
Title: Elements of the Theory and Practice of Chymistry, 5th ed.
Author: Pierre Joseph Macquer
Release date: September 29, 2014 [eBook #46998]
Most recently updated: October 24, 2024
Language: English
Credits: Produced by The Online Distributed Proofreading Team at
http://www.pgdp.net (This file was produced from images
generously made available by The Internet Archive)
Translated from the French of
M. MACQUER,
Member of the Royal Academy of Sciences, and Professor
of Medicine in the University of Paris.
The FIFTH EDITION.
EDINBURGH:
Printed for Alexander Donaldson; and sold at his Shop, No. 48, East Corner of St. Paul's Church-Yard, London; and at Edinburgh.
M. DCC. LXXVII.
An hundred and fifty years are scarce elapsed since the clouds of prejudice, which had long overspread the world, began to clear up, and men were convinced, by cultivating the Sciences, and attending to Nature, that no fanciful hypotheses would ever lead them to the true causes of those various phenomena that incessantly and every where meet the observer's eye; but that the narrow limits of the human understanding confine the course of our researches to one single path; namely, that of Experiment, or the Use of our Senses. Yet, in this short period, Natural Philosophy hath risen to a high pitch of improvement, and may with truth be said to have made much greater advances towards perfection, since the experimental method was introduced, than in the many ages before.
This is true with regard to every branch of Natural Philosophy; but more particularly with regard to Chymistry. Though this Science cannot be said to have ever existed without experiments, yet it laboured under the same disadvantages with the rest; because those who studied it made all their experiments with a view to confirm their own Hypotheses, and in consequence of principles which had no foundation whatever, but in their wild imaginations.
Hence arose that enormous heap, that incongruous jumble of facts, which some time ago constituted all the knowledge of Chymists. Most of them,[Pg iv] and especially those who assumed the pompous title of Alchymists, were persuaded that all the Metals were no other than Nature's rude unfinished essays towards making Gold; which, by means of due coction in the bowels of the earth, advanced gradually towards maturity, till at last they were perfectly converted into that beautiful and precious Metal.
On this principle, which, if not demonstrably false, is at least utterly destitute of proof, and unsupported by a single observation, they attempted to finish what Nature had begun, by procuring to the imperfect Metals this much desired coction. To attain it they made an infinite number of experiments and trials; which all conspired to detect the falsity of their system, and to satisfy men of sense, that the methods they employed were very far from answering the purpose.
However, as facts always promote the knowledge of Nature, it happened that those experiments, though quite useless with regard to the end for which they were originally made, proved the occasion of several curious discoveries.
These lucky consequences of their mistaken labours raised the courage of the Chymists, or rather Alchymists, who looked upon every such instance of success as a new step towards the Grand Work, and greatly increased the fond opinion they entertained of themselves, and of their Art, which, on that account, they set up very high above all other Sciences. Nay, they carried this notion of superiority so far, as to hold the rest of mankind unworthy, or incapable, of rising to such sublime knowledge. In consequence thereof Chymistry became an occult and mysterious Science; its expressions were all tropes and figures, its phrases metaphorical, and its axioms so many enigmas: in short, an obscure unintelligible jargon is the justest character of the Alchymistic Language.
Thus, by endeavouring to conceal their secrets, those gentlemen rendered their Art useless to man[Pg v]kind, and brought it into deserved contempt. But at length the genius of true philosophy prevailed in Chymistry, as well as in the other Sciences. Some great men arose, who had generosity enough to think their knowledge no otherways valuable than as it proved of service to Society. They did their utmost to introduce both the knowledge and the practice of many important secrets, till then of no use; they drew aside the veil which hid the charms of Chymistry; and that Science emerging from the profound obscurity, in which it had for many ages lain concealed, gained the admiration of the world as soon as it appeared in open day. Several societies of ingenious men were formed in the most learned countries of Europe, who vied with one another in their labours to execute the noble scheme, and assisted each other by mutually communicating their discoveries. Chymistry made the most rapid progress, enriching and perfecting the Arts derived from, or depending on it. In a word, it put on a new face, and became truly worthy of the title of Science; founding its principles and its processes on solid experiments, and on just consequences deduced from them.
Since that time the Art is become so extensive, by the numerous discoveries which Chymists have already made, and are daily making, that large volumes are required to contain a complete Treatise on the subject. In short, Chymistry may now, in some degree, be compared to Geometry: each of these Sciences takes in a most ample field of inquiry, which every day enlarges very considerably; from each are derived several Arts, not only useful but even necessary to Society; each hath its Axioms and its undeniable principles, either demonstrated from internal evidence, or founded on constant experience; so that the one, as well as the other, may be reduced to certain fundamental truths, on which all the rest are built. These fundamental truths connected together, and laid down with order and precision, form what[Pg vi] we call the Elements of a Science. It is well known that there are many such works relating to Geometry, but it is not so with regard to Chymistry; there being very few books which treat of this Science in an Elementary manner.
Yet it must be owned, that performances of this kind are exceedingly useful. Many who have a relish for the Sciences, but have not leisure to read elaborate Works which treat of them minutely, are glad to meet with a book from which, without sacrificing too much of their time, or neglecting their ordinary business, they may obtain a taste or just notion of a Science that is not their principal study. Those who incline to go farther, and learn more, may, by reading an elementary tract, be enabled to understand Authors, who, as they commonly write only for proficients in the Art, are obscure and hardly intelligible to mere beginners. Nay, I presume to say, that an Elementary Treatise of Chymistry may prove a very useful book, even to those who have made some progress in the Science: for as it contains only the fundamental propositions, and indeed is an abstract of the whole Art, it may help them to recollect the most important parts of what they have read in many different works, and fix in their memoirs the most essential truths, which might else be either confounded with others, or entirely forgot. And these are the motives which determined me to compose the Work which I now offer to the Public.
The general Plan on which I proceed is to suppose my Reader an absolute Novice in Chymistry; to lead him from the most simple truths, and such as imply the lowest degree of knowledge, to such as are more complex, and require a greater acquaintance with Nature. This order, which I have laid down for my rule, hath obliged me to begin with examining the most simple substances that we know, and which we consider as the elements whereof others are composed; as, by knowing the properties of these elementary parts, we are naturally led to those of their se[Pg vii]veral combinations; and, on the other hand, in order to know the properties of compound bodies, it is necessary we should be first acquainted with the properties of their principles. The same reason induced me, when enquiring into the properties of one substance, to take no notice of those which relate to any other substance not treated of before. For example: as I treat of Acids before Metals, I say nothing under the head of those Acids concerning their power of dissolving metals; that I defer till I come to the subject of Metals: and thus I avoid speaking prematurely of a substance with which I suppose my Reader wholly unacquainted. And this method I was so much the more easily induced to follow, that I know of no Chymical book written on the same Plan.
After discoursing of Elements in general, I treat next of such substances as are immediately composed of them, and are, next to them, the most simple: such are all saline substances. This head comprehends mineral Acids, fixed Alkalis, and their several combinations; the volatile sulphureous spirit, sulphur, phosphorus, and the Neutral salts which have an earth or fixed Alkali for their basis: those which have for their basis either a volatile Alkali, or some metallic substance, are referred, according to my general Plan, to the heads under which I treat of those substances.
Metallic substances are scarcely more compounded than the saline; which induces me to consider them next. I begin with those which are the most simple, or at least seem to be so; because their principles, being very strongly connected together, are separated with the greatest difficulty: such are the Metals properly so called; namely, Gold, Silver, Copper, Iron, Tin, and Lead. After these come the Semi-metals in order; to wit, Regulus of Antimony, Zinc, Bismuth, and Regulus of Arsenic. Mercury being a doubtful substance, which some Chymists rank with the Metals, and others with the Semi-metals, because it actually possesses certain properties in common with[Pg viii] each, I have treated of it in a separate Chapter, which stands between the Metals and Semi-metals.
I next proceed to examine the several sorts of Oils, whether Vegetable, which are divided into fat, essential, and empyreumatic; or Animal, and Mineral Oils.
By examining these substances we obtain ideas of all the principles which enter into the composition of Vegetable and Animal bodies; that is, of those substances that are capable of fermentation: this enables me to treat of fermentation in general; of its three different degrees or kinds, the spirituous, acetous, and putrid; and of the products of those fermentations, ardent spirits, acids analogous to those of vegetables and animals, and volatile alkalis.
The order in which I treat of all those substances being different from that in which they are obtained from compound bodies, I give, in a distinct Chapter, a general idea of Chymical Decomposition, with a view to shew the order in which they are separated, from the several bodies in the composition whereof they are found. This brings them a second time under review, and gives me an opportunity of distinguishing those which exist naturally in compound bodies, from those which are only the result of a new combination of some of their principles produced by the fire.
The succeeding Chapter explains the late Mr Geoffroy's Table of Affinities; which I take to be of great use at the end of an Elementary tract like this, as it collects into one point of view the most essential and fundamental doctrines which are dispersed through the work.
I conclude with an account of the Construction of such Vessels and Furnaces as are usually employed in Chymistry.
In this Part I say nothing of any manual operations, or the several ways of performing Chymical processes; reserving these particulars for my Treatise of Practical Chymistry, to which this must be considered as an Introduction.
Elements of the Theory of Chymistry.
| CHAP. I. | |
| Of the Principles of Bodies | Page 1 |
| Sect. 1. Of Air | 2 |
| Sect. 2. Of Water | 3 |
| Sect. 3. Of Earth | 4 |
| Sect. 4. Of Fire | 5 |
| Sect. 5. Of the Phlogiston | 7 |
| Chap. II. A general View of the Relations or Affinities between Bodies | 9 |
| Chap. III. Of Saline Substances in general | 11 |
| Sect. 1. Of Acids | 12 |
| Sect. 2. Of Alkalis | 14 |
| Sect. 3. Of Neutral Salts | 15 |
| Chap. IV. Of the Several Sorts of Saline Substances. | |
| Sect. 1. Of the Universal Acid | 18 |
| Sect. 2. Of the Nitrous Acid | 22 |
| Sect. 3. Of the Marine Acid | 25 |
| Chap. V. Of Lime | 29 |
| Chap. VI. Of Metallic Substances in general | 34 |
| Chap. VII. Of Metals | 37 |
| Sect. 1. Of Gold | ib. |
| Sect. 2. Of Silver | 39 |
| Sect. 3. Of Copper | 44 |
| Sect. 4. Of Iron | 47 |
| Sect. 5. Of Tin | 52 |
| Sect. 6. Of Lead | 53 |
| Chap. VIII. Of Quick-Silver | 58 |
| Chap. IX. Of the Semi-Metals. | |
| Sect. 1. Of Regulus of Antimony | 62 |
| Sect. 2. Of Bismuth | 69 |
| Sect. 3. Of Zinc | 70 |
| Sect. 4. Of Regulus of Arsenic | 72 |
| Chap. X. Of Oil in general | 76 |
| Sect. 1. Of Charcoal | 77 |
| Sect. 2. Of Soap | 78 |
| Chap. XI. Of the Several Sorts of Oils. | |
| Sect. 1. Of Mineral Oils | 79 |
| Sect. 2. Of Vegetable Oils | 80 |
| Sect. 3. Of Animal Oils | 82 |
| Chap. XII. Of Fermentation in general | 83 |
| Chap. XIII. Of the Spirituous Fermentation | 84 |
| Chap. XIV. Of the Acetous Fermentation | 90[Pg x] |
| Sect. 1. Of Vinegar | 91 |
| Sect. 2. Of Tartar | 93 |
| Chap. XV. Of the Putrid Fermentation, or Putrefaction | 96 |
| Chap. XVI. A general View of Chymical Decomposition | 101 |
| Sect. 1. The Analysis of Vegetable Substances | 102 |
| Emulsions | 104 |
| Sect. 2. The Analysis of Animal Substances | 106 |
| Sect. 3. The Analysis of Mineral Substances | 108 |
| Of the Pyrites | 110 |
| Of Ores | 112 |
| Chap. XVII. Explanation of the Table of Affinities | 119 |
| Chap. XVIII. The Theory of Constructing the Vessels most commonly used in Chymistry | 126 |
| Chap. XIX. The Theory of Constructing the Furnaces most commonly used in Chymistry | 133 |
| Of Lutes | 147 |
| Elements of the Practice of Chymistry. | |
| Introduction | 153 |
| PART I. Of Minerals. | |
| SECTION I. | |
| Operations performed on Saline Mineral Substances. | |
| Chap. I. Of the Vitriolic Acid. | |
| 1. Process. To extract Vitriol from the Pyrites | 159 |
| 2. To extract Sulphur from the Pyrites, and other Sulphureous Minerals | 162 |
| 3. To extract Alum from aluminous Minerals | 165 |
| 4. To extract the Vitriolic Acid from Copperas or Green Vitriol | 170 |
| 5. To decompose Sulphur, and extract its Acid, by burning it | 174 |
| 6. To concentrate the Vitriolic Acid | 176 |
| 7. To decompound Vitriolated Tartar by means of the Phlogiston; or to compose Sulphur by combining the Vitriolic Acid with the Phlogiston | 179 |
| Chap. II. Of the Nitrous Acid. | |
| 1. Process. To Extract Nitre out of Nitrous Earths and Stones. The Purification of Salt-Petre. Mother of Nitre. Magnesia | 181 |
| 2. To decompose Nitre by means of the Phlogiston. Nitre fixed by Charcoal. Clyssus of Nitre. Sal Polychrestum | 186 |
| 3. To decompose Nitre by means of the Vitriolic Acid. The Smoking Spirit of Nitre. Sal de duobus. The Purification of Spirit of Nitre | 191 |
| Chap. III. Of the Marine Acid.[Pg xi] | |
| 1. Process. To extract Sea-salt from Sea-water, and from Brine-springs. Epsom Salt | 195 |
| 2. Experiments concerning the Decomposition of Sea-salt by means of the Phlogiston. Kunckel's Phosphorus | 197 |
| 3. To decompose Sea-salt by means of the Vitriolic Acid. Glauber's Salt. The Purification and Concentration of Spirit of Salt | 211 |
| 4. To decompose Sea-salt by means of the Nitrous Acid. Aqua regis. Quadrangular Nitre | 217 |
| Chap. IV. Of Borax. | 218 |
| SECTION II. | |
| Of Operations on Minerals. | |
| Chap. I. Of Gold. | |
| 1. Process. To separate Gold, by Amalgamation with Mercury, from the Earths and Stones with which it is found mixed | 223 |
| 2. To dissolve Gold in Aqua Regis, and by that means separate it from Silver. Aurum Fulminans. Aurum Fulminans reduced | 227 |
| 3. To dissolve Gold by Liver of Sulphur | 232 |
| 4. To separate Gold from all other Metalline substances by means of Antimony | 233 |
| Chap. II. Of Silver. | |
| 1. Process. To separate Silver from its Ore, by means of Scorification with Lead | 238 |
| 2. The refining of Silver by the Cupel | 243 |
| 3. To purify Silver by Nitre | 248 |
| 4. To dissolve Silver in Aqua Fortis, and thereby separate it from every other Metalline substance. The Purification of Aqua Fortis. Silver precipitated by Copper | 250 |
| 5. To separate Silver from the Nitrous Acid by Distillation. Crystals of Silver. The Infernal Stone | 254 |
| 6. To separate Silver from the Nitrous Acid by Precipitation. Luna Cornea. Luna Cornea reduced | 256 |
| 7. To dissolve Silver, and separate it from Gold, by Cementation | 258 |
| Chap. III. Of Copper. | |
| 1. Process. To separate Copper from its Ore | 262 |
| 2. To purify Black Copper, and render it malleable | 264 |
| 3. To deprive Copper of its Phlogiston by Calcination | 266 |
| 4. To resuscitate the Calx of Copper, and reduce it to Metal, by restoring its Phlogiston | 267 |
| 5. To dissolve Copper in the Mineral Acids | 268 |
| Chap. IV. Of Iron. | |
| 1. Process. To separate Iron from its Ore | 270 |
| 2. To render Pig-iron and brittle Iron malleable | 273 |
| 3. To convert Iron into Steel | 274 |
| 4. The Calcination of Iron. Sundry Saffrons of Mars | 276[Pg xii] |
| 5. Iron dissolved by the mineral Acids | 277 |
| Chap. V. Of Tin. | |
| 1. Process. To extract Tin from its Ore | 279 |
| 2. The Calcination of Tin | 280 |
| 3. The Dissolution of Tin by Acids | 284 |
| Chap. VI. Of Lead. | |
| 1. Process. To extract Lead from its Ore | 286 |
| 2. To separate Lead from Copper | 289 |
| 3. The Calcination of Lead | 292 |
| 4. To prepare Glass of Lead | 293 |
| 5. Lead dissolved by the Nitrous Acid | 295 |
| Chap. VII. Of Mercury. | |
| 1. Process. To extract Mercury from its Ore, or to revivify it from Cinabar | 298 |
| 2. To give Mercury, by the action of Fire, the appearance of a Metalline Calx | 301 |
| 3. To dissolve Mercury in the Vitriolic Acid. Turbith Mineral | 302 |
| 4. To combine Mercury with Sulphur. Æthiop's Mineral | 304 |
| 5. To sublime the Combination of Mercury and Sulphur into Cinabar | 306 |
| 6. To dissolve Mercury in the Nitrous Acid. Sundry Mercurial Precipitates | 307 |
| 7. To combine Mercury with the Acid of Sea-salt. Corrosive Sublimate | 308 |
| 8. Sweet Sublimate | 312 |
| 9. The Panacea of Mercury | 314 |
| SECTION III. | |
| Of Operations on the Semi-Metals. | |
| Chap. I. Of Antimony. | |
| 1. Process. To separate Antimony from its Ore | 315 |
| 2. The common Regulus of Antimony | 316 |
| 3. Regulus of Antimony precipitated by Metals | 318 |
| 4. The Calcination of Antimony | 321 |
| 5. Calx of Antimony reduced to a Regulus | 323 |
| 6. Antimony calcined with Nitre. Liver of Antimony. Crocus Metallorum | 325 |
| 7. Another Calcination of Antimony with Nitre. Diaphoretic Antimony. Materia Perlata. Clyssus of Antimony | 326 |
| 8. Calx of Antimony vitrified | 330 |
| 9. Kermes Mineral | 331 |
| 10. Regulus of Antimony dissolved in the Mineral Acids | 335 |
| 11. Regulus of Antimony combined with the Acid of Sea-salt Butter of Antimony. Cinabar of Antimony | 338 |
| 12. Butter of Antimony decompounded by means of Water only. Pulvis Algaroth, or Mercurius Vitæ. The Philosophic Spirit of Vitriol | 342[Pg xiii] |
| 13. Bezoar Mineral. The Bezoartic Spirit of Nitre | 343 |
| 14. Flowers of Antimony | 347 |
| 15. Regulus of Antimony converted into Flowers | 348 |
| Chap. II. Of Bismuth. | |
| 1. Process. To extract Bismuth from its Ore | 350 |
| 2. Bismuth dissolved by Acids. Magistery of Bismuth. Sympathetic Ink | 352 |
| Chap. III. Of Zinc. | |
| 1. Process. To extract Zinc from its Ore, or Calamine | 357 |
| 2. To sublime Zinc into Flowers | 359 |
| 3. To combine Zinc with Copper. Brass. Prince's Metal, &c. | 361 |
| 4. Zinc dissolved in the Mineral Acids | 365 |
| Chap. IV. Of Arsenic. | |
| 1. Process. To extract Arsenic from its matrix. Zafre or Smalt | 367 |
| 2. To separate Arsenic from Sulphur | 371 |
| 3. To give Arsenic the Metalline Form. Regulus of Arsenic | 374 |
| 4. To distil the Nitrous Acid by the interposition of Arsenic. Blue Aqua Fortis. A new Neutral Salt of Arsenic | 377 |
| 5. To alkalizate Nitre by Arsenic | 379 |
| PART II. Of Vegetables. | |
| SECTION I. Operations on unfermented Vegetables. | |
| Chap. I. Of the Substances obtained from Vegetables by Expression only. | |
| 1. Process. To express and depurate the juice of a Plant, containing its Essential Salt. The crystallization of that Salt | 383 |
| 2. To draw the Oils out of Kernels, Seeds, and Fruits, by Expression | 386 |
| 3. To draw the Essential Oils of certain Fruits by Expression | 387 |
| Chap. II. Of the Substances obtained from Vegetables by Triture. | |
| 1. Process. To make the Extract of a Plant by Trituration | 389 |
| 2. To extract from Seeds and Kernels, by Trituration, the matter of Emulsions | 392 |
| Chap. III. Of Operations on Fat Oils. | |
| 1. Process. To attenuate Fat Oils, and change their nature, by exposing them to the action of fire, and distilling them | 395 |
| 2. To combine Fat Oils with Acids. The decomposition of this combination | 398[Pg xiv] |
| 3. To combine Fat Oils with Fixed Alkalis. Hard and Soft Soap. The decomposition of Soap | 400 |
| 4. To combine Fat Oils with Sulphur | 405 |
| 5. To combine Fat Oils with Lead, and the Calces of Lead. The Basis of Plasters. The decomposition of this combination | 406 |
| Chap. IV. Of the substances obtained from Vegetables with a degree of heat not exceeding that of boiling water. | |
| 1. Process. To obtain from Plants, by distilling them with the mean degree of heat between freezing and boiling water, a liquor impregnated with their Principle of Odour | 408 |
| 2. To extract the Fat Oils of Plants by Decoction in boiling water. Cacao-Butter | 410 |
| 3. To extract the Essential Oils of Plants by Distillation with the heat of boiling water. Distilled Water | 412 |
| 4. To extract the Essential Oils of Plants by distillation per descensum | 418 |
| 5. Infusions, Decoctions, and Extracts of Plants | 419 |
| Chap. V. Of Operations on Essential Oils. | |
| 1. Process. The Rectification of Essential Oils | 422 |
| 2. To fire Oils by combining them with highly concentrated Acids: instanced in Oil of Turpentine | 426 |
| 3. To combine Essential Oils with mineral Sulphur. Balsam of Sulphur. This composition decompounded | 434 |
| 4. To combine Essential Oils with Fixed Alkalis. Starkey's Soap | 438 |
| Chap. VI. Of the Substances obtained from Vegetables by means of a graduated heat, from that of boiling water, to the strongest that can be applied to them in close vessels. | |
| 1. Process. To analyze vegetable substances that yield neither a Fat nor an Essential Oil: instanced in Guaiacum-Wood | 440 |
| 2. To analyze a vegetable substance which yields the same principles as are obtained from Animal matters: instanced in Mustard-seed | 445 |
| Chap. VII. Of the Substances obtained from Vegetables by Combustion. | |
| 1. Process. To procure a Fixed, Caustic, Alkaline Salt from a vegetable substance, by burning it in the open air | 448 |
| 2. To procure the Fixed Salt of a Plant, by burning it after the manner of Tachenius | 453 |
| 3. To render Fixed Alkalis very Caustic by means of Lime. The Caustic Stone | 455 |
| 4. The Analysis of Soot | 457 |
| Chap. VIII. The Analyses of some particular substances belonging to the Vegetable Kingdom. | |
| 1. Process. Analysis of the Native Balsams: instanced in Turpentine | 460[Pg xv] |
| 2. The Analysis of Resins: instanced in Benjamin. The Flowers and Oil of Benjamin | 463 |
| Reflections on the Nature and Properties of Camphor | 465 |
| 3. The Analysis of Bitumens: instanced in Amber. The Volatile Salt and Oil of Amber | 467 |
| 4. The Analysis of Bee's Wax, and such Oily Compounds as are analogous to it | 472 |
| 5. The Saccharine juices of Plants analyzed: instanced in Honey | 474 |
| 6. Gummy substances analyzed: instanced in Gum Arabic | 476 |
| SECTION II. Of Operations on Fermented Vegetable Substances. | |
| Chap. I. Of the Product of Spirituous Fermentation. | |
| 1. Process. To make Wine of Vegetable Substances that are susceptible of Spirituous Fermentation | 478 |
| 2. To draw an Ardent Spirit from substances that have undergone the Spirituous Fermentation. The Analysis of Wine | 482 |
| 3. To dephlegmate Spirit of Wine by the means of Fixed Alkalis. Spirit of Wine analyzed | 486 |
| Chap. II. Spirit of Wine combined with different Substances. | |
| 1. Process. To combine Spirit of Wine with the Vitriolic Acid. This combination decompounded. Rabel's Water. Æther. Sweet Oil of Vitriol. Hoffman's Anodyne Mineral Liquor | 492 |
| 2. Spirit of Wine combined with Spirit of Nitre. Sweet Spirit of Nitre | 503 |
| 3. Spirit of Wine combined with the Acid of Sea-salt. Dulcified Spirit of Salt | 508 |
| 4. Oils, or Oily matters, that are soluble in Spirit of Wine, separated from Vegetables, and dissolved, by means of that Menstruum. Tinctures; Elixirs; Varnishes. Aromatic Strong Waters | 510 |
| Chap. III. Of Tartar. | |
| 1. Process. Tartar analyzed by distillation. The Spirit, Oil, and Alkaline Salt of Tartar | 514 |
| 2. The depuration of Tartar. Cream and Crystals of Tartar | 517 |
| Chap. IV. Crystal of Tartar combined with several substances. | |
| 1. Process. Crystal of Tartar combined with Absorbent Earths. Soluble Tartars | 519 |
| 2. Crystal of Tartar combined with Fixed Alkalis. The Vegetable Salt. Saignette's Salt. The decomposition of Soluble Tartar | 524 |
| 3. Crystal of Tartar combined with Iron. Chalybeated Tartar. Tincture of Steel with Tartar. Soluble Chalybeated Tartar | 528 |
| 4. Crystal of Tartar combined with the reguline part of Antimony. Stibiated or Emetic Tartar | 534[Pg xvi] |
| Chap. V. Of the Product of Acetous Fermentation. | |
| 1. Process. Substances susceptible of the Acetous Fermentation turned into Vinegar | 536 |
| 2. To concentrate Vinegar by Frost | 540 |
| 3. Vinegar analyzed by distillation | 542 |
| Chap. VI. The Acid of Vinegar combined with different Substances. | |
| 1. Process. The Acid of Vinegar combined with Alkaline Substances. Foliated Salt of Tartar, or Regenerated Tartar. Decomposition of that Salt | 547 |
| 2. The Acid of Vinegar combined with Copper. Verdegris. Crystals of Copper. This combination decompounded. Spirit of Verdegris | 550 |
| 3. The Acid of Vinegar combined with Lead. Ceruse. Salt or Sugar of Lead. This combination decompounded | 552 |
| Chap. VII. Of the Putrid Fermentation of Vegetable Substances. | |
| 1. Process. The Putrefaction of Vegetables | 557 |
| 2. Putrefied Vegetable substances analyzed | 559 |
| PART III. Of Operations on Animal Substances. | |
| CHAP. I. Of Milk. | |
| 1. Process. Milk separated into Butter, Curd, and Whey: instanced in Cow's Milk | 562 |
| 2. Butter analyzed by distillation | 566 |
| 3. The Curd of Milk analyzed by distillation | 569 |
| 4. Whey analyzed | 571 |
| Chap. II. Of the Substances which compose an Animal Body. | |
| 1. Process. Blood analyzed: instanced in Bullock's Blood | 574 |
| 2. Flesh analyzed: instanced in Beef | 580 |
| 3. Bones analyzed: instanced in Ox-bones | 583 |
| 4. Animal Fat analyzed: instanced in Mutton-Suet | 584 |
| 5. Eggs analyzed: instanced in Pullet's Eggs | 586 |
| Chap. III. Of Animal Excrements. | |
| 1. Process. Dung analyzed: instanced in Human Excrement. Mr. Homberg's Phosphorus | 588 |
| 2. Human Urine analyzed | 596 |
| Chap. IV. Of Volatile Alkalis. | |
| 1. Process. Volatile Alkalis rectified and depurated | 599 |
| 2. Volatile Alkalis combined with Acids. Sundry Ammoniacal Salts. Sal Ammoniac | 602 |
| 3. Sal Ammoniac decompounded by Acids | 607 |
| 4. Sal Ammoniac decompounded by Fixed Alkalis. Volatile Salt. The Febrifuge of Sylvius | 608 |
| 5. Sal Ammoniac decompounded by Absorbent Earths and Lime. Volatile Spirit of Sal Ammoniac. Fixed Sal Ammoniac. Oil of Lime | 611 |
| 6. Volatile Alkalis combined with oily matters. A Volatile Oily Aromatic Salt | 616 |
| Illustrations | |
| PLATE FIRST. Alembics. | 621 |
| PLATE SECOND. Alembic and other equipment. | 622 |
| PLATE THIRD. Reverberating Furnace. | 623 |
| PLATE FOURTH. Cupelling Furnace. | 624 |
| GEOFFROY'S TABLE of the COMPARATIVE AFFINITIES | 625 |
| INDEX | 629 |

Of the Principles of Bodies.
The object and principal end of Chymistry, is to separate the different substances that enter into the composition of bodies; to examine each of them apart; to discover their properties and relations; to decompose, if possible, those very substances; to compare them together, and combine them with others; to re-unite them again into one body, so as to reproduce the original compound with all its properties; or even to produce new compounds that never existed among the works of nature, from mixtures of other matters differently combined.
But this Analysis, or Decomposition, of bodies is finite; we being unable to carry it beyond a certain limit. In whatever way we attempt to go further, we are always stopped by substances in which we can produce no change, which will not admit of being resolved into others, and which stand as so many firm barriers obstructing our progress.
To these substances we may, in my opinion, give the title of Principles or Elements: at least, with regard to us, they are really such. Of this sort the principal are Earth, Water, Air, and Fire. For though there is ground to believe that these are not the primary component parts, or the most simple elements, of matter; yet, as we know by experience, that our senses cannot possibly discover the principles of which[Pg 2] they are composed, it seems more reasonable to fix upon them, and consider them as simple homogeneous bodies, and the principles of the rest, than to fatigue our minds with vain conjectures about the parts or elements of which they may consist; seeing there is no criterion by which we can know whether we have hit upon the truth, or whether the notions we have formed are mere fancies. We shall therefore consider these four substances as the principles or elements of all the various compounds which nature presents to our inquiries: because, of all those we have as yet discovered, they are in fact the most simple; and because all our decompositions, all our experiments on other bodies, plainly prove that they are at last resolvable into these primary parts.
These principles do not enter in the same proportion into all bodies: there are even some mixts in the composition of which this or that particular principle is not to be found. Thus Air and Water seem to be wholly excluded from the texture of Metals; at least all the experiments that have hitherto been made on them seem to confirm this opinion.
The substances composed immediately of these first Elements we shall call secondary Principles; because in reality their several combinations with each other, the interchangeable coalitions that take place between them, constitute the different natures of all other bodies; which, as they result from the union both of primary and secondary principles, are properly entitled to the name of Compounds or Mixts.
Before we enter upon the examination of Compound Substances, it is necessary to consider with some attention the most Simple ones, or our four first principles, in order to discover their chief properties.
Of Air.
Air is that Fluid which we constantly breathe, and which surrounds the whole surface of the terrestrial globe. Being heavy, like all other bodies, it penetrates into all places that are not either absolutely inaccessible, or filled with some other body heavier than itself. Its principal property is to be susceptible of condensation and rarefaction; so that the very same quantity of Air may occupy a much greater, or a much smaller space, according to the different state it is in. Heat and cold, or, if you will, the presence and the absence of the particles of Fire, are the most usual causes, and indeed[Pg 3] the measures, of its condensation and rarefaction: for if a certain quantity of Air be heated, its bulk enlarges in proportion to the degree of heat applied to it; the consequence of which is, that the same space now contains fewer particles of Air than it did before. Cold again produces just the opposite effect.
On this property which Air has, of being condensed and rarefied by heat, its elasticity or springiness chiefly depends. For if Air were forced by condensation into a less compass than it took up before, and then exposed to a very considerable degree of cold, it would remain quite inactive, without exerting such an effort as it usually makes against the compressing body. On the other hand, the elasticity of heated Air arises only from hence, that being rarefied by the action of Fire, it requires much more space than it occupied before.
Air enters into the composition of many substances, especially vegetable and animal bodies: for by analysing most of them such a considerable quantity thereof is extricated, that some naturalists have suspected it to be altogether destitute of elasticity when thus combined with the other principles in the composition of bodies. According to them, the efficacy of the elastic power of the Air is so prodigious, and its force when compressed so excessive, that it is not possible the other component parts of bodies should be able to confine so much of it, in that state of compression which it must needs undergo, if retaining its elasticity it were pent up among them.
However that be, this elastic property of the Air produces the most singular and important phenomena, observable in the resolution and composition of bodies.
Of Water.
Water is a thing so well known, that it is almost needless to attempt giving a general idea of it here. Every one knows that it is a transparent, insipid substance, and usually fluid. I say it is usually so; for being exposed to a certain degree of cold it becomes solid: solidity therefore seems to be its most natural state.
Water exposed to the Fire grows hot; but only to a limited degree, beyond which its heat never rises, be the force of Fire applied to it ever so violent: it is known to have acquired this degree of heat by its boiling up with great tumult.[Pg 4] Water cannot be made hotter, because it is volatile, and incapable of enduring the heat, without being evaporated and entirely dissipated.
If such a violent and sudden heat be applied to Water, as will not allow it time to exhale gently in vapours, as when, for instance, a small quantity thereof is thrown upon a metal in fusion, it is dissipated at once with vast impetuosity, producing a most terrible and dangerous explosion. This surprising effect may be deduced from the instantaneous dilatation of the parts of the Water itself, or rather of the Air it contains. Moreover, Water enters into the texture of many bodies, both compounds and secondary principles; but, like Air, it seems to be excluded from the composition of all metals and most minerals. For although an immense quantity of Water exists in the bowels of the Earth, moistening all its contents, it cannot be thence inferred, that it is one of the principles of minerals. It is only interposed between their parts; for they may be entirely divested of it, without any sort of decomposition: indeed it is not capable of an intimate connection with them.
Of Earth.
We observed that the two principles above treated of are volatile; that is, the action of Fire separates them from the bodies they help to compose, carrying them quite off, and dissipating them. That of which we are now to speak, namely Earth, is fixed, and, when it is absolutely pure, resists the utmost force of Fire. So that, whatever remains of a body, after it hath been exposed to the power of the fiercest Fire, must be considered as containing nearly all its earthy principle, and consisting chiefly thereof. I qualify my expression thus for two reasons: the first is, because it often happens, that this remainder does not actually contain all the Earth which existed originally in the mixt body decomposed by Fire; since it will afterwards appear that Earth, though in its own nature fixed, may be rendered volatile by being intimately united with other substances which are so; and that, in fact, it is common enough for part of the Earth of a body to be thus volatilized by its other principles: the second is, that what remains after the calcination of a body is not generally its earth in perfect purity, but combined with some of its other principles, which, though volatile in[Pg 5] their own natures, have been fixed by the union contracted between it and them. We shall, in the sequel, produce some examples to illustrate this theory.
Earth, therefore, properly so called, is a fixed principle, which is permanent in the Fire. There is reason to think it very difficult, if not impossible, to obtain the earthy principle entirely free from every other substance: for after our utmost endeavours to purify them, the Earths we obtain from different compounds are found to have different properties, according to the different bodies from which they are procured; or else, if those Earths be pure, we must allow them to be essentially different, seeing they have different properties.
Earth, in general, with regard to its properties, may be distributed into fusible, and unfusible; that is, into Earth that is capable of melting or becoming fluid in the Fire, and Earth that constantly remains in a solid form, never melting in the strongest degree of heat to which we can expose it.
The former is also called vitrifiable, and the second unvitrifiable Earth; because, when Earth is melted by the force of Fire, it becomes what we call Glass, which is nothing but the parts of Earth brought into nearer contact, and more closely united by the means of fusion. Perhaps the Earth, which we look upon as incapable of vitrification, might be fused if we could apply to it a sufficient degree of heat. It is at least certain, that some Earths, or stones, which separately resist the force of Fire, so that they cannot be melted, become fusible when mixed together. Experience convinced Mr. du Hamel that lime-stone and slate are of this kind. It is however undoubtedly true, that one Earth differs from another in its degree of fusibility: and this gives ground to believe, that there may be a species of Earth absolutely unvitrifiable in its nature, which, being mixed in different proportions with fusible Earths, renders them difficult to melt.
Whatever may be in this, as there are Earths which we are absolutely unable to vitrify, that is a sufficient reason for our division of them. Unvitrifiable Earths seem to be porous, for they imbibe Water; whence they have also got the name of Absorbent Earths.
Of Fire.
The Matter of the Sun, or of Light, the Phlogiston, Fire, the Sulphureous Principle, the Inflammable Matter,[Pg 6] are all of them names by which the Element of Fire is usually denoted. But it should seem, that an accurate distinction hath not yet been made between the different states in which it exists; that is, between the phenomena of Fire actually existing as a principle in the composition of bodies, and those which it exhibits when existing separately and in its natural state: nor have proper distinct appellations been assigned to it in those different circumstances. In the latter state we may properly give it the names of Fire, Matter of the Sun, of Light, and of Heat; and may consider it as a substance composed of infinitely small particles, continually agitated by a most rapid motion, and, of consequence, essentially fluid.
This substance, of which the sun may be called the general reservoir, seems to flow incessantly from that source, diffusing itself over the world, and through all the bodies we know; but not as a principle, or essential part of them, since they may be deprived thereof, at least in a great measure, without suffering any decomposition. The greatest change produced on them, by its presence or its absence, is the rendering them fluid or solid: so that all other bodies may be deemed naturally solid; Fire alone essentially fluid, and the principle of fluidity in others. This being presupposed, Air itself might become solid, if it could be entirely deprived of the Fire it contains; as bodies of most difficult fusion become fluid, when penetrated by a sufficient quantity of the particles of Fire.
One of the chief properties of this pure Fire is to penetrate easily into all bodies, and to diffuse itself among them with a sort of uniformity and equality: for if a heated body be contiguous to a cold one, the former communicates to the latter all its excess of heat, cooling in exact proportion as the other warms, till both come to have the very same degree of heat. Heat, however, is naturally communicable soonest to the upper parts of a body; and consequently, when a body cools, the under parts become soonest cold. It hath been observed, for instance, that the lower extremity of a heated body, freely suspended in the air, grows cold sooner than the upper; and that, when a bar of iron is red-hot at one end, and cold at the other, the cold end is much sooner heated by placing the bar so that the hot end may be undermost, than, when that end is turned uppermost. The levity of the matter of Fire, and the vicinity of the Earth, may possibly be the causes of this phenomenon.
Another property of Fire is to dilate all bodies into which[Pg 7] it penetrates. This hath already been shewn with regard to Air and Water; and it produces the same effect on Earth.
Fire is the most powerful agent we can employ to decompose bodies; and the greatest degree of heat producible by man, is that excited by the rays of the sun collected in the focus of a large burning-glass.
Of the Phlogiston.
From what hath been said concerning the nature of Fire, it is evidently impossible for us to fix and confine it in any body. Yet the phenomena attending the combustion of inflammable bodies shew, that they really contain the matter of Fire as a constituent principle. By what mechanism then is this fluid, which is so subtle, so active, so difficult to confine, so capable of penetrating into every other substance in nature; how comes it, I say, to be so fixed as to make a component part of the most solid bodies? It is no easy matter to give a satisfactory answer to this question. But, without pretending to guess the cause of the phenomenon, let us rest contented with the certainty of the fact, the knowledge of which will undoubtedly procure us considerable advantages. Let us therefore examine the properties of Fire thus fixed, and become a principle of bodies. To this substance, in order to distinguish it from pure and unfixed Fire, the Chymists have assigned the peculiar title of the Phlogiston, which indeed is no other than a Greek word for the Inflammable Matter; by which latter name, as well as by that of the Sulphureous Principle, it is also sometimes called. It differs from elementary Fire in the following particulars. 1. When united to a body, it communicates to it neither heat nor light. 2. It produces no change in its state, whether of solidity or fluidity; so that a solid body does not become fluid by the accession of the Phlogiston, and vice versa; the solid bodies to which it is joined being only rendered thereby more apt to be fused by the force of the culinary fire. 3. We can convey it from the body with which it is joined into another body, so that it shall enter into the composition thereof, and remain fixed in it.
On this occasion both these bodies, that which is deprived of the Phlogiston and that which receives it, undergo very considerable alterations; and it is this last circumstance, in particular, that obliges us to distinguish the Phlogiston from[Pg 8] pure Fire, and to consider it as the element of Fire combined with some other substance, which serves it as a basis for constituting a kind of secondary principle. For if there were no difference between them, we should be able to introduce and fix pure Fire itself, wherever we can introduce and fix the Phlogiston: yet this is what we can by no means do, as will appear from experiments to be afterwards produced.
Hitherto, Chymists have never been able to obtain the Phlogiston quite pure, and free from every other substance: for there are but two ways of separating it from a body of which it makes a part; to wit, either by applying some other body with which it may unite the moment it quits the former; or else by calcining and burning the compound from which you desire to sever it. In the former case it is evident that we do not get the Phlogiston by itself, because it only passes from one combination into another; and in the latter, it is entirely dissipated in the decomposition, so that no part of it can possibly be secured.
The inflammability of a body is an infallible sign that it contains a Phlogiston; but from a body's not being inflammable, it cannot be inferred that it contains none; for experiments have demonstrated that certain metals abound with it, which yet are by no means inflammable.
We have now delivered what is most necessary to be known concerning the principles of bodies in general. They have many other qualities besides those above-mentioned; but we cannot properly take notice of them here, because they pre-suppose an acquaintance with some other things relating to bodies, of which we have hitherto said nothing; intending to treat of them in the sequel as occasion shall offer. We shall only observe in this place, that when animal and vegetable matters are burnt, in such a manner as to hinder them from flaming, some part of the Phlogiston contained in them unites intimately with their most fixed earthy parts, and with them forms a compound, that can be consumed only by making it red-hot in the open air, where it sparkles and wastes away, without emitting any flame. This compound is called a Coal. We shall inquire into the properties of this Coal under the head of Oils: at present it suffices that we know in general what it is, and that it readily communicates to other bodies the Phlogiston it contains.
A general View of the Relations or Affinities between Bodies.
Before we can reduce compound Bodies to the first principles above pointed out, we obtain, by analysing them, certain substances which are indeed more simple than the bodies they helped to compose, yet are themselves composed of our primary principles. They are therefore at one and the same time both principles and compounds; for which reason we shall, as was before said, call them by the name of Secondary Principles. Saline and oily matters chiefly constitute this class. But before we enter upon an examination of their properties, it is fit we lay before the reader a general view of what Chymists understand by the Relations or Affinities of Bodies; because it is necessary to know these, in order to a distinct conception of the different combinations we are to treat of.
All the experiments hitherto made concur with daily observation to prove, that different bodies, whether principles or compounds, have such a mutual Conformity, Relation, Affinity, or Attraction, if you will call it so, as disposes some of them to join and unite together, while they are incapable of contracting any union with others. This effect, whatever be its cause, will enable us to account for, and connect together, all the phenomena that Chymistry produces. The nature of this universal affection of matter is distinctly laid down in the following propositions.
First, If any substance hath any Affinity or conformity with another, the two will unite together, and form one compound.
Secondly, It may be laid down as a general rule, that all similar substances have an Affinity with each other, and are consequently disposed to unite; as water with water, earth with earth, &c.
Thirdly, Substances that unite together lose some of their separate properties; and the compounds resulting from their union partake of the properties of those substances which serve as their principles.
Fourthly, The simpler any substances are, the more perceptible and considerable are their Affinities: whence it follows, that the less bodies are compounded, the more difficult it is to analyse them; that is, to separate from each other the principles of which they consist.
Fifthly, If a body consist of two substances, and to this compound be presented a third substance, that has no Affinity at all with one of the two primary substances aforesaid, but has a greater Affinity with the other than those two substances have with each other, there will ensue a decomposition, and a new union; that is, the third substance will separate the two compounding substances from each other, coalesce with that which has an Affinity with it, form therewith a new combination, and disengage the other, which will then be left at liberty, and such as it was before it had contracted any union.
Sixthly, It happens sometimes that when a third substance is presented to a body consisting of two substances, no decomposition follows; but the two compounding substances, without quitting each other, unite with the substance presented to them, and form a combination of three principles: and this comes to pass when that third substance has an equal, or nearly equal, Affinity with each of the compounding substances. The same thing may also happen even when the third substance hath no Affinity but with one of the compounding substances only. To produce such an effect, it is sufficient that one of the two compounding substances have to the third body a Relation equal, or nearly equal, to that which it has to the other compounding substance with which it is already combined. Thence it follows, that two substances, which, when apart from all others, are incapable of contracting any union, may be rendered capable of incorporating together in some measure, and becoming parts of the same compound, by combining with a third substance with which each of them has an equal Affinity.
Seventhly, A body, which of itself cannot decompose a compound consisting of two substances, because, as we just now said, they have a greater affinity with each other than it has with either of them, becomes nevertheless capable of separating the two by uniting with one of them, when it is itself combined with another body, having a degree of Affinity with that one, sufficient to compensate its own want thereof. In that case there are two Affinities, and thence ensues a double decomposition and a double combination.
These fundamental truths, from which we shall deduce an explanation of all the phenomena in Chymistry, will be confirmed and illustrated by applying them, as we shall do, to the several cases, of which our design in this treatise obliges us to give a circumstantial account.
Of Saline Substances in general.
If a particle of water be intimately united with a particle of earth, the result will be a new compound, which, according to our third proposition of Affinities, will partake of the properties of earth and of water; and this combination principally forms what is called a Saline Substance. Consequently every Saline Substance must have an affinity with earth and with water, and be capable of uniting with both or either of them, whether they be separate or mixed together: and accordingly this property characterizes all Salts, or Saline Substances, in general.
Water being volatile and Earth fixed, Salts in general are less volatile than the former, and less fixed than the latter; that is, fire, which cannot volatilize and carry off pure earth, is capable of rarefying and volatilizing a Saline Substance; but then this requires a greater degree of heat than is necessary for producing the same effects on pure water.
There are several sorts of Salts, differing from one another, in respect either of the quantity, or the quality of the earth in their composition; or, lastly, they differ on account of some additional principles, which not being combined with them in sufficient quantity to hinder their Saline properties from appearing, permit them to retain the name of Salts, though they render them very different from the simplest Saline Substances.
It is easy to infer, from what has been said of Salts in general, that some of them must be more, some less, fixed or volatile than others, and some more, some less, disposed to unite with water, with earth, or with particular sorts of earth, according to the nature or the proportion of their principles.
Before we proceed further, it is proper just to mention the principle reasons, which induce us to think that every[Pg 12] Saline Substance is actually a combination of earth and water, as we supposed at our entering on this subject. The first is, the conformity Salts have with earth and water, or the properties they possess in common with both. Of these properties we shall treat fully, as occasion offers to consider them, in examining the several sorts of Salts. The second is, that all Salts may be actually resolved into earth and water by sundry processes; particularly by repeated dissolution in water, evaporation, desiccation, and calcination. Indeed the Chymists have not yet been able to procure a Saline Substance, by combining earth and water together. This favours a suspicion, that, besides these two, there is some other principle in the composition of salts, which escapes our researches, because we cannot preserve it when we decompose them; but it is sufficient to our purpose, that water and earth are demonstrably amongst the real principles of Saline Substances, and that no experiment hath ever shewn us any other.
Of Acids.
Of all Saline Substances, the simplest is that called an Acid, on account of its taste; which is like that of verjuice, sorrel, vinegar, and other sour things, which, for the same reason, are also called Acids. By this peculiar taste are Acids chiefly known. They have moreover the property of turning all the blue and violet colours of vegetables red, which distinguishes them from all other salts.
The form, under which Acids most commonly appear, is that of a transparent liquor; though solidity is rather their natural state. This is owing to their affinity with water; which is so great, that, when they contain but just as much of it as is necessary to constitute them Salts, and consequently have a solid form, they rapidly unite therewith the moment they come into contact with it: and as the air is always loaded with moisture and aqueous vapours, its contact alone is sufficient to liquify them; because they unite with its humidity, imbibe it greedily, and by that means become fluid. We therefore say, they attract the moisture of the air. This change of a salt from a solid to a fluid state, by the sole contact of the air, is also called Deliquium; so that when a salt changes in this manner from a solid into a fluid form, it is said to run per deliquium. Acids being the sim[Pg 13]plest species of Saline bodies, their affinities with different substances are stronger than those of any other sort of salt with the same substances; which is agreeable to our fourth proposition concerning Affinities.
Acids in general have a great affinity with earths: that with which they most readily unite is the unvitrifiable earth to which we gave the name of absorbent earth. They seem, not to act at all upon vitrifiable earths, such as sand; nor yet upon some other kinds of earths, at least while they are in their natural state. Yet the nature of these earths may be in some measure changed, by making them red-hot in the fire, and then quenching them suddenly in cold water: for, by repeating this often, they are brought nearer to the nature of absorbent earths, and rendered capable of uniting with Acids.
When an acid liquor is mixed with an absorbent earth, for instance with chalk, these two substances instantly rush into union, with so much impetuosity, especially if the acid liquor be as much dephlegmated, or contain as little water, as may be, that a great ebullition is immediately produced, attended with considerable hissing, heat, and vapours, which rise the very instant of their conjunction.
From the combination of an acid with an absorbent earth there arises a new compound, which some Chymists have called Sal Salsum; because the Acid by uniting with the earth loses its sour taste, and acquires another not unlike that of the common sea-salt used in our kitchens; yet varying according to the different sorts of Acids and earths combined together. The Acid at the same time loses its property of turning vegetable blues and violet colours red.
If we inquire what is become of its propensity to unite with water, we shall find that the earth, which of itself is not soluble in water, hath, by its union with the Acid, acquired a facility of dissolving therein; so that our Sal Salsum is soluble in water. But, on the other hand, the Acid hath, by its union with the earth, lost part of the affinity it had with water; so that if a Sal Salsum be dried, and freed of all superfluous humidity, it will remain, in that dry solid form, instead of attracting the moisture of the air, and running per deliquium, as the Acid would do if it were pure and unmixed with earth. However, this general rule admits of some exceptions; and we shall have occasion in another place to take notice of certain combinations of Acids with earths, which still continue to attract the moisture of the air, though not so strongly as a pure Acid.
Acids have likewise a great affinity with the Phlogiston. When we come to treat of each Acid in particular, we shall examine the combinations of each with the Phlogiston: they differ so widely from one another, and many of them are so little known, that we cannot at present give any general idea of them.
Of Alkalis.
Alkalis are Saline combinations, in which there is a greater proportion of earth than in Acids. The principal arguments that may be adduced to prove this fact are these: First; if they be treated in the manner proposed above for analyzing Saline Substances, we obtain from them a much greater quantity of earth than we do from Acids. Secondly; by combining certain Acids with certain earths we can produce Alkalis; or at least such saline compounds as greatly resemble them. Our third and last argument is drawn from the properties of those Alkalis which, when pure and unadulterated with any other principle, have less affinity with water than Acids have, and are also more fixed, resisting the utmost force of fire. On this account it is that they have obtained the title of Fixed, as well as to distinguish them from another species of Alkali, to be considered hereafter, which is impure and volatile.
Though fixed Alkalis, when dry, sustain the utmost violence of fire without flying off in vapours, it is remarkable that, being boiled with water in an open vessel, considerable quantities of them rise with the steam: an effect which must be attributed to the great affinity between these two substances, by means whereof water communicates some part of its volatility to the fixed salt.
Alkalis freed of their superfluous humidity by calcination attract the moisture of the air, but not so strongly as Acids: so that it is easier to procure and preserve them in a solid form.
They flow in the fire, and are then capable of uniting with vitrifiable earths, and of forming therewith true glass, which, however, will partake of their properties, if they be used in sufficient quantity.
As they melt more readily than vitrifiable earth, they facilitate its fusion; so that a weaker fire will reduce it to glass,[Pg 15] when a fixed Alkali is joined with it, than will melt it without that addition.
Alkalis are known by their taste, which is acrid and fiery; and by the properties they possess of turning vegetable blues and violet colours green; particularly syrop of violets.
Their affinity with acids is greater than that of absorbent earths; and hence it comes to pass, that if an Alkali be presented to a combination of an Acid with an absorbent earth, the earth will be separated from the Acid by the Alkali, and a new union between the Acid and the Alkali will take place. This is both an instance and a proof of our fifth proposition concerning Affinities.
If a pure Alkali be presented to a pure Acid, they rush together with violence, and produce the same phenomena as were observed in the union of an absorbent earth with an Acid; but in a greater and more remarkable degree.
Fixed Alkalis may in general be divided into two sorts: one of these hath all the above-recited properties; but the other possesses some that are peculiar to itself. We shall consider this latter sort more particularly under the head of Sea-Salt.
Of Neutral Salts.
The Acid and the Alkali thus uniting mutually rob each other of their characteristic properties; so that the compound resulting from their union produces no change in the blue colours of vegetables, and has a taste which is neither sour nor acrid, but saltish. A saline combination of this kind is for that reason named Sal Salsum, Sal Medium, of a Neutral Salt. Such combinations are also called by the plain general name of Salts.
It must be observed that, in order to make these Salts perfectly Neutral, it is necessary that neither of the two saline principles of which they are compounded be predominant over the other; for in that case they will have the properties of the prevailing principle. The reason is this: neither of these saline substances can unite with the other but in a limited proportion, beyond which there can be no further coalition between them. The action by which this perfect union is accomplished is termed Saturation; and the instant when such proportions of the two saline substances are mixed together, that the one is incorporated with as much of the[Pg 16] other as it can possibly take up, is called the Point of Saturation. All this is equally applicable to the combination of an Acid with an absorbent earth.
The combination is known to be perfect, that is, the Point of Saturation is known to be obtained, when, after repeated affusions of an Acid in small quantities to an Alkali, or an absorbent earth, we find those phenomena cease, which in such cases constantly attend the conflict of union, as we said above, namely, ebullition, hissing, &c. and we may be assured the Saturation is complete when the new compound hath neither an acid nor an acrid taste, nor in the least changes the blue colours of vegetables.
Neutral salts have not so great an affinity with water as either Acids or Alkalis have; because they are more compounded: for we observed before, that the affinities of the most compounded bodies are generally weaker than those of the most simple. In consequence hereof few Neutral Salts, when dried, attract the moisture of the air; and those that do, attract it more slowly, and in less quantity, than either Acids or Alkalis do.
All Neutral Salts are soluble in water; but more or less readily, and in a greater or smaller quantity, according to the nature of their component principles.
Water made boiling hot dissolves a greater quantity of those salts which do not attract the moisture of the air, than when it is cold; and indeed it must be boiling hot to take up as much of them as it is capable of dissolving: but as for those which run in the air, the difference, if there be any, is imperceptible.
Some Neutral Salts have the property of shooting into crystals, and others have it not.
The nature of Crystallization is this: Water cannot dissolve, nor keep in solution, more than a determinate quantity of any particular Salt: when therefore such a quantity of water is evaporated from the solution of a Salt capable of crystallization, that the remainder contains just as much Salt as it can dissolve, then by continuing the evaporation the Salt gradually recovers its solid form, and concretes into several little transparent masses called Crystals. These crystals have regular figures, all differing from one another according to the species of salt of which they are formed. Different methods of evaporating saline solutions have different effects on the figure and regularity of the crystals; and each particular sort of salt requires a peculiar method of evaporation to make its crystals perfectly regular.
A solution of salt designed for crystallization is usually evaporated by means of fire to a pellicle; that is, till the Salt begin to concrete; which is perceived by a kind of thin dark skin that gathers on the surface of the liquor, and is formed of the crystallized particles of Salt. When this pellicle appears the solution is suffered to cool, and the crystals form therein faster or slower, according to the sort of salt in hand. If the evaporation be carried on briskly to perfect dryness, no crystals will be formed, and only an irregular mass of salt will be obtained.
The reasons why no crystals appear when the evaporation is hastily performed, and carried on to dryness, are, first, that the particles of salt, being always in motion while the solution is hot, have not time to exert their mutual affinities, and to unite together as crystallization requires: Secondly, that a certain quantity of water enters into the very composition of crystals; which is therefore absolutely necessary to their formation, and in a greater or smaller proportion according to the nature of the Salt[1].
If these crystallized salts be exposed to the fire, they first part with that moisture which is not necessary to a saline concretion, and which they retained only by means of their crystallization: afterwards they begin to flow, but with different degrees of fusibility.
It must be observed, that certain salts melt as soon as they are exposed to the fire; namely, those which retain a great deal of water in crystallizing. But this fluor which they so readily acquire must be carefully distinguished from actual fusion: for it is owing only to their superfluous humidity, which heat renders capable of dissolving and liquifying them; so that when it is evaporated the Salt ceases to be fluid, and requires a much greater degree of fire to bring it into real fusion.
The Neutral Salts that do not crystallize may, indeed, be dried by evaporating the water which keeps them fluid; but by becoming solid they acquire no regular form; they again attract the moisture of the air, and are thereby melted into a liquor. These may be called Liquescent Salts.
Most of the Neutral Salts, that consist of an Acid joined with a fixed Alkali, or with an absorbent earth, are them[Pg 18]selves fixed and resist the force of fire; yet several of them, if they be dissolved in water, and the solution boiled and evaporated, fly off along with the steams.
Of the several Sorts of Saline Substances.
Of the Universal Acid.
The Universal Acid is so called, because it is in fact the Acid which is most universally diffused through all nature, in waters, in the atmosphere, and in the bowels of the earth. But it is seldom pure; being almost always combined with some other substance. That from which we obtain it with most ease, and in the greatest quantity, is Vitriol, a mineral which we shall consider afterwards: and this is the reason why it is called the Vitriolic Acid; the name by which it is best known.
When the Vitriolic Acid contains but little phlegm, yet enough to give it a fluid form, it is called Oil of Vitriol, on account of a certain unctuosity belonging to it. In truth this name is very improperly bestowed on it; for we shall afterwards see that, bating this unctuousness, it has none of the properties of oils. But this is not the only impropriety in names that we shall have occasion to censure.
If the Vitriolic Acid contain much water, it is then called Spirit of Vitriol. When it does not contain enough to render it fluid, and so is in a solid form, it is named the Icy Oil of Vitriol.
When Oil of Vitriol highly concentrated is mixed with water, they rush into union with such impetuosity, that, the moment they touch each other, there arises a hissing noise, like that of red-hot iron plunged in cold water, together with a very considerable degree of heat, proportioned to the degree to which the Acid was concentrated.
If, instead of mixing this concentrated Acid with water, you only leave it exposed to the air for some time, it attracts the moisture thereof, and imbibes it most greedily. Both its bulk and its weight are increased by this accession; and if it[Pg 19] be under an icy form, that is, if it be concreted, the phlegm thus acquired will soon resolve it into a fluid.
The addition of water renders the Vitriolic Acid, and indeed all other Acids, weaker in one sense; which is, that when they are very aqueous they leave on the tongue a much fainter taste of acidity, and are less active in the solution of some particular bodies: but that occasions no change in the strength of their affinities, but in some cases rather enables them to dissolve several substances, which, when well dephlegmated, they are not capable of attacking.
The Vitriolic Acid combined to the point of saturation with a particular absorbent earth, the nature of which is not yet well known, forms a Neutral Salt that crystallizes. This Salt is called Alum, and the figure of its crystals is that of an octahedron, or solid of eight sides. These octahedra are triangular pyramids, the angles of which are so cut off that four of the surfaces are hexagons, and the other four triangles.
There are several sorts of Alum, which differ according to the earths combined with the Vitriolic Acid. Alum dissolves easily in water, and in crystallization retains a considerable quantity of it; which is the reason that being exposed to the fire it readily melts, swelling and puffing up as its superfluous moisture exhales. When that is quite evaporated, the remainder is called Burnt Alum, and is very difficult to fuse. The Acid of the Alum is partly dissipated by this calcination. Its taste is saltish, with a degree of roughness and astringency.
The Vitriolic Acid combined with certain earths forms a kind of Neutral Salt called Selenites, which crystallizes in different forms according to the nature of its earth. There are numberless springs of water infected with dissolved Selenites; but when this Salt is once crystallized, it is exceeding difficult to dissolve it in water a second time. For that purpose a very great quantity of water is necessary, and moreover it must boil; for as it cools most of the dissolved Selenites takes a solid form, and falls in a powder to the bottom of the vessel.
If an Alkali be presented to the Selenites, or to Alum, these Salts, according to the principles we have laid down, will be thereby decomposed; that is, the Acid will quit the earths, and join the Alkali, with which it hath a greater affinity. And from this conjunction of the Vitriolic Acid with a fixed Alkali there results another sort of Neutral Salt, which is called Arcanum duplicatum, Sal de duobus, and[Pg 20] Vitriolated Tartar, because one of the fixed Alkalis most in use is called Salt of Tartar.
Vitriolated Tartar is almost as hard to dissolve in water as the Selenites. It shoots into eight-sided crystals, having the apices of the pyramids pretty obtuse. Its taste is saltish, inclining to bitter; and it decrepitates on burning coals. It requires a very great degree of fire to make it flow.
The Vitriolic Acid is capable of uniting with the Phlogiston, or rather it has a greater affinity with it than with any other body: whence it follows, that all compounds, of which it makes a part, may be decomposed by means of the Phlogiston.
From the conjunction of the Vitriolic Acid with the Phlogiston arises a compound called Mineral Sulphur, because it is found perfectly formed in the bowels of the earth. It is also called Sulphur vivum, or simply Sulphur.
Sulphur is absolutely insoluble in water, and incapable of contracting any sort of union with it. It melts with a very moderate degree of heat, and sublimes in fine light downy tufts called Flowers of Sulphur. By being thus sublimed, it suffers no decomposition, let the operation be repeated ever so often; so that Sublimed Sulphur, or Flower of Sulphur, hath exactly the same properties as Sulphur that has never been sublimed.
If Sulphur be exposed to a brisk heat in the open air, it takes fire, burns, and is wholly consumed. This deflagration of Sulphur is the only means we have of decomposing it, in order to obtain its Acid in purity. The Phlogiston is destroyed by the flame, and the Acid exhales in vapours: these vapours collected have all the properties of the Vitriolic Acid, and differ from it only as they still retain some portion of the Phlogiston; which, however, soon quits them of its own accord, if the free access of the common air be not precluded.
The portion of Phlogiston retained by the Acid of Sulphur is much more considerable when that mineral is burnt gradually and slowly: in that case the vapours which rise from it have such a penetrating odour, that they instantaneously suffocate any person who draws in a certain quantity of them with his breath. These vapours constitute what is called the Volatile Spirit of Sulphur. There is reason to think this portion of Phlogiston which the Acid retains is combined therewith in a manner different from that in which these two are united in the Sulphur itself; for, as has just been observed, nothing but actual burning is capable of separating the Vi[Pg 21]triolic Acid and the Phlogiston, which by their union form Sulphur; whereas in the Volatile Spirit of Sulphur they separate spontaneously when exposed to the open air; that is, the Phlogiston flies off and leaves the Acid, which then becomes in every respect similar to the Vitriolic Acid.
That the Volatile Spirit of Sulphur is a compound, as we have asserted it to be, appears evidently from hence, that whenever the Vitriolic Acid touches any substance containing the Phlogiston, provided that Phlogiston be disengaged or opened to a certain degree, a Volatile Spirit of Sulphur is infallibly and immediately generated. This Spirit hath all the properties of Acids, but considerably weakened, and of course less perceptible. It unites with absorbent earths or fixed Alkalis; and with them forms Neutral Salts; but when combined therewith it may be separated from them by the Vitriolic Acid, and indeed by any of the mineral Acids, because its affinities are weaker. Sulphur hath the property of uniting with absorbent earths, but not near so intimately as with fixed Alkalis.
If equal parts of Sulphur and an Alkali be melted together, they incorporate with each other; and from their conjunction proceeds a compound of a most unpleasant smell, much like that of rotten eggs, and of a red colour nearly resembling that of an animal liver, which has occasioned it to bear the name of Hepar Sulphuris, or Liver of Sulphur.
In this composition the fixed Alkali communicates to the Sulphur the property of dissolving in water: and hence it comes that Liver of Sulphur may be made as well when the Alkali is dissolved by water into a fluid, as when it is fused by the action of fire.
Sulphur has less affinity than any Acid with the fixed Alkalis: and therefore Liver of Sulphur may be decompounded by any Acid whatever; which will unite with the fixed Alkali, form therewith a Neutral Salt, and separate the Sulphur.
If Liver of Sulphur be dissolved in water, and an Acid poured thereon, the liquor, which was transparent before, instantly turns to an opaque white; because the Sulphur, being forced to quit its union with the Alkali, loses at the same time the property of dissolving in water, and appears again in its own opaque form. The liquor thus made white by the Sulphur is called Milk of Sulphur.
If this liquor be suffered to stand still for some time, the particles of Sulphur, now most minutely divided, gradually[Pg 22] approach each other, unite, and fall insensibly to the bottom of the vessel; and then the liquor recovers its transparency. The Sulphur thus deposited on the bottom of the vessel is called the Magistery or Precipitate of Sulphur. The names of Magistery and Precipitate are also given to all substances whatever, that are separated from another by this method; which is the reason that we use the expression of precipitating one substance by another, to signify the separating one of them by means of the other.
Of the Nitrous Acid.
It is not certainly known what constitutes the difference between the Nitrous Acid and the Vitriolic Acid, with regard to the constituent principles of each. The most probable opinion is, that the Nitrous Acid is no other than the Vitriolic Acid combined with a certain quantity of Phlogiston by the means of putrefaction. If it be so, the Phlogiston must be united with the Universal Acid in another manner than it is in sulphur, and in its volatile spirit: for the Nitrous Acid differs from them both in its properties. What gives ground for this opinion is, that the Nitrous Acid is never found but in earths and stones which have been impregnated with matters subject to putrefaction, and which therefore must contain the Phlogiston. For it is necessary just to observe here, though it be not yet proper to enter particularly into the subject, that all substances susceptible of putrefaction really contain the Phlogiston.
The Nitrous Acid combined with certain absorbent earths, such as chalk, marle, boles, forms Neutral Salts which do not crystallize; and which, after being dried, run in the air per deliquium.
All those Neutral Salts which consist of the Nitrous Acid joined to an earth, may be decomposed by a fixed Alkali, with which the Acid unites, and deserts the earth; and from this union of the Nitrous Acid with a fixed Alkali results a new Neutral Salt which is called Nitre, or Salt-peter. This latter name signifies the Salt of Stone; and, in fact, Nitre is extracted from the stones and plaster, in which it forms, by boiling them in water saturated with a fixed Alkali.
Nitre shoots in long crystals adhering sideways to each o[Pg 23]ther; it has a saltish taste, which produces a sensation of cold on the tongue.
This Salt easily dissolves in water; which, when boiling hot, takes up still a greater quantity thereof.
It flows with a pretty moderate degree of heat, and continues fixed therein; but being urged by a brisk fire, and in the open air, it lets go some part of its Acid, and indeed flies off itself in part.
The most remarkable property of Nitre, and that which characterizes it, is its fulmination or explosion; the nature of which is as follows:
When Nitre touches any substance containing a Phlogiston, and actually ignited, that is, actually on fire, it bursts out into a flame, burns, and is decompounded with much noise.
In this deflagration the Acid is dissipated, and totally separated from the Alkali, which now remains by itself.
Indeed the Acid, at least the greatest part of it, is by this means quite destroyed. The Alkali which is left when Nitre is decompounded by deflagration, is called in general Fixed Nitre, and, more particularly, Nitre fixed by such and such a substance as was used in the operation. But if Nitre be deflagrated with an inflammable substance containing the vitriolic Acid, as sulphur, for instance, the fixed Salt produced by the deflagration is not a pure Alkali, but retains a good deal of the vitriolic Acid, and, by combining therewith, hath now formed a neutral Salt.
Hitherto Chymists have been at a loss for the reason why Nitre flames, and is decompounded in the manner above-mentioned, when it comes in contact with a Phlogiston properly circumstanced. For my part, I conjecture it to be for the same reason that vitriolated tartar is also decompounded by the addition of a Phlogiston; viz. the Nitrous Acid, having a greater affinity with the Phlogiston than with the fixed Alkali, naturally quits the latter to join with the former, and so produces a kind of sulphur, differing probably from the common sulphur, formed by the vitriolic Acid, in that it is combustible to such a degree, as to take fire and be consumed in the very moment of its production; so that it is impossible to prevent its being thus destroyed, and consequently impossible to save it. In support of this opinion let it be considered, that the concurrence of the Phlogiston is absolutely necessary to produce this deflagration, and that the matter of pure fire is altogether incapable of effecting it: for though Nitre be exposed to the most violent degree of fire,[Pg 24] even that in the focus of the most powerful burning-glass, it will not flame; nor will that effect ever happen till the Nitre be brought into contact with a Phlogiston properly so called, that is, the matter of fire existing as a principle of some body; and it is moreover necessary that this Phlogiston be actually on fire, and agitated with the igneous motion, or else that the Nitre itself be red hot, and so penetrated with fire as to kindle any inflammable matter that touches it.
This experiment, among others, helps to shew the distinction that ought to be made between pure elementary fire, and fire become a principle of bodies, to which we have given the name of Phlogiston.
Before we leave this subject, we shall observe, that Nitre deflagrates only with such substances as contain the Phlogiston in its simplest and purest form; such as charcoal, sulphur, and the metalline substances; and that, though it will not deflagrate without the addition of some combustible matter, it is nevertheless the only known body that will burn, and make other combustibles burn with it, in close vessels, without the admission of fresh air.
The Nitrous Acid hath not so great an affinity with earths and Alkalis as the vitriolic Acid hath with the same substances; whence it follows that the vitriolic Acid decomposes all neutral salts arising from a combination of the Nitrous Acid with an earth or an Alkali. The vitriolic Acids expells the Nitrous Acid, unites with the substance which served it for a basis, and therewith forms a neutral salt, which is an Alum, a Selenites, or a vitriolated Tartar, according to the nature of that basis.
The Nitrous Acid, when thus separated from its basis by the vitriolic Acid, is named Spirit of Nitre, or Aqua Fortis. If it be dephlegmated, or contain but little superfluous water, it exhales in reddish vapours; these vapours, being condensed and collected, form a liquor of a brownish yellow, that incessantly emits vapours of the same colour, and of a pungent disagreeable smell. These characters have procured it the names of Smoaking Spirit of Nitre, and Yellow Aqua Fortis. This property in the Nitrous Acid, of exhaling in vapours, shews it to be less fixed than the vitriolic Acid; for the latter, though ever so thoroughly dephlegmated, never yields any vapours, nor has it any smell.
Of the Acid of Sea-Salt.
The Acid of Sea-salt is so called because it is in fact obtained from such Sea-salt as is used in our kitchens. It is not certainly known in what this Acid differs from the vitriolic and the nitrous, with regard to its constituent parts. Several of the ablest Chymists, such as Becher and Stahl, are of opinion that the Marine Acid is no other than the Universal Acid united to a particular principle which they call a Mercurial Earth. Concerning this earth we shall have occasion to say more, when we come to treat of metallic substances: but in the mean time it must be owned, that the truth of this opinion is so far from being proved by a sufficient number of experiments, that the very existence of such a mercurial earth is not yet well established; and therefore, that we may not exceed the bounds of our knowledge, we shall content ourselves with delivering here the properties which characterize the Acid in question, and by which it is distinguished from the two others considered above.
When it is combined with absorbent earths, such as lime and chalk, it forms a neutral salt that does not crystallize, and, when dried, attracts the moisture of the air. If the absorbent earth be not fully saturated with the Marine Acid, the salt thereby formed has the properties of a fixed Alkali: and this is what made us say, when we were on the subject of those salts, that they might be imitated by combining an earth with an Acid. The Marine Acid, like the rest, hath not so great an affinity with earths as with fixed Alkalis.
When it is combined with the latter, it forms a neutral salt which shoots into cubical crystals. This salt is inclined to grow moist in the air, and is consequently one of those which water dissolves in equal quantities, at least as to sense, whether it be boiling hot or quite cold.
The affinity of this Acid with Alkalis and absorbent Earths is not so great as that of the vitriolic and nitrous Acids with the same substances: whence it follows, that, when combined therewith, it may be separated from them by either of those Acids.
The Acid of Sea-salt, thus disengaged from the substance which served it for a basis, is called Spirit of Salt. When it contains but little phlegm it is of a lemon colour, and continually emits many white, very dense, and very elastic[Pg 26] vapours; on which account it is named the Smoaking Spirit of Salt. Its smell is not disagreeable, nor much unlike that of saffron; but extremely quick and suffocating when it smokes.
The Acid of Sea-salt, like the other two, seems to have a greater affinity with the Phlogiston, than with fixed Alkalis. We are led to this opinion by a very curious operation, which gives ground to think that Sea-salt may be decomposed by the proper application of a substance containing the Phlogiston.
From the Marine Acid combined with a Phlogiston results a kind of Sulphur, differing from the common sort in many respects; but particularly in this property, that it takes fire of itself upon being exposed to the open air. This combination is called English Phosphorus, Phosphorus of Urine, because it is generally prepared from urine; or, only Phosphorus.
This combination of the Marine Acid with a Phlogiston is not easily effected; because it requires a difficult operation in appropriated vessels. For these reasons it does not always succeed; and Phosphorus is so scarce and dear, that hitherto Chymists have not been able to make on it the experiments necessary to discover all its properties. If Phosphorus be suffered to burn away in the air, a small quantity of an acid liquor may be obtained from it, which seems to be spirit of salt, but either altered, or combined with some adventitious matter; for it has several properties that are not to be found in the pure Marine Acid; such as, leaving a fixed fusible substance behind it when exposed to a strong fire, and being easily combined with the Phlogiston so as to reproduce a Phosphorus.
Phosphorus resembles sulphur in several of its properties: it is soluble in oils; it melts with a gentle heat; it is very combustible; it burns without producing soot; and its flame is vivid and bluish.
From what has been said of the union of the Acid of Sea-salt with a fixed Alkali, and of the neutral salt resulting therefrom, it may be concluded that this neutral salt is no other than the common kitchen-salt. But it must be observed, that the fixed Alkali, which is the natural basis of the common salt obtained from sea-water, is of a sort somewhat differing from fixed Alkalis in general, and hath certain properties peculiar to itself. For,
1. The basis of Sea-salt differs from other fixed Alkalis in this, that it crystallizes like a neutral salt.
2. It does not grow moist in the air; on the contrary, when exposed to the air, it loses part of the water that united with it in crystallization, by which means its crystals lose their transparency, become, as it were, mealy, and fall into a fine flour.
3. When combined with the vitriolic Acid to the point of saturation, it forms a neutral salt differing from vitriolated tartar, first, in the figure of its crystals, which are oblong six-sided solids; secondly, in its quantity of water, which in crystallization unites therewith in a much greater proportion than with vitriolated tartar; whence it follows, that this salt dissolves in water more readily than vitriolated tartar; thirdly, in that it flows with a very moderate degree of heat, whereas vitriolated tartar requires a very fierce one.
If the Acid of Sea-salt be separated from its basis by means of the vitriolic Acid, it is easy to see that, when the operation is finished, the salt we have been speaking of must be the result. A famous Chymist, named Glauber, was the first who extracted the Spirit of Salt in this manner, examined the neutral salt resulting from his process, and, finding it to have some singular properties, called it his Sal mirabile, or wonderful Salt: on this account it is still called Glauber's Sal mirabile, or plainly Glauber's Salt.
4. When the basis of Sea-salt is combined with the nitrous Acid to the point of saturation, there results a neutral salt, or a sort of nitre, differing from the common nitre, first, in that it attracts the moisture of the air pretty strongly; and this makes it difficult to crystallize; secondly, in the figure of its crystals, which are parallelopipeds; and this has procured it the name of Quadrangular Nitre.
Common salt, or the neutral salt formed by combining the Marine Acid with this particular sort of fixed Alkali, has a taste well known to every body. The figure of its crystals is exactly cubical. It grows moist in the air, and, when exposed to the fire, it bursts, before it melts, into many little fragments, with a crackling noise; which is called the Decrepitation of Sea-salt.
That neutral salt mentioned above, which is formed by combining the Marine Acid with a common fixed Alkali, and called Sal febrifugum Sylvii, hath also this property.
India furnishes us with a saline substance, known by the name of Borax, which flows very easily, and then takes the form of glass. It is of great use in facilitating the fusion of metallic substances. It possesses some of the properties of[Pg 28] fixed Alkalis, which has induced certain Chymists to represent it, through mistake, as a pure fixed Alkali.
By mixing borax with the vitriolic Acid, Mr. Homberg obtained from it a salt, which sublimes in a certain degree of heat, whenever such a mixture is made. This salt has very singular properties; but its nature is not yet thoroughly understood. It dissolves in water with great difficulty; it is not volatile, though it rises by sublimation from the borax. According to Mr. Rouelle's observation, it rises then only by means of the water which carries it up: for, when once made, it abides the fiercest fire, flows and vitrifies just as borax does: provided care be taken to free it previously from moisture by drying it properly. Mr. Homberg called it Sedative Salt, on account of its medical effects. The sedative salt hath the appearance, and some of the properties, of a neutral salt; for it shoots into crystals, and does not change the colour of violets; but it acts the part of an Acid with regard to Alkalis, uniting with them to the point of saturation, and thereby forming a true neutral salt. It also acts, like the Acid of vitriol on all neutral salts; that is, it discharges the Acid of such as have not the vitriolic Acid in their composition.
Since Mr. Homberg's time it hath been discovered, that a sedative salt may be made either with the nitrous or with the marine Acid; and that sublimation is not necessary to extract it from the borax, but that it may be obtained by crystallization only. For this latter discovery we are indebted to Mr. Geoffry, as we are to Mr. Lemery for the former.
Since that time M. Baron d'Henouville, an able Chymist, hath shewn that a sedative salt may be obtained by the means of vegetable Acids; and hath lately demonstrated, in some excellent papers published in the collection of Memoirs written by the correspondents of the Academy of Sciences, that the sedative salt exists actually and perfectly in the borax, and that it is not produced by mixing Acids with that saline substance, as it seems all the Chymists before him imagined. This he proves convincingly from his analysis of borax, (which thereby appears to be nothing else but the sedative salt united with that fixed Alkali which is the basis of Sea-salt) and from his regenerating the same borax by uniting together that Alkali and the sedative salt: a proof the most complete that can possibly be produced in natural philosophy, and equivalent to demonstration itself.
In order to finish what remains to be said upon the several sorts of saline substances, we should now speak of the Acids[Pg 29] obtained from vegetables and animals, and also of the volatile Alkalis: but, seeing these saline substances differ from those of which we have already treated, only as they are variously altered by the unions they have contracted with certain principles of vegetables and animals, of which nothing has been yet said, it is proper to defer being particular concerning them, till we have explained those principles.
Of Lime.
Any substance whatever, that has been roasted a considerable time in a strong fire without melting, is commonly called a Calx. Stones and metals are the principal subjects that have the property of being converted into Calces. We shall treat of Metalline Calces in a subsequent chapter, and in this confine ourselves to the Calx of Stone, known by the name of Lime.
In treating of earths in general we observed that they may be divided into two principal kinds; one of which actually and properly flows when exposed to the action of fire, and turns to glass; whence it is called a fusible or vitrifiable earth; the other resists the utmost force of fire, and is therefore said to be an unfusible or unvitrifiable earth. The latter is also not uncommonly called calcinable earth; though sundry sorts of unfusible earths are incapable of acquiring by the action of fire all the qualities of calcined earth, or Lime properly so called: such earths are particularly distinguished by the denomination of refractory earths.
As the different sorts of stones are nothing more than compounds of different earths, they have the same properties with the earths of which they are composed, and may, like them, be divided into fusible or vitrifiable, and unfusible or calcinable. The fusible stones are generally denoted by the name of Flints; the calcinable stones, again, are the several sorts of marbles, cretaceous stones, those commonly called free-stones, &c. some of which, as they make the best Lime, are, by way of eminence, called Lime-stones. Sea-shells, also, and stones that abound with fossile shells, are capable of being burnt to Lime.
All these substances, being exposed, for a longer or shorter time, as the nature of each requires, to the violent action of fire, are said to be calcined. By calcination they lose a considerable part of their weight, acquire a white colour, and become friable though ever so solid before; as, for instance, the very hardest marbles. These substances, when thus calcined, take the name of Quick Lime.
Water penetrates Quick Lime, and rushes into it with vast activity. If a lump of newly calcined Lime be thrown into water, it instantly excites almost as great a noise, ebullition, and smoke, as would be produced by a piece of red-hot iron; with such a degree of heat too, that, if the Lime be in due proportion to the water, it will set fire to combustible bodies; as hath unfortunately happened to vessels laden with Quick Lime, on their springing a small leak.
As soon as Quick Lime is put into water, it swells, and falls asunder into an infinite number of minute particles: in a word, it is in a manner dissolved by the water, which forms therewith a sort of white paste called Slacked Lime.
If the quantity of water be considerable enough for the Lime to form with it a white liquor, this liquor is called Lac Calcis; which, being left some time to settle, grows clear and transparent, the Lime which was suspended therein, and occasioned its opacity, subsiding to the bottom of the vessel. Then there forms on the surface of the liquor a crystalline pellicle, somewhat opaque and dark-coloured, which being skimmed off is reproduced from time to time. This matter is called Cremor Calcis.
Slacked Lime gradually grows dry, and takes the form of a solid body, but full of cracks and destitute of firmness. The event is different when you mix it up, while yet a paste, with a certain quantity of uncalcined stony matter, such as sand, for example: then it takes the name of Mortar, and gradually acquires, as it grows drier and older, a hardness equal to that of the best stones. This is a very singular property of Lime, nor is it easy to account for it: but it is a beneficial one; for every body knows the use of Mortar in building.
Quick Lime attracts the moisture of the air, in the same manner as concentrated acids, and dry fixed alkalis; but not in such quantities as to render it fluid: it only falls into extremely small particles, takes the form of a fine powder, and the title of Lime slacked in the air.
Lime once slacked, however dry it may afterwards appear, always retains a large portion of the water it had imbibed;[Pg 31] which cannot be separated from it again but by means of a violent calcination. Being so recalcined it returns to be Quick Lime, recovering all its properties.
Besides this great affinity of Quick Lime with water, which discovers a saline character, it has several other saline properties, to be afterwards examined, much resembling those of fixed alkalis. In Chymistry it acts very nearly as those salts do, and may be considered as holding the middle rank between a pure absorbent earth and a fixed alkali: and this hath induced many Chymists to think that Lime contains a true salt, to which all the properties it possesses in common with salts may be attributed.
But as the chymical examination of this subject hath long been neglected, the existence of a saline substance in Lime hath been long doubtful. Mr. du Fay, author of some excellent chymical experiments, was one of the first who obtained a salt from Lime, by lixiviating it with a great deal of water, which he afterwards evaporated. But the quantity of salt he obtained by that means was very small; nor was it of an alkaline nature, as one would think it should have been, considering the properties of Lime. Mr. du Fay did not carry his experiments on this subject any further, probably for want of time; nor did he determine of what nature the salt was.
Mr. Malouin had the curiosity to examine this salt of Lime, and soon found that it was nothing else but what was above called Cremor Calcis. He found, moreover, that, by mixing a fixed alkali with lime-water, a vitriolated tartar was formed; that, by mixing therewith an alkali like the basis of sea-salt, a Glauber's salt was produced; and, lastly, by combining lime with a substance abounding in phlogiston, he obtained a true sulphur. These very ingenious experiments prove to a demonstration, that the vitriolic acid constitutes the salt of Lime: for, as hath been shewn, no other acid is capable of forming such combinations. On the other hand, Mr. Malouin, having forced the vitriolic acid of this salt to combine with a phlogiston, found its basis to be earthy, and analogous to that of the selenites: whence he concluded, that the salt of Lime is a true neutral salt, of the same kind as the selenites. Mr. Malouin tells us he found several other salts in Lime. But as none of them was a fixed alkali, and as all the saline properties of Lime have an affinity with those of that kind of salt, there is great reason to think that all those salts are foreign to Lime, and that their union with it is merely accidental.
I myself have made several experiments in order to get some insight into the saline nature of Lime, and shall here produce the result with all possible conciseness. I took several stones of different kinds, some of which produced by calcination a very strong Lime, and others but a very weak one. These I impregnated with different saline substances, acids, alkalis, and neutrals, and then exposed them all to the same degree of fire, which was a pretty strong one, and long enough continued to have made very good Lime of stones the most difficult to calcine. The consequence was, that, in the first place, those stones which naturally made but a weak Lime were not, by this process, converted into a stronger Lime; and, moreover, that none of these stones, even such as would naturally have produced the most active Lime, had acquired the properties of Lime. These experiments I varied many ways, employing different proportions of saline matters, and almost every possible degree of fire, and constantly observed, after calcination, that all those stones were so much the farther from the nature of Lime, as they had been combined with larger doses of salts. Among those which were impregnated with the greatest proportion of salts, and had suffered the greatest violence of fire, I observed some that had begun to flow, and were in a manner vitrified. Now, as the same subject cannot be, at one and the same time, in the state of glass and of Lime too; as a body cannot approach to one of these states but in proportion as it recedes from the other; and as salts in general dispose those bodies to fusion and vitrification which are in themselves the most averse to either, I concluded from my experiments, that the saline substances I used, had, by acting as fluxes upon the stones, prevented their calcination; that consequently we may suspect there is no saline matter in the composition of Lime, as Lime; and that Lime does not owe its saline and alkaline properties to any salt; or at least that, if it does owe those properties to a salt, such salt must be naturally and originally combined with the matter of the stone in so just a proportion, that it is impossible to increase the quantity thereof without prejudicing the Lime, and depriving it in some measure of its virtue. This theory agrees perfectly with the illustrious Stahl's opinion; for he thinks, as we observed in discoursing of salts in general, that every saline substance is but an earth combined in a certain manner with water. This notion he applies to Lime, and says, that fire only subtilizes and attenuates the earthy matter, and thereby renders it capable of uniting with water in such a manner, that the re[Pg 33]sult of their combination shall be a substance having saline properties; and that Lime accordingly never acquires these properties till it be combined with water.
I have dwelt longer on the Salt of Lime than I shall on any other particular; because the subject, though in itself of great importance, has hitherto been but little attended to, and because the experiments here recited are entirely new.
Lime unites with all acids, and in conjunction with them exhibits various phenomena.
The vitriolic acid poured upon Lime dissolves it with effervescence and heat. From this mixture there exhales a great quantity of vapours, in smell and colour perfectly like those of sea-salt; from which, however, they are found to be very different when collected into a liquor. From this combination of the vitriolic acid with Lime arises a neutral salt, which shoots into crystals, and is of the same kind with the selenetic salt obtained from Lime by Mr. Malouin.
The nitrous acid poured upon Lime dissolves it in like manner with effervescence and heat: but the solution is transparent, and therein differs from the former, which is opaque. From this mixture there arises a neutral salt, which does not crystallize, and has withal the very singular property of being volatile, and rising wholly by distillation in a liquid form. This phenomenon is so much the more remarkable, as Lime, the basis of this salt, is one of the most fixed bodies known in Chymistry.
With the acid of sea-salt Lime forms also a singular sort of salt, which greedily imbibes the moisture of the air. We shall have occasion to take further notice of it in another place.
These experiments made on Lime with acids are likewise quite new. We are indebted for them to Mr. Du Hamel of the Academy of Sciences, whose admirable Memoirs on several subjects shew his extensive knowledge in all parts of Natural Philosophy.
Lime applied to fixed alkalis adds considerably to their caustic quality, and makes them more penetrating and active. An alkaline lixivium in which Lime hath been boiled, being evaporated to dryness, forms a very caustic substance, which flows in the fire much more easily, attracts and retains moisture much more strongly, than fixed alkalis that have not been so treated. An alkali thus acuated by Lime is called the Caustic Stone, or Potential Cautery; because it is employed by surgeons to produce eschars on the skin and cauterize it.
Of Metallic Substances in general.
Metallic Substances are heavy, glittering, opaque, fusible bodies. They consist chiefly of a vitrifiable earth united with the phlogiston.
Several Chymists insist on a third principle in these bodies, and have given it the name of Mercurial Earth; which, according to Becher and Stahl, is the very same that being combined with the vitriolic acid forms and characterizes the acid of sea-salt. The existence of this principle hath not yet been demonstrated by any decisive experiment; but we shall shew that there are pretty strong reasons for admitting it.
We shall begin with mentioning the experiments which prove Metallic Substances to consist of a vitrifiable earth united with the phlogiston. The first is this: if they be calcined in such a manner as to have no communication with any inflammable matter, they will be spoiled of all their properties, and reduced to an earth or calx, that has neither the splendour nor the ductility of a metal, and in a strong fire turns to an actual glass, instead of flowing like a metal.
The second is, that the calx or the glass resulting from a metal thus decomposed, recovers all its metalline properties by being fused in immediate contact with an inflammable substance, capable of restoring the phlogiston of which calcination had deprived it.
On this occasion we must observe, that Chymists have not yet been able, by adding the phlogiston, to give the properties of metals to all sorts of vitrifiable earths indiscriminately; but to such only as originally made a part of some metallic body. For example, a compound cannot be made with the phlogiston and sand that shall have the least resemblance of a metal: and this is what seems to point out the reality of a third principle, as necessary to form the metalline combination. This principle may probably remain united with the vitrifiable earth of a metallic substance, when reduced to a glass; whence it follows, that such vitrified metals require only the addition of a phlogiston to enable them to appear again in their pristine form.
It may be inferred from another experiment, that the calx and the glass of a metal are not its pure vitrifiable earth, properly so called: for by repeated or long-continued calcinations, such a calx or glass may be rendered incapable of ever resuming the metalline form, in whatever manner the phlogiston be afterwards applied to it; so that by this means it is brought into the condition of a pure vitrifiable earth, absolutely free from any mixture. Those Chymists who patronize the Mercurial earth, produce many other proofs of the existence of that principle in Metallic Substances; but they would be misplaced in an elementary treatise like this.
When by adding the phlogiston to a metallic glass we restore it to the form of a metal, we are said to reduce, resuscitate, or revivify that metal.
Metallic Substances are of different kinds, and are divided into Metals and Semi-metals.
Those are called Metals which, besides their metalline splendour and appearance, are also malleable; that is, have the property of stretching under the hammer, and by that means of being wrought into different forms without breaking.
Those which have only the metalline splendour and appearance, without malleability, are called Semi-metals.
Metals are also further subdivided into two sorts; viz. Perfect and Imperfect Metals.
The Perfect Metals are those which suffer no damage or change whatever by the most violent and most lasting action of fire.
The Imperfect Metals are those which by the force of fire, may be deprived of their phlogiston, and consequently of their metalline form.
When but a moderate degree of fire is employed to deprive a Metal of its phlogiston, the metal is said to be calcined; and then it appears in the form of a powdered earth, which is called a Calx: and this metalline calx being exposed to a more violent degree of fire melts and turns to glass.
Metallic Substances have an affinity with acids: but not equally with all; that is, every Metallic Substance is not capable of uniting and joining with every acid.
When an acid unites with a Metallic Substance there commonly arises an ebullition, attended with a kind of hissing noise and fuming exhalations. By degrees, as the union becomes more perfect, the particles of the metal combining with the acid become invisible: this is termed Dissolution; and when a metalline mass thus appears in an acid, the me[Pg 36]tal is said to be dissolved by that acid. It is proper to observe, that acids act upon Metalline Substances, in one respect, just as they do upon alkalis and absorbent earths: for an acid cannot take up above such a certain proportion thereof as is sufficient to saturate it, to destroy several of its properties, and weaken others. For example, when an acid is combined with a metal to the point of saturation, it loses its taste, does not turn the blue colour of a vegetable red, and its affinity with water is considerably impaired. On the other hand, Metalline Substances, which when pure are incapable of uniting with water, by being joined with an acid acquire the property of dissolving in water. These combinations of Metalline Substances with acids form different sorts of neutral salts; some of which have the property of shooting into crystals, while others have it not: most of them, when thoroughly dried, attract the moisture of the air.
The affinity which Metalline Substances have with acids is less than that which absorbent earths and fixed alkalis have with the same acids; so that all metalline salts may be decompounded by one of these substances, which will unite with the acid, and precipitate the metal.
Metalline Substances thus separated from an acid solvent are called Magisteries and Precipitates of metals. None of these precipitates, except those of the perfect metals, retain the metalline form: most of their phlogiston hath been destroyed by the solution and precipitation, and must be restored before they can recover their properties. In short, they are nearly in the same state with Metalline Substances deprived of their phlogiston by calcination; and accordingly such a precipitate is called a Calx.
A metalline calx prepared in this manner loses a greater or a less portion of its phlogiston, the more or less effectually and thoroughly the Metalline Substance, of which it made a part, was dissolved by the acid.
Metallic Substances have affinities with each other which differ according to their different kinds: but this is not universal; for some of them are incapable of any sort of union with some others.
It must be observed, that Metallic Substances will not unite, except they be both in a similar state; that is, both in a Metalline form, or both in the form of a Glass; for a Metalline Substance retaining its phlogiston cannot contract an union with any metallic glass, even its own.
Of Metals.
There are six Metals, of which two are Perfect and four Imperfect. The perfect Metals are Gold and Silver; the others are Copper, Tin, Lead, and Iron. Some Chymists admit a seventh Metal, to wit, Quick-silver: but as it is not malleable, it has been generally considered as a metallic body of a particular kind. We shall soon have occasion to examine it more minutely.
The ancient Chymists, or rather the Alchymists, who fancied a certain relation or analogy between Metals and the Heavenly Bodies, bestowed on the seven Metals, reckoning Quick-silver one of them, the names of the seven Planets of the Ancients, according to the affinity which they imagined they observed between those several bodies. Thus Gold was called Sol, Silver Luna, Copper Venus, Tin Jupiter, Lead Saturn, Iron Mars, and Quick-silver Mercury. Though these names were assigned for reasons merely chimerical, yet they still keep their ground; so that it is not uncommon to find the Metals called by the names, and denoted by the characters, of the Planets, in the writings even of the best Chymists. Metals are the heaviest bodies known in nature.
Of Gold.
Gold is the heaviest of all Metals. The arts of wire-drawing and gold-beating shew its wonderful ductility. The greatest violence of fire is not able to produce any alteration in it. Indeed Mr. Homberg, a famous Chymist, pretended that he had made this metal fume, and even vitrified it, by exposing it to the focus of one of the best burning-glasses, known by the name of the Lens of the Palais Royal: but, there are very good reasons for calling in question the experiments he made on this occasion, or rather for thinking that he was quite mistaken. For,
1. No man hath since been able to vitrify Gold, though several good Experimenters have assiduously tried to effect it, by exposing it to the focus of the same lens, and of other burning-glasses still stronger.
2. It hath been observed, that though Gold, when exposed to the focus of those glasses, did indeed emit some vapours and decrease in weight; yet, those vapours being carefully collected on a piece of paper, proved to be true Gold, in no degree vitrified, and which consequently had suffered no change but that of being carried away by the violence of the heat, its nature not being in the least altered.
3. The small portion of vitrified matter, which was formed on the arm that supported the Gold in Mr. Homberg's experiment, may have come either from the arm itself, or rather from some heterogeneous particles contained in the Gold; for it is almost impossible to have it perfectly pure.
4. Neither Mr. Homberg, nor any that have repeated his experiment, ever reduced this pretended glass of Gold by restoring its Phlogiston, as is done with other metallic glasses.
5. To render the experiment decisive, the whole mass of Gold employed ought to have been vitrified; which was not the case.
Nevertheless, I do not pretend that this metal is in its own nature absolutely indestructible, and unvitrifiable: but there is reason to think that no body hath hitherto found the means of producing those effects on it, probably for want of a sufficient degree of fire; at least the point is very doubtful.
Gold cannot be dissolved by any pure acid: but if the acid of nitre be mixed with the acid of sea-salt, there results a compound acid liquor, with which it has so great an affinity that it is capable of being perfectly dissolved thereby. The Chymists have called this solvent Aqua Regis, on account of its being the only acid that can dissolve Gold, which they consider as the King of Metals. The solution of gold is of a beautiful orange colour.
If Gold dissolved in aqua regis be precipitated by an alkali or an absorbent earth, the precipitate gently dried, and then exposed to a certain degree of heat, is instantly dispersed into the air, with a most violent explosion and noise: Gold thus precipitated is therefore called Aurum Fulminans. But if the precipitated Gold be carefully washed in plenty of water, so as to clear it of all the adhering saline particles, it will not fulminate, but may be melted in a crucible without[Pg 39] any additament, and will then appear in its usual form. The acid of vitriol being poured on aurum fulminans likewise deprives it of its fulminating quality.
Gold does not begin to flow till it be red-hot like a live coal. Though it be the most malleable and most ductile of all metals, it has the singular property of losing its ductility more easily than any of them: even the fumes of charcoal are sufficient to deprive it thereof, if they come in contact with it while it is in fusion.
The malleability of this metal, and indeed of all the rest, is also considerably diminished by exposing it suddenly to cold when it is red-hot; for example, by quenching it in water, or even barely exposing it to the cold air.
The way to restore ductility to gold, when lost by its coming in contact with the vapour of coals, and in general to any metal rendered less malleable by being suddenly cooled, is to heat it again, to keep it red hot a considerable time, and then to let it cool very slowly and gradually; this operation frequently repeated will by degrees much increase the malleability of a metal.
Pure sulphur hath no effect on Gold; but being combined with an alkali into a hepar sulphuris, it unites therewith very readily. Nay, so intimate is their union, that the Gold, by means thereof, becomes soluble in water; and this new compound of Gold and liver of sulphur, being dissolved in water, will pass through the pores of brown paper without suffering any decomposition; which does not happen, at least in such a manifest degree, to other metallic substances dissolved by liver of sulphur.
Aurum fulminans, mixed and melted with flower of sulphur, loses its fulminating quality: which arises from hence, that on this occasion the sulphur burns, and its acid, which is the same with the vitriolic, being thereby set at liberty becomes capable of acting upon the Gold as a vitriolic acid would; which, as was said above, deprives the Gold of its fulminating quality.
Of Silver.
Next to Gold, Silver is the most perfect metal. Like Gold it resists the utmost violence of fire, even that in the focus of a burning-glass. However, it holds only the second place among metals; because it is lighter than Gold by al[Pg 40]most one half; is also somewhat less ductile; and, lastly, because it is acted upon by a greater number of solvents.
Yet Silver hath one advantage over gold, namely that of being a little harder; which makes it also more sonorous.
This metal, like Gold, begins to flow when it is so thoroughly penetrated by the fire as to appear ignited like a live coal.
While this metal is in fusion, the immediate contact of the vapour of burning coals deprives it almost entirely of its malleability, in the same manner as we observed happens to Gold: but both these metals easily recover that property by being melted with nitre.
The nitrous acid is the true solvent of Silver, and being somewhat dephlegmated will very readily and easily take up a quantity of Silver equal in weight to itself.
Silver thus combined with the nitrous acid forms a metallic salt which shoots into crystals, called by the name of Lunar Crystals, or Crystals of Silver.
These crystals are most violently caustic: applied to the skin they quickly affect it much as a live coal would; they produce a blackish eschar, corroding and entirely destroying the parts they touch. Surgeons use them to eat away the proud fungous flesh of ulcers. As Silver united with the nitrous acid hath the property of blackening all animal substances, a solution of this metallic salt is employed to dye hair, or other animal matters, of a beautiful and durable black.
These crystals flow with a very moderate heat, and even before they grow red. Being thus melted they form a blackish mass; and in this form they are used by Surgeons, under the title of Lapis Infernalis, Infernal Stone, or Lunar Caustic.
Silver is also dissolved by the vitriolic acid: but then the acid must be concentrated, and in quantity double the weight of the Silver; nor will the solution succeed without a considerable degree of heat.
Spirit of salt and aqua regis, as well as the other acids, are incapable of dissolving this metal; at least in the ordinary way.
Though Silver be not soluble in the acid of sea-salt, nor easily in the acid of vitriol, as hath just been observed, it doth not follow that it hath but a weak affinity with the latter, and none at all with the former: on the contrary, it appears from experiment that it hath with these two acids a much greater affinity than with the acid of nitre: which is[Pg 41] singular enough, considering the facility with which this last acid dissolves it.
The experiment which proves the fact, is this. To a solution of Silver in the nitrous acid, add the acid either of vitriol or of sea-salt, and the Silver will instantly quit its nitrous solvent to join with the superadded acid.
Silver thus united with the vitriolic or the marine acid is less soluble in water than when combined with the nitrous acid; and for this reason it is, that when either of these two acids is added to a solution of Silver, the liquor immediately becomes white, and a precipitate is formed, which is no other than the Silver united with the precipitating acid. If the precipitation be effected by the vitriolic acid, the precipitate will disappear upon adding a sufficient quantity of water, because there will then be water enough to dissolve it. But the case is not the same when the precipitation is made by the marine acid; for Silver combined therewith is scarce soluble in water.
This Precipitate of Silver, procured by means of the marine acid, is very easily fused, and when fused changes to a substance in some measure transparent and flexible, which hath occasioned it to be called by the name of Luna Cornea. If it be proposed to decompound this luna cornea, that is, to separate the marine acid from the Silver with which it is united, the luna cornea must be melted along with fatty and absorbent matters, with which the acid will unite, and leave the metal exceeding pure.
It must be observed, that if, instead of the marine acid, sea-salt in substance be added to a solution of Silver in the nitrous acid, a Precipitate is also produced, which by fusion appears to be a true luna cornea. The reason is, that the sea-salt is decomposed by the nitrous acid, which seizes its basis, as having a greater affinity therewith than its own acid hath; and this acid being consequently disengaged and set at liberty unites with the Silver, which, as has been shewn, has a greater affinity with it than with the nitrous acid. This is an instance of decomposition effected by means of one of those double affinities mentioned by us in our seventh proposition concerning Affinities.
From what hath been already said it is clear, that all these combinations of Silver with acids may be decompounded by absorbent earths and by fixed alkalis; it being a general law with regard to all metallic substances. We shall not therefore repeat this observation when we come to treat of the other metals; unless some particular occasion require it.
With regard to Silver I must take notice that, when separated by these means from the acids in which it was dissolved, it requires nothing but simple fusion to restore it to its usual form; because it does not, any more than Gold, lose its Phlogiston by those solutions and precipitations.
Silver unites with sulphur in fusion. If this metal be only made red-hot in a crucible, and sulphur be then added, it immediately flows; the sulphur acting as a flux to it. Silver thus united with sulphur forms a mass that may be cut, is half malleable, and hath nearly the colour and consistence of Lead. If this sulphurated Silver be kept a long time in fusion, and in a great degree of heat, the sulphur flies off and leaves the Silver pure. But if the sulphur be evaporated by a violent heat, it carries off with it part of the Silver.
Silver unites and mixes perfectly with Gold in fusion. The two metals thus mixed form a compound with properties partaking of both.
Metallurgists have hitherto sought in vain for a perfectly good and easy method of separating these two metals by the dry way only: (this term is used to signify all operations performed by fusion): but they are conveniently enough parted by the moist way, that is, by acid solvents. This method is founded on the above-mentioned properties of Gold and Silver with respect to acids. It hath been shewn that aqua regis only will dissolve Gold; that Silver, on the contrary, is not soluble by aqua regis, and that its proper solvent is the acid of nitre; consequently, when Gold and Silver are mixed together, if the compound mass be put into aqua fortis, this acid will take up all the Silver, without dissolving a particle of the Gold, which will therefore remain pure; and by this means the desired separation is effected. This method, which is commonly made use of by Goldsmiths, and in Mints, is called the Parting Assay.
It is plain, that if aqua regis were employed instead of aqua fortis, the separation would be equally effected; and that the only difference between this process and the former would consist in this, that now the Gold would be dissolved, and the Silver remain pure. But the operation by aqua fortis is preferable; because aqua regis does take up a little Silver, whereas aqua fortis hath not the least effect on Gold.
It must be observed, that, when Gold and Silver are mixed together in equal parts, they cannot be parted by the means of aqua fortis. To enable the aqua fortis to act duly on the Silver, this metal must be, at least, in a triple[Pg 43] proportion to the Gold. If it be in a less proportion, you must either employ aqua regis to make the separation, or, if you prefer the use of aqua fortis, melt the metalline mass, and add as much Silver as is necessary to make up the proportion above-mentioned: and hence this Process is called Quartation.
This effect, which is pretty singular, probably arises from hence, that when the Gold exceeds or even equals the Silver in quantity, the parts of both being intimately united, the former are capable of coating over the latter, and covering them so as to defend them from the action of the aqua fortis; which is not the case when there is thrice as much Silver as Gold.
There is one thing more to be taken notice of with regard to this process; which is, that perfectly pure aqua fortis is rarely to be met with, for two reasons; first, it is difficult in making it wholly to prevent the rising of the medium employed to disengage the nitrous acid; that is, a little of the vitriolic acid will mix with the vapours of the aqua fortis: secondly, unless the salt-petre be very well purified it will always hold some small portion of sea-salt, the acid of which, we know, is very readily set loose by the vitriolic acid, and consequently rises together with the vapours of the aqua fortis. It is easy to see that aqua fortis mixed either with the one or the other is not proper for the Parting Process; because, as has just been said, the vitriolic and the marine acid equally precipitate Silver dissolved in the nitrous acid; by which means, when they are united with that acid, they weaken its action upon the Silver, and hinder the dissolution. Add, that aqua fortis adulterated with a mixture of spirit of salt becomes an aqua regis, and consequently is rendered capable of dissolving Gold, in proportion as its action upon Silver is diminished.
In order to remedy this inconvenience, and free aqua fortis from the vitriolic or marine acid with which it is tainted, Silver must be dissolved therein: by degrees as the metal dissolves, those heterogeneous acids lay hold of it, and precipitate with it in the form of a white powder, as we observed before. This precipitate being wholly fallen, the liquor grows clear; after which, if it be found capable of dissolving more silver, without turning milky, it may be depended on as a perfectly pure aqua fortis. Then filtre it, dissolve more Silver in it, as long as it will take up any, and you will have a solution of Silver in a very pure aqua fortis. By means of this solution may other aqua fortis be[Pg 44] purified: for pour a few drops thereof into a very impure aqua fortis, and immediately the vitriolic or marine acid, with which that aqua fortis is contaminated, will join the Silver and fall therewith to the bottom. When the solution of Silver, prepared as above, does not in the least affect the transparency of the aqua fortis, it is then very pure, and fit for the purposes of Quartation.
This operation of purifying aqua fortis by a solution of Silver is called the Precipitation of Aqua Fortis, and aqua fortis thus purified is called Precipitated Aqua Fortis.
When Silver is dissolved in aqua fortis it may be separated therefrom, as hath been shewn, by absorbent earths and fixed alkalis.
We shall see by and by that there are other means of effecting this: but whatever way it be separated from its solvent it recovers its metalline form, as Gold does, by being simply fused without any additament.
Of Copper.
Of all the imperfect metals Copper comes the nearest to Gold and Silver. Its natural colour is a deep-red yellow. It resists a very violent degree of fire for a considerable time; but losing its phlogiston at last, it changes its metalline form for that of a calx, or a pure reddish earth. This calx is hardly, if at all, reducible to glass, without the addition of something to promote its fusion; all that the fiercest heat can do being only to render it soft. Copper, even while it retains its metalline form, and is very pure, requires a considerable degree of fire to melt it, and does not begin to flow till long after it is red-hot. When in fusion, it communicates a greenish colour to the flame of the coals.
This metal is inferior to Silver in point of gravity; nor is its ductility so great, though it be pretty considerable: but, on the other hand, it exceeds that metal in hardness. It unites readily with Gold and Silver; nor does it greatly lessen their beauty when added to them in a small quantity: nay, it even procures them some advantages; such as making them harder, and less subject to lose their ductility, of which those metals are often liable to be deprived, by the mixture of the smallest heterogeneous particle. This may probably arise from hence, that the ductility of Copper has the peculiarity[Pg 45] of resisting most of those causes which rob the perfect metals of theirs.
The property, which other metalline substances have in common with Copper, of losing the phlogiston by calcining and then vitrifying, furnishes us with a method of separating them from Gold and Silver, when they are combined therewith. Nothing more is required than to expose the mass compounded of the perfect metals and other metalline substances to a degree of heat sufficient to calcine whatever is not either Gold or Silver. It is evident, that, by this means, these two metals will be obtained as pure as is possible; for, as hath already been said, no metalline calx or glass is capable of uniting with metals possessed of their phlogiston. On this principle is formed the whole business of refining Gold and Silver.
When the perfect metals have no other alloy but Copper, as this metal is not to be calcined or vitrified without great difficulty, which is increased by its union with the unvitrifiable metals, it is easy to see that it is almost impossible to separate them without adding something to facilitate the vitrification of the Copper. Such metals as have the property of turning easily to glass are very fit for this purpose; and it is necessary to add a certain quantity thereof, when Gold or Silver is to be purified from the alloy of Copper. We shall have occasion to be more particular on this subject when we come to treat of Lead.
Copper is soluble in all the acids, to which it communicates a green colour, and sometimes a blue. Even the neutral salts, and water itself, act upon this metal. With regard to water indeed, as the procuring it absolutely pure and free from any saline mixture is next to an impossibility, it remains a question whether the effect it produces on Copper be not owing to certain saline particles contained in it. It is this great facility of being dissolved that renders Copper so subject to rust; which is nothing else but some parts of its surface corroded by saline particles contained in the surrounding air and water.
The rust of Copper is always green or blue, or of a colour between these two. Internally used it is very noxious, being a real poison, as are all the solutions of this metal made by any acid whatever. The blue colour which Copper constantly assumes, when corroded by any saline substance, is a sure sign by which it may be discovered wherever it exists, even in a very small quantity.
Copper dissolved in the vitriolic acid forms a kind of metalline salt, which shoots into rhomboidal crystals of a most beautiful blue colour. These crystals are called Blue Vitriol, or Vitriol of Copper. They are sometimes found ready formed in the bowels of the earth; and may be artificially made by dissolving Copper in the vitriolic acid; but the solution will not succeed unless the acid be well dephlegmated. The taste of this vitriol is saltish and astringent. It retains a considerable quantity of water in crystallizing, on which account it is easily rendered fluid by fire.
It must be observed, that, when it is exposed to a certain degree of heat, in order to free it of its humidity, a great part of its acid flies off at the same time: and hence it is that, after calcination, there remains only a kind of earth, or metalline calx, of a red colour, which contains but very little acid. This earth cannot be brought to flow but with the greatest difficulty.
A solution of Copper in the nitrous acid forms a salt which does not crystallize, but, when dried, powerfully attracts the moisture of the air. The same thing happens when it is dissolved in the spirit of salt, or in aqua regis.
If the Copper thus dissolved by any of these acids be precipitated by an earth or an alkali, it retains nearly the colour it had in the solution: but these precipitates are scarce any thing more than the earth of Copper, or Copper deprived of most of its phlogiston; so that if they were exposed to a violent fire, without any additament, a great part of them would be converted into an earth that could never be reduced to a metalline form. Therefore, when we intend to reduce these precipitates to Copper, it is necessary to add a certain quantity of a substance capable of restoring to them the phlogiston they have lost.
The substance which hath been found fittest for such reductions is charcoal-dust; because charcoal is nothing but a phlogiston closely combined with an earth, which renders it exceedingly fixed, and capable of resisting a violent force of fire. But as charcoal will not melt, and consequently is capable of preventing rather than forwarding the flux of a metalline calx or glass, which nevertheless is essentially necessary to complete the reduction, it hath been contrived to mix it, or any other substance containing the phlogiston, with such fixed alkalis as easily flow, and are fit to promote the flux of other bodies. These mixtures are called Reducing Fluxes; because the general name of Fluxes is given to all salts or mixtures of salts, which facilitate fusion.
If Sulphur be applied to Copper made perfectly red-hot, the metal immediately runs; and these two substances uniting form a new compound much more fusible than pure Copper.
This compound is destroyed by the sole force of fire, for two reasons: the first is, that, sulphur being volatile, the fire is capable of subliming a great part of it, especially when it is in a great proportion to the Copper with which it is joined; the second is, that the portion of sulphur which remains, being more intimately united with the Copper, though it be rendered less combustible by that union, is nevertheless burnt and consumed in time. Copper being combined with sulphur, and together with it exposed to the force of fire, is found to be partly changed into a blue vitriol; because the vitriolic acid, being disengaged by burning the sulphur, is by that means qualified to dissolve the Copper. The affinity of Copper with sulphur is greater than that of Silver.
This metal, as well as the other imperfect metals and the semi-metals, being mingled with nitre and exposed to the fire, is decomposed and calcined much sooner than by itself; because the phlogiston which it contains occasions the deflagration of the nitre, and consequently the two substances mutually decompose each other. There are certain metalline substances whose phlogiston is so abundant, and so weakly connected with their earth, that when they are thus treated with nitre, there arises immediately a detonation, accompanied with flame, and as violent as if sulphur or charcoal-dust had been employed; so that in a moment the metalline substance loses its phlogiston, and is calcined. The nitre, after these detonations, always assumes an alkaline character.
Of Iron.
Iron is lighter and less ductile than Copper; but it is much harder, and of more difficult fusion.
It is the only body that has the property of being attracted by the magnet, which therefore serves to discover it wherever it is. But it must be observed, that it hath this property only when in its metalline state, and loses it when converted to an earth or calx. Hence very few Iron-ores are attracted by the load-stone: because, for the most part, they are only sorts of earths, which require a phlogiston to be added before they can be brought to the form of true Iron.
When Iron hath undergone no other preparation but the fusion which is necessary to smelt it from its ore, it is usually quite brittle, and flies to pieces under the hammer: which arises in some measure from its containing a certain portion of unmetallic earth interposed between its parts. This we call Pig Iron.
By melting this a second time it is rendered purer, and more free from heterogeneous matters: but still, as its proper parts are probably not brought sufficiently near, or closely enough united, till the Iron hath undergone some further preparation besides that of fusion, it seldom hath any degree of malleability.
The way to give it this property is to make it just red-hot, and then hammer it for some time in all directions; to the end that its parts may be properly united, incorporated, and welded together, and that the heterogeneous matters which keep them asunder may be separated. Iron made by this means as malleable as possible we call Bar Iron, or Forged Iron.
Bar Iron is still harder to fuse than Pig Iron: to make it flow requires the utmost force of fire.
Iron has the property of imbibing a greater quantity of phlogiston than is necessary to give it the metalline form. It may be made to take in this superabundant phlogiston two ways: the first is by fusing it again with matters that contain the phlogiston; the second is, by encompassing it with a quantity of such matters, charcoal-dust, for instance, and then exposing it so encompassed, for a certain time, to a degree of fire barely sufficient to keep it red-hot. This second method, whereby one substance is incorporated with another by means of fire, but without fusing either of them, is in general called Cementation.
Iron thus impregnated with an additional quantity of phlogiston is called Steel. The hardness of Steel may be considerably augmented by tempering it; that is, by making it red-hot, and suddenly quenching it in some cold liquor. The hotter the metal, and the colder the liquor in which it is quenched, the harder will the Steel be. By this means tools are made, such as files and sheers, capable of cutting and dividing the hardest bodies, as glass, pebbles, and Iron itself. The colour of Steel is darker than that of Iron, and the facets which appear on breaking it are smaller. It is also less ductile and more brittle, especially when tempered.
As Iron may be impregnated with an additional quantity of phlogiston, and thereby converted into Steel, so may Steel[Pg 49] be again deprived of that superabundant phlogiston, and brought back to the condition of Iron. This is effected by cementing it with poor earths, such as calcined bones and chalk. By the same operation Steel may be untempered; nay, it will lose the hardness it had acquired by tempering, if it be but made red-hot, and left to cool gradually. As Iron and Steel differ only in the respects we have here taken notice of, their properties being in all other respects the same, what follows is equally applicable to both.
Iron being exposed to the action of fire for some time, especially when divided into small particles, such as filings, is calcined and loses its phlogiston. By this means it turns to a kind of reddish yellow earth, which, on account of its colour, is called Crocus Martis, or Saffron of Mars.
This calx of Iron has the singular property of flowing in the fire with somewhat less difficulty than Iron itself; whereas every other metalline calx flows with less ease than the metal that produced it. It has moreover the remarkable property of uniting with the phlogiston, and of being reduced to Iron without fusion; requiring for that purpose only to be made red-hot.
Iron may be incorporated with Silver, and even with Gold, by means of certain operations. Under the article of Lead we shall see how it may be separated from these metals.
The acids produce on it much the same effects as on Copper; every one of them acts upon it. Certain neutral salts, alkalis, and even water itself, are capable of dissolving it; and hence it is also very subject to rust. The vitriolic acid dissolves it with the greater ease: but the circumstances which attend the solution thereof are different from those with which the same Acid dissolves Copper: for, 1. whereas the vitriolic acid must be concentrated to dissolve Copper, it must on the contrary be diluted with water to dissolve Iron, which it will not touch when well dephlegmated. 2. The vapours which rise in this dissolution are inflammable; so that if it be made in a small-necked bottle, and the flame of a candle be applied to the mouth thereof, the vapours in the bottle take fire with such rapidity as to produce a considerable explosion.
This solution is of a beautiful green colour; and from this union of the vitriolic acid with Iron there results a neutral metalline salt, which has the property of shooting into crystals of a rhomboidal figure, and a green colour. These crystals are called Green Vitriol, Vitriol of Mars, and Copperas.
Green Vitriol hath a saltish and astringent taste. As it retains a great deal of water in crystallizing, it quickly flows by the action of fire: but this fluidity is owing to its water only, and is not a real fusion; for, as soon as its moisture is evaporated, it resumes a solid form. Its green transparent colour is now changed into an opaque white: and, if the calcination be continued, its acid also exhales and is dissipated in vapours; and as it loses that, it turns gradually to a yellow colour, which comes so much the nearer to a red the longer the calcination is continued, or the higher the force of the fire is raised; which being driven to the utmost, what remains is of a very deep red. This remainder is nothing but the body of the Iron, which having lost its phlogiston is now no more than an earth, nearly of the same nature with that which is left after calcining the metal itself.
Green Vitriol dissolved in water spontaneously lets fall a yellowish earthy sediment. If this solution be defecated by filtration, it still continues to deposite some of the same substance, till the vitriol be wholly decomposed. This sediment is nothing but the earth of Iron, which is then called Ochre.
The nitrous acid dissolves Iron with great ease. This solution is of a yellow colour, inclining more or less to a russet, or dark-brown, as it is more or less saturated with Iron. Iron dissolved by this acid, also, falls spontaneously in a kind of calx, which is incapable of being dissolved a second time; for the nitrous acid will not act upon Iron that has lost its phlogiston. This solution does not crystallize, and if evaporated to dryness attracts the moisture of the air.
Spirit of salt likewise dissolves Iron, and this solution is green. The vapours which rise during the dissolution are inflammable, like those which ascend when this metal is attacked by the vitriolic acid. Aqua regis makes a solution of Iron, which is of a yellow colour.
Iron hath a greater affinity than either Silver or Copper with the nitrous and vitriolic acids: so that if iron be presented to a solution of either in one of these two acids, the dissolved metal will be precipitated; because the acid quits it for the Iron, with which it has a greater affinity.
On this occasion it must be observed, that if a solution of Copper in the vitriolic acid be precipitated by means of Iron, the precipitate has the form and splendour of a metal, and does not require the addition of a phlogiston to reduce it to true Copper; which is not the case, as has been shewn, when the precipitation is effected by earths or alkaline salts.
The colour of this metalline precipitate hath deceived several persons, who being unacquainted with such phenomena, and with the nature of blue vitriol, imagined that Iron was transmuted into Copper, when they saw a bit of Iron laid in a solution of that vitriol become, in form and external appearance, exactly like Copper: whereas the surface only of the Iron was crusted over with the particles of Copper contained in the vitriol, which had gradually fallen upon and adhered to the Iron, as they were precipitated out of the solution.
Among the solvents of Iron we mentioned fixed alkalis; and that they have such a power is proved by the following phenomenon. If a large proportion of alkaline salts be suddenly mixed with a solution of Iron in an acid, no precipitation ensues, and the liquor remains clear and pellucid; or if at first it look a little turbid, that appearance lasts but a moment, and the liquor presently recovers its transparency. The reason is, that the quantity of alkali is more than sufficient to saturate all the acid of the solution, and the superabundant portion thereof, meeting with the Iron already finely divided by the acid, dissolves it with ease as fast as it falls, and so prevents its muddying the liquor. To evince that this is so in fact, let the alkali be applied in a quantity that is not sufficient, or but barely sufficient, to saturate the acid, and the Iron will then precipitate like any other metal.
Water also acts upon Iron; and therefore Iron exposed to moisture grows rusty. If iron-filings be exposed to the dew, they turn wholly to a rust, which is called Crocus Martis Aperiens.
Iron exposed to the fire together with nitre makes it detonate pretty briskly, sets it in a flame, and decomposes it with rapidity.
This metal hath a greater affinity than any other metalline substance with sulphur; on which account it is successfully used to precipitate and separate all metalline substances combined with sulphur.
Sulphur uniting with Iron communicates to it such a degree of fusibility, that if a mass of this metal heated red-hot be rubbed with a bit of sulphur, it incessantly runs into as perfect a fusion as a metal exposed to the focus of a large burning-glass.
Of Tin.
Tin is the lightest of all metals. Though it yields easily to the impression of hard bodies, it has but little ductility. Being bent backwards and forwards it makes a small crackling noise. It flows with a very moderate degree of fire, and long before it comes to be red-hot. When it is in fusion, its surface soon grows dusty, and there forms upon it a thin dark-coloured dusty pellicle, which is no other than a part of the Tin that has lost its phlogiston, or a calx of Tin. The metal thus calcined easily recovers its metalline form on the addition of a phlogiston. If the calx of Tin be urged by a strong fire it grows white, but the greatest violence of heat will not fuse it; which makes some Chymists consider it as a calcinable or absorbent earth, rather than a vitrifiable one. Yet it turns to glass, in some sort, when mixed with any other substance that vitrifies easily. However, it always produces an imperfect glass only, which is not at all transparent, but of an opaque white. The calx of Tin thus vitrified is called Enamel. Enamels are made of several colours by the addition of this or that metalline calx.
Tin unites easily with all the metals; but it destroys the ductility and malleability of every one of them, Lead excepted. Nay, it possesses this property of making metals brittle in such an eminent degree, that the very vapour of it, when in fusion, is capable of producing this effect. Moreover, which is very singular, the most ductile metals, even Gold and Silver, are those on which it works this change with the most ease, and in the greatest degree. It has also the property of making Silver mixed with it flow over a very small fire.
It adheres to, and in some measure incorporates with, the surface of Copper and of Iron; whence arose the practice of coating over those metals with Tin. Tin plates are no other than thin plates of Iron tinned over.
If to twenty parts of Tin one part of Copper be added, this alloy renders it much more solid, and the mixed mass continues tolerably ductile.
If, on the contrary, to one part of Tin ten parts of Copper be added, together with a little Zink, a semi-metal to be considered hereafter, from this combination there results a metalline compound which is hard, brittle, and very sonor[Pg 53]ous; so that it is used for casting bells: this composition is called Bronze and Bell-metal.
Tin hath an affinity with the vitriolic, nitrous, and marine acids. All of them attack and corrode it; yet none of them is able to dissolve it without great difficulty: so that if a clear solution thereof be desired, particular methods must be employed for that purpose; for the acids do but in a manner calcine it, and convert it to a kind of white calx or precipitate. The solvent which has the greatest power over it is aqua regis, which has even a greater affinity therewith than with Gold itself; whence it follows, that Gold dissolved in aqua regis may be precipitated by means of Tin; but then the aqua regis must be weakened. Gold thus precipitated by Tin is of a most beautiful colour, and is used for a red in enameling and painting on porcelain, as also to give a red colour to artificial gems. If the aqua regis be not lowered, the precipitate will not have the purple colour.
Tin hath the property of giving a great lustre to all red colours in general; on which account it is used by the dyers for striking a beautiful scarlet, and tin vessels are employed in making fine syrup of violets. Water does not act upon this metal, as it does upon Iron and Copper; for which reason it is not subject to rust: nevertheless, when it is exposed to the air, its surface soon loses its polish and splendour.
Tin mixed with nitre and exposed to the fire deflagrates with it, makes it detonate, and is immediately converted to a refractory calx: for so all substances are called which are incapable of fusion.
Tin readily unites with sulphur, and with it becomes a brittle and friable mass.
Of Lead.
Next to Gold and Mercury Lead is the heaviest of all metalline substances, but in hardness is exceeded by every one of them. Of all metals also it melts the easiest except Tin. While it is in fusion there gathers incessantly on its surface, as on that of Tin, a blackish dusty pellicle, which is nothing but a calx of Lead.
This calx further calcined by a moderate fire, the flame being reverberated on it, soon grows white. If the calcination be continued it becomes yellow, and at last of a beautiful red. In this state it is called Minium, and is used as a[Pg 54] pigment. Minium is not easily made, and the operation succeeds well in large manufactures only.
To convert Lead into Litharge, which is the metal in a manner half vitrified, you need only keep it melted by a pretty strong fire; for then as its surface gradually calcines, it tends more and more to fusion and vitrification.
All these preparations of Lead are greatly disposed to perfect fusion and vitrification, and for that purpose require but a moderate degree of fire; the calx or earth of Lead being of all metalline earths that which vitrifies the most easily.
Lead hath not only the property of turning into glass with the greatest facility, but it hath also that of promoting greatly the vitrification of all the other imperfect metals; and, when it is actually vitrified, procures the ready fusion of all earths and stones in general, even those which are refractory, that is, which could not be fused without its help.
Glass of Lead, besides its great fusibility, hath also the singular property of being so subtile and active as to corrode and penetrate the crucibles in which it is melted, unless they be of an earth that is exceeding hard, compact, and withal very refractory: for Glass of Lead being one of the most powerful fluxes that we know, if the earth of the crucible in which it is melted be in the smallest degree fusible, it will be immediately vitrified; especially if there be any metallic matter in its composition.
The great activity of Glass of Lead may be weakened by joining it with other vitrifiable matters: but unless these be added in a very great proportion, it will still remain powerful enough to penetrate common earths, and carry off the matters combined with it.
On these properties of Lead, and of the Glass of Lead, depends the whole business of refining Gold and Silver. It hath been shewn, that as these two metals are indestructible by fire, and the only ones which have that advantage, they may be separated from the imperfect metals, when mixed therewith, by exposing the compound to a degree of fire sufficiently strong to vitrify the latter; which, when once converted into glass, can no longer remain united with any metal that has its metalline form. But it is very difficult to procure this vitrification of the imperfect metals, when united with Gold and Silver; nay, it is in a manner impossible to vitrify them entirely, for two reasons: first, because most of them are naturally very difficult to vitrify; secondly, because the union they have contracted with the perfect metals defends them, in a manner, from the action of the fire, and that so[Pg 55] much the more effectually as the proportion of the perfect metals is greater; which being indestructible, and in some sort coating over those with which they are alloyed, serve them as a preservative and impenetrable shield against the utmost violence of fire.
It is therefore clear, that a great deal of labour may be saved, and that Gold and Silver may be refined to a much greater degree of purity than can otherwise be obtained, if to a mixture of these metals with Copper, for instance, or any other imperfect metal be added a certain quantity of Lead. For the Lead, by its known property, will infallibly produce the desired vitrification; and as it likewise increases the proportion of the imperfect metals, and so lessens that of the perfect metals, in the mass, it evidently deprives the former of a part of their guard, and so effects a more complete vitrification. In conclusion, as the Glass of Lead hath the property of running through the crucible, and carrying with it the matters which it has vitrified, it follows, that, when the vitrification of the imperfect metals is effected by its means, all those vitrified matters together penetrate the vessel containing the fused metalline mass, disappear, and leave only the Gold and Silver perfectly pure, and freed, as far as is possible, from all admixture of heterogeneous parts.
The better to promote the separation of such parts it is usual to employ, in this process, a particular sort of small crucibles, made of the ashes of calcined bones, which are exceedingly porous and easily pervaded. They are called cupels, on account of their figure, which is that of a wide-mouthed cup: and from hence the operation takes its name; for when we refine Gold and Silver in this manner we are said to cupel those metals. It is easy to perceive that the more Lead is added the more accurately will the Gold and Silver be refined; and that so much the more Lead ought to be added as the perfect metals are alloyed with a greater proportion of the imperfect. This is the most severe trial to which a perfect metal can be put; and consequently any metal that stands it may be fairly considered as such.
In order to denote the fineness of Gold, it is supposed to be divided into twenty-four parts called carats; and Gold which is quite pure and free from all alloy is said to be twenty-four carats fine; that which contains 1/24 part of alloy is called Gold of twenty-three carats; that which contains 2/24 of alloy is but twenty-two carats; and so on. Silver again is supposed to be divided into twelve parts only, which are[Pg 56] called penny-weights: so that when absolutely pure it is said to be twelve penny-weights fine; when it contains 1/12 of alloy, it is then called eleven penny-weights fine; when it contains 2/12 of alloy, it is called ten penny-weights fine, and so on.
In treating of Copper we promised to shew, under the article of Lead, how to separate it from Iron. The process is founded on that property of Lead which renders it incapable of mixing and uniting with Iron, though it readily dissolves all other metalline substances. Therefore, if you have a mass compounded of Copper and Iron, it must be fused with a certain quantity of Lead, and then the Copper, having a greater affinity with Lead than with Iron, will desert the latter and join the former, which being incapable of any union with Iron, as was said, will wholly exclude it from the new compound. The next point is to separate the Lead from the Copper; which is done by exposing the mass compounded of these two metals to a degree of fire strong enough to deprive the Lead of its metalline form, but too weak to have the same effect on the Copper: and this may be done; since, of all the imperfect metals, Lead is, next to Tin, the easiest to be calcined, and Copper on the contrary resists the greatest force of fire longest, without losing its metalline form. Now what we gain by this exchange, viz. by separating Copper from Iron and uniting it with Lead, consists in this, that as Lead is calcined with less fire than Iron, the Copper is less exposed to be destroyed: for it must be observed that, however moderate the fire be, it is hardly possible to prevent a certain quantity thereof from being calcined in the operation.
Lead melted with a third part of Tin forms a compound, which being exposed to a fire capable of making it thoroughly red-hot, swells, puffs up, seems in some sort to take fire, and is presently calcined. These two metals mixed together are much sooner calcined than either of them separately.
Both Lead and Tin are in some measure affected by water, and by a moist air; but they are both much less subject than Iron or Copper to be corroded by these solvents, and of course are much less liable to rust.
The vitriolic acid acts upon and dissolves Lead, much in the same manner as it doth Silver.
The nitrous acid dissolves this metal with much ease, and in great quantities; and from this solution a small portion of Mercury may be obtained. On this subject see our Elements of the Practice of Chymistry.
When this solution of Lead is diluted with a good deal of water, the Lead precipitates in the form of a white powder; which happens because the acid is rendered too weak to keep the Lead dissolved.
If this solution of Lead be evaporated to a certain degree, it shoots into crystals formed like regular pyramids with square bases. These crystals are of a yellowish colour, and a saccharine taste: they do not easily dissolve in water. This nitrous metalline salt has the singular property of detonating in a crucible, without any additament, or the contact of any other inflammable substance. This property it derives from the great quantity of phlogiston contained in, and but loosely connected with, the Lead which is one of its principles.
If spirit of salt, or even sea-salt in substance, be added to a solution of Lead in the nitrous acid, a white precipitate immediately falls; which is no other than the Lead united with the marine acid. This precipitate is extremely like the precipitate of Silver made in the same manner, and that being called Luna cornea hath occasioned this to be named Plumbum corneum. Like the luna cornea it is very fusible, and being melted hardens like it into a kind of horny substance: it is volatile, and may be reduced by means of inflammable matters combined with alkalis. But it differs from the luna cornea in this chiefly, that it dissolves easily in water; whereas the luna cornea, on the contrary, dissolves therein with great difficulty, and in a very small quantity.
As this precipitation of Lead from its solution in spirit of nitre is procured by the marine acid, Lead is thereby proved to have a greater affinity with the latter acid than with the former. Yet, if you attempt to dissolve Lead directly by the acid of sea-salt, the solution is not so easily effected as by the spirit of nitre, and it is always imperfect; for it wants one of the conditions essential to every solution in a liquor, namely transparency.
If Lead be boiled for a long time in a lixivium of fixed alkali, part of it will be dissolved.
Sulphur renders this metal refractory and scarce fusible; and the mass they form when united together is friable. Hence it appears that sulphur acts upon Lead much in the same manner as upon Tin; that is, it renders both these metals less fusible, which are naturally the most fusible of any, while it exceedingly facilitates the fusion of Silver, Copper,[Pg 58] and Iron, metals which of themselves flow with the greatest difficulty.
Of Quick-Silver.
We treat of Quick-silver in a chapter apart, because this metallic substance cannot be classed with the metals properly so called, and yet has some properties which will not allow us to confound it with the semi-metals. The reason why Quick-silver, by the Chymists commonly called Mercury, is not reputed a metal, is, that it wants one of the essential properties thereof, to wit, malleability. When it is pure and unadulterated with any mixture, it is always fluid, and of course unmalleable. But as, on the other hand, it eminently possesses the opacity, the splendour, and, above all, the gravity of a metal, being next to Gold the heaviest of all bodies, it may be considered as a true metal, differing from the rest no otherwise than by being constantly in fusion; which we may suppose arises from its aptness to flow with such a small degree of heat, that be there ever so little warmth on earth, there is still more than enough to keep Mercury in fusion; which would become solid and malleable if it were possible to apply to it a degree of cold considerable enough for that purpose. These properties will not allow us to confound it with the semi-metals. Add, that we are not yet assured by any undoubted experiment that it can be wholly deprived of its phlogiston, as the imperfect metals may. Indeed we cannot apply the force of fire to it as could be wished: for it is so volatile that it flies off and exhales in vapours, with a much less degree of fire than is necessary to make it red-hot. The vapours of Mercury thus raised by the action of fire, being collected and united in a certain quantity, appear to be no other than true Mercury, retaining every one of its properties; and no experiment hath ever been able to shew the least change thus produced in its nature.
If Mercury be exposed to the greatest heat that it can bear without sublimation, and continued in it for several months, or even a whole year together, it turns to a red powder, which the Chymists call Mercurius Præcipitatus per se.[Pg 59] But, to succeed in this operation, it is absolutely necessary that the heat be such as is above-specified; for this metallic substance may remain exposed to a weaker heat for a considerable number of years, without undergoing any sensible alteration.
Some Chymists fancied, that by this operation they had fixed Mercury and changed its nature; but without any reason: for if the Mercury thus seemingly transmuted be exposed to a somewhat stronger degree of fire, it sublimes and exhales in vapours as usual; and those vapours collected are nothing else but running Mercury, which has recovered all its properties without the help of any additament.
Mercury has the property of dissolving all the metals, Iron only excepted. But it is a condition absolutely necessary to the success of such dissolution, that the metalline substances be possessed of their phlogiston; for if they be calcined, Mercury cannot touch them: and hence it follows, that Mercury doth not unite with substances that are purely earthy. Such a combination of a metal with Mercury is called an Amalgam. Trituration alone is sufficient to effect it; however, a proper degree of heat also is of use.
Mercury amalgamated with a metal gives it a consistence more or less soft, and even fluid, according to the greater or smaller proportion of Mercury employed. All amalgams are softened by heat, and hardened by cold.
Mercury is very volatile; vastly more so than the most unfixed metals; moreover, the union it contracts with any metal is not sufficiently intimate to entitle the new compound resulting from that union to all the properties of the two substances united: at least with regard to their degree of fixity and volatility. From all which it follows, that the best and surest method of separating it from metals dissolved by it, is to expose the amalgam to a degree of heat sufficient to make all the Quick-silver rise and evaporate; after which the metal remains in the form of a powder, and being fused recovers its malleability. If it be thought proper to save the Quick-silver, the operation must be performed in close vessels, which will confine and collect the mercurial vapours. This operation is most frequently employed to separate Gold and Silver from the several sorts of earths and sands with which they are mixed in the ore; because these two metals, Gold especially, are of sufficient value to compensate the loss of Mercury, which is inevitable in this process: besides, as they very readily amalgamate with it, this way of separat[Pg 60]ing them from every thing unmetallic is very facile and commodious.
Mercury is dissolved by acids; but with circumstances peculiar to each particular sort of acid.
The vitriolic acid, concentrated and made boiling hot, seizes on it, and presently reduces it to a kind of white powder, which turns yellow by the affusion of water, but does not dissolve in it; it is called Turbith Mineral. However, the vitriolic acid on this occasion unites with a great part of the Mercury, in such a manner that the compound is soluble in water. For if to the water which was used to wash the Turbith a fixed alkali be added, there falls instantly a russet-coloured precipitate, which is no other than Mercury separated from the vitriolic acid by the intervention of the alkali.
This dissolution of Mercury by the vitriolic acid is accompanied with a very remarkable phenomenon; which is, that the acid contracts a strong smell of volatile spirit of sulphur: a notable proof that part of the phlogiston of the Mercury hath united therewith. And yet, if the Mercury be separated by means of a fixed alkali, it does not appear to have suffered any alteration. Turbith mineral is not so volatile as pure Mercury.
The nitrous acid dissolves Mercury with ease. The solution is limpid and transparent, and as it grows cold shoots into crystals, which are a nitrous mercurial salt.
If this solution be evaporated to dryness, the Mercury remains impregnated with a little of the acid, under the form of a red powder, which hath obtained the names of Red Precipitate, and Arcanum Corallinum. This Precipitate, as well as Turbith, is less volatile than pure Mercury.
If this solution of Mercury be mixed with a solution of Copper, made likewise in the nitrous acid, and the mixture evaporated to dryness, there will remain a green powder called Green Precipitate. These precipitates are caustic and corrosive; and are used as such in surgery.
Though Mercury be dissolved more easily and completely by the nitrous acid than by the vitriolic, yet it has a greater affinity with the latter than with the former: for if a vitriolic acid be poured into a solution of Mercury in spirit of nitre, the Mercury will quit the latter acid in which it was dissolved, and join the other which was added. The same thing happens when the marine acid is employed instead of the vitriolic.
Mercury combined with spirit of salt forms a singular body; a metalline salt which shoots into long crystals, pointed like daggers. This salt is volatile, and sublimes easily without decomposition. It is moreover the most violent of all the corrosives hitherto discovered by Chymistry. It is called Corrosive Sublimate, because it must absolutely be sublimed to make the combination perfect. There are several ways of doing this: but the operation will never fail, if the Mercury be rarefied into vapours, and meet with the marine acid in a similar state.
Corrosive Sublimate is dissolved by water, but in very small quantities only. It is decompounded by fixed alkalis, which precipitate the Mercury in a reddish yellow powder, called, on account of its colour, Yellow Precipitate.
If Corrosive Sublimate be mixed with tin, and the compound distilled, a liquor comes over which continually emits abundance of dense fumes, and, from the name of its inventor, is called the Smoking Liquor of Libavius. This liquor is no other than the tin combined with the marine acid of the Corrosive Sublimate, which therefore it hath actually decompounded: whence it follows, that this acid hath a greater affinity with tin than with Mercury.
The marine acid in Corrosive Sublimate is not quite saturated with Mercury; but is capable of taking up a much greater quantity thereof. For if Corrosive Sublimate be mixed with fresh Mercury, and sublimed a second time, another compound will be produced containing much more Mercury, and less acrimonious; for which reason it is named Sweet Sublimate of Mercury, Mercurius dulcis, Aquila alba. This compound may be taken internally, and is purgative or emetic according to the dose administered. It may be rendered still more gentle by repeated sublimations, and then it takes the title of Panacea Mercurialis. No way hath hitherto been found to dissolve Mercury in aqua regis without great difficulty, and even then it is but imperfectly dissolved.
Mercury unites easily and intimately with sulphur. If these two substances be only rubbed together in a gentle heat, or even without any heat, they will contract an union, though but an incomplete one. This combination takes the form of a black powder, which has procured it the name of Æthiops Mineral.
If a more intimate and perfect union be desired, this compound must be exposed to a stronger heat; and then a red ponderous substance will be sublimed, appearing like a mass of shining needles: this is the combination desired, and is[Pg 62] called Cinabar. In this form chiefly is Mercury found in the bowels of the earth. Cinabar finely levigated acquires a much brighter red colour, and is known to painters by the name of Vermilion.
Cinabar rises wholly by sublimation, without suffering any decomposition; because the two substances of which it consists, viz. Mercury and Sulphur, are both volatile.
Though Mercury unites and combines very well with sulphur, as hath been said, yet it hath less affinity with that mineral than any other metal, Gold only excepted: whence it follows, that any of the other metals will decompound Cinabar, by uniting with its sulphur, and so setting the Mercury at liberty to appear in its usual form. Mercury thus separated from sulphur is esteemed the purest, and bears the name of Mercury revivified from Cinabar.
Iron is generally used in this operation, preferably to the other metals, because among them all it has the greatest affinity with sulphur, and is the only one that has none with Mercury.
Cinabar may also be decompounded by means of fixed alkalis; the affinity of these salts with sulphur being generally greater than that of any metalline substance whatever.
Of the Semi-Metals.
Of Regulus of Antimony.
Regulus of Antimony is a metallic substance of a pretty bright white colour. It has the splendour, opacity, and gravity of a metal: but it is quite unmalleable, and crumbles to dust, instead of yielding or stretching, under the hammer; on which account it is classed with the Semi-metals.
It begins to flow as soon as it is moderately red; but, like the other Semi-metals, it cannot stand a violent degree of fire; being thereby dissipated into smoke and white vapours, which adhere to such cold bodies as they meet with, and so[Pg 63] are collected into a kind of farina called Flowers of Antimony.
If Regulus of Antimony, instead of being exposed to a strong fire, be only heated so moderately that it shall not even melt, it will calcine, lose its phlogiston, and take the form of a greyish powder destitute of all splendour: this powder is called Calx of Antimony.
This calx is not volatile like the Regulus, but will endure a very violent fire; and being exposed thereto will flow, and turn to a glass of the yellowish colour of a hyacinth.
It is to be observed, that the more the Regulus is deprived of its phlogiston by continued calcination, the more refractory is the calx obtained from it. The glass thereof has also so much the less colour, and comes the nearer to common glass.
The calx and the Glass of Antimony will recover their metalline form, like every other Calx and Glass of a metal, if reduced by restoring to them their lost phlogiston. Yet if the calcination be carried too far, their reduction will become much more difficult, and a much smaller quantity of Regulus will be resuscitated.
Regulus of Antimony is capable of dissolving the metals: but its affinities with them are various, and differ according to the following order. It affects Iron the most powerfully, next Copper, then Tin, Lead, and Silver. It promotes the fusion of metals, but makes them all brittle and unmalleable.
It will not amalgamate with Mercury; and though by certain processes, particularly the addition of water and continued trituration, a sort of union between these two substances may be produced, yet it is but apparent and momentary; for, being left to themselves and undisturbed, they quickly disunite and separate[2].
The vitriolic acid, assisted by heat, and even by distillation, dissolves Regulus of Antimony. The nitrous acid likewise attacks it: but the solution can by no art be made clear[Pg 64] and limpid: so that the Regulus is only calcined, in a manner, by this acid.
The marine acid dissolves it well enough; but then it must be exceedingly concentrated, and applied in a peculiar manner, and especially by distillation. One of the best methods of procuring a perfect union between the acid of sea-salt and Regulus of Antimony, is to pulverize the latter, mix it with corrosive sublimate, and distil the whole. There rises in the operation a white matter, thick, and scarce fluid, which is no other than the Regulus of Antimony united and combined with the acid of sea-salt. This compound is extremely corrosive, and is called Butter of Antimony.
It is plain that the corrosive sublimate is here decompounded; that the Mercury is revivified, and that the acid which was combined therewith hath quitted it to join the Regulus of Antimony, with which its affinity is greater. This Butter of Antimony by repeated distillations acquires a considerable degree of fluidity and limpidness.
If the acid of nitre be mixed with Butter of Antimony, and the whole distilled, there rises an acid liquor, or a sort of aqua regis, which still retains some of the dissolved Regulus, and is called Bezoardic Spirit of Nitre. After the distillation there remains a white matter, from which fresh spirit of nitre is again abstracted, and which being then washed with water is called Bezoar Mineral. This Bezoar Mineral is neither so volatile nor so caustic as Butter of Antimony; because the nitrous acid hath not the property of volatilizing metallic substances, as the marine acid does, and because it remains much more intimately combined with the reguline part.
If Butter of Antimony be mixed with water, the liquor immediately becomes turbid and milky, and a precipitate falls, which is nothing but the metallic matter partly separated from its acid, which is too much weakened by the addition of water to keep it dissolved. Yet this precipitate still retains a good deal of acid; for which reason it continues to be a violent emetic, and in some degree corrosive. It hath therefore been very improperly called Mercurius Vitæ.
The proper solvent of Regulus of Antimony is aqua regis; by means whereof a clear and limpid solution of this Semi-metal may be obtained.
Regulus of Antimony mixed with nitre, and projected into a red-hot crucible, sets the nitre in a flame, and makes it detonate. As it produces this effect by means of its phlogiston, it must needs at the same time be calcined, and lose[Pg 65] its metallic properties, which accordingly happens, and when the nitre is in a triple proportion to the Regulus, the latter is so perfectly calcined as to leave only a white powder, which is fused with great difficulty, and then turns to a faintly coloured glass, not very different from common glass, and which is not reducible to a Regulus by the addition of inflammable matter; at least it yields but a very small quantity thereof. If less nitre be used, the calx is not so white; the glass it produces is more like a metalline glass, and is more easily reduced. The calx of the Regulus thus prepared by nitre is called, on account of the medicinal virtue ascribed to it, Diaphoretic Antimony, or Diaphoretic Mineral.
Nitre always becomes an alkali by deflagration, and in the present case retains part of the calx, which it even renders soluble in water. This calx may be separated from the alkali, if an acid be employed to precipitate it; and then it is called Materia Perlata. This pearly matter is a calx of Antimony, so completely deprived of its phlogiston as to be altogether incapable of reduction to a Regulus.
Regulus of Antimony readily joins and unites with sulphur, forming therewith a compound which has a very faint metallic splendour. This compound appears like a mass of long needles adhering together laterally; and under this form it is usually found in the ore, or at least when only separated by fusion from the stones and earthy matters with which the ore is mixed. It is called Crude Antimony.
Antimony flows with a moderate heat, and becomes even more fluid than other metallic substances. The action of fire dissipates or consumes the sulphur it contains, and its phlogiston also, so as to convert it into a calx and a glass, as it does the Regulus.
Aqua regis, which we observed to be the proper solvent of the Regulus, being poured on Antimony, attacks and dissolves the reguline part, but touches not the sulphur; in consequence whereof it decomposes the Antimony, and separates its sulphur from its Regulus.
There are several other ways of effecting this decomposition, and obtaining the reguline part of Antimony by itself: they consist either in destroying the sulphureous part of the Antimony by combustion, or in melting the Antimony with some substance which has a greater affinity than its reguline part with sulphur. Most metals are very fit for this latter purpose: for though the Regulus has a considerable affinity with sulphur, yet all the metals, except Gold and Mercury, have a greater.
If therefore Iron, Copper, Lead, Silver, or Tin, be melted with Antimony, the metal employed will unite with the sulphur, and separate it from the Regulus.
It must be observed, that, as these metals have some affinity with the Regulus of Antimony, the Regulus will be joined in the operation by some of the metal employed as a Precipitant, (so those substances are called which serve as the means of separating two bodies from each other); and therefore the Regulus procured in this manner will not be absolutely pure: on this account care is taken to distinguish each by adding the name of the metal employed in its precipitation; and thence come these titles, Martial Regulus of Antimony, or only Martial Regulus, Regulus Veneris; and so of the rest.
Antimony is employed with advantage to separate Gold from all the other metals with which it may be alloyed. It has been shewn, that all the metals have a greater affinity than the reguline part of Antimony with sulphur, Gold only excepted; which is incapable of contracting any union therewith: and therefore, if a mass compounded of Gold and several other metals be melted with Antimony, every thing in that mass which is not Gold will unite with the sulphur of the Antimony. This union occasions two separations, to wit, that of the sulphur of the Antimony from its reguline part, and that of the Gold from the metals with which it was adulterated; and from the whole two new compounds arise; namely, a combination of the metals with the sulphur, which being lightest rises to the surface in fusion; and a metalline mass, formed of the Gold and the reguline part of the Antimony united together, which being much the heaviest sinks to the bottom. There is no difficulty in parting the Gold from the Regulus of Antimony with which it is alloyed: for the metalline mass needs only be exposed to a degree of fire capable of dissipating into vapours all the Semi-metal it contains; which being very volatile, the operation is much easier, and more expeditiously finished, than if the metals with which the Gold was debased were to be vitrified on the cupel; without taking into the account, that, if Silver were one of them, recourse must needs be had to the process of quartation after that of the cupel.
If equal parts of nitre and Antimony be mixed together, and the mixture exposed to the action of fire, a violent detonation ensues; the nitre deflagrating consumes the sulphur of the Antimony, and even a part of its phlogiston. After the detonation there remains a greyish matter which contains fix[Pg 67]ed nitre, vitriolated tartar, and the reguline part of the Antimony in some measure deprived of its phlogiston, and half vitrified by the action of the fire, which is considerably increased by the deflagration. This matter is called Liver of Antimony.
If, instead of equal parts of nitre and Antimony, two parts of the former be used to one of the latter, then the reguline part loses much more of its phlogiston, and remains in the form of a yellowish powder.
Again, if three parts of nitre be taken to one of Antimony, the Regulus is thereby entirely robbed of its phlogiston, and converted to a white calx, which bears the name of Diaphoretic Antimony, or Diaphoretic Mineral. The pearly matter may be precipitated by pouring an acid on the saline substances which here remain after the detonation, in the same manner as we shewed above was to be done with regard to the Regulus.
In the two last operations, where the nitre is in a double or triple proportion to the Antimony, the reguline part is found after the detonation to be converted into a calx, and not into a half-vitrified matter, which we have seen is the effect when equal parts only of nitre and Antimony are used. The reason of this difference is, that in these two cases the reguline part, being wholly, or almost wholly, deprived of its phlogiston, becomes, as was observed, more difficult to fuse, and consequently cannot begin to vitrify in the same degree of heat as that which hath not lost so much of its phlogiston. If, instead of performing the operation with equal parts of nitre and Antimony alone, a portion of some substance which abounds with phlogiston be added, in that case the sulphur only of the Antimony will be consumed, and the Regulus will remain united with its phlogiston and separated from its sulphur.
The Regulus prepared in this manner is absolutely pure, because no metalline substance being employed, none can mix with and adulterate it. It is called Regulus of Antimony per se, or only Regulus of Antimony.
It is true indeed that in this operation much of the reguline part unavoidably loses its phlogiston and is calcined, and consequently a much smaller quantity of Regulus is obtained than when metalline precipitants are employed: but this loss is easily repaired, if it be thought proper, by restoring to the calcined part its lost phlogiston.
Antimony melted with two parts of fixed alkali yields no[Pg 68] Regulus, but is entirely dissolved by the salt, and forms with it a mass of a reddish yellow colour.
The reason why no precipitate is produced on this occasion is, that the alkali uniting with the sulphur of the Antimony forms therewith the combination called Liver of Sulphur, which by its nature is qualified to keep the reguline part dissolved. This mass formed by the union of the Antimony with the alkali is soluble in water. If any acid whatever be dropt into this solution, there falls a precipitate of a reddish yellow colour; because the acid unites with the alkali, and forces it to quit the matters with which it was combined. This precipitate is called Golden Sulphur of Antimony.
As in the operation for preparing Regulus of Antimony per se, some of the nitre is, by the inflammable matters added thereto, turned to an alkali, this alkali seizes on part of the Antimony, and therewith forms a compound like that just described. Hence it comes, that if the scoria formed in this process be dissolved in water, and an acid dropped into the solution, a true golden sulphur of Antimony is thereby separated.
This union of Antimony with an alkali may also be brought about by the humid way; that is, by making use of an alkali resolved into a liquor, and boiling the mineral in it. The alkaline liquor, in proportion as it acts upon the Antimony, gradually becomes reddish and turbid. If left to settle and cool when well saturated therewith, it gradually deposites the Antimony it had taken up, which precipitates in the form of a red powder; and this precipitate is the celebrated remedy known by the name of Kermes Mineral. It is plain that the kermes is nearly the same thing with the golden sulphur: yet it differs from it in some respects; and especially in this, that being taken inwardly it operates much more gently than the golden sulphur, which is a violent emetic. Nitre fixed by charcoal, and resolved into a liquor, is the only alkali employed in preparing the kermes.
It was shewn above, that Regulus of Antimony mixed and distilled with corrosive sublimate decompounds it, disengages the Mercury, and joining itself to the marine acid forms therewith a new combination, called Butter of Antimony. If the same operation be performed with crude Antimony instead of its Regulus, the same effects are produced: but then the Antimony itself is also decomposed; that is, the reguline part is separated from the sulphur, which being set free unites with the Mercury, now also at liberty, and these[Pg 69] two together form a true cinabar, called Cinabar of Antimony.
Of Bismuth.
Bismuth, known also by the name of Tin-glass, is a semi-metal, having almost the same appearance as Regulus of Antimony; yet it has a more dusky cast, inclining somewhat to red, and even presents some changeable streaks, especially after lying long in the air.
When exposed to the fire it melts long before it is red, and consequently with less heat than Regulus of Antimony, which does not flow, as was shewn above, till it begin to be red-hot. It becomes volatile, like all the other semi-metals, when acted on by a violent fire: being kept in fusion by a proper degree of heat it loses its phlogiston with its metallic form, and turns to a powder or a calx; and that again is converted into glass by the continued action of fire. The calx and glass of Bismuth may be reduced, like any other metallic calx, by restoring their phlogiston.
Bismuth mixes with all the metals in fusion, and even facilitates the fusion of such as do not otherwise flow readily. It whitens them by its union, and destroys their malleability.
It amalgamates with Mercury, if they be rubbed together with the addition of water: yet after some time these two metalline substances desert each other, and the Bismuth appears again in the form of a powder. Hence it is plain, that the union it contracts with Mercury is not perfect; and yet it has the singular property of attenuating Lead, and altering it in such a manner that it afterwards amalgamates with Mercury much more perfectly, so as even to pass with it through shamoy leather without any separation. The Bismuth employed in making this amalgama afterwards separates from it spontaneously, as usual; but the Lead still continues united with the Mercury, and always retains the property thus acquired.
The vitriolic acid does not dissolve Bismuth: its proper solvent is the nitrous acid, which dissolves it with violence, and abundance of fumes.
Bismuth dissolved in the nitrous acid is precipitated not only by alkalis, but even by the bare addition of water. This[Pg 70] precipitate is extremely white, and known by the name of Magistery of Bismuth.
The acid of sea-salt and aqua regis likewise act upon Bismuth, but with less violence.
This semi-metal does not sensibly deflagrate with nitre; yet it is quickly deprived of its phlogiston, and turned into a vitrifiable calx, when exposed with it to the action of fire.
It readily unites with sulphur in fusion, and forms therewith a compound which appears to consist of needles adhering laterally to each other.
It may be separated from the sulphur with which is combined, by only exposing it to the fire, without any additament; for the sulphur is either consumed or sublimed, and leaves the Bismuth behind.
Of Zinc.
Zinc to appearance differs but little from Bismuth, and has even been confounded with it by several authors. Nevertheless, besides that it has something of a blueish cast, and is harder than Bismuth, it differs from it essentially in its properties, as will presently be shewn. These two metallic substances scarce resemble each other in any thing, but the qualities common to all semi-metals.
Zinc melts the moment it grows red in the fire, and then also begins to turn to a calx, which, like any other metallic calx, may be reduced by means of the phlogiston: but if the fire be considerably increased, it sublimes, flames, and burns like an oily matter; which is a proof of the great quantity of phlogiston in its composition. At the same time abundance of flowers rise from it in the form of white flakes, flying about in the air like very light bodies; and into this form may the whole substance of the Zinc be converted. Several names have been given to these flowers, such as Pompholyx, Philosophic Wool. They are supposed to be no other than the Zinc itself deprived of its phlogiston; yet no body has hitherto been able to resuscitate them in the form of Zinc, by restoring their phlogiston according to the methods used in the reduction of metals. Though they rise in the air with very great ease while the Zinc is calcining, yet when once formed they are very fixed; for they withstand the utmost violence of fire, and are capable of being vitrified,[Pg 71] especially if joined with a fixed alkali. They are soluble in acids.
Zinc unites with all metalline substances, except Bismuth. It has this singular property, that being mixed with Copper, even in a considerable quantity, such as a fourth part, it does not greatly lessen the ductility thereof, and at the same time communicates to it a very beautiful colour not unlike that of Gold: on which account the composition is frequently made, and produces what is called Brass. This metal melts much more easily than Copper alone, because of the Zinc with which it is alloyed. If it be exposed to a great degree of heat, the Zinc which it contains takes fire, and sublimes in white flowers, just as when it is pure.
It is to be observed, that Brass is ductile only while it is cold, and not then, unless the Zinc used in making it was very pure; otherwise the composition will prove but a Tombac or Prince's Metal, having very little malleability.
Zinc is very volatile, and carries off with it any metallic substance with which it is fused, making a kind of sublimate thereof. In the furnaces where they smelt ores containing Zinc, the matter thus sublimed is called Cadmia Fornacum, to distinguish it from the native Cadmia called also Calamine, or Lapis Calaminaris; which, properly speaking, is an ore of Zinc, containing a great deal of that semi-metal, together with some Iron, and a stony substance. The name of Cadmia Fornacum is not appropriated solely to the metallic sublimates procured by means of Zinc, but is given in general to all the metallic sublimates found in smelting houses.
If a violent and sudden heat be applied to Zinc, it sublimes in its metalline form; there not being time for it to burn and be resolved into flowers.
This semi-metal is soluble in all the acids, but especially in spirit of nitre, which attacks and dissolves it with very great violence.
Zinc has a greater affinity than iron or copper with the vitriolic acid; and therefore it decompounds the green and blue vitriols, precipitating those two metals by uniting with the vitriolic acid, with which it forms a metallic salt, or vitriol, called White Vitriol, or Vitriol of Zinc.
Nitre mixed with Zinc, and projected into a red-hot crucible, detonates with violence, and during the detonation there rises a great quantity of white flowers, like those which appear when it is calcined by itself.
Sulphur has no power over Zinc. Even liver of sulphur,[Pg 72] which dissolves all other metallic substances, contracts no union with this semi-metal.
Messrs. Hellot and Malouin have bestowed a great deal of pains on this semi-metal. An account of their experiments is to be found in the Memoirs of the Academy of Sciences.
Of Regulus of Arsenic.
Regulus of Arsenic is the most volatile of all the semi-metals. A very moderate heat makes it wholly evaporate, and fly off in fumes; on which account it cannot be brought to fusion, nor can any considerable masses thereof be obtained. It has a metallic colour, somewhat resembling Lead; but it soon loses its splendour when exposed to the air.
It unites readily enough with metallic substances, having the same affinities with them as Regulus of Antimony hath. It makes them brittle, and unmalleable. It hath also the property of rendering them volatile, and greatly facilitates their scorification.
It very easily parts with its phlogiston and its metallic form. When exposed to the fire it rises in a kind of shining crystalline calx, which, on that account, looks more like a saline matter than a metallic calx. To this calx or these flowers are given the names of White Arsenic, Crystalline Arsenic, and most commonly plain Arsenic.
The properties of this substance are very singular, and extremely different from those of any other metallic calx. Hitherto it hath been but little examined; and this led me to make some attempts towards discovering its nature, which may be seen in the Memoirs of the Academy of Sciences.
Arsenic differs from every other metalline calx, first, in being volatile; whereas the calces of all other metallic substances, not excepting those of the most volatile semi-metals, such as Regulus of Antimony and Zinc, are exceeding fixed; and, secondly, in having a saline character, which is not found in any other metalline calx.
The saline character of Arsenic appears, first, from its being soluble in water; secondly, from its corrosive quality, which makes it one of the most violent poisons: a quality from which the other metallic substances are free, when they are not combined with some saline matter. Regulus of Antimony must however be excepted. But then the best Chymists agree that this semi-metal is either nearly of the same[Pg 73] nature with Arsenic, or contains a portion thereof in its composition: besides, its noxious qualities never discover themselves so plainly as when it is combined with some acid. Lastly, Arsenic acts just like the vitriolic acid upon nitre; that is, it decompounds that neutral salt, by expelling its acid from its alkaline basis, of which it takes possession, and therewith forms a new saline compound.
This combination is a species of salt that is perfectly neutral. When the operation is performed in a close vessel, the salt shoots into crystals in the form of right-angled quadrangular prisms, terminated at each extremity by pyramids that are also quadrangular and right angled; some of which however, instead of ending in a point, are obtuse as if truncated. The consequence is different when the operation is performed in an open vessel; for then nothing is obtained but an alkaline salt impregnated with Arsenic, which cannot be crystallized.
The cause of this different effect is, that, when the Arsenic is once engaged in the alkaline basis of the nitre, it can never be separated from it by the utmost force of fire, so long as it is kept in a close vessel; whereas, if you expose it to the fire without that precaution, it readily separates from it. This property of arsenic was never before observed by any Chymist, and therefore this our new species of Neutral arsenical salt was absolutely unknown till lately.
This new salt possesses many singular properties, the chief of which are these. First, it cannot be decompounded by the intervention of any acid, even the strongest acid of vitriol; and this, joined to its property of expelling the nitrous acid from its basis, shews that it has a very great affinity with fixed alkalis.
Secondly, this very salt, on which pure acids have no effect, is decompounded with the greatest ease by acids united with metallic substances. The reason of this phenomenon is curious, and furnishes us with an instance of what we advanced concerning double affinities.
If to a resolution of any metallic substance whatever, made by any acid whatever, (except that of Mercury by the marine acid, and that of Gold by aqua regis), a certain quantity of our New Salt dissolved in water be added, the metallic substance is instantaneously separated from the acid in which it was dissolved, and falls to the bottom of the liquor.
All metallic precipitates obtained in this manner are found to be a combination of the metal with Arsenic; whence it[Pg 74] necessarily follows that the new Neutral Salt is by this means decompounded, its arsenical part uniting with the metallic substance, and its alkaline basis with the acid in which that substance was dissolved.
The affinities of these several bodies must be considered as operating on this occasion in the following manner: The acids which tend to decompound the Neutral Salt of Arsenic, by virtue of their affinity with its alkaline basis, are not able to accomplish it, because this affinity is powerfully counteracted by that which the Arsenic has with the same alkaline basis, and which is equal or even superior to theirs. But if these acids happen to be united with a substance which naturally has a very great affinity with the arsenical part of the Neutral Salt, then, the two parts of which this Salt consists being drawn different ways by two several affinities tending to separate them from each other, the Salt will undergo a decomposition, which could not have been effected without the help of this second affinity. Now, as metallic substances have a great affinity with Arsenic, it is not surprising that the Neutral Salt of Arsenic, which cannot be decompounded by a pure acid, should nevertheless yield to an acid combined with a metal. The decomposition of this Salt, therefore, and the precipitation which of course it produces in metallic solutions, are brought about by the means of a double affinity; namely, that of the acid with the alkaline basis of the Neutral Salt, and that of the metal with the arsenical part of that salt.
Arsenic has not the same effect on sea-salt as on nitre, and cannot expel its acid: a very singular phenomenon, for which it is hard to assign a reason; for the nitrous acid is known to have a greater affinity than the marine acid with alkalis, and even with the basis of sea-salt itself.
Yet Arsenic may be combined with the basis of sea-salt, and a Neutral Salt thereby obtained, like that which results from the decomposition of nitre by Arsenic: but for that purpose a quadrangular nitre must be first prepared, and Arsenic applied thereto as to common nitre.
The Salt produced by uniting Arsenic with the basis of sea-salt very much resembles the Neutral Salt of Arsenic above treated of as well in the figure of its crystals as in its several properties.
Arsenic presents another singular phenomenon, both with the alkali of nitre and with that of sea-salt; which is, that if it be combined with these salts in a fluid state, it forms with them[Pg 75] a saline compound, quite different from the Neutral Salts of Arsenic which result from the decomposition of nitrous salts.
This saline compound, which I call Liver of Arsenic, takes up a much greater quantity of Arsenic than is necessary for the perfect saturation of the alkali. It has the appearance of a glue, which is so much the thicker the more Arsenic it contains. Its smell is disagreeable; it attracts the moisture of the air, and does not crystallize; it is easily decompounded by any acid whatever, which precipitates the Arsenic and unites with the alkali. Lastly, the effects it produces on metallic solutions are different from those of our neutral arsenical salts. But the bounds which I have set myself in this treatise will not allow me to be more particular. Such as have the curiosity to inquire further into the subject may consult my Dissertations on Arsenic, published among the Memoirs of the Academy of Sciences.
Arsenic is easily reduced to a Regulus. It need only be mixed with any matter containing the phlogiston, and by the help of a moderate heat a true Regulus will sublime. This Regulus, as was said, is very volatile, and calcines with the greatest ease; which is the reason why it cannot be obtained but in small quantities, and also why, in order to obtain masses of it, some have thought of adding thereto some metal with which it has a great affinity, such as Copper or Iron; because, by joining with the metal, it is partly fixed and restrained from flying off. But it is plain the Regulus obtained by this means is not pure, as it must partake considerably of the metal employed.
Arsenic readily unites with sulphur, and rises with it in a yellow compound, called Orpiment.
Sulphur cannot be separated from Arsenic but by the intervention of two bodies only; to wit, a fixed alkali and Mercury.
The property which Mercury possesses of separating sulphur from Arsenic is founded on this, that these two metallic substances are incapable of contracting any union; whereas, though most of the other metals and semi-metals have a greater affinity with sulphur than Mercury hath, as was shewn in treating of the decomposition of Cinabar, nevertheless they are all unable to decompound Orpiment; because some of them have as great an affinity with Arsenic as with sulphur; others have no affinity with either; and lastly, sulphur hath as great an affinity with Arsenic as with any of them.
It must be observed that, if fixed alkalis be employed to purify Arsenic in this manner, no more must be used than is necessary to absorb the sulphur or the phlogiston, of which also it is their nature to deprive Arsenic; for otherwise, as it has been shewn that Arsenic readily unites with alkalis, they would absorb a considerable quantity thereof.
Of Oil in general.
Oil is an unctuous body, which burns and consumes with flame and smoke, and is not soluble in water. It consists of the phlogiston united with water by means of an acid. There is, moreover, in its composition a certain proportion of earth, more or less, according to each several sort of Oil.
The inflammability of Oil evidently proves that it contains the phlogiston. That an acid is one of its constituent principles many experiments demonstrate, of which these are the chief: If certain Oils be long triturated with an alkaline salt, and the alkali afterwards dissolved in water, crystals of a true neutral salt will be produced: some metals, and particularly Copper, are corroded and rusted by Oils, just as they are by acids: again, acid crystals are found in some Oils that have been long kept. This acid in Oil serves undoubtedly to unite its phlogiston with its water; because these two substances having no affinity with each other cannot be united without the intervention of such a medium as an acid, which has an affinity with both. As to the existence of water in Oils, it appears plainly when they are decomposed by repeated distillations, especially after mixing them with absorbent earths. Lastly, when an Oil is destroyed by burning, a certain quantity of earth is constantly left behind.
We are very sure that the above-mentioned principles enter into the composition of Oils; for they may be obtained from every one of them: but it is not absolutely certain that they consist of these only, and that they do not contain some other principle which may escape our notice in decomposing them; for hitherto it doth not appear, by any experiment we can depend on, that Oil was ever produced by combining together the principles here specified: yet such redintegrations[Pg 77] are the only means we have of satisfying ourselves that we know all the principles which constitute a body.
Oils exposed to the fire in close vessels pass over almost wholly from the containing vessel into any other applied to receive them. There remains, however, a small quantity of black matter, which is extremely fixed, and continues unalterable as long as it hath no communication with the external air, be the force of the fire ever so violent. This matter is no other than part of the phlogiston of the Oil united with its most fixed and grossest earth; and this is what we called Charcoal, or plainly a Coal.
Of Charcoal.
When Oil happens to be united to much earth, as it is in vegetable and animal bodies, it leaves a considerable quantity of Coal or charred matter.
This Coal, exposed to the fire in the open air, burns and wastes, but without blazing like other combustible matters: there appears only a small blueish flame, but not the least smoke. Most commonly it only glows and sparkles, and so gradually falls into ashes, which are nothing but the earth of the body, combined with an alkaline salt in burning. This alkaline salt may be separated from the earth, by lixiviating the ashes with water, which dissolves all the salt, and leaves the earth quite pure.
Charcoal is unalterable and indestructible by any other body but fire; whence it follows, that when it is not actually kindled and ignited, the most powerful agents, such as the acids, though ever so strong and concentrated, have not the least effect on it.
The case is otherwise when it is lighted, that is, when its phlogiston begins to separate from its earth; for then the pure acid of vitriol being joined therewith, contracts an instantaneous union with its phlogiston, and evaporates in a volatile sulphureous spirit. If the vitriolic acid, instead of being applied quite pure, be first clogged with some basis, especially an alkaline one, it quits that basis, enters into a more intimate union with the phlogiston of the burning Coal, and so forms an actual sulphur, with which the alkali now unites and forms a hepar.
The pure acid of sea-salt hath not been observed to act in the least upon Charcoal, especially when it is not on fire.[Pg 78] But when this acid is incorporated with an alkaline or metallic basis, and combined according to a peculiar process with burning Charcoal, it in like manner quits its basis, unites with the phlogiston, and therewith forms a phosphorus, of which we have already taken notice.
Nor has the pure nitrous acid any effect on a charred Coal, even when ignited: and so far is it from being able to kindle a cold one, that when poured on a live one, it extinguishes it like water. But when this acid is united with a basis, it quits it rapidly as soon as it touches a burning coal, and rushes violently into an union with the phlogiston thereof. From this union there probably arises, as we said before, a kind of sulphur or phosphorus, which is so inflammable as to be destroyed by the fire the very moment it is generated.
The acids of nitre and vitriol act upon Oils; but very differently, according to the quantity of phlegm they contain. If they be weakened with much water, they have no effect at all upon Oils; if they contain little water, or be dephlegmated to a certain degree, they dissolve them with heat, and with them form compounds of a thick consistence. Acids, thus combined in a considerable proportion with Oils, render them soluble in water.
Of Soap.
Alkalis also have the same property. When an Oil is combined with an acid or an alkali in such a manner, that the compound resulting from their union is soluble in water; such a compound may in general be called a Soap. Soap itself hath the property of rendering fat bodies in some measure soluble in water; on which account it is very useful for scouring or cleansing any thing greasy.
Oily and saline substances, combined together, observe the same general rules as all other combinations; that is, they mutually communicate the properties belonging to each: thus Oils, which naturally are not soluble in water, acquire, by their union with saline matters, the property of dissolving therein; and salts lose, by their conjunction with Oils, part of their natural tendency to incorporate with water; so that, while they serve to constitute soap, they do not, as before, attract the moisture of the air, &c. and, in like manner, as they are not inflammable, they considerably lessen the inflammability of the Oils combined with them.
Acid Soaps are decompounded by alkalis, as alkaline Soaps are by acids, according to the general rules of affinities.
The acids of nitre and vitriol, when highly concentrated, dissolve Oils with such violence as to heat them, make them black, burn them, and even set them on fire. How sea-salt affects Oils is not yet sufficiently ascertained.
All Oils have the property of dissolving sulphur; which is not at all surprising, seeing each of its component principles hath an affinity with Oil.
It is also a property common to all Oils to become more fluid, subtile, light, and limpid, the oftener they are distilled. On the contrary, by being incorporated with saline substances they acquire a greater consistence, and sometimes form compounds that are most solid.
Of the several Sorts of Oils.
Oils are distinguished by the substances from which they are drawn: and as Oils are extracted from minerals, from vegetables, and from animals, there are of course Mineral, Vegetable, and Animal Oils.
Of Mineral Oils.
In the bowels of the earth we find but one sort of Oil, called Petroleum: its smell is strong and not disagreeable, and its colour sometimes more sometimes less yellow. There are certain mineral substances which yield by distillation a great deal of Oil very like Petroleum. This sort of substance is called a Bitumen, and is, indeed, nothing but an Oil rendered consistent and solid by being combined with an acid; as appears from hence, that by uniting Petroleum with the acid of vitriol we can produce an artificial Bitumen very like the native.
Of Vegetable Oils.
Vegetable substances yield a very great quantity and variety of oils: for there is not a plant, or part of a plant, that does not contain one or more sorts thereof, generally peculiar to itself, and different from all others.
By expression only, that is, by bruising and squeezing vegetable substances, particularly certain fruits and seeds, a sort of oil is obtained which has scarce any smell or taste. Oils of this sort are very mild and unctuous; and, because in this respect they resemble animal fat more than the rest do, they are called Fat Oils.
These Oils, being exposed to the air for some time, sooner or latter grow thick, acquire an acrid taste, and a strong disagreeable smell. Some of them congeal with the smallest degree of cold. This sort of Oil is well adapted to dissolve those preparations of Lead called Litharge and Minium, with which they form a thick tenacious substance, that is used for the basis of almost all plasters. They also dissolve Lead in its metalline form; but not so easily as the sorts of calx above-mentioned; probably because its body is not so much opened, nor its parts so divided.
By expression alone we also procure from certain vegetable substances another sort of Oil, which is thin, limpid, volatile, of a pungent taste, and retains the smell of the vegetable that yielded it; on which account it is called an Essential Oil. Of this there are several sorts, differing from one another, like the Fat Oils, according to the subjects from which they are obtained.
We must observe, that it is very difficult, or rather in most cases impossible, to force from the greatest part of vegetables, by expression only, all the essential Oil they contain. For this purpose therefore recourse must be had to fire: a gentle heat, not exceeding that of boiling water, will extract all the essential Oils of vegetables; and this is the most usual and most convenient way of procuring them.
The fat Oils cannot be obtained by the same method: these being much less volatile than the essential Oils, require a much greater degree of heat to raise them; which nevertheless they cannot bear without being much spoiled and entirely changed in their nature, as shall presently be shewn. All[Pg 81] Oils, therefore, which rise with the heat of boiling water, and such alone, should be called Essential Oils.
Essential Oils, in a longer or shorter time, according to the nature of each, lose the fragrant smell they had when newly distilled, and acquire another, which is strong, rancid, and much less agreeable: they also lose their tenuity, becoming thick and viscid; and in this state they greatly resemble those substances abounding in Oil which flow from certain trees, and which are called Balsams or Resins, according as they are less or more consistent.
Balsams and Resins are not soluble in water. But there are other Oily compounds which likewise run from trees; and, though not unlike Resins, are however soluble in water. These are called Gums; and their property of dissolving in water arises from their containing more water and more salt than Resins have; or at least their saline parts are less clogged and more disengaged.
Balsams and Resins distilled with the heat of boiling water yield great quantities of a limpid, subtile, odoriferous, and, in one word, essential Oil. In the still there remains a substance thicker and more consistent than the Balsam or Resin was before distillation. The same thing happens to essential Oils which by length of time have acquired a consistence and are grown resinous. If they be re-distilled, they recover their former tenuity, leaving behind them a remainder thicker and more resinous than they themselves were. This second distillation is called the Rectification of an Oil.
It must be observed, that an essential Oil, combined with an acid strong enough to dissolve it, immediately becomes as thick and resinous, in consequence of this union, as if it had been long exposed to the air: which proves the consistence an Oil acquires by long keeping to be owing to this, that its lightest and less acid parts being evaporated, the proportion of its acid to the remainder is so increased, that it produces therein the same change, as an additional acid mixed with the Oil would have wrought before the evaporation.
This also shews us, that Balsams and Resins are only essential Oils combined with a great proportion of acid, and thereby thickened.
If vegetable substances, from which no more essential Oil can be drawn by the heat of boiling water, be exposed to a stronger heat, they yield an additional quantity of Oil; but it is thicker and heavier than the essential Oil. These Oils are black, and have a very disagreeable burnt smell, which[Pg 82] hath made them be called Fetid or Empyreumatic Oils. They are moreover very acrid.
It must be observed, that, if a vegetable substance be exposed to a degree of heat greater than that of boiling water, before the fat or the essential Oil is extracted from it, an empyreumatic Oil only will then be obtained; because both the fat and essential Oils, when exposed to the force of fire, are thereby burnt, rendered acrid, acquire a smell of the fire, and, in a word, become truly empyreumatic. There is ground to think, that an empyreumatic Oil is nothing else but an essential or fat Oil burnt and spoiled by the fire, and that no other Oil besides these two exists naturally in vegetables.
Empyreumatic Oils, distilled and rectified several times by a gentle heat, acquire by every distillation a greater degree of tenuity, lightness, and limpidity. By this means also they lose something of their disagreeable odour; so that they gradually come nearer and nearer to the nature of essential Oils: and if the rectifications be often enough repeated, ten or twelve times for instance, they become perfectly like those Oils; except that their smell will never be so agreeable, nor like that of the substances from which they were obtained.
Fat Oils may also be brought by the same means to resemble essential Oils: but neither essential nor empyreumatic Oils are capable of acquiring the properties of fat Oils.
Of Animal Oils.
Distillation procures us considerable quantities of Oil from all the parts of animal bodies, and especially from their fat. This Oil at first is not very fluid, and is extremely fetid: but by many rectifications it gradually acquires a great degree of clearness and tenuity, and at the same time loses much of its disagreeable odour. Animal Oils, thus rendered thin and fluid by a great number of rectifications, have the reputation of being an excellent medicine, and a specific in the epilepsy.
Of Fermentation in general.
By Fermentation is meant an intestine motion, which, arising spontaneously among the insensible parts of a body, produces a new disposition and a different combination of those parts.
To excite a Fermentation in a mixt body, it is necessary, first, that there be in the composition of that mixt a certain proportion of watery, saline, oily, and earthy parts: but this proportion is not yet sufficiently ascertained. Secondly, it is requisite that the body to be fermented be placed in a certain degree of temperate heat: for much cold obstructs fermentation; and too much heat decomposes bodies. Lastly, the concurrence of the air is also necessary to fermentation.
All vegetable and animal substances are susceptible of Fermentation, because all of them contain in a due proportion the principles above specified. However, many of them want the proper quantity of water, and cannot ferment while they remain in such a state of dryness. But it is easy to supply that defect, and so render them very apt to ferment.
With respect to minerals properly so called, (that is, excluding such vegetable and animal substances as may have lain long buried in the earth), they are not subject to any Fermentation; at least, that our senses can perceive.
There are three sorts of Fermentation, distinguished from one another by their several productions. The first produces wines and spirituous liquors; for which reason it is called the Vinous or Spirituous Fermentation: the result of the second is an acid liquor; and therefore it is called the Acetous Fermentation: and the third generates an alkaline salt; which, however, differs from the alkaline salts hitherto treated of, in this respect chiefly, that, instead of being fixed, it is extremely volatile: this last sort takes the name of the Putrid or Putrefactive Fermentation. We shall now consider these three sorts of Fermentation and their effects a little more particularly.
These three sorts of Fermentation may take place successively in the same subject; which proves them to be only three different degrees of fermentation, all proceeding from[Pg 84] one and the same cause, rather than three distinct fermentations. These degrees of fermentation always follow the order in which we have here placed them.
Of the Spirituous Fermentation.
The juices of almost all fruits, all saccharine vegetable matters, all farinaceous seeds and grains of every kind, being diluted with a sufficient quantity of water, are proper subjects of Spirituous Fermentation. If such liquors be exposed, in vessels slightly stopped, to a moderate degree of heat, they begin in some time to grow turbid; there arises insensibly a small commotion among their parts, attended with a hissing noise; this by little and little increases, till the grosser parts appear, like little seeds or grains, moving to and fro, agitated among themselves, and thrown up to the surface. At the same time some air bubbles rise, and the liquor acquires a pungent, penetrating smell, occasioned by the very subtile vapours which exhale from it.
These vapours have never yet been collected, in order to examine their nature; and they are known only by their noxious effects. They are so actively pernicious, that if a man comes rashly into a close place, where large quantities of liquors are fermenting, he suddenly drops down and expires, as if he were knocked on the head.
When these several phenomena, begin to go off, it is proper to stop the fermentation, if a very spirituous liquor be required: for if it be suffered to continue longer, the liquor will become acid, and from thence proceed to its last stage, that is, to putrefaction. This is done by stopping the containing vessels very close, and removing them into a cooler place. Then the impurities precipitate, and settling at the bottom leave the liquor clear and transparent: and now the palate discovers that the sweet saccharine taste it had before fermentation is changed to an agreeable pungency, which is not acid.
Liquors thus fermented are in general called Wines: for though in common life that word properly signifies the fermented juice of grapes only, and particular names are given to the fermented juices of other vegetable substances; as that[Pg 85] obtained from Apples is called Cyder; that made from malt is called Beer: yet in Chymistry it is of use to have one general term denoting every liquor that has undergone this first degree of fermentation.
By distillation we draw from Wine an inflammable liquor, of a yellowish white colour, light, and of a penetrating, pleasant smell. This liquor is the truly spirituous part of the wine, and the product of fermentation. That which comes off in the first distillation is commonly loaded with much phlegm and some oily parts, from which it may be afterwards freed. In this state it goes by the name of Brandy; but when freed from these heterogeneous matters by repeated distillations, it becomes still clearer, lighter, more fragrant, and much more inflammable, and then is called Spirit of Wine, and Rectified Spirit of Wine, or an Ardent Spirit, if considerably purified. The properties which distinguish an Ardent Spirit from all other substances are its being inflammable; its burning and consuming entirely, without the least appearance of smoke or fuliginosity; its containing no particles reducible to a coal; and its being perfectly miscible with water. Ardent Spirits are lighter and more volatile than any of the principles of the mixts from which they were produced, and consequently more so than the phlegm, the acid, and the oil of which they themselves consist. This arises from a particular disposition of these principles, which are in a singular manner attenuated by fermentation, and thereby rendered more susceptible of expansion and rarefaction.
Ardent spirits are supposed to be the natural solvents of oils and oily matters. But it is very remarkable that they dissolve essential oils only, without touching the fat of animals, or the fat oils obtained from vegetables by expression; yet when these oils have once undergone the action of fire, they become soluble in spirit of wine, and even acquire a new degree of solubility every time they are distilled. It is not so with essential oils, which can never be rendered more soluble in ardent spirits than they are at first; and are so far from acquiring a new degree of solubility every time they are distilled, that on the contrary they even in some measure lose that property by repeated rectifications.
I have taken some pains to find out the causes of these singular effects, and the result of my inquiries is published among the Memoirs of the Academy of Sciences for the year 1745. I therein consider ardent spirits as consisting of an oil, or at least a phlogiston, mixed with a portion of wa[Pg 86]ter, in which it is rendered soluble by means of an acid. This being laid down, I shew that the inability of spirit of wine to dissolve some oils must be imputed to its aqueous part, in which oils are not naturally soluble without the intervention of a salt: and that the power which this spirit exerts in dissolving other oils with ease, such as essential oils, must in all probability be owing to this, that in these oils it meets with the necessary saline medium, that is, with an acid, which numberless experiments shew they actually contain.
On the other hand, I there prove, that the acid in essential oils is superabundant, and in some sort foreign to their nature, or that it is but slightly connected with them, and in part deserts them every time they are distilled; which renders them less soluble after every new rectification: whereas, on the contrary, the fat expressed oils in their natural state give not the least sign of acidity, but the action of fire upon them discovers an acid which was not perceivable before. Hence I conjecture, that these oils contain no more acid than is just necessary to constitute them oils; that this acid is intimately blended with their other component parts; that it is so sheathed and entangled by these parts as to be incapable of exerting any of its properties; and that on this account these oils in their natural state are not soluble in spirit of wine: but that the disposition of their parts being gradually changed by the fire, and their acid, being by that means set more and more at liberty, at length recovers its properties, and particularly that of rendering the oily parts soluble in an aqueous menstruum: and hence it follows, that the fat oils become so much the more soluble in spirit of wine the oftener they are exposed to the action of fire.
Spirit of wine doth not dissolve fixed alkalis; or at least it takes up but a very small quantity thereof; and hence ardent spirits may be freed from much of their phlegm by means of these salts thoroughly dried: for as they strongly imbibe moisture, and have even a greater affinity than ardent spirits with water, if a fixed alkali, well exsiccated, be mixed with spirit of wine that is not perfectly dephlegmated, the alkali immediately attracts its superfluous moisture, and is thereby resolved into a liquor, which, on account of its gravity, descends to the bottom of the vessel. The spirit of wine, which swims at top, is by this means as much dephlegmated, and as dry, as if it had been rectified by several distillations. As it takes up some alkaline particles in this operation, it is thereby qualified to dissolve oily matters with the greater fa[Pg 87]cility. When rectified in this manner, it is called Alcoholized Spirit of Wine.
Yet spirit of wine, even when rectified to an alcohol, is not capable of dissolving all oily matters. Those named Gums will by no means enter into any sort of union therewith; but it readily dissolves most of those which are known by the appellation of Resins. When it has dissolved a certain proportion of resinous particles it acquires a greater consistence, and forms what is called a Spirit Varnish, or a Drying Varnish, because it soon dries. This Varnish is subject to be damaged by water. Many sorts thereof are prepared, differing from each other according to the different resins employed, or the proportions in which they are used. Most of these Varnishes are transparent and colourless.
Such bitumens or resins, as spirit of wine will not touch, are dissolved in oils by means of fire, and then form another kind of Varnish, which water does not hurt. These Varnishes are usually coloured, and require much longer time to dry than the Spirit Varnishes: they are called Oil Varnishes.
Spirit of wine hath a much greater affinity with water than with oily matters: and therefore if a solution of any oil or resin in spirit of wine be mixed with water, the liquor immediately grows turbid, and acquires a whitish milky colour, owing entirely to the oily parts being separated from the spirituous menstruum by the accession of water, and too finely divided to appear in their natural form. But if the liquor stand some time quiet, several of these particles unite together, and gradually acquire a bulk sufficient to render them very perceptible to the eye.
Acids have an affinity with spirit of wine, and may be combined with it. By this union they lose most of their acidity, and on that account are said to be Dulcified. But as these combinations of acids, especially of the vitriolic acid, with spirit of wine furnish some new productions of very singular properties, and as an examination thereof may throw much light on the nature of ardent spirits, it will not be amiss to take notice of them in this place, and consider each of them particularly.
One part of highly concentrated oil of vitriol being mixed with four parts of well dephlegmated spirit of wine, there arises immediately a considerable ebullition and effervescence, attended with great heat, and abundance of vapours, which smell pleasantly, but are hurtful to the lungs. At the same time is heard a hissing like that produced by a piece of red-hot[Pg 88] iron plunged into water. Indeed it is proper to mix the liquors very gradually; for otherwise the vessels in which the operation is performed will be in great danger of breaking.
If the two liquors thus mixed be distilled with a very gentle heat, there rises first a spirit of wine of a most penetrating and grateful odour: when about half thereof is come over, what follows has a quicker and more sulphureous smell, and is also more loaded with phlegm. When the liquor begins to boil a little, there comes off a phlegm which smells very strong of sulphur, and grows gradually more acid. On this phlegm floats a small quantity of a very light and very limpid oil. In the still there remains a thick blackish substance, somewhat like a resin or bitumen. From this substance may be separated a good deal of a vitriolic but sulphureous acid. When that is extracted, there remains a black mass like a charred coal, which being put into a crucible, and exposed to a violent heat, leaves a small portion of earth, very fixed, and even vitrifiable.
By rectifying the ardent spirit, which came over in distilling the above-mentioned mixture, a very singular liquor is obtained, which differs essentially both from oils and from ardent spirits, though in certain respects it resembles them both. This liquor is known in Chymistry by the name of Æther, and its chief properties are as follow.
Æther is lighter, more volatile, and more inflammable, than the most highly rectified spirit of wine. It quickly flies off when exposed to the air, and suddenly catches fire when any flame approaches it. It burns like spirit of wine without the least smoke, and consumes entirely without leaving the smallest appearance of a coal or of ashes. It dissolves oils and oily matters with great ease and rapidity. These properties it has in common with an ardent spirit. But it resembles an oil in that it is not miscible with water; and this makes it essentially different from spirit of wine, the nature of which is to be miscible with all aqueous liquors.
Another very singular property of Æther is its great affinity with gold, exceeding even that of aqua regis. It does not indeed dissolve gold when in a mass, and in its metalline form; but if a small quantity of Æther be added to a solution of gold in aqua regis, and the whole shaken together, the gold separates from the aqua regis, joins the Æther, and remains dissolved therein.
The reason of all the phenomena above-mentioned, resulting from the mixture of spirit of wine with oil of vitriol,[Pg 89] is founded on the great affinity between this acid and water. For if the vitriolic acid be weak, and as it were over-dosed with watery parts, neither oil nor Æther can be obtained by means thereof: but when highly concentrated, it attracts the aqueous parts very powerfully; and therefore, being mixed with spirit of wine, lays hold of most of the water contained in it, and even robs it of some portion of that which is essential to its nature, and necessary to constitute it spirit of wine: whence it comes to pass, that a certain quantity of the oily particles in its composition being separated from the watery particles, and so brought nearer to each other, they unite and assume their natural form; and thus the oil that swims at top of the sulphureous phlegm is produced.
The vitriolic acid moreover thickens and even burns some of this oil; and hence comes the bituminous residuum left at the bottom of the still, which looks like the result of a vitriolic acid combined with common oil. Lastly, the vitriolic acid becomes sulphureous, as it always doth when united with oily matters, and also very aqueous, on account of the quantity of phlegm which it attracts from the spirit of wine.
Æther may be considered as a spirit of wine exceedingly dephlegmated, even to such a degree that its nature is thereby changed; so that the few aqueous particles left in it are not sufficient to dissolve the oily particles and keep them asunder; which therefore being now much nearer to one another than in common spirit of wine, the liquor hath lost its property of being miscible with water.
Spirit of nitre well dephlegmated, and combined with spirit of wine, presents likewise some very singular appearances.
First, in the very instant of its mixture with spirit of wine, it produces a greater and more violent effervescence than the vitriolic acid occasions.
Secondly, this mixture, without the help of distillation, and only by stopping the bottle in which the liquors are contained, affords a sort of Æther, produced probably by the vapours which ascend from, and swim at top of the mixture. This is a very singular liquor. Dr. Navier was the first that took notice of it, and gave a description thereof, which may be seen in the Memoirs of the Academy of Sciences.
Thirdly, some authors pretend that, by distilling the mixture under consideration, an oil is obtained greatly resembling that which, as we observed above, rises from spirit of[Pg 90] wine combined with the vitriolic acid: others again deny this. For my part, I believe the thing depends on the different concentration of the spirit of nitre, as well as on the quality of the spirit of wine, which is sometimes more sometimes less oily.
Fourthly, the two liquors we are speaking of, being intimately mixed by distillation, form a liquor slightly acid, used in medicine, and known by the name of Sweet or Dulcified Spirit of Nitre: a very proper name, seeing the nitrous acid, by uniting with the spirit of wine, actually loses almost all its acidity and corrosive quality.
Fifthly and lastly, when the distillation is finished, there remains in the bottom of the vessel a thick blackish substance, nearly resembling that which is found after distilling oil of vitriol and spirit of wine.
Spirit of salt hath likewise been combined with spirit of wine; but it does not unite therewith so easily or so intimately as the two acids above-mentioned. To mix them thoroughly, the spirit of salt must be highly concentrated, and smoking, and moreover the assistance of the still must be called in. Some authors pretend that from this mixture also a small quantity of oil may be obtained; which probably happens when the liquors have the qualities above-specified. The marine acid likewise, by uniting with spirit of wine, loses most of its acidity; on which account it is in like manner called Sweet or Dulcified Spirit of Salt. A thick residuum is also found here after distillation.
Of the Acetous Fermentation.
Besides an ardent spirit, wine affords a great deal of water, oil, earth, and a sort of acid which shall be considered presently. When the spirituous part is separated from these other matters, they undergo no further change. But if all the constituent parts of wine remain combined together, then, after some time, shorter or longer as the degree of heat in which the wine stands is greater or less, the fermentation begins afresh, or rather arrives at its second stage. The liquor once more grows turbid, a new intestine motion arises, and, after some days, it is found changed into[Pg 91] an acid; which, however, is very different from those hitherto treated of. The liquor then takes the name of Vinegar. The acetous fermentation differs from the spirituous, not only in its effect, but also in several of its concomitant circumstances. Moderate motion is of service to this, whereas it obstructs the spirituous; and it is attended with much more warmth than the spirituous. The vapours it produces are not noxious, like those of fermenting wine. Lastly, Vinegar deposites no tartar, even when the wine employed in this operation is quite new, and hath not had time to discharge its tartar: instead of tartar, Vinegar deposites a viscid matter which is very apt to putrify.
It must be observed, that wine is not the only substance that is susceptible of the acetous fermentation: for several vegetable and even animal matters, which are not subject to the spirituous fermentation, turn sour before they putrify. But as vinous liquors possess in a very eminent degree the property of being susceptible of the acetous fermentation, and likewise of producing the strongest acids that can result from such fermentation, their acid shall be more particularly considered in this place.
Of Vinegar.
If wine, which has gone through this second stage of fermentation, be distilled, instead of an ardent spirit, only an acid liquor is obtained, which is called Distilled Vinegar.
This acid has the same properties as the mineral acids of which we have already treated; that is, it unites with alkaline salts, absorbent earths, and metallic substances, and therewith forms neutral saline combinations.
Its affinity with these substances observes the same order as that observed by the mineral acids with regard to the same substances; but in general it is weaker; that is, any mineral acid is capable of expelling the acid of Vinegar out of all matters with which it is united.
Vinegar hath likewise a greater affinity than sulphur with alkalis: whence it follows, that it is capable of decompounding that combination of sulphur with an alkali called Liver of Sulphur, and of precipitating the sulphur it contains.
The acid of Vinegar is always clogged with a certain proportion of oily parts, which greatly weaken it, and deprive it of much of its activity; and for this reason it is not near[Pg 92] so strong as the mineral acids, which are not entangled with any oil. By distillation, indeed, it may be freed from this oil, and at the same time from the great quantity of water which in a manner suffocates it, and by that means may be brought much nearer to the nature of the mineral acids: but this attempt hath not yet been prosecuted with the assiduity it deserves. Besides distillation, there is another way of freeing Vinegar from a good deal of its phlegm; and that is, by exposing it to a hard frost, which readily congeals the watery part into ice, while the acid retains its fluidity.
Vinegar, saturated with a fixed alkali, forms a neutral oily salt, of a dark colour, which is semi-volatile, melts with a very gentle heat, flames when thrown upon burning coals, and dissolves in spirit of wine, of which, however, it requires six parts to complete the solution. This solution being evaporated to dryness leaves a matter in the form of leaves lying on each other; on which account it hath obtained the name of Terra Foliata. The same foliated matter will be obtained, though the salt be not previously dissolved in spirit of wine; but not so readily. This salt is also called Regenerated Tartar. Under the head of Tartar we shall see the reason of these different appellations. Regenerated Tartar is also in some degree capable of crystallizing: for this purpose a solution thereof in water must be slowly evaporated to the consistence of a syrup, and then suffered to stand quiet in a cool place; by which means it will shoot into clusters of crystals, lying one upon another, not unlike the feathers on a quill.
With Vinegar and several absorbent earths, such as calcined pearls, coral, shells of fish, &c. are also formed neutral saline compounds, each of which takes the name of the particular earth employed in its composition.
Vinegar perfectly dissolves Lead, and converts it to a neutral metallic salt, which shoots into crystals, and has a sweet saccharine taste. This compound is called Sugar of Lead, or Sal Saturni.
If Lead be exposed to the bare vapour of Vinegar, it will be thereby corroded, calcined, and converted into a white matter much used in painting, and known by the name of Ceruse; or, when it is finer than ordinary, White Lead.
Vinegar corrodes Copper likewise, and converts it into a beautiful green rust, which also is used in painting; and distinguished by the name of Verdegris. However, Vinegar is not commonly employed to make Verdegris: for this pur[Pg 93]pose they use wine, or the rape of wine, from which fire extricates an acid analogous to that of Vinegar.
In treating of the several substances which constitute wine, we mentioned an acid matter, but did not then enter into a particular examination thereof; because as that matter greatly resembles the acid of Vinegar, we thought it more proper to defer the consideration of its properties till we had treated of the acetous fermentation, and its effects.
Of Tartar.
This substance is a saline compound, consisting of earthy, oily, and especially acid parts. It is found in the form of crusts, adhering to the inner sides of vessels in which wines have stood for some time, particularly acid wines, such as those of Germany.
Tartar derives its origin from the superabundant quantity of acid contained in the juice of the grape. This superfluous acid, being more than is requisite to constitute the ardent spirit, unites with some of the oil and earth contained in the fermented liquor, and forms a kind of salt; which for some time continues suspended in that liquor, but, when the wine stands undisturbed in a cool place, is deposited, as hath been said, on the sides of the cask.
Tartar in this state contains many earthy parts, which are superfluous, and foreign to its nature. From these it may be freed by boiling it repeatedly with a sort of earth found in the neighbourhood of Montpelier, as may be seen in the Memoirs of the Academy of Sciences.
When it is purified, there appears on the surface of the liquor a sort of white, crystalline pellicle, which is skimmed off as it forms. This matter is called Cream of Tartar. The same liquor which produces this Cream, and in which the purified Tartar is dissolved, being set to cool, yields a great number of white semi-transparent crystals, which are called Crystals of Tartar. The Cream and the Crystals of Tartar are therefore no other than purified Tartar, and differ from each other in their form only.
Though the Crystals of Tartar have every appearance of a neutral salt, yet they are far from being such; for they have all the properties of a true acid, which scarce differs from that of vinegar, except that it contains less water, and more earth and oil; to which it owes its solid form, as well as its pro[Pg 94]perty of not being soluble in water without much difficulty: for a very great quantity of water is requisite to keep the Crystals of Tartar in solution; and it must moreover be boiling hot; otherwise as soon as it cools most of the Tartar dissolved in it separates from the liquor, and falls to the bottom in the form of a white powder.
Tartar is decomposed by calcination in the open fire. All its oily parts are consumed or dissipated in smoke, together with most of its acid. The other part of its acid, uniting intimately with its earth, forms a very strong and very pure fixed alkali, called Salt of Tartar.
It will be shewn in its proper place, that almost every vegetable matter, as well as Tartar, leaves a fixed alkali in its ashes: yet Tartar has these peculiar properties; first, it assumes an alkaline character even when burnt or calcined in close vessels, whereas other substances acquire it only by being burnt in the open air; secondly, the alkali of Tartar is stronger and more saline than almost any that is obtained from other matters.
This alkali, when thoroughly calcined, powerfully attracts the moisture of the air, and melts into an unctuous alkaline liquor, improperly called Oil of Tartar per deliquium. This is the alkali generally used in making the Terra Foliata, mentioned under the head of Vinegar; for which reason this combination is called Terra Foliata Tartari; a name suitable enough. But the same cannot be said of the other name, Regenerated Tartar, which is also given it. It is true, that on this occasion an oily acid is restored to the earth of the Tartar, analagous to that of which the fire had deprived it: but the compound thence resulting is a neutral salt which very readily dissolves in water; whereas Tartar is manifestly acid, and not soluble, or at least hardly soluble, in water.
Crystals of Tartar combined with alkali of Tartar produce a great effervescence while they are mixing, as all acids usually do; and if the combination be brought exactly up to the point of saturation, a perfectly neutral salt is formed, which shoots into crystals, and easily dissolves in water; and this hath procured it the name of Soluble Tartar. It is also called the Vegetable Salt, as being obtained from vegetables only; and again, Tartarised Tartar, because it consists of the acid and the alkali of Tartar combined together.
Crystals of Tartar combined with alkalis procured from the ashes of maritime plants, such as Soda, which alkalis resemble the basis of sea-salt, form likewise a neutral salt, which crystallizes well, and dissolves easily in water. This[Pg 95] salt is another sort of soluble Tartar. It is called Saignette's Salt, from the inventor's name.
Both the Vegetable Salt and Saignette's Salt are gently purgative soaps, and much used in Medicine.
Tartar likewise dissolves the absorbent earths, as lime, chalk, &c. and with them forms neutral salts which are soluble in water[3]. It even attacks metallic bodies, and when combined with them becomes soluble. A soluble Tartar for medical use is prepared with Crystals of Tartar and Iron: the metallic salt thereby produced hath the name of Chalybeated Soluble Tartar. This salt attracts the moisture of the air, and is one of those which do not crystallize.
Crystallized Tartar acts also upon several other metallic substances: for instance, it dissolves the Regulus, Liver, and Glass of Antimony, and thence acquires an emetic quality: it is then called Stibiated or Emetic Tartar. It likewise dissolves Lead, and therewith forms a salt which, in the figure of its crystals, resembles Tartarised Tartar.
It is very extraordinary that Tartar, which of itself is not soluble in water, should be soluble therein when become a neutral salt by uniting either with alkalis or with absorbent earths, or even with metals. With respect to alkalis, indeed, it may be urged, that, having themselves a great affinity with water, they communicate to Tartar some of that facility with which they naturally unite therewith: but the same cannot be alledged concerning absorbent earths, and metallic substances, which water dissolves not at all, or at least with great difficulty, and in small quantity. This effect, therefore, must be attributed wholly to some change in the disposition of its parts which is to us unknown.
All the Soluble Tartars are easily decompounded by exposing them to a certain degree of heat. In distillation they yield the same principles which are obtained from Tartar; and what remains fixed in the fire, after they are thoroughly burnt, is a compound of the alkali which Tartar naturally produces, and of the alkaline or metallic substance with which it was converted into a neutral salt.
As Crystal of Tartar is the weakest of all acids, on account of the oily and earthy matters with which it is combined, Soluble Tartars are decompounded by all the acids; by any of which crystal of Tartar may be separated from the substance that serves it for a basis and renders it a neutral salt.
The other acids which are procured from vegetables, and even those which are obtainable from some animal substances, may all be referred to and compared with either Vinegar or Tartar, according to the quantities of oil or earth with which they are combined.
After all, these acids have not yet been thoroughly examined. There is great reason to think that they are no other than the mineral acids, which, in passing through the bodies of vegetables, and even of animals, undergo a considerable change, especially by contracting an union with oily matters. For, as we said before in treating of Vinegar, by freeing them from their oil they are brought very near to the nature of mineral acids; and so likewise the mineral acids acquire many of the properties of vegetable acids by being combined with oils.
Of the Putrid Fermentation, or Putrefaction.
Every body which hath gone through the two stages of fermentation above described, that is, the spirituous and the acetous fermentation, being left to itself in a due degree of warmth, which varies according to the subject, advances to the last stage of fermentation; that is, to putrefaction.
It is proper to observe, before we go any further, that the converse of this proposition is not true; that is, it is not necessary that a body should successively pass through the spirituous and the acetous fermentation, before it can arrive at the putrid; but that, as certain substances fall into the acetous without having gone through the spirituous fermentation, so others begin to putrify without having undergone either the spirituous or the acetous fermentation; of which last kind are, for instance, most animal substances. When therefore we represented these three sorts of fermentation as three different degrees or stages of one and the same fermentation, we supposed it to be excited in a body susceptible of fermentation in its full extent.
However, there is still room to think that every substance which is capable of fermenting always passes necessarily through these three different stages; but that the substances[Pg 97] most disposed thereto pass with such rapidity through the first, and even the second, that they arrive at the third before our senses can perceive the least signs of either of the two former. This opinion is not destitute of probability: yet it is not supported by proofs sufficiently strong and numerous to compel our assent.
When a body is in a putrefying state it is easy to discover (as in the two sorts of fermentation already treated of) by the vapours which rise from it, by the opacity which invades it, if a pellucid liquor, and frequently even by a greater degree of heat than is found in the two other sorts of fermentation, that an intestine motion is begun among its constituent parts, which lasts till the whole be entirely putrefied.
The effect of this intestine motion is in this, as in the two other sorts of fermentation, to break the union, and change the disposition, of the particles constituting the body in which it is excited, and to produce a new combination. This is brought about by a mechanism to which we are strangers, and concerning which nothing beyond conjectures can be advanced: but these we neglect, resolving to keep wholly to facts, as the only things in Natural Philosophy that are positively certain.
If, then, we examine a substance that has undergone putrefaction, we shall soon perceive that it contains a principle which did not exist in it before. If this substance be distilled, there rises, first, by means of a very gentle heat, a saline matter which is exceedingly volatile, and affects the organ of smelling briskly and disagreeably. Nor is the aid of distillation necessary to discover the presence of this product of putrefaction: it readily manifests itself in most substances where it exists, as any one may soon be convinced by observing the different smell of fresh and of putrefied urine; for the latter not only affects the nose, but even makes the eyes smart, and irritates them so as to draw tears from them in abundance.
This saline principle which is the product of putrefaction, when separated from the other principles of the body which affords it, and collected by itself, appears either in the form of a liquor, or in that of a concrete salt, according to the different methods used to obtain it. In the former state it is called a Volatile Urinous Spirit; and in the latter a Volatile Urinous Salt. The qualification of urinous is given it, because, as was said, a great deal thereof is generated in putrefied urine, to which it communicates its smell. It goes[Pg 98] also by the general name of a Volatile Alkali, whether in a concrete or in a liquid form. The enumeration of its properties will shew why it is called an alkali.
Volatile Alkalis, from whatever substance obtained, are all alike, and have all the same properties; differing only according to their degrees of purity. The Volatile Alkali, as well as the Fixed, consists of a certain quantity of acid combined with and entangled by a portion of the earth of the mixt body from which it was obtained; and on that account it has many properties like those of a Fixed Alkali. But there is moreover in its composition a considerable quantity of a fat or oily matter, of which there is none in a Fixed Alkali; and on this account again there is a great difference between them. Thus the Volatility of the Alkali produced by putrefaction, which is the principal difference between it and the other kind of Alkali whose nature it is to be Fixed, must be attributed to the portion of oil which it contains: for there is a certain method of volatilizing Fixed Alkalis by means of a fatty substance.
Volatile Alkalis have a great affinity with acids, unite therewith rapidly and with ebullition, and form with them neutral salts, which shoot into crystals, but differ from one another according to the kind of acid employed in the combination.
The neutral salts which have a Volatile Alkali for their basis are in general called Ammoniacal Salts. That whose acid is the acid of sea-salt is called Sal Ammoniac. As this was the first known, it gave name to all the rest. Great quantities of this salt are made in Egypt, and thence brought to us. They sublime it from the soot of cow's dung, which is the fuel of that country, and contains sea-salt, together with a Volatile Alkali, or at least the materials proper for forming it; and consequently all the ingredients that enter into the composition of Sal Ammoniac. See the Memoirs of the Academy of Sciences.
The neutral salts formed by combining the acids of nitre and of vitriol with a Volatile Alkali are called, after their acids, Nitrous Sal Ammoniac, and Vitriolic Sal Ammoniac: the latter, from the name of its inventor, is also called Glauber's Secret Sal Ammoniac.
A Volatile Alkali, then, has the same property as a Fixed Alkali with regard to acids: yet they differ in this, that the affinity of the former with acids is weaker than that of the latter: and hence it follows, that any Sal Ammoniac may be[Pg 99] decompounded by a Fixed Alkali, which will lay hold of the acid, and discharge the Volatile Alkali.
A Volatile Alkali will decompound any neutral salt which has not a Fixed Alkali for its basis; that is, all such as consist of an acid combined with an absorbent earth or a metallic substance. By joining with the acids in which they are dissolved, it disengages the earths or metallic substances, takes their place, and, in conjunction with their acids, forms Ammoniac Salts.
Hence it might be concluded, that, of all substances, next to the Phlogiston and the Fixed Alkalis, Volatile Alkalis have the greatest affinity with acids in general. Yet there is some difficulty in this matter: for absorbent earths, and several metallic substances, are also capable of decompounding Ammoniacal Salts, discharging their volatile Alkali, and forming new compounds by uniting with their acids. This might induce us to think, that these substances have nearly the same affinity with acids.
But it is proper to observe, that a Volatile Alkali decompounds such neutral salts as have for their basis either an absorbent earth or a metallic substance, without the aid of fire; whereas absorbent earths or metallic substances will not decompound an Ammoniacal Salt, unless they be assisted by a certain degree of heat.
Now, as all these matters are extremely fixed, at least in comparison with a Volatile Alkali, they have the advantage of being able to resist the force of fire, and so of acting in conjunction therewith; and fire greatly promotes the natural action of substances upon one another: whereas the Volatile Alkali in the Ammoniacal Salt, being unable to abide the force of fire, is compelled to desert its acid; and that so much the more quickly, as its affinity therewith is considerably weakened by the presence of an earthy or metallic substance, both of which have a great affinity with acids.
These considerations oblige us to conclude, that Volatile Alkalis have a somewhat greater affinity, than absorbent earths and metallic substances, with acids.
Ammoniacal Salts projected upon nitre in fusion make it detonate; and the Nitrous Sal Ammoniac detonates by itself, without the addition of any inflammable matter. This singular effect evidently demonstrates the existence of an oily matter in Volatile Alkalis; for it is certain that nitre will never deflagrate without the concurrence, and even the immediate contact, of some combustible matter.
This oily substance is often found combined with Volatile Alkalis in such a large proportion as to disguise it, in some measure, and render it exceedingly foul. The salt may be freed from its superfluous oil by repeated sublimations; and particularly by subliming it from absorbent earths, which readily drink up oils. This is called the Rectification of a Volatile Alkali. The salt, which before was of a yellowish or dirty colour, by being thus rectified becomes very white, and acquires an odour more pungent and less fetid than it had at first, that is, when obtained by one single distillation from a putrid substance.
It is proper to observe, that the rectification of a Volatile Alkali must not be carried too far, or repeated too often; for by that means it may be entirely decomposed at length; and particularly if an absorbent earth, and especially chalk, be employed for that purpose, the salt may be converted into an oil, an earth, and water.
Volatile Alkalis act upon several metallic substances, and particularly on copper; of which they make a most beautiful blue solution. On this property depends a pretty singular effect, which happens sometimes when we attempt, by means of a Volatile Alkali, to separate copper from any acid with which it is combined. Instead of seeing the liquor grow turbid, and the metal fall, both which generally happen when any Alkali whatever is mixed with a metallic solution, we are surprised to observe the solution of copper, upon adding a Volatile Alkali, retain its limpidity, and let fall no precipitate; or at least, if the liquor does grow turbid, it remains so but for a moment, and instantly recovers its transparency.
This is occasioned by adding such a quantity of Volatile Alkali as is more than sufficient fully to saturate the acid of the solution, and considerable enough to dissolve all the copper as fast as it is separated from the acid. On this occasion the liquor acquires a deeper blue than it had before; which arises from the property which Volatile Alkalis have of giving this metal, when combined with them, a fuller blue than any other solvent can: hence we have a touchstone to discover copper wherever it is; for let the quantity of this metal combined with other metals be ever so small, a Volatile Alkali never fails to discover it, by making it appear of a blue colour.
Though a Volatile Alkali be constantly the result of putrefaction, yet it must not therefore be imagined that none can be produced by any other means; on the contrary, most of[Pg 101] those substances which contain the ingredients necessary to form it, yield no inconsiderable quantity thereof in distillation. Tartar, for example, which by being burnt in an open fire is converted, as was shewn, into a Fixed Alkali, yields a Volatile Alkali when it is decomposed in close vessels; that is, when it is distilled; because, in this latter case, the oily part is not dissipated or burnt, as it is by calcination in a naked fire, but has time to unite with some of the earth and acid of the mixt, in such a manner as to form a true Volatile Alkali.
To prove that on this occasion, as well as on all others, where unputrefied bodies yield a Volatile Alkali, this salt is the product of the fire, we need only observe, that in these distillations it never rises till after some part of the phlegm, of the acid, and even of the thick oil of the mixt, is come over; which never is the case when it is formed beforehand in the body which is the subject of the operation, as it is in those which have undergone putrefaction: for this salt, being much lighter and more volatile than those other substances, rises of course before them in distillation.
A General View of Chymical Decomposition.
Though we have considered all the substances which enter into the composition of Vegetables, Animals, and Minerals, whether as primary or as secondary principles, it will not be improper to shew in what order we obtain these principles from the several mixts; and especially from Vegetables and Animals, because they are much more complicated than Minerals. This is called Analysing a compound.
The method most commonly taken to decompose bodies is by applying to them successive degrees of heat, from the gentlest to the most violent, in appropriated vessels, so contrived as to collect what exhales from them. By this means the principles are gradually separated from each other; the most volatile rise first, and the rest follow in order, as they come to be acted on by the proper degree of heat: and this is called Distillation.
But it being observed that fire, applied to the decomposition of bodies, most commonly alters their secondary principles very sensibly, by combining them in a different manner with each other, or even partly decomposing them, and reducing them to their primitive principles; other means have been used to separate those principles without the help of fire.
With this view the mixts to be decomposed are forcibly compressed, in order to squeeze out of them all such parts of their substance as they will by this means part with: or else those mixts are for a long time triturated, either along with water, which carries off all their saline and saponaceous contents, or with solvents, such as ardent spirits, capable of taking up every thing in them that is of an oily or resinous nature.
We shall here give a succinct account of the effects of these different methods, as applied to the principal substances among Vegetables and Animals, and likewise to some Minerals.
The Analysis of Vegetable Substances.
A vast many vegetable substances, such as kernels and seeds, yield, by strong compression, great quantities of mild, fat, unctuous Oils, which are not soluble in ardent spirits: these are what we called Expressed Oils. They are also sometimes called Fat Oils, on account of their unctuousness, in which they exceed all other sorts of Oil. As these oils are obtained without the aid of fire, it is certain that they existed in the mixt just as we see them, and that they are not in the least altered: which could not have been the case had they been obtained by distillation; for that never produces any Oils but such as are acrid and soluble in spirit of wine.
Some vegetable matters, such as the rind of citrons, lemons, oranges, &c. also yield, only by being squeezed between the fingers, a great deal of Oil. This spirts out in fine small jets, which being received upon any polished surface, such as a looking glass, run together and form a liquor that is a real Oil.
But it must be carefully noted, that this sort of Oil, though obtained by expression only, is nevertheless very different from the Oils mentioned before, to which the title of[Pg 103] Expressed Oils peculiarly belongs: for this is far lighter and thinner; moreover, it retains the perfect odour of the fruit which yields it, and is soluble in spirit of wine; in a word, it is a true essential Oil, but abounds so in the fruits which produce it, and is lodged therein in such a manner, occupying a vast number of little cells provided in the peel for its reception, that a very slight pressure discharges it; which is not the case with many other vegetables that contain an essential Oil.
Succulent and green plants yield by compression a great deal of liquor or juice, which consists of most of the phlegm, of the salts, and a small portion of the oil and earth of the plant. These juices, being set in a cool place for some time, deposite saline crystals, which are a combination of the acid of the plant with part of its oil and earth, wherein the acid is always predominant. These salts, as is evident from the description here given, bear a great resemblance to the tartar of wine treated of above. They are called Essential Salts; so that Tartar might likewise be called the Essential Salt of Wine.
Dried plants, and such as are of a ligneous, or acid nature, require to be long triturated with water, before they will yield their essential salts. Trituration with water is an excellent way to get out of them all their saline and saponaceous contents.
A vegetable matter that is very oily yields its essential salt with much difficulty, if at all; because the excessive quantity of oil entangles the salt so that it cannot extricate itself or shoot into crystals. Mr. Gerike, in his Principles of Chymistry, says, that if part of the oil of a plant be extracted by spirit of wine, its essential salt may be afterwards obtained with more ease and in greater quantity. This must be a very good method for such plants as have an excessive proportion of essential oil; but will not succeed if the essential salt be hindered from crystallizing by a redundancy of fat oil, because fat oils are not soluble in spirit of wine.
Essential Salts are among those substances which cannot be extracted from mixts by distillation: for the first impression of fire decomposes them.
Though the acid which predominates in the Essential Salts of plants, be most commonly analogous to the vegetable acid, properly so called, that is, to the acid of vinegar and tartar, which is probably no other than the vitriolic acid disguised; yet it sometimes differs therefrom, and somewhat resembles the nitrous or the marine acid. This depends on[Pg 104] the places where the plants grow which produce these salts: if they be maritime plants, their acid is akin to the acid of sea-salt; if on the contrary they grow upon walls, or in nitrous grounds, their acid is like that of nitre. Sometimes one and the same plant contains salts analogous to all the three mineral acids; which shews that the vegetable acids are no other than the mineral acids variously changed by circulating through plants.
Liquors containing the Essential Salts of plants being evaporated by a gentle heat to the consistence of honey, or even further, are called Extracts. Hence it is plain, that an Extract is nothing but the essential salt of a plant, combined with some particles of its oil and earth, that remained suspended in the liquor, and are now incorporated by evaporation.
Extracts of plants are also prepared by boiling them long in water, and then evaporating some part of it. But these Extracts are of inferior virtue; because the fire dissipates many of the oily and saline parts.
Emulsions.
Substances which abound much in Oil, being bruised and triturated with water for some time, afford a liquor of an opaque dead-white colour, like milk. This liquor consists of such juices as the water is capable of dissolving, together with a portion of the oil, which being naturally indissoluble in water, is only divided and dispersed in the liquor, the limpidity whereof is by that means destroyed. This sort of oily liquor, in which the oil is only divided, not dissolved, is called an Emulsion. The oily particles in Emulsions spontaneously separate from the water, when left at rest, and uniting into greater masses rise, on account of their lightness, to the surface of the liquor, which by that means recovers a degree of transparency.
If vegetables abounding in essential oils and resins be digested in spirit of wine, the menstruum takes up these oily matters, as being capable of dissolving them; and they may afterwards be easily separated from it by the affusion of water. The water, with which spirit of wine has a greater affinity than with oily matters, separates them by this means from their solvent, agreeably to the common laws of affinities.
Without the help of fire, scarce any thing, besides the substances already mentioned, can be obtained from a plant:[Pg 105] but, by the means of distillation, we are enabled to analyse them more completely. In prosecuting this method of extracting from a plant the several principles of which it consists, the following order is to be observed.
A plant being exposed to a very gentle heat, in a distilling vessel set in the balneum mariæ, yields a water which retains the perfect smell thereof. Some Chymists, and particularly the illustrious Boerhaave, have called this liquor the Spiritus Rector. The nature of this odoriferous part of plants is not yet thoroughly known; because it is so very volatile that it is difficult to subject it to the experiments necessary for discovering all its properties.
If, instead of distilling the plant in the balneum mariæ, it be distilled over a naked fire, with the precaution of putting a certain quantity of water into the distilling vessel along with it, to prevent its suffering a greater heat than that of boiling water, all the essential oil contained in that plant will rise together with that water, and with the same degree of heat.
On this occasion it must be observed, that no essential oil can be obtained from a plant after the Spiritus Rector hath been drawn off; which gives ground to think that the volatility of these oils is owing to that spirit.
The heat of boiling water is also sufficient to separate from vegetable matters the fat oils which they contain. That, however, is to be done by the way of decoction only, and not by distillation: because, though these oils will swim on water, yet they will not rise in vapours without a greater degree of heat.
When the essential oil is come over, if the plant be exposed to a naked fire, without the addition of water, and the heat be increased a little, a phlegm will rise that gradually grows acid; after which, if the heat be increased as occasion requires, there will come over a thicker and heavier oil; from some a volatile alkali; and last of all, a very thick, black, empyreumatic oil.
When nothing more rises with the strongest degree of heat, there remains of the plant a mere coal only, called the Caput Mortuum, or Terra Damnata. This coal when burnt falls into ashes, which, being lixiviated with water, give a fixed alkali.
It is observable, that in the distillation of plants which yield an acid and a volatile alkali, these two salts are often found quite distinct and separate in the same receiver; which seems very extraordinary, considering that they are naturally[Pg 106] disposed to unite, and have a great affinity with one another. The reason of this phenomenon is, that they are both combined with much oil, which embarrasses them so that they cannot unite to form a neutral salt, as they would not fail to do were it not for that impediment.
All vegetables, except such as yield a great deal of volatile alkali, being burnt in an open fire, and so as to flame, leave in their ashes a large quantity of an acrid, caustic, fixed alkali. But if care be taken to smother them, so as to prevent their flaming while they burn, by covering them with something that may continually beat down again what exhales, the salt obtained from their ashes will be much less acrid and caustic; the cause whereof is, that some part of the acid and oil of the plant being detained in the burning, and stopped from being dissipated by the fire, combines with its alkali. These salts crystallize, and, being much milder than the common fixed alkalis, may be used in medicine, and taken internally. They are called Tachenius's Salts, because invented by that Chymist.
Marine plants yield a fixed alkali analogous to that of sea-salt. As for all other plants or vegetable substances, the fixed alkalis obtained from them, if rightly prepared and thoroughly calcined, are all perfectly alike, and of the very same nature.
The last observation I have to make on the production of fixed alkalis is, that if the plant you intend to work upon be steeped or boiled in water before you burn it, a much smaller quantity of salt will be obtained from it; nay, it will yield none at all, if repeated boilings have robbed it entirely of those saline particles which must necessarily concur with its earth to form a fixed Alkali.
The Analysis of Animal Substances.
Succulent animal substances, such as new-killed flesh, yield by expression a juice or liquid, which is no other than the phlegm, replete with all the principles of the animal body, except the earth, of which it contains but little. The hard or dry parts, such as the horns, bones, &c. yield a similar juice, by boiling them in water. These juices become thick, like a glue or jelly, when their watery parts are evaporated; and, in this state, they are truly extracts of animal matters. These juices afford no crystals of essential salt,[Pg 107] like those obtained from vegetables, and shew no sign either of an acid or an alkali.
Great part of the oil which is in the flesh of animals may be easily separated without the help of fire; for it lies in a manner by itself: it is commonly in a concrete form, and is called Fat. This oil somewhat resembles the fat oils of vegetables; for like them it is mild, unctuous, indissoluble in spirit of wine, and is subtilized and attenuated by the action of fire. But there is not in animals, as in vegetables, any light essential oil, which rises with the heat of boiling water; so that, properly speaking, animals contain but one sort of oil.
Few animal substances yield a perceptible acid. Ants and bees are almost the only ones from which any can be obtained: and indeed the quantity they yield is very small, as the acid itself is extremely weak.
The reason thereof is, that as animals do not draw their nourishment immediately from the earth, but feed wholly either on vegetables or on the flesh of other animals, the mineral acids, which have already undergone a great change by the union contracted between them and the oily matters of the vegetable kingdom, enter into a closer union and combination with these oily parts while they are passing through the organs and strainers of animals; whereby their properties are destroyed, or at least so impaired, that they are no longer sensible.
Animal matters yield in distillation, first, a phlegm, and then, on increasing the fire, a pretty clear oil, which gradually becomes thicker, blacker, more fetid, and empyreumatic. It is accompanied with a great deal of volatile alkali; and if the fire be raised and kept up till nothing more comes over, there will remain in the distilling vessel a coal like that of vegetables; except that when it is reduced to ashes, no fixed alkali, or at least very little, can be obtained from them, as from the ashes of vegetables. This arises from hence, that, as we said before, the saline principle in animals being more intimately united with the oil than it is in plants, and being consequently more attenuated and subtilized, is too volatile to enter into the combination of a fixed alkali; on the contrary, it is more disposed to join in forming a volatile alkali, which on this occasion does not rise till after the oil, and therefore must certainly be the production of the fire. It must be observed, that all we have hitherto said concerning the analysis of bodies must be under[Pg 108]stood of such matters only as have not undergone any sort of Fermentation.
The chyle and milk of animals which feed on plants still retain some likeness to vegetables; because the principles of which these liquors are composed have not gone through all the changes which they must suffer before they enter into the animal combination.
Urine and sweat are excrementitious aqueous liquors, loaded chiefly with the saline particles which are of no service towards the nourishment of the animal, but pass through its strainers without receiving any alteration; such as the neutral salts which have a fixed alkali for their basis, and particularly the sea-salt, which happens to be in the food of animals, whether it exist therein naturally, as it does in some plants, or whether the animals eat it to please their palates.
The saliva, the pancreatic juice, and especially the bile, are saponaceous liquors, that is, they consist of saline and oily particles combined together: so that being themselves dissolved in an aqueous liquor, they are capable of dissolving likewise the oily parts, and of rendering them miscible with water.
Lastly, the blood being the receptacle of all these liquors partakes of the nature of each, more or less in proportion to the quantity thereof which it contains.
The Analysis of Mineral Substances.
Minerals differ greatly from vegetables, and from animals; they are not near so complex as those organized bodies, and their principles are much more simple; whence it follows, that these principles are much more closely connected, and that they cannot be separated without the help of fire; which not having on their parts the same action and the same power as on organized bodies, hath not the same ill effect on them; I mean the effect of changing their principles, or even destroying them entirely.
I do not here speak of pure, vitrifiable, or refractory earths; of mere metals and semi-metals; of pure acids; or even of their simplest combinations, such as sulphur, vitriol, alum, sea-salt: of all these we have said enough.
We are now to treat of bodies that are more complex, and therefore more susceptible of decomposition. These bodies[Pg 109] are compound masses, or combinations of those above-mentioned; that is, metallic substances as they are found in the bowels of the earth, united with several sorts of sand, stones, earths, semi-metals, sulphur, &c. When the metallic matter is combined with other matters, in such a proportion to the rest that it may be separated from them with advantage and profit, these compounds are called Ores; when the case is otherwise, they are called Pyrites, and Marcasites; especially if sulphur or arsenic be predominant therein, which often happens.
In order to analyse an ore, and get out of it the metal it contains, the first step is to free it from a great deal of earth and stones, which commonly adhere to it very slightly and superficially. This is effected by pounding the ore, and then washing it in water; to the bottom of which the metalline parts presently sink, as being the heaviest, while the small particles of earth and stone remain suspended some time longer.
Thus the metallic part is left combined with such matters only as are most intimately complicated with it. These substances are most commonly sulphur and arsenic. Now, as they are much more volatile than other mineral matters, they may be dissipated in vapours, or the sulphur may be consumed, by exposing the ore which contains them to a proper degree of heat. If the sulphur and arsenic be desired by themselves, the fumes thereof may be catched and collected in proper vessels and places. This operation is called Roasting an Ore.
The metal thus depurated is now fit to be exposed to a greater force of fire, capable of melting it.
On this occasion the semi-metals and the imperfect metals require the addition of some matter abounding in phlogiston, particularly charcoal-dust; because these metallic substances lose their phlogiston by the action of the fire, or of the fluxes joined with them, and therefore without this precaution would never acquire either the splendour or the ductility of a metal. By this means the metallic substance is more accurately separated from the earthy and stony parts, of which some portion always remains combined therewith till it is brought to fusion. For, as we observed before, a metallic glass or calx only will contract an union with such matters; a metal possessed of its phlogiston and metalline form being utterly incapable thereof.
We took notice of the cause of this separation above, where we shewed that a metal possessed of its phlogiston and metal[Pg 110]line form will not remain intimately united with any calcined or vitrified matter, not even with its own calx or glass.
The metal therefore on this occasion gathers into a mass, and lies at the bottom of the vessel, as being most ponderous; while the heterogeneous matters float upon it in the form of a glass, or a semi-vitrification. These floating matters take the name of Scoriæ, and the metalline substance at bottom is called the Regulus.
It frequently happens, that the metalline regulus thus precipitated is itself a compound of several metals mixed together, which are afterwards to be separated. We cannot at present enter into a detail of the operations necessary for that purpose: they will appear in our Treatise of Practical Chymistry: but the principles on which they are founded may be deduced from what we have said above, concerning the properties of the several metals and of acids.
It is proper to observe, before we quit this subject, that the rules here laid down for analysing ores are not absolutely general: for example, it is often adviseable to roast the ore before you wash it; for by that means some ores are opened, attenuated, and made very friable, which would cost much trouble and expence, on account of their excessive hardness, if you should attempt to pound them without a previous torrefaction.
It is also frequently necessary to separate the ore from part only of its stone; sometimes to leave the whole; and sometimes to add more to it, before you smelt it. This depends on the quality of the stone, which always helps to promote fusion when it is in its own nature fusible and vitrifiable. It is then called the Fluor of the ore: but of this we must say, as we did of the preceding article, it is sufficient for our present purpose to lay down the fundamental principles on which the reason of every process is built; the description of the operations themselves being reserved for our second Part.
We shall now give a succinct account of the principal ores and mineral bodies, contenting ourselves with just pointing out the particulars of which they severally consist.
Of the Pyrites.
The yellow Pyrites.
The yellow Pyrites is a mineral consisting of sulphur, iron, an unmetallic earth, and frequently a little copper:[Pg 111] the sulphur, which is the only one of these principles that is volatile, may be separated from the rest by sublimation: it usually makes a fourth, and sometimes a third, of the whole weight of these Pyrites. The other principles are separated from one another by fusion and reduction with the phlogiston, which, by metallizing the ferruginous and cupreous earths, parts them from the unmetallic earth: for this earth vitrifies, and cannot afterwards continue united with metallic matters possessed of their metalline form, as hath been said before.
There is yet another way of decomposing the yellow Pyrites, which is to let it ly till it effloresces, or begins to shoot into flowers; which is nothing but a sort of slow accension of the sulphur it contains. The sulphur being by this means decomposed, its acid unites with the ferruginous and cupreous parts of the Pyrites, and therewith forms green and blue vitriols; which may be extracted by steeping in water the Pyrites which has effloresced or been burnt, and then evaporating the lixivium to a pellicle; for by this means the vitriol will shoot into crystals.
Sometimes the Pyrites contains also an earth of the same nature with that of alum; a Pyrites of this sort, after flowering, yields alum as well as vitriol.
The white Pyrites.
The white Pyrites contains much arsenic, a ferruginous earth, and an unmetallic earth. The arsenic, being a volatile principle, may be separated by sublimation or distillation from the rest, which are fixed: and these again may be disjoined from each other by fusion and reduction, as was said in relation to the yellow Pyrites.
The Copper Pyrites.
The Copper Pyrites contains sulphur, copper, and an unmetallic earth. A great deal thereof likewise holds arsenic, and its colour approaches more or less to orange, yellow, or white, according to the quantity of arsenic in it. It may be decomposed by the same means as the yellow and white Pyrites.
Of Ores.
Of Gold Ores.
Gold being constantly found in its metalline form, and never combined with sulphur and arsenic, its matrices are not, properly speaking, ores; because the metal contained in them is not mineralized. The gold is only lodged between particles of stone, earth, or sand, from which it is easily separated by lotion, and by amalgamation with quick-silver. The gold thus found is seldom pure, but is frequently alloyed with more or less silver, from which it is to be separated by quartation.
It is also very common to find gold in most ores of other metals or semi-metals, and even in the Pyrites; but the quantity contained therein is generally so small, that it would not pay the cost of extracting it. However, if any should incline to attempt it, merely out of curiosity, it would be necessary to begin with treating these ores in the manner proper for separating their metalline part; then to cupel the metalline regulus so obtained; and, lastly, to refine it by quartation.
Of Silver Ores.
It is no rare thing to find silver, as well as gold, in its metalline form, only lodged in sundry earths and stony matters, from which it may be separated in the same manner as gold. But the greatest quantities of this metal are usually dug out of the bowels of the earth in a truly mineral state: that is, combined with different substances, and particularly with sulphur and arsenic.
Several silver ores are distinguished by peculiar characteristics, and are accordingly denoted by particular names. That which is called the Vitreous Silver Ore, is scarce any thing else but a combination of silver and sulphur. Another is known by the name of the Horny Silver Ore, because when in thin plates it is semi-transparent: in this ore the silver is mineralized by sulphur and a little arsenic. The Red Silver Ore is of the colour which its name imports, sometimes more, sometimes less vivid; and is chiefly composed of silver, arsenic, and sulphur: it also contains a little iron.
These three ores are very rich in silver: the first contains nearly three fourths of its weight, and the others about two thirds of theirs.
There is a fourth, called the White Silver Ore, which, though it be heavier, is not so rich in silver, because it contains much copper. Many other minerals contain silver, yet are not, properly speaking, silver ores; because a much greater quantity of other metals than of silver is found in them.
When a silver ore is to be decomposed, in order to have the silver pure, or when silver is to be extracted out of any ore that contains it, the first thing to be done is to roast the ore, in order to clear it of the volatile minerals: and as silver cannot be had pure without the operation of the cupel, which requires more or less lead to be joined with it, it is usual to mix with the torrified silver ore a quantity of lead, proportioned to that of the heterogeneous matters combined with the silver, and to melt the whole together. Part of the added lead vitrifies during the fusion, and at the same time converts some of the heterogeneous matters also into glass, with which it forms a scoria that rises to the surface of the matter. The other part of the lead, with which the silver is mixed, falls to the bottom in the form of a regulus, which must be cupelled in order to have the silver pure.
Of Copper Ores.
Copper is much seldomer found in a metalline form than gold or silver: it is commonly in a mineral state: it is mineralized by sulphur and arsenic: almost all its ores contain also more or less of iron; sometimes a little silver, or even gold, together with unmetallic earths and stones, as all ores do.
Most copper ores are of a beautiful green or blue, or else in shades blended of these two colours. The minerals called mountain green, and mountain blue, are true copper ores; not in the form of hard stones, like other ores, but crumbly and friable like earth.
Nevertheless, there are several copper ores of different colours, as ash-coloured, whitish, and shaded with yellow or orange; which colours arise from the different proportions of arsenic, sulphur, and iron, which these ores contain.
In order to decompose a copper ore, and to extract the copper it contains, it is first of all to be freed from as many of its earthy, stony, sulphureous, and arsenical parts, as is possible, by roasting and washing; then what remains is to be mixed with a flux, compounded of a fixed alkali and some inflammable matter; a little sea-salt is to be put over all, and[Pg 114] the whole melted by a strong fire. The salts facilitate the fusion and scorification of the unmetallic matters, and therewith form a slag, which being the lightest rises to the surface. The metalline matters are collected below in the form of a shining regulus of copper; which, however, is not usually fine copper, but requires to be purified in the manner to be shewn in our second part.
In order to separate the copper from the unmetallic matters, it is absolutely necessary to melt its ore along with inflammable substances abounding in phlogiston. For, as this metal is not possessed of its metalline form while it is in a mineral state, as it is destitute of the true quantity of phlogiston, and, though it were not, would lose it by the action of the fire, it would come to pass, that if its ore were melted without the addition of any inflammable matter, the cupreous earth or calx would be scorified and confounded with the unmetallic matters; and as all metallic matters, except gold and silver, are subject to this inconvenience as well as copper, the addition of an inflammable substance, in fluxing all ores that contain them, is a general rule that ought constantly to be observed.
Of Iron Ores.
Iron is seldom found pure and malleable in the earth; yet it is much seldomer found in the mineral state, properly so called, than any of the other metals: for most iron ores are scarce any thing more than a ferruginous earth mixed in different proportions with unmetallic earths and stones. Some of them, however, contain also volatile minerals, such as sulphur and arsenic; and therefore it is necessary to roast the iron ores, like all others, before you attempt to extract the metal out of them. That being done, they are to be smelted with a flux consisting of fusible and inflammable matters, as the general rule directs.
Iron is the commonest of all metals: nay, it is so universally diffused through the earth, that it is difficult to find any stone, earth, or sand, that does not contain some of it; and therefore none of these are usually considered and treated as iron ores, except such as contain a great deal of that metal, and melt easily. The hematites, emery, yellow pyrites, calamine, all contain a pretty considerable quantity of iron; but no body attempts to extract it from them, because they are very hard to melt.
Ferruginous earth being naturally of an orange colour, a stone or earth may be judged to contain iron, if either naturally, or after roasting, it appears to have one shade of yellow or red.
The singular property which iron has of being attracted by the magnet, and of being the only body, exclusive of all others, that is so, likewise affords us an easy method of discovering the presence of this metal among other matters, where it often exists in such a small quantity that it could not otherwise be found out. For this purpose the body in which iron is suspected to lurk, must be pulverised and torrefied with some inflammable matter; and then the powder thus roasted being touched with a magnet, or an animated bar, if it contains any particles of iron they will infallibly adhere to the magnet or bar.
Of Tin Ores.
Tin is never found in the earth pure and malleable, but always in a mineral state, and always mineralized by arsenic. Tin ores are not sulphureous; whence it comes, that though tin be the lightest of all metals, its ores are nevertheless heavier than those of other metals, as arsenic greatly exceeds sulphur in gravity. Some tin ores contain also a little iron. The ores of tin are to be washed, roasted, and smelted with a reducing flux, according to the general rules.
Of Lead Ores.
Lead, like tin, is never found but in a mineral state. It is most commonly mineralized by sulphur; yet there are some lead ores which also contain arsenic.
Lead ores, as well as others, must be roasted and smelted with a reducing flux: however, as it is difficult to free them from all their sulphur by torrefaction only, the reducing flux employed in their fusion may be made up with a quantity of iron filings, which being incapable of any union with lead, and having a much greater affinity than that metal with sulphur, will, on this occasion, be of great service by interposing between them.
Of Quick-silver Ores.
Running Mercury is sometimes found in certain earths, or grey, friable stones; but most commonly in a mineral[Pg 116] state. It is always mineralized by sulphur, and by sulphur alone: so that cinabar is the only ore of quick-silver that we know of; and a very rich one it is, seeing it contains six or seven times as much mercury as sulphur.
Roasting can be of no use towards decomposing the ore of mercury, and separating its sulphur; because mercury being itself very volatile would be carried off by the fire together with the sulphur. In order, therefore, to part the two substances of which cinabar consists, recourse must necessarily be had to some third body, which will unite with one of them, and by that means separate it from the other. Now all the metals, except gold, having a greater affinity than mercury with sulphur, such a body is easily found: any metal but gold may be employed with success in this decomposition; but as iron hath a greater affinity with sulphur than any of the rest, and is moreover the only one that cannot unite with mercury, it must, on account of these two qualities, be preferred to all the rest.
Fixed alkalis are also well qualified to absorb the sulphur of cinabar. Cinabar must be decomposed in close vessels, and by the way of distillation; otherwise the mercury, as soon as it separates from the sulphur, will be dissipated in vapours and entirely lost.
In this operation it is needless to add either flux or phlogiston; because the cinabar is decomposed without melting, and the mercury, though in a mineral state, contains, like gold and silver, all the phlogiston requisite to secure its metalline properties.
Of the Ores of Regulus of Antimony.
Regulus of Antimony is always found in a mineral state: it is mineralized by sulphur; but sometimes, though rarely, it is also combined with a little arsenic.
When the ore of regulus of antimony is to be decomposed, the first thing to be done is to expose it to a degree of heat too weak to melt its earthy and stony parts, but strong enough to fuse its reguline, together with its sulphureous parts, which by this means are separated from the earth, and united into one mass, known by the name of Antimony.
It is plain that this first operation, which is founded on the great fusibility of antimony, produces, with regard to the ore of regulus of antimony, the same effect that washing hath on other ores: so that after this first fusion nothing more is requisite to the obtaining of a pure regulus of antimony, but[Pg 117] to separate it from its sulphur by roasting, and to melt it with some matter abounding in phlogiston, in the same manner as other metallic matters are treated. The term Calcination is generally used to express this torrefaction of antimony, by means whereof the metallic earth of the regulus of antimony is separated from its sulphur.
As regulus of Antimony hath, like Mercury, much less affinity with sulphur than the other metals have, it follows that antimony may be decomposed by the same means as cinabar; but the regulus, so obtained, is adulterated with a portion of the additament made use of, which combines therewith.
There is still another process employed for obtaining the regulus of antimony: it consists, as was mentioned in its place, in detonating the mineral with a mixture of nitre and tartar, applied in such a proportion that, after the detonation has consumed the sulphur, there may remain so much inflammable matter as will be sufficient to furnish the metalline earth of the antimony with the phlogiston necessary to preserve its metallic properties. But by this method less regulus is produced, than by calcining, or torrefying, and reducing as usual.
Of the Ores of Bismuth.
The ore of Bismuth consists of the semi-metal mineralized by arsenic, and of an unmetallic earth. It is very easy to decompose this ore, and to extract the bismuth it contains: for this purpose it need only be exposed to a moderate heat, whereby the arsenic will be dissipated in vapours, and the bismuth melted, which will then separate from the unmetallic earth. This earth, at least, in several ores of bismuth, possesses the property of tinging all vitrifiable matters, with which it is melted, of a beautiful blue colour.
To decompose the ore of bismuth no flux or inflammable matter is used; because this semi-metal is possessed, even in its mineral state, of all the phlogiston requisite to maintain its metalline properties; and its great fusibility makes it unnecessary to melt the unmetallic earth contained in its ore.
Of the Ores of Zinc.
Zinc is not generally obtained from a particular ore of its own; but sublimes during the fusion of a mineral, or rather[Pg 118] a confused mass of minerals, that contains this semi-metal together with iron, copper, lead, sulphur, arsenic, and, like all other ores, an unmetallic earth.
Nevertheless, there is a substance which may be considered as the proper ore of zinc, because it contains a pretty large quantity of that semi-metal, a little iron, and an unmetallic earth. It is called Calamine, or Lapis Calaminaris; but hitherto the art of procuring zinc directly from this mineral hath no where been practised. Calamine is commonly employed only to convert copper into brass, or a yellow metal, by cementing it therewith. Indeed, till lately, no easy or practicable method of obtaining pure zinc from calamine was publicly known; for that semi-metal being volatile and very inflammable, its ore cannot be fused like others. Mr. Margraaf was the first who, by mixing powdered charcoal with calamine in close vessels, obtained a perfect zinc from it, by the means of distillation or sublimation, as shall be shewn in our Practical Chymistry.
Of Arsenical Minerals.
Arsenic, as well as sulphur, is naturally combined with almost all ores, or minerals containing metallic substances. As it is very volatile, while the matters with which it is united are fixed, at least in comparison therewith, it is easily separated by sublimation.
The minerals that contain most arsenic are the white pyrites, orpiment, and cobalt. We have already considered the white pyrites: as to orpiment, it consists of sulphur and arsenic. Both these substances being very volatile, it is difficult to separate them by sublimation: yet, with proper management, and a due regulation of the fire, this separation may be effected; because sulphur sublimes a little more easily than arsenic. But it is more convenient, as well as more expeditious, to make use of some additament that hath a greater affinity with one of those substances than with the other. Fixed alkalis and mercury, both of which have more affinity with sulphur than with arsenic, may be very properly employed on this occasion.
Cobalt is a mineral composed of arsenic, an unmetallic earth, and frequently bismuth: and as none of these are very volatile, except the arsenic, this may be easily separated from the rest by sublimation. The unmetallic earth which remains has, like that of the ore of bismuth, the property of giving a blue colour to any vitrifiable matters melted with[Pg 119] it; whence it is conjectured, that cobalt and the ore of bismuth have a great resemblance, or are often blended with each other. Nevertheless, Mr. Brant, an ingenious Swedish Chymist, insists that they are very different: he pretends that the metallic substance contained in the true cobalt is a semi-metal of a peculiar nature, which hath been erroneously confounded with bismuth: and indeed he proves by a great number of curious experiments, related in the Memoirs of the Academy of Upsal, that these two metallic substances have properties that are essentially different: to that which is obtained from cobalt, he gives the name of Regulus of Cobalt.
Besides the minerals already recited, there is found in the bowels of the earth another species of compound body, of which we have already taken notice; but which is supposed, with some degree of probability, to belong as much to the vegetable as to the mineral kingdom: I mean the Bitumens; which the best observations oblige us to consider as vegetable oils, that by lying long in the earth have contracted an union with the mineral acids, and by that means acquired the thickness, consistence, and other properties observable in them.
By distillation they yield an oil, and an acid not unlike a mineral acid. Mr. Bourdelin has even demonstrated, by a very artful and ingenious process, that amber contains a manifest acid of sea-salt. See the Memoirs of the Royal Academy of Sciences.
Explanation of the Table of Affinities.
It hath been shewn in the course of this work, that the causes of almost all the phenomena, which Chymistry exhibits, are deducible from the mutual affinities of different substances, especially the simplest. We have already explained (Chap. II.) what is meant by affinities, and have laid down the principal laws to which the relations of different bodies are subject. The late Mr. Geoffroy, one of the best Chymists we have had, being convinced of the advantages which all who cultivate Chymistry would receive from having constantly before their eyes a state of the best ascertained[Pg 120] relations between the chief agents in Chymistry, was the first who undertook to reduce them into order, and unite them all in one point of view, by means of a table. We are of opinion, with that great man, that this Table will be of considerable use to such as are beginning to study Chymistry, in helping them to form a just idea of the relations which different substances have with one another; and that the practical Chymist will thereby be enabled to account for what passes in several of his operations, otherwise difficult to be understood, as well as to judge what may be expected to result from mixtures of different compounds. These reasons have induced us to insert it at the end of this Elementary Treatise, and to give a short explanation of it here; especially as it will serve, at the same time, for a recapitulation of the whole work, in which the several axioms of this Table are dispersed.
You have it here just as it was drawn up by Mr. Geoffroy, without any addition or alteration. I own, however, that it might be improved both ways: for since the death of that great Chymist many experiments have been made, some of which have discovered new affinities, and others have raised exceptions to some of those laid down by him. But several reasons dissuade me from publishing a new Table of Affinities, containing all the emendations and innovations that might be made in the old one.
The first is, that many of the affinities lately discovered are not yet sufficiently verified, but, on the contrary, subject to be contested: in short, they are perhaps liable to more considerable objections, and exceptions, than the other.
The second is, that as Mr. Geoffroy's Table contains all the fundamental affinities, it is more suitable to an Elementary Treatise than a much fuller one would be; seeing this would necessarily suppose the knowledge of many things not treated of by us, and of which it was not proper to say any thing in such a book as this.
However, as it is essential to our purpose that we lead none into error, we shall take care in explaining the affinities delivered by Mr. Geoffroy, to mention the principal objections and exceptions to which they are liable: we shall, moreover, add a very few new ones, confining ourselves to such only as are elementary and well ascertained.
The upper line of Mr. Geoffroy's Table, comprehends several substances used in Chymistry. Under each of those substances are ranged in distinct columns several matters compared with them, in the order of their relation to[Pg 121] that first substance; so as that which is the nearest to it is that which hath the greatest affinity with it, or that which none of the substances standing below it can separate therefrom; but which, on the contrary, separates them all when they are combined with it, and expels them in order to join itself therewith. The same is to be understood of that which occupies the second place of affinity; that is, it has the same property with regard to all below it, yielding only to that which is above it: and so of all the rest.
At the top of the first column stands the character which denotes an Acid in general. Immediately under this stands the mark of a Fixed Alkali, being placed there as the substance which has the greatest affinity with an Acid. After the Fixed Alkali appears the Volatile Alkali, whose affinity with Acids yields only to the Fixed Alkali. Next come the Absorbent Earths; and last of all Metallic Substances. Hence it follows, that when a Fixed Alkali is united with an acid it cannot be separated therefrom by any other substance; that a Volatile Alkali united with an Acid cannot be separated from it by any thing but a Fixed Alkali; that an Absorbent Earth combined with an acid may be separated from it either by a Fixed or by a Volatile Alkali; and lastly, that any Metallic Substance combined with an Acid may be separated from it by a Fixed Alkali, a Volatile Alkali, or an Absorbent Earth.
There are many important remarks to be made on this first column. First, it is making the rule too general to say that any Acid whatever has a greater affinity with a Fixed Alkali, than with any other substance. And indeed Mr. Geoffroy himself hath made an exception with respect to the Vitriolic Acid; for in the fourth column, at the head of which stands that Acid, we find the sign of the Phlogiston placed above that of the Fixed Alkali, as having a greater affinity than the Fixed Alkali with the Vitriolic Acid. This is founded on the famous experiment, wherein Vitriolated Tartar and Glauber's Salt are decompounded by means of the Phlogiston, which separates the Fixed Alkalis of these Neutral Salts, and uniting with the Vitriolic Acid contained in them forms therewith a Sulphur.
Secondly, Nitre deflagrates, and is decomposed, by the contact of any inflammable matter whatever that is actually ignited; and the operation which produces Phosphorus is no other than a decomposition of sea-salt, whose Acid quits its Alkaline basis to join with the Phlogiston: now these facts furnish very strong reasons for believing that both these[Pg 122] Acids, as well as the Vitriolic, have a stronger affinity with the Phlogiston than with a Fixed Alkali. Lastly, as several experiments shew the Vegetable Acids to be only the Mineral Acids disguised and mortified, there are sufficient grounds for suspecting that Acids in general have a greater affinity with the Phlogiston than with Fixed Alkalis: so that instead of making an exception with regard to the Vitriolic Acid, it would perhaps be better to lay down this greater affinity as common to all Acids whatever, and to place the Phlogiston in the first column, immediately under the character which denotes an Acid in general. This theory, however, stands in need of confirmation from other experiments[4].
Thirdly, in this same column the character of a Volatile Alkali is set above that of an Absorbent Earth, as having a greater affinity with Acids; and yet these Absorbent Earths decompose the Ammoniacal salts, drive away the Volatile Alkali from the Acids, and assume its place. This is one of the first objections made against Mr. Geoffroy's Table. His answer thereto is printed in the Memoirs of the Academy of Sciences for 1718, where his Table also is to be found. We have already declared our opinion about this matter in treating of a Volatile Alkali.
Fourthly, in 1744, Mr. Geoffroy, brother to the author of the Table, who hath done no less honour to Chymistry than that eminent physician, gave in a Memoir containing an exception to the last affinity in the first column; namely, that which places Absorbent Earths above Metallic substances. He therein shews, that Alum may be converted into Copperas by boiling it in iron vessels; that, on this occasion, the iron precipitates the Earth of the Alum, separates it from its Acid, and assumes its place; so that of course it must have a greater affinity, than the Absorbent Earth of Alum, with the Vitriolic Acid.
At the head of the second column stands the character of the Marine Acid, which signifies that the affinities of this Acid are the subject of the column. Immediately below it is placed the mark of Tin. As this is a metalline substance, and as the first column places metalline substances in the[Pg 123] lowest degree of affinity with all Acids, it is plain we must suppose Fixed Alkalis, Volatile Alkalis, and Absorbent Earths, to be placed here in order after the Marine Acid, and before Tin. Tin, then, is of all Metalline substances that which has the greatest affinity with the Marine Acid; and then follow Regulus of Antimony, Copper, Silver, Mercury. Gold comes last of all; and there are no less than two vacant places above it. By this means it is in some sort excluded from the rank of substances that have an affinity with the Marine Acid. The reason thereof is, that this Acid alone is not capable of dissolving Gold and combining therewith, necessarily requiring for that purpose the aid of the Nitrous Acid, or at least of the Phlogiston.
The third column exhibits the affinities of the Nitrous Acid, the character whereof stands at its head. Immediately below it is the sign of Iron, as the metal which has the greatest affinity with this Acid; and then follow other metals, each according to the degree of its relation; to wit, Copper, Lead, Mercury, and Silver. In this column, as in the preceding one, we must suppose the substances, which in the first column stand above Metallic substances, to be placed in their proper order before Iron.
The fourth column is intended to represent the Affinities of the Vitriolic Acid. Here Mr. Geoffroy has placed the Phlogiston as the substance which has the greatest affinity with this Acid, for the reason given in our explanation of the first column. Below it he has ranked Fixed Alkalis, Volatile Alkalis, and Absorbent Earths, to shew that this is an exception to the first column. As to Metalline substances, he has set down but three, being those with which the Vitriolic Acid has the most perceptible affinity: these metals, placed in the order of their affinities, are Iron, Copper, and Silver.
The fifth column shews the affinities of Absorbent Earths. As these Earths have no sensible affinity but with Acids, this column contains only the characters of the Acids ranked according to the degree of their strength, or affinity with the Earths; to wit, the Vitriolic, the Nitrous, and the Marine Acids. Underneath this last might be placed the Acid of Vinegar, or the Vegetable Acid.
The sixth column expresses the Affinities of Fixed Alkalis with Acids, which are the same with those of Absorbent Earths. Moreover, we find Sulphur placed here below all the Acids; because Liver of Sulphur, which is a combination of Sulphur with a Fixed Alkali, is actually decom[Pg 124]pounded by any Acid: for any Acid precipitates the Sulphur and unites with the Alkali.
Immediately over the Sulphur, or in the same square with it, might be set a mark denoting the Volatile Sulphureous Spirit; because, like Sulphur, it has less affinity than any other Acid with Fixed Alkalis. Oils might also be ranked with Sulphur, because they unite with Fixed Alkalis, and therewith form Soaps, which are decompounded by any acid whatever.
The seventh column points out the affinities of Volatile Alkalis, which are likewise the same as those of Absorbent Earths; and the Vegetable Acid might be placed here also under the Marine Acid.
The eighth column specifies the affinities of Metallic substances with Acids. The affinities of the Acids, which, with respect to Fixed Alkalis, Volatile Alkalis, and Absorbent Earths, succeeded each other uniformly, do not appear in the same order here. The Marine Acid, instead of being placed below the Vitriolic and Nitrous Acids, stands, on the contrary, at their head; because, in fact, this Acid separates Metalline substances from all the other Acids with which they happen to be united, and, forcing these Acids to quit possession, intrudes into their place. Nevertheless, this is not a general rule; for several Metalline substances must be excepted, particularly Iron and Copper.
The ninth column declares the affinities of Sulphur. Fixed Alkalis, Iron, Copper, Lead, Silver, Regulus of Antimony, Mercury, and Gold, stand below it in the order of their affinities. With regard to Gold it must be observed, that it will not unite with pure Sulphur: it suffers itself to be dissolved only by the Liver of Sulphur, which is known to be a composition of Sulphur and Fixed Alkali.
At the head of the tenth column appears Mercury, and beneath it several Metalline substances, in the order of their affinities with it. Those Metalline substances are Gold, Silver, Lead, Copper, Zinc, and Regulus of Antimony.
It is proper to remark on this column, that Regulus of Antimony, which stands the lowest, unites but very imperfectly with Mercury; and that after a seeming union of these two Metallic substances hath been obtained, by a tedious triture with the addition of water, they do not continue long united, but spontaneously separate from each other in a short time. Iron and Tin are here excluded; the former with great reason, because hitherto it hath not been clearly proved, by any known experiment, that ever Mercury was united[Pg 125] with Iron: but the same objection cannot be made to Tin, which amalgamates very well with Mercury, and might therefore be placed in this column nearly between Lead and Copper. I use the word nearly, because the different degrees of affinity between Metalline substances and Mercury are not so exactly determined, as the other relations before considered; seeing they generally unite with it, without excluding one another. We can therefore scarce judge of the degree of affinity that belongs to each, but by the greater or less readiness of each to amalgamate therewith.
The eleventh column shews, that Lead has a greater affinity with Silver than with Copper.
The twelfth, that Copper has a greater affinity with Mercury than with Calamine.
The thirteenth, that Silver has a greater affinity with Lead than with Copper.
The fourteenth contains the affinities of Iron. Regulus of Antimony stands immediately underneath it, as being the Metallic substance which has the greatest affinity with it. Silver, Copper, and Lead, are placed together in the next square below, because the degrees of affinity which those metals have with Iron are not exactly determined.
The same is to be said of the fifteenth column: Regulus of Antimony stands at its head; Iron is immediately below it; and below the Iron the same three metals occupy one square as before.
Lastly, the sixteenth column indicates that Water has a greater affinity with Spirit of Wine than with Salts. By this general expression must not be understood any Saline substance whatever; but only the Neutral Salts, which Spirit of Wine frees from the water that kept them in solution. Fixed Alkalis, on the contrary, as well as the Mineral Acids, have a greater affinity than Spirit of Wine with water: so that these Saline substances, being well dephlegmated, and mixed with Spirit of Wine; imbibe the water it contains and rectify it.
To these might be added another short column, having Spirit of Wine at its head: immediately below it should be the character of Water, and below that the mark of Oil. This column would shew that the Spirit of Wine has a greater affinity with Water than with Oils; because any Oily matter whatever, that is dissolved in Spirit of Wine, may be actually separated from it by the affusion of Water. This rule admits of no exception but in one case; which is when the oily substance partakes of the nature of soap, by having[Pg 126] contracted an union with some saline matter. But as this must be imputed wholly to that adventitious saline matter being superadded to the oily substance, it is no just foundation for an exception, and the affinity in question is nevertheless general.
We have now delivered every thing material that we had to say concerning Mr. Geoffroy's Table of Affinities. It is, as we observed before, of exceeding great service, as it collects into one view the principal truths laid down in this Treatise. Indeed the most advantageous way of using it is, not to delay consulting it till you have read the book through, but to turn to it while you are reading, as oft as any affinity between bodies is treated of; which it will imprint more strongly on your mind, by representing it in a manner before your eyes.
The Theory of Constructing the Vessels most commonly used in Chymistry.
Chymists cannot perform the operations of their art without the help of a considerable number of vessels, instruments, and furnaces, adapted to contain the bodies on which they intend to work, and to apply to them the several degrees of heat required by different processes. It is therefore proper, before we advance to the operations themselves, to consider particularly and minutely what relates to the instruments with which they are to be performed.
Vessels intended for Chymical Operations should, to be perfect, be able to bear, without breaking, the sudden application of great heat and great cold; be impenetrable to every thing, and unalterable by any solvent; unvitrifiable, and capable of enduring the most violent fire without melting: but hitherto no vessels have been found with all these qualities united.
They are made of sundry materials; namely, of metal, of glass, and of earth. Metalline vessels, especially those made of Iron or Copper, are apt to be corroded by almost every saline, oily, or even aqueous substance. For this reason, in order to render the use of them a little more extensive, they are tinned on the inside. But, notwithstanding[Pg 127] this precaution, they are on many occasions not to be trusted; and should never be employed in any nice operations which require great accuracy: they are, moreover, incapable of resisting the force of fire.
Earthen vessels are of several sorts. Some, that are made of a refractory earth, are capable of being suddenly exposed to a strong fire without breaking, and even of sustaining a great degree of heat for a considerable time: but they generally suffer the vapours of the matters which they contain, as well as vitrified metals, to pass through them, especially the glass of lead, which easily penetrates them and runs through their pores as through a sieve. There are others made of an earth that, when well baked, looks as if it were half vitrified: these being much less porous, are capable of retaining the vapours of the matters which they contain, and even glass of lead in fusion; which is one of the severest trials a vessel can be put to: but then they are more brittle than the other sort.
Good glass vessels should constantly be employed in preference to all others, whenever they can possibly be used: and that not only because they are no way injured by the most active solvents, nor suffer any part of what they contain to pass through, but also because their transparency allows the Chymist to observe what passes within them: which is always both curious and useful. But it is pity that vessels of this sort should not be able to endure a fierce fire without melting. We shall take care, when we come to describe the several sorts of chymical instruments, and the manner of using them, to note what vessels are to be preferred to others on different occasions.
Distillation, as hath been already said, is an operation by which we separate from a body, by the help of a gradual heat, the several principles of which it consists.
There are three methods of distilling. The first is performed by applying the heat over the body whose principles are to be extracted. In this case, as the liquors, when heated and converted into vapours, constantly endeavour to fly from the center of heat, they are forced to re-unite in the lower part of the vessel, that contains the matter in distillation, and so passing through the pores or holes of that vessel, they fall into another cold vessel applied underneath to receive them. This way of distilling is on this account called distilling per Descensum. It requires no other apparatus than two vessels figured like segments of hollow spheres, whereof that which is pierced with little holes, and intended[Pg 128] to contain the matter to be distilled, should be much less than the other, which is to contain the fire, and to fill its aperture exactly; the whole together to be supported vertically upon a third vessel, which is to serve the purpose of a recipient, admitting into its mouth the convex bottom of the vessel containing the matter to be distilled, which must accurately fill it. This method of distilling is but little used.
The second method of distilling is performed by applying the heat underneath the matter to be decomposed. On this occasion the liquors being heated, rarefied, and converted into vapours, rise, and are condensed in a vessel contrived for that purpose, which we shall presently describe. This way of distilling is called distilling per Ascensum, and is much used.
The vessel in which this distillation per Ascensum is performed we call an Alembic.
There are several sorts thereof, differing from one another both in the matter of which, and the manner in which, they are made.
Those employed to draw the odoriferous waters and essential oils of plants are generally made of copper, and consist of several pieces. The first, which is designed to contain the plant, is formed nearly like a hollow cone, the vertex whereof is drawn out in the shape of a hollow cylinder or tube: this part is named the Cucurbit, and its tube the Neck of the Alembic. To the upper end of this tube another vessel is soldered: this is called the Head, and commonly has likewise the form of a cone, joined to the neck of the alembic by its base, round which, on the inside, is hollowed a small groove, communicating with an orifice that opens at its most depending part. To this orifice is soldered a small pipe in a direction sloping downwards, which is called the Nose, Spout, or Beak of the alembic.
As soon as the matters contained in the alembic grow hot, vapours begin to arise from them, and ascending through the neck of the alembic into the head, are by the sides thereof stopped and condensed: from thence they trickle down in little streams to the groove, which conveys them to the spout; and by that they pass out of the alembic into a glass vessel with a long neck, the end of the spout being introduced into that neck, and luted thereto.
To facilitate the refrigeration and condensation of the vapours circulating in the head, all alembics of metal are moreover provided with another piece, which is a kind of large pan of the same metal, fitted and soldered round the head.[Pg 129] This piece serves to keep cold water in, which incessantly cools the head, and therefore it is called the Refrigeratory. The water in the refrigeratory itself grows hot after some time, and must therefore be changed occasionally; the heated water being first drawn off by means of a cock fixed near the bottom of the refrigeratory. All copper alembics should be tinned on the inside for the reasons already given.
When saline spirits are to be distilled, alembics of metal must not be used; because the saline vapours would corrode them. In this case recourse must be had to alembics of glass. These consist of two pieces only; namely, a Cucurbit, whose superior orifice is admitted into and exactly luted with its Head, which is the second piece.
In general, as alembics require that the vapours of the matter to be distilled should rise to a considerable heighth, they ought to be used only when the most volatile principles are to be drawn from bodies: and the lighter and more volatile the substances to be separated by distillation are, the taller must the alembic be; because the most ponderous parts, being unable to rise above a certain heighth, fall back again into the cucurbit as soon as they arrive there, leaving the lighter to mount alone, whose volatility qualifies them to ascend into the head.
When a matter is to be distilled, that requires a very tall alembic, and yet does not admit of a metalline vessel, the end will be best answered by a glass vessel of a round or oval shape, having a very long neck, with a small head fitted to its extremity. Such a vessel serves many purposes: it is sometimes employed as a receiver, and at other times as a digesting vessel; on which last occasion it goes under the name of a Matrass. When one of these, provided with a head, is applied to the purpose of distilling, it forms a sort of alembic.
There are some alembics of glass, blown in such a manner by the workmen, that the body and head form but one continued piece. As these alembics do not stand in need of having their several pieces luted together, they are very useful on some occasions, when such exceeding subtile vapours rise as are capable of transpiring through lutes. The head must have an aperture at the top, provided with a short tube, through which, by means of a funnel with a long pipe, the matter to be distilled may be introduced into the cucurbit. This is to be exactly closed with a glass stopple, the surface whereof must be made to fit the inside of the tube in every[Pg 130] point, by rubbing those two pieces well together with emery.
Another sort of alembic hath also been invented, which may be used with advantage when Cohobation is required; that is, when the liquor obtained by distillation is to be returned upon the matter in the cucurbit; and especially when it is intended that this cohobation shall be repeated a great number of times. The vessel we are speaking of is constructed exactly in the same manner as that last described; except that its beak, instead of being in a straight line, as in the other alembics, forms a circular arch, and re-enters the cavity of the cucurbit, in order to convey back again the liquor collected in the head. This instrument hath commonly two beaks opposite to each other, both turned in this manner, and is called a Pelican: it saves the artist the trouble of frequently unluting and reluting his vessels, as well as the loss of a great many vapours.
There are certain substances which in distillation afford matters in a concrete form, or rise wholly in the form of a very light powder, called Flowers. When such substances are to be distilled, the cucurbit which contains them is covered with a head without a nose, which is named a Blind-head.
When the flowers rise in great quantities and very high, a number of heads is employed to collect them; or rather a number of a kind of pots, consisting of a body only without any bottom, which fitting one into the other form a canal, that may be lengthened or shortened at pleasure, according as the flowers to be sublimed are more or less volatile. The last of the heads, which terminates the canal, is quite close at one end, and makes a true blind-head. These vessels are called Aludels; they are usually of earthen or stone ware.
All the vessels above-mentioned are fit only for distilling such light volatile matters as can be easily raised and brought over; such as phlegm, essential oils, fragrant waters, acid oily spirits, volatile alkalis, &c. But when the point is to procure by distillation principles that are much less volatile, and incapable of rising high, such as the thick fetid oils, the vitriolic, the nitrous, and the marine acids, &c. we are under a necessity of having recourse to other vessels, and another manner of distilling.
It is easy to imagine, that such a vessel must be much lower than the alembic. It is indeed no more than a hollow globe, whose upper part degenerates into a neck or tube, that[Pg 131] is bent into a horizontal position; for which reason this instrument is called a Retort: it is always of one single piece.
The matter to be distilled is introduced into the body of the retort by means of a ladle with a long tubular shank. Then it is set in a furnace built purposely for this use, and so that the neck of the retort coming out of the furnace may, like the nose of the alembic, stand in a sloping position, to facilitate the egress of the liquors, which by its means are conveyed to a receiver, into which it is introduced, and with which it is luted. This way of distilling, in which the vapours seem rather to be driven out of the vessel horizontally and laterally, than raised up and sublimed, is for that reason called Distillation per Latus.
Retorts are, of all the instruments of distillation, those that must sustain the greatest heat, and resist the strongest solvents; and therefore they must not be made of metal. Some, however, which are made of iron may do well enough on certain occasions: the rest are either of glass or earth. Those of glass, for the reasons above given, are preferable to the other sort, in all cases where they are not to be exposed to such a force of fire as may melt them. The best glass, that which stands both heat and solvents best, is that in which there are fewest alkaline salts. Of this sort is the green German glass: the beautiful white crystal glass is far from being equally serviceable.
Retorts, as well as alembics, may be of different forms. For example, some matters are apt to swell, and rise over the neck of the retort in substance, without suffering any decomposition; when such matters are to be distilled in a retort, it is proper that the body of the vessel, instead of being globular, be drawn out into the form of a pear, so as nearly to resemble that of a cucurbit. In a retort of this kind, the distance between the bottom and the neck being much greater than in those whose bodies are spherical, the matters contained have much more room for expansion; so that the inconvenience here mentioned is thereby prevented. Retorts of this form are called English retorts. As they hold the middle place between alembics and common retorts, they may be used to distil such matters as have a mean degree of volatility between the greatest and the least.
It is moreover proper to have, in a laboratory, sundry retorts with necks of different diameters. Wide necks will be found the fittest for conveying thick matters, and such as readily become fixed; for instance, some very thick fetid oils, butter of antimony, &c.; for as these matters acquire a con[Pg 132]sistence as soon as they are out of the reach of a certain degree of heat, they would soon choak a narrow neck, and by stopping the vapours which rise at the same time from the retort, might occasion the bursting of the vessels.
Some retorts are also made with an opening on their upper side, like that of tubulated glass alembics, which is to be closed in the same manner with a glass stopple. These retorts are also called Tubulated retorts, and ought always to be used whenever it is necessary to introduce fresh matter into the retort during the operation; seeing it may be done by means of this invention, without unluting and reluting the vessels; which ought always to be avoided as much as possible.
One of the things that most perplexes the Chymists, is the prodigious elasticity of many different vapours, which are frequently discharged with impetuosity during the distillation, and are even capable of bursting the vessels with explosion, and with danger to the artist. On such occasions it is absolutely necessary to give these vapours vent, as we shall direct in its proper place: but as that can never be done without losing a great many of them; as some of them in particular are so elastic that scarce any at all would remain in the vessel; for instance, those of the spirit of nitre, and especially those of the smoking spirit of salt; the practice is to make use of very large receivers, of about eighteen or twenty inches diameter, that the vapours may have sufficient room to circulate in, and by applying to the wide surface presented them by the extensive inside of such a large vessel, may be condensed into drops. These huge receivers are commonly in the form of hollow globes, and are called Ballons.
To give these vapours still more room, ballons have been contrived with two open gullets in each, diametrically opposite to one another; whereof one admits the neck of the retort, and the other is received by one of the gullets of a second ballon of the same form, which is joined in like manner to a third, and so on. By this artifice the space may be enlarged at pleasure. These ballons with two necks are called Adopters.
Operations on bodies that are absolutely fixed, as metals, stones, sand, &c. require only such vessels as are capable of containing those bodies, and resisting the force of fire. These vessels are little hollow pots, of different dimensions, which are called Crucibles. Crucibles can hardly be made of any thing but earth; they ought to have a cover of the same material fitted to shut them close. The best earth we know is[Pg 133] that whereof those pots are made in which butter is brought from Bretagne: these pots themselves are exceeding good crucibles; and they are almost the only ones that are capable of holding glass of lead in fusion, without being penetrated by it.
For the roasting of ores, that is, freeing them, by the help of fire, from their sulphureous and arsenical parts, little cups of the same material with crucibles are used; but they are made flat, shallow, and wider, above than below, that these volatile matters may the more freely exhale. These vessels are called Tests, or Scorifiers: they are scarce ever used but in the Docimastic art, that is, in making small Assays of ores.
The Theory of Constructing the Furnaces most commonly used in Chymistry.
Skill in conducting and applying fire properly, and determining its different degrees, is of very great consequence to the success of Chymical operations.
As it is exceeding difficult to govern and moderate the action of fire, when the vessels in which any operation is performed are immediately exposed to it, Chymists have contrived to convey heat to their vessels, in nice operations, through different mediums, which they place occasionally between those vessels and the fire.
Those intermediate substances in which they plunge their vessels are called Baths. They are either fluid or solid: the fluid baths are water or its vapours. When the distilling vessel is set in water, the bath is called Balneum Mariæ, or the Water Bath; and the greatest degree of heat of which it is susceptible is that of boiling water. When the vessel is exposed only to the vapours which exhale from water, this forms the Vapour Bath; the heat of which is nearly the same with that of the Balneum Mariæ. These baths are useful for distilling essential oils, ardent spirits, sweet-scented waters; in a word, all such substances as cannot bear a greater heat, without prejudice either to their odour, or to some of their other qualities.
Baths may also be made of any other fluids, such as oils, mercury, &c. which are capable of receiving and communicating much more heat: but they are very seldom used. When a more considerable degree of heat is required, a bath is prepared of any solid matter reduced to a fine powder, such as sand, ashes, filings of iron, &c. The heat of these baths may be pushed so far as to make the bottom of the vessel become faintly red. By plunging a thermometer into the bath, by the side of the vessel, it is easy to observe the precise degree of heat applied to the substance on which you are working. It is necessary that the thermometers employed on this occasion be constructed on good principles, and so contrived as to be easily compared with those of the most celebrated natural philosophers. Those of the illustrious Réaumur are most used and best known, so that it would not be amiss to give them the preference. When a greater heat is required than any of those baths can give, the vessels must be set immediately on live coals, or in a flaming fire: this is called working with a naked fire; and, in this case it is much more difficult than in the other to determine the degrees of heat.
There are several ways of applying a naked fire. When the heat or flame is reflected upon the upper part of a vessel which is exposed to the fire, this is called a Reverberated heat. A Melting heat is that which is strong enough to fuse most bodies. A Forging heat is that of a fire which is forcibly excited by the constant blast of a pair of bellows, or more.
There is also another sort of fire which serves very commodiously for many operations, because it does not require to be fed or frequently mended: this is afforded by a lamp with one or more wicks, and may be called a Lamp-heat. It is scarce ever employed but to heat baths, in operations which require a gentle and long continued warmth: if it hath any fault, it is that of growing gradually hotter.
All the different ways of applying fire require Furnaces of different constructions: we shall therefore describe such as are of principal and most necessary use.
Furnaces must be divided into different parts or stories, each of which has its particular use and name.
The lower part of the furnace, designed for receiving the ashes and giving passage to the air, is called the Ash-hole. The ash-hole is terminated above by a grate, the use of which is to support the coals and wood, which are to be burnt thereon: this part is called the Fire-place. The fire[Pg 135]-place is in like manner terminated above by several iron bars, which lie quite across it from right to left, in lines parallel to each other: the use of these bars is to sustain the vessels in which the operations are to be performed. The space above these bars to the top of the furnace is the upper story, and may be called the Laboratory of the furnace. Lastly, some furnaces are quite covered above by means of a kind of vaulted roof called the Dome.
Furnaces have moreover several apertures: one of these is at the ash-hole, which gives passage to the air, and through which the ashes that fall through the grate are raked out; this aperture is called the ash-hole Door: another is at the fire-place, through which the fire is supplied with fuel, as occasion requires; this is called the mouth or door of the Fire-place, or the Stoke-hole: there is a third in the upper story, through which the neck of the vessel passes; and a fourth in the dome for carrying off the fuliginosities of combustible matters, which is called the Chimney.
To conclude, there are several other openings in the several parts of the furnace, the use whereof is to admit the air into those places, and also, as they can be easily shut, to incite or slacken the activity of the fire, and so to regulate it; which has procured them the title of Registers. All the other openings of the furnace should be made to shut very close, the better to assist in governing the fire; by which means they likewise do the office of registers.
In order to our forming a just and general idea of the construction of furnaces, and of the disposition of the several apertures in them, with a view to increase or diminish the activity of the fire, it will be proper to lay down, as our ground-work, certain principles of natural philosophy, the truth of which is demonstrated by experience.
And first, every body knows that combustible matters will not burn or consume unless they have a free communication with the air; insomuch that if they be deprived thereof, even when burning most rapidly, they will be extinguished at once: that consequently combustion is greatly promoted by the frequent accession of fresh air, and that a stream of air, directed so as to pass with impetuosity through burning fuel, excites the fire to the greatest possible activity.
Secondly, it is certain that the air which touches, or comes near ignited bodies is heated, rarefied, and rendered lighter than the air about it, that is, farther distant from the center of heat; and consequently that this air, so heated and become lighter, is necessarily determined thereby to ascend and[Pg 136] mount aloft, in order to make room for that which is less heated and not so light, which by its weight and elasticity tends to occupy the place quitted by the other. Another consequence hereof is, that if fire be kindled in a place enclosed every where but above and below, a current of air will be formed in that place, running in a direction from the bottom to the top; so that if any light bodies be applied to the opening below, they will be carried up towards the fire; but, on the contrary, if they be held at the opening above, they will be impelled by a force which will drive them up, and carry them away from the fire.
Thirdly and lastly, it is a truth demonstrated in hydraulics, that the velocity of a given quantity of any fluid, determined to flow in any direction whatever, is so much the greater the narrower the channel is to which that fluid is confined; and consequently that the velocity of a fluid will be increased by making it run from a wider through a narrower passage.
These principles being established, it is easy to apply them to the construction of furnaces. First, if a fire be kindled in the fire-place of a furnace, which is open on all sides, it burns nearly as if it were in the open air. It has with the surrounding air a free communication; so that fresh air is continually admitted to facilitate the entire combustion of the inflammable matters employed as fuel. But there being nothing to determine that air to pass with rapidity through the fire in this case, it does not at all augment the activity thereof, but suffers it to waste away quietly.
Secondly, if the ash-hole or dome of a furnace, in which a fire is burning, be shut quite close, then there is no longer any free communication between the air and the fire: if the ash-hole be shut, the air is debarred from having free access to the fire; if the dome be stopt, the egress of the air rarefied by the fire is prevented; and consequently the fire must in either case burn very faintly and slowly, gradually die away, and at last go quite out.
Thirdly, if all the openings of the furnace be wholly closed, it is evident that the fire will be very quickly extinguished.
Fourthly, if only the lateral openings of the fire-place be shut, leaving the ash-hole and upper part of the furnace open; it is plain that the air entering by the ash-hole will necessarily be determined to go out at top, and that consequently a current of air will be formed, which will pass through the fire, and make it burn briskly and vigorously.
Fifthly, if both the ash-hole and the upper story of the furnace be of some length, and form canals either cylindric or prismatic, then the air being kept in the same direction through a longer space, the course of its stream will be both stronger and better determined, and consequently the fire will be more animated by it.
Sixthly and lastly, if the ash-hole and the upper part of the furnace, instead of being cylindric or prismatic canals, have the form of truncated cones or pyramids, standing on their bases, and so ordered that the upper opening of the ash-hole, adjoining to the fire-place, may be wider than the base of the superiour cone or pyramid, then the stream of air, being forced to pass incessantly from a larger channel through a smaller, must be considerably accelerated, and procure to the fire the greatest activity which it can receive from the make of a furnace.
The materials fittest for building furnaces are, 1. Bricks, joined together with potters clay mixed with sand and moistened with water. 2. Potters clay mingled with potsherds, moistened with water, and baked in a violent fire. 3. Iron; of which all furnaces may be made, with this precaution, that the inside be provided with a great many prominent points, as fastenings for a coat of earth, with which the internal parts of the furnace must necessarily be covered to defend it from the action of the fire.
The reverberating furnace is one of those that are most employed in Chymistry: it is proper for distillations by the retort, and should be constructed in the following manner.
First, the use of the ash-hole being, as was said, to give passage to the air and to receive the ashes, no bad consequence can attend its being made pretty high: it may have from twelve to twenty or twenty-four inches in heighth. Its aperture should be wide enough to admit billets of wood, when a great fire is to be made.
Secondly, the ash-hole must be terminated at its upper part by an iron grate, the bars of which should be very substantial, that they may resist the action of the fire: this grate is the bottom of the fire-place, and destined to support the coals. In the lateral part of the fire-place, and nearly about the same heighth with the grate, there should be a hole of such a size that it may easily admit charcoal, as well as little tongs and shovels for managing the fire. This aperture or mouth of the fire-place should be perpendicularly over the mouth of the ash-hole.
Thirdly, from six to eight or ten inches high above the grate over the ash-hole, little apertures must be made in the walls of the furnace, of eight or ten lines in diameter, an inch from one another, and those in one side must be diametrically opposite to those in the other. The use of these holes is to receive bars of iron for the retort to rest on; which should be, as I said, at different heights, in order to accommodate retorts of different sizes. At the upper extremity of this part of the furnace, which reaches from the iron bars to the top, the heighth whereof should be somewhat less than the width of the furnace, must be cut a semi-circular aperture for the neck of the retort to come through. This hole must by no means be over the doors of the fire-place and ash-hole; for then, as it gives passage to the neck of the retort, it must of course be opposite to the receiver, and in that case the receiver itself would stand over against those two apertures; which would be attended with this double inconvenience, that the receiver would not only grow very hot, but greatly embarrass the operator, whose free access to the fire-place and ash-hole would be thereby obstructed. It is proper therefore that the semi-circular cut we are speaking of be so placed that when the greatest ballons are luted to the retort they may leave an open passage to the fire-place and ash-hole.
Fourthly, in order to cover in the laboratory of the reverberating furnace, there must be a roof made for it in the form of a cupola, or concave hemisphere, having the same diameter as the furnace. This dome should have a semi-circular cut in its rim, answering to that above-directed to be made in the upper extremity of the furnace, so that, when adjusted to each other, the two together may form a circular hole for the neck of the retort to pass through. At the top of this dome there must also be a circular hole of three or four inches diameter, carrying a short tapering funnel of the same diameter, and three inches high, which will serve for a chimney to carry off all fuliginosities, and accelerate the current of the air. This passage may be shut at pleasure with a flat cover. Moreover, as it is necessary that the dome should be taken off and put on with ease, it should have two ears or handles for that purpose: a portative or moveable furnace should also have a pair of handles, fixed opposite to each other, between the ash-hole and the fire-place.
Sixthly and lastly, a conical canal must be provided of about three feet long, and sufficiently wide at its lower end[Pg 139] to admit the funnel of the aperture at the top of the dome. This conical tube is to be applied to the dome when the fire is required to be extremely active: it tapers gradually from its base upwards, and breaks off as if truncated at top, where it should be about two inches wide.
Besides the apertures already mentioned as necessary to a reverberating furnace, there must also be many other smaller holes made in its ash-hole, fire-place, laboratory, and dome, which must all be so contrived as to be easily opened and shut with stopples of earth: these holes are the registers of the furnace, and serve to regulate the activity of the fire, according to the principles before laid down.
When the action of the fire is required to be exactly uniform and very brisk, it is necessary to stop carefully with moist earth all the little chinks in the juncture of the dome with the furnace, between the neck of the retort and the circular hole through which it passes, and which it never fills exactly, and, lastly, the holes which receive the iron bars that sustain the retort.
It is proper to have, in a laboratory, several reverberating furnaces of different magnitudes; because, they must be proportioned to the size of the retorts employed. The retort ought to fill the furnace, so as to leave only the distance of an inch between it and the inside of the furnace.
Yet when the retort is to be exposed to a most violent fire, and especially when it is required that the heat shall act with equal force on all parts of the furnace, and as strongly on its vault as on its bottom, a greater distance must be left between the retort and the inside of the furnace; for then the furnace may be filled with coals, even to the upper part of the dome. If moreover some pieces of wood be put into the ash-hole, the conical canal fitted on to the funnel of the dome, and all the apertures of the furnace exactly closed, except the ash-hole and the chimney, the greatest heat will then be excited that this furnace can produce.
The furnace now described may also be employed in many other chymical operations. If the dome be laid aside, an alembic may very well be placed therein: but then the space, which will be left between the body of the alembic and the top of the upper part of the furnace, must be carefully filled up with Windsor-loam moistened; for without that precaution the heat will soon reach the very head, which ought to be kept as cool as possible, in order to promote the condensation of the vapours. On this occasion therefore it will be proper to leave no holes open in the fire-place, but the la[Pg 140]teral ones; of which also those over-against the receiver must be stopped.
A pot, or broad-brimmed earthen pan, may be placed over this furnace, and being so fitted to it as to close the upper part thereof accurately, and filled with sand, may serve for a sand-heat to distil with.
The bars designed to support distilling vessels being taken out, a crucible may stand therein, and many operations be performed that do not require the utmost violence of fire. In a word, this furnace is one of the most commodious that can be, and more extensively useful than any other.
The Melting furnace is designed for applying the greatest force of heat to the most fixed bodies, such as metals and earths. It is never employed in distilling: it is of no use but for calcination and fusion; and consequently need not admit any vessels but crucibles.
The ash-hole of this furnace differs from that of the reverberating furnace only in this, that it must be higher, in order to raise the fire-place to a level with the artist's hand; because in that all the operations of this furnace are performed. The ash-hole therefore must be about three feet high: and this heighth procures it moreover the advantage of a good draught of air. For the same reason, and in consequence of the principles we laid down, it should be so built that its width lessening insensibly from the bottom to the top, it may be narrower where it opens into the fire-place than any where below.
The ash-hole is terminated at its upper end, like that of the reverberating furnace, by a grate, which serves for the bottom of the fire-place, and ought to be very substantial, that it may resist the violence of the fire. The inside of this furnace is commonly an elliptic curve; because it is demonstrated by mathematicians that surfaces having that curvature reflect the rays of the sun, or of fire, in such a manner, that meeting in a point, or a line, they produce there a violent heat. But, to answer this purpose, those surfaces must be finely polished; an advantage hardly procurable to the internal surface of this furnace, which can be made of nothing but earth: besides, if it were possible to give it a polish, the violent action of the fire that must be employed in this furnace would presently destroy it. Yet the elliptical figure must not be entirely disregarded: for, if care be taken to keep the internal surface of the furnace as smooth as possible, it will certainly reflect the heat pretty strongly, and collect it about the center.
The fire-place of this furnace ought to have but four apertures.
First, that of the lower grate, which communicates with the ash-hole.
Secondly, a door in its fore-side, through which may be introduced coals, crucibles, and tongs for managing them: this aperture should be made to shut exactly with a plate of iron, having its inside coated with earth, and turning on two hinges fixed to the furnace.
Thirdly, over this door a hole slanting downwards, towards the place where the crucible is to stand. The use of this hole is to give the operator an opportunity of examining the condition of the matters contained in his crucible without opening the door of the fire-place: this hole should be made to open and shut easily, by means of a stopple of earth.
Fourthly, a circular aperture of about three inches wide in the upper part or vault of the furnace, which should gradually lessen and terminate, like that of the dome of the reverberating furnace, in a short conical funnel of about three inches long, and fitted to enter the conical pipe before described, which is applied when the activity of the fire is to be increased.
When this furnace is to be used, and a crucible to be placed in it, care must be taken to set on the grate a cake of baked earth, somewhat broader than the foot of the crucible. The use of this stand is to support the crucible, and raise it above the grate, for which purpose it should be two inches thick. Were it not for this precaution the bottom of the crucible, which would stand immediately on the grate, could never be thoroughly heated, because it would be always exposed to the stream of cold air which enters by the ash-hole. Care should also be taken to heat this earthen bottom red-hot before it be placed in the furnace, in order to free it from any humidity, which might otherwise happen to be driven against the crucible during the operation, and occasion its breaking.
We omitted to take notice, in speaking of the ash-hole, that, besides its door, it should have about the middle of its heighth a small hole, capable of receiving the nosel of a good perpetual bellows, which is to be introduced into it and worked, after the door is exactly shut, when it is thought proper to excite the activity of the fire to the utmost violence.[Pg 142] The Forge is only a mass of bricks of about three feet high, along whose upper surface is directed the nose or pipe of a pair of large perpetual bellows, so placed that the operator may easily blow the fire with one hand. The coals are laid on the hearth of the forge near the nose of the bellows; they are confined, if necessary, to prevent their being carried away by the wind of the bellows, within a space inclosed by bricks; and then by pulling the bellows the fire is continually kept up in its greatest activity. The forge is of use when there is occasion to apply a great degree of heat suddenly to any substance, or when it is necessary that the operator be at liberty to handle frequently the matters which he proposes to fuse or calcine.
The Cupelling furnace is that in which gold and silver are purified, by the means of lead, from all alloy of other metallic substances. This furnace must give a heat strong enough to vitrify lead, and therewith all the alloy which the perfect metals may contain. This furnace is to be built in the following manner.
First, of thick iron plates, or of some such composition of earth as we recommended for the construction of furnaces, must be formed a hollow quadrangular prism, whose sides may be about a foot broad, and from ten to eleven inches high; and extending from thence upwards may converge towards the top, so as to form a pyramid truncated at the heighth of seven or eight inches, and terminated by an aperture of the width of seven or eight inches every way. The lower part of the prism is terminated, and closed, by a plate of the same materials of which the furnace is constructed.
Secondly, in the fore-side or front of this prism there is an opening of three or four inches in heighth, by five or six inches in breadth: this opening, which should be very near the bottom, is the door of the ash-hole. Immediately over this opening is placed an iron grate, the bars of which are quadrangular prisms of half an inch square, laid parallel to each other, and about eight or nine inches asunder, and so disposed that two of their angles are laterally opposite, the two others looking one directly upwards and the other downwards. As in this situation the bars of the grate present to the fire-place very oblique surfaces, the ashes and very small coals do not accumulate between them, or hinder the free entrance of the air from the ash-hole. This grate terminates the ash-hole at its upper part, and serves for the bottom of the fire-place.
Thirdly, three inches, or three and a half, above the grate, there is in the fore-side of the furnace another opening, terminated by an arch for its upper part, which consequently has the figure of a semi-circle: it ought to be four inches wide at bottom, and three inches and an half high at its middle. This opening is the door of the fire-place; yet it is not intended for the same uses as the door of the fire-place in other furnaces: the purpose for which it is actually destined shall be explained when we come to shew how the furnace is to be used. An inch above the door of the fire-place, still in the fore-side of the furnace, are two holes of about an inch diameter, and at the distance of three inches and a half from each other, to which answer two other holes of the same size, made in the hinder part, directly opposite to these. There is, moreover, a fifth hole of the same width about an inch above the door of the fire-place. The design of all these holes shall be explained when we describe the manner in which these furnaces are to be used.
Fourthly, the fore-part of the furnace is bound by three iron braces, one of which is fixed just below the door of the ash-hole; the second occupies the whole space between the ash-hole door and the door of the fire-place, and has two holes in it, answering to those which we directed to be made in the furnace itself about this place; and the third is placed immediately over the door of the fire-place. These braces must extend from one corner of the front of the furnace to the other, and be fastened thereto with iron pins, in such a manner that their sides next to the doors may not lie quite close to the body of the furnace, but form a kind of grooves for the iron plates to slide in, that are designed to shut the two doors of the furnace when it is necessary. Each of these iron plates should have a handle, by which it may be conveniently moved; and to each door there should be two plates, which meeting each other, and joining exactly in the middle of the door-place, may shut it very close. Each of the two plates belonging to the door of the fire-place ought to have a hole in its upper part; one of these holes should be a slit of about two lines wide, and half an inch long; the other may be a semi-circular opening of one inch in heighth and two in breadth. These holes should be placed so that neither of them may open into the fire-place when the two plates are joined together in the middle of the door to shut it close.
Fifthly, to terminate the furnace above, there must be a pyramid formed of the same materials with the furnace, hollow, quadrangular, three inches high on a base of seven[Pg 144] inches, which base must exactly fit the upper opening of the furnace: the top of this pyramidal cover must end in a tube of three inches in diameter and two in heighth, which must be almost cylindrical, and yet a little inclining to the conical form. This tube serves, as in the furnaces already described, to carry the conical funnel, which is fitted to the upper part when a fire of extraordinary activity is wanted.
The furnace thus constructed is fit to serve all the purposes for which it is designed: yet before it can be used another piece must be provided, which, though it does not properly belong to the furnace, is nevertheless necessary in all the operations performed by it; and that is a piece contrived to contain the cupels, or other vessels which are to be exposed to the fire in this furnace. It is called a Muffle, and is made in the following manner.
On an oblong square, of four inches in breadth, and six or seven in length, a concave semi-cylinder is erected, in the form of a vault, which makes a semi-circular canal, open at both ends. One of these is almost entirely closed, except that near the bottom two small semi-circular holes are left. In each of its sides likewise two such holes are made, and the other end is left quite open.
The Muffle is intended to bear and communicate the fiercest heat; and therefore it must be made thin, and of an earth that will resist the violence of fire, such as that of which crucibles are made. The Muffle being thus constructed, and then well baked, is fit for use.
When it is to be used it must be put into the furnace by the upper opening, and set upon two iron bars, introduced through the holes made for that purpose below the door of the fire-place. The Muffle must be placed on these bars in the fire-place in such a manner that its open end shall stand next to, and directly against the door of the fire-place, and may be joined to it with lute. Then the cupels are ranged in it, and the furnace is filled up, to the heighth of two or three inches above the Muffle, with small coals not bigger than a walnut, to the end that they may lie close round the Muffle, and procure it an equal heat on every side. The chief use of the Muffle is to prevent the coals and ashes from falling into the cupels, which would be very prejudicial to the operations carrying on in them: for the lead would not vitrify as it ought, because the immediate contact of the coals would continually restore its phlogiston; or else the glass of lead, which ought to penetrate and pass through the cupels, would be rendered[Pg 145] incapable of so doing; because the ashes mixing therewith would give it such a consistence and tenacity as would destroy that property, or at least considerably lessen it. The openings, therefore, which are left in the lower part of the Muffle, should not be so high as to admit coals or ashes to get into the cupels; the use of them is to procure an easier passage for the heat and the air to those vessels. The Muffle is left quite open in its fore-part, that the operator may be at liberty to examine what passes in the cupels, to stir their contents, to remove them from one place to another, to convey new matters into them, &c. and also to promote the free access of the air, which must concur with the fire towards the evaporation necessary to the vitrification of lead; which air, if fresh were not often enough admitted, would be incapable of producing that effect; because it would soon be loaded with such a quantity of vapours that it could not take up any more.
The government of the fire in this furnace is founded on the general principles above laid down for all furnaces. Yet as there are some little differences, and as it is very essential to the success of the operations for which this furnace is intended, that the artist should be absolutely master of his degree of heat, we shall in few words shew how that may be raised or lowered.
When the furnace is filled with coals and kindled, if the door of the ash-hole be set wide open, and that of the fire-place shut very close, the force of the fire is increased; and if, moreover, the pyramidal cover be put on the top, and the conical funnel added to it, the fire will become still more fierce.
Seeing the matters contained in this furnace are encompassed with fire on all sides, except in the fore-part opposite to the door of the fire-place, and as there are occasions which require that the force of the fire should be applied to this part also, an iron box, of the shape and size of the door, hath been contrived to answer that purpose. This box is filled with lighted coals, and applied immediately to the door-place, by which means the heat there is considerably augmented. This help may be made use of at the beginning of the operation, in order to accelerate it, and bring the heat sooner to the desired degree; or in case a very fierce heat be required; or at a time when the air being hot and moist will not make the fire burn with the necessary vigour.
The heat may be lessened by removing the iron box, and shutting the door of the fire-place quite close. It may be still further and gradually diminished, by taking off the conical funnel from the top; by shutting the door of the fire-place with one of its plates only, that which has the least, or that which has the greatest aperture in it; by taking off the pyramidal cover; by shutting the ash-hole door wholly or in part; and, lastly, by setting the door of the fire-place wide open: but, in this last case, the cold air penetrates into the cavity of the Muffle, and refrigerates the cupels more than is almost ever necessary. If it be observed, during the operation, that the Muffle grows cold in any particular part, it is a sign there is a vacuity left by the coals in that place: in this case an iron wire must be thrust into the furnace, through the hole which is over the door of the fire-place, and the coals stirred therewith, so as to make them fall into their places and fill up the vacant interstices.
It is proper to observe, that, besides what has been said concerning the ways of increasing the activity of the fire in the cupelling furnace, several other causes also may concur to procure to the matters contained in the Muffle a greater degree of heat: for example, the smaller the Muffle is, the wider and more numerous the holes in it are; the nearer to its bottom, or further end, the cupels are placed, the more will the matters therein contained be affected with heat.
Besides the operations to be performed by the cupel, this furnace is very useful, and even necessary, for many chymical experiments; such, for instance, as those relating to sundry vitrifications and enamelling. As it is pretty low, the best way is to place it, when it is to be used, on a base of brick-work that may raise it to a level with the operator's hand.
A Lamp-furnace is exceeding useful for all operations that require only a moderate, but long-continued, degree of heat. The furnace for working with a lamp-heat is very simple: it consists only of a hollow cylinder, from fifteen to eighteen inches high, and five or six in diameter, having at its bottom an aperture large enough for a lamp to be introduced and withdrawn with ease. The lamp must have three or four wicks, to the end that by lighting more or fewer of them a greater or less degree of heat may be produced. The body of the furnace must moreover have several small holes in it, in order to supply the flame of the lamp with air enough to keep it alive.
On the top of this furnace stands a bason five or six inches[Pg 147] deep, which ought to fill the cavity of the cylinder exactly, and to be supported at its circumference by a rim which may entirely cover and close the furnace: the use of this bason is to contain the sand through which the lamp-heat is usually conveyed.
Besides this, there must be a kind of cover or dome made of the same material with the furnace, and of the same diameter with the sand-bath, without any other opening than a hole, nearly circular, cut in its lower extremity. This dome is a sort of reverberatory, which serves to confine the heat and direct it towards the body of the retort; for it is used only when something is to be distilled in a vessel of this fashion; and then the hole at its bottom serves for a passage to the neck of the retort. This dome should have an ear or handle, for the conveniency of putting it on and taking it off with ease.
Chymical vessels, especially such as are made of glass, and the earthen vessels commonly called stone-ware, are very subject to break when exposed to sudden heat or cold: whence it comes, that they often crack when they begin to heat, and also when being very hot they happen to be cooled, either by fresh coals thrown into the furnace, or by the access of cold air. There is no way to prevent the former of these accidents, but by taking the pains to warm your vessel very slowly, and by almost insensible degrees. The second may be avoided by coating the body of the vessel with a paste or lute, which being dried will defend it against the attacks of cold.
The fittest stuff for coating vessels is a composition of fat earth, Windsor-loam, fine sand, filings of iron, or powdered glass, and chopped cow's hair, mixed and made into a paste with water. This lute serves also to defend glass vessels against the violence of the fire, and to prevent their melting easily.
In almost all distillations it is of great consequence, as hath been said, that the neck of the distilling vessel be exactly joined with that of the receiver into which it is introduced, in order to prevent the vapours from escaping into the air and so being lost: and this junction is effected by means of a lute.
A few slips of paper applied round the neck of the vessels with common size will be sufficient to keep in such vapours as are aqueous or not very spirituous.
If the vapours are more acrid, or more spirituous, recourse may be had to slips of bladder long steeped in water, which containing a sort of natural glue, close the junctures of the vessels very well.
If it be required to confine vapours of a still more penetrating nature, it will be proper to employ a lute that quickly grows very hard; particularly a paste made with quick-lime and any sort of jelly, whether vegetable or animal; such as the white of an egg, stiff size, &c. This is an excellent lute, and not easily penetrated. It is also used to stop any cracks or fractures that happen to glass vessels. But it is not capable of resisting the vapours of mineral acid spirits, especially when they are strong and smoking: for that purpose it is necessary to incorporate the other ingredients thoroughly with fat earth softened with water; and even then it frequently happens that this lute is penetrated by acid vapours, especially those of the spirit of salt, which of all others are confined with the greatest difficulty.
In such cases its place may be supplied with another, which is called Fat Lute, because it is actually worked up with fat liquors. This lute is composed of a very fine cretaceous earth, called tobacco-pipe clay, moistened with equal parts of the drying oil of lint-seed, and a varnish made of amber and gum copal. It must have the consistence of a stiff paste. When the joints of the vessels are closed up with this lute, they may, for greater security, be covered over with slips of linen smeared with the lute made of quick-lime and the white of an egg.
Chymical vessels are liable to be broken in an operation by other causes besides the sudden application of heat or cold. It frequently happens, that the vapours of the matters exposed to the action of fire rush out with such impetuosity, and are so elastic, that, finding no passage through the lute with which the joints of the vessels are closed, they burst the vessels themselves, sometimes with explosion and danger to the operator.
To prevent this inconvenience, it is necessary that in every receiver there be a small hole, which being stopped only with a little lute may easily be opened and shut again as occasion requires. It serves for a vent-hole to let out the vapours, when the receiver begins to be too much crowded with them. Nothing but practice can teach the artist when it is requisite to open this vent. If he hits the proper time, the vapours commonly rush out with rapidity, and a considerable hissing noise; and the vent should be stopped again[Pg 149] as soon as the hissing begins to grow faint. The lute employed to stop this small hole ought always to be kept so ductile, that, by taking the figure of the hole exactly, it may entirely stop it. Besides, if it should harden upon the glass, it would stick so fast that it would be very difficult to remove it without breaking the vessel. This danger is easily avoided by making use of the fat lute, which continues pliant for a long time, when it is not exposed to an excessive heat.
This way of stopping the vent-hole of the receiver has yet another advantage: for if the hole be of a proper width, as a line and half, or two lines, in diameter, then, when the vapours are accumulated in too great a quantity, and begin to make a great effort against the sides of the receiver, they push up the stopple, force it out, and make their way through the vent-hole: so that, by this means, the breaking of the vessels may always be certainly prevented. But great care must be taken that the vapours be not suffered to escape in this manner, except when absolute necessity requires it; for it is generally the very strongest and most subtile part of a liquor which is thus dissipated and lost.
Heat being the chief cause that puts the elasticity of the vapours in action, and prevents their condensing into a liquor, it is of great consequence in distillation that the receiver be kept as cool as possible. With this view a thick plank should be placed between the receiver and the body of the furnace, to intercept the heat of the latter, and prevent its reaching the former. As the vapours themselves rise very hot from the distilling vessel, they soon communicate their heat to the receiver, and especially to its upper part, against which they strike first. For this reason it is proper that linen cloths, dipt in very cold water, be laid over the receiver, and frequently shifted. By this means the vapours will be considerably cooled, their elasticity weakened, and their condensation promoted.
By what hath been said in this first part, concerning the properties of the principal agents in Chymistry, the construction of the most necessary vessels and furnaces, and the manner of using them, we are sufficiently prepared for proceeding directly to the operations, without being obliged to make frequent and long stops, in order to give the necessary explanations on those heads.
Nevertheless, we shall take every proper occasion to extend the theory here laid down, and to improve it by the addition of several particulars, which will find their places in our Treatise of Chymical Operations.
WHEREIN
The Fundamental Operations are described, and illustrated by Observations on each Process.

ELEMENTS
OF THE
PRACTICE OF CHYMISTRY.
As the Elements of the Theory of Chymistry, delivered in the former part of this work, were intended for the use of persons supposed to be altogether unacquainted with the art, they could not properly admit of any thing more than fundamental principles, so disposed as constantly to lead from the simple to the compound, from things known to things unknown: for which reason I could not therein observe the usual order of Chymical Decomposition, which is not susceptible of such a method. I therefore supposed all the analyses made, and bodies reduced to their simplest principles; to the end that, by observing the chief properties of those primary elements, we might be enabled to trace them through their several combinations, and to form some sort of judgment a priori of the qualities of such compounds as may result from their junctions.
But this latter part is of a different nature. It is a practical Treatise, intended to contain the manner of performing the principal Operations of Chymistry; the operations which serve as standards for regulating all the rest, and which confirm the fundamental truths laid down in the Theory.
As these operations consist almost wholly of analyses and decompositions, there can be no doubt concerning the order[Pg 154] proper to be observed in giving an account of them: it evidently coincides with that of the analysis itself.
But as all bodies, which are the subjects of Chymical operations, are divided by nature into three classes or kingdoms, the mineral, the vegetable, and the animal, the analysis thereof may naturally be divided into three branches: some difference may also arise from the different order in which these three may be treated of.
As the reasons assigned for beginning with one kingdom rather than with another have never been thoroughly canvassed, and may perhaps seem equally good when viewed in a particular light, Chymical writers differ in their opinions on this point. For my part, without entering into a discussion of the motives which have determined others to follow a different order, I shall only produce the reasons that led me to begin with the mineral kingdom, to examine the vegetable in the second place, and to conclude with the animal.
First, then, seeing vegetables draw their nourishment from minerals, and animals derive theirs from vegetables, the bodies which constitute these three kingdoms seem to be generated the one by the other, in a manner that determines their natural rank.
Secondly, this disposition procures us the advantage of tracing the principles, from their source in the mineral kingdom, down to the last combinations into which they are capable of entering, that is, into animal matters; and of observing the successive alterations they undergo in passing out of one kingdom into another.
Thirdly and lastly, I look upon the analysis of minerals to be the easiest of all; not only because they consist of fewer principles than vegetables and animals, but also because almost all of them are capable of enduring the most violent action of fire, when that is necessary to their decomposition, without any considerable change or diminution of their principles, to which those of other substances are frequently liable.
Besides, I am not singular in this distribution of the three classes of bodies, which are the subjects of the chymical analysis: as it is the most natural, it has been adopted by several authors, or rather by most who have published Treatises of Chymistry. But there is something peculiarly my own in the manner wherein I have treated the analysis of each kingdom. In the mineral kingdom, for instance, will be found a considerable number of operations not to be met with in other Treatises of Chymistry; the authors having[Pg 155] probably considered them as useless, or in some measure foreign, to the purpose of Elementary Books, and as constituting together a distinct art. I mean the processes for extracting saline and metallic substances from the minerals containing them.
Yet, if it be considered that salts, metals, and semi-metals are far from being produced by nature in a state of perfection, or in that degree of purity which they are commonly supposed to have when they are first treated of in Books of Chymistry; but that, on the contrary, these substances are originally blended with each other, and adulterated with mixtures of heterogeneous matters, wherewith they form compound minerals; I imagine it will be allowed, that the operations by which these minerals are decomposed, in order to extract the metals, semi-metals, and other simpler substances, especially as they are founded on the most curious properties of these substances, are so far from being useless or foreign to the purposes of an Elementary Treatise, that they are, on the contrary, absolutely necessary thereto.
After I had made these reflections, I could not help thinking that an analysis of minerals, which should treat of saline and metallic substances, without taking any notice of the manner in which their matrices must be analysed, in order to extract them, would be no less defective than a treatise of the analysis of vegetables, in which Oils, essential Salts, fixed and volatile Alkalis, should be amply treated of, without saying one word of the manner of analysing the plants from which these several substances are obtained. I therefore thought myself indispensably obliged to describe the manner of decomposing every ore or mineral, before I attempted to treat of the saline or metallic substance which it yields.
For example: as the Vitriolic Acid, with the consideration of which I begin my Mineral Analysis, is originally contained in Vitriol, Sulphur, and Alum; and as these substances again derive their origin from the sulphureous and ferruginous Pyrites, the first operations I describe under this head are the processes for decomposing the Pyrites in order to extract its Vitriol, Sulphur, and Alum. I then proceed to the particular analysis of each of these substances, with a view to extract their Vitriolic Acid; and afterwards deliver, in their order, the other operations usually performed on this Acid. Thus it appears, that this saline substance occasions my describing the analyses of the Pyrites, Vitriol, Sulphur,[Pg 156] and Alum. The whole of the Treatise on Minerals proceeds on the same plan.
The operations by which we decompose ores and minerals are of two sorts: those employed in working by the great, and those for trying in small the yield of any ore. These two manners of operating are sometimes a little different; yet in the main they are the same, because they are founded on the same principles, and produce the same effects.
As my chief design was to describe the operations that may be conveniently performed in a laboratory, I have preferred the processes for small assays: especially as they are usually performed with more care and accuracy than the operations in great works: and here I must acknowledge, that I am obliged to M. Cramer's Docimasia, or Art of Assaying, for all the operations of this kind in my analysis of minerals. As M. Hellot's work on that subject did not appear till after I had finished this, M. Cramer's Docimasia, in which sound Theory is joined with accurate practice, was the best book of the kind I could at that time consult. I therefore preferred it to all others; and as I have not quoted it in my analysis of minerals, because the quotations would have been too frequent, let what I say here serve for a general quotation. I have been careful to name, as often as occasion required, the other authors whose processes I have borrowed: it is a tribute justly due to those who have communicated their discoveries to the public.
Though I have told the reader that in my analysis of minerals he will find the processes for extracting out of each the saline or metallic substances contained in it, yet he must not expect that this book will instruct him in all that is necessary he should know to be able to determine, by an accurate assay, the contents of every mineral. My intention was not to compose a Treatise of Assaying; and I have taken in no more than was absolutely necessary to make the analysis of minerals perfectly understood, and to render it as complete as it ought to be in an Elementary Treatise. I have therefore described only the principal operations relating thereto; the operations which are fundamental, and which, as I said before, are to serve as standards for the rest, abstracted from such additional circumstances as are of consequence only to the Art of Assaying, properly so called.
Such therefore as are desirous of being fully instructed in that Art, must have recourse to those works which treat pro[Pg 157]fessedly of the subject; and particularly to that published by M. Hellot: a performance most esteemed by such as are best skilled in Chymistry, and rendered so complete by the numerous and valuable observations and discoveries of the Author, that nothing better of the kind can be wished for. I thought it proper to give these notices in relation to my analysis of minerals; and shall now proceed to shew the plan of my analyses of vegetables and of animals.
Seeing all vegetable matters are susceptible of fermentation, and when analysed after fermentation, yield principles different from those we obtain from them before they are fermented, I have divided them into two classes; the former including vegetables in their natural state, before they have undergone fermentation; and the latter those only which have been fermented. This analysis opens with the processes by which we extract from vegetables all the principles they will yield without the help of fire: and then follow the operations for decomposing plants by degrees of heat, from the gentlest to the most violent, both in close vessels, and in the open air.
I have not made the same division in the animal kingdom, because the substances that compose it are susceptible only of the last degree of fermentation, or putrefaction; and moreover the principles they yield, whether putrefied or unputrefied, are the very same, and differ only with regard to their proportions, and the order in which they are extricated during the analysis.
I begin this analysis with an examination of the milk of animals that feed wholly on vegetables; because, though this substance be elaborated in the body of the animal, and by that means brought nearer to the nature of animal matters, yet it still retains a great similitude to the vegetables from which it derives its origin, and is a sort of intermediate substance between the vegetable and animal. Then I proceed to the analysis of animal matters properly so called, those which actually make a part of the animal body. I next examine the excrementitious substances, that are thrown out of the animal body as superfluous and useless. And then I conclude this latter part with operations on the Volatile Alkali; a saline substance of principal consideration in the decomposition of animal matters.
Though, in the general view here given of the order observed in this Treatise of Practical Chymistry, I have mentioned only such processes as serve for analysing bodies, yet I have also inserted some other operations of different kinds.[Pg 158] The book would be very defective if it contained no more: for the design of Chymistry is not only to analyse the mixts produced by nature, in order to obtain the simplest substances of which they are composed, but moreover to discover by sundry experiments the properties of those elementary principles, and to recombine them in various manners, either with each other, or with different bodies, so as to reproduce the original mixts with all their properties, or even form new compounds which never existed in nature. In this book therefore the reader will find processes for combining and recompounding, as well as for resolving and decomposing bodies. I have placed them next to the processes for decomposition, taking all possible care not to interrupt their order, or break the connection between them.
Operations performed on Saline Mineral Substances.
Of the Vitriolic Acid.
To extract Vitriol from the Pyrites.
Take any quantity you please of Iron Pyrites; leave them for some time exposed to the air: they will crack, split, lose their brightness, and fall into powder. Put this powder into a glass cucurbit, and pour upon it twice its weight of hot water; stir the whole with a stick, and the liquor will grow turbid. Pour it, while it is yet warm, into a glass funnel lined with brown filtering paper; and having placed your funnel over another glass cucurbit, let the liquor drain into it. Pour more hot water on the powdered Pyrites, filter as before, and so go on, every time lessening the quantity of water, till that which comes off the Pyrites appears to have no astringent vitriolic taste.
Put all these waters together into a glass vessel that widens upwards; set it on a sand-bath, and heat the liquor till a considerable smoke arises; but take care not to make it boil. Continue the same degree of fire till the surface of the liquor[Pg 160] begins to look dim, as if some dust had fallen into it; then cease evaporating, and remove the vessel into a cool place: in the space of four and twenty hours will be formed therein a quantity of crystals, of a green colour and a rhomboidal figure: these are Vitriol of Mars, or Copperas. Decant the remaining liquor; add thereto twice its weight of water; filter, evaporate, and crystallize as before; repeat these operations till the liquor will yield no more crystals, and keep by themselves the crystals obtained at each crystallization.
OBSERVATIONS.
The Pyrites are minerals which, by their weight and shining colours, frequently impose on such as are not well acquainted with ores. At first sight they may be taken for very rich ones; and yet they consist only of a small quantity of metal combined with much sulphur or arsenic, and sometimes with both.
They strike fire with a steel as flints do, and emit a sulphureous smell: so that they may be known by this extemporaneous proof. The metal most commonly and most abundantly found in the Pyrites is iron; the quantity whereof sometimes equals, or even exceeds, that of the sulphur. Besides metallic and sulphureous matters, the Pyrites contain also some unmetallic earth.
There are several sorts of Pyrites: some of them contain only iron and arsenic. They have not all the property of efflorescing spontaneously in the air, and turning into vitriol: none do so but such as consist only of iron and sulphur, or at least contain but a very small portion of copper, or of arsenic: and even amongst those that are composed of iron and sulphur alone, there are some that will continue for years together exposed to the air without shooting, and indeed without suffering the least sensible alteration.
The efflorescence of the Iron Pyrites, and the changes they undergo, are phenomena well worth our notice. They depend on the singular property which iron possesses of decomposing sulphur by the help of moisture. If very fine iron-filings be accurately mingled with flowers of sulphur, this mixture, being moistened with water, grows very hot, swells up, emits sulphureous vapours, and even takes fire; what remains is found converted into Vitriol of Mars. On this occasion, therefore, the sulphur is decomposed; its inflammable part is dissipated or consumed; its acid combines with the iron, and a Vitriol arises from that conjunction.
This is the very case with the Pyrites that consist only of iron and sulphur; yet some of them, as we said before, do not effloresce spontaneously and turn to Vitriol. The reason probably is, that, in such minerals, the particles of iron and sulphur are not intimately mixed together, but separated by some earthy particles.
In order to procure Vitriol from Pyrites of this kind, they must be for some time exposed to the action of fire, which, by consuming part of their sulphur, and rendering their texture less compact, makes way for the air and moisture, to which they must be afterwards exposed, to penetrate their substance, and produce in them the changes with which those others are affected that germinate spontaneously.
The Pyrites which contain copper and arsenic, and for that reason do not effloresce, must likewise undergo the action of fire; which, besides the effects it produces on Pyrites that consist of iron and sulphur only, dissipates also the greatest part of the arsenic. These Pyrites being first roasted, and then exposed to the air for a year or two, do also yield Vitriol; but then it is not a pure Vitriol of Iron, but is combined with a portion of blue Vitriol, the basis of which is Copper.
Sometimes also there is Alum in the vitriolic waters drawn off the Pyrites. It was on account of this mixture of different salts that we recommended the keeping apart the crystals obtained from each different crystallization: for by this means they may be examined separately, and the species to which they belong discovered.
When Vitriol of Iron is adulterated with a mixture of the Vitriol of Copper only, it is easy to purify it and bring it to be entirely martial, by dissolving it in water, and setting plates of iron in the solution: for iron having a greater affinity than copper with the vitriolic acid, separates the latter from it, and assuming its place produces a pure Vitriol of Mars.
In large works for extracting Vitriol from the Pyrites they proceed thus. They collect a great quantity of Pyrites on a piece of ground exposed to the air, and pile them up in heaps of about three feet high. There they leave them exposed to the action of the air, sun, and rain, for three years together; taking care to turn them every six months, in order to facilitate the efflorescence of those which at first lay undermost. The rain-water which has washed those Pyrites is conveyed by proper channels into a cistern; and when a sufficient quantity thereof is gathered, they evaporate it to a[Pg 162] pellicle in large leaden boilers, having first put into it a quantity of iron, some part of which is dissolved by the liquor, because it contains a vitriolic acid that is not fully saturated therewith. When it is sufficiently evaporated, they draw it off into large leaden or wooden coolers, and there leave it to shoot into crystals. In these last vessels several sticks are placed, crossing each other in all manner of directions, in order to multiply the surfaces on which the crystals may fasten.
The Pyrites are not the only minerals from which Vitriol may be procured. All the ores of iron and copper that contain sulphur may also be made to yield green or blue Vitriol, according to the nature of each, by torrefying them, and leaving them long exposed to the air: but this use is seldom made of them, as there is more profit to be got by extracting the metals they contain. Besides, it is easier to obtain Vitriol from the Pyrites than from those other mineral substances.
To extract Sulphur from the Pyrites, and other sulphureous Minerals.
Reduce to a coarse powder any quantity of yellow Pyrites, or other Mineral containing Sulphur. Put this powder into an earthen or glass retort, having a long wide neck, and so large a body that the matter may fill but two thirds of it. Set the retort in a sand-bath fixed over a reverberating furnace: fit to it a receiver half full of water, and so placed that the nose of the retort may be about an inch under the water: give a gradual fire, taking care you do not make it so strong as to melt the matter. Keep the retort moderately red for one hour, or an hour and half, and then let the vessels cool.
Almost all the Sulphur separated by this operation from its matrix will be found at the extremity of the neck of the retort, being fixed there by the water. You may get it out either by melting it with such a gentle heat as will not set it on fire, or by breaking the neck of the retort.
OBSERVATIONS.
Of all minerals the Pyrites contain the most Sulphur; those especially which have the colour of fine brass, a regular[Pg 163] form, such as round, cubical, hexagonal, and being broken present a number of shining needles, all radiating, as it were, from a center.
A very moderate heat is sufficient to separate the Sulphur they contain. We directed that the retort employed should have a long and wide neck, with a view to procure a free passage for the Sulphur: the water set in the receiver detains the Sulphur, fixes it, and prevents it from flying off; so that it is unnecessary to close the joints of the vessels. But it is proper to take notice, that whenever you use an apparatus for distilling, which requires the beak of the retort to be under water, it is of very great consequence that the fire be constantly so regulated, that the retort may not cool in the least; for, in that case, as the rarefied air contained therein would be condensed, the water in the receiver would rise into the retort and break it.
If in distilling Sulphur, according to the present process, the matter contained in the retort should happen to melt, the operation would be thereby considerably protracted, and it would require a great deal more time to extract all the Sulphur; because all evaporation is from the surface only, and the matter, while it remains in a coarse powder, presents a much more extensive surface than when it is melted.
This remark holds with regard to all other distillations. Any quantity of liquor, set to distil in its fluid state, will take much more time to rise in vapours, and pass from the retort into the receiver, than if it be incorporated with some solid body reduced to minute parts, so that the whole shall make a moist powder; and this though the very same degree of fire be applied in both cases.
If the matter from which it is proposed to extract Sulphur be such as will melt with the degree of fire necessary to this operation; that is, with a heat which will make the retort but faintly red, it must be mixed with some substance that is not so fusible. Very pure coarse sand, or clean gravel, may be used with success: but absorbent earths are altogether improper for this purpose, because they will unite with the Sulphur.
The sulphureous minerals which are most apt to fuse are the cupreous Pyrites, or yellow copper ores: common lead ores are also very fusible.
The Pyrites are by this operation deprived of almost all the Sulphur they contain; and consequently little is left behind, but the particles of iron and copper, together with a portion of unmetallic earth, which we shall shew how to se[Pg 164]parate from these metals, when we come to treat of them. I say that by this operation the Pyrites are deprived of almost all, and not entirely of all their Sulphur; because, this separation being made in close vessels only, there always remains a certain quantity of Sulphur, which adheres so obstinately to the metals, that it would be almost impossible to get it all out, even though a much stronger fire than that directed in the process were applied for this purpose, and though choice had been, as it ought to be, made of such Pyrites, or other sulphureous Minerals as part most easily with their sulphur. Nothing but a very strong fire in the open air is capable of carrying it wholly off, or consuming it entirely.
In several places are found great quantities of native Sulphur. The Volcanoes abound with it, and people gather it at the foot of those burning mountains. Several springs of mineral waters also yield Sulphur, and it is sometimes found sublimed to the vaulted roofs of certain wells, and among others in one at Aix-la-Chapelle.
The Germans and Italians have large works for extracting Sulphur in quantities out of Pyrites, and other minerals which abound therewith. The process they work by is the same with that here delivered; but with this difference only, that Sulphur being but of small value they do not use so many precautions. They content themselves with putting the sulphureous minerals into large crucibles, or rather earthen cucurbits, which they place in the furnace in such a manner that, when the sulphureous part melts, it runs into vessels filled with water, and is thereby fixed.
The Sulphur obtained, either by distillation or by simple fusion, is not always pure.
When it is obtained by distillation, if the matters from which you extract it contain moreover some other minerals of nearly the same volatility, such, for instance, as Arsenic, or Mercury, these minerals will come over with it. This is easily perceived: for pure sublimed Sulphur is always of a beautiful yellow, inclining to a lemon colour. If it look red, or have a reddish cast, it is a sign that some Arsenic hath risen along with it.
Mercury sublimed with Sulphur likewise gives it a red colour; but Sulphur is very seldom adulterated with this metallic substance: for Arsenic is frequently found combined with the Pyrites, and other sulphureous minerals; whereas, on the contrary, we very rarely meet with any Mercury in them.
But if Mercury should happen to rise with the Sulphur in distillation, it may be discovered by examining the sublimate; which, in that case, will have the properties of Cinabar: on being broken its inside will appear to consist of needles adhering laterally to each other; its weight will be very considerable; and, lastly, the great heat of the place where it is collected will furnish another mark to know it by; for, as Cinabar is less volatile than Arsenic or Sulphur, it fastens on places too hot for either Sulphur or Arsenic to bear.
Sulphur may also be adulterated with such fixed matters, either metallic or earthy, as it may have carried up along with it in the distillation, or as may have been sublimed by the Arsenic, which has a still greater power than Sulphur to volatilize fixed bodies.
If you desire to free the Sulphur from most of these heterogeneous matters, it must be put into an earthen cucurbit, and set in a sand-bath. To the cucurbit must be fitted one or more aludels, and such a degree of heat applied as shall but just melt the Sulphur; which is much less than that necessary to separate the Sulphur from its matrix. As soon as the Sulphur is melted it will sublime in lemon-coloured flowers, that will stick to the insides of the aludels.
When nothing more appears to rise with this degree of heat, the vessels must be suffered to cool. At the bottom of the cucurbit will be found a sulphureous mass, containing the greatest part of the adventitious matters that were mixed with the Sulphur, and more or less red or dark-coloured, according to the nature of those matters.
When we come to treat of Arsenic and Mercury, we shall give the methods of separating Sulphur entirely from those metallic substances.
To extract Alum from aluminous Minerals.
Take such minerals as are known or suspected to contain Alum. Expose them to the air, that they may effloresce. If they remain there a year without any sensible change, calcine them, and then leave them exposed to the air, till a bit thereof being put on the tongue imparts an astringent aluminous taste.
When your matters are thus prepared, put them into a leaden or glass vessel; pour upon them thrice their weight[Pg 166] of hot water; boil the liquor; filter it; and repeat these operations till the earth be so edulcorated that the water which comes off it hath no taste. Mix all these solutions together, and let them stand four and twenty hours, that the gross and earthy parts may settle to the bottom; or else filter the liquor: then evaporate till it will bear a new-laid egg. Now let it cool, and stand quiet four and twenty hours: in that time some crystals will shoot, which are most commonly vitriolic; for Alum is rarely obtained by the first crystallization. Remove these vitriolic crystals: if any crystals of Alum be found amongst them, these must be dissolved anew, and set to crystallize a second time in order to their purification; because they partake of the nature as well as of the colour of vitriol. By this method extract all the Alum that the liquor will yield.
If you get no crystals of Alum by this means, boil your liquor again, and add to it a twentieth part of its weight of a strong alkaline lixivium, or a third part of its weight of putrefied urine, or a small quantity of quick-lime. Experience and repeated trials must teach you which of these three substances is to be preferred, according to the particular nature of the mineral on which you are to operate. Keep your liquor boiling, and if there be any alum in it, there will appear a white precipitate: in that case let it cool and settle. When the white precipitate is entirely fallen, decant the clear, and leave the crystals of Alum to shoot at leisure, till the liquor will yield no more: it will then be exceeding thick.
OBSERVATIONS.
Alum is obtained from several sorts of Minerals. In some parts of Italy, and in sundry other places, it effloresces naturally on the surface of the earth. There it is swept together with brooms, and thrown into pits full of water. This water is impregnated therewith till it can dissolve no more. Then it is filtered, and set to evaporate in large leaden vessels; and when it is sufficiently evaporated, and ready to shoot into crystals, it is drawn off into wooden coolers, and there left for the salt to crystallize.
In aluminous soils there are often found springs strongly impregnated with Alum: so that to obtain it, the water need only be evaporated.
In the country about Rome there is a very hard stone, which is hewn out of the quarry just like other stones for[Pg 167] building; this stone yields a great deal of Alum. In order to extract it, the stones are calcined for twelve or fourteen hours; after which they are exposed to the air in heaps, and carefully watered three or four times a-day for forty days together. In that time they begin to effloresce, and to throw out a reddish matter on their surface. Then they are boiled in water, which dissolves all the Alum they contain, and, being duly evaporated, gives it back in crystals. This is the Alum called Roman Alum.
Several sorts of Pyrites also yield a great deal of Alum. The English have a stone of this kind, which, in colour, is very like a slate. This stone contains much Sulphur, which they get rid of by roasting it. After this they steep the calcined stone in water, which dissolves the Alum it contains, and to this solution they add a certain quantity of a lye made of the ashes of sea-weeds.
The Swedes have a Pyrites of a bright golden colour, variegated with silver spots, from which they procure Sulphur, Vitriol, and Alum. They separate from it the Sulphur and the Vitriol by the methods above prescribed. When the liquor which hath yielded Vitriol is become thick, and no more vitriolic crystals shoot in it, they add an eighth part of its weight of putrefied urine, mixed with a lye made of the ashes of green wood. Upon this there appears and falls to the bottom a copious red sediment. They decant the liquor from this precipitate, and, when it is duly evaporated, find it shoot into beautiful crystals of Alum.
What hath been said, concerning the several matrices from which Alum is obtained, sufficiently shews, that it is seldom solitary in the waters with which aluminous subjects have been lixiviated. It is almost always accompanied with a certain quantity of Vitriol, or other saline mineral matters, which obstruct its crystallization, and prevent its being pure. It is with a view to free it from these matters, that the waters impregnated with Alum are mixed with a certain quantity of the lye of some fixed Alkali, or with putrefied urine, which contains much volatile Alkali. These Alkalis have the property of decompounding all the Neutral salts which have for their basis either an absorbent earth or a metallic substance; and such as have a metallic substance for their basis more readily than those whose basis is an earth. Consequently, if they are mixed with a liquor in which both these sorts of salts are dissolved, they must decompound that sort whose basis is metallic sooner than the other whose basis is an earth. This is what comes to pass in a solution of[Pg 168] Alum and Vitriol. The metallic part of the latter is separated from its acid by the Alkalis when mixed with that solution; and it is this metallic part, which is generally iron, that appears in the form of a reddish precipitate, as above-mentioned.
But because Alkalis decompound also those Neutral salts which have an earth for their basis, care must be taken that too much thereof be not added; else what you put in, more than is necessary to decompound the vitriolic salts in your liquor, will attack the Alum, and decompound it likewise.
The Alkali made use of to promote the crystallization of the Alum joins with the Vitriolic Acid, which had dissolved the substances now precipitated, and therewith forms different Neutral salts according to its particular nature. If the Alkali be a lixivium of common wood-ashes, the Neutral salt will be a vitriolated Tartar; if a lixivium of the ashes of a maritime plant like Soda, the Neutral salt will be a Glauber's salt; if putrefied urine, the Neutral salt will be a vitriolic Ammoniacal salt. Some of these salts incorporate with the Alum, which in large works crystallizes in vast lumps: and hence it comes that some sorts of Alum when mixed with a fixed Alkali smell like a volatile Alkali.
The crystals of Alum are octaedral, that is, they are solids with eight sides. These octaedral solids are triangular pyramids, having their angles cut away, so that four of their surfaces are hexagons, and the other four triangles.
Sulphur, Vitriol, and Alum are the three principal subjects in which we certainly know that the universal or Vitriolic Acid particularly resides, and from which we extract it when we want to have it pure. For this reason we thought it proper, before we treated of the extraction of this Acid, to shew the method of separating those matters themselves from the other minerals out of which we obtain them.
Moreover, all the other matrices, in which the Vitriolic Acid is most commonly lodged, may be referred to one or other of the matters which serve as bases to these three minerals.
To Sulphur we may refer all combinations of the Vitriolic Acid with an inflammable matter: but we must take care not to confound Sulphur with those Bitumens in which the Vitriolic Acid may be found: for the basis of those bitumens is a real Oil; whereas the basis of Sulphur is the pure Phlogiston. Yet as Oils themselves contain the Phlogiston, which, in union with the Vitriolic Acid forms a true[Pg 169] Sulphur, it follows that such bitumens may in a certain respect be classed with Sulphur.
The same is to be said of Vitriol. The name is usually given to such combinations only as are formed of the Vitriolic Acid with Iron or Copper, which make the green and blue Vitriol; and to a third species of Vitriol, which is white, and has Zinc for its basis: but as the Vitriolic Acid may, by particular combinations, be united with many other metallic substances, all such Metallic Salts must be referred to the class of Vitriols.
The same may also be said of Alum, which is no other than a combination of the Vitriolic Acid with a particular kind of absorbent earth; so that all combinations of this Acid with any earth whatever may be placed in the same class.
This last class of mixts is the most extensive of all that contain the Vitriolic Acid; because there are a vast many earths, all differing from one another, with which that Acid may be united. Alum properly so called, the Gypsums, Talcs, Selenites, Boles, and all the other compounds of this kind, differ from each other only in their particular earths.
The different properties of these earthy salts depend on the nature of their bases. Those which are of the aluminous kind retain much water in crystallizing, which makes them very soluble in water, and gives them the property of acquiring readily the aqueous fluor when exposed to the fire. Those which are of the nature of the Selenites admit but very little water in their crystals, and consequently are almost insoluble in water; nor does the fire give them an aqueous fluor. Lastly, the Gypsums and Talcs are still more destitute of these properties. The natures of the earths in these several compounds are hitherto but very imperfectly known, and may give the Chymists occasion for inquiries equally curious and useful.
The Vitriolic Acid is sometimes found complicated with a fixed alkaline basis. This is almost always the Alkali of Sea-salt; so that the compound is a Glauber's Salt. Some mineral waters are impregnated therewith; which happens when these waters contain Vitriol or Alum, together with Sea-salt.
From the principles laid down, in our Elements of the Theory, it appears that the Vitriolic Acid hath not so great an affinity with earthy and metallic substances as with fixed Alkalis; and also that it is stronger than the Marine Acid,[Pg 170] and hath a greater affinity with fixed Alkalis. This being allowed, the generation of native Glauber's Salts is easily accounted for. The Acid of aluminous or vitriolic Salts quits the earth or the metal with which it was combined, and expelling the Acid of Sea-salt unites with its basis. Warmth greatly promotes these decompositions.
If the common fossil salt, usually called Sal Gem, or any other kind of Sea-salt, should happen to be near a Volcano, when it discharges flaming Sulphur, as is frequently the case, and if this Sulphur should run among the Sea-salt, a Glauber's Salt would instantly be formed in that place; because when Sulphur burns, its Acid is separated and set at liberty.
Lastly, if aluminous or vitriolic matters, or burning Sulphur, should meet with the ashes of plants or trees consumed by fire, a vitriolated Tartar would be formed, because these ashes contain a fixed Alkali of the same nature with that of Tartar.
The Vitriolic Acid when combined with an earthy basis adheres strongly thereto; so that the force of fire is able to expel very little or none of it. There is no way of separating it from such a basis, but by presenting to it an Alkaline Salt, with which it will unite: nor is it ever extracted from such matters when it is required pure. It does not adhere so firmly to metallic substances; but is separated from them by the force of fire: so that it may be obtained from the several sorts of Vitriol. It is usually drawn from Green Vitriol; that being the commonest sort.
As to Sulphur, the Phlogiston which is its basis being the substance wherewith the Vitriolic Acid hath the greatest affinity, it would be altogether impossible to decompose it, and to separate its Acid, if it were not inflammable; but by burning it the Phlogiston is destroyed, and leaves the Acid at liberty. By this means therefore it may be separated. We shall now give the processes for extracting the Acid from Vitriol and Sulphur.
To extract the Vitriolic Acid from Green Vitriol.
Take any quantity of Green Vitriol: put it in an unglazed earthen vessel, and heat it gradually. Vapours will soon begin to rise. Increase the fire a little, and it will[Pg 171] liquefy by means of the water contained in it, and acquire what we called an aqueous fluor. Continue the calcination, and it will become less and less fluid, grow thick, and turn of a greyish colour. Now raise your fire, and keep it up till the salt recover its solidity, acquire an orange colour, and begin to grow red where it immediately touches the sides of the vessel. Then take it out, and reduce it to powder.
Put the Vitriol thus calcined and pulverized into a good earthen retort, of which one half at least must remain empty. Set the retort in a reverberatory furnace: fit thereto a large glass receiver, and, having luted the joint well, give fire by degrees. You will soon see white clouds rise into the receiver, which will render it opaque, and heat it. Continue the same degree of fire till these clouds disappear: they will be succeeded by a liquor which will trickle down the sides of the receiver in veins. Still keep up the fire to the same degree as long as these veins appear. When they begin to abate, increase the fire, and push it to the utmost extremity: upon this, there will come over a black, thick liquor: it will even be found congealed, and prove the icy Oil of Vitriol, if care hath been taken to change the receiver, keep the vessels perfectly close, and give a sufficient degree of heat. Proceed thus till nothing more comes over, or at least very little. Let the vessels cool, unlute them, pour the contents of the receiver into a bottle, and seal it hermetically.
OBSERVATIONS.
Green Vitriol retains much water in crystallizing; and, in order to free it from that superfluous phlegm, it must be calcined before you distil it. Without this precaution the operation will be exceedingly protracted, and a great deal of time wasted in distilling such a quantity of water; which will moreover greatly weaken the Acid by commixing with it, unless care be taken to change the recipient as soon as the water is all come over.
But there is also another advantage in calcining the Vitriol before you put it into the retort: for otherwise this salt would melt on the first application of heat, and run into a mass; which would prove a great hindrance to its distillation. This inconvenience is avoided by a previous calcination, in consequence whereof the Vitriol is easily reduced to a powder which never becomes fluid.
Vitriol calcined as directed in the process grows so hard, and adheres so firmly to the vessel in which the calcination is performed, that it requires no small pains to separate and pulverize it. Care must be taken to put it into the retort as soon as it is pulverized, and to stop that vessel very close if you do not begin the distillation immediately: for otherwise it will naturally attract from the air almost all the moisture it hath lost.
The Acid which Vitriol yields by distillation is sulphureous; probably because it still retains some of the Phlogiston, with which it was united when under the form of sulphur in the Pyrites; or else hath laid hold on a portion of that belonging to the iron which served for its basis in Vitriol. But this sulphureous part is volatile, and flies off in time.
This decomposition of Vitriol in close vessels is a difficult and laborious process. To carry the operation to its utmost perfection requires a fire of extreme violence, kept up without intermission during four or five days; such in short as few vessels are able to bear. Of course this operation is seldom performed in laboratories. The French Chymists fetch their Oil of Vitriol from Holland, where it is extracted from Vitriol in large quantities, by means of furnaces erected for the purpose, in which many retorts are employed at once.
In the Memoirs of the Academy of Sciences M. Hellot hath given us the most material circumstances of a very fine experiment of this kind, in which he pushed the distillation of Green Vitriol to the utmost. Into a German retort[5] he put six pounds of Green English Vitriol calcined to redness, which he exposed to a fire of the extremest violence, constantly kept up during four days and four nights. At the expiration of that time he found in the vessels employed as receivers an Icy Oil of Vitriol, which was altogether in a crystalline form and black. The precautions necessary to make this experiment succeed, he represents, in the following terms.
"The success of this operation, which produces an Oil of Vitriol perfectly Icy and without any liquor, depends on the care taken to prevent the acid vapours, driven by the fire out of Vitriol calcined to redness, from having any communication with the external air while they are distilling: for otherwise they will attract from it a moi[Pg 173]sture which will keep them fluid in the receiver. The receiver must be at such a distance from the furnace that it may remain cool enough for the vapours to condense in it. There must also be sufficient room for those vapours to circulate in, and to prevent the sulphureous explosions, which are every now and then discharged out of the retort, from bursting the vessels: for though the previous calcination of the Vitriol hath carried off the most volatile, yet there still remains enough of the inflammable principle, even in the iron itself, to form a Sulphur with the Acid as it is extricated, or at least a mixt that would be as apt to take fire as common Sulphur, if it were not over-dosed with the Acid.
"As the best means of gaining these ends, M. Hellot contrived to adapt to the neck of his retort a receiver with two necks, the lowermost of which was inserted into a large ballon. Receivers applied to each other in this manner are called Adopters.
"It is no easy matter to get this Icy Oil out of the ballon: for as soon as the air touches it such a thick cloud of sulphureous fumes arises, that it is absolutely necessary to place the vessel on some shelf over head, because a man cannot stand exposed thereto for a single minute without being suffocated."
This Icy Acid must be shut up with all possible expedition in a crystal bottle accurately closed with a glass stopple, which should be ground with emery in its neck so as to fit it exactly: for it attracts moisture so powerfully, that, unless exceeding great care be taken to prevent all communication with the external air, it will soon dissolve into a fluid.
"The Icy Oil is black; because the acid vapours carry over with them something of a greasy matter, from which Vitriol is seldom free, and which always appears, after repeated solutions and crystallizations of this Salt, in the mother-water which will shoot no more. Now the smallest portion of inflammable matter presently blackens the most highly rectified Oil of Vitriol, which is perfectly clear.
"The Vitriolic Acid, when forced over by a violent heat, carries along with it some ferruginous particles also, that want nothing but to be united with a phlogiston to become true iron. They are easily discovered, either in the common black Oil of Vitriol, or in the blackish crystals of the Icy Oil, by only dissolving them in a large quantity of[Pg 174] distilled water: for after seven or eight days digestion a light powder or downy sediment precipitates, which being calcined in a violent fire is partly attracted by the magnet; and being again calcined with bees-wax becomes almost entirely iron."
The Caput mortuum of this distillation of Vitriol is the ferruginous earth of this Salt, and is called Colcothar. When this Colcothar hath undergone a violent fire, as in the experiment now related, scarce any Acid remains therein. Out of six pounds of Vitriol that M. Hellot used, he could recover no more, by lixiviating what was left in the retort, than two ounces of a Vitriolic Salt; and even that was very earthy.
If Vitriol be exposed to a fire neither so violent nor so long continued, its Colcothar will yield a greater quantity of Vitriol that hath not been decomposed. A white crystalline salt is also obtained from it, and called Salt of Colcothar; which is no other than the small portion of Alum usually contained in Vitriol, and not so easily decomposed by the the action of fire.
To decompose Sulphur, and extract its Acid, by burning it.
Take any quantity of the purest Sulphur: fill therewith a crucible or other earthen dish: heat it till it melts; then set it on fire, and, when its whole surface is lighted, place it under a large glass head, taking care that the flame of the Sulphur do not touch either its sides or bottom; that the air have free access, in order to make the Sulphur burn clear; and that the head incline a little toward the side on which its beak is, that, as the vapours condense therein, the liquor may run off with ease. To the beak of this vessel fit a receiver: the fumes of the lighted sulphur will be condensed, and gather into drops in the head, out of which they will run into the receiver. There, when the Sulphur has done burning, you will find an Acid liquor, which is the Spirit of Sulphur.
OBSERVATIONS.
In the burning of Sulphur, the Phlogiston which serves for its basis is dissipated, and separated from the acid which[Pg 175] is left at liberty. The acid fumes which rise from the lighted sulphur strike against the inside of the head placed over it, are there condensed, and appear in the form of a liquor. But as Sulphur, like all other inflammable bodies, Nitre excepted, will not burn in close vessels, it is necessary that the air be freely admitted here; which occasions the loss of a great deal of the Acid of the Sulphur, as is evident from the pungent suffocating smell perceived in the laboratory during the operation.
This Acid, while combined with the Phlogiston, is incapable of contracting any union with water; but when alone is very apt to mix therewith: it is even proper to put some in its way, that it may incorporate therewith as soon as it is discharged from the Sulphur; for it is then very free from phlegm, very volatile, and consequently very little disposed to condense into a liquor, but, on the contrary, very apt to fly off in vapours. The water, which it imbibes with a kind of avidity, fixes and detains it; so that by this means a much greater quantity thereof is obtained from Sulphur, than if it were distilled without this precaution.
It is proper, therefore, now and then, to introduce a dish full of hot water under the head which receives the fumes of the Sulphur. The vapours that exhale from the water be-dew the inside of the head, and procure the advantage we are speaking of.
The same thing may be effected several other ways: thus, the crucible containing the Sulphur may be set on a foot placed in an earthen dish with some water in it; which, however, must not rise above the foot; for if it should reach the crucible, it might cool and fix the sulphur. The dish thus prepared must be placed on a sand-bath hot enough to make the water smoke continually; and over all is to be placed the head as directed in the process.
The size and form of the vessel which immediately receives the sulphureous fumes may also contribute to increase the quantity of the Acid Spirit. A very large vessel, with a hole at bottom no wider than is just sufficient to admit the vapours, is the properest for this operation.
After the Sulphur has burnt for some time, it often happens that a sort of skin or crust forms on its surface, which is not inflammable, but gradually lessens the quantity and vigour of the flame as it increases in thickness, and at last puts it quite out. This crust proceeds from the impurities, and heterogeneous uninflammable particles contained in the sul[Pg 176]phur. Care must be taken to remove it with an iron wire as fast as it forms.
Two quantities of sulphur may also be kept in two crucibles, and heated alternately. That in which the Sulphur is hot and melted may be substituted for the other in which the Sulphur is grown cold and fixed; because cold Sulphur does not burn well.
The Spirit of Sulphur is at first pungent and volatile, because it still retains a small portion of the Phlogiston: but that sulphureous part flies off, especially if the bottle in which the Spirit is kept be left for some time unstopped.
The Acid obtained from Sulphur appears by all chymical proofs perfectly like that obtained from Vitriol: they differ in this only, that the former is the purest; for the Acid obtained from Vitriol carries over with it some metallic parts, as we observed before, which can never happen to that obtained from Sulphur.
If linen rags dipped in a solution of Fixed Alkali be exposed to the fumes of burning brimstone, the Spirit of Sulphur joins with the Alkali, and therewith forms a Vitriolated Tartar. This Salt is known to be formed when the rags grow stiff, and appear spangled with a vast many glittering points, which are nothing but little crystals of the Salt we are speaking of.
When the Sulphur burns very gently and slowly the Spirit that exhales from it is so much the more sulphureous and volatile: and hence the Salt formed by the combination of this Spirit with the Alkali exposed to it in linen rags, as in the above-mentioned experiment, is not at first a Vitriolated Tartar; but a Neutral Salt of a particular kind, which is capable of being decomposed by any other Mineral Acid, the sulphureous Acid having less affinity than any of the rest with Alkalis. Nevertheless, this Salt becomes in time a true Vitriolated Tartar, because the sulphureous part which weakened its Acid easily quits it and flies off.
To concentrate the Vitriolic Acid.
Take the Vitriolic Acid you intend to concentrate, that is, to dephlegmate and make stronger: pour it into a good glass retort, of such a size that your quantity of Acid may but half fill it: set this retort in the sand-bath of a re[Pg 177]verberating furnace: fit to it a receiver; lute it on, and give a gradual fire. There will come over into the receiver a clear liquor, the first drops of which will be but faintly acid: this is the most aqueous part.
When the drops begin to follow one another much more slowly, raise your fire, till the liquor begin to bubble a little in the middle. Keep it thus gently boiling, till one half or two thirds thereof be come over into the receiver. Then let your vessels cool; unlute them; what remains in the retort pour into a crystal bottle, and stop it exactly with a glass stopple rubbed with emery.
OBSERVATIONS.
The Acid obtained from Sulphur is generally very aqueous; either because in preparing it water must necessarily be administered, that it may unite therewith as it separates from the Sulphur; or because it is so greedy of moisture as to attract a great deal from the air, which must needs be admitted to make the Sulphur burn.
The Acid obtained from Vitriol, excepting that which rises last, is also mixed with a pretty considerable quantity of phlegm; because the Vitriol, though calcined, still retains a great deal thereof, which rises with the Acid in distillation. Now, as there are many chymical experiments that will not succeed without Acids exceedingly dephlegmated, it is proper to have in a laboratory all the Acids thus conditioned; because if they happen to be too strong for particular operations, as is sometimes the case, it is very easy to lower them to the desired degree, by adding a sufficient quantity of water.
The Vitriolic Acid is much heavier and much less volatile than water. If therefore a mixture of these two liquors be exposed to the fire, the aqueous part will rise with a degree of heat which is not able to carry up the Acid: by this means they may be separated from each other; and thus is the Vitriolic Acid concentrated.
Nevertheless, as this Acid combines most closely with water, and is in a manner strongly connected with it, the water carries up some portion thereof along with it; and hence it comes, that the liquor which rises into the receiver is acid: it is called Spirit of Vitriol.
As the fire carries off the most aqueous part, the other which remains in the retort increases in specific gravity. The Acid particles are brought nearer together, retain the aque[Pg 178]ous particles more obstinately, and therefore to separate them the degree of heat must be increased.
It is usual to draw off one half or two thirds of the liquor that was put into the retort: but this depends on the degree of strength the Acid was of before concentration, and the degree of concentration intended to be given it.
If the Acid to be concentrated be Oil of Vitriol, from being brown or black it grows clearer as the operation advances, and at last becomes perfectly colourless and transparent; because the fat matter which tinged it black is dissipated during the process. Some of it deposites a white crystalline earth.
A sulphureous smell is generally perceived about the vessels in this operation. This arises from a small portion of the Phlogiston from which the Acid is not free; and it is this inflammable matter which gives the Oil of Vitriol its black colour: for the clearest and best rectified Oil of Vitriol will become brown, and even black, in a short time, if any inflammable matter, though in a very small quantity, be dissolved therein.
The vessels are luted in this operation, to prevent any loss of the Spirit of Vitriol, which being very acid is of use in many chymical experiments, and may itself also be again concentrated.
We observed, that in this operation it is necessary the retort should be of very good glass. Indeed the Acid is so active, and so strong, that if the glass be tender and have a little too much salt in its composition, it will be so corroded thereby that it will fall to pieces.
Though we directed the retort to be set in a sand-bath for this operation, it does not follow that it may not also be placed in a naked fire: on the contrary, when the heat is not conveyed through a bath the operation advances faster, and is much less tedious. But then great caution must be used, and the closest attention given to the management of the fire, which must be raised by almost imperceptible degrees, especially at the beginning of the operation; otherwise it is next to a certainty that the vessels will break. In general, a naked fire may be employed in almost all distillations which require a greater degree of heat than that of boiling water, or the balneum mariæ: the operation will be sooner finished; but it requires an experienced hand, that has by practice acquired a habit of governing the fire with judgment.
There is moreover another advantage in not using the sand-bath; which is, that if in the time of the operation you[Pg 179] perceive the fire too fierce, you can quickly check it, either by stopping close all the apertures of the furnace, or by drawing out all or part of the lighted coals. This inconvenience is not near so easily remedied when you use the sand-bath; because when once heated it retains its heat very long after the fire is quite extinguished.
To decompose Vitriolated Tartar by means of the Phlogiston; or to compose Sulphur by combining the Vitriolic Acid with the Phlogiston.
Take equal parts of Vitriolated Tartar, and very dry Salt of Tartar, separately reduced to powder; add an eighth part of their weight of charcoal-dust; and mix the whole together very accurately. Throw this mixture into a red-hot crucible, placed in a furnace filled with burning coals. Cover it very close, and keep it very hot; till the mixture melt, which may be known by uncovering the crucible from time to time. There will then appear a blueish flame, accompanied with a pungent smell of Sulphur.
Take the crucible out of the fire: dissolve its contents in hot water: filter the solution through brown paper supported by a glass funnel: drop into the filtered liquor by little and little any Acid whatever. As you add the acid the liquor will grow more and more turbid, and let fall a grey precipitate. Continue dropping in more Acid till the liquor will yield no more precipitate. Filter it a second time, to separate it from the precipitate: what remains on the filter is a true inflammable Sulphur, which you may either melt or sublime into flowers.
OBSERVATIONS.
All bodies that contain the Vitriolic Acid may contribute, as well as Vitriolated Tartar, to the generation of Sulphur: so that all the neutral salts in which this Acid is a principle, the Alums, Selenites, Gypsums, Vitriols, may be substituted for it in this experiment. All these matters, with the addition of charcoal-dust only, being fused in a crucible, constantly produce Sulphur; because the Vitriolic Acid having a greater affinity with the Phlogiston than with any thing[Pg 180] else, will quit its basis, whatever it be, to join with the Phlogiston of the charcoal, and therewith form a Sulphur.
The fixed Alkali added thereto helps to promote the fusion of the ingredients, which is necessary for effecting the desired combination. It also serves to unite with the Sulphur, when formed; and thus makes the combination called Liver of Sulphur, which prevents the Sulphur from being consumed as soon as formed: for the fixed Alkalis, which are incombustible, hinder Sulphur from burning so easily as it would do if they were not joined with it. They may afterwards be separated from each other, by the means of any Acid whatever.
This process, in which Sulphur is regenerated by recombining together the principles of which it was originally composed, is one of the most beautiful experiments that modern Chymistry hath produced. We are indebted for it to M. Stahl; and Dr. Geoffroy hath given a particular account of it in the Memoirs of the Academy of Sciences.
Before these gentlemen Glauber and Boyle had indeed published methods of producing Sulphur, Glauber made use of his Sal mirabile and powdered charcoal: Boyle employed the Vitriolic Acid and Oil of Turpentine. But neither of those Chymists understood the true theory of their operations: they did not thoroughly know the principles of Sulphur: they did not imagine they had composed Sulphur: they thought they only extracted what they supposed to exist previously in the matters they employed in their experiments.
M. Stahl was the first who discovered and explained the nature of Sulphur, and proved that in Glauber's and Boyle's experiments Sulphur was actually produced, by uniting together the principles of which it is constituted. This beautiful experiment gives the strongest lustre of evidence to the theory of the composition of that mixt, which acts such a capital part in Chymistry; and it can no longer be doubted, that Sulphur is actually a combination of the Vitriolic Acid with the Phlogiston.
Besides this important truth, our process for composing Sulphur by art proves several others that are equally essential and fundamental.
The first is, that the Vitriolic Acid hath a greater affinity with the Phlogiston than with any other thing, seeing it quits metallic and earthy substances, as well as Alkaline salts, in order to combine therewith.
The second is, that Sulphur combines with fixed Alkalis without suffering any decomposition; seeing it may be sepa[Pg 181]rated from them entire and unaltered; and seeing that very Sulphur, which is naturally indissoluble in water, is rendered soluble therein by the union it hath contracted with the fixed alkali.
The third is, that the Vitriolic Acid, which, when it is pure, hath the greatest affinity with Alkalis of any Acid whatever, loses a great deal of that affinity by contracting an union with the Phlogiston; seeing the weakest acids are capable of decomposing the Liver of Sulphur, and separating the Sulphur from the Alkali. And this also confirms one of the general propositions concerning affinities advanced in our theory; to wit, that the affinities of compound or mixed substances are weaker than those of the same substances in a purer or more simple state.
Of the Nitrous Acid.
To extract Nitre out of nitrous Earths and Stones. The Purification of Salt-petre. Mother of Nitre. Magnesia.
Take any quantity of nitrous earths or stones; reduce them to powder; and therewith mix a third part of the ashes of green-wood and quick-lime. Put this mixture into a barrel or vat, and pour on it hot water to about twice the weight of the whole mass. Let it stand thus for twenty-four hours, stirring it from time to time with a stick. Then filter the liquor through brown paper, or pass it through a flannel bag, till it come clear: it will then have a yellowish colour. Boil this liquor, and evaporate till you perceive that a drop of it let fall on any cold body coagulates. Then stop the evaporation, and set your liquor in a cool place. In the space of four and twenty hours crystals will be formed in it, the figure of which is that of an hexagonal prism, having its opposite planes generally equal, and terminated at each extremity by a pyramid of the same number of sides. These crystals will be of a brownish colour, and deflagrate on a live coal.
Decant the liquor from these crystals; mix it with twice its weight of hot water; evaporate and crystallize as before. Repeat the same operation till the liquor will yield no more crystals: it will then be very thick, and goes by the name of Mother of Nitre.
OBSERVATIONS.
Earths and stones that have been impregnated with animal or vegetable juices susceptible of putrefaction, and have been long exposed to the air, but sheltered from the sun and rain, are those which yield the greatest quantity of Nitre. But all sorts of earths and stones are not equally fit to produce it. None is ever found in flints or sands of a crystalline nature.
Some earths and stones abound so with Nitre, that it effloresces spontaneously on their surface, in the form of a crystalline down. This Nitre may be collected with brooms, and accordingly has the name of Salt-petre Sweepings. Some of this sort is brought from India.
Hitherto we are much in the dark as to the origin and generation of Nitre. Some Chymists pretend that the Nitrous Acid is diffused through the air, and gradually deposited in such earths and stones as are qualified to receive it.
Others, considering that none of it is ever obtained but from earths that have been impregnated with vegetable or animal juices, have from thence concluded those two kingdoms to be the general repositories of the Nitrous Acid; that if we do not perceive it to exist in such matters at all, or at least in any great quantity, till they have undergone putrefaction, and are in some measure incorporated with suitable earths and stones, it is because the Acid is so entangled with heterogeneous particles that it requires the assistance of putrefaction, and much more of filtration through an earth, to disengage it, and enable it to appear in its proper nature.
Lastly, others are of opinion that this Acid is no other than the universal or Vitriolic Acid; disguised indeed by a portion of the Phlogiston, which is combined with it in a peculiar manner by the means of putrefaction. They ground this opinion chiefly on the analogy or resemblance which they find between the Nitrous Acid and the Volatile Sulphureous Spirit. Its volatility, its pungent smell, its properties of taking fire, and of destroying the blue and violet colours of vegetables, serve them as so many proofs.
Their opinion is the more probable on this account, that even though the Nitrous Acid should actually be produced by vegetable and animal substances, yet as these substances themselves draw all their component principles from the earth, and as the Vitriolic Acid is diffused through all the soils which afford them nourishment, there is great reason to think that the Nitrous Acid is no other than the Vitriolic Acid altered by the changes and combinations it hath undergone in its passage into and through those substances. In 1750 the Royal Academy of Sciences at Berlin proposed an account of the generation of Nitre as the subject for their prize, which was conferred on a Memoir wherein this last opinion was supported by some new and very judicious experiments.
The process by which our Salt-petre makers extract Nitre in quantities, out of rubbish and nitrous earths, is very nearly the same with that here set down; so that I shall not enter into a particular account of it. I shall only take notice of one thing, which it is of some consequence to know; namely, that there is no nitrous earth which does not contain sea-salt also. The greatest quantities of this salt are to be found in those earths which have been drenched with urine, or other animal excrements. Now as the rubbish of old houses in great cities is in this class, it comes to pass that when the Salt-petre workers evaporate a nitrous lixivium drawn from that rubbish, as soon as the evaporation is brought to a certain pitch, a great many little crystals of sea-salt form in the liquor, and fall to the bottom of the vessel.
The Salt-petre workers in France call these saline particles the Grain, and take care to separate them from the liquor (which, as long as it continues hot, keeps the salt-petre dissolved) before they set it to crystallize. This fact seems a little singular, considering that sea-salt dissolves in water more easily than salt-petre, and crystallizes with more difficulty.
In order to discover the cause of this phenomenon, we must recollect some truths delivered in our theoretical Elements. The first is, that water can keep but a determinate quantity of any salt in solution, and that if water fully saturated with a salt be evaporated, a quantity of salt will crystallize in proportion to the quantity of water evaporated. The second is, that those salts which are the most soluble in water, particularly those which run in the air, will dissolve in cold and in boiling water equally; whereas much greater quantities of the other salts will dissolve in hot and boiling[Pg 184] water than in cold water. These things being admitted, when we know that sea-salt is one of the first sort, and salt-petre of the second, the reason why sea-salt precipitates in the preparation of salt-petre appears at once. For,
When the solution of Salt-petre and Sea-salt comes to be evaporated to such a degree that it contains as much Sea-salt as it possibly can, this salt must begin to crystallize, and continue to do so gradually as the evaporation advances. But because at the same time it does not contain as much salt-petre as it can hold, seeing it is capable of dissolving a much greater quantity thereof when it is boiling hot than when it is cold, this last-named salt will not crystallize so soon. If the evaporation were continued till the case of the Salt-petre came to be the same with that of the Sea-salt, then the salt-petre also would begin to crystallize gradually in proportion to the water evaporated, and the two salts will continue crystallizing promiscuously together: but it is never carried so far; nor is it ever necessary: for, as the water cools, it becomes more and more incapable of holding in solution the same quantity of salt-petre as when it was boiling hot.
And then comes the very reverse, with regard to the crystallizing of the two salts; for then the Salt-petre shoots, and not the Sea-salt. The reason of this fact also is founded on what has just been said. The Sea-salt, of which cold water will dissolve as much as boiling water, and which owed its crystallizing before only to the evaporation, now ceases to crystallize as soon as the evaporation ceases; while the Salt-petre, which the water kept dissolved only because it was boiling hot, is forced to crystallize merely by the cooling of the water.
When the solution of Salt-petre has yielded as many crystals of that Salt as it can yield by cooling, it is again evaporated, and being then suffered to cool yields more crystals. And thus they continue evaporating and crystallizing, till the liquor will afford no more crystals. It is plain that as the Salt-petre crystallizes, the proportion of Sea-salt to the dissolving liquor increases; and as a certain quantity of water evaporates also during the time employed in crystallizing the Salt-petre, a quantity of Sea-salt, proportioned to the water so evaporating, must crystallize in that time: and this is the reason why Salt-petre is adulterated with a mixture of Sea-salt. It likewise follows that the last crystals of Nitre, obtained from a solution of Salt-petre and Sea-salt, contain much more Sea-salt than the first.
From all that has been said concerning the crystallization of Salt-petre and Sea-salt, it is easy to deduce the proper way of purifying the former of these two Salts from a mixture of the latter. For this purpose the Salt-petre to be refined need only be dissolved in fair water. The proportion between the two salts in this second solution is very different from what it was in the former; for it contains no more Sea-salt than what had crystallized along with the Salt-petre under favour of the evaporation, the rest having been left dissolved in the liquor that refused to yield any more nitrous crystals.
As there is therefore a much greater quantity of Salt-petre than of Sea-salt in this second solution, it is easy to evaporate it to such a degree that a great deal of Salt-petre shall crystallize, while much more of the water must necessarily be evaporated before any of the Sea-salt will crystallize.
However, the Salt-petre is not yet entirely freed from all mixture of Sea-salt by this first purification; for the crystals obtained from this liquor, in which Sea-salt is dissolved, are still encrusted, and, as it were, infected therewith: hence it comes, that, to refine the Salt-petre thoroughly, these crystallizations must be repeated four or five times.
The Salt-petre men commonly content themselves with crystallizing it thrice, and call the produce Salt-petre of the first, second, or third shoot, according to the number of crystallizations it has undergone. But their best refined Salt-petre, even that of the third shooting, is not yet sufficiently pure for Chymical experiments that require much accuracy: so that it must be further purified; but still by the same method.
The Nitrous Acid is not pure in the earths and stones from which it is extracted. It is combined partly with the very earth in which it is formed, and partly with the Volatile Alkali produced by the putrefaction of the vegetable or animal matters that concurred to its generation. A Fixed Alkali and Quick-lime are added to the lixivium of a nitrous earth, in order to decompose the nitrous Salt formed in that earth, and to separate the Acid from the Volatile Alkali and the absorbent earth with which it is united: thence comes that copious sediment which appears in the lye at the beginning of the evaporation. These matters form with that Acid a true Nitre, much more capable than the original Nitrous Salts of crystallization, detonation, and the other pro[Pg 186]perties which are essential thereto. The basis of Nitre is therefore a Fixed Alkali mixed with a little lime.
The Mother of Nitre, which will yield no more crystals, is brown and thick: by evaporation over a fire it is further inspissated, and becomes a dry, solid body; which, however, being left to itself soon gives, and runs into a liquor. This water still contains a good deal of Nitre, Sea-salt, and the Acids of these Salts united with an absorbent earth. It contains moreover a great deal of a fat, viscid matter, which prevents its crystallizing.
All saline solutions in general, after having yielded a certain quantity of crystals, grow thick, and refuse to part with any more, though they still contain much Salt. They are all called Mother-waters, as well as that which hath yielded Nitre. The Mother-waters of different Salts may prove the subjects of curious and useful inquiries.
If a Fixed Alkali be mixed with the Mother of Nitre, a copious white precipitate immediately falls, which being collected and dried is called Magnesia. This precipitate is nothing but the absorbent earth that was united with the Nitrous Acid, together with a good deal of the lime that was added, and was also united with that Acid, from which they are now separated by the Fixed Alkali, according to the usual laws of affinities.
The Vitriolic Acid poured upon Mother of Nitre causes many Acid vapours to rise, which are a compound of the Nitrous and Marine Acids, that is, an Aqua Regia. On this occasion also there falls a large quantity of a white powder, which is still called Magnesia; yet it differs from the former in that it is not, like it, a pure absorbent earth, but combined with the Vitriolic Acid.
An Aqua regis may also be drawn from nitrous earths by the force of fire only, without the help of any additament.
To decompose Nitre by means of the Phlogiston. Nitre fixed by Charcoal. Clyssus of Nitre. Sal Polychrestum.
Take the purest Salt-petre in powder; put it into a large crucible, which it may but half fill; set the crucible in a common furnace, and surround it with coals. When it is red-hot the Nitre will melt, and become as fluid[Pg 187] as water. Then throw into the crucible a small quantity of charcoal-dust: the Nitre and the Charcoal will immediately deflagrate with violence; and a great commotion will be raised, accompanied with a considerable hissing, and abundance of black smoke. As the charcoal wastes, the detonation will abate, and cease entirely as soon as the coal is quite consumed.
Then throw into the crucible the same quantity of charcoal-dust as before, and the same phenomena will be repeated. Let this coal also be consumed: then add more, and go on in the same manner till you can excite no further deflagration; always observing to let the burning coal be entirely consumed before you add any fresh. When no deflagration ensues, the matter contained in the crucible will have lost much of its fluidity.
OBSERVATIONS.
Nitre will not take fire, unless the inflammable matter added to it be actually burning, or the Nitre itself red-hot, and so thoroughly ignited, as immediately to kindle it. Therefore, if you would procure the detonation of Nitre with charcoal, and make use of cold charcoal, as in the process, the Nitre in the crucible must be red-hot, and in perfect fusion: but you may also use live coals, and then the Nitre need not be red-hot.
It is proper that the crucible used in this experiment should be only half full; for during the detonation its contents swell, and might run over without this precaution. For the same reason the charcoal-dust is to be thrown in by little and little; and that first put in must be entirely consumed before any fresh be added.
The matter remaining in the crucible after the operation is a very strong Fixed Alkali. Being exposed to the air it quickly attracts the moisture thereof, and runs into a liquor. It is called Alkalizated Nitre, or, to distinguish it from Nitre alkalizated by other inflammable matters, Nitre fixed by charcoal.
However, this Alkali is not absolutely pure. It still contains a portion of the Nitre that hath not been decomposed. For when there remains but a little of this salt mixed with a great quantity of Alkali, which is not inflammable, the Alkali in some measure shelters it, coats it over, and obstructs that immediate contact with the inflammable matters applied, which is necessary to make it deflagrate.
If the Fixed Alkali be desired perfectly free from any mixture of undecomposed Nitre, the fire about the crucible must be considerably increased as soon as the detonation is entirely over; the matter must be made to flow, which requires a much stronger heat than would melt Nitre, and kept thus in fusion for about an hour. After this no perfect Nitre will be found therein: for the little that was left, being unable to abide the force of the fire, as not being extremely fixed, either is entirely dissipated, or loses its Acid, which is carried off by the violence of the heat.
Fixed Nitre contains also a portion of the earth that constituted the basis of the Nitre, which is no other than the lime employed in its crystallization, or else some of the earth with which its Acid was originally combined, and which it retained in crystallizing. When Nitre is deflagrated with such matters as produce ashes, these ashes likewise furnish a certain quantity of earth, which mixes with the Fixed Alkali. To separate these several earths from the Alkali, nothing more is requisite than to let it run per deliquium, or to dissolve it in water, and filter the solution through brown paper. Whatever is saline will pass through the filtre with the water, and the earthy part will be left upon it.
The Nitrous Acid is not only dissipated during the deflagration of the Nitre, but is even destroyed, and perfectly decomposed. The smoke that rises during the operation has not the least odour of an Acid. Its nature may be accurately examined by catching it in proper vessels, and condensing it into a liquor.
Nitre differs from Sulphur, and from all other inflammable bodies whatever, in this, that the free access of the air is indispensably necessary to make any of the others burn; whereas Nitre, and Nitre only, is capable of burning in close vessels: and this property furnishes us with the means of collecting the vapours which it discharges in deflagration.
For this purpose, to a tubulated earthen retort you must fit two or three large adopters: set the retort in a furnace; and under it make a fire sufficient to keep its bottom moderately red. Then take a small quantity, two or three pinches for example, of a mixture of three parts of Nitre with one of charcoal-dust, and drop it into the retort through its tube, which must be uppermost, and immediately stopped close. A detonation instantly ensues, and the vapours that rise from the inflamed mixture of Nitre and charcoal, passing out[Pg 189] through the neck of the retort into the adopters, circulate therein for a while, and at last condense into a liquor.
When the detonation is over, and the vapours condensed, or nearly so, drop into the retort another equal quantity of the mixture; and repeat this till you find there is liquor enough in the recipients to be examined with ease and accuracy. This liquor is almost insipid, and shews no tokens of acidity; or at most but very slight ones. It is called Clyssus of Nitre.
It is easy to perceive why several adopters are required in this experiment, and why a very small quantity of the mixture must be introduced into the retort at once. The explosion, and the quantity of air and vapours discharged on this occasion, would quickly burst the vessels, if all these precautions were not attended to. This plainly appears from the terrible effects of gun-powder, which is nothing but a composition of Nitre, Sulphur, and Charcoal.
Nitre is also decomposed and takes fire by the means of Sulphur; but the circumstances and the result differ widely from those produced therewith by charcoal, or any other inflammable body.
Nitre deflagrates with Sulphur on account of the Phlogiston which the latter contains. If one part of Sulphur be mixed with two or three parts of Nitre, and the mixture thrown by little and little into a red-hot crucible, upon every projection there arises a detonation accompanied with a vivid flame.
The vapours discharged on this occasion have the mingled smell of a Sulphureous Spirit and Spirit of Nitre; and if they be collected by means of a tubulated retort, and such an apparatus of vessels as was used in the preceding experiment, the liquor contained in the recipients is found to be an actual mixture of the Acid of Sulphur, the Sulphureous Spirit, and the Acid of Nitre; the first being in greater quantity than the other two, and the second greater than the last.
Nor is the remainder after detonation a Fixed Alkali, as in the former experiments; but a Neutral Salt, consisting of the Acid of Sulphur combined with the Alkali of Nitre; a sort of Vitriolated Tartar known in medicine by the name of Sal Polychrestum.
There are evidently two essential differences between this last experiment and the preceding one. What remains after the deflagration of Nitre with Sulphur is not a Fixed Alkali: and, moreover, the vapours emitted in the operation are impregnated with a quantity of the Nitrous Acid; which is not[Pg 190] the case when Nitre is decomposed by any other inflammable matter which contains no Vitriolic Acid.
The reason of these differences is naturally deducible from what hath been already said concerning the properties of the Vitriolic and Nitrous Acids. We have seen that by burning Sulphur its Acid is not decomposed, but only separated from its Phlogiston. We also know, that its Acid has a great affinity with Fixed Alkalis. These things being granted, it follows that, as soon as the Nitrous Acid quits its Alkaline basis, by deflagrating with the Phlogiston of the Sulphur, the Acid of this very Sulphur, being set at liberty by that deflagration, must unite with the Alkaline basis deserted by the Acid of Nitre, and therewith form a Neutral Salt. Hence, instead of a Fixed Alkali, we find at the end of the operation a sort of Vitriolated Tartar; the Acids of Sulphur and of Vitriol being the same, as is evident from what hath been above said concerning them.
In order to discover the cause of the other phenomenon, we must recollect two things advanced in our Elements of the Theory; to wit, that the affinity of the Vitriolic Acid with Fixed Alkalis is greater than that of the Nitrous Acid; and again, that the Nitrous Acid is not capable of combining and taking fire with the Phlogiston, but when it is in the form of a Neutral Salt, that is, when it is united with some alkaline, earthy, or metallic basis. If these two principles be applied to the effect in question, the solution is easy and natural. For, in the deflagration of Nitre with Sulphur, the Phlogiston is not the only substance capable of separating the Nitrous Acid from its basis: the Acid of the Sulphur, more and more of which is set at liberty as the Phlogiston is consumed, is also capable of producing the same effect; but with this difference, that the portion of the Nitrous Acid which is detached from its Alkali by the Phlogiston is at the same instant set on fire and decomposed by that union; whereas the portion thereof which is separated by the Vitriolic Acid, being when so separated incapable of uniting with the Phlogiston, and of consuming therewith, is preserved entire, and rises in vapours, together with that portion of the Vitriolic Acid which could not unite with the basis of the Nitre.
To decompose Nitre by means of the Vitriolic Acid. The Smoking Spirit of Nitre. Sal de duobus. The Purification of Spirit of Nitre.
Take equal parts of well purified Nitre and Green Vitriol: dry the Nitre thoroughly, and bruise it to a fine powder. Calcine the Vitriol to redness: reduce it likewise to a very fine powder; and mingle these two substances well together. Put the mixture into an earthen long-neck, or a good glass retort coated, of such a size that it may be but half full.
Set this vessel in a reverberating furnace covered with its dome; apply a large glass receiver, having a small hole in its body, stopped with a little lute. Let this receiver be accurately luted to the retort with the fat lute, and the joint covered with a slip of canvas smeared with lute made of quick-lime and the white of an egg. Heat the vessels very gradually. The receiver will soon be filled with very dense red vapours, and drops will begin to distil from the nose of the retort.
Continue the distillation, increasing the fire a little when you observe the drops to follow each other but slowly, so that above two thirds of a minute passes between them; and, in order to let out the redundant vapours, open the small hole in the receiver from time to time. Towards the end of the operation raise the fire so as to make the retort red. When you find that, even when the retort is red-hot, nothing more comes over, unlute the receiver, and without delay pour the liquor it contains into a crystal bottle, and close it with a crystal stopple ground in its neck with emery. This liquor will be of a reddish yellow colour, smoking exceedingly, and the bottle containing it will be constantly filled with red fumes like those observed in the receiver.
OBSERVATIONS.
The Vitriolic Acid having a greater affinity with Fixed Alkalis than with any other substance, the Phlogiston excepted, and being in the Vitriol united with a ferruginous basis, will naturally quit that basis to join with the Fixed Alkali of the Nitre; the Acid whereof being weaker than the Vitrio[Pg 192]lic, as we have already observed on several occasions, must needs be thereby expelled from its basis. The Nitre therefore is decomposed by the Vitriol, and its Acid being set at liberty, is carried up by the force of the fire.
Indeed the Nitrous Acid, being thus separated from its alkaline basis, might be expected to combine with the ferruginous basis of the Vitriol: but as it has, like all other Acids, much less affinity with Metallic substances than with Alkalis, even a moderate degree of fire is sufficient to separate it from them. Moreover, this Acid hath either no effect, or very little, upon iron that has lost much of its Phlogiston by contracting an union with any Acid; which is the case of the ferruginous basis of Vitriol.
By the process here delivered a very strong, perfectly dephlegmated, and vastly smoking Spirit of Nitre is obtained. If the precautions of drying the Nitre and calcining the Vitriol be neglected, the Acid that comes over, greedily attracting the water contained in these salts, will be very aqueous, will not smoke, and will be almost colourless, with a very slight tinge of lemon.
The fumes of highly concentrated Spirit of Nitre, such as that obtained by the above process, are light, corrosive, and very dangerous to the lungs; being no other than the most dephlegmated part of the Nitrous Acid. The person therefore who unlutes the vessels, or pours the liquor out of the receiver into the bottle, ought with the greatest caution to avoid drawing them in with his breath; and for that reason ought to place himself so that a current of air, either natural or artificial, may carry them off another way. It is also necessary that care be taken, during the operation, to give the vapours a little vent every now and then, by opening the small hole in the recipient; for they are so elastic, that, if too closely confined, they will burst the vessels.
When the operation is over, you will find a red mass at the bottom of the retort, cast, as it were, in a mould. This is a Neutral Salt of the nature of Vitriolated Tartar, resulting from the union of the Acid of the Vitriol with the Alkaline basis of the Nitre.
The ferruginous basis of the Vitriol, which is mixed with this salt, gives it the red colour. To separate it therefrom, you must pulverise it, dissolve it in boiling water, and filter the solution several times through brown paper; because the ferruginous earth of the Vitriol is so fine, that some of it will pass through the first time. When the solution is very clear, and deposites no sediment, let it be set to shoot, and it will[Pg 193] yield crystals of Vitriolated Tartar; to which Chymists have given the peculiar title of Sal de duobus.
In this Caput mortuum we frequently find, besides the ferruginous earth of Vitriol, a portion of Nitre and Vitriol not decomposed; either because the two salts were not thoroughly mingled, or because the fire was not raised high enough towards the end of the operation.
Nitre may also be decomposed, and its acid obtained, by the interposition of any of the other Vitriols, Alums, Gypsums, Boles, Clays; in short, by means of any compound in which the Vitriolic Acid is found, provided it have not a Fixed Alkali for its basis.
The distillers of Aqua fortis, who make large quantities at a time, and who use the least chargeable methods, do their business by the means of earths impregnated with the Vitriolic Acid; such as Clays and Boles. With these earths they accurately mix the Nitre from which they intend to draw their Spirit: this mixture they put into large oblong earthen pots, having a very short curved neck, which enters a recipient of the same matter and form. These vessels they place in two rows opposite to each other in long furnaces, and cover them over with bricks cemented with Windsor-loam, which serves for a reverberatory: then they light the fire in the furnace, making it at first very small, only to warm the vessels; after which they throw in wood, and raise the fire till the pots grow quite red-hot, in which degree they keep it up till the distillation is entirely finished.
The Acid of Nitre may also be separated from its basis by means of the pure Vitriolic Acid. For this purpose the Nitre from which you mean to extract the Acid must be finely pulverized, put into a glass retort, and a third of its weight of concentrated Oil of Vitriol poured on it: the retort must be placed in a reverberating furnace, and a receiver, like that used in the preceding operation, expeditiously applied.
As soon as the Oil of Vitriol touches the Nitre the mixture grows hot, and copious red fumes begin to appear: some drops of the Acid come over even before the fire is kindled in the furnace.
On this occasion the fire must be moderate; because the Vitriolic Acid, being clogged by no basis, acts upon the Nitre much more briskly, and with much greater effect, than when it is not pure.
This operation may be performed by a sand-heat; which is a speedy and commodious way of obtaining the Nitrous Acid. In other respects the precautions recommended in[Pg 194] the preceding experiment must be carefully observed here, both in distilling the Acid and in taking it out of the receiver.
The Spirit of Nitre extracted by this method is as strong, and smokes as much, as that obtained by calcined Vitriol, provided the Oil of Vitriol made use of be well concentrated; but it is generally tainted by the admixture of a small portion of the Vitriolic Acid, which, having no basis of its own to restrain it, is carried up by the heat before it can lay hold of the basis of the Nitre.
There are several experiments in Chymistry that succeed equally well whether the Nitrous Acid be or be not thus adulterated with a mixture of the Vitriolic Acid; but there are some, as we shall see, that will not succeed without a Spirit of Nitre so mixed. If the Acid be distilled with a view to such experiments, it must be kept as it is. But most experiments require the Spirit of Nitre to be absolutely pure; and if it be intended for such, it must be perfectly cleansed from the Vitriolic taint.
This is easily effected by mixing your Spirit with very pure Nitre, and distilling it a second time. The Vitriolic Acid, with which this Spirit of Nitre is adulterated, coming in contact with a great quantity of undecomposed Nitre, unites with its Alkaline basis, and expels a proportionable quantity of the Nitrous Acid.
In the retort made use of to distil the Nitrous Acid, by means of the pure Vitriolic Acid, is found a Caput mortuum, differing from that left after the distillation of the same Acid by the interposition of Vitriol, in as much as it contains no red ferruginous earth. This is a very white saline mass, moulded in the bottom of the retort: if you pound it, dissolve it in boiling water, and evaporate the solution, it will shoot into crystals of Vitriolated Tartar: sometimes also it contains a portion of undecomposed Nitre, which shoots after the Vitriolated Tartar, because it is much more soluble in water.
Of the Marine Acid.
To extract Sea-salt from Sea-water, and from Brine-springs. Epsom Salt.
Filter the salt-water from which you intend to extract the salt: evaporate it by boiling till you see on its surface a dark pellicle: this consists wholly of little crystals of salt just beginning to shoot: now slacken the fire, that the brine may evaporate more slowly, and without any agitation. The crystals, which at first were very small, will become larger, and form hollow truncated pyramids, the apices whereof will point downwards, and their bases be even with the surface of the liquor.
These pyramidal crystals are only collections of small cubical crystals concreted into this form. When they have acquired a certain magnitude they fall to the bottom of the liquor. When they come to be in such heaps as almost to reach the surface of the liquor, decant it from them, and continue the evaporation till no more crystals of Sea-salt will shoot.
OBSERVATIONS.
The Acid of Sea-salt is scarce ever found, either in sea-water or in the earth, otherwise than united with a fixed alkali of a particular kind, which is its natural basis; and consequently it is in the form of a Neutral Salt. This salt is plentifully dissolved in the waters of the ocean, and when obtained therefrom bears the name of Sea-salt. It is also found in the earth in vast crystalline masses, and is then called Sal-gem: so that Sea-salt and Sal-gem are but one and the same sort of salt, differing very little from each other, except as to the places where they are found.
In the earth are also found springs and fountains, whose waters are strong brines, a great deal of Sea-salt being dissolved in them. These springs either rise directly from the[Pg 196] sea, or run through some mines of Sal-gem, of which they take up a quantity in their passage.
As the same, or at least nearly the same, quantity of Sea-salt will continue dissolved in cold water as boiling water will take up, it cannot shoot, as Nitre does, by the mere cooling of the water in which it is dissolved: it crystallizes only by the means of evaporation, which continually lessens the proportion of the water to the salt; so that it is always capable of containing just so much the less Sea-salt the more there is crystallized.
The brine should not boil after you perceive the pellicle of little crystals beginning to form on its surface; for the calmness of the liquor allows them to form more regularly, and become larger. Nor after this should the evaporation be hurried on too fast; for a saline crust would form on the liquor, which, by preventing the vapours from being carried off, would obstruct the crystallization.
If the evaporation be continued after the liquor ceases to yield any crystals of Sea-salt, other crystals will be obtained of an oblong four-sided form, which have a bitter taste, and are almost always moist. This sort of salt is known by the name of Epsom Salt, which it owes to a salt spring in England, from the water of which it was first extracted. This salt, or rather saline compound, is a congeries of Glauber's salt and Sea-salt, in a manner confounded together, and mixed with some of the Mother of Sea-salt, in which is contained a kind of bituminous matter. These two Neutral Salts, which constitute the Epsom Salt, may be easily separated from each other, by means of crystallization only. Epsom Salt is purgative and bitter; and therefore named Sal Catharticum Amarum, or bitter purging Salts.
There are different methods used in great works for obtaining Sea-salt out of water in which it is dissolved. The simplest and easiest is that practised in France, and in all those countries which are not colder. On the sea-shore they lay out a sort of broad shallow pits, pans, or rather ponds, which the sea fills with the tide of flood. When the ponds are thus filled, they stop their communication with the sea, and leave the water to evaporate by the heat of the sun; by which means all the salt contained in it necessarily crystallizes. These pits are called Salt Ponds. Salt can be made in this way in the summer-time only; at least in France, and other countries of the same temperature: for during the winter, when the sun has less power and rains are frequent, this method is not practicable.
For this reason, as it often rains in the province of Normandy, the inhabitants take another way to extract Salt from sea-water. The labourers employed for this purpose raise heaps of sand on the shore, so that the tide waters and drenches them when it flows, and leaves the sand dry when it ebbs. During the interval between two tides of flood the sun and the air easily carry off the moisture that was left, and so the sand remains impregnated with all the salt that was contained in the evaporated water. Thus they let it acquire as much salt as it can by several returns of flood, and then wash it out with fresh water, which they evaporate over a fire in leaden boilers.
To obtain the Salt from brine-springs, the water need only be evaporated: but as several of these springs contain too little salt to pay the charges that would be incurred, if the evaporation were effected by the force of fire only, the manufacturers have fallen upon a less expensive method of getting rid of the greatest part of the water, and preparing the brine for crystallization, in much less time, and with much less fire, than would otherwise have been necessary.
The method consists in making the water fall from a certain heighth on a great many small spars of wood, which divide it into particles like rain. This is performed under sheds open to all the winds, which pass freely through this artificial shower. By this means the water presents to the air a great extent of surface, being indeed reduced almost entirely to surface, and the evaporation is carried on with great ease and expedition. The water is raised by pumps to the heighth from which it is intended to fall[6].
Experiments concerning the decomposition of Sea-salt, by means of the Phlogiston. Kunckel's Phosphorus.
"Of pure urine that has fermented five or six days take a quantity in proportion to the quantity of phosphorus you intend to make: it requires about one third[Pg 198] part of a hogshead to make a dram of Phosphorus. Evaporate it in iron pans, till it become clotted, hard, black, and nearly like chimney-soot; at which time it will be reduced to about a sixtieth part of its original weight before evaporation.
"When the urine is brought to this condition put it in several portions into so many iron pots, under which you must keep a pretty brisk fire so as to make their bottoms red, and stir it incessantly till the volatile salt and the fetid oil be almost wholly dissipated, till the matter cease to emit any smoke, and till it smell like peach-blossoms. Then put out the fire, and pour on the matter, which will now be reduced to a powder, somewhat more than twice its weight of warm water. Stir it about in this water, and leave it to soak therein for twenty-four hours. Pour off the water by inclination; dry the drenched matter, and pulverize it. The previous calcination carries off from the matter about a third of its weight, and the lixiviation washes out half the remainder.
"With what remains thus calcined, washed, and dried, mix half its weight of gravel, or yellow freestone rasped, having sifted out and thrown away all the finest particles. River sand is not proper on this occasion, because it flies in a hot fire. Then add to this mixture a sixteenth part of its weight of charcoal, made of beech, or of any other wood except oak, because that also flies. Moisten the whole with as much water as will bring it to a stiff paste, by working and kneading it with your hands: now introduce it into your retort, taking care not to daub its neck. The retort must be of the best earth, and of such a size, that when your matter is in it, a full third thereof shall still be empty.
"Place your retort, thus charged, in a reverberating furnace, so proportioned, that there may be an interval of two inches all round between the sides of the furnace and the bowl of the retort, even where it contracts to form the neck, which should stand inclined at an angle of sixty degrees. Stop all the apertures of the furnace, except the doors of the fire-place and ash-hole.
"Fit on to the retort a large glass ballon two thirds full of water, and lute them together, as in distilling the Smoking Spirit of Nitre. In the hinder part of this ballon, a little above the surface of the water, a small hole must be bored. This hole is to be stopped with a small peg of birch-wood, which must slip in and out very easily,[Pg 199] and have a small knob to prevent its falling into the ballon. This peg is to be pulled out from time to time, that by applying the hand to the hole it may be known whether the air, rarefied by the head of the retort, issues out with too much or too little force.
"If the air rushes out with too much rapidity, and with a hissing noise, the door of the ash-hole must be entirely shut, in order to slacken the fire. If it do not strike pretty smartly against the hand, that door must be opened wider, and large coals thrown into the fire-place to quicken the fire immediately.
"The operation usually lasts four and twenty hours; and the following signs shew that it will succeed, provided the retort resist the fire.
"You must begin the operation with putting some unlighted charcoal in the ash-hole, and a little lighted charcoal at the door thereof, in order to warm the retort very slowly. When the whole is kindled, push it into the ash-hole, and close the door thereof with a tile. This moderate heat brings over the phlegm of the mixture. The same degree of fire must be kept up four hours, after which some coals may be laid on the grate of the fire-place, which the fire underneath will kindle by degrees. With this second heat brought nearer the retort, the ballon grows warm, and is filled with white vapours, which have the smell of fetid oil. In four hours after, this vessel will grow cool and clear; and then you must open the door of the ash-hole one inch, throw fresh coals into the fire-place every three minutes, and every time shut the door of it, lest the cold air from without should strike against the bottom of the retort and crack it.
"When the fire has been kept up to this degree for about two hours, the inside of the ballon begins to be netted over with a volatile salt of a singular nature, which cannot be driven up but by a very violent fire, and which smells pretty strong of peach-kernels. Care must be taken that this concrete salt do not stop the little hole in the ballon: for in that case it would burst, the retort being then red-hot, and the air exceedingly rarefied. The water in the ballon, being heated by the vicinity of the furnace, exhales vapours which dissolve this sprigged salt, and the ballon clears up in half an hour after it has ceased rising.
"In about three hours from the first appearance of this salt, the ballon is again filled with new vapours, which[Pg 200] smell like Sal Ammoniac thrown upon burning coals. They condense on the sides of the receiver into a salt which is not branched like the former, but appears in long perpendicular streaks, which the vapours of the water do not dissolve. These white vapours are the fore-runners of the Phosphorus, and a little before they cease to rise they lose their first smell of Sal Ammoniac, and acquire the odour of garlic.
"As they ascend with great rapidity, the little hole must be frequently opened, to observe whether the hissing be not too strong: for, in that case, it would be necessary to shut the door of the ash-hole quite close. These white vapours continue two hours. When you find they cease rising, make a small passage through the dome, by opening some of its registers, that the flame may just begin to draw. Keep up the fire in this mean state till the first volatile Phosphorus begin to appear.
"This appears in about three hours after the white vapours first begin to rise. In order to discover it, pull out the little birchen peg once every minute, and rub it against some hot part of the furnace, where it will leave a trail of light, if there be any Phosphorus upon it.
"Soon after you observe this sign, there will issue out through the little hole of the ballon a stream of blueish light, which continues of a greater or shorter extent to the end of the operation. This stream or spout of light does not burn. If you hold your finger against it for twenty or thirty seconds, the light will adhere to it; and if you rub that finger over your hand, the light will besmear it, and render it luminous.
"But from time to time this streamer darts out to the length of seven or eight inches, snapping and emitting sparks of fire; and then it burns all combustible bodies that come in its way. When you observe this, you must manage the fire very warily, and shut the door of the ash-hole quite close, yet without ceasing to throw coals into the fire-place every two minutes.
"The Volatile Phosphorus continues two hours; after which the little spout of light contracts to the length of a line or two: and now is the time for pushing your fire to the utmost: immediately set the door of the ash-hole wide open, throw billets of wood into it, unstop all the registers of the reverberatory, supply the fire-place with large coals every minute: in short, for six or seven hours all[Pg 201] the inside of the furnace must be kept of a white heat, so that the retort shall not be distinguishable.
"In this fierce extremity of heat the true Phosphorus distils like an oil, or like melted wax: one part thereof floats on the water in the recipient, the other falls to the bottom. At last, the operation is known to be quite over when the upper part of the ballon, in which the volatile Phosphorus appears condensed in a blackish film, begins to grow red: for this shews that the Phosphorus is burnt where the red spot appears. You must now stop all the registers, and shut all the doors of the furnace, in order to smother the fire; and then close up the little hole in the ballon with fat lute or bees-wax. In this condition the whole must be left for two days; because, the vessels must not be separated till they are perfectly cold, lest the Phosphorus should take fire.
"As soon as the fire is out, the ballon, which is then in the dark, presents a most agreeable object: all the empty part thereof above the water seems filled with a beautiful blue light: which continues for seven or eight hours, or as long as the ballon keeps warm, never disappearing till it is cooled.
"When the furnace is quite cold take out the vessels, and separate them from each other as neatly as possible. With a linen cloth wipe away all the black stuff you find in the mouth of the ballon; for if that filth should mix with the Phosphorus, it would hinder it from being transparent when moulded. This must be done with great expedition: after which pour into the ballon two or three quarts of cold water, to accelerate the precipitation of the Phosphorus that swims at top. Then agitate the water in the ballon, to rinse out all the Phosphorus that may stick to the sides: pour out all the water thus shaken and turbid, into a very clean earthen pan, and let it stand till it grows clear. Then decant this first useless water, and on the blackish sediment, left at the bottom of the pan, pour some boiling water to melt the Phosphorus; which thereupon unites with the fuliginous matter, or volatile Phosphorus, that precipitated with it, both together forming a mass of the colour of slate. When this water, in which you have melted the Phosphorus, is cool enough, take out the Phosphorus, throw it into cold water, and therein break it into little bits in order to mould it.
"Then take a matras, having a long neck somewhat wider next the body than at its mouth: cut off half the[Pg 202] body, so as to make a funnel of the neck-part, the smaller end of which must be stopped with a cork. The first mould being thus prepared, plunge it endwise, with its mouth uppermost, in a vessel full of boiling water, and fill it with that water. Into this funnel throw the little bits of your slate-like mass, which will melt again in this hot water, and fall so melted to the bottom of the tube. Stir this melted matter with an iron wire, to promote the separation of the Phosphorus from the fuliginous matter with which it is fouled, and which, being less ponderous than the Phosphorus, will gradually rise above it towards the upper part of the cylinder.
"Keep the water in the vessel as hot as at first, till, on taking out the tube, you see the Phosphorus clean and transparent. Let the clear tube cool a little, and then set it in cold water, where the Phosphorus will congeal as it cools. When it is perfectly congealed, pull out the cork, and with a small rod, near as big as the tube, push the cylinder of Phosphorus towards the mouth of the funnel, where the feculency lies. Cut off the black part of the cylinder, and keep it apart: for when you have got a quantity thereof, you may melt it over again in the same manner, and separate the clean Phosphorus which it still contains. As to the rest of the cylinder which is clean and transparent, if you intend to mould it into smaller cylinders, you may cut it in slices, and melt it again by the help of boiling water in glass tubes of smaller dimensions."
OBSERVATIONS.
This process for making Phosphorus is copied from the Memoirs of the Academy of Sciences for the Year 1737; where it is described by M. Hellot, with so much accuracy, clearness, and precision, that I thought I could not do better than transcribe it, without departing from the author's own expressions, for the sake of such as may not have those Memoirs. We shall take occasion, in these observations, to point out some essential circumstances which I have omitted in the description of the Process, that I might not break the connection between the phenomena that happen in the course of this experiment.
It is proper to observe, in the first place, that one of the most usual causes of miscarriage in this operation is a defect of the requisite qualities in the retort employed. It is abso[Pg 203]lutely necessary to have that vessel made of the best earth, and so well made that it shall be capable of resisting the utmost violence of fire, continued for a very long time; as appears by the description of the process. The retorts commonly sold by potters, and other earthen-ware men, are not fit for this operation; and M. Hellot was obliged to send to Hesse-Cassel for such as he wanted.
We shall, in the second place, observe with M. Hellot, that, "before you set your retort in the furnace, it is proper to make an essay of your matter, to see if there be reason to hope for success. For this purpose put about an ounce thereof into a small crucible, and heat it till the vessel be red. The mixture, after having smoked, ought to chop or crack without puffing up, or even rising in the least. From these cracks will issue undulating flames, white and blueish, darting upwards with rapidity. This is the first volatile Phosphorus, which occasions all the danger of the operation. When these first flashes are over, increase the heat of your matter by laying a large live coal upon the crucible. You will then see the second Phosphorus, like a luminous, steady vapour, of a colour inclining to violet, covering the whole surface of the matter: it continues for a very long time, and diffuses a smell of garlic, which is the distinguishing odour of the Phosphorus you are seeking.
"When this luminous vapour is entirely gone, pour the red hot matter out of the crucible upon an iron plate. If you do not find one drop of salt in fusion, but that, on the contrary, the whole falls readily into powder, it is a proof that your matter was sufficiently lixiviated, and that it contains no more fixed Salt, or Sea-salt, if you will, than is requisite. If you find on the plate a drop of salt coagulated, it shews that there is too much left in, and that there is danger of your miscarrying in the operation; because the redundant salt would corrode, and eat through the retort. In this case your matter must be washed again, and then sufficiently dried."
Our third observation shall be concerning the furnace proper to be employed in this operation. This furnace must be so constructed, that, within a narrow compass it may give a heat at least equal to that of a glass-house furnace, or rather greater, especially during the last seven or eight hours of the operation. M. Hellot in his Memoir gives an exact description of such a furnace.
"As certain accidents may happen in the course of the operation, some precautions are to be taken against them. For instance, if the ballon should break while the Phosphorus is distilling, and any of it should fall on combustible bodies, it would set them on fire, and probably burn the laboratory, because it is not to be extinguished without the greatest difficulty. The furnace must therefore be erected under some vault, or upon a bed of brick-work raised under some chimney that draws well: nor must any furniture or utensil of wood be left near it. If a little flaming Phosphorus should fall on a man's legs or hands, in less than three minutes it would burn its way to the very bone. In such a case nothing but urine will stop its progress.
"If the retort crack while the Phosphorus is distilling, there is an unsuccessful end of your operation. It is easy to perceive this by the stink of garlic which you will smell about the furnace; and moreover, the flame that issues through the apertures of the reverberatory will be of a beautiful violet colour. The Acid of Sea-salt always gives this colour to the flame of such matters as are burnt along with it. But if the retort break before the Phosphorus hath made its appearance, its contents may be saved by throwing a number of cold bricks into the fire-place, and upon them a little water to quench the fire at once." All these useful observations we owe also to M. Hellot.
The Phosphorus here described was first discovered by a citizen of Hamburgh, named Brandt, who worked upon urine in search of the Philosopher's stone. Afterwards two other skilful Chymists, who knew nothing more of the process, than that Phosphorus was obtained from urine, or, in general, from the human body, likewise endeavoured to discover it; and each of them separately did actually make the discovery. These two Chymists were Kunckel and Boyle.
The former perfected the discovery, and found out a method of making it in considerable quantities at a time; which occasioned it to be called Kunckel's Phosphorus. The other, who was an English gentleman, had not time to bring his discovery to perfection, and contented himself with lodging a voucher of his having discovered it in the hands of the Secretary of the Royal Society of London, who gave him a certificate thereof.
"Though Brandt," says M. Hellot, "who had before this sold his secret to a Chymist named Krafft, sold it afterwards to several other persons, and even at a very low rate; and though Mr. Boyle published the process for making it; yet it is extremely probable that both of them kept in their own hands the master-key; I mean, the particular management necessary to make the operation succeed: for, till Kunckel found it out, no other Chymist ever made any considerable quantity thereof, except Mr. Godfrey Hankwitz, an English Chymist, to whom Mr. Boyle revealed the whole mystery.
"Nevertheless," continues he, "we are very far from alledging that all those who have described this operation meaned to impose upon the world: but we conceive that most of them having observed luminous vapours in the ballon, and some sparks about the juncture of the vessels, were contented with those appearances. And thus it came to pass, that, after Kunckel and Boyle died, Mr. Godfrey Hankwitz was the only Chymist that could supply Europe therewith; on which account it is likewise very well known by the name of English Phosphorus."
Almost all the Chymists consider Phosphorus as a substance consisting of the Acid of Sea-salt combined with the Phlogiston, in the same manner as Sulphur consists of the Vitriolic Acid combined with the Phlogiston. This opinion is founded on the following principles.
First, Urine abounds with Sea-salt, and contains also a great deal of Phlogiston; now these are the ingredients of which they conjecture Phosphorus to be composed.
Secondly, Phosphorus has many of the properties of Sulphur; such as being soluble in oils; melting with a gentle heat; being very combustible; burning without any soot; giving a vivid and blueish flame; and lastly, leaving an acid liquor when burnt: sensible proofs that it differs from Sulphur in nothing but the nature of its Acid.
Thirdly, this Acid of Phosphorus, being mixed with a solution of silver in Spirit of Nitre, precipitates the silver, and this precipitate is a true Luna cornea, which appears to be more volatile even than the common sort; as M. Hellot tells us, who made the experiment. This fact proves incontestably that the Acid of Phosphorus is of the same nature with that of Sea-salt: for all Chymists know that the property of precipitating silver in a Luna cornea belongs to the Marine Acid only.
Fourthly, M. Stahl observes, that, if Sea-salt be cast on live coals, they instantly burn with great activity; then they emit a very vivid flame, and are much sooner consumed than if none of this salt had touched them; that Sea-salt in substance, which will bear the violence of fire a considerable time when fused in a crucible, without sustaining any sensible diminution, yet evaporates very quickly, and is reduced to white flowers, by the immediate contact of burning coals; and, lastly, that the flame which rises on this occasion is of a blue colour inclining to violet, especially if it be not thrown directly on the coals themselves, but kept in fusion amidst burning coals, in a crucible so placed that the vapour of the Salt may join with the enflamed Phlogiston as it rises from the coals.
These experiments of M. Stahl's prove, that the Phlogiston acts upon the acid of Sea-salt, even while it is combined with its alkaline basis. The flame that appears on this occasion may be considered as an imperfect Phosphorus: and indeed its colour is exactly like that of Phosphorus.
All the facts above related evince, that the Acid of Phosphorus is akin to that of Sea-salt; or rather that it is the very same. But there are other facts which prove that this Acid undergoes some change at least, some peculiar preparation, before it enters into the composition of a true Phosphorus, and that, when extricated therefrom by burning, it is not a pure Acid of Sea-salt, but is still adulterated with a mixture of some other substance, which makes it considerably different from that Acid. For these observations we are obliged to M. Marggraff, of the Academy of Sciences at Berlin, a celebrated Chymist. I shall presently give an account of his principal experiments as succinctly as possible.
M. Marggraff hath also published a process for making Phosphorus, and assures us, that by means thereof we may obtain in less time, with less heat, less trouble, and less expence, a greater quantity of Phosphorus than by any other method. His operation is this:
He takes two pounds of Sal Ammoniac in powder, which he mixes accurately with four pounds of Minium. This mixture he puts into a glass retort, and with a graduated fire draws off a very sharp, volatile, urinous spirit.
We observed in our theoretical Elements, that some metallic substances have the property of decomposing Sal Ammoniac, and separating its volatile Alkali; concerning which phenomenon we there gave our opinion. Minium, which is a calx of lead, is one of those metallic substances. In this[Pg 207] experiment it decomposes the Sal Ammoniac, and separates its volatile Alkali; what remains in the retort is a combination of the Minium with the Acid of the Sal Ammoniac, which is well known to be the same with the Marine Acid; and consequently the residue of this operation is a sort of Plumbum corneum.
The quantity thereof is four pounds eight ounces. Of this he mixes three pounds with nine or ten pounds of urine, that has stood putrefying for two months, evaporated to the consistence of honey. These he mixes by little and little in an iron pan over the fire, stirring the mixture from time to time. Then he adds half a pound of charcoal-dust, and evaporates the matter, kept continually stirring, till the whole be brought to a black powder. He next distils the mixture in a glass retort with degrees of fire, which he raises towards the end so as to make the retort red-hot, in order to expel all the urinous spirit, superfluous oil, and ammoniacal salt. The distillation being finished, there remains nothing in the retort but a very friable caput mortuum.
This remainder he pulverises again, and throws a pinch thereof on live coals, thereby to discover whether or no the matter be rightly prepared, and in order for yielding Phosphorus. If it be so, it presently emits an arsenical odour, and a blue undulating flame, which passes over the surface of the coals like a wave.
Being thus assured of the success of his operation, he puts one half of his matter in three equal parts, into three small earthen German retorts, capable of holding about eighteen ounces of water a-piece. These three retorts, none of which is above three quarters full, he places together in one reverberatory furnace, built much like those we have described, except that it is so constructed as to hold the three retorts disposed in one line. To each retort he lutes a recipient something more than half full of water, ordering the whole in such a manner, that the noses of his retorts almost touch the surface of the water.
He begins the distillation with warming the retorts slowly, for about an hour, by a gentle heat. When that time is elapsed he raises the fire gradually, so that in half an hour more the coals begin to touch the bottoms of the retorts. He continues throwing coals into the furnace by little and little, till they rise half way the heighth of the retorts; and in this he employs another half hour. Lastly, in the next half hour he raises the coals above the bowls of the retorts.
Then the Phosphorus begins to ascend in clouds: on this he instantly increases the heat of the fire as much as possible, filling the furnace quite up with coals, and making the retorts very red. This degree of fire causes the Phosphorus to distil in drops, which fall to the bottom of the water. He keeps up this intense heat for an hour and half, at the end of which the operation is finished; so that it lasts but four hours and an half in all: nay, he further assures us, that an artist well versed in managing the fire, may perform it in four hours only. In the same manner he distils the second moiety of his mixture in three other such retorts.
The advantage he finds in making use of several small retorts, instead of a single large one, is, that the heat penetrates them with more ease, and the operation is performed with less fire, and in less time. He purifies and moulds his Phosphorus much in the same manner as M. Hellot does. From the quantity of ingredients above-mentioned, he obtains two ounces and a half of fine crystalline moulded Phosphorus.
M. Marggraff considering, as a consequence of the experiments above related, that a highly concentrated Acid of Sea-salt contributes greatly towards the formation of Phosphorus, proceeded to try several other experiments, in which he employed that Acid in a state of combination with other bases. He mixed, for instance, an ounce of Luna cornea with an ounce and half of putrefied and inspissated urine, and from the mixture obtained a very beautiful Phosphorus.
In short, the several experiments mentioned having thoroughly persuaded him that the Acid of Sea-salt, provided it were highly concentrated, would combine with the Phlogiston as readily as the Vitriolic Acid does, he resolved to try whether he could not make Phosphorus with matters containing that Acid and the Phlogiston, without making use of any urine.
With this view he made a great number of different trials, wherein he employed Sea-salt in substance, Sal Ammoniac, Plumbum corneum, Luna cornea, fixed Sal Ammoniac, otherwise called Oil of Lime. These several substances, all of which contain the Acid of Sea-salt, he mixed with sundry matters abounding in Phlogiston, different vegetable coals, and even animal matters, such as the oil of hartshorn, human blood, &c. varying the proportions of these substances many different ways, without ever being able to produce a single atom of Phosphorus: which gave this able Chymist just cause to suspect, that the Marine Acid, while pure and[Pg 209] crude, is not capable of combining with the Phlogiston in the manner requisite to form a Phosphorus; that for this purpose it is necessary the Acid would have contracted a previous union with some other matter; and that the Acid found in urine hath probably undergone the necessary change. M. Marggraff is of opinion that the matter, which by its union renders the Marine Acid capable of entering into the composition of Phosphorus, is a sort of exceedingly subtle vitrifiable earth. The experiments he made upon the Acid of Phosphorus, will shew that his notion is not altogether groundless. M. Marggraff having let some urine, evaporated to the consistence of honey, stand quiet in a cool place, obtained from it, by crystallization, a Salt of a singular nature. By distilling this urine afterwards, he satisfied himself that it yielded him much less Phosphorus than urine from which no Salt had been extracted; and as it cannot be entirely deprived of this Salt, he thinks that the small quantity of Phosphorus, which this urine yielded him, came from the Salt that was still left in it.
Further, he distilled this salt separately with lamp-black, and obtained from it a considerable quantity of very fine Phosphorus. He even mixed Luna cornea with this Salt, in order to see whether it would not increase the quantity of his Phosphorus; but without success: whence he concluded, that in this Saline matter resides the true Acid that is fit to enter into the composition of Phosphorus. This opinion is confirmed by several experiments on the Acid of Phosphorus, which he found to have some properties resembling those of this Salt.
The Acid of Phosphorus seems to be more fixed than any other: and therefore if you would separate it, by burning, from the Phlogiston with which it is united, there is no occasion for such an apparatus of vessels as is employed for obtaining the Spirit of Sulphur. For this Acid will remain at the bottom of the vessel in which you burn your Phosphorus: indeed, if it be urged by the force of fire, its most subtile part evaporates, and the remainder appears in the form of a vitrified matter.
This Acid effervesces with fixed and volatile Alkalis, and therewith forms Neutral Salts; but very different from Sea-salt, and from Sal Ammoniac. That which has a fixed Alkali for its basis does not crackle when thrown on burning coals; but swells and vitrifies like Borax. That which has a volatile Alkali for its basis shoots into long pointed crystals; and, being urged by fire in a retort, lets go its volatile alkali,[Pg 210] a vitrified matter remaining behind. This Salt is like that above-mentioned, as obtained from urine and yielding Phosphorus.
It appears from the experiments adduced, that the Acid of Phosphorus tends always to vitrification; which proves that it is not pure, and gave M. Marggraff cause to think that it is altered by the admixture of a very subtile vitrifiable earth.
M. Marggraff also obtained Phosphorus from several vegetable substances which we use every day for food. This gives him occasion to conjecture, that the Salt requisite to the formation of Phosphorus exists in vegetables, and passes from thence into the animals that feed upon them.
Lastly, he concludes his dissertation by informing us of a very important truth, viz. That the Acid obtained from Phosphorus, by burning it, will serve to form Phosphorus anew; for which purpose it need only be combined with some charred coal, such as lamp-black, and distilled.
From what hath been said on this subject it is plain, that the Chymists have a great many curious and interesting inquiries to make concerning Phosphorus, and particularly concerning its Acid.
I shall conclude this article with an account of certain properties of Phosphorus which I have not yet mentioned.
Phosphorus dissolves by lying exposed to the air. What water cannot effect, says M. Hellot, or at least requires eight or ten years to bring about, the moisture of the air accomplishes in ten or twelve days; whether it be that the Phosphorus takes fire in the air, and the inflammable part evaporating, almost entirely, leaves the Acid of the Phosphorus naked, which, like all other Acids, when exceedingly concentrated, is very greedy of moisture; or else that the moisture of the air, being water divided into infinitely fine particles, is so subtile as to find its way through the pores of the Phosphorus, into which the grosser particles of common water can by no means insinuate themselves.
Phosphorus heated by the vicinity of fire, or by being any way rubbed, soon takes fire and burns fiercely. It is soluble in all Oils and in Ether, giving to those liquors the property of appearing luminous when the bottle containing the solution is opened. Being boiled in water, it likewise communicates thereto this luminous quality. M. Morin, Professor at Chartres, is the author of this observation.
The late Mr. Grosse, a celebrated Chymist of the Academy of Sciences, observed, that Phosphorus being dissolved[Pg 211] in essential oils crystallizes therein. These crystals take fire in the air, either when thrown into a dry vessel, or wrapt up in a piece of paper. If they be dipped in Spirit of Wine, and taken out immediately, they do not afterwards take fire in the air: they smoke a little, and for a very short time, but hardly waste at all. Though some of them were left in a spoon for a fortnight, they did not seem to have lost any thing of their bulk: but when the spoon was warmed a little they took fire, just like common Phosphorus that had never been dissolved and crystallized in an essential Oil.
M. Marggraff, having put a dram of Phosphorus, with an ounce of highly concentrated Spirit of Nitre, into a glass retort, observed, that, without the help of fire, the Acid dissolved the Phosphorus; that part of the Acid came over into the recipient which was luted to the retort; that, at the same time, the Phosphorus took fire, burnt furiously, and burst the vessels with explosion. Nothing of this kind happens when any of the other Acids, though concentrated, are applied to Phosphorus.
To decompose Sea-salt by means of the Vitriolic Acid. Glauber's Salt. The Purification and Concentration of Spirit of Salt.
Put the Sea-salt from which you mean to extract the Acid into an unglazed earthen pipkin, and set it amidst live coals. The Salt will decrepitate, grow dry, and fall into a powder. Put this decrepitated Salt into a tubulated glass retort, leaving two thirds thereof empty. Set the retort in a reverberating furnace; apply a receiver like that used in distilling the Smoking Spirit of Nitre, and lute it on in the same manner, or rather more exactly if possible. Then through the hole, in the upper convexity of the retort, pour a quantity of highly concentrated Oil of Vitriol, equal in weight to about a third part of your Salt, and immediately shut the hole very close with a glass stopple, first ground therein with emery so as to fit it exactly.
As soon as the Oil of Vitriol touches the Salt, the retort and receiver will be filled with abundance of white vapours; and soon after, without lighting any fire in the furnace, drops of a yellow liquor will distil from the nose of the retort. Let the distillation proceed in this manner without fire, as long[Pg 212] as you perceive any drops come: afterwards kindle a very small fire under the retort, and continue distilling and raising the fire by very slow degrees, and with great caution, to the end of the distillation; which will be finished before you have occasion to make the retort red-hot. Unlute the vessels, and without delay pour the liquor, which is a very smoking Spirit of Salt, out of the receiver into a crystal bottle, like that directed for the smoking Spirit of Nitre.
OBSERVATIONS.
Sea-Salt, as hath been already said, is a Neutral Salt composed of an Acid, which differs from those of Vitriol and Nitre, combined with a fixed Alkali that has some peculiar properties; but does not vary from the others in its affinities. This Salt therefore, as well as Nitre, must be decomposed by the Vitriolic Acid; which accordingly is the case in the process here described. The Vitriolic Acid unites with the Alkaline basis of the Sea-salt, and separates its Acid; and that with much greater facility than it expels the Nitrous Acid from its Alkaline basis, because the Acid of Sea-salt has not so great an affinity as the Nitrous Acid with Fixed Alkalis.
As a highly concentrated Oil of Vitriol is used on this occasion, and as the Sea-salt is previously dried and decrepitated, the Acid obtained from it by distillation is very free from phlegm, and always smokes, even more violently than the strongest Acid of Nitre. The vapours of this Acid are also much more elastic and more penetrating than those of the Nitrous Acid: on which account this distillation of the smoking Spirit of Salt is one of the most difficult, most laborious, and most dangerous operations in Chymistry.
This process requires a tubulated retort, that the Oil of Vitriol may be mixed with the Sea-salt after the receiver is well luted to the retort, and not before: for, as soon as these two matters come together, the Spirit of Salt rushes out with so much impetuosity, that, if the vessels were not luted at the time, the copious vapours that would issue through the neck of the ballon would so moisten it, as well as the neck of the retort, that it would be impracticable to apply the lute and secure the joint as the operation requires. Moreover, the operator would be exposed to those dangerous fumes, which, on this occasion, rush out, and enter the lungs, with such incredible activity as to threaten instant suffocation.
Having said so much of the elasticity and activity of the fumes of Spirit of Salt, it is needless to insist upon the necessity of giving vent to the vessels from time to time, by opening the little hole of the ballon: indeed the best way to prevent the loss of a great many vapours, on this occasion, is to employ adopters, and cover them with wet canvas, which will cool and condense the vapours they contain.
When the operation is finished, we find a white, saline mass at the bottom of the retort as in a mould. If this mass be dissolved in water, and the solution crystallized, it yields a considerable quantity of Sea-salt that hath not been decomposed, and a Neutral Salt consisting of the Vitriolic Acid united with the Alkaline basis of that part which hath been decomposed. This Neutral Salt, which bears the name of Glauber its inventor, differs from Vitriolated Tartar, or the Sal de duobus, which remains after distilling the Nitrous Acid, especially in that it is more fusible, more soluble in water, and hath its crystals differently figured. But as in these two Salts the Acid is the same, the differences that appear between them must be attributed to the peculiar nature of the basis of Sea-salt.
Spirit of Salt drawn by the process above described is tainted with a small mixture of the Vitriolic Acid, carried up by the force of fire before it had time to combine with the Alkali of the Sea-salt; which happens likewise to the Nitrous Acid procured in the same manner. If you desire to have it pure, and absolutely free from the Acid of Vitriol, it must be distilled a second time from Sea-salt, as the Acid of Nitre was before directed to be distilled again from fresh Nitre, in order to purify it from any Vitriolic taint.
Sea-salt, as well as Nitre, may be decomposed by any combination of the Vitriolic Acid with a metallic or earthy substance: but it is proper to observe, that if you distil Spirit of Salt by means of Green Vitriol, the operation will not succeed so well as when Spirit of Nitre is distilled in the same manner: less Spirit is obtained, and a much fiercer fire is required.
The cause of this lies in the property which the Acid of Sea-salt possesses of dissolving Iron, even when deprived of a part of its Phlogiston by having contracted an union with another Acid; so that it is no sooner dislodged from its own basis by the Vitriolic Acid, than it unites with the ferruginous basis of the Vitriol, from which it cannot be separated but by a most violent fire. This is the consequence more[Pg 214] especially when calcined Vitriol is made use of: for moisture, as we shall presently see, greatly facilitates the separation of the Marine Acid from those substances with which it is united.
When you do not desire a highly dephlegmated and smoking Spirit of Salt, you may distil with the additament of any earth containing the Vitriolic Acid; as Clay, for instance, or Bole. To this end one part of Sea-salt, slightly dried and reduced to a fine powder, must be accurately mingled with two parts of the earth you intend to employ likewise pulverized; of this mixture make a stiff paste with a proper quantity of rain water, and having formed little balls thereof about the size of a hazel nut, let them dry in the sun; when dry, put them into a stone or coated glass retort, leaving a third part thereof empty; set this vessel in a reverberating furnace, covered with its dome; apply a receiver, which need not be luted on for some time; and heat the vessels very slowly. At first an insipid water will rise, which must be thrown away: afterwards the Spirit of Salt will appear in white clouds. Now lute your vessels, and raise the fire by degrees; which, towards the end must be pushed to the utmost extremity. The operation is known to be finished when no drops fall from the nose of the retort, the receiver cools, and the white vapours that filled it are seen no more.
The Spirit of Salt obtained by the process here delivered does not smoke, and contains much more phlegm than that which is distilled by means of the concentrated Oil of Vitriol; because the earth, though dried in the sun, still retains a great deal of moisture, which commixes with the Acid of the Sea-salt. Consequently it is much easier to collect its vapours; so that this operation is attended with much less trouble than the other. Nevertheless it is adviseable to proceed gently; to apply but little heat at first, and to unstop every now and then the small hole of the receiver: for a quantity of the vapours of Spirit of Salt, even when weakened by the admixture of water, is very apt to burst the vessels.
A much greater degree of fire is necessary to raise the Spirit of Salt by this latter process, than by that in which the pure Vitriolic Acid is employed: for, as fast as the Spirit of Salt is dislodged from its own basis, by the Vitriolic Acid contained in the earth made use of, part of it joins that earth, and cannot be separated from it without the most violent heat.
A Spirit of Salt that shall not smoke may also be obtained by means of the pure Vitriolic Acid. Spirit of Vitriol, or Oil of Vitriol, lowered with a good deal of water, will do the business.
Some Chymists direct a little water to be placed in the receiver, when Spirit of Salt is to be distilled by the intermedium of concentrated Oil of Vitriol, in order to make the acid vapours condense more readily. By this means indeed some of the inconveniencies attending the distillation of smoking Spirit of Salt may be avoided: but, on the other hand, the acid vapours being absolutely suffocated by the water as fast as they come over, the Spirit of Salt obtained by this method will be no less aqueous than that procured by the interposition of earths: so that here is an expence to no manner of purpose. Therefore, when a Spirit of Salt is desired that shall not smoke, it is best to employ an additament of earth; and that so much the rather as the Marine Acid obtained by this means is purer and freer from any Vitriolic taint, for the reasons already assigned.
Part of the Acid of Sea-salt may be separated from its Alkaline basis by the force of fire alone, without the intervention of any other body. With this view the Salt must be put into the retort without being dried. At first an insipid water rises; but it gradually becomes acid, and hath all the properties of Spirit of Salt. When the Salt in the retort is grown perfectly dry, nothing more can be forced over by any degree of heat whatever. If you would obtain more Acid from the same Salt, you must take it out of the retort, where you will find it in a lump, reduce it to powder, and expose it to the air for some time, that it may attract the moisture thereof; or else wet it at once with some rain water, and distil as before. You will again have an insipid water, and a little Spirit of Salt; which will in like manner cease to rise when the Salt in the retort becomes dry. This operation may be repeated as often as shall be thought proper: and perhaps it may be possible to decompose Sea-salt entirely by means thereof, without the interposition of any other body. The Spirit of Salt thus obtained is exceeding weak, in small quantity, and loaded with much water.
This experiment proves, that moisture greatly facilitates the separation of the Acid of Sea-salt from the matters with which it is united: and this is the reason that, in distilling Spirit of Salt with the additament of an earth, the operation requires much less fire at the beginning, while the earth and[Pg 216] salt retain a great deal of humidity, than towards the end, when they begin to grow dry.
After the operation there remains in the retort a saline and earthy mass, which contains, 1. Some entire Sea-salt that has suffered no decomposition; 2. A Glauber's Salt which is, as we said before, a Neutral Salt consisting of the Vitriolic Acid united with the Alkaline basis of the Sea-salt, from which it hath expelled its proper Acid; 3. Part of the earth used as an intermedium, still retaining a portion of its original Vitriolic Acid, which happening not to lie near enough to any particles of Sea-salt, could not exert its power in decomposing them, and so remains united with its earthy basis; 4. Another part of the same earth, impregnated with some of the Marine Acid, which combined therewith upon being expelled from its Alkaline basis by the Vitriolic Acid, and which the force of fire was unable to separate from it when the matters were grown perfectly dry. In consequence of what remains in this caput mortuum, if the whole mass be triturated, moistened with a little water, and distilled a second time, considerably more Spirit of Salt will be obtained from it: and the same is to be said of all distillations of this sort.
Spirit of Salt obtained by the means of any other additament than concentrated Oil of Vitriol is generally very weak: but it may be dephlegmated and concentrated, if required, much in the same manner as Oil of Vitriol. For this purpose you must put it into a glass cucurbit, set it in a balneum mariæ, fit thereto a head and a receiver, and with a moderate degree of heat draw off one third or one half of the liquor. What comes over into the receiver will be the most aqueous part, which being the lightest will rise first, impregnated however with a little acid: in the cucurbit will be left a concentrated Spirit of Salt, or the most acid part, which being the heaviest will not rise with the degree of heat that is capable of carrying up the phlegm. Spirit of Salt thus concentrated, called also Oil of Salt, does not smoke: it is of a yellow colour inclining to green, and an agreeable smell, not unlike that of saffron.
To decompose Sea-salt by means of the Nitrous Acid. Aqua regis. Quadrangular Nitre.
Take dried Sea-salt; bruise it to powder; put it into a glass retort, leaving one half of the vessel empty. Pour upon it a third of its weight of good Spirit of Nitre. Place your retort in the sand-bath of a reverberating furnace; put on the dome; lute to the retort a receiver having a small hole in it, and heat the vessels very slowly. There will come over into the receiver some vapours, and an acid liquor. Increase the fire gradually till nothing more rises. Then unlute the vessels, and pour the liquor out of the receiver into a crystal bottle, stopped like others containing Acid Spirits.
OBSERVATIONS.
The Nitrous Acid hath a greater affinity than the Marine Acid with fixed Alkalis. When therefore Spirit of Nitre and Sea-salt are mixed together, the same consequences, in some measure, will follow, as when the Vitriolic Acid is mixed with that salt; that is, the Nitrous Acid will, like the Vitriolic, decompose it, by dislodging its Acid from its Alkaline basis, and assuming its place. But as the Nitrous Acid is considerably weaker, and much lighter, than the Vitriolic Acid, a good deal of it rises along with the Acid of Sea-salt during the operation. The liquor found in the receiver is therefore a true Aqua regis.
If decrepitated Salt, and a right smoking Spirit of Nitre, be employed in this process, the Aqua regis obtained will be very strong; and, during the operation, very elastic vapours will rush out and burst the vessels, if those precautions be not taken which we pointed out as necessary in distilling the Spirit of Nitre, and the smoking Spirit of Salt.
The operation being finished, there is left in the retort a saline mass, containing Sea-salt not decomposed, and a new species of Nitre, which having for its basis the Alkali of Sea-salt, that is, as we have several times observed, an Alkali of a peculiar nature, differs from the common Nitre, 1. In the figure of its crystals; which are solids of four sides, formed like lozenges: 2. In that it crystallizes with more[Pg 218] difficulty, retains more water in its crystals, attracts the moisture of the air, and dissolves in water with the same circumstances as Sea-salt.
Of Borax.
To decompose Borax by the means of Acids, and to separate from it the Sedative Salt by sublimation and by crystallization.
Reduce to a fine powder the Borax from which you intend to extract the Sedative Salt. Put this powder into a wide-necked glass retort. Pour upon it an eighth part of its weight of common water, to moisten the powder; and then add concentrated Oil of Vitriol to the weight of somewhat more than a fourth part of the weight of Borax. Set the retort in a reverberatory, make a moderate fire at first, and augment it gradually till the retort become red-hot.
A little phlegm will first come over, and then with the last moisture that the heat expels the Sedative Salt will rise; by which means some of it will be dissolved in this last phlegm, and pass therewith into the receiver; but most of it will adhere in the form of saline flowers to the fore-part of the neck of the retort, just where it is clear of the groove of the furnace. There they collect into a heap, which the succeeding flowers push insensibly forward till they slightly stop the passage. Those which rise after the neck is thus stopped stick to the after-part of it which is hot, vitrify in some measure, and form a circle of fused Salt. In this state the flowers of the Sedative Salt seem to issue out of the circle, as from their basis: they appear like very thin, light, shining scales, and must be brushed off with a feather.
At the bottom of the retort will be left a saline mass: dissolve this in a sufficient quantity of hot water; filter the solution in order to free it from a brown earth which it deposites; set the liquor to evaporate, and crystals of Sedative Salt will form in it.
OBSERVATIONS.
Though Borax is of great use in many chymical operations, especially in the fusion of metals, as we shall have occasion to see, yet, till of late years, Chymists were quite ignorant of its nature, as they still are of its origin; concerning which we know nothing with certainty, but that it comes rough from the East Indies, and is purified by the Dutch.
M. Homberg was one of the first that attempted to analyse this Salt. He shewed, that on mixing it with the Vitriolic Acid, and distilling the mixture, a salt sublimes in little fine needles. This product of Borax he called by the name of Sedative Salt, because he found it had the property of moderating the great tumult and heat of the blood in fevers.
After M. Homberg, other Chymists also exercised themselves on Borax. M. Lemery discovered that the Vitriolic is not the only Acid by means of which the Sedative Salt may be obtained from Borax; but that either of the other two Mineral Acids, the Nitrous or the Marine, may be used in its stead.
M. Geoffroy hath greatly facilitated the means of obtaining the Sedative Salt from Borax; having shewn that it may be extracted by crystallization as well as by sublimation; and that the Sedative Salt so obtained is in no respect inferior to that which was procured before by sublimation only. To him also we are indebted for the discovery, that in the composition of Borax there is an Alkaline Salt of the same nature as the basis of Sea-salt. This he found by observing that he got a Glauber's Salt from a solution of Borax into which he had poured some Vitriolic Acid with a view to obtain its Sedative Salt.
Lastly, M. Baron, whom we mentioned before on occasion of this Salt, hath proved, by a great number of experiments, that a Sedative Salt may be procured from Borax by the help of Vegetable Acids, which was never done by any body before him; that the Sedative Salt is not a combination of an Alkaline matter with the Acid made use of in extracting it, as some of its properties seemed to indicate; but that it exists previously and completely formed in the Borax; that the Acid employed to extract it only helps to disengage it from the Alkali with which it is united; that this Alkali is actually of the same nature as the basis of Sea-salt, because that after extracting the Sedative Salt, which by its union therewith forms the Borax, a Neutral Salt is found, of the[Pg 220] same sort with that which would be produced by combining the basis of Sea-salt with the particular Acid made use of; that is, if with the Vitriolic Acid, a Glauber's Salt; if with the Nitrous Acid, a quadrangular Nitre; and if with the Marine Acid, a true Sea-salt; and, lastly, that the Sedative Salt may be re-united to its Alkali, and reproduce a Borax.
Nothing therefore now remains, to give us all the insight we can desire into the nature of Borax, but to know what the Sedative Salt is. M. Baron hath already given us certain negative notices concerning it, by shewing what it is not; that is, that the Acid employed in its extraction doth not enter into its composition. We have great reason to hope, that he will carry his inquiries still further, and clear up all our doubts on this subject.
The Sedative Salt may be extracted from Borax, not only by the means of pure and simple Acids, but also by the same Acids combined with a metallic basis. Thus Vitriols, for instance, may be employed for this purpose with good success. It is easy to see, that the Vitriol must be decomposed on this occasion, and that its Acid cannot unite with the Alkali in which the Sedative Salt is lodged, without quitting its metallic basis, which must of course precipitate.
The Sedative Salt actually sublimes, when a liquid containing it is distilled; but it does not therefore follow, that it is naturally volatile. It rises only by the aid of the water with which it is mixed. The proof of this assertion is, that, when all the humidity of the mixture containing this Salt is dissipated, no more Salt will rise, be the fire ever so violent; and that by adding more water to moisten the dried mass containing it, more Salt will every time be obtained, through many repeated distillations. In the same manner, if some Sedative Salt be moistened, and exposed to a proper degree of heat, a small quantity thereof will rise at first by the help of the water; but as soon as it grows dry it remains exceedingly fixed. This observation we owe to M. Rouelle.
The Sedative Salt hath the appearance and the taste of a Neutral Salt: it does not change the colour of the juice of violets; nor does it easily dissolve in water; for it requires a quart of boiling water to dissolve two ounces of it: yet, with regard to Alkalis, it has the properties of an Acid; it unites with those salts, forms therewith a saline compound which crystallizes, and even expels the Acids that happen to be combined with them; so that it decomposes the same Neutral Salts that are decomposed by the Vitriolic Acid.
The Sedative Salt, when suddenly exposed to the violent heat of a naked fire, loses near half its weight, melts, puts on and retains the appearance of glass; but its nature still remains unchanged. This glass dissolves in water, and shoots anew into crystals of Sedative Salt. This Salt communicates to the Alkaline salt with which it is united, when in the form of Borax, the property of melting with a moderate heat, and forming a kind of glass; and it is this great fusibility that recommends the frequent use of Borax as a flux for assaying ores. It is also employed sometimes as an ingredient in the composition of glass; but, in time, it always communicates thereto the fault which its own glass hath, namely that of tarnishing with the air. The Sedative Salt hath, moreover, the singular property of dissolving in Spirit of Wine, and of giving to its flame, when set on fire, a beautiful green colour. All these observations we owe to Mess. Geoffroy and Baron.
M. Geoffroy prepares the Sedative Salt by crystallization only, in the following manner. "He dissolves four ounces of refined Borax in a sufficient quantity of warm water, and then pours into the solution one ounce and two drams of highly concentrated Oil of Vitriol, which makes a crackling noise as it falls in. When this mixture has stood evaporating for some time, the Sedative Salt begins to make its appearance in little, fine, shining plates floating on the surface of the liquor. The evaporation is then to be stopped, and the plates will by little and little increase in thickness and breadth. They unite together into little tufts, forming with each other sundry different groups. If the vessel be ever so little stirred, the regularity of the crystals will be disturbed; so that it must not be touched till the crystallization appears to be finished. The crystalline clusters, being grown too bulky and too heavy, will then fall of themselves to the bottom of the vessel. This being observed, the saline liquor must be gently decanted from those little crystals, which, as they are not easily dissolved, must be washed clean, by pouring cold water slowly on the sides of the pan, three or four times successively, in order to rinse out all remains of the saline liquor, and then set first to drain, and afterwards to dry in the sun. This Salt, in the form of light flakes of snow, is now soft to the touch, cool in the mouth, slightly bitter, crackling a little between the teeth, and leaving a small impression of acidity on the tongue. It will keep long without giving or calcining, if managed according[Pg 222] to the preceding directions; that is, if it be exactly freed from its saline liquor.
"It differs from the Sedative Salt obtained by sublimation in this respect only, that notwithstanding its seeming lightness it is a little heavier than the other. M. Geoffroy supposes the cause of this weight to be, that, as several of the thin plates adhere together in crystallizing, they retain between them some small matter of humidity; or, if you will, that, as they form larger crystals, they present less surface to the air which elevates light bodies: whereas, on the contrary, the other Sedative Salt, being driven up by the force of fire, rises into the head of the cucurbit in a more subtile form, having its particles much more expanded and divided.
"M. Geoffroy, having put his Sedative Salt made by crystallization to all the same trials with that made by sublimation, satisfied himself that there is no other difference between the two. If the Sedative Salt made by crystallizations happens to calcine in the sun; that is, if its lustre tarnishes, and its surface grows mealy, it is a sign that it still contains either a little Borax or some Glauber's Salt: for these two Salts are apt to calcine in this manner, and pure Sedative Salt should not be subject to this inconvenience. In order to purify it, and free it entirely from those Salts, it must be dissolved once more in boiling water. As soon as the water cools, the Sedative Salt reappears in light, shining, crystalline plates, swimming in the liquor. After standing four and twenty hours, the liquor must be decanted, and the salt washed with fresh water; by which means it will be very pure and beautiful."
Glauber's Salt and Borax dissolve in water with vastly more ease than the Sedative Salt, and consequently do not crystallize so readily by much: so that the small portion of those salts which may have been left on the surface of the Sedative Salt, being diffused through a large quantity of water, continues in a state of solution, while the Sedative Salt crystallizes; which being also washed afterwards with fair water, it is impossible that the smallest particle of those other Salts should remain adhering to it; and consequently this must be deemed an excellent way of purifying it.
Of Operations on Metals.
Of Gold.
To separate Gold, by Amalgamation with Mercury, from the Earth and Stones with which it is found mixed.
Pulverize the earths and stones containing Gold. Put the powder into a little wooden tray; dip this tray in water, gently shaking it and its contents. The water will grow muddy, by taking up the earthy parts of the ore. Continue washing it in this manner till the water cease to appear turbid. Upon the ore thus washed pour strong vinegar, having first dissolved therein, by the help of heat, about a tenth part of its weight of alum. The powder must be quite drenched and covered with this liquor, and so left to stand for twice twenty-four hours.
Decant the vinegar, and wash your powder with warm water, till the last that comes off hath no taste: then dry it, and put it into an iron mortar, with four times its weight of Quick-silver: triturate the whole with a heavy wooden pestle, till all the powder be of a blackish colour: then pour in a little water, and continue rubbing for some time longer. More earthy and heterogeneous particles will be separated from the metalline parts by means of this water, which will look dirty: it must then be decanted, and more fair water added. Repeat this several times; then dry what remains in the mortar with a sponge, and by the help of a gentle heat: you will find it an Amalgam of the Mercury with the Gold.
Put this Amalgam into a chamoy bag: tie a knot on its neck, and squeeze it hard between your fingers, over some[Pg 224] wide-mouthed vessel; there will issue through the pores of the leather numberless little jets of Mercury, forming a sort of shower, that will collect into large globules in the vessel placed underneath. When you can force out no more Mercury by this means, open the bag, and in it you will find the Amalgam freed from the superfluous Mercury; the Gold retaining only about as much thereof as nearly equals itself in weight.
Put this Amalgam into a glass retort; set this retort in the sand-bath of a reverberating furnace; cover it quite over with sand; apply a glass receiver half full of water, so that the nose of the retort may be under the water. The receiver need not be luted to the retort. Give a gradual heat, and raise the fire till drops of the sublimed Mercury appear in the neck of the retort, and fall into the water with a hissing noise. If you hear any noise in the retort slacken your fire a little. Lastly, when you observe, that, though you raise the fire still higher than before, nothing more will come over, take out your retort, break it, and there you will find the Gold, which must be melted in a crucible with Borax.
OBSERVATIONS.
Gold is a perfect metal, which can by no means be deprived of its Phlogiston, and on which few, even of the most powerful chymical solvents, have any effect: and therefore it almost always hath its metalline form when found in the earth; from which it may sometimes be separated by simple lotion. The Gold dust found in the sands of certain rivers is of this kind. When it resides in stones, or tenacious earths, it may be extracted by the process here delivered; to wit, by Amalgamation, or combination of Mercury with Gold. Mercury is incapable of uniting with any earthy substances, not even with the metallic earths, when they are deprived of their Phlogiston, and consequently have not the metalline form.
Hence it follows, that when Mercury is triturated with particles of Gold, of earth, and of stone, mingled together, it unites with the Gold, and separates it from those heterogeneous matters. Yet, if there be along with the Gold any other metal, in its metalline form, except Iron, the Mercury will amalgamate with that also. This often happens to Silver, which being a perfect metal as well as Gold, is for that reason sometimes dug up in its metalline form, and even incorporated with Gold. When this is the case, the mass[Pg 225] that remains in the retort, after abstracting the Mercury of the Amalgama, is a compound of Gold and Silver, which are to be separated from each other by the methods we shall give for that purpose. The present process is therefore applicable to Silver as well as Gold.
Sometimes Gold is intimately combined with such mineral matters as hinder the Mercury from acting upon it. In that case the mixed mass must be roasted before you proceed to Amalgamation: for if the matters be volatile, such, for instance, as antimony or arsenic, the fire will carry them off; so that, after roasting, the Amalgamation will succeed. But sometimes these matters are fixed, and require fusion; if so, recourse must be had to some particular methods, which we shall describe when we come to treat of Silver, as these two perfect metals are to be treated in the same manner.
Ores containing Gold must be washed before an Amalgam is attempted; that the metalline parts, being freed from the numerous particles of earth with which they are encompassed, may the more readily incorporate with the Mercury. Besides, it is the property of Mercury to take the form of a dark unmetallic powder, after being long rubbed with other matters, so that it cannot be easily distinguished from the particles of earth. And hence, if you still continue to grind the matters together, after the Amalgamation is completed, and wash them again and again, the water that comes off will always look turbid, being impregnated with some particles of the Amalgam. This is easily proved: for if you let the turbid water settle, and distil the sediment, you will obtain Quick-silver from it.
The ore is to be steeped in vinegar charged with alum, in order to cleanse the surface of the Gold, which is often covered with a thin coat of earth that obstructs the Amalgamation.
Great care must be taken that the Mercury employed in this operation be very pure. If it be adulterated with any metallic substance, it must be freed therefrom by the methods which we shall propose in their proper place.
The way of separating Mercury from Gold is founded on the different properties of these two metallic substances; the one being exceedingly fixed, and the other very volatile. The union which Mercury contracts with the metals is not intimate enough, to give the new compound which results therefrom all the properties of either of the two united substances; at least so far as concerns their degrees of fixity and volatility. Hence it comes, that, in our Amalgam, the Gold[Pg 226] communicates but very little of its fixity to the Mercury, and the Mercury communicates to the Gold but very little of its volatility. Yet if the Mercury be distilled off with a much greater degree of heat than is necessary to elevate it, a pretty considerable quantity of Gold will most certainly be carried up along with it.
It is also of consequence, on another account, that the fire be duly governed on this occasion. For if too great a degree of heat be applied, and the fire afterwards lowered, the water in the receiver, which covers the nose of the retort, will rise into its body, break it to pieces, and spoil the operation.
The cause of this phenomenon depends on the property which air possesses of rarefying with heat and condensing with cold, joined to its weight. As soon as the retort is acted on by a less degree of heat than acted on it the instant before, the air contained therein is condensed, and leaves a vacuum, which the external air, by virtue of its weight, tends to occupy; but, the orifice of the retort being under water, the external air can no way gain admittance, but by pushing in before it the water which intercepts its passage. This caution, as we observed above, must be applied to all distillations, where the vessels are disposed as they are in this.
Care must also be taken that the nose of the retort be not placed too deep under water: for as the neck grows very warm during the operation, because the degree of heat required to raise Mercury is about three times greater than that which raises water, it may easily be broken by the contact of the cold water in the receiver.
This method of extracting Gold and Silver from their ores, by Amalgamation with Mercury, is not to be absolutely depended on as a sure proof of the quantity of those metals that may be contained in the earth assayed by this means: for some small part of the Amalgam is always lost in washing it; and, moreover, the Mercury, when squeezed through chamoy, always carries with it a small portion of Gold. So that if you desire to know more exactly, by this method, the quantity of Gold or Silver contained in any earth, the Amalgam must not be squeezed through chamoy, but distilled altogether. Much the surest method of making an accurate assay is that by fusion and scorification, which we shall describe under the head of Silver.
In some countries, and especially in America, the method of Amalgamation is used for extracting Gold and Silver in[Pg 227] large quantities, from the matrices which contain them in their metalline form. Agricola and other metallurgists have described the machines by means whereof such Amalgamations are managed.
To dissolve Gold in Aqua regis, and by that means to separate it from Silver. Aurum Fulminans. Aurum Fulminans reduced.
Take Gold that is perfectly pure, or alloyed with Silver only. Reduce it to little thin plates, by hammering it on an anvil. If it be not sufficiently tough, neal it till it be red in a moderate, clear fire, quite free from smoking coals, and then let it cool gradually, which will restore its ductility.
When the plates are thin enough, make them red hot once more, and cut them into small bits with a pair of sheers. Put these bits into a tall, narrow-mouthed cucurbit, and pour on them twice their weight of good Aqua regis, made of one part Sal Ammoniac, or Spirit of Salt, and four parts Spirit of Nitre. Set the cucurbit in a sand-bath moderately heated, stopping its orifice slightly with a paper coffin, to prevent any dirt from falling in. The Aqua regis will presently begin to smoke. Round the little bits of Gold will be formed an infinite number of small bubbles, which will rise to the surface of the liquor. The Gold will totally dissolve, if it be pure, and the solution will be of a beautiful yellow colour: if the Gold be alloyed with a small quantity of Silver, the latter will remain at the bottom of the vessel in the form of a white powder. If the Gold be alloyed with much Silver, when the Gold is dissolved the Silver will retain the form of the little metalline plates put into the vessel.
When the dissolution is completed, gently pour off the liquor into another low, wide-mouthed, glass cucurbit, taking care that none of the Silver, which lies at the bottom in the form of a powder, escape with the liquor. On this powder of Silver pour as much fresh Aqua regis as will cover it entirely; and repeat this till you are sure that nothing more can be taken up by it. Lastly, having decanted the Aqua regis from the Silver, wash the Silver with a little Spirit of Salt weakened with water, and add this Spirit of Salt to the[Pg 228] Aqua regis in which your Gold is dissolved. Then to the body containing these liquors fit a head and a receiver, and distil with a gentle heat, till the matter contained in the cucurbit become dry.
OBSERVATIONS.
It is certain that aqua regis is the true solvent of Gold, and that it does not touch Silver: so that if the Gold dissolved in it were alloyed with Silver, which is often the case, the two metals would by this means be pretty accurately separated from each other. But if you desire to obtain from this solution a Gold absolutely pure, you must free it, before you dissolve it, from every other metallic substance but Silver; because aqua regis acts upon most of the other metals and the semi-metals. We shall shew under the head of Silver, as we promised before, how to purify a mass of Gold and Silver from every other metallic alloy. Thither also we refer the common parting assay performed by means of aqua fortis: because in that operation the Silver is dissolved, and not the Gold.
If the Gold put to dissolve in aqua regis be pure, the dissolution is easily and readily effected. But if, on the contrary, it be alloyed with Silver, the aqua regis finds more difficulty in dissolving it. Nay, if the Silver exceed the Gold in quantity, the dissolution will not take place at all, for the reasons adduced in our Theoretical Elements; of which we shall speak more fully when we come to treat of the Parting Assay.
In the process we directed the Gold to be dissolved in a tall body. This precaution is necessary to prevent the loss of some part thereof: for it is the property of aqua regis to carry off along with it some of the Gold, especially when there is any Sal Ammoniac in its composition, if the vessel be heated while the dissolution is going on, or if the aqua regis be very strong. Yet it is proper to make use of aqua regis that is too strong rather than too weak: for if it prove too strong, and be observed not to act upon the metal for that reason, it is easy to weaken it, by gradually adding small quantities of pure water, till you perceive it begin to act with vigour. This is a general rule regarding all metallic dissolutions in Acids.
When the solution of Gold is evaporated to dryness, if you desire to reduce into a mass the Gold dust left at the bottom of the cucurbit, you must put it into a crucible, and[Pg 229] cover it with pulverized borax, mixed with a little nitre and calcined wine-lees; then cover the crucible close, heat it with a moderate fire, which must be afterwards increased so as to melt the contents. At the bottom of the crucible you will find a lump of Gold, over which the salts you added will be as it were vitrified. These salts are added chiefly to promote the fusion of the metal.
The Gold may, if you will, be separated from its solvent without evaporating the solution as above directed. You need only mix with the solution a fixed or volatile Alkali by little and little, till you see no more precipitate fall, and then let the liquor stand to settle, at the bottom of which you will find a sediment: filter the whole, and dry what is left on the filter.
Both fixed and volatile Alkalis possessing, as hath been frequently repeated, a greater affinity with Acids than metallic substances have, they precipitate the Gold, and separate it from the Acids in which it is dissolved: but it is of great consequence to take notice, that, if you attempt to melt this precipitated Gold in a crucible, it will fulminate as soon as it feels the heat, with such a terrible explosion, that, if the quantity be at all considerable, it may prove fatal to the operator: even rubbing it a little hard will make it blow up. This preparation is therefore called Aurum Fulminans.
Hitherto no satisfactory explanation hath been given of this phenomenon. Some Chymists considering, that, in the precipitation of the Gold, a Nitre is regenerated by the union of the Alkali with the Nitrous Acid which enters into the composition of the aqua regis, imagine that some of this regenerated Nitre, combining with the precipitated Gold, takes fire and detonates, either by means of some small portion of Phlogiston that may be contained in the Alkali, or by means of that which constitutes the Gold itself. But, in the first place, it is well known that Fixed Alkalis do not contain Phlogiston enough to make Nitre detonate. Indeed, if a Volatile Alkali be employed in the precipitation, a Nitrous Ammoniacal Salt will be formed, containing Phlogiston enough to be capable of detonating without the concourse of any additional Phlogiston: but this detonation of the Nitrous Ammoniacal Salt is not to be compared, as to the violence of its effects, with the fulmination of Gold. Besides, we do not find that Gold precipitated by a Volatile Alkali explodes with greater force than that precipitated by a Fixed Alkali. As for the Gold, it is certain that it suffers no decomposition at all by fulminating. When fulminated under a glass bell,[Pg 230] in such small quantities as not to endanger the operator, the Gold is found scattered about under the bell in very fine particles, without having undergone any alteration.
Others have fancied this fulmination of the Gold to be nothing but the decrepitation of the Sea-salt that is regenerated, in the precipitation of the metal, by the Fixed Alkali uniting with the Acid of Sea-salt which makes part of the aqua regis. But to this it may be said, that Gold precipitated by a Volatile Alkali fulminates as violently as that precipitated by a Fixed Alkali; and yet no sea-salt can be formed in the liquor by the addition of a Volatile Alkali, but only a Sal Ammoniac which has not the property of decrepitating. Moreover, there is no comparison, as to the effects, between the decrepitation of Sea-salt and the fulmination of Gold.
Nor, lastly, can this fulmination be attributed, as it is by some, to the effort made by the Salts to escape from amidst the particles of Gold, in which they are supposed by them to be imprisoned: for then we might deprive this Gold entirely of its fulminating quality by only boiling it in water, and so washing off all the saline particles, which probably adhere to its surface only. It is plain there is great room for very beautiful discoveries on this subject. In Walerius's Mineralogy we find some observations that may throw a little light on the point before us.
"The quantity," says he, "of fulminating Gold precipitated exceeds that of the Gold dissolved: if the aqua regis be made with Sal Ammoniac the explosion will be stronger; it will also be more violent if the solution be precipitated with a Volatile Alkali, than if a Fixed Alkali be used for that purpose."
One of the speediest and easiest methods to deprive this Gold of its fulminating quality, is to grind in a mortar twice as much flowers of Sulphur as you have Gold to reduce, mixing your fulminating Gold therewith by little and little, as you grind them together; then to put the mixture into a crucible, and heat it just enough to melt the Sulphur. Part of the Sulphur will be dissipated in vapours, and the rest will burn away. When it is quite consumed, increase the fire so as to make the crucible red-hot. When you perceive no more smell of Sulphur, pour on the Gold a little Borax, previously melted in another crucible with a Fixed Alkali, as calcined Wine-lees, or Nitre fixed with Tartar; and then raise the fire sufficiently to make the whole flow. After the[Pg 231] fusion is completed, you will find a button of Gold at the bottom of the crucible under the Salts.
Fulminating Gold may also be reduced by pouring on it a sufficient quantity of Fixed Alkali reduced to a liquor, or of oil of Vitriol, evaporating all the moisture, and gradually throwing what remains, mixed up with some pinguinous matter, into a crucible kept red-hot in a furnace. The reason why these substances deprive the Gold of its fulminating quality, depends on the causes that produce the fulmination.
Gold may also be separated from aqua regis, and precipitated by the means of several metallic substances that have a greater affinity, either with aqua regis, or with one of the two Acids that compose it. Mercury is one of the fittest for this purpose. On dropping a solution of Mercury in the Nitrous Acid by little and little into a solution of Gold, the mixture becomes turbid, and a precipitate is formed. Continue dropping in more of the solution of Mercury till no more precipitate falls; then let the liquor stand to settle, and at the bottom of it you will find a sediment, which is the precipitated Gold: pour off the liquor by inclination, and wash the precipitate with fair water.
Mercury hath a greater affinity with the Marine than with the Nitrous Acid. The affinity which Mercury hath with the Marine Acid is also greater than that of Gold with the Marine Acid; for unless this Acid be associated either with the Nitrous Acid, or at least with a certain proportion of Phlogiston, it will not dissolve Gold. Hence it comes, that when a solution of Mercury in the Nitrous Acid is dropped into a solution of Gold in aqua regis, the Mercury unites with the Acid of Sea-salt, which is an ingredient in the aqua regis: but the Marine Acid cannot on this occasion join the Mercury, without deserting the Gold and the Nitrous Acid with which it was united; and then the Gold, which cannot be kept in solution by the Nitrous Acid alone, is forced to quit its solvent and precipitate. The liquor, therefore, that now floats over the Gold thus precipitated, must contain Mercury united with the Acid of Sea-salt: and in fact it yields a true Corrosive Sublimate, which is known to be a combination of Mercury with the Marine Acid.
Mercury dissolved in Spirit of Nitre is employed to procure the precipitation we are speaking of; because metallic substances, when so comminuted by an Acid, are much fitter for such experiments than when they are in a concrete form.
Gold precipitated in this manner by a metallic substance doth not fulminate.
To dissolve Gold by Liver of Sulphur.
Mix together equal parts of common Brimstone, and a very strong Fixed Alkali; for instance, Nitre fixed by Charcoal. Put them in a crucible, and melt the mixture, stirring it from time to time with a small rod. There is no occasion to make the fire very brisk; because the Sulphur facilitates the fusion of the Fixed Alkali. Some sulphureous vapours will rise from the crucible; the two substances will mix intimately together, and form a reddish compound. Then throw into the crucible some little pieces of Gold beat into thin plates, so that the whole do not exceed in weight one third part of the Liver of Sulphur: raise the fire a little. As soon as the Liver of Sulphur is perfectly melted, it will begin to dissolve the Gold with ebullition; and will even emit some flashes of fire. In the space of a few minutes the Gold will be entirely dissolved, especially if it was cut and flatted into small thin leaves.
OBSERVATIONS.
The process here delivered is taken from M. Stahl. The design of that ingenious Chymist's inquiry was to discover how Moses could burn the golden calf, which the Israelites had set up and worshipped while he was on the mount; how he could afterwards reduce that calf to powder, throw it into the water which the people used, and make all who had apostatized drink thereof, as related in the Book of Exodus.
M. Stahl, having first observed that Gold is absolutely unalterable and indestructible by the force of fire alone, be it ever so violent, concludes, that without a miracle Moses could not possibly perform the above-mentioned operations on the golden calf any way but by mixing with the Gold some matter qualified to alter and dissolve it. He then takes notice, that pure Sulphur does not act upon Gold at all, and that many other substances, which are thought capable of dividing and dissolving it, cannot however do it so completely as is necessary to render that metal susceptible of the effects related. He then gives the method of dissolving it by Liver of Sulphur, described in the process.
Liver of Sulphur dissolves likewise all the other metals: but M. Stahl observes, that it attenuates Gold more than any other metallic substance, and unites with it much more intimately than with the rest. This appears from what happens, on attempting to dissolve in water any of the mixts resulting from the union of another metal with the Liver of Sulphur: for then the metal separates, and appears in the form of a powder or fine calx; whereas, when Gold is united with Liver of Sulphur, the whole compound dissolves in water so perfectly, that the Gold even passes with the Liver of Sulphur through the pores of filtering paper.
If an Acid be poured into a solution of this combination of Gold with Liver of Sulphur, the Acid unites with the Alkali of the Hepar, and the Gold falls to the bottom of the liquor along with the Sulphur, which doth not quit it. The Sulphur thus precipitated with the Gold is easily carried off by a slight torrefaction, after which the Gold remains exceedingly comminuted. The Sulphur of this compound may also be destroyed by torrefaction, without the trouble of a previous solution and precipitation, and then also the Gold remains so attenuated as to be miscible with liquors, and floats on them, or swims in them, in such a manner that it may easily be swallowed with them in drinking. From all this M. Stahl concludes there is great reason to believe it was by means of the Liver of Sulphur that Moses divided, and in a manner calcined, the golden calf, so that he could mingle it with water, and make the Israelites drink it.
To separate Gold from all other metallic Substances by means of Antimony.
Having put the Gold you intend to purify into a crucible, set it in a melting furnace, cover it, and make the Gold flow. When the metal is in fusion cast upon it, by a little at a time, twice its weight of pure crude Antimony in powder, and after each projection cover the crucible again immediately: this done keep the matter in fusion for a few minutes. When you perceive that the metallic mixture is perfectly melted, and that its surface begins to sparkle, pour it out into a hollow iron cone, previously heated, and smeared on the inside with tallow. Immediately strike with a hammer the floor on which the cone stands;[Pg 234] and when all is cold, or at least sufficiently fixed, invert the cone and strike it: the whole metallic mass will fall out, and the under part thereof, which was at the point of the cone, will be a Regulus more or less yellow as the Gold was more or less pure. On striking the metallic mass the Regulus will freely part from the sulphureous crust at top.
Return this Regulus into the crucible, and melt it. Less fire will do now than was required before. Add the same quantity of Antimony, and proceed as at first. Repeat the same operation a third time, if your Gold be very impure.
Then put your Regulus into a good crucible, much larger than is necessary to hold it. Set your crucible in a melting furnace, and heat the matter but just enough to make it flow, with a smooth, brilliant surface. When you find it thus conditioned, point towards it the nose of a long-snouted pair of bellows, and therewith keep gently and constantly blowing. There will arise from the crucible a considerable smoke, which will abate greatly when you cease to blow, and increase as soon as you begin again. You must raise the fire gradually as you approach towards the end of the operation. If the surface of the metal lose its brilliant polish, and seem covered with a hard crust, it is a sign the fire is too weak; in which case it must be increased, till the surface recover its shining appearance. At last, when no more smoke rises, and the surface of the Gold looks neat and greenish, cast on it, by little and little, some pulverized Nitre, or a mixture of Nitre and Borax. The matter will swell up. Continue thus adding more Nitre gradually, till no commotion is thereby produced in the crucible; and then let the whole cool. If you find, when the Gold is cold, that it is not tough enough, melt it over again; when it begins to melt, cast in the same Salts as before; and repeat this till it be perfectly ductile.
OBSERVATIONS.
Antimony is a compound, consisting of a semi-metallic part united with about a fourth part of its weight of common Sulphur. It appears, in the ninth column of the Table of Affinities, that all the metals, Mercury and Gold excepted, have a greater affinity than the reguline part of Antimony with Sulphur. If therefore Gold, adulterated with a mixture of Copper, Silver, or any other metal, be melted with Antimony, those metals will unite with the Sulphur of[Pg 235] the Antimony, and separate it from the reguline part, which being thus set free will combine and be blended with the Gold. These two metallic substances, forming a mass far heavier than the other metals mixed with the sulphur, fall together to the bottom of the crucible in the form of a Regulus, while the others float over them like a sort of scoria or flag: and thus the Gold is freed from all alloy but the reguline part of the Antimony.
As all the other metals have a great affinity with Sulphur, and Gold is the only one that is capable of resisting its action, one would think Sulphur alone might be sufficient to free it from the metals combined with it, and that it would therefore be better to employ pure Sulphur, in this operation, than to make use of Antimony; the reguline part of which remaining united with the Gold requires another long and laborious operation to get rid of it.
Indeed, strictly speaking, Sulphur alone would be sufficient to produce the desired separation: but it is proper to observe, that, as Sulphur alone is very combustible, most of it would be consumed in the operation before it could have an opportunity to unite with the metallic substances; whereas, when it is combined with the Regulus of Antimony, it is thereby enabled to bear the action of the fire much longer without burning, and consequently is much fitter for the purpose in question. Besides, if we were to make use of pure Sulphur, a great part of the Gold, which is kept in perfect fusion, and its precipitation facilitated, by the Regulus of Antimony, would remain confounded with the Sulphureous scoria.
Nevertheless, seeing the metals with which Gold is alloyed cannot be separated from it by Antimony, but that a quantity of Regulus proportioned to the quantity of the metals so separated will unite with the Gold, and that the more Regulus combines with the Gold, the more tedious, chargeable, and laborious will the operation prove, this consideration ought to have some influence in directing our process. Therefore, if the Gold be very impure, and worse than sixteen carats, we must not mix it with crude Antimony alone, but add two drams of pure Sulphur for every carat the Gold wants of sixteen, and lessen the quantity of Antimony in proportion to that of the real Gold.
It is necessary to keep the crucible close covered, after mixing the Antimony with the Gold, to prevent any coals from falling into it: for, if that should happen, the melted[Pg 236] mass would puff up considerably, and might perhaps run over.
The inside of the cone, into which you pour the melted metallic mass, must be greased with tallow, to prevent its sticking thereto, and that it may come easily out. Striking the floor, on which the cone with the melted metal stands, helps the precipitation and descent of the Regulus of Gold and Antimony to the bottom of the cone.
Less fire is requisite to melt this compound Regulus, in order to add fresh Antimony, than was necessary before the Gold was mixed with the reguline part of the Antimony; because this metallic substance, being much more fusible than Gold, promotes its melting. The Antimony is mixed with the Gold by repeated projections, that the separation of the metals may be accomplished with the greater ease and accuracy. Yet the operation might be successfully performed, by putting in all the Antimony at once, and with one melting only.
The metalline mass found at the bottom of the cone after all these operations, is a mixture of Gold with the reguline part of the Antimony. All the rest of the process consists only in separating this reguline part from the Gold. As Gold is the most fixed of all metals, and as the Regulus of Antimony cannot bear the violence of fire without flying off in vapours, nothing more is necessary for this purpose but to expose the compound, as directed in the process, to a heat strong enough and long enough continued, to dissipate all the Regulus of Antimony. This semi-metal exhales in the form of a very thick white smoke. It is proper to blow gently into the crucible during the whole operation; because the immediate contact of the fresh air incessantly thrown in promotes and considerably increases the evaporation: and this is a general rule applicable to all evaporations.
The fire must be gradually raised as the Regulus of Antimony is dissipated, and the operation draws towards an end; because the mixed mass of Regulus of Antimony and Gold becomes so much the less fusible as the proportion of the Regulus is lessened. Though the Regulus of Antimony be separated from the Gold in this operation, because the latter is of such a fixed nature that it cannot be volatilized by the degree of fire which dissipates the Regulus; yet, as the Regulus is very volatile, it will undoubtedly carry up some of the Gold along with it, especially if you hurry on the evaporation too fast, by applying too great a degree of fire, by blowing too briskly into the crucible, and still more if[Pg 237] you evaporate your mixture in a broad flat vessel instead of a crucible. All these things must therefore be avoided, if you would lose no more Gold than you needs must.
However, unless the evaporation be carried to the utmost, by the means above pointed out, a small portion of the Regulus of Antimony will always remain combined with the Gold, which defends it from the action of the fire. This small portion of Regulus hinders the Gold from being perfectly pure and ductile. In order therefore to consume and scorify it, we cast Nitre into the crucible when we perceive it to emit no more white vapours.
We know that Nitre has the property of reducing all metallic substances to a calx, Gold and Silver excepted; because it deflagrates with the phlogiston to which their metalline form is owing: but as this accension of the Nitre occasions a tumid effervescence, care must be taken to throw it in but by little and little at a time; for if too much be projected at once the melted matter will run over.
This operation might be considerably abridged by taking advantage of the property which Nitre possesses of thus consuming the phlogiston of metallic substances; as by means thereof we might destroy all the Regulus of Antimony incorporated with the Gold, without having recourse to a long and tedious evaporation. But then we should at the same time lose a much greater quantity of Gold, by reason of the tumult and ebullition which are inseparable from the detonation of Nitre. On the whole, therefore, if Nitre be made use of to purify Gold, great care must be taken to apply but very little of it at a time.
All the Silver that was mixed with the Gold, and indeed a little of the Gold itself, remains confounded with the sulphureous scoria, which floats upon the Golden Regulus after the addition of the Antimony: we shall shew in the Chapter on Silver how these two metals are to be separated from the Sulphur.
Of Silver.
To separate Silver from its Ore, by means of Scorification with Lead.
Beat to powder in an iron mortar the ore from which you mean to separate the Silver, having first roasted it well in order to free it from all the Sulphur and Arsenic that it may contain. Weigh it exactly: then weigh out by itself eight times as much granulated Lead. Put one half of this Lead into a test, and spread it equally thereon: upon this Lead lay your ore, and cover it quite over with the remaining half of the Lead.
Place the test thus loaded under the further end of the muffle in a cupelling furnace. Light your fire, and increase it by degrees. If you look through one of the apertures in the door of the furnace you will perceive the ore, covered with calcined Lead, swim upon the melted Lead. Presently afterwards it will grow soft, melt, and be thrown towards the sides of the vessel, the surface of the Lead appearing in the midst thereof bright and shining like a luminous disc: the Lead will then begin to boil, and emit fumes. As soon as this happens, the fire must be a little checked, so that the ebullition of the Lead may almost entirely cease, for about a quarter of an hour. After this it must be excited to the degree it was at before, so that the Lead may begin again to boil and smoke. Its shining surface will gradually lessen, and be covered with scoriæ. Stir the whole with an iron hook, and draw in towards the middle what you observe towards the sides of the vessel; to the end that, if any part of the ore should still remain undissolved by the Lead, it may be mixed therewith.
When you perceive that the matter is in perfect fusion, that the greatest part of what sticks to the iron hook, when you dip it in the melted matter, separates from it again, and drops back into the vessel; and that the extremity of this in[Pg 239]strument, when grown cold, appears varnished over with a thin, smooth, shining crust; you may look on these as marks that the business is done, and the more uniform and evenly the colour of the crust is, the more perfect may you judge the scorification to be.
Matters being brought to this pass, take the test with a pair of tongs from under the muffle, and pour its whole contents into an iron cone, first heated and greased with tallow. This whole operation lasts about three quarters of an hour. When all is cold, the blow of a hammer will part the Regulus from the scoria; and as it is not possible, how perfect soever the scorification be, to avoid leaving a little Lead containing Silver in the scoria, it is proper to pulverize this scoria, and separate therefrom whatever extends under the hammer, in order to add it to the Regulus.
OBSERVATIONS.
Silver, as well as Gold, is often found quite pure, and under its metalline form, in the bowels of the earth; and in that case it may be separated from the stones or sand in which it is lodged by simple washing, or by Amalgamation with Mercury, in the same manner as before directed for Gold. But it also happens frequently, that Silver is combined in the ore with other metallic substances and minerals, which will not admit of this process, but force us to employ other methods of separating it from them.
Sulphur and Arsenic are the substances to which Silver and the other metals usually owe their mineral state. These two matters are never very closely united with Silver; but may be pretty easily separated from it by the action of fire, and the addition of Lead. If Arsenic be predominant in a Silver ore, it will unite with the Lead by the help of a pretty moderate heat, and quickly convert a considerable quantity thereof into a penetrating fusible glass, which has the property of scorifying with ease all substances that are capable of scorification.
When Sulphur predominates, the scorification proceeds more slowly, and doth not always succeed; because that mineral combined with Lead lessens its fusibility, and retards its vitrification. In this case, part of the Sulphur must be dissipated by roasting: the other part unites with the Lead; and that, being rendered lighter by this union, floats on the rest of the mixture, which chiefly contains the Silver. At last, the joint action of the air and of the fire dissipates the[Pg 240] portion of Sulphur that had united with the Lead: the Lead vitrifies and reduces to a scoria whatever is not either Silver or Gold: and thus the Silver being disentangled from the heterogeneous matters with which it was united, one part thereof being dissipated and the other vitrified, combines with the portion of Lead which is not vitrified, and falls through the scoria, which, to favour its descent, must be in perfect fusion.
The whole process, therefore, consists of three distinct operations. The first is Roasting, which dissipates some of the volatile substances found united with the Silver: the second is Scorification, or the Vitrification of the fixed matters also united with the Silver, such as sand, stones, metals, &c. and the third is precipitation, or the separation of the Silver from the scoria. The two first are, as hath been shewn, preparatives for the last, and indeed produce it.
As every thing we said concerning Gold, when we treated of the process of Amalgamation, is to be applied to Silver, which may be extracted by the same method when it is in its metalline form; in the same manner, all we now advance touching the method of extracting Silver by Scorification, when it is depraved with a mixture of heterogenous matters, is equally applicable to Gold in the same circumstances: and indeed Silver almost always contains more or less Gold naturally.
In the process we directed, that the ore should be pulverized before it be exposed to the fire, with a view to enlarge its surface, and by that means facilitate the action of the Lead upon it, as well as the evaporation of its volatile parts.
We recommended the precaution of slackening the fire a little at the beginning of the operation, only to prevent the Lead from being too hastily converted into litharge, lest it should penetrate and corrode the test before it had wholly dissolved the ore: but if we were perfectly certain of the vessel's being so good as to be in no danger of penetration by the Lead, this precaution would be needless.
It is proper to add eight parts of Lead for one of ore; though so much is not always absolutely necessary, especially when the ore is very fusible. The success of this operation depends chiefly on the completeness of the Scorification; and therefore the addition of more Lead than enough is attended with no inconvenience: for, as it always promotes the Scorification, it can never do any harm.
If the ore be mixed with such earthy and stony parts as cannot be separated from it by washing, it is the more diffi[Pg 241]cult of fusion, even though the stones should be such as are most disposed to vitrify; because the most fusible earths and stones are always less so than most metallic substances. In that case it will be necessary, for effecting the Scorification, to mix thoroughly with the pulverized ore an equal quantity of Glass of Lead, to add twelve times as much granulated Lead, and then to proceed as directed for a fusible ore; exposing the mixture to a degree of fire strong enough, and long enough kept up, to give the scoria all the properties above required as signs of a perfect scorification.
Silver ore is sometimes mixed with Pyrites, and the ore of Arsenic, or Cobalt, which also make it refractory. As the Pyrites contain a large quantity of Sulphur, which is very volatile as well as Arsenic; in this case it is proper to begin with freeing the ore from these two extraneous substances. This is easily done by roasting: only be sure, when you first expose the ore to the heat, to cover the vessel in which you roast it, for some minutes, with an inverted vessel of the same width; because such sorts of ore are very apt to fly when they first feel the heat.
After this uncover it, and leave it exposed to the fire till no more sulphureous or arsenical matters rise. Then mix it with the same quantity of Glass of Lead as we ordered for ores rendered refractory by the admixture of earths or stones, and proceed in the same manner.
It is the more necessary to roast Silver ore infected with Sulphur and Arsenic, because, as Sulphur obstructs the fusion of Lead, it cannot but do hurt, and protract the operation; and Arsenic does mischief, on the other hand, by scorifying a very great quantity of Lead too hastily.
When the Sulphur and Arsenic are dissipated by roasting, the ore must be treated like that which is rendered refractory by stony and earthy matters; for as the pyrites contain much iron, there remains, after the Sulphur is evaporated, a considerable quantity of martial earth, which is difficult to scorify. The pyrites, as well as the cobalts, contain moreover an unmetallic earth, which is hard to fuse.
The general rule therefore is, when the ore is rendered refractory by any cause whatever, to mix it with Glass of Lead, and to add a larger quantity of granulated Lead. Yet some ores are so refractory that Lead alone will not do the business, and recourse must be had to some other flux. That which is fittest for the present purpose is the Black Flux, composed of one part of Nitre and two parts of Tartar deflagrated together. The Phlogiston contained in this quantity[Pg 242] of Tartar is more than sufficient to alkalizate the Nitre. This Flux, therefore, is nothing more than Nitre alkalizated by Tartar, mixed with some of the same Tartar that hath not lost its Phlogiston, and is only reduced to a sort of coal.
The White Flux is also very fit to promote fusion; but on this occasion the Black Flux is preferable, because the Phlogiston of the Black Flux prevents the Lead from being too soon converted to litharge, and so gives it time to dissolve the metallic matters. The White Flux, which is the result of equal parts of Tartar and Nitre alkalizated together, being no more than an Alkali destitute of Phlogiston, or containing but very little, doth not possess this advantage.
If Silver should be combined in the ore with Iron in its metalline state, which however does not commonly happen, then, in order to separate them, the Iron must be deprived of its Phlogiston, and converted to a crocus before the mixed mass be melted with Lead; which may be done by dissolving it in the Vitriolic Acid, and then evaporating the Acid.
We are necessitated to make use of this contrivance, because Iron in its metalline form cannot be dissolved either by Lead or by the Glass of Lead; but when it is reduced to a calx, litharge unites with it and scorifies it.
If you have not at hand the utensils necessary for performing the operation we have been describing in a test, and under the muffle; or if you have a mind to work on a greater quantity of ore at a time, you may make use of a crucible for the purpose, and perform the operation in a melting furnace.
In this case the ore must be prepared, as above directed, according to its nature, and mixed with a proper quantity of Lead and Glass of Lead; the whole put into a good crucible, leaving two thirds thereof empty, and covered with a mixture of Sea-salt and a little Borax, both very dry, to the thickness of a full half inch.
This being done, set the crucible in the midst of a melting furnace, raise the coals quite to the lip of the crucible; light the fire; cover the furnace with its dome; but do not urge the fire more than is necessary to bring the mixture to perfect fusion: leave it thus in fusion for a good quarter of an hour; stir the whole with a bit of strong iron wire; then let it cool; break the crucible, and separate the Regulus from the scoria.
The Salts added on this occasion are fluxes, and their use is to procure a perfect fusion of the scoria.
If the melted matters be left exposed to the fire, either in a test or in a crucible, longer than is above prescribed, the portion of Lead, that hath united and precipitated with the Silver, will at last vitrify, and at the same time scorify all the alloy with which that metal may be mixed. But as there are no vessels that can long endure the action of litharge, without being pierced like a sieve, some of the Silver may escape through the holes or fissures of the vessel, and so be lost. It is better, therefore, to complete the purification of your Silver by the operation of the Cupel, the description of which follows.
The refining of Silver by the Cupel.
Take a cupel capable of containing one third more matter than you have to put into it: set it under the muffle of a furnace, like that described in our Theoretical Elements, as peculiarly appropriated to this sort of operation. Fill the furnace with charcoal; light it; make the cupel red-hot, and keep it so till all its moisture be evaporated; that is, for about a good quarter of an hour, if the cupel be made wholly of the ashes of burnt bones; and for a whole hour, if there be any washed wood-ash in its composition.
Reduce the Regulus which remained after the preceding operation to little thin plates, flatting them with a small hammer, and separating them carefully from all the adherent scoria. Wrap these in a bit of paper, and with a small pair of tongs put them gently into the cupel. When the paper is consumed the Regulus will soon melt, and the scoria, which will be gradually produced by the Lead as it turns to litharge, will be driven to the sides of the cupel, and immediately absorbed thereby. At the same time the cupel will assume a yellow, brown, or blackish colour, according to the quantity and nature of the scoria imbibed by it.
When you see the matter in the cupel in a violent ebullition, and emitting much smoke, lower the fire by the methods formerly prescribed. Keep up such a degree of heat only that the smoke which ascends from the matter may not rise very high, and that you may be able to distinguish the colour which the cupel acquires from the scoria.
Increase the fire by degrees, as more and more litharge is formed and absorbed. If the Regulus examined by this assay contain no Silver, you will see it turn wholly into scoria, and at last disappear. When it contains Silver, and the quantity of Lead is much diminished, you will perceive little vivid irises, or beautiful rain-bow colours, shooting swiftly along its surface, and crossing each other in many different directions. At last, when all the Lead is destroyed, the thin dark skin, that is continually protruded by the Lead while it is turning into litharge, and which hitherto covered the Silver, suddenly disappears; and, if at this moment the fire happen not to be strong enough to keep the Silver in fusion, the surface of that metal will at once dart out a dazzling splendour: but, if the fire be strong enough to keep the Silver in fusion, though freed from all mixture of Lead, this change of colour, which is called its fulguration, will not be so perceptible, and the Silver will appear like a bead of fire.
These phenomena shew that the operation is finished. But the cupel must still be left a minute or two under the muffle, and then drawn slowly out with the iron hook towards the door of the furnace. When the Silver is so cooled as to be but moderately red, you may take the cupel from under the muffle with your little tongs, and in the middle of its cavity you will find an exceeding white bead of Silver, the lower part whereof will be unequal, and full of little pits.
OBSERVATIONS.
The Regulus obtained by the former process consists altogether of the Silver contained in the ore, alloyed with the other metals that happened to be mixed therewith in its mineral state, and a good deal of the Lead that was added to precipitate the Silver. The operation of the cupel may be considered as the sequel of that process, being intended only to reduce into a scoria whatever is not Gold or Silver. Lead being of all metals that which vitrifies the most easily, which most promotes the vitrification of the rest, and the only one which, when vitrified, penetrates the cupel, and carries along with it the other metals which it hath vitrified, is consequently the fittest for that purpose. We shall see in its place, that Bismuth hath the same properties with Lead, and may be substituted for it in this operation.
Care must be taken to chuse a cupel of a proper capacity. Indeed it should rather be too big than too little: because the operation is no way prejudiced by an excess in its size;[Pg 245] whereas, if it be too small, it will be over-dosed with Lead, and at last the litharge, which destroys every thing, will corrode its cavity, and eat holes through the very body of the vessel. Add, that the ashes, of which the cupel is made, being once glutted with litharge, absorb it afterwards but slowly, and that the quantity of this vitrified litharge, becoming too great to be contained in the substance of the vessel, exsudes through it, and drops on the floor of the muffle, which it corrodes and renders unequal; and moreover solders to it the vessels set thereon. It may be laid down as a general rule for determining the size of a cupel, that it weigh, at least, half as much as the metallic mass to be refined in it.
It is also of the utmost consequence that the cupel be well dried before the metal be put into it. In order to make sure of this point, it must be kept red-hot for a certain time, as is above directed: for though to the sight and to the touch it may appear very dry, it nevertheless obstinately retains a small matter of moisture, sufficient to occasion the loss of some of the metal; which, when it comes to melt, will be thereby spirited up, in the form of little globules, to the very roof of the muffle. The cupels that stand most in need of an intense heat to dry them, are those chiefly in whose composition wood-ashes are employed: for whatever care be taken to lixiviate those ashes before they are used, they will still retain a little alkaline salt; and that, we know, is very greedy of moisture, will not part entirely with it, but by the means of a violent calcination, and presently re-imbibes it when exposed to the air.
A little Phlogiston also may still be left in the ashes of which the cupels are made; and that is another reason for calcining them before they are used. By this means the remaining Phlogiston is dissipated, which might otherwise combine with the litharge during the operation, reduce it, and occasion such a ferment in the matter as to make some of it run over; to these inconveniencies, which any remainder of moisture or Phlogiston may produce, we must add the cracks and flaws, which are very incident to cupels not perfectly freed from both those matters.
It is of no less importance to the success of this operation, that a due degree of heat be kept up. In the process we have described the marks which shew the heat to be neither too strong nor too weak; when it exceeds in either of these respects it may be known by the following signs.
If the fume emitted by the Lead rise like a spout to the roof of the muffle; if the surface of the melted metal be extremely convex, considering the quantity of the mass: if the[Pg 246] cupel appear of such a white heat, that the colour communicated thereto by the imbibed scoria cannot be distinguished: all these shew that the heat is too great, and that it ought to be diminished. If, on the contrary, the vapours only hover, as it were, over the surface of the metal; if the melted mass be very flat, considering its quantity; if its ebullition appear but faint; if the scoriæ, that appear like little fiery drops of rain, have but a languid motion; if the scoria gather in heaps, and do not penetrate the cupel; if the metal be covered with it as with a glassy coat; and, lastly, if the cupel look dull; these are proofs that the heat is too weak, and ought to be increased.
The design of this operation being to convert the Lead into litharge, and to give it sufficient time and opportunity to scorify and carry off with it whatever is not Gold or Silver; the fire must be kept up to such a degree that the Lead may easily be turned into litharge, and yet that litharge not be absorbed too hastily by the cupel, but that a small quantity thereof may all along remain, like a ring, round the melted metal.
The fire is to be gradually increased as the operation draws nearer to its end: for, as the proportion of the Lead to the Silver is continually lessening, the metallic mass gradually becomes less fusible; while the Silver defends the Lead mixed with it from the action of the fire, and prevents its being easily converted into litharge.
When the operation is finished, the cupel must still be left under the muffle, till it has imbibed all the litharge, to the end that the bead of Silver may be easily taken out: for, without this precaution, it would stick so fast as not to be removed, but by breaking off part of the cupel along with it. Care must also be taken to let this bead of Silver cool gradually, and be perfectly fixed, before you draw it from under the muffle; for if you expose it at once to the cold air, before it be fixed, it will swell, shoot into sprigs, and even dart out several little grains to a considerable distance, which will be lost.
If the bead appear to have a yellowish tinge, it is a sign that it contains a great deal of Gold, which must be separated from it by the methods to be hereafter shewn.
It is proper to observe, that there is scarce any Lead that does not contain some Silver; too little perhaps to defray the charges necessary to separate it, yet considerable enough to lead us into an error, by mixing with the Silver obtained from an ore, and increasing its weight. And therefore,[Pg 247] when the operations above described are applied to the assaying of an ore, in order to know how much Silver it yields, it is previously necessary to examine the Lead to be used, and to ascertain the quantity of Silver it contains, which must be deducted from the total weight of the bead of Silver obtained by purifying it in this manner.
Silver may be separated from its ore, and at the same time refined, by the single operation of the cupel, without any previous scorification with Lead. In order to do this, you must pound the ore; roast it, to dissipate all its volatile parts; mix it with an equal quantity of litharge, if it be refractory; divide it into five or six parcels, wrapping each in a bit of paper; weigh out eight parts of granulated Lead for one of ore, if it be fusible, and from twelve to sixteen, if it be refractory; put one half of the Lead into a very large cupel under the muffle; add thereto one of the little parcels of ore, when the Lead begins to smoke and boil; immediately slacken the fire a little; continue the same degree of heat till you perceive that the litharge formed round the metal, and on its surface, begins to look bright; then raise the fire; add a fresh parcel of ore; continue proceeding in the same manner till you have put in all the ore; then add the remaining half of the granulated Lead, and conduct the succeeding part of the operation in the same manner as that of cupelling.
In this operation it is necessary that the fire be not too strongly urged, and that it be diminished every time you add a fresh parcel of ore; that so the Lead and the litharge may have time to dissolve, scorify, and carry off into the pores of the cupel, all the adventitious matters with which your Silver may be mixed. Notwithstanding this precaution, when the ore is refractory, there often gathers in the cupel a great quantity of scoria, together also with some of the ore that could not be dissolved and scorified. It is with a view to remedy this inconvenience that the second moiety of the Lead is added towards the end, which completes the dissolution and scorification of the whole; so that by means thereof no scoria, or very little, is left in the cupel at the end of the operation.
The operation of the cupel is chiefly used to purify Silver from the alloy of Copper; because this metal, being more fixed and harder to calcine than other metallic substances, is the only one that remains united with Silver and Lead, after roasting and scorification with Lead. It requires no less than sixteen parts of the Lead to destroy it in the cupel, and separate it from Silver. It melts into one mass with the Lead;[Pg 248] and the glass produced by these two metals, deprived of their phlogiston, inclines to a brown or a black colour; by which appearance chiefly we know that our Silver was alloyed with Copper.
To purify Silver by Nitre.
Granulate the Silver you intend to purify, or reduce it to thin plates; put it into a good crucible; add thereto a fourth part in weight of very dry pulverized Nitre, mixed with half the weight of the Nitre of calcined Wine-lees, and about a sixth part of the same weight of common glass in powder. Cover this crucible with another crucible inverted; which must be of such a size that its mouth may enter a little way into that of the lower one, and have its bottom pierced with a hole of about two lines in diameter. Lute the two crucibles together with clay and Windsor-loam. When the lute is dry, place the crucibles in a melting furnace. Fill the furnace with charcoal, taking care however that the fuel do not rise above the upper crucible.
Kindle the fire, and make your vessels of a middling-red heat. When they are so, take up with the tongs a live-coal, and hold it over the hole of the upper crucible. If you immediately perceive a vivid splendour round the coal, and at the same time hear a gentle hissing noise, it is a sign that the fire is of a proper strength; and it must be kept up at the same degree till this phenomenon cease.
Then increase the fire to the degree requisite to keep pure Silver in fusion; and immediately after take your vessels out of the furnace. You will find the Silver at the bottom of the lower crucible, covered with a mass of Alkaline scoria of a greenish colour. If the metal be not rendered perfectly pure and ductile by this operation, it must be repeated a second time.
OBSERVATIONS.
The purification of Silver by Nitre, as well as the process for refining it on the cupel, is founded on the property which this metal possesses of resisting the force of the strongest fire, and the power of the most active solvents, without losing its phlogiston. The difference between these two ope[Pg 249]rations consists wholly in the substances made use of to procure the scorification of the imperfect metals, or semi-metals, that may be combined with the Silver. In the former this was obtained by Lead, and here it is effected by Nitre. This Salt, as we have shewn, hath the property of calcining and quickly destroying all metallic substances, by consuming their phlogiston, except the perfect metals, Gold and Silver, which alone are able to resist its force. This method may therefore be employed to purify Gold as well as Silver, or indeed both the two mixed together.
In this operation the Nitre is gradually alkalizated, as its Acid is consumed with the phlogiston of the metallic substances. The Alkaline Salt and pounded glass are added, with a view to promote the fusion of the metalline calces, as fast as they are formed, and to fix and retain the Nitre, which, as we shall presently see, is apt to fly off in a certain degree of heat.
The precaution of covering the crucible with another crucible inverted, which hath only a small hole in its bottom, is designed to prevent any of the Silver from being lost in the operation: for when the Nitre comes to be acted on by a certain degree of heat, and especially when it deflagrates with any inflammable matter, part of it flies off, and so rapidly too as to be capable of carrying off with it a good deal of the Silver. The little hole left in the covering crucible is necessary for giving vent to the vapours, which rise during the deflagration of the Nitre, as they would otherwise open themselves a passage by bursting the vessels. After the operation this vent-hole is found beset with many little particles of Silver, which would have been lost if the crucible had not been covered.
If you should observe, during the detonation of the Nitre, that a great many vapours issue through the vent-hole with a considerable hissing noise, even without applying the coal, you must take it for a sign that the fire is too brisk, and accordingly check it; else a great deal of the Nitre will be dissipated, and with it much Silver.
You must observe to take the Silver out of the fire as soon as it is in fusion: for if you neglect this, the Nitre being entirely dissipated or alkalizated, the calces of the metals destroyed by it may possibly recover a little phlogiston, communicated either by the vapours of the charcoal, or by little bits of coal accidentally falling into the crucible; by which means some portion of those metals being reduced will mix again with the Silver, prevent its having the desired degree[Pg 250] of purity and ductility, and oblige you to begin the operation afresh.
To dissolve Silver in Aqua Fortis, and thereby separate it from every other metalline substance. The Purification of Aqua Fortis. Silver precipitated by Copper.
The Silver you intend to dissolve being beaten into thin plates, put it into a glass cucurbit; pour on it twice its weight of good precipitated aqua fortis; cover the cucurbit with a piece of paper, and set it on a sand-bath moderately heated. The aqua fortis will begin to dissolve the Silver as soon as it comes to be a little warm. Red vapours will rise; and from the upper surfaces of the Silver there will seem to issue streams of little bubbles, ascending to the top of the liquor, between which and the Silver they will form, as it were, a number of fine chains: this is a sign that the dissolution proceeds duly, and that the degree of heat is such as it ought to be. If the liquor appear to boil and be agitated, a great many red vapours rising at the same time, it is a sign that the heat is too great, and should be lessened till it be reduced to the proper degree indicated above: having obtained that, keep it equally up till no more bubbles or red vapours appear.
If your Silver be alloyed with Gold, the Gold will be found, when the dissolution is finished, at the bottom of the vessel in the form of a powder. This solution must now be decanted while it is yet warm; on the powder pour half as much fresh aqua fortis as before, and make it boil; again decant this second aqua fortis, and repeat the same a third time; then with fair water wash the remaining powder well: it will be of a brown colour inclining to red. In the observations we shall show how the Silver is to be separated from the aqua fortis.
OBSERVATIONS.
All the processes on Silver already delivered, whether for extracting it from its ores, or for refining it, either by the Cupel or by Nitre, are applicable to Gold also. And if Silver be alloyed with Gold before it undergo those several operations, it will still remain alloyed therewith after them,[Pg 251] in the same manner, and in the same quantity; because both metals bear them equally. All therefore that can be expected from those several assays, is the separation of every thing that is neither Silver nor Gold from these two metals. But in order to separate these two from each other, recourse must be had either to the process laid down under the head of Gold, or to that here described, which is the most commodious, the most usual, and known by the names of Quartation and the Parting Assay.
Aqua fortis is the true solvent of Silver, and is utterly incapable of dissolving the least atom of Gold. If therefore a mass consisting of Gold and Silver be exposed to the action of aqua fortis, that Acid will dissolve the Silver contained in the compound, without touching the Gold, and the two metals will be separated from each other. This method of parting them is just the reverse of that described before under the head of Gold, which is effected by the means of aqua regis.
To the success of this separation, by means of aqua fortis, several conditions are essentially necessary. The first is, that the Gold and Silver be in due proportion to each other; that is, there must be at least twice as much Silver as Gold in the metalline mass, otherwise the aqua fortis will not be able to dissolve it, for the reason formerly given. If therefore the mass contain too little Silver, it must either be melted down again, and a proper quantity of Silver added; or else, if the Gold be in a sufficient proportion to the Silver, they may be parted by means of aqua regis.
Secondly, it is necessary that the aqua fortis employed in this operation be absolutely pure, and free from any taint of the Vitriolic or Marine Acid: for, if it be adulterated with the Vitriolic Acid, the Silver will precipitate as fast as it dissolves, and so the precipitated Silver will again mix with the Gold. If the aqua fortis contain any of the Marine Acid, the Silver will be precipitated in that case also; and this inconvenience will be attended with another, namely, that the menstruum, being partly an aqua regis, will dissolve some of the Gold. You must therefore be very sure that your aqua fortis is pure, before you set about the operation. In order to discover its quality, you must try it by dissolving, in a small portion thereof, as much Silver as it will take up: if the aqua fortis grow opaque and milky as it dissolves the Silver, it is a sign it contains some foreign Acid, from which it must be purified.
In order to effect this, let the portion of aqua fortis used for the above trial stand to settle: the white milky part will gradually fall to the bottom of the vessel. When it is all fallen, gently decant the clear liquor, and pour a few drops of this decanted solution of Silver into the aqua fortis which you want to purify. It will instantly become milky. Let the white particles precipitate as before, and then add a few more drops of your solution of Silver. If the aqua fortis still become milky, let it precipitate again, and repeat this till you find that a drop of your solution of Silver, let fall into this aqua fortis, does not make it in the least turbid. Then filter it through brown paper, and you will have an aqua fortis perfectly fit for the Parting Assay.
The white particles that appear and settle to the bottom, on dissolving silver in an aqua fortis adulterated with a mixture of some foreign Acid, are no other than that very Silver, which is no sooner dissolved by the Nitrous Acid than it deserts that solvent to unite with the Vitriolic or Marine Acid, wherewith it has a greater affinity, and falls to the bottom with them. And this happens as long as there remains in the aqua fortis a single atom of either of those two Acids.
When therefore your aqua fortis hath dissolved as much Silver as it is capable of taking up, and when all the white particles formed during the dissolution are settled to the bottom, you may be assured that the portion which remains clear and limpid is a solution of Silver in an exceeding pure aqua fortis. But if the solution of Silver thus depurated be mixed with an aqua fortis adulterated with the Vitriolic or Marine Acid, a like precipitation will immediately ensue, for the reasons above given, till the very last particle of the heterogeneous Acid contained in the aqua fortis be precipitated.
Aqua fortis purified by this method contains no extraneous substance whatever, except a small portion of Silver; so that it is very fit for the parting process. But if it be intended for other chymical purposes, it must be rectified in a glass retort with a moderate heat, in order to separate it from the small portion of Silver it contains, which will remain at the bottom of the retort.
The third condition necessary to the success of this operation is, that your aqua fortis be neither too aqueous, nor too highly concentrated. If too weak, it will not act upon the Silver: and the consequence will be the same if it be too strong. Both these inconveniencies are easily remedied: for in the former case part of the superfluous phlegm may be[Pg 253] drawn off by distillation; or a sufficient quantity of much stronger aqua fortis may be mixed with that which is too weak: and, in the latter case, very pure rain water, or a weaker aqua fortis, may be mixed with that which is too strong.
You may satisfy yourself whether or no your aqua fortis hath the requisite degree of strength, by dissolving therein a thin plate consisting of one part Gold and two or three parts Silver; which plate must be rolled up in form of a paper coffin. If, when all the Silver contained in the plate is dissolved, the Gold remains in the form of the coffin, it is a sign that your solvent has a due degree of strength. If, on the contrary, the Gold be reduced to a powder, it is a proof that your aqua fortis is too strong, and ought to be weakened.
The Gold remaining after the dissolution of the Silver must be melted in a crucible with Nitre and Borax, as hath already been said under the process for parting Gold and Silver by means of aqua regis. As to the Silver which remains dissolved in the aqua fortis, there are several ways to recover it.
The most usual is to precipitate it by the interposition of Copper, which hath a greater affinity than Silver with the Nitrous Acid[7]. For this purpose the solution is weakened by adding twice or thrice as much very pure rain water. The cucurbit containing the solution is set on a sand-bath gently heated, and very clean plates of copper put into it. The surfaces of these plates are soon covered with little white scales, which gradually fall to the bottom of the vessel, as they come to be collected in quantities. It is even proper to strike the cucurbit gently now and then, in order to shake the scales of Silver from the copper plates, and so make room for a new crop.
The aqua fortis parts with the Silver by degrees only, as it dissolves the Copper; and therefore the liquor gradually acquires a blueish green colour as the precipitation advances. This precipitation of the Silver is to be continued as long as any remains dissolved in the aqua fortis: you may be sure that your liquor contains no more Silver, if the surface of a fresh plate of Copper laid therein remain clean and free from ash-coloured or greyish particles: or if one drop of a solution of Sea-salt let fall into it produce no white or milky cloud.
The precipitation being finished, the liquor is to be gently poured off from the precipitated Silver, which must be rinsed[Pg 254] in several waters, and even made to boil therewith, in order to free it wholly from the dissolved Copper. The Silver thus well washed must be thoroughly dried, mixed with a fourth part of its weight of a flux compounded of equal parts of Nitre and calcined Borax, and then melted in a crucible. On this occasion care must be taken to raise the fire gently and gradually, till the Silver be brought to fusion.
With what accuracy soever the precipitated Silver be washed, in order to free it from the solution of Copper, yet the Silver will always be found alloyed with a small portion of the Copper: but then this Copper is easily destroyed by the Nitre, with which the Silver is afterwards melted; so that the latter metal remains perfectly pure after the operation.
Though the Silver be not previously cupelled, but be alloyed with other metallic substances at the time it is thus dissolved, yet the dissolving, precipitating, and fusing it with Nitre, would be sufficient to separate it accurately from them all, and refine it to a degree of purity equal to that obtained by the cupel.
The Copper that remains dissolved in the aqua fortis, after the precipitation of the Silver, may in like manner be precipitated by Iron, and, as it retains a small portion of Silver, ought not to be neglected when these operations are performed on considerable quantities.
In the two next processes we shall shew two other methods of separating Silver from aqua fortis.
To separate Silver from the Nitrous Acid by Distillation. Crystals of Silver. The Infernal Stone.
Into a large, low, glass body put the solution of Silver, from which you intend to separate the Silver by distillation. To this body fit a tubulated head provided with its stopple. Set this alembic in a sand-bath, so that the body may be almost covered with sand: apply a receiver, and distil with a moderate heat, so that the drops may succeed each other at the distance of some seconds. If the receiver grow very hot, check the fire. When red vapours begin to appear, pour into the alembic, through the hole in its head, a fresh quantity of your solution of Silver, first made very hot. Continue distilling in this manner, and repeating the addition of fresh liquor, till all your solution be put into the a[Pg 255]lembic. When you have no more fresh solution to put in, and when, the phlegm being all come over, red vapours begin again to appear, convey into the alembic half a dram or a dram of tallow, and distil to dryness; which being done, increase your fire so as to make the vessel containing the sand-bath red-hot. In the alembic you will find a calx of Silver, which must be melted in a crucible with some soap and calcined wine-lees.
OBSERVATIONS.
A low cucurbit is recommended for this operation, to the intent that the particles of the Nitrous Acid, which are ponderous, may the more easily be carried up and pass over into the receiver. For the same reason the cucurbit is directed to be almost wholly covered with sand, lest otherwise the acid vapours should be condensed about that part of the cucurbit, which, being out of the sand, would be much cooler than that which is encompassed therewith, and from thence should fall back again to the bottom; by which means the distillation would certainly be retarded, and the vessel probably be broken.
Notwithstanding these precautions the vessels are liable to break in such distillations; especially when they contain a great deal of liquor. With a view, therefore, to prevent this accident, we ordered that the whole quantity of the solution of Silver to be distilled should not be put at once into the alembic. The little bit of tallow, added towards the end of the operation, is intended to hinder the metal from adhering closely to the vessel, as it would otherwise do, when all the moisture is dissipated.
The Soap and Fixed Alkali mixed with the Silver to flux it, after its separation from the aqua fortis in this way, serve to absorb such of the most fixed particles of the Acid as may still remain united with the metal.
If the distillation be stopped when part of the phlegm is drawn off, and the liquor be then suffered to cool, many crystals will shoot therein, which are a Neutral Salt constituted of the Nitrous Acid and Silver. If the distillation be carried further, and stopped when near its conclusion, the liquor being then suffered to cool will wholly coagulate into a blackish mass called the Infernal Stone.
This way of separating Silver from its solvent is attended with the advantage of saving all the aqua fortis, which is excellent, and fit to be employed in other operations.
To separate Silver from the Nitrous Acid by Precipitation. Luna Cornea. Luna Cornea reduced.
Into your solution of Silver pour about a fourth part in weight of Spirit of Salt, solution of Sea-salt, or solution of Sal Ammoniac. The liquor will instantly become turbid and milky. Add twice or thrice its weight of fair water, and let it stand some hours to settle. It will deposite a white powder. Decant the clear liquor, and on the precipitate pour fresh aqua fortis, or Spirit of Salt, and warm the whole on a sand-bath with a gentle heat for some time. Pour off this second liquor, and boil your precipitate in pure water, shifting it several times, till the precipitate and the water be both quite insipid. Filter the whole, and dry the precipitate, which will be a Luna Cornea, and must be reduced in the following manner.
Smear the inside of a good crucible well with soap. Put your Luna Cornea into it; cover it with half its weight of Salt of Tartar, thoroughly dried and pulverized; press the whole hard down; pour thereon as much oil, or melted tallow, as the powder is capable of imbibing; set the crucible thus charged, and close covered, in a melting furnace, and, for the first quarter of an hour, make no more fire than is necessary to make the crucible moderately red: after that raise it so as to melt the Silver and the Salt, throwing into the crucible from time to time little bits of tallow. When it ceases to smoke, let the whole cool; or pour it into a hollow iron cone, warmed and tallowed.
OBSERVATIONS.
The process here delivered furnishes us with the means of procuring Silver in a degree of purity which is not to be obtained by any other method of treating it whatever. That which is refined on the cupel always retains a small portion of Copper, from which it cannot possibly be separated in that way: but if it be dissolved in aqua fortis, and precipitated thence in a Luna Cornea by the Marine Acid, the precipitate will be an absolutely pure Silver, unalloyed with that small portion of Copper which is retained on the cupel. The reason of this effect is, that the Copper remains as per[Pg 257]fectly dissolved in Spirit of Salt and in aqua regia as in aqua fortis: so that when the Silver, and the Copper with which it is alloyed, are dissolved together in the Nitrous Acid, if the Acid of Sea-salt be mixed with the solution, part of this latter Acid unites with the Silver, and therewith forms a new compound, which not being soluble in the liquor, falls to the bottom. The other part of the Acid mixing with the Nitrous, forms an aqua regis, in which the Copper remains dissolved, without separating from it.
Fresh Acid is poured on the precipitated calx of Silver, in order to complete the solution of the small portion of Copper that may have escaped the action of the first solvent. It is indifferent whether the Spirit of Salt or the Spirit of Nitre be employed for this purpose, because they both dissolve Copper alike, and because Silver precipitated by Spirit of Salt is not soluble in either.
After this it is necessary to wash the precipitate well with pure water, in order to free it entirely from the particles of aqua fortis adhering to the Silver; because they may possibly contain something of Copper, which would mix with the Silver in melting, and taint its purity.
If this precipitate of Silver be exposed to the fire, unmixed with any other substance, it melts as soon as it begins to be red; and, if the fire be increased, part thereof will be dissipated in vapours, and the rest will make its way through the crucible. But being poured out as soon as melted, it coagulates into a cake of a purplish red colour, semi-transparent, ponderous, and in some degree pliable, especially if it be very thin. It bears some resemblance to horn, which hath occasioned it to be called Luna Cornea.
As Luna Cornea is not soluble in water, recourse must be had to fusion, in order to reduce it, by separating from the Silver those acids which give it the above-mentioned properties. Fixed Alkalis and fatty matters are very fit to produce that separation.
We directed that the inside of the crucible, in which the reduction is to be made, should be carefully smeared with soap, and that the Luna Cornea should be quite covered with a Fixed Alkali and fat, to the end that when the heat is strong enough to dissipate it in vapours, or to attenuate it so as to render it capable of penetrating the crucible, it may be forced to pass through matters qualified to absorb its Acid, and reduce it.
Luna Cornea may also be reduced by being melted with such metalline substances as have a greater affinity than Sil[Pg 258]ver with the Acids wherewith it is impregnated. Of this kind are Tin, Lead, Regulus of Antimony: but the Luna Cornea rushes so impetuously into conjunction with those metalline substances, that a vast many vapours arise, and carry off with them part of the Silver: if therefore you chuse to effect the reduction by the interposition of such metalline substances, you must employ a retort instead of a crucible.
But this method is attended with another inconvenience; which is, that some part of those metalline substances may unite with the Silver, and adulterate it: for which reason it is best to keep to the method first proposed.
To dissolve Silver, and separate it from Gold, by Cementation.
Mix thoroughly together fine brick-dust four parts, Vitriol calcined to redness one part, and Sea-salt or Nitre one part. Moisten this powder with a little water. With this cement cover the bottom of a crucible half an inch thick; on this first bed lay a thin plate of the mass of Gold and Silver you intend to cement, and which you must previously take care to beat into such thin plates. Cover this plate with a second layer of cement, of the same thickness as the former; on this second bed lay another plate of your metal; cover it in like manner with cement; and so proceed till the crucible be filled to within half an inch of its brim. Fill up the remaining space with cement, and close the crucible with a cover, luted with a paste made of Windsor-loam and water: set your crucible thus charged in a furnace, whose fire-place is deep enough to let it be entirely surrounded with coals, quite up to its mouth. Light some coals in the furnace, taking care not to make the fire very brisk at first; increase it by degrees, but only so far as to make the crucible moderately red; keep up the fire in this degree for eighteen or twenty hours: then let the fire go out; open the crucible when it is cold, and separate the cement from your plates of Gold. Boil the Gold repeatedly in fair water, till the water come off quite insipid.
OBSERVATIONS.
It cannot but seem strange, that, after having so often declared the Acid of Sea-salt to be incapable of dissolving[Pg 259] Silver, we should direct either Nitre or Sea-salt indifferently to be employed in composing a cement, which is to produce an Acid capable of eating out all the Silver mixed with Gold. It is easy to conceive how the Nitrous Acid extricated from its basis by means of the Vitriolic Acid may produce this effect: but if Sea-salt instead of Nitre be made an ingredient in the cement, its Acid, though set at liberty in the same manner by the Vitriolic Acid, must at first sight appear unable to answer the end.
In order to remove this difficulty, we must here observe, that there are two very essential differences between the Marine Acid collected in a liquor, as it is when distilled in the usual manner, and the same Acid separated from its basis in a crucible, as it is in cementation.
The first of these two differences is, that the Acid being reduced into vapours when it acts on the Silver in cementation, its activity is thereby greatly increased: the second is, that in the crucible it sustains a vastly greater degree of heat than it can ever bear when it is in the form of a liquor. For, after it is once distilled and separated from its basis, it cannot sustain any extraordinary degree of heat without being volatilized and entirely dissipated: whereas, while it continues united with its basis, it is much more fixed, and cannot be separated but by a very intense heat. Consequently, if it meet with any body to dissolve, at the very instant of its separation from its basis, while it is actuated by a much fiercer heat than can ever be applied to it on any other occasion, it must operate upon that body with so much the more efficacy: and thus it comes to pass, that in cementation it has the power of dissolving Silver, which it would be incapable of touching if it were not so circumstanced.
But herein Gold differs from Silver: for, whatever force the Nitrous or the Marine Acid may exert, when extricated from their bases in the cementing crucible, this metal obstinately refuses to yield to either of those Acids separately, and can never be dissolved by them, unless both be united together.
Our cementation, therefore, is actually a parting process in the dry way. The Silver is dissolved, and the Gold remains unaltered. Nay, as the action of the Acids is much stronger when they are applied this way, than when they are used for dissolution in the moist way, the Nitrous Acid, which in the common parting process will not dissolve Silver unless its weight be double that of the Gold, is able in ce[Pg 260]mentation to dissolve a very small quantity of Silver diffused through a large quantity of Gold.
It sometimes happens, that after the operation the cement proves extremely hard, so that it is very troublesome to separate it entirely from the Gold. In this case it must be softened by moistening it with hot water. This hardness which the cement acquires is occasioned by the fusion of the Salts, which is the effect of too strong a heat. It was in order to prevent this, and that a due degree of heat might be applied, without the danger of melting the salts, that we directed the cement to be mixed with a considerable quantity of earthy matter incapable of fusion, such as brick-dust. A greater inconvenience will ensue, if the fire be made so strong as to melt the Gold: for then it will partly commix again with the other metalline substances dissolved by the cement, and consequently will not be purified.
The crucible is covered, and its cover luted on, to prevent the acid vapours from being too soon dissipated, and to force them to circulate the longer in the crucible. However, it is necessary that those vapours should find a vent at last, otherwise they would burst the vessel: and for this reason we directed the crucible to be luted only with Windsor-loam, which does not grow very hard by the action of fire, and so is capable of yielding and giving passage to the vapours, when a certain quantity of them is collected in the crucible, and they begin to struggle for an escape on every side.
When the operation is finished, the Silver dissolved by the Acid of the cement is partly distributed through the cement, and partly in the Gold itself, which is impregnated therewith. For this reason the Gold must be washed several times in boiling water, till the water become absolutely insipid: for, if the Gold be melted without this precaution, it will mix again with the Silver: the cement also may be washed in the same manner to recover the Silver it contains.
Though this cementation be, properly speaking, a purification of Gold, yet we have placed it among the processes on Silver, because it is the Silver that is dissolved on this occasion, and because this is a particular way of dissolving that metal. Moreover, most of the processes hitherto delivered, either on Gold or Silver, are equally applicable to both these metals.
If the Gold do not appear quite pure after the cementation, the process must be repeated.
There are several ways to know the fineness of Gold, the quantity of Silver with which it is alloyed, and the propor[Pg 261]tion in which these two metals are mixed in a mass purified by the cupel.
One of the simplest is the trial by the Touch-stone; which indeed is hardly any more than judging by the eye only, from the colour of the compound metal, what proportion of Gold and Silver it contains.
The Touch-stone is a sort of black marble, whose surface ought to be half polished. If the metalline mass which you want to try be rubbed on this stone, it leaves thereon a thin coat of metal, the colour of which may be easily observed. Such as are accustomed to see and handle Gold and Silver can at once judge very nearly from this sample in what proportion the two metals are combined: but, for greater accuracy, those who are in the way of having frequent occasion for this trial are provided with a sufficient number of small bars or needles, of which one is pure Gold, another pure Silver, and all the rest consist of these two metals mixed together in different proportions, varied by carats, or even by fractions of carats, if greater exactness be required.
The fineness of each needle being marked on it, that needle whose colour seems to come nearest the colour of the metalline streak on the Touch-stone, is rubbed on the stone by the side of that streak. This needle likewise leaves a mark; and if there appear to be no difference between the two metalline streaks, the metalline mass is judged to be of the same fineness as the needle thus compared with it. If the eye discovers a sensible difference, another needle is sought for whose colour may come nearer to that of the metal to be tried. But though a man be ever so well versed in judging thus of the fineness of Gold by the eye only, he can never be perfectly and accurately sure of it by this means alone. If such certainty be required, recourse must be had to the parting assay; and yet when you have gone through it, there always remains a small quantity of the metal, which should have been dissolved, and yet escaped the action of the solvent. For example, if you make use of aqua regis, the Silver that remains after the operation still contains a little Gold; and, if you make use of aqua fortis, the Gold that remains after the operation still contains a little Silver. And therefore if you resolve to carry the separation of these two metals still further by solvents, it will be necessary, after you have gone through one parting process, to perform a second the contrary way. For example, if you begin with aqua fortis, then, after it has dissolved all the Silver in the metalline mass that it is capable of taking up, dissolve the remaining Gold in[Pg 262] aqua regis: by which means you will separate the small portion of Silver left in it by the aqua fortis. The contrary is to be done if you made use of aqua regis first.
Of Copper.
To separate Copper from its Ore.
Beat your Copper ore to a fine powder, having first freed it as accurately as possible, by washing and roasting, from all stony, earthy, sulphureous, and arsenical parts. Mix your ore thus pulverized with thrice its weight of the black flux; put the mixture into a crucible; cover it with common salt to the thickness of half an inch, and press the whole down with your finger. With all this the crucible must be but half full. Set it in a melting furnace; kindle the fire by degrees, and raise it insensibly till you hear the Sea-salt crackle. When the decrepitation is over, make the crucible moderately red-hot for half a quarter of an hour. Then give a considerable degree of heat, exciting the fire with a pair of good perpetual bellows, so that the crucible may become very red-hot, and be perfectly ignited. Keep the fire up to this degree for about a quarter of an hour; then take out the crucible, and with a hammer strike a few blows on the floor whereon you set it. Break it when cold. If the operation hath been rightly and successfully performed, you will find at the bottom of the vessel a hard Regulus, of a bright yellow colour, and semi-malleable; and over it a scoria of a yellowish brown colour, hard and shining, from which you may separate the Regulus with a hammer.
OBSERVATIONS.
Copper in the ore is often blended with several other metallic substances, and with volatile minerals, such as Sulphur and Arsenic. Copper ores also frequently participate[Pg 263] of the nature of the pyrites, containing a martial and an unmetallic earth, both of which are entirely refractory, and hinder the ore from melting. In this case you must add equal parts of a very fusile glass, a little borax, and four parts of the black flux, to facilitate the fusion. The black flux is moreover necessary to furnish the Copper with the Phlogiston it wants, or restore so much thereof as it may lose in melting. For the same reason, when any ore, but that of Gold or Silver, is to be smelted, it is a general rule to add some black flux, or other matter abounding with Phlogiston.
The Regulus produced by this operation is not malleable, because it is not pure Copper, but a mixture of Copper with all the other metallic substances that were in the ore; except such as were separated from it by roasting, of which it contains but little.
According to the nature of the metallic matters that remain combined with the Copper after this fusion, the colour of the Regulus is either like that of pure Copper, or a little more whitish: it is also frequently blackish, which has procured it the name of Black Copper. In this state, and even in general, it is usual enough to call this Regulus by the name of Black Copper, when alloyed with other metallic substances that render it unmalleable, whatever its colour be.
Hence it appears that there may be several different sorts of Black Copper. Iron, Lead, Tin, Bismuth, and the reguline part of Antimony, are almost always combined with the ores of Copper, in a multitude of different proportions; and all these substances, being reduced by the black flux in the operation, mix and precipitate with the Copper. If the ore contain any Gold or Silver, as is pretty often the case, these two metals also are confounded with the rest in the precipitation, and become part of the Black Copper.
Pyritose, sulphureous, and arsenical Copper ores may be fused, in order to get rid of the grosser heterogeneous parts, without previously roasting them: but in this case no alkaline flux must be mixed with the ore; because the Alkali in combination with the Sulphur would produce a Liver of Sulphur, and so dissolve the metalline part; by which means all would be confounded together, and no Regulus, or very little, be precipitated. On this occasion therefore nothing must be added to promote the fusion, but some tender fusile glass, together with a small quantity of borax.
This first fusion may also be performed amidst the coals, by casting the ore upon them in the furnace, without using a crucible; and then an earthen vessel, thoroughly heated, or even made red-hot, must be placed under the grate of the fire-place, to receive the metal as it runs from the ore.
The Regulus obtained by this means is much more impure and brittle than Black Copper, because it contains moreover a large quantity of Sulphur and Arsenic; as these volatile substances have not time to evaporate during the short space requisite to melt the ore, and as they cannot be carried off by the action of the fire after the ore is once melted, whatever time be allowed for that purpose. However, some part thereof is dissipated; and the Iron which is in pyritose ores, having a much greater affinity than Copper, and indeed than any other metallic substance, with Sulphur and Arsenic, absorbs another part thereof, and separates it from the Regulus.
This Regulus, it is plain, still contains all the same parts that were in the ore, but in different proportions; there being more Copper, combined with less Sulphur, Arsenic, and unmetallic earth, which have been either dissipated or turned to slag. Therefore, if you would make it like Black Copper, you must pound it, roast it over and over, to free it from its Sulphur and Arsenic, and then melt it with the black flux.
If this Regulus contain much Iron, it will be adviseable to melt it once or twice more, before all the Sulphur and Arsenic are separated from it by roasting; for as the Iron, by uniting with these volatile substances, separates them from the Copper, with which they have not so great an affinity; so also the Sulphur and Arsenic, by uniting with the Iron, help in their turn to separate it from the Copper.
To purify Black Copper, and render it malleable.
Break into small bits the Black Copper you intend to purify; mix therewith a third part in weight of granulated Lead, and put the whole into a cupel set under the muffle in a cupelling furnace, and previously heated quite red. As soon as the metals are in the cupel raise the fire considerably, making use, if it be needful, of a pair of perpetual bellows, to melt the Copper speedily. When it is[Pg 265] thoroughly melted, lower the fire a little, and continue it just high enough to keep the metalline mass in perfect fusion. The melted matter will then boil, and throw up some scoriæ, which will be absorbed by the cupel.
When most of the Lead is consumed, raise the fire again, till the face of the Copper become bright and shining, thereby shewing that all its alloy is separated. As soon as your Copper comes to this state, cover it with charcoal-dust conveyed into the cupel with an iron ladle: then take the cupel out of the furnace and let it cool.
OBSERVATIONS.
Of all the metals, next to Gold and Silver, Copper bears fusion the longest without losing its phlogiston; and on this property is founded the process here delivered for purifying it.
It is necessary the Copper should melt as soon as it is in the cupel, because its nature is to calcine much more easily and much sooner, when it is only red-hot, than when it is in fusion. For this reason the fire is to be considerably raised, immediately on putting the Copper under the muffle, that it may melt as soon as possible. Yet too violent a degree of fire must not be applied to it: for when it is exposed to such a degree of heat only as is but just necessary to keep it in fusion, it is then in the most favourable condition for losing as little as may be of its phlogiston; and if the heat be stronger, a greater quantity thereof will be calcined. As soon therefore as it flows it is proper to weaken the fire, and reduce it to the degree just requisite to keep up the fusion.
The Lead added on this occasion is intended to facilitate and expedite the scorification of the metallic substances combined with the Copper. So that the event is here nearly the same as when Gold or Silver is refined on the cupel. The only difference between this refining of Copper, and that of the perfect metals, is that the latter as hath been shewn, absolutely resist the force of fire and the action of Lead, without suffering the least alteration; whereas a good deal of Copper is calcined and destroyed, when it is purified in this manner on the cupel. Indeed it would be wholly destroyed, if a greater quantity of Lead were added, or if it were left too long in the furnace. It is with a view to save as much of it as possible that we order it to be covered with charcoal-dust as soon as the scorification is finished.
The Lead serves moreover to free the Copper expeditiously from the Iron with which it may be alloyed. Iron and Lead are incapable of contracting any union together: so that as fast as the Lead unites with the Copper, it separates the Iron, and excludes it out of the mixture. For the same reason if Iron were combined in a large proportion with Copper, it would prevent the Lead from entering into the composition. Now, as it is necessary to give the more heat, and to keep the Copper to be incorporated with Lead the longer in fusion, as that Copper is alloyed with a greater proportion of Iron, some black flux must be added on this occasion, to prevent the Copper and the Lead from being calcined before their association can be effected.
Copper purified in the manner here directed is beautiful and malleable. It is now alloyed with no other metalline substance but Gold or Silver, if there were any in the mixed mass. If you desire to extract this Gold or Silver, recourse must be had to the operation of the cupel. The process here given for purifying Copper is not used in large works, because it would be much too chargeable. In order to purify their Black Copper, and render it malleable, the smelters content themselves with roasting it, and melting it repeatedly, that the metallic substances, which are not so fixed as Copper, may be dissipated by sublimation, and the rest scorified by fusion.
To deprive Copper of its Phlogiston by calcination.
Put your Copper in filings into a test, and set it under the muffle of a cupelling furnace; light the fire, and keep up such a degree of heat as may make the whole quite red, but not enough to melt the Copper. The surface of the Copper will gradually lose its metalline splendour, and put on the appearance of a reddish earth. From time to time stir the filings with a little rod of copper or iron, and leave your metal exposed to the same degree of fire till it be entirely calcined.
OBSERVATIONS.
In our observations on the preceding process we took notice that Copper, in fusion, calcines more slowly, and less[Pg 267] easily, than when it is exposed to a degree of fire barely sufficient to keep it red-hot, without melting it; and therefore, the design here being to calcine it, we have directed that degree of heat only to be applied.
The cupelling furnace is the fittest for this operation, because the muffle is capable of receiving such a flat vessel as ought to be used on this occasion, and communicating to it a great deal of heat; while, at the same time, it prevents the falling in of any coals, which, by furnishing the Copper with fresh phlogiston, would greatly prejudice and protract the operation.
As Copper calcines with great difficulty, this operation is extremely tedious: nay, though Copper hath stood thus exposed to the fire for several days and nights, and seems perfectly calcined, yet it frequently happens that, when you try afterwards to melt it, some of it resumes the form of Copper: a proof that all the Copper had not lost its phlogiston. Copper is much more expeditiously deprived of its phlogiston by calcining it in a crucible with Nitre.
The calx of Copper perfectly calcined is with great difficulty brought to fusion: yet, in the focus of a large burning-glass, it melts and turns to a reddish and almost opaque glass.
By the process here delivered, you may likewise calcine all other metalline substances, which do not melt till they are thoroughly red-hot. As to those which melt before they grow red, they are easily enough calcined, even while they are in fusion.
To resuscitate the Calx of Copper, and reduce it to Copper, by restoring its Phlogiston.
Mix the Calx of Copper with thrice as much of the black flux; put the mixture into a good crucible, so as to fill two thirds thereof, and over it put a layer of Sea-salt a finger thick. Cover the crucible, and set it in a melting furnace; heat it gradually, and keep it moderately red till the decrepitation of the Sea-salt be over. Then raise the fire considerably by means of a good pair of perpetual bellows; satisfy yourself that the matter is in perfect fusion, by dipping into the crucible an iron wire; continue the fire in this degree for half a quarter of an hour. When the cruci[Pg 268]ble is cold, you will find at its bottom a button of very fine Copper, which will easily separate from the saline scoria at top.
OBSERVATIONS.
What hath been said before on the smelting of Copper ores may be applied to this process, as being the very same. The observations there added should therefore be consulted on this occasion.
To dissolve Copper in the Mineral Acids.
On a sand-bath, in a very gentle heat, set a matrass containing some Copper filings; pour on them twice their weight of Oil of Vitriol. That Acid will presently attack the Copper. Vapours will rise, and issue out of the neck of the matrass. A vast number of bubbles will ascend from the surface of the metal to the top of the liquor, and the liquor will acquire a beautiful blue colour. When the Copper is dissolved, put in a little and a little more, till you perceive the Acid no longer acts upon it. Then decant the liquor, and let it stand quiet in a cool place. In a short time great numbers of beautiful blue crystals will shoot in it. These crystals are called Vitriol of Copper, or Blue Vitriol. They dissolve easily in water.
OBSERVATIONS.
The Vitriolic Acid perfectly dissolves Copper, which is also soluble in all the Acids, and even in many other menstruums.
This Acid may be separated from the Copper which it hath dissolved by distillation only: but the operation requires a fire of the utmost violence. The Copper remaining after it must be fused with the black flux, to make it appear in its natural form; not only because it still retains a portion of the Acid, but also because it hath lost part of its phlogiston by being dissolved therein. The black flux is very well adapted both to absorb the Acid that remains united with the Copper, and to restore the phlogiston which the metal hath lost.
The most usual method of separating Copper from the Vitriolic Acid is by presenting to that Acid a metal with which it hath a greater affinity than with Copper. Iron being so qualified is consequently very fit to bring about this separation. When therefore plates of Iron well cleaned are laid in a solution of Blue Vitriol, the Acid soon begins to act upon them, and by degrees, as it dissolves them, deposites on their surfaces a quantity of Copper in proportion to the quantity of Iron it takes up. The Copper thus precipitated hath the appearance of small leaves or scales, exceeding thin, and of a beautiful copper-colour. Care must be taken to shake the Iron-plates now and then, to make the scales of Copper fall off, which will otherwise cover them entirely, hinder the Vitriolic Acid from attacking the Iron, and so put a stop to the precipitation of the remaining Copper.
When these scales of Copper cease to settle on the clean Iron plates, you may be sure all the Copper that was in the liquor is precipitated, and that this liquor, which was a solution of Copper before the precipitation, is a solution of Iron after it. So that here two operations are performed at one and the same time; to wit, the precipitation of the Copper, and the dissolution of the Iron.
The Copper thus precipitated requires only to be separated from the liquor by filtration, and melted with a little black flux, to become very fine malleable Copper.
The Copper may also be precipitated out of a solution of Blue Vitriol by the interposition of a Fixed Alkali. This precipitate is of a greenish blue colour, and requires a much greater quantity of the black flux to reduce it.
Copper dissolves in the Nitrous Acid, in the Marine Acid, and in Aqua regis; from all of which it may be separated by the same methods as are here ordered with regard to the Vitriolic Acid.
Of Iron.
To separate Iron from its Ore.
Pound into a coarse powder the martial stones or earths out of which you design to extract the Iron: roast this powder in a test under the muffle for some minutes, and let your fire be brisk. Then let it cool, beat it very fine, and roast it a second time, keeping it under the muffle till it emit no more smell.
Then mix with this powder a flux composed of three parts of Nitre fixed with Tartar, one part of fusile glass, and half a part of Borax and charcoal-dust. The dose of this reducing flux must be thrice the weight of the ore.
Put this mixture into a good crucible; cover it with about half a finger thick of Sea-salt; over the crucible put its cover, and lute it on with Windsor-loam made into a paste with water. Having thus prepared your crucible, set it in a melting furnace, which you must fill up with charcoal. Light the fire, and let it kindle by gentle degrees, till the crucible become red-hot. When the decrepitation of the Sea-salt is over, raise your fire to the highest by the blast of a pair of perpetual bellows, or rather several. Keep up this intense degree of heat for three quarters of an hour, or an whole hour, taking care that during all this time the furnace be kept constantly filling up with fresh coals as the former consume. Then take your crucible out of the furnace; strike the pavement on which you set it several times with a hammer, and let it stand to cool: break it, and you will find therein a Regulus of Iron covered with slag.
OBSERVATIONS.
Iron ore, like all others, requires roasting, to separate from it, as much as possible, the volatile minerals, Sulphur and Arsenic, which being mixed with the Iron would render[Pg 271] it unmalleable. Indeed it is so much the more necessary to roast these ores, as Iron is, of all metallic substances, that which has the greatest affinity with those volatile minerals; on which account no metallic substance whatever is capable of separating it from them by fusion and precipitation.
Fixed Alkalis, it is true, have a greater affinity than Iron with Sulphur; but then the composition which a Fixed Alkali forms with Sulphur is capable of dissolving all metals. Consequently, if you do not dissipate the Sulphur by roasting, but attempt to separate it from the Iron by melting the ore with a Fixed Alkali, the Liver of Sulphur formed in the operation will dissolve the martial part; so that after the fusion you will find little or no Regulus.
All Iron ores in general are refractory, and less fusible than any other; for which reason a much greater proportion of flux, and a much more violent degree of fire, is required to smelt them. One principal cause why these ores are so refractory is the property which Iron itself has of being extremely difficult to fuse, and of resisting the action of the fire so much the more as it is purer, and further removed from its mineral state. Among all the metallic substances it is the only one that is less fusible when combined with that portion of phlogiston which gives it the metalline form, than when it is deprived thereof, and in the form of a calx.
In smelting-houses Iron ore is fused amidst charcoal, the phlogiston of which combines with the martial earth, and gives it the metalline form. The Iron thus melted runs down to the bottom of the furnace, from whence it is let out into large moulds, in which it takes the shape of oblong blocks, called Pigs of Iron. This Iron is still very impure, and quite unmalleable. Its want of ductility after the first melting arises partly from hence, that, notwithstanding the previous roasting which the ore underwent, there still remains, after this first fusion, a considerable quantity of Sulphur or Arsenic combined with the metal.
A certain quantity of quick-lime, or of stones that will burn to lime, is frequently mixed with Iron ore on putting it into the smelting furnace. The lime being an absorbent earth, very apt to unite with Sulphur and Arsenic, is of use to separate those minerals from the Iron.
It is also of use to mix some such matters with the ore, when the stones or earths which naturally accompany it are very fusible; for, as the Iron is of difficult fusion, it may happen that the earthy matters mixed with the Iron shall[Pg 272] melt as easily as the metal, or perhaps more easily. In such a case there is no separation of the earthy from the metalline part, both of which melt and precipitate together promiscuously; now quick-lime, being extremely refractory, serves on this occasion to check the melting of those matters which are too fusible.
Yet quick-lime, notwithstanding its refractory quality, may sometimes be of use as a flux for Iron. This is the case when the ore happens to be combined with substances which, being united with lime, render it fusible: such are all arsenical matters, and even some earthy matters, which being combined with quick-lime make a fusible compound.
When the ore of an Iron mine is found difficult to reduce, it is usually neglected even though it be rich: because Iron being very common, people chuse to work those mines only whose ores are smelted with the most ease, and require the least consumption of wood.
Yet refractory ores are not to be altogether rejected, when another Iron ore of a different quality is found near them. For it often happens, that two several Iron ores, which being worked separately are very difficult to manage, and yield at last but bad metal, become very tractable, and yield excellent Iron, when smelted together: and accordingly such mixtures are often made at Iron-works.
The Iron obtained from ores by the first fusion may be divided into two sorts. The one, when cold, resists the hammer, doth not easily break, and is in some measure extensible on the anvil; but, if struck with a hammer when red-hot, flies into many pieces: this sort of Iron hath always a mixture of Sulphur in it. The other sort, on the contrary, is brittle when cold, but somewhat ductile when red-hot. This Iron is not sulphurated, is naturally of a good quality, and its brittleness arises from its metalline parts not being sufficiently compacted together.
Iron abounds so much, and is so universally diffused through the earth, that it is difficult to find a body in which there is none at all: and this hath led several Chymists, even men of great fame, into the error of thinking that they had transmuted into Iron several sorts of earths in which they suspected no Iron, by combining them with an inflammable matter; whereas, in fact, all they did was to give the metalline form to a true martial earth which happened to be mixed with other earths.
To render Pig-iron and brittle Iron malleable.
Into an earthen vessel widening upwards put some charcoal-dust, and thereon lay the Pig-iron which you propose to render ductile; cover it all over with a quantity of charcoal; excite the fire violently with a pair, or more, of perpetual bellows till the Iron melt. If it do not readily flow and form a great deal of slag on its surface, add some flux, such as a very fusible sand.
When the matter is in fusion keep stirring it from time to time, that all the parts thereof may be equally acted on by the air and the fire. On the surface of the melted Iron scoriæ will be formed, which must be taken off as they appear. At the same time you will see a great many sparkles darted up from the surface of the metal, which will form a sort of fiery shower. By degrees, as the Iron grows purer, the number of these sparkles diminishes, though they never vanish entirely. When but few sparkles appear, remove the coals which cover the Iron, and let the slag run out of the vessel; whereupon the metal will grow solid in a moment. Take it out while it is still red-hot, and give it a few strokes with a hammer, to try if it be ductile. If it be not yet malleable, repeat the operation a second time, in the same manner as before. Lastly, when it is thus sufficiently purified by the fire, work it for a long time on the anvil, extending it different ways, and making it red-hot as often as there is occasion. Iron thus brought to the necessary degree of ductility, so as to yield to the hammer, and suffer itself to be extended every way, either hot or cold, without breaking to bits, or even cracking in the least, is very good and very pure. If it cannot be brought to this degree by the method here prescribed, it is a proof that the ore from which this Iron was extracted ought to be mixed with other ores; but it frequently requires a great number of trials to obtain an exact knowledge of the quality and proportion of those other ores with which it is to be mixed.
OBSERVATIONS.
The brittleness and shortness of Pig-iron arises from the heterogeneous parts which it contains, and which could not[Pg 274] be separated from it by the first fusion. These extraneous matters are usually Sulphur, Arsenic, and unmetallic earth, and also a ferruginous earth; but such as could not be combined with the phlogiston as it ought to be, in order to have the properties of a metal, and must therefore be considered as heterogeneous, with respect to the other well-conditioned martial particles.
The Pig-iron, by undergoing repeated fusions, is freed from those heterogeneous matters; those which are volatile, such as Sulphur and Arsenic, being dissipated, and the unmetallic matters being scorified. As to the ferruginous earth, which did not at first acquire the metalline form, it becomes true Iron at last; because, among the coals with which it is encompassed, it meets with a sufficient quantity of phlogiston to reduce it to metal. Charcoal is also necessary on this occasion, that it may continually furnish phlogiston to the Iron, which would otherways be converted into a calx.
Hammering the red-hot Iron, after each fusion, serves to force out from amongst the martial parts such earthy matters as may happen to remain there, and so bring into closer contact the metalline parts which were separated before by the interposition of those heterogeneous matters.
To convert Iron into Steel.
Take small bars of the best Iron; that is, of such as is malleable both hot and cold; set them on their ends in a cylindrical earthen vessel, whose depth is equal to the length of the bars, and in such a manner that they may be an inch distant from each other, and from the sides of the crucible. Fill the vessel with a cement compounded of two parts of charcoal, one part of bones burnt in a close vessel till they become very black, and one half part of the ashes of green wood; having first pulverized and thoroughly mixed the whole together. Take care to lift up the Iron bars a little, to the end that the cement may cover the bottom of the vessel, and so that there be about the depth of half an inch thereof under every bar: cover the crucible and lute on the cover.
Set the crucible thus prepared in a furnace, so contrived that the crucible may be surrounded with coals from top to bottom: for eight or ten hours keep up such a degree of fire[Pg 275] that the vessel may be moderately red; after this take it out of the furnace; plunge your little Iron bars into cold water, and you will find them converted into Steel.
OBSERVATIONS.
The principal difference between Iron and Steel consists in this, that the latter is combined with a greater quantity of phlogiston than the former.
It appears by this experiment, that, to make Iron unite with an inflammable matter, it is not necessary it should be in fusion; it is sufficient that it be so red-hot as to be opened and softened by the fire.
Every kind of charcoal is fit to be an ingredient in the composition of the cement employed to make Steel, provided it contain no Vitriolic Acid. However, it hath been observed, that animal coals produce a speedier effect than others: for which reason it is proper to mix something of that kind with charcoal-dust, as above directed.
The following signs shew that the operation hath succeeded, and that the Iron is changed into good Steel.
This metal being quenched in cold water, as proposed above, acquires such an extraordinary degree of hardness, that it will by no means yield to any impression of the file or hammer, and will sooner break in pieces than stretch upon the anvil. And here it is proper to observe, that the hardness of Steel varies with the manner in which it is quenched. The general rule is, that the hotter the Steel is when quenched, and the colder the water is in which you quench it, the harder it becomes. It may be deprived of the temper thus acquired, by making it red-hot, and letting it cool slowly; for it is thereby softened, rendered malleable, and the file will bite upon it. For this reason the artisans who work in Steel begin with untempering it, that they may with more ease shape it into the tool they intend to make. They afterwards new-temper the tool when finished, and by this second temper the Steel recovers the same degree of hardness it had acquired by the first temper.
The colour of Steel is not so white as that of Iron, but darker, and the grains, facets, or fibres, which appear on breaking it, are finer than those observed in Iron.
If the bars of Iron thus cemented in order to convert them into Steel be too thick, or not kept long enough in cementation, they will not be turned into Steel throughout their whole thickness: their surfaces only will be Steel to a certain depth,[Pg 276] and the center will be mere Iron; because the phlogiston will not have thoroughly penetrated them. On breaking a bar of this sort, the difference in colour and grain between the Steel and the Iron is very visible.
It is easy to deprive Steel of the superabundant quantity of phlogiston which constitutes it Steel, and thereby reduce it to Iron. For this purpose it need only be kept red-hot some time, observing that no matter approach it all the while that is capable of refunding to it the phlogiston which the fire carries off. The same end is still sooner obtained by cementing it with meagre hungry matters, capable of absorbing the phlogiston; such as bones calcined to whiteness, and cretaceous earths.
Steel may also be made by fusion; or Pig-iron may be converted into Steel. For this purpose the same method must be employed as was above directed for reducing Pig-iron into malleable Iron; with this difference, that, as Steel requires more phlogiston than is necessary to Iron, all the means must be made use of that are capable of introducing into the Iron a great deal of phlogiston; such as melting but a small quantity of Iron at a time, and keeping it constantly encompassed with abundance of charcoal; reiterating the fusions; taking care that the blast of the bellows directed along the surface of the metal do not remove the coals that cover it, &c. And here it must be observed, that there are some sorts of Pig-iron which it is very difficult to convert into Steel by this method, and that there are others which succeed very readily, and with scarce any trouble at all. The ores which yield the last-mentioned sort of Pig-iron are called Steel Ores. Steel made by this means must be tempered in the same manner as that made by cementation[8].
The Calcination of Iron. Sundry Saffrons of Mars.
Take filings of Iron, in what quantity you please; put them into a broad unglazed earthen vessel; set it under the muffle of a cupelling furnace; make it red-hot; stir[Pg 277] the filings frequently; and keep up the same degree of fire till the Iron be wholly turned into a red powder.
OBSERVATIONS.
Iron easily loses its phlogiston by the action of fire. The calx that remains after its calcination is exceeding red; which makes this be thought the natural colour of the earth of that metal. It hath accordingly been observed, that all the earths and stones which either are naturally red, or acquire that colour by calcination, are ferruginous.
The yellowish red colour which every calx of Iron hath, in whatever manner it be prepared, hath procured the name of Crocus or Saffron to every preparation of this kind. That made in the manner above directed is called in Medicine Crocus Martis astringens.
The rust produced on the surface of Iron is a sort of calx of Iron made by way of dissolution. The moisture of the air acts upon the metal, dissolves it, and robs it of some of its phlogiston. This rust is called in Medicine Crocus Martis Aperiens; because it is thought that the saline parts, by means whereof the humidity dissolves the Iron, remain united with the metal after its dissolution, and give it an aperitive virtue. The Apothecaries prepare this sort of Saffron of Mars by exposing Iron filings to the dew, till they be turned entirely to rust: which is then called Saffron of Mars by dew.
Another Saffron of Mars is also prepared in a much shorter manner, by mixing filings of Iron with pulverized Sulphur, and moistening the mixture, which after some time ferments and grows hot. It is then set on the fire; the Sulphur burns away, and the mass is kept stirring till it become a red matter. This Saffron is nothing but Iron dissolved by the Acid of Sulphur, which is known to be of the same nature with that of Vitriol; and consequently this Saffron of Mars is no way differing from Vitriol calcined to redness.
Iron dissolved by the mineral Acids.
Put any mineral Acid whatever into a matrass with some water; set the matrass on a sand-bath gently heated; drop into the vessel some filings of Iron: the phenomena[Pg 278] which usually accompany metalline dissolutions will immediately appear. Add more filings, till you observe the Acid hath lost all sensible action upon them: then remove your matrass from the sand-bath; you will find in it a solution of Iron.
OBSERVATIONS.
Iron is very easily dissolved by all the Acids. If you make use of the Vitriolic Acid, care must be taken to weaken it with water, in case it be concentrated; because the dissolution will succeed the better. The vapours that rise on this occasion are inflammable; and if a lighted paper be held to the mouth of the matrass, especially after keeping it stopt for some time and shaking the whole gently, the sulphureous vapours take fire with such rapidity as to produce a considerable explosion; which is sometimes strong enough to burst the vessel into a thousand pieces. This solution hath a green colour, and is in fact a fluid Green Vitriol, which wants nothing but rest to make it shoot into crystals.
If you make use of the Nitrous Acid, you must cease adding more filings when the liquor, after standing still some moments, becomes turbid; for, when this Acid is impregnated with Iron to a certain degree, it lets fall some of that which it had dissolved, and becomes capable of taking up fresh filings. Thus, by constantly adding new supplies of Iron, this Acid may be made to dissolve a much greater quantity thereof than is necessary to saturate it entirely. This solution is of a russet colour, and doth not crystallize.
If the weather be not extremely cold, and the Acids have a proper degree of strength, the sand-bath is unnecessary, as the dissolution will succeed very well without it.
Iron dissolved by Acids may be separated therefrom, like all other metallic substances in the same circumstances, either by the action of fire, which carries off the Acid and leaves the Martial Earth, or by the interposition of substances which have a greater affinity than metallic substances have with Acids; that is, by Absorbent Earths and Alkaline Salts. By whatever means you separate Iron from an Acid solvent, it constantly appears, after the separation, in the form of a yellowish red powder; because it is then deprived of most of the phlogiston to which it owed its metalline form; whence it is reasonable to think, that this is the proper colour of Martial earth.
All these precipitates of Iron are true Saffrons of Mars, which, as well as those prepared by calcination, are so much the further removed from the nature of a metal, the more they are deprived of their phlogiston. Thence it comes that they are more or less soluble by Acids, and more or less attracted by the magnet: as no ferruginous earth, perfectly deprived of all inflammable matter, is at all attracted by the magnet, or soluble by Acids.
Of Tin.
To extract Tin from its Ore.
Break your Tin ore into a coarse powder, and by washing carefully separate from it all the heterogeneous matters, and ores of a different kind, that may be mixed therewith. Then dry it, and roast it in a strong degree of fire, till no more Arsenical vapour rise from it. When the ore is roasted, reduce it to a fine powder, and mix it thoroughly with twice its weight of the black flux well dried, a fourth part of its weight of clean iron filings, together with as much borax and pitch; put the mixture into a crucible; over all put Sea-salt to the thickness of four fingers, and cover the crucible close.
Set the crucible thus prepared in a melting furnace: apply at first a moderate and slow degree of fire, till the flame of the pitch, which will escape through the joint of the cover, disappear entirely. Then suddenly raise your fire, and urge it with rapidity to the degree necessary for melting the whole mixture. As soon as the whole is in fusion take the crucible out of the furnace, and separate the Regulus from the scoria.
OBSERVATIONS.
All Tin ores contain a considerable quantity of Arsenic, and no Sulphur at all, or at most very little. Hence it comes that, though Tin be the lightest of all metals, its ore is never[Pg 280]theless much heavier than any other; Arsenic being much heavier than Sulphur, of which the ores of every other kind always contain a pretty large proportion. This ore is moreover very hard, and is not brought to a fine powder with so much ease as the rest.
These properties of Tin ore furnish us with the means of separating it easily by lotion, not only from earthy and stony parts, but even from the other ores which may be mixed with it. And this is of the greater advantage on two accounts, viz. because Tin cannot endure, without the destruction of a great part thereof, the degree of fire necessary to scorify the refractory matters which accompany its ore; and again because this metal unites so easily with Iron and Copper, the ores of which are pretty commonly blended with Tin ore, that, after the reduction, it would be found adulterated with a mixture of these two metals, if they were not separated from it before the fusion.
But sometimes the Iron ore confounded with that of Tin is very heavy, and is not easily pulverized; whence it comes to pass that it cannot be separated therefrom by washing only. In that case the magnet must be employed to separate it, after the ore hath been roasted.
Roasting is moreover necessary for Tin ore, in order to dissipate the Arsenic which volatilizes, calcines, or destroys one part of the Tin, and reduces the rest to a short, brittle substance, like a Semi-metal. The ore is known to be sufficiently roasted when no more fumes rise from it; when it has lost the smell of garlic; and when it does not whiten a clean plate of Iron held over it.
Tin being one of those metals which are most easily calcined, it is necessary in reducing its ore to employ such matters as may furnish it with phlogiston. In order to defend it from the contact of the air, which always accelerates the calcination of metallic substances, the mixture is to be covered with Sea salt; and the addition of pitch helps to increase the quantity of phlogiston.
The Calcination of Tin.
Into an unglazed earthen dish put the quantity of Tin you intend to calcine; melt it, and keep stirring it from time to time. Its surface will be covered with a greyish[Pg 281] white powder: continue the calcination till all your Tin be converted into such a powder, which is the Calx of Tin.
OBSERVATIONS.
Though the calcination of metalline substances is promoted by exposing them, in powder, or in filings, to the action of fire, and by ordering it so that they may not melt, because they present a much smaller surface when melted than when unmelted; yet we have not directed this precaution to be used in calcining Tin. The reason is, this metal is so fusible that it cannot endure the degree of fire requisite to destroy its phlogiston without melting, and of course, though Tin calcines easily, the operation is nevertheless tedious, because the melted metal presents but a small surface to be acted on by the fire and the air. This inconvenience may be partly remedied, and the operation greatly expedited, by dividing the quantity of Tin to be calcined into several small parcels, and exposing them to the fire in separate vessels, so that they may not re-unite when melted, and form one single mass.
Leaf Tin cast on Nitre in actual fusion causes it to deflagrate and fulminate; and from this mixture there rises a white vapour, which is converted into flowers when it meets with any obstacle to impede its flying off entirely.
Mr. Geoffroy, who went through a course of experiments on Tin, an account whereof may be seen in the Memoirs of the Academy of Sciences, found that from the colour of the calx of that metal a judgment may be formed of its degree of purity, and nearly of the quantity and quality of the metallic substances with which it is alloyed. The experiments tried on this subject by that eminent Chymist are very curious.
He performed the calcination in a crucible, which he heated to a cherry-red, and kept up the same degree of fire from the beginning to the end of the operation. The calx which formed upon his metal, in that degree of heat, appeared like small white scales, a little reddish on the under side. He pushed it to one side as it formed, to the end that it might not cover the surface of the metal, which, like all others, requires the contact of the air to turn it into a calx.
"While he was making these calcinations, he had an opportunity of observing a curious fact, of which no body before him had ever taken notice; probably because no body had ever calcined Tin by the same method. The[Pg 282] fact is, that during the calcination of the Tin, whether you break the pellicle which forms on the surface of the metal while in red-hot fusion, or whether you let it remain without touching it, you perceive in several places a small swell of a certain matter, which bursts and makes its way through the pellicle. This matter puffs up, grows red, at the same instant takes fire, and darts out a small whitish flame, as vivid and as brilliant as that of Zinc, when urged by a fire strong enough to sublime it into flowers. The vividness of this flame may be further compared to that of several small grains of phosphorus of urine fired and gently dropped on boiling water. From this bright flame a white vapour exhales; after which the swelled mass partly crumbles down, and turns to a light white powder, sometimes spotted with red, according to the force of the fire. After this momentary ignition, there arise stronger, more numerous, or more frequent heavings of matter, out of which issues a good deal of white fume, that may be intercepted by a cover of tin-plate or copper fitted to the crucible, and appears to be the flowers of Tin, which in some measure corrode these metals. Hence Mr. Geoffroy conjectures, with a great deal of probability, that their sublimation is promoted by a portion of Arsenic. When the crust formed by this calx comes to be too thick, or in too great a quantity, to be pushed on one side, so as to leave part of the metal uncovered, Mr. Geoffroy puts out the fire, because no more calx would be formed: the communication of the external air with the Tin in fusion being absolutely necessary thereto, as hath been already said. In this operation it is to be observed that, if the fire be too slow, neither the inflammation of the sulphureous particles, nor the white fumes that rise, will be so distinctly perceived, as when the fire is of the degree requisite to keep the crucible just of a cherry-red heat.
"Mr. Geoffroy having taken off this first calx began the calcination anew. In this second heat the buddings or heavings were more considerable, and shot up in the form of cauli-flowers; but were still composed of little scales. The thoroughly calcined portion of this vegetation was likewise white and red; and the inferior surfaces of some little bits thereof were wholly red. When these calcinations are continued, sulphureous vapours rise seemingly of another kind than those which appeared in the beginning; for all the calx made by the first heat was perfectly white:[Pg 283] whereas in the second it begins to be spotted here and there with a tinge of black. Mr. Geoffroy was obliged to go through a course of twelve several calcinations before he could convert two ounces of Tin into a calx. He had the opportunity, during these several calcinations, to observe that after the fourth, and sometimes after the third, the red spots of the calx decrease, and the black increase; that the germinations cease; that the crust of the calx remains flat; that in the twelfth fire the Tin yields no more of this scaly crust; that towards the end the undulations of the fused metal appear no longer; and that the small remainder of calx is mixed with several very minute grains of metal, which seem much harder than Tin. Mr. Geoffroy could not collect a sufficient quantity thereof to cupel them, and satisfy himself whether or no they were Silver."
Though Tin, and all the imperfect metals in general, seem converted to a calx, and lose the metalline form, by one single calcination, and that a slight one; yet they are not wholly deprived of their phlogiston: for if the calx of Tin, for instance, prepared according to the process above delivered, be cast upon Nitre in fusion, it will make that salt deflagrate very perceptibly; a convincing proof that it still contains much inflammable matter. If therefore a calx be required absolutely free from phlogiston, this first calx must be recalcined by a more violent fire, and the calcination continued till all the phlogiston be dissipated.
"Mr. Geoffroy, being desirous of having his calx of Tin very pure and perfectly calcined, exposed once more to the action of fire the twelve portions of calx obtained by his former calcinations. But, as it would have been too tedious to re-calcine them all separately, he made four parcels of the whole, each consisting of three taken according to the order in which they were first calcined; and gave to each a fire sufficiently strong, and long enough continued, to calcine them as thoroughly as was possible. After this second calcination he found them all of a most beautiful white, except the first parcel: as that consisted of the portions obtained by the three first heats, in all of which there were scales tinged with red, it still retained a stain of carnation, though hardly perceptible. Agreeably to the general rule, the two ounces of Tin gained in weight by being thus calcined; and the increase was two drams and fifty seven grains.
"Mr. Geoffroy observes, that no Tin, but what is absolutely pure, will yield a perfectly white calx. He calcined in this manner several other parcels of Tin that were impure and variously alloyed; each of which produced a calx differently coloured, according to the nature and quantity of its alloy: whence he justly concludes, that calcination is a very good method of trying the fineness of Tin, or its degree of purity." The particulars of Mr. Geoffroy's experiments on this subject, which are very curious, may be seen in the Memoirs of the Academy for 1738.
It is proper to take notice that a man should be very cautious how he exposes himself to the vapours of Tin, because they are dangerous; this metal being very justly suspected by Chymists of containing something Arsenical.
The dissolution of Tin by Acids, The Smoking Liquor of Libavius.
Put into a glass vessel what quantity you please of fine Tin cut into little bits. Pour on it thrice as much aqua regis, compounded of two parts aqua fortis weakened with an equal quantity of very pure water, and one part Spirit of Salt. An ebullition will arise, and the Tin will be very rapidly dissolved; especially if the quantities of metal and of aqua regis be considerable.
OBSERVATIONS.
Tin is soluble by all the Acids; but aqua regis dissolves it best of any. Yet in this dissolution it comes to pass that part of the dissolved Tin precipitates of its own accord to the bottom of the vessel, in the form of a white powder. This solution of Tin is very fit for preparing the purple-coloured precipitate of Gold. For this purpose the solution of Tin must be let fall, drop by drop, into a solution of Gold. Spirit of Nitre dissolves Tin nearly as aqua regis does; but it occasions a greater quantity of calx.
If two or three parts of Oil of Vitriol be poured on one part of Tin, and if the vessel in which the mixture is made be exposed to such a degree of heat as to evaporate all the moisture, there will remain a tenacious matter[Pg 285] sticking to the sides of the vessel. If water be poured on this matter, and it be then exposed a second time to the fire, it will dissolve entirely, excepting a small portion of a glutinous substance, which also may be dissolved in fresh Oil of Vitriol.
The Acid of Sea-salt may be combined with Tin by the following process. Mix perfectly, by trituration in a marble mortar, an amalgam of two ounces of fine Tin, and two ounces and a half of Quick-silver, with as much Corrosive Sublimate. As soon as the mixture is completed, put it into a glass retort, and distil with the same precautions as we directed to be used in preparing concentrated and smoking Acids. There will first come over into the receiver some drops of a limpid liquor, which will be soon followed by an elastic spirit that will issue out with impetuosity. At last some flowers, and a saline tenacious matter, will rise into the neck of the retort. Then stop your distillation, and pour into a glass bottle the liquor you will find in the receiver. This liquor continually exhales a considerable quantity of dense, white fumes, as long as it is allowed to have a free communication with the air.
The product of this distillation is a combination of the Acid of Sea-salt with Tin. As the affinity of Tin with this Acid is greater than that of Mercury, the Acid contained in the Corrosive Sublimate quits the Mercury, wherewith it was united, to join the Tin; which it volatilizes so as to make it rise with itself in a limpid form. We make use of the amalgam of Tin with Quick-silver, because we are thereby enabled to mix the Corrosive Sublimate perfectly therewith, as the success of the operation requires it should be.
In this experiment the Tin is volatilized, and the Acid of Sea-salt, which is exceedingly concentrated, flies off incessantly in the form of white vapours. This compound is known in Chymistry by the name of Smoking Liquor of Libavius; a name derived from its quality, and from its Inventor. Tin dissolved by Acids is easily separated from them by Alkalis. It always precipitates in the form of a white calx.
Of Lead.
To extract Lead from its Ore.
Having roasted your Lead ore reduce it to a fine powder; mix it with twice its weight of the black flux, and one fourth of its weight of clean iron filings and borax; put the whole into a crucible capable of containing at least thrice as much; over all put Sea-salt four fingers thick; cover the crucible; lute the juncture; dry the whole with a gentle heat, and set it in a melting furnace.
Make the crucible moderately red: you will hear the Sea-salt decrepitate, and after the decrepitation a small hissing in the crucible. Keep up the same degree of fire till that be over.
Then throw in as many coals as are necessary to complete the operation entirely, and raise the fire suddenly, so as to bring the whole mixture into perfect fusion. Keep up this degree of fire for a quarter of an hour, which is time sufficient for the precipitation of the Regulus.
When the operation is finished, which may be known by the quietness of the matter in the crucible, and by a bright vivid flame that will rise from it, take the crucible out of the furnace, and separate the Regulus from the scoria.
OBSERVATIONS.
All Lead ore contains a good deal of Sulphur, which must be first separated from it by roasting: and as this kind of ore is apt to fly when first exposed to the fire, it is proper to keep it covered till it be thoroughly heated. Another precaution to be used, in roasting this ore, is not to give it too great a heat, but to keep the vessel which contains it just moderately red; because it easily turns clammy, which occasions it to stick to the vessel.
The Iron that is added, and mixed with the flux, absorbs the Sulphur which may happen to remain, even after roasting: it helps also to separate from the Lead some portions of semi-metal, especially of Antimony, which are frequently mixed with this ore.
There is no fear least the Iron mix with the Lead in fusion, and adulterate it: for these two metals are incapable of contracting any union together, when each has its metalline form.
Nor is there any reason to apprehend lest the Iron should, by its refractory quality, obstruct the fusion of the mixture; for though this metal be not fusible when alone, yet, by the union it contracts with the matters it is designed to absorb, it becomes so to such a degree as in some measure to perform, on this occasion, the office of a flux.
The government of the fire is a point of great consequence in this operation. It is necessary to apply but a moderate degree of heat at first: for, when the metallic earth of the Lead, combining with the phlogiston, acquires the metalline form, it swells up in such an extraordinary manner, that there is great danger least the matter should overflow, and run all out of the containing vessel. With a view therefore to avoid this inconvenience, we direct a very large crucible to be used. This heaving of the Lead, at the instant of its reduction, is attended with a noise like the whistling of wind.
Notwithstanding all the precautions that can be used to prevent the reduction from taking place too hastily, and so occasioning the effusion of the matter, it often happens that, on raising the fire in order to bring the mixture into fusion, the hissing suddenly begins again, and is very loud. In that case all the apertures of the furnace must immediately be shut close, in order to choak and suffocate the fire: for, if this be neglected, the matter in the crucible will swell up, make its way through the luting of the juncture, nay, push up the cover, and run over. This accident is to be apprehended during the first five or six minutes after you raise the fire in order to melt the mixture. This effusion of the matter is accompanied with a dull flame, a thick, grey and yellow smoke, and a noise like that of some boiling liquor. When you observe these several phenomena you may be sure the matter is run out of the crucible, either in the manner above described, or by making its way through some cracks in the vessel, and consequently that the operation is spoiled.
Moreover, this event infallibly follows whenever a bit of coal happens to fall into the crucible; and this is one reason why it is necessary to cover it.
You may be certain that the operation hath succeeded if the scoria be smooth when cold, and have not in part escaped through the lute; if the Lead be not dispersed in globules through the whole mass of the matter contained in the crucible, but is, on the contrary, collected at the bottom, in the form of a solid Regulus, not very shining, but of a blueish cast, and ductile. Moreover, the scoria ought, in the present case, to be hard and black, and should not appear full of holes like a sieve, except only in that part which was contiguous to the Salt.
Here it is proper to observe, that the Sea-salt doth not mix with the scoria, but floats upon it. After the operation it is black; which colour it gets, no doubt, from the charred parts of the flux. The absence of these signs shews the operation to have miscarried.
When the ore to be smelted is pyritose and refractory, it may be roasted at first with a much stronger degree of fire than is used for ores that are fusible; because the martial earth, and the unmetallic earth, which are always mixed in pyritose matters, hinder it from growing readily soft in the fire. Besides, such an ore requires a greater quantity of the black flux and of borax to be mixed with it, and a higher degree of fire to fuse it.
It is generally needless to mix iron filings with this sort of ore; because the martial earth, with which pyritose matters are always accompanied, is reduced during the operation by the help of the black flux, which for that purpose is mixed with it in a large proportion, and furnishes a quantity of iron sufficient to absorb the heterogeneous minerals mixed with the Lead.
Yet, if it should be observed that the pyrites which accompany the Lead ore are arsenical, then, as such pyrites contain but a small quantity of ferruginous earth, iron filings must be added; which are, on this occasion, so much the more necessary for absorbing the Arsenic, as this mineral remains in part confounded with the ore, is reduced to a Regulus during the operation, unites with the Lead, and destroys a great deal of it by procuring its vitrification.
The Lead obtained from such pyritose ores is commonly not very pure; it is blackish and scarce ductile; qualities communicated to it by a small mixture of Copper in the py[Pg 289]rites, which always contain more or less thereof. We shall presently shew the method of separating Lead from Copper.
Lead ore may also be reduced by melting it amidst coals. For that purpose first kindle a fire in the furnace in which you intend to melt your ore; then put a layer of your ore immediately upon the lighted coals, and cover it with another layer of coals.
Though the melting furnace used for this operation be capable of giving a considerable heat, yet it is necessary further to increase the force of the fire by the means of a good pair of perpetual bellows, which will produce an effect like that of a forge. The ore melts, the earth of the Lead unites with the phlogiston of the coals, and so is reduced to metal, which runs through the coals, and falls into an earthen vessel placed at the bottom of the furnace to receive it. Care must be taken to keep this vessel well filled with charcoal-dust, to the end that the Lead may be in no danger of calcination while it continues there; the charcoal-dust constantly furnishing it with phlogiston to preserve its metalline form.
The earthy and stony matters that accompany the ore are scorified by this fusion, just as they are by the other which is performed in a close vessel. With regard to the Sulphur and Arsenic, they are supposed to have been first accurately separated from the ore by roasting. This is the method commonly employed for smelting Lead ore at the works.
To separate Lead from Copper.
With luting earth and charcoal-dust make a flat vessel, widening upwards, and large enough to contain your metalline mass. Set it shelving downwards from the back towards the fore-part; and in the fore-part, at the bottom, make a little gutter communicating with another vessel of the same nature, placed near the former and a little lower. Let the mouth of the gutter within side the upper vessel be narrowed, by means of a small iron plate fixed across it, while the loam is yet soft; so as to leave a very small aperture, in the lower part of this canal, sufficient to discharge the Lead as it melts. Dry the whole by placing lighted coals around it.
When this apparatus is dry, put your mixed mass of Copper and Lead into the upper vessel: both in that, and in the other vessel, light a very gentle fire of wood or charcoal, so as not to exceed the degree of heat necessary to melt Lead. In such a degree of heat the Lead contained in the mixed mass will melt, and you will see it run out of the upper vessel into the lower; at the bottom of which it will unite into a Regulus. When in this degree of heat no more Lead flows, increase the fire a little, so as to make the vessel moderately red.
When no more will run, collect the Lead contained in the lower vessel. Melt it over again in an iron ladle, with a degree of fire sufficient to make the ladle red; throw into it a little tallow or pitch, and while it burns keep stirring the metal, in order to reduce any part of it that may be calcined. Remove the pellicle or thin crust which will form on the surface; squeeze out all the Lead it contains, and then put it to the mass of Copper left in the upper vessel. Check the fire, and in the same manner take off a second skin that will form on the surface of the Lead. Lastly, when the metal is ready to fix, take off the skin that will then appear on it. The Lead remaining after this will be very pure, and free from all alloy of Copper.
With regard to the Copper itself, you will find it in the upper vessel covered with a thin coat of Lead: and if the Lead mixed with it was in the proportion of a fourth or a fifth part only, and the fire applied was gentle and slow, it will retain nearly the same form after the operation that the mixed mass had before.
OBSERVATIONS.
Lead frequently remains mixed with Copper after the reduction of its ore, especially if the ore was pyritose. Though Copper be a much more beautiful and more ductile metal than Lead, yet the latter by being alloyed with the former is rendered eager and brittle. This bad quality is easily discovered by the eye on breaking it: for the surface of the broken part appears all granulated; whereas when it is pure it is more evenly, and resembles a congeries of solid angles. If the Lead be alloyed with a considerable quantity of Copper, its colour hath a yellowish cast.
Considering the bad qualities which Copper communicates to Lead, it is necessary to separate these two metals from each other. The method above laid down is the simplest and the[Pg 291] best. It is founded on two properties belonging to Lead: the first is that of being much more fusible than Copper; so that it will melt and run in a degree of heat that is not capable of making the Copper even red-hot, which yet is very far from being able to melt it: the second is, that Lead, though it hath an affinity with Copper, and unites very perfectly therewith, yet is not able to dissolve it without a greater heat than the degree barely necessary to fuse Lead. Hence it comes that Lead may be melted in a Copper vessel, provided no greater degree of heat be applied than that purpose requires. But when the Lead becomes so hot as to be red, fume, and boil, it instantly begins to dissolve the Copper. For this reason, it is essential to the success of our operation that a moderate degree of heat only be applied, and no greater than is requisite to keep the Lead in fusion.
Charcoal-dust is made an ingredient in the composition of the vessels used on this occasion, in order to prevent the calcination of the Lead.
The iron plate, with which the entrance of the gutter within the upper vessel is narrowed, serves to prevent the larger pieces of Copper, which the Lead may carry along with it, from passing through: it stops them, and allows the Lead to run off alone.
But as these parcels of Copper may entirely choak the passage, care must be taken, when any happen to be stopt, to remove them from the entrance of the gutter, and push them back into the middle of the vessel. It is also necessary to observe whether or no the Lead fixes any where in the passage; and, if it does, the heat of that part must be increased, in order to melt it and make it run off.
Notwithstanding all the precautions that can be taken, to hinder the melted Lead from carrying off any Copper with it, it is impossible to prevent this inconvenience entirely; and therefore the Lead is melted over again, in order to separate the small portion of Copper with which it is still adulterated.
As Copper is much lighter than Lead, if these two metals happen to be so blended together that the Copper, without being in fusion and dissolved by the Lead, is only interposed between the parts of the melted Lead, so as to swim therein, it is then precisely in the case of a solid body plunged into a fluid heavier than itself, and must rise to the surface, like wood thrown into water. It is proper to burn some inflammable matter on this melted Lead, in order to reduce such parts thereof as are constantly calcining on its surface while[Pg 292] it is in fusion; for without this precaution they would be taken off together with the Copper.
The Copper remaining after this separation is, as we took notice before, still mixed with a little Lead. If you desire to separate it entirely therefrom, you must put it into a cupel, and expose it under the muffle to such a degree of fire as may convert all the Lead into litharge. This cannot be so done but that some of the Copper also will be scorified by the heat of the fire, and by the action of the Lead: but as there is a very great difference between the facility and readiness with which these two metals calcine, the portion of Copper that is calcined, while the whole Lead is turning into litharge, is scarce worth considering.
The Lead, though carefully separated from the Copper by the process here delivered, is not yet absolutely pure: sometimes it is alloyed with Gold, and almost always contains some Silver. If you would free the Lead as much as possible from any mixture of these two metals, you must convert it into glass, separate the remaining bead, and afterwards reduce this glass of Lead. But, as these two perfect metals are of no prejudice to the Lead, it is not usual to separate them from it, unless they be in a sufficient proportion to defray the charge, and produce some profit besides.
When we examine by the cupel the just proportion of Gold and Silver that an ore or a mixed metalline mass will yield, we make a previous assay of the Lead to be employed in the operation, and afterwards, in our estimate, deduct a proper allowance for the quantity of fine metal due to the Lead made use of.
The Calcination of Lead.
Take what quantity of Lead you please; melt it in one or more unglazed earthen pans: a dark grey powder will be found on its surface. Keep stirring the metal incessantly till it be wholly converted into such a powder, which is the Calx of Lead.
OBSERVATIONS.
As Lead is a very fusible metal, and in that respect greatly resembles Tin, most of the observations we made on the calcination of Tin may be applied here.
In the calcination of all metals, and particularly in this of Lead, there appears a singular phenomenon which is not easily accounted for. It is this: though these matters lose a great deal of their substance, either by the dissipation of their phlogiston, or because some of the metal, perhaps, exhales in vapours, yet when the calcination is over their calces are found to be increased in weight, and this increase is very considerable. An hundred pounds of Lead, for example, converted into Minium, which is nothing but a calx of Lead brought to a red colour by continuing the calcination, are found to gain ten pounds weight; so that for an hundred pounds of Lead we have one hundred and ten pounds of Minium: a prodigious and almost incredible augmentation, if it be considered that, far from adding any thing to the Lead, we have on the contrary dissipated part of it.
To account for this phenomenon Natural Philosophers and Chymists have invented several ingenious hypotheses, but none of them entirely satisfactory. As we have no established theory to proceed upon, we shall not undertake to explain this extraordinary fact.
To prepare Glass of Lead.
Take two parts of Litharge, and one part of pure crystalline Sand; mingle them together as exactly as possible, adding a little Nitre and Sea-salt: put this mixture into a crucible of the most solid and most compact earth. Shut the crucible with a cover that may perfectly close it.
Set the crucible thus prepared in a melting furnace; fill the furnace with coals; light the fire gradually, so that the whole may be slowly heated: then raise the fire so as to make the crucible very red, and bring the matter it contains into fusion; keep it thus melted for a quarter of an hour.
Then take the crucible out of the furnace, and break it: in the bottom thereof you will most commonly find a small button of Lead, and over it a transparent Glass, of a yellow colour nearly resembling that of amber. Separate this Glass from the little button of metal, and from the saline matters which you will find above it.
OBSERVATIONS.
Pure Lead, being exposed to a strong fire without any additament, turns to Litharge; which is a scaly sort of substance, more or less yellowish, shining, and soft to the touch. This is the first advance to the Vitrification of Lead. The large refineries of Gold and Silver by the means of Lead furnish a great quantity of this material. It is sometimes whitish, and is then called Litharge of Silver; sometimes yellow, and then bears the name of Litharge of Gold. The difference of its colour depends on the degree of fire it hath undergone, and on the metalline substances vitrified with it.
Litharge alone is very fusible, and being exposed to the fire is easily converted into glass: but this Glass of Lead, made without additament, is so active, so penetrating, and so apt to swell, that it can scarcely be made use of when pure. We are obliged in some sort to clog it, by uniting it with some vitrifiable matter that is not so subtile, such as sand; and it is for this reason, not to render the mixture more fusible, that we have directed the addition of one third part of Sand to two thirds of Litharge.
The Nitre and Sea-salt, prescribed as ingredients in the mixture, are designed to procure an equal fusion of the whole. For, as the sand is lighter and less fusible than the Litharge, it will partly rise towards the upper part of the crucible when that matter first begins to flow; in consequence whereof the contents of the upper part will be much more difficult to melt, and form a Glass much more compact than that below: but the Nitre and Sea-salt possessing the upper part of the crucible, because they are still lighter than the Sand, and being in their own nature very efficacious fluxes, on account of their great fusibility, they quickly bring about the fusion of those particles of sand, which, having escaped the action of the Litharge, may have risen unvitrified to its surface.
The most difficult thing to procure, and yet the most necessary to the success of this operation, is a crucible of earth so firm and compact as not to be penetrated by the Glass of Lead, which corrodes and makes its way through every thing.
The precaution of chusing a crucible, that shall contain a good deal more than the matter to be vitrified, is a necessary one, because Litharge and Glass of Lead are very apt to swell.
The rule to keep the crucible close shut is also indispensably necessary, to prevent any bit of charcoal, or other inflammable matter, from falling into it: for when this happens it occasions a reduction of the Lead, which is always attended with a sort of effervescence, and such a considerable heaving, that commonly most of the mixture runs over the crucible. For the same reason it is very proper, before you expose the mixture to the fire, to examine whether or no it contains any matter capable of furnishing a phlogiston during the operation; and if it does, to remove that matter with great care.
The little button of Lead, found at the bottom of the crucible after the operation, comes from a small portion of Lead that is commonly left in Litharge, unless you prepare it carefully yourself, and do not take it from the fire till you are sure of having destroyed all the Lead. Besides, this small portion of Lead can be of no prejudice to the operation, because it cannot communicate its phlogiston to the rest of the matter.
The revivifying of Litharge, of the Calx, and of the Glass of Lead, may be obtained by the same processes as the reduction of its ore.
Lead dissolved by the Nitrous Acid.
Put into a matrass some aqua fortis precipitated like that used to dissolve Silver; weaken it by mixing therewith an equal quantity of common water; set the matrass in a hot sand-bath; throw into it, little by little, small bits of Lead, till you see that no more will dissolve. Aqua fortis thus lowered will dissolve about a fourth of its weight of Lead.
There is gradually formed upon the Lead, as it dissolves, first a grey powder, and afterwards a white crust, which at last hinder the solvent from acting on the remaining part of the metal; and therefore the liquor should be made to boil, and the vessel should be shaken to remove those impediments, by which means all the Lead will be dissolved.
OBSERVATIONS.
Lead very much resembles Silver, with respect to the phenomena which attend its dissolution in Acids. For ex[Pg 296]ample, the Nitrous Acid must be very pure and uncontaminated with the Vitriolic or Marine Acid, to qualify it for keeping the Lead in solution: for, if it be mixed with either the one or the other of these Acids, the Lead will precipitate in the form of a white powder as fast as it dissolves; which is just the case with Silver.
If the Vitriolic Acid be mixed with the Nitrous, the precipitate will be a combination of the Vitriolic Acid with Lead; that is, a Neutral Metallic Salt, or Vitriol of Lead. If the Acid of Sea-salt be mixed therewith, the precipitate will be a Plumbum corneum; that is, a Metallic Salt resembling the Luna cornea.
When all the Lead is dissolved as above described, the liquor appears milky. If it be kept warm over the fire till little crystals begin to appear on its surface, and afterwards left to stand quiet, in a certain time there will be found at the bottom a greyish powder, which being tried on Gold is Mercurial enough to whiten it. Little globules of Quick-silver are even discernible in it.
We owe this observation, together with this manner of proving the existence of Mercury in Lead, and of procuring it from thence, to M. Grosse, who hath given an account of his process in the Memoirs of the Academy of Sciences, from whence we have copied the description of the operation in hand.
The solution being quickly poured off by inclination from the grey mercurial precipitate is still milky, and deposites another white sediment. When this second precipitate falls the liquor becomes clear and limpid, and is then of a fine yellow colour, like a solution of Gold. On this gold-coloured solution, and on the two precipitates above-mentioned, M. Grosse made several observations, the chief of which we shall here insert.
The yellow liquor affects the tongue at first with a taste of sweetness; but afterwards vellicates it very smartly, and leaves on it a strong sensation of acrimony, which continues for a long time.
Alkalis precipitate the Lead suspended in this liquor, just as they do all other metals dissolved by Acids; and this precipitate of Lead is white.
Sea-salt, or Spirit of Salt, separates the Lead from its solvent, and precipitates it, as we observed before, into a Plumbum corneum: but this precipitate differs from the Luna cornea, as being very soluble in water; whereas the Luna cornea will not dissolve in it at all; or at least dissolves there[Pg 297]in with great difficulty, and in a very small quantity. This Plumbum corneum dissolved in water is again precipitated by the Vitriolic Acid. M. Grosse observes, that this forms an exception to the eighth column of Mr. Geoffroy's Table of Affinities; in which the Acid of Sea-salt is marked as having a greater affinity than any other Acid with Metallic substances.
Our solution of Lead is also precipitated in a white powder by several Neutral Salts; such as Vitriolated Tartar, Alum, and common Vitriol. It is by the means of double affinities that these Neutral Salts effect this precipitation.
Even pure water alone is capable of precipitating the Lead of our solution, by weakening the Acid, and thereby disabling it from keeping the metal suspended.
Lastly, as all the solutions of metals in Acids are nothing but Neutral Metallic Salts in a fluid form, so if the solution of Lead be evaporated over the fire, it will shoot into very beautiful crystals, about the bigness of hemp-seed, shaped like regular pyramids having square bases. These crystals are yellowish, and have a sweet saccharine taste: but what is most singular in them is, that, as they consist of the Nitrous Acid combined with Lead, which manifestly contains a great deal of phlogiston, they constitute a Nitrous Metallic Salt, which has the property of deflagrating in a crucible, without the addition of any other inflammable matter. It is extremely hard to dissolve this Salt in water.
The grey mercurial precipitate which whitens Gold, and in which little globules of running Mercury are perceivable, is far from being pure Mercury. This metallic substance makes but a small part thereof: for it is an assemblage, 1. of little crystals of the same nature with those afforded by the evaporated solution; 2. of a portion of the white matter, or powder, which renders the solution milky; 3. of a grey powder, which M. Grosse considers as the only mercurial part; 4. and lastly, of little particles of Lead that have escaped the action of the solvent; especially if a little more Lead than the Acid is capable of dissolving were added with a view to saturate it entirely, as in the present process.
By means of motion and heat the small parcels of Mercury may be amalgamated with the Lead.
That Mercury should be found entire and in globules in the Spirit of Nitre, which very easily dissolves that metallic substance, will not be surprizing to those who reflect that, in the present case, the Acid is saturated with Lead, with which it has a greater affinity than with Mercury; as ap[Pg 298]pears by M. Geoffroy's Table of Affinities, where, in the column that hath the Nitrous Acid at top, Lead is placed above Mercury. Agreeably to this, if Lead be presented to a solution of Mercury in Spirit of Nitre, the Lead will be dissolved, and as the dissolution thereof advances the Mercury will precipitate.
Hence it appears that, in order to find any Mercury in the spontaneous precipitate of Lead dissolved by the Nitrous Acid, it is necessary that the Acid be entirely saturated with Lead; or else that portion of the Acid which remains unsaturated will dissolve the Mercury.
With regard to the white powder that renders the solution milky, and afterwards precipitates, it is nothing but a portion of the Lead, which, not being intimately united with the Acid, falls in part of its own accord. It is a sort of calx of Lead, which being exposed to the fire becomes partly glass, and partly Lead, because it still retains some of its phlogiston.
Of Mercury.
To extract Mercury from its Ore, or to revivify it from Cinabar.
Pulverize the Cinabar from which you would extract the Mercury; with this powder mix an equal part of clean iron filings; put the mixture into a retort of glass or iron, leaving at least one third part thereof empty. Set the retort thus prepared in a sand-bath, so that its body may be quite buried in the sand, and its neck decline considerably downwards: fit on a receiver half filled with water, and let the nose of the retort enter about half an inch into the water.
Heat the vessels so as to make the retort moderately red. The Mercury will rise in vapours, which will condense into little drops, and fall into the water in the receiver. When you see that nothing more comes over with this degree of heat, increase it, in order to raise what Mercury may still be[Pg 299] left. When all the Mercury is thus brought over, take off the receiver, pour out the water contained in it, and collect the Mercury.
OBSERVATIONS.
Mercury is never mineralized in the bowels of the earth by any thing but Sulphur; with which it forms a compound of a brownish red colour, known by the name of Cinabar.
Sometimes it is only mixed with earthy and stony matters that contain no Sulphur; but, as this metallic substance is never destitute of its phlogiston, it then has its metalline form and properties. When it is found in this condition, nothing is more easy than to separate it from those heterogeneous matters. For that purpose no more is requisite than to distil the whole with a fire strong enough to raise the Mercury in vapours. This mineral is volatile; the earthy and stony matters are fixed; and a certain degree of heat will effect a complete separation of what is volatile from what is fixed.
This is not the case when Mercury is combined with Sulphur: for this latter mineral is volatile as well as Mercury; and the compound resulting from the union of them both is also volatile: so that if Cinabar were exposed to the fire in close vessels, as it must be to save the Mercury, it would be sublimed in substance, without being decomposed at all.
In order therefore to separate these two substances from each other, we must have recourse to the interposition of some third, which hath a greater affinity with one of them than the other hath, and no affinity with that other.
Iron hath all the conditions requisite for this purpose; seeing it hath, as may be seen in the Table, a much greater affinity with Sulphur than Mercury hath, and is incapable of contracting any union with Mercury.
Iron, however, is not the only substance that may be employed on this occasion: Fixed Alkalis, Absorbent earths, Copper, Lead, Silver, Regulus of Antimony, have all, as well as Iron, a greater affinity than Mercury with Sulphur. Nay, several of these substances, namely, the saline and earthy Alkalis, as well as Regulus of Antimony, cannot contract any union with Mercury: the rest, to wit, Copper, Lead, and Silver, are indeed capable of amalgamating with Mercury; but then the union which these metals contract[Pg 300] with the Sulphur prevents it; and even though they should unite with this metallic substance, the degree of heat to which the whole mixture is exposed would soon carry up the Mercury, and separate it with ease from those fixed substances.
In this distillation the same cautions must be observed as in all others: that is, the vessels must be slowly heated, especially if a glass retort be used; the fire must be raised by degrees, and a much stronger one applied at last than at first. This operation particularly requires a very strong degree of fire, when there is but a small quantity of Mercury left.
After the operation there remains in the retort a compound of Iron and Sulphur, which may easily be converted into a crocus, by calcining it and burning away the Sulphur.
If a Fixed Alkali be employed, a Liver of Sulphur will be found in the retort after the distillation.
If the Cinabar from which you extract the Mercury be good, you will generally obtain seven eighths of its weight in Quick-silver.
In the present operation it is not necessary to lute on the receiver, because the water, in which the nose of the retort is plunged, is sufficient to fix the Mercurial vapours. In case the Cinabar, from which you intend to separate the Mercury, be mixed with a great quantity of heterogeneous, but fixed, matters, such as earths, stones, &c. it may be separated from them by subliming it with a proper degree of heat, because it is volatile.
The vapours of Mercury are prejudicial, and may excite a salivation, tremors, and palsies; they should therefore be always avoided by such as work on this mineral.
The oldest and richest mine of Mercury is that of Almaden in Spain. It is a singular property of that mine that, though the Mercury found in it is combined with Sulphur, and in the form of Cinabar, yet no additament is required to procure the separation of these two; the earthy and stony matter, with which the particles of the ore are incorporated, being itself an excellent absorbent of Sulphur.
In the Quick-silver works carried on at this mine they make no use of retorts. They place lumps of the ore on an iron grate, which stands immediately over the furnace. The furnaces which serve for this operation are closed at the top by a sort of dome, behind which stands the shaft of a chimney that communicates with the fire-place, and gives vent to the smoke. These furnaces have in their fore-side sixteen apertures, to each of which is luted an aludel in a horizon[Pg 301]tal position, communicating with a long row of other aludels placed likewise in an horizontal direction; which aludels so connected together form one long pipe or canal, the further end whereof opens into a chamber destined to receive and condense all the mercurial vapours. These rows of aludels are supported from end to end by a terrass, which runs from the body of the building, wherein the furnaces are erected, to that where the chambers are built that perform the office of receivers.
This is a very ingenious contrivance and saves much labour, expence, and trouble, that would be unavoidable if retorts were employed.
That part of the furnace which contains the lumps of ore, serves for the body of the retort; the row of aludels for its neck; and the little chambers in which these canals terminate are actual receivers. The terrass of communication, which reaches from the one building to the other, is formed of two inclined planes, the lower edges of which, meeting in the middle of the terrass, rise from thence insensibly; the one quite to the building where the furnaces are, and the other to that which forms the recipient chambers. By this means, when any Mercury escapes through the joints of the aludels, it naturally runs down along these inclined planes, and so is collected in the middle of the terrass, where the inferior sides of the planes meeting together form a sort of canal, out of which it is easily taken up.
The celebrated M. de Jussieu having viewed the whole himself, in a journey he made to this mine, furnished us with this description of the work.
To give Mercury, by the action of Fire, the appearance of a Metalline Calx.
Put Mercury into several little glass matrasses with long and narrow necks. Stop the matrasses with a little paper, to prevent any dirt from falling into them. Set them all in one sand-bath, so that they may be surrounded with sand as high as two thirds of their length. Apply the strongest degree of heat that Mercury can bear without subliming: continue this heat without interruption, till all the Mercury be turned to a red powder. The operation lasts about three months.
OBSERVATIONS.
Mercury treated according to the process here delivered hath all the appearance of a metalline calx, but it hath no more: for, if it be exposed to a pretty strong degree of fire, it sublimes, and is wholly reduced to running Mercury, without the addition of any other inflammable matter; which proves that during this long calcination it lost none of its phlogiston.
The volatile nature of Mercury, which permits it not to bear a heat of any strength without subliming, prevents our examining all the effects that fire is capable of producing on it. Yet there is reason to believe that, as this metallic substance resembles the perfect metals in its weight, its splendour, and a brilliancy which resists all the impressions of the air without alteration, it would like them be unchangeable by the greatest force of fire, if it were fixed enough to bear it.
In order to give Mercury the form of a metalline calx, it must necessarily be exposed for about three months together, to the utmost heat it can bear without subliming, as is above directed. Boerhaave kept it digesting in a less heat for fifteen years successively, both in open and in close vessels, without observing it to suffer the least change; except that there was formed upon its surface a small quantity of a black powder, which was reduced to running Mercury by trituration alone.
Mercury thus converted to a red powder is known in chymistry and medicine by the name of Mercury precipitated per se: a title proper enough, as it is actually reduced to the form of a precipitate, and that without any additament; but very improper on the other hand, considering, that in reality this Mercury is not a precipitate, as not having been separated from any menstruum in which it was dissolved.
To dissolve Mercury in the Vitriolic Acid. Turbith Mineral.
Put Mercury into a glass retort, and pour on it thrice its weight of good Oil of Vitriol. Set the retort in a sand-bath; fit on a recipient; warm the bath by degrees till[Pg 303] the liquor just simmer. With this heat the Mercury will begin to dissolve. Continue the fire in this degree till all the Mercury be dissolved.
OBSERVATIONS.
The Vitriolic Acid dissolves Mercury pretty well: but for this purpose the Acid must be very hot, or even boil; and then too it is a very long time before the dissolution is completed. We have directed the operation to be performed in a retort; because this solution is usually employed to make another preparation called Turbith Mineral, which requires that as much as possible of the Acid solvent be abstracted by distillation. Having therefore dissolved your Mercury in the Vitriolic Acid, if you will now prepare the Turbith, you must, by continuing to heat the retort, drive over all the liquor into the receiver, and distil till nothing remains but a white powdery matter: then break the retort; pulverize its contents in a glass mortar, and thereon pour common water, which will immediately turn the white matter of a lemon-colour; wash this yellow matter in five or six warm waters, and it will be what is called in medicine Turbith Mineral; that is, a combination of the Vitriolic Acid with Mercury, five or six grains whereof is a violent purgative, and also an emetic; qualities which it possesses in common with the Vegetable Turbith, whose name it hath therefore taken.
There rises out of the retort, both while the Mercury is dissolving, and while the solvent is abstracting, a weak Spirit of Vitriol; because a great part of the Acid remains united with the Quick-silver, which at last appears in the form of a white powder: so that, if you do not incline to save the Acid which rises on this occasion, you may, instead of drawing off the liquor in a retort, evaporate it in a glass bason set on a sand-bath, which will be much sooner done.
It is very remarkable that, on this occasion, the Mercury may be exposed, without any danger of subliming, to a much greater heat than it is capable of bearing when not combined with the Vitriolic Acid; which shews that this Acid hath the property of fixing Mercury to a certain degree.
The white matter, that remains after the evaporation of the fluid, is one of the most violent corrosives, and would prove an actual poison if taken internally. By washing it several times in warm water it is freed from a great deal of its Acid, and so considerably sweetened. The proof is this;[Pg 304] if the water used in washing the Turbith be evaporated, there remains after the evaporation a matter in form of a Salt, that being set in a cellar runs into a liquor called Oil of Mercury, which is a powerful corrosive. Several authors further direct Spirit of Wine to be burnt on the Turbith, to sweeten it still more.
If, instead of washing the white matter that remains after the moisture is drawn off, fresh Oil of Vitriol be poured on it, and then abstracted as before; this treatment being repeated two or three times, there will at last remain in the retort a matter having the appearance of an oil, which resists the action of the fire, and cannot be desiccated: qualities which are owing to the great quantity of Acid particles thus united with the Mercury. This Oil of Mercury is one of the most violent corrosives. The Mercury may be separated therefrom, by precipitating it with an Alkali, or a metallic substance that hath more affinity than Mercury with the Vitriolic Acid: Iron, for instance, may be employed in this precipitation. Mercury thus separated from the Vitriolic Acid need only be distilled to recover the form of Quick-silver.
To combine Mercury with Sulphur. Æthiops Mineral.
Mix a dram of Sulphur with three drams of Quick-silver, by triturating the whole in a glass mortar with a glass pestle. By degrees, as you triturate, the Mercury will disappear, and the matter will acquire a black colour. Continue the triture till you cannot perceive the least particle of running Mercury. The black matter you will then have in the mortar is known in medicine by the name of Æthiops Mineral. An Æthiops may also be made by fire in the following manner.
In a shallow unglazed earthen pan melt one part of flowers of Sulphur: add three parts of running Mercury, making it fall into the pan in the form of small rain, by squeezing it through chamoy leather. Keep stirring the mixture with the shank of a tobacco-pipe all the while the Mercury is falling: you will see the matter grow thick and acquire a black colour. When the whole is thoroughly mixed, set fire to it with a match, and let as much of the Sulphur burn away as will flame.
OBSERVATIONS.
Mercury and Sulphur unite together with great ease; cold triture alone is sufficient to join them. By this means the Mercury is reduced into exceeding small atoms, and combines so perfectly with the Sulphur that the least vestige thereof is not to be seen.
Sulphur is not the only matter which being rubbed with Mercury will destroy its form and fluidity: all fat substances that have any degree of consistence, such as the fat of animals, balsams, and resins, are capable of producing the same effect. This metallic substance, being triturated for some time in a mortar with these matters, becomes at last invisible, and communicates to them a black colour. When thus divided by the interposition of heterogeneous particles, it is said to be Killed. But Mercury doth not contract such an intimate union with these other matters as it doth with Sulphur.
The Æthiops prepared by fusion is a more perfect and accurate combination of Mercury and Sulphur than the other: for, the quantity of Sulphur directed to be used in making it being much greater than is absolutely necessary to fix the Mercury, the redundant Sulphur is destroyed by burning, and none left but what is most intimately united with the Mercury; and hindered by the union it hath contracted with that metallic substance from being so easily consumed. The Æthiops therefore, which is prepared by fusion and burning the Sulphur, contains a much greater proportion of Mercury than that which is made by simple triture; so that in Medicine it ought to be prescribed in different cases, and in smaller doses.
If no more Sulphur than is just necessary to kill the Mercury be added to it at first, it will be difficult to obtain a perfect mixture; because that quantity is very small: it is better, therefore, to employ at once the quantity above directed.
To sublime the Combination of Mercury and Sulphur into Cinabar.
Grind to powder Æthiops mineral prepared by fire. Put it into a cucurbit; fit thereto a head; place it in a sand-bath, and begin with applying such a degree of heat as is requisite to sublime Sulphur. A black matter will rise, and adhere to the sides of the vessel. When nothing more will rise with this degree of heat, raise the fire so as to make the sand and the bottom of the cucurbit red; and then the remaining matter will sublime in the form of a brownish red mass, which is true Cinabar.
OBSERVATIONS.
Æthiops Mineral requires nothing but sublimation to become true Cinabar, like that found in Quick-silver mines: but our Æthiops contains still more Sulphur than ought to be in the composition of Cinabar; for which reason we have directed the degree of fire applied at first to be no greater than that which is capable of subliming Sulphur. As Cinabar, though consisting of Mercury and Sulphur, is yet much less volatile than either of these substances alone; which probably arises from the Vitriolic Acid contained in the Sulphur; therefore, if there be any redundant Sulphur in the Æthiops, which hath not contracted an intimate union with the Mercury, it will sublime by itself in this first degree of heat. Some mercurial particles also will rise with it, and give it a black colour.
Cinabar contains no more Sulphur than about a sixth or seventh part of its weight: so that, instead of employing the common Æthiops to make it, it would be better to prepare one on purpose that should contain much less Sulphur; because too much Sulphur prevents the success of the operation by blackening the Sublimate. Indeed in whatever manner you go about it, the Cinabar always appears black at first: but when it is well prepared, and contains no more than its due proportion of Sulphur, the blackness is only external. This black coat therefore may be taken off; and then the internal part will appear of a fine red, and, if sublimed a second time, will be very beautiful.
As artificial Cinabar hath the same properties with the native, it may be decomposed by the same means: so that, if you want to extract the Mercury out of it, recourse must be had to the process above delivered for working on Cinabar ores.
To dissolve Mercury in the Nitrous Acid. Sundry Mercurial Precipitates.
Put into a matrass the quantity of Mercury you intend to dissolve: pour on it an equal quantity of good Spirit of Nitre, and set the matrass in a sand-bath moderately heated. The Mercury will dissolve with the phenomena that usually attend the dissolutions of metals in this Acid. When the dissolution is completed let the liquor cool. You will know that the Acid is perfectly saturated, if there remain at the bottom of the vessel, notwithstanding the heat, a little globule of Mercury that will not dissolve.
OBSERVATIONS.
Mercury dissolves in the Nitrous Acid with much more facility, and in much greater quantity, than in the Vitriolic; so that it is not necessary, on this occasion, to make the liquor boil. This solution when cold yields crystals, which are a Nitrous Mercurial Salt. If you desire to have a clear limpid solution of Mercury, you must employ an aqua fortis that is not tainted with the Vitriolic or Marine Acid: for, the affinity of these two Acids with Mercury being greater than that of the Nitrous Acid, they precipitate it in the form of a white powder, when they are mixed with the solvent.
Mercury thus precipitated in a white powder, out of a solution thereof in the Spirit of Nitre, is used in Medicine. To obtain this precipitate, which is known by the name of the White Precipitate, Sea-salt dissolved in water together with a little Sal Ammoniac is used; and the precipitate is washed several times in pure water, without which precaution it would be corrosive, on account of the great quantity of the Marine Acid which it would contain.
The preparation known by the name of Red Precipitate is also obtained from our solution of Mercury in Spirit of[Pg 308] Nitre. It is made by abstracting all the moisture of the solution, either by distillation in a retort, or by evaporation in a glass bason set on a sand-bath. When it begins to grow dry it appears like a white ponderous mass. Then the fire is made strong enough to drive off almost all the Nitrous Acid, which, being now concentrated, rises in the form of red vapours. If these vapours be catched in a receiver, they condense into a liquor, which is a very strong and vastly smoking Spirit of Nitre.
By degrees, as the Nitrous Acid is forced up by the fire, the mercurial mass loses its white colour, and becomes first yellow, and at last very red. When it is become entirely of this last colour the operation is finished. The red mass remaining is a Mercury that contains but very little Acid, in comparison of what it did while it was white: and indeed the first white mass is such a violent corrosive, that it cannot be used in Medicine; whereas, when it is become red, it makes an excellent escharotic, which those who know how to use it properly apply with very great success, particularly to venereal ulcers.
This preparation is very improperly called a Precipitate: for the Mercury is not separated from the Spirit of Nitre by the interposition of any other substance, but only by evaporating the Acid. It is also called Arcanum Corallinum.
It must be observed that Mercury, by its union with the Nitrous Acid, acquires a certain degree of fixity: for the red precipitate is capable of sustaining, without being volatilized, a stronger degree of heat than pure Mercury can; which, as we observed before, is the property of Turbith Mineral also.
To combine Mercury with the Acid of Sea-salt. Corrosive Sublimate.
Evaporate a solution of Mercury in the Nitrous Acid till there remain only a white powder, as mentioned in our observations on the preceding process. With this powder mix as much Green Vitriol calcined to whiteness, and as much decrepitated Sea-salt, as there was Mercury in the solution. Triturate the whole carefully in a glass mortar. Put this mixture into a matrass, so that two thirds thereof may remain empty, having first cut off the neck to[Pg 309] half its length: or instead thereof you may use an apothecary's phial. Set your vessel in a sand-bath, and put sand round it as high as the contents can reach. Apply a moderate fire at first, and raise it by slow degrees. Vapours will begin to ascend. Continue the fire in the same degree till they cease. Then stop the mouth of the vessel with paper, and increase the fire till the bottom of the sand-bath be red-hot. With this degree of heat a Sublimate will rise, and adhere to the inside and upper part of the vessel, in the form of white, semi-transparent crystals. Keep up the fire to the same degree till nothing more will sublime. Then let the vessel cool; break it, and take out what is sublimed, which is Corrosive Sublimate.
OBSERVATIONS.
In this operation the mineral Acids act, and are acted upon, in a remarkable manner. Every one of the three is at first neutralized, or united with a different basis; the Vitriolic being combined with Iron; the Nitrous with Mercury, forming therewith a Nitrous Mercurial Salt; and the Marine with its natural Alkaline basis. The Vitriolic and Nitrous Acids, which are united with metalline substances, being both stronger than the Acid of Sea-salt, strive to expel it from its basis, in order to combine with it themselves; but the Vitriolic Acid, being the strongest of the two, would take sole possession of this basis exclusive of the Nitrous, which would continue united with the Mercury, if the Marine Acid had not a greater affinity than the Nitrous with this metallic substance. This Acid therefore being expelled from its basis by the Vitriolic Acid, and so set at liberty, must unite with the Mercury, and separate the Nitrous Acid from it; which now hath no resource but to unite with the Iron deserted by the Vitriolic Acid. But as all these changes are brought about by the means of a considerable heat, and as the Nitrous Acid hath not a very firm connection with the Iron, it is driven off by the force of the fire; and this it is which we see rise in vapours during the operation. It also carries off with it some parts of the other two Acids, but in a very small quantity. After the operation therefore there remains, 1. A combination of the Vitriolic Acid with the basis of Sea-salt; that is, a Glauber's Salt: 2. A red martial earth, being that which was the basis of the Vitriol: these two substances are blended together, and remain at the bottom of the vessel because of their fixity: 3. A combina[Pg 310]tion of the Marine Acid with Mercury; both of which being volatile, they ascend together into the upper part of the vessel, and there form a Corrosive Sublimate.
If we reflect on this process with attention, and recollect distinctly the affinities of the different substances employed in it, we shall perceive that it is not necessary to make use of all those matters, and that the operation would succeed though several of them were left out.
First, the Nitrous Acid may be omitted; since, as hath been shewn, it is not an ingredient in the Sublimate, but is dissipated in vapours during the operation. From an accurate mixture therefore of Vitriol, Sea-salt, and Mercury, a Corrosive Sublimate must be obtained: for as the Acid of the Vitriol will disengage the Acid of Sea-salt, the latter will be at liberty to combine with the Mercury, and so form the compound we are in quest of.
Secondly, if we make use of Mercury dissolved by the Nitrous Acid, we may omit the Vitriol; because the Nitrous Acid having a greater affinity than the Marine Acid itself with the basis of Sea-salt, and the Acid of Sea-salt having a greater affinity than the Nitrous Acid with Mercury, these two Acids will naturally make an exchange of the bases with which they are united: the Nitrous will lay hold on the basis of Sea-salt, and form a quadrangular Nitre, while the Marine Acid will join the Mercury, and with it form a Corrosive Sublimate.
Thirdly, instead of Sea-salt its Acid only may be employed; which being mixed with the solution of Mercury in the Spirit of Nitre, will, by virtue of its greater affinity with that metallic substance, separate it from the Nitrous Acid, unite with it, and form a white mercurial precipitate, which need only be sublimed to become the combination required.
Fourthly, instead of Mercury dissolved in the Nitrous Acid, Mercury dissolved by the Vitriolic Acid, or Turbith, may be used; only mixing Sea-salt therewith: for these two saline substances will mutually decompound each other, by virtue of the affinities of their Acids, and for the same reasons that Sea-salt and the Mercurial Nitrous Salt decompound each other. The Vitriolic Acid quits the Mercury with which it is combined, to unite with the basis of the Sea-salt; and the Acid of this Salt being expelled by the Vitriolic, combines with the Mercury, and consequently forms our Corrosive Sublimate. In this case a Glauber's Salt remains after the sublimation.
These several methods of preparing Corrosive Sublimate are never used, because each of them is attended with some inconvenience; such as requiring too long triture, yielding a Sublimate less corrosive than it should be, or a smaller quantity of it. We must, however, except the last; which was invented by the late Mr. Boulduc, of the Academy of Sciences, who found none of these inconveniencies attending it[9].
Corrosive Sublimate may also be made only by mixing Mercury with Sea-salt, without any additament. This may appear surprizing when we consider that, as Acids have a greater affinity with Alkalis than with metallic substances, the Acid of Sea-salt ought not to quit its basis, which is Alkaline, to unite with Mercury.
In order to explain this phenomenon it must be remembered that Sea-salt, when exposed to the fire without additament, suffers a little of its Acid to escape. Now this portion of the Marine Acid unites with the Mercury, and forms a Corrosive Sublimate. Moreover, as there is a pretty strong affinity between the Marine Acid and Mercury, this may help to detach from the Sea-salt a greater quantity of Acid than it would otherwise part with. Nevertheless, the quantity of Sublimate obtained by this means is not considerable, nor is it very corrosive.
On this occasion we must also mention another combination of the Marine Acid with Mercury; which is made by mixing that metallic substance perfectly with Sal Ammoniac, by the means of triture. Mercury, like all other metals except Gold, possesses the property of decompounding Sal Ammoniac, separating the volatile Alkali which serves it for a basis, and combining, by the help of a very gentle heat, with its Acid, which is well known to be the same with that of Sea-salt. This decomposition of Sal Ammoniac, by the metalline substances, is a full exception to the first column of Mr. Geoffroy's Table of Affinities, and is the basis of several new Medicines invented by the late Comte de la Garaye[10].
Corrosive Sublimate is the most violent and the most active of all corrosive poisons. It is never used in Medicine, but in external applications. It is a powerful escharotic; it de[Pg 312]stroys proud flesh, and cleans old ulcers: but it must be used by those only who know how to apply it properly, and requires an able hand to manage it. It is not commonly applied by itself, but mixed in the proportion of half a dram to a pound of lime-water. This mixture is yellowish, and bears the name of Aqua Phagadenica.
Water dissolves Corrosive Sublimate, but in a small quantity. If a Fixed Alkali be mixed with this solution, the Mercury precipitates in the form of a red powder. If the precipitate be procured by a Volatile Alkali, it is white; if by Lime-water, it is yellow. This Mercurial Salt dissolves pretty easily in boiling Spirit of Wine.
Sweet Sublimate.
Take four parts of Corrosive Sublimate; pulverize it in a glass or marble mortar; add by little and little three parts of Mercury revivified from Cinabar; triturate the whole carefully, till the Mercury be perfectly killed, so that no globule thereof can be perceived. The matter will then be grey. Put this powder into an apothecary's phial, or into a matrass, whose neck is not above four or five inches long, leaving two thirds thereof empty. Set the vessel in a sand-bath, and put sand round it to one third of its heighth. Apply a moderate fire at first; and afterwards raise it gradually till you perceive that the mixture sublimes. Keep it up to this degree till nothing more will rise, and then break the vessel. Reject, as useless, a small quantity of earth which you will find at the bottom; separate also what adheres to the neck of the vessel, and carefully collect the matter in the middle, which will be white. Pulverize it; sublime it a second time, in the same manner as before; and in the same manner separate the earthy matter left at the bottom of the vessel, and what you find sublimed into the neck. Pulverize, and sublime a third time, the white matter you last found in the middle. The white matter of this third sublimation is the Sweet Sublimate, called also Aquila Alba.
OBSERVATIONS.
The Acid of Sea-salt in the Corrosive Sublimate is very far from being perfectly saturated with Mercury; and thence[Pg 313] comes the corrosive quality of this saline compound. But though Mercury, as appears by this combination, is capable of imbibing a much greater quantity of Acid than is necessary to dissolve it; nay, though it naturally takes up this superabundant quantity of Acid, yet it doth not follow from thence that this redundant Acid may not combine with Mercury to the point of perfect saturation, so as to lose its corrosive acidity.
This is the case in the operation here described. A fresh quantity of running Mercury is mixed with Corrosive Sublimate; and the fresh Mercury, combining with the super-abounding Acid, deprives the Sublimate of its acrimony, and forms a compound which comes much nearer the nature of a Neutral Metallic Salt.
Trituration alone is not sufficient to produce an union between the newly added Mercury and the Acid of the Corrosive Sublimate: because, generally speaking, the Acid of Sea-salt cannot dissolve Mercury without the help of a certain degree of heat, and unless it be reduced into vapours.
Thus, though the newly added Mercury becomes invisible by trituration, and seems actually combined with the Corrosive Sublimate, yet the union is not intimate. There is only an interposition of parts, but no true dissolution of the newly added Mercury by the super-abundant Acid of the Corrosive Sublimate. For this reason the mixture must be sublimed; and by this sublimation only is the true union effected. Nor is one single sublimation sufficient: no less than three are necessary to deprive the Sublimate of the corrosive quality which renders it poisonous. After the third sublimation, the Sublimate being put upon the tongue gives no considerable sensation of acrimony; nor doth it retain any more of its former activity than is requisite to make it a gentle purgative, when administered from six to thirty grains for a dose.
If a less quantity of Mercury than that above directed be mixed with the Corrosive Sublimate, the super-abundant Acid will not be sufficiently saturated; and the less Mercury is added, the more of its corrosive virtue will the Sublimate retain.
If, on the contrary, a greater quantity of Mercury be added, there will be more than the Acid can possibly dissolve, and the superfluous quantity will remain in its natural form of Quick-silver. It is better therefore to err in the excess than in the defect of the proportion of Mercury to be added; because the Corrosive Sublimate will take up no more than is necessary to dulcify it.
Part of the Acid of the Corrosive Sublimate is also dissipated in vapours during the operation; and it is necessary to allow room for these vapours to circulate, and a vent to give them passage, or else they will burst the vessels. These are our reasons for leaving an empty space in the subliming vessels, and for having their necks no more than five or six inches long.
The matter which sublimes into the neck of the vessel is always very acrid; for which reason it must be separated from the Sweet Sublimate. There remains also at the bottom of the matrass an earthy, reddish matter; which probably comes from the Vitriol employed in making the Corrosive Sublimate. This matter must likewise be rejected as useless after every sublimation.
The Panacea of Mercury.
Pulverize some Sweet Sublimate, and sublime it in the same manner as you did thrice before. Repeat this nine times. After these sublimations it will make no impression on the tongue. Then pour on it aromatic Spirit of Wine, and set the whole in digestion for eight days. After that decant the Spirit of Wine, and dry what remains, which is the Panacea of Mercury.
OBSERVATIONS.
The great number of sublimations, which the Sweet Sublimate is made to undergo, sweeten it still more, and to such a degree that it leaves no sensation on the tongue, nor hath any purgative virtue.
The Spirit of Wine in which it is digested after all the sublimations, is designed to blunt still more the sharpness of any acid particles that may not have been sufficiently dulcified by the preceding sublimations.
As Mercury is the specific remedy for venereal disorders, sundry preparations thereof have been attempted with a view to produce different effects. Sweet Sublimate is purgative; and for that reason is not quite proper for procuring a salivation, because it carries off the humours by stool. The Panacea of Mercury, which, on the contrary, is not purgative, may raise a salivation when taken inwardly.
Of Operations on the Semi-Metals.
Of Antimony.
To Separate Antimony from its Ore by Fusion.
Having drilled some small holes, of about two lines in diameter, in the bottom of a crucible, put into it your Antimonial Ore broken into little bits, about the size of a hazel nut; lute on its cover; set the crucible thus prepared in the mouth of another crucible, and close the joints with lute.
At the distance of half a foot from this compound vessel place bricks all round, so as to form a furnace; the sides of which must rise as high as the brim of the uppermost crucible.
Let the bottom of this furnace be filled with ashes, up to the top of the lower crucible, and the rest of the furnace with lighted coals. Blow the fire, if it be necessary, with bellows, till the upper crucible become red. Keep it up in this degree for about a quarter of an hour. Then take your vessels out of the furnace, and you will find the Antimony collected in the bottom of the lower crucible, having run through the holes of the upper one.
OBSERVATIONS.
The ore of Antimony is one of the most fusible: it always contains a great deal of Sulphur, and cannot sustain a fire of any force without being dissipated into vapours. It requires no additament to flux it: for it is not necessary, on this occasion,[Pg 316] that the earthy and stony matters mixed therewith be brought to fusion. It is sufficient that the Antimonial part be melted; which, as soon as it becomes fluid, is carried by its weight to the lower part of the crucible. Thus it is separated from all heterogeneous matters; which are left in the upper crucible, while it passes through the holes in its bottom, and forms a mass in the lower.
The precaution of closing all the apertures of both crucibles is necessary, on account of the volatility of this mineral: and that the Antimony, when once melted, may not continue exposed to a great heat, it is made to run down into a vessel surrounded with ashes only, and by that means very little affected with heat; ashes being one of those solid mediums that transmit least of it.
The common Regulus of Antimony.
Reduce crude Antimony to powder. Mix it with three fourths of its weight of white Tartar, and half its weight of refined Salt-petre, both pulverized. Into a large crucible made red-hot in the fire, throw a spoonful of your mixture, and cover it. There will be a very considerable detonation. When it is over, throw in a second spoonful of your mixture, and cover the crucible as before: this will produce a second detonation. Go on thus, till you have thrown in all your mixture.
When the whole has thus fulminated, increase the fire so as to bring the matter into fusion; that being done, take the crucible out of the furnace, and immediately pour its contents into an iron cone heated and greased with tallow. Strike the floor and the cone some gentle blows with a hammer, to make the Regulus precipitate: and when the matter is fixed and cold, invert the cone and turn it out. You will see it consist of two distinct substances; the uppermost of which is a saline scoria, and the undermost the reguline part. Strike this mass a blow with a hammer, in the place where these substances join, and you will by this means separate the scoria from the Regulus; the latter of which will have the form of a metallic cone, on whose base you will observe the signature of a bright star.
OBSERVATIONS.
Antimony, though separated by a former fusion from the earthy and stony parts of its ore, must nevertheless be still considered as an ore, on account of the great quantity of Sulphur it contains, which mineralizes the metalline part or Regulus. Therefore, if you desire to have this Regulus pure, you must separate it from the Sulphur that is combined with it. This may be done several ways. The method above proposed is one of the readiest and easiest, though not altogether free from inconveniencies, as we shall shew.
The Salt-petre in the mixture detonates by means of the Sulphur of the Antimony, which it consumes, and from which it separates the reguline part: but lest it should also consume some of the phlogiston which gives the Regulus its metalline form, Tartar is added; because it contains a great deal of inflammable matter, and so is capable of furnishing enough for the detonation of the Nitre, or rather for restoring to the metallic earth of the Antimony, the phlogiston that may be consumed by the Nitre.
If we consider what passes in this operation we shall soon be convinced that a great deal must be lost in it, and that we do not thereby obtain near the whole of the Regulus that the Antimony is capable of yielding: for, 1. the Regulus of Antimony being a volatile substance, much of it must be dissipated during the detonation; and so much the more as the detonation is frequently repeated, and continued for a considerable time. The flowers that may be collected by presenting cold bodies to the smoke that rises in the operation, and which may be reduced to a Regulus by the addition of a phlogiston, sufficiently prove what is here advanced.
2. All the Sulphur of the Antimony is not consumed by the Nitre on this occasion; and moreover, the Acid of that part thereof which is burnt, uniting with some of the Alkali produced by the deflagration of the Nitre and Tartar, forms a Vitriolated Tartar, which meeting with a sufficient quantity of phlogiston in the mixture produces new Sulphur. Now this Sulphur, whether not consumed, or reproduced, in the operation, combining with the Alkali forms a Liver of Sulphur; and that dissolves part of the Regulus, which by this means remains confounded with the scoria. The proof of this is, that, if the scoria be mixed with filings of iron, and fused a second time, you will find at the bottom of the cru[Pg 318]cible a button of Regulus, which it contained, and which is separated therefrom by the interposition of the Iron. We shall say more on this subject in the process for making the Martial Regulus, which immediately follows this. If, instead of melting the scoria with iron filings, we pulverize it, boil it in water, and then pour an acid into that water; the liquor will instantly grow turbid, and a Sulphureous Precipitate will fall, commonly called the Golden Sulphur of Antimony; which is nothing else but common Sulphur still combined with some particles of the Regulus; a new proof of what we advanced concerning the production of Liver of Sulphur in this operation.
As Regulus of Antimony is of no great value, the loss sustained in this process is seldom regarded. However, we shall have occasion, in the sequel, to point out a method of obtaining this Regulus with less disadvantage.
Regulus of Antimony precipitated by Metals.
Put one part of small iron nails into a crucible, and set it amidst burning coals, in a melting furnace. When the iron is thoroughly red-hot, and begins to grow white, add thereto little by little, and at several times, two parts of crude Antimony in powder. The Antimony will immediately flow and unite with the Iron. When the Antimony is entirely melted, add thereto, at several times, the fourth of its weight of pulverized Nitre: a detonation will ensue, and the whole mixture will be in fusion.
After you have kept the matter in this condition for some minutes, pour it into an iron cone, first heated and tallowed. Strike the sides of the cone with a hammer, that the Regulus may fall to the bottom; and, when all is cold, separate it from the scoria by a blow with a hammer. Melt this first Regulus again in another crucible, adding a fourth part of its weight of crude Antimony. Keep the crucible close shut, and give no more heat than is necessary to melt the matter. When it is in perfect fusion, add to it at several times, as you did before, the sixth part of its weight of pulverized Nitre; and, in half a quarter of an hour after this, pour the whole into a cone as you did the first time.
Lastly, melt your Regulus over again a third, or even a fourth time, always adding a little Nitre, which will deto[Pg 319]nate as before. If after all these fusions you pour the Regulus into an iron cone, you will find it very beautiful, and the star well formed: it will be covered with a semi-transparent, lemon-coloured scoria. This scoria is extremely acrid and caustic.
OBSERVATIONS.
Though Regulus of Antimony unites very readily with Sulphur, and is always found combined therewith in the earth, we must not thence conclude that it hath a greater affinity than other substances with that mineral: on the contrary, all the metals, except Gold, have a greater affinity than this Semi-metal with Sulphur. Hence it follows that all the metals, except Gold, are capable of decomposing Antimony, and separating the sulphureous part from the metalline; so that, instead of employing Iron, as in our process, Copper, Lead, Tin, or Silver, may be used, and a Regulus obtained by means thereof.
But as Iron is, of all the metallic substances, that which hath the greatest affinity with Sulphur, it is on this occasion preferred to the rest. And from hence two advantages arise: the first is, that the operation is performed sooner and with greater ease: the second, that the Regulus is purer, and contains less of the precipitating metal. For it is a general rule, that, when one metallic substance is employed to precipitate another, the precipitated substance is always a little adulterated by the admixture of some particles of the precipitant. Now, the greater affinity the precipitant hath with the matter united to that which is to be precipitated, the less doth the precipitate retain of the precipitant.
In this process the Iron melts very easily by means of the union it contracts with the Sulphur; which, as we observed before, hath the property of rendering this metal very fusible, though of itself the most refractory of all.
The scoria found on the Regulus of the first fusion is a combination of Iron with the sulphureous part of the Antimony. This scoria is extremely hard, and not to be separated from the Regulus without some trouble. The Nitre added, being alkalizated and united therewith, renders it a little softer, and gives it the property of relenting in the air. Any Alkaline Salt may be substituted for the Nitre.
The Nitre that is alkalizated in the operation, or the Alkali that is added, procures moreover another advantage; namely, that, by uniting with part of the Sulphur of the Antimony, it produces a Liver of Sulphur, which dissolves[Pg 320] the Iron, retains it, and hinders that which is not yet combined with pure Sulphur from uniting so readily with the Regulus as it otherwise would do.
Lastly, the addition of Nitre, or an Alkali, contributes greatly to promote the fusion, to render it more perfect, and to procure a more complete precipitation of the Regulus.
The second fusion which the Regulus is made to undergo is intended to purify it from any mixture of Iron. When the fresh Antimony added on that occasion comes to melt with the Regulus, the Sulphur contained in the Antimony joins with the ferruginous parts in the Regulus; and the Iron becoming lighter by this union is thrown up to the surface of the matter. There it forms a sort of scoria, with which a good deal of Antimony is mixed; the Regulus not being wholly precipitated, because there is not Iron enough in the mixture for that purpose. The Salt-petre added here produces the same effect as in the first fusion.
But if, on one hand, the Regulus precipitated in the first fusion be purified, by this addition of fresh Antimony, from most of the Iron with which it was alloyed; on the other hand, this same Regulus cannot be kept from re-uniting with some sulphureous parts.
In order therefore to separate it entirely from these, it must be melted over again once or twice more, and a little Nitre added each time, to consume them by deflagration. But this cannot be done without consuming also some of the very phlogiston which gives the Regulus its metalline form: whence it comes to pass that part of the Regulus is converted to a calx, which, by means of the alkalizated Nitre, is turned into glass; and it is this glass which mixing with the scoria gives it the yellow colour observed therein. This yellow colour may also be in part produced by some ferruginous particles, of which a small quantity always remains combined with the Regulus, notwithstanding its former depuration by Antimony.
It is of no use to repeat the fusions of the Regulus oftener than is above proposed, or to add fresh Nitre with a view to consume the Sulphur it may still contain: for after the second fusion it contains none at all, and retains only the phlogiston necessary to give it the metalline form. So that, by prosecuting the matter further, you would only calcine and destroy the Regulus to no manner of purpose.
From what hath been said it is plain that, even by this process, we do not obtain all the Regulus which our Antimony is capable of yielding; seeing part of it is destroyed[Pg 321] by the fusions it must necessarily undergo with Nitre, in order to its purification. We shall give a process for obtaining from Antimony the greatest quantity of Regulus it can possibly be made to yield, after we have treated of its Calcination, which is in some sort the first step of that process.
The Calcination of Antimony.
Take an unglazed earthen vessel, wider at top than at bottom; put into it two or three ounces of crude Antimony finely pulverized. Set this vessel over a weak charcoal fire, and increase the heat till you see the Antimony begin to smoke a little. Continue the fire in this degree, and keep incessantly stirring the Antimony with the shank of a tobacco-pipe all the while it is upon the fire.
The powder of Antimony, which, before calcination, was of a brilliant colour inclining to black, will become dull, and look like an earth. When it comes to have this appearance raise your fire till the vessel be red-hot, and keep it up in this degree till the matter cease entirely to smoke.
OBSERVATIONS.
Antimony, as hath been already said, is a sort of ore consisting of a metalline or reguline part mineralized by Sulphur.
The design of this calcination is, by the action of fire, to dissipate the sulphureous part, which is the most volatile, in order to separate it from the metalline part. It is evidently a real torrefaction; but the operation is very difficult, and requires a good deal of attention; for Antimony very easily melts, while at the same time it is necessary to our success that it do not melt; because when the matter is in fusion the Sulphur requires a much greater degree of heat to carry it off. Now, as Regulus of Antimony itself is very volatile, a good deal of it would be dissipated along with the Sulphur, if it were exposed to the degree of heat necessary to carry off the Sulphur when the mass is melted.
Therefore if it happen that the Antimony begin to melt during the calcination, which is easily perceived by its running into clots, it must be taken off the fire, and the clotted[Pg 322] parts be again pulverized; after which the calcination is to be prosecuted with a less degree of heat.
When the Antimony has lost all its brightness, and is become like an earth, it is time to augment the degree of heat, in order to complete the calcination; because the last portions of the Sulphur are the most difficult to raise. Moreover, the inconveniences just mentioned are not now to be apprehended: for, as the great fusibility of the reguline part is owing to the Sulphur, what remains, after you have dissipated the greatest part of the Sulphur, is much less fusible; and, as the redundant Sulphur of the Antimony cannot be driven off, without dissipating at the same time a good deal of the phlogiston necessary to metallize its Regulus, the matter that remains comes much nearer to the nature of a calx, than to that of a metalline substance; and consequently partakes of the nature of all metallic calces, which is to be very fixed.
Antimony may also be calcined by mixing with that mineral an equal quantity of charcoal-dust. As charcoal is incapable of fusion, it prevents the Antimony from clotting, as well as from losing so much of its metallizing phlogiston as it otherwise would: and hence it comes to pass that the calx of Antimony, prepared in this manner, comes nearer to the nature of a Regulus, than that which is prepared without addition.
If you happen to raise the fire too much, in this calcination with charcoal-dust, the calx will be partly reduced to a Regulus, by means of the phlogiston which the charcoal furnishes; and then the Regulus will be dissipated in vapours, especially as this calx, which comes very near the nature of a Regulus, is not so fixed as that prepared without addition. For this reason it always continues to smoke, even when it contains no superfluous Sulphur: and therefore you must not wait till it cease to smoke before you put an end to your calcination; for you will lose a great deal of it in vapours. It is time to stop when the vapours that rise from it, while it is moderately red, do not smell of burning Sulphur.
Calx of Antimony reduced to a Regulus.
Mix the calx of Antimony, which you intend to reduce, with an equal quantity of black soap. This mixture will make a thin paste. Put it little by little into a crucible, previously made red-hot amidst live coals. Thus let the soap burn till it cease to emit any oily smoke. Then cover the crucible; make the fire strong enough to melt the matter, and you will hear it effervesce and boil. When this noise is over let the crucible cool, and then break it: you will find in it a beautiful scoria, marked with circles of several colours; and under that a button of Regulus, which is not yet quite pure, and must be purified in the following manner.
Pound this Regulus, and mix it with half its weight of an antimonial calx as perfectly desulphurated as possible. Put it into a crucible, and cover it: melt the whole, so that the surface of the melted matter may be smooth and uniform. Let the crucible cool, and then break it: you will find in it a beautiful button of very pure Regulus, covered with a scoria having the appearance of an opaque glass, or a kind of greyish enamel, moulded on the finely radiated surface of the Regulus.
OBSERVATIONS.
Of all the metalline calces that of Antimony is most easily reduced. Any matter that contains the phlogiston, even charcoal-dust alone, is sufficient to procure it the form of a Regulus, without the addition of any thing to facilitate its fusion; because this calx, which is not of itself altogether refractory, becomes still more fusible as it combines with the phlogiston, and approaches to the reguline state.
Though all inflammable matters are capable of procuring the reduction of the calx of Antimony, yet there are some with which the operation succeeds better, and produces a greater quantity of Regulus, than it does with others. Fatty matters, joined with Alkalis, are those which answer best in this reduction, as they do in most others. The black flux, for instance, is very proper for this purpose: but Mr. Geoffroy, who made many experiments on Antimony, found[Pg 324] by repeated trials that black soap is still fitter for it, and that a greater quantity of Regulus was obtained by its means, than by any other reducing flux whatever. The process here given is taken from one of the Memoirs on this subject, which he laid before the Academy of Sciences.
Black soap is made of the lye of a Fixed Alkali, such as potash for instance, with quick-lime, incorporated by boiling with oil of lint-seed, rape-seed, or hemp-seed, and sometimes also with animal fat. The oily matters, contained in this reducing flux, are first burnt and charred to a coal in the crucible. As soon as they are brought to this state, the crucible is covered, and the fire is increased, till the matters melt. At that instant the reduction begins to take place; and the bubbling noise observed is an effect thereof.
The Regulus obtained by this first fusion is not yet very pure, being adulterated with the mixture of some unmetallic earth that was contained in the Antimony, and with a portion of the calcarious earth of the soap.
Mr. Geoffroy found that his Regulus was contaminated with this substance, by putting it into water: for on that occasion he observed a very brisk ebullition about the reguline buttons, which sometimes lasted above four and twenty hours; and on examining them with a glass, he discovered some little holes, imperceptible to the naked eye, through which the water entered, to unite with the lime retained in the internal parts of the Regulus, which having been recalcined in the operation required to be slaked.
This Regulus may be purified by simple fusion, without any additament, because the particles of lime, being lighter than those of the Regulus, will be thrown up to the surface, on which they will form a sort of scoria. But Mr. Geoffroy took notice that, in this case, the surface of the Regulus is never very neat; that it is always sullied with a very adhesive scoria, and that no star is formed upon it. Besides, the Regulus must be kept a long while in very thin fusion, that the heterogeneous matters, which hinder the perfect re-union of its parts, may have time to rise to the surface by their lightness. But the longer the Regulus is kept in fusion, the more of it evaporates, because of its volatility. He was therefore obliged to have recourse to other means.
We have in the process described the method which succeeded best with Mr. Geoffroy. It consists in melting the Regulus over again, with the addition of a little fresh calx of Antimony thoroughly freed from its Sulphur. This calx[Pg 325] being in its nature easily vitrifiable, and combining with the earthy parts that deprave the Regulus, and which cannot be vitrified without addition, scorifies these matters, and with them forms the opaque glass, or kind of enamel which is found over the Regulus purified in this manner.
The star on that part of the Regulus of Antimony, which was contiguous to the scoria, is a mark of its purity, and a proof that the operation was well performed. This star is nothing but a particular disposition of the parts of the Antimony, which have the property of running naturally into facets and needles. The perfect fusion, both of the Regulus and the scoria that covers it, leaves the parts of the Regulus at liberty to range themselves in this order. This disposition appears not only on the upper surface of the Regulus, but, if you break the button, you find the same in its internal parts. There are some round pyrites whose insides have nearly the same appearance, and seem to consist of rays issuing from a common center.
The quantity of Regulus obtained by Mr. Geoffroy's process is more than double of what is procured in the common way, which yields but about four ounces in the pound; whereas this gives from eight to ten ounces.
When Antimony is calcined with charcoal-dust, what remains after the dissipation of all the Sulphur is not, properly speaking, a calx of Antimony; but a sort of Regulus quite formed, and differing from the common Regulus only in that its parts are disunited, and not collected into a mass. For if this pretended calx of Antimony be melted, it directly coalesces into a Regulus, without the addition of any inflammable matter fit to procure its reduction. Indeed less Regulus is obtained by this means than when a reductive is added: but nevertheless this experiment still proves what I advanced; seeing Regulus of Antimony cannot be melted without losing more or less thereof, either because some of it is dissipated in vapours, or because part of it loses its phlogiston in the fusion, and so is converted into a calx.
Antimony calcined with Nitre. Liver of Antimony. Crocus Metallorum.
Pulverize and mix perfectly together equal parts of Nitre and Antimony: put the mixture into an iron mortar, and cover it with a tile, which however must not[Pg 326] shut it quite close. With a live coal set fire to the matter in the mortar, and immediately withdraw it. The mixture will flame, with great detonation; which being over, and the mortar cooled, invert it, and strike its bottom to make all the matter fall out. Then, by a blow with a hammer, separate the scoria from the shining part, which is the Liver of Antimony.
OBSERVATIONS.
In this operation the Nitre takes fire and detonates with the Sulphur of the Antimony; and nothing remains but the metallic earth of the mineral, which, meeting with no substance to restore its phlogiston, cannot take the form of a Regulus; but, being combined with a large quantity of fused saline matters, begins itself to flow, and forms a sort of vitrification; which, however, is not a complete one, because the matters do not continue long enough in fusion, but cool too soon. This preparation of Antimony is a violent Emetic. It is used to make Emetic Wine and Tartar Emetic: it is also given in substance to horses.
The saline matters found after the operation in the form of a scoria, or perhaps confounded with the Liver of Antimony, are combinations of Fixed Nitre, partly with the Acid of the burnt Sulphur, forming a Neutral Salt of the same kind as Vitriolated Tartar, and partly with some unburnt Sulphur, forming a sort of Liver of Sulphur that contains a little Regulus. It is usual to pulverize this Liver of Antimony and wash it with water, in order to dissolve and carry off all the Salts. When thus pulverized and washed it is called Crocus Metallorum. If Liver of Antimony be melted with any inflammable matter, it will be reduced to a Regulus; because it is nothing but a metalline calx half vitrified.
Another Calcination of Antimony with Nitre. Diaphoretic Antimony. Materia Perlata. Clyssus of Antimony.
Mix one part of Antimony with three parts of Nitre; project this mixture by spoonfuls into a crucible kept red-hot in a furnace. Each projection will be attended with a detonation. Continue doing this till you have used all[Pg 327] your mixture: then raise the fire, and keep it up for two hours; after which throw your matter into a pan full of hot water. Let it lie steeping in water kept hot for a whole day. Then pour off the liquor: wash the white powder you find at bottom in warm water; and repeat the ablutions till the powder become insipid. Dry it, and you have Diaphoretic Antimony.
OBSERVATIONS.
This operation differs from the preceding one, in respect of the quantity of Nitre deflagrated with the Antimony. In the former we added one part only of Nitre to one part of Antimony; but in this three parts of Nitre are put to one of the mineral; and the calx resulting from this mixture is of course very different from the other.
In the first place, Liver of Antimony hath a reddish colour; whereas Diaphoretic Antimony is very white. Secondly, Liver of Antimony is in a manner half vitrified; Diaphoretic Antimony is, on the contrary, in the form of a powder, the parts of which have no connection together.
The reason of these differences will easily appear, if we consider, that, Liver of Antimony being the result of calcination with one part of the Nitre only, which is not sufficient to consume all the Sulphur of the mineral, what remains after the detonation is not entirely deprived of its phlogiston; from whence arise the colour it retains and the ease with which it flows in the fire: but that, when three parts of Nitre are added instead of one, this quantity is not only sufficient to consume all the Sulphur and the phlogiston of the Antimony, but even more than enough; seeing that, after the operation, some Nitre is still found undecomposed.
The calx of Antimony, prepared by calcining it with three parts of Nitre, is therefore deprived of all its phlogiston. This is the cause of its whiteness, and the reason why it is not half vitrified by the operation, as Liver of Antimony is: for we know that the more a metallic calx is deprived of its phlogiston, the less fusible and the less vitrifiable it is. This calx of Antimony bears the name of Diaphoretic Antimony, or Diaphoretic Mineral: because, being neither emetic nor purgative, it is thought to have the virtue of promoting perspiration.
Antimony may be calcined with various proportions of Nitre, between that used to make Liver of Antimony, and this with which Diaphoretic Antimony is prepared; and[Pg 328] from these calcinations will result calces possessed of properties both chymical and medical, of an intermediate nature between the extremes of those two preparations. The nearer the proportion of Nitre comes to that employed in preparing Liver of Antimony, the more will the resulting calx resemble that preparation; and in the same manner, a calx prepared with a greater proportion of Nitre will so much the more resemble Diaphoretic Antimony, as the proportion of Nitre used comes nearer three parts of Nitre for one of Antimony.
It is not necessary that Antimony in substance be employed to make the Diaphoretic Mineral: you may, if you please, make use of its Regulus. But as the Regulus contains no Sulphur, nor any more phlogiston than is requisite to secure its metalline form, it is needless to put three parts of Nitre to one of Regulus; an equal quantity thereof being sufficient.
The matter is projected by spoonfuls, to the end that, by gradual and repeated detonations, the Antimony may be more perfectly calcined: it is also with a view to destroy entirely the small remainder of phlogiston, which may have escaped the action of the Nitre, that the matter is kept red-hot in the crucible for two hours.
The whole is afterwards thrown into hot water, and left steeping therein for several hours, with design to give the water time to dissolve all the saline matters that are mixed with the Diaphoretic Calx. When crude Antimony is used in making this preparation, these saline matters are, 1. an Alkalizated Nitre; 2. a Neutral Salt formed by the union of the Acid of Sulphur with part of that Alkali, as in the preparation of Liver of Antimony; 3. a portion of undecomposed Nitre.
The water in which the Diaphoretic is washed takes up moreover a portion of the calx of Antimony, which is exceeding finely attenuated, and continues united with the fixed Nitre, and suspended therewith in the liquor. This matter is to be separated from the Fixed Nitre, by mixing the water wherein it is dissolved with an Acid, which unites with the Alkali, and precipitates this matter in the form of a white powder, to which the name of Materia Perlata hath been given. Because it is precipitated in the same manner as the Golden Sulphur of Antimony, and, like that, is found in the water with which the saline matters are washed out, after the detonation of Nitre with Antimony, some[Pg 329] Chymists have given it, though very improperly, the name of the Fixed Sulphur of Antimony.
This matter is a true Calx of Antimony, and differs from Diaphoretic Antimony in nothing but its being still more perfectly calcined. It is so indeed to such a degree that it is impossible to restore its metalline form, or reduce it to a Regulus, by the addition of an inflammable matter. Diaphoretic Antimony, on the contrary, may be re-metallized, by supplying it with phlogiston: but it must be observed that, in whatever manner you go about this, you will obtain a much smaller quantity of Regulus, than when you use a Calx of Antimony prepared with a smaller quantity of Nitre.
If you attempt to reduce either Liver of Antimony or Diaphoretic Antimony, great care must be taken to wash them thoroughly, in order to free them from every thing saline: for, without this precaution, the Acid of the Sulphur, having, as was observed, formed a Neutral Salt with the Alkali of the Nitre, will combine with the inflammable matter added to revivify the calx of Antimony and reproduce a Sulphur; which, uniting afterwards with the same Alkali, will produce a Liver of Sulphur, that will dissolve part of the Regulus, hinder its precipitation, and greatly lessen the quantity which might otherwise be expected.
A particular sort of Diaphoretic Antimony is sometimes prepared for Medical uses, which hath a purgative quality: it is not washed at all, and is therefore called Unwashed Diaphoretic Mineral.
Diaphoretic Antimony may also be prepared in close vessels, by means of which the vapours that rise during the operation are retained. For this purpose a tubulated retort is employed, having a series of adopters fitted to it. The retort is placed in a furnace, and heated till its bottom become red: then a very small quantity of the mixture, for making Diaphoretic Antimony, is introduced through the tube in the upper part of the retort, and the tube immediately stopped. A detonation ensues, and the vapours expand themselves into the adopters, where they condense. This is repeated till the intended quantity of matter be used. After the operation some white flowers are found sublimed in the neck of the retort, and a small quantity of liquor in the recipients. This liquor is acid. It consists of some of the Acid of the Nitre, which the Acid of the Sulphur hath expelled from its basis, and also a little of the Acid of the Sulphur carried up by the heat before it could combine with the[Pg 330] basis of the Nitre. This liquor is called Clyssus of Antimony. The name of Clyssus is given to all liquors in general that are prepared by this method.
The white flowers found in the neck of the retort are flowers of Antimony; that is, a calx of Antimony forced up by the heat, and by the impetus of the detonation. These flowers may be reduced to a Regulus. What remains in the retort is the same with the matter that remains in the crucible, wherein the mixture of Nitre and Antimony for making Diaphoretic Antimony hath been deflagrated.
Neither Diaphoretic Antimony nor the Pearly matter are soluble in any Acid.
Calx of Antimony Vitrified.
Take any quantity you please of calx of Antimony, made without addition; put it into a good crucible, which set in a melting furnace: kindle the fire gradually, and leave the crucible uncovered at the beginning.
A quarter of an hour after the matter is red-hot, cover the crucible, and excite the fire vigorously till the calx melt. You may know when it is thoroughly melted, by dipping into the crucible an iron wire, to the end of which a little knob of glass will adhere, if the matter be in perfect fusion. Keep it in fusion for a quarter of an hour, or rather longer if your crucible can bear it. Then take it out of the furnace, and immediately pour out the melted matter on a smooth stone, made very hot for the purpose: it will presently fix into a yellow Glass.
OBSERVATIONS.
All the calces of Antimony, when exposed to a violent fire, are converted into Glass; but not all with the same facility. In general, the more of their phlogiston they have lost in the calcination, the more difficult is their vitrification. This causes also a difference in the colour of the Glass; which will be of so much a deeper yellow, and the nearer to a red, the less the Antimony was calcined.
It frequently happens, when we employ a calx of Antimony which is not sufficiently deprived of its phlogiston, that we find in the crucible a button of Regulus, which, be[Pg 331]ing heavier than the Glass, always lies at the bottom. With a view to avoid this inconvenience, and to dissipate completely the excess of phlogiston that may still be left in the calx of Antimony, we direct the crucible to be left uncovered for some time, at the beginning of the operation. If, notwithstanding this precaution, any Regulus be still found at the bottom of the crucible, and you resolve to vitrify it, the crucible must be returned to the furnace, and the fusion continued; by which means the Regulus will at last be converted into Glass.
If, on the contrary, you meet with any difficulty in effecting the vitrification, on account of your having employed a calx that hath lost too much of its phlogiston, such as Diaphoretic Antimony, or the Pearly matter, the fusion may be greatly facilitated by throwing into the crucible a little crude Antimony.
Glass of Antimony is a most violent emetic. This Glass, as well as Liver of Antimony, is employed in preparing Emetic Wine and Emetic Tartar.
It may be resuscitated, like the calces of Antimony, into a Regulus, by re-uniting it with a phlogiston. For this purpose it must be finely pulverized, thoroughly mixed with some black flux, and melted in a covered crucible. This Glass, as well as that of Lead, hath the property of greatly promoting the vitrification of matters that are to be scorified.
Kermes Mineral.
Break any quantity you will of Hungarian Antimony into little bits: put it into a good earthen coffee-pot: pour on it twice its weight of rain-water, and a fourth part of its weight of well filtered liquor of Nitre fixed by charcoal. Boil the whole briskly for two hours, and then filter the liquor. As it cools it will acquire a red colour, grow turbid, and leave a red powder on the filter.
Return your Antimony into the coffee-pot. Pour on it as much rain-water as before, and three fourths of the former quantity of the liquor of Fixed Nitre. Boil it again for two hours, and then filter the liquor. It will again deposite a red sediment. Return your Antimony into the coffee-pot: pour on it the same quantity of rain-water, and half the first[Pg 332] quantity of the liquor of Fixed Nitre. Boil it again for two hours, and then filter the liquor as formerly. Wash all these sediments with warm water, till they become insipid; then dry them, and you have the Kermes Mineral.
OBSERVATIONS.
If you recollect what we said concerning the property which Fixed Alkalis possess of uniting with Sulphur, both by fusion, and, when those Salts are resolved into a liquor, by boiling, and of forming therewith a Liver of Sulphur, which dissolves all metalline substances, you will readily comprehend the nature of this Kermes.
Antimony consists of a sulphureous and a reguline part. Therefore, if this mineral be boiled in a solution of a Fixed Alkali, such as Nitre fixed by charcoal, the Alkali will dissolve the Sulphur of the Antimony, and form therewith a Liver of Sulphur, which, in its turn, will dissolve the reguline part. Now, Kermes Mineral, prepared as above directed, is no other than a Liver of Sulphur combined with a certain quantity of Regulus of Antimony.
Mr. Geoffroy hath set this truth in the clearest light, by his accurate analysis of the Kermes Mineral. The experiments he made on that subject are circumstantially related in several Memoirs printed in the volumes of the Academy for 1734 and 1735. By combining Acids with the Kermes he demonstrated, 1. the existence of Sulphur in this compound; having separated from it a burning Sulphur, which cannot be mistaken for any other than the Sulphur of Antimony. In order to obtain this Sulphur pure, an Acid must be employed that will not only absorb the Alkali, but also perfectly dissolve the reguline part that might otherwise remain united with the Sulphur. Aqua regia was the Acid which succeeded best with Mr. Geoffroy. 2. He also proved that there is a Fixed Alkali in the composition of the Kermes; seeing the Acids with which he precipitated the Sulphur became Neutral Salts, and just such as those very Acids combined with a Fixed Alkali would have constituted: that is, the Vitriolic Acid produced a Sal de duobus; the Nitrous Acid a regenerated Nitre; and the Marine Acid a regenerated Sea-salt. 3. Mr. Geoffroy demonstrated the reguline part of Antimony to be an ingredient in the Kermes; having procured therefrom an actual Regulus of Antimony, by fusing it with the black flux.
In preparing the Kermes it is necessary to renew the liquor from time to time, as above directed; because, when it is once impregnated with Kermes to a certain degree, it can take up no more; and consequently the same liquor cannot operate again on the Antimony. Experience hath shewn, that, if the doses above prescribed be applied, the liquor will after two hours boiling be sufficiently saturated with Kermes.
If the liquor in which the Kermes is dissolved be filtered while it is very hot, and almost boiling, it leaves nothing on the filter; the Kermes passing through with it: but as it cools it grows turbid, and gradually deposites the Kermes. Therefore it ought not to be filtered till it be cold; or, if it be filtered while it is boiling hot, in order to separate from it some coarse particles of Antimony not yet converted into Kermes, it must be filtered a second time when it is cold, in order to get the Kermes.
Though in the method usually practised for making Kermes, the Antimony is boiled only thrice, yet it does not follow that more Kermes may not be obtained from it, or that but little more would be obtained by a fourth and fifth boiling; on the contrary, it would yield considerably more. Mr. Geoffroy observed, that he got more Kermes by the second boiling than by the first, and still more by the third than by the second; and that the yield goes on increasing in this manner to a very great number of times, which he hath not determined. This increased effect arises from hence, that by multiplying the frictions of the little bits of Antimony against each other, new surfaces are exposed to the action of the Alkaline liquor, and furnish it with more Sulphur; while the addition of this sulphur renders the hepar more active and more penetrating; or, if you please, produces a new hepar every time the matters are boiled. When the Alkaline liquor is once saturated with Kermes, it ceases to act, and forms no new hepar; but it does not follow that its virtue is quite exhausted. To restore its ability of acting as well as at first, or nearly so, you need only let it cool, and deposite the Kermes dissolved in it. We owe this singular observation also to Mr. Geoffroy: he had the patience to go through no less than threescore and ten boilings with the same liquor, without adding any thing but rain water, to supply the place of what was dissipated by evaporation: and he always obtained a pretty considerable quantity of Kermes by each boiling, for the reason given above.
Boiling is not the only means of making Kermes. Mr. Geoffroy found the way of making it by fusion. For this[Pg 334] purpose you must mix accurately one part of very pure Fixed Alkali, dried and pulverized, with two parts of Hungarian Antimony also pulverized, and melt the mixture. Mr. Geoffroy made use of a retort. When the mass is melted, it must again be pulverized, while it is still hot, and then put into, and kept in, boiling hot water for an hour or two; after which the liquor, now become saline and antimonial, must be filtered into another vessel filled with boiling water. Every ounce of Antimony treated in this manner yields, by thrice boiling the melted mass, from six drams to six drams and a half of Kermes; which differs from the Kermes made by boiling, only in that it is not quite so soft to the touch, having in every other respect the same qualities.
As Liver of Sulphur is made two different ways, to wit, by boiling and by fusion, and as the Kermes is nothing but a Liver of Sulphur in which the reguline part is dissolved; it follows that Kermes may be made by fusion as well as by boiling. It is necessary to pulverize the melted mass, and to steep it in boiling hot water for an hour or two, that the water may dissolve and divide it sufficiently to make the Kermes fine and beautiful.
With the same view, that is, to make it finer and more perfect, Mr. Geoffroy orders the water saturated with the Kermes made by fusion, to be received, when filtered, in a vessel full of other boiling hot water. He observed, that when the liquor impregnated with Kermes cools too fast, the Kermes that precipitates is much coarser. The warm solution of Kermes is diffused through the boiling-hot water into which it is filtered, and is thereby enabled to retain its heat so much the longer.
From what hath been said on the nature of Kermes, it plainly appears that there must be a great resemblance between it and the Golden Sulphur of Antimony, obtained from the scoria, either of plain Regulus of Antimony, or of the Liver of Antimony; this Golden Sulphur being no other than a portion of the Antimony combined with the Nitre alkalizated during the operation.
Yet there is a difference in the manner of precipitating these two compounds: for the Kermes precipitates spontaneously, on the bare cooling of the water in which it is dissolved; whereas an Acid is employed to precipitate the Golden Sulphur suspended in the water, with which the scoria of the plain Regulus of Antimony, or that of Liver of Antimony, hath been washed. This gives some ground to suspect that the reguline part is not so intimately united with the[Pg 335] Liver of Sulphur in the Kermes, as in the scoriæ from which the Golden Sulphur is obtained.
Regulus of Antimony dissolved in the Mineral Acids.
Compound an aqua regis by mixing together four measures of Spirit of Nitre, and one measure of Spirit of Salt: on a sand-bath moderately heated place a matrass, into which pour sixteen times as much of this aqua regis as you have Regulus to dissolve. Break your Regulus into little bits; and throw them successively one after another into the matrass, observing not to add a new one till that put in before is entirely dissolved: continue this till your Regulus be all used. By degrees, as the dissolution advances, the liquor will acquire a beautiful golden colour; which, however, will insensibly disappear, as the white fumes that continually ascend from it evaporate.
OBSERVATIONS.
Regulus of Antimony is one of those metalline substances that dissolve with the greatest difficulty. Not but that most of the Acids attack and corrode it; but they do not make a clear, limpid solution thereof: they in some sort only calcine it, and this semi-metal, as fast as it dissolves, precipitates of its own accord in the form of a white magistery. In order to effect a complete dissolution thereof, it is necessary to employ an aqua regis compounded as directed, and in the dose prescribed in the process, which is wholly taken from Mr. Geoffroy's Memoirs on Antimony mentioned above.
If, instead of the Regulus, small bits of crude Antimony be thrown into the aqua regis, the Acid will attack and dissolve the reguline part, and so separate it from the sulphureous part which it will not touch. When the dissolution is finished, the particles of Sulphur being now become lighter, because no longer united with the metalline part, will float upon the liquor. Being collected they form a true Sulphur, which seems no way different from common brimstone. This operation, you see, is a sort of Parting Process.
The Vitriolic Acid, whether concentrated or much weakened with water, does not act when cold either on Antimony[Pg 336] or on its Regulus. This Acid only dims the splendour of the facets of the Regulus; but if one part of exceeding pure Regulus of Antimony be put into a retort, and four parts of clear concentrated Oil of Vitriol poured on it, as soon as the Acid is heated it turns brown, and emits a most suffocating smell of Sulphur, which increases as the Regulus is penetrated and corroded by the Acid.
On raising the fire, there separates from it a matter that seems mucilaginous; and when the Acid hath boiled some time, the Regulus is converted into a white saline mass, as Mercury is in the preparation of Turbith mineral. At the same time a little Sulphur sublimes into the neck of the retort. At last all the Oil of Vitriol passes over into the receiver, and leaves the Regulus in a white, spungy, saline mass in the retort. When the fire is out, the vessels unluted, and the receiver separated from the retort, there rises a white vapour like that of the smoking liquor of Libavius.
The saline mass left in the retort, after the operation, is found increased to near double its weight: this increased weight is owing to the Acid that hath united with the Regulus.
This combination of the Vitriolic Acid with the Regulus of Antimony is excessively caustic, and cannot, for that reason, be administered internally.
The purest Spirit of Salt hath no sensible effect either on Antimony or its Regulus: but if Antimony be coarsely pounded, it separates therefrom, though slowly, some light, sulphureous flakes.
The action of Spirit of Nitre on this metallic substance is more perceptible: by little and little it attacks the plates of the Antimony, which discharge a great number of air-bubbles. As the dissolution advances, the Acid acquires a greenish colour inclining to blue; and if there be not too much of it, it will be almost entirely imbibed by the Antimony, penetrate between its laminæ, and exfoliate them in the direction of the needles that compose them. If there be too much of the Acid, that is, if it rise above the Antimony, it will destroy these plates, and reduce them to a white powder.
But when the Acid is imbibed slowly, we discover between the swelled laminæ little saline transparent crystals, that vegetate much in the same manner as those of the pyrites, in which small crystals of Vitriol are frequently observed, whose figures are not very well determined. These little crystals between the Antimonial plates are intermixed with yellow[Pg 337] particles, which being carefully separated burn like common Sulphur.
All these useful observations, concerning the action of the Acids on Antimony and its Regulus, we owe likewise to Mr. Geoffroy; who advises the collecting a quantity of these little crystals in time; because they disappear soon after they are formed, being probably covered by the white powder, or magistery, which is continually produced as fast as the Nitrous Acid disunites and separates the needle-like fibres of the Antimony.
Mr. Geoffroy observed the same sort of crystals on the Regulus of Antimony, when substituted for crude Antimony in this experiment; but it requires a great deal of care to separate these crystals; for as soon as the air comes into contact with them they lose their transparency; and if you wait till the Regulus be in some measure converted into a magistery, they are not then to be distinguished.
In order therefore to have a good view of these crystals, the Regulus must be broken to pieces; these pieces put in a glass bason, and Spirit of Nitre poured on them to half their heighth, but not to cover them. This Acid penetrates them, exfoliates them in white scales; and on the surface of these scales the crystals shoot of a dead white colour. In two or three days time these crystals vegetate and grow in the form of cauli-flowers: they must then be gathered, to prevent their being confounded in the white magistery which continues to be produced, and would not suffer them to be distinguished. If you attempt to dissolve the reguline part of Antimony by an aqua regis compounded in different proportions, and applied in a different dose from what is prescribed in the process, the Regulus of Antimony will only be calcined, as it is by the other Acids, and will precipitate in the form of a white magistery as fast as it dissolves, so that no part thereof will remain united with the solvent. The proof of this is, that if an alkaline liquor be poured, even to the point of saturation, upon the aqua regis that hath thus dropt the Antimony, no new precipitate will be deposited.
Regulus of Antimony combined with the Acid of Sea-salt. Butter of Antimony. Cinabar of Antimony.
Pulverize and mix thoroughly six parts of Regulus of Antimony, and sixteen parts of Corrosive Sublimate. Put this mixture into a glass retort that hath a wide short neck, and let one half of its body at least be left empty. Set it in a reverberatory furnace, and having fitted a recipient thereto, and luted the joint, make a very small fire at first, to heat it slowly. Increase it afterwards by degrees, till you see a liquor ascend from the retort that grows thick as it cools. Keep up the fire to this degree as long as you see any of this matter come over.
When no more rises with this degree of fire, unlute your vessels, take off the receiver, and in its place substitute another filled with water. Then increase your fire by degrees till the retort be red-hot. Some running Mercury will fall into the water, which you may dry and keep for use; it being very pure.
OBSERVATIONS.
In our observations on the preceding process, we took notice that the purest Marine Acid, in the form of a liquor, will not dissolve the reguline part of Antimony. Here this very Acid combined with Mercury, and applied in a dry form to the Regulus of Antimony, quits the Mercury with which it was united, in order to join this very Regulus, as having a greater affinity therewith. This operation is a further proof of what we advanced on the subject of Mercury; to wit, that several metallic substances, which are not soluble by certain Acids when in a fluid state, may be dissolved by those Acids when most highly concentrated; as they are when combined with any other substance in a dry form, and are separated from it by the force of fire. Their efficacy is also further promoted by their being reduced, on this occasion, into subtile vapours.
The Marine Acid combined with the reguline part of Antimony doth not form a hard, solid compound; but a kind of soft substance, that melts in a very gentle heat, and also becomes fixed by the least cold, much in the same manner as butter; and from this property it hath its name.
Soon after mixing the Regulus with the Corrosive Sublimate, the matter sometimes grows considerably hot: this is occasioned by the Marine Acid's beginning to act on the reguline part, and to desert its Mercury.
The Butter of Antimony rises with a very moderate heat; because the Acid of Sea salt hath the property of volatilizing, and carrying up along with it, the metallic substances with which it is combined: and for this reason a very gentle heat only is required at the beginning of the operation.
It is absolutely necessary that the neck of the retort be wide and short: for otherwise if the Butter of Antimony should fix and be accumulated therein, it might stop up the passage entirely, and occasion the bursting of the vessels. By this operation we obtain eight parts and three quarters of fine Butter of Antimony, and ten parts of running Mercury; there being left in the retort one part and a half of a rarefied matter, black, white, and red. This is probably the most earthy and most impure part of the Regulus of Antimony.
It is of the utmost consequence to the operator that he avoid with the greatest care the vapours that issue from the vessels, because they are extremely noxious, and may occasion mortal disorders. The Butter of Antimony is a most violent Corrosive and Caustic.
When all the Butter is risen, the receiver is shifted in order to receive the Mercury; which, being disengaged from the Acid that gave it a saline form, appears in its natural form of Quick-silver: but it requires a much greater degree of heat than the Butter of Antimony to raise it by distillation.
If crude Antimony, instead of Regulus of Antimony, be mixed with Corrosive Sublimate, a Butter of Antimony will be obtained in the same manner; but, instead of having a running Mercury after the Butter, you will find a Cinabar sublimed into the neck and upper concavity of the retort.
The reason of this difference is easily conceived: for when the Regulus is used, the Mercury being deserted by its Acid finds no other substance to unite with, and so rises in the form of Quick-silver; but when crude Antimony is employed instead of its Regulus, as the reguline part thereof cannot combine with the Acid without quitting its Sulphur, so this Sulphur, being at liberty, unites with the Mercury, which is so likewise, and therewith forms a Cinabar; which from its origin is named Cinabar of Antimony. When you intend to make both Butter and Cinabar of Antimony at the same time, six parts of Antimony must be mixed with[Pg 340] eight of Corrosive Sublimate; and care must be taken, while the Butter is coming over, to warm the neck of the retort by holding some live coals near it, with the precautions necessary to avoid breaking it. This warmth makes the Butter melt and run into the receiver; whereas, being thicker and of a much denser consistence than that made with the Regulus, it would otherwise gather in the neck of the retort, choak it entirely, and burst the vessel.
When the Butter is drawn from crude Antimony, more circumspection is necessary to make it of a beautiful white colour, than when it is obtained from the Regulus: for, if the fire be too strong during the distillation, or if the receiver be not soon enough separated from the neck of the retort, certain red sulphureous vapours, the fore-runners of the Cinabar, will at last ascend, and mixing with the Butter give it a brown colour.
In order to restore its beauty it must be put into a clean retort, and rectified by distilling it over again with a gentle sand-heat. By this rectification the Butter of Antimony becomes more fluid; and by re-distilling it a second time you may give it the thinness and fluidity of an oil.
After the operation there will be found in the receiver three parts and three quarters of Butter of Antimony, and some small crystals adhering to its inside, in the form of sprigs. When you break the retort there exhales from it a sulphureous odour; and you will find in it seven parts of Cinabar of Antimony, the greatest part of which is usually in compact glebes, that are heavy, smooth, shining, blackish throughout most of the mass, but in some places red: another part thereof appears in shining needles, and the rest in powder.
When all the Butter of Antimony is come over, and you begin to see the red vapours that predict the approaching ascent of the Cinabar, the receiver containing the Butter must be removed, lest the colour of the Butter should be spoiled by those sulphureous vapours. Another receiver is usually fitted on, without luting; in which a small quantity of running Mercury is sometimes found, when the operation is finished.
There remains, at the bottom of the retort, a fixed, shining, crystalline, black mass, which may be reduced to a Regulus by the common method.
Butter of Antimony may also be obtained from a mixture of Antimony with any of the other preparations of Mercury in which the Acid of Sea-salt is an ingredient; such as sweet[Pg 341] Sublimate, the Mercurial Panacea, and White Precipitate: but as none of these combinations contain so great a proportion of that Acid as is in the Corrosive Sublimate, the Butter obtained by their means is far from being so caustic and so fiery as that which rises from a mixture of Antimony, or its Regulus, with Corrosive Sublimate.
Silver precipitated by the Acid of Sea-salt, and ready to be melted into a Luna cornea, being mixed with powdered Regulus of Antimony yields likewise a Butter of Antimony.
If you propose to make it by this means, you must mingle one part of the Regulus of Antimony in powder with two parts of the Precipitate; put this mixture into a glass retort of such a size that it may fill but one half thereof; set it in a furnace; apply a receiver; begin with a gentle heat, which will make a clear liquor come over; and then increase your fire by degrees. White vapours will rise and condense into a liquid Butter; and in the mean time there will be a slight ebullition in the receiver, attended with a little heat. Continue the fire till nothing more will come over; then let your vessels cool and unlute them.
You will find in the receiver an Oil or Butter of Antimony, partly fluid and partly congealed, somewhat inclined to yellow, weighing an eighth part more than the Regulus of Antimony made use of.
The inside of the retort will be carpeted over with small white flowers, of a brilliant silver colour, and an acid taste; and in the bottom of the retort will be found a hard, compact, ponderous mass, difficult to break, yet falling of itself to a powder; its colour externally grey, white, and blueish; internally black, and shining much like Regulus of Antimony; having a saltish taste on its surface, and weighing about a sixteenth less than the Precipitate of Silver employed in the operation.
This experiment demonstrates that the Acid of Sea-salt hath a greater affinity with Regulus of Antimony than with Silver.
The Butter of Antimony prepared by this method is somewhat less caustic than that made with Corrosive Sublimate. It is called the Lunar Butter of Antimony.
The effervescence that arises in the receiver is remarkable. Probably the Acid of Sea-salt, though reduced into vapours when it ascends out of the retort, is not yet perfectly combined with the reguline part of the Antimony, which it nevertheless carries over with it, and the union is completed in the receiver; which occasions the effervescence observed.
The little white silvery flowers, adhering to the inside of the retort, are flowers of Regulus of Antimony, which sublime towards the end of the distillation.
The compact mass, found at the bottom of the retort, is no other than the Silver separated from its Acid, and combined with a portion of the Regulus of Antimony. The colours and the saltish taste of its surface are occasioned by a remainder of the Marine Acid. This Silver is rendered brittle and eager by the union it hath contracted with some of the Regulus of Antimony.
It is easy to purify it, and restore its ductility, by separating it from the Regulus of Antimony. There are several ways of doing this: one of the most expeditious is to flux it with Nitre, which burns and converts to a calx the semi-metal with which the Silver is adulterated.
Butter of Antimony decompounded by means of Water only. The Pulvis Algaroth, or Mercurius Vitæ. The Philosophic Spirit of Vitriol.
Melt with a gentle heat as much Butter of Antimony as you please. When it is melted, pour it into a large quantity of warm water. The water will immediately grow turbid, but whitish, and let fall a great quantity of white powder. When all the precipitate is settled, decant the water: pour on fresh warm water; and having thus edulcorated it by several ablutions, dry it, and you have the Pulvis Algaroth, or Mercurius Vitæ.
OBSERVATIONS.
In the preceding processes we observed that the Marine Acid will not dissolve the reguline part of Antimony, unless it be very highly concentrated, and more so than it can possibly be while in the form of a liquor. Of this the experiment before us is a further proof. Whilst the Marine Acid is so perfectly dephlegmated, as it is in Corrosive Sublimate and Butter of Antimony, it remains combined with the reguline part of Antimony; but if this combination be dissolved in water, the moment the Acid is weakened by the interposition of the particles of water, it becomes incapable of continuing united with the semi-metal which it had be[Pg 343]fore dissolved; deserts it, and lets it fall in the form of a white powder.
The Pulvis Algaroth is therefore no other than the reguline part of Antimony, attenuated and divided by the union it had contracted with the Acid of Sea-salt, and afterwards separated from that Acid by the intervention of water alone. The proof is, that this powder retains none of the properties of the Butter of Antimony: it is neither so fusible nor so volatile; on the contrary, it is capable of sustaining a very strong degree of fire, without subliming and without melting: it may be reduced to a Regulus: it hath not now the same caustic nature: it is only an emetic; which however is extremely violent, and on that account is never prescribed by any prudent physician.
Another proof, that the Marine Acid is separated from the Regulus of Antimony in the precipitation of the Pulvis Algaroth, is, that the water in which this precipitation is made becomes acid, or a sort of weak Spirit of Salt. If it be evaporated, and concentrated by distillation, a very strong acid liquor may be obtained from it. This Acid goes, very improperly, by the name of the Philosophic Spirit of Vitriol; for it is rather a Spirit of Salt.
The Pulvis Algaroth, made with Butter of Antimony procured from the Regulus, is whiter than that made with Butter of Antimony procured from crude Antimony; probably because the latter always retains some sulphureous particles.
Butter of Antimony exposed to the air attracts the moisture thereof, and partly runs into a liquor; but, as fast as this liquor is produced, it deposites a white sediment, which is an actual Pulvis Algaroth. This also is very agreeable to what we advanced touching the decomposition of Butter of Antimony by the addition of water. The Butter attracts the moisture of the air, because the Acid it contains is exceedingly concentrated; and this moisture produces the same effect as water purposely added.
Bezoar Mineral. The Bezoartic Spirit of Nitre.
Melt Butter of Antimony over warm ashes, and put it into a phial or matrass. Gradually pour on it good Spirit of Nitre, till the matter be entirely dissolved.[Pg 344] This usually requires as much Spirit of Nitre as there is Butter of Antimony. During the dissolution fumes will rise, which must be carefully avoided. Pour your solution, which will be clear and of a reddish colour, into a glass cucurbit, or a pan of stone-ware: set it in a sand-bath, and evaporate to dryness with a moderate heat. There will be left a white mass, weighing a fourth part less than the whole quantity used, both of the Butter and the Spirit of Nitre. Let it cool, and again pour on it as much Spirit of Nitre as you used the first time. Place the vessel again in the sand-bath, and evaporate the moisture as before. You will have a white mass that hath neither gained nor lost in weight. On this pour, for the third time, the same quantity of Spirit of Nitre as you did the first time. Again evaporate the moisture to perfect dryness: then increase your fire, and calcine the matter for half an hour. You will have a dry, friable, light, white matter, of an agreeable acid taste; which will fall into a coarse powder, and must be kept in a phial carefully stopt. This is Bezoar Mineral: it is neither caustic nor emetic, and has only a sudorific virtue. It obtained the name it bears, because, like the animal Bezoar, it was imagined to have the property of resisting poison.
OBSERVATIONS.
It is not surprising that the Nitrous Acid poured on Butter of Antimony should dissolve it, and unite with it: for with the Marine Acid, which makes a part of this combination, it forms an aqua regis, which we know is the true solvent of the reguline part of Antimony. But in this dissolution, and the changes it produces, there are some things very remarkable and worthy of attention. 1. The Nitrous Acid, by uniting with the Butter of Antimony, deprives it of its property of rising with a very gentle heat, and makes it much more fixed: it can now be dried, and suffer all its moisture to be evaporated; which is not to be done with pure Butter of Antimony: for that, being exposed to a certain degree of heat, instead of letting go its moisture and remaining dry, rises wholly, without the least appearance of any separation of parts.
2. The Butter of Antimony, which, before its combination with the Nitrous Acid, is a most violent Caustic and Corrosive, becomes so mild after it, that it may not only be taken internally without danger, but hath scarce any sensible operation.
The following considerations will lead us to a reasonable explanation of these phenomena. 1. The Nitrous Acid, when combined with metallic substances, doth not communicate to them the same volatility as they acquire from the Marine Acid. Hence it follows, that, if the Nitrous Acid be added to any combination of a metallic substance with the Marine Acid, this new compound will be rendered less volatile, and consequently more able, without rising in vapours, to bear a degree of heat sufficient to carry off part of its Acid. This is the case with Butter of Antimony, after Spirit of Nitre is mixed with it; especially considering, 2. That the Nitrous Acid cannot unite with the reguline part of the Butter of Antimony without weakening the connection between it and the Marine Acid; whence it follows, that the combination of the Nitrous Acid further facilitates the separation of the Marine Acid from the Regulus. Now as soon as the Marine Acid quits the reguline part, that part becomes more fixed, and consequently more capable of enduring the degree of heat requisite to discharge all the adhering Acid; and not only the Marine, but even the Nitrous also. It is not therefore surprizing that, after the Antimony which remains combined with the Nitrous Acid is dried, it should not possess that corrosive power which it derives only from the Acids wherewith it is armed. In order to free it more perfectly from all Acid, we order the fire to be increased after the third desiccation; and the remainder of the Butter of Antimony to be calcined for a full half-hour longer.
That the Marine Acid is separated from the reguline part of the Butter of Antimony, by the desiccations it undergoes in converting it into Bezoar, is proved by this, that, when these desiccations are performed in close vessels, the liquor drawn off is a true aqua regis, known by the name of the Bezoartic Spirit of Nitre.
It remains to be considered why the Bezoar mineral, though freed from all acid, is not emetic; while the Pulvis Algaroth, which is likewise the reguline part of the Butter of Antimony deprived of its Acid, is such a violent emetic, and even to be dreaded for its remaining causticity.
In order to discover the reason of this difference, it is proper to observe that, when we say Bezoar mineral and the Pulvis Algaroth contain no Acid, we must not be understood in too strict a sense: on the contrary, there is reason to think that a certain quantity of Acid still remains in each of them; which however is scarce worth notice, in comparison of the quantity each contained at first. This being allowed,[Pg 346] it will not be hard to find the difference between these two preparations of Antimony. The Pulvis Algaroth is deprived of its Acid by the addition of water alone, which only carries off all the loose Acid it can take up, without making any change in the nature of that which continues in combination with the reguline part. Now, as the Marine Acid is not intimately united with the reguline part in Butter of Antimony; as it still retains some of its properties, such as attracting the moisture of the air, giving manifest tokens of its Acid nature, &c.; and as the corrosive quality of this compound depends on this last in particular; the small portion of Acid left in the Pulvis Algaroth will in some degree preserve its former character: and hence comes the effect of this powder, which still retains a little of the corrosive quality that belonged to the Butter of Antimony.
But this is not the case with the small remainder of Acid, which possibly still continues united with the Bezoar mineral prepared as here directed. This compound hath been exposed to a fire sufficient, not only to dry it, but even to calcine it. Now fire is capable of producing great changes in the texture of bodies. It must have forced off from the Bezoar all the Acid that was not intimately combined with it; and that part which it could not drive off, because of its obstinate adhesion, it must have further united and combined more closely with the metallic earth: for we see that fire greatly promotes the action of solvents on the matters with which they are united.
With regard to the properly emetic quality of the Pulvis Algaroth, it cannot be imputed to the combination of any Acid with that powder; since we see that the most powerfully emetic preparations of Antimony, viz. its Regulus and Glass, contain no Acid: it must therefore be attributed to some cause different from that on which its corrosive quality depends. This cause we shall easily find by attending to the different manners in which the Marine Acid, when alone and in aqua regia, operates on the reguline part of Antimony.
The Marine Acid alone dissolves the Regulus of Antimony, but with great difficulty; nor doth it effect a complete dissolution thereof, as is evident from what hath been already said: whereas the Marine Acid, combined with the Nitrous Acid, and therewith forming an aqua regis, as in the preparation of Bezoar, dissolves the reguline part of Antimony completely and radically. Now, it is certain that, the more efficaciously Acids operate on metallic substances, the[Pg 347] more of their phlogiston do they destroy; and we cannot but recollect that the preparations of Antimony are so much the less emetic the less phlogiston they contain, or the further they recede from the nature of a Regulus, and the nearer they approach to that of Diaphoretic Antimony: consequently it is plain how Bezoar mineral, which is a sort of calx of Antimony entirely deprived of its phlogiston by the intimate dissolution thereof made by the Acids of the aqua regis, may be in no degree emetic; while the Pulvis Algaroth, being a true Regulus of Antimony, on which the Marine Acid hath operated but very superficially, and which still contains a great deal of phlogiston, is a most violent emetic.
Flowers of Antimony.
Take an unglazed earthen pot, having an aperture in its side, with a stopple to shut it close. Set this pot in a furnace, the cavity whereof it may fit as exactly as possible; and fill up with lute the space, if any, left between the vessel and the furnace. Over this vessel fix three aludels, with a blind-head at the top; and light a fire in the furnace under the pot.
When the bottom of the pot is thoroughly red, throw into the lateral aperture a small spoonful of powdered Antimony. Stir the matter immediately with an iron spatula made a little bending, in order to spread it over the bottom of the vessel, and then stop the hole. The flowers will rise and adhere to the insides of the aludels. Keep up the fire so that the bottom of the pot may always continue red; and, when nothing more sublimes, put in a like quantity of Antimony, and operate as before. In this manner go on subliming your Antimony, till you have as many flowers as you want. Then let the fire go out; and when the vessels are cold unlute them. You will find flowers adhering all round the insides of the aludels and the head, which you may collect with a feather.
OBSERVATIONS.
Antimony is a volatile mineral, capable of being sublimed into flowers; but this cannot be effected without oc[Pg 348]casioning a notable change in its parts. The reguline and the sulphureous parts are not united so intimately, or in the same proportion, in the flowers as in the Antimony itself; and accordingly we find these flowers have a strong emetic quality, which Antimony hath not. They are of divers colours; which probably arises from their containing more or less Sulphur. Three or four aludels are placed one over another, not only with a view to provide a greater surface, to which the flowers may adhere, but also to give them room enough to circulate, without which they might burst the vessels.
If you introduce the nosle of a pair of bellows into the pot that contains the Antimony, and blow upon it, the sublimation of the flowers will be much sooner effected. This is a general rule with regard to all matters that are to be sublimed or evaporated; the reason of which we have already given.
It is proper that no interval be left between the furnace and the pot containing the Antimony, lest the heat should be thereby communicated to the aludels, on which the flowers fasten best when they are cold.
After the operation, there remains at the bottom of the pot a portion of Antimony half calcined; which being pulverized, and thoroughly calcined till it emit no fume, may be employed to make the Glass of Antimony.
Regulus of Antimony converted into Flowers.
Pulverize your Regulus of Antimony: put the powder into an unglazed earthen pot: three or four fingers breadth above the powder, fit into the pot a little cover, made of the same earth, and having a small hole in its middle, so that it may with ease be placed in the pot, and taken out when there is occasion: cover the mouth of the pot with a common lid; set it in a furnace, and kindle a fire under it sufficient to make the bottom of the pot red, and to melt the Regulus. When it hath been thus kept in fusion for about an hour, let the fire go out, and the whole cool. Then remove the two covers. You will find adhering to the surface of the Regulus, which will be in a mass at the bottom of the pot, white flowers resembling snow, intermixed with beautiful, brilliant, silver-coloured needles. Take them out, and you will find them make about one part in sixty-two of the whole Regulus employed.
Put the covers again in their places, and proceed in the same manner as before; when the vessels are cold you will find half as many more flowers as you got the first time.
Proceed thus till you have converted all your Regulus into flowers. This will require a considerable number of sublimations, which, as you advance, will always yield you a greater portion of flowers; respect, however, being had to the quantity of Regulus remaining in the pot.
OBSERVATIONS.
We must here repeat what we said just before, in our observations on the preceding process; viz. that Regulus of Antimony is capable of being wholly elevated and sublimed by the action of fire; but that it must at the same time undergo a considerable change and alteration. These flowers of Regulus of Antimony are very different from every other Antimonial preparation. They resemble the Pearly Matter in this, that they cannot be reduced to a Regulus by any means whatever: but they differ from it, 1. in that they are not fixed; for, when melted by fire, they fly wholly away in vapours: 2. in that they are capable of being dissolved by aqua regis, much in the same manner as the Regulus; whereas the Pearly Matter is known to be indissoluble by any Acid.
As soon as Regulus of Antimony is in fusion, it begins to sublime into flowers; so that it is needless to apply a greater degree of heat than is just sufficient to melt it.
A pan of some width is preferable to a crucible for this operation; because the upper surface of the Regulus melted therein is larger, and, the larger that surface is, the more considerable is the quantity sublimed from it.
The two covers which are applied within and over the pot are designed to check, as much as possible, the dissipation of the melted Regulus; yet without absolutely excluding the free access of the air, the concourse of which is useful in all metallic sublimations. Notwithstanding these precautions, it is impossible to prevent the escape of some of the Regulus, in vapours that cannot be confined. Somewhat less than three fourths of the Regulus made use of is nearly the yield in flowers: the rest evaporates through the interstices left by the covers, which must not be luted for the reason just assigned.
Of Bismuth.
To extract Bismuth from its Ore.
Break the ore of Bismuth into small pieces, and therewith fill a crucible either of earth or iron. Set the crucible in a furnace, and light such a fire that the bits of ore may become moderately red. Stir the ore from time to time, and, if you perceive it crackle and fly, keep the crucible covered. At the bottom you will find a button of Bismuth.
OBSERVATIONS.
The extraction of Bismuth from its ore requires nothing but simple fusion, without the addition of any inflammable matter, because it is naturally possessed of its metalline form. Nor does it require any flux; because it is very fusible: which allows us to melt it, and collect it in a mass, without the necessity of fusing likewise the earthy and stony matters in which it is lodged. These matters remain in their first state; and the melted Bismuth descends by its gravity to the bottom of the crucible. No greater degree of heat must be applied, on this occasion, than is necessary to melt the semi-metal: for, as it is volatile, part of it would be dissipated; so that much less thereof would be obtained, if the fire were made too strong, and so much the less as another portion thereof would be converted into a calx. For the same reason, the crucible must be taken out of the furnace as soon as you perceive that all the Bismuth contained in the ore is melted, and that the button doth not increase.
The ore of Bismuth may also be treated like the ores of Lead and Tin; that is, it may be reduced into a fine powder, mixed with the black flux, a little Borax, and Sea-salt; put into a close crucible, and fused in a melting furnace. In that case you will find a button of Regulus covered with scoria. By this method rather more Bismuth is obtained;[Pg 351] and it is best to make use of it when the ore is poor, because, in such a case, none at all would be obtained by the other process. But here care must be taken to apply at once the degree of fire necessary to melt the mixture: for, if it remain long in the fire, much Bismuth will be lost, on account of the great volatility of this semi-metal, and the facility with which it turns to a calx.
Bismuth is pretty frequently found pure in its earthy and stony matrices; and when mineralized it is usually so by Arsenic, which, being still more volatile, flies off in vapours while the ore is melting, provided it be but in a small quantity: if there be much of it, and the ore be smelted by fusing it with the black flux, the Arsenic also is reduced to a Regulus, unites more intimately with the Bismuth, becomes a little more fixed by that union, and increases the quantity of the semi-metallic mass found after the fusion.
Though Bismuth be not usually mineralized by Sulphur, that is not because it is incapable of uniting therewith; for, if equal parts of Bismuth and Sulphur be melted together, after the fusion the Bismuth will be found increased near an eighth part, and formed into a mass disposed in needles much like Antimony.
When we come to treat of the ore of Arsenic, we shall have occasion to say a good deal more concerning Bismuth and its ore; because these minerals resemble each other very much.
Mr. Geoffroy, son of the Academician, hath shewn in a Memoir read before the Academy of Sciences, that there is a great resemblance between Bismuth and Lead. That Memoir, which contains only the beginning of Mr. Geoffroy's course of experiments, proves that the author supports with dignity the glory of his name. It is there demonstrated, by a very great number of experiments, that fire produces the same effects on Bismuth as on Lead. This semi-metal is converted into a calx, into litharge, and into glass, as Lead is; and these productions have the same properties as the preparations of Lead made with the same degree of fire. Bismuth is capable of vitrifying all the imperfect metals, and of carrying them off through the pores of the crucible. So that Gold and Silver may be purified and cupelled by its means, as well as with Lead. You may on this occasion turn to what we have said concerning Lead.
Bismuth dissolved by Acids. Magistery of Bismuth. Sympathetic Ink.
Into a matrass put Bismuth broken into little bits: pour on it, by little and little, twice as much aqua fortis. This Acid will attack the semi-metal briskly, and dissolve it entirely, with heat, effervescence, vapours, and puffing up. The solution will be clear and limpid.
OBSERVATIONS.
Of all Acids the Nitrous is that which best dissolves Bismuth. It is not necessary, on this occasion, to place the phial, in which the dissolution is performed, on a sand-heat, as in most other metallic dissolutions: on the contrary, care must be taken not to pour on all the aqua fortis at once; because it operates with so much activity that the mixture will heave up and run over the vessel.
The bare addition of water is sufficient to precipitate the solution of Bismuth. If this solution be mixed with a very large proportion of water, the liquor grows turbid, appears milky, and deposites a precipitate of a very beautiful white. This is that white which the ladies use at their toilets.
Water produces this precipitation by weakening the Acid; which probably is incapable of keeping the Bismuth dissolved, unless it have a certain degree of strength.
If you would have a Magistery of Bismuth beautifully white, you must perform the dissolution with an aqua fortis that is not tainted with any mixture of the Vitriolic Acid; for this gives the precipitate a dirty white colour, inclining to grey. Several authors advise the use of a solution of Sea-salt, instead of pure water, for precipitating the Bismuth, imagining that this Salt will effect a precipitation here as it does in the cases of Silver and Lead. But Mr. Pott, a German Chymist, who hath published a long dissertation on Bismuth, pretends, on the contrary, that neither Sea-salt, nor its Acid, is capable of precipitating this semi-metal; and that when a precipitation takes place on mixing them with our solution, it is brought about only by means of the water in which those substances are diffused.
Bismuth may also be precipitated by the means of Fixed[Pg 353] or Volatile Alkalis; but the precipitate is not of so fine a white as when procured by the means of pure water only.
If a greater quantity of aqua fortis, than that prescribed in the process, be made use of to dissolve the Bismuth, a great deal more water will also be required to precipitate the Magistery; because there will be much more Acid to weaken. This white ought to be well washed, in order to free it from any remainder of acidity; and it should be kept in a bottle well stopped; because the access of the air makes it turn brown, and if any of the Acid be left it will turn it yellow.
A solution of Bismuth prepared with the proper quantity of aqua fortis, that is, with two parts of the Acid to one of the semi-metal, concretes into little crystals almost as soon as made.
Aqua fortis not only acts on Bismuth when separated from its ore, and reduced to a Regulus, but attacks it even in its ore, and likewise dissolves at the same time some portion of the ore itself. With this solution of the ore of Bismuth Mr. Hellot makes a very curious Sympathetic Ink, differing from all that were known before.
Mr. Hellot prepares the liquor in the following manner: "He bruises the ore of Bismuth to a coarse powder. On two ounces of this powder he pours a mixture of five ounces of common water with five ounces of aqua fortis. He does not heat the vessel till the first ebullitions are over. He then sets it in a gentle sand-heat, and lets it digest there till he sees no more air bubbles rise. When none appear in this heat, he increases it so as to make the solvent boil slightly for a full quarter of an hour. It takes up a tincture nearly of the colour of brown beer. The ore that gives the aqua fortis this colour is the best. He then lets the solution cool, laying the matrass on its side, that he may decant the liquor more conveniently when all is precipitated that is not taken up by the solvent.
"The second vessel, into which the liquor is first decanted, he also lays declining, that a new precipitation of the undissolved matters may be obtained; after which he pours the liquor into a third vessel. This liquor must not be filtered, if you would have the rest of the process succeed perfectly; because the aqua fortis would dissolve some of the paper, and that would spoil the colour of your paper.
"When this solution, which Mr. Hellot calls the Impregnation, is thoroughly clarified by being decanted three or[Pg 354] four times, he puts it into a glass bason with two ounces of very pure Sea-salt. The fine white salt made by the sun succeeded best with Mr. Hellot. If that cannot be had, common bay-salt, purified by solution, filtration, and crystallization, may be used instead of it. But as it is rare to meet with any of the sort that is not a little tainted with iron, the white bay-salt is to be preferred. The glass bason he sets in a gentle sand-heat, and keeps it there till the mixture be reduced by evaporation to an almost dry saline mass.
"If you desire to save the aqua regis, the impregnation must be put into a retort, and distilled with the gentle heat of a sand-bath. But there is an inconveniency, as Mr. Hellot observes, in employing a retort; which is, that, as the saline mass cannot be stirred while it coagulates in the retort, it is reduced to a compact cake of coloured Salt, which presents but one single surface to the water in which it must be dissolved; so that the dissolution thereof takes up sometimes no less than five or six days. In the bason, on the contrary, the saline mass is easily brought to a granulated Salt, by stirring it with a glass rod; and, when thus granulated, it has a great deal more surface; it dissolves more easily, and yields its tincture to water in four hours time. Indeed one is more exposed to the vapours of the solvent, which would be dangerous, if the operation were to be often performed, without proper precautions.
"When the bason, or little vessel, containing the mixture of the Impregnation and Sea-salt is heated, the liquor, which was of an orange-coloured red, becomes a crimson red; and, when all the phlegm of the solvent is evaporated, it acquires a beautiful emerald colour. By degrees it thickens, and acquires the colour of a mass of Verdegris. It must then be carefully stirred with the glass rod, in order to granulate the Salt, which must not be kept over the fire till it be perfectly dry; because you run a risk of losing irrecoverably the colour you are seeking. You may be sure you have lost it, if by too much heat the Salt that was of a green colour become of a dirty yellow. If it be once brought to this state, it will continue without changing when cold: but if care be taken to remove it from the fire while it is still green, you will see it gradually grow pale, and become of a beautiful rose colour as it cools.
"Mr. Hellot removes it from this vessel, and throws it into another containing distilled rain water: and this se[Pg 355]cond vessel he keeps in gentle digestion till he observes that the powder which falls to the bottom is perfectly white. If, after three or four hours digesting, this powder still continues tinged with a rose colour, it is a proof that water enough was not added to dissolve all the Salt impregnated with the tincture of the solution. In this case, the first tinged liquor must be poured off, and fresh water added, in proportion to the quantity of tinged Salt, that is supposed to remain mixed with the precipitate.
"When the ore is pure, and doth not contain a great deal of fusible stone, commonly called Fluor or Quartz, an ounce of it generally yields tincture enough for eight or nine ounces of water, and the liquor is of a beautiful colour like that of the lilach or pipe-tree blossom. In order to prove the effect of this tincture, you must write with this lilach-coloured liquor on good well-gummed paper, that does not sink: or you may use it to shade the leaves of some tree or plant, having first drawn the outlines thereof lightly, with China-ink or with a black-lead pencil. Let this coloured drawing, or writing, dry in a warm air. You will perceive no colour while it is cold; but if it be gently warmed before the fire, you will see the writing, or the drawing, gradually acquire a blue or greenish-blue colour, which is visible as long as the paper continues a little warm, and disappears entirely when it cools."
The singularity of this sympathetic ink consists in its property of disappearing entirely and becoming invisible, though it be not touched with any thing whatever: and this distinguishes it from all others; which, when once rendered visible by the application of proper means, do not again disappear, or at least not without touching the strokes on the paper with some other liquor.
Mr. Hellot made a vast variety of experiments on this subject, and gave his sympathetic ink successively the properties of all others that are known.
It follows from Mr. Hellot's experiments, that it is the Acid of Sea-salt which makes this saline magma of a green colour while it is hot: that without this Acid the saline matter continues red; and that the solution of Bismuth-ore in aqua fortis may therefore serve as a touchstone, to discover whether or no any unknown Salt under examination contains Sea-salt, or a portion of the Marine Acid.
He also proves, in the Memoirs he hath given in on this subject, that the Nitrous Acid is the true solvent of those ores[Pg 356] of Bismuth which contain moreover Smalt and Arsenic. That Acid dissolves all the metallic and colouring matters contained in those ores, sparing nothing but the sulphureous and arsenical portion, the greatest part of which remains precipitated; and from this colouring matter the sympathetic ink derives its virtue.
Under the head of Arsenic we shall speak more amply of this matter in Cobalt, or the ore of Arsenic, that gives a blue colour to the sand with which it is vitrified.
The Vitriolic Acid does not, properly speaking, dissolve Bismuth. If to one part and an half of this semi-metal you add one part of Oil of Vitriol; distil the whole to dryness; and then lixiviate with water what remains in the retort; the liquor you obtained by this means will be of a reddish yellow colour, but will let nothing fall when mixed with an Alkali: and this shews that the Vitriolic Acid acts only upon the inflammable part of Bismuth, and doth not dissolve its metallic earth.
It dissolves the ore of Bismuth more perceptibly than Bismuth itself; because the ore contains, besides the reguline part, an arsenical matter, and a coloured matter, over which perhaps it hath more power.
The Acid of Sea-salt attacks and dissolves Bismuth in some small measure, but slowly and with difficulty. That this Acid dissolves a portion of our semi-metal may be proved, by mixing a Fixed or Volatile Alkali with Spirit of Salt in which Bismuth hath lain some time digesting; for then a precipitate falls.
But, though the Marine Acid be capable of dissolving Bismuth, it doth not follow that it hath a greater affinity than the Nitrous Acid with this metallic substance, as some Chymists have thought; who imagined that, in the precipitation of the Magistery of Bismuth by a solution of Sea-salt, the Acid of that Salt quits its basis to unite with the Bismuth which it precipitates, as is the case in the precipitations of Lead and of Silver by the same Salt, and that it forms, on this occasion, a Bismuthum corneum.
On this subject, Mr. Pott observed, 1. that, when only a small quantity of the solution of Sea-salt is mixed with the solution of Bismuth in the Nitrous Acid, no precipitate is formed: now it is certain that when the smallest quantity whatever of Sea-salt is mixed with the solution either of Lead or of Silver, a precipitate is immediately deposited, in a quantity proportioned to that of the Salt used.
2. Mr. Pott, having examined the precipitate of Bismuth thrown down by a solution of Sea-salt, found it not to have the properties of a metallic substance rendered horny: on the contrary, that precipitate being exposed to a very violent fire appeared refractory, and could not be melted.
Of Zinc.
To extract Zinc from its Ore, or Calamine.
Take eight parts of Calamine reduced to a powder; mix this powder accurately with one part of fine charcoal-dust, previously calcined in a crucible to free it from all moisture: put this mixture into a stone retort coated with lute, leaving a third part of it empty: set your retort in a reverberatory furnace, capable of giving a very fierce heat. To the retort apply a receiver, with a little water in it. Kindle the fire, and raise it by degrees till the heat be strong enough to melt Copper. With this degree of fire the Zinc being metallized will separate from the mixture, and sublime into the neck of the retort, in the form of metallic drops. Break the retort when it is cold, and collect the Zinc.
OBSERVATIONS.
The process here given for smelting Zinc out of Calamine is taken from the Memoirs of the Academy of Sciences at Berlin. The author of it is Mr. Marggraff, a skilful Chymist, whom we have already had occasion to mention under the article of Phosphorus.
Till this process was published, we knew no method of obtaining pure Zinc directly from the Lapis Calaminaris.
Most of the Zinc we have comes from an ore of difficult fusion that is worked at Goslar, and yields, at one and the same time, Lead, Zinc, and another metallic matter called[Pg 358] Cadmia Fornacum, which also contains much Zinc, as we shall afterwards see.
The furnace used for smelting this ore is closed on its fore-side with thin plates or tables of stone, not above an inch thick. This stone is greyish, and bears a violent fire.
In this furnace the ore is melted amidst charcoal, by the help of bellows. Each melting takes twelve hours, during which time the Zinc flowing with the Lead is resolved into flowers and vapours, great part of which adheres to the sides of the furnace in the form of a very hard crust of earth. The workmen take care to remove this crust from time to time; for it would otherwise grow so thick at last as to lessen the cavity of the furnace very considerably.
There adheres moreover to the fore-part of the furnace, which is formed, as we said before, of thin plates of stone, a metallic matter, which is the Zinc, and is carefully collected at the end of each melting, by removing from this part all the live coals. A quantity of small coal is laid unlighted at the bottom; and on this small coal, by striking the stone plates gently with a hammer, the Zinc is made to fall out of the other matter, known by the Latin name of Cadmia Fornacum, among which it appears fixed in a radiated form. To this other matter we may properly enough give the name of Furnace-Calamine. The Zinc falls in the form of a melted metal, all on fire, and in a bright flame. It would soon be entirely burnt and reduced to flowers, as we shall see, if it were not extinguished, and easily cooled and fixed, by being hid under the unlighted small-coal placed below on purpose to receive it.
The Zinc adheres to the fore-part of the furnace preferably to any other, because that being the thinnest is therefore the coolest: and, in order further to promote its fixing on this part, they take care to keep the thin stone plates cool during the operation, by throwing water on them.
Hence it appears, that Zinc is not extracted from its ore by fusion and the precipitation of a Regulus, like other metallic substances. This is owing to the great volatility of our semi-metal, which cannot, without subliming, bear the degree of fire necessary to melt its ore. It is at the same time so combustible, that a great part of it rises in flowers which have not the metalline form.
Mr. Marggraff provides against these inconveniences by working the ore of Zinc in close vessels. By this means he prevents the Zinc from taking fire, and being converted into flowers; so that it sublimes in its metalline form. The wa[Pg 359]ter in the recipient serves to receive and cool the drops of Zinc that may be forced quite over the helm. As the operation requires a most violent fire, these drops must needs issue exceeding hot, and, without this precaution, break the recipient.
Mr. Marggraff by the same process extracted Zinc out of the Furnace-Calamine procured from ores containing Zinc; from Tutty, which is a sort of furnace-calamine; from the flowers and from the calx of Zinc; and from the precipitate of White Vitriol; all of them matters known to be Zinc, that wanted nothing but the phlogiston to give it a semi-metalline form, and from which nevertheless no body could ever before him procure any Zinc.
Mr. Marggraff observes, that the Zinc obtained by his process bears being flatted under the hammer into pretty thin plates; which the common Zinc will not do. The cause of this probably is, that the Zinc obtained by his method is more intimately combined with the phlogiston, and contains a greater quantity thereof, than that which is procured in the ordinary way.
To sublime Zinc into Flowers.
Take a very deep, large crucible: place it in a furnace, so that it may stand inclining in an angle of forty-five degrees nearly. Throw some Zinc into it, and kindle a fire in the furnace somewhat stronger than would be necessary to keep Lead in fusion. The Zinc will melt. Stir it with an iron wire, and there will appear on its surface a very bright white flame: two inches above this flame a thick smoke will be formed, and with this smoke exceeding white Flowers will rise, and remain some time adhering to the sides of the crucible, in the form of a very fine light down. When the flame slackens, stir your melted matter again with the iron wire: you will see the flame renewed, and the flowers begin again to appear in greater abundance. Go on thus till you observe that the matter will not flame, nor any more Flowers rise.
OBSERVATIONS.
Zinc takes fire very easily as soon as it is affected by a certain degree of heat; which proves, that in the composition[Pg 360] of this semi-metal there is very much phlogiston, united but slightly with its metallic earth. The Flowers into which Zinc resolves, during its combustion, are of a perfectly singular nature, and differ greatly from all the other productions obtainable out of metallic substances.
They may be considered as the very calx of Zinc, or its metallic earth robbed of its phlogiston, and sublimed during the combustion of this semi-metal, being probably carried up by the phlogiston in flying off. For these Flowers, when once sublimed, are afterwards exceedingly fixed: they sustain the greatest violence of fire without rising, and are converted by it into a sort of glass.
None of the methods hitherto employed, for restoring to the Flowers of Zinc their metalline form, have ever succeeded. When treated like other metalline calces in a crucible, with every kind of inflammable matter, and different sorts of reducing fluxes, they never can be re-metallized: they only melt with the flux, and produce a kind of Glass.
Mr. Marggraff indeed, as mentioned before, obtained Zinc from these Flowers, by treating them as he did Calamine in a retort with charcoal-dust: but as the Flowers often carry up with them little particles of undecomposed Zinc, there still remains some doubt concerning the reduction of these Flowers, even by this method.
If the crucible, into which you put the Zinc to be converted into Flowers, instead of being left open, as directed, be covered with another crucible inverted, the two vessels luted together, placed in a melting furnace, and a strong fire immediately kindled and kept up for about half an hour; you will find, when the vessels are cold, that all the Zinc hath left the lower crucible, and is sublimed into the upper one, in its metalline form, without suffering any decomposition. This experiment proves, that Zinc, to be converted into Flowers, must necessarily be set on fire and burnt. As it cannot burn in close vessels, any more than other combustible bodies, and as it is volatile, it sublimes without suffering any decomposition. Regulus of Antimony and Bismuth may be sublimed in the same manner; but not so easily as Zinc, which is still more volatile than those other semi-metals.
It is necessary to stir the Zinc in fusion from time to time with an iron wire, when you intend to convert it into Flowers: for there forms on its surface a grey crust that obstructs its deflagration, and beneath which it is gradually[Pg 361] converted into a clotted calx. In order, therefore, to promote the rising of the flowers, care must be taken to break this crust, as oft as it begins to form. On this there immediately appears a very bright white flame: two inches above the flame is seen a thick smoke, and with this smoke very white Flowers rise, that continue some time adhering to the inside of the crucible, in the form of a fine down.
M. Malouin, who, in sundry Memoirs on Zinc, hath endeavoured to discover what resemblance there is between this semi-metal and Tin, tried to calcine Zinc in the same manner as Tin; but found it somewhat more difficult. Zinc, while it is not in fusion, doth not calcine; but it begins to turn to a calx the moment it begins to melt. M. Malouin, having repeated the fusion of Zinc a great number of times, by that means collected at last a quantity of the calx of this semi-metal, resembling other metalline calces. This calx of Zinc he melted in a crucible with animal fat; whereby the calx was re-metallized, and reduced to Zinc. There is great reason to believe that the calx of Zinc made by this method is not so much burnt as the Flowers, and that it still contains a portion of phlogiston.
To combine Zinc with Copper. Brass. Prince's Metal, &c.
Pound one part and an half of Calamine, and an equal quantity of charcoal: mingle these two powders together, and moisten them with a little water. Put this mixture into a large crucible, or some other earthen vessel that will bear a melting heat. Amongst and over this mixture put one part of very pure Copper in thin plates, and then put fresh charcoal-dust over all: cover the crucible; set it in a melting furnace; put coals all round it, and let them kindle gradually. Raise the fire so as to make the crucible very red-hot. When you observe that the flame hath acquired a purple or bluish-green colour, uncover the crucible, and dip into it an iron wire, to examine whether or no the copper be in fusion under the charcoal-dust. If you find it is, moderate the force of the fire a little, and let your crucible remain in the furnace for a few minutes. Then take it out and let it cool: you will find your Copper of a gold colour, increased in weight a fourth, or perhaps a third part, and yet very malleable.
OBSERVATIONS.
The Lapis Calaminaris is not the only substance with which Copper may be converted into brass: all other ores containing Zinc, the Furnace-Calamine that sublimes where such ores are worked, Tutty, Zinc in substance, may be substituted for it, and, like it, will make very fine Brass; but, in order to succeed, sundry precautions are necessary which we shall now lay before you.
This process is a sort of cementation: for the Calamine doth not melt; only the Zinc is converted into vapours, and then combines with the Copper. On this the success of the operation partly depends, as it is the means of the Copper's preserving its purity and malleability; because the other metallic substances that may be united with the ore of Zinc, or with the Zinc itself, not having the same volatility, cannot be reduced to vapours. If you are apprised that the Calamine, or other ore of Zinc used on this occasion, is contaminated with a mixture of any other metallic matter, you must mingle luting earth with the charcoal-dust and the matter containing the Zinc; make it into stiff paste with water; of this make a bed at the bottom of your crucible, and ram it hard down; lay the Copper plates thereon, cover them with charcoal-dust, and then proceed as before. By this means when the Copper melts it cannot fall to the bottom of the crucible, nor mix with the ore; but is borne up by the mixture, and cannot combine with any thing but the Zinc, that rises in vapours, and, passing through the lute, fixes in the Copper.
Lapis Calaminaris, or other ore of Zinc, may also be purified before it be used for making Brass; especially if adulterated with Lead ore, which is often the case. For this purpose the ore must be roasted in a fire strong enough to give a small degree of fusion to the leaden matter; which will thereby be reduced into larger, heavier, and tougher masses. The most subtile particles are dissipated in the torrefaction, together with some of the Calamine. The Calamine, on the contrary, is by roasting made more tender, lighter, and much more friable. When it is in this condition, put it into a washing tray or van; dip the tray in a vessel full of water, and bruise the matter it contains. The water will carry off the lightest powder, which is the Calamine, and leave nothing at the bottom of the tray but the heaviest substance; that is the leaden matter, which is to be[Pg 363] rejected as useless. The powder of the Calamine will settle at the bottom of the vessel, where, after pouring off the water, it may be found, and used as above directed.
In this operation the charcoal-dust serves to prevent both the Copper and the Zinc from being calcined: and for this reason, when you work on a great quantity of materials at once, it is not necessary to use so much charcoal-dust, in proportion, as when you work but on a small quantity; because, the greater the mass of metal, the less easily will it calcine.
Though the Copper melts in this operation, yet it is far from being necessary to apply such a strong fire as Copper usually requires to melt it: for the accession of the Zinc, on this occasion, communicates to it a great deal of fusibility. The increase of its weight is also owing to the quantity of Zinc combined with it. Copper acquires still another advantage by its association with this semi-metal; for it remains longer in the fire without calcining.
Brass well prepared ought to be malleable when cold. But in whatever manner it be made, and whatever proportion of Zinc there be in it, it is constantly found quite unmalleable when red-hot.
Brass melted in a crucible, with a fierce heat, takes fire almost like Zinc, and from its surface many white flowers ascend, dancing about in flakes like the flowers of Zinc. They are indeed the flowers of Zinc, and the flame of Brass urged by a strong fire is no other than the flame of the Zinc that is united with the Copper, and at that time burns. If Brass be thus kept long in fusion it will lose almost all the Zinc it contains. It will also lose much of its weight, and its colour will be nearly that of Copper. It is therefore necessary, towards performing this operation aright, to seize the moment when the Copper is sufficiently impregnated with Zinc, when it hath acquired the most weight and the finest colour, with the least detriment to its ductility, that is possible, and that instant to put out the fire; because, if the Copper be left longer in fusion, it will only lose the Zinc already united with it. Skill acquired by much practice, and an acquaintance with the particular Calamine employed, are necessary to guide the artist surely through this operation; for there are very considerable differences between the sundry ores of Zinc. Some of them contain Lead, as was said above, and in others there is Iron. When these heterogeneous metals come to be mixed with the Copper, they do indeed augment its weight, but they render it at the same[Pg 364] time pale, and make it very harsh. Some Calamines require to be roasted before they can be used for this purpose, and in the torrefaction emit vapours of a Volatile Alkali, succeeded by vapours of a Sulphureous Spirit: others exhale no vapours while roasting, and may be employed without any antecedent preparation. These different qualities must evidently produce great differences in the operation.
Brass may also be made as Prince's metal and other imitations of Gold are actually made, by using Zinc in substance, instead of the ores that contain it. But these compositions have not, when cold, the ductility of Brass prepared with Lapis Calaminaris, because Zinc is seldom pure, or free from a mixture of Lead. Perhaps also the different manner in which the Zinc unites with the Copper may contribute to this variation.
To obviate this inconvenience, the Zinc must be refined from all alloy of Lead. The property of being indissoluble by Sulphur, which this semi-metal possesses, points out a very practicable method of doing it. The Zinc must be melted in a crucible, and stirred briskly with a strong iron wire, while tallow and mineral Sulphur are alternately projected upon it; but so that the quantity of Sulphur may greatly exceed that of the tallow. If the Sulphur do not burn entirely away, but form a kind of scoria on the surface of the Zinc, it is a sign that your semi-metal contains Lead. In this case you must continue throwing in more Sulphur, and keep stirring the Zinc incessantly, till you perceive that the Sulphur ceases to unite any more with a metallic substance, but burns freely on the surface of the Zinc. The semi-metal is then refined, because the Sulphur, which cannot dissolve it, unites very readily with the Lead, or other metallic substance, contained in it.
If Zinc thus refined be mixed with pure Copper, in the proportion of a fourth or a third part, and the mixture be kept in fusion and constantly stirring for some time, the Brass produced will be as ductile, when cold, as that made by cementation with the Lapis Calaminaris.
With regard to Prince's metal, and other imitations of Gold, they are made either with Copper or Brass re-combined with more Zinc. As it is necessary, for giving them a fine golden colour, to mix with them other proportions of Zinc than that acquired to make Brass only, they are generally much less ductile. In 1725, M. Geoffroy gave a Memoir on this subject in which he examined the effects of[Pg 365] incorporating both Copper and Brass with Zinc, from a small to a very large quantity.
Zinc dissolved in the Mineral Acids.
Weaken concentrated Oil of Vitriol by mixing with it an equal quantity of water. Into a matrass put the Zinc you intend to dissolve, first broken to small pieces. Pour on it six times its weight of the Vitriolic Acid, lowered as above directed, and set the matrass in a sand-bath gently heated. The Zinc will dissolve entirely, without any sediment. The Neutral Metallic Salt resulting from this dissolution shoots into crystals, which go by the name of White Vitriol, or Vitriol of Zinc.
OBSERVATIONS.
Though Zinc be soluble in all the Acids, and when combined with those Acids exhibits some uncommon phenomena, yet M. Hellot is the first that ever gave a particular account of what happens in those dissolutions: so that all we have to say on this head is extracted from that Gentleman's Memoirs. If a solution of Zinc in the Vitriolic Acid, prepared according to the directions in the process, be distilled from a retort placed in a sand-bath with a graduated heat, almost half the liquor presently comes over in pure phlegm. A small quantity of a Sulphureous Acid Spirit rises next. A greater force of fire is now requisite: the retort must therefore be removed into a reverberatory, and the distillation continued with a naked fire. On the first impression of this heat an odour of Liver of Sulphur discovers itself, which becomes sharp and suffocating towards the end of the distillation. In two hours time white vapours begin to appear, as in the rectification of common Oil of Vitriol. If the receiver be then shifted, you will obtain an Oil of Vitriol, in quantity about the eighteenth part of the whole used in the distillation, which, though sulphureous, is yet so concentrated, that, if a few drops thereof be poured into a weak Oil of Vitriol, they fall to the bottom with as much noise as if they were so many bits of red-hot iron, and heat this Oil of Vitriol as much as common Oil of Vitriol heats water.
At the bottom of the retort there remains a dry, white, crystalline, saline mass, exceeding in weight the Zinc that was dissolved, about a twelfth part of the whole weight of the liquor. The increase of its weight is owing to a portion of the Vitriolic Acid that remains concentrated in the Zinc, and could not be expelled by the fire. This portion of Acid adheres to it most tenaciously: for, though M. Hellot kept the retort containing it during two whole hours in so violent a fire that the vessel began to melt, the smallest vapour did not rise from it.
This saline caput mortuum is in the form of needles, much like the Sedative Salt. It is caustic, grows considerably hot when water is poured on it, and gives in the air, but slowly. Spirit of wine, digested with this Salt for eight or ten days, acquires the same smell as that which is mixed with concentrated Oil of Vitriol in preparing Æther.
Zinc is dissolved by the Nitrous and Marine Acids, much in the same manner as by the Vitriolic; except that the Marine Acid does not touch a black, spungy, rarefied matter, which it separates from the Zinc. M. Hellot found upon trial that this matter is not Mercury, and that it cannot be reduced to a metallic substance.
That ingenious Chymist distilled likewise Solutions of Zinc in the Nitrous and Marine Acids. There came over at first, as there did from the solution made by the Vitriolic Acid, an aqueous, and then an acidulated liquor. At last, by exciting the fire with great violence, towards the end of the distillation, he obtained a small quantity of the Acid that hath been employed in the dissolution: but the small portion of Acid thus obtained was exceeding strong; and the quantity of the Nitrous much more considerable than that of the Marine Acid.
A solution of Zinc in the Marine Acid, being distilled to dryness, yields a Sublimate on applying a violent heat to it.
All the Acids dissolve with ease, not only Zinc, but its Flowers also; and that nearly in the same quantity, and with almost all the same phenomena. M. Hellot, observing that the residues of most of the solutions of Zinc have a great resemblance with its flowers, is of opinion that this semi-metal may be reduced, by the means of solvents, to the same state into which it is brought by the fire when sublimed in Flowers.
Of Arsenic.
To extract Arsenic from its Matrices. Zaffre or Smalt.
Powder some Cobalt, white Pyrites, or other Arsenical matters. Put this powder into a retort with a short wide neck, leaving a full third thereof empty. Set your retort in a reverberating furnace; lute on a receiver; heat your vessel by degrees, and increase the fire till you see a powder sublime into the neck of the retort. Keep up the fire in this degree as long as the sublimation continues: when this begins to slacken, raise your fire, and make it as strong as the vessels will bear. When nothing more ascends, let it go out. On unluting the vessels, you will find in the receiver a little Arsenic in the form of a fine light farina. The neck of the retort will be full of white flowers, not quite so fine, some of which will appear like little crystals; and if a good deal of Arsenic be sublimed, a ponderous matter, like a white, semi-transparent glass, will be found adhering to that part of the neck of the retort which is next its body.
OBSERVATIONS.
Arsenic is a metallic substance still more volatile than Zinc; so that it cannot be separated from the matters with which it is mixed otherwise than by sublimation. It is proper, however, to take notice, that it is not naturally in a metallic form, and that, properly speaking, the whole Sublimate obtained from Cobalt, as above directed, is nothing but a metallic calx, that cannot be brought to the form and gloss of a metal, till it be worked up with fatty matters, as we shall shew in its place.
This calx is of a very singular nature, and differs from every other metallic calx, in that this is volatile, and all the rest extremely fixed; even those procured from the semi-metals: for the Flowers of Zinc, which are justly considered as a calcined Zinc, though obtained by a sort of sublimation,[Pg 368] are not for all that of a volatile nature, but rather exceedingly fixed; seeing they are capable of sustaining the most violent fire, and melt instead of subliming. Arsenic, on the contrary, is not only extracted from its ore by sublimation, but when once sublimed continues to be volatile, and flies off in vapours as soon as it is exposed even to a moderate degree of heat.
This metallic matter, before it is combined with the phlogiston, is called White Arsenic, or plain Arsenic: it acquires the title of Regulus of Arsenic when it is united with the phlogiston, and glitters like a metal.
Though Arsenic be volatile, yet it requires a pretty strong fire to separate it from the minerals containing it, especially in close vessels; because it adheres very close to earthy and vitrifiable matters. This adhesion is so firm, that, when thus combined, it is capable of bearing a melting heat, and vitrifies with metallic calces, and other fusible matters. On this account it is impossible to extract from Cobalt, or other Arsenical matters, all the Arsenic they contain by working them only in close vessels. If such matters are to be freed from all their Arsenic, you must, after you have extracted all they will yield by distillation, put them into a crucible, and set it uncovered in the midst of a strong fire. Many Arsenical vapours will still rise; and care must be taken to stir the contents of the crucible frequently with an iron rod, to facilitate the discharge of the remaining Arsenic.
It often happens that the Arsenic, obtained from minerals by sublimation, is not very white, but of a lighter or darker grey colour. This is owing to some particles of inflammable matter, from which Arsenical minerals are seldom quite free. A very small quantity of phlogiston is sufficient to deprive much Arsenic of its whiteness, and to give it a grey colour. But when fouled in this manner, it may easily be brought to its due degree of whiteness: it need only be sublimed once more, after mixing it with some substance on which it doth not act; Sea-salt, for instance. If the matters from which Arsenic is extracted contain Sulphur also, as some pyrites do, the Arsenic sublimes with much less heat, than when it is united with earthy matters only; because it combines with the Sulphur, wherewith it hath a great affinity, and the Sulphur serves to separate the Arsenic, by this interposition, from the earth. In consequence hereof, Sulphur may be employed to extract Arsenic out of the earths in which it is fixed. In this case, the Sulphur changes the colour of the Arsenic, which it makes of a lighter or deeper yellow, or even red, in pro[Pg 369]portion to the quantity there is of it, and to the degree of fire that hath acted on both together.
The consistence of Arsenic is different, according to the degree of heat applied in subliming it. If the Arsenical vapour meet with a cold place, it gathers there in the form of a powder, as the Flowers of Sulphur do: this is the case with that which falls into the receiver in distilling it. But if it be stopped in a hot place, and cannot escape from that heat, it condenses into a heavy, compact, semi-transparent body, having undergone the first degree of fusion.
Yet it cannot be perfectly melted, so as to flow like other fused matters: not that it is refractory; for, on the contrary, the degree of heat in which it begins to melt is very moderate, and it is in its own nature very fit to promote the fusion of refractory matters: but the reason is this; it is necessarily converted into vapours by the degree of heat necessary to fuse it, and these vapours burst the vessels, if they find no vent.
Arsenic made yellow by a mixture of Sulphur, which is also called Orpiment, is reducible to the form of a solid Sublimate with more ease; because it is alloyed with a twentieth, or perhaps a tenth part, of its weight of Sulphur, which renders it more fusible.
Red Arsenic, which contains still more Sulphur, melts also more easily. It then becomes of a transparent red, like a ruby: and hence, when it is in this form, it is called Ruby of Arsenic.
When a combination of Sulphur and Arsenic is wanted, it is better to mingle and distil together such minerals as contain Sulphur and Arsenic, the white and the yellow pyrites, for instance, than to mingle pure Arsenic with pure Sulphur: for the great volatility of these two substances is a hindrance to their uniting; whereas, when combined with other matters, they are capable of sustaining a much greater degree of heat, which favours and promotes their union.
Those who work by the grate do not extract Arsenic out of Cobalt by distillation: they throw the ore mixed promiscuously with wood and charcoal into a great furnace, from whence a flue carries the vapours into a long winding passage, across which beams of wood are fixed at proper distances from each other. The Arsenical vapours being conducted into this passage, adhere both to the sides thereof and to the joists that lye across it. The fuliginous parts of the combustible matters being lighter ascend higher, and go out through a chimney at the farther end of this passage.
The Arsenic sublimed by this method is not white, but of a grey colour; owing to the inflammable matter of the wood and charcoal with which the ore is torrefied.
When all the Arsenic the Cobalt will yield is thus separated, the earthy fixed matter left behind is mixed with divers fusible matters and vitrified, and produces a glass of a beautiful blue colour. It is called Smalt. This glass is to be prepared in the following manner.
Take four parts of fine fusible sand, an equal quantity of any Fixed Alkali perfectly depurated, and one part of Cobalt from which the Arsenic hath been sublimed by torrefaction. Pulverize these different substances very finely, and mix them thoroughly together; put the mixture into a good crucible, cover it, and set it in a melting furnace. Make a strong fire, and keep it up constantly in the same degree for some hours. Then dip an iron wire into the crucible; to the end of which a glassy matter will stick, in the form of threads, if the fusion and vitrification be perfect. In this case take the crucible out of the fire; cool it by throwing water on it, and then break it. You will find in it a glass, which will be of an exceeding deep blue, and almost black, if the operation hath succeeded. This glass, when reduced to a fine powder, acquires a much brighter and more lively blue colour.
If you find after the operation that the glass hath too little colour, the fusion must be repeated a second time, with twice or thrice the quantity of Cobalt. If, on the contrary, the glass be too dark, less Cobalt must be used.
Instead of the mixture here prescribed you may employ a ready-made glass, providing it be white and fusible. But as glass is always hard to melt, and as the mixing Cobalt with it renders it still more refractory, therefore though an Alkaline Salt be one of the ingredients in its composition, it is proper to promote the fusion, by mixing therewith calcined wine-lees, in the quantity of one third part of the weight of the Cobalt.
In order to make the assay of a particular Cobalt, with a view to know what quantity of blue glass it will yield, it is necessary to perform the operation in the manner here set down; a great deal of time and trouble may be saved by melting one part of Cobalt with two or three parts of Borax. This Salt is very fusible, and turns, when melted, into a substance which, for a time, possesses all the properties of glass. In this trial the glass of Borax will be nearly of the same colour as the true glass, or Smalt, made with the same Cobalt.
The ores of Bismuth, as well as Cobalt, yield a matter that colours glass blue; nay, the Smalt made with these ores is more beautiful than that procured from the ore of pure Arsenic. Some Cobalts yield both Arsenic and Bismuth. When such Cobalts are used, it is common to find at the bottom of the crucible a little button of metallic matter, which is called Regulus of Cobalt. This Regulus is a sort of Bismuth, generally adulterated with a mixture of ferruginous and arsenical parts.
The heaviest and most fixed Flowers of Arsenic, procured from Cobalt, have likewise the property of giving a blue colour to glass. But this colour is faint: it is owing to a portion of the colouring matter carried up along with the Arsenic. These Flowers may be made an ingredient in the composition of blue glass, not only because of the colouring principle they contain, but also because they greatly promote fusion; Arsenic being one of the most efficacious fluxes known.
In short, all those blue glasses, or Smalts, contain a certain quantity of Arsenic; for a portion of this semi-metal always remains united with the fixed matter of the Cobalt, though roasted for a long time, and in a very hot fire. The portion of Arsenic that is thus fixed vitrifies with the colouring matter, and enters into the composition of the Smalt.
The blue glass made with the fixed part of Cobalt hath several names, according to the condition in which it is. When it hath undergone the first imperfect degree of fusion only it is called Zaffre. It takes the name of Smalt when perfectly vitrified: and this again being pulverized is called Powder-blue, or, if finely levigated, Blue Enamel; because it is used in enamelling, as well as in painting earthen ware and porcelain.
To separate Arsenic from Sulphur.
Powder the yellow or red Arsenic which you intend to separate from its Sulphur. Moisten this powder with a Fixed Alkali resolved into a liquor. Dry the mixture gently; put it into a very tall glass cucurbit, and fit on a blind-head. Set this cucurbit in a sand-bath; warm the vessels gently, and increase the fire by degrees, till you perceive that no more Arsenic sublimes. The Arsenic, which before[Pg 372] was yellow or red, rises into the head partly in white flowers, and partly in a compact, white, semi-transparent matter, which looks as if it were vitrified. The Sulphur combined with the Fixed Alkali remains at the bottom of the cucurbit.
OBSERVATIONS.
A Fixed Alkali hath more affinity than any metallic substance with Sulphur: so that it is not surprising Sulphur should be separated from Arsenic by its interposition. Yet there is an inconvenience attends the use of it: for it hath a great affinity with the Arsenic also, and so always retains some part thereof, which continues fixed with it. For this reason care should be taken not to mix, with sulphurated Arsenic, a greater quantity of Alkali than is necessary to absorb the Sulphur it contains. Nothing, however, but experience and repeated trials can teach us the exact quantity of Alkali that ought to be employed; because the quantity of Sulphur that may be contained in yellow or red Arsenic is indefinite.
The vessel ought to be tall, that the upper part of the head, where the Arsenical particles condense, may be the less exposed to heat. Towards the end of the operation the fire must be strongly excited, so as to make the sand red-hot; because the last portions of Arsenic that rise are strongly retained by the Fixed Alkali.
Arsenic that is grey or blackish may be depurated and whitened by the same means; because a Fixed Alkali absorbs the phlogiston likewise with great avidity. Mercury, as well as a Fixed Alkali, is an excellent additament for separating Arsenic from Sulphur. If you will use it for that purpose, reduce the sulphurated Arsenic to a very fine powder, by rubbing it a long time in a glass mortar; when it is well pulverized, let a few drops of Mercury fall upon it, by squeezing it through chamoy, and continue the trituration. The yellow or red colour of the Arsenic will insensibly change, and gradually grow darker as the Mercury incorporates with it. When the Mercury is perfectly killed, add a little more of it than you did the first time, and in the same manner: continue to triturate till it disappear; and thus go on adding more and more till the Mercury you add remain quick, and you can kill no more of it. Neither the red nor the yellow colour will then appear in the mixture; which will be grey, if it contain but a little Sulphur, and black, if a great deal.
Put this mixture into a very tall glass cucurbit; fit on a blind-head; set it in a sand-bath, and bury it in the sand as far as the contained mixture reaches. Heat the vessels, and, during the whole operation, keep up a degree of fire a little weaker than that required for subliming Cinabar. White Arsenical Flowers will adhere to the upper part of the head, amongst which will be some beautiful crystals of Arsenic; and underneath them you will find some Cinabar sublimed, but not entirely free from Arsenic. If you desire to have your Cinabar and your Arsenic purer, and more unmixed with each other, separate the upper sublimate, which is Arsenical, from the lower, which consists chiefly of Cinabar. Powder each of them coarsely, and sublime them separately each in a different cucurbit.
On this occasion the Mercury separates the Sulphur from the Arsenic, because it hath a greater affinity than Arsenic with that mineral. It is not the only metallic substance of this character: for, as hath been shewn, there are several others that have a greater affinity than Mercury with Sulphur, being able to decompose Cinabar by their interposition. Yet those metallic substances must not be substituted for Mercury in the present operation: because there is none of them but hath at the same time a very great affinity with Arsenic, or even as strong an one as they have with Sulphur; whereas Mercury will by no means unite with Arsenic.
This method of separating Arsenic from Sulphur hath two advantages over that in which a Fixed Alkali is the medium. The first is, that by this means all the Arsenic contained in the mixture is extracted out of it; and the second, that, as Mercury doth not absorb Arsenic, we are not put to the trouble of groping out, as it were, by trials the quantity necessary to be added; and that, though more be added than is necessary to absorb all the Sulphur, it will be of no prejudice to the operation. But then it is attended with the inconvenience of being much more tedious and more laborious than the other. For, in the first place, it requires previously a very tiresome trituration, in order to procure an union between the Sulphur and the Mercury, and so to form an Æthiops; without which the Mercury and the sulphurated Arsenic will sublime separately, so that no decomposition will be effected. Secondly, though the Mercury be sufficiently united with the Sulphur of the Arsenic by the long trituration that precedes the sublimation, this doth not prevent, as we took notice above, the sublimed Arsenic and[Pg 374] Cinabar from being in some measure blended together; so that each requires a second separate sublimation to render it very pure.
These inconveniencies cause a Fixed Alkali to be used preferably to Mercury; the loss of a small quantity of the Arsenic, which remains united with the Alkali, being little regarded; as that metallic substance is neither scarce nor precious.
When Arsenic is united with a great quantity of Sulphur, it may be freed from a part thereof without the intervention of any third body: it is sufficient for the purpose to sublime it with a very gentle fire, increased by insensible degrees. The most sulphureous part ascends first; what rises afterwards is more Arsenical, and less sulphureous; and the last flowers of all are pure Arsenic, or at least nearly so.
To give Arsenic the Metalline Form. Regulus of Arsenic.
Take two parts of white Arsenic in fine powder, one part of the black flux, half a part of Borax, and as much clean iron filings. Rub the whole together, in order to mix them thoroughly. Put this mixture into a good crucible, and over it put Sea-salt three fingers thick. Cover the crucible; set it in a melting furnace; and begin with a gentle fire to heat the crucible equally.
When arsenical vapours begin to ascend from the crucible, raise the fire immediately so as to melt the mixture. Examine whether or no the matter be thoroughly melted, by introducing an iron wire into the crucible; and if the fusion be perfect, take the crucible out of the furnace. Let it cool; break it; and you will find in it a Regulus of a white and livid metallic colour, very brittle, scarcely hard, but rather friable.
OBSERVATIONS.
White Arsenic is, as hath been said, a metallic calx; and consequently wants no more, in order to its acquiring the metalline properties, than to be combined with the phlogiston: this is effected by the operation before us.
The Iron added doth not serve here, as in making the Regulus of Antimony, to precipitate the Regulus of Arsenic,[Pg 375] by separating it from some other substance with which it was united: on this occasion it does nothing but join the Regulus of Arsenic, to which it gives solidity and consistence. This is the only reason of its being made an ingredient in the mixture; as the Regulus of Arsenic, without it, would have such a tender consistence, that it could scarce be handled without falling asunder into little bits. The Iron procures a further advantage in this process; which is, that it prevents a great quantity of Arsenic from being lost in vapours: for the Arsenic, with which it combines, is restrained, and, in some measure, fixed by it.
Copper may be substituted for Iron, and procures the same advantages.
It is very necessary to remove the crucible from the fire as soon as the matter is melted, and indeed to cool it as expeditiously as possible, to prevent the Arsenic from flying off in vapours: for, when once the Regulus is formed, the proportion of Arsenic, with respect to that of the metal mixed with it, is continually lessening while it stays in the fire; so that, after some time, there will be left in the crucible, not a Regulus of Arsenic, but only Iron or Copper, alloyed with a little Arsenic. On this occasion the Copper turns white, and assumes the colour of Silver; but it soon tarnishes in the air.
It is easy to perceive, by what hath been said, that the Regulus of Arsenic made according to this process is never pure, but contains always a considerable quantity of Iron or Copper, whatever precautions be used: but it is difficult to avoid this inconvenience, for the reasons above assigned; and if we attempt to fuse Arsenic alone, with reducing fluxes, the greatest part thereof is dissipated in vapours, long before the very flux begins to melt: and that part of it, which is found metallized, is not collected in one mass at the bottom of the crucible, as in other metallic reductions; but in small particles, dispersed and mixed among the scoriæ. There are nevertheless several expedients for obtaining a Regulus of Arsenic absolutely pure, and unalloyed with any metallic substance.
First: into a little low cucurbit, covered with a blind-head, put Regulus of Arsenic made with Iron or Copper; set this cucurbit in a sand-bath; heat it till the sand begins to grow red, and you will see part of the Regulus sublime into the head, still retaining its metalline splendour. The portion of Regulus thus sublimed is pure Arsenic, or at least contains but a very small portion of the adventitious metal,[Pg 376] which may have been carried up with it. What is left in the bottom of the cucurbit is the metal that was added, still containing a little Arsenic, which continues obstinately fixed with it, and which the violence of fire is unable to force away from it in close vessels.
Secondly: mix your Arsenic in equal parts with the black flux; put the mixture into such a cucurbit as that last mentioned; and apply to it the strongest degree of heat that can be procured by a sand-bath; arsenical flowers, of a blackish grey colour, will first sublime into the head, and after them a Regulus of Arsenic of a white metalline colour, which is pretty glossy, but tarnishes very soon in the air. This Regulus hath no solidity: it is exceedingly friable; but it is pure.
Thirdly: I have also made a Regulus of pure Arsenic by another method, which produces a much greater quantity thereof, with a much smaller degree of heat. For this purpose I powder the Arsenic, and mix with it any Fat Oil; so that the mixture may be like a liquid paste: this paste I put into a little phial of thin glass, like one of those used by apothecaries; I set this phial in a sand-bath, and gradually heat it, till the bottom of the pot containing the sand begin to be red. Part of the Oil first rises out of the phial in vapours, which must be suffered to pass off. After this the upper part of the phial is gradually lined, on the inside, with a glittering metallic crust, which makes it look like a quick-silvered glass. This crust is the Regulus of Arsenic. When it begins to sublime, the mouth of the phial must be slightly stopped with a bit of paper, and the heat increased a little, till you see that nothing more rises.
If you break the bottle after the operation, you will find its upper part crusted over with a coat of Regulus, thicker or thinner in proportion to the quantity of Arsenic employed. The Regulus is in a mass, of a beautiful brilliant colour, which to me seems to stand the air better than that of any Regulus made by other methods; probably because of the great quantity of fat matter with which it is united, and by which it is defended.
This Regulus of Arsenic is absolutely pure, and a much greater quantity thereof is obtained, by this method, than by treating it with the black flux; because the Arsenic is much sooner and more easily combined with the inflammable matter: and hence it comes to pass that part of the Arsenic doth not rise at first in grey flowers, as in operating with the black flux. Moreover, by our process, all the Arsenic is[Pg 377] sublimed in Regulus: whereas, when the black flux is employed, a pretty considerable part of the Arsenic unites with the alkaline part of the flux, and remains fixed therewith. In our operation there is nothing left at the bottom of the phial, except an oily, light, but very fixed coal.
Regulus of Arsenic, in whatever manner made, may be easily reduced into white, crystalline Arsenic, by the means of a Fixed Alkali, or of Mercury, applied in the same manner as for separating Arsenic from Sulphur.
To distil the Nitrous Acid by the interposition of Arsenic. Blue Aqua Fortis. A new Neutral Salt of Arsenic.
Pulverize finely any quantity you please of refined Salt-petre. Mix it accurately with an equal weight of white crystalline Arsenic, well pulverized, or else with very white and very fine flowers of Arsenic. Put this mixture into a glass retort, leaving one half of it empty. Set your retort in a reverberating furnace; apply a receiver having a small hole drilled in it, and containing a little filtered rain-water; lute the receiver to the retort with stiff lute. Begin with putting two or three small live coals in the ash-hole of the furnace, and replace them with others when they are ready to go out. Go on thus warming your vessels by insensible degrees, and put no coals in the fire-place, till the retort begin to be very warm. You will soon see the receiver filled with vapours of a dark-red, inclining to a russet colour. With a bit of lute stop the little hole of the receiver. The vapours will be condensed in the water of this vessel, and give it a very fine blue colour, that will grow deeper and deeper as the distillation advances. If your Salt-petre was not very dry, some drops of Acid will also come over, and falling from the nose of the retort mix with the water in the receiver. Continue your distillation, increasing the fire little by little as it advances, but exceeding slowly, till you see that when the retort is red-hot nothing more comes off; and then let your vessels cool.
When the vessels are cold, unlute the receiver, and, as expeditiously as you can, pour the blue aqua fortis it contains into a crystal bottle; which you must seal hermetically, because this colour disappears in a short time when the liquor takes air. You will find in the retort a white saline mass[Pg 378] moulded in its bottom, and some flowers of Arsenic sublimed to its upper cavity, and into its neck.
Pulverize the saline mass, and dissolve it in warm water. Filter the solution, in order to separate some arsenical parts that will be left on the filter. Let the filtered liquor evaporate of itself in the open air; when it is sufficiently evaporated, crystals will shoot in it representing quadrangular prisms, terminated at each extremity by pyramids, that are also quadrangular. These crystals will be in confused heaps at the bottom of the vessel: over them will be other crystals in the form of needles; a saline vegetation creeping along the sides of the vessel; and the surface of the liquor will be obscured by a thin dusty pellicle.
OBSERVATIONS.
Arsenic, as we took notice in our Elements of the Theory, besides the properties it hath in common with metallic substances, possesses others also in common with saline substances. One of the most remarkable among the latter is that of decomposing Nitre; of expelling the Acid of that Salt from its Alkaline basis, assuming its place, and forming with that Alkali a Neutral Salt, which is very soluble in water, and shoots into regular crystals.
To inquire into what passes in the decomposition of Nitre by Arsenic, and into the new Salt resulting from thence, was the design of the first Memoir given in by me to the Academy of Sciences on this subject, and from that the present process is copied. Though the whole quantity of Arsenic prescribed in the process doth not enter into the composition of the new Neutral Salt, seeing some of it sublimes in flowers, that quantity must not therefore be thought too great: for we see, on the other hand, that part of the Nitre is not decomposed. The needle-like Salt is no other than Nitre that hath not suffered any decomposition, and actually deflagrates on live coals like common Nitre.
The precaution of putting some water in the receiver is absolutely necessary, to condense the nitrous vapours that rise in the distillation: for they are so elastic, so volatile, so dephlegmated, that a very small part of them will otherwise be condensed into a liquor, while the rest will remain in the form of vapours, to which vent must be given through the small hole in the receiver, as without that they will burst the vessels with impetuosity: and consequently scarce any[Pg 379] Acid will be obtained; especially if the Nitre employed be very dry, as it must be to be reducible into a fine powder.
The blue colour communicated by the Nitrous Acid to the water is very remarkable. The cause that produces this colour is not yet known.
Though the Acid is, on this occasion, mortified by a great quantity of water, yet, when it rises out of the retort, it is so concentrated as to form, even with that water, if too much be not put in, a most active and even smoking aqua fortis.
It is necessary in this operation, and more so than in any other, to warm the vessels gradually, and to proceed exceeding slowly; otherwise the artist runs the risque of seeing his vessels burst to pieces with violence, and with great danger to his person: for Arsenic acts on Nitre with incredible vivacity; insomuch that, if a mixture of Nitre and Arsenic be heated to a certain degree, the Nitre is decomposed almost as rapidly, and with as great an explosion, as when it is made to fulminate with an inflammable matter. In short, the appearances are such, that one would be almost induced to think the Nitre really takes fire on this occasion: though it be only decomposed just as it is by the Vitriolic Acid.
The solution of the caput mortuum of this distillation contains, at the same time, several sorts of Salts: to wit, 1. the Neutral Salt of Arsenic, formed by the union of the Arsenic with the basis of the Nitre; this shoots into the prismatic crystals above-mentioned: 2. some Nitre that hath not been decomposed; this forms the needles and part of the vegetations: 3. a small portion of Arsenic, that is known to be soluble in water; this forms the thin dark pellicle that covers the surface of the liquor when it begins to evaporate.
For the properties of this new Neutral Salt of Arsenic you may consult what we have said thereupon in our Elements of the Theory, and in the Memoirs of the Academy of Sciences.
To alkalizate Nitre by Arsenic.
Melt in a crucible the Nitre you intend to alkalizate. When it is melted, and moderately red, project upon it two or three pinches of pulverized Arsenic. A consider[Pg 380]able effervescence and ebullition will immediately be produced in the crucible, attended with a noise like that which Nitre makes, when it detonates with an inflammable matter. At the same time a thick smoke will rise, which at first will smell like garlic, the odour peculiar to Arsenic; it will also smell afterwards like Spirit of Nitre. When the effervescence in the crucible is over, throw again upon the Nitre as much pulverized Arsenic as you did the first time; and all the same phenomena will be repeated. Continue thus throwing in Arsenic in small parcels, till it produce no more effervescence; taking care to stir the matter at every projection with an iron wire, the better to mix the whole together. Then increase your fire, and melt what remains. Keep it thus in fusion for a quarter of an hour, and then take the crucible out of the fire. It will contain a Nitre alkalizated by Arsenic.
OBSERVATIONS.
This operation, as well as the preceding one, is a decomposition of Nitre by Arsenic; yet the result is very different: for, instead of a Salt capable of crystallizing, and discovering no tokens either of Acid or Alkali, we obtain, on this occasion, only a Salt that runs into a liquor by the moisture of the air, doth not crystallize, and hath all the properties of an Alkali.
These differences arise only from the different manner in which the decomposition of the Nitre, and the union of the Arsenic with the basis of that Salt, is brought about. When the Nitrous Acid is distilled by the interposition of Arsenic, with a view to obtain the Arsenical Salt, the operation must be performed in close vessels; no greater degree of heat must be applied to the mixture than is necessary for enabling the Arsenic to act; and that heat must be administered very slowly and by insensible degrees. But, when the business is to alkalizate Nitre by the means of Arsenic, the operation is performed in a crucible, in a naked fire, with a strong degree of heat, and that suddenly applied. The violence of the heat, the suddenness with which it is applied, the vivacity wherewith the Arsenic unites with the basis of the Nitre; and, still more than all these, the free access of the air, occasion the greatest part of the Arsenic, which at first combines with the basis of the Nitre after having expelled its Acid, to be presently carried off and dissipated in vapours;[Pg 381] and consequently the basis of the Nitre, not being sufficiently saturated, discovers its Alkaline properties.
I say, the concurrence of the air contributes, still more than all the rest, to separate the Arsenic from the Alkaline basis of the Nitre; experience having taught me that the Neutral Salt of Arsenic is not to be alkalizated by the most violent force of heat, as long as it continues in close vessels, and the external air hath no communication with it; but that some of the Arsenic contained in that Salt is dissipated, by exposing it to a strong heat in open vessels.
The tumult and effervescence that arise, when Arsenic is projected on Nitre fused in a crucible, are so considerable, and so nearly resemble the detonation of Nitre with an inflammable matter, that we should be tempted to think, if we trusted appearances only, that Arsenic furnishes a combustible matter, and that the Alkalization of the Nitre is effected, on this occasion, in the same manner as when it is fixed by charcoal: but, by examining attentively what passes, we easily discover that there is no inflammation at all, and that the Nitre is alkalizated in the manner and by the means above pointed out.
The first vapours that rise, when Arsenic is projected on Nitre, are purely arsenical; and, if any cold body be put in their way, they adhere to it in the form of flowers. These vapours are actual particles of Arsenic, carried up by the heat before they could come to act on the Nitre; but they are soon after mixed with Nitrous vapours, consisting of the Acid of the Nitre, which the Arsenic expels from its basis as fast as it comes to act on that Salt.
The nearer you come to the end of the operation, the more does the matter in the crucible lose of its fluidity, though an equal fire be constantly kept up in the furnace. At last it becomes quite like a paste, and the fire must be made much stronger to put it again in fusion. The reason of this is, that Nitre when alkalizated is much less fusible than when it is not so. The case is the same when this Salt is alkalizated by deflagration.
Though the Nitre, when alkalizated, makes no more effervescence with Arsenic, and though, when kept in fusion, it emits no more arsenical vapours, it doth not thence follow that it is a pure Alkali, and that it contains no Arsenic: it still contains a large quantity thereof, but so strongly united that the force of fire is not able to separate them; which hath led some authors to give this Salt the title of Fixed Arsenic.
The existence of Arsenic in this saline compound is easily discovered, by fusing it with metallic substances, on which it produces the same effects as Arsenic.
With solutions of metals in the Acids, it also presents almost the same phenomena as the Neutral Salt of Arsenic. Particularly it precipitates Silver dissolved by the Nitrous Acid in a red powder, as that Salt does; and the differences observed between the precipitations made by our new Neutral Salt of Arsenic, and those made by Nitre alkalizated with Arsenic, can be attributed only to the alkaline quality of the latter. See the Memoirs of the Academy for 1746.
Of VEGETABLES.
Operations on unfermented Vegetables.
Of the Substances obtained from Vegetables by Expression only.
To express and depurate the Juice of a Plant, containing its Essential Salt. The Crystallization of that Salt.
Before sun-rise gather a good quantity of the plant from which you design to express the juice, in order to obtain its Salt. Wash it well in running water, to clear it of earth, insects, and other adventitious matters. Bruise it in a marble mortar; put it into a bag of new, strong, thick linen cloth; tye the bag tight, and commit it to a press. By pressing it strongly you will squeeze out a great quantity of green, thick juice, which will have the same taste as the plant. Dilute this juice with six times as much pure rain-water, and filter it repeatedly through a woollen bag, till it pass clear and limpid. Evaporate the filtered juice with a gentle heat, till it be almost as thick as before it was mixed with water. Put this inspissated juice into a jar, or other vessel of earth or glass; on its surface pour olive oil to the depth of a line, and set it in a cellar. Seven or eight[Pg 384] months after this, pour off gently the liquor contained in the vessel, the inside of which you will find covered with a crystallized Salt. Separate the crystals gently; wash them quickly with a little fair cold water, and dry them: this is the Essential Salt of the plant.
OBSERVATIONS.
Every plant is not equally disposed to yield its Essential Salt, by the method here proposed. Succulent vegetables only, whose juices are aqueous and not too viscous, are fit for this purpose. Such, for example, as sorrel, brook-lime, succory, fumitory, water-cresses, plantain, &c. An Essential Salt cannot be procured from those that yield thick, viscid, mucilaginous juices, such as the seeds of flea-wort; unless their juices be previously attenuated by fermentation, and that viscosity destroyed which obstructs the Crystallization of this Salt.
Nor can the Essential Salt be obtained in any quantity from vegetable matters abounding in Oil. Most kernels and seeds are of this sort: they all contain a great quantity of fat oil, which so entangles and clogs this Salt, that the particles thereof cannot shoot away from the tenacious juices into crystals.
The same is to be said of dry aromatic plants; because they contain much essential oil, or resinous matters that produce the same effect. It is true the Essential Salt itself contains a certain portion of oil; for it is no other than the Acid of the plant incorporated and crystallized with part of its oil and of its earth: but then the oil must not be in too great a quantity: because it sheaths the Acid, renders it clammy, as it were, and hinders it from extricating itself, so as to be able to exert its qualities, and appear in the form of Salt.
The plants, from which you intend to extract this Salt, should be gathered in the morning before sun-rise; because they are then most succulent, not being yet dried up or withered by the heat of the sun.
The juice of plants obtained by expression is very thick; because it contains many particles of the bruised plant, that are unavoidably squeezed out along with it. In order to clear it of these superfluous parts, it is proper to filter it; but as that would be difficult, on account of the thickness of the juice, it must be thinned, by diluting it with a quantity of water, sufficient to give it the requisite degree of fluidity.
Instead of thus diluting the expressed juice, the plant may be ground with water, before it is put into the press: it will by this means furnish a more fluid juice, that will easily pass through the filter. This method may be employed with success on dry plants, or such as are not very succulent. For this operation rain-water is to be preferred to any other; because it is the purest: for all waters that have run some time through the earth, or on its surface, are to be suspected of containing some saline or selenetic matter, which would mix with and deprave the Essential Salt.
The juice of the plant, when diluted with the quantity of water sufficient to facilitate its filtration, is too aqueous to let the Salt it contains unite into crystals: it must therefore be evaporated, till it hath recovered a somewhat thicker consistence. The heat applied for that purpose must be gentle; lest the acid and oily parts, that are to form the Salt, be spoiled or dissipated, as they are not very fixed. In summer, the heat of the sun is sufficient to effect this evaporation: but if you make use of this method, the juice to be evaporated must be put into several broad flat pans; that, a larger surface being exposed to the action of the air and sun, the evaporation may be the sooner completed: for if the juice should continue too long in the degree of heat requisite for its evaporation, it might begin to ferment; which would be very detrimental.
The oil poured on the liquor prevents its fermenting, putrefying, or growing mouldy, during the long space of time required for the crystallization of the Essential Salt.
These Salts are excellent medicines, being endued with the same virtues as the plants from which they were obtained.
They cannot be procured from plants by distillation, though they consist in a great measure of volatile principles: nor are they obtainable by any other process that requires much heat; because they are easily decomposed, and the fire changes their natures entirely. The oily Acids extracted from plants by distillation do not crystallize, and always have an empyreumatic acrimony, that makes them very different from the Essential Salts, which are very mild and saponaceous.
To draw the Oils out of Kernels, Seeds, and Fruits, by Expression.
Pound in a marble mortar, or grind in a mill, the kernels, seeds, or fruits, out of which you intend to express the Oil. If your matters be meagre, and grind to meal, suspend that meal in the steam of boiling water, in order to moisten it a little, and then dry it.
Tye up your matter thus prepared in a new, strong, thick, canvass bag, and put it into a press, between two iron plates previously heated in boiling water: squeeze it strongly, and you will see the Oil run in streams into the receiving vessel.
OBSERVATIONS.
The Fat Oil of Plants is particularly found in kernels, seeds, and some fruits; some kernels contain such a vast quantity thereof, that, on being very slightly bruised in a mortar, they discharge it in great abundance. Sweet and bitter Almonds, Walnuts, and Lint-seed, are all of this kind; and require no other management but to be pounded and pressed, to make them yield a great deal of Oil. But there are others more meagre, that being ground produce an almost dry flower. In order to facilitate the expression of the Oil out of such, they must be expressed, when ground, to the steam of boiling water. For this purpose the meal may be put into a fine sieve, and that suspended over a pan half-full of water kept boiling on the fire. The ascending vapours will moisten the flower, render it more unctuous, and facilitate the expression of the Oil.
It is proper to dry it a little before it be put into the press, that it may yield as little water as possible along with the Oil. Nevertheless, so much water happens now and then to be left in it, that some is expressed together with the Oil: but as oil and water do not incorporate, they are easily separated after the operation is finished.
The extraction of the Oil is also greatly facilitated by heating the plates, between which the oleaginous matters are squeezed: but they must not be made too hot, if you mean to have a very mild Oil, designed either for aliment or for medicine; such as the Oil of Olives, and that of sweet al[Pg 387]monds. For this reason the plates must be warmed in boiling water only: if you heat them to a greater degree, you run the risk of giving an acrimony to the Oils you express. But, when these Oils are intended for other uses, the plates may be made hotter, because their heat increases the yield of Oil.
It is remarkable, that all the Oils obtained by expression, with the precautions above recommended, are constantly very mild; even though the matters from which they are extracted be in themselves very acrid. Mustard-seed, which is so acrid that it is even caustic, yields, by expression, an Oil as mild as that of sweet almonds. But then the kernels, seeds, and fruits, from which the Oils are extracted, must not be old; because these Oils, which are perfectly mild when fresh and new, become intolerably acrid when they grow old, and acquire this acrimony even in the fruit itself; for it is observed that these fruits turn rancid as they grow old.
The Fat Oils obtained by expression are used in medicine, both internally and externally, as Lenitives and Emollients. Every body knows the great use of Oil of sweet Almonds, in inflammatory distempers of the breast and intestines. But it must be carefully noted, that these Oils can produce no good effects, unless they be fresh expressed, and from fruits, kernels, or seeds, that have not been long kept: for they not only lose their lenient virtue by growing old, but they even acquire an opposite quality, and contract such a sharp acrimony, that far from procuring any salutary relief or mitigation to the inflamed parts, they are capable of irritating and inflaming the sound.
It is therefore of the last importance to administer them only when they are quite fresh: they ought never to be above two or three days old. Those that are old are generally more limpid and transparent than the fresh, which look a little more cloudy. The best way to distinguish them is to taste them, and to try whether or no they leave any sensation of rancidity on the palate and in the throat.
To draw the Essential Oils of certain Fruits by Expression.
Take the rind of a Citron, Lemon, Orange, Bergamot-pear, or other fruit of that kind; cut it in slices, and, doubling the slices, squeeze them between your fingers, over[Pg 388] against a polished glass set upright, with its lower end in a vessel of earth or porcelain. Every time you squeeze the peel in a new ply, there will squirt out of it several fine jets of liquor, which, meeting with the surface of the glass, will be condensed into drops, and trickle down in small streams into the recipient. This liquor is the Essential Oil of the fruit.
OBSERVATIONS.
No fruits but those of the kind above-mentioned will yield an essential Oil by expression. The rind of the fruit is the reservoir of this Oil: it is contained in little vesicles, which may be seen by the naked eye, spread all over the surface of the peel, and which, bursting when the peel is squeezed, discharge the Oil in the form of very fine slender spouts. Every body knows, that these little oily streams instantly take fire, when spirted through the flame of a candle: the Oil in this case is entirely consumed.
The Essential Oil, thus obtained by expression, hath a very sweet and most agreeable scent. It is in every respect the same as when it made a part of the fruit that yielded it, seeing it hath not undergone the action of fire. Yet this method, however good it may be, can hardly be practised but in the countries where those fruits are in great plenty; because we cannot by this means obtain any thing near the quantity of Oil they contain.
This inconvenience may be remedied by rubbing the rind, which contains the Essential Oil, on the surface of a sugar-loaf. The inequalities of that surface produce the effects of a rasp, by tearing all the oily vesicles. The Oil, which issues in abundance, is imbibed by the sugar and moistens it. When the sugar is sufficiently impregnated therewith, it may be scraped off with a knife, and put into a well-stopped bottle. The sugar does not alter the nature of the Oil; which may be kept in this manner for years, and used, though combined with the sugar, for almost all the same purposes as when in a fluid state; that is, to aromatize the several matters with which you incline to mix it. We owe these observations to Mr. Geoffroy.
This experiment, in which the Essential Oil of a vegetable is obtained by expression alone, and without the aid of fire, proves that the Oils of this kind exist naturally in vegetables; and that the Oils of the same kind obtained by distillation, as shall be shewn in its place, are not the product of the fire. Essential Oils drawn by expression do not very sensibly differ from those procured by distillation.
Of the Substances obtained from Vegetables by Trituration.
To make the Extract of a Plant by Trituration.
Bruise the vegetable substance of which you intend to make the Extract; or, if it be hard and dry, grind it to a powder: put the matter thus prepared, together with seven or eight times as much rain-water, into an earthen vessel; and into this vessel fit a churning staff, so that it may be continually whirled round with a rotatory motion, by means of a cord, a wheel, and a winch. Ply this machine for ten or twelve hours; and then filter the liquor through two linen cloths spread on a hair-sieve. Let your filtered liquor stand quiet for twelve hours more: then pour it off by inclination from the sediment you will find at bottom; and filter it a second time through a flannel bag.
Pour fresh water, but in a smaller quantity, on the mass left after trituration with the machine. Triturate it again for four or five hours. Treat the liquor of this second triture just as you did that of the first, and mix them both together. Distribute all the liquor you now have among a sufficient number of shallow earthen plates, and evaporate it by a gentle warmth, such as that of the sun, or of a vapour-bath, to the consistence of an Extract, or even to dryness, as you think proper.
OBSERVATIONS.
In trituration the water takes up, not only the Salts of plants, but also a pretty considerable quantity of their oily and earthy parts, which those Salts have rendered soluble therein, by communicating to them a saponaceous and mucilaginous quality. After trituration, therefore, nothing remains but the grossest particles of oil and earth. Hence it is evident, that the water, in which plants have been triturated, contains nearly the same principles as the juices of those[Pg 390] plants drawn by expression; and that it is also impregnated with their Essential Salts: so that, by evaporating it to a due consistence, we have a well made Extract of the triturated plant.
The Count de la Garaye, who hath long cultivated with great assiduity those parts of Chymistry by which Medicine may be improved, hath made a great number of experiments for obtaining from plants, by triture with water, the matters in which their virtues chiefly reside, and hath also published a work, entitled Hydraulic Chymistry, in which he gives a particular account of all the processes for making such Extracts of the chief mineral, vegetable, and animal substances, as are most frequently used in the Practice of Physic. His way of evaporating, by a gentle heat, the liquor containing the Extract of a triturated substance is a very good one: for we know that heat, if but a very little too strong, is capable of changing the natures of compound bodies, by disuniting their principles, and exhaling some of them.
If all vegetable matters were fat and succulent, as most pot-herbs are, triture would not be necessary for the making an Extract of them, even without the help of fire. We should have nothing to do, for that purpose, but to express their juices, as before, clarify them, and evaporate with a gentle heat to the consistence of an Extract. But many vegetable substances, such as woods, barks, roots, &c. are dry, hard, and compact. These matters will not give out their Extract, without such an application of water as shall dissolve their saline, saponaceous, and mucilaginous parts. Now this must be effected either by triture or by fire. Trituration has the advantage of procuring Extracts, in which the principles are perfectly unaltered, and retain the same proportions, with respect to each other, as in the plant: but then it is attended with the inconveniencies of being very tedious, troublesome, and chargeable. When we come to deliver the methods of making extracts by decoction and by infusion, we shall see what are the advantages and disadvantages of preparing Extracts by heat.
The matters, from which an extract is to be made by triture, must be previously bruised and reduced into small parts, in order to facilitate the action of water upon them. The several filtrations and decantations here directed are intended to separate the grosser parts of the plant, that were only suspended in the liquor, but not truly dissolved, by means of the agitation and motion: for this reason also, the longer the liquor is left to settle, the purer will the Extract be.
Though the plant be triturated the first time with a great deal of water, and for a good while too, yet it is not by that means wholly exhausted: M. de la Garaye therefore directs the remainder to be triturated again with fresh water: but this second operation requires only half the water used in the former, and need be continued only half the time; the plant having been already opened by the former triture, and having fewer parts to give out. It is better to add fresh water, and triturate a second time, than to triturate but once, and for a greater length of time: for when the water is impregnated with the principles of the plant to a certain degree, it is less capable of acting, and of dissolving more, than when it is pure.
As the water impregnated with the principles of the plant by triture must be almost wholly evaporated, in order to bring those principles nearer together, and that the whole may lie in the smallest compass possible; and, moreover, as this evaporation must be effected by the gentlest heat, it is necessary to spread the liquor so, by distributing it among a great number of plates, that it shall be reduced in a manner entirely to surface. By this means the Extract may be evaporated even to dryness; and this is M. de la Garaye's practice. As the Extracts, thus evaporated to dryness, cannot be taken up otherwise than in little scales, the lower surfaces whereof, by adhering to the glazing of the plate, are smooth and shining, they in some measure resemble a crystallized Salt; which led M. de la Garaye into an error, and induced him to give the title of Essential Salts to the Extracts prepared in this manner. The Essential Salt is indeed contained in them; but still they are only Extracts, as Mr. Geoffroy hath shewn, in a memoir on this subject given in by him to the Academy; since, besides the Essential Salt, they contain moreover, as was said before, a great deal of the oil and earth of the matters from which they were extracted. This, in the main, is no objection, but rather an advantage to them; considering that such saline Extracts are, on that account, so much the more like the substances from which they were obtained; especially with regard to their medicinal properties.
To extract from Seeds and Kernels, by Trituration, the Matter of Emulsions.
Blanch the kernels of which you desire to make an Emulsion; put them into a marble mortar; add a very little water; and pound them with a wooden pestle. Continue pounding and triturating till the matter become like a white paste. From time to time pour on it, by little and little, more fair water warmed, still continuing the trituration; by which means the paste will grow thinner. Go on thus till every particle of your kernels be crushed to pap. Then add, still rubbing the mixture, enough of water to make the whole an actual fluid; and you will have a liquor of a dead-white colour, resembling milk. Strain it through a clean linen cloth; it will leave on the filter some coarse parts, which must be returned to those left in the mortar. Again triturate and rub the remainder of the kernels, with the addition of water as before. This second liquor will not be so white nor so rich as the former: filter it in the same manner, and again grind with water the solid parts remaining. In this manner proceed, repeatedly rubbing and adding fresh water, till it appear no longer milky, but come off clear. The white milky waters thus obtained go by the name of an Emulsion.
OBSERVATIONS.
All the matters, from which a Fat Oil is obtainable by expression, produce Emulsions when triturated with water.
An Emulsion consists chiefly of two substances. One of these is mucilaginous, and soluble in water. This substance by itself would not give a milky appearance to the Emulsion, which, with it alone, would be limpid. The other is a Fat Oil, which of itself is not soluble in water; but being divided by the means of trituration into very small globules, it is dispersed through the whole liquor, and suspended therein by the aid of the mucilaginous part. It is this oily part that gives the Emulsion its dead-white, milky colour; because it is not actually dissolved in the water, but only diffused through it.
If Oil be mixed with water in a phial, and the mixture strongly shaken for some time, with a rapid and continued[Pg 393] motion, the Oil will be divided into a vast number of little globules, which intervening between the parts of the water will destroy its transparency, and give it a dead-white colour, like that of our Emulsion. But, as the Oil is not so minutely divided by this means, as by triturating the matters containing it; and again, there being no mucilage in this liquor, as there is in Emulsions, the Oil soon separates from the water when it is left at rest, re-unites into round globules, and these joining together rise to the surface of the liquor, which then recovers its transparency.
The case is not exactly the same with Emulsions; but something like it happens to them also. If they be left to stand quiet in a long bottle, the liquor, which at first appeared homogeneous, separates into two manifestly different parts. The upper part retains its dead-white colour, but is thicker and more opaque; while the lower part becomes perfectly transparent. This is the beginning of an entire separation of the oily from the aqueous parts. The former, being the lighter, ascend and gain the upper part of the liquor; while the lower, being freed from that which obstructed its translucence, recovers its proper limpidity: but the oily parts do not re-unite into masses large enough to form one homogeneous whole, with the appearance and limpidness of Oil; their being minutely divided and entangled in the mucilage impeding their natural tendency.
Emulsions first begin to spoil, as they grow old, not by turning rancid and acrimonious like the Fat Oils drawn by expression, but by turning sour; which is owing to the great quantity of mucilage they contain. As there is a Fat Oil in their composition, they have the same virtues with that sort of Oil; but they are, moreover, incrassating, cooling, and emollient; qualities which render them extremely useful in acute and inflammatory disorders. They grow sour in a very short time, especially in the heat of summer; nay, they sometimes do so in two hours: and therefore they ought to be prepared from time to time as they are to be used.
The matter that is left when all the substance of the Emulsion is extracted, and from which the water comes off clear and limpid, is scarce any thing but the earthy part of the seed or kernel that was triturated; which, however, still retains a portion of tenacious and gross Oil, adhering to it so firmly as not to be separable by water.
The chyle and milk of animals resemble an Emulsion in several respects, and particularly in their dead-white colour; which arises, in the same manner, from the very minute par[Pg 394]ticles of Oil contained in them, and distributed through an aqueous gelatinous fluid, but not dissolved therein. In general, whenever any Oil of any kind happens to be lodged in this manner between the parts of an aqueous liquor, it always makes the whole of an opaque white: for Oil will not mix with water, so as to produce a liquor that shall appear homogeneous and transparent, unless it be intimately dissolved in the water; which cannot be effected but by means of an union previously contracted between it and some saline matter: as is the case of mucilages, certain saponaceous matters, and some other combinations of which we shall have occasion to treat in the sequel.
The methods we have hitherto proposed, for extracting from vegetable substances all that they will yield without the assistance of fire, are not capable of analyzing those substances accurately, as you may have observed; since by expression and trituration we obtain only the liquid parts, impregnated indeed with almost all the principles of plants, which, however are still combined with each other, and barely separated from the grossest earthy and oily parts. We must therefore necessarily have recourse to a more effectual expedient for carrying our analysis further. This expedient consists in making them undergo the action of fire, successively graduated, from the gentlest to the most violent heat.
But, before we enter on this Analysis of Vegetables, it is proper to describe the different operations that may be performed on Oils, the only pure principle we have been able to obtain without the help of fire. As we shall have occasion, when we come to treat of the analysis of plants by fire, to say a great deal more concerning Essential Oils, we reserve till then what relates to the operations that may be performed on them; and confine ourselves here to the operations on Fat Oils.
Of Operations on Fat Oils.
To attenuate Fat Oils, and change their Nature, by exposing them to the Action of Fire, and distilling them.
Mix thoroughly three or four pounds of any Fat Oil whatever, with twice its weight of lime flaked in the air. Put this mixture into a large earthen retort, leaving a third part of it empty. Set it in a reverberating furnace, and lute on a receiver. Heat the vessel with a very gentle fire. A little phlegm will rise first, and will soon be followed by an Oil that will fall in drops from the nose of the retort. Continue the distillation very slowly, till you perceive the Oil that comes over begin to be not quite so fluid as before, but rather a little thicker.
Then unlute your receiver, and put another in its place. Continue the distillation, increasing your fire by degrees. The Oil that comes over will grow thicker and thicker, its fluidity will decrease, and it will acquire a dark-brown colour, which at last will become blackish. The Oil will then be very thick. Push the operation till nothing more will come off, though the retort be red-hot. During the whole time this distillation lasts, there rises a good deal of water, in company with the Oil. Keep the second thick Oil by itself.
Mix the Oil that came over first, in this operation, with an equal part of fresh lime flaked in the air. Put the mixture into an earthen or glass retort, of a size so proportioned to the quantity, that a third part thereof may remain empty. Distil as before. The same phenomena will appear: a clear Oil will first come over, and be succeeded by one a little thicker. Then shift your receiver, and distil off all the rest of the Oil with an increased fire. The first Oil obtained by this second distillation will be clearer and thinner than that of the first distillation; and the second Oil will not be so thick, nor of so deep a colour as before.
Distil over again, in the same manner, the thin Oil of this second distillation, and go on thus repeatedly distilling, till the first clear oil come over with a degree of heat not exceeding that of boiling water. Then, instead of mixing your Oil with lime, put it with some water into a glass retort, or into a body with its head fitted on, and distil it, keeping the water just in a simmer. Your Oil will be more and more attenuated, and, after being thus distilled twice or thrice with water, will be so limpid, so thin, and so clear, that you will scarce be able to distinguish it from water itself.
OBSERVATIONS.
Fat Oils, which are naturally mild, unctuous, inodorous, or have at most a scarce perceptible smell, resembling that of the fruit or kernel from which they were extracted, change their natures totally when exposed to the action of fire. If they be but heated so as to boil, they become acrid, lose much of their unctuosity, and acquire a very pungent odour. From several analogies, and by several experiments, recited in a Memoir on Oils which I read to the Academy, I shewed that these alterations of Fat Oils are produced by the fire's extricating an Acid in them, which before lay concealed and inactive. What I advanced on this subject may be seen in the Memoirs of the Academy for 1745, and in my Elements of the Theory of Chymistry. I shall take occasion to add something more, in my Observations on the following process, by which these Oils are combined with Acids. In this place I shall only examine what passes in the repeated distillations they are here made to undergo.
Fat Oils do not rise in distillation without a degree of heat greater than that of boiling water; and therefore they must be distilled in a sand-bath, or with a naked fire. We prefer the latter method, for reasons elsewhere assigned, and chiefly because the operator is more master of his fire; it being absolutely necessary, in this operation, that he have it in his power to suppress it in an instant, when he finds it too strong: for, in such a case, it will impetuously raise the thin Oil mixed with the thick; nay, the whole will be burnt, as it were, to a coal, if a degree of fire ever so little too strong be kept up but for a few moments. When this accident happens, it is always predicted by a great quantity of white vapours ascending with impetuosity out of the retort, and by drops of Oil following each other very fast, that are scarce limpid[Pg 397] at first, and soon become of a dark colour. All this may be prevented by distilling very slowly, and with great patience.
Fat Oils may be distilled and attenuated without any additament: but then the operation, which is tedious and troublesome enough, even when lime is used, as appears from our description of the process, would be much more so if the Oil were distilled alone, without the addition of any thing to divide it, spread it, and enlarge its surface.
Lime is one of the best additaments that can be employed on this occasion; not only because it procures the advantages just mentioned, but also by reason that, being an absorbent of fat matters, it unites with the grosser parts of the Oil, retains them, and so allows the thinnest and lightest parts to be readily separated from the rest. By this means it greatly expedites the operation: and, the more of it is added, with respect to the oil, the sooner is a considerable quantity of thin limpid Oil obtained: and this is the reason of our directing a double quantity of lime to be mixed with the Oil in the first distillation.
Lime slaked in the air is employed preferably to quick-lime; because it is naturally divided into a very fine powder, and capable of mixing perfectly with all sorts of matters.
The water that first appears in the distillation comes from the lime: it is part of the humidity which the lime had imbibed from the air. This water continues to rise with the Oil during the whole distillation, according as the degree of heat is increased: and, if the distillation be finished by keeping the retort red-hot for some time after all is come over, the lime in it will have a greyish cast, and, when water is poured on it, grow almost as hot as quick-lime.
If you resolve to carry on these distillations of a Fat Oil, till it becomes as light as an Essential Oil, it is necessary to begin with a pretty large quantity thereof, as three or four pounds: for the quantity of the Oil is considerably lessened by every distillation; not only because the thickest and grossest part is separated from it every time; but also because a portion of the Oil remains so strongly united with the lime, that the force of fire is not able to separate them. Moreover, there is reason to believe that some of it is decomposed every time it is distilled.
If Oil be distilled by itself, the thickest and heaviest part remains charred, as it were, in the retort, the inside of which is lined with a crust of coal, that is to the last degree fixed: this therefore always occasions a diminution of the Oil.
A Fat Oil must be distilled eight or nine times, even with lime, before it become as light as an Essential Oil, and capable of rising wholly with the heat of boiling water: by that time therefore it must be considerably diminished; and if, at least, the quantity prescribed be not taken at first, there will scarce remain a few ounces capable of being distilled with water.
The portion of thick heavy Oil, obtained in the several distillations, may, if you will, be rectified again. For this purpose you must mix it with fresh lime, and distil it as you did the clear Oil. A portion of this also will be attenuated, and come over first. Thus all the Fat Oil may be subtilized by the action of fire; an absolutely charred black part excepted, that remains fixed, and appears susceptible of no change, but by burning it in the open air, and thereby reducing it to ashes, from which a little Fixed Alkali may be obtained. In this fixed part of the Oil the acid and earthy parts are combined therewith, in a greater proportion than they ought to be in pure Oil.
The portion of Oil that hath become light and thin is nothing but the purest oily part, separated from the gross acids, and from a certain quantity of earth, which made it thick and heavy. This Oil resembles the Essential Oils in lightness, fluidity, and a penetrating agreeable odour: it dissolves in Spirit of Wine. We shall have occasion in the sequel to enlarge further on the qualities of the several sorts of Oils, and their solubility in Spirit of Wine, when we come to treat of Ardent Spirits and of Æther.
To combine Fat Oils with Acids. The Decomposition of this Combination.
Put any Fat Oil whatever into a glass bason, and set it in a sand-bath very moderately heated. Pour on this Oil an equal quantity of concentrated Oil of Vitriol, which will immediately dissolve it with violence; a considerable ebullition and effervescence will arise, attended with great heat, and a prodigious quantity of black, thick vapours, in which may be easily perceived the smell of burnt Oil, together with that of a Sulphureous Acid. The mixture will become of a deep-red, black, and thick. Stir it with a small stick, till you observe that all is quiet.
OBSERVATIONS.
The Vitriolic and Nitrous Acids unite with Fat Oils, and dissolve them with violence; but these Acids must be sufficiently strong and concentrated, otherwise they will not act upon the Oils. The Vitriolic Acid, in particular, dissolves them pretty thoroughly. If hot water be poured on the mixture described in our process, this water will become cloudy and milky, by dissolving some of it: so that Oils may be rendered soluble in water by the means of Acids. Spirit of Wine, which doth not attack Fat Oils in their natural state, unites perfectly with them, and makes a clear limpid solution of them, when they are thus combined with Acids.
The Acids also suffer a considerable alteration by contracting an union with Oils. They become much milder, and lose almost all their strength. If the mixture described in the process be distilled, there will come over a great quantity of an empyreumatic acidulated phlegm, that smells strong of Sulphureous Spirit; an Oil thinner than the original saponaceous mixture; a weak Oily Acid, and a very thick, black Oil. If the fire be made very strong, when the Oil ceases to rise, it sometimes happens that a little Sulphur sublimes into the neck of the retort.
By this analysis it appears, that the strong concentrated Acid, which was an ingredient in the combination, is not now to be found. The Vitriolic Acid hath changed its nature, and is considerably weakened by the union it hath contracted with the principles of the Oil. The aqueous part of this latter substance weakens the other, and loads it with phlegm; the inflammable part thereof renders it sulphureous, and even converts it into Sulphur.
Hence it follows, that same part of the Oil is decomposed, by the union it contracts with the Vitriolic Acid; for its phlogiston and its aqueous principle cannot be disunited, so as to form a Sulphureous Spirit, or an actual Sulphur, and an aqueous Acid, without the decomposition of a certain quantity of the Oil, in proportion to the two disjoined principles. Another portion of the Oil remains united with the Vitriolic Acid, without suffering any decomposition, and communicates to that portion of the Acid, with which it is combined, a somewhat saponaceous quality, which makes it resemble the Vegetable Acids.
Thus we see, that when the Vitriolic Acid and a Fat Oil are combined together, they both suffer considerable changes; the Acid by the new alliances into which it enters, and the[Pg 400] Oil by the decomposition it undergoes. In consequence hereof a much smaller quantity of Oil is obtained, by decompounding this combination, than was at first put in.
If the Oil abstracted by distillation be combined again with a fresh quantity of the concentrated Acid, the same effects will again follow; and by this means any quantity of Oil at pleasure may be entirely decomposed. This single experiment affords an evident proof of many important truths advanced in our Elements of the Theory.
Spirit of Nitre likewise dissolves expressed Oils. With Oil of Olives it forms a white paste, resembling a fine pomatum. This compound is perfectly soluble in Spirit of Wine. The Acid must be very strong and smoking to unite with this, or with any other Fat Oil: but it dissolves some of them with more rapidity than others; in which number is the Oil of Walnuts. It acts on these Oils with so much vehemence that it burns them, in some measure, making them black and thick.
To combine Fat Oils with Fixed Alkalis. Hard and soft Soap. The Decomposition of Soap.
Take a lixivium of Alicant kelp made more caustic by lime, as we shall shew when we come to speak of Alkalis. Evaporate this lye till it be capable of bearing a new-laid egg. Divide it into two parts; and to one of these put just water enough to weaken it so, that a new-laid egg will not swim in it, but fall to the bottom. With the lye thus weakened mix an equal quantity of fresh-drawn Olive Oil. Stir and agitate the mixture well, till it become very white. Set it over a gentle fire, and continue stirring it incessantly, that the two ingredients of which it is compounded may gradually combine together, as part of the water evaporates. When you perceive they begin to unite, pour into the mixture thrice as much of the first strong lye as you took of Olive Oil. Continue the coction with a gentle fire, always stirring the matter, till it becomes so thick that a drop of it fixes, as it cools, into the consistence that Soap ought to have. By dissolving a little of this Soap in water, you will discover whether or no it contains more Oil than ought to be in the composition. If it dissolves therein wholly and perfectly, without the appearance of the least little drop of Oil[Pg 401] floating on the water, it is a sign that it doth not contain too much Oil. If, on the contrary, you perceive any of these little globules, you must pour into the vessel, containing your matter, a little more of the strong lye, to absorb the redundant Oil. If there be too much of the Alkali it may be discovered by the taste. If the Soap leave on your tongue the sensation of an Alkaline Salt, and produce an urinous savour, it is a sign that there is too much Salt in proportion to the Oil. In this case a little Oil must be added to the mixture, to saturate the super-abundant Alkali. An excess in the quantity of Alkali discovers itself likewise by the Soap's growing moist in the air, on being exposed to it for some time.
OBSERVATIONS.
Fixed Alkalis, even when resolved into a liquor, that is, when loaded with much water, unite easily with Fat Oils, as appears from the experiment just recited, and require but a moderate heat to perfect that union. This combination may even be completely effected without the aid of fire, and by the heat of the sun only, provided sufficient time be allowed for that purpose; as Mr. Geoffroy found upon trial. It only requires the mixture of the Oil and Alkali to be kept five or six days in digestion, and stirred from time to time. A lixivium of pure Alkali, not acuated by lime, may also be used to make Soap: but it is observed, that the combination succeeds better, and that the Alkali unites sooner and more perfectly with the Oil, when it is sharpened by lime.
The Oil is first mixed with a weaker and more aqueous lye, to the end that the combination may not take place too hastily, but that all the particles of the two substances to be compounded together may unite equally. But as soon as the Alkali begins to dissolve the Oil gradually and quietly, the dissolution may then be accelerated; and that is done by adding the remaining lye, which is stronger and less diluted than the other.
Soap made with Olive Oil is white, hard, and hath not a very disagreeable smell: but as that Oil is dear, others, even the fat and oils of animals, are sometimes substituted for it. The Soaps made with most of these other matters are neither so hard, nor so white, as that made of Olive Oil: they are called Soft Soaps.
Oils thus associated with Fixed Alkalis are by that means rendered soluble in water; because the Alkaline Salts, having a great affinity with water, communicate part thereof to the[Pg 402] Oils with which they are now incorporated. Yet the Oil is not for all that rendered thoroughly miscible with water, or perfectly soluble therein; for the water in which Soap is dissolved hath always a milky cast: now there is no other criterion of a perfect solution but transparency.
Alkalis also lose part of their affinity with water, by the union they thus contract with Oils: for, when the combination is properly made, they no longer attract the moisture of the air, nor doth water dissolve them in such quantities as before. The composition of Soap is plainly a saturation of an Alkali with an Oil; and, in order to make perfect Soap, we are forced, as was said in the process, to grope, in a manner, by repeated trials, for this point of saturation; just as when we prepare a Neutral Salt by saturating an Alkali with an Acid. The union which the Oil contracts with the Alkali makes it lose, in part, the readiness with which it naturally takes fire; because the Salt is not inflammable: the water also, which enters in pretty considerable quantities into the composition of Soap, as we shall presently see, contributes a good deal to hinder the accension of the Oil.
Soap may be decompounded either by distilling it, or by mixing it with some substance that hath a greater affinity than Oil with Alkalis.
If we decompound it by distillation, a phlegm, or transparent spirit, of a somewhat yellowish colour, first comes over. This liquor is the aqueous part of the Soap, quickened by a little of its Alkali, which gives it an acrid taste. It is followed by a red Oil, which at first is pretty thin and limpid, but thickens as the distillation advances, grows black, and has a very disagreeable empyreumatic smell. This Oil is soluble in Spirit of Wine.
When the distillation is finished, that is, when the retort being kept red-hot for some time will discharge no more, there is left in it a saline mass; which is the Alkali of the Soap, crusted over with some of the most fixed parts of the Oil, that are charred to a coal. This Salt may be restored to the same degree of purity it had before its combination with the Oil, by calcining it in a crucible with a naked fire, that may consume this burnt part of the Oil, and reduce it to ashes.
It is plain that the Oil contained in Soap is affected by distillation, much in the same manner as that which we mixed with lime and distilled.
Mr. Geoffroy, by analysing Soap with care, discovered that two ounces thereof contain ninety-six grains of Salt of[Pg 403] kelp, freed from all Oil and moisture; or two drams and forty-eight grains of that Salt, as it is used in manufacturing Soap; that is, containing water enough to make it crystallize; one ounce three drams twenty grains of Olive Oil; and about two drams four grains of water.
As Acids have a greater affinity than any other substance with Alkalis, they may be very effectually employed to decompound Soap.
If you propose to decompound Soap by means thereof, you must first dissolve it in a sufficient quantity of water. Mr. Geoffroy, who made this experiment likewise, dissolved two ounces thereof in about three gallons of warm water, and to the solution added Oil of Vitriol, which he let fall into it drop by drop. Every time a drop of Acid falls into it, a coagulum is formed in the liquor. The vessel in which the solution is contained must then be shaken, that the Acid may equally attack all the Alkali diffused in it. When no new coagulation is produced by a drop of the Acid, it is a sign you have added enough. The liquor then begins to grow clear: and if another quart of water be added, in order to facilitate the separation of the oily particles, you will see them rise and unite together on the surface of the liquor.
This is a pure, clear, true Olive Oil, hath its taste, its smell, and, like it, is fluid in warm weather, and becomes fixed by cold. Yet it differs in some respects from that which never hath been united with an Alkali in order to form a Soap; for it burns more vividly and more rapidly, and is soluble in Spirit of Wine. We shall account for these differences when we come to treat of Ardent Spirits.
Not only the Vitriolic Acid, but all others, even those obtained from vegetables, are capable of decompounding Soap, and separating the Oil from the Alkali. In the liquor wherein Soap is thus decompounded is found a Neutral Salt, consisting of the Acid made use of, united with the Alkali of the Soap. If the Vitriolic Acid be used, you will have a Glauber's Salt; a quadrangular Nitre, if the Nitrous Acid be used; and so of the rest.
The facility with which Acids decompound Soap is the reason that no water, but what is very pure, will dissolve it, or is fit to be used in washing with it.
Water that doth not dissolve Soap well is usually called Hard Water. Such waters contain a certain quantity of saline matters, washed out of the earths through which they pass. The hardness of water is generally occasioned by selenitic particles.
The hardness of all the well-water in and about Paris is owing to a considerable quantity of Selenetic Gypsum with which the Soil abounds. The Selenites, we know, are Neutral Salts, consisting of the Vitriolic Acid united with an earthy basis. If therefore Soap be put into water in which a Salt of this kind is dissolved, it is evident that the Vitriolic Acid in the Selenites, having a greater affinity with the fixed Alkali of the Soap than with its own earthy basis, will quit the latter to unite with the former; and thus the Soap will be decompounded instead of being dissolved. Accordingly we see, that, when we attempt to dissolve Soap in our well-water, the surface of the liquor is in a short time covered with a fat oily pellicle. However, this decomposition of Soap is not complete; at least, but a small part of it is perfectly decompounded; because the great quantity of Selenites, with which the water is impregnated, hinders the Soap from mixing so thoroughly with it, as is requisite to produce a total decomposition thereof.
All mineral waters are likewise hard, with regard to Soap; for as most of them owe their virtues to the efflorescences they have washed off from pyrites, that have grown hot and begun to be decomposed, they are impregnated with the saline matters produced by pyrites in that state: that is, with aluminous, vitriolic, and sulphureous substances, which have the same effect on Soap as the Selenites have.
Mineral waters containing Neutral Salts only, such as Sea-salt, Epsom Salt, Glauber's Salt, are nevertheless hard with regard to Soap, though the Acids of those Salts, being united with Fixed Alkalis, are incapable of decompounding it. The reason is, that those Neutral Salts are more soluble in water than Soap is; so much indeed as even to exclude it: because each of the two principles that composed them hath a very great affinity with water; whereas only one of the principles of Soap, namely, its Alkali, hath that affinity; the other, to wit, the oily principle, having none at all. Thus water impregnated with an Acid, or with any Neutral Salt, is hard with regard to Soap, and incapable of dissolving it; and hence it follows, that Soap is a sort of touchstone for trying the purity of water.
Wine dissolves Soap; but imperfectly, because it contains an acid or tartarous part. Spirit of Wine also dissolves it: but neither is this dissolution perfect; because it contains too little water: for its spirituous part can dissolve nothing but the Oil of the Soap; and the Alkali is not at all, or at least in a very small quantity, soluble in this menstruum. The[Pg 405] true solvent of Soap is therefore a liquor that is partly spirituous, partly aqueous, and not acid.
Brandy has these qualities: and accordingly it is the solvent that unites best with Soap, dissolves the greatest quantity, and makes the most limpid solution thereof. Yet even this solution hath something of a milky cast, occasioned by its not being entirely free from an Acid, or the tartarous principle. This fault may be easily corrected, by mixing with it a little Alkali to absorb the Acid. A dram of crystallized salt of kelp mixed with three ounces and a half of good brandy, renders it capable of dissolving an ounce and two drams of good hard Soap, into a perfectly limpid liquor. This experiment also we owe to Mr. Geoffroy.
Some years ago it was discovered that Soap might be used with great success in Medicine, and that it possesses the property of dissolving the stony concretions that form in several parts of the body, particularly in the kidneys and bladder. Soap is the basis of the composition known by the name of Mrs. Stephen's Remedy, and in this one ingredient its whole virtue resides.
From what hath been said on the nature of this compound, as well as on the cause and phenomena of its dissolution, it plainly appears to be of the last consequence, in administering it to a patient, that his constitution be considered, and a proper regimen ordered. All Acids should be absolutely forbid him; as we know they hinder the Soap from dissolving, and decompound it; and if the patient have any acidities in the first passages, matters capable of neutralizing them should be prescribed him: as prepared crabs eyes, and other absorbents known in Medicine: in such cases those with which the Soap is compounded in Mrs. Stephen's remedy may be of use.
To combine Fat Oils with Sulphur.
Put any Fat Oil whatever into an earthen vessel; add to it about the fourth part of its weight of Flower of Sulphur, and set the vessel in a furnace, with lighted coals under it. When the Oil hath acquired a certain degree of heat, the Sulphur will melt, and you will see it fall immediately to the bottom of the Oil, in the form of a very red fluid. The two substances will remain thus separated, without mix[Pg 406]ing together, while the heat is no greater than is necessary to keep the Sulphur in fusion. Increase it therefore; but slowly and with circumspection, lest the matter take fire. When the Oil begins to smoke, the two liquors will begin to mix and look turbid: at last they will unite so as to appear one homogeneous whole. If you keep up the heat so that the mixture shall always continue smoking and ready to boil, you may add more Sulphur, which will perfectly incorporate with it: and thus may a pretty considerable quantity thereof be introduced into this composition.
OBSERVATIONS.
The Phlogiston and the Vitriolic Acid have each an affinity with Oils. It is not therefore surprising that Sulphur, which is a compound of these two substances, should be soluble in oily matters. Yet it is remarkable, that Essential Oils, which are much thinner than the Fat Oils, dissolve Sulphur with much more difficulty; as will be shewn when we come to treat of those Oils; and that Spirit of Wine, which contains an exceeding subtile Oil, doth not act upon Sulphur at all.
Oil, by contracting an union, with Sulphur, produces a considerable alteration in that mineral: a phenomenon so much the more surprising, that we know it to be in some sort unalterable by any other solvent, of what kind soever, add, that its nature admits of no change but by burning. We shall say more on this subject under the head of Essential Oils.
To combine Fat Oils with Lead, and the Calces of Lead. The Basis of Plasters. The Decomposition of this Combination.
Into an earthen vessel put granulated Lead, Litharge, Ceruse, or Minium; and pour thereon twice its weight of any Fat Oil whatever. If you set the vessel over a brisk fire, the Lead at bottom will melt before the Oil begin to boil. When it boils, stir the matter with a stick: the Lead, or the Calx of Lead, will gradually disappear, and at last be totally dissolved by the Oil, to which it will give a very thick consistence.
OBSERVATIONS.
Fat Oils dissolve not only Lead, but its calces also: nay, they dissolve the latter more readily than Lead in substance; probably because they are more divided. The result of a combination of these matters is a thick, tenacious mass, that grows in some degree hard in the cold, and soft by heat. This composition is known in Pharmacy by the name of Plaster. It is made up with several drugs into plasters, which partake of the virtues of those drugs; so that it is the basis of almost all plasters.
Lead itself is seldom used to make plasters: Ceruse, Litharge, or Minium, are preferred to it; because these matters unite, as hath been said, more readily and more easily with Oils.
It sometimes happens, that the Oil is burnt in the operation, and that the calx of Lead is partly resuscitated: and this gives the plaster a black colour, which however it ought not to have. This accident is occasioned by an excess of heat: and as it is very difficult to keep the Oil and the Lead in the proper degree of heat, seeing both these matters are apt to grow very hot, it hath been contrived to put into the vessel, in which the coction is to be performed, a pretty large quantity of water; which being susceptible only of a much smaller and a certain degree of heat, that is constantly the same when it boils, procures the advantage of having the composition very uniform and very white.
It is necessary to stir the mixture incessantly, in order to prevent the burning of the combined Oil and Lead; which, as they unite, sink in the water by their greater weight. If the water happen to be wasted before the Oil hath dissolved all the Lead, or before the plaster hath acquired a proper degree of consistence, you must remove the vessel from the fire, and let the mixture cool, before you add more: for, if this precaution be neglected, the heat of the matter, which is now much greater than that of boiling water, will occasion a considerable explosion and extravasation thereof, though the water poured into it be as hot as possible.
The combination of Fat Oil with a Calx of Lead may be considered as a sort of metallic Soap, having a metalline Calx, instead of a Fixed Alkali, for its basis. Mr. Geoffroy hath observed, that if a pound of Litharge, rubbed very fine and well washed, be incorporated with two pounds of Olive Oil, in the same manner as plaster is made, keeping water enough in the vessel to hinder the mixture from burning,[Pg 408] there rises a smoke, while the Oil is uniting with the Calx of Lead, smelling much like that which rises from Soap.
The Oil may be separated from the Calx of Lead, by the methods used to separate it from a Fixed Alkali: and when it is so separated, it hath the same properties as that separated from common Soap.
This species of metallic Soap, formed by the union of a Fat Oil with the Calx of Lead, is not soluble in water, and communicates nothing to it but a greasy taste. Therefore, if you would decompound it by the means of an Acid, you must pour that Acid immediately on the compound. The Acid will attack and dissolve the Calx of Lead; and the Oil, being thus set at liberty, will rise clear and limpid to the surface of the acid liquor. Distilled vinegar effects this separation better than any other Acid, because it is the true solvent of Lead.
Of the Substances obtained from Vegetables with a Degree of Heat not exceeding that of boiling Water.
To obtain from Plants, by distilling them with the mean Degree of Heat between freezing and boiling Water, a Liquor impregnated with their Principle of Odour.
In the morning, before sun-rise, gather the plant from which you design to extract its odoriferous water. Chuse the plant in its full vigour, perfectly sound, and free from all adventitious matters, except dew. Put this plant, without squeezing it, into the body of a tinned copper alembic, and set it in a water-bath. Fit on its head, and to the nose thereof lute a glass receiver with wet bladder.
Warm the bath to the mean degree between freezing and boiling water. You will see a liquor distil and fall drop by drop into the receiver. Continue the distillation with this degree of heat, till no more drops fall from the nose of the alembic. Then unlute the vessels; and if you have not as much liquor as you want, take out of the cucurbit the plant[Pg 409] already distilled, and put a fresh one in its place. Distil as before, and go on thus till you have a sufficient quantity of odoriferous liquor. Put it into a bottle; stop it close; and set it in a cool place.
OBSERVATIONS.
The liquor obtained from plants, with the degree of heat here prescribed, consists of the dew that was on the plant, and some of the phlegm of the plant itself, together with its odorous principle. Mr. Boerhaave, who examined this odoriferous part of plants with great care, calls it the Spiritus Rector. The nature of this Spirit is not yet thoroughly ascertained; because it is so very volatile, that it cannot easily be subjected to the experiments that are necessary to analyze it, and to discover all its properties. If the bottle containing the liquor, which may be considered as the vehicle of this Spirit, be not exceeding carefully stopped, it flies quite off: so that in a few days nothing will be found but an insipid inodorous water.
Great part of the virtue of plants resides in this their principle of odour; and to it must be ascribed the most singular and the most wonderful effects we every day see produced by them. Every body knows, that a great number of odorous plants affect, in a particular manner, by their scent only, the brain and the genus nervosum, of such especially whose nerves are very sensible, and susceptible of the slightest impression; such as hypochondriacal or melancholy men, and hysterical women. The smell of the Tuberose, for instance, is capable of throwing such persons into fits, so as to make them drop down and swoon away. The smell of Rue, again, which is equally strong and penetrating, but of a different kind, is a specific remedy against the ill effects of the Tuberose; and brings those persons to life again, with as quick and as surprising an efficacy, as that by which they were reduced to a state not unlike death. This is Mr. Boerhaave's observation.
The odorous exhalations of plants must be considered as a continual emanation of their Spiritus Rector: but as growing plants are in a condition to repair, every instant, the losses they sustain by this means, as well as by transpiration, it is not surprising that they are not soon exhausted, while they continue in vigour. Those, on the contrary, which we distil, having no such resource, are very soon entirely deprived of this principle.
The separation of the Spiritus Rector from plants requires but a very gentle heat, equally distant from the freezing point and from the heat of boiling water. Accordingly the heat of the sun in summer is sufficient to dissipate it almost entirely. This shews why it is dangerous to stay long in fields, or woods, where many noxious plants grow. The virtues of plants residing chiefly in their exhalations, which the heat of the sun increases considerably, a sort of atmosphere is formed round them, and carried by the air and the wind to very great distances.
For the same reason the air of a country may be rendered salutary and medicinal, by the exhalations of wholesome plants growing therein. From the facility with which the odorous principle of plants evaporates, we learn what care ought to be taken in drying those intended for medical uses, so as to preserve their virtues. They must by no means be exposed to the sun, or laid in a warm place: a cool, dry place, into which the rays of the sun never penetrate, is the properest for drying plants, with as little loss of their virtue as possible.
Though there is reason to believe that every vegetable matter hath a Spiritus Rector, seeing each hath its particular scent, yet this principle is not very perceptible in any but those which have a very manifest odour: and accordingly it is extracted chiefly from aromatic plants, or the most odoriferous parts of plants. I say the most odoriferous parts; because, in most plants and trees, there are generally certain parts that have a much more sensible, and much stronger scent than the rest. The odour of a plant, or of a tree, hath its principal residence sometimes in the root, sometimes in the leaves, at other times in the bark or wood, and very frequently in the flowers and seeds. Therefore, when you design to extract the principle of odour from a vegetable that is not equally odoriferous in every part, you must chuse those parts that have the most perceptible and strongest scent.
To extract the Fat Oils of Plants by Decoction in boiling Water. Cacao Butter.
Pound or bruise in a marble mortar your vegetable substances, abounding with the Fat Oil which you intend to extract by decoction: tie them up in a linen cloth;[Pg 411] put this packet into a pan, with seven or eight times as much water, and make the water boil. The Oil will be separated by the ebullition, and float on the surface of the water. Skim it off carefully with a ladle, and continue boiling till no more Oil appear.
OBSERVATIONS.
The heat of boiling water is capable of separating the Fat Oils from vegetable matters that contain any: but this is to be effected by actual decoction only, and not by distillation; because these Oils will not rise in an alembic with the heat of boiling water. We are therefore necessitated to collect them from the surface of the water, as above directed. By this means a much greater quantity of Fat Oil may be obtained than by expression alone; because the degree of heat applied greatly facilitates the separation of the Oil. For a convincing proof of this truth, take the remains of any vegetable matters, from which the Oil hath been so thoroughly expressed that they would yield no more; boil them in this manner, and you will obtain a great deal more Oil.
The water used in this coction generally becomes milky, like an emulsion; because it contains many oily particles, that are dispersed in it just as in an emulsion. Nevertheless, this way of obtaining the Fat Oils is not generally practised; because the heat, to which they are exposed in the operation, occasions their being less mild than they naturally are: but it is an excellent method, and indeed the only one that can be employed, for extracting from particular vegetables certain concrete oily matters, in the form of Butter or Wax; which matters are no other than Fat Oils in a fixed state. The Cacao yields, by this means, a very mild butter; and in the same manner is a Wax obtained from a certain shrub in America.
The heat of boiling water melts these oily matters, which then ascend to the surface of the liquor, and float on it like other Oils. They afterwards fix as they cool, and resume their natural consistence. We shall see in the sequel, that they cannot be extracted in a concrete form by distillation, which requires a greater degree of heat than that of boiling water; because distillation changes their nature, partly decomposes them, and prevents their returning to their proper consistence as they cool.
To extract Essential Oils of Plants by Distillation with the Heat of boiling Water. Distilled Waters.
Put into a cucurbit the plant from which you design to extract the Essential Oil. Add as much water as will fill two thirds of your vessel, and dissolve therein half an ounce of Sea-salt for every quart of water you use. To this body fit on an alembic-head, and to the nose thereof lute a receiver, with sized paper, or wet bladder. Set it in a furnace, and let the whole digest together, in a very gentle warmth, for twenty-four hours.
This being done, light a wood-fire under your vessel, brisk enough to make the water in it boil immediately. Then slacken your fire, and leave it just strong enough to keep the water simmering. There will come over into the receiver a liquor of a whitish colour, somewhat milky; on the surface of which, or at the bottom, will be found an Oil; which is the Essential Oil of the vegetable you put into the cucurbit. Continue your distillation with the same degree of heat, till you perceive the liquor come off clear, and unaccompanied with any Oil.
When the distillation is finished, unlute the receiver; and, if the Essential Oil be of that sort that it is lighter than water, fill the vessel up to the top with water. On this occasion a long-necked matrass should be used for a receiver; that the Oil which floats on the water may collect together in its neck, and rise up to its mouth. Then in the neck of this vessel put the end of a thread of cotton-twine, so that the depending part without the vessel may be longer than that in the Oil, and the extremity thereof hang within the mouth of a little phial, just big enough to contain your quantity of Oil. The Oil will rise along the yarn as in a siphon, filter through it, and fall drop by drop into the little phial. When all the Oil is thus come over, stop your little bottle very close, with a cork coated over with a mixture of wax and a little pitch.
If your Oil be ponderous, and of the sort that sinks in water, pour the whole contents of the receiver into a glass funnel, the pipe of which must terminate in a very small aperture that may be stopped with your fore-finger. All the Oil will be collected in the lower part of the funnel: then remove your finger, and let the Oil run out into a little bottle through[Pg 413] another small funnel. When you see the water ready to come, stop the pipe of the funnel, and cork the bottle containing your Oil.
OBSERVATIONS.
Essential Oils, though they all resemble each other in their principal properties, are nevertheless very different in some respects: for which reason almost every one of them requires a particular management, for obtaining it with the greatest advantage possible, both as to quality and quantity.
One of the first things requisite is, to chuse the proper time for distilling the plant, from which you desire to extract the Essential Oil; because the quantity of Oil varies considerably, according to the season of the year, as well as the age of the plant. For example, the most favourable time for obtaining these Oils from the leaves of ever-green plants or trees, such as Thyme, Sage, Rosemary, the Orange, the Bay, the Fir, &c. is the end of Autumn; because these vegetables contain a great deal more Oil at that season than at any other. With regard to annual plants, they must be chosen when in their prime, and just before they begin to decline. The time therefore of gathering them is when they begin to flower: and if you want to extract the Oil from the flowers themselves, you must pull them just when they are newly blown.
Secondly, it must be observed, that the Essential Oils of plants are, as it were, the chief residence and reservoir of their odorous principle; that they are to be found wherever that principle exists, and never where it is not: so that what we said concerning the Spiritus Rector of plants is applicable here. It must be remembered, that all the parts of some vegetables are odoriferous. Such plants may be put into the alembic all together, and the Essential Oil distilled from all their parts at once. But others, and indeed the greatest number, have no odour, or at least none that is very perceptible, except in some particular parts; as in their leaves, flowers, roots, or seeds: therefore, when you want to have the Essential Oil of such a plant, you must chuse that part in which the Odour resides. The sense of smelling must be the artist's principal guide on this occasion.
Thirdly, all vegetables, and all the parts of vegetables, have not the same texture: some are hard and compact, as woods, barks, and some roots; others are tender and succulent, as most annual plants, and some fruits. For this rea[Pg 414]son, they must be differently prepared for distillation. It may be laid down as a general rule, that the closer and more compact their texture is, the more they require to be opened and divided, either by comminuting them into small particles, or by digesting them a considerable time in water acuated with Salt.
Fourthly, though all Essential Oils be capable of rising in distillation with the heat of boiling water, yet they have not all an equal degree of levity and weight: on the contrary, they vary exceedingly in this respect: some, as, for instance, those of all our European aromatics, being lighter than water, so that they always float on its surface; whereas others, such as those of Cloves, Sassafras, &c. which are Indian aromatics, are heavier than water, and always sink in it by their specific gravity. These differences therefore require different methods of distillation. It is proper, for example, to make use of a low alembic in distilling such Essential Oils as are heavier than water; and, moreover, to facilitate their separation, by applying a degree of heat somewhat stronger than that of boiling water. This is easily done by impregnating the water with a proper quantity of Sea-salt, or the Vitriolic Acid; for, the more saline matters are contained in water, the more will the degree of heat it acquires, by being brought to boil, exceed that of pure boiling water.
Fifthly, Essential Oils differ from one another in point of fluidity. Some are as thin and as fluid as Spirit of Wine: of this number is the Essential Oil of Turpentine. Others, again, are thick, and even congeal as they cool: such, for instance, is the Oil of Roses. In distilling Oils of this latter sort, care must be taken that the spout of the alembic head do not grow too cold, but be kept always in such a degree of warmth as may prevent the Oil from fixing in it, and stopping it up; which would interrupt the distillation, and might also occasion some other more considerable inconveniencies, of which we shall take notice presently.
From what hath been said it appears, that the distillation of Essential Oils cannot be regulated by any one general rule; but that the manner of operating must be a little varied, according to the nature of the Oil to be distilled, and to that of the vegetable from which it is to be drawn.
The time of day fittest to gather plants for this distillation is the morning before sun-rise; because the coolness of the night hath shut all their pores, and concentrated their odour: whereas in the evening, after the plants have been exposed all day to the heat of the sun, their odorous principle is in a[Pg 415] great measure dissipated, and they are left almost quite exhausted of it. Now, the more of the odorous principle the plants contain, the more Essential Oil will they yield, and the more virtue will that Oil have.
Plants fresh gathered, and as yet full of moisture, do not yield so much Oil in distillation as they do when dried; because the oily particles in a very moist plant are more diffused, and even separated from each other, by the interposition of the aqueous parts: whence it comes to pass that, in distillation, they ascend in a state of separation from each other; so that being dispersed through the water they give it a milky colour, like that of an emulsion; and cannot unite together but in small quantities, which hinders their being easily separated from the water.
This inconvenience doth not happen, or at least is considerably less, when the greatest part of the humidity of the plant is evaporated by desiccation: for the oily particles, being thus delivered from the intervening aqueous parts, which kept them separated from each other, are brought nearer together, unite, and form little visible globules of Oil, which easily emerge from the water employed in the distillation. But, in drying plants from which the Essential Oil is to be extracted, great care must be taken that they be neither exposed to the sun, nor laid in a warm place; because the heat would carry off part of their odour, and even, from some plants, a pretty considerable quantity of their Essential Oil.
Plants of a loose texture, that easily give out their Essential Oils, need not be comminuted, or macerated in water with Salt. But this method must unavoidably be taken with such as are hard, and do not readily part with their Oil. Woods, barks, roots, for instance, must be first rasped, then set to macerate in water impregnated with Salt, as before directed; and this sometimes for several weeks before they be distilled.
On this occasion Salt procures three different advantages. In the first place, it prevents the matters, that must stand in maceration for some time, from running into fermentation: an inconvenience that would considerably diminish the quantity of Essential Oil, or perhaps rob us of the whole, by converting it into an Ardent Spirit, if the fermentation were spirituous; or into a Volatile Alkali, if it went on to the last stage, and as far as putrefaction. In the next place, it acuates the water, and renders it more capable of penetrating and properly dividing, during the maceration, the texture of the plant which requires to be thus prepared.[Pg 416] Lastly, it adds a little to the heat of the boiling water, and so promotes the ascent of the heaviest Oils.
Nevertheless, when you find it necessary, for the reasons assigned above, to mix Salt with the water to be employed in distilling your Essential Oil, you must be cautious of putting in too much. You will indeed obtain, by means thereof, much more Oil than if you distilled it without Salt: but, as a great quantity of Salt will make the water acquire a much greater degree of heat than that of pure boiling water, a good deal of the heavy Oil of the vegetable will be raised by such a heat, mix with the Essential Oil, deprave it, and make it like those that are adulterated with a mixture of some heterogeneous Oil, as will be afterwards shewn.
When every thing is prepared for distillation, it is proper, as directed in the process, to apply at once a flaming fire, brisk enough to make the liquor boil immediately: for, if the water be kept long heating before it be made to boil, the Essential Oil, which cannot rise without the heat of boiling water, will, by a less degree of heat, be only agitated, dashed about every way, and churned as it were; by which means it will be divided into very minute particles, and dispersed in the water, which will thence acquire a milky colour: and consequently we shall fall into the inconvenience that was pointed out above, as happening when we distil plants without having dried them, and while they are loaded with all the moisture and sap that was in them when fresh gathered.
When the water in the cucurbit boils, it will be known by the noise that boiling water usually makes, which is produced by the numerous bubbles that rise and burst on its surface. The spout of the alembic is then so hot, that a man cannot lay his finger on it, without such a sensation of burning heat as is not to be endured. With this degree of heat the water distils in drops, which succeed each other so fast, that they seem to form a continued small stream; and this water is replete with much Essential Oil.
And now it is proper to weaken the fire considerably, so as to leave it but just strong enough to keep the liquor gently boiling: for if the distillation be urged too precipitately, the aqueous and oily vapours, being forcibly hurried up by too great a heat, may carry along with them some parts of the plant, which may stick in the spout, stop it up, and endanger the bursting of the vessel, or at least the forcing off its head, by the exceedingly rarefied particles of water, oil, and air, all striving to escape at the same time; and these burn[Pg 417]ing hot vapours, being discharged with impetuosity, may not only scald the operator, but injure his lungs.
In such distillations it is of consequence to keep constantly cooling the head of the alembic, by frequency renewing the water in the refrigeratory, in order to facilitate the condensation of the oily particles. The water in the cooler ought to be renewed when it begins to smoke very perceptibly.
Whatever care be taken to save as much of the Oil as possible, and to prevent its being left dispersed in the water, yet some loss of this kind cannot be totally avoided: and thus the water that rises in distilling the Oil is always more or less milky, and strongly scented, even after it is separated from the Essential Oil. Yet this portion of the Oil and of the odorous principle, which is retained by the water employed in such distillation, is not therefore lost: the water impregnated with these principles partakes of the properties of the plant from which the Essential Oil was drawn, and may be used medicinally: it is known in Pharmacy by the title of the Distilled Water of the plant.
The same water may be used again, with advantage, in distilling the Essential Oil of a fresh plant of the same sort; because the oily and odorous particles, with which it is impregnated, joining with those afforded by the fresh plant, form larger moleculæ, capable of uniting more easily, and emerging better from the water; and consequently they increase the quantity of Oil. Thus the same water may be always employed in new distillations; and, the oftener it is used, with the greater advantage may it be used again.
After all the Essential Oil is risen, if the distillation be continued, and the receiver changed, the liquor that will then come off will not be milky, but limpid. It will have no odour at all of the plant, but a kind of sourish smell; and indeed it is a part of the Acid of the vegetable in the still, which is elevated by the heat of boiling water, after all the Essential Oil is come over.
If you intend to keep the distilled water which hath served as a vehicle to the Essential Oil, and design it for medicinal use, great care must be taken to stop the distillation before this acid phlegm begin to rise: for, if it should mix with the distilled water, it would spoil it, and hinder it from keeping; probably because it contains some mucilaginous parts, which are apt to putrify.
To extract the Essential Oils of Plants by Distillation per Descensum.
Reduce to a powder, or a paste, the vegetable substances from which you intend to extract the Essential Oil by the method proposed. Lay this matter about half an inch thick on a fine, close, linen cloth. If it be dry and hard, expose the cloth containing it to the steam of boiling water, till the matter become moist and soft. Then lay the cloth, with its contents, over the mouth of a very tall cylindrical glass vessel, which is to do the office of a receiver in this distillation; and, by means of a piece of small pack-thread, fasten down the extremities of the cloth, by winding the thread several times over them and round the vessel; in such a manner, however, that the cloth be not tight, but may yield to a small weight, and sink about five or six lines deep into the vessel over which it is fastened. Set this recipient in a larger vessel, containing so much cold water as will reach half way up the cylindrical vessel; which, having little in it but air, must be ballasted with as much lead as will sink it to the bottom of the water.
On the cloth containing the substance to be distilled set a flat pan of iron or copper, about five or six lines deep, that may just fit the mouth of the glass vessel over which the cloth is fastened, so as to shut it quite close. Fill this pan with hot ashes, and on these lay some live coals. Soon after this, you will see vapours descend from the cloth, which will fill the recipient, and drops of liquor will be formed on the under side of the cloth, from whence they will fall into the vessel. Keep up an equal gentle heat till you perceive nothing more discharged. Then uncover the recipient: you will find in it two distinct liquors; one of which is the phlegm, and the other the Essential Oil of the substance distilled.
OBSERVATIONS.
The apparatus for distilling above described is very convenient, when we have not the vessels necessary for distilling with water, or when we want to obtain the Essential Oil of any vegetable substance in much less time. The aqueous and oily parts of the substances distilled in this manner, be[Pg 419]ing rarefied by the heat of the fire placed over them, cannot ascend upwards, because they are close confined on that side; and, moreover, the fire which rarefies them possessing all the upper part of the vessel in which they are contained, they are forced to fly from it to the place which most favours their condensation: and this determines them to descend in the recipient, where they meet with a coolness that condenses and fixes them. It was with a view to promote this condensation, that we ordered the lower part of the recipient to be sunk in cold water.
Cloves are one of those substances whose Essential Oil is best obtained by this method. In the same way also may be drawn the Essential Oil of Lemon-peel, Citron-peel, Orange-peel, Nutmegs, and several other vegetable substances: but you must be cautious of applying too strong a heat; for in that case the Oil, instead of being white and limpid, acquires a red, dark-brown, blackish colour, is burnt, and smells of empyreuma: and, on the other hand, if you do not apply a proper degree of heat, you will scarce get any Oil at all. It is the surest, and therefore the best, way to distil these Oils with water in an alembic. And indeed the distillation per descensum is seldom used, but out of curiosity to try its effect, or on such pressing occasions as allow no choice.
Infusions, Decoctions, and Extracts of Plants.
Make some water boiling-hot, and then take it off the fire. When it ceases to boil, pour it on the plant of which you desire to have the Infusion; taking care there be enough of it to cover the plant entirely. Cover the vessel, and let your plant lie in the hot water for the space of half an hour, or longer, if it be of a firm close texture. Then pour off the water by inclination: it will have partly acquired the colour, the smell, the taste, and the virtues of the plant. This liquor is called an Infusion.
To make the Decoction of a vegetable substance, put it into an earthen pan, or into a tinned copper vessel, with a quantity of water sufficient to bear being boiled for several hours, without leaving any part of the plant dry. Boil your plant more or less according to its nature; and then pour off the water by inclination. This water is impregnated with[Pg 420] several of the principles of the plant, of which we shall take notice in the following observations.
OBSERVATIONS.
Water, especially when boiling hot, is capable of dissolving not only all that is purely saline in vegetables, but also a pretty considerable quantity of their Oil and of their earth, which, by contracting an union with the saline parts, have formed saponaceous, gummy, and mucilaginous compounds, that are soluble in water. After violent and long-continued boiling, therefore, there remains nothing in the plant but the purest oily part, and such as is the most fixed, that is, the most closely united with the earth of the plant. I say, the most fixed: for some part of the oily matters, though not soluble in water, may be separated by the action of boiling water, when those matters abound greatly in the vegetable decocted; as we have seen happen to the Fat Oils of certain vegetable matters; but in that case these oily matters float upon the Decoction, and do not constitute a part of it.
From what we have already said, touching the analysis of plants, it seems evident, that, if those decocted be odoriferous and contain an Essential Oil, the Decoction will contain none, or at most but very little, of their Essential Oil, or their odorous principle; seeing we know that these substances cannot bear the heat of boiling water, without being carried off and entirely dissipated by it. Therefore, when we make a decoction of an aromatic plant, containing an Essential Oil, we may be assured that it will not possess the virtues, either of the odorous part, or of the Essential Oil, and that it will have none but those of the other more fixed principles of the plant, with which it may be impregnated. The Decoction of such a plant perfectly resembles the water left in the cucurbit, after distilling its Essential Oil. But for those plants in which there are no such volatile parts, or whose virtue doth not reside in those principles, such as astringent and emollient plants, for example, that owe their properties wholly to an earthy Salt, or to a mucilage, they are capable of communicating their whole virtue to the water in which they are infused or decocted.
If, on one hand, the Salts of plants render some portion of the principles of those plants soluble in water, such as part of their Oil and their earth, which if they were pure would not dissolve therein; on the other hand, these principles,[Pg 421] being of their own nature indissoluble in water, hinder the Salts, by the union they have contracted together, from dissolving in it so easily, so soon, and in such quantities, as if they were pure. This is so true, that water, though boiled long and violently, is far from extracting out of plants all those parts that it is capable of dissolving. If, after boiling a plant in water, as directed in the process, this water be poured off, fresh water added, and a second decoction made in the same manner as the first, the water of this latter decoction will, by that means, be almost as strongly impregnated with the principles of the plant as the former was. Mr. Boerhaave was obliged to make twenty successive decoctions of the same plant, to wit, Rosemary, before the water came off the plant colourless and insipid; in a word, just as it was before the plant was boiled in it.
Mr. Boerhaave observes, that a plant, after having thus given out all that water can dissolve, still retains exactly the same form that it had before it underwent any of the many boilings necessary to exhaust it; that its colour, from being green at first, becomes brown; and that the plant, which when green is lighter than water, or at least doth not sink in it, is heavier after this operation, and falls to the bottom. This is a proof that the water hath extracted out of the plant its lightest substances, assuming their places itself, and that it hath left nothing but its heaviest principles, namely, its fixed oil and its earth. We shall afterwards examine more particularly these remains of plants exhausted by water.
If the Infusions and Decoctions of plants be filtered, and evaporated in a gentle heat, they become Extracts, that may be kept for whole years, especially if they be evaporated to a thick consistence; and better still if they be evaporated to dryness.
From what hath been said concerning the Infusions, Decoctions, and Extracts of plants, it follows, 1. That Infusions and Decoctions of aromatic plants do not furnish a complete Extract of those plants; because they do not contain the volatile and odorous parts, in which the principal virtue of such plants usually resides. If therefore you desire to make Extracts of such vegetables, that shall have no defect, you must employ their juices drawn by expression, or water impregnated with their principles by the means of trituration, and evaporate the liquor by spreading it over a great number of plates, in order to enlarge its surface, and quick[Pg 422]en the evaporation, which must be effected by the heat of the sun alone, or the well-tempered warmth of a stove.
2. It may also be inferred, that water alone, aided by the degree of heat it is capable of acquiring by being made to boil, is not sufficient to effect the complete analysis of a plant; since not only some of its principles are still left combined in it, though exhausted as much as it can be by boiling water; but also several of the substances extracted from it by water are compounds of some of the principles of the plant, and susceptible of a much more accurate analysis; as we shall be convinced when we come to examine the effects which a degree of heat superior to that of boiling water is able to produce on entire plants, on their Extracts, and on their remains exhausted as much as they can be by boiling water.
But before we enter on that part of the analysis, it is proper to consider the experiments and combinations that may be made with the principles we have already obtained; in order to discover their nature, and in some measure analyze even them. Essential Oils in particular deserve to be thus examined.
We also obtain from certain plants, with a degree of heat less than that of boiling water, a Volatile Alkali, which exists formally in them: but as these plants, when analyzed, yield principles different from these we obtain out of all other vegetable substances, and as they resemble animal matters, we shall refer their analysis to a distinct chapter.
Of Operations on Essential Oils.
The Rectification of Essential Oils.
Put into a cucurbit the Essential Oil you propose to rectify. Set the cucurbit in a balneum mariæ; fit to it a head of tin, or of copper tinned, together with its refrigeratory; and lute on a receiver. Make the water in the bath boil, and keep up this degree of heat till nothing more will come over. When the distillation is finished, you will find[Pg 423] in the receiver a rectified Essential Oil, which will be clearer, thinner, and better scented, than before it was thus re-distilled; and in the bottom of the cucurbit will be left a matter of a deeper colour, more tenacious, more resinous, and of a less grateful smell.
OBSERVATIONS.
Essential Oils, even the purest, the best prepared, and the thinnest, suffer great changes, and are much impaired by growing old: they gradually turn thick and resinous; their sweet grateful scent is lost, and succeeded by a more disagreeable smell, somewhat like that of Turpentine. The cause of these changes is, that their finest and most volatile part, that which contains most of the odorous principle, is dissipated and separated from that which contains least of it; which therefore grows thicker, and comes so much the nearer to the nature of a resin, as the quantity of Acid, that was distributed through the whole Oil before the dissipation of the more volatile part is, after such dissipation, united and concentrated in the heaviest part; the Acid in Oils being much less volatile than the odorous part, to which alone they owe their levity.
Hence it appears what precautions are to be used for preserving Essential Oils, as long as possible, without spoiling. They must be kept in a bottle perfectly well stopped, and always in a cool place, because heat quickly dissipates the volatile parts. Some authors direct the bottle to be kept under water.
If these Oils should grow thick and resinous by age, yet they are not to be thrown away. We shall shew, in the analysis of Balsams and Resins, that, from these thick and even solid substances, Essential Oils may be drawn, as thin and as limpid as from plants. Essential Oils, thickened by time, may therefore be treated like Balsams, and actually analyzed, by separating all the subtile odorous matter they contain from their thick acid parts. For this purpose they need only be distilled with a degree of heat just sufficient to elevate the thin odorous parts, without raising the thick matter.
The residue left at the bottom of the vessel, because it could not rise in distillation, is much thicker and less odorous than the Oil was before rectification. The reason of this is evident, and follows from what hath just been said. This remainder dissolves in Spirit of Wine more readily, and in greater quantity, than the light Oil drawn from it; be[Pg 424]cause it contains more Acid, and because Oils owe their solubility in this menstruum to their Acid part, as is proved in our Memoir on Oils already quoted.
When we come to treat of Resins, we shall inquire more particularly what this remainder is, and what principles it yields when analyzed: in this place it is sufficient to take notice, that though all the Oil of which it made a part came over at first with the heat of boiling water, yet it cannot now be raised by the same degree of heat in distillation; because it is not now combined with the principle of odour which gives the Oil its volatility, and because it is rendered sluggish by being clogged with too great a proportion of Acid.
From what hath been already said, it must be concluded, that Essential Oils suffer great diminution by being rectified; and that in proportion to the quantity of resinous matter left behind. All this resinous matter, while combined with a proper quantity of the odorous principle of the plant, (that is, at the time of its being distilled, and a little while after), was really an Essential Oil: the change of its nature, therefore, is entirely owing to its having left that principle.
An Essential Oil, though rectified, is still as apt to change and be spoiled as before, because it still continues to lose its odorous principle by degrees. After some time, therefore, it requires a second rectification, which again lessens its quantity. In short, it is plain that Oils will, in a number of years, greater or smaller according to their nature, and the manner in which they are kept, be wholly changed, and metamorphosed into a resinous matter, from which no thin Oil can be drawn with the heat of boiling water: and this is a proof of the fugacity of that odorous principle, or Spiritus Rector, of plants, which, when united with their lightest Oil, gives it the character of an Essential Oil.
This resinous matter, to which Essential Oils are finally reduced, being subjected to repeated distillations, with a degree of heat superior to that of boiling water, is still capable of yielding a certain portion of a thin, limpid, sweet-scented Oil, which is as light as an Essential Oil; as we observed before is the case with Fat Oils drawn by expression: but the thin Oil obtained by this means, though it possesses almost all the properties of an Essential Oil, is not for all that a genuine one; seeing it hath not the same odour with the plant from which it was originally drawn.
Essential Oils must be rectified in the balneum mariæ, as ordered in the process: for, as some of the Oil touches the sides of the vessel in the operation, if that vessel be made[Pg 425] hotter than boiling water, the thick matter will rise with the thin Oil, which therefore will not be rectified.
Rectification is of use not only for procuring to Essential Oils the tenuity and levity they may have lost by age, but also to separate them from other oily matters with which they may be adulterated. If, for instance, an Essential Oil be not properly distilled; if, by the addition of too much Salt, the water have acquired a degree of heat greater than that of pure boiling water, and if, in consequence thereof, some of the heavy Oil of the plant have risen with the Essential Oil, and mixed therewith, the Essential Oil may, by rectification, be separated from this heterogeneous Oil; which, being heavier and incapable of rising with the heat of pure boiling water, will remain at the bottom of the vessel.
The effect will be the same, if your Essential Oil be falsified with a mixture of any Fat Oil, as is often the case: for, some of them being extremely dear, the vender frequently adds a portion of Fat Oil to increase the quantity. For this purpose Oil of Ben is generally used.
When an Essential Oil is thus falsified with a mixture of any Fat Oil, it may be discovered by letting a few drops of it fall into rectified Spirit of Wine; which will dissolve the Essential Oil only, leaving the Fat Oil quite untouched.
Essential Oils are sometimes falsified by mixing them with a certain quantity of Spirit of Wine. This fraud doth not render their smell less fragrant: on the contrary, it becomes rather more agreeable and quicker. In order to try an Oil suspected of being falsified in this manner, drop a little of it into very clear water. If a milky cloud appear in the water, be assured the Oil is mixed with Spirit of Wine: for as this liquor unites more readily with water than with Oil, it quits the Oil with which it was mixed to incorporate with the water: mean time a good deal of the Oil that was dissolved by the Spirit of Wine, and is now separated from it by the intervention of water, necessarily remains dispersed through this water in very small particles; and these form the milky cloud produced on this occasion.
An Essential Oil may also be adulterated with another Essential Oil that is much more common, and of much less value. Those who practise this fraud generally employ Oil of Turpentine for that purpose, on account of its cheapness and tenuity. The cheat is easily discovered, by moistening a linen rag with the Oil supposed to be thus falsified, and then holding the rag a little before the fire, which presently dissipates the odorous part of the falsified Oil. This odour,[Pg 426] which prevented our distinguishing that of the Oil of Turpentine, being vanished, the peculiar smell of the Turpentine, which is much more permanent, remains alone; and is so perceptible that it cannot easily be mistaken.
Those who are much accustomed to see and examine Essential Oils, have seldom occasion to make the experiments here proposed for discovering their qualities. A certain degree of thickness, partaking of unctuosity, in an Essential Oil, convinces them that it is falsified with a Fat Oil: on the other hand, a greater degree of tenuity, together with a quicker smell, than a pure Essential Oil ought to have, discovers the admixture of Spirit of Wine. Lastly, any one, whose sense of smelling is not very dull, will easily discover the odour of the Oil of Turpentine, though disguised by that of the Essential Oil with which it is mixed.
To fire Oils by combining them with highly concentrated Acids: instanced in Oil of Turpentine.
Mix together, in a glass, equal parts of concentrated Oil of Vitriol, and highly smoking fresh-drawn Spirit of Nitre: pour this mixture at several times, but suddenly, on three parts of Oil of Turpentine, set for that purpose in a glass bason. By a part here must be understood a dram at least. A most violent commotion, accompanied with smoke, will immediately be raised in the liquors, and the whole will take fire in an instant, flame, and be consumed.
OBSERVATIONS.
There is not in Chymistry a phenomenon more extraordinary, and more surprising, than the firing of Oils by mixing them with Acids. It could never have been suspected that a mixture of two cold liquors would produce a sudden, violent, bright, and lasting flame, like that we are at present considering. Beccher gave notice, in his Physica subterranea, that highly rectified Spirit of Wine would be set on fire by mixing it with highly concentrated Oil of Vitriol.
Afterwards Borrichius, a Danish Chymist, published a process for kindling Oil of Turpentine, by mixing it with the Nitrous Acid, as we find in the Philosophical Transactions of Copenhagen for the year 1671. Most Chymists have since[Pg 427] tried to repeat those experiments, and particularly to fire the Oil of Turpentine by mixing it with Oil of Vitriol, or Spirit of Nitre; but to no purpose, when they made use of the Oil of Vitriol, till Mr. Homberg told us, in the Memoirs of the Academy of Sciences for 1701, that he had fired Oil of Turpentine by mixing it with Oil of Vitriol.
To make the experiment succeed he requires, "That the Oil of Vitriol be dephlegmated as much as possible, and that the Oil of Turpentine be the last that comes over in distillation, which is thick like a syrop, and of a dark-brown colour; for that which is white, and rises at the beginning of the distillation, never takes fire." These are his own words: but no body else hath ever succeeded in making the experiment.
Tournefort had succeeded, a little before Homberg, in firing, not Oil of Turpentine indeed, in which he always failed, but the Oil of Sassafras, by mixing it with an equal quantity of well dephlegmated Spirit of Nitre. Homberg came afterwards, as appears by the Memoirs of the Academy for the year 1702, to fire with Spirit of Nitre the Essential Oils of the aromatic plants of India; and in 1706 Mr. Rouviere fired, with Spirit of Nitre, the empyreumatic Oil of Guaiacum. While this Oil of Guaiacum is burning, a porous spongy body rises from the midst of the flame, to the height of about two feet above the vessel.
Lastly, several years after all these discoveries, Messrs. Geoffroy and Hoffman, the one at Paris, and the other at Hall in Saxony, found a way to fire the Æthereal Oil of Turpentine, each by a different process; yet agreeing in this, that they both combined the Vitriolic Acid with the Nitrous, and with this compound Acid fired that Æthereal Essential Oil, which is one of the thinnest, and, probably for that very reason, the most unfit to produce a flame with Acids.
The most celebrated Chymists, as appears from this short account, have employed themselves in firing Essential Oils; but no body attempted the experiment on Fat Oils. It was not so much as suspected that they were capable of taking fire after this manner, till in 1745 I read before the Academy a Memoir on Oils, which I have already mentioned, and in which I express myself thus:
"I put two ounces and a half of Walnut Oil into the bottom part of a broken retort, having the figure of a cap, or concave hemisphere; and poured thereon two ounces of smoking Spirit of Nitre. It was scarce put in when a considerable ebullition arose, with a very thick smoke. As[Pg 428] I found it continually increasing, and very fast too, I retired a little, that I might observe the event without danger. This caution was not unnecessary: for immediately the whole mixture blew up as high as the ceiling, with a noise like the discharge of a musket. Nothing was left in the vessel but a black matter, which still continued to boil a little and run over, and at last remained very rare, spungy, and as full of holes as a honeycomb: its consistence also was such that it did not stick to my fingers when I handled it.
"As Mr. Geoffroy, who first found the means of firing the natural Balsams, observed in them a similar explosion on that occasion, it appears that my Oil was very near taking fire in this experiment: which makes me presume that we may at last succeed in firing Fat Oils likewise, and consequently all others; seeing these have always been looked upon as the most unlikely to produce that phenomenon. I imagine that, to accomplish this, nothing more is necessary than to make use of sufficiently great quantities, and to order it so that the surfaces of the liquors, where they come into contact, may be of a large extent."
Afterwards, in 1747, Mr. Rouelle read before the Academy a Memoir on the accension of Oils by Acids. That Memoir contains a great number of curious experiments, and peculiar manual operations described very distinctly, from which there results a general method of firing without fail, not only Essential Oils, but even any Fat Oil whatever: so that my conjecture, concerning the possibility of firing these latter Oils, mentioned in my above-cited Memoir of 1745, is now changed into a certainty. I shall proceed to explain how I conceive these accensions are brought about, and endeavour to account for the phenomenon from such causes as to me seem the most probable.
A due attention to the phenomena produced by mixing Oils with Acids will enable us, I imagine, to discover the natural cause why the Oils take fire. It is certain, and demonstrated by the most decisive experiments, that the friction of several bodies rubbing against each other produces heat; and that when these bodies are combustible, and the heat produced by their friction rises to a certain degree, they take fire. This, in my opinion, is what happens to Oils when mixed with concentrated Acids. When these two sorts of substances rush into union with rapidity, as in the experiments under consideration, there must necessarily be a great friction among their parts. This friction produces the heat[Pg 429] observed at the time of their union. The more concentrated the Acids are, with the greater violence and rapidity do they act upon the Oils, and the greater is the heat raised. If the Acids be concentrated to such a degree as to produce, by uniting with the Oils, a heat equal to that of an ignited body, the combustible substances that are exposed to it, which in this case are Oils, must needs take fire and flame.
The heat produced on this occasion is so great, that, even when the inflammation doth not take place, if you touch the surface of the Oil with your finger, as soon as the Acid hath had its effect, you will find it burn you like a live coal.
Two pieces of wood, rapidly and violently rubbed against each other, take fire. What is it that is kindled in this case? It can be nothing but their Oil: for they contain no other combustible principle. Why doth this Oil take fire? I do not think it possible to assign any reason for it, but the heat produced by the friction of the pieces of wood containing the Oil. If, when Oil is dispersed in a body, of which it is only one component principle, and consequently mixed with many saline, aqueous, and earthy parts, that are not inflammable, but, on the contrary, make the Oil less so, the Oil nevertheless takes fire, and burns when agitated by a sufficient degree of heat; why shall not this very Oil, when separated from the mixt of which it made a part, when united into one distinct mass, and entirely, or almost entirely, freed from the heterogeneous, incombustible parts with which it was combined, and consequently now more inflammable than before; why, I say, shall it not take fire, when exposed to a degree of heat equal, or rather superior, to that which is produced by rubbing two pieces of wood together?
Let us now examine the phenomena produced when Oils are fired by Acids, all the circumstances that favour or hinder their accension, and see if they agree with the explanation here offered.
First, no sort of Oil will take fire with any Acid whatever that is not highly concentrated; for weak Acids act but feebly on Oils, and dissolve them slowly; so that the friction is neither quick nor violent, and consequently produces too faint a heat, far below the degree of ignition.
Secondly, no inflammation is produced when Acids and Oils are mixed in too small quantities; but the more Acid and Oil you mix together, the greater is the certainty of succeeding: for the heat is exactly in proportion to the friction that produces it; and the total quantity, or amount, of this friction is so much the greater, as there are more particles[Pg 430] rubbing against each other at the same time. So that if a very small quantity of Acid and Oil be mixed together, there will be but a very small quantity of friction, and consequently a very small quantity of heat; and in that case no inflammation. It was with a view to avoid these inconveniencies, and to procure the opposite advantages in as great a degree as possible, that, in the passage above quoted from my Memoir of Oils, I proposed mixing together large doses of Acid and of Oil, as one of the means by which we might succeed in the accension of Fat Oils.
Thirdly, the figure of the vessel, in which the two liquors are mixed together, is not a matter of indifference. A wide-spreading vessel, of a large diameter with respect to the quantity of liquor it is to contain, favours the inflammation much more than one of a small diameter. Nay, it may not succeed at all in too narrow a vessel, though all other circumstances be properly attended to.
The reason of this is, that the activity of heat produced by friction is not in proportion to the successive, but to the simultaneous frictions: for the heat actually produced by the frictions of an hundred particles, rubbing successively against each other, with intervals sufficient to let the heat go off, almost as fast as it is generated, would be equal to the friction of a single particle only; whereas the heat actually produced by the friction of the same number of particles, all rubbing against each other at the same instant, would be equal to the frictions of all the particles taken together, and consequently an hundred times more active than the other[11]. This being laid down, it is easy to conceive how a large vessel favours the accension more than a small one. It is certain that two liquors which mutually present large surfaces to each other, at the instant of their being mixed together, touch each other at one and the same time in a much greater number of points, than if each had but a small surface; and consequently that they must unite much sooner, and with greater rapidity, in the former case than in the latter.
With these views, and in order to give the liquors this advantageous disposition, I recommended it as what would greatly promote the inflammation of Fat Oils, to order the liquors so, that, at the moment of their mixture, a large surface of each might come into contact with the other.
Fourthly, if we reflect on the experiments hitherto made for kindling Oils by Acids, we shall easily be convinced that all Oils are not equally apt to be fired; and that light, æthereal, very thin, Essential Oils do not produce this phenomenon so readily and so surely, as those of the same kind that are heavy and thick, or at least soon grow thick upon being mixed with Acids.
Mr. Homberg says positively in the above-cited passage of his Memoir, that he never could succeed in setting fire with the Acid of Vitriol to the white, æthereal Oil of Turpentine; that is, to the lightest which comes over first in distillation; but that the very same Acid set fire to "that which comes over last in distillation, which is thick like a syrop, and of a dark-brown colour."
All the experiments by which Oils have been fired, from those of Beccher and Borrichius down to those of Geoffroy and Hoffman, were made on the Essential Oils of the aromatic plants of India, which are the heaviest we know, and on the empyreumatic Oil of Guaiacum, which, besides being very ponderous, is also very thick.
Now these singular effects likewise agree perfectly well with our explanation. It is certain that the parts of a heavy fluid do not yield to any impulse or shock, so easily as those of a lighter fluid; just as the parts of a thick, viscous fluid undoubtedly resist any attempt to separate them, so much the more the nearer the consistence of that fluid is to solidity, or the further it is removed from the state of fluidity. Now, the more resistance the Acid meets with in separating and dividing the parts of the Oil, as it must do to dissolve them, the more considerable will be the force and motion with which it must necessarily act to surmount those obstacles; besides, as experience teaches us that the density and viscidity of the Oils do not, at least to sense, diminish the quickness and activity which the Acid exerts in uniting with them; the greater therefore must be the collisions, frictions, and heat produced: and this plainly shews why heavy, thick Oils take fire, in this case, more readily than those which are fluid and light.
It may here be objected, that Fat Oils, which are thicker and heavier than the light Essential Oils, take fire nevertheless[Pg 432] with greater difficulty. This objection is easily answered, by observing, that when we say Acids fire heavy thick Oils with more ease than thin light Oils, this position must be restricted to Oils of the same kind, on which Acids have an equal, or nearly equal, action; that is, to such Oils as differ from each other in no other respect but their thickness and weight.
For example, Mr. Homberg, who could by no means set fire, with Oil of Vitriol, to the Oil that rises first in the distillation of Turpentine, found that the same Acid would fire the Oil that comes last over: and therefore it is reasonable to attribute his success, in firing this last Oil, to its being thicker and heavier than the former; seeing these two Oils are in other respects of the same nature; that Acids have an equal action on both; and that they differ from each other only in the qualities specified above.
But it is evident, that, if the Oils compared together be of different kinds, and differ from each other, not only in weight and thickness, but also by containing different principles, or, at least, the same principles combined differently, and in different proportions, the action of any Acid on those Oils must also be different; and that regard must be had thereto in determining their degrees of inflammability.
Now all this is applicable to Fat Oils, when compared with light Essential Oils, in point of inflammability. If all these Oils were of the same nature, and differed from each other in weight and thickness only, the objection drawn from Fat Oils, which though thicker than Essential Oils do not take fire so easily, would be a very good one, and fact would be against our reasoning. But this is far from being the case: the properties, as well as the analysis, of Fat Oils shew their nature to be very different from that of Essential Oils; that there is more water in their composition; and that they are full of a mucilaginous or gummy principle, which must greatly obstruct their inflammability, and the action of Acids upon them.
None of the effects, therefore, that attend the firing of Oils with Acids, is repugnant to our way of accounting for the phenomenon, which is one of the most beautiful in all Natural Philosophy. To conclude this important subject, nothing now remains but to consider the effects produced by the Vitriolic Acid in these accensions.
This Acid, though of a stronger nature, and capable of being more highly concentrated than the Nitrous Acid, seems however less qualified to produce a flame with Oils. Indeed[Pg 433] Mr. Homberg fired Oil of Turpentine by mixing it with Oil of Vitriol: but I do not know that the experiment hath succeeded with any other Chymist; on the contrary, most of those who have tried it affirm, that they never could fire any Oil with that Acid alone.
Oils are probably in the same case as metallic substances, with regard to these two Acids. We know that the Nitrous Acid dissolves those substances with vastly more activity and violence than the Vitriolic Acid exerts upon them; which may depend, either on the disposition and configuration of their parts, or on the portion of phlogiston which, according to the opinion of most Chymists, is united with the Nitrous Acid, is its peculiar characteristic, and the cause of the great vivacity with which it dissolves almost all matters that contain the phlogiston.
I say almost all matters that contain the phlogiston; because there are some substances that contain a great deal thereof, and yet are not at all acted on by the pure Nitrous Acid. These substances are matters perfectly charred: that is, such as are capable of enduring the greatest violence of fire in close vessels, without yielding a single atom of Oil; that burn almost quite away, yet only grow red hot without flaming; or at least produce but a very small, slight flame, from which it is impossible to obtain the least particle of soot or fuliginosity; in a word, that contain an inflammable matter, but such as is fit to be an ingredient in the composition of metallic substances, to which the peculiar title of the Phlogiston is appropriated.
I say, then, that if the Nitrous Acid be poured on a mere coal, perfectly charred, it is impossible for the Acid, be it ever so highly concentrated, to set the coal on fire, though heated before to the greatest degree that it can possibly admit of without kindling; and, which is still more remarkable, if a live coal be plunged into the most highly smoking Spirit of Nitre, it will be extinguished as if dipt in pure water.
But to return to the Vitriolic Acid: it is singular enough that this Acid, which attacks Oils with less activity, and for that reason seems less fit to set them on fire, than the Nitrous Acid, yet greatly promotes their accension, when mixed with that very Acid. This may be owing to its rendering the Oils with which it mixes heavier and thicker; or else, as Mr. Rouelle conjectures with great probability, being more concentrated than the Nitrous Acid, and having a greater affinity with water, it dephlegmates the other, and thereby increases its activity; or, lastly, this may arise from some[Pg 434] other cause yet unknown to us, and perhaps from that by which the Acids of Nitre and of Sea-salt, which, when separate and perfectly pure, can neither of them dissolve Gold, are enabled, when combined together, to make a perfect solution of that metal.
To combine Essential Oils with Mineral Sulphur. Balsam of Sulphur. This Composition decompounded.
Put into a matrass one part of Flowers of Sulphur; pour on them six parts of the Essential Oil of Turpentine, for instance; set the matrass in a sand-bath, and heat it gradually till the Oil boil. The Sulphur, which at first lay at the bottom of the matrass, will begin to melt, and appear to dissolve in the Oil. When it hath boiled in this manner for about an hour, take the matrass from the fire, and let the liquor cool. A great deal of the Sulphur that was dissolved therein will separate from it as it cools, and fall to the bottom of the vessel in the form of needles, much like a Salt shooting in water.
When the liquor is perfectly cold, decant it from the Sulphur that lies at the bottom of the vessel: to that Sulphur put fresh Oil of Turpentine, and proceed as before: the Sulphur will again disappear, and be dissolved in the Oil: but when the mixture is cold, you will find new crystals of Sulphur deposited at the bottom. Decant once more this Oil from the crystals, and pour on fresh Oil to dissolve them: continue the same method, and you will find that about sixteen parts of Essential Oil are required to keep one part of Sulphur dissolved when cold. This combination is called Balsamum Sulphuris Terebinthinatum, if made with Oil of Turpentine; Anisatum, if with Oil of Anise-seeds; and so of others.
OBSERVATIONS.
Essential Oils do not dissolve Sulphur, in such quantities, and with so much ease, as Fat Oils do. It was shewn above, that a Fat Oil is capable of keeping a considerable quantity of Sulphur in solution; whereas no less than sixteen parts of Essential Oil are required to dissolve one part only of Sulphur, as in this process.
The property which Sulphur hath of separating, in part, from the Essential Oil in which it is dissolved, and falling to the bottom of the vessel in the form of crystals, as the Oil cools, proves that it is a kind of Neutral Salt, which, being insoluble in water, because of the great quantity of inflammable matter that serves it for a basis, is not to be dissolved but by substances that actually contain themselves a great deal of inflammable matter; such as Oils and Metallic substances.
Though the latter are almost always solid, it nevertheless unites with several of them into regular forms, resembling saline crystals in every thing but pellucidity; as appears, for example, in several Pyrites, Antimony, and some other sulphureous minerals. But when it is dissolved in Oils, especially in such as are capable of keeping but a small quantity thereof in solution, and consequently drop a good deal of it as they cool, it is precisely in the case of one of those Salts whereof hot water dissolves more than cold; that is, the Oil, that is saturated with as much Sulphur as it can possibly take up when boiling hot, lets some part thereof precipitate as it cools; while the Sulphur thus separated from the Oil unites into little glebes of a regular figure, and actually crystallizes; in the same manner as Nitre, when boiling water hath dissolved as much thereof as it can possibly take up, partly separates from it when it cools, and falls to the bottom of the vessel in small crystalline moleculæ, of the form peculiar to that Salt.
Mr. Homberg made some very curious experiments on this combination of Sulphur with an Essential Oil. In the Memoirs of the Academy he gives the following analysis thereof.
"Put your Sulphur dissolved by Oil of Turpentine into a pretty large retort, because the matter puffs up towards the end, and distil with a very gentle heat for twelve or fifteen days and nights. There will come over about two thirds of the quantity of a colourless Oil of Turpentine, and at the same time a pretty considerable quantity of a whitish ponderous water, as acid as good Spirit of Vitriol. After this, the drops of Oil that come off will begin to be red. Then change your receiver, and increase the fire gradually; and in seven or eight hours time, with a very great heat, force off all that will rise, using a glass retort for your recipient. At last, most of the Oil will come over into the receiver very thick and high-coloured, still accompanied with a whitish and very acid water. In the retort will be left a black caput mortuum, spongy, or fo[Pg 436]liated, shining, and insipid.... This caput mortuum neither grows white, nor flames, nor wastes considerably in a strong fire.
"The matter that comes over into the receiver must be distilled again, with a very gentle heat continued for several days and nights, in order to separate once more the colourless Oil and the remaining acid water, till the Oil begin to come off red. Then take the retort from the fire, and on the black gummy matter left in it pour good Spirit of Wine; mix the whole well together, and distil with a very gentle heat. When this Spirit of Wine is come off, pour some fresh on the black gum left in the retort, and distil as before. Repeat this till the Spirit of Wine cease to have a bad smell."
There is great reason to believe, that, by the union which the Sulphur contracts with the Oil, the cohesion of the Acid and the Phlogiston, which constitute that mineral, is considerably weakened; and that this is what occasions the decomposition of the Sulphur so manifest in Mr. Homberg's analysis. The inflammable matter of the Sulphur is so incorporated with that of the Oil in the solution, that they form together one homogeneous whole; by which means the Acid of the Sulphur, which is of course dispersed through the whole liquor, is not now combined with the Phlogiston, as it was in the Sulphur before it was blended with the Oil; that is, with the pure Phlogiston; but with that Phlogiston which constitutes the oily mixture, or, which is the same thing, with actual Oil. And this is the reason that a composition of Oil and Sulphur yields, in distillation, nearly the same principles that a combination of the same Oil with the Vitriolic Acid would yield.
We have already seen, under the head of Fat Oils, that when Oils are combined with Acids, if this combination be again decompounded by distillation, those two substances cannot be obtained in their original state; but that they are changed and partly decomposed. The case is the same in the experiment before us. We first get, by distillation, a pretty considerable quantity of Oil of Turpentine, that seems to have suffered no change at all. This first Oil is that which the action of fire separates from the Acid; and this it effects with so much the more ease, that, a great quantity thereof having been necessarily used to dissolve a little Sulphur, it greatly exceeds the quantity of Acid in the mixture, and that the distillation is ordered to be made with a very weak degree of heat: for M. Homberg says, it ought to be conti[Pg 437]nued twelve or fifteen days and nights. Now this manner of distilling, with a very gentle heat, is the most effectual means of separating Oils, especially light Essential Oils, from Acids; because these Oils rise in distillation with very little heat; whereas the Acids, being much more ponderous, require a great deal more.
The Oil that rises first in distillation, appears indeed to be the same with that which was originally used in the mixture; but the quantity is much smaller: first, because some part of it, being combined with the Acid of the Sulphur, is thereby rendered thick and heavy, which hinders it from rising in this first distillation with a very gentle heat, and is the reason that it cannot be elevated without a much stronger degree of fire. It is this part that afterward comes over in the form of a red liquor upon increasing the fire.
The second cause why the quantity of Oil is lessened, is, that part of it is decomposed in the operation. This decomposed part of the Oil furnishes that considerable quantity of water which ascends at the same time with the Oil, or a little after it, and serves for a vehicle to the Acid that rises with it in this first distillation; which Acid, though pretty strong, is now much more loaded with water than when it was an ingredient in the combination of Sulphur. This acid water is of a milky white colour, because many oily particles are suspended and diffused in it, but not perfectly dissolved.
The caput mortuum that is left in the retort, after all the red thick Oil is driven up by a very strong degree of fire, is a sort of charred matter, consisting of some of the earth of the Sulphur, and of the decomposed Oil, united with a phlogiston, which is probably furnished by both these substances. This matter contains also a little Acid fixed with it. This Acid reproduces Sulphur, or at least becomes sulphureous, and flies off in vapours, when the coal is urged by a violent forge-heat: for Mr. Homberg observed, that by this means it exhaled an odour of Sulphur, and lost in weight.
This charred matter is of a singular nature: for, by being exposed to a forge-heat, and even to the heat in the focus of a burning glass, it seemed to suffer no other change than some loss of weight, occasioned by the evaporation of the acid effluvia carried off by the heat; for it still retained its black colour, and was neither consumed nor vitrified. In order to melt it, Mr. Homberg was forced to mix it with Borax. This Salt converted it into a glass of a dark-grey colour: and, as there appeared a little verdegris on the sur[Pg 438]face of this glass after keeping it in a moist place, he thereby found that the Sulphur he had used contained a little Copper.
We know that the earth of Copper is refractory, and that it communicates a dark colour to matters vitrified along with it: and perhaps it was the cause why the fixed matter in question retained its blackish colour so obstinately, notwithstanding the phlogiston that must have been in it at first was, in all probability, consumed by the violent ignitions it underwent.
As to the thick oily matter, called gummy by Mr. Homberg, from which he directs Spirit of Wine to be repeatedly distilled, till it cease to have a disagreeable smell, there is great reason for thinking it to be, as we said before, a portion of the Oil which the Acid hath rendered thick and heavy. The Spirit of Wine dissolves and carries up the most acid part, which always hath a disagreeable smell.
Mr. Homberg says, that "the part remaining after this, which he calls the Gum of common Sulphur, hath a pleasant balsamic odour; that it partly dissolves in Spirit of Wine, a hard resinous matter being left, which will not dissolve, either in Spirit of Wine, or in the strongest lixivium." Of consequence, therefore, it is neither a resinous matter nor a sulphur; "yet it dissolves perfectly in distilled Oils." What then is this singular body? It is certainly a subject for very curious inquiries. In general, Mr. Homberg's whole process is full of interesting facts, and well deserves to be repeated, carried further, and carefully attended to.
To combine Essential Oils with Fixed Alkalis. Starkey's Soap.
Take Salt of Tartar, or any other Alkali, thoroughly calcined. Heat it in a crucible till it be red, and in that condition throw it into a hot iron mortar: rub it quickly with a very hot iron pestle; and as soon as it is powdered pour on it, little by little, nearly an equal quantity of Oil of Turpentine. The Oil will enter into the Salt, and unite intimately with it, so as to form a hard paste. Continue rubbing this composition with the pestle, in order to complete the union of the two substances; and, as your Oil of Tur[Pg 439]pentine disappears, add more, which will unite in the same manner, and give a softer consistence to the soapy mass. You may add still more Oil, according to the consistence you intend to give your Soap.
OBSERVATIONS.
Essential Oils do not unite near so easily as Fat Oils with Alkalis. For this reason, to make a Soap with an Essential Oil, we must take a method different from that used in common soaperies. For if an Essential Oil be substituted for the Fat Oil, in the ordinary way of making Soap, far from combining with the alkaline lixivium, though ever so strong, it will be wholly dissipated and vanish: so that, after boiling some time, you will find nothing but the lye, just as when first put in, only a little more concentrated.
The water, in which the Alkali is dissolved when in the form of a lye, is the principal thing that hinders the Salt from uniting with the Essential Oil. Water is such an enemy to this union, that, if the Alkali be ever so little moist, the operation will not succeed; even though all the other precautions mentioned in the process should be exactly observed.
In order, therefore, to free the Alkali from all humidity, it is necessary to begin with making it red-hot; and then, that this Salt, which is very greedy of moisture, may not imbibe any from the air, before it be mixed with the Essential Oil, it must not be suffered to cool; but the mixture must be made in a hot vessel, as soon as the Salt is reduced to powder. When every particle of the Salt is once covered with Oil, you need not fear its attracting any moisture, at least very quickly, because the Oil opposes its admission.
Starkey, the first Chymist who found the means of making Soap with an Essential Oil, and by whose name this kind of Soap is therefore called, made use of a much more tedious method than that proposed in our process. He began with mixing a very small quantity of Oil with this Salt, and waited till all the Oil united therewith of its own accord, so as to disappear entirely, before he added any more; and thus protracted his operation exceedingly, though in the main it was the same with ours. The method here proposed is more expeditious, and was invented by Dr. Geoffroy.
Starkey's Soap dissolves in water much as common Soap does, without any separation of the Oil: and by this mark[Pg 440] it is known to be well made. It may also be decompounded, either by distillation, or by mixing it with an Acid: and its decomposition, in either of these ways, is attended with nearly the same phenomena as the decomposition of common Soap.
Of the Substances obtained from Vegetables by Means of a GRADUATED HEAT, from that of boiling Water, to the strongest that can be applied to them in close Vessels.
To analyze Vegetable Substances that yield neither a Fat nor an Essential Oil. Instanced in Guaiacum-Wood.
Take thin shavings of Guaiacum-wood, and put them into a glass or stone retort, leaving one half thereof empty. Set your retort in a reverberating furnace, and lute on a large glass receiver having a small hole drilled in it; such as is used for distilling the Mineral Acids. Put a live coal or two in the furnace, to warm the vessels gently and slowly.
With a degree of heat below that of boiling water, you will see drops of a clear insipid phlegm fall into the receiver. If you raise the fire a little, this water will come slightly acid, and begin to have a pungent smell. With a degree of fire somewhat stronger, a water will continue to rise which will be still more acid, smell stronger, and become yellowish. When the heat comes to exceed that of boiling water, the phlegm that rises will be very acid, high coloured, have a strong pungent smell, like that of matters long smoked with wood in a chimney, and will be accompanied with a red, light Oil, that will float on the liquor in the receiver.
And now it is necessary that the operation be carried on very cautiously, and vent frequently given to the rarefied air by opening the small hole in the receiver: such an incredible quantity thereof rushing out of the Wood, with this degree of heat, as may burst the vessels to pieces, if not discharged from time to time.
When this red, light Oil is come over, and the air ceases to rush out with impetuosity, raise your fire gradually, till the retort begin to redden. The receiver will be filled with dense vapours; and, together with the watery liquor, which will then be extremely acid, there will rise a black, thick, ponderous Oil, which will fall to the bottom of the receiver, and lye under the liquor.
Then give the utmost degree of heat; that is, the greatest your furnace will allow, and your vessels bear. With this excessive heat a little more Oil will rise, which will be very ponderous, as thick and black as pitch; and the vessels will continue full of vapours that will not condense.
At last, when you have kept the retort exceeding red for a long time in this extremity of heat, so that it begins to melt, if it be of glass, and you perceive nothing more come over, let the fire go out and the vessel cool. Then take off your receiver: from the black oil at bottom decant the acid liquor with the red Oil floating on it, and pour them both into a glass funnel, lined with brown filtering paper, and placed over a bottle. The acid liquor will pass through the filter into the bottle, and the Oil will be left behind, which must be kept by itself in a separate bottle. Lastly, into another funnel, prepared as the former, pour the thick Oil remaining with a little of the acid liquor at the bottom of the receiver. This liquor will filter off in the same manner, and thus be separated from the heavy Oil.
In the retort you will find your Guaiacum-shavings, not in the least altered as to their figure, but light, friable, very black, scentless, and tasteless, easily taking fire, and consuming without flame or smoke; in short, you will find them charred to a perfect coal.
OBSERVATIONS.
Hitherto we have examined the substances that may be obtained from vegetables, either without the help of fire, or with a degree of heat not exceeding that of boiling water. The analysis of plants can be carried no further without a greater degree of heat: for, when the principle of odour, and the essential oil of an aromatic plant, are wholly extracted by the preceding processes, if the distillation be afterward continued without increasing the heat, nothing more will be obtained but a little Acid; which will soon cease, as a small part only of the quantity contained in the plant will be elevated; the rest being either too ponderous, or too much en[Pg 442]tangled with the other principles of the body, to rise with so small a degree of heat.
In order, therefore, to carry on the decomposition of a plant, from which you have, by the methods before proposed, extracted all the principles it is capable of yielding when so treated; or, which comes to the same thing, in order to analyze a vegetable matter, which affords neither an expressed nor an essential oil, it must be distilled in a retort with a naked fire, as directed in the process, and be made to undergo all the degrees of heat successively, from that of boiling water, to the highest that can be raised in a reverberating furnace.
A heat inferior to that of boiling water, with which we must begin in order to warm the vessel gradually, brings nothing over, as hath been said, but an insipid water, destitute of all acidity. By increasing it nearly to the degree of boiling water, the distilled water comes to be slightly acid.
When the heat is made a little stronger than that which is necessary for the elevation of an Essential Oil, the acidity of the water that comes off is much more considerable. It hath now both colour and smell, and there rises with it a red, light Oil, that floats on the liquor in the receiver. This is not an Essential Oil; it hath none of the odour of the plant. Though so light as to float on water, yet it will not rise with the degree of heat that raises Essential Oils; even those that much surpass it in gravity, and will not swim on water as this does. This proves that the ease or difficulty, with which a particular degree of heat raises any substance in distillation, doth not depend altogether on its gravity: its dilatability, or the volatile nature of the matters, with which it is so closely united as not to be separated from them by distillation, may probably contribute greatly to produce this effect.
It is very surprising that a substance so hard, so compact, so dry, in appearance, as Guaiacum-wood, should yield such a large quantity of water by distillation; and it is equally so, that it should discharge so much air, and with so much impetuosity, as nothing but experience could render credible. We have, in the process, directed the precautions to be taken when this air, from being prodigiously condensed in the body of which it made a part, is set at large, rushes out of confinement, and expands with all its natural elasticity. From this air arises the greatest danger attending the operation.
It hath been remarked, that the heaviest and most compact[Pg 443] woods yield the most air in distillation: and accordingly Guaiacum-wood, which we have chosen for an instance, as exceeding almost all others in hardness and weight, discharges a vast quantity of air when analyzed.
The thick, burnt, empyreumatic Oil, that comes over last in this distillation, is heavier than water; on account, probably, of the great quantity of Acid with which it is replete. The two kinds of Oil obtained in this analysis may be rectified, by distilling them a second time, or rather several times; by which means they will become lighter and more fluid, as we have seen happen to Fat and Essential Oils. In general, all thick, heavy Oils constantly owe these qualities to an Acid united with them; and it is by being freed from some of that Acid in distillation, that they always acquire a greater degree of lightness and fluidity from that operation. To these laws all vegetable Oils are subject, of what nature soever they be.
The analysis of a vegetable substance, exhibited above, shews what may be obtained from them, when distilled in close vessels, with a graduated heat, from that of boiling water, to that which converts the mixt to a perfect coal; viz. Phlegm, an Acid, a light Oil, much Air, and a thick Oil. But this analysis is far from being a complete one: it may be carried much farther, and made more perfect.
None of the principles obtained by this analysis are pure, simple, and thoroughly separated from the rest. They are still in some measure blended all together: their separation is but begun; and each requires a second and more accurate analysis, to reduce it to the greatest degree of purity of which it is capable. The Oil and the Acid chiefly merit so much pains.
A great deal of the Acid of the plant remains, as was said, combined with the two sorts of Oil here obtained; which we have reason to think differ no otherwise from one another, than as there is more or less Acid united with each. The best way of freeing these Oils from their redundant Acid is to distil them frequently from Alkalis and Absorbents. Some of our best Chymists have taken this pains with several sorts of Oils; but the method might be still extended, and the operation carried further than hath yet been done.
The Acid is in the same circumstances nearly as the Oil. The first that rises is mortified with much water, to which it owes a good deal of its volatility. That which comes over last is much more concentrated, and consequently heavier; yet it is still very aqueous. It might be freed in a great[Pg 444] measure from this adventitious water, and so rendered much stronger; which would give us a better opportunity to discover its nature and properties, of which we know but very little.
Water is not the only heterogeneous substance that disguises the vegetable Acid: a pretty considerable quantity of the Oil of the plant is also combined with it, and contaminates its purity. The proof of this is, that, when these Acids are kept, in the same condition in which they first come over, for any length of time, in a glass vessel, they gradually deposite, on the bottom and sides of the vessel, an oily incrustation, which grows thicker and thicker the longer it stands; and, as this oily matter separates from it, the Acid liquor appears less unctuous and saponaceous.
A very good way to separate this Oil more effectually from the Acid is to combine the whole with absorbents, and abstract the Oil again by distillation. By this means a very sensible quantity of Oil may be separated that was not perceived before. On this occasion it is proper to remark, that the Oil thus united with the vegetable Acid is perfectly dissolved by it; seeing it is thereby rendered miscible with water, so that it doth not, like Alkaline soaps, in the least obscure its limpidity, or give it a milky cast: for these aqueous, oily Acids are very transparent, especially after they have stood for some time.
The air that is discharged with impetuosity in the operation, and must be let out, is loaded with many particles of Acid and Oil reduced to vapours, which it carries off; and by this means the quantity of the principles extracted from the mixt cannot be accurately determined: nor are the vapours, of which the vessels remain full after the operation, any other than particles of Acid and Oil, which the violence of the fire hath rarefied exceedingly, and which do not easily condense.
If we distil in this manner a vegetable aromatic substance, which of course contains an Essential Oil, provided it hath not been previously extracted by the appropriated process, this Essential Oil will rise first, as soon as the distilling vessel acquires the heat of boiling water: but its scent will not be near so sweet or grateful, as if it were distilled in the manner before directed as properest for it. On the contrary, it will have an empyreumatic smell: because in this way it is impossible to avoid scorching and half-burning some of the matter distilled; especially that part of it which touches the sides of the retort. Moreover, the very same equable degree of heat can hardly be kept up with a naked fire. The Es[Pg 445]sential Oil, therefore, though it rises first, will not be pure, but contaminated with a mixture of the empyreumatic Oil that first comes over, and will be confounded therewith.
If a substance abounding with Fat Oil, that hath not been expressed from it, be distilled according to the present process, it will yield no Fat Oil by distillation; but only much more of the first clear Oil, and of the second thick Oil, than if all the Fat Oil it would have afforded had been first drawn off by expression: for as the Fat Oil will not rise in distillation, without a degree of heat greater than that of boiling water, neither can it endure such a degree of heat without changing its nature, without losing that mildness, and, in a great measure, that unctuosity which is natural to it. It will therefore be confounded with the other empyreumatic Oil, which, in all probability, would itself be no other than a Fat Oil, if it could be wholly extracted, without the aid of fire, from the vegetable substances containing it.
Most vegetable substances, when distilled with a strong fire, yield the same principles with that which we have chosen for an instance. Entire plants of this kind, those from which the odorous principle, the Essential Oil, or the Fat Oil, hath been drawn, those of which extracts have been made by infusion or decoction, or the extracts themselves; all such matters being distilled yield a Phlegm, an Acid, a thin Oil, Air, and a thick Oil, and the products of their several analyses differ from each other, only on account of the different quantity or proportion that each contains of the principles here enumerated.
But there are many other plants, which, besides these substances, yield also a considerable quantity of a Volatile Alkaline Salt. This property is possessed chiefly by that tribe of plants which is distinguished by having cruciform flowers; among which there are some that being analyzed greatly resemble animal matters. We shall now analyze one of these; Mustard-seed, for instance.
To analyze a vegetable Substance which yields the same Principles as are obtained from Animal Matters; instanced in Mustard-seed.
With an apparatus like that of the preceding process, and with the same fire, distil Mustard-seed. With a degree of heat inferior to that of boiling water, there will[Pg 446] come over a phlegm somewhat coloured, and impregnated with a Volatile Alkaline Salt. With a degree of heat greater than that of boiling water, the same kind of phlegm, impregnated with the same Salt, will continue to come over; but it will be much higher coloured, and will be accompanied with a light Oil. At this time a considerable quantity of air is discharged; with regard to which the same precautions must be taken as in distilling Guaiacum.
If the fire be gradually raised, there will come over a black thick Oil, lighter however than water; and at the same time vapours will rise, and, condensing on the sides of the receiver, form into sprigs or ramifications. This is a Volatile Alkaline Salt, in a concrete form, like that of animals, as we shall hereafter see. These vapours are much whiter than those of Guaiacum.
When you have thus drawn off, with a very strong fire, all the Volatile Alkali and thick Oil contained in the subject, there will be nothing left in the retort but a sort of coal, from which a small quantity of phosphorus may be obtained, provided the retort you employ for that purpose be good enough to stand a very violent heat.
OBSERVATIONS.
Mustard-seed furnishes us with an instance of a vegetable, from which we obtain, by analyzing it, the very same principles that animal matters yield. Instead of getting an Acid from it, we obtain only a Volatile Alkali; probably because the Acid, which originally enters into the composition of this kind of vegetables, as well as of all others, undergoes in passing through their strainers, and mixing with their juices, such alterations as it suffers when it enters into the composition of animals: that is, it combines with some of their Earth and of their Oil, in such a manner as to be changed into a Volatile Alkali, or at least disposed to be converted into one with the aid of fire.
We shall not here speak of the manner of separating and depurating the principles obtained by this process; but reserve it for the analysis of animals, which is absolutely the same. We shall content ourselves with observing, that the first Volatile Alkali which rises at the beginning of the operation together with the phlegm, in a degree of heat below that of boiling water, differs from that which doth not come over till towards the end of the distillation, when the last thick Oil ascends. The different times, and different de[Pg 447]grees of heat, in which these two Alkalis rise, shew that the former exists actually and perfectly in the plant; but that the latter is generated during the distillation, and is the product of the fire, which combines together the materials whereof it is composed.
Vegetables that thus yield a Volatile Alkali with a heat less than that of boiling water, irritate the organ of smelling, affecting it with a sensation of acrimony; and the effluvia, which rise from them when bruised, make the eyes smart so as to draw tears from them in abundance. Several of these matters, being only bruised, effervesce with Acids: effects producible only by a very Volatile Alkaline principle.
This is that Alkali, the lightest of all the principles that can be extracted from bodies, which rises first in our distillation along with the phlegm, and with a degree of heat much inferior to that of boiling water. As the phlegm with which it rises is very copious, it is dissolved thereby; which is the reason it doth not appear in a concrete form. To this water it gives a slight yellowish tinge, because it is impure and oily. The saline Alkaline properties of this liquor have procured it the title of a Volatile Spirit. This Volatile Alkali, which exists naturally and perfectly formed in Mustard-seed, Onions, Garlic, Cresses, and other such vegetables, constitutes a difference between them and animal substances, which contain only the materials requisite to form a Volatile Alkali, but none ready formed, unless they have undergone the putrid fermentation.
The second Volatile Alkali which rises in our distillation, but not without a very strong degree of fire, and at the same time with the last thick Oil, seems to be a production of the fire; for if it were already formed in the mixt, as the other is, it would rise with the same heat, and at the same time, being equally volatile. It is not impossible, however, that it may exist perfectly formed in the plant; but, having contracted an union with some Acid, and therewith composing an Ammoniacal Salt, it may by that means be hindered from rising so readily as is agreeable to its natural volatility.
The Phosphorus obtained by a violent fire, from the caput mortuum of this distillation, seems to throw a light of probability on this conjecture. There is certainly a great deal of Acid in the composition of Phosphorus. Perhaps this Acid was originally combined with our second Volatile Alkali, and formed therewith, as was said, a sort of Sal Ammoniac. Moreover, almost all the plants that yield a Volatile Alkali by distillation, yield also a considerable quantity[Pg 448] of Acid: which may perhaps be the remains of such a Sal Ammoniac decomposed by the operation. This is a subject for curious and useful inquiries. This second Volatile Alkali appears in a concrete form, because very little phlegm comes over along with it; so that the vapours thereof are not sufficient to dissolve it, as they did the first.
Of the Substances obtained from Vegetables by Combustion.
To procure a Fixed Caustic Alkaline Salt from a Vegetable Substance, by burning it in the open Air.
Take any vegetable matter whatever; set it on fire, and let it burn in the open air till it be wholly reduced to ashes. On these ashes pour a quantity of boiling water sufficient to drench them thoroughly. Filter the liquor in order to separate the earthy parts; and evaporate your lye to dryness, stirring it incessantly; and you will have a yellowish-white Salt.
Put this Salt in a crucible; set it in a melting furnace, and make a moderate fire, so as not to fuse the Salt. It will turn first of a blue-grey colour, afterwards of a blue-green, and at last reddish. Put on the dome of the furnace; fill it with coals; make your fire strong enough to melt the Salt, and keep it in fusion for an hour, or an hour and half. Then pour it into a heated metal mortar; pound it while it is red-hot; put it, as soon as possible, into a glass bottle, first made very hot and dry, and shut it up close with a glass stopple rubbed with emery. By this means you will have the pure Fixed Alkali of the vegetable substance you burnt.
OBSERVATIONS.
Burning a vegetable substance in the open air is a kind of violent and rapid analysis made by fire, which separates, resolves, and decomposes, several of its principles.
When any wood or plant is laid on a quick fire, there ascends from it immediately an aqueous smoke, which consists of little more than phlegm; but this smoke soon becomes thicker and blacker: it is then pungent, draws tears from one's eyes, and excites a cough if drawn into the lungs with the breath. These effects arise from its being replete with the Acid, and some of the Oil, of the vegetable converted into vapours. Soon after this the smoke grows exceeding black and thick: it is now still more acrid, and the plant turns black. Its strongest Acid and last thick Oil are now discharged with impetuosity.
This rarefied Oil being heated red-hot suddenly takes fire and flames. The vegetable burns and deflagrates rapidly, till all its Oil is consumed. Then the flame ceases; and nothing remains but a coal, like that found in a retort after all the principles of a plant have been extracted by the force of fire. But this coal having a free communication with the air, which is absolutely necessary to keep a combustible burning, continues to be red, sparkles, and wastes, till all its phlogiston is dissipated and destroyed. After this nothing remains but the Earth and Fixed Salt of the vegetable; which, mixed together, form what we call the Ashes. Water, which is the natural solvent of Salts, takes up every thing of that kind that is contained in the ashes; so that, by lixiviating them, as directed, all the Salt is extracted, and nothing left but the pure earth of the mixt which is thus decomposed.
The phenomena observed in the burning of a vegetable substance, and the production thereby of a Fixed Alkali, seem to prove that this salt is the work of the fire; that it did not exist in the plant before it was burnt; that the plant only contained materials adapted to form this Salt; and that this Salt is no other than a combination of some of the Acid, united with a portion of Earth, by means of the igneous motion.
In the first place; a Fixed Alkali may be obtained by lixiviation from the ashes of all vegetable matters that contain an Acid, Earth, and Phlogiston, in due proportion. Thus Essential Salts; the substance of extracts made by trituration, infusion, or decoction; wood coals burnt to ashes; all yield a quantity of this Salt in proportion to the quantity of Acid and Earth contained in them.
Secondly; Fat, Essential, and Empyreumatic Oils afford, when burnt, such a small quantity of Fixed Alkali as is scarce perceptible; because they contain but a little Acid, and still less Earth: and these same Oils, when rectified by repeated distillations, and then burnt, leave still less of this[Pg 450] Salt; because they are separated by rectification from most of the Acid, together with, the small matter of Earth contained in them.
Thirdly; those vegetable matters which, being analyzed, furnish a great deal of Volatile Alkali, yield but very little Fixed Alkali; because a great deal of their Acid is employed in forming the Volatile Alkali, which is dissipated by burning the plant: and, for the same reason, those which in distillation afford only a Volatile Alkali, and no Acid, leave in their ashes little or no Fixed Alkali, as is also the case with animal matters.
Fourthly, and lastly; the ashes of such plants as have been long steeped in water, and from which infusions and decoctions have been made, always contain the less Alkali the longer they have been infused or boiled, and the more water they were infused or boiled in; because water dissolves and carries off their Acid. It is for this reason that the ashes of float-wood are much less saline than those of green wood. Boerhaave assures us, in his Chymistry, that having exhausted Rosemary by repeated decoctions, and having afterwards boiled the plant thus treated, the ashes produced by it shewed not the least sign of a Fixed Alkali. He says, that, in order to exhaust thoroughly all the saline matters contained in Rosemary, he was obliged to decoct it no less than twenty times successively, with fresh water every time, and never ceased boiling it in this manner, till he was sure that the water, by boiling the plant in it for a long time, took up from it no kind of matter whatever that in the least affected its purity: so that the water of his last decoction had absolutely no smell, taste, or colour; but was in short precisely the same as before he used it for the decoction. The same author observes, that his plant, after having been exhausted in this manner, and having suffered such continued boiling, retained nevertheless its perfect external form; that from being green at first it became brown, and sunk to the bottom of the water, instead of floating thereon as it did before decoction.
If, in reiterating this beautiful experiment of Mr. Boerhaave's, you should not succeed as you expect, you must not therefore accuse this great man of having been mistaken on this occasion; seeing it is very difficult, not to say impossible, to ascertain exactly, from the account he hath given of his experiment, all that is necessary to its perfect success: for he hath not specified either the duration of the coctions which he made the Rosemary undergo, or the quantity of water he employed in each; whereas a difference in either of these[Pg 451] may occasion a vast difference in the result. It is evident, that if five or six pounds of water be used for each coction of a pound of Rosemary, and be kept boiling for two or three hours, the plant will not be near so much exhausted by being so treated, as if the same quantity thereof were kept boiling for several days, in forty or fifty quarts of water.
Indeed, these points seem, in some measure, to be determined, by what he says of the quality which the water of the last decoction ought to have. But the same objections occur here also; nay, the two circumstances of the quantity of water and the duration of the boiling, have the greatest influence here: for the more a plant is exhausted of its Salts, the more difficult it becomes for the water to dissolve and separate the small quantity thereof that remains united with the tenacious Oil; and consequently it may happen, that this last water, after the plant hath boiled in it five or six hours, shall appear insipid, scentless, colourless; and yet that a much greater quantity of water, but reduced by longer boiling to the same quantity with that which hath been boiled but five or six hours, shall have acquired both taste and colour; in a word, shew that it hath taken up some of the principles of the plant. It may also happen, that, a small portion of saline matter being diffused through a large quantity of water, after long continued coction, shall not be perceptible either to the taste or to the eye; but that the very same portion of saline matter shall become very sensible, when the quantity of water in which it is lost, as it were, is sufficiently lessened by evaporation.
Hence, if we would make sure of fulfilling the conditions required by Mr. Boerhaave, the last decoction of the plant must be made in a much greater quantity of water, and continued for a much longer time, than may perhaps be imagined, or perhaps easily determined; and this decoction being evaporated to any degree you please, must have neither taste, smell, nor colour: in short, it must from first to last remain perfectly like pure water. In other words, it is very difficult to attain to any certainty in this matter.
Though what hath hitherto been said, about procuring the Fixed Alkali of plants by combustion, seems to prove that this Salt is wholly the production of the fire, yet it must not be asserted that no part thereof pre-existed formally in the plant before it was burnt. On the contrary, it is certain that, amongst the saline matters found in the composition of plants, there are true Neutral Salts whose basis is a Fixed Alkali; but this Alkali being combined with an Acid discovers none of its properties, and never appears in its true[Pg 452] form till the Neutral Salt, of which it makes a part, is decomposed by combustion. The case of Sea-plants, all of which contain Sea-salt, and when burnt yield an Alkaline Salt perfectly resembling the basis of Sea-salt, seems to decide this point.
If, in lixiviating the ashes of a plant, to dissolve and wash out its Alkali, you intend that nothing should be left but an absolutely pure earth, fit for making cupels, you must not be contented with one ablution only, even with a large quantity of water; because the ashes continue drenched with the water in which the Salts are dissolved, and consequently, when this water is evaporated, some of the Salts will be left with the earth. Therefore, if this be your view, you must wash it three or four several times, using fresh water every time.
The water impregnated with the Alkali cannot be evaporated without a considerable loss of Salt, especially if it be violently boiled; because the water, with which it is closely united, carries off part of it. In consequence of this intimate union, it is very difficult, when the evaporation is near finished, and but a little water left, to dry the Salt perfectly, because it pertinaciously retains this last portion of humidity.
The Alkali obtained from the ashes of a burnt plant is not perfectly pure: it is contaminated with a small mixture of fatty matters, which were probably defended thereby against the action of the fire, and which render it somewhat saponaceous. In order to free it from this extraneous matter, and to render it very caustic, it must be calcined a long time in a crucible, but without melting it at first: because it is with this Salt as with most metallic matters, which are sooner and more easily deprived of their phlogiston by being calcined without melting, provided they be comminuted into small particles, than when they are in fusion; all melted matters having but a small surface exposed to the air, by the contact of which the evaporation or anything whatever is exceedingly promoted. It was for this reason we directed the Salt to be calcined for a long time in a crucible before melting it.
Mr. Boerhaave was very sensible of the utility of this calcination of the Alkali previous to its being melted, when in his Chymistry he ordered the ashes containing this Salt to be put into a large earthen vessel, kept red-hot for a considerable time, taking great care that the Salt do not melt. He takes notice, that, the longer the ashes are calcined in this manner, the stronger is the Alkali obtained from them.[Pg 453] This method is, in the main, the very same with that here prescribed, and produces the same effect; because the Alkali is equally well freed of the extraneous fatty matter, whether it be calcined before or after its separation, provided it be not suffered to melt.
Mr. Boerhaave gives another reason for recommending care to be taken that the Fixed Alkali do not melt, while the ashes are calcining to render it stronger and more caustic: for, if that should happen, the melted mixture of the Salt and ashes would produce a vitrified mass, which would have none of the properties of the Salt.
To procure the Fixed Salt of a Plant by burning it after the manner of Tachenius.
Into an iron pot put the plant whose Salt you desire to obtain in the manner of Tachenius, and set it over a fire, strong enough to make its bottom red-hot; at the same time cover your plant with a plate of iron, that may lie immediately upon it in the pot. The plant will grow black, and smoke considerably; but will not flame, because it hath not a sufficient communication with the air. The black smoke only will escape through the interstice left between the side of the pot and the rim of the plate; which, for that purpose, should be made so as not to fit exactly into the pot. From time to time take up the iron plate, stir the plant, and cover it again immediately, to prevent its taking fire, or to smother it if it should happen to flame: go on thus till the black smoke cease.
Then take off the iron plate: the upper part of the half-burnt plant will take fire as soon as the air is admitted, consume gradually, and be reduced to a white ash. Stir your matter with an iron wire, that the undermost parts, which are still black, may be successively brought uppermost, take fire, and burn to white ashes. Go on thus as long as you perceive the least blackness remaining. After this, leave your ashes some time longer on the fire; but stir them frequently, to the end that, if any black particles should still be left, they may be entirely consumed.
Your ashes being thus prepared, lixiviate them with seven times their quantity of water, made to simmer over the fire, and keep stirring it with an iron ladle. Then filter the li[Pg 454]quor, and evaporate it to dryness in an iron pot, stirring it incessantly towards the end, lest the matter, when it grows stiff, should adhere too closely to the vessel. When all the humidity is evaporated, you will have a Salt of a darkish colour, and alkaline nature; which you may melt in a crucible, and mould into cakes. This is the Fixed Salt of plants, prepared in the manner of Tachenius.
OBSERVATIONS.
The Fixed Salt obtained from plants in the manner invented by Tachenius, and here described, is in many respects different from the Caustic Fixed Alkali extracted out of the ashes of plants that have been consumed by flaming in the open air. Tachenius's Salt is indeed of an Alkaline nature; but much weaker than a pure Fixed Alkali. It is not by far so caustic; it attracts the moisture of the air much more feebly and slowly; it melts with a much smaller degree of heat; and it doth not make so strong an effervescence with Acids. In short, if you dissolve it in water, evaporate the solution to a pellicle, and set it in a cool place, it will shoot into small crystals; which is not the case with a pure Fixed Alkali.
These several different effects, which characterize Tachenius's Salt, and distinguish it from the Caustic Fixed Alkali produced by burning a plant in the open air, prove that it is not a pure Alkali, but combined with certain substances that bring it nearer to the nature of a Neutral Salt, and place it, as it were, in the mid-way between such a Salt and a true Alkali. If we reflect on the manner in which it is produced, it is easy to perceive what those substances are that must be combined with it. It hath been shewn that plants, when analyzed, yield a great deal of Oil and of Acid. When they are burnt in the open air, all their Oil is dissipated in smoke, or consumed in flame. Great part of the Acid is likewise dissipated, and the remainder combining with the Earth of the plant forms a Fixed Alkali.
When the same plants are analyzed, by distilling them in close vessels, the same principles are carried up by the action of the fire, forced to separate from the fixed parts, and pass over into the receiver in the form of vapours and of a liquid: but, when they are burnt in the manner of Tachenius, the Acid and Oil of the plant, as fast as they are expelled by the action of the fire, are repelled by the iron cover, which, at the same time that it prevents the Oil from being entirely[Pg 455] consumed in flame, obliges these two substances to circulate, reverberates them on the rest of the plant, and, in a manner, forces them to re-unite, in part, with that from which they were just before separated.
A considerable quantity, therefore, of the Oil and Acid of the plant, must evidently combine, in this operation, with its Fixed Salt, as fast as it is produced; and the properties above specified are owing to these two substances. Tachenius's Salt is, therefore, a Fixed Alkali, partly neutralized by some of the Acid of the plant, and rendered a little saponaceous by a portion of its Oil; whence it is much milder than a pure Fixed Alkali, and proper to be given internally, as an excellent remedy in several disorders.
For the medicinal virtues of this Salt Mr. Boerhaave's Chymistry ought to be consulted, as the author was a very good judge of such matters.
Tachenius's Salt may be converted into a Caustic Fixed Alkali, by freeing it from the Acid and from the Oil to which its peculiar properties are owing. For this purpose nothing more is requisite than to calcine it for a long time in a crucible, stirring it frequently with an iron wire, and taking care not to melt it, till it have undergone the same changes, and successively acquired the same colours, as our Fixed Alkali; and, when it becomes reddish, melting it and keeping it in fusion for an hour or two.
Hitherto no sensible difference hath been observed between the Caustic Fixed Alkalis obtained from different plants, when equally calcined; except that those produced by Sea-plants have, as we said before, the same properties as the Alkaline basis of Sea-salt. Much the same thing may be said of the Fixed Salts obtained from plants by Tachenius's method: for, though they be combined with a portion of the Acid and Oil of the plant, yet, as these principles have-been exposed to the action of a strong fire, they are exceedingly altered, and almost wholly reduced to one and the same condition.
To render Fixed Alkalis very caustic by means of Lime. The Caustic Stone.
Take a lump of newly burnt quick-lime, that hath not yet begun to flake in the air: put it into a stone pan, and cover it with twice its weight of the unwashed ashes of[Pg 456] some plant, that are full of the Salt you design to render caustic. Pour on them a great quantity of hot water; let them steep in it five or six hours, and then boil them gently. Filter the liquor through a thick canvas bag, or through brown filtering paper supported by a linen cloth.
Evaporate the filtered liquor in a copper bason set over the fire; and there will remain a Salt, which must be put into a crucible set in the fire. It will melt, and boil for some time; after which it will be still, and look like an Oil, or melted Fat. When it comes to this condition, pour it out on a very hot copper plate, and cut it into oblong tapering slips, before it grow hard by cooling. Put these slips, while they are still hot, into a very dry glass bottle, and seal it hermetically. This is the Caustic Stone, or common Caustic.
OBSERVATIONS.
The design of this operation is to combine with the Fixed Alkali all the saline acrid parts of the quick-lime. This is to be effected only by dispersing and diffusing both those substances in water, which is the proper solvent of all saline matters. Seeing, therefore, we must have an actual lixivium, it is needless to employ an Alkali already prepared and separated from ashes; for which reason we directed ashes that are still replete with Alkali to be used instead of a pure Alkali. By this means two ends are answered at once: the Salt contained in the ashes is extracted from them, and combined with the most acrid, subtile, and saline parts of the lime.
The lye, when saturated with these two saline matters together, is vastly more acrid and caustic than if it contained but one of the two in a quantity equal to both. With this lye Soap is usually made; because the acuated Alkali contained in it hath a much greater effect on Oils than any other kind of Alkali. It also acts with incredible violence on all animal matters; which it dissolves, divides, and, in some measure, destroys, with surprising efficacy and quickness.
For this reason it is impossible to filter it through a woollen or silken bag; for it will eat holes in them, or even reduce them to a pap, almost as soon as it touches them. Besides, as the lye would dissolve some part thereof, it would thence acquire a saponaceous quality, and so lose much of its caustic nature. We must, therefore, necessarily use a filter made of vegetable matters, which resist this destroying Salt much better than animal matters.
An Alkali thus acuated by quick-lime attracts and retains humidity more strongly than any other kind of Alkali, even the perfectest and best calcined. For this reason it is almost impossible to dry it thoroughly in the bason wherein you evaporate the lixivium.
To the moisture still left in it must be attributed its boiling when it begins to melt in the crucible. When all the humidity is dissipated, the fused Salt remains smooth and unruffled, like wax melted with a gentle heat.
This caustic Salt is vastly more fusible than the common Alkalis. It scarce grows red before it flows like wax. When it is once in quiet fusion, all the humidity that occasioned the boiling observed at first being dissipated, it is as caustic as it can be made. It is then time to pour it out, and to cut it into long narrow sticks, fit for the use of Surgeons, who apply it to eat away callosities and excrescences, and to open tissues. On this account it is called the Caustic Stone. The operation of this Salt is so quick, that, in a very short time, it produces on the skin a sensation like that of fire.
As this Salt grows surprisingly soon moist in the air, and loses its virtue when so moistened, it is necessary to shut it up, while it is still hot, in a very dry bottle, which must be immediately stopped with a glass stopple rubbed with emery, or else with a round cork and then dipt in pitch. In spite of all these precautions, it can scarce be kept five or six months in full vigour; especially if the bottle be sometimes opened in the mean while. We shall not attempt to explain here why an Alkali becomes so violently caustic by being combined with quick-lime. This question seems to be one of the most subtile, and the most difficult to answer, in all Chymistry. It depends on the cause of the Alkaline properties of lime; and can hardly be resolved, till we attain a further insight into the nature of that substance than we have yet got.
The Analysis of Soot.
Take wood-soot from a chimney under which no animal matter hath been dressed or burnt: put it into a glass retort set in a reverberating furnace; lute on a receiver, and begin to distil with a degree of heat somewhat less than that of boiling water. A considerable quantity of limpid[Pg 458] phlegm will come over. Keep the fire in the same degree as long as any of this phlegm rises; but increase it when the drops begin to come slow: and then there will ascend a good deal of a milky water. When this water ceases to run, change the receiver, and increase your fire a little: a yellow Volatile Salt will rise, and stick to the sides of the receiver. The fire ought now to be very fierce, and, if so, will force up at the same time a very thick black Oil. Let the vessels cool: you will find a saline matter risen into the neck of the retort, which could not pass over into the receiver: in the bottom of the retort will be a caput mortuum, or black charred substance, the upper part of which will be crusted over with a saline matter, like that in the neck of the retort.
OBSERVATIONS.
The preceding analysis shewed what principles are obtained from vegetable substances without the aid of fire; those which the heat of fire raises and carries over out of one close vessel into another; and, lastly, those that continue fixed after the vegetable hath been thoroughly charred, either in a close vessel, or in the open air: nothing therefore remained, to finish the subject of vegetable principles, but to examine those which fire raises, in the form of vapours, smoke, and flame, from a vegetable matter burnt and consumed in the open air. Every body knows that Soot consists only of these principles, collected in the shafts of chimneys, which serve as alembics for this sort of distillation in the open air. By analysing Wood-soot, therefore, we shall discover the principles we are in quest of. The process we have given for that purpose is taken from Boerhaave's Chymistry, where we find it described with great exactness and precision.
As we are at present inquiring into the nature of vegetables only, it is evidently necessary that we chuse a Soot produced by burning vegetables alone. Soot, though dry in appearance, contains nevertheless much humidity, as appears from this analysis; seeing there comes over at first a considerable quantity of phlegm, that doth not seem to be impregnated with any principle, except perhaps an extremely subtile, saline, and oily matter, that communicates to it a disagreeable smell, from which it cannot by any means be entirely freed.
The white milky liquor, which follows this first phlegm, is still water, but much, more impregnated with saline and oily parts than the former. By its smell, which is exceeding[Pg 459] quick and pungent, we may judge it contains much Volatile Alkali; and accordingly, when re-distilled by itself, it yields a Volatile Spirit, and a Volatile Salt in a concrete form. With regard to its white colour, it is occasioned by the oily parts which are diffused and suspended, but not dissolved, in the water. When this second liquor is come off, there ascends a Volatile Alkali in a dry form, and a very thick black Oil; because there is not moisture enough left to dissolve these principles, or rather to divide and disperse them.
The Volatile Alkali obtained from Soot is, in a double respect, the product of the fire. In the first place, though it derives its origin wholly from wood, or other vegetables, which, when distilled in close vessels, yield no Volatile Alkali at all, yet it produces such a Salt when analyzed in the present manner: whence it must be inferred, that the principles of those vegetables are metamorphosed into a Volatile Alkali, by being burnt in the open air, and sublimed in the form of Soot. Secondly, though Soot when analyzed yields a great deal of this Salt, yet this Salt doth not formally pre-exist therein; for it doth not rise till after the phlegm, nor without a very considerable degree of heat: therefore Soot contains only the materials necessary to form this Salt; therefore the perfect combination of this Salt requires that the force of fire be applied a second time; therefore it is, as was said, doubly the product of the fire.
The saline matter which we find sublimed into the neck of the retort, and which also forms the crust that covers the caput mortuum of the Soot, appears by all Chymical trials to be an Ammoniacal Salt; that is, a Neutral Salt consisting of an Acid and a Volatile Alkali. This Ammoniacal Salt rises only into the neck of the retort, and doth not come over into the receiver: because it is but semi-volatile. We shall treat more at large of the production of a Volatile Alkali, and of this Ammoniacal Salt, when we come to the analysis of Animals, and the article of Sal Ammoniac.
The charred matter that remains in the retort after distillation, being burnt in the open air, is reduced to an exceeding fixed white earth. As this fixed matter was part of that very Soot, which was sublimed to a great height whilst the vegetable was burning; this is a proof of what we advanced before, that the most fixed matters are capable of sublimation, when united with volatile substances; especially when they are exposed at the same time to the combined action of air and of fire.
The Analysis of some particular Substances belonging to the Vegetable Kingdom.
Analysis of the natural Balsams: instanced in Turpentine.
Into a cucurbit put as much rain-water as will fill about a fourth part of its cavity, and pour into it the Turpentine you intend to analyze. Cover the cucurbit with its head, and lute it on with slips of sized paper or wet bladder. Set your alembic in a sand-heat; lute on a long-necked receiver; and give a gradual fire till the water in the cucurbit boil. There will come over into the receiver a good deal of phlegm, which, by little and little, will become more and more acid; and at the same time there will rise a great quantity of an æthereal Oil, extremely light, fluid, and as limpid and colourless as water,
When you observe that no more Oil comes off, unlute your vessels; and in the receiver you will find an acidulated water, and the æthereal Oil floating on it. These two liquors may be easily separated from each other, by means of a glass funnel.
In the cucurbit will be left some of the water you put in, together with the remainder of your turpentine; which, when cold, instead of being fluid as it was before distillation, will be solid, and of the consistence of a resin, and is then called Rosin.
Put this residuum into a glass retort, and distil it in a reverberatory with a naked fire, gradually increased according to the general rule for all distillations. At first, with a degree of heat a little greater than that of boiling water, you will see two liquors come over into the recipient; one of which will be aqueous and acid, the other will be a transparent, limpid, yellowish Oil, floating on the acid liquor.
Continue your distillation, increasing your fire from time to time, by slow degrees. These two liquors will continue to come off together: and the nearer the operation draws to its end, the more acid will the aqueous liquor become, and[Pg 461] the thicker and deeper coloured will the Oil grow. At last the Oil will be very thick, and of a deep reddish-yellow colour. When nothing more ascends, unlute your vessels: in the retort you will find only a very small quantity of a charred, light, friable substance.
OBSERVATIONS.
All Natural Balsams, as well as Turpentine, are oily, aromatic matters, which flow in great quantities from the trees containing them, either spontaneously, or through incisions made on purpose. As these matters have a strong scent, it is not surprising that they should greatly abound with Essential Oils. They may even be considered as Essential Oils, that naturally, and of their own accord, separate from the vegetables in which they exist.
Indeed these Natural Balsams differ from the Essential Oils obtained out of plants by distillation, in this alone, that the former contain a greater proportion of Acid; and, for that reason, are thicker than Essential Oils distilled with the heat of boiling water. But it hath been shewn, that these same distilled Essential Oils, though ever so fluid and light at first, gradually lose their tenuity as they grow old, and at last become considerably thick. On that occasion we observed that they are thus changed, because the lightest, most fluid, and least acid parts, are little by little dissipated and evaporated; so that at last there remains only the thickest and heaviest part, which owes these qualities to the Acid wherewith it is over-dosed.
Hence it follows, that Natural Balsams, and Essential Oils grown thick with age, are exactly one and the same thing. Accordingly we see that fire and distillation produce the same effects on both. The rectification of an Essential Oil, thickened by keeping, is nothing but a decomposition thereof, by separating, with the heat of boiling water, all those parts that are light enough to rise with that degree of heat, from what is so loaded with Acid as to remain fixed therein.
This operation is therefore precisely the same as our first distillation of Balsams with the heat of boiling water, by which the Essential Oil contained in them is drawn off. The residues of these two operations are also the same: each of them is a thick Oil, loaded with Acid, that is wholly, or nearly, deprived of the principle of odour peculiar to the original vegetable, and requires a degree of heat greater than that of boiling water to decompose it, by separating part of[Pg 462] the Acid from the Oil; which will be rendered still the more fluid, the more the thickening Acid is separated from it by repeated distillations.
The newer Natural Balsams are, the thinner they are, and the more Essential Oil do they yield; and this Essential Oil, like all others, grows thick in time, and at last turns again to an actual Balsam.
These Balsams, by being long exposed to the heat of the sun, acquire such a consistence as to become solid. They then take another name, and are called Resins. Resins yield much less Essential Oil, when distilled, than Balsams do. Hence it follows, that Resins are to Balsams, what Balsams are to Essential Oils. All these effects are produced by the causes assigned above, and confirm the analogy we have established.
We have no other observations to make on this analysis of Turpentine, except that when Rosin is distilled in a retort with a naked fire, the operation must be carried on very slowly, and the fire duly governed: for the matter is apt to swell, and to rise in substance into the receiver, without being at all decomposed. In order to avoid this inconvenience, it is adviseable to make use of a long-bodied retort, such as is known by the name of the English Retort.
If you stop the distillation of Rosin about mid-way, or when the Oil that comes over begins to grow thick, you may, by changing the receiver, keep the first Oil apart: it is pretty fluid, and of a middle nature between the æthereal Oil, obtained with the heat of boiling water, and the last thick Oil, that doth not rise till towards the end of the distillation. This last thick Oil is that which Mr. Homberg fired with concentrated Oil of Vitriol.
If we examine the matter contained in the retort, when the distillation is thus stopped short, it appears, when cold, in the form of a solid substance, almost perfectly diaphanous, of a deep reddish-yellow colour, and friable: It is known by the name of Colophony.
This analysis of boiled Turpentine, is a specimen of the analysis of almost all other resins; so that what hath been said on this occasion is in a manner general, and applicable to other decompositions of the same kind. We shall now proceed to examine some other oily matters, which exhibit peculiar phenomena, and do not come under the general rules.
The Analysis of Resins: instanced in Benjamin: The Flowers and Oil of Benjamin.
Into a pretty deep earthen pot, having a border or rim round its mouth, put the Benjamin you intend to analyze. Cover the pot with a large conical cap of very thick white paper, and tye it on under the rim. Set your pot in a sand-bath, and warm it gently till the Benjamin melt. Continue the heat in this degree for an hour and half. Then untie the paper cap and take it off, shaking it as little as possible. You will find all the inside of the cap covered with a great quantity of beautiful, white, shining Flowers, in the form of little needles. Brush them off gently with a feather, put them into a bottle, and stop it close.
As soon as you take off the first cap, cover your pot immediately with a second like the former. In this manner go on till you perceive the Flowers begin to grow yellowish; and then it is proper to desist.
The matter left in the pot will be blackish and friable when cold. Pulverize it; mix it with sand; and distil it in a glass retort with a graduated heat. There will come over a light Oil, of a fragrant scent, but in very small quantity; a little of an acid liquor, and a great quantity of a red thick Oil. There will be left in the retort a charred, spongy substance.
OBSERVATIONS.
All oily matters, that are naturally thick and in a concrete form, resemble each other in this, that they derive these qualities from an Acid combined with them. But they nevertheless differ greatly from one another in many respects. The quality, the quantity, of the Acid to which they owe their consistence, and the manner in which it is united with them, diversify them a thousand ways.
In the preceding process we advanced, that Natural Balsams are distinguished from Resins by their containing so much more Oil, in proportion to their Acid, as suffices to render them almost fluid. For this reason they yield an essential Oil: whereas Resins, on the contrary, are solid; all their Oil being loaded and weighed down with a great quantity of Acid, so that no Essential Oil can be drawn from them.
We observed at the same time, that, when all the Essential Oil contained in a Natural Balsam is drawn off, with the heat of boiling water, the residue takes a solid consistence, and resembles a Resin. In fact, almost all Resins yield, by distillation, the same principles as that residue; that is, an Oil of a middling nature between Essential Oils and thick Oils, in point of lightness and fluidity; the whole being always accompanied with an Acid diffused in phlegm.
In consequence hereof, the analysis of Benjamin, described in the process, appears to vary much from that of other Resins: for here we see a volatile matter in a concrete form; namely, the white Flowers that rise first; which doth not usually occur in the analysis of Resins. Yet, if we examine the matter, we shall be convinced that it is very analagous to one of the principles obtainable from all Resins; that indeed it differs therefrom in some of its properties, particularly in its external form; but that it is in reality the very same.
In fact, the Flowers of Benjamin are no other than an Oily Acid, nearly of the same nature with those obtained from all other vegetable substances; but which, instead of being liquid like them, appears in a dry concrete form, and in a manner crystallized. It probably derives this property from its Oil being combined with its Acid, either in greater quantity, or in a more intimate manner, than in the rest, and so strongly united therewith as not to be separated from it by a subliming heat; or from hence, that the compound, of which it is a part, contains too little phlegm to dissolve it; or else, that it is hindered from dissolving therein by the Oil with which it is combined. Perhaps all these causes may concur together in producing its concrete form.
The saline character of this substance appears chiefly from its being soluble in water: but the water must be very hot, and even boiling, before it will effect this solution; and when it cools, the Salt shoots into fine needles at the bottom. This phenomenon directs us to a method of separating it from Benjamin without sublimation.
For this purpose the Resin must be boiled in water: the water will then dissolve the Salt; and, as it cools, the Salt will crystallize, and may be easily collected. But as the Oil, with which the Acid is combined, hinders the water from dissolving it so easily as it otherwise would, we cannot obtain quite so much of it, from the same quantity of Benjamin, by decoction as by sublimation; the last portions thereof being united with a great quantity of Oil, which defends them against the action of the water. This Salt dissolves readily[Pg 465] in Spirit of Wine, on account of the Oil combined with it. A course of well connected experiments might give us a far greater insight into its natural properties than we can now boast of.
Benjamin yields a much smaller quantity of fluid Oil by distillation than other Resins do; because the greatest part of its Oil is employed in the composition of its oily, volatile, acid Salt. The thick Oil drawn from this Resin, is thicker than that obtained from any other Resin, and even fixes like butter when cold; nor can we get more than a very small quantity of Acid in a distinct liquor. All these effects depend on what we mentioned above, in relation to its saline flowers: to wit, the peculiar and intimate union between the Acid and Oily part of this Resin, so that the fire cannot so easily or so perfectly disjoin them, as it doth those of other Resins.
Benjamin, when distilled, leaves in the retort much more of a charred coal than is left by most other resinous matters. This may be owing to the considerable quantity of earthy matter which it contains, and which, perhaps, may also be one of the causes that contribute to give its Salt a concrete form.
REFLECTIONS
On the Nature and Properties of Camphor.
We do not propose to give an analysis of this singular body; because hitherto there is no process known in Chymistry by which it can be decomposed. We shall therefore content ourselves with reciting its principal properties, and making a few reflections on its nature.
Camphor is an oily concrete substance; a kind of Resin, brought to us from the island of Borneo, but chiefly from Japan. This substance resembles Resins, in being inflammable, and burning much as they do; it is not soluble in water, but dissolves entirely and perfectly in Spirit of Wine; it is easily separated again from this menstruum, as all other oily matters are, by the addition of water; it dissolves both in expressed and in distilled Oils; it hath a very strong aromatic smell. These are the chief properties which Camphor possesses in common with Resins: but in other respects it differs totally from them; especially in the following particulars.
Camphor takes fire and flames with vastly more ease than any other Resin. It is so very volatile, that it vanishes entirely in the air, without any other heat than that of the atmosphere. In distillation it rises entire, without any decomposition, or even the least alteration. It dissolves in concentrated mineral Acids; but with circumstances very different from those that attend other oily or resinous substances. The dissolution is accompanied with no effervescence, no sensible heat; and consequently can produce no inflammation. Acids do not burn, blacken, or thicken it, as they do other oily matters; on the contrary, it becomes fluid, and runs with them into a liquor that looks like Oil.
Camphor doth not, like other oily matters, acquire a disposition to dissolve in water by the union it contracts with Acids; though its union with them seems to be more intimate than that of many oily matters with the same Acids. On the contrary, if a combination of Camphor and an Acid be diluted with water, these two substances instantly separate from each other: the Acid unites with the water, and the Camphor, being entirely disengaged from it, swims on the surface of the liquor. Neither Volatile Alkalis, nor the most caustic Fixed Alkalis, can be brought into union with it; for it always eludes their power.
Notwithstanding these wide differences between Camphor and all other oily and resinous substances, the rule, that Acids thicken Oils, seems to be universal, and so constantly observed by nature, that we cannot help thinking this substance, like all the rest, is an Oil thickened by an Acid. But what Oil? What Acid? and how are they united? This is a subject for very curious inquiries.
With a yellow Oil drawn from wine, and an acid vinous Spirit, of which we shall say more under the article of Æther, Mr. Hellot made a kind of artificial Camphor; a substance having the odour, favour, and inflammability of Camphor; an imperfect Camphor. True Camphor hath the levity, the volatility, and the inflammability of Æther. Can it be a substance of the same nature with Æther, a kind of solid Æther, an Æther in a concrete form?
The Analysis of Bitumens: instanced in Amber, The Volatile Salt and Oil of Amber.
Into a glass retort put some small bits of Amber, so as to fill but two thirds of the vessel. Set your retort in a furnace covered with its dome; fit on a large glass receiver; and, beginning with a very gentle heat, distil with degrees of fire. Some phlegm will first come off, which will gradually grow more acid, and be succeeded by a Volatile Salt, figured like fine needles, that will stick to the sides of the receiver.
Keep the fire up to this degree, in order to drive over all the Salt. When you perceive that little or none rises, change the receiver, and increase your fire a little. A light, clear, limpid Oil will ascend. As the distillation advances, this Oil will grow higher coloured, less limpid, and thicker, till at last it will be opaque, black, and have the consistence of Turpentine.
When you perceive that nothing more comes off, though the retort be red-hot, let the fire go out. You will have in the retort a black, light, spongy coal. If you have taken care to shift the receiver, from time to time, during the distillation of your Oil, you will have sundry separate portions thereof, each of which will have a different degree of tenuity or thickness, according as it came over at the beginning, or towards the end of the distillation.
OBSERVATIONS.
The substance of which we have here given the analysis, together with all others of the same, that is, of the Bituminous kind, is by most Chymists and Naturalists classed with Minerals: and so far they are right, that we actually get these mixts, like other minerals, out of the bowels of the earth, and never procure them immediately from any vegetable or animal compound. Yet we have our reasons for proceeding otherwise, and for thinking that we could not, in this work, place them better, than immediately after those vegetable substances which we call Resins.
Several motives determine us to act in this manner. The analysis of Bitumens demonstrates, that, with regard to the principles of which they consist, they are totally different[Pg 468] from every other kind of mineral; and that, on the contrary, they greatly resemble vegetable Resins in almost every respect. In short, though they are not immediately procured from vegetables, there is the greatest reason for believing that they were originally of the vegetable kingdom, and that they are no other than resinous and oily parts of trees or plants, which, by lying long in the earth, and there contracting an union with the mineral Acids, have acquired the qualities that distinguish them from Resins.
Mineralogists know very well that we find, every where in the earth, many vegetable substances, that have lain very long buried under it, and frequently at a considerable depth. It is not uncommon to find, under ground, vast beds of fossile trees, which seem to be the remains of immense forests: and Bitumens, particularly Amber, are often found among this subterraneous wood.
These considerations, joined to proofs drawn from their analysis, make this opinion more than probable: nor are we singular in maintaining it, as it is adopted by many able modern Chymists.
The analysis of Amber, above described, may serve as a general specimen of the decomposition of other Bitumens: with this single difference, that Amber is the only one among them which yields the Volatile Salt aforesaid; and this determined us to examine it preferably to any other. As for the rest, they all yield a phlegm, an acid liquor, and an Oil; which is thin at first, but grows thicker and thicker, as the distillation draws towards an end. It must be understood, however, that these Acids and these Oils may differ, according to the nature of the Bitumens from which they are drawn; just as the Phlegm, the Acid, and the Oil, resulting from the decomposition of Resins, differ in quantity and quality, according to the nature of the Resins from which they are procured.
The principal differences observed between Resins and Bitumens are these: the latter are less soluble in Spirit of Wine; have a peculiar scent, which cannot be accurately described, and of which the sense of smelling only can judge; and their Acid is stronger and more fixed. This last property is one of the motives which induce us to think, that, besides the vegetable Acid, originally combined with the resinous or oily matter now become a Bitumen, a certain quantity of mineral Acid hath, in a course of time, been superadded to constitute this mixt. We shall presently see that the fact is certainly so, in the case of Amber at least.
Almost all authors, who mention the analysis of Amber, have given different accounts of the volatility of its Salt, and of the time of the distillation when it begins to rise. Some make it ascend immediately after the first acid phlegm. Others say, that it doth not begin to appear till after the first thin Oil; and others again affirm, that it comes over with the last thick Oil. Mr. Bourdelin, who hath examined this matter to the bottom, in a Memoir on the analysis of Amber given in to the Academy, very judiciously remarks, that the different results which those Chymists met with in analyzing our mixt, arose wholly from the different manner wherein each conducted his fire during the operation.
It is certain that such a cause is capable of producing vast differences: for when fire is hastily applied, or made too violent, it not only confounds and tumultuously mingles the principles of the body to be analyzed, but it even frequently drives up the entire substance itself out of the retort into the receiver, without decomposing it at all. This is really so in the case of Amber, and of almost all compound substances that are not extremely fixed.
It ought therefore to be observed, as a general and important rule in every analysis, to administer the fire exceeding slowly and cautiously, as one can never err on that side; and to increase it only by such degrees as appear necessary for carrying on the distillation. By observing this method, an accurate analysis will be attained: by this means the Salt of Amber will rise before the Oil; whereas, if a degree of heat sufficient to raise the thin Oil, or even the thick Oil, be applied at first, the Salt will accordingly come over with the one or the other of these Oils.
Chymists remained a long time unacquainted with the nature of this Salt of Amber, and authors of the greatest name agreed as little on this point as on that just mentioned. Some asserted it to be a Volatile Salt of the same kind with that which is obtained from animal substances; that is, a Volatile Alkali: others, on the contrary, pretended that it was an Acid of a singular nature.
It is very surprising that such authors should disagree on such a point, considering how easily it may be ascertained whether this Salt be really an Acid or an Alkali. Mr. Bourdelin justly decides the question in favour of those who affirm it to be an Acid. In fact it hath all the properties of an Acid: it hath the taste of one, forms Neutral Salts with Alkalis, and differs from the most unquestionable Acids in this alone, that, being combined with a portion of Oil and a[Pg 470] small quantity of earth, these give it a concrete form; which is not a solitary case in Chymistry, as is evident from Cream of Tartar. With regard to its Volatility, there is nothing in that repugnant to the properties of its constituent principles; seeing the Acid and the Oil predominant therein may easily be supposed to communicate their volatile nature to the small portion of earth with which they are combined.
Those Chymists who looked upon the Salt of Amber as a Volatile Alkali, either did not examine it thoroughly, but contented themselves with its first appearance, in which it resembles the Volatile Salt of animals, or else were led into the error by some particular circumstances. We know, for example, that animal as well as vegetable substances are dug out of the earth. The insects, sometimes found inclosed in lumps of Amber, sufficiently prove this. Perhaps they made their experiments on such pieces of Amber; or else, that which they used might be mixed with some animal substance not very perceptible. In such a case, it would be no wonder if the Volatile Salt obtained should shew some tokens of an Alkali: for the Volatile Alkali arising from the animal matter would only be mixed, not combined, with the Salt of the Amber; as the great quantity of the Oil, in which both these Salts are entangled, would hinder them from dissolving each other, and forming such a Neutral Salt as would be produced in other circumstances.
The acid or alkaline nature of the Salt of Amber was not the only point that remained to be discussed on this occasion. Its acid quality being once clearly ascertained, the nature of this Acid was next to be determined. This is the object chiefly aimed at in Mr. Bourdelin's Memoirs, and his discovery thereof is unquestionably one of the finest, and at the same time one of the most difficult, that could be attempted with regard to this Bitumen.
It appears plainly from several experiments, of which we have given an account in the course of this work, that the strongest mineral Acids, by being combined with an oily matter, are so vastly altered, and so strangely disguised, that we not only are incapable of distinguishing what they are, but even can hardly avoid decomposing, and partly destroying them, by those very operations which seem the best adapted to separate them from the Oil in which they are inviscated. Mr. Bourdelin had all these difficulties to surmount, and incessantly met with new obstacles in that troublesome fatty matter, which, like an impenetrable veil, concealed from his view the Acid whose nature he wanted to discover.[Pg 471] But at last, by dint of manifold experiments, he happily gained his end. Two parts of pure Nitre, unadulterated with the lead particle of Sea-salt, and one part of Amber, pulverized and mingled together, procured him, by deflagration, a Salt partly neutral and partly alkaline; which being lixiviated, and set to evaporate spontaneously, there formed at the bottom a residue of a mucilaginous, pappy, whitish matter, amongst which he could distinguish crystals, that were very transparent, regularly figured, of a cubical form, but rather oblong; so that they represented little oblong squares most exactly formed, and about half a line thick.
As these crystals perfectly resembled, in their figure, the Neutral Salt produced by a combination of the Acid of Sea-salt with the alkaline basis of Nitre; this was a proof to Mr. Bourdelin that the Acid of Amber is of the same kind, or rather exactly the same, with that of Sea-salt. The Nitre being alkalizated by means of the phlogiston of the Amber, the Acid of the Bitumen, finding this Alkali a proper basis to fix in, unites with it, and by that means is enabled to resist the action of the fire, so as not to be carried off by it.
On the other hand, it is separated from the fat matter by which it was masked before; for by the help of this fat matter the Nitre is alkalizated. The Acid, having by this means recovered all its properties, begins to discover them, as hath been said, by the figure it constantly gives to the crystals of the Neutral Salt which it helps to constitute.
Moreover, this Neutral Salt hath all the essential properties of Sea-salt. It hath its taste; it decrepitates in the same manner on live coals; if Oil of Vitriol be poured on it, white vapours arise, which have the smell of Spirit of Salt, and are an actual Spirit of Salt. Lastly, it makes a white precipitate of Mercury dissolved in Spirit of Nitre, and a luna cornea of Silver dissolved in the same Spirit; which last proofs would alone be sufficient to establish Mr. Bourdelin's opinion, though we had no other.
It were to be wished that the experiments which Mr. Bourdelin hath made on Amber were also tried on other Bitumens. There is reason to think they would be found to contain either the Marine or the Vitriolic Acid: for though they do not yield a Volatile Salt, as Amber doth, in distillation, yet the Acids obtained from them are very strong, and appear, as we said before, to have a mineral origin. Mr. Geoffroy observed, that Amber, being pulverized and infused in hot water, parts with its Salt in the same manner as Benjamin[Pg 472] does; which gives room to suspect that Amber is to Bitumens what Benjamin is to Resins.
The Analysis of Bees-Wax, and such Oily Compounds as are analogous to it.
Melt the Wax you intend to analyze, and mix with it as much fine sand as will make it into stiff paste. Put this paste in little bits into a retort, and distil as usual, with a graduated fire, beginning with a very gentle heat. An acid phlegm will come over, and be followed by a liquor which at first will look like an Oil, but will soon congeal in the receiver, and have the appearance of a butter or grease. Continue the distillation, increasing the fire by insensible degrees, till nothing more will come off. Then separate the butter from the acid phlegm in the receiver, mix it with fresh sand, and distil it again just as you did the Wax before. Some acid phlegm will still come off, and an Oil will ascend, which will not fix in the receiver, though it be still thick. Continue the distillation, with a fire so governed that the drops may succeed each other at the distance of six or seven seconds of time. Do not increase it, till you perceive the drops fall more slowly; and then increase it no more than is necessary to make the drops follow each other as above directed. When the distillation is finished, you will find in the receiver the Oil come wholly over, and a little acid phlegm. Separate the Oil from this liquor; and, if you desire to have it more fluid, re-distil it a third time in the same manner.
OBSERVATIONS.
Bees-Wax, like all other oily matters in a concrete form, is an Oil thickened by an Acid. Its decomposition furnishes us with a very convincing proof of this truth; which, you see, is confirmed more and more, by every new analysis we make of such substances.
Wax doth not part with all its Acid in the first distillation: and this is the reason that it doth not then become a fluid Oil, but a butter, which hath only a degree of softness proportioned to the quantity of Acid separated from it. The same thing holds with regard to its butter; which losing, by a second distillation, a great part of the remaining Acid[Pg 473] which caused its consistence, is by that means turned to an Oil. Lastly, this Oil, from being thick, becomes very fluid by a third distillation, and so follows the general rule of Oils; which always become the more fluid the oftener they are distilled or rectified.
What is here said concerning Wax is applicable to Resins, also; which it further resembles in its consistence, and its refusing to dissolve in water: yet it differs from them essentially in several respects; and for this reason we thought proper to treat of it in particular. The properties in which it differs from Resins are these:
First, It hath no aromatic scent, nor acrid taste, as Resins have.
Secondly, It doth not yield a thin limpid Oil, in the first distillation, as they do.
Thirdly, Its Oil, or its butter, doth not grow sensibly thicker with age. Mr. Boerhaave kept some butter of Bees-Wax for twenty years, in a vessel that was not stopt, but only covered with a bit of paper; yet it did not grow hard. An Essential Oil, though kept much closer shut up, would in much less time have acquired the consistence of a Balsam; and a Balsam, in that time, would have become a Resin.
Fourthly, Bees-Wax is not soluble in Spirit of Wine; whereas it is the very nature of Resins to dissolve in that menstruum.
Fifthly, I have observed that Spirit of Wine acts faintly on the butter of Bees-Wax; dissolves that butter when distilled to an Oil; unites more readily with that Oil when rectified by a third distillation; and dissolves it still the more readily the oftener it is distilled. Resins, on the contrary, are more soluble in Spirit of Wine than the thin Oils drawn from them; and those Oils acquire the property of resisting that menstruum more and more obstinately the oftener they are rectified.
By these differences we may judge whether it be proper to confound Bees-Wax with Resins, or whether it ought not rather to be considered as an oily compound of a singular species, which deserves to be ranked in a different class, or at least in some other division.
If we take the most cursory view of the properties of Essential Oils, and compare them with those of Fat Oils, we cannot avoid being struck with a resemblance between the properties of Essential Oils and those of Resins, as well as with the apparent conformity between the properties of Fat Oils and those of Bees-Wax: from all which we may con[Pg 474]clude with good reason, in my opinion, that the Oil of Bees-Wax is not of the same nature with that of Resins. The Oil of Resins hath all the properties of an Essential Oil, and is justly allowed to be an Essential Oil rendered thick and ponderous by an Acid. The Oil of Bees-Wax, on the contrary, hath all the properties of Fat Oils; and there is great room to think, that this substance is really no other than a Fat Oil hardened by an Acid.
Bees-Wax is not the only oily compound that appears to have a Fat Oil for its basis. Certain shrubs in America yield, by decoction, a substance that hath all the properties of Bees-Wax, differing therefrom only in its colour, which is green. The Butter of Cacao is also a substance analogous to Bees-Wax, and would be really Wax, if it were but as hard; for it contains the same principles, but in different proportions: in short, it is to Bees-Wax what Balsams are to Resins.
The Saccharine Juices of Plants analyzed: instanced in Honey.
Put into a stone cucurbit the Honey you intend to distil; set it in a moderate sand-heat, and evaporate the greatest part of its humidity, till you perceive the phlegm begin to be acid. Then take out the matter remaining in the cucurbit, put it into a retort, leaving a full third thereof empty, and distil in a reverberatory with degrees of fire. An acid, amber-coloured liquor will come over. As the operation advances, this liquor will continually become deeper coloured and more acid, and at the same time a little black Oil will ascend. When the distillation is over, you will find in the retort a pretty large charred mass, which being burnt in the open air, and lixiviated, affords a Fixed Alkali.
OBSERVATIONS.
If we consider nothing but the nature of the principles obtained from Honey, we may be induced to think that this substance is of the same kind with Resins; for we get from each a Phlegm, an Acid, an Oil, and a Coal. Yet there is a very great difference between these two sorts of compounds. Oily matters of the resinous kind are very inflammable, and[Pg 475] by no means soluble in water: Honey, on the contrary, is not inflammable in its natural state; will not flame till it be half consumed, or turned almost to a coal, by the fire; and mixes readily and perfectly with water. Now whence can this difference arise? Since it is not owing to the nature of the principles that constitute these mixts, it must necessarily be attributed to the proportions in which those principles are united. And indeed if we attend to the quantities obtained from each by analyzing them severally, we shall find that, in this respect, there is a very great difference between them. Oily compounds of the nature of Resins, which are not soluble in water, yield in distillation a little phlegm, a quantity of Oil vastly exceeding that of their Acid, and a very small matter of coal, which, when burnt, scarce leaves any token of a Fixed Alkali. Honey, on the contrary, and all other juices of the same nature, give out, when analyzed, a great deal of phlegm, a quantity of Acid much superior to that of their Oil, and a considerable mass of coal; from which, when burnt in the open air and lixiviated, a very perceptible Alkali may be obtained.
If the quantity of the principles procured by these two analyses be compared together, it will be easy to deduce from thence the causes of the different properties observed in the mixts that afforded them. In the large quantity of Oil, of which resinous substances consist almost entirely, we see the cause of their being so inflammable, and so indissoluble in water. When such bodies are decomposed, there remains but little coal, and very little Fixed Alkali; because their Oil carries off with it almost all their Acid, leaving a scarce perceptible portion thereof fixed in the coal. Now we know that this Acid is an essential requisite to the formation of an Alkali. Honey, on the contrary, and the analogous mixts, are so unapt to take fire, and mix so readily with water, only because there is very little Oil in their composition, in comparison of the Acid, which is their predominant principle. For the same reason they leave, when decomposed, a greater quantity of coal, which also yields much more Fixed Alkali than we find in the coals of Resins. Perhaps these mixts may also contain a little more earth. The cause of this greater quantity of Fixed Alkali will be found in what we delivered above concerning the combination and production of that Salt.
Sugar, Manna, and the Saccharine juices of fruits and plants, are of the same nature as Honey, yield the same principles, and in the same proportions. All these substan[Pg 476]ces must be considered as native Soaps; because they consist of an Oil rendered miscible with water, by means of a saline substance. They differ from the common artificial Soaps in several respects; but chiefly in this, that their saline part is an Acid, whereas that of common Soap is an Alkali. The natural Soaps are not for that reason the less perfect: on the contrary, they dissolve in water without destroying its transparency, and without giving it a milky colour: which proves that Acids are not less proper than Alkalis, or rather that they are more proper additaments, for bringing Oils into a saponaceous state.
But it must be owned, that we are not yet able to imitate by art the Acid Soaps which are prepared and so perfectly combined by nature, and that the detersive quality of these is not near so strong as that of the Soaps which have an Alkali for their saline principles.
Though Honey, and the other vegetable substances analogous to it, contain much Acid, yet they have no taste of sourness, nor any of the other properties of Acids; but, on the contrary, their taste is soft and saccharine: the cause of this is, that their Acid is intimately mixed and perfectly combined with their Oil, which entirely sheathes and blunts it.
Gummy Substances analyzed: instanced in Gum Arabic.
Distil Gum Arabic in a retort with degrees of fire. A limpid, scentless, and tasteless phlegm will first come over; and then a russet-coloured acid liquor, a little Volatile Alkali, and an Oil, which will first be thin and afterwards come thick. In the retort will be left a good deal of a charred substance, which being burnt and lixiviated will give a Fixed Alkali.
OBSERVATIONS.
Gums have at first sight some resemblance of Resins; which hath occasioned many resinous matters to be called Gums, though very improperly: for they are two distinct sorts of substances, of natures absolutely different from each other. It hath been shewn, that Resins have an aromatic odour; that they are indissoluble in water, and soluble in[Pg 477] Spirit of Wine; that they are only an Essential Oil grown thick. Gums, on the contrary, have no odour, are soluble in water, indissoluble in Spirit of Wine, and, by being analyzed as in the process, are converted almost wholly into a phlegm and an Acid. The small portion of Oil contained in them is so thoroughly united with their Acid, that it dissolves perfectly in water, and the solution is clear and limpid. In this respect Gums resemble Honey, and the other vegetable juices analogous to it. They are all fluid originally; that is, when they begin to ooze out of their trees. At that time they perfectly resemble mucilages, or rather they are actual mucilages, which grow thick and hard in time by the evaporation of a great part of their moisture: just as Resins are true Oils, which, losing their most fluid parts by evaporation, at last become solid. Infusions or slight decoctions of mucilaginous plants, when evaporated to dryness, become actual Gums.
Some trees abound both in Oil and in mucilage: these two substances often mix and flow from the tree blended together. Thus they both grow dry and hard together in one mass, which of course is at the same time both gummy and resinous: and accordingly such mixts are named Gum-resins.
But it must be observed, that these resinous and gummy parts suffer no alteration by being thus mixed; but each preserves its properties, as if it were alone. The reason is, that they are not truly united together: Gums being indissoluble by Oils or by Resins, the parts of each are only entangled among those of the other, by means of their viscosity. Hence, if the Gum-resin be put into water, the water will dissolve only the gummy part, without touching the resinous. On the contrary, if the same Gum-resin be put into Spirit of Wine, this menstruum will dissolve the Resin, and leave the Gum. We shall treat more particularly of this dissolution under the head of Spirit of Wine.
If a Gum-resin, instead of being only infused in water, be triturated with water, it will be thereby wholly diffused through it: but the resinous part, which is only divided by the triture, and not dissolved in the water, gives the liquor a milky colour, like that of an emulsion. It is indeed an actual emulsion; that which is made with kernels being, like this, no other than a divided oil, dispersed in small particles by triture, and suspended in the water by means of a mucilage.
Of Operations on Fermented Vegetable Substances.
Of the Product of Spirituous Fermentation.
To make Wine of Vegetable Substances that are susceptible of Spirituous Fermentation.
Let a liquor susceptible of, and prepared for, the Spirituous Fermentation be put into a cask. Set this cask in a temperately warm cellar, and cover the bung-hole with a bit of linen cloth only. In more or less time, according to the nature of the liquor to be fermented, and to the degree of heat in the air, the liquor will begin to swell, and be rarefied. There will arise an intestine motion, attended with a small hissing and effervescence, throwing up bubbles to the surface, and discharging vapours: while the gross, viscous, and thick parts, being driven up by the fermenting motion, and rendered lighter by little bubbles of air adhering to them, will rise to the top, and there form a kind of soft, spongy crust, which will cover the liquor all over. The fermenting motion still continuing, this crust will, from time to time, be lifted up and cracked by vapours making their escape through it; but those fissures will presently close again, till, the fermentation gradually going off, and at last entirely ceasing, the crust will fall in pieces to the bottom of the liquor, which will insensibly grow clear. Then stop the cask close with its bung, and set it in a cooler place.
OBSERVATIONS.
Matters that are susceptible of the Spirituous Fermentation are seldom so perfectly prepared for it by nature as[Pg 479] they require to be. If we except the juices that flow naturally from certain trees, but oftener from incisions made on purpose in them, all other substances require some previous preparation.
Boerhaave, who hath handled this subject excellently well in his Chymistry, divides the substances that are fit for Spirituous Fermentation into five classes. In the first he places all the mealy seeds, the legumens, and the kernels of almost all fruits. The second class includes the juices of all fruits that do not tend to putrefaction. In the third class stand the juices of all the parts of plants which tend rather to acidity than to putrefaction; and consequently those which yield much Volatile Alkali are to be excluded. The fourth class comprehends the juices or saps that spontaneously distil from several trees and plants, or flow from them when wounded. He forms his fifth and last class of the saponaceous, saccharine, and concrete or thick juices of vegetables. Resinous or purely gummy matters are excluded, as not being fermentable.
These five classes may be reduced to two; one comprehending all the Juices, and another all the Mealy parts, of vegetables that are susceptible of fermentation. The juices want nothing to fit them for fermentation, but to be expressed out of the substances containing them, and to be diluted with a sufficient quantity of water. If they be very thick, the best way is to add so much water as shall render the mixed liquor just capable of bearing a new-laid egg. With respect to farinaceous substances, as they are almost all either oily or mucilaginous, they require a little more management. The method of brewing malt-liquors will furnish us with examples of such management. It is thus described by Mr. Boerhaave.
In warm weather the grain is put into large vats, and a considerable quantity of rain-water, or very clean river-water, is poured thereon, in which it lies till it be well soaked and swelled. This first operation is called the Steeping.
When the grain is by this means grown very plump, it is taken out of the steep, and laid on great heaps in an open place, yet not too much exposed to the wind. In a very little time those heaps grow hot, the grain begins to sprout, and shoot out little buds of leaves and roots. The art of managing this operation properly consists in seizing the exact point of time when the germination should be stopt: on this in a great measure depends the success of the business. For, if the grain be left too long in this hot bed, it may begin to[Pg 480] rot, or else the leaves and roots, by growing too much, may consume most of the mealy substance, which, in this case, is the only subject of fermentation; and, if the germination be checked too soon, the advantage expected from it will be lost; that is, the mucid matters will not be sufficiently attenuated.
As soon therefore as the germination is observed to have attained its proper stage, it must be stopt with all possible expedition. For this purpose the grain is carried into an open place exposed to the north wind, where it is spread on a boarded floor and dried; by which means it is hindered from sprouting any more. It is next made to run slowly down through a long tunnel made very hot, which at once dries it thoroughly to the very heart, and in some measure scorches it, though very slightly. Grain thus prepared is called Malt.
By this germination, exsiccation, and slight torrefaction of the grain, the farinaceous substance is considerably attenuated, and its natural viscosity destroyed, which would otherwise hinder the meal, when boiled in water, from mixing with it and dissolving in it, as it must in some measure do to form a liquor fit for Spirituous Fermentation.
Mr. Boerhaave takes notice, that if grain, which hath not been thus prepared, be chewed in the mouth, its meal makes a paste that is not easily attenuated, or entirely dissolved, by the spittle; whereas the meal of the same grain, after malting, mixes immediately and perfectly with the spittle: it hath moreover a sweet agreeable taste, which common grain hath not.
The grain being thus malted, is ground: then hot water is poured thereon, in which it is left to infuse for three or four hours. In that time the water takes up all the attenuated flour of the Malt; whereas it would not dissolve the farina of grain that had not undergone the above described preparations. The Wort is then drawn off the grains, and boiled to a proper degree of inspissation; the decoction is suffered to cool, and afterwards put into casks to be fermented as the process directs.
As Malt-liquor is apt to grow sour, and will not keep so long as Wine, some bitter plants are usually boiled in the decoction, to make it keep the longer, and hinder it from turning sour so soon as it otherwise would. For this purpose such plants are chosen as have an agreeable bitter taste; and the preference is generally given to Hops.
Besides these preparations, relating chiefly to Malt-liquors,[Pg 481] there are many other things to be observed relating to Spirituous Fermentation in general, and to all matters susceptible of that fermentation. For example; all grains and fruits designed for that fermentation must be perfectly ripe; for otherways they will not ferment without difficulty, and will produce little or no inflammable Spirit. Such matters as are too austere, too acrid, or astringent, are for the same reason unfit for Spirituous Fermentation; as well as those which abound too much in Oil.
In order to make the fermentation succeed perfectly, so as to produce the best Wine that the fermented liquor is capable of affording, it is necessary to let it stand quiet without stirring it, lest the crust that forms on its surface should be broken to little fragments, and mix with the liquor. This crust is a kind of cover, which hinders the spirituous parts from exhaling as fast as they are formed. The free access of the air is another condition necessary to fermentation: and for this reason the vessel that contains the fermenting liquor must not be close stopped; the bung-hole is only to be covered with a linen cloth, to hinder dirt and insects from falling into it. Nor must the bung-hole be too large, lest too much of the spirituous parts should escape and be lost.
Lastly, a just degree of warmth is one of the conditions most necessary for fermentation: for in very cold weather there is no fermentation at all; and too much heat precipitates it in such a manner, that the whole liquor becomes turbid, and many fermenting and fermented particles are dissipated.
If, notwithstanding the exactest observance of every particular requisite to excite a successful fermentation, the liquor cannot, without difficulty, be brought to effervesce, which scarce ever happens but to Malt-liquor, it may be accelerated by mixing therewith some matter that is very susceptible of fermentation, or actually fermenting. Such matters are called Ferments. The crust, or Yest, that forms on the surface, of fermenting liquors is a most efficacious ferment, and on that account very much used.
It sometimes happens, that there is occasion to check the fermentation excited in the liquor, before it ceases of itself. To effect this, such means must be used as are directly opposite to those mentioned above for promoting fermentation. The end is obtained by mixing with the liquor a quantity of Alkali, sufficient to absorb the Acid contained therein: but this method is seldom made use of, because it spoils the liquor: which, after being thus treated, is incapa[Pg 482]ble of any spirituous fermentation, but on the contrary will certainly putrefy.
Spirituous fermentation may also be stopped by mixing with the liquor a great quantity of some mineral Acid. But this likewise alters its nature; because these Acids, being fixed, always remain confounded therewith, and never separate from it.
The best method yet found out for checking this fermentation, without injury to the fermenting liquor, is to impregnate it with the fumes of burning sulphur. These fumes are known to be acid, and it is that quality in them which suspends the fermentation. But, at the same time, this Acid is extremely volatile: so that it separates spontaneously from the liquor, after some time, and leaves it in a condition to continue its fermentation.
For this reason, when a Wine is desired that shall be but half fermented, and shall partly retain the sweet taste it had in the state of Must, (the proper name for the unfermented juice of the grape), it is put into casks in which Sulphur hath been previously burnt, and the vapours thereof confined by stopping the bung-hole. These are called Matched Wines. If the same operation be performed on Must, its fermentation will be absolutely prevented: it will retain all its saccharine taste, and is then called Stum. As the sulphureous Acid evaporates spontaneously, in no long space, it is necessary to fumigate matched wines, or stums, from time to time, when they are intended to be kept long without fermenting.
To draw an Ardent Spirit from Substances that have undergone the Spirituous Fermentation. The Analysis of Wine.
Fill a large copper cucurbit half full of Wine. Fit on its head and refrigeratory. Lute on a receiver with wet bladder, and distil with a gentle fire; yet so that the drops which fall from the nose of the alembic may succeed one another pretty quick, and form a sort of small continued stream. Go on thus till you perceive that the liquor which comes over ceases to be inflammable; and then desist. You will find in the receiver a clear liquor, somewhat inclining to an amber-colour, of a pleasant quick smell, and which being thrown into the fire instantly flames. The quantity thereof will be nearly a fourth part of the Wine you put into the[Pg 483] alembic; and this is what is called Brandy; that is, the Ardent Spirit of Wine loaded with much phlegm.
In order to rectify it, and reduce it to Spirit of Wine, put it into a long-necked matrass, capable of holding double the quantity. Fit a head to the matrass, and lute on a receiver: place your matrass over a pot half full of water; set this pot over a moderate fire; and with this vapour-bath distil your Spirit, which will rise pure. Continue this degree of heat till nothing more will come over. You will find in, the receiver a very clear colourless Spirit of Wine, of a quick but agreeable smell, which will catch fire at once by the bare contact of any flaming substance.
OBSERVATIONS.
It hath been shewn, that Honey, and the vegetable juices analogous to it, such as Must, and the juices of all saccharine fruits and plants, yield by distillation no other principles than Phlegm, an Acid, and a small quantity of Oil. The analysis of Wine, and of all substances that have undergone the spirituous fermentation, shews us that this fermentation produces, and in some sense creates, in those mixts, a principle that did not exist in them before; I mean the Ardent Spirit, which is an inflammable liquor that is miscible with water. This liquor results from a closer combination of the Acid and the Oil, which are attenuated and united together by fermentation. To this Oil, which is one of its constituent parts, its inflammability is owing; and the Acid imparts to this Oil the property of mixing with water, more perfectly and more intimately than when it makes a part of any other compound. Nay, there is, in the very composition of an Ardent Spirit, a certain quantity of water which is necessary to it, which is one of its essential parts, and without which it would not have the properties that characterise it. We shall presently have occasion to see, that, when Spirit of Wine is dephlegmated to a certain pitch, we cannot deprive it of any more of its aqueous parts, without decomposing a quantity of the Spirit, proportioned to the quantity of water drawn from it.
Ardent Spirits are more volatile than any of the principles of the mixt from which they are produced, and consequently more volatile than the phlegm, the Acid, or the Oil thereof, though they wholly consist of these. This cannot be attributed to any thing but a peculiar disposition of these principles, which are attenuated in a singular manner by the fer[Pg 484]menting motion, and thereby rendered more susceptible of expansion and rarefaction.
The great volatility of the Ardent Spirit procures us an easy method of separating it from the other principles of Wine, and of dephlegmating it. For this purpose it need only be distilled with such a gentle heat as is just capable of raising the Spirit, but too weak to produce the same effect on the other matters from which you desire to free it. For this reason the more slowly, and with the less heat, you distil your Wine, the stronger and more spirituous will your Brandy be. The same is to be said of the second distillation, by which Brandy is changed into Spirit of Wine, or, in other words, dephlegmated. The Spirit of Wine thus drawn from it will be so much the better, the more exactly you observe the conditions here proposed.
If Spirit of Wine be treated in the same manner as Brandy, that is, if it be rectified by distillation with the same precautions, it will be thereby dephlegmated as much as possible; and then it is called Alkohol. By this rectification it is not only freed from its redundant phlegm, but also from some particles of Acid and of Oil, which, though much less volatile than itself, yet ascend with it in the first distillation: nor is it possible wholly to avoid this inconvenience.
Mr. Boerhaave proposes to dephlegmate Spirit of Wine more easily, and more accurately, by distilling it from decrepitated Sea-salt mixed, while very hot, with the Spirit. This must certainly be a very good method; because decrepitated Sea-salt powerfully attracts moisture, and consequently is very apt to imbibe and retain that which is in the Ardent Spirit: and Spirit of Wine doth not dissolve Sea-salt; so that there is no reason to fear its being in the least contaminated therewith.
All fermented liquors do not yield near an equal quantity of Ardent Spirit; because they do not all, before fermentation, equally contain the principles necessary to produce an Ardent Spirit, in the most advantageous proportion or disposition.
There are several ways of proving whether or no Spirit of Wine be as highly rectified as it possibly can be, that is, whether or no it contain any more phlegm than is precisely necessary to constitute it Spirit of Wine; and many Chymists have judged that worthy of the title which burns away entirely, without leaving behind it the least token of humidity; or that which, being burnt on gun-powder, fires it at last.
But Mr. Boerhaave justly observes, that neither of these is a sufficient proof; because, though there should be a small quantity of unnecessary phlegm in Spirit of Wine, yet it may very well be evaporated and dissipated by the deflagration in either way. He therefore proposes another proof, which is much more to be depended on; that is, by mixing and shaking with the Spirit of Wine a small quantity of a very dry pulverized Alkali. If this Salt, when thus agitated, and even warmed, with Spirit of Wine, continue as dry as it was at first, it is a sign that the Spirit is perfectly dephlegmated.
Mr. Boerhaave tried in this manner some Spirit of Wine that had fired gun-powder, and found it to contain so much phlegm that it moistened his Salt very perceptibly: nay, one single drop of water, being mixed with a considerable quantity of Spirit of Wine, which before left the Alkali perfectly dry, discovered itself in this way by the moisture it communicated to the very same Salt.
Spirit of Wine may also be contaminated with some heterogeneous substances; such as acid, alkaline, or oily matters. These are to be discovered by very easy experiments proper to each: for an acid or alkalious Spirit of Wine being mixed with syrop of violets will give it a red or a green colour, according to the nature of the saline matter contained in it; and, if it be combined with an Oil, that will shew itself by the white milky colour which a drop of it will give to water.
Besides the Ardent Spirit, Wine contains an Acid united with a portion of earth and of Oil, which give the Acid a concrete form. This substance generally separates spontaneously from the Wine, and adheres, in the form of a strong crust, to the sides of the cask. It is called Tartar, and is, properly speaking, the Essential Salt of Wine. We shall exhibit the analysis of Tartar, and treat of it more at length, in a chapter apart.
Wine-lees consist of the grossest parts of the fermented liquor; which being uncapable of remaining dissolved, sink to the bottom, and form a sediment, which contains also some Tartar and a little Ardent Spirit.
The residue left in the cucurbit, after the Spirit is drawn off, is a sort of Extract of Wine. This liquor hath an exceeding rough, or rather acid taste. When distilled it yields an acid phlegm, which comes more and more acid as the distillation advances, and a fetid empyreumatic Oil. From the caput mortuum, when burnt, a considerable quantity of a Fixed Alkali may be extracted.
From all this it follows, that Wine consists of an Ardent Spirit, and a Tartarous Acid, diffused through a great quantity of water, together with some oily and earthy parts.
Malt-liquor contains much less Tartar than Wine; but, instead thereof, it is impregnated with a mucilaginous matter, which becomes very perceptible when any body is smeared with it and dried; for then it makes a kind of Varnish. This mucilaginous matter, which is not sufficiently attenuated, especially when the Malt-liquor is new, makes it very apt to swell up and rise over the helm with rapidity, in the distillation of an Ardent Spirit from it: for which reason it is necessary to proceed more cautiously, and more slowly, in distilling a Spirit from this liquor than from Wine.
To dephlegmate Spirit of Wine by the means of Fixed Alkalis. Spirit of Wine analyzed.
Into a glass cucurbit pour the Spirit of Wine you intend to dephlegmate, and add to it about a third part of its weight of Fixed Alkali, newly calcined, perfectly dry, heated, and pulverized. Shake the vessel, that the two matters may be mixed and blended together. The Salt will gradually grow moist, and, if the Spirit of Wine be very aqueous, melt into a liquor, that will always lie at the bottom of the vessel, without uniting with the Spirit of Wine which will swim at top.
When you perceive that the Alkali attracts no new moisture, and that no more of it melts, decant your Spirit of Wine from the liquor beneath it, and add to your Spirit fresh Salt thoroughly dried as before. This Salt will also imbibe a little moisture; but it will not grow liquid, because the Alkali, with which it was mixed before, hath left too little phlegm to melt this. Decant it from this Salt as at first, and continue to mix and make it in the same manner with fresh Salt, till you observe that the Salt remains as dry after as it was before mixing it with the Spirit of Wine. Then distil your Spirit in a small alembic with a gentle heat, and you will have it as much dephlegmated as it can be.
OBSERVATIONS.
Next to the Mineral Acids, Fixed Alkalis perfectly calcined are the substances which have the greatest affinity with[Pg 487] water, and therefore it is no wonder they are so very fit to dephlegmate Spirit of Wine, and to free it from all its redundant humidity. Indeed Spirit of Wine cannot be perfectly dephlegmated without their assistance: for when distillation alone is made use of for that purpose, it is impossible to prevent some phlegm from rising with the Spirit of Wine, whatever precautions we take to avoid it. Hence it comes to pass, that Spirit of Wine, though ever so highly rectified by distillation, always imparts a little moisture to an Alkali, when mixed with it in order to prove its goodness.
But, while the Alkali attracts the super-abundant phlegm of the Spirit of Wine, it produces in that liquor, and undergoes itself, remarkable changes.
Spirit of wine, when so highly dephlegmated by an Alkali that, being kept in digestion therewith, it leaves the Salt perfectly dry, hath a red colour, an odour somewhat different from that which is peculiar to it when perfectly pure, a taste in which that of the Fixed Alkali may be distinguished; and it makes a slight effervescence with Acids: which manifestly proves, that it is united with a portion of the Alkali employed to rectify it.
Mr. Boerhaave thinks, with great probability, that this portion of the Alkali unites with the Spirit of Wine, much in the same manner as with Oils, viz. that it forms with the Spirit a kind of liquid Soap. He observes, that this alkalizated Spirit cleans the fingers; and that things wetted with it do not dry so speedily as those wetted with pure Spirit of Wine. This alkalizated Spirit is also called Tincture of Salt of Tartar.
In making this Alkaline Tincture, great care is to be taken that the Spirit of Wine you use be as highly rectified as possible: for, as long as it communicates any phlegm to the Alkali, it doth not acquire from the Salt mixed with it either the red colour, or the other properties which shew it to have dissolved part thereof. It is also a rule, to throw the Alkali exceeding hot into the Spirit of Wine, which being heated beforehand boils on the addition of the hot Salt. In order to render the Tincture still stronger, they are left to digest together for some time; after which, if part of the Spirit of Wine be drawn off by distillation, the remainder will have a redder colour and a more acrid taste.
The Spirit drawn off by distillation is clear, colourless, and doth not give the same tokens of an alkaline quality as the Tincture; and for that reason, as the design of the present process is only to dephlegmate and rectify Spirit of Wine[Pg 488] by means of a Fixed Alkali, we have directed it to be distilled as soon as all its phlegm is absorbed by the Salt.
However, Spirit of Wine rectified in this manner must not be considered as absolutely pure; for a small degree of an alkaline quality is still perceptible in it: but that doth not hinder its being employed with success in several chymical operations, where the property chiefly required in Spirit of Wine is that it be perfectly dephlegmated.
In order to free Spirit of Wine from the small portion of Alkali remaining in it after distillation, Mr. Boerhaave proposes to mix with it a few drops of the Vitriolic Acid, before the last distillation. But there is great reason to apprehend an opposite inconvenience from this practice: that is, instead of an alkaline character, we may give the Spirit an acid taint. Indeed this cannot be avoided, but by mixing with the Spirit of Wine exactly as much Acid, as suffices to saturate the Alkali contained in it, and no more; which is a point very difficult to hit.
Van Helmont tells us, that having distilled Spirit of Wine from Salt of Tartar perfectly calcined, half of it came over pure water; and Mr. Boerhaave, to whom this appeared very surprising, resolved to repeat Van Helmont's experiment, in order to satisfy himself of the truth, and see with his own eyes what would be the result. With this view he made a tincture of Salt of Tartar in the manner above described, as strong and as fully impregnated as he possibly could. He set it in digestion with the Alkali for several months, and afterwards let it stand four years without touching it. He then poured the whole into a cucurbit, and drew off the Spirit of Wine from the Salt by distillation. The Spirit of Wine, which was before very red, became clear on being distilled, having left its colour in the Salt which remained at the bottom of the cucurbit. This Spirit he returned upon the Salt, and distilled as before. He observed, that, in this second distillation, the Spirit of Wine rose with a little more difficulty, and that the remaining Salt was of a more saturated colour, and become of a dark red. In this manner he cohobated and distilled his Spirit twenty times, with the same Salt. He then found that the Spirit of Wine had acquired a caustic, fiery taste, and that the saline mass in the bottom of the cucurbit was grown black. This saline residue he distilled with a stronger fire, and obtained from it a liquor, which was water, and not Spirit of Wine.
Though Mr. Boerhaave seems, by this tedious labour, to have made Van Helmont's experiment succeed, at least in[Pg 489] part, yet that famous and accurate Philosopher did not flatter himself with the notion of having solved the problem. He first observes, that he was far from getting the quantity of water which Van Helmont says he obtained, viz. half the weight of the Spirit of Wine. Secondly, he could scarce think that the quantity he did obtain actually came from the Spirit of Wine. The thing appeared to him so singular, and so hard to be accounted for, that he inclined to believe the water was quite extraneous both to his Spirit of Wine and to his Salt, and that it came from the air, which could not but be admitted in the frequent cohobations of the Spirit of Wine with the Alkali.
When Mr. Boerhaave undertook this long laborious course of operations, he had it also in his view to try whether he could not, by the same means, solve another problem famed among the Chymists, namely, the Volatilization of the Salt of Tartar. He acquaints us, that in this also he failed; which may easily be believed: but, in my opinion, he was more successful with regard to the first point, than he himself imagined; for I think the water he obtained came immediately from the Spirit of Wine. We shall easily be convinced of this, if we carefully consider all the circumstances attending his experiments.
It hath been shewn, that Spirit of Wine consists of an Oil, of an Acid, and of water, with which the Oil is intimately mixed by means of the Acid; that Spirit of Wine, which is not perfectly dephlegmated, may be deprived of a pretty considerable quantity of Water, which is superfluous and unnecessary to its composition; and that it suffers no change thereby, except that it becomes lighter, stronger, more inflammable, in short, more Spirit of Wine: but that, when it is once freed of this super-abundant phlegm, it would be in vain to attempt separating a greater quantity of water from it. All the water then left in it is essential to its composition, and necessary to give it its properties; for, without that, it would not be Spirit of Wine, but only an Oil loaded with an Acid.
This being laid down, the water which cannot be separated from Spirit of Wine while it continues Spirit of Wine, must become sensible when it is decomposed. And this actually comes to pass: for if you rob Spirit of Wine of one of its principles, its Oil, for instance, and for that purpose burn it under a glass bell, as you do Sulphur, you will by this means collect a great quantity of water, even though you make use of the most highly rectified Spirit of Wine;[Pg 490] which proves that this water was one of the essential parts that constituted the Spirit.
If, instead of depriving this mixt of its oily principle, you separate from it one of its other principles, such as its Acid, it is plain that it will in like manner be decomposed, and that then the Oil and the water, which were combined together only by means of that Acid, will separate from one another, and appear each in its natural form. Now this is exactly the case in Van Helmont's experiment, as repeated by Boerhaave. The Fixed Alkali, on which the Spirit of Wine is cohobated, hath a greater affinity with the Acid of this mixt than with its phlegm or its Oil. It therefore unites with part of that Acid; by which means a proportional quantity of its Oil and water must needs separate from each other, and of course a portion of the Spirit of Wine will be decomposed. Accordingly Boerhaave observed, that, in dephlegmating Spirit of Wine by a Fixed Alkali, a portion of Oil is always separated from it, and that the Alkali employed in this operation is impregnated with an Acid, so that, when it hath been several times used for this purpose, it is almost changed into a Neutral Salt, and hath acquired the properties of the Foliated Salt of Tartar. That on which Spirit of Wine hath been cohobated a great number of times must consequently be impregnated with a great quantity of Acid; and, as the Acid carries with it a great deal of water, it is not surprising that when the Alkali, thus impregnated with Acid and phlegm, is exposed to a strong fire, the phlegm should be separated from it: seeing the union between them is but weak.
Thus it appears that the water obtained by Mr. Boerhaave, in his experiment, came immediately from the Spirit of Wine, agreeably to Van Helmont's notion; whose most intelligent followers have clearly explained his sentiments on this subject, telling us, as their author's positive assertion, that, "in his experiment, the purest Spirit of Wine deposites one of its principles in the Salt of Tartar; that another of them is turned into water, and so separated from that Spirit, and from the principle attracted by the Salt of Tartar; that consequently Spirit of Wine certainly consists of these two principles, which may be separated from each other; and that the principle which unites with the Alkali of the Tartar changes this Salt into a medicament, or Balsam, of admirable virtue in curing wounds, known by the title of the Samech of Paracelsus."
It may here be asked, why Boerhaave obtained but a small quantity of water in this experiment, seeing Van Helmont pretends that it ought to be equal to half the weight of the Spirit of Wine. The most natural answer to this question is, that, as Van Helmont did not publish all the circumstances of his experiment, there is reason to think Boerhaave did not go about it in the same manner as Van Helmont did.
In my opinion he would have succeeded perfectly, and have obtained from his Spirit of Wine the whole quantity of water he desired, if, instead of cohobating it always on the same Alkali, he had taken fresh Alkali every time; had drawn a tincture from it; had distilled his Spirit of Wine from this Salt; and, after collecting all the parcels of Alkali remaining after those distillations, he had exposed them to a strong fire, in order to separate all the moisture contained in them. Perhaps also such a great number of cohobations and distillations would not have been necessary to decompose the Spirit of Wine totally by this method; especially if he had employed a greater quantity of Alkali in each operation. For it is evident, that a Fixed Alkali, by being impregnated with a certain quantity of the Acid and water of the Spirit of Wine, loses thereby a great deal of its strength and activity, and at last becomes incapable of absorbing any more; so that, when it is entirely saturated, it is no more able to act upon Spirit of Wine, so as to decompose it, than so much Vitriolated Tartar, or common Sand. Hence you see, that there are still many beautiful experiments to be made on this subject, and that we may hope by a regular course of them to obtain a perfect solution of Van Helmont's problem.
In the following processes we shall treat of another method of decomposing Spirit of Wine, which consists in depriving it of its essential water, or aqueous principle, by the means of highly concentrated Acids.
Spirit of Wine combined with different Substances.
To combine Spirit of Wine with the Vitriolic Acid. This combination decompounded. Rabel's Water. Æther. Sweet Oil of Vitriol. Hoffman's Anodyne Mineral Liquor.
Into an English glass retort put two pounds of Spirit of Wine perfectly dephlegmated, and pour on it at once two pounds of highly concentrated Oil of Vitriol: shake the retort gently several times, in order to mix the two liquors. This will produce an ebullition, and considerable heat; vapours will ascend, with a pretty loud hissing noise, which will diffuse a very aromatic smell, and the mixture will be of a deeper or lighter red colour, according as the Spirit of Wine was more or less oily. Set the retort on a sand-bath, made nearly as hot as the liquor; lute on a tubulated ballon, and distil the mixture with a fire strong enough to keep the liquor always boiling: a very aromatic Spirit of Wine will first come over into the ballon, after which the Æther will rise. When about five or six ounces of it are come off, you will see in the upper concavity of the retort a vast number of little points in a veined form, which will appear fixed, and which are nevertheless so many little drops of Æther, rolling over one another, and trickling down into the receiver. These little points continue to appear and succeed each other to the end of the operation. Keep up the same degree of fire, till upon opening the little hole in the ballon you perceive that the vapours, which instantly fill the receiver, have the suffocating smell of volatile Spirit of Sulphur[12].
Then unlute the ballon, pour the liquor it contains into a crystal bottle, and stop it close: there will be about eighteen ounces of it. Lute on your receiver again, and continue the distillation with a greater degree of fire. There will come over an aqueous, acid liquor, smelling strong of a sulphureous spirit, which is not inflammable. It will be accompanied with undulating vapours; which being condensed will form an oil, most commonly yellow, one part of which will float on the surface of the liquor, and another will sink to the bottom.
Towards the end of the distillation of this acid liquor, and of the yellow Oil of which it is the vehicle, that part of the mixture, which is left in the retort and grown black, will begin to rise in froth. Then suppress your fire at once: stop the distillation, and change your receiver once more. When the vessels are grown pretty cool, finish your distillation with a lamp-heat, kept up for twelve or fifteen days, which in all that time will raise but a very little sulphureous spirit. Then break your retort, in which you will find a black, solid mass, like a Bitumen. It will have an acid taste, arising from a remainder of the Acid imperfectly combined with Oil.
This artificial Bitumen may be freed from its redundant Acid, by washing it in several waters. Then put it into a glass retort, and distil it with a strong reverberated fire. You will obtain a reddish Oil that will swim on water, much like the Oil obtained by distilling the natural Bitumens. This Oil also will be accompanied with an aqueous acid liquor. In the retort will be left a charred matter, which, being put into an ignited crucible in the fire, burns for some time, and, when well calcined, leaves a white earth.
The liquors that rise first in this distillation, and which we directed to be kept by themselves, are a mixture consisting, 1. of a highly dephlegmated Spirit of Wine, of a most fragrant smell; 2. of Æther, which the Spirit of Wine wherewith it is united renders miscible with water; 3. of a portion of Oil, which commonly rises with the Æther, towards the end of the operation; 4. and sometimes of a little sulphureous acid, if the receiver be not changed soon enough.
In order to separate the Æther from these other substances, put the whole into an English retort, with a little Oil of[Pg 494] Tartar per deliquium to absorb the Sulphureous Acid, and distil very slowly in a sand-bath heated by a lamp, till near half the liquor be come over. Then cease distilling; put the liquor in the receiver into a phial with some water and shake it; you will see it rise with rapidity to the upper part of the phial, and float on the surface of the water: this is the Æther.
OBSERVATIONS.
This operation is only a decomposition of Spirit of Wine by means of Oil of Vitriol. In the preceding process we saw that this Spirit, which consists of three essential principles, viz. an Oil, an Acid, and Water, cannot be deprived of one of them without being at the same time decomposed; the two others that remain having, by such separation, lost the bond of intimate union and connection that was between them. We saw also that Spirit of Wine, when mixed and digested with a very caustic Fixed Alkali, and several times distilled from it, deposites its Acid in that Salt: and hence it comes that the Oil and the Water, being deprived of the principle which was the bond of their union, separate from each other, and appear in their natural forms.
In the present experiment, the Vitriolic Acid decomposes the Spirit of Wine in a different manner. We know that this Acid acts powerfully on Oils; and that, when it is highly concentrated, as the operation requires it should be, it seizes and attracts with surprising force the moisture of all bodies that touch it. So that, when it is mixed with Spirit of Wine, it acts at the same time both on the aqueous and on the oily principle of that mixt. The rapidity and activity, wherewith it rushes into union with these substances, produce the heat, the ebullition, and the hissing noise, which we observe during the first moments after their mixture.
The red colour, which the two liquors confounded together acquire after some time, is owing to the combination of the acid with the oily part; for it is known that Oils, as colourless as Spirit of Wine, such as the Essential Oil of Turpentine, become of a brownish red when dissolved by a concentrated Acid: and Kunckel observed, that, the more Oil there is in Spirit of Wine mixed with Oil of Vitriol, the deeper is the red colour it acquires on being so mixed. He even gives this experiment as the certain means of discovering whether Spirit of Wine be more or less oily; and he adds, that Spirit of Wine, which hath lost part of its Oil by[Pg 495] being rectified with Lime, acquires less redness than any other by being mixed with Oil of Vitriol.
When the mixture hath acquired this colour, and before it undergoes distillation, it appears like a homogeneous liquor. There is yet no decomposition; or at least none that is perceptible; and the Vitriolic Acid is united at the same time with the Oil, the Acid, and the Water of the Spirit of Wine; that is, with the whole Spirit of Wine in substance. This mixture, when made with three parts of Spirit of Wine to one of Oil of Vitriol, is an astringent remedy much used in hemorrhages, and known by the name of Rabel's Water.
The actual decomposition of the Spirit of Wine is effected by the distillation. The first liquor, or the first portion of the liquor that rises before the rest, hath the smell and all the properties of Spirit of Wine. It is indeed part of the Spirit of Wine employed as an ingredient in the mixture; but, being abstracted from a highly concentrated Oil of Vitriol, which, of all known substances, attracts moisture with the greatest power, it is perfectly freed of all its unnecessary phlegm, and retains no more than what is a constituent part thereof, as one of its principles, without which it would not be Spirit of Wine.
The liquor that succeeds this first Spirit of Wine is of a different nature. It may be considered as an Æther: for, though it be not a pure Æther, it contains the whole of it: from this liquor only can it be obtained; it is no other than an Æther mixed with some of the Spirit of Wine that comes over first, and a little of the acid liquor which comes afterward. Now the production of Æther is the effect of a beginning decomposition of the Spirit of Wine: it is Spirit of Wine degenerated, half decomposed; Spirit of Wine too highly dephlegmated; that is, Spirit of Wine which hath lost a part of its essential phlegm, of that phlegm which as a necessary principle made it Spirit of Wine: it is a liquor still composed of oily parts mixed with aqueous parts, and on that account must retain a resemblance of Spirit of Wine; but such that its oily parts, not being dissolved and diffused among a sufficient number of aqueous particles, are brought nearer to each other than they should be to constitute perfect Spirit of Wine; on which account it is not now miscible with water, but is as much nearer to the nature of Oil, as it is removed from the nature of Spirit of Wine: it is a liquor, in short, which, being neither Spirit of Wine nor pure Oil, yet possesses some properties in common with both, and is consequently to be ranked in the middle between them.
This explanation of the nature of Æther, which I imagine was never before given by any other, is the same that we proposed in our Elements of the Theory of Chymistry, which may be consulted on this occasion.
An objection against this opinion may, perhaps, be drawn from an experiment well known in Chymistry. It may be said, that, if Æther were nothing but depraved Spirit of Wine, which ceases to be miscible with water, because the loss it hath sustained of a portion of the water necessary to its constitution hath disordered the proportion which ought to subsist between its aqueous and oily parts, from which proportion it derives that property, it would be very easy to change Spirit of Wine into Æther by a method quite contrary to the usual one; viz. by mixing Spirit of Wine with a sufficient quantity of superfluous Oil: for it seems to be a matter of indifference whether the proportion, between the aqueous and the oily parts of Spirit of Wine, be changed by lessening the quantity of the former, as in the common operation for Æther, or by increasing the quantity of the latter, as is here proposed; and we can, by the last method, put these two principles together in what proportion we please. Now it is certain that, whatever quantity of Oil be dissolved in Spirit of Wine, it will still remain miscible with water; and that, if Spirit of Wine thus replete with Oil be mixed with water, it will unite therewith as usual, and quit the Oil which it had dissolved.
This objection, though seemingly a very specious one, will be removed with the utmost ease, if we reflect but ever so little on some of the principles already laid down. We said, and we gave some instances of it, that certain substances may be united together in sundry different manners: so that from these combinations, though made in the same proportions, there shall result compounds of very dissimilar properties. The combination we are now considering is another evidence of this truth. It is allowed that the proportion between the oily and the aqueous parts may be exactly the same in Æther and in Spirit of Wine replete with Oil; but it must also be owned that the manner in which the Oil is combined in these two cases is very different.
That Oil, which at first is a constituting part of the Spirit of Wine, and afterwards becomes a part of the Æther, is united with the other principles of those mixts, that is, with their Acid and their Water, by the means of fermentation, whereby it is much more attenuated, and much more closely combined, than that with which Spirit of Wine is impreg[Pg 497]nated by dissolution only. And accordingly this adventitious Oil is so slightly connected with Spirit of Wine, that it is easily separable from it by barely distilling it, or even mixing it with water: whereas that which makes a part of the Spirit of Wine, as one of its constituent principles, is united therewith in such a manner as not to be separable from it by either of these methods, nor indeed without employing the most vigorous and powerful agents for that purpose. So that the chief differences between Æther and Oily Spirit of Wine must be ascribed to the different manner in which the Oil is combined in these two mixts: and, if a sufficient quantity of superfluous Oil could be united with Spirit of Wine, in such a manner that, without being soapy, it should not be separable therefrom by the affusion of water, I make no doubt but such a Spirit of Wine would be perfectly like Æther, so far as not to be miscible with water.
But let us return to our distillation, and trace the decomposition of the Spirit of Wine by the Vitriolic Acid. We have shewn that the Acid begins with attracting part of the Water which constitutes the Spirit of Wine, by which means it changes the nature of this compound, destroys its miscibility with water, and brings it as much nearer to the nature of an Oil as it thereby removes it from the nature of Spirit of Wine.
According to the theory laid down it is evident, that, if the Acid continue to act in the same manner on Spirit of Wine thus depraved and become Æther; that is, if it continue to draw from it the small remaining quantity of the aqueous principle, to which it owes the properties it still retains in common with Spirit of Wine, this must produce a total decomposition thereof; so that the oily parts, being no longer dissolved and divided by the aqueous parts, will be collected together, unite, and appear under their natural form, with all their properties. Now this is exactly the case. The Vitriolic Acid rises in the distillation after the Æther; but considerably changed, because it is loaded with the scattered remains of the decomposed Spirit of Wine. It is in a manner suffocated by the Water it hath attracted from the Spirit; which is the reason why it appears in the form of a very aqueous acid liquor. It carries up along with it the Oil which it hath separated from that Water: this is the Oil we took notice of in the process, and it is consequently that very Oily principle which actually constituted the Spirit of Wine. Lastly, by acting on this Oil also, it takes up a portion of phlogiston, which renders it sulphureous.
What remains in the retort is also a portion of the Oil, that was contained in the Spirit of Wine, now combined with some of the Acid; which is the reason why it is black and thick. It is a compound much resembling a Bitumen, and when analyzed yields the same principles we obtain from native Bitumens, or from an Essential Oil thickened and half burnt by its combination with concentrated Oil of Vitriol.
As to the Acid of the Spirit of Wine, some of it remains combined with the Æther: but there is great reason to think, that, when the Vitriolic Acid robs the Spirit of Wine of its aqueous part, it takes up at the same time most of its Acid, which being itself very aqueous, may be considered as pure water with respect to the concentrated Oil of Vitriol, by which it is attracted, and with which it is confounded.
The properties which characterise Æther agree perfectly well with what we have said of its nature, and of the manner in which it is produced. It is one of the lightest liquors we know; it evaporates so suddenly, that, if a little of it be dropt on the palm of your hand, you will scarce perceive the part it touches to be wet by it; it is more volatile than Spirit of Wine; which is not at all surprising, seeing it differs therefrom only by containing less water, which is the heaviest principle in Spirit of Wine.
Æther is more inflammable than Spirit of Wine; for, if any flame be brought but near it, it immediately catches fire. The reason of this is, that the oily parts of which it consists are not only as much attenuated, and as subtile, as those of Spirit of Wine, but also in a greater proportion with regard to its aqueous parts. To the same cause must be attributed the facility with which it dissolves any oily matters whatever.
Æther burns without smoke, as Spirit of Wine does, and without leaving any coal or earthy matter behind; because the inflammable or oily parts contained in it are, in this respect, disposed like those of Spirit of Wine.
The properties of not being miscible with water, and of taking up Gold dissolved in aqua regis, it possesses in common with Essential Oils; but the latter property it possesses in a much more sensible degree than any Oil: for Essential Oils sustain the Gold they thus take up but a little while; whereas the Æther never lets it fall. It seems the ancient Chymists were unacquainted with the Æther: or at least, if they did know it, they made a mystery of it, according to custom, and spoke of it only in enigmatical terms. Amongst the moderns Frobenius, a German Chymist, seems to have[Pg 499] been the first who brought it to perfection. Godfrey Hankwitz, also a German, but settled in England, made mention of it much about the same time in the Philosophical Transactions. According to the latter, Mr. Boyle and Sir Isaac Newton both knew the preparation of Æther, for which they had each a different process. But none of these Chymists ever published an exact and circumstantial account of a method by which this liquor might be prepared: so that Messrs. Duhamel, Grosse, and Hellot, who have since made several experiments for that purpose, and have discovered, and communicated to the public, easy and certain methods of procuring Æther, had no assistance in their labours but from their own skill and sagacity; which gives them a just title to the honour of the invention. Mr. Beaumé also, a very ingenious Artist in Paris, who hath bestowed a great deal of pains on this subject, lately communicated to the Academy a Memoir, which, among several very important observations, contains the commodious and expeditious process above inserted. As there are many experiments in Mr. Hellot's Memoir, agreeing perfectly well with what hath been said concerning the decomposition of Spirit of Wine by the Vitriolic Acid, we think it will be proper to take notice of them here, and to examine them briefly at least.
The quantity, the colour, and the weight of the Oil, which rises in the distillation at the same time with the aqueous acid liquor, are various, according to the different proportions of Spirit of Wine and Oil of Vitriol that are mixed together. Mr. Hellot observed that by increasing the quantity of the Vitriolic Acid he obtained more of this Oil, and less of the Ardent Spirit containing the Æther. The reason is this: the more Oil of Vitriol you put in the mixture, the more Spirit of Wine must be totally decomposed, and consequently the more of this Oil will be obtained; which, as we have shewn, is one of the principles resulting from the decomposition of Spirit of Wine.
"This Oil is also lighter or heavier, in proportion to the quantity of Oil of Vitriol poured on the Spirit of Wine. That which arises from mixing six, five, four, or even three parts of Spirit of Wine with one part of concentrated Oil of Vitriol, always floats on the water, and continues white. That which ascends from two parts of Spirit of Wine is yellow, and most commonly sinks; and, lastly, that which is produced from equal parts of these two liquors is greenish, and constantly falls to the bottom."
Mr. Hellot remarks, on this occasion, that part of the Acid, by the intervention of which this Oil is separated, unites therewith; and, to the greater or smaller quantity of the Acid thus combined with the Oil, he imputes its being more or less ponderous: which is the more probable, as the heaviest Oil is always obtained from a mixture in which the Acid bears the greatest proportion, and vice versa. Perhaps the different specific gravity of Essential Oils is wholly owing to the greater or smaller quantity of Acid they contain.
Mr. Hoffman hath made several observations on this Oil, which evidently prove that it contains much Acid. He says, that, if it be kept for some time in a bottle, it grows red, and loses its transparency; that its agreeable aromatic taste becomes acid and corrosive; and that if you hold it over the fire in a silver spoon, it corrodes it, and leaves a black spot on it; and that it also corrodes Mercury, when heated therewith in a matrass. To this Mr. Pott adds, that it makes a very perceptible effervescence with Fixed Alkalis; and that being rectified by those salts it loses all the acid properties observed by Mr. Hoffman.
Mr. Hellot obtained a still more considerable quantity of this Oil, by adding three or four ounces of a Fat Oil to the mixture of Spirit of Wine with the Vitriolic Acid. Now, as the Oil we are speaking of hath the properties of Essential Oils, and is soluble in Spirit of Wine, Mr. Hellot observes, that Oil of Vitriol by uniting with Fat Oils converts them into Essential Oils: which agrees very well with our opinion concerning the cause of the solubility of Oils in Spirit of Wine; which, in the Memoir already referred to on other occasions, we attribute to an Acid superficially and slightly united with Oils.
The Oil which thus rises, in distilling Spirit of Wine mixed with the Vitriolic Acid, is known by the name of the Sweet Spirit of Vitriol. This name is very improper, because it may suggest a notion that this Oil derives its origin from the Vitriolic Acid, as some Chymists have erroneously thought; whereas it comes entirely from the Spirit of Wine, as we have shewn. If any reason can be assigned for keeping up the name, it must be because of the considerable quantity of the Vitriolic Acid that remains in the combination, and is dulcified by its union with the Spirit of Wine.
This Oil is an ingredient in Hoffman's famous Anodyne Mineral Liquor. That liquor is thought to be nothing but this very Oil dissolved, and combined with the two liquors that rise first in the distillation, and immediately before the[Pg 501] sulphureous acid phlegm. It dissolves very easily and quickly in those spirituous menstrua; so that, if you intend to have it by itself, and to prevent its recombining with the liquors that come off before, (which should be prevented, because it hinders the separation of the Æther), you must take great care to change the receiver as soon as the acid phlegm with which it rises begins to appear.
We have seen that, by the methods which Mr. Hellot hath pointed out, this Sweet Oil of Vitriol may be increased, both in weight and quantity. In that ingenious Chymist's Memoir we also find some methods of preventing it from rising in the distillation. They consist wholly in the addition of some Absorbent bodies, which, he tells us, divert the action of the Vitriolic Acid, at least in some measure, from the inflammable part of the Spirit of Wine. One of these methods is as follows.
"Put into Spirit of Wine as much soft Soap as it can dissolve: filter it, and pour on it some of the heaviest and most concentrated Oil of Vitriol: shake the mixture. The Soap will be instantly decompounded, and its Oil will float on the surface; because the Vitriolic Acid robs it of the Alkali, which renders it miscible with Spirit of Wine. Distil it, and you will obtain but a very little of Rabel's water; which, moreover, will have the disagreeable smell of a most rancid Oil. There will afterwards ascend a great quantity of Spirit of Wine having the same smell; then an aqueous, acid, and sulphureous liquor; but not a drop of yellow Oil. Mean time there forms a bituminous fungus, of some confidence, rising above the Oil of the Soap which floats on the rest of the liquid."
Most of the Vitriolic Acid having been absorbed by the Alkali of the Soap, in this experiment, as Mr. Hellot observes, it is not surprising that it should not act upon the Spirit of Wine with so much efficacy as to decompose it, and separate its Oil. For the same reason but a little of Rabel's Water comes over, and almost all the Spirit of Wine rises without undergoing any sensible alteration. The disagreeable smell of those liquors comes from the Oil of the Soap, which, being naturally heavy, remains behind in the retort, where it grows rancid and is partly burnt.
The last experiment in Mr. Hellot's Memoir, of which we shall take notice, is a peculiar process for preparing Æther; by means whereof, with the help of an earthy medium, it is easy to distil the vinous acid Spirit containing the Æther, without any sensible change of smell from the beginning to[Pg 502] the end of the operation; without its being succeeded by an acid sulphureous liquor, oil, black scum, resin, or bitumen; and without the necessity of taking any great care about the management of the fire, as the liquor may always be kept boiling in the retort, and distilled to dryness without any danger. This medium is common potter's earth. Mr. Hellot puts six ounces thereof, well dried and pulverized, into a large retort, with one pound of Spirit of Wine and eight ounces of Oil of Vitriol. These he digests together three or four days. The mixture acquires no sensible colour. He sets the retort in a sand-bath, and continues the distillation to dryness with a moderate charcoal fire. Excepting a few drops that rise first, and which are pure Spirit of Wine, all the rest of the liquor that distils hath constantly the smell of Æther: which is even somewhat more penetrating than that of the vinous acid Spirit obtained without the intervention of this earthy medium.
We have shewn, that the production of the æthereal liquor is owing to a semi-decomposition of the Spirit of Wine effected by the Vitriolic Acid during the distillation; that this Acid continuing to act, produces a total decomposition, or perfect separation of the Oil and Phlegm of the Spirit of Wine from each other; and that the Vitriolic Acid, uniting with these two principles, forms the sulphureous phlegm, the fluid oil, and the bituminous matter, all frequently mentioned above. Why then, in this experiment of Mr. Hellot's, do we obtain only a Spirit of Wine replete with Æther, while none of the other productions appear? The reason is a very natural one, and very clear: it is this; the potter's clay containing an earth of that kind which we called Absorbent, because it possesses the property of uniting with Acids, that earth joins with the Vitriolic Acid in the mixture, reduces it to a Neutral Salt, and thereby prevents its continuing to act upon the Spirit of Wine, as is necessary to the total decomposition thereof.
Mr. Hellot says on this occasion, "that part of the Vitriolic Acid turning its action on this soluble earth or bole, which it finds in the potter's clay, ceases to act on the inflammable principle of the Spirit of Wine; that, consequently, as there is not an immediate and continuous combination of these two substances, neither a resin nor a bitumen can result therefrom. This is so true, that a great part of the Oil of Vitriol may be afterwards recovered from the potter's clay as colourless as when it was first used."
Mr. Hellot makes use of the following method for procuring the Æther from the acid vinous Spirit obtained by this distillation. "You must," says he, "put all this liquor into a glass body, made of one piece with its head; pour upon it, through the hole in the upper part of the head, twice or thrice as much well-water, the hardest to the taste, and the most impregnated with gypsum, that can be got. Very pure water, he observes, produces much less Æther.
"If the vinous acid Spirit have such a sulphureous smell, as to occasion a suspicion that it contains a little too much of a Volatile Vitriolic Acid, you must add to the water two or three drams of Salt of potash to absorb that Acid; and then distil with a lamp-heat.
"While any true Æther remains in the mixture, you will see it ascend like a white pillar issuing from the midst of the liquor, and consisting of an infinite number of air bubbles inexpressibly small. Nothing seems to condense in the cavity of the head, which always remains clear, and without any visible humidity. The gutts which light on the sides of the receiver, instead of forming a net-work thereon, as Spirit of Wine doth when it is a little aqueous, spread to the breadth of two inches or more, when they consist of true Æther. As soon as you perceive this track begin to grow considerably narrower, the fire must be put out; for what rises afterwards will be mixed with water, and communicate that fault to the Æther already collected in the receiver.
"Then pour this ætherial liquor into a long bottle, and add to it an equal quantity of well-water. Shake the bottle; the liquor will become milky, and the true Æther will instantly separate, float upon the water, and mix no more with it. Separate it then by a siphon, and keep it in a glass bottle shut close with a glass stopple."
Spirit of Wine combined with Spirit of Nitre. Sweet Spirit of Nitre.
Into an English retort of crystal glass put some highly rectified Spirit of Wine; and, by means of a glass funnel with a long pipe, let fall into your Spirit of Wine a few drops of the Smoking Spirit of Nitre. There will arise in[Pg 504] the retort an effervescence attended with heat, red vapours, and a hissing noise like that of a live coal quenched in water. Shake the vessel a little, that the liquors may mix thoroughly, and that the heat may be equally communicated to the whole. Then add more Spirit of Nitre, but in a very small quantity, and with the same precautions as before. Continue thus adding Spirit of Nitre, by little and little at a time, till you have put into the retort a quantity equal to a third part of your Spirit of Wine. Let this mixture stand quiet, in a cool place, for ten or twelve hours; then set it to digest in a very gentle warmth for eight or ten days, having first luted on a receiver to the retort.
During this time a small quantity of liquor will come over into the receiver, which must be poured into the retort. Then distil with a somewhat stronger degree of heat, but still very gently, till nothing be left in the retort but a thick matter. In the receiver you will find a spirituous liquor, of a quick grateful smell, which will excite a very smart sensation on the tongue, but without any corrosive acrimony. This is The Sweet Spirit of Nitre.
OBSERVATIONS.
By this operation Spirit of Nitre is combined with Spirit of Wine; these two liquors being united with each other, much in the same manner as the Vitriolic Acid is with Spirit of Wine in Rabel's Water.
The proportion of the liquors which form this combination is not absolutely determined, and the several authors who have written on the subject differ much about it. Some require equal parts of the ingredients; others again from two as far as ten parts of Spirit of Wine to one of Spirit of Nitre. This depends on the degree to which the Spirit of Nitre made use of is concentrated, and on the greater or less acidity which your dulcified Spirit of Nitre is intended to have.
The Dispensatory of the College of Paris orders one part of Spirit of Nitre distilled from dried clay, that is, of Spirit which doth not smoke, to be mixed with two parts of rectified Spirit of Wine, and the whole to stand in digestion for a month, without distilling the mixture at all. This is a very good method: because the long digestion supplies the place of distillation, and the Spirit of Nitre, not being highly concentrated, doth not greatly alter the Spirit of Wine; besides that many inconveniences, to be presently taken notice of, are by this means avoided.
But as our design is not to describe such Chymical preparations only as are commonly used in medicine, our plan requiring us to treat particularly of those which may give any light into the fundamental properties of bodies, the process here set down appeared the fittest for our purpose; because the action which Spirit of Nitre exerts upon Spirit of Wine is therein stronger and more perceptible.
One of the first particularities attending the mixture of those two liquors, is the great effervescence, accompanied with violent heat, abundance of fumes, and loud hissing, which arises as soon as the Spirit of Nitre and the Spirit of Wine come into contact with each other. There is great reason to think, that these phenomena are produced only by the rapidity and vigour with which the Nitrous Acid rushes into union with the inflammable part of the Spirit of Wine. We observed, in treating of the Æther, that phenomena of the same kind appear at the instant when the Vitriolic Acid unites with Spirit of Wine: but on that occasion, how highly soever the Vitriolic Acid be concentrated, all these effects are in a less degree than those produced in the present experiment; because the Nitrous Acid, though weaker than the Vitriolic, generally acts much more vigorously and violently on the bodies with which it unites, than any other sort of Acid.
Concerning these mixtures of Acids with Spirit of Wine, Mr. Pott observes, that it is not a matter of indifference whether you pour the Spirit of Wine upon the Acid, or the Acid on the Spirit of Wine; but that every thing passes much more quietly, when the Acid is poured to the Spirit of Wine, than when the contrary is done: and he gives the true reason thereof; to wit, that when the Acid is poured on the Spirit of Wine it finds in that liquor a great quantity of water, with which it immediately unites; that this weakens it, and hinders it from acting on the inflammable part with so much impetuosity as it otherwise would; and therefore he advises that such mixtures be always made in this manner. But it is evident that this advantage is gained only by mixing the Acid with the Spirit of Wine very gradually, and drop by drop, as directed in the process after Mr. Pott. For, if the two liquors were to be mixed together suddenly, and all at once, it is certain that the Acid would not meet with a single drop of phlegm more or less in that way than in the other.
Therefore the chief, and, in some measure, the only precaution necessary to be taken, in the making of such mix[Pg 506]tures, to prevent the violent effervescence and other inconveniences that may attend it, such as explosion, and the bursting of the vessels, is to pour but a very small quantity of one liquor into the other at a time, and to add no more till the effervescence, and even the heat, produced by the first portion, be entirely ceased. With these precautions you may proceed either way, and be always sure that the vessels will not burst; because it is in your power to add such a small quantity of liquor at a time, as shall scarce produce a sensible effervescence. We own, however, that Mr. Pott's observation is a very just one. There is even an advantage in pouring the Acid to the Spirit of Wine, as he directs; which is, that the mixture is a little sooner made, and without any danger.
We have shewn, that the Vitriolic Acid becomes aqueous and sulphureous by mixing Spirit of Wine with it: the Nitrous Acid is changed by this mixture in a manner no less remarkable. Mr. Pott observes, that when Spirit of Nitre is dulcified, that is, when it is perfectly combined with Spirit of Wine, it loses the disagreeable odour peculiar to it, and acquires another that is quick and fragrant; it doth not afterwards emit any red fumes; it rises with a less degree of heat than when pure; it acts with less vigour on Fixed Alkalis and Absorbent earths. Lastly, we shall here relate an experiment made by that Chymist, which seems to prove that the Nitrous Acid loses its most characteristic properties, and entirely changes its nature, by being combined with Spirit of Wine.
Mr. Pott examined the thick liquor left in the retort, when the dulcified Spirit of Nitre is distilled off. By analyzing it he obtained an acid liquor, of a yellow colour, and of a somewhat empyreumatic smell. This Acid was followed by some drops of a red empyreumatic Oil; and there remained, at the bottom of the distilling vessel, a black, shining, charred matter, like that which remains after the rectification of a fetid Oil.
The Oil extracted from this residue is a portion of that which helped to constitute the Spirit of Wine; being separated therefrom by the Nitrous Acid, in the same manner as that treated of in the preceding process, and called Sweet Oil of Vitriol, is separated by the Vitriolic Acid. But as the Nitrous Acid, which is weaker than the Vitriolic, doth not so effectually decompose the Spirit of Wine, the Oil, obtained in the present experiment, is in smaller quantity than that[Pg 507] procured in the distillation of a mixture of the Vitriolic Acid with Spirit of Wine.
As to the Acid which Mr. Pott drew off in his experiment, there is great reason to think it a part of that which was an ingredient in the mixture; namely, of the Nitrous Acid. And yet Mr. Pott having saturated with a Fixed Alkali one part of the residuum, which he had a mind to examine before the Acid was separated from it by distillation, and expecting this matter to contain a regenerated Nitre, he threw it on a live coal; but was surprised to see it burn without the least sign of detonation; and thence concluded, that the Nitrous Acid had changed its nature. This experiment, he thinks, may furnish hints for the transmutation of Acids; and he is of opinion, that the Nitrous Acid loses its virtue of detonating, in the present case, only because its inflammable part, to which it owes its distinguishing properties, hath deserted it, and joined with that of the Spirit of Wine.
Indeed if the Acid obtained by Mr. Pott, which being reduced to a Neutral Salt doth not detonate, derives its origin from the Nitrous Acid that was combined with the Spirit of Wine, there is no doubt of its being depraved in a peculiar manner, and having entirely changed its nature. But may we not suppose it to have another origin? May it not be the Acid of the Spirit of Wine itself, resulting from the decomposition of that mixt in the distillation?
Mr. Navier, whom we mentioned in our Elements of the Theory, extracted a very singular oily liquor from the mixture of Spirit of Wine and Spirit of Nitre, without distillation, and even without the help of fire. He put equal parts of the two liquors, by measure, not by weight, into a bottle, which he stopped close with a good cork, fastened down with pack-thread. Nine days afterwards he found about a sixth part of the mixture separated from, and floating on, the rest of the liquor. This was a very fine æthereal Oil, very limpid, and almost as colourless as water.
In another experiment Mr. Navier substituted a solution of Iron in the Nitrous Acid for pure Spirit of Nitre; and with this solution he mixed an equal weight of Spirit of Wine. From the mixture, after a fermentation which appeared in it, he obtained by the same method an æthereal Oil, like that of his former experiment; except that the latter, which was at first as colourless as the other, acquired a redness in the space of about three weeks. He conjectures, with probability, that this colour proceeded from some parti[Pg 508]cles of Iron which were united with it, and which gradually exhaled.
If a few drops of Oil of Tartar per deliquium be poured on this Oil, as soon as it is separated, there appears at first no sensible change therein: but after some time needle-like crystals shoot in it, which are a true regenerated Nitre; and if the bottle be then unstopped, the liquor emits a most pungent nitro-sulphureous odour; which leaves no doubt of this Oil's containing a Nitrous Acid. When it is thus freed of its Acid, by means of the Oil of Tartar, it is much more volatile than before.
Neither the Vitriolic nor the Marine Acid is capable of separating such an Oil from Spirit of Wine: but the Nitrous Acid always produces it, even when it is not concentrated, and doth not smoke.
It is very certain that this Oil derives its origin from the Spirit of Wine: but there are not yet experiments enough made upon it, to enable us to speak very accurately about the manner in which this liquor is formed, or of the cause of its separation from the Spirit of Wine.
Spirit of Wine combined with the Acid of Sea-salt. Dulcified Spirit of Salt.
Mix together, little by little, in a glass retort, two parts of Spirit of Wine with one part of Spirit of Salt. Set this mixture to digest for a month in a gentle heat, and distil it, till nothing remain in the retort but a thick matter.
OBSERVATIONS.
The Acid of Sea-salt is much less disposed to unite with inflammable matters than the other two mineral Acids; and therefore, though it be ever so highly concentrated, when mixed with Spirit of Wine, it never produces an effervescence comparable to that which is produced by the Spirit of Nitre. Neither the proportion nor strength of the Spirit of Salt, requisite to prepare the Sweet Spirit of Salt, are unanimously agreed upon by Authors. Some direct equal parts of the two liquors; while others prescribe from two to four or five parts of Spirit of Wine to one part of Spirit of Salt. Some use only common Spirit of Salt; others require the[Pg 509] Smoking Spirit, distilled by means of Spirit of Vitriol. Lastly, some order the mixture to be distilled, after some days digestion; and others content themselves with barely digesting it. The whole depends on the degree of strength which the Sweet Spirit of Salt is intended to have. This composition, as well as the Sweet Spirit of Nitre, is esteemed in medicine to be very aperitive and diuretic.
When the mixture of Spirit of Salt and Spirit of Wine is distilled, there comes over but one liquor, which appears homogeneous. This is the Sweet Spirit of Salt. The nature of the Marine Acid is not changed in this combination: the Acid is only weakened and rendered more mild; but in other respects it retains its characteristic properties.
Some authors pretend, that an Oil is obtained by distilling the mixture for the Sweet Spirit of Salt; but others expressly deny the fact. This variety may be occasioned by the quality of the Spirit of Wine employed. It would not be surprising if a Spirit of Wine, which contains much Oil that is unnecessary to its nature, and, as it were, adventitious to it, should yield an Oil when distilled with Spirit of Salt.
The thick residue, found in the retort after distillation, contains the most ponderous part of the Acid, united with part of the Spirit of Wine. If the distillation be continued to dryness, there remains in the retort a black charred matter, much like that which is left by the combinations of Spirit of Wine with the other Acids.
A sweet Spirit of Salt may also be prepared by digesting Spirit of Wine with, or distilling it from, metallic compositions replete with the Marine Acid adhering but slightly to them; such as Corrosive Sublimate, and Butter of Antimony. Part of this Acid, which is very highly concentrated, quits the metallic substance with which it is but superficially combined, in order to unite with the Spirit of Wine. If Butter of Antimony be used for this purpose, Mr. Pott, the author of these experiments, observes, that a Mercurius Vitæ precipitates; which is nothing else, as we observed in its place, but the reguline part of the Butter of Antimony deserted by its Acid.
Oils, or Oily Matters, that are soluble in Spirit of Wine, separated from Vegetables, and dissolved by means of that Menstruum. Tinctures; Elixirs; Varnishes. Aromatic Strong Waters.
Put into a matrass the substances from which you intend to extract a Tincture, having first pounded them, or pulverized them if they are capable of it. Pour upon them Spirit of Wine to the depth of three fingers breadth. Cover the matrass with a piece of wet bladder, and tye it on with pack-thread. Make a little hole in this bit of bladder with a pin, leaving it in the hole to keep it stopped. Set the matrass in a sand-bath very gently heated. If the Spirit of Wine dissolve any part of the body, it will accordingly acquire a deeper or lighter colour. Continue the digestion till you perceive that the Spirit of Wine gains no more colour. From time to time pull out the pin, to give vent to the vapours, or rarefied air, which might otherwise burst the matrass. Decant your Spirit of Wine, and keep it in a bottle well corked. Pour on some fresh Spirit in its stead; digest as before; and go on in this manner, pouring on and off fresh Spirit of Wine, till the last come off colourless.
OBSERVATIONS.
It is commonly said, that Spirit of Wine is the solvent of Oils and oily matters: but this proposition is too general; for there are several sorts of Oils and oily matters which this menstruum will not dissolve. Of this number are the Fat Oils, Bees-Wax, and the other Oily compounds of that kind. Properly speaking, it dissolves but two sorts of oily substances; namely, Essential Oils, and Balsams or Resins, which are matters of the same kind, differing from each other only as they are more or less thick; and Oils that are in a saponaceous state.
In our Elements of the Theory we have explained our opinion on this head, from a Memoir on the subject printed among those of the Academy for 1745. To repeat it in a few words: we take the cause of the solubility of Oils in Spirit of Wine to be an Acid, which is but superficially united with them, and so as still to retain its properties.
The principal proofs on which we found this opinion are drawn from that property of Essential Oils, Balsams, and Resins, which are naturally soluble in Spirit of Wine, that they become so much the less soluble in this menstruum, the oftener they are distilled or rectified; and from that property which Fat Oils, or other Oily matters, naturally indissoluble in Spirit of Wine, possess, of becoming more and more soluble therein the oftener they are distilled. We shewed that distillation lessens the solubility of Essential Oils, Balsams, and Resins, only by depriving these substances of part of the manifest Acid which they contain, and which is the cause of their solubility; and that Fat Oils, and other oily matters, naturally indissolvable in Spirit of Wine, are by the same operation rendered capable of dissolving therein, only because it discovers, and partly extricates, an Acid, which is naturally combined with them so intimately that it is entirely deprived of action, and all its properties perfectly masked. If these principles be well attended to, and if it be recollected withal, that Spirit of Wine unites with Water preferably to Oils; insomuch that, if it be mixed with water when it hath dissolved an Oil, it quits the Oil to unite with the Water; that for the same reason it is not capable, when very aqueous, of dissolving any Oil, seeing that, as Oil and water are not susceptible of contracting any union, it must then desert its phlegm to unite with the Oil; which it cannot do, because it hath a greater affinity with phlegm than with Oil; and, lastly, that if Oil be combined with any saline substance, which makes it soluble in water; that is, if it be in a saponaceous state, it will then remain dissolved in Spirit of Wine, without being precipitated by water; or will be dissolved by a very aqueous Spirit of Wine, and frequently much better than by a highly rectified Spirit: if these things, I say, be considered, we shall easily perceive what must be the effect of digesting Spirit of Wine with any vegetable substance whatever.
Spirit of Wine dissolves all the Essential Oil, Balsam, and Resin contained in any vegetable; and as these matters are not soluble in water, they may be separated from the Spirit in which they are dissolved, by lowering it with much water. It instantly becomes white and opaque, like milk; the oily parts gradually unite, and form considerable masses, especially if they be resinous. This is the method commonly made use of to extract the Resin of Scammony, Jalap, Guaiacum, and several other vegetable substances, which it would be difficult to procure by any other means.
If the matters digested with Spirit of Wine contain any saponaceous juices, the Spirit will take up those juices also. But as soaps are soluble in water, as well as in Spirit of Wine, they cannot be separated, by the addition of water, from the Spirit in which they are dissolved. Whatever quantity of water therefore you mix with a Spirit that is impregnated with such juices, no separation thereof will be produced; and for the same reason the saponaceous matters will be dissolved by a very aqueous Spirit of Wine.
Spirit of Wine impregnated with such parts of any vegetable substance, as it is capable of dissolving, is commonly called a Tincture. Several Tinctures mixed together, or a Tincture drawn from sundry vegetable substances at the same time, and in the same vessel, take the name of an Elixir. Tinctures or Elixirs impregnated with Resinous matters only are true Varnishes. All these preparations are made in the same manner; to wit, as directed in our process. We shall only add here, that if the substances from which a Tincture or Elixir is to be made contain too much moisture, it is proper to free them from it by a gentle desiccation; especially if you design that the Tincture should be well impregnated with the oily and resinous parts: for their excess of moisture uniting with the Spirit of Wine would weaken it, and render it unable to act on those matters, which it cannot dissolve when it is aqueous.
Vegetable substances which have been repeatedly digested with different parcels of Spirit of Wine, till the last would extract nothing, are deemed to be exhausted of all their Essential Oils, and saponaceous juices: but if they contain moreover any Fat Oil, Wax, or Gum, these principles will still remain therein after the digestion, in the same quantity as before; because Spirit of Wine is incapable of dissolving them.
With regard to the Fat Oil and Wax, this is not at all surprising: we have explained in another place why these matters are indissoluble by Ardent Spirits: but as for the Gum, it would seem, according to the general principles above-mentioned, that it should be soluble in that menstruum, even with more ease than Resins; as it consists almost entirely of water, with which Spirit of Wine is known to unite more easily than with Oils. Indeed there is also a little Oil in its composition: but this Oil seems to be in a perfectly saponaceous state; for Gum dissolves wholly and easily in water, without lessening its transparency in the least.
I own that it is extremely difficult to give a very satisfactory account of this matter. We may however venture to throw out some conjectures concerning it, deduced from what hath been already said, relating to the cause of the solubility of Oils in Spirit of Wine. We shewed that the Oils which dissolve in this menstruum derive that property from a manifest Acid, which is united with them but superficially, and in such a manner as to retain all its virtue; but that if this same Acid be too intimately united with the Oil, so as to have no manifest power, but be in a manner destroyed, and converted as it were into a Neutral Salt, it will not then produce this effect.
A modern author[13] relates two experiments which agree very well with this opinion, and indeed confirm it. He mixed together Oil of Vitriol and Oil of Turpentine, with a view to imitate by art a bituminous matter; which, we know, is not at all, or at least scarcely, soluble in water. These two matters being united together produced a red, thick compound, which by evaporation became like a natural Bitumen.
The author observes, that when this mixture is just made it dissolves in Alcohol; but that in some time it changes its nature, and communicates scarce any part of its substance to that solvent. Now whence can such a difference arise, but from this, that when the mixture is new, the Acid is as yet but superficially united with the Oil, and combines with it more and more intimately, as the mixture grows older.
The same author, having repeated the experiment with Spirit of Vitriol, obtained a compound which continued always very soluble in Spirit of Wine: because Spirit of Vitriol being much weaker and more aqueous than Oil of Vitriol, was incapable of combining so closely with the Oil of Turpentine, as that concentrated Acid did in the former experiment. By the by, there is great reason to believe that the very intimate union of a mineral Acid with an oily matter is the true cause why Bitumens will not dissolve in Spirit of Wine.
It seems therefore pretty probable, that the Acid which makes the Oil of Gummy matters soluble in water, and reduces it to a saponaceous state, is so intimately united with that Oil, that it loses its properties, and is in a manner converted into a Neutral Salt. Now we know that such Salts[Pg 514] are soluble in water, but are not so, for the most part, in Spirit of Wine.
If your Tinctures or Elixirs be not so strong or so saturated as you desire, you may by distillation abstract part of the Spirit of Wine which they contain, and by that means give them such a degree of thickness as you judge proper. But the Spirit of Wine thus drawn off constantly carries along with it a good deal of the aromatic principle. It is a truly Aromatic Strong Water. This Spirit of Wine also carries up with it a portion of thin Oil, which is so much the more considerable as the degree of heat employed is greater: and this is the reason why it becomes of a milky colour when mixed with water.
If you intend to make an Aromatic Strong Water only, you need not previously extract a Tincture from the vegetable substance with which you mean to prepare your water: you need only put it in a cucurbit, pour Spirit of Wine upon it, and distil with a gentle heat. By this means you will obtain a Spirit of Wine impregnated with all the odour of the plant.
Of Tartar.
Tartar analyzed by distillation. The Spirit, Oil, and Alkaline Salt of Tartar.
Into a stone retort, or a glass one coated with lute, put some white Tartar broken into small bits, observing that one half, or at least a full third, of the vessel be left empty. Set your retort in a reverberating furnace. Fit on a large ballon, having a small hole drilled in it; lute it exactly with fat lute, and secure the joint with a linen cloth smeared with lute made of quick-lime and the white of an egg. Apply at first an exceeding gentle heat, which will raise a limpid, sourish, pungent water, having but little smell, and a bitterish taste.
When this first phlegm ceases to come off, increase your fire a little, and make the degree of heat nearly equal to that of boiling water. A thin limpid Oil will rise, accompanied with white vapours, and with a prodigious quantity of air, which will issue out with such impetuosity, that if you do not open the little hole in the receiver time enough to give it vent, it will burst the vessels with explosion. An acid liquor will rise at the same time. Continue the distillation, increasing the heat by insensible degrees, and frequently unstopping the little hole of the receiver, till the elastic vapours cease to issue, and the oil to distil.
Then raise your fire more boldly. The acid Spirit will continue to rise, and will be accompanied with a black, fetid, empyreumatic, ponderous, and very thick Oil. Urge the fire to the utmost extremity, so that the retort may be of a perfect red heat. This violent fire will raise a little Volatile Alkali, besides a portion of Oil as thick as pitch. When the distillation is finished, you will find in the retort a black, saline, charred matter, which grows hot when wetted, attracts the moisture of the air, runs per deliquium, and hath all the properties of a Fixed Alkali.
This mass, being exposed to a naked fire in the open air, burns, consumes, and is reduced to a white ash, which is a fiery, caustic, Fixed Alkali.
OBSERVATIONS.
The matters qualified to produce a spirituous liquor by fermentation, do not all contain the just and accurate proportion of Acid necessary to constitute an Ardent Spirit. Many of them, the juices of fruits for instance, and especially that of the Grape, are replete with a super-abundant quantity of Acid, more than concurs to form that product of fermentation. This super-abundant Acid, combined with some of the Oil and earth contained in the fermented liquor, produces a sort of Salt, which hangs for a while suspended in that liquor, but after some time, when the Wine stands quiet in a cool place, separates from it, and forms a stone-like incrustation on the inside of the vat in which the Wine is kept. This matter is called Tartar.
The Lees of Wine resemble Tartar, in as much as they contain, and yield when analyzed, the same principles; but they differ from it in this, that they contain, moreover, a greater quantity of earth, of phlegm, and a little Ardent[Pg 516] Spirit, which are only mixed, but not united, with the tartarous Acid.
The residue, or sort of extract, which remains in the cucurbit after Wine hath been deprived of its Ardent Spirit by distillation, hath also a great conformity with Tartar. It even contains that portion of Tartar which remained suspended in the Wine at the time of its distillation: and accordingly this residue of Wine, being analyzed, yields the same principles with Tartar.
Hence we see, that liquors, which have undergone the spirituous fermentation, consist of an Ardent Spirit and a Tartarous Acid suspended in a certain quantity of Water.
In the analysis of Tartar there are several things worthy of notice. The first is, the vast quantity of Air that this mixt body yields when it begins to be decomposed. The chief difficulty attending its analysis arises from this air; which issues out and exerts its elastic force with such impetuosity, that all the precautions above-mentioned are no more than necessary to prevent the bursting of the vessels.
The singular nature of the thin limpid Oil, which rises with this air, after the first acid phlegm, deserves likewise our particular attention. This Oil is one of the most penetrating we know. Boerhaave, who distilled Tartar without having a vent-hole in his receiver, was obliged, in order to prevent its bursting, to apply it to his retort with a lute so weak that most of the elastic vapours might perspire through it; and he observed, that, though the neck of his retort entered above five inches into the mouth of his receiver, and was luted on as closely as possible with such a lute, yet this light Oil of Tartar constantly returned back again, as it were, and pervaded the substance of the lute, so that a good deal of it dropped in a dish placed on the outside on purpose to receive it. This Oil is probably rendered so active and subtile, only by having been exceedingly attenuated by the fermenting motion. This experiment is one of those which sufficiently prove the necessity of employing receivers having a small vent-hole, that may be opened and shut as occasion requires.
The last remark we shall make, on the productions of Tartar by distillation, relates to the caput mortuum found in the retort when the operation is finished. This residue is very different from that which other vegetable matters afford: for, when they are decomposed in close vessels, they leave nothing but a mere charred matter, in which no saline property appears, and from which no Fixed Alkali can be ob[Pg 517]tained, but by carrying their analysis to the utmost; that is, by burning them in the open air. Tartar, on the contrary, only by being distilled in close vessels, without burning it afterwards in the open air, is changed into a substance which hath all the properties of a Fixed Alkali. This is probably owing to the Tartar's containing the principles requisite to form a Fixed Alkali in a much greater quantity than they are to be found in any other substance. As Tartar thus alkalizated in close vessels still contains much inflammable matter, it might be employed with advantage as a reducing flux, in several operations of metallurgy.
Of all the vegetable matters we know, calcined Tartar yields the greatest quantity of Fixed Alkali; which is likewise very pure, and therefore much used in Chymistry.
Burnt Lees of Wine also afford a great quantity of Fixed Alkali, which is of the same nature with that of Tartar. This Salt is used in different trades, and particularly in Dying. The French Vinegar-makers collect quantities of these Lees, which they make up into cakes and dry: while it is in this state they call it Gravelle or Gravelée; and Cendre Gravelée when it is burnt.
If the extract of Wine, which remains after the Spirit is drawn off, be gently evaporated to dryness, and that dry matter burnt like Tartar or Gravelle, it will make a sort of Cendre Gravelée very rich in alkaline Salt.
The Depuration of Tartar. Cream and Crystals of Tartar.
Reduce to a fine powder the Tartar you intend to purify, and boil it in twenty-five or thirty times as much water. Filter the boiling liquor through a flannel-bag, and then gently evaporate some part of it: there will soon form on its surface a saline crust, which is the Cream of Tartar. Let your liquor cool, and there will adhere to the sides of the vessel a great quantity of a crystallized saline matter, which is Crystal of Tartar.
OBSERVATIONS.
Tartar, when taken out of the vats in which it forms, is mixed with a considerable quantity of earthy parts, which are not intimately united therewith, but adulterate it. This[Pg 518] extraneous earth makes about two fifths of the whole weight of common Tartar; but white Tartar, which is the best, contains but about a third part of earth.
The method of refining Tartar, and freeing it from this adventitious earth, is very simple, as appears from the process. Earthy matters, which are not intimately combined with an Acid in the form of a Neutral Salt, are not dissoluble in water: for which reason the water, in which crude Tartar is boiled, dissolves the saline part only, which passes with it through the filter; but doth not dissolve the earth of the Tartar, because that earth is not combined with the saline part, and so being only suspended in the liquor remains on the filter.
The saline parts of the Tartar, though they are now separated from the gross earth with which they were mixed, are not yet perfectly pure. These first Crystals of Tartar have a disagreeable russet colour, and are not transparent: this is owing to their being coated over, as it were, with a fatty matter, which also is foreign to their nature, and may be separated from them without decomposing them in the least.
The crystals of Tartar are but seldom perfectly depurated in Chymical Laboratories; because the operation doth not usually succeed well on small quantities: but there are manufactories which do it by the great, and supply the Chymists, as well as the several tradesmen, with very fine and very pure Crystals of Tartar. These manufactories are chiefly set up in the neighbourhood of Montpelier. Mr. Fifes, a celebrated Professor of Medicine, hath in the Memoirs of the Academy for 1725 described the operation as performed in one of these works. He tells us, that having separated the earthy part from the Crystals of Tartar, by boiling and filtering, they dissolve them again, and boil them in large caldrons, mixed with a white saponaceous earth, which cleanses and whitens them to perfection.
The saponaceous earth is found near the works; but it is not the only one that may be employed for this purpose; since, as Mr. Fifes observed, they have successively made use of several different earths in that very work, and that the earth they now use hath not been long employed. There is reason to think that most saponaceous earths might answer the purpose of refining Crystal of Tartar: but one necessary condition is, that they be altogether indissoluble by Crystal of Tartar, which being acid dissolves many sorts of earth; for, if they have not this quality, they will form a Neutral Salt with the saline part of the Tartar, the nature of which they[Pg 519] will entirely change, and convert it into soluble Tartar, as will appear by the experiments that follow.
Crystal of Tartar combined with several Substances.
Crystal of Tartar combined with Absorbent Earths. Soluble Tartars.
Boil an Absorbent Earth, such as Chalk, in a pan with water; and, when you perceive the Earth thoroughly divided, and equally distributed through the water, throw into a pan, from time to time, some pulverized Crystal of Tartar, which will excite a considerable effervescence. Continue those projections, till you observe no effervescence excited thereby. All the Absorbent Earth, which obscured the transparency of the water, and gave it an opaque white colour, will gradually disappear as the Crystal of Tartar combines with it; and when the combination is perfected, the liquor will be clear and limpid. Then filter it, and there will be left on the filter but a very small quantity of earth. Evaporate all the filtered liquor with a gentle heat; and then set it in a cool place to shoot. Crystals will form therein, having the figure of flat quadrangular prisms, with almost always one, sometimes two, of the angles of the prism shaved down, as it were; and then the surfaces at each end are oblique, answering to those depressed angles. These crystals are a Neutral Salt, which readily dissolves in water; a true Soluble Tartar.
OBSERVATIONS.
Crystal of Tartar is a saline substance of a singular nature. Though it crystallizes like a Neutral Salt, yet it is not one: it hath only the form of one; its principal properties being those of an Acid. Nevertheless it is not a pure Acid; for it is united with a certain quantity of Oil and of earth, which give it the property of crystallizing, and it is scarce[Pg 520] dissolvable in water. It is a middle substance between an Acid and a Neutral Salt. It is an Acid half-neutralized; on which account it is capable of acting like an Acid on all substances soluble by Acids, and so of being converted into a perfectly Neutral Salt by combining with them to the point of saturation.
In the experiments made to neutralize Crystal of Tartar, Fixed Alkaline Salts alone were formerly used. Messrs. Duhamel and Grosse were the first who discovered that Absorbent Earths might be substituted for Alkalis, and would produce nearly the same effects on Crystal of Tartar. The experiments made by these two Academicians in conjunction are circumstantially related in two curious Memoirs on this subject, given in by them jointly, and printed with those of the Academy for 1732 and 1733. From these Memoirs we took the process here given, and shall also borrow from thence most of the remarks we are now going to make.
Stone-lime holds, as it were, the middle place between mere Absorbent Earths and Fixed Alkalis. Now, seeing Crystal of Tartar may be converted into a Neutral Salt by either of these two substances, it follows, that lime ought to produce the same effect upon it. Accordingly Messrs. Duhamel and Grosse found it to be so upon trial: having formed, with Lac calcis and Crystal of Tartar, a Neutral Salt perfectly like that which results from the union of that saline matter with Chalk. Cremor calcis, or that salino-terrene pellicle which forms on lime-water, produced the same effect: but, what is most singular is, that lime-water itself, though it be clear and limpid, and consequently doth not seem to contain any earthy particles, produced nevertheless a great effervescence with Crystal of Tartar, and neutralized it as perfectly as Cremor calcis, or water ever so much impregnated with Chalk. This arises from hence, that a great quantity of the salino-terrene matter, which forms the Cremor calcis is dissolved in the lime-water.
Though lime-water neutralizes Crystal of Tartar as perfectly as Chalk does, and though the Crystals of soluble Tartar, or neutralized Tartar, thereby produced, be like those which have Chalk for their basis, yet Messrs. Duhamel and Grosse observed some differences, worthy of notice, between the phenomena accompanying the production of these two Neutral Salts, which resemble each other so much that they seem but one and the same species of Salt. The principal difference consists in this, that the water containing the Tartar neutralized by Chalk is very limpid, and leaves but a very[Pg 521] small quantity of earth on the filter; whereas the lime-water, with which Tartar hath been neutralized, leaves on the filter a considerable quantity of earth.
This must appear the more surprising, that the water replete with Chalk was, before its union with the Crystal of Tartar, turbid and opaque; whereas the Lime water was at first clear and limpid. Messrs. Duhamel and Grosse suspect this to arise from hence, that the effervescence excited, while the Crystal of Tartar dissolves the matter contained in lime-water, is greater than that which is produced by its union with Chalk suspended in water.
"If we consider," say they, "that in a great effervescence a considerable quantity of the acid Spirit is evaporated, we shall easily perceive, that, the more of that Spirit escapes, the more of the earth of the Tartar will be precipitated. Now, as the effervescence with lime-water is more considerable, and as there is less alkaline earth to check, as it were, and restrain the Acid, than in the experiment with Chalk, a greater quantity of the acid Spirit may escape; which being entirely lost will cause more earth to precipitate in this case than in the other, where the Acid is all at once attracted by a great deal of alkaline earth: and accordingly this was the reason that our Tartar dissolved by Chalk deposited, in crystallizing, a grey earth, which was scarce perceivable in the experiment made with Lime-water.
"Yet perhaps," say they, "an Acid, which we suspect to be contained in lime, may have partly occasioned the precipitation of this earth." The existence of this Acid, which these gentlemen at that time only suspected, hath been since demonstrated by several experiments, and particularly by those which Mr. Malouin hath published. This Acid is the Vitriolic, which, in combination with some of the earth of the lime, forms a sort of Selenitic Salt; which adds greatly to the probability of Messrs. Duhamel and Grosse's last conjecture. I shall now explain how I conceive the Vitriolic Acid in lime may occasion the copious precipitate which falls in lime-water, when Crystal of Tartar is neutralized by it.
The quantity of Vitriolic Acid contained in lime is very inconsiderable; so that to convert it into a Neutral Salt requires its intimate union with a very small quantity of the earthy and absorbent parts. Hence it comes to pass, that, when water is poured upon quick-lime, in order to make the Lime-water, it in some sort divides the lime into two parts.[Pg 522] All the particles of Absorbent Earth, which had not contracted an union with the Acid, are at first barely suspended in the liquor, the transparency of which they destroy, giving it an opaque white colour; and this is what makes the Lac calcis: but they soon separate from it, and fall to the bottom, in the form of a precipitate; because they are not soluble in water. By this precipitation the liquor becomes limpid, and remains impregnated only with such of the earthy parts as are united with the Vitriolic Acid, in the form of a kind of Neutral Salt, and have by that union acquired solubility. But the Vitriolic Acid finding many more Absorbent parts in the lime than were necessary to neutralize it, in a manner over-dosed itself with earthy parts, and thereby exceeded the bounds of a perfect Neutrality.
On the other hand, it hath been shewn, that Crystal of Tartar is an imperfect Neutral Salt. Now these two Salts, which are neither of them perfectly Neutral, differ from a perfectly Neutral Salt by properties directly opposite to each other; seeing the Selenitic matter in Lime exceeds in its absorbent or alkaline quality, and Crystal of Tartar exceeds, on the contrary, in acidity.
What must be the consequence, therefore, of mixing these two saline matters together? The same as when an Acid is mixed with a Fixed Alkali; that is, the Salt which exceeds in acidity will combine with the super-abundant alkaline earth of the Selenitic Salt; so that these two saline matters will both become perfectly Neutral Salts. Yet these two Neutral Salts have not the same degree of solubility in water. The neutralized Crystal of Tartar dissolves very readily in water, and is for that reason called Soluble Tartar: the Selenitic Salt, on the contrary, is hardly dissolvable in it at all. Now it is a rule that, when two Salts of this nature meet together, the most soluble always remains united with the water, exclusive of the other, which is forced to precipitate. This I imagine to be what happens in the present case; and the precipitate which we see fall, in the lime-water employed to neutralize Crystal of Tartar, seems to me to be no other than the Selenitic Salt of the Lime; which, being less soluble than the neutralized Tartar, gives place to it, and separates from the liquor.
Indeed we cannot, in my opinion, account for the precipitate under consideration, any other way, than by supposing it to be a portion either of the earth of the Crystal of Tartar, or a portion of the Lime. Now, either of these earths is dissolvable by Acids; whereas the precipitate in question, ac[Pg 523]cording to the observations of Messrs. Duhamel and Grosse, is not so: and this ought to be the case, if the precipitate be nothing but the selenitic Salt of the Lime, which being a Neutralized Salt, partly constituted by the most powerful of all the Acids, must be unalterable by any Acid whatever.
Messrs. Duhamel and Grosse made a great many experiments on the combinations of Crystal of Tartar with different sorts of earths. The result of the whole is, that there are some earths which this Acid dissolves, and which contract such an union with Crystal of Tartar, that they not only change its external character, that is, its tendency to crystallize, and its indissolubleness in cold water, but also entirely alter its taste and other qualities. In a word, those earths produce on this Salt all the effects of alkaline Salts. These earths are such as are called Absorbent Earths; stone-lime, animal-lime, cretaceous earths, a portion of calcined gypsum, and of potash; in short, all such as distilled vinegar is capable of dissolving: this is the mark by which those earths, which are qualified to neutralize Crystal of Tartar, and to render it soluble, may be distinguished.
Messrs. Duhamel and Grosse found also upon trial, that there are other earths, on the contrary, which are, in a manner, inaccessible to the Acid of Crystal of Tartar; that they take up, indeed, the grossest and redundant Oil of the Tartar, but without affecting its saline part at all: and if these earths are ever observed to form any union with the Crystals of Tartar, as happens in the refineries near Montpelier, that union is only superficial, not intimate; and therefore it alters none of the characters of the Salt. Among these earths are the clayey, bolar, sandy earths, and others of that kind. Hence Messrs. Duhamel and Grosse conclude, that these are the earths which ought to be employed in the purification and whitening of Crystal of Tartar. Vinegar is here also the test by which it may be known whether an earth intended for this purpose be fit for it: for you may be sure that it will form no union with Crystal of Tartar, if the Acid of Vinegar be incapable of dissolving it.
Crystal of Tartar combined with Fixed Alkalis. The Vegetable Salt. Saignette's Salt. The Decomposition of Soluble Tartars.
In eight parts of water dissolve one part of a very pure alkaline Salt, perfectly freed from the phlogiston by calcination. Heat this lixivium in a stone pan set on a sand-bath, and from time to time throw into it a little powdered Cream or Crystal of Tartar. Each projection will excite a great effervescence, attended with many bubbles, which will rise to a considerable height one over the other. Stir the liquor when the effervescence ceases, and you will see it begin again.
When no effervescence appears upon stirring the liquor, add a little more Cream of Tartar, and the same phenomena will be renewed. Go on thus till you have obtained the point of perfect saturation.
Then filter your liquor. If the Alkali you made use of was the Salt of Soda, evaporate your liquor quickly to a pellicle, and there will shoot in it crystals of nine sides, resembling a coffin; the bottom part thereof being concave, and streaked with a great many parallel lines; and this is Saignette's Salt. If you have employed any other Alkali but Soda, or the basis of Sea-salt, evaporate your liquor slowly to the consistence of a syrup: let it stand quiet, and there will form in it crystals having the figure of slatted parallellopipeds; and this is the Vegetable Salt, or Tartarized Tartar.
OBSERVATIONS.
Seeing pure Absorbent Earths are capable of neutralizing Crystal of Tartar, and converting it into Soluble Tartar, there is still more reason to expect that Fixed Alkalis should possess the same property, as they have a much greater affinity with Acids: and accordingly Crystal of Tartar always forms, with every species of these salts, a Neutral Salt which is a Soluble Tartar.
A Soluble Tartar, formed by the union of Crystal of Tartar with Tartar converted into an Alkali by fire, hath been long used in medicine as a gentle saponaceous purgative,[Pg 525] known by the names of Tartarized Tartar, or the Vegetable Salt. But the Soluble Tartar, prepared by combining Crystal of Tartar with the Alkali of Soda, which, as we remarked before, is analogous to the basis of Sea-salt, and different from all other Alkalis, was not well known to Chymists till the year 1731, when M. Boulduc published the preparation in a Memoir printed in the Academy's collection for that year[14].
Not but that it was very much used before that time: for it had been for several years in high reputation, and prescribed instead of Tartarized Tartar, which became almost quite neglected. But M. Saignette, a physician of Rochelle, who was the first inventor and vender of this Salt, did not publish the preparation of it, which he kept as a secret: and this probably contributed not a little to the great esteem which this medicine had acquired; for men are naturally inclined to put a much greater value on secrets, than on what is universally known. He gave it the name of Sal Polychrestum; and the public called it also Saignette's Salt, and Rochelle Salt. Since the discoveries of M. Geoffroy and M. Boulduc were published, the method of preparing this Salt hath been no secret; it was described in Dispensatories, and every apothecary hath made it ever since.
Saignette's Salt, as well as every other Soluble Tartar, melts when laid on live coals, boils up, emits smoke, and leaves a black charred matter behind. This resemblance of Saignette's Salt to Tartarized Tartar, joined to the smell of the vapour which exhaled in burning it, and is the same with that of Tartar, were the first notices that led M. Boulduc to suppose this Salt to be a soluble Tartar. On examining the alkaline coal produced by the calcination, and comparing it with that left by Tartarized Tartar, he perceived there was some difference between them. At last his friend, M. Grosse, having advised him, as he tells us in his Memoir, to combine Crystal of Tartar with the Salt of Soda, and to examine the new Salt that would result from their union, M. Boulduc immediately suspected that it must produce a species of Soluble Tartar, which might possibly prove to be the Salt in question. Nor was he mistaken in his conjecture: for with these two saline substances he actually composed a Salt perfectly like Saignette's.
Under the head of Borax we remarked that it contains an Alkali like the basis of Sea-salt. This Alkali is not perfectly neutralized by the sedative Salt, which is also contained in Borax: for its alkaline properties are so perceptible as to have led some Chymists to think that Borax was only an Alkali of a particular kind. This induced M. le Fevre, a Physician at Uzes, and one of the Academy's correspondents, to combine Crystal of Tartar with Borax, and to examine the result. He communicated to the Academy his experiments on this subject; by which he found that the combination of these two saline matters forms a Soluble Tartar, but greatly different from Saignette's Salt; especially in that it doth not crystallize, but remains in the form of a gummy matter, and retains all the acidity natural to pure Cream or Crystal of Tartar: a circumstance which is very remarkable.
Mr. Lemery had the curiosity to repeat M. le Fevre's experiment, and found that this singular Soluble Tartar had the properties ascribed to it by the inventor. The process he recommends for making the experiment with success is as follows:
"Take four ounces of Crystal of Tartar finely pulverized, and two ounces of Borax carefully powdered, and put these two Salts into a flint-glass body. Pour on them two ounces of water, and set the cucurbit into a sand-bath. Warm it with a gentle fire, and then increase the heat so as to make the liquor boil for a quarter of an hour; which will produce a perfect dissolution of the Cream of Tartar and Borax. After the dissolution of these two Salts united together, the liquor will remain clear and limpid, though the boiling hath dissipated a good deal of it. If the liquor be still further evaporated, the remainder will have the consistence of Honey, or Turpentine: and, if the evaporation be carried still farther, with a gentle heat, the matter remaining will in colour resemble the gum of a plumb-tree, and yield to pressure as that does; and, if it be exposed to the air in a damp place, it will grow moist and run, almost like Salt of Tartar:" a new and singular property, which belongs neither to Borax nor to Crystal of Tartar, when they are not combined together.
All Soluble Tartars are easily decompounded, by means of a certain degree of heat. They yield in the distillation the same principles as Tartar; and the Alkali that remains, when they are perfectly calcined, consists of that which the Tartar naturally affords, and of the alkaline matter with which it was converted into a Neutral Salt.
These Neutral Salts, resulting from the union of Crystal of Tartar with any alkaline matter, are also decompounded by all the Acids, even by vinegar, which nevertheless is an Oily Vegetable Acid, and consequently of the same kind with Crystal of Tartar. The reason of this is that the Acid of Vinegar, though blunted by much phlegm and oil, must be considered as a free and pure Acid, when compared with Crystal of Tartar; which is still more embarrassed with heterogeneous matters, so as to be a semi-neutral Salt.
When Soluble Tartar is decompounded by an Acid, the Crystal of Tartar, which helped to constitute the Neutral Salt, is then wholly recovered. This saline matter, being separated from that which rendered it soluble in water, ceases now to be so, and for that reason precipitates to the bottom of the liquor.
The Neutral Salts, resulting from the decomposition of Soluble Tartar by an Acid, differ according to the Acid made use of. From Saignette's Salt decompounded by the Vitriolic Acid M. Boulduc obtained a true Glauber's Salt, and a precipitate of Crystal of Tartar: and this he justly adduces as a demonstrative proof, that Saignette's Salt is no other than Crystal of Tartar neutralized by a Fixed Alkali analogous to the basis of Sea-salt.
Though all Soluble Tartars may be decompounded by Acids, as hath just been said, yet they do not all forsake their bases with equal facility. Messrs. Duhamel and Grosse found that, in this respect, they observe the following order, beginning with those which afford the readiest and most copious precipitate: viz. Soluble Tartar made 1. with Potash; 2. with Chalk; 3. with uncalcined Oyster-shells; 4. with Stone-Lime; 5. with calcined Oyster-shells; 6. with Salt of Tartar; 7. with Salt of Soda; 8. and lastly, Tartar made soluble with Borax is not precipitated by distilled vinegar.
It is not easy to account for this difference between Soluble Tartars. If the Salt of Soda were more alkaline than Salt of Tartar, and Borax more alkaline than the Salt of Soda, it might be conjectured that the more alkaline the matters are with which Crystal of Tartar is neutralized, the closer is the union it contracts with them; since it is plain, from what hath been said on this subject, that though Soluble Tartars, which have for their basis Absorbent Earths only, not converted into Lime, are more easily decompounded than those which are rendered soluble by Limes; and these again more easily than those which have a Fixed Alkali for their basis. But, on the contrary, the Salt of Soda is less[Pg 528] alkaline than Salt of Tartar, and Borax still less than the Salt of Soda.
Crystal of Tartar combined with Iron. Chalybeated Tartar. Tincture of Steel with Tartar. Soluble Chalybeated Tartar.
Mix four ounces of Iron, in filings, with one pound of white Tartar, finely pulverized. Boil the mixture in about twelve times as much water as you took of Tartar. When the saline part of the Tartar is dissolved, filter the liquor boiling-hot through a flannel bag, and then set it in a cool place. In a very little time crystals of a russet colour will shoot therein. Decant the liquor from these crystals; evaporate it to a pellicle, and set it again to crystallize. Go on in this manner till it will shoot no more. Collect all the Salt you have thus obtained, and keep it under the name of Chalybeated Tartar.
To make the Tincture of Steel with Tartar, mix together six ounces of clean Iron filings, and one pound of white Tartar in powder. Put this mixture into a large iron kettle, and pour thereon as much rain-water as will moisten it.
Make a paste of this matter, and leave it thus in a mass for twenty-four hours. Then pour on it twelve pounds of rain-water, and boil the whole for twelve hours at least, stirring the mixture frequently, and adding from time to time some hot water, to supply the place of what evaporates. When you have thus boiled the liquor, let it stand quiet for some time, and then pour it off from the sediment at bottom. Filter, and evaporate to the consistence of a syrup; and you have the Tincture of Mars with Tartar. The Dispensatories generally order an ounce of rectified Spirit of Wine to be poured on this Tincture, in order to preserve it, and to keep it from growing mouldy, as it is very apt to do.
Soluble Chalybeated Tartar is prepared by mixing four ounces of Tartarized Tartar, with one pound of the Tincture of Mars with Tartar, and evaporating them together in an iron vessel to dryness; after which it is kept in a well stopped phial, to prevent its growing moist in the air.
OBSERVATIONS.
The three preparations of this process are medicines very well known and much used. There is even reason to think that those, who first thought of combining Tartar in this manner with Iron, had it in their view to prepare compositions useful in medicine, rather than merely to produce new combinations for the improvement of Chymistry. Indeed, were we to consider only the account here given of the manner in which these three compositions are made, we should be inclined to think Crystal of Tartar incapable of dissolving Iron so thoroughly and radically, that, from the union of these two substances, a Neutral Metallic Salt should arise, a Tartar neutralized and made soluble by Iron. For it is very certain that the first of these preparations, which is called Chalybeated Tartar, is nothing but the saline part of Tartar dissolved by boiling water, and then precipitated and crystallized along with particles of iron, that are reduced, at most, into a rust, or a crocus only, but have contracted no union with the crystal of Tartar, which remains as Acid and as indissoluble after this preparation as before. Accordingly it is called only Chalybeated Tartar, and not Soluble Chalybeated Tartar: and, as this latter name hath been given only to the Tartarized Tincture of Mars compounded with Tartarized Tartar; that is, with Tartar rendered soluble by a Fixed Alkali, and not by Iron; there is reason to presume, that the Tincture of Mars alone was not thought worthy of being called a Soluble Chalybeated Tartar; but that the name, importing Tartar rendered soluble by Mars, belongs to that Tincture only when compounded with a true Soluble Tartar.
It is nevertheless very certain, that the Tincture of Mars made with Tartar contains a true Soluble Chalybeated Tartar; that is, a Neutral Salt consisting of Crystal of Tartar united with Iron, and rendered soluble by that union. The long boiling, necessary to prepare this Tincture, gives the Acid of Tartar time to dissolve the Iron radically, and to unite very closely therewith: but this is not the case in the preparation of Chalybeated Tartar; to make which the Tartar is boiled in water only as long as is necessary for the dissolution of its saline parts; that is, about a quarter or half an hour; in which space the Acid of the Tartar can scarce begin to act on the surface of the Iron: for Acids have not so quick an effect on metals, as on Alkalis and Absorbent Earths. Metallic substances, being vastly more compact, are[Pg 530] not near so soon dissolved by Acids, and especially by vegetable Acids, weakened with heterogeneous matters, as the Acid of Tartar is.
I thought the dissolution of Iron by Tartar a point of sufficient importance to deserve a little more attention than hath commonly been given to it; and for that reason resolved to examine, and trace with care, the phenomena observable in this operation.
As the crude Tartar, employed in making the Tartarized Tincture of Mars, is replete with many oily and earthy parts, which cannot but obstruct the dissolution of the Iron, and prevent our seeing clearly how that dissolution is carried on, I thought it better to make use of Cream, or Crystals, of Tartar, which, being pure and freed from all those heterogeneous parts, dissolve in boiling water without prejudicing its transparency.
I therefore pulverized Cream of Tartar, and dissolved as much thereof in boiling water as it would take up. This solution I poured boiling hot into a matrass, at the bottom of which I laid some fine iron wire cut into small pieces. I set the matrass in a sand-bath; and having heated it so as to make the liquor boil, I observed that, the instant before it boiled, the liquor began to act very perceptibly upon the Iron, in the same manner as other Acids act upon metallic substances; that is, there appeared on the surfaces of the little bits of Iron small bubbles, which immediately rose to the surface of the liquor, and succeeded each other so fast, that they formed lines, or jets, seemingly continued from the surface of the Iron to the surface of the liquor, which, little by little, acquired a faint tinge of yellow.
When the liquor was heated so as to boil, the dissolution still went on, but much more briskly, and the liquor acquired a deeper colour. After boiling about an hour, the liquor, which at first was very clear, became turbid, and of an opaque white; which made me think, that some of the Cream of Tartar, dissolved therein, began to precipitate.
I let the whole boil some time longer, and the white precipitate becoming more considerable, I resolved to filter the liquor, which passed through clear, and tinged with a greenish yellow. There remained on the filter a whitish sediment, which I found to be true Cream of Tartar. The filtered liquor tasted much like a solution of Copperas. I evaporated it in a glass bason, set in a sand-heat, but no pellicle appeared; which made me conclude that it would produce no crystals: accordingly, having taken some of it out of the bason,[Pg 531] when it was considerably reduced by evaporation, and set it in a cool place, no crystal shot in it.
The rest of the liquor I evaporated to dryness: it left a blackish brown residuum, which had the same taste with the liquor before evaporation, but much stronger. This residuum melts very readily in the mouth, without leaving on the tongue the least gritty particle. Being exposed very dry to the air, it grows moist, and runs into a liquor in a very little time. It dissolves easily and readily in a very small quantity of cold water. This solution being mixed with Fixed Alkalis, in various proportions, doth not grow turbid, nor drops any precipitate; but with a decoction of galls it makes ink. Acids give it a much clearer colour, and at first produce no precipitation; but, in a quarter of an hour, there appears a precipitate much of the same colour with the solution. This precipitate is no other than Cream of Tartar, tinged of a russet colour by the liquor, which grows turbid, and a little whitish, when the precipitate begins to form.
These experiments, and the circumstances attending them, will not allow us to doubt the truth of what I advanced concerning the Tincture of Mars made with Tartar, viz. that it is nothing but Crystal of Tartar by which Iron is dissolved, and which is rendered soluble by that metal. We see at the very first that Crystal of Tartar acts upon Iron, just as other Acids do. Indeed this metallic solution is not precipitated by Alkalis: but we know that Alkalis possess the property of dissolving Iron, especially when the metal is previously divided by an Acid; so that there is reason to think this may be the case, when an Alkali is mixed with our Soluble Chalybeated Tartar.
As this Soluble Tartar is a saponaceous and oily Salt, it is also possible that it may be dissolved entirely by the Alkali, without suffering any decomposition; especially as Alkalis decompound Neutral Metallic Salts, by means only of the stronger affinity which they have with the Acids, than with the Metals, of which those Salts are compounded. Now, as our Soluble Chalybeated Tartar is compounded of that Metal which the Alkali dissolves with the greatest ease, and of that Acid with which it hath the least affinity of any, it is very possible that it may not have a greater affinity with the Acid than with the metallic basis of this Salt, and so be uncapable of decompounding it. However, as this Soluble Chalybeated Tartar makes a black liquor with a decoction of Galls, and as nothing but Iron dissolved by an Acid hath[Pg 532] that property, it may be safely concluded, that this Salt really consists of Iron dissolved by the Acid of Tartar.
The precipitate which a solution of this Salt lets fall, on the addition of an Acid, is another proof that it consists of these two principles: for this precipitate can be no other than the Tartarous Acid, which, being the weakest of all Acids, is separated from the Iron by the Acid added to the solution; which Acid unites with the Martial basis, and forms another Neutral Metallic Salt, according to the Acid employed. Lastly, the great solubility of the desiccated residuum of the Tincture of Mars, made with Tartar, is a very strong and decisive proof, that this residuum is no other than Iron dissolved by the Acid of Tartar: for what else can it be? Nothing but Iron and Crystal of Tartar is made use of in the operation; and neither of these two substances singly is so soluble as this new body.
We know, moreover, that Crystal of Tartar, which itself is indissoluble, forms a Soluble Tartar when combined with pure Absorbent Earths, though these matters be still more indissoluble than it, or rather, are not soluble at all. Hence it is very natural to conclude, that our residuum is a Tartar rendered soluble by Iron. This Chalybeated Tartar is even more soluble than any other sort of Soluble Tartar; for it very readily grows moist in the air, and runs wholly into a liquid; on which account it is not susceptible of crystallization.
I return to one of the circumstances attending my experiment, which it is proper I should account for; though I have hitherto only mentioned it, without more particular notice, that I might not break the connection between facts, and the consequences resulting from them. The circumstance I mean is the precipitation of the Cream of Tartar dissolved in the liquor, which, I said, happens when the saline solution hath boiled upon the Iron about an hour. This precipitation of the Cream of Tartar may be partly occasioned by the evaporation of the water in which it is dissolved: for the water having taken up, as was said, as much Cream of Tartar as it was capable of dissolving, when the quantity of water comes to be lessened, a proportional quantity of Cream of Tartar must precipitate.
But some other cause must also contribute to produce this precipitation: for, as I boiled my liquor in a matrass, the evaporation of the liquor could not be considerable, and yet the precipitate was very copious. Moreover, I replenished the matrass with much more water than was necessary to re[Pg 533]place what had evaporated; yet I could not re-dissolve the precipitated Cream of Tartar, nor even sensibly lessen its quantity.
The true cause of this effect I take to be as follows. When the solution of Cream of Tartar hath boiled for some time upon the Iron, and dissolved a certain quantity thereof, a proportional quantity of Soluble Chalybeated Tartar is formed. Now as this Salt is much more soluble in water than Cream of Tartar, and as water always takes up the more soluble Salts, preferably to the less soluble, it is not surprising that Cream of Tartar, being one of those saline substances which dissolve with the greatest difficulty, should on this occasion separate from the liquor, and precipitate; yielding its place to a Salt which hath a much greater affinity with water.
Hence it appears, that to re-dissolve the Cream of Tartar, and render it capable of continuing to dissolve the iron as efficaciously as before, it is not sufficient that fresh water be added; but the solution of the Soluble Chalybeated Tartar already formed must be entirely decanted, and fresh water poured on the residue; and then this water, not being impregnated with any Soluble Chalybeated Tartar, will be capable of re-dissolving the Cream of Tartar, and every thing will go on as at the beginning of the operation, till the Cream of Tartar come to precipitate again, for the same reason as before, and make a repetition of the same management necessary. The liquor is far from being saturated with Soluble Chalybeated Tartar, when the precipitation of the Cream of Tartar renders it necessary to decant it: so that the water must be often renewed, if you carry the operation to the utmost; and then all these solutions must be added together, and evaporated, either to dryness, if you desire to have the salt in a dry form, or to any other degree you think proper.
This method I followed at first: but as it is exceeding long and tedious, though perhaps the best; and as I wanted to have a moderate quantity of Soluble Chalybeated Tartar, with less trouble, and in less time, if possible, I resolved to try whether or no Cream of Tartar, though separated from the liquor and undissolved, were still capable of acting on the iron with such efficacy as to dissolve it. I therefore continued to boil the tartarous solution on the filings of Iron, notwithstanding the precipitation of the Cream of Tartar, taking care only to add fresh water from time to time, as directed in the process for the Tartarized Tincture of Mars, to[Pg 534] replace what evaporated; and I observed that, in fact, the Cream of Tartar, though not perfectly dissolved, but only divided and agitated by the motion of boiling, still continued to act upon the Iron; so that the liquor, after boiling seven or eight hours, was so impregnated as to yield by evaporation a reasonable quantity, in bulk, of Salt in a dry form.
Crystal of Tartar combined with the reguline Part of Antimony. Stibiated or Emetic Tartar.
Pulverize and mix together equal parts of the Glass and of the Liver of Antimony. Put this mixture, with the same quantity of pulverized Cream of Tartar, into a vessel capable of containing as much water as will dissolve the Cream of Tartar. Boil the whole for twelve hours, from time to time adding warm water, to replace what is dissipated by evaporation. Having thus boiled your liquor, filter it while boiling hot; evaporate to dryness; and you will have a saline matter which is Emetic Tartar.
OBSERVATIONS.
The Glass and Liver of Antimony are no other, as was said in its place, than the metallic earth of Antimony separated from the redundant Sulphur of that mineral; but still retaining such a quantity of phlogiston as to possess, excepting its metalline colour, nearly the same properties with Regulus of Antimony, and especially its emetic quality, and its solubility in Acids. Indeed these two preparations seem to have more of an emetic quality than the Regulus itself, and therefore are employed preferably to all others in the preparation of Emetic Tartar.
It is not yet ascertained in which of the principles of Antimony its emetic virtue resides. We are sure, however, that it cannot be ascribed to its earthy part: for the calx of Antimony, when entirely deprived of all phlogiston, is not emetic, nor even purgative; as is evident from the effects of Diaphoretic Antimony and the Pearly Matter.
Some authors think Antimony contains an arsenical principle, to which they impute its emetic quality; nor is their opinion altogether void of probability. For this arsenical part seems to be indicated by several of the properties of An[Pg 535]timony, and particularly by its affinities with other metallic substances, in which it very nearly resembles Arsenic. But this doth not amount to a positive proof: for we can draw nothing but probable conjectures, at most, from such analogies.
Other Chymists think the emetic virtue of Antimony depends on the union of its metallic earth with its phlogiston. This opinion seems to me much more probable than the other: for by only recombining a phlogiston with the earth of Antimony, deprived by calcination of all its emetic virtue, that virtue is perfectly restored, and the Regulus thus revivified is no less emetic than that which never underwent calcination.
However this be, it is certain that Cream of Tartar acquires an emetic quality, not by barely uniting with one of the principles of Antimony, but by dissolving entirely the reguline, or semi-reguline, part thereof; and that its emetic quality is so much the stronger, the more of that substance it hath dissolved. This is the result of several experiments made on the subject by Mr. Geoffroy.
That gentleman collected several parcels of Emetic Tartar, having different degrees of strength. "I employed," says he[15], "an ounce of each of those Emetic Tartars: I rubbed them separately with an equal weight, or something more, of a black flux, made of two parts of red Tartar, and one part of Nitre calcined together. These mixtures I put into different crucibles, formed like inverted cones: I kept them in a melting heat till the Salts in fusion sunk, and appeared like a smooth oil at the bottom of each crucible. I then let the fire go out, broke the crucibles when cold, and found the resuscitated Regulus in a mass at bottom.
"Out of one ounce of the weakest Emetic Tartars I obtained from thirty grains to one dram eighteen grains of Regulus. From one ounce of such as were of a middling strength I got one dram and an half; and the most violent yielded me two drams and ten grains.
"The power, therefore, of the strongest Emetic Tartars," continues he, "depends on the quantity of Regulus of Antimony dissolved by the Cream of Tartar, and the nearer the preparations of Antimony, on which the solution of Cream of Tartar is boiled, are to the form of a Regulus or a Glass, the more violent is the Emetic Tartar; because the Vegetable Acid of the Tartar acts then more imme[Pg 536]diately upon the Emetic part of the Antimony, and dissolves more of it."
Mr. Geoffroy found upon trial, that Cream of Tartar boiled for a due time on Crude Antimony, doth indeed dissolve a little of the reguline part thereof; but that the quantity of Regulus dissolved thereby is so very small, that the Emetic Tartar produced is extremely weak. The gross Sulphur, in this case, hinders the Cream of Tartar from acting on the reguline part with so much efficacy, as when the Antimony is properly prepared by freeing it entirely from its redundant Sulphur.
Nothing can be added to what Mr. Geoffroy hath said on this subject. His experiments are decisive, and set the truth he intended to prove in the clearest light.
Mr. Hoffman affirms, that Emetic Tartar loses part of its virtue by being boiled too long. A very able Chymist goes so far as to say, that Tartar ought not to boil above six or seven minutes with prepared Antimony; because longer boiling destroys part of its Emetic quality. Can this arise from hence, that Cream of Tartar, after dissolving a certain quantity of the reguline substance, separates from it afterwards? Or is the Cream of Tartar itself decomposed by too long boiling? This deserves to be particularly inquired into, as well as the nature of the Metallic Salt, which results from the union of the Acid of Tartar with the Regulus of Antimony.
Crystal of Tartar acts also on several other metallic substances, and particularly on Lead; with which it forms a Salt, resembling Tartarized Tartar in the figure of its crystals.
Of the Product of Acetous Fermentation.
Substances susceptible of the Acetous Fermentation turned into Vinegar.
The Wine, the Cyder, or the Malt-liquor, which you intend to convert into Vinegar, being first thoroughly mixed with its lees, and with the Tartar it may have depo[Pg 537]sited, put your liquor into a vat used before, either for making or for holding Vinegar. This vessel must not be quite full, and the external air must have access to the liquor contained in it. Set it where the air may have a degree of warmth answering nearly to the twentieth degree above 0 in Mr. de Réaumur's Thermometer. Stir the liquor from time to time. There will arise in it a new fermentative motion, accompanied with heat: its vinous odour will gradually change, and turn to a sour smell, which will become stronger and stronger, till the fermentation be finished, and cease of itself. Then stop your vessel close; the liquor it contains will be found converted into Vinegar.
OBSERVATIONS.
All substances that have undergone the spirituous fermentation are capable of being changed into an Acid, by passing through this second fermentation, or this second stage of fermentation. Spirituous liquors, such as Wine, Cyder, Beer, being exposed to a hot air, grow sour in a very short time. Nay, these liquors, though kept with all possible care, in very close vessels, and in a cool place, degenerate at last, change their natures, and insensibly turn sour. Thus the product of spirituous fermentation naturally and spontaneously degenerates to an Acid.
For this reason it is of great importance, in making Wine, or any other vinous liquor, to stop the fermentation entirely, if you desire the Wine should contain as much Spirit as possible. It is even more advantageous to check the fermentation a little before it comes to the height, than afterwards: because the fermentation, though slackened, and in appearance totally ceased, still continues in the vessels; but in a manner so much the less perceptible, as it proceeds more slowly. Thus those liquors, in which the fermentation is not quite finished, but checked, continue for some time to gain more Spirit: whereas, on the contrary, they degenerate and gradually turn sour, if you let the spirituous fermentation go on till it be entirely finished.
The production of the second fermentation, which we are now to consider, is an Acid of so much the greater strength, the stronger and more generous the spirituous liquor, in which it is excited, originally was. The strength of this Acid, commonly called Vinegar, depends likewise, in a great measure, on the methods used in fermenting the vinous liquor, in order to convert it into Vinegar: for[Pg 538] if it be fermented in broad, flat vessels, and left to grow sour of itself, the spirituous part will be dissipated, and the liquor, though sour indeed, will be vapid and effete.
The Vinegar-makers, to increase the strength of their Vinegar, use certain methods of which they make a mystery, keeping them very secret. However, Mr. Boerhaave gives us, from some Authors, the following description of a process for making Vinegar.
"Take two large oaken Vats or Hogsheads, and in each of these place a wooden grate or hurdle, at the distance of a foot from the bottom. Set the vessel upright, and on the grates place a moderately close layer of green twigs, or fresh cuttings of the vine. Then fill up the vessel with the foot-stalks of grapes, commonly called the Rape, to within a foot of the top of the vessel, which must be left quite open.
"Having thus prepared the two vessels, pour into them the Wine to be converted into Vinegar, so as to fill one of them quite up, and the other but half full. Leave them thus for twenty-four hours, and then fill up the half-filled vessel, with liquor from that which is quite full, and which will now in its turn be left only half-full. Four and twenty hours afterwards repeat the same operation, and thus go on, keeping the vessels alternately full and half-full, during every twenty-four hours, till the Vinegar be made. On the second or third day there will arise, in the half-filled vessel, a fermentative motion, accompanied with a sensible heat, which will gradually increase from day to day. On the contrary, the fermenting motion is almost imperceptible in the full vessel; and as the two vessels are alternately full and half-full, the fermentation is by that means, in some measure, interrupted, and is only renewed every other day, in each vessel.
"When this motion appears to be entirely ceased, even in the half-filled vessel, it is a sign that the fermentation is finished; and therefore the vinegar is then to be put into common casks, close stopped, and kept in a cool place.
"A greater or less degree of warmth accelerates or checks this, as well as the spirituous fermentation. In France it is finished in about fifteen days, during the summer; but if the heat of the air be very great, and exceed the twenty-fifth degree of Mr. de Réaumur's Thermometer, the half-filled vessel must be filled up every twelve hours; be[Pg 539]cause, if the fermentation be not so checked in that time, it will become so violent, and the liquor will be so heated, that many of the spirituous parts, on which the strength of the Vinegar depends, will be dissipated; so that nothing will remain, after the fermentation, but a vapid wash, sour indeed, but effete. The better to prevent the dissipation of the spirituous parts, it is a proper and usual precaution to close the mouth of the half-filled vessel, in which the liquor ferments, with a cover made also of oak-wood. As to the full vessel, it is always left open, that the air may act freely on the liquor it contains: for it is not liable to the same inconveniencies, because it ferments but very slowly."
The vine-cuttings and grape-stalks, which the Vinegar-makers put into their vessels, serve to increase the strength of the liquor. These matters contain a very manifest and perceptible Acid. They also serve as a ferment; that is, they dispose the Wine to become eager more expeditiously, and more vigorously. They are the better, and the more efficacious, for having been once used, because they are thereby thoroughly drenched with the fermented Acid: and therefore the Vinegar-makers lay them by, for preparing other Vinegar, after washing them nimbly in running water, in order to free them from a viscid oily matter, which settles on them during the fermentation. This matter must by all means be removed; because it is disposed to grow mouldy and rot; so that it cannot but be prejudicial to any liquor into which you put it.
As the Acetous fermentation differs from the Spirituous in its production, so it doth in many circumstances attending it. 1. Motion and agitation are not prejudicial to the Acetous fermentation, as they are to the Spirituous; on the contrary, moderate stirring, provided it be not continual, is of service to it. 2. This fermentation is accompanied with remarkable heat; whereas, the warmth of the spirituous fermentation is scarce sensible. 3. I do not believe there ever was an instance of the vapour that rises from a liquor in Acetous fermentation proving noxious, and producing either disorders or sudden death, as the vapour of fermenting Wine doth. 4. Vinegar deposites a viscid oily matter, as hath just been observed, very different from the Lees and Tartar of Wine. Vinegar never deposites any Tartar; even though new Wine, that hath not yet deposited its Tartar, should be used in making it.
The following processes will give us occasion to treat of the nature of Vinegar, and the principles of which it consists.
To concentrate Vinegar by Frost.
Expose to the air, in frosty weather, the Vinegar you desire to concentrate. Icicles will form in it; but the whole liquor will not freeze. Take out those icicles: and if you desire a further concentration of your Vinegar by this method, the liquor which did not freeze the first time must be exposed to a stronger frost. More icicles will form therein, which must likewise be separated, and kept by themselves. The liquor which doth not freeze this second time will be a very strong concentrated Vinegar.
OBSERVATIONS.
Liquors, replete with an Acid, freeze with much more difficulty than pure water. Thus, if a very aqueous acid liquor be exposed to frost, some of the water in the liquor will presently freeze; while the rest, being rendered more acid by the separation of the frozen phlegm, will remain fluid, and resist the degree of cold which freezes water. Now Vinegar, being an acid liquor containing much water, may therefore be highly concentrated by freezing its phlegm in this manner; and the more icicles you get from it, the stronger and more active will the remaining Vinegar be.
Mr. Stahl was the first, I believe, who thus made use of congelation, for procuring a very strong Acid of Vinegar. Mr. Geoffroy hath since taken the same method. A curious and circumstantial account of his experiments, on this subject, are printed in the Memoirs of the Academy for 1739.
As it was excessive cold in the winter of that year, Mr. Geoffroy took the opportunity of exposing to the frost several Vinegars of different strengths; and he determined the degree of Acidity in each, both before and after their concentration, in order to compare them, and discover how much stronger each Vinegar was rendered by the freezing of the aqueous part. To determine the strength of the Vinegars,[Pg 541] he made use of the method pointed out by Mr. Homberg and Mr. Stahl. This method consists in combining to the exact point of saturation, a certain quantity of Vinegar with well-dried Salt of Tartar. The more Salt of Tartar is required, to absorb and perfectly neutralize the Vinegar, the stronger it must be reckoned; because the quantity of Alkali necessary to constitute a Neutral Salt is always proportioned to the quantity of Acid in that Salt.
One of the Vinegars employed in Mr. Geoffroy's experiments, two drams of which were entirely absorbed by six grains of Salt of Tartar, having been concentrated by once freezing, and thereby reduced from eighteen quarts to six, he found it so increased in strength, that two drams thereof required twenty-four grains of Salt of Tartar to absorb them.
The first icicles that separate from Vinegar, in this process, are perfectly clear, and as insipid as water. As the Vinegar becomes more concentrated, the plates of ice becoming thinner, more spongy, and flaky like snow, retain between them some portion of the Acid; and it is proper to begin to save them as soon as they appear to be sensibly acid.
Mr. Geoffroy carried the concentration of Vinegar as far as the cold of that winter in 1739 would allow him; and eight quarts of Vinegar, already concentrated by frost in the preceding years, being reduced to two quarts and a half by the frost of the 19th of January, the coldest day of that year, was found to be so strong, that two drams thereof required forty-eight grains of Salt of Tartar to absorb them. The icicles of this Vinegar, being thawed, retain so much strength as to require thirteen grains of the Salt of Tartar to absorb them.
Vinegar suffers no decomposition by the congelation of its phlegm, and the consequent concentration of its Acid. What is left still contains all the principles of which Vinegar consists. Its principles are only brought nearer together, and into a smaller compass: and for this reason it grows the thicker the more it is concentrated. When therefore you desire to concentrate the Acid of Vinegar, and at the same time to purify it, that is, to free it from some of its oil and earth, you must have recourse to distillation.
Wine, as well as Vinegar, may be concentrated by freezing. Mr. Stahl exposed several sorts of Wine to the frost, and by that means separated from them about two thirds, or three quarters, of almost pure phlegm. The remainders of[Pg 542] the Wines so concentrated were of a somewhat thickish consistence. They were very strong, and kept for several years without altering, in places where the free access of the air, alternately cold and hot according to the seasons, would have soured, or spoiled, any other kind of Wine in the space of a few weeks.
Wine thus concentrated by freezing is not thereby decomposed, any more than Vinegar: it is only dephlegmated. By the addition of as much water as was separated from it, you may restore it to its former condition; in which respect it differs greatly from the residue of Wine whose spirituous part, with a proportion of its phlegm, hath been drawn off by distillation: for though you mix that residue again with the principles you separated from it, you can never make Wine of it again; the spirituous part being no longer in a capacity to combine with the other principles of the Wine, in the same manner as before that separation. And this shews that heat, besides separating the most volatile parts, produces moreover a considerable change in the disposition of those which did not rise in the first distillation.
Since the above experiments were made by Messrs. Stahl and Geoffroy, concentration by freezing is pretty frequently practised in laboratories; but on Vinegar only, seldom on Wine: because, when Vinegar is thus concentrated, a much stronger Acid is more easily and more expeditiously obtained from it, as will be shewn in the following process; whereas the distillation, as well as the quality, of Spirit of Wine is much the same, whether the Wine it is obtained from be concentrated or no. The reason of this difference is, that Spirit of Wine, being very light, rises in distillation before the phlegm; whereas the Acid of Vinegar, being much more ponderous, rises only at the same time with the aqueous part, or even after it.
Vinegar analyzed by Distillation.
Into a glass or stone cucurbit put the Vinegar to be distilled; fit to it a glass head; place your alembic in the sand-bath of a distilling furnace, and lute on a receiver. Apply a very gentle heat at first. A clear, limpid, light liquor[Pg 543] will rise, and fall in distinct drops, like water, from the nose of the alembic.
Continue distilling this first liquor, till the vinegar contained in the cucurbit be diminished about a fourth part. Then shift your receiver, and increase the fire a little. A clear liquor will still come over, but heavier and more acid than the former. Distil in this manner, till you have drawn off, into your second receiver, two-thirds of the liquor that was left in the cucurbit.
A thick matter will now remain at the bottom of the still: put it into a retort; lute on a receiver; set your retort in a reverberating furnace, and distil with degrees of fire. There will come over a limpid liquor, very acid and sharp, yet ponderous, and requiring a great degree of fire to raise it; on which account it makes the receiver very hot. It hath a strong empyreumatic smell. When the distillation begins to slacken, increase your fire. There will rise an Oil of a fetid, quick smell. At last, when nothing more will rise with the strongest fire, break the retort, and in it you will find a black charred matter: burn it, and from the ashes lixiviated with water you will obtain a Fixed Alkali.
OBSERVATIONS.
None of the liquors that come over in this operation, before the last fetid Oil, seem to have any other properties than those of an oily Acid; none of them is inflammable, none of them resembles Spirit of Wine; but all of them being thrown into the fire extinguish it. Mr. Boerhaave however takes notice, that a Chymist, named Vigani, affirms the first portion of the liquor which rises in the distillation of Vinegar to be inflammable, and no other than Spirit of Wine. Mr. Boerhaave suspected that this might happen from Vigani's having distilled Vinegar too newly made; and found upon trial that Vinegar, being distilled soon after it was made, yielded at first in distillation a certain quantity of an Ardent Spirit; but that the same thing did not happen in the distillation of old Vinegar. And this proves that fermentation hath the same effect on Vinegar as on Wine; that is, that though the fermentation which produces these liquors seems to be over in a certain time, when the violent intestine commotion ceases, yet it still continues in the vessels for a considerable time after, though it be imperceptible. Thus, the portion of Ardent Spirit, obtained from some Vinegars, comes from a small quantity of Wine, which[Pg 544] still remains unchanged in these Vinegars, not having had time enough to turn sour. For it is certain, from the experiments of all other Chymists as well as Mr. Boerhaave, that Vinegar, when old enough, yields no Ardent Spirit in distillation.
But though old and well-made Vinegar yields no Ardent Spirit in distillation, we cannot thence conclude that it contains none. On the contrary, there are experiments which demonstrate that some of the Ardent Spirit, which was in the Wine before it was turned into Vinegar, still remains; but probably so combined and blended with the acid part, that it cannot be separated and rendered perceptible but by peculiar processes.
Mr. Geoffroy obtained an Ardent Spirit from Vinegar, by distilling it as soon as it was concentrated by freezing. "This spirit," says he[16], "is the first liquor that rises. At first it hath only the same degree of inflammability as brandy; but, when re-distilled in the balneum mariæ, it fires gun-powder, like the best rectified Spirit of Wine: with this difference, that our Spirit is impregnated with an oil of an acrid taste and empyreumatic smell, which makes it yellow, and imparts its odour to it. This Spirit, at least that which comes over first, retains none of the Acid of the Vinegar; seeing it neither changes the tincture of violets, nor effervesces with Salt of Tartar."
Mr. Geoffroy observes, that, if Vinegar concentrated by freezing be afterwards kept for several years, no Ardent Spirit will then be obtained from it by distillation. And this confirms what we said of unconcentrated Vinegar, and gives reason to think that the Ardent Spirit obtained from Vinegar, either by distilling it after concentration by freezing, or by other processes of which we shall treat in the sequel, is foreign to the Vinegar, and is only found therein, as was said above, because Vinegar contains a certain quantity of Wine which hath not altered its nature. For the Spirit of Wine we obtain from Vinegar doth not hinder our obtaining from it a great deal of Acid, which being more ponderous rises after it. Mr. Geoffroy gives the following account of the sequel of his analysis of Vinegar by distillation.
"Continuing to distil in a balneum mariæ the concentrated Vinegar, of which I had employed four pounds two ounces, there was left, after the distillation, a resi[Pg 545]duum of fourteen ounces; which could not rise, because it was too thick. I found it covered with a saline crust, which is the true Essential Salt of Vinegar, and not of the same nature with Tartar: for Tartar of Wine is scentless; whereas the Salt of Vinegar hath a pungent smell, being the Acid of Tartar subtilized by its union with the Sulphureous parts. If a sand-bath be now used, instead of the balneum mariæ, to carry on the distillation without burning the matter, part of this Salt will be resolved, and yield the last Acid Spirit, which is the strongest that can be obtained.
"After I had, by a sand-heat, extracted all the Acid Spirit that the several residuums put together would yield, I found at the bottom of the cucurbit a brown mass, of the consistence of a pretty solid extract. Of this I put into a retort two pounds, together with six pounds of sand well washed and very dry; and, applying a graduated heat, I first obtained six ounces of an Acid Spirit, that smelt very strong of the empyreuma, and was a little coloured with some portion of oil; seven ounces of Spirit, having a volatile urinous smell, came over next: at last the white vapours appeared more and more dense. A volatile concrete Salt adhered to the sides of the ballon, and I found four ounces of a thick fetid Oil floating on the Spirit. The concrete volatile Salt, when collected, weighed two drams. The black matter remaining in the bottom of the retort, being calcined and lixiviated, yielded a fat alkaline Salt, which it is almost impossible to dry."
I have given this account of Mr. Geoffroy's analysis of Vinegar at length, only because it differs in several respects from that described in the process, which is Mr. Boerhaave's, as well as from those delivered by several other Authors, who make no mention either of the saline matter, which Mr. Geoffroy found on the residuum of Vinegar, after its first distillation in the balneum mariæ, or of the volatile urinous Spirit and Salt, which he obtained from that residuum.
These differences may arise either from the manner of distilling the Vinegar, or from Mr. Geoffroy's Vinegar having been concentrated by freezing, or rather from the quantity, and, above all, from the age of the Vinegar, examined by those different Chymists.
The distillation of Vinegar serves not only to separate its Acid from a considerable quantity of earth and oily parts,[Pg 546] with which it is entangled, but also to dephlegmate and concentrate it. Yet Mr. Lemeri affirms, that Vinegar is not distilled with a view to dephlegmate it. He condemns the common method of throwing away the first runnings as useless phlegm, and saving only what comes off afterwards; having, he says, observed, that the phlegm of Vinegar cannot be abstracted, like that of many other acid liquors, and that what comes over first is almost as sharp as what rises afterwards, be the fire applied at first ever so small.
There is reason to think that Mr. Lemeri did not carefully enough examine the strength of his Spirit of Vinegar, at the different stages of his distillation: for Mr. Geoffroy, in the Memoir above cited, gives an account of a distillation of Vinegar, the product whereof he examined with care, having for that purpose divided it into five different portions: and his experiments put it beyond all doubt, that the first portions of Spirit of Vinegar are far from being so acid as the last. This Vinegar was so strong before distillation, that it required six grains of Salt of Tartar to absorb two drams of it. Two drams of the first portion of his Spirit were absorbed by three grains only of Salt of Tartar: the Acid of the second portion took five grains to absorb it. (Each experiment was made with two drams of Vinegar). The third portion was absorbed by ten grains; the fourth by thirteen, and the fifth took no less than nineteen: which proves that Vinegar, like most other Acids, may be concentrated by distilling off the most aqueous part, which is lighter than the Acid.
There are therefore two ways of concentrating Vinegar, and separating its most acid part, namely distillation and congelation. These two methods may be successively applied to the same Vinegar, and a very powerful Acid obtained by their concurrence. Mr. Geoffroy having exposed to the frost, on the 19th of January 1739, the last russet-coloured liquor, drawn from the residuum of distilled Vinegar, found it so concentrated thereby, that it required sixty grains of Salt of Tartar to absorb two drams of it.
The Acid of Vinegar combined with different SUBSTANCES.
The Acid of Vinegar combined with alkaline Substances. Foliated Salt of Tartar, or Regenerated Tartar. Decomposition of that Salt.
Into a glass cucurbit put some very pure and well-dried Salt of Tartar; and pour on it some good distilled Vinegar, by little and little at a time. An effervescence will arise. Pour on more Vinegar, till you attain the point of saturation. Then fit a head to the cucurbit; set it in a sand-bath; and having luted on a receiver, distil with a gentle heat, and very slowly, till nothing remain but a dry matter. On this residuum drop a little of the same Vinegar; and if any effervescence appears, add more Vinegar till you attain the point of saturation, and distil again as before. If you observe no effervescence, the operation was rightly performed.
OBSERVATIONS.
It is not easy to hit the exact point of saturation in preparing this Neutral Salt; because the oily parts, with which the Acid of Vinegar is loaded, hinder it from acting so briskly and readily as it would do, if it were as pure as the Mineral Acids: and for this reason it often happens, that, when we have nearly attained the point of saturation, the addition of an Acid makes no sensible effervescence, though the Alkali be not yet entirely saturated; which deceives the operator, and makes him conclude erroneously that he hath attained the true point of saturation.
But he easily perceives his mistake, when, after having separated from this saline compound all its superfluous moisture by distillation, he drops fresh Vinegar upon it: for then the Salts being more concentrated, and consequently[Pg 548] more active, produce an effervescence, which would not have been sensible if this last portion of Acid, instead of coming into immediate contact with the dried Alkali, could not have mixed therewith till diffused through, and in a manner suffocated by, that phlegm from which the Acid of the Vinegar, before neutralized, was gradually separated by its combining with the Alkali; that phlegm keeping in solution both the Neutral Salt already formed, and the Alkali not yet saturated. And for this reason it is necessary to try, after the first desiccation of this Salt, which is called Regenerated Tartar, whether or no the just point of saturation hath been attained.
It may also happen, that, though the point of saturation was exactly hit at first, this compound Salt shall nevertheless, after desiccation, effervesce with fresh Vinegar, and therefore not be in a perfectly neutral state at that time. In this case the Salt must have been dried by too violent a fire, and partly decompounded by an excess of heat carrying off some of the Acid, which does not adhere very strongly to the Alkali. This is one of the reasons why it is necessary that Regenerated Tartar be desiccated with a very gentle heat.
From what hath been said, concerning the desiccation of this Neutral Salt, it is plain, that the use of it is only to free the Salt from the great quantity of superfluous moisture wherein it is dissolved: which proves that the Acid of Vinegar, like all other Acids dissolved in much water, is separated from most of this redundant phlegm by being combined with a Fixed Alkali. And hence we must conclude, that the Acid of Vinegar, contained in Regenerated Tartar desiccated, is vastly stronger and more concentrated than it was before: and accordingly Mr. Geoffroy, having decompounded this Salt, by the means of concentrated Oil of Vitriol, obtained a Spirit of Vinegar in white vapours, which was very volatile and very strong, but perhaps somewhat depraved with a taint of the Vitriolic Acid.
Though the Acid of Vinegar be freed, by combining with a Fixed Alkali, from a great quantity of superfluous phlegm, as was shewn above; yet the oily parts with which it is entangled still cleave to it: these parts are not separated from it by its conversion into a Neutral Salt, but, without quitting it, combine also with the Fixed Alkali; and this gives Regenerated Tartar a saponaceous quality, and several other peculiar properties.
Regenerated Tartar, when dried, is of a brown colour. It is semi-volatile; melts with a very gentle heat, and then resembles an unctuous liquor; which indicates its containing an Oil: when cast upon live coals it flames; and, when distilled with a strong heat, yields an actual oil; all which evidently prove the existence of that Oil.
This Salt is soluble in Spirit of Wine; a quality which it probably owes also to its Oil. It requires about six parts of Spirit of Wine to dissolve it; and the dissolution succeeds very well in a matrass, with the help of a gentle warmth. If the Spirit of Wine be abstracted from this solution, by distilling with a small fire, the Salt remains at the bottom of the cucurbit, in the form of a dry substance composed of leaves lying one upon another; which hath procured it the name of Terra Foliata Tartari, or Foliated Salt of Tartar.
It is not absolutely necessary that Regenerated Tartar be dissolved in Spirit of Wine to make the Foliated Salt: for it may be procured in this form, by only evaporating the water in which it is dissolved. But the operation succeeds better with Spirit of Wine; probably because the success thereof depends on using an exceeding gentle warmth: now Spirit of Wine evaporates with much less heat than water.
Regenerated Tartar may also be crystallized. If you desire to have it in this form, combine the Acid with the Alkali to the point of saturation; evaporate the liquor slowly to the consistence of a syrop, and set it in a cool place; where it will shoot into clusters of crystals lying one upon another like feathers.
Vinegar perfectly dissolves absorbent matters also, and particularly those of the animal kingdom; such as Coral, Crabs-eyes, Pearls, &c. In order to a dissolution of such matters, you must pulverize them, put them into a matrass, and pour on them Spirit of Vinegar to the depth of four fingers breadth: an effervescence will arise: when that is over, set the mixture to digest two or three days in a sand-bath; then decant the liquor, filter it, and evaporate it to dryness with a very gentle heat. The matter which remains is called Salt of Coral, of Pearls, of Crabs-eyes, &c. according to the substances dissolved. If, instead of evaporating the liquor, a Fixed Alkali be mixed therewith, the absorbent matter, that was dissolved by the Acid, will precipitate in the form of a white powder, which is called the Magistery of Coral, of Pearls, &c.
The Acid of Vinegar combined with Copper. Verdegris. Crystals of Copper. This combination decompounded. Spirit of Verdegris.
Into a large matrass put Verdegris in powder. Pour on it distilled Vinegar to the depth of four fingers breadth. Set the matrass in a moderate sand-heat, and leave the whole in digestion, shaking it from time to time. The Vinegar will acquire a very deep blue-green colour. When the liquor is sufficiently coloured, pour it off by inclination. Put some fresh Vinegar into the matrass; digest as before; and decant the liquor again when it is sufficiently coloured. Proceed in this manner till the Vinegar will extract no more colour. There will remain in the matrass a considerable quantity of undissolved matter. The Vinegar thus impregnated with Verdegris is called Tincture of Copper.
Mix these several Tinctures, and evaporate them with a gentle heat to a pellicle. Then set the liquor in a cool place: in the space of a few days a great many crystals of a most beautiful green colour will shoot therein, and stick to the sides of the vessel. Pour off the liquor from the crystals; evaporate it again to a pellicle, and set it by to crystallize. Continue these evaporations and crystallizations, till no more crystals will shoot in the liquor. These are called Crystals of Copper, and are used in painting. To this combination of the Acid of Vinegar with Copper the painters and dealers have given the title of Distilled Verdigris.
OBSERVATIONS.
Verdegris is prepared at Montpelier. To make it they take very clean plates of Copper, which they lay, one over another, with husks of grapes between, and after a certain time take them out. Their surfaces are then covered all over with a very beautiful green crust, which is Verdegris. This Verdegris is nothing but Copper corroded by the Acid of Tartar, analagous to the Acid of Vinegar, which abounds in the Wines of Languedoc, and especially in the rape, husks, and stones of grapes that have a very austere taste. Verdegris is a sort of rust of Copper; or Copper corroded[Pg 551] and opened by the Acid of Wine, but not yet converted intirely into a Neutral Salt: for it is not soluble in water, nor does it crystallize. This arises from its not being united with a sufficient quantity of Acid. The design of the operation here described is to furnish the Verdegris with the quantity of Acid requisite to make it a true Metallic Salt: for which purpose distilled Vinegar is very fit.
Crystals of Copper may be obtained, without employing Verdegris, by making use of Copper itself dissolved by the Acid of Vinegar, according to the method practised with respect to Lead, as shall be shewn hereafter. But Verdegris is generally used, because it dissolves soonest; it being a Copper already half-dissolved by an Acid correspondent to that of Vinegar.
Crystals of Copper are decompounded by the action of fire alone, without any additament; because the Acid of Vinegar adheres but loosely to Copper. In order to decompound this Salt and extract its Acid, it must be put into a retort, and distilled in a reverbatory furnace with degrees of fire. An insipid phlegm rises first, which is the water retained by the Salt in crystallizing. This phlegm is succeeded by an acid liquor, which rises in the form of white vapours that fill the receiver. Towards the end of the distillation the fire must be violently urged, in order to raise the strongest and most fixed Acid. At last there remains in the retort a black matter, which is nothing but Copper, that may be reduced by melting it in a crucible with one part of Salt-petre and two parts of Tartar. A similar Acid, but more oily, and in a much smaller quantity, may be obtained from Verdegris by distillation.
The Acid, which in this distillation comes over after the first phlegm, is an exceeding strong and concentrated Vinegar. It is known by the title of Spirit of Verdegris. Zwelfer, and after him M. le Fevre in his Chymistry, bestows extraordinary praises on this Spirit; pretending that it will produce the Salt of Coral, and others of the same kind, without losing any of its virtue, or ceasing to be acid; so as to remain still capable of performing other operations of the same nature. But Mr. Boerhaave and Mr. Lemeri positively deny the fact; and with good reason, having formed their judgments on their own experiments.
Yet I can hardly think both Zwelfer and le Fevre would have affirmed a thing of this nature, in such a positive and confident manner, if they had been convinced in their minds that it was false. We must suppose that those Chymists exa[Pg 552]mined the matter with too little attention, and were misled by some fallacious appearance. Probably they may have compared this concentrated Vinegar with common distilled Vinegar; they may have put to their Coral an equal dose thereof; and, after saturation, they may have distilled off the superfluous liquor, which may have effervesced with fresh Coral and dissolved it. Surprised at this effect, they may have imagined that their Acid had lost none of its strength, and that it had the virtue of converting into Salt any quantity of Coral, or such other matters, without any prejudice to its Acidity. A rash conclusion: which certainly they never would have made, if they had carried the experiment far enough; if they had dissolved a third or a fourth quantity of Coral in their Vinegar: for they would have been thereby convinced that the Spirit of Verdegris, like all other acid Spirits, deposites and leaves its Acid in absorbent matters; and that if the liquor, which they drew off by distillation from their first Salt of Coral, was still acid, and capable of dissolving fresh Coral, nothing could be inferred from thence but that Spirit of Verdegris is an exceedingly concentrated Vinegar, which, in the same quantity of liquor, contains much more Acid than the strongest distilled Vinegar prepared in the common way; that therefore a much smaller dose thereof is required to convert a given quantity of Coral into Salt; and that the liquor, which they distilled from their first Salt, still retained some of its virtue, only because it was replete with much more Acid than could be neutralized by the Coral. But a love of the marvellous so prepossesses the mind of man, that it often hinders him from perceiving the most obvious facts. This is the fault of all the ancient Chymists in general: and I believe the only reason why we find their books stuffed with so many unsucceeding experiments was, that their heated imaginations frequently represented things to them otherwise than they really were.
The Acid of Vinegar combined with Lead. Ceruse. Salt or Sugar of Lead. This combination decompounded.
Into the glass head of a cucurbit, put thin plates of Lead, and secure them so that they may not fall out when the head is put upon the cucurbit. Fit on this head to a wide-mouthed cucurbit containing some Vinegar. Set it in a[Pg 553] sand-bath; lute on a receiver, and distil with a gentle heat for ten or twelve hours. Then take off the head: in it you will find the leaden plates covered, and, in a manner, crusted over with a white matter. This being brushed off with a hare's foot is what we call Ceruse. The leaden plates thus cleansed may be employed again for the same purpose, till they be wholly converted into Ceruse by repeated distillations. During the operation there will come over into the receiver a liquor somewhat turbid and whitish. This is a distilled Vinegar in which some Lead is dissolved.
Reduce a quantity of Ceruse into powder; put it into a matrass; pour on it twelve or fifteen times as much distilled Vinegar; set the matrass in a sand-bath; leave the matter in digestion for a day, shaking it from time to time: then decant your liquor, and keep it apart. Pour fresh Vinegar on what is left in the matrass, and digest as before. Proceed thus till you have dissolved one half, or two thirds, of the Ceruse.
Evaporate to a pellicle the liquors you poured off from the Ceruse, and set them in a cool place. Greyish crystals will shoot therein. Decant the liquor from the crystals; evaporate it again to a pellicle, and set it by to crystallize. Proceed thus evaporating and crystallizing, as long as any crystals will shoot. Dissolve your crystals in distilled Vinegar, and evaporate the solution, which will then shoot into whiter and purer crystals. This is the Salt or Sugar of Lead.
OBSERVATIONS.
Lead is easily dissolved by the Acid of Vinegar. If it be barely exposed to the vapour of that Acid, its surface is corroded, and converted into a kind of calx or white rust, much used in painting, and known by the name of Ceruse or White Lead. But this preparation of Lead is not combined with a sufficient quantity of Acid to convert it into a Salt: it is no more than lead divided and opened by the Acid of Vinegar; a matter which is to Lead what Verdegris is to Copper. And therefore if you desire to combine Ceruse with the quantity of Acid necessary to convert it into a true Neutral Salt, you must treat it in the same manner as we did Verdegris, in order to procure Crystals of Copper; that is, you must dissolve it in distilled Vinegar, as the process directs.
The Salt of Lead is not very white when it first shoots; and for this reason it is dissolved again in distilled Vinegar, and crystallized a second time. If salt of Lead be repeated[Pg 554]ly dissolved in distilled Vinegar, and the liquor evaporated, it will grow thick; but cannot be desiccated without great difficulty. If the same operation be oftener repeated, this quality will be thereby more and more increased; till at last it will remain on the fire like an Oil, or melted Wax: it coagulates as it cools, and then looks, at first sight, like a metallic mass, somewhat resembling Silver. This matter runs with a very gentle heat, almost as easily as wax.
The Salt of Lead hath a saccharine taste, which hath procured it the name also of Sugar of Lead. For this reason when Wine begins to turn sour, the ready way to cure it of that disagreeable taste is, to substitute a sweet one which is not disagreeable to the taste, by mixing therewith Ceruse, Litharge, or some such preparation of Lead: for the Acid of the Wine dissolves the Lead, and therewith forms a Sugar of Lead, which remains mixed with the Wine, and hath a taste which, joined with that of the Wine, is not unpleasant. But, as Lead is one of the most dangerous poisons we know, this method ought never to be practised; and whoever employs such a pernicious drug deserves to be most severely punished. Yet something very like this happens every day, and must needs have very bad consequences; while there is nobody to blame, and those to whom the thing may prove fatal can have no mistrust of it.
All the retailers of Wine have a custom of filling their bottles on a counter covered with Lead, having a hole in the middle, into which a leaden pipe is soldered. The Wine which they spill on the counter, in filling the bottles, runs through this pipe into a leaden vessel below. In that it usually stands the whole day, or perhaps several days; after which it is taken out of the leaden vessel, and mixed with other Wine, or put into the bottle of some petty customer. But, alas for the man to whose lot such Wine falls! He must feel the most fatal effects from it; and the danger to which he is exposed is so much the greater, the longer the Wine hath stood in the leaden vessel, and thereby acquired more of a noxious quality. We daily see cruel distempers among the common people, occasioned by such causes, which are not sufficiently attended to.
Wine that is not kept in close vessels is apt to turn sour very soon, especially in the summer; and the retailers of Wine have observed that their drippings, thus collected in vessels of Lead, are not liable to this inconvenience. This is what hath established among them the practice I am speaking against. As they see only the good effects there[Pg 555]of, and know nothing of its ill consequences, we cannot be angry with them. It is natural to think, that, as Lead hath the property of keeping Wine cool, it may by that means prevent its growing sour for some time; and persons who are not versed in Chymistry can hardly suspect that Wine is preserved from being pricked, only by being converted into a kind of poison. Yet this is the very case: for Lead doth not hinder the Wine from growing sour; but, uniting with its Acid, as soon as it appears, and forming therewith a Sugar of Lead, changes the taste thereof as hath been said, and hinders the Acid from affecting the palate.
Hence it appears how much it were to be wished that the use of those counters covered with Lead were abolished entirely. I am informed, by a Chymist zealous for the public good[17], that he represented this matter to the Magistrates several years ago. It is not to be doubted, that, when the dealers in Wine know the ill consequences attending this practice, they will with pleasure sacrifice the small benefit they receive from it to the public safety.
It is easy to prove whether or no a suspected Wine contains Lead. You need only pour into it a little Oil of Tartar per deliquium; or, if you have not that at hand, a lye of the ashes of green wood. If there be any Lead dissolved in it, the liquor will immediately grow turbid, and the Lead will precipitate in the form of a white powder; because the Sugar of Lead it contains, being a Neutral Salt, whose basis is a metal, is decompounded by the Fixed Alkali, which separates that metal from the Acid. Lead thus separated from the Acid of Vinegar by an Alkali is called Magistery of Lead.
Ceruse, or White Lead, is also a very dangerous poison. It is a pigment very much used, being the only White that can be applied with Oil. This White is the most common, or, perhaps, the only cause of those dreadful colics with which painters, and all that work in colours, are frequently afflicted. This induced me to examine all the substances[Pg 556] capable of affording a White, in order to find one, if possible, which might be substituted for White Lead: but, after a vast number of experiments, I had the mortification to be convinced, that all Whites, even the brightest and most beautiful, which are not metallic, produce nothing, when ground with Oil, but greys, or dirty yellows. There is still something to be hoped for in Whites obtainable from certain metallic substances: but, as every one of those matters may be suspected of some noxious quality, long experience alone will remove our just apprehensions of danger from every thing afforded by such substances.
To return to the Salt of Lead: it may be decompounded by distillation without addittament. In order to perform this, you must put the Salt of Lead into a glass or stone retort, leaving a full third thereof empty, and distil in a reverberating furnace with degrees of fire. A spirit rises, which fills the receiver with clouds. When nothing more will come over with a fire that makes the retort red-hot, let the vessels cool, and then unlute them. You will find in the receiver, an austere liquor, which is inflammable, or, at least, an inflammable Spirit may be obtained from it, if about one half thereof be drawn off by distillation in a glass alembic. The retort in which the Salt of Lead was decompounded contains at the end of the operation, a blackish matter: this is Lead, which will resume its metallic form on being melted in a crucible; because the Acid by which it was dissolved, and from which it hath been separated, being of a very oily nature, hath left in it a sufficient quantity of phlogiston.
What is most remarkable in this decomposition of Salt of Lead is the inflammable Spirit which it yields, though the Vinegar which entered into the composition of the Salt seemed to contain none at all.
Of the Putrid Fermentation of Vegetable Substances.
The Putrefaction of Vegetables.
Fill a hogshead with green plants, and tread them down a little; or, if the vegetables be dry and hard substances, divide them into minute parts, and steep them a little in water to moisten them: then leave them, or the green plants, in the vessel, uncovered and exposed to the open air. By degrees a heat will arise in the center of the vessel, which will continue increasing daily, at last grow very strong, and be communicated to the whole mass. As long as the heat is moderate, the plants will retain their natural smell and taste. As the heat increases, both these will gradually alter, and at last become very disagreeable, much like those of putrid animal substances. The plants will then be tender as if they had been boiled; or even be reduced to a kind of pap, more or less liquid according to the quantity of moisture they contained before.
OBSERVATIONS.
Almost all vegetable matters are susceptible of putrefaction; but some of them rot sooner, and others more slowly. As putrefaction is only a species of fermentation, the effect whereof is to change entirely the state of the Acid, by combining it with a portion of the earth and Oil of the mixt, which are so attenuated that from this union there results a new saline substance in which no Acid is discernible; which on the contrary hath the properties of an Alkali, but rendered Volatile; it is plain, that, the nearer the Acid of a plant set to putrefy is to this state, the sooner will the putrefaction of that plant be completed. Accordingly all plants that contain a Volatile Alkali ready formed, or from which it can be obtained by distillation, are the most disposed to putrefaction.
Those plants, in which the Acid is very manifest and sensible, are less apt to putrefy; because all their Acid must undergo the change above specified. But vegetable matters, whose Acid is entangled and clogged by several of their other principles, must be still longer elaborated, before they can be reduced to the condition into which complete putrefaction brings all vegetables. The earthy and oily parts, in which the Acids of these substances are sheathed, must be attenuated and divided by a previous fermentation, which, from those parts subtilized and united with the Acid, forms an Ardent Spirit, wherein the Acid is more perceptible than in the almost insipid, or saccharine juices, out of which it is produced. The Acid contained in the Ardent Spirit must be still further disengaged, before it can enter into the combination of a Volatile Alkali: consequently the Ardent Spirit must undergo a sort of decomposition; its Acid must be rendered more sensible, and be brought to the same condition as the Acid of plants in which it manifests all its properties.
Hence it appears, that the spirituous and acetous fermentations are only preparatives, which nature makes use of, for bringing certain vegetable matters to putrefaction. These fermentations therefore must be considered as advances towards that putrefaction, in which they terminate, or rather as the first stages of putrefaction itself. This is the opinion of Mr. Stahl, who hath treated this subject with great sagacity, and thrown much light upon it.
Mr. Boerhaave is not altogether of the same mind. He considers putrefaction as something foreign to fermentation; as an operation independent of it, and very different from it. He gives the title of fermentation to that intestine and spontaneous motion only which produces an Ardent Spirit, and changes it into an Acid. He founds his opinion on this, that the circumstances attending putrefaction are different from those which accompany spirituous and acetous fermentation; that the product of putrefaction is very different from the products of these fermentations; and lastly, that all vegetable and animal substances are susceptible of putrefaction, whereas only some kinds of them are capable of fermentation properly so called.
Mr. Boerhaave is so far right, that we ought not to confound together operations which differ in several respects, and result in different productions; but Mr. Stahl's opinion must nevertheless be looked on as highly probable, or rather absolutely true. For it doth not necessarily follow, from the[Pg 559] difference between the circumstances and productions of fermentative motions, that the operations have no relation to, or connection with, each other. They may nevertheless be considered as different steps of one and the same operation: and if all vegetable and animal matters are not susceptible of the three degrees of fermentation, we can only infer from thence that there are mixts, in which the whole work of fermentation is yet to do; and that there are others whose principles are so disposed that they are in the same condition as if they had already undergone the first, or even the second, degree of fermentation; and consequently such mixts are susceptible only of the second, or perhaps of the third, degree of fermentation.
Mr. Stahl therefore says very judiciously, that, far from denying putrefaction to be a fermentation, we ought on the contrary to consider all fermentation as no other than putrefaction. Matters susceptible of the spirituous and acetous fermentation do but pass through these previous alterations in their way to complete putrefaction. On this principle, Wine and Vinegar are only liquors that had begun to putrefy, but were stopt at the first or second stage of their putrefaction. This is so true, that, if a fermenting liquor be left to itself in the open air, and in a due degree of heat, it will proceed directly, without any stop, to perfect putrefaction.
The acetous fermentation is attended with more heat than the spirituous, and the putrid with still more than the acetous. The heat of putrefying plants is sometimes so considerable, that, when they are not too moist, and are stacked up in great heaps, they take fire and burn violently. Of this there are frequent instances in hay-ricks.
Putrefied Vegetable Substances analyzed.
Put the putrefied plants you mean to analyze into a glass cucurbit, and set it in a sand-bath. Fit to it a head; lute on a receiver; distil with a gentle fire, and a limpid fetid liquor will come over. Continue the distillation till the matter contained in the retort be almost dry.
Then unlute your vessels, and keep the liquor you find in the receiver by itself. Put the matter remaining in the cucurbit into a retort, and distil with a graduated heat. There will rise white vapours; a pretty considerable quantity of li[Pg 560]quor nearly like that of the former distillation; a Volatile Salt in a concrete form; and a black oil, which towards the end will be very thick. In the retort there will remain a black charred matter, which being burnt in the open air will fall into ashes, from which no Fixed Alkali can be extracted.
By means of a funnel separate your oil from the aqueous liquor. Distil this liquor with a gentle heat. You will by this means obtain a Volatile Salt like that of animals; of which you may also get some, by the same means, from the liquor which came over in the first distillation.
OBSERVATIONS.
This analysis shews the changes which putrefaction produces in vegetable matters. Scarce any of their principles are now to be discerned. They now yield no aromatic liquor; no Essential Oil; no Acid; and consequently no Essential Salt, Ardent Spirit, or Fixed Alkali: in a word, whatever their natures were before putrefaction, they are all alike when they have once undergone this fermentative motion in its full extent. Nothing can then be obtained from them but Phlegm, a Volatile Alkali, a fetid Oil, and an insipid Earth.
Almost all these changes are owing to the transmutation of the Acid, which is depraved by putrefaction, and combined with a portion of the Oil and subtilized Earth of the mixt; so that the result of their union is a Volatile Alkali. Now, as the Fixed Alkali, found in the ashes of unputrefied plants, is only the most fixed part of their earth and of their Acid, closely united together by the igneous motion, it is not surprising that, when all the Acid, with a part of the earth, is subtilized and volatilized by putrefaction, no Fixed Alkali can be found in the ashes of putrefied Vegetables. The alteration which the Acid suffers by the putrefactive motion is, in my opinion, the greatest it can undergo, without being entirely destroyed and decomposed, so as to be no longer a Salt.
We have seen it, in the Mineral kingdom, in its greatest purity and strength. Its combination with Oil, and the other alterations its undergoes, in the Vegetable kingdom, have shewn it weakened and disguised. The changes it suffers by the spirituous and acetous fermentation, have exhibited it in other forms. And lastly, putrefaction disfigures it completely, and, in some sort, changes its very nature, so that it cannot be distinguished. In the animal kingdom we[Pg 561] find it nearly in the same condition: for though the Vegetable substances, on which animals feed, do not undergo direct putrefaction, in its full extent, before they are converted into animal juices, yet they suffer most of the alterations produced by putrefaction; so that when they have acquired the qualities necessary to their becoming an actual nutritious animal juice, they are within one step of complete putrefaction. For this reason all animal substances are very apt to putrefy, and are unsusceptible of the first degrees of fermentation. But this discussion belongs to the animal kingdom, of which we are now going to treat in the third part of these Elements; the theory of putrefaction serving to introduce it, and naturally leading us to it.
Of OPERATIONS on ANIMAL SUBSTANCES.
Of Milk.
Milk separated into Butter, Curd, and Whey; instanced in Cow's Milk.
Put new Cow's milk into a flat earthen pan, and set it in a temperate heat. In ten or twelve hours time there will gather on its surface a thick matter, of a somewhat yellowish white: this is called Cream. Gently skim off this Cream with a spoon, letting the milk you take up with it run off. Put all this Cream into another vessel, and keep it. The milk thus skimmed will not be quite so thick as before: nor will it be of such a dead white, but have a little blueish cast. If all the Cream be not separated from it, more will gather on its surface after some time, which must be taken off as the former. In two or three days the skimmed milk will coagulate into a soft mass called Curd, and then it tastes and smells sour.
Cut this Curd across in several places. It will immediately discharge a large quantity of Serum. Put the whole into a clean linen cloth; hang it up, and underneath it set a vessel to receive the Serum as it drops. When the aqueous part hath done dripping, there will remain in the filter a white substance somewhat harder than the curdled milk. This substance is called Cheese, and the Serum separated from it is known by the name of Whey.
OBSERVATIONS.
The milk of animals, that feed only on vegetables, is of all animal matters the least removed from the vegetable nature. The truth of this will be demonstrated by the experiments we shall produce by and by, for the further analysis of milk. For this reason we judged, with Mr. Boerhaave, that it was proper to begin the analysis of animals by examining this liquor.
Most Chymists justly consider Milk as of the same nature with Chyle. Indeed there is great reason to think, that, except some small differences to be afterwards taken notice of, these two matters are nearly the same. They are both of a dead white colour, like that of an emulsion; which proves that, like emulsions, they consist of an oily matter divided, diffused, and suspended, but not perfectly dissolved, in an aqueous liquor.
It is not surprising that these liquors should resemble emulsions; for they are produced in the same manner, and may very justly be called Animal Emulsions. For how are vegetable substances converted into Chyle and Milk in an animal body? They are bruised, divided, and triturated by mastication and digestion, as perfectly, at least, as the matters pounded in a mortar to make an emulsion; and must thereby undergo the same changes as those matters; that is, their oily parts, being attenuated by those motions, must be mixed with and lodged between the aqueous parts, but not dissolved therein; because they do not, in the bodies of animals, meet with saline matters, sufficiently disentangled and active, to unite intimately with them, and by that means render them soluble in water.
Nevertheless Chyle and Milk, though produced in the same manner as emulsions, and very much resembling them, differ greatly from them in some respects; owing chiefly to the time they remain in the bodies of animals, their being heated while there, the elaborations they undergo therein, and the animal juices commixed with them.
New Milk hath a mild agreeable taste, without any saline pungency; nor hath any Chymical trial discovered in it either an Acid or an Alkali. Yet it is certain that the juices of plants, out of which milk is formed, contain many saline matters, and especially Acids: accordingly Milk also contains the same; but the Acids are so sheathed and combined, that they are not perceptible. The case is the same[Pg 564] with all the other liquors intended to constitute part of an animal body: there is no perceptible Acid in any of them.
Hence it may be inferred that one of the principal changes which vegetables undergo, in order to their being converted into an animal substance, consists in this, that their Acids are combined, entangled, and sheathed in such a manner that they become imperceptible, and exert none of their properties.
Milk left to itself, without the help of distillation, or any additament whatever, undergoes a sort of decomposition. It runs into a kind of spontaneous analysis; which doth not indeed reduce it to its first principles, yet separates it into three distinct substances, as the process shews; namely, into Cream, or the buttery fat part, into Curd or Cheese, and into Serum or Whey: which shews that those three substances of which Milk consists, are only mixed and blended together, but not intimately united.
The first parts, being the lightest, rise gradually to the surface of the liquor as they separate from the rest: and this forms the Cream.
Cream, as skimmed from the surface of Milk, is not however the pure buttery or fat part; it is still mixed with many particles of Cheese and Whey, which must be separated in order to reduce it into Butter. The most simple, and at the same time the best method of effecting this, is daily practised by the country people. It consists in beating or churning the Cream, in a vessel contrived for that purpose, with the flat side of a circular piece of wood, in the center of which a staff is fixed. One would think that the motion, impressed on the Cream by this instrument, should rather serve to blend more intimately the particles of Butter, Cheese, and Whey, of which it consists, than to separate them from each other; as this motion seems perfectly adapted to divide and attenuate those particles. But, if we consider what passes on this occasion, we shall soon perceive that the motion by which Butter is churned is nothing like triture: for churning is no other, properly speaking, than a continually repeated compression, the effect whereof is to squeeze out from amongst the buttery particles those of Cheese and Whey mixed therewith; by which means the particles of Butter are brought into contact with each other, unite, and adhere together.
Milk, whether skimmed or no, grows sour of itself, and curdles in a few days. When it is newly curdled, the Cheese and Whey seem to be united, and to make but one mass:[Pg 565] but these two matters separate spontaneously from each other, with the greatest ease, and in a very short time.
The acidity, which Milk naturally contracts in the space of a few days, must be considered as the effect of a fermenting motion, which discovers in that liquor an Acid that was not perceptible before. This, properly speaking, is an acetous fermentation, which Milk passes through in its way to putrefaction; and it soon follows, especially if the Milk be exposed to a hot air.
If, instead of leaving Milk to grow sour and curdle of itself, an Acid be mixed therewith, while it is yet sweet and newly milked, it immediately coagulates; which gives reason to think, that its curdling naturally is the effect of the Acid, which discovers itself therein as it grows stale.
The coagulation of Milk may also be considerably accelerated, by setting it in a sand-bath gently heated; or by mixing therewith a little of what, in the language of the Dairy, is called Runnet; which is nothing but some curdled and half-digested Milk taken from the stomach of a Calf: or both these methods may be employed at once, which will produce the effect still more expeditiously.
It is not difficult to find out the cause of these effects. The Runnet, which is Milk already curdled and grown sour, is an actual ferment to sweet Milk, disposing it to turn sour, much more readily: for though Milk, when thus hastily curdled by the Runnet, hath not a manifestly acid taste, yet it is certain that this Acid begins to exert itself. The proof thereof is, that, being exposed to the same degree of heat with Milk equally new, that is not mixed with this ferment, it turns sour much sooner. As to the effect of heat in coagulating Milk, there is nothing extraordinary in it: we know how much it promotes and accelerates all fermentative motion. The whole of this perfectly agrees with what we said before concerning fermentation.
Fixed Alkalis also coagulate Milk; but at the same time they separate the Whey from the Cheese, which floats on the liquor in clots. They give the Milk a russet-colour inclining to red; which may arise from their attacking the fat part.
The separation of Milk into Butter, Cheese, and Whey, is a kind of imperfect analysis thereof, or rather the beginning of one. In order to render it complete, we must examine each of these substances separately, and find the principles of which they consist. This we shall endeavour to do in the following process.
Butter analyzed by Distillation.
Into a glass retort put the quantity of fresh Butter you intend to distil. Set the retort in a reverberatory; apply a receiver, and let your fire be very gentle at first. The Butter will melt, and there will come over some drops of clear water, which will have the peculiar smell of fresh Butter, and shew some tokens of Acidity. If the fire be increased a little, the Butter will seem to boil: a froth will gather on its surface, and the phlegm, still continuing to run, will gradually come to smell just like Butter clarefied in order to be preserved. Its Acidity will be stronger and more manifest than that of the first drops that came over.
Soon after this, by increasing the fire a little more, there will rise an Oil, having nearly the same degree of fluidity as fat Oils; but it will grow thicker as the distillation advances, and at last will fix in the receiver when it cools. It will be accompanied with some drops of liquor, the Acidity whereof will always increase, while its quantity decreases, as the distillation advances.
While this thick Oil is distilling, the Butter contained in the retort, which at first seemed to boil, will be calm and smooth, without the least appearance of ebullition; though the heat be then much greater than when it boiled. Continue the distillation, constantly increasing the fire by degrees as you find it necessary for the elevation of the thick Oil. This Oil, or rather this kind of Butter, will be at last of a russet-colour. There will rise along with it some white vapours exceeding sharp and pungent.
When you observe that nothing more comes over, though the retort be quite red-hot, let the vessels cool, and unlute them. You will find in the receiver an aqueous acid liquor, a fluid Oil, and a kind of fixed brown Butter. Break the retort, and you will find therein a charred matter; the surface of which, where it touched the glass, will be of a shining black, and have a fine polish.
OBSERVATIONS.
The analysis of Butter proves that this substance, which is an oily matter in a concrete form, owes its consistence to the Acid only, with which the oily part is combined: that[Pg 567] is, it follows the general rule frequently mentioned above in treating of other oily compounds; the consistence whereof we shewed to be so much the firmer, the more Acid they contain. The first portions of Oil that come over in the distillation of Butter are fluid, because a pretty considerable quantity of Acid rose before them, which being mixed with the phlegm gives it the Acidity we took notice of.
This Oil, being freed from its Acid, and by that means rendered fluid, rises first; because it is by the same means rendered lighter. The kind of Butter that comes over afterwards, though it be fixed, is nevertheless far from having the same consistence as it had before distillation; because it loses much of its Acid in the operation. This Acid is what rises in the form of white vapours. These vapours are, at least, as pungent and irritating as the Sulphureous Acid or Volatile Alkalis: but their smell is different: it hath a resemblance, or rather is the same, with that which rises from Butter, when it is burnt and browned in an open vessel. But, when concentrated and collected in close vessels, as in the distillation of Butter, they are vastly stronger: they irritate the throat so as to inflame it; they are exceeding sharp and pungent to the smell, and are so hurtful to the eyes that they quickly inflame them, as in an ophthalmy, and make them shed abundance of tears. The great volatility of this Acid is entirely owing to a portion of the phlogiston of the Butter with which it is still combined.
It may be asked why Butter, or the oily part of Milk which hath the consistence of a fixed Oil, is more replete with an Acid than the Oils of the vegetables whereof the Milk was formed; as these Oils are almost all fluid, which indicates their containing less Acid before than after they were digested in the body of an animal. This must appear the more extraordinary, because the Acid contained in the liquors of animals is sheathed and imperceptible, and consequently incapable of combining with the Oils of vegetables so as to give them this consistence.
I think it will be easy to give a satisfactory answer to this question, if it be considered, that the Oils, which exist in the vegetable juices whereof the Milk is formed, are far from being combined with the whole Acid of those vegetables; because there is hardly a plant that doth not yield a great deal of Acid, even without the help of fire. Now, there is reason to think, that one of the principal effects of digestion is, to combine and unite this Acid, with the oily parts of vegetables, more intimately than it was before.
The further we advance in the analysis of animals, the more we shall be convinced, that, in the different elaborations, which vegetable substances undergo in order to their being changed into the nutritious juices of animals, nature employs all her powers to expel, destroy, or at least, weaken and blunt the Acids, so as to render them absolutely imperceptible. One of the best means by which she can effect this, is the combining and uniting them intimately with the oily parts: and this operation she probably begins in digestion. She gets rid of most part of the Acids contained in the aliments, by thus uniting them with the Oils contained in those aliments. Hence arises the consistence of Butter, which is the fat part of Milk, that is, of a liquor half-changed into an animal juice.
This explication furnishes us also with the reason why Acids agree so ill with people of weak and delicate constitutions. The motion and heat in their bodies is not sufficient to effect a due combination of the Acids with the Oils. Hence it comes to pass, that, during and after digestion, they find in their bowels the bad effects of those Acids, in the disorder commonly called the Heart-burn. Hence also it is that such people receive great benefit from the use of Absorbents, which uniting with the Acids neutralize them, and relieve nature when she has not strength enough herself to get the better of them.
To return to our analysis of Butter: we took notice in the process that Butter seems to boil with a very moderate heat at the beginning of the distillation, and that in the course of the operation the ebullition ceases entirely, though the heat be then greatly increased; which is contrary to the general rule. The reason is, that butter, though a seemingly homogeneous mass, contains nevertheless some particles of Cheese and Whey. The particles of Whey, being much the lightest, endeavour, on the first application of heat, to extricate themselves from amongst the particles of Butter, and to rise in distillation. Thus they form the drops of acidulated phlegm which come over at first, and, in struggling to get free, lift up the buttery parts, or actually boil, which occasions the ebullition observable at the beginning of the process. When they are once separated, the melted Butter remains calm and smooth without boiling. If you want to make it boil you must apply a much greater degree of heat; which you cannot do in close vessels, without spoiling the whole operation: because the degree of heat necessary for that purpose would force up the Butter in substance, which would[Pg 569] rush over into the receiver, without any decomposition. Indeed if the vessels were luted they would be in danger of bursting.
As to the caseous parts, which are mixed with fresh Butter, they also separate at the beginning of the distillation, when the Butter is melted, and gather on its surface in a scum. These particles of Cheese and Whey, which are heterogeneous to Butter, help to make it spoil the sooner. And for this reason those who want to keep Butter a long time, without the use of salt, melt it, and thereby evaporate the aqueous parts. The lightest portion of the particles of Cheese rises to the surface, and is skimmed off; the rest remains at the bottom of the vessel, from which the Butter is easily separated, by decanting it while it is yet fluid.
Butter may also be distilled, by incorporating it with some additament which will yield no principle itself, nor retain any of those of the Butter. I have distilled it in this manner with the additament of fine sand: the operation succeeds very well, is sooner finished, and more easily conducted: but I chose to describe here the manner of doing it without additament; because the several changes, which the Butter undergoes in the retort during the operation, may be better observed.
If you desire to convert the Butter wholly into Oil, you must take the fixed matter you find in the receiver, and distil it once more, or oftener, according to the degree of fluidity you want to give it. The case is the same with this matter as with all other thick Oils, which, the oftener they are distilled, grow always the more fluid, because in every distillation they are separated from part of the Acid, to which alone they owe their consistence.
The Curd of Milk analyzed by distillation.
Into a glass retort put some new Curd, having first drained it thoroughly of all its Whey, and even squeezed it in a linen cloth to express all its moisture. Distil it as you did Butter. There will come over at first an acidulated phlegm, smelling like Cheese or Whey. As the distillation advances, the Acidity of this phlegm will increase.
When it begins to run but very slowly raise your fire. There will come over a yellow Oil, somewhat empyreuma[Pg 570]tic. Continue the distillation, still increasing the fire by degrees as occasion requires. The Oil and acid Phlegm will continue to rise; the Phlegm growing gradually more acid, and the Oil deeper coloured, and more empyreumatic. At last, when the retort is almost red-hot, there comes off a second black Oil, of the consistence of Turpentine, very empyreumatic, and so heavy as to sink in water. In the retort will be left a considerable quantity of charred matter.
OBSERVATIONS.
Cheese-curd barely drained, till no more Whey will drip from it, is not entirely freed thereof; and for this reason we directed it to be pressed in a linen cloth, before it be put into the retort to be distilled. Without this precaution, the remaining Whey would rise in a considerable quantity on the first application of heat; and, instead of analyzing the Curd only, we should at the same time analyze the Whey also. This is to be understood of green Curd and new-made Cheese; for, if it be suffered to grow old, it will at length dry of itself: but then we should not obtain from it the same principles by distillation; as it corrupts and begins to grow putrid after some time, especially if it be not mixed with some seasoning to preserve it.
The first Phlegm that rises in this distillation, as in that of Butter, is a portion of the Whey that was left in the Cheese, notwithstanding its being well pressed. This Phlegm grows gradually more acid, being the vehicle of the Acids of the Cheese, which are forced up along with it by the fire.
The Acid obtained from this matter is less in quantity, and weaker, than that of Butter: and accordingly the Oil distilled from Cheese is not fixed like that of Butter. Yet it is remarkable that the last empyreumatic Oil, which is as thick as Turpentine, is heavier than water: a property which it probably derives from the quantity of Acid it retains.
The quantity of charred matter, which remains in the retort after the distillation of Cheese, is much greater than that left by Butter; which proves that the former contains a much greater quantity of earth. These coals are exceeding difficult to burn and reduce to ashes. I have kept them red-hot, in the open air, and in a very strong fire, about six hours, continually stirring them, in order to bring the under parts to the surface, that they might be burnt, yet I could not consume them entirely. They even deflagrated afterwards[Pg 571] with Nitre, as if they had not been burnt at all; and yet, during the whole time of their calcination, there appeared constantly a small flame, like that of charcoal, on the surface of the matter.
Whey analyzed.
Evaporate two or three quarts of Whey almost to dryness in a balneum mariæ; and distil the extract, or residuum, in a retort set in a reverberating furnace, with degrees of fire, according to the general rule. At first some Phlegm will come over; then a lemon-coloured acid Spirit; and afterwards a pretty thick Oil. There will remain in the retort a charred matter, which being exposed to the air grows moist. Lixiviate it with rain water, and evaporate the lixivium: it will yield you crystals of Sea-salt. Dry the charred matter, and burn it in the open air with a strong fire, till it be reduced into ashes. A lixivium of these ashes will shew some tokens of a Fixed Alkali.
OBSERVATIONS.
Milk, as was said before, separates naturally and spontaneously into three sorts of substances, the analyses whereof being put together make a complete analysis of this animal liquor. I know no Author that hath delivered the analyses of Butter and Cheese; so that the processes here given for analyzing these two substances are taken from the experiments I thought proper to make, in order to obtain the necessary lights in this matter. As for the analysis of Whey, it is taken from one of Mr. Geoffroy's Memoirs, containing experiments on several animal substances, which was published in 1732. It is there so particularly and so well described, that it was needless for me to attempt it anew.
It will appear, on examining the three analyses of the substances whereof Milk consists, that none of them yields a Volatile Alkali: which I think very worthy of notice; as it is, I believe, the only animal matter from which such a Salt cannot be obtained. It is true, the milk of animals that feed on vegetables may be considered as an intermediate liquor between vegetable and animal substances; as an imperfect animal juice, which still retains much of the vege[Pg 572]table nature: and we actually find that Milk almost always hath, at least in part, the properties of those plants with which the animals that yield it are fed. Yet, as it cannot be formed in the body of the animal, without mixing with several of its juices that are entirely perfected, and become purely animal, it must appear strange that the analysis thereof should not afford the least vestige of that principle, which all other animal matters yield in the greatest plenty.
I imagine the reason of this may be found in the use to which Milk is destined. It is intended for the nourishment of animals of the same species with those in whose bodies it is produced. Consequently it ought as much as possible to resemble the juices of the food which is proper for those animals. Now, as animals that live only on vegetables could not be properly nourished by animal matters, for which nature itself hath even given them an aversion, it is not surprising that the Milk of such animals should be free from any mixture of such things as are unsuitable to the young ones whom it is designed to nourish. There is reason therefore to think that nature hath disposed the organs, in which the secretion of Milk is performed, so as to separate it entirely from all the animal juices first mixed with it: and this I take to be the principal difference between Milk and Chyle; the latter being necessarily blended with the saliva, the gastric and pancreatic juices, the bile and lymph, of the animals in which it is formed. Hence it may be concluded, that, if a quantity of Chyle could be collected sufficient to enable us to analyze it, the analysis thereof would differ from that of Milk, in this chiefly that it would yield a great deal of Volatile Alkali, of which Milk, as hath been said, yields none at all.
The same thing probably takes place in carnivorous animals. It is certain that those animals chuse to eat the flesh of such others only as feed upon vegetables; and that nothing but extreme hunger, and the absolute want of more agreeable food, will force them to eat the flesh of other carnivorous animals. Wolves, which greedily devour sheep, goats, &c. seldom eat Foxes, Cats, Polecats, &c. though these animals are not strong enough to resist them. Foxes, Cats, and Birds of prey, that make such terrible havock among wild fowl, and other sorts of game, do not devour one another. This being laid down, there is reason to think that the Milk of carnivorous animals is something of the nature of the flesh of those animals that feed on vegetables, and which they chuse to eat, and not of the nature of their[Pg 573] own flesh; as the Milk of animals that feed on vegetables is analagous to the juice of vegetables, and when analyzed yields no Volatile Alkali, though every other part of their body does.
But whatever be the nature of Milk, and of whatever ingredients it be formed, it always contains the three several substances above-mentioned; namely, the fat, or Buttery part, properly so called, the Cheesy, and the Serous part, the last of which we are now examining. It is, properly speaking, the Phlegm of the Milk, and consists almost entirely of water. For this reason it is proper to lessen the quantity thereof considerably by evaporation, so that its other principles, being concentrated and brought nearer together, may become much more sensible. There is no danger of losing any essential part of the Whey in the evaporation, if it be performed in the balneum mariæ, with such a gentle heat as may carry off the aqueous parts only: this greatly shortens the analysis, which will prove exceeding long and tedious, if all the water be distilled off in close vessels.
As Whey is chiefly the aqueous part of Milk, as said above, it must contain all the principles thereof that are soluble in water; that is, its saline and saponaceous parts. And accordingly the analysis thereof shews that it contains an Oil, rendered perfectly saponaceous by an Acid; that is, made perfectly miscible with water. This quality of the Oil contained in Whey appears from the perfect transparency of that liquor, which we know is the mark of a complete dissolution. In the distillation of Whey, the saponaceous matter contained therein is decomposed; the saline part rises first, as being the lightest; this is the Acid taken notice of in the process; after which the Oil, now separated from the principle which rendered it miscible with water, comes over in its natural form, and doth not afterwards mix with the aqueous part.
Besides the saponaceous matter, Whey contains also another saline substance; namely, Sea-Salt: this is obtained by lixiviating the caput mortuum left in the retort, which, because of its fixedness, cannot rise with the other principles in distillation. To this Salt it is owing that what remains in the retort after distillation grows moist in the air; for we know that Sea-salt thoroughly dried hath this property.
The fixed Alkaline Salt, obtained from the caput mortuum burnt to ashes, proves that Milk still retains something of the vegetable nature: for the following analysis will shew us that matters purely animal yield none at all.
Of the Substances which compose an Animal Body.
Blood analyzed. Instanced in Bullock's Blood.
In a balneum mariæ evaporate all the moisture of the Blood that the heat of boiling water will carry off. There will remain an almost dry matter. Put this dried Blood into a glass retort, and distil with degrees of heat, till nothing more will come over, even when the retort is quite red-hot, and ready to melt. A brownish phlegm will rise at first: this will soon be impregnated with a little Volatile Alkali, and then will come over a yellow Oil, a very pungent Volatile Spirit, a volatile Salt in a concrete form, which will adhere to the sides of the receiver; and, at last, a black Oil, as thick as pitch. There will be left in the retort a charred matter, which being burnt yields no Fixed Alkali.
OBSERVATIONS.
Blood, which is carried by the circulation into all the parts of the animal body, and furnishes the matter of all the secretions, must be considered as a liquor consisting of almost all the fluids necessary to the animal machine: so that the analysis thereof is a sort of general, though imperfect, analysis of an animal.
Blood drawn from the body of an animal, and set by in a vessel, coagulates as it grows cold; and sometime afterwards the coagulum discharges a yellowish Serum or lymph; and in the midst thereof swims the red part, which continues curdled. These two substances, when analyzed, yield nearly the same principles; and in that respect seem to differ but little from each other. Though the Serum of Blood be naturally in a fluid form, yet it hath also a great tendency to coagulate, and a certain degree of heat applied to it, either by water, or by a naked fire, will curdle it. Spirit of Wine mixed with this liquor produces on it the same effect as heat.
Blood, while circulating in the body of a healthy animal, and when newly taken from it, hath a mild taste, which discovers nothing like either an Acid or an Alkali; nor doth it shew any sign of either the one or the other in Chymical trials. When tasted with attention it betrays something like a savour of Sea-salt; because it actually contains a little thereof, which is found in the charred matter left in the retort after distillation, when carefully examined.
We shewed that Milk also contains a little of this Salt. It enters the bodies of animals with the food they eat, which contains more or less thereof according to its nature. It plainly suffers no alteration by undergoing the digestions, and passing through the strainers, of the animal body. The case is the same with the other Neutral Salts which have a Fixed Alkali for their basis: we find them unchanged in the juices of animals into whose bodies they have been introduced. They are incapable of combining, as Acids do, with the oily parts; and so are dissolved by the aqueous fluids, of which nature makes use to free herself from those Salts, and discharge them out of the body; as shall be shewn when we come to speak of Urine and Sweat.
Blood, like all other animal matters, is, properly speaking, susceptible of no fermentation but that of putrefaction. Yet it turns somewhat sour before it putrefies. This small degree of acetous fermentation is most sensible in flesh; and especially in the flesh of young animals, such as calves, lambs, chickens, &c.
The quantity of pure water, which Blood, in its natural state, contains, is very considerable, and makes almost seven eighths thereof. If it be distilled, without being first dried, the operation will be much longer; because it will be necessary to draw off all this insipid phlegm with a gentle fire. There is no reason to apprehend that, by drying Blood in open vessels as directed, any of its other principles will be carried off with its Phlegm: for it contains no other substance that is volatile enough to rise with the warmth of a balneum mariæ. This may be proved by putting some undried Blood into a glass cucurbit, fitting thereto a head and receiver, and distilling, in a balneum mariæ, all that the heat of the bath, not exceeding the heat of boiling water, will raise: for, when nothing more will come over, you will find in the receiver an insipid phlegm only, scarce differing from pure water, except in having a faint smell like that of Blood; wherein it resembles all the phlegms that rise first in distillation, which always retain something of the smell of[Pg 576] the matters from which they were drawn. That part of the Blood, which remains in the cucurbit after this first distillation, being put into a retort, and distilled with a stronger fire, yields exactly the same principles, and in the same proportion, as Blood dried in open vessels in the balneum mariæ: so that, if this Phlegm of Blood contain any principles, the quantity thereof is so small as to be scarce perceptible.
The Volatile Alkali that rises with the Oil, when Blood is distilled in a retort with a degree of heat greater than that of boiling water, is either the production of the fire, or arises from the decomposition of an Ammoniacal Salt, of which it made a part. For we shall see, when we come to treat of this saline substance, that it is so extremely volatile as to exceed, in that respect, almost all other bodies that we know: and therefore if this Volatile Alkali pre-existed formally in the Blood, uncombined with any other matter capable, in some measure, of fixing it, it would rise at first almost spontaneously, or at least, on the first application of the gentlest heat. We have an instance of this in Blood, or any other animal matter, that is perfectly putrefied; which containing a Volatile Alkali, either formed or extricated by putrefaction, lets go this principle when distilled, even before the first phlegm: and, for this reason, when putrefied Blood is to be analyzed, it must by no means be dried, like fresh Blood, before distillation; for all the Volatile Alkali would by that means be dissipated and lost at once.
The Volatile Alkali obtained from Blood that hath not undergone putrefaction, affords matter of some speculation. Indeed the separation of this Salt from Blood requires a degree of heat, vastly greater than that which is necessary to make it rise, when it is perfectly formed and disentangled: and this gives room to think that it is the result of a combination formed by the fire, during the distillation. But then this same degree of heat neither separates nor forms any Volatile Alkali in a great number of plants, or in milk, as hath been shewn. Yet it cannot be supposed that the blood of animals, which feed only on those plants or on milk, is any other than these very matters digested and rendered perfectly animal substances: whence it must be concluded, that, when vegetable substances are converted into animal substances, they undergo such alterations as render them capable of yielding, when analyzed, a principle that was not discoverable in them before. Now we know that this same principle, that is, the Volatile Alkali, is the product of putrefaction, or, which is the same thing, of the[Pg 577] last degree of fermentation: and this, I think, makes the opinion of those more than probable, who believe that trituration and mechanical motion are not the only causes, that effect the conversion of food into an animal juice, but that fermentation hath a great share in this change. It is true, we do not find, in animal matters, any manifest token of an Ardent Spirit, an Acid, or a Volatile Alkali; nor, consequently, any substance that is an evident production of any of the three different degrees of fermentation: and yet, as substances perfectly animalized are exactly in the same state with vegetables that have undergone the first, and even the second, degree of fermentation, so that they are susceptible of putrefaction only, (or, at least, if they shew at first some faint tokens of acidity, they run immediately and rapidly into complete putrefaction); it is nevertheless probable, that vegetable matters, in order to their becoming animal substances, undergo certain changes and alterations, which have some resemblance with those produced by fermentation.
This opinion is further confirmed by two other analogies, between animal matters, and vegetables advanced to the last stage of fermentation; which is, that they yield neither an Essential Oil nor a Fixed Alkali: for the coal, that remains in the retort after the distillation of Blood, being burnt in an open fire, discovers no Fixed Alkali in its ashes.
The want of a Fixed Alkali in animal matters arises from hence, that their Acid is nearly in the same state with the Acid of vegetable matters which have undergone putrefaction; that is, it is so subtilized and attenuated, as to be fit to enter into the combination of a Volatile Alkali, and is no longer so intimately united with the fixed earth as to produce therewith a Fixed Alkali in the fire.
Though Blood and other animal matters afford no Fixed Alkali, but, on the contrary, yield much Volatile Alkali, it doth not therefore follow that all the Acid, which those substances contained before they were analyzed, is employed in the production of a Volatile Alkali. We shall hereafter take notice of an animal matter which contains a great deal of Acid: and, not to depart from our present subject, it doth not appear to me to be a settled point among Chymists, whether or no Blood, when analyzed, yields a portion manifestly acid, and possessing all the properties of an Acid.
Mr. Boerhaave, with some other Chymists, makes no mention of any Acid in his analysis of Blood. Mr. Homberg, on the contrary, says[18] expressly, that he constantly ob[Pg 578]tained an Acid from the Blood and flesh of different sorts of animals, of which he analyzed a great number. Mr. Boerhaave's authority is very respectable, and of great weight: on the other hand, Mr. Homberg's experiments are very conclusive, seem to be made with great care, and are all affirmative. This apparent diversity in the same analysis, delivered by these two great men, determined me to analyze Blood myself, and to examine scrupulously all the principles I could obtain from it.
I therefore distilled some Bullock's Blood in a retort with degrees of fire. Some Phlegm came over first, and then a Volatile Spirit. I changed my receiver; and on increasing the fire there arose, with the Volatile Spirit, a yellow Oil, a Volatile Salt in a concrete form, a russet liquor which smelled strong of Volatile Alkali, and seemed at first to be only a Spirit impregnated with much of that Salt: at last came a very thick fetid Oil.
In this brown liquor, which comes off towards the end of the distillation, Mr. Homberg affirms the Acid to be contained: but, as it certainly is replete with a Volatile Alkali also, he alledges that it contains, at the same time, both a Volatile Alkali and the animal Acid; that these two Salts are distinct from each other, and not combined together in the form of an Ammoniacal Salt; that each of consequence possesses its peculiar properties; and that this liquor is at the same time both Acid and Alkaline; that it effervesces with Acids, and also changes the blue colours of plants to red.
The Alkaline quality of this liquor is very evident, and discovers itself in every Chymical trial; but the same cannot be said of its Acid property. I dropped some of it on blue paper, the colour of which did not at first change in the least, nor acquire the faintest shade of redness. This experiment almost determined me to conclude that Mr. Homberg was mistaken: but some time afterwards I perceived that the blue paper began to turn red where it had been wetted, and that the red colour grew deeper and deeper as the paper dried: and this convinced me, that this liquor actually contains an Acid, as Mr. Homberg asserted; but, that the Volatile Alkali in this liquor, being much more copious than the Acid, had first entered the paper, and hindered the Acid from turning it red as usual; and that, as the Alkali evaporated, the Acid began to act, and produce the customary effect. Hence we see that the Acid of Blood, though extricated by distillation, is not easily perceived at first, because of the great proportion of Volatile Alkali, with which the liquor containing it[Pg 579] is impregnated. This is probably what prevented its being discovered by several Chymists, who, it seems, did not suspect its existence, and therefore did not look for it.
Mr. Homberg takes no notice of this little difficulty in his Memoir: but he relates an experiment which might have given occasion to suspect it. It is in his analysis of Human Blood. As the Acid in Human Blood is in less quantity, and less perceptible, than in the Blood of animals that live wholly on vegetables, he directs a second distillation of the brown liquor, which contains at once both the Volatile Alkali and the Acid, till very little thereof be left in the retort. This residuum, says he, contains a very perceptible and distinct Acid. There is reason to believe, from Mr. Homberg's directing the saline liquor to be distilled again, that he did not find the Acid sufficiently perceptible in it at first. Now a second distillation is a very good way to render it much more sensible. For though this animal Acid be volatile, the Volatile Alkali is still vastly more so; and therefore if the liquor containing both these saline substances be distilled, the Volatile Alkali must needs rise first, and leave the Acid alone, or almost alone, at the bottom of the retort. This is exactly the case in our experiment on blue paper; the operation being here performed with a small quantity, and much more expeditiously, as appears from our account of it.
It is not at all surprising that the Volatile Alkali and animal Acid, though confounded in the same liquor, should not be united together and converted into a Neutral Ammoniacal Salt. Mr. Homberg pretends that these two saline matters do not act upon each other, because they are too much dephlegmated. The oily parts, with which they are both loaded, may also contribute thereto: nor is this unprecedented; the same thing being observed of the Acid and the Volatile Alkali of several vegetable substances.
Mr. Homberg, justly suspecting that there might be some difference between the condition of the Acid in the Blood of animals that feed altogether on vegetables, and that in the blood of those that feed only on flesh, examined likewise, by decomposition, the Blood and the flesh of some carnivorous animals. In these also he found an Acid; and it doth not appear that he observed any great difference, in this respect, between their Blood and that of other animals. The difference he found between the Blood of young, and that of grown, or old, animals, with respect to the Acid, seems, by his account, to be more considerable; the Blood of the former containing much more of it than that of the latter: and[Pg 580] this is so much the more probable, as we know that the flesh of young animals grows sour, before it putrefies, more sensibly than that of old ones.
We shall conclude this head with a remark concerning the management required in distilling Blood. When the operation is advanced to a certain point, the matter contained in the retort often swells so as to stop the neck of that vessel entirely, and by that means makes it burst with an explosion. To avoid this inconvenience, a very small quantity of Blood must be put into the retort, and the fire must be governed very warily. I have also found that this accident may generally be prevented by mixing the Blood with some matter that can afford no principle by distillation; such as pounded glass or fine sand.
Flesh analyzed. Instanced in Beef.
Into an alembic or retort, placed in a sand-bath, put some lean Beef, from which you have carefully separated all the fat. Distil till nothing more will rise. In this first distillation a phlegm will come over, weighing at least half the mass of the distilled flesh. In the retort you will find a matter almost dry, which you must afterwards distil, with a naked fire, in a reverberating furnace, taking the usual precautions. There will come over at first a little phlegm replete with Volatile Alkali; then a Volatile Alkali in a dry form, which will stick to the sides of the vessel; and also a thick Oil. After the distillation there will be left in the retort a black, shining, light coal. Burn it to ashes in the open air, and lixiviate those ashes: the water of the lixivium will have no Alkaline property, but will shew some tokens of its containing a little Sea-salt.
OBSERVATIONS.
This analysis of Beef is taken from a Memoir given in by Mr. Geoffroy in 1730, the purpose of which was a Chymical examination of the meat commonly used to make broth. The flesh of an animal, as appears from the process, yields much the same principles with its Blood: and it cannot be otherwise; because it is formed all together of materials furnished by the Blood.
Mr. Geoffroy observes, that the first phlegm, drawn off from it in the balneum mariæ, produces a white precipitate in a solution of Corrosive Sublimate; which shews it to contain a little Volatile Alkali: but the quantity thereof must be very small; seeing the phlegm that contains it smells only like broth, and not like a Volatile Alkali; one particle of which, we know, is capable of affecting the organ of smelling very sensibly. As to the Acid of flesh, there is great reason to believe that it is conditioned exactly like that of Blood.
The ashes of the caput mortuum of flesh, burnt in an open fire, attract the moisture of the air, as Mr. Geoffroy remarks, and increase in weight, though they contain no Fixed Alkali. However, this is not at all surprising; since they contain some Sea-salt, the known property whereof is to grow moist in the air.
The flesh of animals contains much matter that is soluble in water. Mr. Geoffroy examined separately that part of flesh which water is capable of dissolving. With this view he boiled four ounces of beef with three pints of water, in a very close vessel, and repeated the operation six times with equal quantities of fresh water; in order to extract, as far as possible, all the juices of the meat. These broths he put all together, the last of them having but a faint smell of very weak veal broth: he evaporated them over a slow fire, filtering them towards the end of the evaporation, to separate an earthy part; and there remained in the vessel a moderately solid extract, which soon grew moist in the air. This extract, being analyzed, yielded a dram and two grains of Volatile Salt, which adhered to the sides of the receiver; not in ramifications, as Volatile Salts usually do, but in flat crystals, mostly in the form of parallelopipeds. The Spirit and the Oil, which came over together after the Volatile Salt, weighed thirty-eight grains. Salt of Tartar being mixed with this Volatile Salt seemed to increase its strength; which gives room to suspect that the latter contains an Ammoniacal Salt.
The charred matter left in the retort weighed but six grains. Its lixivium gave some tokens of Sea-salt, by making a white precipitate in a solution of quick-silver. The mass of fleshy fibres, that was exhausted by boiling, being dried and analyzed in the same manner, yielded a Volatile Spirit, a Volatile Salt in a concrete form, which stuck to the sides of the receiver in ramifications as usual; and a thick fetid Oil. There now remained in the retort a[Pg 582] charred matter, which being burnt in the open air or not burnt, shewed not the least sign of its containing any saline matter.
This method of analyzing flesh, by boiling it at first in water, in order to extract all that can be dissolved by this menstruum, shews us that animal flesh contains an Oil, which is in a saponaceous state: for the extract made therefrom, by water, yields in distillation a considerable quantity of Oil, which was perfectly dissolved in the water, while that extract was in the diluted state of broth, and before it was analyzed.
It is remarkable that the Volatile Salt, yielded by the extract of flesh, is different from that which is obtained out of the flesh itself, when nothing hath been extracted from it. This Salt, as Mr. Geoffroy observed, differs from the common Volatile Alkalis in the form of its crystals; which made that Chymist justly consider it as a Salt of a somewhat Ammoniacal nature; a kind of Essential Salt of flesh.
There is reason to think that this Salt, when dissolved in the water in which we boil flesh, is separated therefrom, by the action of fire, with more ease than while it remains combined with the other principles, in the substance of the flesh; that its separation, in the latter case, requiring a greater degree of heat, it is thereby decomposed; and that the Volatile Alkali, which is obtained from flesh distilled in the usual manner, is only one of the parts that constituted the Ammoniacal Salt thereby decomposed.
The charred matter remaining, after the distillation of flesh first exhausted by boiling, yields nothing saline; because the Sea-salt, which is the only Fixed Salt it could contain, was dissolved by the water together with the matter of the extract.
Mr. Geoffroy likewise examined what parts of flesh Spirit of Wine is capable of dissolving. For this purpose he took four ounces of Beef, dried in the balneum mariæ, poured on it an equal weight of well rectified Spirit of Wine, and left the whole in digestion for a considerable time. The Spirit extracted from the Beef a weak tincture, and separated from it some drops of Oil: it acquired a brown colour, and a faint smell. Mr. Geoffroy found, by several experiments, that the Spirit of Wine had taken up a portion of the Ammoniacal, or Essential, Salt of the flesh. With respect to the Oil, if any at all were dissolved, it could be but very little; for that which the Spirit separated, and which retained its natural form, was certainly not dissolved: seeing in that[Pg 583] case it would not have been perceived, but would have made a homogeneous liquor, to appearance, with the Spirit of Wine.
Bones analyzed. Instanced in Ox-bones.
Cut into pieces the Bones of a leg of beef, carefully separating all the marrow. Put them into a retort, and distil them in a reverberating furnace, as usual. A phlegm will come over first; then a Volatile Spirit, which will become stronger and stronger; afterwards a Volatile Salt in a dry form, with some Oil; and, lastly, a black Oil, with a little more Volatile Salt. There will be left in the retort a charred matter, from which a little Sea salt may be extracted. Reduce this charred matter to ashes, by burning it in the open air. These ashes will give some slight tokens of a Fixed Alkali.
OBSERVATIONS.
The analysis of Bones proves that they consist of the same principles with flesh and blood; and the same may be said, in general, of all matters that are truly animal, or that actually constitute any part of an animal.
Nevertheless, we find in the ashes of Bones somewhat of an Alkaline quality; seeing they make a red precipitate in a solution of Corrosive Sublimate: and yet a true Fixed Alkali cannot be obtained from them. These ashes are probably in the same case with quick lime; which hath certain properties of Alkaline Salts, though no Salt of that kind can be extracted from it.
Mr. Geoffroy analyzed Bones in the same manner as he did flesh; that is, he at first made a strong decoction of them with water, and then examined and distilled apart the extract afforded him by that decoction, and the Bones deprived of that extract. On this analysis he made two remarkable observations.
The first is, that Bones yielded to boiling water their principles and their Volatile Salts, both sooner and more copiously than flesh did: for in the analysis which Mr. Geoffroy made of several sorts of flesh, though he robbed them in a manner of all their principles by boiling, yet their dried fibres[Pg 584] afterwards yielded a considerable quantity of Volatile Salt; whereas the Bones, of which he had made an extract by boiling, afforded him but a very small quantity thereof when analyzed.
The second observation worthy of notice which Mr. Geoffroy made on his analysis of Bones is this; the Salt, which, as was shewn in the analysis of flesh, was resolved by the water wherein he boiled the flesh, and consequently arose when he distilled the extract obtained from that decoction, and crystallized in the form of parallelopipeds, took a quite different turn in the analysis of Bones. None of it appeared in distilling the extract made by decoction, but arose in distilling the boiled Bones, that were exhausted of almost all their other principles by the decoction with water. These differences probably arise from the different contexture of the animal matters in which they are observed.
This analysis of Bones may serve as a pattern for analyzing all the solid parts of animals, such as horns, hoofs, ivory, &c.
Animal Fat analyzed. Instanced in Mutton-Suet.
Put as much Mutton-Suet as you please into a glass retort, only taking care that the vessel be but half-full; and distil with degrees of fire as usual. A phlegm smelling of the Suet will rise first, and soon grow very acid. After this some drops of Oil will come over, and be followed by a matter like Oil, in appearance, when it comes over; but it will fix in the receiver, and acquire a consistence somewhat softer than Suet. This kind of Butter of Suet will continue to rise to the end of the distillation; and there will be left in the retort a small quantity of charred matter.
OBSERVATIONS.
Though animal Fat be a substance that hath passed through all the strainers of the body; though it hath undergone all the elaborations necessary to form an animal matter, and become itself part of the animal: it contains, nevertheless, as its analysis shews, principles differing greatly from those of all other animal matters: so that it must be classed, in some sort, by itself.
It consists almost entirely of Oil: but this Oil is in a concrete form, and observes the general rule of all concreted oily matters, which owe their consistence wholly to the Acid that is combined with them. The rule is evidently so general, that it extends even to the animal kingdom, where, in all other instances, Acids seem to be almost annihilated.
All we said above on the subject of Butter must be applied here: for animal Fat, properly so called, and Butter, do not, in my opinion, differ sensibly from each other, with respect to their analysis. And therefore there is great reason to believe, that what is Butter in Chyle, or Milk, becomes Fat when fixed in the animal body. It is a kind of repository, in which nature lays up and confines the Acid that is unnecessary to the animal composition, and which she could not any other way eliminate.
I made choice of Mutton-Suet for an instance of the analysis of Fat; because this Fat, being the firmest of any, must contain a stronger and more perceptible Acid.
When it is thus distilled, the part which remains fixed hath much less consistence than the Suet had before; which arises from its having lost part of its Acid. Repeated distillations will deprive it of a much greater quantity thereof, and so reduce it into an Oil that will always remain clear and fluid.
Not one particle of Volatile Alkali is obtained by distilling Suet: but then the experiment will not succeed as it ought, unless care be taken to free the Suet perfectly from all the membranes, and all the particles of flesh and blood that may be mixed with it; for, if it should be distilled without this precaution, those heterogeneous matters mingled with it would yield a great deal of Volatile Alkali in distillation; which might impose on the Artist, and make him think the Salt came actually from the Suet. Suet that hath been often melted, as the tallow, for instance, of which candles are made, is sufficiently purified: of this I made use in my analysis, and it yielded me no Volatile Alkali; at least I could perceive none.
In conclusion, all that hath been said, on several occasions, touching the properties of concreted oily matters, may be applied to Suet. I shall only observe here, that it is one of those that manifest no Acidity, and consequently that in its natural state it is not soluble in Spirit of Wine, and only becomes soluble in that menstruum by degrees, as its Acid is extricated by repeated distillations: and on this ac[Pg 586]count it ought to be classed with Bees-Wax, and other oily compounds of that kind.
Eggs analyzed. Instanced in Pullet's Eggs.
Put some Hen's Eggs in water, and boil them till they be hard. Then separate the Yelks from the Whites. Cut the Whites into little bits; put them into a glass cucurbit; fit on a head and receiver; distil in a balneum mariæ with degrees of fire, raising it towards the end to the strongest heat which that bath can give; that is, to the heat of boiling water. There will come over an aqueous liquor, or insipid phlegm; the quantity whereof will be very considerable, seeing it will make about nine-tenths of the whole mass of the Whites of the Eggs. Continue your distillation, and keep the water in the bath constantly boiling, till not a drop more of liquor will ascend from the alembic. Then unlute your vessels. In the cucurbit you will find your Whites of Eggs considerably shrunk in their bulk. They will look like little bits of brown glass, and be hard and brittle.
Put this residuum into a glass retort, and distil, as usual, in a reverberating furnace with degrees of heat. There will come over a Volatile Oily Spirit, a yellow Oil, a Volatile Salt in a dry form, and, at last, a black thick Oil. There will be left in the retort a charred matter.
Reduce also into the smallest pieces you can the hard Yelks of the Eggs which you separated from the Whites. Set them in a pan over a gentle fire: stir them with a stick till they turn a little brown, and discharge a substance like melted marrow. Then put them into a new strong canvass bag, and press them between two iron plates well heated; whereby you will obtain a considerable quantity of a yellow Oil.
Let what remains in the bag be distilled in a retort set in a reverberating furnace: it will give you the same principles as you got from the Whites.
OBSERVATIONS.
Of the two perfectly distinct substances that constitute the Egg, the Yelk contains the embryo of the chick, and is des[Pg 587]tined to hatch it: the White is to serve for the nourishment of the chick when it is formed.
These two matters, though they contain the very same principles, yet differ considerably from each other; and chiefly in this, that their principles are not in the same proportions.
The White of an Egg contains so much phlegm, that it seems to consist almost totally thereof. All the aqueous liquor, obtained by distilling it in the balneum mariæ, is, properly speaking, nothing but pure water; for no Chymical trial can discover in it either an Acid or a Volatile Alkali; or any very perceptible Oily part. And yet it must contain some Oil, because the liquor that rises last is a little bitterish to the taste, and smells somewhat of empyreuma. But the principles from which it derives these properties are in too small quantities to be distinctly perceived.
If, instead of distilling the hard White of an Egg, with a view to draw off the great quantity of water it contains, you leave it some time in an air that is not too dry, the greatest part of its moisture separates spontaneously, and becomes very sensible. In all probability this is the effect of a beginning putrefaction, which attenuates this substance, and breaks its contexture. The liquor thus discharged by the White of an Egg thoroughly dissolves Gum-Resins, and particularly Myrrh. If you desire to dissolve Myrrh in this manner, cut a hard-boiled Egg in halves; take out the Yelk; put the powdered Gum-Resin into the cavity left by the Yelk; join the two halves of the White; fasten them together with a thread, and hang them up in a cellar. In a few days time the Myrrh will be dissolved by the moisture that issues from the White of the Egg, and will drop into the vessel placed underneath to receive it. This liquor is improperly called Oil of Myrrh per deliquium.
All the properties of the Whites of Eggs, as well as the principles obtained by analyzing them, are the same with those of the lymphatic part of the blood; so that there is a great resemblance between these two substances.
As to the Yelk, it is plain from its analysis that Oil is the predominant principle thereof. If the Yelk of an Egg be mixed with water, the Oil with which it is replete, and which is by nature very minutely divided, diffuses itself through the whole liquor, and remains suspended therein by means of its viscosity. The liquor at the same time becomes milk-white like an emulsion, and is in fact a true animal emulsion.
In order to obtain the Oil of Eggs by expression with the more ease, care must be taken to chuse Eggs that are seven or eight days old; because they are then a little less viscous. Nevertheless, their viscosity is still so great that they will not easily yield their Oil by expression: and therefore, in order to attenuate and destroy entirely this viscosity, they must be torrefied before they are put to be pressed.
The Oil of Eggs, like all other oily animal matter, seems analagous to the Fat Oils of vegetables. It hath all the properties that characterise those Oils. Its colour is yellow, and it smells and tastes a little of the empyreuma, occasioned by torrefying the Yelks. It is rendered somewhat less disagreeable by being exposed to the dew for thirty or forty nights, if care be taken to stir it often in the mean time.
To conclude: all the principles, both in the Yelk and the White of an Egg, are the same as those found in Blood, Flesh, and all other matters that are perfectly animal.
Of the Excrements of Animals.
Dung analyzed. Instanced in Human Excrement. Mr. Homberg's Phosphorus.
Take any quantity you please of human Excrement, and distil it in a glass alembic set in the balneum mariæ. You will obtain an aqueous, clear, insipid liquor; which will nevertheless have a disagreeable odour. Having urged the distillation as far as is possible, with the heat of this bath, unlute your vessels, and you will find at the bottom of the cucurbit a dry matter, making about an eighth part only of what you put into it. Put this residuum into a glass retort, and distil in a reverberating furnace, with degrees of heat. You will obtain a Volatile Spirit, and a Volatile Salt, with a fetid Oil; and a charred matter will be left in the retort.
OBSERVATIONS.
Mr. Homberg made a great many experiments on the dung of animals; concerning which he composed two Memoirs published in the Academy's collection for 1711. That Chymist tells us, that, in distilling Excrement, he aimed not so much at discovering the principles of which it consists, as he was desirous to satisfy a friend of his, who had earnestly entreated him to try whether he could not extract therefrom a clear Oil, having no bad smell; because he had seen, as he said, Mercury fixed into pure Silver by such an Oil.
Mr. Homberg's labour had the usual fate of all enterprises of this nature. He actually found the art of drawing from Excrement a clear scentless Oil; but, in whatever way he applied it to Mercury, it produced no change in that metallic substance. However, as Mr. Homberg was a man of sagacity, and knew how to improve every hint offered by his experiments, he made several curious discoveries on this occasion; of which we shall give a concise account, after we have made some remarks on the principles obtained from Excrement by the method described in the process.
This substance, consisting of matters subject to putrefaction, hath constantly a fetid smell, like that of all putrid matters; having been for some time confined in a warm, moist place, which we know promotes putrefaction, and even quickly produces it. Yet the analysis thereof proves that it is not putrefied, or at least not entirely so: for all putrefied matters contain a Volatile Alkali perfectly formed and extricated; and, as this principle rises with less heat than that of boiling water, it always comes over first in distillation. Now we have seen that, with the heat of boiling water, it parts with nothing but an insipid phlegm, containing no Volatile Alkali: a sure proof that the fecal matter is not completely putrefied.
There is nothing remarkable in the Volatile Salt and fetid Oil, which rise with a degree of heat greater than that of boiling water. They are common productions, of which we have made frequent mention in several of the preceding analyses; and therefore they need not now detain us from proceeding to give a summary account of Mr. Homberg's chief discoveries.
One of the methods by which Mr. Homberg endeavoured to obtain from Excrement a clear Oil, without any bad smell, was to separate its earthy and gross parts, by filtering[Pg 590] it before he distilled it. "For this purpose he diluted Excrement newly discharged with hot water, using a quart of water to an ounce of feces. Then he let the mixture stand to cool, and, the gross parts falling to the bottom, he poured off the water by inclination. This liquor he filtered through brown paper, and evaporated to a pellicle over a gentle fire. There shot in it long crystals of four, five, and six sides, which Mr. Homberg thinks may be called the Essential Salt of Excrement. They resemble Salt-petre, in some measure, and deflagrate in the fire much like it; with this difference, that their flame is red, and they burn slowly; whereas the flame of Salt-petre is white and very vivid: probably, says Mr. Homberg, because there is too much of an oily matter in the one, and less in the other.
"Mr. Homberg distilled this Salt in a glass retort with degrees of fire, and at last with a very violent one. At first there came over an aqueous liquor, sharp and acid, which was followed by a brown fetid oil, smelling very strong of empyreuma. This distillation he attempted four several times; and each time the matter in the retort took fire, just when the Oil began to come off."
The Salt which Mr. Homberg obtained from excrement is very remarkable. We shall have occasion to speak of it in another place, and shall only observe here, that its Nitrous character is by no means ambiguous: its deflagrating on live coals convinced Mr. Homberg of its being a true Nitre. But its constantly taking fire in the retort, as oft as distilled, is a sure proof that it is a Nitrous Salt: for Nitre only hath the property of thus taking fire in close vessels, and making other combustible matters burn along with it.
The process by which Mr. Homberg at last obtained from Excrement a clear oil without any bad smell is curious, and worthy of a place here; on account of the views and occasions of reflection which it may open.
"Mr. Homberg having tried in vain, by distilling Excrement a great many different ways, to obtain from it such an Oil as he wanted, resolved to employ fermentation, the effect whereof is to change the disposition of the principles of mixts. With this view he dried some Excrement in the water-bath, and, having pulverized it, poured thereon six times its weight of phlegm that had been separated from it by distillation, and put the whole into a large glass cucurbit, covered with an inverted vessel that fitted exactly into it, and was close luted. This vessel he set[Pg 591] in a balneum mariæ for six weeks, keeping up such a gentle heat as would not burn one's hand; after which he uncovered the cucurbit, and having fitted thereto a head and a receiver, distilled off all the aqueous moisture in the balneum mariæ with a very gentle heat. It had now lost almost all its bad smell, which was changed into a faint one. It came over somewhat turbid, whereas it was very clear when put into the cucurbit. Mr. Homberg found this water to have a cosmetic virtue: He gave some of it to persons whose complexion, neck, and arms, were quite spoiled, being turned brown, dry, rough, and like a goose skin: they washed with it once a day, and, by continuing the use of this water, their skin became very soft and white."
The dry matter, that remained in the bottom of the cucurbit after distillation, had lost about a twentieth part of its weight; that is, of twenty ounces, put at one time into the cucurbit, somewhat less than nineteen ounces remained. Mr. Homberg suspects that it was not so dry when put into the cucurbit as when it was taken out. Perhaps also the species of fermentation which the matter underwent had attenuated and volatilized some part of it; so that it came over with the phlegm in distillation. The turbidness of that phlegm, which was clear and limpid before, seems to countenance this conjecture.
"The dry matter left in the cucurbit after the first distillation, had not the least smell of feces: on the contrary, it had an agreeable aromatic odour; and the vessel in which Mr. Homberg had digested it, being left open in a corner of his laboratory, acquired in time a strong smell of Ambergris. It is surprising, as Mr. Homberg justly observes, that digestion alone should change the abominable smell of Excrement into an odour as agreeable as that of Ambergris.
"This dry matter he powdered coarsely, and put two ounces thereof at once into a glass retort, that would hold about a pound or a pound and half of water. This he distilled in a sand-bath with a very gentle heat. A small quantity of an aqueous liquor came over first, and then an Oil as colourless as spring-water. Mr. Homberg continued the same gentle degree of heat, till the drops began to come off a little reddish; and then he changed the receiver, stopping that which contained the clear Oil very close with a cork. Having carried on the distillation with a fire gradually augmented, there came over a considerable[Pg 592] quantity of red Oil; and there remained in the retort a charred matter which burnt very readily."
The clear Oil, without any ill smell, which Mr. Homberg obtained from the fecal matter by this process, was the very thing he was in search of, and which he had been assured would convert Mercury into fine fixed Silver: yet he ingenuously owns, that, whatever way he applied it, he could never produce any change in that metallic substance. We shall now proceed to the other discoveries made by Mr. Homberg on this occasion.
In his attempt to obtain a clear Oil from Excrement, he distilled it with different additaments, and amongst the rest with Vitriol and Alum. He found that the matters left in the retort, when he made use of these Salts, being exposed to the open air, took fire of themselves; that they kindled combustible matters; in a word, that they were a true Phosphorus, of a species different from all then known. Pursuing these first hints, he sought and found the means of preparing this Phosphorus by a way much more expeditious, certain, and easy. His process is this.
"Take four ounces of Feces newly excreted: Mix therewith an equal weight of Roch-Alum coarsely powdered: put the whole into a little iron pan that will hold about a quart of water, and set it over a gentle fire under a chimney. The mixture will melt, and become as liquid as water. Let it boil with a gentle fire, constantly stirring it; breaking it into little crumbs, and scraping off with a spatula whatever sticks to the bottom or sides of the pan, till it be perfectly dry. The pan must from time to time be removed from the fire, that it may not grow red-hot, and the matter must be stirred, even while it is off the fire, to prevent too much of it from sticking to the pan. When the matter is perfectly dried, and in little clots, let it cool, and powder it in a metal mortar. Then put it again into the pan, set it over the fire, and stir it continually. It will again grow a little moist, and adhere together in clots, which must be continually bruised and roasted till they be perfectly dry; after which they must be suffered to cool, and then be pulverized. This powder must be returned a third time to the pan, set on the fire, roasted and perfectly dried: after which it must be reduced to a fine powder, and kept in a paper in a dry place. This is the first or preparatory operation.
"Take two or three drams of this powder. Put it into a little matrass, the belly of which will hold an ounce or[Pg 593] an ounce and half of water, and having a neck about six or seven inches long. Order it so that your powder shall take up no more than about a third part of the matrass. Stop the neck of the matrass slightly with paper: then take a crucible four or five inches deep: in the bottom of the crucible put three or four spoonfuls of sand: set the matrass on this sand, and in the middle of the crucible, so as not to touch its sides. Then fill up the crucible with sand, so that the belly of the matrass may be quite buried therein. This done, place your crucible, with the matrass, in the midst of a little earthen furnace, commonly called a Stove, about eight or ten inches wide above, and six inches deep from the mouth to the grate. Round the crucible put lighted coals about half way up, and when it hath stood thus half an hour, fill up with coals to the very top of the crucible. Keep up this fire a full half-hour longer, or till you see the inside of the matrass begin to be red. Then increase your fire, by raising your coals above the crucible. Continue this strong heat for a full hour, and then let the fire go out.
"At the beginning of this operation dense fumes will rise out of the matrass, through the stopple of paper. These fumes issue sometimes in such abundance as to push out the stopple; which you must then replace, and slacken the fire. The fumes cease when the inside of the matrass begins to grow red; and then you may increase the fire without any fear of spoiling your operation.
"When the crucible is so cold that it may be safely taken out of the furnace with one's hand, you must gradually draw the matrass out of the sand, that it may cool slowly, and then stop it close with a cork.
"If the matter at the bottom of the matrass appear to be in powder when shaken, it is a sign the operation hath succeeded: but if it be in a cake, and doth not fall into powder on shaking the matrass, it shews that your matter was not sufficiently roasted and dried in the iron pan, during the preparatory operation."
Since Mr. Homberg, Mr. Lemeri the younger hath made a great many experiments on this Phosphorus, which may be seen in the Memoirs of the Academy for 1714 and 1715. In those Memoirs Mr. Lemeri hath shewn, that Excrement is not the only matter capable of producing this Phosphorus with Alum; but that, on the contrary, almost all animal and even vegetable matters are fit for this combination; that[Pg 594] though Mr. Homberg mixed Alum in equal quantities only with the fecal matter, it may be used in a much greater proportion, and, in certain cases, will succeed the better; that, according to the nature of the substances to be worked on, the quantity of that Salt may be more or less increased; and that whatever is added, more than the dose requisite for each matter, serves only to lessen the virtue of the Phosphorus, or even destroys it entirely: that the degree of fire applied must be different according to the nature of those matters; and, lastly, that Salts containing exactly the same Acid with that of Alum, or the Acid of those Salts separated from its basis and reduced into Spirit, do not answer in the present operation: which shews, says Mr. Lemeri, that many sulphureous matters may be substituted for Excrement in this operation; but that there are no Salts, or very few if any, that will succeed in the place of Alum. Nevertheless, a Chymist, who lately communicated to the Academy a great number of experiments on this Phosphorus, found that any Salt containing the Vitriolic Acid may be substituted for Alum.
This Phosphorus, made either by Mr. Homberg's or by Mr. Lemeri's method, shines both by day and by night. Besides emitting light, it takes fire soon after it is exposed to the air, and kindles all combustible matters with which it comes in contact; and this without being rubbed or heated.
Mess. Homberg and Lemeri have given the most probable and the most natural explanation of the cause of the accension and other phenomena of this Phosphorus. What they say amounts in short to what follows.
Alum is known to be a Neutral Salt, consisting of the Vitriolic Acid and a calcareous earth. When this Salt is calcined with the fecal matter, or other substances abounding in Oil, the volatile principles of these substances, such as their Phlegm, their Salts, and their Oils, exhale in the same manner as if they were distilled; and there is nothing left in the matrass, when those principles are dissipated, but a charred matter, like that which is found in retorts wherein such mixts have been decomposed by distillation.
This remainder therefore is nothing but a mixture of Alum and charcoal. Now, as the Acid of this Salt, which is the Vitriolic, hath a greater affinity with the phlogiston than with any other substance, it will quit its basis to unite with the phlogiston of the coal, and be converted by that union into a Sulphur. And this is the very case; of which we[Pg 595] have certain proofs in the operation for preparing this Phosphorus: for when, after the volatile principles of the oily matter are drawn off, the fire is increased, in order to combine closely together the fixed parts that remain in the matrass, that is, the Alum and the charred matter, we perceive at the mouth of the matrass a small blue sulphureous flame, and a pungent smell of burning Sulphur. Nay, when the operation is finished, we find a real Sulphur sticking in the neck of the matrass; and, while the Phosphorus is burning, it hath plainly a strong sulphureous smell. It is therefore certain that this Phosphorus contains an actual Sulphur; that is, a matter disposed to take fire with the greatest ease. But though Sulphur be very inflammable, it never takes fire of itself, without being either in contact with some matter that is actually ignited, or else being exposed to a considerable degree of heat. Let us then see what may be the cause of its accension, when it is a constituent part of this Phosphorus.
We mentioned just now that the Acid of the Alum quits its basis, in order to form a Sulphur by combining with the Phlogiston of the coal. This basis we know to be an earth capable of being converted into Lime; and that it is actually converted into Quick-lime by the calcination necessary to produce the Phosphorus. We know that new-made Lime hath the property of uniting with water so readily, that it thereby contracts a very great degree of heat. Now when this Phosphorus, which is partly constituted of the basis of the Alum converted into Quick-lime, is exposed to the air, the Lime instantly attracts the moisture of which the air is always full, and by this means, probably, grows so hot as to fire the Sulphur with which it is mixed. Perhaps also the Acid of the Alum is not totally changed into Sulphur; some part thereof may be only half disengaged from its basis, and in that condition be capable of attracting strongly the humidity of the air, of growing very hot likewise by imbibing the moisture, and so of contributing to the accension of the Phosphorus.
There is also room to think that all the Phlogiston of the charred matter is not employed in the production of Sulphur in this Phosphorus, but that some part of it remains in the state of a true coal. The black colour of the unkindled Phosphorus, and the red sparkles it emits while burning, sufficiently prove this. The explanation of the accension of this Phosphorus, as here given by Mess. Homberg and Lemeri, is very ingenious, and in the main just; but yet, in my opinion, the subject deserves a more thorough examination.
Human Urine analyzed.
Put some Human Urine into a glass Alembic; set it in a water-bath, and distil till there remain only about a fortieth part of what you put in; or else evaporate the Urine, in a pan set in the balneum mariæ, till it be reduced to the same quantity. With this heat nothing will exhale but an insipid Phlegm, smelling however like Urine. The residuum will, as the evaporation advances, become of a darker and darker russet, and at last acquire an almost black colour. Mingle this residuum with thrice its weight of sand, and distil it in a retort set in a reverberating furnace, with the usual precautions. At first there will come over a little more insipid Phlegm like the former. When the matter is almost dry, a Volatile Spirit will rise. After this Spirit, white vapours will appear on increasing the fire; a yellow oily liquor will come off, trickling down in veins; and together with this liquor a concrete Volatile Salt, which will stick to the sides of the receiver. At last there will come over a deep-coloured fetid Oil. In the retort there will remain a saline earthy residuum, which being lixiviated will yield some Sea-salt.
OBSERVATIONS.
Urine must be considered as an aqueous liquor replete with all the saline matters which are of no use to the body, either for nourishment or health: it is a lixivium of animal matters, prepared by nature for dissolving and separating from them all the unnecessary Salts. It contains a very large quantity of almost pure phlegm, which evaporates with the heat of a water-bath.
The residue of the Urine, from which this phlegm is separated by the first distillation, though thereby rendered considerably thicker, doth not coagulate, or curdle in the least, like Milk or Blood; which shews that it contains no parts analagous to those of these two nutritious liquors. Yet it contains oily and saline parts, disposed like those of truly animal matters; as appears from the Spirit, the Volatile Salt, and the Oil, obtained from it by distillation; which are, in every respect, perfectly like the same principles yielded by other animal substances. But, if the animal that[Pg 597] made the Urine took in with its food any of the Neutral Salts, which cannot be decompounded by digestion; that is, of those chiefly which consist of Acids and Alkalis, the Urine will contain, over and above the other parts of that animal, almost all the Neutral Salt that entered into its body. Accordingly human Urine is replete with a considerable quantity of Sea-salt, because men eat a great deal of it. It is found, after the distillation of the Urine, united with the caput mortuum left in the retort; because, being of a fixed nature, it doth not rise with the volatile principles in distillation.
Besides this Sea-salt, Urine contains another Salt of a singular nature, which crystallizes differently from Sea-salt. In this Salt, according to Mr. Marggraff's experiments mentioned on the subject of Phosphorus, is contained the Acid necessary to produce the Phosphorus of Urine. There is reason to think that this Salt is a Sea-salt, disguised by the fat matters with which it combines during its stay in the animal body.
Mr. Boerhaave calls it the Essential Salt of Urine. If you desire to have it by itself, you must evaporate the Urine, with a gentle heat, to the consistence of fresh cream, filter it, and let it stand quiet in a cool place. Crystals will at length shoot therein, and adhere to the sides of the vessel. These crystals are the Salt you want: they are brown and oily. If you desire to have them purer, you must dissolve them in warm water, filter the solution, and set it by to shoot. This operation repeated several times will render them clear and transparent. Mr. Schlosser, a young and very promising Chymist, is the last who hath made any experiments on this curious Salt of Urine. Those who are desirous of a particular account of its properties may consult his dissertation, printed at Leyden in 1753, as well as Mr. Marggraff's excellent Memoirs, printed among those of the Academy of Berlin.
The chief result of Mr. Schlosser's experiments is, first, that this Salt may be obtained from recent Urine, and even in greater quantities than from putrid Urine, and that too in very little time: seeing it crystallizes in twenty-four hours, after due evaporation.
Secondly, that this Salt is a Neutral Ammoniacal Salt, consisting of a Volatile Alkali, (which can never be extracted from it but in a liquid form, like that which is separated from Urine by the addition of Lime); and of an Acid of a very singular nature, the most remarkable property of which[Pg 598] is, its being so fixed as to resist the violence of fire, and turn into a sort of glass rather than exhale in vapours. This is that Acid which, according to Mr. Marggraff's experiments, forms the combination of Phosphorus when united with the Phlogiston. The other properties of this singular Acid are the principal objects of Mr. Marggraff's inquiries.
It follows, in the third place, from Mr. Schlosser's experiments, that this Acid, being combined to the point of saturation with a common Volatile Alkali, forms a true, regenerated Salt of Urine; and that, by this union, the nature of the Volatile Alkali is so changed, that it cannot afterwards appear by itself in a concrete form, but is always fluid, like that which is extricated by the additament of lime.
If Fixed Alkalis be mixed with fresh Urine, they immediately separate from it a Volatile Alkali; and, if the mixture be quickly put into an alembic, and distilled, the first liquor that rises is a Volatile Spirit: or else a Volatile Alkali in a concrete form will rise first, provided the Fixed Alkali made use of be not liquid, and the Urine be dephlegmated.
Herein Urine resembles other animal matters: for Fixed Alkalis produce the same effect on them. This affords us good grounds for believing that all animal matters contain a Neutral Salt of an Ammoniacal nature, which the Fixed Alkali decomposes, as it doth all other Ammoniacal Salts. Quick-lime also extricates from Urine a Volatile Alkali, still more quick and pungent than that which is separated by a Fixed Alkali, and which constantly remains liquid without ever putting on a concrete form: and this is another proof of the existence of the Ammoniacal Salt above-mentioned; for quick-lime hath just the same effect on Sal-Ammoniac, as we shall see in its place. Mr. Schlosser's experiments, compared with those now mentioned, seem to shew that the Urine contains several distinct sorts of Ammoniacal Salts.
Of all the liquors which animals afford, Urine putrefies the most easily, and by putrefaction parts with, or forms, the greatest quantity of Volatile Alkali. If it be distilled when putrefied, there comes over first a Spirit impregnated with much Volatile Alkali; then an aqueous liquor, which Van Helmont assures us is a medicine of wonderful efficacy in dissolving the stone in the bladder. When all this water is come over, and the remaining matter is almost dry, there[Pg 599] ascends, on increasing the fire, a yellow Oil, together with a Volatile Salt.
After this there remains in the retort a black charred earthy matter, containing a great deal of Sea-salt. If this matter be calcined in the open air, in order to consume its Phlogiston, and be afterwards lixiviated, all the Sea-salt it contains may by this means be easily separated; nothing but its earth being left behind. This caput mortuum contains also the materials proper for forming Kunckel's Phosphorus; and if, instead of calcining it in the open air, it be urged with a violent fire, in close vessels, it will yield a Phosphorus: but then all the precautions recommended on the subject of Phosphorus must be used; and, in particular, the caput mortuum must be lixiviated before it be distilled, in order to free it from part of the Sea-salt contained therein; because too much of that Salt might defeat the operator, by not only melting itself, but melting also the containing vessel during the operation.
Of the Volatile Alkali.
Volatile Alkalis rectified and depurated.
Mix together the Spirit, the Volatile Salt, the Phlegm, and the Oil, obtained from any substance whatever. Put the whole into a large wide-mouthed glass body, and thereto fit a head with a large beak. Set this alembic in a water-bath, lute on a receiver, and distil with a very gentle heat. There will ascend a Spirit, strongly impregnated with a Volatile Alkali, and a Volatile Salt in a concrete form, which must be kept by itself. Then increase your heat to the degree of boiling water; whereupon there will rise a second Volatile Spirit, somewhat more ponderous than the former, with a light Oil that will swim on its surface, and a little concrete Volatile Salt. Proceed till nothing more will rise with this degree of heat. Keep by itself what came[Pg 600] over into the receiver. At the bottom of the cucurbit you will find a thick fetid Oil.
Into such another distilling vessel put the Spirit and Salt that rose first in this distillation, and distil them in the balneum mariæ with a heat still gentler than before. A whiter, purer, Volatile Salt will sublime. Continue the distillation till an aqueous moisture rise, which will begin to dissolve the Salt. At the bottom of the vessel will be left a phlegm, with a little Oil floating on it. Keep your Salt in a bottle well stopped.
OBSERVATIONS.
In the analysis of any substance that yields a Volatile Alkali, this Salt is generally found in the receiver, blended with the other principles of the mixt; which, ascending from the retort in the form of liquors and vapours, dissolve the Salt, or at least moisten it, and render it very impure. So that, if you desire to have it without any mixture, recourse must be had to a second distillation, in order to separate it from the heterogeneous matters with which it is confounded.
It is of consequence in this distillation to apply but a very weak degree of heat; because on that depends the success of the operation, insomuch that, the less heat you employ to sublime the Salt, the purer it will be. For, being far more volatile than any of the other principles with which it is mixed, it must evidently rise by itself, if no more heat be applied than is just necessary to elevate it; such a heat being much too weak to raise the Oil and phlegm with which it is blended.
Nevertheless, whatever care be taken to govern the heat, it is not possible to hinder this Volatile Salt from carrying up some portions of the principles mixed with it; those, to wit, with which it is most closely united, and to which it hath by that means communicated a share of its volatility. For this reason it requires a second rectification, which is performed in the same manner as the former. But, seeing it is more volatile and lighter after the first rectification than before, being thereby freed from part of the heterogeneous matters with which it was loaded, a still less degree of heat must be applied in this second rectification.
The Oil with which the Volatile Salt is loaded, when but once distilled, is perceivable only by the yellow colour and weight it communicates thereto; because it is closely united therewith, and in a perfectly saponaceous state. This ap[Pg 601]pears from the facility with which Volatile Salts, even the most oily, dissolve in water, without discovering in the solution any separation of the oily parts, and even without giving it a milky colour. But, in the second rectification, this Oil becomes very perceptible; for it then separates, in a great measure, from the Salt, and remains at the bottom of the cucurbit, floating on the phlegm, which is also separated from the Salt.
The Salt is then whiter, more volatile, and purer; yet it is still far from being brought to the utmost degree of purity, even by this second rectification. It requires a third, a fourth, and even many more rectifications, to purify it perfectly: every rectification separates from it some oily particles: and if you should resolve to go on rectifying till you can separate no more Oil, there is reason to think this Salt would be entirely decomposed; because there is necessarily a certain quantity of Oil in its composition, without which it would not be a Volatile Alkali. You must therefore desist from rectifying it any further, when you find it very white, and very light; and shut it up in bottles hermetically sealed.
It often happens that Volatile Salts, though of a beautiful white after rectification, grow yellow after being kept some time in close bottles. This is occasioned by the Oil they contain disengaging, and discovering itself by degrees. To remedy this inconvenience, Mr. Boerhaave proposes to mingle the Volatile Salt, which you intend to purify, with four times its weight of pulverized chalk, thoroughly dried, and even heated; to put the mixture into a glass alembic, and distil it with a gentle heat. By this means the Salt rises exceeding pure and very white; because the chalk absorbs most of its Oil, and frees it therefrom. He adds, that Volatile Salt thus purified may be kept a long time, and will retain all its whiteness.
If a Volatile Alkali thus purified be combined, to the point of saturation, with an Acid, such as the Marine Acid for instance; the result of this union, as we shall afterwards see, will be a Sal Ammoniac, from which the Volatile Alkali may be separated by the intervention of a Fixed Alkali. A Volatile Alkali that hath passed through all these trials will then be in the highest degree of purity that Chymistry can bring it to, and appears constantly the same, from whatever substance it was originally obtained: which proves that if Volatile Alkalis, extracted from different vegetable and animal substances, seem to differ from each other in some[Pg 602] respects, this can arise only from the heterogeneous matters with which they are mixed; but that, at bottom, they are all constituted of one single principle, which is constantly the same, and exactly alike in them all.
It is of the last consequence, on all occasions where a Volatile Alkali is to be distilled in a concrete form, to make use of subliming vessels with very large necks, that it may have room enough to make its way to the receiver with ease; for otherwise it may choak up the passage, and burst the vessels.
Volatile Alkalis combined with Acids. Sundry Ammoniacal Salts. Sal Ammoniac.
On a Volatile Spirit or Salt pour gradually any Acid whatever. An effervescence will arise, and be more or less violent according to the nature of the Acid. Go on adding more Acid in the same manner, till no effervescence be thereby excited, or at least till it be very small. The liquor will now contain a semi-volatile Neutral Salt, called an Ammoniacal Salt; which may be obtained in a dry form by crystallizing as usual, or by subliming it in close vessels, after the superfluous moisture hath been drawn off.
OBSERVATIONS.
Volatile Alkalis have the same properties with Fixed Alkalis, fixity only excepted: so that a Volatile Alkali must produce an effervescence when mixed with Acids, and form therewith Neutral Salts, differing from each other in nothing but the nature of the Acid in their composition.
It must be observed, that, on this occasion, the point of saturation is very difficult to hit; owing probably to the Volatility of the Alkali, which, being much lighter than the Acid, tends always to possess the uppermost part of the mixture, while the Acid sinks to the bottom: whence it comes to pass, that the lower part of the liquor is sometimes over-charged with Acid, while the upper part is still very Alkaline. But it is most eligible that the Alkali should predominate in the mixture; because the excess of this principle easily flies off, while the moisture is evaporating, in order to the crystallization or sublimation of the Ammoniacal Salt;[Pg 603] which being only semi-volatile resists the heat longer, and remains perfectly Neutral.
If the Vitriolic Acid be combined with a Volatile Alkali, and the mixture distilled in a retort to draw off the superfluous moisture, a liquor comes over into the receiver, which smells strong of a Sulphureous Acid. Now, as the Acid of Vitriol never becomes sulphureous, but when it is combined with an inflammable matter, this experiment is one of those which demonstrate that Volatile Alkalis contain a very sensible quantity of inflammable matter. This same liquor tastes of an Ammoniacal Salt; which proves that it carries up with it some of the Neutral Salt contained in the mixture. The rest of this Salt, which is called Glauber's Secret Sal Ammoniac, or Vitriolic Sal Ammoniac, sublimes into the neck of the retort. It is very pungent on the tongue; it crackles a little when thrown on a red hot shovel, and then flies off in vapours.
The Ammoniacal Salt formed by the Acid of Nitre exhibits much the same phenomena; but it requires greater care in drying and subliming it, because it hath the property of detonating all alone, without the addition of any other inflammable matter: and it will infallibly do so, if too strong a fire be applied towards the end of the operation, when it begins to be very dry. This property of detonating by itself it derives from the inflammable matter contained in the Volatile Alkali which serves for its basis: and this is another demonstrative proof of the existence of such an inflammable matter in the Volatile Alkali. This Salt is called Nitrous Sal Ammoniac.
With the vegetable Acids, that of Vinegar for instance, is formed an Ammoniacal Salt of a singular nature, and which can scarce be brought to a dry form.
A Volatile Alkali, combined to the point of saturation with the Acid of Sea-salt, forms another Neutral Salt, which takes a concrete form either by sublimation or crystallization. The crystals of this Salt are so very soft and fine, that a parcel of it looks like cotton or wool. This is the Salt properly called Sal Ammoniac. It is of great use in Chymistry and in manufactures: but that which is daily consumed in great quantities is not made in the manner above mentioned. It would come extremely dear if we had no other way of procuring it, but by forming it thus with the Acid of Sea-salt and a Volatile Alkali. This Salt, or at least the materials of which it is formed, may be found in the fuliginosities and soots of most animal, and of some vegetable substances.[Pg 604] The greatest part of what we use comes from Egypt, where vast quantities thereof are made.
The method of preparing Sal Ammoniac in Egypt was not known among us, till Mess. Lemaire and Granger, two of the Academy's correspondents, gave in several Memoirs in which that business is described with great accuracy, from their own view on the spot. Their Memoirs inform us, that chimney-soot alone, without any additament, is the matter from which they obtain their Sal Ammoniac; that those chimneys under which nothing is burnt but Cow's-dung furnish the best Soot. Six and twenty pounds of that Soot yield usually six pounds of Sal Ammoniac.
"The operation takes up about fifty, or two and fifty hours. The vessels in which they put the soot are ballons of very thin glass, terminating in a neck of fifteen or sixteen lines long, and an inch in diameter: but they are not all of the same size. The least contain twelve pounds of Soot, and the greatest fifty; but they fill them only three quarters full, in order to leave room for the sublimation of the Salt.
"The furnace, in which they place these ballons, consists of four walls, built in a quadrangular form. The two front walls are ten, and the sides nine feet long: but they are all five feet high, and ten inches thick. Within the quadrangle formed by these walls three arches run lengthwise from end to end thereof, at the distance of ten inches asunder. The mouth of this furnace is in the middle of one of its fronts, and of an oval form; two feet four inches high, and sixteen inches wide.
"The ballons lie in the spaces between the arches of the furnace, which serve instead of a grate to support them. Four of them are usually placed in each interval; which makes sixteen for one furnace. They are set at the distance of about half a foot from each other, and secured in their places with brick and earth. But they leave about four inches on the upper part of the ballon uncovered, with a view to promote the sublimation, as they also do six inches of the inferior part, that the heat may the better act on the matters to be sublimed. Things being thus prepared they first make a fire with straw, which they continue for an hour. Afterwards they throw in Cow's-dung made up in square cakes like bricks. (The want of wood in this country is the reason that they generally make use of this fuel). These cakes of dung add to the violence of the fire, which they continue in this manner[Pg 605] for nineteen hours; after which they increase it considerably for fifteen hours more; and then they slacken it by little and little.
"When the matter contained in the vessels begins to grow hot, that is, after six or seven hours baking, it emits a very thick and ill-scented smoke, which continues for fifteen hours. Four hours after that, the Sal Ammoniac is observed to rise in white flowers, which adhere to the inside of the neck of the vessel; and those who have the direction of the operation take care, from time to time, to pass an iron rod into the neck of the ballon, in order to preserve a passage through the saline vault, for giving vent to some blueish vapours, which constantly issue out of the vessel during the whole operation."
From this history of the preparation of Sal Ammoniac it appears that Soot, and particularly the Soot of animal matters, either contains abundance of this Salt perfectly formed, and waiting only for sublimation to separate it therefrom, or, at least, that it contains the proper materials for forming it; and that during the operation, which is a kind of distillation of Soot, these materials combine together and sublime.
We shewed, in our analysis of Soot, that this substance yields by distillation a great deal of Volatile Alkali; and this is an ingredient which makes at least one-half of Sal Ammoniac. As to the other principle of this Salt, I mean the Marine Acid, this also must needs exist in Soot: but it is not so easy to conceive how it should come there.
It is very true that vegetable and animal substances, the only ones that produce Soot in burning, contain some portion of Sea-salt: but then this Salt is very fixed, and seems unfit to rise with the Acid, the Oil, and the subtile Earth, of which the Volatile Alkali is formed. Therefore we must suppose either that its elevation is procured by the force of the fire, aided by the volatility of the matters that exhale in burning; or that, being decomposed by the violence of the combustion, its Acid alone rises with the other principles above-mentioned. The latter seems probable enough: for though in the common operations of Chymistry the bare force of fire doth not seem sufficient to decompose Sea salt; yet the example of Sea-plants, which, before burning, contain this Salt in abundance, and whole ashes contain scarce any at all, but are replete with its fixed part, that is, with its Alkaline basis, seems to prove that, when this Salt is intimately mixed with inflammable matters, it may be destroyed[Pg 606] by burning; so that its Acid shall desert its basis, and fly off with the Soot.
Before the exact method of procuring Sal Ammoniac was known, it was generally imagined that the manufacturers, mixed Sea-salt, and even Urine, with the Soot; because these two substances contain the principles of which this Salt consists. But, besides that the contrary now certainly appears from the above-mentioned Memoirs, it hath been shewn by Mr. Duhamel, who hath published several Memoirs and experiments concerning the composition and decomposition of Sal Ammoniac, from which we have partly taken what we have already said on this subject, and which will furnish us with some more curious observations; it hath been shewn, I say, in the first of Mr. Duhamel's Memoirs, printed with those of the Academy for 1735, that the addition of Sea-salt to the Soot, from which Sal Ammoniac is to be extracted, contributes nothing to its production, and cannot increase its quantity. That alone, therefore, which was originally contained in the matters that produced the Soot, enters as a principle into the composition of Sal Ammoniac. We observed also, in treating of the analysis of Soot, that Mr. Boerhaave obtained from it a considerable quantity of an Ammonical Salt without any additament.
Sal Ammoniac is sometimes found perfectly formed in the neighbourhood of Volcanoes. This Salt is probably produced from the fuliginosities of vegetable or animal matters consumed by the fire of the Volcano.
Sal Ammoniac is often impure, because it carries up with it, in sublimation, some of the black charred matter which ought to be left at the bottom of the vessel: but it is easily purified. For this purpose you need only dissolve it in water, filter the solution, then evaporate and crystallize; by which means you will have a very white and very pure Sal Ammoniac. You may, if you please, sublime it again in a cucurbit and blind head, with a fire not too brisk. Some of it will rise in the form of a light white powder, called Flowers of Sal Ammoniac. These Flowers are no other than true Sal Ammoniac, which hath suffered no decomposition; because the bare action of fire is not capable of separating the Acid and the Volatile Alkali, of which this Neutral Salt consists. When you intend to decompose it, you must use the means to be mentioned hereafter.
Though Sal Ammoniac be only semi-volatile, and requires a considerable heat to sublime it, yet it hath the property of carrying up with it matters that are very fixed and ponderous;[Pg 607] such as metallic substances, and some kinds of earths. For medicinal uses we sublime therewith Iron, Lapis Hæmatites, the Copper in blue Vitriol, &c. and then it takes different names, as Martial Flowers of Sal Ammoniac, Ens veneris, and other such denominations, which it borrows from the matters sublimed with it.
Sal Ammoniac decompounded by Acids.
Into a large tubulated glass retort put a small quantity of Sal Ammoniac in powder: set your retort in a furnace, and lute on a large ballon, as in the distillation of the smoaking Acids of Nitre and Sea-salt. Through the hole in your retort pour a quantity of Oil of Vitriol, or Spirit of Nitre, equal in weight to your Sal Ammoniac. An effervescence will instantly follow. The mixture will swell, and discharge white vapours, which will come over into the receiver. Stop the hole in the retort immediately, and let the first vapours pass over, together with some drops of liquor, which will distil without fire. Then put a few coals into the furnace, and continue the distillation with a very gentle heat; which however must be increased, little by little, till nothing more will come off. When the operation is finished, you will find in the receiver a Spirit of Salt, if you made use of Oil of Vitriol; or an Aqua regis, if Spirit of Nitre was employed: and in the retort will be left a saline mass, which will be either a Glauber's Secret Sal Ammoniac, or a Nitrous Sal Ammoniac, according to the nature of the Acid used to decompound the Sal Ammoniac.
OBSERVATIONS.
Sal Ammoniac, which consists of the Marine Acid united to a Volatile Alkali, is, with respect to the Vitriolic and Nitrous Acids, just the same as Sea-salt is with respect to those Acids; that is, the Vitriolic and Nitrous Acids, having a greater affinity, than the Marine Acid, with Volatile as well as Fixed Alkalis, will decompound the Sal Ammoniac, by expelling the Acid from its basis, and assuming its place, just as they do with regard to Sea-salt. Most therefore of what was said concerning the decomposition of Sea[Pg 608]-salt, and the distillation of its Acid, by the two other Acids, must be applied here.
We shall only observe, that, when the Acid of Sal Ammoniac is to be distilled from it by the interposition of the Vitriolic or Nitrous Acid, great care must be taken to put but a very small quantity of this Salt into the retort; especially if the Acids to be added are concentrated: for, as soon as they mix with the Sal Ammoniac, a great effervescence arises, and the mixture swells to such a degree, that, unless the quantity in the retort be very small, it may run over altogether into the receiver. It is also proper to take notice, that this operation admits of but a small degree of heat, for two reasons; first, because the Acid of the Sal Ammoniac, being very easily dislodged by an Acid stronger than itself, rises also very easily; secondly, because the Sal Ammoniac which is to be decompounded, as well as the Ammoniacal Salts which result from its decomposition, are semi-volatile, and will sublime in substance if they be exposed to the smallest excess of heat. Moreover, the Nitrous Sal Ammoniac would be in danger of taking fire and exploding, for a reason frequently mentioned above.
The Nitrous Sal Ammoniac may be decompounded, as well as Sal Ammoniac, by the Vitriolic Acid. But, as the Nitrous Acid contained in the Salt is the strongest of all Acids next to the Vitriolic, no other Acid but this is able to expel it from its basis; in which respect this Salt resembles Nitre.
Instead of employing the Acids of Vitriol and Nitre to decompound Sal Ammoniac, we might make use of Neutral Salts consisting of these Acids combined with metallic or earthy bases: but then, as this decomposition cannot be effected without a greater degree of heat, there is reason to apprehend that some of the Sal Ammoniac would be thereby sublimed, before it could be decompounded.
Sal Ammoniac decompounded by Fixed Alkalis. Volatile Salt. The Febrifuge of Sylvius.
Into a glass alembic or retort put Sal Ammoniac and Salt of Tartar, pulverized and mixed together in equal quantities. Set your vessel in a proper furnace, and imme[Pg 609]diately lute on a large receiver. A little volatile Spirit will ascend; and a volatile Alkali, in a concrete form, very white and beautiful, will sublime into the head, and come over into the receiver, in quantity near two thirds or three fourths of the Sal Ammoniac used. Continue the distillation, increasing the fire by degrees, till nothing more will sublime. Then unlute the vessels. Put up your Volatile Salt immediately into a wide-mouthed bottle, and stop it close with a crystal stopple. At the bottom of the retort, or cucurbit, you will find a saline mass, which, being dissolved and crystallized, will form a Salt nearly cubical, having the taste and other properties of Sea-salt. This is the Sal Febrifugum Sylvii.
OBSERVATIONS.
This decomposition of Sal Ammoniac is the reverse of that in the preceding process. In the former operation it was shewn that the Acid of Sal Ammoniac may be separated from its basis, by applying to that basis a stronger Acid: in the present operation, on the contrary, the basis of this Salt is separated from its Acid, by presenting to that Acid a Fixed Alkali, wherewith it hath a greater affinity than with the Volatile Alkali which serves it for a basis.
The action of Fixed Alkalis upon Sal Ammoniac is so vigorous and sudden, that, as soon as these two matters are mixed together, the Volatile urinous Salt rushes out with great activity, even without the help of heat; so that much of it will be lost, if care be not taken to confine the mixture immediately in those vessels by means of which it is to be distilled.
The Volatile Salt obtained by this operation is white, pure, and very active; having been freed from the greatest part of its superfluous fat matter, both by the union it had contracted with the Marine Acid, and by the Fixed Alkali employed to separate it therefrom. This Salt is so quick and volatile, that if, on taking out the receiver, it be left a little too long exposed to the air, before it be put into the bottle in which it is to be kept, a great deal of it will exhale and be lost. For the same reason care should be taken, while the vessels are unluting, that the vapour of this Salt do not strike the organ of smelling, or be drawn into the lungs in respiration; for it affects those organs so powerfully, and makes such a quick impression on them, that the operator would be in danger of suffocation. Yet it is of great service, when[Pg 610] cautiously smelled to, for exciting the vibrations of the Genus Nervosum, in Apoplexies, Fainting fits, and Hysterical disorders. But it must always be administered with great caution; for it hath a corrosive quality, and is no less caustic than a Fixed Alkali. This is proved by applying it to the bare skin, and keeping it on by means of a pitch-plaster, so that it cannot fly off in vapours: for, as soon as it begins to grow warm, it produces on the skin a smarting sensation, like that of burning, attended with much pain, and in a very short time makes an eschar like a caustic.
The Volatile Spirit, obtained in the decomposition of Sal Ammoniac by a Fixed Alkali, derives its origin from the Phlegm contained in the saline matters that are mixed together on that occasion. The moister those matters are, the more Spirit there will be. This also is very active and penetrating. But as it owes these qualities wholly to the Volatile Salt dissolved in it, the more of this Spirit comes off, the less Salt will there be.
If you desire to have much Volatile Spirit, a quantity of water, proportioned to the quantity of Spirit you want, must be mixed with the Salts. In this case the distillation begins with a humid vapour, which coagulates on the sides of the receiver into a concrete Salt, almost as soon as it comes over. There rises afterwards an aqueous vapour, not so saline or volatile as the former. This liquor dissolves the Salt that was coagulated before; and, if the water added was in sufficient quantity, it will dissolve the Salt entirely; otherwise it will dissolve but a part thereof, and then it is certain that the liquor is a Volatile Spirit as strongly impregnated with Salt as it can be. The reason why the liquor that rises first contains a great deal more Volatile Salt than the other, in so much that it coagulates and becomes solid, is because the Volatile Salt rises in distillation much more easily than water.
In whatever manner the Volatile Spirit or Salt be distilled from Sal Ammoniac, by means of a Fixed Alkali, we always find at the bottom of the retort, or cucurbit, when the operation is finished, a new Neutral Salt compounded of the Acid of the Sal Ammoniac, and of the Alkali used in the distillation. If the Salt of Tartar be used, this new Neutral Salt will be perfectly like that produced by combining this Alkali with the Acid of Sea-salt, to the point of saturation. The figure of the crystals of this Salt, though much like that of the crystals of Sea-salt, is nevertheless a little[Pg 611] different. However, this Salt possesses the chief properties of Sea-salt. It bears the name of Sal Febrifugum Sylvii, because that Physician attributed to it the virtue of curing intermitting fevers. But its title to this virtue is very doubtful, at least in this country.
If the Salt of Soda be used, instead of Salt of Tartar, to decompound Sal Ammoniac, a Volatile Spirit and Salt will in like manner be obtained; and the Neutral Salt left in the retort, after distillation, will be a true regenerated Sea-salt, perfectly like native Sea-salt; because, as we have said before, the Salt of Soda is of the same kind with the natural basis of Sea-salt; and the inconsiderable differences, observable between the Sal Febrifugum and Sea-salt, can be attributed only to such as may be found between the Alkaline bases of those two Salts.
Sal Ammoniac decompounded by Absorbent Earths and Lime. The Volatile Spirit of Sal Ammoniac. Fixed Sal Ammoniac. Oil of Lime.
Let one part of Sal Ammoniac, and three parts of Lime, slaked in the air, be pulverized separately, and expeditiously mixed together. Put this mixture immediately into a glass retort, so large that half of it may remain empty. Apply thereto a capacious receiver, with a small hole in it to give vent to the vapours, if needful. Let your retort stand in the furnace about a quarter of an hour, without any fire under it. While it stands thus, a great quantity of invisible vapours will rise, condense into drops, and form a liquor in the receiver. Then put two or three live coals in your furnace, and gradually increase the fire till no more liquor will rise. Now unlute your vessels, taking all possible care to avoid the vapours, and quickly pour the liquor out of the receiver into a bottle, which you must stop with a crystal stopple rubbed with emery. There will remain, at the bottom of the retort, a white mass, consisting of the Lime employed in the distillation, together with the Acid of the Sal Ammoniac: this is called Fixed Sal Ammoniac.
OBSERVATIONS.
In our Elements of the Theory, we explained how we imagine that Lime and other substances, which, according to the Table, have less affinity than Volatile Alkalis with Acids, are nevertheless capable of decompounding Sal Ammoniac, by uniting with its Acid, after expelling it from its basis, which is a Volatile Alkali. To recapitulate our opinion in two words: we conceive this to depend on the fixedness of these earthy and metallic additaments, which enables them to resist the force of fire, and on the volatility of the basis of Sal Ammoniac, which proves a great disadvantage to it when it comes to struggle, as it were, with those fixed additaments, aided by a considerable degree of heat. We shall only observe, that we are not singular in this opinion, nor indeed did we deliver it as a new one; that several modern Chymists concur with us therein, and particularly Mr. Baron, whom we have already mentioned more than once on the subject of Borax; and who, we think, was the first that ever took particular notice of it in print, viz. in his Memoirs on Borax, communicated to the Academy before the publication of our Elements. For the explanation of this phenomenon, therefore, we refer to those Memoirs, which are actually published, and to what we have already said on the subject in our treatise above-mentioned.
Another phenomenon, which is equally singular and curious, furnishes us with matter for several reflections, and gives us occasion to relate, in few words, the result of Mr. Duhamel's most sagacious experiments and speculations tending to discover the cause thereof. The point under consideration is the different forms and properties which the Volatile Alkali assumes, when separated from Sal Ammoniac by the means of a Fixed Alkali, and by the means of Lime. We know that the former is always in a concrete form, unless the mixture, from which it is distilled, be absolutely drenched with water; and that the latter, on the contrary, is always in a fluid form, and constantly liquid, whatever method be taken to distil it.
Some Chymists imagine, that the Volatile Salt of Sal Ammoniac appears in a concrete form, only because it still contains some Acid; whence they conclude that the reason why no concrete Volatile Salt can be obtained by the means of Lime is, because it absorbs all the Acid of the Sal Ammoniac; which is not the case, they say, with Fixed Alkalis. Others impute the constant fluidity of the Volatile Spi[Pg 613]rit of Sal Ammoniac, obtained with Lime, to the particles of fire which they suppose communicated thereto by that substance. Mr. Duhamel equally refutes both these opinions, by proving from experiments that Fixed Alkalis are capable of absorbing as much Acid as Lime can, and even more; and that, having been calcined as long, and with as violent a fire, as Lime, they must contain and communicate as many particles of fire; if indeed it be possible that the particles of fire should actually be lodged, and continue imprisoned, in calcined substances, as these gentlemen suppose. Yet this is contrary to experience; seeing the Volatile Salt distilled by the means of a Fixed Alkali, though ever so long and ever so violently calcined, is always in a concrete form, and doth not resemble the Volatile Spirit of Sal Ammoniac prepared with Lime.
In order to throw the necessary lights on this point, Mr. Duhamel had recourse to the only method that can be depended on in Natural Philosophy; namely, Experiments. He accordingly made several, of which these are the chief.
First, he distilled a Volatile Salt, by the means of well desiccated Salt of Tartar, and Salt of Soda; and, urging the fire with great violence towards the end of the operation, he thus obtained a quantity of Volatile Salt equal to, or even exceeding, that of the Sal Ammoniac he used: whence he justly concluded that, on this occasion, the Volatile Salt carried up, and volatilized some of the Fixed Salt.
Secondly, he found upon trial that the Volatile Spirit, obtained from Sal Ammoniac by the means of Lime, appears in the form of a liquor, only because it is mixed with some water which was contained in the Lime. Of this truth he had the following decisive proof: having attempted to prepare a Volatile Spirit of Sal Ammoniac with Lime, which had not been slaked, either in the air or by water, he could not obtain any Volatile Spirit: or, at least, the quantity was so small that it might be reckoned as nothing; and even that was wholly due to the moisture which Sal Ammoniac necessarily contains, together with that which Lime imbibes from the air, if ever so little exposed thereto.
From these two experiments Mr. Duhamel draws the following consequences: viz. that the Volatile Salt cannot be separated from the Sal Ammoniac and sublimed, without carrying along with it some of the additament which serves to extricate it; or, instead thereof, some other body with which it is capable of uniting: that Fixed Alkalis have the property of being thus carried up by the Volatile Alkali,[Pg 614] and subliming with it: that the case is not the same with Lime, which therefore cannot, when alone, separate and sublime the Volatile Alkali of the Sal Ammoniac; but becomes capable thereof when it hath imbibed any moisture, which joins with the Volatile Salt, and rises therewith in distillation. And hence it must be concluded, that, seeing the Volatile Salt carries up with it some of the Fixed Alkali, by the means of which it is separated, it will be in a concrete form; what it carries up along with it being dry and solid: whereas, when it is distilled with Lime, it cannot but be liquid; seeing it must needs be dissolved by the moisture it gets from the Lime, without which it would not rise.
But to what must we attribute these effects produced by Lime, so different from those produced by Fixed Alkalis? Are they owing to its quality of Lime? or would it produce the same, if it were only a mere Absorbent Earth? Mr. Duhamel hath answered this question by a third sort of experiment. He tried to decompound Sal Ammoniac, and to separate its Volatile Alkali, by a pure Absorbent of Earth, without mixing any water with it, or calcining it.
For this purpose he made use of Chalk; and his experiment succeeded. By means of this additament he decompounded Sal Ammoniac, and by the experiment obtained the lights he wanted. The Volatile Alkali, being extricated by the dry but uncalcined Chalk, rose in a concrete form, as with Fixed Alkalis; and in like manner carried up with it some of the earthy additament. The same Chalk when calcined, and converted into Lime, produced the very effect of Lime on Sal Ammoniac. It is therefore from calcination alone that Absorbent Earths derive the property of retaining obstinately the Volatile Alkali, and preventing its sublimation by refusing to rise with it as Fixed Alkalis do.
Though these ingenious experiments evidently furnish us with great lights, for discovering the cause of the solidity or fluidity of the Volatile Alkali, when separated from Sal Ammoniac by different additaments, as they fully determine several preliminary questions immediately relating thereto; yet they still leave us, in some measure, at a loss with regard to the chief point. For we do not yet know why Fixed Alkalis and Absorbent Earths, which, in all Chymical trials, shew that they have certainly as much fixity as Lime, are carried up by the Volatile Alkali, while Lime resists, instead of rising with it as those other substances do, obstinately retains it, and even fixes it in some measure, so that it is im[Pg 615]possible for it to sublime. This question, in my opinion, depends on the theory of Lime; nor can we hope to resolve it in its full extent, till we get a further insight into the nature of that singular substance than we have at present.
On this subject, however, Mr. Duhamel hath offered some conjectures, founded on the known properties of Lime, and supported by experiments. "Lime," says he, "is an earth freed by calcination from almost all its humidity, almost all its Acid, and all the fat it contained; whether that fat came from some animal parts, as is the case of those stones which consist of shells; or whether it were a bituminous fat, as may happen to be the case with some others: this substance is withal acrid and fiery; it is very greedy of moisture, and imbibes it when exposed thereto. It absorbs Acids, and retains them strongly; and, lastly, it unites with fat matters, and therewith makes a kind of soap."
All these properties are verified by experiments; and therefore Mr. Duhamel thinks he hath a right to say, that Lime acts not only on the Acid of Sal Ammoniac, but also on the fatty matter which always accompanies Volatile Alkalis, and is essential to their nature; and therefore it decompounds them. Of this Mr. Duhamel gives the following convincing proof, founded on experiment. He took some Volatile Spirit distilled with Lime, and abstracted it several times from a fresh parcel of Quick-Lime. The quantity of the Spirit diminished sensibly every time; and the Lime was at last so replete with fat, that the Vitriolic Acid, when poured thereon, became very sulphureous; and moreover, when calcined in a crucible, it emitted a very perceptible smell of burnt grease.
Indeed Fixed Alkalis are also capable of absorbing and retaining fat matters; but not near so strongly as Lime: because these Salts are never entirely freed from that which they contain originally; whereas Lime seems much poorer, and absolutely void of any oily matter.
On these principles Mr. Duhamel resolved to try if he could not obtain a Volatile Alkali in a concrete form, by distilling the Volatile Spirit from Lime, brought nearly to the condition of a Fixed Alkali, by imbibing a portion of fat matter. With this view he distilled a great quantity of Volatile Spirit from a little Lime, and actually obtained a small portion of Volatile Salt; because the great quantity of Volatile Spirit had, in some measure, saturated the Lime with fat matter.
Mr. Duhamel tried also to bring Lime back to the condition of a pure Absorbent Earth, to decalcine it, if I may use the term; in order to try whether he could not by this means make it produce the same effect as Chalk. For this purpose he lixiviated some Lime four months successively, pouring every day fresh water on it, and removing that of the preceding day, together with the crystalline crust which always formed on it; and after leaving this Lime two years in the shade, he applied it to Sal Ammoniac. It produced a moderate quantity of Volatile Salt, which was very transparent, and seemed to be crystallized in cubes. Thus we see Lime rendered very like Chalk. Yet it was pretty acrid on the tongue, and the Volatile Salt, obtained by its means, was more disposed to run into a liquid than that separated by Chalk: which shews that this Lime still retained some part of its former character, and that its transformation was not complete.
To conclude what relates to the Volatile Alkali of Sal Ammoniac, it only remains that we say a word or two of that portion of the earthy or saline additament, which, though fixed in its nature, sublimes nevertheless with the Volatile Alkali, and gives it a concrete form.
Mr. Duhamel, who, in every subject that he handles, omits nothing worthy of attention, made several other experiments, with a view to discover whether or no the Salt of Tartar, and the Chalk, carried up by the Volatile Alkali, be truly volatilized; and whether or no there be such a strict union contracted, between the Urinous Salt and these fixed substances, that the whole results in what is called a Concrete Volatile Salt; or if those fixed substances be united but superficially with the Urinous Salt, which only carries them up along with itself in sublimation, as Sal Ammoniac carries up several very fixed metallic matters.
The result of the experiments made by Mr. Duhamel for this purpose is, that the fixed substances carried up by the Volatile Alkali of the Sal Ammoniac are actually volatilized; that they make, as it were, one whole with it; and are so closely combined therewith, that almost all the most efficacious means of separating fixed from volatile matters are unsuccessful with regard thereto. Nothing, for instance, is fitter to separate a volatile substance from a fixed one, than to mix the compound with a great quantity of water, and to distil the whole, with such a degree of heat as shall be exactly sufficient to elevate the volatile part. In this manner Mr. Duhamel treated Volatile Alkalis replete with Fixed[Pg 617] Salt, and with Chalk: but though he applied no more than the gentlest degree of heat; nay, exposed his mixture to the air only, fearing lest he should make the heat too strong if he used fire; yet the fixed part, which the Volatile Salt had carried up with it, continued still united therewith; so that the whole passed over in distillation, or was dissipated by evaporation, without leaving any thing fixed at the bottom of the vessel.
He also justly looked on Acids as an effectual means of procuring the separation, or decomposition, he was in quest of. We know that, with the Volatile Alkali, they form Ammoniacal Salts, which, though they are not so light as the Volatile Alkali, sublime nevertheless with a moderate heat; and that, on the contrary, the same Acids with Fixed Alkalis, or Absorbent Earths, form Neutral Salts, which resist the violence of fire. On this principle Mr. Duhamel poured Acids, to the point of saturation, upon Volatile Alkalis containing much Fixed Alkali, or Chalk. But this experiment succeeded no better than the foregoing; for the mixture being put to distil, sublimed wholly in Sal Ammoniac. Indeed a little fixed matter was left at the bottom of the retort; but the quantity thereof was too small to merit notice.
At last, the only way Mr. Duhamel could think of, for separating, from a Concrete Volatile Alkali, the fixed parts which that Salt had rendered Volatile, was to expose it to the air, covered with a piece of gauze only; but in its dry state, without dissolving it in water. The Volatile Urinous Salt was by this means dissipated; having deserted the fixed part, which remained at the bottom of the bason, and, being exposed to the fire, retained its fixed nature. But it took more than a year to effect this separation; nor are we sure that it was complete; for it is not certain that all the fixed part was left behind, and that some of it was not dissipated with the Volatile Urinous Salt.
This volatilization, this kind of metamorphosis of a Fixed Alkali and an Absorbent Earth into a Volatile Alkali, is a very curious phenomenon, and deserves to be considered by the best Chymists.
We shall finish our observations on the decomposition of Sal Ammoniac by Lime, with some reflections on the nature of the caput mortuum that remains after this distillation.
This residuum is only Lime impregnated, but not saturated, with the Acid of Sea-salt. If the distillation be urged[Pg 618] at last with a violent fire, the caput mortuum will be found formed into a mass, seeming to have been half-melted. This matter is a kind of Phosphorus, and emits light in the dark, when struck with any hard body. Mr. Homberg was the first who discovered it to have this property. Having calcined, and melted together in a crucible, one part of Sal Ammoniac and two parts of Lime, with a design to fix that Salt, he observed the mass remaining after the fusion to have the property just mentioned.
Lime, thus impregnated with the Acid of Sal Ammoniac, is very improperly called by the name of Fixed Sal Ammoniac. This compound attracts the moisture of the air, and even runs wholly into a liquid, if it be impregnated with much Acid. It hath almost all the properties of Fixed Alkalis. This liquid is called Oil of Lime, for the same reason that deliquated Salt of Tartar is called Oil of Tartar.
Volatile Alkalis combined with Oily matters. A Volatile Oily Aromatic Salt.
Pulverise and mix together equal parts of Sal Ammoniac and Salt of Tartar: put the mixture into a glass or stone cucurbit: pour on it good Spirit of Wine, till it rise half an inch above the matter. Mix the whole with a wooden spatula; apply a head and a receiver, and distil in a sand-bath, gently heated, for two or three hours. A Volatile Salt will rise into the head; and then the Spirit of Wine will distil into the receiver, carrying with it a portion of the Volatile Salt.
When nothing more will come over, let your vessels cool; then unlute them, separate the Volatile Salt, and weigh it directly. Return it into a glass cucurbit, and for every ounce thereof add a dram and a half of Essential Oil, drawn from one or more sorts of aromatic plants. Stir the whole with a wooden spatula, that the Essence may incorporate thoroughly with the Volatile Salt. Cover the cucurbit with a head, fit on a receiver, and, having luted it exactly, distil in a sand-bath, as before, with a very gentle heat. All the Volatile Salt will rise, and stick to the head. Let the fire go out, and when the vessels are cooled take your Salt out of the head. It will have an odour compounded of its own[Pg 619] proper smell, and the smell of the Essence with which it is combined. This is an Aromatic Oily Salt. Put it into a bottle stopped close with a crystal stopple.
OBSERVATIONS.
The design of this operation is to incorporate and unite an Oil with a Volatile Alkali. Spirit of Wine is added in the distillation of the Volatile Salt, intended for this purpose, in order to prepare it for receiving the Oil, and combining more easily therewith. This Salt hath the property, as was shewn in the preceding operation, to carry up with it part of the substances with which it is distilled. On this occasion therefore, it is impregnated with a little of the Spirit of Wine; and this Spirit, which contains in itself an oily matter, and is the solvent of Oils, cannot fail to facilitate the union of the Oil with the Volatile Salt, as it serves for a medium between them. Yet it must not be considered as a necessary one. A Volatile Salt, sublimed with Salt of Tartar alone, would also very readily take up any Oil with which it should be distilled. We have seen that Volatile Alkalis are originally impregnated with much Oil, which is radically dissolved in them; and consequently they have a great affinity with that substance. So that if we distil them with Spirit of Wine, at the beginning of this operation, we do it not out of any necessity, but only with a view to accelerate or facilitate the intended union.
In this distillation the Volatile Alkali always rises first, and before the Spirit of Wine; which proves that it is much more volatile, though it be more ponderous than the Spirit.
If the Spirit of Wine used in this distillation be very aqueous, it will dissolve the Salt as it comes over, and will reduce it into a Spirit: but if, on the contrary, it be well dephlegmated, the Volatile Alkali will remain in a concrete form, and will not be dissolved in this first distillation.
If you desire to have the Volatile Salt entirely dissolved in the Spirit of Wine, though highly dephlegmated, it must be repeatedly distilled a great number of times with the same Spirit of Wine: for, though the small quantity of Spirit of Wine, with which it unites in the first distillation, be not capable of reducing it into a liquid, yet, as it takes up more and more every time it is distilled, it dissolves at last, and then with the Spirit of Wine forms a fluid that appears perfectly homogeneous. The Volatile Alkali is now rendered considerably milder by the union thus contracted, and is[Pg 620] accordingly called the Dulcified Volatile Spirit of Sal Ammoniac.
When well dephlegmated Spirit of Wine is mixed with a Volatile Spirit of Sal Ammoniac, perfectly saturated with Volatile Salt, these two liquors together immediately form a white opaque coagulum. But for this purpose you must not use a Volatile Spirit distilled with Lime; for then the experiment will not succeed.
This coagulum does not seem to be the effect of an intimate union between the two substances mixed together, like that which results from the union of a Fixed Alkali with an Oil. It hath just now been shewn that Spirit of Wine and a Volatile Alkali do not readily unite together. I believe the effect rather depends on this, that Spirit of Wine hath a greater affinity than the Volatile Salt with water; and therefore the Spirit, which ought to be perfectly dephlegmated, attracts the water wherein the Volatile Salt was dissolved, which thereupon recovers its concrete form; and being at that time mixed with the Spirit of Wine, it keeps that Spirit locked up among its parts, and hinders it from appearing with its natural fluidity.
What confirms this notion is, that the coagulum, which at first seems to make but one whole, soon separates into two parts, whereof one, which is solid, and nothing but the Volatile Salt concreted, lies at the bottom of the vessel; and the other, which is fluid, cannot be mistaken for any thing but the Spirit of Wine, which, being disengaged from the particles of Salt, recovers the form of a liquid, and, being the lightest, floats over the Salt. Yet these two substances, though now very distinct from each other, are not so pure as before they were mixed together. The Spirit of Wine hath dissolved a little of the Volatile Salt; and, on the other hand, the Volatile Salt retains a little of the Spirit of Wine. They may indeed be perfectly united and blended with each other, by the method above delivered; that is, by being frequently distilled and cohobated together, till they form one mixt; but then that mixt will be in a liquid form.
The first time this mixture is distilled, a great deal of Volatile Salt rises first, which is very fit to unite with an Essential Oil, and so to become a Volatile Oily Aromatic Salt.
THE END.
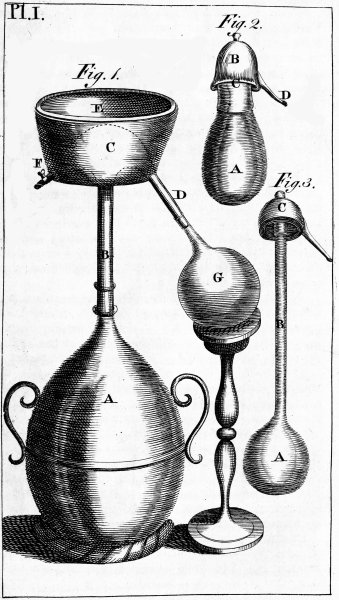
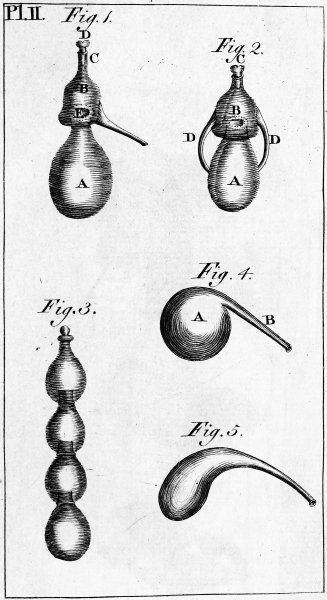
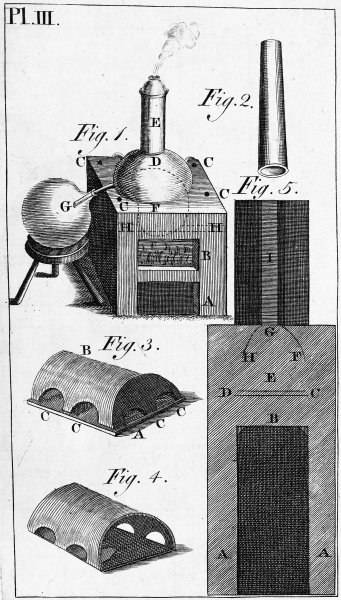
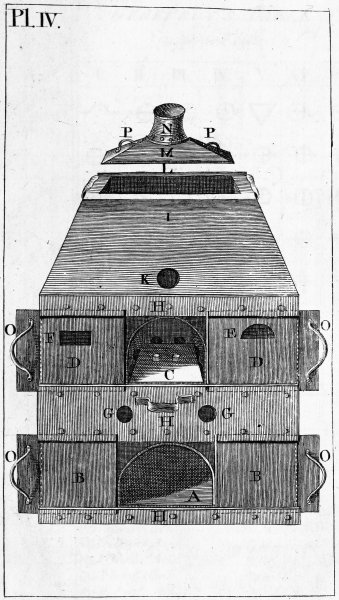
N. B. The Furnaces, as represented in the two last Plates, are not in due proportion to each other. The Cupelling Furnace is much larger than it should be, with respect to the Melting Furnace. These dimensions are here given it, only that all its parts might be more distinctly expressed, than could have been done if we had made it less.
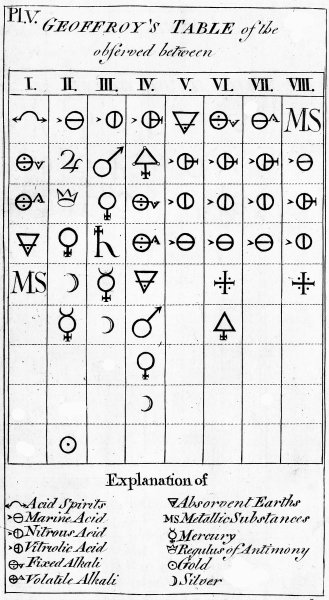
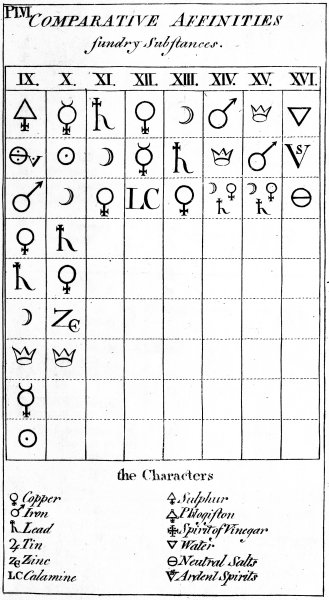
| GEOFFROY'S TABLE of the COMPARATIVE AFFINITIES | ||
|---|---|---|
| observed between sundry substances. | ||
| I. | II. | III. |
| Acid Spirits | Marine Acid | Nitrous Acid |
| Fixed Alkali | Tin | Iron |
| Volatile Alkali | Regulus of Antimony | Copper |
| Absorbent Earths | Copper | Lead |
| Metallic Substances | Silver | Mercury |
| Mercury | Silver | |
| Gold | ||
| IV. | V. | VI. |
| Vitriolic Acid | Absorbent Earths | Fixed Alkali |
| Phlogiston | Vitriolic Acid | Vitriolic Acid |
| Fixed Alkali | Nitrous Acid | Nitrous Acid |
| Volatile Alkali | Marine Acid | Marine Acid |
| Absorbent Earths | Spirit of Vinegar | |
| Iron | Sulphur | |
| Copper | ||
| Silver | ||
| VII. | VIII. | IX. |
| Volatile Alkali | Metallic Substances | Sulphur |
| Vitriolic Acid | Marine Acid | Fixed Alkali |
| Nitrous Acid | Vitriolic Acid | Iron |
| Marine Acid | Nitrous Acid | Copper |
| Lead | ||
| Silver | ||
| Regulus of Antimony | ||
| Mercury | ||
| Gold | ||
| X. | XI. | XII. |
| Mercury | Lead | Copper |
| Gold | Silver | Mercury |
| Silver | Copper | Calomine |
| Lead | ||
| Copper | ||
| Zinc | ||
| Regulus of Antimony | ||
| XIII. | XIV. | XV. |
| Silver | Iron | Regulus of Antimony |
| Lead | Regulus of Antimony | Iron |
| Copper | Silver, Copper, Lead | Silver, Copper, Lead |
| XVI. | ||
| Water | ||
| Ardent Spirits | ||
| Neutral Salts | ||
FINIS.
[1] Those who have the curiosity to see a more particular account of the Crystallization of Neutral Salts, may read Mr. Rouelle's excellent Memoir on that subject, among those of the Academy of Sciences for 1744.
[2] M. Malouin, however, hath found a way to unite these two metallic substances: but then he does it by the interposition of sulphur; that is, he combines crude Antimony with Mercury. This combination is brought about in the same way that Æthiops Mineral is made; viz. either by fusion, or by trituration only without fire. It resembles the common Æthiops, and M. Malouin calls it Æthiops of Antimony. He observed that Mercury unites with Antimony much more intimately, by melting, than by rubbing them together.
[3] See Mr. Duhamel's Essays on this subject in the Memoirs of the Academy of Sciences.
[4] Mr. Margraaf, an able German Chymist, has made several experiments, which induce him to think that the Acid of Phosphorus is of a particular kind, and different from that of sea-salt. May it not be the Marine Acid, but altered by the union it has contracted with the Phlogiston? Or may it not be, with respect to Phosphorus, what the volatile sulphureous spirit is, with respect to Sulphur? See the Memoirs of the Royal Academy of Sciences of Berlin.
[5] They are much the best, and bear a very fierce heat.
[6] The Marquis de Montalembert, in a Memoir read before the Academy of Sciences, proposes a new method of effecting these evaporations, together with some considerable improvements in the structure and disposition of the buildings necessary for that purpose. They are called by the French Batiments de Graduation; which may properly enough be rendered Brine-houses.
[7] See the Table of Affinities, Column IV.
[8] M. Réaumur hath obliged the public with a treatise on the means of converting Iron into Steel, in which he hath exhausted the subject. Such as desire the amplest and most useful instructions on that part of metallurgy, would do well to consult his Work.
[9] See the Memoirs of the Academy for 1730.
[10] See the Memoir given in by me on this subject to the Academy of Sciences in the Memoires l'Acadamie 1754.
[11] I believe this proposition is not strictly true: for it appears to me, that, in order to make the heat, produced by the simultaneous frictions of an hundred particles, an hundred times more active than that produced by the successive frictions of the same number of particles, it is necessary that the simultaneous frictions should act all together in one point or center; which is impossible. But, as the particles that rub against each other, in the present case, are very near and contiguous, it is still true that the heat, resulting from their simultaneous frictions, is much more active than that produced by successive frictions only: which is sufficient for our present purpose.
[12] These white vapours do not appear when the vessels are perfectly close. Mr. Hellot, to whom we owe the remark, having performed this operation in a crystal retort procured from London, the neck of which had been rubbed with emery in the mouth of its receiver, so that these two vessels fitted each other exactly, saw the ætherial liquor distil pretty fast, but without white vapours. He then loosened the receiver, by turning it a little upon the neck of the retort, so that the external air might get in; whereupon the white vapours appeared immediately. When the receiver was close fitted on again, the vapours disappeared. He repeated the same thing five times from half hour to half hour, and these vapours as often appeared and disappeared.
[13] Mr. Eadows, in a little English book, entitled The Modern Apothecary.
[14] It could not be any longer concealed; for M. Geoffroy having made some experiments on the same subject, without knowing any thing of what M. Boulduc had done, likewise discovered it. See the History of the Academy for 1731, p. 35.
[15] Memoirs of the Academy for 1734, p. 421.
[16] Memoirs of the Academy for 1729.
[17] Mr. Rouelle, whom I have had occasion to mention several times in this work with the honour which he deserves, and with whom I went through a course of Chymistry, when I was a Student in Medicine. It must be observed, to the praise of this ingenious Artist, that he is the first Frenchman that ever gave Courses of Chymistry. In these he explains the operations according to the true and sound Theory of the Science, drawn from the writings of Beccher, Stahl, Juncker, Boyle, Boerhaave, Hoffman, and many other excellent Chymists, whom it would be tedious to mention here, as well as from the Memoirs of the most celebrated Academies, particularly those of the Academy of Sciences at Paris.
[18] Memoirs of the Academy of Sciences for 1712.
Transcriber's Notes
Obvious typographical errors, including missing punctuation have been corrected and hyphenation has been standardised, but variations in spelling in the original have been retained.
Chap. II., Part II., Section I. is wrongly headed Chap. I. in the text. This has been corrected.
A reference to Mr. Fifes, on page 518, could possibly be Mr. Fises.
On page 547 "fit for the use of Surgeons, who apply it to eat away callosities and excrescences, and to open issues." issues has been changed to tissues.
Colophony, 462 has been placed in correct alphabetical order in the index.
Illustrations and Index have been added to the Table of Contents.
In the original the final section "Explanation of the Plates" was a separate section following the plates. The individual explanations have been moved so that they are immediately below the relevant plate.
A text version of Geoffroy's Table has been appended as an alternative to the illustration.