kilogrammes of pitch. For the purpose of making a crude
acetate of lead (pyrolignite), he dries pyrolignite of lime upon iron plates, mixes it with
the equivalent decomposing quantity of sulphuric acid, previously diluted with its
own weight of water, and cooled; and transfers the mixture as quickly as possible into a
cast-iron cylindric still, built horizontally in a furnace; the under half of the mouth
of the cylinder being always cast with a semicircle of iron. The acetic acid is received
into large salt-glazed stone bottles. From 100 parts of acetate of lime, he obtains 133
of acetic acid, at 38° Baumé. It contains always a little sulphurous acid from the
reaction of the tar and the sulphuric acid.
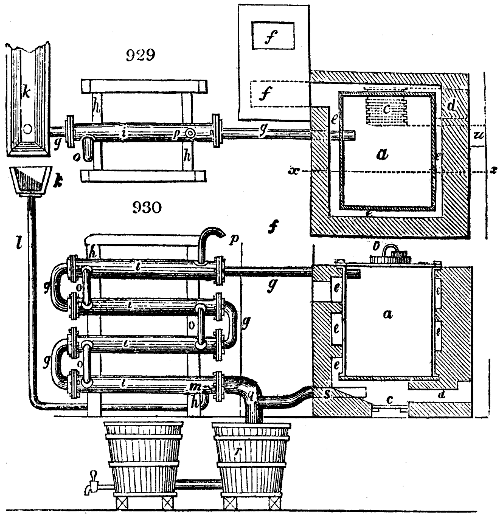
The apparatus represented in figs. 929. and 930. is a convenient modification of that
exhibited under acetic acid, for producing pyrolignous acid. Fig. 929. shows the furnace
in a horizontal section
drawn through the
middle of the flue which
leads to the chimney.
Fig. 930. is a vertical
section taken in the dotted
line x, x, of fig. 929. The
chest a is constructed with
cast-iron plates bolted together,
and has a capacity
of 100 cubic feet. The
wood is introduced into it
through the opening b, in
the cover, for which purpose
it is cleft into billets
of moderate length. The
chest is heated from the
subjacent grate c, upon
which the fuel is laid,
through the fire-door d.
The flame ascends spirally
through the flues e, e, round
the chest, which terminate
in the chimney f. An iron
pipe g conveys the vapours
and gaseous products from
the iron chest to the condenser.
This consists of a series of pipes laid zigzag over each other, which rest upon a
framework of wood. The condensing tubes are enclosed in larger pipes i, i; a stream
of cold water being caused to circulate in the interstitial spaces between them. The
water passes down from a trough k, through a conducting tube l, enters the lowest
cylindrical case at m, flows thence along the series of jackets i, i, i, being transmitted
from the one row to the next above it, by the junction tubes o, o, o, till at p it runs off
in a boiling-hot state. The vapours proceeding downwards in an opposite direction to
the cooling stream of water, get condensed into the liquid state, and pass off at q,
through a discharge pipe, into the first close receiver r, while the combustible gases flow
off through the tube s, which is provided with a stopcock to regulate the magnitude of
their flame under the chest. As soon as the distillation is fully set agoing, the stopcock
upon the gas-pipe is opened; and after it is finished, it must be shut. The fire should be[1053]
supplied with fuel at first, but after some time the gas generated keeps up the distilling
heat. The charcoal is allowed to cool during 5 or 6 hours, and is then taken out
through an aperture in the back of the chest, which corresponds to the opening u, fig.
929., in the brickwork of the furnace. About 60 per cent. of charcoal may be obtained
from 100 feet of fir-wood, with a consumption of as much brush-wood for fuel.
Stoltze has ascertained, by numerous experiments, that one pound of wood yields from
6 to 71⁄2 ounces of liquid products; but in acetic acid it affords a quantity varying from 2
to 5, according to the nature of the wood. Hard timber, which has grown slowly upon
a dry soil, gives the strongest vinegar. White birch and red beech afford per pound 71⁄3
ounces of wood vinegar, 11⁄3 ounce of combustible oil, and 4 ounces of charcoal. One
ounce of that vinegar saturates 110 grains of carbonate of potassa. Red pine yields per
pound 61⁄2 ounces of vinegar, 21⁄4 ounces of oil, 33⁄4 ounces of charcoal; but one ounce of
the vinegar saturates only 44 grains of carbonate of potassa, and has therefore only two-fifths
of the strength of the vinegar from the birch. An ounce of the vinegar from the
white beech, holly oak (Ilex), common ash, and horse chesnut, saturates from 90 to 100
grains of the carbonate. In the same circumstances, an ounce of the vinegar of the
alder and white pine saturates from 58 to 60 grains.
PYROLIGNOUS or PYROXILIC SPIRIT, improperly called naphtha. This
is employed, as well as pyroacetic ether, to dissolve the sandarach, mastic, and other
resinous substances, which, under the name of gums, are used for stiffening the bodies
of hats. I have already described, in the article Pyrolignous Acid, how this spirit is
obtained. Berzelius has found that the crude spirit may be best purified by agitating
it with a fat oil, in order to abstract the empyreumatic oil; then to decant the spirit,
distil it, first with fresh calcined charcoal, and next with chloride of calcium. The
pyrolignous spirit, thus purified, is colourless, and limpid like alcohol; has an ethereous
smell, somewhat resembling that of ants. Its taste is hot, and analogous to that of oil
of peppermint. Its specific gravity, by my experiments, is 0·824. It readily takes
fire, and burns with a blue flame, without smoke. It combines with water in any proportion;
a property which distinguishes it from pyroacetic ether and spirit.
It is not easy to say what is the real chemical nature of pyroxilic spirit. There is no
ultimate analysis of it that can be depended upon. The properties of the spirit
examined by MM. Marcet and Macaire, differ from those of our spirit, in refusing to
combine with water, like alcohol. The article on sale in this country readily unites
with water, and in all proportions with alcohol.
PYROMETER, is the name of an instrument for measuring high degrees of heat
above the range of the mercurial thermometer. Wedgewood’s is the one commonly
referred to by writers upon porcelain and metallurgy; but a better one might be easily
contrived.
PYROPHORUS, is the generic name of any chemical preparation, generally a
powder, which inflames spontaneously when exposed to the air.
PYROTECHNY. See Fire-works.
PYROXILINE, is a name which I have ventured to give to a substance detected
in pyroxilic spirit, by Mr. Scanlan, while residing in Dublin, and therefore called by
him Eblanin. I am indebted to that ingenious chemist for the following facts.
If potash water be added to raw wood-spirit (pyrolignous), as long as it throws down
any thing, a precipitate is produced, which is pyroxiline, mixed with tarry matter. This
precipitate is to be collected on a filter cloth, and submitted to strong pressure between
folds of blotting-paper; it is next to be washed with cold alcohol, spec. grav. 0·840, in
order to free it from any adhering tarry matter; when the pyroxiline is left nearly pure.
If it be dissolved in boiling alcohol, or hot oil of turpentine, it crystallizes regularly on
cooling, in right square prisms, of a fine yellow colour, that look opaque to the naked
eye, but when examined under the microscope, have the transparency and colour of
ferroprussiate of potash. Its turpentine solution affords crystals of a splendid orange-red
colour, having the appearance of minute plates, whose form is not discernible by
the naked eye, but when examined by the microscope, they are seen to be thin right rectangular
prisms. The orange-red colour is only the effect of aggregation; for when
ground to powder, these crystals become yellow; and under the microscope, the difference
in colour between the two is very slight. Its melting point is 318° F. It sublimes
at 300° in free air; heated in a close tube in a bath of mercury, it emits vapour
at 400°; it then begins to decompose, and is totally decomposed at 500°. Sulphuric
acid decomposes it, producing a beautiful blue colour, which passes into crimson, as the
acid attracts water from the atmosphere, and it totally disappears on plentiful dilution
with water, leaving carbon of a dirty-brown colour. Its alcoholic or turpentine solution
imparts a permanent yellow dye to vegetable or animal matter.
Pyroxiline consists, according to the analysis of Drs. Apjohn and Gregory, of—carbon,
75·275; hydrogen, 5·609; oxygen, 19·116, in 100 parts.
[1054]
QUARTATION, is the alloying of one part of gold that is to be refined, along
with three parts of silver, so that the gold shall constitute one quarter of the whole, and
thereby have its particles too far separated to be able to protect the other metals originally
associated with it, such as silver, copper, lead, tin, palladium, &c., from the action of the
nitric or sulphuric acid employed in the subsequent parting process. See Refining.
QUARTZ, has been described in the article Lapidary.
QUASSIA, is the wood of the root of the Quassia excelsa, a tree which grows in
Surinam, the East Indies, &c. It affords to water an intensely bitter decoction, which
is occasionally used in medicine, and was formerly substituted by some brewers for hops,
but is now prohibited under severe penalties. It affords a safe and efficacious fly-water,
or poison for flies.
QUEEN’s WARE. See Pottery.
QUEEN’s YELLOW, is an antient name of Turbith Mineral, or yellow subsulphate
of mercury.
QUERCITRON, is the bark of the Quercus nigra, or yellow oak, a tree which grows
in North America, The colouring principle of this yellow dye-stuff has been called
Quercitrin, by its discoverer Chevreul. It forms small pale yellow spangles, like those
of Aurum musivum, has a faint acid reaction, is pretty soluble in alcohol, hardly in ether,
and little in water. Solution of alum developes from it, by degrees, a beautiful yellow
dye. See Calico-printing and Yellow Dye.
QUICKSILVER; see Mercury.
QUININA. This medicine is now prepared in such quantities as to constitute a
chemical manufacture. Quinina and cinchonina are two vegetable alkalis, which exist
in Peruvian bark or cinchona; the pale or gray bark contains most cinchonina, and
the yellow bark most quinina. The methods of extracting these bases are very various.
In general, water does not take them out completely, because it transforms the neutral
salts in the barks into more soluble acidulous salts, and into less soluble sub-salts. To
exhaust the bark completely, one or other of the following solvents is employed:
1. Alcohol.—An extract by this menstruum, is to be treated with very dilute
warm muriatic acid, in order to dissolve every thing thus soluble; the acid liquor is to
be saturated with magnesia, by boiling it with an excess of this earth; the precipitate
is to be dried, filtered, and then exhausted by boiling-hot alcohol.
2. Dilute acids.—Boil the bark, coarsely pounded, with eight times its weight of
water, containing 5 per cent. of the weight of the bark of sulphuric acid. This treatment
is to be repeated with a fresh quantity of dilute acid. The whole liquors must
be filtered, the residuum strained, and the solution mixed with quicklime, equal to one
fourth of the bark employed. This mixture, after having been well stirred, is to be
strained, whenever it acquires an alkaline reaction, that is, tinges reddened litmus paper
blue, or turmeric brown. The calcareous mass is to be now washed with a little
water, and dried, and then boiled thrice with spirit of wine of sp. grav. 0·836. This
solution being filtered, is to be mixed with a little water, and distilled. The bases,
cinchonina and quinina, remain under the form of a brown viscid mass, and must be
purified by subsequent crystallization, after being converted into sulphates.
3. An alkali, and then an acid.—The object of this process is, to retain the
vegetable alkalis in the bark, while with the alkaline water we dissolve out the acids,
the colouring matters, the extractive, the gum, &c. Boil for an hour one pound of
the bark with six pounds of water, adding by degrees a little solution of potash, so
that the liquor may have still an alkaline taste when the boiling is over. Allow it to cool,
filter, wash the residuum with a little water, and squeeze it. Diffuse it next in tepid
water, to which add by degrees a little muriatic acid, till after a prolonged digestion
the mixture shall perceptibly redden litmus paper. Filter the liquor, and boil it with
magnesia. The precipitate being washed and dried, is to be treated with hot alcohol,
which dissolves the quinina and cinchonina.
Obtained by any of the above methods, the quinina and cinchonina are more or less
coloured, and may be blanched by dissolving them in dilute muriatic acid, and treating
the solution with animal charcoal.
There are several methods of separating these two vegetable alkalis.
1. When their solution in spirit of wine is evaporated by heat to a certain point, the
greater part of the cinchonina crystallizes on cooling, while the quinina remains dissolved.
[1055]
2. Digestion in ether dissolves the quinina, and leaves the cinchonina.
3. We may supersaturate slightly the two bases with sulphuric acid. Now as the
supersulphate of quinina is sparingly soluble, the liquor need only to be evaporated to a
proper point to crystallize out that salt, while the supersulphate of cinchonina continues
in solution with very little of the other salt. Even this may be separated by precipitating
the bases, and treating them, as above prescribed, with alcohol or ether.
One pound of bark rarely yields more than 2 drams of the bases. One pound of red bark
afforded, to Pelletier and Caventou, 74 grains of cinchonina, and 107 grains of quinina.
Quinina is composed of 75·76 carbon, 7·52 hydrogen, 8·11 azote, and 8·61 oxygen.
The salts of quinina are distinguished by their strong taste of Peruvian bark, and if
crystallized, by their pearly lustre. Most of them are soluble in water, and some also
in ether and alcohol. The soluble salts are precipitated by the oxalic, gallic, and tartaric
acids, and by the salts of these acids. Infusion of nutgalls also precipitates them.
The sulphate of quinina is the only object of manufacturing operations. Upon the
brownish viscid mass obtained in any of the above processes for obtaining quinina, pour
very dilute sulphuric acid, in sufficient quantity to produce saturation. The solution
must be then treated with animal charcoal, filtered, evaporated, allowed to cool, when it
deposits crystals. 1000 parts of bark afford, upon an average, 12 parts of sulphate.
The sulphate of cinchonina, which is formed at the same time, remains dissolved in the
mother-waters.
The neutral sulphate of quinina occurs in small transparent right prismatic needles.
By spontaneous evaporation of their solution, larger crystals may be procured. They
contain 242⁄3 per cent. of water; and, therefore, melt when exposed to heat. They dissolve
in 11 parts of water at ordinary temperatures; are much more soluble in hot spirit
of wine, somewhat dilute, than in cold; and are nearly insoluble in anhydrous alcohol.
If they be well dried, they possess the property of becoming luminous when heated a little
above the boiling point of water, especially when they are rubbed. The sulphate is,
in this case, charged with vitreous electricity.
There is a sub-sulphate, but it is applied to no use. The effloresced sulphate, called
by some bisulphate, is preferred for medical practice. The extensive sale and high
price of sulphate of quinina, have given rise to many modes of adulteration. It has
been mixed with boracic acid, margaric acid, sugar, sugar of manna, gypsum, &c. By
incinerating a little of the salt upon a slip of platina, the boracic acid and gypsum remain,
while the quinine is dissipated; sugar and margaric acid exhale their peculiar
smoke and smell; or they may be dissolved out by a few drops of water. Cinchonina
may be detected by adding ammonia to the solution, and treating the precipitate
with ether, which leaves that vegeto-alkali.
QUINTESSENCE. The alchemists understood by this term, now no longer in
scientific use, the solution in alcohol of the principles which this menstruum can extract
from aromatic plants or flowers, by digestion, during some days, in the sun, a stove, or upon
a sand-bath slightly warmed. A quintessence, therefore, corresponds to the alcoholic
tincture or essence (not essential oil) of the present day. See Perfumery.
RAISINS, are grapes allowed to ripen and dry upon the vine. The best come
from the south of Europe, as from Roquevaire in Provence, Calabria, Spain, and Portugal.
Fine raisins are also imported from Smyrna, Damascus, and Egypt. Sweet
fleshy grapes are selected for maturing into raisins, and such as grow upon the sunny
slopes of hills sheltered from the north winds. The bunches are pruned, and the vine
is stripped of its leaves, when the fruit has become ripe; the sun then beaming full
upon the grapes, completes their saccharification, and expels the superfluous water. The
raisins are plucked, cleansed, and dipped for a few seconds in a boiling lye of wood ashes and
quicklime, at 12 or 13 degrees of Baumé’s areometer. The wrinkled fruit is lastly drained,
dried, and exposed in the sun upon hurdles of basket-work during 14 or 15 days.
The finest raisins are those of the sun, so called; being the plumpest bunches, which
are left to ripen fully upon the vine, after their stalks have been half cut through.
The amount of raisins imported for home consumption was, in the year 1836,
156,495 cwts.; in 1837, 152,635 cwts.
RAPE-SEED, imported for home consumption in 1836, 561,457 bushels; in 1837,
937,526 bushels. See Oils, unctuous.
RASP, MECHANICAL, is the name given by the French to an important machine
much used for mashing beet-roots. See Sugar.
[1056]
RATAFIA, is the generic name, in France, of liqueurs compounded with alcohol,
sugar, and the odoriferous or flavouring principles of vegetables. Bruised cherries with
their stones are infused in spirit of wine to make the ratafia of Grenoble de Teyssère.
The liquor being boiled and filtered, is flavoured, when cold, with spirit of noyau, made
by distilling water off the bruised bitter kernels of apricots, and mixing it with alcohol.
Syrup of bay laurel and galango are also added.
REALGAR, Red Orpiment. (Arsenic rouge sulphuré, Fr.; Rothes schwefelarsenik,
Germ.) This ore occurs in primitive mountains, associated sometimes with native
arsenic, under the form of veins, efflorescences, very rarely crystalline; as also in volcanic
districts; for example, at Solfatara near Naples; or sublimed in the shape of stalactites,
in the rents and craters of Etna, Vesuvius, and other volcanoes. Its spec. grav. varies
from 3·3 to 3·6. It has a fine scarlet colour in mass, but orange-red in powder, whereby
it is distinguishable from cinnabar. It is soft, sectile, readily scratched by the nail;
its fracture is vitreous and conchoidal. It volatilizes easily before the blowpipe, emitting
the garlic smell of arsenic, along with that of burning sulphur. It consists of, arsenic 70,
sulphur 30, in 100 parts. It is employed sometimes as a pigment. Factitious orpiment
is made by distilling, in an earthen retort, a mixture of sulphur and arsenic, of orpiment
and sulphur, or of arsenious acid, sulphur, and charcoal. It has not the rich colour of the
native pigment, and is much more poisonous; since, like factitious orpiment, it always
contains more or less arsenious acid.
RECTIFICATION, is a second distillation of alcoholic liquors, to free them from
whatever impurities may have passed over in the first.
RED LIQUOR, is a crude acetate of alumina, employed in calico-printing, and prepared
from pyrolignous acid; which see.
REED, is the well-known implement of the weaver, made of parallel slips of
metal or reeds, called dents. A thorough knowledge of the adaptation of yarn
of a proper degree of fineness to any given measure of reed, constitutes one of the
principal objects of the manufacturer of cloths; as upon this depends entirely the
appearance, and in a great degree the durability, of the cloth when finished. The
art of performing this properly, is known by the names of examining, setting, or
sleying, which are used indiscriminately, and mean exactly the same thing. The
reed consists of two parallel pieces of wood, set a few inches apart, and they are of
any given length, as a yard, a yard and a quarter, &c. The division of the yard being
into halves, quarters, eighths, and sixteenths; the breadth of a web is generally expressed
by a vulgar fraction, as 1⁄4, 4⁄4, 5⁄4, 6⁄4; and the subdivisions by the eighths or sixteenths,
or nails, as they are usually called, as 7⁄8, 9⁄8, 11⁄8, &c., or 13⁄16, 15⁄16, 19⁄16, &c. In Scotland,
the splits of cane which pass between the longitudinal pieces or ribs of the reed,
are expressed by hundreds, porters, and splits. The porter is 20 splits, or 1⁄5th of an hundred.
In Lancashire and Cheshire a different mode is adopted, both as to the measure and
divisions of the reed. The Manchester and Bolton reeds are counted by the number of
splits, or, as they are there called, dents, contained in 241⁄4 inches of the reed. These
dents, instead of being arranged in hundreds, porters, and splits, as in Scotland, are calculated
by what is there termed hares or bears, each containing 20 dents, or the same
number as the porter in the Scotch reeds. The Cheshire or Stockport reeds, again, receive
their designation from the number of ends or threads contained in one inch, two
ends being allowed for every dent, that being the almost universal number in every
species and description of plain cloth, according to the modern practice of weaving, and
also for a great proportion of fanciful articles.
The number of threads in the warp of a web is generally ascertained with considerable
precision by means of a small magnifying glass, fitted into a socket of brass, under which
is drilled a small round hole in the bottom plate of the standard. The number of threads
visible in this perforation, ascertains the number of threads in the standard measure
of the reed. Those used in Scotland have sometimes four perforations, over any one of
which the glass may be shifted. The first perforation is 1⁄4 of an inch in diameter, and
is therefore well adapted to the Stockport mode of counting; that is to say, for ascertaining
the number of ends or threads per inch; the second is adapted for the Holland
reed, being 1⁄200th part of 40 inches; the third is 1⁄700th of 37 inches, and is adapted for
the now almost universal construction of Scotch reeds; and the fourth, being 1⁄200th of
34 inches, is intended for the French cambrics. Every thread appearing in these
respective measures, of course represents 200 threads, or 100 splits, in the standard
breadth; and thus the quality of the fabric may be ascertained with considerable precision,
even after the cloth has undergone repeated wettings, either at the bleaching-ground
or dye-work. By counting the other way, the proportion which the woof bears
to the warp is also known, and this forms the chief use of the glass to the manufacturer
and operative weaver, both of whom are previously acquainted with the exact measure
of the reed.
[1057]
Comparative Table of 37-inch reeds, being the standard used throughout Europe, for
linens, with the Lancashire and Cheshire reeds, and the foreign reeds used for holland
and cambric.
| Scotch. |
Lanca-
shire. |
Chesh-
ire. |
Dutch
holland. |
French
cambric. |
| 600 |
20 |
34 |
550 |
653 |
| 700 |
24 |
38 |
650 |
761 |
| 800 |
26 |
44 |
740 |
870 |
| 900 |
30 |
50 |
832 |
979 |
| 1000 |
34 |
54 |
925 |
1089 |
| 1100 |
36 |
60 |
1014 |
1197 |
| 1200 |
40 |
64 |
1110 |
1300 |
| 1300 |
42 |
70 |
1202 |
1414 |
| 1400 |
46 |
76 |
1295 |
1464 |
| 1500 |
50 |
80 |
1387 |
1602 |
| 1600 |
52 |
86 |
1480 |
1752 |
| 1700 |
56 |
92 |
1571 |
1820 |
| 1800 |
58 |
96 |
1665 |
1958 |
| 1900 |
62 |
104 |
1757 |
2067 |
| 2000 |
66 |
110 |
1850 |
2176 |
In the above table, the 37-inch is placed first. It is called Scotch, not because it
either originated or is exclusively used in that country. It is the general linen reed of
all Europe; but in Scotland it has also been adopted as the regulator of her cotton
manufactures.
REFINING OF GOLD AND SILVER; called also Parting. (Affinage d’argent,
Départ, Fr.; Scheidung in die quart, Germ.) For several uses in the arts, these precious
metals are required in an absolutely pure state, in which alone they possess their
malleability and peculiar properties in the most eminent degree. Thus, for example,
neither gold nor silver leaf can be made of the requisite fineness, if the metals contain
the smallest portion of copper alloy. Till within these ten or twelve years, the parting
of silver from gold was effected every where by nitric acid; it is still done so in all the
establishments of this country, except the Royal Mint; and in the small refining-houses
abroad. The following apparatus may be advantageously employed in this
operation. It will serve the double purpose of manufacturing nitric acid of the utmost
purity, and of separating silver from gold by its means.
1. On procuring nitric acid for parting.—a is a platinum retort or alembic; b is
its capital, terminating above in a tubulure, to which a kneed tube of platinum,
about 2 feet long, is adapted; c is the tubulure of the retort, for supplying acid
during the process, and for inspecting its progress. It is furnished with a lid ground
air-tight, which may be secured in its place by a weight. e is a stoneware pipe, about
two inches diameter, and several feet long, according to the locality in which the
operation is to be carried on. It is made in lengths fitted to one another, and secured
at the joints with loam-lute. The one bend of this earthenware hard salt-glazed
pipe is adapted to receive the platinum tube, and the other bend is inserted into a tubulure
in the top of the stoneware drum f. The opening l, l, in the middle of the top of f, is[1058]
for inspecting the progress of the condensation of acid; and the third tubulure terminates
in a prolonged pipe i, i, consisting of several pieces, each of which enters from above
conically into the one below. The joinings of the upper pieces need not be tightly
luted, as it is desirable that some atmospherical oxygen should enter, to convert the
relatively light nitrous gas into nitrous or nitric acid vapour, which when supplied with
moisture will condense and fall down in a liquid state. To supply this moisture in the most
diffusive form, the upright stoneware pipes i, i, l, l, (at least 3 inches diameter, and 12 feet
high,) should be obstructed partially with flint nodules, or with siliceous pebbles; and
water should be allowed to trickle upon the top pebble from a cistern placed above.
Care must be taken to let the water drop so slowly as merely to preserve the pebbles
in a state of humidity. h is a stopcock, of glass or stoneware, for drawing off the acid
from the cistern f. k is a section of a small air-furnace, covered in at top with an iron
ring, on which the flat iron ring of the platinum frame rests.
g, g, is a tub in which the stoneware cistern stands, surrounded with water, kept constantly
as cold as possible by passing a stream through it; the spring water entering by a
pipe that dips near to the bottom, and the hot water escaping at the upper edge.
With the above apparatus, the manufacture of pure nitric acid is comparatively easy
and economical. Into the alembic a, 100 pounds (or thereby) of pure nitre, coarsely
bruised if the crystals be large, are to be put; the capital is then to be adapted, and the
platinum tube (the only movable one) luted into its place. Twenty pounds of strong
sulphuric acid are now to be introduced by the tubulure c, and then its lid must be put
on. No heat must yet be applied to the alembic. In about an hour, another ten pounds
of acid may be poured in, and so every hour, till 60 pounds of acid have been added.
A few hours after the affusion of the last portion of acid, a slight fire may be kindled in
the furnace k.
By judicious regulation of the heat, the whole acid may be drawn off in 24 hours;
its final expulsion being aided by the dexterous introduction of a quart or two of boiling
water, in small successive portions, by the tubulure c, whose lid must be instantly shut
after every inspersion. The most convenient strength of acid for the parting process, is
when its specific gravity is about 1·320, or when a vessel that contains 16 ounces of
pure water, will contain 211⁄8 of the aquafortis. To this strength it should be brought
very exactly by the aid of a hydrometer.
Its purity is easily ascertained by letting fall into it a few drops of solution of silver;
and if no perceptible milkiness ensues, it may be accounted good. Should a white
cloud appear, a few particles of silver may be introduced, to separate whatever muriatic
acid may be present, in the form of chloride of silver. Though a minute quantity of
sulphuric acid should exist in the nitric, it will be of no consequence in the operation of
parting.
2. On parting by the nitric acid, called by the Mexicans, “Il apartado.”—The principle
on which this process is founded, is the fact of silver being soluble in nitric acid,
while gold is insoluble in that menstruum. If the proportion of gold to that of silver
be greater than one to two, then the particles of the former metal so protect or envelop
those of the latter, that the nitric acid, even at a boiling heat, remains quite inactive on the
alloy. It is indispensable, therefore, that the weight of the silver be at least double that
of the gold. 100 pounds of silver take 38 pounds of nitric acid, of specific gravity
1·320, for oxidizement, and 111 for solution of the oxide; being together 149; but
the refiner often consumes, in acid of the above strength, more than double the weight
of silver, which shows great waste, owing to the imperfect means of condensation
employed for recovering the vapours of the boiling and very volatile acid.
By the apparatus above delineated, the 38 pounds of acid expended in oxidizing the silver,
become nitrous gas in the first place, and are afterwards reconverted in a great measure
into nitric acid by absorption of atmospherical oxygen; so that not one-fifth need be lost,
under good management. As the acid must be boiled on the granulated garble, or alloy,
to effect the solution of the silver, by proper arrangements the vapours may be entirely
condensed, and nearly the whole acid be recovered, except the 111 parts indispensable to
constitute nitrate of silver. Hence, with economical management, 120 pounds of such
acid may be assigned as adequate to dissolve 100 of silver associated with 50 of gold.
It must here be particularly observed, that 100 pounds of copper require 130 pounds
of the above acid for oxidizement; and 390 for solution of the oxide; being 520 pounds
in whole, of which less than 1⁄4 part could be recovered by the above apparatus. It is
therefore manifest that it is desirable to employ silver pretty well freed from copper by
a previous process; and always, if practicable, a silver containing some gold.
These data being assumed as the bases of the parting operation, 60 pounds of gold and
silver alloy or garble finely granulated, containing not less than 40 pounds of silver, are to
be introduced into the ten-gallon alembic of platinum, fig. 931., and 80 pounds of nitric
acid, of 1·320, is to be poured over the alloy; a quantity which will measure 6 gallons
imperial. As for the bulk of the alloy, it is considerably less than half a gallon. Abundance[1059]
of space therefore remains in the alembic for effervescence and ebullition, provided
the fire be rightly tempered.
By the extent of stoneware conducting pipe e, which should not be less than 40 feet,
by the dimensions and coldness of the cistern f, and by the regenerating influence of the
vertical aerial pipe filled with moist pebbles i, i, it is clear, that out of the 80 pounds
of nitric acid, specific gravity 1·320, introduced at first, from 20 to 30 will be recovered.
Whenever the effervescence and disengagement of nitrous red fumes no longer appear
on opening the orifice c, the fire must be removed, and the vessel may be cooled by the
application of moist cloths. The alembic may be then disengaged from the platinum
tube, and lifted out of its seat. Its liquid contents must be cautiously decanted off,
through the orifice c, into a tub nearly filled with soft water. On the heavy pulverulent
gold which remains in the vessel, some more acid should be boiled, to carry off
any residuary silver. This metallic powder, after being well washed with water, is to
be dried, fused along with a little nitre or borax, and cast into ingots.
Plates of copper being immersed in the nitric solution contained in wooden or stoneware
cisterns, will throw metallic silver down, while a solution of nitrate of copper,
called blue water, will float above. The pasty silver precipitate is to be freed from the
nitrate of copper, first, by washing with soft water, and next, by strong hydraulic pressure
in cast-iron cylinders. The condensed mass, when now melted in a crucible along with
a little nitre and borax, is fine silver.
The above apparatus has the further advantage of enabling the operator to recover a
great portion of his nitric acid, by evaporating the blue water to a state approaching to
dryness, with the orifices at c, and at the top of the capital, open. In the progress of
this evaporation, nothing but aqueous vapour escapes. Whenever the whole liquid is
dissipated, the pipe d is to be re-adjusted, and the lid applied closely to c. The heat
being now continued, and gradually increased, the whole nitric acid will be expelled
from the copper oxide, which will remain in a black mass at the bottom of the alembic.
The contrivance for letting water trickle upon the pebbles, must be carefully kept in
play, otherwise much of the evolved acid would be dissipated in nitrous fumes. With
due attention to the regenerative plan, a great part of the acid may be recovered, at no
expense but that of a little fuel.
The black oxide of copper thus obtained, is an economical form of employing that
metal for the production of the sulphate; 100 pounds of it, with 1221⁄2 of sulphuric
acid diluted with water, produce 3121⁄2 pounds of crystallized sulphate of copper. A
leaden boiler is best adapted for that operation. 100 pounds of silver are precipitable
from its solution in nitric acid, by 29 of copper. If more be needed, it is a proof that
a wasteful excess of acid has existed in the solution.
In parting by nitric acid, the gold generally retains a little silver; as is proved by
the cloud of chloride of silver which it affords, at the end of some hours, when dissolved
in aqua regia. And on the other hand, the silver retains a little gold. These facts
induced M. Dizé, when he was inspector of the French mint, to adopt some other process,
which would give more accurate analytical results; and after numerous experiments,
he ascertained that sulphuric acid presented great advantages in this point of
view, since with it he succeeded in detecting, in silver, quantities of gold which had
eluded the other plan of parting. The suggestion of M. Dizé has been since universally
adopted in France. M. Costell, about nine or ten years ago, erected in Pomeroy-street,
Old Kent-road, a laboratory upon the French plan, for parting by sulphuric
acid; but he was not successful in his enterprise; and since he relinquished the business,
Mr. Matheson introduced the same system into our Royal Mint, under the management
of M. Costell’s French operatives. In the Parisian refineries, gold, to the amount of
one-thousandth part of the weight, has been extracted from all the silver which had
been previously parted by the nitric acid process; being 3500 francs in value upon
every thousand kilogrammes of silver.
I shall give first a general outline of the method of parting by sulphuric acid, and
then describe its details as I have lately seen them executed upon a magnificent scale
in an establishment near Paris.
The most suitable alloy for refining gold, by the sulphuric-acid process, is the compound
of gold, silver, and copper, having a standard quality, by the cupel, of from 900 to 950
millièmes, and containing one-fifth of its weight of gold. The best proportions of the
three metals are the following:—silver, 725; gold, 200; copper, 75; = 1000. It has
been found that alloys which contain more copper, afford solutions that hold some
anhydrous sulphate of that metal in solution, which prevents the gold from being readily
separated; and that alloys containing more gold, are not acted on easily by the sulphuric
acid. The refiner ought, therefore, when at all convenient, to reduce the alloys that
he has to treat, to the above-stated proportions. He may effect this purpose either by
fusing the coarser alloys with nitre in a crucible, or by adding finer alloy, or even fine
silver, or finally, by subjecting the coarser alloys to a previous cupellation with lead on[1060]
the great scale. As to gold or silver bullion, which contains lead and other easily oxidizable
metals besides copper, the refiner ought always to avoid treating them by sulphuric
acid; and should separate, first of all, these foreign metals by the agency of nitre, if
they exist in minute quantity; but if in larger, he should have recourse to the cupel.
Great advantage will therefore be derived from the judicious preparation of the alloy to
be refined.
For an alloy of the above description, the principal Parisian refiners are in the habit
of employing thrice its weight of sulphuric acid, in order to obtain a clear solution of
sulphate of silver, which does not too suddenly concrete on cooling, so as to obstruct its
discharge from the alembic by decantation. A small increase in the quantity of copper,
calls for a considerable increase in the quantity of acid.
Generally speaking, one-half of the sulphuric acid strictly required for converting the
silver and copper into sulphates, is decomposed into sulphurous acid, which is lost to
the manufacturer, unless he has recourse to the agency of nitrous acid.
The process for silver containing but little gold, consists of five different operations.
1. Upon several furnaces, one foot in diameter, egg-shaped alembics of platinum are
mounted, into each of which are put 3 kilogrammes (8 lbs. troy) of the granulated
silver, containing a few grains of gold per pound, and 6 kilogrammes of concentrated
sulphuric acid. The alembics are covered with conical capitals, ending in bent tubes,
which conduct the acid vapours into lead pipes of condensation; and the furnaces are
erected under a proper hood. As the cold acid is inoperative, it must be set a boiling,
at which temperature it gives up one atom of its oxygen to the metal, and is transformed
into sulphurous acid, which escapes in a gaseous state. Some of the undecomposed
sulphuric acid immediately combines with the oxide into a sulphate, which subsides,
in the state of a crystalline powder, to the bottom of the vessel. The solution
goes on vigorously, with a copious disengagement of sulphurous acid gas, only during
the two or three first hours; after which it proceeds slowly, and is not completed till
after a digestion of nearly twelve hours more. During the ebullition a considerable
quantity of sulphuric acid vapour escapes along with the sulphurous acid gas; the former
of which is readily condensed in a large leaden receiver immersed in a cistern of cold
water, if need be. It has been proposed to condense the sulphurous acid, by leading
it over extensive surfaces of lime-pap, as in the coal-gas purifiers.
2. When the whole silver has been converted into sulphate, this is to be emptied out
of the alembic into water contained in a round-bottomed receiver lined with lead, and
diluted till the density of the solution marks from 15° to 20° Baumé. The small portion
of gold, in the form of a brown powder, which remains undissolved, having been
allowed to settle to the bottom, the supernatant solution of silver is to be decanted carefully
off into a leaden cistern, and the powder being repeatedly edulcorated with water,
the washings are to be added to it. The silver is now to be precipitated by plunging
plates of copper in the solution, and the magma which falls is to be well washed, and
freed from the residuary particles of sulphate of copper by powerful compression.
3. The silver, precipitated and dried as above described, is melted in a crucible, and
cast into an ingot.
4. The gold powder is also dried and cast into an ingot, a little nitre being added in
the fusion, to oxidize and separate any minute particles of copper that may perchance
have been protected from the solvent action of the acid.
5. As the sulphate of copper is of considerable value, its solution is to be neutralized,
evaporated in leaden pans to a proper strength, and set aside to crystallize in leaden
cisterns. The farmers throughout France consume an immense quantity of this salt.
They sprinkle a weak solution of it (at 2° or 3° Baumé) over their grain before sowing
it, in order to protect it against the ravages of birds and insects.
The pure gold, at the instant of its separation from the alloy by the action of sulphuric
acid, being in a very fine powder, and lying in close contact with the platinum, under the
influence of a boiling menstruum, which brightens the surfaces of the two metals, and
raises their temperature to fully the 600th degree of Fahrenheit’s scale, tends to become
partially soldered to the platinum, and may thus progressively thicken the bottom of the
still. The importance of preserving this vessel entire, and of economizing the fuel requisite
to heat its contents, induces the refiner to detach the crust of gold from time to
time, by passing over the bottom of the still, in small quantities, a dilute nitro-muriatic
acid, which acts readily on gold, but not on platinum. But as this operation
is a very delicate one, it must be conducted with great circumspection. The danger of
such adhering deposits is much increased by using too high a heat, and too small a
body of acid, relatively to the metals dissolved. Hence it is advantageous to employ
alembics of large size. Should any lead or tin get into the platinum still, while the hot
acid is in it, the precious vessel would be speedily destroyed; an accident which has not
unfrequently happened. Each operation may be conveniently finished in twelve hours;[1061]
so that each alembic may refine with ease 160 marcs daily. Some persons work more
rapidly, but such haste is hazardous.
The Parisian refiners restore to the owners the whole of the gold and silver contained
in the ingots, reserving to themselves the copper which formed the alloy, and charging
only the sum of 51⁄2 francs per kilogramme (2·68 lbs. troy) for the expense of the
parting of the metals.
If they are employed to refine an ingot of silver containing less than one-tenth of gold,
they retain for themselves a two-thousandth part of the gold, and all the copper, existing
in the alloy; return all the rest of the gold, with the whole of the silver, in the ingot; and
give, besides, to the owners a premium or bonus, which amounted lately to 3⁄4 of a franc
on the kilogramme of metal. Should the owner desire to have the whole of the gold and
silver contained in his ingot, the refiner then demands from him 2 francs and 68 centimes
per kilogramme, retaining the copper of the alloy. As to silver ingots of low
standard, the perfection of the refining processes is such, that the mere copper contained
in them pays all the costs; for in this case, the refiner restores to the proprietor of the
ingot as much fine silver as the assay indicated to exist in the ingot, contenting himself
with the copper of the alloy. See infrà.
The chemical works of M. Poizat, called affinage d’argent, on the bank of the canal
de l’Ourcq, in the vicinity of Paris, are undoubtedly the most spacious and best arranged
for refining the precious metals, which exist in the world. On being introduced to this
gentleman, by my friend and companion M. Clement-Desormes, he immediately expressed
his readiness to conduct me through his fabrique, politely alluding to the
French translation of my Dictionary of Chemistry, which lay upon the desk of his
bureau. The principal room is 240 feet long, 40 feet wide, and about 30 feet high. A
lofty chimney rises up through the middle of the apartment, and another at each of its
ends. The one space, 120 feet long, to the right of the central chimney, is allotted to
the processes of dissolving the silver, and parting the gold; the other, to the left, to the
evaporation and crystallization of the sulphate of copper, and the concentration of the
recovered sulphuric acid.
M. Poizat melts his great masses of silver in pots made of malleable iron, capable of
holding several cwts. each; and granulates it by pouring it into water contained in large
iron pans. The granulated silver is dried with heat, and carried into a well lighted office
enclosed by glazed casements, to be weighed, registered, and divided into determinate portions.
Each of these is put into a cast-iron pot, of a flattened hemispherical shape, about
2 feet in diameter, covered with an iron lid, made in halves, and hinged together in the
middle line. From the top of the fixed lid a bent pipe issues, and proceeds downwards
into an oblong leaden chest sunk beneath the floor. Four of the above cast-iron pots
stand in a line across the room, divided into two ranges, with an intervening space for
passing between them. The bottoms of the pots are directly heated by the flame, one
fire serving for two pots. Two parts of concentrated sulphuric acid by weight are poured
upon every part of granulated silver, and kept gently boiling till the whole silver be
converted into a pasty sulphate.
From the underground leaden chests, a leaden pipe, 4 inches in diameter, rises vertically,
and enters the side of a leaden chamber, which is supported upon strong cross-beams
or rafters, a little way beneath the roof of the apartment. This chamber, which is 30
feet long, 10 feet wide, and 6 feet high, is intended to condense the sulphuric acid
vapours, along with some of the sulphurous acid; that of the latter being promoted by the
admission of nitrous gas and air, which convert it into sulphuric acid. From the further
end of this chamber, a large square leaden pipe returns with a slight slope towards the
middle of the room, and terminates at the right-hand side of the central chimney, in
a small leaden chest, for receiving the drops of acid which are condensed in the pipe.
From that chest a pipe issues, to discharge into the high central chimney the incondensable
gases, and also to maintain a constant draught through the whole series of
leaden chambers back to the cast-iron hemispherical pots.
Besides the above cast-iron pots, destined to dissolve only the coarse cupreous silver,
containing a few grains of gold per pound, there are, in the centre of the apartment, at
the right-hand side of the chimney, 6 alembics of platinum, in which the rich alloys of
gold and silver are treated in the process of refining gold.
The pasty sulphate of silver obtained in the iron pots, is transferred by cast-iron ladles
with long handles into large leaden cisterns, adjoining the pots, and there diluted with
a little water to the density of 36° Baumé. Into this liquor, steam is admitted through
a series of upright leaden pipes arranged along the side of the cistern, which speedily
causes ebullition, and dilutes the solution eventually to the 22d degree of Baumé. In
this state, the liquid supersulphate is run off by leaden syphons into large oblong leaden
cisterns, rounded at the bottom; and is there exposed to the action of ribands of copper,
like thin wood shavings. The metallic silver precipitates in a pasty form; and the[1062]
supernatant sulphate of copper is then run off into a cistern, upon a somewhat lower
level, where it is left to settle and become clear.
The precipitate of silver, called by the English, water-silver, and by the French, chaux
d’argent, is drained, then strongly squeezed in a square box of cast iron, by the action of
a hydraulic press; in which 60 pounds of silver are operated upon at once.
The silver lumps are dried, melted in black lead crucibles, in a furnace built near the
silver end of the room, where the superintendent sits in his bureau—a closet enclosed by
glazed casements, like a green-house. The whole course of the operations is so planned,
that they are made to commence near the centre with the mixed metals, and progressively
approach towards the office end of the apartment as the parting processes
advance. Here the raw material, after being granulated and weighed, was given out,
and here the pure gold and silver are finally eliminated in a separate state.
In the other half of the hall, the solutions of sulphate of copper are evaporated
in large shallow leaden pans, placed over a range of furnaces; from which, at the proper
degree of concentration, they are run off by syphons into crystallizing pans of the same
metal. From the mother-waters, duly evaporated, a second crop of crystals is obtained;
and also a third, the last being anhydrous, from the great affinity for water possessed by
the strong sulphuric acid with which they are now surrounded. The acid in this way
parts with almost the whole of the cupreous oxide, and is then transferred into a large
alembic of platinum (value 1000l.), to be rendered fit, by re-concentration, for acting
upon fresh portions of granulated silver. The capital of that alembic is connected
with a leaden-worm, which traverses an oblong vessel, through which a stream of cold
water flows.
The crystallized sulphate of copper fetched, two years ago, 30l. a ton. It is almost
all sold to the grocers in the towns of the agricultural districts of France. In the above
establishment of M. Poizat, silver to the value of 10,000l. can be operated upon daily.
There is a steam engine of 6-horse power placed in a small glazed chamber at one side
of the parting hall, which serves to work all his leaden pumps for lifting the dilute
sulphuric acid and acidulous solutions of copper into their appropriate cisterns of concentration,
as also to grind his old crucibles, and drive his amalgamation mill, consisting of
a pair of vertical round-edged wheels, working upon one shaft, in a groove formed round a
central hemisphere—of cast iron. After the mercury has dissolved out of the ground
crucibles all the particles of silver which it can find, the residuary earthy matter is sold
to the sweep-washers. The floor of the hall around the alembics, pots, and cisterns, is
covered with an iron grating, made of bars having one of their angles uppermost, to act
as scrapers upon the shoes of the operatives. The dust collects in a vacant space left
beneath the grating, whence it is taken to the amalgamation mill. The processes are
so well arranged and conducted by M. Poizat, that he can execute as much business in
his establishment with 10 workmen as is elsewhere done with from 40 to 50; and with less
than 3 grains of gold, in one Paris pound or 7561 grains of silver, he can defray the
whole expenses of the parting or refining.
Since 26 parts of copper afford 100 of the crystallized sulphate, the tenth of copper
present in the dollars, and most foreign coins, will yield nearly four times its weight of
blue vitriol; a subsidiary product of considerable value to the refiner.
The works of M. Poizat are so judiciously fitted up as to be quite salubrious, and have
not those “very mischievous effects upon the trachea,” which Mr. Matheson states as
being common in his refinery works in the Royal Mint.[48] But, in fact, as refining
by sulphuric acid is always a nuisance to a neighbourhood, it is not suffered in the
Monnaie Royale of Paris; but is best and most economically performed by private
enterprise and fair competition, which is impossible in London, on account of the
anomalous privilege, worth at least 2000l. a year, possessed by Mr. Matheson, who
works most extensively for private profit on a public plant, fitted up with a lofty
chimney, platinum vessels to the value of 3000l., and other apparatus, at the cost of
the government. His charge to the crown for refining gold per lb. troy, is 6s. 6d.;
that of the refiners in London, who are obliged, for fear of prosecution, to employ the
more expensive, but more condensable, nitric acid, is only 4s. That of the Parisian
refiners is regulated as follows. For the dealers in the precious metals:—
For gold bullion containing silver, and more than 100⁄1000 of gold, 6 fr. 12 c. per kilogramme,
= 2 fr. 29 c. per lb. troy.
For silver bullion, containing from 1⁄1000 to 100⁄1000 of gold (called dorés), 3 fr. 27 c. per
kilogramme, = 1 fr. 22 c. per lb. troy.
For the Monnaie, the charges are—
For gold refined by sulphuric acid, when alloyed with copper only, from 898⁄1000 to 1⁄1000,
5 fr. per kilogramme, = 1 fr. 86 c. per lb. troy.
For gold alloyed with copper and silver, whatever be the quantity of silver, 5 fr. 75 c.
per kilogramme, = 2 fr. 12 c. per lb. troy.
[1063]
There are about ten bullion refiners by sulphuric acid in the environs of Paris;
two of whom, M. Poizat St. André, and M. Chauvière, are by far the most considerable;
the former working about 300 kilogrammes (= 804 lbs. troy) daily, and the
latter about two-thirds of that quantity. In former times, when competition was open
in London, Messrs. Browne and Brinde were wont to treat 6 cwts. of silver, or 9 cwts.
of gold alloy, daily, for several months in succession.
The result of free trade in refining bullion at Paris is, that the silver bars imported
into London from South America, &c., are mostly sent off to Paris to be stripped of the
few grains of gold which they may contain, and are then brought back to be sold here.
Three grains of gold in one Paris lb. of silver, pay the refiners there for taking them
out. What a disgrace is thus brought upon our manufacturing industry and skill, by
the monopoly charges in refining and assaying granted to two individuals in our Royal
Mint.
Mr. Bingley’s charges for assaying at the Royal Mint in London, are—
For an assay of gold, 4s.; for a parting assay of gold and silver, 6s.; for a silver
assay, 2s. 6d.—charges which absorb the profits of many a transaction.
The charges at the Royal Mint of Paris, for assays made under the following distinguished
chemical savants—Darcet, Directeur; Bréant, Verificateur; Chevillot and
Pelouze, Essayeurs; are—
| For an assay of |
gold, |
or doré, (a parting assay,) |
3 francs. |
| — |
silver |
—— |
0. 80 c. = 8d. English. |
M. Gay Lussac is the assayer of the Bureau de Garantie at the Monnaie Royale, an
office which corresponds to the Goldsmiths’ Hall at London. The silver assays in all
the official establishments of Europe, except the two in London, are made by the humid
method, and are free from those errors and blunders which daily annoy and despoil the
British bullion merchant, who is compelled by the Mint and Bank of England to buy
and sell by the cupellation assay of Mr. Bingley. See Assay and Silver.
REFRIGERATION OF WORTS, &c. In August, 1826, Mr. Yandall obtained
a patent for an apparatus designed for cooling worts and other hot fluids, without
exposing them to evaporation. Utensils employed for this purpose, are generally called
refrigerators, and are so constructed, that a quantity of cold water shall be brought in
contact with the vessel which contains the heated fluid. But in every construction of
refrigerator heretofore used, the quantity of cold water necessarily employed in the
operation, greatly exceeded the quantity of the fluid cooled, which, in some situations,
where water cannot be readily obtained, was a serious impediment and objection to the
use of such apparatus.
The inventor has contrived a mode of constructing a refrigerator, so that any quantity
of wort or other hot fluid may be cooled by an equal quantity of cool water; the
process being performed with great expedition, simply by passing the two fluids through
very narrow passages, in opposite directions, the result of which is, that the cold liquor
imbibes the heat from the wort, or other fluid, and the temperature of the hot fluid is
reduced in the same ratio.
Figs. 932, 933, and 934. represent different forms in which the apparatus is proposed to
be made. The two first have zigzag passages; the third, channels running in convolute
curves. These channels or passages are of very small capacity in thickness, but of
great length, and of any breadth that may be required, according to the quantity of
fluid intended to be cooled or heated.
Fig. 935. is the section of a portion of the apparatus shown at figs. 932. and 933.
upon an enlarged scale; it is made by connecting three sheets of copper or any other
thin metallic plates together, leaving parallel spaces between each plate for the passage
of the fluids, represented by the black lines.
These spaces are formed by occasionally introducing between the plates thin straps,
ribs, or portions of metal, by which means very thin channels are produced, and
through these channels the fluids are intended to be passed, the cold liquor running in
one direction, and the hot in the reverse direction.
Supposing that the passages for the fluids are each one-eighth of an inch thick, then
the entire length for the run of the fluid should be about 80 feet, the breadth of the
apparatus being made according to the quantity of fluid intended to be passed through
it in a given time. If the channels are made a quarter of an inch thick, then their
length should be extended to 160 feet; and any other dimensions in similar proportions:
but a larger channel than one quarter of an inch, the patentee considers would
be objectionable. It is, however, to be observed, that the length here recommended, is
under the consideration, that the fluids are driven through the apparatus by some degree
of hydrostatic pressure from a head in the delivery-vats above; but if the fluids flow
without pressure, then the lengths of the passages need not be quite so great.
In the apparatus constructed as shown in perspective at fig. 932., and further[1064]
developed by the section, fig. 935., cold water is to be introduced at the funnel a,
whence it passes down the pipe b, and
through a long slit or opening in the side
of the pipe, into the passage c, c (see fig.
935.), between the plates, where it flows
in a horizontal direction through the
channel towards the discharge-pipe d.
When such a quantity of cold water has
passed through the funnel a, as shall have
filled the channel c, c, up to the level of
the top of the apparatus, the cock e being
shut, then the hot wort or liquor intended
to be cooled, may be introduced at the
funnel f, and which, descending in the
pipe g, passes in a similar manner to the
former, through a long slit or opening in
the side of the pipe g, into the extended
passage h, h (see fig. 935.), and from thence
proceeds horizontally into the discharge-pipe
i.
The two cocks e and k, being now
opened, the wort or other liquor is drawn off, or otherwise conducted away through the
cock k, and the water through e. If the apertures of the two cocks e and k, are equal,
and the channels equal also, it follows that the same quantity of wort, &c., will flow
through the channel h, h, h, in a given time, as of water through the channel c, c;
and by the hot fluid passing through the apertures in contact with the side of the
channel which contains the cold fluid, the heat becomes abstracted from the former,
and communicated to the latter; and as the hot fluid enters the apparatus at that
part which is in immediate contact with the part where the cooling fluid is discharged,
and the cold fluid enters the apparatus at that part where the wort is discharged, the
consequence is, that the wort or other hot liquor becomes cooled down towards its
exit-pipe nearly to the temperature of cold water; and the temperature of the water, at
the reverse end of the apparatus, becomes raised nearly to that of the boiling wort.
It only remains to observe, that by partially closing either of the exit-cocks, the
quantity of heat abstracted from one fluid, and communicated to the other, may be regulated;
for instance, if the cock e of the water-passage be partially closed, so as to
diminish the quantity of cold water passed through the apparatus, the wort or other hot
fluid conducted through the other passages will be discharged at a higher temperature,
which in some cases will be desirable, when the refrigerated liquor is to be fermented.
Fig. 933. exhibits an apparatus precisely similar to the foregoing, but different in its
position; for instance, the zigzag channels are made in obliquely descending planes.
a is the funnel for the hot liquor, whence it
descends through the pipe d into the channel
c, c (see fig. 935.), and ultimately is discharged
through the pipe b, at the cock e. The cold
water being introduced into the funnel f, and
passing down the pipe i, enters the zigzag
channel h, h, and, rising through the apparatus,
runs off by the pipe g, and is discharged at
the cock below.
The passages of this apparatus for heating
and cooling fluids, may be bent into various
contorted figures; one form found particularly
convenient under some applications, is that
represented at fig. 934., which is contained
in a cylindrical case. The passages here run
in convolute curves, the one winding in a
spiral to the centre, the other receding from
the centre.
The wort or other hot liquor intended to
be cooled, is to be introduced at the funnel a,
and passing down the pipe b, is delivered into
the open passage c, which winds round to the
central chamber d, and is thence discharged
through the pipe e, at the cock f. The cold
water enters the apparatus at the funnel g,
and proceeding down the pipe h, enters the[1065]
closed channel i, and after traversing round through the apparatus, is in like manner
discharged through the pipe k, at the cock l. Or the hot liquor may be passed through
the closed channel, and the cold through
the open one; or these chambers may be
both of them open at top, and the apparatus
covered by a lid when at work,
the principal design of which is to afford
the convenience of cleaning them more
readily than could be done if they were
closed; or they may be both closed.
A similar ingenious apparatus for cooling
brewers’ worts, or wash for distillers,
and also for condensing spirits in place
of the ordinary worm tub, is called by
the inventor, Mr. Wheeler, an Archimedes
condenser, or refrigerator, the
peculiar novelty of which consists in
forming the chambers for the passage of
the fluids in spiral channels, winding
round a central tube, through which
spiral channels the hot and cold fluids
are to be passed in opposite directions.
Fig. 936. represents the external appearance of the refrigerator, enclosed in a cylindrical
case; fig. 937., the same, one-half of the case being removed to show the form
of the apparatus within; and fig. 938., a
section cut through the middle of the apparatus
perpendicularly, for the purpose of
displaying the internal figure of the spiral
channels.
The apparatus is proposed to be made of
sheet copper, tinned on its surface, and is
formed by cutting circular pieces of thin copper,
or segments of circles, and connecting
them together by rivets, solder, or by any other convenient means, as coppersmiths
usually do; these circular pieces of copper being united to one another, in the way of
a spiral or screw, form the chambers through which the fluids are to pass within, in an
ascending or descending inclined plane.
In figs. 937. and 938., a, a, is the central tube or standard (of any diameter that may
be found convenient), round which the spiral chambers are to be formed; b, b, are the
sides of the outer case, to which the edges of the spiral fit closely, but need not be
attached; c, c, are two of the circular plates of copper, connected together by rivets at
the edges, in the manner shown, or by any other suitable means; d, is the chamber,
formed by the two sheets of copper, and which is carried round from top to bottom in a
spiral or circular inclined plane, by a succession of circular plates connected to each
other.
[1066]
The hot fluid is admitted into the spiral chamber d, through a trumpet or wide-mouthed
tube e, at top, and is
discharged at bottom by an
aperture and cock f. The cold
water which is to be employed
as the cooling material, is to be
introduced through the pipe g,
in the centre, from whence discharging
itself by a hole at bottom,
the cold water occupies
the interior of the cylindrical
case b, and rises in the spiral
passage h, between the coils of
the chamber, until it ascends to
the top of the vessel, and then
it flows away by a spout i, seen
in fig. 936.
It will be perceived that the
hot fluid enters the apparatus
at top, and the cold fluid at
bottom, passing each other, by
means of which an interchange
of temperatures takes place
through the plates of copper,
the cooling fluid passing off at
top in a heated state, by means
of the caloric which it has abstracted
from the hot fluid; and
the hot fluid passing off through
the pipe and cock at bottom, in
a very reduced state of temperature,
by reason of the caloric
which it held having been given
out to the cooling fluid.
Fig. 939. is a side view and
section of Wagenmann’s apparatus for cooling worts; fig. 940., a view from above.
The preceding contrivances seem to be far preferable.
a, a, is the tub for receiving the apparatus, whose central upright shaft b, rests upon a
step c, in the bottom, and revolves at top in a bush at d, made fast to a bar e, fixed flat
across the mouth of the tub. The shaft may be driven by the two bevel wheels f, f, at
right angles to each other, and the horizontal rod turned by hand; or the whole may
be impelled by any power. g, is an iron basin for receiving the cold water from the
spout h, supplied by a well; it flows out of the basin through two tubes i i, down into
the lower part of the cooler k k. The cooler consists of two flat vessels, both of which
are formed of a flat interior plate, and an arched exterior one, so that their transverse
section is plano-convex. The water which flows along the tubes i i, spreads itself upon
the bottom of the cooler, and then rises through the scabbard-shaped tubes l l, &c., into
the upper annular vessel m m; whence it is urged by hydrostatic pressure, in a now
heated state, through the slanting tubes n n, which terminate in the common pipe o, of
the annular basin p p, and is thence discharged by the pipe q. The basin p p, is
supported by the two bearers r, made fast to the cross-beam e. There is in the lowest
part of the hollow ring at bottom, a screw plug, which may be opened when it is desired
to discharge the whole contents, and to wash it with a stream of water.
REGULUS, is a term introduced, by the alchemists, now nearly obsolete. It means
literally a little king, and refers to the metallic state as one of royalty, compared
with the native earthy condition. Antimony is the only metal now known by the
name of regulus.
RESINS (Résines, Fr.; Harze, Germ.); are proximate principles found in most
vegetables, and in almost every part of them; but the only resins which merit a particular
description, are those which occur naturally in such quantities as to be easily
collected or extracted. They are obtained chiefly in two ways, either by spontaneous
exudation from the plants, or by extraction by heat and alcohol. In the first case, the
discharge of resin in the liquid state is sometimes promoted by artificial incisions made
in summer through the bark into the wood of the tree.
Resins possess the following general properties:—They are soluble in alcohol, insoluble
in water, and melt by the application of heat, but do not volatilize without
partial decomposition. They have rarely a crystalline structure, but, like gums, they[1067]
seldom affect any peculiar form. They are almost all translucid, not often colourless,
but generally brown, occasionally red or green. Any remarkable taste or smell, which
they sometimes possess, may be ascribed to some foreign matter, commonly an essential
oil. Their specific gravity varies from 0·92 to 1·2. Their consistence is also very
variable. The greater part are hard, with a vitreous fracture, and so brittle as to be
readily pulverized in the cold. Some of them are soft, a circumstance probably dependent
upon the presence of a heterogeneous substance. The hard resins do not
conduct electricity, and they become negatively electrical by friction. When heated,
they melt more or less easily into a thick viscid liquid, and concrete, on cooling, into a
smooth shining mass, of a vitreous fracture, which occasionally flies off into pieces, like
Prince Rupert’s drops; especially after being quickly cooled, and scratched with a
sharp point. They take fire by contact of an ignited body, and burn with a bright
flame, and the diffusion of much sooty smoke. When distilled by themselves in close
vessels, they afford carbonic acid and carburetted gases, empyreumatic oil of a less
disagreeable smell than that emitted by other such oils, a little acidulous water, and a
very little shining charcoal. See Rosin Gas.
Resins are insoluble in water, but dissolve in considerable quantities in alcohol, both
hot and cold. This solution reddens tincture of litmus, but not syrup of violets; it is
decomposed by water, and a milkiness ensues, out of which the particles of the resin
gradually agglomerate. In this state it contains water, so as to be soft, and easily
kneaded between the fingers; but it becomes hard and brittle again when freed by
fusion from the water. The resins dissolve in ether and the volatile oils, and, with the
aid of heat, combine with the unctuous oils. They may be combined by fusion with
sulphur, and with a little phosphorus. Chlorine water bleaches several coloured
resins, if they be diffused in a milky state through water. The carburet of sulphur
dissolves them.
Resins are little acted upon by acids, except by the nitric, which converts them into
artificial tan. They combine readily with the alkalis and alkaline earths, and form
what were formerly reckoned soaps: but the resins are not truly saponified; they rather
represent the acid constitution themselves, and, as such, saturate the salifiable bases.
Every resin is a natural mixture of several other resins, as is the case also with oils;
one principle being soluble in cold alcohol, another in hot, a third in ether, a fourth
in oil of turpentine, a fifth in naphtha, &c. The soft resins, which retain a certain
portion of volatile oil, constitute what are called balsams. Certain other balsams contain
benzoic acid. The solid resins are, amber, animé, benzoin, colophony (common rosin),
copal, dammara, dragon’s blood, elemi, guaiac, lac, resin of jalap, ladanum, mastic, sandarach,
storax, takamahac.
RESIN, KAURI or COWDEE, is a new and very peculiar substance, recently imported
in considerable quantities from New Zealand, which promises to be useful in the
arts. It oozes from the trunk of a noble tree called Dammara australis, or Pinus kauri,
which rises sometimes to the height of 90 feet without a branch, with a diameter of 12 feet,
and furnishes a log of heart timber of 11 feet. The resin, which is called Cowdee gum
by the importers, is brought to us in pieces varying in size from that of a nutmeg to a
block of 2 or 3 cwts. The colour varies from milk-white to amber, or even deep brown;
some pieces are transparent and colourless. In hardness it is intermediate between copal
and resin. The white milky pieces are somewhat fragrant, like elemi. Specific gravity,
1·04 to 1·06. It is very inflammable, burns all away with a clear bright flame, but does
not drop. When cautiously fused, it concretes into a transparent hard tough mass, like
shellac. It affords a fine varnish with alcohol, being harder and less coloured than mastic,
while it is as soluble, and may be had probably at one-tenth of the price. A solution in
alcohol, mixed with one-fourth of its bulk of a solution in oil of turpentine, forms an
excellent varnish, which dries quickly, is quite colourless, clear, and hard. It is insoluble
in pyro-acetic (pyroxilic?) spirit. Combined with shellac and turpentine, it forms a good
sealing-wax.
REVERBERATORY FURNACE; see Copper, Iron, and Soda.
RETORT. For producing coal gas, there are many modifications, varying in
dimension and shape with the caprice of the constructor, and in many cases, without
any definite idea of the principle to be aimed at.
They may be divided into three general classes:
1st. The circular retort, from twelve to twenty inches in diameter, and from six to
nine feet in length. This retort is used in Manchester and some other places, in general
for the distillation of cannel, or Scotch parrot coal. It answers for the distillation
of a coal which retains its form in lumps, and is advantageous only from the facility
with which its position is changed, when partially destroyed by the action of fire on
the under side.
2nd. The small or London D retort, so called in consequence of its having first been
used by the chartered company in London, being still in use at their works, and recommended[1068]
by their engineer. This retort is 12 inches broad on the base, 11 inches
high, and 7 feet long, carbonizing one and a half to two bushels at a charge.
3rd. The York D retort, (so called in consequence of its having been introduced by
Mr. Outhit, of York,) and the modifications of it, among which I should include the
elliptic retort, as having the same general purpose in view. The difference between
the London and York D retorts, consists only in an extension of surface upon which
the coal is spread. See Gas-light.
RHODIUM, is a metal discovered by Dr. Wollaston in 1803, in the ore of platinum.
It is contained to the amount of three per cent. in the platinum ore of Antioquia in
Colombia, near Barbacoas; it occurs in the Ural ore, and, alloyed with gold, in Mexico.
The palladium having been precipitated from the muriatic solution of the platinum ore
previously saturated with soda, by the cyanide of mercury, muriatic acid is to be poured
into the residuary liquid, and the mixture is to be evaporated to dryness, to expel the
hydrocyanic acid, and convert the metallic salts into chlorides. The dry mass is to be
reduced to a very fine powder, and washed with alcohol of specific gravity 0·837. This
solvent takes possession of the double chlorides which the sodium forms with the platinum,
iridium, copper, and mercury, and does not dissolve the double chloride of rhodium
and sodium, but leaves it in the form of a powder, of a fine dark-red colour. This salt
being washed with alcohol, and then exposed to a very strong heat, affords the rhodium.
But a better mode of reducing the metal upon the small scale, consists in heating the
double chloride gently in a glass tube, while a stream of hydrogen passes over it, and
then to wash away the chloride of sodium with water.
Rhodium resembles platinum in appearance. Any heat which can be produced in a
chemical furnace is incapable of fusing it; and the only way of giving it cohesive solidity,
is to calcine the sulphuret or arseniuret of rhodium in an open vessel at a white
heat, till all the sulphur or arsenic be expelled. A button may thus be obtained, somewhat
spongy, having the colour and lustre of silver. According to Wollaston, the
specific gravity of rhodium is 11. It is insoluble by itself in any acid; but when an
alloy of it with certain metals, as platinum, copper, bismuth, or lead, is treated with
aqua regia, the rhodium dissolves along with the other metals; but when alloyed with
gold or silver, it will not dissolve along with them. It may, however, be rendered very
soluble by mixing it in the state of a fine powder with chloride of potassium or sodium,
and heating the mixture to a dull-red heat, in a stream of chlorine gas. It thus forms a
triple salt, very soluble in water. The solutions of rhodium are of a beautiful rose colour,
whence its name. In the dry way, it dissolves by heat in bisulphate of potassa; and
disengages sulphurous acid gas in the act of solution. There are two oxides of rhodium.
Rhodium combines with almost all the metals; and, in small quantity, melted with steel,
it has been supposed to improve the hardness, closeness, and toughness of this metal.
Its chief use at present is for making the inalterable nibs of the so-named rhodium pens.
RIBBON MANUFACTURE, is a modification of Weaving, which see.
RICE, of Carolina, analyzed by Braconnot, was found to be composed of starch
85·07, of gluten 3·60, of gum 0·71, of uncrystallizable sugar 0·29, of a colourless rancid
fat like suet 0·13, of vegetable fibre 4·8, of salts with potash and lime bases 0·4, and
5·0 of water.
The quantity of rice entered for home consumption in the year 1836, was—
| |
Cwts. |
81,610. |
In 1837, |
126,739. |
| Ditto in the husk, |
Bushels |
292,444. |
282,377. |
Rice Paper, as it is called, on which the Chinese and Hindoos paint flowers so
prettily, is a membrane of the bread-fruit tree, the Artocarpus incisifolia of naturalists.
RICE CLEANING. Various machines have been contrived for effecting this
purpose, of which the following, secured by patent to Mr. Melvil Wilson, in 1826, may
be regarded as a good specimen. It consists of an oblong hollow cylinder, laid in an
inclined position, having a great many teeth stuck in its internal surface, and a central
shaft also furnished with teeth. By the rapid revolution of the shaft, its teeth are
carried across the intervals of those of the cylinder with the effect of parting the grains
of rice, and detaching whatever husks or impurities may adhere to them. A hopper
is set above to receive the rice, and conduct it down into the cleansing cylinder.
About 80 teeth are supposed to be set in the cylinder, projecting so as to reach
very nearly the central shaft; in which there is a corresponding number of teeth, that
pass freely between the former.
The cylinder is shown inclined in the figure which accompanies the specification;
but it may be placed also upright or horizontal, and may be mounted in any convenient
frame-work. The central shaft should be put in rapid rotation, while the cylinder
receives a slow motion in the opposite direction. The rice, as cleaned by that action,
is discharged at the lower end of the cylinder, where it falls into a shute (shoot), and
is conducted to the ground. The machine may be driven by hand, or by any other convenient
power.
[1069]
Rice consists chiefly of starch, and therefore cannot by itself make a proper bread. It
is used in the cotton factories to form weavers’ dressings for warps. The Chinese
reduce its flour into a pulp with hot water, and mould it into figures and plates,
which they afterwards harden, and ornament with engravings, resembling those of
mother-of-pearl. When a decoction of rice is fermented and distilled, it affords the
sort of ardent spirit called arrack in the East Indies.
RINSING MACHINE, is one of those ingenious automatic contrivances for
economizing labour, and securing uniformity of action, now so common in the factories
of Lancashire. Fig. 941. is a longitudinal middle
section of an approved mechanism for rinsing pieces
of calico dyed with spirit or fancy colours, and
which require more delicate treatment than is compatible
with hand-washing. A, E, F, B, is a wooden
cistern, about 12 feet long, 4 feet high at one end,
2 feet at the other, and of the ordinary width of
calico cloth. It is divided transversely into a series
of equal compartments by partitions, decreasing in
height from the upper to the lower end, the top of
each of them, however, being an inch at least under
the top of the enclosing side at its line of junction.
Above the highest end of the trough, a pair of
squeezing rollers is mounted at B; the lower one
having a pulley upon the end of its shaft, for turning
it, by means of a band from one of the driving-shafts
of the factory; and the upper one is pressed
down upon it by weighted levers acting on the ends
of its axis. The roller above the second highest
partition has also a pair of squeezing rollers, with a
weighted lever D. The pieces of cloth, stitched
endwise, being laid upon a platform to the right
hand of the cistern, are introduced over the roller
A, passed down under the roller beneath it, and so
up and down in a serpent-like path, from the lowest
compartment of the cistern to the uppermost, being
drawn through the series by the traction of the
rotatory roller at B. While the long web is thus
proceeding upwards from A to B, a stream of pure
water is made to flow along in the opposite direction
from B to A, running over the top of each partition
in a thin sheet. By this contrivance, the goods
which enter at A, having much loose colour upon
their surface, impregnate the water strongly, but
as they advance they continually get cleaner by the
immersion, and pressure of the successive rollers,
being exposed to purer water, till at last they
reach the limpid stream, and are discharged at B
perfectly bright. The rinsing operation may be modified by varying the quantity of
water admitted, the speed with which the pieces are drawn through the cells, or the
pressure upon the series of top rollers.
ROCKETS. M. de Montgery, captain of a frigate in the French service, has
written a Traité sur les Fusées de Guerre, in which he discusses the merits of the Congreve
rockets, and describes methods of imitating them. As the subject of military
projectiles is foreign to this Dictionary, I refer my readers to the above work, which is
commended by the editor of the Dictionnaire Technologique.
ROLLING-MILL. See Iron, Mint, and Plated Manufacture.
ROPE-MAKING. The fibres of hemp which compose a rope, seldom exceed in
length three feet and a half, at an average. They must, therefore, be twined together so
as to unite them into one; and this union is effected by the mutual circumtorsion of the
two fibres. If the compression thereby produced be too great, the strength of the fibres
at the points where they join will be diminished; so that it becomes a matter of great
consequence to give them only such a degree of twist as is essential to their union.
The first part of the process of rope-making by hand, is that of spinning the yarns or
threads, which is done in a manner analogous to that of ordinary spinning. The spinner
carries a bundle of dressed hemp round his waist; the two ends of the bundle being
assembled in front. Having drawn out a proper number of fibres with his hand, he
twists them with his fingers, and fixing this twisted part to the hook of a whirl, which[1070]
is driven by a wheel put in motion by an assistant, he walks backwards down the rope
walk, the twisted part always serving to draw out more fibres from the bundle round
his waist, as in the flax-spinning wheel. The spinner takes care that these fibres are
equably supplied, and that they always enter the twisted parts by their ends, and never
by their middle. As soon as he has reached the termination of the walk, a second spinner
takes the yarn off the whirl, and gives it to another person to put upon a reel, while
he himself attaches his own hemp to the whirl hook, and proceeds down the walk.
When the person at the reel begins to turn, the first spinner, who has completed his yarn,
holds it firmly at the end, and advances slowly up the walk, while the reel is turning,
keeping it equally tight all the way, till he reaches the reel, where he waits till the
second spinner takes his yarn off the whirl hook, and joins it to the end of that of the
first spinner, in order that it may follow it on the reel.
The next part of the process previous to tarring, is that of warping the yarns, or
stretching them all to one length, which is about 200 fathoms in full-length rope-grounds,
and also in putting a slight turn or twist into them.
The third process in rope-making, is the tarring of the yarn. Sometimes the yarns
are made to wind off one reel, and, having passed through a vessel of hot tar, are wound
upon another, the superfluous tar being removed by causing the yarn to pass through a
hole surrounded with spongy oakum; but the ordinary method is to tar it in skains or
hanks, which are drawn by a capstan with a uniform motion through the tar-kettle. In
this process, great care must be taken that the tar is boiling neither too fast nor too slow.
Yarn for cables requires more tar than for hawser-laid ropes; and for standing and running
rigging, it requires to be merely well covered. Tarred cordage has been found to
be weaker than what is untarred, when it is new; but the tarred rope is not so easily
injured by immersion in water.
The last part of the process of rope-making, is to lay the cordage. For this purpose
two or more yarns are attached at one end to a hook. The hook is then turned the contrary
way from the twist of the individual yarn, and thus forms what is called a strand.
Three strands, sometimes four, besides a central one, are then stretched at length, and
attached at one end to three contiguous but separate hooks, but at the other end to a
single hook; and the process of combining them together, which is effected by turning
the single book in a direction contrary to that of the other three, consists in so regulating
the progress of the twists of the strands round their common axis, that the three
strands receive separately at their opposite ends just as much twist as is taken out of
them by their twisting the contrary way, in the process of combination.
Large ropes are distinguished into two main classes, the cable-laid and hawser-laid.
The former are composed of nine strands, namely, three great strands, each of these
consisting of three smaller secondary strands, which are individually formed with an
equal number of primitive yarns. A cable-laid rope eight inches in circumference, is
made up of 333 yarns or threads, equally divided among the nine secondary strands. A
hawser-laid rope consists of only three strands, each composed of a number of primitive
yarns, proportioned to the size of the rope; for example, if it be eight inches in circumference,
it may have 414 yarns, equally divided among three strands. Thirty fathoms
of yarn are reckoned equivalent in length to eighteen fathoms of rope cable-laid, and to
twenty fathoms hawser-laid. Ropes of from one inch to two inches and a half in circumference
are usually hawser-laid; of from three to ten inches, are either hawser or
cable laid; but when more than ten inches, they are always cable-laid.
Every hand-spinner in the dock-yard is required to spin, out of the best hemp, six
threads, each 160 fathoms long, for a quarter of a day’s work. A hawl of yarn, in the
warping process, contains 336 threads.
The following are Captain Huddart’s improved principles of the rope manufacture:—
1. To keep the yarns separate from each other, and to draw them from bobbins revolving
upon skewers, so as to maintain the twist while the strand or primary cord is forming.
2. To pass them through a register, which divides them by circular shells of holes;
the number in each concave shell being conformable to the distance from the centre of
the strand, and the angle which the yarns make with a line parallel to it, and which
gives them a proper position to enter.
3. To employ a tube for compressing the strand, and preserving the cylindrical figure
of its surface.
4. To use a gauge for determining the angle which the yarns in the outside shell
make with a line parallel to the centre of the strand, when registering; because according
to the angle made by the yarns in this shell, the relative lengths of all the yarns in
the strand will be determined.
5. To harden up the strand, and thereby increase the angle in the outside shell;
which compensates for the stretching of the yarns, and the compression of the strands.
A great many patents have been obtained, and worked with various degrees of success,
for making ropes. Messrs. Cartwright, Fothergill, Curr, Chapman, Balfour, and Huddart,[1071]
have been the most conspicuous inventors in this country; but the limits of this
work preclude us doing justice to their respective merits.
All improvements in the manufacture of cordage at present in use, either in her
Majesty’s yards or in private rope-grounds, owe their superiority over the old method
of making cordage to Captain Huddart’s invention of the register plate and tube.
Mr. Balfour took out a patent for the manufacture of cordage about a month before
Captain Huddart; but the formation of his strand was to be accomplished by what he
called a top minor, (in the form of a common top, with pins to divide the yarns,) which
upon trial could not make cordage so good as by the common mode. On seeing Captain
Huddart’s specification, Mr. Balfour, five years after, procured another patent, in
which he included a plate and tube, but which was not sufficiently correct, and experience
in the navy proved the insufficiency of the cordage. Captain Huddart’s
plate and tube were then adopted in the king’s yards, and he gave his assistance for
the purpose.
Captain Huddart then invented and took a patent for a machine, which by registering
the strand at a short length from the tube, and winding it up as made, preserved
an uniformity of twist, or angle of formation, from end to end of the rope, which cannot
be accomplished by the method of forming the strands down the ground, where the
twist is communicated from one end to the other of an elastic body upwards of 300
yards in length. This registering-machine was constructed with such correctness, that
when some were afterwards required, no alteration could be made with advantage by the
most skilful and scientific mechanic of that day, Mr. Rennie. Thus the cold register
was carried to the greatest perfection.
A number of yarns cannot be put together in a cold state, without considerable
vacancies, into which water may gain admission; Captain Huddart, therefore, formed
the yarns into a strand immediately as they came from the tar-kettle, which he was
enabled to do by his registering-machine, and the result was most satisfactory. This
combination of yarns was found by experiment to be 14 per cent. stronger than the cold
register; it constituted a body of hemp and tar impervious to water, and had great advantage
over any other cordage, particularly for shrouds, as after they were settled on the
mast-head, and properly set up, they had scarcely any tendency to stretch, effectually
secured the mast, and enabled the ship to carry the greatest press of sail.
In order more effectually to obtain correctness in the formation of cables and large
cordage, Captain Huddart constructed a laying-machine, which has carried his inventions
in rope-making to the greatest perfection, and which, founded on true mathematical
principles, and the most laborious calculations, is one of the noblest monuments of
mechanical ability since the improvement of the steam-engine by Mr. Watt. By this
machine, the strands receive that degree of twist only which is necessary, and are laid
at any angle with the greatest regularity; the pressure is regulated to give the required
elasticity, and all parts of the rope are made to bear equally. In no one instance has a
rope or cable thus formed, been found defective in the lay, or stiff, or difficult to coil.
Such a revolution in the manufacture of cordage could not be accomplished without
great expense, as the works at Limehouse fully testify; and considerable opposition
necessarily arose. Captain Huddart’s first invention was, however, generally adopted, as
soon as the patent expired; and experience has established the great importance of his
subsequent improvements.
His cordage has been supplied in large quantities to her Majesty’s navy, and has
received the most satisfactory reports.
The following description of one of the best modern machines for making ropes on
Captain Huddart’s plan, will gratify the intelligent reader.
Fig. 942. exhibits a side elevation of the tackle-board and bobbin-frame at the head
of the ropery, and also of the carriage or rope-machine in the act of hauling out and
twisting the strands.
Fig. 943. is a front elevation of the carriage.
Fig. 944. is a yarn-guide, or board, or plate, with perforated holes for the yarns to
pass through before entering the nipper.
Figs. 945. and 946. are side and front views of the nipper for pressing the rope-yarns.
[1072]
a is the frame for containing the yarn bobbins. The yarns are brought from the
frame, and pass through a yarn-guide at b. c is a small roller, under which the rope-yarns
pass; they are then brought over the reel d, and through another yarn-guide e,
after which they enter the nippers at v, and are drawn out and formed into strands by the
carriage. The roller and reel may be made to traverse up and down, so as to regulate
the motion of the yarns.
The carriage runs on a railway. f, f, is the frame of the carriage; g, g, are the small
wheels on which it is supported; k, k, is an endless rope, reaching from the head to
the bottom of the railway, and is driven by a steam-engine;
m, m, is a wheel with gubs at the back of it, over which the
endless rope passes, and gives motion to the machinery of the
carriage. n, is the ground rope for taking out the carriage,
as will be afterwards described. On the shaft of m, m, are
two bevel wheels 3, 3, with a shifting catch between them;
these bevel wheels are loose upon the shaft, but when the
catch is put into either of them, this last then keeps motion
with the shaft, while the other runs loose. One of these
wheels serves to communicate the twist to the strand in
drawing out; the other gives the opposite or after turn to
the rope in closing. 4, 4, is a lever for shifting the catch
accordingly. 5, is a third bevel wheel, which receives its
motion from either of the other two, and communicates the
same to the two spur wheels 6, 6, by means of the shaft x.
These can be shifted at pleasure; so that by applying wheels
of a greater or less number of teeth above and beneath, the
twist given to the strands can be increased or diminished
accordingly. The upper of these two communicates motion,
by means of the shaft o, to another spur wheel 8, which working in the three pinions
above, 9, 9, gives the twist to the strand hooks.
The carriage is drawn out in the following manner. On the end of the shaft of m, m,
is the pinion 3, which, working in the large wheel R, gives motion to the ground-rope
shaft upon its axis. In the centre of this shaft is a curved pulley or drum t, round
which the ground rope takes one turn. This rope is fixed at the head and foot of the
ropery; so that when the machinery of the carriage is set a-going by the endless rope k, k,
and gives motion to the ground-rope shaft, as above described, the carriage will necessarily
move along the railway; and the speed may be regulated either by the diameter of
the circle formed by the gubs on the wheel m, m, or by the number of teeth in the pinion
3. At T, is a small roller, merely for preventing the ground rope from coming up among
the machinery. At the head of the railway, and under the tackle-board, is a wheel
and pinion Z, with a crank for tightening the ground rope. The fixed machinery at the
head, for hardening or tempering the strands, is similar to that on the carriage, with the
exception of the ground-rope geer, which is unnecessary. The motion is communicated
by another endless rope, (or short band, as it is called, to distinguish it from the other,)
which passes over gubs at the back of the wheel 1, 1.
When the strands are drawn out by the carriage to the requisite length, the spur
wheels 3, R, are put out of geer. The strands are cut at the tackle-board, and fixed to
the hooks 1, 1, 1; after which they are hardened or tempered, being twisted at both
ends. When this operation is finished, three strands are united on the large hook h, the
top put in, and the rope finished in the usual way.
In preparing the hemp for spinning an ordinary thread or rope-yarn, it is only
heckled over a large keg or clearer, until the fibres are straightened and separated, so as
to run freely in the spinning. In this case, the hemp is not stript of the tow, or cropt,
unless it is designed to spin beneath the usual grist, which is about 20 yarns for the
strand of a three-inch strap-laid rope. The spinning is still performed by hand, being
found not only to be more economical, but also to make a smoother thread, than has yet
been effected by machinery. Various ways have been tried for preparing the yarns
for tarring. That which seems now to be most generally in use, is, to warp the yarns
upon the stretch as they are spun. This is accomplished by having a wheel at the foot,
as well as the head of the walk, so that the men are able to spin both up and down,
and also to splice their threads at both ends. By this means, they are formed into a
haul, resembling the warp of a common web, and a little turn is hove into the haul, to
preserve it from getting foul in the tarring. The advantages of warping from the
spinners, as above, instead of winding on winches, as formerly, are, 1st, the saving of
this last operation altogether; 2dly, the complete check which the foreman has of the
quantity of yarn spun in the day; 3dly, that the quality of the work can be subjected
to the minutest inspection at any time. In tarring the yarn, it is found favourable to
the fairness of the strip, to allow it to pass around or under a reel or roller in the[1073]
bottom of the kettle while boiling, instead of coiling the yarn in by hand. The tar is
then pressed from the yarn, by means of a sliding nipper, with a lever over the upper
part, and to the end of which the necessary weight is suspended. The usual proportion
of tar in ordinary ropes, is something less than a fifth. In large strap-laid ropes, which
are necessarily subjected to a greater press in the laying of them, the quantity of tar can
scarcely exceed a sixth, without injuring the appearance of the rope when laid.
For a long period, the manner of laying the yarns into ropes, was by stretching the
haul on the rope-ground, parting the number of yarns required for each strand, and
twisting the strands at both ends, by means of hand-hooks, or cranks. It will be
obvious that this method, especially in ropes of any considerable size, is attended with
serious disadvantages. The strand must always be very uneven; but the principal disadvantage,
and that which gave rise to the many attempts at improvement, was, that
the yarns being all of the same length before being twisted, it followed, when the rope
was finished, that while those which occupied the circumference of the strand were perfectly
tight, the centre yarns, on the other hand, as they were now greatly slackened by
the operation of hardening or twisting the strands, actually would bear little or no part
of the strain when the rope was stretched, until the former gave way. The method
displayed in the preceding figures and description, is among the latest and most
improved; Every yarn is given out from the bobbin frame as it is required in twisting
the rope; and the twist communicated in the out-going of the carriage, can be increased
or diminished at pleasure. In order to obtain a smooth and well-filled strand,
it is necessary also, in passing the yarns through the upper board, to proportion the
number of centre to that of outside yarns. In ordinary sized ropes, the strand seems to
have the fairest appearance, when the outside yarns form from 2⁄3ds to 3⁄4ths of the whole
quantity, in the portion of twist given by the carriage in drawing out and forming the
strands.
In laying cables, torsion must be given both behind and before the laying top. Figs.
947, 948, 949. represent the powerful patent apparatus employed for this purpose. A, is
a strong upright iron pillar,
supported upon the great
horizontal beam N, N, and
bearing at its upper end the
three-grooved laying top M.
H, H, are two of the three
great bobbins or reels round
which the three secondary
strands or small hawsers are
wound. These are drawn up
by the rotation of the three
feeding rollers I, I, I, thence
proceed over the three guide
pulleys K, K, K, towards the
laying top M, and finally
pass through the tube O, to
be wound upon the cable-reel
D. The frames of the
three bobbins H, H, H, do not
revolve about the fast pillar
A, as a common axis; but
each bobbin revolves round
its own shaft Q, which is
steadied by a bracing collet
at N, and a conical step at
its bottom. The three bobbins
are placed at an angle
of 120 degrees apart, and
each receives a rotatory motion
upon its axis from the
toothed spur wheel B, which
is driven by the common
central spur wheel C. Thus
each of the three secondary
cords has a proper degree of
twist put into it in one direction,
while the cable is laid,
by getting a suitable degree of twist in an opposite direction, from the revolution of the
frame or cage G, G, round two pivots, the one under the pulley E, and the other over O.[1074]
The reel D has thus, like the bobbins H, H, two movements; that in common with its
frame, and that upon its axis, produced by the action of the endless band round the pulley
E, upon one of its ends, and the pulley E′ above its centre of rotation. The pulley E is
driven by the bevel mill-geering P, P, P, as also the under spur wheel C. L, in fig. 949., is
the place of the ring L, fig. 947., which bears the three guide pulleys K, K, K. Fig. 948.
is an end view of the bobbin H, to show the worm or endless screw J, of fig. 949., working
into the two snail-toothed wheels, upon the ends of the two feed-rollers I, I, which serve
to turn them. The upright shafts of J, J, receive their motion from pulleys and cords
near their bottom. Instead of these pulleys, and the others E, E′, bevel-wheel geering has
been substituted with advantage, not being liable to slip, like the pulley-band mechanism.
The axis of the great reel is made twice the length of the bobbin D, in order to allow
of the latter moving from right to left, and back again alternately, in winding on the
cable with uniformity as it is laid. The traverse mechanism of this part is, for the sake
of perspicuity, suppressed in the figure.
Mr. William Norvell, of Newcastle, obtained a patent in May, 1833, for an improvement
adapted to the ordinary machines employed for twisting hempen yarns into
strands, affording, it is said, a simpler and more eligible mode of accomplishing that
object, and also of laying the strands together, than has been hitherto effected by
machinery. The yarns spun from the fibres of hemp, are wound upon bobbins, and
these bobbins are mounted upon axles, and hung in the frame of the machine, as shown
in the elevation, fig. 950., from which bobbins the several ends of yarn are passed upwards
through slanting tubes; by the rotation of which tubes, and of the carriages in which
the bobbins are suspended, the yarns become twisted into strands, and also the strands
are laid so as to form ropes.
His improvements consist, first, in the application of three or more tubes, two of
which are shown in fig. 950, placed in inclined positions, so as to receive the strands
immediately above the press-block a, a, and nearly in a line with A, the point of closing
or laying the rope. B1, and B3, are opposite side views; B2, an edge view; and B, a side
section of the same. He does not claim any exclusive right of patent for the tubes
themselves, but only for their form and angular position.
Secondly, in attaching two common flat sheaves, or pulleys, C, C, fig. 950., to each
of the said tubes, nearly
round which each
strand is lapped or
coiled, to prevent it
from slipping, as shown
in the section B1. The
said sheaves or pulleys
are connected by a
crown or centre wheel
D, loose upon b, b, the
main or upright axle;
E, E, is a smaller wheel
upon each tube, working
into the said crown
or centre wheel, and
fixed upon the loose
box I, on each of the
tubes.
F, F, is a toothed
or spur wheel, fixed
also upon each of the
loose boxes I, and
working into a smaller
wheel G, upon the axis
2, of each tube; H, is
a bevel wheel fixed
upon the same axis
with G, and working
into another bevel
wheel J, fixed upon the cross axle 3, of each tube; K, is a spur wheel attached to the
same axis with J, at the opposite end, and working into L, another spur wheel of the
same size upon each of the tubes. By wheels thus arranged and connected with the
sheaves or pulleys, as above described, a perfectly equal strain or tension is put upon
each strand as drawn forward over the pulley C.
Thirdly, the invention consists in the introduction of change wheels M, M, M, M,
fig. 950., for putting the forehard or proper twist into each strand before the rope is[1075]
laid; this is effected by small spindles on axles 4, 4, placed parallel with the line of
each tube B.
Upon the lower end of each spindle the bevel wheels N, N, are attached, and driven
by other bevel wheels O, O, fixed immediately above each press-block a, a. On the
top end of each spindle or axle 4, 4, is attached one of the change wheels, working
into the other change wheel fixed upon the bottom end of each of the tubes,
whereby the forehard or proper twist in the strands for all sizes of ropes, is at once
attained, by simply changing the sizes of those two last described wheels, which can be
very readily effected, from the manner in which they are attached to the tubes B, B, and
4, 4.
From the angular position of the tubes towards the centre, the strands are nearly
in contact at their upper ends, where the rope is laid, immediately below which the
forehard or proper twist is given to the strands.
Fourthly, in the application of a press-block P, of metal, in two parts, placed
directly above and close down to where the rope is laid at A, the inside of which is
polished, and the under end is bell-mouthed; to prevent the rope from being chafed in
entering it, a sufficient grip or pressure is put upon the rope by one or two levers and
weights 5, 5, acting upon the press-block, so as to adjust any trifling irregularity in the
strand or in the laying; the inside of which being polished, gives smoothness, and by
the said levers and weights, a proper tension to the rope, as it is drawn forward through
the press-block. By the application of this block, ropes may be made at once properly
stretched, rendering them decidedly preferable and extremely advantageous, particularly
for shipping, inclined planes, mines, &c.
The preceding description includes the whole of Mr. Norvell’s improvements; the
remaining parts of the machine being similar to those now in use, may be briefly
described as follows:—A wheel or pulley c, is fixed independently of the machine, over
which the rope passes to the drawing motion represented at the side; d, d, is a grooved
wheel, round which the rope is passed, and pressed into the groove by means of the
lever and weight e, e, acting upon the binding sheaf f, to prevent the rope from slipping.
After the rope leaves the said sheave, it is coiled away at pleasure. g, g, are two change
wheels, for varying the speed of the grooved wheel d, d, to answer the various sizes of
ropes; h, is a spiral wheel, driven by the screw k, fixed upon the axle l; m, is a band-wheel,
which is driven by a belt from the shaft of the engine, or any other communicating
power; n, n, is a friction strap and striking clutch. The axle q, is driven by two
change wheels p, p; by changing the sizes of those wheels, the different speeds of the
drum R, R, for any sizes of ropes, are at once effected.
The additional axle s, and wheels t, t, shown in fig. 951., are applied occasionally for
reversing the motion of the
said drums, and making what
is usually termed left-hand
ropes; u, figs. 950. and 951.,
show a bevelled pinion, driving
the main crown wheel v, v,
which wheel carries and gives
motion to the drums R, R;
w, w, is a fixed or sun wheel,
which gives a reverse motion
to the drums, as they revolve round the same, by means of the intervening wheels
x, x, x, whereby the reverse or retrograding motion is produced, and which gives to the
strands the right twist. The various retrograding motions, or right twists for all sizes
and descriptions of ropes, may be obtained by changing the diameters of the pinions
y, y, y, on the under ends of the drum spindles; the carriages of the intervening
wheels x, x, x, being made to slide round the ring z, z; W, W, is the framework of the
machine and drawing motion; T, T, T, are the bobbins containing the yarns; their number
is varied to correspond with the different sizes of the machines.
The machine here described, in elevation and plan, is calculated to make ropes from
three to seven and one-half inches in circumference, and to an indefinite length.
Messrs. Chapman of Newcastle, to whom the art of rope-making is deeply indebted,
having observed that rope yarn is considerably weakened by passing through the tar-kettle,
that tarred cordage loses its strength progressively in cold climates, and so rapidly in
hot climates as to be scarcely fit for use in three years, discovered that the deterioration
was due to the reaction of the mucilage and acid of the tar. They accordingly proposed
the following means of amelioration. 1. Boiling it with water, in order to remove
these two soluble constituents. 2. Concentrating the washed tar by heat, till it becomes
pitchy, and then restoring the plasticity which it thereby loses, by the addition of
tallow, or animal or expressed oils.
In 1807, the same able engineers obtained a patent for a method of making a[1076]
belt or flat band, of two, three, or more strands of shroud or hawser-laid rope, placed
side by side, so as to form a band of any desired breadth, which may be used for hoisting
the kibbles and corves in mine-shafts, without any risk of its losing twist by rotation.
The ropes should be laid with the twist of the one strand directed to the right hand,
that of the other to the left, and that of the yarns the opposite way to the strands,
whereby perfect flatness is secured to the band. This parallel assemblage of strands
has been found also to be stronger than when they are all twisted into one cylinder.
The patentees at the same time contrived a mechanism for piercing the strands transversely,
in order to brace them firmly together with twine. Flat ropes are usually
formed of hawsers with three strands, softly laid, each containing 33 yarns, which with four
ropes, compose a cordage four and a half inches broad, and an inch and a quarter thick, being
the ordinary dimensions of the grooves in the whim-pulleys round which they pass.
Relative Strength of Cordage, shroud laid.
| Size. |
Warm Register. |
Cold Register. |
Common Staple. |
| |
Tons. |
Cwt. |
Qrs. |
Lbs. |
Tons. |
Cwt. |
Qrs. |
Lbs. |
Tons. |
Cwt. |
Qrs. |
Lbs. |
| 3 |
|
inches bore |
3 |
17 |
— |
16 |
3 |
5 |
3 |
16 |
2 |
9 |
1 |
24 |
| 3 |
1⁄2 |
— |
5 |
5 |
— |
— |
4 |
9 |
2 |
21 |
3 |
6 |
1 |
27 |
| 4 |
|
— |
6 |
17 |
— |
16 |
5 |
17 |
— |
4 |
4 |
5 |
3 |
7 |
| 4 |
1⁄2 |
— |
8 |
13 |
2 |
8 |
7 |
5 |
3 |
1 |
5 |
1 |
2 |
6 |
| 5 |
|
— |
10 |
14 |
1 |
4 |
9 |
3 |
— |
4 |
6 |
9 |
2 |
8 |
| 5 |
1⁄2 |
— |
12 |
19 |
2 |
4 |
11 |
1 |
1 |
25 |
7 |
12 |
— |
22 |
| 6 |
|
— |
14 |
15 |
2 |
24 |
13 |
3 |
2 |
8 |
8 |
17 |
1 |
20 |
| 6 |
1⁄2 |
— |
18 |
2 |
— |
10 |
15 |
9 |
1 |
9 |
9 |
16 |
3 |
14 |
| 7 |
|
— |
21 |
— |
— |
— |
17 |
18 |
3 |
8 |
11 |
4 |
1 |
21 |
| 7 |
1⁄2 |
— |
24 |
2 |
— |
16 |
20 |
11 |
3 |
9 |
12 |
8 |
3 |
6 |
| 8 |
|
— |
27 |
8 |
1 |
26 |
23 |
8 |
2 |
8 |
13 |
2 |
3 |
12 |
The above statement is the result of several hundred experiments.
ROSIN, or COLOPHANY (Galipot, Fr.; Fichtenharz, Germ.); is the rosin left
after distilling off the volatile oil from the different species of turpentine. Yellow rosin
contains some water, which black rosin does not. See Turpentine.
ROSIN GAS. Fig. 952. exhibits the retort and its appendages, as erected by Messrs.
Taylor and Martineau, under the direction of the patentee, Professor Daniel, F.R.S.
I have introduced this manufacturing project, not as a pattern to imitate, but as an
example to deter; as affording a very instructive lesson of the danger of rushing headlong
into most extensive enterprises, without fully verifying, upon a moderate scale, the
probability of their ultimate success. The capital, labour, and time annually wasted
upon visionary schemes of this sort, got up by chamber chemists, are incalculably great.
No more essential service could be rendered to the cause of productive industry, than
to unmask the thousand and one chimerical inventions which disgrace our lists of
patents during the last thirty years. These remarks have been suggested by the circumstance,
that 50,000l. were squandered upon the rosin-gas concern; a fact communicated
to me by an eminent capitalist, who was induced by fallacious statements to embark
largely in the speculation. Had 100l. been employed beforehand, by a dispassionate
practical man, in making judicious trials, and in calculating the chances of eventual
profit and loss, it would have been demonstrated, as clearly as noonday, that rosin could
never compete with pitcoal in the production of gas-light. Whatever ingenuity was
expended in getting up the following apparatus, may be regarded as an additional ignis
fatuus to mislead the public, and divert their thoughts from the abyss that lay before
them. The main preliminary to be settled, in all new undertakings, is the soundness
of the principle. By neglecting this point, projectors perpetually realize the expiatory
fable of the Danaïds.
The retort e, e, fig. 952., is seen charged with coke, which is in the first instance
raised to a bright red heat, by means of the furnace beneath. The common brown
rosin of commerce, which is deposited in the tank a, is to be mixed with the essential
oil (condensed from the rosin vapours in a preceding operation) in the proportion of one
hundred pounds of the former to ten gallons of the latter. The influence of the flame
and heated air beneath serves to preserve this in a fluid state, and by a damper passing
across the aperture in the chimney the temperature of the fluid may be exactly regulated.
A wire-gauze screen at f, reaches to the bottom of the tank, and prevents the solid rosin,
or any impurity with which it may be mixed, from choking the stopcock.
The melted rosin having passed by the stopcock b, funnel c, and syphon d, into the
retort, falls on the coke, and in its passage through the ignited mass, becomes decomposed.
On arriving at the other end of the retort, a large portion of the oil of turpentine, in[1077]
the form of condensable vapour, is separated by the refrigerator g; this is supplied with
water from a cistern above, and the non-condensable vapour or gas passes up the tube
h, and dips beneath the surface
of the fluid in the vessel i.
This completes the condensation;
and the gas proceeds in
a perfectly pure state, by the
pipe k, to the gasometer, or
rather to the floating reservoir,
for use.
The essential oil, when it
leaves the refrigerator, is conveyed,
by the syphon l, to a
cistern beneath. The necessity
for employing a syphon
will be apparent, when it is
borne in mind that the tube
prevents the escape of the gas,
which would otherwise pass
away from the box with the
essential oil. Another pipe
and syphon m, n, serve to convey
the condensed essential oil
from the top cistern.
ROTTEN-STONE. See
Tripoli.
ROUGE. (Fard, Fr.) The only cosmetic which can be applied without injury to
brighten a lady’s complexion, is that prepared, by the following process, from safflower
(Carthamus tinctorius). The flowers, after being washed with pure water till it comes
off colourless, are dried, pulverized, and digested with a weak solution of crystals of soda,
which assumes thereby a yellow colour. Into this liquor a quantity of finely carded
white cotton wool is plunged, and then so much lemon juice or pure vinegar is added
as to supersaturate the soda. The colouring matter is disengaged, and falls down in an
impalpable powder upon the cotton filaments. The cotton, after being washed in cold
water, to remove some yellow colouring particles, is to be treated with a fresh solution of
carbonate of soda, which takes up the red colouring matter in a state of purity. Before
precipitating this pigment a second time by the acid of lemons, some soft powdered talc
should be laid in the bottom of the vessel, for the purpose of absorbing the fine rouge,
in proportion as it is separated from the carbonate of soda, which now holds it dissolved.
The coloured mixture must be finally triturated with a few drops of olive-oil, in order
to make it smooth and marrowy. Upon the fineness of the talc, and the proportion of
the safflower precipitate which it contains, depend the beauty and value of the
cosmetic. The rouge of the above second precipitation is received sometimes upon
bits of fine-twisted woollen stuff, called crepons, which ladies rub upon their cheeks.
RUM, is a variety of ardent spirits, distilled in the West Indies, from the fermented
skimmings of the sugar teaches, mixed with molasses, and diluted with water to the
proper degree. A sugar plantation in Jamaica or Antigua, which makes 200 hogsheads
of sugar, of about 16 cwt. each, requires, for the manufacture of its rum two
copper stills; one of 1000 gallons for the wash, and one of 600 gallons for the low
wines, with corresponding worm refrigeratories. It also requires two cisterns, one of
3000 gallons for the lees or spent wash of former distillations, called dunder (Quasi
redundar, Span.), another for the skimmings of the clarifiers and teaches of the sugar-house;
along with twelve, or more, fermenting cisterns or tuns.
Lees that have been used more than three or four times, are not considered to be
equally fit for exciting fermentation, when mixed with the sweets, as fresher lees. The
wort is made, in Jamaica, by adding to 1000 gallons of dunder, 120 gallons of molasses,
720 gallons of skimmings (= 120 of molasses in sweetness), and 160 gallons of water;
so that there may be in the liquid nearly 12 per cent. of solid saccharum. Another
proportion, often used, is 100 gallons of molasses, 200 gallons of lees, 300 gallons of
skimmings, and 400 of water; the mixture containing, therefore, 15 per cent. of sweets.
These two formulæ prescribe so much spent wash, according to my opinion, as would
be apt to communicate an unpleasant flavour to the spirits. Both the fermenting and
flavouring principles reside chiefly in the fresh cane juice, and in the skimmings of the
clarifier; because, after the syrup has been boiled, they are in a great measure dissipated.
I have made many experiments upon fermentation and distillation from West India
molasses, and always found the spirits to be perfectly exempt from any rum flavour.
[1078]
The fermentation goes on most uniformly and kindly in very large masses, and
requires from 9 to 15 days to complete; the difference of time depending upon the
strength of the wort, the condition of its fermentable stuff, and the state of the weather.
The progress of the attenuation of the wash should be examined from day to day with
a hydrometer, as I have described in the article Distillation. When it has reached
nearly to its maximum, the wash should be as soon as possible transferred by pumps into
the still, and worked off by a properly regulated heat; for if allowed to stand over, it
will deteriorate by acetification. Dr. Higgins’s plan, of suspending a basket full of
limestone in the wash-tuns, to counteract the acidity, has not, I believe, been found to be
of much use. It would be better to cover up the wash from the contact of atmospheric
air, and to add perhaps a very little sulphite of lime to it, both of which means would tend
to arrest the acetous fermentation. But one of the best precautions against the wash
becoming sour, is to preserve the utmost cleanliness among all the vessels in the distillery.
They should be scalded at the end of every round with boiling water and quicklime.
About 115 gallons of proof rum are usually obtained from 1200 gallons of wash.
The proportion which the product of rum bears to that of sugar, in very rich moist
plantations, is rated, by Edwards, at 82 gallons of the former to 16 cwt. of the latter;
but the more usual ratio is 200 gallons of rum to 3 hogsheads of sugar. But this
proportion will necessarily vary with the value of rum and molasses in the market,
since whichever fetches the most remunerating price, will be brought forward in the
greatest quantity. In one considerable estate in the island of Grenada, 92 gallons of
rum were made for every hogshead (16 cwts.) of sugar. See Still.
| |
Rum imported, in |
Retained for Home Consumption.—Duty
9s. per Imp. Gallon. |
| |
1835. |
1836. |
1837. |
1835. |
1836. |
1837. |
| Galls. |
5,540,170; |
4,993,942; |
4,612,416. |
3,416,966; |
3,325,068; |
3,184,599. |
RUST, is the orange-yellow coat of peroxide which forms upon the surface of iron exposed
to moist air. Oil-paint, varnish, plumbago, or a film of caoutchouc, may be
employed, according to circumstances, to prevent the rusting of iron utensils.
RYE, consists, according to the analysis of Einhof, of 24·2 of husk, 65·6 of flour,
and 10·2 of water, in 100 parts. This chemist found in 100 parts of the flour, 61·07 of
starch, 9·48 of gluten, 3·28 of vegetable albumen, 3·28 of uncrystallizable sugar, 11·09
of gum, 6·38 of vegetable fibre, and the loss was 5·62, including a vegetable acid not
yet investigated. Some phosphate of lime and magnesia are also present. See Gin.
SAFETY LAMP. I have reserved for this place an account of the patented improvement
made upon Davy’s lamp by Messrs. Upton and Roberts; the latter of
whom, having worked in coal mines from a boy, and having observed, that in peculiar
circumstances, the Davy was insecure, was led to contrive certain modifications of it, for
which he received, some years ago, a reward from the Society of Arts. It appears from
undoubted experiments, that if a jet of carburetted hydrogen (coal gas for example) be
impelled with very moderate force against the side of the Davy, it will first fill the wire
cylinder of the burning lamp with flame, and then take fire itself exteriorly. This
passage of the flame of explosive gases through the meshes of wire gauze of the fineness
prescribed for safety lamps by Sir H. Davy, was demonstrated in several trials before
the select committee of the House Commons on accidents in mines, by Mr. Pereira,
at the London University.[49] While the gas is at rest, relatively to Davy’s lamp, the
explosion has never been known to pass; but “if,” says Mr. Pereira, “a lamp be held
before a jet of gas until it becomes hot (a red heat is not essential), and then gently moved,
the flame will pass, and the experiment may be repeated successively a number of times
in the minute.” Two layers of wire gauze, though they greatly impede the transmission
of light, will still permit that of flame, in the above circumstances. In Upton
and Roberts’ lamp, there is but one coat of wire gauze, but it is enclosed in a glass cylinder,
in such a manner as to admit the air which feeds the flame only under its bottom, first
through an annular range of holes, and next through one disc, or several, of wire gauze,
fixed a little way below the wick. The explosive air, after passing up through these
wire-gauze discs, enters a little brass cupola, and is reflected inwards from the orifice at
its top upon the flame, whereby it is completely burned before it reaches the cavity of
the surmounting cylinder. By this reverberatory action of the air upon the wick, the
intensity of the light is at the same time greatly augmented. Since the feed orifices of the
lamp are small in comparison with the capacity of the surmounting cage, the latter does not
get filled with flame on being plunged in an explosive gaseous mixture, as happens to the
naked cage of Davy. The wire gauze can never, therefore, become very hot, far less
ignited, in the new lamp. There are, in fact, three impediments to the passage of the flame[1079]
out of the lamp; first, the stratum of carbonic acid round the light; secondly, the wire-gauze
cylinder; and thirdly, the glass cylinder. The entrance at the bottom may be
made secure in any desired degree, by multiplying the layers of wire cloth. The top is
protected, moreover, by a brass hood, through which the currents of carbonic acid and
nitrogen gases, continually ascending from the burning wick, oppose certain obstacles to
the transmission of flame downwards. Even should the glass be accidentally broken, the
lamp is still a complete Davy.
In the experiments made before the honourable committee at the London University,
Mr. Pereira showed, first, that when a jet of coal-gas alone, or an explosive mixture of
coal-gas and air, impinged upon the wire-gauze cylinder of one of Davy’s lamps with a
certain force, the flame generally passed through the meshes, of which there were from
950 to 1024 in the square inch. When a mixture of four parts of hydrogen, and one
of coal-gas was directed in a jet upon the lighted lamps of Davy, Stevenson, Dillon,
Wood of Killingworth (called the refrigerating lamp), Robson, and Clanny, the flame
readily passed; but when thrown upon the lamp of Upton and Roberts, it did not once
pass, causing merely slight detonations within the lamp. When the force of the jet was
augmented, it extinguished the light. This lamp was finally subjected to the still
severer test of a mixture of four parts of atmospherical air, and one of hydrogen; yet it
did not explode it. When exposed to a mixture of two-thirds of air, and one of hydrogen,
the lamp was immediately extinguished.
The following, out of many certificates, appears to me decisive in favour of this improvement
of Davy’s lamp. It comes from an experienced pitman, in a very deep and
extensive coal mine, which I know to be replete with explosive gas, as I have myself
visited it in company with its accomplished engineer, John Buddle, Esq.
“I hereby certify that I have this day tried Messrs. Upton and Roberts’ new patent
safety lamp, in the Jarrow colliery; and I state, as an experienced pitman, having been
thirty-two years master wasteman in that colliery, that I greatly prefer this new lamp
to the common Davy lamp. I had it between five and six hours on trial in the pit. I
consider that it gives about three times the light of the Davy lamp, as I could see at
least ten yards before me in a straight line; and of its great safety I can have no doubt,
as it does not fill with flame, as the Davy does. And although I had this extra light,
there was much less oil consumed. I consider it a good working lamp.
“Jarrow Colliery, near Newcastle on Tyne, March 31, 1836.” (Signed) “ROBERT FAIRLY.”
Fig. 953., is a vertical section through the middle of the lamp. a, a, is the oil-cistern,
showing the fold of the wick; it is covered at top with b, b,
several layers of wire gauze; c, c, is the perforated brass
ring, under these layers, for admitting air, which is reverberated
upon the burning wick by the cupola c; d, d, is the
cylinder of glass, surrounding the wire-cloth one; e, e, is
the safety brass hood, which screws down in the frame, so
as to cover in the top of the glass chimney; f, is the arched
wire for suspending the lamp to the girdle of the miner;
g, is the bent tube for supplying oil to the cistern; and h
is the safety-trimmer, shown more distinctly in the figure
illustrative of the Lamp of Davy.
Between the glass and the cage there should be a
space of about one-tenth of an inch, forming an annular
chimney for the free ventilation of the flame; and between
the under edge of the hood e, and the upper rim of the
glass, there should likewise be an interval, as also vent-holes
in the top of the hood, for the free escape of the
smoke. The orifice of the little tube g, should be rather
lower than the ring of holes c, otherwise the oil, when
incautiously poured into it, might overflow them, and
prevent the lamp from burning. The figure is drawn somewhat
in perspective.
As the naked cage of Davy often gets red-hot with
flame; as it is sometimes used for hours by miners in
this most hazardous state; as this lamp gives so little
light as to tempt rash men to remove its safety-cage;[50]
as “it is upon record, that taking the average of ten
years previous to the introduction of Sir H. Davy’s
safety lamp, and allowing one clear year for its introduction,
and of ten years after it was properly introduced,
there had been double the number of accidents,
and at least double the number of deaths, of[1080]
what took place in the ten years previous to its introduction;[51] as his lamp in explosive
air-courses needs to be carried close upon the bosom, or under the coat of the miner;
as it was declared by its illustrious inventor to be dangerous when exposed to such
currents of explosive gas; and as the above described modification of it is free from all
these defects and dangers,—I humbly apprehend that no conscientious proprietor or
viewer of coal-mines will delay to substitute the lamp of Upton and Roberts for the
naked Davy, for otherwise he will certainly stand in a very painful predicament before
a coroner’s inquest, at the next mortal casualty from explosion.”
The patentees have, I am told, been put to so much trouble and expense in trying to
introduce this life-protector into our coal-mines, that they have in a great measure
abandoned the business. Messrs. Smith of Birmingham have meanwhile undertaken to
make the lamps.
SAFFLOWER. This dye-stuff has been fully described under Carthamus and Rouge.
SAFFRON (Saffran, Fr. and Germ.); is a filamentous cake, composed of the
stigmata of the flowers of the Crocus sativus. It contains a yellow matter called polychroïte,
because a small quantity of it is capable of colouring a great body of water. This
is obtained by evaporating the watery infusion of saffron to the consistence of an extract,
digesting the extract with alcohol, and concentrating the alcoholic solution. The
polychroïte remains in the form of a brilliant mass, of a reddish-yellow colour, transparent,
and of the consistence of honey. It has the agreeable smell, with the bitter pungent
taste, of saffron. It is very soluble in water; and if it be stove-dried, it deliquesces
speedily in the air. According to M. Henry père, polychroïte consists of 80 parts of
colouring matter, combined with 20 parts of a volatile oil, which cannot be separated
by distillation till the colouring matter has been combined with an alkali. By mixing
one part of shred saffron with eight parts of saturated brine, and one-half part of
caustic lye, and distilling the mixture, the oil comes over into the receiver, and leaves
the colouring matter in the retort, which may be precipitated from the alkaline
solution by an acid. The pure colouring matter, when dried, is of a scarlet hue, and
then readily dissolves in alcohol, as also in the fat and volatile oils, but sparingly in
water. Light blanches the reddish-yellow of saffron, even when it is contained in a full
phial well corked. Polychroïte, when combined with fat oil, and subjected to dry distillation,
affords ammonia, which shows that azote is one of its constituents. Sulphuric acid
colours the solution of polychroïte indigo blue, with a lilac cast; nitric acid turns it
green, of various shades, according to the state of dilution. Protochloride (muriate) of
tin produces a reddish precipitate.
Saffron is employed as a seasoning in French cookery. It is also used to tinge confectionary
articles, liqueurs, and varnishes; but rarely as a pigment.
SAGO (Sagou, Fr. and Germ.); is a species of starch, extracted from the pith of the
sago palm, a tree which grows to the height of 30 feet in the Moluccas and the Philippines.
The tree is cut down, cleft lengthwise, and deprived of its pith, which being
washed with water upon a sieve, the starchy matter comes out, and soon forms a deposit.
This is dried to the consistence of dough, pressed through a metal sieve to corn it (which
is called pearling), and then dried over a fire with agitation in a shallow copper pan.
Sago is sometimes imported in the pulverulent state, in which it can be distinguished from
arrow-root only by microscopic examination of its particles. These are uniform and
spherical, not unequal and ovoid, like those of arrow-root.
SAL AMMONIAC. The manufacture of this salt may be traced to the remotest
era. Its name is derived from Ammonia, or the temple of Jupiter Ammon, in Egypt,
near to which the salt was originally made. Sal ammoniac exists ready formed in several
animal products. The dung and urine of camels contain a sufficient quantity to
have rendered its extraction from them a profitable Egyptian art in former times, in order
to supply Europe with the article. In that part of Africa, fuel being very scarce, recourse
is had to the dung of these animals, which is dried for that purpose, by plastering it upon
the walls. When this is afterwards burned in a peculiar kind of furnace, it exhales a thick
smoke, replete with sal ammoniac in vapour; the soot of course contains a portion of
that salt, condensed along with other products of combustion. In every part of Egypt,
but especially in the Delta, peasants are seen driving asses loaded with bags of that soot,
on their way to the sal ammoniac works.
Here it is extracted in the following manner. Glass globes coated with loam are
filled with the soot pressed down by wooden rammers, a space of only two or three inches
being left vacant, near their mouths. These globes are set in round orifices formed in
the ridge of a long vault, or large horizontal furnace flue. Heat is gradually applied by
a fire of dry camels’ dung, and it is eventually increased till the globes become obscurely[1081]
red. As the muriate of ammonia is volatile at a temperature much below ignition, it
rises out of the soot in vapour, and gets condensed into a cake upon the inner surface of
the top of the globe. A considerable portion, however, escapes into the air; and another
portion concretes in the mouth, which must be cleared from time to time by an iron rod.
Towards the end, the obstruction becomes very troublesome, and must be most carefully
attended to and obviated, otherwise the globes would explode by the uncondensed
vapours. In all cases, when the subliming process approaches to a conclusion, the
globes crack or split; and when they come to be removed, after the heat has subsided,
they usually fall to pieces. The upper portion of the mass is separated, because to it the
white salt adheres; and on detaching the pieces of glass with a hatchet, it is ready for
the market. At the bottom of each balloon a nucleus of salt remains, surrounded with
fixed pulverulent matter. This is reserved, and after being bruised, is put in along
with the charge of soot in a fresh operation.
The sal ammoniac obtained by this process is dull, spongy, and of a grayish hue;
but nothing better was for a long period known in commerce. Forty years ago, it
fetched 2s. 6d. a pound; now, perfectly pure sal ammoniac may be had at one-fifth
part of that price.
Various animal offals develope during their spontaneous putrefactive fermentation, or
their decomposition by heat, a large quantity of free or carbonated ammonia, among
their volatile products. Upon this principle many sal ammoniac works have been established.
In the destructive distillation of pitcoal, there is a considerable quantity of
ammoniacal products, which are also worked up into sal ammoniac.
The first attempts made in France to obtain sal ammoniac profitably in this manner,
failed. A very extensive factory of the kind, which experienced the same fate, was
under the superintendence of the celebrated Baumé, and affords one out of a thousand
instances where theoretical chemists have shown their total incapacity for conducting
operations on the scale of manufacturing economy. It was established at Gravelle near
Charenton, and caused a loss to the shareholders in the speculation of upwards of
400,000 francs. This result closed the concern in 1787, after a foolish manipulation of
27 years. For ten years after that event, all the sal ammoniac consumed in France was
imported into it from foreign countries. Since then the two works of MM. Payen
and Pluvinet were mounted, and seem to have been tolerably successful. Coal soot
was, prior to the introduction of the gas-works, a good deal used in Great Britain for
obtaining sal ammoniac. In France, bones and other animal matters are distilled in
large iron retorts, for the manufacture of both animal charcoal and sal ammoniac.
These retorts are iron cylinders, 2 or 3 feet in diameter, and 6 feet long. Figs. 954.
and 955. show the form of the furnace, and the manner in which the cylinders are
arranged; the first being a longitudinal, the second a transverse section of it. A, the ash-pits
under the grates; B, the fireplaces, arched over at top; C, the vault or bench of fire-bricks,
perforated inside with eight flues for distributing the flame; D, a great arch, with
a triple voussoir D, d′, d′′, under which the retorts are set. The first arch D, is perforated
with twenty vent-holes; the second, with four vent-holes; through which the flame
passes to the third arch, and thence to the common chimney-stalk. The retorts e, are
shut by the door e′ (fig. 955.), luted, and made fast with screw-bolts. Their other ends e′′
terminate in tubes f, f, f, which all enter the main pipe h. The condensing pipe proceeds
slantingly downwards from the further end of h, and dips into a large sloping iron cylinder
immersed in cold water. See Gas-light and Stove, for a better plan of furnace.
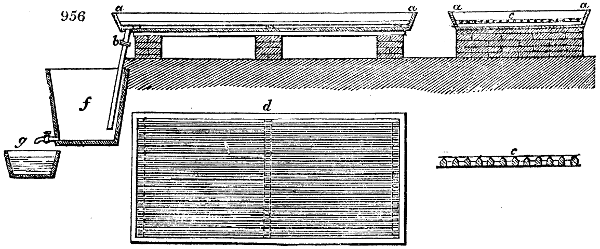
The filters used in the large sal ammoniac works in France are represented in fig.
956. The apparatus consists——1. of a wooden chest a, lined with lead, and which is
turned over at the edges; a socket of lead b, soldered into the lowest part of the bottom,
serves to discharge the liquid; 2. of a wooden crib or grating formed of rounded rods,[1082]
as shown in the section c, c, and the plan d; this grating is supported one inch at least
above the bottom, and set truly horizontal, by a series of wedges; 3. of an open
fabric of canvas or strong calico, laid on the grating, and secured over the edges,
so as to keep it tense. A large wooden reservoir f, lined with lead, furnished with a
cover, is placed under each of the filters; a pump throws back once or twice upon the
filters what has already passed through. A common reservoir g, below the others, may
be made to communicate at pleasure with one of them, by means of intermediate stopcocks.
The two boilers for evaporating and decomposing are made of lead, about one quarter
of an inch thick, set upon a fire-brick vault, to protect them from the direct action
of the flame. Through the whole extent of their bottoms above the vault, horizontal
cast-iron plates, supported by ledges and brick compartments, compel the flame and
burned air, as they issue from the arch, to percur many sinuosities before they pass up
the chimney. This floor of cast iron is intended to support the bottom of the boiler,
and to diffuse the heat more equably. The leaden boilers are surrounded with brickwork,
and supported at their edges with a wooden frame. They may be emptied at
pleasure into lower receivers, called crystallizers, by means of leaden syphons and long-necked
funnels.
The crystallizers are wooden chests lined with lead, 15 inches deep, 3 or 4 feet
broad, and from 6 to 8 feet long; and may be inclined to one side at pleasure. A
round cistern receives the drainings of the mother-waters. The pump is made of
lead, hardened with antimony and tin.
The subliming furnace is shown in figs. 957. and 958. by a transverse and longitudinal
section. a is the ash-pit; b, the grate and fireplace; c, the arch above them. This
arch, destined to protect the bottles from the direct action of the fire, is perforated with
vent-holes, to give a passage to the products of combustion between the subliming vessels.
d, d, are bars of iron, upon which the bottoms of the bottles rest; e, stoneware bottles,
protected by a coating of loam from the flame.
Fig. 959. shows the cast-iron plates, a, b, c, which, placed above the vaults, receive
each two bottles in a double circular opening.
At the extremity of the above furnace, a second one, called the drier, fig. 960., receives the
products of the combustion of the first, at A, under horizontal cast-iron plates, and upon
which the bottom of a rather shallow boiler B, rests. After passing twice under these
plates, round a longitudinal brick partition b, b′, b′′, the products of combustion enter
the smoke chimney C. See plan, fig. 961.
The boiler set over this furnace should have no soldered joints. It may be 31⁄2 feet[1083]
broad, 9 or 10 feet long, and 1 foot deep. The concrete sal ammoniac may be crushed
under a pair of edge mill-stones, when it is to be sold in powder.
Bones, blood, flesh, horns, hoofs, woollen rags, silk, hair, scrapings of hides and
leather, &c., may be distilled for procuring ammonia. When bones are used, the
residuum in the retort is bone black. The charcoal from the other substances will serve
for the manufacture of prussian blue. The bones should undergo a degree of calcination
beyond what the ammoniacal process requires, in order to convert them into the best
bone black; but the other animal matters should not be calcined up to that point,
otherwise they are of little use in the prussian blue works. If the bones be calcined,
however, so highly as to become glazed, their decolouring power on syrups is nearly
destroyed. The other substances should not be charred beyond a red-brown heat.
The condensed vapours from the cylinder retorts afford a compound liquor holding
carbonate of ammonia in solution, mixed with a large quantity of empyreumatic oil,
which floats at top. Lest incrustations of salt should at any time tend to obstruct the
tubes, a pipe should be inserted within them, and connected with a steam boiler, so as to
blow steam through them occasionally.
The whole liquors mixed have usually a density of 8° or 9° Baumé (1·060). The
simplest process for converting their carbonate of ammonia into muriate, is to saturate
them with muriatic acid, to evaporate the solution in a leaden boiler till a pellicle
appears, to run it off into crystallizers, and to drain the crystals. Another process is, to
decompose the carbonate of ammonia, by passing its crude liquor through a layer of
sulphate of lime, 3 or 4 inches thick, spread upon the filters, fig. 956. The liquor may
be laid on with a pump; it should never stand higher than 1 or 2 inches above the
surface of the bruised gypsum, and it should be closely covered with boards, to prevent
the dissipation of the volatile alkali in the air. When the liquor has passed through
the first filter, it must be pumped upon the second; or the filters being placed in a terrace
form, the liquor from the first may flow down upon the second, and thus in succession.
The last filter should be formed of nearly fresh gypsum, so as to ensure the
thorough conversion of the carbonate into sulphate. The resulting layers of carbonate
of lime should be washed with a little water, to extract the sulphate of ammonia interposed
among its particles. The ammoniacal liquor thus obtained must be completely
saturated, by adding the requisite quantity of sulphuric acid; even a slight excess of acid
can do no harm. It is then to be evaporated, and the oil must be skimmed off in the
course of the concentration. When the liquid sulphate has acquired the density of about
1·160, sea salt should be added, with constant stirring, till the whole quantity equivalent
to the double decomposition be introduced into the lead boiler.
The fluid part must now be drawn off by a syphon into a somewhat deep reservoir,
where the impurities are allowed to subside; it is then evaporated by boiling, till the
sulphate of soda falls down in granular crystals, as the result of the mutual reaction of the
sulphate of ammonia and muriate of soda; while the more soluble muriate of ammonia
remains in the liquor. During this precipitation, the whole must be occasionally
agitated with wooden paddles; the precipitate being in the intervals removed to the
cooler portion of the pan, in order to be taken out by copper rakes and shovels, and thrown
into draining-hoppers, placed near the edges of the pan. The drained sulphate of soda
must be afterwards washed with cold water, to extract all the adhering sal ammoniac.
The liquor thus freed from the greater part of the sulphate, when sufficiently concentrated,
is to be drawn off by a lead syphon, into the crystallizers, where, at the end
of 20 or 30 hours, it affords an abundant crop of crystals of sal ammoniac. The mother-water
may then be run off, the crystallizers set aslope to drain the salt, and the salt itself
must be washed, first by a weak solution of sal ammoniac, and lastly with water. It
must be next desiccated, by the apparatus fig. 960., into a perfectly dry powder, then
put into the subliming stoneware balloons, by means of a funnel, and well rammed down.
The mouth of the bottle is to be closed with a plate or inverted pot of any kind. The
fire must be nicely regulated, so as to effect the sublimation of the pure salt from the
under part of the bottle, with due regularity, into a white cake in the upper part. The
neck of the bottle should be cleared from time to time with a long steel skewer, to
prevent the risk of choking, and consequent bursting; but in spite of every precaution,
several of the bottles crack almost in every operation. In Scotland, sal ammoniac is
sublimed in cast-iron pots lined with thin fire-tiles, made in segments accommodated to
the internal surface of the pots; the vapour being received and condensed into cakes,
within balloons of green glass set over their mouths. The salt, when taken out, and freed
by scraping from any adhering ochreous or other impurities, is ready for the market,
being sold in hollow spherical masses. The residuum in the pots or bottles may be
partially worked up in another operation. The greatest evil is produced by the mixture
or even contact of iron, because its peroxide readily rises in vapour with the sal ammoniac,
and tinges it of a red or yellow colour.
The most ordinary process for converting the ammoniacal liquor of the gas works into[1084]
sal ammoniac, is to saturate it with sulphuric acid, and to decompose the sulphate, thus
formed, by the processes above described. But muriatic acid will be preferred, where it
is as cheap as sulphuric of equivalent saturating power; because a tolerably pure sal
ammoniac is thereby directly obtained. As the coal-gas liquor contains a good deal of
sulphuretted hydrogen, the saturation of it with acid should be so conducted as to burn
the disengaged noxious gases in a chimney. Formerly human urine was very extensively
employed, both in this country and in France, in the manufacture of sal ammoniac;
but since the general establishment of gas-works it has been, I believe, abandoned. The
process was exceedingly offensive.
The best white sal ammoniac is in spheroidal cakes of about one foot diameter, three
or four inches thick in the middle, somewhat thinner at the edges, and is semi-transparent
or translucent. Each lump weighs about one quarter of a cwt. As it is
easily volatilized by heat, it may be readily examined as to its sophistication with other
salts. Sal ammoniac has a certain tenacity, and is flexible under the hammer or pestle.
It is principally used in tinning of cast-iron, wrought iron, copper, brass, and for making
the various ammoniacal preparations of pharmacy.
In a chemical factory near Glasgow, 7200 gallons of ammoniacal liquor, obtained weekly
from the gas-works, are treated as follows:—The liquor is first rectified by distillation
from a waggon-shaped wrought-iron boiler, into a square cistern of iron lined with lead.
4500 lbs. of sulphuric acid, of specific gravity 1·625, are then slowly added to the somewhat
concentrated distilled water of ammonia. The produce is 2400 gallons of sulphate
of ammonia, slightly acidulous, of specific gravity 1·150, being of such strength as to
deposit a few crystals upon the sides of the lead-lined iron tank in which the saline
combination is made. It is decomposed by common salt.
From the 7200 gallons of the first crude liquor, 900 gallons of tar are got by subsidence,
and 200 gallons of petroleum are skimmed off the surface. The tar is converted,
by a moderate boiling in iron pans, into good pitch.
SALAMSTONE. See Lapidary.
SALEP, or SALOUP, is the name of the dried tuberous roots of the Orchis, imported
from Persia and Asia Minor, which are the product of a great many species of the
plant, but especially of the Orchis mascula. Salep occurs in commerce in small oval
grains, of a whitish-yellow colour, at times semi-transparent, of a horny aspect, very,
hard, with a faint peculiar smell, and a taste like that of gum tragacanth, but slightly
saline. These are composed almost entirely of starchy matter, well adapted for making
a thick pap with water or milk, and are hence in great repute in the Levant, as
restorers of the animal forces. Their aphrodisiacal properties are apocryphal. If the
largest roots of the Orchis mascula of our own country were cleaned, scraped, steeped
for a short time in hot, and then for a few minutes in boiling water, to extract their rank
flavour, afterwards suspended upon strings to dry in the air, they would afford as
nourishing and palatable an article as the Turkey saloup, and at a vastly lower price.
SALICINE, is a febrifuge substance, which may be obtained in white pearly crystals
from the bark of the white willow (Salix alba), of the aspen tree (Salix helix), as also
of some other willows, and some poplars. It has a very bitter taste.
SAL PRUNELLA, is fused nitre cast into cakes or balls.
SAL VOLATILE, is sesquicarbonate of ammonia.
SALT, MICROCOSMIC, is the triple phosphate of soda and ammonia.
SALT OF AMBER, is succinic acid.
SALT OF LEMONS, is citric acid.
SALT OF SATURN, is acetate of lead.
SALT OF SODA, is carbonate of soda.
SALT OF SORREL, is bi-oxalate of potassa.
SALT OF TARTAR, is carbonate of potassa.
SALT OF VITRIOL, is sulphate of zinc.
SALT PERLATE, is phosphate of soda.
SALTPETRE, is nitre, or nitrate of potassa.
SALT, SEDATIVE, is boracic acid.
SALTS, are an important class of chemical compounds, antiently studied under the
Greek title of Halurgy. At one period every inorganic substance readily soluble in
water, was regarded as a salt; and afterwards, every substance soluble in five hundred
times its weight of water. Thus both acid and alkaline bodies came to be enrolled among
salts; but latterly, the combinations of the acids with alkalis, earths, and metallic calces
(now styled oxides), were alone thought to be entitled to the denomination of salts, in
consequence of their resemblance in appearance, and supposed analogy in composition,
to culinary salt. Since Sir H. Davy demonstrated that this substance contained neither
acid nor alkaline matter, but that it consisted of chlorine and the metal sodium, the
generality of chemists found it impossible to include salts under one category of constitution;[1085]
while a few have rashly offered to cut the knot, by excluding from the saline
family, chloride of sodium, the patriarch of the whole.
Salts, may be justly divided into three orders:
1. The binary, consisting of two single members; such as the bromides, chlorides,
cyanides, fluorides, iodides, carburets, phosphurets, sulphurets, &c.
2. The bi-binary, consisting of two double members; such as the borates, bromates,
carbonates, chlorates, sulphates, sulphites, hyposulphites, sulphohydrates, &c.
3. The ternary, consisting of two single members of one genus, and one member of
another; such as the boro-fluorides, silico-fluorides, sulpho-cyanides, chloriodides, &c.
The species of each order may exist in three states, constituting neutral salts; supersalts;
and subsalts; as for example, the chloride of sodium, the bisulphate of potassa,
the subnitrate of lead, &c.
In the above arrangement, cyanogen is allowed to represent a simple substance, from
its forming analogous compounds with chlorine and iodine. The neutral state of salts is
commonly indicated by their solutions not changing the colours of litmus, violets, or red
cabbage; the sub-state of salts, by their turning the violet and cabbage green; and the
super-state of salts, by their changing the purple of litmus, violets, and cabbage, red; but
to the generality of this criterion there are some exceptions. The atomic theory may be
advantageously resorted to, in this predicament. 1. When one prime equivalent of the one
member (whether single or double) of a salt, combines with one prime of the other
member, a neutral salt is the result, as in chloride of sodium or nitrate of potassa. 2.
When two primes of the electro-negative member combine with one prime of the
electro-positive, a supersalt is formed, as bichloride of tin, or bisulphate of potassa.
3. When one prime of the electro-negative member combines with two or more primes
of the electro-positive, a subsalt is produced, as the subacetate and subchromate of
lead, &c.
SALT, SEA, or CULINARY; Chloride of sodium; muriate of soda. (Hydrochlorate
de soude, Fr.; Chlornatrium, Germ.) Sea salt, or rock salt, in a state of purity, consists
of 60 of chlorine + 40 of sodium, in 100 parts.
This important species of the saline class possesses, even in mass, a crystalline structure,
derived from the cube, which is its primitive form. It has generally a foliated
texture, and a distinct cleavage; but it has also sometimes a fibrous structure. The
massive salt has a vitreous lustre. It is not so brittle as nitre; it is nearly as hard as
alum, a little harder than gypsum, and softer than calcareous spar. Its specific gravity
varies from 2·0 to 2·25. When pure, it is colourless, translucent, or transparent. On
exposure to heat, it commonly decrepitates; but some kinds of rock salt enter quietly
into fusion at an elevated temperature, a circumstance which has been ascribed to their
having been originally subjected to the action of fire.
According to M. Gay Lussac, 100 parts of water dissolve—
| 35·81 |
parts of the salt, at temperature |
57·0° |
Fahr. |
| 35·88 |
— |
62·5° |
|
| 37·14 |
— |
140·0° |
|
| 40·38 |
— |
229·5° |
|
Native chloride of sodium, whether obtained from the waters of the ocean, from saline
lakes, from salt springs, or mineral masses, is never perfectly pure. The foreign
matters present in it vary with its different origins and qualities. These are, the
sulphates of lime, magnesia, soda, muriates of magnesia and potash, bitumen, oxide of
iron, clay in a state of diffusion, &c.
Muriate of potash has been detected, in the waters of the ocean, in the sal-gem of
Berchtesgaden in Bavaria, of Hallein in the territory of Salzbourg, and in the salt
springs of Rosenheim.
The more heterogeneous the salt, the more soluble is it, by the reciprocal affinity of its
different saline constituents; and thus a delicate hydrometer, plunged in saturated brine,
may serve to show approximately the quality of the salt. I find that the specific gravity
of a saturated solution of large-grained cubical salt, is 1·1962 at 60° F. 100 parts of
this brine contain 251⁄2 of salt, (100 w. + 34·2 s.) From mutual penetration, 100
volumes of the aqueous and saline constituents form rather less than 96 of the
solution.
Among the varieties in the form of this salt, the octahedral, the cubo-octahedral, and
the dodecahedral, have been mentioned; but there is another, called the funnel or hopper-shaped,
which is very common. It is a hollow rectangular pyramid, which forms at
the surface of the saline solution in the course of its evaporation, commencing with a
small floating cube, upon which lines of other little cubes attach themselves to the
edges of the upper face; whereby they form and enlarge the sides of a hollow pyramid,
whose apex, the single cubic crystal, is downward. This sinks by degrees as the aggregation
goes on above, till a pyramidal boat of considerable size is constructed.
[1086]
A Table of the results of the Analyses of several varieties of Culinary Salt.
| Origin of the Salt. |
Chloride
of
Sodium. |
Muriate
of
Mag-
nesia. |
Muriate
of
Lime. |
Sulphate
of
Soda. |
Sulphate
of
Mag-
nesia. |
Sulphate
of
Lime. |
Clay and
other
insoluble
bodies. |
Oxide
of
Iron. |
| |
|
|
|
|
|
|
|
|
| Sal-gem of Vic |
- |
|
white |
99 |
·30 |
— |
— |
— |
— |
0 |
·005 |
0 |
·020 |
|
| red |
99 |
·80 |
— |
— |
— |
— |
— |
0 |
·002 |
|
| —— Cheshire, crushed |
98 |
·33 |
0 |
·02 |
— |
— |
— |
0 |
·65 |
— |
0 |
·002 |
| Salt from Salt Springs: |
|
|
|
|
|
|
|
|
| Schönbeck, Westphalia |
93 |
·90 |
0 |
·30 |
— |
1 |
·00 |
— |
0 |
·80 |
|
|
| Moutiers |
- |
|
des cordes |
97 |
·17 |
0 |
·25 |
— |
2 |
·00 |
0 |
·58 |
|
|
|
| boilers |
93 |
·59 |
0 |
·61 |
— |
5 |
·55 |
0 |
·25 |
|
|
|
| Château Salins |
97 |
·82 |
2 |
·12 |
|
|
|
|
|
|
| White of Sulz |
96 |
·88 |
3 |
·12 |
|
|
|
|
|
|
| Ludwigshall, middle grained |
99 |
·45 |
— |
— |
0 |
·05 |
— |
0 |
·28 |
|
|
| Kœnigsborn, Westphalia |
95 |
·90 |
— |
0 |
·27 |
— |
— |
1 |
·10 |
|
|
| Sea salt, half white |
97 |
·20 |
0 |
·004 |
— |
— |
0 |
·050 |
0 |
·120 |
0 |
·070 |
|
| ——, of Saint Malo |
96 |
· |
0 |
·30 |
— |
— |
0 |
·45 |
2 |
·35 |
|
|
| Common Scottish salt |
93 |
·55 |
2 |
·80 |
— |
— |
1 |
·75 |
1 |
·50 |
|
|
| Lymington, common |
93 |
·7 |
1 |
·1 |
— |
— |
3 |
·50 |
1 |
·50 |
2 |
·00 |
|
| ——, cat |
98 |
·8 |
0 |
·5 |
— |
— |
0 |
·5 |
0 |
·1 |
|
|
| Cheshire, stoved |
98 |
·25 |
0 |
·075 |
0 |
·025 |
— |
— |
1 |
·55 |
|
|
The geological position of rock salt is between the coal formation and the lias. The
great rock-salt formation of England occurs within the red marl, or new red sandstone,
the bunter-sandstein of the Germans, so called, because its colours vary from red to
salmon and chocolate. This mineral stratum frequently presents streaks of light blue,
verdigris, buff, or cream colour; and is chiefly remarkable for containing considerable
masses or beds of gypsum. At Northwich, in the vale of the Weaver, the rock salt
consists of two beds, together not less than 60 feet thick, which are supposed to constitute
large insulated masses, about a mile and a half long, and nearly 1300 yards
broad. There are other deposits of rock salt in the same valley, but of inferior importance.
The uppermost bed occurs at 75 feet beneath the surface, and is covered
with many layers of indurated red, blue, and brown clay, interstratified more or less
with sulphate of lime, and interspersed with argillaceous marl. The second bed of rock
salt lies 311⁄2 feet below the first, being separated from it by layers of indurated clay,
with veins of rock salt running through them. The lowest bed of salt was excavated
to a depth of 110 feet, several years ago.
The beds or masses of rock salt are occasionally so thick, that they have not been yet
bored through, though mined for many centuries. This is the case with the immense
mass of Wieliczka, and the lower bed at Northwich. But in ordinary cases, this
thickness varies from an inch or two to 12 or 15 yards. When the strata are thin,
they are usually numerous; but the beds, layers, or masses never exhibit throughout a
great extent any more than an illusory appearance of parallelism; for when they are
explored at several points, enlargements are observed, and such diminutions as cause
the salt to disappear sometimes altogether. This mineral is not deposited, therefore, in
a geological stratum, but rather in lenticular masses, of very variable extent and thickness,
placed alongside of each other at unequal distances, and interposed between the
courses of the other formations.
Sometimes the rock salt is disseminated in small masses or little veins among the calcareous
and argillaceous marls which accompany or overlie the greater deposits. Bitumen,
in small particles, hardly visible, but distinguishable by the smell, occurs in all the
minerals of the saliferous system.
It has been remarked, that the plants which grow generally on the sea shores, such as
the Triglochinum maritimum, the Salicornia, the Salsola kali, the Aster trifolium, or farewell
to summer, the Glaux maritima, &c., occur also in the neighbourhood of salt mines and
salt springs, even of those which are most deeply buried beneath the surface.
The interior of rock-salt mines, after digging through the strata of clay marl, &c. is
extremely dry; so that the dust produced in the workings becomes an annoyance to
the miners, though in other respects the excavations are not at all insalubrious.
Salt springs occur nearly in the same circumstances, and in the same geological formation[1087]
as the salt rock. It has been noticed that salt springs issue, in general, from the
upper portion of the saliferous strata, principally from the saline clay marls. Cases
however occur, where the salt springs are not accompanied by rock salt, and where the
whole saline matter is derived from the marls themselves, which thus constitute the only
saliferous beds.
It has been imagined that there are two other periods of geological formation of
this substance; one much more antient, belonging to the transition series of rocks; the
other relatively modern, among secondary strata. To the former has been referred the
salt formation of Bex, that of Cardonne, &c. But M. Brongniart assigns valid reasons
for rejecting this supposition. M. Beudant, indeed, refers to the secondary strata above
the chalk, the rock-salt formation of Wieliczka, and of the base of the Carpathians;
placing these among the plastic clay and lignites.
The mines of rock salt do not appear to possess any determinate elevation upon the surface
of the earth. Immense masses of it are met with at very great depths below the level
of the sea, (the mine of Wieliczka is excavated 860 feet beneath the soil,) and others exist
at a considerable altitude, as that of Hallein near Salzbourg, which is 3300 feet above
the level of the sea, and the saline rock of Arbonne in Savoy, which is nearly 4000 feet
higher, situated at the great elevation of 7200 feet above the level of the sea, and
consequently in the region of perpetual snow. The rock is a mass of saccharoid and
anhydrous gypsum, imbued with common salt, which is extracted by lixiviation; after
which the gypsum remains porous and light.
The inland seas, salt lakes, and salt marshes, have their several localities obviously
independent of peculiar geological formations. The ocean is, however, the most magnificent
mine of salt, since this chloride constitutes about one-thirtieth part of its weight;
being pretty evenly diffused throughout its waters, when no local cause disturbs the equilibrium.
The largest proportion of salt held in solution in the open sea, is 38 parts in
1000, and the smallest 32. In a specimen taken by Mr. Wilkinson, out of the Red Sea, at
Berenice, I found 43 parts of salt in 1000. The specific gravity of the water was 1·035.
Were it requisite to extract the chloride of sodium from sea-water by fuel alone,
many countries, even maritime, would find the process too costly. The salt is
therefore obtained from it in two different manners; 1. by natural evaporation alone;
2. by natural and artificial evaporation combined. The first method is employed in
warm regions, under the form of saline tanks, or brine reservoirs, called also brine-pits.
These are large shallow basins, the bottom of which is very smooth, and formed of clay.
They are excavated along the sea-shore, and consist of—
1st. A large reservoir, deeper than the proper brine-pits, which is dug between them
and the sea. This reservoir communicates with the sea by means of a channel provided
with a sluice. On the sea-shore, these reservoirs may be filled at high water, though the
tides are rather inconvenient than advantageous to brine-pits.
2dly. The brine-pits, properly so called, which are divided into a number of compartments
by means of little banks. All these compartments have a communication
with each other, but so that the water frequently has a long circuit to make, from one
set to another. Sometimes it must flow 400 or 500 yards, before it reaches the
extremity of this sort of labyrinth. The various divisions have a number of singular
names, by which they are technically distinguished. They should be exposed to the
north, north-east, or north-west winds.
The water of the sea is let into these reservoirs in the month of March, where it is
exposed on a vast surface to evaporation. The first reservoir is intended to detain the
water till its impurities have subsided, and from it the other reservoirs are supplied,
as their water evaporates. The salt is considered to be on the point of crystallizing
when the water begins to grow red. Soon after this, a pellicle forms on the surface,
which breaks, and falls to the bottom. Sometimes the salt is allowed to subside in
the first compartment; at others, the strong brine is made to pass on to the others,
where a larger surface is exposed to the air. In either case the salt is drawn out,
and left upon the borders to drain and dry.
The salt thus obtained, partakes or the colour of the bottom on which it is formed;
and is hence white, red, or gray.
Sea water contains, in 1000 parts, 25 of chloride of sodium, 5·3 sulphate of magnesia,
3·5 chloride of magnesium, 0·2 carbonate of lime and magnesia, 0·1 sulphate of
lime, besides 1⁄2000 of sulphate and muriate of potash. It also contains iodide of sodium,
and bromide of magnesium. Its average spec. grav. is from 1·029 to 1·030.
Sea-water and weak brines may be concentrated either by the addition of rock salt,
by spontaneous evaporation in brine-pits (see suprà), or by graduation. Houses for the
last purpose are extensively employed in France and Germany. The weak brine is
pumped into an immense cistern on the top of a tower, and is thence allowed to flow
down the surface of bundles of thorns built up in regular walls, between parallel wooden
frames. At Salza, near Schönebeck, the graduation-house is 5817 feet long, the thorn[1088]
walls are from 33 to 52 feet high, in different parts, and present a total surface of
25,000 square feet. Under the thorns, a great brine cistern, made of strong wooden
planks, is placed, to receive the perpetual shower of water. Upon the ridge of the
graduation-house there is a long spout, perforated on each side with numerous holes, and
furnished with spigots or stopcocks for distributing the brine, either over the surface of
the thorns, or down through their mass; the latter method affording larger evaporation.
The graduation-house should be built lengthwise in the direction of the prevailing
wind, with its ends open. An experience of many years at Salza and Dürrenberg has
shown, that in the former place graduation can go on 258, and in the latter 207 days, on
an average, in the year; the best season being from May till August. At Dürrenberg,
3,596,561 cubic feet of water are evaporated annually. According to the weakness of
the brine, it must be the more frequently pumped up, and made to flow down over the
thorns in different compartments of the building, called the 1st, 2d, and 3d graduation.
A deposit of gypsum incrusts the twigs, which requires them to be renewed at the end
of a certain time. Figs. 962. and 963. represent the graduation-house of the salt-works
at Dürrenberg. a, a, a, are low stone pillars for supporting the brine cistern b, called
the soole-schiff. c, c are the inner, d, d the outer, walls of thorns; the first have perpendicular
sides, the last sloping. The spars e, e, which support the thorns, are longer
than the interval between two thorn walls from f to g, fig. 963, whereby they are readily
fastened by their tenons and mortises. The spars are laid at a slope of 2 inches in the
foot, as shown by the line h, i. The bundles of thorns are each 11⁄2 foot thick, from
5 to 7 feet long, and are piled up in the following way:——Guide-bars are first placed
in the line k, l, to define the outer surface of the thorn wall, the undermost spars m, n,
are fastened upon them; and the thorns are evenly spread, after the willow-withs of the
bundles have been cut. Over the top of the thorn walls are laid, through the whole length
of the graduation-house, the brine spouts o, o, which are secured to the upper beams; and
at both sides of these spouts are the drop-spouts p, p, for discharging the brine by the
spigots s, s, as shown upon a larger scale in fig. 964. The drop-spouts are 6 feet long,
have on each side small notches, 5 inches apart, and are each supplied by a spigot.
The space above the ridge of the graduation-house is covered with boards, supported at
their ends by binding-beams q. r, r show the tenons of the thorn-spars. Over the
soole schiff b, inclined planes of boards are laid for conducting downwards the innumerable
showers. The brine, which contains at first 7·692 per cent. of salt, indicates, after the
first shower, 11·473; after the second, 16·108; and after the third, 22. The brine, thus
concentrated to such a degree as to be fit for boiling, is kept in great reservoirs, of which
the eight at Salza, near Schönebeck, have a capacity of 2,421,720 cubic feet, and are furnished
with pipes leading to the sheet-iron salt-pans. The capacity of these is very different
at different works. At Schönebeck there are 22, the smallest having a square surface
of 400 feet, the largest of 1250, and are enclosed within walls, to prevent their being
affected by the cold external air. They are covered with a funnel-formed or pyramidal
trunk of deals, ending in a square chimney, to carry off the steam.
Figs. 965, 966, 967. represent the construction of a salt-pan, its furnace, and the
salt store-room of the works at Dürrenberg; fig. 967. being the ground plan, fig. 966.[1089]
the longitudinal section, and fig. 965. the transverse section, a is the fire-grate, which
slopes upwards to the back part, and is 311⁄2 inches distant from the bottom of the pan.
The ratio of the surface of the grate to that of the bottom of the pan, is as 1 to 59·5;
that of the air-hole into the ash-pit, as 1 to 306. The bed under the pan is laid with
bricks, smoothly plastered over, from b to c, in fig. 966. Upon this bed the pillars d, d,
&c., are built in a radiated direction, being 6 inches broad at the bottom, and tapering to
11⁄2 inch at top. The pan is so laid that its bottom has a fall towards the middle of
21⁄2 inches: see e, f, fig. 966. The fire
diffuses itself in all directions under the
pan, proceeds thence through several
holes g, g, g, into flues h, h, h, which run
round three sides of the pan; the burnt
air then passes through i, fig. 967., under
other pans, from which it is collected
in the chimneys k, k, to be conducted into
the drying-room. At l, l, there is a
transverse flue, through which, by means
of dampers, the fire-draught may be conducted into an extra chimney m. From the flues
k, k, four square iron pipes n, n, issue and conduct the burnt air into the main chimneys
in the opposite wall.
The bottoms of the several flues have a gradual ascent above the level of the fire-grate.
A special chimney o, rises above the ash-pit, to carry off the smoke, which may chance to
regurgitate in certain states of the wind. p, p, are iron pipes laid upon each side of
the ash-pit (see figs. 966. and 967.), into which cold air is admitted by the flue q, r,
where, becoming heated, it is conducted through iron pipes s, and thence escapes at t,
into the stove-room. Upon both sides of the hot flues in the stove-room, hurdle-frames
u, u, are laid, each of which contains 11 baskets, and every basket, except the undermost,
holds 60 pounds of salt, spread in a layer 2 inches thick. v, v, show the pipes
by which the pan is supplied with graduated brine.
Description of the Steam-trunk, in fig. 968.
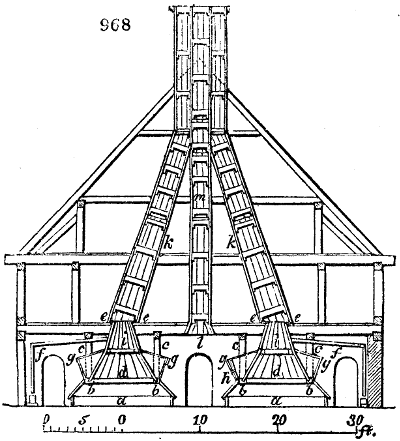
In front of the pan a, a, there are two upright posts, upon which, and in holes of
the back wall, two horizontal beams b, b, are supported. The pillars c, c, are sustained
upon the bearers d, d. At e, e, a deep quadrangular groove is made in the beams,
for fixing down the four boards which form the bottom of the steam-way. In this
groove any condensed water from the steam collects, and is carried off by a pipe f, to
prevent it falling back into the pan. Upon the three sides of the pan not in contact
with the wall, there are three rows of boards hinged upon planks b, b. Behind the
upper one, a board is hung on at g, upon which the boiled salt is laid to drain. The[1090]
two other rows of boards are hooked on so as to cover the pan, as shown at h.
Whenever the salt is sufficiently drained, the upper shelves are placed in a horizontal
position; the salt is put into small baskets,
and carried into the stove-room. i, k, is
the steam-trunk; l, m, is a tunnel for carrying
off the steam from the middle of the
pan, when this is uncovered by lifting the
boards.
In proportion as the brine becomes concentrated
by evaporation, more is added
from the settling reservoir of the graduation-house,
till finally small crystals appear
on the surface. No more weak brine
is now added, but the charge is worked
off, care being taken to remove the scum,
as it appears. In some places the first
pan is called a schlot-pan, in which the
concentration is carried only so far as to
cause the deposition of the sludge, from
which the saline solution is run into another
pan, and gently evaporated, to produce
the precipitation of the fine salt.
This salt should be continually raked towards
the cooler and more elevated sides
of the pan, and then lifted out with cullender-shovels into large conical baskets, arranged
in wooden frames round the border of the pan, so that the drainage may flow back into
the boiling liquor. The drained salt is transferred to the hurdles or baskets in the stove-room,
which ought to be kept at a temperature of from 120° to 130°, Fahr. The salt
is then stowed away in the warehouse.
The graduation range should be divided lengthwise into several sections: the first
to receive the water of the spring, the lake, or the sea; the second, the water from the
first shower-receiver; the third, the water from the second receiver; and so on. The
pumps are usually placed in the middle of the building, and lift the brine from the
several receivers below into the alternate elevated cisterns. The square wooden spouts
of distribution may be conveniently furnished with a slide-board, attached to each of
their sides, to serve as a general valve for opening or shutting many trickling orifices
at once. The rate of evaporation at Moutiers is exhibited by the following table:—
Number of
Showers. |
Total Surface
of the Fagots. |
Specific Gravity
of the Brine. |
Water
evaporated. |
| |
|
1·010 |
0·000 |
| 1 and 2 |
5158 |
square feet |
1·023 |
0·540 |
| 3, 4, 5, 6, 7, 8, and 9 |
2720 |
|
1·072 |
0·333 |
| 10 |
550 |
|
1·140 |
0·062 |
| Total evaporation |
0·935 |
| Water remaining in the brine at the density of 1·140 |
1·065 |
| Water assigned at the density of 1·010 |
1·000 |
From the above table it appears that no less than 10 falls of the brine have been
required to bring the water from the specific gravity 1·010 to 1·140, or 18° Baumé.
The evaporation is found to proceed at nearly the same rate with the weaker water, and
with the stronger, within the above limits. When it arrives at a density of from 1·140
to 1·16, it is run off into the settling cisterns. M. Berthier calculates, that upon an
average, in ordinary weather, at Moutiers, 60 kilogrammes of water (13 gallons, imp.)
are evaporated from the fagots, in the course of 24 hours, for every square foot of their
surface. Without the aid of currents of air artificially warmed, such an amount of
evaporation could not be reckoned upon in this country. In the schlotting, or throwing
down of the sediment, a little bullock’s blood, previously beaten up with some cold brine,
promotes the clarification. When the brine acquires, by brisk ebullition, the density of
1·200, it should be run off from the preparation, to the finishing or salting pans.
The mother-water contains a great deal of chloride of magnesium, along with chloride
of sodium, and sulphate of magnesia. Since the last two salts mutually decompose
each other at a low temperature, and are transformed into sulphate of soda, which
crystallizes, and muriate of magnesia, which remains dissolved, the mother-water with[1091]
this view may be exposed in tanks to the frost during winter, when it affords three successive
crystalline deposits, the last being sulphate of soda, nearly pure.
The chloride of magnesium, or bittern, not only deteriorates the salt very much, but
occasions a considerable loss of weight. It may, however, be most advantageously got
rid of, and converted into chloride of sodium by the following simple expedient:—Let
quicklime be introduced in equivalent quantity to the magnesia present, and it will
precipitate this earth, and form chloride of calcium, which will immediately react upon
the sulphate of soda in the mother-water, with the production of sulphate of lime and
chloride of sodium. The former being sparingly soluble, is easily separated. Lime, moreover,
decomposes directly the chloride of magnesium, but with the effect of merely substituting
chloride of calcium in its stead. But in general there is abundance of sulphate
of soda in brine springs to decompose the chloride of calcium. A still better way of
proceeding with sea-water, would be to add to it, in the settling tank, the quantity of
lime equivalent to the magnesia, whereby an available deposit of this earth would be
obtained, at the same time that the brine would be sweetened. Water thus purified
may be safely crystallized by rapid evaporation.
In summer, the saturated boiling brine is crystallized by passing it over vertical
ropes; for which purpose 100,000 metres (110,000 yards) are mounted in an apartment
70 metres (77 yards) long. When the salt has formed a crust upon the ropes about
21⁄2 inches thick, it is broken off, allowed to fall upon the clean floor of the apartment,
and then gathered up. The salting of a charge, which would take 5 or 6 days in the
pan, is completed in this way in 17 hours; but the mother-waters are more abundant.
The salt is, however, remarkably pure.
The boilers constructed at Rosenheim, in Bavaria, evaporate 31⁄2 pounds of water for
every pound of wood burned; which is reckoned a favourable result; but some of those
described under Evaporation, would throw off much more.
“The rock salt mines and principal brine springs are in Cheshire; and the chief part
of the Cheshire salt, both fossil and manufactured, is sent by the river Weaver to Liverpool,
a very small proportion of it being conveyed elsewhere, by canal or land carriage.
There are brine springs in Staffordshire, from which Hull is furnished with white salt;
and in Worcestershire, from which Gloucester is supplied. If to the quantity shipped
by the Weaver, 100,000 tons of white salt are added annually for internal consumption
and exports, exclusive of Liverpool, the total manufacture will be approached very
nearly; but as there is now no check from the excise, it is impossible to ascertain it
exactly. Fossil salt is used in small quantities at some of the Cheshire manufactories,
to strengthen the brine, but is principally exported; some to Ireland, but chiefly to
Belgium and Holland.”[52] The average quantity of rock salt sent annually down the river
Weaver, from the mines in Cheshire, between the years 1803 and 1834 inclusive, was
86,000 tons, of 2,600 lbs. each; the greatest being 125,658, in the year 1823, and the
least 47,230, in the year 1813. The average quantity of white salt sent annually down
the Weaver from the manufactories in Cheshire during the same period, was 221,351; the
greatest being 383,669, in the year 1832, and the least being 120,486, in the year 1811.
M. Clement-Desormes, engineer and chief actionnaire of the great salt-works of Dieuze,
in France, informs me that the internal consumption of that kingdom is rather more than
200,000 tons per annum, being at the rate of 61⁄2 kilogrammes for each individual of a
population estimated at 32,000,000. As the retail price of salt in France is 10 sous
per kilogramme (of 21⁄5 lbs. avoird.), while in this country it is not more than 2 sous
(1 penny), its consumption per head will be much greater with us; and, taking into
account the immense quantity of salted provisions that are used, it may be reckoned at
22 lbs.; whence our internal consumption will be 240,000 tons, instead of 100,000, as
quoted above, from the tables published by the Board of Trade.
In 1836, 9,622,427 bushels, of 56 lbs. = 240,560 tons of salt, value 173,923l., were
exported from the United Kingdom, of which 1,350,849 bushels went to Russia;
1,235,086 to Belgium; 314,132 to the Western coast of Africa; 1,293,560 to the
British North American colonies; 2,870,808 to the United States of America; 53,299
to New South Wales, Van Diemen’s Land, and other Australian settlements; 58,735
to the British West Indies; and 90,655 to Guernsey, Jersey, Alderney, and Man.
SAND (Eng. and Germ.; Sable, Fr.); is the name given to any mineral substance in
a hard granular or pulverulent form, whether strewed upon the surface of the ground,
found in strata at a certain depth, forming the beds of rivers, or the shores of the sea.
The siliceous sands seem to be either original crystalline formations, like the sand of
Neuilly, in 6-sided prisms, terminated by two 6-sided pyramids, or the débris of granitic,
schistose, quartzose, or other primitive crystalline rocks, and are abundantly distributed
over the globe; as in the immense plains known under the names of downs, deserts,
steppes, landes, &c., which, in Africa, Asia, Europe, and America, are entirely covered with[1092]
loose sterile sand. Valuable metallic ores, those of gold, platinum, tin, copper, iron,
titanium, often occur in the form of sand, or mixed with that earthy substance. Pure
siliceous sands are very valuable for the manufacture of glass, for making mortars,
filters, ameliorating dense clay soils, and many other purposes. For moulder’s sand,
see Founding. Lynn and Ryegate furnish our purest siliceous sand.
SANDAL or RED SAUNDERS WOOD (Santal Fr.; Sandelholz Germ.); is the
wood of the Pterocarpus santalinus, a tree which grows in Ceylon, and on the coast of
Coromandel. The old wood is preferred by dyers. Its colouring matter is of a resinous
nature; and is, therefore, quite soluble in alcohol, essential oils, and alkaline lyes; but
sparingly in boiling water, and hardly if at all in cold water. The colouring matter
which is obtained by evaporating the alcoholic infusion to dryness, has been called
santaline; it is a red resin, which is fusible at 212° F. It may also be obtained by
digesting the rasped sandal wood in water of ammonia, and afterwards saturating the
ammonia with an acid. The santaline falls, and the supernatant liquor, which is yellow
by transmitted, appears blue by reflected light. Its spirituous solution affords a fine
purple precipitate with the protochloride of tin, and a violet one with the salts of lead.
Santaline is very soluble in acetic acid, and the solution forms permanent stains upon
the skin.
Sandal wood is used in India, along with one-tenth of sapan wood (the Cæsalpinia
sapan of Japan, Java, Siam, Celebes, and the Philippine isles), principally for dyeing
silk and cotton. Trommsdorf dyed wool, cotton, and linen a carmine hue by dipping
them alternately in alkaline solution of the sandal wood, and in an acidulous bath.
Bancroft obtained a fast and brilliant reddish-yellow, by preparing wool with an alum and
tartar bath, and then passing it through a boiling bath of sandal wood and sumac.
Pelletier did not succeed in repeating this experiment. According to Togler, wool,
silk, cotton, and linen, mordanted with salt of tin, and dipped in a cold alcoholic tincture
of the wood, or the same tincture mixed with 8 parts of boiling water, become of
a superb ponceau-red colour. With alum, they took a scarlet-red; with sulphate of iron,
a deep violet, or brown-red. Unluckily these dyes do not stand exposure to light well.
SANDARACH, is a peculiar resinous substance, the product of the Thuya articulata,
a small tree of the coniferous family, which grows in the northern parts of Africa, especially
round Mount Atlas.
The resin comes to us in pale yellow, transparent, brittle, small tears, of a spherical
or cylindrical shape. It has a faint aromatic smell, does not soften, but breaks between
the teeth, fuses readily with heat, and has a specific gravity of from 1·05 to 1·09. It
contains three different resins; one soluble in spirit of wine, somewhat resembling pinic
acid (see Turpentine); one not soluble in that menstruum; and a third, soluble only in
alcohol of 90 per cent. It is used as pounce-powder for strewing over paper erasures, as
incense, and in varnishes.
SAPAN WOOD, is a species of the Cæsalpinia genus, to which Brasil wood belongs.
It is so called by the French, because it comes to them from Japan, which
they corruptly pronounce Sapan. As all the species of this tree are natives of either
the East Indies or the New World, one would imagine that they could not have been
used as dye-stuffs in Europe before the beginning of the 16th century. Yet the author
of the article “Brasil,” in Rees’ Cyclopædia, and Mr. Southey, in his History of Brasil,
say that Brasil wood is mentioned nearly one hundred years before the discoveries of
Columbus and Vasco de Gama, by Chaucer, who died in 1400; that it was known many
ages before his time; and that it gave the name to the country, instead of the country
giving the name to the wood, as I have stated, with Berthollet and other writers on
dyeing. The Cæsalpinia sappan, being a native of the Coromandel coast, may possibly
have been transported along with other Malabar merchandise to the Mediterranean marts
in the middle ages; but the importation of so lumbering an article in any considerable
quantity by that channel, is so improbable, that I am disposed to believe that Brasil wood
was not commonly used by the dyers of Europe before the discovery of the New World.
SATIN (Eng., Fr., and Germ.); is the name of a silk stuff, first imported from China,
which is distinguished by its very smooth, polished, and glossy surface. It is woven
upon a loom with at least five-leaved healds or heddles, and as many corresponding
treddles. These are so mounted as to rise and fall four at a time, raising and depressing
alternately four yarns of the warp, across the whole of which the weft is thrown by the
shuttle, so as to produce a uniform smooth texture, instead of the chequered work resulting
from intermediate decussations, as in common webs. See Textile Fabrics.
Satins are woven with the glossy or right side undermost, because the four-fifths of the
warp, which are always left there during the action of the healds, serve to support the
shuttle in its race. Were they woven in the reverse way, the scanty fifth part of the
warp threads could either not support, or would be too much worn by the shuttle.
[1093]
SATURATION, is the term at which any body has taken its full dose or chemical
proportion of any other with which it can combine; as water with a salt, or an acid with
an alkali in the neutro-saline state.
SCALIOLA, is merely ornamental plaster-work, produced by applying a pap made
of finely-ground calcined gypsum, mixed with a weak solution of Flanders’ glue, upon
any figure formed of laths nailed together, or occasionally upon brickwork, and bestudding
its surface, while soft, with splinters (scagliole) of spar, marble, granite, bits of concrete,
coloured gypsum, or veins of clay, in a semi-fluid state. The substances employed to
colour the spots and patches, are the several ochres, boles, terra di Sienna, chrome yellow,
&c. The surface of the column is turned smooth upon a lathe, polished with stones of
different fineness, and finished with some plaster-pap, to give it lustre. Pillars and other
flat surfaces are smoothed by a carpenter’s plane, with the chisel finely serrated, and
afterwards polished with plaster by friction. The glue is the cause of the gloss, but
makes the surface apt to be injured by moisture, or even damp air.
SCARLET DYE. (Teinture en écarlate, Fr.; Scharlachfärberei, Germ.) Scarlet
is usually given at two successive operations. The boiler (see figs. 364, 365., article
Dyeing,) is made of block tin, but its bottom is formed occasionally of copper.
1. The bouillon, or the colouring-bath.—For 100 pounds of cloth, put into the water,
when it is little more than lukewarm, 6 pounds of argal, and stir it well. When the
water becomes too hot for the hand, throw into it, with agitation, one pound of cochineal
in fine powder. An instant afterwards, pour in 5 pounds of the clear mordant G,
(see Tin Mordants,) stir the whole thoroughly as soon as the bath begins to boil,
introduce the cloth, and wince it briskly for two or three rotations, and then more slowly.
At the end of a two-hours’ boil, the cloth is to be taken out, allowed to become perfectly
cool, and well washed at the river, or winced in a current of pure water. (See an automatic
plan of washing described under the article Rinsing Machine.)
2. The rougie, or finishing dye.—The bouillon bath is emptied, and replaced with water
for the rougie. When it is on the point of boiling, 51⁄2 pounds of cochineal in fine powder
are to be thrown in, and mixed with care; when the crust, which forms upon the surface,
opens of itself in several places, 14 pounds of solution of tin (as above) are to be
added. Should the liquor be likely to boil over the edges of the kettle, it must be
refreshed with a little cold water. When the bath has become uniform, the cloth is to
be put in, taking care to wince it briskly for two or three turns; then to boil it bodily
for an hour, thrusting it under the liquor with a rod whenever it rises to the surface.
It is lastly taken out, aired, washed at the river, and dried.
As no person has done more for the improvement of the scarlet dyes than Poërner, I
shall here give his processes in detail.
Bouillon, or colouring.—For every pound of cloth or wool, take 14 drams of cream of
tartar. When the bath is boiling, and the tartar all dissolved, pour in successively 14 drams
of solution of tin, (Mordant F, Tin,) and let the whole boil together during a few minutes.
Now introduce the cloth, and boil it for 2 hours; then take it out, and let it drain and cool.
Rougie, or dye.—For every pound of woollen stuff, take 2 drams of cream of tartar.
When the bath begins to boil, add 1 ounce of cochineal reduced to fine powder, stir the
mixture well with a rod of willow or any white wood, and let it boil for a few minutes.
Then pour in, by successive portions, 1 ounce of solution of tin (Mordant F), stirring
continually with the rod. Lastly, dye as quickly as possible. The colour will be
a beautiful scarlet.
Second scarlet process of Poërner, the bouillon being the same as above given, and
always estimated for 1 pound of cloth or wool. Rougie.—Take one ounce of cochineal
in fine powder, and two ounces of solution of tin without tartar.
Third scarlet process of Poërner; the bouillon being as above. Rougie for a pound of
cloth.—Take two drams of cream of tartar, one ounce of cochineal, one ounce of
solution of tin, and two ounces of sea salt: dye as in process 1. The salt helps the
dye to penetrate into the cloth.
Tables of the Composition of the Bouillon and Rougie, by different Authors,
for 100 pounds of Cloth or Wool.
Composition of the Bouillon.
Names of the
Authors. |
Starch. |
Cream of
Tartar. |
Cochi-
neal. |
Solution
of Tin. |
Common
Salt. |
| |
lb. |
oz. |
lb. |
oz. |
lb. |
dr. |
lb. |
oz. |
lb. |
oz. |
| Berthollet |
0 |
0 |
6 |
0 |
8 |
0 |
5 |
0 |
0 |
0 |
| Hellot |
0 |
0 |
12 |
8 |
18 |
6 |
12 |
8 |
0 |
0 |
| Scheffer |
9 |
6 |
9 |
6 |
12 |
4 |
9 |
6 |
0 |
0 |
| Poërner |
0 |
0 |
10 |
15 |
0 |
0 |
10 |
15 |
0 |
0 |
[1094]
Composition of the Rougie.
Names of the
Authors. |
Starch. |
Cream of
Tartar. |
Cochi-
neal. |
Solution
of Tin. |
Common
Salt. |
| |
lb. |
oz. |
lb. |
oz. |
lb. |
oz. |
lb. |
oz. |
lb. |
oz. |
| Berthollet |
0 |
0 |
0 |
0 |
5 |
8 |
|
14 |
0 |
0 |
0 |
| Hellot |
3 |
2 |
0 |
0 |
7 |
4 |
|
12 |
8 |
0 |
0 |
| Scheffer |
3 |
2 |
3 |
2 |
5 |
7 |
1⁄2 |
4 |
11 |
0 |
0 |
| Poërner |
- |
|
|
0 |
0 |
1 |
8 |
6 |
4 |
|
6 |
4 |
0 |
0 |
| 0 |
0 |
0 |
0 |
6 |
4 |
|
12 |
8 |
0 |
0 |
| 0 |
0 |
1 |
8 |
6 |
4 |
|
6 |
4 |
12 |
8 |
| |
|
|
|
|
|
M. Lenormand states that he has made experiments of verification upon all the
formulæ of the preceding tables, and declares his conviction that the finest tint may be
obtained by taking the bouillon of Scheffer, and the rougie No. 4. of Poërner. The solution
which produced the most brilliant red, is that made according to the process of
mordant B (Tin). M. Robiquet has given the following prescription for making a
printing scarlet, for well-whitened woollen cloth.
Boil a pound of pulverized cochineal in four pints of water down to two pints, and
pass the decoction through a sieve. Repeat the boiling three times upon the residuum,
mix the eight pints of decoction, thicken them properly with two pounds of starch, and
boil into a paste. Let it cool down to 104° F., then add four ounces of the subjoined
solution of tin, and two ounces of ordinary salt of tin (muriate). When a ponçeau red
is wanted, two ounces of pounded curcuma (turmeric) should be added.
The solution of tin above prescribed, is made by taking—one ounce of nitric acid,
of specific gravity 36° B. = 1·33; one ounce of sal ammoniac; four ounces of grain
tin. The tin is to be divided into eight portions, and one of them is to be put into the
acid mixture every quarter of an hour.
A solution of chlorate of potassa (chloride?) is said to beautify scarlet cloth in a
remarkable manner.
Bancroft proposed to supplant the nitro-muriatic acid, by a mixture of sulphuric and
muriatic acids, for dissolving tin; but I do not find that he succeeded in persuading scarlet-dyers
to adopt his plans. In fact the proper base is, in my opinion, a mixture of the
protoxide and peroxide of tin; and this cannot be obtained by acting upon the metal
with the murio-sulphuric acid. He also prescribed the extensive use of the quercitron
yellow to change the natural crimson of the cochineal into scarlet, thereby economizing
the quantity of this expensive dye-stuff. See Lac Dye.
SCHEELE’S GREEN, is a pulverulent arsenite of copper, which may be prepared
as follows:—Form, first, an arsenite of potassa, by adding gradually 11 ounces of
arsenious acid to 2 pounds of carbonate of potassa, dissolved in 10 pounds of boiling
water; next, dissolve 2 pounds of crystallized sulphate of copper in 30 pounds of
water; filter each solution, then pour the first progressively into the second, as long as
it produces a rich grass-green precipitate. This being thrown upon a filter-cloth, and
edulcorated with warm water, will afford 1 pound 6 ounces of this beautiful pigment.
It consists of, oxide of copper 28·51, and of arsenious acid 71·46. This green is
applied by an analogous double decomposition to cloth. See Calico-printing.
SCHWEINFURTH GREEN, is a more beautiful and velvety pigment than the
preceding, which was discovered in 1814, by MM. Rusz and Sattler, at Schweinfurth,
and remained for many years a profitable secret in their hands. M. Liebig having made
its composition known, in 1822, it has been since prepared in a great many colour-works.
Braconnot published, about the same time, another process for manufacturing
the same pigment. Its preparation is very simple; but its formation is accompanied
with some interesting circumstances. On mixing equal parts of acetate of copper and
arsenious acid, each in a boiling concentrated solution, a bulky olive-green precipitate
is immediately produced; while much acetic acid is set free. The powder thus
obtained, appears to be a compound of arsenious acid and oxide of copper, in a peculiar
state; since when decomposed by sulphuric acid, no acetic odour is exhaled. Its
colour is not changed by drying, by exposure to air, or by being heated in water.
But, if it be boiled in the acidulous liquor from which it was precipitated, it soon
changes its colour, as well as its state of aggregation, and forms a new deposit in the
form of a dense granular beautiful green powder. As fine a colour is produced by
ebullition during five or six minutes, as is obtained at the end of several hours by
mixing the two boiling solutions, and allowing the whole to cool together. In the latter
case, the precipitate, which is slight and flocky at first, becomes denser by degrees; it
next betrays green spots, which progressively increase, till the mass grows altogether of
a crystalline constitution, and of a still more beautiful tint than if formed by ebullition.
When cold water is added to the mixed solutions, immediately after the precipitate[1095]
takes place, the development of the colour is retarded, with the effect of making it
much finer. The best mode of procedure, is to add to the blended solutions, their own
bulk of cold water, and to fill a globe up to the neck with the mixture in order to prevent
the formation of any such pellicle on the surface, as might, by falling to the bottom,
excite premature crystallization. Thus the reaction continues during two or three
days with the happiest effect. The difference of tint produced by these variations,
arises merely from the different sizes of the crystalline particles; for when the several
powders are levigated upon a porphyry slab to the same degree, they have the same
shade. Schweinfurth green, according to M. Ehrmann’s researches, in the 31st Bulletin
de la Société Industrielle de Mulhausen, consists of, oxide of copper 31·666, arsenious acid
58·699, acetic acid 10·294. Kastner has given the following prescription for making
this pigment:—For 8 parts of arsenious acid, take from 9 to 10 of verdigris; diffuse the
latter through water at 120° F., and pass the pap through a sieve; then mix it with
the arsenical solution, and set the mixture aside, till the reaction of the ingredients shall
produce the wished-for shade of colour. If a yellowish tint be desired, more arsenic must
be used. By digesting Scheele’s green in acetic acid, a variety of Schweinfurth green
may be obtained.
Both of the above colours are rank poisons. The first was detected a few years ago,
as the colouring-matter of some Parisian bonbons, by the conseil de salubrité; since which
the confectioners were prohibited from using it, by the French government.
SCOURING, or renovating articles of dress. This art has been much more studied
by Frenchmen, who wear the same coats for two or three years, than by Englishmen,
who generally cast them off after so many months. The workmen who remove greasy
stains from dress, are called, in France, teinturiers-degraisseurs, because they are often
obliged to combine dyeing with scouring operations. The art of cleansing clothes being
founded upon the knowledge of solvents, the practitioner of it should, as we shall
presently illustrate by examples, be acquainted with the laws of chemical affinity.
Among the spots which alter the colours fixed upon stuffs, some are caused by a
substance which may be described as simple, in common language; and others by a substance
which results from the combination of two or more bodies, that may act
separately or together upon the stuff, and which may therefore be called compound.
Simple stains.—Oils and fats are the substances which form the greater part of
simple stains. They give a deep shade to the ground of the cloth; they continue to
spread for several days; they attract the dust, and retain it so strongly, that it is not
removable by the brush; and they eventually render the stain lighter coloured upon a
dark ground, and of a disagreeable gray tint upon a pale or light ground.
The general principle of cleansing all spots, consists in applying to them a substance
which shall have a stronger affinity for the matter composing them, than this has for the
cloth, and which shall render them soluble in some liquid menstruum, such as water,
spirits, naphtha, oil of turpentine, &c. See Bleaching.
Alkalis would seem to be proper in this point of view, as they are the most powerful
solvents of grease; but they act too strongly upon silk and wool, as well as change too
powerfully the colours of dyed stuffs, to be safely applicable in removing stains. The
best substances for this purpose are—1. Soap. 2. Chalk, fuller’s earth, soap-stone or
steatite (called in this country French chalk). These should be merely diffused
through a little water into a thin paste, spread upon the stain, and allowed to dry. The
spot requires now to be merely brushed. 3. Ox-gall and yolk of egg have the property
of dissolving fatty bodies without affecting perceptibly the texture or colours of cloth,
and may therefore be employed with advantage. The ox-gall should be purified, to
prevent its greenish tint from degrading the brilliancy of dyed stuffs, or the purity of
whites. Thus prepared (see Gall), it is the most precious of all substances known for
removing these kinds of stains. 4. The volatile oil of turpentine will take out only recent
stains; for which purpose it ought to be previously purified by distillation over quicklime.
Wax, rosin, turpentine, pitch, and all resinous bodies in general, form stains of greater
or less adhesion, which may be dissolved out by pure alcohol. The juices of fruits, and
the coloured juices of all vegetables in general, deposit upon clothes marks in their
peculiar hues. Stains of wine, mulberries, black currants, morellos, liquors, and weld,
yield only to soaping with the hand, followed by fumigation with sulphurous acid;
but the latter process is inadmissible with certain coloured stuffs. Iron mould or rust
stains may be taken out almost instantaneously with a strong solution of oxalic acid.
If the stain is recent, cream of tartar will remove it.
Compound spots.—That mixture of rust of iron and grease called cambouis by the
French, is an example of this kind, and requires two distinct operations; first, the
removal of the grease, and then of the rust, by the means above indicated.
Mud, especially that of cities, is a compound of vegetable remains, and of ferruginous
matter in a state of black oxide. Washing with pure water, followed if necessary with
soaping, will take away the vegetable juices; and then the iron may be removed with[1096]
cream of tartar, which itself must, however, be well washed out. Ink stains, when
recent, may be taken out by washing, first with pure water, next with soapy water, and
lastly with lemon juice; but if old, they must be treated with oxalic acid. Stains occasioned
by smoke, or by sauces browned in a frying-pan, may be supposed to consist of a
mixture of pitch, black oxide of iron, empyreumatic oil, and some saline matters
dissolved in pyrolignous acid. In this case several reagents must be employed to
remove the stains. Water and soap dissolve perfectly well the vegetable matters, the
salts, the pyrolignous acid, and even the empyreumatic oils in a great measure; the
essence of turpentine will remove the rest of the oils and all the pitchy matter; then
oxalic acid may be used to discharge the iron. Coffee stains require a washing with
water, with a careful soaping, at the temperature of 120° F., followed by sulphuration.
The two latter processes may be repeated twice or thrice. Chocolate stains may be
removed by the same means, and more easily.
As to those stains which change the colour of the stuff, they must be corrected by appropriate
chemical reagents or dyes. When black or brown cloth is reddened by an
acid, the stain is best counteracted by the application of water of ammonia. If delicate
silk colours are injured by soapy or alkaline matters, the stains must be treated with
colourless vinegar of moderate force. An earthy compound for removing grease spots
is made as follows:—Take fuller’s earth, free it from all gritty matter by elutriation
with water; mix with half a pound of the earth so prepared, half a pound of soda, as
much soap, and eight yolks of eggs well beat up with half a pound of purified ox-gall.
The whole must be carefully triturated upon a porphyry slab; the soda with the soap
in the same manner as colours are ground, mixing in gradually the eggs and the ox-gall
previously beat together. Incorporate next the soft earth by slow degrees, till a uniform
thick paste be formed, which should be made into balls or cakes of a convenient size, and
laid out to dry. A little of this detergent being scraped off with a knife, made into a
paste with water, and applied to the stain, will remove it. Purified ox-gall is to be diffused
through its own bulk of water, applied to the spots, rubbed well into them with
the hands till they disappear, after which the stuff is to be washed with soft water. It
is the best substance for removing stains on woollen clothes.
The redistilled oil of turpentine may also be rubbed upon the dry clothes with a sponge
or a tuft of cotton, till the spot disappear; but it must be immediately afterwards covered
with some plastic clay reduced to powder. Without this precaution, a cloud would be
formed round the stain, as large as the part moistened with the turpentine.
Oxalic acid may be applied in powder upon the spot previously moistened with water,
well rubbed on, and then washed off with pure water.
Sulphurous acid is best generated at the moment of using it. If the clothes be
much stained, they should be suspended in an ordinary fumigating chamber. For
trifling stains, the sulphur may be burned under the wide end of a small card or paper
funnel, whose upper orifice is applied near the cloth.
Manipulations of the scourer.—These consist, first, in washing the clothes in clear soft
water, or in soap-water. The cloth must be next stretched on a sloping board, and
rubbed with the appropriate reagent as above described, either by a sponge or a small
hard brush. The application of a redhot iron
a little way above a moistened spot often
volatilizes the greasy matter out of it. Stains
of pitch, varnish, or oil paint, which have become
dry, must first be softened with a little
fresh butter or lard, and then treated with the
powder of the scouring ball. When the gloss
has been taken from silk, it may be restored
by applying the filtered mucilage of gum
tragacanth; stretching it upon a frame to
dry. Ribbons are glossed with isinglass.
Lemon juice is used to brighten scarlet spots,
after they have been cleaned.
SEAL ENGRAVING. The art of engraving
gems is one of extreme nicety. The
stone having received its desired form from
the lapidary, the engraver fixes it by cement
to the end of a wooden handle, and then
draws the outline of his subject, with a brass
needle or a diamond, upon its smooth surface.
Fig. 969. represents the whole of the seal
engraver’s lathe. It consists of a table on
which is fixed the mill, a small horizontal[1097]
cylinder of steel, into one of whose extremities the tool is inserted, and which is made
to revolve by the usual fly-wheel, driven by a treddle. The tools that may be fitted
to the mill-cylinder, are the following: fig. 970. a hollow cylinder, for describing circles,
and for boring; fig. 971. a knobbed tool, or rod terminated by a small ball; fig. 972.
a stem terminated with a cutting disc, whose edge may be either rounded, square, or
sharp; being in the last case called a saw.
Having fixed the tool best adapted to his style of work in the mill, the artist applies
to its cutting point, or edge, some diamond-powder, mixed up with olive oil; and turning
the wheel, he holds the stone against the tool, so as to produce the wished-for
delineation and erosion. A similar apparatus is used for engraving on glass.
In order to give the highest degree of polish to the engraving, tools of boxwood,
pewter, or copper, bedaubed with moistened tripoli or rotten-stone, and lastly, a brush,
are fastened to the mill. These are worked like the above steel instruments. Modern
engravings on precious stones, have not in general the same fine polish as the antient.
The article Gems, in Rees’ Cyclopædia, contains a variety of valuable information on
this subject, equally interesting to the artist and the scholar.
SEALING-WAX. (Cire à cacheter, Fr.; Siegellack, Germ.) The Hindus from
time immemorial have possessed the resin lac, and were long accustomed to use it for
sealing manuscripts before it was known in Europe. It was first imported from the
East into Venice, and then into Spain; in which country sealing-wax became the
object of a considerable commerce, under the name of Spanish wax.
If shellac be compounded into sealing-wax, immediately after it has been separated
by fusion from the palest qualities of stick or seed lac, it then forms a better and less
brittle article, than when the shellac is fused a second time. Hence sealing-wax,
rightly prepared in the East Indies, deserves a preference over what can be made in
other countries, where the lac is not indigenous. Shellac can be restored in some degree,
however, to a plastic and tenacious state by melting it with a very small portion of turpentine.
The palest shellac is to be selected for bright-coloured sealing-wax, the dark
kind being reserved for black.
The following prescription may be followed for making red sealing-wax:—Take 4
ounces of shellac, 1 ounce of Venice turpentine (some say 11⁄2 ounces), and 3 ounces of
vermillion. Melt the lac in a copper pan suspended over a clear charcoal fire, then
pour the turpentine slowly into it, and soon afterwards add the vermillion, stirring
briskly all the time of the mixture with a rod in either hand. In forming the round
sticks of sealing-wax, a certain portion of the mass should be weighed while it is ductile,
divided into the desired number of pieces, and then rolled out upon a warm marble
slab, by means of a smooth wooden block, like that used by apothecaries for rolling a
mass of pills. The oval sticks of sealing-wax are cast in moulds, with the above compound
in a state of fusion. The marks of the lines of junction of the mould-box may
be afterwards removed by holding the sticks over a clear fire, or passing them over a
blue gas-flame. Marbled sealing-wax is made by mixing two, three, or more coloured
kinds of it, while they are in a semi-fluid state. From the viscidity of the several
masses, their incorporation is left incomplete, so as to produce the appearance of marbling.
Gold sealing-wax is made simply by stirring gold-coloured mica spangles into the
melted resins. Wax may be scented by introducing a little essential oil, essence of
musk, or other perfume. If 1 part of balsam of Peru be melted along with 99 parts of
the sealing-wax composition, an agreeable fragrance will be exhaled in the act of sealing
with it. Either lamp black or ivory black serves for the colouring-matter of black
wax. Sealing-wax is often adulterated with rosin; in which case it runs into thin
drops at the flame of a candle.
SEA WATER, is composed as follows, according to the author of the article
Salines, in the Dictionnaire Technologique:—Chloride of sodium, 2·50; chloride of
magnesium, 0·35; sulphate of magnesia, 0·58; carbonates of lime and magnesia,
0·02; sulphate of lime, 0·01; water, 96·54, in 100 parts. See Salt, Sea.
SEGGAR, or SAGGER, is the cylindric case, of fire-clay, in which fine stoneware
is enclosed while being baked in the kiln.
SELENIUM, from Σελἡνη, the moon, is a metalloid principle, discovered by Berzelius,
in 1817. It occurs sparingly in combination with several metals, as lead, cobalt,
copper, and quicksilver, in the Harz, at Tilkerode; with copper and silver (Eukairite)
in Sweden, with tellurium and bismuth in Norway, with tellurium and gold in
Siebenbürgen, in several copper and iron pyrites, and with sulphur in the volcanic products
of the Lipari Islands. Selenium has been found likewise in a red sediment which
forms upon the bottoms of the lead chambers in which oil of vitriol has been made from
peculiar pyrites, or pyritous sulphur. The extraction of selenium from that deposit, is
a very complex process.
Selenium, after being fused and slowly cooled, appears of a bluish-gray colour, with a
glistening surface; but it is reddish brown, and of metallic lustre when quickly cooled,[1098]
It is brittle, not very hard, and has little tendency to assume the crystalline state.
Selenium is dark-red in powder, and transparent, with a ruby cast, in thin scales. Its
specific gravity is 4·30. It softens at the temperature of 176° F., is of a pasty consistence
at 212°, becomes liquid at a somewhat higher heat, forming in close vessels dark-yellow
vapours, which condense into black drops; but in the air, the fumes have a cinnabar-red
colour.
This singular substance, apparently intermediate in its constitution between sulphur
and metals, has not hitherto been applied to any use in the arts.
SELTZER WATER. See Soda-water, and Waters, Mineral.
SEPIA, is a pigment prepared from a black juice secreted by certain glands of the
cuttle-fish, which the animal ejects to darken the water when it is pursued. One part
of it is capable of making 1000 parts of water nearly opaque. All the varieties of this
mollusca secrete the same juice; but the Sepia officinalis, the Sepia ioligo, and the Sepia
tunicata, are chiefly sought after for making the pigment. The first, which occurs abundantly
in the Mediterranean, affords most colour; the sac containing it being extracted,
the juice is to be dried as quickly as possible, because it runs rapidly into putrefaction.
Though insoluble in water, it is extremely diffusible through it, and is very slowly
deposited. Caustic alkalis dissolve the sepia, and turn it brown; but in proportion as
the alkali becomes carbonated by exposure to air, the sepia falls to the bottom of the
vessel. Chlorine blanches it slowly. It consists of carbon in an extremely divided
state, along with albumine, gelatine, and phosphate of lime.
The dried native sepia is prepared for the painter, by first triturating it with a little
caustic lye, then adding more lye, boiling the liquid for half an hour, filtering, next
saturating the alkali with an acid, separating the precipitate, washing it with water, and
finally drying it with a gentle heat. The pigment is of a brown colour, and a fine grain.
SEPTARIA, called antiently ludus Helmontii, (the quoits of Van Helmont, from
their form,) are lenticular concretions of clay ironstone, intersected by veins of calc-spar,
which, when calcined, and ground to powder, form an excellent hydraulic cement. See
Mortar, Hydraulic.
SERPENTINE, is a mineral of the magnesian family, of a green colour; it is
scratched by calcareous spar, is sectile, tough, and therefore easily cut into ornamental
forms. It occurs in Unst and Fetlar, in Shetland; at Portsoy, in Banffshire; in Cornwall;
and the Isle of Holyhead. The floors of bakers’ ovens are advantageously laid
with slabs of serpentine.
SHAFT, in mining, signifies a perpendicular or slightly inclined pit.
SHAGREEN. (Chagrin, Fr. and Germ.) The true oriental shagreen is essentially
different from all modifications of leather and parchment. It approaches the
latter somewhat, indeed, in its nature, since it consists of a dried skin, not combined
with any tanning or foreign matter whatever. Its distinguishing characteristic is having
the grain or hair side covered over with small rough round specks or granulations.
It is prepared from the skins of horses, wild asses, and camels; of strips cut along
the chine, from the neck towards the tail, apparently because this stronger and thicker
portion of the skin is best adapted to the operations about to be described. These fillets
are to be steeped in water till the epidermis becomes loose, and the hairs easily come away
by the roots; after which they are to be stretched upon a board, and dressed with the
currier’s fleshing-knife. They must be kept continually moist, and extended by cords
attached to their edges, with the flesh side uppermost upon the board. Each strip now
resembles a wet bladder, and is to be stretched in an open square wooden frame by
means of strings tied to its edges, till it be as smooth and tense as a drum-head. For
this purpose it must be moistened and extended from time to time in the frame.
The grain or hair side of the moist strip of skin must next be sprinkled over with a
kind of seeds called Allabuta, which are to be forced into its surface either by tramping
with the feet, or with a simple press, a piece of felt or other thick stuff being laid upon
the seeds. These seeds belong probably to the Chenapodium album. They are lenticular,
hard, of a shining black colour, farinaceous within, about the size of poppy seed, and are
sometimes used to represent the eyes in wax figures.
The skin is exposed to dry in the shade, with the seeds indented into its surface;
after which it is freed from them by shaking it, and beating upon its other side with a
stick. The outside will be then horny, and pitted with small hollows corresponding to
the shape and number of the seeds.
In order to make the next process intelligible, we must advert to another analogous
and well-known operation. When we make impressions in fine-grained dry wood with
steel punches or letters of any kind, then plane away the wood till we come to the level
of the bottom of these impressions, afterwards steep the wood in water, the condensed or
punched points will swell above the surface, and place the letters in relief. Snuff-boxes
have been sometimes marked with prominent figures in this way. Now shagreen is
treated in a similar manner.
[1099]
The strip of skin is stretched in an inclined plane, with its upper edge attached to hooks,
and its under one loaded with weights, in which position it is thinned off with a proper
semi-lunar knife, but not so much as to touch the bottom of the seed-pits or depressions.
By maceration in water, the skin is then made to swell, and the pits become prominent
over the surface which had been shaved. The swelling is completed by steeping the
strips in a warm solution of soda, after which they are cleansed by the action of salt
brine, and then dyed.
In the East the following processes are pursued. Entirely white shagreen is obtained
by imbuing the skin with a solution of alum, covering it with the dough made with
Turkey wheat, and after a time washing this away with a solution of alum. The strips
are now rubbed with grease or suet, to diminish their rigidity, then worked carefully in
hot water, curried with a blunt knife, and afterwards dried. They are dyed red with
decoction of cochineal or kermes, and green with fine copper filings and sal ammoniac,
the solution of this salt being first applied, then the filings being strewed upon the skin,
which must be rolled up and loaded with weights for some time; blue is given with
indigo, quicklime, soda, and honey; and black, with galls and copperas.
SHALE, or SLATE-CLAY, is an important stratiform member of the coal-measures.
See Pitcoal.
SHAMOY LEATHER. See Leather.
SHEATHING OF SHIPS. For this purpose many different metals and metallic
alloys have been lately proposed. From a train of researches which I made for an eminent
copper company, a few years ago, upon various specimens of sheathing which had been
exposed upon ships during many voyages, it appeared that copper containing a minute
but definite proportion of tin, was by far the most durable.
SHELLAC. See Lac, and Sealing-wax.
SIENITE, is a granular aggregated compound rock, consisting of felspar and hornblende,
sometimes mixed with a little quartz and mica. The hornblende is the characteristic
ingredient, and serves to distinguish sienite from granite, with which it has been
sometimes confounded; though the felspar, which is generally red, is the more abundant
constituent. The Egyptian sienite, containing but little hornblende, with a good
deal of quartz and mica, approaches most nearly to granite. It is equally metalliferous
with porphyry; in the island of Cyprus, it is rich in copper; and in Hungary, it contains
many valuable gold and silver mines.
Sienite forms a considerable part of the Criffle, a hill in Galloway. It takes its name
from the city of Syene, in the Thebaid, near the cataracts of the Nile, where this
rock abounds. It is an excellent building-stone, and was imported in large quantities
from Egypt by the Romans, for the architectural and statuary decorations of their
capital.
SILICA and SILICON. (Silice, silicium, Fr.; Kieselerde, kiesel, Germ.) Silica was
till lately ranked among the earths proper; but since the researches of Davy and Berzelius,
it has been transferred to the chemical class of acids. It constitutes the principal
portion of most of the hard stones and minerals which compose the crust of the globe;
occurring nearly pure in rock crystal, quartz, agate, calcedony, flint, &c. Silica or
silicic acid may be obtained perfectly pure, and also in the finest state of comminution,
by taking the precipitate formed by passing silicated fluoric gas through water, filtering,
washing, and igniting it, to expel the last traces of the fluoride of silicon. The
powder thus obtained is so light as to be blown away with the least breath of air. Silica
may be more conveniently procured, however, by fusing ground flint with four times its
weight of a mixture, in equal parts, of dry carbonate of potassa and carbonate of soda, in
a platinum or silver crucible. The alkaline carbonates should be first fused, and the flint
powder sprinkled into the liquid, as long as it dissolves with effervescence. The mass is
to be then allowed to cool, dissolved in dilute muriatic acid; the solution is to be filtered,
and evaporated to dryness; the dry crust is to be pulverized, digested for two hours
with a little muriatic acid, to remove any iron and alumina that may be present, next
washed with hot water, drained, dried, and ignited.
The above silicate of potassa and soda is the compound called soluble glass, which
applied in solution to the surface of wood, calico, paper, &c., renders them unsusceptible
of taking fire on the contact of an ignited body.
Silica, as thus prepared, is a white powder, rough to the touch, gritty between the
teeth, absolutely insoluble in water, acids, and most liquids. Its specific gravity is
2·66. It cannot be fused by the most intense heat of our furnaces, but at the flame of
the oxy-hydrogen blowpipe it melts into a limpid colourless glass. By peculiar chemical
methods, an aqueous solution of it may be made artificially, similar to what nature
presents us with in many thermal springs, as in those of Reikum and of Geyser in Iceland,
and of most mineral waters, in minute quantity. There is no acid except the
fluoric which can directly dissolve dry or calcined silica. Silica is composed of 48·04
silicon, and 51·96 oxygen.
[1100]
SILICATES, are compounds of silicic acid (silica), with the bases alumina, lime,
magnesia, potassa, soda, &c. They constitute the greater number by far of the hard
minerals which encrust the terrestrial globe. Thus cyanite is a subsilicate of alumina;
felspar and leucite, are silicates of alumina and potassa; albite and analcime, are silicates
of alumina and soda; stilbite, prehnite, mesolite, labradorite, tourmaline, mica, &c., are
silicates of alumina and lime; chrysolite, steatite, serpentine, and meerschaum, are silicates
of magnesia; augite and hornblende, are silicates of lime and magnesia, &c.
SILICON, called also silicium, may be obtained by burning potassium in silicated
fluoric gas. The product of the combustion is a brown cinder, which, on being thrown
into water, disengages hydrogen with violence, and lets fall a dark liver-brown powder,
upon which water exercises no action. This matter is silicon mixed with a salt of
difficult solution, which is composed of fluorine, potassium, and silicon. This salt may,
however, be removed by a great deal of washing. The further details of this curious
subject will be given in my forthcoming system of chemistry.
SILK MANUFACTURE. (Fabrique de soie, Fr.; Seidenfabrik, Germ.) This
may be divided into two branches: 1. the production of raw silk; 2. its filature and
preparation in the mill, for the purposes of the weaver and other textile artisans. The
threads, as spun by the silkworm, and wound up in its cocoon, are all twins, in consequence
of the twin orifice in the nose of the insect through which they are projected.
These two threads are laid parallel to each other, and are glued more or less evenly
together by a kind of glossy varnish, which also envelopes them, constituting nearly
25 per cent. of their weight. Each ultimate filament measures about 1⁄2000 of an inch
in average fine silk, and the pair measures of course fully 1⁄1000 of an inch. In the raw
silk, as imported from Italy, France, China, &c., several of these twin filaments are
slightly twisted and agglutinated to form one thread, called a single.
The specific gravity of silk is 1·300, water being 1·000. It is by far the most tenacious
or the strongest of all textile fibres, a thread of it of a certain diameter being
nearly three times stronger than a thread of flax, and twice stronger than hemp. Some
varieties of silk are perfectly white, but the general colour in the native state is a
golden yellow.
The production of silk was unknown in Europe till the sixth century, when two
monks, who brought some eggs of the silkworm from China or India to Constantinople,
were encouraged to breed the insect, and cultivate its cocoons, by the Emperor Justinian.
Several silk manufactures were in consequence established in Athens, Thebes,
and Corinth, not only for rearing the worm upon mulberry-leaves, but for unwinding its
cocoons, for twisting their filaments into stronger threads, and weaving these into robes.
The Venetians having then and long afterwards intimate commercial relations with the
Greek empire, supplied the whole of western Europe with silk goods, and derived great
riches from the trade.
About 1130, Roger II., king of Sicily, set up a silk manufacture at Palermo, and
another in Calabria, conducted by artisans whom he had seized and carried off as
prisoners of war in his expedition to the Holy Land. From these countries, the silk
industry soon spread throughout Italy. It seems to have been introduced into Spain
at a very early period, by the Moors, particularly in Murcia, Cordova, and Granada.
The last town, indeed, possessed a flourishing silk trade when it was taken by Ferdinand
in the 15th century. The French having been supplied with workmen from Milan,
commenced, in 1521, the silk manufacture; but it was not till 1564 that they began
successfully to produce the silk itself, when Traucat, a working gardener at Nismes,
formed the first nursery of white mulberry-trees, and with such success, that in a few
years he was enabled to propagate them over many of the southern provinces of France.
Prior to this time, some French noblemen, on their return from the conquest of Naples,
had introduced a few silkworms with the mulberry into Dauphiny; but the business
had not prospered in their hands. The mulberry plantations were greatly encouraged
by Henry IV.; and since then they have been the source of most beneficial employment
to the French people. James I. was most solicitous to introduce the breeding of
silkworms into England, and in a speech from the throne he earnestly recommended his
subjects to plant mulberry-trees; but he totally failed in the project. This country does
not seem to be well adapted for this species of husbandry, on account of the great prevalence
of blighting east winds during the months of April and May, when the worms
require a plentiful supply of mulberry-leaves. The manufacture of silk goods, however,
made great progress during that king’s peaceful and pompous reign. In 1629 it had
become so considerable in London, that the silk-throwsters of the city and suburbs
were formed into a public corporation. So early as 1661 they employed 40,000 persons.
The revocation of the edict of Nantes, in 1685, contributed in a remarkable manner to
the increase of the English silk trade, by the influx of a large colony of skilful French
weavers, who settled in Spitalfields. The great silk-throwing mill mounted at Derby, in
1719, also served to promote the extension of this branch of manufacture; for soon[1101]
afterwards, in the year 1730, the English silk goods bore a higher price in Italy than
those made by the Italians, according to the testimony of Keysler.
Till the year 1826, however, our silk manufactures in general laboured under very
grievous fiscal burdens. Foreign organzine, or twisted raw silk, paid an import duty of
14s. 71⁄2d. per pound; raw Bengal silk, 4s.; and that from other places, 5s. 71⁄2d. Mr.
Huskisson introduced a bill at that time, reducing the duty on organzine to 5s., and the
duty on other raw silk to 3d. per pound. The total prohibition of the import of French
manufactured silks, which gave rise to so much contraband trade, was also converted
into a duty of 30 per cent. ad valorem. During the reign of the prohibitory system,
when our silk weavers had no variety of patterns to imitate, and no adequate stimulus
to excel, on account of the monopoly which they possessed in the home market, the
inferiority of their productions was a subject of constant pride and congratulation
among the Lyonnais; and accordingly the English could not stand their competition
any where. At that time, the disadvantage on English silk goods, compared to French,
was estimated in foreign markets at 40 per cent.; of late years it certainly does not exceed
20, notwithstanding the many peculiar facilities which France enjoys for this her
favourite staple.
The silkworm, called by entomologists Phalæna bombyx mori, is, like its kindred
species, subject to four metamorphoses. The egg, fostered by the genial warmth of
spring, sends forth a caterpillar, which, in its progressive enlargement, casts its skin
either three or four times, according to the variety of the insect. Having acquired its
full size in the course of 25 or 30 days, and ceasing to eat during the remainder
of its life, it begins to discharge a viscid secretion, in the form of pulpy twin
filaments, from its nose, which harden in the air. These threads are instinctively
coiled into an ovoid nest round itself, called a cocoon, which serves as a defence against
living enemies and changes of temperature. Here it soon changes into the chrysalis or
nymph state, in which it lies swaddled, as it were, for about 15 or 20 days. Then it
bursts its cearments, and comes forth furnished with appropriate wings, antennæ, and
feet, for living in its new element, the atmosphere. The male and the female moths
couple together at this time, and terminate their union by a speedy death, their whole
existence being limited to two months. The cocoons are completely formed in the course
of three or four days; the finest being reserved as seed worms. From these cocoons,
after an interval of 18 or 20 days, the moth makes its appearance, perforating its tomb
by knocking with its head against one end of the cocoon, after softening it with saliva,
and thus rendering the filaments more easily torn asunder by its claws. Such moths or
aurelias are collected and placed upon a piece of soft cloth, where they couple and lay
their eggs.
The eggs, or grains as they are usually termed, are enveloped in a liquid which
causes them to adhere to the piece of cloth or paper on which the female lays them.
From this glue they are readily freed, by dipping them in cold water, and wiping them
dry. They are best preserved in the ovum state at a temperature of about 55° F. If
the heat of spring advances rapidly in April, it must not be suffered to act on the eggs,
otherwise it might hatch the caterpillars long before the mulberry has sent forth its
leaves to nourish them. Another reason for keeping back their incubation is, that they
may be hatched together in large broods, and not by small numbers in succession. The
eggs are made up into small packets, of an ounce, or somewhat more, which in the south
of France are generally attached to the girdles of the women during the day, and placed
under their pillows at night. They are, of course, carefully examined from time to
time. In large establishments, they are placed in an appropriate stove-room, where
they are exposed to a temperature gradually increased till it reaches the 86th degree of
Fahrenheit’s scale, which term it must not exceed. Aided by this heat, nature completes
her mysterious work of incubation in eight or ten days. The teeming eggs are
now covered with a sheet of paper pierced with numerous holes, about one-twelfth of an
inch in diameter. Through these apertures the new-hatched worms creep upwards instinctively,
to get at the tender mulberry leaves strewed over the paper.
The nursery where the worms are reared, is called by the French a magnanière; it
ought to be a well-aired chamber, free from damp, excess of cold or heat, rats and other
vermin. It should be ventilated occasionally, to purify the atmosphere from the
noisome emanations produced by the excrements of the caterpillars and the decayed
leaves. The scaffolding of the wicker-work shelves should be substantial; and they
should be from 15 to 18 inches apart. A separate small apartment should be allotted
to the sickly worms. Immediately before each moulting, the appetite of the worms
begins to flag; it ceases altogether at that period of cutaneous metamorphosis, but
revives speedily after the skin is fairly cast, because the internal parts of the
animal are thereby allowed freely to develop themselves. At the end of the second
age, the worms are half an inch long; and should then be transferred from the small
room in which they were first hatched, into the proper apartment where they are to[1102]
be brought to maturity and set to spin their balls. On occasion of changing their
abode, they must be well cleansed from the litter, laid upon beds of fresh leaves, and
supplied with an abundance of food every six hours in succession. In shifting their
bed, a piece of network being laid over the wicker plates, and covered with leaves, the
worms will creep up over them; when they may be transferred in a body upon the net.
The litter, as well as the sickly worms, may thus be readily removed, without handling
a single healthy one. After the third age, they may be fed with entire leaves; because
they are now exceedingly voracious, and must not be subsequently stinted in their
diet. The exposure of chloride of lime, spread thin upon plates, to the air of the
magnanière, has been found useful in counteracting the tendency which sometimes
appears of an epidemic disease among the silkworms, from the fetid exhalations of the
dead and dying.
When they have ceased to eat, either in the fourth or fifth age, agreeably to the variety
of the bombyx, and when they display the spinning instinct by crawling up among
the twigs of heath, &c., they are not long of beginning to construct their cocoons, by
throwing the thread in different directions, so as to form the floss, filoselle, or outer
open network, which constitutes the bourre or silk for carding and spinning.
The cocoons destined for filature, must not be allowed to remain for many days with
the worms alive within them; for should the chrysalis have leisure to grow mature
or come out, the filaments at one end would be cut through, and thus lose almost all
their value. It is therefore necessary to extinguish the life of the animal by heat, which
is done either by exposing the cocoons for a few days to sunshine, by placing them in
a hot oven, or in the steam of boiling water. A heat of 202° F. is sufficient for effecting
this purpose, and it may be best administered by plunging tin cases filled with the
cocoons into water heated to that pitch.
80 pounds French (88 Eng.) of cocoons, are the average produce from one ounce of
eggs, or 100 from one ounce and a quarter; but M. Folzer of Alsace obtained no less
than 165 pounds. The silk obtained from a cocoon is from 750 to 1150 feet long. The
varnish by which the coils are glued slightly together, is soluble in warm water.
The silk husbandry, as it may be called, is completed in France within six weeks
from the end of April, and thus affords the most rapid of agricultural returns, requiring
merely the advance of a little capital for the purchase of the leaf. In buying up cocoons,
and in the filature, indeed, capital may be often laid out to great advantage.
The most hazardous period in the process of breeding the worms, is at the third and fourth
moulting; for upon the 6th day of the third age, and the seventh day of the fourth, they
in general eat nothing at all. On the first day of the fourth age, the worms proceeding
from one ounce of eggs will, according to Bonafons, consume upon an average twenty-three
pounds and a quarter of mulberry leaves; on the first of the fifth age, they will
consume forty-two pounds; and on the sixth day of the same age, they acquire their
maximum voracity, devouring no less than 223 pounds. From this date their appetite
continually decreases, till on the tenth day of this age they consume only fifty-six
pounds. The space which they occupy upon the wicker tables, being at their birth only
nine feet square, becomes eventually 239 feet. In general the more food they consume,
the more silk will they produce.
A mulberry-tree is valued, in Provence, at from 6d. to 10d.; it is planted out of the
nursery at four years of age; it is begun to be stripped in the fifth year, and affords an
increasing crop of leaves till the twentieth. It yields from 1 cwt. to 30 cwt. of
leaves, according to its magnitude and mode of cultivation. One ounce of silkworm
eggs is worth in France about 21⁄2 francs; it requires for its due development into
cocoons about 15 cwt. of mulberry leaves, which cost upon an average 3 francs per
cwt. in a favourable season. One ounce of eggs is calculated, as I have said, to produce
from 80 to 100 pounds of cocoons, of the value of 1 fr. 52 centimes per pound, or
125 francs in whole. About 8 pounds of reeled raw silk, worth 18 francs a pound, are
obtained from these 100 pounds of cocoons.
There are three denominations of raw silk; viz., organzine, trame (shute or tram), and
floss. Organzine serves for the warp of the best silk stuffs, and is considerably twisted;
tram is made usually from inferior silk, and is very slightly twisted, in order that it may
spread more, and cover better in the weft; floss, or bourre, consists of the shorter broken
silk, which is carded and spun like cotton. Organzine and trame may contain from 3
to 30 twin filaments of the worm; the former possesses a double twist, the component
filaments being first twisted in one direction, and the compound thread in the opposite;
the latter receives merely a slender single twist. Each twin filament gradually diminishes
in thickness and strength, from the surface of the cocoon, where the animal
begins its work in a state of vigour, to the centre, where it finishes it, in a state of debility
and exhaustion; because it can receive no food from the moment of its beginning
to spin by spouting forth its silky substance. The winder is attentive to this progressive
attenuation, and introduces the commencement of some cocoons to compensate for the[1103]
termination of others. The quality of raw silk depends, therefore, very much upon the
skill and care bestowed upon its filature. The softest and purest water should be used
in the cocoon kettle.
The quality of the raw silk is determined by first winding off 400 ells of it, equal to
475 metres, round a drum one ell in circumference, and then weighing that length.
The weight is expressed in grains, 24 of which constitute one denier; 24 deniers constitute
one ounce; and 16 ounces make one pound, poids de marc. This is the Lyons
rule for valuing silk. The weight of a thread of raw silk 400 ells long, is two grains
and a half, when five twin filaments have been reeled and associated together.
Raw silk is so absorbent of moisture, that it may be increased ten per cent. in weight
by this means. This property has led to falsifications; which are detected by enclosing
weighed portions of the suspected silk in a wire-cloth cage, and exposing it to a stove-heat
of about 78° F. for 24 hours, with a current of air. The loss of weight which it thereby
undergoes, demonstrates the amount of the fraud. There is an office in Lyons called
the Condition, where this assay is made, and by the report of which the silk is bought and
sold. The law in France requires, that all the silk tried by the Condition must be
worked up into fabrics in that country.
In the Journal of the Asiatic Society of Bengal, for January, 1837, there are two
very valuable papers upon silkworms; the first, upon those of Assam, by Mr. Thomas
Hugon, stationed at Nowgong; the second by Dr. Heifer, upon those which are indigenous
to India. Besides the Bombyx mori, the Doctor enumerates the following
seven species, formerly unknown:—1. The wild silkworm of the central provinces,
a moth not larger than the Bombyx mori. 2. The Joree silkworm of Assam, Bombyx
religiosæ, which spins a cocoon of a fine filament, with much lustre. It lives upon the
pipul tree (Ficus religiosa), which abounds in India, and ought therefore to be turned
to account in breeding this valuable moth. 3. Saturnia silhetica, which inhabits the
cassia mountains in Silhet and Dacca, where its large cocoons are spun into silk.
4. A still larger Saturnia, one of the greatest moths in existence, measuring ten inches
from the one end of the wing to the other; observed by Mr. Grant, in Chirra Punjee.
5. Saturnia paphia, or the Tusseh silkworm, is the most common of the native species,
and furnishes the cloth usually worn by Europeans in India. It has not hitherto been
domesticated, but millions of its cocoons are annually collected in the jungles, and
brought to the silk factories near Calcutta and Bhagelpur. It feeds most commonly
on the hair-tree (Zizyphus jujuba), but it prefers the Terminalia alata, or Assam tree,
and the Bombax heptaphyllum. It is called Koutkuri mooga, in Assam. 6. Another
Saturnia, from the neighbourhood of Comercolly. 7. Saturnia assamensis, with a cocoon
of a yellow-brown colour, different from all others, called mooga, in Assam; which,
although it can be reared in houses, thrives best in the open air upon trees, of which
seven different kinds afford it food. The Mazankoory mooga, which feeds on the Adakoory
tree, produces a fine silk, which is nearly white, and fetches 50 per cent. more
than the fawn-coloured. The trees of the first year’s growth produce by far the most
valuable cocoons. The mooga which inhabits the soom-tree, is found principally in the
forests of the plains, and in the villages. The tree grows to a large size, and yields
three crops of leaves in the year. The silk is of a light fawn colour, and ranks next in
value to the Mazankoory. There are generally five breeds of mooga worms in the year;
1. in January and February; 2. in May and June; 3. in June and July; 4. in August
and September; 5. in October and November; the first and last being the most
valuable.
The Assamese select for breeding, such cocoons only as have been begun to be
formed in the largest number on the same day, usually the second or third after the
commencement; those which contain males being distinguishable by a more pointed
end. They are put in a closed basket suspended from the roof; the moths, as they
come forth, having room to move about, after a day, the females (known only by their
large body) are taken out, and tied to small wisps of thatching-straw, selected always from
over the hearth, its darkened colour being thought more acceptable to the insect.
If out of a batch, there should be but few males, the wisps with the females tied to
them are exposed outside at night; and the males thrown away in the neighbourhood
find their way to them. These wisps are hung upon a string tied across the roof, to
keep them from vermin. The eggs laid after the first three days are said to produce
weak worms. The wisps are taken out morning and evening, and exposed to the sunshine,
and in ten days after being laid, a few of them are hatched. The wisps being
then hung up to the tree, the young worms find their way to the leaves. The ants,
whose bite is fatal to the worm in its early stages, are destroyed by rubbing the trunk
of the tree with molasses, and tying dead fish and toads to it, to attract these rapacious
insects in large numbers, when they are destroyed with fire; a process which needs to be
repeated several times. The ground under the trees is also well cleared, to render it easy
to pick up and replace the worms which fall down. They are prevented from coming to[1104]
the ground by tying fresh plantain-leaves round the trunk, over whose slippery surface
they cannot crawl; and they are transferred from exhausted trees to fresh ones, on
bamboo platters tied to long poles. The worms require to be constantly watched and
protected from the depredations of both day and night birds, as well as rats and other
vermin. During their moultings, they remain on the branches; but when about
beginning to spin, they come down the trunk, and being stopped by the plantain-leaves,
are there collected in baskets, which are afterwards put under bunches of dry leaves,
suspended from the roof, into which the worms crawl, and form their cocoons—several
being clustered together: this accident, due to the practice of crowding the worms
together, which is most injudicious, rendering it impossible to wind off their silk in continuous
threads, as in the filatures of Italy, France, and even Bengal. The silk is, therefore,
spun like flax, instead of being unwound in single filaments. After four days the
proper cocoons are selected for the next breed, and the rest are uncoiled. The total
duration of a breed varies from 60 to 70 days; divided into the following periods:—
| Four moultings, with one day’s illness attending each |
20 |
| From fourth moulting to beginning of cocoon |
10 |
| In the cocoon 20, as a moth 6, hatching of eggs 10 |
36 |
| |
66 |
On being tapped with the finger, the body renders a hollow sound; the quality of
which shows whether they have come down for want of leaves on the tree, or from
their having ceased feeding.
As the chrysalis is not soon killed by exposure to the sun, the cocoons are put on
stages, covered up with leaves, and exposed to the hot air from grass burned under
them; they are next boiled for about an hour in a solution of the potash, made from
incinerated rice-stalks; then taken out, and laid on cloth folded over them to keep
them warm. The floss being removed by hand, they are then thrown into a basin of
hot water to be unwound; which is done in a very rude and wasteful way.
The plantations for the mooga silkworm in Lower Assam, amount to 5000 acres,
besides what the forests contain; and yield 1500 maunds of 84 lbs. each per annum.
Upper Assam is more productive.
The cocoon of the Koutkuri mooga is of the size of a fowl’s egg. It is a wild species,
and affords filaments much valued for fishing-lines. See Silkworm Gut.
8. The Arrindy, or Eria worm, and moth, is reared over a great part of Hindustan,
but entirely within doors. It is fed principally on the Hera, or Palma
christi leaves, and gives sometimes 12 broods of spun silk in the course of a year.
It affords a fibre which looks rough at first; but when woven, becomes soft and silky,
after repeated washings. The poorest people are clothed with stuff made of it, which is
so durable as to descend from mother to daughter. The cocoons are put in a closed
basket, and hung up in the house, out of reach of rats and insects. When the moths
come forth, they are allowed to move about in the basket for twenty-four hours; after
which the females are tied to long reeds or canes, twenty or twenty-five to each, and
these are hung up in the house. The eggs that are laid the first three days, amounting
to about 200, alone are kept; they are tied up in a cloth, and suspended to the roof
till a few begin to hatch. These eggs are white, and of the size of turnip-seed. When
a few of the worms are hatched, the cloths are put on small bamboo platters hung up
in the house, in which they are fed with tender leaves. After the second moulting,
they are removed to bunches of leaves suspended above the ground, beneath which a
mat is laid to receive them when they fall. When they cease to feed, they are thrown
into baskets full of dry leaves, among which they form their cocoons, two or three
being often found joined together. Upon this injudicious practice I have already
animadverted.
9. The Saturnia trifenestrata, has a yellow cocoon of a remarkably silky lustre. It
lives on the soom-tree in Assam, but seems not to be much used.
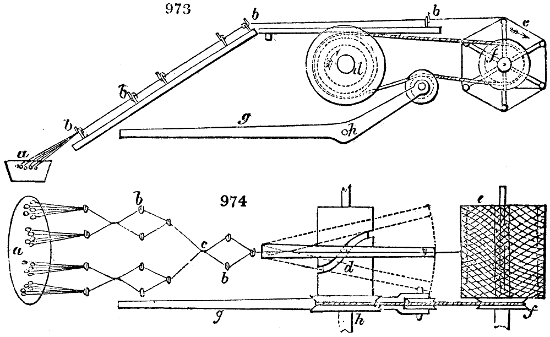
The mechanism of the silk filature, as lately improved in France, is very ingenious.
Figs. 973. and 974. exhibit it in plan and longitudinal view. a is an oblong copper
basin containing water heated by a stove or by steam. It is usually divided by transverse
partitions into several compartments, containing 20 cocoons, of which there are 5 in
one group, as shown in the figure. b, b, are wires with hooks or eyelets at their ends,
through which the filaments run, apart, and are kept from ravelling. c, c, the points
where the filaments cross and rub each other, on purpose to clean their surfaces. d, is
a spiral groove, working upon a pin point, to give the traverse motion alternately to
right and left, whereby the thread is spread evenly over the surface of the reel e. f, f, are
the pulleys, which by means of cords transmit the rotatory movement of the cylinder d,
to the reel e. g, is a friction lever or tumbler, for lightening or slackening the endless[1105]
cord, in the act of starting or stopping the winding operation. Every apartment of a
large filature contains usually a series of such reels as the above, all driven by one prime
mover; each of which, however, may by means of the tumbling lever be stopped at
pleasure. The reeler is careful to remove any slight adhesions, by the application of
a brush in the progress of her work.
The expense of reeling the excellent Cevennes silk is only 3 francs and 50 centimes
per Alais pound; from 4 to 5 cocoons going to one thread. That pound is 92 hundredths
of our avoirdupois pound. In Italy, the cost of reeling silk is much higher,
being 7 Italian livres per pound, when 3 to 4 cocoons go to the formation of one
thread; and 6 livres when there are from 4 to 5 cocoons. The first of these raw silks
will have a titre of 20 to 24 deniers; the last, of 24 to 28. If 5 to 6 cocoons go to
one thread, the titre will be from 26 to 32 deniers, according to the quality of the cocoons.
The Italian livre is worth 71⁄2d. English. The woman employed at the
kettle receives one livre and five sous per day; and the girl who turns the reel, gets
thirteen sous a day; both receiving board and lodging in addition. In June, July, and
August, they work 16 hours a day, and then they wind a rubo or ten pounds weight of
cocoons, which yield from 1-5th to 1-6th of silk, when the quality is good. The whole
expenses amount to from 6 to 7 livres upon every ten pounds of cocoons; which is
about 2s. 8d. per English pound of raw silk.
The raw silk, as imported into this country in hanks from the filatures, requires to
be regularly wound upon bobbins, doubled, twisted, and reeled in our silk-mills. These
processes are called throwing silk, and their proprietors are called silk throwsters; terms
probably derived from the appearance of swinging or tossing which the silk threads
exhibit during their rapid movements among the machinery of the mills.
A representation of a French mill for throwing silk, is given in the Dictionnaire
Technologique, under the article Moulinage de Soie. But it is a most awkward, operose,
and defective piece of machinery, quite unworthy of being presented to my readers.
It was in Manchester that throwing-mills received the grand improvement upon the
antient Italian plan, which had been originally introduced into this country by Sir Thomas
Lombe, and erected at Derby. That improvement is chiefly due to the eminent factory
engineers, Messrs. Fairbairn and Lillie, who transferred to silk the elegant mechanism of
the throstle, so well known in the cotton trade. Still, throughout the silk districts of
France the throwing mills are generally small, not many of them turning off more than
1000 pounds of organzine per annum, and not involving 5000l. of capital. The average
price of throwing organzine in that country, where the throwster is not answerable for
loss, is 7 francs; of throwing trame, from 4 fr. to 5 fr. (per kilogramme?) Where the
throwster is accountable for loss, the price is from 10 fr. to 11 fr. for organzine, and
from 6 to 7 for trame. In Italy, throwing adds 3s. 9d. to the price of raw silk, upon an
average. I should imagine, from the perfection and speed of the silk-throwing machinery
in this country, as about to be described, that the cost of converting a pound of raw silk
either into organzine or trame must be considerably under any of the above sums.
SILK-THROWING MILL.
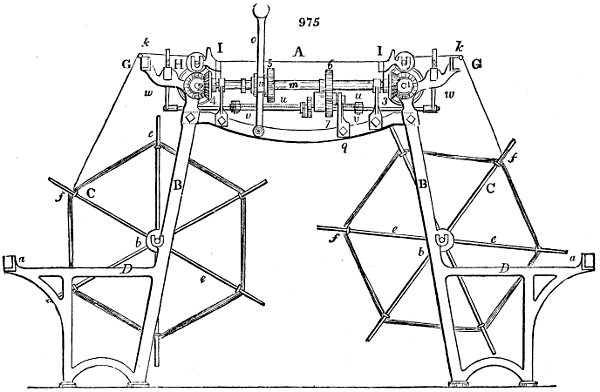
The first process to which the silk is subjected, is winding the skeins, as imported, off
upon bobbins. The mechanism which effects this winding off and on, is technically
called the engine, or swift. The bobbins to which the silk is transferred, are wooden[1106]
cylinders, of such thickness as may not injure the silk by sudden flexure, and which
may also receive a great length of thread without having their diameter materially
increased, or their surface velocity changed. Fig. 975. is an end view of the silk-throwing
machine, or engine, in which the two large hexagonal reels, called swifts, are
seen in section, as well as the table between them, to which the bobbins and impelling
mechanism are attached. The skeins are put upon these reels, from which the silk is
gradually unwound by the traction of the revolving bobbins. One principal object of
attention, is to distribute the thread over the length of the bobbin-cylinder in a spiral
or oblique direction, so that the end of the slender semi-transparent thread may be
readily found when it breaks. As the bobbins revolve with uniform velocity, they would
soon wind on too fast, were their diameters so small at first as to become greatly
thicker when they are filled. They are therefore made large, are not covered thick, but
are frequently changed. The motion is communicated to that end of the engine shown
in the figure.
The wooden table A, shown here in cross section, is sometimes of great length, extending
20 feet, or more, according to the size of the apartment. Upon this the skeins
are laid out. It is supported by the two strong slanting legs B, B, to which the bearings
of the light reel C are made fast. These reels are called swifts, apparently by the same
etymological casuistry as lucus à non lucendo; for they turn with reluctant and irregular
slowness; yet they do their work much quicker than any of the old apparatus,
and in this respect may deserve their name. At every eighth or tenth leg there is a
projecting horizontal piece D, which carries at its end another horizontal bar a, called
the knee rail, at right angles to the former. This protects the slender reels or swifts
from the knees of the operatives.
These swifts have a strong wooden shaft b, with an iron axis passing longitudinally
through it, round which they revolve, in brass bearings fixed near to the middle of the
legs B. Upon the middle of the shaft b, a loose ring is hung, shown under c, in fig. 976.,
to which a light weight d, is suspended, for imparting friction to the reel, and thus
preventing it from turning round, unless it be drawn with a gentle force, such as the
traction of the thread in the act of winding upon the bobbin.
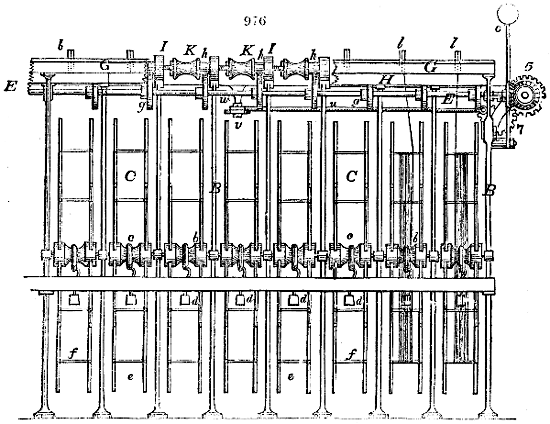
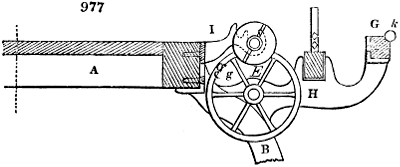
Fig. 976. is a front view of the engine. B, B, are the legs, placed at their appropriate
distances (scale 11⁄2 inch to the foot); C, C, are the swifts. By comparing figs. 975. and
976., the structure of the swifts will be fully understood. From the wooden shaft b,
six slender wooden (or iron) spokes e, e, proceed, at equal angles to each other; which
are bound together by a cord f, near their free ends, upon the transverse line f of
which cord, the silk thread is wound, in a hexagonal form; due tension being given
to the circumferential cords, by sliding them out from the centre. Slender wooden rods[1107]
are set between each pair of spokes, to stay them, and to keep the cord tight. E is one
of the two horizontal shafts, placed upon each side of the engine, to which are affixed
a number of light iron pulleys g, g (shown on a double scale in fig. 977.) These serve,
by friction, to drive the bobbins which rest upon their peripheries.
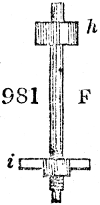
To the table A, fig. 975., are screwed the light cast-iron slot-bearings I, I, wherein
the horizontal spindles or skewers rest, upon which the bobbins revolve. The spindles
(see F, fig. 981.) carry upon one end a little wooden pulley h, whereby they press and
revolve upon the larger driving pulleys g, of the shaft E. These pulleys are called
stars by our workmen. The other ends of the spindles, or skewers, are cut into screws,
for attaching the swivel nuts i (fig. 981.), by which the bobbins K, K, are made fast to
their respective spindles.
Besides the slots, above described,
in which the spindles
rest when their friction
pulleys h, are in contact
with the moving stars g,
there is another set of slots
in the bearings, into which
the ends of the spindles may
be occasionally laid, so as
to be above the line of contact
of the rubbing periphery of the star g, in case the thread of any bobbin breaks.
Whenever the girl has mended the thread, she replaces the bobbin-spindle in its deeper
slot-bearings, thereby
bringing its pulley
once more into
contact with the star,
and causing it to revolve.
G is a long ruler
or bar of wood, which
is supported upon
every eighth or
twelfth leg B, B.
(The figure being,
for convenience of
the page, contracted in length, shows it at every sixth leg.) To the edge of that bar
the smooth glass rods k, are made fast, over which the threads glide from the swifts, in[1108]
their way to the bobbins. H is the guide bar, which has a slow traverse or seesaw
motion, sliding in slots at the top of the legs B, where they support the bars G. Upon
the guide bar H, the guide pieces l, l, are made fast. These consist of two narrow, thin,
upright plates of iron, placed endwise together, their contiguous edges being smooth,
parallel, and capable of approximation to any degree by a screw, so as to increase or
diminish at pleasure the ordinary width of the vertical slit that separates them.
Through this slit the silk thread must pass, and, if rough or knotty, will be either
cleaned or broken; in the latter case, it is neatly mended by the attendant girl.
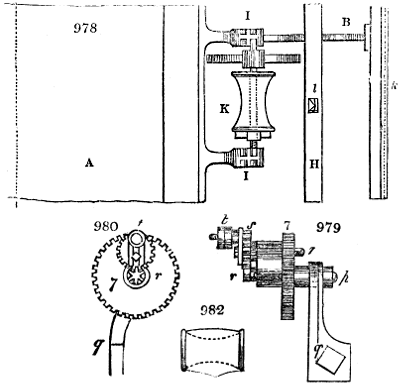
The motions of the various parts of the engine are given as follows. Upon the end of
the machine, represented in fig. 975., there are attached to the shafts E (fig. 976.), the
bevel wheels 1 and 2, which are set in motion by the bevel wheels 3 and 4, respectively.
These latter wheels are fixed upon the shaft m, fig. 975. m is moved by the main steam
shaft which runs parallel to it, and at the same height, through the length of the engine
apartment, so as to drive the whole range of the machines. 5 is a loose wheel or pulley
upon the shaft m, working in geer with a wheel upon the steam shaft, and which may
be connected by the clutch n, through the hand lever or geering rod o (figs. 975. and
976.), when the engine is to be set at work. 6 is a spur wheel upon the shaft m, by
which the stud wheel 7, is driven, in order to give the traverse motion to the guide bar
H. This wheel is represented, with its appendages, in double size, figs. 979. and
980., with its boss upon a stud p, secured to the bracket q. In an eccentric hole
of the same boss, another stud r, revolves, upon
which the little wheel s, is fixed. This wheel s,
is in geer with a pinion cut upon the end of
the fixed stud p; and upon it is screwed the
little crank t, whose collar is connected by two
rods u (figs. 975. and 976.), to a cross-piece v,
which unites the two arms w, that are fixed upon
the guide bar H, on both sides of the machine.
By the revolution of wheel 7, the wheel s will
cause the pinion of the fixed stud p to turn
round. If that wheel bear to the pinion the
proportion of 4 to 1, then the wheel s will make, at each revolution of the wheel 7, one-fourth
of a revolution; whereby the crank t will also rotate through one-fourth of a turn,
so as to be brought nearer to the centre of the stud, and to draw the guide bar so much
less to one side of its mean position. At the next revolution of wheel 7, the crank t will
move through another quadrant, and come still nearer to the central position, drawing
the guide bars still less aside, and therefore causing the bobbins to wind on more
thread in their middle than towards their ends. The contrary effect would ensue,
were the guide bars moved by a single or simple crank. After four revolutions of the
wheel 7, the crank t will stand once more as shown in fig. 980., having moved the bar
H through the whole extent of its traverse. The bobbins, when filled, have the appearance
represented in fig. 982.; the thread having been laid on the mall the time
in diagonal lines, so as never to coincide with each other.
Doubling is the next operation of the silk throwster. In this process, the threads of
two or three of the bobbins, filled as above, are wound together in contact upon a single
bobbin. An ingenious device is here employed to stop the winding-on the moment
that one of these parallel threads happens to break. Instead of the swifts or reels,
a creel is here mounted for receiving the bobbins from the former machine, two or
three being placed in one line over each other, according as the threads are to be
doubled or trebled. Though this machine is in many respects like the engine, it has
some additional parts, whereby the bobbins are set at rest, as above mentioned, when
one of the doubling threads gets broken.
Fig. 983. is an end view, from which it will be perceived that the machine is, like the
preceding, a double one, with two working sides.
Fig. 984. is a front view of a considerable portion of the machine.
Fig. 985. shows part of a cross section, to explain minutely the mode of winding
upon a single bobbin.
Fig. 986. is the plan of the parts shown in fig. 985.; these two figures being drawn
to double the scale of figs. 983. and 984.
A, A, figs. 983. and 984. are the end frames, connected at their tops by a wooden
stretcher, or bar-beam, a, which extends through the whole length of the machine; this
bar is shown also in figs. 985. and 986.
B, B, are the creels upon each side of the machine, or bobbin bearers, resting upon
wooden beams or boards, made fast to the arms or brackets C, about the middle of the
frames A.
D, D, are two horizontal iron shafts, which pervade the whole machine, and carry a
series of light movable pulleys, called stars, c, c, (figs. 985, 986.) which serve to drive the[1109]
bobbins E, E, whose fixed pulleys rest upon their peripheries, and are therefore turned
simply by friction. These bobbins are screwed by swivel nuts e, e, upon spindles, as in
the silk engine. Besides
the small friction
pulley or boss, d, seen
best in fig. 986., by
which they rest upon
the star pulleys c, c, a
little ratchet wheel f,
is attached to the other
end of each bobbin.
This is also shown by
itself at f, in fig. 987.
The spindles with
their bobbins revolve
in two slot-bearings
F, F, fig. 986., screwed
to the bar-beam a,
which is supported by
two or three intermediate
upright frames,
such as A′. The slot-bearings
F, have also
a second slot, in which
the spindle with the
bobbin is laid at rest, out of contact of the star wheel, while its broken thread is being
mended. G is the guide bar (to which the cleaner slit pieces g, g, are attached), for
making the thread traverse to the right and the left, for its proper distribution over
the surface of the bobbin. The guide bar of the doubling machine is moved with a
slower traverse than in the engine; otherwise, in consequence of the different obliquities
of the paths, the single threads would be readily broken, h, h, is a pair of smooth rods of
iron or brass, placed parallel to each of the two sides of the machine, and made fast to the
standards H, H, which are screwed to brackets projecting from the frames A, A′. Over these
rods the silk threads glide, in their passage to the guide wires g, g, and the bobbins E, E.
I, I, is the lever board upon each side of the machine, upon which the slight brass
bearings or fulcrums i, i, one for each bobbin in the creel, are made fast. This board
bears the balance-lever k, l, with the fullers n, n, n, which act as dexterous fingers, and
stop the bobbin from winding-on the instant a thread may chance to break. The levers
k, l, swing upon a fine wire axis, which passes through their props i, i, their arms being
shaped rectangularly, as shown at k, k′, fig. 986. The arm l, being heavier than the arm k,
naturally rests upon the ridge bar m, of the lever board I. n, n, n, are three wires, resting
at one of their ends upon the axis of the fulcrum i, i, and having each of their other hooked
ends suspended by one of the silk threads, as it passes over the front steel rod h, and under[1110]
h′. These faller wires, or stop fingers, are guided truly in their up-and-down motions
with the thread, by a cleaner-plate o, having a vertical slit in its middle. Hence, whenever
any thread happens to break, in its way to a winding-on bobbin E, the wire n,
which hung by its eyelet end
to that thread, as it passed
through between the steel rods
in the line of h, h′, falls upon
the lighter arm of the balance
lever k, l, weighs down that
arm k, consequently jerks up
the arm l, which pitches its tip
or end into one of the three
notches of the ratchet or catch
wheel f (figs. 986. and 987.),
fixed to the end of the bobbin.
Thus its motion is instantaneously
arrested, till the girl has
had leisure to mend the thread,
when she again hangs up the
faller wire n, and restores the
lever k, l, to its horizontal position.
If, meanwhile, she took occasion to remove the winding bobbin out of the sunk
slot-bearing, where pulley d touches the star wheel c, into the right-hand upper slot of
repose, she must now shift it into its slot of rotation.
The motions are given to the doubling
machine in a very simple way. Upon the
end of the framing, represented in fig. 983.,
the shafts D, D, bear two spur wheels 1 and
2, which work into each other. To the
wheel 1, is attached the bevel wheel 3,
driven by another bevel wheel 4 (fig.
984.), fixed to a shaft that extends the
whole length of the apartment, and serves,
therefore, to drive a whole range of machines.
The wheel 4 may be put in geer
with the shaft, by a clutch and geer-handle,
as in the silk engine, and thereby it
drives two shafts, by the one transmitting
its movement to the other.
The traverse motion of the guide bar G,
is effected as follows:—Upon one of the shafts D, there is a bevel wheel 5, driving the
bevel wheel 6, upon the top of the upright shaft p (fig. 984., to the right of the
middle); whence the motion is transmitted to the horizontal shaft q, below, by means of
the bevel wheels 7 and 8. Upon this shaft q, there is a heart-wheel r, working against
a roller which is fixed to the end of the lever s, whose fulcrum is at t, fig. 983. The
other end of the lever s, is connected by two rods (shown by dotted lines in fig. 984.)
to a brass piece which joins the arms u (fig. 984.), of the guide bars G. To the same
cross piece a cord is attached, which goes over a roller v, and suspends a weight w, by
means of which the lever s, is pressed into contact with the heart-wheel r. The fulcrum
t, of the lever s, is a shaft which is turned somewhat eccentric, and has a very slow
rotatory motion. Thus the guide bar, after each traverse, necessarily winds the silk in
variable lines, to the side of the preceding threads.
The motion is given to this shaft in the following way. Upon the horizontal
shaft q, there is a bevel wheel g (figs. 983. and 984.), which drives the wheel 10 upon
the shaft x; on whose upper end, the worm y works in the wheel 11, made fast to the
said eccentric shaft t; round which the lever s, swings or oscillates, causing the guide
bars to traverse.
The spinning silk-mill.—The machine which twists the silk threads, either in their
single or doubled state, is called the spinning mill. When the raw singles are first
twisted in one direction, next doubled, and then twisted together in the opposite
direction, an exceedingly wiry, compact thread, is produced, called organzine. In the
spinning mill, either the singles or the doubled silk, while being unwound from one set
of bobbins, and wound upon another set, is subjected to a regular twisting operation;
in which process the thread is conducted as usual through guides, and coiled diagonally
upon the bobbins by a proper mechanism.
Fig. 988. exhibits an end view of the spinning mill; in which four working lines
are shown; two tiers upon each side, one above the other. Some spinning mills have[1111]
three working tiers upon each side; but as the highest tier must be reached by a ladder
or platform, this construction is considered by many to be injudicious.
Fig. 989. is a front view, where, as
in the former figure, the two working
lines are shown.
Fig. 990. is a cross section of a part
of the machine, to illustrate the construction
and play of the working
parts; figs. 996, 997. are other views
of fig. 990.
Fig. 991. shows a single part of the
machine, by which the bobbins are
made to revolve.
Figs. 992. and 993. show a different
mode of giving the traverse to
the guide bars, than that represented
in fig. 990.
Figs. 994. and 995. show the shape
of the full bobbins, produced by the
action of these two different traverse
motions.
The upper part of the machine
being exactly the same as the under
part, it will be sufficient to explain
the construction and operation of one
of them.
A, A, are the end upright frames or
standards, between which are two or
three intermediate standards, according
to the length of the machine.
They are all connected at their sides
by beams B and C, which extend the
whole length of the machines. D, D,
are the spindles, whose top bearings a, a, are made fast to the beams B, and their
bottoms turn in hard brass steps, fixed to the bar C. These two bars together are[1112]
called, by the workmen, the spindle box. The standards A, A, are bound with cross
bars N, N.
c, c, are the wharves or whorls, turned by a band from the horizontal tin cylinder in
the lines of E, E, fig. 988., lying in the middle line between the two parallel rows of
spindles D, D. F, F, are the bobbins containing the untwisted doubled silk, which are
simply pressed down upon the taper end of the spindles. d, d, are little flyers, or
forked wings of wire, attached to washers of wood, which revolve loose upon the tops
of the said bobbins F, and round the spindles. One of the wings is sometimes bent
upwards, to serve as a guide to the silk, as shown by dotted lines in fig. 990. e, e, are
pieces of wood pressed upon the tops of the spindles, to prevent the flyers from starting
off by the centrifugal force. G, are horizontal shafts bearing a number of little spur
wheels f, f. H, are slot-bearings, similar to those of the doubling-machine, which are
fixed to the end and middle frames. In these slots, the light square cast-iron shafts or
spindles g, fig. 989., are laid, on whose end the spur wheel h is cast; and when the shaft g
lies in the front slot of its bearing, it is in geer with the wheel f, upon the shaft G; but
when it is laid in the back slot, it is out of geer, and at rest. See F, F, fig. 986.
Upon these little cast-iron shafts or spindles g, fig. 991., the bobbins or blocks I, are
thrust, for receiving, by winding-on, the twisted
or spun silk. These blocks are made of a large
diameter, in order that the silk fibres may not be
too much bent; and they are but slightly filled,
at each successive charge, lest, by increasing their
diameter too much, they should produce too rapid
an increase in the rate of winding, with proportional
diminution in the twist, and risk of stretching
or tearing the silk. They are therefore the more
frequently changed. K, K, are the guide bars, with
the guides i, i, through which the silk passes, being
drawn by the revolving bobbins I, and delivered
or laid on by the flyers d, d, from the rotatory
twisting-bobbins F. The operation of the machine
is therefore simple, and the motions are
given to the parts in a manner equally so.
Upon the shaft of the tin cylinder or drum,
exterior to the frame, the usual fast and loose
pulleys, or riggers, L, L′, are mounted, for driving
the whole machine. These riggers are often called
steam-pulleys by the workmen, from their being
connected by bands with the steam-driven shaft
of the factory. In order to allow the riggers upon
the shafts of the upper and the under drums to
be driven from the same pulley upon the main
shaft, the axis of the under drum is prolonged at
L, L, and supported at its end, directly from the
floor, by an upright bearing. Upon the shafts
of the tin cylinders there is also a fly-wheel M,
to equalize the motion. Upon the other ends of
these shafts, namely at the end of the spinning-mill,
represented in fig. 988., the pinions 1 are
fixed, which drive the wheels 3, by means of the
intermediate or carrier wheel 2; called also the
plate wheel, from its being hollowed somewhat
like a trencher. 1, is called the change-pinion, because
it is changed for another, of a different size
and different number of teeth, when a change in
the velocity of wheels 2 and 3 is to be made. To allow a greater or smaller pinion to
be applied at 1, the wheel 2 is mounted upon a stud k, which is movable in a slot concentric
with the axis of the wheel 3. This slot is a branch from the cross bar N. The
smaller the change-pinion is, the nearer will the stud k approach to the vertical line
joining the centres of wheels 1 and 3; and the more slowly will the plate wheel 2 be
driven. To the spur wheel 3, a bevel wheel 4, is fixed, with which the other also
revolves loose upon a stud. The bevel wheel 5, upon the shaft l, is driven by the bevel
wheel 4; and it communicates motion, by the bevel wheels 6 and 7, to each of the
horizontal shafts G, G, extending along the upper and under tiers of the machine. At
the left-hand side of the top part of fig. 988. the two wheels 6 and 7 are omitted, on
purpose to show the bearings of the shaft G, as also the slot-bearings for carrying the
shafts or skewers of the bobbins.
[1113]
If it be desired to communicate twist in the opposite direction to that which would
be given by the actual arrangement of the wheels, it is necessary merely to transpose
the carrier wheel 2, from its present position on the right hand of pinion 1, to the
left of it, and to drive the tin cylinder by a crossed or close strap, instead of a straight
or open one.
The traverse motion of the guide is given here in a similar way to that of the engine,
(fig. 975.) Near one of the middle or cross-frames of the machine (see fig. 990.) the
wheel f, in geer with a spur wheel h, upon one of the block-shafts, drives also a spur
wheel m, that revolves upon a stud, to which wheel is fixed a bevel wheel n, in geer
with the bevel wheel o. To wheel o, the same mechanism is attached as was described
under figs. 979. and 980., and which is here marked with the same letters.
To the crank-knob r, fig. 990., a rod x, is attached, which moves or traverses the guide
bar belonging to that part of
the machine; to each machine
one such apparatus is
fitted. In figs. 992. and 993.
another mode of traversing
the guide bar is shown, which
is generally used for the
coarser qualities of silk.
Near to one of the middle
frames, one of the wheels f,
in geer with the spur wheel
m, and the bevel wheel n,
both revolving on one stud,
gives motion also to the
wheel o, fixed upon a shaft
a′, at whose other end the
elliptical wheel b′ is fixed, which drives a second elliptical wheel c′, in such a way that
the larger diameter of the one plays in geer with the smaller diameter of the other; the
teeth being so cut as to take into each other in all positions. The crank-piece d′ is screwed
upon the face of the wheel c′, at such a distance from its centre
as may be necessary to give the desired length of traverse motion
to the guide bar for laying the silk spirally upon the blocks.
The purpose of the elliptical wheel is to modify the simple crank
motion, which would wind on more silk at the ends of the bobbins
than in their middle, and to effect an equality of winding-on
over the whole surface of the blocks. In fig. 993. the elliptical wheels are shown in
front, to illustrate their mode of operating upon each other. Fig. 994. is a block filled
by the motion of
the eccentric, fig.
900.; and fig.
995. is a block
filled by the elliptical
mechanism.
As the length of
the motions of the
bar in the latter
construction remains
the same during the whole operation, the silk, as it is wound on the blocks, will
slide over the edges, and thereby produce the flat ends of the barrel in fig. 995. The
conical ends of the block (fig. 994.) are produced by the continually
shortened motions of the guide bar, as the stud approaches,
in its sun-and-planet rotation, nearer to the general
centre.
Figs. 996, 997. are two different views of the differential
mechanism described under fig. 990.
The bent wire x, fig. 990., is called the guider iron. It is
attached at one end to the pivot of the sun-and-planet wheel-work
t, s, o, and at
the other to the
guide bar f, f,
fig. 989. The
silk threads pass
through the guides, as already explained. By the motion communicated to the guide
bar (guider), the diamond pattern is produced, as shown in fig. 994.
[1114]
THE SILK AUTOMATIC REEL.
In this machine, the silk is unwound from the blocks of the throwing-mill, and
formed into hanks for the market. The blocks being of a large size, would be productive
of much friction, if made to revolve upon skewers thrust through them, and would cause
frequent breakage of the silk. They are, therefore, set with their axes upright upon a
board, and the silk is drawn from their surface, just as the weft is from a cop in the
shuttle. On this account the previous winding-on must be executed in a very regular
manner; and preferably as represented in fig. 994.
Fig. 998. is a front view of the reel; little more than one-half of it being shown.
Fig. 999. is an end view. Here the steam-pulleys are omitted, for fear of obstructing the
view of the more essential parts. A, A, are the two end framings, connected by mahogany
stretchers, which form the table B, for receiving the bobbins C, C, which are sometimes
weighted at top with a lump of lead, to prevent their tumbling. D is the reel, consisting
of four long laths of wood, which are fixed upon iron frames, attached to an octagonal
wooden shaft. The arm which sustains one of these laths is capable of being bent
inwards, by loosening a tightening hook, so as to permit the hanks, when finished, to be
taken off, as in every common reel.
The machine consists of two equal parts, coupled together at a, to facilitate the
removal of the silk from either half of the reel; the attendant first lifting the one part,
and then the other. E is the guide bar, which by a traverse motion causes the silk to
be wound on in a cross direction. b and c are the wire guides, and d are little levers
lying upon the cloth-covered guide bar E. The silk in its way from the block to the
reel, passes under these levers, by which it is cleaned from loose fibres.
On the other end of the shaft of the reel, the spur wheel 1 is fixed, which derives
motion from wheel 2, attached to the shaft of the steam-pulley F. Upon the same shaft
there is a bevel wheel 3, which impels the wheel 4 upon the shaft e; to whose end a
plate is attached, to which the crank f is screwed, in such a way as to give the proper
length of traverse motion to the guide bar E, connected to that crank or eccentric stud by
the jointed rod g. Upon the shaft of the steam-pulleys F, there is a worm or endless
screw, to the left of f, fig. 999., which works in a wheel 5; attached to the short upright
shaft h (fig. 998.). At the end of h, there is another worm, which works in a wheel 6;
at whose circumference there is a stud i, which strikes once at every revolution against
an arm attached to a bell, seen to the left of G; thus announcing to the reel-tenter that a
measured length of silk has been wound upon her reel. e is a rod or handle, by which
the fork l, with the strap, may be moved upon the fast or loose pulley, so as to set
on or arrest the motion at pleasure.
Throwsters submit their silk to scouring and steaming processes. They soak the[1115]
hanks, as imported, in lukewarm soap-water in a tub; but the bobbins of the twisted
single silk from the spinning mill are enclosed within a wooden chest, and exposed to
the opening action of steam for about ten minutes.
They are then immersed in a cistern of warm water,
from which they are transferred to the doubling
frame.
The wages of the workpeople in the silk-throwing
mills of Italy are about one half of their wages
in Manchester; but this difference is much more
than counterbalanced by the protecting duty of
2s. 10d. a pound upon thrown silk, and the superior
machinery of our mills. In 1832, there was
a power equal to 342 horses engaged in the silk-throwing
mills of Manchester; and of about 100
in the mills of Derby. The power employed in
the other silk mills of England and Scotland has
not been recorded.
There is a peculiar kind of silk called marabout,
containing generally three threads, made from the
white Novi raw silk. From its whiteness, it takes
the most lively and delicate colours without the
discharge of its gum. After being made into tram
by the single twist upon the spinning mill, it is
reeled into hanks, and sent to the dyer without
further preparation. After being dyed, the throwster
re-winds and re-twists it upon the spinning
mill, in order to give it the whipcord hardness
which constitutes the peculiar feature of marabout.
The cost of the raw Novi silk is 19s. 6d. a pound;
of throwing it into tram, 2s. 6d.; of dyeing, 2s.;
of re-winding and re-twisting, after it has been dyed, about 5s.; of waste, 2s., or 10 per
cent.; the total of which sum is 31s.; being the price of one pound of marabout in
1832.
An Estimate of the Annual Quantities of Silk produced or exported from the several
Countries in the World, exhibiting also the Countries to which exported.
Countries whence
exported. |
Quantities. |
Countries to
which exported. |
Quanti-
ties. |
| Italy exports |
34,000 |
bales |
of |
225 |
|
small lbs. |
|
Bales. |
| France produces |
10,500 |
|
- |
|
73 |
1⁄8 |
kils., or |
|
|
- |
England |
28,000 |
| India and Bengal export |
9,500 |
|
128 |
1⁄2 |
Vienna lbs. |
France |
22,000 |
| Persia |
7,500 |
|
|
|
162 |
|
lbs. English |
|
Prussia |
7,600 |
| China |
4,000 |
|
|
Russia |
6,400 |
| Asia Minor |
3,500 |
|
|
Austria and Germany |
5,000 |
| Levant, Turkey, and Archipelago export |
3,500 |
|
|
Switzerland |
5,000 |
| Spain |
1,500 |
|
|
|
| Total |
74,000 |
bales. |
|
Total |
74,000 |
Note.—These estimates exclude the silk manufactured in Italy.
The declared value of the silk manufactures exported from the United Kingdom in
1836, was 917,822l.; and in 1837, only 494,569. The deficit in the last year was owing
to the commercial crisis in the United States; which country took, the preceding year,
our silk goods to the value of 524,301l.
SILKWORM GUT, for angling, is made as follows:—Select a number of the
best and largest silkworms, just when they are beginning to spin; which is known by
their refusing to eat, and having a fine silk thread hanging from their mouths.
Immerse them in strong vinegar, and cover them closely for twelve hours, if the
weather be warm, but two or three hours longer, if it be cool. When taken out, and
pulled asunder, two transparent guts will be observed, of a yellow green colour, as thick
as a small straw, bent double. The rest of the entrails resembles boiled spinage, and
therefore can occasion no mistake as to the silk-gut. If this be soft, or break upon
stretching it, it is a proof that the worm has not been long enough under the influence
of the vinegar. When the gut is fit to draw out, the one end of it is to be dipped[1116]
into the vinegar, and the other end is to be stretched gently to the proper length.
When thus drawn out, it must be kept extended on a thin piece of board, by putting its
extremities into slits in the end of the
wood, or fastening them to pins, and then
exposed in the sun to dry. Thus genuine
silk-gut is made in Spain. From the
manner in which it is dried, the ends are
always more or less compressed or attenuated.[53]
Fig. 1000. a, is the silkworm;
b, the worm torn asunder; c, c, the guts;
d, d, a board slit at the ends, with the gut
to dry; f, f, a board with wooden pegs,
for the same purpose.
SILVER (Argent, Fr.; Silber, Germ.;)
was formerly called a perfect metal, because
heat alone revived its oxide, and because it
could pass unchanged through fiery trials,
which apparently destroyed most other
metals. The distinctions, perfect, imperfect,
and noble, are now justly rejected.
The bodies of this class are all equal in metallic nature, each being endowed merely
with different relations to other forms of matter, which serve to characterize it, and to
give it a peculiar value.
When pure and planished, silver is the brightest of the metals. Its specific gravity
in the ingot is 10·47; but, when condensed under the hammer or in the coining press, it
becomes 10·6. It melts at a bright red heat, a temperature estimated by some as equal to
1280° Fahr., and by others to 22° Wedgewood. It is exceedingly malleable and ductile;
affording leaves not more than 1⁄100000 of an inch thick, and wire far finer than a human
hair.
By Sickingen’s experiments, its tenacity is, to that of gold and platinum, as the numbers
19, 15, and 261⁄4; so that it has an intermediate strength between these two metals.
Pure atmospheric air does not affect silver, but that of houses impregnated with sulphuretted
hydrogen, soon tarnishes it with a film of brown sulphuret. It is distinguished
chemically from gold and platinum by its ready solubility in nitric acid, and from almost
all other metals, by its saline solutions affording a curdy precipitate with a most minute
quantity of sea salt, or any soluble chloride.
Silver occurs under many forms in nature:—
1. Native silver, possesses the greater part of the above properties; yet, on account of
its being more or less alloyed with other metals, it differs a little in malleability,
lustre, density, &c. It sometimes occurs crystallized in wedge-form octahedrons, in
cubes, and cubo-octahedrons. At other times it is found in dendritic shapes, or arborescences,
resulting from minute crystals implanted upon each other. But more usually it
presents itself in small grains without determinable form, or in amorphous masses of
various magnitude.
The gangues (mineral matrices) of native silver are so numerous, that it may be said
to occur in all kinds of rocks. At one time it appears as if filtered into their fissures,
at another as having vegetated on their surface, and at a third, as if impasted in their
substance. Such varieties are met with principally in the mines of Peru.
The native metal is found in almost all the silver mines now worked; but especially
in that of Kongsberg in Norway, in carbonate and fluate of lime, &c.; at Schlangenberg
in Siberia, in a sulphate of barytes; at Allémont, in a ferruginous clay, &c. In
the article Mines, I have mentioned several large masses of native silver that have been
discovered in various localities.
The metals most usually associated with silver in the native alloy, are gold, copper,
arsenic, and iron. At Andreasberg and Guadalcanal it is alloyed with about 5 per cent.
of arsenic. The auriferous native silver is the rarest; it has a brass-yellow colour.
2. Antimonial silver.—This rare ore is yellowish-blue; destitute of malleability; even
very brittle; spec. grav. 9·5. It melts before the blowpipe, and affords white fumes of
oxide of antimony; being readily distinguished from arsenical iron, and arsenical cobalt,
by its lamellar fracture. It consists of from 76 to 84 of silver, and from 24 to 16
of antimony.
3. Mixed antimonial silver.—At the blowpipe it emits a strong garlic smell. Its constituents
are, silver 16, iron 44, arsenic 35, antimony 4. It occurs at Andreasberg.
4. Sulphuret of silver.—This is an opaque substance, of a dark-gray or leaden hue;
slightly malleable, and easily cut with a knife, when it betrays a metallic lustre. The
silver is easily separated by the blowpipe. It consist of, 13 of sulphur to 89 of silver,[1117]
by experiment; 13 to 87 are the theoretic proportions. Its spec. grav. is 6·9. It occurs
crystallized in most silver mines, but especially in those of Freyberg, Joachimsthal in
Bohemia, Schemnitz in Hungary, and Mexico.
5. Red sulphuret of silver; silver glance.—Its spec. grav. is 5·7. It contains from 84
to 86 of silver.
6. Sulphuretted silver, with bismuth.—Its constituents are, lead 35, bismuth 27, silver
15, sulphur 16, with a little iron and copper. It is rare.
7. Antimoniated sulphuret of silver, the red silver of many mineralogists, is an ore
remarkable for its lustre, colour, and the variety of its forms. It is friable, easily
scraped by the knife, and affords a powder of a lively crimson red. Its colour in mass,
is brilliant red, dark red, or even metallic reddish-black. It crystallizes in a variety of
forms. Its constituents are,—silver from 56 to 62; antimony from 16 to 20; sulphur
from 11 to 14; and oxygen from 8 to 10. The antimony being in the state of a purple
oxide in this ore, is reckoned to be its colouring principle. It is found in almost all
silver mines; but principally in those of Freyberg, Sainte-Marie-aux-Mines, and Guadalcanal.
8. Black sulphuret of silver; is blackish, brittle, cellular, affording globules of silver
at the blowpipe. It is found only in certain mines, at Allémont, Freyberg; more
abundantly in the silver mines of Peru and Mexico. The Spaniards call it negrillo.
9. Chloride of silver, or horn silver.—In consequence of its semi-transparent aspect, its
yellowish or greenish colour, and such softness that it may be cut with the nail, this ore
has been compared to horn, and may be easily recognised. It melts at the flame of a
candle, and may be reduced when heated along with iron or black flux, which are
distinctive characters. It is seldom crystallized; but occurs chiefly in irregular forms,
sometimes covering the native silver as with a thick crust, as in Peru and Mexico. Its
density is only 4·74.
Chloride of silver sometimes contains 60 or 70 per cent. of clay; and is then called
butter-milk ore, by the German miners. The blowpipe causes globules of silver to
sweat out of it. This ore is rather rare. It occurs in the mines of Potosi, of Annaberg,
Freyberg, Allémont, Schlangenberg, in Siberia, &c.
10. Carbonate of silver, a species little known, has been found hitherto only in the
mine of S. Wenceslas, near Wolfache.
Table of the Quantities of Silver brought into the Market every year, on an average,
from 1790 to 1802.
| Old Continent. |
Lbs.
Avoird. |
New Continent. |
Lbs.
Avoird. |
| ASIA. |
|
|
|
| Siberia |
38,500 |
Central America |
1,320,000 |
| EUROPE. |
|
South America |
605,000 |
| Hungary |
44,000 |
|
|
| Austrian States |
11,000 |
|
|
| Hartz and Hessia |
11,000 |
|
|
| Saxony |
22,000 |
|
|
| Norway |
22,000 |
|
|
| Sweden |
|
- |
11,000 |
|
|
| France |
|
|
| Spain |
|
|
| Total of the Old Continent |
159,500 |
Total of the New Continent |
1,925,000 |
Thus the New Continent furnished twelve times more silver than the Old. For
more detailed statistics of silver, see the end of the article.
The following is Mr. Ward’s description of the treatment of silver ores in Mexico:—
“After returning from San Augustin,” says he, “I passed the whole of the afternoon
at the hacienda (metallurgic works) of Salgado, in which the ores of the Valenciana
mine are reduced. The hacienda, of which a representation is given below, fig. 1001.
contains forty-two crushing-mills, called arrastres, and thirty-six stampers. The ore,
on being extracted from the mine, is placed in the hands of the pepenadores, men and
women, who break all the larger pieces with hammers, and after rejecting those in
which no metallic particles are contained, divide the rest into three classes” (inferior,
middling, and rich). “These are submitted to the action of the morteros (stamps),
one of which, of eight stampers, is capable of reducing to powder ten cargas of ore (each
of 350 lbs.) in twenty-four hours. This powder not being thought sufficiently fine for
the quicksilver to act upon with proper effect, it is transferred from the morteros to the
arrastres (crushing-mills, see wood-cut), in which water is used. Each of these
reduces to a fine impalpable metalliferous mud, six quintals (600 lbs.) of powder in[1118]
24 hours. At Guanajuato, where water-power cannot be obtained, the arrastres
are worked by mules (see fig. 1001.), which are kept constantly in motion at a slow
pace, and are changed every 6 hours. The grinding-stones, as well as the sides and
bottom of the mill itself, are composed of granite; four blocks of which revolve in
each crushing-mill, attached to cross-bars of wood. This part of the operation is
thought of great importance, for it is upon the perfection of the grinding that the
saving of the quicksilver is supposed in a great measure to depend, in the subsequent
amalgamation. The grinding is performed usually in a covered shed or gallery
which in a large hacienda, like Salgado, from the number of arrastres at work at the
same time, is necessarily of considerable extent.”
1001 The Gallera of the Hacienda of Salgado

Fig. 1002. represents the rude grinding apparatus used at the lavaderos, or gold washings,
in Chile. The streamlet of water conveyed to the hut of the gold washer, is received upon
a large rude stone, whose flat surface
has been hollowed out into a
shallow basin, and in the same
manner into 3 or 4 others in succession;
the auriferous particles
are thus allowed to deposit themselves
in these receptacles, while
the lighter earthy atoms, still
suspended, are carried off by the
running water. The gold thus collected is mixed with a quantity of ferruginous black
sand and stony matter, which requires the process of trituration, effected by the very rude
and simple trapiche shown in the figure; consisting of two stones, the under one being
about three feet in diameter, and slightly concave. The upper stone is a large
spherical boulder of syenitic granite, about two feet in diameter, having on its upper
part two iron plugs fixed oppositely, to which is secured, by lashings of hide, a transverse
horizontal pole of canela (cinnamon) wood, about 10 feet long; two men seated on
the extremities of this lever, work it up and down alternately, so as to give to the stone a
rolling motion, which is sufficient to crush and grind the materials placed beneath it.
The washings thus ground, are subjected to the action of running water, upon inclined
planes formed of skins, by which process the siliceous particles are carried off, while a
portion of the ferruginous matter, mixed with the heavier grains of gold, is extracted by a
loadstone; it is again washed, till nothing but pure gold-dust remain. The whole process
is managed with much dexterity; and if there were much gold to be separated, it[1119]
would afford very profitable employment; but generally the small quantity collected is
sufficient only to afford subsistence to a few miserable families.
The trapiche, ingenio, or mill, for grinding the ores of silver, is a very simple piece of
mechanism. A place is chosen where a small current of water, whose section will
present a surface of six inches diameter, can be brought to a spot where it can fall
perpendicularly ten or twelve feet; at this place a well is built of this depth, about
6 feet in diameter; in its centre is fixed an upright shaft, upon a central brass pin; it
is confined above by a wooden collar. A little above its foot, the shaft has a small
wheel affixed to it, round which are fixed a number of radiating spokes, shaped at the
end somewhat like cups, and forming altogether a horizontal wheel, four feet in diameter.
Upon the slanting edges of the cups, the water is made to strike with the force it has
acquired in falling down a nearly perpendicular trough, scooped out of the solid trunk of
a tree. This impression makes the wheel turn with a quick rotatory motion. The upright
axis rises about 6 feet above the top of the well, at about half which height is
inserted a small horizontal arm, four feet long, which serves as an axle to a ponderous
mill-stone of granite, of from four to six feet diameter, which is made to roll on its edge
in a circular trough, sometimes made of the same material, and sometimes of hard wood.
The weight of this quickly rolling stone effects the pulverization of the ore. In some
cases, it is taken out in the dry state, and sifted; but more generally the separation of
the finely ground particles is accomplished by the action of running water. For this
purpose a small stream is made to trickle into the circular trough, by which the
pounded ore is worked up into a muddy consistence, and the finer particles flow off with
the excess of water, through a notch cut in the margin of the trough. This fine matter
is received in little pools, where the pounded ore is left to settle; and the clear water
being run off, the powder is removed
from the bottom, and carried
to the place of amalgamation.
The ingenios, or stamping-mills,
are driven by a small breast water-wheel,
of five feet diameter, and
one foot broad. Fig. 1003. will
give a sufficient idea of their construction.
The long horizontal
shaft, fixed on the axis of the wheel,
is furnished with 5 or 6 cams placed
at different situations round the
shaft, so as to act in succession on
the projecting teeth of the upright
rods or pestles. Each of these
weighs 200 pounds, and works in a
corresponding oblong mortar of
stone or wood.
The patio, or amalgamation floor, fig. 1004., is a large flat space, open to the sky,
312 feet in length, by 236 in breadth, and securely surrounded by strong walls. It is
paved with large unhewn
blocks of porphyry,
and is capable of
containing 24 tortas, or
flat circular collections
of lama, of about 50 feet
diameter, and 7 inches
deep, when the patio is
not filled, (but of somewhat
smaller dimensions
when nearly so,) ranged
in 4 rows, and numbered
from the left-hand corner.
At one end a small space is generally set apart for the assays, which are made
each on one monton.
The following description of Mexican amalgamation is given by Captain Lyon.
A torta of Zacatecas contains 60 montons of 20 quintals each, and is thus formed:—In
the first instance, a square space, of the requisite size for a torta, is marked out, and
enclosed by a number of rough planks, which are propped in their places on the patio
floor by large stones, and dried horse-dung and dust are piled round their edges to prevent
the escape of the lama. A heap of saltierra (salt mixed with earthy impurities) is
then piled in the centre, in the proportion of 2 fanegas (each = 1·6 English bushels) and
a half to the monton, = 150 for the torta. After this, the lama, or ore ground into a[1120]
fine paste, is poured in. When the last or 60th monton is delivered, the saltierra is
shovelled down and well mixed with the lama, by treading it with horses, and turning it
with shovels; after which the preparation is left at rest for the remainder of the day.
On the following day comes the el incorporo. After about one hour’s treading by
horses, the magistral or roasted and pulverized copper ore is mixed with the lama, (the
repaso or treading-mill still continuing,) in summer in the proportion of 15 cargas of 12
arrobas (25 lbs. each) to the torta, if the ore be of 6 marcs to the monton, and in
winter in only half the quantity. For it is a singular fact, that in summer the mixture
cools, and requires more warmth; while in winter it acquires of itself additional heat.
With poorer ores, as for instance those of 4 marcs to the monton, 12 cargas are applied
in summer, and 6 in winter. From November to February, lime is also occasionally
used to cool the lama, in the proportion of about a peck per monton.
The repaso, or treading out, is continued by six horses, which are guided by one man,
who stands in the lama, and directs them all by holding all their long halters. This
operation is much more effectual in a morning than an evening, and occupies about five
or six hours. When the magistral is well mixed, the quicksilver is applied, by being
sprinkled through pieces of coarse cloth doubled up like a bag, so that it spurts out in
very minute particles. The second treading of the horses then follows; after which the
whole mixture is turned over by six men with wooden shovels, who perform the
operation in an hour. The torta is then smoothed and left at rest for one entire day,
to allow the incorporation to take place. It undergoes the turning by shovels and
treading by horses every other day, until the amalgamator ascertains that the first admixture
of quicksilver is found to be all taken up by the silver; and this he does by
vanning or washing a small quantity of the torta in a little bowl. A new supply is then
added, and when this has done its duty, another is applied to catch any stray particles
of silver. On the same day, after a good repaso, the torta is removed on hand-barrows
by the labourers, to the lavaderos, in order that it may receive its final cleansing. The
general method of proportioning the quicksilver to the tortas, is by allowing that every
marco of silver which is promised by trial of the ores as the probable produce of a
monton, will require in the whole process 4 lbs.
In metals of five to six marcs and a half per monton (of the average richness of Zacatecas),
16 lbs. of quicksilver were incorporated for every monton, = 900 lbs. for the torta.
On the day of the second addition, the proportion is 5 lbs. the monton; and when the
torta is ready to receive the last dose of quicksilver, it is applied at the rate of 7 lbs.
the monton, = 420 lbs.; making a total of 1620 lbs. of quicksilver. With poorer ores,
less quicksilver and less magistral are required.
The usual time for the completion of the process of amalgamation, is from 12 to 15
days in the summer, and 20 to 25 in the winter. This is less than a third of the time
taken at some other mines in Mexico. This rapidity is owing to the tortas being spread
very flat, and receiving thereby the stronger influence of the sun. In the Mexican mines,
only one monton is commonly mixed at a time; and the lama is then piled in a small
conical heap or monton.
Lavadero, or washing vat.—Here the prepared tortas are washed, in order to carry off
the earthy matters, and favour the deposition of the amalgam at the bottom. Each vat
is about 8 feet deep, and 9 in diameter; and solidly built in masonry.
A large horizontal wheel, worked by mules, drives a vertical one, which turns a horizontal
wheel fitted round a perpendicular wooden shaft, revolving upon an iron pivot at
the bottom of the vat. To the lower end of this shaft, four cross-beams are fitted, from
which long wooden teeth rise to the height of 5 feet. Their motion through the water
being rapid, keeps all the lighter particles afloat, while the heavier sink to the bottom.
The large wheel is worked by four mules, two at each extremity of the cross-beam.
Water is supplied from an elevated tank. It requires 12 hours’ work of one tub to wash
a torta. Eight porters are employed in carrying the prepared lama of the torta in hand-barrows
to the vats. The earthy matter receives a second washing.
The amalgam is carried in bowls into the azogueria, where it is subjected to straining
through the strong canvas bottom of a leather bag. The hard mass left in the bag is
moulded into wedge-shaped masses of
30 lbs., which are arranged in the burning-house,
(fig. 1005.), to the number
of 11, upon a solid copper stand, called
baso, having a round hole in its centre.
Over this row of wedges several others
are built; and the whole pile is called
pina. Each circular range is firmly
bound round with a rope. The base is
placed over a pipe which leads to a small tank of water for condensing the quicksilver;
a cylindrical space being left in the middle of the pina, to give free egress to the mercurial
vapours.
[1121]
A large bell-shaped cover, called capellina, is now hoisted up, and carefully lowered
over the pina, by means of pulleys. A strong lute of ashes, saltierra, and lama is applied
to its lower edge, and made to fit very closely to the plate on which the base stands.
A wall of fire-bricks is then built loosely round the capellina, and this space is filled
with burning charcoal, which is thrice replenished, to keep it burning all night. After
the heat has been applied 20 hours, the bricks and ashes are removed, the luting broken,
and the capellina hoisted up. The burned silver is then found in a hard mass, which is
broken up, weighed, and carried to the casting-house, to be formed into bars of about
1080 ounces each. The loss of silver in burning, is about 5 ounces to each bar (barra),
and the loss of quicksilver, from 21⁄2 upon the good metals, to 9 upon the coarse.
Molina told Mr. Miers, that the produce of the galena ores of Uspaltata did not
average more than 2 marcs per caxon of 5000 lbs., which is an excessively poor ore.
The argentiferous galena ores of Cumberland afford 11 marcs per caxon; while the
average produce of the Potosi silver ores is only 5 or 6 marcs in the same quantity.
These comparisons afford the clearest evidence that the English mode of smelting can never
be brought into competition with the process of amalgamation as practised in America.
Humboldt, Gay Lussac, Boussingault, Karsten, and several other chemists of note,
have offered solutions of the amalgamation enigma of Mexico and Peru. The following
seems to be the most probable rationale of the successive steps of the process:—
The addition of the magistral (powder of the roasted copper pyrites), is not for the
purpose of disengaging muriatic acid from the sea salt (saltierra), as has been supposed,
since nothing of the kind actually takes place; but, by reciprocal or compound affinity,
it serves to form chloride of copper, and chloride of iron, upon the one hand, and sulphate
of soda, upon the other. Were sulphuric acid to be used instead of the magistral, as
certain novices have prescribed, it would certainly prove injurious, by causing muriatic
acid to exhale. Since the ores contain only at times oxide of silver, but always a great
abundance of oxide of iron, the acid would carry off both partly, but leave the chloride of
silver in a freer state. A magistral, such as sulphate of iron, which is not in a condition
to generate the chlorides, will not suit the present purpose; only such metallic sulphates
are useful as are ready to be transformed into chlorides by the saltierra. This is peculiarly
the case with sulphate of copper. Its deuto-chloride gives up chlorine to the
silver, becomes in consequence a protochloride, while the chloride of silver, thus formed,
is revived, and amalgamated with the quicksilver present, by electro-chemical agency
which is excited by the saline menstruum; just as the voltaic pile of copper and silver is
rendered active by a solution of sea salt. A portion of chloride of mercury will be simultaneously
formed, to be decomposed in its turn by the sulphate of silver resulting from
the mutual action of the acidified pyrites, and the silver or its oxide in the ore. An
addition of quicklime counteracts the injurious effect of too much magistral, by decomposing
the resulting sulphate of copper. Quicksilver being an excellent conductor of
heat, when introduced in too great quantities, is apt to cool the mass too much, and
thereby enfeebles the operation of the deuto-chloride of copper upon the silver.
There is a method of extracting silver from its ores by what is called imbibition. This
is exceedingly simple, consisting in depriving, as far as possible, the silver of its gangue,
then melting it with about its own weight of lead. The alloy thus procured, contains
from 30 to 35 per cent. of silver, which is separated by cupellation on the great scale, as
described under ores of Lead. In this way the silver is obtained at Kongsberg in Norway.
The amalgamation works at Halsbrücke, near Freyberg, for the treatment of silver
ores by mercury, have been justly admired as a model of arrangement, convenience, and
regularity; and I shall conclude this subject with a sketch of their general distribution.
Fig. 1006. presents a vertical section of this great usine or hüttenwerk, subdivided into
four main departments. The first, A, B, is devoted to the preparation and roasting of the
matters intended for amalgamation. The second, B, C, is occupied with two successive[1122]
siftings and the milling. The third, C, D, includes the amalgamation apartment above,
and the wash-house of the residuums below. And in the fourth, D, E, the distilling
apparatus is placed, where the amalgam is finally delivered.
Thus, from one extremity of this building to the other, the workshops follow in the
order of the processes; and the whole, over a length of 180 feet, seems to be a natural
laboratory, through which the materials pass, as it were of themselves, from their crude
to their refined condition; so skilfully economized and methodical are the labours of
the workmen; such are the regularity, precision, concert, and facility, which pervade
this long series of combinations, carriages, movements, and metamorphoses of matter.
Here we distinguish the following objects:—
1. In division A, B; a, a, is the magazine of salt; b, b, is the hall of preparation of the
ores; on the floor of which they are sorted, interstratified, and mixed up with salt;
c, c, are the roasting furnaces; in each of which we see, 1, the fireplace; 2, 3, the
reverberatory hearth, divided into two portions, one a little higher than the other, and
more distant from the fireplace, called the drier. The materials to be calcined fall into
it, through a chimney 6. The other part 2, of the hearth is the calcining area. Above
the furnace are chambers of sublimation 4, 5, for condensing some volatile matters
which escape by the opening 7. e is the main chimney.
2. In the division B, C, we have d, the floor for the coarse sifting; beneath, that for
the fine sieves; from which the matters fall into the hopper, whence they pass down to
g, the mill-house, in which they are ground to flour, exactly as in a corn-mill, and are
afterwards boulted through sieves, p, f, is the wheel machinery of the mill.
3. The compartment C, D, is the amalgamation work, properly speaking, where the
casks are seen in their places. The washing of the residuums is effected in the shop
l, below. k, k, is the compartment of revolving casks.
4. In the division D, E, the distillation process is carried on. There are four similar
furnaces, represented in different states, for the sake of illustration. The wooden
drawer is seen below, supporting the cast-iron basin, in which the tripod with its
candelabra for bearing the amalgam saucers is placed. q is a store chamber.
At B, are placed the pulleys and windlass for raising the roasted ore, to be sifted and
ground; as also for raising the milled flour, to be transported to the amalgamation casks.
At D, the crane stands for raising the iron bells that cover the amalgamation candelabra.
Details of the Amalgamation Process, as practised at Halsbrücke.—All ores which
contain more than 7 lbs. of lead, or 1 lb. of copper, per cent., are excluded from this
reviving operation (anquickverfahren); because the lead would render the amalgam very
impure, and the copper would be wasted. They are sorted for the amalgamation, in
such a way that the mixture of the poorer and richer ores may contain 71⁄2, or, at most,
8 loths (of 1⁄2 oz. each) of silver per 100 lbs. The most usual constituents of the ores
are, sulphur, silver, antimonial silver (speissglanzsilber), bismuth, sulphurets of arsenic,
of copper, iron, lead (nickel, cobalt), zinc, with several earthy minerals. It is essential
that the ores to be amalgamated shall contain a certain proportion of sulphur, in order
that they may decompose enough of sea salt in the roasting to disengage as much
chlorine as to convert all the silver present into a chloride. With this view, ores poor
in sulphur are mixed with those that are richer, to make up a determinate average.
The ore-post is laid upon the bed-floor, in a rectangular heap, about 17 ells long, and
41⁄2 ells broad (13 yards and 31⁄2); and upon that layer the requisite quantity of salt is
let down from the floor above, through a wooden tunnel; 40 cwts. of salt being allotted
to 400 cwts. of ore. The heap being made up with alternate strata to the desired
magnitude, must be then well mixed, and formed into small bings, called roast-posts,
weighing each from 31⁄2 to 41⁄2 cwts. The annual consumption of salt at Halsbrücke is
6000 cwts.; it is supplied by the Prussian salt-works.
Roasting of the Amalgamation Ores.—The furnaces appropriated to the roasting of
the ore-posts are of the reverberatory class, provided with soot chambers. They are
built up alongside of the bed-floor, and connected with it by a brick tunnel. The
prepared ground ore (erzmehl) is spread out upon the hearth, and dried with incessant
turning over; then the fire is raised so as to kindle the sulphur, and keep the ore redhot
for one or two hours; during which time, dense white-gray vapours of arsenic, antimony,
and water, are exhaled. The desulphuration next begins, with the appearance of a blue
flame. This continues for three hours, during which the ignition is kept up; and the
mass is diligently turned over, in order to present new surfaces, and to prevent any
caking. Whenever sulphurous acid ceases to be formed, the finishing calcination is to
be commenced with increased firing; the object being now to decompose the sea salt by
means of the metallic sulphates that have been generated, to convert them into chlorides,
with the simultaneous production of sulphate of soda. The stirring is to be continued
till the proofs taken from the hearth no longer betray the smell of sulphurous, but only
of muriatic acid gas. This roasting stage lasts commonly three quarters of an hour,
13 or 14 furnaces are worked at the same time at Halsbrücke; and each turns out in a[1123]
week 5 tons upon an average. Out of the nicht chambers or soot vaults of the furnaces,
from 96 to 100 cwts. of ore-dust are obtained, containing 32 marcs (16 lbs.) of silver.
This dust is to be treated like unroasted ore. The fuel of the first fire is pitcoal; of the
finishing one, fir-wood. Of the former 1151⁄2 cubic feet, and of the latter, 2941⁄4, are, upon
an average, consumed for every 100 cwts. of ore.
During the last roasting, the ore increases in bulk by one fourth, becomes in consequence
a lighter powder, and of a brown colour. When this process is completed, the ore
is raked out upon the stone pavement, allowed to cool, then screened in close sieve-boxes,
in order to separate the finer powder from the lumps. These are to be bruised, mixed
with sea salt, and subjected to another calcination. The finer powder alone is taken to
the millstones, of which there are 14 pairs in the establishment. The stones are of
granite, and make from 100 to 120 revolutions per minute. The roasted ore, after it has
passed through the boulter of the mill, must be as impalpable as the finest flour.
The Amalgamation.—This (the verquicken) is performed in 20 horizontal casks,
arranged in 4 rows, each turning upon a shaft which passes through its axis; and all
driven by the water-wheel shown in the middle of fig. 1006. The casks are 2 feet 10 inches
long, 2 feet 8 inches wide, inside measure, and are provided with iron ends. The staves
are 31⁄2 inches thick, and are bound together with iron hoops. They have a double bung-hole,
one formed within the other, secured by an iron plug fastened with screws.
They are filled by means of a wooden spout terminated by a canvas hose; through
which 10 cwts. of the boulted ore-flour (erzmehl) are introduced after 3 cwts. of water
have been poured in. To this mixture, from 3⁄4 to 7⁄8 of a cwt. of pieces of iron, 11⁄2 inch
square, and 3⁄8 thick, are added. When these pieces get dissolved, they are replaced by
others from time to time. The casks being two thirds full, are set to revolve for 11⁄2 or
2 hours, till the ore-powder and water become a uniform pap; when 5 cwts. of quicksilver
are poured into each of them. The casks being again made tight, are put in geer
with the driving machinery, and kept constantly revolving for 14 or 16 hours, at the
rate of 20 or 22 turns in the minute. During this time they are twice stopped and
opened, in order to see whether the pap be of the proper consistence; for if too thick,
the globules of quicksilver do not readily combine with the particles of ore; and if too
thin, they fall and rest at the bottom. In the first case, some water must be added;
in the second, some ore. During the rotation, the temperature rises, so that even in
winter it sometimes stands so high as 104° F.
The chemical changes which occur in the casks are the following:—The metallic
chlorides present in the roasted ore are decomposed by the iron, whence results muriate
of iron, whilst the deutochloride of copper is reduced partly to protochloride, and partly
to metallic copper, which throw down metallic silver. The mercury dissolves the
silver, copper, lead, antimony, into a complex amalgam. If the iron is not present in
sufficient quantity, or if it has not been worked with the ore long enough to convert
the copper deutochloride into a protochloride, previously to the addition of the mercury,
more or less of the last metal will be wasted by its conversion into protochloride
(calomel). The water holds in solution sulphate of soda, undecomposed sea salt, with
chlorides of iron, manganese, &c.
As soon as the revivification is complete, the casks must be filled with water, set to
revolve slowly (about 6 or 8 times in the minute), whereby in the course of an hour, or
an hour and a half at most, a great part of the amalgam will have collected at the
bottom; and in consequence of the dilution, the portion of horn silver held in solution
by the sea salt will fall down and be decomposed. Into the small plug in the centre
of the bung, a small tube with a stopcock is now to be inserted, to discharge the
amalgam into its appropriate chamber. The cock must be stopped whenever the
brown muddy residuum begins to flow. The main bung being then opened, the
remaining contents of the casks are emptied into the wash-tun, while the pieces of iron
are kept back. The residuary ore is found to be stripped of its silver within 5⁄32 or 7⁄40
of an ounce per cwt. The emptying of all the casks, and charging them again, takes 2
hours; and the whole process is finished within 18 or 20 hours; namely, 1 hour for
charging, 14 to 16 hours for amalgamating; 11⁄2 hour for diluting; 1 hour for emptying.
In 14 days, 3200 cwts. of ore are amalgamated. For working 100 cwts. of ore,
141⁄2 lbs. of iron, and 2 lbs. 121⁄2 ounces of mercury, are required; whence, for every pound
of silver obtained, 0·95 of an ounce of mercury are consumed.
Trials have been made to conduct the amalgamation process in iron casks, heated to
150° or 160° Fahrenheit, over a fire; but though the de-silvering was more complete, the
loss by mercury was so much greater as to more than counterbalance that advantage.
Treatment of the Amalgam.—It is first received in a moist canvas bag, through
which the thin uncombined quicksilver spontaneously passes. The bag is then tied up
and subjected to pressure. Out of 20 casks, from 3 to 31⁄2 cwts. of solid amalgam are thus
procured, which usually consist of 1 part of an alloy, containing silver of 12 or 13 loths
(in 16), and 6 parts of quicksilver. The foreign metals in that alloy are, copper, lead,[1124]
gold, antimony, cobalt, nickel, bismuth, zinc, arsenic, and iron. The filtered quicksilver
contains moreover 2 to 3 loths of silver in the cwt.
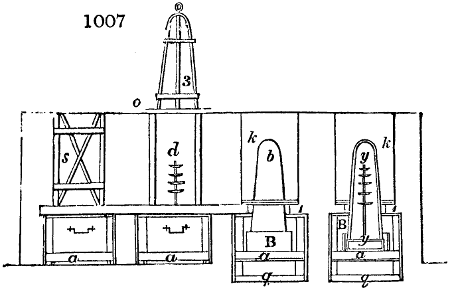
Fig. 1007. represents the apparatus for distilling the amalgam in the Halsbrücke
works, marked m in fig. 1006. a is the wooden drawer, sliding in grooves upon the
basis q; B is an open basin or box of cast iron, laid in the
wooden drawer; y is a kind of iron candelabra, supported upon
four feet, and set in the basin B; under d are five dishes or
plates, of wrought iron, with a hole in the centre of each, whereby
they are fitted upon the stem of the
candelabra, 3 inches apart, each plate
being successively smaller than the
one below it. 3 indicates a cast-iron
bell, furnished with a wrought-iron
frame and hook, for raising it by
means of a pulley and cord. s is a
sheet-iron door for closing the stove,
whenever the bell has been set in its
place.
The box a, and the basin B, above it, are filled with water, which must be continually
renewed, through a pipe in the side of the wooden box, so that the iron basin may be
kept always submersed and cool. The drawer a, being properly placed, and the plates
under d being charged with balls of amalgam (weighing altogether 3 cwts.), the bell 3
is to be let down into the water, as at y, and rested upon the lower part of the candelabra.
Upon the ledge 1, which defines the bottom of the fireplace, a circular plate of iron is
laid, having a hole in its middle for the bell to pass through. Upon this plate chips of
fir-wood are kindled, then the door s, which is lined with clay, is closed and luted
tight. The fuel is now placed in the vacant space k, round the upper part of the bell.
The fire must be fed in most gradually, first with turf, then with charcoal; whenever
the bell gets red, the mercury volatilizes, and condenses in globules into the bottom of
the basin B. At the end of 8 hours, should no more drops of mercury be heard to fall
into the water, the fire is stopped. When the bell has become cool, it is lifted off; the
plates are removed from the candelabra d; and this being taken out, the drawer a is
slid away from the furnace. The mercury is drained, dried, and sent again into the
amalgamation works. The silver is fused and refined by cupellation.
The solid amalgam which is distilled in the above apparatus, would be distilled more
profitably out of iron trays set in the mercurial retorts described and figured in pages
809, 810.
From 3 cwts. of amalgam, distilled under the bell, from 95 to 100 marcs (1⁄2 lbs.) of
teller silver (dish silver) are procured, containing from 10 to 131⁄2 parts of fine silver out
of 16; one-fifth part of the metal being copper. The teller silver is refined in quantities
of 160 or 170 marcs, in black-lead crucibles filled within two inches of their brims, and
submitted to brisk ignition. The molten mass exhales some vapours, and throws up a
liquid slag, which being skimmed off, the surface is to be strewed over with charcoal
powder, and covered with a lid. The heat having been briskly urged for a short time,
the charcoal is then removed along with any fresh slag that may have risen, in order to
observe whether the vapours have ceased. If not, fresh charcoal must be again applied,
the crucible must be covered, and the heat increased, till fumes are no longer produced,
and the surface of the silver becomes tranquil. Finally, the alloy, which contains a
little gold, and much copper, being now from 11 to 13 löthig (that is, holding from 11
to 13 parts of fine silver in 16 parts), is cast into iron moulds, in ingots of 60 marcs.
The loss of weight by evaporation and skimming of the slag amounts to 2 per cent.;
the loss in silver is quite inconsiderable.
The dust from the furnace (tiegelöfen) is collected in a large condensation chamber
of the chimney, and affords from 40 to 50 marcs of silver per cwt. The slags and old
crucibles are ground and sent to the small amalgamation mill.
The earthy residuum of the amalgamation casks being submitted to a second amalgamation,
affords out of 100 cwts. about 2 lbs. of coarse silver. This is first fused
along with three or four per cent. of a mixture of potashes and calcined quicksalz,
(impure sulphate of soda), and then refined. The supernatant liquor that is drawn out
of the tanks in which the contents of the casks are allowed to settle, consists chiefly of
sulphate of soda, along with some common salt, sulphates of iron and manganese, and a
little phosphate, arseniate, and fluate of soda. The earthy deposit contains from 1⁄4 to 9⁄32
of a loth of silver per cwt., but no economical method of extracting this small quantity
has yet been contrived.
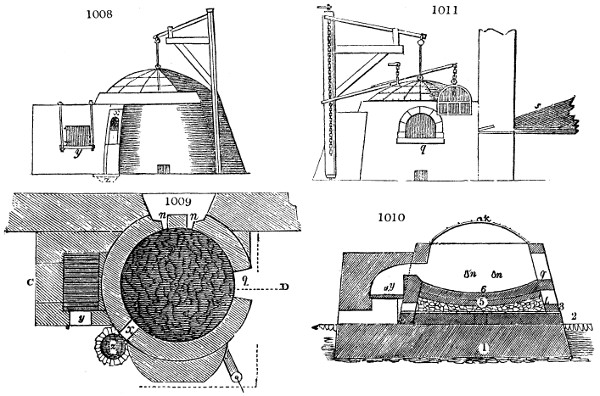
The argentiferous or rich lead is treated in Germany by the cupellation furnace
represented in figs. 1008, 1009, 1010, and 1011. These figures exhibit the cupellation
furnace of the principal smelting work in the Hartz, where the following parts must be[1125]
distinguished; (fig. 1010.) 1, masonry of the foundation; 2, flues for the escape of
moisture; 3, stone covers of the flues; 4, bed of hard rammed scoriæ; 5, bricks set on
edge, to form the permanent area of the furnace; 6, the sole, formed of wood ashes,
washed, dried, and beaten down; k, dome of iron plate, movable by a crane, and susceptible
of being lined two inches thick with loam; n, n, tuyères for two bellows s;
having valves suspended before their orifices to break and spread the blast; q, door for
introducing into the furnace the charge of lead, equal to 84 quintals at a time; s, fig.
1011., two bellows, like those of a smith’s forge; y, door of the fireplace, through which
billets of wood are thrown on the grate; x, small aperture or door, for giving issue to the
frothy scum of the cupellation, and
the litharge; z, basin of safety,
usually covered with a stone slab,
over which the litharge falls: in
case of accident the basin is laid
open to admit the rich lead.
The following is the mode of
conducting the cupellation. Before
putting the lead into the furnace,
a floor is made in it of ashes beat
carefully down (see 6, fig. 1010.);
and there is left in the centre of
this floor a circular space, somewhat lower than the rest of the hearth, where the silver
ought to gather at the end of the operation. The cupel is fully six feet in diameter.
In forming the floor of a cupel,
35 cubic feet of washed wood
ashes, usually got from the soap
works, are employed. The preparation
of the floor requires 21⁄2
hours’ work; and when it is completed,
and the movable dome of
iron plate has been lined with
loam, 84 quintals (cwt.) of lead
are laid on the floor, 42 quintals
being placed in the part of the
furnace farthest from the bellows,
and 42 near to the fire-bridge; to
these, scoriæ containing lead and
silver are added, in order to lose
nothing. The movable lid is now luted on the furnace, and heat is slowly applied in the
fireplace, by burning fagots of fir-wood, which is gradually raised. Section 1010. is in
the line C, D, of 1009.
At the end of three hours, the whole lead being melted, the instant is watched for
when no more ebullition can be perceived on the surface of the bath or melted metal;
then, but not sooner, the bellows are set a-playing on the surface at the rate of 4 or 5
strokes per minute, to favour the oxidizement.
In five hours, reckoned from the commencement of the process, the fire is smartly
raised; when a grayish froth (abstrich) is made to issue from the small aperture x of
the furnace. This is found to be a brittle mixture of oxidized metals and impurities.
The workman now glides the rake over the surface of the bath, so as to
draw the froth out of the furnace; and, as it issues, powdered charcoal is strewed upon
it, at the aperture x, to cause its coagulation. The froth skimming lasts for about an
hour and a half.
[1126]
After this time, the litharge begins to form, and it is also let off by the small opening
x; its issue being aided by a hook. In proportion as the floor of the furnace gets
impregnated with litharge, the workman digs in it a gutter for the escape of the liquid
litharge: it falls in front of the small aperture, and concretes in stalactitic forms.
By means of the two movable valves suspended before the tuyères n, n, (fig. 1010.)
the workman can direct the blast as he will over the surface of the metal. The wind
should be made to cause a slight curl on the liquid, so as to produce circular undulations,
and gradually propel a portion of the litharge generated, towards the edges of
the cupel, and allow this to retain its shape till the end of the operation. The stream
of air should drive the greater part of the litharge towards the small opening x, where
the workman deepens the outlet for it, in proportion as the level of the metal bath descends,
and the bottom of the floor rises by the apposition of the litharge formed. Litharge
is thus obtained during about 12 hours; after which period the cake of silver
begins to take shape in the centre of the cupel.
Towards the end of the operation, when no more than four additional quintals of
litharge can be looked for, and when it forms solely in the neighbourhood of the silver
cake in the middle of the floor, great care must be taken to set apart the latter portions,
because they contain silver. About this period, the fire is increased, and the workman
places before the little opening x a brick, to serve as a mound to the efflux of litharge.
The use of this brick is,—1, to hinder the escape of the silver in case of any accident;
for example, should an explosion take place in the furnace; 2, to reserve a magazine of
litharge, should that still circulating round the silver cake be suddenly absorbed by the
cupel, for in this dilemma the litharge must be raked back on the silver; 3, to prevent
the escape of the water that must be thrown on the silver at the end of the process.
When the argentiferous litharge, collected in the above small magazine, is to be
removed, it is let out in the form of a jet, by the dexterous use of the iron hook.
Lastly, after 20 hours, the silver cake is seen to be well formed, and nearly circular.
The moment for stopping the fire and the bellows is indicated by the sudden disappearance
of the coloured particles of oxide of lead, which, in the latter moments of
oxidation, undulate with extreme rapidity over the slightly convex surface of the silver
bath, moving from the centre to the circumference. The phenomenon of their total
disappearance is called the lightning, or fulguration. Whenever this occurs, the plate of
silver being perfectly clean, there is introduced into the furnace, by the door q, a
wooden spout, along which water, previously heated, is carefully poured on the silver.
The cupellation of 84 quintals of argentiferous lead takes in general 18 or 20 hours’
working. The promptitude of the operation depends on the degree of purity of the
leads employed, and on the address of the operator, with whom also lies the economy
of fuel. A good workman completes the cupellation of 84 quintals with 300 billets,
each equivalent to a cubic foot and eight-tenths of wood (Hartz measure); others consume
400 billets, or more. In general, the cupellation of 100 quintals of lead, executed
at the rate of 84 quintal charges, occasions a consumption of 790 cubic feet of resinous
wood billets.
The products of the charge are as follow:—
| 1. |
Silver, holding in 100 marcs, 7 marcs and 3 loths of alloy |
24 |
to |
30 |
marcs. |
| 2. |
Pure litharge, containing from 88 to 90 per cent. of lead |
50 |
- |
60 |
quintals. |
| 3. |
Impure litharge, holding a little silver |
2 |
- |
6 |
— |
| 4. |
Skimmings of the cupellation |
4 |
- |
8 |
— |
| 5. |
Floor of the furnace impregnated with litharge |
22 |
- |
30 |
— |
Note.—The marc is 7 oz. 2 dwts. 4 gr. English troy; and the loth is half an ounce.
16 loths make a marc. 100 pounds Cologne are equal to 103 pounds avoirdupois; and the
above quintal contains 116 Cologne pounds.
The loss of lead inevitable by this operation, is estimated at 4 parts in 100. It has
been diminished as much as possible in the Frankenscharn works of the Hartz, by leading
the smoke into long flues, where the lead fumes are condensed into a metallic soot.
The silver cake receives a final purification at the Mint, in a cupel on a smaller scale.
From numerous experiments in the great way, it has been found that not more than
100 quintals of lead can be profitably cupelled at one operation, however large the
furnace, and however powerful and multiplied the bellows and tuyères may be; for the
loss on either the lead or the silver, or on both, would be increased. In one attempt,
no less than 500 quintals were acted on, in a furnace with two fireplaces, and four
escapes for the litharge; but the silver remained disseminated through the lead, and
the lightning could not be brought on. The chief object in view was economy of fuel.
Reduction of the Litharge.—This is executed in a slag-hearth, with the aid of wood
charcoal.
Such is the train of operations by which the cupriferous galena schlich, or ground ore[1127]
is reduced, in the district of Clausthal, into lead, copper, and silver. The works of
Frankenscharn have a front fully 400 feet long.
Fig. 1012. exhibits the plan and elevation of these smelting-works, near Clausthal,
in the Hartz, for lead ores containing copper and silver, where about 84,000 cwts. of
schlich (each of 123 Cologne pounds) are treated every year. This quantity is the
produce of 30 distinct mines, as also of nearly as many stamp and preparation works.
All these different schlichs, which belong to so many different joint-stock companies,
are confounded and worked up together in the same series of metallurgic operations;
the resulting mixture being considered as one and the same ore belonging to a single
undertaking; but in virtue of the order which prevails in this royal establishment, the
rights of each of the companies, and consequently of each shareholder, are equitably
regulated. A vigorous control is exercised between the mines and the stamps, as
also between the stamps and the smelting-houses; while the cost of the metallurgic
operations is placed under the officers of the crown, and distributed, upon just principles,
among the several mines, according to the quantities of metal furnished by
each.
From these arrangements, the following important advantages flow:—
1. The poor ores may be smelted with profit, without putting the companies to
any risk or expense in the erection of new works; 2, by the mixture of many different
ores, the smelting and metallic product become more easy and abundant; 3, the train
of the operations is conducted with all the lights and resources of science; and 4, the
amount of metal brought into the market is not subject to such fluctuations as might
prove injurious to their sale.
The following is the series of operations:—
1, The fusion of the schlich (sludge); 2, the roasting of the mattes under a shed,
and their treatment by four successive re-meltings; 3, the treatment of the resulting
black copper; 4, the liquation; 5, the reliquation (ressuage); 6, the refining of the
copper; 7, the cupellation of the silver; 8, the reduction of the litharge into lead.
The 5th and 6th processes are carried on at the smelting works of Altenau.
The buildings are shown at A, B, C, and the impelling stream of water at D; the
upper figure being the elevation; the lower, the plan of the works.
a, is the melting furnace, with a cylinder bellows behind it; b, c, d, furnaces similar
to the preceding, with wooden bellows, such as fig. 1013; e, is a furnace for the same
purpose, with three tuyères,
and a cylinder bellows; f, the
large furnace of fusion, also
with three tuyères; g, a furnace
with seven tuyères, now seldom
used; h, low furnaces, like the
English slag-hearths (krummofen),
employed for working the
last mattes; k, slag-hearths for
reducing the litharge; m, the
area of the liquation; n, p, cupellation
furnaces.
x, y, a floor which separates[1128]
the principal smelting-house into two stories; the materials destined for charging the
furnaces being deposited in beds upon the upper floor, to which they are carried by
means of two inclined planes, terraced in front of the range of buildings.
Here 89,600 quintals of schlich are annually smelted, which furnish—
| Marketable lead |
20,907 |
quintals. |
| Marketable litharge, containing 90 per cent. of lead |
7,555 |
|
| Silver, about |
67 |
|
| Copper (finally purified in the works of Altenau) |
35 |
|
| Total product |
28,564 |
|
This weight amounts to one twenty-fifth of the weight of ore raised for the service of
the establishment. Eight parts of ore furnish, on an average, about one of schlich.
The bellows are constructed wholly of wood, without any leather; an improvement
made by a bishop of Bamberg, about the year 1620. After receiving different modifications,
they were adopted, towards 1730, in almost all the smelting-works of the continent,
except in a few places, as Carniola, where local circumstances permitted a water
blowing-machine to be erected. These pyramidal shaped bellows, composed of movable
wooden boxes, have, however, many imperfections: their size must often be inconveniently
large, in order to furnish an adequate stream of air; they do not drive into the
furnace all the air which they contain; they require frequent repairs; and, working with
great friction, they waste much mechanical power.
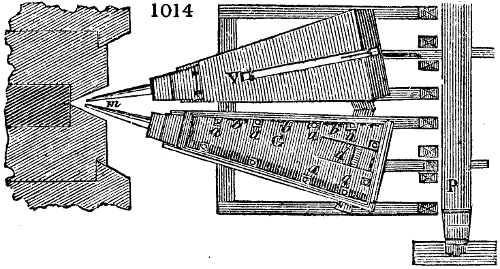
Fig. 1014. represents such wooden bellows, consisting of two chests or boxes, fitted
into each other; the upper or moving one being called the fly, the lower or fixed
one, the seat (gite). In the bottom
of the gite, there is an
orifice furnished with a clack-valve
d, opening inwards when
the fly is raised, and shutting
when it falls. In order that the
air included in the capacity of
the two chests may have no
other outlet than the nose-pipe
m, the upper portion of the gite
is provided at its four sides with
small square slips of wood c, c, c,
which are pressed against the sides of the fly by strong springs of iron wire b, b, b, while
they are retained upon the gite by means of small square pieces of wood a, a, a, a. The
latter a, a, are perforated in the centre, and adjusted upon rectangular stems, called
buchettes; they are attached, at their lower ends, to the upright sides of the gite G.
P is the driving-shaft of a water-wheel, which, by means of cams or tappets, depresses
the fly, while the counterweight Q, fig. 1013., raises it again.
Figs. 1015, 1016, 1017, 1018. represent the moderately high (demihauts, or half-blast,)
furnaces employed in the works of the lower Hartz, near Goslar, for smelting the silvery
lead ores extracted from the mine of Rammelsberg. See its section, in fig. 737.
Fig. 1015. is the front elevation of the twin furnaces, built in one body of masonry;
fig. 1016. is a plan taken at the level of the tuyères, in the line v, l, 6. of fig. 1017.;
figs. 1017. and 1018.
exhibit two vertical sections;
the former in the
line A, B, the latter in
the line C, D, of fig.
1016. In these four
figures the following
objects may be distinguished.
a, b, c, d, a balcony or
platform, which leads to
the place of charging n;
e, f, wooden stairs, by
which the charging
workmen mount from
the ground p, q, of the
works, to the platform;
g, h, brickwork of the furnaces; i, k, wall of the smelting-works, against which they are
supported; l, upper basin of reception, hollowed out of the brasque (or ground charcoal
bed) 6; m, arch of the tuyère v, by which each furnace receives the blast of two[1129]
bellows; n, place of charging, which takes place through the upper orifice n, o, of the
basin n, o, v, t, of the furnace; t, a slab of clay, placed in such a way that, during the
treatment of the lead, a little
metallic zinc may run together
in a sloping gutter,
seen in fig. 1001., formed of
slates cemented together
with clay.
In figs. 1015 and 1017.,
l, z, is the brickwork of the
foundations; m, conduits
(called evaporatory), for the
exhalation of the moisture;
4, a layer of slags, rammed
above; 5, a bed of clay, rammed above the slags; 6, a brasque, composed of one part
of clay, and two parts of ground charcoal, which forms the sole of the furnace.
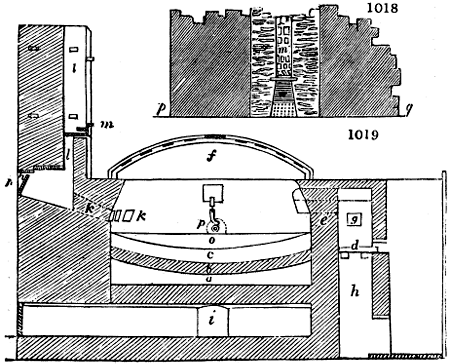
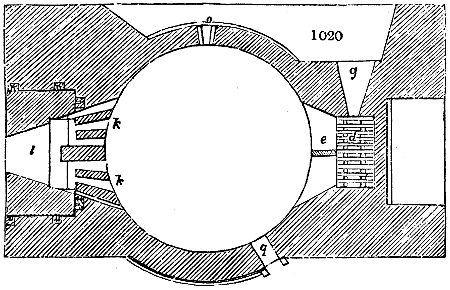
The excellent refinery furnace, or treibheerd, of Frederickshütte, near
Tarnowitz, in Upper Silesia, is represented in figs. 1019. and 1020. a,
is the bottom, made of slag or cinders;
b, the foundation, of fire-bricks; c, the
body of the hearth proper, composed of
a mixture of 7 parts of dolomite, and
1 of fire-clay, in bulk; d, the grate of
the air furnace; e, the fire-bridge; f,
the dome or cap, made of iron plate
strengthened with bars, and lined with
clay-lute, to protect the metal from burning;
g, the door of the fireplace; h, the
ash-pit; i, the tap-hole; k, k, the flue,
which is divided by partitions into several
channels; l, the chimney; m, a damper-plate
for regulating the draught; n, a
back valve, for admitting air to cool the
furnace, and brushes to sweep the flues;
o, tuyère of copper, which by means of an
iron wedge may be sloped more or less
towards the hearth; p, the schnepper, a
round piece of sheet iron, hung before
the eye of the tuyère, to break and spread
the blast; q, the outlet for the glassy
litharge.
Lime-marl has been found to answer
well for making the body of the hearth-sole,
as it absorbs the vitrified litharge freely, without combining with it. A basin-shaped
hollow is formed in the centre, for receiving the silver at the end of the process;
and a gutter is made across the hearth for running off the glätte or
fluid litharge.
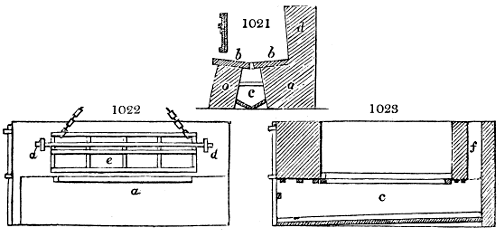
Figs. 1021, 1022. represent the eliquation hearth of Neustadt.
Fig. 1021. is a cross section; fig. 1022. is a front view; and fig. 1023.
a longitudinal section. It is formed by two walls a, a, 31⁄2 feet high,
placed from 1⁄2 to 1 foot apart, sloped off at top with iron plates, 3
inches thick, and
18 inches broad,
called saigerscharten, or refining
plates, b, b, inclined
3 inches
towards each other
in the middle, so
as to leave at the
lowest point a slit
21⁄2 inches wide
between them,
through which the
lead, as it sweats
out by the heat, is
allowed to fall into[1130]
the space between the two walls c, called the saigergasse (sweating-gutter). The sole
of this channel slopes down towards the front, so that the liquefied metal may run off
into a crucible or pot.
Upon one of the long
sides, and each of the
shorter ones, of the
hearth, the walls d, d,
are raised two feet
high, and upon these
the liquation lumps
rest; upon the other
long side, where there
is no wall, there is
an opening for admitting
these lumps
into the hearth. The
openings are then
shut with a sheet or
cast iron plate e,
which, by means of
a chain, pulley, and counterweight, may be easily raised and lowered. f, is a passage
for increasing the draught of air.
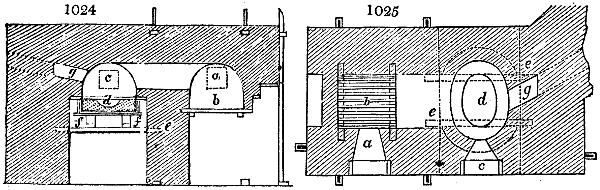
Figs. 1024. and 1025. represent the refining furnaces of Frederickshütte
by Tarnowitz; a, is the fire-door; b, the grate; c, the
door for introducing the silver; d, the movable test, resting upon
a couple of iron rods e, e, which are let at their ends into the
brickwork. They lie lower than would seem to be necessary;
but this is done in order to be able to place the surface of the
test at any desired level, by placing tiles f, f, under it; g, the
flue, leading to a chimney 18 feet high. For the refining of 100
marks of blicksilber, of the fineness of 151⁄2 loths (half ounces) per cwt., 3 cubic feet of
pitcoal are required. The test or cupel must be heated before the impure silver and soft
lead are put into it.
At these smelting-houses from 150 to 160 cwt. of very pure workable lead (lead containing
merely a little silver) are put into the furnace at once, and from 10 to 14
cwt. run off in vitrified oxide; the remainder is then refined with some pure lead, when
an alloy containing from 141⁄2 to 151⁄2 loths of blicksilber per cwt. is obtained.
English refining furnaces.—The refining of lead is well performed in some works in
the neighbourhood of Alston-moor, in reverberatory furnaces, figs. 1026. and 1027., whose
fireplace is 22 inches square, and is separated from the sole by a fire-bridge, 14 inches
in breadth. The flame, after having passed over the surface of the lead in the cupel,
enters two flues e, e, on the opposite side of the furnace, which terminate in a chimney
i, i, i, i, 40 feet high. At the bottom of the chimney are openings f, f, for taking out
the metallic dust deposited within. These openings are shut during the process.
The cupel or test, which constitutes, in fact, the sole of the hearth in which the
operation takes place, is movable. It consists of a vertical elliptical ring of iron,[1131]
A, B, C, D, figs. 1028. and 1029., 33⁄4 inches high, the greatest diameter of the ellipse being
4 feet, and the smallest 21⁄2. Four iron bars (A D, m, m′, B C, n, n′,) are fixed across its
bottom, which are
also 33⁄4 inches broad,
and an inch thick.
The first of these bars
is placed 9 inches
from the end of the
elliptic ring nearest
the fireplace, and
the three others are
equally distributed
between this bar and
the back end.
In forming the
cupel, several layers
of a mixture of moistened
bone ashes, and
fern ashes, in very
fine powder, are put
into the test-frame.
The bone ash constitutes
from 1⁄8 to 1⁄16
of the bulk of the
mixture, according
to the purity of the
fern ashes employed, estimated by the proportion of potash they contain, which has the
property of semi-vitrifying the powder of burnt bones, of thus removing its friability, and
rendering it more durable. The layers of ashes are strongly beat down, till the frame is
entirely filled. The mass thus formed is then hollowed out by means of a little spade,
made on purpose, till it is only three quarters of an inch thick above the iron bars near
the centre of the bottom. A flange, 2 inches broad, is made at the upper part, and 21⁄2
inches at the lower part, except on the front or breast, which is 5 inches thick. In
this anterior part, there is hollowed out an opening of an inch and a quarter broad, and
6 inches long, with which the outlet or gateway of the litharge communicates.
The cupel thus prepared is placed in the refining furnace. It rests in an iron ring
built into the brickwork. The arched roof of the furnace is 12 inches above the cupel
near the fire-bridge, and 9 inches near the flue at the other end.
The tuyère is placed in the back of the furnace, opposite to the side at which the litharge
is allowed to overflow.
Openings g, g, are left at the sides of each cupel, either for running off or for introducing
melted lead.
Refining of lead to extract its silver.—This operation, which the lead of Derbyshire
cannot be submitted to with advantage, is performed in a certain number of the smelting-houses
at Alston-moor, and always upon leads reduced in the Scotch furnace.
The cupel furnace above described, must be slowly heated, in order to dry the cupel
without causing it to crack, which would infallibly be produced by sudden evaporation
of the moisture in it. When it has been thus slowly brought to the verge of a red
heat, it is almost completely filled with lead previously melted in an iron pot. The
cupel may be charged with about 5 cwt. At the temperature at which the lead is introduced,
it is immediately covered with a gray pellicle of oxide; but when the heat of
the furnace has been progressively raised to the proper pitch, it becomes whitish-red,
and has its surface covered over with litharge. Now is the time to set in action the
blowing-machine, the blast of which, impelled in the direction of the great axis of the
cupel, drives the litharge towards the breast of the cupel, and makes it flow out by the
gateway prepared for it, through which it falls upon a cast-iron plate, on a level with the[1132]
floor of the apartment, and is dispersed into tears. It is carried in this state to the furnace
of reduction, and revived. As by the effect of the continual oxidization which it
undergoes, the surface of the metal necessarily falls below the level of the gateway of
the litharge, melted lead must be added anew by ladling it into the furnace from the
iron boiler, as occasion may require. The operation is carried on in this manner till
84 cwt. or 4 Newcastle fodders of lead have been introduced, which takes from 16 to 18
hours, if the tuyère has been properly set. The whole quantity of silver which this
mass of lead contains, is left in combination with about 1 cwt. of lead, which, under
the name of rich lead, is taken out of the cupel.
When a sufficient number of these pieces of rich lead have been procured, so that by
their respective quality, as determined by assaying, they contain in whole from 1000 to
2000 ounces of silver, they are re-melted to extract their silver, in the same furnace,
but in a cupel which differs from the former in having at its bottom a depression
capable of receiving at the end of the process the cake of silver. In this case a portion
of the bottom remains uncovered, on which the scoriæ may be pushed aside with a little
rake, from the edges of the silver.
The experiments of MM. Lucas and Gay Lussac have proved that fine silver,
exposed to the air in a state of fusion, absorbs oxygen gas, and gives it out again in the
act of consolidation. The quantity of oxygen thus absorbed may amount to twenty-two
times the volume of the silver. The following phenomena are observed when the
mass of metal is considerable; for example, from 40 to 50 pounds.
The solidification commences at the edges, and advances towards the centre. The
liquid silver, at the moment of its passage to the solid state, experiences a slight
agitation, and then becomes motionless. The surface, after remaining thus tranquil
for a little, gets all at once irregularly perturbed, fissures appear in one or several
lines, from which flow, in different directions, streams of very fluid silver, which increase
the original agitation. The first stage does not yet clearly manifest the presence of
gas, and seems to arise from some intestine motion of the particles in their tendency to
group, on entering upon the process of crystallization, and thus causing the rupture of
the envelop or external crust, and the ejection of some liquid portions.
After remaining some time tranquil, the metal presents a fresh appearance, precisely
analogous to volcanic phenomena. As the crystallization continues, the oxygen gas is
given out with violence at one or more points, carrying with it melted silver from the
interior of the surface, producing a series of cones, generally surmounted by a small
crater, vomiting out streams of the metal, which may be seen boiling violently within
them.
These cones gradually increase in height by the accumulation of metal thrown up,
and that which becomes consolidated on their sloping sides. The thin crust of metal
on which they rest, consequently experiences violent impulses, being alternately raised
and depressed by such violent agitation, that were it not for the tenacity and elasticity
of the metal, there would evidently arise dislocation, fissures, and other analogous
accidents. At length several of the craters permanently close, while others continue to
allow the gas a passage. The more difficult this is, the more the craters become
elevated, and the more their funnels contract by the adhesion or coagulation of a
portion of the metal. The projection of globules of silver now becomes more violent;
the latter being carried to great distances, even beyond the furnace, and accompanied by
a series of explosions, repeated at short intervals. It is generally the last of these little
volcanoes that attains the greatest altitude, and exhibits the foregoing phenomena with
the greatest energy. It is, moreover, observable, that these cones do not all arise at the
same time, some having spent their force, when others commence forming at other
points. Some reach the height of an inch, forming bases of two or three inches in
diameter. The time occupied by this exhibition is at least from half to three quarters
of an hour.
During the formation of these cones, by the evolution of gas, portions of silver are
shot forth, which assume, on induration, a form somewhat cylindrical, and often very
fantastic, notwithstanding the incompatibility which appears to exist between the
fluidity of the silver and these elongated figures. Their appearance is momentary, and
without any symptoms of gas, although it is impossible to decide whether they may not
arise from its influence; they seem, in fact, to resemble the phenomena of the first
volcanic period.
Till very recently the only operations employed for separating silver from lead in the
English smelting-works, were the following:—
1. Cupellation, in which the lead was converted into a vitreous oxide, which was
floated off from the surface of the silver.
2. Reduction of that oxide, commonly called litharge.
3. Smelting the bottoms of the cupels, to extract the lead which had soaked into
them, in a glassy state.
[1133]
Cupellation and its two complementary operations were, in many respects, objectionable
processes; from the injurious effects of the lead vapours upon the health of the
workmen; from the very considerable loss of metallic lead, amounting to 7 per cent. at
least; and, lastly, from the immense consumption of fuel, as well as from the vast amount
of manual labour incurred in such complicated operations. Hence, unless the lead
were tolerably rich in silver, it would not bear the expense of cupellation.
The patent process lately introduced by Mr. Pattinson, of Newcastle, is not at all
prejudicial to the health of workmen; it does not occasion more than 2 per cent. of loss
of lead, and in other respects it is so economical, that it is now profitably applied in
Northumberland to alloys too poor in silver to be treated by cupellation. This process
is founded upon the following phenomena.
After melting completely an alloy of lead and silver, if we allow it to cool very slowly,
continually stirring it meanwhile with a rake, we shall observe at a certain period a
continually increasing number of imperfect little crystals, which may be taken out with
a drainer, exactly as we may remove the crystals of sea salt deposited during the concentration
of brine, or those of sulphate of soda, as its agitated solution cools. On submitting
to analysis the metallic crystals thus separated, and also the liquid metal deprived
of them, we find the former to be lead almost alone, but the latter to be rich in silver,
when compared with the original alloy. The more of the crystalline particles are drained
from the metallic bath, the richer does the mother liquid become in silver. In practice,
the poor lead is raised by this means to the standard of the ordinary lead of the litharge
works; and the better lead is made ten times richer. This very valuable alloy is then
submitted to cupellation; but as it contains only a tenth part of the quantity of lead
subjected to crystallization, the loss in the cupel will be obviously reduced to one-tenth of
what it was by the former process; that is, 7⁄10 of a per cent., instead of 7.
These nine-tenths of the lead separated by the drainer, are immediately sent into the
market, without other loss than the trifling one, of about 1⁄2 per cent., involved in reviving
a little dross skimmed off the surface of the melted metal at the beginning of the
operation. Hence the total waste of lead in this method does not exceed 2 per cent.
And as only a small quantity of lead requires to be cupelled, this may be done with the
utmost slowness and circumspection; whereby loss of the precious metal, and injury to
the health of the workpeople, are equally avoided.
The crystallization refinery of Mr. Pattinson is an extremely simple smelting-house.
It contains 3 hemispherical cast-iron pans, 41 inches in diameter, and 1⁄4 of an inch thick.
The three pans are built in one straight line, the broad flange at their edge being supported
upon brickwork. Each pan has a discharge pipe, proceeding laterally from one
side of its bottom, by which the melted metal may be run out when a plug is withdrawn,
and each is heated by a small separate fire.
Three tons of the argentiferous lead constitute one charge of each pan; and as soon
as it is melted, the fire is withdrawn; the flue, grate-door, and ash-pit, are immediately
closed, and made air-tight with bricks and clay-lute. The agitation is now commenced,
with a round bar of iron terminated with a chisel point, the workman being instructed
merely to keep moving that simple rake constantly in the pan, but more especially
towards the edges, where the solidification is apt to begin. He must be careful to take
out the crystals, progressively as they appear, with an iron drainer, heated a little higher
than the temperature of the metal bath. The liquid metal lifted in the drainer, flows
readily back through its perforations, and may be at any rate effectually detached by
giving the ladle two or three jogs. The solid portion remains in the form of a spongy,
semi-crystalline, semi-pasty mass.
The proportion of crystals separated at each melting, depends upon the original
quality of the alloy. If it be poor, it is usually divided in the proportion of two-thirds
of poor crystals, and one-third of rich liquid metal; but this proportion is reversed
if the alloy contain a good deal of silver.
Let us exemplify, by the common case of a lead containing 10 ounces of silver per
ton. Operating upon three tons of this alloy, or 60 cwt., containing 30 oz. of silver,
there will be obtained in the first operation—
| (a) |
40 cwt. at |
4 |
1⁄4 |
ounces of silver per ton; |
in whole |
9 |
oz. |
|
- |
30 oz. |
| (b) |
20 cwt. at |
21 |
|
— |
— |
21 |
|
Each of these alloys (a) and (b) will be joined to alloys of like quality obtained in
the treatment of one or several other portions of three tons of the primitive alloy.
Again, three tons of each of these rich alloys are subjected to the crystallization process,
and thus in succession. Thus poorer and poorer lead is got on the one hand,
and richer and richer alloys on the other. Sometimes the mother metal is parted from
a great body of poor crystals, by opening the discharge-pipe, and running off the liquid,
while the workman keeps stirring, to facilitate the separation of the two.
25 fodders, 15 cwts., 49 lbs. = 540 cwts., 49 lbs. of alloy, holding 5 oz. of silver per
fodder, in the whole 130 oz., afforded, after three successive crystallizations—
[1134]
| |
oz. |
| 440 |
cwts. of |
poor lead, |
holding 1⁄2 oz. of silver per fodder; in all |
10 |
1⁄2 |
| 15 |
cwt. 49 |
— |
holding the original quantity, nearly |
3 |
1⁄2 |
| 84 |
cwts. of |
lead for the cupel, holding 29 oz. |
116 |
|
| |
Total |
|
130 |
|
| 1 cwt. of loss, principally in the reduction of dross. |
The expenses of the new method altogether, including 3s. per fodder of patent dues
are about one-third of the old; being 17l. 13s. and 54l. 16s. respectively, upon 84 cwts.
of lead, at 29 oz. per fodder.
In the conditions above stated, the treatment of argentiferous lead occasions the following
expenses:—
| FOR ONE FODDER. |
£ |
s. |
d. |
| By the |
new process |
0 |
13 |
7 |
| |
old process |
2 |
2 |
2 |
Admitting that the treatment of silver holding lead is economically possible only
when the profit is equal to one-tenth of the gross expenses of the process, we may easily
calculate, with the preceding data, that it is sufficient for the lead to have the following
contents in silver:—
| With the new process, 3 ounces per fodder; or, |
0·000078 |
| With the old process, 84⁄10 ounces per fodder; or, |
0·000218 |
To conclude, the refining by crystallization reduces the cost of the parting of lead and
silver, in the proportion of 3 to 1; and allows of extracting silver from a lead which
contains only about 3 oz. per ton. In England, the new method produces at present
very advantageous results, especially in reference to the great masses to which it may be
applied. In 1828, the quantity of lead annually extracted from the mines in the United
Kingdom had been progressively raised to 47,000 tons. Reduced almost to one-half of
this amount in 1832, by the competition of the mines of la Sierra de Gador, the English
production began again to increase in 1833. In 1835, 35,000 tons of lead were obtained,
one-half of which only having a mean content of 81⁄2 oz. of silver per ton, was subjected to
cupellation, and produced 14,000 oz. of that precious metal. The details of this production
are—
| Silver extracted from 17,500 tons of lead, holding upon the average 81⁄2 oz. per ton. |
140,000 |
oz. |
| Silver extracted from silver ores, properly so called, in Cornwall |
36,000 |
|
| |
176,000 |
|
In 1837, the production of lead amounted probably to 40,000 tons; upon which
the introduction of the new method would have the effect not only of reducing considerably
the cost of parting the 20,000 tons of lead containing 8 oz. of silver per ton,
but of permitting the extraction of 4 or 5 oz. of silver, which may be supposed to exist
upon an average in the greater portion of the remaining 20,000 tons. Otherwise, this
mass of the precious metal would have had no value, or have been unproductive.
There are two oxides of silver; called argentic oxide, and suroxide, by Berzelius.
1. The first is obtained by adding solution of caustic potassa, or lime-water, to a solution
of nitrate of silver. The precipitate has a brownish-gray colour, which darkens when
dried, and contains no combined water. Its specific gravity is 7·143. On exposure
to the sun, it gives out a certain quantity of oxygen, and becomes a black powder.
This oxide is an energetic base; being slightly soluble in pure water, reacting like the
alkalis upon reddened litmus paper, and displacing, from their combinations with the
alkalis, a portion of the acids, with which it forms insoluble compounds. It is insoluble
in the caustic lyes of potassa or soda. By combination with caustic ammonia, it forms
fulminating silver. This formidable substance may be prepared by precipitating the
nitrate of silver with lime-water, washing the oxide upon a filter, and spreading it upon
gray paper, to make it nearly dry. Upon the oxide, still moist, water of ammonia is to
be poured, and allowed to remain for several hours. The powder which becomes black,
is to be freed from the supernatant liquor by decantation, divided into small portions
while moist, and set aside to dry upon bits of porous paper. Fulminating silver may
be made more expeditiously by dissolving the nitrate in water of pure ammonia, and
precipitating by the addition of caustic potassa lye in slight excess. If fulminating
silver be pressed with a hard body in its moist state, it detonates with unparalleled
violence; nay, when touched even with a feather, in its dry state, it frequently explodes.
As many persons have been seriously wounded, and some have been killed, by these
explosions, the utmost precautions should be taken, especially by young chemists, in its
preparation. This violent phenomenon is caused by the sudden production of water
and nitrogen, at the instant when the metallic oxide is reduced. The quiescent and[1135]
divellent affinities seem to be so nicely balanced in this curious compound, that the
slightest disturbance is sufficient to incite the hydrogen of the ammonia to snatch the
oxygen from the silver. The oxide of silver dissolves in glassy fluxes, and renders them
yellow. It consists, according to Berzelius, of 93·11 parts of silver, and 6·89 of oxygen.
2. The suroxide of silver is obtained by passing a voltaic current through a weak solution
of the nitrate; it being deposited, of course, at the positive or oxygenating pole.
It is said to crystallize in needles of a metallic lustre, interlacing one another, which are
one-third of an inch long. When thrown into muriatic acid, it causes the disengagement
of chlorine, and the formation of chloride of silver; into water of ammonia, it
occasions such a rapid production of nitrogen gas, with a hissing sound, as to convert
the whole liquid into froth. If a little of it, mixed with phosphorus, be struck with a
hammer, a loud detonation ensues. With heat it decrepitates, and becomes metallic
silver.
Sulphuret of silver, which exists native, may be readily prepared by fusing the
constituents together; and it forms spontaneously upon the surface of silver exposed to
the air of inhabited places, or plunged into eggs, especially rotten ones. The tarnish
may be easily removed, by rubbing the metal with a solution of cameleon mineral, prepared
by calcining peroxide of manganese with nitre. Sulphuret of silver is a powerful
sulpho-base; since though it be heated to redness in close vessels, it retains the volatile
sulphides, whose combinations with the alkalis are decomposed at that temperature. It
consists of 87·04 of silver, and 12·96 of oxygen.
A small quantity of tin, alloyed with silver, destroys its ductility. The best method
of separating these two metals, is to laminate the alloy into thin plates, and distil them
along with corrosive sublimate. The bichloride of tin comes over in vapours, and
condenses in the receiver. Silver and lead, when combined, are separated by heat alone
in the process of cupellation, as described in the article Assay, and in the reduction of
silver ores. See suprà.
An alloy, containing from one-twelfth to one-tenth of copper, constitutes the silver
coin of most nations; being a harder and more durable metal under friction than pure
silver. When this alloy is boiled with a solution of cream of tartar and sea-salt, or
scrubbed with water of ammonia, the superficial particles of copper are removed, and a
surface of fine silver is left.
Chloride of silver is obtained by adding muriatic acid, or any soluble muriate, to a
solution of nitrate of silver. A curdy precipitate falls, quite insoluble in water, which
being dried and heated to dull redness, fuses into a semi-transparent gray mass, called,
from its appearance, horn-silver. Chloride of silver dissolves readily in water of
ammonia, and crystallizes in proportion as the ammonia evaporates. It is not decomposed
by a red heat, even when mixed with calcined charcoal; but when hydrogen or
steam is passed over the fused chloride, muriatic acid exhales, and silver remains.
When fused along with potassa (or its carbonate), the silver is also revived; while
oxygen (or also carbonic acid) gas is liberated, and chloride of potassium is formed.
Alkaline solutions do not decompose chloride of silver. When this compound is
exposed to light, it suffers a partial decomposition, muriatic acid being disengaged.
See Assay by the humid method.
The best way of reducing the chloride of silver, says Mohr, is to mix it with one-third
of its weight of colophony (black rosin), and to heat the mixture moderately in a crucible
till the flame ceases to have a greenish-blue colour; then suddenly to increase the fire,
so as to melt the metal into an ingot.
The subchloride may be directly formed, by pouring a solution of deuto-chloride of
copper or iron upon silver leaf. The metal is speedily changed into black spangles,
which, being immediately washed and dried, constitute subchloride of silver. If the
contact of the solutions be prolonged, chloride would be formed.
The bromide, cyanide, fluoride, and iodide of silver, have not been applied to any use
in the arts. Sulphate of silver may be prepared by boiling sulphuric acid upon the
metal. See Refining of Gold and Silver. It dissolves in 88 parts of boiling water,
but the greater part of the salt crystallizes in small needles, as the solution cools. It
consists of 118 parts of oxide, combined with 40 parts of dry acid. Solutions of the
hyposulphite of potassa, soda, and lime, which are bitter salts, dissolve chloride of
silver, a tasteless substance, into liquids possessed of the most palling sweetness, but not
at all of any metallic taste.
The iodide of silver is remarkable, like some other metallic compounds, for changing
its colour alternately with heat and cold. If a sheet of white paper be washed over with
a solution of nitrate of silver, and afterwards with a somewhat dilute solution of hydriodate
of potash, it will immediately assume the pale yellow tint of the cold silver iodide.
On placing the paper before the fire, it will change colour from a pale primrose to a
gaudy brilliant yellow, like the sun-flower; and on being cooled, it will again resume
the primrose hue. These alternations may be repeated indefinitely, like those with the[1136]
salts of cobalt, provided too great a heat be not applied. The pressure of a finger upon
the hot yellow paper makes a white spot, by cooling it quickly.
Fulminate of silver is prepared in the same way as Fulminate of Mercury, which see.
On the 10th of February, 1798, the Lords of the Privy Council appointed the Hon.
Charles Cavendish, F. R. S., and Charles Hatchett, Esq., F. R. S., to make investigations
upon the wear of gold coin by friction. Their admirable experiments were begun in
the latter end of 1798, and completed in April 1801, having been instituted and conducted
with every mechanical aid, as devised by these most eminent chemical philosophers,
and provided, at no small expense, by the government. The following are the important
conclusions of their official report:—[54]
“Gold made standard by a mixture of equal parts of silver and copper, is not so soft
as gold alloyed only with silver; neither is it so pale; for it appears to be less removed
from the colour of fine gold, than either the former or the following metal.
“Gold, when alloyed with silver and copper, when annealed, does not become black,
but brown; and this colour is more easily removed by the blanching liquor, or solution
of alum, than when the whole of the alloy consists of copper. It may also be rolled
and stamped with great facility; and, under many circumstances, it appears to suffer
less by friction than gold alloyed by silver or copper alone.
“If copper alone forms the alloy, it must be dissolved and separated from the surface
of each piece of coin, in the process of annealing and blanching.
“Upon a comparison of the different qualities of the three kinds of standard gold, it
appears (strictly speaking) that gold made standard by silver and copper is rather to
be preferred for coin.”
It will, undoubtedly, seem not a little strange to the uninitiated, that this report, and
its important deductions, should have been of late years entirely set at nought, without
any scientific reason or research, apparently for the purpose of giving a certain official
in our Mint a good job, in sweating out all the silver from our sovereigns, and replacing
it, in the new coinage, with copper, taking on an average 3d. worth of silver out of each
ounce of our excellent gold coin, and charging the country 61⁄2d. for its extraction,
besides the very considerable expense in providing fine copper to replace the
silver. The pretence set up for this extraordinary degradation of the gold, was, that
our coin might peradventure be exported, in order to be de-silvered abroad, a danger
which could have been most readily averted, by leaving out as much gold in every
sovereign as was equivalent to the silver introduced, and thus preserving its intrinsic
value in precious metal. When the film of fine gold which covers each of our present
pieces has been rubbed off from the prominent parts, these must appear of a very different
and deeper colour than the flat part or ground of the coin. “The reason,
therefore, is sufficiently apparent, says Mr. Hatchett, why gold which is alloyed with
silver only, cannot be liable to this blemish;” and with one-half of silver alloy, it must
be much less liable to it, than with copper alone. Why did the political economists
in the recent Committee of the House of Commons on the Mint, blink this question, of
public economy and expediency?
Gold, as imported from America, Asia, and Africa, contains on an average nearly the
right proportion of silver for making the best coin; and were it alloyed to our national
standard, of 22 parts of gold, 1 of silver, and 1 of copper, as defined by Messrs. Cavendish
and Hatchett, then by simply adding the deficient quantities of one or two of these
metals, by the rule of alligation, the very considerable expense would be saved to the
nation, and sulphureous nuisance to the Tower Hamlets, now foolishly incurred in de-silvering
and cuprifying sovereigns at the Royal Mint.
It was long imagined in Europe, that the average metallic contents of the silver ores
of Mexico and Peru, were considerably greater than those of Saxony and Hungary.
Much poorer ores, however, are worked among the Cordilleras than in any part of
Europe. The mean product of the whole silver ores that are annually reduced in
Mexico, amounts only to from 0·18 to 0·25 of a per cent.; that is, from 3 to 4 ounces
in 100 lbs.; the true average being, perhaps, not more than 21⁄2. It is by their greater
profusion of ores, not their superior richness, that the mines of South America surpass
those of Europe.
Gold and Silver produced in Forty Years, from 1790 to 1830.
| |
Gold. |
Silver. |
| Mexico |
£6,436,453 |
£139,818,032 |
| Chile |
2,768,488 |
1,822,924 |
| Buenos Ayres |
4,024,895 |
27,182,673 |
| Russia |
3,703,743 |
1,502,981 |
[1137]
Returns of the Dollars coined at the different Mints in Mexico.
| |
1829. |
1830. |
1831. |
1834. |
| Mexico |
1,280,000 |
1,090,000 |
1,386,000 |
952,000 |
| Guanajuato |
2,406,000 |
2,560,000 |
2,603,000 |
2,703,000 |
| Zacatecas |
4,505,000 |
5,190,000 |
4,965,000 |
5,527,000 |
| Guadalaxara |
596,000 |
592,000 |
590,000 |
715,000 |
| Durango |
659,000 |
453,000 |
358,000 |
1,215,000 |
| San Luis |
1,613,000 |
1,320,000 |
1,497,000 |
928,000 |
| Ilalpan |
728,000 |
90,000 |
323,000 |
— |
| Total |
11,787,000 |
11,295,000 |
11,722,000 |
12,040,000 |
| The returns for 1832 and 1833 are wanting. |
Peru.—Returns of Gold and Silver coined at the Mints of Lima and Casco.
| |
Gold. |
Silver. |
Total, in
Dollars. |
| 1830 |
180,000 |
2,015,000 |
2,195,000 |
| 1831 |
92,000 |
2,384,000 |
2,476,000 |
| 1832 |
94,000 |
3,210,000 |
3,284,000 |
| 1833 |
150,000 |
2,990,000 |
3,140,000 |
| 1834 |
110,000 |
3,150,000 |
3,260,000 |
Returns of Silver in Bars produced at the different Smelting-works in Peru.
| |
Lima. |
Truxillo. |
Pasco. |
Aya-
cucho. |
Puno. |
Are-
quipa. |
Total, in
Dollars. |
| 1830 |
270,000 |
190,000 |
780,000 |
120,000 |
250,000 |
150,000 |
1,760,000 |
| 1831 |
270,000 |
60,000 |
1,110,000 |
70,000 |
310,000 |
110,000 |
1,930,000 |
| 1832 |
290,000 |
100,000 |
1,800,000 |
70,000 |
345,000 |
25,000 |
2,640,000 |
| 1833 |
222,000 |
70,000 |
2,130,000 |
50,000 |
25,000 |
65,000 |
2,562,000 |
Returns of Silver in Dollars exported from the Provinces of Chili.
| |
Coquimbo. |
Huasco. |
Copiano. |
| 1831 |
785,000 |
115,000 |
670,000 |
| 1832 |
316,000 |
— |
36,000 |
| 1833 |
490,000 |
100,000 |
585,000 |
| |
1,591,000 |
215,000 |
1,291,000 |
Santiago—Mint Coinage.
| Gold. |
Silver. |
Total. |
| 1832, 174,000; 1833, 392,500 |
1832, 42,000; 1833, 92,000 |
700,500 |
The production of Silver in the kingdom of Saxony, amounted to—
| 59,231 |
marcs |
and |
8 |
loths, |
in the year |
1825 |
| 55,023 |
— |
|
— |
1826 |
| 60,034 |
— |
|
— |
1827 |
| 61,361 |
— |
|
— |
1828 |
| 65,176 |
— |
and |
10 |
loths |
— |
1830 |
| 65,886 |
— |
|
— |
1832 |
The mine of Himmelsfürst alone produces annually 10,000 marcs.
The quantity of Silver produced in the Prussian states was—
| 22,135 |
marcs in |
1825 |
| 20,071 |
— |
1826 |
| 18,631 |
— |
1827 |
| 21,731 |
— |
1828 |
| 20,612 |
— |
1829 |
| 20,887 |
— |
1830 |
| 19,031 |
— |
1831 |
| 22,083 |
— |
1832 |
The whole annual production of Europe, and Asiatic Russia, has been rated by
Humboldt at 292,000 marcs; by other authorities, at 310,000; while at the beginning
of the present century, that of the Spanish colonies in America was 3,349,160 marcs, or[1138]
nearly twelve times as much. The sum total is 3,704,160 marcs, of 3609 grains troy
each; which is nearly 1,900,000 lbs. avoirdupois; that is, little less than 9000 tons.
The English Mint silver contains 222 pennyweights of fine silver, and 18 of copper, in
the troy pound of 240 pennyweights; or 92·5 in 100 parts. 1 pound troy = 5760 grains,
contains 65·8 shillings, each weighing 87·55 grains. The French silver coin contains one-tenth
of copper, and a franc weighs 5 grammes = 77·222 grains troy. The Prussian
dollar, (thaler), is the standard coin; 101⁄2 thaler weigh 1 marc; hence, 1 thaler weighs 343·7
grains troy, and contains 257·9 grains of fine silver; being 75 per cent. of silver, and 25
of alloy. The Austrian coin contains 13⁄288 of alloy, according to Wasserberg; which is
only 41⁄2 per cent.
SILVER LEAF, is made in precisely the same way as Gold Leaf, to which article I
must therefore refer the reader.
SILVERING, is the art of covering the surfaces of bodies with a thin film of silver.
When silver leaf is to be applied, the methods prescribed for gold leaf are suitable.
Among the metals, copper or brass are those on which the silverer most commonly
operates. Iron is seldom silvered; but the processes for both metals are essentially the
same.
The principal steps of this operation are the following:—
1. The smoothing down the sharp edges, and polishing the surface of the copper;
called émorfiler by the French artists.
2. The annealing; or, making the piece to be silvered redhot, and then plunging it
in very dilute nitric acid, till it be bright and clean.
3. Pumicing; or, clearing up the surface with pumice-stone and water.
4. The warming, to such a degree merely as, when it touches water, it may make a
slight hissing sound; in which state it is dipped in the very weak aquafortis, whereby
it acquires minute insensible asperities, sufficient to retain the silver leaves that are to
be applied.
5. The hatching. When these small asperities are inadequate for giving due solidity
to the silvering, the plane surfaces must be hatched all over with a graving tool; but
the chased surfaces need not be touched.
6. The bluing, consists in heating the piece till its copper or brass colour changes to
blue. In heating, they are placed in hot tools made of iron, called mandrins in France.
7. The charging, the workman’s term for silvering. This operation consists in
placing the silver leaves on the heated piece, and fixing them to its surface by burnishers
of steel, of various forms. The workman begins by applying the leaves double.
Should any part darken in the heating, it must be cleared up by the scratch-brush.
The silverer always works two pieces at once; so that he may heat the one, while
burnishing the other. After applying two silver leaves, he must heat up the piece to
the same degree as at first, and he then fixes on with the burnisher four additional
leaves of silver; and he goes on charging in the same way, 4 or 6 leaves at a time, till he
has applied, one over another, 30, 40, 50, or 60 leaves, according to the desired solidity
of the silvering. He then burnishes down with great pressure and address, till he has
given the surface a uniform silvery aspect.
Silvering by the precipitated chloride of silver.—The white curd obtained by adding
a solution of common salt to one of nitrate of silver, is to be well washed and dried.
One part of this powder is to be mixed with 3 parts of good pearlash, one of washed
whiting, and one and a half of sea salt. After clearing the surface of the brass, it is to be
rubbed with a bit of soft leather, or cork moistened with water, and dipped in the above
powder. After the silvering, it should be thoroughly washed with water, dried, and
immediately varnished. Some use a mixture of 1 part of the silver precipitate, with 10
of cream of tartar, and this mixture also answers very well.
Others give a coating of silver by applying with friction, in the moistened state, a
mixture of 1 part of silver-powder precipitated by copper, 2 parts of cream of tartar, and
as much common salt. The piece must be immediately washed in tepid water very
faintly alkalized, then in slightly warm pure water, and finally wiped dry before the
fire. See Plated Manufacture.
The inferior kinds of plated buttons get their silver coating in the following way:—
2 ounces of chloride of silver are mixed up with 1 ounce of corrosive sublimate,
3 pounds of common salt, and 3 pounds of sulphate of zinc, with water, into a paste.
The buttons being cleaned, are smeared over with that mixture, and exposed to a
moderate degree of heat, which is eventually raised nearly to redness, so as to expel the
mercury from the amalgam, formed by the reaction of the horn silver and the corrosive
sublimate. The copper button thus acquires a silvery surface, which is brightened by
clearing and burnishing.
Leather is silvered by applying a coat of parchment size, or spirit varnish, to the surface,
and then the silver leaf, with pressure.
SIMILOR, is a golden-coloured variety of brass.
[1139]
SINGEING OF WEBS. The old furnace for singeing cotton goods is represented
in longitudinal section, fig. 1030., and in a transverse one in fig. 1031. a is the
fire-door; b, the
grate; c, the ash-pit;
d, a flue, 6
inches broad, and 21⁄2
high, over which a
hollow semi-cylindrical
mass of cast
iron e, is laid, one
inch thick at the
sides, and 21⁄2 thick
at the top curvature.
The flame passes
along the fire-flue d, into a side opening f, in the chimney. The goods are swept
swiftly over this ignited piece of iron, with considerable friction, by means of a wooden
roller, and a swing frame for raising them at any moment out of contact.
In some shops, semi-cylinders of copper, three quarters of an inch thick, have been
substituted for those of iron, in singeing goods prior to bleaching them. The former
last three months, and do 1500 pieces with one ton of coal; while the latter, which are
an inch and a half thick, wear out in a week, and do no more than from 500 to 600
pieces with the same weight of fuel.
In the early part of the year 1818, Mr. Samuel Hall enrolled the specification of a
patent for removing the downy fibres of the cotton thread from the interstices of bobbin-net
lace, or muslins, which he effected by singeing the lace with the flame of a gas-burner.
The second patent granted to Mr. Hall, in April, 1823, is for an improvement in
the above process; viz., causing a strong current of air to draw the flame of the gas through
the interstices of the lace, as it passes over the burner, by means of an aperture in a
tube placed immediately above the row of gas-jets, which tube communicates with an
air-pump or exhauster.
Fig. 1032. shows the construction of the apparatus complete, and manner in which it
operates; a, a, is a gas-pipe, supplied by an ordinary gasometer; from this pipe,
several small ones
extend upwards to
the long burner b, b.
This burner is a
horizontal tube, perforated
with many
small holes on the
upper side, through
which, as jets, the
gas passes; and when
it is ignited, the
bobbin-net lace, or
other material intended
to be singed,
is extended and
drawn rapidly over
the flame, by means
of rollers, which are
not shown in the
figure.
The simple burning
of the gas, even with a draught chimney, as in the former specification, is found
not to be at all times efficacious; the patentee, therefore, now introduces a hollow
tube c, c, with a slit or opening, immediately over the row of burners; and this tube,
by means of the pipes d, d, d, communicates with the pipe e, e, e, which leads to the
exhausting apparatus.
This exhausting apparatus consists of two tanks, f and g, nearly filled with water,
and two inverted boxes or vessels, h and i, which are suspended by rods to the vibrating
beam k; each of the boxes is furnished with a valve opening upwards; l, l, are pipes
extending from the horizontal part of the pipe e, up into the boxes or vessels h and i,
which pipes have valves at their tops, also opening upward. When the vessel h descends,
the water in the tank forces out the air contained within the vessel at the valve
m; but when that vessel rises again, the valve m being closed, the air is drawn from
the pipe e, through the pipe l. The same takes place in the vessel i, from which the
air in its descent is expelled through the valve n, and, in its ascent, draws the air[1140]
through the pipe l, from the pipe e. By these means, a partial exhaustion is effected
in the pipe e, e, and the tube c, c; to supply which, the air rushes with considerable
force through the long opening of the tube c, c, and carries with it the flame of the
gas-burners. The bobbin-net lace, or other goods, being now drawn over the flame
between the burner b, b, and the exhausted tube c, c, by means of rollers, as above said,
the flame of the gas is forced through the interstices of the fabric, and all the fine
filaments and loose fibres of the thread are burnt off, without damaging the substance of
the goods.
To adjust the draught from the gas-burners, there are stopcocks introduced into several
of the pipes d; and to regulate the action of the exhausting apparatus, an air vessel o, is
suspended by a cord or chain passing over pulleys, and balanced by a weight p.
There is also a scraper introduced into the tube c, which is made, by any convenient
contrivance, to revolve and slide backwards and forwards, for the purpose of removing
any light matter that may arise from the goods singed, and which would otherwise
obstruct the air passage. Two of these draught tubes c, may be adapted and united to the
exhausting apparatus, when a double row of burners is employed, and the inclination of
the flame may be directed upwards, downwards, or sideways, according to the position
of the slit in the draft tube, by which means any description of goods may, if required,
be singed on both sides at one operation.
The greater part of the bobbin-net lace made in England, is sent to Mr. Hall’s works,
at Basford, near Nottingham, to be singed; and at a reduction of price truly wonderful.
He receives now only one farthing for what he originally was paid one shilling.
SKIN (Peau, Fr.; Haut, Germ.); the external membrane of animal bodies, consists
of three layers: 1. the epidermis, scarf-skin, (Oberhaut, Germ.); 2. the vascular
organ, or papillary body, which performs the secretions; and 3. the true skin,
(Lederhaut, Germ.), of which leather is made. The skin proper, or dermoid substance,
is a tissue of innumerable very delicate fibres, crossing each other in every possible
direction, with small orifices between them, which are larger on its internal than on its
external surface. The conical channels thus produced, are not straight, but oblique, and
filled with cellular membrane; they receive vessels and nerves which pass out through
the skin (cutis vera), and are distributed upon the secretory organ. The fibrous texture
of the skin is composed of the same animal matter as the serous membranes, the
cartilages, and the cellular tissue; the whole possessing the property of dissolving in boiling
water, and being, thereby, converted into glue. See Glue, Leather, and Tan.
SLAG (Laitier, Fr.; Schlacke, Germ.); is the vitreous mass which covers the
fused metals in the smelting-hearths. In the iron-works it is commonly called cinder.
Slags consist, in general, of bi-silicates of lime and magnesia, along with the oxides of
iron and other metals; being analogous in composition, and having the same crystalline
form as the mineral, pyroxene. See Copper and Iron.
SLATES (Ardoises, Fr.: Schiefern, Germ.) The substances belonging to this class
may be distributed into the following species:—
- 1. Mica-slate, occasionally used for covering houses.
- 2. Clay-slate, the proper roofing-slate.
- 3. Whet-slate.
- 4. Polishing-slate.
- 5. Drawing-slate, or black chalk.
- 6. Adhesive slate.
- 7. Bituminous shale.
- 8. Slate-clay.
1. Mica-slate.—This is a mountain rock of vast continuity and extent, of a schistose
texture, composed of the minerals mica and quartz, the mica being generally predominant.
2. Clay-slate.—This substance is closely connected with mica; so that uninterrupted
transitions may be found between these two rocks in many mountain chains. It is a
simple schistose mass, of a bluish-gray or grayish-black colour, of various shades, and
a shining, somewhat pearly internal lustre on the faces, but of a dead colour in the cross
fracture.
Clay-slate is extensively distributed in Great Britain. It skirts the Highlands of
Scotland, from Lochlomond by Callender, Comrie, and Dunkeld; resting on, and
gradually passing into mica-slate throughout the whole of that territory. Roofing-slate
occurs, on the western side of England, in the counties of Cornwall and Devon;
in various parts of North Wales and Anglesea; in the north-east parts of Yorkshire,
near Ingleton, and in Swaledale; as also in the counties of Cumberland and Westmorland.
It is likewise met with in the county of Wicklow and other mountainous districts
of Ireland.
All the best beds of roofing-slate improve in quality as they lie deeper under the
surface; near to which, indeed, they have little value.
A good roofing-slate should split readily into thin even laminæ; it should not be
absorbent of water either on its face or endwise, a property evinced by its not increasing
perceptibly in weight after immersion in water; and it should be sound, compact, and[1141]
not apt to disintegrate in the air. The slate raised at Eisdale, on the west coast of
Argyllshire, is very durable.
Cleaving and dressing of the slates.—The splitter begins by dividing the block, cut
lengthwise, to a proper size, which he rests on end, and steadies between his knees. He
uses a mallet and a chisel, which he introduces into the stone in a direction parallel to
the folia. By this means he reduces it into several manageable pieces, and he gives to
each the requisite length, by cutting cross grooves on the flat face, and then striking the
slab with the chisel. It is afterwards split into thinner sections, by finer chisels dexterously
applied to the edges. The slate is then dressed to the proper shape, by being
laid on a block of wood, and having its projecting parts at the ends and sides cut off
with a species of hatchet or chopping-knife. It deserves to be noticed, that blocks of
slate may lose their property of divisibility into thin laminæ. This happens from long
exposure to the air, after they have been quarried. The workmen say, then, that they
have lost their waters. For this reason, the number of splitters ought to be always
proportioned to the number of block-hewers. Frost renders the blocks more fissile;
but a supervening thaw renders them quite refractory. A new frost restores the faculty
of splitting, though not to the same degree; and the workmen therefore avail themselves
of it without delay. A succession of frosts and thaws renders the quarried blocks quite
intractable.
3. Whet-slate, or Turkey hone, is a slaty rock, containing a great proportion of quartz,
in which the component particles, the same as in clay-slate and mica-slate, but in different
proportions, are so very small as to be indiscernible.
4. Polishing slate. Colour, cream-yellow, in alternate stripes; massive; composition
impalpable; principal fracture, slaty, thin, and straight; cross fracture, fine earthy; feels
fine, but meagre; adheres little, if at all, to the tongue; is very soft, passing into
friable; specific gravity in the dry state, 0·6; when imbued with moisture, 1·9. It is
supposed to have been formed from the ashes of burnt coal. It is found at Planitz,
near Zwickau, and at Kutschlin near Bilin in Bohemia.
5. Drawing-slate, or black chalk; has a grayish-black colour; is very soft, sectile,
easily broken, and adheres slightly to the tongue; spec. grav. 2·11. The streak is
glistening. It occurs in beds in primitive and transition clay-slate; also in secondary
formations, as in the coal-measures of most countries. It is used in crayon drawing.
Its trace upon paper is regular and black. The best kinds are found in Spain, Italy, and
France. Some good black chalk occurs also in Caernarvonshire and in the island of Islay.
6. Adhesive slate, has a light greenish-gray colour, is easily broken or exfoliated, has
a shining streak, adheres strongly to the tongue, and absorbs water rapidly, with the
emission of air-bubbles and a crackling sound.
7. Bituminous shale, is a species of soft, sectile slate-clay, much impregnated with
bitumen, which occurs in the coal-measures.
8. Slate-clay, has a gray or grayish-yellow colour; is massive, with a dull glimmering
lustre from spangles of mica interspersed. Its slaty fracture approaches at times to
earthy; fragments, tabular; soft, sectile, and very frangible; specific gravity, 2·6. It
adheres to the tongue, and crumbles down when immersed for some time in water. It
is found as an alternating bed in the coal-measures. (See the sections of the strata
under Pitcoal.) When breathed upon, it emits a strong argillaceous odour. When
free from lime and iron, it forms an excellent material for making refractory fire-bricks,
being an infusible compound of alumina and silica; one of the best examples of which
is the schist known by the name of Stourbridge clay.
SMALL WARES, is the name given in this country to textile articles of the tape
kind, narrow bindings of cotton, linen, silk, or woollen fabric; plaited sash cord, braid,
&c. Tapes are woven upon a loom like that for weaving ribbons, which is now generally
driven by mechanical power. Messrs. Worthington and Mulliner obtained a
patent, in June, 1825, for improvements in such a loom, which have answered the
purposes of their large factory in Manchester very well; and in May, 1831, Mr. Whitehead,
of the same town, patented certain improvements in the manufacture of small
wares. The objects of the latter patent are, the regular taking up of the tape or cloth,
as it is woven, a greater facility of varying the vibration of the lay, together with the
saving of room required for a range of looms to stand in.[55] See Braiding Machine.
SMALT, see Azure and Cobalt.
Imported for home consumption in 1834, 162,232 lbs.; in 1835, 96,649; in 1836,
79,531; duty, 4d. per lb.
SMELTING, is the operation by which the ores of iron, copper, lead, &c., are reduced
to the metallic state. See Metallurgy, Ores, and the respective metals.
SOAP (Savon, Fr.; Seife, Germ.); is a chemical compound, of saponified fats or
oils with potash or soda, prepared for the purposes of washing linen, &c. Fatty[1142]
matters, when subjected to the action of alkaline lyes, undergo a remarkable change,
being converted into three different acids, called stearic, margaric, and oleic; and it is
these acids, in fact, which combine with the bases, in definite proportions, to form
compounds analogous to the neutro-saline. Some chemical writers describe under the
title soap, every compound which may result from the union of fats with the various
earths and metallic oxides—a latitude of nomenclature which common language cannot
recognise, and which would perplex the manufacturer.
Soaps are distinguished into two great classes, according to their consistence; the
hard and the soft; the former being produced by the action of soda upon fats, the latter
by that of potash. The nature of the fats contributes also somewhat to the consistence
of soaps; thus tallow, which contains much stearine and margarine, forms with potash
a more consistent soap than liquid oils will do, which consist chiefly of oleine. The
drying oils, such as those of linseed and poppy, produce the softest soaps.
1. Of the manufacture of hard soap.—The fat of this soap, in the northern countries
of Europe, is usually tallow, and in the southern, coarse olive oil. Different species of
grease are saponified by soda, with different degrees of facility; among oils, the olive,
sweet almond, rapeseed, and castor oil; and among solid fats, tallow, bone grease, and
butter, are most easily saponified. According to the practice of the United Kingdom,
six or seven days are required to complete the formation of a pan of hard soap, and a
day or two more for settling the impurities, if it contains rosin. From 12 to 13 cwt.
of tallow are estimated to produce one ton of good soap. Some years ago, in many
manufactories the tallow used to be saponified with potash lyes, and the resulting soft
soap was converted, in the course of the process, into hard soap, by the introduction of
muriate of soda, or weak kelp lyes, in sufficient quantity to furnish the proper quantity
of soda by the reaction of the potash upon the neutral salts. But the high price of
potash, and the diminished price as well improved quality of the crude sodas, have
led to their general adoption in soap-works. The soda-ash used by the soap-boiler, contains
in general about 36 per cent. of real soda, in the state of dry carbonate, mixed with
muriate of soda, and more or less undecomposed sulphate. I have met lately with soda-ash,
made from sulphate of soda, in which the materials had been so ill worked, and so
imperfectly decomposed, as to contain 16 per cent. of sulphate, a circumstance equally
disgraceful, as it was ruinous to the soda manufacturer. The barillas from Spain and
Teneriffe contain from 18 to 24 per cent. of real soda. The alkali in both states is
employed in England; barilla being supposed by many to yield a finer white or curd
soap, on account of its freedom from sulphur.
The crude soda of either kind being ground, is to be stratified with lime in
cylindrical cast-iron vats, from 6 to 7 feet wide, and from 4 to 5 feet deep; the lowest
layer consisting, of course, of unslaked or shell quicklime. The vats have a false bottom,
perforated with holes, and a lateral tubulure under it, closed commonly with a wooden
plug, similar to the épine of the French soap pans, by which the lyes trickle off clear
and caustic, after infiltration through the beds of lime. The quantity of lime must be
proportional to the carbonic acid in the soda.
Upon 1 ton of tallow put into the soap pan, about 200 gallons of soda lye, of
specific gravity 1·040, being poured, heat is applied, and after a very gentle ebullition
of about 4 hours, the fat will be found to be completely saponified, by the test of the
spatula, trowel, or pallet knife; for the fluid lye will be seen to separate at once upon
the steel blade, from the soapy paste. Such lyes, if composed of pure caustic soda,
would contain 4 per cent. of alkali; but from the presence of neutro-saline matter, they
seldom contain so much as 2 per cent.; in fact, a gallon may be estimated to contain
not more than 2 ounces; so that 200 gallons contain 25 pounds of real soda. The fire
being withdrawn from the soap pan, the mass is allowed to cool during one hour, or a
little more, after which the spent lyes, which are not at all alkaline, are run off by a spigot
below, or pumped off above, by a pump set into the pan. A second similar charge of lye is
now introduced into the pan, and a similar boiling process is renewed. Three such boils
may be given in the course of one day’s work, by an active soap-maker. Next day the same
routine is resumed with somewhat stronger lyes, and so progressively, till, towards the sixth
day, the lye may have the density of 1·160, and will be found to contain 6 per cent. of real
soda.[56] Were the lye a solution of pure caustic soda, it would contain at this density
no less than 143⁄4 per cent. of alkali. The neutro-saline matter present in the spent lye
is essential to the proper granulation and separation of the saponaceous compound; for
otherwise the watery menstruum would dilute and even liquefy the soap. Supposing
121⁄2 cwt. of tallow to yield upon an average 20 cwt. of hard soap, then 20 cwt. of tallow
will produce 32 cwt.; and as its average contents in soda are 6 per cent., these 32 cwt.
should require 1·52 cwt. of real soda for their production. If barilla at 20 per cent.
be the alkali employed, then 7·6 cwt. of barilla must be consumed in the said process.[1143]
If the alkali be soda-ash of 40 per cent., half the weight will of course suffice. I have
reason to believe that there is great waste of alkali incurred in many soap-works, as
6 cwt. of soda-ash, of at least 30 per cent., is often expended in making 1 ton of soap,
being 50 per cent. more than really enters into the composition of the soap.
The barillas always contain a small proportion of potash, to which their peculiar
value, in making a less brittle or more plastic hard soap than the factitious sodas, may
with great probability be ascribed. Chemistry affords many analogies, especially in
mineral waters, where salts, apparently incompatible, co-exist in dilute solutions. We
may thus conceive how a small quantity of stearate or oleate of potash may resist the
decomposing action of the soda salts. The same modification of the consistence of hard
soap may, however, be always more conveniently produced by a proper admixture
of oleine with stearine.
Soda which contains sulphurets is preferred for making the mottled or marbled soap,
whereas the desulphuretted soda makes the best white curd soap. Mottling is usually
given in the London soap-works, by introducing into the nearly finished soap in the
pan a certain quantity of the strong lye of crude soda, through the rose spout of a
watering-can. The dense sulphuretted liquor, in descending through the pasty mass,
causes the marbled appearance. In France a small quantity of solution of sulphate
of iron is added during the boiling of the soap, or rather with the first service of
the lyes. The alkali seizes the acid of the sulphate, and sets the protoxide of iron
free, to mingle with the paste, to absorb more or less oxygen, and to produce thereby
a variety of tints. A portion of oxide combines also with the stearine to form a
metallic soap. When the oxide passes into the red state, it gives the tint called manteau
Isabelle. As soon as the mottler has broken the paste, and made it pervious in all directions,
he ceases to push his rake from right to left, but only plunges it perpendicularly, till he
reaches the lye; then he raises it suddenly in a vertical line, making it act like the
stroke of a piston in a pump, whereby he lifts some of the lye, and spreads it over the
surface of the paste. In its subsequent descent through the numerous fissures and
channels, on its way to the bottom of the pan, the coloured lye impregnates the soapy
particles in various forms and degrees, whence a varied marbling results.
Three pounds of olive oil afford five pounds of marbled Marseilles soap of good quality,
and only four pounds four ounces of white soap; showing that more water is
retained by the former than the latter. Oil of grains, as linseed and rapeseed, do not
afford so solid a soda soap as oil of olives; but tallow affords a still harder soap with
soda. Some of the best Windsor soap made in London contains one part of olive oil
(gallipoli) for every nine parts of tallow. Much of the English hard soap is made
with kitchen and bone fat, of a very coarse quality; the washing of the numerous
successive lyes, however, purifies the foul fats, and deprives them of their offensive smell
in a great degree. It is common now at Marseilles to mix ten per cent. of the oil of
grains with olive oil; for which purpose a large proportion of the oils extracted from
seeds in the mills of the Department du Nord is sent to Marseilles; but five per cent. of
poppy-seed oil, mixed with tallow, renders the soap made with the mixture stringy and
unfit for washing; because the two species of fat refuse to amalgamate.
The affinity between the stearine of tallow and the alkali, is so great that a soap
may be speedily made from them in the cold. If we melt tallow at the lowest
possible temperature, and let it cool to the fixing point, then add to it half its weight of
caustic lye, at 36° B., agitating meanwhile incessantly with a pallet knife, we shall
perceive, at the end of some hours of contact, the mixture suddenly acquire a very solid
consistence, and at the same moment assume a marked elevation of temperature, proving
the phenomenon to be due to chemical attraction. In some trials of this kind, the
thermometer has risen from 54° to 140° F.
According to recent experiments made in Marseilles, 100 pounds of olive oil take, for
their conversion into soap, 54 pounds of crude soda, of 36 per cent. alkaline strength.
One part of lime is employed for rendering three parts of the soda caustic. The richer
the oil is in stearine, the more dilute should be the lye used in the saponification; and
vice versâ when it abounds in oleine. For oil of the former kind, the first lyes added
have a density of from 8° to 9° B.; but for the latter kind, the density is from 10° to 11°.
When four parts of olive oil are mixed with one part of poppy, rape, or linseed oil, as
is now the general practice at Marseilles, then for such a mixture the first lyes have
usually a specific gravity of from 20° to 25°, the second from 10° to 15°, and the third
from 4° to 5°, constituting a great difference from the practice in Great Britain, where
the weaker lyes are generally employed at the commencement. The chief reason for this
practice is, however, to be found in the more complete causticity of the weak than of
the strong lyes, according to the slovenly way in which most of our soap-boilers prepare
them. Indeed, one very extensive manufacturer of soap in London assured me that the
lyes should not be caustic; an extraordinary assertion, upon which no comment need be
made. In common cases, I would recommend the first combination of the ingredients[1144]
to be made with somewhat weak, but perfectly caustic lye, and when the saponification
is fairly established, to introduce the stronger lye.
In a Marseilles soap-house, there are four lye-vats in each set: No. 1. is the fresh vat,
into which the fresh alkali and lime are introduced; No. 2. is called the avançaire,
being one step in advance; No. 3. is the small avançaire, being two steps in advance,
and therefore containing weaker liquor; No. 4. is called the water vat, because it receives
the water directly.
Into No. 3. the moderately exhausted or somewhat spent lyes are thrown. From
No. 3. the lye is run or pumped into No. 2., to be strengthened; and in like manner
from No. 3. into No. 1. Upon the lime paste in No. 4., which has been taken from
No. 3., water is poured; the lye thus obtained is poured upon the paste of No. 3., which
has been taken from No. 2. No. 3. is twice lixiviated; and No. 2., once. The receiver
under No. 1. has four compartments; into No. 1. of which the first and strongest lye is
run; into No. 2. the second lye; into No. 3. the third lye; and into No. 4. the fourth
lye, which is so weak as to be used for lixiviation, instead of water; (pour d’avances).
The lime of vat No. 4., when exhausted, is emptied out of the window near to which
it stands; in which case the water is poured upon the contents of No. 3.; and upon No.
2. the somewhat spent lyes.
No. 1. is now the avançaire of No. 4; because this has become, in its turn, the fresh
vat, into which the fresh soda and quicklime are put. The lye discharged from No. 3.
comes, in this case, upon No. 2.; and after being run through it, is thrown upon No. 1.
144 pounds of oil yield at Marseilles, upon an average, not more than from 240 to 244
pounds of soap; or 100 pounds yield about 168; so that in making 100 pounds of soap,
at this rate nearly 60 pounds of oil are consumed.
OF YELLOW OR ROSIN SOAP.
Rosin, although very soluble in alkaline menstrua, is not however susceptible, like fats,
of being transformed into an acid, and will not of course saponify, or form a proper soap by
itself. The more caustic the alkali, the less consistence has the resinous compound which
is made with it. Hence fat of some kind, in considerable proportion, must be used along
with the rosin, the minimum being equal parts; and then the soap is far from being good.
As alkaline matter cannot be neutralized by rosin, it preserves its peculiar acrimony in a
soap poor in fat, and is ready to act too powerfully upon woollen and all other animal
fibres to which it is applied. It is said that rancid tallow serves to mask the strong odour
of rosin in soap, more than any oil or other species of fat. From what we have just
said, it is obviously needless to make the rosin used for yellow soaps pass through all the
stages of the saponifying process; nor would this indeed be proper, as a portion of the
rosin would be carried away, and wasted with the spent lyes. The best mode of proceeding,
therefore, is first of all to make the hard soap in the usual manner, and at the last
service or charge of lye, namely, when this ceases to be absorbed, and preserves in the
boiling-pan its entire causticity, to add the proportion of rosin intended for the soap.
In order to facilitate the solution of the rosin in the soap, it should be reduced to coarse
powder, and well incorporated by stirring with the rake. The proportion of rosin is
usually from one-third to one-fourth the weight of the tallow. The boil must be kept
up for some time with an excess of caustic lye; and when the paste is found, on cooling
a sample of it, to acquire a solid consistence, and when diffused in a little water, not
to leave a resinous varnish on the skin, we may consider the soap to be finished. We
next proceed to draw off the superfluous lyes, and to purify the paste. For this
purpose, a quantity of lyes at 80° B. being poured in, the mass is heated, worked
well with a rake, then allowed to settle, and drained of its lyes. A second service of
lyes, at 4° B., is now introduced, and finally one at 2°; after each of which, there is the
usual agitation and period of repose. The pan being now skimmed, and the scum
removed for another operation, the soap is laded off by hand-pails into its frame-moulds.
A little palm oil is usually employed in the manufacture of yellow soap, in order to
correct the flavour of the rosin, and brighten the colour. This soap, when well made,
ought to be of a fine wax-yellow hue, be transparent upon the edges of the bars, dissolve
readily in water, and afford, even with hard pump-water, an excellent lather.
The frame-moulds for hard soap are composed of strong wooden bars, made into the
form of a parallelogram, which are piled over each other, and bound together by screwed
iron rods, that pass down through them. A square well is thus formed, which in large soap
factories is sometimes 10 feet deep, and capable of containing a couple of tons of soap.
Mr. Sheridan some time since obtained a patent for combining silicate of soda with
hard soap, by triturating them together in the hot and pasty state with a crutch in
an iron pan. In this way from 10 to 30 per cent. of the silicate may be introduced.
Such soap possesses very powerful detergent qualities, but it is apt to feel hard and be
somewhat gritty in use. The silicated soda is prepared by boiling ground flints in a
strong caustic lye, till the specific gravity of the compound rises to nearly double the[1145]
density of water. It then contains about 35 grains of silica, and 46 of soda-hydrate, in
100 grains[57].
Hard soap, after remaining two days in the frames, is at first divided horizontally
into parallel tablets, 3 or 4 inches thick, by a brass wire; and these tablets are again cut
vertically into oblong nearly square bars, called wedges in Scotland.
The soap-pans used in the United Kingdom are made of cast iron, and in three separate
pieces joined together by iron-rust cement. The following is their general form:—The
two upper frusta of cones are called curbs; the third, or undermost, is the pan, to
which alone the heat is applied, and which, if it gets cracked in the course of boiling,
may easily be lifted up within the conical pieces, by attaching chains or cords for raising
it, without disturbing the masonry, in which the curbs are firmly set. The surface of
the hemispherical pan at the bottom, is in general about one-tenth part of the surface
of the conical sides.
The white ordinary tallow soap of the London manufacturers, called curd soap, consists,
by my experiments, of—fat, 52; soda, 6; water, 42; = 100. Nine-tenths of the
fat, at least, is tallow.
I have examined several other soaps, and have found their composition somewhat
different.
The foreign Castile soap of the apothecary
has a specific gravity of 1·0705, and
consists of—
| Soda |
9 |
|
| Oily fat |
76 |
·5 |
| Water and colouring-matter |
14 |
·5 |
| |
100 |
·0 |
English imitation of Castile soap, spec.
grav. 0·9669, consists of—
| Soda |
10 |
·5 |
| Pasty-consistenced fat |
75 |
·2 |
| Water, with a little colouring-matter |
14 |
·3 |
| |
100 |
·0 |
A perfumer’s white soap was found to
consist of—
| Soda |
9 |
·0
|
| Fatty matter |
75 |
|
| Water |
16 |
|
| |
100 |
|
Glasgow white soap—
| Soda |
6 |
·4 |
| Tallow |
60 |
·0 |
| Water |
33 |
·6 |
| |
100 |
·0 |
Glasgow brown rosin soap—
| Soda |
6 |
·5 |
| Fat and rosin |
70 |
·0 |
| Water |
23 |
·5 |
| |
100 |
·0 |
A London cocoa-nut oil soap was found to
consist of—
| Soda |
4 |
·5 |
| Cocoa-nut lard |
22 |
·0 |
| Water |
73 |
·5 |
| |
100 |
·0 |
This remarkable soap was sufficiently
solid; but it dissolved in hot water with
extreme facility. It is called marine soap,
because it washes linen with sea water.
A poppy-nut-oil hard soap consisted
of—
| Soda |
7 |
|
| Oil |
76 |
|
| Water |
17 |
|
| |
100 |
[58] |
The soap known in France by the name
of soap in tables consists, according to
M. Thenard’s analysis, of—
| Soda |
4 |
·6 |
| Fatty matter |
50 |
·2 |
| Water |
45 |
·2 |
| |
100 |
·0 |
M. D’Arcet states the analysis of Marseilles
soap at—
| Soda |
6 |
|
| Oil |
60 |
|
| Water |
34 |
|
| |
100 |
|
SOFT SOAP.
The principal difference between soaps with base of soda, and soaps with base of potash,
depends upon their mode of combination with water. The former absorb a large
quantity of it, and become solid; they are chemical hydrates. The others experience a
much feebler cohesive attraction; but they retain much more water in a state of mere
mixture.
Three parts of fat afford, in general, fully five parts of soda soap, well dried in the open
air; but three parts of fat or oil will afford from six to seven parts of potash soap of
moderate consistence. This feebler cohesive force renders it apt to deliquesce, especially
if there be a small excess of the alkali. It is, therefore, impossible to separate it from
the lyes; and the washing or relargage, practised on the hard-soap process, is inadmissible
in the soft. Perhaps, however, this concentration or abstraction of water might be
effected by using dense lyes of muriate of potash. Those of muriate or sulphate of
soda change the potash into a soda soap, by double decomposition. From its superior[1146]
solubility, more alkaline reaction, and lower price, potash soap is preferred for many
purposes, and especially for scouring woollen yarns and stuffs.
Soft soaps are usually made in this country with whale, seal, olive, and linseed oils,
and a certain quantity of tallow; on the continent, with the oils of hempseed, sesame, rapeseed,
linseed, poppy-seed, and colza; or with mixtures of several of these oils. When
tallow is added, as in Great Britain, the object is to produce white and somewhat solid
grains of stearic soap in the transparent mass, called figging, because the soap then resembles
the granular texture of a fig.
The potash lyes should be made perfectly caustic, and of at least two different
strengths; the weakest being of specific gravity 1·05; and the strongest, 1·20, or even
1·25. Being made from the potashes of commerce, which contain seldom more than
60 per cent., and often less, of real alkali, the lyes correspond in specific gravity to
double their alkaline strength; that is to say, a solution of pure potash, of the same
density, would be fully twice as strong. The following is the process followed by respectable
manufacturers of soft soap (savon vert, being naturally or artificially green,)
upon the continent.
A portion of the oil being poured into the pan, and heated to nearly the boiling point
of water, a certain quantity of the weaker lye is introduced; the fire being kept up so
as to bring the mixture to a boiling state. Then some more oil and lye are added alternately,
till the whole quantity of oil destined for the pan is introduced. The ebullition
is kept up in the gentlest manner possible, and some stronger lye is occasionally
added, till the workman judges the saponification to be perfect. The boiling becomes
progressively less tumultuous, the frothy mass subsides, the paste grows transparent, and
it gradually thickens. The operation is considered to be finished when the paste ceases
to affect the tongue with an acrid pungency, when all milkiness and opacity disappear,
and when a little of the soap placed to cool upon a glass-plate, assumes the proper
consistency.
A peculiar phenomenon may be remarked in the cooling, which affords a good criterion
of the quality of the soap. When there is formed around the little patch, an
opaque zone, a fraction of an inch broad, this is supposed to indicate complete saponification,
and is called the strength; when it is absent, the soap is said to want its strength.
When this zone soon vanishes after being distinctly seen, the soap is said to have false
strength. When it occurs in the best form, the soap is perfect, and may be secured in
that state by removing the fire, and then adding some good soap of a previous round, to
cool it down, and prevent further change by evaporation.
200 pounds of oil require for their saponification—72 pounds of American potash of
moderate quality, in lyes at 15° B.; and the product is 460 pounds of well-boiled soap.
If hempseed oil have not been employed, the soap will have a yellow colour, instead
of the green, so much in request on the continent. This tint is then given by the addition
of a little indigo. This dye-stuff is reduced to fine powder, and boiled for some
hours in a considerable quantity of water, till the stick with which the water is stirred,
presents, on withdrawing it, a gilded pellicle over its whole surface. The indigo paste
diffused through the liquid, is now ready to be incorporated with the soap in the pan,
before it stiffens by cooling.
M. Thenard states the composition of soft soap at—potash 9·5, + oil 44·0, + water
46·5, = 100.
Good soft soap of London manufacture, yielded to me—potash 8·5, + oil and tallow
45, + water 46·5.
Belgian soft or green soap afforded me—potash 7, + oil 36, + water 57, = 100.
Scotch soft soap, being analyzed, gave me—potash 8, + oil and tallow 47, + water 45.
Another well-made soap—potash 9, + oil and fat 34, + water 57.
A rapeseed-oil soft soap, from Scotland, consisted of—potash 10, + oil 51·66, +
water 38·33.
An olive-oil (gallipoli) soft soap, from ditto, contained—potash with a good deal of
carbonic acid 10, oil 48, water 42, = 100.
A semi-hard soap, from Verviers, for fulling woollen cloth, called savon économique,
consisted of, potash 11·5, + fat (solid) 62, + water 26·5, = 100.
The following is a common process, in Scotland, by which good soft soap is made:—
273 gallons of whale or cod oil, and 4 cwt. of tallow, are put into the soap-pan, with
250 gallons of lye from American potash, of such alkaline strength that 1 gallon contains
6600 grains of real potash. Heat being applied to the bottom pan, the mixture
froths up very much as it approaches the boiling temperature, but is prevented from
boiling over by being beat down on the surface, within the iron curb or crib which surmounts
the cauldron. Should it soon subside into a doughy-looking paste, we may infer
that the lye has been too strong. Its proper appearance is that of a thin glue. We
should now introduce about 42 gallons of a stronger lye, equivalent to 8700 gr. of potash
per gallon; and after a short interval, an additional 42 gallons; and thus successively[1147]
till nearly 600 such gallons have been added in the whole. After suitable
boiling to saponify the fats, the proper quality of soap will be obtained, amounting in
quantity to 100 firkins of 64 pounds each, from the above quantity of materials.
It is generally supposed, and I believe it to be true, from my own numerous experiments
upon the subject, that it is a more difficult and delicate operation to make a
fine soft soap of glassy transparency, interspersed with the figged granulations of stearate
of potash, than to make hard soap of any kind.
Soft soap is made in Belgium as follows:—For a boil of 18 or 20 tons, of 100
kilogrammes each, there is employed for the lyes—1500 pounds of American potashes,
and 500 to 600 pounds of quicklime.
The lye is prepared cold in cisterns of hewn stone, of which there are usually five in
a range. The first contains the materials nearly exhausted of their alkali; and the last
the potash in its entire state. The lye run off from the first, is transferred into the
second; that of the second into the third; and so on to the fifth.
In conducting the empatage of the soap, they put into the pan, on the eve of the
boiling-day, six aimes (one ohm, = 30 gallons imperial,) of oil of colza, in summer,
but a mixture of that oil with linseed oil in winter, along with two aimes of potash lye
at 13° B., and leave the mixture without heat during eight hours. After applying the
fire, they continue to boil gently till the materials cease to swell up with the heat;
after which, lye of 16° or 17° must be introduced successively, in quantities of one
quarter of an aime after another, till from 2 to 4 aimes be used. The boil is finished by
pouring some lye of 20° B., so that the whole quantity may amount to 91⁄2 aimes.
It is considered that the operation will be successful, if from the time of kindling the
fire till the finish of the boil, only five hours elapse. In order to prevent the soap from
boiling over, a wheel is kept revolving in the pan. The operative considers the soap
to be finished, when it can no longer be drawn out into threads between the finger
and thumb. He determines if it contains an excess of alkali, by taking a sample
out during the boil, which he puts into a tin dish; where if it gets covered with a skin,
he pours fresh oil into the pan, and continues the boil till the soap be perfect. No
wonder the Belgian soap is bad, amid such groping in the dark, without one ray of
science!
SOFT TOILET SOAPS.
The soft fancy toilet soaps are divisible into two classes: 1. good potash soap, coloured
and scented in various ways, forms the basis of the Naples and other ordinary soft soaps
of the perfumer; 2. pearl soap (savon nacré), which differs from the other both in physical
aspect and in mode of preparation.
Ordinary soft Toilet Soap.—Its manufacture being conducted on the principles
already laid down, presents no difficulty to a man of ordinary skill and experience; the
only point to be strictly attended to, is the degree of evaporation, so as to obtain soap
always of uniform consistence. The fat generally preferred is good hog’s lard; of which
30 pounds are to be mixed with 45 pounds of a caustic lye marking 17° on Baumé’s
scale; the temperature is to be gradually raised to ebullition, but the boil must not be
kept up too long or too briskly, till after the empatage or saponification is completed, and
the whole of the lye intimately combined with the fatty particles; after this, the evaporation
of the water may be pushed pretty quickly, by a steady boil, till copious vapours cease to
rise. This criterion is observed when the paste has become too stiff to be stirred freely.
The soap should have a dazzling snowy whiteness, provided the lard has been well refined,
by being previously triturated in a mortar, melted by a steam heat, and then strained.
The lard soap so prepared, is semi-solid, and preserves always the same appearance. If
the paste is not sufficiently boiled, however, it will show the circumstance very soon; for
in a few days the soap will become gluey and stringy, like a tenacious mass of birdlime.
This defect may not only be easily avoided, but easily remedied, by subjecting the
paste to an adequate evaporation. Such soaps are in great request for shaving, and
are most convenient in use, especially for travellers. Hence their sale has become
very considerable.
Pearl soft Soap.—It is only a few years since the process for making this elegant soap
became known in France. It differs little from the preceding, and owes its beautiful
aspect merely to minute manipulations, about to be described. Weigh out 20 pounds
of purified hog’s lard on the one hand; and 10 pounds of potash lye at 36° B. on the
other. Put the lard into a porcelain capsule, gently heated upon a sand-bath, stirring
it constantly with a wooden spatula; and when it is half melted, and has a milky
appearance, pour into it only one-half of the lye, still stirring, and keeping up the same
temperature, with as little variation as possible. While the saponification advances
gradually, we shall perceive, after an hour, some fat floating on the surface, like a film of
oil, and at the same time the soapy granulations falling to the bottom. We must then
add the second portion of the lye; whereon the granulations immediately disappear[1148]
and the paste is formed. After conducting this operation during four hours, the paste
becomes so stiff and compact, that it cannot be stirred; and must then be lightly beaten.
At this time the capsule must be transferred from the sand-bath into a basin of warm
water, and allowed to cool very slowly.
The soap, though completely made, has yet no pearly appearance. This physical
property is developed only by pounding it strongly in a marble mortar; whereby all its
particles, which seemed previously separated, combine to form a homogeneous paste.
The perfume given to it, is always essence of bitter almonds; on which account the
soap is called almond cream, crème d’amandes.
HARD SOAPS FOR THE TOILET.
The soaps prepared for the perfumer, are distinguished into different species, according
to the fat which forms their basis. Thus there is soap of tallow, of hog’s lard, of
oil of olives, of almonds, and palm oil.
It is from the combination of these different sorts, mingled in various proportions,
and perfumed agreeably to the taste of the consumer, that we owe the vast number of
toilet soaps sold under so many fantastic names. One sort is rarely scented by itself,
as a mixture of several is generally preferred; in which respect every perfumer has his
peculiar secret. Some toilet soaps, however, require the employment of one kind more
than of another.
Formerly the Windsor soap was made in France, wholly with mutton suet; and it
was accordingly of inferior value. Now, by mixing some olive oil or lard with the
suet, a very good Windsor soap is produced. I have already stated, that the fat of the
London Windsor is, nine parts of good ox tallow, and one of olive oil. A soap made
entirely with oil and soda, does not afford so good a lather as when it contains a considerable
proportion of tallow.
The soaps made with palm oil are much used; when well made, they are of excellent
quality, and ought to enter largely into all the coloured sorts. They naturally
possess the odour of violets.
The soaps made with oil of almonds are very beautiful, and preserve the agreeable
smell of their perfume; but being expensive, are introduced sparingly into the mixtures
by most manufacturers.
Some perfumers are in the habit of making what may be called extempore soaps, employing
lyes at 36° Baumé in their formation. This method, however, ought never to
be adopted by any person who prefers quality to beauty of appearance. Such soap is,
indeed, admirably white, glistening, contains no more water than is necessary to its constitution,
and may therefore be sold the day after it is made. But it has counterbalancing
disadvantages. It becomes soon very hard, is difficultly soluble in water, and,
if not made with tallow, does not lather well. Hog’s lard is very commonly used, for
making that soap. Twenty kilogrammes of the fat are taken, to ten kilogrammes of
soda lye, at 36° B. (specific gravity 1·324); as soon as the former is nearly fluid, 5 kilogrammes
of the lye are introduced, and the mixture is continually agitated during an
hour with a wooden spatula. The temperature should never be raised above 150° Fahr.
at the commencement of the operation; at the end of one hour, 5 other kilogrammes of
lye are to be added, with careful regulation of the heat. The paste thus formed by the
union of the fat and alkali, ought to be perfectly homogeneous, and should increase in
consistence every hour, till it becomes firm enough to be poured into the frame; during
which transfer, the essential oils destined to scent it, should be introduced. Next day
the soap is hard enough; nor does it differ in appearance from ordinary soap, only it
requires prompt manipulation to be cut into bars and cakes; for when neglected a day
or two, it may become too brittle for that purpose, and too hard to take the impression
of the stamps in relief. Such an article gets the name of little-pan soap, on account of
the small quantity in which it is usually manufactured. Hard soap, made in the common
way, is, on the contrary, called large-pan soap. This extemporaneous compound is
now seldom or never made by respectable manufacturers. In making Windsor soap, the
admixture of olive oil is advantageous; because, being richer in oleine than suet, it saponifies
less readily than it, and thus favours the formation of a more perfect neutral combination.
When the soap cuts, or parts from the lye, when the paste becomes clotty, or, in the
language of the operative, when the grain makes its appearance, the fire should be immediately
withdrawn, that the impurities may be allowed to subside. This part of the
operation lasts 12 hours at least; after which, the soap, still hot, becomes altogether fluid
and perfectly neutral.
For every 1000 pounds of the paste, there must be introduced 9 pounds of essences,
mingled in the following proportions:—6 pounds of essence of carui; 11⁄2 ditto lavender
(finest); 11⁄2 ditto rosemary.
The mixture must be well stirred, in order to get completely saturated with the
perfumes; and this may be readily done without at all touching or stirring up the[1149]
subjacent lyes; in the course of two hours, the soap may be transferred into the ordinary
frames. In twenty-four hours, the mass is usually solidified enough for cutting into
bars and cakes, ready to be stamped for sale.
The above method of scenting Windsor soap is practised only in the largest establishments;
in the smaller, the soap is pailed out of the soap-pans, into a pan provided with
a steam case or jacket, and there mixed with the essential oils, by means of appropriate
heat and agitation.
The most fashionable toilet soaps are, the rose, the bouquet, the cinnamon, the orange-flower,
the musk, and the bitter almond or peach blossom.
Soap à la rose.—This is made of the following ingredients: 30 pounds of olive-oil
soap; 20 of good tallow soap.
Toilet soaps must be reduced to thin shavings, by means of a plane, with its under
face turned up, so that the bars may be slid along it. These shavings must be put into an
untinned copper pan, which is surrounded by a water-bath, or steam. If the soap be old
and hard, 5 pounds of water must be added to them; but it is preferable to take fresh-made
soaps, which may melt without addition, as soap some time kept does not readily
form a homogeneous paste. The fusion is commonly completed in an hour, or thereby,
the heat being applied at 212° F., to accelerate the progress, and prevent the dissolution
of the constituent water of the soap. For this purpose the interior pan may be covered.
Whenever the mass is sufficiently liquefied, 11⁄2 ounces of finely ground vermillion are to
be introduced, and thoroughly mixed, after which the heat may be taken off the pan;
when the following perfumes may be added with due trituration:—3 ounces of essence
of rose; 1 ditto cloves; 1 ditto cinnamon; 21⁄2 ditto bergamot; = 71⁄2.
The scented soap being put into the frames, speedily consolidates. Some recommend
to pass the finished fused soap through a tammy cloth, in order to free it from all clots and
impurities; a very proper precaution in the act of transferring it to the frame. If the
preceding instructions be observed, we obtain a soap perfect in every point of view;
possessing a delicious fragrance, equally rich and agreeable, a beautiful roseate hue, and
the softest detergent qualities, which keeping cannot impair. Such a soap has, in fact,
been known to retain every property in perfection during four or five years. When the
essential oils are particularly volatile, they should not be added to the soap till its temperature
has fallen to about 140° Fahr.; but in this case a more careful trituration is
required. The economy is, however, ill bestowed; for the cakes made of such cooler
soap, are never so homogeneous and glossy.
Soap au bouquet.—30 pounds of good tallow soap; 4 ounces of essence of bergamot;
oil of cloves, sassafras, and thyme, 1 ounce each; neroli, 1⁄2 ounce. The colour is given
with 7 ounces of brown ochre.
Cinnamon Soap.—30 pounds of good tallow soap; 20 ditto of palm-oil soap. Perfumes:—7
ounces of essence of cinnamon; 11⁄4 ditto sassafras; 11⁄4 ditto bergamot.
Colour:—1 pound of yellow ochre.
Orange-flower Soap.—30 pounds of good tallow soap; 20 ditto palm-oil soap.
Perfumes:—71⁄2 ounces essence of Portugal; 71⁄2 ditto amber. Colour:—91⁄2 ounces,
consisting of 81⁄4 of a yellow-green pigment, and 11⁄4 of red lead.
Musk Soap.—30 pounds of good tallow soap; 20 ditto palm-oil soap. Perfumes:—Powder
of cloves, of pale roses, gilliflower, each 41⁄2 ounces; essence of bergamot, and
essence of musk, each 31⁄2 ounces. Colour:—4 ounces of brown ochre, or Spanish
brown.
Bitter Almond Soap.—Is made by compounding, with 50 pounds of the best white
soap, 10 ounces of the essence of bitter almonds.
LIGHT SOAPS.
The apparatus employed for making these soaps, is a copper pan, heated by a water-bath;
in the bottom of the pan there is a step, to receive the lower end of a vertical shaft, to
which arms or paddles are attached, for producing constant agitation, by causing them to
revolve among the liquefied mass. Into a pan so mounted, 50 pounds of a good oil
soap of any kind are put (for a tallow soap does not become frothy enough), and melted
by proper heat, with the addition of 3 or 4 pounds of water. By the rapid rotation of
the machine, an abundant thick lather is produced, beginning first at the bottom, and
creeping gradually upwards to the top of the pan, when the operation should be stopped;
the soap having by this time doubled its volume. It must now be pailed off into the
frame, allowed to cool, and then cut into cakes. Such soap is exceedingly pleasant
at the wash-stand, feeling very soft upon the skin, affording a copious thick lather,
and dissolving with the greatest ease.
TRANSPARENT SOAPS.
These soaps were for a long time manufactured only in England, where the process
was kept a profound secret. They are now made every where.
[1150]
Equal parts of tallow soap, made perfectly dry, and spirit of wine, are to be put into a
copper still, which is plunged in a water-bath, and furnished with its capital and
refrigeratory. The heat applied to effect the solution should be as slight as possible, to
avoid evaporating too much of the alcohol. The solution being effected, must be suffered
to settle; and after a few hours’ repose, the clear supernatant liquid is drawn off
into tin frames, of the form desired for the cakes of soap. These bars do not acquire their
proper degree of transparency till after a few weeks’ exposure to dry air. They are now
planed, and subjected to the proper mechanical treatment for making cakes of any form.
The soap is coloured with strong alcoholic solution of archil for the rose tint, and of turmeric
for the deep yellow. Transparent soaps, however pleasing to the eye, are always
of indifferent quality; they are never so detergent as ordinary soaps, and they eventually
acquire a disagreeable smell.
| Soap charged with duty in |
1834. |
1835. |
1836. |
| |
lbs. |
lbs. |
lbs. |
| Hard |
144,344,043 |
143,806,207 |
146,539,210 |
| Soft |
10,401,281 |
12,103,109 |
13,358,894 |
| Amount of duty |
at 11⁄2d. |
per lb. on |
hard soap |
£902,150 |
£930,039 |
£915,861 |
| do. |
at 1d. |
|
soft soap |
43,339 |
50,429 |
55,662 |
SODA, Caustic soda (Hydrate de soude, Fr.; Aetznatron, Germ.); is an alkaline substance,
used in chemical researches, in bleaching, and in the manufacture of soap. It is
prepared by boiling a solution of crystallized carbonate of soda in 4 or 5 parts of water,
with half its weight of recently slaked and sifted lime. At the end of half an hour, the
vessel of iron, porcelain, or preferably silver, may be removed from the fire, and covered
carefully, till the calcareous matter has settled into a solid magma at the bottom. The
clear supernatant lye may be then decanted into bottles for use in the liquid state, or
evaporated, out of contact of air, till it assumes an oily appearance, then poured upon an
iron or marble slab, broken into pieces, and put up in phials secured with greased
stoppers or corks.
Caustic soda is a white brittle mass, of a fibrous texture, a specific gravity of 1·536,
melting at a heat under redness, having a most corrosive taste and action upon animal
matters, dissolving readily in both water and alcohol, attracting carbonic acid when
exposed to the atmosphere, but hardly any water, and falling thereby into an
efflorescent carbonate; it forms soaps with tallow, oils, wax, rosin; dissolves wool,
hair, silk, horn, alumina, silica, sulphur, and some metallic sulphurets. It consists of
77·66 soda, and 22·34 water. A solution of caustic soda affords no precipitate with
solution of chloride of platinum, or tartaric acid, as a solution of caustic potash never
fails to do.
The following Table of the quantity of Caustic Soda contained in Lyes of different
densities, has been given by Richter:—
Spec.
grav. |
Soda
per
cent. |
| 1·00 |
0·00 |
| 1·02 |
2·07 |
| 1·04 |
4·02 |
| 1·06 |
5·89 |
| 1·08 |
7·69 |
| 1·10 |
9·43 |
| 1·12 |
11·10 |
| 1·14 |
12·81 |
| 1·16 |
14·73 |
| 1·18 |
16·73 |
| 1·20 |
18·71 |
| 1·22 |
20·66 |
| 1·24 |
22·58 |
| 1·26 |
24·47 |
| 1·28 |
26·33 |
| 1·30 |
28·16 |
| 1·32 |
29·96 |
| 1·34 |
31·67 |
| 1·35 |
32·40 |
| 1·36 |
33·08 |
| 1·38 |
34·41 |
Soda free from water, can be obtained only by the combustion of sodium, which see.
SODA, CARBONATE OF (Kohlensaures natron, Germ.): is the soda of commerce
in various states, either crystallized, in lumps, or in a crude powder called soda-ash. It
exists in small quantities in certain mineral waters; as, for example, in those of Seltzer,
Seydschutz, Carlsbad, and the volcanic springs of Iceland, especially the Geyser; it frequently
occurs as an efflorescence in slender needles upon damp walls, being produced
by the action of the lime upon the sea salt present in the mortar. The mineral soda is
the sesquicarbonate, to be afterwards described.
Of manufactured soda, the variety most antiently known is barilla, the incinerated
ash of the Salsola soda. This plant is cultivated with great care by the Spaniards,
especially in the vicinity of Alicant. The seed is sown in light low soils, which are
embanked towards the sea shore, and furnished with sluices, for admitting an occasional
overflow of salt water. When the plants are ripe, the crop is cut down and dried; the[1151]
seeds are rubbed out and preserved; the rest of the plant is burned in rude furnaces, at
a temperature just sufficient to cause the ashes to enter into a state of semi-fusion, so
as to concrete on cooling into cellular masses moderately compact. The most valuable
variety of this article is called sweet barilla. It has a grayish-blue colour and gets
covered with a saline efflorescence when exposed for some time to the air. It is hard
and difficult to break; when applied to the tongue, it excites a pungent alkaline taste.
I have analyzed many varieties of barilla. Their average quantity of free or alkalimetrical
soda, is about 17 per cent.; though several contain only 14 parts in the
hundred, and a few upwards of 20. This soda is chiefly a carbonate, with a little
sulphuret and sulphite; and is mixed with sulphate and muriate of soda, carbonate of
lime, vegetable carbon, &c.
Another mode of manufacturing crude soda, is by burning sea-weed into kelp. Formerly
very large revenues were derived by the proprietors of the shores of the Scottish
islands and Highlands, from the incineration of sea-weed by their tenants, who usually
paid their rents in kelp; but since the tax has been taken off salt, and the manufacture
of a crude soda from it has been generally established, the price of kelp has fallen
extremely low.
The crystals of soda-carbonate, as well as the soda-ash of British commerce, are now
made altogether by the decomposition of sea salt.
SODA MANUFACTURE.
The manufacture divides itself into three branches:—1. The conversion of sea salt,
or chloride of sodium, into sulphate of soda. 2. The decomposition of this sulphate into
crude soda, called black balls by the workmen. 3. The purification of these balls, either
into a dry white soda-ash or into crystals.
1. The preparation of the sulphate of soda.—Figs. 1033, 1034, 1035. represent the
furnace for converting the muriate of soda into the sulphate. The furnace must be
built interiorly of the most refractory fire-bricks, such as are used for glasshouses, but
of the ordinary brick size; except the bridges C, G, N, which should be formed of
one mass, such as what is called a Welsh lump. A is the ash-pit; B, the grate; C, the
first bridge, between the fire and the first calcining hearth, D, D; F, F, is its roof; G, the
second bridge, between the calcining hearth and the decomposing hearth I, I, I; the
roof of which is K, K. This hearth I, I, is lined with a lead square pan, 5 or 6 inches
deep, sloped at the back opening, in fig. 1035., marked M′; which deficient part of the
upright side is filled up with two bricks placed one over the other, as shown at m, m,
fig. 1034., and luted with clay, to confine the semi-liquid mass in the pan, I, I. Some
manufacturers make this pan 8 inches deep, and line its bottom and sides with bricks
or siliceous sandstone, to protect the
lead from the corrosive action of the
acid. There are others who consider
this precaution troublesome, as the
points of the pan which become leaky
are thereby concealed. In the roof of
the decomposing hearth, one or two
syphon funnels R, of lead, are inserted
when the charge of acid (sulphuric)
is to be poured down upon
the salt in I, I, to save the risk of any
annoyance from the fumes of the muriatic acid. O, O, is a chimney filled with round
flint nodules, which are kept continually moist by the trickling of a streamlet of water
upon the topmost layer. The muriatic gas
meeting this descending film of water upon
so extensive a surface, becomes absorbed, and
runs out below in a liquid form. When the
acid is required in a somewhat concentrated
state, this chimney should be made both high
and capacious. Such a plan, moreover, is
very valuable for abating the nuisance caused
by the disengagement of the muriatic acid
gas; which is otherwise apt to sterilize the surrounding vegetation.
A fire being kindled in the grate B, figs. 1033. and 1034., 3 cwt. of salt in powder
are to be thrown by a shovel into the pan I, through the door M, fig. 1035., or m, m, fig.
1034. Two hundred weight and a half of oil of vitriol, of specific gravity 1·844 having
been diluted with from 25 to 30 per cent. of water, and well mixed, or 3 cwts. at 56°
Baumé, are to be slowly poured in by the funnel, and diffused among the muriate of
soda, by an occasional stir with an iron rake cased with sheet lead. Fumes of muriatic
acid will now plentifully escape, and, passing up the condensing-shaft O, will flow down[1152]
in the form of liquid spirit of salt, and escape by the stoneware stopcock P, into the
pipe of a sunk cistern. The fire having been steadily kept up at a moderate degree, the
chemical reaction will be tolerably complete in the course of two hours; but as this is
relative to the nature of the fuel, and the draught of the furnace, no very precise rule
in point of time can be laid down; but it is sufficient for this stage of the process, when
the fumes cease to be very dense and copious, as may be ascertained by opening the door
M, and looking in, or by the appearance at the top of the shaft O. Over the door M′, in
the opposite side of the decomposing hearth, fig. 1035., there must be an arch or hood
terminating in a small chimney, 15 or 20 feet high, for the ascent of the muriatic vapours,
when the charge is drawn or run out of the hearth, and allowed to fall into a
square shallow iron tray, placed on the ground at the back of the furnace. For this discharge,
the two bricks which serve as stoppers to that orifice, must be unluted and removed.
As soon as that charge is taken out, (the fire being meanwhile checked by opening
the door T, fig. 1034., and shutting partially the ash-pit opening at A,) a fresh charge
must be introduced as above described. The nearly decomposed saline matter during
the second charging of the hearth I, will have grown cool and concrete. It must be
shovelled into the calcining hearth D, D, fig. 1033., by the back door Q, fig. 1035., where
it will receive a higher degree of heat; and, by the expulsion of the remaining part of
the muriatic acid, it will become a
perfect sulphate of soda. It should
be finally brought into a state of semi-fusion.
When a sample of it, taken
out on the end of the rake or trowel-shaped
scraper, emits no fumes, the
conversion is accomplished.
From 3 cwts. of common salt, or
muriate of soda, rather more than 31⁄2
cwts. of perfect sulphate should be obtained, quite free from metallic impurity.
The next step is the conversion of the sulphate into a crude soda.
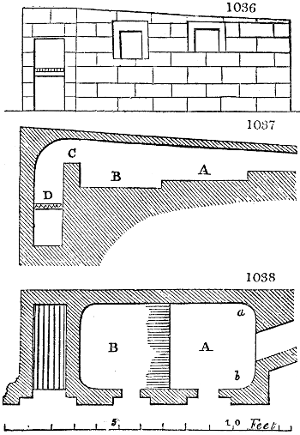
One of the most improved soda furnaces is that, employed in a few factories, represented
in figs. 1036, 1037, and 1038. In the section fig. 1037., there are two hearths
in one furnace, the one elevated above the level of the other by the thickness of a brick,
or about 3 inches. A is the preparatory shelf, where the mixture to be decomposed is
first laid in order to be thoroughly heated, so that when transferred to the lower or
decomposing hearth B, it may not essentially chill it, and throw back the operation.
C is the fire-bridge, and D is the grate.
In the horizontal section, or ground
plan, fig. 1038., we see an opening in
the front corresponding to each hearth.
This is a door, as shown in the side
view or elevation of the furnace,
fig. 1036.; and each door is shut by
an iron square frame filled with a
fire-tile or bricks, and suspended
by a chain over a pulley fixed in
any convenient place. See Pitcoal,
coking of, p. 1041. The workman,
on pushing up the door lightly,
makes it rise, because there is a counterweight
at the other end of each
chain, which balances the weight of the
frame and bricks. In the ground plan,
only one smoke-flue is shown; and
this construction is preferred by many
manufacturers; but others choose to
have two flues, one from each shoulder,
as at a, b; which two flues afterwards
unite in one vertical chimney, from
25 to 40 feet high; because the draught
of a soda-furnace must be very sharp.
Having sufficiently explained the
construction of this improved furnace,
I shall now proceed to describe
the mode of making soda with it.
The materials with which the sulphate is decomposed into a rough carbonate of soda,
are chalk or ground limestone, and ground coal or charcoal. The proportions in which
these three substances are mixed, influence in a remarkable degree the success of the[1153]
decomposing process. I have known a false proportion introduced, and persevered in,
at a factory, with the most prejudicial effect to the product; the soda-ash produced, being
in a small quantity relatively to the sulphate employed, and being much charged with
sulphur. After very numerous trials which I have made on the great scale, and many
inquiries at the most successful soda-works, both in this country and abroad, I am
warranted to offer the following proportions as the most profitable:—
Sulphate of soda, 100 parts: carbonate of lime (chalk or limestone), from 110 to
120 parts; if pure, 110; if a little impure or damp, 120: pit coal, 50 parts.
These materials must be separately ground by an edge-stone mill, and sifted into a
tolerably fine powder. They must be then very carefully mixed. Attention to these
particulars is of no little importance to the success of the soda process.
One hundred parts or pounds of sulphate of soda are equivalent to 75 parts of carbonate,
and when skilfully decomposed, will generally yield fully 70 pounds. A charge
for the decomposing furnace with the preparatory shelf should not exceed 200 lbs., or
perhaps 180; therefore if 75 pounds of ground sulphate of soda, with 80 pounds of chalk
or limestone (ground), and 37 pounds of ground coal; be well mixed, they will constitute
one charge. This charge must be shovelled in upon the hearth A, or shelf of preparation,
(fig. 1037.); and whenever it has become hot (the furnace having been previously brought
to bright ignition), it is to be transferred to the decomposing hearth or laboratory B, by
an iron tool, shaped exactly like an oar, called the spreader. This tool has the flattened
part from 2 to 3 feet long, and the round part, for laying hold of and working by, from
6 to 7 feet long. Two other tools are used; one, a rake, bent down like a garden hoe at
the end; and another, a small shovel, consisting of a long iron rod terminated with a piece
of iron plate, about 6 inches long, 4 broad, sharpened and tipped with steel, for cleaning
the bottom of the hearth from adhering cakes or crusts. Whenever the charge is shoved
by the sliding motion of the oar down upon the working hearth, a fresh charge should
be thrown into the preparation shelf, and evenly spread over its surface.
The hot and partially carbonized charge being also evenly spread upon the hearth B,
is to be left untouched for about ten minutes, during which time it becomes ignited, and
begins to fuse upon the surface. A view may be taken of it through a peep-hole in
the door, which should be shut immediately, in order to prevent the reduction of the
temperature. When the mass is seen to be in a state of incipient fusion, the workman
takes the oar and turns it over breadth by breadth in regular layers, till he has reversed
the position of the whole mass, placing on the surface the particles which were formerly
in contact with the hearth. Having done this, he immediately shuts the door, and lets
the whole get another decomposing heat. After five or six minutes, jets of flame begin
to issue from various parts of the pasty-consistenced mass. Now is the time to incorporate
the materials together, turning and spreading by the oar, gathering them together
by the rake, and then distributing them on the reverse part of the hearth; that is, the oar
should transfer to the part next the fire-bridge the portion of the mass lying next the
shelf, and vice versâ. The dexterous management of this transposition characterizes a
good soda-furnacer. A little practice and instruction will render this operation easy to a
robust clever workman. After this transposition, incorporation, and spreading, the door
may be shut again for a few minutes, to raise the heat for the finishing off. Lastly, the
rake must be dexterously employed to mix, shift, spread, and incorporate. The jets,
called candles, are very numerous, and bright at first; and whenever they begin to fade,
the mass must be raked out into cast-iron moulds, placed under the door of the laboratory
to receive the ignited paste.
One batch being thus worked off, the other, which has lain undisturbed on the shelf,
is to be shoved down from A to B, and spread equally upon it, in order to be treated as
above described. A third batch is then to be placed on the shelf.
The article thus obtained should contain at least 22 per cent. of real soda, equivalent
to 37 per cent. of dry carbonate, or to 100 of crystals. A skilful workman can turn
out a batch in from three quarters of an hour to an hour, producing a perfect carbonate,
which yields on solution an almost colourless liquid, nearly destitute of sulphur, and
containing hardly any decomposed sulphate.
In some soda-works, where the decomposing furnace is very large, and is charged
with a ton of materials at a time, it takes two men to work it, and from five to six
hours to complete a batch. Having superintended the operation of the above-described
small furnace, and examined its products, I feel warranted to recommend its adoption.
The following materials and products show the average state of this soda process:—
Materials—100 parts of sulphate of soda, ground, equivalent to 7·5 of
carbonate; 110 of chalk or ground limestone; 55 of ground coal: in the whole, 265.
Products—168 parts of crude soda, at 33 per cent. = 55·5 of dry carbonate.
| Or, |
- |
|
130 — crystals of carbonate of soda = 48 of dry carbonate; and |
| 100 — insoluble matter. |
But these products necessarily vary with the skill of the workman.
[1154]
In another manufactory the following proportions are used:—Six stones, of 14 lbs.
each, of dry ground sulphate of soda, are mixed with 3 of chalk and 3 of coal. This
mixture, weighing 11⁄2 cwt., forms a batch, which is spread upon the preparation shelf
of the furnace (figs. 1037. and 1038.), as above described, and gradually heated to incipient
ignition. It is then swept forwards to the lower area B, by the iron oar, and
spread evenly by the rake. Whenever it begins to soften under the rising heat of the
laboratory (the side doors being meanwhile shut), the mass must be laboriously turned
over and incorporated; the small shovel, or paddle, being employed to transfer, by the
interchange of small portions at a time, in rapid but orderly succession, the whole materials
from the colder to the hotter, and from the hotter to the colder parts of the hearth.
The process of working one batch takes about an hour, during the first half of which
period it remains upon the preparation shelf. The average weight of the finished ball
is 1 cwt., and its contents in alkalimetrical soda are 33 pounds.
Where the acidulous sulphate of iron from pyrites may be had at a cheap rate, it has
been long ago employed, as at Hurlett in Scotland, instead of sulphuric acid, for decomposing
the chloride of sodium. Mr. Turner’s process of preparing soda, by decomposing
sea salt with litharge and quicklime, has been long abandoned, the resulting patent
yellow, or sub-chloride of lead, having a very limited sale.
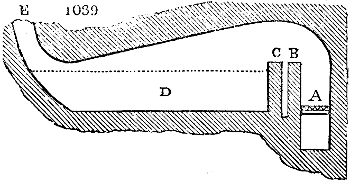
2. The extraction of pure soda from the crude article.—The black balls must
be broken into fragments, and thrown into large square iron cisterns, furnished
with false bottoms of wooden spars; when the cisterns are nearly full of these lumps,
water is pumped in upon them, till they are all covered. After a few days, the
lixiviation is effected, and the lye is drawn off either by a syphon or by a plug-hole
near the bottom of the cistern, and run into evaporating vessels. These may be
of two kinds. The surface-evaporating furnace, shown in fig. 1039., is a very
admirable invention for economizing
vessels, lime, and fuel. The grate A,
and fireplace, are separated from the
evaporating laboratory D, by a double
fire-bridge B, C, having an interstitial
space in the middle, to arrest the
communication of a melting or igniting
heat towards the lead-lined
cistern D. This cistern may be 8,
10, or 20 feet long, according to the
magnitude of the soda-work, and 4
feet or more wide. Its depth should be about 4 feet. It consists of sheet lead, of about
6 pounds weight to the square foot, and it is lined with one layer of bricks, set in
roman or hydraulic cement, both along the bottom and up the sides and ends. The
lead comes up to the top of C, and the liquor, or lye, may be filled in to nearly that
height. Things being thus arranged, a fire is kindled upon the grate A; the flame and
hot air sweep along the surface of the liquor, raise its temperature there rapidly to
the boiling point, and carry off the watery parts in vapour up the chimney E, which
should be 15 or 20 feet high, to command a good draught. But, indeed, it will be most
economical to build one high capacious chimney stalk, as is now done at Glasgow,
Manchester, and Newcastle, and to lead the flues of the several furnaces above
described into it. In this evaporating furnace the heavier and stronger lye goes to
the bottom, as well as the impurities, where they remain undisturbed. Whenever
the liquor has attained to the density of 1·3, or thereby, it is pumped up into
evaporating cast-iron pans, of a flattened somewhat hemispherical shape, and evaporated
to dryness while being diligently stirred with an iron rake and iron scraper.
This alkali gets partially carbonated by the above surface-evaporating furnace, and
is an excellent article.
When pure carbonate is wanted, that dry mass must be mixed with its own bulk of
ground coal, sawdust, or charcoal, and thrown into a reverberatory furnace, like fig. 1038.,
but with the sole all upon one level. Here it must be exposed to a heat not exceeding
650° or 700° F.; that is, a little above the melting heat of lead; the only object being to
volatilize the sulphur present in the mass, and carbonate the alkali. Now, it has been
found, that if the heat be raised to distinct redness, the sulphur will not go off, but will
continue in intimate union with the soda. This process is called calking, and the furnace
is called a calker furnace. It may be six or eight feet long, and four or five feet
broad in the hearth, and requires only one door in its side, with a hanging iron frame
filled with a fire-tile or bricks, as above described.
This carbonating process may be performed upon several cwts. of the impure soda,
mixed with sawdust, at a time. It takes three or four hours to finish the desulphuration;
and it must be carefully turned over by the oar and the rake, in order to burn the coal
into carbonic acid, and to present the carbonic acid to the particles of caustic soda diffused
through the mass, so that it may combine with them.
[1155]
When the blue flames cease, and the saline matters become white, in the midst of the
coaly matter, the batch may be considered as completed. It is raked out, and when
cooled, lixiviated in great iron cisterns with false bottoms, covered with mats. The
watery solution being drawn off clear by a plug-hole, is evaporated either to dryness, in
hemispherical cast-iron pans, as above described, or only to such a strength that it
shows a pellicle upon its surface, when it may be run off into crystallizing cisterns of
cast iron, or lead-lined wooden cisterns. The above dry carbonate is the best article
for the glass manufacture.
Crystallized carbonate of soda, contains 623⁄4 per cent. of water. The crystals are colourless
transparent rhomboids, which readily effloresce in the air, and melt in their own water
of crystallization. On decanting the liquid from the fused mass, it is found that one
part of the salt has given up its water of crystallization to another. By evaporation of
that fluid, crystals containing one-fifth less water than the common carbonate are
obtained. These do not effloresce in the air.
Mineral soda, the sesquicarbonate, (Anderthalb kohlensaures natron, Germ.); is found
in the province of Sukena, in Africa, between Tripoli and Fezzan. It forms a stratum
no more than an inch thick, just below the surface of the soil. Its texture is striated
crystalline, like fibrous gypsum. Several hundred tons of it are collected annually,
which are chiefly consumed in Africa. This species of soda does not effloresce like the
Egyptian, or the manufactured soda crystals, owing to its peculiar state of composition
and density. It was analyzed by Klaproth, under its native name of trona, and was
found to consist, in 100 parts, of—soda, 37; carbonic acid, 38; sulphate of soda, 2·5;
water, 22·5, in 100.
This soda is, therefore, composed of—3 atoms of carbonic acid, associated with 2 atoms
of soda, and 4 of water; while our commercial soda crystals are composed of—1 atom
of carbonic acid, 1 atom of soda, and 10 atoms of water.
There are six natron lakes in Egypt. They are situated in a barren valley, called
Bahr-bela-ma, about thirty miles to the west of the Delta.
There are natron lakes also in Hungary, which afford in summer a white saline
efflorescent crust of carbonate of soda, mixed with a little sulphate.
There are several soda lakes in Mexico, especially to the north of Zacatecas, as also
in many other provinces. In Columbia, 48 English miles from Merida, mineral soda is
extracted from the earth in great abundance, under the name of urao.
Bicarbonate of soda (Doppelt kohlensaures natron, Germ.); is prepared, like
bicarbonate of potassa, by transmitting carbonic acid gas through a cold saturated
solution of pure carbonate of soda, till crystalline crusts be formed. The bicarbonate
may also be obtained in four-sided tables grouped together. It has an alkaline taste
and reaction upon litmus paper, dissolves in 13 parts of cold water, and is converted by
boiling water into the sesquicarbonate, with the disengagement of one fourth of its
carbonic acid. It consists of—37 of soda, 52·35 carbonic acid, and 10·65 water.
SODA-WATER, is the name given to water containing a minute quantity of soda,
and highly charged with carbonic acid gas, whereby it acquires a sparkling appearance,
an agreeable pungent taste, an exhilarating quality, and certain medicinal powers. It
constitutes a considerable object of manufacture in this kingdom. The following
figure represents, I understand, the best system of apparatus for preparing it. A very
dilute solution of soda is put into the globular vessel H, and the carbonic acid gas is
forced into it from the gasometer E, by means of the powerful pump-work, as will be
understood from the subjoined explanation.
The same apparatus may serve for making any species of aerated water, in imitation
of any natural spring. All that is necessary for this purpose, is to put into the cistern
Q, the neutro-saline matter, earths, metallic oxides, pure water, &c., each in due proportion,
according to the most accredited analysis of the mineral water to be imitated,
to agitate that mixture, to suck it into the condenser H, through the pipe R, and then to
impregnate it to the due degree, by pumping in the appropriate gas, previously contained
in the gasometer F.
Thus, to make Seltzer water, for each 12 pounds troy, = 69,120 grains, or 1 gallon
imperial very nearly, take 55 grains of dry carbonate of soda, 17 of carbonate of lime,
18 of carbonate of magnesia, 31⁄2 of subphosphate of alumina, 3 of chloride of potassium,
155 of chloride of sodium, and 3 of finely precipitated silica. Put these materials into
the cistern Q, and charge the gasometer F with 353 cubic inches of carbonic acid gas.
Then work the machine by the handle of the wheel X, as explained below, and regulate
the introduction of the liquid and the gas in aliquot portions; for example, if the
condenser H admits half a gallon of water at a time, that quantity of liquid should be
charged with 176 cubic inches of the gas, being one half of the whole quantity. The
sulphuretted mineral waters may be imitated in like manner, by taking the proportions
of their constituents, as given in Table II. of Waters, Mineral.
[1156]
IMPROVED SODA-WATER APPARATUS, AS MADE BY MR. HAYWARD TYLER,
OF MILTON STREET.
Fig. 1040. front view of the soda-water machine. Fig. 1041. end view of the same.
A, lead generator, for making the gas. B, lead pot, for
holding sulphuric acid. C, handle for moving the agitator
of the receiver, which stirs up the ingredients in the lead
generator. a, cap and screw, for charging the lead pot with
sulphuric acid. b, swivel-joint, which is movable, for occasionally
throwing in portions of sulphuric acid for generating
gas. c, stuffing-box for agitator. d, large cap and
screw, for charging the lead generator with whiting and
water. e, cap and screw, for emptying contents of ditto.
D, lead pipe, to convey the gas from the lead generator to
gasometer. E, wood tub, filled with water, for gasometer
to work in. F, copper gasometer. G, strong iron frame,
for gasometer and tub to stand on, firmly fixed together by
three wrought-iron rods, f, f. g, g, two pulleys, for carrying
rope and counterbalance weight h, for balancing copper gasometer.
i, cock for discharging atmospheric air contained in
the gasometer before making the gas. k, cock for occasionally
emptying the water out of the tub. l, union joint, to which is fixed a copper
pipe, passing through the water in the tub, to deliver the gas as generated
into the copper gasometer. m, another union joint, with a similar
copper pipe, passing through the water in the tub, and projecting two or
three inches above the surface of the water, to convey the gas from the
copper gasometer to the soda-water machine. H, H, condenser for aerating
the soda-water. I, safety valve. K, K, bottling valve. L, bottling nipple.
M, M, soda-water pump. N, valve-piece. O, O, piston of the pump. P,
pipe for conducting gas from the gasometer to pump. Q, copper pan for
holding the solution of soda. R, copper pipe for conducting the solution
of soda to the force pump. S, S, two cocks for regulating the admission of
the solution and gas to the pump. T, copper pipe through which the soda-water
is forced to the condenser. U, pinion wheel, to give motion to the
agitator revolving inside the condenser. V, V, wheel for driving ditto.
W, W, cast-iron frame for carrying machinery. X, X, cast-iron fly-wheel.
Z, wrought-iron crank. Y, Z, Z, wood stools and curb, upon which the
whole of the machinery is fixed.
[1157]
SODIUM, the metallic basis of soda, is obtained by processes similar to those by
which potassium is procured. By fusing hydrate of soda with a little hydrate of
potassa, a mixture is obtained, which yields more readily than soda by itself to the decomposing
action of iron-turnings at a high heat, in a bent gun-barrel. The portion
of potassium produced, may be got rid of, by digesting the alloy for a few days in some
naphtha or oil of turpentine contained in an open vessel. The sodium remains at the
bottom of the liquid. Pure sodium may, however, be prepared at once, by subjecting
incinerated tartrate of soda to heat in the apparatus of Brunner, described under
Potassium. It is white, like silver; softer and more malleable than any other metal,
and may be readily reduced into very thin leaves. It preserves its malleability till it
approaches the melting point. Its specific gravity is 0·970. It softens at the
temperature of 122° F., and at 200° it is perfectly fluid; but it will not rise in vapour
until heated to nearly the melting point of glass. In the air it oxidizes slowly, and
gets covered with a crust of soda; but it does not take fire till it is made nearly red-hot;
and then it emits brilliant scintillations. When thrown upon water, it is rapidly
oxidized, but without kindling, like potassium. If a drop of water be thrown upon it, it
becomes so hot by the chemical action as to take fire. There are three oxides of sodium;
1. the suboxide; 2. the oxide, or the basis of common soda; and, 3. the suroxide; the
last being formed when sodium is heated to redness upon a plate of silver.
SOLDERING (Souder, Fr.; Löthen, Germ.); is the process of uniting the surfaces
of metals, by the intervention of a more fusible metal, which being melted upon each
surface, serves, partly by chemical attraction, and partly by cohesive force, to bind them
together. The metals thus united may be either the same or dissimilar; but the
uniting metal must always have an affinity for both. Solders must be, therefore,
selected in reference to their appropriate metals. Thus tin-plates are soldered with
an alloy consisting of from 1 to 2 parts of tin, with 1 of lead; pewter is soldered with
a more fusible alloy, containing a certain proportion of bismuth added to the lead and
tin; iron, copper, and brass are soldered with spelter, an alloy of zinc and copper, in
nearly equal parts; silver, sometimes with pure tin, but generally with silver-solder, an
alloy consisting of 5 parts of silver, 6 of brass, and 2 of zinc; zinc and lead, with an alloy
of from 1 to 2 parts of lead with 1 of tin; platinum, with fine gold; gold, with an
alloy of silver and gold, or of copper and gold; &c.
In all soldering processes, the following conditions must be observed: 1. the surfaces
to be united must be entirely free from oxide, bright, smooth, and level; 2. the contact
of air must be excluded during the soldering, because it is apt to oxidize one or other
of the surfaces, and thus to prevent the formation of an alloy at the points of union.
This exclusion of air is effected in various ways. The locksmith encases in loam the
objects of iron, or brass, that he wishes to subject to a soldering heat; the silversmith
and brazier mix their respective solders with moistened borax powder; the coppersmith
and tinman apply sal ammoniac, rosin, or both, to the cleaned metallic surfaces, before
using the soldering-iron to fuse them together with the tin alloy. The strong solder of
the coppersmith consists of 8 parts of brass and 1 of zinc; the latter being added to the
former, previously brought into a state of fusion. The crucible must be immediately
covered up for two minutes till the combination be completed. The melted alloy is to
be then poured out upon a bundle of twigs held over a tub of water, into which it falls
in granulations. An alloy of 3 parts of copper and 1 of zinc forms a still stronger
solder for the coppersmiths. When several parts are to be soldered successively upon
the same piece, the more fusible alloys, containing more zinc, should be used first. A
softer solder for coppersmiths is made with 6 parts of brass, 1 of tin, and 1 of zinc; the
tin being first added to the melted brass, then the zinc; and the whole well incorporated
by stirring.
The edges of sheet lead for sulphuric acid chambers, and its concentration pans, are
joined together by melted lead itself, because any solder containing tin would soon be
corroded. With this view, the two edges being placed in contact, are flattened down
into a long wooden groove, and secured in their situation by a few brass pins driven
into the wood. The surfaces are next brightened with a triangular scraper, rubbed
over with candle grease, and then covered with a stream of hot melted lead. The
riband of lead thus applied is finally equalized by being brought into partial fusion with
the plumber’s conical iron heated to redness; the contact of air being prevented by
sprinkling rosin over the surface. The sheets of lead are thus burned together, in the
language of the workmen.
SOOT (Noir de fumée, Suie, Fr.; Rus, Flatterrus, Germ.); is the pulverulent
charcoal condensed from the smoke of wood or coal fuel. A watery infusion of the
former is said to be antiseptic, probably from its containing some creosote.
The soot of pitcoal has not been analyzed with any minuteness. It contains some
sulphate and carbonate of ammonia, along with bituminous matter.
[1158]
SORBIC ACID, is the same with malic acid; which see.
SOY, is a liquid condiment, or sauce, imported chiefly from China. It is prepared
with a species of white haricots, wheat flour, common salt, and water; in the proportions
respectively of 50, 60, 50, and 250 pounds. The haricots are washed, and boiled
in water till they become so soft as to yield to the fingers. They are then laid in a flat
dish to cool, and kneaded along with the flour, a little of the hot water of the decoction
being added from time to time. This dough is next spread an inch or an inch and a
half thick upon the flat vessel (made of thin staves of bamboo), and when it becomes hot
and mouldy, in two or three days, the cover is raised upon bits of stick, to give free access
of air. If a rancid odour is exhaled, and the mass grows green, the process goes on
well; but if it grows black, it must be more freely exposed to the air. As soon as all
the surface is covered with green mouldiness, which usually happens in eight or ten days,
the cover is removed, and the matter is placed in the sunshine for several days. When it
has become as hard as a stone, it is cut into small fragments, thrown into an earthen
vessel, and covered with the 250 pounds of water having the salt dissolved in it. The
whole is stirred together, and the height at which the water stands is noted. The vessel
being placed in the sun, its contents are stirred up every morning and evening; and a
cover is applied at night, to keep it warm and exclude rain. The more powerful the
sun, the sooner the soy will be completed; but it generally requires two or three of the
hottest summer months. As the mass diminishes by evaporation, well water is added;
and the digestion is continued till the salt water has dissolved the whole of the flour and
the haricots; after which the vessel is left in the sun for a few days, as the good quality
of the soy depends on the completeness of the solution, which is promoted by regular
stirring. When it has at length assumed an oily appearance, it is poured into bags, and
strained. The clear black liquid is the soy, ready for use. It is not boiled, but is put up
into bottles, which must be carefully corked. Genuine soy was made in this way at Canton,
by Michael de Grubbens. See Memoirs of Academy of Sciences of Stockholm for 1803.
SPECIFIC GRAVITY, designates the relative weights of different bodies under
the same bulk; thus a cubic foot of water weighs 1000 ounces avoirdupois; a cubic
foot of coal, 1350; a cubic foot of cast iron, 7280; a cubic foot of silver, 10,400; and
a cubic foot of pure gold, 19,200; numbers which represent the specific gravities of the
respective substances, compared to water = 1·000. See Alloy.
SPECULUM METAL, is an alloy of copper and tin; described under Copper.
SPERMACETI; the Cetine of Chevreul. In certain species of the cachalot whale,
as the Physeter macrocephalus, tursio, microps, and orthodon, as also the Delphinus edentulus,
the fat of some parts of their bodies contains a peculiar kind of stearine, called spermaceti.
The oil obtained from cavities in the bones of the cranium of the above
cetaceæ is the richest in this kind of stearine. This being thrown into great filter-bags,
the spermaceti oil passes through, and is subsequently purified by the addition of a
small quantity of potash lye, which precipitates certain matters by neutralizing the acid
that held them in solution. The solid which remains on the filter is next squeezed in
bags, by means of a horizontal hydraulic press encased in steam, then digested with a
weak potash lye, in order to dissolve out any oil which may continue to adhere to it,
washed with water, finally dissolved in a tub by the agency of steam, laded into tin pans,
and allowed slowly to concrete into a white semi-transparent brittle lamellar crystalline
mass, which forms elegant candles.
At 60° its specific gravity is 0·943. It melts at 112·5°; 100 parts of alcohol at
0·821 dissolve 31⁄2 of it, of which 0·9 are deposited on cooling. Warm ether dissolves it
in very large quantities. It is soluble also in the fat of volatile oils; and if the solutions
have been saturated while hot, the greater part of the spermaceti crystallizes on
cooling. When this substance has been purified by digesting alcohol upon it repeatedly,
what remains is the cetine of Chevreul, or pure spermaceti. Its melting point has
now become 116° F., and its boiling point 616° F., at which it distils without alteration.
Caustic alkaline lyes saponify it with difficulty.
SPIRIT OF AMMONIA, is, properly speaking, alcohol combined with ammonia
gas; but the term is often applied to water of ammonia.
SPIRITS, VINOUS. This subject has been fully discussed in the articles Alcohol,
Distillation, and Fermentation. I have shown that the progressive increase of alcohol
in the wash tends progressively to prevent the conversion of the wort into spirit, or
checks the fermenting process, though a great deal of fermentable matter remains unchanged.
Mr. Sheridan has sought to remove this obstacle to the thorough transmutation
of saccharine matter into alcohol, by drawing off the spirit as it is formed.
For this purpose he ferments his wash in close tuns, connected with a powerful air-pump
worked by machinery, thus continually removing the carbonic acid as it is formed,
and maintaining a diminished pressure under which the alcohol readily distils at a
temperature of 120° or 130° F. He finds that this degree of heat is not injurious to the[1159]
fermentation, provided that it be communicated by the air of a stove-room, and not by
water or steam pipes traversing the liquid, which would inevitably scald or seeth the particles
in succession, and thereby extinguish the fermenting principle.
By the above ingenious plan, Mr. Sheridan tells me he has obtained 28 gallons of
proof spirit from a quarter of grain, instead of the average product 21, being an increase
of 25 per cent. The experiment was tried upon a considerable scale at Messrs. Currie’s
great distillery near London; but could not be established as a mode of manufacture, on
account of the excise laws, which prohibit the distillers from carrying on the two processes
of fermentation and distillation at the same time.
SPONGE (Eponge, Fr.; Schwamm, Germ.); is a cellular fibrous tissue produced by
small animals, almost imperceptible, called polypi by naturalists, which live in the sea.
This tissue is said to be covered in its recent state with a kind of semi-fluid thin coat
of animal jelly, susceptible of a slight contraction or trembling on being touched; which
is the only symptom of vitality displayed by the sponge. After death, this jelly disappears,
and leaves merely the sponge; formed by the combination of a multitude of
small capillary tubes, capable of receiving water in their interior, and of becoming
thereby distended. Sponges occur attached to stones at the bottom of the sea; and
abound particularly upon the shores of the islands in the Grecian Archipelago. Although
analogous in their origin to coral, sponges are quite different in their nature; the
former being composed almost entirely of carbonate of lime; while the latter are formed
of the same elements as animal matters, and afford, on distillation, a considerable quantity
of ammonia.
Dilute sulphuric acid has been recommended for bleaching sponges, after the calcareous
impurities have been removed by muriatic acid. Chlorine water answers better.
SPOON MANUFACTURE. See Stamping of Metals.
STAINED GLASS. When certain metallic oxides or chlorides, ground up with
proper fluxes, are painted upon glass, their colours fuse into its surface at a moderate
heat, and make durable pictures, which are frequently employed in ornamenting the
windows of churches as well as of other public and private buildings. The colours of
stained glass are all transparent, and are therefore to be viewed only by transmitted
light. Many metallic pigments, which afford a fine effect when applied cold on canvas
or paper, are so changed by vitreous fusion as to be quite inapplicable to painting in
stained glass.
The glass proper for receiving these vitrifying pigments, should be colourless, uniform,
and difficult of fusion; for which reason crown glass, made with little alkali,
or with kelp, is preferred. When the design is too large to be contained on a single
pane, several are fitted together, and fixed in a bed of soft cement while painting, and
then taken asunder to be separately subjected to the fire. In arranging the glass
pieces, care must be taken to distribute the joinings so that the lead frame-work may
interfere as little as possible with the effect.
A design must be drawn upon paper, and placed beneath the plate of glass; though the
artist cannot regulate his tints directly by his pallet, but by specimens of the colours
producible from his pallet pigments after they are fired. The upper side of the glass
being sponged over with gum-water, affords, when dry, a surface proper for receiving
the colours, without the risk of their running irregularly, as they would be apt to do,
on the slippery glass. The artist first draws on the plate, with a fine pencil, all the
traces which mark the great outlines and shades of the figures. This is usually done
in black, or, at least, some strong colour, such as brown, blue, green, or red. In
laying on these, the painter is guided by the same principles as the engraver, when he
produces the effect of light and shade by dots, lines, or hatches; and he employs that
colour to produce the shades, which will harmonize best with the colour which is to be
afterwards applied; but for the deeper shades, black is in general used. When this is
finished, the whole picture will be represented in lines or hatches similar to an engraving
finished up to the highest effect possible; and afterwards, when it is dry, the
vitrifying colours are laid on by means of larger hair pencils; their selection being
regulated by the burnt specimen tints. When he finds it necessary to lay two colours
adjoining, which are apt to run together in the kiln, he must apply one of them to the
back of the glass. But the few principal colours to be presently mentioned, are all fast
colours, which do not run, except the yellow, which must therefore be laid on the
opposite side. After colouring, the artist proceeds to bring out the lighter effects by
taking off the colour in the proper place, with a goose quill cut like a pen without a
slit. By working this upon the glass, he removes the colour from the parts where the
lights should be the strongest; such as the hair, eyes, the reflection of bright surfaces
and light parts of draperies. The blank pen may be employed either to make the
lights by lines, or hatches and dots, as is most suitable to the subject.
[1160]
By the metallic preparations now laid upon it, the glass is made ready for being
fired, in order to fix and bring out the proper colours. The furnace or kiln best
adapted for this purpose, is similar to that used by enamellers. See Enamel, and the
Glaze-kiln; under Pottery. It consists of a muffle or arch of fire-clay or pottery, so
set over a fireplace, and so surrounded by flues, as to receive a very considerable heat
within, in the most equable and regular manner; otherwise some parts of the glass
will be melted; while, on others, the superficial film of colours will remain unvitrified.
The mouth of the muffle, and the entry for introducing fuel to the fire, should be on
opposite sides, to prevent as much as possible the admission of dust into the muffle,
whose mouth should be closed with double folding-doors of iron, furnished with small
peep-holes, to allow the artist to watch the progress of the staining, and to withdraw
small trial slips of glass, painted with the principal tints used in the picture.
The muffle must be made of very refractory fire-clay, flat at its bottom, and only 5
or 6 inches high, with such an arched top as may make the roof strong, and so close
on all sides as to exclude entirely the smoke and flame. On the bottom of the muffle
a smooth bed of sifted lime, freed from water, about half an inch thick, must be prepared
for receiving the pane of glass. Sometimes several plates of glass are laid over
each other with a layer of dry pulverulent lime between each. The fire is now lighted,
and most gradually raised, lest the glass should be broken; and after it has attained to
its full heat, it must be kept up for 3 or 4 hours, more or less, according to the indications
of the trial slips; the yellow colour being principally watched, as it is found to be
the best criterion of the state of the others. When the colours are properly burnt in,
the fire is suffered to die away, so as to anneal the glass.
STAINED-GLASS PIGMENTS.
Flesh colour.—Take an ounce of red lead, two ounces of red enamel (Venetian glass
enamel, from alum and copperas calcined together), grind them to fine powder, and
work this up with spirits (alcohol) upon a hard stone. When slightly baked, this
produces a fine flesh colour.
Black colour.—Take 141⁄2 ounces of smithy scales of iron, mix them with two ounces
of white glass (crystal), an ounce of antimony, and half an ounce of manganese; pound
and grind these ingredients together with strong vinegar. A brilliant black may also
be obtained by a mixture of cobalt blue with the oxides of manganese and iron.
Another black is made from three parts of crystal glass, two parts of oxide of copper,
and one of (glass of) antimony worked up together, as above.
Brown colour.—An ounce of white glass or enamel, half an ounce of good manganese;
ground together.
Red, rose, and brown colours, are made from peroxide of iron, prepared by nitric acid.
The flux consists of borax, sand, and minium in small quantity.
Red colour, may be likewise obtained from one ounce of red chalk pounded, mixed
with two ounces of white hard enamel, and a little peroxide of copper.
A red, may also be composed of rust of iron, glass of antimony, yellow glass of lead,
such as is used by potters (or litharge), each in equal quantity; to which a little sulphuret
of silver is added. This composition, well ground, produces a very fine red colour on
glass. When protoxide of copper is used to stain glass, it assumes a bright red or green
colour, according as the glass is more or less heated in the furnace, the former corresponding
to the orange protoxide, the latter having the copper in the state of peroxide.
Bistres and brown reds, may be obtained by mixtures of manganese, orange oxide of
copper, and the oxide of iron called umber, in different proportions. They must be
previously fused with vitreous solvents.
Green colour.—Two ounces of brass calcined into an oxide, two ounces of minium,
and eight ounces of white sand; reduce them to a fine powder, which is to be enclosed
in a well luted crucible, and heated strongly in an air-furnace for an hour. When the
mixture is cold, grind it in a brass mortar. Green may, however, be advantageously
produced by a yellow on one side, and a blue on the other. Oxide of chrome has
been also employed to stain glass green.
A fine yellow colour.—Take fine silver laminated thin, dissolve in nitric acid, dilute
with abundance of water, and precipitate with solution of sea salt. Mix this chloride
of silver, in a dry powder, with three times its weight of pipe-clay well burnt and
pounded. The back of the glass pane is to be painted with this powder; for when
painted on the face, it is apt to run into the other colours.
Another yellow can be made by mixing sulphuret of silver with glass of antimony,
and yellow ochre previously calcined to a red-brown tint. Work all these powders
together, and paint on the back of the glass. Or silver laminæ melted with sulphur,
and glass of antimony, thrown into cold water, and afterwards ground to powder, afford
a yellow.
[1161]
A pale yellow may be made with the powder resulting from brass, sulphur, and glass
of antimony, calcined together in a crucible, till they cease to smoke; and then mixed
with a little burnt yellow ochre.
The fine yellow of M. Merand, is prepared from chloride of silver, oxide of zinc,
white-clay, and rust of iron. This mixture, simply ground, is applied on the glass.
Orange colour.—Take 1 part of silver powder, as precipitated from the nitrate of
that metal by plates of copper, and washed; mix it with 1 part of red ochre and 1 of
yellow, by careful trituration; grind into a thin pap with oil of turpentine or lavender,
and apply this with a brush, dry, and burn in.
In the Philosophical Magazine, of December, 1836, the anonymous author of an
ingenious essay, “On the Art of Glass-painting,” says, that if a large proportion of ochre
has been employed with the silver, the stain is yellow; if a small proportion, it is
orange-coloured; and by repeated exposure to the fire, without any additional colouring-matter,
the orange may be converted into red; but this conversion requires a nice management
of the heat. Artists often make use of panes coloured throughout their
substance in the glass-house pots, because the perfect transparency of such glass
gives a brilliancy of effect, which enamel painting, always more or less opaque,
cannot rival. It was to a glass of this kind that the old glass-painters owed their
splendid red. This is, in fact, the only point in which the modern and antient processes
differ; and this is the only part of the art which was ever really lost. Instead
of blowing plates of solid red, the old glass-makers (like those of Bohemia, for some time
back,) used to flash a thin layer of brilliant red over a substratum of colourless glass;
by gathering a lump of the latter upon the end of their iron rod in one pot, covering it
with a layer of the former in another pot, then blowing out the two together into a globe
or cylinder, to be opened into circular tables, or into rectangular plates. The elegant
art of tinging glass red by protoxide of copper, and flashing it on common crown glass,
has become general within these few years.
That gold melted with flint glass stains it purple, was originally discovered and practised,
as a profitable secret, by Kunckel. Gold has been recently used at Birmingham
for giving a beautiful rose-colour to scent bottles. The proportion of gold should be
very small, and the heat very great, to produce a good effect. The glass must contain
either the oxide of lead, bismuth, zinc, or antimony; for crown glass will take no colour
from gold. Glass combined with this metal, when removed from the crucible is generally
of a pale rose-colour; nay, sometimes is as colourless as water, and does not assume its
ruby colour till it has been exposed to a low red heat, either under a muffle or at the
lamp. This operation must be nicely regulated; because a slight excess of fire destroys
the colour, leaving the glass of a dingy brown, but with a blue (green?) transparency,
like that of gold leaf. It is metallic gold which gives the colour; and, indeed,
the oxide is too easily reduced, not to be converted into the metal by the intense heat
which is necessarily required.
Upon the kindred art of painting in enamel, Mr. A. Essex has published an interesting
paper in the same journal, for June, 1837, in which he says that the antient
ruby glass, on being exposed to the heat of a glass-kiln, preserves its colour unimpaired,
while the modern suffers considerable injury, and in some cases becomes almost
black. Hence the latter cannot be painted upon, as the heat required to fix the fresh
colour would destroy the beauty of the original basis. To obviate this difficulty, the
artist paints upon a piece of plain glass the tints and shadows necessary for blending
the rich ruby glow with the other parts of his picture, leaving those parts untouched
where he wishes the ruby to appear in undiminished brilliancy, and fixes the ruby
glass in the picture behind the painted piece, so that in such parts, the window is
double-glazed. Mr. Essex employs, as did the late Mr. Muss, chrome oxide alone for
greens; and he rejects the use of iron and manganese in his enamel colours.
Coloured transparent glass is applied as enamel in silver and gold bijouterie, previously
bright-cut in the metal with the graver or the rose-engine. The cuts, reflecting
the rays of light from their numerous surfaces, exhibit through the glass, richly
stained with gold, silver, copper, cobalt, &c., a gorgeous play of prismatic colours,
varied with every change of aspect. When the enamel is to be painted on, it should
be made opalescent by oxide of arsenic, in order to produce the most agreeable effect.
The artist in enamel has obtained from modern chemistry, preparations of the metals
platinum, uranium, and chromium, which furnish four of the richest and most useful
colours of his palette. Oxide of platinum produces a substantive rich brown, formerly
unknown in enamel painting; a beautiful transparent tint, which no intensity or repetition
of firing can injure. Colours proper for enamel painting, he says, are not to be
purchased; those sold for the purpose, are adapted only for painting upon china. The
constituents of the green enamel used by his brother, Mr. W. Essex, are, silica, borax,
oxide of lead, and oxide of chrome.
[1162]
Mr. Essex’s enamelling furnace is a cubic space of about 12 inches, and contains a
fire-clay muffle, without either bottom or back, which is surrounded with coke, except
in front. The entire draught of air which supplies the furnace, passes through the
muffle; the plates and paintings being placed on a thin slab, made of tempered fire-clay,
technically termed planche, which rests on the bed of coke-fuel. As the greatest
heat is at the back of the muffle, the picture must be turned round while in the fire, by
means of a pair of spring tongs. The above furnace serves for objects up to five inches
in diameter; but for larger works a different furnace is required, for the description of
which I must refer to the original paper.
Relatively to the receipts for enamel colours, and for staining and gilding on glass,
for which twenty guineas were voted by the Society for the Encouragement of Arts, in
the session of 1817, to Mr. R. Wynn, Mr. A. Essex says, in p. 446. of his essay—“the
unfortunate artist who shall attempt to make colours for the purpose of painting
in enamel from these receipts, will assuredly find, to his disappointment, that they are
utterly useless.” In page 449. he institutes a comparison between Mr. Wynn’s complex
farrago for green, as published in the Transactions of the Society, with the simple
receipt of his brother, as given above. It is a remarkable circumstance, that not one of
our enamel artists, during a period of twenty years, should have denounced the fallacy
of these receipts, and the folly of sanctioning imposture by a public reward. Should
Mr. Essex’s animadversions be just, the well-intentioned Society in the Adelphi may,
from the negligence of its committee, come to merit the sobriquet, “For the Discouragement
of Arts.”
STAMPING OF METALS. The following ingenious machine for manufacturing
metal spoons, forks, and other articles, was made the subject of a patent by Jonathan
Hayne, of Clerkenwell, in May, 1833. He employs a stamping-machine with dies, in
which the hammer is raised to a height between guides, and is let fall by a trigger.
He prefers fixing the protuberant or relief portion of the die to the stationary block or
bed of the stamping-machine, and the counterpart or intaglio to the falling hammer
or ram.
The peculiar feature of improvement in this manufacture consists in producing the
spoon, ladle, or fork perfect at one blow in the stamping-machine, and requiring no
further manipulation of shaping, but simply trimming off the barb or fin, and polishing
the surface, to render the article perfect and finished.
Heretofore, in employing a stamping-machine, or fly-press, for manufacturing spoons,
ladles, and forks, it has been the practice to give the impressions to the handles, and to
the bowls or prongs, by distinct operations of different dies, and after having so partially
produced the pattern upon the article, the handles had to be bent and formed by
the operations of filing and hammering.
By his improved form of dies, which, having curved surfaces and bevelled edges, allow
of no parts of the faces of the die and counter-die to come into contact, he is enabled to
produce considerable elevations of pattern and form, and to bring up the article perfect
at one blow, with only a slight barb or fin upon its edge.
In the accompanying drawings, fig. 1042. is the lower or bed die for producing a
spoon, seen edgewise; fig. 1043. is the face of the upper or counter-die, corresponding;
fig. 1044. is a section,
taken through the
middle of the pair of
dies, showing the
space in which the
metal is pressed to
form the spoon.
To manufacture
spoons, ladles, or forks
according to his improved
process, he
first forges out the
ingot into flat pieces,
of the shape and dimensions of the die of the intended article; and if a spoon or ladle
is to be made, gives a slight degree of concavity to the bowl part; but, if necessary,
bends the back, in order that it may lie more steadily, and bend more accurately, upon
the lower die; if a fork, he cuts or otherwise removes portions of the metal at those
parts which will intervene between the prongs; and, having thus produced the rude
embryo of the intended article, scrapes its entire surface clean and free from oxidation-scale
or fire-strain, when it is ready to be introduced into the stamping-machine.
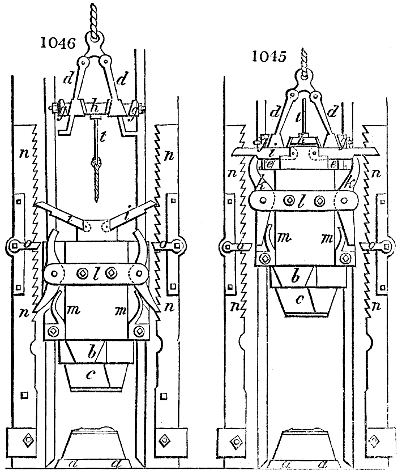
He now fixes the lower die in the bed of the stamping-machine, shown at a, a, in the
elevations figs. 1045. and 1046., and fixes, in the hammer b, the upper or counter-die[1163]
c, accurately adjusting them both, so that they may correspond exactly when brought
together. He then places the rudely-formed article above described upon the lower
die, and having drawn up the
hammer to a sufficient elevation
by a windlass and rope, or other
ordinary means, lets go the trigger,
and allows the hammer with the
counter-die to fall upon the under
die, on which the article is placed;
when, by the blow thus given to
the metal, the true and perfect
figure and pattern of the spoon,
ladle, or fork is produced, and
which, as before said, will only
require the removal of the slight
edging of barb or fin, with polishing,
to finish it.
On striking the blow, in the
operation of stamping the article,
the hammer will recoil and fly up
some distance, and if allowed to
fall again with reiterated blows,
would injure both the article and
the dies; therefore, to avoid this
inconvenience, he causes the hammer
on recoiling to be caught by
a pair of palls locking into racks
on the face of the standards, seen
in figs. 1045. and 1046. In fig. 1045. the hammer b, of the stamping-machine, is seen
raised and suspended by a rope attached to a pair of jointed hooks or holders d, d, the
lower ends of which pass into eyes e, e, extending from the top of the hammer. When
the lever or trigger t is drawn forward, as in fig. 1046., the two inclined planes g, g,
on the axle h, press the two legs of the holders d, d, inward, and cause their hooks or
lower ends to be withdrawn from the eyes e, e, when the hammer instantly falls, and
brings the dies together: such is the ordinary construction of the stamping-machine.
On the hammer falling from a considerable elevation, the violence of the blow causes
it to recoil and bound upwards, as before mentioned; it therefore becomes necessary
to catch the hammer when it has rebounded, in order to prevent the dies coming again
together; this is done by the following mechanism:—
Two latch levers i, i, are connected by joints to the upper part of the hammer, and
two pall levers k, k, turning upon pins, are mounted in the bridge l, affixed to the hammer.
Two springs m, m, act against the lower arms of these levers, and press them
outwards, for the purpose of throwing the palls at the lower ends of the levers into the
teeth of the ratchet racks n, n, fixed on the sides of the upright standards.
Previously to raising the hammer, the upper ends of the pall levers k, are drawn
back, and the latches i, being brought down upon them, as in fig. 1045., the levers k are
confined, and their palls prevented from striking into the side racks; but as the hammer
falls, the ends of the latches i strike upon the fingers o, o, fixed to the side standards,
and liberate the palls, the lower ends of which, when the hammer rebounds, after
stamping, catch into the teeth of the racks, as in fig. 1046., and thereby prevent the
hammer from again descending.
STARCH; (Amidon, Fecule, Fr.; Stärke, Germ.); is a white pulverulent substance,
composed of microscopic spheroids, which are bags containing the amylaceous matter.
It exists in a great many different plants, and varies merely in the form and size of its
microscopic particles; as found in some plants, it consists of spherical particles 1⁄1000 of
an inch in diameter; and in others, of ovoid particles, of 1⁄300 or 1⁄400 of an inch. It occurs,
1. in the seeds of all the acotyledinous plants, among which are the several species of
corns, and those of other gramineæ; 2. in the round perennial tap roots, which shoot
up an annual stem; in the tuberose roots, such as potatos, the Convolvulus batatas and
edulis, the Helianthus tuberosus, the Jatropha manihot, &c., which contain a great quantity
of it; 3. in the stems of several monocotyledinous plants, especially of the palm
tribe, whence sago comes; but it is very rarely found in the stems and branches of
the dicotyledinous plants; 4. it occurs in many species of lichen. Three kinds of
starch have been distinguished by chemists; that of wheat, that called inuline, and lichen
starch. These three agree in being insoluble in cold water, alcohol, ether, and oils, and in
being converted into sugar by either dilute sulphuric acid or diastase. The main
difference between them consists in their habitudes with water and iodine. The first[1164]
forms with hot water a mucilaginous solution, which constitutes, when cold, the paste
of the laundress, and is tinged blue by iodine; the second forms a granular precipitate,
when its solution in boiling-hot water is suffered to cool, which is tinged yellow by iodine;
the third affords, by cooling the concentrated solution, a gelatinous mass, with a clear
liquor floating over it, that contains little starch. Its jelly becomes brown-gray with iodine.
1. Ordinary starch.—This may be extracted from the following grains:—wheat, rye,
barley, oats, buckwheat, rice, maize, millet, spelt; from the siliquose seeds, as peas, beans,
lentiles, &c.; from tuberous and tap roots, as those of the potato, the orchis, manioc; arrowroot,
batata, &c. Different kinds of corn yield very variable quantities of starch.
Wheat differs in this respect, according to the varieties of the plant, as well as the soil,
manure, season, and climate. See Bread.
Wheat partly damaged by long keeping in granaries, may be employed for the manufacture
of starch, as this constituent suffers less injury than the gluten; and it may be
used either in the ground or unground state.
1. With unground wheat.—The wheat being sifted clean, is to be put into cisterns,
covered with soft water, and left to steep till it becomes swollen and so soft as to be
easily crushed between the fingers. It is now to be taken out, and immersed in clear
water of a temperature equal to that of malting-barley, whence it is to be transferred
into bags, which are placed in a wooden chest containing some water, and exposed to
strong pressure. The water rendered milky by the starch being drawn off by a
tap, fresh water is poured in, and the pressure is repeated. Instead of putting the
swollen grain into bags, some prefer to grind it under vertical edge-stones, or between
a pair of horizontal rollers, and then to lay it in a cistern, and separate the starchy
liquor by elutriation with successive quantities of water well stirred up with it. The
residuary matter in the sacks or cisterns contains much vegetable albumen and gluten,
along with the husks; when exposed to fermentation, it affords a small quantity of
starch of rather inferior quality.
The above milky liquor, obtained by expression or elutriation, is run into large cisterns,
where it deposits its starch in layers successively less and less dense; the uppermost
containing a considerable proportion of gluten. The supernatant liquor being drawn
off, and fresh water poured on it, the whole must be well stirred up, allowed again to
settle, and the surface-liquor again withdrawn. This washing should be repeated as
long as the water takes any perceptible colour. As the first turbid liquor contains a
mixture of gluten, sugar, gum, albumen, &c., it ferments readily, and produces a certain
portion of vinegar, which helps to dissolve out the rest of the mingled gluten, and
thus to bleach the starch. It is, in fact, by the action of this fermented or soured
water, and repeated washing, that it is purified. After the last deposition and
decantation, there appears on the surface of the starch a thin layer of a slimy mixture
of gluten and albumen, which, being scraped off, serves for feeding pigs or oxen; underneath
will be found a starch of good quality. The layers of different sorts are then taken
up with a wooden shovel, transferred into separate cisterns, where they are agitated with
water, and passed through fine sieves. After this pap is once more well settled, the
clear water is drawn off, the starchy mass is taken out, and laid on linen cloths in wicker
baskets, to drain and become partially dry. When sufficiently firm, it is cut into pieces,
which are spread upon other cloths, and thoroughly desiccated in a proper drying-room,
which in winter is heated by stoves. The upper surface of the starch is generally
scraped, to remove any dusty matter, and the resulting powder is sold in that state.
Wheat yields, upon an average, only from 35 to 40 per cent. of good starch. It should
afford more by skilful management.
2. In this country, wheat crushed between iron rollers is laid to steep in as much water
as will wet it thoroughly; in four or five days the mixture ferments, soon afterwards
settles, and is ready to be washed out with a quantity of water into the proper fermenting
vats. The common time allowed for the steep, is from 14 to 20 days. The
next process consists in removing the stuff from the vats into a stout round basket
set across a back below a pump. One or two men keep going round the basket, stirring
up the stuff with strong wooden shovels, while another keeps pumping water, till
all the farina is completely washed from the bran. Whenever the subjacent back is
filled, the liquor is taken out and strained through hair sieves into square frames or
cisterns, where it is allowed to settle for 24 hours; after which the water is run off from
the deposited starch by plug taps at different levels in the side. The thin stuff, called
slimes, upon the surface of the starch, is removed by a tray of a peculiar form. Fresh
water is now introduced, and the whole being well mixed by proper agitation, is then
poured upon fine silk sieves. What passes through is allowed to settle for 24 hours;
the liquor being withdrawn, and then the slimes, as before, more water is again poured
in, with agitation, when the mixture is again thrown upon the silk sieve. The milky
liquor is now suffered to rest for several days, 4 or 5, till the starch becomes settled
pretty firmly at the bottom of the square cistern. If the starch is to have the blue tint,[1165]
called Poland, fine smalt must be mixed in the liquor of the last sieve, in the proportion
of 2 or 3 lbs. to the cwt. A considerable portion of these slimes may, by good management,
be worked up into starch by elutriation and straining.
The starch is now fit for boxing, by shovelling the cleaned deposit into wooden chests,
about 4 feet long, 12 inches broad, and 6 inches deep, perforated throughout, and
lined with thin canvas. When it is drained and dried into a compact mass, it is turned
out by inverting the chests upon a clean table, where it is broken into pieces 4 or 5
inches square, by laying a ruler underneath the cake, and giving its surface a cut with a
knife, after which the slightest pressure with the hand will make the fracture. These
pieces are set upon half-burned bricks, which by their porous capillarity imbibe the
moisture of the starch, so that its under surface may not become hard and horny. When
sufficiently dried upon the bricks, it is put into a stove (which resembles that of a sugar
refinery), and left there till tolerably dry. It is now removed to a table, when all the
sides are carefully scraped with a knife; it is next packed up in the papers in which it
is sold; these packages are returned into the stove, and subjected to a gentle heat during
some days; a point which requires to be skilfully regulated.
Mr. Samuel Hall obtained a patent for bleaching starch by chloride of lime in 1821.
Chlorine water would probably be preferable, and might prove useful in operating upon
damaged wheat.
The sour water of the starch manufacture contains, according to Vauquelin, acetic
acid, acetate of ammonia, alcohol, phosphate of lime, and gluten.
During the drying, starch splits into small prismatic columns, of considerable regularity.
When kept dry, it remains unaltered for a very long period. When it is
heated to a certain degree in water, the envelopes of its spheroidal particles burst, and
the farina forms a mucilaginous emulsion, magma, or paste. When this apparent solution
is evaporated to dryness, a brittle horny-looking substance is obtained, quite different
in aspect from starch, but similar in chemical habitudes. When the moist paste
is exposed for 2 or 3 months to the air in summer, the starch is converted into sugar
to the amount of one-third or one-half of its weight, into gum, and gelatinous starch
called amidine by De Saussure, with occasionally a resinous matter. This curious change
goes on even in close vessels.
Starch from potatos.—From the following table of analyses, it appears that potatos
contain from 24 to 30 per cent. of dry substance:—
| |
Starch. |
Fibrous
paren-
chyma. |
Veg.
Albumen. |
Gum,
Sugar,
and Salts. |
Water. |
| Red potato |
15·0 |
7·0 |
1·4 |
9·2 |
75·0 |
| Germinating potatos |
15·2 |
6·8 |
1·3 |
3·7 |
73·0 |
| Kidney potatos |
9·1 |
8·8 |
0·8 |
— |
81·3 |
| Large red potatos |
12·9 |
6·0 |
0·7 |
— |
78·0 |
| Sweet potatos |
15·1 |
8·2 |
0·8 |
— |
74·3 |
| Peruvian potatos |
15·0 |
5·2 |
1·9 |
1·9 |
76·0 |
| English potatos |
12·9 |
6·8 |
1·1 |
1·7 |
77·5 |
| Parisian potatos |
13·3 |
6·8 |
0·9 |
4·8 |
73·1 |
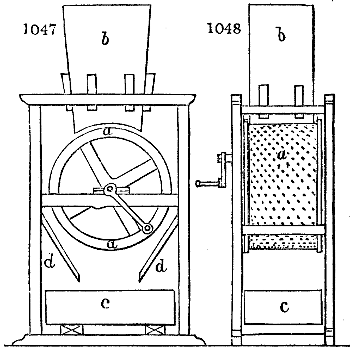
Manufacture of potato starch.—The potatos are first washed in a cylindrical cage
formed of wooden spars, made to revolve upon a horizontal axis, in a trough filled with
water to the level of the axis. They are then reduced to a pulp by a rasping machine,
similar to that represented in figs. 1047, 1048., where a is a wooden drum covered with
sheet-iron, roughened outside with numerous prominences, made by punching out holes
from the opposite side. It is turned by a winch fixed upon each end of the shaft. The
drum is enclosed in a square wooden box, to prevent the potato-mash from being scattered
about. The hopper b is attached to the upper frame, has its bottom concentric with
the rasp-drum, and nearly in contact with it. The pulp chest c is made to slide out, so
as when full to be readily replaced by another. The two slanting boards d, d, conduct the
pulp into it. A moderate stream of water should be made to play into the hopper upon
the potatos, to prevent the surface of the rasp from getting foul with fibrous matter.
Two men, with one for a relay, will rasp, with such a machine, from 21⁄2 to 3 tons of
potatos in 12 hours.
The potato pulp must be now elutriated upon a fine wire or hair sieve, which is set
upon a frame in the mouth of a large vat, while water is made to flow upon it from a
spout with many jets. The pulp meanwhile must be stirred and kneaded by the hand,
or by a mechanical brush-agitator, till almost nothing but fibrous particles are left upon
the sieve. These, however, generally retain about 5 per cent. of starch, which cannot be
separated in this way. This parenchyma should therefore be subjected to a separate
rasping upon another cylinder. The water turbid with starch is allowed to settle for some[1166]
time in a back; the supernatant liquor is then run by a cock into a second back, and after
some time into a third, whereby the whole starch will be precipitated. The finest powder
collects in the last vessel. The starch
thus obtained, containing 33 per cent.
of water, may be used either in the moist
state, under the name of green fecula,
for various purposes, as for the preparation
of dextrine, and starch syrup; or
it may be preserved under a thin layer
of water, which must be renewed from
time to time, to prevent fermentation;
or lastly, it may be taken out and
dried.
In trials made with St. Etienne’s rasp
and starch machinery, in Paris, which
was driven by two horses, nearly 18 cwt.
of potatos were put through all the
requisite operations in one hour, including
the pumping of the water.
The product in starch amounted to
from 17 to 18 per cent. of the potatos.
The quicker the process of potato-starch
making, the better is its quality.
Starch from certain foreign plants.—1. From the pith of the sago palm. See Sago.
2. From the roots of the Maranta arundinacea, of Jamaica, the Bahamas, and other
West India islands, the powder called arrow-root is obtained, by a process analogous to
that for making potato starch.
3. From the root of the Manioc, which also grows in the West Indies, as well as in
Africa, the cassava is procured, by a similar process. The juice of this plant is poisonous,
from which the wholesome starch is deposited. When dried with stirring upon hot
iron plates, it agglomerates into small lumps, called tapioca; being a gummy fecula.
The characters of the different varieties of starch can be learnt only from microscopic
observation; by which means also their sophistication or admixture may be readily
ascertained.
Starch, from whatever source obtained, is a white soft powder, which feels crispy,
like flowers of sulphur, when pressed between the fingers; it is destitute of taste and
smell, unchangeable in the atmosphere, and has a specific gravity of 1·53. I have already
described the particles as spheroids enclosed in a membrane. The potato contains some
of the largest, and the millet the smallest. Potato starch consists of truncated ovoids,
varying in size from 1⁄300 to 1⁄3000 of an inch; arrow-root, of ovoids varying in size from 1⁄800
to 1⁄2000 of an inch; flower starch, of insulated globules about 1⁄1000 of an inch; cassava,
of similar globules assembled in groups. These measurements I have made with a
good achromatic microscope, and a divided glass-slip micrometer of Tully.
For the saccharine changes which starch undergoes by the action of diastase, see Fermentation.
Lichenine, a species of starch obtained from Iceland moss (Cetraria islandica), as well
as inuline, from elecampane (Inula Helenium), are rather objects of chemical curiosity,
than of manufactures.
There is a kind of starch made in order to be converted into gum for the calico-printer.
This conversion having been first made upon the great scale in this country, has
occasioned the product to be called British gum. The following is the process pursued
in a large and well conducted establishment near Manchester. A range of four wooden
cisterns, each about 7 or 8 feet square, and 4 feet deep, is provided. Into each of them
2000 gallons of water being introduced, 121⁄2 loads of flour are stirred in. This mixture
is set to ferment upon old leaven left at the bottom of the backs, during 2 or 3 days. The
contents are then stirred up, and pumped off into 3 stone cisterns, 7 feet square and 4
feet deep; as much water being added, with agitation, as will fill the cisterns to the brim.
In the course of 24 hours the starch forms a firm deposit at the bottom; and the water is
then syphoned off. The gluten is next scraped from the surface, and the starch is
transferred into wooden boxes pierced with holes, which may be lined with coarse cloth,
or not, at the pleasure of the operator.
The starch, cut into cubical masses, is put into iron trays, and set to dry in a
large apartment, two stories high, heated by a horizontal cylinder of cast-iron traversed
by the flame of a furnace. The drying occupies two days. It is now ready for conversion
into gum, for which purpose it is put into oblong trays of sheet iron, and heated to the
temperature of 300° F. in a cast-iron oven, which holds four of these trays. Here it
concretes into irregular semi-transparent yellow-brown lumps, which are ground into[1167]
fine flour between mill-stones, and in this state brought to the market. In this roasted
starch, the vesicles being burst, their contents become soluble in cold water. British
gum is not convertible into sugar, as starch is, by the action of dilute sulphuric acid; nor
into mucic acid, by nitric acid; but into the oxalic; and it is tinged purple-red by iodine.
It is composed, in 100 parts, of 35·7 carbon, 6·2 hydrogen, and 58·1 oxygen; while
starch is composed of, 43·5 carbon, 6·8 hydrogen, and 49·7 oxygen.
To prove whether starch be quite free from gluten, or whether it be mixed with any
wheat flour, diffuse
12 grains of
it through six
ounces of water,
heat the mixture
to boiling, stirring
it meanwhile
with a glass slip.
If the starch be
pure, no froth
will be seen upon
the surface of the
pasty fluid; or if
any be produced
during the stirring,
it will immediately
subside
after it; but if
the smallest portion
of gluten be
present, much
froth will be permanently
formed,
which may be
raised by stirring
into the appearance
of soap-suds.
STARCHING
and Steam-drying
Apparatus.
The system of
hollow cylinders,
for drying goods
in the processes
of bleaching or
calico-printing, is
represented in
fig. 1049. in a
longitudinal section,
and in fig.
1050. in a top
view; but the
cylinders are supposed
to be
broken off in the
middle, as it was
needless to repeat
the parts at the
other end, which
are sufficiently
shown in the section.
A is the box
containing the
paste, when the
goods are to be
starched or stiffened:
a, a winch,
when it is desired
to turn the machine[1168]
by hand, though it is always moved by power in considerable factories; b, is the
driving pinion; d, d′, two brass rollers with iron shafts, the undermost of which is
moved by the wheel c, in geer with the pinion b. The uppermost roller d′, is turned by
the friction with the former, d, being pressed upon it by the weighted lever h; e is the
trough filled with the paste, which rests upon the bars f, and may be placed higher or
lower by means of the adjusting screws g, according as the roller d is to be plunged
more or less deeply. A brass roller i serves to force down the cloth into the paste.
B, is the drying part of the machine: k, k, its iron framing; l, l, &c., five drums, or
hollow copper cylinders, heated with steam: m, m, m, &c., small copper drums, in
pairs, turning freely on shafts under the former, for stretching the goods, and airing
them, during their passage through the machine: n, n, is the main steam-pipe, from
which branch off small copper tubes, o, o, &c., which conduct the steam through
stuffing-boxes into the cavity of the drying-drums. There are similar tubes upon the
other ends of the drums, for discharging the condensed water through similar stuffing-boxes:
q, q, are valves, opening internally, for admitting the air whenever the steam is
taken off, or becomes feeble, to prevent the drums from being crushed by the
unbalanced pressure of the atmosphere upon their external surfaces.
C, is the cloth-beam, from which the starching roller draws forward the goods; d, d, are
two rollers, of which the lower is provided with a band-pulley or rigger, driven by a
similar pulley fixed upon the shaft of the starching roller d. These two rollers pull
the goods through the drying machine, and then let them fall either upon a table or the
floor.
STEAM, is the vapour of hot water; the discussion of which belongs to chemistry,
physics, and engineering. Certain practical applications of the subject will be found
in the article Evaporation.
STEARIC ACID, improperly called Stearine (Talgsaüre, Germ.), is the solid constituent
of fatty substances, as of tallow and olive oil, converted into a crystalline mass
by saponification with alkaline matter, and abstraction of the alkali by an acid. By this
process, fats are convertible into three acids, called Stearic, Margaric, and Oleic; the
first two being solid, and the last liquid. The stearine, of which factitious wax candles
are made, consists of the stearic and margaric acids combined. These can be separated
from each other only by the agency of alcohol, which holds the margaric acid in
solution after it has deposited the stearic in crystals. Pure stearic acid is prepared,
according to its discoverer, Chevreul, in the following way:—Make a soap, by boiling
a solution of potash and mutton-suet in the proper equivalent proportions (see Soap);
dissolve one part of that soap in 6 parts of hot water, then add to the solution 40
or 50 parts of cold water, and set the whole into a place whose temperature is about
52° Fahrenheit. A substance falls to the bottom, possessed of pearly lustre, consisting
of the bi-stearate and bi-margarate of potash; which is to be drained and washed upon
a filter. The filtered liquor is to be evaporated, and mixed with the small quantity of
acid necessary to saturate the alkali left free by the precipitation of the above bi-salts.
On adding water to it afterwards, the liquor affords a fresh quantity of bi-stearate and
bi-margarate. By repeating this operation with precaution, we finally arrive at a point
when the solution contains no more of these solid acids, but only the oleic. The precipitated
bi-salts are to be washed and dissolved in hot alcohol, of specific gravity 0·820,
of which they require about 24 times their weight. During the cooling of the solution,
the bi-stearate falls down, while the greater part of the bi-margarate, and the remainder
of the oleate, remain dissolved. By once more dissolving in alcohol, and crystallizing,
the bi-stearate will be obtained alone; as may be proved by decomposing a little of it
in water at a boiling heat, with muriatic acid, letting it cool, washing the stearic acid
obtained, and exposing it to heat, when, if pure, it will not fuse in water under the 158th
degree of Fahrenheit’s scale. If it melts at a lower heat, it contains more or less margaric
acid. The purified bi-stearate being decomposed by boiling in water along with any
acid, as the muriatic, the disengaged stearic acid is to be washed by melting in water,
then cooled and dried.
Stearic acid, prepared by the above process, contains combined water, from which it
cannot be freed. It is insipid and inodorous. After being melted by heat, it solidifies
at the temperature of 158° Fahrenheit, and affects the form of white brilliant needles
grouped together. It is insoluble in water, but dissolves in all proportions in boiling
anhydrous alcohol, and on cooling to 122°, crystallizes therefrom, in pearly plates;
but if the concentrated solution be quickly cooled to 112°, it forms a crystalline mass.
A dilute solution affords the acid crystallized in large white brilliant scales. It dissolves
in its own weight of boiling ether of 0·727, and crystallizes on cooling in beautiful
scales, of changing colours. It distils over in vacuo without alteration; but if the
retort contains a little atmospheric air, a small portion of the acid is decomposed during
the distillation; while the greater part passes over unchanged, but slightly tinged
brown, and mixed with traces of empyreumatic oil. When heated in the open air, and[1169]
kindled, stearic acid burns like wax. It contains 3·4 per cent. of water, from which
it may be freed by combining it with oxide of lead. When this anhydrous acid is
subjected to ultimate analysis, it is found to consist of—80 of carbon, 12·5 hydrogen,
and 7·5 oxygen, in 100 parts. Stearic acid displaces, at a boiling heat in water,
carbonic acid from its combinations with the bases; but in operating upon an alkaline
carbonate, a portion of the stearic acid is dissolved in the liquor before the
carbonic acid is expelled. This decomposition is founded upon the principle, that
the stearic acid transforms the salt into a bicarbonate, which is decomposed by the
ebullition.
Stearic acid put into a strong watery infusion of litmus, has no action upon it in the
cold; but when hot, the acid combines with the alkali of the litmus, and changes its
blue colour to red; so that it has sufficient energy to abstract from the concentrated
tincture all the alkali required for its neutralization. If we dissolve bi-stearate of potash
in weak alcohol, and pour litmus water, drop by drop, into the solution, this will become
red, because the litmus will give up its alkali to a portion of the bi-stearate, and will convert
it into neutral stearate. If we now add cold water, the reddened mixture will resume
its blue tint, and will deposit bi-stearate of potash in small spangles. In order that the
alcoholic solution of the bi-stearate may redden the litmus, the alcohol should not be very
strong.
From the composition of stearate of potash, the atomic weight of the acid appears to be
106·6; hydrogen being 1; for 18 : 48 × 2 ∷ 100 : 533·3 = 5 atoms of acid.
From the stearate of soda, it appears to be 104; and from that of lime, 102. The
stearate of lead, by Chevreul, gives 109 for the atomic weight of the acid.
The margaric and oleic acids seem to have the same neutralizing power, and the
same atomic weight.
The preceding numbers will serve to regulate the manufacture of stearic acid for the
purpose of making candles. Potash and soda were first prescribed for saponifying
fat, as may be seen in M. Gay Lussac’s patent, under the article Candle; and were
it not for the cost of these articles, they are undoubtedly preferable to all others in a
chemical point of view. Of late years lime has been had recourse to, with perfect
success, and has become subservient to a great improvement in candle-making. The
stearine block now made by many London houses, though containing not more than
2 or 3 per cent. of wax, is hardly to be distinguished from the purified produce of the bee.
The first process is to boil the fat with quicklime and water in a large tub, by means of perforated
steam pipes distributed over its bottom. From the above statements we see that
about 11 parts of dry lime are fully equivalent to 100 of stearine and oleine mixed: but
as the lime is in the state of hydrate, 14 parts of it will be required when it is perfectly
pure; in the ordinary state, however, as made from average good limestone, 16 parts may
be allowed. After a vigorous ebullition of 3 or 4 hours, the combination is pretty complete.
The stearate being allowed to cool to such a degree as to allow of its being
handled, becomes a concrete mass, which must be dug out with a spade, and transferred
into a contiguous tub, in order to be decomposed with the equivalent quantity of sulphuric
acid diluted with water, and also heated with steam. Four parts of concentrated
acid will be sufficient to neutralize three parts of slaked lime. The saponified fat now
liberated from the lime, which is thrown down to the bottom of the tub in the state of
sulphate, is skimmed off the surface of the watery menstruum into a third contiguous
tub, where it is washed with water and steam.
The washed mixture of stearic, margaric, and oleic acids, is next cooled in tin pans;
then shaved by large knives, fixed on the face of a fly-wheel, called a tallow cutter,
preparatory to its being subjected in canvas or caya bags to the action of a powerful
hydraulic press. Here a large portion of the oleic acid is expelled, carrying with it a little
of the margaric. The pressed cakes are now subjected to the action of water and steam
once more, after which the supernatant stearic acid is run off, and cooled in moulds. The
cakes are then ground by a rotatory rasping-machine to a sort of mealy powder, which
is put into canvas bags, and subjected to the joint action of steam and pressure in a
horizontal hydraulic press of a peculiar construction, somewhat similar to that which has
been long used in London for pressing spermaceti. The cakes of stearic acid thus freed
completely from the margaric and oleic acids, are subjected to a final cleansing in a tub
with steam, and then melted into hemispherical masses called blocks. When these blocks
are broken, they display a highly crystalline texture, which would render them unfit for
making candles. This texture is therefore broken down or comminuted by fusing the
stearine in a plated copper pan, along with one thousandth part of pulverized arsenious
acid, after which it is ready to be cast into candles in appropriate moulds. See Candle.
1051Scale 3-20ths of an inch to the foot.
STEARINE COLD PRESS. The cold hydraulic press, as mounted by Messrs.
Maudslay and Field, for squeezing out the oleic acid from saponified fat, or the oleine
from coco-nut lard, is represented in plan in fig. 1051.; in side view of pump in fig.
1052.; and in elevation, fig. 1053.; where the same letters refer to like objects.
[1170]
A, A, are two hydraulic
presses; B the
frame; C, the cylinder;
D, the piston or
ram; E, the follower;
F, the recess in the bottom
to receive the oil;
G, twilled woollen bags
with the material to be
pressed, having a thin
plate of wrought iron
between each; H, apertures
for the discharge
of the oil; I, cistern in
which the pumps are
fixed; K, framing for
machinery to work in;
L, two pumps, large
and small, to inject the
water into the cylinders; M, a
frame containing three double
branches; N, three branches, each
having two stops or plugs, by which
the action of one of the pumps
may be intercepted from, or communicated
to, one or both of the
presses; the large pump is worked
at the beginning of the operation,
and the small one towards the end;
by these branches, one or both
presses may be discharged when
the operation is finished; O, two
pipes from the pumps to the
branches; P, pipe to return the
water from the cylinders to the
cisterns; Q, pipes leading from the
pumps through the branches to the
cylinders; R, conical drum, fixed
upon the main shaft Y, driven by
the steam-engine of the factory;
S, a like conical
drum to work the
pumps; T, a narrow
leather strap
to communicate
the motion from R
to S; U, a long
screw bearing a
nut, which works
along the whole
length of the drum;
V, the fork or guide
for moving the
strap T; W, W, two
hanging bearings to
carry the drum S;
X, a pulley on the
spindle of the drum
S; Y, the main
shaft; Z, fly-wheel
with groove on the
edge, driven by the
pulley X; on the
axis of S, is a double
crank, which
works the two
pumps L. a, is a[1171]
pulley on the end of the long screw U; an endless cord passes twice round this pulley,
and under a pulley fixed in the weight b; by laying hold of both sides of his cord,
and raising or lowering it, the forked guide V, and the leather strap T, are moved
backwards or forwards, by means of the nut fixed in the guide, so as to accelerate
or retard at pleasure the speed of the working of the pumps; c, is a piece of iron,
with a long slit, in which a pin, attached to the fork V, travels, to keep it in the vertical
position.
STEATITE (Soapstone; Craie de Briançon, Fr.; Speckstein, Germ.); is a mineral
of the magnesian family. It has a grayish-white or greenish-white colour, often marked
with dendritic delineations, and occurs massive, as also in various supposititious crystalline
forms; it has a dull or fatty lustre; a coarse splintery fracture, with translucent
edges; a shining streak; it writes feebly; is soft, and easily cut with a knife; but somewhat
tough; does not adhere to the tongue; feels very greasy; infusible before the
blowpipe; specific gravity from 2·6 to 2·8. It consists of—silica, 44; magnesia, 44;
alumina, 2; iron, 7·3; manganese, 1·5; chrome, 2; with a trace of lime. It is found
frequently in small contemporaneous veins that traverse serpentine in all directions, as
at Portsoy, in Shetland, in the limestone of Icolmkiln, in the serpentine of Cornwall,
in Anglesey, in Saxony, Bavaria (at Bayruth), Hungary, &c. It is used in the manufacture
of porcelain. It makes the biscuit semi-transparent, but rather brittle, and apt
to crack with slight changes of heat. It is employed for polishing serpentine, marble,
gypseous alabaster, and mirror glass; as the basis of cosmetic powders; as an ingredient
in anti-attrition pastes; it is dusted in powder upon the inside of boots, to make the
feet glide easily into them; when rubbed upon grease-spots in silk and woollen clothes,
it removes the stains by absorption; it enters into the composition of certain crayons,
and is used itself for making traces upon glass, silk, &c. The spotted steatite, cut into
cameos and calcined, assumes an onyx aspect. Soft steatite forms excellent stoppers
for the chemical apparatus used in distilling or subliming corrosive vapours. Lamellar
steatite is Talc.
STEEL (Acier, Fr.; Stahl, Germ.); as a carburet of iron, has already been considered
under that metal. I shall treat in this article more particularly of its manufacture
and technical relations.
1. Steel of cementation, bar or blistered steel.—With the exception of the Ulverstone
charcoal iron, no bars are manufactured in Great Britain capable of conversion into
steel at all approaching in quality to that made from the Madras, Swedish, and Russian
irons, so largely imported for that purpose. The first rank is assigned to the Swedish
iron stamped with a circle enclosing the letter L (hence called hoop L); which fetches the
high price of 36l. 10s. per ton, while excellent English coke-iron may be had for one-fifth
of the price. The other Swedish irons are sold at a much lower rate, though said
to be manufactured in the same way; and therefore the superiority of the Dannemora
iron must be owing to some peculiarity in the ore from which it is smelted. The
steel recently made in the Indian steel-works at Chelsea, from Mr. Heath’s Madras iron,
rivals that from the hoop L.
The Sheffield furnace for making bar or blistered steel, called the furnace of cementation,
is represented in fig. 1054. in a cross section, and in fig. 1055. in a ground plan.
The hearth of this oblong quadrangular furnace, is divided
by a grate into two parts,
upon each side of which
there is a chest a, called a
trough, made of fire-clay, or
fire-tiles. The breadth of the
grate varies according to the
quality of the fuel. b, b, are
air-holes; c, c, flues leading
to the chimney d, d. To aid
the draught of the smoke and
the flame, an opening e, is
made in the middle of the flat
arch of the furnace. In one
of its shorter sides (ends),
there are orifices f, f, through which the long bars of iron may be put in and taken
out; g, is the door by which the steel-maker enters, in filling or emptying the
trough; h, is a proof hole, at which small samples of the steel, in the act of its conversion,
may be drawn out. The furnace is built under a conical hood or chimney,
from 30 to 50 feet high, for aiding the draught, and carrying off the smoke.
The two chests are built of fire-stone grit. They are 8, 10, or even 15 feet long, and
from 26 to 36 inches in width and depth; the lower and smaller they are, the more
uniform will the quality of the steel be. A great breadth and height of trough are incompatible[1172]
with equability of the cementing temperature. The sides are a few inches
thick. The space between them is at least a foot wide. They should never rest
directly upon the sole of the furnace, but must have their bottom freely played upon
by the flame, as well as the sides and top. The degree of heat is regulated by openings
in the arch, or upon the long sides of the furnace, which lead to the chimney; as also
by the greater or less quantity of air admitted below the grate, as in glass-house
furnaces.
The cement consists of ground charcoal (sometimes of soot), mixed with one-tenth
of ashes, and some common salt; the charcoal of hard wood being preferred. Ground
coke is inadmissible, on account of the sulphur, silica, and clay which it generally
contains. Possibly the salt serves to vitrify the particles of silica in the charcoal, and
thus to prevent their entering into combination with the steel. As for the ashes, it is
difficult to discover their use. The best steel may be made without their presence.
The bottom of the trough being covered with two inches of the powder of cementation,
the bars are laid along in it, upon their narrow edge, the side bar being one inch
from the trough, and the rest being from 1⁄2 to 3⁄4 of an inch apart. Above this first
layer of iron bars, fully half an inch depth of the powder is spread, then a new series of
bars is stratified, and so on till the trough is filled within six inches of the top. This
space is partially filled with old cement powder, and is covered with refractory
damp sand. Sometimes the trough is filled to the surface with the old cement, and
then closely covered with fire-tiles. The bars should never be allowed to touch each
other, or the trough. The fire must be carefully urged from 2 to 4 days, till it acquires
the temperature of 100° Wedgewood; which must be steadily maintained during
the 4, 6, 8, or 10 days requisite for the cementation; a period dependent on the size of
the furnace, and which is determined by the examination of the proof pieces, taken out
from time to time.
In the front or remote end of the furnace, fig. 1054., a door is left in the outer building,
corresponding to a similar one in the end of the interior vault, through which the
workman enters for charging the furnace with charcoal and iron bars, as also for taking
out the steel after the conversion. Small openings are likewise made in the ends of
the chests, through which the extremities of a few bars are left projecting, so that they
may be pulled out and examined, through small doors opposite to them in the exterior
walls. These tap holes, as they are called, should be placed near the centre of the end
stones of the chests, that the bars may indicate the average state of the process. The
joinings of the fire-stones are secured with a finely ground Stourbridge clay.
The interval between the two chests (in furnaces containing two, for many have
only one,) being covered with an iron platform, the workman stands on it, and sifts a
layer of charcoal on the bottom of the chests evenly, about half an inch thick; he then
lays a row of bars, cut to the proper length, over the charcoal, about an inch from each
other; he next sifts on a second stratum of charcoal-dust, which, as it must serve for
the bars above, as well as below, is made an inch thick; thus, he continues to stratify,
till the chest be filled within two inches of the top; and he covers the whole with the
earthy detritus found at the bottom of grindstone troughs, or any convenient fire-loam.
It is obvious that the second series of bars should correspond vertically with the interstices
between the first series, and so in succession. The trial-rods are left longer
than the others, and their projecting ends are encrusted with fire-clay, or imbedded
in sand. The iron platform being removed, and all the openings into the vault closed,
the fire is lighted, and very gradually increased, to avoid every risk of cracking the gritstone
by too sudden a change of temperature; and the ignition being finally raised to
about 100° Wedgewood, but not higher, for fear of melting the metal, must be
maintained at a uniform pitch, till the iron have absorbed the desired quantity of
carbon, and have been converted as highly as the manufacturer intends for his peculiar
object. From six to eight days may be reckoned a sufficient period for the production
of steel of moderate hardness, and fit for tilting into shear steel. A softer steel, for
saws and springs, takes a shorter period; and a harder steel, for fabricating chisels used
in cutting iron, will need longer exposure to the ignited charcoal. But, for a few
purposes, such as the bits for boring cast iron, the bars are exposed to two or three
successive processes of cementation, and are hence said to be twice or thrice converted
into steels. The higher the heat of the furnace, the quicker is the process of conversion.
The furnace being suffered to cool, the workman enters it again, and hands out the
steel bars, which being covered with blisters, from the formation and bursting of vesicles
on the surface filled with gaseous carbon, is called blistered steel. This steel is very
irregular in its interior texture, has a white colour, like frosted silver, and displays
crystalline angles and facettes, which are larger the further the cementation has been
urged, or the greater the dose of carbon. The central particles are always smaller than
those near the surface of the bar.
In such a furnace as the above, twelve tons of bar iron may be converted at a charge.[1173]
But other furnaces are constructed with one chest, which receives six or eight tons at a
time; the small furnaces, however, consume more fuel in proportion than the larger.
The absorption and action of the carbonaceous matter, to the amount of about a half
per cent., occasions fissures and cavities in the substance of the blistered bars, which
render the steel unfit for any useful purpose in tool-making, till it be condensed and
rendered uniform by the operation of tilting, under a powerful hammer driven by
machinery. See Iron.[59]
The heads of the tilt-hammers for steel weigh from one and a half to two hundred
pounds. Those in the neighbourhood of Sheffield are much simpler than the one referred
to in the note. They are worked by a small water-wheel, on whose axis is another wheel,
bearing a great number of cams or wipers on its circumference, which strike the tail of
the hammer in rapid succession, raise its head, and then let it fall smartly on the hot
metal rod, dexterously presented on its several parts to the anvil beneath it, by the
workman. The machinery is adapted to produce from 300 to 400 blows per minute;
which on this plan requires an undue and wasteful velocity of the float-boards. Were
an intermediate toothed wheel substituted between the water-wheel and the wiper-wheel,
so that while the former made one turn, the latter might make three, a much
smaller force of water would do the work. The anvils of the tilt-hammer are placed
nearly on a level with the floor of the mill-house; and the workman sits in a fosse, dug
on purpose, in a direction perpendicular to the line of the helve, on a board suspended
from the roof of the building by a couple of iron rods. On this swinging seat, he can
advance or retire with the least impulse of his feet, pushing forward the steel bar, or
drawing it back with equal rapidity and convenience.
At a small distance from each tilt, stands the forge-hearth, for heating the steel. The
bellows for blowing the fire are placed above-head, and are worked by a small crank
fixed on the end of the axis of the wheel, the air being conveyed by a copper pipe down
to the nozzle. Each workman at the tilt has two boys in attendance, to serve him with
hot rods, and to take them away after they are hammered. In small rods, the bright
ignition originally given at the forge soon declines to darkness; but the rapid impulsions
of the tilt revive the redness again in all the points near the hammer; so that the rod,
skilfully handled by the workman, progressively ignites where it advances to the strokes.
Personal inspection alone can communicate an adequate idea of the precision and celerity
with which a rude steel rod is stretched and fashioned into an even, smooth, and sharp-edged
prism, under the operation of the tilt-hammer. The heat may be clearly referred
to the prodigious friction among the particles of so cohesive a metal, when they are
made to slide so rapidly over each other in every direction during the elongation and
squaring of the rod.
2. Shear steel derives its name from the accidental circumstance of the shears for
dressing woollen cloth being usually forged from it. It is made by binding into a bundle,
with a slender steel rod, four parallel bars of blistered steel, previously broken into lengths
of about 18 inches, including a fifth of double length, whose projecting end may serve
as a handle. This faggot, as it is called, is then heated in the forge-hearth to a good
welding heat, being sprinkled over with sand to form a protecting film of iron slag,
carried forthwith to the tilt, and notched down on both sides to unite all the bars
together, and close up every internal flaw or fissure. The mass being again heated,
and the binding rings knocked off, is drawn out into a uniform rod of the size required.
Manufacturers of cutlery are in the habit of purchasing the blistered bars at the conversion
furnaces, and sending them to tilt-mills to have them drawn out to the proper
size, which is done at regular prices to the trade; from 5 to 8 per cent. discount
being allowed on the rude bars for waste in the tilting. The metal is rendered so compact
by the welding and hammering, as to become susceptible of a much finer polish than
blistered steel can take; while the uniformity of its body, tenacity, and malleability
are at the same time much increased; by which properties it becomes well adapted for
making table knives and powerful springs, such as those of gun-locks. The steel is
also softened down by this process, probably from the expulsion of a portion of its
carbon during the welding and subsequent heats; and if these be frequently or awkwardly
applied, it may pass back into common iron.
3. Cast steel is made by melting, in the best fire-clay crucibles, blistered steel, broken
down into small pieces of convenient size for packing; and as some carbon is always
dissipated in the fusion, a somewhat highly converted steel is used for this purpose.
The furnace is a square prismatic cavity, lined with fire-bricks, 12 inches in each side,
and 24 deep, with a flue immediately under the cover, 31⁄2 inches by 6, for conducting
the smoke into an adjoining chimney of considerable height. In some establishments
a dozen such furnaces are constructed in one or two ranges, their tops being on a level
with the floor of the laboratory, as in brass-foundries, for enabling the workmen more[1174]
conveniently to inspect, and lift out, the crucibles with tongs. The ash-pits terminate
in a subterraneous passage, which supplies the grate with a current of cool air, and
serves for emptying out the ashes. The crucible, stands of course, on a sole-piece of
baked fire-clay; and its mouth is closed with a well-fitted lid. Sometimes a little
bottle-glass, or blast-furnace slag, is put into the crucible, above the steel pieces, to form
a vitreous coating, that may thoroughly exclude the air from oxidizing the metal. The
fuel employed in the cast-steel furnace is a dense coke, brilliant and sonorous, broken
into pieces about the size of an egg, one good charge of which is sufficient. The
tongs are furnished at the fire end with a pair of concave jaws, for embracing the
curvature of the crucible, and lifting it out whenever the fusion is complete. The
lid is then removed, the slag or scoriæ cleared away, and the liquid metal poured
into cast-iron octagonal or rectangular moulds, during which it throws out brilliant
scintillations.
Cast-steel works much harder under the hammer than shear steel and will not, in
its usual state, bear much more than a cherry-red heat without becoming brittle; nor
can it bear the fatigue incident to the welding operation. It may, however, be firmly
welded to iron, through the intervention of a thin film of vitreous boracic acid, at a
moderate degree of ignition. Cast steel, indeed, made from a less carburetted bar steel,
would be susceptible of welding and hammering at a higher temperature; but it would
require a very high heat for its preparation in the crucible.
Iron may be very elegantly plated with cast steel, by pouring the liquid metal from
the crucible into a mould containing a bar of iron polished on one face. In this circumstance
the adhesion is so perfect as to admit of the two metals being rolled out
together; and in this way the chisels of planes and other tools may be made, at a
moderate rate and of excellent quality, the cutting-edge being formed in the steel side.
Such instruments combine the toughness of iron with the hardness of steel.
For correcting the too high carbonization of steel, or equalizing the too highly converted
exterior of a bar with the softer steel of the interior, the metal requires merely to
be imbedded, at a cementing heat, in oxide of iron or manganese; the oxygen of which
soon abstracts the injurious excess of carbon, so that the outer layers may be even converted
into soft iron, while the axis continues steely; because the decarbonizing advances
far more rapidly than the carbonizing.
Fig. 1056. represents the mould for making crucibles for the cast-steel works. M, M,
is a solid block of wood, to support the two-handled outside mould N, N. This being
rammed full of the proper clay dough or compost (see Crucible),
the inner mould is to be then pressed vertically into
it, till it reaches the bottom P, being directed and facilitated
in its descent by the point K. A cord passes through O, by
which the inner mould is suspended over a pulley, and
guided in its motions.
When a plate of polished steel is exposed to a progressive
heat, it takes the following colours in succession: 1. a faint
yellow; 2. a pale straw-colour; 3. a full yellow; 4. a brown
yellow; 5. a brown with purple spots; 6. a purple; 7. a
bright blue; 8. a full blue; 9. a dark blue, verging on black;
after which the approach to ignition supersedes all these
colours. If the steel plate has been previously hardened by
being dipped in cold water or mercury when red-hot, then
those successive shades indicate or correspond to successive degrees of softening or
tempering. Thus, No. 1. suits the hard temper of a lancet, which requires the finest
edge, but little strength of metal; No. 2. a little softer, for razors and surgeons’ amputating
instruments; No. 3. somewhat more toughness, for penknives; No. 4. for cold
chisels and shears for cutting iron; No. 5. for axes and plane-irons; No. 6. for table
knives and cloth shears; No. 7. for swords and watch springs; No. 8. for small fine
saws and daggers; No. 9. for large saws, whose teeth need to be set with pliers, and
sharpened with a file. After ignition, if the steel be very slowly cooled, it becomes
exceedingly soft, and fit for the engraver’s purposes. Hardened steel may be tempered
to the desired pitch, by plunging it in metallic baths heated to the proper thermometric
degree, as follows: for No. 1. 430° Fahr.; No. 2. 450°; No. 3. 470°; No. 4.
490°; No. 5. 510°; No. 6. 530°; No. 7. 550°; No. 8. 560°; No. 9. 600°.
Small steel tools are most frequently tempered, after hardening, by covering their
surface with a thin coat of tallow, and heating them in the flame of a candle till the
tallow diffuses a faint smoke, and then thrusting them into the cold tallow. Rinman long
ago defined steel to be any kind of iron which, when heated to redness, and then plunged
in cold water, becomes harder. But several kinds of cast iron are susceptible of such
hardening. Every malleable and flexible iron, however, which may be hardened in that
way, is a steel. Moreover, steel may be distinguished from pure iron by its giving a[1175]
dark-gray spot when a drop of dilute nitric acid is let fall on its surface, while iron
affords a green one. Exposed to the air, steel rusts less rapidly than iron; and the
more highly carburetted, the more slowly does it rust, and the blacker is the spot left
by an acid.
After hardening, steel seems to be quite a different body; even its granular texture
becomes coarser or finer according to the degree of heat to which it was raised; it
grows so hard as to scratch glass, and resist the keenest file, while it turns exceedingly
brittle. When a slowly cooled steel rod is forged and filed, it becomes capable of affording
agreeable and harmonious sounds by its vibrations; but hard-tempered steel
affords only dull deafened tones, like those emitted by a cracked instrument.
The good quality of steel is shown by its being homogeneous; being easily worked
at the forge; by its hardening and tempering well; by its resisting or overcoming
forces; and by its elasticity. To ascertain the first point, the surface should be ground
and polished on the wheel; when its lustre and texture will appear. The second test
requires a skilful workman to give it a heat suitable to its nature and state of conversion.
The size and colour of the grain are best shown by taking a bar forged into
a razor form; hardening and tempering it; and then breaking off the thin edge in
successive bits with a hammer and anvil. If it had been fully ignited only at the end,
then, after the hardening, it will display, on fracture, a succession in the aspect of its
grains from that extremity to the other; as they are whiter and larger at the former
than the latter. The other qualities become manifest on filing the steel; using it as a
chisel for cutting iron; or bending it under a heavy weight.
Much interest was excited a few years back by the experiments of Messrs. Stodart
and Faraday on the alloys of steel with silver, platinum, rhodium, and iridium. Steel
refuses to take up in fusion more than one five-hundreth part of silver; but with this
minute quantity of alloy, it is said to bear a harder temper, without losing its tenacity.
When pure iron is substituted for steel, the alloys so formed are much less subject to
oxidation in damp air than before. With three per cent. of iridium and osmium, an
alloy was obtained which had the property of tempering like steel, and of remaining
clean and bright, in circumstances when simple iron became covered with rust. “Upon
the whole,” says the editor of the Quarterly Journal of Science, giving a report of these
experiments in his 14th volume, p. 378, “though we consider these researches upon
the alloys of steel as very interesting, we are not sanguine as to their important influence
upon the improvement of the manufacture of cutlery, and suspect that a bar of
the best ordinary steel, selected with precaution, and most carefully forged, wrought,
and tempered, under the immediate inspection of the master, would afford cutting instruments
as perfect and excellent as those composed of wootz, or of the alloys.”
Case-hardening of iron, is a process for converting a thin film of the outer surface into
steel, while the interior remains as before. Fine keys are generally finished in this
way. See Case-hardening.
So great is the affinity of iron for carbon, that, in certain circumstances, it will absorb
it from carburetted hydrogen, or coal-gas, and thus become converted into steel. On this
principle, Mr. Macintosh of Glasgow obtained a patent for making steel. His furnace
consists of one cylinder of bricks built concentrically within another. The bars of iron
are suspended in the innermost, from the top; a stream of purified coal-gas circulates
freely round them, entering below and escaping slowly above, while the bars are maintained
in a state of bright ignition by a fire burning in the annular space between the
cylinders. The steel so produced is of excellent quality; but the process does not seem
to be so economical as the ordinary cementation with charcoal powder.
Damasking of steel, is the art of giving to sabre blades a variety of figures in the
style of watering. See Damascus Blades.
Several explanations have been offered of the change in the constitution of steel,
which accompanies the tempering operation; but none of them seems quite satisfactory.
It seems to be probable that the ultimate molecules are thrown by the sudden cooling
into a constrained state, so that their poles are not allowed to take the position of
strongest attraction and greatest proximity; and hence the mass becomes hard, brittle,
and somewhat less dense. An analogous condition may be justly imputed to hastily
cooled glass, which, like hardened steel, requires to be annealed by a subsequent nicely
graduated heat, under the influence of which the particles assume the position of repose,
and constitute a denser, softer, and more tenacious body. The more sudden the cooling
of ignited steel, the more unnatural and constrained will be the distribution of its particles,
and also the more refractory, an effect produced by plunging it into cold mercury.
This excess of hardness is removed in any required degree by judicious annealing or
tempering. The state of the carbon present in the steel may also be modified by the
rate of refrigeration, as Mr. Karsten and M. Bréant conceive happens with cast iron
and the damask metal. If the uniform distribution and combination of the carbon
through the mass, determine the peculiarity of white cast iron, which is a hard and[1176]
brittle substance, and if its transition to the dark-gray and softer cast metal be effected
by a partial formation of plumbago during slow cooling, why may not something similar
be supposed to occur with steel, an analogous compound?
Mr. Oldham, printing engineer of the Bank of England, who has had great experience
in the treatment of steel for dies and mills, says that, for hardening it, the fire
should never be heated above the redness of sealing-wax, and kept at that pitch for a
sufficient time. On taking it out, he hardens it by plunging it, not in water, but in
olive oil, or rather naphtha, previously heated to 200° F. It is kept immersed only
till the ebullition ceases, then instantly transferred into cold spring water, and kept
there till quite cold. By this treatment the tools come out perfectly clean, and as
hard as it is possible to make cast-steel, while they are perfectly free from cracks, flaws,
or twist. Large tools are readily brought down in temper by being suspended in the
red-hot muffle till they show a straw-colour; but for small tools, he prefers plunging
them in the oil heated to 400 degrees; and leaves them in till they become cold.
Mr. Oldham softens his steel dies by exposing them to ignition for the requisite time,
imbedded in a mixture of chalk and charcoal.
“The common mode of softening steel,” says Mr. Baynes, “is to put it into an iron
case, surrounded with a paste made of lime, cow’s gall, and a little nitre and water; then
to expose the case to a slow fire, which is gradually increased to a considerable heat, and
afterwards allowed to go out, when the steel is found to be soft and ready for the
engraver.”[60]
Indian steel, or wootz.—The wootz ore consists of the magnetic oxide of iron, united
with quartz, in proportions which do not seem to differ much, being generally about 42
of quartz and 58 of magnetic oxide. Its grains are of various size, down to a sandy texture.
The natives prepare it for smelting by pounding the ore, and winnowing away the stony
matrix, a task at which the Hindoo females are very dexterous. The manner in which
iron ore is smelted and converted into wootz or Indian steel, by the natives at the present
day, is probably the very same that was practised by them at the time of the invasion of
Alexander; and it is a uniform process, from the Himalaya mountains to Cape Comorin.
The furnace or bloomery in which the ore is smelted, is from 4 to 5 feet high; it is
somewhat pear-shaped, being about 2 feet wide at bottom, and one foot at top; it is
built entirely of clay, so that a couple of men can finish its erection in a few hours,
and have it ready for use the next day. There is an opening in front about a foot or
more in height, which is built up with clay at the commencement, and broken down
at the end, of each smelting operation. The bellows are usually made of a goat’s skin,
which has been stripped from the animal without ripping open the part covering the belly.
The apertures at the legs are tied up, and a nozzle of bamboo is fastened in the opening
formed by the neck. The orifice of the tail is enlarged and distended by two slips of
bamboo. These are grasped in the hand, and kept close together in making the stroke
for the blast; in the returning stroke they are separated to admit the air. By working
a bellows of this kind with each hand, making alternate strokes, a pretty uniform
blast is produced. The bamboo nozzles of the bellows are inserted into tubes of clay,
which pass into the furnace at the bottom corners of the temporary wall in front.
The furnace is filled with charcoal, and a lighted coal being introduced before the
nozzles, the mass in the interior is soon kindled. As soon as this is accomplished, a
small portion of the ore, previously moistened with water, to prevent it from running
through the charcoal, but without any flux whatever, is laid on the top of the coals,
and covered with charcoal to fill up the furnace.
In this manner ore and fuel are supplied; and the bellows are urged for 3 or 4 hours,
when the process is stopped; and the temporary wall in front being broken down, the
bloom is removed by a pair of tongs from the bottom of the furnace. It is then beaten
with a wooden mallet, to separate as much of the scoriæ as possible from it, and, while
still red-hot, it is cut through the middle, but not separated, in order merely to show
the quality of the interior of the mass. In this state it is sold to the blacksmiths,
who make it into bar iron. The proportion of such iron made by the natives from 100
parts of ore, is about 15 parts. In converting the iron into steel, the natives cut it into
pieces, to enable it to pack better in the crucible, which is formed of refractory clay,
mixed with a large quantity of charred husk of rice. It is seldom charged with more
than a pound of iron, which is put in with a proper weight of dried wood chopped
small, and both are covered with one or two green leaves; the proportions being in
general 10 parts of iron to 1 of wood and leaves. The mouth of the crucible is then
stopped with a handful of tempered clay, rammed in very closely, to exclude the air. The
wood preferred is the Cassia auriculata, and the leaf that of the Asclepias gigantea, or[1177]
the Convolvulus laurifolius. As soon as the clay plugs of the crucibles are dry, from
20 to 24 of them are built up in the form of an arch, in a small blast furnace; they are
kept covered with charcoal, and subjected to heat urged by a blast for about two hours
and a half, when the process is considered to be complete. The crucibles being now
taken out of the furnace and allowed to cool, are broken, and the steel is found in
the form of a cake, rounded by the bottom of the crucible. When the fusion has been
perfect, the top of the cake is covered with striæ, radiating from the centre, and is free
from holes and rough projections; but if the fusion has been imperfect, the surface of
the cake has a honeycomb appearance, with projecting lumps of malleable iron. On an
average, four out of five cakes are more or less defective. These imperfections have
been tried to be corrected in London by re-melting the cakes, and running them into
ingots; but it is obvious, that when the cakes consist partially of malleable iron and of
unreduced oxide, simple fusion cannot convert them into good steel. When care is taken,
however, to select only such cakes as are perfect, to re-melt them thoroughly, and
tilt them carefully into rods, an article has been produced which possesses all the requisites
of fine steel in an eminent degree. In the Supplement to the Encyclopædia
Britannica, article Cutlery, the late Mr. Stodart, of the Strand, a very competent judge,
has declared “that for the purposes of fine cutlery, it is infinitely superior to the best
English cast steel.”
The natives prepare the cakes for being drawn into bars by annealing them for several
hours in a small charcoal furnace, actuated by bellows; the current of air being made
to play upon the cakes while turned over before it; whereby a portion of the combined
carbon is probably dissipated, and the steel is softened; without which operation the
cakes would break in the attempt to draw them. They are drawn by a hammer of a
few pounds weight.
The natives weld two pieces of cast steel, by giving to each a sloping face, jagged all
over with a small chisel; then applying them with some calcined borax between, and tying
them together with a wire, they are brought to a full red heat, and united by a few smart
blows of a hammer.
The ordinary bar iron of Sweden and England, when converted by cementation into
steel, exhibits upon its surface numerous small warty points, but few or no distinct
vesicular eruptions; whereas the Dannemora and the Ulverston steels present, all over
the surface of the bars, well raised blisters, upwards of three-eighths of an inch in
diameter horizontally, but somewhat flattened at top. Iron of an inferior description,
when highly converted in the cementing-chest, becomes gray on the outer edges of the
fracture; while that of Dannemora acquires a silvery colour and lustre on the edges,
with crystalline facets within. The highly converted steel is used for tools that require
to be made very hard; the slightly converted, for softer and more elastic articles, such
as springs and sword blades.
STEREOTYPE PRINTING, signifies printing by fixed types, or by a cast typographic
plate. This plate is made as follows:—The form, composed in ordinary types,
and containing one, two, three, or more pages, inversely as the size of the book, being
laid flat upon a slab, with the letters looking upwards, the faces of the types are brushed
over with oil, or preferably, with plumbago (black lead). A heavy brass rectangular
frame of three sides, with bevelled borders, adapted exactly to the size of the pages, is
then laid down upon the chase,[61] to circumscribe three sides of its typography; but the
fourth side, which is one end of the rectangle, is formed by placing near the types, and
over the hollows of the chase, a single brass bar, having the same inwards sloping bevel
as the other three sides. The complete frame resembles that of a picture, and serves to
define the area and thickness of the cast, which is made by pouring the pap of Paris
plaster into its interior space, up to a given line on its edges. The plaster mould, which
soon sets, or becomes concrete, is lifted gently off the types, and immediately placed
upright on its edge in one of the cells of a sheet-iron rack mounted within the cast-iron
oven. An able workman will mould ten sheets octavo in a day, or 160 pages. The
moulds are here exposed to air heated to fully 400° F., and become perfectly dry in the
course of two hours. As they are now friable and porous, they require to be delicately
handled. Each mould, containing generally two pages octavo, is laid, with the impression
downwards, upon a flat cast-iron plate, called the floating-plate; this plate
being itself laid on the bottom of the dipping-pan, which is a cast-iron square tray,
with its upright edges sloping outwards. A cast-iron lid is applied to the dipping-pan,
and secured in its place by a screw. The pan having been heated to 400° in a cell of
the oven, under the mould-rack, previous to receiving the hot mould, is ready to be
plunged into the bath of melted alloy contained in an iron pot placed over a furnace,
and it is dipped with a slight deviation from the horizontal plane, in order to facilitate
the escape of the air. As there is a minute space between the back or top surface of[1178]
the mould and the lid of the dipping-pan, the liquid metal, on entering into the pan
through the orifices in its corners, floats up the plaster along with the iron plate on
which it had been laid, thence called the floating-plate, whereby it flows freely into every
line of the mould, through notches cut in its edge, and forms a layer or lamina upon its
face, of a thickness corresponding to the depth of the border. Only a thin metal film is
left upon the back of the mould. The dipping-pan is suspended, plunged, and removed
by means of a powerful crane, susceptible of vertical and horizontal motions in all directions.
When lifted out of the bath, it is set in a water-cistern, upon bearers so placed
as to allow its bottom only to touch the surface. Thus the metal first concretes below,
while, by remaining fluid above, it continues to impart hydrostatic pressure during
the shrinkage attendant upon refrigeration. As it thus progressively contracts in volume,
more melted metal is fed into the corners of the pan by a ladle, in order to keep up the
hydrostatic pressure upon the mould, and to secure a perfect impression, as well as a solid
cast. Were the pan more slowly and equably cooled, by being left in the air, the thin
film of metal upon the back of the inverted plaster cake would be apt to solidify first,
and intercept the hydrostatic action indispensable to the purpose of filling all the lines in
its face. A skilful workman makes five dips, containing two pages octavo each, in the
course of an hour, or about nine and a half octavo sheets per day. The pan being taken
asunder, the compound cake of mould and metal is removed, and beat upon its edges
with a wooden mallet, to detach the superfluous metal. The stereotype plate is then
handed over to the picker, who planes its edges truly square, turns its back flat upon
a lathe to a determinate thickness, and carefully removes the little imperfections occasioned
by dirt or air left among the letters when the mould was cast. Should any of
them be damaged in the course of the operation, they must be cut out, and replaced by
soldering in separate types of the same size and form.
STILL (Alambic, Fr.; Blase, Germ.); is a chemical apparatus, for vaporizing
liquids by heat in one part, called the cucurbit, and condensing the vapours into liquids
in another part, called the refrigeratory; the general purpose of both combined being to
separate the more volatile fluid particles from the less volatile. In its simplest form, it
consists of a retort and a receiver, or of a pear-shaped matrass and a capital, furnished
with a slanting tube for conducting away the condensed vapours in drops; whence the
term still, from the Latin verb stillare, to drop. Its chief employment in this country
being to eliminate alcohol, of greater or less strength, from fermented wash, I shall
devote this article to a description of the stills best adapted to the manufacture of
British spirits, referring to chemical authors[62] for those fitted for peculiar objects.
In respect of rapidity and extent of work, stills had attained to an extraordinary pitch
of perfection in Scotland about thirty years ago, when legislative wisdom thought fit to
levy the spirits duty, per annum, from each distiller, according to the capacity of his
still. It having been shown, in a report presented to the House of Commons in 1799,
that an 80-gallon still could be worked off in eight minutes, this fact was made the
basis of a new fiscal law, on the supposition that the maximum of velocity had been
reached. But, instigated by the hopes of enormous gains at the expense of the revenue,
the distillers soon contrived to do the same thing in three minutes, by means of broad-bottomed
shallow stills, with stirring-chains, and lofty capitals. In the year 1815,
that preposterous law, which encouraged fraud and deteriorated the manufacture, was
repealed. The whiskey duties having been since levied, independently of the capacity
of the still, upon the quantity produced, such rapid operations have been abandoned,
and processes of economy in fuel, and purity in product, have been sought after.
One of the greatest improvements in modern distilleries, is completing the analysis
of crude spirit at one operation. Chemists had been long familiar with the contrivance
of Woulfe, for impregnating with gaseous matter, water contained in a range of bottles;
but they had not thought of applying that plan to distillation, when Edouard Adam,
an illiterate workman of Montpellier, after hearing accidentally a chemical lecture
upon that apparatus, bethought himself of converting it into a still. He caused
the boiling-hot vapours to chase the spirits successively out of one bottle into another,
so as to obtain in the successive vessels alcohol of any desired strength and purity, “at
one and the same heat.” He obtained a patent for this invention in 1801, and was soon
afterwards enabled, by his success on the small scale, to set up in his native city a magnificent
distillery, which excited the admiration of all the practical chemists of that
day. In November, 1805, he obtained a certificate of certain improvements for extracting
from wine, at one process, the whole of its alcohol. Adam was so overjoyed,
after making his first experiments, that he ran about the streets of Montpellier, telling
every body of the surprising results of his invention. Several competitors soon entered
the lists with him, especially Solimani, professor of chemistry in that city, and Isaac[1179]
Berard, distiller in the department of Gard; who, having contrived other forms of continuous
stills, divided the profits with the first inventor.
The principles of spirituous distillation may be stated as follows:—The boiling
point of alcohol varies with its density or strength, in conformity with the numbers
in the following table:—
Specific
gravity. |
Boiling point,
by
Fahrenheit’s
scale. |
| 0·7939 |
168·5° |
| 0·8034 |
168·0 |
| 0·8118 |
168·5 |
| 0·8194 |
169·0 |
| 0·8265 |
172·5 |
| 0·8332 |
173·5 |
| 0·8397 |
175·0 |
| 0·8458 |
177·0 |
| 0·8518 |
179·0 |
| 0·8875 |
181·0 |
| 0·8631 |
183·0 |
| 0·8765 |
187·0 |
| 0·8892 |
190·0 |
| 0·9013 |
194·0 |
| 0·9126 |
197·0 |
| 0·9234 |
199·0 |
| 0·9335 |
201·0 |
See also the table under Alcohol, page 16.
Hence, the lower the temperature of the spirituous vapour which enters the refrigeratory
apparatus, the stronger and purer will the condensed spirit be; because the
offensive oils, which are present in the wash or wine, are less volatile than alcohol, and
are brought over chiefly with the aqueous vapour. A perfect still should, therefore,
consist of three distinct members; first, the cucurbit, or kettle; second, the rectifier,
for intercepting more or less of the watery and oily particles; and third, the refrigerator,
or condenser of the alcoholic vapours.
These principles are illustrated in the construction of the still represented in figs. 1057,
1058, 1059, 1060, 1061.; in which the resources of the most refined French stills are
combined with a simplicity and solidity suited to the grain distilleries of the United
Kingdom. Three principal objects are obtained by the arrangement here shown; first,
the extraction from fermented wort or wine, at one operation, of a spirit of any desired
cleanness and strength; second, great economy of time, labour, and fuel; third, freedom
from all danger of blowing up or boiling over, by mismanaged firing. When a combination
of water, alcohol, and essential oil, in the state of vapour, is passed upwards
through a series of winding passages, maintained at a determinate degree of heat,
between 170° and 180°, the alcohol alone, in any notable proportion, will retain the
elastic form, and will proceed onwards into the refrigeratory tube, in which the said
passages terminate; while the water and the oil will be in a great measure condensed,
arrested, and thrown back into the body of the still, to be discharged with the effete
residuum.
The system of passages or channels, represented in fig. 1058., is so contrived as to
bring the mingled vapours which rise from the alembic a, into ample and intimate
contact with metallic surfaces, maintained, in a water-bath, at a temperature self-regulated
by a heat-governor. See Thermostat.
The neck of the alembic tapers upwards, as shown at b, fig. 1057.; and at c, fig. 1058.,
it enters the bottom, or ingress vestibule, of the rectifier c, f. f is its top or egress
vestibule, which communicates with the bottom one by parallel cases or rectangular
channels d, d, d, of which the width is small, compared with the length and height.
These cases are open at top and bottom, where they are soldered or riveted into a general
frame within the cavity, enclosed by the two covers f, c, which are secured round their
edges e, e, e, e, with bolts and packing. Each case is occupied with a numerous series
of shelves or trays, placed at small distances over each other, in a horizontal or slightly
inclined position, of which a side view is given in fig. 1059., and cross sections at d, d, d,
fig. 1058. Each shelf is turned up a little at the two edges, and at one end, but sloped
down at the other end, that the liquor admitted at the top may be made to flow slowly
backwards and forwards in its descent through the system of shelves or trays, as indicated
by the darts and spouts in fig. 1059. The shelves of each case are framed
together by two or more vertical metallic rods, which pass down through them, and
are fixed to each shelf by solder, or by screw-nuts. By this means, if the cover f, be
removed, the sets of shelves may be readily lifted out of the cases and cleaned; for
which reason they are called movable.
The intervals i, i, i, fig. 1058., between the cases, are left for the free circulation of
the water contained in the bath-vessel g, g; these intervals being considerably narrower
than the cases.
Fig. 1060. represents in plan the surface of the rectifying cistern, shown in two
different sections in figs. 1058. and 1059. h, k, figs. 1058. and 1060., is the heat-governor,[1180]
shaped somewhat like a pair of tongs. Each leg is a compound bar, consisting of a
flat bar or ruler of steel, and one of brass alloy, riveted facewise together, having their
edges up and down. The links, at k, are joined to the free ends of these compound bars,
which, receding by increase and approaching by decrease of temperature, act by a lever on
the stopcock l, fixed to the pipe of a cold-water back, and are so adjusted by a screw-nut,
that whenever the water in the bath vessel g, g, rises above the desired temperature,
cold water will be admitted, through the stopcock l, and pipe n, into the bottom of the
cistern, and will displace the over-heated water by the overflow-pipe m. Thus a perfect
equilibrium of caloric may be maintained, and alcoholic vapour of correspondent
uniformity transmitted to the refrigeratory.
Fig. 1061. is the cold condenser, of similar construction to the rectifier, fig. 1058.; only
the water cells should be here larger in proportion to the vapour channels d, d. This
refrigeratory system will be found very powerful, and it presents the great advantage of
permitting its interior to be readily inspected and cleansed. It is best made of laminated
tin, hardened with a little copper alloy.
The mode of working the preceding apparatus will be understood by the following
instructions. Into the alembic, a, let as much fermented liquor be admitted as will protect
its bottom from being injured by the fire, reserving the main body in the charging-back.
Whenever the ebullition in the alembic has raised the temperature of the water-bath
g, g, to the desired pitch, whether that be 170°, 175°, or 180°, the thermostatic
instrument is to be adjusted by its screw-nut, and then the communication with the
charging-back is to be opened by moving the index of the stopcock o, over a proper
portion of its quadrantal arch. The wash will now descend in a slender equable
stream, through the pipe o, f, thence spread into the horizontal tube p, p, and issue
from the orifices of distribution, as seen in the figure, into the respective flat trays or
spouts. The manner of its progress is seen for one set of trays, in fig. 1059. The direction
of the stream in each shelf is evidently the reverse of that in the shelf above and
below it; the turned-up end of one shelf corresponding to the discharge slope of its
neighbour.
By diffusing the cool wash or wine in a thin film over such an ample range of surfaces,
the constant tendency of the bath to exceed the proper limit of temperature is
counteracted to the utmost, without waste of time or fuel; for the wash itself, in
transitu, becomes boiling-hot, and experiences a powerful steam distillation. By this
arrangement a very moderate influx of cold water, through the thermostatic stopcock,
suffices to temper the bath; such an extensive vaporization of the wash producing a far
more powerful refrigerant influence than its simple heating to ebullition. It deserves
to be remarked, that the maximum distillatory effect, or the bringing over the greatest
quantity of pure spirits in the least time, and with the least labour and fuel, is here
accomplished without the least steam pressure in the alembic; for the passages are[1181]
all pervious to the vapour; whereas, in almost every wash-still heretofore contrived for
similar purposes, the spirituous vapours must force their way through successive layers
of liquid, the total pressure produced by which causes undue elevation of temperature,
and obstruction to the process. Whatever supplementary refrigeration of the vapours
in their passage through the bath may be deemed proper, will be administered by the
thermostatic regulator.
Towards the end of the process, after all the wash has entered the alembic, it may
be sometimes desirable, for the sake of despatch, to modify the thermostat, by its adjusting-screw,
so that the bath may take a higher temperature, and allow the residuary
feints to run rapidly over, into a separate cistern. This weak fluid may be pumped
back into the alembic, as the preliminary charge of a fresh operation.
The above plan of a water-bath regulated by the thermostat, may be used simply as
a rectifying cistern, without transmitting the spirit or wash down through it. The
series of shelves will cause the vapours from the still to impinge against a most extensive
system of metallic surfaces, maintained at a steady temperature, whereby their
watery and crude constituents will be condensed and thrown back, while their fine
alcoholic particles will proceed forwards to the refrigeratory. Any ordinary still may
be readily converted into this self-rectifying form, by merely interposing the cistern,
fig. 1058., between the alembic and the worm-tub. The leading novelty of the present
invention is the movable system of shelves or trays, enclosed in metallic cases, separated
by water, combined with the thermostatic regulator. By this combination, any quality
of spirits may be procured at one step from wash or wine, by an apparatus, simple,
strong and easily kept in order.
The empyreumatic taint which spirits are apt to contract from the action of the naked
fire on the bottom of the still, may be entirely prevented by the use of a bath of potash
lye, p, p, fig. 1057.; for thus a safe and effectual range of temperature, of 300° F., may
be conveniently obtained. The still may also be used without the bath vessel.
Mr. D. T. Shears, of Southwark, obtained a patent in March, 1830, for certain improvements
and additions to stills, which are ingenious. They are founded upon a
previous patent, granted to Joseph Corty, in 1818; a section of whose contrivance is
shown in fig. 1062., consisting of a first still a, a second still b, a connecting tube c, from
the one end to the other, and the tube d, which leads
from the second still-head down through the bent tube
e, e, to the lower part of the condensing apparatus.
The original improvements described under Corty’s
patent, consisted further, in placing boxes f, f, f, of the
condensing apparatus in
horizontal positions, and
at a distance from each
other, in order that the
vapour might ascend
through them, for the
purpose of discharging
the spirit by the top tube
g, and pipe h, into the
worm, in a highly rectified
or concentrated
state. In each of the
boxes f, there is a convex
plate or inverted dish i,
i, i, and the vapour in
rising from the tube e,
strikes against the concave
or under part of
the first dish, and then
escapes round its edges,
and over its convex surface,
to the under part
of the second dish, and
so on to the top, the
condensed part of the vapour flowing down again into the still, and the spirit passing
off by the pipe h, at top; and as the process of condensation will be assisted by cooling
the vapour as it rises, cold water is made to flow over the tops of the boxes f, from
a cock k, and through small channels or tubes on the sides of the boxes, and is ultimately
discharged by the pipe l, at bottom.
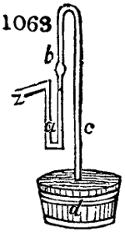
Fig. 1063. represents a peculiarly shaped tube a, through which the spirit is described
as passing after leaving the end of the worm at b, which tube is open to the atmospheric[1182]
air at z; c, is the passage through which the carbonic acid gas is described as escaping
into the vessel of water d.
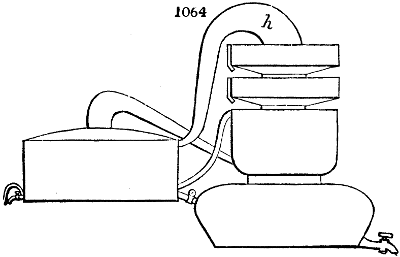
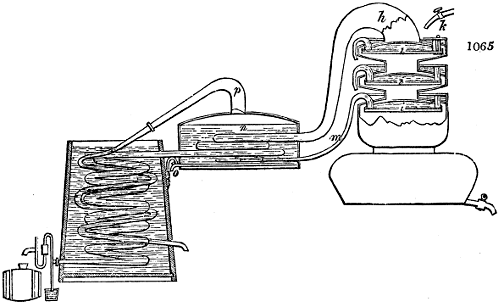
Now the improvements claimed under the present patent, are exhibited in figs. 1064,
1065, and 1066. Fig. 1064. represents the external appearance of a still, the head
of which is made very capacious, to guard against over-boiling by any mismanagement
of the fire; fig. 1065. is the same, partly in section. On the top of the still-head is
formed the first-described rectifying apparatus, or series of condensing boxes. The
vapour from the body of the still filling the head, meets with the first check from
the dish or lower vessel i, and after passing under its edges, ascends and strikes against
the lower part of the second dish or vessel i, and so on, till it ultimately leaves the still-head
by the pipe at top.
This part of the apparatus is slightly altered from the former, by the substitution of
hollow convex vessels, instead of the inverted dishes before described, which vessels have
rims descending from their under surfaces, for the purpose of retaining the vapour.
The cold water, which, as above described, flowed over the tops of the boxes f, for the
purpose of cooling them, now flows also through the hollow convex vessels i, within
the boxes, and by that means greatly assists the refrigerating process, by which the
aqueous parts of the vapour are more readily condensed, and made to fall down and
flow back again into the body of the still, while the spirituous parts pass off at top to
the worm, in a very high state of rectification.
After the water employed for the refrigeration has passed over all the boxes, and
through all the vessels, it is carried off by the pipe m, through the vessel n, called the
wash-heater; that is, the vessel in which the wash is placed previous to introducing it
into the still. The pipe m, is coiled round in the lower part of the vessel n, in order
that the heated water may communicate its caloric to the wash, instead of losing the
heat by allowing the water to flow away. After the heated water has made several
turns round the wash heater, it passes out at the curved pipe o, which is bent up, in
order to keep the coils of the pipe within always full of water.
Instead of the coiled pipe n, last described, the patentee proposes sometimes to pass
the hot water into a chamber in a tub or wooden vessel, as at n, in fig. 1061., in which the
wash to be heated occupies the upper part of the vessel, and is separated from the lower
part by a thin metallic partition.
The swan-neck h, figs. 1064. and 1065., which leads from the head of the still, conducts
the spirit from the still through the wash-heater, where it becomes partially cooled, and
gives out its heat to the wash; and from thence the
spirit passes to the worm tub, and being finally condensed,
is passed through a safety tube, as (fig. 1058.)
before described, and by the funnel is conducted into the
cask below.
Should any spirit rise in the wash-heater during the above operation, it will be
carried down to the worm by the neck p, and coiled pipe, and discharged at its lower
end; or it may be passed into the still-head, as shown in fig. 1062.
A patent was obtained by Mr. Æneas Coffey, in August, 1830, for a still, which has
been since mounted in several distilleries. It is economical in fuel, labour, and time,
but is said not to produce a clean spirit, without peculiar attention.
The apparatus is represented in fig. 1067. a, b, c, d, is a sectional view of that part
of the still wherein the wash is deprived of its alcohol, and the vapours analyzed. It is
described as consisting of a chamber or vessel a, with the vertical chamber b, c,
placed above it; the lower half of this chamber is divided into compartments by horizontal[1183]
plates e, e, e, of thin copper or other metal; each of these plates is turned down at one
side, until it nearly touches the plate next underneath it, as shown in the figure; thus leaving
a passage throughout the
whole of them, by which any
liquid falling on the top plate
may descend into the next under
it, and from that to the
third, and so on, from plate
to plate, at the alternate ends,
until it arrives at the last
plate, wherein it falls into the
vessel a, by the pipe f; each
of these plates is furnished with
several light valves, opening
upwards, through which any
steam or vapour may ascend; it
may also be perforated with
holes, but they must not be so
numerous or so large as to allow
of all the steam passing
through them without raising
the valves; c, is a pipe by which the alcoholic vapour, after it has been analyzed, and
has acquired the proper strength, is conducted into the vessel d, which is made perfectly
close; the vapour will here be condensed on the surface of the pipe g, g, g; from
this chamber it will descend in a liquid state into the pipe h, whence it may be conducted
to a worm or refrigerator, to be cooled in the ordinary way; i, is a vessel through
which the spent wash flows, after being operated upon in the distilling apparatus, and is
discharged in a state of ebullition; j, is a vessel or chamber containing the wash to be
distilled. A force pump may be substituted, to force the wash through the pipes k, and
distilling apparatus, with the velocity required.
The patentee states that it is requisite the wash should be passed through the pipe
k, with sufficient velocity and force, so as to prevent the deposition of sediment in the
pipe; the wash in its passage through the pipe k, will gradually become increased in
temperature as it passes through the spent wash in the chamber, and the close vessel d,
until it is discharged nearly at the boiling point on the upper plate in the chamber,
where it comes in contact with the vapours arising from the vessel a.
It is to be observed, that the wort does not reach the boiling point while in the pipe
k, k; to ascertain which, a thermometer is placed on the pipe, and by increasing or
diminishing the quantity of wash, its temperature may be regulated. The wash, after
being discharged from the pipe k, descends from plate to plate as before mentioned, at
which time a supply of steam from a boiler, or generator is admitted into the apparatus,
through the pipe.
The lower part of this pipe in the vessel a, is pierced with a number of small holes,
so as to spread the steam over the vessel; it then rises upwards, passing through the
plate by the small holes and valves, and through the stratum or sheet of wash flowing
over them; the wash, as it descends, gives out a portion of its alcohol to the steam, as it
passes over every plate, until it is entirely deprived of its spirit, which it will generally
do by the time it arrives at the 7th or 8th plate; but it is better to employ a greater
number, to guard against accidents or neglect.
A small steam pipe rises from the chamber a, with its upper end opening into the
box or chamber; into this chamber the end of a worm projects from the cistern of cold
water; the steam rising up the pipe is nearly all condensed in the worm, and flows back
into the chamber a, by the pipe. The small portion of the steam uncondensed, is
allowed to escape at the upper end of the worm, and the flame of a small lamp or taper
is to be constantly kept over the orifice; when, should the least quantity of alcohol
descend with the wash into the chamber a, it will rise with the steam through the pipe
and worm, and immediately take fire from the flame of the lamp or taper, thereby
warning the attendant to increase the supply of steam or diminish the quantity of wash,
as may seem necessary.
[1184]
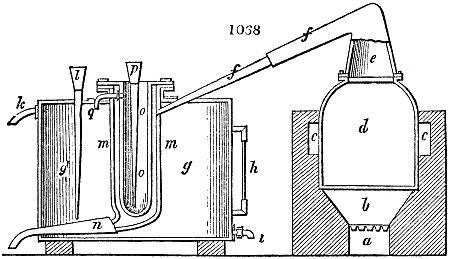
I shall conclude this article with a description of the cheap still which is commonly
employed by the chemists in Berlin for rectifying alcohol. a, is the ash-pit; b, the
fireplace; c, c, the
flues, which go spirally
round the
sides of the cucurbit
d; e, the capital,
made of block tin,
and furnished with
a brass edge, which
fits tight to a corresponding
edge on
the mouth of d; f, f,
the slanting pipes
of the capital; g,
the oval refrigeratory,
made of copper;
h, the water-gauge
glass tube; i,
a stopcock for emptying the vessel; k, do., for drawing off the hot water from the surface;
l, tube for the supply of cold water. A double cylinder of tin is placed in the
refrigeratory, of which the outer one m, m, stands upon three feet, and is furnished
with a discharge pipe n. The inner one o, o, which is open above, receives cold water
through the pipe p, and lets the warm water flow off through the short tube q, into
the refrigeratory. In the narrow space between the two cylinders, the vapours proceeding
from the capital are condensed, and pass off in the liquid state through n. The
refrigeratory is made oval, in order to receive two condensers alongside of each other
in the line of the longer axis; though only one, and that in the middle, is represented in
the figure.
STOCKING MANUFACTURE. See Hosiery.
STONE, is earthy matter, condensed into so hard a state as to yield only to the blows
of a hammer, and therefore well adapted to the purposes of building. Such was the
care of the antients to provide strong and durable materials for their public edifices, that
but for the desolating hands of modern barbarians, in peace and in war, most of the
temples and other public monuments of Greece and of Rome would have remained
perfect at the present day, uninjured by the elements during 2000 years. The contrast,
in this respect, of the works of modern architects, especially in Great Britain, is very
humiliating to those who boast so loudly of social advancement; for there is scarcely a
public building of recent date, which will be in existence one thousand years hence.
Many of the most splendid works of modern architecture are hastening to decay, in what
may be justly called the very infancy of their existence, if compared with the date of
those erected in antient Italy, Greece, and Egypt. This is remarkably the case with
the three bridges of London, Westminster, and Blackfriars; the foundations of which
began to perish most visibly in the very lifetime of their constructors. Every stone
intended for a durable edifice, ought to be tested as to its durability, by immersion in a
saturated solution of sulphate of soda, and exposure during some days to the air. The
crystallization which ensues in its interior, will cause the same disintegration of its substance
which frost would occasion in a series of years.
STONE, ARTIFICIAL, for statuary and other decorations of architecture, has
been made for several years with singular success at Berlin, by Mr. Feilner. His
materials are nearly the same with those of English pottery; and the plastic mass is
fashioned either in moulds, or by hand. His kilns, which are peculiar in form, and
economical in fuel, deserve to be generally known. Figs. 1069. and 1070. represent his
round kiln; fig. 1069. being an oblique section in the line A, B, C, of fig. 1070., which is
a ground plan in the line D, a, b, E, of fig. 1069. The inner circular space c, covered
with the elliptical arch, is filled with the figures to be baked, set upon brick supports.
The hearth is a few feet above the ground; and there are steps before the door d, for
the workmen to mount by, in charging the kiln. The fire is applied on the four sides
under the hearth. The flame of each passes along the straight flues f i, f i, and f k. In
the second annular flue g, g, as also in the third l, l, the flame of each fire is kept apart,
being separated from the adjoining, by the stones h and m. In the fourth flue n, the
flames again come together, as also in o, and ascend by the middle opening. Besides
this large orifice, there are several small holes, p, p, in the hearth over the above flues,
to lead the flames from the other points into contact with the various articles. There
are also channels q, q, in the sides, enclosed by thin walls r, to promote the equable
distribution of the heat; and these are placed right over the first fire-flues e. The[1185]
partitions r, are perforated with many holes, through which, as well as from their tops,
the flame may be directed inwards and downwards; s are the vents for carrying off the
flames into the upper space u, which is usually left empty. These vents can be closed
by iron damper-plates, pushed in through the side-slits of the dome. t, t, are peep-holes,
for observing the state of ignition in the furnace; but they are most commonly
bricked up. Fig. 1071. is a vertical section, and fig. 1072. a plan, of an excellent kiln for
baking clay to a stony consistence, for the above purpose, or for burning fire-bricks.
A, is the lower; B, the middle; C, the upper kiln; and D, the
hood, terminating in the chimney E. a, a, is the ash-pit; b, b, the
vault for raking out the ashes; it is covered with an iron door c.
d, is the peep-hole, filled with a clay stopper; e, is the fireplace;
f, f, a vent in the middle of each arch; g, g, flues at
the sides of the arches, situated between the two fireplaces;
h, i, k, are apertures for introducing the articles to be baked; l, a
grate for the fire in the uppermost kiln; m, the ash-pit; n, the
fire-door; o, openings
through which
the flames of a second
fire are thrown
in. At first, only the
ground kiln A, is
fired, with cleft billets
of pine-wood,
introduced at the
opening e; when
this is finished, the
second is fired; and
then the third, in
like manner. This kiln is very like the porcelain kiln of Sèvres, and is employed
in many places for baking stoneware.
Mr. Keene obtained a patent, about a year ago, for making a factitious stone-paste
in the following way:—He dissolves one pound of alum in a gallon of water, and
in this solution he soaks 84 pounds of gypsum calcined in small lumps. He exposes
these lumps in the open air for about eight days, till they become apparently dry, and
then calcines them in an oven at a dull-red heat. The waste heat of a coke oven is
well adapted for this purpose. (See Pitcoal, coking of.) These lumps, being ground
and sifted, afford a fine powder, which, when made up into a paste with the proper
quantity of water, forms the petrifying ground. The mass soon concretes, and after
being brushed over with a thin layer of the petrifying paste, may be polished with
pumice, &c., in the usual way. It then affords a body of great compactness and durability.
If half a pound of copperas be added to the solution of the alum, the gypsum
paste, treated as above, has a fine cream or yellow colour. This stone stands the
weather well.
STONEWARE. (Fayence, Fr.; Steingut, Germ.) See Pottery.
STORAX, STYRAX, flows from the twigs and the trunk of the Liquidambar
styraciflua, a tree which grows in Louisiana, Virginia, and Mexico. Liquidamber, as
this resin is also called, is a brown or ash-gray substance, of the consistence of turpentine,
which dries up rapidly, has an agreeable smell, like benzoin, and a bitterish, sharp,
burning taste. It dissolves in four parts of alcohol, and affords 1·4 per cent. of benzoic
acid.
[1186]
STOVE (Poële, Calorifère, Fr.; Ofen, Germ.); is a fireplace, more or less close,
for warming apartments. When it allows the burning coals to be seen, it is called a
stove-grate. Hitherto stoves have rarely been had recourse to in this country for
heating our sitting-rooms; the cheerful blaze and ventilation of an open fire being
generally preferred. But last winter, by its inclemency, gave birth to a vast multitude
of projects for increasing warmth and economizing fuel, many of them eminently
insalubrious, by preventing due renewal of the air, and by the introduction of noxious
fumes into it. When coke is burned very slowly in an iron box, the carbonic acid gas
which is generated, being half as heavy again as the atmospherical air, cannot ascend in
the chimney at the temperature of 300° F.; but regurgitates into the apartment through
every pore of the stove, and poisons the atmosphere. The large stoneware stoves of
France and Germany are free from this vice; because, being fed with fuel from the outside,
they cannot produce a reflux of carbonic acid into the apartment, when their draught
becomes feeble, as inevitably results from the obscurely burning stoves which have the
doors of the fireplace and ash-pit immediately above the hearth-stone.
I have recently performed some careful experiments upon this subject, and find that
when the fuel is burning so slowly in the stove as not to heat the iron surface above the
250th or 300th degree of Fahr., there is a constant deflux of carbonic acid gas from
the ash-pit into the room. This noxious emanation is most easily evinced by applying
the beak of a matrass, containing a little Goulard’s extract (solution of subacetate
of lead), to a round hole in the door of the ash-pit of a stove in this languid
state of combustion. In a few seconds the liquid will become milky, by the reception
of carbonic acid gas. I shall be happy to afford ocular demonstration of this fact to
any incredulous votary of the pseudo-economical, anti-ventilation, stoves now so much
in vogue. There is no mode in which the health and life of a person can be placed in
more insidious jeopardy, than by sitting in a room with its chimney closed up with
such a choke-damp-vomiting stove.
That fuel may be consumed by an obscure species of combustion, with the emission
of very little heat, was clearly shown in Sir H. Davy’s Researches on Flame. “The
facts detailed on insensible combustion,” says he, “explain why so much more heat is
obtained from fuel when it is burned quickly, than slowly; and they show that, in all
cases, the temperature of the acting bodies should be kept as high as possible; not
only because the general increment of heat is greater, but likewise because those combinations
are prevented, which, at lower temperatures, take place without any considerable
production of heat. These facts likewise indicate the source of the great
error into which experimenters have fallen, in estimating the heat given out in the combustion
of charcoal; and they indicate methods by which the temperature may be increased,
and the limits to certain methods.” These conclusions are placed in a
strong practical light by the following simple experiments:—I set upon the top orifice
of a small cylindrical stove, a hemispherical copper pan, containing six pounds of
water, at 60° F., and burned briskly under it 31⁄2 pounds of coke in an hour; at the
end of which time, 41⁄2 pounds of water were boiled off. On burning the same
weight of coke slowly in the same furnace, surmounted by the same pan, in the course
of 12 hours, little more than one-half the quantity of water was exhaled. Yet, in the
first case, the aerial products of combustion swept so rapidly over the bottom of the
pan, as to communicate to it not more than one-fourth of
the effective heat which might have been obtained by one
of the plans described in the article Evaporation; while,
in the second case, these products moved at least 12 times
more slowly across the bottom of the pan, and ought therefore
to have been so much the more effective in evaporation,
had they possessed the same power or quantity of
heat.
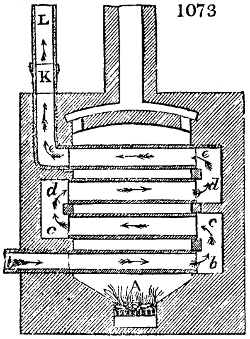
Stoves, when properly constructed, may be employed
both safely and advantageously to heat entrance-halls upon
the ground story of a house; but care should be taken not
to vitiate the air by passing it over ignited surfaces, as is
the case with most of the patent stoves now foisted upon
the public. Fig. 1073. exhibits a vertical section of a stove
which has been recommended for power and economy; but
it is highly objectionable, as being apt to scorch the air.
The flame of the fire A, circulates round the horizontal
pipes of cast iron, b b, c c, d d, e e, which receive the external air at the orifice b,
and conduct it up through the series, till it issues highly heated at K, L, and may be
thence conducted wherever it is wanted. The smoke escapes through the chimney B.
This stove has evidently two prominent faults; first, it heats the air-pipes very unequally,[1187]
and the undermost far too much; secondly, the air, by the time it has ascended through
the zigzag range to the pipe e e, will be nearly of the same temperature with it, and
will therefore abstract none of its heat. Thus the upper pipes, if there be several in the
range, will be quite inoperative, wasting their warmth upon the sooty air.
Fig. 1074. exhibits a transverse vertical section of a far more economical and powerful
stove, in which the above evils are avoided. The products of combustion of the fire A,
rise up between two brick walls, so
as to play upon the bed of tiles B,
where, after communicating a moderate
heat to the series of slanting
pipes whose areas are represented
by the small circles a, a, they turn
to the right and left, and circulate
round the successive rows of pipes
b b, c c, d d, e e, and finally escape
at the bottom by the flues g, g, pursuing
a somewhat similar path to
that of the burned air among a
bench of gas-light retorts. It is
known, that two-thirds of the fuel
have been saved in the gas-works by
this distribution of the furnace. For
the purpose of heating apartments,
the great object is to supply a vast
body of genial air; and, therefore,
merely such a moderate fire should
be kept up in A, as will suffice to
warm all the pipes pretty equably to
the temperature of 220° Fahr.; and, indeed, as they are laid with a slight slope, are
open to the air at their under ends, and terminate at the upper in a common main pipe
or tunnel, they can hardly be rendered very hot by any intemperance of firing. I can
safely recommend this stove to my readers. If the tubes be made of stoneware, its
construction will cost very little; and they may be made of any size, and multiplied so
as to carry off the whole effective heat of the fuel, leaving merely so much of it in the
burned air, as to waft it fairly up the chimney.
I shall conclude this article by a short extract of a paper which was read before the
Royal Society, on the 16th of June, 1836, upon warming and ventilating apartments; a
subject to which my mind had been particularly turned at that time, by the Directors
of the Customs Fund of Life Assurance, on account of the very general state of indisposition
and disease prevailing among those of their officers (nearly 100 in number)
engaged on duty in the Long Room of the Custom House, London.
“The symptoms of disorder experienced by the several gentlemen (about twenty in
number), whom I examined, out of a great many who were indisposed, were of a very
uniform character. The following is the result of my researches:—
“A sense of tension or fulness of the head, with occasional flushings of the countenance,
throbbing of the temples, and vertigo, followed, not unfrequently, with a confusion
of ideas, very disagreeable to officers occupied with important and sometimes
intricate calculations. A few are affected with unpleasant perspiration on their sides.
The whole of them complain of a remarkable coldness and languor in their extremities,
more especially the legs and feet, which has become habitual, denoting languid circulation
in these parts, which requires to be counteracted by the application of warm
flannels on going to bed. The pulse is, in many instances, more feeble, frequent, sharp,
and irritable, than it ought to be, according to the natural constitution of the individuals.
The sensations in the head occasionally rise to such a height, notwithstanding the most
temperate regimen of life, as to require cupping, and at other times depletory remedies.
Costiveness, though not a uniform, is yet a prevailing symptom.
“The sameness of the above ailments, in upwards of one hundred gentlemen, at very
various periods of life, and of various temperaments, indicates clearly sameness in the
cause.
“The temperature of the air in the Long Room ranged, in the three days of my experimental
inquiry, from 62° to 64° of Fahrenheit’s scale; and in the Examiner’s Room it
was about 60°, being kept somewhat lower by the occasional shutting of the hot-air
valve, which is here placed under the control of the gentlemen; whereas that of the
Long Room is designed to be regulated in the sunk story, by the fireman of the stove,
who seems sufficiently careful to maintain an equable temperature amidst all the vicissitudes
of our winter weather. Upon the 7th of January, the temperature of the open[1188]
air was 50°; and on the 11th it was only 35°; yet upon both days the thermometer in
the Long Room indicated the same heat, of from 62° to 64°.
“The hot air discharged from the two cylindrical stove-tunnels into the Long Room
was at 90° upon the 7th, and at 110° upon the 11th. This air is diluted, however, and
disguised, by admixture with a column of cold air, before it is allowed to escape. The
air, on the contrary, which heats the Examiner’s Room, undergoes no such mollification,
and comes forth at once in an ardent blast of fully 170°; not unlike the simoom of the
desert, as described by travellers. Had a similar nuisance, on the greater scale, existed
in the Long Room, it could not have been endured by the merchants and other visitors
on business: but the disguise of an evil is a very different thing from its removal.
The direct air of the stove, as it enters the Examiner’s Room, possesses, in an eminent
degree, the disagreeable smell and flavour imparted to air by the action of red-hot
iron; and, in spite of every attention on the part of the fireman to sweep the stove
apparatus from time to time, it carries along with it abundance of burned dusty
particles.
“The leading characteristic of the air in these two rooms, is its dryness and disagreeable
smell. In the Long Room, upon the 11th, the air indicated, by Daniell’s hygrometer,
70 per cent. of dryness, while the external atmosphere was nearly saturated with
moisture. The thermometer connected with the dark bulb of that instrument stood at
30° when dew began to be deposited upon it; while the thermometer in the air stood at
64°. In the court behind the Custom-house, the external air being at 35°, dew was
deposited on the dark bulb of the hygrometer by a depression of only 3°; whereas in
the Long Room, on the same day, a depression of 34° was required to produce that
deposition. Air, in such a dry state, would evaporate 0·44 in. depth of water from a
cistern in the course of twenty-four hours; and its influence on the cutaneous exhalents
must be proportionably great.
“As cast iron always contains, beside the metal itself, more or less carbon, sulphur,
phosphorus, or even arsenic, it is possible that the smell of air passed over it in an
incandescent state, may be owing to some of these impregnations; for a quantity of
noxious effluvia, inappreciably small, is capable of affecting not only the olfactory
nerves, but the pulmonary organs. I endeavoured to test the air as it issued from the
valve in the Examiner’s Room, by presenting to it pieces of white paper moistened
with a solution of nitrate of silver, and perceived a slight darkening to take place, as if
by sulphurous fumes. White paper, moistened with sulphuretted hydrogen water,
was not in the least discoloured. The faint impression on the first test paper, may be,
probably, ascribed to sulphurous fumes, proceeding from the ignition of the myriads of
animal and vegetable matters which constantly float in the atmosphere, as may be seen
in the sunbeam admitted into a dark chamber: to this cause, likewise, the offensive
smell of air, transmitted over red-hot iron, may in some measure be attributed, as well
as to the hydrogen resulting from the decomposition of aqueous vapour, always present
in our atmosphere in abundance; especially close to the banks of the Thames, below
London Bridge.
“When a column of air sweeps furiously across the burning deserts of Africa and
Arabia, constituting the phenomenon called simoom by the natives, the air becomes
not only very hot and dry, but highly electrical, as is evinced by lightning and thunder.
Dry sands, devoid of vegetation, cannot be conceived to communicate any noxious gas
or vapour to the atmosphere, like the malaria of marshes, called miasmata: it is, hence,
highly probable that the blast of the simoom owes its deadly malignity, in reference to
animal as well as vegetable life, simply to extreme heat, dryness, and electrical disturbance.
Similar conditions, though on a smaller scale, exist in what is called the bell, or
cockle, apparatus for heating the Long Room and the Examiner’s apartment in the
Custom-house. It consists of a series of inverted, hollow, flattened pyramids of cast
iron, with an oblong base, rather small in their dimensions, to do their work sufficiently
in cold weather, when moderately heated. The inside of the pyramids is exposed to
the flames of coke furnaces, which heat them frequently to incandescence, while currents
of cold air are directed to their exterior surfaces by numerous sheet-iron channels.
The incandescence of these pyramids, or bells, as they are vulgarly called, was proved
by pieces of paper taking fire when I laid them on the summits. Again, since air
becomes electrical when it is rapidly blown upon the surfaces of certain bodies, it
occurred to me that the air which escapes into the Examiner’s Room might be in this
predicament. It certainly excites the sensation of a cobweb playing round the head,
which is well known to all who are familiar with electrical machines. To determine
this point, I presented a condensing gold-leaf electrometer to the said current of hot
air, and obtained faint divergence with negative electricity. The electricity must be
impaired in its tension, however, in consequence of the air escaping through an iron
grating, and striking against the flat iron valves, both of which tend to restore the
electric equilibrium. The air blast, moreover, by being diffused round the glass of the[1189]
condenser apparatus, would somewhat mask the appearances. Were it worth while, an
apparatus might be readily constructed for determining this point, without any such
sources of fallacy. The influence of an atmosphere charged with electricity in exciting
headache and confusion of thought in many persons, is universally known.
“The fetid burned odour of the stove air, and its excessive avidity for moisture, are of
themselves, however, sufficient causes of the general indisposition produced among the
gentlemen who are permanently exposed to it in the discharge of their public duties.
“From there being nearly a vacuum, as to aqueous vapour, in the said air, while there
is nearly a plenum in the external atmosphere round about the Custom-house, the
vicissitudes of feeling in those who have occasion to go out and in frequently, must be
highly detrimental to health. The permanent action of an artificial desiccated air on
the animal economy may be stated as follows:—
“The living body is continually emitting a transpirable matter, the quantity of which,
in a grown up man, will depend partly on the activity of the cutaneous exhalents, and
partly on the relative dryness or moisture of the circumambient medium. Its average
amount, in common circumstances, has been estimated at 20 ounces in twenty-four
hours.
“When plunged in a very dry air, the insensible perspiration will be increased; and, as
it is a true evaporation or gasefaction, it will generate cold proportionably to its amount.
Those parts of the body which are most insulated in the air, and furthest from the
heart, such as the extremities, will feel this refrigerating influence most powerfully.
Hence the coldness of the hands and feet, so generally felt by the inmates of the apartment,
though its temperature be at or above 60°. The brain, being screened by the
skull from this evaporating influence, will remain relatively hot, and will get surcharged,
besides, with the fluids which are repelled from the extremities by the condensation,
or contraction, of the blood-vessels, caused by cold. Hence the affections of the head,
such as tension, and its dangerous consequences. If sensible perspiration happen, from
debility, to break forth from a system previously relaxed, and plunged into dry air, so
attractive of vapour, it will be of the kind called a cold clammy sweat on the sides and
back, as experienced by many inmates of the Long Room.
“Such, in my humble apprehension, is a rationale of the phenomena observed at the
Custom-house. Similar effects have resulted from hot-air stoves of a similar kind in
many other situations.
“After the most mature physical and medical investigation, I am of opinion that the
circumstances above specified cannot act permanently upon human beings, without
impairing their constitutions, and reducing the value of their lives. The Directors of
the Customs Fund are therefore justified in their apprehensions, ‘that the mode of
heating the Long Room is injurious to the health of persons employed therein, and that
it must unduly shorten the duration of life.’
“It may be admitted, as a general principle, that the comfort of sedentary individuals,
occupying large apartments during the winter months, cannot be adequately secured by
the mere influx of hot air from separate stove-rooms: it requires the genial influence
of radiating surfaces in the apartments themselves, such as of open fires, of pipes, or
other vessels filled with hot water or steam. The clothing of our bodies, exposed to
such radiation in a pure, fresh, somewhat cool and bracing air, absorbs a much more
agreeable warmth than it could acquire by being merely immersed in an atmosphere
heated even to 62° Fahr., like that of the Long Room. In the former predicament,
the lungs are supplied with a relatively dense air, say at 52° Fahr.; while the external
surface of the body or the clothing is maintained at, perhaps, 70° or 75°. This distinctive
circumstance has not, I believe, been hitherto duly considered by the stove
doctors, each intent on puffing his own pecuniary interest; but it is obviously one of
great importance, and which the English people would do well to keep in view; because
it is owing to our domestic apartments being heated by open fires, and our factories by
steam pipes, that the health of our population, and the expectation of life among all
orders in this country, are so much better than in France and Germany, where hot-air
stoves, neither agreeable nor inoffensive, and in endless variety of form, are generally
employed.
“In conclusion, I take leave to state to you my firm conviction that the only method
of warming your Long Room and subsidiary apartments, combining salubrity, safety,
and economy, with convenience in erection and durable comfort in use, is by a series
of steam pipes laid along the floor, at the line of the desk partitions, in suitable lengths,
with small arched junction-pipes rising over the several doorways, to keep the passages
clear, and at the same time to allow a free expansion and contraction in the pipes,
thereby providing for the permanent soundness of the joints.”
It would not be difficult to construct a stove or stove-grate which should combine
economy and comfort of warming an apartment, with briskness of combustion and durability
of the fire, without any noxious deflux of carbonic acid. See Chimney.
[1190]
STRAW-HAT MANUFACTURE. The mode of preparing the Tuscany or
Italian straw, is by pulling the bearded wheat while the ear is in a soft milky state, the
corn having been sown very close, and of consequence produced in a thin, short, and
dwindled condition. The straw, with its ears and roots, is spread out thinly upon the
ground in fine hot weather, for three or four days or more, in order to dry the sap; it
is then tied up in bundles and stacked, for the purpose of enabling the heat of the mow
to drive off any remaining moisture. It is important to keep the ends of the straw air-tight,
in order to retain the pith, and prevent its gummy particles from passing off by
evaporation.
After the straw has been about a month in the mow, it is removed to a meadow and
spread out, that the dew may act upon it, together with the sun and air, and promote
the bleaching, it being necessary frequently to turn the straw while this process is going
on. The first process of bleaching being complete, the lower joint and root is pulled
from the straw, leaving the upper part fit for use, which is then sorted according to
qualities; and after being submitted to the action of steam, for the purpose of extracting
its colour, and then to a fumigation of sulphur, to complete the bleaching, the straws
are in a condition to be platted or woven into hats and bonnets, and are in that state
imported into England in bundles, the dried ears of the wheat being still on the straw.
Straw may be easily bleached by a solution of chloride of lime, and also by sulphuring.
For the latter purpose, a cask open at both ends, with its seams papered, is to be set
upright a few inches from the ground, having a hoop nailed to its inside, about six
inches beneath the top, to support another hoop with a net stretched across it, upon
which the straw is to be laid in successive handfuls loosely crossing each other. The
cask having been covered with a tight overlapping lid, stuffed with lists of cloth, a
brazier of burning charcoal is to be inserted within the bottom, and an iron dish containing
pieces of brimstone is to be put upon the brazier. The brimstone soon takes
fire, and fills the cask with sulphurous acid gas, whereby the straw gets bleached in the
course of three or four hours. Care should be taken to prevent such a violent combustion
of the sulphur as might cause black burned spots, for these cannot be afterwards removed.
The straw, after being aired and softened by spreading it upon the grass for a night,
is ready to be split, preparatory to dyeing. Blue is given by a boiling-hot solution of
indigo in sulphuric acid, called Saxon blue, diluted to the desired shade; yellow, by
decoction of turmeric; red, by boiling hanks of coarse scarlet wool in a bath of weak
alum water, containing the straw; or directly, by cochineal, salt of tin, and tartar. Brazil
wood and archil are also employed for dyeing straw. For the other colours, see their
respective titles in this Dictionary.
STRETCHING MACHINE. Cotton goods and other textile fabrics, either
white or printed, are prepared for the market by being stretched in a proper machine,
which lays all their warp and woof yarns in truly parallel positions. A very ingenious
and effective mechanism of this kind was made the subject of a patent by Mr. Samuel
Morand, of Manchester, in April, 1834, which serves to extend the width of calico pieces,
or of other cloths woven of cotton, wool, silk, or flax, after they have become shrunk
in the processes of bleaching, dyeing, &c. I regret that the limits of this volume will
not admit of its description. The specification of the patent is published in Newton’s
Journal, for December, 1835.
STRONTIA, one of the alkaline earths, of which strontium is the metallic basis,
occurs in a crystalline state, as a carbonate, in the lead mines of Strontian in Argyleshire,
whence its name. The sulphate is found crystallized near Bristol, and in several
other parts of the world; but strontitic minerals are rather rare. The pure earth is
prepared exactly like baryta, from either the carbonate or the sulphate. It is a grayish-white
powder, infusible in the furnace, of a specific gravity approaching that of baryta,
having an acrid, burning taste, but not so corrosive as baryta, though sharper than
lime. It becomes hot when moistened, and slakes into a pulverulent hydrate, dissolves
in 150 parts of water at 60°, and in much less at the boiling point, forming an alkaline
solution called strontia water, which deposits crystals in four-sided tables as it cools.
These contain 68 per cent. of water, are soluble in 52 parts of water at 60°, and in
about 2 parts of boiling water; when heated they part with 53 parts of water, but
retain the other 15 parts, even at a red heat. The dry earth consists of 84·55 of base,
and 15·45 of oxygen. It is readily distinguished from baryta, by its inferior solubility,
and by its soluble salts giving a red tinge to flame, while those of baryta give a yellow
tinge. Fluosilicic acid and iodate of soda precipitate the salts of the latter earth, but
not those of the former. The compounds of strontia are not poisonous, like those of
baryta. The only preparation of strontia used in the arts is the Nitrate, which see.
STRYCHNIA, is an alkaline base, extracted from the Strychnos nux vomica, Strychnos
ignatia, and the Upas tiente; which has been employed in medicine by some of the
poison doctors, but is of no use in any of the arts. When introduced into the stomach,[1191]
strychnia acts with fearful energy, causing lock-jaw immediately, and the death of the
animal in a very short time. Half a grain, blown into the throat of a rabbit, proves
fatal in five minutes.
SUBERIC ACID, is prepared by digesting grated cork with nitric acid. It forms
crystals, which sublime in white vapours when heated.
SUBLIMATE, is any solid matter resulting from condensed vapours, and,
SUBLIMATION, is the process by which the volatile particles are raised by
heat, and condensed into a crystalline mass. See Calomel and Sal-ammoniac, for
examples.
SUBSALT, is a salt in which the base is not saturated with acid; as subacetate of
lead.
SUCCINIC ACID, Acid of amber, (Acide succinique, Fr.; Bernsteinsaüre, Germ.)
is obtained by distilling coarsely pounded amber in a retort by itself, with a heat
gradually raised; or mixed with one-twelfth of its weight of sulphuric acid, diluted with
half its weight of water. The acid which sublimes is to be dissolved in hot water,
to be saturated with potassa or soda, boiled with bone black, to remove the foul empyreumatic
oily matter, filtered, and precipitated by nitrate of lead, to convert it into an insoluble
succinate; which being washed, is to be decomposed by the equivalent quantity of
sulphuric acid. Pure succinic acid forms transparent prisms. The succinate of ammonia
is an excellent reagent for detecting and separating iron.
SUGAR (Sucre, Fr.; Zucker, Germ.); is the sweet constituent of vegetable and
animal products. It may be distinguished into two principal species. The first, which
occurs in the sugar-cane, the beet-root, and the maple, crystallizes in oblique four-sided
prisms, terminated by two-sided summits; it has a sweetening power which may be
represented by 100; and in circumpolarization it bends the luminous rays to the right.
The second occurs ready formed in ripe grapes and other fruits; it is also produced by
treating starch with diastase or sulphuric acid. This species forms cauliflower concretions,
but not true crystals; it has a sweetening power which may be represented by
60, and in circumpolarization it bends the rays to the left. Besides these two principal
kinds of sugar, some others are distinguished by chemists; as the sugar of milk, of
manna, of certain mushrooms, of liquorice-root, and that obtained from sawdust and
glue by the action of sulphuric acid; but they have no importance in a manufacturing
point of view.
Sugar, extracted either from the cane, the beet, or the maple, is identical in its
properties and composition, when refined to the same pitch of purity; only that of the
beet seems to surpass the other two in cohesive force, since larger and firmer crystals of
it are obtained from a clarified solution of equal density. It contains 5·3 per cent. of
combined water, which can be separated only by uniting it with oxide of lead, into
what has been called a saccharate; made by mixing syrup with finely ground litharge,
and evaporating the mixture to dryness upon a steam-bath. When sugar is exposed to
a heat of 400° F., it melts into a brown pasty mass, but still retains its water of composition.
Sugar thus fused is no longer capable of crystallization, and is called caramel
by the French. It is used for colouring liqueurs. Indeed sugar is so susceptible of
change by heat, that if a colourless solution of it be exposed for some time to the
temperature of boiling water, it becomes brown and partially uncrystallizable. Acids
exercise such an injurious influence upon sugar, that after remaining in contact with it
for a little while, though they be rendered thoroughly neutral, a great part of the
sugar will refuse to crystallize. Thus, if 3 parts of oxalic or tartaric acid be added to
sugar in solution, no crystals of sugar can be obtained by evaporation, even though
the acids be neutralized by chalk or carbonate of lime. By boiling cane sugar with
dilute sulphuric acid, it is changed into starch sugar. Manufacturers of sugar should be,
therefore, particularly watchful against every acidulous taint or impregnation. Nitric
acid converts sugar into oxalic and malic acids. Alkaline matter is likewise most detrimental
to the grain of sugar; as is always evinced by the large quantity of molasses
formed, when an excess of temper lime has been used in clarifying the juice of the cane
or the beet. When one piece of lump sugar is rubbed against another in the dark, a
phosphorescent light is emitted.
Sugar is soluble in all proportions in water; but it takes four parts of spirits of wine,
of spec. grav. 0·830, and 80 of absolute alcohol, to dissolve it, both being at a boiling
temperature. As the alcohol cools, it deposits the sugar in small crystals. Caramelized
and uncrystallizable sugar dissolves readily in alcohol. Pure sugar is unchangeable in
the air, even when dissolved in a good deal of water, if the solution be kept covered
and in the dark; but with a very small addition of gluten, the solution soon begins to
ferment, whereby the sugar is decomposed into alcohol and carbonic acid, and ultimately
into acetic acid.
Sugar forms chemical compounds with the salifiable bases. It dissolves readily in[1192]
caustic potash lye, whereby it loses its sweet taste, and affords on evaporation a mass
which is insoluble in alcohol. When the lye is neutralized by sulphuric acid, the sugar
recovers its sweet taste, and may be separated from the sulphate of potash by alcohol,
but it will no longer crystallize.
That syrup possesses the property of dissolving the alkaline earths, lime, magnesia,
strontites, barytes, was demonstrated long ago by Mr. Ramsay of Glasgow, by experiments
published in Nicholson’s Journal, vol. xviii. page 9, for September 1807. He
found that syrup is capable of dissolving half as much lime as it contains of sugar; and
as much strontites as sugar. Magnesia dissolved in much smaller quantity, and barytes
seemed to decompose the sugar entirely. These results have been since confirmed by
Professor Daniell. Mr. Ramsay characterized sugar treated with lime as weak, from its
sweetening power being impaired; from its solution he obtained, after some time, a
deposit of calcareous carbonate. M. Pelouze has lately shown that the carbonic acid
in this case is derived from the atmosphere, and is not formed at the expense of the elements
of the sugar, as Mr. Daniell had asserted.
Sugar forms with oxide of lead two combinations; the one soluble, the other insoluble.
Oxide of lead digested in syrup dissolves to a certain amount, forms a yellowish
liquor, which possesses an alkaline reaction, and leaves after evaporation an uncrystallizable,
viscid, deliquescent mass. If syrup be boiled with oxide of lead in excess, if
the solution be filtered boiling hot, and if the phial be corked in which it is received,
white bulky flocks will fall to its bottom in the course of 24 hours. This compound is best
dried in vacuo. It is in both cases light, tasteless, and insoluble in cold and boiling water;
it takes fire like German tinder (Amadou), when touched at one point with an ignited
body, and burns away, leaving small globules of lead. It dissolves in acids, and also in
neutral acetate of lead, which forms with the oxide a subsalt, and sets the sugar free.
Carbonic acid gas passed through water, in which the above saccharate is diffused, decomposes
it with precipitation of carbonate of lead. It consists of 58·26 parts of oxide
of lead, and 41·74 sugar, in 100 parts. From the powerful action exercised upon sugar
by acids and oxide of lead, we may see the fallacy and danger of using these chemical
reagents in sugar-refining. Sugar possesses the remarkable property of dissolving the
oxide, as well as the subacetate of copper (verdigris), and of counteracting their poisonous
operation. Orfila found that a dose of verdigris, which would kill a dog in an hour
or two, might be swallowed with impunity, provided it was mixed with a considerable
quantity of sugar. When a solution of sugar is boiled with the acetate of copper, it
causes an abundant precipitate of protoxide of copper; when boiled with the nitrates of
mercury and silver, or the chloride of gold, it reduces the respective bases to the metallic
state.
The following Table shows the quantities of Sugar contained in Syrups of the annexed
specific gravities.[63] It was the result of experiments carefully made.
Experimental
spec. grav.
of solution
at 60° F. |
Sugar in 100.
by weight. |
| 1 |
·3260 |
66 |
·666 |
| 1 |
·2310 |
50 |
·000 |
| 1 |
·1777 |
40 |
·000 |
| 1 |
·440 |
33 |
·333 |
| 1 |
·1340 |
31 |
·250 |
| 1 |
·1250 |
29 |
·412 |
| 1 |
·1110 |
26 |
·316 |
| 1 |
·1045 |
25 |
·000 |
| 1 |
·0905 |
21 |
·740 |
| 1 |
·0820 |
20 |
·000 |
| 1 |
·0685 |
16 |
·666 |
| 1 |
·0500 |
12 |
·500 |
| 1 |
·0395 |
10 |
·000 |
If the decimal part of the number denoting the specific gravity of syrup, be multiplied
by 26, the product will denote very nearly the quantity of sugar per gallon in pounds
weight, at the given specific gravity.[64]
Sugar has been analyzed by several chemists; the following Table exhibits some of
their results:—
| |
Gay
Lussac
and
Thenard. |
Berze-
lius. |
Prout. |
Ure. |
|
| Oxygen |
56·63 |
49·856 |
53·35 |
50·33 |
in 100. |
| Carbon |
42·47 |
43·265 |
39·99 |
43·38 |
— |
| Hydrogen |
6·90 |
6·875 |
6·66 |
6·29 |
— |
[1193]
Of the sugar cane, and the extraction of sugar from it.—Humboldt, after the most
elaborate historical and botanical researches in the New World, has arrived at the conclusion,
that before America was discovered by the Spaniards, the inhabitants of that
continent and the adjacent islands were entirely unacquainted with the sugar canes, with
any of our corn plants, and with rice. The progressive diffusion of the cane has been
thus traced out by the partisans of its oriental origin. From the interior of Asia it was
transplanted first into Cyprus, and thence into Sicily, or possibly by the Saracens directly
into the latter island, in which a large quantity of sugar was manufactured in
the year 1148. Lafitau relates the donation made by William the Second, king of Sicily,
to the convent of St. Benoit, of a mill for crushing sugar canes, along with all its privileges,
workmen, and dependencies: which remarkable gift bears the date of 1166.
According to this author, the sugar cane must have been imported into Europe at the
period of the Crusades. The monk Albertus Aquensis, in the description which he has
given of the processes employed at Acre and at Tripoli to extract sugar, says, that in
the Holy Land, the Christian soldiers being short of provisions, had recourse to sugar
canes, which they chewed for subsistence. Towards the year 1420, Dom Henry, regent
of Portugal, caused the sugar cane to be imported into Madeira from Sicily. This plant
succeeded perfectly in Madeira and the Canaries; and until the discovery of America
these islands supplied Europe with the greater portion of the sugar which it consumed.
The cane is said by some to have passed from the Canaries into the Brazils; but
by others, from the coast of Angola in Africa, where the Portuguese had a sugar colony.
It was transported in 1506, from the Brazils and the Canaries, into Hispaniola or Hayti,
where several crushing-mills were constructed in a short time. It would appear,
moreover, from the statement of Peter Martyr, in the third book of his first Decade,
written during the second expedition of Christopher Columbus, which happened between
1493 and 1495, that even at this date the cultivation of the sugar cane was widely spread
in St. Domingo. It may therefore be supposed to have been introduced here by Columbus
himself, at his first voyage, along with other productions of Spain and the Canaries,
and that its cultivation had come into considerable activity at the period of his second
expedition. Towards the middle of the 17th century, the sugar cane was imported into
Barbadoes from Brazil, then into the other English West Indian possessions, into the
Spanish Islands on the coast of America, into Mexico, Peru, Chile, and, last of all, into
the French, Dutch, and Danish colonies.
The sugar cane, Arundo saccharifera, is a plant of the graminiferous family, which varies
in height from 8 to 10, or even to 20 feet. Its diameter is about an inch and a half; its
stem is dense, brittle, and of a green hue, which verges to yellow at the approach of
maturity. It is divided by prominent annular joints of a whitish-yellow colour, the plane
of which is perpendicular to the axis of the stem. These joints are placed about
3 inches apart; and send forth leaves, which fall off with the ripening of the plant.
The leaves are 3 or 4 feet long, flat, straight, pointed, from 1 to 2 inches in breadth, of
a sea-green tint, striated in their length, alternate, embracing the stem by their base.
They are marked along their edges with almost imperceptible teeth. In the 11th or
12th month of their growth, the canes push forth at their top a sprout 7 or 8 feet in
height, nearly half an inch in diameter, smooth, and without joints, to which the name
arrow is given. This is terminated by an ample panicle, about 2 feet long, divided into
several knotty ramifications, composed of very numerous flowers, of a white colour,
apetalous, and furnished with 3 stamens, the anthers of which are a little oblong. The
roots of the sugar cane are jointed and nearly cylindrical; in diameter they are about
one twelfth of an inch; in their utmost length 1 foot, presenting over their surface a
few short radicles.
The stem of the cane in its ripe state is heavy, very smooth, brittle, of a yellowish-violet,
or whitish colour, according to the variety. It is filled with a fibrous, spongy,
dirty-white pith, which contains very abundant sweet juice. This juice is elaborated
separately in each internodary portion, the functions of which are in this respect independent
of the portions above and below. The cane may be propagated by seeds or
buds with equal facility; but it is usually done by cuttings or joints of proper lengths,
from 15 to 20 inches, in proportion to the nearness of the joints, which are generally
taken from the tops of the canes, just below the leaves.
There are several varieties of the sugar-cane plant. The first, and longest known, is the
creole, or common sugar cane, which was originally introduced at Madeira. It
grows freely in every region within the tropics, on a moist soil, even at an elevation of
3000 feet above the level of the sea. In Mexico, among the mountains of Caudina-Masca,
it is cultivated to a height of more than 5000 feet. The quantity and quality
of sugar which it yields, is proportional to the heat of the place where it grows, provided
it be not too moist and marshy.
[1194]
The second variety of this plant is the Otaheitan cane. It was introduced into the
West Indies about the end of the 18th century. This variety, stronger, taller, with
longer spaces between the joints, quicker in its growth, and much more productive in
sugar, succeeds perfectly well in lands which seem too much impoverished to grow the
ordinary cane. It sends forth shoots at temperatures which chill the growth and development
of the creole plant. Its maturation does not take more than a year, and is
accomplished sometimes in nine months. From the strength of its stem, and the
woodiness of its fibres, it better resists the storms. It displays a better inflorescence,
weighs a third more, affords a sixth more juice, and a fourth more sugar, than the
common variety. Its main advantage, however, is to yield four crops in the same time
that the creole cane yields only three. Its juice contains less feculency and mucilage,
whence its sugar is more easily crystallized, and of a fairer colour.
Besides these two varieties, another kind is described by Humboldt and Bonpland,
under the name of the violet sugar-cane, for its haum and leaves are of this colour. It
was transported from Batavia in 1782. It flowers a month sooner than the rest, that
is, in August, but it yields less solid sugar, and more liquid, both of which have a violet
tint.
In saying that the cane may be propagated by seeds as well as buds, we must remark,
that in all the colonies of the New World, the plant flowers, indeed, but it then sends
forth a shoot (arrow), that is, its stem elongates, and the seed-vessel proves abortive.
For this reason, the bud-joints must there be used for its propagation. It grows to seed,
however, in India. This circumstance occurs with some other plants, which, when propagated
by their roots, cease to yield fertile seeds; such as the banana, the bread-fruit,
the lily, and the tulip.
In the proper season for planting, the ground is marked out by a line into rows three
or four feet asunder, in which rows the canes are planted about two feet apart. The
series of rows is divided into pieces of land 60 or 70 feet broad, leaving spaces of
about 20 feet, for the convenience of passage, and for the admission of sun and air
between the stems. Canes are usually planted in trenches, about 6 or 8 inches deep,
made with the hand-hoe, the raised soil being heaped to one side, for covering-in the
young cane; into the holes a negro drops the number of cuttings intended to be
inserted, the digging being performed by other negroes. The earth is then drawn
about the hillocks with the hoe. This labour has been, however, in many places
better and more cheaply performed by the plough; a deep furrow being made, into
which the cuttings are regularly planted, and the mould then properly turned in.
If the ground is to be afterwards kept clear by the horse-hoe, the rows of canes
should be 5 feet asunder, and the hillocks 21⁄2 feet distant, with only one cane left in
one hillock. After some shoots appear, the sooner the horse-hoe is used, the more will
the plants thrive, by keeping the weeds under, and stirring up the soil. Plant-canes
of the first growth have been known to yield, on the brick-mould of Jamaica, in very
fine seasons, 21⁄2 tons of sugar per acre. The proper season for planting the cane-slips,
containing the buds, namely, the top part of the cane, stripped of its leaves,
and the two or three upper joints, is in the interval between August and the beginning
of November. Favoured by the autumnal weather, the young plants become
luxuriant enough to shade the ground before the dry season sets in; thereby keeping the
roots cool and moderately moist. By this arrangement the creole canes are ripe for the mill
in the beginning of the second year, so as to enable the manager to finish his crop early
in June. There is no greater error in the colonist than planting canes at an improper
season of the year, whereby his whole system of operations becomes disturbed and, in a
certain degree, abortive.
The withering and fall of a leaf afford a good criterion of the maturity of the cane-joint
to which it belonged; so that the eight last leafless joints of two canes, which are
cut the same day, have exactly the same age and the same ripeness, though one of the
canes be 15 and the other only 10 months old. Those, however, cut towards the end
of the dry season, before the rains begin to fall, produce better sugar than those cut in
the rainy season, as they are then somewhat diluted with watery juice, and require
more evaporation to form sugar. It may be reckoned a fair average product, when one
pound of sugar is obtained from one gallon (English) of juice.
Rattoons (a word corrupted from rejettons) are the sprouts or suckers that spring from
the roots or stoles of the canes that have been previously cut for sugar. They are
commonly ripe in 12 months; but canes of the first growth are called plant-canes, being
the direct produce of the original cuttings or germs placed in the ground, and require a
longer period to bring them to maturity. The first yearly return from the roots that
are cut over, are called first rattoons; the second year’s growth, second rattoons; and so
on, according to their age. Instead of stocking up his rattoons, holing, and planting
the land anew, the planter suffers the stoles to continue in the ground, and contents[1195]
himself, as the cane-fields become thin and impoverished, with supplying the vacant
places with fresh plants. By these means, and with the aid of manure, the produce of
sugar per acre, if not apparently equal to that from plant-canes, gives perhaps in the
long run as great returns to the owner, considering the relative proportion of the labour
and expense attending the different systems. The common yielding on proper land,
such as the red soil of Trelawney, in Jamaica, is 7 hogsheads, of 16 cwt. each, to 10
acres of rattoons cut annually; and such a plantation lasts from 6 to 10 years.
When the planted canes are ripe, they are cut close above the ground, by an oblique
section, into lengths of 3 or 4 feet, and transported in bundles to the mill-house. If the
roots be then cut off, a few inches below the surface of the soil, and covered up with fine
mould, they will push forth more prolific offsets or rattoons, than when left projecting
in the common way.
OF SUGAR MILLS.
The first machines employed to squeeze the canes, were mills similar to those which
serve to crush apples in some cider districts, or somewhat like tan-mills. In the centre
of a circular area, of about 7 or 8 feet in diameter, a vertical heavy wheel was made to
revolve on its edge, by attaching a horse to a cross beam projecting horizontally from
it, and making it move in a circular path. The cane pieces were strewed on the somewhat
concave bed in the path of the wheel, and the juice expressed flowed away through
a channel or gutter in the lowest part. This machine was tedious and unproductive.
It was replaced by the vertical cylinder-mill of Gonzales de Velosa; which has continued
till modern times, with little variation of external form, but is now generally
superseded by the sugar-mill with horizontal cylinders.
SUGAR-CANE MILL.
Specification of, and Observations on, the Construction and Use of the best
Horizontal Sugar-mill.
Fig. 1075. Front elevation of the entire mill. Fig. 1076. Horizontal plan. Fig.
1077. End elevation. Fig. 1078. Diagram, showing the dispositions of the feeding
and delivering rollers, feeding board, returner, and delivering board.
Fig. 1075. A, A, solid foundation of masonry; B, B, bed plate; C, C, headstocks or
standards; D, main shaft (seen only in fig. 1076.); E, intermediate shaft; F, F, plummer-blocks
of main shaft D, (seen only in fig. 1076.); H, driving pinion on the fly-wheel
shaft of engine; I, first motion mortise wheel driven by the pinion; K, second motion
pinion, on the same shaft; L, second motion mortise-wheel, on the main shaft; M, brays
of wood, holding the plummer-blocks for shaft D; N, wrought-iron straps connecting
the brays to the standards C, C; O, O, regulating screws for the brays; P, top roller and
gudgeons; Q and R, the lower or feeding and delivering rollers; S, clutch for the connexion
of the side of lower rollers Q and R, to the main shaft (seen only in fig. 1076.);
T, T, the drain gutters of the mill-bed (seen only in fig. 1076.).
The same letters of reference are placed respectively on the same parts of the mill
in each of figs. 1075, 1076, and 1077.
The relative disposition of the rollers is shown in the diagram, fig. 1078., in which A
is the top roller; B, the feeding roller; C, the delivering roller; D, the returner; E, the
feed board; F, the delivering board.
The rollers are made two inches and a quarter to two inches and a half thick,
and ribbed in the centre. The feeding and delivering rollers have small flanges at
their ends (as shown in fig. 1075.), between which the top roller is placed; these flanges
prevent the pressed canes or begass from working into the mill-bed. The feeding and
top rollers are generally fluted, and sometimes diagonally, enabling them the better to
seize the canes from the feed-board. It is, however, on the whole, considered better to
flute the feeding roller only, leaving the top and delivering rollers plane; when the top
roller is fluted, it should be very slightly, for, after the work of a few weeks, its surface
becomes sufficiently rough to bite the canes effectively. The practical disadvantage of
fluting the delivering rollers, is in the grooves carrying round a portion of liquor, which
is speedily absorbed by the spongy begass, as well as in breaking the begass itself, and
thus causing great waste.
The feed board is now generally made of cast iron, and is placed at a considerable
inclination, to allow the canes to slip the more easily down to the rollers. The returner
is also of cast iron, serrated on the edge, to admit the free flowing of the liquor to the
mill-bed. The concave returner, formerly used, was pierced with holes to drain off the
liquor, but it had the serious disadvantage of the holes choking up with the splinters of
the cane, and has therefore been discarded. The delivering board is of cast iron, fitted
close to the roller, to detach any begass that may adhere to it, and otherwise mix with
the liquor.
[1196]
In Demerara, Surinam, Cayenne, and the alluvial district of Trinidad, it is usual to
attach to the mill a liquor-pump, with two barrels and three adjustments of stroke. This
is worked from the gudgeon of the top roller. In action, the liquor from the gutter of
the mill-bed runs into the cistern of the pump, and is raised by the pump to the
gutter which leads to the clarifier or coppers. Such pumps have brass barrels and
copper discharging pipes, are worked with a very slow motion, and require to be
carefully adjusted to the quantity of liquor to be raised, which, without such precaution,
is either not drawn off sufficiently quick, or is agitated with air in the barrels, and
delivered to the gutter in a state of fermentation.
In working this mill, the feeding roller is kept about half an inch distant from the
upper roller, but the delivering roller is placed so close to it, as to allow the begass to pass
through unbroken.
The practice with this mill is to cut the sugar canes into short lengths of about
three feet, and bring them to the mill tied up in small bundles; there the feeder unties
them, throws them on the feed board, and spreads them so that they may cross each[1197]
other as little as possible. They are taken in by the feed rollers, which split and slightly
press them; the liquor flows down, and, the returner guiding the canes between the top and
delivering rollers, they receive the final pressure, and are turned out on the mill-floor,
while the liquor runs back and falls into the mill-bed. The begass, then in the state of
pith, adhering to the skin of the cane, is tied up in bundles, and after being exposed a
short time to the sun, is finally stored in the begass-house for fuel. By an important
improvement in this stage of the process, recently introduced, the begass is carried to the
begass-house by a carrier chain, worked by the engine.
The relative merits of horizontal and vertical sugar-mills on this construction, may
be thus stated:—The horizontal mill is cheaper in construction, and is more easily
fixed; the process of feeding is performed at about one-half of the labour, and in a
much superior manner; the returner guides the canes to receive the last pressure more
perfectly; and the begass is not so much broken as in the vertical mill; but left tolerably
entire, so as to be tied, dried, and stored, with less trouble and waste.
The vertical mill has a considerable advantage, in being more easily washed; and it
can be readily and cheaply mounted in wooden framing; but the great labour of feeding
the vertical mill, renders it nearly inapplicable to any higher power than that of about
ten horses. In situations where the moving power is a windmill, or a cattle gin, the
vertical mill may be preferred.
The scale of produce of such mills varies according to the climate and soil.
In Demerara, a well constructed engine and mill will produce about 100 gallons of
liquor per hour for each horse power.
The dimensions of the most approved horizontal mills are these:—
Horse-
power
of
Engine. |
Length
of
Rollers. |
Diameter
of
Rollers. |
| |
ft. |
in. |
inches. |
| 8 |
4 |
0 |
25 |
| 10 |
4 |
6 |
27 |
| 12 |
4 |
8 |
28 |
The surface speed of the rollers is 3·4 or 3·6 feet per minute; and to provide for the
varying resistance arising from irregular feeding, or the accidental crossing of the canes,
by which the engine is often brought up so suddenly as to break the fly-wheel shaft, it is
necessary to make both the shaft and the fly-wheel of unusual strength and weight.
Sugar is manufactured in the East Indies by two distinct classes of persons; the
ryots, who raise the sugar cane, extract its juice, and inspissate it to a syrupy consistence;
and the goldars, who complete the conversion into sugar.
The ryots are the farmers, or actual cultivators of the soil; but, properly speaking,
they are merely peasants, toiling under oppressive landlords, and miserably poor.
After they cut the canes, they extract the juice by one or other of the rude mills or
mortars presently to be described, and boil it down to an entire mass, which is generically
called goor, without making any attempt to clarify it, or separate the granular[1198]
sugar from the uncrystallizable molasses. This goor is of various qualities; one of
which, in most common use for making sugar, is known amongst the English settlers
under the name of jaggery. There is a caste in Ceylon, called jaggeraros, who
make sugar from the produce of the Caryota urens, or Kitul tree; and the sugar is
styled jaggery. Sugar is not usually made in Ceylon from the sugar cane; but either
from the juice of the Kitul, from the Cocos nucifera, or the Borassus flabelliformis (the
Palmyra tree).
Several sorts of cane are cultivated in India.
The Cadjoolee (fig. 1079.) is a purple-coloured cane; yields a sweeter and richer juice
than the yellow or light coloured, but in less quantities, and is harder to press. It grows
in dry lands. When eaten raw, it is somewhat dry and pithy in the
mouth, but is esteemed very good for making sugar. It is not
known to the West India planter. The leaves rise from a point 6
feet above the ground. An oblique and transverse section of the
cane is represented by the parts near the bottom of the figure.
The Pooree is a light-coloured cane, yellow, inclining to white,
deeper yellow when ripe and on rich ground. West India planters
consider it the same sort as one of theirs. It is softer and more
juicy than the preceding, but the juice is less rich, and produces a
weaker sugar. It requires seven parts of pooree juice to make as
much goor as is produced from six of the cadjoolee. Much of this
cane is brought to the Calcutta market, and eaten raw.
The Cullorah thrives in swampy lands, is light-coloured, and grows
to a great height. Its juice is more watery, and yields a weaker
sugar also than the cadjoolee. However, since much of Bengal
consists of low grounds, and since the upland canes are apt to suffer
from drought, it deserves encouragement in certain localities.
It is only large farms that cut an acre of cane in a year; one mill,
therefore, and one set of the implements used in inspissating the
juice, although very rude and simple, serve for several farms, and
generally belong to some wealthy man, who lets them out for hire to
his poorer neighbours, the whole of whom unite to clear each other’s
fields by turns; so that though many people and cattle are employed
at one of these miserable sets of works, very few indeed are
hired, and the greater part of the labour is performed by the common
stock of the farms.
The inspissated juice, or extract of cane, called by the natives
goor, is of two kinds; one of which may be termed cake extract,
and the other pot extract; both being often denominated jaggery, as above stated,
by the English residents.
One-third of an acre of good land in the southern districts, is reckoned by the
farmers to produce 18,891
pounds of cane, and 1,159
pounds of pot extract. Its
produce in cake extract is
about 952 pounds.
I shall now describe the
primitive rude mill and
boiler used in preparing the
extract of sugar cane, and
which are usually let to
the ryots by the day. The
mill in Dinajpur, fig. 1080.
is on the principle of a
pestle and mortar. The
pestle, however, does not
beat the canes, but is rubbed
against them, as is done
in many chemical triturations;
and the moving force
is two oxen. The mortar is
generally a tamarind tree,
one end of which is sunk
deep in the ground, to give
it firmness. The part projecting
a, a, a, a, may be about two feet high, and a foot and a half in diameter; and in
the upper end a hollow is cut, like the small segment of a sphere. In the centre of this, a[1199]
channel descends a little way perpendicularly, and then obliquely to one side of the mortar,
so that the juice, as squeezed from the cane, runs off, by means of a spout b, into
a strainer c, through which it falls into an earthen pot, that stands in a hole
d, under the spout. The pestle e, is a tree about 18 feet in length, and 1 foot in
diameter, rounded at its bottom, which rubs against the mortar, and which is secured
in its place by a button or knob, that goes into the channel of the mortar.
The moving force is applied to a horizontal beam f, about 16 feet in length, which
turns round about the mortar, and is fastened to it by a bent bamboo b. It is
suspended from the upper end of the pestle by a bamboo g, which has been cut
with part of the root, in which is formed a pivot that hangs on the upper point of the
pestle. The cattle are yoked to the horizontal beam, at about ten feet from the mortar,
move round it in a circle, and are driven by a man, who sits on the beam, to increase
the weight of the triturating power. Scarcely any machine more miserable can be
conceived; and it would be totally ineffectual, were not the cane cut into thin slices.
This is a troublesome part of the operation. The grinder sits on the ground, having
before him a bamboo stake, which is driven into the earth, with a deep notch formed
in its upper end. He passes the canes gradually through this notch, and at the same
time cuts off the slices with a kind of rude chopper.
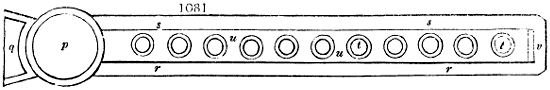
The boiling apparatus is somewhat better contrived, and is placed under a shed,
though the mill is without shelter. The fireplace is a considerable cavity dug in the
ground, and covered with an iron boiler p, fig. 1081. At one side of this, is an opening
q, for throwing in fuel; and opposite to this, is another opening, which communicates
with the horizontal flue. This is formed by two parallel mud walls r, r, s, s, about 20
feet long, 2 feet high, and 18 inches distant from each other. A row of eleven earthen
boilers t, is placed on these walls, and the interstices u, are filled with clay, which
completes the furnace-flue, an opening v, being left at the end, for giving vent to the
smoke.
The juice, as it comes from the mill, is first put into the earthen boiler that is most
distant from the fire, and is gradually removed from one boiler to another, until it reaches
the iron one, where the process is completed. The fireplace is manifestly on the
same model as the boiler range in the West Indies, and may possibly have suggested it,
since the Hindostan furnace is, no doubt, of immemorial usage. The execution of its
parts is very rude and imperfect. The inspissated juice that can be prepared in 24
hours by such a mill, with 16 men and 20 oxen, amounts to no more than 476 lbs.; and
it is only in the southern parts of the district, where the people work night and day,
that the sugar-works are so productive. In the northern districts, the people work only
during the day, and inspissate about one-half the quantity of juice. The average daily
make of a West India sugar-house, is from 2 to 3 hogsheads, of 16 cwts each.
The Indian manufacturers of sugar purchase the above inspissated juice or goor from the
farmers, and generally prefer that of a granular honey consistence, which is offered for
sale in pots. As this, however, cannot conveniently be brought from a distance, some
of the cake kind is also employed. The boilers are of two sizes; one adapted for
making at each operation about ten cwt.; the other, about eight and a half. The latter
is the segment of a sphere, nine feet diameter at the mouth; the former is larger. The
boiler is sunk into a cylindrical cavity in the ground, which serves as a fireplace, so
that its edge is just above the floor of the boiling-house. The fuel is thrown in by an
aperture close to one side of the boiler, and the smoke escapes by a horizontal chimney
that passes out on the opposite side of the hut, and has a small round aperture, about
ten feet distant from the wall, in order to lessen the danger from fire. Some manufacturers
have only one boiler; others as many as four; but each boiler has a separate hut,
in one end of which is some spare fuel; and in the other, some bamboo stages, which
support cloth strainers, that are used in the operation. This hut is about twenty-four
cubits long, and ten broad; has mud walls, six cubits high; and is raised about one
cubit above the ground.
For each boiler, two other houses are required: one in which the cane extract is
separated by straining from the molasses, is about twenty cubits long by ten wide;
another, about thirty cubits long, by eight wide, is that in which, after the extract has
been strained, boiled and clarified, the treacle is separated from the sugar by an operation
analogous to claying.
[1200]
Each sugar manufacturer has a warehouse besides, of a size proportional to the
number of his boilers.
About 960 pounds of pot extract being divided into four parts, each is put into a bag
of coarse sackcloth, hung over an equal number of wide-mouthed earthen vessels, and
is besprinkled with a little water. These drain from the bags about 240 lbs. of a
substance analogous to West Indian molasses. The remainder in the bags is a kind of
coarse muscovado sugar; but is far from being so well drained and freed from molasses
as that of the Antilles. The 720 lbs. of this substance are then put into a boiler with
270 pounds of water, and the mixture is boiled briskly for 144 minutes, when 180
additional pounds of water are added, and the boiling is continued for 48 minutes more.
An alkaline solution is prepared from the ashes of the plantain tree, strewed over straw
placed in the bottom of an earthen pot perforated with holes. Ninety pounds of water
are passed through; and 6 pounds of the clear lixivium are added to the boiling syrup,
whereby a thick scum is raised, which is removed. After 24 minutes, four and a half
pounds of alkaline solution, and about two-fifths of a pound of raw milk, are added;
after which the boiling and skimming are continued 24 minutes. This must be repeated
from five to seven times, until no more scum appears. 240 pounds of water being now
added, the liquor is to be poured into a number of strainers. These are bags of coarse
cotton cloth, in the form of inverted quadrangular pyramids, each of which is suspended
from a frame of wood, about 2 feet square. The operation of straining occupies about
96 minutes. The strained liquor is divided into three parts: one of these is put into a boiler,
with from half a pound to a pound and a half of alkaline solution, one-twelfth of a
pound of milk, and 12 pounds of water. After having boiled for between 48 and 72
minutes, three quarters of a pound of milk are added, and the liquor is poured, in equal
portions, into four refining pots. These are wide at the mouth, and pointed at the bottom;
but are not conical, for the sides are curved. The bottom is perforated, and the
stem of a plantain leaf forms a plug for closing the aperture. The two remaining portions
of the strained liquor are managed in exactly the same manner; so that each refining
pot has its share of each portion. When they have cooled a little, the refining pot
is removed to the curing-house, and placed on the ground for 24 hours; next day
they are placed on a frame, which supports them at some distance from the ground. A
wide-mouthed vessel is placed under each, to receive the viscid liquor that drains from
them. In order to draw off this more completely, moist leaves of the Valisneria spiralis
are placed over the mouth of the pot, to the thickness of two inches; after 10 or 12
days, these are removed; when a crust of sugar, about half an inch in thickness is found
on the surface of the boiled liquor. The crust being broken and removed, fresh leaves
are repeatedly added, until the whole sugar has formed; which requires from 75 to 90
days. When cake extract is used, it does not require to be strained before it be put
into the boiler.
On the above-described operose and preposterous process, it is needless to make any
remarks. While it is adhered to with the tenacity of Hindu habit, the West Indies
has no reason to fear the competition of the East, in the manufacture of sugar, provided
the former avail themselves of the aids which chemical and mechanical science are ready
to supply.
In every part of the Behar and Putna districts, several of the confectioners prepare
the coarse article called shukkur, which is entirely similar in appearance to the inferior
Jamaica sugars. They prepare it by putting some of the thin extract of sugar cane
into coarse sackcloth bags, and by laying weights on them, they squeeze out the molasses;
a process perfectly analogous to that
contemplated in several English patents.
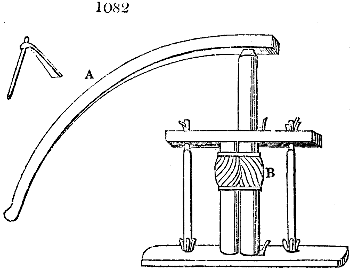
The sugar-mill at Chica Ballapura
is worked by a single pair of buffaloes
or oxen, fig. 1082., going round with
the lever A, which is fixed on the top
of the right-hand roller. The two
rollers have endless screw heads B,
which are formed of 4 spiral grooves
and 4 spiral ridges, cut in opposite
directions, which turn into one another,
when the mill is working.
These rollers and their heads are of
one piece, made of the toughest and
hardest wood that can be got, and such
as will not impart any bad taste to the juice. They are supported in a thick strong
wooden frame, and their distance from each other is regulated by means of wedges,
which pass through mortises in the frame planks, and a groove made in a bit of some[1201]
sort of hard wood, and press upon the axis of one of the rollers. The axis of the
other presses against the left-hand side of the hole in the frame-boards. The cane juice
runs down the rollers, and through a hole in the lower frame-board, into a wooden conductor,
which carries it into an earthen pot. Two long-pointed stakes or piles are
driven into the earth, to keep the mill steady, which is all the fixing it requires. The
under part of the lowermost plank of the frame rests upon the surface of the ground,
which is chosen level and very firm, that the piles may hold the faster. A hole is dug
in the earth, immediately below the spout of the conductor, to receive the pot.
The mill used in Burdwan and near Calcutta, is simply two small wooden cylinders,
grooved, placed horizontally, close to each other, and turned by two men, one at each
end. This simple engine is said completely, but slowly, to express the juice. It is very
cheap, the prime cost not being two rupees; and being easily moved from field to field,
it saves much labour in the carriage of the cane. Notwithstanding this advantage, so
rude a machine must leave a large proportion of the richest juice in the cane-trash.
It is curious to find in the antient arts of Hindostan exact prototypes of the sugar-rollers,
horizontal and upright, of relatively modern invention in the New World.
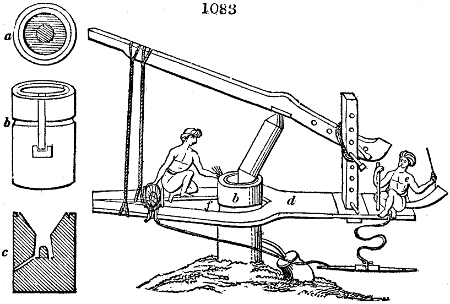
The sugar-mill of Chinapatam, fig. 1083., consists of a mortar, lever, pestle, and regulator.
The mortar is a tree about 10 feet in length, and 14 inches in diameter: a is
a plan of its upper
end; b is an outside
view; and c is a vertical
section. It is
sunk perpendicularly
into the earth, leaving
one end two feet
above the surface.
The hollow is conical,
truncated downwards,
and then becomes cylindrical,
with a hemispherical
projection
in its bottom, to
allow the juice to run
freely to the small
opening that conveys
it to a spout, from
which it falls into an earthen pot. Round the upper mouth of the cone is a circular
cavity, which collects any of the juice that may run over from the upper ends of
the pieces of cane; and thence a canal conveys this juice, down the outside of the
mortar, to the spout. The beam d, is about sixteen feet in length, and six inches in
thickness, being cut out from a large tree that is divided by a fork into two arms.
In the fork an excavation is made for the mortar b, round which the beam turns
horizontally. The surface of this excavation is secured by a semicircle of strong wood.
The end towards the fork is quite open, for changing the beam without trouble. On
the undivided end of the beam sits the bullock-driver e, whose cattle are yoked by a rope
which comes from the end of the beam; and they are prevented from dragging out of
the circle by another rope, which passes from the yoke to the forked end of the beam.
On the arms f, a basket is placed, to hold the cuttings of cane; and between this and the
mortar sits the man who feeds the mill. Just as the pestle comes round, he places the
pieces of cane sloping down into the cavity of the mortar; and after the pestle has
passed, he removes those that have been squeezed.
OF THE MANUFACTURE OF SUGAR IN THE WEST INDIES.
Cane-juice varies exceedingly in richness, with the nature of the soil, the culture, the
season, and variety of the plant. It is an opaque fluid, of a dull gray, olive, or olive-green
colour; in taste, balmy and saccharine; exhaling the balsamic odour of the cane;
slightly viscid; and of a specific gravity varying from 1·033 to 1·106, according to
circumstances. When fresh, it consists of two parts; the one liquid, the other solid;
the latter of which being merely suspended in the former, and, therefore, separable in a
great measure by filtration or repose. The solid matter consists of fragments of the
cellular parenchyma of the cane, its fibres, and bark, mechanically protruded through
the mill; mixed with a very abundant greenish substance, like that called chlorophyle
by chemists.
When left to itself in the colonial climates, the juice runs rapidly into the acetous
fermentation; twenty minutes being, in many cases, sufficient to bring on this destructive
change. Hence arises the necessity of subjecting it immediately to clarifying[1202]
processes, speedy in their action. When deprived of its green fecula and glutinous
extractive, it is still subject to fermentation; but this is now of the vinous kind. The
juice flows from the mill through a wooden gutter lined with lead, and being conducted
into the sugar-house, is received in a set of large pans or caldrons, called clarifiers.
On estates which make on an average, during crop time, from 15 to 20 hogsheads of
sugar a week, three clarifiers, of from 300 to 400 gallons’ capacity each, are sufficient. With
pans of this dimension, the liquor may be drawn off at once by a stopcock or syphon, without
disturbing the feculencies after they subside. Each clarifier is hung over a separate
fire, the flue being furnished with a damper for checking the combustion, or extinguishing
it altogether. The clarifiers are sometimes placed at one end, and sometimes in
the middle of the house, particularly if it possesses a double set of evaporating pans.
Whenever the stream from the mill cistern has filled the clarifier with fresh juice, the
fire is lighted, and the temper, or dose of slaked lime, diffused uniformly through a little
juice, is added. If an albuminous emulsion be used to promote the clarifying, very
little lime will be required; for recent cane-liquor contains no appreciable portion of
acid to be saturated. In fact, the lime and alkalies in general, when used in small
quantity, seem to coagulate the glutinous extractive matter of the juice, and thus tend
to brighten it up. But if an excess of temper be used, the gluten is taken up again by
the strong affinity which is known to exist between sugar and lime. Excess of lime
may always be corrected by a little alum-water. Where canes grow on a calcareous
marly soil, in a favourable season the saccharine matter gets so thoroughly elaborated,
and the glutinous mucilage so completely condensed, that a clear juice and a fine sugar
may be obtained without the use of lime.
As the liquor grows hot in the clarifier, a scum is thrown up, consisting of the
coagulated feculencies of the cane-juice. The fire is now gradually urged till the temperature
approaches the boiling point; to which, however, it must not be suffered to
rise. It is known to be sufficiently heated, when the scum rises in blisters, which
break into white froth; an appearance observable in about forty minutes after kindling
the fire. The damper being shut down, the fire dies out; and after an hour’s repose,
the clarified liquor is ready to be drawn off into the last and largest in the series of
evaporating pans. In the British colonies, these are merely numbered 1, 2, 3, 4, 5,
beginning at the smallest, which hangs right over the fire, and is called the teache; because
in it the trial of the syrup, by touch, is made. The flame and smoke proceed
in a straight line along a flue to the chimney-stalk at the other end of the furnace.
The area of this flue proceeds, with a slight ascent from the fire, to the aperture at the
bottom of the chimney; so that between the surface of the grate and the bottom of the
teache, there is a distance of 28 inches; while between the bottom of the flue and that
of the grand, No. 5., at the other end of the range, there are barely 18 inches.
In some sugar-houses there is planted, in the angular space between each boiler, a
basin, one foot wide and a few inches deep, for the purpose of receiving the scum which
thence flows off into the grand copper, along a gutter scooped out on the margin of the
brickwork. The skimmings of the grand are thrown into a separate pan, placed at its
side. A large cylindrical cooler, about 6 feet wide and 2 feet deep, has been placed in
certain sugar-works near the teache, for receiving successive charges of its inspissated
syrup. Each finished charge is called a skipping, because it is skipped or laded out.
The term striking is also applied to the act of emptying the teache. When upon one skipping
of syrup in a state of incipient granulation in the cooler, a second skipping is
poured, this second congeries of saccharine particles agglomerates round the first as nuclei
of crystallization, and produces a larger grain; a result improved by each successive
skipping. This principle has been long known to the chemist, but does not seem to
have been always properly considered or appreciated by the sugar-planter.
From the above described cooler, the syrup is transferred into wooden chests or boxes,
open at top, and of a rectangular shape; also called coolers, but which are more properly
crystallizers or granulators. These are commonly six in number; each being
about one foot deep, seven feet long, and five or six feet wide. When filled, such a
mass is collected, as to favour slow cooling, and consequent large-grained crystallization.
If these boxes be too shallow, the grain is exceedingly injured, as may be easily
shown by pouring some of the same syrup on a small tray; when, on cooling, the sugar
will appear like a muddy soft sand.
The criterion by which the negro boilers judge of the due concentration of the syrup
in the teache, is difficult to describe, and depends almost entirely on the sagacity and
experience of the individual. Some of them judge by the appearance of the incipient
grain on the back of the cooling ladle; but most decide by “the touch,” that is, the feel
and appearance of a drop of the syrup pressed and then drawn into a thread between
the thumb and fore-finger. The thread eventually breaks at a certain limit of extension,
shrinking from the thumb to the suspended finger, in lengths somewhat proportional[1203]
to the inspissation of the syrup. But the appearance of granulation in the
thread must also be considered; for a viscid and damaged syrup may give a long
enough thread, and yet yield almost no crystalline grains when cooled. Tenacity and
granular aspect must therefore be both taken into the account, and will continue to
constitute the practical guides to the negro boiler, till a less barbarous mode of concentrating
cane-juice be substituted for the present naked teache, or sugar frying-pan.
That weak sugars are such as contain an inferior proportion of carbon in their composition,
was first deduced by me from my experiments on the ultimate analysis of
vegetable and animal bodies; an account of which was published in the Philosophical
Transactions of the Royal Society for 1822. Since then Dr. Prout has arrived at results
comfirmatory of my views. See Philosophical Transactions for 1827. Thus, he found
pure sugar-candy, and the best refined sugar, to contain 42·85 parts of carbon per cent.;
East India sugar-candy, 41·9 parts; East India raw sugar in a thoroughly dry state,
but of a low quality, 40·88; manna sugar, well refined, 28·7; sugar from Narbonne
honey, 36·36; sugar from starch, 36·2. Hence, by caramelizing the syrup in the teache,
not only is the crystallizable sugar blackened, but its faculty of crystallizing impaired,
and the granular portion rendered weaker.
A viscous syrup containing much gluten and sugar, altered by lime, requires a higher
temperature to enable it to granulate, than a pure saccharine syrup; and therefore the
thermometer, though a useful adjuvant, can by no means be regarded as a sure guide,
in determining the proper instant for striking the teache.
The colonial curing-house is a capacious building, of which the earthen floor is excavated
to form the molasses reservoir. This is lined with sheet lead, boards, tarras, or other retentive
cement; its bottom slopes a little, and it is partially covered by an open massive
frame of joist-work, on which the potting casks are set upright. These are merely empty
sugar hogsheads, without headings, having 8 or 10 holes bored in their bottoms, through
each of which the stalk of a plantain leaf is stuck, so as to protrude downwards 6 or
8 inches below the level of the joists, and to rise above the top of the cask. The act
of transferring the crude concrete sugar from the crystallizers into these hogsheads, is
called potting. The bottom holes, and the spongy stalks stuck in them, allow the molasses
to drain slowly downwards into the sunk cistern. In the common mode of procedure,
sugar of average quality is kept from 3 to 4 weeks in the curing-house; that
which is soft-grained and glutinous, must remain 5 or 6 weeks. The curing-house
should be close and warm, to favour the liquefaction and drainage of the viscid
caramel.
Out of 120 millions of pounds of raw sugar, which used to be annually shipped by
the St. Domingo planters, only 96 millions were landed in France, according to the
authority of Dutrone, constituting a loss by drainage in the ships of 20 per cent. The
average transport waste at present in the sugars of the British colonies cannot be estimated
at less than 12 per cent., or altogether upwards of 27,000 tons! What a tremendous
sacrifice of property!
Within these few years a very considerable quantity of sugar has been imported into
Great Britain in the state of concentrated cane-juice, containing nearly half its weight
of granular sugar, along with more or less molasses, according to the care taken in the
boiling operations. I was at first apprehensive that the syrup might undergo some
change on the voyage; but among more than a hundred samples which I have analyzed
for the custom-house, I have not perceived any traces of fermentation. Since sugar softens
in its grain at each successive solution, whatever portion of the crop may be destined
for the refiner, should upon no account be granulated in the colonies; but should be
transported in the state of a rich cane-syrup to Europe, transferred at once into the
blowing-up cistern, subjected there to the reaction of bone black, and passed through
bag-filters, or through layers of the coarsely ground black, previously to its final concentration
in the vacuum pan. Were this means generally adopted, I am convinced
that 30 per cent. would be added to the amount of home-made sugar loaves corresponding
to a given quantity of average cane-juice; while 30 per cent., would be taken from
the amount of molasses. The saccharine matter now lost by drainage from the hogsheads
in the ships, amounting to from 10 to 15 per cent., would, also be saved. The
produce of the cane would, on this plan, require less labour in the colonies, and might
be exported 5 or 6 weeks earlier than at present, because the period of drainage in the
curing-house would be spared.
It does not appear that our sugar colonists have availed themselves of the proper chemical
method of counteracting that incipient fermentation of the cane-juice, which sometimes
supervenes, and proves so injurious to their products. It is known that grape-must,
feebly impregnated with sulphurous acid, by running it slowly into a cask in
which a few sulphur matches have been burned, will keep without alteration for a year;
and if must, so muted, is boiled into a syrup within a week or ten days, it retains no
sulphureous odour. A very slight muting would suffice for the most fermentable cane-juice:[1204]
and it could be easily given, by burning a sulphur match within the cistern
immediately before charging it from the mill. The cane-juice should, in this case, be
heated in the clarifier, so as to expel the sulphurous acid, before adding the temper lime;
for otherwise a little calcareous sulphite might be introduced into the sugar. Thus the
arescence so prejudicial to the saccharine granulation would be certainly prevented.
An Account of Sugar Imported into the United Kingdom during the years ending
5th January, 1837, and 5th January, 1838.
| |
Quantities imported. |
Quantities entered for
Home Consumption. |
Gross amount
of Duty received. |
| |
1837. |
1838. |
1837. |
1838. |
1837. |
1838. |
| Sugar, unrefined; viz.— |
Cwt. |
qr. |
lb. |
Cwt. |
qr. |
lb. |
Cwt. |
qr. |
lb. |
Cwt. |
qr. |
lb. |
£. |
£. |
| of the British possessions in America |
3,600,516 |
3 |
2 |
3,304,092 |
2 |
2 |
3,296,641 |
1 |
19 |
3,562,703 |
1 |
24 |
3,956,879 |
4,275,207 |
| Of Mauritius |
497,303 |
0 |
8 |
537,054 |
1 |
21 |
518,228 |
0 |
5 |
522,348 |
3 |
11 |
621,596 |
626,131 |
| East India British possessions |
152,229 |
1 |
13 |
296,677 |
2 |
12 |
110,236 |
2 |
0 |
270,146 |
1 |
2 |
176,376 |
368,672 |
| East India Foreign possessions |
71,464 |
2 |
0 |
77,090 |
0 |
18 |
20 |
3 |
18 |
3 |
3 |
11 |
66 |
12 |
| Other sorts |
327,647 |
1 |
12 |
266,559 |
2 |
24 |
31 |
1 |
6 |
37 |
3 |
10 |
41 |
95 |
| Total |
4,649,161 |
0 |
7 |
4,481,474 |
1 |
21 |
3,925,140 |
0 |
20 |
4,355,240 |
1 |
2 |
4,754,958 |
5,270,117 |
| |
Quantities imported. |
| |
1837. |
1838. |
| Sugar, unrefined; viz.— |
Cwt. |
qr. |
lb. |
Cwt. |
qr. |
lb. |
| of the British possessions in America |
3,600,516 |
3 |
2 |
3,304,092 |
2 |
2 |
| Of Mauritius |
497,303 |
0 |
8 |
537,054 |
1 |
21 |
| East India British possessions |
152,229 |
1 |
13 |
296,677 |
2 |
12 |
| East India Foreign possessions |
71,464 |
2 |
0 |
77,090 |
0 |
18 |
| Other sorts |
327,647 |
1 |
12 |
266,559 |
2 |
24 |
| Total |
4,649,161 |
0 |
7 |
4,481,474 |
1 |
21 |
| |
Quantities entered for
Home Consumption. |
| |
1837. |
1838. |
| Sugar, unrefined; viz.— |
Cwt. |
qr. |
lb. |
Cwt. |
qr. |
lb. |
| of the British possessions in America |
3,296,641 |
1 |
19 |
3,562,703 |
1 |
24 |
| Of Mauritius |
518,228 |
0 |
5 |
522,348 |
3 |
11 |
| East India British possessions |
110,236 |
2 |
0 |
270,146 |
1 |
2 |
| East India Foreign possessions |
20 |
3 |
18 |
3 |
3 |
11 |
| Other sorts |
31 |
1 |
6 |
37 |
3 |
10 |
| Total |
3,925,140 |
0 |
20 |
4,355,240 |
1 |
2 |
| |
Gross amount
of Duty received. |
| |
1837. |
1838. |
| Sugar, unrefined; viz.— |
£. |
£. |
| of the British possessions in America |
3,956,879 |
4,275,207 |
| Of Mauritius |
621,596 |
626,131 |
| East India British possessions |
176,376 |
368,672 |
| East India Foreign possessions |
66 |
12 |
| Other sorts |
41 |
95 |
| Total |
4,754,958 |
5,270,117 |
An Account of Sugar Exported in the year ended 5th January, 1838, compared with
the Exports of the preceding Year.
| |
1837. |
1838. |
| |
Cwts. |
qrs. |
lbs. |
Cwts. |
qrs. |
lbs. |
| Sugar, |
of |
the British possessions in America |
8,774 |
1 |
15 |
9,267 |
0 |
21 |
| |
Mauritius |
2,687 |
3 |
14 |
3,065 |
0 |
19 |
| |
East India, of British possessions |
22,290 |
3 |
16 |
13,283 |
0 |
22 |
| |
East India, of Foreign possessions |
52,384 |
0 |
4 |
68,252 |
2 |
18 |
| |
Other sorts |
191,961 |
0 |
20 |
354,513 |
1 |
23 |
Syrup intended for forming clayed sugar must be somewhat more concentrated in the
teache, and run off into a copper cooler, capable of receiving three or four successive
skippings. Here it is stirred to ensure uniformity of product, and is then transferred by
ladles into conical moulds, or formes, made of coarse pottery, having a small orifice at
the apex, which is stopped with a plug of wood wrapped in a leaf of maize. These
pots are arranged with the base upwards. As their capacity, when largest, is greatly
less than that of the smallest potting-casks, and as the process lasts several weeks, the
claying-house requires to have very considerable dimensions. Whenever the syrup is
properly granulated, which happens usually in about 18 or 20 hours, the plugs are
removed from the apices of the cones, and each is set on an earthen pot to receive the
drainings. At the end of 24 hours, the cones are transferred over empty pots, and the
molasses contained in the former ones is either sent to the fermenting-house or sold.
The claying now begins, which consists in applying to the smoothed surface of the
sugar at the base of the cone, a plaster of argillaceous earth, or tolerably tenacious loam
in a pasty state. The water diffused among the clay escapes from it by slow infiltration,
and descending with like slowness through the body of the sugar, carries along
with it the residuary viscid syrup which is more readily soluble than the granulated
particles. Whenever the first magma of clay has become dry, it is replaced by a
second; and this occasionally in its turn by a third, whereby the sugar cone gets tolerably
white and clean. It is then dried in a stove, cut transversely into frusta, crushed
into a coarse powder on wooden trays, and shipped off for Europe. Clayed sugars are
sorted into different shades of colour according to the part of the cone from which they
were cut; under the denomination in French commerce of premier, second, troisième,
petit, commun, and tête; the last or the tip being an indifferent article. The clayed
sugar of Cuba is called Havannah sugar, from the name of the shipping port.
Clayed sugar can be made only from the ripest cane-juice, for that which contains
much gluten would be apt to get too much burned by the ordinary process of boiling,
to bear the claying operation. The syrups that run off from the second, third, and
fourth application of the clay-paste, are concentrated afresh in a small building apart,
called the refinery, and yield tolerable sugars. Their drainings go to the molasses cistern.
The cones remain for 20 days in the claying-house, before the sugar is taken
out of them.
Claying is seldom had recourse to in the British plantations, on account of the
increase of labour, and diminution of weight in the produce, for which the improvement[1205]
in quality yields no adequate compensation. Such, however, was the esteem in which
the French consumers held clayed sugar, that it was prepared in 400 plantations of
St. Domingo alone.
SUGAR REFINING.
Raw, or muscovado sugar, as imported from the colonies, is contaminated more or
less with gluten, lime, but particularly caramel, which give its grains a yellow brown
tint, an empyreumatic odour, and a soft clammy feel in the hand. If such sugar be dissolved
in water, and the syrup be evaporated by a gentle heat, it will afford a sugar of
still inferior quality and appearance. This rapid deterioration is in some measure
owing to the injurious operation of a prolonged heat upon the crystalline structure, but
chiefly to the chemical reaction of the glutinous ferment and lime upon the sugar.
The first care of the refiner should therefore be the immediate abstraction of these
noxious alteratives, which he effects by the process called meltings; that is, mixing up
the sugar in a pan with hot water or steam into a pap, and transferring this pap into large
sugar-moulds. Whenever these become cool, their points are unplugged, and they are
set to drain for a few days in a warm apartment. Sugar thus cleansed is well prepared
for the next refining process; which consists in putting it into a large square
copper cistern along with some lime-water, (a little bullock’s blood,) and from 5 to 20
per cent. of bone black, and blowing it up with steam; or, in other words, injecting
steam through the mixture from numerous orifices in copper pipes laid along the
bottom and sides of the vessel. Under the influence of the heat and agitation thus
occasioned, the saccharine matter is perfectly dissolved and incorporated with the albumen
of the blood and the bone black. Instead of the blood, many refiners employ a mixture of
gelatinous alumina and gypsum, called finings, prepared by adding a solution of alum to
a body of lime-water, collecting, washing, and draining the precipitate upon a filter.
Other refiners use both the blood and finings, with advantage.
Bone black is now very frequently employed by the sugar-refiner,
not in a fine meal, but in a granular state, like corned gunpowder,
for the purpose of decolouring his syrups; in which case, he places
it in a box, in a stratum 8 or 10 inches thick, and makes the
syrup percolate downwards through it, into a cistern placed beneath.
By this means it is deprived of colour, and forms the
claircé of the French refiner. When the blowing up cistern is
charged with sugar, finely ground bone black, and blood, the mixture
must be passed through a proper system of filters. That
now most in use is the creased bag filter, represented in figs. 1084,
1085, 1086.
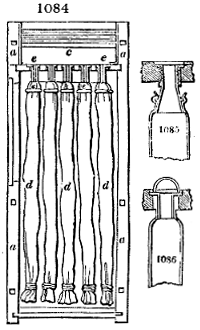
The apparatus consists of an upright square wooden case a, a,
about 6 or 8 feet high, furnished with a door of admission to arrange
the interior objects; beneath is a cistern with an educting-pipe for
receiving and carrying off the filtered liquor; and above the case
is another cistern e, which, like the rest, is lined with tinned sheet
copper. Into the upper cistern, the syrup mixed with animal
charcoal is introduced, and passes thence into the mouths e, e, of
the several filters d, d. These consist, each of a bag of thick
tweeled cotton cloth, about 12 or 15 inches in diameter, and 6 or
8 feet long, which is inserted into a narrow bottomless bag of
canvas, about 5 inches in diameter, for the purpose of folding the
filter-bag up into a small space, and thus enabling a great extent of
filtering surfaces to be compressed into one box. The orifice of
each compound bag is tied round a conical brass month-piece or
nozzle e, which screws tight into a corresponding opening in the
copper bottom of the upper cistern. From 40 to 60 bags are mounted
in each filter case. The liquor which first passes is generally tinged
a little with the bone black, and must be pumped back into the upper cistern, for refiltration.
In cold weather the interior of the case may be kept warm by a proper distribution
of steam-pipes. Fig. 1085. shows one mode of forming the funnel-shaped
nozzles of the bags, in which they are fixed by a bayonet catch. Fig. 1086. shows the
same made fast by means of a screwed cap, which is more secure.
The next process in sugar-refining is the evaporation of the clarified syrup to the
granulating or crystallizing pitch. The more rapidly this is effected, and with the less
scorching injury from fire, the better and greater is the product in sugar-loaves. No
apparatus answers the refiner’s double purpose of safety and expedition so well as the
vacuum-pan of Howard.
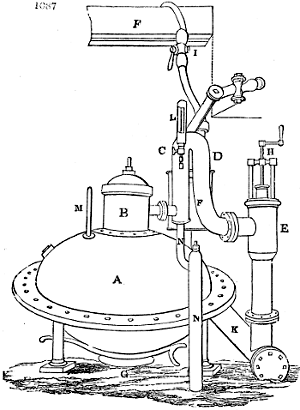
Fig. 1087. shows the structure of a single vacuum-pan. The horizontal diameter of
the copper spheroid A, is not less than 5 feet; the depth of the under hemisphere is at[1206]
least 18 inches from the level of the plane; and the height of the dome-cover is 2 feet.
The two hemispheres (of which the inferior one is double, or has a steam-jacket,) are
put together by bolts and screws, with packing between the flanges to preserve the
joints tight against atmospheric pressure. The jacket of the lower hemisphere forms
the case of the steam, which communicates heat to the syrup enclosed in the inner
hemisphere. In general, the pans contain, when filled to the flange, 100 gallons of
syrup, and yield about 11 cwt. of granulated sugar, at every charge.
A, represents the vacuum spheroid; B, the neck with the lid. From the side of B, a
pipe passes into the lower extremity of the bent pipe C, D, which terminates in the
vertical pipe E, connected
with the vacuum
main-pipe K, proceeding
horizontally from the
air-pump (not shown in
the figure). At the top
of E, a valve, movable by
a screw H, is placed for
establishing or cutting
off the connexion with
the air-pump at pleasure.
Behind F, is the measure
cistern, from which
the successive charges
are admitted into the
pan. This measure is
filled with the clear syrup,
by opening the
stopcock I, on the pipe
under the ceiling, which
communicates with the
filter-cistern placed above.
G is the valve or
plug-hole, at the bottom
of the pan, for discharging
the granulating syrup.
This plug is opened
by means of a powerful
lever attached to it;
the connexion with the
air-pump being previously
intercepted. L, is
the barometer, or manometer,
for showing
the state of the vacuum
corresponding to the
temperature. N, N, is a
cistern-pipe for receiving any little syrup which may accidentally boil over the neck B.
Its contents are let off by a stopcock at its bottom from time to time. M shows the
place of the proof-stick, an ingenious brass rod for taking out a sample of syrup without
admitting air. See infrà.
The charging-cistern contains about 20 gallons. This quantity of syrup being first
admitted, and brought to a certain pitch of concentration, a second measure is introduced,
the inspissation of which is supposed by some refiners to cause an agglomeration
of saccharine matter round the first crystalline particles. The repetition of this process
for two or three times is imagined to produce the large brilliant grain of vacuum-pan
sugar. This hypothesis is more specious than sound, because the granulating syrup
discharged from the pan is subjected to a heat of 180° or 190° in the subjacent steam-cased
receiver, whereby the granulations are again reduced to a very small size. Into
this receiver, two or three skippings or discharges of the pan are admitted in succession,
and the whole are diligently mixed and agitated by a stirring oar. It is by this process
that the granulating tendency is promoted and determined. From this receiver
(absurdly enough called a cooler) the moulds are filled in the usual way, by means of
copper basins or large ladles.
The case of the under hemisphere of the vacuum-pan is filled with steam, generated under
a pressure of four or five pounds on the square inch; the heat of which causes the interior
syrup to boil rapidly while the air-pump is kept in action. A small escape-pipe for
waste steam must be placed at the opposite side of the case or jacket, to ensure its equal[1207]
distribution; as also a stopcock below, to let off the water of condensation. The pans
are mounted on iron feet, or short pillars, which insulate them from the floor, and allow
their whole surface to be inspected, and any flaw to be repaired. The air-pump usually
stands in a cold-water cistern, to favour the condensation of the aqueous vapour, which
it draws out of the pans; and it is kept in constant action by the steam-engine, being
attached to the working-beam of its piston.
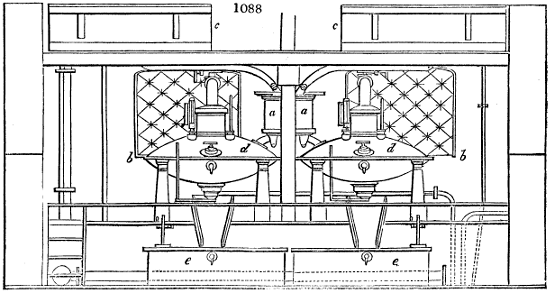
Fig. 1088. exhibits the general arrangement of the vacuum-pans, and their subsidiary
apparatus. Here are shown, on the ground floor, the heaters e, e, (miscalled coolers), into
which the concentrated syrup is let down. These heaters are made of copper, in one piece,
surrounded with a cast-iron jacket, bolted at the flange or brim to it. Each pan contains,
when full, about 350 gallons, equivalent to nearly 35 cwt. of crystallized sugar. They
are furnished with steam-cocks and waste steam-pipes. Under the level of the spheroids
d, d, the horizontal main-pipe is seen, for supplying the cases with steam. In the face
of each pan, above the line b, b, the handle of the proof-stick appears, like that of a stop-cock.
The distribution of the measure cisterns, and some other parts of the pans, is
slightly varied in this representation from the former. From the bottom of the liquor
cisterns C, C, pipes descend to the charging measures a, a, below. The cisterns C, C, are
made of copper, and contain each about 400 gallons. Six tons of refined sugar can be
turned out daily in a three-pan house.
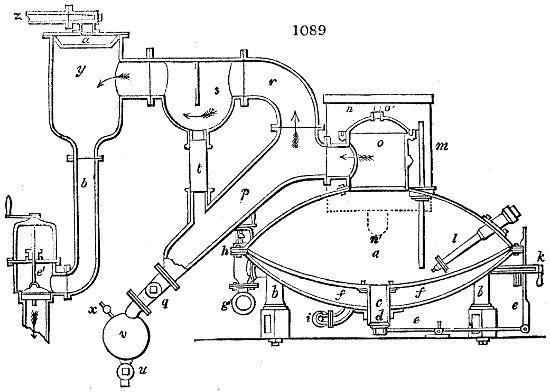
Fig. 1089. represents in section another form of the vacuum-pan, a is the spheroidal
copper vessel, supported by four iron columns b, b. It may be discharged by means of
the pipe c, which is secured with a conical valve d. This may be opened or shut, by
acting on the lever e. The lower of the two hemispheres of which the pan is composed[1208]
is double, and the interstitial space f, f, is filled with steam by the pipe g, as the heating
and evaporating agent. h, is the steam valve; i, the pipe for the efflux of the condensed
water. k, a tube for the escape of the air at the commencement of the operation. l, is
an apparatus inserted air-tight into the cover of the vacuum-pan, and which dips down
into the syrup; serving to take out a sample of it, without allowing air to enter, and
hence called the proof-stick. The construction of this instrument is exhibited in figs.
1091, 1092, 1093, 1094, 1095., which will be presently explained. m, is the thermometer,
which is also plunged into the sugar; behind it, is the barometer. n, is the charger or
gauge-vessel, filled with the filtered syrup, which it discharges by the pipe n′. o, is the
cover or capital of the vacuum-pan. o′, is a safety-valve, through which the air may be
admitted, after the completion of the process. p, is a bent pipe, slanting downwards,
with a stopcock q, at its end, to receive the superfluous syrup. The vapour, which is
disengaged from the syrup during its concentration, is extracted from the top of the
pan into the pipe r, passes from this into the vessel s, which is divided by a plate of
copper into two compartments. The syrup forced over accidentally in the ebullition,
goes into the vessel s, and passes by the glass tube t, into the pipe p. The glass tube
serves to show the quantity of the syrup that has boiled over, so that it may be drawn
off when necessary. For this purpose, the stopcock u, of the vessel v, must be closed,
and q must be opened, in order to fill v, while the air contained in it escapes into the
pan. The stopcock q, being then shut, and u, with the little air-cock x, opened, the syrup
will flow into the large receiver placed beneath it, commonly but erroneously called a
cooler; because it is a double copper basin, with steam in the interstitial space. The
hot steam rushes from s, into the cast-iron vessel y, where it is condensed. z, is a pipe
for introducing the water of condensation through the copper rose a′. The condensed
water flows through the pipe b′, and the valve e′, to the air-pump, which receives motion
from the shaft of the steam-engine.
The vacuum-pan was originally heated solely by the admission of steam between the
double bottom; but of late years the heat
has been also applied to the syrup through
several coils of pipe placed within the pan,
filled with steam at a temperature many
degrees above 212° F., sometimes so high
as 250°. By this double application of
heat, the evaporating power of a pan has
been vastly increased. The latest made
pans have a considerably flat bottom, fig.
1090.; a spiral pipe, laid close upon it; and
between the under hemisphere and the
upper one, there is a space a, a, 21⁄2 feet
high, to give the syrup room for frothing
up without boiling over. The space b, of
the bottom receives steam of common
pressure, and the spiral tubes, of high
pressure. A pan like this is now making
for a house in London, which is to work
off 16 tons of sugar-loaves daily.
The proof-stick, fig. 1095., consists of a cylindrical rod, capable of being screwed air-tight
into the pan in an oblique direction downwards. The upper or exterior end is open;
the under, which dips into the syrup, is closed, and has on one side a slit a (figs. 1091, 1092.),
or notch, about 1⁄2 inch wide. In this external tube, there is another shorter tube b, capable
of moving round in it, through
an arc of 180°. An opening
upon the under end e, corresponds
with the slit in the outer
tube, so that both may be made
to coincide, fig. 1091. A. A
wooden plug d, is put in the
interior tube, but so as not to
shut it entirely. Upon the
upper end there is a projection
or pin, which catches in a slit
of the inner tube, by which
this may be turned round at
pleasure. In the lower end of
the plug there is a hole e, which
can be placed in communication with the lateral openings in both tubes. Hence it is
possible, when the plug and the inner tube are brought into the proper position, A,
fig. 1091., to fill the cavity of the wooden rod with the syrup, and to take it out without[1209]
allowing any air to enter. In order to facilitate the turning of the inner tube within
the outer, there is a groove in the under part, into which a little grease may be
introduced.
Whenever a proof has been taken, the wooden plug must be placed in reference to
the inner tube, as shown in fig. 1091. c, and then be turned into the position A; when
the cavity of the plug will again be filled with syrup. c must be now turned back to
the former position, whereby all intercourse with the vacuum-pan is cut off; the plug
being drawn out a little, and placed out of communication with the inner tube. The plug
is then turned into the position B, drawn out, and the proof examined by the fingers.
Table showing the boiling point of syrup, at the corresponding atmospheric pressure
within the vacuum-pan:—
| Height of the mercury (inches) in one leg of the syphon, above that in the other— |
| 0·74 |
0·86 |
1·01 |
1·17 |
1·36 |
1·57 |
1·80 |
2·05 |
2·36 |
2·72 |
3·10 |
3·52 |
4·00. |
| Boiling point, Fahr.— |
| 115° |
120° |
125° |
130° |
135° |
140° |
145° |
150° |
155° |
160° |
165° |
170° |
175°. |
The large double steam-basin, which receives several successive skippings of the
concentrated granulating syrup, serves to heat it from the temperature of 160° or 170°,
at which it leaves the vacuum-pan, up to 200° or thereby, before it is filled out into the
moulds; for were it introduced in the cooler state, it would not concrete into sufficiently
compact loaves.
The following apparatus is used in many French sugar-houses, for concentrating
syrups, called the swing pan, or chaudière à bascule. It is represented in fig. 1096. in
elevation, and in fig. 1097. in ground plan. a, is the pan;
b, its spout; c, the axis or pivot round which it swings,
so as to empty itself, when raised behind by the chain
d; e, is the furnace door; f, the passage to the fireplace
and grate g; h, h, h, side flues for conducting the smoke
into the chimney.
The duly clarified, concentrated, granulated, and reheated
syrup, is transferred, by means of copper basins, from
the coolers into conical moulds, made either of brown and
somewhat porous earthenware, or of sheet iron, strongly
painted. The sizes of the moulds vary, from a capacity
of 10 pound loaves, to that of 56 pound bastards—a kind
of soft brown sugar obtained by the concentration of the
inferior syrups. These moulds have the orifices at their
tips closed with bits of twisted paper, and are set up in
rows close to each other, in an airy apartment adjoining
the coolers. Here they are left several hours, commonly
the whole night, after being filled, till their contents
become solid, and they are lifted next morning into an
upper floor, kept at a temperature of about 80° by means of
steam pipes, and placed each over a pot to receive the syrup
drainings—the paper plug being first removed, and a steel
wire, called a piercer, being thrust up to clear away any
concretion from the tip. Instead of setting the lower
portion of the inverted cones in pots, some refiners arrange them in wooden racks, with
their apices suspended over longitudinal gutters of lead or zinc, laid with a slight slope
upon the floor, and terminating in a sunk cistern. The syrup which flows off spontaneously
is called green syrup. It is kept separate. In the course of two or three
days, when the drainage is nearly complete, some finely clarified syrup, made from loaf
sugar, called liquor by the refiners, is poured to the depth of about an inch upon the
base of each cone, the surface having been previously rendered level and solid by an
iron tool, called a bottoming trowel. The liquor, in percolating downwards, being
already a saturated syrup, can dissolve none of the crystalline sugar, but only the
coloured molassy matter; whereby, at each successive liquoring, the loaf becomes whiter,
from the base to the apex. A few moulds, taken promiscuously, are emptied from time
to time, to inspect the progress of the blanching operation; and when the loaves appear
to have acquired as much colour, according to the language of refiners, as is wanted for
the particular market, they are removed from the moulds, turned on a lathe at the tips, if
necessary, set for a short time upon their bases, to diffuse their moisture equally through
them, and then transferred into a stove heated to 130° or 140° by steam pipes, where
they are allowed to remain for two or three days, till they be baked thoroughly dry.
They are then taken out of the stove, and put up in blue paper for sale.
In the above description of sugar-refining, I have said nothing of the process of claying
the loaves, because it is now nearly obsolete, and abandoned in all well-appointed[1210]
sugar-houses. Those of my readers who desire to become acquainted with sugar-refining
upon the old plan, may consult my Report made upon the subject to the
Honourable House of Commons in July 1833; where they will find every step detailed,
and the numerical results stated with minute accuracy. The experiments subservient
to that official report were instituted purposely to determine the average yield or product,
in double and single refined loaves, lumps, bastards and treacle, which different
kinds of sugar would afford per cwt., when refined by decolouring with not more than 5
per cent. of bone black, boiling in an open pan, and clearing the loaves with clay-pap.
BEET-ROOT SUGAR.
The physical characters which serve to show that a beet-root is of good quality, are
its being firm, brittle, emitting a creaking noise when cut, and being perfectly sound
within; the degree of sweetness is also a good indication. The 45th degree of latitude
appears to be the southern limit of the successful growth of beet in reference to the
extraction of sugar.
Extraction of Sugar from the Beet.—The first manipulations to which the beets are
exposed, are intended to clear them from the adhering earth and stones, as well as the
fibrous roots and portions of the neck. It is desirable to expose the roots, after this
operation, to the action of a cylinder washing-machine.
The parenchyma of the beet is a spongy mass, whose cells are filled with juice. The
cellular tissue itself, which forms usually only a twentieth or twenty-fifth of the whole
weight, consists of ligneous fibre. Compression alone, however powerful, is inadequate
to force out all the liquor which this tissue contains. To effect this object, the roots
must be subjected to the action of an instrument which will tear and open up the
greatest possible number of these cells. Experiments have, indeed, proved, that by the
most considerable pressure, not more than 40 or 50 per cent. in juice from the beet can
be obtained; whilst the pulp procured by the action of a grater produces from 75 to
80 per cent.
The beet-root rasp of Moulfarine is represented in figs. 1098, 1099. a, a, is the frame-work
of the machine; b, the feed-plate made of cast iron, divided by a ridge into two
parts; c, the hollow drum; d, its shaft, upon either side of whose periphery nuts are
screwed for securing the saw blades e, e, which are packed tight against each other by means
of laths of wood; f, is a pinion upon the shaft of the drum, into which the wheel g works,
and which is keyed upon the shaft h; i, is the driving rigger; k, pillar of support; l,
blocks of wood, with which the workman pushes the beet-roots against the revolving-rasp;
m, the chest for receiving the beet-pap; n, the wooden cover of the drum, lined
with sheet iron. The drum should make 500 or 600 turns in the minute.
A few years ago, M. Dombasle introduced a process of extracting the juice from the
beet without either rasping or hydraulic pressure. The beets were cut into thin slices,
by a proper rotatory blade-machine; these slices were put into a macerating cistern,
with about their own bulk of water, at a temperature of 212° F. After half an hour’s
maceration, the liquor was said to have a density of 2° B., when it was run off into a
second similar cistern, upon other beet-roots; from the second, it was let into a third,
and so on to a fifth; by which time, its density having risen to 51⁄2°, it was ready for the
process of defecation. Juice procured in this way is transparent, and requires little
lime for its purification; but it is apt to ferment, or to have its granulating power impaired[1211]
by the watery dilution. The process has been accordingly abandoned in most
establishments.
I have seen the following operations successfully executed in a beet-root factory near
Lille, and have since verified their propriety in my own laboratory upon white beets,
grown near Mitcham in Surrey. My product was nearly 5 per cent.; it was very fair,
and large grained, like the vacuum-pan sugar of Demerara, but without its clamminess.
The roots were washed by a rotatory movement upon a grating made like an Archimedes’
screw, formed round the axis of a squirrel-cage cylinder, which was laid horizontally
beneath the surface of water in an oblong trough. It was turned by hand rapidly, with
the intervention of a toothed wheel and pinion. The roots, after being sufficiently
agitated in the water, were tossed out by the rotation at the end of the cylinder furthest
from the winch. They were next hoisted in a basket up through a trap hole into the
floor above, by means of a cord and pulley moved by mechanical power; a six-horse steam
engine, upon Woolfe’s expansive principle, being employed to do all the heavy work.
They were here subjected to the mechanical grater (rape mécanique), see fig. 1098, 1099.,
which had, upon its sloping feed-table, two square holes for receiving at least two beets at
a time, which were pushed forwards by a square block of wood held in the workman’s
hand by means of a strap. The rasp was a drum, having rows of straight saws placed
half an inch apart round its periphery, parallel to the axis, with teeth projecting about
1⁄8 of an inch. The space between each pair of saws was filled with a wedge of wood. The
steel slips, or saw plates, were half an inch broad, twelve inches long, and serrated on both
their longitudinal edges, so that when the one line of teeth was blunted, the other
could be turned out. The drum made 750 turns per minute.
The pulp from the rasp fell into a flat trough placed beneath, whence it was shovelled
into small bags. Each bag had its mouth folded over, was laid upon a wicker plate,
and spread flat with a rolling-pin. The bags and hurdles were then piled in the
hydraulic press. There were three presses, of which the two allotted to the first
pressure were charged alternately, and the third was reserved for a final and more durable
pressure of the marc. See Press, hydraulic, and Stearine Press.
The juice flowed over the edges of the wicker plates, and fell into the sill-plate of
the press, which was furnished with upright borders, like a tray, through whose front
side a pipe issued, that terminated in a leathern hose, for conducting the juice into an
elevated cistern in the boiling-house. Here one pound of slaked lime was mixed with
every four hectolitres (about 88 gallons imp.) of juice. The mixture was made to boil for
a little while in a round pan alongside, whence it was decanted into oblong flat filters, of
blanket stuff. The filtered liquor, which had in general a spec. gravity of 15° Baumé,
(about double that of the fresh juice), was now briskly concentrated by boiling, in an
oblong pan, till it acquired the density of 28° B. The fire being damped with raw
coal, the syrup was run off rapidly by a stopcock into a large basin with a swing handle,
and immediately replaced by fresh defecated liquor. The basin was carried by two men
to the opposite side of the boiling-house, and emptied into a cistern set on a high
platform, whose horizontal discharge-pipe was provided with a series (five) of stopcocks,
placed respectively over five copper chests (inverted truncated pyramids), containing a
thick bed of granular bone black, covered with a perforated copper plate. The hot
syrup thus filtered had a pale straw-colour, and was subsequently evaporated in swing
pans, figs. 1096, 1097., over a brisk fire, in quantities equivalent to half a cwt. of sugar,
or four hectolitres of average juice.
MAPLE SUGAR.
The manufacture of sugar from the juice of a species of maple tree, which grow
spontaneously in many of the uncultivated parts of North America, appears to have
been first attempted about 1752, by some of the farmers of New England, as a branch
of rural economy.
The sugar maple, the Acer saccharinum of Linnæus, thrives especially in the states of
New York and Pennsylvania, and yields a larger proportion of sugar than that which
grows upon the Ohio. It is found sometimes in thickets which cover five or six acres
of land; but it is more usually interspersed among other trees. They are supposed to
arrive at perfection in forty years.
The extraction of maple sugar is a great resource to the inhabitants of districts far
removed from the sea; and the process is very simple. After selecting a spot among
surrounding maple trees, a shed is erected, called the sugar-camp, to protect the boilers
and the operators from the vicissitudes of the weather. One or more augers, three-fourths
of an inch in diameter; small troughs for receiving the sap; tubes of elder or
sumach, 8 or 10 inches long, laid open through two-thirds of their length, and corresponding
in size to the auger-bits; pails for emptying the troughs, and carrying the sap
to the camp; boilers capable of holding 15 or 16 gallons; moulds for receiving the
syrup inspissated to the proper consistence for concreting into a loaf of sugar; and,[1212]
lastly, hatchets to cut and cleave the fuel, are the principal utensils requisite for this
manufacture. The whole of February and beginning of March are the sugar season.
The trees are bored obliquely from below upwards, at 18 or 20 inches above the
ground, with two holes 4 or 5 inches asunder. Care must be taken that the auger
penetrates no more than half an inch into the alburnum, or white bark; as experience
has proved that a greater discharge of sap takes place at this depth than at any other.
It is also advisable to perforate in the south face of the trunk.
The trough, which contains from two to three gallons, and is made commonly of
white pine, is set on the ground at the foot of each tree, to receive the sap which flows
through the two tubes inserted into the holes made with the auger; it is collected
together daily, and carried to the camp, where it is poured into casks, out of which the
boilers are supplied. In every case, it ought to be boiled within the course of two or
three days from flowing out of the tree, as it is liable to run quickly into fermentation,
if the weather become mild. The evaporation is urged by an active fire, with careful
skimming during the boiling; and the pot is continually replenished with more sap, till
a large body has at length assumed a syrupy consistence. It is then allowed to cool,
and passed through a woollen cloth, to free it from impurities.
The syrup is transferred into a boiler to three-fourths of its capacity, and it is urged
with a brisk fire, till it acquires the requisite consistence for being poured into the
moulds or troughs prepared to receive it. This point is ascertained, as usual, by its
exhibiting a granular aspect, when a few drops are drawn out into a thread between the
finger and the thumb. If in the course of the last boiling, the liquor froth up considerably,
a small bit of butter or fat is thrown into it. After the molasses have been
drained from the concreted loaves, the sugar is not at all deliquescent, like equally
brown sugar from the cane. Maple sugar is in taste equally agreeable with cane sugar,
and it sweetens as well. When refined, it is equally fair with the loaf sugar of Europe.
The period during which the trees discharge their juices is limited to about six weeks.
Towards the end of the flow, it is less abundant, less saccharine, and more difficult to
be crystallized.
Quantity of Sugar brought into the Markets of the World, in the year 1838.
| |
Tons. |
|
| British West Indies |
160,000 |
|
| Mauritius, 35,000; and British East Indies, 20,000 |
55,000 |
|
| Java |
36,000 |
|
| Manilla and Siam |
30,000 |
|
| Dutch West Indies |
25,000 |
|
| St. Thomas and St. Croix |
7,000 |
|
| Martinique and Guadaloupe |
80,000 |
|
| Bourbon |
20,000 |
|
| Cuba |
100,000 |
|
| Brazils |
95,000 |
|
| From Beet-root, in France and Belgium |
65,000 |
|
| United States |
65,000 |
|
| |
738,000 |
[65] |
SUGAR OF LEAD, properly Acetate of lead, (Acetate de plomb; Sel de Saturne,
Fr.; Essigsaures Bleioxyd, Bleizucker, Germ.) is prepared by dissolving pure litharge,
with heat, in strong vinegar, made of malt, wood, or wine, till the acid be saturated.
A copper boiler, rendered negatively electrical by soldering a strap of lead within it, is
the best adapted to this process on the great scale. 325 parts of finely ground and
sifted oxide of lead, require 575 parts of strong acetic acid, of spec. grav. 7° Baumé,
for neutralization, and afford 960 parts of crystallized sugar of lead. The oxide should
be gradually sprinkled into the moderately hot vinegar, with constant stirring, to prevent
adhesion to the bottom; and when the proper quantity is dissolved, the solution
may be weakened with some of the washings of a preceding process, to dilute the acetate,
after which the whole should be heated to the boiling point, and allowed to cool slowly,
in order to settle. The limpid solution is to be drawn off by a syphon, concentrated
by boiling to the density of 32° B., taking care that there be always a faint excess of
acid, to prevent the possibility of any basic salt being formed, which would interfere with
the formation of regular crystals. Should the concentrated liquor be coloured, it may
be whitened by filtration through granular bone black.
Stoneware vessels, with salt glaze, answer best for crystallizers. Their edges should
be smeared with candle-grease, to prevent the salt creeping over them by efflorescent
vegetation. The crystals are to be drained, and dried in a stove-room very slightly
heated. It deserves remark, that linen, mats, wood, and paper, imbued with sugar of
lead, and strongly dried, readily take fire, and burn away like tinder. When the
motherwaters cease to afford good crystals, they should be decomposed by carbonate of
soda, or by lime skilfully applied, when a carbonate or an oxide will be obtained, fit for
treating with fresh vinegar. The supernatant acetate of soda may be employed for the
extraction of pure acetic acid.
[1213]
A main point in the preparation of sugar of lead, is to use a strong acid; otherwise
much time and acid are wasted in concentrating the solution. This salt crystallizes in
colourless, transparent, four and six sided prisms, from a moderately concentrated
solution; but from a stronger solution, in small needles, which have a yellow cast if the
acid has been slightly impure. It has no smell, a sweetish astringent metallic taste, a
specific gravity of 2·345; it is permanent in the air at ordinary temperatures, but
effloresces when heated to 95°, with the loss of its water of crystallization and some
acid, falling into a powder, which passes, in the air, slowly into carbonate of lead.
The crystals dissolve in 11⁄2 times their weight of water at 60°, but in much less of boiling
water, and in 8 parts of alcohol. The solution feebly reddens litmus paper, but has
an alkaline reaction upon the colours of violets and turmeric. The constituents of
the salt are, 58·71 oxide of lead, 27·08 acetic acid, and 14·21 water, in 100.
Acetate of lead is much used in calico-printing. It is poisonous, and ought to be
prepared and handled with attention to this circumstance.
There are two subacetates of lead; the first of which, the ter-subacetate, has three
atoms of base to one of acid, and is the substance long known by the name of Goulard’s
extract. It may be obtained by digesting with heat a solution of the neutral acetate,
upon pure litharge or massicot. The solution affords white crystalline scales,
which do not taste so sweet as sugar of lead, dissolve in not less than 30 parts of
water, are insoluble in alcohol, and have a decided alkaline reaction upon test
paper. Carbonic acid, transmitted through the solution, precipitates the excess of the
oxide of lead, in the state of a carbonate, a process long ago prescribed by Thenard
for making white-lead. This subacetate consists of 88·66 of oxide, and 13·34 acid, in
100 parts. It is employed for making the orange sub-chromate of lead, as also sometimes
in surgery.
A sex-subacetate, containing six atoms of base, may be obtained by adding ammonia
in excess to a solution of the preceding salt, and washing the precipitate with dilute
water of ammonia. A white powder is thus formed, that dissolves sparingly in cold
water, but gives a solution in boiling water, from which white silky needles are deposited.
It consists of 92·86 oxide, and 7·14 acid.
SULPHATES, are saline compounds of sulphuric acid with oxidized bases. The
minutest quantity of them present in any solution, may be detected by the precipitate,
insoluble in nitric or muriatic acid, which they afford with nitrate or muriate of baryta.
They are mostly insoluble in alcohol.
SULPHATE OF ALUMINA AND POTASSA, is alum.
SULPHATE OF AMMONIA, is a salt sometimes formed by saturating the
ammonia liquor of the gas-works with sulphuric acid; and it is employed for making
carbonate of ammonia. See Ammonia and Sal Ammoniac.
SULPHATE OF BARYTA, is the mineral called heavy-spar, which frequently
forms the gangue or vein-stone of lead and other metallic ores.
SULPHATE OF COPPER, Roman or Blue Vitriol (Vitriol de Chypre, Fr.; Kupfervitriol,
Germ.); is a salt composed of sulphuric acid and oxide of copper, and may
be formed by boiling the concentrated acid upon the metal, in an iron pot. It is,
however, a natural product of many copper mines, from which it flows out in the form
of a blue water, being the result of the infiltration of water over copper pyrites, which
has become oxygenated by long exposure to the air in subterranean excavations. The
liquid is concentrated by heat in copper vessels, then set aside to crystallize. The
salt forms in oblique four-sided tables, of a fine blue colour; has a spec. gravity of 2·104;
an acerb, disagreeable, metallic taste; and, when swallowed, it causes violent vomiting.
It becomes of a pale dirty blue, and effloresces slightly, on long exposure to the
air; when moderately heated, it loses 36 per cent. of water, and falls into a white
powder. It dissolves in 4 parts of water, at 60°, and in 2 of boiling water, but not in
alcohol; the solution has an acid reaction upon litmus paper. When strongly ignited,
the acid flies off, and the black oxide of copper remains. The constituents of crystallized
sulphate of copper are—oxide, 31·80; acid, 32·14; and water, 36·06. Its chief
employment in this country is in dyeing, and for preparing certain green pigments.
See Scheele’s and Schweinfurth Green. In France, the farmers sprinkle a weak
solution of it upon their grains and seeds before sowing them, to prevent their being
attacked by birds and insects.
SULPHATE OF IRON, Green vitriol, Copperas (Couperose verte, Fr.; Eisenvitriol,
Schwefelsaures Eisenoxydul, Germ.); is a crystalline compound of sulphuric acid
and protoxide of iron; hence called, by chemists, the protosulphate; consisting of, 26·10
of base, 29·90 of acid, and 44·00 of water, in 100 parts; or of 1 prime equivalent
of protoxide, 36, + 1 of acid, 40, + 7 of water, 63, = 139. It may be prepared by
dissolving iron to saturation in dilute sulphuric acid, evaporating the solution till a
pellicle forms upon its surface, and setting it aside to crystallize. The copperas of
commerce is made in a much cheaper way, by stratifying the pyrites found in the coal[1214]
measures (Vitriolkies and Strahlkies of the Germans), upon a sloping puddled platform
of stone, leaving the sulphuret exposed to the weather, till, by the absorption of oxygen,
it effloresces, lixiviating with water the supersulphate of iron thus formed, saturating
the excess of acid with plates of old iron, then evaporating and crystallizing. The other
pyrites, which occurs often crystallized, called by the Germans Schwefelkies or Eisenkies,
must be deprived of a part of its sulphur by calcination, before it acquires the property
of absorbing oxygen from the atmosphere, and thereby passing from a bisulphuret into
a bisulphate. Alum schist very commonly contains vitriolkies, and affords, after being
roasted and weather-worn, a considerable quantity of copperas, which must be carefully
separated by crystallization from the alum.
This liquor used formerly to be concentrated directly in leaden vessels; but the first
stage of the operation is now carried on in stone canals of considerable length, vaulted
over with bricks, into which the liquor is admitted, and subjected at the surface to the
action of flame and heated air, from a furnace of the reverberatory kind, constructed at
one end, and discharging its smoke by a high chimney raised at the other. See Soda
Manufacture. Into this oblong trough, resting on dense clay, and rendered tight in
the joints by water-cement, old iron is mixed with the liquor, to neutralize the excess of
acid generated from the pyrites, as also to correct the tendency to superoxidizement in
copperas, which would injure the fine green colour of the crystals. After due concentration
and saturation in this surface evaporator, the solution is run off into leaden
boilers, where it is brought to the proper density for affording regular crystals, which it
does by slow cooling, in stone cisterns.
Copperas forms sea-green, transparent, rhomboidal prisms, which are without
smell, but have an astringent, acerb, inky taste; they speedily become yellowish-brown
in the air, by peroxidizement of the iron, and effloresce in a warm atmosphere: they
dissolve in 1·43 parts of water at 60°, in 0·27 at 190°, and in their own water of crystallization
at a higher heat. This salt is extensively used in dyeing black, especially
hats, in making ink and prussian blue, for reducing indigo in the blue vat, in the China
blue dye, for making the German oil of vitriol, and in many chemical and medicinal
preparations.
There is a persulphate and subpersulphate of iron, but they belong to the domain of
chemistry. The first may be formed, either by dissolving with heat one part of red
oxide of iron (colcothar) in one-and-a-half of concentrated sulphuric acid, or by adding
some nitric acid to a boiling-hot solution of copperas. It forms with galls and logwood
a very black ink, which is apt to become brown-black. When evaporated to dryness,
it appears as a dirty white pulverulent substance, which is soluble in alcohol. It consists,
in 100 parts, of 39·42 of red oxide of iron, and 60·58 sulphuric acid.
Hydrated peroxide of iron, prepared by precipitation with alkali from solution of the
persulphate, is an excellent antidote against poisoning by arsenic. A French peruquier,
who had swallowed two drams of arsenious acid, was, after an interval of twenty
minutes, treated with the oxide precipitated from 6 ounces of that salt by caustic potash.
It was diffused in 20 quarts of weak syrup, and administered in successive doses. After
repeated vomiting and purging, the patient felt no more pain, and was pronounced by
the physician to be quite convalescent.
In the copperas and alum works, a very large quantity of ochrey sediment is obtained;
which is a peroxide of iron, containing a little sulphuric acid and alumina. This
deposit, calcined in reverberatory hearths, becomes of a bright-red colour; and when
ground and elutriated, in the same way as is described under white lead, forms a cheap
pigment, in very considerable demand, called English red, in the French market.
Colcothar of Vitriol, and Crocus of Mars, are old names for red oxide of iron.
This brown-red powder is obtained in its purest state, by calcining dried sulphate of iron
in a furnace till all its acid be expelled, and its base become peroxidized. It must be
levigated, elutriated, and dried. This powder is employed extensively in the steel
manufacture, for giving the finishing lustre to fine articles; it is used by silversmiths
under the name of plate powder and rouge; and by the opticians for polishing the
specula of reflecting telescopes. Much of the crocus in the market, is made, however,
from the copperas and alum sediments, and is greatly inferior to the article prepared by
the last process. The finest rouge is made by precipitating the oxide with soda, then
washing and calcining the powder.
An excellent powder for applying to razor-strops, is made by igniting together in a
crucible equal parts of well-dried copperas and sea salt. The heat must be slowly
raised and well regulated, otherwise the materials will boil over in a pasty state, and
the product will be in a great measure lost. When well made, out of contact of air, it
has the brilliant aspect of plumbago. It has a satiny feel, and is a true fer olegiste,
similar in composition to the Elba iron ore. It requires to be ground and elutriated;
after which it affords, on drying, an impalpable powder, that may be either rubbed
on a strop of smooth buff leather, or mixed up with hog’s-lard or tallow into a stiff
cerate.
[1215]
SULPHATE OF LIME. See Gypsum.
SULPHATE OF MAGNESIA, Epsom Salt (Sel amer, Fr.; Bittersalz, Germ.);
exists in sea-water, as also in the waters of Saidschütz, Sedlitz, and Püllna; and in many
saline springs, besides Epsom in Surrey, whence it has derived its trivial name, and from
which it was first extracted, in the year 1695, and continued to be so, till modern chemistry
pointed out cheaper and more abundant sources of this useful purgative salt.
The sulphate of magnesia, occasionally found effloresced on the surface of minerals
in crystalline filaments, was called haarsalz (hair salt) by the older writers. The bittern
of the Scotch sea-salt works is muriate of magnesia, mixed, with a little sulphate of
magnesia and chloride of sodium. If the proper decomposing quantity (found by trial)
of sulphate of soda be added to it, and the mixed solution be evaporated at the temperature
of 122° F., chloride of sodium will form by double affinity, and fall down in
cubical crystals; while the solution of sulphate of magnesia which remains, being
evaporated to the proper point, will afford regular crystals in four-sided prisms with
four-sided acuminations. Or, if bittern be treated in a retort with the equivalent
quantity of sulphuric acid, the muriatic acid may be distilled off into a series of Woulfe’s
bottles, and the sulphate of magnesia, soda, and lime, will remain in the retort, from
which mixture the sulphate of magnesia may be separated by filtration and crystallization.
Magnesian limestone being digested with as much muriatic acid as will dissolve out its
lime only, will, after washing, afford, with the equivalent quantity of sulphuric acid, a pure
sulphate of magnesia; and this is certainly the simplest and most profitable process for
manufacturing this salt upon the great scale. Many prepare it directly, by digesting
upon magnesian limestone the equivalent saturating quantity of dilute sulphuric acid.
The sulphate of lime being separated by subsidence, the supernatant solution of sulphate
of magnesia is evaporated and crystallized.
This salt is composed of, magnesia 16·72, sulphuric acid 32·39, and water 50·89.
When free from muriate, it tends to effloresce in the air. It dissolves in 4 parts of
water at 32°, in 3 parts at 60°, in 1·4 at 200°, and in its own water of crystallization
at a higher heat.
SULPHATE OF MANGANESE, is prepared on the great scale for the calico-printers,
by exposing the peroxide of the metal and pitcoal ground together, and made
into a paste with sulphuric acid, to a heat of 400° F. On lixiviating the calcined
mass, a solution of the salt is obtained, which is to be evaporated and crystallized. It
forms pale amethyst-coloured prisms, which have an astringent bitter taste, dissolve
in 21⁄2 parts of water, and consist of, protoxide of manganese 31·93, sulphuric acid 35·87,
and water 32·20, in 100 parts.
SULPHATE OF MERCURY, is a white salt which is used in making corrosive
sublimate. See Mercury. The subsulphate, called Turbith Mineral, is a pale yellow
pigment, and may be prepared by washing the white sulphated peroxide with hot water,
which resolves it into the soluble supersulphate, and the insoluble subsulphate, or Turbith.
It is poisonous.
SULPHATE OF POTASSA, is obtained by first igniting and then crystallizing
the residuum of the distillation of nitric acid from nitre.
SULPHATE OF SODA, is commonly called Glauber’s salt, from the name of the
chemist who first prepared it. It is obtained by igniting and then crystallizing the
residuum of the distillation of muriatic acid from common salt. It crystallizes in
channelled 6-sided prisms. See Soda Manufacture.
SULPHATE OF ZINC, called also White Vitriol, is commonly prepared in the
Harz, by washing the calcined and effloresced sulphuret of zinc or blende, on the same
principle as green and blue vitriol are obtained from the sulphurets of iron and copper.
Pure sulphate of zinc may be made most readily by dissolving the metal in dilute
sulphuric acid, evaporating and crystallizing the solution. It forms prismatic crystals,
which have an astringent, disagreeable, metallic taste; they effloresce in a dry air,
dissolve in 2·3 parts of water at 60°, and consist of—oxide of zinc, 28·29; acid, 28·18;
water, 43·53. Sulphate of zinc is used for preparing drying oils for varnishes, and in
the reserve or resist pastes of the calico-printer.
SULPHITES, are a class of salts, consisting of sulphurous acid, combined in equivalent
proportions with the oxidized bases.
SULPHOSELS, is the name given by Berzelius to a class of salts which may be
prepared as follows:—1. Dissolve a salt consisting of an oxide and an acid (an oxisalt),
in a very small quantity of water, and pass through the solution a stream of sulphuretted
hydrogen, till the salt be entirely decomposed. In this operation, the oxisalt
is transformed into a sulphosalt, by the sulphur of the compound gas; while its hydrogen
forms water with the oxygen of the saline base. This process is applicable only to the
metallic salts; and among these, not to the nitrates, carbonates, or phosphates. 2. Another
method of preparing sulphosalts is, to add to a watery solution of sulphuret of[1216]
potassium, an electro-negative metallic sulphuret, which will dissolve in the liquid till
the sulphuret of potassium be saturated. This saline compound is to be employed
to effect double decompositions with the oxisalts; that is, to convert the radical of
another base, combined with an oxacid, into a sulphosalt. 3. If the electro-negative
sulphuret be put in powder into a solution of the hydrosulphuret of potassa, it will
dissolve and expel the sulphuretted hydrogen with effervescence; just as carbonic acid
is displaced by a stronger acid. For his other three methods of preparing sulphosalts,
see his Elements, vol. iii. p. 336, Fr. translation.
SULPHUR; Brimstone (Soufre, Fr.; Schwefel, Germ.); is a simple combustible,
solid, non-metallic, of a peculiar yellow colour, very brittle, melting at the temperature
of 226° Fahr., and possessing, after it has been fused, a specific gravity of 1·99.
When held in a warm hand, a roll of sulphur emits a crackling sound, by the fracture
of its interior parts; and when it is rubbed, it emits a peculiar well-known smell, and
acquires at the same time negative electricity. When heated to the temperature of
560° F. it takes fire, burns away with a dull blue flame of a suffocating odour, and
leaves no residuum. When more strongly heated, sulphur burns with a vivid white
flame. It is not affected by air or water.
Sulphur is an abundant product of nature; existing sometimes pure or merely mixed,
and at others in intimate chemical combination with oxygen, and various metals, forming
sulphates and sulphurets. See Ores of Copper, Iron, Lead, &c., under these
metals.
Fig. 1100. represents one of the cast-iron retorts used at Marseilles for refining sulphur,
wherein it is melted and converted into vapours, which are led into a large
chamber for condensation. The body a, of the retort is an iron pot, 3 feet in diameter
outside, 22 inches deep, half an inch thick, which weighs 14 cwt., and receives a charge of
8 cwt. of crude sulphur. The grate is 8 inches under its bottom, whence the flame
rises and plays round its sides. A cast-iron capital b, being luted to the pot, and
covered with sand, the opening in front is shut with an iron plate. The chamber d, is
23 feet long, 11 feet wide, and 13 feet high, with walls 32 inches thick. In the roof,
at each gable, valves or flap-doors,
e, 10 inches square, are
placed at the bottom of the
chimney c. The cords for opening
the valves are led down to
the side of the furnace. The
entrance to the chamber is shut
with an iron door. In the wall
opposite to the retorts, there are
two apertures near the floor, for
taking out the sulphur. Each
of the two retorts belonging to
a chamber is charged with 71⁄2 or
8 cwts. of sulphur; but one is
fired first, and with a gentle
heat, lest the brimstone froth
should overflow; but when the
fumes begin to rise copiously,
with a stronger flame. The distillation
commences within an
hour of kindling the fire, and is
completed in six hours. Three hours after putting fire to the first retort, the second is
in like manner set in operation.
When the process of distillation is resumed, after having been some time suspended,
explosions may be apprehended, from the presence of atmospherical air; to obviate the
danger of which, the flap-doors must be opened every 10 minutes; but they should
remain closed during the setting of the retorts, and the reflux of sulphurous fumes or
acid should be carried off by a draught-hood over the retorts. The distillation is carried
on without interruption during the week, the charges being repeated four times in the
day. By the third day, the chamber acquires such a degree of heat as to preserve the
sulphur in a liquid state; on the sixth, its temperature becoming nearly 300° F., gives
the sulphur a dark hue, on which account the furnace is allowed to cool on the Sunday.
The fittest distilling temperature is about 248°. The sulphur is drawn off through two
iron pipes cast in the iron doors of the orifices on the side of the chamber opposite to the
furnace. The iron stoppers being taken out of the mouths of the pipes, the sulphur is
allowed to run along an iron spout placed over red-hot charcoal, into the appropriate
wooden moulds.
Native sulphur in its pure state is solid, brittle, transparent, yellow, or yellow bordering[1217]
on green, and of a glassy lustre when newly broken. It occurs frequently in
crystalline masses, and sometimes in complete and regular crystals, which are all derivable
from the rhomboidal octahedron. The fracture is usually conchoidal and shining.
Its specific gravity is 2·072, exceeding somewhat the density of melted sulphur. It
possesses a very considerable refractive power; and doubles the images of objects even
across two parallel faces. Sulphur, crystallized by artificial means, presents a very
remarkable phenomenon; for by varying the processes, crystals are obtained whose
forms belong to two different systems of crystallization. The red tint, so common in
the crystals of Sicily, and of volcanic districts, has been ascribed by some mineralogists
to the presence of realgar, and by others to iron; but Stromeyer has found the sublimed
orange-red sulphur of Vulcano, one of the Lipari islands, to result from a natural combination
of sulphur and selenium.
It is extracted from the minerals containing it, at Solfatara, by the following process:—
Ten earthen pots, of about a yard in height, and 41⁄2 gallons imperial in capacity,
bulging in the middle, are ranged in a furnace called a gallery; five being set on the one
side, and five on the other. These are so distributed in the body of the walls of the
gallery, that their belly projects partly without, and partly within, while their top rises
out of the vault of the roof. The pots are filled with lumps of the sulphur ore of
the size of the fist; their tops are closed with earthenware lids, and from their
shoulder proceeds a pipe of about 2 inches diameter, which bends down, and enters into
another covered pot, with a hole in its bottom, standing over a tub filled with water.
On applying heat to the gallery, the sulphur melts, volatilizes, and runs down in a
liquid state into the tubs, where it congeals. When one operation is finished, the pots
are re-charged, and the process is repeated.
In Saxony and Bohemia, the sulphurets of iron and copper are introduced into large
earthenware pipes, which traverse a furnace-gallery; and the sulphur exhaled flows into
pipes filled with cold water, on the outside of the furnace. 900 parts of sulphuret
afford from 100 to 150 of sulphur, and a residuum of metallic protosulphuret. See
Metallurgy and Copper.
Volcanic sulphur is purer than that extracted from pyrites; and as the latter is
commonly mixed with arsenic, and some other metallic impregnations, sulphuric acid
made of it would not answer for many purposes of the arts; though a tolerably good
sulphuric acid may be made directly from the combustion of pyrites, instead of sulphur,
in the lead chambers. The present high price of the Sicilian sulphur is a great
encouragement to its extraction from pyrites. It is said that the common English
brimstone, such as was extracted from the copper pyrites of the Parys mine of
Anglesey, contained fully a fifteenth of residuum, insoluble in boiling oil of turpentine,
which was chiefly orpiment; while the fine Sicilian sulphur, now imported in vast
quantities by the manufacturers of oil of vitriol, contains not more than 3 per cent. of
foreign matter, chiefly earthy, but not at all arsenical.
Sulphur has been known from the most remote antiquity. From its kindling at a
moderate temperature, it is employed for readily procuring fire, and lighting by its
flame other bodies not so combustible. At Paris, the preparation of sulphur matches
constitutes a considerable branch of industry. The sulphurous acid formed by the
combustion of sulphur in the atmospheric air, is employed to bleach woollen and silken
goods, as also cotton stockings; to disinfect vitiated air, though it is inferior in power
to nitric acid vapour and chlorine; to kill mites, moths, and other destructive insects
in collections of zoology; and to counteract too rapid fermentation in wine-vats, &c.
As the same acid gas has the property of suddenly extinguishing flame, sulphur has
been thrown into a chimney on fire, with the best effect; a handful of it being sometimes
sufficient. Sulphur is also employed for cementing iron bars in stone; for taking
impressions from seals and cameos, for which purpose it is kept previously melted for
some time, to give the casts an appearance of bronze. Its principal uses, however, are
for the manufactures of vermillion, or cinnabar, gunpowder, and sulphuric acid.
See Metallurgy, page 823, for the description of Gahn’s furnace for extracting sulphur
from pyrites.
Pyrites as a bi-sulphuret, consisting of 45·5 parts of iron, and 54·5 of sulphur, may,
by proper chemical means, be made to give off one half of its sulphur, or about 27 per
cent.; but great care must be taken not to generate sulphurous acid, as is done very
wastefully by the Fahlun and the Goslar processes. By the latter, indeed, not more
than 1 or 2 parts of sulphur are obtained, by roasting 100 parts of the pyritous ores of
the Rammelsberg mines. In these cases, the sulphur is burned, instead of being sublimed.
The residuum of the operation, when it is well conducted, is black sulphuret of
iron, which may be profitably employed for making copperas. The apparatus for
extracting sulphur from pyrites should admit no more air than is barely necessary to
promote the sublimation.—Sicily produced last year 70,000 tons of sulphur, and Tuscany[1218]
1200; of which Great Britain consumed 46,000; France, 18,000; other places, 6000.
In 1820, Great Britain consumed only 5000 tons.
SULPHURATION, is the process by which woollen, silk, and cotton goods are
exposed to the vapours of burning sulphur, or to sulphurous acid gas. In the article
Straw-hat Manufacture, I have described a simple and cheap apparatus, well adapted
to this operation.
Sulphuring-rooms are sometimes constructed upon a great scale, in which blankets,
shawls, and woollen clothes may be suspended freely upon poles or cords. The floor
should be flagged with a sloping pavement, to favour the drainage of the water that
drops down from the moistened cloth. The iron or stoneware vessels, in which the
sulphur is burned, are set in the corners of the apartment. They should be increased
in number according to the dimensions of the place, and distributed uniformly over it.
The windows and the entrance door must be made to shut hermetically close. In the
lower part of the door, there should be a small opening, with a sliding shutter, which
may be raised or lowered by the mechanism of a cord passing over a pulley.
The aperture by which the sulphurous acid and azotic gases are let off, in order to
carry on the combustion, should be somewhat larger than the opening at the bottom.
A lofty chimney carries the noxious gases above the building, and diffuses them over a
wide space, their ascension being promoted by means of a draught-pipe of iron, connected
with an ordinary stove, provided with a valve to close its orifice when not kindled.
When the chamber is to be used, the goods are hung up, and a small fire is made in
the draught-stove. The proper quantity of sulphur being next put into the shallow
pans, it is kindled, the entrance door is closed, as well as its shutter, while a vent-hole
near the ground is opened by drawing its cord, which passes over a pulley. After a
few minutes, when the sulphur is fully kindled, that vent-hole must be almost entirely
shut, by relaxing the cord; when the whole apparatus is to be let alone for a sufficient
time.
The object of the preceding precautions is to prevent the sulphurous acid gas escaping
from the chamber by the seams of the principal doorway. This is secured by closing it
imperfectly, so that it may admit of the passage of somewhat more air than can enter
by the upper seams, and the smallest quantity of fresh air that can support the combustion.
The velocity of the current of air may be increased at pleasure, by enlarging
the under vent-hole a little, and quickening the fire of the draught-stove.
Before opening the entrance door of the apartment, for the discharge of the goods,
a small fire must be lighted in the draught furnace, the vent-hole must be thrown
entirely open, and the sliding shutter of the door must be slid up, gradually more and
more every quarter of an hour, and finally left wide open for a proper time. By this
means the air of the chamber will become soon respirable.
SULPHURETTED HYDROGEN, is a gas, composed of one part of hydrogen
and sixteen parts of sulphur, by weight. Its specific gravity is 1·1912, compared to
air = 1·0000. It is the active constituent of the sulphureous mineral waters. When
breathed, it is very deleterious to animal life; and being nearly twice as dense as air, it
may be poured from its generating bottle into cavities; a scheme successfully employed
by M. Thenard to destroy rats in their holes.
SULPHURIC ACID, Vitriolic Acid, or Oil of Vitriol (Acid sulfurique, Fr.;
Schwefelsaüre, Germ.). This important product, the agent of many chemical operations,
was formerly procured by the distillation of dried sulphate of iron, called green
vitriol, whence the corrosive liquid which came over, having an oily consistence, was
denominated oil of vitriol. This method has been superseded in Great Britain, France,
and most other countries, by the combustion of sulphur along with nitre, in large
leaden chambers; but as the former process, which is still practised at Bleyl in Bohemia,
and Nordhausen in Saxony, gives birth to some interesting results, I shall describe it
briefly.
Into a long horizontal furnace, or gallery of brickwork, a series of earthenware retorts,
of a pear shape, is arranged, with curved necks fitted into stoneware bottles or condensers.
Each retort is charged with sulphate of iron, which has been previously heated
to moderate redness. The first product of the distillation, a slightly acidulous phlegm,
is allowed to escape; then the retort and receiver are securely luted together. The fire
is now raised, and urged briskly for 36 hours, whereby the strong sulphuric acid is
expelled, in the form of heavy white vapours, which condense in the cold receiver into
an oily-looking liquid. The latter portions, when received in a separate refrigerator,
frequently concrete into a crystalline mass, formerly called glacial oil of vitriol.
About 64 pounds of strong acid may be obtained from 600 pounds of copperas. It is
brown-coloured; and varies in specific-gravity from 1·842 to 1·896. Its boiling point
is so low as 120° Fahr. When re-distilled in a glass retort, into a receiver surrounded
with ice, a very moderate heat sends over white fumes, which condense into a soft solid,
in silky filaments, like asbestos, tough, and difficult to cut. When this is exposed to[1219]
the air, it emits copious fumes of sulphuric (not sulphurous) acid. It burns holes in
paper as rapidly as a red-hot iron. Dropped in small quantities into water, it excites a
hissing noise, like ignited metal; and in larger quantities, it occasions an explosion.
By dropping a fragment of it into a poised phial containing water, and stoppering instantly,
to prevent the ejection of liquid, by the ebullition which always ensues, I got
a dilute acid, containing a known portion of the solid acid, from the specific gravity of
which, as well as from its saturating power, I ascertained that the above solid sulphuric
acid was truly anhydrous (void of water), consisting of 1 equivalent proportion of sulphur,
and 3 of oxygen; or, by weight, of 16 of the former, and 24 of the latter. This
acid makes a red solution of indigo.
The production of sulphuric acid from sulphur and nitre may be elegantly illustrated
by means of a glass globe with a stoppered hole at its side, and four bent glass
tubes inserted into a leaden cap in its upper orifice. The first tube is to be connected
with a heated matrass, disengaging sulphurous acid from copper filings and sulphuric
acid; the second with a retort, disengaging more slowly deutoxide of azote (nitric
oxide) from copper filings and nitric acid; the third with a vessel for furnishing
steam in a moderate current towards the end of the process, when no water has been
previously admitted into the balloon; the fourth tube may be upright, and terminate in
a small funnel. Through the opening in the side of the globe, atmospherical air is to
be admitted from time to time, by removing the stopper; after which, the residuary
lighter azote may be allowed to escape by the funnel orifice.
The nitric oxide first absorbs oxygen from the air, becomes, in consequence, nitrous
acid vapour, which giving up one third of its oxygen to the sulphurous acid, converts
this, with the aid of water, into sulphuric acid, while itself returning to the state of nitric
oxide, is again qualified to take oxygen from the air, and to transfer it to the sulphurous
acid gas; and thus in perpetual rotation. These oxygenating and disoxygenating processes
continue until nearly the whole oxygen of the atmospheric air contained in the
globe is consumed. Were there little aqueous vapour present, those gases would soon
cease to operate upon each other; for though the nitric oxide became nitrous acid, this
would oxygenate little of the sulphurous acid, because the three substances would condense
into white crystals upon the sides of the balloon, like hoar frost upon a window-pane
in winter. These indicate a deficiency of aqueous vapour, and an excess of nitrous
acid. On the admission of steam, the crystals disappear, the sulphuric acid is liquefied,
the nitrous acid is converted into nitric acid and nitric oxide; the former of which combines
with the water, while the latter is converted by the atmospheric oxygen into nitrous
acid vapour. A certain quantity of water is therefore requisite to prevent the formation
of that crystalline compound, which condenses the nitrous acid, and renders it inoperative
in transforming fresh portions of sulphurous acid into sulphuric. On these
principles alone is it possible to oxygenate the sulphurous acid, by the nitrous acid
resuming and surrendering a dose of oxygen, in perpetual alternation.
It was MM. Clement and Desormes who first had the sagacity to trace these complicated
changes. They showed that nitrous acid gas and sulphurous acid gas mixed, react on each
other through the intervention of moisture; that there resulted thence a combination of sulphuric
acid, deutoxide of azote (nitrous gas), and water; that this crystalline compound
was instantly destroyed by more water, with the separation of the sulphuric acid in a
liquid state, and the disengagement of nitrous gas; that this gas re-constituted nitrous
acid at the expense of the atmospheric oxygen of the leaden chamber, and thus brought
matters to their primary condition. From this point, starting again, the particles of
sulphur in the sulphurous acid, through the agency of water, became fully oxygenated
by the nitrous acid, and fell down in heavy drops of sulphuric acid, while the nitrous
gas derived from the nitrous acid, had again recourse to the air for its lost dose of
oxygen. This beautiful interchange of the oxygenous principle was found to go on, in
their experiments, till either the sulphurous acid, or oxygen in the air, was exhausted.
They verified this proposition, with regard to what occurs in sulphuric acid chambers,
by mixing in a crystal globe the three substances, deutoxide of azote, sulphurous acid,
and atmospheric air. The immediate production of red vapours indicated the transformation
of the deutoxide into nitrous acid gas; and now the introduction of a very
little water caused the proper reaction, for opaque vapours rose, which deposited
white star-form crystals on the surface of the glass. The gases were once more transparent
and colourless; but another addition of water melted these crystals with effervescence,
when ruddy vapours appeared. In this manner the phenomena were
made to alternate, till the oxygen of the included air was expended, or all the sulphurous
acid was converted into sulphuric. The residuary gases were found to be nitrous acid
gas, and azote, without sulphurous acid gas; while unctuous sulphuric acid bedewed the
inner surface of the globe. Hence, they justly concluded their new theory of the
manufacture of oil of vitriol to be demonstrated.
In consequence of their discovery, the manufacture of this acid has received such[1220]
improvements, that a nearly double product of it may now be obtained from the same
weight of materials. Indeed, the economy may be reckoned to be much greater; for one
half of the more costly ingredient, the nitre, formerly employed with a given weight of
sulphur, suffices at present.
In the manufacture of sulphuric acid upon the great scale, two different systems of
working were long prevalent; the intermittent or periodical, and the continuous or
uniform. Both were carried on in large leaden chambers. In the former, the chambers
were closed during the period of combustion and gaseous combination, but were
opened from time to time to introduce fresh atmospheric air. This method is, I believe,
generally abandoned now, on account of the difficulties and delays attending it, though
it afforded large products in skilful hands. In the latter, a continuous current of air is
allowed to enter at the oven in front of the chamber for the combustion of the sulphur,
and there is a constant escape of nitrogen gas, with a little sulphurous acid gas, at the
remote end of the roof.
Fig. 1101. represents a sulphuric acid chamber, a, a, are the brick or stone pillars upon
which it rests; b, b, are the sustaining wooden beams or joists; c, is the chimney for the
discharge of the nitrogen; d, is the roof, and e, the sole of the hearth for the combustion
of the sulphur; f, is the cylindrical tunnel, or pipe of lead or cast iron, for conducting
the gasiform materials into the chamber; g, is the steam-boiler; and h, the steam-pipe.
That plan is variously modified, by different oil-of-vitriol makers in this country
and in France. Very frequently, the oven e, d, is not situated under the chamber,
but is built at the end of it, as at i, and arched over with brick, the crown being
9 inches thick. The pipe f, 18 inches in diameter, is then placed outside of the chamber,
being inserted into a brick chimney, and, turning rectangularly, enters it opposite k.
The sole of the hearth e, is a thick plate of cast iron (not hollowed as shown in the
figure), 5 or 6 feet long, and 3 or 4 broad, with a small fireplace constructed beneath
it, whose smoke-flue runs outwards, under the floor, to the side wall of the building.
The oven is in this case about 2 feet in height, from the sole to the roof; and it has
an iron door, about 12 inches by 15, which slides up and down in a tightly-fitted iron
frame. This
door is frequently
placed in the
side of the oven,
parallel to the
long side of the
leaden chamber.
A stout collar
of lead is bolted
to the chamber,
where the pipe
enters it. At
the middle of
the side of the
chamber, about
2 feet above the
ground, a leaden
trough is fixed,
which serves as
a syphon-funnel and water-trap for introducing water to the acid gases.
Several manufacturers divide the chamber into a series of rectangular compartments,
by parallel leaden screens, 10 or 12 feet asunder, and allow these compartments to communicate
by a narrow opening, or a hole 1 foot square, in the top and bottom of each
screen alternately. Thus the fumes, which enter from the chimney-pipe over k, will be
forced, by the screen at b, to descend to 1, and pass through the opening there, to get into
the second compartment, whence they will escape near the top at 2, thus circulating up
and down, so as to occasion a complete agitation and intermixture of their heterogeneous
particles. Into the side of the chamber, opposite to the centre of each compartment,
a lead pipe enters, and proceeds towards the middle of the area, terminating
in a narrow orifice, for discharging a jet of high-pressure steam from a boiler loaded
with 40 pounds upon the square inch. This boiler should be placed under a shed
exterior to the building. It deserves to be noted, that the incessant tremors produced
in this pipe by the escape of the steam, cause the orifice to contract, and eventually to
close almost entirely, just as the point of a glass tube does when exposed directly to the
flame of a blowpipe. Provision should therefore be made against this event, by the
chemical engineer.
Equidistant between the middle point and each end of the chamber, two round holes
are cut out in its side, about 16 inches in diameter, and 2 feet from the floor; the sheet[1221]
lead being folded back over the face of the strong deals which strengthen the chamber
in that place. The edges of the holes are bevelled outwards, so as to fit a large conical
plug of wood faced with lead, called a man-hole door. One or other of these doors is
opened from time to time, to allow the superintendent to inspect the process, or workmen
to enter, after the chamber is well ventilated, for the purpose of making repairs.
The joists or tie-beams, that bind the rafters of the roof of both the leaden chamber and
the house, must be at least 7 inches deep, by 3 broad, and of such length as to have
their ends supported upon the outer wall, or the columnar supports of the roof, in case
a number of chambers are enclosed together in parallel ranges under a vast shed. These
beams, which lie two feet apart, suspend the leaden roof, by means of leaden straps,
soldered to its upper surface and edges. The sides of the chamber are sustained by
means of similar leaden straps affixed to the wooden posts (uprights), 4 inches broad by 3
thick, placed two or three feet apart along the sides of the chamber; resting on the ground
below, and mortised into the tie-beams above. Some chambers rest upon a sand-floor;
but they are preferably placed upon wooden joists, supported by pillars stretching over
an open area, as shown in the figure, into which the workmen may descend readily,
to examine the bottom.
The outlet c, on the top of the chamber, is sometimes joined to a long pipe of lead
laid nearly horizontally, with a slight inclination upwards, along the roof, for favouring
the condensation and return of acid matter.
At the extremity l, of the chamber, which, having a downward slope of 1 inch in
every 20 feet, should stand from 3 to 6 inches (according to its length) lower than i,
one leg of an inverted syphon pipe is fixed by fusion, into which the liquid of the chamber
passing, will show by its altitude the depth on the bottom within. From the cup-shaped
orifice of that bent-up pipe, the acid of the chamber is drawn off by an ordinary
leaden syphon into the concentration pans.
The sheet lead of which the sides and top are made, should weigh from 5 to 6 pounds
per square foot; that of the bottom should be nearly of double thickness.
Having now detailed, with sufficient minuteness, the construction of the chamber, I
shall next describe the mode of operating with it. There are at least two plans at present
in use for burning the sulphur continuously in the oven. In the one, the sulphur is laid
on the hearth e, (or rather on the flat hearth in the separate oven, above described,) and is
kindled by a slight fire placed under it; which fire, however, is allowed to go out after
the first day, because the oven becomes by that time sufficiently heated by the sulphur
flames to carry on the subsequent combustion. Upon the hearth, an iron tripod is set,
supporting, a few inches above it, a hemispherical cast-iron bowl (basin) charged with
nitre and its decomposing proportion of strong sulphuric acid. In the other plan, 12 parts
of bruised sulphur, and 1 of nitre, are mixed in a leaden trough on the floor with 1 of
strong sulphuric acid, and the mixture is shovelled through the sliding iron door upon
the hot hearth. The successive charges of sulphur are proportioned, of course, to the
size of the chamber. In one of the largest, which is 120 feet long, 20 broad, and 16
high, 12 cwt. are burned in the course of 24 hours, divided into 6 charges, every fourth
hour, of 2 cwt. each. In chambers of one-sixth greater capacity, containing 1400
metres cube, 1 ton of sulphur is burned in 24 hours. This immense production was
first introduced at Chaunay and Dieuze, under the management of M. Clement-Desormes.
The bottom of the chamber should be covered at first with a thin stratum of sulphuric
acid, of spec. grav. 1·07, which decomposes nitrous acid into oxygen and nitrous gas;
but not with mere water, which would absorb the nitrous acid vapours, and withdraw
them from their aerial sphere of action. The vapour of nitric acid, disengaged from
the nitre on the hearth of the oven, when brought into intimate contact with the
sulphurous acid, either gives up oxygen to it, becomes itself nitrous gas, and converts
it into sulphuric acid; or combines with the sulphurous acid into the crystalline
compound above described, which, the moment it meets with moisture, is decomposed
into sulphuric acid and nitrous gas. The atmospherical oxygen of the chamber immediately
reconverts this gas into nitrous or nitric acid fumes, which are again ready, with
the co-operation of sulphurous acid gas and aqueous vapour, to produce fresh quantities
of hydrous sulphuric acid (oil of vitriol) and nitrous gas. At low temperatures, this
curious play of chemical affinities has a great tendency to form the crystalline compound,
and to deposit it in a crust of considerable thickness (from one-half to one inch) on the
sides of the chamber, so as to render the process inoperative. A circumstance of this
kind occurred, in a very striking manner, during winter, in a manufacture of oil of
vitriol in Russia; and it has sometimes occurred, to a moderate extent, in Scotland. It
is called, at Marseilles, the maladie des chambres. It may be certainly prevented, by
maintaining the interior of the chamber, by a jet of steam, at a temperature of 100° F.
When these crystals fall into the dilute acid at the bottom, they are decomposed with a
violent effervescence, and a hissing gurgling noise, somewhat like that of a tun of beer
in brisk fermentation.
[1222]
M. Clement-Desormes demonstrated the proposition relative to the influence of temperature
by a decisive experiment. He took a glass globe, furnished with three tubulures,
and put a bit of ice into it. Through the first opening he then introduced sulphurous
acid gas; through the second, oxygen; and through the third, nitrous gas (deutoxide of
azote). While the globe was kept cool, by being plunged in iced water, no sulphuric
acid was formed, though all the ingredients essential to its production were present.
But on exposing the globe to a temperature of 100° Fahr., the four bodies began immediately
to react on each other, and oil of vitriol was condensed in visible striæ.
The introduction of steam is a modern invention, which has vastly facilitated and
increased the production of oil of vitriol. It serves, by powerful agitation, not only
to mix the different gaseous molecules intimately together, but to impel them against
each other, and thus bring them within the sphere of their mutual chemical attraction.
This is its mechanical effect. Its chemical agency is still more important. By supplying
moisture at every point of the immense included space, it determines the formation of
hydrous sulphuric acid, from the compound of nitric, nitrous, sulphurous, and dry sulphuric
acids. No sooner is this reaction accomplished, than the nitrous gas resumes its
oxygen, from the continuous atmospherical current, and becomes again fit to operate a
like round of transmutations with sulphurous acid, steam, and oxygen. The nitrogen
(azote), which ought to be the only residuum in a perfectly regulated vitriol chamber,
escapes, by its relative lightness, at the opening c, in the roof, or, more properly speaking,
is displaced by the influx of the heavier gases at the entrance-pipe.
On the intermittent plan, after the consumption of each charge, and condensation of
the product, the chamber was opened, and freely ventilated, so as to expel the residuary
azote, and replenish it with fresh atmospheric air. In this system there were four
distinct stages or periods:—1. Combustion for two hours; 2. Admission of steam,
and settling, for an hour and a half; 3. Conversion, for three hours, during which
interval the drops of strong acid were heard falling like heavy hailstones on the bottom;
4. Purging of the chamber, for three quarters of an hour.
By the continuous method, sulphuric acid may be currently obtained in the chambers,
of the specific gravity 1·350, or 1·450 at most; for, when stronger, it absorbs and
retains permanently much nitrous acid gas; but by the intermittent, so dense as 1·550,
or even 1·620; whence in a district where fuel is high priced, as near Paris, this
method recommended itself by economy in the concentration of the acid. In Great
Britain, and even in most parts of France, however, where time, workmen’s wages, and
interest of capital, are the paramount considerations, manufacturers do not find it for
their interest in general to raise the density of the acid in the chambers above 1·400, or
at most 1·500; as the further increase goes on at a retarded rate, and its concentration
from 1·400 to 1·600, in leaden pans, costs very little.
At about the specific gravity of 1·35, in Great Britain, the liquid of the chambers is run
off, by the syphon above described, into a leaden gutter or spout, which discharges it into
a series of rectangular vessels made of large sheets of lead, of 12 or 14 lbs. to the square
foot, simply folded up at the angles into pans 8 or 10 inches deep, resting upon a grate
made of a pretty close row of wrought-iron bars of considerable strength, under which
the flame of a furnace plays. Where coals are very cheap, each pan may have a separate
fire; but where they are somewhat dear, the flame, after passing under the lowest
pan of the range, which contains the strongest acid (at about 1·600), proceeds upwards
with a slight slope to heat the pans of weaker acid, which, as it concentrates, is gradually
run down by syphons to replenish the lower pans, in proportion as their aqueous
matter is dissipated. The 3 or 4 pans constituting the range are thus placed in a
straight line, but each at a different level, terrace-like; en gradins, as the French say.
When the acid has thereby acquired the density of 1·650, or 1·700 at most, it must be
removed from the leaden evaporators, because, when of greater strength, it would begin to
corrode them; and it is transferred into leaden coolers, or run through a long refrigeratory
worm-pipe surrounded by cold water. In this state it is introduced into glass or
platinum retorts, to undergo a final concentration, up to the specific gravity of 1·842, or
even occasionally 1·845, in consequence of slight saline impurities. When glass retorts
are used, they are set in a long sand-bath over a gallery furnace, resting on fire tiles,
under which a powerful flame plays; and as the flue gradually ascends from the fireplace,
near to which it is most distant from the tiles; to the remoter end, the heat acts
with tolerable equality on the first and last retort in the range. When platinum stills
are employed, they are fitted into the inside of cast-iron pots, which protect the thin
bottom and sides of the precious metal. The fire being applied directly to the iron,
causes a safe, rapid, and economical concentration of the acid. The iron pots,
with their platinum interior, filled with concentrated boiling-hot oil of vitriol, are
lifted out of the fire-seat by tackle, and let down into a cistern of cold water, to effect
the speedy refrigeration of the acid, and facilitate its transvasion into carboys packed in
osier baskets lined with straw. Sometimes, however, the acid is cooled by running it[1223]
slowly off through a long platinum syphon, surrounded by another pipe filled with cold
water. Fig. 1102. shows my contrivance for this purpose.
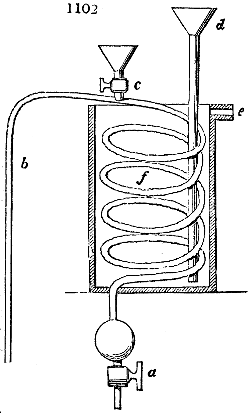
The under stopcock a, being shut, and the leg
b, being plunged to nearly the bottom of the still,
the worm is to be filled with concentrated cold
acid through the funnel c. If that stopcock is
now shut, and a opened, the acid will flow out in
such quantity as to rarefy the small portion of air
in the upper part of the pipe b, sufficiently to make
the hot acid rise up over the bend, and set the
syphon in action. The flow of the fluid is to be so
regulated by the stopcock a, that it may be greatly
cooled in its passage by the surrounding cold
water in the vessel f, which may be replenished by
means of the tube and funnel d, and overflow at e.
A manufacturer of acid in Scotland, who burns in
each chamber 210 pounds of sulphur in 24 hours,
being at the rate of 420 pounds for 20,000 cubic feet
(= nearly 2000 metres cube) has a product of nearly
3 pounds of concentrated oil of vitriol for every
pound of sulphur and twelfth of a pound of nitre.
The advantage of his process results, I conceive,
from the lower concentration of the acid in the
chambers, which favours its more rapid production.
The platinum retort admits of from 4 to 6
operations in a day, when it is well mounted and
managed. It has a capital of platinum, furnished
with a short neck, which conducts the disengaged vapours into a lead worm of condensation;
and the liquid thus obtained is returned into the lead pans. Great care
must be taken to prevent any particles of lead from getting into the platinum vessel,
since at the temperature of boiling sulphuric acid, the lead unites with the precious
metal, and thus causes holes in the retort. These must be repaired by soldering-on
a plate of platinum with gold.
Before the separate oven or hearth for burning the sulphur in contact with the nitre
was adopted, this combustible mixture was introduced into the chamber itself, spread on
iron trays or earthen pans, supported above the water on iron stands. But this plan was
very laborious and unproductive. It is no longer followed.
One of the characters of the good quality of sulphuric acid, is its dissolving indigo
without altering its fine blue colour.
Sulphuric acid, when well prepared, is a colourless and inodorous liquid, of an oily
aspect, possessing a specific gravity, in its most concentrated state, of 1·842, when redistilled,
but as found in commerce, of 1·845. It is eminently acid and corrosive, so
that a single drop will communicate the power of reddening litmus to a gallon of water,
and will produce an ulcer of the skin when allowed to remain upon it. If swallowed
in its strongest state, in even a small quantity, it acts so furiously on the throat and
stomach as to cause intolerable agony and speedy death. Watery diluents, mixed with
chalk or magnesia, are the readiest antidotes. At a temperature of about 600° F., or
a few degrees below the melting point of lead, it boils and distils over like water. This
is the best method of procuring sulphuric acid free from the saline and metallic matters
with which it is sometimes contaminated.
The affinity of sulphuric acid for water is so strong that, when exposed in an open
saucer, it imbibes one-third of its weight from the atmosphere in 24 hours, and fully
six times its weight in a few months. Hence it should be kept excluded from the air.
If four parts, by weight, of the strongest acid be suddenly mixed with one part of water,
both being at 50° F., the temperature of the mixture will rise to 300°; while, on the other
hand, if four parts of ice be mixed with one of sulphuric acid, they immediately liquefy
and sink the thermometer to 4° below zero. From the great attraction existing between
this acid and water, a saucer of it is employed to effect the rapid condensation of
aqueous vapour as it exhales from a cup of water placed over it; both standing under
the exhausted receiver of an air-pump. By the cold produced by this unchecked
evaporation in vacuo, the water is speedily frozen.
To determine the purity of sulphuric acid, let it be slowly heated to the boiling point
of water, and if any volatile acid matter be present, it will evaporate, with its characteristic
smell. The presence of saline impurity, which is the common one, is discovered
by evaporating a given weight of it in a small capsule of platinum placed on red-hot
cinders. If more than two grains remain out of 500, the acid may be reckoned to be[1224]
impure. The best test for sulphuric acid, and the soluble salts into which it enters, is
the nitrate of baryta, of which 182 parts are equivalent to 49 of the strongest liquid
acid, or to 40 of the dry, as it exists in crystallized sulphate of potassa. One twenty
thousandth part of a grain of the acid may be detected by the grayish-white cloud which
baryta forms with it. 100 parts of the concentrated acid are neutralized by 143 parts
of dry carbonate of potassa, and by 110 of dry carbonate of soda, both perfectly pure.
Of all the acids, the sulphuric is most extensively used in the arts, and is, in fact, the
primary agent for obtaining almost all the others, by disengaging them from their saline
combinations. In this way, nitric, muriatic, tartaric, acetic, and many other acids, are
procured. It is employed in the direct formation of alum, of the sulphates of copper,
zinc, potassa, soda; in that of sulphuric ether, of sugar by the saccharification of starch,
and in the preparation of phosphorus, &c. It serves also for opening the pores of skins
in tanning, for clearing the surfaces of metals, for determining the nature of several salts
by the acid characters that are disengaged, &c.
According to the analysis of Dr. Thomson, the crystalline compound deposited
occasionally in the leaden chambers above described consists of—
| Sulphurous acid |
0·6387, |
or |
3 |
atoms. |
| Sulphuric acid |
0·5290, |
|
2 |
|
| Nitric acid |
0·3450, |
1 |
atom. |
| Water |
0·0733, |
1 |
| Sulphate of lead |
0·0140. |
|
He admits that the proportion of water is a little uncertain; and that the presence of
sulphurous acid was not proved by direct analysis. When heated with water, the
crystalline matter disengages nitrous gas in abundance; lets fall some sulphate of lead;
and the liquid is found to be sulphuric acid. When heated without water, it is decomposed
with emission of nitrous gas and fuming nitric acid; leaving a liquid which,
mixed with water, produces a brisk effervescence, consisting chiefly of nitrous gas.
The following Table shows the quantity of concentrated and dry sulphuric acid in
100 parts of dilute, at different densities, by my experiments, published in the Quarterly
Journal of Science, for October, 1817:—
| Liquid. |
Sp. grav. |
Dry. |
| 100 |
1 |
·8460 |
81 |
·54 |
| 99 |
1 |
·8438 |
80 |
·72 |
| 98 |
1 |
·8415 |
79 |
·90 |
| 97 |
1 |
·8391 |
79 |
·09 |
| 96 |
1 |
·8366 |
78 |
·28 |
| 95 |
1 |
·8340 |
77 |
·46 |
| 94 |
1 |
·8288 |
76 |
·65 |
| 93 |
1 |
·8235 |
75 |
·83 |
| 92 |
1 |
·8181 |
75 |
·02 |
| 91 |
1 |
·8026 |
74 |
·20 |
| 90 |
1 |
·8070 |
73 |
·39 |
| 89 |
1 |
·7986 |
72 |
·57 |
| 88 |
1 |
·7901 |
71 |
·75 |
| 87 |
1 |
·7815 |
70 |
·94 |
| 86 |
1 |
·7728 |
70 |
·12 |
| 85 |
1 |
·7640 |
69 |
·31 |
| 84 |
1 |
·7540 |
68 |
·49 |
| 83 |
1 |
·7425 |
67 |
·68 |
| 82 |
1 |
·7315 |
66 |
·86 |
| 81 |
1 |
·7200 |
66 |
·05 |
| 80 |
1 |
·7080 |
65 |
·23 |
| 79 |
1 |
·6972 |
64 |
·42 |
| 78 |
1 |
·6860 |
63 |
·60 |
| 77 |
1 |
·6744 |
62 |
·78 |
| 76 |
1 |
·6624 |
61 |
·97 |
| 75 |
1 |
·6500 |
61 |
·15 |
| 74 |
1 |
·6415 |
60 |
·34 |
| 73 |
1 |
·6321 |
59 |
·52 |
| 72 |
1 |
·6204 |
58 |
·71 |
| 71 |
1 |
·6090 |
57 |
·89 |
| 70 |
1 |
·5975 |
57 |
·08 |
| 69 |
1 |
·5868 |
56 |
·26 |
| 68 |
1 |
·5760 |
55 |
·45 |
| 67 |
1 |
·5648 |
54 |
·63 |
| 66 |
1 |
·5503 |
53 |
·82 |
| 65 |
1 |
·5390 |
53 |
·00 |
| 64 |
1 |
·5280 |
52 |
·18 |
| 63 |
1 |
·5170 |
51 |
·37 |
| 62 |
1 |
·5066 |
50 |
·55 |
| 61 |
1 |
·4960 |
49 |
·74 |
| 60 |
1 |
·4860 |
48 |
·92 |
| 59 |
1 |
·4760 |
48 |
·11 |
| 58 |
1 |
·4660 |
47 |
·29 |
| 57 |
1 |
·4560 |
46 |
·48 |
| 56 |
1 |
·4460 |
45 |
·66 |
| 55 |
1 |
·4360 |
44 |
·85 |
| 54 |
1 |
·4265 |
44 |
·03 |
| 53 |
1 |
·4170 |
43 |
·22 |
| 52 |
1 |
·4073 |
42 |
·40 |
| 51 |
1 |
·3977 |
41 |
·58 |
| 50 |
1 |
·3884 |
40 |
·77 |
| 49 |
1 |
·3788 |
39 |
·95 |
| 48 |
1 |
·3697 |
39 |
·14 |
| 47 |
1 |
·3612 |
38 |
·32 |
| 46 |
1 |
·3530 |
37 |
·51 |
| 45 |
1 |
·3440 |
36 |
·69 |
| 44 |
1 |
·3345 |
35 |
·88 |
| 43 |
1 |
·3255 |
35 |
·06 |
| 42 |
1 |
·3165 |
34 |
·25 |
| 41 |
1 |
·3080 |
33 |
·43 |
| 40 |
1 |
·2999 |
32 |
·61 |
| 39 |
1 |
·2913 |
31 |
·80 |
| 38 |
1 |
·2826 |
30 |
·98 |
| 37 |
1 |
·2740 |
30 |
·17 |
| 36 |
1 |
·2654 |
29 |
·35 |
| 35 |
1 |
·2572 |
28 |
·54 |
| 34 |
1 |
·2490 |
27 |
·72 |
| 33 |
1 |
·2409 |
26 |
·91 |
| 32 |
1 |
·2334 |
26 |
·09 |
| 31 |
1 |
·2260 |
25 |
·28 |
| 30 |
1 |
·2184 |
24 |
·46 |
| 29 |
1 |
·2108 |
23 |
·65 |
| 28 |
1 |
·2032 |
22 |
·83 |
| 27 |
1 |
·1956 |
22 |
·01 |
| 26 |
1 |
·1876 |
21 |
·20 |
| 25 |
1 |
·1792 |
20 |
·38 |
| 24 |
1 |
·1706 |
19 |
·57 |
| 23 |
1 |
·1626 |
18 |
·75 |
| 22 |
1 |
·1549 |
17 |
·94 |
| 21 |
1 |
·1480 |
17 |
·12 |
| 20 |
1 |
·1410 |
16 |
·31 |
| 19 |
1 |
·1330 |
15 |
·49 |
| 18 |
1 |
·1246 |
14 |
·68 |
| 17 |
1 |
·1165 |
13 |
·86 |
| 16 |
1 |
·1090 |
13 |
·05 |
| 15 |
1 |
·1019 |
12 |
·23 |
| 14 |
1 |
·0953 |
11 |
·41 |
| 13 |
1 |
·0887 |
10 |
·60 |
| 12 |
1 |
·0809 |
9 |
·78 |
| 11 |
1 |
·0743 |
8 |
·97 |
| 10 |
1 |
·0682 |
8 |
·15 |
| 9 |
1 |
·0614 |
7 |
·34 |
| 8 |
1 |
·0544 |
6 |
·52 |
| 7 |
1 |
·0477 |
5 |
·71 |
| 6 |
1 |
·0405 |
4 |
·89 |
| 5 |
1 |
·0336 |
4 |
·08 |
| 4 |
1 |
·0268 |
3 |
·26 |
| 3 |
1 |
·0206 |
2 |
·446 |
| 2 |
1 |
·0140 |
1 |
·63 |
| 1 |
1 |
·0074 |
0 |
·8154 |
[1225]
SUMACH (Eng. and Fr.; Schmack, Germ.); is the powder of the leaves, peduncles,
and young branches of the Rhus coriaria, and Rhus cotinus, shrubs which grow in Hungary,
the Bannat, and the Illyrian provinces. Both kinds contain tannin, with a little yellow
colouring-matter, and are a good deal employed for tanning light-coloured leathers;
but the first is the best. With mordants, it dyes nearly the same colours as galls. In
calico-printing, sumach affords, with a mordant of tin, a yellow colour; with acetate of
iron, weak or strong, a gray or black; and with sulphate of zinc, a brownish-yellow. A
decoction of sumach reddens litmus paper strongly; gives white flocks with the protomuriate
of tin; pale-yellow flocks with alum; blue flocks with red sulphate of iron,
with an abundant precipitate. In the south of France, the twigs and leaves of the
Coriaria myrthifolia are used for dyeing, under the name of rédoul, or rodou.
SWEEP-WASHER, is the person who extracts from the sweepings, potsherds, &c.,
of refineries of silver and gold, the small residuum of precious metal.
SYNTHESIS, is a Greek word, which signifies combination, and is applied to the
chemical action which unites dissimilar bodies into a uniform compound; as sulphuric
acid and lime, into gypsum; or chlorine and sodium, into culinary salt.
SYRUP, is a solution of sugar in water. Cane-juice, concentrated to a density of
1·300, forms a syrup which does not ferment in the transport home from the West
Indies, and may be boiled and refined at one step into superior sugar-loaves, with eminent
advantage to the planter, the refiner, and the revenue.
TABBYING, or WATERING, is the process of giving stuffs a wavy appearance
with the calender.
TACAMAHAC, is a resin obtained from the Fagura octandra, a tree which grows
in Mexico and the West Indies. It occurs in yellowish pieces, of a strong smell, and
a bitterish aromatic taste. That from the island of Madagascar has a greenish tint.
TAFFETY, is a light silk fabric, with a considerable lustre or gloss.
TAFIA, is a variety of rum.
TALC, is a mineral genus, which is divided into two species, the common and the
indurated. The first occurs massive, disseminated in plates, imitative, or crystallized in
small six-sided tables. It is splendent, pearly, or semi-metallic, translucent, flexible,
but not elastic. It yields to the nail; spec. gray. 2·77. Before the blowpipe, it first
whitens, and then fuses into an enamel globule. It consists of—silica, 62; magnesia, 27;
alumina, 1·5; oxide of iron, 3·5; water, 6. Klaproth found 21⁄2 per cent. of potash in it.
It is found in beds of clay-slate and mica-slate, in Aberdeenshire, Banffshire, Perthshire,
Salzburg, the Tyrol, and St. Gothard. It is an ingredient in rouge for the toilette,
communicating softness to the skin. It gives the flesh polish to soft alabaster
figures, and is also used in porcelain paste.
The second species, or talc-slate, has a greenish-gray colour; is massive, with tabular
fragments, translucent on the edges, soft, with a white streak; easily cut or broken, but
is not flexible; and has a greasy feel. It occurs in the same localities as the preceding.
It is employed in the porcelain and crayon manufactures; as also as a crayon itself, by
carpenters, tailors, and glaziers.
TALLOW (Suif, Fr.; Talg, Germ.); is the concrete fat of quadrupeds and man.
That of the ox consists of 76 parts of stearine, and 24 of oleine; that of the sheep contains
somewhat more stearine. See Fat and Stearine.
Tallow imported into the United Kingdom, in 1836, 1,186,364 cwts. 1 qr. 4 lbs.;
in 1837, 1,308,734 cwts. 1 qr. 4 lbs. Retained for home consumption, in 1836,
1,318,678 cwts. 1 qr. 25 lbs.; in 1837, 1,294,009 cwts. 2 qrs. 21 lbs. Duty received, in
1836, £208,284; in 1837, £204,377.
TALLOW, PINEY. See Piney Tallow.
TAMPING, is a term used by miners to express the filling up of the hole which
they have bored in a rock, for the purpose of blasting it with gunpowder. See Mines.
TAN, or TANNIC ACID. (Tannin, Fr.; Gerbstoff, Germ.) See its preparation
and properties described under Galls.
The barks replete with this principle should be stripped with hatchets and bills, from
the trunk and branches of trees, not less than 30 years of age, in spring, when their sap
flows most freely. Trees are also sometimes barked in autumn, and left standing, whereby
they cease to vegetate, and perish ere long; but afford, it is thought, a more compact
timber. This operation is, however, too troublesome to be generally practised, and
therefore the bark is commonly obtained from felled trees; and it is richer in tannin
the older they are. The bark mill is described in Gregory’s Mechanics, and other similar
works.
[1226]
The following Table shows the quantity of extractive matter and tan in 100 parts of
the several substances:—
| Substances. |
In 480, by
Davy. |
In about
8 oz., by
Biggins. |
In 100 parts,
by Cadet de
Gassincourt. |
| White inner bark of old |
72 |
|
21 |
| Do. young oak |
77 |
|
|
| Do. Spanish chestnut |
63 |
30 |
|
| Do. Leicester willow |
79 |
|
|
| Coloured or middle bark of oak |
19 |
|
|
| Do. Spanish chestnut |
14 |
|
|
| Do. Leicester willow |
16 |
|
|
| Entire bark of oak |
29 |
|
|
| Do. Spanish chestnut |
21 |
|
|
| Do. Leicester willow |
33 |
109 |
|
| Do. Elm |
13 |
28 |
|
| Do. Common willow |
11 |
boughs, 31 |
|
| Sicilian sumach |
78 |
158 |
|
| Malaga sumach |
79 |
|
|
| Souchong tea |
48 |
|
|
| Green tea |
41 |
|
|
| Bombay catechu |
261 |
|
|
| Bengal catechu |
231 |
|
|
| Nut-galls |
127 |
|
46 |
| Bark of oak, cut in winter |
— |
30 |
|
| Do. beech |
— |
31 |
|
| Do. Elder |
— |
41 |
|
| Do. Plum-tree |
— |
58 |
|
| Bark of the trunk of Willow |
— |
52 |
|
| Do. Sycamore |
— |
53 |
16 |
| Bark of Birch |
— |
54 |
|
| Bark of Cherry-tree |
— |
59 |
24 |
| Do. Sallow |
— |
59 |
|
| Do. Poplar |
— |
76 |
|
| Do. Hazel |
— |
79 |
|
| Do. Ash |
— |
82 |
|
| Do. trunk of Span. chestnut |
— |
98 |
|
| Do. Smooth oak |
— |
104 |
|
| Do. Oak, cut in spring |
— |
108 |
|
| Root of Tormentil |
— |
|
46 |
| Cornus sanguinea of Canada |
— |
|
44 |
| Bark of Alder |
— |
|
36 |
| Do. Apricot |
— |
|
32 |
| Do. Pomegranate |
— |
|
32 |
| Do. Cornish cherry-tree |
— |
|
19 |
| Do. Weeping willow |
— |
|
16 |
| Do. Bohemian olive |
— |
|
14 |
| Do. Tan shrub with myrtle leaves |
— |
|
13 |
| Do. Virginian sumach |
— |
|
10 |
| Do. Green oak |
— |
|
10 |
| Do. Service-tree |
— |
|
8 |
| Do. Rose chestnut of Amer. |
— |
|
8 |
| Do. Rose chestnut |
— |
|
6 |
| Do. Rose chestnut of Carolina |
— |
|
6 |
| Do. Sumach of Carolina |
— |
|
5 |
TANNING (Tanner, Fr.; Gärberei, Germ.); is the art of converting skin into
Leather, which see. It has been ascertained, beyond a doubt, that “the saturated
infusions of astringent barks contain much less extractive matter, in proportion to their
tannin, than the weak infusions; and when skin is quickly tanned (in the former),
common experience shows that it produces leather less durable than leather slowly
formed.”[66] The older tanners, who prided themselves on producing a substantial article,
were so much impressed with the advantages of slowly impregnating skin with astringent
matter, that they employed no concentrated infusion (ooze) in their pits, but
stratified the skins with abundance of ground bark, and covered them with soft water,
knowing that its active principles are very soluble, and that, by being gradually extracted,
they would penetrate uniformly the whole of the animal fibres, instead of acting
chiefly upon the surface, and making brittle leather, as the strong infusions never fail to
do. In fact, 100 pounds of skin, quickly tanned in a strong infusion of bark, produce
137 of leather; while 100 pounds, slowly tanned in a weak infusion, produce only 1171⁄2.
The additional 191⁄2 pounds weight in the former case serve merely to swell the tanner’s
bill, while they deteriorate his leather, and cause it to contain much less of
the textile animal solid. Leather thus highly charged with tannin, is, moreover, so
spongy as to allow moisture to pass readily through its pores, to the great discomfort
and danger of persons who wear shoes made of it. That the saving of time, and the
increase of product, are temptations strong enough to induce many modern tanners
to steep their skins in a succession of strong infusions of bark, is sufficiently intelligible;
but that any shoemaker should be so ignorant or so foolish as to proclaim that his
leather is made by a process so injurious to its quality, is unaccountably stupid.
TANTALUM, is the rare metal; also called Columbium.
TAPESTRY, is an ornamental figured textile fabric of worsted or silk, for lining the
walls of apartments; of which the most famous is that of the Gobelins Royal Manufactory,
near Paris.
TAPIOCA, is a modification of starch, partially converted into gum, by heating
and stirring cassava upon iron plates. See Cassava and Starch.
TAR (Goudron, Fr.; Ther, Germ.); is the viscid, brown-black, resino-oleaginous
compound, obtained by distilling wood in close vessels, or in ovens of a peculiar construction.
See Charcoal, Pitcoal, coking of, and Pyrolignous Acid. According to
Reichenbach, tar contains the peculiar proximate principles, paraffine, eupion, creosote,
picamar, pittacal, besides pyrogenous resin, or pyretine, pyrogenous oil, or pyroleine, and
vinegar. The resin, oil, and vinegar are called empyreumatic, in common language.
Tar imported into the United Kingdom, in 1836, 9,797 lsts. 8 brls.; in 1837,[1227]
11,480 lsts. 1 brl. Retained for home consumption, in 1836, 9,639 lsts. 8 brls.; in
1837, 11,686 lsts. 8 brls. Duty received, in 1836, £7,231; in 1837, £8,775.
TARTAR (Tartre, Fr.; Weinstein, Germ.); called also argal or argol; is the
crude bitartrate of potassa, which exists in the juice of the grape, and is deposited
from wines in their fermenting casks, being precipitated in proportion as the alcohol is
formed, in consequence of its insolubility in that liquid. There are two sorts of argal
known in commerce, the white, and the red; the former, which is of a pale-pinkish colour,
is the crust let fall by white wines; the latter is a dark-red, from red wines.
The crude tartar is purified, or converted into cream of tartar, at Montpellier, by
the following process:—
The argal having been ground under vertical mill-stones, and sifted, one part of it is
boiled with 15 of water, in conical copper kettles, tinned on the inside. As soon as it is
dissolved, 31⁄2 parts of ground pipe-clay are introduced. The solution being well stirred,
and then settled, is drawn off into crystallizing vessels, to cool; the crystals found concreted
on the sides and bottom are picked out, washed with water, and dried. The
mother-water is employed upon a fresh portion of argal. The crystals of the first crop
are re-dissolved, re-crystallized, and exposed upon stretched canvas to the sun and air,
to be bleached. The clay serves to abstract the colouring-matter. The crystals formed
upon the surface are the whitest, whence the name cream of tartar is derived.
Purified tartar, the bitartrate of potassa, is thus obtained in hard clusters of small colourless
crystals, which, examined by a lens, are seen to be transparent 4-sided prisms. It
has no smell, but a feebly acid taste; is unchangeable in the air, has a specific gravity
of 1·953, dissolves in 16 parts of boiling water, and in 200 parts at 60° F. It is insoluble
in alcohol. It consists of 24·956 potassa, 70·276 tartaric acid, and 4·768 water. It
affords, by dry distillation, pyrotartaric acid, and an empyreumatic oil; while carbonate
of potassa remains associated with much charcoal in the retort, constituting black flux.
Tartar is used in dyeing, medicine, and for extracting—
TARTARIC ACID. (Acide tartarique, Fr.; Weinsteinsäure, Germ.) This is prepared
by adding gradually to a boiling-hot solution of 100 parts of tartar, in a large
copper boiler, 26 of chalk, made into a smooth pap with water. A brisk effervescence ensues,
by the disengagement of the carbonic acid of the chalk, while its base combines
with the acid excess in the tartar, and forms an insoluble precipitate of tartrate of lime.
The supernatant liquor, which is a solution of neutral tartrate of potassa, must be
drawn off by a syphon, and decomposed by a solution of chloride of calcium (muriate
of lime). 281⁄2 parts of the dry chloride are sufficient for 100 of tartar. The tartrate
of lime, from both processes, is to be washed with water, drained, and then subjected, in
a leaden cistern, to the action of 49 parts of sulphuric acid, previously diluted with 8
times its weight of water: 100 of dry tartrate take 75 of oil of vitriol. This mixture,
after digestion for a few days, is converted into sulphate of lime and tartaric acid. The
latter is to be separated from the former by decantation, filtration through canvas, and
edulcoration of the sulphate of lime upon the filter.
The clear acid is to be concentrated in leaden pans, by a moderate heat, till it acquires
the density of 40° B. (spec. grav. 1·38), and then it is run off, clear from any sediment,
into leaden or stoneware vessels, which are set in a dry stove-room for it to crystallize.
The crystals, being re-dissolved and re-crystallized, become colourless 6-sided prisms.
In decomposing the tartrate of lime, a very slight excess of sulphuric acid must be employed;
because pure tartaric acid would dissolve any tartrate of lime that may escape decomposition.
Bone black, previously freed from its carbonate and phosphate of lime, by
muriatic acid, is sometimes employed to blanch the coloured solutions of the first crystals.
Tartaric acid contains nearly 9 per cent. of combined water. It is soluble in two parts
of water at 60°, and in its own weight of boiling water. In its dry state, as it exists in
the tartrate of lime or lead, it consists of 36·8 of carbon, 3 of hydrogen, and 60·2 of
oxygen. It is much employed in calico-printing, and for making sodaic powders.
TARTRATES, are salts composed of tartaric acid, and oxidized bases, in equivalent
proportions.
TAWING, is the process of preparing the white skins of the sheep doe, &c. See
Leather.
TEA, green, contains 34·6 parts of tannin, 5·9 of gum, 5·7 of vegetable albumine,
51·3 of ligneous fibre, with 2·5 of loss; and black tea contains 40·6 of tannin,
6·3 of gum, 6·4 of vegetable albumine, 44·8 of ligneous fibre, with 2 of loss. The ashes
contain silica, carbonate of lime, magnesia, and chloride of potassium.—Frank. Davy
obtained 32·5 of extract from Souchong tea; of which 10 were precipitated by gelatine.
He found 8·5 only of tannin in green tea. The latter chemist is most to be depended
upon. Chemical analysis has not yet discovered that principle in tea, to which its exciting
property is due.
[1228]
The Chinese method of making Black Tea in Upper Assam.[67]—In the first place, the
youngest and most tender leaves are gathered; but when there are many hands and a
great quantity of leaves to be collected, the people employed nip off with the forefinger
and thumb the fine end of the branch with about four leaves on, and sometimes even
more, if they look tender. These are all brought to the place where they are to be
converted into tea; they are then put into a large, circular, open-worked bamboo basket,
having a rim all round, two fingers broad. The leaves are thinly scattered in these
baskets, and then placed in a framework of bamboo, in all appearance like the side of an
Indian hut without grass, resting on posts, 2 feet from the ground, with an angle of
about 25°. The baskets with leaves are put in this frame to dry in the sun, and are
pushed up and brought down by a long bamboo with a circular piece of wood at the
end. The leaves are permitted to dry about two hours, being occasionally turned; but
the time required for this process depends on the heat of the sun. When they begin to
have a slightly withered appearance, they are taken down and brought into the house,
where they are placed on a frame to cool for half an hour. They are then put into
smaller baskets of the same kind as the former, and placed on a stand. People are now
employed to soften the leaves still more, by gently clapping them between their hands,
with their fingers and thumb extended, and tossing them up and letting them fall, for
about five or ten minutes. They are then again put on the frame during half an hour,
and brought down and clapped with the hands as before. This is done three successive
times, until the leaves become to the touch like soft leather; the beating and putting
away being said to give the tea the black colour and bitter flavour. After this the tea
is put into hot cast-iron pans, which are fixed in a circular mud fireplace, so that the
flame cannot ascend round the pan to incommode the operator. This pan is well heated
by a straw or bamboo fire to a certain degree. About two pounds of the leaves are then
put into each hot pan, and spread in such a manner that all the leaves may get the same
degree of heat. They are every now and then briskly turned with the naked hand, to
prevent a leaf from being burnt. When the leaves become inconveniently hot to the
hand, they are quickly taken out and delivered to another man with a close-worked
bamboo basket ready to receive them. A few leaves that may have been left behind are
smartly brushed out with a bamboo broom; all this time a brisk fire is kept up under
the pan. After the pan has been used in this manner three or four times, a bucket of
cold water is thrown in, and a soft brickbat and bamboo broom used, to give it a good
scouring out; the water is thrown out of the pan by the brush on one side, the pan
itself being never taken off. The leaves, all hot on the bamboo basket, are laid on a
table that has a narrow rim on its back, to prevent these baskets from slipping off when
pushed against it. The two pounds of hot leaves are now divided into two or three
parcels, and distributed to as many men, who stand up to the table with the leaves right
before them, and each placing his legs close together; the leaves are next collected into
a ball, which he gently grasps in his left hand, with the thumb extended, the fingers
close together, and the hand resting on the little finger. The right hand must be extended
in the same manner as the left, but with the palm turned downwards, resting on
the top of the ball of tea leaves. Both hands are now employed to roll and propel the
ball along; the left hand pushing it on, and allowing it to revolve as it moves; the right
hand also pushes it forward, resting on it with some force, and keeping it down to
express the juice which the leaves contain. The art lies here in giving the ball a circular
motion, and permitting it to turn under and in the hand two or three whole
revolutions, before the arms are extended to their full length, and drawing the ball of
leaves quickly back without leaving a leaf behind, being rolled for about five minutes in
this way. The ball of tea leaves is from time to time gently and delicately opened with
the fingers, lifted as high as the face, and then allowed to fall again. This is done two
or three times, to separate the leaves; and afterwards the basket with the leaves is lifted
up as often, and receives a circular shake to bring these towards the centre. The leaves
are now taken back to the hot pans, and spread out in them as before, being again
turned with the naked hand, and when hot taken out and rolled; after which they are
put into the drying basket, and spread on a sieve which is in the centre of the basket,
and the whole placed over a charcoal fire. The fire is very nicely regulated; there
must not be the least smoke, and the charcoal should be well picked.
When the fire is lighted, it is fanned until it gets a fine red glare, and the smoke is all
gone off; being every now and then stirred and the coals brought into the centre, so as
to leave the outer edge low. When the leaves are put into the drying basket, they are
gently separated by lifting them up with the fingers of both hands extended far apart,
and allowing them to fall down again; they are placed 3 or 4 inches deep on the sieve,
leaving a passage in the centre for the hot air to pass. Before it is put over the fire, the
drying basket receives a smart slap with both hands in the act of lifting it up, which is
done to shake down any leaves that might otherwise drop through the sieve, or to prevent[1229]
them from falling into the fire and occasioning a smoke, which would affect and
spoil the tea. This slap on the basket is invariably applied throughout the stages of the
tea manufacture. There is always a large basket underneath to receive the small leaves
that fall, which are afterwards collected, dried, and added to the other tea; in no case
are the baskets or sieves permitted to touch or remain on the ground, but always laid on
a receiver with three legs. After the leaves have been half dried in the drying basket,
and while they are still soft, they are taken off the fire and put into large open-worked
baskets, and then put on the shelf, in order that the tea may improve in colour.
Next day the leaves are all sorted into large, middling, and small; sometimes there
are four sorts. All these, the Chinese informed me, become so many different kinds of
teas; the smallest leaves they called Pha-ho, the second Pow-chong, the third Su-chong,
and the fourth, or the largest leaves, Toy-chong. After this assortment they are again
put on the sieve in the drying basket (taking great care not to mix the sorts), and on
the fire, as on the preceding day; but now very little more than will cover the bottom of
the sieve is put in at one time, the same care of the fire is taken as before, and the same
precaution of tapping the drying basket every now and then. The tea is taken off the
fire with the nicest care, for fear of any particle of the tea falling into it. Whenever
the drying basket is taken off, it is put on the receiver, the sieve in the drying basket
taken out, the tea turned over, the sieve replaced, the tap given, and the basket placed
again over the fire. As the tea becomes crisp, it is taken out and thrown into a large
receiving basket, until all the quantity on hand has become alike dried and crisp; from
which basket it is again removed into the drying basket, but now in much larger
quantities. It is then piled up eight and ten inches high on the sieve in the drying
basket; in the centre a small passage is left for the hot air to ascend; the fire that was
before bright and clear, has now ashes thrown on it to deaden its effect, and the shakings
that have been collected are put on the top of all; the tap is given, and the basket with
the greatest care is put over the fire. Another basket is placed over the whole, to throw
back any heat that may ascend. Now and then it is taken off, and put on the receiver;
the hands, with the fingers wide apart, are run down the sides of the basket to the sieve,
and the tea gently turned over, the passage in the centre again made, &c., and the basket
again placed on the fire. It is from time to time examined, and when the leaves have
become so crisp that they break by the slightest pressure of the fingers, it is taken off,
when the tea is ready. All the different kinds of leaves underwent the same operation.
The tea is now little by little put into boxes, and first pressed down with the hands and
then with the feet (clean stockings having been previously put on).
There is a small room inside of the tea-house, 7 cubits square and 5 high, having
bamboos laid across on the top to support a net work of bamboo, and the sides of
the room smeared with mud to exclude the air. When there is wet weather, and the
leaves cannot be dried in the sun, they are laid out on the top of this room, on the network,
on an iron pan, the same as is used to heat the leaves; some fire is put into it,
either of grass or bamboo, so that the flame may ascend high; the pan is put on a square
wooden frame, that has wooden rollers on its legs, and pushed round and round this
little room by one man, while another feeds the fire, the leaves on the top being
occasionally turned; when they are a little withered, the fire is taken away, and the
leaves brought down and manufactured into tea, in the same manner as if it had been
dried in the sun. But this is not a good plan, and never had recourse to, if it can possibly
be avoided.
Tea imported into the United Kingdom, in 1836, 49,307,701 lbs.; in 1837,
36,765,735 lbs. Retained for home consumption, in 1836, 49,841,507 lbs.; in 1837,
31,872 lbs. Duty received, in 1836, £4,728,600; in 1837, £3,319,665.
TEASEL, the head of the thistle (Dipsacus), is employed to raise the nap of cloth.
See Woollen Manufacture.
TELLURIUM, is a metal, too rare and high-priced to be used in the arts.
TERRA-COTTA, literally baked clay, is the name given to statues, architectural
decorations, figures, vases, &c., modelled or cast in a paste made of pipe or potter’s clay and
a fine-grained colourless sand, from Ryegate, with pulverized potsherds, slowly dried in the
air, and afterwards fired to a stony hardness in a proper kiln. See Stone, artificial.
TERRA DI SIENA, is a brown ferruginous ochre, employed in painting.
TESTS, are chemical reagents of any kind, which indicate, by special characters,
the nature of any substance, simple or compound. See Assay, the several metals,
acids, &c.
TEXTILE FABRICS. The first business of the weaver is to adapt those parts of
his loom which move the warp, to the formation of the various kinds of ornamental figures
which the cloth is intended to exhibit. This subject is called the draught, drawing or reading
in, and the cording of looms. In every species of weaving, whether direct or cross, the
whole difference of pattern or effect is produced, either by the succession in which the[1230]
threads of warp are introduced into the heddles, or by the succession in which those heddles
are moved in the working. The heddles being stretched between two shafts of wood, all
the heddles connected by the same shafts are called a leaf; and as the operation of introducing
the warp into any number of leaves is called drawing a warp, the plan of succession
is called the draught. When this operation has been performed correctly, the next
part of the weaver’s business is to connect the different leaves with the levers or treddles
by which they are to be moved, so that one or more may be raised or sunk by every
treddle successively, as may be required to produce the peculiar pattern. These connections
being made by coupling the different parts of the apparatus by cords, this operation
is called the cording. In order to direct the operator in this part of his business,
especially if previously unacquainted with the particular pattern upon which he is employed,
plans are drawn upon paper, specimens of which will be found in figs. 1103, 1104., &c.
These plans are horizontal sections of a loom, the
heddles being represented across the paper at a, and
the treddles under them, and crossing them at right
angles, at b. In figs. 1103. and 1104. they are represented
as if they were distinct pieces of wood,
those across being the under shaft of each leaf of
heddles, and those at the left hand the treddles.
See Weaving. In actual weaving, the treddles are
placed at right angles to the heddles, the sinking
cords descending perpendicularly as nearly as possible
to the centre of the latter. Placing them at the
left hand, therefore, is only for ready inspection, and
for practical convenience. At c a few threads of warp
are shown as they pass through the heddles, and the
thick lines denote the leaf with which each thread is connected. Thus, in fig. 1103., the
right-hand thread, next to a, passes through the eye of a heddle upon the back leaf, and is
disconnected with all the other leaves; the next thread passes through a heddle on the second
leaf; the third, through the third leaf; the fourth, through the fourth leaf; and the fifth,
through the fifth or front leaf. One set of the draught being now completed, the weaver
recommences with the back leaf, and proceeds in the same succession again to the front.
Two sets of the draught are represented in this figure, and the same succession, it is
understood by weavers (who seldom draw more than one set), must be repeated until all
the warp is included. When they proceed to apply the cords, the right-hand part of the
plan at b serves as a guide. In all the plans shown by these figures, excepting one which
shall be noticed, a connexion must be formed, by cording, between every leaf of heddles and
every treddle; for all the leaves must either rise or sink. The raising motion is effected
by coupling the leaf to one end of its correspondent top lever; the other end of this
lever is tied to the long march below, and this to the treddle. The sinking connexion
is carried directly from under the leaf to the treddle. To direct a weaver which of
these connexions is to be formed with each treddle, a black spot is placed when a leaf is
to be raised, where the leaf and treddle intersect each other upon the plan, and the
sinking connexions are left blank. For example, to cord, the treddle 1, to the back
leaf, put a raising cord, and to each of the other four, sinking cords; for the treddle 2,
raise the second leaf, and sink the remaining four, and so of the rest; the spot always
denoting the leaf or leaves to be raised. The figs. 1103. and 1104. are drawn for the
purpose of rendering the general principle of this kind of plans familiar to those who
have not been previously acquainted with them; but those who have been accustomed
to manufacture and weave ornamented cloths, never consume time by representing
either heddles or treddles as solid or distinct bodies. They content themselves with
ruling a number of lines across a piece of paper, sufficient to make the intervals between
these lines represent the number of leaves required. Upon these intervals, they merely
mark the succession of the draught, without producing every line to resemble a thread
of warp. At the left hand, they draw as many lines across the former as will afford an
interval for each treddle; and in the squares produced by the intersections of these lines,
they place the dots, spots, or ciphers which denote the raising cords. It is also common
to continue the cross lines which denote the treddle a considerable length beyond the
intersections, and to mark by dots, placed diagonally in the intervals, the order or
succession in which the treddles are to be pressed down in weaving. The former of
these modes has been adopted in the remaining figs. to 1112.; but to save room, the
latter has been avoided, and the succession marked by the order of the figures under the
intervals which denote the treddles.
Some explanation of the various kinds of fanciful cloths represented by these plans,
may serve further to illustrate this subject, which is, perhaps, the most important of any
connected with the manufacture of cloth, and will also enable a person who thoroughly
studies them, readily to acquire a competent knowledge of the other varieties in weaving,[1231]
which are boundless. Figs. 1103. and 1104. represent the draught and cording of the
two varieties of tweeled cloth wrought with five leaves of heddles. The first is the regular
or run tweel, which, as every leaf rises in regular succession, while the rest are
sunk, interweaves the warp and woof only at every fifth interval, and as the succession
is uniform, the cloth when woven, presents the appearance of parallel diagonal lines, at
an angle of about 45° over the whole surface. A tweel may have the regularity of its
diagonal lines broken by applying the cording as in fig. 1104. It will be observed, that
in both figures the draught of the warp is precisely the same, and that the whole difference
of the two plans consists in the order of placing the spots denoting the raising
cords, the first being regular and successive, and the second alternate.
Figs. 1105. and 1106. are the regular and broken tweels which may be produced with
eight leaves. This properly is the tweel denominated satin in the silk manufacture,
although many webs
of silk wrought with
only five leaves receive
that appellation.
Some of the
finest Florentine
silks are tweeled
with sixteen leaves.
When the broken tweel of eight leaves is used, the effect is much superior to what
could be produced by a smaller number; for in this, two leaves are passed in every interval,
which gives a much nearer resemblance to plain cloth than the others. For this
reason it is preferred in weaving the finest damasks. The draught of the eight-leaf
tweel differs in nothing from the others, excepting in the number of leaves. The difference
of the cording in the broken tweel, will appear by inspecting the cyphers which
mark the raising cords, and comparing them with those of the broken tweel of five
leaves. Fig. 1107. represents the draught and cording of striped dimity of a tweel of
five leaves. This is the most simple species of fanciful tweeling. It consists of ten
leaves, or double the number of the common tweel.
These ten leaves are moved by only five treddles, in
the same manner as a common tweel. The stripe is
formed by one set, of the leaves flushing the warp,
and the other set, the woof. The figure represents
a stripe formed by ten threads, alternately drawn
through each of the two sets of leaves. In this case,
the stripe and the intervals will be equally broad,
and what is the stripe upon one side of the cloth, will be the interval upon the other,
and vice versâ. But great variety of patterns may be introduced by drawing the warp
in greater or smaller portions through either set. The tweel is of the regular kind,
but may be broken by placing the cording as in fig. 1104. It will be observed that
the cording-marks of the lower or front leaves are exactly the converse of the other
set; for where a raising mark is placed upon one, it is marked for sinking in the other;
that is to say, the mark is omitted; and all leaves which sink in the one, are marked
for raising in the other: thus, one thread rises in succession in the back set, and
four sink; but in the front set, four rise, and only
one sinks. The woof, of course, passing over the
four sunk threads, and under the raised one, in the
first instance, is flushed above; but where the reverse
takes place, as in the second, it is flushed
below; and thus the appearance of a stripe is formed.
The analogy subsisting between striped dimity and
dornock, is so great, that before noticing the plan
for fancy dimity, it may be proper to allude to the dornock, the plan of which is represented
by fig. 1108.
The draught of dornock is precisely the same in every respect with that of striped
dimity. It also consists of two sets of tweeling-heddles, whether three, four, or five
leaves are used for each set. The right-hand set of treddles is also corded exactly in the
same way, as will appear by comparing them. But as the dimity is a continued stripe
from the beginning to the end of the web, only five treddles are required to move ten
leaves. The dornock being checker-work, the weaver must possess the power of reversing
this at pleasure. He therefore adds five more treddles, the cording of which is
exactly the reverse of the former; that is to say, the back leaves, in the former case,
having one leaf raised, and four sunk, have, by working with these additional treddles,
one leaf sunk and four leaves raised. The front leaves are in the same manner reversed,
and the mounting is complete. So long as the weaver continues to work with either
set, a stripe will be formed, as in the dimity; but when he changes his feet from one set[1232]
to the other, the whole effect is reversed, and the checkers formed. The dornock pattern
upon the design-paper, fig. 1108., may be thus explained: let every square of the
design represent five threads upon either set of the heddles, which are said by weavers
to be once over the draught, supposing the tweel to be one of five leaves; draw three
parallel lines, as under, to form two intervals, each representing one of the sets; the
draught will then be as follows:—
| |
4 |
|
1 |
|
4 |
|
1 |
|
1 |
|
4 |
|
1 |
|
| |
4 |
|
4 |
|
1 |
|
1 |
|
1 |
|
4 |
|
4 |
|
The above is exactly so much of the pattern as is there laid down, to show its appearance;
but one whole range of the pattern is completed by the figure 1, nearest to
the right hand upon the lower interval between the lines, and the remaining figures,
nearer to the right, form the beginning of a second range or set. These are to be repeated
in the same way across the whole warp. The lower interval represents the five
front leaves; the upper interval, the five back ones. The first figure 4, denotes that five
threads are to be successively drawn upon the back leaves, and this operation repeated
four times. The first figure 4, in the lower interval, expresses that the same is to be
done upon the front leaves; and each figure, by its diagonal position, shows how often,
and in what succession, five threads are to be drawn upon the leaves which the interval
in which it is placed represents.
Dornocks of more extensive patterns are sometimes woven with 3, 4, 5, and even 6
sets of leaves; but after the leaves exceed 15 in number, they both occupy an inconvenient
space, and are very unwieldy to work. For these reasons the diaper harness is
in almost every instance preferred.
Fig. 1109. represents the draught and cording of a fanciful species of dimity, in
which it will be observed that the warp is not drawn directly from the back
to the front leaf, as in the former examples; but
when it has arrived at either external leaf, the draught
is reversed, and returns gradually to the other. The
same draught is frequently used in tweeling, when it
is wished that the diagonal lines should appear upon
the cloth in a zigzag direction. This plan exhibits
the draught and cording which will produce the pattern
upon the design-paper in fig. 1103. a. Were all
the squares produced by the intersection of the lines denoting the leaves and treddles,
where the raised dots are placed, filled the same as on the design, they would produce the
effect of exactly one-fourth of that pattern. This is caused by the reversing of the
draught, which gives the other side reversed as on the design; and when all the treddles,
from 1 to 16, have been successively used in the working, one-half of the pattern will
become complete. The weaver then goes again over his treddles, in the reversed
order of the numbers, from 17 to 30, when the other half of the pattern will be completed.
From this similarity of the cording to the design, it is easy, when a design is
given, to make out the draught and cording proper to work it; and when the cording is
given, to see its effect upon the design.
Fig. 1110. represents the draught of the diaper mounting, and the cording of the front
leaves, which are moved by treddles. From the plan,
it will appear that 5 threads are included in every
mail of the harness, and that these are drawn in
single threads through the front leaves. The cording
forms an exception to the general rules, that when one
or more leaves are raised, all the rest must be sunk;
for in this instance, one leaf rises, one sinks, and three
remain stationary. An additional mark, therefore, is used in this plan. The dots, as
formerly, denote raising cords; the blanks, sinking cords; and where the cord is to be
totally omitted, the cross marks × are placed.
Fig. 1111. is the draught and cording of a spot whose two sides are similar, but reversed.
That upon the plan forms a diamond, similar to the one drawn upon the design
paper in the diagram, but smaller in size. The draught here is reversed, as in the
dimity plan, and the treading is also to be reversed, after arriving at 6, to complete the
diamond. Like it, too, the raising marks form one-fourth of the pattern. In weaving
spots, they are commonly placed at intervals, with a portion of plain cloth between
them, and in alternate rows, the spots of one row being between those of the
other. But as intervals of plain cloth must take place, both by the warp and woof, 2
leaves are added for that purpose. The front, or ground leaf, includes every second
thread of the whole warp; the second, or plain leaf, that part which forms the intervals[1233]
by the warp. The remaining leaves form the spots; the first six being allotted to one
row of spots, and the second six to the next row; where each spot is in the centre between
the former. The reversed draught of the first
is shown entire, and is succeeded by 12 threads of
plain. One-half of the draught of the next row is
then given, which is to be completed exactly like the
first, and succeeded by 12 threads more of plain;
when, one set of the pattern being finished, the same
succession is to be repeated over the whole warp.
As spots are formed by inserting woof of coarser
dimensions than that which forms the fabric, every second thread only is allotted for the
spotting. Those included in the front, or ground leaf, are represented by lines, and the
spot threads between them, by marks in the intervals, as in the other plans.
The treddles necessary to work this spot are, in number, 14. Of these, the two in the
centre, a, b, when pressed alternately, will produce plain cloth; for b raises the front leaf,
which includes half of the warp, and sinks all the rest; while a exactly reverses the operation.
The spot-treddles on the right hand work the
row contained in the first six spot-leaves; and those
upon the left hand, the row contained in the second
six. In working spots, one thread, or shot of spotting-woof,
and two of plain, are successively inserted, by
means of two separate shuttles.
Dissimilar spots, are those whose sides are quite
different from each other. The draught only of these is represented by fig. 1112.
The cording depends entirely upon the figure.
Fig. 1113. represents any solid body composed of parts lashed together. If the
darkened squares be supposed to be beams of wood, connected by cordage, they will
give a precise idea of textile fabric. The beams cannot come into actual contact,
because, if the lashing cords were as fine even as human hairs, they must still require
space. The thickness is that of one
beam and one cord; but if the cords
touch each other, it may then be one
beam and two cords; but it is not
possible in practical weaving to bring every thread of weft into actual contact. It
may therefore be assumed, that the thickness is equal to the diameter of one thread of
the warp, added to that of one yarn of the weft; and when these are equal, the thickness
of the cloth is double of that diameter.
Denser cloth would not be
sufficiently pliant or flexible.
Fig. 1114. is a representation of a
section of cloth of an open fabric, where the round dots which represent the warp
are placed at a considerable distance from each other.

Fig. 1115. may be supposed a plain fabric of that description which approaches the
most nearly to any idea we can form of the most dense or close contact of which yarn
can be made susceptible. Here the warp is supposed to be so tightly stretched in
the loom as to retain entirely the
parallel state, without any curvature,
and the whole flexure is therefore
given to the woof. This mode of
weaving can never really exist; but if the warp be sufficiently strong to bear any tight
stretching, and the woof be spun very soft and flexible, something very near it may be
produced. This way of making cloth is well fitted for those goods which require to
give considerable warmth; but they are sometimes the means of very gross fraud and
imposition; for if the warp is made of very slender threads, and the woof of slackly
twisted cotton or woollen yarn, where the fibrils of the stuff, being but slightly brought
into contact, are rough and oozy, a great appearance of thickness and strength may be
given to the eye, when the cloth is absolutely so flimsy, that it may be torn asunder as
easily as a sheet of writing-paper. Many frauds of this kind are practised.
In fig. 1116. is given a representation of the position of a fabric of cloth in section, as
it is in the loom before the warp has been closed upon the woof, which still appears as a
straight line. This figure may usefully
illustrate the direction and ratio
of contraction which must unavoidably
take place in every kind of cloth, according
to the density of the texture,
the dimensions of the threads, and the description of the cloth. Let A, B, represent one
thread of woof completely stretched by the velocity of the shuttle in passing between[1234]
the threads of warp which are represented by the round dots 1, 2, &c., and those
distinguished by 8, 9, &c. When these threads are closed by the operation of the
heddles to form the inner texture, the first tendency will be to move in the direction
1 b, 2 b, &c., for those above, and in that of 8 a, 9 a, &c., for those below; but the
contraction for A, B, by its deviation from a straight to a curved line, in consequence
of the compression of the warp threads 1 b, 2 b, &c., and 1 a, 2 a, &c., in closing, will
produce, by the action of the two powers at right angles to each other, the oblique or
diagonal direction denoted by the lines 1, 8-2, 9, to the left, for the threads above,
and that expressed by the lines 2, 8-3, 9, &c., to the right, for the threads below.
Now, as the whole deviation is produced by the flexure of the thread A, B, if A is supposed
to be placed at the middle of the cloth, equidistant from the two extremities, or
selvages as they are called by weavers, the thread at 1 may be supposed to move really
in the direction 1 b, and all the others to approach to it in the directions represented,
whilst those to the right would approach in the same ratio, but the line of approximation
would be inverted. Fig. 1117.
represents that common fabric used
for lawns, muslins, and the middle
kind of goods, the excellence of which neither consists in the greatest strength, nor in
the greatest transparency. It is entirely a medium between fig. 1114. and fig. 1115.

In the efforts to give great strength and thickness to cloth, it will be obvious that the
common mode of weaving, by constant intersection of warp and woof, although it may
be perhaps the best which can be devised for the former, presents invincible obstructions
to the latter, beyond a certain limit. To remedy this, two modes of weaving are
in common use, which, while they add to the power of compressing a great quantity of
materials in a small compass, possess the additional advantage of affording much facility
for adding ornament to the superficies of the fabric. The first of these is double cloth,
or two webs woven together, and joined by the operation. This is chiefly used for
carpets; and its geometrical principles
are entirely the same as those
of plain cloth, supposing the webs
to be sewed together. A section of the cloth will be found in fig. 1118. See Carpet.
Of the simplest kind of tweeled fabrics, a section is given in fig. 1119.
The great and prominent advantage of the tweeled fabric, in point of texture, arises
from the facility with which a very great quantity of materials may be put closely together.
In the figure, the warp is
represented by the dots in the
same straight line as in the plain
fabrics; but if we consider the
direction and ratio of contraction,
upon principles similar to those laid down in the explanation given of fig. 1116., we
shall readily discover the very different way in which the tweeled fabric is affected.
When the dotted lines are drawn at a, b, c, d, their direction of contraction,
instead of being upon every second or alternate thread, is only upon every fifth
thread, and the natural tendency would consequently be, to bring the whole into
the form represented by the lines and dotted circles at a, b, c, d. In point, then,
of thickness, from the upper to the under superficies, it is evident that the whole
fabric has increased in the ratio of nearly three to one. On the other hand, it will
appear, that four threads or cylinders being thus put together in one solid mass, might
be supposed only one thread, or like the strands of a rope before it is twisted; but, to
remedy this, the thread being shifted every time, the whole forms a body in which
much aggregate matter is compressed; but where, being less firmly united, the accession
of strength acquired by the accumulation of materials is partially counteracted by the
want of equal firmness of junction.
The second quality of the tweeled fabric, susceptibility of receiving ornament, arises
from its capability of being inverted
at pleasure, as in fig. 1120. In
this figure we have, as before, four
threads, and one alternately intersected;
but here the four threads marked 1 and 2 are under the woof, while those
marked 3 and 4 are above.

Fig. 1121. represents that kind of tweeled work which produces an ornamental effect,
and adds even to the strength of a fabric, in so far as accumulation of matter can be
considered in that light. The figure
represents a piece of velvet cut in
section, and of that kind which, being
woven upon a tweeled ground,
is known by the name of Genoa velvet. 1st. Because, by combining a great quantity[1235]
of material in a small compass, they afford great warmth. 2nd. From the great
resistance which they oppose to external friction, they are very durable. And, 3rd.
Because, from the very nature of the texture, they afford the finest means of rich ornamental
decoration.
The use of velvet cloths in cold weather is a sufficient proof of the truth of the first.
The manufacture of plush, corduroy, and other stuffs for the dress of those exposed to
the accidents of laborious employment, evinces the second; and the ornamented velvets
and Wilton carpeting, are demonstrative of the third of these positions.
In the figure, the diagonal form which both the warp and woof of cloth assume, is
very apparent from the smallness of the scale. Besides what this adds to the strength
of the cloth, the flushed part, which appears interwoven at the darkly shaded intervals
1, 2, &c., forms, when finished, the whole covering or upper surface. The principle, in
so far as regards texture, is entirely the same as any other tweeled fabric.
Fig. 1122., which represents corduroy, or king’s cord, is merely striped velvet.
The principle is the same, and the figure shows that the one is a copy of the other.
The remaining figures represent
those kinds of work which are of
the most flimsy and open description
of texture; those in which neither
strength, warmth, nor durability
are much required, and of which openness and transparency are the chief recommendations.
Fig. 1123. represents common gauze, or linau, a substance very much used for various
purposes. The essential difference between this description of cloth and all others,
consists in the warp being turned or
twisted like a rope during the operation
of weaving, and hence it bears
a considerable analogy to lace. The twining of gauze is not continued in the same
direction, but is alternately from right to left, and vice versâ, between every intersection
of the woof. The fabric of gauze is always open, flimsy, and transparent; but, from the
turning of the warp, it possesses an uncommon degree of strength and tenacity in
proportion to the quantity of material which it contains. This quality, together with
the transparency of the fabric, renders it peculiarly adapted for ornamental purposes of
various kinds, particularly for flowering or figuring, either in the loom; or by the
needle. In the warp of gauze; there arises a much greater degree of contraction during
the weaving, than in any other species of cloth; and this is produced by the turning.
The twisting between every intersection of weft amounts precisely to one complete
revolution of both threads; hence this difference exists between this and every other
species of weaving, namely, that the one
thread of warp is always above the woof,
and the contiguous thread is always below.

Fig. 1124. represents a section of another species of twisted cloth, which is known by
the name of catgut, and which differs from the gauze only, by being subjected to a
greater degree of twine in weaving; for in place of one revolution between each intersection,
a revolution and a half is always given; and thus the warp is alternately above
and below, as in other kinds of weaving.
Fig. 1125. is a superficial representation of the most simple kind of ornamental network
produced in the loom. It is called a whip-net by weavers, who use the term whip
for any substance interwoven in
cloth for ornamental purposes,
when it is distinct from the
ground of the fabric. In this,
the difference is merely in the
crossing of the warp; for it is
very evident that the crossings at 1, 2, 3, 4, and 5, are of different threads from those at
6, 7, 8, and 9.
Fig. 1126. represents, superficially, what is called the mail-net, and is merely a combination
of common gauze and the whip-net in the same fabric. The gauze here being
in the same direction as the dotted line
in the former figure, the whole fabric
is evidently a continued succession of
right-angled triangles, of which the
woof forms the basis, the gauze part
the perpendiculars, and the whip part
the hypothenuses. The contraction here being very different, it is necessary that the
gauze and whip parts should be stretched upon separate beams.
In order to design ornamental figures upon cloths, the lines which are drawn from the[1236]
top to the bottom of the paper may be supposed to represent the warp; and those drawn
across, the woof of the web; any number of threads being supposed to be included between
every two lines. The paper thus forms a double scale, by which, in the first instance,
the size and form of the pattern may be determined with great precision; and
the whole subsequent operations of the weaver regulated, both in mounting and working
his loom. To enable the projector of a new pattern to judge properly of its effects,
when transferred from the paper to the cloth, it will be essentially necessary that he
should bear constantly in his view the comparative scale of magnitude which the design
will bear in each, regulating his ideas always by square or superficial measurement.
Thus, in the large design, fig. 1127., representing a bird perched upon the branch of a
tree, it will be proper,
in the first place, to
count the number of
spaces from the point
of the bill to the extremity
of the tail;
and to render this the
more easy, it is to be
observed that every
tenth line is drawn
considerably bolder
than the others. This
number in the design
is 135 spaces. Counting
again, from the stem of the branch to the upper part of the bird’s head, he will
find 76 spaces. Between these spaces, therefore, the whole superficial measure of the
pattern is contained. By the measure of the paper, this may be easily tried with a pair
of compasses, and will be found to be nearly 65⁄10 inches in length, by 33⁄16 inches in
breadth. Now, if this is to be woven in a reed containing 800 intervals in 37 inches,
and if every interval contains five threads, supposed to be contained between every
two parallel lines, the length will be 6·24 inches, and the breadth 3·52 inches nearly;
so that the figure upon the cloth would be very nearly of the same dimensions as
that upon the paper; but if a 1200 reed were used, instead of an 800, the dimensions
would be proportionally contracted.
A correct idea being formed of the design, the weaver may proceed to mount his
loom according to the
pattern; and this is
done by two persons,
one of whom takes
from the design the
instructions necessary
for the other to follow in tying his cords.
Fig. 1128. is a representation of the most simple species of table-linen, which is merely
an imitation of checker-work of various sizes; and is known in Scotland, where the
manufacture is chiefly practised, by the name of Dornock. When a pattern is formed
upon tweeled cloth, by reversing the flushing, the two sides of the fabric being dissimilar,
one may be supposed to be represented by the black marks, and the other by the
part of the figure which is left uncoloured. For such a pattern as this, two sets of
common tweel-heddles, moved in the ordinary way, by a double succession of heddles,
are sufficient. The other part of fig. 1128. is a design of that intermediate kind of
ornamental work which is called diaper, and which partakes partly of the nature of the
dornock, and partly of that of the damask and tapestry. The principle upon which all
these descriptions of
goods are woven is
entirely the same,
and the only difference
is in the extent
of the design, and
the means by which
it is executed. Fig.
1129. is a design for
a border of a handkerchief
or napkin, which may be executed either in the manner of damask, or as the
spotting is practised in the lighter fabrics.
THENARD’S BLUE, or COBALT BLUE, is prepared by digesting the oxide
of cobalt used in the potteries, with nitric acid, evaporating the nitrate almost to dryness,
diluting it with water, and filtering, to separate some arseniate of iron, which usually[1237]
precipitates. The clear liquor is to be poured into a solution of phosphate of soda,
whence an insoluble phosphate of cobalt falls. This being well washed, is to be intimately
mixed in its soft state with eight times its weight of well-washed gelatinous
alumina, which has been obtained by pouring a solution of alum into water of ammonia
in excess. The uniformly coloured paste is to be spread upon plates, dried in a stove,
then bruised dry in a mortar, enclosed in a crucible, and subjected to a cherry-red heat
for half an hour. On taking out the crucible, and letting it cool, the fine blue pigment
is to be removed into a bottle, which is to be stoppered till used.
The arseniate of cobalt may be substituted, in the above process, for the phosphate, but
it must be mixed with sixteen times its weight of the washed gelatinous alumina. The
arseniate is procured by pouring the dilute nitrate of cobalt into a solution of arseniate
of potassa. If nitrate of cobalt be mixed with the alumina, and the mixture be treated
as above described, a blue pigment will also be obtained, but paler than the preceding,
showing that the colour consists essentially of alumina stained with oxide of cobalt.
THERMOMETER, signifies the measure of heat. Its description belongs to a
treatise on chemical physics.
THERMOSTAT, is the name of an apparatus for regulating temperature, in vaporization,
distillation, heating baths or hothouses, arid ventilating apartments, &c.;
for which I obtained a patent in the year 1831. It operates upon the physical principle,
that when two thin metallic bars of different expansibilities are riveted or soldered
facewise together, any change of temperature in them will cause a sensible movement
of flexure in the compound bar, to one side or other; which movement may be made to
operate, by the intervention of levers, &c., in any desired degree, upon valves, stopcocks,
stove-registers, air-ventilators, &c.; so as to regulate the temperature of the media in
which the said compound bars are placed. Two long rulers, one of steel, and one of hard
hammered brass, riveted together, answer very well; the object being not simply to
indicate, but to control or modify temperature. The following diagrams will illustrate
a few out of the numerous applications of this instrument:—
Fig. 1130. a, b, is a single thermostatic bar, consisting of two or more bars or rulers
of differently expansible solids (of which, in certain cases, wood may be one): these bars
or rulers are firmly riveted or soldered together,
face to face. One end of the compound bar is
fixed by bolts at a, to the interior of the containing
cistern, boiler, or apartment, a, l, m, b,
whereof the temperature has to be regulated,
and the other end of the compound bar at b, is
left free to move down towards c, by the flexure
which will take place when its temperature is
raised.
The end b, is connected by a link, b, d, with a
lever d, e, which is moved by the flexure into the
dotted position b, g, causing the turning-valve,
air-ventilator, or register, o, n, to revolve with
a corresponding angular motion, whereby the
lever will raise the equipoised slide-damper k, i,
which is suspended by a link from the end e, of
the lever e, d, into the position k, h. Thus a
hothouse or a water-bath may have its temperature
regulated by the contemporaneous admission
of warm, and discharge of cold air, or
water.
Fig. 1131. a, b, c, is a thermostatic hoop, immersed
horizontally beneath the surface of the water-bath
of a still. The hoop is fixed at a, and the
two ends b, c, are connected by two links b d, c d,
with a straight sliding rod d, h, to which the hoop will give an endwise motion, when
its temperature is altered; e; is an adjusting screw-nut on the rod d, h, for setting the
lever f, g, which is fixed on the axis of the turning-valve or cock f; at any desired
position, so that the valve may be opened or shut at any desired temperature, corresponding
to the widening of the points b, c, and the consentaneous retraction of
the point d, towards the circumference a, b, c, of the hoop. g, h, is an arc graduated by a
thermometer, after the screw-piece e has been adjusted. Through a hole at h, the guide-rod
passes. i, is the cold-water cistern; i, f, k, the pipe to admit cold water; l, the overflow
pipe, at which the excess of hot water runs off.
Fig. 1132. shows a pair of thermostatic bars, bolted fast together at the ends a. The free
ends b, c, are of unequal length, so as to act by the cross links d, f, on the stopcock e.
The links are jointed to the handle of the turning plug of the cock, on opposite sides[1238]
of its centre; whereby that plug will be turned round in proportion to the widening of
the points b, c. h, g, is the pipe communicating with the stopcock.
Suppose that for certain purposes in pharmacy, dyeing, or any other chemical art,
a water-bath is required to be maintained steadily at a temperature of 150° F.: let the
combined thermostatic bars, hinged together at e, f, fig. 1133., be placed in the bath,
between the outer and inner vessels
a, b, c, d, being bolted fast to the inner
vessel at g; and have their sliding rod k,
connected by a link with a lever fixed
upon the turning plug of the stopcock i,
which introduces cold water from a
cistern m, through a pipe m, i, n, into the
bottom part of the bath. The length
of the link must be so adjusted that the
flexure of the bars, when they are at a
temperature of 150°, will open the said
stopcock, and admit cold water to pass
into the bottom of the bath through the
pipe i, n, whereby hot water will be displaced
at the top of the bath through
an open overflow-pipe at q. An oil
bath may be regulated on the same
plan; the hot oil overflowing from q, into a refrigeratory worm, from which it may be
restored to the cistern m. When a water bath is heated by the distribution of a tortuous
steam pipe through it, as i, n, o, p, it will be necessary to connect the link of the
thermostatic bars with the lever of the turning plug of the steam-cock, or of the throttle
valve i, in order that the bars, by their flexure, may shut or open the steam passage
more or less, according as the temperature of the water in the bath shall tend more or
less to deviate from the pitch to which the apparatus has been adjusted. The water of
the condensed steam will pass off from the sloping winding-pipe i, n, o, p, through the
sloping orifice p. A saline, acid, or alkaline bath has a boiling temperature proportional
to its degree of concentration, and may therefore have its heat regulated by immersing
a thermostat in it, and connecting the working part of the instrument with a stopcock i,
which will admit water to dilute the bath whenever by evaporation it has become concentrated,
and has acquired a higher boiling point. The space for the bath, between
the outer and inner pans, should communicate by one pipe with the water-cistern m;
and by another pipe, with a safety cistern r, into which the bath may be allowed to
overflow during any sudden excess of ebullition.
Fig. 1136. is a thermostatic apparatus, composed of three pairs of bars d, d, d, which
are represented in a state of flexure by heat; but they become nearly straight and parallel
when cold, a, b, c, is a guide rod, fixed
at one end by an adjusting screw e, in the
strong frame f, e, having deep guide grooves
at the sides. f, g, is the working-rod,
which moves endways when the bars d, d, d,
operate by heat or cold. A square register-plate
h, g, may be affixed to the rod
f, g, so as to be moved backwards and forwards
thereby, according to the variations
of temperature; or the rod f, g, may cause
the circular turning air-register i, to revolve
by rack and wheel-work, or by a chain
and pulley. The register-plate h, g, or
turning register i, is situated at the ceiling
or upper part of the chamber, and serves
to let out hot air. k, is a pulley, over which
a cord runs to raise or lower a hot-air
register l, which may be situated near the
floor of the apartment or hothouse, to admit
hot air into the room. c, is a milled head, for adjusting the thermostat, by means of
the screw at e, in order that it may regulate the temperature to any degree.
Fig. 1137. represents a chimney, furnished with a pyrostat a, b, c, acting by the links
b, d, e, c, on a damper f, h, g. The more expansible metal is in the present example
supposed to be on the outside. The plane of the damper-plate will, in this case, be
turned more directly into the passage of the draught through the chimney by increase
of temperature.
Fig. 1135. represents a circular turning register, such as is used for a stove, or stove-grate,[1239]
or for ventilating apartments; it is furnished with a series of spiral thermostatic
bars, each bar being fixed fast at the circumference of the circle b, c, of the fixed plate
of the air-register; and all the bars act in concert at the centre a, of the
twining part of the register, by their ends being inserted between the
teeth of a small pinion, or by being jointed to the central part of the
turning plate by small pins.
Fig. 1134. represents another arrangement of my thermostatic apparatus
applied to a circular turning register, like the preceding, for ventilating
apartments. Two pairs of compound bars are applied so as to act in concert,
by means of the links a c, b c, on the opposite ends of a short lever, which
is fixed on the central part of the turning plate of the air-register. The
two pairs of compound bars a, b, are fastened to the circumference of
the fixed plate of the turning register, by two sliding rods a d, b e, which
are furnished with adjusting screws. Their motion or flexure is transmitted
by the links a c, and b c, to the turning plate, about its centre, for
the purpose of shutting or opening the ventilating sectorial apertures,
more or less, according to the temperature of the air which surrounds the thermostatic
turning register. By adjusting the screws a d, and b c, the turning register is made
to close all its apertures at any desired degree of temperature; but whenever the air is
above that temperature, the flexure of the compound bars will open the apertures.
THIMBLE (Dé à coudre, Fr.; Fingerhut (fingerhat), Germ.); is a small truncated
metallic cone, deviating little from a cylinder, smooth within, and symmetrically pitted
on the outside with numerous rows of indentations, which is put upon the tip of the
middle finger of the right hand, to enable it to push the needle readily and safely
through cloth or leather, in the act of sewing. This little instrument is fashioned in
two ways; either with a pitted round end, or without one; the latter, called the open
thimble, being employed by tailors, upholsterers, and, generally speaking, by needle-men.
The following ingenious process for making this essential implement, the contrivance of
M. M. Rouy and Berthier, of Paris, has been much celebrated, and very successful.
Sheet-iron, one twenty-fourth of an inch thick, is cut into strips, of dimensions suited
to the intended size of the thimbles. These strips are passed under a punch-press,
whereby they are cut into discs of about 2 inches diameter, tagged together by a tail.
Each strip contains one dozen of these blanks. A child is employed to make them
red-hot, and to lay them on a mandril nicely fitted to their size. The workman now
strikes the middle of each with a round-faced punch, about the thickness of his finger,
and thus sinks it into the concavity of the first mandril. He then transfers it successively
to another mandril, which has five hollows of successively increasing depth; and, by
striking it into them, brings it to the proper shape.
A second workman takes this rude thimble, sticks it in the chuck of his lathe, in order
to polish it within, then turns it outside, marks the circles for the gold ornament, and
indents the pits most cleverly with a kind of milling tool. The thimbles are next annealed,
brightened, and gilt inside, with a very thin cone of gold leaf, which is firmly
united to the surface of the iron, simply by the strong pressure of a smooth steel mandril.
A gold fillet is applied to the outside, in an annular space turned to receive it,
being fixed, by pressure at the edges, into a minute groove formed on the lathe.
Thimbles are made in this country by means of moulds in the stamping-machine.
See Stamping of Metals.
THORINA, is a primitive earth, with a metallic basis, discovered in 1828, by Berzelius.
It was extracted from the mineral thorite, of which it constitutes 58 per cent.,
and where it is associated with the oxides of iron, lead, manganese, tin, and uranium,
besides earths and alkalis, in all 12 substances. Pure thorina is a white powder, without
taste, smell, or alkaline reaction on litmus. When dried and calcined, it is not affected
by either the nitric or muriatic acid. It may be fused with borax into a transparent
glass, but not with potash or soda. Fresh precipitated thorina is a hydrate, which
dissolves readily in the above acids, as well as in solutions of the carbonates of potash,
soda, and ammonia, but not in these alkalis in a pure state. This earth consists of
74·5 parts of the metal thorinum, combined with 100 of oxygen. Its hydrate contains
one equivalent prime of water. It is hitherto merely a chemical curiosity, remarkable
chiefly for a density of 9·402, far greater than that of all the earths, and even of copper.
THREAD MANUFACTURE. The doubling and twisting of cotton or linen
yarn into a compact thread, for weaving bobbin-net, or for sewing garments, is performed
by a machine resembling the throstle of the cotton-spinner. Fig. 1138. shows the
thread-frame in a transverse section, perpendicular to its length. a, is the strong
framing of cast iron; b, is the creel, or shelf, in which the bobbins of yarn l, l, are set
loosely upon their respective skewers, along the whole line of the machine, their lower
ends turning in oiled steps, and their upper in wire eyes; c, is a glass rod, across which
the yarn runs as it is unwound; d, d, are oblong narrow troughs, lined with lead, and[1240]
filled with water, for moistening the thread during its torsion; the threads being made
to pass through eyes at the bottom of the fork e, which has an upright stem for lifting
it out, without wetting the fingers, when any thing goes amiss; f, f, are the pressing
rollers, the under one g, being of smooth iron, and the upper one h, of box-wood; the
former extends from end to end of the frame, in lengths comprehending 18 threads,
which are joined by square pieces, as in the drawing-rollers of the mule-jenny. The
necks of the under rollers are supported, at the ends and the middle, by the standards i,
secured to square bases j, both made of cast iron. The upper cylinder has an iron axis,
and is formed of as many rollers as there are threads; each roller being kept in its place
upon the lower one by the guides k, whose vertical slots receive the ends of the axes.
The yarn delivered by the bobbin l, glides over the rod c, and descends into the trough
d, e, where it gets wetted; on emerging, it goes along the bottom of the roller g, turns
up, so as to pass between it and h, then turns round the top of h, and finally proceeds
obliquely downwards, to be wound upon the bobbin m, after traversing the guide-eye n.
These guides are fixed to the end of a plate, which may be turned up by a hinge-joint
at o, to make room for the bobbins to be changed.
There are three distinct simultaneous movements to be considered in this machine:
1. that of the rollers, or rather of the under roller, for the upper one revolves merely by
friction; 2. that of the spindles m, s′; 3. the up-and-down motion of the bobbins upon
the spindles.
The first of these motions is produced by means of toothed wheels, upon the right
hand of the under set of rollers. The second motion, that of the spindles, is effected by
the drum z, which extends the whole length of the frame, turning upon the shaft v, and
communicating its rotatory movement (derived from the steam pulley) to the whorl b′,
of the spindles, by means of the endless band or cord a′. Each of these cords turns four
spindles, two upon each side of the frame. They are kept in a proper state of tension[1241]
by the weights c′, which act tangentially upon the circular arc d′, fixed to the extremity
of the bell-crank lever e′ f′ g′, and draw in a horizontal direction the tension pulleys
h, embraced by the cords. The third movement, or the vertical traverse of the bobbins,
along the spindles m, takes place as follows:—
The end of one of the under rollers carries a pinion, which takes into a carrier wheel,
that communicates motion to a pinion upon the extremity of the shaft m′, of the heart-shaped
pulley n′. As this eccentric revolves, it gives a reciprocating motion to the
levers o′, o′, which oscillate in a vertical plane round the points, p′, p′. The extremities
of these levers, on either side, act by means of the links q′, upon the arms of the sliding
sockets r′, and cause the vertical rod s′, to slide up and down in guide-holes at t′, u′, along
with the cast-iron step v′, which bears the bottom washer of the bobbins. The periphery
of the heart-wheel n′, is seen to bear upon friction wheels x, x′, set in frames adjusted
by screws upon the lower end of the bent levers, at such a distance from the point p′,
as that the traverse of the bobbins may be equal to the length of their barrel.
By adapting change pinions and their corresponding wheels to the rollers, the delivery
of the yarn may be increased or diminished in any degree, so as to vary the degree of
twist put into it by the uniform rotation of the drum and spindles. The heart motion
being derived from that of the rollers, will necessarily vary with it.
Silk thread is commonly twisted in lengths of from 50 to 100 feet, with hand reels,
somewhat similar to those employed for making ropes by hand.
TILTING OF STEEL. See Steel. Rees’s Cyclopædia contains an excellent article
on this subject.
TIN (Etain, Fr.; Zinn, Germ.); in its pure state, has nearly the colour and
lustre of silver. In hardness it is intermediate between gold and lead; it is very
malleable, and may be laminated into foil less than the thousandth of an inch in
thickness; it has an unpleasant taste, and exhales on friction a peculiar odour; it is
flexible in rods or straps of considerable strength, and emits in the act of bending a
crackling sound, as if sandy particles were intermixed, called the creaking of tin.
A small quantity of lead, or other metal, deprives it of this characteristic quality. Tin
melts at 442° Fahr., and is very fixed in the fire at higher heats. Its specific gravity
is 7·29. When heated to redness with free access of air, it absorbs oxygen with
rapidity, and changes first into a pulverulent gray protoxide, and by longer ignition,
into a yellow-white powder, called putty of tin. This is the peroxide, consisting of 100
of metal + 27·2 of oxygen.
Tin has been known from the most remote antiquity; being mentioned in the books
of Moses. The Phœnicians carried on a lucrative trade in it with Spain and Cornwall.
There are only two ores of tin; the peroxide, or tin-stone, and tin pyrites; the
former of which alone has been found in sufficient abundance for metallurgic purposes.
The external aspect of tin-stone has nothing very remarkable. It occurs sometimes in
twin crystals; its lustre is adamantine; its colours are very various, as white, gray,
yellow, red, brown, black; specific gravity 6·9 at least; which is, perhaps, its most
striking feature. It does not melt by itself before the blowpipe; but is reducible in
the smoky flame or on charcoal. It is insoluble in acids. It has somewhat of a greasy
aspect; and strikes fire with steel.
Tin-stone occurs disseminated in the antient rocks, particularly granite; also in
beds and veins, in large irregular masses, called stockwerks; and in pebbles, an assemblage
of which is called stream-works, where it occasionally takes a ligneous aspect, and
is termed wood-tin.
This ore has been found in few countries in a workable quantity. Its principal
localities are, Cornwall, Bohemia, Saxony, in Europe; and Malacca and Banca, in
Asia. The tin-mines of the Malay peninsula lie between the 10th and 6th degree
of south latitude; and are most productive in the island of Junck-Ceylon, where
they yield sometimes 800 tons per annum, which are sold at the rate of 48l. each.
The ores are found in large caves near the surface; and though actively mined for
many centuries, still there is easy access to the unexhausted parts. The mines in the
island of Banca, to the east of Sumatra, discovered in 1710, are said to have furnished,
in some years, nearly 3500 tons of tin. Small quantities occur in Gallicia in Spain, in
the department of Haute Vienne in France, and in the mountain chains of the Fichtel
and Riesengebürge in Germany. The columnar pieces of pyramidal tin-ore from
Mexico and Chile, are products of stream-works. Small groups of black twin crystals
have been lately discovered in the albite rock of Chesterfield in Massachusetts.
The Cornish ores occur—1. in small strata or veins, or in masses; 2. in stockwerks,
or congeries of small veins; 3. in large veins; 4. disseminated in alluvial deposits.
The stanniferous small veins, or thin flat masses, though of small extent, are sometimes
very numerous, interposed between certain rocks, parallel to their beds, and are
commonly called tin-floors. The same name is occasionally given to stockwerks. In[1242]
the mine of Bottalack, a tin-floor has been found in the killas (primitive schistose rock),
thirty-six fathoms below the level of the sea; it is about a foot and a half thick, and
occupies the space between a principal vein and its ramification; but there seems to be
no connexion between the floor and the great vein.
2. Stockwerks occur in granite and in the felspar porphyry, called in Cornwall,
elvan. The most remarkable of these in the granite, is at the tin-mine of Carclase,
near St. Austle. The works are carried on in the open air, in a friable granite, containing
felspar disintegrated into kaolin, or china clay, which is traversed by a great
many small veins, composed of tourmaline, quartz, and a little tin-stone, that form black
delineations on the face of the light-gray granite. The thickness of these little veins
rarely exceeds 6 inches, including the adhering solidified granite, and is occasionally
much less. Some of them run nearly east and west, with an almost vertical dip;
others, with the same direction, incline to the south at an angle with the horizon of
70 degrees.
Stanniferous stockwerks are much more frequent in the elvan (porphyry); of
which the mine of Trewidden-ball is a remarkable example. It is worked among
flattened masses of elvan, separated by strata of killas, which dip to the east-north-east
at a considerable angle. The tin ore occurs in small veins, varying in thickness from
half an inch to 8 or 9 inches, which are irregular, and so much interrupted, that it is
difficult to determine either their direction or their inclination.
3. The large and proper metalliferous veins are not equally distributed over the surface
of Cornwall and the adjoining part of Devonshire; but are grouped into three districts;
namely, 1. In the south-west of Cornwall, beyond Truro; 2. In the neighbourhood
of St. Austle; and 3. In the neighbourhood of Tavistock in Devonshire.
The first group is by far the richest, and the best explored. The formation most
abundant in tin mines is principally granitic; whilst that of the copper mines is most
frequently schistose or killas; though with numerous exceptions. The great tin veins
are the most antient metalliferous veins in Cornwall; yet they are not all of one
formation, but belong to two different systems. Their direction is, however, nearly
the same, but some of them dip towards the north, and others towards the south. The
first are older than the second; for in all the mines where these two sets of veins are
associated, the one which dips to the north, cuts across and throws out the one which
dips to the south. See Mines, p. 835.
At Trevannance mines, the two systems of tin veins are both intersected by the oldest
of the copper veins; indicating the prior existence of the tin veins. In fig. 1139.
b, marks the first system of tin veins; c, the second;
and d, the east and west copper veins. Some of
these tin veins, as at Poldice, have been traced
over an extent of two miles; and they vary in
thickness from a small fraction of an inch to
several feet, the average width being from 2 to 4
feet; though this does not continue uniform for
any length, as these veins are subject to continual
narrowings and expansions. The gangue is
quartz, chlorite, tourmaline, and sometimes decomposed
granite and fluor spar.
4. Alluvial tin ore, stream tin.—Peroxide of tin occurs disseminated both in the
alluvium which covers the gentle slopes of the hills adjoining the rich tin-mines, and
also in the alluvium which fills the valleys that wind round their base; but in these
numerous deposits the tin-stone is rarely distributed in sufficient quantities to make it
worth the working. The most important explorations of alluvial tin ore are grouped
in the environs of St. Just and St. Austle; where they are called stream-works; because
water is the principal agent employed to separate the metallic oxide from the sand and
gravel.
The tin mine of Altenberg, in Saxony (fig. 1140., which is a vertical projection in
a plane passing from west to east,) is remarkable for a stockwerke, or interlaced mass
of ramifying veins, which has been worked ever since the year 1458. The including
rock is a primitive porphyry, superposed upon gneiss; becoming very quartzose as it
approaches the lode. This is usually disseminated in minute particles, and accompanied
with wolfram, copper and arsenical pyrites, fer oligiste, sulphuret of molybdenum,
and bismuth, having gangues of lithomarge, fluor spar, mica, and felspar. The space
which the ore occupies in the heart of the quartz, is a kind of dædalus, the former being
often so dispersed among the latter as to seem to merge into it; whence it is called by
the workmen zwitter, or ambiguous. In 1620, the mine was worked by 21 independent
companies, in a most irregular manner, whereby it was damaged to a depth of 170 fathoms
by a dreadful downfall of the roofs. This happened on a Sunday, providentially, when the
pious miners were all at church. The depth of this abyss, marked by the curved line[1243]
b, b, b, is 66 fathoms; but the devastation is manifest to a depth of 95 fathoms below that
curve, and 35 fathoms below the actual workings, represented at the bottom of the shaft
under B. The parts excavated are shaded black in the
figure. There are two masses of ore, one under the
shaft B, and another under the shaft C; which at the
levels 5 and 10 are in communication, but not at
6, 7. There is a direct descent from 8 to 9. The
deposits are by no means in one vertical plane, but at
a considerable horizontal distance from each other.
A is the descending shaft; B is the extraction shaft,
near the mouth of which there is a water-wheel; C is
another extraction shaft, worked also by means of a
water-wheel. A and C are furnished with ladders, but
for B the ladders are placed in an accessory shaft b′;
under D, a shaft is sunk for pumping out the water,
by means of an hydraulic wheel at D; E is the gallery
or drift for admitting the water which drives the
wheels. This falls 300 feet, and ought to be applied
to a water-pressure engine, instead of the paddles of a
wheel. At D, is the gallery of discharge for the waters,
which serves also to ventilate the mine, being cut to
the day, through 936 toises of syenitic porphyry and
gneiss. J, is a great vaulted excavation. The mine
has 13 stages of galleries, of which 11 serve for extracting
the ore; 1 is the mill-course; the rest are
marked with the numbers 2, 3, 4, &c.; each having
besides a characteristic German name. The rare
mineral called topaz pycnite is found in this mine,
above 10, between the shafts C and D.
The only rule observed in taking ore from this
mine, has been to work as much out of each of these
levels as is possible, without endangering the superincumbent
or collateral galleries; on which account
many pillars are constructed to support the roofs.
The mine yields annually 1600 quintals (Leipzick)
of tin, being four-fifths of the whole furnished by the
district of Altenberg; to produce which, 400,000 quintals of ore are raised. 1000
parts of the rock yield 8 of concentrated schlich, equivalent to only 4 of metal; being
only 1 in 250 parts.
But the most extensive and productive stream-works, are those of Pentowan, near
St. Austle.
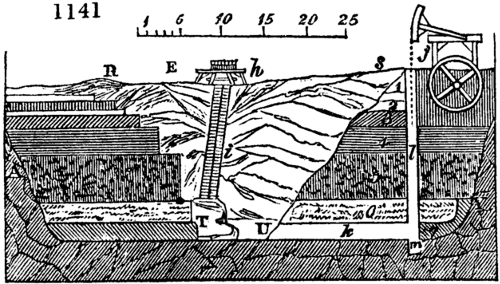
Fig. 1141. represents a vertical section of the Pentowan mine, taken from the
stream-work, Happy Union. A vast excavation, R, T, U, S, has been hollowed out in
the open air, in quest of the alluvial tin
ore T, which occurs here at an unusual
depth, below the level of the strata R, S.
Before getting at this deposit, several
successive layers had to be sunk through;
namely, 1, 2, 3; the gravel, containing
in its middle a band of ochreous earth
2, or ferruginous clay; 4, a black peat,
perfectly combustible, of a coarse texture,
composed of reeds and woody
fibres, cemented into a mass by a fine
loam; 5, coarse sea-sand, mingled with
marine shells; 6, a blackish marine mud, filled with shells. Below these the deposit of
tin-stone occurs, including fragments of various size, of clay slate, flinty slate, quartz,
iron ore, jasper; in a word, of all the rocks and gangues to be met with in the surrounding
territory, with the exception of granite. Among these fragments there
occur, in rounded particles, a coarse quartzose sand, and the tin-stone, commonly in
small grains and crystals. Beneath the bed T, the clay slate occurs, called killas (A, X, Y),
which supports all the deposits of more recent formation.
The system of mining is very simple. The successive beds, whose thickness is shown
in the figure, are visibly cut out into steps or platforms. By a level or gallery of efflux
k, the waters flow into the bottom of the well l, m, which contains the drainage pumps;
and these are put in action by a machine j, moved by a water-wheel. The extraction
of the ore is effected by an inclined plane i, cut out of one of the sides of the excavation,[1244]
at an angle of about 45 degrees. At the lower end of this sloping pathway there is a
place of loading; and at its upper end h, a horse-gin, for alternately raising and lowering
the two baskets of extraction on the pathway i.
Mine tin requires peculiar care in its mechanical preparation or dressing, on account
of the presence of foreign metals, from which, as we have stated, the stream tin is free.
1. As the mine tin is for the most part extremely dispersed through the gangue, it
must be all stamped and reduced to a very fine powder, to allow the metallic particles
to be separated from the stony matters.
2. As the density of tin-stone is much greater than that of most other metallic ores,
it is less apt to run off in the washing; and may, therefore, be dressed so as to be completely
stripped of every matter not chemically combined.
3. As the peroxide of tin is not affected by a moderate heat, it may be exposed to calcination;
whereby the specific gravity of the associated sulphurets and arseniurets is so
diminished as to facilitate their separation.
We may therefore conclude, that tin ore should be first of all pounded very fine in
the stamp-mill, then subjected to reiterated washings, and afterwards calcined. The
order of proceeding in Cornwall is as follows:—
1. Cleaning the ore.—This is usually done at the mouth of the gallery of efflux, by
agitating the ore in the stream of water as it runs out. Sometimes the ore is laid on a
grating, under a fall of water.
2. Sorting.—The ore thus cleaned, is sorted on the grate, into four heaps: 1. stones
rich in tin; 2. stones containing both tin and copper ore; 3. copper ore; 4. sterile
pieces, composed in a great measure of stony gangue, with iron and arsenical pyrites.
In those veins where there is no copper ore, the second and third heaps are obviously
absent. When present, the compound ore is broken into smaller pieces with a mallet,
and the fragments are sorted anew.
3. Stamping.—The stanniferous fragments (No. 1.) are stamped into a sand, of greater
or less fineness, according to the dissemination of the tin-stone in the gangue. The
determination of the size of the sand, is an object of great importance. It is
regulated by a copper plate pierced with small holes, through which every thing from
the stamping-mill must run off with the rapid stream introduced for this purpose. This
plate forms the front of the stamp cistern.
Several years ago, all the stamp mills were driven by water-wheels, which limited the
quantity of ore that could be worked to the hydraulic power of the stream or waterfall;
but since the steam engine has been applied to this purpose, the annual product of tin
has been greatly increased. On the mine of Huel Vor, there are three steam engines
appropriated to the stamping-mills. Their force is 25 horses at least. One of these
machines, called south stamps, drives 48 pestles; a second, called old stamps, drives 36;
and a third, 24. The weight of these pestles varies from 370 to 387 pounds; and
they generally rise through a space of 101⁄2 inches. The machine called south stamps, the
strongest of the three, gives 171⁄2 blows in the minute, each pestle being lifted twice for
every stroke of the piston. The steam engine of this mill has a power of 25 horses, and
it consumes 1062 bushels of coals in the month. Three pestles constitute a battery, or
stamp-box.
Washing and stamping of tin ores at Polgooth, near St. Austle.—The stamps or pestles
are of wood, 6 inches by 51⁄2 in the square: they carry lifting bars b, secured with a
wooden wedge and a bolt of iron, and they terminate below in a lump of
cast iron A, called the head, which is fastened to them by a tail, and
weighs about 21⁄2 cwts. The shank of the pestle is strengthened with iron hoops.
A turning-shaft communicates motion to the stamps by cams stuck round
its circumference, so arranged that the second falls while the first and third
of each set are uplifted. There are 4 cams on one periphery, and the
shaft makes 7 turns in the minute. Each stamp, therefore, gives 28
strokes per minute, and falls through a space of 71⁄2 inches. The stamp
chest is open behind, so that the ore slips away under the pestles, by its
weight, along the inclined plane with the stream of water. The bottom
of the troughs consists of stamped ores. With 6 batteries of 6 pestles
each, at Poldice, near Redruth, 120 bags of ore are stamped in 12
hours; each bag containing 18 gallons of 282 cubic inches; measuring
altogether 352 cubic feet, and 864 cubic inches.
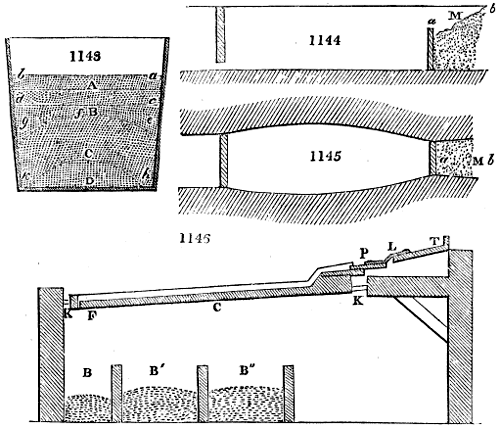
The openings in the front sides of the troughs are nearly 8 inches by 71⁄2: they are
fitted with an iron frame, which is closed with sheet iron, pierced with about 160 holes
in the square inch, bored conically, being narrower within. The ore, on issuing,
deposits its rough in the first basin, and its slimes in the following basins. The rough is
washed in buddles (see Lead, page 751), and in tossing tubs; the slimes in trunks, and upon
a kind of twin tables, called racks. Into the tossing-tub, or dolly, fig. 1143., the stamped
ore is thrown, along with a certain quantity of water, and a workman stirs it about[1245]
with an iron shovel for three or four minutes. He then removes a little of the water
with a handled pitcher, and strikes the sides of the tub for 8 or 10 minutes with a hammer,
which hastens the subsidence of the denser parts. The water
is next poured off by inclining the tub to one side. In one
operation of this kind, four distinct strata of the ores may be
procured, as indicated by the lines a b, c d, e f g, h i k, in
the figure. The portion A is to be washed again in the
trunking-box, figs. 1144, 1145.; B is to be washed upon the
German chests or racks, fig. 1146.; C, the most considerable,
is put aside, as schlich fit for the market; D, forming a nucleus
the centre of the tub, is to be passed through sieves of
copper wire, having 18 meshes in the square inch. This
product thus affords a portion D′, which passes through
the sieve, and D′′ which remains upon it; the latter is sometimes thrown away, and
at others is subjected to the operation called the tie, viz., a washing upon the sloping
bottom of a long trough.
The slimes are freed from the lighter mud in the trunking-box, figs. 1144, 1145.;
which is from 7 to 8 feet long. Being accumulated at M, the workman pushes them
back with a shovel from a towards b.
The metallic portion is carried off, and
deposited by the stream of water upon
the table; but the earthy matters are
floated along into a basin beyond it. The
product collected in the chest is divided
into two portions; the one of
which is washed once, and the other
twice, upon the rack, fig. 1146. This
is composed of a frame C, which carries
a sloping board or table, susceptible of
turning round to the right or left upon
two pivots, K, K. The head of the
table is the inclined plane T. A small
board P, which is attached by a band
of leather L, forms the communication with the lower table C, whose slope is generally
5 inches in its whole length of 9 feet; but this may vary with the nature of the ore,
being somewhat less when it is finely pulverized. The ore is thrown upon T, in small
portions of 20 or
25 lbs. A woman
spreads it with
a rake, while a
stream of water
sweeps a part of
it upon the table,
where it gets
washed. The fine
mud falls through
a cross slit near
the lower end,
into a basin B.
After working for a few minutes, should the schlich seem tolerably rich, the operative
turns the table round its axis K, K, so as to tumble it into the boxes below. The mud
is in B; an impure schlich in B′, which must be washed again upon the rack; and a
schlich fit for roasting in B′′.
The slope of the rack-table for washing the roasted tin ore, is 73⁄4 inches in the
9 feet.
Crushing rolls at the Pembroke mines.—Waggons, moved on a railway by an endless
rope, bring the ore to be crushed, immediately over the rolls, as shown in fig. 1147.
A trap being opened in the side of the waggon, the ore falls into the hopper T, whence
it passes directly between the twin cylinders C, C, and next upon the sieve D, which
receives a seesaw motion horizontally, by means of the rod L, and the crank of the
upright turning-shaft. The finer portion of ore, which passes through that sieve,
forms the heap S. The coarser portion is tossed over the edge of the sieve, and falls
between the cylinders C′ C′, upon a lower level, and forms the second heap S′ of sifted,
and S′′ of unsifted, ore.
The holes of the sieves D, D′, being of the same size, the products S, S′, are of the same
fineness. S′′ is ground again, being mixed, in the uppermost hopper T, along with the
lumps from the waggons.
[1246]
The diameter and length of the under rolls (see fig. 1148.) are each 16 inches.
a b, is the square end of the gudgeon t, which prevents the shaft shifting laterally out
of its place. The diameter
of the upper
rolls is 18 inches, but
their length is the
same. Both are made
of white cast iron,
chilled or case-hardened
by being cast in
iron moulds instead of
sand; and they last a
month, at least, when
of good quality. They
make from 10 to 15
turns in a minute, according
to the hardness
of the ores of tin
or copper; and can
grind about 50 tons of rich copper ore in 12 hours; but less of the poorer sort.
The next process is the calcination in the burning-house; which includes several
reverberatory furnaces. At the mine of Poldice, they are 4 or
5 yards long, by from 21⁄2 to 3 yards wide. Their hearth is horizontal;
the elevation, about 26 inches high near the fireplace,
sinks slightly towards the chimney. There is but one opening,
which is in the front; it is closed by a plate-iron door, turning
on hinges. Above the door there is a chimney, to let the sulphureous and arsenical
vapours fly off, which escape out of the hearth, without annoying the workmen. This
chimney leads to horizontal flues, in which the arsenious acid is condensed.
Six hundred weight of ore are introduced; the calcination of which takes from 12 to
18 hours, according to the quantity of pyrites contained in the ore. At the beginning
of the operation, a moderate heat is applied, after which it is pushed to a dull red, and
kept so during several hours. The door is shut; the materials are stirred from time to
time with an iron rake, to expose new surfaces, and prevent them from agglutinating or
kerning, as the workmen say. The more pyrites is present, the more turning is necessary.
Should the ore contain black oxide of iron, it becomes peroxidized, and is then
easily removed by a subsequent washing.
Figs. 1149, 1150. represent the furnace employed at Altenberg, in Saxony, for roasting
tin ores. a is the grate; b, the sole of the roasting hearth; c, an opening in the arched
roof for introducing the dried schlich (the ground
and elutriated ore); d, is the smoke-mantle or
chimney-hood, at the end of the furnace, under
which the workmen turn over the spread schlich,
with long iron rods bent at their ends; e, is the
poison vent, which conducts the arsenical vapours
to the poison chamber (gifthaus) of condensation.
When the ore is sufficiently calcined, as is
shown by its ceasing to exhale vapours, it is
taken out, and exposed for some days to the
action of the air, which decomposes the sulphurets,
or changes them into sulphates. The
ore is next put into a tub filled with water,
stirred up with a wooden rake, and left to settle;
by which means the sulphate of copper that may
have been formed, is dissolved out. After some
time, this water is drawn off into a large tank,
and its copper recovered by precipitation with
pieces of old iron. In this way, almost all the
copper contained in the tin ore is extracted.
The calcined ore is sifted, and treated again on the racks, as above described.
The pure schlich, called black tin, is sold under this name to the smelters; and that
which collects on the middle part of the inclined wash-tables, being much mixed
with wolfram, is called mock lead. This is passed once more through the stamps, and
washed; when it also is sold as black tin.
Stream tin is dressed by similar methods: 1. by washing in a trunking-box, of such
dimensions that the workman stands upon it in thick boots, and makes a skilful use[1247]
of the rake; 2. by separating the larger conglomerate pebbles from the smaller pure
ones; picking, stamping, and washing, on a kind of sleeping-tables. See Metallurgy,
figs. 677, 678.
The tin ores of Cornwall and Devonshire are all reduced within the counties where
they are mined, as the laws prohibit their exportation out of them. Private interests
suffer no injury from this prohibition; because the vessels which bring the fuel from
Wales, for smelting these ores, return to Swansea and Neath loaded with copper ores.
The smelting-works belong in general to individuals who possess no tin mines, but
who purchase at the cheapest rate the ores from the mining proprietors. The ores are
appraised according to their contents in metal, and its fineness; conditions which they
determine by the following mode of assay. When a certain number of bags of ore, of
nearly the same quality, are brought to the works, a small sample is taken from
each bag, and the whole are well blended. Two ounces of this average ore are
mixed with about 4 per cent. of ground coal, put into an open earthen crucible,
and heated in an air furnace (in area about 10 inches square) till reduction takes
place. As the furnace is very hot when the crucible is introduced, the assay is
finished in about a quarter of an hour. The metal thus revived, is poured into a
mould, and what remains in the crucible is pounded in a mortar, that the grains of tin
may be added to the ingot.
This method, though imperfect in a chemical point of view, serves the smelter’s purpose,
as it affords him a similar result to what he would get on the great scale. A more
exact assay would be obtained by fusing, in a crucible lined with hard-rammed charcoal,
the ore mixed with 5 per cent. of ground glass of borax. To the crucible a gentle
heat should be applied during the first hour, then a strong heat during the second hour,
and, lastly, an intense heat for a quarter of an hour. This process brings out from
4 to 5 per cent. more tin than the other; but it has the inconvenience of reducing the
iron, should any be present; which by subsequent solution in nitric acid will be readily
shown. This assay would be too tedious for the smelter, who may have occasion to try
a great many samples in one day.
The smelting of tin ores is effected by two different methods:—
In the first, a mixture of the ore with charcoal is exposed to heat on the hearth of a
reverberatory furnace fired with coal.
In the second, the tin ore is fused in a blast furnace, called a blowing-house, supplied
with wood charcoal. This method is practised in only a few works, in order to obtain
a very pure quality of tin, called grain tin in England, and étain en larmes in France;
a metal required for certain arts, as dyeing, &c. This method is applied merely to
stream tin.
In the smelting-houses, where the tin is worked in reverberatories, two kinds of furnaces
are employed; the reduction and the refining furnaces.
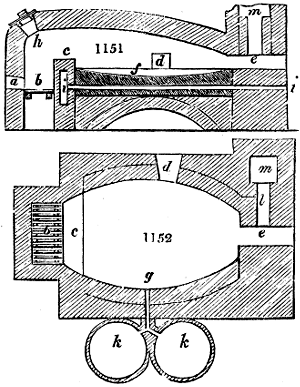
Figs. 1151, 1152. represent the furnaces for smelting tin at St. Austle, in Cornwall;
the former being a longitudinal section, the latter a ground plan, a is the fire-door,
through which pitcoal is laid upon the
grate b; c is the fire-bridge; d, the
door for introducing the ore; e, the
door through which the ore is worked
upon the hearth f; g, the stoke-hole;
h, an aperture in the vault or roof, which
is opened at the discharge of the waste
schlich, to secure the free escape of the
fumes up the chimney; i, i, air channels,
for admitting cold air under the
fire-bridge and the sole of the hearth,
with the view of protecting them from
injury by the intensity of the heat above.
k, k, are basins into which the melted
tin is drawn off; l, the flue; m, the
chimney, from 35 to 50 feet high. The
roasted and washed schlich is mixed
with small coal or culm, along with a
little slaked lime, or fluor spar, as a
flux; each charge of ore amounts to
from 15 to 24 cwt., and contains from
60 to 70 per cent. of metal.
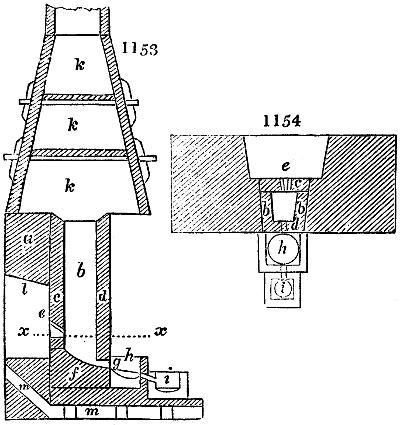
Fig. 1153. represents in a vertical
section through the tuyère, and fig.
1154. in a horizontal section, in the
dotted line x, x, of fig. 1153., the furnace[1248]
employed for smelting tin at the Erzgebirge mines, in Saxony. a, are the furnace
pillars, of gneiss; b, b, are shrouding or casing walls; c, the tuyère wall; d, front
wall, both of granite; as also the tuyère e. f, the sole-stone,
of granite, hewn out basin-shaped; g, the eye, through which
the tin and slag are drawn off into the fore-hearth h; i, the
stoke-hearth; k, k, the light ash chambers; l, the arch of the
tuyère; m, m, the common flue, which is placed under the
furnace and the hearths, and has its outlet under the vault of
the tuyère.
In the smelting furnaces at Geyer the following dimensions
are preferred:—Length of the tuyère wall, 11 inches; of the
breast wall, 11 inches; depth of the furnace, 17 inches. High
chimney-stalks are advantageous where a great quantity of
ores is to be reduced, but not otherwise.
The refining furnaces are similar
to those which serve for
reducing the ore; only, instead
of a basin of reception, they
have a refining basin placed
alongside, into which the tin is
run. This basin is about 4 feet
in diameter, and 32 inches deep;
it consists of an iron pan, placed
over a grate, in which a fire may
be kindled. Above this pan
there is a turning gib, by means of which a billet of wood may be thrust down into the
bath of metal, and kept there by wheeling the gibbet over it, lowering a rod, and fixing
it in that position.
The works in which the blast furnaces are employed, are called blowing-houses. The
smelting furnaces are 6 feet high, from the bottom of the crucible (concave hearth) to
the throat, which is placed at the origin of a long and narrow chimney, interrupted by
a chamber, where the metallic dust, carried off by the blast, is deposited. This chamber
is not placed vertically over the furnace; but the lower portion of the chimney has an
oblique direction from it. The furnace is lined with an upright cylinder of cast iron,
coated internally with loam, with an opening in it for the blast. This opening, which
corresponds to the lateral face opposite to the charging side, receives a tuyère, in which
the nozzles of two cylinder single bellows, driven by a water-wheel, are planted. The
tuyère opens at a small height above the sole of the furnace. On a level with the sole, the
iron cylinder presents a slope, below which is the hemispherical basin of reception, set
partly beneath the interior space of the furnace, and partly without. Near the corner of
the building there is a second basin of reception, larger than the first, which can discharge
itself into the former by a sloping gutter. Near this basin there is another, for
the refining operation. These are all made either of brick or cast iron.
The quality of the average ground-tin ore prepared for smelting is such, that 20 parts
of it yield from 121⁄2 to 13 of metallic tin, (621⁄2 to 65 per cent.) The treatment consists
of two operations, smelting and refining.
First operation; deoxidization of the ore, and fusion of the tin.—Before throwing the
ore into the smelting furnace, it is mixed with from one-fifth to one-eighth of its weight
of blind coal, in powder, called culm; and a little slaked lime is sometimes added, to
render the ore more fusible. These matters are carefully blended, and damped with
water, to render the charging easier, and to prevent the blast from sweeping any of it
away at the commencement. From 12 to 16 cwt. are introduced at a charge; and the
doors are immediately closed and luted, while the heat is progressively raised. Were
the fire too strong at first, the tin oxide would unite with the quartz of the gangue,
and form an enamel. The heat is applied for 6 or 8 hours, during which the doors are
not opened; of course the materials are not stirred. By this time the reduction is, in
general, finished; the door of the furnace is removed, and the melted mass is worked
up to complete the separation of the tin from the scoriæ, and to ascertain if the operation
be in sufficient forwardness. When the reduction seems to be finished, the scoriæ
are taken out at the same door, with an iron rake, and divided into three sorts; those
of the first class A, which constitute at least three-fourths of the whole, are as poor as
possible, and may be thrown away; the scoriæ of the second class B, which contain some
small grains of tin, are sent to the stamps; those of the third class C, which are last removed
from the surface of the bath of tin, are set apart, and re-smelted, as containing a
considerable quantity of metal in the form of grain tin. These scoriæ are in small
quantity. The stamp slag contains fully 5 per cent. of metallic tin.
As soon as the scoriæ are cleared away, the channel is opened which leads to the[1249]
basin of reception, into which the tin consequently flows out. Here it is left for some
time, that the scoriæ which may be still mixed with the metal, may separate, in virtue
of the difference of their specific gravities. When the tin has sufficiently settled, it is
lifted out with ladles, and poured into cast-iron moulds, in each of which a bit of wood is
fixed, to form a hole in the ingot, for the purpose of drawing it out when it becomes cold.
Refining of tin.—The object of this operation is to separate from the tin, as completely
as possible, the metals reduced and alloyed along with it. These are, principally,
iron, copper, arsenic, and tungsten; to which are joined, in small quantities, some sulphurets
and arseniurets that have escaped decomposition, a little unreduced oxide of
tin, and also some earthy matters which have not passed off with the scoriæ.
Liquation.—The refining of tin consists of two operations; the first being a liquation,
which, in the interior, is effected in a reverberatory furnace, similar to that employed in
smelting the ore. (figs. 1151, 1152.) The blocks being arranged on the hearth of the
furnace, near the bridge, are moderately heated; the tin melts, and flows away into the
refining-basin; but, after a certain time, the blocks cease to afford tin, and leave on the
hearth a residuum, consisting of a very ferruginous alloy.
Fresh tin blocks are now arranged on the remains of the first; and thus the liquation
is continued till the refining-basin be sufficiently full, when it contains about 5 tons.
The residuums are set aside, to be treated as shall be presently pointed out.
Refining proper.—Now begins the second part of the process. Into the tin-bath,
billets of green wood are plunged, by aid of the gibbet above described. The disengagement
of gas from the green wood produces a constant ebullition in the tin;
bringing up to its surface a species of froth, and causing the impurest and densest
parts to fall to the bottom. That froth, composed almost wholly of the oxides of
tin and foreign metals, is successively skimmed off, and thrown back into the furnace.
When it is judged that the tin has boiled long enough, the green wood is lifted out, and
the bath is allowed to settle. It separates into different zones, the upper being the
purest; those of the middle are charged with a little of the foreign metals; and the
lower are much contaminated with them. When the tin begins to cool, and when a
more complete separation of its different qualities cannot be looked for, it is lifted
out in ladles, and poured into cast-iron moulds. It is obvious, that the order in which
the successive blocks are obtained, is that of their purity; those formed from the bottom
of the basin being usually so impure, that they must be subjected anew to the refining
process, as if they had been directly smelted from the ore.
The refining operation takes 5 or 6 hours; namely, an hour to fill the basin, three hours
to boil the tin with the green wood, and from one to two hours for the subsidence.
Sometimes a simpler operation, called tossing, is substituted for the above artificial
ebullition. To effect it, a workman lifts some tin in a ladle, and lets it fall back into
the boiler, from a considerable height, so as to agitate the whole mass. He continues
this manipulation for a certain time; after which, he skims with care the surface of
the bath. The tin is afterwards poured into moulds, unless it be still impure. In this
case, the separation of the metals is completed by keeping the tin in a fused state
in the boiler for a certain period, without agitation; whereby the upper portion of the
bath (at least one-half) is pure enough for the market.
The moulds into which the tin blocks are cast, are usually made of granite. Their
capacity is such, that each block shall weigh a little more than three hundred weight.
This metal is called block tin. The law requires them to be stamped or coined by
public officers, before being exposed to sale. The purest block tin is called refined tin.
The treatment just detailed gives rise to two stanniferous residuums, which have to
be smelted again. These are—
1. The scoriæ B and C, which contain some granulated particles of tin.
2. The dross found on the bottom of the reverberatory furnace, after re-melting the
tin to refine it.
The scoriæ C, are smelted without any preparation; but those marked B, are stamped
in the mill, and washed, to concentrate the tin grains; and from this rich mixture, called
prillion, smelted by itself, a tin is procured of very inferior quality. This may be
readily imagined, since the metal which forms these granulations is what, being less
fusible than the pure tin, solidified quickly, and could not flow off into the metallic bath.
Whenever all the tin blocks have thoroughly undergone the process of liquation, the
fire is increased, to melt the less fusible residuary alloy of tin with iron and some other
metals, and this is run out into a small basin, totally distinct from the refining basin.
After this alloy has reposed for some time, the upper portion is lifted out into block
moulds, as impure tin, which needs to be refined anew. On the bottom and sides of
the basin there is deposited a white, brittle alloy, with a crystalline fracture, which contains
so great a proportion of foreign metals, that no use can be made of it. About
31⁄2 tons of coal are consumed in producing 2 of tin.
Smelting of tin by the blast furnace.—This mode of reduction employs only wood[1250]
charcoal, and its object is to obtain tin of the maximum purity to which it can be
brought by manufacturing processes. The better ores of the stream-works, and the
finer tin sands, are selected for this operation. The washings being always well performed,
the oxide of tin is exempt from every arsenical or sulphureous impurity, and is associated
with nothing but a little hematite. It is therefore never calcined.
The smelting is effected without addition; only, in a few cases, some of the residuary
matters of a former operation are added to the ore. About a ton and six-tenths of wood
charcoal are burned for one ton of fine smelted tin. The only rule is, to keep the furnace
always full of charcoal and ore. The revived tin is received immediately in the first
basin; then run off into the second, where it is allowed to settle for some time. The
scoriæ that run off into the first basin, are removed as soon as they fix. These scoriæ
are divided into two classes; namely, such as still retain tin oxide, and such as hold
none of the metal in that state, but only in granulations. The metallic bath is
divided, by repose, into horizontal zones, of different degrees of purity; the more
compound and denser matters falling naturally to the bottom of the basin. The
tin which forms the superior zones, being judged to be pure enough, is transvased by
ladles into the refining basin, previously heated, and under which, if it is of cast-iron,
a moderate fire is applied. The tin near the bottom of the receiving basin is always
laded out apart, to be again smelted; sometimes, indeed, when the furnace is turning
out very impure tin, none of it is transvased into the second basin; but the whole is
cast into moulds, to be again treated in the blast furnace.
In general they receive no other preparation, but the green wood ebullition, before
passing into the market. Sometimes, however, the block of metal is heated till it becomes
brittle, when it is lifted to a considerable height, and let fall, by which it is broken
to pieces, and presents an agglomeration of elongated grains or tears; whence it is
called grain tin.
On making a comparative estimate of the expense by the blowing-house process, and
by the reverberatory furnace, it has been found that the former yields about 66
per cent. of tin, in smelting the stream or alluvial ore, whose absolute contents are
from 75 to 78 parts of metal in the hundred. One ton of tin consumes a ton and
six-tenths of wood charcoal, and suffers a loss of 15 per cent. In working with the
reverberatory furnace, it is calculated that ore whose mean contents by an exact
analysis are 70 per cent., yields 65 per cent. on the great scale. The average value of
tin ore, as sold to the smelter, is 50 pounds sterling per ton; but it fluctuates, of course,
with the market prices. In 1824, the ore of inferior quality cost 30l., while the
purest sold for 60l. One ton of tin, obtained from the reverberatory furnace, cost—
| 11⁄2 tons of ore, worth |
£75 |
0 |
0 |
| 13⁄4 tons of coals, at 10s. per ton |
0 |
17 |
6 |
| Wages of labour, interest on capital, &c. |
3 |
0 |
0 |
| |
78 |
17 |
6 |
On comparing these results with the former, we perceive that in a blowing-house the
loss of tin is 15 per cent., whereas it is only 5 in the reverberatory furnace. The expense
in fuel is likewise much less relatively in the latter process; for only 13⁄4 tons of
coals are consumed for one ton of tin; while a ton and six-tenths of wood charcoal are
burned to obtain the same quantity of tin in the blowing-house; and it is admitted that
one ton of wood charcoal is equivalent to two tons of coal, in calorific effect. Hence every
thing conspires to turn the balance in favour of the reverberatory plan. The operation
is also, in this way, much simpler, and may be carried on by itself. The scoriæ, besides,
from the reverberatory hearth, contain less tin than those derived from the same
ores treated with charcoal by the blast, as is done at Altenberg. It must be remembered,
however, that the grain tin procured by the charcoal process is reckoned to be
finer, and fetches a higher price; a superiority partly due to the purity of the ore reduced,
and partly to the purity of the fuel.
To test the quality of tin, dissolve a certain weight of it with heat in muriatic acid;
should it contain arsenic, brown-black flocks will be separated during the solution, and
arseniuretted hydrogen gas will be disengaged, which, on being burned at a jet, will
deposit the usual gray film of metallic arsenic upon a white saucer held a little way
above the flame. Other metals present in the tin, are to be sought for, by treating the
above solution with nitric acid of spec. grav. 1·16, first in the cold, and at last with heat
and a small excess of acid. When the action is over, the supernatant liquid is to be decanted
off the peroxidized tin, which is to be washed with very dilute nitric acid, and both
liquors are to be evaporated to dissipate the acid excess. If, on the addition of water
to the concentrated liquor, a white powder falls, it is a proof that the tin contains
bismuth; if on adding sulphate of ammonia, a white precipitate appears, the tin contains
lead; water of ammonia added to supersaturation, will occasion reddish-brown[1251]
flocks, if iron is present; and on evaporating the supernatant liquid to dryness, the copper
will be obtained.
The uses of tin are very numerous. Combined with copper, in different proportions,
it forms bronze, and a series of other useful alloys; for an account of which see
Copper. With iron, it forms tin-plate; with lead, it constitutes pewter, and solder
of various kinds (see Lead). Tin-foil coated with quicksilver makes the reflecting
surface of glass mirrors. (See Glass.) Nitrate of tin affords the basis of the scarlet dye
on wool, and of many bright colours to the calico-printer and the cotton-dyer. (See
Scarlet and Tin Mordants.) A compound of tin with gold, gives the fine crimson
and purple colours to stained glass and artificial gems. (See Purple of Cassius.)
Enamel is made by fusing oxide of tin with the materials of flint glass. This oxide is
also an ingredient in the white and yellow glazes of pottery-ware.
An Account of Tin coined in Cornwall and Devon, from 1817 to 1829 inclusive:—
| Years. |
Blocks. |
Tons. |
| 1817 |
25,379 |
4,120 |
|
| 1818 |
23,048 |
3,745 |
1⁄3 |
| 1819 |
18,881 |
3,065 |
|
| 1820 |
17,084 |
2,773 |
1⁄2 |
| 1821 |
19,273 |
3,128 |
|
| 1822 |
18,732 |
3,137 |
|
| 1823 |
24,077 |
4,031 |
|
| 1824 |
28,602 |
4,819 |
|
| 1825 |
24,902 |
4,170 |
|
| 1826 |
26,299 |
4,406 |
|
| 1827 |
31,744 |
5,316 |
|
| 1828 |
28,179 |
4,696 |
|
| 1829 |
26,344 |
4,396 |
|
Tin
imported.
Duty, 50s.
per cwt. |
Tin
exported. |
| |
Cwts. |
Cwts. |
| 1827 |
2,217 |
2,938 |
| 1828 |
3,386 |
3,258 |
| 1829 |
2,674 |
2,581 |
| 1830 |
15,539 |
10,426 |
| 1831 |
8,099 |
12,226 |
| 1832 |
29,203 |
21,720 |
| 1833 |
35,124 |
39,850 |
| 1834 |
46,769 |
46,685 |
| 1835 |
17,705 |
23,796 |
| 1836 |
23,236 |
17,231 |
The principal importations are from the East India Company’s territories and
Ceylon:—they amounted in 1832 to 24,585 cwts.; in 1833 to 27,928; in 1834 to
33,611; in 1835 to 10,104; and in 1836 to 17,729. From Sumatra and Java
1961 cwts. were imported in 1832, and 1145 in 1834, but in the other years
greatly less.
| Declared value of tin and pewter wares and tin-plates exported in |
|
- |
1827. |
1829. |
1831. |
1833. |
1835. |
| 302,255l. |
235,178l. |
239,143l. |
282,176l. |
381,076l. |
| 1828. |
1830. |
1832. |
1834. |
1836. |
| 266,651l. |
249,657l. |
243,259l. |
337,056l. |
387,951l. |
Of these goods, from two-fifths to three-fifths go to the United States of America.
Abstract of Tin coined in Cornwall and Devon, in the year ending June 30, 1835;
from the Mining Review, vol. iii.
| Smelters. |
Blocks of
Grain Tin. |
Blocks of
Common Tin. |
Totals. |
| 1834. |
1835. |
1834. |
1835. |
1834. |
1835. |
| Daubuz and Co. |
728 |
875 |
6114 |
4494 |
6842 |
5369 |
| Grenfell and Boase |
344 |
196 |
3776 |
3097 |
4120 |
3293 |
| Bolitho and Sons |
229 |
153 |
3829 |
3099 |
4058 |
3252 |
| R. and J. Michell |
101 |
75 |
709 |
575 |
810 |
650 |
| Wheal Vor Adventurers |
— |
— |
3925 |
4069 |
3925 |
4069 |
| Taylor, Sons and Co. |
— |
112 |
— |
1250 |
— |
1362 |
| John Batten and Son |
28 |
49 |
2352 |
2351 |
2380 |
2400 |
| Joseph Carne |
— |
— |
896 |
851 |
896 |
851 |
| William Cornish |
— |
— |
622 |
574 |
622 |
574 |
| Gill and Co. |
(at Morwelham) |
— |
— |
758 |
— |
758 |
— |
| Ditto |
(at Calstock) |
60 |
— |
605 |
— |
665 |
— |
| Rundle, Paul and Co. |
— |
12 |
— |
1545 |
— |
1557 |
| |
Total |
1490 |
1472 |
23586 |
21905 |
25076 |
23377 |
| Total, in 1834, 4180 tons; in 1835, 3899 tons. (6 blocks = 1 ton.) |
[1252]
TINCTORIAL MATTER. One of the most curious and valuable facts ascertained
upon this subject, is, that madder kept in casks, in a warm place, undergoes
a species of fermentation, which, by ripening or rather deoxidizing the colouring-matter,
increases its dyeing power by no less than from 20 to 50 per cent. See M. H. Schlumberger’s
memoir read to the Société Industrielle de Mulhausen, 24 November, 1837.
TINCTURE is a title used by apothecaries to designate alcohol, in a somewhat
dilute state, impregnated with the active principles of either vegetable or animal substances.
TIN-GLASS, is a name of bismuth.
TIN MORDANTS, for dyeing scarlet:—
Mordant A, as commonly made by the dyers, is composed of 8 parts of aquafortis,
1 part of common salt or sal ammoniac, and 1 of granulated tin. This preparation
is very uncertain.
Mordant B.—Pour into a glass globe with a long neck, 3 parts of pure nitric acid at
30° B.; and 1 part of muriatic acid at 17°; shake the globe gently, avoiding the corrosive
vapours, and put a loose stopper in its mouth. Throw into this nitro-muriatic
acid, one-eighth of its weight of pure tin, in small bits at a time. When the solution
is complete, and settled, decant it into bottles, and close them with ground stoppers.
It should be diluted only when about to be used.
Mordant C, by Dambourney.—In two drams Fr. (144 grs.) of pure muriatic acid,
dissolve 18 grains of Malacca tin. This is reckoned a good mordant for brightening
or fixing the colour of peachwood.
Mordant D, by Hellot.—Take 8 ounces of nitric acid, diluted with as much water;
dissolve in it half an ounce of sal ammoniac, and 2 drams of nitre. In this acid
solution dissolve one ounce of granulated tin of Cornwall, observing not to put in a
fresh piece till the preceding be dissolved.
Mordant E, by Scheffer.—Dissolve one part of tin in four of a nitro-muriatic acid,
prepared with nitric acid diluted with its own weight of water, and one thirty-secondth
of sal ammoniac.
Mordant F, by Poërner.—Mix one pound of nitric acid with one pound of water, and
dissolve in it an ounce and a half of sal ammoniac. Stir it well, and add, by very slow
degrees, two ounces of tin turned into thin ribbons upon the lathe.
Mordant G, by Berthollet.—Dissolve in nitric acid of 30° B., one-eighth of its
weight of sal ammoniac, then add by degrees one-eighth of its weight of tin, and dilute
the solution with one-fourth of its weight of water.
Mordant K, by Dambourney.—In one dram (72 grs.) of muriatic acid at 17°,
one of nitric acid at 30°, and 18 grains of water, dissolve, slowly and with some heat,
18 grains of fine Malacca tin.
Mordant L, is the birch bark prescribed by Dambourney.—This bark, dried and
ground, is said to be a very valuable substance for fixing the otherwise fugitive colours
produced by woods, roots, archil, &c.
TIN-PLATE. The only alloy of iron interesting to the arts, is that with tin, in the
formation of tin-plate, or white-iron.
The sheet iron intended for this manufacture is refined with charcoal instead of coke,
subsequently rolled to various degrees of thinness, and cut into rectangles of different
sizes, by means of a shearing-machine driven by a water-wheel, which will turn out 100
boxes a day, or four times the number cut by hand labour. The first step towards tinning,
is to free the metallic surface from every particle of oxide or impurity, for any such
would inevitably prevent the iron from alloying with the tin. The plates are next bent
separately by hand into a saddle or Λ shape, and ranged in a reverberatory oven, so that the
flame may play freely among them, and heat them to redness. They are then plunged
into a bath, composed of four pounds of muriatic acid diluted with three gallons of
water, for a few minutes, taken out and drained on the floor, and once more exposed to
ignition in a furnace, whereby they are scaled, that is to say, cast their scales. The above
bath will suffice for scaling 1800 plates. When taken out, they are beat level and
smooth on a cast-iron block, after which they appear mottled blue and white, if the
scaling has been thoroughly done. They are next passed through chilled rolls or cast-iron
cylinders, rendered very hard by being cast in thick iron moulds, as has been long
practised by the Scotch founders in casting bushes for cart-wheels. After this process
of cold rolling, the plates are immersed, for ten or twelve hours, in an acidulous lye,
made by fermenting bran-water, taking care to set them separately on edge, and to turn
them at least once, so that each may receive a due share of the operation. From this lye-steep
they are transferred into a leaden trough, divided by partitions, and charged with
dilute sulphuric acid. Each compartment is called a hole by the workmen, and is calculated
to receive about 225 plates, the number afterwards packed up together in a box.
In this liquid they are agitated about an hour, till they become perfectly bright, and free[1253]
from such black spots as might stain their surface at the time of immersion. This process,
called pickling, is both delicate and disagreeable, requiring a good workman, at
high wages. The temperature of the two last steeps should be at least 90° or 100° F.,
which is kept up by stoves in the apartments. The plates are finally scoured with
hemp and sand in a body of water, and then put aside for use in a vessel of pure water,
under which they remain bright and free from rust for many months, a very remarkable
circumstance.
The tinning follows these preparatory steps. A range of rectangular cast-iron pots
is set over a fire-flue in an apartment called the stow, the workmen stationing themselves
opposite to the narrow ends. The first rectangle in the range is the tin-pot; the
second is the wash-pot, with a partition in it; the third is the grease-pot; the fourth is
the pan, grated at bottom; the fifth is the list-pot, and is greatly narrower than any of
the rest: they are all of the same length.
The prepared plates, dried by rubbing bran upon them, are first immersed one by one
in a pot filled with melted tallow alone, and are left there for nearly an hour. They
are thence removed, with the adhering grease, into pot No. 1., filled with a melted
mixture of block and grain tin, covered with about four inches of tallow, slightly carbonized.
This pot is heated by a fire, playing under its bottom and round its sides,
till the metal becomes so hot as nearly to inflame the grease. Here about 340
plates are exposed, upright, to the action of the tin for an hour and a half, or more,
according to their thickness. They are next lifted out, and placed upon an iron grating,
to let the superfluous metal drain off; but this is more completely removed in the next
process, called washing.
Into the wash-pot, No. 2., filled with melted grain tin, the workman puts the above
plates, where the heat detaches the ribs, and drops. There is a longitudinal partition
in it, for keeping the drop of tin that rises in washing from entering the vessel where
the last dip is given. Indeed, the metal in the wash-pot, after having acted on 60 or
70 boxes, becomes so foul, that the weight of a block (300 cwt.) of it, is transferred
into the tin-pot, No. 1., and replaced by a fresh block of grain tin. The plates being
lifted out of the wash-pot, with tongs held in the left hand of the workman, are scrubbed
on each side with a peculiar hempen brush, held in his right hand, then dipped for a
moment in the hot tin, and forthwith immersed in the adjoining grease-pot, No. 3. This
requires manual dexterity; and though only three-pence be paid for brushing and tin-washing
225 plates, yet a good workman can earn six shillings and three-pence in twelve
hours, by putting 5625 plates through his hands. The final tin-dip is useful to remove
the marks of the brush, and to make the surface uniformly bright. To regulate the
temperature of the tallow-pot, and time during which the plates are left in it, requires
great skill and circumspection on the part of the workman. If kept in it too long,
they would be deprived to a certain extent of their silvery lustre; and if too short,
streaks of tin would disfigure their surface. As a thick plate retains more heat after
being lifted out of the washing-pot, it requires a proportionally cooler grease-pot.
This pot has pins fixed within it, to keep the plates asunder; and whenever the workman
has transferred five plates to it, a boy lifts the first out into the cold adjoining pan,
No. 4.; as soon as the workman transfers a sixth plate, the boy removes the second; and
so on. The manufacture is completed by removing the wire of tin left on the under
edge of the plates, in consequence of their vertical position in the preceding operations.
This is the business of the list-boy, who seizes the plates when they are cool enough to
handle, and puts the lower edge of each, one by one, into the list-pot, No. 5., which
contains a very little melted tin, not exceeding a quarter of an inch in depth. When
he observes the wire-edge to be melted, he takes out the plate, and, striking it smartly
with a thin stick, detaches the superfluous metal, which leaves merely a faint stripe
where it lay. This mark may be perceived on every tin-plate in the market.
The plates are finally prepared for packing up in their boxes, by being well cleansed
from the tallow, by friction with bran.
Mr. Thomas Morgan obtained a patent, in September, 1829, for clearing the sheet-iron
plates with dilute sulphuric acid in a hole, instead of scaling them in the usual way,
previous to their being cold rolled, annealed, and tinned; whereby, he says, a better
article is produced at a cheaper rate.
Crystallized tin-plate, see Moirée Metallique. It would seem that the acid merely
lays bare the crystalline structure really present on every sheet, but masked by a film of
redundant tin. Though this showy article has become of late years vulgarized by its
cheapness, it is still interesting in the eyes of the practical chemist. The English tin-plates
marked F, answer well for producing the Moirée, by the following process. Place
the tin-plate, slightly heated, over a tub of water, and rub its surface with a sponge
dipped in a liquor composed of four parts of aquafortis, and two of distilled water,
holding one part of common salt or sal ammoniac in solution. Whenever the crystalline
spangles seem to be thoroughly brought out, the plate must be immersed in water,[1254]
washed either with a feather or a little cotton (taking care not to rub off the film of
tin that forms the feathering), forthwith dried with a low heat, and coated with a lacquer
varnish, otherwise it loses its lustre in the air. If the whole surface is not plunged at
once in cold water, but if it be partially cooled by sprinkling water on it, the crystallization
will be finely variegated with large and small figures. Similar results will be
obtained by blowing cold air through a pipe on the tinned surface, while it is just passing
from the fused to the solid state; or a variety of delineations may be traced, by
playing over the surface of the plate with the pointed flame of a blowpipe.
The following Table shows the several sizes of tin-plates, the marks by which they
are distinguished, and their current wholesale prices in London:—
| Names. |
Sizes. |
No.
in a
box. |
Weight of
each box. |
Marks on the boxes. |
Prices per
box, in |
| 1823. |
1838. |
| |
Inches. |
|
cwt. |
qrs. |
lbs. |
|
s. |
|
s. |
d. |
| Common, |
No. |
1. |
13 |
3⁄4 |
by |
10 |
|
225 |
1 |
0 |
0 |
CI. |
47 |
|
35 |
|
| Ditto |
2. |
13 |
1⁄4 |
— |
9 |
1⁄4 |
0 |
3 |
21 |
CII. |
45 |
|
33 |
6 |
| Ditto |
3. |
12 |
3⁄4 |
— |
9 |
1⁄2 |
0 |
3 |
16 |
CIII. |
43 |
|
32 |
9 |
| Cross, |
No. |
1. |
13 |
3⁄4 |
— |
10 |
|
1 |
1 |
0 |
XI. |
53 |
|
40 |
2 |
| Two crosses, |
1. |
1 |
1 |
21 |
XXI. |
58 |
|
43 |
2 |
| Three crosses, |
1. |
1 |
2 |
14 |
XXX. I. |
63 |
|
47 |
|
| Four crosses, |
1. |
1 |
3 |
7 |
XXXX. I. |
|
|
| Common doubles |
16 |
3⁄4 |
— |
12 |
1⁄2 |
100 |
0 |
3 |
21 |
CD. |
64 |
-6 |
|
- |
150 sheets in each. |
48 |
6 |
| Cross doubles |
1 |
0 |
14 |
XD. |
73 |
-6 |
56 |
|
| Two cross do. |
1 |
1 |
7 |
XXD. |
81 |
|
60 |
6 |
| Three cross do. |
1 |
2 |
0 |
XXXD. |
88 |
-6 |
65 |
|
| Four cross do. |
1 |
2 |
21 |
XXXXD. |
|
|
| Com. small doubles |
5 |
|
— |
11 |
|
200 |
1 |
2 |
0 |
CSD. |
69 |
|
51 |
6 |
| Cross do. do. |
1 |
2 |
21 |
XSD. |
75 |
|
56 |
0 |
| Two cross do. |
1 |
3 |
14 |
XXSD. |
80 |
|
59 |
6 |
| Three do. do. |
2 |
0 |
7 |
XXXSD. |
|
|
| Four do. do. |
2 |
1 |
0 |
XXXXSD. |
|
|
| Waster’s com. No. 1. |
3 |
3⁄4 |
— |
10 |
|
225 |
1 |
0 |
0 |
WCI. |
44 |
|
32 |
9 |
| Ditto |
cross, |
1. |
ditto |
1 |
1 |
0 |
WXI. |
50 |
|
47 |
3 |
These are the cash prices of one wholesale warehouse in Thames-street; an immediately
adjoining warehouse charges fully 1s. more upon the standard CI, and proportionally
upon the others.
TITANIUM, is a rare metal, discovered by Klaproth, in menachanite, in 1794.
It has been detected since in the form of small cubes of a copper-red colour, in some of
the blast furnaces in Yorkshire. According to Hassenfratz, its presence in small
quantity does not impair the malleability of iron. It is very brittle, so hard as to
scratch steel, and very light, having a specific gravity of only 5·3. It will not melt
in the heat of any furnace, nor dissolve, when crystallized, even in nitro-muriatic acid;
but only when in fine powder. By calcination with nitre, it becomes oxygenated, and
forms titanate of potassa. Traces of this metal may be detected in many irons, both
wrought and cast. The principal ores of titanium are sphene, common and foliated,
rutile, iserine, menachanite, and octahedrite or pyramidal titanium ore. None of them
has been hitherto applied to any use.
TOBACCO. It is said that the name tobacco was given by the Spaniards to the
plant, because it was first observed by them at Tabasco, or Tabaco, a province of Yucatan
in Mexico. In 1560, Nicot, the French ambassador to Portugal, having received some
tobacco from a Flemish merchant, showed it, on his arrival in Lisbon, to the grand
prior, and, on his return into France, to Catherine of Medicis, whence it has been called
Nicotiana by the botanists. Admiral Sir Francis Drake having, on his way home
from the Spanish Main, in 1586, touched at Virginia, and brought away some forlorn
colonists, is reported to have first imported tobacco into England. But, according to
Lobel, this plant was cultivated in Britain before the year 1570; and was consumed
by smoking in pipes by Sir Walter Raleigh, and companions, so early as the year 1584.
The plants are hung up to dry during four or five weeks; taken down out of the
sheds in damp weather, for in dry they would be apt to crumble into pieces; stratified in
heaps, covered up, and left to sweat for a week or two, according to their quality and the
state of the season; during which time they must be examined frequently, opened up,
and turned over, lest they become too hot, take fire, or run into putrefactive fermentation.
This process needs to be conducted by skilful and attentive operatives. An
experienced negro can form a sufficiently accurate judgment of the temperature, by
thrusting his hand down into the heap.
[1255]
The tobacco thus prepared, or often without fermentation, is sent into the market;
but, before being sold, it must undergo the inspection of officers, appointed by the state
with very liberal salaries, who determine its quality, and brand an appropriate stamp
upon its casks, if it be sound; but if it be bad, it is burned.
Our respectable tobacconists are very careful to separate all the damaged leaves, before
they proceed to their preparation, which they do by spreading them in a heap upon a stone
pavement, watering each layer in succession, with a solution of sea salt, of spec. grav. 1·107,
called sauce, till a ton or more be laid; and leaving their principles to react on each other for
three or four days, according to the temperature, and the nature of the tobacco. It is highly
probable that ammonia is the volatilizing agent of many odours, and especially of those
of tobacco and musk. If a fresh green leaf of tobacco be crushed between the fingers, it
emits merely the herbaceous smell common to many plants; but if it be triturated in a
mortar, along with a little quicklime or caustic potash, it will immediately exhale the
peculiar odour of snuff. Now analysis shows the presence of muriate of ammonia in
this plant, and fermentation serves further to generate free ammonia in it; whence, by
means of this process, and lime, the odoriferous vehicle is abundantly developed. If, on
the other hand, the excess of alkaline matter in the tobacco of the shops be saturated by
a mild dry acid, as the tartaric, its peculiar aroma will entirely disappear.
Tobacco contains a great quantity of an azotized principle, which by fermentation
produces abundance of ammonia; the first portions of which saturate the acid juices of
the plant, and the rest serve to volatilize its odorous principles. The salt water is useful
chiefly in moderating the fermentation, and preventing it from passing into the putrefactive
stage; just as salt is sometimes added to saccharine worts in tropical countries,
to temper the fermentative action. The sea salt, or concentrated sea water, which contains
some muriate of lime, tends to keep the tobacco moist, and is therefore preferable
to pure chloride of sodium for this purpose. Some tobacconists mix molasses with the
salt sauce, and ascribe to this addition the violet colour of the macouba snuff of Martinique;
and others add a solution of extract of liquorice. The following prescription is
that used by a skilful manufacturer:—In a solution of the liquorice juice, a few figs are
to be boiled for a couple of hours; to the decoction, while hot, a few bruised anise-seeds
are to be added, and when cold, common salt to saturation. A little silent spirit of
wine being poured in, the mixture is to be equably, but sparingly, sprinkled with the
rose of a watering-pot, over the leaves of the tobacco, as they are successively stratified
upon the preparation floor.
The fermented leaves, being next stripped of their middle ribs by the hands of children,
are sorted anew, and the large ones are set apart for making cigars. Most of the
tobaccos on sale in our shops are mixtures of different growths: one kind of smoking
tobacco, for example, consists of 70 parts of Maryland, and 30 of meagre Virginia; and
one kind of snuff consists of 80 parts of Virginia, and 30 parts of either Humesfort or
Warwick. The Maryland is a very light tobacco, in thin yellow leaves; that of Virginia
is in large brown leaves, unctuous or somewhat gluey on the surface, having a
smell somewhat like the figs of Malaga; that of Havannah is in brownish, light leaves,
of an agreeable and rather spicy smell; it forms the best cigars. The Carolina tobacco
is less unctuous than the Virginian; but in the United States it ranks next to the
Maryland.
The shag tobacco is dried to the proper point upon sheets of copper.
Tobacco is cut into what is called shag tobacco by knife-edged chopping stamps, a machine
somewhat similar to that represented under Metallurgy, fig. 670. For grinding
the tobacco leaves into snuff, conical mortars are employed, somewhat like that used by
the Hindoos for grinding sugar-canes, fig. 1080.; but the sides of the snuff-mill have
sharp ridges from the top to near the bottom.
Mr. L. W. Wright obtained a patent in August, 1827, for a tobacco-cutting
machine, which bears a close resemblance to the well-known machines with revolving
knives, for cutting straw into chaff. The tobacco, after being squeezed into cakes, is
placed upon a smooth bed within a horizontal trough, and pressed by a follower and
screws to keep it compact. These cakes are progressively advanced upon the bed, or
fed in, to meet the revolving blades. The speed of the feeding-screw determines the
degree of fineness of the sections or particles into which the tobacco is cut.
I was employed some years ago by the Excise, to analyze a quantity of snuff, seized
on suspicion of having been adulterated by the manufacturer. I found it to be largely
drugged with pearl-ashes, and to be thereby rendered very pungent, and absorbent of
moisture; an economical method of rendering an effete article at the same time active
and aqueous.
According to the recent analysis of Possett and Reimann, 10,000 parts of tobacco-leaves
contain—6 of the peculiar chemical principle nicotine; 1 of nicotianine; 287 of
slightly bitter extractive; 174 of gum, mixed with a little malic acid; 26·7 of a green
resin; 26 of vegetable albumen; 104·8 of a substance analogous to gluten; 51 of[1256]
malic acid; 12 of malate of ammonia; 4·8 of sulphate of potassa; 6·3 of chloride of
potassium; 9·5 of potassa, which had been combined with malic and nitric acids;
16·6 of phosphate of lime; 24·2 of lime, which had been combined with malic acid;
8·8 of silica; 496·9 of fibrous or ligneous matter; traces of starch; and 88·28 of water.
Nicotine is a transparent colourless liquid, of an alkaline nature. It may be distilled
in a retort plunged into a bath heated to 290° Fahrenheit. It has a pricking,
burning taste, which is very durable; and a pungent disagreeable smell. It burns by
means of a wick, with the diffusion of a vivid light, and much smoke. It may be
mixed with water in all proportions. It is soluble also in acetic acid, oil of almonds,
alcohol, and ether, but not in oil of turpentine. It acts upon the animal economy
with extreme violence; and in the dose of one drop it kills a dog. It forms salts
with the acids. About one part of it may be obtained by very skilful treatment
from one thousand of good tobacco.
Tobacco imported into the United Kingdom, viz.—unmanufactured, in 1836,
52,232,907 lbs.; in 1837, 27,070,448 lbs.;—manufactured, and snuff, in 1836,
182,248 lbs.; in 1837, 642,287 lbs. Retained for home consumption, unmanufactured,
in 1836, 22,309,021 lbs.; in 1837, 22,504,343 lbs.:—manufactured, and snuff, in 1836,
159,226 lbs.; in 1837, 145,045 lbs. Duty received,—on unmanufactured tobacco, in
1836, £3,344,703; in 1837, £3,375,125; on manufactured tobacco, and snuff, in 1836,
£71,560; in 1837, £65,220.
TOBACCO-PIPES. The practice of smoking tobacco has become so general in
many nations as to render the manufacture of tobacco-pipes a considerable branch of
industry. Some seek in the inhalation of tobacco-smoke a pleasurable narcotism; others
imagine it to be beneficial to their health; but, in general, smoking is merely a dreamy
resource against ennui, which ere long becomes an indispensable stimulus. The
filthiness of this habit, the offensive odour which persons under its influence emit from
their mouths and clothes, the stupor it too often occasions, as well as the sallow complexion,
black or carious teeth, and impaired digestion, all prove the great consumption
of tobacco to be akin in evil influence upon mankind to the use of ardent spirits.
Tobacco-pipes are made of a fine-grained plastic white clay, to which they have given
the name. It is worked with water into a thin paste, which is allowed to settle in pits,
or it may be passed through a sieve, to separate the siliceous or other stony impurities;
the water is afterwards evaporated till the clay becomes of a doughy consistence,
when it must be well kneaded to make it uniform. Pipe-clay is found chiefly
in the isle of Purbeck and Dorsetshire. It is distinguished by its perfectly white
colour, and its great adhesion to the tongue after it is baked; owing to the large proportion
of alumina which it contains.
A child fashions a ball of clay from the heap, rolls it out into a slender cylinder upon
a plank, with the palms of his hands, in order to form the stem of the pipe. He sticks
a small lump to the end of the cylinder for forming the bowl; which having done, he
lays the pieces aside for a day or two, to get more consistence. In proportion as he
makes these rough figures, he arranges them by dozens on a board, and hands them to
the pipemaker.
The pipe is finished by means of a folding brass or iron mould, channelled inside of
the shape of the stem and the bowl, and capable of being opened at the two ends. It is
formed of two pieces, each hollowed out like a half-pipe, cut as it were lengthwise; and
these two jaws, when brought together, constitute the exact space for making one pipe.
There are small pins in one side of the mould, corresponding to holes in the other,
which serve as guides for applying the two together with precision.
The workman takes a long iron wire, with its end oiled, and pushes it through the
soft clay in the direction of the stem, to form the bore, and he directs the wire by feeling
with his left hand the progress of its point. He lays the pipe in the groove of one of
the jaws of the mould, with the wire sticking in it; applies the other jaw, brings them
smartly together, and unites them by a clamp or vice, which produces the external
form. A lever is now brought down, which presses an oiled stopper into the bowl of
the pipe, while it is in the mould, forcing it sufficiently down to form the cavity; the
wire being meanwhile thrust backwards and forwards so as to pierce the tube completely
through. The wire must become visible at the bottom of the bowl, otherwise the pipe
will be imperfect. The wire is now withdrawn, the jaws of the mould opened, the pipe
taken out, and the redundant clay removed with a knife. After drying for a day or
two, the pipes are scraped, polished with a piece of hard wood, and the stems being bent
into the desired form, they are carried to the baking kiln, which is capable of firing
fifty gross in from 8 to 12 hours. A workman and a child can easily make five gross of
pipes in a day.
No tobacco-pipes are so highly prized as those made in Natolia, in Turkey, out of
meerschaum, a somewhat plastic magnesian stone, of a soft greasy feel, which is formed
into pipes after having been softened with water. It becomes white and hard in the kiln.
[1257]
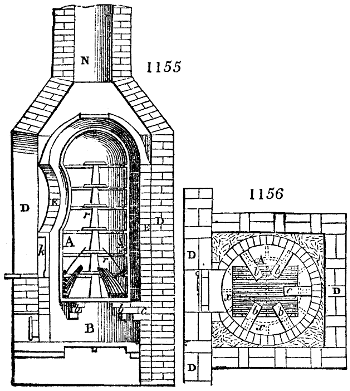
A tobacco-pipe kiln should diffuse an equal heat to every part of its interior, while
it excludes the smoke of the fire. The crucible, or large sagger, A, A, figs. 1155. and
1156., is a cylinder, covered in with a dome. It is placed
over the fireplace B, and enclosed within a furnace of ordinary
brickwork D, D, lined with fire-bricks E, E. Between this lining
and the cylinder, a space of about 4 inches all round is left
for the circulation of the flame. There are 12 supports or
ribs between the cylinder and the furnace lining, which form
so many flues, indicated by the dotted lines x, in fig. 1156.
(the dotted circle representing the cylinder). These ribs are
perforated with occasional apertures, as shown in fig. 1155.,
for the purpose of connecting the adjoining flues; but the main
bearing of the hollow cylinder is given
by five piers, b, b, c, formed of bricks
projecting over and beyond each other.
One of these piers c, is placed at the
back of the fireplace, and the other four
at the sides b, b. These project nearly
into the centre, in order to support and
strengthen the bottom; while the flues
pass up between them, unite at the top
of the cylinder in the dome L, and discharge
the smoke by the chimney N.
The lining F, E, E, of the chimney is
open on one side to form the door, by which the cylinder is charged and discharged.
The opening is permanently closed as high as k, fig. 1155., by an iron plate plastered
over with fire-clay; above this it is left open, and shut merely with temporary brickwork
while the furnace is going. When this is removed, the furnace can be
filled or emptied through the opening, the cylindric crucible having a correspondent
aperture in its side, which is closed in the following ingenious way, while the furnace
is in action. The workman first spreads a layer of clay round the edge of the opening,
he then sticks the stems of broken pipes across from one side to the other, and
plasters up the interstices with clay, exactly like the lath-and-plaster work of a
ceiling. The whole of the cylinder, indeed, is constructed in this manner, the bottom
being composed of a great many fragments of pipe stems, radiating to the centre; these
are coated at the circumference with a layer of clay. A number of bowls of broken
pipes are inserted in the clay; in these other fragments are placed upright to form the
sides of the cylinder. The ribs round the outside, which form the flues, are made in the
same way, as well as the dome L; by which means the cylindric case may be made very
strong, and yet so thin as to require little clay in the building, a moderate fire to heat
it, while it is not apt to split asunder. The pipes are arranged within, as shown in the
figure, with their bowls resting against the circumference and their ends supported on
circular pieces of clay r, which are set up in the centre for that purpose. Six small ribs
are made to project inwards all round the crucible, at the proper heights, to support the
different ranges of pipes, without having so many resting on each other as to endanger
their being crushed by the weight. By this mode of distribution, the furnace may contain
50 gross, or 7200 pipes, all baked within 8 or 9 hours; the fire being gradually
raised, or damped if occasion be, by a plate partially slid over the chimney top.
TODDY, Sura, Mee-ra, sweet juice.—The proprietors of coco-nut plantations in
the peninsula of India, and in the Island of Ceylon, instead of collecting a crop of nuts,
frequently reap the produce of the trees by extracting sweet juice from the flower-stalk.
When the flowering branch is half shot, the toddy-drawers bind the stock round
with a young coco-nut leaf in several places, and beat the spadix with a short baton of
ebony. This beating is repeated daily for ten or twelve days, and about the end of
that period a portion of the flower-stalk is cut off. The stump then begins to bleed,
and an earthen vessel (chatty) or a calabash is suspended under it, to receive the juice,
which is by the Europeans called toddy.
A thin slice is taken from the stump daily, and the toddy is removed twice a day.
A coco-nut frequently pushes out a new spadix once a month; and after each spadix
begins to bleed, it continues to produce freely for a month, by which time another is
ready to supply its place. The old spadix continues to give a little juice for another
month, after which it withers; so that there are sometimes two pots attached to a tree
at one time, but never more. Each of these spadices, if allowed to grow, would produce
a bunch of nuts from two to twenty. Trees in a good soil produce twelve
bunches in the year; but when less favourably situated, they often do not give more
than six bunches. The quantity of six English pints of toddy is sometimes yielded by
a tree daily.
[1258]
Toddy is much in demand as a beverage in the neighbourhood of villages, especially
where European troops are stationed. When it is drunk before sunrise, it is a
cool, delicious, and particularly wholesome beverage; but by eight or nine o’clock fermentation
has made some progress, and it is then highly intoxicating.[68]
TOLU, is a brownish-red balsam, extracted from the stem of the Myroxilon
toluiferum, a tree which grows in South America. It is composed of resin, oil,
and benzoic acid. Having an agreeable odour, it is sometimes used in perfumery.
It has a place in the Materia Medica, but for what good reason I know not.
TOMBAC, is a white alloy of copper.
TONKA BEAN, the fruit of the Dipterix odorata, affords a concrete crystalline
volatile oil (stearoptène), called coumarine by the French. It is extracted by digestion
with alcohol, which dissolves the stearoptène, and leaves a fat oil. It has an
agreeable smell, and a warm taste. It is fusible at 122° Fahrenheit, and volatile at
higher heats.
TORTOISE-SHELL, or rather scales, a horny substance, that covers the hard
strong covering of a bony contexture, which encloses the Testudo imbricata, Linn. The
lamellæ or plates of this tortoise are 13 in number, and may be readily separated from
the bony part by placing fire beneath the shell, whereby they start asunder. They vary
in thickness from one-eighth to one-quarter of an inch, according to the age and size of
the animal, and weigh from 5 to 25 pounds. The larger the animal, the better is the
shell. This substance may be softened by the heat of boiling water; and if compressed
in this state by screws in iron or brass moulds, it may be bent into any shape. The
moulds being then plunged in cold water, the shell becomes fixed in the form imparted
by the mould. If the turnings or filings of tortoise-shell be subjected skilfully to gradually
increased compression between moulds immersed in boiling water, compact
objects of any desired ornamental figure or device may be produced. The soldering of
two pieces of scale is easily effected, by placing their edges together, after they are nicely
filed to one bevel, and then squeezing them strongly between the long flat jaws of hot
iron pincers, made somewhat like a hairdresser’s curling-tongs. The pincers should be
strong, thick, and just hot enough to brown paper slightly, without burning it. They may
be soldered also by the heat of boiling water, applied along with skilful pressure. But
in whatever way this process is attempted, the surfaces to be united should be made very
smooth, level, and clean; the least foulness, even the touch of a finger, or breathing upon
them, would prevent their coalescence. See Horn.
TOUCH-NEEDLES, and TOUCH-STONE, are means of ascertaining the
quality of gold trinkets. See Assay.
TRAGACANTH, GUM. (Gomme adracante, Fr.; Traganth, Germ.) See Gum.
TREACLE, is the viscid brown uncrystallizable syrup which drains from the sugar-refining
moulds. Its specific gravity is generally 1·4, and it contains upon an average
75 per cent. of solid matter, by my experiments.
TRIPOLI (Terre pourrie, Fr.; Tripel, Germ.); rotten-stone; is a mineral of an
earthy fracture, a yellowish-gray or white colour, composition impalpably fine, meagre
to the touch, does not adhere to the tongue, and burns white. Its analogue, the
Polierschiefer, occurs in thin flat foliated pieces, of the above colours, occasionally
striped; soft, absorbent of water; spec. grav. 1·9. to 2·2.
M. Ehrenberg has shown that both of these friable homogeneous rocks, which consist
almost entirely of silica, are actually composed of the exuviæ or rather the skeletons of
infusoria (animalcula) of the family of Barcillariæ, and the genera Cocconema, Gonphonema,
&c. They are recognised with such distinctness in the microscope, that their analogies
with living species may be readily traced; and in many cases there are no appreciable
differences between the living and the petrified. The species are distinguished by the
number of partitions or transverse lines upon their bodies. The length is about 1⁄288 of a
line. M. Ehrenberg made his observations upon the tripolis of Billen in Bohemia of
Santafiora in Tuscany, of the Isle of France, and of Francisbad, near Eger.
The meadow iron ore (Fer limoneux des marais) is composed almost wholly of the
Gaellonella ferruginea. Most of these infusoria are lacustrine; but others are marine,
particularly the tripolis of the Isle of France.
According to the chemical analysis of Bucholz, tripoli consists of—silica, 81; alumina,
1·5; oxide of iron, 8; sulphuric acid, 3·45; water, 4·55. This specimen was probably
found in a coal-field. The tripoli of Corfu is reckoned the best for scouring or brightening
brass and other metals. Mr. Phillips found in the Derbyshire rotten-stone (near[1259]
Bakewell), 86 of alumina, 4 of silica, and 10 of carbon—being a remarkable difference
in composition from the Bohemian.
TUFA, or TUF, is a gray deposit of calcareous carbonate, from springs and
streams.
TULA METAL, is an alloy of silver, copper, and lead.
TUNGSTEN (Eng. and Fr.; Wolfram, Germ.); is a peculiar metal, which occurs
in the state of an acid (the tungstic), combined with various bases, as with lime, the
oxides of iron, manganese, and lead. The metal is obtained by reduction of the ore, or
the deoxidizement of the acid, in the form of a dark steel-gray powder, which assumes
under the burnisher a feeble metallic lustre. Its specific gravity is 17·22.
TURBITH MINERAL, is the yellow subsulphate of mercury.
TURF (Peat, Scotch; Tourbe, Fr.; Torf, Germ.); consists of vegetable matter,
chiefly of the moss family, in a state of partial decomposition by the action of water.
Cut, during summer, into brick-shaped pieces, and dried, it is extensively used as fuel
by the peasantry in every region where it abounds. The dense black turf, which forms
the lower stratum of a peat-moss, is much contaminated with iron, sulphur, sand, &c.,
while the lighter turf of the upper strata, though nearly pure vegetable matter, is too
bulky for transportation, and too porous for factory fuel. These defects have been
happily removed by Mr. Williams, managing director of the Dublin Steam Navigation
Company, who has recently obtained a patent for a method of converting the lightest
and purest beds of peat-moss, or bog, into the four following products: 1. A brown
combustible solid, denser than oak; 2. A charcoal, twice as compact as that of hard
wood; 3. A factitious coal; and 4. A factitious coke; each of which possesses very
valuable properties.
Mr. D’Ernst, artificer of fire-works to Vauxhall, has proved, by the severe test of
coloured fires, that the turf charcoal of Mr. Williams is 20 per cent. more combustible
than that of oak. Mr. Oldham, engineer of the Bank of England, has applied it in
softening his steel plates and dies, with remarkable success. But one of the most important
results of Mr. Williams’s invention is, that with 10 cwts. of pitcoal, and 21⁄2 cwts.
of his factitious coal, the same steam power is now obtained, in navigating the Company’s
ships, as with 171⁄2 cwts. of pitcoal alone; thereby saving 30 per cent. in the
stowage of fuel. What a prospect is thus opened up of turning to admirable account
the unprofitable bogs of Ireland; and of producing, from their inexhaustible stores, a
superior fuel for every purpose of arts and engineering.
The turf is treated as follows:—Immediately after being dug, it is triturated under
revolving edge-wheels, faced with iron plates perforated all over their surface, and is
forced by the pressure through these apertures, till it becomes a species of pap, which is
freed from the greater part of its moisture by squeezing in a hydraulic press between
layers of caya cloth, then dried, and coked in suitable ovens.—(See Charcoal, and Pitcoal,
coking of.) Mr. Williams makes his factitious coal by incorporating with pitch
or rosin, melted in a cauldron, as much of the above charcoal, ground to powder, as
will form a doughy mass, which is moulded into bricks in its hot and plastic state.
From the experiments of M. Le Sage, detailed in the 5th volume of “The Repertory of
Arts,” charred ordinary turf seems to be capable of producing a far more intense heat
than common charcoal. It has been found preferable to all other fuel for case-hardening
iron, tempering steel, forging horseshoes, and welding gun-barrels. Since turf is partially
carbonized in its native state, when it is condensed by the hydraulic press, and
fully charred, it must evidently afford a charcoal very superior in calorific power to the
porous substance generated from wood by fire.
TURKEY RED, is a brilliant dye produced on cotton goods by Madder.
TURMERIC, Curcuma, Terra merita, (Souchet, or Safran des Indes, Fr.; Gelbwurzel,
Germ.); is the root of the Curcuma longa and rotunda, a plant which grows in
the East Indies, where it is much employed in dyeing yellow, as also as a condiment in
curry sauce or powder. The root is knotty, tubercular, oblong, and wrinkled; pale-yellow
without, and brown-yellow within; of a peculiar smell, a taste bitterish and
somewhat spicy. It contains a peculiar yellow principle, called curcumine, a brown,
colouring-matter, a volatile oil, starch, &c. The yellow tint of turmeric is changed to
brown-red by alkalis, alkaline earths, subacetate of lead, and several metallic oxides; for
which reason, paper stained with it is employed as a chemical test.
Turmeric is employed by the wool-dyers for compound colours which require an
admixture of yellow, as for cheap browns and olives. As a yellow dye, it is employed
only upon silk. It is a very fugitive colour. A yellow lake may be made by boiling
turmeric powder with a solution of alum, and pouring the filtered decoction upon
pounded chalk.
TURNSOLE. See Archil and Litmus.
[1260]
TURPENTINE (Térébinthine, Fr.; Terpenthin, Germ.); is a substance which
flows out of incisions made in the stems of several species of pines. It has the
consistence and gray-yellow colour of honey. It has a smell which is not disagreeable
to many persons, a warm, sharp, bitterish taste; dries into a solid in the air,
with the evaporation of its volatile oil. It becomes quite fluid at a moderate elevation
of temperature, and burns at a higher heat, with a bright but very fuliginous
flame. There are several varieties of turpentine.
1. Common turpentine, is extracted from incisions in the Pinus abies and Pinus silvestris.
It has little smell; but a bitter burning taste. It consists of the volatile oil
of turpentine to the amount of from 5 to 25 per cent.; and of rosin or colophony.
2. Venice turpentine, is extracted from the Pinus larix (larch), and the French turpentine
from the Pinus maritima. The first comes from Styria, Hungary, the Tyrol,
and Switzerland, and contains from 18 to 25 per cent. of oil; the second, from the south
of France, and contains no more than 12 per cent. of oil. The oil of all the turpentines
is extracted by distilling them along with water. They dissolve in all proportions
in alcohol, without leaving any residuum. They also combine with alkaline lyes,
and in general with the salifiable bases. Venice turpentine contains also succinic acid.
3. Turpentine of Strasbourg is extracted from the Pinus picea and Abies excelsa.
It affords 33·5 per cent. of volatile oil, and some volatile or crystallizable resin, with
extractive matter and succinic acid.
4. Turpentine of the Carpathian mountains, and of Hungary; the first of which
comes from the Pinus cembra, and the second from the Pinus mugos. They resemble
that of Strasbourg.
5. Turpentine of Canada, called Canada balsam, is extracted from the Pinus canadensis
and balsamea. Its smell is much more agreeable than that of the preceding
species.
6. Turpentine of Cyprus or Chio, is extracted from the Pistacea terebinthus. It has
a yellow, greenish, or blue-green colour. Its smell is more agreeable, and taste less
acrid, than those of the preceding sorts.
Common Turpentine imported into the United Kingdom, in 1836, 370,981 cwts.
1 qr. 26 lbs.; in 1837, 415,023 cwts. 1 qr. 10 lbs. Retained for home consumption, in
1836, 341,693 cwts. 18 lbs.; in 1837, 405,772 cwts. 2 qrs. 14 lbs. Duty received, in
1836, £74,052; in 1837, £87,918.
TURPENTINE, OIL OF, sometimes called essence of turpentine. As found
in commerce, it contains more or less rosin, from which it may be freed by re-distillation
along with water. It is colourless, limpid, very fluid, and possessed of a very peculiar
smell. Its specific gravity, when pure, is 0·870; that of the oil commonly sold in
London, is 0·875. It always reddens litmus paper, from containing a little succinic
acid. According to Oppermann, the oil which has been repeatedly rectified over
chloride of calcium, consists of 84·60 carbon, 11·735 hydrogen, and 3·67 oxygen.
When oil of turpentine contains a little alcohol, it burns with a clear flame; but otherwise
it affords a very smoky flame. Chlorine inflames this
oil; and muriatic acid converts it into a crystalline substance,
like camphor. It is employed extensively in varnishes,
paints, &c., as also in medicine.
TUTENAG, is an alloy of copper and zinc.
TYPE, (Caractère, Fr.; Druckbuchstabe, Germ.) The first
care of the letter-cutter is to prepare well-tempered steel
punches, upon which he draws or marks the exact shape of
the letter, with pen and ink if it be large, but with a smooth
blunted point of a needle if it be small; and then, with a
proper sized and shaped graver and sculpter, he digs or scoops
out the metal between the strokes upon the face of the punch,
leaving the marks untouched and prominent. He next works
the outside with files till it be fit for the matrix. Punches
are also made by hammering down the hollows, filing up
the edges, and then hardening the soft steel. Before he proceeds
to sink and justify the matrix, he provides a mould to
justify them by, of which a good figure is shown in plate XV.,
Miscellany, figs. 2. 3. of Rees’s Cyclopædia.
A matrix is a piece of brass or copper, about an inch and
a half long, and thick in proportion to the size of the letter which it is to contain. In
this metal the face of the letter intended to be cast is sunk, by striking it with the
punch to a depth of about one eighth of an inch. The mould, fig. 1157., in which the
types are cast, is composed of two parts. The outer part is made of wood, the inner of
steel. At the top it has a hopper-mouth a, into which the fused type-metal is poured.
The interior cavity is as uniform as if it had been hollowed out of a single piece of steel;[1261]
because each half, which forms two of the four sides of the letter, is exactly fitted to
the other. The matrix is placed at the bottom of the mould, directly under the centre
of the orifice, and is held in its position by a spring b. Every letter that is cast can
be loosened from the matrix only by removing the pressure on the spring.
A good type-foundry is always provided with several furnaces, each surmounted with an
iron pot containing the melted alloy, of 3 parts of lead and 1 of antimony. Into this
pot the founder dips the very small iron ladle, to lift merely as much metal as will cast a
single letter at a time. Having poured in the metal with his right hand, and returned
the ladle to the melting-pot, the founder throws up his left hand, which holds the mould,
above his head, with a sudden jerk, supporting it with his right hand. It is this movement
which forces the metal into all the interstices of the matrix; for without it, the
metal, especially in the smaller moulds, would not be able to expel the air and reach the
bottom. The pouring in the metal, the throwing up the mould, the unclosing it, removing
the pressure of the spring, picking out the cast letter, closing the mould again,
and re-applying the spring to be ready for a new operation, are all performed with such
astonishing rapidity and precision, that a skilful workman will turn out 500 good letters
in an hour, being at the rate of one every eighth part of a minute. A considerable
piece of metal remains attached to the end of the type as it quits the mould. There are
nicks upon the lower edge of the types, to enable the compositor to place them upright,
without looking at them.
From the table of the caster, the heap of types turned out of his mould, is transferred
from time to time to another table, by a boy, whose business it is to break off the superfluous
metal, and that he does so rapidly as to clear from 2000 to 5000 types in an hour;
a very remarkable dispatch, since he must seize them by their edges, and not by their
feeble flat sides. From the breaking-off boy, the types are taken to the rubber, a man
who sits in the centre of the workshop with a grit-stone slab on a table before him, and
having on the fore and middle finger of his right hand a piece of tarred leather, passes
each broad side of the type smartly over the stone, turning it in the movement, and that
so dexterously, as to be able to rub 2000 types in an hour.
From the rubber, the types are conveyed to a boy, who, with equal rapidity sets them
up in lines, in a long shallow frame, with their faces uppermost and nicks outwards.
This frame, containing a full line, is put into the dresser’s hands, who polishes them on
each side, and turning them with their faces downwards, cuts a groove or channel in
their bottom, to make them stand steadily on end. It is essential that each letter be
perfectly symmetrical and square; the least inequality of their length would prevent
them from making a fair impression; and were there the least obliquity in their sides,
it would be quite impossible, when 200,000 single letters are combined, as in one side
of the Times newspaper, that they could hold together as they require to do, when wedged
up in the chases, as securely as if that side of type form a solid plate of metal. Each
letter is finally tied up in lines of convenient length, the proportionate numbers of each
variety, small letters, points, large capitals, small capitals, and figures, being selected,
when the fount of type is ready for delivery to the printer.
The sizes of types cast in this country vary, from the smallest, called diamond, of
which 205 lines are contained in a foot length, to those letters employed in placards, of
which a single letter may be 3 or 4 inches high. The names of the different letters and
their dimensions, or the number of lines which each occupies in a foot, are stated in the
following table:—
| Double Pica |
41 |
1⁄2 |
| Paragon |
44 |
1⁄2 |
| Great Primer |
51 |
1⁄4 |
| English |
64 |
|
| Pica |
71 |
1⁄2 |
| Small Pica |
83 |
|
| Long Primer |
89 |
|
| Bourgeois |
102 |
1⁄4 |
| Brevier |
112 |
1⁄2 |
| Minion |
128 |
|
| Nonpareil |
143 |
|
| Pearl |
178 |
|
| Diamond |
205 |
|
T. Aspinwall, Esq., the American consul, obtained, in May, 1828, a patent for an improved
method of casting printing types by means of a mechanical process, being a communication
from a foreigner residing abroad. The machine is described, with six
explanatory figures, in the second series of Newton’s Journal, vol. v. page 212. The
patentee does not claim, as his invention, any of the parts separately, but the general
process and arrangement of machinery; more particularly the manner of suspending a
swing table (upon which the working parts are mounted) out of the horizontal and perpendicular
position; the mode of moving the table with the parts of the mould towards
the melting-pot; the manner of bringing the parts of the mould together, and keeping
them closed during the operation of casting the types. Several other mechanical schemes
have been proposed for founding types, but I have been informed by very competent
judges, Messrs. Clowes, that none of them can compete in practical utility with that
dexterity and precision of handiwork, which I have often seen practised in their great
printing establishment in Stamford-street.
[1262]
ULTRAMARINE (Outremer, Fr.; Ultramarins, Germ.); is a beautiful blue
pigment obtained from the variegated blue mineral, called lazulite (lapis lazuli), by the
following process:—Grind the stone to fragments, rejecting all the colourless bits,
calcine at a red heat, quench in water, and then grind to an impalpable powder along
with water, in a paint-mill, (see Paints, grinding of,) or with a porphyry slab and
muller. The paste being dried, is to be rubbed to powder, and passed through a silk
sieve. 100 parts of it are to be mixed with 40 of rosin, 20 of white wax, 25 of linseed
oil, and 15 of Burgundy pitch, previously melted together. This resinous compound is to
be poured hot into cold water; kneaded well first with two spatulas, then with the hands,
and then formed into one or more small rolls. Some persons prescribe leaving these pieces
in the water during 15 days, and then kneading them in it, whereby they give out the
blue pigment, apparently because the ultramarine matter adheres less strongly than the
gangue, or merely siliceous matter of the mineral, to the resinous paste. MM. Clement
and Desormes, who were the first to divine the true nature of this pigment, think that
the soda contained in the lazulite, uniting with the oil and the rosin, forms a species of
soap, which serves to wash out the colouring-matter. If it should not separate readily,
water heated to about 150° F. should be had recourse to. When the water is sufficiently
charged with blue colour, it is poured off and replaced by fresh water; and the
kneading and change of water are repeated till the whole of the colour is extracted.
Others knead the mixed resinous mass under a slender stream of water, which runs off
with the colour into a large earthen pan. The first waters afford, by rest, a deposit of
the finest ultramarine; the second, a somewhat inferior article, and so on. Each must
be washed afterwards with several more waters, before they acquire the highest quality
of tone; then dried separately, and freed from any adhering particles of the pitchy
compound by digestion in alcohol. The remainder of the mass being melted with
oil, and kneaded in water containing a little soda or potash, yields an inferior pigment,
called ultramarine ashes. The best ultramarine is a splendid blue pigment, which works
well with oil, and is not liable to change by time. Its price in Italy was five guineas
the ounce, a few years ago, but it is now greatly reduced.
The blue colour of lazulite had been always ascribed to iron, till MM. Clement
and Desormes, by a most careful analysis, showed it to consist of—silica, 34; alumina, 33;
sulphur, 3; soda, 22; and that the iron, carbonate of lime, &c. were accidental ingredients,
essential neither to the mineral, nor to the pigment made from it. By another
analyst, the constituents are said to be—silica, 44; alumina, 35; and soda, 21; and by a
third, potassa was found instead of soda, showing shades of difference in the composition
of the stone.
Till a few years ago, every attempt failed to make ultramarine artificially. At length,
in 1828, M. Guimet resolved the problem, guided by the analysis of MM. Clement and
Desormes, and by an observation of M. Tassaert, that a blue substance like ultramarine
was occasionally produced on the sandstone hearths of his reverberatory soda furnaces.
Of M. Guimet’s finest pigment I received a bottle several years ago, from my friend
M. Merimée, secretary of the Ecole de Beaux Arts, which has been found by artists little,
if any, inferior to the lazulite ultramarine. M. Guimet sells it at 60 francs per pound
French,—which is little more than two guineas the English pound. He has kept
his process secret. But M. Gmelin, of Tübingen, has published a prescription for
making it; which consists in enclosing carefully in a Hessian crucible a mixture of 2
parts of sulphur, and 1 of dry carbonate of soda, heating them gradually to redness till
the mass fuses, and then sprinkling into it by degrees another mixture, of silicate of
soda, and aluminate of soda; the first containing 72 parts of silica, and the second 70
parts of alumina. The crucible must be exposed after this for an hour to the fire.
The ultramarine will be formed by this time; only it contains a little sulphur, which
can be separated by means of water. M. Persoz, professor of chemistry at Strasbourg,
has likewise succeeded in making an ultramarine, of perhaps still better quality than
that of M. Guimet. Lastly, M. Robiquet has announced, that it is easy to form
ultramarine, by heating to redness a proper mixture of kaolin (China clay), sulphur, and
carbonate of soda. It would therefore appear, from the preceding details, that ultramarine
may be regarded as a compound of silicate of alumina, silicate of soda, with
sulphuret of sodium; and that to the reaction of the last constituent upon the former
two, it owes its colour.
UMBER, is a massive mineral; fracture large and flat; conchoidal in the great, very
fine earthy in the small; dull; colour, liver, chestnut,—dark yellowish brown; opaque;
does not soil, but writes; adheres strongly to the tongue, feels a little rough and meagre,
and is very soft; specific gravity 2·2. It occurs in beds with brown jasper in the Island
of Cyprus, and is used by painters as a brown colour, and to make varnish dry quickly.
[1263]
URANIUM, is a rare metal, first discovered by Klaproth, in the black mineral
called pechblende, found in a mine near Johann-Georgen-Stadt, in Saxony, and which is
a sulphuret of uranium. A double phosphate of uranium and copper, called green
uranite, and uran mica, occurs in Cornwall. It has been reduced to the metallic state
by various devices, but it has hardly the appearance of metal to the naked eye, and
from the rarity of its ores is not likely to be of any importance in the arts.
URAO, is the native name of a sesquicarbonate of soda found at the bottom of
certain lakes in Mexico, especially to the north of Zacatecas, and in several other provinces;
also in South America at Columbia, 48 English miles from Merida.
VALONIA, is a kind of acorn, imported from the Levant and the Morea for the use
of tanners, as the husk or cup contains abundance of tannin. The quantity imported
for home consumption in 1836, was 80,511 cwts.; of which Turkey furnished 58,724,
Italy and the Italian islands, 7209.
VANADIUM, is a metal discovered by Sefström, in 1830, in a Swedish iron, remarkable
for its ductility, extracted from the iron mine of Jaberg, not far from Jönköping.
Its name is derived from Vanadis, a Scandinavian idol. This metal has been found in
the state of vanadic acid, in a lead ore from Zimapan, in Mexico. The finery cinders
of the Jaberg iron contain more vanadium than the metal itself. It exists in it as
vanadic acid. For the reduction of this acid to vanadium, see Berzelius’s Traité de
Chimie, vol. iv. p. 644. Vanadium is white, and when its surface is polished, it resembles
silver or molybdenum more than any other metal. It combines with oxygen into two
oxides and an acid.
The vanadate of ammonia, mixed with infusion of nutgalls, forms a black liquid,
which is the best writing-ink hitherto known. The quantity of the salt requisite is so
small as to be of no importance when the vanadium comes to be more extensively extracted.
The writing is perfectly black. The acids colour it blue, but do not remove
it, as they do tannate of iron: the alkalis, diluted so far as not to injure the paper, do not
dissolve it; and chlorine, which destroys the black colour, does not, however, make the
traces illegible, even when they are subsequently washed with a stream of water. It is
perfectly fluent, and, being a chemical solution, stands in want of no viscid gum to suspend
the colour, like common ink. The influence of time upon it remains to be
tried.
VANILLA, is the oblong narrow pod of the Epidendron vanilla, Linn., of the natural
family Orchideæ, which grows in Mexico, Colombia, Peru, and on the banks of the
Oronoco.
The best comes from the forests round the village of Zentila, in the intendancy of
Oaxaca.
The vanilla plant is cultivated in Brazil, in the West Indies, and some other tropical
countries, but does not produce fruit of such a delicious aroma as in Mexico. It clings
like a parasite to the trunks of old trees, and sucks the moisture which their bark derives
from the lichens, and other cryptogamia, but without drawing nourishment from
the tree itself, like the ivy and misletoe. The fruit is subcylindric, about 8 inches
long, one-celled, siliquose, and pulpy within. It should be gathered before it is fully
ripe.
When about 12000 of these pods are collected, they are strung like a garland by their
lower end, as near as possible to the foot-stalk; the whole are plunged for an instant
in boiling water to blanch them; they are then hung up in the open air, and exposed
to the sun for a few hours. Next day they are lightly smeared with oil, by means of a
feather, or the fingers; and are surrounded with oiled cotton, to prevent the valves from
opening. As they become dry, on inverting their upper end, they discharge a viscid
liquid from it, and they are pressed at several times with oiled fingers to promote its
flow. The dried pods lose their appearance, grow brown, wrinkled, soft, and shrink
into one-fourth of their original size. In this state they are touched a second time
with oil, but very sparingly; because, with too much oil, they would lose much of their
delicious perfume. They are then packed for the market, in small bundles of 50 or
100 in each, enclosed in lead foil, or tight metallic cases. As it comes to us, vanilla is
a capsular fruit, of the thickness of a swan’s quill, straight, cylindrical, but somewhat
flattened, truncated at the top, thinned off at the ends, glistening, wrinkled, furrowed
lengthwise, flexible, from 5 to 10 inches long, and of a reddish-brown colour. It contains
a pulpy parenchyma, soft, unctuous, very brown, in which are imbedded black,
brilliant, very small seeds. Its smell is ambrosiacal and aromatic; its taste hot, and[1264]
rather sweetish. These properties seem to depend upon an essential oil, and also upon
benzoic acid, which forms efflorescences upon the surface of the fruit. The pulpy part
possesses alone the aromatic quality; the pericarpium has hardly any smell.
The kind most esteemed in France, is called leq vanilla; it is about 6 inches long,
from 1⁄4 to 1⁄3 of an inch broad, narrowed at the two ends, and curved at the base;
somewhat soft and viscid, of a dark-reddish colour, and of a most delicious flavour, like
that of balsam of Peru. It is called vanilla givrées, when it is covered with efflorescences
of benzoic acid, after having been kept in a dry place, and in vessels not hermetically
closed.
The second sort, called vanilla simarona, or bastard, is a little smaller than the preceding,
of a less deep brown hue, drier, less aromatic, destitute of efflorescence. It
is said to be the produce of the wild plant, and is brought from St. Domingo.
A third sort, which comes from Brazil, is the vanillon, or large vanilla of the French
market; the vanilla pamprona or bova of the Spaniards. Its length is from 5 to 6
inches; its breadth from one-half to three-quarters of an inch. It is brown, soft, viscid,
almost always open, of a strong smell, but less agreeable than the leq. It is sometimes
a little spoiled by an incipient fermentation. It is cured with sugar, and enclosed in
tin-plate boxes, which contain from 20 to 60 pods.
Vanilla, as an aromatic, is much sought after by makers of chocolate, ices, and
creams; by confectioners, perfumers, and liquorists, or distillers. It is difficultly reduced
to fine particles; but it may be sufficiently attenuated by cutting it into small
bits, and grinding these along with sugar. The odorous principle can, for some
purposes, be extracted by alcohol. Their analysis by Bucholz is unsatisfactory, and
refers obviously to the coarsest sort. Berzelius says that the efflorescences are not acid.
VAPOUR (Vapeur, Fr.; Dampf, Germ.); is the state of elastic or aeriform fluidity
into which any substance, naturally solid or liquid at ordinary temperatures, may be
converted by the agency of heat. See Evaporation.
VARNISH. (Vernis, Fr.; Firniss, Germ.); is a solution of resinous matter, which
is spread over the surface of any body, in order to give it a shining, transparent, and hard
coat, capable of resisting, in a greater or less degree, the influences of air and moisture.
Such a coat consists of the resinous parts of the solution, which remain in a thin layer upon
the surface, after the liquid solvent has either evaporated away, or has dried up. When
large quantities of spirit varnish are to be made, a common still, mounted with its capital
and worm, is the vessel employed for containing the materials, and it is placed in a steam or
water bath. The capital should be provided with a stuffing-box, through which a stirring-rod
may pass down to the bottom of the still, with a cross-piece at its lower end, and a
handle or winch at its top. After heating the bath till the alcohol boils and begins to distil,
the heat ought to be lowered, that the solution may continue to proceed in an equable
manner, with as little evaporation of spirit as possible. The operation may be supposed
to be complete when the rod can be easily turned round. The varnish must be passed
through a silk sieve of proper fineness; then filtered through porous paper, or allowed
to clear leisurely in stone jars. The alcohol which has come over should be added to
the varnish, if the just proportions of the resins have been introduced at first. The following
are reckoned good French recipes for varnishes:—
White spirit varnish.—Sandarach, 250 parts; mastic in tears, 64; elemi resin, 32;
turpentine (Venice), 64; alcohol, of 85 per cent., 1000 parts by measure.
The turpentine is to be added after the resins are dissolved. This is a brilliant
varnish, but not so hard as to bear polishing.
Varnish for the wood toys of Spa.—Tender copal, 75 parts; mastic, 12·5; Venice
turpentine, 6·5; alcohol, of 95 per cent., 100 parts by measure; water ounces, for example,
if the other parts be taken in ounces.
The alcohol must be first made to act upon the copal, with the aid of a little oil of
lavender or camphor, if thought fit; and the solution being passed through a linen cloth,
the mastic must be introduced. After it is dissolved, the Venice turpentine, previously
melted in a water-bath, should be added; the lower the temperature at which these
operations are carried on, the more beautiful will the varnish be. This varnish ought
to be very white, very drying, and capable of being smoothed with pumice-stone and
polished.
Varnish for certain parts of carriages.—Sandarach, 190 parts; pale shellac, 95; rosin,
125; turpentine, 190; alcohol, at 85 per cent., 1000 parts by measure.
Varnish for cabinet-makers.—Pale shellac, 750 parts; mastic, 64; alcohol, of 90 per
cent., 1000 parts by measure. The solution is made in the cold, with the aid of
frequent stirring. It is always muddy, and is employed without being filtered.
With the same resins and proof spirit a varnish is made for the bookbinders to do over
their morocco leather.
The varnish of Watin, for gilded articles.—Gum lac, in grain, 125 parts; gamboge,
125; dragon’s blood, 125; annotto, 125; saffron, 32. Each resin must be dissolved in[1265]
1000 parts by measure, of alcohol of 90 per cent.; two separate tinctures must be
made with the dragon’s blood and annotto, in 1000 parts of such alcohol; and a proper
proportion of each should be added to the varnish, according to the shade of golden
colour wanted.
For fixing engravings or lithographs upon wood, a varnish called mordant is used in
France, which differs from others chiefly in containing more Venice turpentine, to make
it sticky; it consists of—sandarach, 250 parts; mastic in tears, 64; rosin, 125; Venice
turpentine, 250; alcohol, 1000 parts by measure.
Copal varnish.—Hard copal, 300 parts; drying linseed or nut oil, from 125 to 250
parts; oil of turpentine, 500; these three substances are to be put into three separate
vessels; the copal is to be fused by a somewhat sudden application of heat; the drying
oil is to be heated to a temperature a little under ebullition, and it is to be added by
small portions at a time to the melted copal. When this combination is made, and the
heat a little abated, the essence of turpentine, likewise previously heated, is to be introduced
by degrees: some of the volatile oil will be dissipated at first; but more being
added, the union will take place. Great care must be taken to prevent the turpentine
vapour from catching fire, which might occasion serious accidents to the operator.
When the varnish is made, and has cooled down to about the 130th degree of Fahr., it
may be strained through a filter, to separate the impurities and undissolved copal.
Almost all varnish-makers think it indispensable to combine the drying oil with the
copal, before adding the oil of turpentine; but in this they are mistaken. Boiling oil of
turpentine combines very readily with fused copal; and, in some cases, it would probably
be preferable to commence the operation with it, adding it in successive small quantities.
Indeed, the whitest copal varnish can be made only in this way; for if the drying oil have
been heated to nearly its boiling point, it becomes coloured, and darkens the varnish.
This varnish improves in clearness by keeping. Its consistence may be varied by
varying the proportions of the ingredients, within moderate limits. Good varnish, applied
in summer, should become so dry in 24 hours that the dust will not stick to it,
nor receive an impression from the fingers. To render it sufficiently dry and hard for
polishing, it must be subjected for several days to the heat of a stove.
Milk of wax, is a valuable varnish, which may be prepared as follows:—Melt in a
porcelain capsule a certain quantity of white wax, and add to it, while in fusion, an
equal quantity of spirit of wine, of sp. gr. 0·830; stir the mixture, and pour it upon a
large porphyry slab. The granular mass is to be converted into a paste by the muller,
with the addition, from time to time, of a little alcohol; and as soon as it appears to be
smooth and homogeneous, water is to be introduced in small quantities successively, to
the amount of four times the weight of the wax. This emulsion is to be then passed
through canvas, in order to separate such particles as may be imperfectly incorporated.
The milk of wax, thus prepared, may be spread with a smooth brush upon the surface
of a painting, allowed to dry, and then fused by passing a hot iron (salamander) over
its surface. When cold, it is to be rubbed with a linen cloth to bring out the lustre. It
is to the unchangeable quality of an encaustic of this nature, that the antient paintings
upon the walls of Herculaneum and Pompeii owe their freshness at the present
day.
The most recent practical account of the manufacture of varnishes, is that communicated
by Mr. J. Wilson Neil to the Society of Arts, and published in the 49th volume
of their “Transactions.”
The building or shed wherein varnish is made, ought to be quite detached from any
buildings whatever, to avoid accidents by fire. For general purposes, a building about
18 feet by 16 is sufficiently large for manufacturing 4000 gallons and upwards annually,
provided there are other convenient buildings for the purpose of holding the utensils,
and warehousing the necessary stock.
Procure a copper pan, made like a common washing-copper, which will contain from
fifty to eighty gallons, as occasion may require; when wanted, set it upon the boiling
furnace, and fill it up with linseed oil within five inches of the brim. Kindle a fire in
the furnace underneath, and manage the fire so that the oil shall gradually, but slowly,
increase in heat for the first two hours; then increase the heat to a gentle simmer; and if
there is any scum on the surface, skim it off with a copper ladle, and put the skimming away.
Let the oil boil gently for three hours longer; then introduce, by a little at a time, one
quarter of an ounce of the best calcined magnesia for every gallon of oil, occasionally
stirring the oil from the bottom. When the magnesia is all in, let the oil boil rather
smartly for one hour; it will then be sufficient. Lay a cover over the oil, to keep out
the dust while the fire is withdrawn and extinguished by water; next uncover the oil, and
leave it till next morning; and then, while it is yet hot, ladle it into the carrying-jack,
or let it out through the pipe and cock; carry it away, and deposit it in either a tin
or leaden cistern, for wooden vessels will not hold it; let it remain to settle for at least
three months. The magnesia will absorb all the acid and mucilage from the oil, and[1266]
fall to the bottom of the cistern, leaving the oil clear and transparent, and fit for use.
Recollect, when the oil is taken out, not to disturb the bottoms, which are only fit for
black paint.
GENERAL OBSERVATIONS AND PRECAUTIONS TO BE OBSERVED IN MAKING VARNISHES.
Set on the boiling-pot with 8 gallons of oil; kindle the fire; then lay the fire in the
gum-furnace; have as many 8lb. bags of gum-copal all ready weighed up, as will be
wanted; put one 8lb. into the pot, put fire to the furnace, set on the gum-pot; in three
minutes (if the fire is brisk) the gum will begin to fuse and give out its gas, steam, and
acid; stir and divide the gum, and attend to the rising of it, as before directed.
8lbs. of copal take in general from sixteen to twenty minutes in fusing, from the
beginning till it gets clear like oil, but the time depends very much on the heat of the
fire, and the attention of the operator. During the first twelve minutes, while the gum
is fusing, the assistant must look to the oil, and bring it to a smart simmer; for it ought
to be neither too hot, nor yet too cold, but in appearance beginning to boil, which he
is strictly to observe, and, when ready, to call out, “Bear a hand!” Then immediately
both lay hold of a handle of the boiling-pot, lift it right up, so as to clear the plate,
carry it out and place it on the ash-bed, the maker instantly returning to the gum-pot,
while the assistant puts three copper ladlefuls of oil into the copper pouring-jack,
bringing it in and placing it on the iron plate at the back of the gum-pot to keep hot
until wanted. When the maker finds the gum is nearly all completely fused, and that
it will in a few minutes be ready for the oil, let him call out, “Ready oil!” The assistant
is then to lift up the oil-jack with both hands, one under the bottom and the other
on the handle, laying the spout over the edge of the pot, and wait until the maker calls
out, “Oil!” The assistant is then to pour in the oil as before directed, and the boiling
to be continued until the oil and gum become concentrated, and the mixture looks
clear on the glass; the gum-pot is now to be set upon the brick-stand until the
assistant puts three more ladlefuls of hot oil into the pouring-jack, and three more into
a spare tin for the third run of gum. There will remain in the boiling-pot still 31⁄2
gallons of oil. Let the maker put his right hand down the handle of the gum-pot near
to the side, with his left hand near the end of the handle, and with a firm grip lift the
gum-pot, and deliberately lay the edge of the gum-pot over the edge of the boiling-pot
until all its contents run into the boiling-pot. Let the gum-pot be held, with its
bottom turned upwards, for a minute right over the boiling-pot. Observe, that whenever
the maker is beginning to pour, the assistant stands ready with a thick piece of
old carpet, without holes, and sufficiently large to cover the mouth of the boiling-pot
should it catch fire during the pouring, which will sometimes happen if the gum-pot is
very hot; should the gum-pot fire, it has only to be kept bottom upwards, and it will
go out of itself; but if the boiling-pot should catch fire, during the pouring, let the
assistant throw the piece of carpet quickly over the blazing pot, holding it down all
round the edges; in a few minutes it will be smothered. The moment the maker has
emptied the gum-pot, he throws into it half a gallon of turpentine, and with the swish
immediately washes it from top to bottom, and instantly empties it into the flat tin jack:
he wipes the pot dry, and puts in 8lbs. more gum, and sets it upon the furnace; proceeding
with this run exactly as with the last, and afterwards with the third run.
There will then be 8 gallons of oil and 24lbs. of gum in the boiling-pot, under which
keep up a brisk strong fire until a scum or froth rises and covers all the surface of the
contents, when it will begin to rise rapidly. Observe, when it rises near the rivets of
the handles, carry it from the fire, and set it on the ash-bed, stir it down again, and
scatter in the driers by a little at a time; keep stirring, and if the frothy head goes
down, put it upon the furnace, and introduce gradually the remainder of the driers,
always carrying out the pot when the froth rises near the rivets. In general, if the fire
be good, all the time a pot requires to boil, from the time of the last gum being poured
in, is about three and a half or four hours; but time is no criterion for a beginner to
judge by, as it may vary according to the weather, the quality of the oil, the quality of
the gum, the driers, or the heat of the fire, &c.; therefore, about the third hour of
boiling, try it on a bit of glass, and keep it boiling until it feels strong and stringy between
the fingers; it is then boiled sufficiently to carry it on the ash-bed, and to be stirred
down until it is cold enough to mix, which will depend much on the weather, varying
from half an hour, in dry frosty weather, to one hour in warm summer weather.
Previous to beginning to mix, have a sufficient quantity of turpentine ready, fill the
pot, and pour in, stirring all the time at the top or surface, as before directed, until
there are fifteen gallons, or five tins of oil of turpentine introduced, which will leave it
quite thick enough if the gum is good, and has been well run; but if the gum was of
a weak quality, and has not been well fused, there ought to be no more than twelve
gallons of turpentine mixed, and even that may be too much. Therefore, when twelve
gallons of turpentine have been introduced, have a flat saucer at hand, and pour into it[1267]
a portion of the varnish, and in two or three minutes it will show whether it is too
thick; if not sufficiently thin, add a little more turpentine, and strain it off quickly.
As soon as the whole is stored away, pour in the turpentine washings, with which the
gum-pots have been washed, into the boiling-pot, and with the swish quickly wash
down all the varnish from the pot sides; afterwards, with a large piece of woollen rag
dipped in pumice-powder, wash and polish every part of the inside of the boiling-pot,
performing the same operation on the ladle and stirrers; rinse them with the turpentine
washings, and at last rinse them altogether in clean turpentine, which also put to
the washings; wipe dry with a clean soft rag the pot, ladle, stirrer, and funnels, and
lay the sieve so as to be completely covered with turpentine, which will always keep it
from gumming up. The foregoing directions concerning running the gum, and pouring
in the oil, and also boiling off and mixing, are, with very little difference, to be
observed in the making of all sorts of copal varnishes, except the differences of the
quantities of oil, gum, &c., which will be found under the various descriptions by name,
which will be hereafter described.
The choice of linseed oil is of peculiar consequence to the varnish-maker. Oil from
fine full-grown ripe seed, when viewed in a phial, will appear limpid, pale, and brilliant;
it is mellow and sweet to the taste, has very little smell, is specifically lighter than
impure oil, and, when clarified, dries quickly and firmly, and does not materially
change the colour of the varnish when made, but appears limpid and brilliant.
Copal varnishes for fine paintings, &c.—Fuse 8 lbs. of the very cleanest pale African
gum copal, and, when completely run fluid, pour in two gallons of hot oil, old measure;
let it boil until it will string very strong; and in about fifteen minutes, or while it is
yet very hot, pour in three gallons of turpentine, old measure, and got from the top of
a cistern. Perhaps during the mixing, a considerable quantity of the turpentine will
escape; but the varnish will be so much the brighter, transparent, and fluid; and will
work freer, dry more quickly, and be very solid and durable when dry. After the
varnish has been strained, if it is found too thick, before it is quite cold, heat as much
turpentine, and mix with it, as will bring it to a proper consistence.
Cabinet varnish.—Fuse 7 lbs. of very fine African gum copal, and pour in half a
gallon of pale clarified oil; in three or four minutes after, if it feel stringy, take it out
of doors, or into another building where there is no fire, and mix with it three gallons
of turpentine; afterwards strain it, and put it aside for use. This, if properly boiled,
will dry in ten minutes; but if too strongly boiled, will not mix at all with the
turpentine; and sometimes, when boiled with the turpentine, will mix, and yet refuse to
incorporate with any other varnish less boiled than itself: therefore it requires a nicety
which is only to be learned from practice. This varnish is chiefly intended for the use
of japanners, cabinet-painters, coach-painters, &c.
Best body copal varnish for coach-makers, &c.—This is intended for the body parts of
coaches and other similar vehicles, intended for polishing.
Fuse 8 lbs. of fine African gum copal; add two gallons of clarified oil (old measure);
boil it very slowly for four or five hours, until quite stringy; mix with three gallons
and a half of turpentine; strain off, and pour it into a cistern. As they are too slow
in drying, coach-makers, painters, and varnish-makers, have introduced to two pots of
the preceding varnish, one made as follows:—
-
- 8 lbs. of fine pale gum animé;
- 2 gallons of clarified oil;
- 31⁄2 gallons of turpentine.
- To be boiled four hours.
Quick drying body copal varnish, for coaches, &c.
- (1.)
- 8 lbs. of the best African copal
- 2 gallons of clarified oil;
- 1⁄2 lb. of dried sugar of lead;
- 31⁄2 gallons of turpentine.
- Boiled till stringy, and mixed and strained.
- (2.)
- 8 lbs. of fine gum animé;
- 2 gallons of clarified oil;
- 1⁄4 lb. of white copperas;
- 31⁄2 gallons of turpentine.
- Boiled as before.
To be mixed and strained while hot into the other pot. These two pots mixed
together will dry in six hours in winter, and in four in summer; it is very useful for
varnishing old work on dark colours, &c.
Best pale carriage varnish.
- (1.)
- 8 lbs. 2d sorted African copal;
- 21⁄2 gallons of clarified oil.
- Boiled till very stringy.
- 1⁄4 lb. of dried copperas;
- 1⁄4 lb. of litharge;
- 51⁄2 gallons of turpentine.
- Strained, &c.
- (2.)
- 8 lbs. of 2d sorted gum animé;
- 21⁄2 gallons of clarified oil;
- 1⁄4 lb. of dried sugar of lead;
- 1⁄4 lb. of litharge;
- 51⁄2 gallons of turpentine.
- Mix this to the first while hot.
[1268]
This varnish will dry hard, if well boiled, in four hours in summer, and in six in
winter. As the name denotes, it is intended for the varnishing of the wheels, springs,
and carriage parts of coaches, chaises, &c.; also, it is that description of varnish which
is generally sold to and used by house-painters, decorators, &c., as from its drying
quality and strong gloss, it suits their general purposes well.
Second carriage varnish.
-
- 8 lbs. of 2d sorted gum animé;
- 23⁄4 gallons of fine clarified oil;
- 51⁄4 gallons of turpentine;
- 1⁄4 lb. of litharge;
- 1⁄4 lb. of dried sugar of lead;
- 1⁄4 lb. of dried copperas.
- Boiled and mixed as before.
Wainscot varnish.
-
- 8 lbs. of 2d sorted gum animé;
- 3 gallons of clarified oil;
- 1⁄4 lb. of litharge;
- 1⁄4 lb. of dried sugar of lead;
- 51⁄2 gallons of turpentine.
- To be well boiled until it strings very strong, and then mixed and strained.
Mahogany varnish is made either with the same proportions, with a little darker
gum; otherwise it is wainscot varnish, with a small portion of gold size.
Black japan, is made by putting into the set-pot 48 pounds of Naples, or
any other of the foreign asphaltums (except the Egyptian). As soon as it is melted, pour
in 10 gallons of raw linseed oil; keep a moderate fire, and fuse 8 pounds of dark
gum animé in the gum-pot; mix it with 2 gallons of hot oil, and pour it into the
set-pot. Afterwards fuse 10 pounds of dark or sea amber in the 10 gallon iron pot;
keep stirring it while fusing; and whenever it appears to be overheated, and rising
too high in the pot, lift it from the fire for a few minutes. When it appears completely
fused, mix in 2 gallons of hot oil, and pour the mixture into the set-pot; continue the
boiling for 3 hours longer, and during that time introduce the same quantity of driers
as before directed: draw out the fire, and let it remain until morning; then boil it until
it rolls hard, as before directed: leave it to cool, and afterwards mix with turpentine.
Pale amber varnish.—Fuse 6 pounds of fine picked, very pale transparent amber in
the gum-pot, and pour in 2 gallons of hot clarified oil. Boil it until it strings very
strong. Mix with 4 gallons of turpentine. This will be as fine as body copal, will
work very free, and flow well upon any work it is applied to: it becomes very hard,
and is the most durable of all varnishes; it is very excellent to mix in copal varnishes,
to give them a hard and durable quality. Observe; amber varnish will always require
a long time before it is ready for polishing.
Best Brunswick black.—In an iron pot, over a slow fire, boil 45 pounds of foreign
asphaltum for at least 6 hours; and during the same time boil in another iron pot 6
gallons of oil which has been previously boiled. During the boiling of the 6 gallons,
introduce 6 pounds of litharge gradually, and boil until it feels stringy between the
fingers; then ladle or pour it into the pot containing the boiling asphaltum. Let the
mixture boil until, upon trial, it will roll into hard pills; then let it cool, and mix it
with 25 gallons of turpentine, or until it is of a proper consistence.
Iron-work black.—Put 48 pounds of foreign asphaltum into an iron pot, and boil for
4 hours. During the first 2 hours, introduce 7 pounds of red lead, 7 pounds of litharge,
3 pounds of dried copperas, and 10 gallons of boiled oil; add 1 eight-pound run of dark
gum, with 2 gallons of hot oil. After pouring the oil and gum, continue the boiling 2
hours, or until it will roll into hard pills like japan. When cool, thin it off with 30
gallons of turpentine, or until it is of a proper consistence. This varnish is intended
for blacking the iron-work of coaches and other carriages, &c.
A cheap Brunswick black.—Put 28 pounds of common black pitch, and 28 pounds of
common asphaltum made from gas tar, into an iron pot; boil both for 8 or 10 hours,
which will evaporate the gas and moisture; let it stand all night, and early next morning,
as soon as it boils, put in 8 gallons of boiled oil; then introduce, gradually, 10
pounds of red lead, and 10 pounds of litharge, and boil for 3 hours, or until it will roll
very hard. When ready for mixing, introduce 20 gallons of turpentine, or more, until
of a proper consistence. This is intended for engineers, founders, ironmongers, &c.; it
will dry in half an hour, or less, if properly boiled.
Axioms observed in the making of copal varnishes.—The more minutely the gum is
run, or fused, the greater the quantity, and the stronger the produce. The more
regular and longer the boiling of the oil and gum together is continued, the more fluid
or free the varnish will extend on whatever it is applied to. When the mixture of oil and
gum is too suddenly brought to string by too strong a heat, the varnish requires more
than its just proportion of turpentine to thin it, whereby its oily and gummy quality is
reduced, which renders it less durable; neither will it flow so well in laying on. The
greater proportion of oil there is used in varnishes, the less they are liable to crack,
because the tougher and softer they are. By increasing the proportion of gum in varnishes,[1269]
the thicker will be the stratum, the firmer they will set solid, and the quicker they will
dry. When varnishes are quite new made, and must be sent out for use before they are
of sufficient age, they must always be left thicker than if they were to be kept the
proper time. Varnish made from African copal alone possesses the most elasticity and
transparency. Too much driers in varnish render it opaque and unfit for delicate colours.
Copperas does not combine with varnish, but only hardens it. Sugar of lead does combine
with varnish. Turpentine improves by age; and varnish by being kept in a warm
place. All copal or oil varnishes require age before they are used.
Concluding observations.—All body varnishes are intended and ought to have 11⁄2 lbs.
of gum to each gallon of varnish, when the varnish is strained off, and cold; but as the
thinning up, or quantity of turpentine required to bring it to its proper consistence, depends
very much upon the degree of boiling the varnish has undergone, therefore, when the
gum and oil have not been strongly boiled, it requires less turpentine for that purpose;
whereas, when the gum and oil are very strongly boiled together, a pot of 20 gallons
will require perhaps 3 gallons above the regular proportionate quantity; and if mixing
the turpentine is commenced too soon, and the pot not sufficiently cool, there will be
frequently above a gallon and a half of turpentine lost by evaporation.
All carriage, wainscot, and mahogany varnish ought to have fully 1 pound of gum for
each gallon, when strained and cold; and should one pot require more than its proportion
of turpentine, the following pot can easily be left not quite so strongly boiled; then
it will require less turpentine to thin it up.
Gold sizes, whether pale or dark, ought to have fully half a pound of good gum copal
to each gallon, when it is finished; and the best black japan, to have half a pound of
good gum, or upwards, besides the quantity of asphaltum.
Fine mastic, or picture varnish.—Put 5 pounds of fine picked gum mastic into a
new 4-gallon tin bottle; get ready 2 pounds of glass, bruised as small as barley; wash
it several times; afterwards dry it perfectly, and put it into the bottle with 2 gallons of
turpentine that has settled some time; put a piece of soft leather under the bung; lay
the tin on a sack upon the counter, table, or any thing that stands solid; begin to agitate
the tin, smartly rolling it backward and forward, causing the gum, glass, and turpentine,
to work as if in a barrel-churn for at least 4 hours, when the varnish may be emptied
out into any thing sufficiently clean, and large enough to hold it. If the gum is not
all dissolved, return the whole into the bottle, and agitate as before, until all the gum
is dissolved; then strain it through fine thin muslin into a clean tin bottle: leave it uncorked,
so that the air can get in, but no dust; let it stand for 9 months, at least, before
it is used; for the longer it is kept, the tougher it will be, and less liable to chill or
bloom. To prevent mastic varnish from chilling, boil 1 quart of river sand with 2
ounces of pearl-ashes; afterwards wash the sand three or four times with hot water,
straining it each time; put the sand on a soup-plate to dry, in an oven; and when it is
of a good heat, pour half a pint of hot sand into each gallon of varnish, and shake it well
for 5 minutes; it will soon settle, and carry down the moisture of the gum and turpentine,
which is the general cause of mastic varnish chilling on paintings.
Common mastic varnish.—Put as much gum mastic, unpicked, into the gum-pot as
may be required, and to every 23⁄4 pounds of gum pour in 1 gallon of cold turpentine;
set the pot over a very moderate fire, and stir it with the stirrer; be careful, when the
steam of the turpentine rises near the mouth of the pot, to cover it with the carpet, and
carry it out of doors, as the vapour is very apt to catch fire. A few minutes’ low heat will
perfectly dissolve 8 pounds of gum, which will, with 4 gallons of turpentine, produce,
when strained, 41⁄2 gallons of varnish; to which add, while yet hot, 5 pints of pale turpentine
varnish, which improves the body and hardness of the mastic varnish.
Crystal varnish, may be made either in the varnish-house, drawing-room, or parlour.
Procure a bottle of Canada balsam, which can be had at any druggist’s; draw
out the cork, and set the bottle of balsam at a little distance from the fire, turning it
round several times, until the heat has thinned it; then have something that will hold
as much as double the quantity of balsam; carry the balsam from the fire, and, while
fluid, mix it with the same quantity of good turpentine, and shake them together until they
are well incorporated: in a few days the varnish is fit for use, particularly if it is poured
into a half-gallon glass or stone bottle, and kept in a gentle warmth. This varnish is
used for maps, prints, charts, drawings, paper ornaments, &c.; and when made upon a
larger scale, requires only warming the balsam to mix with the turpentine.
White hard spirit-of-wine varnish.—Put 5 pounds of gum sandarac into a 4-gallon
tin bottle, with 2 gallons of spirits of wine, 60 over proof, and agitate it until dissolved,
exactly as directed for the best mastic varnish, recollecting, if washed glass is used, that
it is convenient to dip the bottle containing the gum and spirits into a copperful of hot
water every 10 minutes—the bottle to be immersed only 2 minutes at a time—which
will greatly assist the dissolving of the gum; but, above all, be careful to keep a firm hold
over the cork of the bottle, otherwise the rarefaction will drive the cork out with the[1270]
force of a shot, and perhaps set fire to the place. The bottle, every time it is heated,
ought to be carried away from the fire; the cork should be eased a little, to allow
the rarefied air to escape; then driven tight, and the agitation continued in this
manner until all the gum is properly dissolved; which is easily known by having an
empty tin can to pour the varnish into, until near the last, which is to be poured into a
gallon measure. If the gum is not all dissolved, return the whole into the 4-gallon tin,
and continue the agitation until it is ready to be strained, when every thing ought to
be quite ready, and perfectly clean and dry, as oily tins, funnels, strainers, or any thing
damp, or even cold weather, will chill and spoil the varnish. After it is strained off,
put into the varnish 1 quart of very pale turpentine varnish, and shake and mix the two
well together. Spirit varnishes should be kept well corked: they are fit to use the day
after being made.
Brown hard spirit varnish—is made by putting into a bottle 3 pounds of gum
sandarac, with 2 pounds of shellac, and 2 gallons of spirits of wine, 60 over proof; proceeding
exactly as before directed for the white hard varnish, and agitating it when cold,
which requires about 4 hours’ time, without any danger of fire; whereas, making any
spirit varnish by heat is always attended with danger. No spirit varnish ought to be
made either near a fire or by candle light. When this brown hard is strained, add 1
quart of turpentine varnish, and shake and mix it well: next day it is fit for use.
The Chinese varnish, comes from a tree, which grows in Cochin-China, China, and
Siam. It forms the best of all varnishes.
Gold lacker.—Put into a clean 4-gallon tin, 1 pound of ground turmeric, 11⁄2 ounces
of powdered gamboge, 31⁄2 pounds of powdered gum sandarac, 3⁄4 of a pound of shellac,
and 2 gallons of spirits of wine. After being agitated, dissolved, and strained, add 1 pint
of turpentine varnish, well mixed.
Red spirit lacker.
-
- 2 gallons of spirits of wine;
- 1 pound of dragon’s blood;
- 3 pounds of Spanish annotto;
- 31⁄4 pounds of gum sandarac;
- 2 pints of turpentine.
- Made exactly as the yellow gold lacker.
Pale brass lacker.
-
- 2 gallons of spirits of wine;
- 3 ounces of Cape aloes, cut small;
- 1 pound of fine pale shellac;
- 1 ounce gamboge, cut small.
- No turpentine varnish. Made exactly as before.
But observe, that those who make lackers, frequently want some paler, and some
darker, and sometimes inclining more to the particular tint of certain of the component
ingredients. Therefore, if a 4-ounce phial of a strong solution of each ingredient
be prepared, a lacker of any tint can be produced at any time.
Preparation of linseed oil for making varnishes.—Put 25 gallons of linseed oil
into an iron or copper pot that will hold at least 30 gallons; put a fire under, and
gradually increase the heat, so that the oil may only simmer, for 2 hours; during
that time the greatest part of its moisture evaporates; if any scum arises on the
surface, skim it off, and put that aside for inferior purposes. Then increase the
gradually, and sprinkle in, by a little at a time, 3 lbs. of scale litharge, 3 lbs.
of good red lead, and 2 lbs. of Turkey umber, all well dried and free from
moisture. If any moist driers are added, they will cause the oil to tumefy; and,
at the same time, darken it, causing it to look opaque and thick, ropy and clammy,
and hindering it from drying and hardening in proper time; besides, it will lie on the
working painting like a piece of bladder skin, and be very apt to rise in blisters. As
soon as all the driers are added to the oil, keep quietly stirring the driers from the
bottom of the pot; otherwise they will burn, which will cause the oil to blacken and
thicken before it is boiled enough. Let the fire be so regulated that the oil shall only
boil slowly for three hours from the time all the driers were added; if it then ceases to
throw up any scum, and emits little or no smoke, it is necessary to test its temperature
by a few quill tops or feathers. Dip a quill top in the oil every two minutes, for when
the oil is boiled enough, the quill top will crackle or curl up quite burnt; if so, draw out
the fire immediately, and let the oil remain in the pot at least from 10 to 24 hours, or
longer if convenient, for the driers settle much sooner when the oil is left to cool in the
pot, than when it is immediately taken out.
Poppy oil.—Into four pints of pure soft water, put two ounces of foreign white vitriol;
warm the water in a clean copper pan, or glazed earthen jar, until the vitriol is
dissolved; pour the mixture into a clean glass or stone bottle, large enough to contain
three gallons; then add to the solution of vitriol one gallon and a half of poppy oil,
cork and agitate the bottle regularly and smartly for at least two hours; then pour out
the contents into a wide earthenware dish: leave it at rest for eight days, when the oil
will be clean and brilliant on the surface, and may be taken off with a spoon or flat
skimmer, and put up in a glass bottle and exposed to the light, which in a few weeks
renders the oil exceedingly limpid and colourless.
[1271]
Nut-oil, or oil of walnuts, is extracted by expression; and that which is extracted
without heat, is certainly the most pale, pure, and nutritive seasoning, and retains an
exquisite taste of the fruit. That designed for the arts is of inferior quality, and is
plentifully imported to us from France; the heat it undergoes in its torrefaction, previous
to its expression, disposes it to dry more quickly than that expressed by the cold
process; but, at the same time, the heat, though it frees it from its unctuous quality,
gives it more colour. When it has been extracted by the cold process, it may be prepared
in the same way as directed for the poppy oil.
In the above article I have retained the workmen’s names—gum, white vitriol, &c.,
instead of resin, sulphate of zinc, &c.
VEINS (Filons, Fr.; Gänge, Germ.); are the fissures or rents in rocks, which are
filled with peculiar mineral substances, most commonly metallic ores.
VEIN STONES, or GANGUES, are the mineral substances which accompany,
and frequently enclose, the metallic ores.
VELLUM, is a fine sort of Parchment, which see.
VELVET (Velours, Fr.; Sammet, Germ.); a peculiar stuff, the nature of which is
explained under Fustian and Textile Fabrics.
VENETIAN CHALK, is Steatite.
VENUS, is the mythological name of copper.
VENTILATION, or the renewal of fresh air in stagnant places, is nowhere exhibited
to such advantage as in the coal mines of Northumberland and Durham, where
Mr. Buddle has carried well nigh to systematic perfection the plan of coursing the
air through the winding galleries, originally contrived about the year 1760, by Mr. James
Spedding, of Workington, the ablest pitman of his day.[69] He converted the whole of
the passages into air-pipes, so to speak, drew the current of air from the downcast pit,
then traversed it up and down, and round about, through the several sheths of the
workings, so that no particular gallery was left without a current of air. He
thereby succeeded in actually expelling the noxious gases from the mines; those
demons, which in Germany, at no remote era, were wont to be combated by the priests
with impotent exorcisms or pious frauds. Before Mr. Buddle introduced his improvements,
he has known the air to be led through a series of workings, thirty miles
long, before it made its exit. There is in every coal mine an experienced corps, called
wastemen, because they travel over the waste, or the exhausted regions, who can tell at
once, by the whistling sound which the air makes at the crevices in certain partitions and
doors, whether the ventilation be in good condition or not. They hear these stoppings
begin to sing or call, as they say, whenever an interruption takes place in any point of
the labyrinthian line. Another indication of something being wrong, is when the doors
get so heavy, that the boys in attendance upon them find them difficult to shut or
open. The instant such a defect is discovered by any one, he cries aloud, “Holloa,
there is something wrong—the doors are calling!”
In Mr. Spedding’s system, the whole of the return air came in one current to his
rarefying furnace (see letter C, fig. 1158.), whether it was at the explosive point or not.
This distribution was often fraught with such danger, that a torrent of water had to be
kept in readiness, under the name of the waterfall, to be let down to extinguish the fire
in a moment. Many explosions at that time occurred, from the furnaces below, and
also down through tubes from the furnaces above-ground.
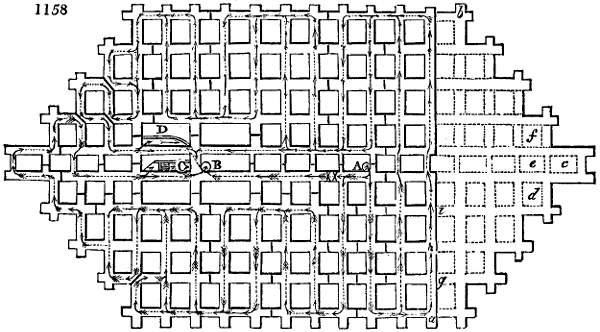
About the year 1807, Mr. Buddle had his attention intensely occupied with this most
important object, and then devised his plan of a divided current, carrying that portion
through the active furnace C, fig. 1158., and the portion of the air from the foul workings
of the air which, descending in the downcast pit A, coursed through the clean workings, up
the dumb furnace D, till it reached a certain elevation in B, the upcast pit, above the
fireplace. The pitmen had a great aversion, however, at first, to adopt this plan, as they
thought that the current of air, by being split, would lose its ventilating power; but
they were, ere long, convinced by Mr. Buddle to the contrary. He divides the main
current into two separate streams, at the bottom of the pit A, as shown by darts in the
figure; the feathered ones, representing that part of the pit in which the course of the
current of air is free from explosive mixture, or does not contain above one-thirtieth
of carburetted hydrogen, as indicated by its effect upon the flame of a candle. The
naked darts denote the portions of the mine where the air, being charged to the firing
point, is led off towards D, the dumb furnace, which communicates with the hot upcast
shaft, out of reach of the flame, and thence derives its power of draught. By suitable alterations
in the stoppings (see the various transverse lines, and the crosses), any portion of
the workings may, by the agency of the furnace, be laid out of, or brought within, the
course of the vitiated current, at the pleasure of the skilful mine-viewer; so that, if he
found it necessary, he could confine, by proper arrangements of his furnace, all the
vitiated current to a mere gas-pipe or drift, and direct it wholly through the dumb furnace.[1272]
During a practice of twenty years, Mr. Buddle has not met with any accident in
consequence of a defect in the stoppings preventing the complete division of the air.
The engineer has it thus within his power to detach or insulate those portions of the
mine in which there is a great exudation of gas, from the rest; and, indeed, he is continually
making changes, borrowing and lending currents, so to speak; sometimes laying
one division or panel upon the one air-course, and sometimes upon the other, just to
suit the immediate emergency. As soon as any district has ceased to be dangerous, by
the exhaustion of the gas-blowers, it is transferred from the foul to the pure air course,
where gunpowder may be safely used, as also candles, instead of Davy’s lamps, which
give less light.
The quantity of air put down into the Wallsend colliery, at the time of the last
dreadful accident, 18th June, 1835, was not less than 5000 cubic feet per minute, whence
it has been justly inferred that the explosion was caused by the rashness of a wasteman
carrying a light through a door into a foul drift.
Till the cutting out of the pillars commences (see the right end of the diagram), the
ventilation of the several passages, boards, &c., may be kept perfect, supposing the
working extended no further than a, or b; because, as long as there are pillars standing,
every passage may be converted into an air-conduit, for leading a current of air in any
direction, either to C, the burning, or D, the dumb furnace. But the first pillar that is
removed deranges the ventilation at that spot, and takes away the means of carrying the
air into the further recess towards c. In taking out the pillars, the miners always work to
windward, that is to say, against the stream of air; so that, whatever gas may be evolved,
shall be immediately carried off from the people at work. When a range of pillars has
been removed, as at d, e, f, no power remains of dislodging the gas from the section of
the mine beyond a, b; and as the pillars are successively cut away to the left hand of the
line a, b, the size of the goaf, or void, is increased. This vacuity is a true gas-holder, or
reservoir, continually discharging itself at the points g, h, i, into the circulating current,
to be carried off by the gas-pipe drift at the dumb furnace, but not to be suffered ever
to come in contact with flame of any description. The next range of working, is the
line of pillars to the left of a, b; the coal having been entirely cleared out of the space
to the right, where the place of the pillars is marked by dotted lines. The roof in the
waste soon falls down, and gets fractured up to the next seam of coal, called the yard-coal
seam, which, abounding in gas, sends it down in large quantities, and keeps the
immense gasometer, or goaf below, continually replenished. See Stove.
VERATRINE, is a vegetable alkali, of a poisonous nature, extracted from the
seeds of the Veratrum sabadilla, the roots of the Veratrum album, or white hellebore, and
of Colchicum autumnale, or meadow saffron, in which plants it exists combined chiefly
with gallic acid. It is obtained in the form of a white powder. It has an acrid, burning
taste, but without any bitterness; it has no smell; but when snuffed into the nostrils,
it excites violent and dangerous sneezing. It melts at a heat of 122° F., and concretes,
on cooling, into a transparent yellowish mass. It restores the blue colour of reddened
litmus paper. It is hardly soluble in water or ether, but abundantly in alcohol. It
consists of—carbon 66·75, hydrogen 8·54, nitrogen 5·04, and oxygen 19·60. Its saline
compounds have an acrid and burning taste. Veratrine resembles strychnine and brucine,
in its effects upon living bodies, producing tetanus and death in a moderate dose; notwithstanding
which, it has been prescribed by some of our poison doctors, especially
mixed with hog’s lard, in the form of frictions on the forehead, for nervous maladies;
but seldom, I believe, with any good effects.
[1273]
VERDIGRIS. (Vert-de-gris, Fr.; Grünspan, Germ.) The copper used in this
manufacture, is formed into round sheets, from 20 to 25 inches diameter, by one twenty-fourth
of an inch in thickness. Each sheet is then divided into oblong squares, from 4
to 6 inches in length, by 3 broad; and weighing about 4 ounces. They are separately
beaten upon an anvil, to smooth their surfaces, to consolidate the metal, and to free it
from scales. The refuse of the grapes, after the extraction of their juice, formerly
thrown on to the dunghill, is now preserved for the purpose of making verdigris. It
is put loosely into earthen vessels, which are usually 16 inches high, 14 in diameter at
the widest part, and about 12 at the mouth. The vessels are then covered with lids,
which are surrounded by straw mats. In this situation the materials soon become
heated, and exhale an acid odour; the fermentation beginning at the bottom of the cask,
and gradually rising till it actuate the whole mass. At the end of two or three days,
the manufacturer removes the fermenting materials into other vessels, in order to check
the process, lest putrefaction should ensue. The copper plates, if new, are now prepared,
by rubbing them over with a linen cloth dipt in a solution of verdigris; and they
are laid up alongside of one another to dry. If the plates are not subjected to this kind
of preparation, they will become black, instead of green, by the first operation. When
the plates are ready, and the materials in a fermenting state, one of them is put into
the earthern vessel for 24 hours, in order to ascertain whether it be a proper period to
proceed to the remaining part of the process. If, at the end of this period, the plate be
covered with an uniform green layer, concealing the whole copper, every thing is right;
but if, on the contrary, liquid drops hang on the surface of the metal, the workmen say
the plates are sweating, and conclude that the heat of the fermented mass has been inadequate;
on which account another day is allowed to pass before making a similar
trial. When the materials are finally found to be ready, the strata are formed in the
following manner. The plates are laid on a horizontal wooden grating, fixed in the
middle of a vat, on whose bottom a pan full of burning charcoal is placed, which heats
them to such a degree, that the women who manage this work are obliged to lay hold
of them frequently with a cloth when they lift them out. They are in this state put
into earthern vessels, in alternate strata with the fermented materials, the uppermost
and undermost layers being composed of the expressed grapes. The vessels are covered
with their straw mats, and left at rest. From 30 to 40 pounds of copper are put into
one vessel.
At the end of 10, 12, 15, or 20 days the vessels are opened, to ascertain, by the
materials having become white, if the operation be completed.
Detached glossy crystals will be perceived on the surface of the plates; in which
case the grapes are thrown away, and the plates are placed upright in a corner of the
verdigris cellar, one against the other, upon pieces of wood laid on the ground. At the
end of two or three days they are moistened by dipping in a vessel of water, after which
they are replaced in their former situation, where they remain seven or eight days, and
are then subjected to momentary immersion, as before. This alternate moistening and
exposure to air is performed six or eight times, at regular intervals of about a week. As
these plates are sometimes dipped into damaged wine, the workmen term these immersions,
one wine, two wines, &c.
By this treatment, the plates swell, become green, and covered with a stratum of
verdigris, which is readily scraped off with a knife. At each operation every vessel
yields from five to six pounds of verdigris, in a fresh or humid state; which is sold to
wholesale dealers, who dry it for exportation. For this purpose, they knead the paste
in wooden troughs, and then transfer it to leathern bags, a foot and a half long, and ten
inches in diameter. These bags are exposed to the sun and air till the verdigris has
attained a sufficient degree of hardness. It loses about half its weight in this operation;
and it is said to be knife-proof, when this instrument, plunged through the leathern bag,
cannot penetrate the loaf of verdigris.
The manufacture of verdigris at Montpellier is altogether domestic. In most wine
farm-houses there is a verdigris cellar; and its principal operations are conducted by the
females of the family. They consider the forming the strata, and scraping off the verdigris,
the most troublesome part. Chaptal says that this mode of making verdigris
would admit of some improvements: for example, the acetification requires a warmer
temperature than what usually arises in the earthen vessels; and the plates, when set
aside to generate the coat of verdigris, require a different degree of heat and moisture
from that requisite for the other operations.
Verdigris is a mixture of the crystallized acetate of copper and the sub-acetate, in
varying proportions. According to Vauquelin’s researches, there are three compounds
of oxide of copper and acetic acid; 1. a subacetate, insoluble in water, but decomposing
in that fluid, at common temperatures changing into peroxide and acetate; 2. a neutral
acetate, the solution of which is not altered at common temperatures, but is decomposed
by ebullition, becoming peroxide and superacetate; and, 3. superacetate, which in[1274]
solution is not decomposed, either at common temperatures or at the boiling point;
and which cannot be obtained in crystals, except by slow spontaneous evaporation, in
air or in vacuo. The first salt, in the dry state, contains 66·51 of oxide; the second,
44·44; and the third, 33·34.
Mr. Phillips has given the following analyses of French and English verdigris;
Annals of Philosophy, No. 21.—
| |
French
Verdigris. |
English
Verdigris. |
| Acetic acid |
29·3 |
29·62 |
| Peroxide of copper |
43·5 |
44·25 |
| Water |
25·2 |
25·51 |
| Impurity |
2·0 |
0·62 |
| |
100·0 |
100·00 |
Distilled verdigris, as it was long erroneously called, is merely a binacetate or superacetate
of copper, made by dissolving, in a copper kettle, one part of verdigris in two of
distilled vinegar; aiding the mutual action by slight heat and agitation with a wooden
spatula. When the liquor has taken its utmost depth of colour, it is allowed to settle,
and the clear portion is decanted off into well glazed earthen vessels. Fresh vinegar is
poured on the residuum, and if its colour does not become deep enough, more verdigris
is added. The clear and saturated solution is then slowly evaporated, in a vessel kept
uniformly filled, till it acquires the consistence of syrup, and shows a pellicle on its surface;
when it is transferred into glazed earthen pans, called oulas in the country. In
each of these dishes, two or three sticks are placed, about a foot long, cleft till within
two inches of their upper end, and having the base of the cleft kept asunder by a bit of
wood. This kind of pyramid is suspended by its summit in the liquid. All these vessels
are transported into crystallizing rooms, moderately heated with a stove, and left in the
same state for 15 days, taking care to maintain an uniform temperature. Thus are obtained
very fine groups of crystals of acetate of copper, clustered round the wooden rods;
on which they are dried, taken off, and sent into the market. They are distinctly rhomboidal
in form, and of a lively deep blue colour. Each cluster of crystals weighs from
five to six pounds; and, in general, their total weight is equal to about one-third of the
verdigris employed.
The crystallized binacetate of commerce consists, by my analysis, of—acetic acid, 52;
oxide of copper, 39·6; water, 8·4, in 100. I have prepared crystals which contain no water.
There is a triple acetate of copper and lime, which resembles distilled verdigris in colour.
It was manufactured pretty extensively in Scotland some years ago, and fetched a high
price, till I published an analysis of it in the Edinburgh Philosophical Journal. It is
much inferior, for all uses in the arts, to the proper binacetate.
VERDITER, or BLUE VERDITER. This is a precipitate of oxide of copper
with lime, made by adding that earth, in its purest state, to the solution of nitrate of
copper, obtained in quantities by the refiners, in parting gold and silver from copper by
nitric acid. The cupreous precipitate must be triturated with lime, after it is nearly dry,
to bring out the fine velvety blue colour. The process is delicate, and readily misgives
in unskilful hands.
The cendres bleues en pâte of the French, though analogous, are in some respects a different
preparation. To make it, dissolve sulphate of copper in hot water, in such proportions
that the liquid may have a density of 1·3. Take 240 pound measures of this
solution, and divide it equally into 4 open-headed casks; add to each of these 45 pound
measures of a boiling-hot solution of muriate of lime, of specific gravity 1·317, whereby
a double decomposition will ensue; with the formation of muriate of copper and sulphate
of lime, which precipitates. It is of consequence to work the materials well together
at the moment of mixture, to prevent the precipitate agglomerating in unequal masses.
After leaving it to settle for 12 hours, a small quantity of the clear liquor may be examined,
to see whether the just proportions of the two salts have been employed, which
is done by adding either sulphate of copper or muriate of lime. Should either cause
much precipitation, some of the other must be poured in till the equivalent decomposition
be accomplished; though less harm results from an excess of sulphate of copper
than of muriate of lime.
The muriate of copper is to be decanted from the subsided gypsum, which must be
drained and washed in a filter; and these blue liquors are to be added to the stronger;
and the whole distributed, as before, into 4 casks; composing in all 670 pound measures
of a green liquor, of 1·151 specific gravity.
Meanwhile, a magma of lime is to be prepared as follows:—100 pounds of quicklime
are to be mixed up with 300 pounds of water, and the mixture is to be passed
through a wire-gauze sieve, to separate the stony and sandy particles, and then to be
ground in a proper mill to an impalpable paste. About 70 or 80 pounds of this mixture
(the beauty of the colour is inversely as the quantity of lime) are to be distributed[1275]
in equal portions between the four casks, strongly stirring all the time with a wooden
spatula. It is then left to settle, and the limpid liquor is tested by ammonia, which
ought to occasion only a faint blue tinge; but if the colour be deep blue, more of the
lime paste must be added. The precipitate is now to be washed by decantation, employing
for this purpose the weak washings of a former operation; and it is lastly to be
drained and washed on a cloth filter. The proportions of material prescribed above,
furnish from 500 to 540 pounds of green paste.
Before making further use of this paste, the quantity of water present in it must be
determined by drying 100 or 200 grains. If it contain 27 per cent. of dry matter, 12
pounds of it may be put into a wooden bucket (and more or less in the ratio of 12 to
to 27 per cent.) capable of containing 171⁄2 pints; a pound (measure) of the lime paste
is then to be rapidly mixed into it; immediately afterwards, a pint and a quarter of a
watery solution of the pearlash of commerce, of spec. grav. 1·114, previously prepared;
and the whole mixture is to be well stirred, and immediately transferred to a colour-mill.
The quicker this is done, the more beautiful is the shade.
On the other hand, two solutions must have been previously made ready, one of sal-ammoniac
(4 oz. troy dissolved in 31⁄2 pints of water), and another of sulphate of copper
(8 oz. troy dissolved in 31⁄2 pints of water).
When the paste has come entirely through the mill, it is to be quickly put into a
jar, and the two preceding solutions are to be simultaneously poured into it; when a
cork is to be inserted, and the jar is to be powerfully agitated. The cork must now be
secured with a fat lute. At the end of four days this jar and three of its fellows are to
be emptied into a large hogshead nearly full of clear water, and stirred well with a
paddle. After repose, the supernatant liquid is run off; when it is filled up again with
water, and elutriated several times in succession, till the liquid no longer tinges turmeric
paper brown. The deposit may be then drained on a cloth filter. The pigment is
sold in the state of a paste; and is used for painting, or printing paper-hangings for the
walls of apartments.
The above prescribed proportions furnish the superfine blue paste: for the second
quality, one-half more quicklime paste is used; and for the third, double of the lime
and sal ammoniac; but the mode of preparation is in every case the same.
This paste may be dried into a blue powder, or into crayons for painters, by exposing
it on white deals to a very gentle heat in a shady place. This is called cendres bleues
en pierre.
VERDITER, or BREMEN GREEN. This pigment is a light powder, like
magnesia, having a blue or bluish green colour. The first is most esteemed. When
worked up with oil or glue, it resists the air very well; but when touched with lime,
it is easily affected, provided it has not been long and carefully dried. A strong heat
deprives it of its lustre, and gives it a brown or blackish-green tint.
The following is, according to M. J. G. Gentele, the process of fabrication in
Bremen, Cassel, Eisenach, Minden, &c.:—
a. 225 lbs. of sea salt, and 222 lbs. of blue vitriol, both free from iron, are mixed in the
dry state, then reduced between mill-stones with water to a thick homogeneous paste.
b. 225 lbs. of plates of old copper are cut by scissors into bits of an inch square,
then thrown and agitated in a wooden tub containing two lbs. of sulphuric acid,
diluted with a sufficient quantity of water, for the purpose of separating the impurities;
they are afterwards washed with pure water in casks made to revolve upon their axes.
c. The bits of copper being placed in oxidation-chests, along with the magma of
common salt and blue vitriol previously prepared in strata of half an inch thick, they
are left for some time to their mutual reaction. The above chests are made of oaken
planks joined without iron nails, and set aside in a cellar, or other place of moderate
temperature.
The saline mixture, which is partially converted into sulphate of soda and chloride
of copper, absorbs oxygen from the air, whereby the metallic copper passes into a
hydrated oxide, with a rapidity proportioned to the extent of the surfaces exposed to
the atmosphere. In order to increase this exposure, during the three months that
the process requires, the whole mass must be turned over once every week, with a
copper shovel, transferring it into an empty chest alongside, and then back into the
former one.
At the end of three months, the corroded copper scales must be picked out, and the
saline particles separated from the slimy oxide with the help of as little water as
possible.
d. This oxidized schlam, or mud, is filtered, then thrown, by means of a bucket containing
30 pounds, into a tub, where it is carefully divided or comminuted.
e. For every six pailfuls of schlam thus thrown into the large tub, 12 pounds of
muriatic acid, at 15° Baumé, are to be added; the mixture is to be stirred, and then
left at rest for 24 or 36 hours.
[1276]
f. Into another tub, called the blue back, there is to be introduced, in like manner,
for every six pailfuls of the acidified schlam, 15 similar pailfuls of a solution of colourless
clear caustic alkali, at 19° Baumé.
g. When the back (e) has remained long enough at rest, there is to be poured into
it a pail of pure water for every pail of schlam.
h. When all is thus prepared, the set of workmen who are to empty the back (e), and
those who are to stir (f), must be placed alongside of each. The first set transfer the
schlam rapidly into the latter back; where the second set mix and agitate it all the
time requisite to convert the mass into a consistent state, and then leave it at rest from
36 to 48 hours.
The whole mass is to be now washed; with which view it is to be stirred about with
the affusion of water, allowed to settle, and the supernatant liquor is drawn off. This
process is to be repeated till no more traces of potash remain among the blue. The
deposit must be then thrown upon a filter, where it is to be kept moist, and exposed
freely to the air. The pigment is now squeezed in the filter-bags, cut into bits, and
dried in the atmosphere, or at a temperature not exceeding 78° Fahr. It is only after
the most complete desiccation that the colour acquires its greatest lustre.
VERMICELLI, is a paste of wheat flour, drawn out and dried in slender cylinders,
more or less tortuous, like worms, whence the Italian name. The gruau of the French
is wheat coarsely ground, so as to free it from the husk; the hardest and whitest part,
being separated by sifting, is preferred for making the finest bread. When this gruau
is a little more ground, and the dust separated from it by the boulting-machine, the
granular substance called semoule is obtained, which is the basic of the best pastes.
The softest and purest water is said to be necessary for making the most plastic vermicelli
dough; 12 pounds of it being usually added to 50 pounds of semoule. It is
better to add more semoule to the water, than water to the semoule, in the act of
kneading. The water should be hot, and the dough briskly worked while still warm.
The Italians pile one piece of this dough upon another, and then tread it well with
their feet for two or three minutes. They afterwards work it for two hours with a
powerful rolling-pin, a bar of wood from 10 to 12 feet long, larger at the one end than
the other, having a sharp cutting edge at the extremity, attached to the large kneading-trough.
When the dough is properly prepared, it is reduced to thin ribands, cylinders, or
tubes, to form vermicelli and macaroni of different kinds. This operation is performed
by means of a powerful press. This is vertical, and the iron plate or follower carried by
the end of the screw fits exactly into a cast-iron cylinder, called the bell, like a sausage-machine,
of which the bottom is perforated with small holes, of the shape and size
intended for the vermicelli. The bell being filled, and warmed with a charcoal fire to
thin the dough into a paste, this is forced slowly through the holes, and is immediately
cooled and dried by a fanner as it protrudes. When the threads or fillets have acquired
the length of a foot, they are grasped by the hand, broken off, and twisted, while still
flexible, into any desired shape upon a piece of paper.
The macaroni requires to be made of a less compact dough than the vermicelli.
The former is forced through the perforated bottom, usually in fillets, which are afterwards
formed into tubes by joining their edges together before they have had time to become
dry. The lazagnes are macaroni left in the fillet or riband shape.
VERMILLION, or Cinnabar, is a compound of mercury and sulphur in the
proportion of 100 parts of the former to 16 of the latter, which occurs in nature as a
common ore of quicksilver, and is prepared by the chemist as a pigment, under the
name of Vermillion. It is, properly speaking, a bisulphuret of mercury. This artificial
compound being extensively employed, on account of the beauty of its colour, in
painting, for making red sealing-wax, and other purposes, is the object of an important
manufacture. When vermillion is prepared by means of sublimation, it concretes in
masses of considerable thickness, concave on one side, convex on the other, of a
needle-form texture; brownish-red in the lump, but when reduced to powder of a lively
red colour. On exposure to a moderate heat, it evaporates without leaving a residuum,
if it be not contaminated with red lead; and at a higher heat, it takes fire, and burns
entirely away, with a blue flame.
Holland long kept a monopoly of the manufacture of vermillion, from being alone in
possession of the art of giving it a fine flame colour. Meanwhile the French chemists
examined this product with great care, under an idea that the failure of other nations
to rival the Dutch, arose from ignorance of its true composition; some, with Berthollet,
imagined that it contained a little hydrogen; and others, with Fourcroy, believed that
the mercury contained in it was oxidized; but, eventually, Seguin proved that both of
these opinions were erroneous; having ascertained, on the one hand, that no hydrogenous
matter was given out in the decomposition of cinnabar, and on the other that[1277]
sulphur and mercury, by combining, were transformed into the red sulphuret in close
vessels, without the access of any oxygen whatever. It was likewise supposed that
the solution of the problem might be found in the difference of composition between
the red and black sulphurets of mercury; and many conjectures were made with this
view, the whole of which were refuted by Seguin. He demonstrated, in fact, that a
mere change of temperature was sufficient to convert the one sulphuret into the other,
without occasioning any variation in the proportion of the two elements. Cinnabar,
moderately heated in a glass tube, is convertible into ethiops, which in its turn is changed
into cinnabar by exposing the tube to a higher temperature; and thence he was led to conclude
that the difference between these two sulphurets was owing principally to the state of
the combination of the constituents. It would seem to result, from all these researches,
that cinnabar is only an intimate compound of pure sulphur and mercury, in the proportions
pointed out by analysis; and it is therefore reasonable to conclude, that in order
to make fine vermillion, it should be sufficient to effect the union of its elements at a
high enough temperature, and to exclude the influence of all foreign matters; but,
notwithstanding these discoveries, the art of making good vermillion is nearly as
much a mystery as ever. M. Seguin, indeed, announced in his Memoirs, that he had
succeeded in obtaining, in his laboratory, as good a cinnabar as that of Holland, and
at a remunerative price; but whatever truth may be in this assertion, or however
much the author may have been excited by the love of honour and profit, no manufacture
on the great scale sprung up under his auspices. France is still as tributary as
ever to foreign nations for this chemical product. At an exposition some years ago,
indeed, a sample of good French vermillion was brought forward to prove that the
problem was nearly solved; but that it is not so completely, may be inferred from the
silence on this subject in M. Dupin’s report of the last exposition, in 1834, where we
see so many chemical trifles honoured with eulogiums and medals by the judges of the
show. The English vermillion is now most highly prized by the French manufacturers
of sealing-wax.
M. Tuckert, apothecary of the Dutch court, published, long ago, in the Annales de
Chimie, vol. iv., the best account we yet have of the manufacture of vermillion in
Holland; one which has been since verified by M. Payssé, who saw the process practised
on the great scale with success.
“The establishment in which I saw, several times, the fabrication of sublimed sulphuret
of mercury,” says M. Tuckert, “was that of Mr. Brand, at Amsterdam, beyond
the gate of Utrecht; it is one of the most considerable in Holland, producing annually,
from three furnaces, by means of four workmen, 48,000 pounds of cinnabar, besides
other mercurial preparations. The following process is pursued here:—
“The ethiops is first prepared by mixing together 150 pounds of sulphur, with 1080
pounds of pure mercury, and exposing this mixture to a moderate heat in a flat polished
iron pot, one foot deep, and two feet and a half in diameter. It never takes fire, provided
the workman understands his business. The black sulphuret, thus prepared, is
ground, to facilitate the filling with it of small earthen bottles capable of holding about
24 ounces of water; from 30 to 40 of which bottles are filled beforehand, to be ready
when wanted.
“Three great subliming pots or vessels, made of very pure clay and sand, have been
previously coated over with a proper lute, and allowed to dry slowly. These pots are set
upon three furnaces bound with iron hoops, and they are covered with a kind of iron
dome. The furnaces are constructed so that the flame may freely circulate and play
upon the pots, over two-thirds of their height.
“The subliming vessels having been set in their places, a moderate fire is kindled in
the evening, which is gradually augmented till the pots become red. A bottle of the
black sulphuret is then poured into the first in the series, next into the second and
third, in succession; but eventually, two, three, or even more, bottles may be emptied
in at once; this circumstance depends on the stronger or weaker combustion of the
sulphuret of mercury thus projected. After its introduction, the flame rises 4 and
sometimes 6 feet high; when it has diminished a little, the vessels are covered with a
plate of iron, a foot square, and an inch and a half thick, made to fit perfectly close.
In this manner, the whole materials which have been prepared are introduced, in
the course of 34 hours, into the three pots; being for each pot, 360 pounds of mercury,
and 50 of sulphur; in all, 410 pounds.”
The degree of firing is judged of, from time to time, by lifting off the cover; for if
the flame rise several feet above the mouth of the pot, the heat is too great; if it be
hardly visible, the heat is too low. The proper criterion being a vigorous flame playing
a few inches above the vessel. In the last of the 36 hours’ process, the mass should
be dexterously stirred up every 15 or 20 minutes, to quicken the sublimation. The
subliming pots are then allowed to cool, and broken to pieces in order to collect all the[1278]
vermillion encrusted within them; and which usually amounts to 400 lbs., being a loss of
only 60 on each vessel. The lumps are to be ground along with water between horizontal
stones, elutriated, passed through sieves, and dried. It is said that the rich tone of the
Chinese vermillion may be imitated by adding to the materials for subliming one per
cent. of sulphuret of antimony, and by digesting the ground article first in a solution of
sulphuret of potassa, and, finally, in diluted muriatic acid.
The humid process of Kirchoff has of late years been so much improved, as to
furnish a vermillion quite equal in brilliancy to the Chinese. The following process
has been recommended. Mercury is triturated for several hours with sulphur, in the
cold, till a perfect ethiops is formed; potash lye is then added, and the trituration is
continued for some time. The mixture is now heated in iron vessels, with constant
stirring at first, but afterwards only from time to time. The temperature must be
kept up as steadily as possible at 130° Fahr., adding fresh supplies of water as it evaporates.
When the mixture which was black, becomes, at the end of some hours,
brown-red, the greatest caution is requisite, to prevent the temperature from being raised
above 114°, and to preserve the mixture quite liquid, while the compound of sulphur
and mercury should always be pulverulent. The colour becomes red, and brightens
in its hue, often with surprising rapidity. When the tint is nearly fine, the process should
be continued at a gentler heat, during some hours. Finally, the vermillion is to be
elutriated, in order to separate any particles of running mercury. The three ingredients
should be very pure. The proportion of product varies with that of the constituents, as
we see from the following results of experiments, in which 300 parts of mercury were
always employed, and from 400 to 450 of water:—
| Sulphur. |
Potash. |
Vermillion
obtained. |
| 114 |
75 |
330 |
| 115 |
75 |
331 |
| 120 |
120 |
321 |
| 150 |
152 |
382 |
| 120 |
180 |
245 |
| 100 |
180 |
244 |
| 60 |
180 |
142 |
The first proportions are therefore the most advantageous; the last, which are those of
M. Kirchoff himself, are not so good.
Brunner found that 300 parts of quicksilver, 114 of sulphur, 75 of caustic potassa,
and from 400 to 450 of water, form very suitable proportions for the moist process;
that the best temperature was 113° F.; and that 122° was the highest limit of heat
compatible with the production of a fine colour.
The theory of this process is by no means clear. We may suppose that a sulphuret
of potassium and mercury is first formed, which is eventually destroyed, in proportion
as the oxygen of the air acts upon the sulphuret of potassium itself. There
may also be produced some hyposulphite of mercury, which, under the same influence,
would be transformed into sulphuret of mercury and sulphate of potash.
Sulphuret of potassium and mercury furnish also vermillion, but it is not beautiful.
Red oxide of mercury, calomel, turbith mineral, and the soluble mercury of Hahnemann,
treated with the sulphuret of potassium, or the hydrosulphuret of ammonia, are
all capable of giving birth to vermillion by the humid way.
The vermillion of commerce is often adulterated with red lead, brickdust, dragon’s
blood, and realgar. The first two, not being volatile, remain when the vermillion is
heated to its subliming point; the third gives a red tincture to alcohol; the fourth
exhales its peculiar garlic smell with heat; and when calcined in a crucible with carbonate
of soda, and nitre in excess, affords arsenic acid, which may be detected by
the usual chemical tests.
VINEGAR MANUFACTORY, BY MALT. Annual produce, 100,000 gallons.
| Expenses for One Month. |
£ |
s. |
d. |
| Cost of material and fuel for 8,333 gallons, at 83⁄4d. |
|
303 |
16 |
2 |
| Wages to 8 workmen, at 25s. per week |
|
40 |
0 |
0 |
| Salaries to clerks, manager, and traveller |
|
83 |
6 |
8 |
| Travelling expenses |
|
30 |
0 |
0 |
| Three horses’ keep |
|
7 |
10 |
0 |
| Rent and taxes |
|
25 |
0 |
0 |
| |
£489 |
12 |
10 |
| Expenses for 5 months, at 489l. 12s. 10d. |
|
2448 |
4 |
2 |
| Duty on 41,665 gallons, at 2d. |
|
347 |
4 |
2 |
| Stock of utensils |
|
1500 |
0 |
0 |
| |
£4295 |
8 |
4 |
| Produce of 100,000 gallons, at 1s. 8d.[1279] |
|
£8333 |
6 |
8 |
| Expenses for 12 months, at 489l. 12s. 10d. |
£5875 |
18 |
0 |
| Duty on 100,000 gallons, at 2d. |
833 |
6 |
8 |
| Interest on capital, 4295l. 8s. 4d. |
214 |
4 |
5 |
| |
6923 |
9 |
1 |
| Net profit |
£1409 |
17 |
7 |
| See Acetic Acid. |
VIOLET DYE, is produced by a mixture of red and blue colouring-matters, which
are applied in succession. Silk is dyed a fugitive violet with either archil or brazil
wood; but a fine fast violet, first by a crimson with cochineal, without tartar or tin mordant,
and after washing, it is dipped in the indigo vat. A finish is sometimes given with
archil. A violet is also given to silk, by passing it through a solution of verdigris, then
through a bath of logwood, and, lastly, through alum water. A more beautiful violet
may be communicated by passing the alumed silk through a bath of brazil wood, and
after washing it in the river, through a bath of archil.
To produce violets on printed calicoes, a dilute acetate of iron is the mordant, and the
dye is madder. The mordanted goods should be well dunged.
A good process for dyeing cottons violet, is—first, to gall, with 18 or 20 pounds of nut-galls
for every 100 pounds of cotton; second, to pass the stuff; still hot, through a mordant
composed of—alum, 10 pounds; iron-liquor, at 11⁄2° B., and sulphate of copper, each 5 or 6
pounds; water, from 24 to 28 gallons; working it well, with alternate steeping, squeezing,
airing, dipping, squeezing, and washing; third, to madder, with its own weight of the root;
and fourth, to brighten with soap. If soda be used at the end, instead of soap, the colour
called prune de monsieur will be produced; and by varying the doses of the ingredients, a
variety of violet tints may be given.
The best violets are produced by dyeing yarn or cloth which has been prepared with
oil as for the Turkey-red process. See Madder.
For the violet pruneau, a little nitrate of iron is mixed with the alum mordant, which
makes a black; but this is changed into violet pruneau, by a madder bath, followed by a
brightening with soap.
VITRIFIABLE COLOURS; see Enamels, Pastes, Pottery, and Stained Glass.
VITRIOL, from vitrum, glass, is the old chemical, and still the vulgar appellation of
sulphuric acid, and of many of its compounds, which in certain states have a glassy
appearance: thus—
Vitriolic acid, or oil of vitriol, is sulphuric acid; blue vitriol, is sulphate of copper;
green vitriol, is green sulphate of iron; vitriol of Mars, is red sulphate of iron; and
white vitriol, is sulphate of zinc.
WACKE, is a massive mineral, intermediate between claystone and basalt. It is of
a greenish-gray colour; vesicular in structure; dull, opaque; streak shining; soft,
easily frangible; spec. grav. 2·55 to 2·9; it fuses like basalt.
WADD, is the provincial name of plumbago in Cumberland; and also of an ore of
manganese in Derbyshire, which consists of the peroxide of that metal, associated with
nearly its own weight of oxide of iron.
WADDING (Ouate, Fr.; Watte, Germ.); is the spongy web which serves to
line ladies’ pelisses, &c. Ouate, or Wat, was the name originally given to the glossy
downy tufts found in the pods of the plant commonly called Apocyn, and by botanists
Asclepias syriaca, which was imported from Egypt and Asia Minor for the purpose of
stuffing cushions, &c. Wadding is now made with a lap or fleece of cotton prepared
by the carding-engine (see Carding, Cotton Manufacture), which is applied to tissue
paper by a coat of size, made by boiling the cuttings of hare-skins, and adding a little
alum to the gelatinous solution. When two laps are glued with their faces together,
they form the most downy kind of wadding.
WAFERS. There are two manners of manufacturing wafers: 1, with wheat flour
and water, for the ordinary kind; and 2, with gelatine. 1. A certain quantity of fine
flour is to be diffused through pure water, and so mixed as to leave no clotty
particles. This thin pap is then coloured with one or other of the matters to be particularly
described under the second head; and which are, vermillion, sulphate of indigo,
and gamboge. The pap is not allowed to ferment, but must be employed immediately
after it is mixed. For this purpose a tool is employed, consisting of two plates of iron,[1280]
which come together like pincers or a pair of tongs, leaving a certain small definite
space betwixt them. These plates are first slightly heated, greased with butter, filled
with the pap, closed, and then exposed for a short time to the heat of a charcoal fire.
The iron plates being allowed to cool, on opening them, the thin cake appears dry,
solid, brittle, and about as thick as a playing-card. By means of annular punches of
different sizes, with sharp edges, the cake is cut into wafers. 2. The transparent wafers
are made as follows:—
Dissolve fine glue, or isinglass, in such a quantity of water, that the solution, when
cold, may be consistent. Let it be poured hot upon a plate of mirror glass, (previously
warmed with steam, and slightly greased,) which is fitted in a metallic frame, with edges
just as high as the wafers should be thick. A second plate of glass, heated and
greased, is laid on the surface, so as to touch every point of the gelatine, resting on the
edges of the frame. By this pressure, the thin cake of gelatine is made perfectly
uniform. When the two plates of glass get cold, the gelatine becomes solid, and may
easily be removed. It is then cut with proper punches into discs of different sizes.
The colouring-matters ought not to be of an insalubrious kind.
For red wafers, carmine is well adapted, when they are not to be transparent; but
this colour is dear, and can be used only for the finer kinds. Instead of it, a decoction
of brazil wood, brightened with a little alum, may be employed.
For yellow, an infusion of saffron or turmeric has been prescribed; but a decoction
of weld, fustic, or Persian berries, might be used.
Sulphate of indigo, partially saturated with potash, is used for the blue wafers; and
this mixed with yellow, for the greens. Some recommend the sulphate to be nearly
neutralized with chalk, and to treat the liquor with alcohol, in order to obtain the best
blue dye for wafers.
Common wafers are, however, coloured with the substances mentioned at the
beginning of the article; and for the cheaper kinds, red lead is used instead of vermillion,
and turmeric instead of gamboge.
WALNUT HUSKS, or PEELS (Brout des noix, Fr.); are much employed by
the French dyers for rooting or giving dun colours.
WARP (Chaine, Fr.; Kette, Auschweif, Zettel, Germ.); is the name of the longitudinal
threads or yarns, whether of cotton, linen, silk, or wool, which being decussated
at right angles by the woof or weft threads, form a piece of cloth. The warp
yarns are parallel, and continuous from end to end of the web. See Weaving, for a
description of the warping-mill.
WASH, is the fermented wort of the distiller.
WASHING. See Bleaching, and Scouring.
WATERING OF STUFFS (Moirage, Fr.); is a process to which silk and
other textile fabrics are subjected, for causing them to exhibit a variety of undulated
reflections, and plays of light. It is produced by sprinkling water upon the goods, and
then passing them through a calender, either with cold or hot rollers, plain or variously
indented.
WATER-PROOF CLOTH. See Caoutchouc, and Gelatine.
A patent was obtained, in August, 1830, by Mr. Thomas Hancock, for rendering
textile fabrics impervious to water and air, by spreading the liquid juice of the caoutchouc
tree upon the surfaces of the goods, and then exposing them to the air to dry. It does
not appear that this project has been realized in our manufactures.
Mr. William Simpson Potter proposes, in his patent, of April, 1835, to render fabrics
water-proof by imbuing them with a solution of isinglass, alum, and soap, by means of
a brush applied to the wrong side of the cloth, distended upon a table. After it is dry,
it must be brushed on the wrong side, against the grain. Then the brush is to be
dipped in clean water, and passed lightly over the cloth. The gloss caused by the
above application can be taken off by brushing the goods when they are dry. Cloth so
prepared is said to be impervious to water, but not to air.
I have examined woollen cloth now on sale in a shop in the Strand, which may be
breathed through with the greatest facility, but which retains water upon its surface, as
is evinced by a body of water standing upon a concave piece of it tied over a show-glass
in the window.
Mr. Sievier’s plan of rendering cloth water-proof, for which he obtained a patent in
December, 1835, consists in spreading over it, with a brush, a solution of India rubber
in spirits of turpentine, at one or more applications, and then applying a similar solution
mixed with acetate of lead, litharge, sulphate of zinc, gum mastic, or other drying
material. He next takes wool, or other textile material, cut into proper lengths, and
spreads it upon the surface of the fabric varnished in this manner, for the purpose of
forming the nap or pile. He then presses the cloth by means of rollers, or brushes, so
as to fix the nap firmly to its surface.
[1281]
WATERS, MINERAL—Table I. Analyses of the principal Mineral Waters
of Germany.
Grains of
Anhydrous Ingredients
in One Pound Troy. |
Carlsbad. |
ms. |
Schlesi-
scher.
Obersalz-
brunnen. |
Marien-
bad.
Kreutzbr. |
Auscho-
witz.
Ferdi-
nands-
brunnen. |
Eger.
Franzens-
brunnen. |
Pyr-
mont. |
Spa.
Pouhon. |
Fach-
ingen. |
Geilnau. |
Seltzer. |
Seid-
schutz. |
Püllna. |
| Carbonate of Soda |
7 |
·2712 |
8 |
·0625 |
6 |
·1133 |
5 |
·3499 |
4 |
·5976 |
3 |
·8914 |
|
0 |
·5531 |
12 |
·3328 |
4 |
·9658 |
4 |
·6162 |
|
|
| Ditto of Lithia |
0 |
·0150 |
0 |
·0405 |
0 |
·0127 |
0 |
·0858 |
0 |
·0507 |
0 |
·0282 |
|
|
|
|
|
|
|
| Ditto of Baryta |
|
0 |
·0022 |
|
|
|
|
|
|
|
|
0 |
·0014 |
|
|
| Ditto of Strontia |
0 |
·0055 |
0 |
·0080 |
0 |
·0165 |
0 |
·0028 |
0 |
·0040 |
0 |
·0023 |
|
|
|
|
0 |
·0144 |
|
|
| Ditto of Lime |
1 |
·7775 |
0 |
·8555 |
1 |
·7497 |
2 |
·9509 |
3 |
·0085 |
1 |
·3501 |
4 |
·7781 |
0 |
·7387 |
1 |
·8667 |
2 |
·2279 |
1 |
·4004 |
5 |
·1045 |
0 |
·5775 |
| Ditto of Magnesia |
1 |
·0275 |
0 |
·5915 |
1 |
·4107 |
2 |
·0390 |
2 |
·2867 |
0 |
·5040 |
|
0 |
·8425 |
1 |
·2983 |
1 |
·6282 |
1 |
·5000 |
0 |
·8235 |
4 |
·8045 |
| Do. (Proto) of Manganese |
0 |
·0048 |
0 |
·0028 |
|
0 |
·0288 |
0 |
·0692 |
0 |
·0322 |
0 |
·0364 |
0 |
·0389 |
|
|
|
0 |
·0032 |
|
| Ditto (Proto) of Iron |
0 |
·0208 |
0 |
·0120 |
0 |
·0480 |
0 |
·1319 |
0 |
·2995 |
0 |
·1762 |
0 |
·3213 |
0 |
·2813 |
|
|
|
0 |
·0095 |
|
| Sub-Phos. of Lime |
0 |
·0012 |
|
|
|
|
0 |
·0172 |
|
0 |
·0102 |
0 |
·0061 |
|
0 |
·0007 |
0 |
·0117 |
0 |
·0026 |
| Ditto of Alumina |
0 |
·0019 |
0 |
·0014 |
0 |
·0045 |
|
0 |
·0040 |
0 |
·0092 |
0 |
·0110 |
0 |
·0064 |
|
|
0 |
·0020 |
0 |
·0088 |
|
| Sulphate of Potassa |
|
0 |
·4050 |
0 |
·2220 |
|
|
|
0 |
·0314 |
0 |
·0598 |
|
0 |
·2154 |
0 |
·2978 |
3 |
·6705 |
3 |
·6000 |
| Ditto of Soda |
14 |
·9019 |
|
2 |
·2095 |
28 |
·5868 |
16 |
·9022 |
18 |
·3785 |
1 |
·6092 |
0 |
·0289 |
0 |
·1267 |
0 |
·0315 |
|
17 |
·6220 |
92 |
·8500 |
| Ditto of Lithia |
|
|
|
|
|
|
0 |
·0067 |
|
|
|
|
|
|
| Ditto of Lime |
|
|
|
|
|
|
5 |
·0265 |
|
|
|
|
1 |
·1287 |
1 |
·9500 |
| Ditto of Strontia |
|
|
|
|
|
|
0 |
·0154 |
|
|
|
|
0 |
·0347 |
|
| Ditto of Magnesia |
|
|
|
|
|
|
2 |
·3684 |
|
|
|
|
62 |
·3535 |
69 |
·8145 |
| Nitr. of Magnesia |
|
|
|
|
|
|
|
|
|
|
|
5 |
·9302 |
|
| Chlor. of Potassium |
|
0 |
·0338 |
|
|
|
|
|
|
|
|
0 |
·2685 |
|
|
| Ditto of Sodium |
5 |
·9820 |
5 |
·7255 |
0 |
·8752 |
10 |
·1727 |
6 |
·7472 |
6 |
·9229 |
|
0 |
·3371 |
3 |
·2337 |
0 |
·4072 |
12 |
·9690 |
|
|
| Ditto of Magnesium |
|
|
|
|
|
|
0 |
·8450 |
|
|
|
|
1 |
·2225 |
14 |
·7495 |
| Fluoride of Calcium |
0 |
·0184 |
0 |
·0014 |
|
|
|
|
|
|
|
|
0 |
·0013 |
|
|
| Alumina |
|
|
|
0 |
·0023 |
|
|
|
|
|
0 |
·0185 |
|
|
|
| Silica |
0 |
·4329 |
0 |
·3104 |
0 |
·2531 |
0 |
·2908 |
0 |
·5023 |
0 |
·3548 |
0 |
·3727 |
0 |
·3739 |
0 |
·0657 |
0 |
·2021 |
0 |
·2265 |
0 |
·0900 |
0 |
·1320 |
| Total |
31 |
·4606 |
16 |
·0525 |
12 |
·9152 |
49 |
·6417 |
34 |
·4719 |
31 |
·6670 |
15 |
·4221 |
3 |
·2691 |
18 |
·9300 |
9 |
·6966 |
21 |
·2982 |
98 |
·0133 |
188 |
·4806 |
Carbonic Acid Gas
in 100 cubic inches |
58 |
51 |
98 |
105 |
146 |
154 |
160 |
136 |
135 |
163 |
126 |
20 |
7 |
| Temperature (F.) |
| Sprud. |
165° |
| Neub. |
138° |
| Mühl. |
128° |
| Ther. |
122° |
|
|
58° |
53° |
46° |
53° |
56° |
50° |
50° |
51° |
58° |
58° |
58° |
| Analyzed by |
Berzelius. |
Struve. |
Struve. |
Berzelius. |
Steinmann. |
Berzelius |
Struve. |
Struve. |
Bischoff. |
Struve. |
Struve. |
Struve. |
Struve. |
Grains of
Anhydrous Ingredients
in One Pound Troy. |
Carlsbad. |
ms. |
Schlesi-
scher.
Obersalz-
brunnen. |
Marien-
bad.
Kreutzbr. |
Auscho-
witz.
Ferdi-
nands-
brunnen. |
| Carbonate of Soda |
7 |
·2712 |
8 |
·0625 |
6 |
·1133 |
5 |
·3499 |
4 |
·5976 |
| Ditto of Lithia |
0 |
·0150 |
0 |
·0405 |
0 |
·0127 |
0 |
·0858 |
0 |
·0507 |
| Ditto of Baryta |
|
0 |
·0022 |
|
|
|
| Ditto of Strontia |
0 |
·0055 |
0 |
·0080 |
0 |
·0165 |
0 |
·0028 |
0 |
·0040 |
| Ditto of Lime |
1 |
·7775 |
0 |
·8555 |
1 |
·7497 |
2 |
·9509 |
3 |
·0085 |
| Ditto of Magnesia |
1 |
·0275 |
0 |
·5915 |
1 |
·4107 |
2 |
·0390 |
2 |
·2867 |
| Do. (Proto) of Manganese |
0 |
·0048 |
0 |
·0028 |
|
0 |
·0288 |
0 |
·0692 |
| Ditto (Proto) of Iron |
0 |
·0208 |
0 |
·0120 |
0 |
·0480 |
0 |
·1319 |
0 |
·2995 |
| Sub-Phos. of Lime |
0 |
·0012 |
|
|
|
|
| Ditto of Alumina |
0 |
·0019 |
0 |
·0014 |
0 |
·0045 |
|
0 |
·0040 |
| Sulphate of Potassa |
|
0 |
·4050 |
0 |
·2220 |
|
|
| Ditto of Soda |
14 |
·9019 |
|
2 |
·2095 |
28 |
·5868 |
16 |
·9022 |
| Ditto of Lithia |
|
|
|
|
|
| Ditto of Lime |
|
|
|
|
|
| Ditto of Strontia |
|
|
|
|
|
| Ditto of Magnesia |
|
|
|
|
|
| Nitr. of Magnesia |
|
|
|
|
|
| Chlor. of Potassium |
|
0 |
·0338 |
|
|
|
| Ditto of Sodium |
5 |
·9820 |
5 |
·7255 |
0 |
·8752 |
10 |
·1727 |
6 |
·7472 |
| Ditto of Magnesium |
|
|
|
|
|
| Fluoride of Calcium |
0 |
·0184 |
0 |
·0014 |
|
|
|
| Alumina |
|
|
|
0 |
·0023 |
|
| Silica |
0 |
·4329 |
0 |
·3104 |
0 |
·2531 |
0 |
·2908 |
0 |
·5023 |
| Total |
31 |
·4606 |
16 |
·0525 |
12 |
·9152 |
49 |
·6417 |
34 |
·4719 |
Carbonic Acid Gas
in 100 cubic inches |
58 |
51 |
98 |
105 |
146 |
| Temperature (F.) |
| Sprud. |
165° |
| Neub. |
138° |
| Mühl. |
128° |
| Ther. |
122° |
|
|
58° |
53° |
46° |
| Analyzed by |
Berzelius. |
Struve. |
Struve. |
Berzelius. |
Steinmann. |
Grains of
Anhydrous Ingredients
in One Pound Troy. |
Eger.
Franzens-
brunnen. |
Pyr-
mont. |
Spa.
Pouhon. |
Fach-
ingen. |
| Carbonate of Soda |
3 |
·8914 |
|
0 |
·5531 |
12 |
·3328 |
| Ditto of Lithia |
0 |
·0282 |
|
|
|
| Ditto of Baryta |
|
|
|
|
| Ditto of Strontia |
0 |
·0023 |
|
|
|
| Ditto of Lime |
1 |
·3501 |
4 |
·7781 |
0 |
·7387 |
1 |
·8667 |
| Ditto of Magnesia |
0 |
·5040 |
|
0 |
·8425 |
1 |
·2983 |
| Do. (Proto) of Manganese |
0 |
·0322 |
0 |
·0364 |
0 |
·0389 |
|
| Ditto (Proto) of Iron |
0 |
·1762 |
0 |
·3213 |
0 |
·2813 |
|
| Sub-Phos. of Lime |
0 |
·0172 |
|
0 |
·0102 |
0 |
·0061 |
| Ditto of Alumina |
0 |
·0092 |
0 |
·0110 |
0 |
·0064 |
|
| Sulphate of Potassa |
|
0 |
·0314 |
0 |
·0598 |
|
| Ditto of Soda |
18 |
·3785 |
1 |
·6092 |
0 |
·0289 |
0 |
·1267 |
| Ditto of Lithia |
|
0 |
·0067 |
|
|
| Ditto of Lime |
|
5 |
·0265 |
|
|
| Ditto of Strontia |
|
0 |
·0154 |
|
|
| Ditto of Magnesia |
|
2 |
·3684 |
|
|
| Nitr. of Magnesia |
|
|
|
|
| Chlor. of Potassium |
|
|
|
|
| Ditto of Sodium |
6 |
·9229 |
|
0 |
·3371 |
3 |
·2337 |
| Ditto of Magnesium |
|
0 |
·8450 |
|
|
| Fluoride of Calcium |
|
|
|
|
| Alumina |
|
|
|
|
| Silica |
0 |
·3548 |
0 |
·3727 |
0 |
·3739 |
0 |
·0657 |
| Total |
31 |
·6670 |
15 |
·4221 |
3 |
·2691 |
18 |
·9300 |
Carbonic Acid Gas
in 100 cubic inches |
154 |
160 |
136 |
135 |
| Temperature (F.) |
53° |
56° |
50° |
50° |
| Analyzed by |
Berzelius |
Struve. |
Struve. |
Bischoff. |
Grains of
Anhydrous Ingredients
in One Pound Troy. |
Geilnau. |
Seltzer. |
Seid-
schutz. |
Püllna. |
| Carbonate of Soda |
4 |
·9658 |
4 |
·6162 |
|
|
| Ditto of Lithia |
|
|
|
|
| Ditto of Baryta |
|
0 |
·0014 |
|
|
| Ditto of Strontia |
|
0 |
·0144 |
|
|
| Ditto of Lime |
2 |
·2279 |
1 |
·4004 |
5 |
·1045 |
0 |
·5775 |
| Ditto of Magnesia |
1 |
·6282 |
1 |
·5000 |
0 |
·8235 |
4 |
·8045 |
| Do. (Proto) of Manganese |
|
|
0 |
·0032 |
|
| Ditto (Proto) of Iron |
|
|
0 |
·0095 |
|
| Sub-Phos. of Lime |
|
0 |
·0007 |
0 |
·0117 |
0 |
·0026 |
| Ditto of Alumina |
|
0 |
·0020 |
0 |
·0088 |
|
| Sulphate of Potassa |
0 |
·2154 |
0 |
·2978 |
3 |
·6705 |
3 |
·6000 |
| Ditto of Soda |
0 |
·0315 |
|
17 |
·6220 |
92 |
·8500 |
| Ditto of Lithia |
|
|
|
|
| Ditto of Lime |
|
|
1 |
·1287 |
1 |
·9500 |
| Ditto of Strontia |
|
|
0 |
·0347 |
|
| Ditto of Magnesia |
|
|
62 |
·3535 |
69 |
·8145 |
| Nitr. of Magnesia |
|
|
5 |
·9302 |
|
| Chlor. of Potassium |
|
0 |
·2685 |
|
|
| Ditto of Sodium |
0 |
·4072 |
12 |
·9690 |
|
|
| Ditto of Magnesium |
|
|
1 |
·2225 |
14 |
·7495 |
| Fluoride of Calcium |
|
0 |
·0013 |
|
|
| Alumina |
0 |
·0185 |
|
|
|
| Silica |
0 |
·2021 |
0 |
·2265 |
0 |
·0900 |
0 |
·1320 |
| Total |
9 |
·6966 |
21 |
·2982 |
98 |
·0133 |
188 |
·4806 |
Carbonic Acid Gas
in 100 cubic inches |
163 |
126 |
20 |
7 |
| Temperature (F.) |
51° |
58° |
58° |
58° |
| Analyzed by |
Struve. |
Struve. |
Struve. |
Struve. |
[1282]
Table II.—The Composition of other celebrated Mineral Waters.
| Names of the Springs. |
Grains
of
water. |
Cubic Inches of Gases. |
Carbonates of |
Sulphates of |
Muriates of |
Silica. |
Alu-
mina. |
Resins. |
Tem-
pera-
ture. |
Oxy-
gen. |
Car-
bonic
acid. |
Sulph.
hydro-
gen. |
Azote. |
Soda. |
Lime. |
Mag-
nesia. |
Iron. |
Soda. |
Lime. |
Mag-
nesia. |
Iron. |
Soda. |
Lime. |
Mag-
nesia. |
Potash. |
| |
|
|
|
|
|
grs. |
grs. |
grs. |
grs. |
grs. |
grs. |
grs. |
grs. |
grs. |
grs. |
grs. |
grs. |
grs. |
grs. |
grs. |
|
| |
Kilburn (1)—acidulous. |
138240 |
|
84 |
·0 |
36 |
·0 |
|
|
2 |
·4 |
1 |
·25 |
0 |
·31⁄4 |
18 |
·2 |
13 |
·0 |
91 |
·0 |
|
6 |
·0 |
0 |
·6 |
2 |
·8 |
|
|
|
6 |
·0 |
cold |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sul-
phu-
rous. |
|
- |
Harrowgate (2) |
103643 |
|
8 |
·0 |
19 |
·0 |
7 |
·0 |
|
18 |
·5 |
5 |
·5 |
|
|
|
0 |
·5 |
|
615 |
·5 |
3 |
·0 |
9 |
·1 |
|
|
|
|
cold |
| Moffat (2) |
103643 |
|
1 |
·0 |
10 |
·0 |
4 |
·0 |
|
|
|
|
|
|
|
|
3 |
·6 |
|
|
|
|
|
|
cold |
| Aix-la-Chapelle (3) |
8940 |
|
|
13 |
·06 |
|
|
15 |
·25 |
5 |
·89 |
|
|
|
|
|
6 |
·21 |
|
|
|
|
|
|
143° |
| Enghein (4) |
92160 |
|
18 |
·5 |
7 |
·0 |
|
|
21 |
·4 |
1 |
·35 |
|
|
33 |
·3 |
5 |
·8 |
|
2 |
·4 |
|
8 |
·0 |
|
|
|
|
cold |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sa-
line. |
|
- |
Seidlitz |
58309 |
|
8 |
·0 |
|
|
|
6 |
·7 |
21 |
·0 |
|
|
41 |
·1 |
14 |
·44 |
|
|
|
36 |
·5 |
|
|
|
|
cold |
| Cheltenham (5) |
103643 |
|
30 |
·3 |
3 |
·0 |
12 |
·0 |
|
|
12 |
·5 |
5 |
·0 |
48 |
·0 |
40 |
·0 |
|
|
5 |
·0 |
|
12 |
·5 |
|
|
|
|
cold |
| Plombieres (6) |
14600 |
|
|
|
|
36 |
·0 |
0 |
·4 |
|
|
1 |
·0 |
|
|
|
2 |
·0 |
|
|
|
|
|
|
cold |
| Dunblane (7) sp. gr. 1·00475 |
7291 |
|
|
|
|
|
0 |
·5 |
|
0 |
·17 |
3 |
·7 |
|
|
|
21 |
·0 |
20 |
·8 |
|
|
|
|
|
cold |
| Pitcaithley (7) |
7291 |
|
1 |
·0 |
|
|
|
0 |
·5 |
|
|
0 |
·9 |
|
|
|
12 |
·7 |
20 |
·2 |
|
|
|
|
|
cold |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cha-
ly-
be-
ate. |
|
- |
Tunbridge (3) |
103643 |
1 |
·4 |
10 |
·6 |
|
4 |
·0 |
|
|
|
1 |
·0 |
|
1 |
·25 |
|
|
0 |
·5 |
|
2 |
·25 |
|
|
|
|
cold |
| Brighton (8) |
58309 |
|
18 |
·0 |
|
|
|
|
|
|
|
32 |
·7 |
|
11 |
·2 |
12 |
·2 |
|
6 |
·0 |
|
1 |
·12 |
|
|
cold |
| Toplitz (9) |
22540 |
|
|
|
|
13 |
·5 |
16 |
·5 |
|
32 |
·5 |
|
|
|
|
61 |
·3 |
28 |
·5 |
|
|
|
15 |
·1 |
|
cold |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cal-
car-
eous,
nearly
pure. |
|
- |
Bath (10) |
15360 |
|
2 |
·4 |
|
|
|
1 |
·6 |
|
0 |
·004 |
3 |
·0 |
18 |
·0 |
|
|
6 |
·6 |
|
|
|
0 |
·4 |
|
|
114° |
| Buxton (11) |
58309 |
|
|
|
2 |
·0 |
|
10 |
·5 |
|
|
|
2 |
·5 |
|
|
1 |
·5 |
|
|
|
|
|
|
82° |
| Bristol (12) |
58309 |
|
30 |
·3 |
|
|
|
13 |
·5 |
|
|
11 |
·2 |
11 |
·7 |
|
|
4 |
·0 |
|
7 |
·25 |
|
|
|
|
74° |
| Matlock |
58309 |
|
|
|
|
|
|
|
|
|
trace |
|
|
|
|
|
|
|
|
|
66° |
| Malvern (13) |
58309 |
|
|
|
|
5 |
·33 |
1 |
·6 |
0 |
·92 |
0 |
·625 |
2 |
·896 |
|
|
|
1 |
·55 |
|
|
|
|
|
|
cold |
| |
Dead Sea |
(14) sp. gr. |
1 |
·211 |
100 |
|
|
|
|
|
|
|
|
|
|
·054 |
|
|
10 |
·676 |
3 |
·8 |
10 |
·1 |
|
|
|
|
|
| Do. |
(15) sp. gr. |
1 |
·245 |
|
|
|
|
|
|
|
|
|
|
|
|
|
7 |
·8 |
10 |
·6 |
24 |
·2 |
|
|
|
|
|
| Do. |
(16) sp. gr. |
1 |
·2283 |
|
|
|
|
|
|
|
|
|
|
|
|
|
6 |
·95 |
4 |
·0 |
15 |
·31 |
|
|
|
|
|
| Sea water, Forth (7) |
7291 |
|
|
|
|
|
|
|
|
25 |
·6 |
|
|
|
159 |
·3 |
5 |
·7 |
35 |
·5 |
trace[70] |
|
|
|
|
| (1) |
Schmesser. |
| (2) |
Garnet. |
| (3) |
Babington. |
| (4) |
Fourcroy. |
| (5) |
Fothergill. |
| (6) |
Vauquelin. |
| (7) |
Dr. Murray. |
| (8) |
Marcet. |
| (9) |
John. |
| (10) |
Phillips. |
| (11) |
Pearson. |
| (12) |
Carrick. |
| (13) |
Dr. Philip. |
| (14) |
Dr. Marcet. |
| (15) |
Klaproth. |
| (16) |
M. Gay Lussac. |
| [70] |
Dr. Wollaston. |
|
|
| Names of the Springs. |
Grains
of
water. |
Cubic Inches of Gases. |
Oxy-
gen. |
Car-
bonic
acid. |
Sulph.
hydro-
gen. |
Azote. |
| |
|
|
|
|
|
| |
Kilburn (1)—acidulous. |
138240 |
|
84 |
·0 |
36 |
·0 |
|
Sul-
phu-
rous. |
|
- |
Harrowgate (2) |
103643 |
|
8 |
·0 |
19 |
·0 |
7 |
·0 |
| Moffat (2) |
103643 |
|
1 |
·0 |
10 |
·0 |
4 |
·0 |
| Aix-la-Chapelle (3) |
8940 |
|
|
13 |
·06 |
|
| Enghein (4) |
92160 |
|
18 |
·5 |
7 |
·0 |
|
| |
|
|
|
|
|
Sa-
line. |
|
- |
Seidlitz |
58309 |
|
8 |
·0 |
|
|
| Cheltenham (5) |
103643 |
|
30 |
·3 |
3 |
·0 |
12 |
·0 |
| Plombieres (6) |
14600 |
|
|
|
|
| Dunblane (7) sp. gr. 1·00475 |
7291 |
|
|
|
|
| Pitcaithley (7) |
7291 |
|
1 |
·0 |
|
|
| |
|
|
|
|
|
Cha-
ly-
be-
ate. |
|
- |
Tunbridge (3) |
103643 |
1 |
·4 |
10 |
·6 |
|
4 |
·0 |
| Brighton (8) |
58309 |
|
18 |
·0 |
|
|
| Toplitz (9) |
22540 |
|
|
|
|
| |
|
|
|
|
|
Cal-
car-
eous,
nearly
pure. |
|
- |
Bath (10) |
15360 |
|
2 |
·4 |
|
|
| Buxton (11) |
58309 |
|
|
|
2 |
·0 |
| Bristol (12) |
58309 |
|
30 |
·3 |
|
|
| Matlock |
58309 |
|
|
|
|
| Malvern (13) |
58309 |
|
|
|
|
| |
Dead Sea |
(14) sp. gr. |
1 |
·211 |
100 |
|
|
|
|
| Do. |
(15) sp. gr. |
1 |
·245 |
|
|
|
|
|
| Do. |
(16) sp. gr. |
1 |
·2283 |
|
|
|
|
|
| Sea water, Forth (7) |
7291 |
|
|
|
|
| (1) |
Schmesser. |
| (2) |
Garnet. |
| (3) |
Babington. |
| (4) |
Fourcroy. |
| (5) |
Fothergill. |
| (6) |
Vauquelin. |
| (7) |
Dr. Murray. |
| (8) |
Marcet. |
| (9) |
John. |
| (10) |
Phillips. |
| (11) |
Pearson. |
| (12) |
Carrick. |
| (13) |
Dr. Philip. |
| (14) |
Dr. Marcet. |
| (15) |
Klaproth. |
| (16) |
M. Gay Lussac. |
|
|
| Names of the Springs. |
Grains
of
water. |
Carbonates of |
| Soda. |
Lime. |
Mag-
nesia. |
Iron. |
| |
|
grs. |
grs. |
grs. |
grs. |
| |
Kilburn (1)—acidulous. |
138240 |
|
2 |
·4 |
1 |
·25 |
0 |
·31⁄4 |
Sul-
phu-
rous. |
|
- |
Harrowgate (2) |
103643 |
|
18 |
·5 |
5 |
·5 |
|
| Moffat (2) |
103643 |
|
|
|
|
| Aix-la-Chapelle (3) |
8940 |
|
15 |
·25 |
5 |
·89 |
|
| Enghein (4) |
92160 |
|
21 |
·4 |
1 |
·35 |
|
| |
|
|
|
|
|
Sa-
line. |
|
- |
Seidlitz |
58309 |
|
6 |
·7 |
21 |
·0 |
|
| Cheltenham (5) |
103643 |
|
|
12 |
·5 |
5 |
·0 |
| Plombieres (6) |
14600 |
36 |
·0 |
0 |
·4 |
|
|
| Dunblane (7) sp. gr. 1·00475 |
7291 |
|
0 |
·5 |
|
0 |
·17 |
| Pitcaithley (7) |
7291 |
|
0 |
·5 |
|
|
| |
|
|
|
|
|
Cha-
ly-
be-
ate. |
|
- |
Tunbridge (3) |
103643 |
|
|
|
1 |
·0 |
| Brighton (8) |
58309 |
|
|
|
|
| Toplitz (9) |
22540 |
13 |
·5 |
16 |
·5 |
|
32 |
·5 |
| |
|
|
|
|
|
Cal-
car-
eous,
nearly
pure. |
|
- |
Bath (10) |
15360 |
|
1 |
·6 |
|
0 |
·004 |
| Buxton (11) |
58309 |
|
10 |
·5 |
|
|
| Bristol (12) |
58309 |
|
13 |
·5 |
|
|
| Matlock |
58309 |
|
|
|
|
| Malvern (13) |
58309 |
5 |
·33 |
1 |
·6 |
0 |
·92 |
0 |
·625 |
| |
Dead Sea |
(14) sp. gr. |
1 |
·211 |
100 |
|
|
|
|
| Do. |
(15) sp. gr. |
1 |
·245 |
|
|
|
|
|
| Do. |
(16) sp. gr. |
1 |
·2283 |
|
|
|
|
|
| Sea water, Forth (7) |
7291 |
|
|
|
|
| (1) |
Schmesser. |
| (2) |
Garnet. |
| (3) |
Babington. |
| (4) |
Fourcroy. |
| (5) |
Fothergill. |
| (6) |
Vauquelin. |
| (7) |
Dr. Murray. |
| (8) |
Marcet. |
| (9) |
John. |
| (10) |
Phillips. |
| (11) |
Pearson. |
| (12) |
Carrick. |
| (13) |
Dr. Philip. |
| (14) |
Dr. Marcet. |
| (15) |
Klaproth. |
| (16) |
M. Gay Lussac. |
|
|
| Names of the Springs. |
Grains
of
water. |
Sulphates of |
| Soda. |
Lime. |
Mag-
nesia. |
Iron. |
| |
|
grs. |
grs. |
grs. |
grs. |
| |
Kilburn (1)—acidulous. |
138240 |
18 |
·2 |
13 |
·0 |
91 |
·0 |
|
Sul-
phu-
rous. |
|
- |
Harrowgate (2) |
103643 |
|
|
0 |
·5 |
|
| Moffat (2) |
103643 |
|
|
|
|
| Aix-la-Chapelle (3) |
8940 |
|
|
|
|
| Enghein (4) |
92160 |
|
33 |
·3 |
5 |
·8 |
|
| |
|
|
|
|
|
Sa-
line. |
|
- |
Seidlitz |
58309 |
|
41 |
·1 |
14 |
·44 |
|
| Cheltenham (5) |
103643 |
5 |
·0 |
48 |
·0 |
40 |
·0 |
|
| Plombieres (6) |
14600 |
1 |
·0 |
|
|
|
| Dunblane (7) sp. gr. 1·00475 |
7291 |
|
0 |
·5 |
|
0 |
·17 |
| Pitcaithley (7) |
7291 |
0 |
·9 |
|
|
|
| |
|
|
|
|
|
Cha-
ly-
be-
ate. |
|
- |
Tunbridge (3) |
103643 |
|
1 |
·25 |
|
|
| Brighton (8) |
58309 |
|
32 |
·7 |
|
11 |
·2 |
| Toplitz (9) |
22540 |
|
|
|
|
| |
|
|
|
|
|
Cal-
car-
eous,
nearly
pure. |
|
- |
Bath (10) |
15360 |
3 |
·0 |
18 |
·0 |
|
|
| Buxton (11) |
58309 |
|
2 |
·5 |
|
|
| Bristol (12) |
58309 |
11 |
·2 |
11 |
·7 |
|
|
| Matlock |
58309 |
|
trace |
|
|
| Malvern (13) |
58309 |
2 |
·896 |
|
|
|
| |
Dead Sea |
(14) sp. gr. |
1 |
·211 |
100 |
|
|
·054 |
|
|
| Do. |
(15) sp. gr. |
1 |
·245 |
|
|
|
|
|
| Do. |
(16) sp. gr. |
1 |
·2283 |
|
|
|
|
|
| Sea water, Forth (7) |
7291 |
25 |
·6 |
|
|
|
| (1) |
Schmesser. |
| (2) |
Garnet. |
| (3) |
Babington. |
| (4) |
Fourcroy. |
| (5) |
Fothergill. |
| (6) |
Vauquelin. |
| (7) |
Dr. Murray. |
| (8) |
Marcet. |
| (9) |
John. |
| (10) |
Phillips. |
| (11) |
Pearson. |
| (12) |
Carrick. |
| (13) |
Dr. Philip. |
| (14) |
Dr. Marcet. |
| (15) |
Klaproth. |
| (16) |
M. Gay Lussac. |
|
|
| Names of the Springs. |
Grains
of
water. |
Muriates of |
| Soda. |
Lime. |
Mag-
nesia. |
Potash. |
| |
|
grs. |
grs. |
grs. |
grs. |
| |
Kilburn (1)—acidulous. |
138240 |
6 |
·0 |
0 |
·6 |
2 |
·8 |
|
Sul-
phu-
rous. |
|
- |
Harrowgate (2) |
103643 |
615 |
·5 |
3 |
·0 |
9 |
·1 |
|
| Moffat (2) |
103643 |
3 |
·6 |
|
|
|
| Aix-la-Chapelle (3) |
8940 |
6 |
·21 |
|
|
|
| Enghein (4) |
92160 |
2 |
·4 |
|
8 |
·0 |
|
| |
|
|
|
|
|
Sa-
line. |
|
- |
Seidlitz |
58309 |
|
|
36 |
·5 |
|
| Cheltenham (5) |
103643 |
5 |
·0 |
|
12 |
·5 |
|
| Plombieres (6) |
14600 |
2 |
·0 |
|
|
|
| Dunblane (7) sp. gr. 1·00475 |
7291 |
21 |
·0 |
20 |
·8 |
|
|
| Pitcaithley (7) |
7291 |
12 |
·7 |
20 |
·2 |
|
|
| |
|
|
|
|
|
Cha-
ly-
be-
ate. |
|
- |
Tunbridge (3) |
103643 |
0 |
·5 |
|
2 |
·25 |
|
| Brighton (8) |
58309 |
12 |
·2 |
|
6 |
·0 |
|
| Toplitz (9) |
22540 |
61 |
·3 |
28 |
·5 |
|
|
| |
|
|
|
|
|
Cal-
car-
eous,
nearly
pure. |
|
- |
Bath (10) |
15360 |
6 |
·6 |
|
|
|
| Buxton (11) |
58309 |
1 |
·5 |
|
|
|
| Bristol (12) |
58309 |
4 |
·0 |
|
7 |
·25 |
|
| Matlock |
58309 |
|
|
|
|
| Malvern (13) |
58309 |
1 |
·55 |
|
|
|
| |
Dead Sea |
(14) sp. gr. |
1 |
·211 |
100 |
10 |
·676 |
3 |
·8 |
10 |
·1 |
|
| Do. |
(15) sp. gr. |
1 |
·245 |
|
7 |
·8 |
10 |
·6 |
24 |
·2 |
|
| Do. |
(16) sp. gr. |
1 |
·2283 |
|
6 |
·95 |
4 |
·0 |
15 |
·31 |
|
| Sea water, Forth (7) |
7291 |
159 |
·3 |
5 |
·7 |
35 |
·5 |
trace[70] |
| (1) |
Schmesser. |
| (2) |
Garnet. |
| (3) |
Babington. |
| (4) |
Fourcroy. |
| (5) |
Fothergill. |
| (6) |
Vauquelin. |
| (7) |
Dr. Murray. |
| (8) |
Marcet. |
| (9) |
John. |
| (10) |
Phillips. |
| (11) |
Pearson. |
| (12) |
Carrick. |
| (13) |
Dr. Philip. |
| (14) |
Dr. Marcet. |
| (15) |
Klaproth. |
| (16) |
M. Gay Lussac. |
| [70] |
Dr. Wollaston. |
|
|
| Names of the Springs. |
Grains
of
water. |
Silica. |
Alu-
mina. |
Resins. |
Tem-
pera-
ture. |
| |
|
grs. |
grs. |
grs. |
|
| |
Kilburn (1)—acidulous. |
138240 |
|
|
6 |
·0 |
cold |
Sul-
phu-
rous. |
|
- |
Harrowgate (2) |
103643 |
|
|
|
cold |
| Moffat (2) |
103643 |
|
|
|
cold |
| Aix-la-Chapelle (3) |
8940 |
|
|
|
143° |
| Enghein (4) |
92160 |
|
|
|
cold |
| |
|
|
|
|
|
Sa-
line. |
|
- |
Seidlitz |
58309 |
|
|
|
cold |
| Cheltenham (5) |
103643 |
|
|
|
cold |
| Plombieres (6) |
14600 |
|
|
|
cold |
| Dunblane (7) sp. gr. 1·00475 |
7291 |
|
|
|
cold |
| Pitcaithley (7) |
7291 |
|
|
|
cold |
| |
|
|
|
|
|
Cha-
ly-
be-
ate. |
|
- |
Tunbridge (3) |
103643 |
|
|
|
cold |
| Brighton (8) |
58309 |
1 |
·12 |
|
|
cold |
| Toplitz (9) |
22540 |
|
15 |
·1 |
|
cold |
| |
|
|
|
|
|
Cal-
car-
eous,
nearly
pure. |
|
- |
Bath (10) |
15360 |
0 |
·4 |
|
|
114° |
| Buxton (11) |
58309 |
|
|
|
82° |
| Bristol (12) |
58309 |
|
|
|
74° |
| Matlock |
58309 |
|
|
|
66° |
| Malvern (13) |
58309 |
|
|
|
cold |
| |
Dead Sea |
(14) sp. gr. |
1 |
·211 |
100 |
|
|
|
|
| Do. |
(15) sp. gr. |
1 |
·245 |
|
|
|
|
|
| Do. |
(16) sp. gr. |
1 |
·2283 |
|
|
|
|
|
| Sea water, Forth (7) |
7291 |
|
|
|
|
| (1) |
Schmesser. |
| (2) |
Garnet. |
| (3) |
Babington. |
| (4) |
Fourcroy. |
| (5) |
Fothergill. |
| (6) |
Vauquelin. |
| (7) |
Dr. Murray. |
| (8) |
Marcet. |
| (9) |
John. |
| (10) |
Phillips. |
| (11) |
Pearson. |
| (12) |
Carrick. |
| (13) |
Dr. Philip. |
| (14) |
Dr. Marcet. |
| (15) |
Klaproth. |
| (16) |
M. Gay Lussac. |
|
|
[1283]
Mineral waters may, in most cases, be artificially prepared, by the skilful application
of the knowledge derived from analysis, with such precision as to imitate very closely
the native springs. When the various earthy or metallic constituents, are held in
solution by carbonic acid, or sulphuretted, they should be placed along with their due
proportions of water, in the receiver of the aerating machine (see Soda Water), and
then the proper quantity of gas should be injected into the water. Sufficient agitation
will be given by the action of the forcing-pump to promote their solution.
WAX (Cire, Fr.; Wachs, Germ.); is the substance which forms the cells of bees.
It was long supposed to be derived from the pollen of plants, swallowed by these
insects, and merely voided under this new form; but it has been proved by the experiments,
first of Mr. Hunter, and more especially of M. Huber, to be the peculiar
secretion of a certain organ, which forms a part of the small sacs, situated on the sides
of the median line of the abdomen of the bee. On raising the lower segments of the
abdomen, these sacs may be observed, as also scales or spangles of wax, arranged in
pairs upon each segment. There are none, however, under the rings of the males and
the queen. Each individual has only eight wax sacs, or pouches; for the first and the
last ring are not provided with them. M. Huber satisfied himself by precise experiments
that bees, though fed with honey, or sugar alone, produced nevertheless a very
considerable quantity of wax; thus proving that they were not mere collectors of this
substance from the vegetable kingdom. The pollen of plants serves for the nourishment
of the larvæ.
But wax exists also as a vegetable product, and may, in this point of view, be
regarded as a concrete fixed oil. It forms a part of the green fecula of many plants,
particularly of the cabbage; it may be extracted from the pollen of most flowers; as
also from the skins of plums, and many stone fruits. It constitutes a varnish upon the
upper surface of the leaves of many trees, and it has been observed in the juice of the
cow-tree. The berries of the Myrica angustifolia, latifolia, as well as the cerifera, afford
abundance of wax.
Bees’ wax, as obtained by washing and melting the comb, is yellow. It has a peculiar
smell, resembling honey, and derived from it, for the cells in which no honey has been deposited,
yield a scentless white wax. Wax is freed from its impurities, and bleached, by
melting it with hot water or steam, in a tinned copper or wooden vessel, letting it
settle, running off the clear supernatant oily-looking liquid into an oblong trough with
a line of holes in its bottom, so as to distribute it upon horizontal wooden cylinders,
made to revolve half immersed in cold water, and then exposing the thin ribbons or
films thus obtained to the blanching action of air, light, and moisture. For this purpose,
the ribbons are laid upon long webs of canvas stretched horizontally between
standards, two feet above the surface of a sheltered field, having a free exposure to the
sunbeams. Here they are frequently turned over, then covered by nets to prevent their
being blown away by winds, and watered from time to time, like linen upon the grass
field in the old method of bleaching. Whenever the colour of the wax seems stationary,
it is collected, remelted, and thrown again into ribbons upon the wet cylinder, in
order to expose new surfaces to the blanching operation. By several repetitions of
these processes, if the weather proves favourable, the wax eventually loses its yellow
tint entirely, and becomes fit for forming white candles. If it be finished under rain,
it will become gray on keeping, and also lose in weight.
In France, where the purification of wax is a considerable object of manufacture, about
four ounces of cream of tartar, or alum, are added to the water in the first melting-copper,
and the solution is incorporated with the wax by diligent manipulation. The
whole is left at rest for some time, and then the supernatant wax is run off into a
settling cistern, whence it is discharged by a stopcock or tap, over the wooden cylinder
revolving at the surface of a large water-cistern, kept cool by passing a stream continually
through it.
The bleached wax is finally melted, strained through silk sieves, and then run into
circular cavities in a moistened table, to be cast or moulded into thin disc pieces, weighing
from two to three ounces each, and three or four inches in diameter.
Neither chlorine, nor even the chlorides of lime and alkalis, can be employed with any
advantage to bleach wax, because they render it brittle, and impair its burning quality.
Wax purified, as above, is white and translucent in thin segments; it has neither
taste nor smell; it has a specific gravity of from 0·960 to 0·966; it does not liquefy
till it be heated to 1541⁄2° F.; but it softens at 86°, becoming so plastic, that it may be
moulded by the hand into any form. At 32° it is hard and brittle.
It is not a simple substance, but consists of two species of wax, which may be easily
separated by boiling alcohol. The resulting solution deposits, on cooling, the waxy
body called cerine. The undissolved wax, being once and again treated with boiling
alcohol, finally affords from 70 to 90 per cent. of its weight of cerine. The insoluble
residuum is the myricine of Dr. John, so called because it exists in a much larger proportion[1284]
in the wax of the Myrica cerifera. It is greatly denser than wax, being of the
same specific gravity as water; and may be distilled without decomposition, which
cerine undergoes. See these two articles.
Wax is adulterated sometimes with starch; a fraud easily detected by oil of turpentine,
which dissolves the former, and leaves the latter substance; and more frequently
with mutton suet. This fraud may be discovered by dry distillation; for wax does not
thereby afford, like tallow, sebacic acid (benzoic), which is known by its occasioning a
precipitate in a solution of acetate of lead. It is said that two per cent. of a tallow
sophistication may be discovered in this way.
Bees’ wax imported for home consumption:—in 1835, unbleached, 4,449 cwts.;
bleached, 243 cwts.;—in 1836, unbleached, 4,673 cwts.; bleached, 121 cwts. Duty,
when from British possessions, 10s.; from foreign, 30s.
WAX, MINERAL, or Ozocerite, is a solid, of a brown colour, of various shades,
translucent, and fusible like bees’ wax; slightly bituminous to the smell, of a foliated
texture, a conchoidal fracture, but wanting tenacity, so that it can be pulverized in
a mortar. Its specific gravity varies from 0·900 to 0·953. Candles have been made
of it in Moldavia, which give a tolerable light. It occurs at the foot of the Carpathians
near Slanik, beneath a bed of bituminous slate-clay, in masses of from 80 to
100 pounds weight. Layers of brown amber are found in the neighbourhood. It
is associated with variegated sandstone, rock salt, and beds of coal (lignite?). It is
analogous to hatchetine. Something similar has been discovered in a trouble at Urpeth
colliery, near Newcastle, 60 fathoms beneath the surface. Ozocerite consists of different
hydro-carburetted compounds associated together; the whole being composed, ultimately,
of—hydrogen 14, carbon 86, very nearly.
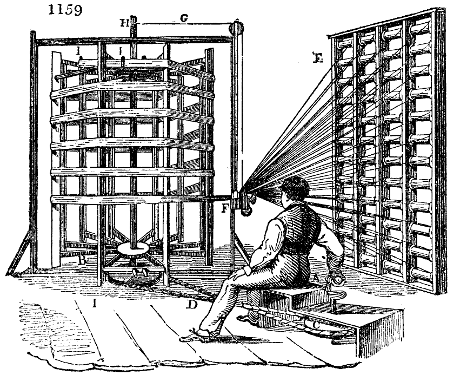
WEAVING (Tissage, Fr.; Weberei, Germ.); is performed by the implement
called loom in English, métier à tisser in French, and weberstuhl in German. The process
of warping must always precede weaving. Its object is to arrange all the longitudinal
threads, which are to form the chain of the web, alongside of each other in one
parallel plane. Such a number of bobbins, filled with yarn, must therefore be taken as
will furnish the quantity required for the length of the intended piece of cloth. One-sixth
of that number of bobbins is usually mounted at once in the warp mill, being set
loosely in a horizontal direction upon wire skewers, or spindles, in a square frame, so
that they may revolve, and give off the yarn freely. The warper sits at A, fig. 1159.,
and causes the reel B to revolve, by turning round with his hand the wheel C, with the
endless rope or band D.
The bobbins filled with
yarn are placed in the
frame E. There is a sliding
piece at F, called the heck
box, which rises and falls
by the coiling and uncoiling
of the cord G, round
the central shaft of the reef
H. By this simple contrivance,
the band of warp-yarns
is wound spirally,
from top to bottom, upon
the reel. I, I, I, are wooden
pins which separate the
different bands. Most
warping mills are of a
prismatic form; having
twelve, eighteen, or more
sides. The reel is commonly
about six feet in
diameter, and seven feet
in height, so as to serve for measuring exactly upon its periphery the total length of
the warp. All the threads from the frame E, pass through the heck F, which consists
of a series of finely-polished hard-tempered steel pins, with a small hole at the upper
part of each, to receive and guide one thread. The heck is divided into two parts,
either of which may be lifted by a small handle below, while their eyes are placed
alternately. Hence, when one of them is raised a little, a vacuity is formed between
the two bands of the warp; but when the other is raised, the vacuity is reversed. In
this way, the lease is produced at each end of the warp, and it is preserved by appropriate
wooden pegs. The lease being carefully tied up, affords a guide to the weaver
for inserting his lease-rods. The warping mill is turned alternately from right to left,
and from left to right, till a sufficient number of yarns are coiled round it to form the[1285]
breadth that is wanted; the warper’s principal care being to tie immediately every
thread as it breaks, otherwise deficiencies would be occasioned in the chain, injurious to
the appearance of the web, or productive of much annoyance to the weaver.
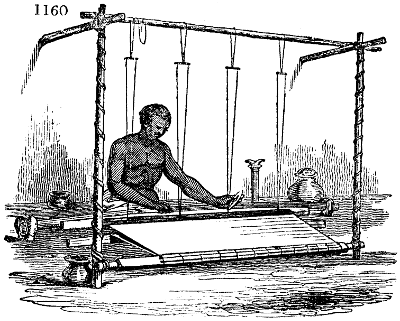
The simplest and probably the most antient of looms, now to be seen in action, is
that of the Hindu tanty, shown in fig. 1160. It consists of two bamboo rollers; one for
the warp, and another for the
woven cloth; with a pair of
heddles, for parting the warp,
to permit the weft to be drawn
across between its upper and
under threads. The shuttle is
a slender rod, like a large netting
needle, rather longer than
the web is broad, and is made
use of as a batten or lay, to
strike home or condense each
successive thread of weft, against
the closed fabric. The Hindu
carries this simple implement,
with his water pitcher, rice pot,
and hooka, to the foot of any
tree which can afford him a
comfortable shade; he there
digs a large hole, to receive his
legs, along with the treddles or lower part of the harness; he next extends his warp,
by fastening his two bamboo rollers, at a proper distance from each other, with pins,
into the sward; he attaches the heddles to a convenient branch of the tree overhead;
inserts his great toes into two loops under the geer, to serve him for treddles; lastly, he
sheds the warp, draws through the weft, and beats it close up to the web with his rod-shuttle
or batten.
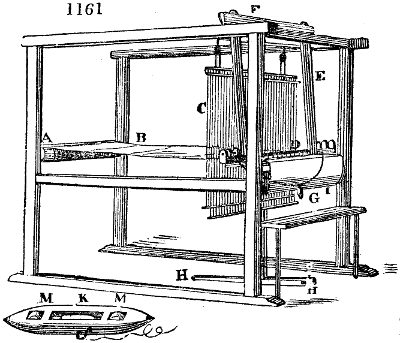
The European loom is represented in its plainest state, as it has existed for several
centuries, in fig. 1161. A is the warp-beam, round which the chain, has been wound; B
represents the flat rods, usually three in number, which pass across between its threads,
to preserve the lease, or the plane of decussation for the weft; C shows the heddles or
healds, consisting of twines looped in the middle, through which loops, the warp yarns
are drawn, one half through the front heddle, and the other through the back one; by
moving which, the decussation is readily
effected. The yarns then pass through
the dents of the REED under D, which
is set in a movable swing-frame E,
called the lathe, lay, and also batten,
because it beats home the weft to the
web. The lay is freely suspended to a
cross-bar F, attached by rulers, called
the swords, to the top of the lateral
standards of the loom, so as to oscillate
upon it. The weaver, sitting on the
bench G, presses down one of the treddles
at H, with one of his feet, whereby
he raises the corresponding heddle,
but sinks the alternate one; thus sheds
the warp, by lifting and depressing each
alternate thread, through a little space,
and opens a pathway or race-course
for the shuttle to traverse the middle of the warp, upon its two friction rollers M, M. For
this purpose, he lays hold of the picking-peg in his right hand, and, with a smart jerk of
his wrist, drives the fly-shuttle swiftly from one side of the loom to the other, between
the shed warp yarns. The shoot of weft being thereby left behind from the shuttle pirn
or cop, the weaver brings home, by pulling, the lay with its reed towards him by his left
hand, with such force as the closeness of the texture requires. The web, as thus
woven, is wound up by turning round the cloth beam I, furnished with a ratchet-wheel,
which takes into a holding tooth. The plan of throwing the shuttle by the picking-peg
and cord, is a great improvement upon the old way of throwing it by hand. It was contrived
exactly a century ago, by John Kay, of Bury in Lancashire, but then resident in
Colchester, and was called the fly-shuttle, from its speed, as it enabled the weaver to
make double the quantity of narrow cloth, and much more broad cloth, in the same time.
The cloth is kept distended, during the operation of weaving, by means of two pieces[1286]
of hard wood, called a templet, furnished with sharp iron points in their ends, which
take hold of the opposite selvages or lists of the web. The warp and web are kept
longitudinally stretched by a weighted cord, which passes round the warp-beam, and
which tends continually to draw back the cloth from its beam, where it is held fast by
the ratchet tooth. See Fustian, Jacquard Loom, Reed, and Textile Fabrics.
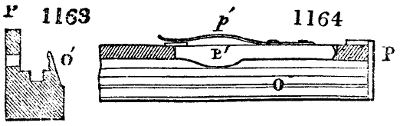
The greater part of plain weaving, and much even of the figured, is now performed by
the power loom, called métier mécanique à tisser, in French. Fig. 1162. represents the
cast-iron power loom of Sharp and Roberts. A, A′, are the two side uprights, or
standards, on the front of the loom. D, is the great arch of cast iron, which binds the
two sides together. E, is the front cross-beam, terminating in the forks e, e; whose[1287]
ends are bolted to the opposite standards A, A′, so as to bind the framework most firmly
together. G′, is the breast beam, of wood, nearly square; its upper surface is sloped a
little towards the front, and its edge rounded off, for the web to slide smoothly over it, in
its progress to the cloth beam. The beam is supported at its end upon brackets, and is
secured by the bolts g′, g′. H, is the cloth beam, a wooden cylinder, mounted with
iron gudgeons at its ends, that on the right hand being prolonged to carry the toothed
winding wheel H′. k′ is a pinion in geer with H′. H′′, is a ratchet wheel, mounted
upon the same shaft h′′′, as the pinion h′. h′, is the click of the ratchet wheel H′′. k′′′,
is a long bolt fixed to the frame, serving as a shaft to the ratchet wheel H′′, and the
pinion h′. I, is the front heddle-leaf, and I′, the back one. J, J, J′, J′, jacks or pulleys
and straps, for raising and depressing the leaves of the heddles. J′′, is the iron shaft
which carries the jacks or system of pulleys J, J, J′, J′. K, a strong wooden ruler, connecting
the front heddle with its treddle. L, L′, the front and rear marches or treddle-pieces,
for depressing the heddle leaves alternately, by the intervention of the rods k,
(and k′, hid behind k). M, M, are the two swords (swing bars) of the lay or batten. N,
is the upper cross-bar of the lay, made of wood, and supported upon the squares of the
levers n, n′, to which it is firmly bolted. N′, is the lay-cap, which is placed higher or
lower, according to the breadth of the reed; it is the part of the lay which the hand-loom
weaver seizes with his hand, in order to swing it towards him. n′, is the reed
contained between the bar N, and the lay-cap N′. O, O, are two rods of iron, perfectly
round and straight, mounted near the ends of the batten-bar N, which serve as guides to
the drivers or peckers o, o, which impel the shuttle. These are made of buffalo hide,
and should slide freely on their guide-rods. O′, O′, are the fronts of the shuttle-boxes;
they have a slight inclination backwards. P, is the back of them. See figs. 1163. and 1164.
O′′, O′′ are iron plates, forming the bottoms of the shuttle-boxes. p, small pegs or pins,
planted in the posterior faces P (fig. 1164.) of the boxes, round which the levers P′ turn.
These levers are sunk in the substance of the faces P, turn round pegs p, being pressed
from without inwards, by the springs p′. P′′, fig. 1162. (to the right of K,) is the whip
or lever, (and Q′′, its centre of motion, corresponding to the right arm and elbow of the
weaver,) which serves to throw the shuttle, by means of the pecking-cord p′′, attached at
its other end to the drivers o, o.
On the axis of Q′′, a kind of eccentric or heart wheel is mounted, to whose concave
part, the middle of the double band or strap r, being attached, receives impulsion; its
two ends are attached to the heads of the bolts r′, which carry the stirrups r′′, that may
be adjusted at any suitable height, by set screws.
S (see the left-hand side of fig. 1162.), is the moving shaft, of wrought iron, resting on
the two ends of the frame, S′ (see the right-hand side), is a toothed wheel, mounted exteriorly
to the frame, upon the end of the shaft S. S′′ (near S′), are two equal elbows, in the
same direction, and in the same plane, as the shaft S, opposite to the swords M, M, of the lay.
Z, is the loose, and Z′, the fast pulley, or riggers, which receive motion from the steam-shaft
of the factory, Z′′, a small fly-wheel, to regulate the movements of the main shaft
of the loom.
T, is the shaft of the eccentric tappets, cams, or wipers, which press the treddle
levers alternately up and down; on its right end is mounted T′, a toothed wheel in geer
with the wheel S′, of one half its diameter. T′′, is a cleft clamping collar, which serves to
support the shaft T.
U, is a lever, which turns round the bolt u, as well as the click h′′. U′, is the click of
traction, for turning round the cloth beam, jointed to the upper extremity of the lever
U; its tooth u′, catches in the teeth of the ratchet wheel H′′. u′′, is a long slender rod,
fixed to one of the swords of the lay M, serving to push the lower end of the lever U, when
the lay retires towards the heddle leaves.
X, is a wrought-iron shaft, extending from the one shuttle-box to the other, supported
at its ends by the bearings x, x.
Y, is a bearing, affixed exteriorly to the frame, against which the spring bar Z, rests,
near its top, but is fixed to the frame at its bottom. The spring falls into a notch in the
bar Y, and is thereby held at a distance from the upright A, as long as the band is upon the
loose pulley z′; but when the spring bar is disengaged, it falls towards A, and carries the
band upon the fast pulley z, so as to put the loom in geer with the steam-shaft of the factory.
Weaving, by this powerful machine, consists of four operations: 1. to shed the warp
by means of the heddle leaves, actuated by the tappet wheels upon the axis Q′, the rods
k, k′, the cross-bar E, and the eyes of the heddle leaves I, I′; 2. to throw the shuttle (see
fig. 1161.), by means of the whip lever P′′, the driver cord p, and the pecker o; 3. to drive
home the weft by the batten N, N′; 4. to unwind the chain from the warp beam, and to
draw it progressively forwards, and wind the finished web upon the cloth beam H, by
the click and toothed wheel mechanism at the right-hand side of the frame. For more
minute details, the reader may consult The Cotton Manufacture of Great Britain, vol. ii.
p. 291.
[1288]
WEFT (Trame, Fr.; Eintrag, Germ.); is the name of the yarns or threads which
run from selvage to selvage in a web.
WELD (Vouëde, Fr.; Wau, Gelbkraut, Germ.); is an annual herbaceous plant,
which grows all over Europe, called by botanists Reseda luteola. The stems and the
leaves dye yellow; and among the dyes of organic nature, they rank next to the Persian
berry for the beauty and fastness of colour. The whole plant is cropped when in seed,
at which period its dyeing power is greatest; and after being simply dried, is brought
into the market.
Chevreul has discovered a yellow colouring principle in weld, which he has called
luteoline. It may be sublimed, and thus obtained in long needle-form, transparent
yellow crystals. Luteoline is but sparingly soluble in water; but it nevertheless dyes
alumed silk and wool of a fine jonquil colour. It is soluble in alcohol and ether; it
combines with acids, and especially with bases.
When weld is to be employed in the dye-bath, it should be boiled for three quarters
of an hour; after which the exhausted plant is taken out, because it occupies too much
room. The decoction is rapidly decomposed in the air, and ought therefore to be made
only when it is wanted. It produces with,
| Solution of isinglass |
a slight turbidity. |
| Litmus paper |
a faint reddening. |
| Potash lye |
a golden yellow tint. |
| Solution of alum |
a faint yellow. |
| Protoxide salts of tin |
a rich yellow |
|
- |
precipitation. |
| Acetate of lead |
ditto |
| Salts of copper |
a dirty yellow-brown |
| Sulphate of red oxide of iron |
a brown, passing into olive. |
A lack is made from decoction of weld with alum, precipitated by carbonate of soda
or potassa. See Yellow Dye.
WELDING (Souder, Fr.; Schweissen, Germ.); is the property which pieces of
wrought iron possess, when heated to whiteness, of uniting intimately and permanently
under the hammer, into one body, without any appearance of junction. The welding
temperature is usually estimated at from 60° to 90° of Wedgewood. When a skilful
blacksmith is about to perform the welding operation, he watches minutely the effect of
the heat in his forge-fire upon the two iron bars; and if he perceives them beginning
to burn, he pulls them out, rolls them in sand, which forms a glassy silicate of iron
upon the surface, so as to prevent further oxidizement; and then laying the one properly
upon the other, he incorporates them by his right-hand hammer, being assisted
by another workman, who strikes the metal at the same time with a heavy forge-hammer.
Platinum is not susceptible of being welded, as many chemical authors have erroneously
asserted.
Mr. T. H. Russell, of Handsworth, near Birmingham, obtained a patent, in May,
1836, for manufacturing welded iron tubes, by drawing or passing the skelp, or fillet of
sheet iron, five feet long, between dies or holes, formed by a pair of grooved rollers,
placed with their sides contiguous; for which process, he does not previously turn up
the skelp from end to end, but he does this so as to bring the edges together at the
time when the welding is performed. He draws the skelp through two or more pairs
of the above pincers or dies, each of less dimension than the preceding. In making
tubes of an inch of internal diameter, a skelp four inches and a half broad is employed.
The twin rollers revolve on vertical axes, which may be made to approach each other
to give pressure; and they are kept cool by a stream of water, while the skelp, ignited
to the welding heat, is passed between them. They are affixed at about a foot in front
of the mouth of the furnace, on a draw-bench; there being a suitable stop within a few
inches of the rollers, against which the workman may place a pair of pincers, having a
bell-mouthed hole or die, for welding and shaping the tube. In the first passage
between the rollers, a circular revolving plate of iron is let down vertically between
them, to prevent the edges of the skelp from overlapping, or even meeting. The welding
is performed at the last passage.
WELLS, ARTESIAN. See also Artesian Wells. The following account of
a successful operation of this kind, lately performed at Mortlake, in Surrey, deserves to
be recorded. The spot at which this undertaking was begun, is within 100 feet of the
Thames. In the first instance, an auger, seven inches in diameter, was used in penetrating
20 feet of superficial detritus, and 200 feet of London clay. An iron tube,
8 inches in diameter, was then driven into the opening, to dam out the land-springs and
the percolation from the river. A 4-inch auger was next introduced through the iron
tube, and the boring was continued until, the London clay having been perforated to
the depth of 240 feet, the sands of the plastic clay were reached, and water of the softest
and purest nature was obtained; but the supply was not sufficient, and it did not reach[1289]
the surface. The work was proceeded with accordingly; and after 55 feet of alternating
beds of sand and clay had been penetrated, the chalk was touched upon. A second
tube, 41⁄2 inches in diameter, was then driven into the chalk, to stop out the water of
the plastic sands; and through this tube an auger, 31⁄2 inches in diameter, was introduced,
and worked down through 35 feet of hard chalk, abounding with flints. To
this succeeded a bed of soft chalk, into which the instrument suddenly penetrated to
the depth of 15 feet. On the auger being withdrawn, water gradually rose to the surface,
and overflowed. The expense of the work did not exceed 300l. The general
summary of the strata penetrated is as follows:—Gravel, 20 feet; London clay, 250;
plastic sands and clays, 55; hard chalk with flints, 35; soft chalk, 15; = 375 feet.
WHALEBONE (Baleine, Fr.; Fischbeine, Germ.); is the name of the horny
laminæ, consisting of fibres laid lengthwise, found in the mouth of the whale, which, by
the fringes upon their edges, enable the animal to allow the water to flow out, as through
rows of teeth (which it wants), from between its capacious jaws, but to catch and
detain the minute creatures upon which it feeds. The fibres of whalebone have little
lateral cohesion, as they are not transversely decussated, and may, therefore, be readily
detached in the form of long filaments or bristles. The blades, or scythe-shaped plates,
are externally compact, smooth, and susceptible of a good polish. They are connected,
in a parallel series, by what is called the gum of the animal, and are arranged along each
side of its mouth, to the number of about 300. The length of the longest blade, which
is usually found near the middle of the series, is the gauge adopted by the fishermen to
designate the size of the fish. The greatest length hitherto known has been 15 feet,
but it rarely exceeds 12 or 13. The breadth, at the root end, is from 10 to 12 inches;
and the average thickness, from four to five tenths of an inch. The series, viewed
altogether in the mouth of the whale, resemble, in general form, the roof of a house.
They are cleansed and softened before cutting, by boiling for two hours in a long
copper.
Whalebone, as brought from Greenland, is commonly divided into portable junks or
pieces, comprising ten or twelve blades in each; but it is occasionally subdivided into
separate blades, the gum and the hairy fringes having been removed by the sailors
during the voyage. The price of whalebone fluctuates from 50l. to 150l. per ton.
The blade is cut into parallel prismatic slips, as follows:—It is clamped horizontally,
with its edge up and down, in the large wooden vice of a carpenter’s bench, and is then
planed by the following tool: fig. 1165. A, B, are its two handles; C, D, is an iron
plate, with a guide-notch E; F, is a semicircular knife, screwed firmly at each end to
the ends of the iron plate C, D, having its cutting edge
adjusted in a plane, so much lower than the bottom of the
notch E, as the thickness of the whalebone slip is intended
to be; for different thicknesses, the knife may be set by
the screws at different levels, but always in a plane parallel
to the lower guide surface of the plate C, D. The workman,
taking hold of the handles A, B, applies the notch
of the tool at the end of the whalebone blade furthest from
him, and with his two hands pulls it steadily along, so as to shave off a slice in the direction
of the fibres; being careful to cut none of them across. These prismatic slips
are then dried, and planed level upon their other two surfaces. The fibrous matter
detached in this operation, is used, instead of hair, for stuffing mattresses.
From its flexibility, strength, elasticity, and lightness, whalebone is employed for
many purposes: for ribs to umbrellas or parasols; for stiffening stays; for the framework
of hats, &c. When heated by steam, or a sand-bath, it softens, and may be bent
or moulded, like horn, into various shapes, which it retains, if cooled under compression.
In this way, snuff-boxes, and knobs of walking-sticks, may be made from the
thicker parts of the blade. The surface is polished at first with ground pumice-stone,
felt, and water; and finished with dry quicklime, spontaneously slaked, and sifted.
WHEAT. (Triticum vulgare, Linn.; Froment, Fr.; Waizen, Germ.) See Bread,
Gluten, and Starch.
WHEEL CARRIAGES. Though this manufacture belongs most properly to
a treatise upon mechanical engineering, I shall endeavour to describe the parts of a
carriage, so as to enable gentlemen to judge of its make and relative merits. The
external form may vary with every freak of fashion; but the general structure of a
vehicle, as to lightness, elegance, and strength, may be judged of from the following
figure and description.
Fig. 1166. shows the body of a chariot, hung upon an iron carriage, with iron wheels,
axletrees, and boxes; the latter, by a simple contrivance, is close at the out-head, by
which means the oil cannot escape; and the fastening of the wheel being at the in-head,
as will be explained afterwards, gives great security, and prevents the possibility of the
wheel being taken off by any other carriage running against it.
[1290]
Fig. 1167. shows the arm of an axletree, turned perfectly true, with two collars in
the solid, as seen at G and H. The parts from G to B are made cylindrical. At K is a
screw nail, the purpose of which will be explained in fig. 1171.
Fig. 1168. is the longitudinal section of a metal nave, which also forms the bush, for
the better fitting of which to the axletree, it is bored out of the solid, and made quite
air-tight upon the pin; and for retaining the oil, it is left close at the out-head D.
Fig. 1169. represents a collet, made of metal, turned perfectly true, the least diameter
of which is made the same with that part of the axletree M, fig. 1167., and its greatest
diameter the same with that of the solid collar G, fig. 1167. This collet is made with a
joint at S, and opens at p. Two grooves are represented at qq, qq, which are seen
at the same letters in fig. 1170., as also the dovetail r, in both figures.
Fig. 1170. is an edge view of the collet, fig. 1169.
Fig. 1171. is a longitudinal section of an axletree arm, nave or bush, and fastening.
A, B, is the arm of the axletree, bored up the centre from B to E. C, C, D, the nave, which
answers also for the bush. P, S, the collet (see figs. 1169. and 1170.), put into its place.
q, q, two steel pins, passing through the in-head of the bush, and filling up the grooves
in the collet. W, W, a caped hoop, sufficiently broad to cover the ends of said pins, and
made fast to the bush by screws. This hoop, when so fastened to the bush, prevents
the possibility of the pins q, q, from getting out of their places. u, u, is a leather
washer, interposed betwixt the in-head of the bush and the larger solid collar of the
axletree, to prevent the escape of oil at the in-head. K, is a screw, the head of which
is near the letter K, in fig. 1167. This screw being undone, and oil poured into the
hole, it flows down the bore in the centre of the axletree arm, and fills the space B, left
by the arm being about one inch shorter than the bore of the bush, and the screw, being
afterwards replaced, keeps all tight. In putting on the wheel, a little oil ought to be[1291]
put into the space betwixt the collet P, S, and the larger collar. The collar P, S, being
movable round the axletree arm, and being made fast to the bush by means of the two
pins q, q, revolves along with the bush, acting against the solid collar G, of the arm, and
keeps the wheel fast to the axletree, until by removing the caped hoop W, W, and driving
out the pins q, q, the collet becomes disengaged from the bush.
The dovetail, seen upon the collet at r, fig. 1170., has a corresponding groove cut in
the bush, to receive it, in consequence of which the wheel must of necessity be put on
so that the collet and pins fit exactly. These wheels very rarely require to be taken off,
and they will run a thousand miles without requiring fresh oiling.
The spokes of the wheel, made of malleable iron, are screwed into the bush or nave at
C, C, figs. 1168. 1171., all round. The felloes, composed merely of two bars of iron, bent
into a circle edgeways, are put on, the one on the front, the other on the back, of the
spokes, which have shoulders on both sides to support the felloes, and all three are
attached together by rivets through them. The space between the two iron rings
forming the felloes, should be filled up with light wood, the tire then put on, and
fastened to the felloes by bolts and glands clasping both felloes.
This is a carriage without a mortise or tenon, or wooden joint of any kind. It is, at
an average, one-seventh lighter than any of those built on the ordinary construction.
The design of Mr. W. Mason’s patent invention, of 1827, is to give any required
pressure to the ends of what are called mail axletrees, in order to prevent their shaking
in the boxes of the wheels. This object is effected by the introduction of leather
collars in certain parts of the box, and by a contrivance, in which the outer cap is
screwed up, so as to bear against the end of the axletree with any degree of tightness,
and is held in that situation, without the possibility of turning round, or allowing the
axletree to become loose.
Fig. 1172. shows the section of the box of a wheel, with the end of the axletree
secured in it. The general form of the box, and of the axle, is the same as other mail
axles, there being
recesses in the box
for the reception of
oil. At the end of
the axle, a cap a, is
inserted, with a leather
collar enclosed
in it, bearing against
the end of the axle;
which cap, when
screwed up sufficiently
tight, is held in that situation by a pin or screw passed through the cap a, into the
end of the iron box; a representation of this end of the iron box being shown at fig. 1173.
In the cap a, there is also a groove for conducting the oil to the interior of the box,
with a screw at the opening, to prevent it running out as the wheel goes round.
The particular claims of improvement are, the leather collar against the end of the
axle; the pin going through one of the holes in the end of the box, to fix it; and the
channel for conducting the oil.
Mr. Mason’s patent, of August, 1830, applies also to the boxes and axles of that
construction of carriage wheels which are fitted with the so called mail-boxes; but
part of the invention applies to other axles.
Fig. 1174. represents the nave of a wheel, with the box for the axle within it, both
shown in section longitudinally; fig. 1175. is a section of the axle, taken in the same
direction; and fig. 1176. represents the screw cap and oil-box, which attaches to the
outer extremity of the axle-box. Supposing the parts were put together, that is, the
axle inserted into the box, then the intention of the different parts will be perceived.
The cylindrical recess a, in the box of the nave, is designed to fit the cylindrical part
of the axle b; and the conical part c, of the axle, to shoulder up against a corresponding
conical cavity in the box, with a washer of leather to prevent its shaking. A collar d,
formed by a metallic ring, fits loosely upon a cylindrical part of the axle, and is kept
there by a flange or rim, fixed behind the cone c. Several strong pins f, f, are cast
into the back part of the box; which pins, when the wheel is attached, pass through
corresponding holes in the collar d; and nuts being screwed on to the ends of the pins
f, behind the collar, keep the wheel securely attached to the axle. The screw-cap g, is
then inserted into the recess h, at the outer part of the box, its conical end and small
tube i, passing into the recess k, in the end of the axle.
The parts being thus connected, the oil contained within the cap g, will flow through
the small tube i, in its end, into the recess or cylindrical channel l, within the axle, and will
thence pass through a small hole in the side of the axle, into the cylindrical recess a, of
the box; and then lodging in the groove and other cavities within the box, will lubricate[1292]
the axle as the wheel goes round. There is also a small groove cut on the outside
of the axle, for conducting the oil, in order that it may be more equally distributed
over the surface and the bearings.
This construction of the box and
axle, as far as the lubrication goes, may
be applied to the axles of wheels in
general; but that part of the invention
which is designed to give greater security
in the attachment of the wheel to
the carriage, applies particularly to mail
axles.
Mr. William Mason’s patent invention
for wheel carriages, of August,
1831, will be understood by reference
to the annexed figures. Fig. 1177. is a
plan showing the fore-axletree bed a, a,
of a four-wheeled carriage, to which
the axletrees b, b, are jointed at each
end; fig. 1178. is an enlarged plan; and
fig. 1179. an elevation, or side view of one
end of the said fore-axletree bed, having
a Collinge’s axletree jointed to the axletree bed, by means of the cylindrical pin or bolt c,
which passes through and turns in a cylindrical hole d, formed at the end of the axletree
bed, shown also in the plan view, fig. 1180., and section, fig. 1181.
The axletree b, is firmly united with the upper end e, of the pin or bolt c; and to the
lower end of it, which is squared, the guide piece f, is also fitted, and secured by the
screw g, and cap or nut h, seen in fig. 1179., and in section in fig. 1182. There are leather
washers i, i, let into recesses made to receive them in the parts a, b, and f, the intent of
which is to prevent the oil from escaping that is introduced through the central perpendicular
hole seen in fig. 1182., which hole is closed by means of a screw inserted into
it. The oil is diffused, or spread over the surface of the cylinder c, by means of a side
branch leading from the bottom of the hole into a groove formed around the cylinder,
and also by means of two longitudinal gaps or cavities made within the hole, as shown
in figs. 1180. and 1181. The guide piece f, is affixed at right angles with the axletree
b, as shown in fig. 1178., and turns freely and steadily in the cylindrical hole d,
made to receive one end of the iron fore-axletree bed a. In like manner, the opposite
fore axletree b, fig. 1177., is jointed to the other end of the iron fore-axletree bed.[1293]
The outer ends of the guide pieces f, f, are jointed to the splinter-bar n, fig. 1181, as
follows:—Fig. 1183. is a plan, and fig. 1184. a section of the joint o, in fig. 1177., shown
on an enlarged scale; a cylindrical pin or bolt c, is firmly secured in the splinter-bar,
and round the lower part of the said pin or bolt the guide piece f, turns, and is
made fast in its place by the screw g, and screwed nut h.
Oil is conveyed to the lower part of the cylindrical pin c, in a similar manner to that
already described, and two leather washers are likewise furnished, to prevent its escape.
The connecting joint at the opposite end of the splinter-bar n, is constructed in a
similar manner. The futchel or socket p, p, for the pole of the carriage, must also be
jointed to the middle of the fore-axletree bed and splinter-bar, in a similar manner. The
swingletrees q, q, fig. 1177., are likewise jointed in the same way to the splinter-bar.
Fig. 1185. is a side view of these parts. The fore wheels of the carriage, fig. 1177., are
furnished with cast-iron boxes, as usual. The dotted lines show the action of the pole
p, p, upon the splinter-bar n, and as communicated through the latter to the guide
pieces f, f, connected with the axletrees b, b, so as to lock the wheels r, r, as shown in
that figure.
The axletree may be incased in the woodwork of the fore-bed of the carriage, as
usual, and as shown by dotted lines in the back end view thereof, fig. 1186.; and the
framing s, fig. 1187., may be affixed firmly upon the said woodwork, in any fit and
proper manner, as well as the fore-springs t, t, shown in figs. 1186. and 1187., and likewise
in the side view, fig. 1188. In certain cases it may be desirable to fix the cylindrical
pin or bolt c, firmly in the splinter-bar n, in the manner shown in figs. 1189. and
1190.; the swingletrees q, q, and guide pieces f, f, turning freely above and below upon
the said pin or bolt, and secured in their places thereon by screws and screwed nuts,
oil being also supplied through holes formed in both ends of the said pin or bolt, and
leather washers provided, as in the above-described instances.
Mr. Gibbs, engineer, and Mr. Chaplin, coach-maker, obtained a patent, in 1832, for
the construction of a four-wheeled carriage which shall be enabled to turn within a
small compass, by throwing the axles of all the four wheels simultaneously into different
positions. They effect this object by mounting each wheel upon a separate jointed
axle, and by connecting the free
ends of the four axles by jointed
rods or chains, with the pole and
splinter-bar in front of the carriage.
To fix the ends of the spokes of
wheels to the felloe or rim, with
greater security than had been effected
by previous methods, is the
object of a contrivance for which
William Howard obtained a patent,
in February, 1830. Fig. 1191. shows
a portion of a wheel constructed on
this new method; a, is the nave,
of wood; b, b, b, wooden spokes,
inserted into the nave in the usual
way; c, c, is the rim or felloe, intended
to be formed by one entire circle of wrought iron; d, and e, e, are the shoes
or blocks, of cast iron, for receiving the ends of the spokes, which are secured by bolts
to the rim on the inner circumference. The cap of the block d, is removed, for the
purpose of showing the internal form of the block; e, e, have their caps fixed on, as
they would appear when the spokes are fitted in. One of the caps or shoes is shown
detached, upon a larger scale, at fig. 1192., by which it will be perceived that the end
of the spoke is introduced into the shoe on the side. It is proposed
that the end of the spoke shall not reach quite to the end of the
recess formed in the block, and that it shall be made tight by a
wedge driven in. The wedge piece is to be of wood, as fig. 1193.,
with a small slip of iron within it; and a hole is perforated in the
back of the block or shoe, for the wedge to be driven through. When this is done, the
ends of the spokes become confined and tight; and the projecting extremities of the
wedges being cut off, the caps are then attached on the face of the block, as at
e, e, by pins riveted at their ends, which secures the spokes, and renders it
impossible for them to be loosened by the vibrations as the wheel passes
over the ground. One important use of the wedges, is to correct the eccentric figure of
the wheel, which may be readily forced out in any part that may be out of the true
form, by driving the wedge up further; and this, it is considered, will be a very important
advantage, as the nearer a wheel can be brought to a true circle, the easier it
will run upon the road. The periphery of the wheel is to be protected by a tire,[1294]
which may be put on in pieces, and bolted through the felloe; or it may be made
in one ring, and attached, while hot, in the usual way.
Mr. Reedhead’s patent improvements in the construction of carriages, are represented
in the following figures. They were specified in July, 1833.
Fig. 1194. is a plan or horizontal view of the fore part of a carriage, intended to be
drawn by horses, showing the fore wheels in their position when running in a straight
course; fig. 1195. is a similar view, showing the wheels as locked, when in the act of
turning; fig. 1196. is a front end elevation of the same; fig. 1197. is a section taken
through the centre of the fore axletree; and fig. 1198. is a side elevation of the general
appearance of a stage-coach, with the improvements appended: a, a, are two splinter-bars,
with their roller-bolts, for connecting the traces of the harness; these splinter-bars
are attached, by the bent irons b, b, to two short axletrees or axle-boxes c, c, which carry
the axles of the fore wheels d, d, and turn upon vertical pins or bolts e, e, passed through
the fore axletree f, the splinter-bars and axle-boxes being mounted so as to move
parallel to each other, the latter partaking of any motion given to the splinter-bars by
the horses in drawing the carriage forward, and thereby producing the locking of the
wheels, as shown fig. 1195.; and in order that the two wheels, and their axles and
axle-boxes, together with the splinter-bars a, a, may move simultaneously, the latter are
connected by pivots to the end of the links or levers g, g, which are attached to the
arms i, i, which receive the pole of the coach by a hinge-joint or pin h; the arms i, i,
turning on a vertical fulcrum-pin k, passed through the main axletree, f, as the pole is
moved from one side to the other.
The axles o, o, are firmly fixed into the naves of the wheels, as represented in the side
view of a wheel detached, at fig. 1200., the axles being mounted so as to revolve within
their boxes in the following manner:—The axle-boxes, which answer the purpose of
short axletrees, are formed of iron, and consist of one main or bottom plate l, seen best
in figs. 1200. and 1199.; upon this bottom plate is formed the chamber m, m, carrying
the two anti-friction rollers n, n, which turn on short axles passed through the sides and
partition at the upper part of the chambers. These anti-friction rollers bear upon the
cylindrical parts of the axle o, of each wheel, and support the weight of the coach; p, is
a bearing firmly secured in the axle-box to the plate l, for the end of the axle o, to run
in, the axle being confined in its proper situation by a collar and screw-nut on its end;
e, is the vertical pin or bolt before mentioned, upon which the axle-bar turns when the[1295]
wheels are locking, which bolt is enlarged within the box, and has an eye for the axle
to pass through, being firmly secured to the plate l, and also to the sides of the box. Fig.
1200. is a plan or horizontal view of an axle and its box, belonging to one of the
fore wheels; a piece q, is fixed to the under side of the main axletree,
which supports the ends of the plates l, and thereby relieves
the pins e, e, of the strain they would otherwise have to withstand.
The axles of the hind wheels are mounted upon similar plates l, l,
with bearings and chambers with anti-friction rollers; but as
these are not required to lock, the plates l, l, are fixed on to the
under side of the hind axletree by screw-nuts; there are small
openings or doors, which can be removed for the purpose of unscrewing
the nuts and collars of the bearings p, when the wheel is
required to be taken off the carriage, when the axle can be
withdrawn from the boxes. If it should be thought necessary,
other chambers with friction rollers, may be placed on the under
side of the plate l, to bear up the end of the axles, and relieve
the bearing p. In order to stop or impede the progress
of a carriage in passing down hills, there is a grooved friction
or brake wheel t, fixed, by clamps or otherwise, on to the spokes of one of the
hind wheels; u, is a brake-band or spring, of metal, encircling the friction wheel, one
end of which band is fixed into the standard v, upon the hind axletree, and the other
end connected by a joint to the shorter end of the lever w, which has its fulcrum in the
standard v; this lever extends up to the hind seat of the coach, as shown in fig. 1198.,
and is intended to be under the command of the guard or passengers of the coach, and
when descending a hill, or on occasion of the horses running away, the longer end of
the lever is to be depressed, which will raise the shorter end, and, consequently, bring
the band or spring u, in contact with the surface of the friction wheel, and thereby
retard its revolution, and prevent the coach travelling too fast; or, instead of attaching
the friction brake to the hind wheel, as represented in fig. 1198., it may be adapted to
the fore wheels, and the end of the lever brought up to the side of the foot-board, or
under it, and within command of the coachman, the standard which carries the fulcrum
being made to move upon a pivot, to accommodate the locking of the wheels. It will
be observed, that by these improved constructions of the carriage, and mode of locking
the patentee is enabled to use much larger fore wheels than in common, and that the
splinter-bars will always be in the position of right angles with the track or way of the
horses in drawing the carriage, by which they are much relieved, and always pull in a
direct and equal manner.
A manifest defect in all four-wheeled carriages, involving vast superfluous friction,
is the small size of the front wheels; a defect which has existed ever since Walter
Rippon made “the first hollow turning coach with pillars and arches for her majesty
Queen Mary, being then her servant,” until the railroad era, when our
engineers remedied the defect by equalizing the wheels, at the expense of another
defect—sacrificing the power of turning, and thus producing great lateral friction;
whence a train of evil consequences result:—necessarily increased strength, and
consequently increased weight of the carriages; increased power and weight of the
engine to draw them, and overcome the friction; and, of course, increased strength of
rails, and greater solidity of railway.
These defects are at last remedied by an invention patented by Mr. William Adams,
author of a work entitled “English Pleasure Carriages.” Instead of placing the perch-bolt,
or turning centre, as is commonly done, over the front axle, he places it at a
convenient distance between the front and hind axles; so that when turning the carriage[1296]
the front wheels, instead of turning beneath the body, as is common, turn outside of it,
and the driver’s seat turns with them; thus giving him a perfect command over his
horses in all positions, instead of the usual dangerous plan, which renders a driver liable
to be pulled off his box by a restive horse, when in the act of turning. A carriage constructed
on Mr. Adams’ plan may also be driven round a corner at full speed, without
any risk of overturning, as the weight is equally poised on the axles in all positions. It
is well known that the oversetting of stage coaches usually takes place when turning a
corner, the momentum urging the vehicle in a right line, while the horses are
pulling at an angle. By the new arrangement the front wheels may be made equal
to the hind ones, or of any desirable height, and at the same time the body may be kept
as low as may be thought convenient, even almost close to the ground, if desired. Thus
two important objects, hitherto deemed incompatible, are combined—high wheels and a
low centre of gravity. These carriages are therefore essentially safety carriages, while the
friction is reduced to a minimum. The principle, in its various modifications, is applicable
to every variety of carriage, both those of the simply useful kind, and those where
beauty of form and colour are prime requisites.
Another most important part of Mr. Adams’ invention, is his new mode of spring
suspension; applying the principle of the bow and string, for the first time, to obviate
the effects of concussion in wheel carriages. All the springs hitherto in use for wheel
carriages, have been friction springs, composed of long sliding surfaces, uncertain in
their action, and liable to quick destruction by rust. But Mr. Adams’ springs are
essentially elastic, being formed of single plates abutting endways, so that all friction is
removed, and they can be hermetically sealed within paint to prevent their corrosion. He
has various modes of applying the bow, either single or double, above or below the axle;
but one most important feature is, that the axle being attached to the flexible cords or
braces, the concussion which affects the wheels, either laterally, vertically, or in the line
of progress, is perfectly intercepted, without the unpleasant oscillation experienced
in carriages where the same purpose is accomplished by the use of the curved or
C spring. Mr. Adams’ brace being, at the same time, a non-conductor of sound, the
rattling of the wheels does not annoy the rider as in ordinary carriages. His springs
are equally applicable to vehicles with two and four wheels.
The advantages of these carriages may be thus summed up:—A great diminution of
the total weight; a diminution of resistance in draught equal to about one third; increase
of safety to the riders; increased durability of the vehicle; absence of noise and
vibration; absence of oscillation.
To these qualities, so desirable to all, and especially those of delicate nervous temperament,
may be added—greater economy, both in the first cost and maintenance.
The whirling public so blindly follows fashionable caprice in the choice of a carriage,
as to have hitherto paid too little attention to this fundamental improvement; but many
intelligent individuals have fully verified its practical reality. Having inspected various
forms of two-wheeled and four-wheeled carriages, in the patentee’s premises in Drury
Lane, I feel justified in recommending them as being constructed on the soundest mechanical
principles; and have no doubt, that if reason be allowed to decide upon their
merits, they will ere long be universally preferred by all who seek for easy-moving,
safe, and comfortable vehicles.
WHETSLATE, is a massive mineral of a greenish-gray colour; feebly glimmering;
fracture, slaty or splintery; fragments tabular; translucent on the edges; feels
rather greasy; and has a spec. grav. of 2·722. It occurs in beds, in primitive and
transition slates. Very fine varieties of whetslate are brought from Turkey, called
honestones, which are in much esteem for sharpening steel instruments.
WHEY (Petit lait, Fr.; Molken, Germ.); is the greenish-gray liquor which exudes
from the curd of milk. Scheele states, that when a pound of milk is mixed with a
spoonful of proof spirit, and allowed to become sour, the whey filtered off, at the end of
a month or a little more, is a good vinegar, devoid of lactic acid.
WHISKEY; is dilute alcohol, distilled from the fermented worts of malt or grains.
WHITE LEAD, Carbonate of lead, or Ceruse. (Blanc de plomb, Fr.; Bleiweiss,
Germ.) This preparation is the only one in general use for painting wood and the
plaster walls of apartments white. It mixes well with oil, without having its bright
colour impaired, spreads easily under the brush, and gives a uniform coat to wood,
stone, metal, &c. It is employed either alone, or with other pigments, to serve as their
basis, and to give them body. This article has been long manufactured with much
success at Klagenfurth in Carinthia, and its mode of preparation has been lately
described with precision by Marcel de Serres. The great white-lead establishments
at Krems, whence, though incorrectly, the terms white of Kremnitz became current
on the continent, have been abandoned.
1. The lead comes from Bleyberg; it is very pure, and particularly free from contamination
with iron, a point essential to the beauty of its factitious carbonate. It is[1297]
melted in ordinary pots of cast iron, and cast into sheets of varying thickness, according
to the pleasure of the manufacturer. These sheets are made by pouring the melted
lead upon an iron plate placed over the boiler; and whenever the surface of the metal
begins to consolidate, the plate is slightly sloped to one side, so as to run off the still
liquid metal, and leave a lead sheet of the desired thinness. It is then lifted off like
a sheet of paper; and as the iron plate is cooled in water, several hundred weight of
lead can be readily cast in a day. In certain white-lead works these sheets are one
twenty-fourth of an inch thick; in others, half that quantity; in some, one of these
sheets takes up the whole width of the conversion-box; in others, four sheets are
employed. It is of consequence not to smooth down the faces of the leaden sheets;
because a rough surface presents more points of contact, and is more readily attacked
by acid vapours, than a polished one.
2. These plates are now placed so as to expose an extensive surface to the acid fumes,
by folding each other over a square slip of wood. Being suspended by their middle,
like a sheet of paper, they are arranged in wooden boxes, from 41⁄2 to 5 feet long,
12 to 14 inches broad, and from 9 to 11 inches deep. The boxes are very substantially
constructed; their joints being mortised; and whatever nails are used, being carefully
covered. Their bottom is made tight with a coat of pitch about an inch thick. The
mouths of the boxes are luted over with paper, in the works where fermenting horsedung
is employed as the means of procuring heat, to prevent the sulphuretted and
phosphuretted hydrogen from injuring the purity of the white lead. In Carinthia it
was formerly the practice, as also in Holland, to form the lead sheets into spiral rolls,
and to place them so coiled up in the chests; but this plan is not to be recommended,
because these rolls present obviously less surface to the action of the vapours, are apt
to fall down into the liquid at the bottom, and thus to impair the whiteness of the lead.
The lower edges of the sheets are suspended about two inches and a half from the
bottom of the box; and they must not touch either one another or its sides, for fear of
obstructing the vapours in the first case, or of injuring the colour in the second.
Before introducing the lead, a peculiar acid liquor is put into the box, which differs in
different works. In some, the proportions are four quarts of vinegar, with four quarts
of wine-lees; and in others, a mixture is made of 20 pounds of wine-lees, with 81⁄2
pounds of vinegar, and a pound of carbonate of potash. It is evident that in the
manufactories where no carbonate of potash is employed in the mixture, and no dung
for heating the boxes, it is not necessary to lute them.
3. The mixture being poured into the boxes, and the sheets of lead suspended within
them, they are carried into a stove-room, to receive the requisite heat for raising round
the lead the corrosive vapours, and thus converting it into carbonate. This apartment
is heated generally by stoves, is about 9 feet high, 30 feet long, and 24 feet wide, or of
such a size as to receive about 90 boxes. It has only one door.
The heat should never be raised above 86° Fahr.; and it is usually kept up for 15
days, in which time the operation is, for the most part, completed. If the heat be too
high, and the vapours too copious, the carbonic acid escapes in a great measure, and the
metallic lead, less acted upon, affords a much smaller product.
When the process is well managed, as much carbonate of lead is obtained, as there
was employed of metal; or, for 300 pounds of lead, 300 of ceruse are procured, besides a
certain quantity of metal after the crusts are removed, which is returned to the melting-pot.
The mixture introduced into the boxes serves only once; and if carbonate of
potash has been used, the residuary matter is sold to the hatters.
4. When the preceding operation is supposed to be complete, the sheets, being
removed from the boxes, are found to have grown a quarter of an inch thick, though
previously not above a twelfth of that thickness. A few pretty large crystals of acetate
of lead are sometimes observed on their edges. The plates are now shaken smartly, to
cause the crust of carbonate of lead formed on their surfaces to fall off. This carbonate
is put into large cisterns, and washed very clean. The cistern is of wood, most commonly
of a square shape, and divided into from seven to nine compartments. These
are of equal capacity, but unequal height, so that the liquid may be made to overflow
from one to the other. Thereby, if the first chest is too full, it decants its excess into
the second, and so on in succession. See Rinsing Machine.
The water poured into the first chest, passes successively into the others, a slight
agitation being meanwhile kept up, and there deposits the white lead diffused in it proportionally,
so that the deposit of the last compartment is the finest and lightest.
After this washing, the white lead receives another, in large vats, where it is always
kept under water. It is lastly lifted out in the state of a liquid paste, with wooden
spoons, and laid on drying-tables to prepare it for the market.
The white lead of the last compartment is of the first quality, and is called on the
continent silver white. It is employed in fine painting.
When white lead is mixed in equal quantities with ground sulphate of barytes, it is[1298]
known in France and Germany by the name of Venice white. Another quality,
adulterated with double its weight of sulphate of barytes, is styled Hamburgh white;
and a fourth, having three parts of sulphate to one of white lead, gets the name of
Dutch white. When the sulphate of barytes is very white, like that of the Tyrol,
these mixtures are reckoned preferable for certain kinds of painting, as the barytes
communicates opacity to the colour, and protects the lead from being speedily darkened
by sulphureous smoke or vapours.
The high reputation of the white lead of Krems was by no means due to the barytes,
for the first and whitest quality was mere carbonate of lead. The freedom from silver of
the lead of Villach, a very rare circumstance, is one cause of the superiority of its carbonate;
as well as the skilful and laborious manner in which it is washed, and separated
from any adhering particle of metal or sulphuret.
In England, lead is converted into carbonate in the following way.—The metal
is cast into the form of a network grating, in moulds about 15 inches long,
and 4 or 5 broad. Several rows of these are placed over cylindrical glazed earthen
pots, about 4 or 5 inches in diameter, containing some treacle-vinegar, which are then
covered with straw; above these pots another range is piled, and so in succession, to a
convenient height. The whole are imbedded in spent bark from the tan-pit, brought
into a fermenting state by being mixed with some bark used in a previous process.
The pots are left undisturbed under the influence of a fermenting temperature for
eight or nine weeks. In the course of this time the lead gratings become, generally
speaking, converted throughout into a solid carbonate, which when removed is levigated
in a proper mill, and elutriated with abundance of pure water. The plan of inserting
coils of sheet lead into earthenware pipkins containing vinegar, and imbedding the
pile of pipkins in fermenting horsedung and litter, is now little used; because the coil
is not uniformly acted on by the acid vapours, and the sulphuretted hydrogen evolved
from the dung is apt to darken the white lead.
In the above processes, the conversion of lead into carbonate, seems to be effected by
keeping the metal immersed in a warm, humid atmosphere, loaded with carbonic and
acetic acids; and hence a pure vinegar does not answer well; but one which is susceptible,
by its spontaneous decomposition in these circumstances, of yielding carbonic
acid. Such are tartar, wine-lees, molasses, &c.
Another process has lately been practised to a considerable extent in France, though
it does not afford a white lead equal in body and opacity to the products of the preceding
operations. M. Thenard first established the principle, and MM. Brechoz and
Leseur contrived the arrangements of this new method, which was subsequently executed
on a great scale by MM. Roard and Brechoz.
A subacetate of lead is formed by digesting a cold solution of uncrystallized acetate,
over litharge, with frequent agitation. It is said that 65 pounds of purified pyrolignous
acid, of specific gravity 1·056, require, for making a neutral acetate, 58 pounds of litharge;
and hence, to form the subacetate, three times that quantity of base, or 174 pounds, must
be used. The compound is diluted with water, as soon as it is formed, and being decanted
off quite limpid, is exposed to a current of carbonic acid gas, which, uniting with the
two extra proportions of oxide of lead in the subacetate, precipitates them in the form of
a white carbonate, while the liquid becomes a faintly acidulous acetate. The carbonic
acid may be extricated from chalk, or other compounds, or generated by combustion of
charcoal, as at Clichy; but in the latter case, it must be transmitted through a solution
of acetate of lead before being admitted into the subacetate, to deprive it of any particles
of sulphuretted hydrogen. When the precipitation of the carbonate of lead is completed,
and well settled down, the supernatant acetate is decanted off, and made to act
on another dose of litharge. The deposit being first rinsed with a little water, this
washing is added to the acetate; after which the white lead is thoroughly elutriated.
This repetition of the process may be indefinitely made; but there is always a small
loss of acetate, which must be repaired, either directly or by adding some vinegar.
In order to obtain the finest white lead by the process with earthen pots containing
vinegar buried in fermenting tan, and covered by a grating of lead, the metal
should be so thin as to be entirely convertible into carbonate; for whenever any
of it remains, it is apt to give a gray tint to the product: if the temperature of the
fermenting mass is less than 90° Fahr., some particles of the metal will resist the action
of the vinegar, and degrade the colour; and if it exceeds 122°, the white verges into
yellow, in consequence of some carbonaceous compound being developed from the
principles of the acetic acid. The dung and tan have been generally supposed to act in
this process by supplying carbonic acid, the result of their fermentation; but it is now said
that this explanation is inexact, because the best white lead can be obtained by the entire
exclusion of air from the pots in which the carbonation of the metal is carried on.
We are thence led to conclude that the lead is oxidized at the expense of the oxygen of
the vinegar, and carbonated by the agency of its oxygen and carbon; the hydrogen of[1299]
the acid being left to associate itself with the remaining oxygen and carbon, so as to
constitute an ethereous compound: thus, supposing the three atoms of oxygen to form,
with one of lead and one of carbon, an atom of carbonate, then the remaining three
atoms of carbon and three of hydrogen would compose olefiant gas.
It is customary on the continent to mould the white lead into conical loaves, before
sending them into the market. This is done by stuffing well-drained white lead into
unglazed earthen pots, of the requisite size and shape, and drying it to a solid mass, by
exposing these pots in stove-rooms. The moulds being now inverted on tables, discharge
their contents, which then receive a final desiccation; and are afterwards put up
in pale-blue paper, to set off the white colour by contrast. Nothing in all the white-lead
process is so injurious as this pot operation; a useless step, fortunately unknown in
Great Britain. Neither greasing the skin, nor wearing thick gloves, can protect the
operators from the diseases induced by the poisonous action of the white lead; and hence
they must be soon sent off to some other department of the work.
It has been supposed that the differences observed between the ceruse of Clichy and
the common kinds, depend on the greater compactness of the particles of the latter, produced
by their slower aggregation; as also, according to M. Robiquet, on the former
containing considerably less carbonic acid. See infrà.
Mr. Ham proposed, in a patent dated June, 1826, to produce white lead with the aid of
the following apparatus, a, a, (fig. 1201.) are the side-walls of a stove-room, constructed of
bricks; b, is the floor of bricks laid in Roman
cement; c, c, are the side-plates, between
which and the walls, a quantity of refuse
tanner’s bark, or other suitable vegetable
matter, is to be introduced. The same material
is to be put also into the lower part
at d (upon a false bottom of grating?) The
tan should rise to a considerable height,
and have a series of strips of sheet lead e, e, e,
placed upon it, which are kept apart by
blocks or some other convenient means,
with a space open at one end of the plates,
for the passage of the vapours; but above
the upper plates, boards are placed, and
covered with tan, to confine them there. In
the lower part of the chamber, coils of steam-pipe f, f, are laid in different directions
to distribute heat; g, is a funnel-pipe, to conduct vinegar into the lower part of the
vessel; and h, is a cock to draw it off, when the operation is suspended. The acid
vapours raised by the heat, pass up through the spent bark, and on coming into contact
with the sheets of lead, corrode them. The quantity of acid liquor should not be
in excess; a point to be ascertained by means of the small tube i, at top, which is
intended for testing it by the tongue. k, is a tube for inserting a thermometer, to watch
the temperature, which should not exceed 170° Fahr. I am not aware of what success
has attended this patented arrangement. The heat prescribed is far too great.
A magnificent factory has been recently erected at West Bromwich, near Birmingham,
to work a patent lately granted to Messrs. Gossage and Benson, for making white
lead by mixing a small quantity of acetate of lead in solution with slightly damped
litharge, contained in a long stone trough, and passing over the surface of the trough
currents of hot carbonic acid, while its contents are powerfully stirred up by a travelling-wheel
mechanism. The product is afterwards ground and elutriated, as usual.
The carbonic acid gas is produced from the combustion of coke. I am told that
40 tons of excellent white lead are made weekly by these chemico-mechanical operations.
Messrs. Button and Dyer obtained a patent about a year and a half ago, for making
white lead by transmitting a current of purified carbonic acid gas, from the combustion
of coke, through a mixture of litharge and nitrate of lead, diffused and dissolved in
water, which is kept in constant agitation and ebullition by steam introduced through a
perforated coil of pipes at the bottom of the tub. The carbonate of lead is formed here
upon the principle of Thenard’s old process with the subacetate; for the nitrate of lead
forms with the litharge a subnitrate, which is forthwith transformed into carbonate and
neutral nitrate, by the agency of the carbonic acid gas. I have discovered that all sorts
of white lead produced by precipitation from a liquid, are in a semi-crystalline condition;
appear, therefore, semi-transparent, when viewed in the microscope; and do not
cover so well as white lead made by the process of vinegar and tan, in which the lead
has remained always solid during its transition from the blue to the white state; and
hence consists of opaque particles.
A patent was obtained, in December, 1833, by John Baptiste Constantine Torassa,[1300]
and others, for making white lead by agitating the granulated metal, or shot, in trays
or barrels, along with water, and exposing the mixture of lead-dust and water to the
air, to be oxidized and carbonated. It is said that upwards of 100,000l. have been
expended at Chelsea, by a joint stock company, in a factory constructed for executing
the preceding most operose and defective process; which has been, many years ago,
tried without success in Germany. I am convinced that the whole of these recent projects
for preparing white lead, are inferior in economy, and quality of produce, to the
old Dutch process, which may be so arranged as to convert sheets of blue lead
thoroughly into the best white lead, within the space of 12 days, at less expense of labour
than by any other plan.
White lead, as obtained by precipitation from the acetate, subacetate, and subnitrate,
is a true carbonate of the metal, consisting of one prime equivalent of lead 104, one of
oxygen 8, and one of carbonic acid 22; whose sum is 134, the atomic weight of the
compound; or, of lead, 77·6; oxygen, 6; carbonic acid, 16·4; in 100 parts. It has
been supposed, by some authors, that the denser and better-covering white lead of
Krems and Holland is a kind of subcarbonate, containing only 9 per cent. of carbonic
acid; but this view of the subject does not accord with my researches.
WICK (Mèche, Fr.; Docht, Germ.); is the spongy cord, usually made of soft spun
cotton threads, which by capillary action, draws up the oil in lamps, or the melted tallow
or wax in candles, in small successive portions, to be burned. In common wax and
tallow candles, the wick is formed of parallel threads; in the stearine candles, the wick
is plaited upon the braiding machine, moistened with a very dilute sulphuric acid, and
dried, whereby as it burns, it falls to one side and consumes without requiring to be
snuffed; in the patent candles of Mr. Palmer, one-tenth of the wick is first imbued with
subnitrate of bismuth ground up with oil, the whole is then bound round in the manner
called gimping; and of this wick, twice the length of the intended candle is twisted
double round a rod, like the caduceus of Mercury. This rod with its coil being inserted
in the axis of the candle mould, is to be enclosed by pouring in the melted tallow; and
when the tallow is set, the rod is to be drawn out at top, leaving the wick in the candle.
As this candle is burned, the ends of the double wick stand out sideways beyond
the flame; and the bismuth attached to the cotton being acted on by the oxygen of the
atmosphere, causes the wick to be completely consumed, and, therefore, saves the trouble
of snuffing it.
WINCING-MACHINE, is the English name of the dyer’s reel, which he suspends
horizontally, by the ends of its iron axis in bearings, over the edge of his vat, so
that the line of the axis, being placed over the middle partition in the copper, will
permit the piece of cloth which is wound upon the reel, to descend alternately into
either compartment of the bath, according as it is turned by hand to the right or the
left. For an excellent self-acting or mechanical wince, see Dyeing.
WINE, is the fermented juice of the grape. In the more southern states of Europe,
the grapes, being more saccharine, afford a more abundant production of alcohol, and
stronger wines, as exemplified in the best port, sherry, and madeira. The influence
of solar heat upon the vines may, however, be mitigated by growing them to moderate
heights on level ground, and by training them in festoons under the shelter of
trees. In the more temperate climates, such as the district of Burgundy, the finer
flavoured wines are produced; and there the vines are usually grown upon hilly
slopes fronting the south, with more or less of an easterly or westerly direction, as
on the Côte d’Or, at a distance from marshes, forests, and rivers, whose vapours
might deteriorate the air. The plains of this district, even when possessing a similar
or analogous soil, do not produce wines of so agreeable a flavour. The influence of
temperature becomes very manifest in countries further north, where, in consequence
of a few degrees of thermometric depression, the production of generous agreeable
wine becomes impossible.
The land most favourable to the vine is light, easily permeable to water, but somewhat
retentive by its composition; with a sandy subsoil, to allow the excess of moisture to
drain readily off. Calcareous soils produce the highly esteemed wines of the Côte
d’Or; a granitic debris forms the foundation of the lands where the Hermitage wines
are grown; siliceous soil interspersed with flints furnishes the celebrated wines of
Château-Neuf, Ferté, and La Gaude; schistose districts afford also good wine, as that
called la Malgue. Thus we see that lands differing in chemical composition, but
possessed of the proper physical qualities, may produce most agreeable wines; and so
also may lands of like chemical and physical constitution produce various kinds of
wine, according to their varied exposure. As a striking example of these effects, we
may adduce the slopes of the hills which grow the wines of Montrachet. The insulated
part towards the top furnishes the wine called Chevalier Montrachet, which is less
esteemed, and sells at a much lower price, than the delicious wine grown on the middle
height, called true Montrachet. Beneath this district, and in the surrounding plains,[1301]
the vines afford a far inferior article, called bastard Montrachet. The opposite side of
the hills produces very indifferent wine. Similar differences, in a greater or less degree,
are observable relatively to the districts which grow the Pomard, Volnay, Beaune,
Nuits, Vougeot, Chambertin, Romanée, &c. Every where it is found, that the reverse
side of the hill, the summit, and the plain, although generally consisting of like soil,
afford inferior wine to the middle southern slopes.
Amelioration of the soil.—When the vine lands are too light or too dense, they may
be modified, within certain limits, by introducing into them either argillaceous or siliceous
matter. Marl is excellent for almost all grounds which are not previously too
calcareous, being alike useful to open dense soils, and to render porous ones more
retentive.
Manure.—For the vine, as well as all cultivated plants, a manure supplying azotized
or animal nutriment may be used with great advantage, provided care be taken to
ripen it by previous fermentation, so that it may not, by absorption in too crude a
state, impart any disagreeable odour to the grape; as sometimes happens to the vines
grown in the vicinity of great towns, like Paris, and near Argenteuil. There is a
compost used in France, called animalized black, of which from 1⁄3 to 1⁄2 of a litre
(old English quart) serves sufficiently to fertilize the root of one vine, when applied every
year, or two years. An excess of manure, in rainy seasons especially, has the effect of
rendering the grapes large and insipid.
The ground is tilled at the same time as the manure is applied, towards the month
of March; the plants are then dressed, and the props are inserted. The weakness of
the plants renders this practice useful; but in some southern districts, the stem of the
vine, when supported at a proper height, acquires after a while sufficient size and
strength to stand alone. The ends of the props or poles are either dipped in tar, or
charred, to prevent their rotting. The bottom of the stem must be covered over with
soil, after the spring rains have washed it down. The principal husbandry of the vineyard
consists in digging or ploughing to destroy the weeds, and to expose the soil to the
influence of the air, during the months of May, June, and occasionally in August.
The vintage, in the temperate provinces, generally takes place about the end of
September; and it is always deteriorated whenever the fruit is not ripe enough before
the 15th or 20th of October; for, in this case, not only is the must more acid, and less
saccharine, but the atmospherical temperature is apt to fall so low during the nights, as to
obstruct more or less its fermentation into wine. The grapes should be plucked in
dry weather, at the interval of a few days after they are ripe; being usually
gathered in baskets, and transported to the vats in dorsels, sufficiently tight to prevent
the juice from running out. Whenever a layer about 14 or 15 inches thick has been
spread on the bottom of the vat, the treading operation begins, which is usually
repeated after macerating the grapes for some time, when an incipient fermentation
has softened the texture of the skin and the interior cells. When the whole bruised
grapes are collected in the vat, the juice, by means of a slight fermentation, reacts,
through the acidity thus generated, upon the colouring-matter of the husks, and also
upon the tannin contained in the stones and the fruit-stalks. The process of fermentation
is suffered to proceed without any other precaution, except forcing down from
time to time the pellicles and pedicles floated up by the carbonic acid to the top; but
it would be less apt to become acetous, were the mouths of the vats covered. With
this view, M. Sebille Auger introduced with success his elastic bung in the manufacture
of wine in the department of the Maine-et-Loire.
With whatever kind of apparatus the fermentation may have been regulated, as soon
as it ceases to be tumultuous, and the wine is not sensibly saccharine or muddy, it
must be racked off from the lees, by means of a spigot, and run into the ripening tuns.
The marc being then gently squeezed in a press, affords a tolerably clear wine, which
is distributed among the tuns in equal proportions; but the liquor obtained by
stronger pressure, is reserved for the casks of inferior wine.
In the south of France the fermentation sometimes proceeds too slowly, on account of
the must being too saccharine; an accident which is best counteracted by maintaining
a temperature of about 65° or 68° F., in the tun-room. When the must, on the other
hand, is too thin, and deficient in sugar, it must be partially concentrated by rapid
boiling, before the whole can be made to ferment into a good wine. By boiling up a
part of the must for this purpose, the excess of ferment is at the same time destroyed.
Should this concentration be inconvenient, a certain proportion of sugar must be introduced,
immediately after racking it off.
The specific gravity of must varies with the richness and ripeness of the grapes which
afford it; being in some cases so low as 1·0627, and in others so high as 1·1283. This
happens particularly in the south of France. In the district of the Necker in Germany,
the specific gravity varies from 1·050 to 1·090; in Heidelberg, from 1·039, to 1·091;
but it varies much in different years.
[1302]
After the fermentation is complete, the vinous part consists of water, alcohol, a
colouring-matter, a peculiar aromatic principle, a little undecomposed sugar, bitartrate
and malate of potash, tartrate of lime, muriate of soda, and tannin; the latter substances
being in small proportion.
It is known that a few green grapes are capable of spoiling a whole cask of wine, and
therefore they are always allowed to become completely ripe, and even sometimes to
undergo a species of slight fermentation, before being plucked, which completes the
development of the saccharine principle. At other times the grapes are gathered whenever
they are ripe, but are left for a few days on wicker-floors, to sweeten, before being
pressed.
In general the whole vintage of the day is pressed in the evening, and the resulting
must is received in separate vats. At the end usually of 6 or 8 hours, if the temperature
be above 50° F., and if the grapes have not been too cold when plucked, a froth
or scum is formed at the surface, which rapidly increases in thickness. After it
acquires such a consistence as to crack in several places, it is taken off with a skimmer,
and drained; and the thin liquor is returned to the vat. A few hours afterwards
another coat of froth is formed, which is removed in like manner, and sometimes a
third may be produced. The regular vinous fermentation now begins, characterized
by air-bubbles rising up the sides of the staves, with a peculiar whizzing as they break
at the surface. At this period all the remaining froth should be quickly skimmed off,
and the clear subjacent must, be transferred into barrels, where it is left to ripen by a
regular fermentation.
The white wines, which might be disposed to become stringy, from a deficient supply
of tannin, may be preserved from this malady by a due addition of the footstalks of ripe
grapes. The tannin, while it tends to preserve the wines, renders them also more easy
to clarify, by the addition of white of egg, or isinglass.
The white wines should be racked off as soon as the first frosts have made them clear,
and at the latest by the end of the February moon. By thus separating the wine from the
lees, we avoid, or render of little consequence, the fermentation which takes place on
the return of spring, and which, if too brisk, would destroy all its sweetness, by decomposing
the remaining portion of sugar.
The characteristic odour possessed by all wines, in a greater or less degree, is produced
by a peculiar substance, which possesses the characters of an essential oil. As it
is not volatile, it cannot be confounded with the aroma of wine. When large quantities
of wine are distilled, an oily substance is obtained towards the end of the operation.
This may also be procured from the wine lees which are deposited in the
casks after the fermentation has commenced. It forms one 40,000th part of the
wine; and consists of a peculiar new acid, and ether, each of which has been called the
œnanthic. The acid is analogous to the fatty acids, and the ether is liquid, but insoluble
in water. The acid is perfectly white when pure, of the consistence of butter at 60°,
melts with a moderate heat, reddens litmus, and dissolves in caustic and carbonated
alkalis, as well as in alcohol and ether. Œnanthic ether is colourless, has an extremely
strong smell of wine, which is almost intoxicating when inhaled, and a powerful
disagreeable taste. Liebig and Pelouze.
Sparkling wines.—In the manufacture of these, black grapes of the first quality are
usually employed, especially those gathered upon the vine called by the French noirien,
cultivated on the best exposures. As it is important, however, to prevent the colouring-matter
of the skin from entering into the wine, the juice should be squeezed as
gently and rapidly as possible. The liquor obtained by a second and third pressing is
reserved for inferior wines, on account of the reddish tint which it acquires. The marc
is then mixed with the grapes of the red-wine vats.
The above nearly colourless must, is immediately poured into tuns or casks, till about
three-fourths of their capacity are filled, when fermentation soon begins. This is allowed
to continue under the control of the elastic bung, above mentioned, for about 15 days,
and then three-fourths of the casks are filled up with wine from the rest. The casks
are now closed by a bung secured with a piece of hoop iron nailed to two contiguous
staves. The casks should be made of new wood, but not of oak—though old white
wine casks are occasionally used.
In the month of January the clear wine is racked off, and is fined by a small quantity
of isinglass dissolved in old wine of the same kind. Forty days afterwards a
second fining is required. Sometimes a third may be useful, if the lees be considerable.
In the month of May the clear wine is drawn off into bottles, taking care to add to
each of them a small measure of what is called liquor, which is merely about 3 per cent.
of a syrup made by dissolving sugar-candy in white wine. The bottles being filled,
and their corks secured by packthread and wire, they are laid on their sides, in this
month, with their mouths sloping downwards at an angle of about 20 degrees, in[1303]
order that any sediment may fall into the neck. At the end of 8 or 10 days, the
inclination of the bottles is increased, when they are slightly tapped, and placed in
a vertical position; so that after the lees are all collected in the neck, the cork is
partially removed for an instant, to allow the sediment to be expelled by the pressure
of the gas. If the wine be still muddy in the bottles, along with a new dose of liquor,
a small quantity of fining should be added to each, and the bottles should be placed again
in the inverted position. At the end of two or three months, the sediment collected
over the cork, is dexterously discharged; and if the wine be still deficient in transparency,
the same process of fining must be repeated.
Sparkling wine (vin mousseux), prepared as above described, is fit for drinking usually
at the end of from 18 to 30 months, according to the state of the seasons. It is in
Champagne that the lightest, most transparent, and most highly flavoured wines, have
been hitherto made. The breakage of the bottles in these sparkling wines amounts
frequently to 30 per cent., a circumstance which adds greatly to their cost of production.
Weak wines of bad growths ought to be consumed within 12 or 15 months after
being manufactured; and should be kept meanwhile in cool cellars. White wines of
middling strength ought to be kept in casks constantly full, and carefully excluded
from contact of air, and the racking off should be done as quickly as possible. As the
most of them are injured by too much fermentation, this process should be so regulated
as always to leave a little sugar undecomposed. It is useful to counteract the absorption
of oxygen, and the consequent tendency to acidity, by burning a sulphur match
in the casks into which they are about to be run. This is done by hooking the match
to a bent wire, kindling and suspending it within the cask through the bung-hole.
Immediately on withdrawing the match, the cask should be corked, if the wine be not
ready for transfer. If the burning sulphur be extinguished on plunging it into the
cask, it is a proof of the cask being unsound, and unfit for receiving the wine; in which
case it should be well cleansed, first with lime-water, then with very dilute sulphuric acid,
and lastly with boiling water.
Wine-cellars ought to be dry at bottom, floored with flags, have windows opening to
the north, be so much sunk below the level of the adjoining ground as to possess a
nearly uniform temperature in summer and winter; and be at such a distance from a
frequented highway or street as not to suffer vibration from the motion of carriages.
Wines should be racked off in cool weather; the end of February being the fittest
time for light wines. Strong wines are not racked off till they have stood a year or
eighteen months upon the lees, to promote their slow or insensible fermentation.
A syphon well managed serves better than a faucet to draw off wine clear from the
sediment. White wines, before being bottled, should be fined with isinglass; red wines
are usually fined with whites of eggs beat up into a froth, and mixed with two or three
times their bulk of water. But some strong wines, which are a little harsh from excess
of tannin, are fined with a little sheep or bullock’s blood. Occasionally a small
quantity of sweet glue is used for this purpose.
The following maladies of wines, are certain accidental deteriorations, to which remedies
should be speedily applied.
La-pousse (pushing out of the cask), is the name given to a violent fermentative
movement, which occasionally supervenes after the wine has been run off into the
casks. If these have been tightly closed, the interior pressure may increase to such a
degree as to burst the hoops, or cause the seams of the staves or ends to open. The
elastic bungs already described, will prevent the bursting of the casks; but something
must be done to repress the fermentation, lest it should destroy the whole of the sugar,
and make the wine unpalatably harsh. One remedy is, to transfer the wine into a cask
previously fumigated with burning sulphur; another is, to add to it about one thousandth
part of sulphite of lime; and a third, and perhaps the safest, is to introduce half
a pound of mustard-seed into each barrel. At any rate the wines should be fined
whenever the movements are allayed, to remove the floating ferment which has been
the cause of the mischief.
Turning sour.—The production of too much acid in a wine, is a proof of its containing
originally too little alcohol, of its being exposed too largely to the air, or to vibrations,
or to too high a temperature in the cellar. The best thing to be done in this
case is, to mix it with its bulk of a stronger wine in a less advanced state, to fine the
mixture, to bottle it, and to consume it as soon as possible, for it will never prove a good
keeping wine. This distemper in wines formerly gave rise to the very dangerous practice
of adding litharge as a sweetener; whereby a quantity of acetate or sugar of lead was
formed in the liquor, productive of the most deleterious consequences to those who drunk
of it. In France, the regulations of police, and the enlightened surveillance of the
council of salubrity, have completely put down this gross abuse. The saturation of the[1304]
acid by lime and other alkaline bases has generally a prejudicial effect, and injures more
or less the vinous flavour and taste.
Ropiness or viscidity of wines.—The cause of this phenomenon, which renders
wine unfit for drinking, was altogether unknown, till M. François, an apothecary of
Nantes, demonstrated that it was owing to an azotized matter, analogous to gliadine
(gluten); and in fact it is the white wines, especially those which contain the least
tannin, which are subject to this malady. He also pointed out the proper remedy, in
the addition of tannin under a rather agreeable form, namely, the bruised berries of the
mountain-ash (sorbier), in a somewhat unripe state; of which one pound, well stirred in, is
sufficient for a barrel. After agitation, the wine is to be left in repose for a day or two,
and then racked off. The tannin by this time will have separated the azotized matter from
the liquor, and removed the ropiness. The wine is to be fined and bottled off.
The taste of the cask, which sometimes happens to wine put into casks which had
remained long empty, is best remedied by agitating the wine for some time with a
spoonful of olive oil. An essential oil, the chief cause of the bad taste, combines with
the fixed oil, and rises with it to the surface.
According to a statement in the Dictionnaire Technologique, the annual produce of a
hectare of vineyard, upon the average of 113 years, in the district of Volnay, is 1779
litres, which fetch 0·877 francs each, or 200 francs the piece of 228 litres, amounting
in all to 1672 francs. Deducting for expenses and taxes (contributions) 572 francs,
there remain 1100 francs of net proceeds; and as the value of the capital may be
estimated at 23,000 francs, the profit turns out to be no more than 5 per cent. The
net proceeds in the growths of Beaune, Nuits, &c., does not exceed 600 francs per
hectare (2·4 acres), and therefore is equivalent to only 21⁄2 per cent. upon the capital.
The quantity of alcohol contained in different wines, has been made the subject of
elaborate experiments by Brande and Fontenelle; but as it must evidently vary with
different seasons, the results can be received merely as approximative. The only
apparatus required for this research, is a small still and refrigeratory, so well fitted up
as to permit none of the spirituous vapours to be dissipated. The distilled liquor should
be received in a glass tube, graduated into one hundred measures, of such capacity as to
contain the whole of the alcohol which the given measure of wine employed is capable
of yielding. In the successive experiments, the quantity of wine used, and of spirit
distilled over, being the same in volume, the relative densities of the latter will show at
once the relative strengths of the wines. A very neat small apparatus has been contrived
for the purpose of analyzing wines in this manner, by M. Gay Lussac. It is constructed,
and sold at a moderate price, by M. Collardeau, No. 56, Rue Faubourg St. Martin,
Paris. The proportion given by Brande (Table I.), has been reduced to the standard
of absolute alcohol by Fesser; and that by Fontenelle (Table II.), to the same
standard by Schubarth; as in the following tables:—
Table I.
| Name of the Wine. |
Sp.
grav. |
100 measures,
contain at 60° F. |
Alcohol
of 0·825. |
Absolute
alcohol. |
| Port Wine |
0 |
·97616 |
21 |
·40 |
19 |
·82 |
| Do. |
0 |
·97200 |
25 |
·83 |
23 |
·92 |
| Mean |
0 |
·97460 |
23 |
·49 |
21 |
·75 |
| Madeira |
0 |
·97810 |
19 |
·34 |
17 |
·91 |
| Do. |
0 |
·97333 |
21 |
·42 |
22 |
·61 |
| Sherry |
0 |
·97913 |
18 |
·25 |
17 |
·00 |
| Do. |
0 |
·97700 |
19 |
·83 |
18 |
·37 |
| Bordeaux, Claret |
0 |
·97410 |
12 |
·91 |
11 |
·95 |
| Do. |
0 |
·97092 |
16 |
·32 |
15 |
·11 |
| Calcavella |
0 |
·97920 |
18 |
·10 |
16 |
·76 |
| Lisbon |
0 |
·97846 |
18 |
·94 |
17 |
·45 |
| Malaga |
0 |
·98000 |
17 |
·26 |
15 |
·98 |
| Bucellas |
0 |
·97890 |
18 |
·49 |
17 |
·22 |
| Red Madeira |
0 |
·97899 |
18 |
·40 |
17 |
·04 |
| Malmsey |
0 |
·98090 |
16 |
·40 |
15 |
·91 |
| Marsala |
0 |
·98190 |
15 |
·26 |
14 |
·31 |
| Do. |
0 |
·98000 |
17 |
·26 |
15 |
·98 |
| Champagne (rose) |
0 |
·98608 |
11 |
·30 |
10 |
·46 |
| ChaDo.agne(white) |
0 |
·98450 |
12 |
·80 |
11 |
·84 |
| Burgundy |
0 |
·98300 |
14 |
·53 |
13 |
·34 |
| Do. |
0 |
·98540 |
11 |
·95 |
11 |
·06 |
| White Hermitage |
0 |
·97990 |
17 |
·43 |
16 |
·14 |
| Red do. |
0 |
·98495 |
12 |
·32 |
11 |
·40 |
| Hock |
0 |
·98290 |
14 |
·37 |
13 |
·31 |
| Do. |
0 |
·98873 |
8 |
·88 |
8 |
·00 |
| Vin de Grave |
0 |
·98450 |
12 |
·80 |
11 |
·84 |
| Frontignac |
0 |
·98452 |
17 |
·79 |
11 |
·84 |
| Côte-Rotí |
0 |
·98495 |
12 |
·27 |
11 |
·36 |
| Roussillon |
0 |
·98005 |
17 |
·24 |
15 |
·96 |
| Cape Madeira |
0 |
·97924 |
18 |
·11 |
16 |
·77 |
| Muscat |
0 |
·97913 |
18 |
·25 |
17 |
·00 |
| Constantia |
0 |
·97770 |
19 |
·75 |
18 |
·29 |
| Tinto |
0 |
·98399 |
13 |
·30 |
12 |
·32 |
| Schiraz |
0 |
·98176 |
15 |
·52 |
14 |
·35 |
| Syracuse |
0 |
·98200 |
15 |
·28 |
14 |
·15 |
| Nice |
0 |
·98263 |
14 |
·63 |
13 |
·64 |
| Tokay |
0 |
·98760 |
9 |
·88 |
9 |
·15 |
| Raisin wine |
0 |
·97205 |
25 |
·77 |
23 |
·86 |
| Drained grape wine |
0 |
·97925 |
18 |
·11 |
16 |
·77 |
| Lachrymæ Christi |
— |
19 |
·70 |
18 |
·24 |
| Currant wine |
0 |
·97696 |
20 |
·55 |
19 |
·03 |
| Gooseberry wine |
0 |
·98550 |
11 |
·84 |
10 |
·96 |
| Elder wine |
|
- |
0 |
·98760 |
9 |
·87 |
9 |
·14 |
| Cyder |
| Perry |
| Brown Stout |
0 |
·99116 |
6 |
·80 |
6 |
·30 |
| Ale |
0 |
·98873 |
8 |
·88 |
8 |
·00 |
| Porter |
— |
4 |
·20 |
3 |
·89 |
| Rum |
0 |
·93494 |
53 |
·68 |
49 |
·71 |
| Hollands |
0 |
·93855 |
51 |
·60 |
47 |
·77 |
| Scotch whiskey |
— |
54 |
·32 |
50 |
·20 |
| Irish whiskey |
— |
53 |
·90 |
49 |
·91 |
[1305]
Table II.
| Name of the Wine. |
Absolute
alcohol. |
| Roussillon (Eastern Pyrenees.) |
|
| Rive-saltes |
18 |
yrs. old |
9·156 |
| Banyulls |
18 |
|
9·223 |
| Collyouvre |
15 |
|
9·080 |
| Salces |
10 |
|
8·580 |
| Department of the Aude. |
|
| Fitou and Leucaté |
10 |
|
8·568 |
| Lapalme |
10 |
|
8·790 |
| Sijeau |
8 |
|
8·635 |
| Narbonne |
8 |
|
8·379 |
| Lezignan |
10 |
|
8·173 |
| Mirepeisset |
10 |
|
8·589 |
| Carcasonne |
8 |
|
7·190 |
| Department of l’Herault. |
|
| Nissau |
9 |
|
7·896 |
| Beziers |
8 |
|
7·728 |
| Montagnac |
10 |
|
8·108 |
| Mèze |
10 |
|
7·812 |
| Montpellier |
5 |
|
7·413 |
| Lunel |
8 |
|
7·564 |
| Frontignan |
5 |
|
7·098 |
| Red Hermitage |
4 |
|
5·838 |
| White do. |
7·056 |
| Burgundy |
4 |
|
6·195 |
| Grave |
3 |
|
5·838 |
| Champagne (sparkling) |
5·880 |
| Do. white do. |
5·145 |
| Do. rose |
4·956 |
| Bordeaux |
6·186 |
| Toulouse |
5·027 |
WINE, FAMILY, may be made by the following recipe:—Take black, red,
white currants, ripe cherries (black hearts are the best), and raspberries, of each an equal
quantity. To 4 pounds of the mixed fruit, well bruised, put 1 gallon of clear soft water;
steep three days and nights, in open vessels, frequently stirring up the magma; then
strain through a hair sieve; press the residuary pulp to dryness, and add its juice to
the former. In each gallon of the mixed liquors dissolve 3 pounds of good yellow
muscovado sugar; let the solution stand other three days and nights, frequently skimming
and stirring it up; then tun it into casks, which should remain full, and purging
at the bung-hole, about two weeks. Lastly, to every 9 gallons, put 1 quart of good
Cognac brandy (but not the drugged imitations made in London with grain whiskey), and
bung down. If it does not soon become fine, a steeping of isinglass may be stirred into the
liquid, in the proportion of about half an ounce to 9 gallons. I have found that the addition
of an ounce of cream of tartar to each gallon of the fermentable liquor, improves the
quality of the wine, and makes it resemble more nearly the produce of the grape.
WINE-STONE, is the deposit of crude tartar, called argal, which settles on the
sides and bottoms of wine casks.
WIRE-DRAWING. (Tréfilerie, Fr.; Draht-ziehen, Drahtzug, Germ.) When an
oblong lump of metal is forced through a series of progressively diminishing apertures
in a steel plate, so as to assume in its cross section the form and dimensions of the last
hole, and to be augmented in length at the expense of its thickness, it is said to be wire-drawn.
The piece of steel called the draw-plate is pierced with a regular gradation of
holes, from the largest to the smallest; and the machine for overcoming the lateral
adhesion of the metallic particles to one another, is called the draw-bench. The
pincers which lay hold of the extremity of the wire, to pull it through the successive
holes, are adapted to bite it firmly, by having the inside of the jaws, cut like a file. For
drawing thick rods of gilt silver down into stout wire, the hydraulic press has been had
recourse to with advantage.
Fig. 1202. represents a convenient form of the draw-bench, where the power is applied by
a toothed wheel, pinion, and rack-work, moved by the hands of one or two men working at
a winch; the motion being so
regulated by a fly-wheel, that it
does not proceed in fits and starts,
and cause inequalities in the wire.
The metal requires to be annealed,
now and then, between successive
drawings, otherwise it would become
too hard and brittle for further
extension. The reel upon which
it is wound is sometimes mounted
in a cistern of sour small beer, for
the purpose of clearing off, or loosening at least, any crust of oxide formed in the
annealing, before the wire enters the draw-plate.
When, for very accurate purposes of science or the arts, a considerable length of
uniform wire is to be drawn, a plate with one or more jewelled holes, that is, filled with
one or more perforated rubies, sapphires, or chrysolites, can alone be trusted to, because
the holes even in the best steel become rapidly wider by the abrasion. Through a hole in
a ruby 0·0033 of an inch in diameter, a silver wire 170 miles long has been drawn, which
possessed at the end, the very same section as at the beginning; a result determined by
weighing portions of equal length, as also by measuring it with a micrometer. The hole in
an ordinary draw-plate of soft steel becomes so wide, by drawing 14,000 fathoms of brass
wire, that it requires to be narrowed before the original sized wire can be again obtained.
Wire, by being diminished one-half, one-third, one-fourth, &c., in diameter, is augmented
in length respectively, four, nine, sixteen times, &c. The speed with which it[1306]
may be prudently drawn out, depends upon the ductility and tenacity of the metal; but
may be always increased the more the wire becomes attenuated, because its particles
progressively assume more and more of the filamentous form, and accommodate themselves
more readily to the extending force. Iron and brass wires, of 0·3 inch in diameter,
bear drawing at the rate of from 12 to 15 inches per second; but when of 0·025 (1⁄40) of
an inch, at the rate of from 40 to 45 inches in the same time. Finer silver and copper
wire may be extended from 60 to 70 inches per second.
By enclosing a wire of platinum within one of silver ten times thicker, and drawing
down the compound wire till it be 1⁄300 of an inch, a wire of platinum of 1⁄3000 of an inch,
will exist in its centre, which may be obtained apart, by dissolving the silver away in
nitric acid. This pretty experiment was first made by Dr. Wollaston.
The French draw-plates are so much esteemed, that one of the best of them used to
be sold in this country, during the late war, for its weight in silver. The holes are
formed with a steel punch; being made large on that side where the wire enters, and
diminishing with a regular taper to the other side. In the act of drawing, they must be
well supplied with grease for the larger kinds of wire, and with wax for the smaller.
WOAD (Vouëde, Pastel, Fr.; Waid, Germ; Isatis tinctoria, Linn.); the glastum
of the antient Gauls and Germans; is an herbaceous plant which was formerly much
cultivated, as affording a permanent blue dye, but it has been in modern times well nigh
superseded by indigo. Pliny says, “A certain plant which resembles plantago, called
glastum, is employed by the women and girls in Great Britain for dyeing their bodies
all over, when they assist at certain religious ceremonies; they have then the colour of
Ethiopians.”—Hist. Nat. cap. xxii. § 2.
When the arts, which had perished with the Roman empire, were revived, in the
middle ages, woad began to be generally used for dyeing blue, and became an object of
most extensive cultivation in many countries of Europe. The environs of Toulouse and
Mirepoix, in Upper Languedoc, produced annually 40,000,000 pounds of the prepared
woad, or pastel, of which 200,000 bales were consumed at Bordeaux. Beruni, a rich
manufacturer of this drug, became surety for the payment of the ransom of his king,
Francis I., then the prisoner of Charles V. in Spain.
The leaves of woad are fermented in heaps, to destroy certain vegetable principles
injurious to the beauty of the dye, as also to elaborate the indigoferous matter present,
before they are brought into the market; but they should be carefully watched during
this process. Whenever the leaves have arrived at maturity, a point judged of very differently
in different countries, they are stripped off the plant, a cropping which is repeated
as often as they shoot, being three or four times in Germany, and eight or ten times in
Italy. The leaves are dried as quickly as possible, but not so much as to become
black; and they are ground before they get quite dry. The resulting paste is laid upon
a sloping pavement, with gutters for conducting the juice which exudes into a tank;
the heap being tramped from time to time, to promote the discharge of the juice. The
woad ferments, swells, and cracks in many places, which fissures must be closed; the
whole being occasionally watered. The fermentation is continued for twenty or thirty
days, in cold weather; and if the leaves have been gathered dry, as in Italy, for four
months. When the fermented heap has become moderately dry, it is ground again, and
put up in cakes of from one to three pounds; which are then fully dried, and packed up
in bundles for the market. Many dyers subject the pastel to a second fermentation.
1,600 square toises (fathoms) of land afford in two cuttings at least 19,000 pounds of
leaves, of which weight four-fifths are lost in the fermentation, leaving 3,880 pounds of
pastel, in loaves or cakes. When good, it has rather a yellow, or greenish-yellow, than a blue
colour; it is light, and slightly humid; it gives to paper a pale-green trace; and improves
by age, in consequence of an obscure fermentation; for if kept four years, it dyes
twice as much as after two years. According to Hellot, 4 pounds of Guatimala indigo
produce the same effect as 210 pounds of the pastel of Albi. At Quins, in Piedmont,
the dyers estimate that 6 pounds of indigo are equivalent to 300 of pastel; but Chaptal
thinks the indigo underrated.
Pastel will dye blue of itself, but it is commonly employed as a fermentative addition
to the proper blue vat, as described under Indigo.
Fresh woad, analyzed by Chevreul, afforded, in 100 parts, 65·4 of juice. After being
steeped in water, the remaining mass yielded, on expression, 29·65 of liquid; being in
whole, 95·05 parts, leaving 4·95 of ligneous fibre. The juice, by filtration, gave 1·95
of green fecula. 100 parts of fresh woad, when dried, are reduced to 13·76 parts. Alcohol,
boiled upon dry woad, deposits, after cooling, indigo in microscopic needles; but these
cannot be separated from the vegetable albumine, which retains a greenish-gray colour.
WOLFRAM, is the native tungstate of iron and manganese, a mineral which
occurs in primitive formations, along with the ores of tin, antimony, and lead, in the
Bohemian Erzgebirge, in Cornwall, Switzerland, North America, &c. It is used by
chemists for obtaining tungstic acid and tungsten.
[1307]
WOOD (Bois, Fr.; Holz, Germ.); is the hard but porous tissue between the pith
and the bark of trees and shrubs, through which the chief part of the juices are conducted
from the root towards the branches and leaves, during the life of the vegetable.
The ligneous fibre is the substance which remains, after the plant has been subjected to
the solvent action of ether, alcohol, water, dilute acids, and caustic alkaline lyes. It
is considered by chemists that dry timber consists, on an average, of 96 parts of fibrous,
and 4 of soluble matter, in 100; but that these proportions vary somewhat with the
seasons, the soil, and the plant. All kinds of wood sink in water, when placed in a
basin of it under the exhausted receiver of an air-pump; showing their specific gravity
to be greater than 1·000. That of fir and maple is stated, by chemical authors, to be
1·46; and that of oak and beech, at 1·53; but I believe them to have all the same
spec. grav. as the fibre of flax; namely, 1·50, as determined by me some years ago.[71]
Wood becomes snow-white, when exposed to the action of chlorine; digested with
sulphuric acid, it is transformed first into gum, and, by ebullition with water, afterwards
into grape-sugar; with concentrated nitric acid, it grows yellow, loses its coherence,
falls into a pulverulent mass, but eventually dissolves, and is converted into oxalic acid;
with strong caustic alkaline lyes, in a hot state, it swells up excessively, dissolves into a
homogeneous liquid, and changes into a blackish-brown mass, containing oxalic and
acetic acids.
The composition of wood has been examined by Gay Lussac and Thenard, and
Dr. Prout. The first two chemists found it to consist, in 100 parts, of—
| |
Oak. |
Beech. |
| Carbon |
52 |
·53 |
51 |
·45 |
| Hydrogen |
5 |
·69 |
5 |
·82 |
| Oxygen |
41 |
·78 |
42 |
·73 |
According to Dr. Prout, the oxygen and hydrogen are in the exact proportions to form
water. Willow contains 50, and box 49·8 per cent. of carbon; each containing, therefore,
very nearly 44·444 of oxygen, and 5·555 of hydrogen. In the analyses of Gay
Lussac and Thenard, there is a great excess of hydrogen above what the oxygen requires
to form water. Authenrieth stated, some years ago, that he found that fine sawdust,
mixed with a sufficient quantity of wheat flour, made a coherent dough with water,
which formed an excellent food for pigs; apparently showing that the digestive organs
of this animal could operate the same sort of change upon wood as sulphuric acid does.
Table of the Distillation of One Pound of Wood, dried, at 86° Fahr.
| Name of the wood. |
Weight
of
wood acid. |
One ounce
of the acid
saturates
of carbonate
of potash. |
Weight
of the
combustible
oil. |
Weight
of the
charcoal. |
| |
Ounces. |
Grains. |
Ounces. |
Ounces. |
| White birch |
7 |
|
44 |
|
1 |
1⁄4 |
3 |
3⁄4 |
| Red beech |
7 |
|
44 |
|
1 |
1⁄2 |
3 |
3⁄4 |
| Prick wood (spindle tree) |
7 |
1⁄2 |
40 |
|
1 |
3⁄4 |
3 |
1⁄2 |
| Large leaved linden |
6 |
3⁄4 |
41 |
|
2 |
|
3 |
3⁄4 |
| Red or scarlet oak |
7 |
|
40 |
|
1 |
1⁄2 |
4 |
1⁄4 |
| White beech |
6 |
1⁄2 |
40 |
|
1 |
3⁄4 |
3 |
3⁄4 |
| Common ash |
7 |
1⁄2 |
34 |
|
1 |
1⁄2 |
3 |
1⁄2 |
| Horse chestnut |
7 |
1⁄2 |
31 |
|
1 |
1⁄2 |
3 |
1⁄2 |
| Italian poplar |
7 |
1⁄4 |
30 |
|
1 |
1⁄2 |
3 |
3⁄4 |
| Silver poplar |
7 |
1⁄4 |
30 |
|
1 |
1⁄4 |
3 |
3⁄4 |
| White willow |
7 |
1⁄4 |
28 |
|
1 |
1⁄2 |
3 |
1⁄2 |
| Root of the sassafras laurel |
6 |
3⁄4 |
29 |
|
1 |
3⁄4 |
4 |
1⁄4 |
| Wild service tree |
7 |
|
28 |
|
1 |
3⁄4 |
3 |
1⁄2 |
| Basket willow |
8 |
|
27 |
|
1 |
1⁄2 |
3 |
1⁄2 |
| Dogberry tree |
7 |
|
27 |
|
2 |
|
3 |
1⁄2 |
| Buckthorn |
7 |
1⁄2 |
26 |
|
1 |
1⁄2 |
3 |
1⁄2 |
| Logwood |
7 |
3⁄4 |
26 |
|
1 |
1⁄2 |
4 |
|
| Alder |
7 |
1⁄4 |
22 |
|
1 |
1⁄2 |
3 |
1⁄2 |
| Juniper |
7 |
1⁄4 |
23 |
|
1 |
3⁄4 |
3 |
1⁄2 |
| White fir (deal) |
6 |
1⁄2 |
23 |
|
2 |
1⁄4 |
3 |
1⁄2 |
| Common pine wood |
6 |
3⁄4 |
22 |
|
1 |
3⁄4 |
3 |
1⁄2 |
| Savine tree |
7 |
|
20 |
|
1 |
3⁄4 |
3 |
3⁄4 |
| Red deal (pine) |
6 |
1⁄2 |
18 |
|
2 |
1⁄4 |
3 |
3⁄4 |
| Guiac wood |
6 |
|
16 |
|
2 |
1⁄2 |
4 |
1⁄4 |
[1308]
WOOF, is the same as Weft.
WOOLLEN MANUFACTURE. In reference to textile fabrics, sheep’s wool is
of two different sorts, the short and the long stapled; each of which requires different
modes of manufacture in the preparation and spinning processes, as also in the treatment
of the cloth after it is woven, to fit it for the market. Each of these is, moreover, distinguished
in commerce by the names of fleece wools and dead wools, according as they
have been shorn at the usual annual period from the living animal, or are cut from its
skin after death. The latter are comparatively harsh, weak, and incapable of imbibing
the dyeing principles, more especially if the sheep has died of some malignant distemper.
The annular pores, leading into the tubular cavities of the filaments, seem, in this case,
to have shrunk and become obstructed. The time of year for sheep-shearing most
favourable to the quality of the wool, and the comfort of the animal, is towards the end
of June and beginning of July;—the period when Lord Leicester holds his celebrated
rural fête for that interesting purpose.
The wool of the sheep has been surprisingly improved, by its domestic culture. The
mouflon (Ovis aries), the parent stock from which our sheep is undoubtedly derived,
and which is still found in a wild state upon the mountains of Sardinia, Corsica, Barbary,
Greece, and Asia Minor, has a very short and coarse fleece, more like hair than wool.
When this animal is brought under the fostering care of man, the rank fibres gradually
disappear; while the soft wool round their roots, little conspicuous in the wild animal,
becomes singularly developed. The male most speedily undergoes this change, and
continues ever afterwards to possess far more power in modifying the fleece of the
offspring, than the female parent. The produce of a breed from a coarse-woolled ewe, and
a fine-woolled ram, is not of a mean quality between the two, but half-way nearer that
of the sire. By coupling the female thus generated, with such a male as the former,
another improvement of one-half will be obtained, affording a staple three-fourths finer
than that of the grandam. By proceeding inversely, the wool would be as rapidly
deteriorated. It is, therefore, a matter of the first consequence in wool husbandry, to
exclude from the flock all coarse-fleeced rams.
Long wool is the produce of a peculiar variety of sheep, and varies in the length of
its fibres from 3 to 8 inches. Such wool is not carded like cotton, but combed like
flax, either by hand or appropriate machinery. Short wool is seldom longer than 3 or
4 inches; it is susceptible of carding and felting, by which processes the filaments
become first convoluted, and then densely matted together. The shorter sorts of the
combing wool are used principally for hosiery, though of late years the finer kinds
have been extensively worked up into merino and mousseline-de-laine fabrics. The
longer wools of the Leicestershire breed are manufactured into hard yarns, for worsted
pieces, such as waistcoats, carpets, bombasines, poplins, crapes, &c.
The wool of which good broad cloth is made, should be not only shorter, but, generally
speaking, finer and softer than the worsted wools, in order to fit them for the fulling
process. Some wool-sorters and wool-staplers acquire by practice great nicety of discernment
in judging of wools by the touch and traction of the fingers. Two years ago, I
made a series of observations upon different wools, and published the results. The
filaments of the finer qualities varied in thickness from 1⁄1100 to 1⁄1500 of an inch; their
structure is very curious, exhibiting, in a good achromatic microscope, at intervals of
about 1⁄300 of an inch, a series of serrated rings, imbricated towards each other, like
the joints of Equisetum, or rather like the scaly zones of a serpent’s skin. See
Philosophy of Manufactures, figs. 11, 12., page 91. second edition.
There are four distinct qualities of wool upon every sheep; the finest being upon the
spine, from the neck to within 6 inches of the tail, including one-third of the breadth of
the back; the second covers the flanks between the thighs and the shoulders; the third
clothes the neck and the rump; and the fourth extends upon the lower part of the neck
and breast down to the feet, as also upon a part of the shoulders and the thighs, to the
bottom of the hind quarter. These should be torn asunder, and sorted, immediately
after the shearing.
The harshness of wools is dependent not solely upon the breed of the animal, or the
climate, but is owing to certain peculiarities in the pasture, derived from the soil. It
is known, that in sheep fed upon chalky districts, wool is apt to get coarse; but in those
upon a rich loamy soil, it becomes soft and silky. The ardent sun of Spain renders the
fleece of the Merino breed harsher than it is in the milder climate of Saxony. Smearing
sheep with a mixture of tar and butter, is deemed favourable to the softness of their
wool.
All wool, in its natural state, contains a quantity of a peculiar potash-soap, secreted
by the animal, called in this country the yolk; which may be washed out by water alone,
with which it forms a sort of lather. It constitutes from 25 to 50 per cent. of the wool,
being most abundant in the Merino breed of sheep; and however favourable to the
growth of the wool on the living animal, should be taken out soon after it is shorn, lest[1309]
it injure the fibres by fermentation, and cause them to become hard and brittle. After
being washed in water, somewhat more than lukewarm, the wool should be well pressed,
and carefully dried. England grows annually about 1,000,000 packs of wool, and
imports 100,000 bags.
Wool imported into the United Kingdom, in 1836, 64,239,977 lbs.; in 1837,
48,356,121 lbs. Retained for home consumption, in 1836, 60,724,795 lbs.; in 1837,
43,148,297 lbs. Duty received, in 1836, £190,075; in 1837, £118,519.
Having premised these general observations on wool, I shall now proceed to treat
of its manufacture, beginning with that of wool-combing, or
THE WORSTED MANUFACTURE.
In this branch of business, a long stapled and firm fibre is required to form a smooth
level yarn, little liable to shrink, curl, or felt in weaving and finishing the cloth. It
must not be entangled by carding, but stretched in lines as parallel as possible, by a
suitable system of combing, manual or mechanical.
When the long wool is brought into the worsted factory, it is first of all washed by
men with soap and water, who are paid for their labour by the piece, and are each
assisted by a boy, who receives the wool as it issues from between the drying squeezers
(see Bleaching). The boy carries off the wool in baskets, and spreads it evenly upon
the floor of the drying-room, usually an apartment over the boilers of the steam-engine,
which is thus economically heated to the proper temperature. The health of the boys
employed in this business is found to be not at all injured.
The wool, when properly dried, is transferred to a machine called the plucker, which
is always superintended by a boy of 12 or 14 years of age, being very light work. He
lays the tresses of wool pretty evenly upon the feed-apron, or table covered with an
endless moving web of canvas, which, as it advances, delivers the ends of the long tufts
to a pair of fluted rollers, whence it is introduced into a fanning apparatus, somewhat
similar to the willow employed in the cotton manufacture, which see. The filaments
are turned out, at the opposite end of this winnowing machine, straightened, cleaned,
and ready for the combing operation. According to the old practice of the trade, and still
for the finer descriptions of the long staple,
according to the present practice, the wool
is carded by hand. This is far more severe
labour than any subservient to machinery,
and is carried on in rooms rendered close
and hot by the number of stoves requisite
to heat the combs, and so enable them to
render the fibres soft, flexible, and elastic.
This is a task at which only robust men
are engaged. They use three implements:
1. a pair of combs for each person; 2. a post, to which one of the combs can be fixed;
3. a comb-pot, or small stove for heating the teeth of the combs. Each comb is composed
either of two or three rows of pointed tapering steel teeth, b,
fig. 1203., disposed in two or three parallel planes, each row being
a little longer than the preceding. They are made fast at the
roots to a wooden stock or head c, which is covered with horn,
and has a handle d, fixed into it at right angles to the lines of the
teeth. The spaces between these two or three planes of teeth,
is about one-third of an inch at their bottoms, but somewhat
more at their tips. The first combing, when the fibres are most
entangled, is performed with the two-row toothed combs; the
second or finishing combing, with the three-row toothed.
In the workshop a post is planted (fig. 1204.) upright, for
resting the combs occasionally upon, during the operation.
An iron stem g, projects from it horizontally, having its end
turned up, so as to pass through a hole in the handle of the
comb. Near its point of insertion into the post, there is another
staple point h, which enters into the hollow end of the
handle; which, between these two catches, is firmly secured
to the post. The stove is a very simple affair, consisting merely
of a flat iron plate, heated by fire or steam, and surmounted with
a similar plate, at an interval sufficient to allow the teeth to be
inserted between them at one side, which is left open, while the space between their
edges, on the other sides, is closed to confine the heat.
In combing the wool, the workman takes it up in tresses of about four ounces each,
sprinkles it with oil, and rolls it about in his hands, to render all the filaments equally[1310]
unctuous. Some harsh dry wools require one-sixteenth their weight of oil, others no
more than a fortieth. He next attaches a heated comb to the post, with its teeth pointed
upwards, seizes one-half of the tress of wool in his hands, throws it over the teeth, then
draws it through them, and thus repeatedly: leaving a few straight filaments each time upon
the comb. When the comb has in this way collected all the wool, it is placed with its points
inserted into the cell of the stove, with the wool hanging down outside, exposed to
the influence of the heat. The other comb, just removed in a heated state from the
stove, is planted upon the post, and furnished in its turn with the remaining two-ounce
tress of wool; after which it supplants the preceding at the stove. Having both combs
now hot, he holds one of them with his left hand over his knee, being seated upon a low
stool, and seizing the other with his right hand, he combs the wool upon the first, by
introducing the teeth of one comb into the wool stuck in the other, and drawing them
through it. This manipulation is skilfully repeated, till the fibres are laid truly parallel,
like a flat tress of hair. It is proper to begin by combing the tips of the tress, and to
advance progressively, from the one end towards the other, till at length the combs are
worked with their teeth as closely together as is possible, without bringing them into collision.
If the workman proceeded otherwise, he would be apt to rupture the filaments,
or tear their ends entirely out of one of the combs. The flocks left at the end of the
process, because they are too short for the comber to grasp them in his hand, are called
noyls. They are unfit for the worsted spinner, and are reserved for the coarse cloth
manufacture.
The wool finally drawn off from the comb, though it may form a uniform tress of
straight filaments, must yet be combed again at a somewhat lower temperature, to prepare
it perfectly for the spinning operation. From ten to twelve slivers are then
arranged in one parcel.
To relieve the workman from this laborious and not very salubrious task, has been
the object of many mechanical inventions. One of these, considerably employed in this
country and in France, is the invention of the late Mr. John Collier, of Paris, for which
a patent was obtained in England, under the name of John Platt, of Salford, in
November, 1827. It consists of two comb-wheels, about ten feet in diameter, having
hollow iron spokes filled with steam, in order to keep the whole apparatus at a proper
combing heat. The comb forms a circle, made fast to the periphery of the wheel, the
teeth being at right angles to the plane of the wheel. The shafts of the two wheels are
mounted in a strong frame of cast iron; not, however, in horizontal positions, but inclined
at acute angles to the horizon, and in planes crossing each other, so that the teeth of one
circular comb sweep with a steady obliquity over the teeth of the other, in a most
ingenious manner, with the effect of combing the tresses of wool hung upon them. The
proper quantity of long wool, in its ordinary state, is stuck in handfuls upon the wheel,
revolving slowly, by a boy, seated upon the ground at one side of the machine. Whenever
the wheel is dressed, the machine is made to revolve more rapidly, by shifting its
driving-band on another pulley; and it is beautiful to observe the delicacy and precision
with which it smoothes the tangled tress. When the wools are set in rapid rotation, the
loose ends of the fleece, by the centrifugal force, are thrown out, in the direction of radii,
upon the teeth of the other revolving comb-wheel, so as to be drawn out and made
truly straight. The operation commences upon the tips of the tresses, where the wheels,
by the oblique posture of their shafts, are at the greatest distance apart; but as the
planes slowly approach to parallelism, the teeth enter more deeply into the wool, till they
progressively comb the whole length of its fibres. The machines being then thrown out
of geer, the teeth are stript of the tresses by the hand of the attendant; the noyls, or
short refuse wool, being also removed, and kept by itself.
This operation being one of simple superintendence, not of handicraft effort and skill,
like the old combing of long wool, is now performed by boys or girls of 13 and 14
years of age; and places in a striking point of view the influence of automatic
mechanism, in so embodying dexterity and intelligence in a machine, as to render the
cheap and tractable labour of children a substitute for the high-priced and often
refractory exertions of workmen too prone to capricious combinations. The chief
precaution to be taken with this machine, is to keep the steam-joints tight, so as not to
wet the apartments, and to provide due ventilation for the operatives.
The following machine, patented by James Noble, of Halifax, worsted-spinner, in
February, 1834, deserves particular notice, as its mode of operation adapts it well also for
heckling flax. In fig. 1205. the internal structure is exhibited. The frame-work a, a,
supports the axle of a wheel b, b, in suitable bearings on each side. To the face of this
wheel is affixed the eccentric or heart-wheel cam c, c. On the upper part of the periphery
of this cam or heart-wheel, a lever d, d, bears merely by its gravity; one end of
which lever is connected by a joint to the crank e. By the rotation of the crank e, it
will be perceived that the lever d, will be slidden to and fro on the upper part of the
periphery of the eccentric or heart-wheel cam c, the outer end of the lever d, carrying[1311]
the upper or working comb or needle-points f, as it moves, performing an elliptical
curve, which curve
will be dependent
upon the position
of the heart-wheel
cam c, that guides
it. A movable
frame g, carries a
series of points h,
which are to constitute
the lower
comb or frame of
needles. Into these
lower needles the
rough uncombed
wool is to be fed by hand, and to be drawn out and combed straight by the movements
of the upper or working comb.
As it is important, in order to prevent waste, that the ends of the wool should be
first combed out, and that the needle-points should be made to penetrate the wool progressively,
the movable frame g, is in the first instance placed as far back as possible;
and the action of the lever d, during the whole operation, is so directed by the varying
positions of the cam-wheel, as to allow the upper comb to enter at first a very little
way only into the wool; but as the operation of combing goes on, the frame with the
lower combs is made to advance gradually, and the relative positions of the revolving
heart cam-wheel c, being also gradually changed, the upper or working needles are at
length allowed to be drawn completely through the wool, for the purpose of combing
out straight the whole length of its fibre.
In order to give to the machine the necessary movements, a train of toothed wheels
and pinions is mounted, mostly on studs attached to the side of the frame; which
train of wheels and pinions is shown by dots in the figure, to avoid confusion. The
driving power, a horse or steam-engine, is communicated by a band to a rigger on the
short axle i; which axle carries a pinion, taking into one of the wheels of the train.
From this wheel the crank e, that works the lever d, is driven; and also, by geer from
the same pinion, the axle of the wheel b, carrying the eccentric or heart-wheel cam,
is also actuated, but slower than the crank-axle.
At the end of the axle of the wheel b, and cam c, a bevel pinion is affixed, which
geers into a corresponding bevel pinion on the end of the lateral shaft k. The reverse
end of this shaft has a worm or endless screw l, taking into a toothed wheel m; and
this last-mentioned toothed wheel geers into a rack at the under part of the frame g.
It will hence be perceived, that by the movements of the train of wheels, a slow
motion is given to the frame g, by which the lower
needles carrying the wool are progressively advanced
as the operation goes on; and also, that by
the other wheels of the train, the heart-wheel cam
is made to rotate, for the purpose of giving such
varying directions to the stroke of the lever which
slides upon its periphery, and to the working
comb, as shall cause the comb to operate gradually
upon the wool as it is brought forward. The construction
of the frames which hold the needles, and
the manner of fixing them in the machine, present
no features of importance; it is therefore unnecessary
to describe them farther, than to say, that
the heckles are to be heated when used for combing
wool. Instead of introducing the wool to be
combed into the lower needles by hand, it is sometimes
fed in, by means of an endless feeding-cloth,
as shown in fig. 1206. This endless cloth is distended
over two rollers, which are made to revolve,
for the purpose of carrying the cloth with the wool
forward, by means of the endless screw and pinions.
A slight variation in the machine is shown at
fig. 1207., for the purpose of combing wool of long
fibre, which differs from the former only in placing
the combs or needle points upon a revolving cylinder
or shaft. At the end of the axle of this shaft,
there is a toothed wheel, which is actuated by an[1312]
endless screw upon a lateral shaft. The axle of the cylinder on which the needles are fixed,
is mounted in a movable frame or carriage, in order that the points of the needles may,
in the first instance, be brought to act upon the ends of the wool only, and ultimately be
so advanced as to enable the whole length of the fibres to be drawn through. The
progressive advancement of this carriage, with the needle cylinder, is effected by the
agency of the endless screw on the lateral shaft before mentioned.
Some combing-machines reduce the wool into a continuous sliver, which is ready
for the drawing-frame; but the short slivers produced by the hand combing, must
be first joined together, by what is called planking. These slivers are rolled up by the
combers ten or twelve together, in balls called tops, each of which weighs half a
pound. At the spinning-mill these are unrolled, and the slivers are laid on a long plank or
trough, with the ends lapping over, in order to splice the long end of one sliver into the
short end of another. The long end is that which was drawn off first from the comb, and
contains the longer fibres; the short is that which comes last from the comb, and
contains the shorter. The wool-comber lays all the slivers of each ball the same way,
and marks the long end of each by twisting up the end of the sliver. It is a curious
circumstance, that when a top or ball of slivers is unrolled and stretched out straight,
they will not separate from each other without tearing and breaking, if the separation
is begun at the short ends; but if they are first parted at the long ends, they will readily
separate.
The machine for combing long wool, for which Messrs. Donisthorpe and Rawson
obtained a patent in April, 1835, has been found to work well, and therefore merits
a detailed description:—
Fig. 1208. is an elevation; fig.
1209. an end view; and fig. 1210.
a plan; in which a, a, is the
framing; b, the main shaft, bearing
a pinion which drives the
wheel and shaft c, in geer with the
wheel d, on the shaft e. Upon
each of the wheels c and d, there
are two projections or studs f,
which cause the action of the
combs g, g, of which h, h, are the
tables or carriages. These are capable
of sliding along the upper
guide rails of the framing a.
Through these carriages or tables
h, h, there are openings or slits,
shown by dotted lines, which act
as guides to the holders i, i, of the
combs g, g, rendering the holders
susceptible of motion at right
angles to the course pursued by the
tables h. The combs are retained in the holders i, i, by means of the lever handles j, j,
which move upon inclined surfaces, and are made to press on the surface of the heads
of the combs g, g, so as to be retained in their places; and they are also held by studs
affixed to the holders, which pass into the comb-heads. From the under side of the
tables, forked projections i, i, stand out, which pass through the openings or slits formed
in the tables h h; these projections are worked from side to side by the frame k, k,
which turning on the axis or shaft l, l, is caused to vibrate, or rock to and fro, by the
arms m, moved by the eccentric groove n, made fast to the shaft e. The tables h, are
drawn inwards, by weights suspended on cords or straps o, o, which pass over friction
pulleys p, p; whereby the weights have a constant tendency to draw the combs into the
centre of the machine, as soon as it is released by the studs f, passing beyond the
projecting arms g, on the tables. On the shaft c, a driving-tooth or catch r, is fixed,
which takes into the ratchet wheel s, and propels one of its teeth at every revolution
of the shaft c. This ratchet wheel turns on an axis at t; to the ratchet the pulley v is
made fast, to which the cord or band w is secured, as also to the pulley x, on the
shaft y. On the shaft y, there are two other pulleys z, z, having the cords or bands A, A,
made fast to them, and also to the end of the gauge-plates B, furnished with graduated
steps, against which the tables h, h, are drawing at each operation of the machine.
In proportion as these gauge-plates are raised, the nearer the carriages or tables h, will
be able to advance to the centre of the machine, and thus permit the combs g, g,
to lay hold of, and comb, additional lengths of the woolly fibres. The gauge-plates B,
are guided up by the bars C, which pass through openings, slots, or guides, made in
the framing a, as shown by D.
[1313]
To the ratchet wheel s, an inclined projection E, is made fast, which in the course of
the rotation of the ratchet wheel, comes under the lever F, fixed to the shaft G, that turns
in bearings H. To this shaft the levers I and J, are also
fixed; I serving to throw out the click or catch K, from the
ratchet wheel, by which the parts of the machine will be released,
and restored to positions ready for starting again. The
lever J, serves to slide the drum upon the driving shaft b, out
of geer, by means of the forked handle L, when the machine
is to be stopped, whenever it has finished combing a certain
quantity of wool. The combs which hold the wool have a
motion upwards, in order to take the wool out of
the way of the combs g, g, as these are drawn into
the centre of the machine; while the holding combs
descend to lay the wool among the points of the
combs g, g. For obtaining this upward and downward
motion, the combs M, M, are placed upon the
frame N, and retained there just as the combs g, g,
are upon the holders i, i. The framing N is made
fast to the bar or spindle O, which moves vertically
through openings in the cross-head P, and the
cross-framing of the machine Q; from the top of
which, there is a strap passes over pulleys with a
weight suspended to it; the cross-head being supported
by the two guide-rods R, fixed to the cross-framing
Q. It is by the guide-rods R, and the spindle
O, that the frame N is made to move up and down;
while the spindle is made to rise by the studs f, as
the wheels c and d come successively under the studs
s, on the spindle O.
A quantity of wool is to be placed on each of the
combs g, g, and M, M, the machine being in the position
shown in fig. 1210. When the main shaft b, is set in motion, it will drive by
its pinion the toothed wheel c, and therefrom the remaining parts of the machine.
The first effect of the movement
will be to raise the combs M, M,
sufficiently high to remove the
wool out of the way of the combs
g, g, which will be drawn towards
the centre of the machine,
as soon as they are released by
the studs f, passing the projecting
arms q, on the tables h;
but the distance between the
combs g, g, and the combs H, H,
will depend on the height to
which the gauge-plates B, have
been raised. These plates are
raised one step at each revolution of the shaft c; the combs g, g, will therefore
be continually approaching more nearly to the combs M, M, till the plates B, are so
much raised as to permit the tables h, to approach the plates B, below the lowest step
or graduation, when the machine will continue to work. Notwithstanding the plates
B, continuing to rise, there being only parallel surfaces against which the tables come,
the combs g, g, will successively come to the same position, till the inclined projection
E, on the ratchet wheel s, comes under the lever F, which will stop the machine. The
wool which has been combed, is then to be removed, and a fresh quantity introduced.
It should be remarked, that the combs g, g, are continually moving from side to side of
the machine, at the same time that they are combing out the wool. The chief object of
the invention is obviously to give the above peculiar motions to the combs g, g, and
M, M; which may be applied also to combing goat-hair.
For the purposes of the worsted manufacture, wool should be rendered inelastic to a
considerable degree, so that its fibres may form long lines, capable of being twisted into
straight level yarn. Mr. Bayliffe, of Kendal, has sought to accomplish this object,
first, by introducing into the drawing machine a rapidly revolving wheel, in contact with
the front drawing roller, by whose friction the filaments are heated, and at the same
time deprived of their curling elasticity; secondly, by employing a movable regulating
roller, by which the extent of surface on the periphery of the wheel that the lengths of[1314]
wool is to act upon, may be increased or diminished at pleasure, and, consequently, the
effect regulated or tempered as the quality of the wool may require; thirdly, the
employment of steam in a rotatory drum,
or hollow wheel, in place of the wheel
first described, for the purpose of heating
the wool, in the process of drawing, in
order to facilitate the operation of straightening
the fibres.
These objects may be effected in several
ways; that is, the machinery may be
variously constructed, and still embrace
the principles proposed. Fig. 1211. shows
one mode:—a, is the friction wheel; b,
the front drawing roller, placed in the
drawing frame in the same way as usual;
the larger wheel a, constituting the lower
roller of the pair of front drawing rollers;
c, and d, are the pair of back drawing
rollers, which are actuated by geer connected to the front rollers, as in the ordinary
construction of drawing machines, the front rollers moving very considerably faster
than the back rollers, and, consequently, drawing or extending the fibres of the sliver
of wool, as it passes through between them; e, is a guide roller, bearing upon the
periphery of the large wheel; f, is a tension roller, which presses the fibres of the wool
down upon the wheel a.
Now, supposing the back rollers c and d to be turned with a given velocity, and the
front roller b to be driven much faster, the effect would be, that the fibres of wool constituting
the sliver, passing through the machine, would be considerably extended between
b and d, which is precisely the effect accomplished in the ordinary drawing frame;
but the wheel a, introduced into the machine in place of the lower front drawing roller,
being made to revolve much faster than b, the sliver of wool extended over the upper
part of its periphery from b, to the tension roller f, will be subjected to very considerable
friction from the contact; and, consequently, the natural curl of the wool will be
taken out, and its elasticity destroyed, which will enable the wool to proceed in a connected
roving down to the spindle or flyer h, where it becomes twisted or spun into a
worsted thread.
In order to increase or diminish the extent to which the fibres of wool are spread
over the periphery of the wheel a, a regulating roller is adapted to the machine, as
shown at g, in place of the tension roller f. This regulating roller g, is mounted by its
pivots in bearings on the circular arms h, shown by dots. These circular arms turn
loosely upon the axle of the wheel a, and are raised or depressed by a rack and a winch,
not shown in the figure; the rack taking into teeth on the periphery of the circular
arms. It will hence be perceived, that by raising the circular arms, the roller g, will be
carried backward, and the fibres of wool pressed upon the periphery of the wheel to a
greater extent. On the contrary, the depression of the circular arms will draw the
roller g, forward, and cause the wool to be acted upon by a smaller portion of the periphery
of the wheel a, and consequently subject it to less friction.
When it is desired to employ steam for the purpose of heating the wool, the wheel a,
is formed as a hollow drum, and steam from a boiler, in any convenient situation, is conveyed
through the hollow axle to the interior of the drum, which, becoming heated by
that means, communicates heat also to the wool, and thereby destroys its curl and
elasticity.
Breaking-frame.—Here the slivers are planked, or spliced together, the long end of
one to the short end of another; after which they are drawn out and extended by the
rollers of the breaking-frame. A sketch of this machine is given in fig. 1212. It consists
of four pairs of rollers A, B, C, D. The first pair A, receives the wool from the inclined
trough E, which is the planking-table. The slivers are unrolled, parted, and
hung loosely over a pin, in reach of the attendant, who takes a sliver, and lays it flat in
the trough, and the end is presented to the rollers A, which being in motion, will draw
the wool in; the sliver is then conducted through the other rollers, as shown in the
figure: when the sliver has passed half through, the end of another sliver is placed
upon the middle of the first, and they pass through together; when this second is
passed half through, the end of a third is applied upon the middle of it, and in this way
the short slivers produced by the combing are joined into one regular and even sliver.
The lower roller C receives its motion from the mill, by means of a pulley upon the
end of its axis, and an endless strap. The roller which is immediately over it, is borne
down by a heavy weight, suspended from hooks, which are over the pivots of the upper
roller. The fourth pair of rollers D, moves with the same velocity as C, being turned[1315]
by means of a small wheel upon the end of the axis of the roller C, which turns a wheel
of the same size upon the axis of the roller D, by means of an intermediate wheel d,
which makes both rollers turn the same way round. The first and second pairs of
rollers, A and B,
move only one-third
as quick as
C and D, in order
to draw out the
sliver between B
and C to three
times the length
it was when put
on the planking-table.
The slow
motion of the rollers
A, is given
by a large wheel
a, fixed upon the
axis of the roller
A, and turned by
the intermediate
cog-wheels b, c,
and d; the latter
communicates between the rollers C and D. The pinions on the rollers C and D being only
one-third the size of the wheel a, C and D turn three times as fast as A, for b, c, and d, are
only intermediate wheels. The rollers B turn at the same rate as A. The upper roller
C is loaded with a heavy weight, similar to the rollers A; but the other rollers, B and
D, are no further loaded than the weight of the rollers.
The two pairs of rollers A, B, and C, D, are mounted in separate frames; and that frame
which contains the third and fourth pairs C, D, slides upon the cast-iron frame F, which
supports the machine, in order to increase or diminish the distance between the rollers
B and C. There is a screw f, by which the frame of the rollers is moved, so as to adjust
the machine according to the length of the fibres of the wool. The space between B
and C should be rather more than the length of the fibres of the wool. The intermediate
wheels b and c, are supported upon pieces of iron, which are movable on
centres; the centre for the piece which supports the wheel b is concentric with the axis
of the roller A; and the supporting piece for the wheel c is fitted on the centre of the
wheel d. By moving these pieces the intermediate wheels b and c can be always kept in
contact, although the distance between the rollers is varied at times. By means of this
breaking-frame, the perpetual sliver, which is made up by planking the sliver together,
is equalized, and drawn out three times in length, and delivered into the can G.
Drawing-frame.—Three of these cans are removed to the drawing-frame, which is
similar to the breaking-frame, except that there is no planking-table E. There are
five sets of rollers, all fixed upon one common frame F, the breaking-frame, which we
have described, being the first. As fast as the sliver comes through one set of rollers, it
is received into a can, and then three of these cans are put together, and passed again
through another set of rollers. In the whole, the wool must pass through the breaker
and four drawing-frames before the roving is begun. The draught being usually four
times at each operation of drawing, and three times in the breaking, the whole will be
3 + 4 + 4 + 4 + 4 = 768; but to suit different sorts of wool, the three last drawing-frames
are capable of making a greater draught, even to five times, by changing the
pinions; accordingly the draught will be 3 + 4 + 5 + 5 + 5 = 1500 times.
The size of the sliver is diminished by these repeated drawings, because only three
slivers are put together, and they are drawn out four times; so that, in the whole, the
sliver is reduced to a fourth or a ninth of its original bulk.
The breaking-frame and drawing-frame which are used when the slivers are prepared
by the combing-machines, are differently constructed; they have no planking-table,
but receive three of the perpetual slivers of the combing-machine from as many
tin cans, and draw them out from ten to twelve times. In this case, all the four
rollers contribute to the operation of drawing: thus the second rollers B, move 21⁄2
times as fast as the rollers A; the third rollers C, move 8 times as fast as A; and the
fourth rollers E, move 101⁄2 times as fast as A. In this case, the motion is given to the
different rollers by means of bevelled wheels, and a horizontal axis, which extends
across the ends of all the four rollers, to communicate motion from one pair of rollers
to another.
There are three of these systems of rollers, which are all mounted on the same
frame; and the first one through which the wool passes, is called the breaking-frame;[1316]
but it does not differ from the others, which are called drawing-frames. The slivers
which have passed through one system of rollers, are collected four or five together, and
put through the drawing-rollers. In all, the slivers pass through three drawings, and
the whole extension is seldom less than 1000 times, and for some kinds of wool much
greater.
After the drawing of the slivers is finished, a pound weight is taken, and is measured
by means of a cylinder, in order to ascertain if the drawing has been properly conducted;
if the sliver does not prove of the length proposed, according to the size of
worsted which is intended to be spun, the pinions of some of the drawing-frames are
changed, to make the draught more or less, until it is found by experiment that one
pound of the sliver measures the required length.
Roving-frame.—This is provided with rollers, the same as the drawing-frames: it
takes in one or two slivers together, and draws them out four times. By this extension,
the sliver becomes so small, that it would break with the slightest force, and it is
therefore necessary to give some twist; this is done by a spindle and flyer. See
Roving, under Cotton Manufacture.
Spinning-frame.—This is so much like the roving-frame, that a short description
will be sufficient. The spindles are more delicate, and there are three pairs of rollers,
instead of two; the bobbins, which are taken off from the spindles of the roving-frame,
when they are quite full, are stuck upon skewers, and the roving which proceeds
from them is conducted between the rollers. The back pair turns round slowly; the
middle pair turns about twice for once of the back rollers; and the front pair makes
from twelve to seventeen turns for one turn of the back roller, according to the degree
of extension which is required.
The spindles must revolve very quickly in the spinning-frame, in order to give the
requisite degree of twist to the worsted. The hardest twisted worsted is called tammy
warp; and when the size of this worsted is such as to be 20 or 24 hanks to the pound
weight, the twist is about 10 turns in each inch of length. The least twist is given
to the worsted for fine hosiery, which is from 18 to 24 hanks to the pound. The
twist is from 5 to 6 turns per inch. The degree of twist is regulated by the size of
the whirls or pulleys upon the spindle, and by the wheel-work which communicates
the motion to the front rollers from the band-wheel, which turns the spindles.
It is needless to enter more minutely into the description of the spinning machinery,
because the fluted roller construction, invented by Sir Richard Arkwright, fully described
under Cotton Manufacture, is equally applicable to worsted. The difference between
the two, is chiefly in the distance between the rollers, which, in the worsted-frame,
is capable of being increased or diminished at pleasure, according to the length of the
fibres of the wool; and the draught or extension of the roving is far greater than in the
cotton.
Reeling.—The bobbins of the spinning-frame are placed in a row upon wires before
a long horizontal reel, and the threads from 20 bobbins are wound off together. The
reel is exactly a yard in circumference, and when it has wound off 80 turns, it rings a
bell; the motion of the reel is then stopped, and a thread is passed round the 80 turns
or folds which each thread has made. The reeling is then continued till another 80
yards is wound off, which is also separated by interweaving the same thread; each of
these separate parcels is called a ley, and when 7 such leys are reeled, it is called a hank,
which contains 560 yards. When this quantity is reeled off, the ends of the binding
thread are tied together, to bind each hank fast, and one of the rails of the reel is
struck to loosen the hanks, and they are drawn off at the end of the reel. These hanks
are next hung upon a hook, and twisted up hard by a stick; then doubled, and the
two parts twisted together to make a firm bundle. In this state, the hanks are weighed
by a small index-machine, which denotes what number of the hanks will weigh a pound,
and they are sorted accordingly into different parcels. It is by this means that the
number of the worsted is ascertained as the denomination for its fineness: thus No. 24.
means, that 24 hanks, each containing 560 yards, will weigh a pound, and so on.
This denomination is different from that used for cotton, because the hank of cotton
contains 840 yards, instead of 560; but in some places the worsted hank is made of the
same length as the cotton.
To pack up the worsted for market, the proper number of hanks is collected to
make a pound, according to the number which has been ascertained; these are weighed
as a proof of the correctness of the sorting, then tied up in bundles of one pound each,
and four of these bundles are again tied together. Then 60 such bundles are packed
up in a sheet, making a bale of 240 pounds, ready for market.
Of the treatment of short wool for the cloth manufacture.—Short wool resembles cotton,
not a little in the structure of its filaments, and is cleaned by the willy, as cotton is by
the willow, which opens up the matted fleece of the wool-stapler, and cleans it from
accidental impurities. Sheep’s wool for working into coarse goods, must be passed repeatedly[1317]
through this machine, both before and after it is dyed; the second last time
for the purpose of blending the different sorts together, and the last for imbuing the
fibres intimately with oil. The oiled wool is next subjected to a first carding operation
called scribbling, whereby it is converted into a broad thin fleece or lap, as cotton is by
the breaker-cards of a cotton mill. The woollen lap is then worked by the cards
proper, which deliver it in a narrow band or sliver. By this process the wool expands
greatly in all its dimensions; while the broken or short filaments get entangled by
crossing in every possible direction, which prepares them for the fulling operation. See
Carding, under Cotton Manufacture.
The slubbing machine, or billy, reduces the separate rolls of cardings into a continuous
slightly twisted spongy cord, which is sometimes called a roving. Fig. 1213. is a perspective
representation of the slubbing machine in most common use. A, A, is the
wooden frame; within which is the movable carriage D, D, which runs upon the lower
side rails at a, a, on friction wheels at 1, 2, to make it move easily backwards and
forwards from one end of the frame to the other. The carriage contains a series of
steel spindles, marked 3, 3, which receive rapid rotation from a long tin drum F, by
means of a series of cords passing round the pulley or whorl of each spindle. This
drum, 6 inches in diameter, is covered with paper, and extends across the whole breadth
of the carriage. The spindles are set nearly upright in a frame, and about 4 inches
apart; their under ends being pointed conically, turn in brass sockets called steps,
and are retained in their position by a small brass collet, which embraces each spindle
at about the middle of its length. The upper half of each spindle projects above the
top of the frame. The drum revolves horizontally before the spindles, having its axis a
little below the line of the whorls; and receives motion, by a pulley at one of its ends,
from an endless band which passes round a wheel E, like the large domestic wheel
formerly used in spinning wool by hand, and of similar dimensions. This wheel is
placed upon the outside of the main frame of the machine, and has its shafts supported
by upright standards upon the carriage D. It is turned by the spinner placed at Q, with
his right hand applied to a winch R, which gives motion to the drum, and thereby causes
the spindles to revolve with great velocity.
Each spindle receives a soft cylinder or carding of wool, which comes through beneath
a wooden roller C, C, at the one end of the frame. This is the billy roller, so much
talked of in the controversies between the operatives and masters in the cotton factories,
as an instrument of cruel punishment to children, though no such machine has been
used in cotton mills for half a century at least. These woollen rolls proceed to the
series of spindles, standing in the carriage, in nearly a horizontal plane. By the
alternate advance and retreat of the carriage upon its railway, the spindles are made to
approach to, and recede from, the roller C, with the effect of drawing out a given length
of the soft cord, with any desired degree of twist, in the following manner:—
The carding rolls are laid down straight, side by side, upon the endless cloth,
strained in an inclined direction between two rollers, one of which is seen at B, and the[1318]
other lies behind C. One carding is allotted to a spindle; the total number of each in
one machine being from 50 to 100. The roller C, of light wood, presses gently with
its weight upon the cardings, while they move onwards over the endless cloth, with
the running out of the spindle carriage. Immediately in front of the said roller,
there is a horizontal wooden rail or bar G, with another beneath it, placed across the
frame. The carding is conducted through between these two bars, the movable upper
one being raised to let any aliquot portion of the roll pass freely. When this bar is
again let down, it pinches the spongy carding fast; whence this mechanism is called the
clasp. It is in fact the clove, originally used by Hargreaves in his cotton-jenny. The
movable upper rail G, is guided between sliders, and a wire 7, descends from it to a
lever C. When the spindle carriage D, D, is wheeled close home to the billy roller, a
wheel 5, lifts the end 6 of the lever, which, by the wire 7, raises the upper bar or rail G,
so as to open the clasp, and release all the card rolls. Should the carriage be now
drawn a little way from the clasp bars, it would tend to pull a corresponding length of
the cardings forward from the inclined plane B, C. There is a small catch, which lays
hold of the upper bar of the clasp G, and hinders it from falling till the carriage has
receded to a certain distance, and has thereby allowed from 7 to 8 inches of the cardings to
be taken out. A stop upon the carriage then comes against the catch, and withdraws it;
thus allowing the upper rail to fall and pinch the carding, while the carriage, continuing
to recede, draws out or stretches that portion of the roll which is between the clasp and
the spindle points. But during this time the wheel has been turned to keep the spindles
revolving, communicating the proper degree of twist to the cardings in proportion to
their extension, so as to prevent them from breaking.
It might be imagined that the slubbing cords would be apt to coil round the spindles;
but as they proceed in a somewhat inclined direction to the clasp, they receive
merely a twisting motion, continually slipping over the points of the spindles, without
getting wound upon them. Whenever the operative or slubber has given a due degree
of twist to the rovings, he sets about winding them upon the spindles into a conical
shape, for which purpose he presses down the faller-wire 8, with his left hand, so as to
bear it down from the points of the spindles, and place it opposite to their middle part.
He next makes the spindles revolve, while he pushes in the carriage slowly, so as to coil
the slubbing upon the spindle into a conical cop. The wire 8, regulates the winding-on
of the whole series of slubbings at once, and receives its proper angle of depression for
this purpose from the horizontal rail 4, which turns upon pivots in its ends, in brasses
fixed on the standards, which rise from the carriage D. By turning this rail on its
pivots, the wire 8 may be raised or lowered in any degree. The slubber seizes the rail
4 in his left hand, to draw the carriage out; but in returning it, he depresses the faller-wire,
at the same time that he pushes the carriage before him.
The cardings are so exceedingly tender, that they would readily draw out, or even
break, if they were dragged with friction upon the endless cloth of the inclined plane.
To save this injurious traction, a contrivance is introduced for moving the apron. A cord
is applied round the groove in the middle part of the upper roller, and after passing over
pulleys, as shown in the figure, it has a heavy weight hung at the one end, and a light
weight at the other, to keep it constantly extended, while the heavy weight tends to
turn the rollers with their endless cloth round in such a direction as to bring forward
the rovings, without putting any strain upon them. Every time that the carriage is
pushed home, the larger weight gets wound up; and when the carriage is drawn out,
the greater weight turns the roller, and advances the endless apron, so as to deliver the
carding at the same rate as the carriage runs out; but when the proper quantity is delivered,
a knot in the rope arrives at a fixed stop, which does not permit it to move any
further; while at the same instant the roller 5 quits the lever 6, and allows the upper
rail G, of the clasp to fall, and pinch the carding fast; the wheel E, being then set in
motion, makes the spindles revolve; and the carriage being simultaneously drawn out,
extends the slubbings while under the influence of twisting. In winding up the
slubbings, the operative must take care to push in the carriage, and to turn the wheel
round at such rates that the spindles will not take up faster than the carriage moves on
its railway, or he would injure the slubbings. The machine requires the attendance of
a child, to bring the cardings from the card-engine, to place them upon the sloping
feed-cloth, and to join the ends of the fresh ones carefully to the ends of the others
newly drawn under the roller. Slubbings intended for warp-yarn must be more twisted
than those for weft; but each must receive a degree of torsion relative to the quality of
the wool and of the cloth intended to be made. In general, however, no more twist
should be given to the slubbings than is indispensable for enabling them to be drawn
out to the requisite slenderness without breaking. This twist forms no part of the twist
of the finished yarn, for the slubbing will be twisted in the contrary direction, when
spun afterwards in the jenny or mule.
I may here remark, that various machines have been constructed of late years for[1319]
making continuous card-ends, and slubbings, in imitation of the carding and roving of
the Cotton Manufacture; to which article I therefore refer my readers. The wool
slubbings are now spun into yarn, in many factories, by means of the mule. Indeed, I
have seen in France the finest yarn, for the mousseline-de-laine fabrics, beautifully spun
upon the self-actor mule of Sharp and Roberts.[72]
Tentering.—When the cloth is returned from the fulling-mill (which see), it is
stretched upon the tenter-frame, and left in the open air till dry.
In the woollen manufacture, as the cloth suffers, by the operation of the fulling-mill,
a shrinkage of its breadth to well nigh one-half, it must at first be woven of
nearly double its intended width when finished. Superfine six-quarter broad cloths
must therefore be turned out of the loom twelve-quarters wide.
Burling is the name of a process, in which the dried cloth is examined minutely in
every part, freed from knots or uneven threads, and repaired by sewing any little rents,
or inserting sound yarns in the place of defective ones.
Teasling.—The object of this operation is to raise up the loose filaments of the
woollen yarn into a nap upon one of the surfaces of the cloth, by scratching it either
with thistle-heads, called teasels, or with teasling-cards or brushes, made of wire. The
natural teasels are the balls which contain the seeds of the plant called Dipsacus fullorum;
the scales which form the balls, project on all sides, and end in sharp elastic
points, that turn downwards like hooks. In teasling by hand, a number of these balls
are put into a small wooden frame, having crossed handles, eight or ten inches long;
and when thus filled, form an implement not unlike a curry-comb, which is used by
two men, who seize the teasel-frame by the handles, and scrub the face of the cloth,
hung in a vertical position from two horizontal rails, made fast to the ceiling of the
workshop. First, they wet the cloth, and work three times over, by strokes in the
direction of the warp, and next of that of the weft, so as to raise all the loose
fibres from the felt, and to prepare it for shearing. In large manufactories, this
dressing operation is performed by a machine called a gig-mill, which originally consisted,
and in most places still consists, of a cylinder bristled all over with the thistle-heads,
and made to revolve rapidly while the cloth is drawn over it in a variety of
directions. If the thistle be drawn in the line of the warp, the points act more efficaciously
upon the weft, being perpendicular to its softer spun yarns. Inventors who
have tried to give the points a circular or oblique action between the warp and the
weft, proceed apparently upon a false principle, as if the cloth were like a plate of
metal, whose substance could be pushed in any direction. Teasling really consists in
drawing out one end of the filaments, and leaving the body of them entangled in the
cloth; and it should seize and pull them perpendicularly to their length, because in
this way it acts upon the ends, which being least implicated, may be most readily
disengaged.
When the hooks of the thistles become clogged with flocks of wool, they must be
taken out of the frame or cylinder, and cleaned by children with a small comb.
Moisture, moreover, softens their points, and impairs their teasling powers; an effect
which needs to be counterbalanced, by taking them out, and drying them from time to
time. Many contrivances have, therefore, been proposed, in which metallic teasels of an
unchangeable nature, mounted in rotatory machines, driven by power, have been substituted
for the vegetable, which being required in prodigious quantities, become sometimes
excessively scarce and dear in the clothing districts. In 1818, several schemes of
that kind were patented in France, of which those of M. Arnold-Merick, and of
MM. Taurin frères, of Elbœuf, are described in the 16th volume of Brevets d’Invention
expirés. Mr. Daniell, cloth manufacturer in Wilts, renewed this invention under
another form, by making his rotatory cards with two kinds of metallic wires, of unequal
lengths; the one set, long, thin, and delicate, representing the points of the thistle;
the other, shorter, stiffer, and blunter, being intended to stay the cloth, and to hinder
the former from entering too far into it. But none of these processes have succeeded
in discarding the natural teasel from the most eminent manufactories.
The French government purchased, in 1807, the patent of Douglas, an English
mechanist, who had, in 1802, imported into France, the best system of gig-mills then
used in the west of England. A working set of his machines having been placed in the
Conservatoire des Arts, for public inspection, they were soon introduced into most of the
French establishments, so as generally to supersede teasling (lainage) by hand.
A description of them was published in the third volume of the Brevets d’Invention.
The following is an outline of some subsequent improvements:—
1. As it was imagined that the seesaw action of the hand operative was in some
respects more effectual than the uniform rotation of a gig-mill, this was attempted
to be imitated by an alternating movement.
[1320]
2. Others conceived that the seesaw motion was not essential, but that it was
advantageous to make the teasels or cards act in a rectilinear direction, as in working
by hand; this action was attempted by placing the two ends of the teasel-frame in
grooves formed like the letter D, so that the teasel should act on the cloth only when
it came into the rectilinear part. Mr. Wells, machine-maker, of Manchester, obtained
a patent, in 1832, for this construction.
3. It was supposed that the teasels should not act perpendicularly to the weft, but
obliquely or circularly upon the face of the cloth. Mr. Ferrabee, of Gloucester,
patented, in 1830, a scheme of this kind, in which the teasels are mounted upon two
endless chains, which traverse from the middle of the web to the selvage or list, one to
the right, and another to the left hand, while the cloth itself passes under them with
such a velocity, that the effect, or resultant, is a diagonal action, dividing into two
equal parts the rectangle formed by the weft and warp yarns. Three patent machines
of Mr. George Oldland—the first in 1830, the second and third in 1832—all proceed
upon this principle. In the first, the teasels are mounted upon discs made to turn flat
upon the surface of the cloth; in the second, the rotating discs are pressed by corkscrew
spiral springs against the cloth, which is supported by an elastic cushion, also pressed
against the discs by springs; and in the third machine, the revolving discs have a larger
diameter, and they turn, not in a horizontal, but a vertical plane.
4. Others fancied that it would be beneficial to support the reverse side of the cloth
by flat hard surfaces, while acting upon its face with cards or teasels. Mr. Joseph
Cliseld Daniell, having stretched the cloth upon smooth level stones, teasels them by hand.
5. Messrs. Charlesworth and Mellor obtained a patent, in 1829, for supporting the
back of the cloth with elastic surfaces, while the part was exposed to the teasling
action. 6. Elasticity has also been imparted to the teasels, in the three patent inventions
of Mr. Sevill, Mr. J. C. Daniell, and Mr. R. Atkinson. 7. It has been thought
useful to separate the teasel-frames upon the drum of the gig-mill, by simple rollers,
or by rollers heated with steam, in order to obtain the combined effect of calendering
and teasling. Mr. J. C. Daniell, Mr. G. Haden, and Mr. J. Rayner, have obtained
patents for contrivances of this kind. 8. Several French schemes have been mounted
for making the gig-drum act upon the two sides of the cloth, or even to mount two
drums on the same machine.
Mr. Jones, of Leeds, contrived a very excellent method of stretching the cloth, so as
to prevent the formation of folds or wrinkles. (See Newton’s Journal, vol. viii., 2nd
series, page 126.) Mr. Collier, of Paris, obtained a patent, in 1830, for a greatly
improved gig-mill, upon Douglas’s plan, which is now much esteemed by the French
clothiers. The following figures and description exhibit one of the latest and best
teasling machines. It is the invention of M. Dubois and Co., of Louviers, and is now
doing excellent work in that celebrated seat of the cloth manufacture.
In the fulling-mill, the woollen web acquires body and thickness, at the expense of
its other dimensions; for being thereby reduced about one-third in length, and one-half
in breadth, its surface is diminished to one-third of its size as it comes out of the
loom; and it has, of course, increased threefold in thickness. As the filaments drawn
forth by teasling, are of very unequal lengths, they must be shorn to make them level,
and with different degrees of closeness, according to the quality of the stuff, and the
appearance it is desired to have. But, in general, a single operation of each kind is
insufficient; whence, after having passed the cloth once through the gig-mill, and once
through the shearing-machine (tondeuse), it is ready to receive a second teasling, deeper
than the first, and then to suffer a second shearing. Thus, by the alternate repetition
of these processes, as often as is deemed proper, the cloth finally acquires its wished-for
appearance. Both of these operations are very delicate, especially the first; and if they
be ill conducted, the cloth is weakened, so as to tear or wear most readily. On the other
hand, if they be skilfully executed, the fabric becomes not only more sightly, but it
acquires strength and durability, because its face is changed into a species of fur, which
protects it from friction and humidity.
Figs. 1214, 1215., represent the gig-mill in section, and in front elevation. A, B, C, D,
A′, B′, C′, D′, being the strong frame of iron, cast in one piece, having its feet enlarged
a little more to the inside than to the outside, and bolted to large blocks in the stone
pavement. The two uprights are bound together below by two cross-beams A′′,
being fastened with screw-bolts at the ears a′′, a′′; and at top, by the wrought-iron
stretcher-rod D, whose ends are secured by screw-nuts at D, D′. The drum is mounted
upon a wrought-iron shaft F, which bears at its right end (fig. 1215.), exterior to the
frame, the usual riggers, or fast and loose pulley, ff′′, f′, which give motion to the
machine by a band from the main shaft of the mill. On its right end, within the frame,
the shaft F, has a bevel wheel F′, for transmitting movement to the cloth, as shall be
afterwards explained. Three crown wheels G, of which one is shown in the section,
fig. 1214., are, as usual, keyed by a wedge to the shaft F. Their contour is a sinuous[1321]
[1322]
band, with six semi-cylindrical hollows, separated alternately by as many portions of
the periphery. One of these three wheels is placed in the middle of the shaft F, and
the other two, towards its extremities. Their size may be judged of, from inspection of
fig. 1214. After having set them so that all their spokes or radii correspond exactly,
the 16 sides H, are made fast to the 16 portions of the periphery, which correspond in
the three wheels. These sides are made of sheet iron, curved into a gutter form, fig. 1214.,
but rounded off at the end, fig. 1215., and each of them is fixed to the three felloes of the
wheels by three bolts h. The elastic part of the plate iron allows of their being sufficiently
well adjusted, so that their flat portions furthest from the centre may lie pretty truly on
a cylindrical surface, whose axis would coincide with that of the shaft F.
Between the 16 sides there are 16 intervals, which correspond to the 16 hollowings
of each of the wheels. Into these intervals are adjusted, with proper precautions,
16 frames bearing the teasels which are to act upon the cloth. These are fitted in as
follows:—Each has the shape of a rectangle, of a length equal to that of the drum,
but their breadth only large enough to contain two thistle-heads set end to end,
thus making two rows of parallel teasels throughout the entire length, (see the contour
in fig. 1214.) A portion of the frame is represented in fig. 1216. The large side I,
against which the tops of the teasels rest, is hollowed out into a semi-cylinder, and its
opposite side is cleft throughout its whole
length, to receive the tails of the teasels,
which are seated and compressed in it. There
are, moreover, cross-bars i, which serve to
maintain the sides of the frame I, at an invariable
distance, and to form short compartments
for keeping the thistles compact. The
ends are fortified by stronger bars k, k, with projecting bolts to fasten the frames
between the ribs. The distance of the sides of the frame I, I′, ought to be such, that
if a frame be laid upon the drum, in the interval of two ribs, the side I will rest upon
the inclined plane of one of the ribs, and the side I′ upon the inclined plane of the
other, (see fig. 1214.); while at the same time the bars k, of the two ends of the frame,
rest upon the flat parts of the ribs themselves. This
point being secured, it is obvious, that if the ends of
the bars k be stopped, the frame will be made fast.
But they need not be fixed in a permanent manner,
because they must be frequently removed
and replaced. They are fastened by the clamp,
(figs. 1217, 1218.), which is shut at the one end, and
furnished at the other with a spring, which can be
opened or shut at pleasure. 2 and 4, in fig. 1215.
(near the right end of the shaft F), shows the place of
the clamp, figs. 1217, 1218. The bar of the right hand is first set in the clamp, by holding
up its other end; the frame is then let down into the left-hand clamp.
The cloth is wound upon the lower beam Q, fig. 1214.; thence it passes in contact
with a wooden cylinder T, turning upon an axis, and proceeds to the upper beam P, on
to which it is wound: by a contrary movement, the cloth returns from the beam P to Q,
over the cylinder T; and may thus go from the one to the other as many times as shall
be requisite. In these successive circuits it is presented to the action of the teasels,
under certain conditions. In order to be properly teasled, it must have an equal
tension throughout its whole breadth during its traverse; it must be brought into
more or less close contact with the drum, according to the nature of the cloth, and
the stage of the operations; sometimes being a tangent to the surface, and sometimes
embracing a greater or smaller portion of its contour, it must travel with a determinate
speed, dependent upon the velocity of the drum, and calculated so as to produce the
best result: the machine itself must make the stuff pass alternately from one winding
beam to the other.
In fig. 1215., before the front end of the machine, there is a vertical shaft L, as high
as the framework, which revolves with great facility, in the bottom step l, the middle
collet l′, and top collet l′′, in the prolongation of the stretcher D. Upon this upright
shaft are mounted—1. a bevel wheel L′; 2. an upper bevel pinion M, with its boss M′;
3. a lower bevel pinion N, with its boss N′. The bevel wheel L′ is keyed upon the shaft
L, and communicates to it the movement of rotation which it receives from the pinion
f, with which it is in geer; but the pinion f, which is mounted upon the shaft F of the
drum, participates in the rotation which this shaft receives from the prime mover, by
means of the fast rigger-pulley f′. The upper pinion M is independent upon the shaft
L; that is to say, it may be slidden along it, up and down, without being driven by it;
but it may be turned in an indirect manner by means of six curved teeth, projecting from[1323]
its bottom, and which may be rendered active or not, at pleasure; these curved teeth,
and their intervals, correspond to similar teeth and intervals upon the top of the boss
M′, which is dependent, by feathered indentations, upon the rotation of L, though it can
slide freely up and down upon it. When it is raised, therefore, it comes into geer
with M. The pinion N, and its boss, have a similar mode of being thrown into and out
of geer with each other. The bosses M′ and N′, ought always to be moved simultaneously,
in order to throw one of them into geer, and the other out of geer. The shaft
L serves to put the cloth in motion, by means of the bevel wheels P′′ and Q′′, upon the
ends of the beams P, Q, which take into the pinions M and N.
The mechanism destined to stretch the cloth is placed at the other end of the
machine, where the shafts of the beams P, Q, are prolonged beyond the frame, and bear
at their extremities P′ and Q′, armed each with a brake. The beam P (fig. 1214.), turns
in an opposite direction to the drum; consequently the cloth is wound upon P, and
unwound from Q. If, at the same time as this is going on, the handle R′, of the brake-shaft,
be turned so as to clasp the brake of the pulley Q′, and release that of the pulley P′,
it is obvious that a greater or smaller resistance will be occasioned in the beam Q, and
the cloth which pulls it in unwinding, will be able to make it turn only when it has
acquired the requisite tension; hence it will be necessary, in order to increase or
diminish the tension, to turn the handle R′ a little more or a little less in the direction
which clasps the brake of the pulley Q′; and as the brake acts in a very equable
manner, a very equable tension will take place all the time that the cloth takes to pass.
Besides, should the diminution of the diameter of the beam Q, render the tension less
efficacious in any considerable degree, the brake would need to be unclamped a very
little, to restore the primitive tension.
When the cloth is to be returned from the beam P, to the beam Q, Z must be
lowered, to put the shaft L out of geer above, and in geer below; then the cloth-beam
Q, being driven by that vertical shaft, it will turn in the same direction as the drum, and
will wind the cloth round its surface. In order that it may do so, with a suitable
tension, the pulley Q′ must be left free, by clasping the brake of the pulley P′, so as to
oppose an adequate resistance.
The cloth is brought into more or less close contact with the drum as follows:—There
is for this purpose a wooden roller T, against which it presses in passing from the one
winding beam to the other, and which may have its position changed relatively to the
drum. It is obvious, for example, that in departing from the position represented in
fig. 1214., where the cloth is nearly a tangent to the drum, if the roller T′ be raised,
the cloth will cease to touch it; and if it be lowered, the cloth will, on the contrary,
embrace the drum over a greater or less portion of its periphery. For it to produce
these effects, the roller is borne at each end, by iron gudgeons, upon the heads of an
arched rack T′′ (fig. 1214.), where it is held merely by pins. These racks have the same
curvature as the circle of the frame, against which they are adjusted by two bolts; and
by means of slits, which these bolts traverse, they may be slidden upwards or downwards,
and consequently raise or depress the roller T. But to graduate the movements,
and to render them equal in the two racks, there is a shaft U, supported by the uprights
of the frame, and which carries, at each end, pinions U′, U′′, which work into the two
racks T′, T′′: this shaft is extended in front of the frame, upon the side of the head of the
machine (fig. 1215.), and there it carries a ratchet wheel u, and a handle u′. The
workman, therefore, requires merely to lay hold of the handle, and turn it in the
direction of the ratchet wheel, to raise the racks, and the roller T, which they carry; or to
lift the click or catch, and turn the handle in the opposite direction, when he wishes to
lower the roller, so as to apply the cloth to a larger portion of the drum.
CLOTH CROPPING.
Of machines for cropping or shearing woollen cloths, those of Lewis and Davis have
been very generally used.
Fig. 1219. is an end view, and fig. 1220. is a side view, of Lewis’s machine, for shearing
cloth from list to list. Fig. 1221. is an end view of the carriage, with the rotatory cutter detached
from the frame of the machine, and upon a larger scale: a, is a cylinder of metal,
on which is fixed a triangular steel wire; this wire is previously bent round the cylinder
in the form of a screw, as represented at a, a, in fig. 1219., and, being hardened, is intended
to constitute one edge of the shear or cutter.
The axis of the cylindrical cutter a, turns in the frame b, which, having proper adjustments,
is mounted upon pivots c, in the standard of the travelling carriage d, d; and
e, is the fixed or ledger blade, attached to a bar f, which constitutes the other edge of
the cutter; that is, the stationary blade, against which the edges of the rotatory cutter act;
f and g, are flat springs, intended to keep the cloth (shown by dots) up against the
cutting edges. The form of these flat springs f, g, is shown at figs. 1222. and 1223.,[1324]
as consisting of plates of thin metal cut into narrow slips (fig. 1222.), or perforated
with long holes, (fig. 1223.) Their object is to support the cloth, which is intended to
pass between them, and operate as a spring bed, bearing the surface of the cloth against
the cutters, so that its
pile or nap may be
cropped off or shorn as
the carriage d is drawn
along the top rails of
the standard or frame
of the machine h, h,
by means of cords.
The piece of cloth
to be shorn, is wound
upon the beam k, and
its end is then conducted
through the
machine, between the
flat springs f and g (as
shown in fig. 1221.), to
the other beam l, and is
then made fast; the
sides or lists of the cloth being held and stretched by small hooks, called habiting
hooks. The cloth being thus placed in the machine, and drawn tight, is held distended
by means of ratchets on the ends of the beams k and l, and palls. In commencing
the operation of shearing, the carriage d, must be brought back, as in fig. 1221., so
that the cutters shall be close to
the list; the frame of the cutters
is raised up on its pivots as it
recedes, in order to keep the
cloth from injury, but is lowered
again previously to being
put in action. A band or winch
is applied to the rigger or pulley
m, which, by means of an
endless cord passed round the
pulley n, at the reverse end of
the axle of m, and round the
other pulleys o and p, and the
small pulley q, on the axle of
the cylindrical cutter, gives the
cylindrical cutter a very rapid
rotatory motion; at the same
time a worm, or endless screw,
on the axle of m and n, taking
into the teeth of the large wheel r, causes that wheel to revolve, and a small drum s,
upon its axle, to coil up the cord, by which the carriage d, with the cutters a and e,
and the spring bed f and g, are slowly, but progressively, made to advance, and to
carry the cutters over the face of the cloth, from list to list; the rapid rotation of the
cutting cylinder a, producing the operation of cropping or shearing the pile.
Upon the cutting cylinder, between the spiral blades, it is proposed to place stripes of
plush, to answer the purpose of brushes, to raise the nap or pile as the cylinder goes
around, and thereby assist in bringing the points of the wool up to the cutters.
The same contrivance is adapted to a machine for shearing the cloth lengthwise.
[1325]
Fig. 1224. is a geometrical elevation of one side of Mr. Davis’s machine. Fig. 1225.
a plan or horizontal representation of the same, as seen in the top; and fig. 1226. a section
taken vertically across the machine near the middle, for the purpose of displaying
the working parts more perfectly than in the two preceding figures. These
three figures represent a complete machine in working condition, the cutters being
worked by a rotatory motion, and the cloth so placed in the carriage as to be cut from
list to list. a, a, a, is a frame or standard, of wood or iron, firmly bolted together by
cross braces at the ends and in the middle. In the upper side-rails of the standard,
there is a series of axles carrying anti-friction wheels b, b, b, upon which the side-rails
c, c, of the carriage or frame that bears the cloth runs, when it is passing under
the cutters in the operation of shearing. The side-rails c, c, are straight bars of iron,
formed with edges v, on their under sides, which run smoothly in the grooves of the
rollers b, b, b. These side-rails are firmly held together by the end stretchers d, d.
The sliding frame has attached to it the two lower rollers e, e, upon which the cloth
intended to be shorn is wound; the two upper lateral rollers f, f, over which the cloth is
conducted and held up; and the two end rollers g, g, by which the habiting rails h, h, are
drawn tight.
In preparing to shear a piece of cloth, the whole length of the piece is, in the first
place, tightly rolled upon one of the lower rollers e, which must be something longer
than the breadth of the cloth from list to list. The end of the piece is then raised,
and passed over the top of the lateral rollers f, f, whence it is carried down to the
other roller e, and its end or farral is made fast to that roller. The hooks of the habiting
rails h, h, are then put into the lists, and the two lower rollers e, e, with the two
end rollers g, g, are then turned, for the purpose of drawing up the cloth, and straining
it tight, which tension is preserved by ratchet wheels attached to the ends of the respective
rollers, with palls dropping into their teeth. The frame carrying the cloth, is
now slidden along upon the top standard rails by hand, so that the list shall be brought[1326]
nearly up to the cutter i, i, ready to commence the shearing operation; the bed is then
raised, which brings the cloth up against the edges of the shears.
The construction of the bed will be seen by reference to the cross section fig. 1226.
It consists of an
iron or other metal
roller k, k, turned
to a truly cylindrical
figure, and
covered with cloth
or leather, to afford
a small degree of
elasticity. This
roller is mounted
upon pivots in a
frame l, l, and is
supported by a
smaller roller m,
similarly mounted,
which roller m, is
intended merely to
prevent any bending
or depression
of the central part
of the upper roller or bed k, k, so that the cloth may be kept in close contact with
the whole length of the cutting blades.
In order to allow the bed k to rise and fall, for the purpose of bringing the cloth up
to the cutters to be shorn, or lowering it away from them after the operation, the frame
l, l, is made to slide up and down in the grooved standard n, n, the movable part enclosed
within the standard being shown by dots. This standard n, is situated about the
middle of the machine, crossing it immediately under the cutters, and is made fast to the
frame a, by bolts and screws. There is a lever o, attached to the lower cross-rail of the
standard, which turns upon a fulcrum-pin, the extremity of the shorter arm of which lever
acts under the centre of the sliding-frame, so that by the lever o, the sliding-frame, with
the bed, may be raised or lowered, and when so raised, be held up by a spring catch j.
It being now explained by what means the bed which supports the cloth is constructed,
and brought up, so as to keep the cloth in close contact with the cutters, while the operation
of shearing is going on; it is necessary, in the next place, to describe the construction
of the cutters, and their mode of working; for which purpose, in addition to
what is shown in the first three figures, the cutters are also represented detached, and
upon a larger scale, in fig. 1227.
In this figure is exhibited a portion of the cutters in the same situation as in fig.
1221.; and alongside of it is a section of
the same, taken through it at right
angles to the former; p, is a metallic
bar or rib, somewhat of a wedge form,
which is fastened to the top part of the
standard a, a, seen best in fig. 1220.
To this bar a straight blade of steel g, is attached by screws, the edge of which stands
forward even with the centre or axis of the cylindrical cutter i, and forms the ledger
blade, or lower fixed edge of the shears. This blade remains stationary, and is in close
contact with the pile or nap of the cloth, when the bed k, is raised, in the manner above
described.
The cutter or upper blade of the shears, is formed by inserting two or more strips of
plate steel r, r, in twisted directions, into grooves in the metallic cylinder i, i, the edges
of which blades r, as the cylinder i revolves, traverse along the edge of the fixed or
ledger blade g, and by their obliquity produce a cutting action like shears; the edges of
the two blades taking hold of the pile or raised nap, as the cloth passes under it, shaves
off the superfluous ends of the wool, and leaves the face smooth.
Rotatory motion is given to the cutting cylinder i, by means of a band leading
from the wheel s, which passes round the pulley fixed on the end of the cylinder i, the
wheel s being driven by a band leading from the rotatory part of a steam-engine, or
any other first mover, and passed round the rigger t, fixed on the axle s. Tension is
given to this band by a tightening pulley u, mounted on an adjustable sliding-piece v,
which is secured to the standard by a screw; and this rigger is thrown in and out of
geer by a clutch-box and lever, which sets the machine going, or stops it.
In order to give a drawing stroke to the cutter, which will cause the piece of cloth
to be shorn off with better effect, the upper cutter has a slight lateral action, produced[1327]
by the axle of the cutting cylinder being made sufficiently long to allow of its sliding
laterally about an inch in its bearings; which sliding is effected by a cam w, fixed at
one end. This cam is formed by an oblique groove, cut round the axle, (see w, fig. 1227.)
and a tooth x, fixed to the frame or standard which works in it, as the cylinder revolves.
By means of this tooth, the cylinder is made to slide laterally, a distance equal to the
obliquity of the groove w, which produces the drawing stroke of the upper shear. In
order that the rotation of the shearing cylinder may not be obstructed by friction, the
tooth x, is made of two pieces, set a little apart, so as to afford a small degree of elasticity.
The manner of passing the cloth progressively under the cutters is as follows:—On
the axle of the wheel s, and immediately behind that wheel, there is a small rigger,
from which a band passes to a wheel y, mounted in an axle turning in bearings on the
lower side-rail of the standard a. At the reverse extremity of this axle, there is another
small rigger 1, from which a band passes to a wheel 2, fixed on the axle 3, which
crosses near the middle of the machine, seen in fig. 1226. Upon this axle there is a
sliding pulley 4, round which a cord is passed several times, whose extremities are made
fast to the ends of the sliding carriage d; when, therefore, this pulley is locked to the
axle, which is done by a clutch box, the previously described movements of the machine
cause the pulley 4 to revolve, and by means of the rope passed round it, to
draw the frame, with the cloth, slowly and progressively along under the cutters.
It remains only to point out the contrivance whereby the machinery throws itself out
of geer, and stops its operations, when the edge of the cloth or list arrives at the cutters.
At the end of one of the habiting rails h, there is a stop affixed by a nut and screw
5, which, by the advance of the carriage, is brought up and made to press against a
lever 6; when an arm from this lever 6, acting under the catch 7, raises the catch up,
and allows the hand-lever 8, which is pressed upon by a strong spring, to throw the
clutch-box 10, out of geer with the wheel 8; whereby the evolution of the machine
instantly ceases. The lower part of the lever 6, being connected by a joint to the top
of the lever j, the receding of the lever 6, draws back the lower catch j, and allows the
sliding frame l, l, within the bed k, to descend. By now turning the lower rollers e, e,
another portion of the cloth is brought up to be shorn; and when it is properly habited
and strained, by the means above described, the carriage is slidden back, and, the
parts being all thrown into geer, the operation goes on as before.
Mr. Hirst’s improvements in manufacturing woollen cloths, for which a patent was
obtained in February, 1830, apply to that part of the process where a permanent lustre
is given usually by what is called roll-boiling; that is, stewing the cloth, when tightly
wound upon a roller, in a vessel of hot water or steam. As there are many disadvantages
attendant upon the operation of roll-boiling, such as injuring the cloths, by overheating
them, which weakens the fibre of the wool, and also changes some colours, he
substituted, in place of it, a particular mode of acting upon the cloths, by occasional
or intermitted immersion in hot water, and also in cold water, which operations may be
performed either with or without pressure upon the cloth, as circumstances may require.
The apparatus which he proposes to employ for carrying on his improved process,
is shown in the accompanying drawing. Fig. 1228. is a front view of the apparatus,
complete, and in working order; fig. 1229. is a section, taken transversely through the
middle of the machine, in the direction of fig. 1230.; and fig. 1230. is an end view
of the same; a, a, a, is a vessel or tank, made of iron or wood, or any other suitable
material: sloping at the back and front, and perpendicular at the ends. This[1328]
tank must be sufficiently large to admit of half the diameter of the cylinder or drum
b, b, b, being immersed into it, which drum is about four feet diameter, and about six
feet long, or something more than the width of the piece of cloth intended to be operated
upon. This cylinder or
drum b, b, is constructed
by combining segments
of wood cut radially on
their edges, secured by
screw-bolts to the rims of
the iron wheels, having
arms, with an axle passing
through the middle.
The cylinder or drum
being thus formed, rendered
smooth on its periphery,
and mounted
upon its axle in the
tank, the piece of cloth
is wound upon it as
tightly as possible, which
is done by placing it in
a heap upon a stool, as at
c, fig. 1229., passing its
end over and between the tension-rollers d, e, and then securing it to the drum,
the cloth is progressively drawn from the heap, between the tension-rollers, which
are confined by a pall and ratchet, on to the periphery of the drum, by causing the
drum to revolve upon its axis, until the whole piece of cloth is tightly wound upon the
drum; it is then bound round with canvas or other wrappers, to keep it secure.
If the tank has not been previously charged with clean and pure water, it is now
filled to the brim, as shown at fig. 1229., and opening the stopcock of the pipe
f, which leads from a boiler, the steam
is allowed to blow through the pipe,
and discharge itself at the lower end, by
which means the temperature of the water
is raised in the tank to about 170° Fahr.
Before the temperature of the water has
got up, the drum is set in slow rotatory
motion, in order that the cloth may be
uniformly heated throughout; the drum
making about one rotation per minute.
The cloth, by immersion in the hot water,
and passing through the cold air, in succession,
for the space of about eight hours,
gets a smooth soft face, the texture not being
rendered harsh, or otherwise injured,
as is frequently the case by roll-boiling.
Uniform rotatory motion to the drum
is shown in fig. 1228., in which g is an
endless screw or worm, placed horizontally,
and driven by a steam-engine or any other first mover employed in the factory.
This endless screw takes into the teeth of, and drives, the vertical wheel h, upon the axle
of which the coupling-box i, i, is fixed, and, consequently, continually revolves with it.
At the end of the shaft of the drum, a pair of sliding clutches k, k, are mounted,
which, when projected forward, as shown by dots in fig. 1228., produce the coupling or
locking of the drum-shaft to the driving wheel, by which the drum is put in motion;
but on withdrawing the clutches k, k, from the coupling-box i, i, as in the figure, the
drum immediately stands still.
After operating upon the cloth in the way described, by passing it through hot water
for the space of time required, the hot water is to be withdrawn by a cock at the
bottom, or otherwise, and cold water introduced into the tank in its stead; in which
cold water the cloth is to be continued turning, in the manner above described, for the
space of twenty-four hours, which will perfectly fix the lustre that the face of the cloth
has acquired by its immersion in the hot water, and leave the pile or nap, to the touch,
in a soft silky state.
In the cold-water operation he sometimes employs a heavy pressing roller l, which,
being mounted in slots in the frame or standard, revolve with the large drum, rolling
over the back of the cloth as it goes round. This roller may be made to act upon the[1329]
cloth with any required pressure, by depressing the screws m, m, or by the employment
of weighted levers, if that should be thought necessary.
Pressing is the last finish of cloth to give it a smooth level surface. The piece is
folded backwards and forwards in yard lengths, so as to form a thick package on the
board of a screw or hydraulic press. Between every fold sheets of glazed paper are
placed to prevent the contiguous surfaces of cloth from coming into contact; and at
the end of every twenty yards, three hot iron plates are inserted between the folds, the
plates being laid side by side, so as to occupy the whole surface of the folds. Thin
sheets of iron not heated are also inserted above and below the hot plates to moderate
the heat. When the packs of cloth are properly folded, and piled in sufficient number
in the press, they are subjected to a severe compression, and left under its influence till
the plates get cold. The cloth is now taken out and folded again, so that the creases
of the former folds may come opposite to the flat faces of the paper, and be removed
by a second pressure. In finishing superfine cloths, however, a very slight pressure is
given with iron plates but moderately warmed. The satiny lustre and smoothness
given by strong compression with much heat is objectionable, as it renders the surface
apt to become spotted and disfigured by rain.
WOOTZ, is the Indian name of steel.
WORT, is the fermentable infusion of malt or grains. See Beer.
WOULFE’S APPARATUS, is a series of vessels, connected by tubes, for the
purpose of condensing gaseous products in water. See Acetic Acid, fig. 1.; also
Muriatic Acid.
XANTHINE, is the name given by Kuhlmann to the yellow dyeing-matter contained
in madder.
YEAST, is the froth of fermenting worts. See Beer and Fermentation.
YELLOW DYE. (Teinture jaune, Fr.; Gelbfarben, Germ.) Annotto, dyer’s-broom
(Genista tinctoria), fustic, fustet, Persian or French berries, quercitron bark,
saw-wort (Serratula tinctoria), turmeric, weld, and willow leaves, are the principal yellow
dyes of the vegetable kingdom; chromate of lead, iron-oxide, nitric acid (for silk), sulphuret
of antimony, and sulphuret of arsenic, are those of the mineral kingdom. Under
these articles, as also under Calico-printing, Dyeing, and Mordants, ample instructions
will be found for communicating this colour to textile and other fibrous substances.
Alumina and oxide of tin are the most approved bases of the above vegetable dyes. A
nankin dye may be given with bablah, especially to cotton oiled preparatory to the
Turkey-red process. See Madder.
YELLOW, KING’S, is a poisonous yellow pigment. See Arsenic and Orpiment.
YTTRIA, is a rare earth, extracted from the minerals gadolinite and yttrotantalite,
being an oxide of the metal yttrium.
ZEDOARY, is the root of a plant which grows in Malabar, Ceylon, &c. It occurs
in wrinkled pieces, externally ash-coloured, internally brownish-red; possessed of a fragrant
odour, somewhat resembling camphor; and of a pungent, aromatic, bitterish taste.
It contains, according to Bucholz, 1·42 of volatile oil, of a burning camphorated taste;
3·60 of a soft, bitter, aromatic resin; 11·75 of a bitter aromatic extract, mixed with a
little resin and potash-salts; 4·5 of gum; 9 of vegetable mucilage; 3·60 of starch;
8·0 of a starchy extract from the woody fibre, by means of caustic potassa, along with
31·2 of another matter, 12·89 of woody fibre, and 15 of water. According to Morin,
this root, contains besides, an azotized substance, analogous to the extract of beef.
ZIMOME, is a principle supposed by Taddei to exist in the gluten of wheat-flour.
Its identity is not recognised by later chemists.
ZIRCON. See Hyacinth and Lapidary.
ZIRCONIA, is a rare earth, extracted from the minerals zircon and hyacinth; it is
an oxide of zirconium, a substance possessing externally none of the metallic characters,
but resembling rather charcoal powder, which burns briskly, and almost with explosive
violence.
[1330]
ZINC, is a metal of a bluish-white colour, of considerable lustre when broken
across, but easily tarnished by the air; its fracture is hackly, and foliated with small
facets, irregularly set. It has little cohesion, and breaks in thin plates before the hammer,
unless it has been previously subjected to a regulated process of lamination, at the temperature
of from 220° to 300° F., whereby it becomes malleable, and retains its malleability
and ductility afterwards. On this singular property, a patent was taken out by Messrs.
Hobson and Sylvester, of Sheffield, many years ago, for manufacturing sheet zinc, for
covering the roofs of houses, and sheathing ships; but the low price of copper at that
time, and its superior tenacity, rendered their patent ineffective. The specific gravity
of zinc varies from 6·9 to 7·2, according to the condensation it has received. It melts
under a red heat, at about the 680th or 700th degree of Fahrenheit’s scale. When
exposed to this heat with contact of air, the metal takes fire, and burns with a brilliant
bluish-white light, while a few flocculi, of a woolly-looking white matter, rise out of the
crucible, and float in the air. The result of the combustion is a white powder, formerly
called flowers, but now oxide of zinc; consisting of 34 of metal, and 8 of oxygen, being
their respective prime equivalents; or, in 100 parts, of 81 and 19.
The principal ores of zinc are, the sulphuret called blende, the silicate called calamine
and the sparry calamine, or the carbonate.
1. Blende crystallizes in the garnet-dodecahedron; its fracture is highly conchoidal;
lustre, adamantine; colours, black, brown, red, yellow, and green; transparent or translucent;
specific gravity, 4. It is a simple sulphuret of the metal; and, therefore, consists,
in its pure state, of 34 of zinc, and 16 of sulphur. It dissolves in nitric acid, with
disengagement of sulphuretted hydrogen gas. It occurs in beds and veins, accompanied
chiefly by galena, iron pyrites, copper pyrites, and heavy spar. There is a radiated
variety found at Przibram, remarkable for containing a large proportion of cadmium.
Blende is found in great quantities in Derbyshire and Cumberland, as also in Cornwall.
2. Calamine, or silicate of zinc, is divided into two species; the prismatic or electric
calamine, and the rhomboidal; though they both agree in metallurgic treatment. The
first has a vitreous lustre, inclining to pearly; colour, white, but occasionally blue,
green, yellow, or brown; spec. grav. 3·38. It often occurs massive, and in botroidal
shapes. This species is a compound of oxide of zinc with silica and water; and its
constituents are—zinc oxide, 66·37; silica, 26·23; water, 7·4; in 100 parts. Reduced
to powder, it is soluble in dilute sulphuric or nitric acid, and the solution gelatinizes
on cooling. It emits a green phosphorescent light before the blowpipe. The second
species, or rhombohedral calamine, is a carbonate of zinc. Its specific gravity is 4·442,
much denser than the preceding. It occurs in kidney-shaped, botroidal, stalactitic, and
other imitative shapes; surface generally rough, composition columnar. Massive, with
a granular texture, sometimes impalpable; strongly coherent. According to Smithson’s
analysis, Derbyshire calamine consists of—oxide of zinc, 65·2; carbonic acid, 34·8;
which coincides almost exactly with a prime equivalent of the oxide and acid, or
42 + 22 = 64.
The mineral genus called zinc-ore, or red oxide of zinc, is denser than either of the
above, its spec. grav. being 5·432. It is a compound of oxide of zinc 88, and oxide of
iron and manganese 12. It is found massive, of a granular texture, in large quantities,
in several localities, in New Jersey. It is set free in several metallurgic processes, and
occurs crystallized in six-sided prisms of a yellow colour, in the smelting-works of
Kœnigshutte in Silesia, according to Mitscherlich.
The zinc ores of England, like those of France, Flanders, and Silesia, occur in two
geological localities.
The first is in veins in the carboniferous or mountain limestone. The blende and the
calamine most usually accompany the numerous veins of galena which traverse that
limestone; though there are many lead mines that yield no calamine; and, on the other
hand, there are veins of calamine alone, as at Matlock, whence a very considerable
quantity of this ore is obtained.
In almost every point of England where that metalliferous limestone appears, there
are explorations for lead and zinc ores. The neighbourhood of Alston-moor in Cumberland,
of Castleton and Matlock in Derbyshire, and the small metalliferous belt of
Flintshire, are peculiarly marked for their mineral riches. On the north side of the last
county, calamine is mined in a rich vein of galena at Holywell, where it presents the
singular appearance of occurring only in the ramifications that the lead vein makes from
east to west, and never in those from north to south; while the blende, abundantly present
in this mine, is found indifferently in all directions.
The second locality of calamine is in the magnesian limestone formation of the
English geologists, the alpine limestone of the French, and the zechstein of the
Germans. The calamine is disseminated through it in small contemporaneous veins,
which, running in all directions, form the appearance of a network. These veins have
commonly a thickness of only a few inches; but in certain cases they extend to four feet,[1331]
in consequence of the union of several small ones into a mass. The explorations of
calamine in the magnesian limestone, are situated chiefly on the flanks of the Mendip
Hills, a chain which extends in a north-west and south-east direction, from the canal
of Bristol to Frome. The calamine is worked mostly in the parishes of Phipham
and Roborough, as also near Rickford and Broadfield-Doron, by means of a great
multitude of small shafts. The miners pay, for the privilege of working, a tax of 1l.
sterling per annum to the Lords of the Treasury; and they sell the ores, mixed with
a considerable quantity of carbonate of lime, for 1l. per ton, at Phipham, after washing
it slightly in a sieve. They are despatched to Bristol, where they receive a new
washing, in order to separate the galena.
OF THE SMELTING OF THE ORES OF ZINC.
The greater part of the zinc works are situated in the neighbourhood of Birmingham
and Bristol. The manufacture of brass, which has been long one of the staple articles
of these towns, was probably the cause of the introduction of this branch of industry,
at the period when brass began to be made by the direct union of copper with metallic
zinc, instead of calamine. A few zinc furnaces exist also in the neighbourhood of
Sheffield, amid the coal-pits surrounding that town. Bristol and Birmingham derive
their chief supply of ores from the Mendip Hills and Flintshire; and Sheffield, from
Alston-moor.
The calamine, freed from the galena by sorting with the hand, is calcined before
its introduction into the smelting-furnaces, by being exposed, coarsely bruised, in
reverberatory ovens, 10 feet long, and 8 broad, in a layer 6 inches thick. In some
establishments the calcination is omitted, and the calamine, broken into pieces about
the size of a pigeon’s egg, is mixed with its bulk of small coal.
Zinc is smelted in England, likewise from blende (sulphuret of zinc). This ore,
after being washed, and broken into pieces of the size of a filbert, was sold a few years
ago at the mine of Holywell for 3l. a ton, or half the price of calamine. It is roasted,
without any other preparation, in reverberatory furnaces; which are about 8 feet wide,
and 10 long; the distance between the roof and the sole being 30 inches, and the
height of the fire-bridge 18. The layer of blende, which is placed on the hearth, is
about 4 or 5 inches thick; and it is continually stirred up with rakes. One ton of it
requires, for roasting, four tons of coals; and it suffers a loss of 20 per cent. The
operation takes from 10 to 12 hours. The mixture of reducing consists of one-fourth
part of the desulphuretted oxide, one-fourth of calcined calamine, and one-half part of
charcoal; which affords commonly 30 per cent. of zinc.
The English furnaces for smelting zinc ores are sometimes quadrangular, sometimes
round; the latter form being preferable. They are mounted with from 6 to 8 crucibles or
pots (see fig. 1231.), arched over with a cupola a, placed under a conical chimney b, which
serves to give a strong draught, and to
carry off the smoke. In this cone there
are as many doors c, c, c, as there are pots
in the furnace; and an equal number of
vents d, d, d, in the cupola, through which
the smoke may escape, and the pots may
be set. In the surrounding walls there are
holes for taking out the pots, when they
become unserviceable; after the pots are
set, these holes are bricked up. The pots
are heated to ignition in a reverberatory furnace
before being set, and are put in by
means of iron tong machinery supported
upon two wheels, as is the case with glass-house
pots, e, is the grate; f, the door for
the fuel; g, the ash-pit. The pots h, h, h,
have a hole in the centre of their bottom,
which is closed with a wooden plug, when
they are set charged with calamine, mixed
with one-seventh of coal; which coal prevents
the mixture from falling through the
orifice, when the heat rises and consumes
the plug. The sole of the hearth i, i, upon
which the crucibles stand, is perforated
under each of them, so that they can be reached from below; to the bottom orifice of the
pot, when the distillation begins, a long sheet-iron pipe k, is joined, which dips at its end
into a water-vessel l, for receiving in drops the condensed vapours of the zinc. The pot[1332]
is charged from above, through an orifice in the lid of the pot, which is left open after
the firing, till the bluish colour of the flame shows the volatilization of the metal;
immediately whereupon the hole is covered with a fire-tile m. The iron tubes are
apt to get obstructed during the distillation, and must therefore be occasionally cleared
out with a redhot rod. When the distillation is finished, the iron pipes must be
removed; the coaly and other contents of the pot cleared away. A pot lasts about four
months upon an average. Five distillations may be made in the course of 14 days,
in which from 6 to 10 tons of calamine may be worked up, and from 22 to 24 tons of
coals consumed, with a product of two tons of zinc. The metal amounts to from 25 to
40 per cent. of the ore.
1, 2, is the level of the upper floor; 3, 4, level of the lower ceiling of the lower floor.
Fig. 1232., ground plan on the level of 1, 2: only one-half is here shown.
The zinc collected in this operation, is in the form of drops, and a very fine powder,
mingled with some oxide. It must be melted in an iron pot or boiler, set in a proper
furnace; and the oxide is skimmed off the surface, to be returned into the crucibles.
The metal is, lastly, cast into square bars or ingots.
The crucibles are discharged at the end of each operation, by withdrawing the condenser,
breaking with a rake the piece of charcoal which shuts their bottom, and then
emptying them completely, by shaking their upper part. In replacing the condenser-pipe
k (see second pot from the right hand, fig. 1231), the flange at its top is covered
with a ring of loam-lute, pressed against the conical bottom of the crucible, and secured
in its place by means of two parallel rods o, o, which can be clamped by screws projecting
horizontally from the vertical tunnel. See their places, indicated by two open dots near o, o.
A smelter and two labourers are employed in conducting a furnace; who make, with
a mixture of equal parts of fire-clay, and cement of old pounds finely ground, the pots or
crucibles, which last about four months. Five charges are made in 15 days; these work
up from 6 to 10 tons of calamine, consume from 22 to 24 tons of coals, and produce 2 tons
of zinc, upon an average. The following estimate of prices was made a few years ago:—
| 3 tons of calamine, at £6. |
£18 |
0 |
0 |
| 24 ditto coal, at 5s. |
6 |
0 |
0 |
| A smelter, at 2 guineas a week |
2 |
2 |
0 |
| Two labourers, each at 4s. per day |
2 |
16 |
0 |
| Incidental expenses |
1 |
0 |
0 |
| |
£29 |
18 |
0 |
The calamine of Alston-moor, used at Sheffield, is not so rich; it produces at most
only 25 per cent. of zinc. The coals are laid down at a cost of 5s. 8d. per ton; and the
calamine laid down there 5l.; whence the zinc will amount to 32l. 14s. per ton. The
considerable importations of zinc from Belgium and Germany, for some years back,
have caused a considerable fall in its price.
At Lüttich, where the calamine of Altenberg, near Aix-la-Chapelle, is smelted, a
reduction furnace, containing long horizontal earthen tubes, is employed. The roasted
calamine is finely ground, and mixed with from one-third to two-thirds its volume of
coke or charcoal, broken to pieces the size of nuts.
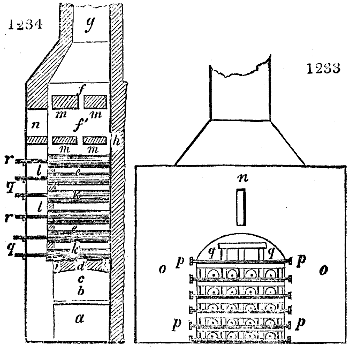
Fig. 1233. represents this zinc furnace in elevation; and fig. 1234. in a vertical
section through the middle. From the hearth to the bottom of the chimney it is
9 feet high, and the chimney itself is
18 or 20 feet high. a, is the ash-pit;
b, the grate; c, the fireplace; d, the
hearth; e, e, the laboratory; f, the
upper arch, which closes in the laboratory;
f, the second arch, which
forms the hood-cap of the furnace; g,
the chimney; h, the fire-wall, which
rests against a supporting wall of the
smelting-house. Through the vaulted
hearth the flame of the fire draws
through ten flues i, i, two placed in
one line; betwixt these 5 pairs of
draught openings, upon the sole of the
hearth, the undermost earthen tubes k,
immediately rest. The second and
third rows of tubes k, k, lie in a
parallel direction over each other, at
about one inch apart; in the sixth
row there are only two tubes; so that
there are 22 tubes altogether in one furnace. At their two ends these tubes rest[1333]
upon fire-tiles, which form, with the side-walls, a kind of checquer-work l, l. The
tubes are 4 feet long, 4 to 5 inches in diameter within, 5⁄4 of an inch thick. The fire,
which arrives at the laboratory through the flues i, i, plays round the tubes, and passes
off through the apertures m, m, in both arches of the furnace, into the chimney. n,
is an opening in the front wall between the two arches, which serves to modify the
draught, by admitting more or less of the external air.
The two slender side walls o, o, of the furnace, are a foot distant from the chequer-work,
so that on the horizontal iron bars q, q, supported by the hooks p, p, the iron
receivers r, r, may have room to rest at their fore part. These receivers are conical
pipes of cast iron, 11⁄2 foot long, posteriorly 11⁄2 inch, and anteriorly 1 inch wide at the
utmost. After the earthen tubes have been filled with the ore to be smelted, these
conical pipes are luted to them in a slightly slanting position. These cones last no
more than three weeks; and are generally lengthened with narrow-mouthed wrought-iron
tubes, to prevent the combustion of the zinc, by contact of air. When the furnace
is in activity, a blue flame is to be seen at the mouths of all these pipes. Every two
hours the liquefied metal is raked out into a
shovel placed beneath; and in 12 hours the charge
is distilled; after which the tubes are cleared out,
and re-charged. 100 pounds of metallic zinc are
the product of one operation. It is remelted at a
loss of 10 per cent., and cast into moulds for sale.
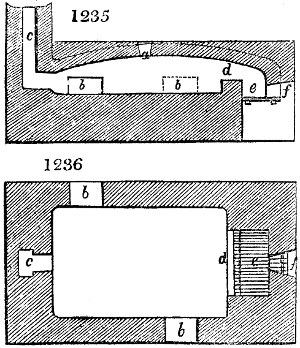
Fig. 1235. is a longitudinal section of the furnace
for calcining calamine in Upper Silesia;
fig. 1236. is a ground-plan of the furnace. a, is the
orifice in the vault or dome, for the introduction
of the ore; b, b, apertures in the side-walls, shut
with doors, through which the matter may be
turned over; c, the chimney; d, the fire-bridge;
e, the grate; f, the feed opening of the fire, the
fuel being pitcoal. The calamine is stirred about
every hour; and after being well calcined during
5 or 6 hours, it is withdrawn; and a new charge
is put in. These Silesian furnaces admit of 30
cwt. at a time; and for roasting every 100 cwt. 15 Prussian bushels of fuel, equal to 23
English bushels, are employed. These calcining furnaces are sometimes built alongside
of the zinc smelting-furnaces, and are heated by
the waste flame of the latter. The roasting is performed
in the Netherlands in shafts, like small blast
iron-furnaces, called schachtofen.
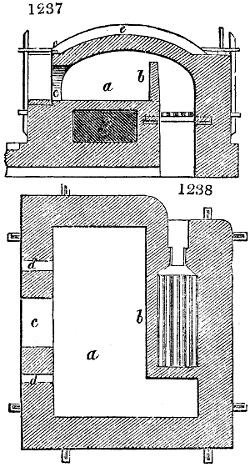
The hearth a, in figs. 1237, 1238., is constructed for
working with 5 muffles, one of which is long, and four
short. The muffles are made upon moulds, of fire-clay
mixed with ground potsherds. The receivers
are stoneware bottles. The grate is 10 inches beneath
the level of the hearth. b, the fire-bridge, is
proportionally high, to diminish the force of the flame
upon the hearth, that it may not strike the muffles.
c, is the opening through which the muffles are put
in and taken out; during the firing it is partly filled
with bricks, so that the smoke and flame may escape
between them; d, d, are openings for adjusting the
positions of the muffles; e, cross hoops of iron, to
strengthen the brick arch; f, is a bed of sand under
the sole of the hearth. During the first two days, the
fire is applied under the grating; the heat must be
very slowly raised to redness, at which pitch it must
be maintained during two days. From 8 to 10 days
are required for the firing of the muffles.
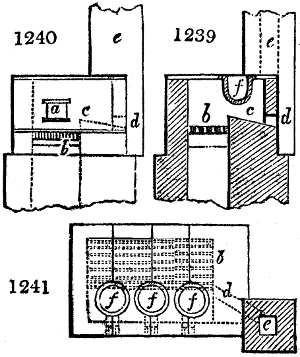
The furnace shown in figs. 1239, 1240, 1241. is for
the melting of the metallic zinc. Fig. 1240. is a
front view; fig. 1239. a transverse section; fig. 1241.
a view from above: a, is the fire-door; b, the grate;
c, the fire-bridge; d, the flue; e, the chimney;
f, f, f, cast-iron melting-pots, which contain each about 10 cwt. of the metal. The heat is
moderated by the successive addition of pieces of cold zinc. The inside of the pots should
be coated with loam, to prevent the iron being attacked by the zinc. When the[1334]
zinc is intended to be laminated, it should be melted with the lowest possible heat, and
poured into hot moulds.
When the zinc ores contain cadmium, this metal distils over in the form of brown
oxide, with the first portions, being more volatile
than zinc.
Under Brass and Copper, the most useful alloys
of zinc are described. The sulphate, vulgarly called
white vitriol, is made from the sulphuret, by roasting
it gently, and then exposing it upon sloping
terraces to the action of air and moisture, as has
been fully detailed under Sulphate of Iron. The
purest sulphate of zinc is made by dissolving the
metal in dilute sulphuric acid, digesting the solution
over some of the metal, filtering, evaporating, and
crystallizing.
Sulphate of zinc is added as a drier to japan
varnishes.
The ordinary zinc found in the market is never
pure; but contains lead, cadmium, arsenic, copper,
iron, and carbon; from some of which, it may be
freed in a great degree by distillation; but even after this process it retains a little
lead, with all the arsenic and cadmium. The separation of the latter is described
under Cadmium. Zinc, free from other metals, may be obtained by distilling a mixture
of charcoal and its subcarbonate, precipitated from the crystallized sulphate by carbonate
of soda. By holding a porcelain saucer over the flame of hydrogen produced
from the action of dilute sulphuric acid upon any sample of the zinc of commerce, the
presence of arsenic in it may be made manifest by the deposit of a gray film of the
latter metal. Antimony, however, produces a somewhat similar effect to arsenic.
Zinc is extensively employed for making water-cisterns, baths, spouts, pipes, plates
for the zincographer, for voltaic batteries, filings for fire-works, covering roofs, and a
great many architectural purposes, especially in Berlin; because this metal, after it gets
covered with a thin film of oxide or carbonate, suffers no further change by long exposure
to the weather. One capital objection to zinc as a roofing material, is its combustibility.
Chloride of zinc has been recently used with great advantage as an escharotic for
removing cancerous tumours, and healing various ill-constitutioned ulcers. It, as also
the nitrate, forms an ingredient in the resist pastes for the pale blues of the indigo vat.
Spelter (zinc) imported for home consumption—in 1835, 52,604 cwts.; in 1836,
47,406 cwts. Duty,—in cakes, 2s.; not in cakes, 10s. per cwt.
THE END.
London:
Printed by A. Spottiswoode
New-Street-Square
Alphabetical List of Articles.
ABB-WOOL —
ACETATE —
ACETATE OF ALUMINA —
ACETIC ACID —
ACETIMETER —
ACETONE —
ACID OF ARSENIC —
ACIDS —
ACROSPIRE —
ADDITIONS —
ADIPOCIRE —
ADIT —
ADULTERATION —
ÆTHER —
AFFINITY —
AGARIC —
AGATE —
AIR —
ALABASTER —
ALBUM GRÆCUM —
ALCARAZZAS —
ALCOHOL —
ALE —
ALEMBIC —
ALEMBROTH —
ALGAROTH —
ALIZARINE —
ALKALI —
ALKALIMETER —
ALKANA —
ALKANET —
ALLIGATION —
ALLOY —
ALMOND —
ALMOND OIL —
ALOE —
ALUDEL —
ALUM —
AMADOU —
AMALGAM —
AMALGAMATION —
AMBER —
AMBERGRIS —
AMIANTHUS —
AMMONIA —
AMMONIAC —
AMORPHOUS —
ANALYSIS —
ANCHOR —
ANIMÉ —
ANKER —
ANNEALING or NEALING —
ANNOTTO —
ANTHRACITE —
ANTIGUGGLER —
ANTIMONY —
ANTISEPTICS —
ANVIL —
AQUA REGIA —
AQUA VITÆ —
AQUAFORTIS —
ARCHIL —
ARDENT SPIRIT —
AREOMETER OF BAUMÉ —
ARGILLACEOUS EARTH —
ARGOL —
ARMS —
ARRACK —
ARROW ROOT —
ARSENIC —
ARTESIAN WELLS —
ASPHALTUM —
ASSAY and ASSAYING —
ATOMIC WEIGHTS or ATOMS —
ATTAR OF ROSES —
AURUM MUSIVUM —
AUTOMATIC —
AUTOMATON —
AXE —
AXLES —
AXUNGE —
AZOTIZED —
AZURE
BABLAH —
BAGASSE —
BAKING —
BALANCE —
BALSAMS —
BANDANNA —
BARBERRY —
BARILLA —
BARIUM —
BARK OF OAK —
BARLEY —
BARM —
BARYTA or BARYTES —
BASSORINE —
BATHS —
BDELLIUM —
BEER —
BEET-ROOT SUGAR —
BELL-METAL —
BELLOWS —
BEN OIL —
BENGAL STRIPES —
BENJAMIN or BENZOIN —
BERLIN BLUE —
BERRIES OF AVIGNON —
BERYL —
BEZOAR —
BILE —
BIRDLIME —
BISMUTH —
BISTRE —
BITTER PRINCIPLE —
BITUMEN, or ASPHALTUM —
BLACK DYE —
BLACK PIGMENT —
BLEACHING —
BLENDE —
BLOCK MANUFACTURE —
BLOOD —
BLOWING MACHINE —
BLOWPIPE —
BLUE DYES —
BLUE PIGMENTS —
BLUE VITRIOL —
BOMBAZINE —
BONE BLACK —
BONES —
BOOKBINDING —
BORAX —
BOTTLE MANUFACTURE —
BOUGIE —
BRACES —
BRAIDING MACHINE —
BRAN —
BRANDY —
BRASS —
BRAZIL-WOOD —
BRAZING —
BREAD —
BRECCIA —
BREWING —
BRICK —
BRIMSTONE —
BRITISH GUM —
BROMINE —
BRONZE —
BROWN DYE —
BRUSHES —
BUTTER —
BUTTER OF CACAO —
BUTTON MANUFACTURE
CABLE —
CACAO, BUTTER OF —
CADMIUM —
CAFEINE —
CAJEPUT OIL —
CALAMANCO —
CALAMINE —
CALC-SINTER —
CALC-TUFF —
CALCAREOUS EARTH —
CALCAREOUS SPAR —
CALCEDONY —
CALCHANTUM —
CALCINATION —
CALCIUM —
CALCULUS —
CALENDER —
CALICO-PRINTING —
CALOMEL —
CALORIC —
CALORIFÈRE OF WATER —
CAMBRIC —
CAMLET OR CAMBLET —
CAMPHOR, or CAMPHIRE —
CAMWOOD —
CANDLE —
CANE-MILL —
CANNON —
CANVASS —
CAOUTCHOUC, GUM-ELASTIC, OR INDIAN-RUBBER —
CAPERS —
CAPSTAN —
CARAT or CARACT —
CARBON —
CARBONATE OF AMMONIA —
CARBONATED WATER —
CARBONATES —
CARBONIC ACID —
CARBONIC OXIDE —
CARBUNCLE —
CARBURET OF SULPHUR —
CARBURETTED HYDROGEN —
CARD CUTTING —
CARDS —
CARDS, PLAYING —
CARMINE —
CARPET —
CARTHAMUS —
CASE-HARDENING —
CASHMERE or CACHEMERE —
CASK —
CASSAVA —
CASSIS —
CASTING OF METALS —
CASTOR —
CASTOR OIL —
CASTOR or CASTOREUM —
CASTORINE —
CATECHU —
CATGUT —
CATHARTINE —
CAUSTIC —
CAVIAR —
CAWK —
CEDRA —
CELESTINE —
CEMENTATION —
CEMENTS —
CERASIN —
CERATE —
CERINE —
CERIUM —
CERUSE —
CETINE —
CHAINWORK —
CHALK —
CHALK—Black —
CHALK—French —
CHALK—Red —
CHARCOAL —
CHICA —
CHIMNEY —
CHINTZ —
CHLORATE OF POTASH —
CHLORATES —
CHLORIC ACID —
CHLORINE —
CHLOROMETRY —
CHOCOLATE —
CHROMATES —
CHROMIC ACID —
CHROMIUM —
CINNABAR —
CINNAMON —
CITRIC ACID —
CIVET —
CLAY —
CLOTH-BINDING —
CLOTH, MANUFACTURE OF —
COBALT —
COCCULUS INDICUS, or Indian berry —
COCHINEAL —
COCOA, STEARINE, AND ELAINE —
COFFEE —
COKE —
COLCOTHAR OF VITRIOL —
COLOPHANY —
COLOURING MATTER —
COLUMBIUM —
COLZA —
COMB —
COMBINATION —
COMBUSTIBLE —
COMBUSTION —
COMPOUND COLOURS —
CONCRETE —
CONGELATION —
COOLING OF FLUIDS —
COPAL —
COPPER —
COPPER, Statistics of —
COPPERAS —
CORAL —
CORK —
CORROSIVE SUBLIMATE —
CORUNDUM; or Telesie —
COTTON DYEING —
COTTON MANUFACTURE —
COURT PLASTER —
CRAPE —
CRAYONS —
CRAYONS, lithographic —
CREOSOTE —
CRUCIBLES —
CRYSTAL —
CUDBEAR —
CUPELLATION —
CURRYING OF LEATHER —
CUTLERY —
CYANATES —
CYANHYDRIC Acid —
CYANIDES —
CYANIDES, FERRO —
CYANOGEN —
CYDER
DAHLINE —
DAMASCUS BLADES —
DAMASK —
DAMASKEENING —
DAMASSIN —
DAMPS —
DAPHNINE —
DATOLITE —
DECANTATION —
DECOCTION —
DECOMPOSITION —
DECREPITATION —
DEFECATION —
DEFLAGRATION —
DELIQUESCENT —
DELPHINIA —
DEPHLEGMATION —
DEPHLOGISTICATED —
DEPILATORY —
DETONATION —
DEUTOXIDE —
DEXTRINE —
DIAMOND —
DIAMOND MICROSCOPES —
DIAMONDS, cutting of —
DIAPER —
DIASTASE —
DIES FOR STAMPING —
DIGESTER —
DIMITY —
DISTILLATION —
DOCIMACY —
DORNOCK —
DRAGON’S BLOOD —
DRUGGET —
DRYING HOUSE —
DUCTILITY —
DUNGING —
DYEING
EARTHS —
EAU DE COLOGNE —
EAU DE LUCE —
EBULLITION —
EDGE-TOOLS —
EDULCORATE —
EFFERVESCENCE —
EFFLORESCENCE —
EGGS, HATCHING —
EIDER-DOWN —
ELAINE —
ELASTIC BANDS —
ELECTIVE AFFINITY —
ELEMENTS —
ELEMI —
ELUTRIATE —
EMBALMING —
EMBOSSING CLOTH —
EMBOSSING WOOD —
EMBROIDERING MACHINE —
EMERALD —
EMERY —
EMPYREUMA —
ENAMELS —
EPSOM SALTS —
EQUIVALENTS, CHEMICAL —
ESSENCE D’ORIENT —
ESSENCES —
ETCHING Varnish —
ETHER —
ETHER, Acetic —
ETHIOPS —
EUDIOMETER —
EVAPORATION —
EXPANSION —
EXTRACTS
FAHLERZ —
FAINTS —
FAN —
FARINA —
FATS —
FAULTS —
FEATHERS —
FECULA —
FELSPAR —
FELTING —
FERMENT —
FERMENTATION —
FERROCYANATE, or, FERROCYANIDE —
FERROPRUSSIATES —
FIBRE, VEGETABLE —
FIBRINE —
FILE —
FILLIGREE —
FILTRATION —
FIRE ARMS, MANUFACTURE OF —
FIRE-DAMP —
FIRE-WORKS —
FISH-HOOKS —
FLAKE WHITE —
FLAME —
FLANNEL —
FLAX —
FLINT —
FLOSS —
FLOSS-SILK —
FLOUR —
FLOUR OF WHEAT, Adulterations of, to Detect —
FLOWERS —
FLOWERS, ARTIFICIAL, MANUFACTURE OF —
FLUATES —
FLUOR SPAR —
FLUX —
FLY POWDER —
FODDER —
FONDUS —
FORGE —
FORMIATES —
FORMIC ACID —
FORMULÆ, CHEMICAL —
FOUNDING —
FOUNTAIN —
FOXING —
FRANKFORT BLACK —
FREEZING —
FRENCH BERRIES —
FRICTION, counteraction of —
FRIT —
FUEL —
FULGURATION —
FULLER’S EARTH —
FULLING —
FULLING MILL —
FULMINATES —
FULMINIC ACID —
FUMIGATION —
FUR —
FURNACE OF ASSAY —
FUSIBILITY —
FUSIBLE METAL —
FUSTET —
FUSTIAN —
FUSTIC
GABRONITE —
GADOLINITE —
GALACTOMETER, or LACTOMETER —
GALBANUM —
GALENA —
GALIPOT —
GALL OF ANIMALS, or OX-GALL, purification of —
GALL OF GLASS —
GALL-NUTS, or GALLS —
GALLATES —
GALLIC ACID —
GALLIPOLI OIL —
GALVANIZED IRON —
GAMBOGE —
GANGUE —
GARNET —
GAS —
GAS-HOLDER —
GAS-LIGHT —
GASOMETER —
GAUZE WIRE CLOTH —
GAY-LUSSITE —
GELATINE —
GEMS —
GEOGNOSY —
GERMAN SILVER —
GERMINATION —
GIG MACHINES —
GILDING —
GIN, or Geneva, —
GINNING —
GLANCE COAL —
GLASS —
GLASS CUTTING AND GRINDING —
GLASS MAKING —
GLAUBER SALT —
GLAZES —
GLAZIER —
GLOVE MANUFACTURE —
GLOVE-SEWING —
GLUCINA —
GLUE —
GLUTEN —
GLYCERINE —
GNEISS —
GOLD —
GONG-GONG; or tam-tam —
GONIOMETER —
GRADUATOR —
GRANITE —
GRANULATION —
GRAPHITE —
GRAUWACKE or GREYWACKE —
GRAY DYE —
GREEN DYE —
GREEN PAINTS —
GREEN VITRIOL —
GUAIAC —
GUANO —
GUM —
GUM RESINS —
GUNPOWDER —
GYPSUM
HADE —
HAIR —
HAIR PENCILS OR BRUSHES —
HALOGENE —
HANDSPIKE —
HARDNESS —
HARTSHORN, SPIRIT OF —
HAT MANUFACTURE —
HATCHING OF CHICKENS —
HEALDS —
HEARTH —
HEAT —
HEAT-REGULATOR —
HEAVY SPAR —
HECKLE —
HELIOTROPE —
HEMATINE —
HEMATITE —
HEMP —
HEPAR —
HEPATIC AIR —
HERMETICAL SEAL —
HIDE —
HIRCINE —
HOG’s LARD —
HONEY —
HONEY-STONE —
HOP —
HORDEINE —
HORN —
HORNSILVER —
HORNSTONE —
HORSE POWER —
HOSIERY —
HOT-FLUE —
HYDRATES —
HYDRAULIC PRESS —
HYDRIODIC ACID —
HYDROCHLORIC ACID —
HYDROGEN —
HYDROMETER —
HYDROSULPHURETS —
HYMEN[OE]A COURBARIL —
HYOSCIAMUS NIGER —
HYPEROXYMURIATES —
HYPOSULPHATES; HYPOSULPHITES
ICEHOUSE —
IMPERMEABLE —
INCOMBUSTIBLE CLOTH —
INCUBATION, ARTIFICIAL —
INDIAN RUBBER —
INDIGO —
INK —
INULINE —
IODINE —
IRIDIUM —
IRON —
ISINGLASS, or Fish-glue —
ISLAND MOSS —
IVORY —
IVORY BLACK
JACK —
JACK and JACK-SINKERS —
JACK-BACK —
JACQUARD —
JADE —
JAPANNING —
JASPER —
JELLY, ANIMAL —
JELLY, VEGETABLE —
JET —
JEWELLERY, Art of.
KALI —
KAOLIN —
KARABÉ —
KELP —
KERMES —
KILLAS —
KILN —
KINIC ACID —
KINO —
KIRSCHWASSER —
KNOPPERN —
KOUMISS
LABDANUM or LADANUM —
LABRADORITE, OPALINE or LABRADORE FELSPAR —
LABYRINTH —
LAC, LAC-DYE —
LACCIC ACID —
LACCINE —
LACE MANUFACTURE —
LACQUER —
LACTIC ACID —
LACTOMETER —
LAKES —
LAMINABLE —
LAMIUM ALBUM —
LAMP OF DAVY —
LAMP-BLACK —
LAMPATES and LAMPIC ACID —
LAMPS —
LAPIDARY, Art of —
LAZULITE —
LEAD —
LEAD-SHOT —
LEATHER —
LEDUM PALUSTRE —
LEGUMINE —
LEMONS —
LEUCINE —
LEUCITE —
LEVIGATION —
LEWIS —
LIAS —
LIBAVIUS, LIQUOR OF —
LICHEN. —
LIGNEOUS MATTER —
LIGNITE —
LILAC DYE —
LIMESTONE —
LINEN —
LINSEED —
LIQUATION —
LIQUEURS, LIQUORISTE —
LIQUID AMBER —
LITHARGE —
LITHIA —
LITHIUM —
LITHOGRAPHY —
LITMUS —
LIXIVIATION —
LOADSTONE, MAGNETIC IRON-STONE —
LOAM —
LODE —
LOGWOOD —
LOOM —
LUBRICATION —
LUPININE —
LUPULINE —
LUTE —
LUTEOLINE —
LYCOPODIUM CLAVATUM —
LYDIAN STONE
MACARONI —
MACE —
MACERATION —
MACLE —
MADDER —
MADREPORES —
MAGISTERY —
MAGISTRAL —
MAGMA —
MAGNANIER —
MAGNESIA —
MAGNESIA, NATIVE —
MAGNESIAN LIMESTONE —
MAGNESITE —
MAGNET, NATIVE —
MAHALEB —
MALACHITE —
MALATES —
MALIC ACID —
MALLEABILITY —
MALT —
MALT KILN —
MALTHA —
MANGANESE —
MANGLE —
MANIOC —
MANNA —
MARBLE —
MARCASITE —
MARGARATES —
MARGARIC ACID —
MARINE ACID —
MARINE SALT —
MARL —
MARQUETRY —
MARTIAL —
MASSICOT —
MASTIC —
MATRASS —
MATTE —
MEADOW ORE —
MEDALS —
MEERSCHAUM —
MELLITE —
MELLITIC ACID —
MELLON —
MENACHANITE —
MERCURY or QUICKSILVER —
MERCURY, BICHLORIDE OF —
MERCURY, PROTOCHLORIDE OF —
METALLURGY —
METALS —
METEORITES —
METHYLÈNE —
MICA —
MICROCOSMIC SALT —
MILK —
MILL-STONE, or BUHR-STONE —
MINERAL WATERS —
MINES —
MINIUM —
MINT —
MIRRORS —
MISPICKEL —
MOHAIR —
MOIRÉE METALLIQUE —
MOLASSE —
MOLASSES —
MOLYBDENUM —
MORDANT (adhesive) —
MORDANT (colouring) —
MOROCCO —
MORPHIA —
MORTAR, HYDRAULIC —
MOSAIC —
MOSAIC GOLD —
MOTHER OF PEARL —
MOTHER-WATER —
MOUNTAIN SOAP —
MUCIC ACID —
MUCILAGE —
MUFFLE —
MUNDIC —
MUNJEET —
MURIATES —
MURIATIC or HYDROCHLORIC ACID —
MUSK —
MUSLIN —
MUST —
MUSTARD —
MUTAGE —
MYRICINE —
MYRRH
NACARAT —
NAILS, MANUFACTURE OF —
NANKIN —
NAPHTHA, or ROCK-OIL —
NAPHTHALINE —
NAPLES YELLOW —
NATRON —
NEALING —
NEB-NEB —
NEEDLE MANUFACTURE —
NEROLI —
NET —
NEUTRALIZATION —
NICARAGUA WOOD —
NICKEL —
NICOTIANINE —
NICOTINE —
NITRATE OF AMMONIA —
NITRATE OF LEAD —
NITRATE OF POTASH —
NITRATE OF SILVER —
NITRATE OF SODA —
NITRATE OF STRONTIA —
NITRIC ACID —
NITRO-MURIATIC ACID —
NITROGEN GAS, or AZOTE —
NITROGEN, DEUTOXIDE OF —
NITROGEN, PROTOXIDE OF —
NITROUS ACID —
NOPAL —
NUT OIL —
NUTMEG —
NUX VOMICA
OAK BARK —
OATS —
OBSIDIAN —
OCHRE —
OIL OF VITRIOL —
OILS —
OILS, VOLATILE OR ESSENTIAL; Manufacture of —
OLEATES —
OLEFIANT GAS —
OLEIC ACID —
OLEINE —
OLIBANUM —
OLIVE OIL —
ONYX —
OOLITE —
OOST, or OAST —
OPAL —
OPERAMETER —
OPIUM —
OPOBALSAM —
OPOPONAX —
ORANGE DYE —
ORCINE —
ORES —
ORPIMENT —
ORYCTNOGNOSY —
OSMIUM —
OSTEOCOLLA —
OXALATES —
OXALIC ACID —
OXIDES —
OXISELS —
OXYGEN
PACKFONG —
PACO, or PACOS —
PADDING MACHINE —
PAINT —
PAINTS, GRINDING OF —
PAINTS, VITRIFIABLE —
PALLADIUM —
PALM OIL —
PAPER CUTTING —
PAPER-HANGINGS —
PAPER, MANUFACTURE OF —
PARAFFINE —
PARCHMENT —
PARTING —
PASTEL (colour) —
PASTEL (crayon) —
PASTES, or FACTITIOUS GEMS —
PASTILLE (perfumery) —
PASTILLE (tablet) —
PE-TUNT-SE —
PEARLASH —
PEARLS —
PEARLS, ARTIFICIAL —
PEARLWHITE —
PECTIC ACID —
PECTINE —
PELTRY —
PENCIL MANUFACTURE —
PENS, STEEL —
PEPPER —
PERFUMERY, ART OF —
PERRY —
PERSIAN BERRIES —
PETROLEUM —
PEWTER, PEWTERER —
PHOSPHORIC ACID —
PHOSPHORUS —
PICAMARE —
PICROMEL —
PICROTOXINE —
PIGMENTS, VITRIFIABLE —
PIMENTO —
PIN MANUFACTURE —
PINCHBECK —
PINE-APPLE YARN and CLOTH —
PINEY TALLOW —
PIPERINE —
PITCH of wood-tar —
PITCH, MINERAL —
PITCOAL —
PITCOAL, COKING OF —
PITTACALL —
PLASTER —
PLASTER OF PARIS —
PLATED MANUFACTURE —
PLATINA-MOHR —
PLATINUM —
PLUMBAGO —
PLUSH —
POINT NET —
PORCELAIN —
PORPHYRY —
PORTER —
PORTLAND STONE —
POTASH, or POTASSA —
POTASSIUM —
POTATO —
POTTERY, PORCELAIN. —
PRECIPITATE —
PRECIPITATION —
PRESS, HYDRAULIC —
PRINCE’S METAL, or Prince Rupert’s metal —
PRINTING INK —
PRINTING MACHINE —
PRUSSIAN BLUE, and PRUSSIATE OF POTASH —
PUMICE-STONE —
PUOZZOLANA —
PURPLE OF CASSIUS, Gold purple —
PURPLE OF MOLLUSCA —
PURPURIC ACID —
PURPURINE —
PUTREFACTION and its prevention —
PYRITES —
PYRO-ACETIC SPIRIT —
PYROLIGNOUS ACID —
PYROLIGNOUS or PYROXILIC SPIRIT —
PYROMETER —
PYROPHORUS —
PYROTECHNY —
PYROXILINE
QUARTATION —
QUARTZ —
QUASSIA —
QUEEN’s WARE —
QUEEN’s YELLOW —
QUERCITRON —
QUICKLIME —
QUICKSILVER —
QUILL —
QUININA —
QUINTESSENCE
RAISINS —
RAPE-SEED —
RASP, MECHANICAL —
RATAFIA —
REALGAR —
RECTIFICATION —
RED LIQUOR —
REED —
REFINING OF GOLD AND SILVER —
REFRIGERATION OF WORTS, &c. —
REGULUS —
RESIN, KAURI or COWDEE —
RESINS —
RETORT —
REVERBERATORY FURNACE —
RHODIUM —
RIBBON MANUFACTURE —
RICE —
RICE CLEANING —
RIFLE —
RINSING MACHINE —
ROCKETS —
ROLLING-MILL —
ROPE-MAKING —
ROSIN GAS —
ROSIN, or COLOPHANY —
ROTTEN-STONE —
ROUGE —
RUBY —
RUM —
RUST —
RYE
SAFETY LAMP —
SAFFLOWER —
SAFFRON —
SAGO —
SAL AMMONIAC —
SAL PRUNELLA —
SAL VOLATILE —
SALAMSTONE —
SALEP, or SALOUP —
SALICINE —
SALT OF AMBER —
SALT OF LEMONS —
SALT OF SATURN —
SALT OF SODA —
SALT OF SORREL —
SALT OF TARTAR —
SALT OF VITRIOL —
SALT PERLATE —
SALT, EPSOM —
SALT, MICROCOSMIC —
SALT, SEA, or CULINARY; Chloride of sodium —
SALT, SEDATIVE —
SALTPETRE —
SALTS —
SAND —
SANDAL or RED SAUNDERS WOOD —
SANDARACH —
SAPAN WOOD —
SARD —
SATIN —
SATURATION —
SCALIOLA —
SCARLET DYE —
SCHEELE’S GREEN —
SCHWEINFURTH GREEN —
SCOURING —
SEA WATER —
SEAL ENGRAVING —
SEALING-WAX —
SEGGAR, or SAGGER —
SELENIUM —
SELTZER WATER —
SEPIA —
SEPTARIA —
SERPENTINE —
SHAFT —
SHAGREEN —
SHALE, or SLATE-CLAY —
SHAMOY LEATHER —
SHEATHING OF SHIPS —
SHELLAC —
SIENITE —
SILICA and SILICON —
SILICATES —
SILICON —
SILK MANUFACTURE —
SILKWORM GUT —
SILVER —
SILVER LEAF —
SILVERING —
SIMILOR —
SINGEING OF WEBS —
SKIN —
SLAG —
SLATES —
SMALL WARES —
SMALT —
SMELTING —
SOAP —
SOAPSTONE —
SODA-WATER —
SODA, Caustic soda —
SODA, CARBONATE OF —
SODIUM —
SOLDERING —
SOOT —
SORBIC ACID —
SOY —
SPECIFIC GRAVITY —
SPECULUM METAL —
SPERMACETI —
SPIRIT OF AMMONIA —
SPIRIT OF WINE —
SPIRITS, VINOUS —
SPONGE —
SPOON MANUFACTURE —
STAINED GLASS —
STAMPING OF METALS —
STARCH —
STARCHING AND STEAM-DRYING APPARATUS —
STEAM —
STEARIC ACID, improperly called STEARINE —
STEARINE COLD PRESS —
STEATITE —
STEEL —
STEREOTYPE PRINTING —
STILL —
STOCKING MANUFACTURE —
STONE —
STONE, ARTIFICIAL —
STONEWARE —
STORAX, STYRAX —
STOVE —
STRASS —
STRAW-HAT MANUFACTURE —
STRETCHING MACHINE —
STRONTIA —
STRYCHNIA —
STUCCO —
SUBERIC ACID —
SUBLIMATE —
SUBLIMATION —
SUBSALT —
SUCCINIC ACID, Acid of Amber —
SUGAR —
SUGAR OF LEAD —
SULPHATE OF ALUMINA AND POTASSA —
SULPHATE OF AMMONIA —
SULPHATE OF BARYTA —
SULPHATE OF COPPER —
SULPHATE OF IRON —
SULPHATE OF LIME —
SULPHATE OF MAGNESIA, Epsom Salt —
SULPHATE OF MANGANESE —
SULPHATE OF MERCURY —
SULPHATE OF POTASSA —
SULPHATE OF SODA —
SULPHATE OF ZINC —
SULPHATES —
SULPHITES —
SULPHOSELS —
SULPHUR; Brimstone —
SULPHURATION —
SULPHURETTED HYDROGEN —
SULPHURIC ACID, Vitriolic Acid, or Oil of Vitriol —
SUMACH —
SWEEP-WASHER —
SYNTHESIS —
SYRUP
TABBYING, or WATERING —
TACAMAHAC —
TAFFETY —
TAFIA —
TALC —
TALLOW —
TALLOW, PINEY —
TAMPING —
TAN, or TANNIC ACID —
TANNING —
TANTALUM —
TAPESTRY —
TAPIOCA —
TAR —
TARRAS —
TARTAR —
TARTARIC ACID —
TARTRATES —
TAWING —
TEA —
TEASEL —
TEETH —
TELLURIUM —
TERRA DI SIENA —
TERRA-COTTA —
TESTS —
TEXTILE FABRICS —
THENARD’S BLUE, or COBALT BLUE —
THERMOMETER —
THERMOSTAT —
THIMBLE —
THORINA —
THREAD MANUFACTURE —
TILES —
TILTING OF STEEL —
TIN —
TIN MORDANTS —
TIN-GLASS —
TIN-PLATE —
TINCAL —
TINCTORIAL MATTER —
TINCTURE —
TITANIUM —
TOBACCO —
TOBACCO-PIPES —
TODDY —
TOLU —
TOMBAC —
TONKA BEAN —
TOPAZ —
TORTOISE-SHELL —
TOUCH-NEEDLES, and TOUCH-STONE —
TOW —
TRAGACANTH, GUM —
TRAVERTINO —
TREACLE —
TRIPOLI —
TUFA, or TUF —
TULA METAL —
TUNGSTEN —
TURBITH MINERAL —
TURF —
TURKEY RED —
TURMERIC, Curcuma, Terra merita —
TURNSOLE —
TURPENTINE —
TURPENTINE, OIL OF —
TURQUOIS —
TUTENAG —
TYPE
ULTRAMARINE —
UMBER —
URANIUM —
URAO
VALONIA —
VANADIUM —
VANILLA —
VAPOUR —
VARNISH —
VEIN STONES, or GANGUES —
VEINS —
VELLUM —
VELVET —
VENETIAN CHALK —
VENTILATION —
VENUS —
VERATRINE —
VERDIGRIS —
VERDITER, or BLUE VERDITER —
VERDITER, or BREMEN GREEN —
VERMICELLI —
VERMILLION, or Cinnabar —
VINEGAR MANUFACTORY, BY MALT —
VIOLET DYE —
VITRIFIABLE COLOURS —
VITRIOL
WACKE —
WADD —
WADDING —
WAFERS —
WALNUT HUSKS, or PEELS —
WARP —
WASH —
WASHING —
WATER-PROOF CLOTH —
WATERING OF STUFFS —
WATERS, MINERAL —
WAX —
WAX, MINERAL, or Ozocerite, —
WEAVING —
WEFT —
WELD —
WELDING —
WELLS, ARTESIAN —
WHALEBONE —
WHEAT —
WHEEL CARRIAGES —
WHETSLATE —
WHEY —
WHISKEY —
WHITE LEAD —
WICK —
WINCING-MACHINE —
WINE —
WINE-STONE —
WINE, FAMILY —
WIRE-DRAWING —
WOAD —
WOLFRAM —
WOOD —
WOOF —
WOOLLEN MANUFACTURE —
WOOTZ —
WORT —
WOULFE’S APPARATUS
XANTHINE
YEAST —
YELLOW DYE —
YELLOW, KING’S —
YTTRIA
ZAFFRE —
ZEDOARY —
ZIMOME —
ZINC —
ZIRCON —
ZIRCONIA
Transcriber’s Notes
General
This e-text follows the text of the original work, including inconsistencies in spelling, hyphenation, capitalisation and typography. In particular, the following have not been standardised or corrected (except when mentioned below):
- the two types of fractions used (21⁄2 and 1-10th, for example);
- errors in calculations (for example, constituent parts that do not add up to the total given);
- articles that are not in alphabetical order have not been moved (note that I and J are considered to be equivalent, as in Jasper — Icehouse — Jelly);
- missing reference letters/figures in illustrations;
- inconsistent numbering of sections and paragraphs (for example, there may be a paragraph number 1, but no number 2 or further; there may be a section number II, but no number I).
Some illustrations are missing from the original and are not referred to in the text (Figs. 57, 372), others are provided in the original work, but not discussed or described. Fig. 93 is not present in the original, but is described in the text. The original has two illustrations numbered 384; the second one (on page 464) has been renamed 384*. Figures 496/497 and 498/499 are remarkably similar, as is their description.
Several illustrations referred to in the text do not show the reference letters, numbers or symbols mentioned in the text.
Hyperlinks have been provided for explicit internal references, and where they seemed useful.
Several wide tables have been optimised for wide (browser) windows and may not look optimal in narrow windows or other formats.
For some illustrations enlarged versions are available in order to make details and reference letters better visible (not available in all formats).
Remarks on the text
Fontanier and Fontanieu probably refer to the same person.
The text occasionally gives ohm and aime as inits of volume; these are probably aams or ames (circa 30-36 imperial gallons).
There are several references to a table of elasticities of vapour; it is not clear which table is meant.
Page 16, table: the temperature 164° stands out from the other temperatures, and may be wrong (possibly error for 194°)
Page 16, The specific gravity of water at 60° being 1000, at 62° it is 99,981. This phrase contains at least one mistake: the intention might be specific gravities 100 and 99·981, respectively (see also the table following on page 21/22).
Page 19, table, first row, column 70°: 9991 does not fit in with the other data, this is possibly an error for 9981.
Page 25, table, first row (17°): 44·9 does not fit with the other data; this is possibly an error for 44·2; last row but one (24°): 693 does not fit with the other data; possibly an error for 993.
Page 26, table, row 20°: the last value was partly illegible in the original, this should probably be 68·4; row 22° the last decimal was illegible in the original, this should probably be 67·7 or 67·8.
Page 29, Lausania inermis: probably Lawsonia inermis.
Page 41, reference to Gems, cutting of: the article Gems refers to the article Lapidary for cutting of gems. Hence there are two hyperlinks here.
Page 46, Fig. 14, a plan of the same: Fig. 14 is a different side view.
Page 67, 1000 of a grain of silver: possibly an error for 1⁄1000 of a grain of silver.
Page 99, reference to Saccharometer: there is no article Saccharometer; the saccharometer is discussed in the article Beer only.
Page 111-112, description of location of elements relative to each other: the author has reversed left and right.
Page 118, See Oil of Ben: there is no such article; the only reference to Oil of Ben occurs on page 895 (table).
Page 150, teint, Germ.: probably error for teint, French.
Page 169, Fernambouc: the 16th century French name for Pernambuco.
Page 178, calculation: 315 loaves at 6d. give 7l. 17s. 6d., making the clear profit 11s. 10d.
Page 225, Chloride of Lime: there is no such article; chloride of lime is discussed under Bleaching, which is where the hyperlink points.
Page 249, reference to Mill: there is no such article; the article Sugar has a part on mills, to which the hyperlink points.
Page 339, first table: the first two columns do not add up to the sub-totals given; it is not clear whether this is due to errors in the data or to miscalculation.
Page 358, Fig. 337 and accompanying text: text and illustration do not seem to fit together, few of the parts mentioned appear in the figure.
Page 382, reference to Prussic Acid: the Dictionary does not have this article.
Page 392, the year 173: the last digit was missing from the original, and has been replaced with a tilde (173~). The 1858 enlarged edition gives 1730 as the year.
Page 396, by of a lever: possibly a word is missing (by means of a lever or similar).
Page 403, footnote [24]: 2198 should possibly read 1829.
Page 512, table Phosphorus: The two values in the last column are incompatible: the first should be one half of the second.
Page 522, about 61⁄2 per cent. upon: possibly a word (such as depending) is missing.
Page 564, second table: the totals do not always add up completely, and the meaning of some of the data is unclear.
Page 605, Abrabanya: should possibly be Abrudbánya
Page 632, dyeing of horse-hair: this is described in the same, not the next article; the hyperlink points to the relevant paragraph.
Page 726, reference to Malt: the hyperlink points to the article Malt Kiln rather than to Malt, as this seemed more appropriate.
Page 742, Bilin in Bohemia: also referred to as Billen and Billin.
Page 784, Houton-Libillardière: probably Jacques-Julien Houtou de La Billardière.
Page 876, The carriage is then again by the rotation ...: there is a verb missing from the text (advanced, moved forward, or similar).
Page 885, Nicaraca: possibly an error for Nicaragua.
Page 895, table, line 41: the specific gravity is likely to be a misprint.
Page 897: strychia: possibly an error for strychnia.
Page 920, three separate sheets: the three knives will cut four sheets.
Page 1093, table: dr. in the column Cochineal may be an error for oz.
Page 1151, reference to page 1041: there is no related information on this page; it has therefore not been linked.
Page 1204, arescence: possibly an error for acescence.
Page 1270, Then increase the gradually: a word is missing from the original (possibly heat or temperature).
Changes and corrections made
Some missing punctuation has been added and obvious minor typographical errors have been corrected silently.
Footnotes have been moved to under the paragraph etc. they refer to.
Illustrations have been moved to where they are first or most fully described, they are therefore not always given in numerical order (the original work does not always show them in numerical order either); some illustrations have been rotated; some hatching has been removed to show reference letters more clearly. Illustration numbers have been added to illustrations that lacked a number.
Multi-page tables have been changed to single tables; tables have been re-arranged or split; some tables are presented here with legends or table notes; in some tables thin lines have been added for ease of reading.
For the sake of consistency, the following changes have been made throughout the text:
- All references to illustration numbers in the text have been italicised (Fig. nnn etc.); reference letters in the text have been standardised to italics (for lower case letters) and small-caps (for upper case letters).
- Apostrophes in French words have been closed with the following word where necessary.
- All decimal points have been changed to mid-dots, commas and dashes have been changed to mid-dots or vice versa where necessary.
- Accents on French words printed in capitals have been moved where necessary (for example E' has been changed to É).
- The Alphabetical List of Articles has been added.
- The German ...saure in nouns has been standardised to ...säure.
- The author uses the single and double dashed pound sign; both are represented here by the same symbol (£). All £/l. s. and d. (pound, shilling, pence) have been italicised for consistency.
Other changes:
| Location |
Original document |
Changed to |
| Page vi, table |
201,773,872 |
204,773,872 |
| Page 14 |
olëine |
oleine as elsewhere |
| Page 15 |
Brongniard |
Brongniart |
| Page 22, table, row ·82 |
·70042 |
·00042 |
| ditto last line |
·100 |
1·00 |
| Page 49 |
αηθραξ |
ανθραξ |
| Page 57 |
a a |
A A as in illustration |
| Page 69, Fig. 87 |
-- |
letter c added |
| Page 75 |
Baume |
Baumé as elsewhere |
| Page 84 |
-- |
2. added before Balsams without benzoic acid |
| Page 92 |
intersticial |
interstitial as elsewhere |
| Page 109, second table |
980 |
0·980 |
| Page 112 |
the troughs u |
the troughs n as in the illustration |
| Page 122 |
Breislack |
Breislak as elsewhere |
| Page 144 |
sp. gr. 0837 |
sp. gr. 0·837 |
| ditto |
sheeps’ wool |
sheep’s wool as elsewhere |
| Page 167 |
fig. 165, between |
fig. 163. Between |
| Fig. 162 |
c |
e |
| Page 171 |
tonne au noir |
tonneau noir |
| Page 178, calculation |
7 17 5 |
7 17 6 |
| ditto |
0 11 61⁄2 |
0 11 91⁄2 |
| Page 183 |
Fig. 170 |
Fig. 171 |
| ditto |
Fig. 171 |
Fig. 170 |
| Page 215 |
Elberfeldt |
Elberfeld |
| Page 220, Fig. 234 |
d |
d′ as in text |
| Page 229 |
see ... page 224 |
see ... page 223 |
| Page 240 |
F′ G′ |
F G as in illustration |
| Page 243 |
tire |
tiré |
| ditto |
k |
K |
| Page 245 |
labiacæ |
labiatæ |
| Page 249 |
-- |
quote marks added after ... for making candles. |
| ditto |
undica |
indica |
| Page 278 |
60·30 feet |
69·39 feet |
| Page 311 |
rhamnus fragula |
rhamnus frangula |
| Page 318 |
Figs. 297, 298. |
Figs. 296, 298. |
| Page 331 |
its bottom, C |
its bottom, c |
| Page 336 |
Gtakamite |
Atakamite |
| Page 345, Fig. 318 |
-- |
′s added as mentioned in text |
| Page 356 |
pulley or whorl g |
pulley or whorl q as in drawing |
| Page 372 |
5·463 lbs. of force |
5463 lbs. of force |
| Page 385 |
45 (or nearly so) |
45° (or nearly so) |
| Page 394 |
Münzstampeln |
Münzstempeln |
| Page 403 |
are constantly making |
are constantly made |
| Page 450 |
1·375 |
137·5 |
| Page 464 |
-- |
Fig. 384 renamed Fig. 384*, (also reference to this illustration in text) |
| Page 480, table header |
3⁄4 to 174 |
3⁄4 to 11⁄4 |
| Page 481 |
Hamecons |
Hameçons |
| ditto |
Fishangeln |
Fischangeln |
| Page 499 |
fig. 524 |
fig. 452 |
| Page 510 |
Bernardiere |
Bernardière |
| ditto |
cachemire |
cachemere as elsewhere |
| Page 515, Peroxide of iron |
2F |
2Fe |
| Page 524, formula (1) |
1·000,000 |
1,000,000 |
| Page 530, Fig. 480* |
lower d |
b |
| Page 534 |
-- |
Opening quotes added before conclusion nr. 1. |
| Page 538, schematic Pillow Fustian |
-- |
2 inserted in second row |
| Page 548, Fig. 482 |
-- |
reference letter s added |
| Page 552 |
30,000 feet of gas |
30,000 cubic feet of gas |
| Page 553 |
fig. 488 |
fig. 489 (second reference in text) |
| Page 554, formula 7 |
) |
( |
| Page 561 |
we have 100·4 |
we have 100 and 4 |
| Page 574, table |
curly bracket embraces lime and silica |
curly bracket embraces silica only |
| Page 580, Fig. 507 and 508 |
-- |
illustration numbers interchanged to conform to text |
| Page 594 |
(3 × 77·6) 232·8 |
(3 × 77·6) = 232·8 |
| Page 608 |
-- |
closing quote mark added after ... 47 millions per annum. |
| Page 618 |
Graufärbe |
Graufarbe |
| Page 645 |
Wohler |
Wöhler |
| Page 650 |
a section of the pressure |
a section of the presser |
| Page 658 |
p, small spiral springs |
P, small spiral springs as in drawing |
| Page 670 |
Liebeg |
Liebig |
| Page 674 |
Elbeuf |
Elbœuf |
| Page 684 |
Huttenberg |
Hüttenberg |
| Page 709 |
shachtofen |
schachtofen |
| Page 713 |
ruckstein |
rückstein as elsewhere |
| Page 740 |
as is shown in fig. 621. |
as is shown in fig. 622. |
| Page 745 |
Himmelsfurst |
Himmelsfürst |
| ditto |
Beschertgluck |
Beschertglück |
| Page 746 |
Huelgoet |
Huelgoët |
| Page 747 |
gelena |
galena |
| ditto |
Huelgöet |
Huelgoët |
| Page 748 |
Kongsburg |
Kongsberg as elsewhere |
| Page 749 |
z′ z′ |
z z as in drawing |
| Page 750 |
Z′ Z′ |
z z as in drawing |
| Page 752 |
Poulläouen |
Poullaouen as elsewhere |
| Page 760 |
Vicenago |
Viconago |
| Page 774 |
Rudersdorf |
Rüdersdorf |
| Page 783 |
Farberröthe |
Färberröthe |
| Page 784 |
Societé |
Société |
| Page 785 |
Kuhlman |
Kuhlmann |
| Page 792 |
silk-works are reared |
silk-worms are reared |
| Page 793 |
magnesien |
magnésien |
| Page 805, Figs. 656 and 657 |
-- |
primes added as described in text |
| Page 814 |
Poullaounen |
Pouallouen as elsewhere |
| Page 818 |
K K′ |
K′ K′′ as in illustration |
| Page 822 |
Ehrenfriedensdorf |
Ehrenfriedersdorf |
| Page 828 |
mlydenum |
molybdenum |
| Page 829, table |
, |
· (2x) |
| ditto |
-- |
blank line inserted under Water |
| ditto |
La Ferte-sous-Jouarre |
La Ferté-sous-Jouarre |
| Page 849 |
rothelie gende |
rothe liegende |
| ditto |
lumachello |
lumachella |
| Page 853 |
Breïtlingerwetterschacht |
Breitlingerwetterschacht |
| ditto |
m, n, o, q, R, s |
m, n, o, q, r, s |
| Page 854/855 (table) |
-- |
table notes [a] through [d] were printed as vertical text in the original work |
| Page 859 |
t t |
t T as in illustration |
| Page 868 |
oölite |
oolite |
| ditto |
Puzzuolo |
Puzzuoli |
| Page 874 |
Braconot |
Braconnot as elsewhere |
| Page 889 (table) |
93·024. |
93,024 |
| Page 891 |
Stichstoffoxyd |
Stickstoffoxyd |
| Page 895 |
Hyociamus |
Hyosciamus as elsewhere |
| Page 897, table |
-- |
the original work has a mixture of plain text (olive oil and spermaceti) and table; this has been changed to a table |
| Page 898 |
Wurtemburg |
Wurtemberg as elsewhere |
| Page 900 |
by passing into a slit-groove the vertical turning shaft |
by passing into a slit-groove in the vertical turning shaft |
| Page 909 |
rosmarinus officialis |
rosmarinus officinalis |
| Page 922 |
a, c, d |
a, b, c |
| Page 929 |
-- |
quote marks added after ... from the invention. |
| Page 930, table |
-- |
2 added before Journeymen |
| Page 931, table, first line |
or 4 vats |
3 or 4 vats |
| Page 933 |
(p. 967.) |
(p. 936.) |
| Page 944, table header above No. I |
grams |
grains |
| Page 989, Fig. 864 |
lower d |
b |
| Page 1000 |
rollers, figs. 885. 886. |
rollers, figs. 886. 887. |
| Page 1001 |
Descotil |
Descotils |
| Page 1002 |
Goro-Blagodatz |
Goroblagodat as elsewhere |
| Page 1029 |
Mintereau |
Montereau as elsewhere |
| Page 1056 |
Solfaterra |
Solfetara |
| Page 1080 |
-- |
quote marks added after ... casualty from explosion. |
| Page 1090 |
schlot-plan |
schlot-pan |
| Page 1074 |
fig. 50 |
fig. 950 |
| Page 1106 |
Fig. 796. |
Fig. 976. |
| Page 1127 |
Fig. 1011. |
Fig. 1012. |
| Page 1129 |
Figs. 1023. and 1026. |
Figs. 1024. and 1025. |
| Page 1137 |
Himmelfürst |
Himmelsfürst |
| Page 1175 |
-- |
quote marks added after ... or of the alloys. |
| Page 1185 |
Liquidamber styraciflua |
Liquidambar styraciflua |
| Page 1196 |
the feeder unites them |
the feeder unties them |
| Page 1245 |
The portion B is to be washed again |
The portion A is to be washed again |
| Page 1250, calculation |
-- |
missing 0 in shillings-column added |
| Page 1252 |
Poerner |
Poërner |
| Page 1253 |
Moiree |
Moirée |
| Page 1281, table, column Carlsbad, row Fluoride of Calcium |
00·184 |
0·0184 |
| ditto column Seltzer, row Alumina |
00·185; |
0·0185 |
| ditto |
Pullna |
Püllna |
| Page 1284 |
In his way |
In this way |
| Page 1332 |
fig. 1230 |
fig. 1231 |