
Title: Technique of Eye Dissections
Author: Frederic A. Woll
Release date: July 2, 2020 [eBook #62544]
Most recently updated: October 18, 2024
Language: English
Credits: Produced by deaurider, Harry Lame and the Online Distributed
Proofreading Team at https://www.pgdp.net (This file was
produced from images generously made available by The
Internet Archive)
Please see the Transcriber’s Notes at the end of this text.

—Wordsworth.
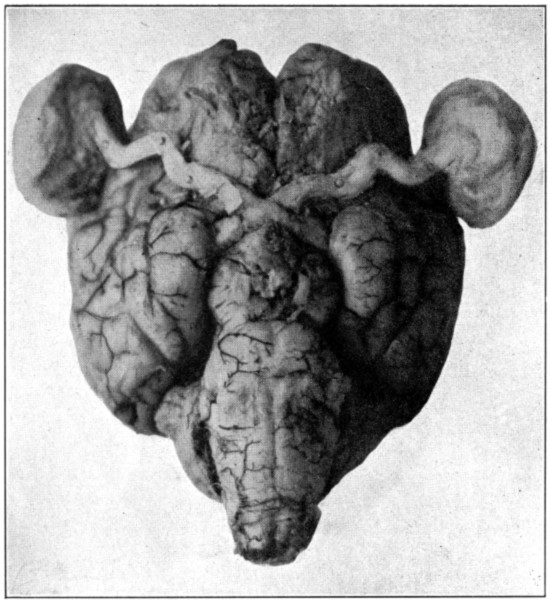
Brain, showing eyes with muscles removed, optic nerves, and chiasm.
BY
FREDERIC A. WOLL, Ph.D.,
Associate Professor, Department of Hygiene, College of the City of New York; Optometry Courses, Columbia University; Member of New York State Board of Examiners in Optometry; Honorary Member: American Optometric Association; State Societies—Alabama, California, Connecticut, Kentucky, Maine, Massachusetts, North Carolina, Rhode Island; Local Societies—Lehigh Valley Society of Optometrists, Mahoning Valley Optometrists’ Society, and Optometrists’ Club of Brooklyn.
SECOND EDITION

NEW YORK
FREDERIC A. WOLL
1924
Copyright, 1914, by
FREDERIC A. WOLL
Printed in the United States of America
First Edition, July, 1914
Second Edition, April, 1924
SCHLUETER PRINTING CO., NEW YORK
DEDICATION
THIS LITTLE BOOK IS DEDICATED WITH AFFECTION
AND ESTEEM TO MY FIRST TEACHER
IN OPTICS, MY FRIEND FOR YEARS
ANDREW JAY CROSS
WHOSE RESEARCH IN THE FIELD OF APPLIED
OPTICS HAS WON FOR HIM RECOGNITION
AND HONOR
[9]
| PAGE | |
|---|---|
| Preface | 13 |
| Introduction | 17 |
| Removal of Hyaloid Membrane with Contents and Attachments Intact | 25 |
| Canal of Petit, The | 35 |
| Interior of the Eye, The | 38 |
| Posterior Half, The | 40 |
| Optic Nerve, The | 47 |
| Anterior Half, The | 49 |
| Iris, The | 51 |
| Cornea, The | 52 |
| Crystalline Lens, The | 53 |
| Choroid, The | 62 |
| Retina, The | 74 |
| Sagittal or Vertical Section of the Eye, The | 86 |
| Papilla, Puncta Lacrimalia, and Nasal Duct, The | 92 |
| Meibomian Glands and Ducts, The | 96 |
| Enucleation of the Orbital Contents, The | 97 |
| Ophthalmoscopic Examinations | 106 |
| Lacrimal Ducts, The | 112 |
| Lacrimal Gland, The | 114 |
| Capsule of Tenon, The | 115 |
| Superior Oblique Muscle and its Pulley | 117 |
| Other Extrinsic Muscles, The | 118 |
| Three Tunics of the Eye, the Hyaloid and its Attachments, The | 120 |
| PAGE | ||
|---|---|---|
| 0. | Brain showing eyes with muscles removed, optic nerves, and chiasm | Frontispiece |
| 1. | Glassware and tools | 22 |
| 2. | The first cut | 28 |
| 3. | How the point of the scissors is kept away from the underlying tissues | 29 |
| 4. | Half of the sclerotic separated | 30 |
| 5. | Picking up the choroid | 31 |
| 6. | Emptying the eye of its contents | 32 |
| 7. | Isolated hyaloid, contents and attachments intact | 33 |
| 8. | Petit’s Canal | 36 |
| 9. | Cutting eye into anterior and posterior sections with safety-razor blade | 39 |
| 10. | Posterior half showing retinal vessels and choroid | 40 |
| 11. | Showing network of vitreous | 42 |
| 12. | Tearing retina away from posterior half of eye | 44 |
| 13. | Posterior half of eye with retina removed | 45 |
| 14. | Excavated posterior half of eye | 46 |
| 15. | Split optic nerve | 47 |
| 16. | Ciliary processes and the lens | 48 |
| 17. | How to pull off vitreous | 50 |
| 18. | Processus Zonuloe | 54 |
| 19. | Onion-like layers of lens removed | 56 |
| 20. | Cross section of lens | 57 |
| 21. | Lenses showing the results of different kinds of treatment | 60 |
| 22. | Puncturing the cornea | 63 |
| 23. | Removing the cornea | 64 |
| 24. | How to separate the choroid from the sclerotic | 65 |
| 25. | Cutting away the separated sclerotic | 67 |
| 26. | Scraping the choroid free from the sclerotic | 68 |
| 27. | The isolated choroid | 69 |
| 28. | Inserting scalpel to loosen lens and cut through vitreous | 70 |
| 29. | Taking out lens and “core” of vitreous | 71 |
| 30. | Squeezing out remaining part of vitreous | 72 |
| 31. | Cutting through the iris | 75 |
| 32. | Cutting around the ciliary ring | 76 |
| 33. | Lens, iris, and part of vitreous removed[12] | 77 |
| 34. | How to force blowpipe into the vitreous | 78 |
| 35. | Bulging out of vitreous caused by blowing air through glass blowpipe | 79 |
| 36. | Showing vitreous removed | 80 |
| 37. | Folding the retina by blowing air at it through blowpipe | 81 |
| 38. | Suspended retina. Sclerotic ready to be cut away | 82 |
| 39. | Showing sclerotic nearly all cut away | 83 |
| 40. | Isolated retina | 84 |
| 41. | The beginning of the cutting of the eye for sagittal sections | 87 |
| 42. | Method of cutting through the crystalline lens | 88 |
| 43. | Cutting through cornea to complete the sagittal sections | 89 |
| 44. | Sagittal section enlarged | 90 |
| 45. | Part of calf’s head showing knitting needles inserted in puncta | 93 |
| 46. | Course of knitting needles showing the course of the canaliculi | 94 |
| 47. | Initial cuts to be made in the skin | 98 |
| 48. | First cut in bones of orbit | 99 |
| 49. | All the cuts to be made in bones of orbit | 100 |
| 50. | How to pry bone loose | 101 |
| 51. | Dissecting close to bones of orbit | 102 |
| 52. | Excavated orbit | 103 |
| 53. | Anterior view of enucleated eye | 104 |
| 54. | Side view of enucleated eye. All parts in situ | 104 |
| 55. | Enlarging pupil for ophthalmoscopic inspection | 107 |
| 56. | How to get rid of the pucker in the cornea | 108 |
| 57. | Window cut in the eye | 110 |
| 58. | Pins inserted in lacrimal ducts | 112 |
| 59. | Capsule of Tenon blown up | 115 |
| 60. | Showing the extrinsic muscles of the eye | 118 |
| 61. | Cutting through the iris | 120 |
| 62. | Scraping ciliary processes free. Choroid cut around ciliary ring | 121 |
| 63. | Cutting away choroid | 122 |
| 64. | Three tunics, hyaloid, and lens | 123 |
[13]
The aim of this booklet is to present to the eye-specialist, the teacher, the student, and others interested in the study of the anatomy and physiology of the eye, some definite methods to follow in the dissection of that organ.
Most dissections of the eye are not made with the same degree of care and skill used in the dissections of other organs. In following the usual method of dissecting eyes, much of the important detail is lost. Often certain membranes are confounded with others, and wrongly demonstrated. Furthermore, an eye is merely divided by some demonstrators into an anterior and a posterior half, a very short time is spent by the students scrutinizing each half; then the text-book is turned to, and the anatomy is studied descriptively.
Not enough time has been given to thoroughly dissecting all parts of the eye. As much time should be given as is necessary to bring[14] out prominently all its parts. Other organs of the body are more thoroughly dissected, and, therefore, the student has better opportunity to gain a clearer comprehension and better understanding of the anatomy and physiology of those organs. Also, as much time should be given, proportionately, to the learning of the technique of the dissection of the eye as is given to the learning of the technique of dissecting other organs of the body.
Many now make a direct specialty of ministering to those suffering from errors of ocular refraction, ocular diseases, and ocular reflexes, and for those specialists, principally, this book is written. It is to fulfil its mission to them by acting as a guide and as a complement to the descriptive matter in the text-book. It is sent forth in the hope that it will tend to create more interest in the study of the practical anatomy of the eye. It is written with a desire to stimulate the ability to make careful and intelligent observation. It carries with it, as a final end, an earnest wish that it may, in some small way, be the means of opening up[15] to the original researcher, a larger field for the further study of the most important of the senses—the eyesight.
Most of the dissections explained in the following pages are original; some, however, are only revisions of old methods.
This opportunity is taken to acknowledge the many helpful suggestions that were made by Dr. Ivin Sickels, of the College of the City of New York, and by the late Dr. Edward C. Spitzka, of New York. Thanks are due Mr. E. F. Howes, of Messrs. Swift & Co., for furnishing the necessary supply of beef eyes; to Messrs. Lee & Beach, photographers, of New York, for their painstaking efforts in producing good photographs of the actual dissections; and to Schlueter Printing Company, of New York, for their many courtesies and interest in the production of the book.
Frederic A. Woll.
New York, July 21, 1914.
In eye dissections it is unnecessary to have either a large equipment of instruments or a special room. To have a laboratory at one’s disposal is but a small added convenience. Not to have it, is no serious hindrance. The work may be carried on and successfully done in one’s office or in the home, as well as in class-room or laboratory. If it is true that the atmosphere of a laboratory adds zeal to the efforts of a worker, but there is no laboratory available, then reverse the order; let the zeal of the worker add to the atmosphere of the place in which he is doing his work.
Two things, among others to be mentioned later, are essential; a table of convenient height, and a good light, natural or artificial. Both are but modest needs. Compared with other dissections, there will be found an absence of offensive odors. Neither are there any repulsive sensations experienced. Such experiences are quite common when making[18] other kinds of dissections. This work is clean and attractive. Indeed, one may even develop a rather keen sense of the æsthetic. Many of the various parts of the eye, when separated and properly preserved, then viewed and inspected, are bound to bring forth exclamations of appreciation and wonderment. One can then better understand the statement: “When Nature perfected the first eye she took a day off so she could admire the result of her finest piece of handiwork.”
This does not imply that dissections of the eye tend to develop art appreciation. Appreciation of the wonderful in Nature’s construction of the special organs is not, however, to be relegated to a distant point. Such appreciation is concomitant with the knowledge that comes from having seen, handled, and examined the object studied.
The orchestra leader must have a good listening and hearing ear. This is developed in him because he has to exercise constantly his power of listening and hearing. The dissector who would become proficient in eye dissections[19] and in anatomical investigations must have a good seeing eye and a dextrous hand. To acquire these two most valuable aids necessary to carry on careful inquiry or research, it is essential to practise using the eye and the hand. Combined with the expertness of these two, must be the ability to continue one’s efforts in the face of failure; to redouble one’s efforts to attain success despite the shortcomings of eye or hand. This simply means practise and patience. And the one who is without that wonderful virtue, patience, will never stay long enough with his problem to gain either an observing eye or an expert hand, or to achieve his end, and thereby reap the full and pleasing results of his efforts. In order of importance, patience really precedes dexterity, skill, and observation; and persistence of effort is a factor not to be entirely outshone by any other virtue. With these attributes, knowledge of the subject in hand naturally follows.
One reason why eye dissections are easily carried on is because material can always be[20] readily procured. Any butcher will furnish sheep, pig, or beef eyes. Or, if one has the time to visit the manager of a slaughter-house, and make known to him one’s needs, he will supply enough eyes to carry through a host of interesting dissections and experiments, and give sufficient material for careful, orderly, and fruitful study. Perhaps in no other kinds of dissections will the investigator find so much of interest, or have his efforts crowned with such abundant and satisfactory results, as in the dissections of the eye. But no one should try to study all parts of the eye with only one specimen. To try to do so is an error, and a common one often committed by both teachers and students. Specimens cost little or nothing, and it is no more trouble to prepare a half-dozen eyes for dissection than one. The cost of preparation, too, is but little more for a number of eyes than it is for one, and may be no more in some instances. Besides, having enough material on hand saves time in case of a failure. Also, one can quickly repeat a dissection, and so procure any number of[21] desired specimens of specific parts, or do over again the same dissection on another eye just for the purpose of practise, and thus add to one’s dexterity. It is, therefore, strongly advocated to have plenty of material on hand before beginning work. Economy here is not even “penny wise.”
The tools, or instruments, needed are but few in number; an ordinary scalpel, a pair of blunt tweezers, or forceps, as they are sometimes called; a pair of sharp-pointed tweezers, a pair of small, sharp-pointed scissors of about three to three and a half inches in length, and a pair of large scissors, about four or five inches in length, having one jaw sharp-pointed and one jaw blunt.
For glassware, any wide-mouthed jar or bottle, such as the ordinary fruit or jelly jar, will do for preparing material. For clarifying tissues, or for preserving and keeping them, small, wide-mouthed bottles or vials should be used. And for temporary keeping, or for purposes of “running through” various fluids, the regular Stender dishes are most convenient.[22] A glass graduate is almost indispensable if accuracy in measuring fluids is desired. (Fig. 1.)

Fig. 1.
The chemicals needed are few in number and small in quantity: Alcohols in varying strength, which can be made by diluting a 95 per cent. alcohol, and keeping an absolute alcohol on hand. A few ounces of formaldehyde will make enough solutions of different strengths to be sufficient for the preparation and keeping of many specimens. Of other chemicals, such as xylol and cedar oil, only small quantities are needed; enough to cover[23] a specimen. Fifteen to twenty cents worth of each will be an ample supply to keep on hand. All of these may be procured at any large drug store, and are the only chemicals required for doing the dissections as explained in this book.
Before further advance is made, it will be best to state that this work deals only with methods for dissecting the various parts of an eye, and is primarily intended to aid in the study of the anatomy and physiology of the eye by being used in conjunction with such books as “Gray’s Anatomy,” “A Text-Book of Physiology,” by Howell, “The Anatomy and the Physiology of the Eye,” by Brown and Zoethout, and similar other works of authority. However, if it is desired to acquire only specimens, then, of course, no other works are necessary, and the matter contained herein is sufficient to enable one to procure just what is wanted.
It is also wise to state here that since human eyes are hard to procure, and not available in large quantities, one must resort to the[24] use of the eyes of animals, which are procurable in large quantities, and which may be used without “feelings” in the matter. Though there is a difference between the eyes of human beings and the eyes of other animals, the difference is slight and of minor importance when compared with the similarity of the more important parts.
[25]
TECHNIQUE
OF EYE DISSECTIONS
One of the easiest and most satisfactory dissections to attempt is the isolating of the hyaloid membrane with its contents and its attachments. The success one meets with in making this dissection will surely prove a strong incentive for making all the rest. For these reasons this has been placed first in this arrangement.
In eye dissections it is quite customary, in giving directions for dissections, merely to[26] mention the hyaloid membrane and its relations with other parts of the eye. Rarely is there any attempt made to isolate it. Often, too, the retina is mistaken for the hyaloid, and the retina then wrongly demonstrated as being attached to the choroid. Of course, it is impossible to separate the hyaloid from the vitreous; but a dissection can be made which, when placed in a glass of some kind, will show the hyaloid. If the following simple technique is carefully observed, the membrane, with all its connections, can be easily separated from certain other parts of the eye. Opportunity for thorough study and observation will then be made extremely easy.
Procure the eye of either a sheep or a bullock. Instead of following the usual procedure of hardening in any one of the several solutions used for the purpose of toughening the ocular tissues, place the eye in a cool place and permit it to collapse a trifle. Usually two or three days is a sufficient length of time to accomplish the result.
Experiments have shown that if an eye is[27] too fresh the ciliary processes will not be easily detached from the hyaloid (zonular processes), and if the eye has been in a preserving fluid, the same result will follow. A sheep’s eye will make a better specimen even if it is small, because the ciliary processes are more easily separated from the zonular processes. If a bullock’s eye is used, it must be left in a cool place a day or two longer than in the case of a sheep’s eye, in order to permit a long enough time to elapse to allow disintegration of the eye to take place sufficient to have the two processes separate easily and cleanly.
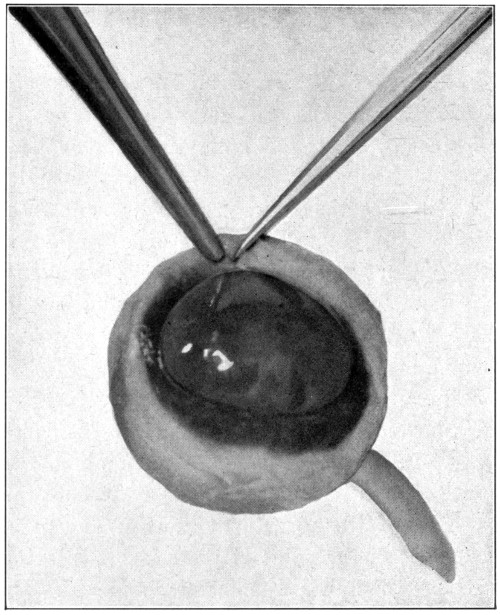
Fig. 2—Making the first cut. (Page 27.)
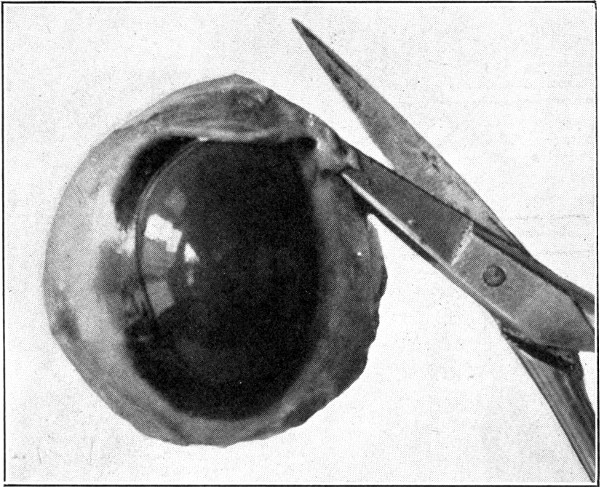
Fig. 3—Showing how the point of the lower jaw of the scissors is to be kept away from the underlying tissues. (Page 27.)
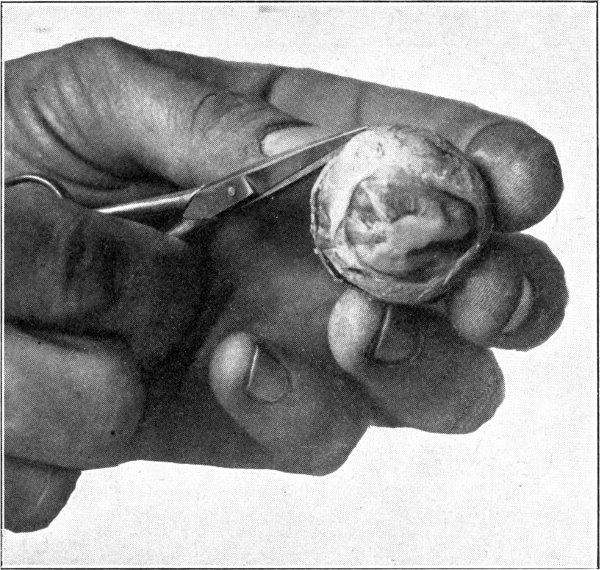
Fig. 4—Showing about half of the sclerotic separated. (Page 29.)
With a pair of dissecting forceps pinch up
the sclerotic about 5 mm. anterior to the equator.
With a pair of small, fine-pointed scissors,
make an incision. (Fig. 2.) Next hold the
eye in the left hand without exerting any pressure.
Insert the point of the scissors into the
incision which has been made, and cut. Be
careful to keep the point of the scissors close
to the sclerotic or an untimely puncturing of
the choroid will occur. (Fig. 3.) Continue
the[28-
29]
cutting on a line parallel to the equatorial meridian
and about 5 mm. anterior to it until
about half the sclerotic has been separated.
In cutting, always move the point of the scissors
forward with a slight oscillating lateral
movement. (Fig. 4.) While doing this, partly
suspend the eyeball from the point of the scissors.
Doing these things will tend to loosen[30]
the choroid from the sclerotic and prevent
puncturing too soon the former mentioned
membrane. Now apply pressure in such a
manner that the lips of the cut sclerotic will
gap. Into this put the point of the scissors
and very carefully pick up the choroid and the
retina with the point of the scissors and cut[31]
them. (Fig. 5.) If the choroid alone has been
picked up and separated, the retina will show
milky white or yellowish white underneath.
The retina must then also be separated. Care
must be taken not to go deeper than the retina
or the hyaloid may be damaged. Continue the
cutting of the choroid and the retina for a
distance of about 20 mm. Apply enough pressure
occasionally so that the vitreous will be[32]
forced upward and above the cut choroid and
the retina. This will show whether any
strands of the two membranes have been left
uncut. If the separation is complete for the
distance specified above, invert the eyeball,
squeeze and shake gently over some receptacle,
such as a Stender dish, three-fourths filled[33]
with a 2½ or 5 per cent. solution of formaldehyde,
and the hyaloid membrane containing
the vitreous, its attachments, suspensory ligament
to the lens capsule, and lens, will drop
out intact, as when one empties the contents
of an egg. (Fig. 6.)
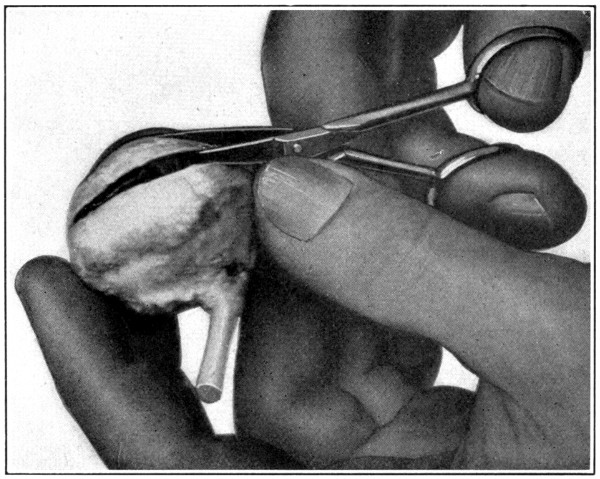
Fig. 5—Picking up the choroid with the point of the scissors.
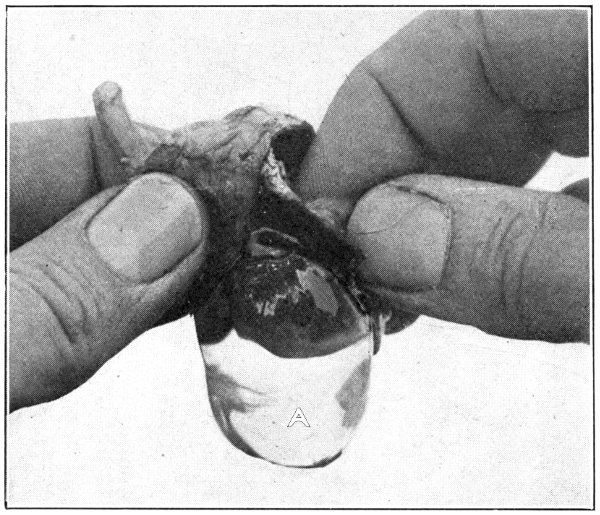
Fig. 6—A. Hyaloid, vitreous, and lens ready to drop out of the eyeball.
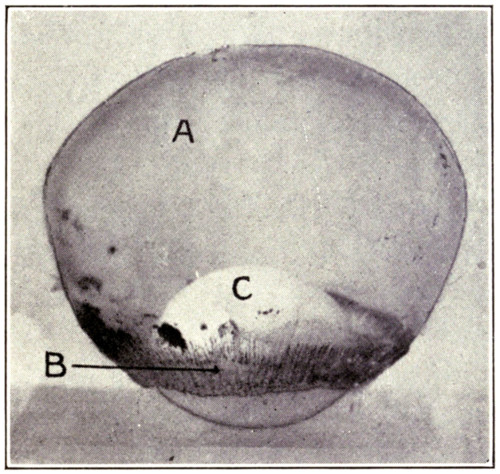
Fig. 7—Photograph of dissected hyaloid membrane (A), with pigmented indentations of the ciliary processes, the suspensory ligament (B), and the crystalline lens in its capsule (C). (Page 34.)
Many times a considerable amount of pigment from the second tunic will remain attached[34] to the processus zonuloe. This pigment may be easily removed by scraping it off with the sharp edge of the scalpel or by brushing it off with a soft, wet tooth-brush.
It is unwise to use alcohol as a preservative because it produces an almost immediate opaqueness and hardness which spoils the specimen for further study.
This description may give the impression that the dissection is a lengthy one; however, it can be done by an expert in two or three minutes; by a beginner in five or six minutes.
For purposes of demonstration or study the specimen should be placed in a small bottle or a vial containing a 5 per cent. solution of formaldehyde. It can then be examined with hand lens or microscope. (Fig. 7.)
[1] Approved as an original article in The Anatomical Record, September, 1912.
[2] This dissection, and several of the following, appeared in The Optical Journal and Review, beginning with the issue of January 16, 1913.
[35]
The canal of Petit is a “triangular space around the circumference of the lens.” That it can be “inflated through a fine blowpipe inserted through the suspensory ligament,” is the usual direction given. However, the ordinary “fine blowpipe” is much too large and too dull to be inserted through the suspensory ligament. Take a long medicine-dropper (5 or 10 cents at a drug store), or a pipette, and heat it until it is red hot over an alcohol lamp or a Bunsen burner; hold one end with one hand and the other end with a pair of tweezers. As the glass becomes white hot pull the tube apart. This will leave the places of separation pointed and sharp-edged. Use the larger of the two pieces. Sometimes the point or tapering end of the tube is too long and the bore too small. All that is necessary is to first mark off with a file the length to be broken off, and then that length may be snapped[36] off, leaving a sharp-edged, tapering point.
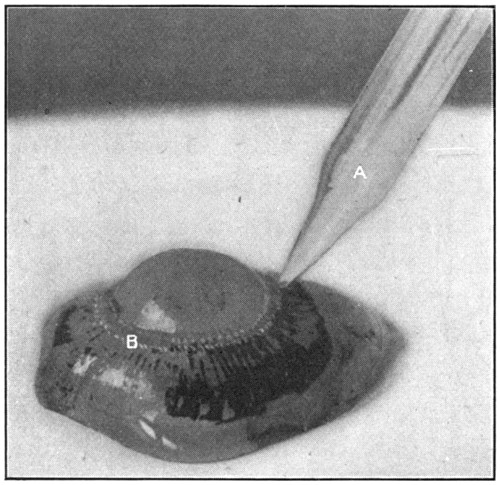
Fig. 8—A. Glass blowpipe. B. Petit’s canal.
After having completed the first dissection (the hyaloid, contents and attachments), and the specimen has been in the formaldehyde solution for ten days or two weeks, it will have become hard and tough enough to stand a considerable amount of rough handling. If the specimen has been kept in a large receptacle, such, for instance, as a jar, remove it with[37] a spoon; if in a small jar or vial, empty out the fluid, then slide the specimen out on whatever has been prepared to receive it. Turn it so that the lens will be uppermost. Find the suspensory ligament in the Zone of Zinn. Insert the pointed end of the glass tube, close to the lens, and blow gently until the canal shows its sacculated construction by filling with air, giving the appearance of a lot of little bubbles surrounding the periphery of the lens. (Fig. 8.) It may be necessary to move the blowpipe in and out in order to find the canal, all the while blowing steadily through the tube.
[38]
For the study of the interior of the eye and its contents in situ either a fresh or a hardened eye will do; a hardened eye is preferable. In the dissection for isolating the hyaloid membrane, vitreous, lens, and other parts, the anterior and posterior halves of the evacuated eye may be separated entirely, and each half studied. However, the choroid and the retina will be more or less mutilated, and the vitreous and other parts will be removed. The absence of these parts will prevent one from receiving a definite idea of their anatomical relationships. Therefore, it is better to work with an entire and complete eye.
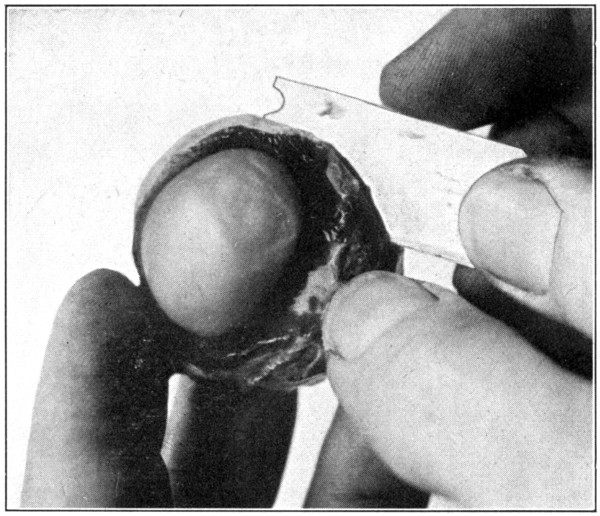
Fig. 9—Showing method of cutting eye into anterior and posterior sections with safety-razor blade.
Remove all the muscles and fatty tissues from the outside of the globe; then cut it in half through the equator, thus dividing it into an anterior and a posterior half. The cutting of the sclerotic, as well as the underlying tissues and the vitreous, should be done with the[39] large scissors; using a knife or scalpel will tend to disturb the positions of those tissues or so tear them that they will not be of much use for purposes of study. An ordinary safety-razor blade makes an excellent instrument for separating the eye into two halves, because it cuts through the tissues without tearing them in any way. (Fig. 9.) The rather dark colored,[40] viscid fluid that escapes when the eye is halved is the perichoroidal lymph, not the aqueous, as is sometimes stated.
The posterior half is taken first because it is the simplest and easiest of the two halves to dissect. In this half of the eye the retina may be readily seen through the vitreous; the choroid and its apparent iridescent colors through both vitreous and retina. (Fig. 10.)[41] Remove the vitreous by simply tilting this half of the eye, and with the finger push out the vitreous.
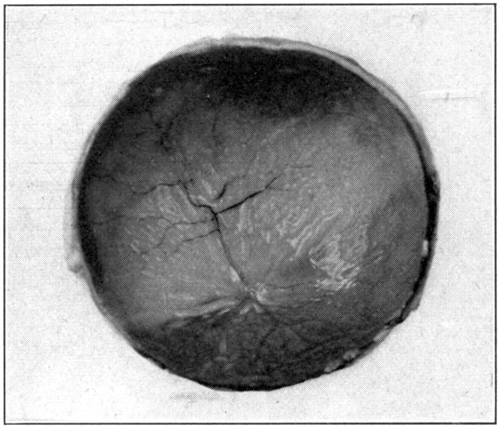
Fig. 10—The retina, retinal vessels, and iridescent choroid showing through the vitreous.
Sometimes the vitreous will adhere very closely to the retina. This occurs especially when the eye has been in formaldehyde for a long time. In such a case the removal of the vitreous without injuring the retina requires patience and care. The use of the scalpel and the scissors may become necessary. Another very good way to remove the vitreous is to take hold of the sclerotic, turn it so that the vitreous is downward, and then shake gently until the vitreous separates itself from the retina and, drops out. After the vitreous has been removed, notice its glassy appearance; hence its name—hyaloid body. Try to pull it apart with the fingers, and it will be noticed that it seems to be held together by more or less of a network of fibres. (Fig. 11.)
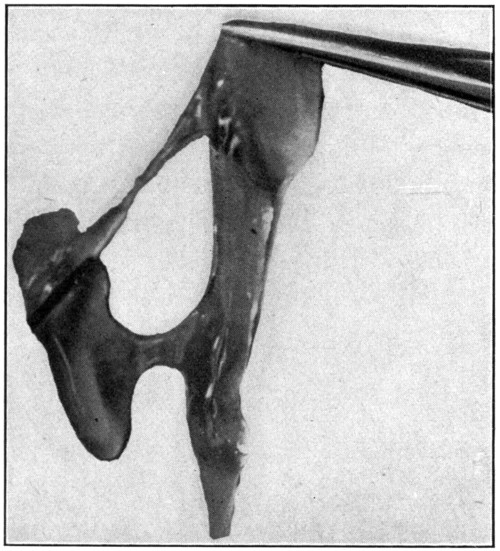
Fig. 11—Showing how vitreous seems to be held together by a network of fibres. (Page 41.)
Whichever method for removing the vitreous is followed, the retina will be left rather badly wrinkled and out of place. If the last-mentioned method, which is really the best of the[42] three described, is the one adopted, the retina will be left in an entirely collapsed and folded form. In any case, to straighten out the retina against the choroid, immerse the whole posterior half in water, inside uppermost. The retina will then slowly unfold itself and lie flat against the choroid. With the tweezers remove[43] the whole half from the water; tilting it slowly to empty it of all the water, and, having done so, turn it down upon the table rather forcibly in order to help it drain itself of all the water.
Notice the thinness of the retina, and, also, that the seeming iridescence of the choroid shows through. The optic disc, which is the point of entrance of the optic nerve, and the optic cup are easily recognized, though neither will be seen as large as when viewed in the living eye with an ophthalmoscope. The blood vessels of the retina, as they ramify outward or forward, after their entrance through the optic nerve through which they pass, are also very plainly seen. A closer inspection will show, in the very centre of the “entrance” of the optic nerve, a whitish, pointed vessel, about 1 or 2 mm. long. That is the sloughed-off and atrophied end of the hyaloid artery, which, when the eye was in an embryonic state, ran forward from the central artery of the retina through the hyaloid canal to the posterior surface of the[44] lens. With the forceps pick up the peripheral edge of the retina, and, by pulling gently upward, tear it away from its apparent place of attachment to the “entrance” of the optic nerve. (Fig. 12.) When this has been done, there will be seen some threads protruding[45] from the optic nerve. Filling the half with water will tend to separate these strands, which are optic-nerve elements.

Fig. 12—Picking up the retina in order to tear it away from the entrance of the optic nerve.
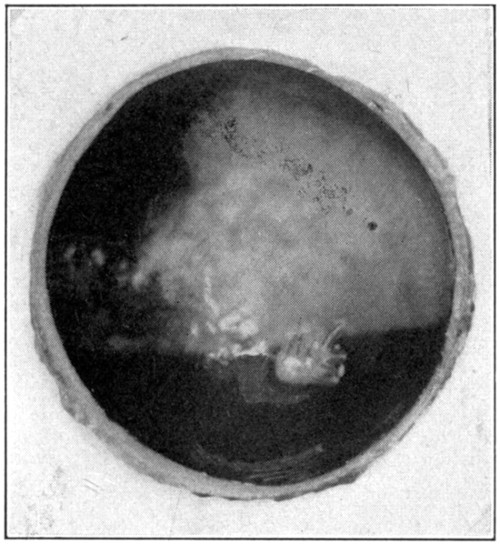
Fig. 13—The lighter area is the field of iridescence of the choroid.
After the removal of the retina, the iridescence of the choroid (tapetum lucidum) (Fig. 13) may be examined with a hand lens, or, after its removal, a piece may be cut and placed under a microscope. This iridescence is, of course, not present in the human[46] eye. (“Physiology of the Senses,” McKendrick & Snodgrass, page 101.)
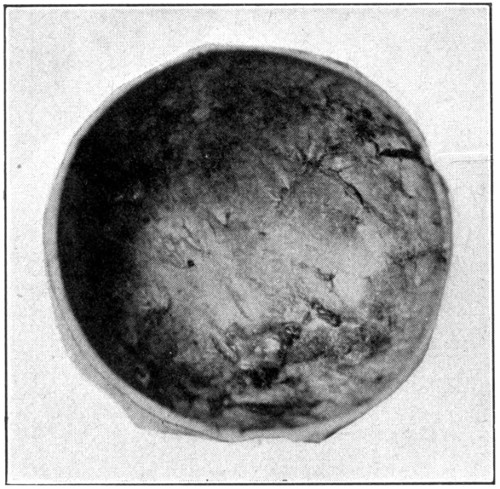
Fig. 14—Excavated posterior half of the sclerotic.
After the choroid is removed, which is accomplished in the same manner that the retina is removed, the inner side of the sclerotic is laid bare to view. The brownish color is mostly due to the presence of a small amount of pigment in the cells of one of the inner layers, it is also due, to a slight extent, to the staining influence of the perichoroidal fluid. (Fig. 14.)
[47]
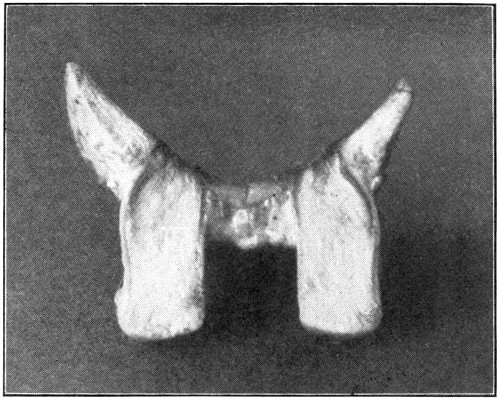
Fig. 15—Enlarged to show the entrance of the optic nerve. (Page 48.)
The excavated posterior half may be used now to show and to study the construction of the optic nerve. In cutting the optic nerve away from the sclerotic leave at least 5 mm. of the sclerotic attached. It will make handling easier. With the thumb and forefinger of the left hand hold the nerve in such a way on the table that it will be straightened out lengthwise, and then, using the scalpel or a safety-razor[48] blade, the latter being preferable, cut the nerve in two longitudinally. (Fig. 15.) The cutting must be done with one movement, otherwise the nerve will be hacked, and will not make a good specimen. This specimen will show the way the nerve fibers are arranged. A cross section should be cut from the optic nerve of another eye, and then the two sections should be compared. The cross section will show the sheath of the nerve a[49] little better than will the longitudinal section.
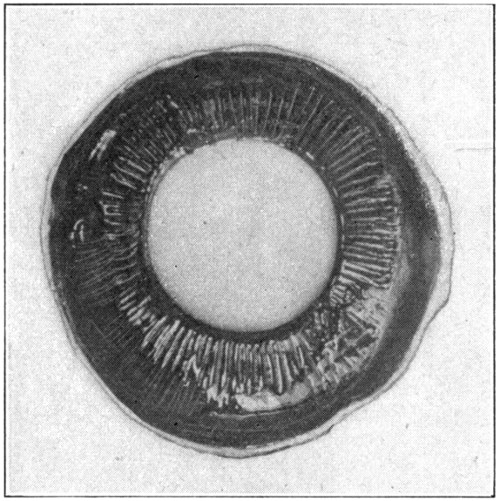
Fig. 16—Showing ciliary processes and crystalline lens.
In cutting the longitudinal section, one is sometimes so fortunate as to cut through the central blood vessels of the retina. These vessels will show up then as a rather thin dark streak about 5 or 6 mm. long.
The anterior half will show the lens in situ, the ciliary processes, the posterior aspects of the iris and the lens, the corona ciliaris, the orbicularis ciliaris, and the ora serrata. (Fig. 16.) If the eye has been cut in two too far forward of its equator, the ora serrata will not be present. The ciliary processes and posterior aspect of the lens may be seen to better advantage when the anterior half of the vitreous is removed. This is done with the dull-pointed tweezers, by catching hold of the vitreous at any part of its free or cut margin, and stripping it off both the ciliary processes and the lens, using a prying, pulling movement to do so. (Fig. 17.) The two layers of the pigment cells, pars ciliaris retinae, which cover[50] the inner surface of the processes, may be removed by picking them away carefully with the tweezers. The processes then will be seen to be a whitish color. The pupillary edge of the iris rests upon the capsule of the lens, but the nearer the approach is to the choroidal edge the farther the iris is from the lens; thus are formed the anterior and the posterior chambers of the eye. The dissection of the sagittal section of the eye, explained further[51] on, will show these two chambers in section. One will gain a much clearer conception of their construction in that section than in the “anterior half” specimen.
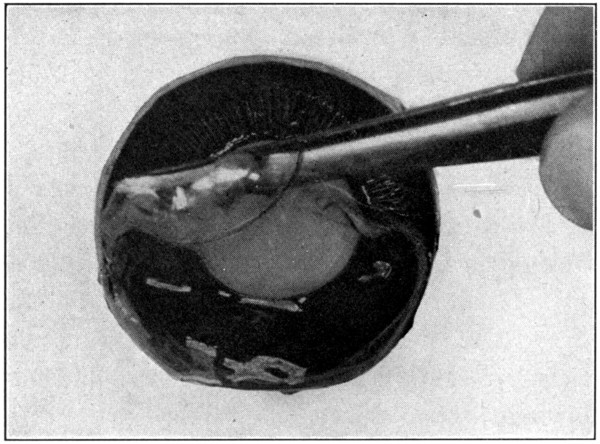
Fig. 17—Anterior half, showing how to pull off vitreous. (Page 49.)
Now, remove the lens, using the point of the scalpel to cut through the suspensory ligament close to the lens. When this has been done there will be seen in the anterior chamber a thin, watery liquid—the aqueous humor.
The corona ciliaris and orbicularis ciliaris may be better seen and studied if viewed through a hand lens.
To see the iris, take hold of the cut edge of the choroid, and, gently pulling, separate it from its attachment to the corneo-scleral junction. The white ring on the anterior surface of this part of the second coat of the eye is the ciliary ring. With a scissors, cut around this ciliary ring at its outer edge. This specimen will show the anterior surface of the iris, and on the posterior side it will show the close relationship between the iris and the ciliary[52] processes. A hand lens will help greatly to bring out the very interesting fine points.
After the anterior portion has had everything removed from it there will be left nothing but the first coat or tunic of the eye—the anterior portion of the sclerotic and the cornea. The way the cornea seems to fit into the sclerotic is not quite as one is led to believe when told that it fits into the sclerotic much the same way in which a watch crystal fits into a watch.[3] Holding this part of the eye up to a strong light one will see that the sclerotic seems to overlap the cornea in the vertical axis.
By using the tweezers the cornea may be split. Nothing in the way of locating its layers can be recognized, however, unless a section is made for microscopic examination. The epithelial may be scraped off when the cornea is a trifle dry. This is the ocular epithelium reduced to a layer of flattened cells.
[53]
If the preceding dissections have been done, the crystalline lens will already have received some notice. To study the lens properly one should use an eye that has not been hardened and also an eye or the lens of an eye that has been in a 5 per cent. solution of formaldehyde for about two weeks.
The lens in the unhardened eye will prove too friable to permit much handling. The dissection should be made, however, in order to give opportunity to notice the crystalline clearness of the lens substance, its great magnifying power, its attachments, its capsule, etc. For this purpose it is necessary to proceed only as in the dissection for the “hyaloid membrane, etc.” That is, use an eye that has been kept in a cool place for several days, and then open it, and remove hyaloid, vitreous, and lens intact, as in the first dissection taken up in this book. To examine the specimen in detail, turn it so the lens will be uppermost. (Fig. 18.)
To remove the lens it is necessary to separate[54] the suspensory ligament, using for this purpose the small-pointed scissors. The capsule may be removed by picking it up on the periphery of the lens, and stripping it off. It will peel off about the same way that the outer skin of a bean or pea does.
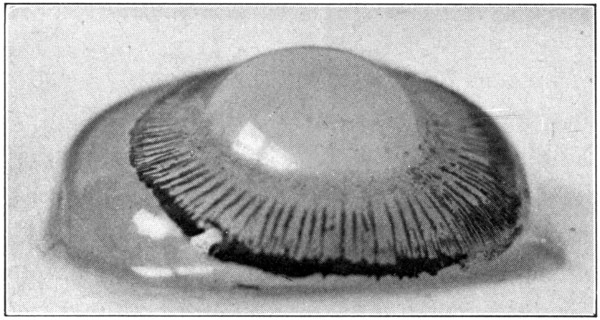
Fig. 18—Enlarged to show the processus zonuloe. (Page 53.)
The tri-radiate lines on the posterior and the anterior surfaces of the lens will not be as clearly discernible as in the lens coming from the hardened eye. Close inspection and the use of a hand lens will help bring them out more clearly.
Now, with the point of the scalpel try to separate the outer layers (cortex) from the[55] harder inner layers (nucleus). This will not prove very successful but is suggested for the purpose of comparison when the same thing is done to the hardened lens.
It will be found that the lens after having been in the formaldehyde solution is no longer crystal like, but more or less translucent. When viewed from either the anterior side or the posterior side, the tri-radiate lines on each surface will be seen to begin at the poles of the lens and radiate outward toward the lens equator. Holding the lens up to a strong light will show that though the lines on either surface form angles of 120 degrees, the angles formed by the lines on one side with the lines on the other side are 60 degrees. On the anterior surface of the lens the vertical line extends upward from the pole; on the posterior surface downward from the pole.
To study the laminated structure of the lens, it is best to boil the lens. The best way to do that is to drop the lens from either a hardened or unhardened eye into boiling water. Let it boil in the water for about two and a half to[56] three minutes. Longer than that time will cause the lens to be put out of shape, and make it so fragile that it can no longer be handled without having it fall apart. If the lens comes from an unhardened eye it might be best to boil it not more than about two minutes.

Fig. 19—Showing the way the onion-like layers of the lens may be peeled off.
Insert the point of the scalpel carefully at one of the poles, and lift gently in the direction of one of the radiating lines. This will tend to raise one of the concentric layers, which can be easily peeled off. Repeat this in the direction of the other two radiating lines. Examining, with a hand lens, the exposed surfaces[57] and the layers, as they are taken off, will show the arrangement of the lens fibres, and will also show plainly their directions. (Fig. 19.) To get another view of the onion-like layers of the lens, cut through it with a safety-razor blade, either longitudinally or equatorially. (Fig. 20.) The better way is to have enough lenses to make one of each kind. Never try to work with only one piece of material. If the lens is first stained with chromic acid the layers may be seen better, or, a simpler way is to drop the lens, before cutting it in two, into a carmine solution; red ink slightly diluted, will do.
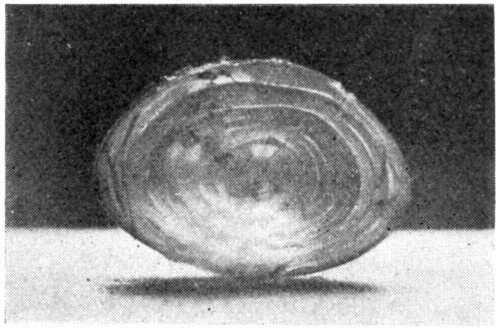
Fig. 20—Section through lens showing its concentric layers.
A lens that has been boiled and partly dissected[58] may be placed in a 5 per cent. formaldehyde solution, and kept indefinitely. The lens fibres, concentric layers, and lens laminae in such a specimen will always be interesting.
A lens that has lost its transparency because of hardening in formaldehyde or boiling may be made clear and nearly transparent again in the following way: First: Place the lens in a 50 per cent. alcohol for several hours. Second: Remove the lens, and let it drain on a piece of blotting-paper; then place it in a 75 per cent. alcohol. Third: Remove the lens, as before, then place it in an 85 per cent. alcohol. The lens may be left in this alcohol from ten to twelve hours, after which length of time it should be removed and drained. Fourth: Place the lens in an absolute alcohol, and leave it there for ten or twelve hours. Several hours longer will not injure the lens, nor interfere with the success of the work. Fifth: Remove the lens from the absolute alcohol. Place it upon a piece of blotting-paper, moving it to another place on the blotting-paper whenever the paper around the lens seems to have taken[59] up as much moisture as it can hold. Be sure that the lens has given up nearly all, if not all, moisture. “Running through the alcohols,” as this process is called, is for the purpose of dehydrating the tissue. It will be on the side of safety to let the lens lie exposed on the blotting-paper for an hour. Sometimes, if the capsule has not been removed, a small quantity of alcohol will remain between the lens and the inner surface of the capsule. This must be removed. It may be done by either puncturing the capsule with a pin or needle, and squeezing out the fluid, or by removing the capsule entirely. The latter is preferable.
Now drop the lens into xylol. Benzine will answer, though it will not produce quite so clear a lens as the xylol does. At the end of 24 or 36 hours the softer cortex will show quite clear, while the harder nucleus will be still cloudy. At the end of a week the whole lens, if it is a small one—pig, calf, sheep—will have become quite clear and transparent; if from a beef eye it will take longer. It sometimes takes nearly two weeks. In the case of a boiled lens[60] it will take much longer to clear; it may take a month.
Cedar oil may also be used for the purpose of clarifying or “clearing” the lens. Harden in the usual way, run through the alcohols, and then place in cedar oil. The oil, however, will stain the lens a yellowish brown, and the lens will not be as transparent and clear as when xylol is used.
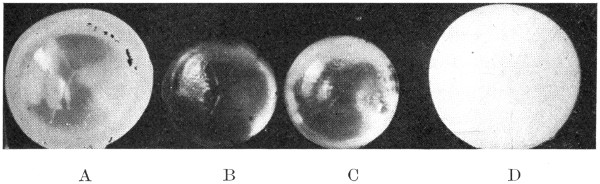
Fig. 21.
A. Lens hardened in formaldehyde.
B. Lens hardened in formaldehyde, run through the alcohols,
and cleared in xylol.
C. Lens hardened in formaldehyde, run through the alcohols,
and cleared in cedar oil.
D. Boiled lens.
The longer a lens is left in either of these two clarifying fluids the harder and smaller it will become. At the end of a month or six[61] weeks the lens will have become so hard that it can no longer be cut through with a knife. If it is desired to halve it, a scroll saw will be found to be the best thing to use for this purpose. (Fig. 21.)
Select an eye that has had a long part of the optic nerve left on it and place it into a 5 per cent. solution of formaldehyde. Leave it in that solution for from two to three weeks. That period of time in the fluid will be sufficient to permit the choroid to become sufficiently toughened and hardened. Leaving it in the solution longer than that length of time will not injure the eye in any way.
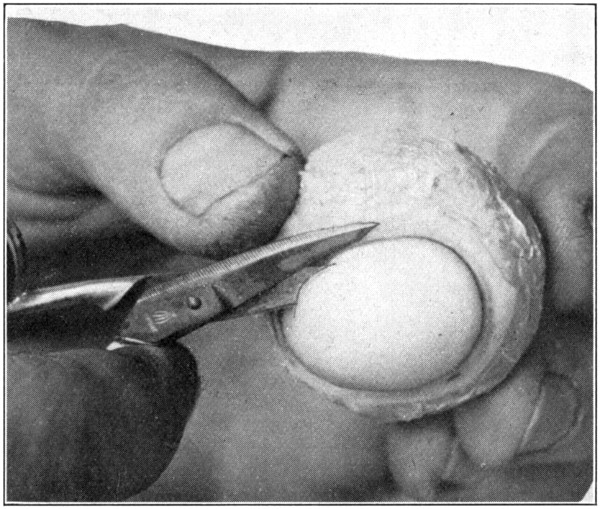
Fig. 22—Showing how to puncture the cornea. (Page 62.)
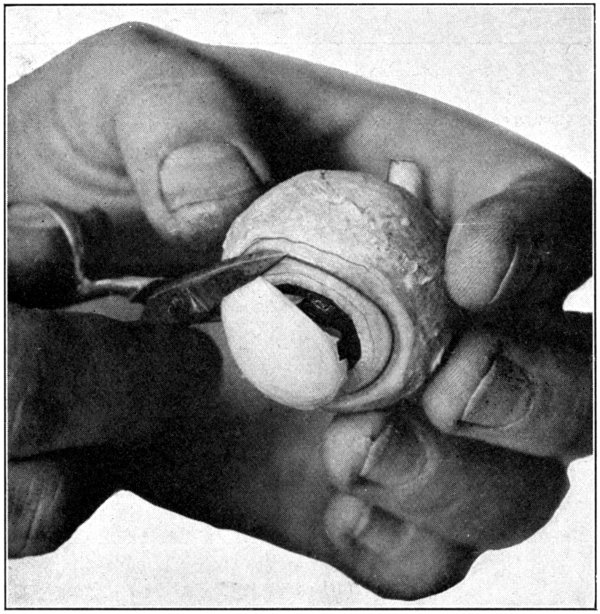
Fig. 23—Removing the cornea. (Page 63.)
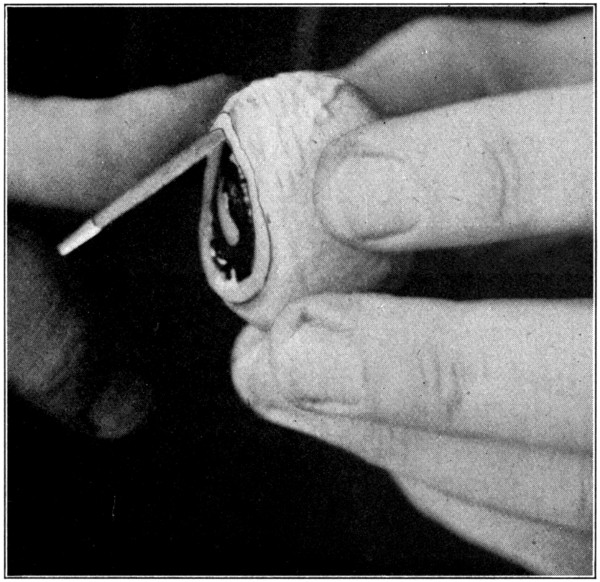
Fig. 24—Showing method of inserting the scalpel to separate the choroid from the sclerotic.
After having removed the eye from the formaldehyde, wash it for a few moments in running water. This will remove the preserving and hardening fluid from the surface, and will save the hands a little from the effects of the fluid. Next remove all the muscles and fatty tissues from the sclerotic. After that has been done, puncture the cornea with the pointed jaw of the scissors about 2 mm. from the corneo-scleral junction. (Fig. 22.) Then proceed to cut the cornea away, being[63] careful not to lacerate the choroid or the iris. (Fig. 23.) The escaping aqueous humor will flow over the eye and make it very slippery, and, therefore, difficult to hold. Dip the eye in water, wash it, and then take it out and thoroughly dry it with a cloth. This procedure is absolutely necessary, and, if omitted, will surely result in the dropping of the eye about[64] the time the work on the specimen is nearly finished. Insert the scalpel between the peripheral edge of the exposed iris and what is left of the cornea. With the back edge of the scalpel, gently loosen the choroid from the inner side of the corneo-scleral junction to[65] which part it is not securely attached. (Fig. 24.) This requires only ordinary care, and but little skill other than that necessary to always keep the scalpel close to the inner surface of the sclerotic. When the choroid-iris edge has been detached from the inner side of the corneo-scleral[66] junction, the weight of the contents of the second tunic will cause it to sag and give opportunity to easily separate, with the back edge of the scalpel, the choroid from the sclerotic for about a distance of from 8 to 10 mm.
It has been the method in the past to force water through a blowpipe between the sclerotic and the choroid, in order to separate the attachments. It has also been the method to work under water when wishing to expose or isolate either the choroid or the retina. It is unnecessary to do either of these two things.
When the sclerotic has been loosened from the choroid for about 10 mm. back from its cut edge around the eye, carefully cut the loosened part away. (Fig. 25.) Then loosen the choroid as far back as to within 1 cm. of the optic nerve. Cut the separated sclerotic away. It will be well to state here that during this dissection the specimen should not be lifted from the table. Keep the eye resting on the table all the time, and never lift it by holding it suspended from the optic nerve.[67] Loosening the choroid from the sclerotic up to this point is a very easy matter; ordinary precaution is all that is necessary to prevent puncturing the choroid with the scalpel, just be sure to remember to keep the point of the scalpel close to the sclerotic.
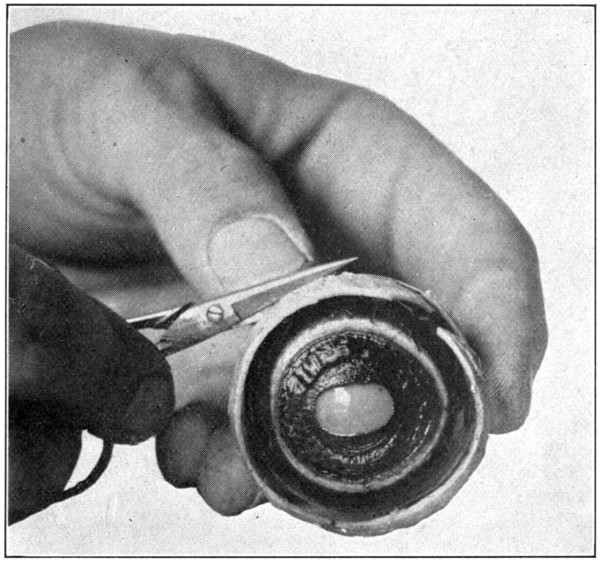
Fig. 25—Cutting away the sclerotic after it has been loosened from the choroid, as shown in Fig. 24.
[68]
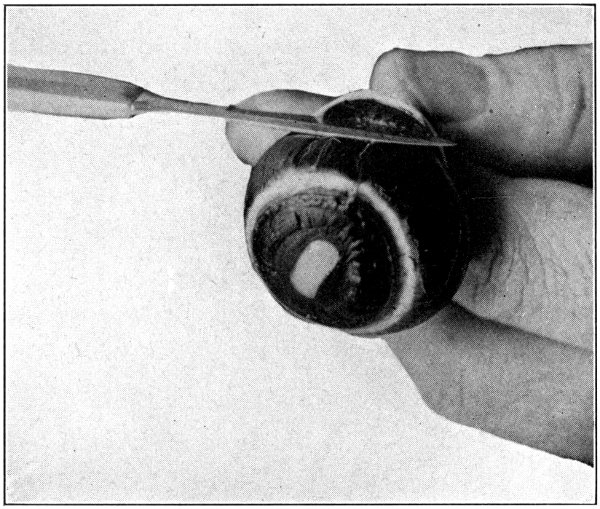
Fig. 26—Showing how to scrape the choroid free from the sclerotic near the optic nerve.
To remove the remaining part of the first coat is a little more difficult, and needs a little more care. Hold the optic nerve in the left hand, and pull it so that the sclerotic will pull away from the choroid. Then, using the cutting edge of the scalpel, scrape the choroid loose from the sclerotic close up to the entrance of the optic nerve. (Fig. 26.) Do not[69] separate the optic nerve from the choroid. Cut away the remainder of the sclerotic close up to the optic nerve and the choroid will be free. (Fig. 27.)
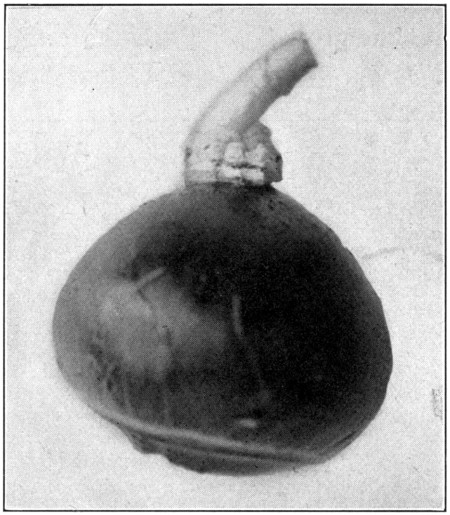
Fig. 27—Showing the choroid, the optic nerve still attached, the ciliary ring, and the ciliary nerves.
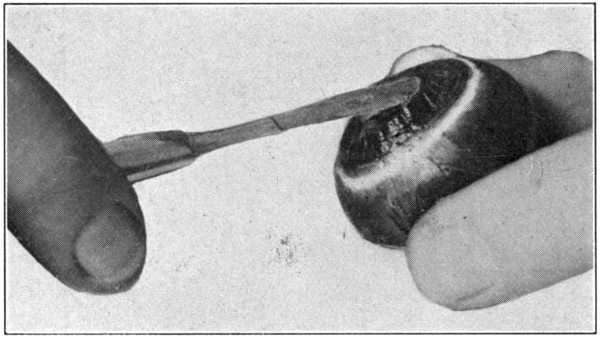
Fig. 28—Showing method of inserting scalpel in order to loosen the lens and cut through the vitreous.
To get a perfect specimen and completely isolated choroid, it must be emptied of its contents. Insert the scalpel between the lens[70] and the iris, force it on through, and in such a manner as to keep the scalpel close to the ciliary processes. (Fig. 28.) Cut the vitreous around the processes. Push the scalpel further into the vitreous, and cut out the central part of it, just as one would cut out the core of an apple. (Fig. 29.) Remove the scalpel, pick out the lens and the cut centre of the vitreous with the broad-point tweezers, holding the choroid a trifle suspended by the optic nerve. The remaining part of the vitreous may be broken down by cutting with the scalpel, and[71] by squeezing and crushing with the fingers of both hands. (Fig. 30.) The choroid will be tough enough to stand this treatment provided the pupil is left clear and open to prevent inter-choroidal pressure. After the vitreous has been removed the choroid will be left in a greatly collapsed condition. Dropping it into[72] water and letting it fill up will make it resume its original shape immediately. The retina does not always come out with the vitreous. In such a case, the tweezers may be used to pick out the retina when the choroid is in a collapsed condition.
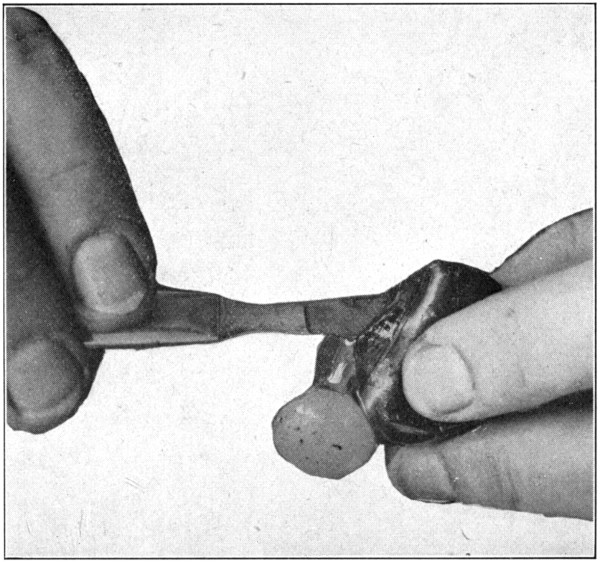
Fig. 29—Taking out the lens and “core” of the vitreous.
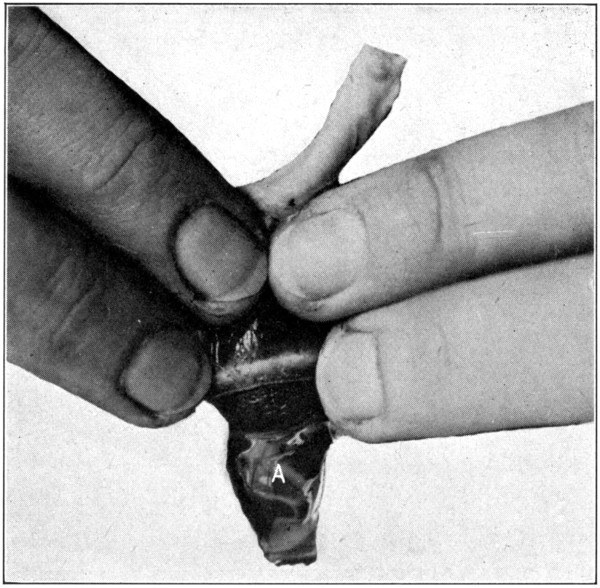
Fig. 30—Showing how to squeeze out the remaining part of the vitreous (A) from the choroid. (Page 71.)
[73]
This specimen will show the vena vorticosa, the ciliary nerves, and their way of ramifying, and the long ciliary arteries, which run opposite each other and which may be recognized by their rather colorless, tubular appearance. The evacuated choroid makes an excellent specimen and one easily examined. Place it in a 3 per cent. solution of formaldehyde, and then examine with a skiascope, an ophthalmoscope, or by “oblique illumination.”
This dissection is wholly original, and may be done in about five minutes. The old technique for doing it required at least an hour of time with the possibility of procuring one perfect specimen in every six or seven. The technique as given here will make it possible to do the work in not longer than five or six minutes for the beginner, and about four minutes for the expert.
[74]
Isolating the retina from the other tissues requires considerable patience and dexterity. When the retina has been removed and placed in a special receptacle, it will be found that the specimen is well worth the little amount of time spent in making it. Previous techniques, even the writer’s own, sometimes took nearly two hours to do, and rarely was the retina isolated without puncturing or tearing it; perfect specimens were almost impossible. The following method will assure one of success in nearly every instance. Failures are almost impossible. Punctures, perforations, tears, etc., are rare. The beginner should isolate the retina in about six to seven minutes; the expert in about four and a half to five minutes.
Select an eye with a long optic nerve, and prepare it for this dissection by placing it in a 10 per cent. solution of formaldehyde for[75] about ten to fourteen days, but no longer. If it is left in the hardening fluid longer than that length of time, it will interfere with the easy removal of the vitreous.

Fig. 31—Cutting through the iris. (Page 77.)
[76]

Fig. 32—Showing how to cut around the ciliary ring. (Page 77.)
The first part of this dissection is the same as the beginning of the dissection for the isolation of the choroid. Remove all the outside tissues first, and then the cornea, and about 10 mm. of the sclerotic, as described in the preceding dissection. (See Figs. 22, 23, 24, and 25.) That will lay bare the iris and a few millimetres of the choroid.
[77]
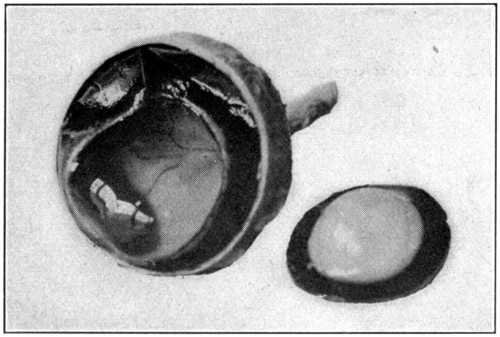
Fig. 33—Lens, iris, and part of vitreous removed. (Page 78.)
After that has been done, turn the eye so the iris will be uppermost. With the tweezers pick up the pupillary margin of the iris, and with the fine-pointed scissors cut through the iris and the ciliary processes (Fig. 31); separate both from the choroid by cutting close to the posterior edge of the processes. (Fig. 32.) In doing that, cut partly through the vitreous also, but be careful not to injure the peripheral edge of the retina—ora serrata. After the iris has been separated from the choroid, cut completely through the vitreous[78] in such a way that the lens will also be removed with the iris. (Fig. 33.)
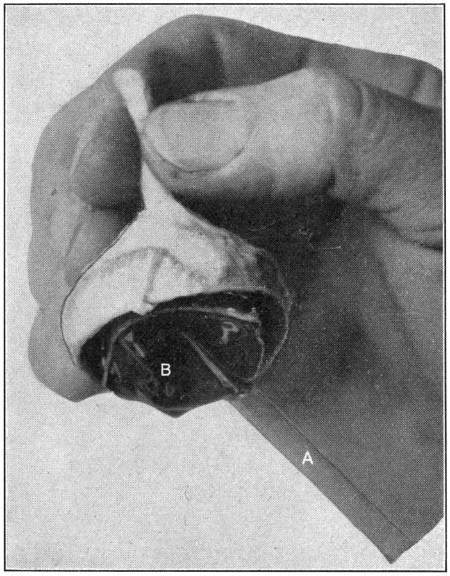
Fig. 34—Showing how to force glass blowpipe (A) into vitreous (B). (Page 80.)
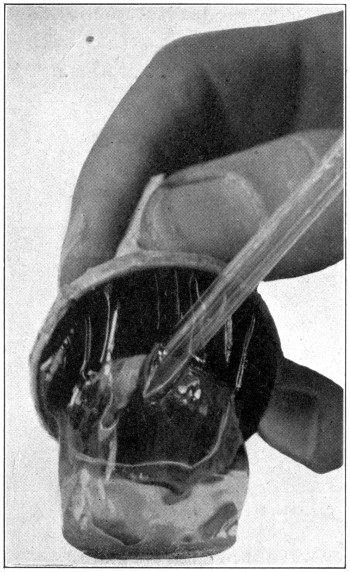
Fig. 35—Showing bulging out of vitreous caused by blowing air through glass blowpipe. (Page 80.)
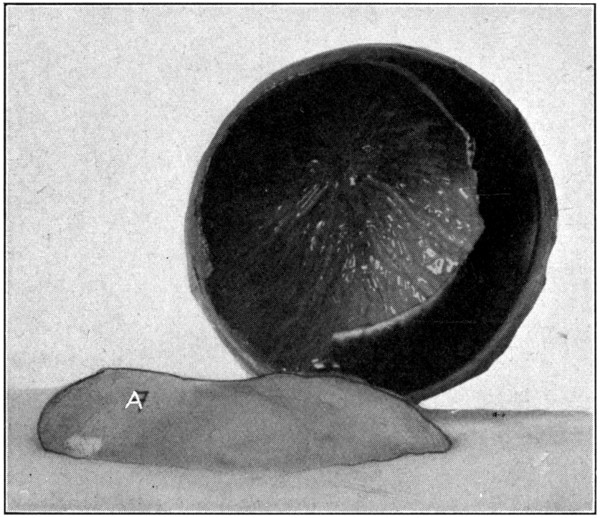
Fig. 36—Showing the vitreous (A) removed.
Holding the eye suspended by its optic
nerve, force the glass blowpipe through the
vitreous until it all but touches the posterior[79-
80]
part of the retina (Fig. 34); blow gently at
first, increasing the pressure until the vitreous
suddenly bulges outward. (Fig. 35.) If the
iris has been cut away close to the ora serrata,
the vitreous will not only bulge forward, but it
will fall out. If, however, it does not detach
itself at once, insert the scalpel close to the
choroid and with its flat side press downward[81]
until a separation occurs. Do not let the
vitreous drop out too suddenly, because it may
tear the retina. Let the vitreous detach itself
slowly by the force of its own weight, though
it will be well to hold some of its weight on the
scalpel. (Fig. 36.)

Fig. 37—A. Showing retina folded upon itself by blowing air at it through the glass blowpipe. (Page 83.)
[82]

Fig. 38—A. Showing folded retina suspended from its attachment, so sclerotic and choroid may be easily cut away. (Page 83.)
After the vitreous has been removed, turn the eye upward, and by blowing strongly through the blowpipe at the marginal edge of the retina, turn the retina upon itself. Repeat this until the retina lies in a small wrinkled lump at the “bottom” of the posterior part[83] of the eye. (Fig. 37.) Invert the eye (Fig. 38) and cut away both the choroid and the sclerotic close to the optic nerve. No care need be taken in doing this until the scissors come close to the optic nerve. (Fig. 39.)
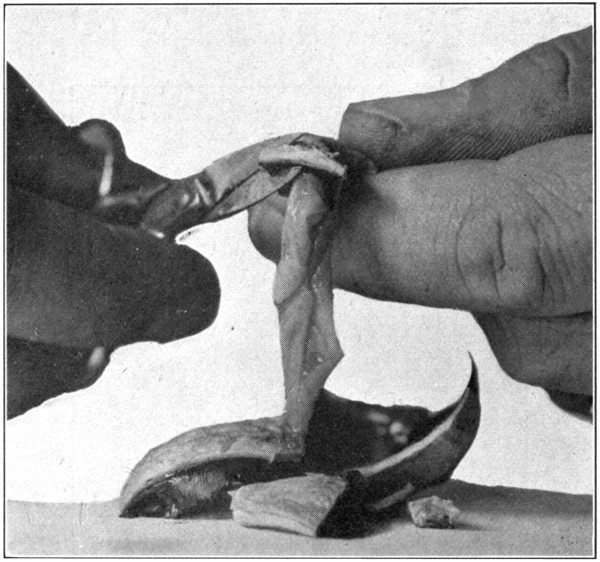
Fig. 39—Showing the sclerotic nearly all cut away.
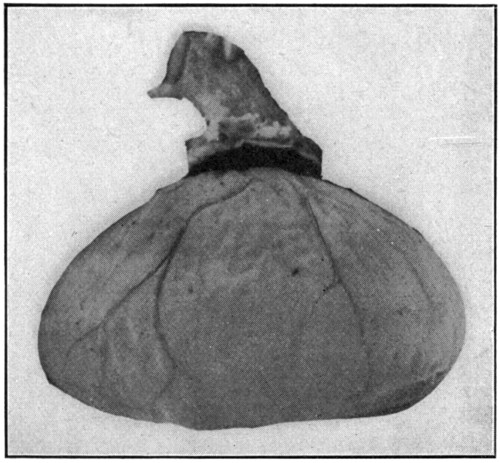
Fig. 40—Isolated retina, with optic nerve attached.
After the choroid and the sclerotic have been cut away, drop the retina into some[84] water, and it will slowly unfold itself by “ballooning” out into a perfect and beautiful specimen. (Fig. 40.) But, if it is desired to study the specimen closely, it is better to suspend it in a jar or bottle made of thin glass, and containing a 5 per cent. solution of formaldehyde. Remember that the retina is a delicate membrane in any state; the slightest rough handling may cause it to be torn, or otherwise damaged. If the vessel, in which[85] the specimen has been placed and suspended, has enough preserving fluid to completely fill it, and it is firmly stoppered, the whole thing may be inverted, and turned in any direction, even abruptly, without fear of damaging the retina. This way of keeping the retina will give opportunity to inspect and study the inside as well as the outside of the membrane; the blood-vessels, and other important parts easily recognized.
[86]
Place an eye in a 5 per cent. solution of formaldehyde for about two weeks. If the eye is kept in that solution longer than that time, the lens is apt to become so hard that in cutting it the capsule and suspensory ligament will be torn, and the lens will then become detached; if for a shorter space of time, the lens and other tissues will be so soft that all may be so badly torn or lacerated, that a perfect specimen will not be possible.
It sometimes happens that in keeping a number of eyes together in a vessel for the purpose of hardening them in the formaldehyde solution, the corneas of some will be crushed in. For this dissection, select an eye that has the cornea in perfect condition.
Remove all the outside tissues with the scissors, being particular to have the region immediately surrounding the optic nerve perfectly[87] clear and clean. If the optic nerve is longer than 5 mm., cut it off to that length.
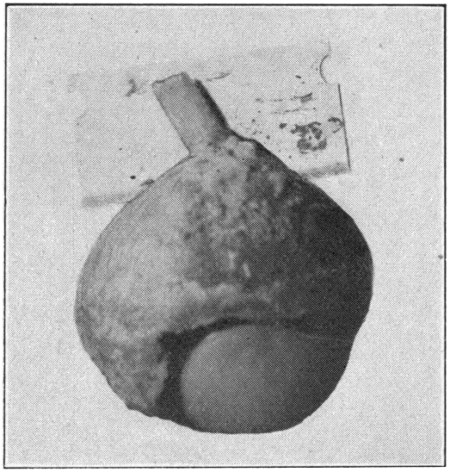
Fig. 41—Showing the beginning of the cutting of the eye for sagittal sections.
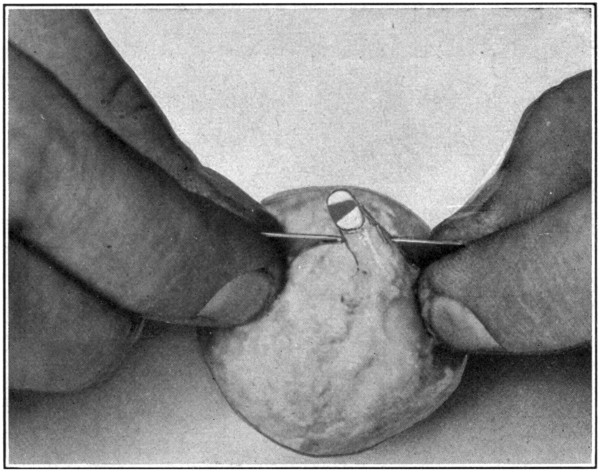
Fig. 42—Showing method of cutting through the crystalline lens.
To cut the eye in two, use a safety-razor blade; never a scalpel. The latter is too thick, too dull, and too clumsy a tool. Begin by cutting through the optic nerve; dividing it as nearly as possible into halves. (Fig. 41.) Continue cutting through the sclerotic and all underlying tissues, stopping at the corneo-scleral junction, but do not, during this procedure,[88] even touch the lens. After the eye has been thus partly separated into, as nearly as possible, two equal parts, lay it down upon the cornea, and, holding the razor blade in the forefingers and thumbs of both hands, cut the lens in two by forcing the blade down through it. (Fig. 42.) Partly open the cut eye to allow one jaw of the large scissors to enter, turn the eye over so the cornea will rest on that[89] jaw, and then cut through the cornea. (Fig. 43.)
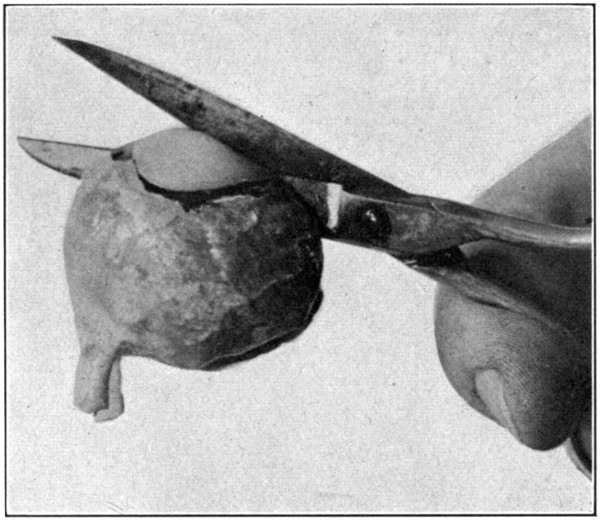
Fig. 43—Showing method of cutting through the cornea and completing the two sagittal sections.
The two specimens may be kept indefinitely by placing them in a 3 per cent. formaldehyde solution. It will be well to remove the lens from one of the specimens, because it will give better opportunity to see the anatomical relationships. Also, these specimens should be mounted, one above the other, between two pieces of glass, before placing them in the receptacle that is to hold them.
[90]
Much can be studied in such specimens. Moreover, they present to view the various parts of the organ of vision in such an impressive way, that one does not soon forget the wonderful appearance of the construction of this, Nature’s perfect camera. (Fig. 44.)
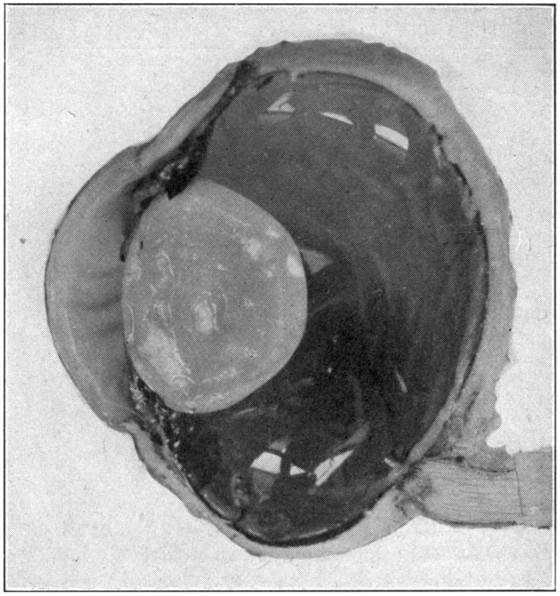
Fig. 44—Sagittal section enlarged.
If another eye is cut into two parts, additional[91] interesting specimens may be procured; for instance, one showing the presence of the second coat only, the retina having been torn out. Another good specimen may be made by removing all of the inner tissues, and leaving only the sclerotic and cornea. This specimen will show that the first coat is almost entirely a coat which affords strength and protection to the parts that lie within.
[92]
The only way to dissect the lacrimal apparatus, other ocular accessories, and the extrinsic muscles, is to procure the head of some animal, preferably a calf’s head, because of its size. Any butcher will supply one for from forty to sixty cents. Have the lower jaw removed. It will make a less bulky piece of material to handle.
Close to the inner canthus, on the inner side of each lid, will be found a little rounded eminence—lacrimal papilla—in the centre of which is a small opening—punctum lacrimalis. Both may be seen better on the lower lid, if it is pulled down, and on the upper lid, if it is pulled up.
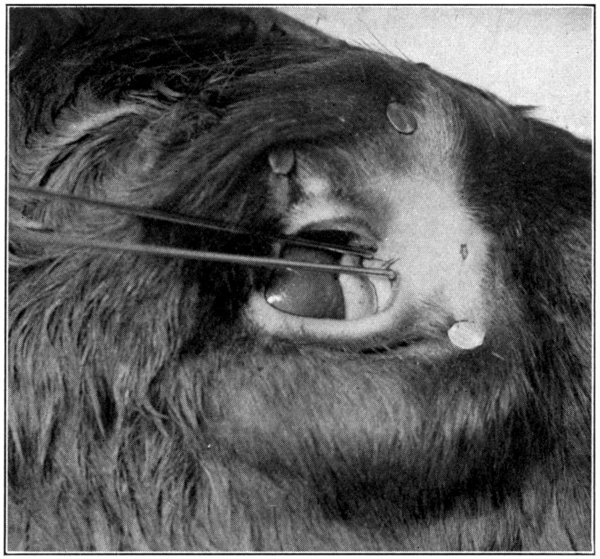
Fig. 45—Showing only a part of a calf’s head and the knitting-needles inserted in the puncta. (Page 94.)
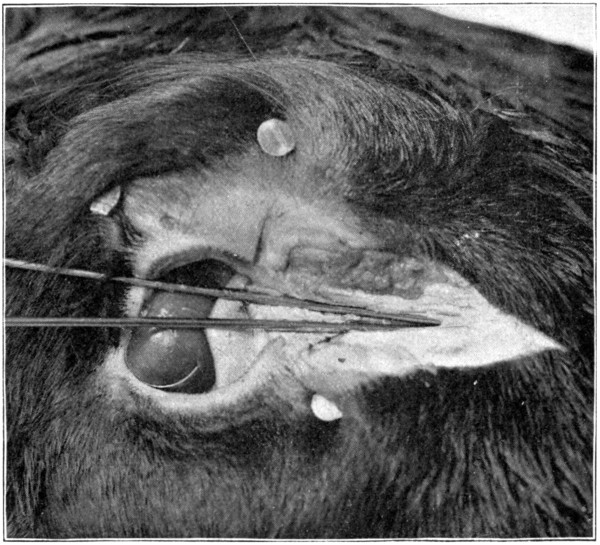
Fig. 46.
Be supplied with two knitting-needles. Take one, lubricate its full length with a little vaseline, lard, oil, or any other lubricant. Insert the needle into the punctum of the[93] lower lid, and push it downward and forward, aiming to come out in the nose a short distance from its end. At first it may be a little difficult to get the needle started; if so, just wiggle the needle, pushing it at the same time as directed, until the nasal duct is found. Do not remove the needle. To insert the other[94] needle into the punctum of the upper lid is rather difficult; for that reason the punctum of the lower lid was chosen, first. Grease the needle, as was done to the first one, and, with a little patience and careful manipulation, the canal opening and its course will soon be found. The needle may then be pushed through until it meets the first one. (Fig. 45.)[95] From the puncta lacrimalia to the place of meeting of the two needles, marks the course of the two canaliculi and their junction before they merge and form the nasal duct. Leave the needles where they are, and begin cutting away the skin. The needles will then mark the course of each canal and the duct very plainly. With the small scissors the canals and the duct may be loosened from the surrounding tissues. Or, the scalpel may be used to lay open the canals, cutting along over the top of the needles. (Fig. 46.)
The cilia, palpebræ, palpebral conjunctiva, ocular conjunctiva, and other superficial ocular accessories may be examined without dissection.
[96]
An examination of the eyelids will show the openings of the ducts of the meibomian glands a short distance back of the cilia. Very fine pins or needles that have been greased may be easily inserted for a short distance into the ducts, and then a dissection made along the course of the duct as outlined by the presence of the inserted pins or needles. Another way to see the glands is to slice through the ducts, with the scalpel or safety-razor blade, the entire width of either eyelid. This will separate the glands into two parts and show their length, breadth and structure.
[97]
The eyes one procures from a butcher or a slaughter house will always have the extrinsic tissues so badly cut and torn that identification of the various parts and their relations is impossible. Therefore, it is best to supply one’s self with the head of an animal, such as a sheep or a calf, and dissect an eye with all its extrinsic tissues intact. For this dissection, a hammer and a chisel are necessary in addition to the tools needed for doing the previous dissections.
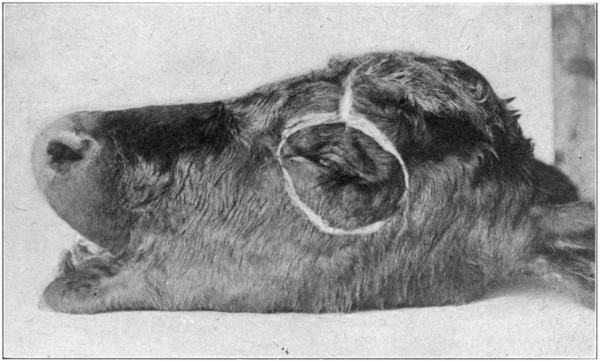
Fig. 47—Showing method of making the initial cuts in the skin. (Page 97.)
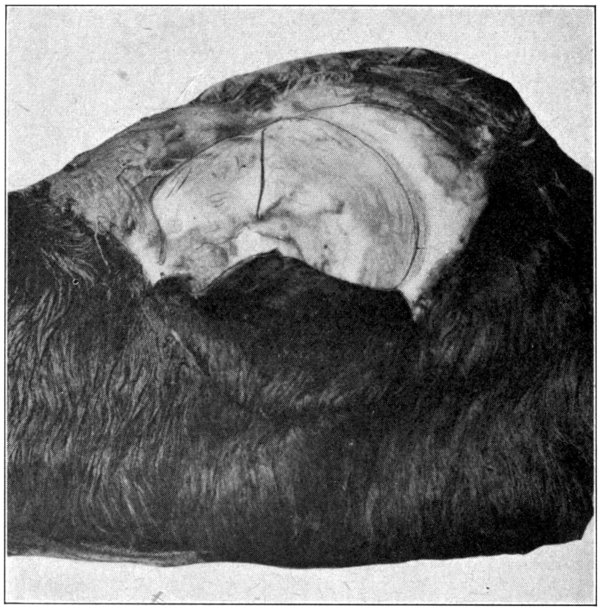
Fig. 48—Part of calf’s head, showing the first cut to be made in the bones of the orbit. (Page 102.)
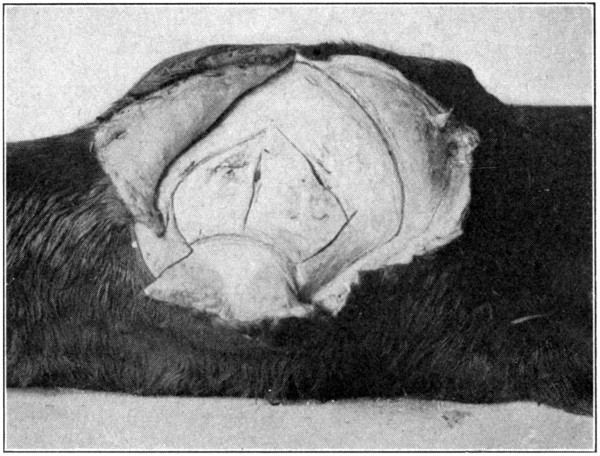
Fig. 49—Showing all the cuts to be made through the bones of the orbit. (Page 102.)
Using the left orbit, begin the dissection by
making an incision directly over the supra-orbital
ridge, extending from over the inner
to the outer canthus. At the middle of that line,
make an incision, and cut at right angles upward
to the top of the head. Next make a cut
below the eye, extending from the outer to
the inner canthus. (Fig. 47.) Loosen the
skin[98-
99]
from the bone with the scalpel, and lay bare
the skull immediately over the orbit. Fold the
flaps of the skin back and fasten them down
to the skull with pins or tacks so they will not
interfere with the work.
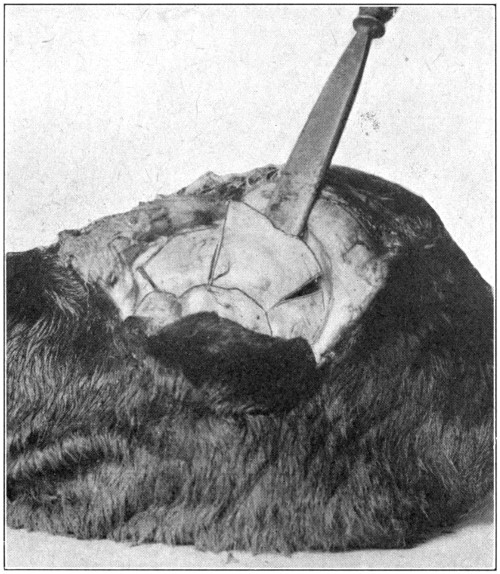
Fig. 50—Showing how to pry the cut bone loose. (Page 102.)
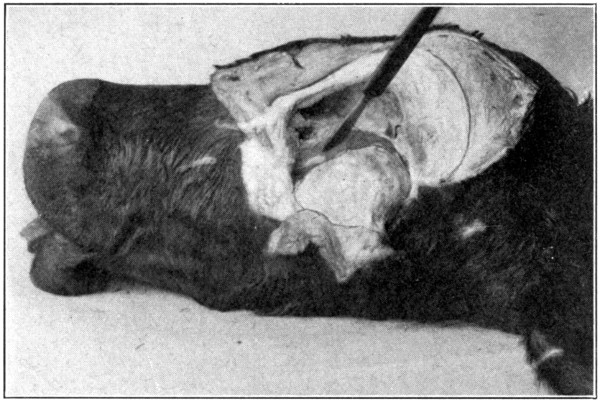
Fig. 51—“In removing orbital contents dissect close to the bone.”
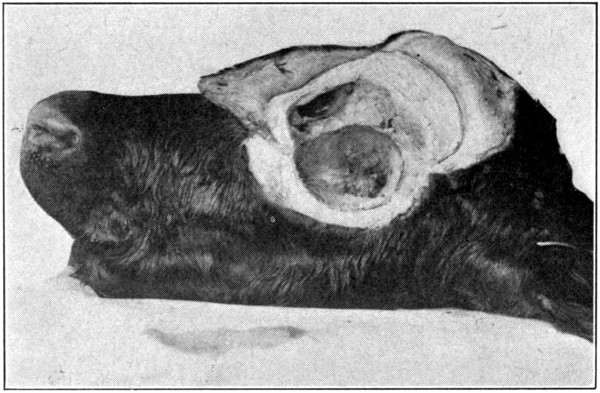
Fig. 52—Showing excavated orbit. (Page 105.)
Using the hammer and the chisel, cut[100]
through the roof of the orbit at the middle of
the supra-orbital ridge, and continue upward
for about two and one-half inches. Do not
strike hard blows, or the chisel may be driven
through the underlying tissues. Listen for
the peculiar sound that is heard when the
bone has been completely penetrated; then remove
the chisel. Continue until the full distance
of two and one-half inches of bone has[101-
102]
been separated. (Fig. 48.) Now, begin at the
upper end, and cut through the bone downward
to the right for about two inches toward the
outer canthus. A similar line should be cut
on the right of the centre line toward the inner
canthus. This will mark out two irregular,
triangular-shaped pieces of bone. (Fig. 49.)
Remove the piece on the right-hand side by
prying it off. (Fig. 50.) The left-hand piece
should be pried loose and then carefully cut
away with the scalpel, so that the pulley through[103]
which the superior oblique muscle runs its
tendon, will not be injured. In removing the
orbital contents, dissect close to the bone (Fig.
51), so that the periosteum will also be removed,
and form a sort of sac or capsule in
which will be contained the eye with all its
extrinsic tissues. If difficulty is experienced
in getting at the posterior parts of the orbit,
it will be best to cut away as much more of
the obstructing bone as is necessary. In this
way the “capsule” containing the eye, its six[104-
105]
muscles, the lacrimal gland, and both eyelids,
all in situ, will be removed. (Fig. 54.) As the
orbital entrance of the optic nerve is neared,
care must be exercised not to cut into this
“capsule,” or sever any of the muscles. (Fig.
52 shows the excavated orbit. Fig. 53 shows
an anterior view of the enucleated eye. Fig. 54
is a side view of the enucleated eye.)
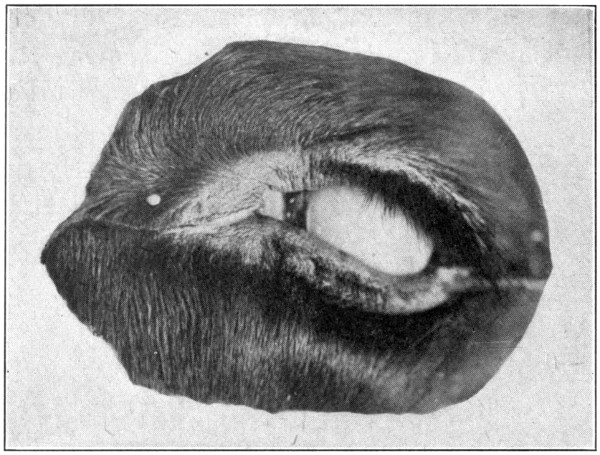
Fig. 53—Anterior view of the enucleated eye.
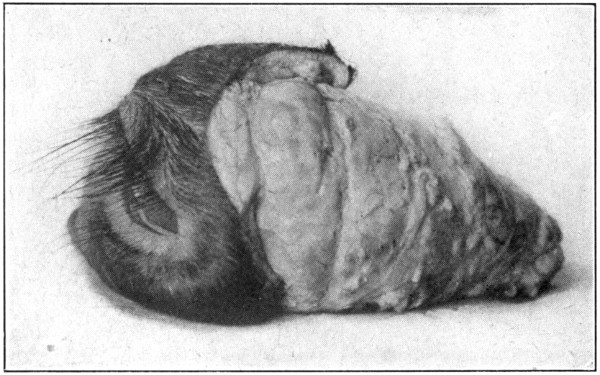
Fig. 54—Showing the enucleated eye, its muscles, and its accessories, all in situ.
[106]
By practising on an enucleated eye, one may gain considerable ability in the use of the ophthalmoscope, and also learn to recognize the blood-vessels and other important parts of the retina. To do this, the eye to be examined must be very fresh, for only in this condition will the cornea and lens be sufficiently clear to permit rays of light to enter the inside of the eye.
However, since the pupil is oblong in shape, and often only a narrow slit—but several millimetres in diameter—the field presented for observation is a rather limited one. To increase the pupillary aperture, take a pin, and force the point through the cornea about three or four millimetres from the corneo-scleral junction, and at right angles to the direction of the parallel edges of the pupil. After the pin has been pushed through until it has reached to within a short distance (one[107] millimetre) of the edge of the iris, carefully pick up the iris by raising the pin into a position perpendicular to the cornea, and force the pin further down into the eye. The pupil will have been enlarged on one side. Do the same thing on the opposite side, and at each extremity of the pupil. (Fig. 55.)
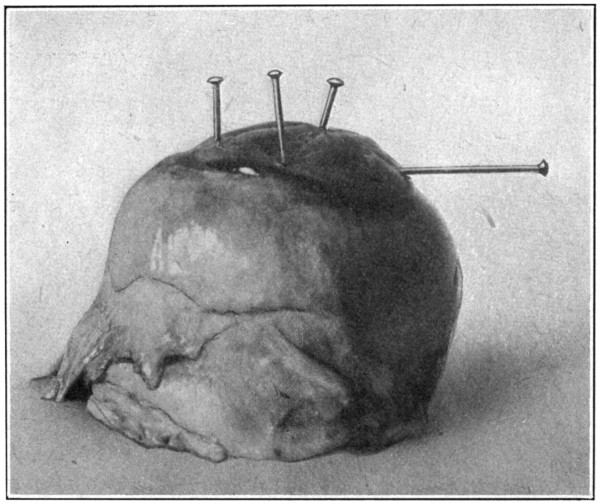
Fig. 55—Showing one pin before the iris has been picked up and pulled back, and three pins after the iris has been picked up and pulled back.
[108]
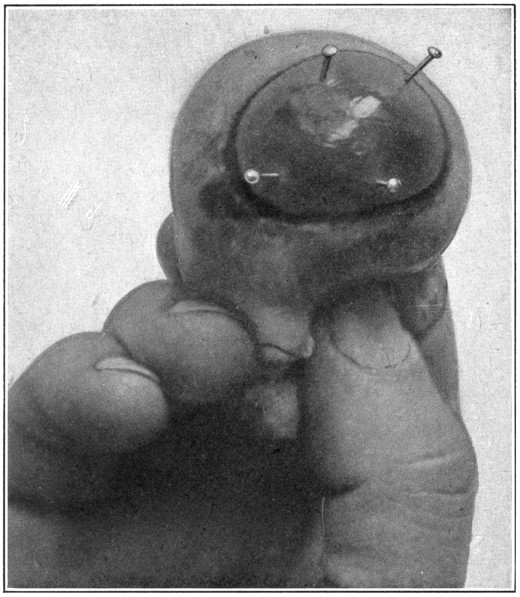
Fig. 56—Showing method of gathering up the extrinsic tissues in order to get rid of the pucker in the cornea.
The pupil will now have been made square, and so large that no difficulty will be experienced in reflecting either light into the eye, or[109] in examining the inside of the eye. Care must be taken not to lacerate the anterior surface of the lens when the iris is drawn back by the pins.
Putting the pins into the cornea, and using them as levers with the point of entrance in the cornea as a fulcrum, will pucker the cornea considerably, and a good clear fundus cannot be obtained. This is easily overcome. Simply gather up all the tissues surrounding the eye, force them backward, and hold them firmly with the fingers of the left hand. (Fig. 56.) The right hand is then free to handle the skiascope or ophthalmoscope, so that the interior of the eye may be thoroughly examined.
Another way to prepare an eye for ophthalmoscopic examination is as follows: Go to a slaughter house and procure a beef eye from an animal that has been killed but a few minutes previously. Placing the eye immediately into an 8 per cent. solution of cocaine and leaving it there for about an hour will dilate the pupil to such an extent that work with the ophthalmoscope will be made very easy. This,[110] as indicated, can be done only with an eye that is very fresh.
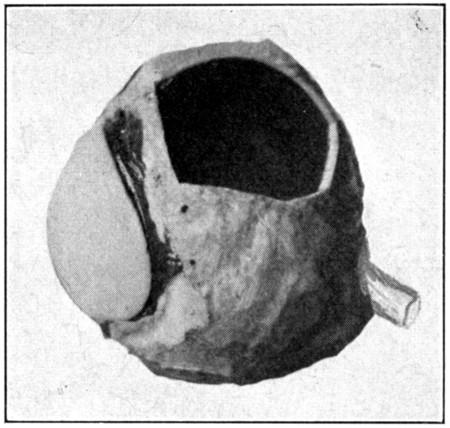
Fig. 57—Showing window cut in sclerotic, choroid, and retina.
Still another way to see the interior is to cut out a piece of the sclerotic about the size of a twenty-five-cent piece; then pinch up and tear out the choroid and the retina under the opening made in the sclerotic. (Fig. 57.) Hold the eye, the cornea forward, close to a bright light, and the image of the light will be seen upon the retina. The closer the light is to the eye, the greater the illumination will be in the[111] interior of the eye. If the opening or “window” is close enough to the optic nerve, the optic papilla can be seen easily. And, if care has been taken to have the opening made midway between the two branches of the retinal artery, the entire course may be followed. The direction of the retinal artery can be determined by ophthalmoscopic examination.
[112]
To find the lacrimal ducts, cut across the outer and inner canthi of the enucleated eye, pushing the eye forward and the lids backward. That will expose the conjunctiva of[113] both eyelids and eye, and also show the conjunctival fornices. On the upper surface of the palpebral conjunctiva, and near the outer canthus, will be seen, upon close inspection, a number of minute openings, usually eight. These are the openings of the lacrimal ducts. Pins or straw that have been lubricated with vaseline, may be inserted and pushed into these openings for a considerable distance, and the course of the ducts then can be traced easily. (Fig. 58.)
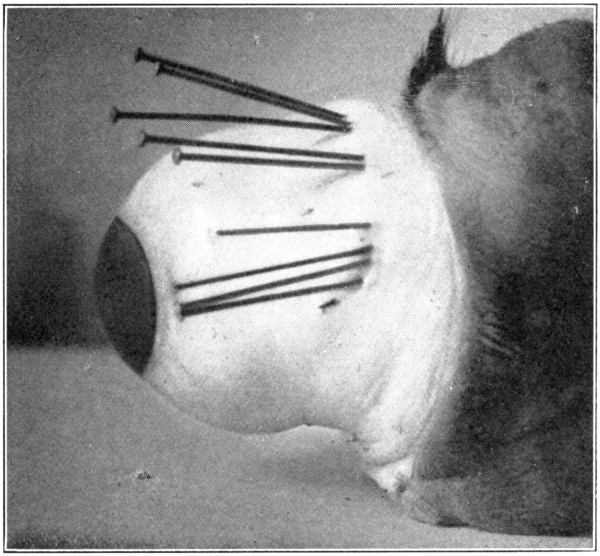
Fig. 58—Showing how pins may be inserted in the lacrimal ducts.
[114]
The lacrimal gland is easily distinguished by its pink appearance. There are two parts, inferior and superior. The gland lies directly over the eye and near the outer angle of the orbit. In the enucleated eye, it will be found to lie near the outer canthus and over the eye. The gland may be easily dissected out of its position and then examined more closely. A hand lens will show the racemose construction of the gland. If the gland is cut in two, the racemose construction may be seen even better.
[115]
To dissect the capsule of Tenon, it is necessary to carefully remove the superficial fat and connective tissue. In text-books and illustrations, the capsule is usually shown as a definite sac-like membrane of considerable thickness, with all its parts well defined. The[116] dissector will soon find that the capsule is not discerned so easily. It will be found to be the thin, semi-transparent, fibrous membrane that surrounds each muscle, as well as the “posterior two-thirds of the eye,” and is continuous anteriorly with the ocular conjunctiva. Portions may be pinched up and inflated through an inserted blow-pipe. This will help to merely demonstrate its location and parts. (Fig. 59.)
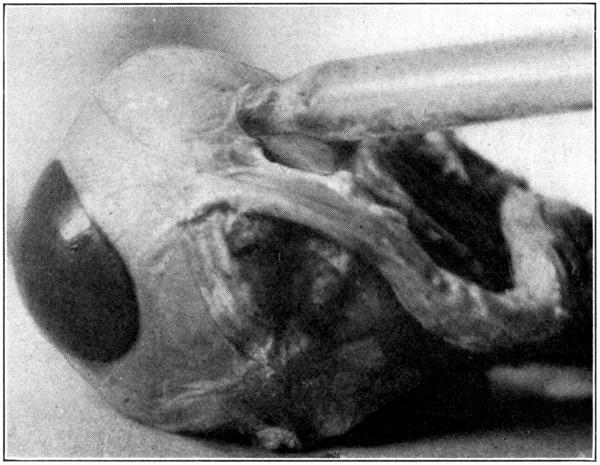
Fig. 59—Enlarged to show part of the Capsule of Tenon blown up. (Page 116.)
[117]
After the lacrimal gland has been dissected away, a beginning will have been made for cutting away the fat and the connective tissue. The first thing to do then is to locate the superior oblique muscle. Try to keep track of which part of the eye is the inner side. Having located the inner side, feel along the top for a little hard eminence. That is the pulley. Begin to dissect around the pulley, not through it, and then follow the muscle along to its origin; do not separate the muscle from its origin. When the superior oblique is completely freed, the action of the muscle may be readily demonstrated by holding the “ring” or tendinous pulley with the fingers of one hand, while the muscle is pulled backward and forward with the other.
[118]
With the dissection of Tenon’s capsule and the superior oblique muscle, the work of isolating the other extrinsic muscles will have begun. This work needs no directions except[119] a warning to be careful not to injure the pulley of the superior oblique, and to be careful not to cut away the inferior oblique. The inferior oblique will be found to be near the “pulley.” If the dissection is not carried too close to the origin of the recti muscles, all the muscles may be kept in place.
If the eye has not been previously subjected to the hardening influence of formaldehyde, it may be put into a 5 per cent. solution, and at the end of ten or twelve hours the muscles will have become rigid. They can then be better studied, and may be kept indefinitely. (Fig. 60.)
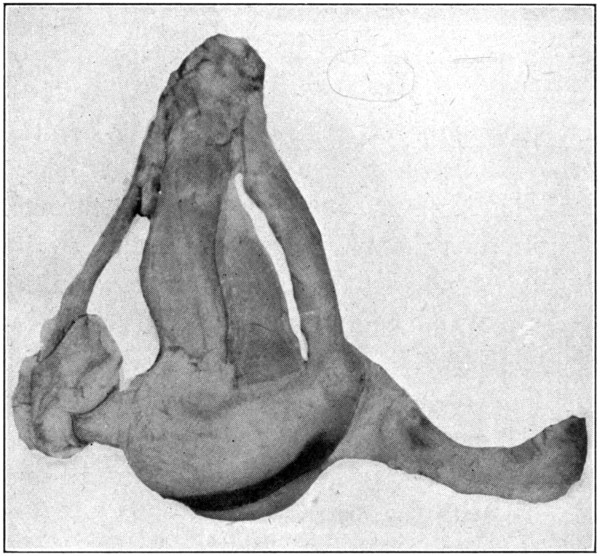
Fig. 60—Showing the tendinous pulley of the superior oblique muscle and the extrinsic muscles.
[120]
This dissection is a rather difficult one to make, and requires patience.
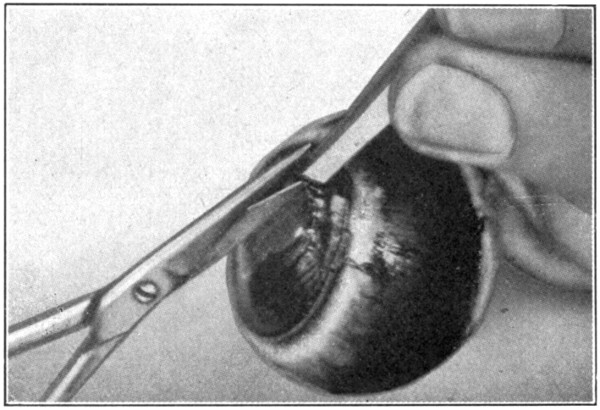
Fig. 61—Cutting through the iris.
Prepare an eye by placing it in a 5 per cent. solution of formaldehyde for about ten days to two weeks. Remove all the outside tissues. Cut away the cornea, as in the dissection for the choroid or the retina. Loosen, as far back as possible, the sclerotic from the choroid. Remove the sclerotic for about 10 mm. back of the equator of the eye. With the tweezers pick up the pupillary edge of the iris. Using the small pointed scissors, cut through[121] the iris. (Fig. 61.) Lift either one of the cut edges of the iris, and, with the sharp edge of the scalpel, gently scrape the processus zonuloe free from the ciliary processes, cutting through the ciliary ring as the ciliary processes are detached from the hyaloid (processus zonuloe). (Fig. 62.)
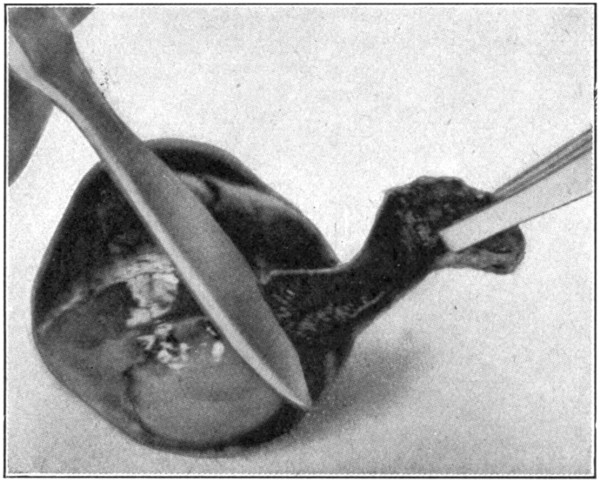
Fig. 62—Scraping the ciliary processes free. Showing, also, the choroid cut around the ciliary ring.
Great care must be taken not to thrust the point of the scissors into the hyaloid, suspensory ligament, or vitreous, else the lens may become detached.
[122]
After the iris with the processes has been removed, pinch up with the tweezers a fold in the choroid. Make an incision with the fine-pointed scissors, and begin removing the choroid to within about 5 mm. of the cut end of the sclerotic. (Fig. 63.) Care must be taken not to penetrate the underlying retina while making this part of the dissection.
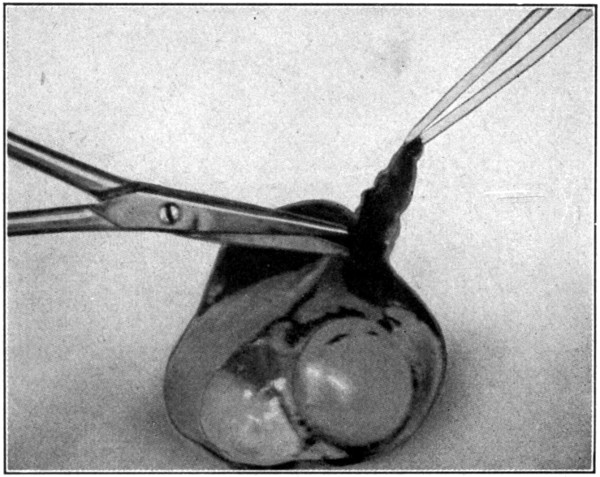
Fig. 63—Cutting away the choroid.
After part of the choroid has been removed, the specimen will show the three coats of the eye in layer-like arrangement, the hyaloid and[123] lens. The lens may now be cut away, if the specimen is preferred without it. Removing the lens before this time is unwise, because it acts as a protection to the other tissues while the specimen is being handled during the dissection.
This specimen will show to the best advantage if it is suspended in a jar containing a 5 per cent. solution of formaldehyde. Figure 64 shows the specimen.
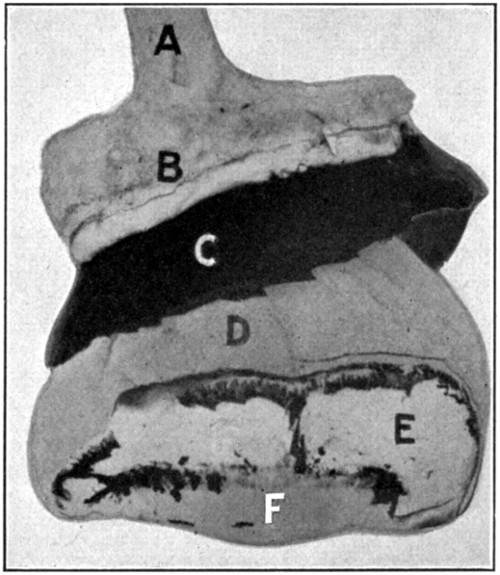
Fig. 64—A. Optic nerve. B. Sclerotic. C. Choroid. D. Retina. E. Hyaloid. F. Lens.
Page 127, cutting window in: either in the wrong place alphabetically, or it is an error for window, cutting in.
The (unusual) spellings zonuloe and zonulœ have been retained.
Changes:
Footnotes have been moved to the end of the chapter in which they are referenced. Illustrations have been moved out of text paragraphs.
Some obvious minor typographical and punctuation errors have been corrected silently.
In some illustrations the reference letters have been accentuated.
Page 11, 12: Illustration numbers added.
Page 127, Gland, racemose construction: page number 114 added.
Page 130: the entries iris and lens have been moved to their proper place; the entry Retinæ, pars ciliaris has been moved to a separate line.