
Title: The Cambridge natural history, Vol. 02 (of 10)
Editor: S. F. Harmer
Author: Frank E. Beddard
W. B. Benham
F. W. Gamble
Marcus Hartog
Lilian Sheldon
Editor: Sir A. E. Shipley
Release date: October 16, 2023 [eBook #71891]
Language: English
Original publication: New York: MacMillan and Co, 1901
Credits: Keith Edkins, Peter Becker and the Online Distributed Proofreading Team at https://www.pgdp.net (This file was produced from images generously made available by The Internet Archive)
THE
CAMBRIDGE NATURAL HISTORY
EDITED BY
S. F. HARMER, M.A., Fellow of King's College, Cambridge; Superintendent of the University Museum of Zoology
AND
A. E. SHIPLEY, M.A., Fellow of Christ's College, Cambridge; University Lecturer on the Morphology of Invertebrates
VOLUME II
FLATWORMS AND MESOZOA
By F. W. Gamble, M.Sc. (Vict.), Owens College
NEMERTINES
By Miss L. Sheldon, Newnham College, Cambridge
THREAD-WORMS AND SAGITTA
By A. E. Shipley, M.A., Fellow of Christ's College, Cambridge
ROTIFERS
By Marcus Hartog, M.A., Trinity College, Cambridge (D.Sc. Lond.), Professor of Natural History in the Queen's College, Cork
POLYCHAET WORMS
By W. Blaxland Benham, D.Sc. (Lond.), Hon. M.A. (Oxon.), Aldrichian Demonstrator of Comparative Anatomy in the University of Oxford
EARTHWORMS AND LEECHES
By F. E. Beddard, M.A. (Oxon.), F.R.S., Prosector to the Zoological Society, London
GEPHYREA AND PHORONIS
By A. E. Shipley, M.A., Fellow of Christ's College, Cambridge
POLYZOA
By S. F. Harmer, M.A., Fellow of King's College, Cambridge
London
MACMILLAN AND CO., Limited
NEW YORK: THE MACMILLAN COMPANY
1901
All rights reserved
'Nous allons faire des vers ensemble'
André de Chénier
First Edition 1896. Reprinted 1901
| PAGE | |
| Scheme of the Classification adopted in this Book | ix |
| PLATYHELMINTHES AND MESOZOA | |
| CHAPTER I | |
| TURBELLARIA | |
| Introduction—description of the Polyclad Leptoplana tremellaris—Appearance—Habits—Structure: Polycladida—Classification—Habits—Anatomy—Development: Tricladida—Occurrence—Structure—Classification: Rhabdocoelida—Occurrence—Habits—Reproduction—Classification | 3 |
| CHAPTER II | |
| TREMATODA | |
| Characters of Trematodes—Habits and Structure of Trematoda Ectoparasitica (Monogenea)—Life-Histories of Polystomum integerrimum, Diplozoon paradoxum, and Gyrodactylus elegans—Trematoda Endoparasitica (Digenea)—Occurrence and Habits of Digenea—Life-History of Distomum macrostomum—Distomum hepaticum and its Effects—Bilharzia haematobia—Bisexual Trematodes—Table of Hosts—Classification | 51 |
| CHAPTER III | |
| CESTODA | |
| Introduction—Nature of Cestodes—Occurrence of Cestodes—The Tape-Worms of Man and Domestic Animals—Table of Life-Histories of Principal Cestodes of Man and Domestic Animals—Structure and Development of Cestodes—Table for the Discrimination of the More Usual Cestodes of Man and Domestic Animals—Classification | 74 |
| {vi}
CHAPTER IV |
|
| MESOZOA | |
| Dicyemidae—Structure—Reproduction—Occurrence: Orthonectidae—Occurrence—Structure: Trichoplax: Salinella | 92 |
| NEMERTINEA | |
| CHAPTER V | |
| NEMERTINEA | |
| Introductory—External Characters—Anatomy—Classification—Development—Habits—Regeneration—Breeding—Geographical Distribution—Land, Fresh-Water, and Parasitic Forms—Affinities | 99 |
| NEMATHELMINTHES AND CHAETOGNATHA | |
| CHAPTER VI | |
| NEMATHELMINTHES | |
| Introduction—Nematoda—Anatomy—Embryology—Classification—Ascaridae—Strongylidae—Trichotrachelidae—Filariidae—Mermithidae—Anguillulidae—Enoplidae—Parasitism: Nematomorpha—Anatomy—Classification—Life-History: Acanthocephala—Anatomy—Embryology—Classification | 123 |
| CHAPTER VII | |
| CHAETOGNATHA | |
| Structure—Reproduction—Habits—Food—Classification—Table of Identification [see also p. 534] | 186 |
| ROTIFERA, GASTROTRICHA, AND KINORHYNCHA | |
| CHAPTER VIII | |
| ROTIFERA, GASTROTRICHA, AND KINORHYNCHA | |
| Rotifera—History—External Features—Movements—Anatomy—Reproduction—Embryology—Classification—Distribution—Affinities: Gastrotricha: Kinorhyncha | 197 |
| {vii}
ARCHIANNELIDA, POLYCHAETA, AND MYZOSTOMARIA |
|
| CHAPTER IX | |
| The Chaetopodous Worms—The Archiannelida—Anatomy of Nereis, as Typical of the Polychaeta | 241 |
| CHAPTER X | |
| Classification of the Polychaeta—Shape—Head—Parapodia—Chaetae—Gills—Internal Organs—Jaws—Sense Organs—Reproduction—Larval Forms—Budding—Fission—Branching—Regeneration | 257 |
| CHAPTER XI | |
| Natural History of Polychaetes—General Habits—Character of Tube and its Formation—Colouring—Protective and Mimetic Devices—Phosphorescence—Food—Uses—Associated Worms—Worms as Hosts—Distribution—Fossil Remains | 284 |
| CHAPTER XII | |
| Characters of the Sub-Orders of Polychaetes—Characters of the Families—Description of British Genera and Species: the Myzostomaria | 303 |
| OLIGOCHAETA (EARTHWORMS, ETC.), AND HIRUDINEA (LEECHES) | |
| CHAPTER XIII | |
| OLIGOCHAETA (EARTHWORMS AND THEIR ALLIES) | |
| Introduction—Anatomy—Reproduction—Bionomics—Distribution—Classification—Microdrili and Megadrili | 347 |
| CHAPTER XIV | |
| HIRUDINEA (LEECHES) | |
| Introduction—Anatomy—Reproduction—Classification—Rhynchobdellae and Gnathobdellae | 392 |
| {viii}
GEPHYREA AND PHORONIS |
|
| CHAPTER XV | |
| GEPHYREA | |
| Introduction—Anatomy—Development—Sipunculoidea—Priapuloidea—Echiuroidea—Epithetosomatoidea—Affinities of the Group | 411 |
| CHAPTER XVI | |
| PHORONIS | |
| History—Habits—Structure—Reproduction—Larva—Metamorphosis—List of Species and Localities—Systematic Position | 450 |
| POLYZOA | |
| CHAPTER XVII | |
| POLYZOA | |
| Introduction—General Characters and Terminology—Brown Bodies—History—Outlines of Classification—Marine Polyzoa—Occurrence—Forms of Colony and of Zooecia—Ovicells—Avicularia—Vibracula—Entoprocta | 465 |
| CHAPTER XVIII | |
| POLYZOA—continued | |
| Fresh-water Polyzoa—Phylactolaemata—Occurrence—Structure of Cristatella—Division of Colony—Movements of Colony—Retraction And Protrusion of Polypides in Polyzoa—Statoblasts—Table for Determination of Genera of Fresh-water Polyzoa—Reproductive Processes of Polyzoa—Development—Affinities—Metamorphosis—Budding | 492 |
| CHAPTER XIX | |
| POLYZOA—continued | |
| Classification—Geographical Distribution—Palaeontology—Methods for the Examination of Specific Characters—Terminology—Key for the Determination of the Genera of British Marine Polyzoa | 515 |
| Addendum to Chaetognatha | 534 |
| Index | 535 |
| PLATYHELMINTHES (p. 3) | |||
| Family. | |||
| TURBELLARIA (p. 3) | Polycladida (p. 7) | Acotylea (p. 16) |
Planoceridae (p. 19). Leptoplanidae (p. 19). Cestoplanidae (p. 19). Enantiidae (p. 19). |
| Cotylea |
Anonymidae (p. 19) Pseudoceridae (p. 19). Euryleptidae (p. 19). Prosthiostomatidae (p. 19). |
||
| Tricladida (p. 30) | Paludicola (p. 30) | Planariidae (p. 42). | |
| Maricola (pp. 30, 32) |
Procerodidae (p. 42). = Gundidae. Bdellouridae (p. 42). |
||
| Terricola (pp. 30, 33) |
Bipaliidae (p. 42). Geoplanidae (p. 42). Rhynchodemidae (p. 42). |
||
| Rhabdocoelida (p. 42) | Acoela (p. 42) |
Proporidae (p. 49). Aphanostomatidae (p. 49). |
|
| Rhabdocoela (p. 43) |
Macrostomatidae (p. 49). Microstomatidae (p. 49). Prorhynchidae (p. 49). Mesostomatidae (p. 49). Proboscidae (p. 49). Vorticidae (p. 50). Solenopharyngidae (p. 50). |
||
| Alloeocoela (p. 43) |
Plagiostomatidae (p. 50). Bothrioplanidae (p. 50). Monotidae (p. 50). |
||
| TREMATODA (pp. 3, 51) | Monogenea (pp. 5, 52) = Heterocotylea + Aspidocotylea (p. 73) |
Aspidobothridae (p. 73). |
|
| Digenea (pp. 5, 52) = Malacocotylea (p. 73) |
Holostomatidae (p. 73). Amphistomatidae (p. 73). Distomatidae (p. 73). Gasterostomatidae (p. 73). Didymozoontidae (p. 73). Monostomatidae (p. 73). |
||
| {x} |
Cestodariidae = Monozoa (p. 91). Bothriocephalidae (p. 91). Tetrarhynchidae (p. 91). Tetraphyllidae (p. 91). Taeniidae (p. 91). |
||
| MESOZOA | |||
| MESOZOA (pp. 3, 92) |
Dicyemidae (p. 93). Orthonectida (p. 94). |
||
| NEMERTINEA (p. 99) | |||
|
HOPLONEMERTEA (p. 110) = Metanemertini (p. 112). SCHIZONEMERTEA (p. 111) = Heteronemertini (ex parte) (p. 113). PALAEONEMERTEA (p. 111) = Protonemertini (p. 112). + Mesonemertini (p. 112). + Heteronemertini (ex parte) (p. 113). |
|||
| NEMATHELMINTHES (p. 123) | |||
| NEMATODA (pp. 123, 124) |
Ascaridae (p. 138). Strongylidae (p. 142). Trichotrachelidae (p. 144). Filariidae (p. 147). Mermithidae (p. 150). Anguillulidae (p. 154). Enoplidae (p. 157). Chaetosomatidae (p. 158). Desmoscolecidae (p. 159). |
||
| NEMATOMORPHA (pp. 123, 164) | Gordiidae (p. 164). | ||
| ACANTHOCEPHALA (pp. 123, 174) |
Echinorhynchidae (p. 182) Gigantorhynchidae (p. 183). Neorhynchidae (p. 184). Arhynchidae (p. 185). |
||
| CHAETOGNATHA (p. 186) | |||
| ROTIFERA (p. 197) | |||
| FLOSCULARIACEAE (p. 220) |
Flosculariidae (p. 221). Apsilidae (p. 221). |
||
| MELICERTACEAE (p. 221) |
Melicertidae (p. 221). Trochosphaeridae (p. 221). |
||
| BDELLOIDA (p. 222) | Philodinidae (p. 222). | ||
| ASPLANCHNACEAE (p. 222) | Asplanchnidae (p. 223). | ||
| SCIRTOPODA (p. 223) | Pedalionidae (p. 223). | ||
| PLOIMA (p. 223) | Illoricata (p. 223) |
Microcodonidae (p. 224). Rhinopidae (p. 224). Hydatinidae (p. 224). Synchaetidae (p. 224). Notommatidae (p. 224). Drilophagidae (p. 224). Triarthridae (p. 224). |
|
| Loricata (p. 224) |
Rattulidae (p. 225). Dinocharididae (p. 225). Salpinidae (p. 225). Euchlanididae (p. 225). Cathypnidae (p. 225). Coluridae (p. 225). Pterodinidae (p. 225). Brachionidae (p. 225). Anuraeidae (p. 225). |
||
| SEISONACEAE (p. 225) | Seisonidae (p. 226). | ||
| {xi}
|
|||
| GASTROTRICHA | |||
| GASTROTRICHA (p. 231). |
Euichthydina (p. 235) Apodina (p. 235) |
||
| KINORHYNCHA (p. 236) | |||
| CHAETOPODA (p. 241) | |||
| ARCHIANNELIDA (p. 241) | |||
| POLYCHAETA (pp. 241, 245) | Phanerocephala (p. 303) | Nereidiformia (p. 303) |
Syllidae (p. 306). Hesionidae (p. 308). Aphroditidae (p. 309). Phyllodocidae (p. 313). Tomopteridae (p. 315). Nereidae (p. 315). Nephthydidae (p. 317). Amphinomidae (p. 318). Eunicidae (p. 318). Glyceridae (p. 320). Sphaerodoridae (p. 320). Ariciidae (p. 321). Typhloscolecidae (p. 321). |
| Spioniformia (p. 304) |
Spionidae (p. 321). Polydoridae (p. 323). Chaetopteridae (p. 323). Magelonidae (325. Ammocharidae (p. 325). |
||
| Terebelliformia (p. 304) |
Cirratulidae (p. 325). Terebellidae (p. 327). Ampharetidae (p. 330). Amphictenidae (p. 330). |
||
| Capitelliformia (p. 305) | Capitellidae (p. 331). | ||
| Scoleciformia (p. 305) |
Opheliidae (p. 331). Maldanidae (p. 332). Arenicolidae (p. 333). Scalibregmidae (p. 334). Chlorhaemidae (p. 334). Sternaspidae (p. 335). |
||
| Cryptocephala (p. 303) | Sabelliformia (p. 305) |
Sabellidae (p. 336). Eriographidae (p. 338). Amphicorinidae (p. 339). Serpulidae (p. 339). |
|
| Hermelliformia (p. 306) | Hermellidae (p. 341). | ||
| MYZOSTOMARIA (pp. 241, 341) | |||
| OLIGOCHAETA (pp. 241, 347) | Microdrili (p. 373) |
Aphaneura (p. 374). Enchytraeidae (p. 375). Discodrilidae (p. 376). Phreoryctidae (p. 376). Naidomorpha (p. 377). Tubificidae (p. 378). Lumbriculidae (p. 379). Moniligastridae (p. 380). |
|
| Megadrili (pp. 373, 374). |
Perichaetidae (p. 380). Cryptodrilidae (p. 382). Acanthodrilidae (p. 384). Eudrilidae (p. 385). Geoscolicidae (p. 386). Lumbricidae (p. 388). |
||
| {xii}
|
|||
| HIRUDINEA (p. 392) | |||
| RHYNCHOBDELLAE (p. 405) |
Ichthyobdellidae (p. 406). Glossiphoniidae (p. 406). |
||
| GNATHOBDELLAE (p. 407) |
Gnathobdellidae (p. 407). Herpobdellidae (p. 407). |
||
| GEPHYREA (p. 411) | |||
| PHORONIS (p. 450) | |||
| POLYZOA (p. 465) | |||
| ENTOPROCTA (pp. 475, 487) | |||
| ECTOPROCTA (p. 475) | Gymnolaemata (p. 476) | Cyclostomata (p. 477) |
Articulata (p. 517). Inarticulata (p. 517). |
| Cheilostomata (p. 477) |
Cellularina (p. 518). Flustrina (p. 518). Escharina (p. 518). |
||
| Ctenostomata (p. 477) |
Alcyonellea (p. 518). Vesicularina (p. 518). |
||
| Phylactolaemata (pp. 476, 493) | |||
| PLATYHELMINTHES (p. 3) | ||||||
| Family. | ||||||
| TURBELLARIA (p. 3) |  |
Polycladida (p. 7) |  |
Acotylea (p. 16) |  |
Planoceridae (p. 19). Leptoplanidae (p. 19). Cestoplanidae (p. 19). Enantiidae (p. 19). |
| Cotylea (p. 16) |  |
Anonymidae (p. 19) Pseudoceridae (p. 19). Euryleptidae (p. 19). Prosthiostomatidae (p. 19). |
||||
| Tricladida (p. 30) |  |
Paludicola (p. 30) | Planariidae (p. 42). | |||
| Maricola (pp. 30, 32) |  |
Procerodidae (p. 42). = Gundidae. Bdellouridae (p. 42). |
||||
| Terricola (pp. 30, 33) |  |
Bipaliidae (p. 42). Geoplanidae (p. 42). Rhynchodemidae (p. 42). |
||||
| Rhabdocoelida (p. 42) |  |
Acoela (p. 42) |  |
Proporidae (p. 49). Aphanostomatidae (p. 49). |
||
| Rhabdocoela (p. 43) |  |
Macrostomatidae (p. 49). Microstomatidae (p. 49). Prorhynchidae (p. 49). Mesostomatidae (p. 49). Proboscidae (p. 49). Vorticidae (p. 50). Solenopharyngidae (p. 50). |
||||
| Alloeocoela (p. 43) |  |
Plagiostomatidae (p. 50). Bothrioplanidae (p. 50). Monotidae (p. 50). |
||||
| TREMATODA (pp. 3, 51) |  |
Monogenea (pp. 5, 52) = Heterocotylea + Aspidocotylea (p. 73) |  |
Aspidobothridae (p. 73). |
||
| Digenea (pp. 5, 52) = Malacocotylea (p. 73) |  |
Holostomatidae (p. 73). Amphistomatidae (p. 73). Distomatidae (p. 73). Gasterostomatidae (p. 73). Didymozoontidae (p. 73). Monostomatidae (p. 73). |
||||
| {xiii} |  |
Cestodariidae = Monozoa (p. 91). Bothriocephalidae (p. 91). Tetrarhynchidae (p. 91). Tetraphyllidae (p. 91). Taeniidae (p. 91). |
||||
| MESOZOA | ||||||
| MESOZOA (pp. 3, 92) |  |
Dicyemidae (p. 93). Orthonectida (p. 94). |
||||
| NEMERTINEA (p. 99) | ||||||
|
HOPLONEMERTEA (p. 110) = Metanemertini (p. 112). SCHIZONEMERTEA (p. 111) = Heteronemertini (ex parte) (p. 113). PALAEONEMERTEA (p. 111) = Protonemertini (p. 112). + Mesonemertini (p. 112). + Heteronemertini (ex parte) (p. 113). |
||||||
| NEMATHELMINTHES (p. 123) | ||||||
| NEMATODA (pp. 123, 124) |  |
Ascaridae (p. 138). Strongylidae (p. 142). Trichotrachelidae (p. 144). Filariidae (p. 147). Mermithidae (p. 150). Anguillulidae (p. 154). Enoplidae (p. 157). Chaetosomatidae (p. 158). Desmoscolecidae (p. 159). |
||||
| NEMATOMORPHA (pp. 123, 164) | Gordiidae (p. 164). | |||||
| ACANTHOCEPHALA (pp. 123, 174) |  |
Echinorhynchidae (p. 182) Gigantorhynchidae (p. 183). Neorhynchidae (p. 184). Arhynchidae (p. 185). |
||||
| CHAETOGNATHA (p. 186) | ||||||
| ROTIFERA (p. 197) | ||||||
| FLOSCULARIACEAE (p. 220) |  |
Flosculariidae (p. 221). Apsilidae (p. 221). |
||||
| MELICERTACEAE (p. 221) |  |
Melicertidae (p. 221). Trochosphaeridae (p. 221). |
||||
| BDELLOIDA (p. 222) | Philodinidae (p. 222). | |||||
| ASPLANCHNACEAE (p. 222) | Asplanchnidae (p. 223). | |||||
| SCIRTOPODA (p. 223) | Pedalionidae (p. 223). | |||||
| PLOIMA (p. 223) |  |
Illoricata (p. 223) |  |
Microcodonidae (p. 224). Rhinopidae (p. 224). Hydatinidae (p. 224). Synchaetidae (p. 224). Notommatidae (p. 224). Drilophagidae (p. 224). Triarthridae (p. 224). |
||
| Loricata (p. 224) |  |
Rattulidae (p. 225). Dinocharididae (p. 225). Salpinidae (p. 225). Euchlanididae (p. 225). Cathypnidae (p. 225). Coluridae (p. 225). Pterodinidae (p. 225). Brachionidae (p. 225). Anuraeidae (p. 225). |
||||
| SEISONACEAE (p. 225) | Seisonidae (p. 226). | |||||
| {xiv}
GASTROTRICHA |
||||||
| GASTROTRICHA (p. 231). |  |
Euichthydina (p. 235) Apodina (p. 235) |
||||
| KINORHYNCHA (p. 236) | ||||||
| CHAETOPODA (p. 241) | ||||||
| ARCHIANNELIDA (p. 241) | ||||||
| POLYCHAETA (pp. 241, 245) |  |
Phanerocephala (p. 303) |  |
Nereidiformia (p. 303) |  |
Syllidae (p. 306). Hesionidae (p. 308). Aphroditidae (p. 309). Phyllodocidae (p. 313). Tomopteridae (p. 315). Nereidae (p. 315). Nephthydidae (p. 317). Amphinomidae (p. 318). Eunicidae (p. 318). Glyceridae (p. 320). Sphaerodoridae (p. 320). Ariciidae (p. 321). Typhloscolecidae (p. 321). |
| Spioniformia (p. 304) |  |
Spionidae (p. 321). Polydoridae (p. 323). Chaetopteridae (p. 323). Magelonidae (325. Ammocharidae (p. 325). |
||||
| Terebelliformia (p. 304) |  |
Cirratulidae (p. 325). Terebellidae (p. 327). Ampharetidae (p. 330). Amphictenidae (p. 330). |
||||
| Capitelliformia (p. 305) | Capitellidae (p. 331). | |||||
| Scoleciformia (p. 305) |  |
Opheliidae (p. 331). Maldanidae (p. 332). Arenicolidae (p. 333). Scalibregmidae (p. 334). Chlorhaemidae (p. 334). Sternaspidae (p. 335). |
||||
| Cryptocephala (p. 303) |  |
Sabelliformia (p. 305) |  |
Sabellidae (p. 336). Eriographidae (p. 338). Amphicorinidae (p. 339). Serpulidae (p. 339). |
||
| Hermelliformia (p. 306) | Hermellidae (p. 341). | |||||
| MYZOSTOMARIA (pp. 241, 341) | ||||||
| OLIGOCHAETA (pp. 241, 347) |  |
Microdrili (p. 373) |  |
Aphaneura (p. 374). Enchytraeidae (p. 375). Discodrilidae (p. 376). Phreoryctidae (p. 376). Naidomorpha (p. 377). Tubificidae (p. 378). Lumbriculidae (p. 379). Moniligastridae (p. 380). |
||
| Megadrili (pp. 373, 374). |  |
Perichaetidae (p. 380). Cryptodrilidae (p. 382). Acanthodrilidae (p. 384). Eudrilidae (p. 385). Geoscolicidae (p. 386). Lumbricidae (p. 388). |
||||
| {xv}
HIRUDINEA (p. 392) |
||||||
| RHYNCHOBDELLAE (p. 405) |  |
Ichthyobdellidae (p. 406). Glossiphoniidae (p. 406). |
||||
| GNATHOBDELLAE (p. 407) |  |
Gnathobdellidae (p. 407). Herpobdellidae (p. 407). |
||||
| GEPHYREA (p. 411)| | ||||||
| PHORONIS (p. 450) | ||||||
| POLYZOA (p. 465) | ||||||
| ENTOPROCTA (pp. 475, 487) | ||||||
| ECTOPROCTA (p. 475) |  |
Gymnolaemata (p. 476) |  |
Cyclostomata (p. 477) |  |
Articulata (p. 517). Inarticulata (p. 517). |
| Cheilostomata (p. 477) |  |
Cellularina (p. 518). Flustrina (p. 518). Escharina (p. 518). |
||||
| Ctenostomata (p. 477) |  |
Alcyonellea (p. 518). Vesicularina (p. 518). |
||||
| Phylactolaemata (pp. 476, 493) | ||||||
BY
F. W. GAMBLE, M.Sc. (Vict.)
Demonstrator and Assistant-Lecturer in Zoology in the Owens College, Manchester.
TURBELLARIA
INTRODUCTION: DESCRIPTION OF THE POLYCLAD LEPTOPLANA TREMELLARIS—APPEARANCE—HABITS—STRUCTURE: POLYCLADIDA—CLASSIFICATION—HABITS—ANATOMY—DEVELOPMENT: TRICLADIDA—OCCURRENCE—STRUCTURE—CLASSIFICATION: RHABDOCOELIDA—OCCURRENCE—HABITS—REPRODUCTION—CLASSIFICATION.
The Platyhelminthes, or Flat Worms, form a natural assemblage of animals, the members of which, however widely they may differ in appearance, habits, or life-history, exhibit a fundamental similarity of organisation which justifies their separation from other classes of worms, and their union into a distinct phylum. Excluding the leeches (Hirudinea), and the long sea-worms (Nemertinea)—which, though formerly included, are now treated independently—the Platyhelminthes may be divided into three branches: (1) Turbellaria (including the Planarians), (2) Trematoda (including the liver-flukes), and (3) Cestoda (tape-worms). The Mesozoa will be treated as an appendix to the Platyhelminthes.
The Turbellaria were so called by Ehrenberg[1] (1831) on account of the cilia or vibratile processes with which these aquatic animals are covered, causing by their incessant action, tiny currents ("turbellae," disturbances) in the surrounding water. The ciliary covering distinguishes this free-living group from the parasitic Trematodes and Cestodes, some of which possess such an investment, but only during their early free {4}larval stage, for the short period when they have left the parental host and are seeking another (Figs. 26, 27, 42).
Some Turbellaria (Rhabdocoelida) resemble Infusoria in their minute size, shape, and movements. Nevertheless they possess an organisation of considerable complexity. The fresh-water Planarians (Fig. 14), abounding in ponds and streams, vary from a quarter to half an inch in length, and are elongated and flattened. Their body is soft, and progresses by a characteristic, even, gliding motion like a snail. The marine Planarians or Polyclads (Fig. 8) are usually broad and leaf-like, sometimes attaining a length of six inches, and swim or creep in a most graceful way. Land Planarians occur in this country (Fig. 15), but far more abundantly in tropical and sub-tropical districts, in moist places, venturing abroad at night in pursuit of prey. They are elongated and cylindrical, in some cases measuring, when fully extended, a foot or more in length, and are often ornamented with brilliantly coloured, longitudinal bands.
Turbellaria are carnivorous, overpowering their prey by peculiar cutaneous offensive weapons, and then sucking out the contents of the victim by the "pharynx." Land Planarians feed on earthworms, molluscs, and wood-lice; fresh-water Planarians on Oligochaet worms, water-snails, and water-beetles; marine forms devour Polychaet worms and molluscs. Some Turbellaria seem to prefer freshly-killed or weakly examples of animals too large to be overpowered when fully active. Certain Rhabdocoelida are messmates of Molluscs and Echinoderms, and a few others are truly parasitic—a mode of life adopted by all Trematodes save Temnocephala.
The Trematodes[2] may be divided into those living on the outer surface of various aquatic animals, usually fish (Ectoparasites); and those which penetrate more or less deeply into the alimentary canal or the associated organs of the host (Endoparasites). They are oval, flattened Platyhelminthes ranging from a microscopic size to a length of three feet (Nematobothrium, Fig. 22), and are provided with organs of adhesion by which they cling to the outer surface, or to the interior, of the animals they inhabit. Trematodes occur parasitically in all groups of Vertebrates, but, with the exception of the liver-flukes of the sheep (Distomum hepaticum and D. magnum), and of Bilharzia haematobia found in man (in the blood-vessels of the urinary bladder) over the greater part {5}of Africa, their attacks are not usually of a serious nature. Ectoparasitic Trematodes are Monogenetic; that is, their larvae grow up directly into mature forms. The Endoparasitic species, however, are usually Digenetic. Their larvae enter an Invertebrate and produce a new generation of different larvae, and these another. The last are immature flukes. They enter a second host, which is swallowed by the final Vertebrate host in which they become mature.
The Cestodes or Tape-worms have undergone more profound modifications both in structure and in mode of development. They are all endoparasitic, and, with one exception (Archigetes), attain maturity solely within the alimentary canal of Vertebrates. In length they range from a few millimetres to several metres, but this great size is attained from the need for the rapid production and accumulation of enormous numbers of eggs. The "head" or "scolex" is attached to the mucous membrane of the host by suckers or hooks, but there is no mouth nor any certain trace of a digestive tract at any stage of the life-history of Cestodes. For nourishment they absorb, through the skin, the previously-digested food (of the host) that bathes them. In a few Cestodes the body is simple and not divided into "proglottides" or generative segments, but in most cases it is jointed in such a way that the last segment is the oldest, and each contains a set of reproductive organs. The life-histories of Cestodes are most remarkable. The proglottides containing the eggs pass out of the final host along with the faeces and enter the intermediate host with the food. The larvae hatch, and boring their way into the blood-vessels, are carried by the circulation to various internal organs. Here they usually become "bladder-worms," and develop the "head" of the future sexual form. Then, if, as is usually the case, the intermediate host is preyed upon by the final host, the larval Cestodes enter the alimentary canal of the latter. The head of the larva alone survives digestion, and from it the mature worm is formed.
Of these three branches of the phylum Platyhelminthes, the Turbellaria possess features of special interest and importance. Not only do they furnish the explanation of the structure of the two parasitic groups (which have probably arisen from Turbellarian-like ancestors), but they occupy the lowest position in the whole group of worms. There are reasons for thinking that this is the simplest group of bilateral animals which adopt the habit of {6}creeping. The Turbellaria are most closely allied to that great extinct group from which they, the Nemertinea, Rotifera, and even the Annelids, offer increasingly convincing evidence of having been derived. Many questions relating to the affinities of, or the origin of organs in, the Annelids, resolve themselves into similar questions about the Turbellaria. For these reasons, this group is here dealt with at greater length than the others, the interest of which is of a more special nature.
The history of our knowledge of the Cestodes dates back to ancient times, as the presence and effects of tape-worms early attracted the attention of physicians. Trematodes are first distinctly referred to in the sixteenth century, while Turbellaria first figure in Trembley's memoir on Hydra (1744).[3] The whole subject of the increase in our knowledge of parasitic Platyhelminthes is dealt with in the standard work, The Parasites of Man, by Leuckart,[4] and a complete list of references in zoological literature to Cestodes and Trematodes is to be found in Bronn's Thierreich.[5] O. F. Müller[6] and Ehrenberg founded our knowledge of the Turbellaria, but for a long time the group remained in a most neglected condition. In this country Montagu, G. Johnston, and in Ireland, William Thompson, discovered several marine species, one of which, Planocera folium (from Berwick), has not again been met with on British shores. Dalyell[7] conducted classical researches on the habits of Planarians, and Faraday[8] made interesting experiments on their power of regenerating lost parts. The credit of assigning the correct interpretation to most of the various organs of fresh-water Planarians belongs to von Baer[9] and Dugès,[10] while Mertens[11] effected a similar service for the marine forms, or Polyclads. The minute Rhabdocoels were first successfully investigated and classified by Oscar Schmidt.[12] The great work on this group is, however, the {7}monograph by von Graff.[13] A similarly comprehensive and indispensable treatise by Lang, on the Polycladida,[14] contains references to all previous publications on the group, among which the papers by Quatrefages, Johannes Müller, Keferstein, Minot, and Hallez stand out conspicuously. Moseley's work[15] on the Land Planarians of Ceylon is undoubtedly the most revolutionary paper referring to this group, and the best contribution towards elucidating the structure of the Tricladida at a time when the subject was very obscure. A monograph on Land Planarians is being prepared by von Graff.
The Turbellaria are divided into: (1) Polycladida, marine forms with multiple intestinal branches; (2) Tricladida, marine, fresh-water, and terrestrial Planarians with three main intestinal branches; (3) the Rhabdocoelida, as varied in habit as the Triclads, but possessing a straight and simple or slightly lobed, intestine. A detailed description of an example of the Polyclads, and then a comparative account of each division, will now be given.
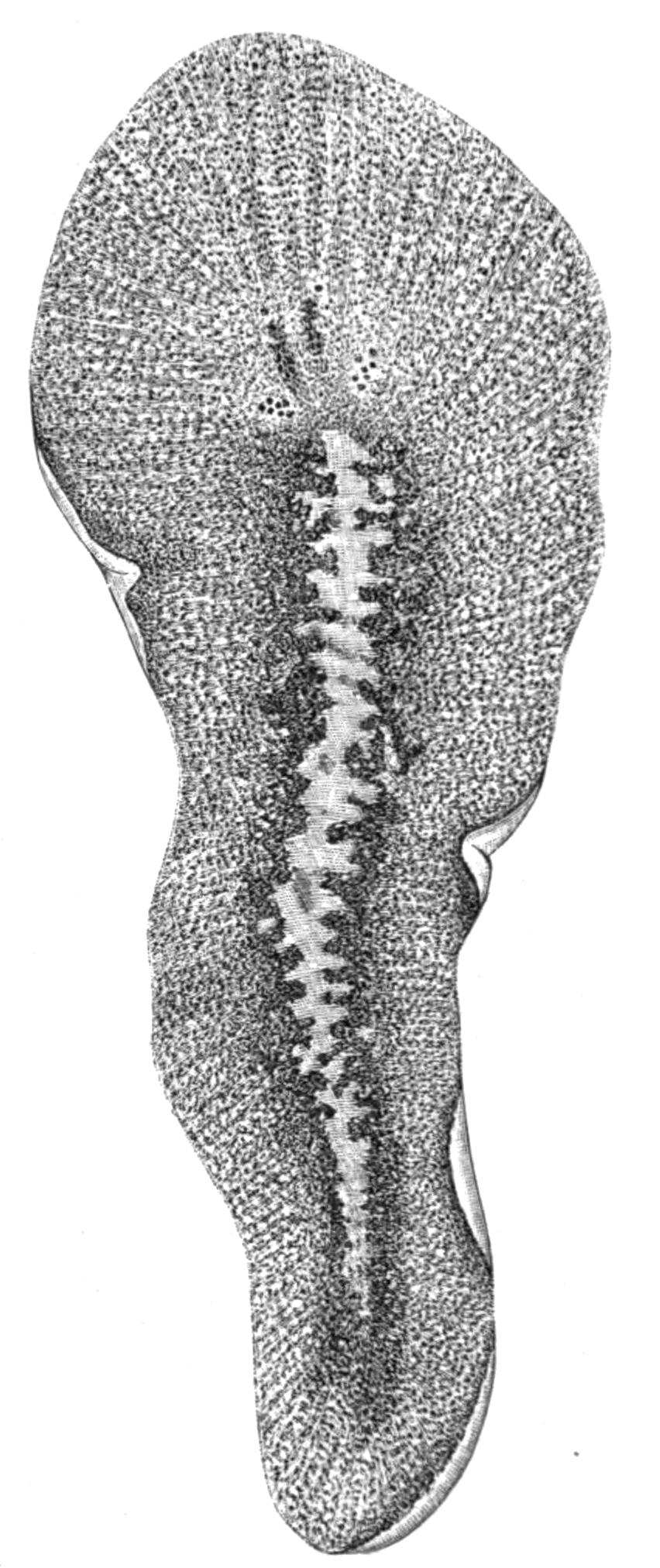
Fig. 1.—Leptoplana tremellaris O. F. M. Seen from the dorsal surface. The alimentary canal runs down the middle line and sends branches to the margin of the body. × 6.
Turbellaria. I. Polycladida.
Description of Leptoplana tremellaris.
Appearance and Habits.—An account of the Polyclad Turbellaria may be fitly prefaced by a description of a very common representative, Leptoplana tremellaris, so called on account of the thin, flat body which executes when disturbed, quivering or tremulous swimming movements.
Like all Polyclads, Leptoplana is marine. It is probably found on all European shores, northwards to Greenland and southwards to the Red Sea, while vertically it ranges from the littoral zone down to fifty fathoms. There is, however, an apparently well-marked difference between the littoral specimens, which vary from three-quarters to one inch in length, are brownish in colour and firm in consistency, and the more delicate examples half an inch long, white with a brown tinge, which occur in deeper water.
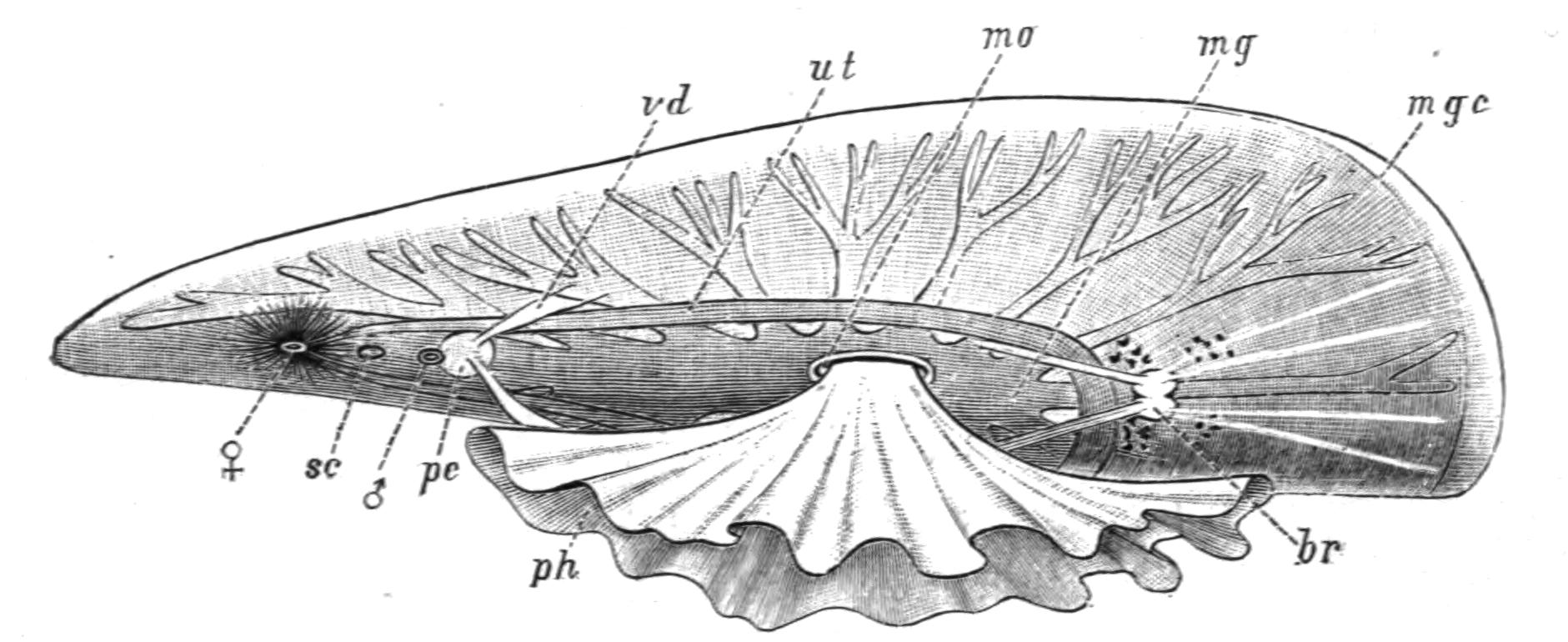
Fig. 2.—Leptoplana tremellaris. Three-quarters view from the ventral surface. The pharynx (ph) is widely protruded through the month (mo) as in the act of attacking prey. br, Brain with nerves, close to which are the four groups of eyes; mg, stomach; mgc, "marginal groove"; pe, penis; sc, sucker; ut, uterus; vd, vasa deferentia; ♀, female genital aperture surrounded by the shell-gland; ♂, male aperture. (Semi-diagrammatic, and × 6.)
At low water Leptoplana may be found buried in mud or on the under surface of stones, in pools where darkness and dampness may be ensured till the return of the tide. It is, however, by no means easy to detect and remove it from the encrusting Polyzoa, Ascidians, or Sponges with which it is usually associated. The flat, soft, unsegmented body is so closely appressed to the substratum that its presence is usually only betrayed by its movement, an even gliding motion of the mobile body, which suggested the apt name "la pellicule animée" to Dicquemare. The creeping surface is called ventral, the upper one dorsal, and as the broader end of the body always goes first, it is anterior as opposed to the more pointed posterior extremity. With a lens the characters shown in Figs. 1 and 2 may be observed. The eyes are seen as black dots near the anterior end, and are placed at the sides of a clear oval space, the brain. Along the transparent margin of the body, the ends of the intestinal branches may be seen. These ramify from a lobed stomach or main-gut, and should the specimen be mature, the "uterus" loaded {9}with eggs forms a dark margin round the latter (Figs. 1 and 2, ut). The ventral surface is whitish, and through it the "pharynx," a frilled protrusible structure, may be dimly observed. The "mouth,"[16] through which the pharynx at the time of feeding is thrust out (Fig. 2, mo), is almost in the centre of the ventral surface. Behind this, a white, V-shaped mark (vd) indicates the ducts of the male reproductive organs, and still further back is the irregular opaque mark of the "shell-gland," by which the egg-shells are formed (Fig. 2, ♀).
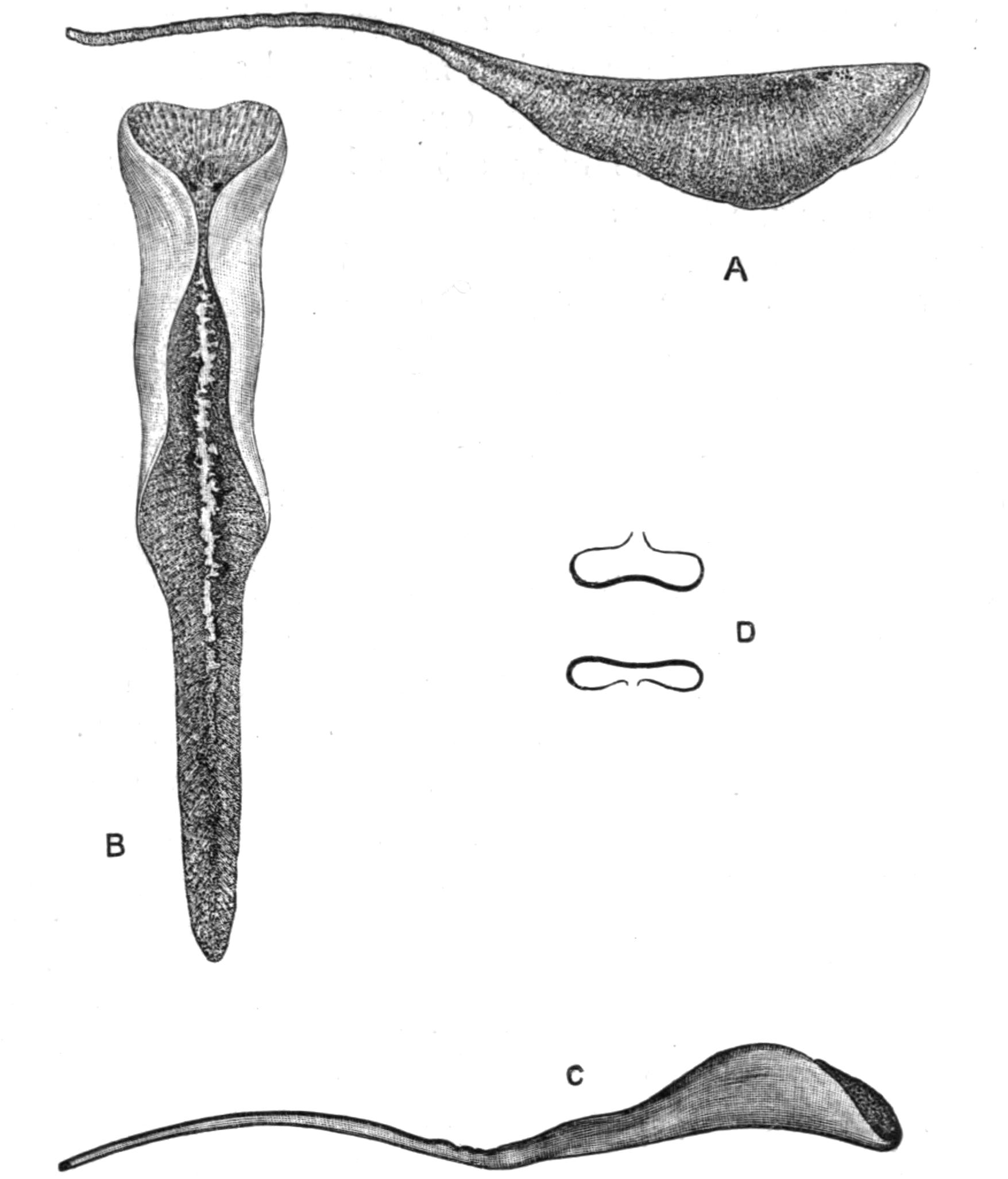
Fig. 3.—Leptoplana tremellaris in the act of swimming. A, Seen from the right side during the downward stroke (the resemblance to a skate is striking); B, from above, showing the upward stroke and longitudinal undulations of the swimming lobes; C, side view during the upward stroke; D, transverse sections of the body during the strokes. × 5.
Leptoplana employs two kinds of movement, creeping and swimming. Creeping is a uniform gliding movement, caused by the cilia of the ventral surface, aided perhaps by the longitudinal muscular layers of this surface, and is effected on the under side of the "surface-film" of water almost as well as on a solid substratum. Swimming is a more rapid and elegant movement, employed when alarmed or in pursuit of prey. The expanded fore-parts of the body act as lobes, which are flapped rapidly up over the body and then down beneath it, undulations running rapidly down them from before backwards. The action in fact is somewhat similar to that by which a skate swims, a resemblance pointed out long ago by Dugès[17] (Fig. 3).
We have few direct observations on the nature of the food of Leptoplana, or the exact mode by which it is obtained. Dalyell,[18] who observed this species very carefully, noticed that it was nocturnal and fed upon a Nereis, becoming greatly distended and of a green colour after the meal, but pale after a long fast. Keferstein[19] noticed a specimen in the act of devouring a Lumbriconereis longer than itself, and also found the radulae of Chiton and Taenioglossate Molluscs in the intestine. That such an apparently weak and defenceless animal does overpower large and healthy Annelids and Mollusca, has not hitherto been definitely proved. Weak or diseased examples may be chiefly selected. The flexible Leptoplana adheres firmly to its prey, and the rapid action of the salivary glands of its mobile pharynx quickly softens and disintegrates the internal parts of the victim. The food passes into the stomach (Fig. 2, mg), and is there digested. It is then transferred to the lateral branches of the intestine, and, after all the nutritious matters have been absorbed, the faeces are ejected with a sudden contraction of the whole body through the pharynx into the water.
Leptoplana probably does not live more than a year. In the spring or summer, batches of eggs are laid and fixed to algae or stones by one individual, after having been fertilised by another. Young Leptoplana hatch out in two to three weeks, and lead a {11}pelagic existence till they are three or four millimetres in length. In late summer, numbers of such immature examples may be found among sea-weeds and Corallina in tide pools. In the succeeding spring they develop first the male and then the female reproductive organs.
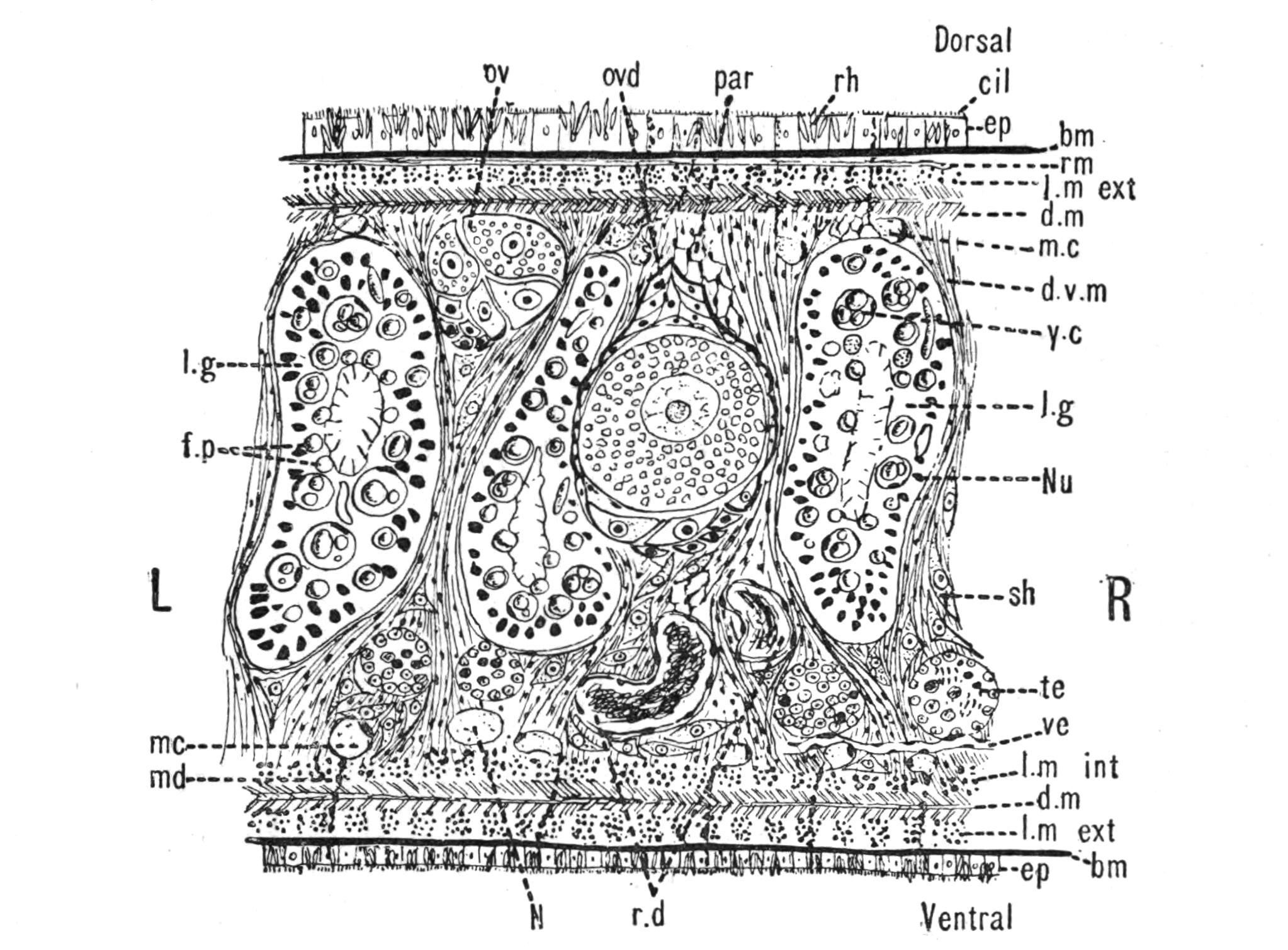
Fig. 4.—Portion of a transverse section of Leptoplana tremellaris in the hinder part of the body. × 100. bm, Basement (skeletal) membrane; cil, cilia; d.m, diagonal muscles; d.v.m, dorso-ventral muscles; ep, epidermis; f.p, food particles; l.g, lateral intestinal branches cut across; l.m ext, external, and l.m int, internal longitudinal muscle layers; m.c, glandular (mucous) cells; md, their ducts; N, longitudinal nerve; Nu, nuclei of the intestinal epithelium; ov, ovary; ovd, oviduct; par, cells of the parenchyma; r.d, vasa deferentia, with spermatozoa; rm, circular musculature; rh, rhabdites; sh, cells of the shell-gland; te, testes; ve, vasa efferentia; y.c, "yellow cells." (After Lang.)
Anatomy of Leptoplana tremellaris.—Leptoplana may be divided into corresponding halves only by a median vertical longitudinal plane. The body and all the systems of organs are strictly bilaterally symmetrical. Excepting the cavities of the organs themselves, the body is solid. A connective "parenchyma" (Fig. 4, par) knits the various internal organs together, while it allows free play of one part on another. These organs are enclosed in a muscular body-wall, clothed externally by the ciliated epidermis, which is separated from the underlying musculature by a strong membrane (Fig. 4, bm), the only skeletal element in the body.
Body-Wall.—The epidermis (Fig. 4, ep) is composed of a single layer of ciliated cells, containing small, highly refractive, pointed rods or "rhabdites" (rh), and gives rise to deeply-placed mucous cells (m.c), which are glandular and pour out on the surface of the body a fluid in which the cilia vibrate. The tenacious hold on a stone which Leptoplana exerts if suddenly disturbed, or when grasping its prey, is probably due to the increased glutinous secretion of these glands, aided perhaps by rhabdites, which on such occasions are shot out in great numbers. The basement membrane is an elastic skeletal membrane composed of stellate cells embedded in a firm matrix. It serves chiefly for the origin and insertion of the dorso-ventral muscles (d.v.m). Under the basement membrane lies a very thin layer of transverse muscular fibres (Fig. 4, rm), which are, however, apparently absent on the ventral surface. Then follows a stout layer of longitudinal fibres (l.m ext), and beneath this a diagonal layer (d.m), the fibres of which intersect along the median line in such a way that the inner fibres of one side become the outer diagonal fibres of the other. Lastly, within this again, on the ventral surface, is a second stout longitudinal layer (l.m int). The sucker (sc, Figs. 2 and 5) is a modification of the body-wall at that point. In addition to the dorso-ventral muscles, there exists a complex visceral musculature regulating the movements of the pharynx, intestine, and copulatory organs.
Parenchyma.—The spaces between the main organs of the body are filled by a tissue containing various kinds of cells, salivary glands, shell-glands, and prostate glands. Besides these, however, we find a vacuolated, nucleated, thick-walled network, and to this the word parenchyma is properly applied. Besides its connective function, the parenchyma confers that elasticity on the body which Leptoplana possesses in such a high degree. Pigment cells are found in the parenchyma in many Polyclads.
Digestive System.—The general arrangement of this system may be seen in Figs. 2, 5, and 7; and may be compared, especially when the pharynx is protruded, as in Fig. 2, with the gastral system of a Medusa. The "mouth" (there is no anus) is placed almost in the centre of the ventral surface. It leads (Fig. 7, B, phs) into a chamber (the peripharyngeal space) divided into an upper and a lower division by the insertion of a muscular collar-fold (the pharynx, ph), which may be protruded, its free lips {13}advancing, through the mouth (Fig. 2), and is then capable of enclosing by its mobile frilled margin, prey as large as Leptoplana itself. The upper division of the chamber communicates by a hole in its roof[20] (the true mouth, Figs. 5 and 7, g.m) with the cavity of the main-gut or stomach (m.g), which runs almost the length of the body in the middle line, forwards over the brain (Fig. 5, up). Seven pairs of lateral gut-branches convey the digested food to the various organs, not directly however, but only after the food mixed with sea-water has been repeatedly driven by peristalsis first towards the blind end of the gut-branches and then back towards the stomach. Respiration is probably largely effected by this means. The epithelium of the intestine (Fig. 4, l.g) of a starving specimen is composed of separate flagellated cells frequently containing "yellow cells."[21] After a meal, however, the cell outlines are invisible. Gregarines, encysted Cercariae, and Orthonectida[22] occur parasitically in the gut-branches.
An excretory system of "flame-cells" and fine vessels has hitherto been seen only by Schultze[23] in this species, which will not, however, resist intact the compression necessary to enable the details to be determined. They are probably similar to those of Thysanozoon described on p. 25.
Nervous System.—The brain, which is enclosed in a tough capsule (Fig. 5, br), is placed in front of the pharynx, but some distance behind the anterior margin of the body. It is of an oval shape, subdivided superficially into right and left halves by a shallow depression, and is provided in front with a pair of granular-looking appendages, composed of ganglion-cells from which numerous sensory nerves arise, supplying the eyes and anterior region. Posteriorly the brain gives rise to a chiefly motor, nervous sheath (Fig. 5, nn), which invests the body just within the musculature. This sheath is thickened along two ventral lines (Fig. 5, ln) and two lateral lines (n.s), but is very slightly developed on the dorsal surface. Ganglion-cells occur on the course of the nerves, and are particularly large at the point of origin of the great motor nerves.
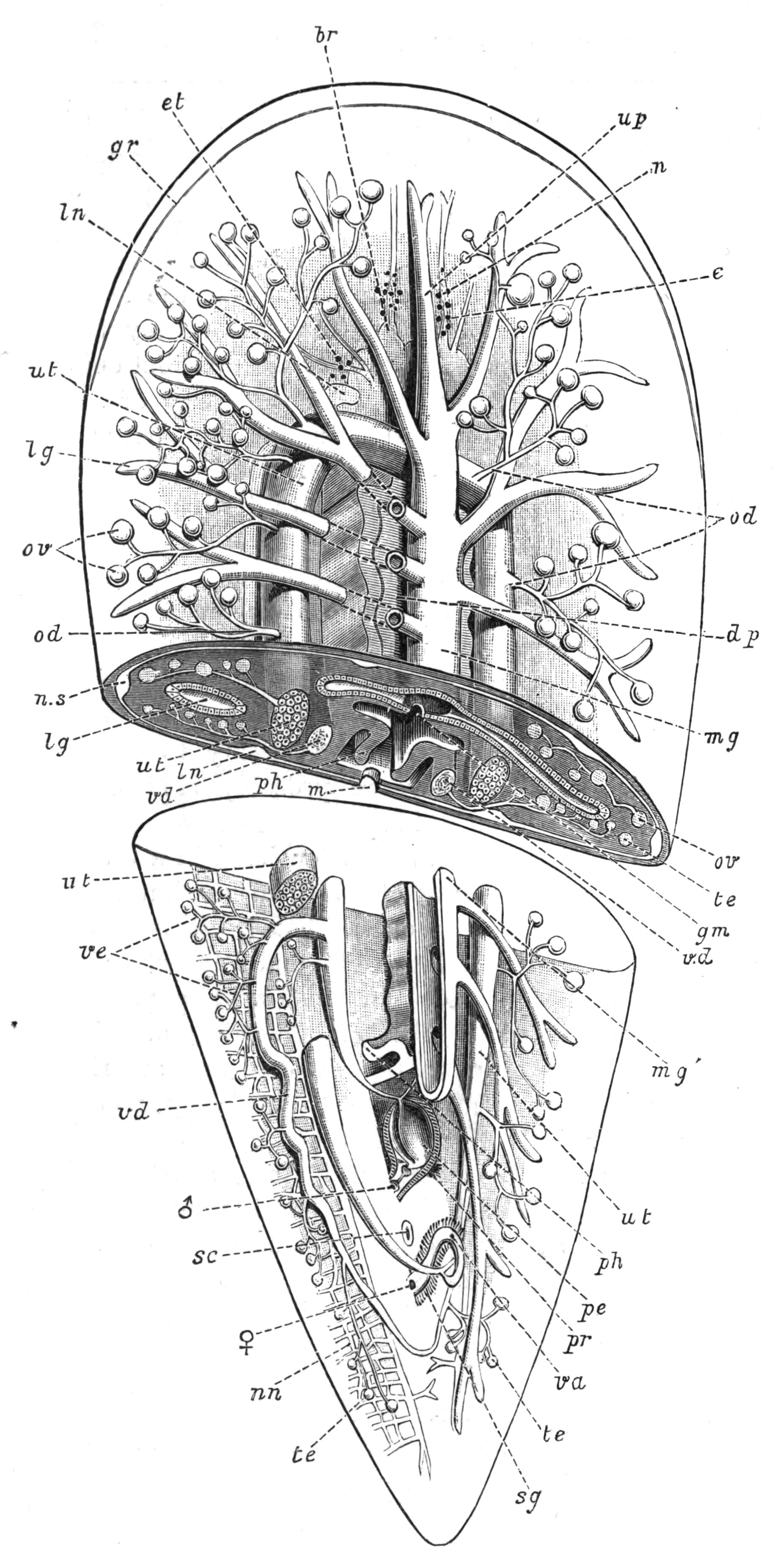
Fig. 5.—Diagrammatic view of the structure of Leptoplana tremellaris as a type of the Polycladida. The body is cut across the middle to show the relative position of organs in transverse section. In the posterior half the alimentary canal has been bisected and removed from the left side, to exhibit the deeply placed nervous sheath (nn) and the male reproductive organs. br, Brain; dp, "diaphragm"; e, cerebral group of eyes; et, tentacular eye-group; gr, marginal groove; gm, true mouth; lg, lateral gut-branch; ln, longitudinal nerve stem; m, external mouth; mg, mg', main-gut, whole, and bisected; n, sensory nerve supplying the eyes; nn, nervous network lying on the ventral musculature; n.s, lateral nerve; od, oviduct; ov, ovary; pe, penis (in section); ph, pharynx; pr, prostate or "granule gland"; sc, sucker; sg, shell-gland; te, testes; up, anterior unpaired gut-branch; ut, uterus; va, vagina (in section); vd, vas deferens; ve, vasa efferentia; ♂, male genital pore; ♀, female pore.
Sense Organs.—Leptoplana possesses eyes, stiff tactile, marginal cilia, and possibly a sense organ in the "marginal groove." The eyes, which are easily seen as collections of black dots lying at the sides of the brain, may be divided into two paired groups: (1) cerebral eyes (Fig. 5, e), and (2) tentacle eyes (et), which indicate the position of a pair of tentacles in allied forms (Fig. 8, A, t and B). Each ocellus consists of a capsule placed at right angles to the surface of the body in the parenchyma, below the dorsal muscles, and with its convex face outwards. It is a single cell in which pigment granules have accumulated. The light, however, can only reach the refractive rods, which lie within it, obliquely at their outer ends. These rods are in connexion with the retinal cells, and thus communicate by the optic nerve with the brain. The cerebral eyes are really paired, and are directed some upwards, some sideways, some downwards.
The "marginal groove" is a shallow depression of the epidermis (Fig. 5, gr) lined by cilia, and containing the ducts of very numerous gland-cells. It runs almost parallel to the anterior margin of the body, a short distance from it, but we have no observations on its functions.
Reproductive Organs.—Leptoplana is hermaphrodite, and, as in most hermaphrodites, the reproductive organs are complicated. The male organs are the first to ripen, but this does not appear to prevent an overlapping of the periods of maturity of the male and female products, so that when the eggs are being laid, the male organs are, apparently, still in a functional state. The principal parts are seen in Fig. 5. The very numerous testes (te) are placed ventrally, and are connected with fine vasa efferentia (ve), which form a delicate network opening at various points into the two vasa deferentia (vd). These tubes, especially when distended with spermatozoa, may easily be seen (Fig. 2, vd) converging at the base of the penis, and connected posteriorly by a loop that runs behind the female genital pore (Fig. 5). The penis (pe) is pyriform and muscular, and is divided into two chambers, a large upper one for the spermatozoa, and a smaller lower one for the secretion of a special {16}"prostate" gland. The apex of the penis is eversible and not merely protrusible, being turned inside out when evaginated. The ovaries (Fig. 5, ov) are numerous and somewhat spherical. They are dorsally placed, but when fully developed extend deeply wherever they can find room to do so, and they not only furnish the ova, but elaborate food-yolk in the ova, as there are no special yolk-glands. The slender oviducts (od) open at several points into the "uterus" (ut) (a misnomer, as no development takes place within it), which encircles the pharynx, and opens by a single duct into the vagina (va). Here the ova are probably fertilised, and one by one invested by the shell-gland (sg) with a secretion which hardens and forms a resistant shell. They are then laid in plate-like masses which are attached to stones or shells. The development is a direct one, and the young Leptoplana, which hatches in about three weeks, has the outline of a spherical triangle, and possesses most of the organs of the adult. After leading a floating life for a few weeks it probably attains maturity in about nine months.
Classification, Habits, and Structure of the Polycladida.
The Polyclads were so called by Lang on account of the numerous primary branches of their intestine. They are free-living, purely marine Platyhelminthes, possessing multiple ovaries, distinct male and female genital pores (Digonopora), but no yolk-glands. The eggs are small, and in many cases give rise to a distinct larval form, known as "Müller's larva" (Fig. 12). The Polyclads, with one exception,[24] fall into two sub-groups, Acotylea and Cotylea:—
| Character. | Acotylea. | Cotylea. |
| Sucker | A sucker absent.[25] | A sucker always present (Figs. 8, D, s; 7, A, sc). |
| Mouth | In the middle, or behind the middle, of the ventral surface. | In the middle, or in front of the middle, of the ventral surface. |
| Pharynx | More or less intricately folded. | Rarely folded. Usually cylindrical or trumpet-shaped. |
| Tentacles | A pair of dorsal tentacles usually present. | A pair of marginal tentacles (except in Anonymus). |
| Development | Usually direct. Larva when present, not a typical Müller's larva. | Müller's larva present. Metamorphosis, however, extremely slight. |
Fig. 8 shows that, starting with a member (A, D) of each division, in which the mouth is almost in the middle of the ventral surface, and the brain and sense organs somewhat remote from the anterior end, we find in the Acotylea a series leading to an elongated form (Cestoplanidae), in which the mouth, pharynx, and genital pores are far back near the hinder end of the body; while in the Cotylea the series leads similarly to the elongated Prosthiostomatidae, in which, however, the pharynx and external apertures are in the front part of the body. This view of the morphology of the Polyclads is due to Lang, and is based on the assumption that the more radially-constructed forms (Fig. 8, A, D) are the primitive ones.
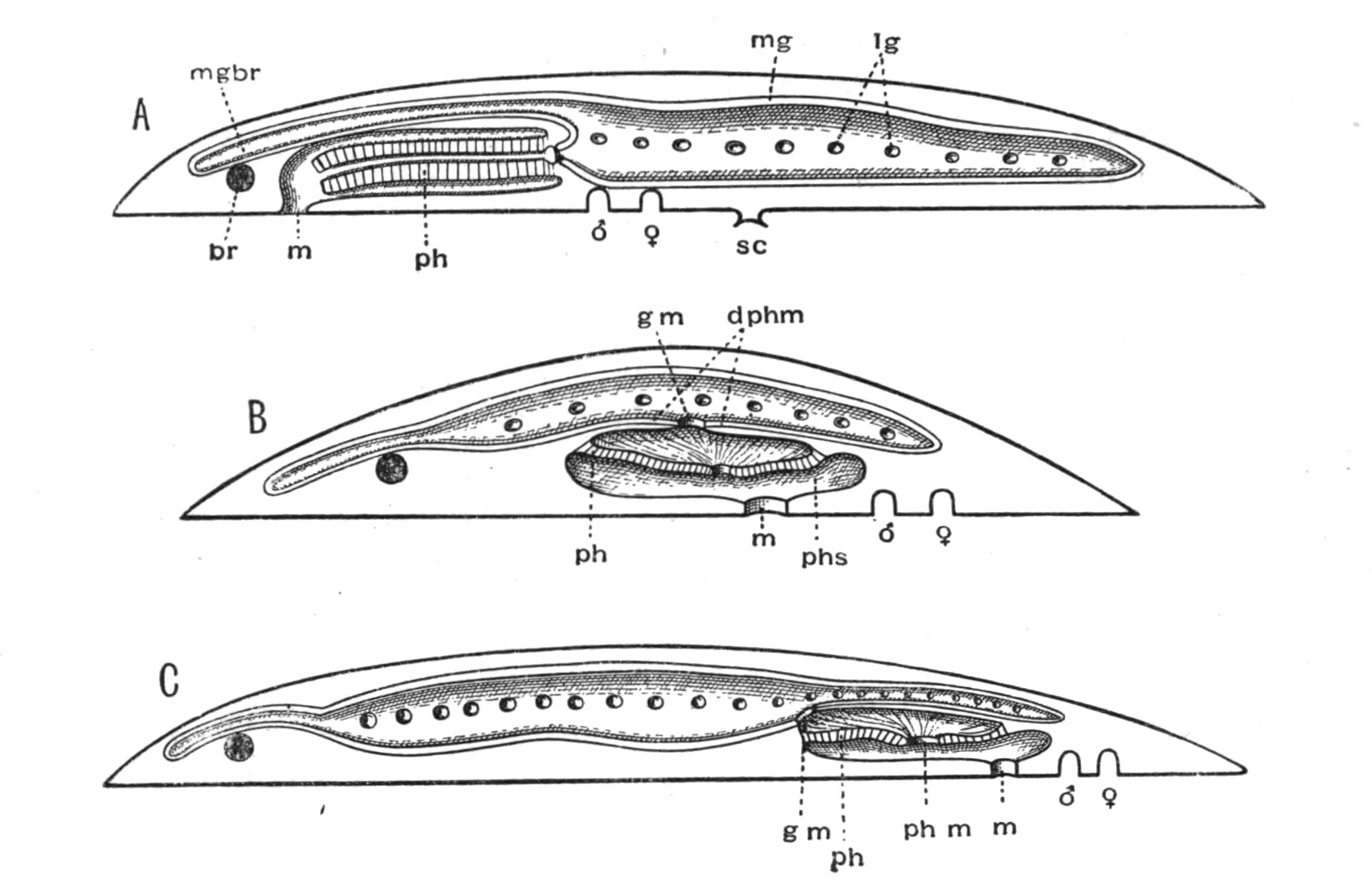
Fig. 7.—Diagrammatic vertical longitudinal sections: A, Of Prosthiostomum (type of Cotylea); B, of Leptoplana; C, of Cestoplana (types of Acotylea). (After Lang.) These figures illustrate the changes which follow the shifting of the mouth from a central position (B) to either end of the body. br, Brain; dphm, "diaphragm"; gm, true mouth; lg, openings of lateral gut-branches; m, mouth; mg, main-gut or stomach; mgbr, median gut-branch; ph, pharynx; ph.m, aperture in pharyngeal fold; phs, peripharyngeal sheath; sc, sucker; ♂, male, and ♀, female, genital aperture.
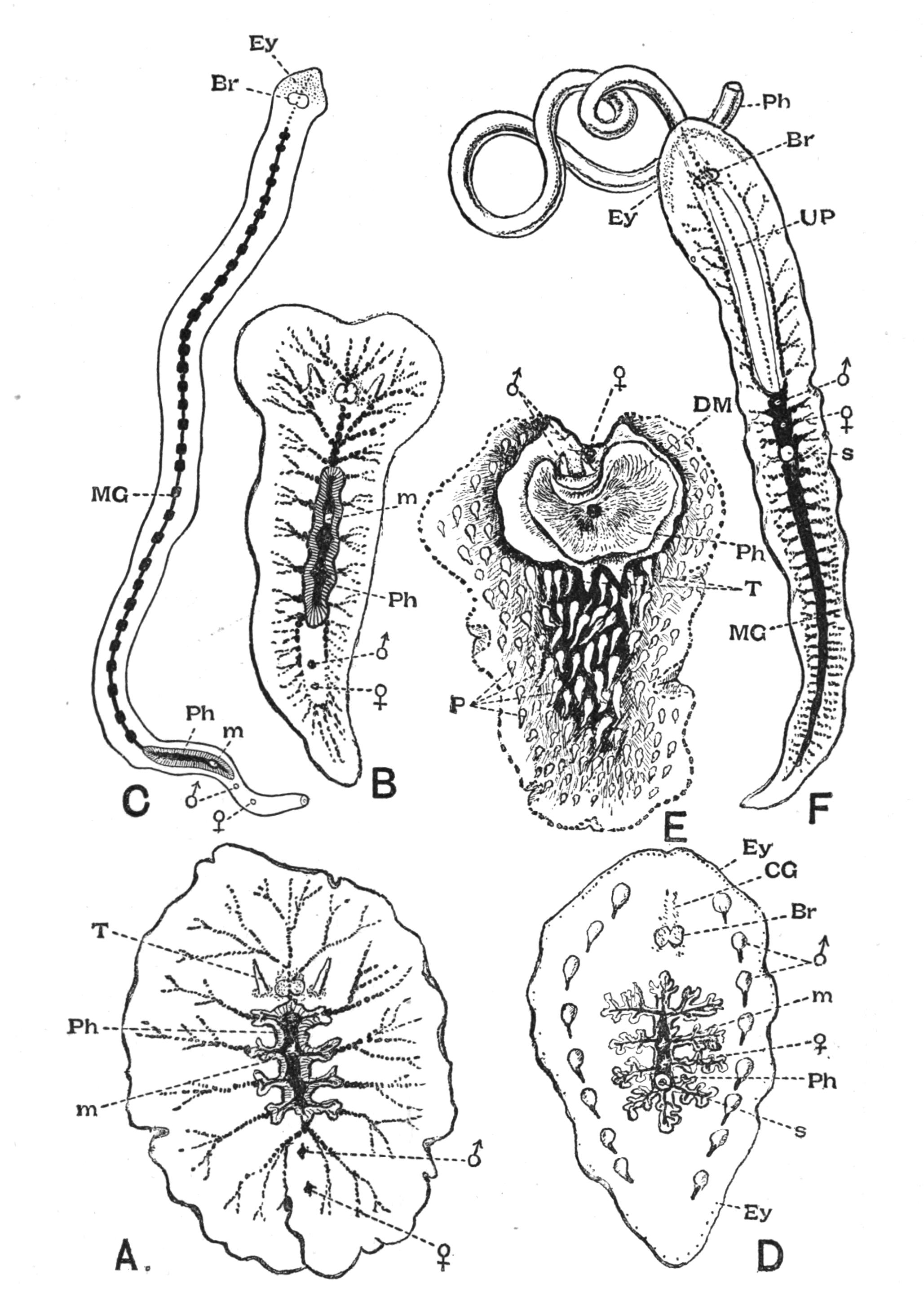
Fig. 8.—Chief forms of Polycladida: A-C, Acotylea; D-F, Cotylea. A, Planocera graffii Lang, nat. size; B, Stylochoplana maculata Stimps, × 7; C, Cestoplana rubrocincta Lang, × 4⁄3; D, Anonymus virilis Lang, × 3, ventral surface; E, Thysanozoon brocchii Grube, nat. size; the head is thrown back and the pharynx (ph) is protruded. F, Prosthiostomum siphunculus Lang, × 3. Br, Brain; CG, cerebral eye group; DM, true mouth; Ey, marginal eyes; m, mouth; MG, main-gut or stomach; P, dorsal papillae; Ph, pharynx; s, sucker (ventral); T, tentacles; UP, dorsal median gut-branch. ♂, male, and ♀, female, genital aperture, except in D, where ♂ refers to the multiple penes. (After Lang and Schmidt.)
Classification of Polycladida.
| ACOTYLEA. | ||
| Family. | Genus. | British Representatives. |
|
Planoceridae. With dorsal tentacles. Mouth sub-central. |
Planocera (Fig. 8, A). Imogine. Conoceros. Stylochus. Stylochoplana (Fig. 8, B). Diplonchus. Planctoplana. |
Planocera folium Grube. Berwick-on-Tweed. Stylochoplana maculata Quatref. Among brown weeds in Laminarian zone. |
|
Leptoplanidae. Without dorsal tentacles. Penis directed backwards. |
Discocelis. Cryptocelis. Leptoplana. Trigonoporus. ?Polypostia (see p. 27). |
Leptoplana tremellaris O. F. Müll. L. fallax Quatref. Plymouth. L. droebachensis Oe. Plymouth Sound. L. atomata O. F. Müll. Doubtful species. |
|
Cestoplanidae. No tentacles. Body elongated. Penis directed forwards. |
Cestoplana (Fig. 8, C). In Mediterranean and on French side of the Channel. |
|
|
Enantiidae. No sucker. No tentacles. Main-gut very short. External apertures as in Euryleptidae. |
Enantia. Adriatic Sea. |
|
| COTYLEA. | ||
|
Anonymidae. Mouth central. No tentacles. With two rows of penes. |
Anonymus (Fig. 8, D). Naples (two specimens). |
|
|
Pseudoceridae. Marginal tentacles folded. Mouth in anterior half. |
Thysanozoon (Fig. 8, E). Pseudoceros. Yungia. |
|
|
Euryleptidae. Tentacles usually present and pointed, or represented by two groups of eyes. Mouth close to anterior end. Pharynx cylindrical. |
Prostheceraeus. Cycloporus. Eurylepta. Oligocladus. Stylostomum. Aceros. |
Prostheceraeus vittatus Mont. On west coast. P. argus Quatref. Guernsey. Cycloporus papillosus Lang. On Ascidians in 2-30 fms. Eurylepta cornuta O.F. Müll. On sponges and shells, 2-10 fms. Oligocladus sanguinolentus Quatref. O. auritus Clap. Doubtful. Stylostomum variabile Lang. |
|
Prosthiostomatidae. Tentacles absent. Body elongated. Pharynx long, cylindrical. Penis with accessory muscular vesicles. |
Prosthiostomum (Fig. 8, F). | |
Appearance and Size of Polyclad Turbellaria.—Polyclads are almost unique amongst animals in possessing a broad and thin, delicate body that glides like a living pellicle over stones and weeds, moulding itself on to any inequalities of the surface over which it is travelling, yet so fragile that a touch of the finger will rend its tissues and often cause its speedy dissolution. The dorsal surface in a few forms is raised into fine processes (Planocera villosa), or into hollow papillae (Thysanozoon brocchii), and in very rare cases may be armed with spines (Acanthozoon armatum,[26] Enantia spinifera); in others, again, nettle-cells (nematocysts) are found (Stylochoplana tarda, Anonymus virilis). Some Polyclads, especially the pelagic forms, are almost transparent; in others, the colour may be an intense orange or velvety black, and is then due to peculiar deposits in the epidermal cells. Between these two extremes the colour is dependent upon the blending of two sources, the pigment of the body itself and the tint of the food. Thus a starved Leptoplana is almost or quite white, a specimen fed on vascular tissue reddish. Many forms are coloured in such a way as to make their detection exceedingly difficult, but this is probably not merely due, as Dalyell supposed, to the substratum furnishing them with food and thus colouring them sympathetically, but is probably a result of natural selection.
The largest Polyclad, the bulkiest Turbellarian, is Leptoplana gigas (6 inches long and 4 in breadth), taken by Schmarda, free-swimming, off the coast of Ceylon. The largest European form is Pseudoceros maximus, 3½ inches in length and stoutly built. A British species, Prostheceraeus vittatus, attains a length of from 2 to 3 inches. These large forms, especially the Pseudoceridae (pre-eminently the family of big Polyclads), are brightly coloured, and usually possess good swimming powers, since, being broad and flat, they are certainly not well adapted for creeping rapidly, and this is well shown by the way these Polyclads take to swimming when in pursuit of prey at night. The size of any individual is determined, amongst other factors, by the period at which maturity sets in, after which probably no increase takes place. Polyclads apparently live about twelve months, and mature specimens of the same species vary from ½ inch to 2½ inches in length (Thysanozoon brocchii), {21}showing that growth is, under favourable conditions, very rapid.
Habits of Polyclad Turbellaria.—Polyclads are exclusively marine, and for the most part littoral, animals. Moreover, there is no evidence of their occurrence in those inland seas where certain marine animals (including one or two species of otherwise characteristically marine Rhabdocoelida, p. 46) have persisted under changed conditions. From half-tide mark down to 50 fathoms, some Polyclads probably occur on all coasts, but as to their relative abundance in different seas we have very little accurate information. The southern seas of Europe possess more individuals and species than the northern, and probably the maximum development of the group takes place on the coasts and coral islands of the tropics.[27] No Polyclads have been taken below 60 fathoms; but their delicacy and inconspicuousness render this negative evidence of little value. Six truly pelagic forms, however, are known,[28] and these are interesting on account of their wide distribution (three occurring in the Atlantic, Pacific, and Indian oceans), and also from the distinct modifications they have undergone in relation to their pelagic existence.
Whatever may be the interpretations of the fact, Polyclads are notoriously difficult to detect, and this fact doubtless explains the scanty references to them by the older naturalists who collected even in tropical seas. Lang, who worked seven years at Naples, added to the Mediterranean fauna as many Polyclads as were previously known for all Europe, in spite of the assiduous labours of his predecessors, Delle Chiaje and Quatrefages. Again Hallez, collecting at Wimereux at low-water, obtained some twenty specimens of Leptoplana tremellaris in an hour, while some other collectors working by his side could only find two or three. Yet, even making allowance for the difficulty of finding Polyclads, few of them appear to be abundant.
Leptoplana tremellaris is frequently associated with colonies of Botryllus, and if separated soon perishes, whereas the free-living individuals are distinctly hardy (Hallez). A closely allied but possibly distinct form lives upon the surface of the Polyzoon {22}Schizoporella, on the French side of the Channel, and cannot long endure separation from its natural habitat, to which it is adaptively coloured. A striking case of protective mimicry is exhibited by Cycloporus papillosus, on the British coasts. This species, eminently variable in colour and in the presence or absence of dorsal papillae, is usually a quarter of an inch in length and of a firm consistency. Fixed by its sucker to Polyclinid and other Ascidians, Cycloporus appears part and parcel of the substratum, an interesting parallel to Lamellaria perspicua,[29] though we are not justified in calling the Polyclad parasitic. Indeed, though a few cases of association between Polyclads and large Gasteropods, Holothurians, and Echinids are known,[30] there is only one case, that of Planocera inquilina,[31] in the branchial chamber of the Gasteropod Sycotypus canaliculatus, which would seem to bear the interpretation of parasitism. The jet-black Pseudoceros velutinus and the orange Yungia aurantiaca of the Mediterranean, are large conspicuous forms with no attempt at concealment, but their taste, which is not known, may protect them. Other habits, curiously analogous with devices employed by Nudibranch Mollusca (compare Thysanozoon brocchii with Aeolis papillosa), emphasise the conclusion that the struggle for existence in the littoral zone has adapted almost each Polyclad to its particular habitat.
As regards the vertical distribution of this group on the British coasts, Leptoplana tremellaris has an extensive range, and appears to come from deeper to shallower water to breed.[32] In the upper part of the Laminarian zone, Cycloporus papillosus, and, among brown weeds, Stylochoplana maculata are found. At and below lowest water-mark Prostheceraeus vittatus, P. argus, and Eurylepta cornuta occur. Stylostomum variabile and Oligocladus sanguinolentus, though occasionally found between tide-marks, especially in the Channel Islands, are characteristic, along with Leptoplana droebachensis and L. fallax, of dredge material from 10 to 20 fathoms.
Locomotion.—Locomotion is generally performed by Polyclads at night when in search of food, and two methods, creeping {23}and swimming, are usually employed—creeping by the cilia, aided possibly, as in the case of some Gasteropod Mollusca, by the longitudinal muscles of the ventral surface; and swimming, by undulations of the expanded margins of the body. In the former case the cilia work in a glandular secretion which bathes the body, and enables them to effect their purpose equally well on different substrata. The anterior region is generally lifted up, exploring the surroundings by the aid of the tentacles, which are here usually present. The rest of the body is closely appressed to the ground.
Swimming is particularly well performed by the Pseudoceridae, certain species of Prostheceraeus, the large Planoceridae, some Stylochoplana, Discocelis, and Leptoplana, and in the same manner as in Leptoplana tremellaris (p. 9). In Cryptocelis, Leptoplana alcinoi, and L. pallida, however, the whole body executes serpentine movements like an active leech (e.g. Nephelis); a cross section of the body would thus present the same appearance during the whole movement. Many Polyclads, notably Anonymus (Lang), if irritated, spread out in all directions, becoming exceeding thin and transparent.
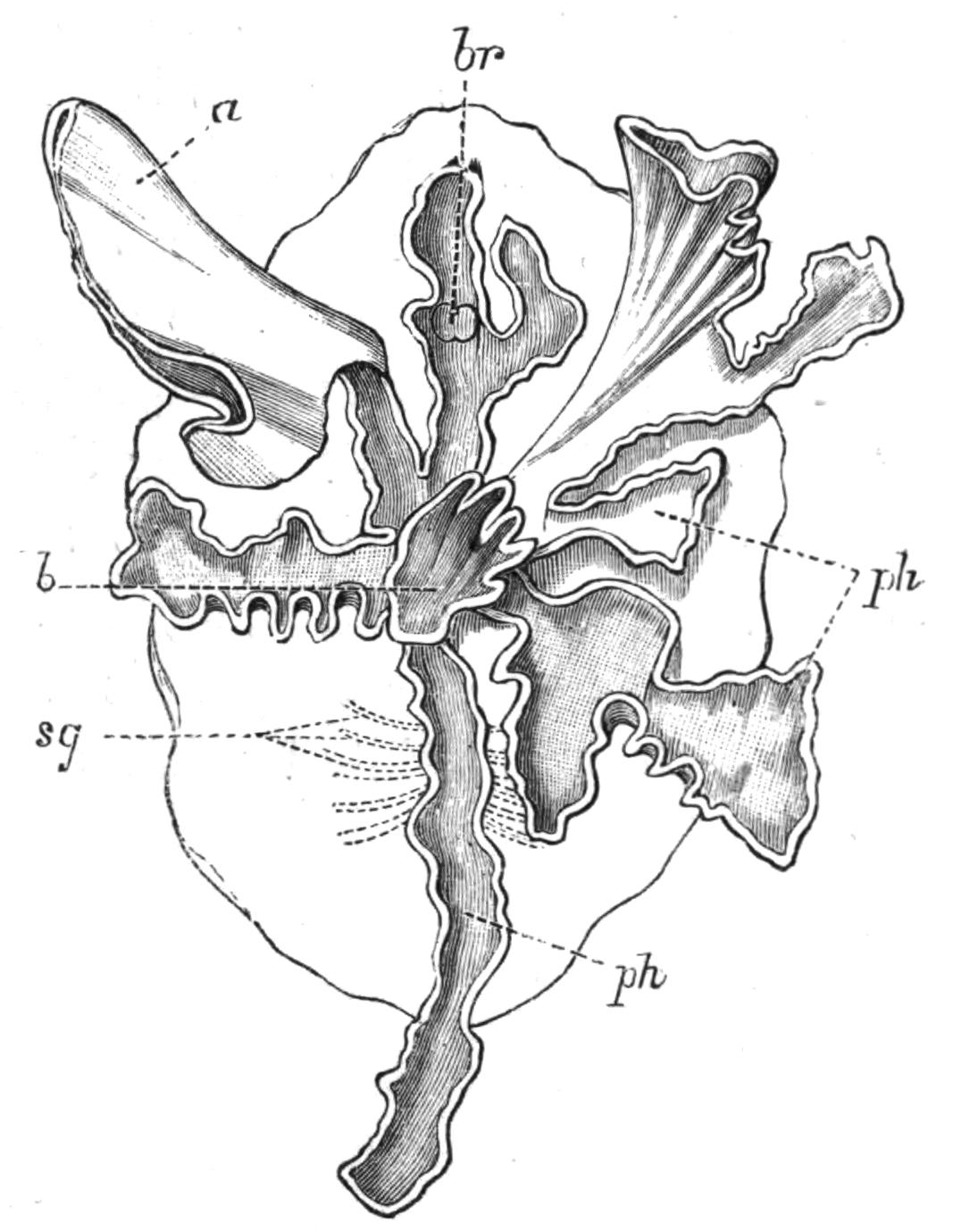
Fig. 9.—Discocelis lichenoides Mert. (after Mertens), creeping on the inner side of a glass vessel by means of the lobes of the extended and exceedingly mobile pharynx (ph). These lobes also serve to enclose Crustacea (a), and one lobe may then be withdrawn independently of the rest, back into the body (b). The brain (br) and shell-gland (sg) are shown by transparency.
Discocelis lichenoides, Planocera graffii, and Anonymus virilis have peculiar modes of progression. The first, according to Mertens, will climb up the sides of a vessel by means of the expanded lobes of the pharynx (Fig. 9, ph), a habit of considerable interest, since we know that certain Ctenophores—Lampetia, for instance—progress when not swimming on the expanded lobes of their "stomach."[33] Planocera and Anonymus {24}creep by extending parts of the anterior margin and dragging the rest of the body behind. In consequence, the brain and dorsal tentacles may come to lie actually behind the middle of the body, and thus no definite anterior end or "head" advances first. Along with this curious habit it may be noticed (Lang) that the radial symmetry of the body is well marked; but even without accepting this author's suggestion of the concurrent development of a "head" with locomotion in a definite direction, the facts, whether these two forms are primitive or not, are highly interesting.
Food.—Though we are probably right in calling Polyclads a carnivorous group, the food of very few forms has been ascertained. Those which possess a large frilled pharynx (most Acotylea) probably enclose and digest large, and, it may be, powerful prey, as appears to be the case in Leptoplana tremellaris. Cryptocelis alba has been seen by Lang with the pharynx so distended, owing to a large Drepanophorus (Nemertine) which it contained, as to resemble a yolk-sac projecting from the under surface of an embryo. The Cotylea such as Thysanozoon, with a bell- or trumpet-shaped pharynx, are fond of fixing this to the side of the aquarium, but whether they thus obtain minute organisms is not clear. Prosthiostomum shoots out its long pharynx with great vehemence (Fig. 8, F) and snaps up small Annelids by its aid (Lang). Those Polyclads which, as Cycloporus and others, are definitely associated with other organisms are not certainly known to feed upon the latter, though "Planaria velellae" has been seen by Lesson[34] devouring the fleshy parts of its host. The salivary glands which open on the lips and the inner surface of the pharynx powerfully disintegrate the flesh of the prey. Digestion takes place in the main-gut, and the circulation of the food is accomplished by the sphinctral musculature of the intestinal branches (conf. Leptoplana, p. 13).
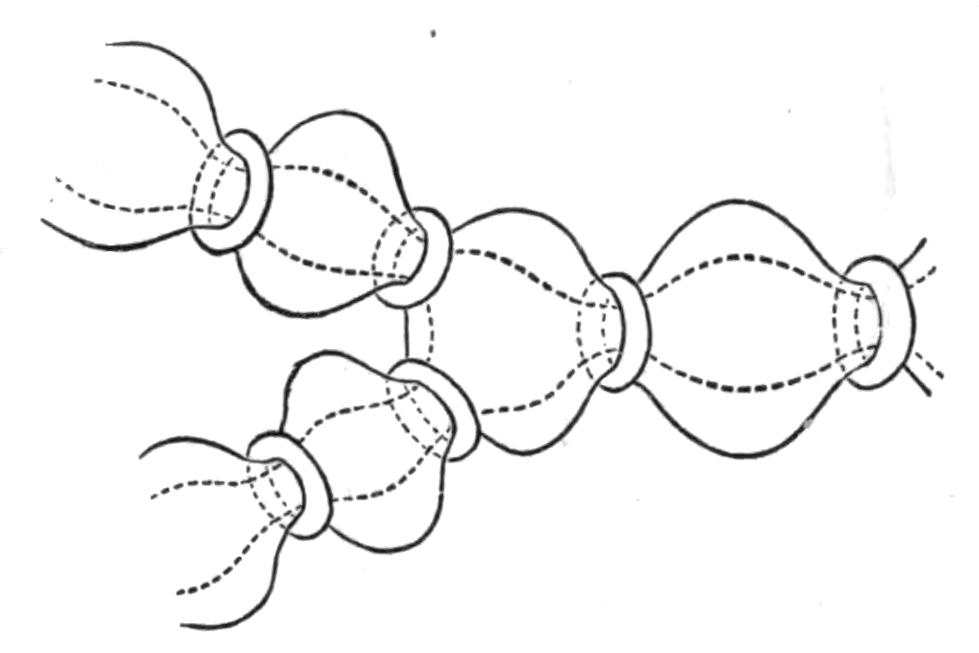
Fig. 10.—Diagram of the musculature, causing peristaltic movements of the intestinal branches of Polyclads. (After Lang.)
A distinct vent or anus is always absent. After a meal the {25}faecal matter collects in the main-gut, and is discharged violently by the pharynx into the water. In a few species, however, the intestinal branches open to the exterior (Lang). Yungia aurantiaca, a large and abundant Neapolitan form, possesses such openings over the greater part of the dorsal surface; Cycloporus papillosus has marginal pores; Oligocladus sanguinolentus apparently possesses an opening at the posterior end of the main-gut; and Thysanozoon brocchii frequently rends at this point, in consequence of the accumulation of food.
Respiration.—The oxygen of the atmosphere dissolved in the sea-water is, in default of a special circulatory fluid, brought to the tissues of Polyclads in two ways. The ciliated epidermis provides a constant change of the surrounding water, by which the superficial organs may obtain their supply; and the peristaltic movements of the digestive system, aided by the cilia of the endoderm cells, ensure a rough circulation of the sea-water, which enters along with the food, to the internal organs. The papillae of Thysanozoon brocchii, containing outgrowths of the intestinal branches, are possibly so much additional respiratory surface, although still larger forms (other Pseudoceridae) are devoid of such outgrowths.
Excretion.—The excretory system of only one Polyclad (Thysanozoon brocchii) is accurately known. Lang, by compressing light-coloured specimens, found the three parts of the system known to occur in many Platyhelminthes: (1) the larger longitudinal canals, and (2) the capillary vessels, which commence with (3) the flame-cells in the parenchyma of the body. The mode of distribution of these parts is not, however, ascertained. The canals are delicate, sinuous, apparently intracellular tubes, coursing close to the margin of the body and sending offsets which suspend the canals to the dorsal surface, where possibly openings may occur. In dilatations of these vessels bunches of cilia, and occasionally flame-cells, are found. Usually, however, flame-cells occur at the commencement or during the course of the capillaries, which are straight, rarely branching, tubes of exceeding tenuity, and appear (Lang) to be outgrowths of the flame-cells, just as the duct is an outgrowth of a gland-cell. In fact there is little doubt that the stellate flame-cells are modified parenchymatous gland-cells, containing a lumen filled with a fluid into which a number of cilia project and vibrate synchronously. The cells excrete {26}nitrogeneous waste substances, which are then discharged into the capillaries, whence the cilia of the main vessels drive them presumably to the exterior, though external openings of the excretory system are not known. Traces of this system have been observed in young Leptoplana (first by Schultze in 1854) and also in Cestoplana.
Sensation.—A nervous sheath, with scattered ganglion cells, everywhere underlies the musculature. It is exceedingly faintly marked on the dorsal surface, but laterally and ventrally forms a dense network with polygonal meshes. Thickenings of this sheath give rise to lateral nerves, and also to a pair of stout longitudinal nerves from which the internal organs are probably innervated. The brain, hardly distinct in pelagic Polyclads, in most forms does not differ greatly from that of Leptoplana (p. 13).
The sense organs of Polyclads have the form of tentacles, eyes, otocysts (in Leptoplana otophora), and stiff tactile cilia. The solid dorsal tentacles of Planoceridae contrast strongly with the folded or pointed hollow processes of the Cotylea. The former (Fig. 8, A, T) are muscular and very contractile, and are placed near the brain some distance from the anterior end. The latter are outgrowths of the front margin of the body, and are sometimes (Yungia) provided superficially with olfactory pits and internally with eyes and intestinal coeca.
The eyes which occur in Polyclads may be divided into (a) a pair of cerebral groups overlying the brain; (b) those embedded in the tentacles (tentacular group); and (c) the marginal eyes, which in Anonymus occur all round the margin. A complex form is sometimes assumed by the cerebral eyes of Pseudoceridae, resulting probably from incomplete fission (Fig. 11). Leptoplana otophora was obtained by Schmarda on the south coast of Ceylon. On each side of the brain is a capsule containing two otoliths. This is the only known case of the occurrence of these organs in Polyclads.
Reproduction.—Although Polyclads are able to repair the result of injuries to a very considerable extent, they are not known to multiply asexually. The two processes are intimately associated, but, though probably all Turbellaria can regenerate certain lost parts, asexual reproduction only occurs sporadically.
All known Polyclads are hermaphrodite. The male organs, scattered, like the testes of Leptoplana, over the ventral surface, develop earlier than the ovaries, though the periods of maturation overlap; hence the possibility of self-fertilisation, though remote, is still worth consideration. The genital apertures, through which, in the male, spermatozoa, and in the female, ova, are emitted, are usually situated as in Leptoplana (Figs. 2 and 5, ♂ and ♀). In Trigonoporus, a genus once found at Naples, a secondary female aperture has been discovered leading into the female genital canal[35]; and in Anonymus, Polypostia, and Thysanozoon (Fig. 7, E, ♂) two or more male pores and penes have been found. Anonymus has several penes (Fig. 7, D, ♂) arranged radially round the body. Polypostia, a remarkable form described by Bergendal,[36] belonging to the Acotylea, possesses about twenty such structures ranged round the female genital aperture. Lang, whose attention was attracted by these singular facts, made the interesting discovery that Thysanozoon uses its penes as weapons of offence rather than as copulating organs, burying them in the skin of another Polyclad (Yungia) that happened to cross its path, spermatozoa being of course left in the wound. Lang further found that Prostheceraeus albocinctus and Cryptocelis alba in this way implanted a spermatophore in the skin of another individual of the same species, and he suggested that from this point the spermatozoa wandered through the tissues till they met with and fertilised the eggs. It is now known that a similar process of "hypodermic impregnation" occurs sporadically in several groups of animals.[37] {28}Nevertheless, in some Polyclads it is probable, and in Stylochus neapolitanus it is certain, that normal copulation takes place. The sperm-masses are transferred to a coecal diverticulum of the female genital canal, and then by a delicate mechanism, of which we know only the effects, one spermatozoon obtains entrance into one matured ovum, which differs from the ova of most Turbellaria in that it contains in its own protoplasm the yolk necessary for the nutrition of the embryo. In other words, there are no special yolk-glands. After fertilisation, the ovum in all Polyclads is coated with a shell formed by the shell-gland, which also secretes a substance uniting the eggs together. They are deposited on stones and shells, either in plate-like masses or in spirals (like those of Nudibranchs). Cryptocelis alba lays masses of an annular shape, with two ova in each shell, and buries them in sand.
Development.[38]—The first stages in the embryology of Polyclads appear to be very uniform. They result, in all Cotylea and in certain Planoceridae, in the formation of a Müller's larva (Fig. 12) about a couple of weeks after the eggs are laid. This larva (1-1.8 mm. long), which is modified in the Planoceridae, is distinguished by the presence of a ciliated band, running somewhat transversely round the body, and usually produced into a dorsal, a ventral, and three pairs of lateral processes. When swimming the body is placed as in Fig. 12, and twists round rapidly about its longitudinal axis by means of the strong locomotor cilia placed in transverse rows upon the processes. The cilia of each row vibrate synchronously, and recall the action of the swimming plates of a Ctenophore. It is noteworthy that whereas Stylochus pilidium passes through a modified or, according to some authors, a primitive larval stage, its near ally, S. neapolitanus, develops directly. Most {29}Acotylea indeed develop directly, and their free-swimming young differ from Müller's larva merely in the absence of the ciliated band and in the mode of swimming.
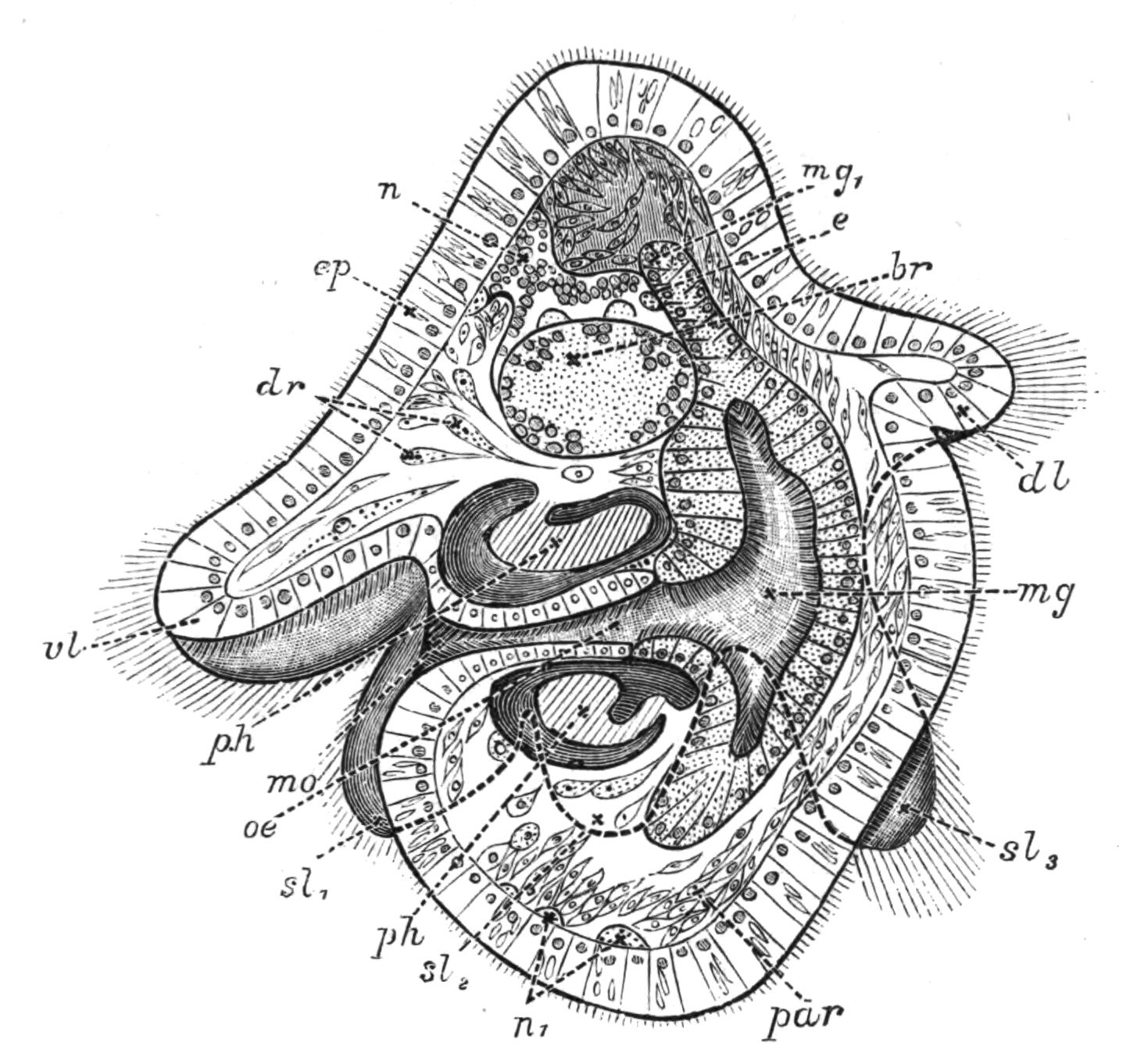
Fig. 12.—Section through Müller's larva of Thysanozoon brocchii (modified from Lang). The right half is seen from inside. × 150. Semi-diagrammatic. br, Brain; dl, dorsal ciliated lobe; dr, salivary gland-cells of pharynx; e, eye; ep, ciliated epidermis containing rhabdites; mg, stomach or main-gut; mg1, unpaired gut branch over the brain; mo, "mouth" of larva; n, n1, section of nerves; oe, ectodermic pit forming oesophagus of larva; par, parenchyma filling the space between the alimentary tract and the body wall; ph, pharynx lying in the cavity of the peripharyngeal sheath, the nuclei of which are visible; sl1, sl2, sl3, lateral ciliated lobes of the right side; vl, ventral ciliated lobe.
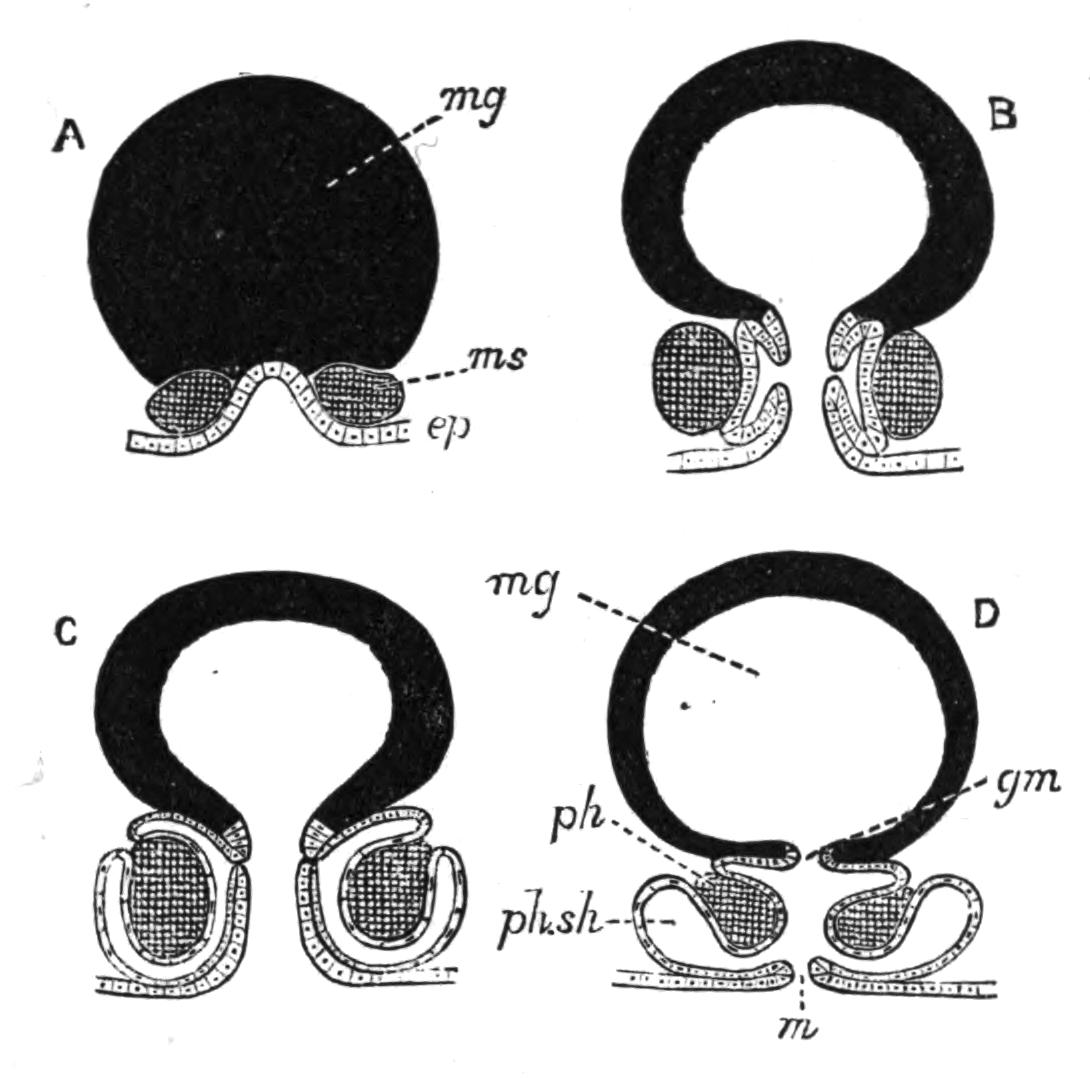
Fig. 13.—Diagrammatic transverse sections of a larval Polyclad at different stages, to illustrate the development of the pharynx. (After Lang.) A, Larva of the eighth day still within the shell. The main-gut (mg) is still solid, the epidermis is slightly invaginated, and a pair of muscular mesodermic thickenings (ms) are present. B, Young pelagic larva. The epidermic invagination has deepened and developed laterally. C, The lateral pouches have formed the wall of the peripharyngeal sheath, enclosing the mesodermic, muscular, thickening or pharyngeal fold (ph). (Compare Fig. 12.) Towards the end of larval life, when the ciliated processes (sl, Fig. 12) have aborted, the stage D is reached. By the opening outwards of the pharyngeal sheath (ph.sh) the two apertures gm, or true mouth, and m, or external mouth, are formed, which together correspond with the oesophageal opening of the younger larva. (Compare the transverse section in Fig. 5.)
Polyclads possess an undoubted mesoderm, which gives rise to the muscles, the pharyngeal fold, and the parenchyma. The ectoderm forms the epidermis, in the cells of which the {30}rhabdites (Fig. 12) arise, apparently as so many condensed secretions. From the ectoderm the brain arises as two pairs of ingrowths, which fuse together, and from these the peripheral nervous system grows out. Three pigmented ectoderm cells give rise, by division, to the eyes—an unpaired cell (Fig. 12, e) to the cerebral group of eyes, and the other two to the marginal and tentacular groups. The copulatory organs apparently arise to a large extent as ingrowths from the ectoderm, from which the accessory glands (prostates, shell-glands) are also formed. The endoderm forms the lining of the main-gut and its branches. The pharynx is developed as in Fig. 13, which shows that the "mouth" of the young larva (C) does not correspond exactly with that of the adult (D). The salivary glands arise from ectoderm cells, which sink deeply into the parenchyma. The reproductive organs (ovaries and testes) possibly arise by proliferation from the gut-cells (Lang, v. Graff). The change from the larva to the adult is gradual, the ciliary band being absorbed and the creeping mode of life adopted.
Turbellaria. II. Tricladida.
The Triclads are most conveniently divided into three groups[39]: (i.) Paludicola, the Planarians of ponds and streams; (ii.) the Maricola, the Triclads of the sea; and (iii.) Terricola or Land Planarians. From the Polyclads they differ in their mode of occurrence; in the elongated form of their body and almost constant, mid-ventral position of the mouth; in possessing a single external genital pore (Monogopora); and in the production of a few, large, hard-shelled eggs provided with food-yolk.
Occurrence of the Paludicola.—The Planarians of our ponds and streams are the most familiar and accessible Turbellaria. Their elongated, flattened bodies, and gliding movements, render them conspicuous objects on the under surface of stones and on the leaves of aquatic plants, where they live gregariously. The variable Polycelis nigra (Fig. 14, H) is very abundant in stagnant water and slowly-moving streams, whereas its ally, P. cornuta (Fig. 14, G), distinguished by a pair of tentacles, is more local. Planaria (Dendrocoelum) lactea (A), P. polychroa (I), P. torva, and P. punctata are not infrequently found together, but the last is at once the largest and rarest.
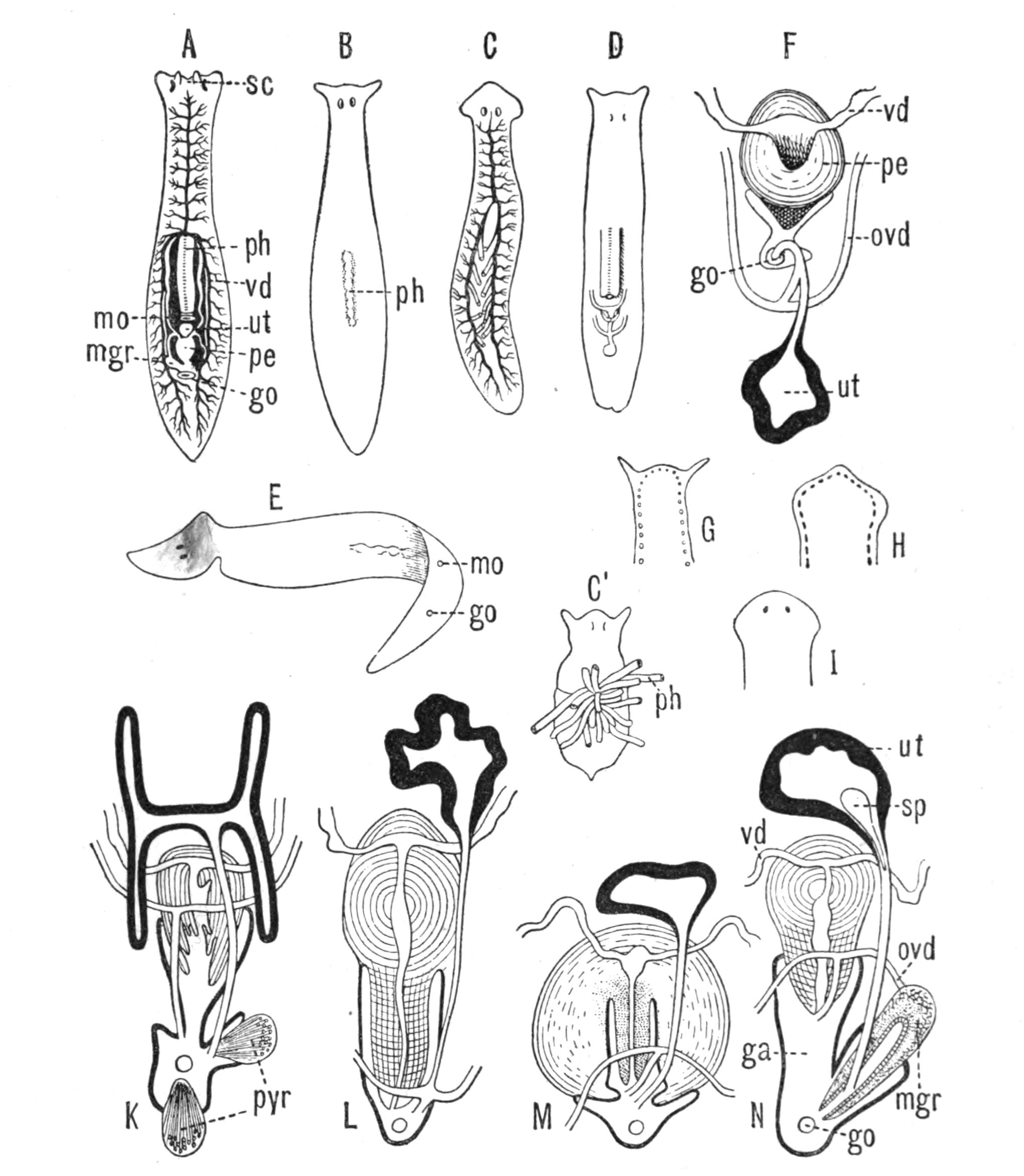
Fig. 14.—Forms of Triclads, with the distinguishing specific characters of certain British forms. A, Planaria lactea O. F. M., × 2; B, Planaria alpina Dana, × 4 (after Kennel); C, Phagocata gracilis Leidy (after Woodworth), × 6; C', the same with the pharynges (ph) extruded; D, Gunda ulvae Oer., × 4; E, Planaria gonocephala Dug. (after Schmidt), × 4; F, genitalia of Gunda ulvae (after Wendt); G, head of Polycelis cornuta Schm.; H, head of Polycelis nigra Ehr.; I, head of Planaria polychroa Schm. K to N show the distinctive characters of the genital ducts in K, Polycelis nigra; L, Planaria polychroa; M, Planaria alpina; N, Planaria torva Schultze (after Iijima and v. Kennel). ga, Genital atrium; go, common genital opening; mgr, "musculo-glandular organ"; mo, "mouth"; ovd, oviduct; pe, penis; ph, pharynx; pyr, pyriform organs of unknown significance; sc, sucker; sp, spermatophore lying in (ut) uterus; vd, vesicula seminalis. (All except C and E are found in England.)
Planaria alpina (Fig. 14, B) is characteristic of cold mountain streams, but occurs down to {32}sea-level in England, the Isle of Man, and Ireland, and from its abundance in spring water, probably enjoys a wide distribution underground. In the Swiss Alps it has been found at altitudes of over 6000 feet, at lower levels in the Rhone, and also in the Lake of Geneva. This wide distribution may perhaps be accounted for, partly, by its faculty for asexual reproduction in summer, and also, by the production, later in the year, of hard-shelled eggs which are laid loosely, not attached to stones or plants.[40] But we have no really direct evidence of the means of dispersal of this or of any of the foregoing species, although they all have a wide distribution in Europe. Of extra-European forms the accounts that exist are very fragmentary. The only indubitable diagnostic character of a Triclad is the structure of its genital ducts, and this is accurately known in only a few cases. Several species such as Dicotylus pulvinar (Fig. 16, B), at present known only from Lake Baikal,[41] and others (Planaria mrazekii, P. albissima) from Bohemia,[42] will doubtless be found elsewhere when they are carefully looked for. Phagocata gracilis is a remarkable North American form, possessing several pharynges (Fig. 14, C and C'), recalling the independent movement of the pharyngeal lobes of Discocelis lichenoides (Fig. 9).[43]
Occurrence of the Maricola.—Little as we accurately know of the distribution of the fresh-water Planariae, our knowledge of the occurrence of the marine forms is still more limited. Gunda (Procerodes) ulvae (Fig. 14, D) is the commonest European form, occurring abundantly in the upper part of the littoral zone, on the shores of the Baltic. G. segmentata from Messina has been carefully described by Lang,[44] but these are almost the only species of Maricola which can be accurately determined. They differ from the Paludicola in the position of the "uterus" behind the genital pore and in the absence of a "musculo-glandular organ" (Fig. 14, F). A special interest attaches to the Bdellouridae, a family containing three species, all parasitic on Limulus from the east coast of America. These remarkable Triclads usually have a sucker at the hinder end of the body, by which they attach themselves firmly to the cephalo-thoracic appendages and to the {33}gill-plates, upon which the eggs may be found in considerable numbers. One species, Syncoelidium pellucidum, possesses a pair of problematical organs in the hinder part of the body, opening to the exterior ventro-laterally by a couple of chitinous mouth-pieces, but having no connexion with the genital ducts.[45]
Occurrence and Distribution of Land Planarians.—The terricolous Triclads or Land Planarians are the most interesting division of the group. Some forms, such as Bipalium kewense, attain large dimensions, being usually 6 to 9 inches in length, and specimens fully extended have measured 18 inches. Their bodies are frequently banded or striped with brilliant colours. Geoplana coerulea Mos. has a blue ventral surface and is olive green or dark Prussian blue above. G. splendens Dendy, is marked dorsally by three stripes of emerald green alternating with four dark brown longitudinal bands. The mode of coloration, though somewhat variable, is an important specific character. Its significance, however, is not clearly understood. The colours may be a warning signal, as some Geoplana at least are disagreeable to the taste of man and some birds[46]; but since Land Planarians are largely nocturnal animals, living by day under logs, banana leaves, and in other moist and dark situations, this explanation is clearly insufficient. Two Geoplana have been noticed by Mr. Dendy which seem to be protectively coloured. G. triangulata var. australis occurs abundantly in the beech forest in the South Island of New Zealand, and its brown back and yellow or orange ventral surface match the leaves around its haunts. G. gelatinosa again looks like a mere slimy patch on the rotten bark where it is found. In arid districts, during the dry season, Land Planarians burrow in the soil and form a cyst, in which they lie coiled up, after the manner of earthworms.[47] The glutinous investment of their delicate bodies forms a moist medium in which the cilia covering the body (and especially the ventral surface) may constantly and evenly vibrate, and by which they adhere firmly to their prey. In some tropical Planarians, in addition to possessing offensive properties, the mucus is so copious in amount and hardens with such rapidity, that {34}these Triclads may creep over bridges of it, and may even be blown from one stem or branch of a plant to another, hanging at the ends of their threads.[48]
Fig. 15.—Some Land Planarians found in Europe. A, Bipalium kewense Mos. × ⅓ (after Bergendal); B, Rhynchodemus terrestris O. F. M., × 2; C, Geodesmus bilineatus Metsch., × 2½ (after Metschnikoff). mr, Region of mouth; gp, region of genital pore.
In Europe there are only two or three indigenous Land Planarians, of which Rhynchodemus terrestris O. F. M. (Fig. 15, B) is the most widely distributed, and has been found in moist situations for the most part wherever it has been carefully looked for. It measures about ¾ inch in length, and is dark grey above, whitish below, and bears a pair of eyes near the anterior extremity (Fig. 15, B). Bipalium kewense (Fig. 15, A), which has been found in the forests of Upolu, Samoa, by Mr. J. J. Lister, has been accidentally imported, from the (unknown) districts where it is indigenous, with plants and soil to various parts of the world—England, Germany, the Cape, and also to Sydney, where it appears to have established itself. In these Bipalia living in hothouses, the genitalia never appear to attain maturity, and apparently multiple fission and subsequent reparation of the missing parts is the only mode of reproduction. Geodesmus bilineatus (Fig. 15, C), which has occurred at Giessen, Würzburg, and Dresden, has, in all probability, been introduced with ferns from the West or East Indies. Microplana humicola, described by Vejdovsky from dunghills in Bohemia, is doubtfully indigenous.
In marked contrast with the poverty of the temperate zones in Land Planarians, is the abundance and great variety of this group in Southern Asia, South America, and especially in Australasia, where the rich Land Planarian fauna has been carefully investigated by Spencer, Dendy, Fletcher, and others, in {35}certain parts of Victoria, New South Wales, and New Zealand.[49] About forty species of Planarians have been discovered on the Australian continent, thirty-five of which belong to the predominant genus Geoplana, distinguished by the presence of numerous eyes along the border of the simple anterior extremity. Of the remaining five, four belong to the genus Rhynchodemus, with, lastly, the introduced Bipalium kewense. The distribution of any one species, however, is so limited that only three forms are common to the two former colonies; and although some of the twenty known New Zealand Planarians (chiefly species of Geoplana), are identical with Australian species, yet only one, or possibly two, varieties of these species are Australian also. In addition to their prevalence in Australasia, the Geoplanidae also occur in South America, South Africa, Japan, and the East Indies. The Bipaliidae are characteristic of the Oriental region, being found in China, Borneo, Bengal, and Ceylon. The Rhynchodemidae are a cosmopolitan family, occurring in Europe, North and South America, the Cape of Good Hope, Ceylon, the East Indies, Australia (particularly Lord Howe Island), and Samoa.[50]
Habits and Structure of Triclads.—The common Planaria (Dendrocoelum) lactea, which usually progresses by ciliary action, aided, it is said, by muscular contractions of the ventral surface, performs, if alarmed, a series of rapid "looping" movements, by affixing a sucker (Fig. 14, A, sc), placed on the under side of the head, to the substratum, and pulling the posterior end close to this. The sucker, discovered by Leydig, is even better developed in P. punctata (Fig. 16, A), P. mrazekii, and P. cavatica, and is an efficient adhering-organ which has probably been developed from a similar but simpler structure found in a considerable number of both fresh-water and marine Triclads (P. alpina, Fig. 16, E). Probably the sucker of the Land Planarian Cotyloplana (D) is the same structure, but the two suckers of Dicotylus (B) are at present unique. Planaria dioica, found by Claparède on the coast of Normandy,[51] is covered with minute adhesive papillae, {36}similar to those of certain Rhabdocoelida (e.g. Monotus, Fig. 19, D), enabling it to cling tightly to the Zostera, and so to resist the loosening action of the waves.
Fig. 16.—Suckers of Triclads. A, Planaria punctata Pall.; a, dorsal surface of head; b, ventral surface (freely moving) showing the sucker; c, sucker contracted (after Hallez): B, ventral surface of head of Dicotylus pulvinar Gr., from Lake Baikal (after Grube): C, dorsal surface of Procotylea fluviatilis Gir. (after Girard): D, sucker of Cotyloplana whiteleggei Sp. (after Spencer): E, ventral view of head of Planaria alpina Dana (preserved specimen); hg, adhering groove; m, thickened musculature forming the margin of the sucker; sc, sucker; t, tentacles.
The movements of Land Planarians are somewhat peculiar. The ventral surface of Bipalium has a median groove, into which the ducts of numerous mucus-glands open. This is bordered by two ridges clothed with long and powerful cilia, which perform the chief part in propelling the animal, aided, according to Lehnert,[52] by muscular waves which pass from the head, backwards, i.e. opposite in direction to those by which a snail slides along. This observation, however, needs confirmation. The whole body executes sinuous movements, during which the crescentic head, lifted slightly above the ground (Fig. 15, A), is constantly altering and regaining its normal shape, somewhat as a Planaria lactea uses the lobes of its head. Further examination shows that the margin of the head of Bipalium is not only provided with eyes, but in addition, with ciliated, (probably) olfactory pits. Such depressions, innervated directly from the cerebral ganglia, have been found in sixteen species of Geoplana, {37}and in one or two species of Rhynchodemus.[53] Some Land Planarians (a species of Rhynchodemus from Ceylon, and a Dolichoplana from the Philippines) wriggle out of a box or the hand with great speed (Moseley).
The skin of Triclads is full of minute rods or rhabdites, which are shot out in great numbers when the animal is irritated, and doubtless serve an offensive purpose. The Terricola possess two kinds of these: (1) needle-like rods; and (2) in Bipalium kewense, flagellated structures, bent into a V-form and with a slender thread attached to one end (Shipley). In Geoplana coerulea these bent rods furnish the blue colour of the ventral surface. The rhabdites arise in all Triclads in cells below the basement-membrane, which they are said to traverse in order to reach the epidermis, thus differing in origin, and also in structure, from the rods of Polyclads.
Food.—Triclads are largely if not wholly carnivorous animals, feeding upon Annelids, Crustacea, Insects, Insect-larvae, and Molluscs. The mouth is usually mid-ventral or behind the middle of the body, but in the anomalous Leimacopsis terricola Schm. from the Andes[54] and in Dolichoplana it is near the anterior end. The pharynx (Figs. 17, 18, ph) is cylindrical or bell-shaped, exceedingly dilatable and abundantly supplied with glands and nervous tissue. It opens into the three main intestinal branches, one of which runs in the median plane forwards, the others backwards right and left, enclosing a space in which the genital ducts lie (Figs. 14, A, 17). The fresh-water Planarians prey upon Oligochaeta, Hydrophilidae (aquatic beetles), and the commoner pond-snails. Bipalium kewense pursues earthworms, seizes the upper surface of the anterior end by the glutinous secretion of its ventral surface, and then proceeds to envelop part or the whole of the worm within its pharynx, which is stretched as a thin skin over the body of its struggling prey (Lehnert). The tissues of the latter pass into the intestine of the Planarian, and distend it greatly. After such a meal, which lasts from one to five hours, a Bipalium may remain for three months without seeking food. Geobia subterranea, a white eyeless form from Brazil, pursues earthworms (Lumbricus corethrurus) in their burrows, and has been seen by Fritz Müller sucking the blood out of a young {38}worm.[55] Geoplana typhlops, a Tasmanian species, is also blind, and pursues worms, as does G. triangulata (Dendy). In Trinidad, von Kennel[56] observed that land-snails (Subulinae) were the food of certain Land Planarians, the name of which, however, he does not state. The pharynx was employed to suck out the soft parts of the snail even from the upper whorls of the shell.
Reproduction.—In Planaria lactea the numerous testes (Fig. 17, te) are placed both above and below the alimentary canal throughout the greater part of its course. The membrane of each gonad is continued into a minute vas efferens, which unites with those of neighbouring testes. Two vasa deferentia (v.d) arise thus on each side, one from the posterior, the other from the anterior testes of the body, and open into the vesiculae seminales (v.s), which may be seen in the living animal as tortuous whitish tubes at the sides of the pharynx (Fig. 14, A). These open into the penis (Figs. 14, A; 17, pe), a large pyriform organ, the apex of which, when retracted, points forwards, projecting into the penial cavity. When this apical portion is evaginated and turned inside out, it is of considerable length, and is able to pass into the long slender duct of the uterus (ut) of another individual. The penial sheath (ps) is part of the genital atrium (gs), which is developed as a pit from the skin, and invests the end of the genital ducts, the mouth of the pit forming the common genital pore (go), through which both male and female genital products are emitted.
There are two ovaries (ov) placed far forwards, between the third and fourth pairs of intestinal coeca. The oviducts (ovd) lie just over the lateral nerves, and have a slightly tortuous course, at each outward bend receiving the duct (yo) of a yolk-gland (yg), so that ova and yolk are already associated when the oviducts open by a short unpaired tube into the genital atrium. The yolk-glands develop rapidly,[57] and when fully formed are massive glands occupying the spaces between the intestinal branches and the testes which are then aborting. The so-called uterus (ut), apparently at first a diverticulum of the genital atrium, expands behind the pharynx into a receptacle lined by long glandular columnar cells, which, however, are not all of the same kind. The uterine duct opens into the atrium just above the aperture of a problematical, eversible, "musculo-glandular organ" (mgr).
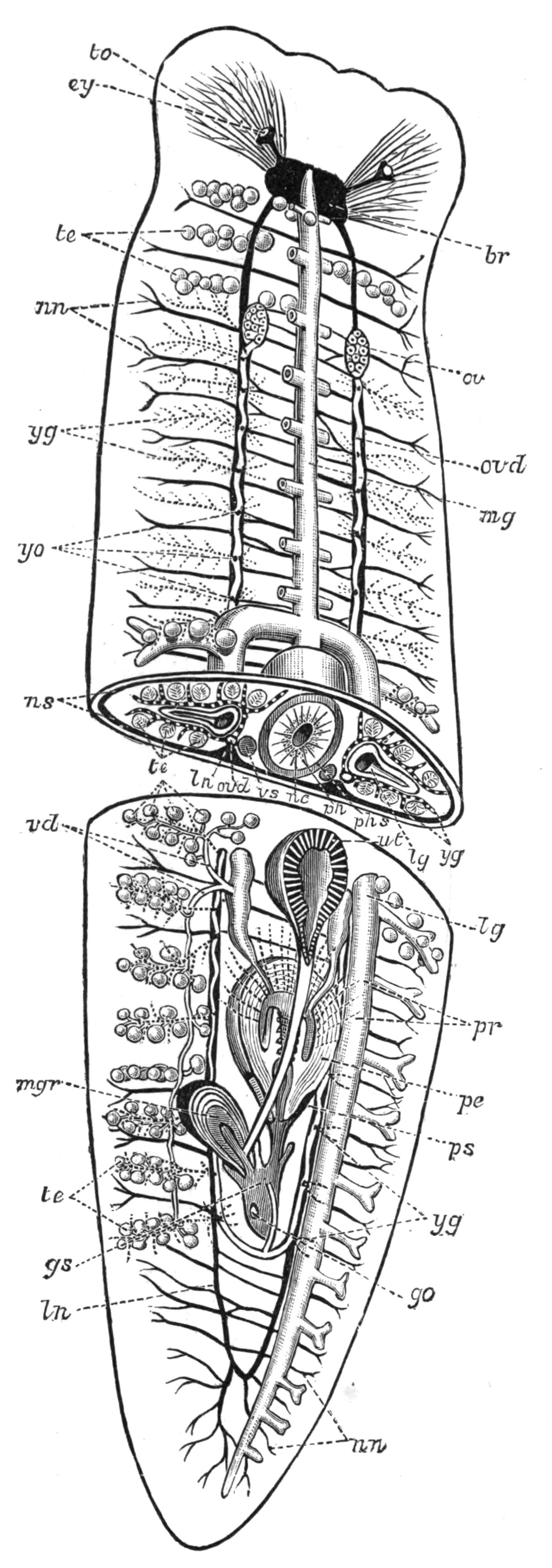
Fig. 17.—Diagrammatic view of the structure of Planaria (Dendrocoelum) lactea. × 7. The body has been cut across and a portion removed. In the posterior half the alimentary tract of the left side is removed and the uterus, penis, and muscular organ sliced open horizontally. The nervous system is represented by black, and the yolk-glands by dotted lines. br, Brain; ey, eye with lens and optic nerve; go, external genital aperture for both male and female products; gs, genital atrium; lg, paired lateral intestinal branch; ln, longitudinal nerve; mg, unpaired anterior intestine, the branches of which are cut off close to the main stem; mgr, eversible "musculo-glandular organ"; nc, nerve-cells in the pharynx; nn, lateral nerve-twigs; ns, nerve-sheath; ov, ovary; ovd, oviduct; pe, the eversible penis, the corrugated inner white portion of which is the apex; ph, pharynx; phs, pharyngeal sheath; pr, "prostate" or granule-gland (represented by dotted lines opening into the penis); ps, penial sheath; te, testes; to, tactile lobe of the head; ut, "uterus" opening into the genital atrium just above mgr; vd, vasa deferentia; vs, vesicula seminalis; yg, yolk-glands; yo, openings of the yolk-ducts into the oviducts.
Fertilisation appears to occur in the uterus, where ova, yolk, and spermatozoa, or (in P. torva) spermatophores (Fig. 14, N, sp), are found. The formation of the cocoon in Planaria lactea is probably begun in the "uterus," but is undoubtedly completed in the genital atrium. In P. polychroa, however, the stalked cocoon is formed wholly in the "uterus." Thus we find two types of cocoons in different species of the genus Planaria associated with two types of reproductive organs (Hallez):—
I. Planariae in which the two oviducts open separately into the posterior part of the duct of the uterus. A musculo-glandular organ is absent. The cocoons are spherical and stalked. Examples—Planaria polychroa (Fig. 14, L), P. albissima, P. gonocephala.
II. Planariae in which the two oviducts open by means of an unpaired duct into the genital atrium. A musculo-glandular organ present (Planaria torva (Fig. 14, N), P. mrazekii, P. lactea, P. cavatica), or absent (P. alpina, Fig. 14, M). The cocoons are sessile.
The genitalia of the Maricola (Fig. 14, F) and Terricola do not differ very much from those of Planaria. The uterus (greatly reduced in the Land Planarians) lies behind the genital pore, and several ova, together with much milky yolk, are enclosed in a capsule which is formed in the genital atrium.
Asexual Reproduction.[58]—It has long been known that fresh-water Planarians have not only great powers of repairing injuries, but that they use this faculty in order to multiply by transverse fission. Planaria alpina and Polycelis cornuta, in summer, separate off the posterior part of the body, and this ultimately becomes an entire individual. P. albissima, and especially P. subtentaculata, anticipate matters so far, that before fission is complete, the new individual has a head nearly fully formed. The new organs are largely regenerated in both parent and young, {41}apparently by the division and specialisation of scattered embryonic cells in the parenchyma. The asexual reproduction of Land Planarians is not fully proved, though it is known that they repair injuries to the body completely, and that Bipalium kewense is often found in hothouses, divided into fragments which regenerate all the organs of the parent, but like the latter, do not mature their sexual organs.
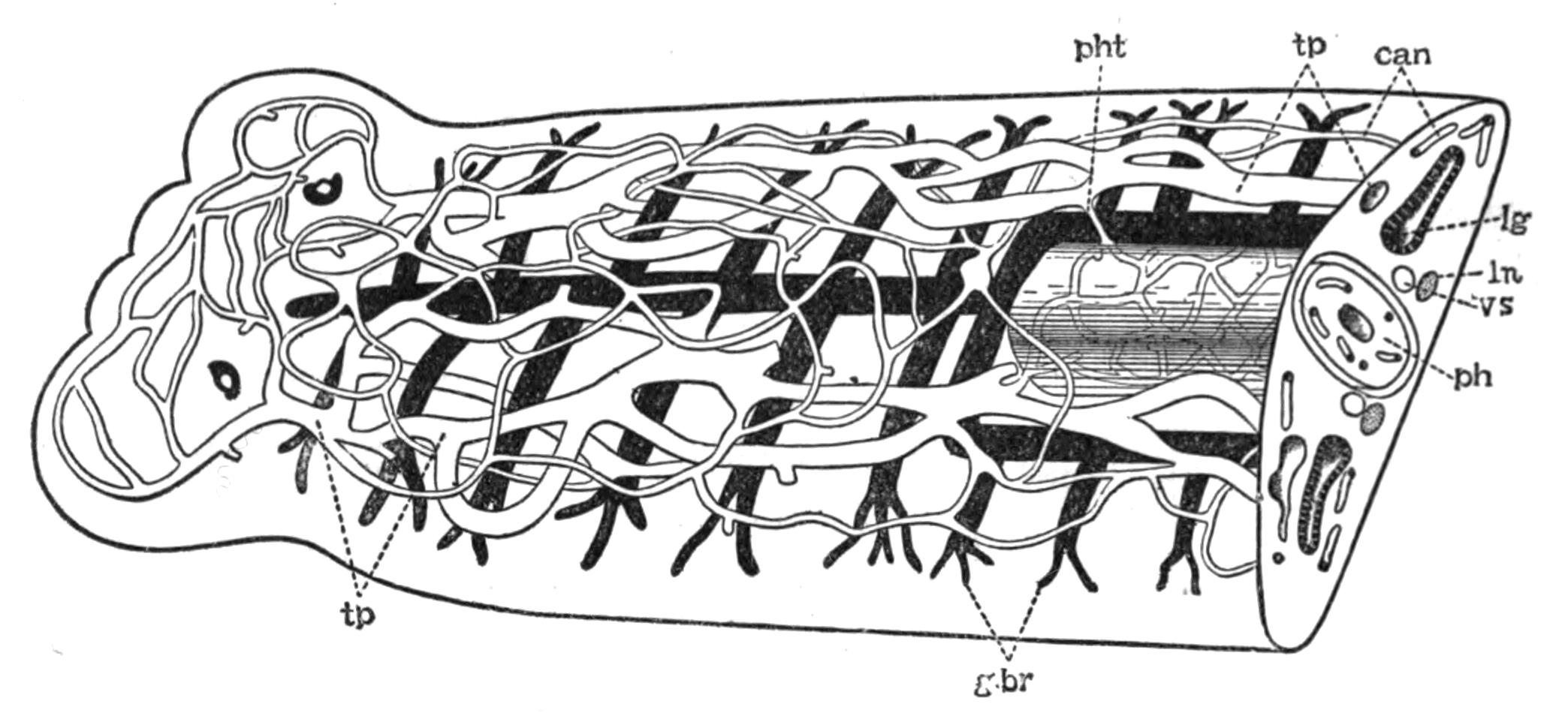
Fig. 18.—Semi-diagrammatic view of the excretory system of Planaria lactea. (Partly after Chickoff.) can, Capillary network on both dorsal and ventral surfaces; g.br, branches of the intestine; lg, lateral branches of the digestive system; ln, longitudinal nerve; ph, pharynx, with intermuscular capillary excretory network arising from the point marked pht; tp, principal vessels of the excretory system, the external opening of which is not certainly known; vs, vesicula seminalis.
Excretion.—The excretory organs of Triclads consist of flame-cells, canaliculi, and a pair of longitudinal canals, the external openings of which, have not been satisfactorily ascertained. The flame-cells are difficult to detect in Planaria lactea, and the latest observer, Chickoff,[59] was unable to see them, although to him we are indebted for figures of this system in P. lactea (Fig. 18) and P. alpina (P. montana). In the latter, the flame-cells are distinct, and may open directly into the two main canals or indirectly through unbranched canaliculi. The pharynx possesses a special supply of excretory tubules communicating with the main canals. A similar system has been described and figured in Gunda segmentata by Lang.[60]
Classification of Tricladida.
| PALUDICOLA. | ||
| Family. | Genus and British Species. | |
| Planariidae |
Planaria lactea O. F. M., P. punctata Pall., P. polychroa Schm., P. torva M. Sch., P. alpina Dana. Polycelis nigra Ehr., P. cornuta Schm. Anocelis. Oligocelis, Procotyla. (Doubtful genera.) Sorocelis. Dicotylus. |
|
| MARICOLA. | ||
| Procerodidae (= Gundidae). |
Procerodes (= Gunda) ulvae Oersted, P. littoralis van Beneden. Cercyra. Uteriporus. |
|
| Bdellouridae |
Bdelloura. Syncoelidium. |
|
| TERRICOLA. | ||
| Bipaliidae | Bipalium kewense Moseley (introduced). | |
| Geoplanidae |
Geoplana. Geodesmus. |
|
| Rhynchodemidae | Rhynchodemus terrestris O. F. M. | |
| Belonging to undetermined Families | 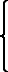 |
Dolichoplana. Polycladus. Microplana. Leimacopsis. |
Turbellaria. III. Rhabdocoelida.
The Rhabdocoelida include a very heterogeneous assemblage of usually minute Turbellaria, distinguished collectively from the Polyclads and Triclads by the form of the digestive tract. This is a simple or slightly lobed sac, except in the Bothrioplanidae, which in this and many other points closely resemble the Triclads. It is to the straight, rod-like nature of the alimentary canal that the name of the group refers. The size and form of the body, and the structure of the pharynx and genitalia, vary within wide limits.
The Rhabdocoelida are subdivided into three tribes:—
(1) Acoela, in which a sub-central mouth and pharynx are present, but lead into the parenchyma of the body, not into an intestine with proper walls. An excretory system has not hitherto been seen. Yolk-glands are absent. An otolith underlies the brain. The Acoela are marine.
(2) Rhabdocoela, which possess a complete alimentary tract separated from the body-wall (except for a few suspensory strands) by a space or body-cavity, filled with fluid. This space is sometimes (Vortex viridis) lined by an endothelium of flattened parenchymatous cells. There are two compact testes, which are enclosed (as are the ovaries and yolk-glands) in a distinct membrane. An otolith is present in some genera and species. Terrestrial, fresh-water, marine.
(3) Alloeocoela, in which the body-cavity is greatly reduced. Except in the Bothrioplanidae, the gonads have no distinct membrane. Testes numerous; yolk-glands present. Marine with a few exceptions.
Occurrence and Habits of the Rhabdocoelida.—The Acoela are usually minute, active Turbellaria abounding amongst weeds throughout the lower half of the Littoral, and the whole of the Laminarian zone, but are most plentiful in the pools exposed during spring-tides on our coasts, especially on the shores of Devonshire. The species of Haplodiscus, however, and Convoluta henseni are modified pelagic forms found in the Atlantic Ocean.[61] Convoluta paradoxa (Fig. 19, B) is the commonest British species. It is from 1 to 9 mm. in length, and of a brown colour, marked above by one or more transverse white bars. The brown colour is due to a symbiotic alga, the nature of which has not been thoroughly investigated. In an allied species, however (C. roscoffensis), from the coast of Brittany, the alga, which is here green, has been carefully examined by Professor Haberlandt,[62] and it appears from his researches that the algae form a special assimilating tissue, enabling the Convoluta to live after the fashion of a green plant. At Roscoff, these elongated green Convoluta live gregariously in the sandy tide-pools, fully exposed to the sun's rays, and have the appearance of a mass of weed floating at the surface of the water. Access to the atmosphere and to sunlight are necessary in order to enable the assimilating tissue to form the carbohydrates, upon which this form lives exclusively. Not only has the alga itself undergone such profound changes (loss of membrane, inability to live independently after the death of the host) as to disguise its true nature (a tissue-cell derived from algal ancestors), but the Convoluta has also undergone {44}concomitant changes, in form, in the loss of a carnivorous habit, and in the development of marked heliotropic movements, thus adapting itself to an holophytic or plant-like mode of nutrition. Nevertheless the Acoela, as a group, are carnivorous, feeding upon Diatoms, Copepoda, and small Rhabdocoela, the absence of a digestive tract indeed being probably more apparent than real.[63]
The Rhabdocoela live under varied conditions. One form, Prorhynchus sphyrocephalus, has been found among plants far from water in the neighbourhood of Leyden, by De Man.[64] With this exception the group is purely aquatic, and though a few genera and even individuals of the same species occur both in salt and fresh water, whole sub-families and genera are either marine or paludicolous. Among the latter, Mesostoma, Castrada, Vortex, and Derostoma are common in brooks and ponds, especially at certain times, often only for one month (May or June) in the year. Species of Macrostoma, Stenostoma, and Microstoma are also abundant in similar places. The two latter occur in chains formed by fission; but the sexual individuals (which are of distinct sexes, contrary to the usual hermaphrodite condition of Flat Worms) only appear at stated times and are not well known. A large number of genera are purely marine, and one family, the Proboscidae (distinguished by having the anterior end invaginated by special muscles and converted into a sensory organ), is entirely so. The most cursory examination of littoral weeds reveals species of Macrorhynchus, Acrorhynchus, Promesostoma, Byrsophlebs, and Proxenetes, the character of which may be gathered from von Graffs great monograph, or from Gamble's paper on the "British Marine Turbellaria."[65] Much, however, still remains to be done before we possess an adequate idea of the occurrence of this group on our coasts.
Fig. 19.—Forms of Rhabdocoelida. A, Mesostoma tetragonum O. F. M. (Rhabdocoela), × 10; B, Convoluta paradoxa Oe. (Acoela), × 10; C, Vorticeros auriculatum O. F. M., × 6; D, Monotus fuscus Oe. (Alloeocoela), × 4. ap, Adhesive papillae; d, intestine; m, pharynx; ot, otolith; rh, rhabdites; te, testes; ut, uterus with eggs; yg, yolk-glands; ♂, male, ♀, female genital pores. (A after Braun.)
Some Rhabdocoels are parasitic. Fecampia erythrocephala, which occurs in the lacunar spaces and alimentary canal of young shore crabs (Carcinus maenas), is a white cylindrical animal ¼ inch long, with a red snout. After attaining maturity it works its way out of the crab and encysts under stones, forming a pyriform mass in shape like a "Prince Rupert's drop." Within this case the eggs develop, and the young probably emerge through the open narrow end of the hard white tube, but how they reach the crab is not known. Graffilla muricicola is found in the kidney of Murex brandaris and M. trunculus, at Naples and Trieste; G. tethydicola in the foot of Tethys. Anoplodium parasiticum occurs among the muscles which attach the cloaca of Holothuria tubulosa to the body-wall; and A. schneideri occurs in the sea-cucumber, Stichopus variegatus. These are truly parasitic forms, constituting a special sub-family. They have no rhabdites in the skin; the nervous system and sense-organs are only slightly developed; and the pharynx has undergone a notable reduction in relation to the simpler mode of obtaining nourishment. Other cases of association between certain Rhabdocoels (closely allied to, if not identical with, certain free-living species) and Lamellibranchs or Sea-urchins, are, however, of another kind. Thus on the gills or in the mantle cavity of species of Mytilus, Cyprina, Tellina, and upon the test of Clypeaster, such forms as Enterostoma mytili, Acmostoma cyprinae, and Provortex tellinae have been found. But it is probable that these Turbellaria here obtain merely a temporary {46}shelter and possibly a supply of the food of the mussel or sea-urchin.
The Alloeocoela afford a well-established case of association. Monotus fuscus (Fig. 19, D), an abundant, active, elongated animal, lives on our coasts in the upper part of the littoral zone among Patella, Balanus, and sometimes Chiton. When the tide is low, the Monotus, to obtain moisture and darkness, creeps between the mantle-folds of these animals, where it may readily be found. Upon the return of the tide it leaves its retreat and creeps or swims about freely. Other Alloeocoela collect in great numbers in tufts of red-seaweeds (Florideae). By placing such tufts in vessels, the sea-water, especially as darkness sets in, begins to swarm with Cylindrostoma 4-oculatum, species of Enterostoma and Plagiostoma; P. vittatum, with three violet bands across the white body, being a particularly obvious form. Vorticeros auriculatum (Fig. 19, C), another abundant species, is remarkable for the long tentacles which can be completely withdrawn, and in this condition it completely resembles a Plagiostoma.
The presence of a species (P. lemani) of the characteristically marine genus Plagiostoma, in the Lake of Geneva, and in one or two other Swiss lakes, at depths varying from 1 to 150 fathoms, is very interesting, and is perhaps the only well-established case of the survival of a once marine Rhabdocoelid under changed conditions. Plagiostoma lemani is by far the biggest of the group to which it belongs, being over half an inch in length. It is usually found in fine mud, sometimes among Chara hispida, and has the general appearance of an inactive white slug. We are indebted to Forel and Duplessis for the discovery of this species, and also of Otomesostoma morgiense, a Mesostoma with an otolith, dredged in 10 to 50 fathoms in the Lake of Geneva, the Lake of Zürich, and found recently also by Zacharias in the Riesengebirge. The genus Bothrioplana, first found by Braun in the water-pipes of Dorpat, has been carefully investigated by Vejdovsky,[66] who places it in a special family, Bothrioplanidae, among the Alloeocoela. One species has recently been found near Manchester.
A comprehensive survey of the Rhabdocoelida shows that, with the chief exception of the Proboscidae, the more lowly organised forms, the Acoela and Alloeocoela, are marine, whereas the fresh-water forms are in most cases the most highly organised {47}genera (Mesostoma, Vortex). But Macrorhynchus helgolandicus, though minute (1.5-2 mm. long), has a more complex structure[67] than any other species of the specialised marine genus to which it belongs, and is a remarkable instance of great complexity being associated with small size.
Reproduction.—The Rhabdocoelida present the greatest diversity in the development of the reproductive system. The Acoela and Alloeocoela have the simplest arrangement. Scattered testes, often without a distinct membrane, form the spermatozoa, which in most cases wander into parenchymatous spaces, but in Monoporus rubropunctatus and Bothrioplana, into distinct vasa deferentia. In both groups a protrusible penis opens independently to the exterior, and may be simply muscular or provided with a chitinous armature. Two ovaries are present, and the oviducts, if distinct, are continuations of the ovarian membrane. In most forms a "bursa seminalis," which receives the spermatozoa of another individual, is appended to the female genital canal. In many of the Alloeocoela, however, a portion of the ovary is sterile, and its cells, forming a yolk-gland, feed the fertile portion, the whole structure being then spoken of as a germ-yolk-gland. In many others (Monotidae) this sterile part has become an independent yolk-gland, which communicates by yolk-ducts with the oviducts. The Acoela form no egg-case, the body of the parent becoming a bag for the ova, which elaborate their own food-yolk. The Alloeocoela lay hard-shelled eggs, which are produced in Bothrioplana and Automolos by the activity and interaction of reproductive organs, resembling closely those of certain Triclads.[68]
The Rhabdocoela exhibit every stage in the development of a complex reproductive system, from the simple ovaries and testes of a Microstoma or Macrostoma, to the intricate system of ducts and glands of a Macrorhynchus (Proboscidae), in which there is still much to be made out. The complications of the copulatory organs chiefly arise from the way in which the spermatozoa are brought into contact with a nutritive prostatic fluid, or are formed into spermatophores; and also from the penial armature, {48}which is often very complex, and may consist of a curved chitinoid hook or a coiled loop (Promesostoma), of hooks (Proboscidae), or of an intricate arrangement of plates (Proxenetes); or the penis may take on a complex corkscrew-like form (Pseudorhynchus). The (frequently armed) female genital canal usually possesses a bursa seminalis for the fertilisation of the eggs, but a receptaculum seminis or spermatheca may serve for the reception, the bursa, for the lodgment of the spermatozoa of another individual. The fertilised ovum is provided with a supply of food-yolk and with a shell, which may be formed in a special diverticulum, the "uterus." The development of these organs strains the resources of the animal to the utmost, and in some Proboscidae the alimentary canal is squeezed out and disintegrates, in order to make room for them.
A few Mesostoma (M. ehrenbergii, M. productum, M. lingua) produce two kinds of eggs—thin- and thick-shelled. The latter are laid throughout the summer, and lie dormant through winter. The young which hatch in spring out of these "winter" eggs develop rapidly, and when only 7 to 8 mm. long (i.e. one-third the size of the parent) already possess functional genital organs; the penis, however, is rudimentary, and incapable of being used for copulation. Hence it is probable that this stunted progeny self-fertilise their thin-shelled or "summer" eggs. After the formation of these eggs the same parent is said (Schneider[69]) to produce thick-shelled or winter eggs, but however that may be, the first young which hatch from the thin-shelled ova are produced in great numbers at a time (April to May) when food is abundant. These grow rapidly to the full size, and then having attained maturity, cross-fertilise one another's ova, which become encased in a thick brown shell; and it is these numerous "winter" eggs that lie dormant throughout the autumn and winter. Many Mesostoma, and practically all other Rhabdocoela, however, produce only thick-shelled eggs, and in all cases it is probable that to these many species owe their wide distribution, the exact range of which is, however, unknown, as is also the means of dispersal.
Classification of Rhabdocoelida.
| ACOELA. | |
| Family. | Genus and British species. |
| Proporidae |
Proporus venenosus O. Sch. Plymouth. Monoporus rubropunctatus O. Sch. Plymouth. Haplodiscus. |
| Aphanostomatidae |
Aphanostoma diversicolor Oe. Common. A. elegans Jen. Plymouth. Convoluta saliens Grff. Plymouth, Millport. C. paradoxa Oe. (Fig. 19, B). Common. C. flavibacillum Jen. Plymouth, Port Erin, Millport. Amphicoerus. Polychoerus. |
| RHABDOCOELA. | |
| Macrostomatidae |
Mecynostoma. Macrostoma hystrix Oe. Stagnant water. Omalostoma. |
| Microstomatidae |
Microstoma lineare Oe. Fresh water. M. groelandicum Lev. Plymouth, among Ulva. Stenostoma (Catenula) lemnae Dug. Near Cork. S. leucops O. Sch. Common in fresh water. Alaurina claparedii Grff. Skye. |
| Prorhynchidae |
Prorhynchus stagnalis M. Sch. In Devonshire rivers. Promesostoma marmoratum M. Sch. Common. P. ovoideum O. Sch., P. agile Lev. Plymouth. P. solea O. Sch. Plymouth, Port Erin. P. lenticulatum O. Sch. Port Erin. |
| Mesostomatidae |
Byrsophlebs graffii Jen. Plymouth, Millport. B. intermedia Grff. Millport, Port Erin. Proxenetes flabellifer Jen. Millport, Plymouth, Port Erin. P. cochlear Grff. Millport. Otomesostoma. Mesostoma productum Leuck., M. lingua O. Sch., M. ehrenbergii O. Sch., M. tetragonum O. F. M. (Fig. 19, A). All at Cambridge. M. rostratum Ehr. Widely distributed. M. viridatum M. Sch. Manchester. M. robertsonii Grff., M. flavidum Grff. Both at Millport. Bothromesostoma personatum O. Sch. Preston. Castrada. |
| Proboscidae |
Pseudorhynchus bifidus M‘Int. Millport, St. Andrews, Port Erin. Acrorhynchus caledonicus Clap. Generally distributed. Macrorhynchus naegelii Köll., M. croceus Fabr. Plymouth, Millport. M. helgolandicus Metsch. West coast. Gyrator hermaphroditus Ehrbg. St. Andrews. Also common in fresh water. Hyporhynchus armatus Jen. Plymouth, Port Erin. H. penicillatus O. Sch. Plymouth. |
| {50}
Vorticidae |
Schultzia. Provortex balticus M. Sch. Generally distributed. P. affinis Jen., P. rubrobacillus Gamb. Plymouth. Vortex truncatus Ehrbg. Abundant in fresh water. V. armiger O. Sch. Millport (fresh water). V. schmidtii Grff., V. millportianus Grff. Millport. V. viridis M. Sch. Generally distributed. Jensenia. Opistoma. Derostoma unipunctatum Oe. Edinburgh. Graffilla. Anoplodium. Fecampia erythrocephala Giard. Plymouth, Port Erin. |
| Solenopharyngidae | Solenopharynx. |
| ALLOEOCOELA. | |
| Plagiostomatidae |
Acmostoma. Plagiostoma dioicum Metsch., P. elongatum Gamb., P. pseudomaculatum Gamb., P. sagitta Ulj., P. caudatum Lev., P. siphonophorum O. Sch., P. ochroleucum Grff. All at Plymouth. P. sulphureum Grff. Port Erin. P. vittatum F. and Leuck. Millport, Plymouth, Port Erin. P. koreni Jen. Plymouth, Millport. P. girardi O. Sch. Plymouth, Port Erin, Valencia. Vorticeros auriculatum O. F. M. (Fig. 19, C). Port Erin, Plymouth. V. luteum Grff. Plymouth. Enterostoma austriacum Grff. Plymouth, Port Erin. E. fingalianum Clap. Skye, Plymouth. E. coecum Grff. Millport. Allostoma pallidum van Ben. Millport. Cylindrostoma 4-oculatum Leuck. Skye, Millport, Plymouth. C. inerme Hall, C. elongatum Lev. Plymouth. Monoophorum striatum Grff. Plymouth. |
| Bothrioplanidae |
Bothrioplana. Bothrioplana sp.? Manchester. Otoplana. |
| Monotidae |
Monotus lineatus O. F. M., M. fuscus Oe. (Fig. 19, D). Both common littoral forms. M. albus Lev. Plymouth. Automolos unipunctatus Oe. Skye, St. Andrews, Plymouth. A. horridus Gamb., A. ophiocephalus O. Sch. Plymouth. |
TREMATODA
CHARACTERS OF TREMATODES—HABITS AND STRUCTURE OF TREMATODA ECTOPARASITICA (MONOGENEA)—LIFE-HISTORIES OF POLYSTOMUM INTEGERRIMUM, DIPLOZOON PARADOXUM, AND GYRODACTYLUS ELEGANS—TREMATODA ENDOPARASITICA (DIGENEA)—OCCURRENCE AND HABITS OF DIGENEA—LIFE-HISTORY OF DISTOMUM MACROSTOMUM—DISTOMUM HEPATICUM AND ITS EFFECTS—BILHARZIA HAEMATOBIA—BISEXUAL TREMATODES—TABLE OF HOSTS—CLASSIFICATION.
From the Turbellaria we now pass on to a consideration of the second great subdivision of the Platyhelminthes, the Trematodes or "flukes," of which the "liver-fluke" is the best known, since it is one of the most dangerous parasites that infest domestic animals.
It has been pointed out that the Polyclads, Triclads, and Rhabdocoels are carnivorous, and that in each of these groups sporadic cases of parasitism occur. In other words, when the prey is much larger than the Turbellarian, the latter tends to become a parasite, and we can trace the development of the parasitic habit from the gradual association of Turbellaria with Ascidians, Crustacea, Molluscs, and Polyzoa merely for protective purposes, through the adoption, not only of the body of the host for shelter, but of its flesh for food; though it is only in some Rhabdocoels (Graffilla, etc.) that there exists a degeneration corresponding to the easier mode of nutrition and simpler life. The Trematodes,[70] however, are wholly parasitic, either on the outer surface, the gills, or internal organs of their host, which is almost always a {52}Vertebrate. Some Trematodes lodge in the mouth; others wander down the oesophagus into the stomach or intestine, where they fix themselves to the mucous membrane. Again, others work their way into the digestive glands by the ducts, and thus become further and further removed from the external world, and more adapted to live in the particular organs of that host in which they best flourish. The most important result of the adoption of this internal habitat by endoparasitic Trematodes is, however, seen in their life-history. If a liver-fluke were to deposit its million or so of eggs in the bile-ducts of the sheep, and these were to develop in situ, the host could not withstand the increased drain upon its vital resources, and host and parasites would perish together. Hence it is clear that the infection of a second host by Trematodes is highly necessary, whether they be ectoparasitic, in which case the infection is easily effected, when two hosts are in contact, by the adult worms, as well as when they are apart, by free-swimming larvae. In endoparasitic Trematodes it is brought about by the migration of the young to the outer world, their entrance into a, usually, Invertebrate host and their asexual multiplication within it, and the capture and deglutition of this "intermediate host" by the final Vertebrate one. Within the latter the immature parasites find out the organ in which their parents flourished, and here they too grow and attain maturity. The chances of any one egg of an endoparasitic Trematode producing eventually an adult are, therefore, far less favourable than in the case of an ectoparasitic form. In other words, while the former must lay a great number of small eggs, the latter need only deposit a (comparatively) few large ones, and this fact has a corresponding influence on the structure of the genitalia in the two cases. The Digenea, which employ two hosts in a lifetime, have accordingly a different generative mechanism from that of the Monogenea. The great need of the latter is a powerful apparatus for adhering to the surface of the body of its host; while the adaptations which the endoparasite requires are, in addition, (1) protection against the solvent action of the glands of its host, (2) the power of firm adhesion to a smooth internal surface, and (3) the ability not only to produce a large quantity of spermatozoa and ova, but in the absence of a fellow-parasite, to fertilise its own ova; and we find these conditions abundantly satisfied.
Trematoda monogenea (ectoparasitica).
There are four subdivisions of the Monogenea:—
I. Temnocephalidae, with four to twelve tentacles, and one sucker posteriorly (Fig. 20).
II. Tristomatidae, with two lateral, anteriorly-placed suckers. Oral suckers are absent, a large posterior sucker is constant, and is often armed with hooks (Fig. 22, C).
III. Polystomatidae, with, usually, two oral suckers and a posteriorly-placed adhesive disc armed with suckers and hooks (Figs. 23 and 24).
IV. Gyrodactylidae (Fig. 29).
Habits and Structure of Ectoparasitic Trematodes.
I. Temnocephalidae.—These interesting forms, of which a good account has lately been written by Haswell,[71] occur on the surface (rarely in the branchial chamber) of fresh-water crayfish and crabs in Australasia, the Malay Archipelago, Madagascar, and Chili. Others have been found on the carapace of a fresh-water tortoise, and in the branchial chamber of the mollusc Ampullaria from Brazil. Wood-Mason discovered others, again, in bottles containing spirit-specimens of Indian fish. Temnocephala is rarely more than a quarter of an inch long, and looks like a minute Cephalopod or a broad flattened Hydra. By the ventral sucker each species adheres to its own particular host, the tentacles being used as an anterior sucker for "looping" movements. The food, consisting of Entomostraca, Rotifera, and Diatoms, is first swallowed whole by the large pharynx (Fig. 20, ph), which can be protruded through the ventrally-placed mouth, and is then received into a simple lobed intestine (d). The skin, especially on the surface of the tentacles, is provided here and there with patches of cilia borne by the cellular epidermis,—the only undoubted case of external cilia occurring in an adult Trematode. Minute rhabdites formed in special gland-cells, occur plentifully on the tentacles, and are another distinctly Turbellarian feature. The excretory system is peculiar (Fig. 21). Fine ducts proceed from the various organs of the body, and open to the exterior by means of a pair of contractile sacs {54}placed on the dorsal surface. Each sac is a single cell, and within it not one merely, but several "flames," or bunches of rhythmically contractile cilia, are present. These are placed on the course of excessively fine canals, which perforate the protoplasm of this cell. The terminal branches of the excretory canals end in branched cells, apparently devoid of "flames."

Fig. 20.—Temnocephala novae-zealandiae Has. × 10. Ventral view to show the digestive and reproductive systems. (After Haswell.)
Fig. 21.—The same from the dorsal surface, to show the excretory system (double line), and the nervous system (black and shaded). (After Haswell.)
d, Intestine; dln, dorso-lateral nerve; dn, dorsal nerve; ex.o, excretory aperture on dorsal surface; ex.s, terminal excretory sac; m, mouth; ov, ovary; ovd, oviduct; ph, pharynx; rh, rhabdites; rh.c, cells in which the rhabdites are formed; rv, yolk receptacle; sc, sucker; sh, shell-gland; te, testes; ut, uterus; vg, vagina; vn, ventral nerve; vs, vesicula seminalis; yd, yolk-duct; yg, yolk-gland. ♀, ♂, common genital pore.
The reproductive system is very similar to that of certain Rhabdocoels. An armed penis and the female genital duct open into a genital atrium, and this by a single aperture (♀, ♂, Fig. 20) to the exterior. The fertilised ovum and yolk are enclosed in a stalked shell formed in the uterus.
The interest and importance of the Temnocephalidae lies in the fact that they are almost as much Turbellaria as Trematodes. {55}In habits, in the character of the skin, the muscular, digestive, and reproductive systems, they find their nearest allies in Rhabdocoels (Vorticidae). But in the excretory and nervous systems, the latter composed of two dorsal, two lateral, and two ventral trunks all connected together (Fig. 21), they are Tristomid Trematodes. Thus they may fitly connect an account of the two great groups.
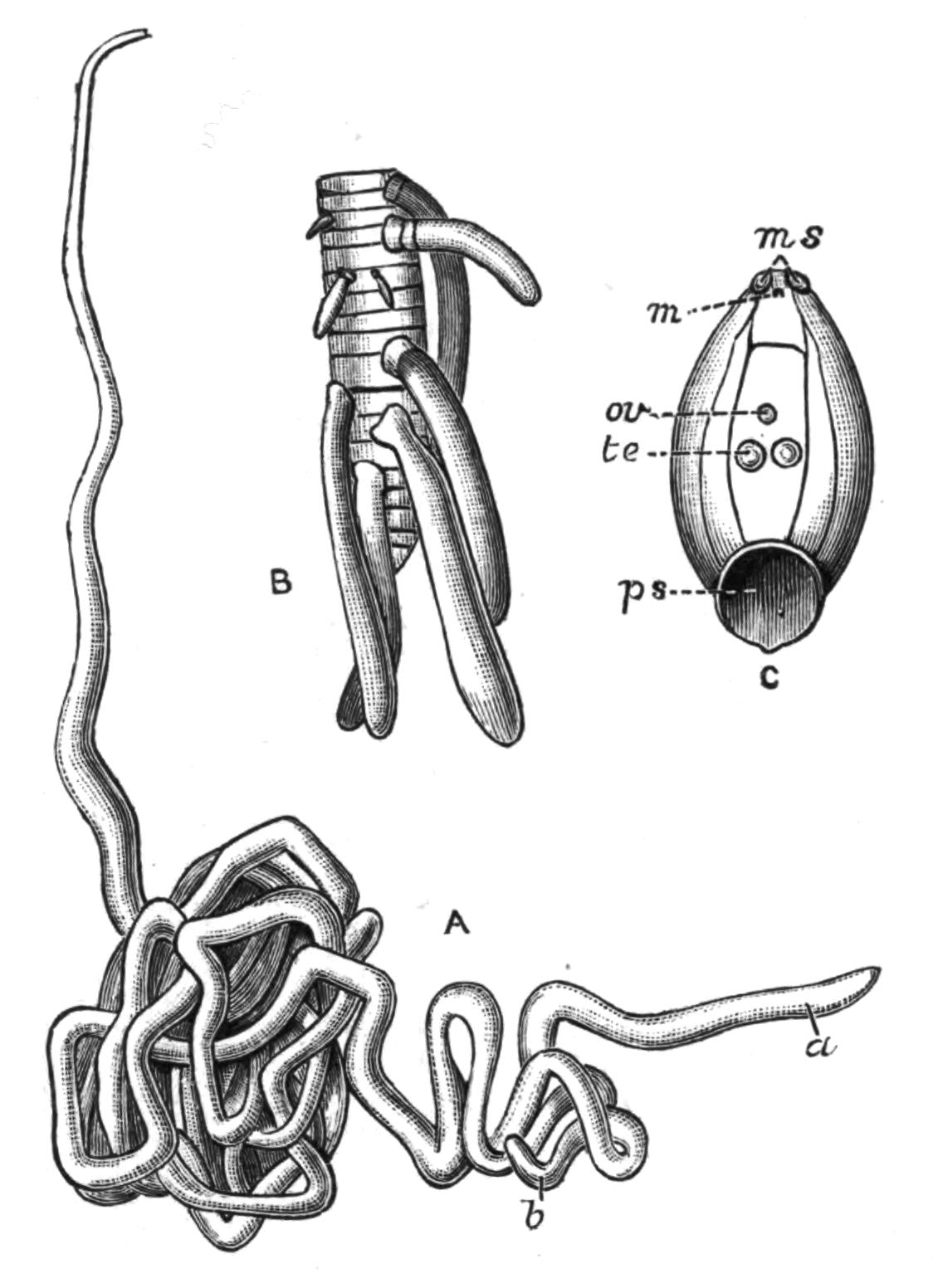
Fig. 22.—A, Nematobothrium filarina van Bened. Nat. size. Two individuals (a and b) are found together, encysted on the branchial chamber of the Tunny. B, Udonella caligorum Johns. A Tristomid, several of which are attached to the ovary of a Copepod (Caligus), itself a parasite on the gills of the Hake. × 8. C, Epibdella hippoglossi O. F. M. A Tristomid found on the body of the Halibut. Nat. size. m, Mouth; ms, lateral suckers; ov, ovary; ps, posterior sucker; te, testes. (All after P. J. van Beneden.)
II. Tristomatidae and III. Polystomatidae.[72]—The members of these families are found on the body, or attached to the gills, of fresh-water and marine fishes. The edible and inedible fish of our coasts have each their particular ectoparasitic Trematodes; while the Minnows, Sticklebacks, and Miller's Thumbs of streams and ponds are attacked by Diplozoon, Gyrodactylus, and other forms. The aquatic Amphibia also harbour a number. Polystomum integerrimum is common in the bladder of Frogs, where it leads a practically aquatic life. Other species of Polystomum inhabit the buccal and nasal cavities of certain Chelonia, but naturally no terrestrial Vertebrates are infested externally by these {56}Trematodes. The blood and epithelia of the host are sucked, and to this end the pharynx has frequently a chitinous armature to aid in the abrasion or inflammation of the tissues upon which the parasite feeds. In the case of a Sturgeon attacked by Nitzschia elongata, a Tristomid, the mouth of the host appeared to be highly inflamed by these attacks (v. Baer).
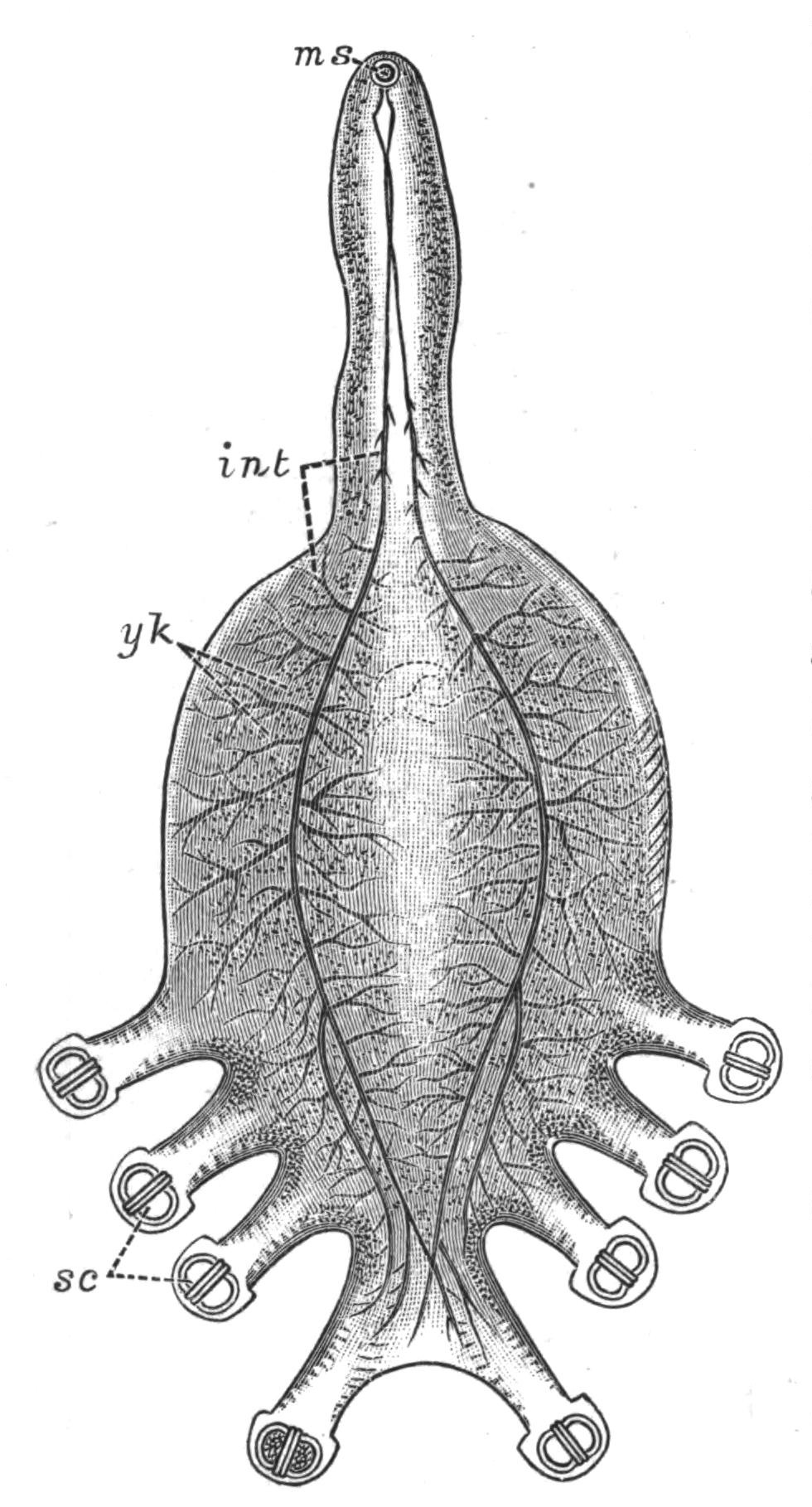
Fig. 23.—Octobothrium merlangi Kuhn, from the gills of the whiting, × 8. int, Intestine; ms, mouth; sc suckers with chitinoid armature; yk yolk-glands. (After v. Nordmann.)
The suckers, in the two families under consideration, vary in number and complexity. There is always a powerful apparatus at the hinder end of the body securing the Trematode firmly to the slimy body or gills of its host, and, usually in the Polystomatidae, a pair of suckers at the sides of the mouth accessory to the pumping action of the pharynx. In Axine, and to a less extent in Octobothrium (Fig. 23), the suckers are strengthened by a complex hingework of chitinoid bars or hooks, which serve as insertions for the muscles of the suckers, and thus increase their efficiency.
The mouth is invariably present just beneath the anterior end of the body. It leads into a muscular, pumping pharynx (Fig. 24, ph), and this into a bifurcated intestine which ends blindly. The two openings of the excretory system lie on the dorsal surface (as in Temnocephala), and the excretory canals branch through the substance of the body, ending usually in "flame-cells." The nervous system is highly developed, and resembles that of Temnocephala (Fig. 21) in detail. Upon the brain one or even two pairs of eye-spots are present in the larvae, and may persist throughout life. Tactile setae occur in Sphyranura, a parasite of the North American Amphibian Necturus, but a cellular epidermis is apparently rendered impossible, perhaps from the nature of {57}the mucus in which the body is bathed, or to the attempts of the host to free itself from these parasites; and hence an investing membrane is present, which morphologically is either a modified epithelium, or a cuticle formed by the glandular secretion of the parenchyma.
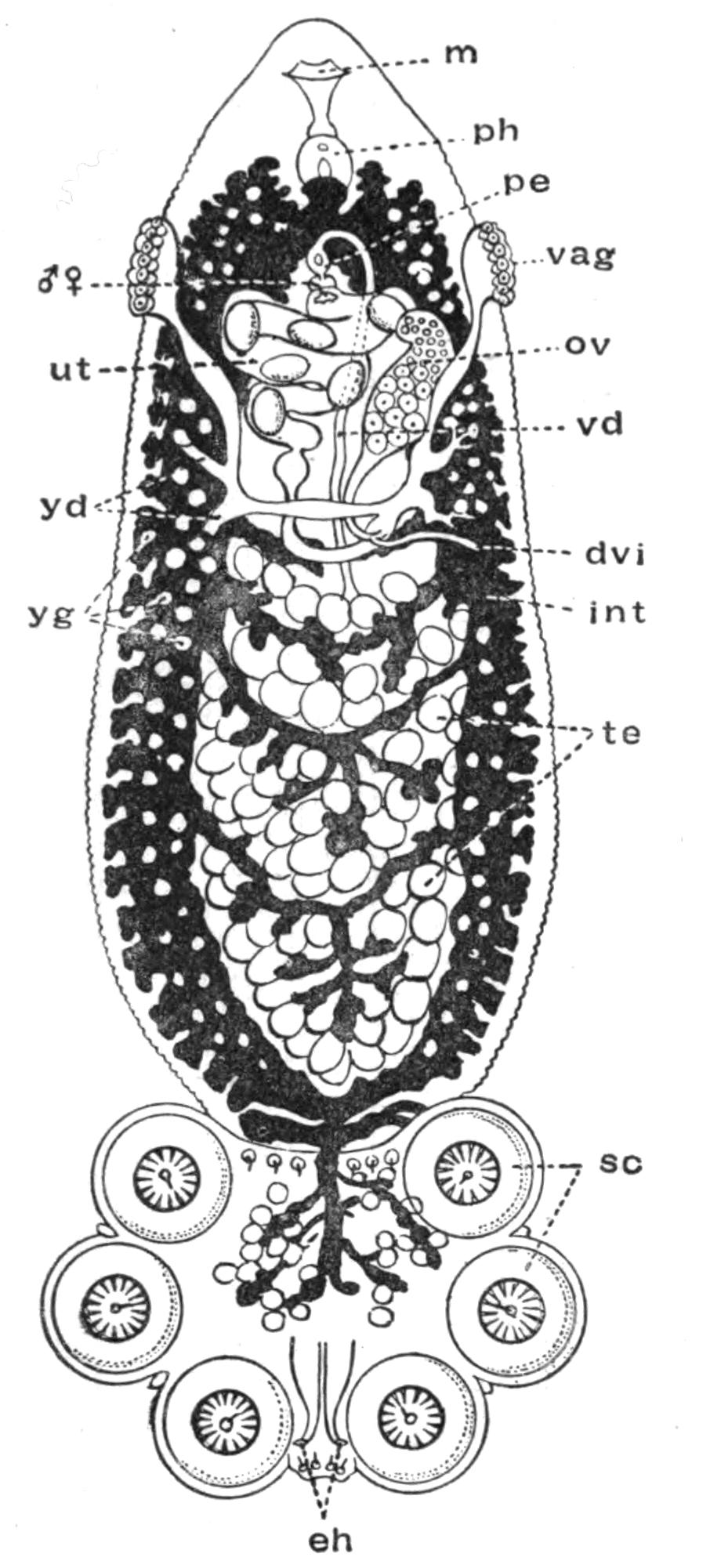
Fig. 24.—Polystomum integerrimum Fröh., from the bladder of the Frog, and seen from the ventral surface. The alimentary canal is black, the white dots upon it being the yolk-glands, dvi, Ductus vitello-intestinalis (probably homologous with the Laurer's canal or "vagina" of Digenea); eh, hooks of sucking disc; int, intestine; m, mouth; ov, ovary; pe, penis; ph, pharynx; sc, suckers with an embryonic hook persisting in each; te, testes; ut, uterus with eggs; vag, left vagina; vd, vas deferens; yd, yolk-duct; yg, yolk-glands; ♂ ♀, common genital aperture. (Modified from Zeller.) × 8.
The reproductive organs of the Polystomatidae may be understood from Figs. 24, 27, and 28. At the point of union of the oviduct (Fig. 28, ovd), the vitelline ducts (yd), and the commencement of the uterus (ut), a slender duct is given off which opens into the intestine, and is known as the "vitello-intestinal canal" (Fig. 24, dvi; Fig. 28, gic). This duct has apparently the same relations as the "canal of Laurer" of Digenea,[73] except only that the latter opens to the exterior directly. In connexion with this vitello-intestinal canal a "vagina" is present, which in Polystomum and most Monogenea is paired (Fig. 24, vag), in Diplozoon and in one {58}or two other forms, however, unpaired. The vagina receives the penis of another individual during copulation (Fig. 26), and does not appear to have an homologue in the liver-fluke or other Digenea.
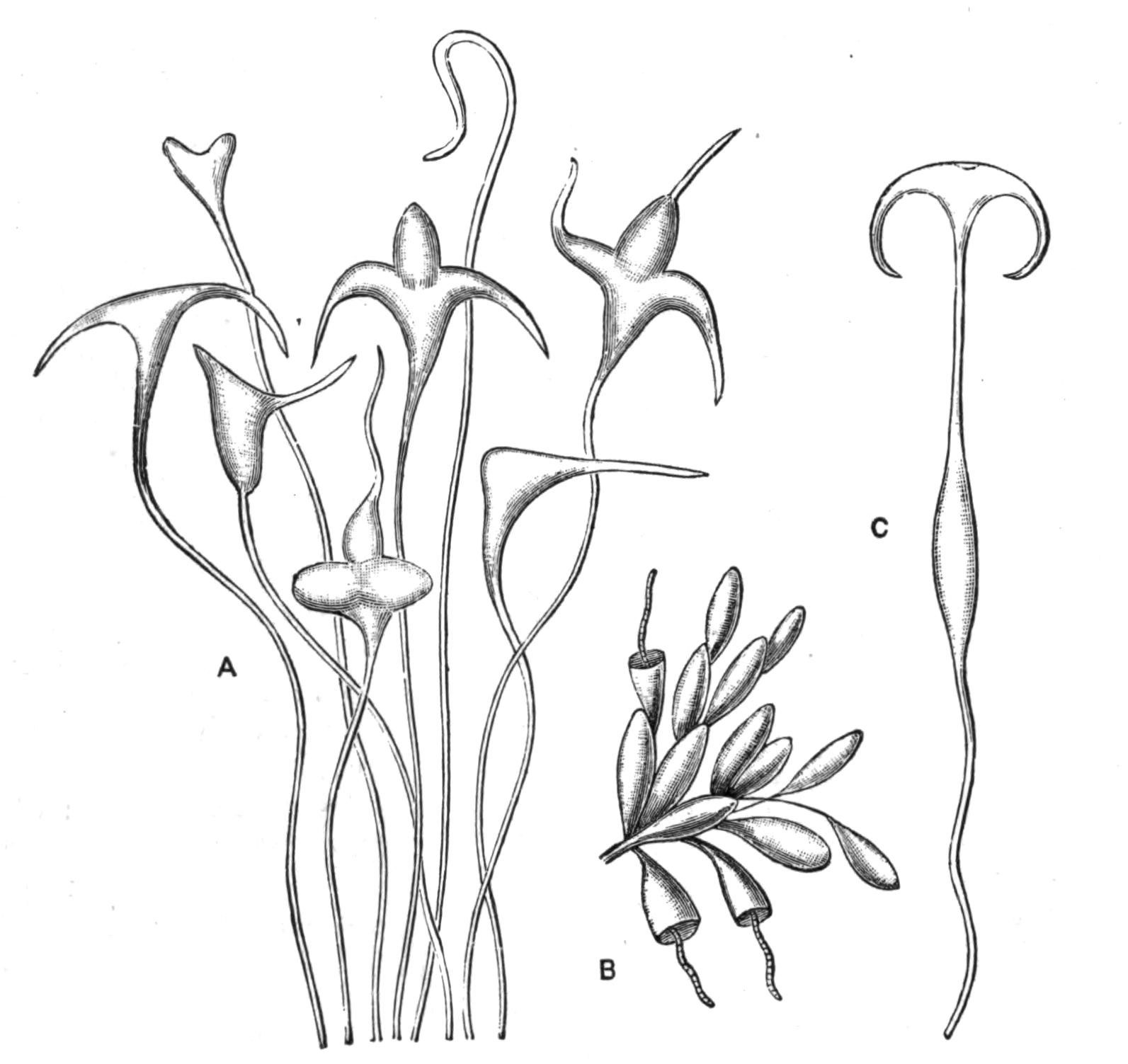
Fig. 25.—Eggs of Monogenea. A, Eggs of Encotylabe pagelli v. Ben.-Hesse; B, eggs of Udonella pollachii v. Ben.-Hesse (with young forms just hatching out); C, egg of Microcotyle labracis v. Ben.-Hesse. (After van Beneden and Hesse.) × 50.
Life-Histories of the Polystomatidae.[74]—Polystomum integerrimum. After the mutual fertilisation of two individuals, the eggs are laid in the water by the protrusion of the body of the parent through the urinary aperture of the Frog. About 1000 eggs are laid in the spring at the rate of 100 a day for ten days. After about six weeks, the larva (.3 mm. long) hatches out, and swims about freely by means of bands of large ciliated cells (Fig. 26, A); but if it does not meet with a tadpole within twenty-four hours, it dies. Should it, however, encounter one, the larva creeps along it in a looping fashion until it approaches the opercular spout, or opening of the branchial chamber, on the left side; into this it darts suddenly, fixes itself, and throws off its cilia. Here it remains eight or ten weeks, feeding, increasing in size, and forming the suckers from behind forwards. {59}At the time of the tadpole's metamorphosis, the young Polystomum works its way down the pharynx into the oesophagus and along the intestine, till it reaches and enters the opening of the bladder. Three years afterwards it becomes mature.
Sometimes, however, Polystomum experiences another fate. The larvae settling down on the external gills of a young, recently-hatched tadpole, and obtaining a richer supply of blood than in the previous case, grow far more rapidly, so that in five weeks they are mature, although still in the branchial chamber of the tadpole. They do not then wander into the alimentary canal, but usually, having discharged their eggs, die at the time of the tadpole's metamorphosis. Still more interesting, however, is the difference between the genitalia in these and in the normal Polystomum. In contrast with the latter, these possess (1) one testis and a rudimentary penis; and their spermatozoa differ in structure and shape from those of the normal Polystomum. (2) The vaginae are absent, a fact connected with the absence of a functional copulatory organ. (3) In compensation for the loss of these, a duct connects the single testis and the point of union of oviduct and yolk-ducts, and by this self-fertilisation occurs. (4) The uterus is absent; the "ootype" or duct into which the shell-gland opens, communicating directly with the exterior. In (1) and (4) these aberrant Polystomum resemble P. ocellatum, from the Tortoise Emys europaea.
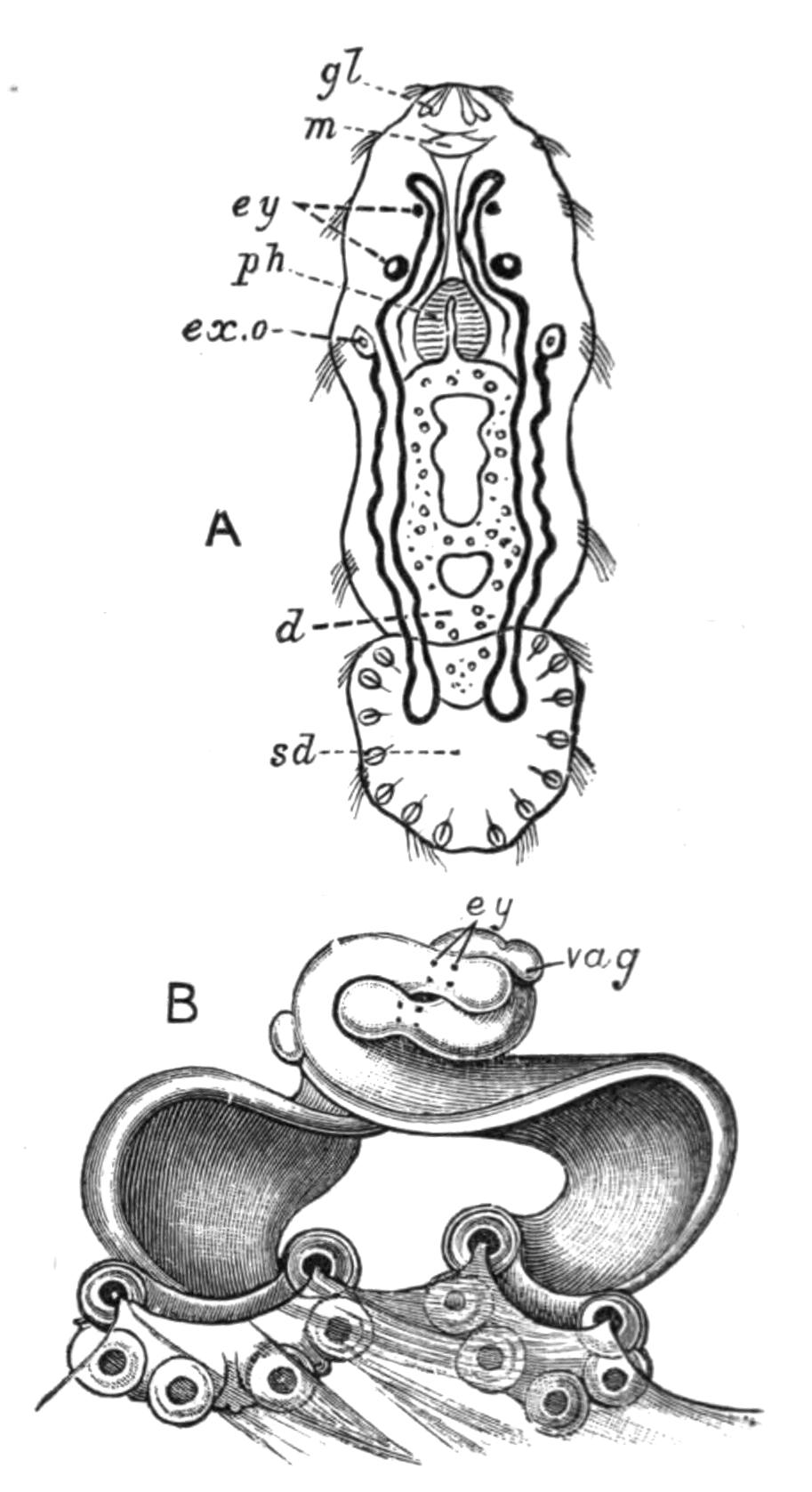
Fig. 26.—Polystomum integerrimum. A, Free-swimming larva, seen from the ventral surface. × 80. B, Two mature individuals in mutual coition attached to the bladder of a Frog. × 5. (After Zeller.) d, Intestine; ex.o, excretory pore, dorsal in position, seen here by transparency; ey, eye-spots; gl, frontal glands; m, mouth; ph, pharynx; sd, adhering disc; vag, vagina.
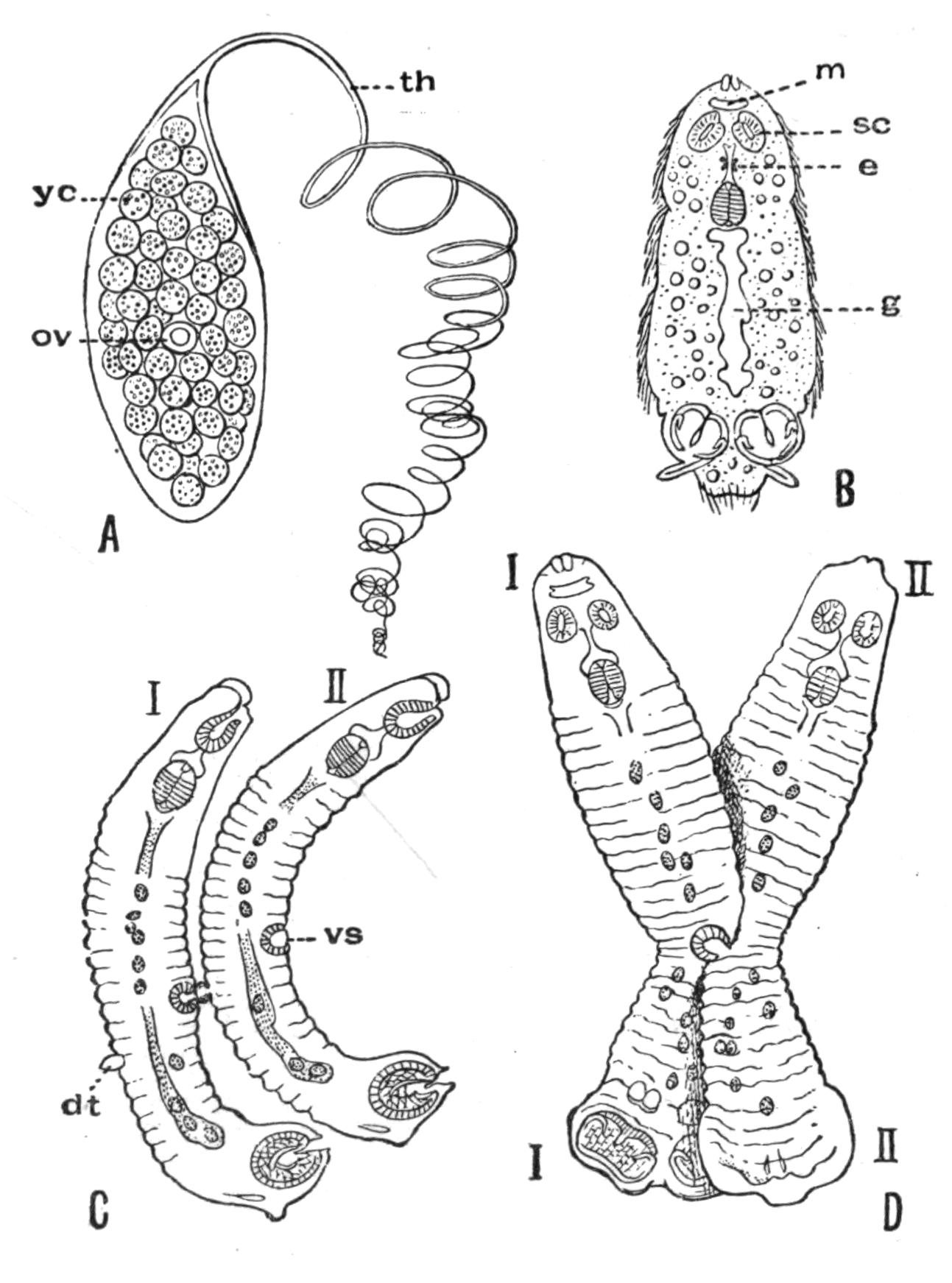
Fig. 27.—A, Egg of Diplozoon paradoxum v. Nord., consisting of a shell enclosing ov, the actual ovum, surrounded by yc, the yolk-cells; B, larva just hatched (× 125); C, two Diporpa (I and II) about to unite; D, conjugation in progress but not yet complete. dt, Dorsal papilla; e, eye; g, intestine; m, mouth; sc, ad-oral sucker; th, spirally-wound thread attaching the egg to the gill of the Minnow; vs, ventral sucker; (in D) I, I, one Diporpa, ventral view; II, II, the other, dorsal view. (After Zeller.)
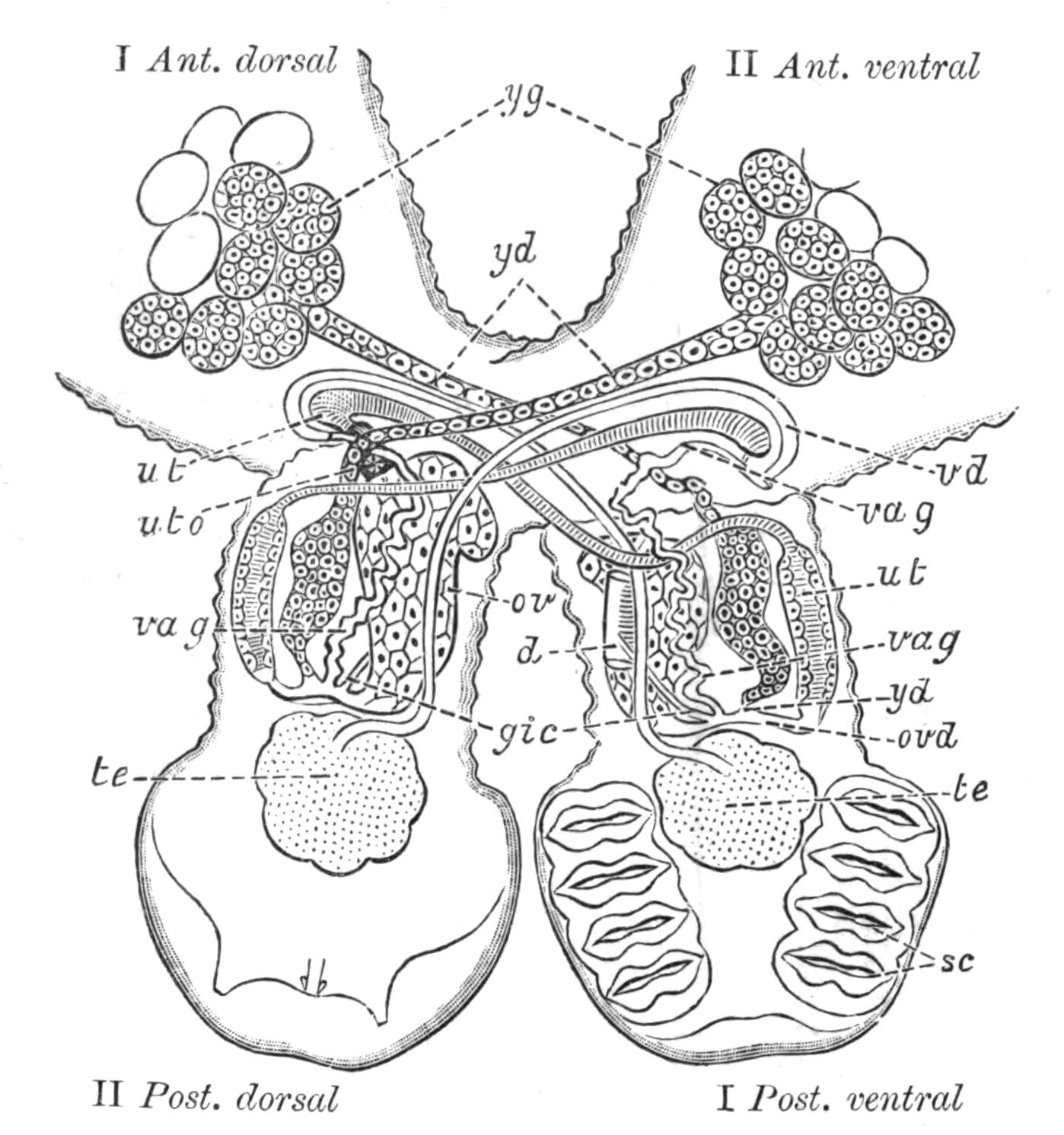
Fig. 28.—Hinder part of the body of Diplozoon paradoxum. The fusion of the two Diporpa, where they come into contact, is now complete. They now cross each other like an X, and are twisted, so that Diporpa I, in front of the point of fusion, is seen from the dorsal surface; behind, from the ventral surface; and the reverse is the case with Diporpa II. The compound animal is seen from the opposite surface to that shown in Fig. 27, D. The digestive and excretory organs are omitted. (After Zeller.) I Ant. dorsal, dorsal surface of Diporpa I, facing the anterior end; I Post. ventral, ventral surface of Diporpa I, posterior end; and similarly for II Ant. ventral and II Post. dorsal. d, Piece of the intestine showing opening of, gic, vitello-intestinal canal; ov, ovary; ovd, point of union of female genital ducts; sc, suckers; te, testis; ut (in Diporpa I), "ootype" or chamber into which shell-glands open. This is continuous with the uterus (ut) of Diporpa I; uto, ventral opening of uterus; vag, vagina, with vd, vas deferens, permanently inserted into it through the genital pore; yd, yolk-ducts; yg, yolk-glands.
Diplozoon paradoxum.—The life-history of Diplozoon is unique. For whereas the larvae of most animals grow up, each into a single adult, in Diplozoon, of the few larvae that survive the dangers of their free-swimming existence, only those become mature which conjugate permanently with another individual. But although there are thus only half as many adult Diplozoon as there were conjugating larvae (or Diporpa, as they were called when they were considered distinct forms), yet the total number of eggs produced is probably as great as if each larva became individually mature.
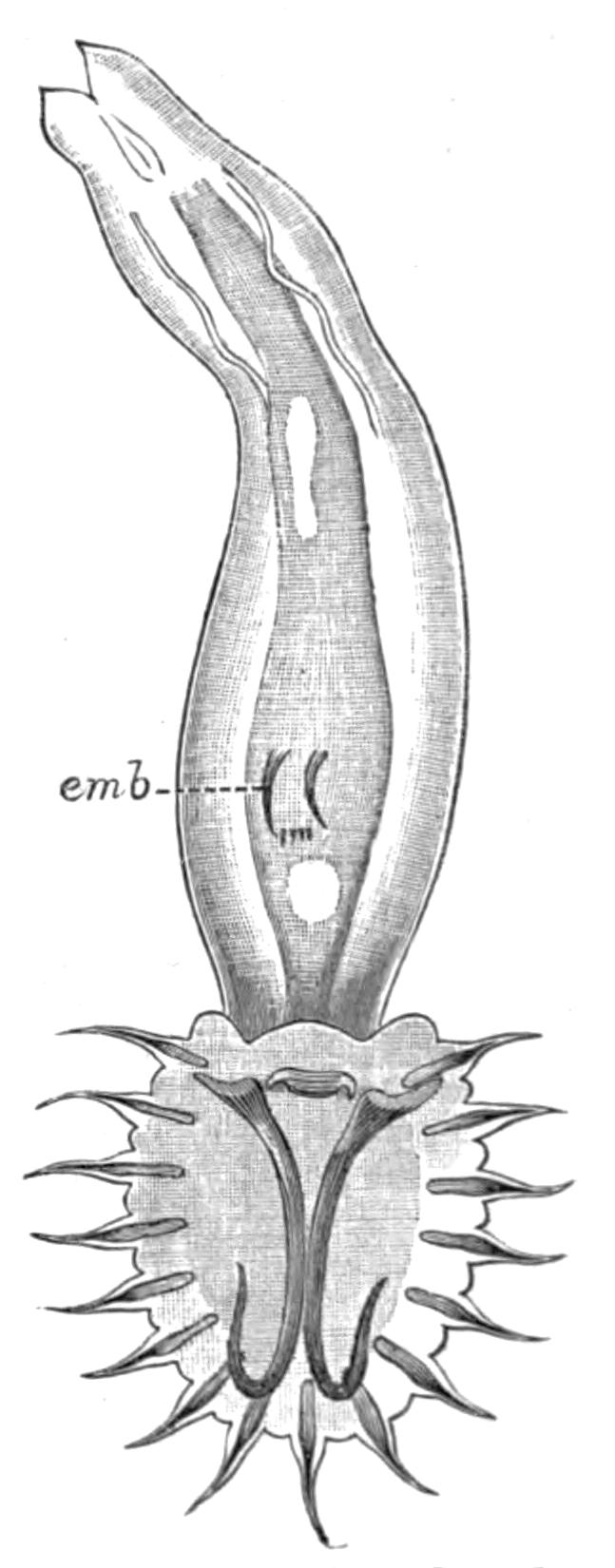
Fig. 29.—Gyrodactylus elegans v. Nord., from the fins of the Stickleback. (After v. Nordmann.) × 125. emb, Embryo.
Diplozoon paradoxum lays its eggs on the gills of the Minnow, which it frequently infests in great numbers. The ovum divides rapidly at the expense of the yolk-cells, and in a fortnight a larva (.2 mm. long) of the shape and complexity shown in Fig. 27, B, hatches out, which, however, succumbs if it does not meet with a Minnow in five or six hours. Should it survive, a dorsal papilla, a median ventral sucker, and a second pair of posterior suckers develop. Thus the Diporpa stage is attained. These Diporpa may acquire a third and even a fourth pair of suckers, and continue to live three months, but they only develop and mature their reproductive organs, if each conjugates with another Diporpa (Fig. 27, C, D), and this only occurs in a small percentage of instances. Each grasps the dorsal papilla of the other by its own ventral sucker, thus undergoing a certain amount of torsion. Where the two bodies touch, complete fusion occurs, and, as shown in Fig. 28, the united Diporpa (or Diplozoon, as the product is now called) decussate, each forming one limb of the X-shaped Diplozoon, within which the two sets of complex genitalia develop (Fig. 28).
IV. Gyrodactylidae.—Gyrodactylus (Fig. 29), the structure of which is in many ways peculiar, produces one large egg at a time. An embryo, in which the large and smaller hooks of the adhesive disc can be seen (emb), develops from this egg while still within the body of the parent, and may give rise to yet another generation within itself. The details of the process have not, however, been well ascertained.
Trematoda digenea (endoparasitica).
Occurrence and Habits of Digenea.—Endoparasitic Trematodes have been found in almost all the organs of Vertebrate hosts excepting in the nervous, skeletal, and reproductive systems. The alimentary canal, however, is the most usual habitat. From the buccal cavity to the large intestine, or even to the cloaca, its different regions are the resorts of various Trematodes. No Digenea have been found in the mouth, pharynx, or oesophagus of Mammals; but in Birds, Reptiles, Amphibia, and especially in Fishes, these parts are largely affected. It is a striking fact that Trematodes should occur in the stomach of (chiefly) large predaceous fishes, such as the Pike, Sharks, the Angler-fish, and others, considering the powerful digestive action of the gastric juice of these carnivores. The peculiar nature of the defence which must be employed by the parasites against this digestive action, becomes still more marked when it is considered that if a Trematode normally living in the stomach of one host be transferred to that of another, it is usually speedily digested, as is shown (p. 65) in the case of Distomum macrostomum. From these considerations the suggestion has been made that the cutaneous secretions of these Trematodes must act, not only as a protection against digestive or other ferments, but that the action in each case must be a specific one (Frenzel, Braun).
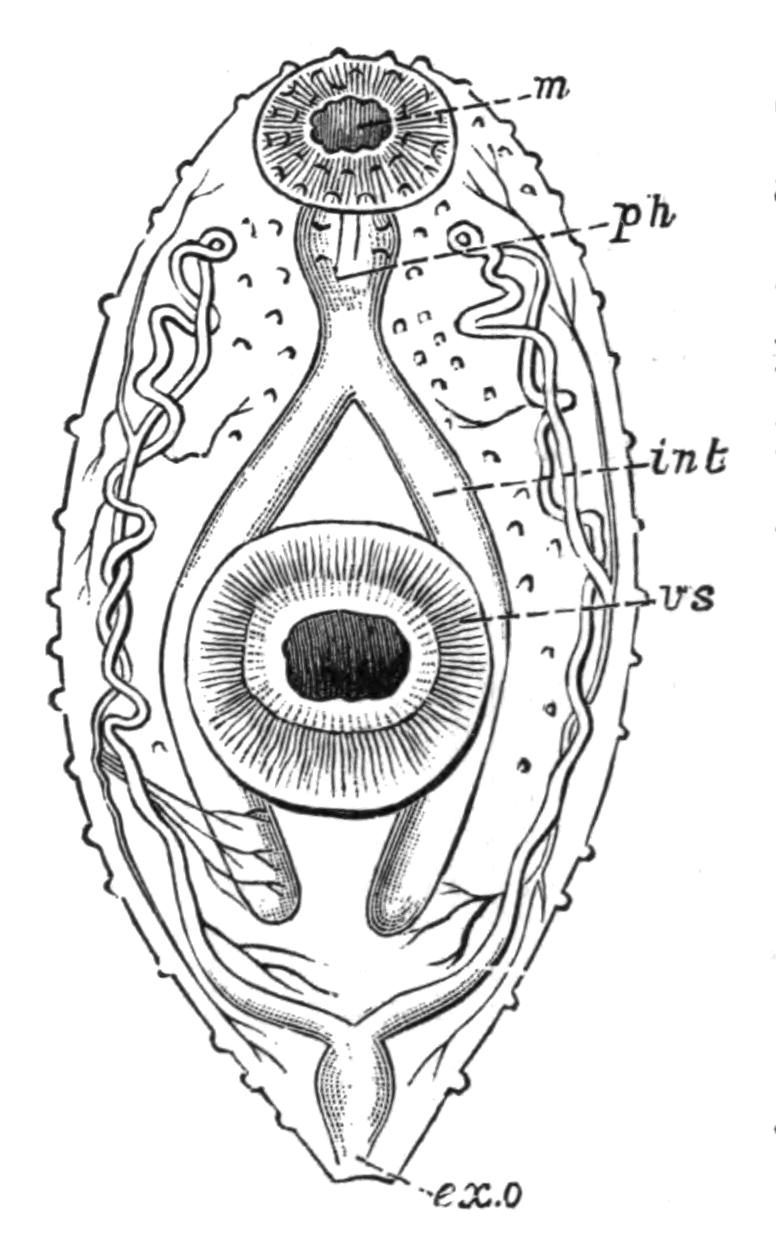
Fig. 30.—Distomum luteum v. Baer (immature), to show the arrangement of the excretory vessels. × 50. ex.o, Excretory aperture by which the terminal contractile duct opens—the finer vessels end in flame-cells; int, intestine; m, mouth-sucker; ph, pharynx; vs, ventral sucker. (After la Valette.)
It is, however, in the small intestine that most Trematodes occur, as the examination of the common Frog[75] will readily demonstrate. Both this and the edible Frog are attacked by a dozen Distomatidae, only a few of which, however, are common {63}to both hosts, and a number of Holostomatidae also pass a stage of their development within these Amphibia. Some idea of the extent to which animals, whose habits lead to infection, may be attacked by Trematodes (to say nothing of Cestodes and Nematodes, which often occur also) may be gathered from the fact that in dissecting a black stork, Nathusius found several hundred Holostomum excavatum and about a hundred Distomum ferox in the small intestine, twenty-two D. hians in the oesophagus, five others in the stomach, and one D. echinatum in the intestine. Snipe, Woodcock, Sandpipers, Dunlin, Gulls, Bittern, Geese, and Wild Ducks are, to mention a few cases, greatly infested by members of this group.
The following Trematodes have occurred in man[76]:—
Distomum hepaticum Abild.
Dist"mum lanceolatum Mehlis.
Dist"mum conjunctum Cobbold.
Dist"mum spathulatum Leuckart (= D. sinense Cobb., D. japonicum R. Blanch.).
Dist"mum rathouisi Poir. (probably = D. crassum Busk, D. buskii Lank.).
Dist"mum heterophyes v. Sieb.
Dist"mum pulmonale Bälz (= D. ringeri Cobb., D. westermanni Kerb.).
Dist"mum oculi humani Ammon (= D. ophthalmobium Dies.).
Monostomum lentis v. Nord.
Amphistomum hominis Lewis and M‘Connell.
Bilharzia haematobia Cobb.
Life-histories of the Digenea.—The classification of Trematodes according to their life-histories, expressed in the divisions Monogenea and Digenea, though a very useful one, breaks down entirely in the case of certain forms. Thus the life-history of Gyrodactylus is probably digenetic rather than monogenetic. Aspidogaster conchicola,[77] which lives in the pericardial cavity of the fresh-water mussel (possibly the only case of a Trematode becoming normally mature in an Invertebrate host, since other species of Aspidogaster live in Chelonia), produces larvae which enter another Anodonta and develop directly into the sexual form. In other words, Aspidogaster, though structurally a digenetic form, possesses a life-history which is direct and simple, i.e. monogenetic.
The Holostomatidae, which live in birds of prey and aquatic birds, give rise to eggs from which a minute larva escapes. The fate of this aquatic larva is not directly known, {64}but in all probability after entering a host (Fish, Amphibian, Mollusc), it undergoes a gradual change into what has long been known as a Tetracotyle, from the frequent presence of four (sometimes only three) adhering organs. Fig. 31 exhibits a species which is abundant in the lens and vitreous humour of the eye of the Perch. Its further history is not known, but presumably the Perch is presently devoured by the final host in which the Diplostomum attains maturity. Thus the Holostomatidae are "metastatic" (Leuckart), their (probably) direct development requiring the presence of two hosts.[78]
The other Digenea, the life-histories of which are known, belong to the Distomatidae and Amphistomatidae, and we may distinguish the steps by which the complex life-history of the liver-fluke (Distomum hepaticum) has been brought about, by a consideration of that of Distomum macrostomum.
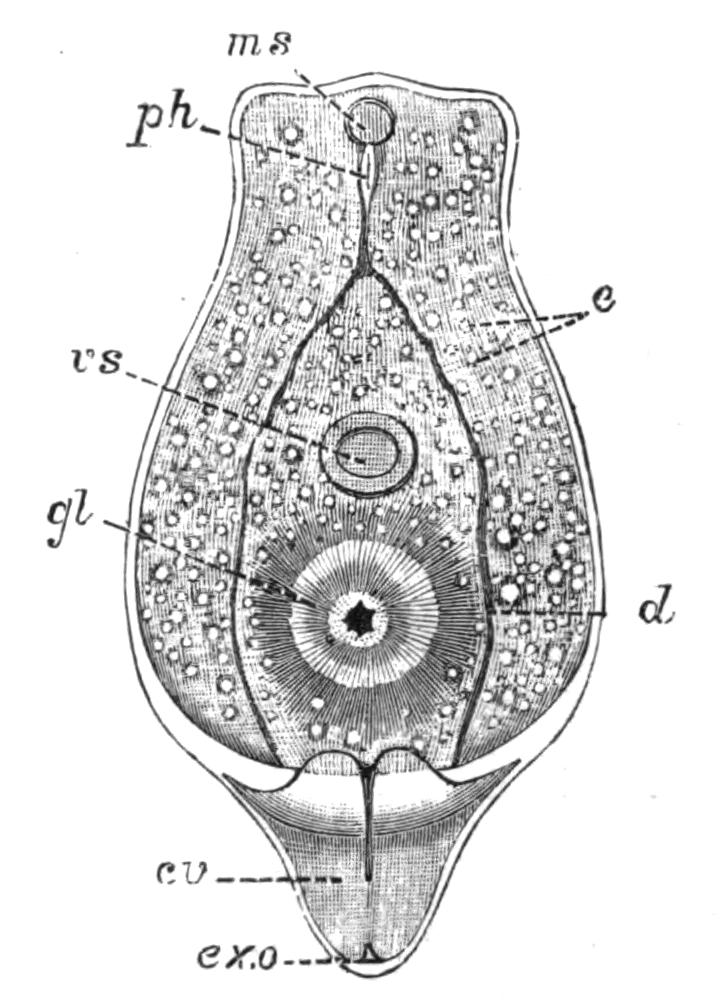
Fig. 31.—Diplostomum (Tetracotyle) volvens. (After v. Nordmann.) × 130. cv, Contractile excretory vesicle; d, intestine; e, calcareous bodies in excretory tubules; ex.o, excretory aperture; gl, glandular adhesive body; ms, oral sucker; ph, pharynx; vs, ventral sucker.
Distomum macrostomum.—This form occurs in the intestine of several common Passerine birds. It is remarkable not only for the large oral sucker, but also on account of the position of the common genital pore at the hinder, and not as usual, at the anterior, end of the body (Fig. 32, A). The eggs pass out through this pore, and are discharged with the bird's excrement. Should a certain snail (Succinea putris) happen to rasp off the epidermis of a leaf upon which the faeces have fallen, the eggs are swallowed and a minute active larva is set free (Fig. 32, B). This penetrates through the thin wall of the digestive tract of the snail, and passing into the connective tissue, throws off its cilia and assumes the shape of Fig. 32, C. This sporocyst, as the larva is now termed, grows rapidly in all directions (Fig. 32, D) at the expense of the snail's tissues, until it becomes impossible to separate parasite and host completely.
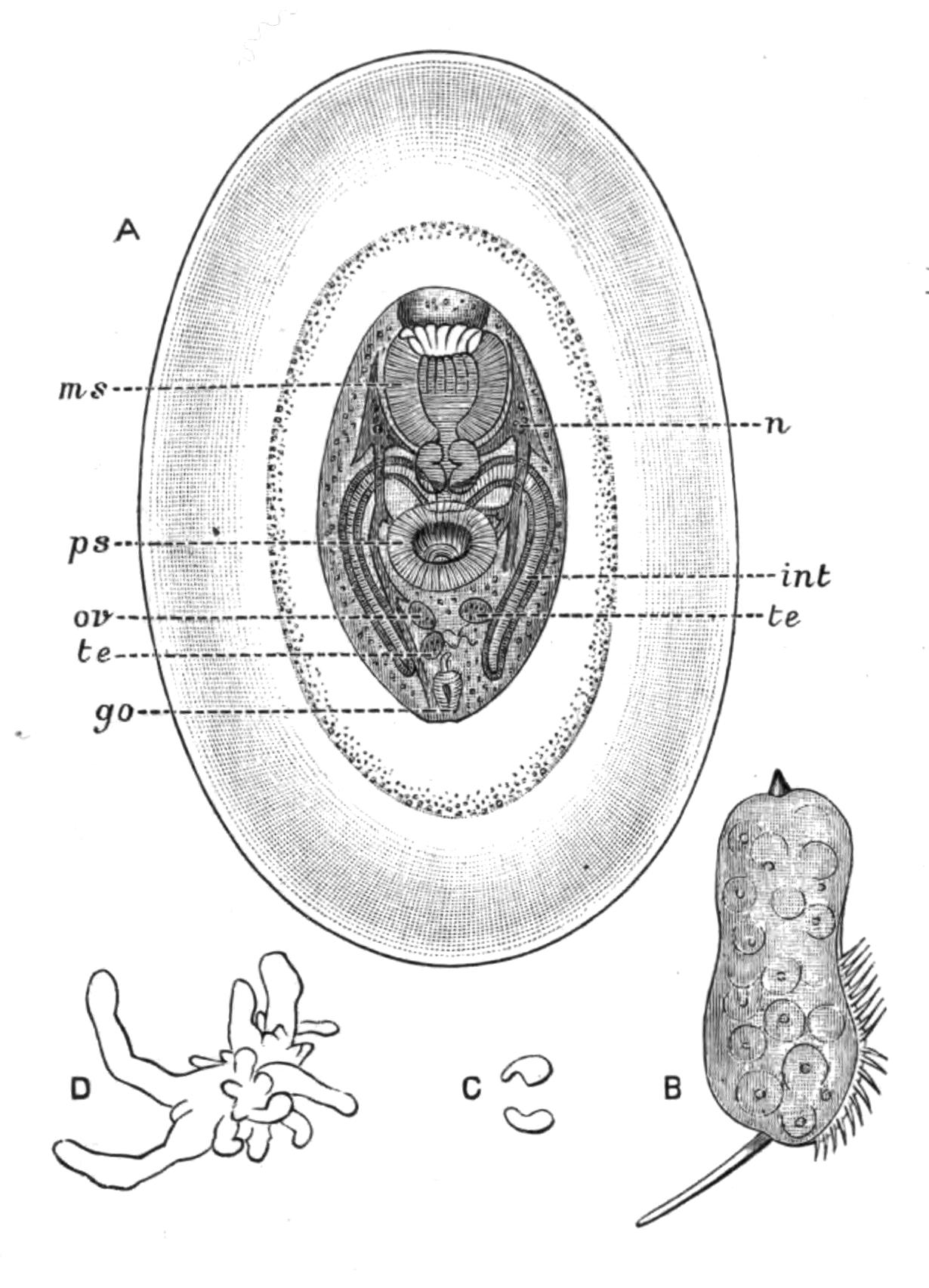
Fig. 32.—Life-history of Distomum macrostomum Rud. A, Immature Distomum (really a tailless Cercaria) found in the swollen terminal parts of Leucochloridium (Fig. 33, B) and enclosed in two protective membranes, × 40; B, larva which hatches out of the egg of D. macrostomum, × 125; C, the metamorphosed larva (sporocyst) fourteen days after having entered Succinea putris, and pierced through its intestinal wall; D, actively growing sporocyst. (After Heckert.) go, Genital aperture; int, intestine; ms, mouth sucker; n, nervous system; ov, ovary; ps, ventral sucker; te, testis.
Those branches which lie superficially in the cephalic region of the snail become greatly swollen, cylindrical, and contractile. They are banded with green and white, ornamented with red terminal spots, and pulsate rapidly. Hence these fertile branches of the sporocyst (which in this condition was known as Leucochloridium paradoxum, Fig. 33, B) naturally attract the attention of insectivorous birds, which peck off the tentacles of the snail, and with it the swollen sporocyst-branch. A sphincter muscle closes the cut end of the fertile sac when the bird's bill nips it off. The sac contains large numbers of young D. macrostomum (Fig. 32, A), produced by the division of embryonic cells of the larva (Fig. 32, B), which are apparently blastomeres of the egg reserved for this future use. It is a remarkable circumstance that the old bird itself is immune from infection, and if it swallows these young Distomes, they are digested. Should, however, the snail's tentacle and its contents be offered as food to the nestlings, their weaker digestive powers merely set the Distomes free from the protective membranes (Fig. 32, A), and thus the Blackcaps, Sparrows, and other birds infested by D. macrostomum have acquired the parasite when they were {66}nestlings by the unintentional agency of their parents.[79] The snail regenerates its lost tentacles only for the sporocyst to again bud off fertile branches into them.
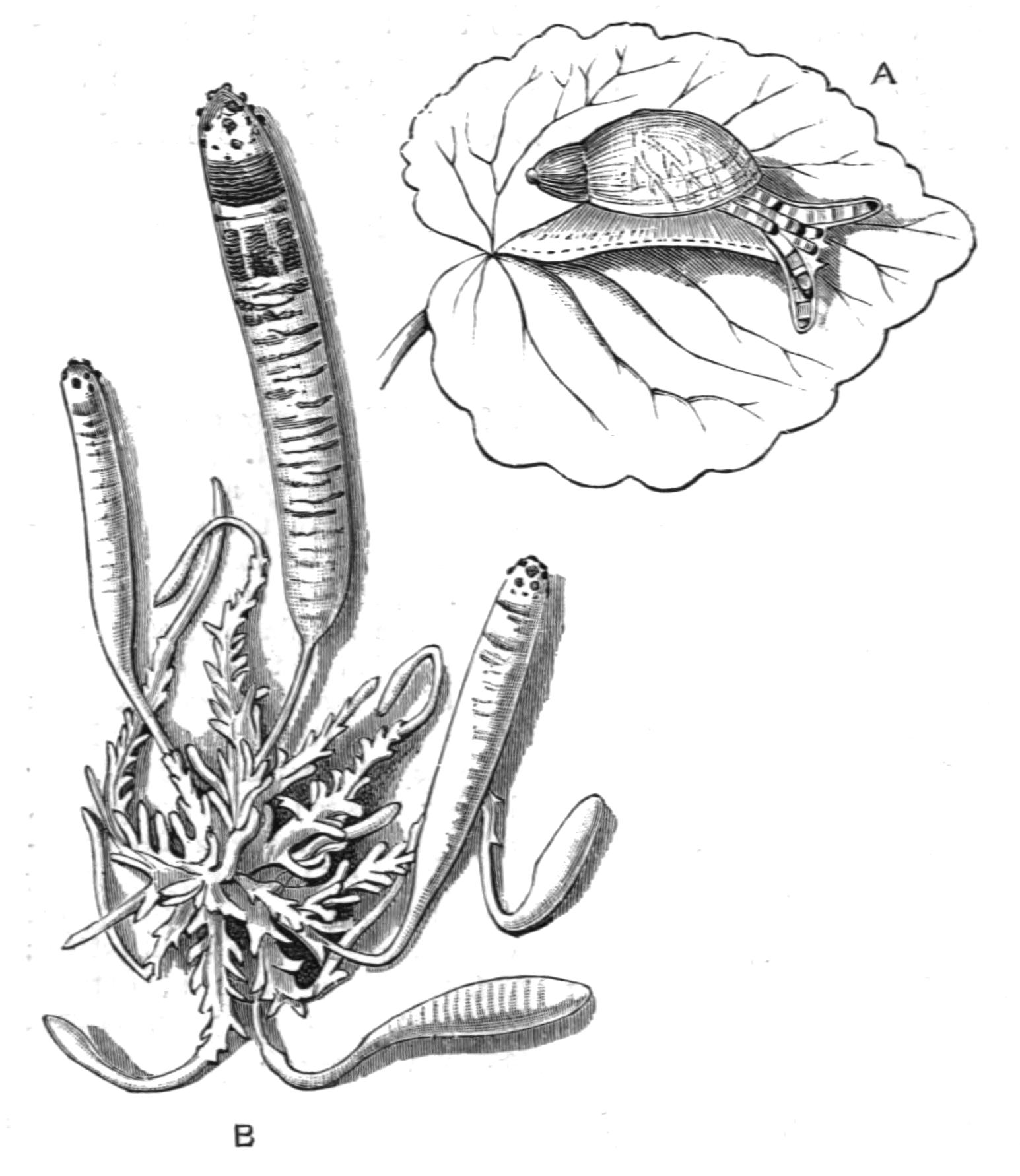
Fig. 33.—A, Succinea putris, infested by B, Leucochloridium paradoxum, or the fully-formed sporocyst of Distomum macrostomum. (After Heckert.) A, Natural size; B, × 7.
The egg of this Distome thus gives rise to a larva which enters the tissues of one particular Mollusc. Here it becomes a branched sporocyst within which the sexual worms are formed, apparently each from a single embryonic blastomere ("Keimzelle"), by a process comparable with the development of a parthenogenetic ovum, and the whole cycle has been termed Alloiogenesis, i.e. alternation of sexual and parthenogenetic generations (Grobben).[80] Leuckart[81] and Looss,[82] however, consider that what was once a metamorphosis of an individual (as in the {67}Holostomatidae) has now become, by maturation of the Cercaria in the comparatively modern warm-blooded bird, a metamorphosis extending over two or more generations.
Distomum (Fasciola) hepaticum.—The liver-fluke of the Sheep, which produces the disastrous disease, liver-rot, has a distribution as wide as that of a small water-snail, Limnaea truncatula, the connexion between the two being, as Thomas[83] and Leuckart discovered, that this snail is the intermediate host in which the earlier larval, sporocyst, and redia stages are passed through, and a vast number of immature flukes (Cercariae) are developed. These leave the snail and encyst upon grass, where they are eaten by the sheep. Over the whole of Europe, Northern Asia, Abyssinia, and North Africa, the Canaries, and the Faroes, the fluke and the snail are known to occur, and recently the former has been found in Australia and the Sandwich Islands, where a snail, apparently a variety of Limnaea truncatula, is also found.[84] Over these vast areas, however, the disease usually only occurs in certain marshy districts and at certain times of the year. Meadows of a clayey soil, liable to be flooded (as in certain parts of Oxfordshire), are the places where this Limnaea occurs most abundantly, and these are consequently the most dangerous feeding-grounds for sheep. The wet years 1816, 1817, 1830, 1853, and 1854—memorable for the occurrence of acute liver-rot in England, Germany, and France—showed that the weather also plays a considerable part in extending the suitable ground for Limnaea over wide areas, which in dry years may be safe pastures. In 1830 England lost from this cause,[85] one and a half million sheep, representing some four millions of money, while in 1879-80 three millions died. In 1862 Ireland lost 60 per cent of the flocks, and in 1882 vast numbers of sheep perished in Buenos Ayres from this cause. In the United Kingdom the annual loss was formerly estimated at a million animals, but is now probably considerably less. After infection during a wet autumn, it is usually in the succeeding winter that the disease reaches its height.
The symptoms of "rot" appear about a month after infection, more acutely in lambs than in sheep, and again, less in oxen than in sheep. At first, death may result from cerebral apoplexy, but if the first few weeks are passed through, a pernicious anaemia sets in, the sheep are less lively and fall at a slight touch, the appetite diminishes, and rumination becomes irregular. The conjunctiva is of a whitish-yellow colour, the dry, brittle wool falls off, and there is sometimes fever and quickened respiration. In January, about three months after infection, the wasting, or fatal, period sets in. Oedemas or swellings, usually visible before, become larger at the dependent parts of the body, a large one in the submaxillary region being especially well marked, and this is considered one of the most characteristic symptoms ("watery poke"). Through this period few of the infected sheep survive, but should they do so, the flukes begin to migrate, though some remain much longer within the liver. Migration is effected through the bile-duct into the duodenum and outwith the faeces, in which the altered remains of the Distomum are sometimes scarcely recognisable. Under these circumstances (or owing to death of the fluke in situ) the sheep recover more or less fully.
The preventive measures seem to be: (1) Destruction of the eggs and of the manure of rotten sheep; (2) slaughter of badly fluked sheep; (3) adequate drainage of pastures; (4) an allowance of salt and a little dry food to the sheep; and (5) dressings of lime or salt on the ground to destroy the embryos.[86]
Distomum hepaticum, contrary to most Trematodes, enjoys a wide range of hosts. Man himself occasionally falls a victim; thus in Dalmatia, in the Narenta Valley, the disease is endemic but slight in its effects. The horse, deer, camel, antelopes, goat, pig, rabbit, kangaroo, beaver, and squirrel have all been known to harbour this fluke occasionally. In the Italian deer-parks at Mandria a large species, D. magnum, decimated the herds some years ago; and this species, probably imported from Italy, is now almost as dangerous a parasite on the western plains of the United States as D. hepaticum.
Bilharzia haematobia.[87]—This formidable parasite was discovered by Bilharz in 1853 in the veins of the bladder of patients {69}at the Cairo Hospital, and is remarkable from its abundance on the east coast and inland countries of Africa from Egypt to the Cape, as well as in the districts bordering Lake Nyassa and the Zambesi river, while westwards it occurs on the Gold Coast. Mecca is a source of infection whence Mohammedans carry the disease to distant places. In Egypt about 30 per cent of the native population is affected by the serious disease known as Haematuria, resulting from the attacks of Bilharzia, so that, of the many scourges from which in Africa man suffers, this one is perhaps the most severe.
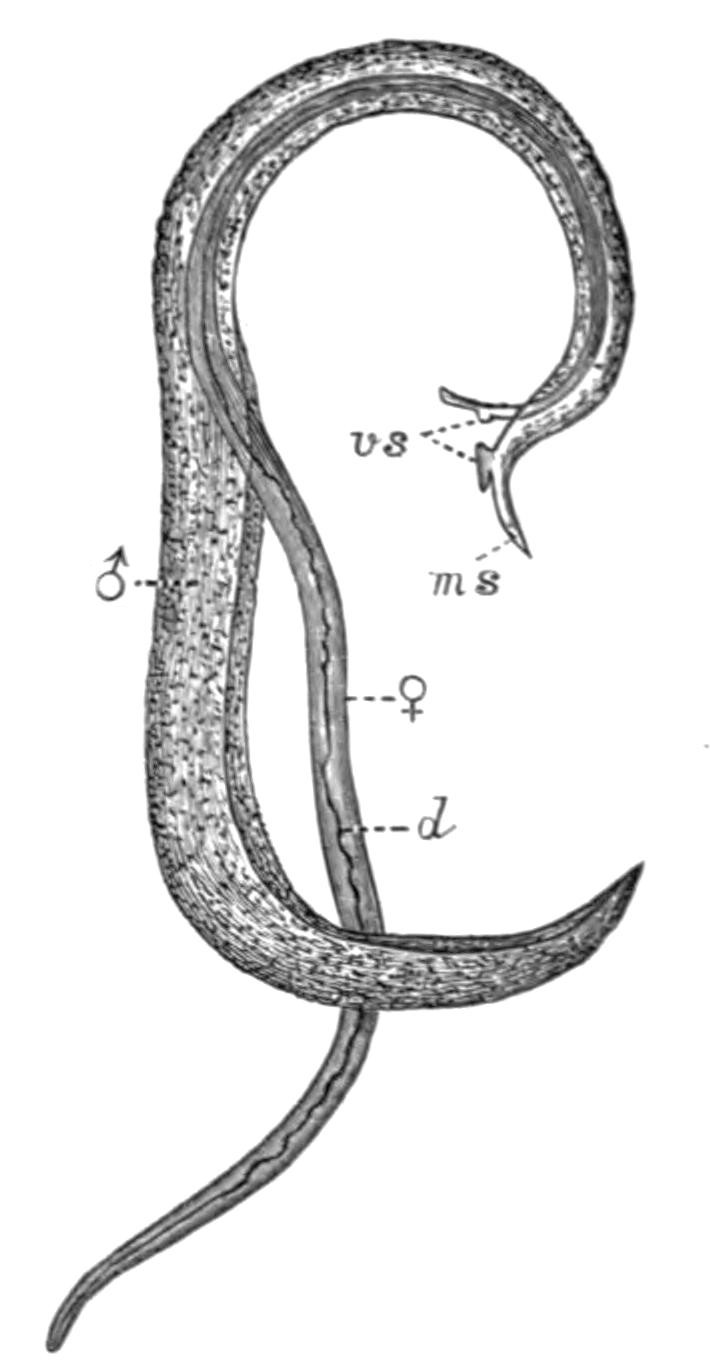
Fig. 34.—Bilharzia haematobia Cobb. × 10. The female (♀) lying in the gynaecophoric canal of the male (♂). d, Alimentary canal; ms, oral sucker of male; vs, ventral suckers. (After Leuckart.)
The worm is found usually in couples, which have been proved to be male and female individuals (Fig. 34), often in considerable numbers in the veins of the pelvic region, chiefly the veins of the bladder and of the large intestine, and it is tolerably certain that Bilharzia enter these vessels from the portal vein. Their long slender bodies enable them to penetrate into the finer vessels, which get partially or entirely choked up, and the circulation accordingly impeded. But the most serious consequences are observed in the urinary bladder. The mucous membrane is swollen and inflamed here and there, chiefly on the dorsal surface, the capillaries appear varicose and covered with mucus, mixed with blood-extravasations in which Bilharzia-eggs are noticeable. The eggs also cause numerous swollen knots in the submucous tissue. Should the disease not pass beyond this stage (and such is usually the case, especially in South Africa), a temporary haematuria ensues. The urine, which is only expelled with great effort, accompanied by intense pain, is mixed with blood, mucous clots, and masses of Bilharzia-eggs, from which some of the embryos have already hatched out. The symptoms, however, may gradually pass away, and a more or less complete recovery accomplished. The disease may indeed be of a far less severe character, and may not interfere with the usual occupations of the patient; but, on {70}the other hand, a far more extensive thickening of the wall of the bladder sometimes occurs; hard masses of eggs, uric acid crystals, and other deposits, may lead to the formation of stones, degeneration of the substance of the ureter, and eventually to that of the kidney itself. The stone, indeed, has long been known to be a prevalent disease in Egypt, and it is now known to arise from concretions formed round masses of Bilharzia eggs. From the portal vein, again, other Bilharzia may gain access to the rectum, or the liver, and it has also been found in the lungs, and may give rise to most serious complications, if indeed the patient lives.
How infection occurs is a question to which at present no satisfactory answer can be made. The attempt to introduce embryos of Bilharzia into the common fresh-water animals of Alexandria has hitherto proved fruitless (Looss[88]), although there seems little doubt that the comparative immunity of Europeans from the disease is in some way owing to their drinking purer water than the natives. Possibly, as Leuckart suggests, the embryo becomes a sporocyst in man himself, somewhat as Taenia murina is known to develop in the rat without an intermediate host.[89] The immense numbers of the parasite in one host would then readily receive an explanation.
A Bilharzia, possibly B. haematobia, was found by Cobbold in the portal vein of Cercopithecus fuliginosus; and B. crassa infests the cattle of Egypt, Sicily, and certain parts of India, but does not produce haematuria.
Of the other Trematodes of man and domestic animals there is not room to speak fully. Distomum pulmonale, which occurs in the lungs of the cat, tiger, and dog, as well as in man, is especially common in Japan, China, Corea, and Formosa. D. sinense and D. rathouisi have been also found in inhabitants of these countries.
Bisexual Trematodes.—Zoologically, Bilharzia is interesting from its bisexual condition. It is not, however, the only bisexual Trematode. In cysts in the branchial chamber of Ray's bream, Brama raii, two worms are found, which are probably the slender male and the swollen female of the same species (Distomum okenii). The only doubt that can arise proceeds from the tendency in all Trematodes for the male organs to ripen before {71}the female organs. Until we certainly know that the swollen egg-bearing form (♀) does not arise from a previously male form (♂), the case is open to suspicion. Since, however, Kölliker[90] never found intermediate hermaphrodite conditions, this Distomum may be almost certainly regarded as of distinct sexes. Didymozoon thynni (Monostomum bipartitum), from cysts on the gills of the Tunny (Thynnus), is another case. Two slender worms flattened posteriorly, come together, and the body of one becomes folded to receive that of the other. They fuse completely except for a small lateral opening through which the anterior parts of both worms may freely protrude. The enclosing individual contains a coiled uterus filled with eggs, and is the female, whereas the smaller individual never possesses eggs, and is probably the male.[91] Nematobothrium (Fig. 22, A), which occurs also in the Tunny, in the form of two immensely long individuals intricately wound about each other in a cyst, is, however, not bisexual.
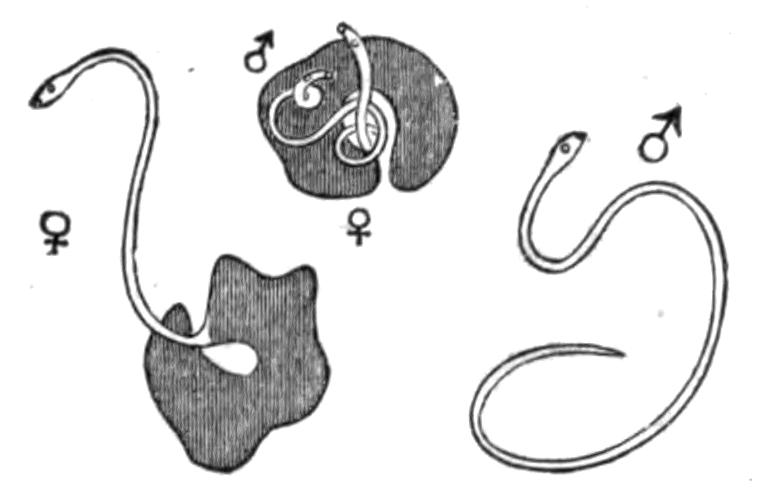
Fig. 35.—Distomum okenii Köll. Showing male and female as they occur together in the branchial cavity of Bramaraii (Ray's bream). (From Bronn, after Kölliker.) Nat. size.
Table of Digenetic Trematodes and their Life-Histories.[92]
| Species. | Final host. | Host into which the larva enters, and in which Cercariae are eventually formed. | Host into which the Cercariae migrate and encyst; eaten by final host. |
| Diplodiscus (Amphistomum) subclavatus Göze | Rana, Bufo, Triton | Smaller species of Planorbis and Cyclas | Insect-larvae, Rana, Bufo, but frequently omitted |
| Distomum advena Duj. (D. migrans Duj.) | Sorex araneus | Not known | Limax |
| D. appendiculatum Rud. | Clupea alosa | Not known | Lucullus acuspes, Centropages hamatus (Copepoda) |
| D. ascidia v. Ben. | Species of Bats |
Limnaea stagnalis Planorbis corneus |
Ephemera, Perla, Chironomus plumosus |
| D. atriventre Weinl. | Frogs and Toads of N. America | Physa heterostropha | Not known |
| D. brachysomum Crepl. | The Dunlin (Tringa alpina) | Not known | Anthura gracilis |
| D. caudatum v. Linst. | Hedgehog (Erinaceus europaeus) | Helix hortensis | |
| {72}
D. clavigerum Rud. |
Rana |
Limnaea ovata Planorbis corneus |
Not known |
| D. cygnoides Zed. | Rana | Pisidium, Cyclas | Limnaea sp. (Cercaria macrocerca Fil.) |
| D. cylindraceum Zed. | Rana | Limnaea ovata | Ilybius fuliginosus |
| D. dimorphum Dies. | Ardea, Ciconia (Brazil) | Not known | Different species of Fishes |
| D. echinatum Zed. | Cygnus, Anser, Anas | Species of Limnaea | Species of Limnaea, Paludina vivipara |
| D. endolobum Duj. | Rana | Limnaea stagnalis | L. stagnalis, Gammarus pulex, larvae of Limnophilus rhombicus |
| D. globiporum Rud. | Perca fluviatilis | Not known | Limnaea stagnalis, L. ovata, Succinea pfeifferi, S. putris, Physa fontinalis, Planorbis marginatus |
| D. hepaticum Abild. | Sheep, Oxen, Man, etc. | Limnaea truncatula | Omitted |
| D. hystrix Duj. | Lophius piscatorius | Not known | Marine Fishes |
| D. macrostomum Rud. | Warblers, Tits, Woodpeckers, etc. | Succinea putris | Omitted |
| D. militare v. Ben. | Common Snipe | Paludina vivipara | P. vivipara |
| D. nodulosum Zed. | Perca fluviatilis | Bithynia tentaculata | Cyprinus, Acerina cernua |
| D. ovocaudatum Vulp. | Rana esculenta | Species of Planorbis | Probably omitted. (Cercaria known as C. cystophora Wag.) |
| D. retusum Duj. | Rana | Limnaea stagnalis | L. stagnalis, larvae of Phryganeidae |
| D. squamula Dies. | Polecat | Unknown | Rana temporaria |
| D. signatum Duj. | Tropidonotus natrix | Unknown | Rana |
| D. trigonocephalum Rud. | Badger, Polecat | Paludina vivipara | Unknown |
| Gasterostomum sp. | Dogfish, Rays | Ostrea edulis, Cardium rusticum, C. edule | Belone vulgaris |
| G. fimbriatum v. Sieb. | Perca, Esox | Unio, Anodonta (Cercaria known as Bucephalus polymorphus) | Leuciscus erythrophthalmus |
| G. gracilescens Rud. | Lophius piscatorius | Unknown | Species of Gadus (e.g. G. aeglefinus), Molva, Lophius |
| Monostomum flavum Mehl. | Anas | Planorbis corneus | Omitted |
Classification of Trematodes.—We have seen (p. 63) that it is hardly possible to carry out fully the division of Trematodes into Monogenea and Digenea. Nevertheless, pending further investigation on the doubtful points, this classification may still be {73}used. Monticelli[93] has proposed the main divisions of a new classification, which has been also adopted by Braun, and is based on the nature of the suckers. These divisions are indicated below in brackets.
| A. Monogenea v. Ben. (Heterocotylea Mont.). | ||
| 1. Fam. | Temnocephalidae Hasw. | |
| Gen. | Temnocephala Hasw. | |
| 2. Fam. | Tristomatidae Tschbg. | |
| Sub-Fam. 1. | Tristomatinae Mont. | |
| Gen. | Tristomum, Nitzschia, Epibdella, Trochopus, Acanthocotyle, Phyllonella, Placunella, Encotylabe. | |
| Sub-Fam. 2. | Monocotylinae Tschbg. | |
| Gen. | Pseudocotyle, Calicotyle, Monocotyle. | |
| Sub-Fam. 3. | Udonellinae v. Ben.-Hesse. | |
| Gen. | Udonella, Echinella, Pteronella. | |
| 3. Fam. | Polystomatidae Tschbg. | |
| Sub-Fam. 4. | Octocotylinae v. Ben.-Hesse. | |
| Gen. | Octobothrium, Pleurocotyle, Diplozoon, Anthocotyle, Vallisnia, Phyllocotyle, Hexacotyle, Platycotyle, Plectanocotyle, Diclidophora. | |
| Sub-Fam. 5. | Polystomatinae v. Ben. | |
| Gen. | Polystomum, Onchocotyle, Erpocotyle, Diplobothrium, Sphyranura. | |
| Sub-Fam. 6. | Microcotylinae Tschbg. | |
| Gen. | Microcotyle, Gastrocotyle, Axine, Pseudaxine. | |
| 4. Fam. | Gyrodactylidae v. Ben. | |
| Sub-Fam. 7. | Gyrodactylinae Par. et Per. | |
| Gen. | Gyrodactylus, Dactylogyrus, Tetraonchus, Diplectanum. | |
| Sub-Fam. 8. | Calceostominae Par. et Per. | |
| Gen. | Calceostomum, Anoplodiscus. | |
| 5. Fam. | Aspidobothridae Burm. (= Aspidocotylea Mont.). | |
| Gen. | Aspidogaster, Platyaspis, Cotylogaster, Macraspis. | |
| B. Digenea v. Ben. (Malacocotylea Mont.). | ||
| 6. Fam. | Holostomatidae Brandes (= Metastatica Leuckart). | |
| Gen. | Diplostomum, Polycotyle, Hemistomum, Holostomum. | |
| 7. Fam. | Amphistomatidae Mont. | |
| Gen. | Amphistomum, Diplodiscus, Gastrodiscus, Homalogaster, Gastrothylax, Aspidocotyle. | |
| 8. Fam. | Distomatidae Mont. | |
| Gen. | Distomum (and sub-genera), Rhopalophorus, Koellikeria, Bilharzia. | |
| 9. Fam. | Gasterostomatidae Braun. | |
| Gen. | Gasterostomum. | |
| 10. Fam. | Didymozoontidae Mont. | |
| Gen. | Didymozoon, Nematobothrium. | |
| 11. Fam. | Monostomatidae Mont. | |
| Gen. | Monostomum, Notocotyle, Ogmogaster, Opisthotrema. | |
CESTODA
INTRODUCTION—NATURE OF CESTODES—OCCURRENCE OF CESTODES—THE TAPE-WORMS OF MAN AND DOMESTIC ANIMALS—TABLE OF THE LIFE-HISTORIES OF THE PRINCIPAL CESTODES OF MAN AND DOMESTIC ANIMALS—STRUCTURE AND DEVELOPMENT OF CESTODES—TABLE FOR THE DISCRIMINATION OF THE MORE USUAL CESTODES OF MAN AND DOMESTIC ANIMALS—CLASSIFICATION.
The Cestodes or Tape-worms are exclusively endoparasitic Platyhelminthes living, in the adult condition, in the alimentary canal of Vertebrates, with the exception of Archigetes (Fig. 37), which may become mature in the body-cavity of Tubifex. In relation with this wholly parasitic existence, the Cestodes exhibit certain characteristic modifications in structure and mode of development, such as the formation, by the segmentation of the "neck," of a (usually) long chain of "proglottides" or joints, which form the "body" of the Cestode; and the entire absence of an alimentary tract, both in the larva and adult. As an adaptation to the fixed mode of life, the anterior end (head, scolex) is modified to form an adhering organ. Various adaptive forms of larvae are known. These live in the internal organs of one or more intermediate hosts, and are transferred to the final host passively during a meal. Lastly, there is the curious metamorphosis by which the adult is formed from a portion (scolex) of the larva.[94]
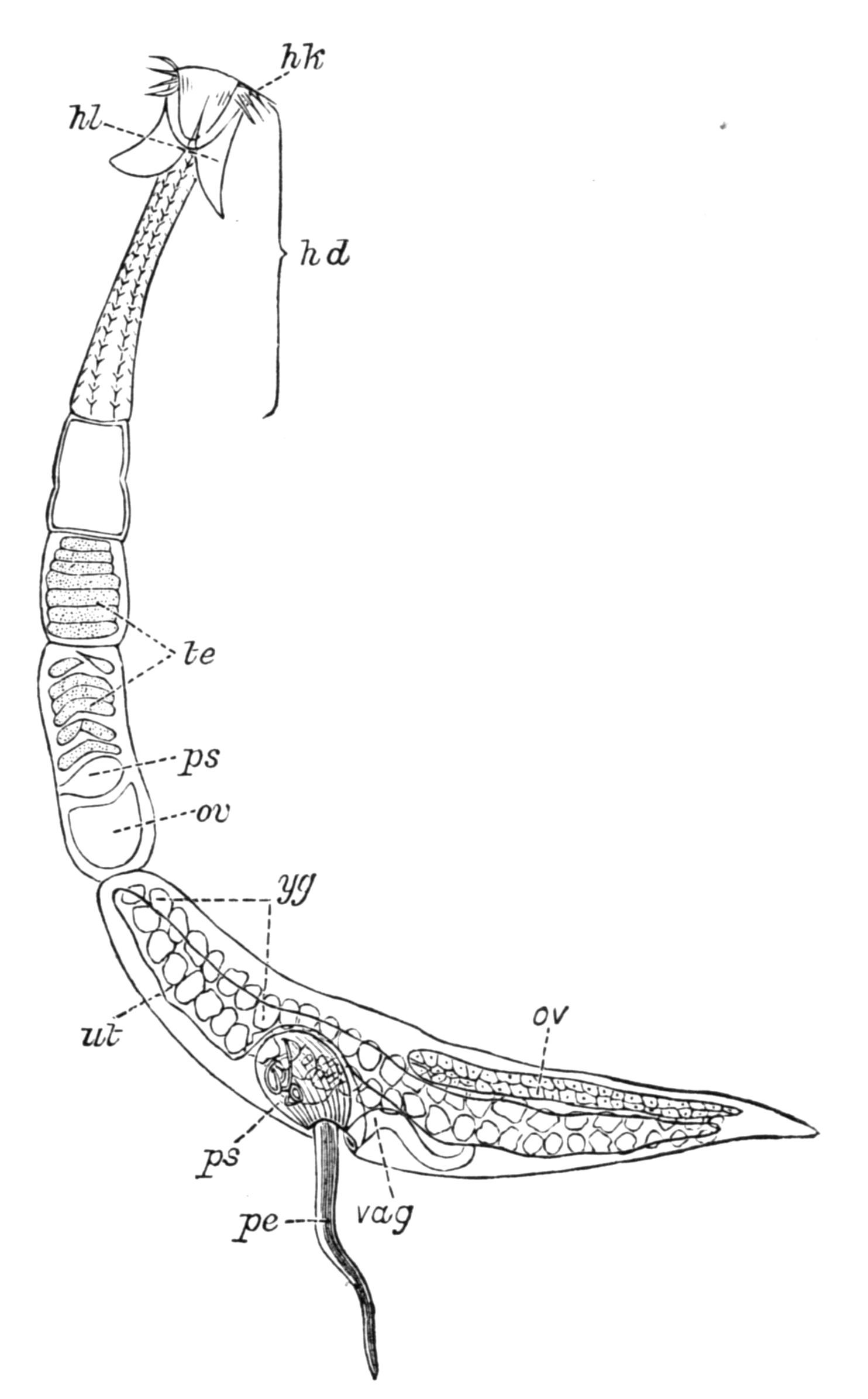
Fig. 36.—Echinobothrium affine Dies., from the intestine of Torpedo, × 43. hd, Head; hk, hooks; hl, lobes of the head; ov, ovary; pe, penis; ps, penis-sheath; te, testes; ut, uterus; vag, vagina; yg, yolk-glands. (After Pintner.[95])
Taenia solium, from man (Fig. 39, B), or Echinobothrium (Fig. 36), from an Elasmobranch fish, is fixed to the mucous lining of the intestine of its host by means of a radially-constructed apparatus of four suckers and a circlet of hooks (Fig. 39), which are borne by the "head" or "scolex," being that part of the worm which is directly derived from part of the larva, and which contains the central, commissural portion of the nervous system. Firm adhesion to the host's intestine is necessary, in order to avoid the loosening action of the peristaltic movements of the intestine as the food passes along. The heads of different Cestodes exhibit a marvellous variety of suckers and hooks, from a mere muscular depression in Schistocephalus, to the compound proboscides of Tetrarhynchus[96] which is found in Elasmobranchs. The jointed body, often of enormous length (up to 20 yards in Bothriocephalus latus), is usually separated from the head by a slender neck, from which the proglottides are segmented off from behind forwards, and become more and more individualised as they recede farther away from the neck by the intercalation of younger joints. Thus in Fig. 36 the mature, distal proglottis has passed through all the stages represented by the other segments.
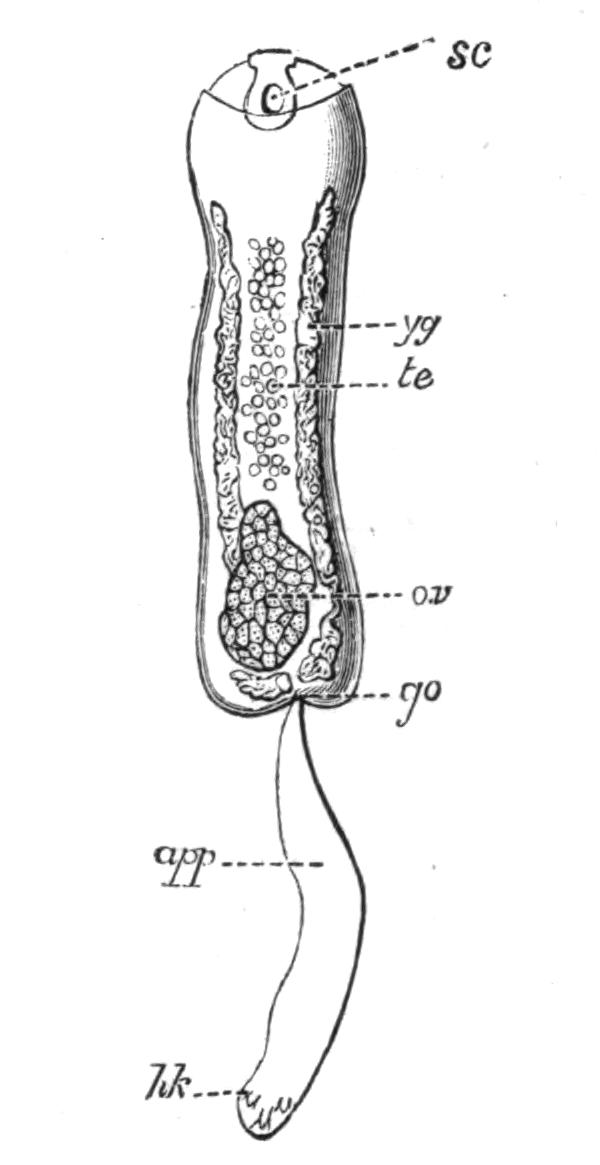
Fig. 37.—Archigetes sieboldii (appendiculatus), from the coelom of Tubifex rivulorum. × 40. app, Persistent larval appendage; go, genital pore; hk, persistent larval hooks; ov, ovary; sc, sucker; te, testes; yg, yolk-glands. (After Leuckart.)
The longitudinal muscles, the nerves, and excretory vessels which supply the proglottides are continuous throughout and with those of the head. Each joint contains at first male genitalia comparable with those of a Trematode; then the female organs develop, and finally self-fertilisation follows. The Cestodes feed through their skin, probably by the aid of fine protoplasmic processes, which penetrate the tough investing membrane and absorb the already digested food which bathes them. When a proglottis of Calliobothrium is approaching maturity it separates from the parent, the broken ends of muscles, nerves, and excretory vessels speedily heal, and it is now capable of continued growth and of fairly active movement if it remains in the intestine of the host. According to van Beneden, it may even attain a size equal to, or exceeding, that of the whole parent or "strobila."[97] These considerations led Leuckart, von Siebold, P. J. van Beneden, and others, to Steenstrup's conclusion that a jointed tape-worm is really a colony composed of two generations—the head and neck derived from the larva, and the proglottides produced by the segmentation of the neck.[98] This view of the colonial nature of jointed Cestodes was generally adopted from 1851 to 1880. During the last fifteen years, however, the varied interpretations of the facts of the ontogeny of this group have led some authors to adopt the monozootic view (that a Cestode is one individual), others are still of the older opinion, and Hatschek (Lehrbuch, p. 349) and Lang take up intermediate positions. Lang considers that the formation of the joints of a tape-worm from a small fixed "scolex," is not only largely comparable with the strobilation of a scyphistoma and the consequent formation of a pile of medusae, as in the life-history of Aurelia, but {77}that both processes have arisen from the power of regenerating the necessary organs in each of the new segments. The result in both cases is the rapid formation of a number of joints, which gradually separate from the parent, to carry the eggs and young to new stations. Just as some Coelenterata (Lucernaria) may be regarded as not having advanced much beyond a scyphistoma stage, so there are unisegmental Cestodes (e.g. Archigetes, Fig. 37) which have remained as a slightly altered but sexual scolex, directly comparable with a Trematode, and, as all authors are agreed, representing one generation only. Such monozootic forms are now classed as a special family, the Cestodaria or Monozoa, of which Caryophylleus mutabilis, from the intestine of various Cyprinoid fish, is the most abundant representative, while Amphiptyches (Gyrocotyle) urna, from Chimaera monstrosa of the northern hemisphere, is paralleled by A. rugosa, found in Callorhynchus antarcticus of the southern seas.
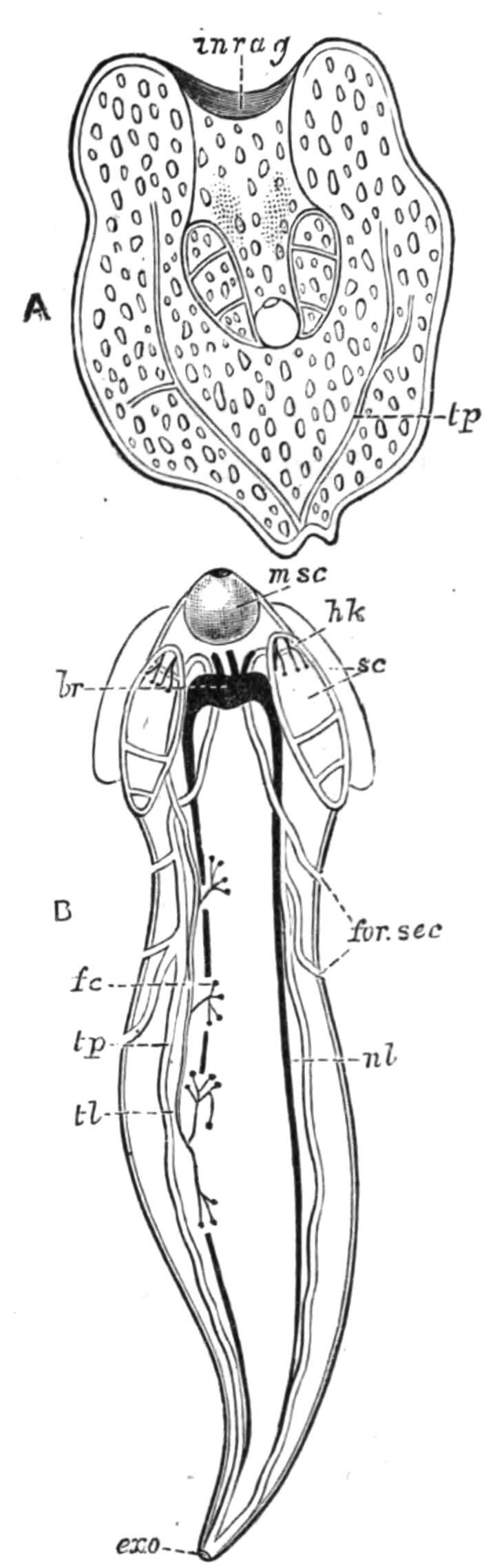
Fig. 38.—Scolex polymorphus Rud. (larva of Calliobothrium filicolle Zschokke), from the muscles of Apogon, a Mediterranean fish; also found in many Invertebrates (e.g. Sepia). A, Inverted scolex, with calcareous bodies; B, everted older larva. br, Brain; exo, terminal excretory aperture; fc, flame-cells; for.sec; secondary excretory pores; hk, hooks of the adult Cestode; inrag, pit at the bottom of which the head is developed; msc, anterior sucker; nl, lateral nerve; sc, suckers; tl, tp, lateral and main excretory vessels. (After Monticelli.)
Occurrence of Cestodes.—The distribution of Cestodes and their larvae is analogous to that of the digenetic Trematodes, although the absence of an alimentary canal limits the habitat of the mature worms to certain sites, such as the blood-vessels, the lymphatic and coelomic spaces, and the digestive system, where their body may be bathed by a nutritive fluid. Almost all groups of Vertebrates are attacked by Cestodes. Those of fishes, and particularly of Elasmobranchs, are distinguished by certain structural and developmental features; those of birds by {78}others; those of mammals, by a third set of characters. The young stages of the Cestodes of Sharks and Rays occur encysted in the body-cavity, or in the pyloric appendages, of Teleosteans, which probably swallow them along with those invertebrate animals upon which they prey. The larvae of the Cestodes of carnivorous mammals or piscivorous birds, live respectively in herbivores and fishes, but how the latter are infected we know in very few instances. Cestode larvae are known to occur in many Invertebrates, and occasionally are taken free swimming in the sea, presumably crossing from one host to the next. Ctenophores, Siphonophores, Copepods, Ostracods, Decapods, various Molluscs especially Cephalopods, Earthworms, and other Annelids, are the intermediate hosts of these larvae (see Fig. 38), the fate of which, however, has been determined in but few cases.
Occurrence of Cestodes in Man.[99]—Tape-worms, either in the adult or larval stages (bladder-worms), have, from ancient times, been known to occur in man, and in the animals that serve him as food. Until comparatively recent times, however, the true nature of these parasites, and particularly of "hydatids" (cystic larvae), was unrecognised. Up to the seventeenth century the larvae were regarded as abscesses or diseased growths of the affected organs, and it was only at the close of that century that their animal nature was even suggested. Even at the beginning of the nineteenth century, three modes of origin of Cestodes—by "generatio aequivoca" from the tissues of the body, or by the union of previously distinct proglottides, or again by metamorphosis of free-living worms drunk with water by cattle or birds (as Linnaeus suggested)—were still variously held, at a time when Malpighi, Pallas, and Goeze had recognised the true connexion between the cystic and segmented states of Taenia crassicollis (the cat tape-worm), and when Goeze had seen the eggs of Taeniae, and Abildgaard[100] had even conducted the first helminthological experiments (conversion of the larval Schistocephalus, Fig. 40, into the adult form).
Fig. 39.—A, Taenia saginata Goeze. Nat. size. (From a specimen in the Cambridge Museum.) The approximate lengths of the portions omitted in the drawing are given. At * (after Leuckart) the branched uterus and the longitudinal and transverse excretory vessels are shown. The genital apertures are seen as a lateral opening on each of the larger proglottides. B, Head (scolex) of T. solium Rud. × 12. (After Leuckart.)
Generally speaking, "a tape-worm" in Western Europe will prove to be Taenia saginata Goeze (the beef tape-worm, Fig. 39, A), exceedingly prevalent also in the East, and indeed cosmopolitan, occurring wherever the infected flesh of the ox is eaten in a raw or half-cooked state. Its attacks are fortunately not usually severe. Taenia solium Rud. (the pork tape-worm) is found wherever the pig is kept as a domestic animal, and has consequently a world-wide distribution. Its size (6-9 feet long) and powers of adhesion would alone render T. solium a formidable parasite. But the danger of its presence in the body of man, or in the flesh of pigs, lies in the fact that the larva or bladder-worm (known as Cysticercus cellulosae) can live in the most varied organs. Thus if by accident a mature proglottis be eaten, the embryos escape, bore their way into the wall of the stomach, and entering the portal vein, may reach in time the muscles, the brain, the eye, or even the heart itself, and attain the cystic condition. Even more disastrous may be the result, should some ripe joints of a mature worm work their way from the intestine back towards the stomach. Should this happen (and though it has not been directly proved, the possibility is to be reckoned with), the result would be the release of vast numbers of embryos capable of inflicting fatal injury on the host. An abnormal Cysticercus of this species is probably the Taenia {80}(Cysticercus) acanthotrias Weinl. (see, however, Leuckart, loc. cit. p. 711).
Taenia (Hymenolepis) nana v. Sieb.[101] is found in man in Egypt, Italy, England, Servia, Argentine Republic, and the United States. Though small (¾-1 inch long), its numbers usually excite digestive and nervous disorders of considerable severity, more serious, indeed, than those caused by the commoner tape-worms. H. diminuta Rud. (flavopunctata Weinl.), normally found in Rodents, has been rarely recorded in man. Taenia (Dipylidium) caninum L. (= T. cucumerina Bloch = T. elliptica Batsch), the commonest parasite of pet cats and dogs, and T. (Davainea) madagascariensis Davaine, have occasionally been recorded from infants and young children. But the attacks of these species are insignificant in comparison with those of the cystic stage (Echinococcus polymorphus) of a tape-worm (T. echinococcus v. Sieb.) which lives when mature in the dog.
Echinococcus is most frequent in Iceland, where it affects 2 to 3 per cent of the population, and a still larger proportion of sheep; while in Copenhagen, Northern Germany, some districts of Switzerland, and Victoria it is not uncommon, but is frequently found during post-mortem examinations when no definite symptoms of its presence had been previously noticed. Echinococcus[102] varies greatly in size, form, and mode of growth, but is distinguished in the formation not of one scolex only, as in the Cysticercus, but in the production of a number of vesicles, usually from the inner wall. Within these, large numbers of scolices may be developed. The whole organism continues to swell by the formation of a watery liquid within it, and if its growth be rapid the fluid tension may cause the rupture of the enclosing connective-tissue capsule formed around the parasite, at the expense of the host, and the protrusion of the daughter vesicles. It is the consequent injury to the surrounding organs of the host, at this critical stage, often only reached after the lapse of several years, that occasions serious or even fatal results. Zoologically, Taenia echinococcus and T. coenurus are interesting, since they exhibit an {81}indubitable alternation of asexual generations in the larval state, with a sexual adult stage.
Bothriocephalus latus Brems., the broad tape-worm, which attains a length of 20-30 feet, or even more, occurs in man endemically in the eastern Baltic provinces, certain parts of Switzerland, generally throughout Russia (especially near Kasan), in North America, and commonly in Japan,—that is, in districts where the population partake largely of pike or other fish in a raw or partially-cooked state. Elsewhere it occurs sporadically, and in Munich, where it was unknown before 1880, its presence has been traced to emigrants from infected districts, who settled on the shores of the Starenberger Lake, from which Munich was supplied with fish. How the pike, the usual but not invariable intermediate host, becomes infested (and its musculature is frequently riddled with the larvae) we do not accurately know, but some Invertebrate, the prey of the pike, is probably the first host into which the free-swimming ciliated larva (Fig. 42) finds its way. In Greenland, B. cordatus is very common in the dog, and probably also in man, though few cases have been recorded. B. mansoni Cobb. (= B. liguloides Leuck.) was, till recently, known only in the larval state from China and Japan. Iijima, however, has found older specimens in the latter country. B. cristatus Dav. is a species founded somewhat doubtfully on two fragments found, one in a child, the other in a man, in France.
Occurrence of Cestodes in Domestic Animals.[103]—Among domestic animals, the dog is, undoubtedly, the most frequently attacked by Taeniae. Six species of Taenia (T. serrata, marginata, coenurus, echinococcus, krabbei, and possibly T. serialis), Dipylidium caninum (the commonest form), Mesocestoides lineatus, and three or four species of Bothriocephalus have been found in the dog. The table of life-histories (p. 83) shows that sheep, rabbits and other Rodents serve as the intermediate hosts, in which the cystic stages of the species of Taenia are found. Hence the prevalence of T. serrata in a given locality is connected with the abundance there of the rabbit and hare, in which the larva (Cysticercus pisiformis) occurs. Bothriocephalus cordatus develops from the young stage present in the fish which the Icelanders give to their dogs. In Iceland and certain parts of {82}Australia T. echinococcus infests one-third to one-half the number of dogs examined; a fact connected with the frequency of Echinococcus in man in these countries.
In sheep the most noteworthy and dangerous parasite is Coenurus cerebralis (or the cystic stage of the dog-taenia, T. coenurus), which gives rise to the disease known as "gid" or "staggers." It is found in various parts of the brain or spinal cord, and the symptoms differ according to the position of the parasite. If this presses upon one hemisphere the sheep describes circles and finally falls: if on the optic lobes, the eyes are affected: if the pressure affects the cerebellum the movements of the sheep are uncertain and incoordinated. Four or six weeks after the appearance of the symptoms, death results from cerebral paralysis, or from general debility, and the loss of sheep incurred by this disease (happily less frequent in England than formerly) has been calculated by Youatt at a million for France annually; at 35 per cent of the flocks for England in bad seasons; and about 2 per cent for Germany. Besides sheep, which are most subject to "gid" during their first year, various ruminants—Goat, Ox, Moufflon, Chamois, Roe, Antelope, Reindeer, Dromedary—are attacked in the same way. A similar form, Coenurus serialis Baill., is common in the wild rabbit in this country, and in Australia in the hare and squirrel. It forms large swellings in the connective tissue of various parts of the body, but usually does not affect the health of the host. It is not known in what carnivore Taenia serialis Baill. normally occurs. Experiments have, however, shown that it develops rapidly in dogs.
The preventive measures which are steadily diminishing the prevalence of the Cestode parasites in man in some parts of Western Europe cannot be dealt with here, but it may be noticed that the Jewish observance with regard to swine is the surest preventive measure against taeniasis and trichinosis. Careful inspection of meat and general cleanliness, are the leading measures that in these hygienic matters secure the greatest immunity from disease.
Table of the Life-Histories of the principal Cestodes of Man and the Domestic Animals.
| Cestode. | Final host. | Larva. | Intermediate host. |
| Taenia serrata Goeze | Dog | Cysticercus pisiformis Zed. | Rabbit, Hare, Mice (liver and peritoneum) |
| T. marginata Batsch | Dog, Wolf | Cyst. tenuicollis Rud. | Monkeys, Ruminants, Ungulates (in peritoneum) |
| T. saginata Goeze (= T. mediocanellata Küch.) | Man | Cyst. bovis Cobb. | Ox, Giraffe (in muscles) |
| T. solium Rud. | Man | Cyst. cellulosae Rud. (? Cyst. acanthotrias Weinl.) | Pig, Man, Monkeys, Bear, Dog, Cat, Black Rat (in various organs) |
| T. crassicollis Rud. | Cat and other Felidae, Stoat | Cyst. fasciolaris Rud. | Rat, Mouse, Bat (liver) |
| T. coenurus Küch. | Dog, Arctic Fox | Coenurus cerebralis Rud. | Brain of Sheep, Ox, Goat, Dromedary, Camel, Antelope, Horse |
| T. serialis Baill. | ? Dog | Coenurus serialis Baill. | Rabbit (connective tissue) |
| T. echinococcus v. Sieb. | Dog, Dingo, Jackal, Wolf | Echinococcus polymorphus Dies.(incl. E. multilocularis found in Man) | Man, Monkeys, many Carnivores, Rodents, Ungulates, Ruminants, and Marsupials; also in Turkey and other birds |
| Moniezia expansa Rud. | Sheep, Ox, Goat, etc. | Unknown | |
| Thysanosoma fimbriata Dies. | Sheep, Cervidae | Unknown | |
| Stilesia globipunctata Riv. | Sheep | Unknown | |
| Anoplocephala perfoliata Goeze | Horse | Unknown | |
| Dipylidium caninum L. (= Taenia cucumerina Bloch = T. elliptica Batsch) | Man, Dog, Cat | Cysticercoid larva (Fig. 43), Cryptocystis trichodectis Vill. | Body-cavity of Trichodectes and Pulex of Dog |
| Hymenolepis murina Duj. | Mouse, Rat | Cercocystis Vill.[104] (develops in parental host) | Usually absent |
| H. nana v. Sieb. | Man | Unknown | |
| H. diminuta Rud. (= Taenia flavopunctata Weinl.) | Man, Mouse, Rat | Cercocystis Vill. | Meal-moth, Asopia (Pyralis) farinalis; also certain Orthoptera and Coleoptera |
| {84}
Drepanidotaenia gracilis Zed. |
Duck, Goose, Wild Duck | Cercocystis Vill. | The Ostracods Candona rostrata and Cypris compressa, and also Cyclops viridis |
| D. anatina Krabbe | Duck | Cerc"cystis V"ll. | Cypris incongruens, and also Perch |
| D. setigera Fröh. | Goose | Cerc"cystis V"ll. | Cyclops brevicaudatus |
| D. infundibuliformis Goeze | Common Fowl | Cerc"cystis V"ll. | House-fly |
| Dicranotaenia coronula Duj. | Duck | Cerc"cystis V"ll. | Cypris ovum |
| Davainea proglottina Dav. | Fowl | Cerc"cystis V"ll. | ? Limax cinereus, L. agrestis |
| D. madagascariensis Dav. | Children | Unknown | |
| D. friedbergeri v. Linst. | Pheasant | Unknown | ? Ants |
| Mesocestoides lineatus Goeze | Dog | Unknown | |
| Bothriocephalus latus Brems. | Man, Dog, ? Cat | Plerocercoid, i.e solid, elongate larva, with no bladder | Probably first enters an Invertebrate host, which is eaten by Pike, Perch, Trout, etc. |
Fig. 40.—A, Stickleback (Gasterosteus aculeatus) infested by an advanced larva of Schistocephalus solidus Crepl. B, The larva. All × 1½. (From specimens in the Cambridge University Museum.)
Structure and Development of Cestoda.[105]—Of the unsegmented Cestodes, Caryophyllaeus mutabilis, from the intestine of carp and other Cyprinoid fishes, is the most easily accessible form. Triaenophorus nodulosus, which is very useful for the study of the excretory system, occurs mature in the pike. In the body-cavity of the Stickleback (Fig. 40) a large, broad, yellow worm may sometimes {85}be found, the larva of Schistocephalus solidus Crepl., which occurs in the intestine of Terns, Storks, Mergansers, and other birds. Species of Ligula are found in the same birds. The intestine of a Lophius or Cyclopterus ("lump-fish") contains, usually, the early and intermediate stages of various Cestodes, while the alimentary canal of Elasmobranchs often contain many peculiar Tetrarhynchidae and other forms. For the study of development, the Taenia anatina from the duck may be used. The ripe proglottides are collected, and the eggs placed with Cypris ovum in an aquarium, with the probability that some of the embryos will enter the Ostracod, and the peculiar Cysticercoid may be bred.[106] Cysticercus pisiformis and Coenurus serialis, which occur commonly in rabbits, are also suitable objects for examination.
A Cestode such as Echinobothrium (Fig. 36) is divisible into head and proglottides. Moniez has suggested that the head is really the morphologically hinder end of the body, in which case the formation of proglottides would closely resemble the mode of segmentation of an Annelid larva. The close similarity, however, between the Cysticercoid larva (Fig. 43, F) and the Cercaria of a liver-fluke, seems to show that the anterior end is the same in both cases, and since it bears the central part of the nervous system, we may reasonably call it the "head." Moreover the hinder end of a Platyhelminth usually possesses the chief excretory pore. Another difficulty is the determination of dorsal and ventral surfaces. Authors are agreed,—on the analogy of Trematodes, in which the testes are usually dorsal and the ovaries ventral,—that the dorsal and ventral aspects of a Cestode are determined by the position of these organs, although the often radially formed "head," the lateral or superficial position of the genital apertures, and the variability of these features, render it a matter of considerable doubt whether "dorsal" and "ventral" are more than useful conventional terms. The suckers and hooks are borne on a muscular cap, the "rostellum," which is only slightly developed in the Ichthyotaeniae. The body is solid, and is divisible into an outer muscular coat—enveloped in a (possibly epidermal) investing membrane—and an inner parenchymatous tissue containing the chief part of the excretory, nervous, and reproductive systems. One or two pairs {86}of longitudinal excretory vessels are present, usually connected by transverse ducts and opening by a single terminal pore. Occasionally a regularly paired arrangement of lateral or secondary pores is present (Figs. 38 and 41, for.sec). Flame-cells occur at the end of the fine tubules (Fig. 38), and the whole system is well developed, but may undergo degenerative changes in the older proglottides. The central nervous system varies according to the degree of differentiation of the rostellum; and, owing to the difficulty of staining the nerves and the contradictory statements of authors, we do not yet possess a fully reliable account of the nervous system even of the commoner Taeniae. Free nerve endings and other sensory terminations have been recently stated to exist in the cuticle of Cestodes and Trematodes. If true, this would tend to show that the parasitic mode of life of these animals demands a complex nervous system comparable with that of the Turbellaria.
Fig. 41.—Diagrammatic transverse section of Schistocephalus solidus Crepl., from the Wild-duck, illustrative of the Cestodes with uterine aperture (uto). × 12. cs, Cirrus-sac; for.sec, one of the paired lateral openings of the excretory vessels; ln, longitudinal nerve; ov, ovary; ovd, oviduct; par.m, parenchymatous muscles; r.sem, receptaculum seminis; sh.gl, shell-gland; te, testes; ut, uterus; uto, uterine pore; vag, vagina; vd, vasa deferentia; yd, yolk-duct; yg, yolk-glands (black); ♂, male, ♀, female genital aperture. (After Riehm.)
The reproductive organs, unlike the preceding systems, are discontinuous from one proglottis to the next. The male and female organs and their mutual connexions, especially in the unsegmented Cestodes, may be compared in detail with those of Trematodes, but the difference between the arrangement of the generative organs of various Cestodes is very great.[107] The penis (Fig. 41, cs) is evaginated through the male pore (Fig. 41, ♂), and inserted far into the vagina (♀, vag) of the same or another segment of the tape-worm.
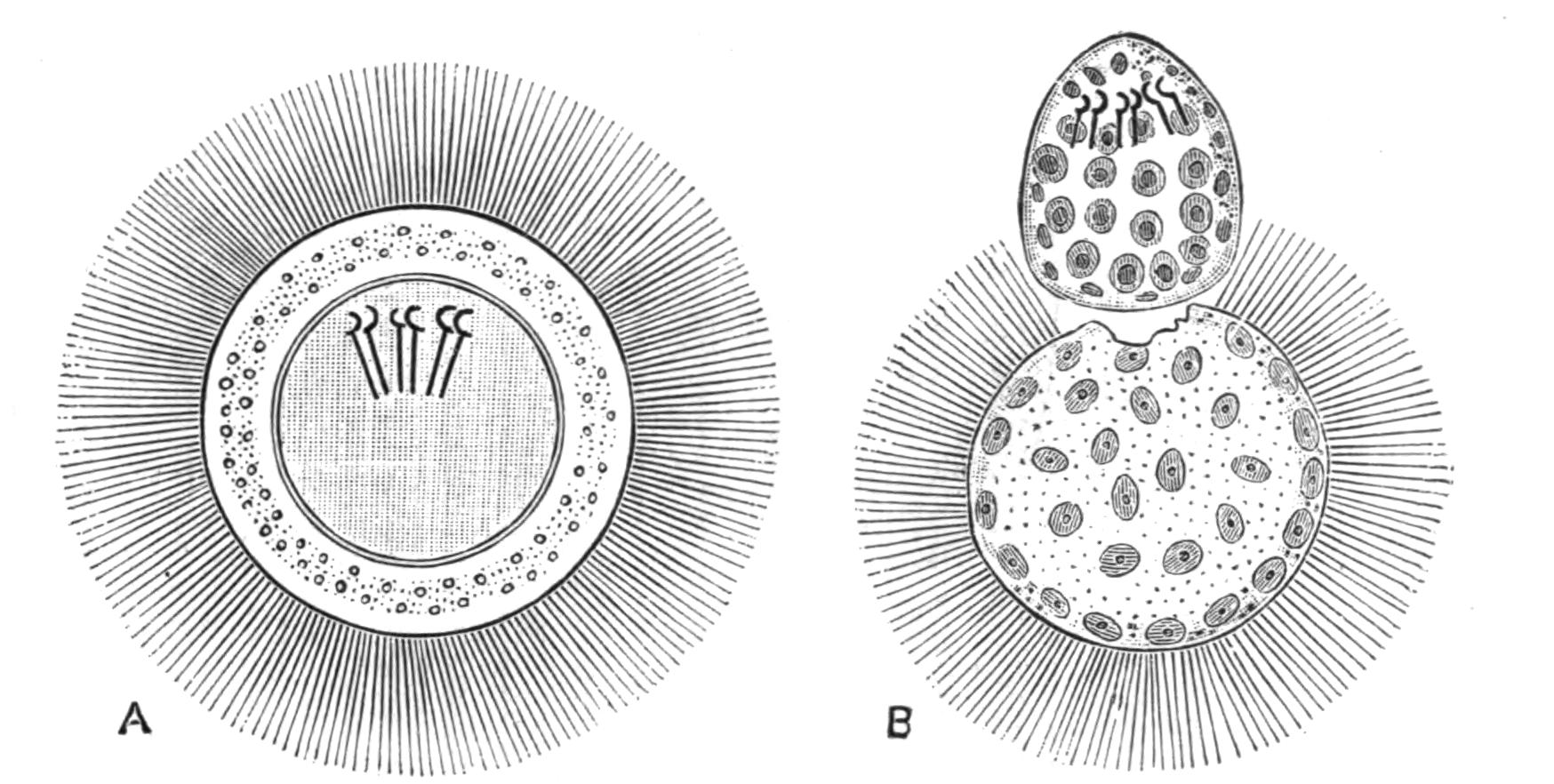
Fig. 42.—A, Free-swimming, six-hooked larva of Bothriocephalus latus Brems. (the broad tape-worm of Man), still enclosed in a ciliated (possibly cellular) double membrane or mantle. In this condition it may continue to live in water for a week or more, but eventually throws off its ciliated coat (as in B) and commences to creep about vigorously by the aid of its hooks, in search of its first host, which is at present unknown. (After Schauinsland.) × 600.
From this fact and the anatomical relations of the vagina, it is becoming increasingly probable that the so-called uterus of Trematodes is an organ corresponding to the vagina of Cestodes, and not to the uterus of Cestodes. The latter opens to the exterior in Schistocephalus, Bothriocephalus, and some other Cestodes of fishes by a special pore (Fig. 41, uto). Through this, some of the eggs (which in these genera give rise to ciliated larvae) are enabled to escape, and need not wait for the detachment of the proglottis, as must happen in the Taeniidae, where the uterus is closed. This uterus, a true physiological one, is probably the homologue of the "canal of Laurer" ("Laurer-Stieda canal," or "vagina") of Trematoda. The fertilised ovum and yolk are brought together into the "ootype," where the shell-gland forms the egg-shell around them (Fig. 41, sh.gl) and the egg is then passed into the uterus. The ovum segments to form a minute six-hooked larva, which may (Bothriidae, Fig. 42) or may not (Taeniidae) be ciliated. Thus in Taenia serrata the proglottides are shed with the faeces of the host (dog), and they protect the young from the desiccating influence of the surroundings. If inadvertently eaten by a rabbit along with herbs, the proglottis and larval envelope are digested, and by its six hooks the tiny larva bores through the gastric wall into the portal vein, and so into the liver. Here the hooks are thrown off, and the solid mass of cells becomes vacuolated.
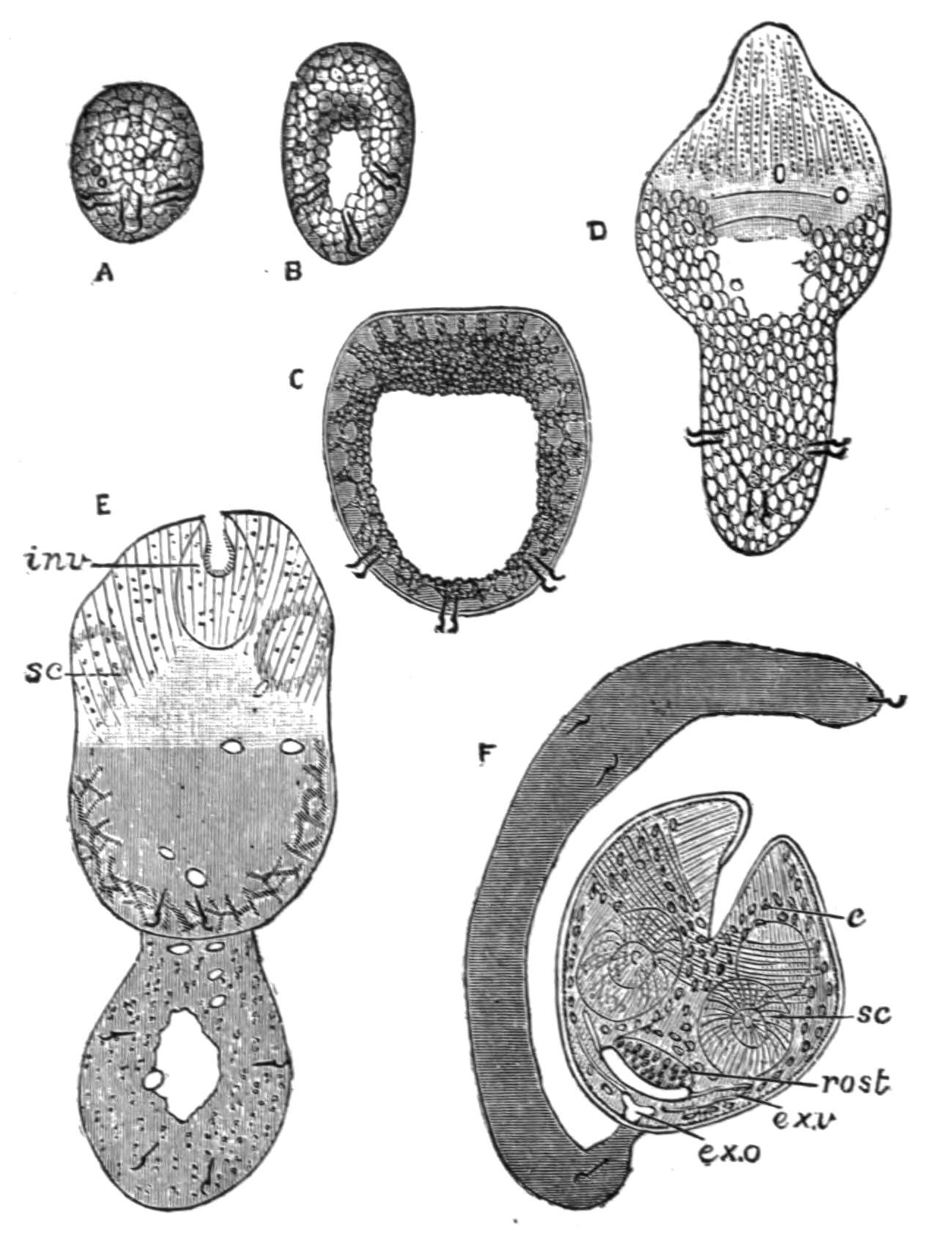
Fig. 43.—Stages in the development of Dipylidium caninum L. (= Taenia elliptica Batsch, T. cucumerina Bloch), the commonest of the Dog-Taeniae; compare Fig. 44. A, Six-hooked larva (now often spoken of as an "Onchosphaera"); B, larva elongating; formation of a central lacuna; C, larva further advanced; D, distinction between body and tail is visible; E, invagination of the rostellum is commencing; F, Cysticercoid larva with four suckers, invaginated rostellum, and excretory vessels. c, Calcareous concretions in cells of the larva; ex.o, excretory aperture; ex.v, excretory vessels; inv, invagination commencing; rost, rostellum; sc, suckers. (After Grassi and Rovelli; highly magnified.)
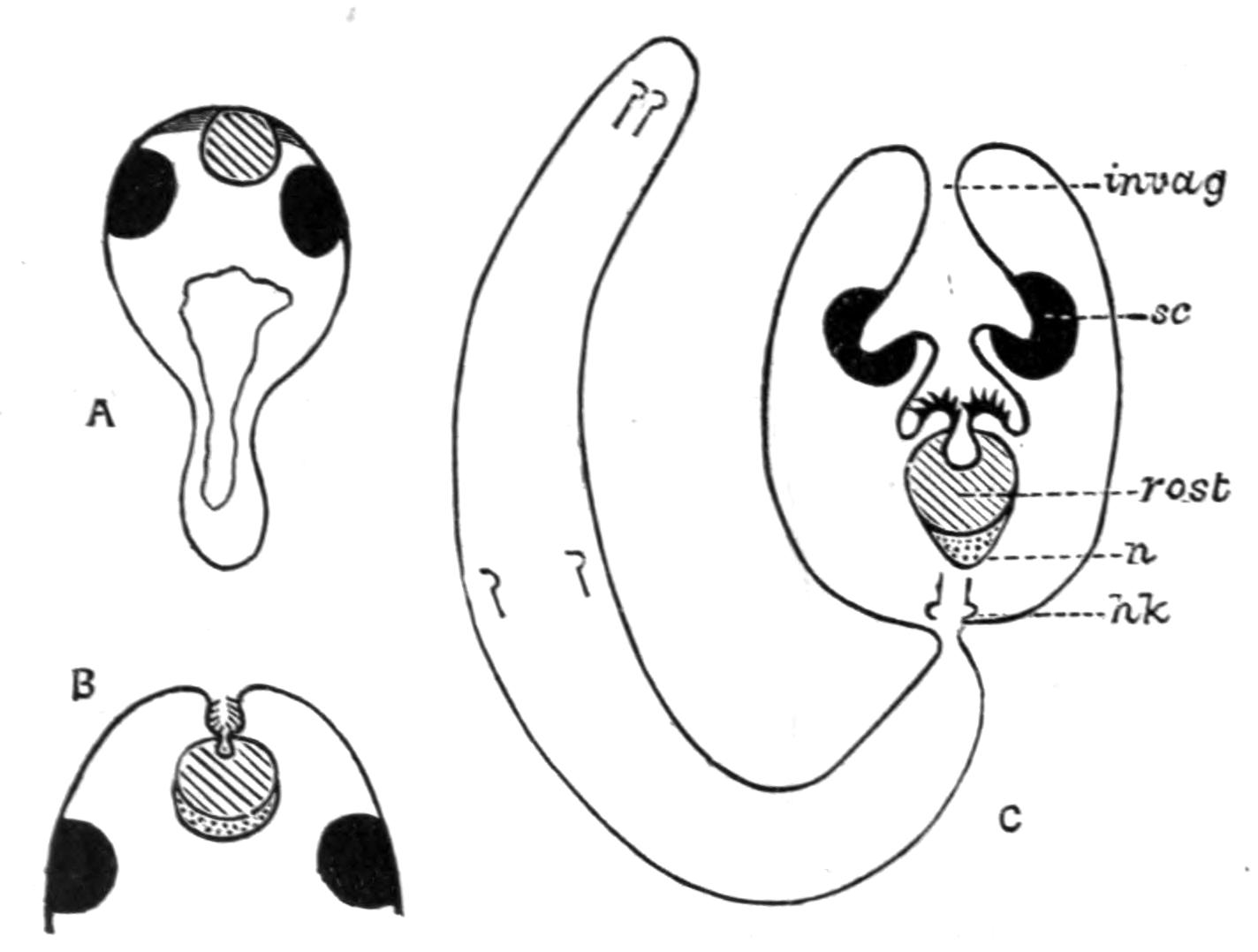
Fig. 44.—Schematic longitudinal sections through the larvae of Dipylidium caninum L. All these stages are passed in the body-cavity of the Dog-flea (Pulex serraticeps). (Compare Fig. 43 for further details.) A, Six-hooked larva with developing rostellum (shaded) and suckers (black). In this species the invagination (C, invag.) occurs after the formation of these organs, and not, as in most Taeniae, before it. B, Invagination commencing; the hooks are developing above the rostellum, while beneath it the nervous system (dotted) is seen. C, The invagination has now carried the suckers inwards. The tail has become distinct, and the whole larva at this stage is known as a Cysticercoid. hk, Larval hooks; invag, mouth of the invagination; n, central nervous system; rost, rostellum and hooks; sc, suckers, of which only two can be seen in a longitudinal section; four are really present. (After Grassi and Rovelli.)
At one pole an invagination occurs, at the bottom of which the rostellum, suckers, and hooks are gradually formed, but inside out as compared with the head of the Taenia serrata. At this stage the larva (Cysticercus pisiformis) has usually issued from the liver and attached itself to the omentum. The invagination projects into the cavity of the bladder, within which a watery fluid accumulates. Thus the "bladder worm" is formed, the head of which is evaginated if the larva be introduced into the digestive system of a dog. The bladder and neck of invagination are digested, while the head, protected by these, remains, and forms the neck, from which the proglottides are afterwards segmented off. In Taenia (Hymenolepis) murina the whole development may take place in the parental host, the larva living in the villi, the adults in the cavity of the same rat's intestine (Grassi). The different forms of Cestode larvae depend largely upon the presence and degree of development of the caudal vesicle or bladder, which in Scolex polymorphus (Fig. 38) (the young stage of Calliobothrium filicolle Zsch.) is practically absent. If the bladder be small, the larva is known as a Cysticercoid. For example, the common Dipylidium caninum, which lives in the dog, has such a larva, the development of which is explained and illustrated by Figs. 43 and 44. The bladder becomes exceeding capacious in Coenurus and Echinococcus.
Table for the Discrimination of the more usual Cestodes of Man and Domestic Animals.[108]
| I. Scolex in most cases with hooks; uterus with a median and lateral branches; yolk-glands simple, median; genital pore single; dorsal excretory vessel narrower than the ventral, without a circular commissural trunk; eggs without pyriform apparatus (processes of the ovarian membrane) | |
| Gen. Taenia L. (s. str.) | |
| A. Genital ducts pass on the ventral side of the nerve and of the two longitudinal excretory vessels | T. crassicollis Rud. |
| A. Genital ducts pass on the ventral side of the nerve and of the two longitudinal excretory vessels | |
| T. crassicollis Rud. | |
| B. Genital ducts pass between the dorsal and ventral longitudinal vessels. | |
| a. Nerve present on dorsal side of genital ducts. | |
| α. Head armed | T. solium Rud. |
| β. Head unarmed | T. saginata Goeze. |
| b. Nerve on ventral side of genital ducts. | |
| {90}
Dog-Taeniae[109] Head armed; genital pore marginal and — Single |
|
| Many proglottides; strobila several centimetres long; small hooks with guard. | |
|
Bifid hooks, which are — 230µ-260µ long[110]; genital pore very distinct |
T. serrata Goeze. |
|
Bifid hooks, which are — 230µ-260µ long[110]; genital pore very distinct |
|
| T. serrata Goeze. | |
| — 136µ-157µ long; genital pore not very salient | T. serialis Ball. |
| — 136µ-157µ long; genital pore not very salient | |
| T. serialis Ball. | |
|
Entire large hooks, which are — 180µ-220µ long; length of mature segments double their width |
T. marginata Batsch. |
|
Entire large hooks, which are — 180µ-220µ long; length of mature segments double their width |
|
| T. marginata Batsch. | |
| — 150µ-170µ long; length of mature segments treble their width | T. coenurus Küch. |
| — 150µ-170µ long; length of mature segments treble their width | |
| T. coenurus Küch. | |
| 3-4 segments; a few mm. long | T. echinococcus v. Sieb. |
| 3-4 segments; a few mm. long | |
| T. echinococcus v. Sieb. | |
| — Double and bilateral | Dipylidium caninum L. |
| Head unarmed; two genital pores on ventral surface | Mesocestoides lineatus Goeze. |
| Head unarmed; two genital pores on ventral surface | |
| Mesocestoides lineatus Goeze. | |
| II. Scolex without hooks; one or two transverse uteri present; one or two genital pores and yolk-glands, the latter never median; genital ducts pass on the dorsal side of the nerve; eggs with pyriform apparatus. | |
| A. One transverse uterus present. | |
| a. Uterus with bullate egg-sacs; pyriform apparatus without horns; genital ducts between dorsal and ventral vessels | |
| Thysanosoma Dies. | |
| α. Head large (1.5 mm.); square lobed testes in median field; posterior margin of segments fimbriated; genital pore double | |
| T. fimbriata Dies. | |
| β. Head small; no fimbriae; pore rarely double | T. giardii Riv. |
| β. Head small; no fimbriae; pore rarely double | |
| T. giardii Riv. | |
| b. Uterus without saccular dilatations; segments short, thick, and slightly imbricate | Anoplocephala E. Blanch. |
| b. Uterus without saccular dilatations; segments short, thick, and slightly imbricate | |
| Anoplocephala E. Blanch. | |
|
Horse-Taeniae. α. Head very large — No posterior lobes |
A. plicata Zed. |
| — Four posterior lobes | A. perfoliata Goeze. |
| β. Head small, without posterior lobes | A. mamillana Mehl. |
| β. Head small, without posterior lobes | |
| A. mamillana Mehl. | |
| B. Two uteri and two genital pores present; horns of pyriform apparatus well developed; genital ducts pass on the dorsal side of the longitudinal vessels | |
| Moniezia R. Bl. | |
| a. Interproglottidal glands[111] arranged in linear series (planissima group) | |
| M. planissima S. and H. M. benedeni Mz. M. neumani Mz. | |
| b. Interproglottidal glands saccular (expansa group) | |
| M. expansa Rud. M. oblongiceps S. and H. M. trigonophora S. and H. | |
| c. Interproglottidal glands absent (denticulata group) | M. denticulata Rud. M. alba Perr. |
| c. Interproglottidal glands absent (denticulata group) | |
| M. denticulata Rud. M. alba Perr. | |
| C. Uterus single or double, without spore-like egg-sacs; eggs with a single shell; genital pores irregularly alternate; strobila narrow; testes absent from median part of the field | |
| Stilesia Raill. | |
| {91}
a. A transverse uterus in middle part of median field; head 2 mm. diameter |
S. centripunctata Riv. |
| a. A transverse uterus in middle part of median field; head 2 mm. diameter | |
| S. centripunctata Riv. | |
| b. Two lateral uteri in each segment; head less than 1 mm. in diameter | S. globipunctata Riv. |
| b. Two lateral uteri in each segment; head less than 1 mm. in diameter | |
| S. globipunctata Riv. | |
| III. Scolex almost invariably provided with hooks; genital pores on left border of segment; eggs with three shells but no cornua. Segments broader than long; posterior angles salient. | |
| Hymenolepis Weinl. | |
| a. Scolex with a single series of 24-30 hooks, each 14-18µ long | |
| H. nana v. Sieb. H. murina Duj. | |
| b. Scolex very small, unarmed | H. diminuta Rud. |
| IV. Scolex provided with two elongated muscular pits. Body segmented; three genital apertures in middle of ventral surface | |
| Bothriocephalus Rud. | |
| Body 2-20 metres in length | |
| B. latus Brems. B. cristatus Dav. (doubtful species). B. cordatus Leuck. B. mansoni Cobb. (= B. liguloides Leuck.) | |
Classification of Cestodes.—The following classification, which, so far as the Taeniidae are concerned, follows that employed by Railliet, Blanchard, and most recent writers, includes only a few representative genera:—
| 1. Fam. | Cestodariidae Mont. (Monozoa Lang). | |
| Gen. | Caryophyllaeus, Archigetes, Gyrocotyle, Amphilina. | |
| 2. Fam. | Bothriocephalidae. | |
| Sub-Fam. 1. | Bothriocephalinae. Gen. Bothriocephalus, Schistocephalus, Triaenophorus (= Tricuspidaria). | |
| Sub-Fam. 2. | Ligulinae. Gen. Ligula. | |
| Sub-Fam. 3. | Solenophorinae. Gen. Solenophorus, Duthiersia. | |
| Sub-Fam. 4. | Diphyllinae. Gen. Echinobothrium. | |
| 3. Fam. | Tetrarhynchidae. | |
| Gen. | Tetrarhynchus. | |
| 4. Fam. | Tetraphyllidae. | |
| Sub-Fam. 1. | Phyllobothrinae. Gen. Phyllobothrium, Echeneibothrium, etc. | |
| Sub-Fam. 2. | Phyllacanthinae. Gen. Calliobothrium, Anthobothrium, etc. | |
| 5. Fam. | Taeniidae. | |
| Sub-Fam. 1. | Cystotaeninae. Gen. Taenia s. str. | |
| Sub-Fam. 2. | Anoplocephalinae. Gen. Moniezia, Thysanosoma, Stilesia, Anoplocephala. | |
| Sub-Fam. 3. | Cystoidotaeninae. Gen. Dipylidium, Hymenolepis, Drepanidotaenia, Dicranotaenia, Echinocotyle, Davainea. | |
| Sub-Fam. 4. | Mesocestoidinae. Gen. Mesocestoides, Dithyridium. | |
| Sub-Fam. 5. | Ichthyotaeninae. Gen. Ichthyotaenia, Corallobothrium. | |
MESOZOA
DICYEMIDAE—STRUCTURE—REPRODUCTION—OCCURRENCE: ORTHONECTIDAE—OCCURRENCE—STRUCTURE: TRICHOPLAX: SALINELLA.
The Mesozoa are an obscure group, the position of which in the animal kingdom is still doubtful. The name Mesozoa was given to the group by its discoverer, E. van Beneden,[112] as he concluded that they were intermediate between the Protozoa and the higher Invertebrates. Recent authors, however, have called attention to the resemblance existing between them and the "sporocysts" of Trematodes, and though we still are ignorant of certain important points in their life-histories, the Mesozoa are most conveniently (and probably rightly) considered as an appendix to the Platyhelminthes.
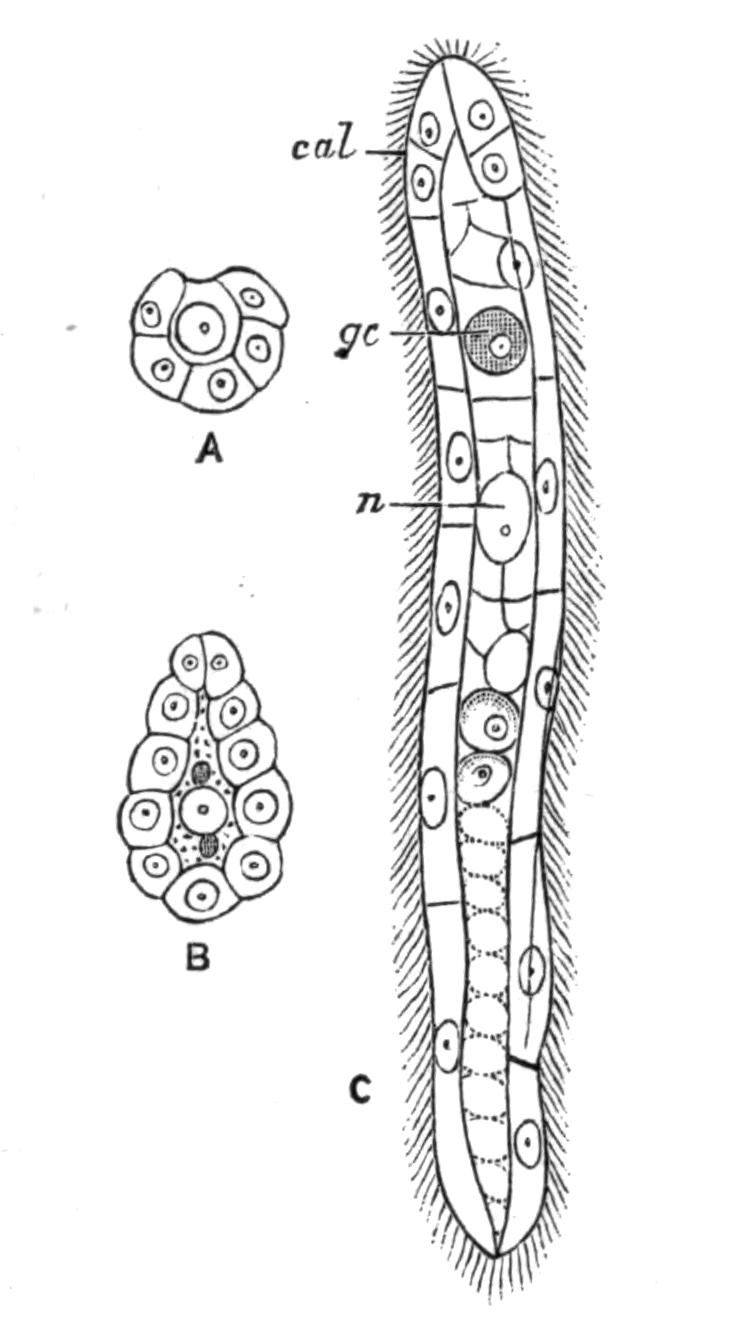
Fig. 45.—A, B, C, Stages in the development of the vermiform larva in Dicyema typus van Ben. (After Ed. van Beneden.) cal, "Calotte"; gc, germinal cell; n, nucleus of endodermal cell.
The animals composing this group are minute and parasitic, and are composed of a small number of cells. They may be divided into two families: the Dicyemidae, which occur exclusively in the kidneys of certain Cephalopods (cuttle-fish); and the Orthonectidae, which live in the brittle-star Amphiura squamata, the Nemertine Nemertes lacteus, or the {93}Polyclad Leptoplana tremellaris. In addition to the undoubted Mesozoa, certain anomalous forms—Trichoplax adhaerens and Salinella salve—may be referred to this group.
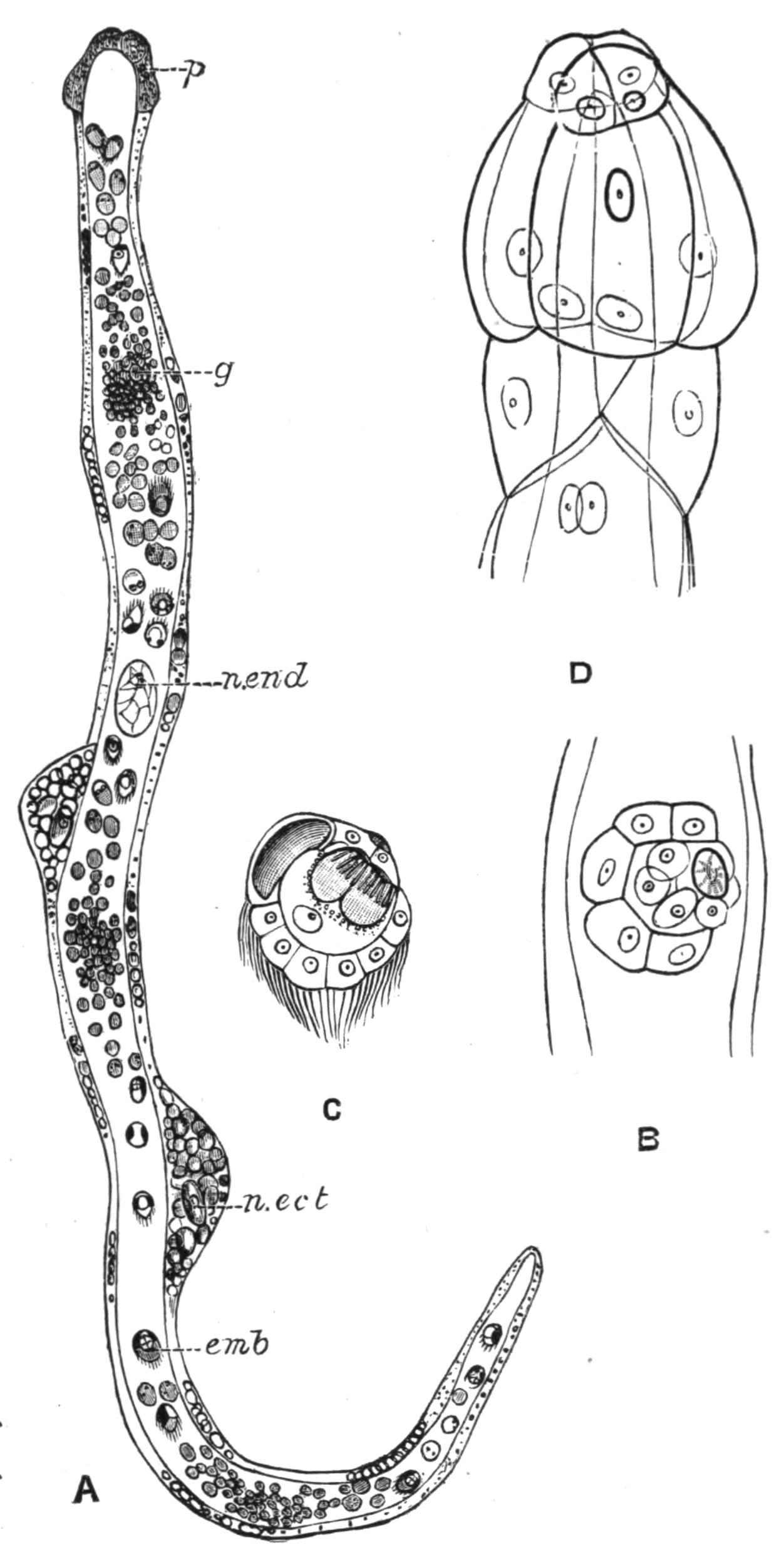
Fig. 46.—Dicyemennea eledones Wag., from the kidney of Eledone moschata. A, Full-grown Rhombogen with infusoriform embryos (emb); B, one of the latter developing; C, fully formed; D, calotte, composed of the upper nine cells shown in the figure. (After Ed. van Beneden and Whitman.) emb, Infusoriform embryo; g, part of endoderm-cell where formation of these embryos is rapidly proceeding; n.ect, nucleus of ectoderm-cell; n.end, nucleus of endoderm-cell; p, "calotte."
Dicyemidae.—If the kidney of Eledone moschata, a Cephalopod common on our south-western shores, be opened, a number of fine, yellowish, hair-like filaments may be seen attached at one end to its inner surface, floating in the fluid contained in the renal cavity. These may be Dicyemennea eledones Wag., although another form, Dicyema moschatum Whit., also occurs in the same host. D. eledones (Fig. 46) is 7 to 9 mm. long, transparent, and is composed of one large inner cell with a simple nucleus (Fig. 46, n.end), and of an outer layer of ciliated cells, nine of which form the "calotte" or pole by which the animal is attached. Within the former (endodermal) cell the formation of urn-shaped "infusoriform embryos" takes place (B and C), the fate of which is not known, but they are possibly the males. The individual which produces these larvae is called a {94}"Rhombogen." Other individuals which produce a more elongated larva ("vermiform larva," Fig. 45) are called "Nematogens," and Whitman has described a third kind, which produce first infusoriform, and then vermiform, larvae (Secondary Nematogens).[113]
The occurrence of the known species of Dicyemids (a group which has not been investigated on our coasts) is as follows:—
| Species. | Host. |
| Dicyema typus van Ben. | Octopus vulgaris. |
| D. clausianum van Ben. | O. macropus. |
| D. microcephalum Whit. | O. de Filippi. |
| D. moschatum Whit. | Eledone moschata. |
| D. macrocephalum van Ben. | Sepiola rondeletii. |
| D. truncatum Whit. | Rossia macrosoma, Sepia elegans, S. officinalis. |
| D. schultzianum van Ben. | S. biseralis, Octopus vulgaris. |
| Dicyemennea eledones Wag. | Eledone moschata, E. aldrovandi. |
| D. mülleri Clap. | E. cirrosa. |
| D. gracile Wag. | Sepia officinalis. |
| Conocyema polymorphum van Ben. | S. officinalis, Octopus vulgaris. |
Orthonectida.[114]—Two species of Orthonectids are fairly well known, Rhopalura giardii Metschn. from Amphiura squamata, and R. intoshii Metschn. from Nemertes lacteus. The latter appears to be very rare, the former occurring in 2 to 5 per cent of the number of hosts examined. The parasites occur in a granular "plasmodium," the nature of which is uncertain. Metschnikoff regards it as formed by the Orthonectids, and he considers that the cellular envelope, by which it is sometimes enclosed, is developed from the neighbouring tissue of the host. These granular, sometimes nucleated, plasmodial masses, which can perform active amoeboid movements in sea-water, occur attached to the ventral part of the body-cavity of Amphiura, and between the gut-branches and body-wall in Nemertes. Should these hosts be infected by great numbers of the Orthonectids, their sexual organs degenerate (as is the case with pond-snails attacked by sporocysts[115]), and it is possible that the remains of these organs may constitute the "plasmodia" (Braun).
Rhopalura giardii is of distinct sexes. Either males or females are found in one Amphiura. Two kinds of females, flattened unsegmented, and cylindrical segmented forms, are known. They consist of a ciliated ectodermal layer enclosing an endodermal mass of eggs, between which is a fibrillar layer usually considered to be of a muscular nature. The cylindrical female gives rise to eggs which develop, probably exclusively, into males. The flattened female produces eggs from which females alone arise, though the origin of the two forms of this sex is not well ascertained. The males contain spermatozoa which fertilise the eggs of the cylindrical female, whereas the ova of the flat form probably develop parthenogenetically.
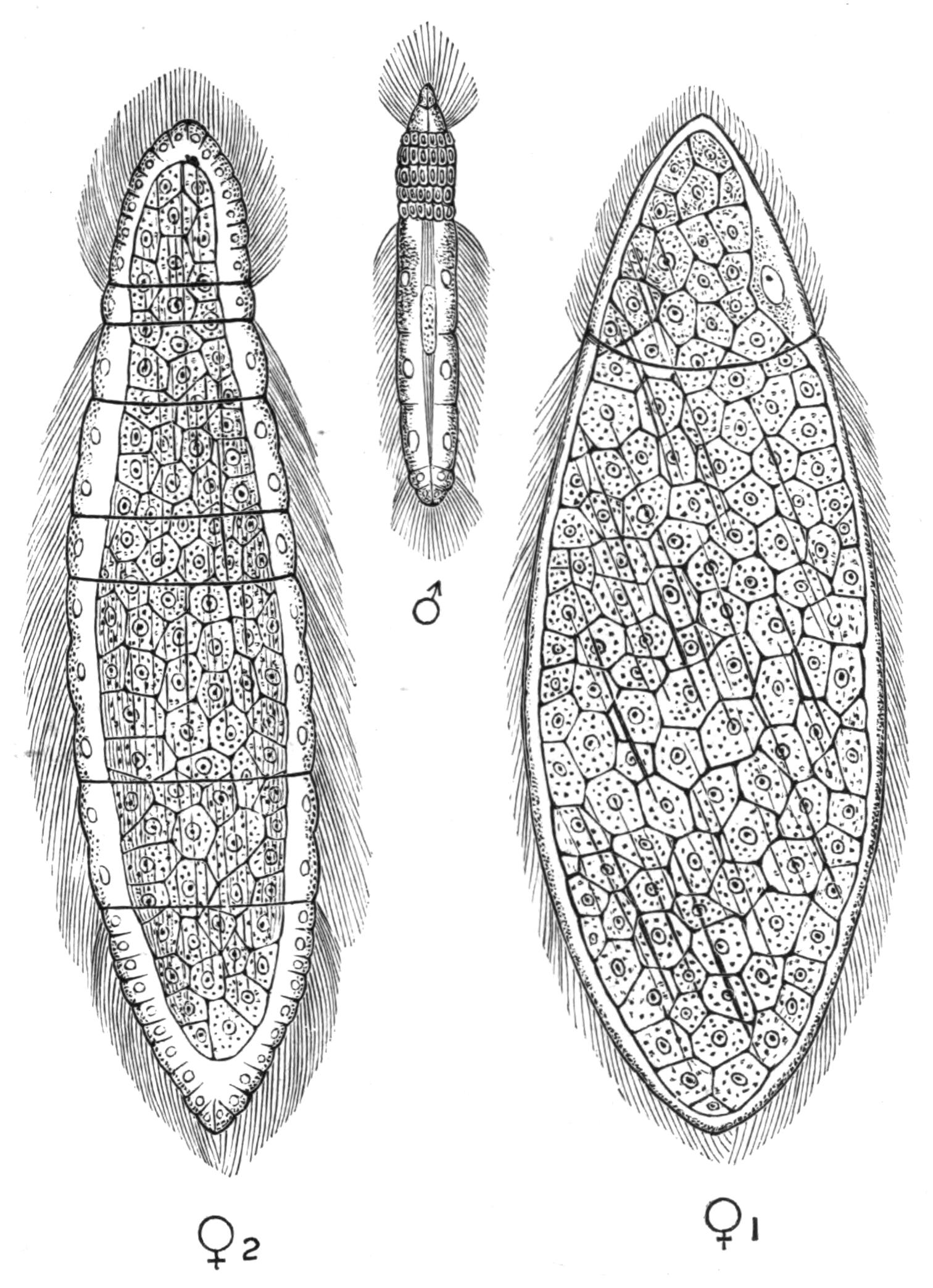
Fig. 47.—Rhopalura giardii Metschn. (from the brittle-star Amphiura squamata). ♂, Full-grown male (× 800); ♀1, flattened form of female (× 510); ♀2, cylindrical female (× 510). (After Julin.)
Trichoplax.[116]—This anomalous animal has only been found in aquaria, originally in the marine aquarium at Graz by {96}Schulze. It has the appearance of a large, flattened, ciliated Amoeba (1.5-3 mm. in diameter), but is distinguished by its structure. The upper surface is composed of a flattened epithelium. The lower surface is made up of cylindrical ciliated cells, which pass imperceptibly into the branched cells, embedded in a hyaline matrix, which compose the middle layer of the body. No distinct organs, and beyond simple fission, no mode of reproduction, have been observed. One species, T. adhaerens, is known, but has never been met with in a free state.
Salinella.[117]—This is another aquarium-animal, found by Frenzel in the Argentine, in an artificial saline solution with which he filled some aquaria. It measures .2 mm. in length, and has a somewhat flattened, barrel-shaped appearance. A single layer of ciliated cells bounds a central cavity opening at each end. Fission, and conjugation followed by encystment, have been observed. One form, S. salve, is known from salines taken from Cordova.
BY
LILIAN SHELDON
Staff Lecturer in Natural Science, Newnham College, Cambridge.
NEMERTINEA
INTRODUCTORY—EXTERNAL CHARACTERS—ANATOMY—CLASSIFICATION—DEVELOPMENT—HABITS—REGENERATION—BREEDING—GEOGRAPHICAL DISTRIBUTION—LAND, FRESH-WATER, AND PARASITIC FORMS—AFFINITIES
The Nemertinea form a compact group, the affinities of which have not been at present clearly determined. Several species were mentioned and described in the works of various naturalists during the latter half of the eighteenth century, though their anatomy was not understood until considerably later. The first mention of any member of the group was made by the Rev. W. Borlase in his Natural History of Cornwall, published in 1758. He gives a short description and a rough figure of Lineus marinus. From that time the increase in the knowledge of the group was very gradual. New species were from time to time described, but few of the descriptions could boast of much completeness, and many erroneous views were held until comparatively recent years. The group was very variously classified, but the general arrangement in early times seems to have been to unite it with the Planarians. Valuable contributions to the history of the development were made in 1848 and the few subsequent years by Desor,[118] Gegenbaur,[119] Krohn,[120] and Leuckart and Pagenstecher[121]; and more recently by Metschnikoff[122] and Salensky.[123]
Nemertines for the most part closely resemble one another in all essential points, though they differ considerably in size, colour, and external details. They vary in length from less than an inch to thirty yards, this extreme size being attained by Lineus marinus.
Fig. 48.—Lineus marinus Mont., from the living specimen in the coiled condition. Plymouth. × 1. a, Anterior end; b, posterior end.
Fig. 49.—L. marinus, from the same specimen as Fig. 48, in the expanded condition. a, Anterior end; b, posterior end.
Nemertines are common on the British coasts; about forty species have been recorded from this area. On turning over a stone on a sandy or muddy shore in a pool left by the receding tide, there may often be seen a coiled mass, having the appearance of a uniform slimy string twisted into a complicated knot. If it be carefully removed, the ends can generally be made out, one bluntly rounded and the other slightly tapering (Fig. 48, a and b). Occasionally there may be seen attached to the blunter end a fine thread, which moves about freely. This thread may, by an instantaneous movement, be drawn into the body, no trace of its existence being left except at the tip of the head, where a small pore is visible; this is the orifice through which it was withdrawn. Shortly afterwards the thread may be again shot out, the process being instantaneous and often accomplished with {101}great force. This thread (Fig. 50, p) is the proboscis, a very important and characteristic organ in Nemertines.
Most Nemertines are marine; they are mostly indifferent to climate and to the nature of the soil on which they live.
A few forms live on land (e.g. Tetrastemma agricola,[124] Geonemertes palaensis,[125] and G. chalicophora[126]) or in fresh water (e.g. Tetrastemma aquarum dulcium[127] and T. lacustre[128]) in various parts of the globe. There are also parasitic forms; the best known of which is Malacobdella.[129] A pelagic form, Pelagonemertes,[130] has been described by Moseley.
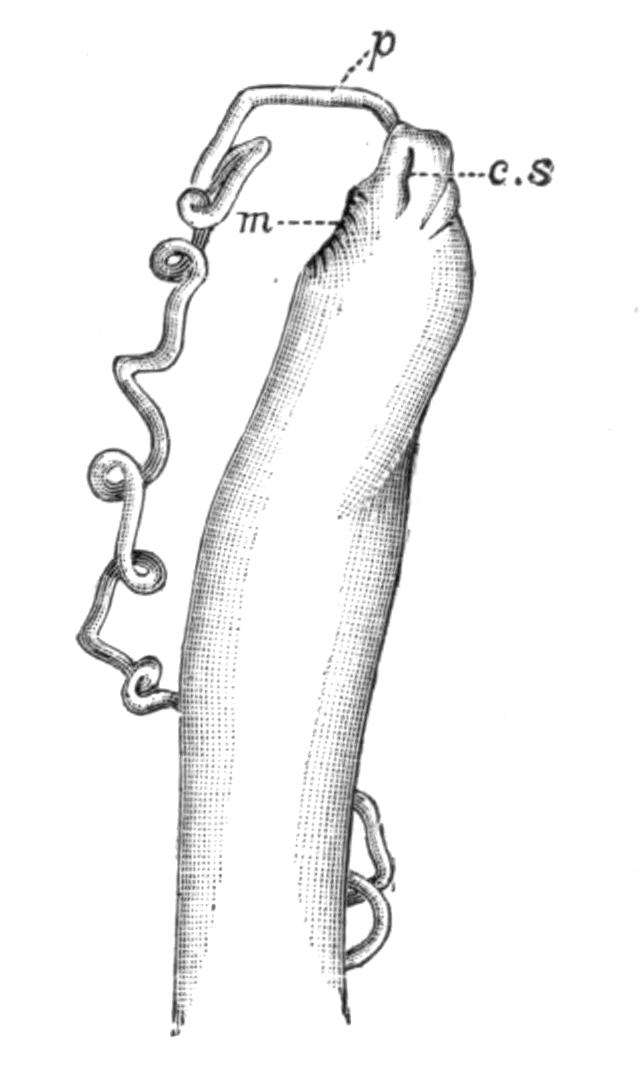
Fig. 50.—Side view of head of Cerebratulus (Micrura) tristis Hubr., showing the everted proboscis. Naples. × 2. Drawn from a spirit specimen. c.s, Cephalic slit; m, mouth; p, proboscis.
External Characters.—A typical Nemertine possesses an elongated worm-like body (Fig. 49), which is usually thrown into numerous close coils (Fig. 48). In section it may be either round or more or less flattened, with the lateral edges in some cases quite thin and almost fin-like. One or two broad, flattened, and leaf-shaped forms are known, but such a condition is exceptional, and the forms in which it occurs have probably assumed it owing to the adoption of special modes of life.
In the ordinary forms the posterior end of the body is pointed either bluntly or sharply. The head is somewhat broader than the rest of the body, and often assumes a spatulate form. Eyes (Fig. 51, e) are usually present either in one or several pairs, or in symmetrically-arranged groups on each side of the head. The mouth (Fig. 58, m) is situated near the front end of the body on the ventral surface, and is usually rendered conspicuous by being surrounded by thick tumid lips. It varies in form from being slit-like to elliptical. At the anterior end of the body a {102}small terminal pore occurs; this is the external opening of the proboscis (Fig. 51, p.p).
Nemertines are often very diversely and brilliantly coloured, the hues most commonly found being white, yellow, green, deep purple, and various shades of red and pink. The ventral surface is usually paler in colour than the dorsal, and the latter is often marked by longitudinal and transverse stripes (Fig. 59) in contrasting colours.
The whole animal is enveloped in a layer of mucus, which sometimes becomes hardened to form a tube, and this may be still further strengthened by an admixture of particles of sand or earth.
The body is capable to a great extent of contraction and extension, a Nemertine many inches long being apt, when irritated or alarmed, to contract itself to the length of not more than half an inch. Hence, unless the animal is kept and carefully watched, a very erroneous idea may be conceived as to its size.
Anatomy.—The body-wall consists of several layers (Fig. 52), which in a typical highly-developed Nemertine are as follows:—
1. An external epidermic layer (ep), consisting of ciliated cells, among which are placed numerous unicellular glands. These glands probably secrete the mucus in which the Nemertine is usually enveloped; their contents when in the body are very highly refracting. The epidermis rests on a basement membrane (b.m).
2. The two or three muscular layers, arranged as either an external circular and an internal longitudinal, or an inner and an outer circular separated by a longitudinal layer, or, as in the figure (c.m and l.m), two longitudinal separated by a circular layer.
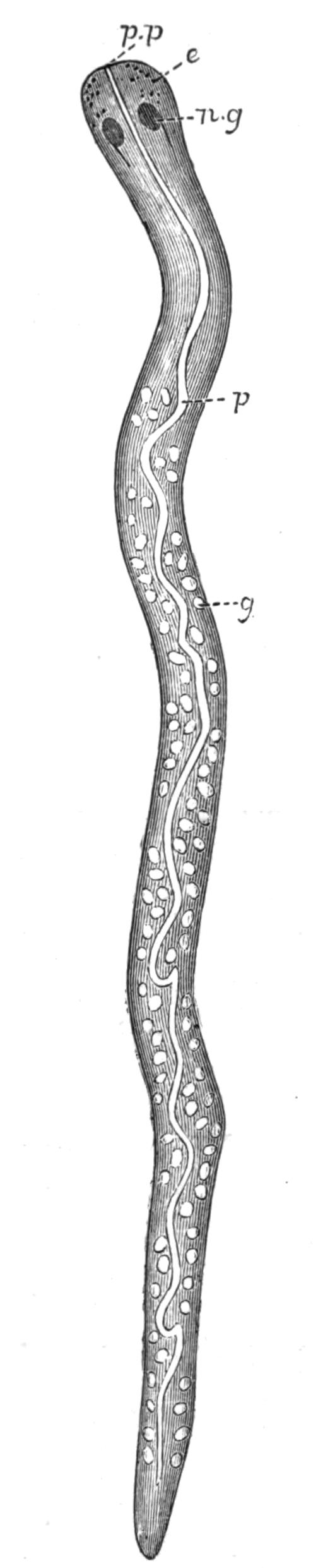
Fig. 51.—Amphiporus lactifloreus Johnst., drawn from the living specimen, from the dorsal surface. Plymouth. × 2. e, Eyes; g, generative organs; n.g, nerve ganglion; p.p, proboscis pore; p, proboscis.
3. A fairly thick connective-tissue layer often found between the epidermis and the muscles, into which latter it gradually merges (s.t).
The Digestive System.—The mouth is placed on the ventral surface near the anterior end of the body (Figs. 53, 58, m). It leads into a straight oesophagus (Fig. 53, oes), whence passes off the intestine (int), which is continued as a straight non-convoluted tube to the anus (a), situated terminally at the posterior end of the body. The intestine is thrown out throughout the greater part of its course into paired lateral pouches.
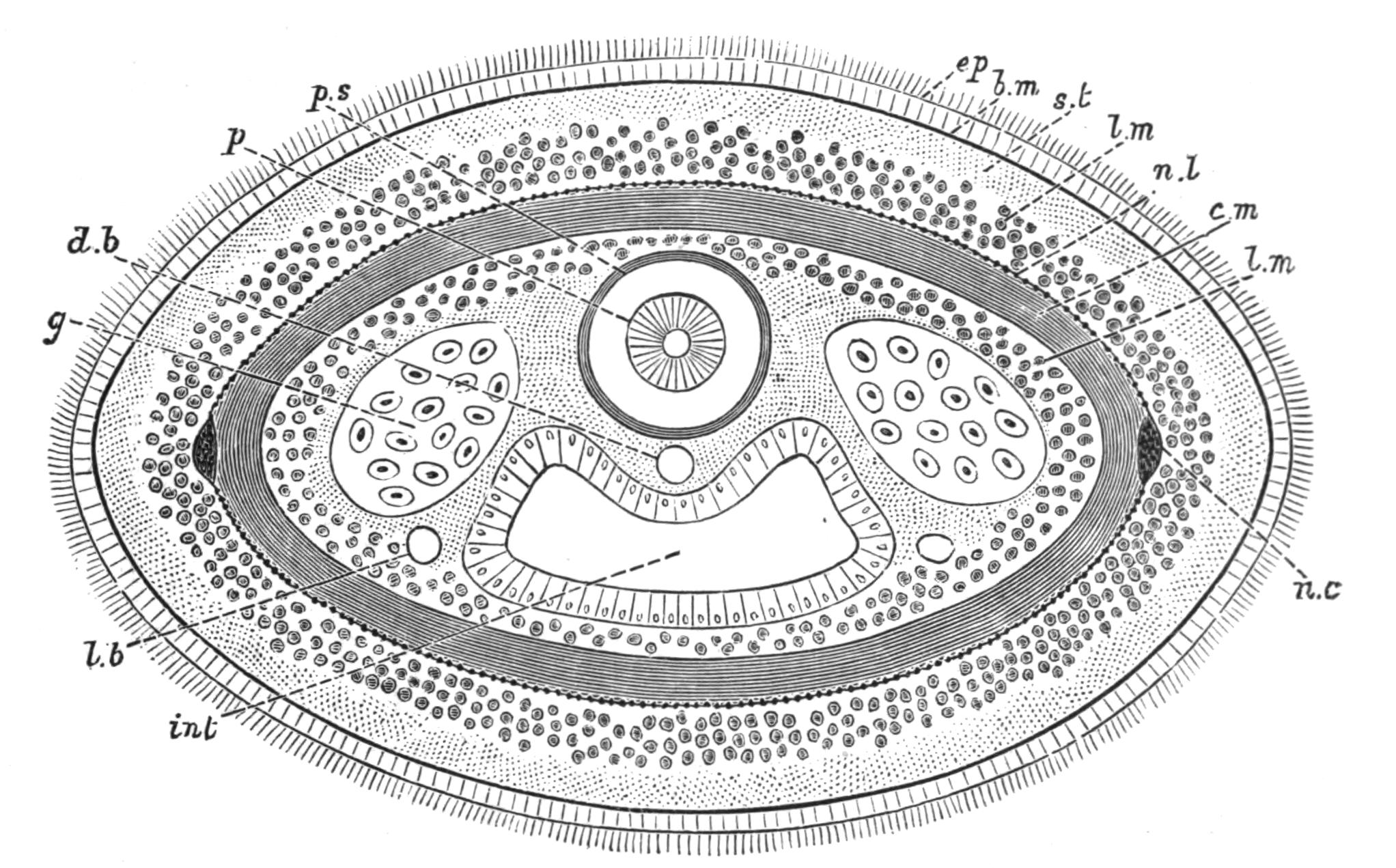
Fig. 52.—Diagrammatic transverse section of a Nemertine (Schizonemertea) through the middle region of the body. b.m, Basement membrane; c.m, circular muscle layer; d.b, dorsal blood-vessel; ep, epidermis; g, generative organs; int, intestine; l.b, lateral blood-vessel; l.m, longitudinal muscle layers; n.c, lateral nerve-cord; n.l, nerve plexus; p, proboscis; p.s, proboscis sheath; s.t, subcutaneous layer.
The alimentary canal is lined throughout by a ciliated epithelium. The oesophagus has, in addition to this layer, an outer thick coat of large granular cells, which probably have a glandular function.
Proboscis.—The most characteristic organ of the Nemertines is the proboscis (Figs. 50, 53, 54). For many years its disposition and function were misunderstood, and it was supposed to be a portion of the digestive system. The proboscis, which lies dorsal to the alimentary canal, opens at the extreme anterior end of the body by a small pore (Figs. 51, 53, 58). When retracted it is sometimes considerably folded, and lies in a long pouch or sheath. To the walls of this sheath it is attached round its anterior {104}end; and strong muscles unite its posterior extremity to the sheath a short distance from the posterior end of the latter.
The proboscis seems to be exclusively a tactile and protective and defensive organ, for which functions it is eminently fitted by the great ease and rapidity with which it is everted or thrust out from the body. It consists of two distinct regions (Fig. 54, g.p and m.p). In the retracted state the anterior part is a hollow tube with very thick muscular walls made up of several layers. At the base of this part in many of the Nemertines there is situated a sharp-pointed spine projecting forward into the lumen, and several smaller stylets situated in a pair of vesicles close to the base of the central spine. The position of the spines in the everted proboscis is shown in Fig. 57. The posterior part of the proboscis is also a tube, but instead of being muscular, its walls are glandular. This posterior glandular part is never everted.

Fig. 53.—Diagrammatic drawing of a Nemertine from the dorsal surface to show the position of some of the principal organs. a, Anus; c.s, cephalic slit; g, generative organs; int, intestine with its lateral diverticula; m, mouth; n.c, lateral nerve-cord; n.g, nerve ganglion; oes, oesophagus; p, proboscis; p.p, proboscis pore; p.s, proboscis sheath.
The eversion is effected by a turning inside out of the anterior part of the proboscis (Fig. 54). The process whereby the proboscis is retracted has been very aptly compared to the effect which would be produced by the inversion of the finger of a glove, accomplished by pulling a string attached to its tip on the inside, the anterior muscular part being comparable to the finger and the glandular part to the string. It is thus obvious that in the everted condition the stylet will form the anterior tip of the {105}proboscis, and will there be in a position for offence or defence (Fig. 57, s).
Nervous System.[131]—The brain is composed of two ganglionic masses (Fig. 53, n.g) lying at the anterior end of the body, one on each side of the proboscis, and united by commissures passing round it (Fig. 55, d.c and v.c). Each ganglionic mass is often partially divided into a dorsal and ventral lobe (n.g.d and n.g.v). From the brain a pair of cords pass off backwards along the sides of the body (n.c); these cords, which have no ganglionic swellings, in some forms unite with one another above the anus. Anteriorly nerves are given off from the brain to the eyes and front part of the head (a.n). A nerve to the proboscis is given off from the commissure which unites the two halves of the brain dorsal to the proboscis (d.n).
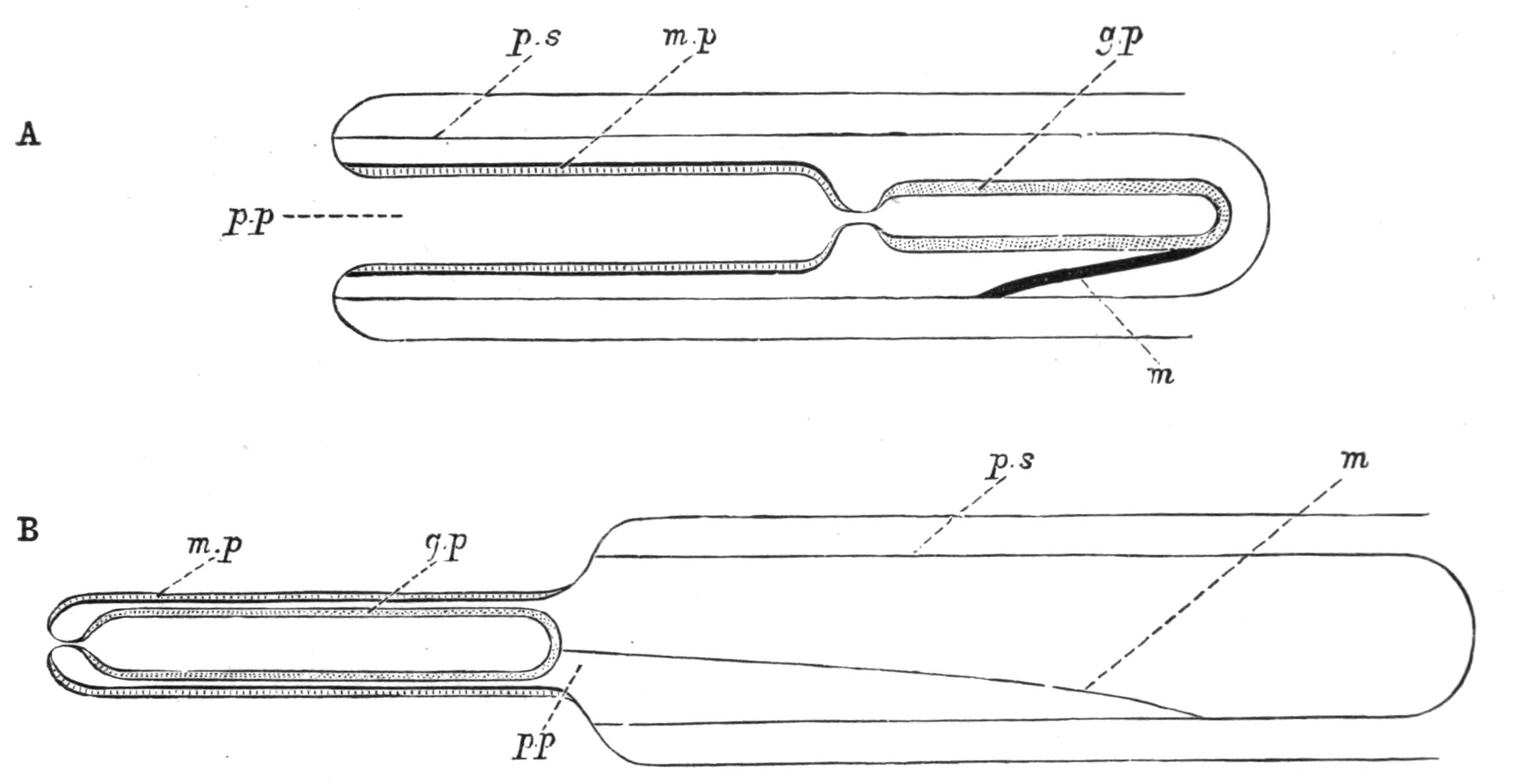
Fig. 54.—Diagrammatic representation of the proboscis, (A) in the retracted condition, (B) in the everted condition. g.p, Glandular portion of the proboscis; m, muscle attaching the proboscis to its sheath; m.p, muscular portion of the proboscis; p.p in A, proboscis pore; p.p in B represents the position of the proboscis pore in the retracted condition of the proboscis; p.s, proboscis sheath.
In two out of the three groups into which the Nemertines are divided, the lateral nerve-cords are in connexion with a network or plexus of nerves lying between the muscular layers of the body-wall (Fig. 52, n.l), and in some forms constituting a comparatively thick layer. In these two groups there are no definite {106}nerve branches except the anterior ones to the head. In the third group of Nemertines the lateral nerve-cords lie within the muscular layers of the body-wall, and in this case paired nerve branches are given off at definite intervals throughout the whole length of the body. These branches divide up among the organs to which they pass, and no nerve plexus is present.
The lateral cords vary in position in different cases. Sometimes they lie laterally, at others the cords tend to approximate to one another in the median dorsal or in the median ventral line, though in every case they remain distinctly separated.
Sense Organs.—Sense organs are usually present in the form of eyes arranged at the sides of the head (Fig. 51, e), sometimes as a single pair and sometimes in one or more groups on each side. The structure of the eyes varies from a simple pigment spot to an organ which receives a special nerve-supply from the brain, and possesses a refracting body answering to a lens, and behind this a pigment layer and a layer of rods. Some forms are devoid of all traces of eyes.
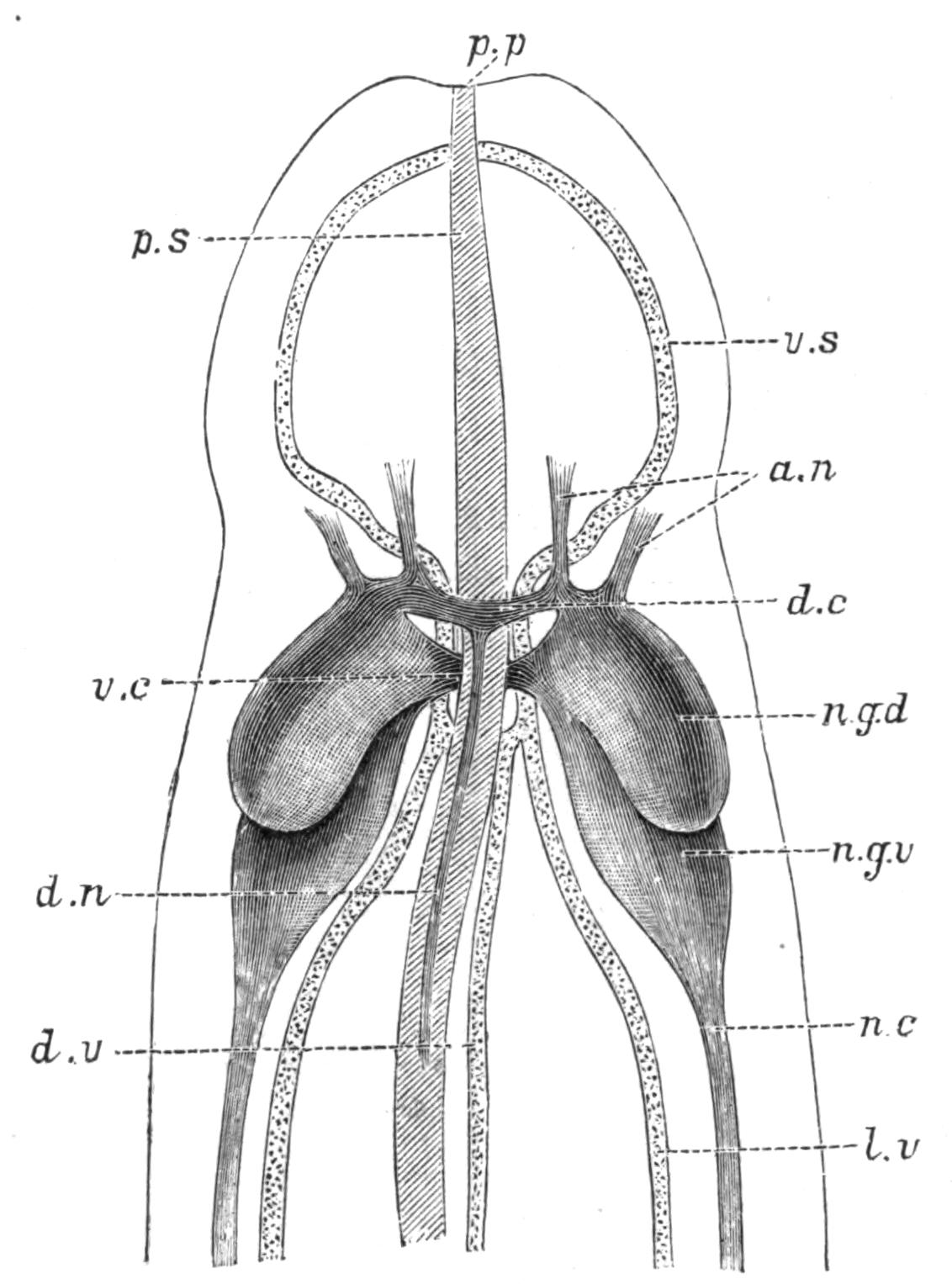
Fig. 55.—Diagram to show the relations of the nervous system, circulatory system, and proboscis sheath in the anterior end of the body in the Hoplonemertea, modified from M‘Intosh. a.n, Nerves to anterior part of body and eyes; d.c, dorsal commissure; d.n, median dorsal nerve; d.v, dorsal vascular trunk; l.v, lateral vascular trunk; n.c, lateral nerve-cord; n.g.d, dorsal lobe of nerve ganglion; n.g.v, ventral lobe of nerve ganglion; p.p, proboscis pore; p.s, proboscis sheath; v.c, ventral commissure; v.s, vascular ring or collar.
A pair of simple auditory capsules has been found in some of the Hoplonemertea, where they occur as small vesicles on the brain.
The whole surface of the body appears to be remarkably sensitive. In a few forms small tufts of tactile hairs are said to be present in the region of the head, while in others there {107}are a few long hairs scattered sparsely among the cilia of the epidermis.
Frontal Organ.—In many Nemertines there is present at the anterior tip of the head a disc-shaped group of cells bearing long hairs or bristles. On this disc open the secreting ducts of a number of gland cells lying in the head. It seems possible that this frontal organ may function as an organ of taste.
Side Organs.—In the Carinellidae there is a pair of circular epithelial patches lying one on each side of the body in the region of the excretory pore. The cells composing them are richly ciliated and provided with a plentiful nerve-supply. The function of these epithelial patches is not known, but it has been suggested that they may be auditory organs.
Cephalic Slits and Cerebral Organs.—In most Nemertines there is a peculiar pair of organs (Figs. 50, 53, c.s), situated in the head and in close connexion with the brain. The function of these organs is not known. Hubrecht has suggested that they may be respiratory, while Bürger[132] conjectures that they may be organs which are used for discriminating the condition of the surrounding medium. In an external examination of the head, the cephalic slits may usually be seen as a pair of lateral furrows or pits. Their form and direction vary considerably; they may take the form of shallow circular depressions, or they may lie longitudinally and be slit-like in shape (Fig. 50), or the slit may lie at right angles to the long axis of the body and be beset with short transverse furrows. In some forms these slits are merely superficial depressions, but in others they are continued into ciliated ducts, which pass inwards and penetrate into special lobes, consisting of glandular tissue and ganglion cells, in close connexion with the brain. These lobes are called the cerebral organs.
In many forms the nervous system is charged with haemoglobin, which gives to it a bright red colour.
Circulatory or Blood-Vascular System.—The circulatory system consists of three main longitudinal vessels, a median dorsal and a pair of lateral ones. These are connected together posteriorly by a transverse trunk, and also throughout the whole length of their course by branches, which are given off at regular intervals. Anteriorly the three longitudinal vessels {108}either all unite and form a collar (Fig. 55, v.s) round the oesophagus, or they break up into a number of lacunar or open spaces in free communication with one another.
The blood is usually colourless, but in some cases the corpuscles are coloured red by haemoglobin.
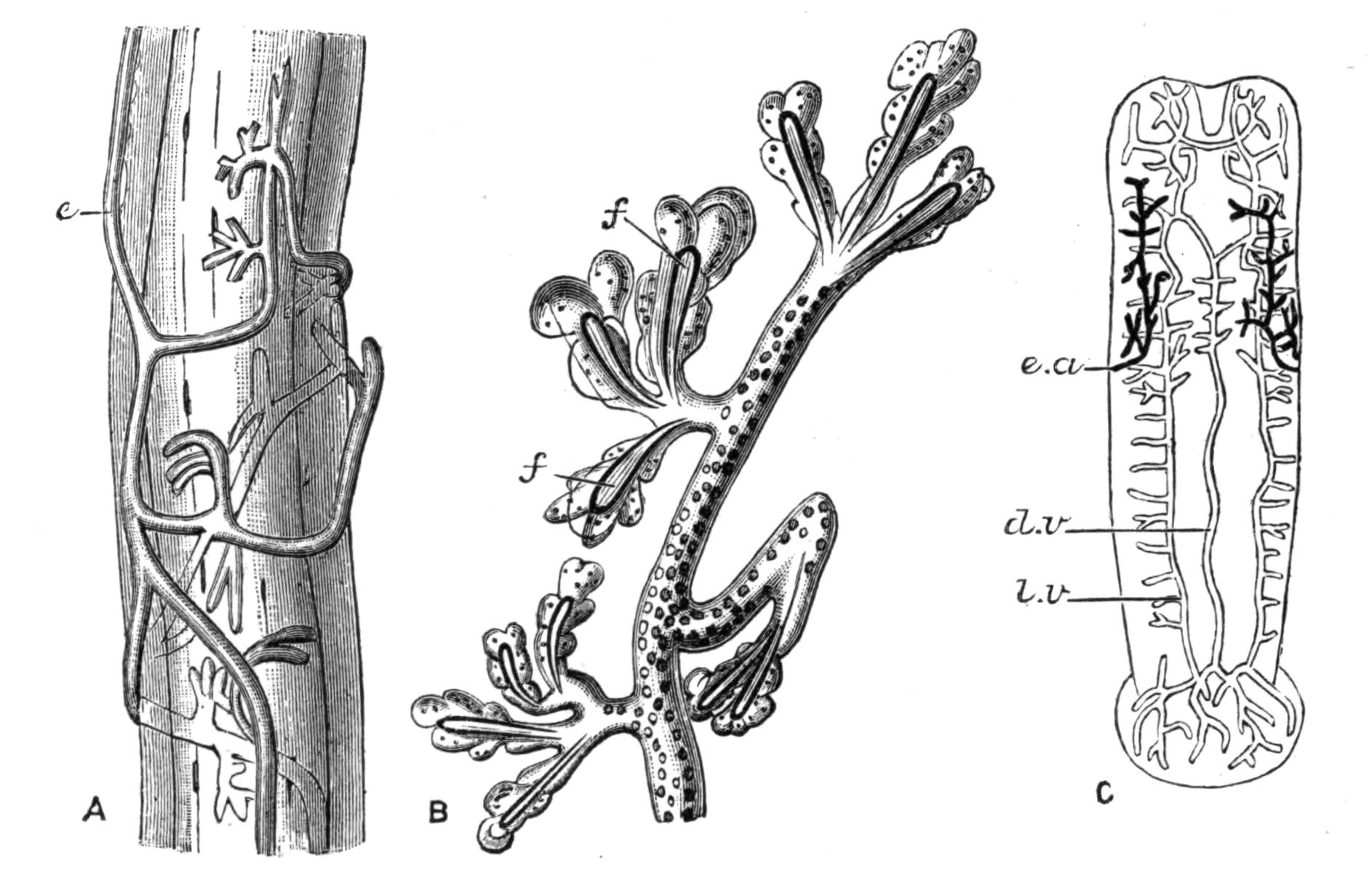
Fig. 56.—Excretory system of Nemertines. A, Drepanophorus spectabilis Qtrf., part of one of the lateral vessels encircled by branches of the excretory organ, × 585; e, main canal of the excretory system: B, D. crassus Qtrf., a terminal branch of the excretory system, × 585; f, ciliated flame: C, Malacobdella grossa O. F. Müll., entire animal, slightly magnified, showing the excretory system (black) and the vascular system; e.a, excretory aperture; d.v, dorsal vessel; l.v, lateral vessel. (From Bürger.)
Excretory System.—Max Schultze[133] found in Tetrastemma obscurum, on the outer side of, but near to the lateral blood-vessels, a pair of canals. He observed ciliary movements in the canals, but could not discover flame cells. Further contributions to our knowledge of the excretory system were made by Semper,[134] von Kennel,[135] Hubrecht,[136] and Oudemans.[137] The latter states that the excretory system consists of a pair of canals situated laterally near the anterior end of the body. Each canal communicates with the exterior by one or more ducts having lateral regularly-arranged apertures. In some cases he was unable to make out any communication with the vascular system, but in others {109}a direct communication, by means of open connexions with the lacunar blood spaces, is said to occur.
Silliman[138] in Tetrastemma aquarum dulcium describes the excretory vessels as ending in numerous capillary branches, at the blind terminations of which cilia are present. He states that there is no important difference between the excretory systems of Rhabdocoeles and Nemertines.
Bürger,[139] as the result of recent investigations on the excretory system in Nemertines, finds that the minute branches end in flame-cells (Fig. 56, B) lying on and among the blood-vessels, but having no open connexion with them.
Generative System.—The Nemertines are for the most part dioecious, only a few certainly hermaphrodite species having been described, e.g. Tetrastemma ("Borlasia") kefersteinii Mar.[140]
The generative products in both cases are contained in sacs (Figs. 52, 53, g) which lie in the lateral region of the body between the pouches of the alimentary canal. The ova and spermatozoa are conveyed to the exterior by short ducts. Most species are oviparous, though a few viviparous species are known (e.g. Prosorhochmus claparedii).
Classification.—Nemertines were divided by M. Schultze[141] into:—
1. Enopla, in which the proboscis is armed with stylets.
2. Anopla, in which the proboscis is unarmed.
Although this classification was fairly correct as far as it went, since many other distinctive features were correlated with the presence or absence of armature in the proboscis, still there are several primitive forms belonging to the Anopla, which possess characters such as render it necessary to class them together in a separate group.
For this reason Hubrecht divided the Nemertinea into three Orders—Hoplonemertea, Schizonemertea, Palaeonemertea; the first of these Orders corresponding with the Enopla, and the other two with the Anopla.
Order I. Hoplonemertea.
The proboscis is armed. The epidermis rests on a thick layer of connective tissue plentifully supplied with glands, below which is a prominent basement membrane. The muscular layers of the body are two in number, an outer circular and an inner longitudinal. The nerve-trunks lie within the muscular layers of the body and give off regularly-arranged branches. There is no nerve plexus. Each of the cephalic slits generally opens by a pore situated in the centre of a transverse groove, which is beset along one side by a row of shorter grooves at right angles to it. The apparatus consists of a ciliated duct surrounded by nerve tissue, and passing into lobes of tissue which are connected with the brain by thick nerve-cords. The mouth opens rather far forward in front of the brain. The intestinal pouches are symmetrically arranged. Auditory organs are said to exist in some forms, consisting of vesicles containing otoliths. The vascular trunks are connected anteriorly by closed vessels and not by lacunar spaces.
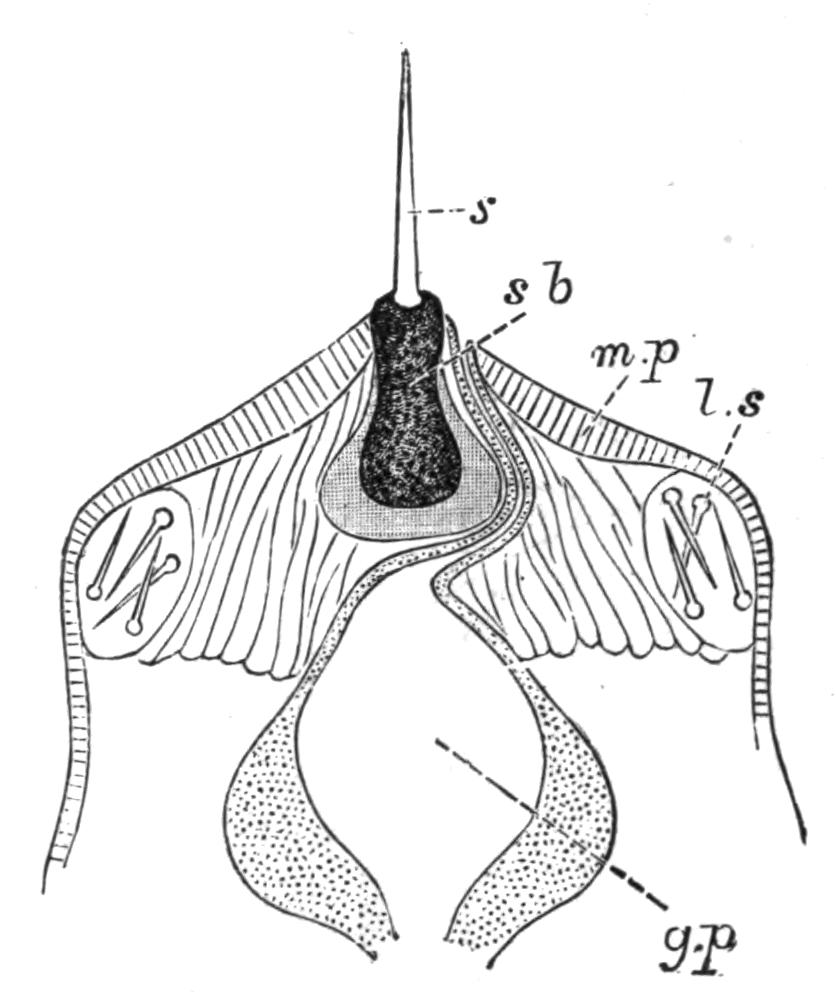
Fig. 57.—Anterior end of the everted proboscis (Hoplonemertea). g.p, Glandular portion of the proboscis; l.s, lateral sacs containing stylets; m.p, muscular portion of the proboscis; s, stylet; s.b, granular basal portion of stylet.
The principal British genera and species[142] are:—
Amphiporus bioculatus M‘Int., A. dissimulans Riches, A. hastatus M‘Int., A. lactifloreus M‘Int., A. pulcher Johnst.
Drepanophorus rubrostriatus Hubr. (= A. spectabilis Qtrf.).
Tetrastemma ambiguum Riches, T. candidum O. F. Müll., T. dorsale Abildg., T. flavidum Ehrenb., T. immutabile Riches, T. melanocephalum Johnst., T. nigrum Riches, T. robertianae M‘Int., T. vermiculatum Qtrf.
Prosorhochmus claparedii Keferstein.
Nemertes carcinophila Köll., N. gracilis Johnst., N. neesii Oerst.
Malacobdella grossa O. F. Müll.
Order II. Schizonemertea.
The proboscis is unarmed. The epidermis is separated from the layer of connective tissue by a thin basement membrane, hence the glands in the connective tissue are more deeply situated and have long ducts. The muscular layers are three in number, an outer and an inner longitudinal layer between which lies a layer of circular muscles. The lateral nerve-cords lie between the outer longitudinal and the circular muscle layers. They are connected throughout the body by a nerve plexus, the only definite nerve branches given off being those to the brain, oesophagus, and proboscis. The cephalic slits are a pair of deep longitudinal grooves at the sides of the head. From each groove a canal passes inwards into a posterior brain-lobe. The mouth opens behind the brain, and is an elongated slit bounded by corrugated lips. Auditory organs have not been observed. The longitudinal vascular trunks are connected anteriorly by lacunar spaces, and not by closed vessels.
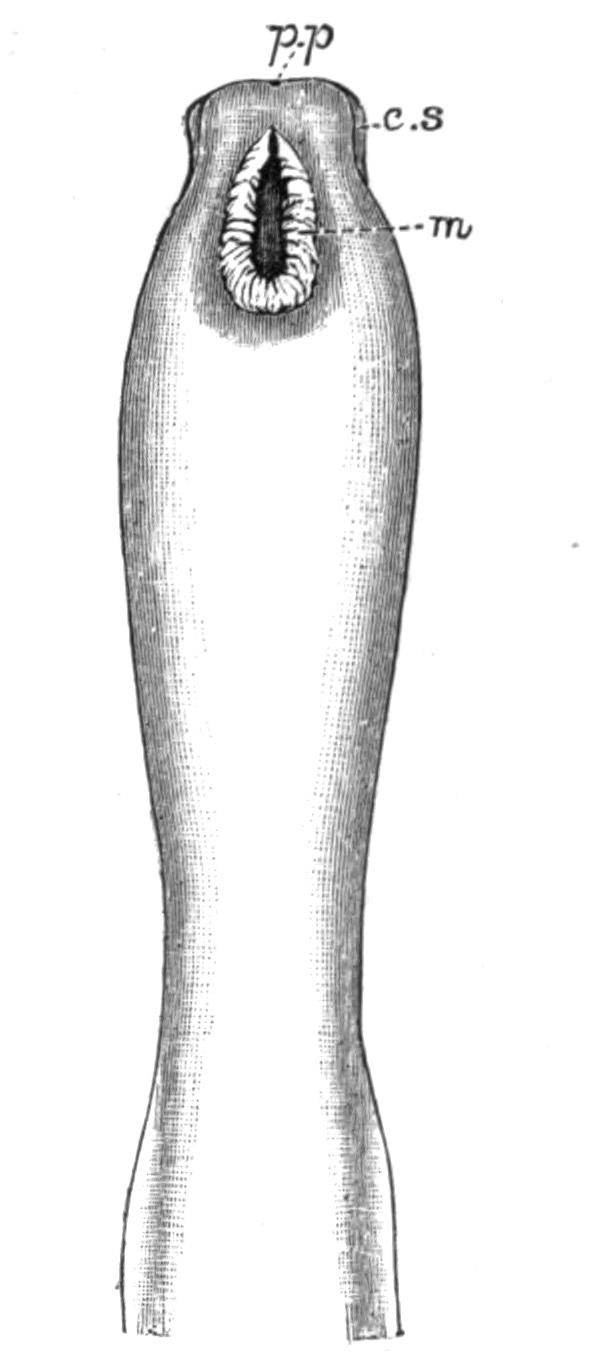
Fig. 58.—Head end of Cerebratulus marginatus Ren., from the ventral surface. Drawn from a spirit specimen. Naples. × 1. c.s, Cephalic slit; m, mouth; p.p, proboscis pore.
Principal British genera and species:—
Lineus bilineatus Ren., L. lacteus Mont., L. marinus Mont. (= L. longissimus Gunnerus), L. gesserensis O. F. Müll. (= L. obscurus Desor and L. sanguineus M‘Int.).
Borlasia elizabethae M‘Int.
Cerebratulus angulatus O. F. Müll., C. fuscus M‘Int., C. pantherinus Hubr.
Micrura aurantiaca Grube, M. candida Bürger, M. fasciolata Ehrenb., M. purpurea J. Müll.
Meckelia asulcata M‘Int.
Order III. Palaeonemertea.
The proboscis is unarmed. The epidermis and connective tissue form one layer, below which is the basement membrane. The muscular layers are three in number, two circular separated by a longitudinal layer. The nerve-cords lie altogether external {112}to the muscular layers, and are connected together throughout by a plexus. No nerve branches are given off. The brain is not divided into lobes. The cephalic slits are only represented by a shallow depression on each side of the head, and no canals have been observed leading from them. The intestine is straight, and the pouches are usually absent or rudimentary. The circulatory system is largely made up of lacunar spaces, the closed system being but little developed.
Principal British genera and species:—
Carinella annulata Mont., C. linearis (Mont., MS.) M‘Int., C. macintoshi Bürger (Fig. 59), C. polymorpha Ren.
Cephalothrix bioculata Oerst., C. linearis Rathke.
Valencinia lineformis M‘Int.
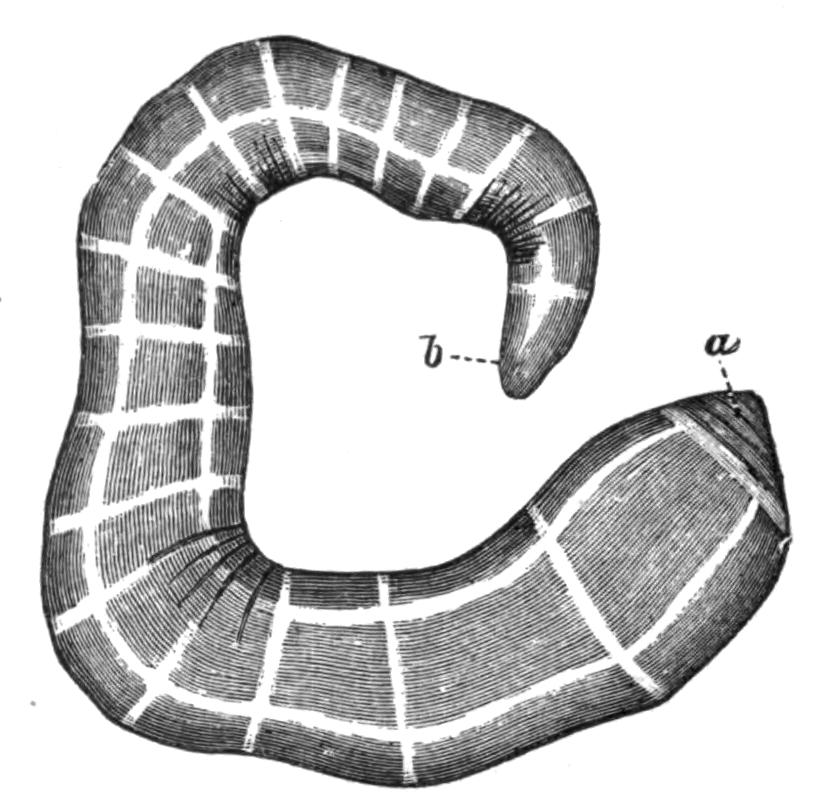
Fig. 59.—Carinella macintoshi Bürger, drawn from the living specimen, slightly contracted. Plymouth. Considerably magnified. a, Anterior end; b, posterior end.
A most important monograph by Bürger[143] on Nemertines has just been published, but unfortunately it appeared too late to be adequately considered here. He gives an elaborate account, illustrated by admirable figures, of the present state of our knowledge of this group, and his work will be indispensable to future students of the subject. The older systems of classification are criticised, and the following scheme is adopted in their place:—
Order I. Protonemertini (= part of the Palaeonemertea, e.g. Carinella).—The brain and lateral nerve-cords lie outside the muscle layers in the epithelium or below the basement membrane. The body-wall consists of the following layers: epidermis, basement membrane, circular muscles, and longitudinal muscles. The mouth lies behind the brain. The proboscis is unarmed.
Order II. Mesonemertini (= part of the Palaeonemertea, e.g. Cephalothrix).—The characters of this Order are similar to those of the Protonemertini except that the brain and lateral nerve-cords lie in the muscle layers.
Order III. Metanemertini (= Hoplonemertea).—The brain and lateral nerve-cords lie in the parenchyma of the body internal to the muscle layers. The layers of the body-wall are {113}similar to those of the Protonemertini. The mouth lies in front of the brain. The proboscis is armed. At the junction of the fore- and mid-gut a diverticulum is given off which projects forwards beneath the fore-gut and ends blindly in front.
Order IV. Heteronemertini (= Schizonemertea, and the genera Eupolia and Valencinia, placed provisionally by Hubrecht in the Palaeonemertea).—The body-wall consists of the following layers: epidermis, thick cutis, and an outer and an inner longitudinal muscle layer separated from one another by a circular muscle layer. The brain and lateral nerve-cords lie between the outer longitudinal and the circular muscle layers. The mouth lies behind the brain. The proboscis is unarmed.
Development of the Nemertinea.—The development of the Palaeonemertea is at present not known: in the Schizonemertea a larval stage occurs; while in the Hoplonemertea the egg develops directly without undergoing any metamorphosis.
There are two forms of larva characteristic of the Schizonemertea, known respectively as Pilidium and the Type of Desor. The Pilidium is hatched early and leads a free-swimming existence, whereas the Type of Desor, though in many respects resembling it, never passes through the free-swimming phase.
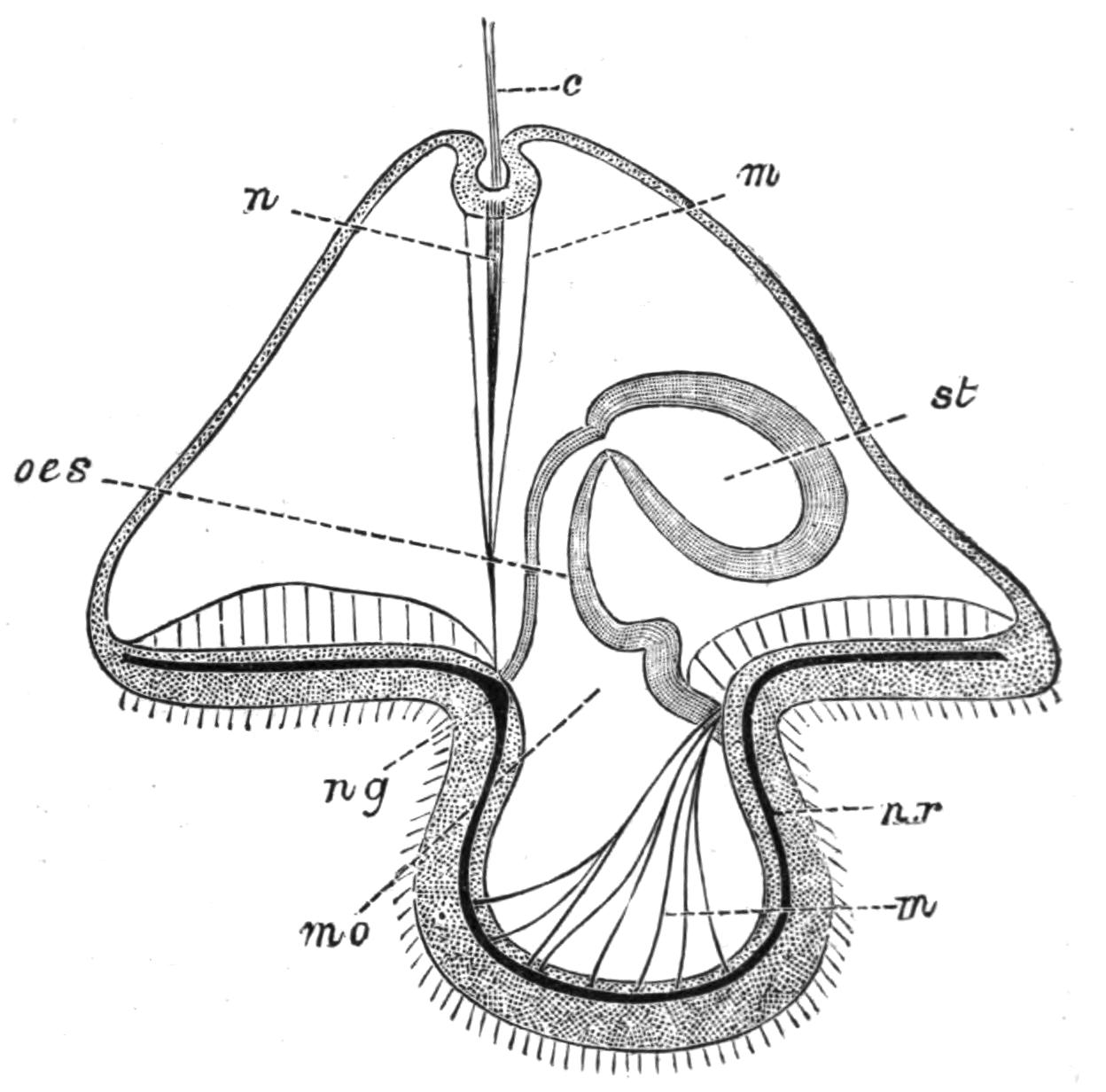
Fig. 60.—Diagram of a Pilidium larva. (After Salensky.) c, Tuft of cilia; m, muscle-fibres; mo, mouth, seen through one of the lateral lobes; n, nerve-fibres; n.r, nerve-ring; n.g, nerve ganglion; oes, oesophagus; st, stomach.
The Pilidium (Fig. 60) is a helmet-shaped larva bearing a tuft or spike dorsally, and prolonged downwards laterally into a pair of lobes. The whole larva is covered with cilia, there being a specially strong band round its ventral surface. The dorsal spike is composed of a bunch of strongly developed cilia or of a long flagellum. The alimentary canal consists of a sac constricted into {114}oesophageal and gastric regions (Fig. 60, oes and st). In this condition the larva swims about freely in the water. The helmet-shaped Pilidium-skin forms no part of the future Nemertine, the skin of which is developed as ingrowths from it; these meet one another and unite to form a complete covering round the alimentary canal; the larval skin is then cast off, and by a series of gradual steps the embryo develops into the adult.
Habits.—Nemertines are often found under stones between high- and low-water marks, lying on sandy or muddy bottoms. They are usually in the form of coiled masses, and are generally in a state of quiescence. Hence it is probable that their period of activity is during high-water, and that when left by the receding tide they subside into a resting condition.
The large kinds, such as Lineus marinus, seem to be always found living alone, but some of the smaller kinds, notably Tetrastemma dorsale and Prosorhochmus claparedii, have gregarious habits and live in masses, the coils of the different individuals being inextricably mixed.
Some species, such as Micrura purpurea, Amphiporus pulcher, and Cerebratulus angulatus, frequent empty bivalve shells, while Nemertines are often found in empty limpet shells adhering to rocks in tidal pools. Other smaller forms resort to no such definite protection, but live among seaweeds; some of these remain naked, while others secrete for themselves tubes of a membranous or gelatinous consistency. Borlasia elizabethae lives in a burrow of clay.
Nemertines are commonly dredged from a depth of six or eight fathoms. They may sometimes be found floating on the surface of the water, and some possess the power of swimming rapidly, propelling themselves by a lateral motion of the tail, the sides of which are in such cases prolonged into a thin fin-like edge. This mode of progression is usually adopted by those which frequent deep water. A pelagic Nemertine (Pelagonemertes) was discovered by Moseley near the southern verge of the South Australian current, being found in a trawl with deep-sea forms from a depth of 1800 fathoms. This animal was leaf-like in shape, bluntly pointed behind and rather square in front.
The power possessed by Nemertines of secreting mucus is very great, their course being often traceable by the tracks which they leave behind them. Many of them glide along with great rapidity, a mode of progression which is probably due to the {115}cilia covering the whole outer skin, and to the extreme contractility of the muscles of the body-wall. In some locomotion is effected by the proboscis; this is protruded and attaches itself by means of its spines to some foreign body, after which the body is drawn up after it. This has been specially observed in a land form, Tetrastemma agricola, discovered by Willemoes-Suhm in the Bermudas. On solid bodies the movement is a kind of crawling action, the head and mouth acting as suckers in much the same way as in many Leeches.
Most Nemertines can be very readily kept in confinement. The chief apparent effect of such a life is a loss of colour, the animal gradually becoming pallid in hue. Owing also to the absence of proper food they diminish very much in size, though even when all food is kept away an animal will sometimes continue to live as long as eighteen months.
Food.—Nemertines are carnivorous in their habits and are very voracious, devouring any prey which comes in their way, whether it be living or dead. No animal food seems to come amiss to them, and they will devour creatures of considerable size. When in contact with its prey, the Nemertine dilates its mouth to a large extent, and the anterior end of the oesophagus is thrust out and engulfs the animal. Chaetopods form a favourite food material, the whole animal being swallowed quite regardless of the hard chitinous bristles and spines with which it is beset. The soft parts are gradually digested, the bristles and other indigestible portions being extruded by the anus. The larger spines often pass out by perforating passages through the wall of the intestine and through the body-wall. The aperture thus formed appears speedily to heal after the foreign body has been extruded.
The carnivorous habits of Nemertines even extend to cannibalism, and when kept in confinement they frequently devour one another. For this reason it is unsafe to keep large and small kinds together, as the small ones speedily disappear, being used as food material by the large. If one be divided into several pieces, the pieces are very rapidly demolished by other individuals.
Regeneration.[144]—This power is, no doubt, of great service to these animals, since injury, or even violent local irritation, often causes complete rupture at the point affected. It seems that the {116}chief power of regeneration is situated in the head, as, if a very short piece be broken off the anterior end of the body, it very rapidly reproduces itself into a new individual. The hind end of the original body often lives for a considerable time, but it does not in most cases appear to possess the power of reproducing a head, and after existing for a time it dies. For a while, however, it so far retains its vital powers that the generative products continue to grow, and actually attain to perfection. Severe wounds also heal very quickly and completely, and all local injuries are speedily repaired.
Owing to the force with which it is shot out, the proboscis is often completely severed from the body, and in such a case the animal grows a new one in an extremely short space of time. The proboscis thus broken off retains its power of movement and contractility for a considerable time, and has been more than once mistaken for a worm. This great vital power is probably due to the great development of nervous tissue, the proboscis being usually richly supplied with nerve plexuses.
One large form, Lineus sanguineus, seems to possess great recuperative powers. It shows a marked tendency to break up into pieces, when not only the head end, but also the other portions develop into perfect animals, each one growing a head and all the organs belonging to it. Thus in this case an animal may multiply by a simple process of transverse fission, and form numerous complete individuals.
Breeding.—The breeding season only appears to cease in the extreme of winter. Different genera and species seem to mature their generative products at different times.
In the armed Nemertines the eggs are deposited separately, and are not connected together except by such accidental mucus as the animal deposits normally; but in the unarmed a special mucous secretion forms a thick investment for the eggs.
M‘Intosh[145] has observed the process of the deposition of the male and female products in Nemertes gracilis. He put into a glass vessel a male and female of this species in which the products were apparently ripe. Soon spermatozoa began to issue in wreath-like jets from the body of the male, at first from the middle region of the body, and afterwards anteriorly and posteriorly, until the animal was enveloped in a dense cloud of {117}spermatozoa. The whole process only lasted a few minutes. When all the spermatozoa had apparently been given out, the female was seen to protrude her head from the sand; she then passed to the side of the vessel and deposited a group of eggs about three inches distant from the spermatozoa.
With only a few exceptions Nemertines are oviparous. Prosorhochmus claparedii, Tetrastemma obscurum, and Monopora vivipara have been observed to contain embryos at certain times of the year. In other forms the eggs are laid when ripe, and development takes place subsequently to their deposition.
Geographical Distribution.—Nemertines have been found in all seas from the arctic to the equatorial regions. Many forms are found in the British Isles both between tide-marks and also at greater depths around our coasts. Some genera seem to be confined to warm climates and others to cold; while others appear to be indifferent to climate, and to subsist equally well under very various degrees of temperature. So far as is known, the land forms are all indigenous to warm countries.
Land Forms.—Land forms, which occur on or in moist earth under stones or decaying vegetable matter, have been discovered and described by Semper,[146] Willemoes-Suhm,[146] and von Graff.[146]
The species found by Semper, and called by him Geonemertes palaensis, lives under damp leaves and the roots of trees on Pelew Island in the North Pacific. It is about 2 inches long, of a reddish-white colour, with narrow, brownish-black, longitudinal stripes on its dorsal surface. It possesses six eyes and very small cephalic slits and cerebral organs. The proboscis is armed, and opens by the mouth instead of by a special pore.
The same peculiarity as to the opening of the proboscis is found in Geonemertes chalicophora, discovered by von Graff in pots of Corypha australis in the palm-house at Frankfurt-on-Main. He found specimens on and beneath the surface of the earth. As it was only found in pots in which this Australian plant was growing, von Graff thought it almost certain that it was a native of Australia. Those found below the surface of the earth were surrounded by a transparent tube in which particles of earth were embedded. The animal is small, only about two-fifths of an inch in length. The colour is milk-white, with a small quantity of red pigment anteriorly: there are four eyes, and the cephalic slits are absent.
The species which was discovered by Willemoes-Suhm, and named by him Tetrastemma agricola, lives under stones in damp earth in the Bermudas. It differs from the other two in that the proboscis opens by a special terminal aperture. It measures nearly an inch and a half in length, and, like G. chalicophora, is milk-white in colour. It resembles it also in possessing four eyes, and in the absence of cerebral organs and cephalic slits.
Fresh-water Forms.—In most cases the descriptions of fresh-water forms are so vague and incomplete that it is difficult to determine whether or not they are different species.
They are probably more numerous than is at present known, and are certainly scattered widely over the face of the earth, since they have been found in Nicaragua, at Tashkend in Turkestan, and at Philadelphia and Monroe in the United States.
A form of which we have a full description is Tetrastemma aquarum dulcium, found by Silliman[147] at Monroe, under stones in brooks in company with Planarians. It is a small worm of a red or pink colour, about half an inch in length, and it possesses usually three pairs of eyes. The proboscis is armed, and opens by a separate aperture. The excretory system consists of a vessel on each side of the body, each opening externally by a pore, and internally dividing into numerous branches which end in ciliated expansions. An individual of the same species was found by Beddard in one of the tanks in the Botanical Gardens in Regent's Park, but as the tank is one in which tropical plants are grown, it had almost certainly been introduced among the roots of the plants, and cannot be considered as a British species.
A fresh-water Nemertine belonging to the genus Tetrastemma was, however, found by Benham[148] on the roots of some water plants in the Cherwell at Oxford. The specimen was of a bright orange colour and measured half an inch in length.
Du Plessis[149] found another fresh-water form on the lower surface of stones in shallow pools on the shores of the Lake of Geneva, and named it Tetrastemma lacustre. It is a small animal, the largest specimens being rather over an inch in length.
Another European genus was found in 1893 by F. E. Schulze in Berlin. It has been fully described by T. H. Montgomery,[150] who has given it the name of Stichostemma eilhardii.
Parasitic Forms.—The genus Malacobdella was found by von Kennel[151] in large numbers living on Cyprina islandica, a Lamellibranch Mollusc, in the harbour at Kiel; and it has also been described by Riches[152] as a British form. It is attached to its host by means of a large round sucker situated at the posterior end of the ventral surface, while the rest of the body waves about freely in the mantle-cavity. It is perhaps hardly correct to describe this animal as parasitic, since it does not appear to obtain its nutriment at the expense of the host by preying on its juices. The advantage of its position is, however, obvious, since a perpetual current of water is kept up in the mantle-cavity of the Mollusc, and from the stream the Nemertine is able to pick out and take for itself any food material which it considers suitable. At the same time it is not subjected to the influence of the winds and waves, as the shell of the mollusc acts as a barrier to prevent the entrance of disturbing elements.
Malacobdella is short and broad, somewhat flattened dorso-ventrally. The anterior end is bluntly rounded. The mouth opens into a wide pharynx, which is constricted behind and then passes into the intestine; this after a few coils opens by the anus situated dorsally immediately above the sucker. The proboscis opens into the pharynx.
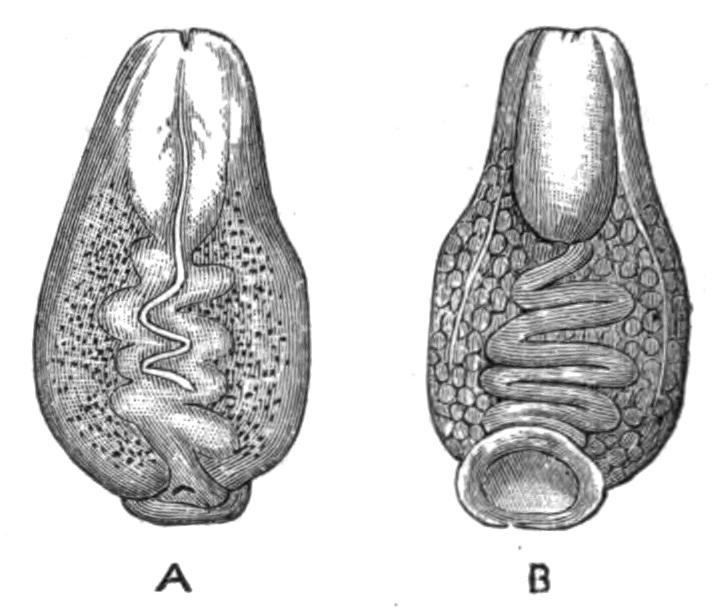
Fig. 61.—Malacobdella grossa O. F. Müll., a large female specimen. Kiel. × 1. (From von Kennel.) A, From the dorsal surface; B, from the ventral surface.
Palaeontology.—Nemertines are unknown in a fossil state; this is probably owing to the softness of their bodies, which would render their preservation extremely improbable.
Affinities.—Until recently the Nemertines were regarded as a sub-order of the Turbellaria. They were afterwards separated from the Turbellaria and placed as a distinct class of the phylum Platyhelminthes.
Some zoologists have considered them to be so different in many respects from the other classes of the Platyhelminthes as to justify their being altogether separated from that phylum, and treated as a distinct group.
If, however, the recent work of Bürger on the excretory system is to be relied upon, the existence of flame cells would be a strong reason for classing them among the Platyhelminthes.
Hubrecht[153] has instituted an interesting comparison between Nemertines and Vertebrates. He compares the median dorsal nerve of Nemertines to the spinal cord of Vertebrates; the lateral nerve-cords to the nerve of the Vertebrate lateral line; and the lateral swellings which constitute the brain in Nemertines to the lateral ganglia of the cephalic region in Vertebrates. This view is strengthened by the existence of transverse nerves connecting the lateral and dorsal nerves of Nemertines, since these may be compared with the spinal nerves of Vertebrates. He suggests that both Nemertines and Vertebrates may have arisen from a vermiform animal possessing a nervous layer in the form of a plexus of nerve-fibres, the nerve tissue having become concentrated along three lines to form a median dorsal and two lateral nerve trunks; the former being specially developed in the Vertebrata and the latter in the Nemertines. Hubrecht further suggests that the notochord of Vertebrates may be a survival of the proboscis sheath of Nemertines, while the proboscis of the latter may be represented by the invagination to form the pituitary body in Vertebrates.
Certain authors[154] have suggested that indications exist of a relationship between Nemertines and Balanoglossus.
The features which are supposed to indicate this are the elongated vermiform shape showing no external signs of segmentation; the ciliated smooth skin and the possession of unicellular mucous glands; and the protrusible proboscis, which may be comparable to the non-retractile proboscis of Balanoglossus, a comparison which is strengthened by the fact that in some Nemertines a sheath of nerve-fibres exists in the wall of the proboscis corresponding to the nerve plexus in the proboscis of Balanoglossus. In both cases an ectodermic nerve plexus exists with local thickenings along definite lines, although these lines are not the same in the two cases. Both possess a straight alimentary canal, ending in a terminal anus and thrown out into paired lateral caeca, between which are the paired metamerically-arranged generative sacs.
BY
ARTHUR E. SHIPLEY, M.A.
Fellow and Tutor of Christ's College, Cambridge.
NEMATHELMINTHES
INTRODUCTION—NEMATODA—ANATOMY—EMBRYOLOGY—CLASSIFICATION—ASCARIDAE—STRONGYLIDAE—TRICHOTRACHELIDAE—FILARIIDAE—MERMITHIDAE—ANGUILLULIDAE—ENOPLIDAE—PARASITISM—NEMATOMORPHA—ANATOMY—CLASSIFICATION—LIFE-HISTORY—ACANTHOCEPHALA—ANATOMY—EMBRYOLOGY—CLASSIFICATION.
The Nemathelminthes include three sub-Orders of very different size and importance. These are—
i. The Nematoda.
ii. The Nematomorpha (Gordiidae).
iii. The Acanthocephala.
Although the members of these groups differ considerably from one another, on the whole there is a closer resemblance between them than between any one of them and any other group of animals, and there is a certain convenience in arranging them under one head.
The following characteristics are common to all three groups of the Nemathelminthes: they are worm-like in form, and with few exceptions are parasitic in the bodies of other animals, either Vertebrate or Invertebrate. Some of them spend their whole existence within the bodies of their hosts, but more commonly they are only parasitic during a certain period of their life; a few, however, lead a free life in water or in damp earth. None of the Nemathelminthes are segmented—that is, their bodies are not divided into a number of parts which serially repeat each other, and which resemble more or less closely the preceding and {124}succeeding parts. They are not provided with any appendages or limbs, but sometimes bear a few bristles or hooks, and in rarer cases suckers. The body, which is elongated and, as a rule, thread-like and tapering at each end, is enclosed in a thick cuticle or hardened secretion of the underlying cells. In no Nemathelminth is there any closed vascular system, nor are special respiratory organs developed.
In many respects the most remarkable peculiarity of these animals is that, with the possible exception of the excretory organs of the Acanthocephala, there is a complete absence of cilia throughout the whole group. In this respect they resemble the Arthropoda. The universal presence of these small flickering processes of cells from man down to the simplest unicellular organisms makes the absence of these structures most remarkable. In many animals they are the sole organs of locomotion, and in almost all they perform very important functions, both in bringing food and oxygen to the body, and in removing waste matter from it. At present there seems to be no adequate explanation for their absence in the two large groups mentioned above.
Nemathelminthes are, with hardly an exception, dioecious—that is to say, their male and female reproductive organs are in different individuals. Their young do not differ markedly from the adults, except in the absence of sexual organs, but the immature stages are usually termed larvae, and not infrequently either inhabit a different host from the adult, or are free when the adults are parasitic, or vice versâ.
Sub-Order I. Nematoda.
Anatomy.—The Nematode worms, or thread-worms, form by far the largest and most important division of the group Nemathelminthes. The number of species is great, and although the conditions under which they live are of the most varied kind, there is, as a rule, little corresponding difference in structure, and hence the determination of the species is attended with no small difficulty.
With few exceptions the shape of the body is filiform (Figs. 66 and 71), the two ends being more or less pointed, and the posterior end of the male, which is generally a smaller animal than the female, is usually slightly recurved. The worms are, as a rule, {125}white, or of the colour of polished ivory; they may be opaque or semi-transparent, but pigment spots are rarely developed.
Minute Nematodes abound in moist soil, around the roots of plants, etc., and may easily be detected with the aid of a lens wriggling about amongst the particles of sand and earth. Of the animal parasites perhaps the most familiar is the "round worm" (Ascaris lumbricoides, Figs. 66 and 67), which inhabits the alimentary canal of man; others are common in domesticated animals, as A. mystax in the cat and dog, and A. megalocephala in the horse and ox. They are also found living parasitically in plants (Fig. 77), causing the formation of galls and other pathological growths; Anguillula (Tylenchus) tritici causes in this way considerable damage to corn, and others attack root-crops, cabbages, etc. The "vinegar eel" (Anguillula aceti), which occurs so often in weak vinegar, is another familiar example of this group.
The Skin.—The body of the worm is encased in a relatively thick, transparent, smooth cuticle, which is turned in at the various apertures, and lines the tubes connected with them for a greater or less distance. The cuticle is in some cases raised to form spikes or hooks, and in certain species, e.g. Ascaris mystax and A. transfuga, it is produced into two lateral fins, which are supported by a thickened triradiate rod of specialised cuticle (Fig. 62); these fins, however, do not run far down the body. As a rule the cuticle is quite smooth, but it may be ringed, as in Filaria laticaudata and in F. denticulata; and the rings may bear backwardly-projecting teeth.
The skin of Nematodes consists of three layers—(i.) the above-mentioned cuticle, which is presumably secreted by (ii.) the sub-cuticle or epidermis which underlies it; the latter surrounds in its turn (iii.) the muscular layer.
The nature of the sub-cuticle is one of the debateable points in the morphology of the Nematoda. No cell outlines have been detected in it, although nuclei are scattered through it; it is in fact a syncytium, or protoplasmic mass in which cell limits cannot be distinguished. Many of the cells forming it have broken down into fibrils, and these form a close meshwork, which is occasionally specialised, as, for instance, round the nerve-cords. Along the median dorsal and ventral lines, and along the lateral lines, this tissue is heaped up in such a way as to divide the {126}enclosed muscle-cells into four quadrants. These thickenings surround dorsally and ventrally a specialised nerve-cord, and laterally the excretory canals.
According to Jammes[155] this lack of differentiation in the sub-cuticular layer is caused by the early appearance of the cuticle, which he thinks is necessitated, at any rate in many of the parasitic forms, by the action which the digestive juices of the host would have on the otherwise unprotected body-wall.
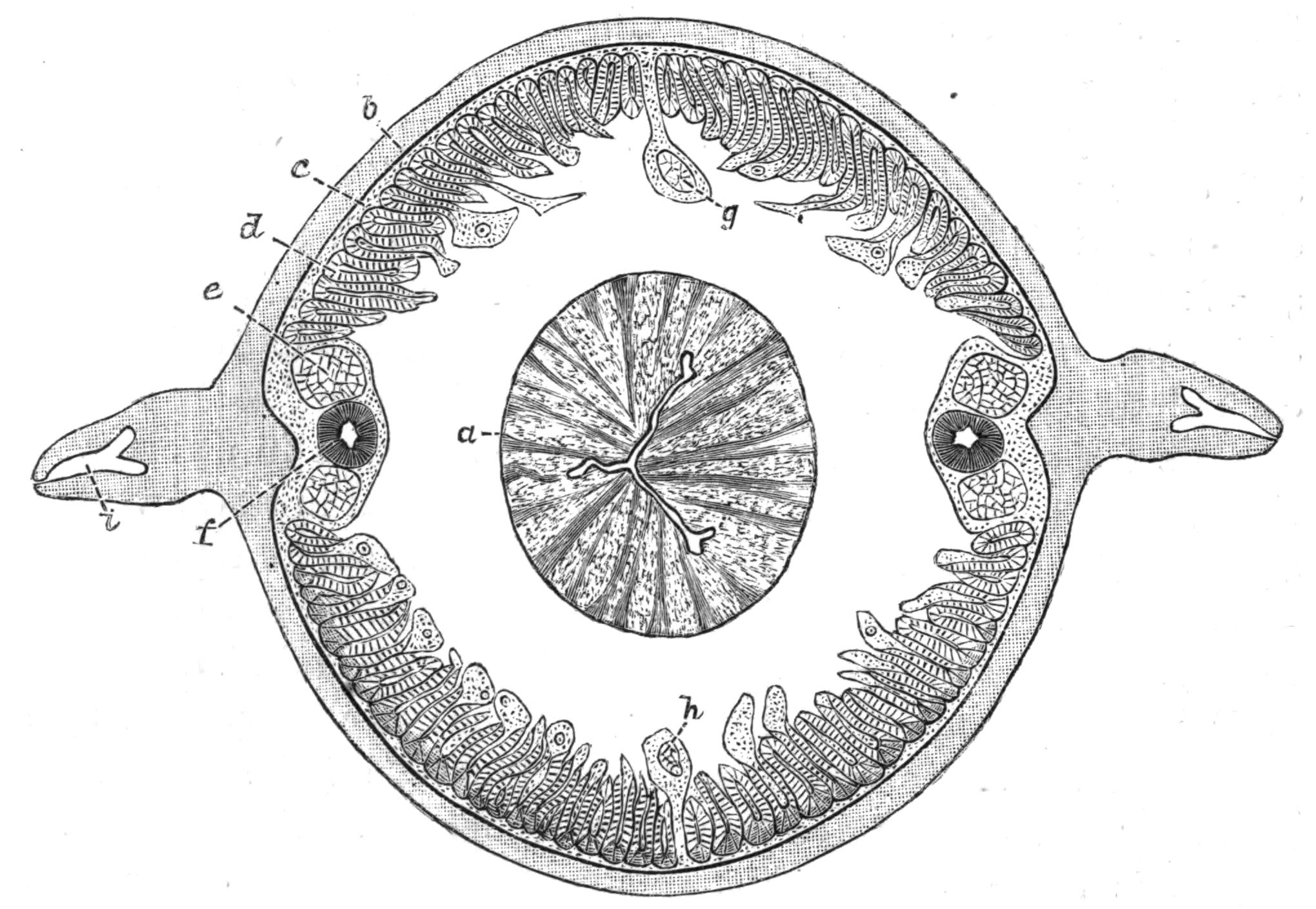
Fig. 62.—A transverse section through the body of Ascaris transfuga Rud., in the region of the oesophagus: a, the muscular oesophagus with its triradiate lumen; b, the cuticle; c, the sub-cuticle; d, the muscular layer; e, the lateral nerves running in the lateral line; f, the excretory canal; g, the dorsal, and h, the ventral nerve; i, the triradiate rod in the fin.
The nervous system, according to the same writer, is of the same nature as this sub-cuticular tissue, only it is more differentiated, or perhaps we should say it has retained more of the primitive cellular character of the embryonic tissue. The fibres of the sub-cuticular tissue are closely connected with the fibrils which compose the spongioplasm (Fig. 64, d) of the muscles,[156] and form also the sheaths of the various nerves; in fact the passage of these fibrils into the nerves is so gradual that it is impossible to make any separation between them.
The Nervous System.—The central organ of the nervous system is the circumoesophageal ring which surrounds the pharynx, close to the anterior end of the body, in A. megalocephala 1½ to 2 mm. behind the mouth.[157] Ganglion cells are found in the ring, but they are not numerous, and are chiefly aggregated round the points of origin of the nerves.
Six short nerves, three on each side of the median line, run forward from the ring, a pair of these ending in each of the three papillae which surround the mouth.
Behind, the nerve-ring gives off six main nerve trunks, of which the dorsal and ventral nerves are usually the largest. These run in the median dorsal and ventral thickenings of the sub-cuticular tissue, and are connected one with another by numerous fine lateral branches running through the sub-cuticle.
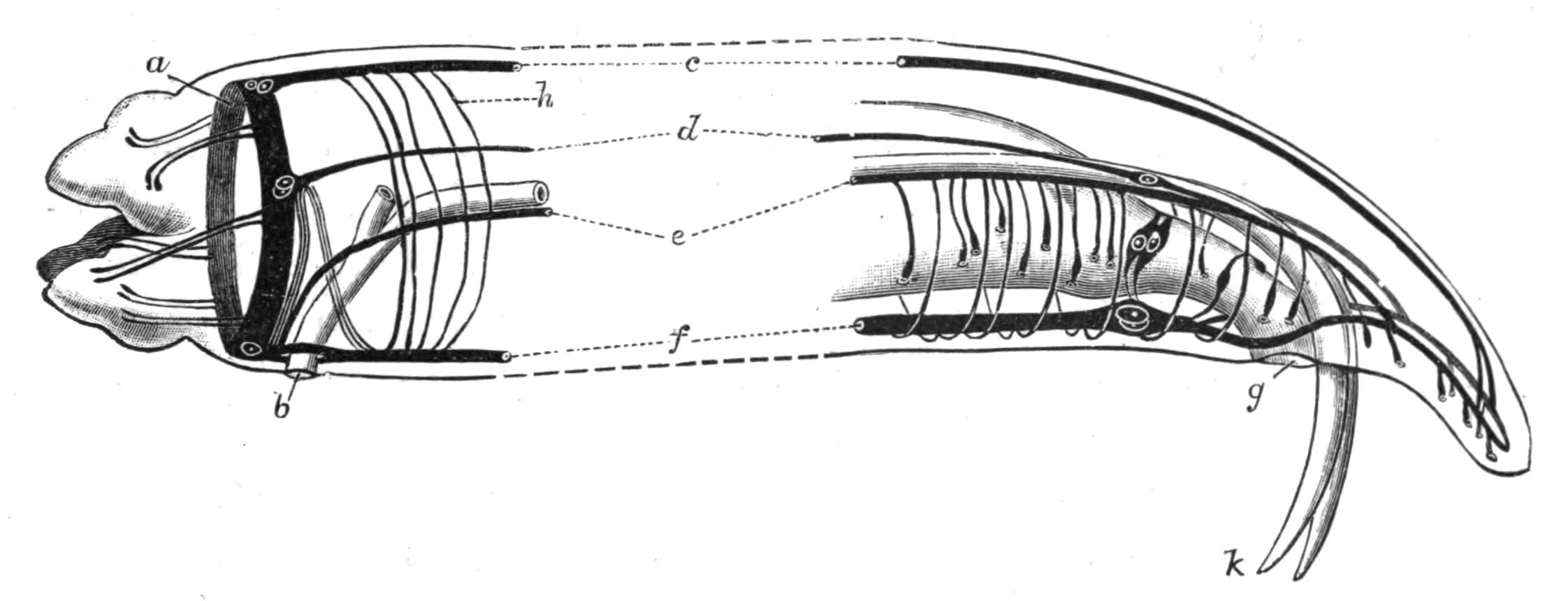
Fig. 63.—Diagram of the nervous system at the two ends of the body in Ascaris megalocephala Cloq., ♂. (After Hesse.) a, Circumoesophageal nerve-ring; b, opening of excretory ducts; c, dorsal nerve; d, dorso-lateral nerve; e, ventro-lateral nerve becoming the bursal nerve posteriorly; f, the ventral nerve; g, cloacal opening; h, sub-cuticular nerves running from c to f; k, spicules.
The lateral nerves, which consist of two or four bundles, one or two lying dorsal and one or two ventral to each excretory canal, have a double origin. The dorsal branches arise directly from the nerve-ring, and at their point of origin there is a considerable accumulation of ganglion cells, from which two commissures on each side run into the ventral nerve (Fig. 63, f). The ventral branches arise from the ventral nerve-cord immediately in front of the excretory pore. At the posterior end the lateral nerves pass into the two branches into which the ventral nerve divides. Just before the point where the ventral {128}nerve splits it swells out into an anal ganglion situated just in front of the anus. In the male[158] this anal ganglion gives off two lateral nerves which pass round the cloaca and form a ring, and in this sex the ventro-lateral nerve, which is much strengthened by fibres from the ventral nerve, and has received, owing to the mistaken impression that it was a special nervus recurrens, the name of the "bursal nerve," gives off numerous branches to the sense papillae which are found in this region of the body and on the tail. The arrangement of these parts is shown in Fig. 63.
Sense organs are but poorly developed in the Nematoda, as is usual in animals which are, as a rule, either parasitic or live underground. Eyes, consisting of masses of dark pigment with or without a lens, occur in the neighbourhood of the circumoesophageal nerve-ring in some free-living forms. Leuckart described as possible auditory organs certain giant-cells lying near the orifice of the excretory ducts. Later research has shown these cells to have some phagocytic action on the contents of the body-cavity. The chief sense organs are the papillae, of which in A. megalocephala there are two kinds, the lip papillae being distinguished from the genital papillae by the fact that the nerve supplying them ends in a fine point and pierces the cuticle in the former case, whilst in the latter it swells out into an "end-organ," which is always covered by a layer of cuticle, though sometimes by a very thin one.
Muscular System.—The muscular system is one of the most characteristic features of the Nematoda, both as regards the histology of the muscle-cells and the way in which the cells are arranged.
Each muscle-cell is of considerable size, and is of the shape of a somewhat flattened spindle produced into a process near the middle. Each end of the spindle cell is said to be continuous with the fibrils of the sub-cuticular layer.[159] The muscle-cell consists of two portions, a contractile part which lies next the sub-cuticle, and which usually, to some extent, wraps round the second or medullary half. The latter consists of a fibrillar spongioplasm, in the meshes of which lies a clear structureless hyaloplasm. The nucleus always lies in the medullary half.
The contractile portion consists of a number of columns, very regularly arranged in two rows and close together, but allowing sufficient space between adjacent columns for fibrils of the spongioplasm to penetrate; and these become continuous with the fibrils of the sub-cuticle, which is thus intimately connected with both nervous and muscular systems.
The medullary portion of the cell varies greatly in size; it may stretch far into the body-cavity, which may be thereby almost occluded, or it may be flattened out, leaving a large space around the alimentary canal. At one point, usually about its middle, it is produced into a process, which bends inwards towards the dorsal or ventral nerve-cord, and by means of this process the muscle receives its nerve supply.
In most Nematodes there are numerous muscle-cells to be seen in any transverse section, forming a layer within the sub-cuticle, and broken up into four quadrants (Fig. 62) by the projection of the dorsal, ventral, and lateral thickenings of the sub-cuticular tissue. In some genera, however, such as Oxyuris, Strongylus, Pelodera, Leptodera, etc., there are but eight muscle-cells in a row, two in each quadrant. Such genera are classed together by Schneider,[160] and termed Meromyarii (vide p. 137).
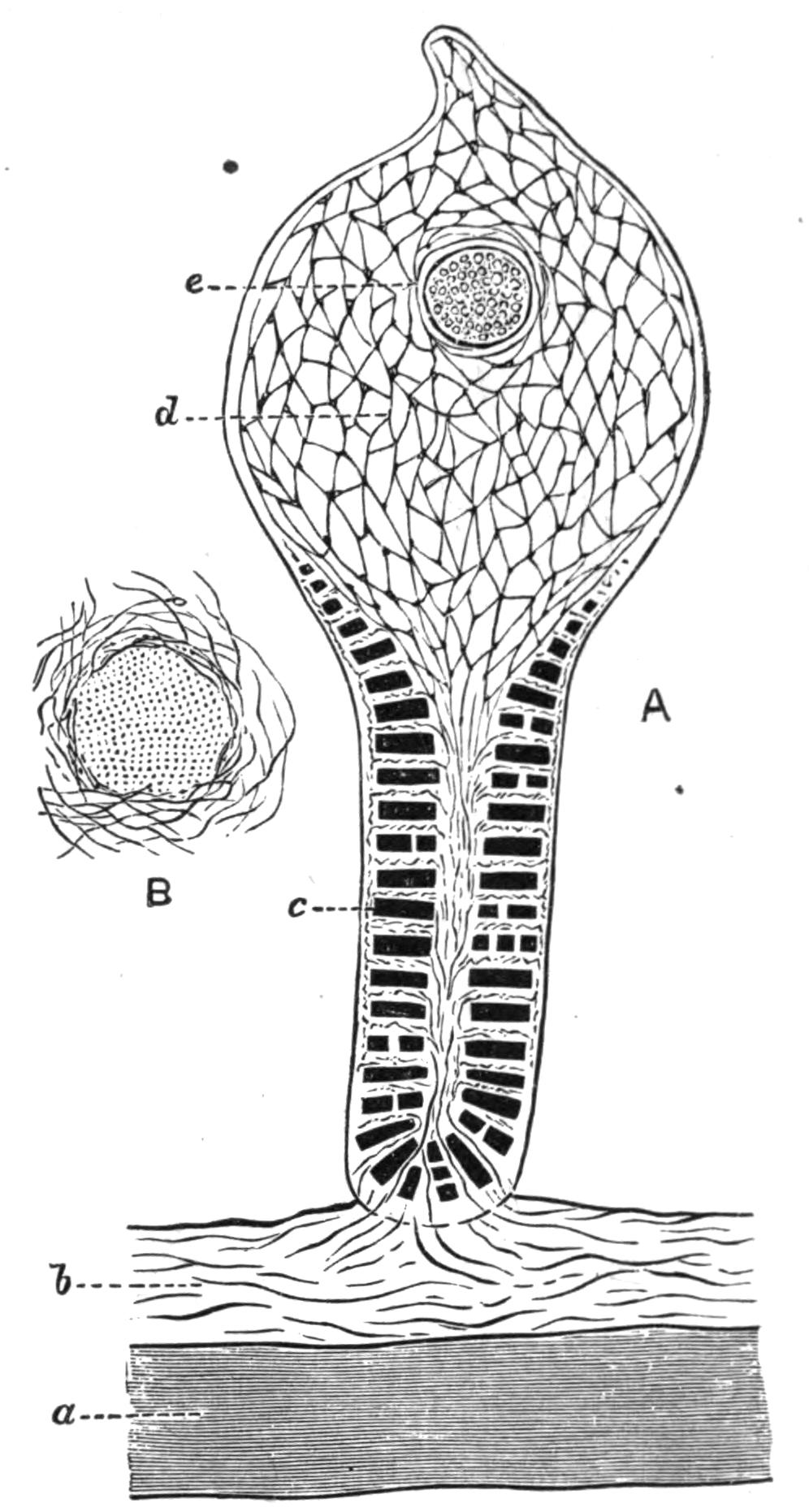
Fig. 64.—A, transverse section through the centre of a muscle-cell; B, the same through a nerve fibre showing the sub-cuticular fibres running into the sheath. (After Rohde.) a, Cuticle; b, sub-cuticular fibres continuous with d; c, contractile columns; d, network of spongioplasm; e, nucleus.
In addition to the characteristic muscles of the body-wall there are others, such as those which move the spicules in the male, which cross the body-cavity obliquely near the anus, and such as sphincter muscles near the latter orifice, which have not {130}the characteristic arrangement of contractile and medullary parts described above.
The Body-Cavity.—The skin of a Nematode, as described above, contains most of the important organs of the body within its thickness. The chief muscular system, the nervous system with its sense organs, and the excretory organs are all embedded in or form part of the skin, which in its turn encloses a cavity—the body-cavity—in which the other two systems of organs which are found in Nematodes lie. These are the digestive system and the reproductive system.
The body-cavity is continuous from one end of the animal to the other, and is in no case divided up into compartments by the presence of septa or mesenteries. It contains a coagulable fluid with numerous corpuscles; this is, as a rule, colourless, but in Syngamus trachealis Sieb. (Fig. 70), which lives on blood, the haemoglobin of its host tinges it red, though the colour is said to disappear if the parasite be isolated and starved.
The morphological nature of this body-cavity affords an interesting problem. It is not a true coelom, such as exists in the earthworm, since it is not surrounded by mesoderm, nor do the excretory organs, with the possible exception of one or two genera, open into it, nor do the generative cells arise from its walls. Essentially it is a space between the mesodermic muscle-cells which line the skin and the endodermic cells of the alimentary canal, and although in many of its functions it resembles the coelom of other animals, its morphological character is quite different.
There are no respiratory or circulatory organs in the Nematoda; possibly the fluid in the body-cavity acts, to some extent, as a carrier of oxygen, but from the inert and almost vegetative life of these animals it seems probable that their respiratory processes are slow, and in fact Bunge[161] has shown that Ascaris mystax, found in the intestine of the cat, will live for four or five days in media quite free from oxygen, and that A. acus from the pike will live and exhibit movements in the same media for from four to six days.
The Digestive System.—The mouth of the Nematoda is usually anterior and terminal, and is surrounded by from two to six projecting lips, the most common number being three. These {131}lips are well provided with sense papillae. The mouth leads into an alimentary canal, which with hardly an exception runs straight through the body to the anus without twists or loops. The anus is usually placed ventrally and is not terminal, but in Trichina and Trichocephalus it is at the end of the body, and in Mermis, where the several parts of the alimentary canal are said not to communicate, it is absent altogether. Ichthyonema, Dracunculus, Allantonema, Atractonema, and other Filariae are also aproctous.
The alimentary canal is divisible into three parts—(i.) the oesophagus, (ii.) the intestine, and (iii.) the rectum. The suctorial oesophagus is a very muscular, thick-walled tube, lined with cuticle continuous with that which covers the body, and like it cast from time to time. Its lumen is usually much reduced, and is almost invariably triangular or triradiate in section (Fig. 62). In many genera the hinder end of the oesophagus is swollen into a muscular bulb, which is armed with teeth in Heterakis, Oxyuris, Pelodera, Leptodera, etc. Other species, such as Tylenchus, Aphelenchus, Dorylaimus, are armed with a spear, which in Onyx,[162] a genus recently described and allied to the last named, is borne on a special bulb. The use of the spear is to pierce the tissue upon the juices of which the animal lives. A gland lies embedded in the thick walls of the oesophagus, and opens into its lumen by a fine tube. This was first described by Schneider[163] in A. megalocephala, and more recently it has been found by Hamann[164] in a number of Ascaridae and Strongylidae from the Adriatic, and also in Lecanocephalus.
With a few exceptions, such as Mermis, where it is blind, the oesophagus opens posteriorly into the intestine. This is a somewhat flattened tube, whose shape and position are often altered by the development of the generative organs. Its wall consists of a single layer of columnar cells, with large nuclei coated internally and externally by a layer of cuticle. The inner layer of cuticle is usually perforated by very numerous minute pores. In some species the intestine is degenerate, in Mermis it is a closed tube opening neither into the oesophagus nor into the rectum; in Trichina spiralis and in the larva of Tylenchus tritici it consists of a single row of cells perforated by a duct, but in the adult of the last named there are many cells in a transverse section.
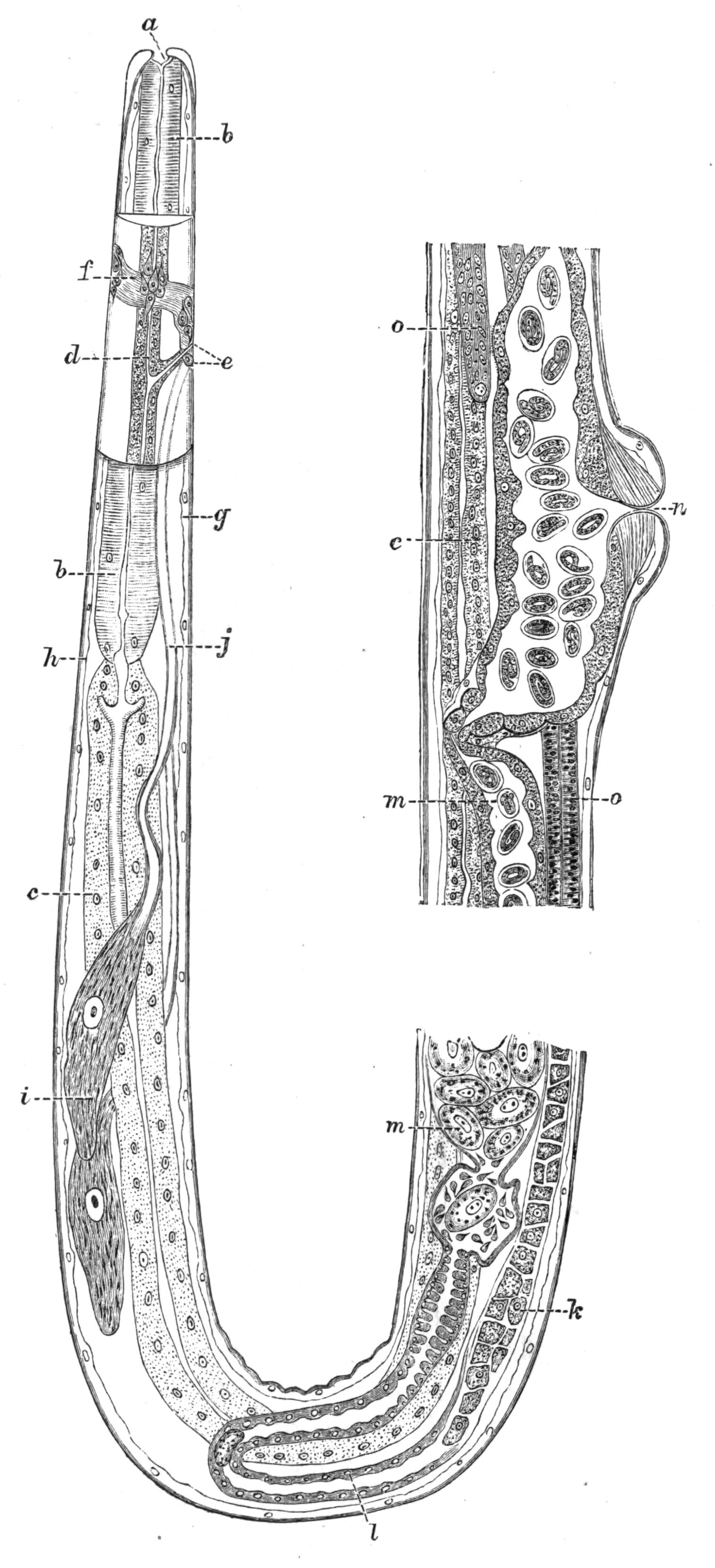
Fig. 65.—A longitudinal section through the body of Strongylus filaria Rud. (From O. Augstein.[165]) A portion of the body, on each side of the excretory pore, is seen in optical section. a, Mouth; b, oesophagus; c, intestine; d, excretory canal; e, excretory pore, and the opening of the poison glands, i; f, circumoesophageal nerve-ring; g, ventral nerve; h, dorsal nerve; i, unicellular poison glands; k, ovary, with the ova separate; l, oviduct; m, uterus, the first egg in the uterus is surrounded by spermatozoa; n, opening of uterus; o, inner end of ovary with the ova undifferentiated.
In some genera, Leptodera and Pelodera, the lumen of the intestine at any one level is bounded by two horseshoe-shaped cells, but by far the commonest arrangement is a tube formed of fairly numerous columnar cells crowded with granules and with large nuclei.
The rectum is usually short; its cuticular lining, like that of the oesophagus, is cast at intervals. At its anterior end there is usually a sphincter muscle, and its walls are divaricated by muscular strands which run from it to the body-wall. The anus is a transverse slit, which in the male Strongylidae is surrounded by a funnel-shaped membrane.
The food of Nematodes seems to be almost entirely fluid, and consists, at any rate in the parasitic forms, of the elaborated juices of their hosts. Little is known about the nutriment of the free-living forms.
The Excretory System.—The excretory organs are peculiar, and, like many other Nematode structures, do not fall readily into line with what is known of similar organs in other animals. They consist of two canals embedded in the lateral thickenings of the sub-cuticular tissue. The canals end blindly behind, but near the anterior end of the body they bend inwards, and after uniting, open by a common pore situated in the middle ventral line, a little way behind the mouth. The lateral canals are in some cases continued in front of the transverse branch, and they then end blindly in the head. The walls of these canals consist of an internal, structureless, refractive layer surrounded by a granular layer with nuclei. They contain a fluid, but nothing is known of its composition.
An interesting divergence from the usual form of excretory organ has been described by Hamann[166] in the genus Lecanocephalus. Here there is only one canal, the right; anteriorly this bends towards the ventral surface and opens by a small median pore close behind the nerve-ring. Posteriorly the canal does not extend much beyond the middle of the body, where it forms a coiled mass, and diminishing in size, opens into the body-cavity. The same author also states that both canals in Dochmius have a similar internal opening; these observations, if confirmed,[167] show a conformity to the ordinary structure of {134}excretory organs which was not supposed to exist in the lateral canals of the Nematoda.
The Reproductive Organs.—With the exception of the genera Angiostomum, Pelodytes, and of Rhabdonema nigrovenosum, which are physiologically hermaphrodite and self-impregnating, the Nematodes have separate sexes. The males are, as a rule, smaller than the females, and may usually be distinguished by the posterior end of the body being curved towards the ventral surface; a genital bursa, and one or more spicules are often found in this sex. Further, the position of the genital opening differs; in the male the vas deferens opens on the ventral surface of the rectum close to the anus, but the oviduct in the female opens in the ventral middle line, usually near the middle of the body, but sometimes close behind the excretory pore, or in some Strongylidae just in front of the anus. The tail of the male bears very numerous papillae, which are of considerable systematic importance.
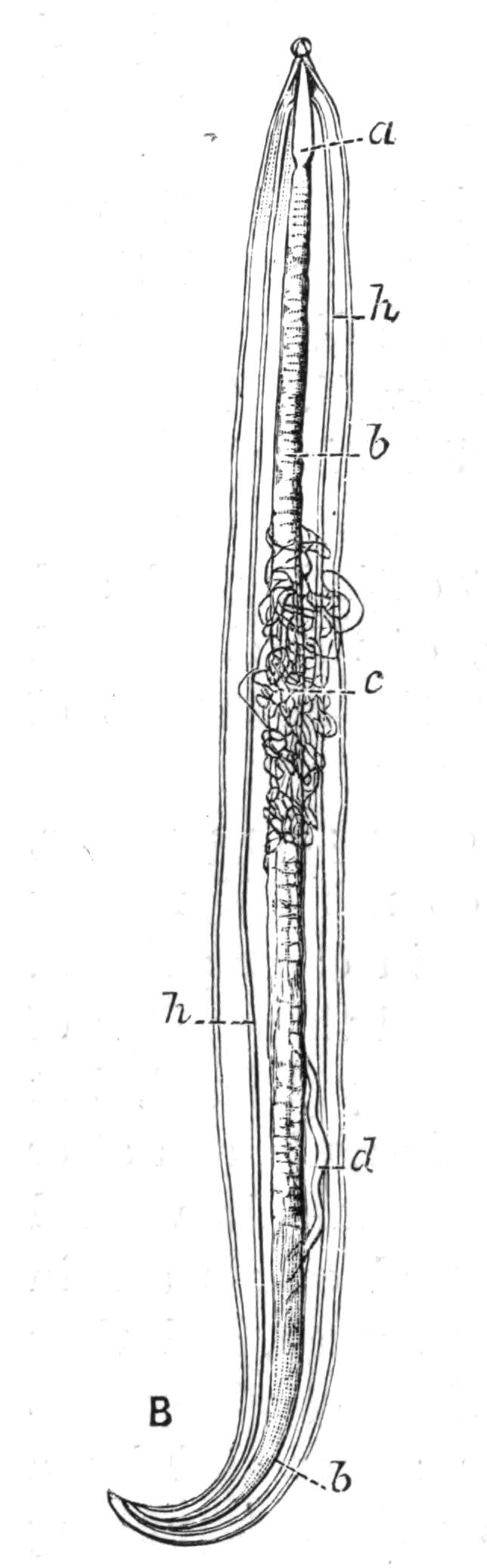
Fig. 66.—Ascaris lumbricoides Cloq. ♂, natural size, cut open along the dorsal middle line. a, Oesophagus; b, intestine; c, testis; d, vas deferens; h, lateral excretory canals.
With rare exceptions, e.g. Filaria attenuata, where it is double, the male reproductive organ consists of a single tube divisible into a testis proper, a vas deferens, a vesicula seminalis, where the spermatozoa are stored up, and a ductus ejaculatorius. The tube stretches through the body in a straight line in the small free-living forms, but is thrown into loops and coils in the larger parasitic Nematodes. Within the testis the mother-cells of the spermatozoa are attached to a rhachis or axial cord; the mother-cells divide, and their products ultimately form spermatozoa. The latter have a very peculiar shape; in accordance with the universal absence of cilia in the Nematoda the spermatozoon has no flagellum, and at first consists of a spherical nucleated cell, on one side of which a cap or covering of some refractive substance appears. The cap elongates and {135}becomes conical, whilst the protoplasmic portion of the spermatozoon throws out pseudopodia and becomes amoeboid, but ultimately rounds itself off again. The spermatozoa do not attain maturity until they reach the uterus of the female.
The internal female reproductive organs are, with few exceptions (Trichina, etc.), double, but the vagina, which is lined with cuticle continuous with that covering the body, is always single. They are usually much coiled, and may be divided into ovary, oviduct, and uterus. The ova arise from a polynucleated mass of protoplasm or syncytium (Fig. 65, o) at the upper end, and acquire distinctness as they approach the oviduct. Fertilisation takes place in the uterus, but the segmentation may not begin until some time after the eggs are laid; in Dochmius, however, it is well advanced at this period, and in many genera, e.g. Pseudalius, Trichina, Dracunculus, etc., the whole development of the larva takes place in the body of the mother.
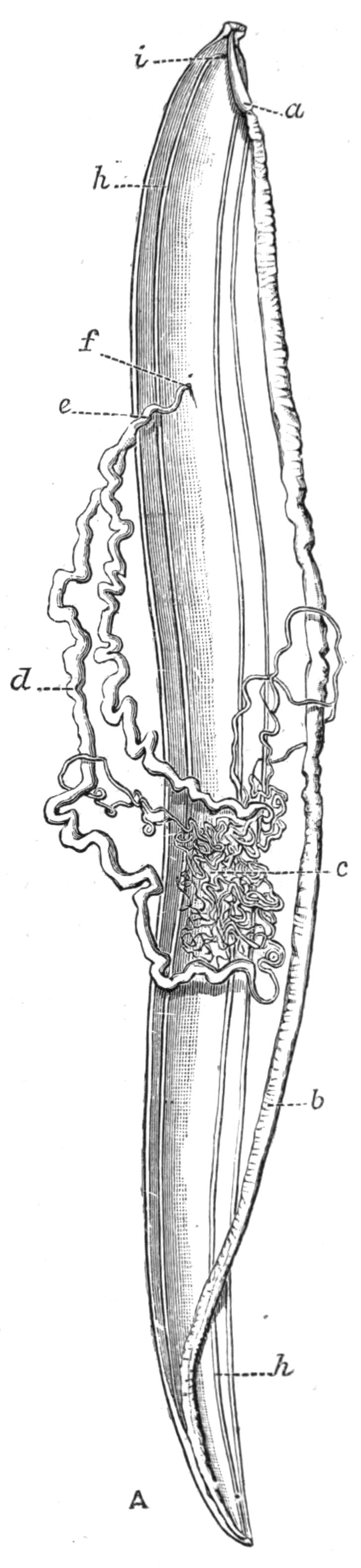
Fig. 67.—Ascaris lumbricoides Cloq. ♀, natural size, cut open along the median dorsal line to show the internal organs. a, The muscular oesophagus; b, the intestine; c, the ovary; d, the uterus; e, the vagina; f, the external opening; h, the excretory canals; i, their opening.
Embryology.—The eggs of many of the parasitic forms require a considerable degree of warmth to develop. Those of Ascaris lumbricoides require a temperature of 20° C., those of Trichocephalus 22.5° C., and those of Oxyuris vermicularis, 40° C. The latter develop in a few hours, the eggs of Dochmius in a few days, whilst those of A. {136}lumbricoides take weeks or even months, and the young of Trichocephalus seldom develop within a year.[168] The ova only develop in a damp atmosphere, and they can be arrested at almost any stage, and for considerable periods, by desiccation.
Our knowledge of the processes by which the fertilised egg-cell develops into the larva is very imperfect. As a rule the segmentation is complete and equal; it results in the formation of a blastula, which may take the form of a hollow sphere of cells—A. megalocephala—or the cavity may be reduced, and the blastula may consist of a double-layered plate, as in Cucullanus.[169] The distinction into cells which will form the three embryonic layers, the ectoderm, mesoderm, and endoderm, is very early evident,—in the eight-cell stage. By the growth of one side of the blastula and the tucking in of the other the blastula becomes converted into a gastrula, which is a two-layered stage with a cavity opening to the exterior by a pore termed the blastopore. In Nematodes the blastopore is elongated and slit-like; it either forms the mouth (Cucullanus) or closes from behind forwards, the mouth ultimately arising at the point where the blastopore finally closed (Rhabdonema nigrovenosum). The mesodermal cells lie between the ectoderm and the endoderm; they ultimately develop into the muscles of the body-wall, the lateral excretory canals, and the reproductive organs; the last-named two systems arise each[170] from a single cell. The nervous system arises from the ectoderm, which also forms the sub-cuticle, and is turned in slightly at the mouth and anus; the remainder of the alimentary canal develops from the endoderm.
The post-embryonic development, which is very variable, and in many cases very extraordinary, will be dealt with under the several families.
Classification.—The classification of the Nematodes is a matter of very considerable difficulty; their structure is unusually monotonous, and, owing perhaps to their largely parasitic mode of life, they show practically none of those external features which are so useful to the systematist in other groups. Schneider in his Monograph divides the group into three subdivisions—(i.) {137}the Polymyarii, in which numerous muscle cells are seen in a transverse section; (ii.) the Meromyarii, in which only eight are seen, two in each quadrant; and (iii.) the Holomyarii, in which the muscles are either not divided, or only divided by longitudinal lines. This grouping has, however, to some extent broken down, since Bütschli[171] and others have shown that the third subdivision is founded on insufficient observation, whilst the first two include, in different subdivisions, Nematodes which are closely allied in all respects except as regards their muscle cells.
The details of the life-history have been used by other writers as a basis of classification. Linstow[172] enumerates fourteen distinct modifications of the post-embryonic development (vide p. 159), and Örley[173] has grouped these under three headings. The animals which fall under each group to some extent resemble one another in structure. Örley's groups are:—
(i.) Nematozoa.—Thread-worms with free larval life, the mature forms being parasitic in animals. Enormous numbers of eggs are produced, and the development is indirect. The genital organs are complicated by many convolutions.
(ii.) Rhabditiformae.—Small, as a rule microscopic, thread-worms, usually living free, but rarely parasitic. They become sexually mature only in decomposing organic substances, or in earth saturated with such substances. They live gregariously and do not produce immense numbers of ova. The metamorphosis is slight, or is complicated by sexual metamorphosis. The oesophagus has two dilatations. The genital tubes are simple and not coiled.
(iii.) Anguillulidae.—Small microscopic thread-worms, with a free existence in mould or water, throughout all stages. They produce large eggs. They are provided with a caudal sucker and bristles, sometimes with eyes and other structures characteristic of a free life. Genital tube simple and not coiled.
The disadvantage of such a system is, that to accurately place a specimen in its proper class we must be acquainted with its life-history, and this is known in but few cases.
The determination of the species to which a Nematode belongs is a matter of considerable difficulty. Amongst the more important features for purposes of classification are the arrangement of the {138}muscles, the character of the tail in the male, especially when papillae are present, the number and the size of the spicules, and the arrangement of the lips and mouth-parts generally.
Cobb[174] has recently devised an ingenious formula in which measurements of different parts of the body appear as percentages of the whole length of the body. The nature of this will be understood by reference to Fig. 68. Such a formula should, however, be used with caution, since it rests on the assumption that the proportions of the various parts of the body are constant in different individuals, and it is by no means certain that this is the case.
Fig. 68.—Diagram to explain the descriptive formula used for Nematodes. (From Cobb.) 6, 7, 8, 10, 6 are the transverse measurements, while 7, 14, 28, 50, 88 are the corresponding longitudinal measurements. The formula in this case is
| 7 | 14 | 28 | 50 | 88 |
| 6 | 7 | 8 | 10 | 6 |
The unit of measurement is the one-hundredth part of the length of the worm. The measurements are therefore percentages of the length.
The measurements are taken with the animal viewed in profile; the first is taken at the base of the oesophagus, the second at the nerve-ring, the third at the cardiac constriction, the fourth at the vulva in females and at the middle in males, the fifth at the anus.
Taking everything into consideration, it has seemed advisable in the following systematic account of the Nematoda to abandon the larger groups, and to deal directly with the families. Claus distinguishes seven of these, and the diagnoses given at the head of each are mainly taken from his Grundzüge der Zoologie.[175]
I. Family Ascaridae.
Body rather stout. A dorsal and two ventro-lateral lips, bearing papillae. Buccal cavity distinct, seldom provided with chitinous armature. The oesophagus often has two dilatations. {139}The tail of the male is ventrally curved, and usually there are two horny spicules. The Ascaridae are found in the intestines of their respective hosts.
Genera: Ascaris, Heterakis, Oxyuris, Nematoxys, Oxysoma, and many others.
Von Linstow[176] enumerates over 250 species of Ascaris, of which it will only be possible to mention here one or two. They are all parasitic in Vertebrata.
A. lumbricoides Linn. is one of the largest known Nematodes ♂ = 4-6 in., ♀ = 10-14 in.; Figs. 66 and 67). It is a common parasite in man, and has been found in the ox. It is now generally recognised as the same parasite which inhabits the pig, and which Dujardin regarded as specifically distinct, and named A. suillae. In the latter host, however, it never attains the dimensions it does in man. It inhabits the upper and middle parts of the small intestine, and has been known to escape into the body-cavity and set up abscesses there, or to make its way into the stomach, and to be voided through the mouth. It is practically cosmopolitan in distribution, and is very common in Japan—Baely found it in twenty-one out of twenty-three post-mortems—and in Tonquin and tropical Africa. Heller[177] states that no one is free from these worms in Finland, and they are common wherever there is a plentiful water supply, as in the marshy districts of Holland and Sweden. In Iceland alone they seem absent. When examined alive they give off an irritating vapour which seriously affects some observers, causing catarrhal symptoms, which in Bastian's case lasted six weeks. The usual number found in one host is small, one to six or eight, but cases are on record where many hundreds occurred in one person.
The details of the life-history of this form are not yet completely worked out. The eggs leave the body of the host with the excreta, and formerly it was thought they re-entered the alimentary canal in drinking-water, etc., and there developed into the adult without change of host. This view has been combated by Leuckart, who failed to rear the Nematodes by direct feeding, and it has been noticed that the youngest parasites found in the {140}intestine are already 2 to 3 mm. long. Von Linstow has recently suggested that the larval stages may be hatched out in the body of the millipede Julus guttulatus, whose habits might easily lead it to eat the eggs of the parasite in manured gardens, etc., and which is itself sometimes unconsciously eaten when hidden in fruit or vegetables. This would account for the frequent presence of the parasite in pigs, and also for the fact that in man it is commonest in children who are apt to eat windfalls, and in maniacs and people with perverted tastes.
A. megalocephala, which is found in the horse, ass, zebra, ox, etc., attains even greater dimensions than the foregoing. The male rarely exceeds 7 inches in length, but the female sometimes reaches 17 inches. They are found in the small intestine of their hosts. Cobbold[178] succeeded in rearing larvae which attained a high degree of organisation when the eggs were placed amongst moist horse-dung, and it seems probable that the larvae pass into the body of their hosts in drinking water; at any rate no intermediate host has yet been found, and Davaine, who fed cows, and Leuckart, who fed horses with the unhatched eggs, both failed to infect the animals they experimented on. A. mystax, which lives in cats, dogs, and other Carnivora, has also been found in man. It is provided with fin-like extensions on the side of its head (cf. Fig. 62), and varies much in size in different hosts. When first found in man it received the name of A. alata. It becomes sexually mature in about three weeks.
One of the most remarkable cycles of development amongst the many curious life-histories met with amongst Nematodes, is that presented by Rhabdonema (Ascaris) nigrovenosum. The free form of this, formerly known as a distinct species, Rhabditis nigrovenosa, lives in the excrement of frogs, and attains sexual maturity in a very short time. The sexes pair, and the fertilised ova give rise to embryos which hatch out within the body of the mother, and then begin to devour her internal organs. After the destruction of the mother, the embryos escape and live in water or slime, and sometimes burrow into water snails, but they undergo no change until swallowed by a frog. Then they make their way into its lungs and grow enormously, attaining a length of almost an inch. This form, parasitic in the frog, is a protandrous hermaphrodite, which first produces spermatozoa and afterwards {141}ova; the latter are fertilised by the spermatozoa, and give rise to rhabditiform embryos, which escape by the alimentary canal and form the free-living sexual stage mentioned above. Thus in the life-history of this form we find an alternation of generation, a sexual free-living form alternating with a hermaphrodite parasitic form.
Of the enormous number of other species of the genus, only a very few can be mentioned. A. transfuga Rud. inhabits bears; A. leptoptera Rud., lions; A. ferox H. and Ehrbg., Hyracoidea; A. depressa Rud., vultures; A. rubicunda Schn., pythons; A. sulcata Rud., turtles; A. mucronata Schn., the cod and pike; A. incurva Rud., the sword-fish.
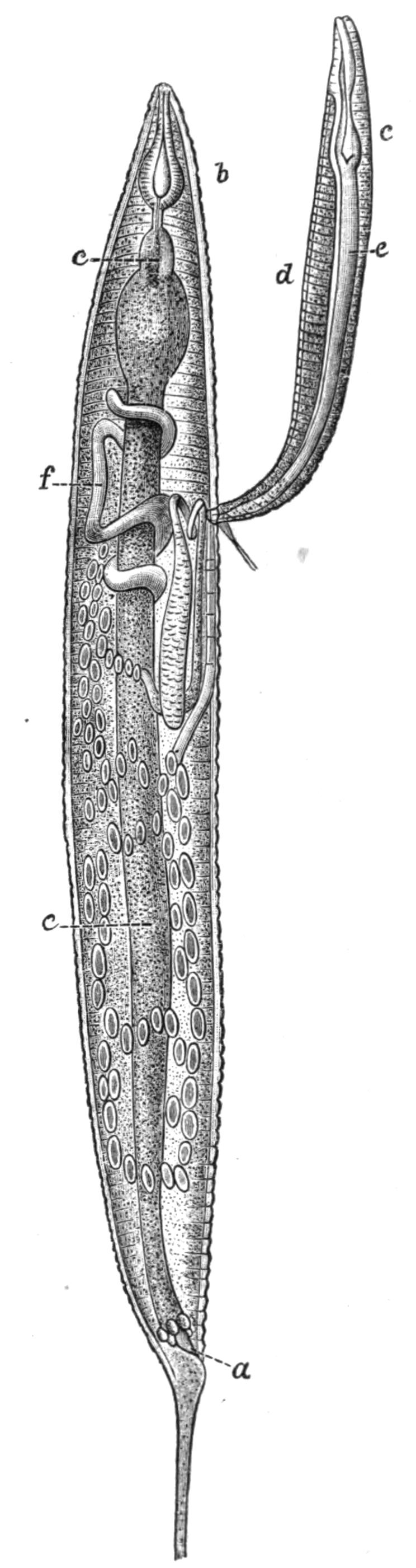
Fig. 69.—A male and female Oxyuris diesingi Ham. in copula, × 60. a, Anus; b, oesophagus; c, bulb; d, testis; e, intestine; f, ovary. (From Galeb.[179])
Oxyuris is Meromyarian (see p. 137), and is characterised by the long capillary tail of the female. It includes another human parasite, O. vermicularis, and it is one which it is difficult to get rid of. The female has the characteristic tail and is about 10 mm. long. The male is smaller. They are found in the caecum and rectum of man, and cause great irritation and sometimes serious functional disturbance. The eggs are laid in immense numbers but perish in water. If whilst still in the egg-shell the larvae are swallowed on fruit or raw vegetables, etc., they are set free in the stomach and small intestine by the action of the digestive secretions. The distribution of this parasite is universal. Besides numerous species that inhabit the alimentary canal of Vertebrates, such as O. ambigua Rud., found in hares and rabbits; O. curvula Rud., in {142}the caecum of horses; O. megatyphlon Rud., in iguanas; several species inhabit the rectum of insects, such as O. blattae, O. diesingi, O. blatticola, found in the cockroach; O. spirotheca, and O. hydrophili in the water beetle Hydrophilus.[180]
The genus Nematoxys has the most complex arrangement of muscles of any Meromyarian, and forms a transition to the Polymyarian type. The whole body of both sexes is covered with numerous irregularly scattered papillae. The members of this genus have hitherto been found in snakes, Amphibia, and eels; there are but few species.
Oxysoma is another small genus with but three species, found in the intestines of opossums, frogs, and turtles respectively.
II. Family Strongylidae.
Mouth surrounded by papillae; an armature of teeth or spines often present. The chitinous lining of the intestine projects into the interior as ridges. No oesophageal bulb. The male orifice at the posterior end of the body is surrounded by a bell-shaped bursa.
Genera: Eustrongylus, Strongylus, Dochmius, Sclerostomum, Cucullanus, Syngamus, Pseudalius, Ollulanus, and others.
The genus Eustrongylus includes two species, E. gigas Rud. and E. tubifex Nitsch. The former attains in the female the gigantic length of 860 mm., with a breadth of 7 mm. and a weight of over 40 grs.[181] The male is a quarter to a third as long as the female. This parasite inhabits the kidney capsules of carnivorous animals, especially of those that eat fish, such as dogs, seals, etc., and has occasionally been found in man, the horse, and the deer. It frequently destroys the substance of the kidney. The worms are red in colour. The eggs die when exposed to desiccation for a few days, but have been kept alive for fifteen months in water; it is believed by Schneider and Leuckart that they are eaten by fish, and that the larvae form the Filaria cystica found in the peritoneal membrane of the fishes Galaxias scriba and Symbranchus laticaudatus, and that they pass into their final host, where they become sexually mature, by the latter eating raw fish. E. tubifex is found in aquatic birds, e.g. ducks, grebes, and divers, etc.
The genus Strongylus is easily recognised by its conspicuous genital bursa, strengthened by variously arranged ridges which are of specific value. There are numerous species, found in man and many other mammals, and also in birds and reptiles. Some species inhabit the intestine, others form aneurisms in the large blood-vessels, and cause considerable mortality amongst horses; others live in the tracheae and lungs of cattle and sheep, their presence often causing great loss to the farmer. No intermediate host has been satisfactorily demonstrated; the larvae live in damp earth, and it seems almost certain that they pass directly into their host with its food.
Dochmius (Ancylostomum) duodenalis, called by Neumann[182] Uncinaria duodenalis, is one of the most dangerous parasites that attack man. It lives in the duodenum and jejunum, and the fertilised eggs leave the body of its host with the excreta, and in damp earth develop into larvae in the course of a few days. These at first eat voraciously, but after undergoing several moults they cease to take food and pass into the resting stage. If now they are swallowed with drinking water, they come to rest in the small intestine of their host, and in a few weeks become sexually mature. They cause great harm by burrowing in the intestinal walls and destroying the capillaries. They are found by hundreds, and even thousands, in the same host, and produce profound anaemia, which is frequently fatal to miners, and was the cause of a great mortality amongst the workers in the St. Gothard Tunnel some fifteen years ago. This species is very widely spread over the face of the globe. Dochmius trigonocephala Rud. and D. stenocephala produce similar diseases in dogs and cats, and D. cernua Crep. is found in sheep and goats.
The genus Cucullanus exists in the adult form in the intestines of fishes, and more rarely of reptiles. C. elegans Zed., which live in fresh-water fish, e.g. the perch, is viviparous; after birth the young pass into the water and make their way into the alimentary canal of the small crustacean Cyclops, and thence into its body-cavity. Here they undergo two moults, accompanied by certain changes in structure. If this second host be swallowed by a fish the parasites are set free, and develop generative organs. {144}Ollulanus tricuspis Leuck., which in the adult state is found in the cat, chiefly in the intestine but also in the bronchi and other parts, gives rise to larvae which are of enormous size compared with the parent; these leave the body, and if eaten by a mouse encyst in its muscles, and if the mouse be devoured by a cat, they complete their life-cycle by becoming sexually mature.
The genus Syngamus infests the trachea and bronchi of birds, more rarely of mammals. The red- or forked-worm, Syngamus trachealis Sieb., is common in poultry and game birds, and causes the disease known as gapes, which is especially common in young birds, and often gives rise to extensive loss. The peculiarity of this genus is that the male is permanently attached to the female, its genital bursa being so closely adherent to the opening of the oviduct that two specimens cannot be separated without tearing the tissues. The ova are not laid, but escape from the body with fully-formed embryos in them, by the decay or rupture of their parent's body. They hatch in damp earth or water in from one to six weeks according to the temperature. When swallowed by a fowl they develop into adults, which reproduce eggs in less than three weeks. No second host is needed, but the embryos remain alive in the alimentary canal of earthworms, and these doubtless to some extent serve to spread the disease.
Fig. 70.—Syngamus trachealis Sieb., natural size and magnified four diameters. The small ♂ is permanently attached to the female. (From Warburton.[183])
III. Family Trichotrachelidae.
This family is characterised by the anterior end of the body being produced into a long whip-like neck. The mouth is small and devoid of papillae. The oesophagus is very long, and it traverses a peculiar strand of cells.
Genera: Trichocephalus, Trichina, Trichosoma, and others.
Trichocephalus dispar Rud. (hominis Gmel.) is common in man, and also occurs in some species of monkey. It does not live freely within the intestine, but buries its long whip-like anterior end in the mucous lining of the caecum or colon. The eggs pass out of the body of the host. The development of the embryo is slow, lasting many months; whilst still in the egg-shell the embryos are swallowed, and give rise to the sexually-mature parasite without the intervention of an intermediate host. They are by no means uncommon. Davaine calculated that about 50 per cent of the inhabitants of Paris were infested with them, but they give rise to little disturbance, and only very occasionally cause serious harm. T. affinis Rud. infests sheep; T. crenatus Rud. the pig; T. depressiusculus Rud. the dog; and T. unguiculatus Rud. the hare and rabbit.
The genus Trichosoma, with many species, is as a rule found in birds, but it occurs also in mammals, as T. plica Rud. in the bladder of the fox and wolf, T. felis cati in the bladder of the cat, T. aerophilum Duj. in the trachea of the fox and marten. The chief interest of this genus is that, at any rate in T. crassicauda Bel., which infests the rat, the dwarf males live two, three, or four at a time within the uterus of the female, a condition of things which recalls the similar arrangement found in the Gephyrean Bonellia.
Trichina spiralis is the cause of the well-known disease trichinosis, which appears in two forms, intestinal and muscular, according to the habitat of the parasite. The mature forms of {146}both sexes are found in the intestine of man and many other mammals. They have been experimentally developed in birds, though in the latter the larval forms have never been observed. By keeping such cold-blooded animals as the salamander at a constant temperature, Goujon and Legros succeeded in infecting them, but the larvae perished as soon as the artificial heat was withdrawn. Muscular trichinosis is unknown in fishes, but the sexual form develops in their intestine.
The adult parasites of the intestine are scarcely visible to the naked eye; the females are 3 to 4 mm. long and more numerous than the males, which measure 1.4 to 1.6 mm. The eggs are very numerous, a single female containing at one time 1200, and probably producing ten times as many during her life. The embryos are hatched out within the uterus, and the larvae leave the body of the mother through the generative pore. The minute larvae bore through the intestinal walls of their host, and then, either burrowing in the tissues or swept along in the stream of blood or lymph, make their way all over the body, and come to rest most usually in the muscles, but occasionally in other parts. When the larva reaches its resting-place, it either pierces the sarcolemma and establishes itself within the substance of the muscle-fibre, or it comes to rest between and not in the fibres. Here its presence sets up the formation of a spindle-shaped cyst which usually contains but one larva, though any number up to seven have been found in one cyst. Within this the larva may remain dormant for years, the walls of the cyst gradually undergoing a fatty or calcareous degeneration. Almost any muscle may be affected; those most usually infested being the muscles of the diaphragm, of the shoulder-blade, and of the lumbar region; the larvae have also been found in the heart. The ends of the muscles near their points of attachment are always the most thoroughly infested.
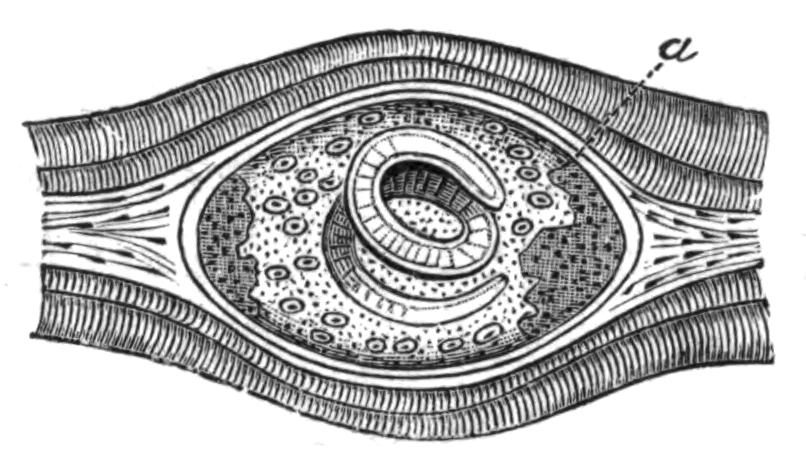
Fig. 72.—Trichina spiralis Owen, encysted in muscle. a, Calcareous deposit. Highly magnified. (From Leuckart.)
The number of the encapsuled larvae in one host is enormous. Leuckart counted between 12,000 and 15,000 in a gramme of muscle, which would give a total of thirty to forty million parasites in one host; other estimates place the total even higher.
When trichinised meat is eaten, unless it has been thoroughly cooked, the cysts are dissolved and the larvae are set free. Within three or four days they become sexually mature and their ova begin to segment. The males after a time leave the body with the excreta and perish, whilst the larvae of the new brood make their way into the tissues of the host.
Man usually acquires trichinosis by eating uncooked or improperly-cooked pork, and the disease is so widely spread and of such a serious nature that most civilised countries have adopted rigorous methods for the detection of trichinised meat. The pigs either acquire the disease by eating uncooked swine's flesh, which is frequently given them in the form of offal, or by devouring rats, which are very susceptible to the disease.
IV. Family Filariidae.
Mouth with two lips, or without lips. Six oral papillae often present, and sometimes a horny oral capsule. Four pre-anal pairs of papillae, and sometimes an unpaired one as well. Two unequal spicula or a single one.
Genera: Filaria, Ichthyonema, Hystrichis, Spiroptera, Dispharagus, and others.
The genus Filaria is a very large one. Like Ascaris, it is confined to Vertebrates, but usually lives in the tissues of the body and not in the intestines. F. (Dracunculus) medinensis Gmel., the guinea-worm, is well known as a human parasite in hot countries; it also occurs in the horse and dog. The female has an average length of 50 to 80 cm., but gigantic forms with a length of 4 metres have been described. The alimentary canal is degenerate. In adult females the body is completely occupied by a uterus crowded with eggs and embryos, which can only escape by the rupture of the mother's body, as the genital ducts have disappeared. Its original home is tropical Asia and Africa, but it has been introduced into South America with the negroes.
The female lives coiled up in the subcutaneous tissues, usually in those of the legs. Its presence gives rise to painful tumours. When these break the female protrudes, and may be withdrawn from the body by very carefully rolling it round a stick or pencil. This must be done very slowly, a few inches a day, as the rupture of the body sets free the contained embryos, and may result in {148}the death of the host. The embryos normally bore their way into the body of the fresh-water Cyclops, and are re-introduced into their Vertebrate hosts with the drinking-water. It is usually stated that the female alone is known, and that it is uncertain whether it is hermaphrodite or whether both sexes are present in the Cyclops. Recently Dr. Charles[184] has described a specimen found in the mesentery of a human subject, from an orifice in the middle of whose body he was able to draw a much smaller specimen, and he thinks this may be the long-sought-for male.
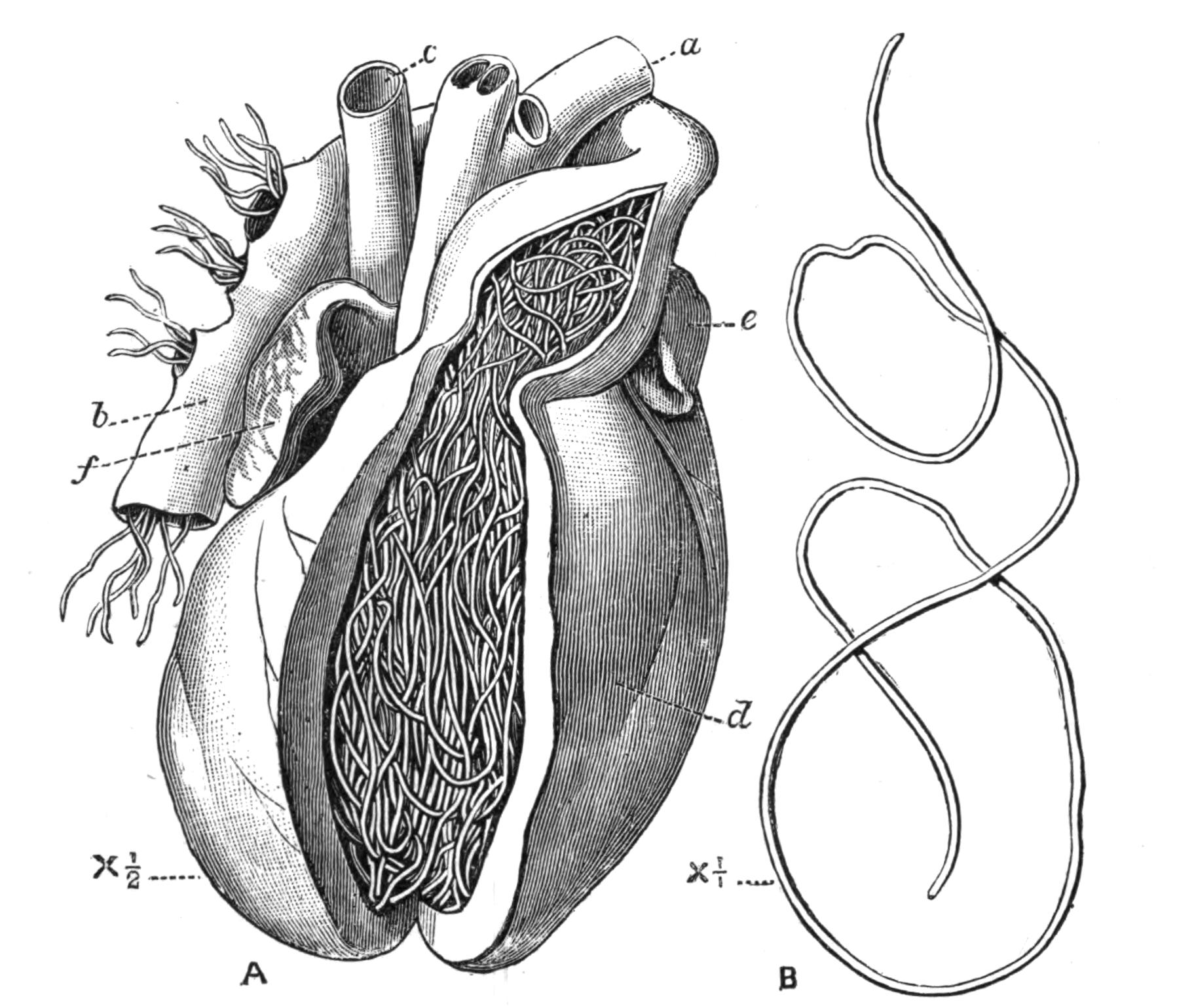
Fig. 73.—A, View of the heart of a dog infested with Filaria immitis[185] Leidy; the right ventricle and base of the pulmonary artery have been opened. a, Aorta; b, pulmonary artery; c, vena cava; d, right ventricle; e, appendix of left auricle; f, appendix of right auricle. B, A female F. immitis removed from the heart to show its length. Natural size.
Filaria immitis Leidy, the cruel worm, is common in dogs in China and the East generally. It is not unknown in America and Europe. It occurs in such large clusters in the right ventricle that it is difficult to see how the circulation can proceed. The intermediate host is unknown, but from the prevalence of the {149}disease in marshy country it is probably some aquatic animal. The larvae are said by Manson to disappear from the peripheral circulation of the dog during the day, but not to such a marked extent as do the F. sanguinis hominis Lew., var. nocturna Man. They were found by Galeb and Pourquier in the foetus of an infested bitch, a fact which establishes the transmission of such parasites through the placenta.
Filaria sanguinis hominis nocturna.—The female of this parasite has been described as living in the lymphatic glands of man. The embryos escape from it into the lymph, and thus reach the blood. According to Manson the intermediate host is the mosquito, in whose stomach the embryos undergo their larval changes. When the mosquito dies the larvae escape into the water, and then make their way into the alimentary canal of man, where they are believed to pair, and whence the female makes its way to the lymphatics. The presence of this Filaria causes great functional disturbance. One of the most remarkable features of it is that the larvae, which are very numerous in the blood during the night, disappear during the day, and are not to be found. Recently Manson[186] has described two new varieties: F. san. hom. diurna, in which the conditions of things are reversed, the larvae being found by day and not by night; and F. san. hom. perstans, in which the larvae occur both by day and by night. The larvae are long-lived, and were found by Manson in the blood of a negro who had not been in Africa, where it is endemic, for six years. The same observer is inclined to associate the presence of F. san. hom. perstans with the fatal disease known as "sleeping sickness." He also suggests that the mature form of the variety diurna is the F. loa, which is not uncommon in the eyes of negroes, and that its intermediate host may be one of the blood-sucking flies so common on the west coast of Africa.
The genus Ichthyonema is confined to fishes. The male is very minute and the female partly degenerate. It has no anus and no external opening to its generative organs. The uterus fills up almost the whole of the body-cavity. I. sanguineum Rud. is found encapsuled in the peritoneum of many fish.
Hystrichis and Dispharagus are confined to birds, where they occur in the oesophagus and stomach. Spiroptera reticulata {150}Crep. occurs in horses, twisted in a spiral round tendons and muscles, forming tumours which require to be opened.
V. Family Mermithidae.
Nematodes without anus and with six mouth papillae. Two spicules in the males and three rows of numerous papillae.
Genera: Mermis, Bradynema, Atractonema, Allantonema, Sphaerularia, and others.
As a rule the Nematoda show but little trace of their parasitic mode of life, but in this family there is considerable degeneration, and in extreme cases the body of the female is reduced to a simple sac crowded with eggs. They are exclusively parasitic in insects. In some respects their structure shows a transition towards Nectonema and the Gordiidae; especially is this the case in the structure of their ventral nerve-cord.
The sexual form of Mermis nigrescens Duj.[187] lives in damp earth, and after storms and in the early morning is sometimes found in such numbers crawling up the stalks of plants, as to give rise to the popular idea that there has been a shower of worms. The male is unknown; the female lays her eggs in the ground, and there they hatch out. It is not known exactly how the larvae make their way into the grasshoppers in whose body-cavity they live, but in an allied species, M. albicans v. Sieb., the larvae have been observed boring their way into small caterpillars through their skin, and it seems probable that the larvae of M. nigrescens burrow in a similar way into young Orthoptera.
Bradynema rigidum Leuck.[188] is found in the adult stage living freely in the body-cavity of a small beetle Aphodius fimetarius, one of the Scarabeidae, from two to three to as many as thirty being found in one host, which does not seem much injured by their presence. The parasite is without mouth, anus, or excretory pore. The eggs hatch out in the uterus of the mother, and the larvae are male and female; they make their way into the body-cavity of the host, and here they pass an unusually long time, five months, soaking in osmotically the nutriment contained in the blood of the insect. Eventually they burrow through the walls of {151}the intestine, and leaving the body of their host through the anus, find their way to the earth. Here, according to zur Strassen, the females die without playing any part in the perpetuation of the species. The males, on the other hand, having developed spermatozoa whilst in the larval stage (paedogenesis), afterwards form ova, and are in fact protandrous hermaphrodites, and become the mature parasites of the beetle, though how they enter the body of the host is unknown.
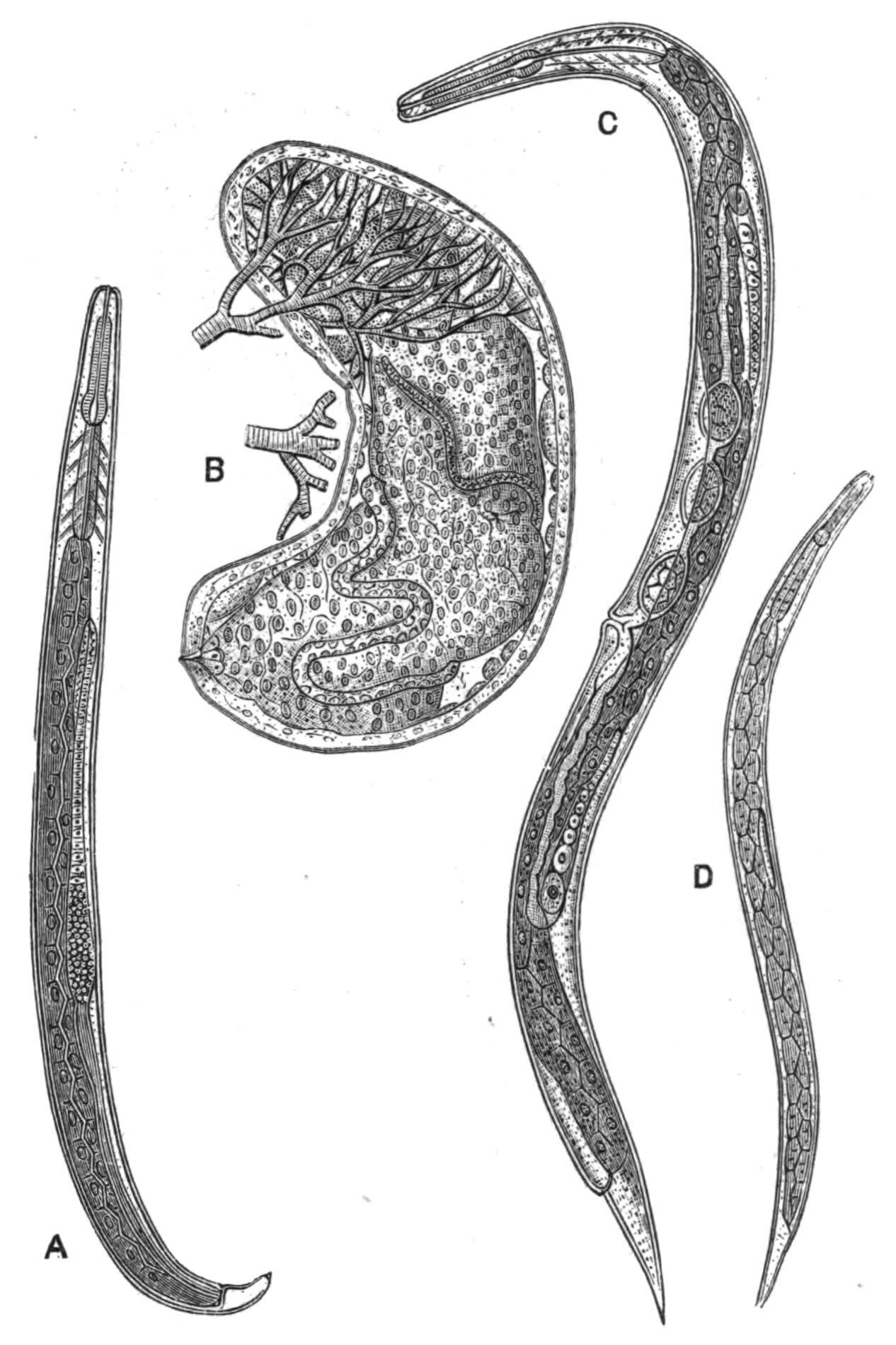
Fig. 74.—Allantonema mirabile Leuck. (From Leuckart.) A, Male Rhabditis stage, sexually mature, × 100; B, the mature female parasitic form, × 17, showing at the upper end part of the capsule richly supplied with the tracheae of the host, a beetle; C, female Rhabditis stage, sexually mature, × 100; D, the larva developed from the Rhabditis form, × 102.
The phenomenon presented by the hermaphroditism of Bradynema is, as far as we know, at present unique, as, though some other Nematodes are hermaphrodite, in their case the hermaphrodite form alternates with a bisexual generation. It is further interesting as showing a means by which hermaphroditism may arise, by the suppression of the females and the assumption of their functions by the male. In the case of Rhabdonema nigrovenosum, no females appear in the alternate generation.
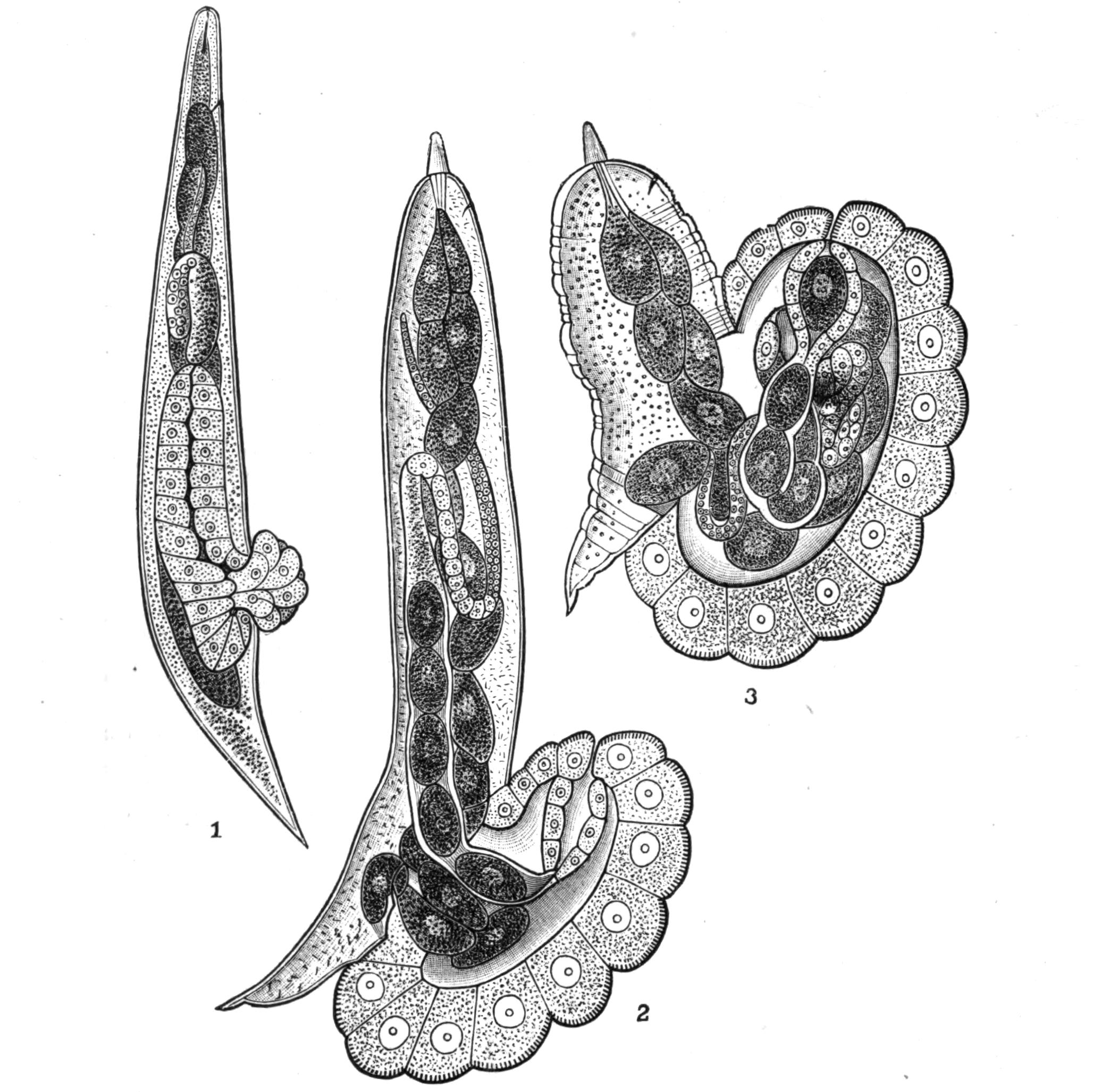
Fig. 75.—Atractonema gibbosum Leuck. (From Leuckart.) 1, Female with commencing prolapsus of the uterus and neighbouring parts, × 130; 2, a further stage, the female being now sexually mature, × 15; 3, a still older stage, with commencing degeneration of the body of the female, × 15.
A similar protandry exists in the parasitic forms of Allantonema,[189] of which there are several species—A. mirabile in Hylobius pini, A. sylvaticum in Geotrupes sylvatica, A. diplogaster in Tomicus typographicus; but in their case the male and female forms which leave their host pair in the damp earth and give rise to larvae which make their way into the body of the beetle-grubs. Here they undergo very extensive retrogressive change. The body of the female, which becomes the shape of a thick sausage, is encapsuled and surrounded by a curious hypertrophied network of tracheae (Fig. 74). As is usually the case with the degenerate parasitic forms, there are practically no organs but the ovary, and this is {153}embedded in a fatty parenchyma which fills all the space within the skin.
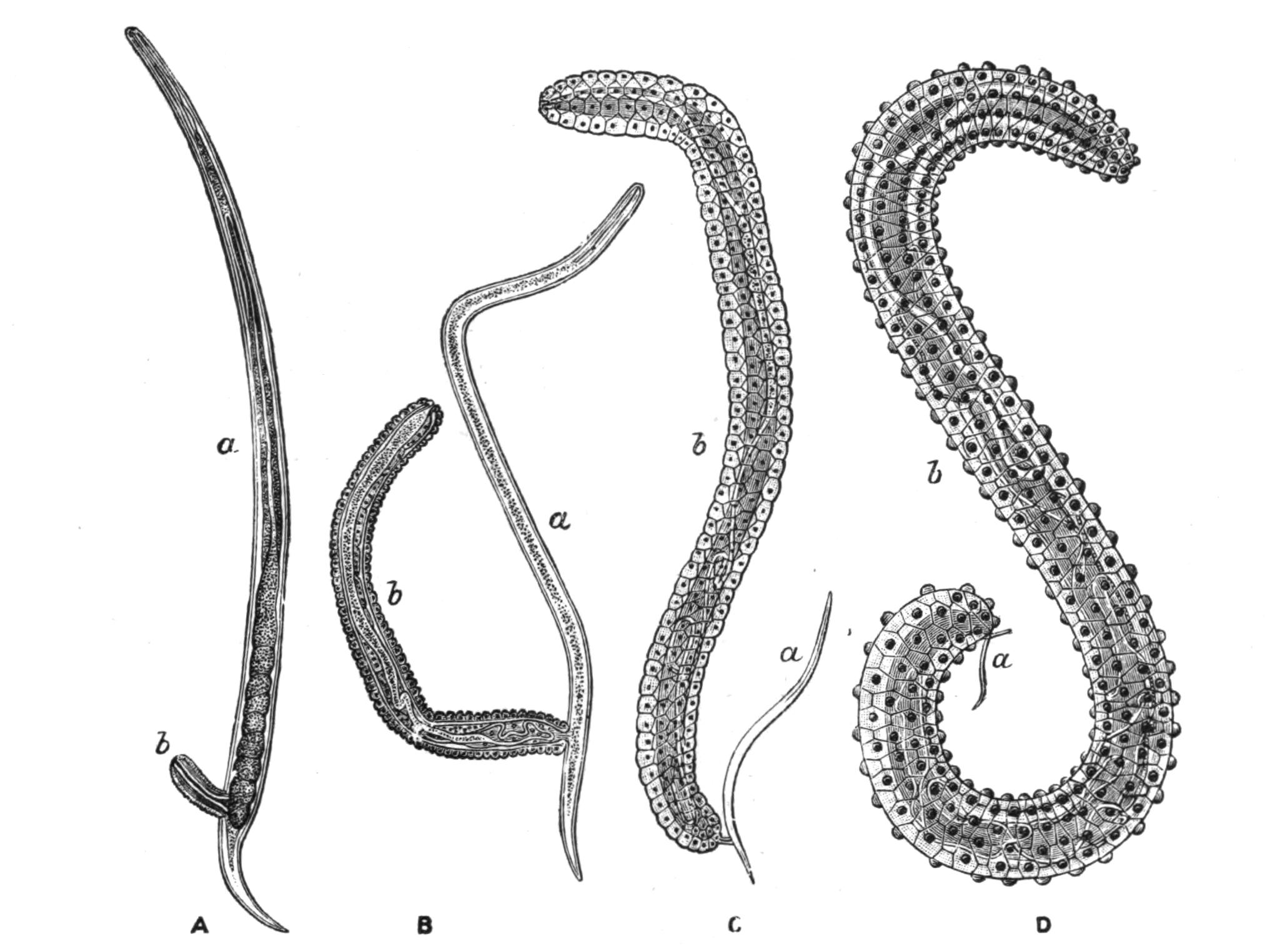
Fig. 76.—Four stages in the life-history of Sphaerularia bombi Dufour, ♀. (From Leuckart.) A, Beginning of the protrusion of the uterus (b), × 66; B, later stage, × 66; C, later stage, × 12; D, the protrusion is complete, × 6. In each case a represents the Nematode, and b its protruded uterus.
Atractonema gibbosum, which lives in the body-cavity of the larva of Cecidomyia pini, has a similar life-history, but the parasitic form has a structural peculiarity which merits attention (Fig. 75). At the time of sexual maturity a swelling, which is caused by the prolapsus of the uterus and vagina, appears at the posterior end of the body; this swelling increases until it equals the rest of the body of the Nematode in size. Even this is far surpassed by a similar protuberance in Sphaerularia bombi, where the evaginated sac grows with such extreme rapidity that in a few weeks its length increases from .25 mm. to 15 mm. and its volume 60,000-fold, the increase being due, according to Leuckart, to the increase in size of the individual cells and not to their multiplication. The Nematode which has produced this enormous growth gets relatively smaller and smaller, and ultimately drops off (Fig. 76). The sexual larvae which arise from the eggs in this sac leave the body of the bee in which this species is parasitic by the anus, {154}and may live in damp earth, moss, etc., for months without taking nourishment, until the autumn, when they become sexually mature and, according to Leuckart, pair. The fertilised female is believed to bore her way into the humble-bee whilst the latter is seeking her underground winter quarters; this accounts for the fact that only queen bees are infected. The parasite is widely distributed both in Europe and North America; it is found in many species of Bombus, but most frequently in B. lapidarius and B. terrestris. The presence of the Sphaerularia affects the reproductive organs of the host, and reduces their fertility, so that an infected queen bee never succeeds in forming a colony.
VI. Family Anguillulidae.
For the most part free living and of small size. The oesophagus has usually a double swelling or two oesophageal bulbs. The male has two equal spicula.
Genera: Diplogaster, Mononchus, Rhabditis, Tylenchus, Anguillula, and many others.
Many species of this family live in humus or decaying matter; others live on, or are parasitic in, plants; some, such as Anguillula aceti, which is found in vinegar and in paste, live in organic fluids.
The part played by the presence of these Nematodes in the soil is not thoroughly understood; sometimes they occur in great numbers, and even when not directly parasitic in plants, probably do them much damage. Cobb[190] has recently described from Australia and Fiji over eighty species, one-half of them new, which occur mostly in the earth, and many of them among plant roots. They frequently crawl up on to plants, especially on to seedlings. An instance of this is given as follows: "The edible part of three bunches of nice-looking celery bought of a Chinaman in Sydney was cut off as far up as it was tender, nearly to the first leaflets. It was washed by hand in a tin dish in tank water, free from Nematodes. The washings gave about 200 to 300 Nematodes, belonging to five different genera."
It is very probable that many of the free-living forms which have received distinct specific names may ultimately turn out to be but stages in the life-history of some of the parasitic species. {155}Von Linstow[191] has pointed out that the free form of A. diplogaster, if found alone, would be placed in the genus Diplogaster; similarly the bisexual form of Ascaris nigrovenosa is known as Rhabditis nigrovenosa.
Those Nematodes which live parasitically in plants, e.g. many of the genera Tylenchus and Aphelenchus and Heterodera, as well as those which only pierce the epidermis of the roots (the remaining species of the above-named genera), are provided with a spine which works to and fro through the mouth and assists the animal to bore into the tissues of the plant. Tylenchus devastatrix lives and reproduces in leaves and stems (never in the roots, except in the case of hops[192]) of many cultivated plants, such as rye, oats, onions, etc. "Clover sickness" is probably caused by this Nematode. The plants become infected by the thread-worms in the soil during the spring; their presence causes swellings and often kills the plant, in which case the worms return to the soil or remain in the straw.
Tylenchus tritici Need. is the cause of "ear-cockles" in corn. These take the form of brown or purple galls, which replace the grains of corn, and which contain hundreds of minute Nematodes. In these galls they are motionless, and are capable of surviving in dryness for at least twenty years; but when moistened,—for instance, by the gall falling on damp earth,—they resume their vitality and make their way to the young wheat plants, and then, wriggling up the leaves and stems, find their way to the ear. Here they pair, and producing a gall-like growth in the flower, lay numerous eggs, from which arise the Nematodes of the ear-cockle.
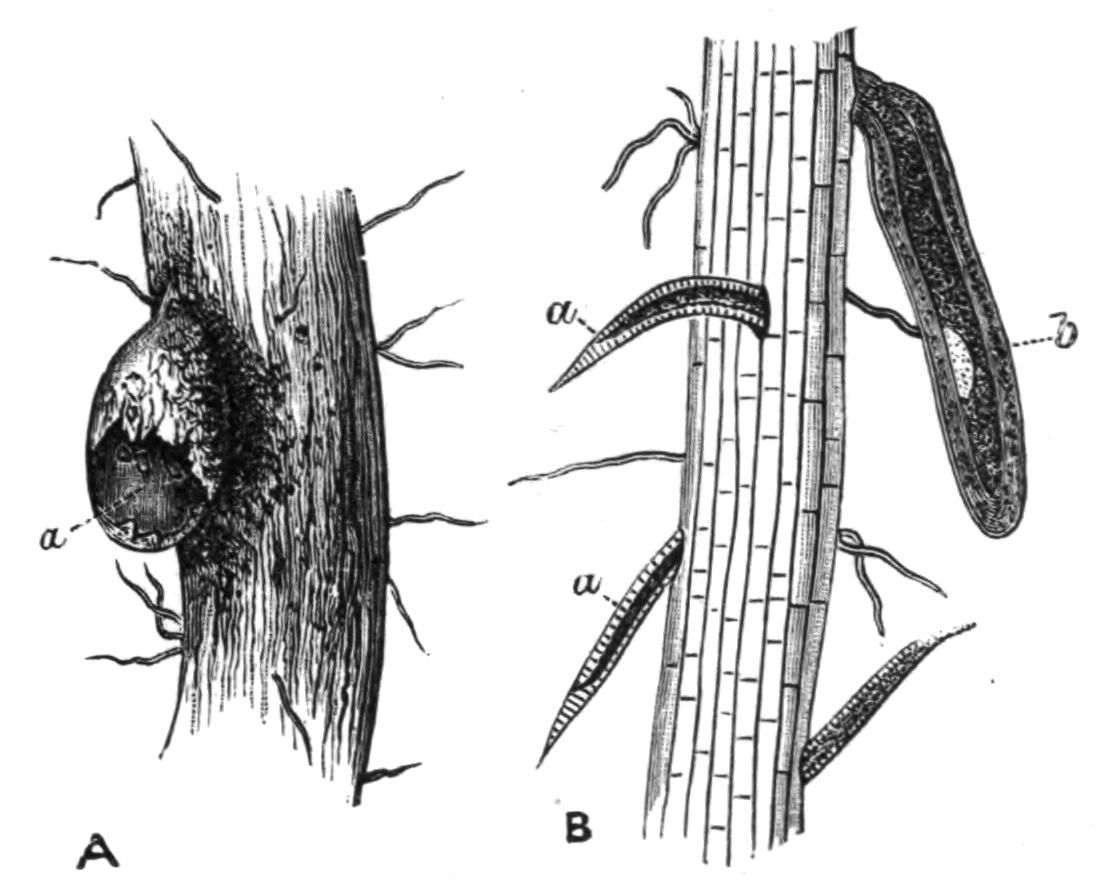
Fig. 77.—A, a, Female Heterodera schachtii Schmidt, breaking through the epidermis of a root; the head is still embedded in the parenchyma of the root: B, a, larvae boring their way into a root; b, larva of the immobile kind surrounded by the old skin, living as an ectoparasite on the outside of the root. (From Strubell.)
Heterodera schachtii[193] Schmidt, is the cause of the "beet {156}sickness," and forms galls or swellings on the roots of many plants, in England especially on the roots of tomatoes and cucumbers. The free larvae live in the earth and make their way into the smaller rootlets; here the female larvae shed their skin, lose their characteristic Nematode form, and become citron-shaped (Fig. 78, D). The male larvae undergo a change, and after a period of rest cast their skin and, leaving the rootlet, seek out the females. The female does not undergo this second ecdysis, but its generative organs grow and mature in what is practically a larval stage. The embryos develop within the body of the mother, and, escaping through the uterus, ultimately cause her death. They then make their way into the earth. The cycle of the development takes but four or five weeks, so that, as in the case of Tylenchus devastatrix, there are several broods in a year; T. tritici, on the other hand, has but one.
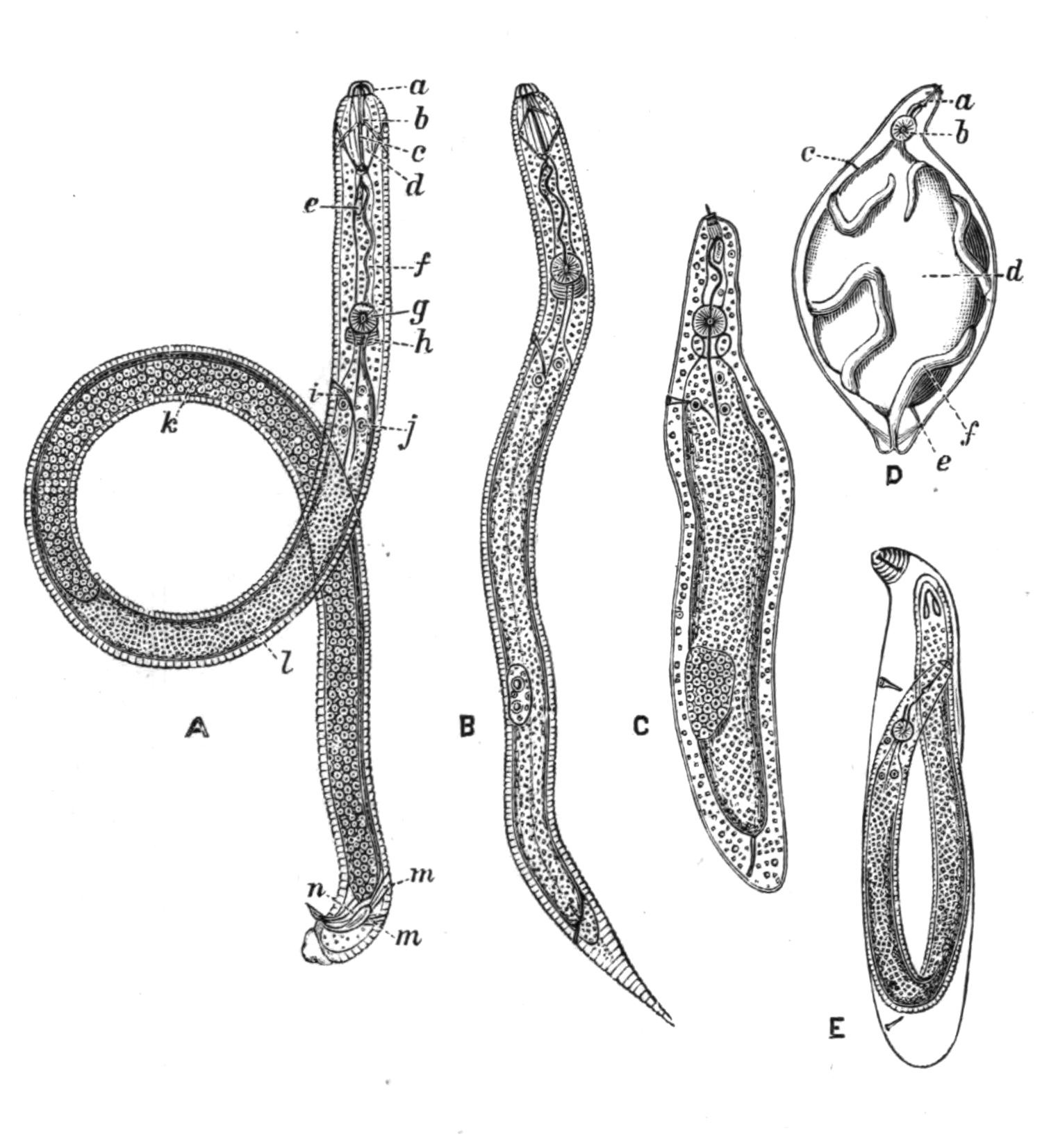
Fig. 78.—A, Male Heterodera schachtii strongly magnified; a, head lappets; b, mouth cavity; c, spine; d, muscle of spine; e, gland; f, oesophagus; g, bulb; h, nerve-ring; i, excretory pore; j, intestine; k, testis; l, intestine; m, muscles moving spicule; n, spicule: B, first motile larva: C, second immovable parasitic larva casting its skin: D, a female with one half of the body-wall taken away to show the coiling generative organs; a, boring apparatus; b, oesophageal bulb; c, excretory pore; d, alimentary canal; e, anus; f, ovary: E, a male shortly before casting its larval skin.
Vuillemin and Legrain[194] point out that while Heterodera is injurious to cultivated plants growing in damp soil, its presence is advantageous to those that grow in deserts. It is very common in the Sahara, and attacks many plants which are {157}immune from it elsewhere. It causes the rootlets to swell out, and the bladder-like extensions thus formed act as reservoirs for water.
Many other species attack plants; Tylenchus millefolii Löw forms galls on Achillea, T. dipsaci Kühn. on the teazle. They all seem to have great powers of resisting desiccation. The former species, when dried and placed in a herbarium in May, gave rise to active worms when moistened the following October; and the corn eel-worm is said to survive twenty-seven years in a state of suspended animation. On the other hand, although these Nematodes like moisture, they cannot withstand submersion in water for any time. They can resist a considerable degree of cold, and a species, Aphelenchus nivalis Auriv.,[195] has been described from Spitzbergen, where it lives in the snow amongst a small red alga, Sphaerella nivalis.
VII. Family Enoplidae.
Small, as a rule free-living, usually marine Nematodes, without a second oesophageal bulb. Eyes and mouth-armature often present. Fine hairs and bristles sometimes surround the mouth.
Genera: Enoplus, Dorylaimus, Enchelidium, and others.
The genus Enoplus is exclusively marine, living amongst Algae and Hydroids in shallow water and moving actively about, but never coiling into spirals. De Man[196] describes Enoplus brevis Bast. as being attacked by a plant parasite, probably a Bacterium, of a greenish colour, which infested the muscles and gave them a peculiar colour.
Numerous other species have been described by De Man from the coast of Holland. It is probable that some of them are the free stages of parasitic forms; a brackish water species found in the East Indies (Dorylaimus palustris) is regarded by Carter as the larva of Filaria medinensis. Oncholaimus echini Leyd. is parasitic in the intestine of the sea-urchin Echinus esculentus. Tricoma cincta[197] has a strongly striated cuticle, which gives it almost the appearance of segmentation. Fimbria tenuis has numerous hairs on the tail, and the mouth is surrounded by bristle-bearing papillae.
Here must be mentioned two families closely allied to the true Nematodes.
(i.) Chaetosomatidae.—This family includes three genera: Chaetosoma, Rhabdogaster, and Tristicochaeta. According to Metschnikoff,[198] although they are not true Nematodes, they have a great likeness to the group. He distinguishes them from the swimming members of the group as "creeping Nematoda." Chaetosoma, of which two species are known, C. ophicephalum and C. claparedii, has a head distinct from the body (Fig. 79). The mouth is at the anterior end, surrounded by a double semicircle of movable spicules; the whole body is covered by fine hairs, and on the ventral surface, just in front of the anus, is a double row of about fifteen cylindrical projections, by whose agency the animal creeps. The female C. claparedii is 1.5 mm. long, the male 1.14 mm. They were found creeping about on sea-weeds in the neighbourhood of Salerno.
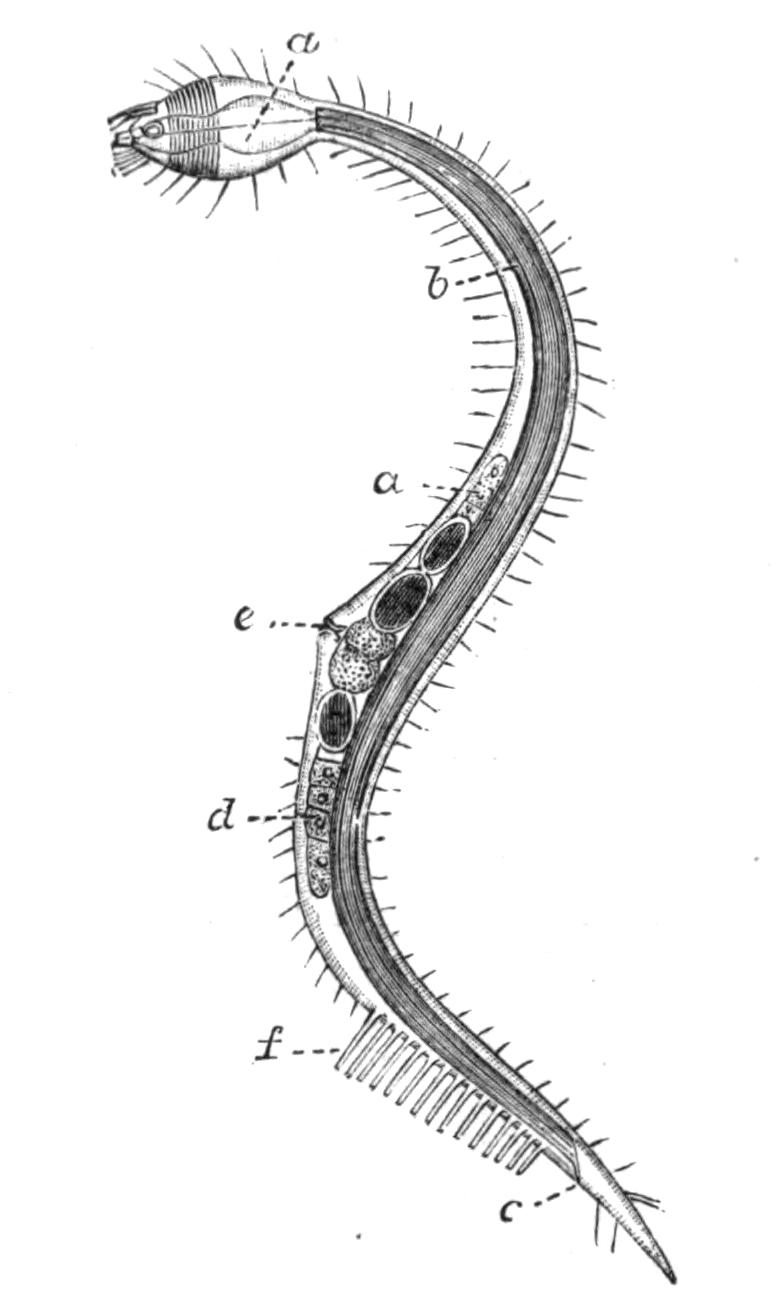
Fig. 79.—Mature female of Chaetosoma claparedii Metschni., × 57. (From Metschnikoff.) a, Oesophagus; b, intestine; c, anus; d, ovary; e, generative pore; f, ventral bristles.
The genus Tristicochaeta[199] differs from the foregoing in having three rows of locomotor projections instead of two.

Fig. 80.—Tristicochaeta inarimense Panceri, in one of its most usual positions, showing the triple row of ventral bristles, × 100. (From Panceri.)
Rhabdogaster has no head distinct from the body, though the anterior part of the body is swollen. A second swelling occurs, as is also the case with Chaetosoma, in the region of the opening of the genital ducts. The female in Rh. cygnoides attains a length of 0.36 mm. In this genus the hairs are confined to {159}the dorsal middle line. The locomotor projections are hooked, and are much finer than those of Chaetosoma, and they are situated farther forward than in the last-named genus. Rhabdogaster occurs in the same surroundings as Chaetosoma. Ch. ophicephalum is recorded from the English Channel.
(ii.) Desmoscolecidae.—The members of this family are minute, and are characterised by the presence of well-marked ridges which surround the body and give it an appearance of segmentation. The head, which is somewhat swollen, bears four bristles, and single pairs are borne by a certain number of the ridges, some on the dorsal and some on the ventral surface. These hairs can be moved independently of one another. Two red eye-spots are described between the fourth and fifth rings. The sexes are distinct, and the internal organs generally have a marked resemblance to those of the true Nematoda. The Desmoscolecidae move by looping their bodies after the manner of the Geometrid caterpillars, as well as by creeping with their bristles. The genus contains numerous species[200]: D. minutus Clap. (English Channel), D. nematoides Greef, D. adelphus Greef, D. chaetogaster Greef, D. elongatus Panceri, and D. lanuginosa Panceri. They are exclusively marine.
Trichoderma oxycaudatum Greef[201] is a minute animal, 0.3 mm. long, which has no head or ventral spines, but whose body is ringed and covered with long hair-like bristles. The male has two spicules, and the internal organisation recalls that of other Nematodes; still its ringed body has induced some authorities to place it near to Desmoscolex.
The Life-History of Nematodes.
Although, considering the enormous number of species of Nematodes and the remarkable diversity of the conditions under {160}which they live, their bodily structure shows a very striking uniformity, the same is by no means the case with their life-history, which exhibits an astounding variety. Von Linstow[202] has arranged the various modifications, which occur under fourteen heads. He includes in his list the Gordian worms, which we have placed under a different heading. The following account has been taken from his paper, with a few alterations:—
1. The embryos develop, with a larval stage and without any change of medium, directly into the mature sexual forms. They live in fresh, brackish, or salt water, in plants, in the earth or in decaying organic matter: examples, Dorylaimus, Enoplus, Plectus, Monhystera.
2. The larvae live in the earth, the sexual forms in plants: examples, Tylenchus tritici and T. devastatrix, Heterodera schachtii (Figs. 77 and 78).
3. The larvae live in animals, after whose death and decay they are set free and develop into the sexual animals in the earth: example, Rhabditis pellio.
4. The bisexual forms live in the earth, and the fertilised females bore into animals (insects), and here produce embryos: example, Sphaerularia bombi (Fig. 76).
5. The bisexual forms live in the earth; the females do not develop, but the males make their way into Insects (Beetles), and becoming hermaphrodite, develop ova which give rise to the bisexual form: example, Bradynema rigidum.
6. The larvae live in the earth, the sexual form in Vertebrates: examples, Dochmius, Strongylus.
7. The Nematode lives as a hermaphrodite in animals, the offspring of this, by an alternation of generations, become sexual in the earth: example, Rhabdonema in Frog.
8. A bisexual free form gives origin to a bisexual parasitic form living in an animal: example, Leptodera appendiculata in Snails.
9. The eggs develop in the earth, and give rise to embryos which are transferred whilst still in the egg-cell to the body of an animal. The embryos hatch out and form bisexual parasites: examples, Oxyuris, Trichocephalus.
10. The larvae live in insects, the sexual worms in water or in the earth: example, Mermis.
11. The larva lives encapsuled and is passively transferred to {161}a second animal: examples, Ollulanus, from Mouse to Cat; Cucullanus elegans, from Cyclops to Perch; Spiroptera obtusa, from Meal-worm to Mouse.
12. The sexual form lives for a short time in the intestine of a Vertebrate, and produces larvae which bore through the intestinal wall and become encapsuled in the tissues: example, Trichina spiralis.
13. The sexual animal lives in the trachea of birds; the ova containing embryos are coughed up and are taken into other birds with food. They quit the egg-shell and wander into the air-sacs, and finally into the trachea: example, Syngamus.
14. There are two larval forms; the first lives in water, the second in the lungs of Amphibia, whence they wander into the intestine and become sexually mature: example, Nematoxys longicauda in Triton alpestris.
Parasitism.
1. Effect of Parasitism on the Parasite.—The usual effect of parasitism on the parasitic organism is that the various organs necessary for a free life tend to degenerate, whilst there is a multiplication and development of organs of adhesion, by means of which the parasite maintains its hold on its host. There is further an immense increase in the powers of reproduction, which may take the form of an increase in the number of fertilised eggs produced, or the parasite may at some time of its life reproduce asexually, by budding, or fission, or parthenogetically.
Of the various classes of animals which are more or less parasitic, the Nematodes show less difference between the free-living and parasitic members of the group than obtains in any other class. With few exceptions, such as Sphaerularia, Allantonema, and one or two others, the parasitic forms have undergone but little degeneration. It is true that they have no eyes such as the free forms often possess, but in other respects, such as in the nervous, muscular, and digestive systems, they do not show any marked retrogression; further, the mouth-armature is developed in many free forms, and is not confined to the parasites.
The group has developed no methods of asexual reproduction by budding or fission, such as are found in Platyhelminthes; and the cases of an alternation of generations in which a sexual form alternates with a parthenogenetic form, are rare, e.g. {162}Rhabdonema nigrovenosum; and it seems possible that even when parthenogenesis has been described, further observation may show that the parthenogenetic stage is really a protandrous hermaphrodite, in which case the alternation of generations in Nematodes, i.e. the hermaphrodite alternating with the dioecious form, is a case of heterogamy or the alternation of two sexual generations.
On the other hand, parasitic Nematodes produce enormous numbers of eggs. Van Beneden states that 60,000,000 have been computed in a single Nematode, and this multiplication of ova is absolutely necessary, for the chance of the embryo reaching the right host, in which alone it can develop, is always a small one.
It is a common thing to find that parasites are either hermaphrodite or that the male is degenerate, as is the case with many of the parasitic Crustacea, but with one or two exceptions the Nematoda are bisexual, and although, as a rule, the males are smaller than the females, they show no other trace of degeneracy.
In spite of the fact that the class as a whole shows but few special modifications consequent on a parasitic mode of life, it is clear that the Nematoda are peculiarly adapted for such a mode of life. Their elongated thread-like bodies afford little resistance to the passage of the food, which, as it passes through the intestine of the host, might tend to carry the parasites out of the body. At the same time their shape enables them to pierce and wriggle through the various tissues without making any very serious lesions such as might prove fatal to their host. Their extraordinary power of resisting desiccation both in the egg and in the adult state vastly increases their chances of ultimately hitting on the right host. They are capable of living in a state of suspended animation for months, and even years when dried (vide p. 136), and of resuming their activity on being moistened.
The great faculty this group shows for living parasitically is evinced by the extraordinary variety of life-history presented by the different species. There is scarcely a stage which may not be parasitic; the eggs, the larvae, the adults are all in some cases free, in others parasitic, and in many cases first the one and then the other.
2. Occurrence and Effect of the Parasite on the Host.—Von Linstow states that the only law that can be derived inductively from the study of the life-history of Nematodes is that those that live in animals never pass through all their stages of development in the same organ; consequently, in considering the distribution of {163}the parasites within the body of their host we have a double habitat to consider. Many forms, such as Trichina spiralis, wander from the intestine to the muscles; others, such as Filaria medinensis, from the alimentary canal to the lymphatics or blood vessels or subcutaneous tissues. Others pass from the body-cavity to the intestine, as the Mermithidae, which infest Insects, or from the stem and leaves of a plant to its flower, as in the case of Tylenchus tritici.
With regard to their occurrence in the different classes of the animal kingdom, they have been most frequently observed in Vertebrates and in Insects. They are comparatively rare in the other large divisions. Many genera are confined to certain hosts: thus Ascaris, Filaria, Trichosoma occur only in Vertebrates; Spiroptera (with one exception) in Mammals and Birds; Cucullanus in Fishes and Amphibia; Strongylus and Physaloptera in Mammals, Birds, and Reptiles; Dochmius, Pseudalius, Trichocephalus in Mammals; Dispharagus, Hystrichis, Syngamus in Birds; Nematoxys, Hedruris in Amphibia and Reptiles; Ichthyonema in Fishes; and Isacis and Mermis in Insects.
Twenty-two species have been described as parasitic in man, of which perhaps the most dangerous are Filaria medinensis, the three varieties of F. sanguinis hominis; Dochmius (Ancylostomum) duodenalis, and Trichina spiralis. The Ascaridae, as Ascaris lumbricoides and Oxyuris vermicularis, though painful, seldom cause death.
The enormous number of parasites harboured by one host is shown by the fact mentioned in Leuckart's Parasites of Man, that Nathusius[203] took from a single black stork 24 specimens of Filaria labiata from the lungs, 16 Syngamus trachealis from the trachea, more than 100 Spiroptera alata from the coats of the stomach, besides several hundred Trematodes belonging to several different species (see p. 63). Even this has been surpassed in the case of a young horse, in whose body Krause found 500 Ascaris megalocephala, 190 Oxyuris curvula, several millions of Strongylus tetracanthus, 214 Sclerostomum armatum, 287 Filaria papillosa, 69 Taenia perfoliata, and 6 Cysticercus forms.
It is impossible here to enter into a full description of the destruction caused to domesticated animals and crops by the presence of these parasites; full details will be found in books dealing {164}especially with this question, such as Neumann's Parasites and Parasitic Diseases of Domesticated Animals. A couple of cases will show how important this matter is to the farmer. Crisp estimates that Syngamus trachealis causes the death of half a million pullets in England every year, and Mégnin states that in a single pheasantry 1200 victims died daily; again, the loss of one-third the crop of beetroot is by no means uncommon when it is infested with Heterodera schachtii. These show the practical importance of what at first sight seem quite insignificant animals, and the necessity for the minutest observation, for only when we are fully acquainted with all the details of the life-history of a parasite are we in a position to successfully combat it.
Sub-Order II. Nematomorpha.
Until the last few years it has been customary to regard the Gordiidae as a family of Nematodes. Although in external appearance and life-history they closely resemble the members of this group, yet recent research has shown so many important morphological differences between them and the Nematoda, that most zoologists are now agreed in placing them in a different sub-Order, the Nematomorpha, a name first suggested by Vejdovsky.[204]
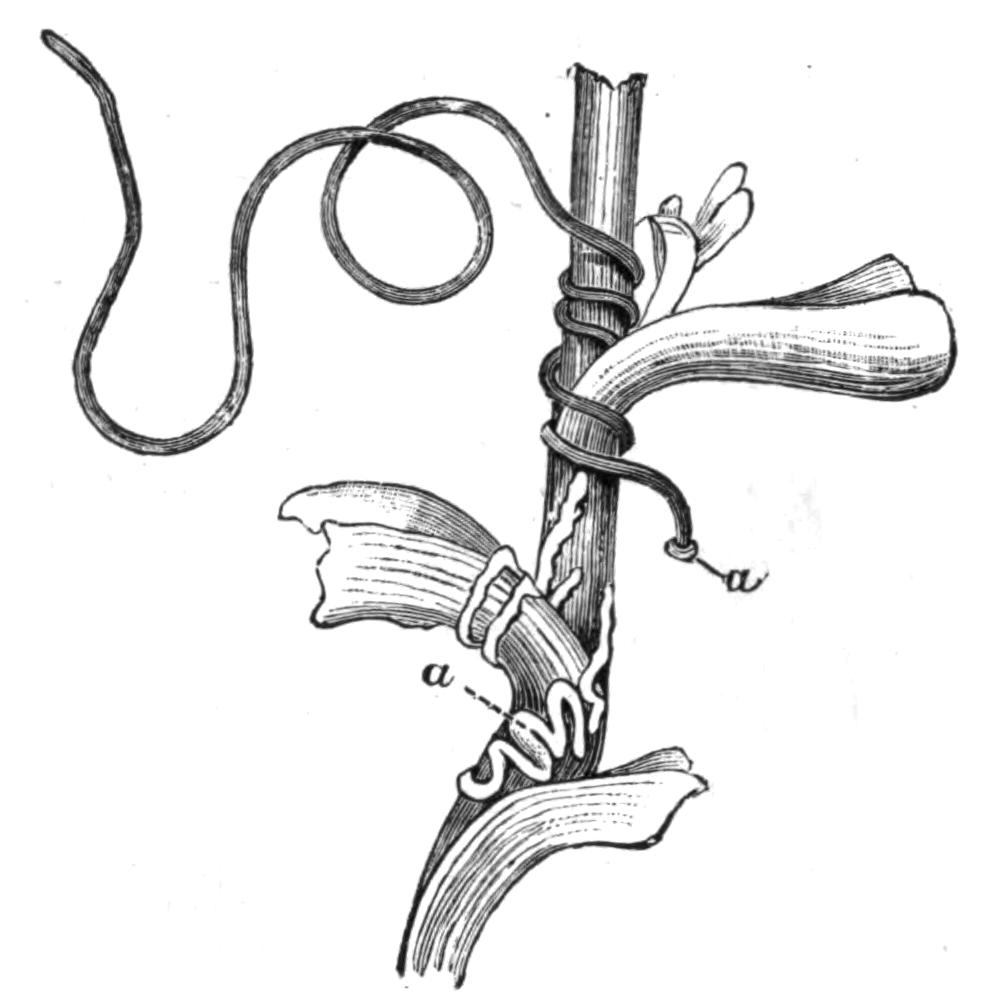
Fig. 82.—A water plant around which a female Gordius is twining and laying eggs. a, a, Clump and string of eggs. (From von Linstow.[205])
The Gordiidae comprise but two genera, Gordius and Nectonema. The latter has but one species, N. agile Verr., and is marine; the former, on the other hand, is exclusively fresh-water, and contains a very large number of species. Gordian worms are frequently to be found in ditches, ponds, or large puddles, moving with an undulating motion through the water, or twining and writhing round water-plants; they are scarcer in running water. In shape they are like a piece of thin whip-cord, slightly tapering {165}at each end; the male, however, is easily distinguished from the female by its forked tail (Fig. 89). Not unfrequently a considerable number are found inextricably tangled together into a knot, and the name of the genus refers to this fact. Where numbers have suddenly appeared in water hitherto free from them, legends have sprung up which attribute their presence to a rain of worms; in reality they have come out of the bodies of Insects in which they are parasitic for the greater part of their life.
The genus Gordius passes through three distinct stages, of which the first two are larval and parasitic; the third is sexually mature and lives in water. The second larval stage closely resembles the adult, but the reproductive organs are not developed. The following account of the structure of this larval form and of the adult is in the main taken from von Linstow.[206]
The whole body is covered with a well-developed two-layered cuticle, which in the adult is marked out into areas, and bears numerous minute sensory bristles, which are especially developed in the neighbourhood of the cloaca of the male. Beneath this is a hypodermis which differs markedly from the sub-cuticle of Nematodes, inasmuch as it consists of a single layer of polygonal nucleated cells. Within this lies a single layer of longitudinal muscle-cells, which differ from the corresponding layer of Nematodes in having that part of their medulla which is not surrounded by the contractile portion directed outwards towards the hypodermis, and not inwards towards the body-cavity.
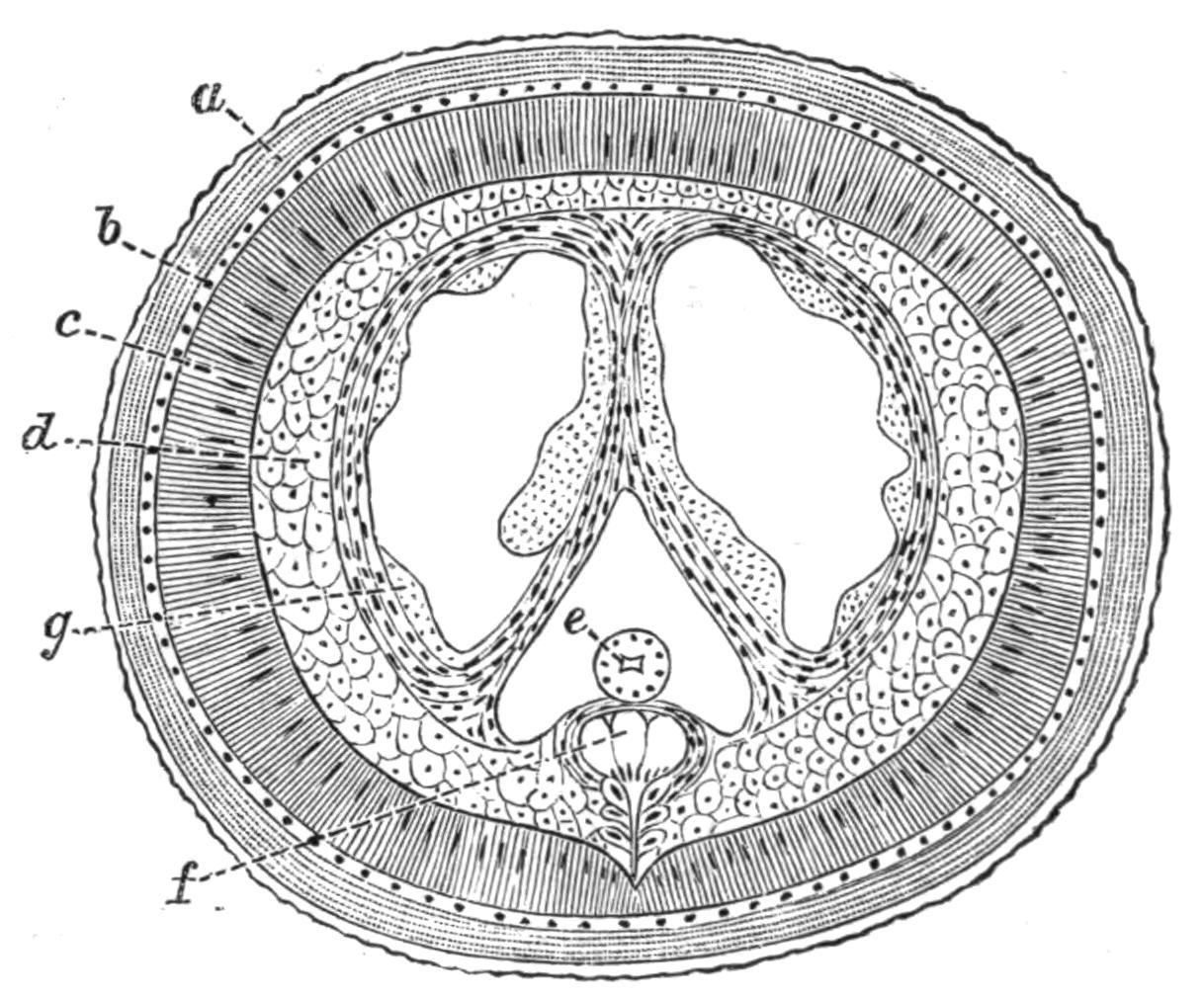
Fig. 83.—Transverse section through a young male Gordius tolosanus Duj. (From von Linstow.) Highly magnified. a, Cuticle; b, hypodermis; c, muscular layer; d, parenchyma; e, alimentary canal; f, nervous system; g, cells of the testis.
The body is in the younger stages practically solid, the interior being filled with clearly defined polygonal cells which are arranged in definite rows; in later life certain splits arise in this tissue which {166}subserve various functions; between these splits strands of tissue are left which form mesenteries, and some of the cells remain lining the muscular layer (Fig. 86). These cells have been described by Vejdovsky as a definite somatic, peritoneal epithelium, but this was not found by von Linstow. Besides forming the mesenteries, and acting as packing between the various organs of the body, these cells also form the ova and the spermatozoa.
The splits which have appeared when the animal has reached the second larval stage, are two dorsal and a ventral; the latter contains the alimentary canal, and may be termed the body-cavity, the former will develop the generative organs. The mouth is occluded in the older larvae, and in the adults there is a distinct but solid oesophagus which passes into a tubular intestine. The intestine consists of a single layer of cells surrounding a lumen; it runs straight to the hinder end of the body, where it opens in both sexes with the ducts of the reproductive organs.
The nervous system consists of a well-defined circumoesophageal ring with two dorsal swellings, and, arising from this, a median ventral cord which runs the whole length of the body. The cord consists of three longitudinal strands with ganglionic cells below them; the latter, though they lie within the muscle layer, maintain a connexion with the hypodermis. Behind, the nerve-cord splits in the male, one half passing into each caudal fork. In the adult a pair of black eyes can be detected on the head; the only other sense organs are the tactile bristles mentioned above. Excretory organs are unknown.
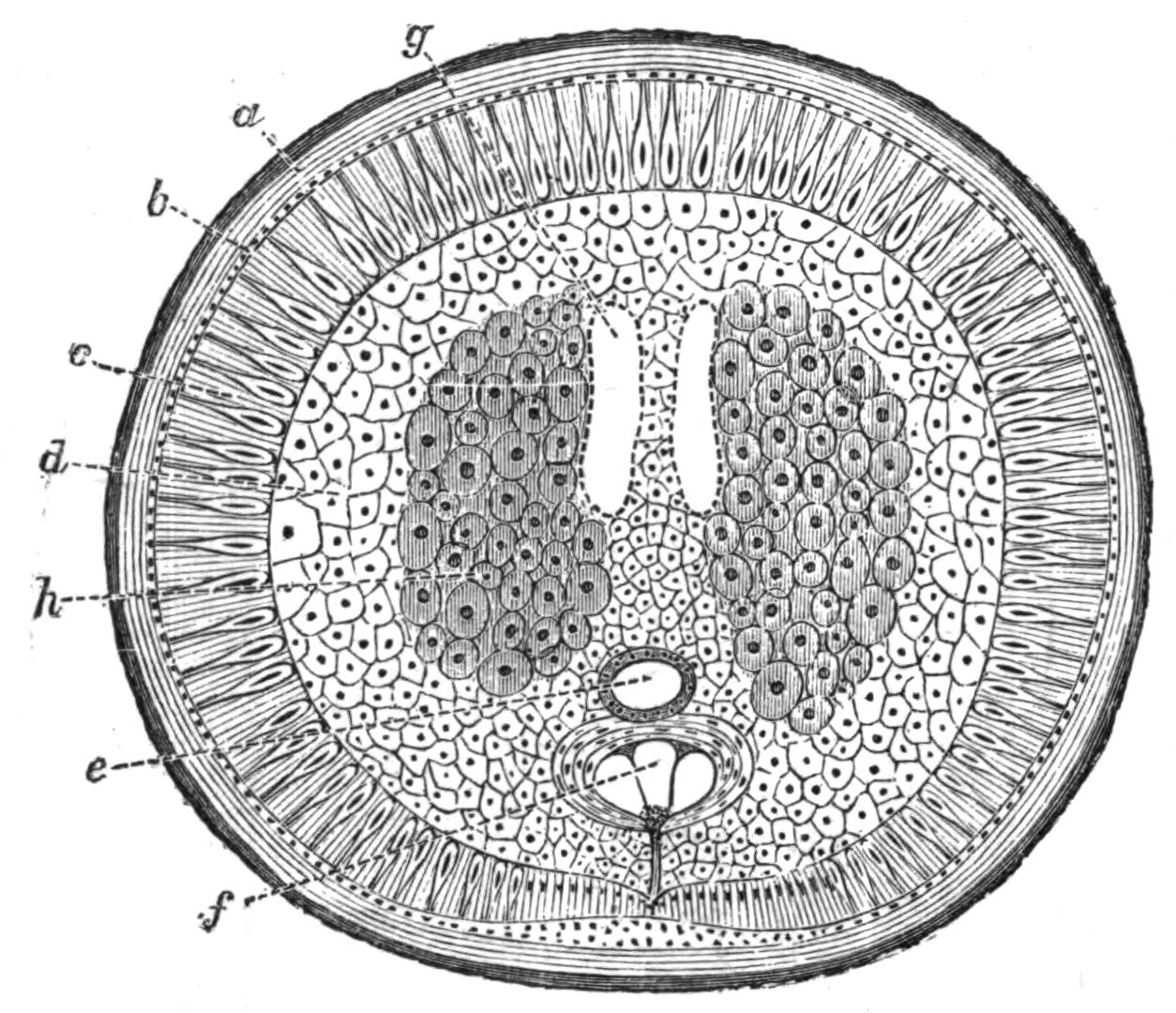
Fig. 84.—Section through a young female Gordius tolosanus. (From von Linstow.) a, Cuticle; b, hypodermis; c, muscular layer; d, parenchyma; e, alimentary canal; f, nervous system; g, egg-sac; h, ovary.
The generative organs only attain maturity in the adult, which is, in fact, exclusively devoted to reproduction. No trace of testes {167}is found in the larva, though the two dorsal splits from the walls of which the spermatozoa will arise are present. They are lined by a definite epithelium (Fig. 83), and this serves at once to distinguish them from the body-cavity. Posteriorly the splits narrow and become the two vasa deferentia which open one on each side into the cloaca. The cells lining the lumen give rise to secondary cells, and these become spermatozoa, the process extending from behind forwards. The external organs—bursa, etc.—described by Vejdovsky were not found by von Linstow.
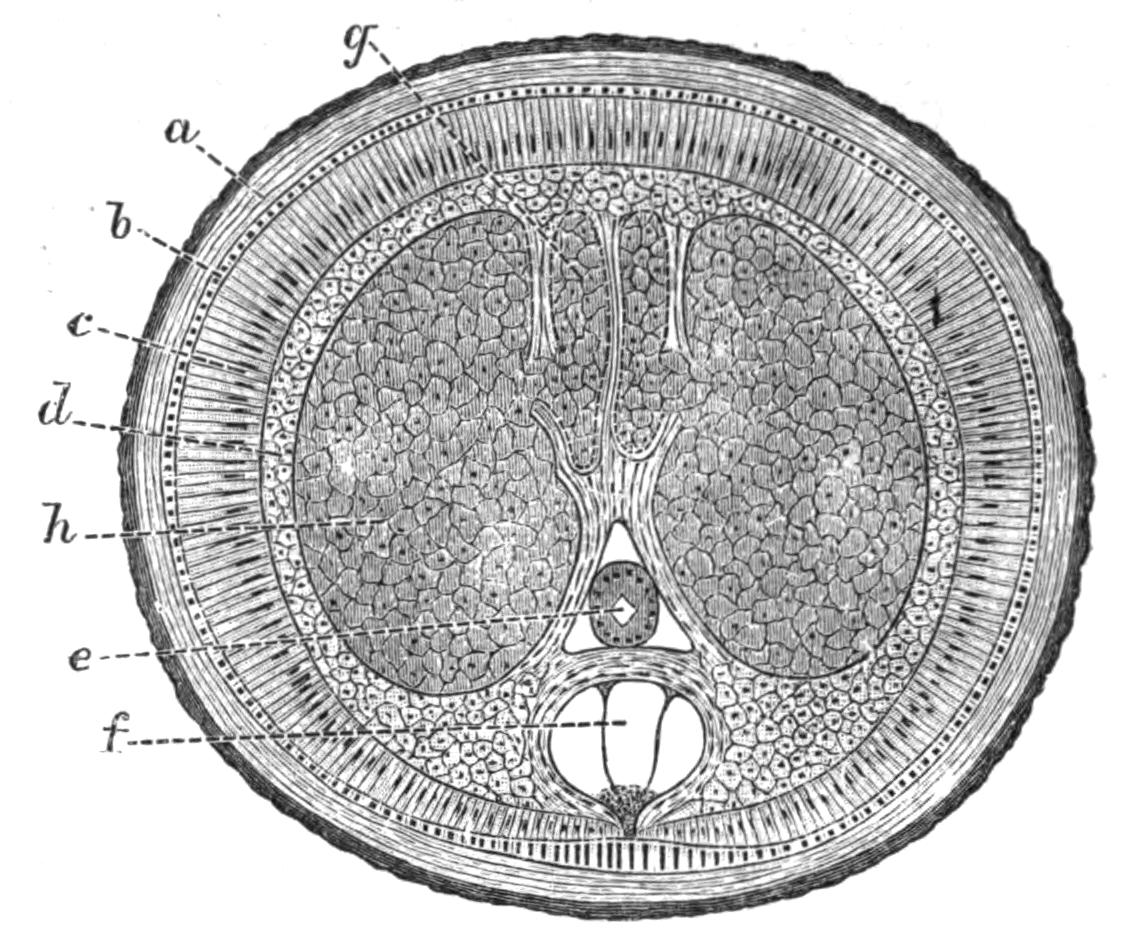
Fig. 85.—Section through a mature female Gordius tolosanus. (From von Linstow.) Lettering as in Fig. 84; g, egg-sac; h, ovary.
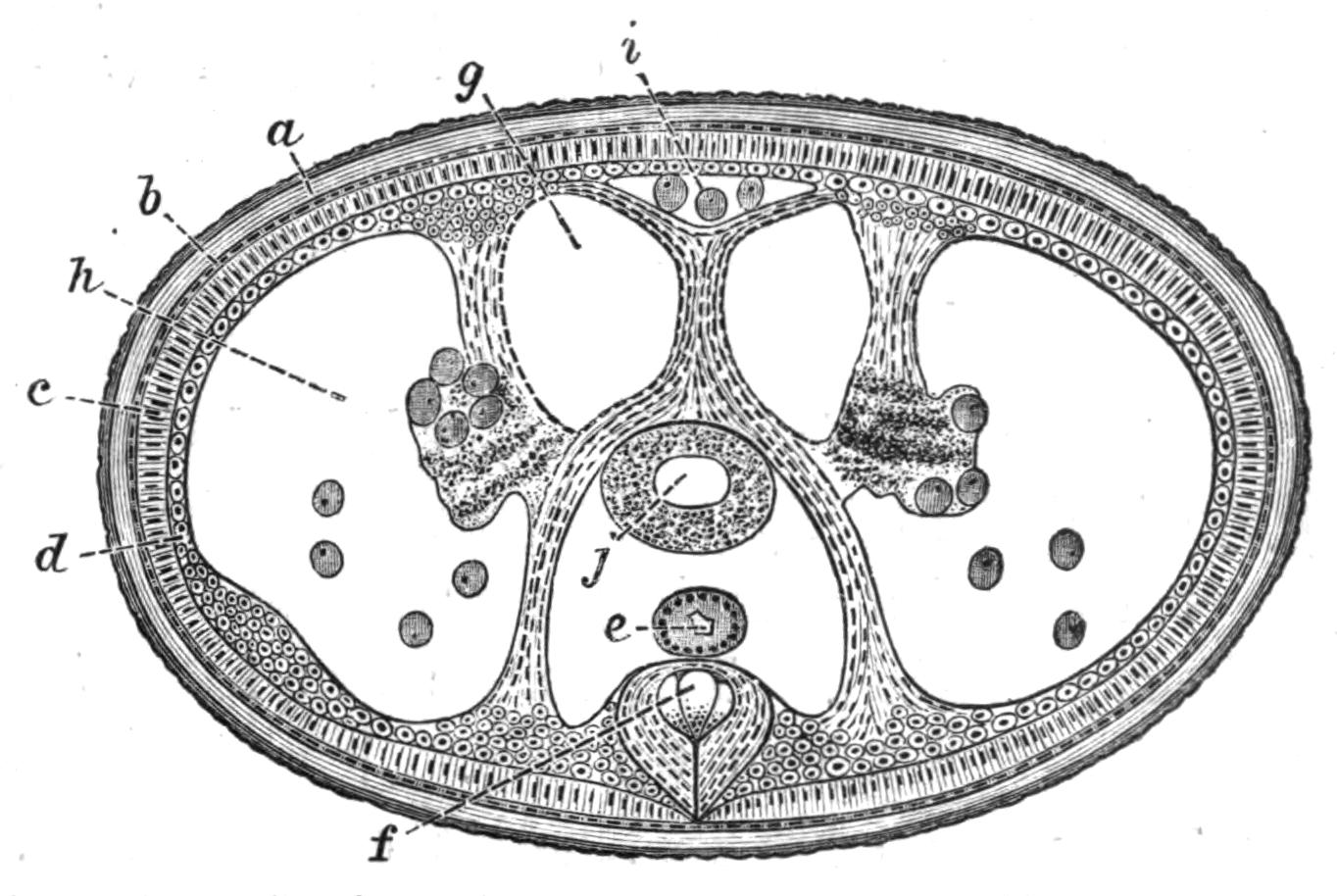
Fig. 86.—Section through a female Gordius tolosanus when the deposition of ova is almost complete. a, b, c, d, e, and f, as in Fig. 84; g, egg-sac; h, ovary almost empty; i, dorsal canal containing eggs; j, receptaculum seminis.
In the female larva two similar splits are present; these form the egg-sacs. Posteriorly they end in two short oviducts which open into a uterus, in which fertilisation takes place, and in which the secretion arises which cements the eggs together. In the adult the ovaries and a receptaculum seminis are found, in addition to the organs present in the larva. The ovaries are formed from modifications of the packing tissue; they begin close behind the head, and soon attain such dimensions as to compress the egg-sacs and body-cavity to small slits. After a time the wall between the ovary and the egg-sacs becomes absorbed, and the eggs grow into the latter. In the old females, where the egg sacs are empty, there is a considerable space round the exhausted ovary, into which eggs continue to fall off; there {168}is also a median dorsal canal which contains a few eggs. By this time the wall between the ovary and the egg-sac has again appeared.
One of the most interesting points about the female is that, according to Vejdovsky, the ovary is segmented, the cells which form the ova being heaped up in segmentally-arranged masses. This observation, if correct, is almost the only instance of segmentation recorded in the group Nemathelminthes.
Fig. 87.—Nectonema agile Verrill. A, The adult. Magnified. (After Fewkes.) B, Longitudinal section through the head. × about 20. (From Bürger.) a, Mouth; b, circumoesophageal commissure (dorsal); c, cell of salivary gland; d, septum cutting off head from rest of body; e, testis; f, ventral cord; g, oesophageal cells; h, lumen of oesophagus; i, cerebral ganglion (ventral).
The only other genus which is associated with Gordius in the group Nematomorpha is Nectonema, of which there is as yet but one species known, Nectonema agile Verr.[207] Our knowledge of the anatomy of this worm is due mainly to Bürger[208] and Ward.[209] Nectonema is a marine worm found swimming near the surface of the sea with rapid undulatory motion. The males are from 50 to 200 mm. long, the females from 30 to 60 mm. The body is faintly ringed, and bears two rows of fine bristles on each side. Owing to a curious torsion of the body through a right angle, the lateral bristles of the anterior third seem to be placed in the ventral and dorsal middle line. They are very easily broken off. The body is divided into a small anterior and a large posterior {169}chamber by a transverse septum placed a little way behind the head. The anterior chamber contains the brain and is lined by a definite epithelium, the posterior is not. The layers of the skin correspond with those of Nematodes or of Gordius, but the hypodermal cells show no cell outlines; still they are not so modified as in the former group. The hypodermis is thickened in the median dorsal and ventral line, and the single nerve-cord lies in the latter.
The alimentary canal is degenerate, as in Gordius. A mouth exists, but it is minute, and opens into a very fine tube lined with chitin, which pierces through the substance of a single elongated cell. This minute oesophagus, with its coextensive cell, reaches back to the transverse partition, but behind this a few other cells become associated with it, and ultimately the lumen of the alimentary canal is surrounded by four cells; but the number diminishes behind, and soon only two cells surround the tube at any one level, and the intestine dwindles away some little distance in front of the tail. There is no sign of an anus. A circumoesophageal nerve-ring exists, of which the ventral part is by far the larger (Fig. 87); it gives off a ventral nerve-cord, which swells posteriorly in the male into a large anal ganglion, far bigger than the brain, and larger in the male than in the female.
The testes consist of a dorsally placed sac, continuous behind with a vas deferens; this opens at the posterior end, which is pointed and slightly curved ventrally. The ovary is unknown; but females have been found with their body-cavity crammed with ova; these escape, like the spermatozoa, from a genital pore at the posterior end of the body.
Classification.—The separation of the Nematomorpha from the Nematoda depends mainly on the character of the nervous system, the absence of the lateral lines and of the dorsal line, the character of the contents of the body-cavity, and the character of the reproductive organs. In Gordiidae the latter are always placed dorsal to the intestine, and ovaries and testes open alike at the hinder end of the body. The importance of the differences in the organs just enumerated has been considered sufficient to justify the removal of the Gordiidae from the Nematoda, and the establishment of the special sub-Order Nematomorpha for their reception; and although Nectonema has a dorsal line, and is in some other respects intermediate between the two groups, there can be little doubt that it is more closely allied to Gordius than to any member {170}of the Nematoda, and it must therefore be placed with it in the Nematomorpha.
On the other hand, it ought to be mentioned that Camerano[210] found that the chief details of the fertilisation and development of the egg in Gordius closely conform with what is known of the same processes in Nematodes, and he is of opinion that these resemblances are sufficiently important to justify the retention of the group among the Nematoda.
Life-History.—The life-history of Gordius comprises four stages—the early development of the egg, the first larval form, the second larval form, and the sexually mature form. Both larval forms are parasitic, and during their life they are actively engaged in feeding; the free form, on the other hand, takes in no nourishment, and is exclusively engaged in reproduction.
Fig. 88.—Abdomen of Pterostichus niger with the terga removed to expose the Gordius larva within. Slightly magnified. (From von Linstow.)
Von Linstow[211] gives the following account of the life-history of G. tolosanus, a form which has been more fully worked out than any other. In the month of April numerous specimens of the beetle Pterostichus niger were found floating on the surface of the ditches and small ponds in the fields surrounding Göttingen. Some were found dead or dying; others appeared quite healthy, and these were swimming actively, endeavouring to reach land. Within the abdomen of these beetles, in about 20 per cent of those collected, the second larval form of the G. tolosanus was found. The longest larvae were 122 mm. in length, and very soft, partly snow-white and partly brown in colour; traces of the boring apparatus of the first larval form were still to be seen, but in other respects the larva only differed from the free form in the immaturity of its sexual organs. Besides the parasite hardly anything was to be found in the abdomen of the beetle, the larva having eaten up all trace of the fat body and the generative organs of its host. The larvae bored their way out of the body of the beetle and became adult animals.
It is rather difficult to say what brings these essentially {171}terrestrial beetles to the water, but von Linstow suggests that, as they live partly on snails, and at this time of year there are not many land-snails about, they may be in search of water-snails such as Limnaea. They may also be sometimes blown into the water by wind storms, but, whatever the cause is, their presence in water is essential for the continuance of the life of their parasites.
Once free in the water the Gordius is soon sexually mature; the fertilisation takes place in April, and then the female may be seen twisting and writhing round the stems of water-plants and laying the long bead-like strands of eggs (Fig. 82). The first deposition observed by von Linstow took place on 14th April, the last on 2nd August, and the period of egg-laying for each female extended over four weeks. At first the eggs are snow-white, but within twenty-four hours they turn brown in colour.
The development of the first larva within the egg takes about a month. When it emerges from the egg-shell it is minute, .065 mm. long, ringed anteriorly, and provided with a protrusible and retractile boring apparatus consisting of three chitinous rods; round the base of this piercing proboscis is a double crown of papillae, each bearing a spine (Fig. 90).
Fig. 89.—The tail ends of a female Gordius (a) and a male (b) in copula. × 1.5. (From G. Meissner.[212])
This first larval form breaks through the egg-shell and sinks to the bottom of the water, where it moves about sluggishly and awaits the arrival of the right host in which to take up its abode. This host is the larva of the Alder-fly, Sialis lutaria Lin. (vide vol. v. p. 444), and into this it bores and comes to rest in the muscles or the fat body. It does not form distinct capsules. It remains in this larva during the following winter, and in the spring passes over into the imago Sialis. The complete insect frequents the small plants growing along the water's edge, and falls an easy prey to the predaceous beetle Pt. niger. The larva is eaten, and undergoing a change becomes the second larval form mentioned above. It remains in the body of the beetle during the second winter, and finally returns to the water {172}as the adult some eighteen or twenty months after it has been hatched from the egg.
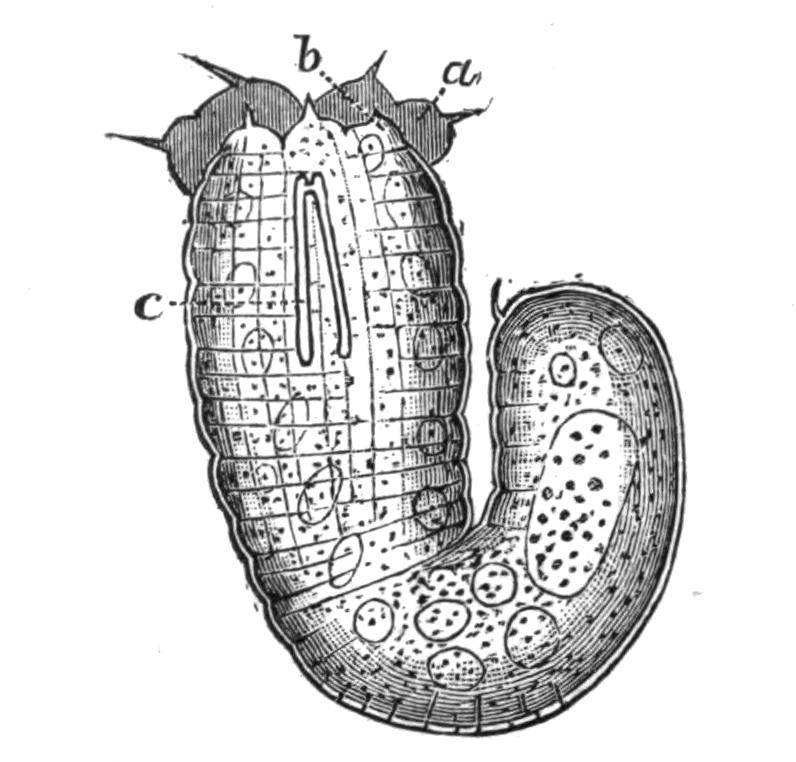
Fig. 90.—Embryo or first larval form of Gordius tolosanus taken from the egg. Highly magnified. a and b, The bristle-bearing papillae on the head; c, the boring apparatus. (From von Linstow.)
From the above account of the life-history of Gordius it will be seen that the chances of an egg reaching maturity are comparatively small, and to compensate for this a very large number of eggs are laid. In addition to the risk of the larvae not finding the right host at the right time, and of the first host not being eaten by the second, and the second not being drowned, there is the danger that the ditches and ponds in which the adults live may dry up, and, in fact, great numbers of worms perish by this taking place.
The sex of the adults may be told from their colour, the males being of a blackish brown, the females of a light clay brown; the former average 120 mm. in length, the latter 170 mm. The males are also more numerous, the proportion being seven to three. Camerano[213] has drawn attention to the fact that there is a certain polymorphism in size, form, and colour which is especially common amongst the males; dwarf forms with mature reproductive organs exist, and he is of opinion that these differences depend both on the size of the second host and on the duration of the parasitic life.
In addition to the larva of Sialis lutaria, the first larval stage has also been found in the larva of Ephemera, Tanypus, Corethra, and Chironomus; the second in Carabus hortensis Fabr., Procerus (Carabus) coriaceus Linn., Calathus fuscipes Goeze, Molops elatus Fabr., several species of Pterostichus, and a number of other beetles. It is probable that its normal hosts are S. lutaria and Pt. niger, but it is clear that it often comes to rest in other insects. The view that the Gordiidae have no special hosts, but may either pass the whole of their life-history within one and the same animal, or, on the other hand, may inhabit animals belonging to very different groups, is held by Villot, who has paid great attention to the subject. He finds the first larval form encysted in the walls of the alimentary canal in fishes, such {173}as Leuciscus phoxinus, the minnow, Cobitis barbatula, the loach, and Petromyzon planeri, the lamprey; in the larvae of Diptera, Ephemera, and beetles, in Planorbis (a water snail), in Enchytraeus (an Oligochaet); the second larval form in all kinds of insects, spiders, Crustacea, fish, frogs, birds (Otis), and in man, and these various habitats lead him to the conclusion that "Les Gordiens n'ont pas d'hôtes spéciaux." On the other hand, as von Linstow points out, it is contrary to our knowledge of parasites that a single species should develop equally well in the body of warm and cold-blooded Vertebrates and of Insects, and the explanation of the presence of the larvae in these various forms may either be that they belong to different species of Gordius or, more probably, that they are accidentally present, having passed into their hosts with drinking water.
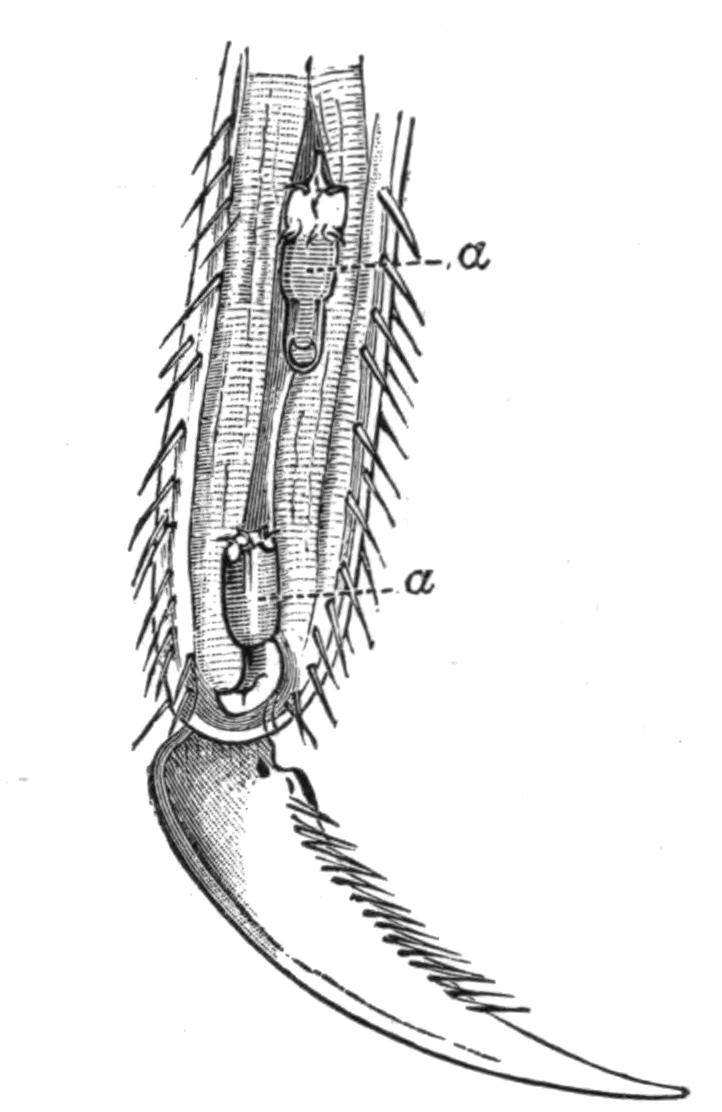
Fig. 91.—Tarsal joint of an Ephemerid larva into which two Gordius larvae (a, a) have penetrated. Magnified. (From G. Meissner.)
The number of species of Gordius is large; over 100 are enumerated in the Compendium der Helminthologie,[214] the great majority of which inhabit insects.
The life-history of Nectonema is practically unknown; the adults have been found swimming near the surface of the sea at two places only: Newport, R.I., and Wood's Holl, Mass., on the south coast of New England. It has been fished close to the shore, from the end of June to the beginning of October, when the tide is going out at evening and there is no moon. This seems to indicate that it avoids the light. When first caught the worms move actively about, coiling themselves into figures of eight and then uncoiling; at the same time there is a rhythmical movement caused by waves of muscular contraction passing down each side of the body alternately; by this kind of motion they make rapid and definite progress through the water.
It seems probable that the adult Nectonema is preceded by one or more larval stages, and what appears to be a young form has {174}been obtained from the thoracic cavity of a prawn, Palaemonetes,[215] which has thus some claim to be regarded as the host of this species, but nothing is known about its early life-history.
Sub-Order III. Acanthocephala.
The Acanthocephala, which form the third class of the Nemathelminthes, consists of but few genera; there are, however, numerous species of very different size, varying from 10 to 65 cm. long in the female Gigantorhynchus (Echinorhynchus) gigas, to quite minute forms a few millimetres in length. The adult stage occurs in the alimentary canal of Vertebrates, as a rule in those which live in, or frequent water; the larvae are found in the bodies of certain Invertebrates, very frequently small Crustacea.
Fig. 92.—Two specimens of Echinorhynchus proteus Westrumb., with their anterior ends embedded in the wall of the intestine of a Pike. Magnified with a lens. (From Hamann.)
Anatomy.—The body of the mature forms can usually be divided into three sections—the proboscis, the neck, and the trunk, but the middle region is not always discernible. The proboscis is armed with rings of hooks (Fig. 93) arranged in longitudinal rows; they are usually of two kinds, but in E. proteus of three. They have a certain specific value, but not much stress can be laid on the number of rings, e.g. in E. angustatus the number varies from eight to twenty-four. The recurved hooks serve to fasten the parasite very firmly to the tissues of the host. The proboscis is hollow and retractile; it can be withdrawn into the body by means of muscles attached internally to its tip. It does not, however, pass straight into the body-cavity, but is retracted into a special cavity—the proboscis sheath—with a double muscular wall. The proboscis sheath may perhaps be looked upon as a septum, such as is found in some of the Nematomorpha, dividing the body-cavity into two parts. It is inserted into the body-wall at the junction of the neck and trunk or of the proboscis and trunk. In addition to the muscles which withdraw the proboscis into its sheath, there are two retractors running from the {175}outside of the sheath to the body-wall; these serve to retract the whole sheath and its contents into the body-cavity of the trunk.
The structure of the skin is essentially like that of Nematodes, but the details are much more complicated. The whole body is covered by a thin cuticle secreted by the epidermis, which, as in the other groups, breaks down and forms a syncytium called the sub-cuticle. The minute fibrils which penetrate this layer are much more definitely arranged than in Nematodes; the largest of them run from without inwards, others run concentrically round the body. Large oval or spherical nuclei are scattered in the sub-cuticle, which is further honeycombed by a number of lacunae or spaces which are described below.
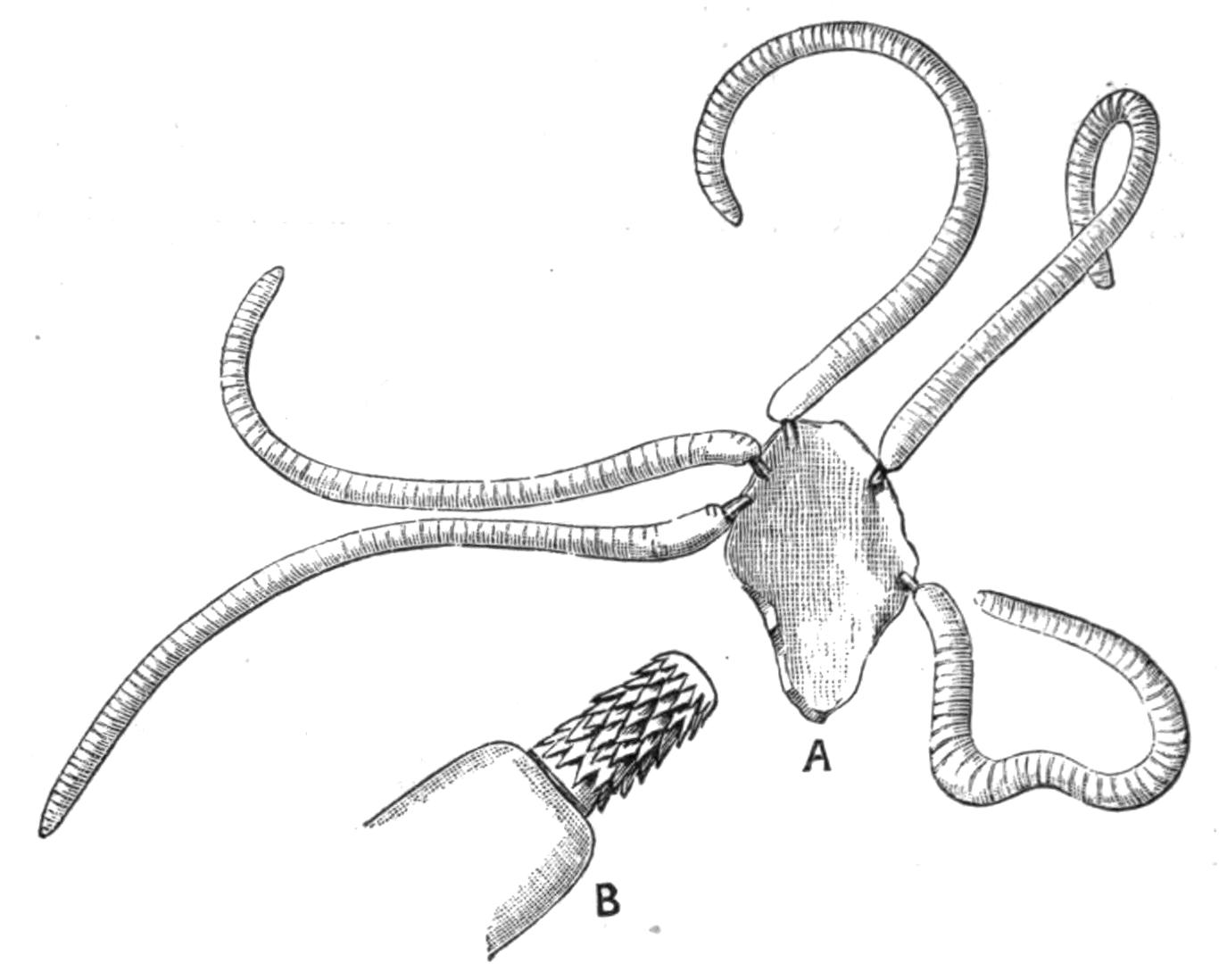
Fig. 93.—A, Five specimens of Echinorhynchus acus Rud. attached to a piece of intestinal wall, × 4; B, the proboscis of one still more highly magnified.
Within the sub-cuticular layer is found a sheath of circularly-arranged muscle-fibres, and within this again a sheath of longitudinal muscles which do not extend into the proboscis; this inner layer lines the body-cavity, there being no epithelium within it. In their minute structure the muscle-cells resemble those of Nematodes.
The canals in the sub-cuticle form a very curious system of anastomosing spaces, in which a clear fluid containing fat globules circulates. The extent to which the system is developed varies in different species, but in all there is a pair of longitudinal canals which are situated laterally, and which give off the subsidiary channels in their course. The above description applies to the lacunar spaces in the skin of the trunk; those of the proboscis are quite distinct, and there is no communication between the two sets of spaces; in fact, the sub-cuticle in which the lacunae are formed is not continuous across the line of junction of the proboscis and the neck, or, when the latter is absent, of the proboscis and the trunk, but it is interrupted by the ingrowth of a thin ring of cuticle which reaches down to the muscular layers (Fig. 94).
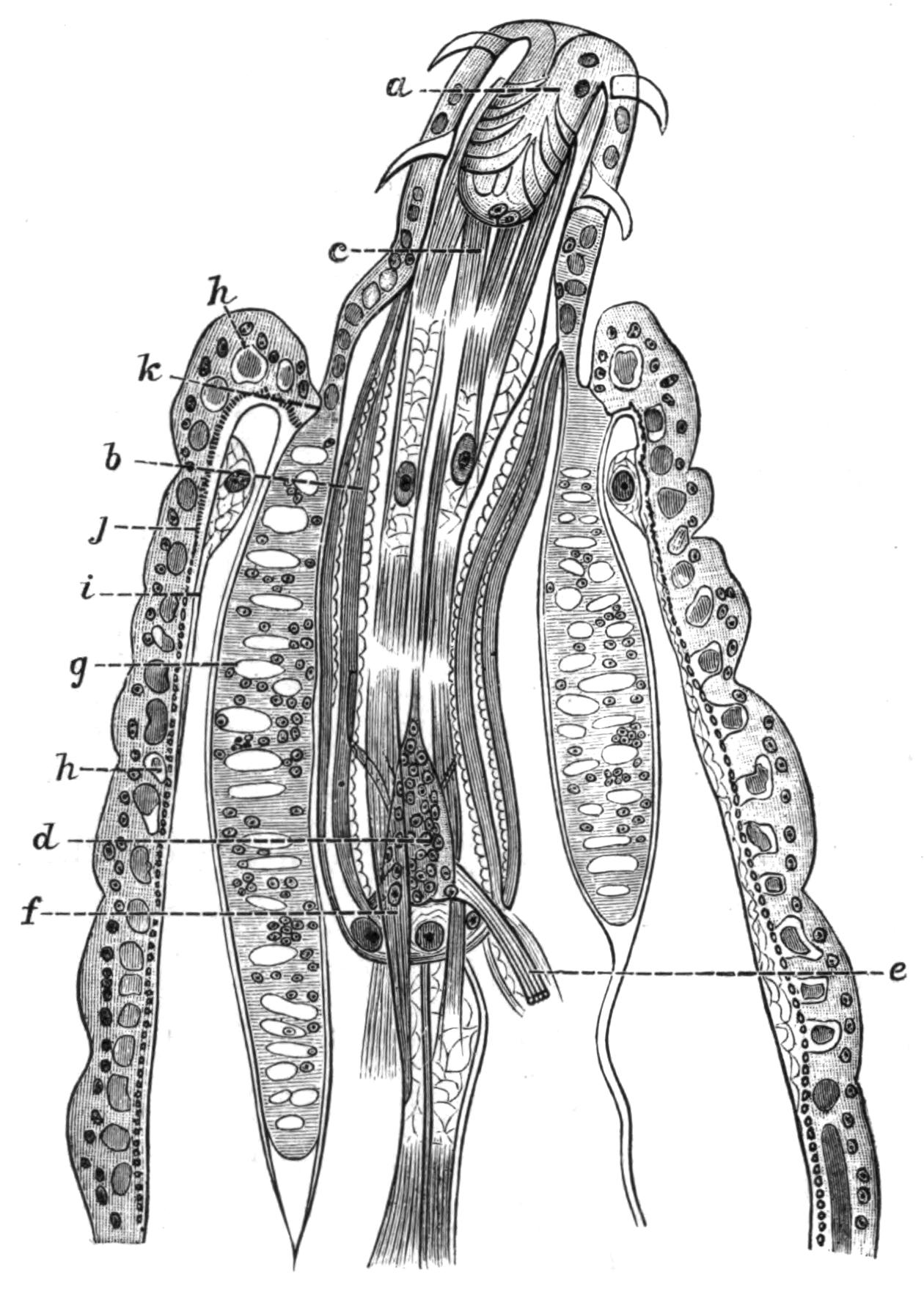
Fig. 94.—A longitudinal section through the anterior end of Echinorhynchus haeruca Rud. (From Hamann.) a, The proboscis not fully expanded; b, proboscis-sheath; c, retractor muscles of the proboscis; d, cerebral ganglion; e, retinaculum enclosing a nerve; f, one of the retractors of the sheath; g, a lemniscus; h, one of the spaces in the sub-cuticular tissue; i, longitudinal muscular layer; j, circular muscular layer; k, line of division between the sub-cuticular tissue of the trunk and that of the proboscis with the lemnisci.
All the spaces in the skin of the proboscis open ultimately into a circular canal situated round its base; on each side the canal opens into a sac-like structure which extends through the body-cavity towards the posterior end of the animal. These two lateral diverticula are termed the lemnisci. They have always attracted considerable attention from the workers at the group, and numerous functions have from time to time been attributed to them. They are more or less hollow, and their walls consist of sub-cuticular tissue surrounded with a scanty muscular coat; they contain the same fluid as the lacunae of the skin of the proboscis, with which they are placed in communication by means of the circular canal; and it seems most probable that, as Hamann[216] suggests, they act as reservoirs into which the lacunar fluid retires when the proboscis is retracted, {177}and which, by means of the contractions of their muscular coat, force the fluid into the lacunae when the proboscis is everted, and thus aid in its protrusion.
The parasitic habits of Echinorhynchus have had a deeper influence on the structure of the body than is the case with the Nematoda. All traces of an alimentary canal have disappeared, and the animals live entirely by the imbibition through the skin of the already elaborated fluids of their hosts. The power of absorbing fluids is shown by the fact that they swell up and become tense when placed in fresh water.
Until recently no definite excretory organs had been recognised, and the function of excreting the nitrogenous matter was by some assigned to the lemnisci. In 1893 Kaiser[217] described in G. gigas two organs which he called nephridia, placed dorsally to the ducts of the male and female reproductive organs. Each nephridium, which somewhat resembles a cauliflower, consists of a stalk or duct, opening at one end into the reproductive ducts, and at the other branching and breaking up into a number of secondary and tertiary twigs. The end of each twig is closed by a membrane pierced with a number of most minute pores, by means of which it communicates with the body-cavity; on the inner side the membrane bears a number of long cilia, which keep up an active flickering. The presence of these cilia is interesting, as elsewhere they are unknown throughout the Nemathelminthes.
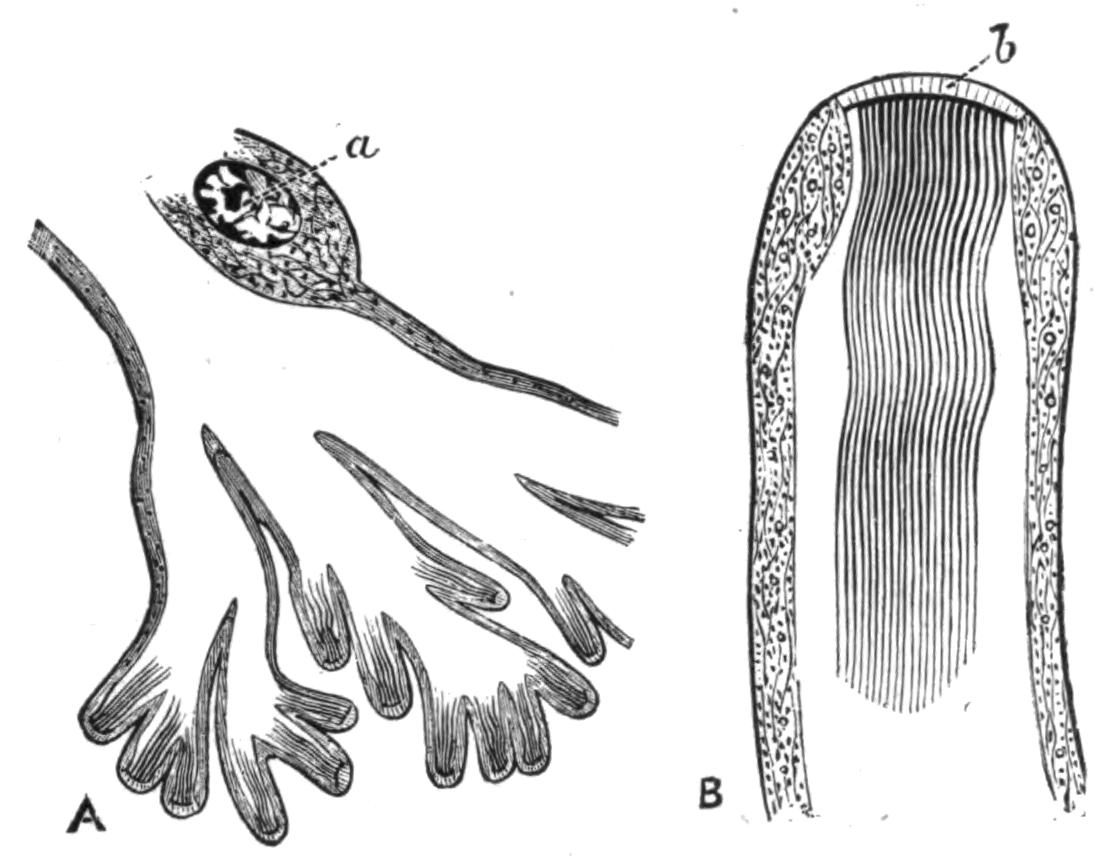
Fig. 95.—A, A longitudinal section through the terminal twigs of the nephridium of Gigantorhynchus gigas. (From J. E. Kaiser.) Highly magnified. a, Nucleus. B, A terminal twig more highly magnified; b, the porous membrane.
The nervous system consists of a central ganglion situated in the proboscis sheath; it is oval and flattened in shape. The ganglion gives off nerves to the proboscis, and two main trunks which pierce the proboscis-sheath and run backward surrounded by a cluster of muscle-fibres, the whole being termed the {178}retinaculum; in the male they are in connexion with a special genital ganglion which lies near the ductus ejaculatorius.
With the exception of certain sensory papillae in the neighbourhood of the male genital orifice, and of three similar papillae mentioned by Kaiser on the proboscis, the Acanthocephala are devoid of sense organs.
The Acanthocephala are dioecious; their generative organs are developed in connexion with the ligament, a cord-like structure which arises between the inner and outer layer of the hinder end of the proboscis sheath and traverses the body-cavity, ending posteriorly in connexion with the genital ducts. The testes lie in this ligament; they are paired oval bodies which open each into a vas deferens. The vasa deferentia each bear three lateral diverticula, the vesiculae seminales; and three pairs of cement glands pour their secretion into a duct which opens into the vasa deferentia; the latter unite and open by a penis which is withdrawn into a genital bursa, but is capable of being extruded.
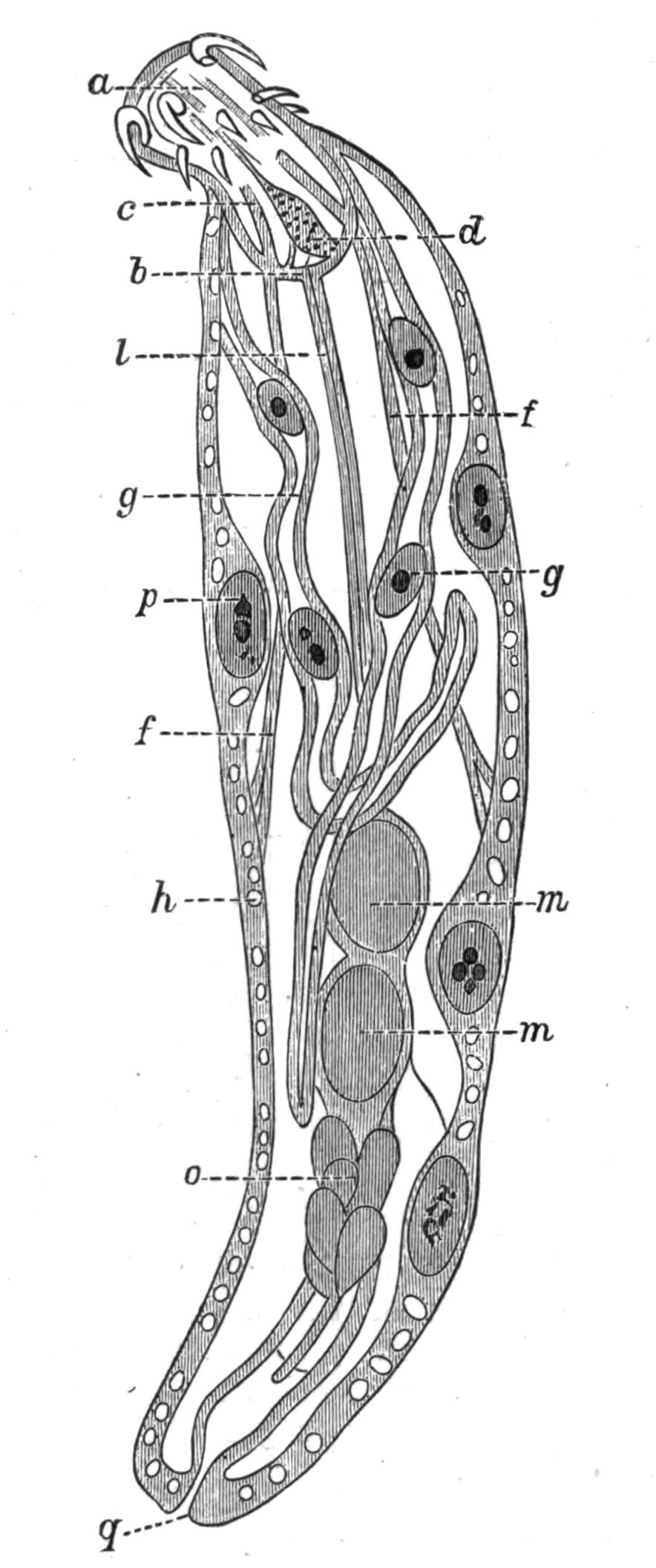
Fig. 96.—An optical section through a male Neorhynchus clavaeceps Zed. (From Hamann.) a, Proboscis; b, proboscis sheath; c, retractor of the proboscis; d, cerebral ganglion; f, f, retractors of the proboscis sheath; g, g, lemnisci, each with two giant nuclei; h, space in sub-cuticular layer of the skin; l, ligament; m, m, testes; o, glands on vas deferens; p, giant nucleus in skin; q, opening of vas deferens.
The two ovaries are formed in the ligament of the female in a corresponding position to that occupied by the testes in the male, but at an early stage they break down into packets of cells, of which those of the peripheral layer develop into ova at the cost of the central cells, which serve them as a food supply. As these masses grow and increase in number they rupture the walls of the ligament, and escape into the body-cavity, in which they float. The ova are {179}fertilised whilst floating in the fluid of the body-cavity. The eggs segment and the embryo is formed whilst still in the body of the mother.
The embryos escape by means of a complicated apparatus the details of which vary in the different species, but which, like many of the organs in these animals, consists of very few cells with very large nuclei. This apparatus consists of three parts: the bell, the uterus, and the oviduct. The bell is a large funnel-shaped structure, which opens into the body-cavity, and is connected with the end of the ligament; near its lower end, where it is continuous with the uterus, is a second smaller opening situated dorsally. By the contraction and expansion of its lips the oval embryos are swallowed and pass on through the uterus to the oviduct, which opens at the posterior end of the body. If the bell takes in any of the less mature eggs which are spherical in shape, they are passed back into the body-cavity through the above-mentioned dorsal opening, and the same orifice permits the passage of the spermatozoa even when the bell is full of embryos.
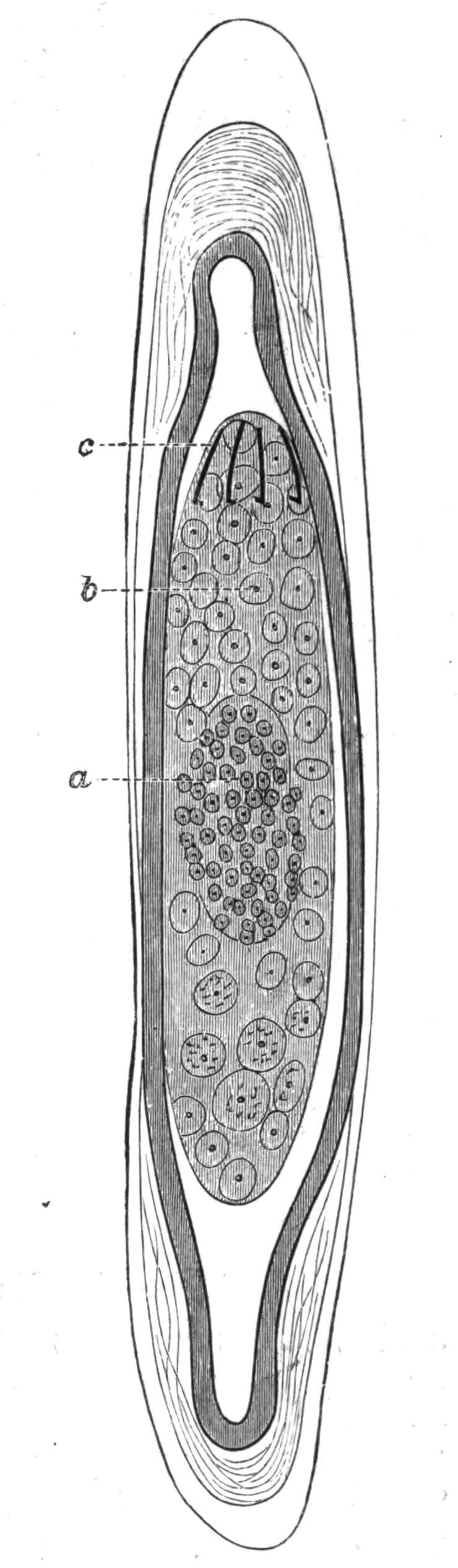
Fig. 97.—An egg of Echinorhynchus acus Rud. surrounded by three egg-shells. Highly magnified. The egg has segmented, and the cells are differentiated into a, the entoblast, and b, the ectoblast; c, spines. (From Hamann.)
Embryology.—After fertilisation the egg surrounds itself with several egg-shells, three of which are usually distinguished; the embryo is already far advanced in its development by the time it leaves the body of the mother and passes out into the alimentary canal of the Vertebrate host. It leaves the body of this second host with the faeces, and is eaten by the first or larval host, usually a small Crustacean or water-insect, but in some cases a fish, within whose alimentary canal it casts its membranes and {180}becomes actively mobile. By means of a ring of hooks developed round the anterior end it bores its way through the wall of the alimentary canal, and after some time—three weeks in E. proteus—comes to rest in the body-cavity of its host. By this time most of the organs of the adult, with the exception of the reproductive glands, are already well established; the latter only attain maturity when the first host is eaten by the second, and the larvae find themselves in the intestine of a Vertebrate.
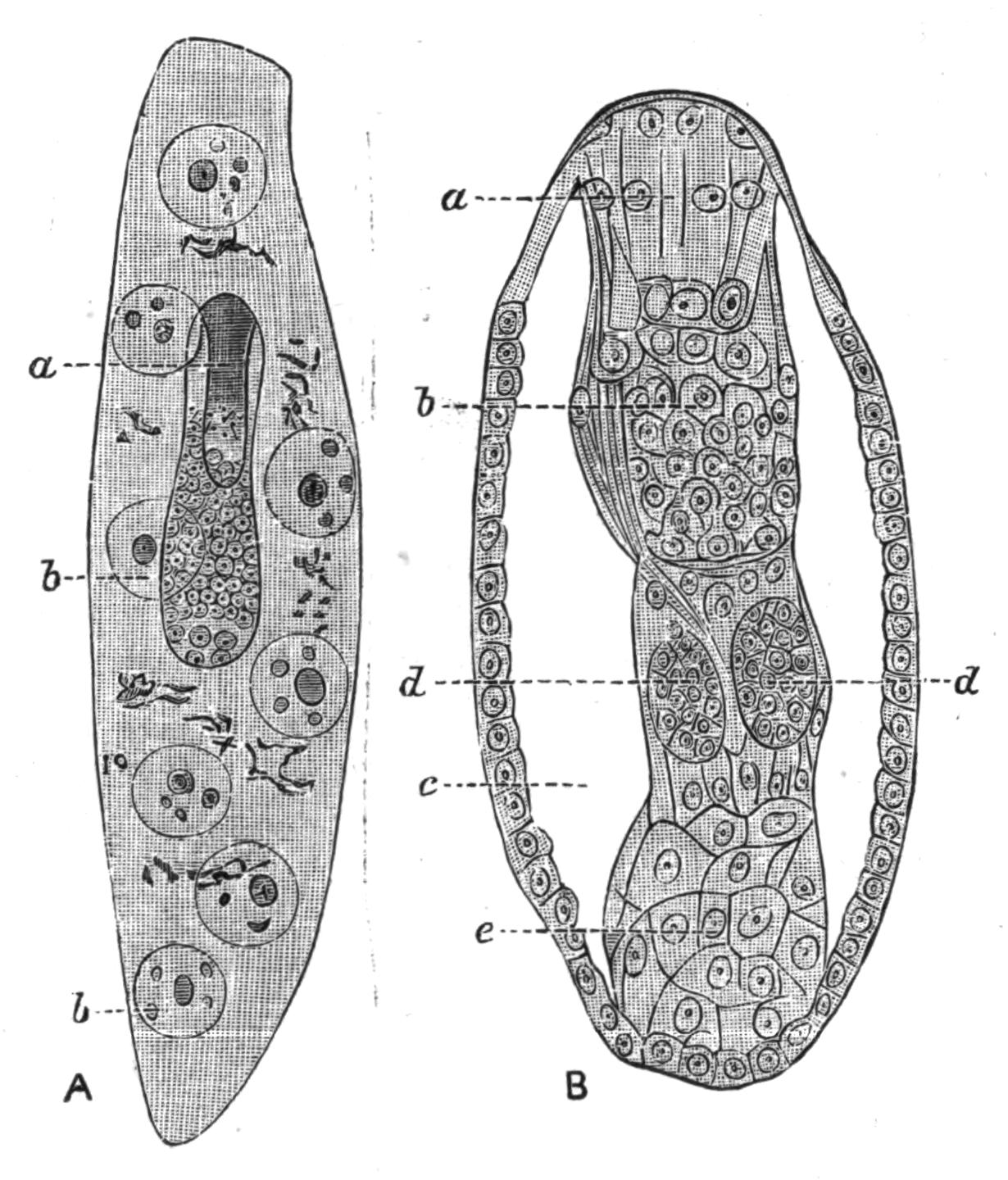
Fig. 98.—A, A larval Echinorhynchus proteus Westrumb. further developed than in Fig. 97. Highly magnified. The entoblast has developed inside it the proboscis a; b, b, the giant nuclei of the ectoblast. B, The entoblast at a more advanced stage, the ectoblast is not shown. The outermost layer of cells will form the muscles of the body-wall; the body-cavity has appeared; a, proboscis; b, cerebral ganglion; c, body-cavity; d, d, the testes beginning to appear in the ligament; e, cells which will form the generative ducts.
Some of the details of the development are very remarkable, and a short account of them may be given. The segmentation of the egg is unequal; it results in the formation of a central biscuit-shaped mass of small cells and a peripheral mass of larger cells; the former is called by Hamann[218] the entoblast, the latter the ectoblast. From the entoblast arise all the organs of the body but the sub-cuticle and the associated lemnisci, which are formed from the ectoblast. The latter has a remarkable history; the cells begin to break down and lose their outlines, whilst their nuclei fuse together and form a small number of giant nuclei, which lie scattered throughout the syncytium thus formed. The syncytium surrounds the entoblast on all sides; by this time the anteriorly-placed hooks have appeared; in E. proteus there {181}are ten of these, but the number is not the same in all species. The syncytium is in a fluid state, with a few gigantic nuclei floating in it; these now lose their spherical shape, and throwing out processes become amoeboid; in this way they bud off small portions of their substance, and from these the oval nuclei of the sub-cuticle and the lemnisci arise. The rest of the syncytium hardens into the fibrillar matrix of the sub-cuticle, leaving, however, scattered spaces which form the sub-cuticular sinuses of the adult. An interesting feature of N. clavaeceps and Arhynchus hemignathi is that the skin of the adult retains the larval features, and it and the lemnisci consist of a syncytium with a very few giant nuclei scattered through it. Hamann counted only eight in the skin and two in each lemniscus in the example figured on p. 178.
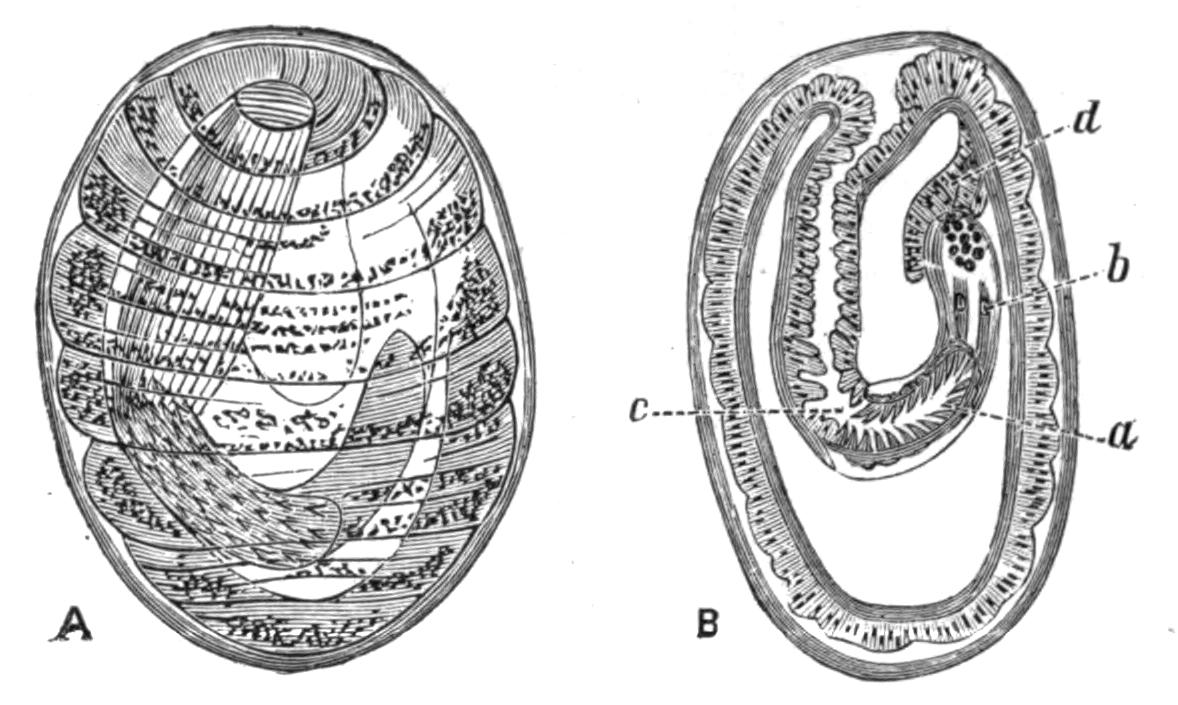
Fig. 99.—A, The larva of Echinorhynchus proteus from the body-cavity of Phoxinus laevis, with the proboscis retracted and the whole still enclosed in a capsule. B, A section through the same; a, the invaginated proboscis; b, proboscis sheath; c, beginning of the neck; d, lemniscus. Highly magnified. (Both from Hamann.)
The whole of the rest of the body is formed by the entoblast. Within the latter a circular split arises which separates a single layer of outermost cells from an axial strand of many cells (Fig. 98, B). The split is the future body-cavity; the axial strand forms the proboscis, its sheath, the cerebral ganglion, muscles, etc., and the ligament with the contained generative organs; the outermost layer of cells forms the muscular lining to the skin. It is interesting to note that these cells destined to become muscle-fibres are at first arranged as a single layer of cubical epithelial cells lining the body-cavity; most of them become circular muscle-fibres, but a few are pushed inwards so as to lie next the body-cavity, and these become the longitudinal fibres.
Classification.—Until recently the Acanthocephala were supposed to include but one genus, Echinorhynchus, with several hundred species, but Hamann[219] has pointed out that these species {182}present differences which enabled him to divide the group into three families, each with a corresponding genus. To these I have ventured to add a fourth family, to include a remarkable species, Arhynchus hemignathi, described below. The characters of the first three families in the account given below are taken from Hamann's paper.
Fig. 100.—Fully formed larva of Echinorhynchus proteus from the body-cavity of Phoxinus laevis. (From Hamann.) Highly magnified. a, Proboscis; b, bulla; c, neck; d, trunk; e, e, lemnisci.
Family I. Echinorhynchidae.—The body is elongated and smooth. The proboscis-sheath has a double wall, and the proboscis is invaginated into it. The central nerve-ganglion lies in the middle line, as a rule on the posterior blind end of the proboscis-sheath. The papillae which bear the hooks are only covered with a chitinous cap at their apex, and the hooks have a process below. This family is by far the largest; a few species only can be mentioned. Echinorhynchus proteus lives in its mature form in fishes; the young forms, up to a centimetre in length, are found living freely in the intestine of numerous fresh-water fishes. Those found in Gobio fluviatilis, the gudgeon; Leuciscus virgo; Lota vulgaris, the burbot or eel-pout; young trout; Thymallus vulgaris, the grayling, seldom surpass this size, but those found in Acerina cernua, the pope fish; in Abramis bipunctatus; in Esox lucius,the pike, and in older trout, attain or surpass double the length. As the parasites grow older they bury their proboscis and neck in the wall of the intestine, the inner surface of which is studded with the orange-coloured bodies of the parasites. The proboscis is so deeply sunk in the wall of the alimentary canal as to form a papilla on its outer surface (Fig. 92). The larvae of E. proteus are found in the body-cavity of Gammarus pulex, one of the Amphipod Crustacea, and also in the same position in numerous fresh-water fishes; they must have passed into this first host by the {183}mouth and alimentary canal. If the liver of an infested minnow, Leuciscus phoxinus, be examined, it will be found to contain on its surface numerous spherical or egg-shaped capsules of an orange colour, 2 to 2.5 mm. in length; these contain the larval forms of the parasite. They develop into the adult form when the first host is eaten by a carnivorous fish, but a complication may take place when the larval form is found in Gammarus, as the latter, the first host, may be eaten by a fish (intermediate host) in which the larva does not become mature, and only develops sexual organs when eaten by a carnivorous fish (second host). The larval form is also found in Nemachilus barbatulus, Gobio fluviatilis, and the sticklebacks Gasterosteus aculeatus and G. pungitius.
E. clavula Duj. is found in Salmo fario, Abramis brama, Cyprinus carpio, Gobius niger, Lepadogaster gouanii, etc.; E. linstowi Ham. in Leuciscus idus, Abramis ballerus, Abramis bipunctatus, and Acipenser huso; E. lutzii Ham. was found by Dr. Lutz in Brazil in the intestine of Bufo agua; E. angustatus Rud. occurs in such numbers in the perch, Perca fluviatilis, as to almost occlude the lumen of the intestine, and one out of every three or four fish in certain districts is infested by it. It is also found in the pike, Esox lucius, and the barbel, Barbus vulgaris. The first or larval host of this species is the Isopod Asellus aquaticus. E. moniliformis Brews. is stated to attain maturity in the human intestine. Except for the fact that G. gigas has once been observed in the same place, this is the only human parasite amongst the Acanthocephala. Its normal second hosts are Mus decumanus and Myoxus quercinus, and its first or larval host, the larvae of the beetle Blaps mucronata. E. porrigens Rud. is found in considerable numbers in the small intestine of a fin-whale (Balaenoptera sibbaldii), and E. strumosus Rud., in the small intestine of a seal (Phoca vitulina), and in the body-cavity of the angler fish (Lophius piscatorius). E. acus is common in the whiting, Gadus merlangus.
Family II. Gigantorhynchidae.—Large forms with ringed, flattened, and Taenia-like bodies. The hook-papillae are covered all over with transparent chitinous sheaths with two root-like processes. The proboscis-sheath is muscular and without a lumen. The central nervous system is excentrically placed below the middle of the so-called sheath. The lemnisci are long twisted tubes with a central canal.
Hamann places three species in this family: Gigantorhynchus echinodiscus, G. spira, and G. taenioides; but as he points out that E. gigas resembles these in its more important structural features, it seems advisable to include it here under the name G. gigas. The members of the first family often present a transversely ringed appearance after death, but the Gigantorhynchidae are ringed when alive, and the circular canals in the skin show a certain regularity, being arranged one between each two rings. There is no lumen in the proboscis-sheath, which is not attached to the boundary between the proboscis and the trunk, but to the inner surface of the proboscis, and the whole can be retracted within the anterior portion of the body, which is invaginable. There are always eight cement-glands, and other differences exist in the musculature, hooks, and position of the nervous system.
G. gigas occurs in the adult state in the small intestine of swine; in Europe its first or larval host is believed to be the grubs of Melolontha vulgaris and Cetonia aurata, but these beetles are absent from America, though the parasite infests American hogs. Stiles[220] has recently made some experiments which tend to show that in the United States the source of infection is some species of the beetle Lachnosterna, and he has succeeded in infecting the grub of L. arcuata by feeding it on the eggs of the parasite; from one larva he took 300 parasites six weeks after feeding it. L. arcuata is, like M. vulgaris, phytophagous, but the grubs of both the beetles are fond of frequenting manure heaps and patches of dung, and thus are much exposed to the dangers of infection.
G. echinodiscus inhabits the intestine of ant-eaters, having been found in Myrmecophaga jubata and Cycloturus didactylus. G. spira lives in the king vulture Sarcorhampus papa, and G. taenioides in Dicholophus cristatus, a species of Cariama.
Family III. Neorhynchidae.—Sexual maturity is reached in the larval stage. The proboscis-sheath has a single wall. A few giant nuclei only are found in the sub-cuticle and in the lemnisci. The circular muscle layer is very simply developed. The longitudinal muscle-cells are only present in certain places.
This family includes two species, Neorhynchus clavaeceps and N. agilis, which afford interesting examples of paedogenesis. The sub-cuticle and the lemnisci are dominated by a few giant {185}nuclei, which remain in the embryonic state and do not break up into numerous nuclei as in other forms. The musculature is but little developed and the longitudinal sheath hardly exists. The proboscis-sheath consists of a simple muscular layer, and the short proboscis has few hooks and presents an embryonic appearance.
The sexually-mature form lives in the carp, Cyprinus carpio; the larval form is found, according to Villot,[221] encysted in the fat bodies of the larva of Sialis lutaria, one of the Neuroptera, and in the alimentary canal of the leech Nephelis octocula, and successful experiments have been made in infecting some species of the water snail Limnaea. N. agilis occurs in Mugil auratus and M. cephalus.
Family IV. Arhynchidae.—Short forms with the body divided into three well-marked regions—head, collar, and trunk. The head is pitted, the collar smooth, and the trunk wrinkled, not annulated, in spirit specimens. There is no eversible introvert, and no introvert sheath and no hooks. The sub-cuticle and the lemnisci have a few giant nuclei, and the lemnisci are long and coiled.[222]
This family resembles the Gigantorhynchidae in the length and curvature of its lemnisci, and the Neorhynchidae in the persistence of the embryonic condition of the nuclei in the sub-cuticle and the lemnisci; but in the shape of the body, its division into three well-marked regions, the absence of eversible proboscis, proboscis sheath, and hooks it stands alone, though it is nearer to the Neorhynchidae than to either of the other families.
The single species Arhynchus hemignathi was found attached to the skin around the anus of a Sandwich Island bird, Hemignathus proceros. The bird is a member of a family Drepanididae, which is entirely confined to the Sandwich Island group. Professor Newton tells me that it is probable that the "food of Hemignathus consists entirely of insects which it finds in or under the bark of trees," hence it is probable that the second host of this parasite, if such exists, must be looked for amongst the Insecta.
CHAETOGNATHA
STRUCTURE—REPRODUCTION—HABITS—FOOD—CLASSIFICATION TABLE OF IDENTIFICATION
At certain seasons and at certain times of the day the naturalist who is investigating the fauna of the surface of the sea is apt to find his tow-net crammed with innumerable transparent spindle-shaped animals, which by their number and the way in which they become entangled with rarer objects, often render useless the result of his labours. These animals belong to the class Chaetognatha, which includes three genera, Sagitta, Spadella, and Krohnia. Amongst them are divided about twenty species, some of which, however, are of doubtful value.
Anatomy.—The body of these animals is as transparent as crystal; it is elongated, and bears a resemblance to certain torpedos, except that the head forms a somewhat blunt termination to the spindle-shaped body. The tail bears a caudal fin, and Spadella and Krohnia have a single pair, and Sagitta two pairs, of lateral fins; all of which are flattened horizontally.
The body is externally divisible into three regions—head, trunk, and tail—and these correspond with the arrangement of the internal organs.
The head is surrounded by a fold of skin, forming a hood, {187}which is most prominent at the sides (Fig. 102, g); within the hood the head bears from two to four rows of short spines, and outside these a right and left row of sickle-shaped hooks, the free ends of which in a state of rest converge round the mouth, but when disturbed these hooks can be widely divaricated.
The cavity of the body, or coelom, is divided into three distinct chambers by the presence of two thin transverse walls or septa, one situated between the head and the trunk, the other between the trunk and the tail (Figs. 104, 105). In the head, this cavity is much reduced by the presence of special muscles which move the spines, hooks, etc.; and in the small species, such as Spadella cephaloptera, the other two cavities are almost entirely occupied by the digestive and reproductive organs[223]; but in the large species, e.g. Sagitta hexaptera, a considerable space is left between the internal organs and the skin, and this is occupied by a coelomic fluid. If the skin of one of these larger species be punctured the fluid escapes and the animal shrivels up. A longitudinal partition or mesentery, with numerous pores in it, runs through these spaces, dividing the body-cavity into a right and left half; in the region of the trunk this mesentery supports the alimentary canal.
In addition to certain muscles in the head, which move the hooks, etc., there is a muscular lining to the body-wall. This is divided into two dorsal and two ventral bands, much in the same way as in Nematodes. The muscle fibres are striated.
The mouth, situated either terminally—Spadella marioni[224]—or below the head, leads into a pharynx; this passes into an intestine lined by a single layer of ciliated cells with a few glandular ones intermingled. The intestine runs straight through the body without loop or coil, and opens by an anus situated at the junction of the trunk and the tail. In most cases the anus is ventral or on the lower surface, but Gourret asserts that in Spadella marioni it is on the upper surface.
There are no special respiratory, excretory, or circulatory organs, unless a glandular structure described by Gourret in the head of Spadella marioni be a real kidney.
The nervous system consists of a supra-oesophageal ganglion {188}or brain situated in the head, and of a ventral ganglion lying in the trunk; both these nerve centres are embedded in the epidermis, and are connected with one another by means of two stout peri-oesophageal nerves (Figs. 102, 104). The brain also gives off a pair of nerves to the eyes, another pair to the olfactory organ, and a pair which ultimately meet one another and so form a ring; on this are certain ganglia giving off nerves which supply the muscles of the head. Both the chief ganglia give off numerous nerves, which divide and split up into a network of fibres which permeate the whole skin.
The sense organs are comparatively simple. A pair of very small eyes lie in the skin of the head; they are of complex structure, and to some extent remind one of the simple eyes of certain Crustacea. Behind the eyes and also on the upper surface of the animal is an unpaired organ which is usually described as olfactory in function (Figs. 103, 105). This is a ring-shaped modification of the epidermis drawn out into different shapes in the various species. The modified epidermal cells bear long cilia. The remaining sensory organs found in the group consist of clumps of modified cells scattered in round groups over the surface of the body and of the fins. The central cells of each group bear long tactile hairs, and are surrounded by supporting cells.
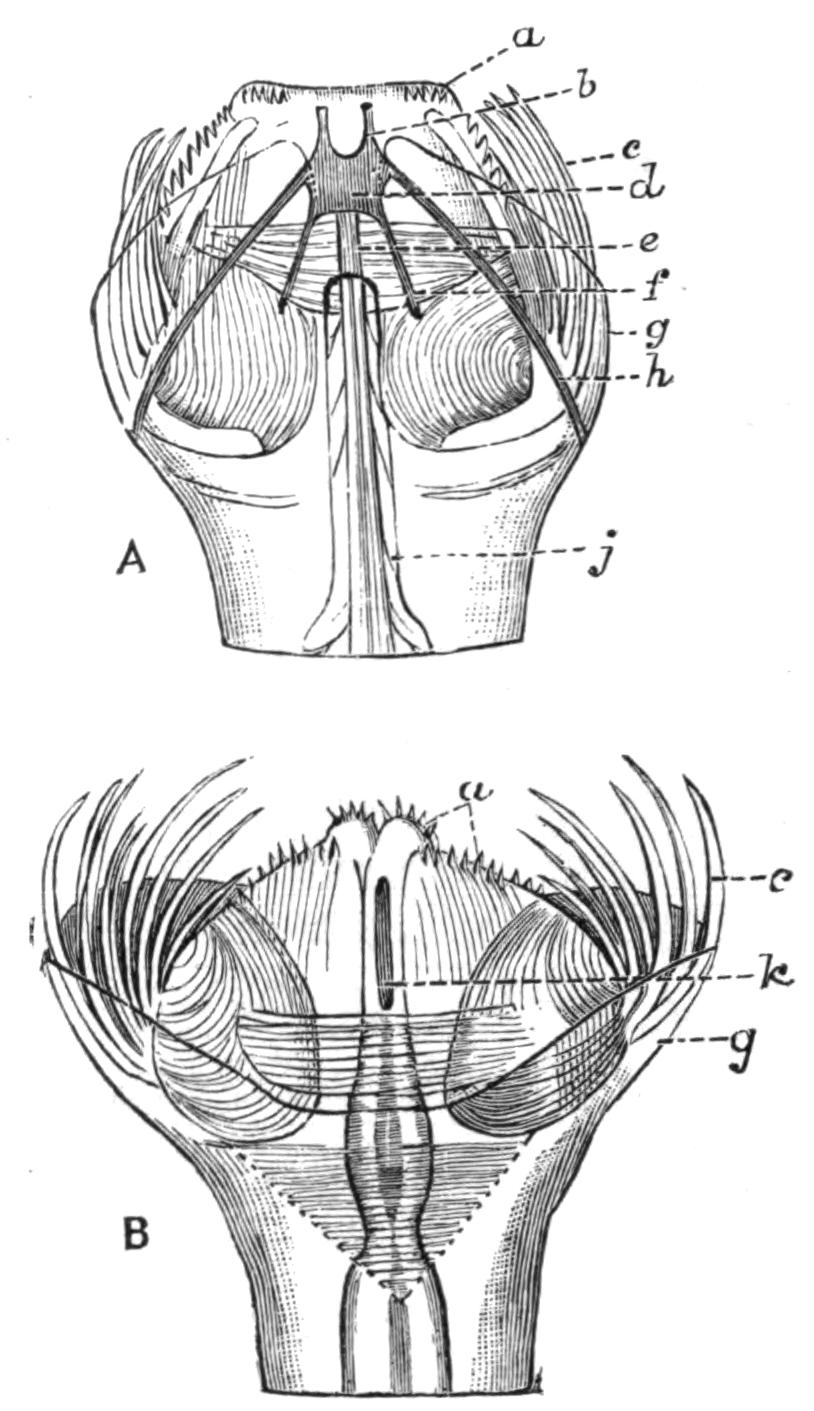
Fig. 102.—Head of Sagitta bipunctata. A, Dorsal view; B, ventral view. × about 33. (From Hertwig.) A, a, spines; b, nerves to lateral cephalic ganglia; c, hooks; d, cephalic ganglion; e, olfactory nerve; f, optic nerve; g, hood; h, commissure to ventral ganglion; j, olfactory organ: B, a, c, and g as in A; k, mouth.
The Chaetognatha are hermaphrodite, and carry the female organs in the trunk, the male in the tail. In a mature specimen the two ovaries occupy almost all the space in the trunk between the alimentary canal and the skin, and each is supported by a narrow lateral mesentery. The ovary is traversed by a oviduct which often contains spermatozoa; it is not clear how the eggs make their way into the oviduct, which seems to have {189}no internal opening and to act largely as a receptaculum seminis. The oviducts open externally on the upper side at the base of the lateral fin, close to the junction of the tail and the trunk.
The cavity of the tail is divided into two lateral chambers by the extension backward of the median vertical mesentery. In each of these a testis and a vas deferens are found. The testes are solid ridges formed by the growth of the lining cells of this part of the body-cavity; the cells mature into spermatozoa, which break off and float freely in the coelomic fluid. At the breeding season the whole tail may be crowded with masses of spermatozoa, which are kept in a more or less regular circulation by the ciliated cells lining the body-wall. The vas deferens opens internally into the space where the spermatozoa lie, and at the other end into a vesicula seminis, which opens to the exterior. The position of the latter structure varies, and is of some systematic value.
The eggs are laid in the water and as a rule float at the surface of the sea. Spadella cephaloptera is, however, an exception to this rule, as it attaches its eggs by means of a gelatinous stalk to sea-weeds. The segmentation of the ovum is regular, and gives rise to a two-layered stage or gastrula, which opens by a pore, the blastopore. This does not, however, become the mouth, but closes up and the mouth arises at the opposite pole. Perhaps the most interesting feature of the development of Sagitta is that the cells destined to form the reproductive organs separate from the other cells of the embryo at a very early date, whilst it is still in the gastrula stage. There is no larval form, but the young hatch out from the egg in a state resembling the adult in all respects but that of size.
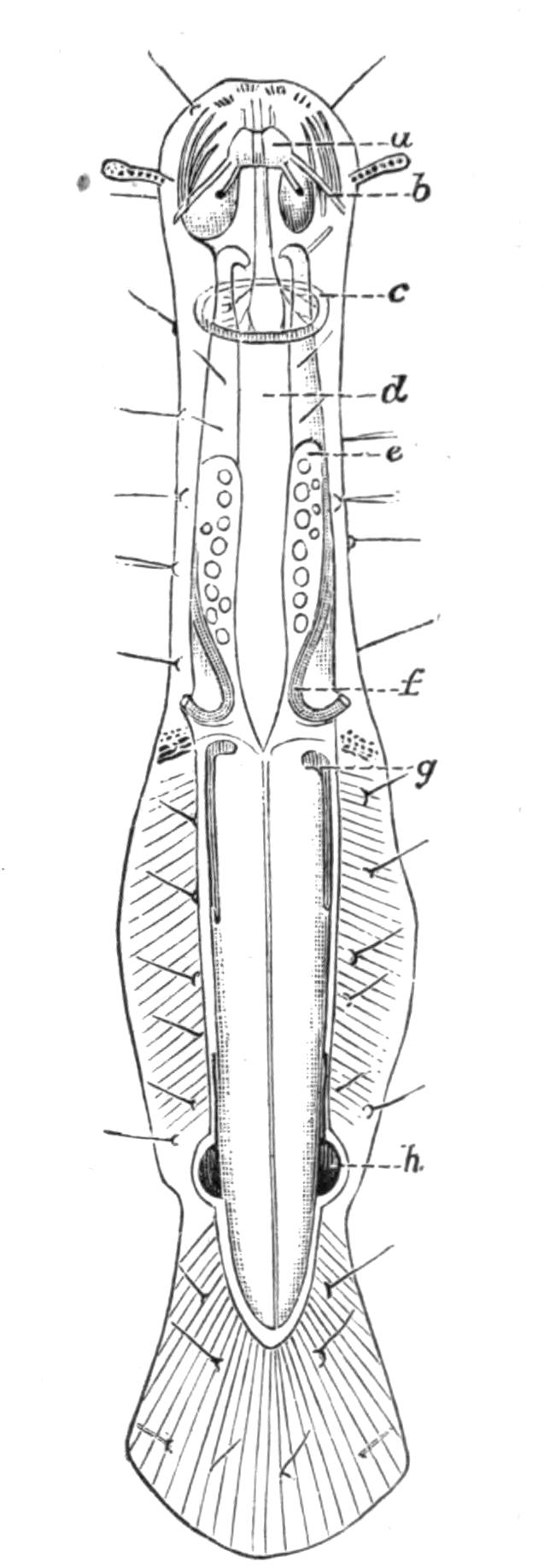
Fig. 103.—Spadella cephaloptera. Dorsal view. x 30. (From Hertwig.) a, Cephalic ganglion; b, commissure to ventral ganglion; c, olfactory organ; d, alimentary canal; e, ovary; f, oviduct; g, testis; h, vesicula seminalis.
Habits.—The Chaetognatha are essentially pelagic, and {190}resemble many other creatures that dwell at the surface of the ocean in being almost completely transparent. Most species have been taken far out at sea, but some are perhaps rather more numerous near the coast, and one species, Spadella cephaloptera, is littoral. They swim by means of muscular movements of the whole body; the fins have no movement of their own, and seem to serve as balancers, and not as locomotory organs. Although usually found at the surface of the water, many species have been taken at considerable depths. Chun[225] states that they are found in countless numbers at depths of from 100 metres to 1300 metres. The commonest species at these depths are Sagitta hexaptera and Sagitta serratodentata. Sagitta bipunctata, according to the same authority, confines itself to the surface. Whether the change of depth is diurnal, or whether it has any relation to sexual maturity, or to any other cause, has not been satisfactorily determined.
The food of the Chaetognatha consists of floating diatoms, Infusoria, small larvae, and such Copepods as Calanus finmarchicus, and small Amphipods as Phoxus plumosus.[226] At times they also devour small larval or post-larval fishes, and owing to their incredible numbers, they doubtless do considerable damage to sea fisheries. It is also recorded that they eat one another, and specimens have been taken which have ingested the whole body of another Sagitta except the head, which hangs out of the mouth of the eater, and gives it the appearance of a double-headed monster.[227] It has been said that they attack hydroid polypes, but here at any rate they do not have it all their own way. Masterman[228] has figured the apical group of five polypes of Obelia, three of which are engaged in ingesting as many young Sagitta.
They exist in incredible numbers; Grassi describes the surface of the sea at Messina on certain days as being literally covered with them, and they must form the food supply of numerous animals which prey upon the pelagic fauna. The immense number of individuals is probably accounted for to some extent by the fact that they lay eggs all the year round, and pass {191}through a very short and rapid development. They are not known to be phosphorescent.
Classification.—The features of the Chaetognatha which have most systematic value are the size of the adult, the relations of the length to the breadth, and of the three divisions to one another; the size, number, and position of the lateral fins, and of the hooks and spines on the head; the thickness of the epidermis, and the structure of the olfactory organ; and, finally, the form of the reproductive organs.
Strodtmann,[229] who gives the latest and most complete account of the species of Chaetognatha, arranges them under three genera, which he characterises as follows:—
(i.) Sagitta Slabber.—Two pairs of lateral fins, two rows of spines on the head. The lateral thickening of the epidermis absent or insignificant.
Under this genus are included nine definite species and five others—S. gracilis Verrill, S. elegans Verrill, S. darwini Grassi, S. diptera d'Orbigny, and S. triptera d'Orbigny—whose position, owing to the inadequacy of their description, is of doubtful validity.
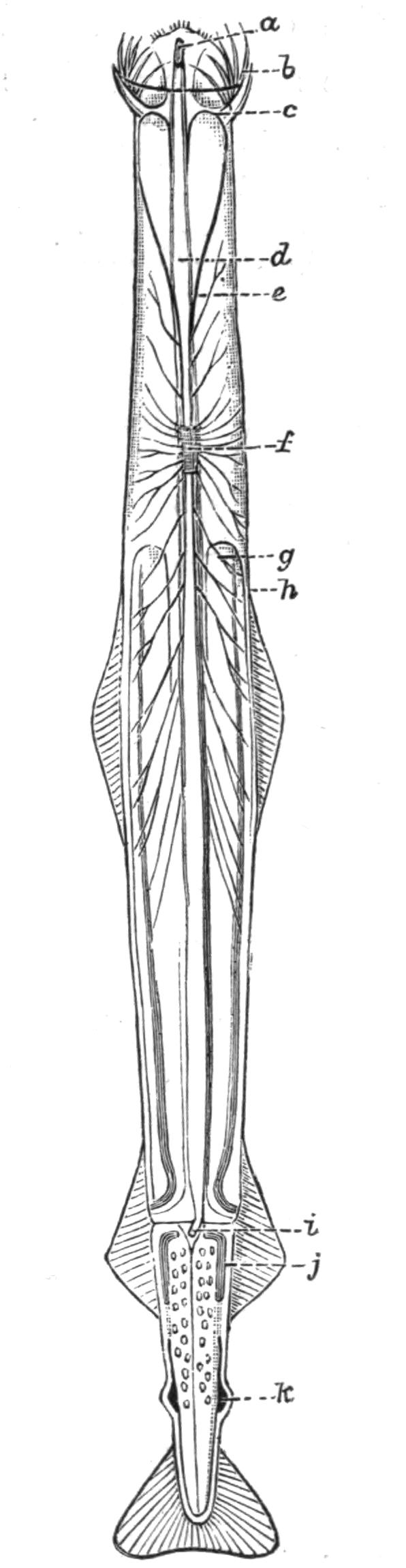
Fig. 104.—Sagitta hexaptera. Ventral view. × 4. (From Hertwig.) a, Mouth; b, hooks; c, anterior septum; d, alimentary canal; e, commissure from the brain to the ventral ganglion; f, ventral ganglion; g, ovary; h, oviduct; i, posterior septum; j, testis; k, vesicula seminalis.
The distribution of the other species may be mentioned. S. hexaptera is the largest Chaetognath known, and reaches in the adult stage a length of 7 cm. It is very widely distributed, being found in practically all the temperate and warm seas, usually at the surface of the water, though at times it is found at a depth of one metre, or even deeper. S. lyra, Mediterranean, very rare. S. tricuspidata, widely distributed. S. magna, Mediterranean and Madeiran, living at the surface. S. bipunctata, the most frequently described form, smaller than the preceding species, 1-2 {192}cm. in length, widely distributed, and as a rule living near the coast line. S. serratodentata, Mediterranean. S. enflata, on the surface of the sea, Mediterranean and Madeiran. S. minima, a very small species, 1 cm. in length, Mediterranean. S. falcidens, Atlantic, off the coast of New Jersey.
(ii.) Krohnia Langerhans.—A single lateral fin extending on to both trunk and tail segment, no lateral epidermal extensions behind the head, only one row of spines on the head. Trunk longer than the tail.
Krohnia has but two species: K. hamata Möbius, with a length of 3-4 cm., found in the North Atlantic and at considerable depths, 200 to 300 fathoms; and K. subtilis Grassi, 1.5 cm. long, with an extraordinary slender body and a relatively large head, found at Messina, but very rare; as a rule only one specimen has been found at a time.
(iii.) Spadella Langerhans.—A single pair of lateral fins; these are situated on the tail segment. Behind the head a thickening of the epidermis extends down each side of the body to the fin, or even farther. Two rows of spines on the head. Small animals, not longer than 1 cm.
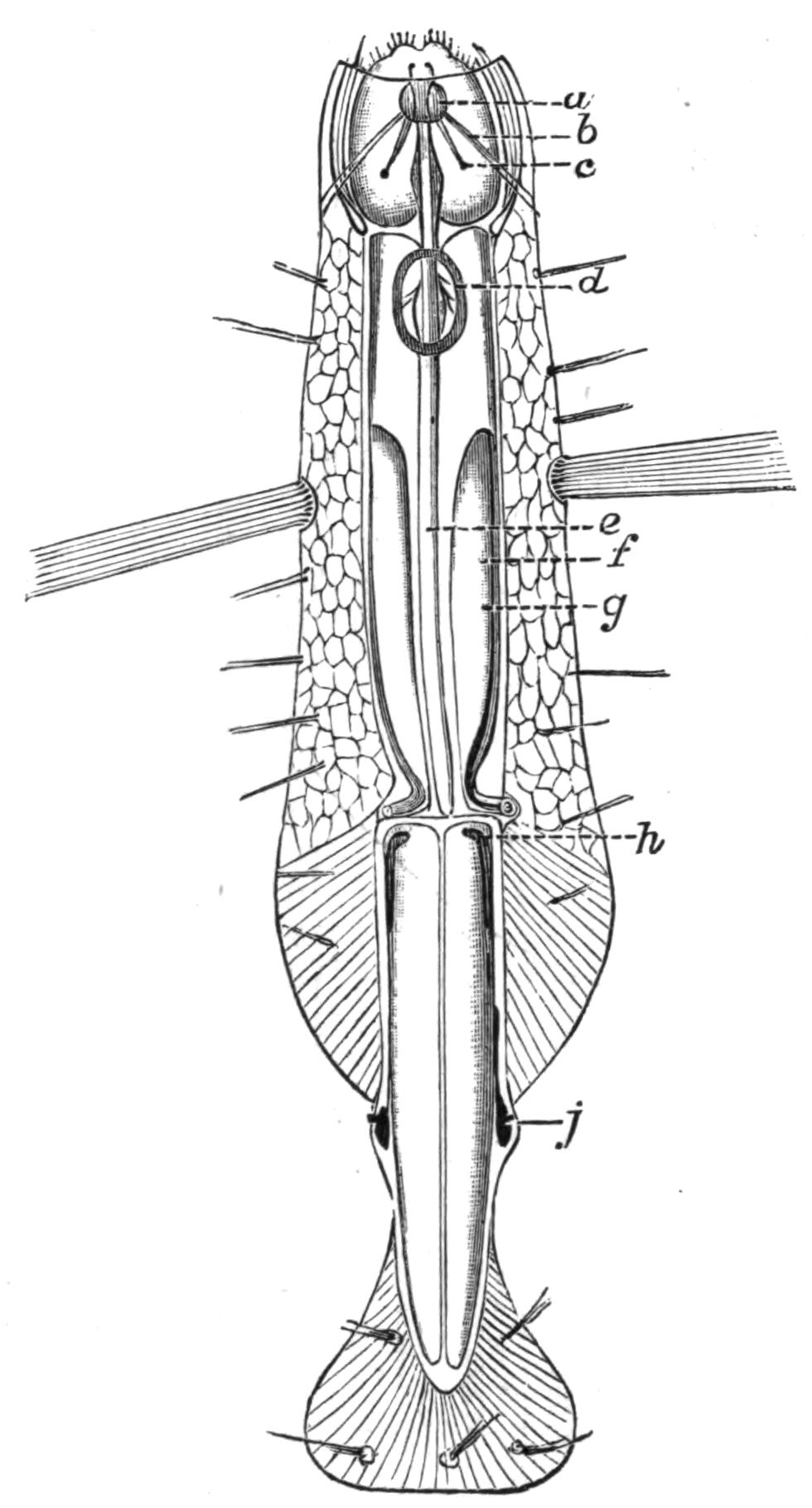
Fig. 105.—Spadella draco. Dorsal view. × 12. (From Hertwig.) a, Cephalic ganglion; b, commissure between the cephalic ganglion and the ventral; c, eye; d, olfactory organ; e, alimentary canal; f, ovary; g, oviduct (the line goes a little beyond the duct); h, testis; j, vesicula seminalis.
S. cephaloptera Busch is the smallest species of Chaetognatha, attaining at most a length of .5 cm. The body is not so transparent as in other species, and is of a yellowish colour. It has been found from the Orkney Islands to the Mediterranean. Strodtmann is of the opinion that the three species S. mariana Lewes, S. batziana Giard, and S. gallica Pagenstecher differ from the above-named only in size, or that their description is too indefinite to permit of accurate {193}characterisation. He recognises three other distinct species: S. pontica Uljanin, from the Black Sea; S. marioni Gourret, from the Gulf of Lyons; and S. draco Krohn, Mediterranean and Madeiran, and from the Canaries.
Much confusion has been introduced into the classification of the Chaetognatha by Grassi,[230] who calls some—but not all—of what other writers term Sagitta, Spadella, and vice versâ. The following table was compiled by Strodtmann,[231] but I have incorporated in it two species recently described from Amboyna by Béraneck,[232] and called by him Sagitta bedoti and Spadella vougai respectively:—
CHAETOGNATHA
I. Two pairs of lateral fins; two rows of spines on the head; slender forms.
(i.) Number of spines in posterior row greater than in anterior.
a. Border of hooks smooth, their point not curved.
α. No interval between the two fins on each side. 3.5 cm. long; 4-7 anterior spines, 8-11 posterior spines; olfactory organ lying entirely on the trunk. The anterior nerves of the ventral ganglion lie close to one another as far as the head.—Sagitta lyra.
β. A distinct interval between the two fins on each side.
aa. Adult animals large; hooks 6-7; anterior spines 3-4; posterior spines 5-7; tail ¼ or ⅕ of the total length; lateral areas relatively larger.—Sagitta hexaptera.
bb. Greatest length 1-2 cm.
αα. Thickening of the epidermis behind the head; prominently projecting vesiculae seminales; olfactory organ very long; hooks 8-10; anterior spines 4-6; posterior spines 10-15.—Sagitta bipunctata.
ββ. No epidermal thickening; two caeca on the anterior end of intestine; length 1 cm.; hooks 6-9; anterior spines 3-4; posterior spines 7-8; point of the hooks somewhat bent round.—Sagitta minima.
γγ. Epidermis thin; no caeca; hooks 8-9, their ends not bent; anterior spines 3-4; posterior spines 7-8; length 2 cm.; small head; trunk proportionately thick.—Sagitta enflata.
δδ. Hooks 11-14, usually 12; length 1.8 cm.; anterior spines 6-7; posterior spines 18.—Sagitta falcidens.
εε. Hooks 7 on each side; length 1.3 cm.; anterior spines 8-10, posterior spines 18-22; no olfactory organ.—Sagitta bedoti.
b. Edge of hooks toothed and their point bent round; hooks 6-8; anterior spines 6-8; posterior spines 10-12; length 1.5 cm.; slender; conspicuously projecting vesiculae seminales.—Sagitta serratodentata.
(ii.) Number of the spines in posterior row smaller than in anterior.
a. Anterior spines 3; posterior spine 1; hooks 8; length 3.5 cm.—Sagitta tricuspidata.
b. Anterior spines 4; posterior spines 3; hooks 10-13; length 4.1 cm.; tail ⅕ of the total length.—Sagitta magna.
II. One pair of lateral fins lying on the trunk and tail; one row of spines; body slender; epidermis not thickened.
(i.) Hooks 8-9, bent like an elbow at the point, serrated in the young; 20-25 spines in a row; ovary reddish; length 3-4 cm.—Krohnia hamata.
(ii.) Hooks 8, broad at their base but very sharply pointed; spines in a curved row, about 18, with a constriction below like the neck of a bottle; body thin; length 1-1.5 cm.—Krohnia subtilis.
III. One pair of lateral fins, these lie on the tail; body relatively very broad in consequence of the thickening of the epidermis lying behind the head; two rows of spines; greatest length 1 cm.; tail and trunk usually the same length.
(i.) A great extension of the epidermis behind the head, consisting of very large cells; amongst these, at the level of the ventral ganglion, lies a bundle of stiff hairs; tactile organ on papillae; hooks 9-10; anterior spines 6-8; posterior spines 12-18.—Spadella draco.
(ii.) Lateral extension of the epidermis not so conspicuous, and the cells composing it smaller. Tactile organs in little depressions. Transverse as well as longitudinal muscles in the trunk. Adhesive cells on the ventral surface of the body. No interval between the lateral fins and the tail fin. Two papillae on the head-hood elongated into club-shaped tentacles. Hooks 8-9, slightly serrated; anterior spines 3-4; posterior spines 3-4.—Spadella cephaloptera.
(iii.) Similar to the last-mentioned species, but the tail segment is larger than the trunk; in the above it is of the same size. No adhesive cells. The fins are covered with papillae, and with a number of serrated spines pointed at both ends.—Spadella pontica.
(iv.) Tactile organs and adhesive cells are unmodified epidermal cells. Anus dorsal. Orifice of oviducts ventral. No olfactory organ. Epidermis colourless. Lateral fins without rays. A pair of ganglia at the postero-lateral angle of the brain.—Spadella marioni.
(v.) Tactile organs well developed on the head, trunk, and fins; tail segment a little shorter than the trunk. Body short, length 3-4 mm. Hooks 9; anterior spines 4-5, posterior spines 6-7.—Spadella vougai.
BY
MARCUS HARTOG, M.A., Trinity College (D.Sc. Lond.)
Professor of Natural History in the Queen's College, Cork.
ROTIFERA, GASTROTRICHA, AND KINORHYNCHA
ROTIFERA—HISTORY—EXTERNAL FEATURES—MOVEMENT—ANATOMY—REPRODUCTION—EMBRYOLOGY—CLASSIFICATION—DISTRIBUTION—AFFINITIES—GASTROTRICHA—KINORHYNCHA
The Rotifera are microscopic animals, the largest not exceeding one-eighth of an inch in length. According to Hudson and Gosse,[233] they are first recorded in an observation of the Rev. John Harris, in 1696, of "an Animal like a large Maggot which could contract itself into a Spherical Figure, and then stretch itself out again; the end of its Tail appeared with a Forceps like that of an Ear-wig."[234] This was certainly a Bdelloid Rotifer.
In 1703 Leeuwenhoek[235] gave a fuller description of a tubicolous form, probably Limnias, and noted the peculiar appearance of the ciliary wreath as "two wheels thickset with teeth as the wheel of a watch." He also noted a little later[236] the way in which Melicerta (see p. 206) builds its tube, and was the first to observe the revivification of certain species after drying.[237] Joblot, a French professor of mathematics, in 1718 figured and described a large number of new genera and species with more or less fantastic details. Baker's figures[238] are a considerable advance on Joblot's, and his descriptions of habits are still fresh and accurate. Eichhorn found a number of new and interesting forms; and O. F. Müller, influenced by the new discipline of Linnaeus, not {198}only figured many species, but gave good short diagnoses of their characters. Ehrenberg in 1838 brought out his magnificent Infusionsthierchen, which contains descriptions and figures of what are now divided into Protophyta, Protozoa, Rotifera, and Gastrotricha. Dujardin's monograph on the "Infusoires," in the Suites à Buffon,[239] was in several respects an advance on Ehrenberg, whose power of observation was so great as to render his mistakes the more inexplicable. But Ehrenberg ever adhered to his errors as firmly as to his facts.
The occurrence of Rotifers among microscopic plants induced the botanists Cohn and Williamson[240] to work at their structure; the group has been studied by men engrossed in other professional cares, such as Gosse, Bedwell, Moxon, Rousselet, and Maupas. Huxley,[241] Leydig,[242] and Cohn[243] studied Rotifers in the '50's and early '60's with a precision the more remarkable when we remember the imperfect methods then available. This period was closed by the valuable monograph published in Arlidge's (4th) edition of Pritchard's Infusoria,[244] under the supervision of W. C. Williamson. Leidy began the study of the American Rotifers. Eckstein[245] gave a careful and interesting account of the species about Giessen in a richly illustrated paper. In recent times the modern methods of histological and embryological research have been applied by Vallentin,[246] Plate,[247] Tessin,[248] and Zelinka,[249] the three Studien ueber Rotatorien of the last author being indispensable to every student, and containing a full bibliography.
Hudson and Gosse's Monograph (1886-89) contains a history of the class to which, as to the whole book, we are deeply indebted; and a full systematic account of all published species.[250] C. Rousselet has introduced a method[251] of preparation of Rotifers in microscopic slides which enables workers to preserve the types they figure and describe for future identification and comparison. Gunson Thorpe has collected and studied Rotifera in China and {199}Australia. It would be unfair not to record here the invaluable services of the late Thomas Bolton, and his son of the same name, both of Birmingham, and of J. Hood of Dundee, who have found and widely distributed living specimens of new, rare, and interesting species.
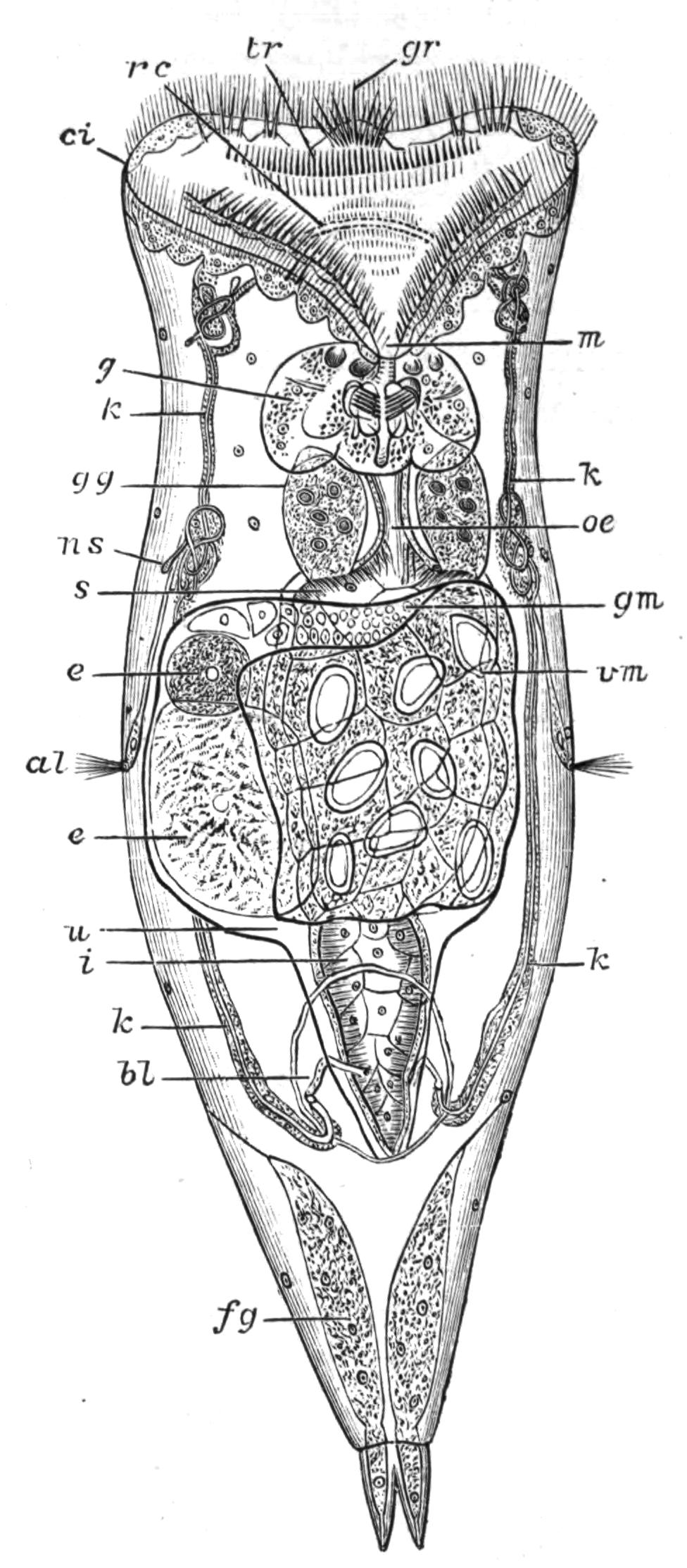
Fig. 106.—Hydatina senta, ventral view. (After Plate.) al, Lateral antenna; bl, bladder; ci, cingulum; e, e, eggs in uterus; fg, foot gland; g, gizzard; gg, gastric gland; gm, germarium or ovary; gr, ciliated lobes of "groove"; i, intestine; k, k, kidneys; m, mouth; ns, nephrostome; oe, oesophagus; rc, renal commissure, transverse tube uniting kidneys above mouth; s, stomach overlaid by reproductive organs; tr, trochus; u, uterus; vm, vitellarium or yolk-gland.
Definition of the Class.—We may define Rotifera as a class of minute bilaterally symmetrical animals, with a chitinous integument, a soft terminal "disc" fringed by a complex ciliary "wreath," an anterior or subventral mouth, and a dorsal cloacal aperture, beyond which the body is usually prolonged into the "foot" or process bearing cement glands, and serving for attachment, temporary or permanent. The body-cavity has no epithelial lining, and is traversed by nerves and muscles. The alimentary canal possesses a chitinous gizzard or mastax of peculiar arrangement, and it usually opens into a cloaca. The nervous centre consists of a ganglion on the dorsal side of the pharynx, to which a second one on the ventral side is sometimes connected to form a complete ring; eyes and bristle-bearing feelers are usually present as sense-organs. A paired system of renal tubes serves for excretion, opening through a median contractile bladder into the ventral side of the cloaca. The sexes are distinct; but the males (Fig. 107), which mostly lack digestive organs, occur {200}rarely, and the females are usually viviparous, or carry about the eggs till they are hatched; while, owing to the rarity of the males, parthenogenesis is habitual. Fission and budding are alike unknown. The fertilised eggs are of the kind termed "winter" or "resting" eggs, and resist conditions adverse to life.
The Rotifera are of cosmopolitan distribution; most of the species inhabit fresh water, whilst some are brackish, and a few are marine; 84 genera and about 700 species have been described.
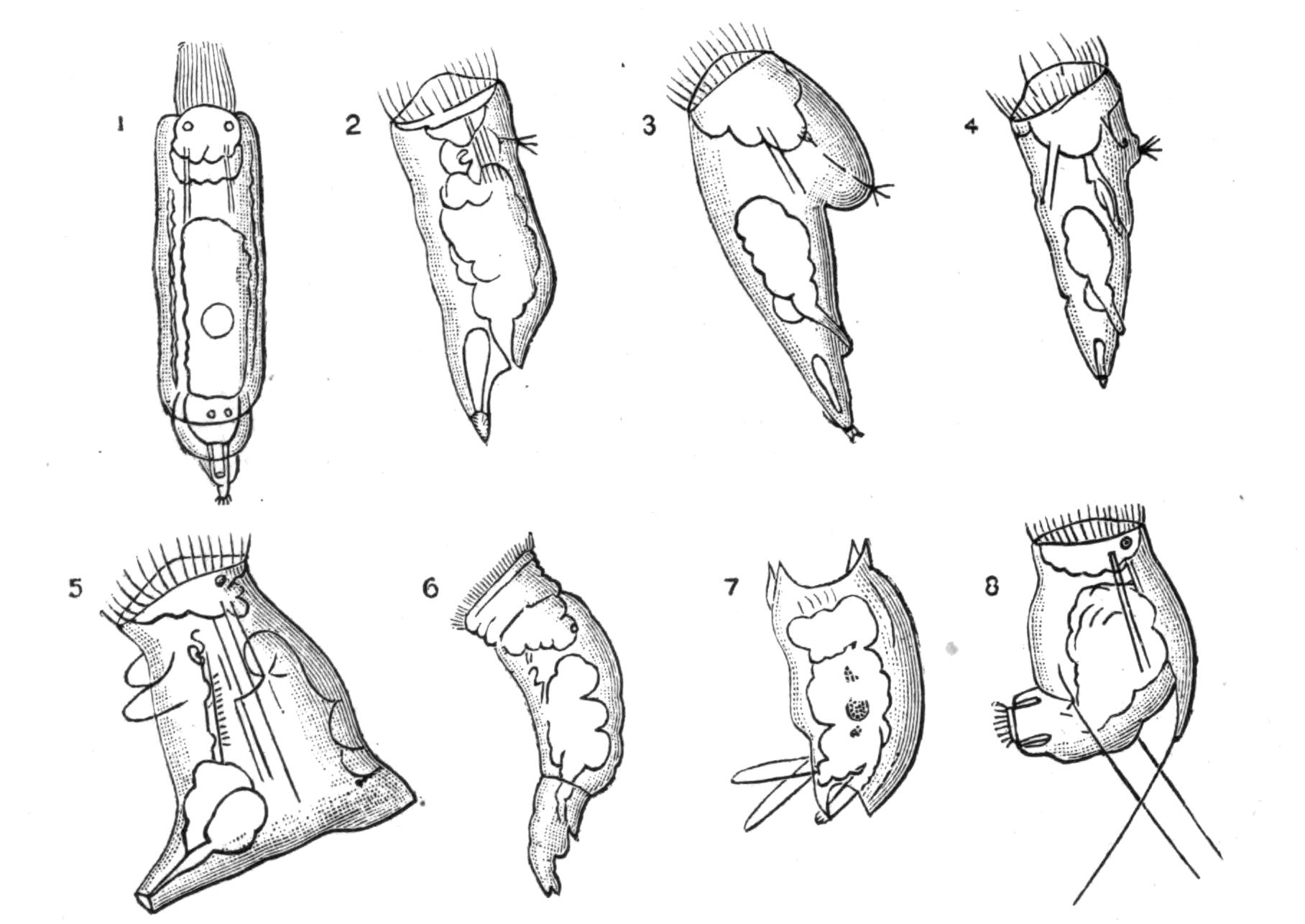
Fig. 107.—Male Rotifers. (After Hudson.[252]) 1, Floscularia campanulata; 2, Lacinularia socialis; 3, Notops brachionus; 4, Synchaeta tremula; 5, Asplanchna ebbesbornii; 6, Brachionus urceolaris; 7, Salpina mucronata; 8, Pedalion mirum.
External Features.[253]—The body is divided into three regions: (1) the head, ending in the disc, which bears the ciliary wreath; (2) the trunk, containing the viscera; (3) the foot, which only contains muscles, nerves, and cement-glands. The general form of the BODY varies greatly: it is spherical in Trochosphaera, ovoid in Asplanchnidae, conical in Scirtopoda, Triarthridae, and Synchaeta; moderately elongated in the majority of the Ploima, among which some forms are very flat, like Pterodina, Metopidia, and Brachionus; shortly elongated and cylindrical in Hydatina (Fig. 106), Notommatidae, and many others. In Taphrocampa it is cylindrical and segmented, while the segments are {201}telescopic in the Bdelloida, both ends being retractile into the middle segment. In most attached, tube-dwelling forms the body is ovate, tapering behind into the elongated stalk-like foot.
The FOOT at the hinder end of the body is usually more or less jointed; in Pterodina and Brachionus it is long, transversely wrinkled, and retractile. Usually it terminates in a couple of acute, mobile toes, perforated at the tips by the ducts of the pedal glands (Fig. 106, fg), whose viscid secretion serves to anchor the animal. In Rotifer there are three of these toes, which are retractile, and in addition there are in this genus, as in most of the Bdelloida, toe-like pointed spurs in pairs on the more proximal joints of the foot. In Callidina the spurs are often perforated, and the toes are replaced by numerous openings on the last joint of the foot (Fig. 109, A); while in Discopus the end of the foot expands into a large disc, with numerous pores for the exudation of the pedal cement, and there are no spurs. In Pedalion mirum the foot is represented by two tubular processes ciliated at the apex and at the outer side near the base (Fig. 117, f). These are inconstant in size and form, that of one side being sometimes reduced or absent, while both are absent in the closely allied species P. fennicum.
In Melicertidae and Flosculariidae the long foot ends in an expanded disc, which is cupped and ciliated in the larva (Fig. 112, B) and in the larva-like male (Fig. 107); but in two species it is prolonged into a long flexible thread which is not contractile. The foot is also elongated in the Bdelloid genus Actinurus and the Ploimal genus Scaridium. It forms a mere ventral disc in Apsilus (and Atrochus?), and is absent in Asplanchnidae (except Asplanchnopus), Triarthridae, and Anuraeidae, and in the genera Trochosphaera (Melicertaceae) and Pompholyx (Pterodinidae).
The fringed spines of Triarthridae are jointed appendages moved by powerful muscles; in Triarthra one is median and ventral, the others being attached to the shoulders. In Polyarthra, there are twelve flattened and serrated spines, a bunch of three being attached to the dorsal and ventral faces of either shoulder. An easy transition leads to the hollow appendages of Scirtopoda, which end in a fringe of bristly hairs, themselves feathered with finer hairs (Fig. 117). These processes are in Pedalion six in number, two median (respectively dorsal and ventral), two antero-lateral, and two postero-lateral. As they contain proper muscles, {202}and the postero-lateral pair contain part of the nephridia and bear the lateral antennae, they are true outgrowths of the body, and are not homologous with the spines of Triarthridae.
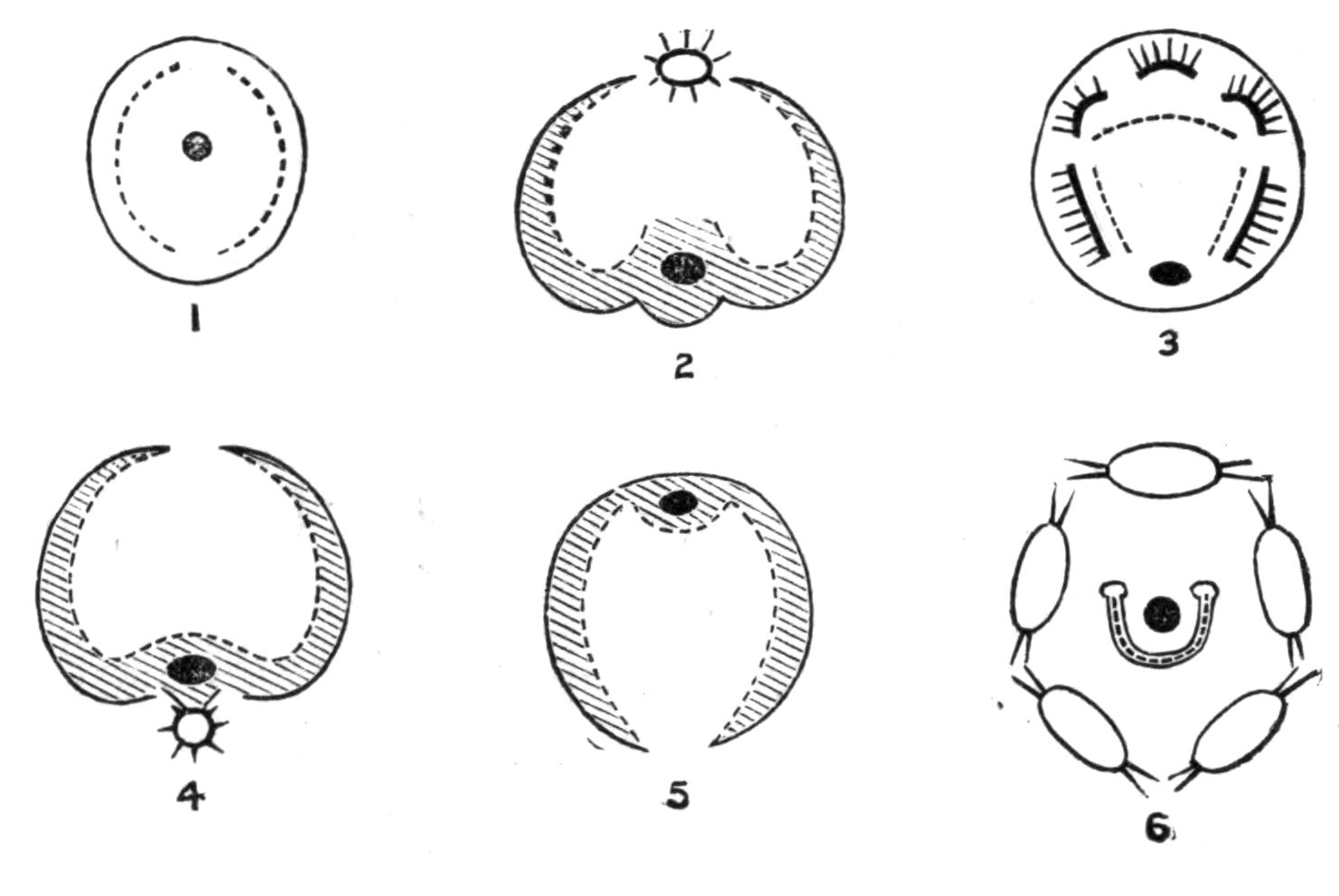
Fig. 108.—Diagrammatic views of disc of Rotifers. Cingulum represented by a black line, groove shaded; trochus dotted; the black spot represents the mouth. 1, Simple disc of Microcodon; 2, Bdelloid disc of Rotifer or Callidina, the star represents the ciliated proboscis; 3, disc of Hydatina, groove represented by lobes bearing ciliated styles; 4, disc of Melicerta, the star represents the ciliated ventral cup with openings into it from the groove; 5, disc of Conochilus; 6, disc of Stephanoceros, cingulum (?) of setose lobes, trochus horseshoe-shaped, mouth central.
The front of the body constitutes the HEAD, which is scarcely distinct, though usually separated by a slight neck-like constriction. The DISC, which terminates the head, varies greatly in shape and in the arrangement of its parts. Imagine a circular funnel, finely ciliated within, and with the mouth at the bottom, the prominent rim bearing two zones of cilia, the inner or anterior being the coarser, and termed the "trochus" or hoop; the outer finer, and termed the "cingulum" or girdle, while a very finely ciliated groove lies between the two zones. Either or both of these zones may be interrupted on the dorsal or ventral median line, or both; and the funnel-shaped mouth may be shifted—usually ventrally, so that it forms only a dilatation of the ciliated groove. Again, the wreath as a whole may be festooned or lobed; or the lobing may be confined to the area between the cingulum and trochus, as in most Ploima (Figs. 106 and 108, 3). Very frequently on these lobes adjacent cilia are fused together during life, producing "vibratile styles," whose true nature is only revealed after death. In Microcodonidae the structure of the disc (Fig. 108, 1) nearly conforms to the primitive type; but the ciliated groove is absent, and the "trochus" is in two separate half-elliptical bands. In the Flosculariaceae (Fig. 108, 6) the mouth is also central, the disc is funnel-shaped, {203}and the trochus is a horseshoe-shaped ridge, with its ends dorsal and raised into prominent knobs. The margin of the funnel is in Flosculariidae (Fig. 115) usually lobed, and furnished either with exceptionally strong cilia, or else with very long bristles which are usually passive. However, by the retraction of the lobes that bear them they are clasped together like casting-nets to enclose prey brought into the funnel by the action of the trochal cilia. An external ring of cilia in Floscularia mutabilis and F. pelagica serves for swimming. In Apsilidae the margin of the disc bears neither cilia nor bristles, but is either simple and ring-like, or is produced into tentacles (Fig. 112, C). The oral funnel is probably represented in Flosculariaceae by the continuation of the small central mouth into a ciliated tube (Fig. 115, C, tf), open below, and hanging freely down into the crop.
In all other cases the mouth is displaced, and lies in the groove and on its ventral side (except in Conochilus, where it is dorsal, Fig. 108, 5). In the Bdelloida the disc is prolonged into two great lobes like kettle-drums, round the posterior, external, and ventral edges of which run the trochus, cingulum, and ciliated groove (Fig. 108, 2). All three are interrupted behind in the median line; ventrally the groove widens into the oral funnel, the cingulum is continued into a sort of spout-like lower lip (Fig. 109, C, D, l), and the trochus is absent. The body is prolonged dorsally above the lobes into a two-jointed proboscis, ending in a ciliated cup overhung by two dorsal flaps: this we regard as a detached portion of the wreath.
This "Bdelloid" type of wreath occurs also in Scirtopoda (Fig. 117), and in the Ploimal genera Triarthra, Pterodina, and Pompholyx. A simpler wreath of essentially the same type occurs in Asplanchnaceae and Melicertaceae; the disc is not prolonged into drum-shaped lobes, but is thin at the rim, where it bears the triple ciliated zone, interrupted on the dorsal median line and depressed ventrally into the oral funnel. In the Melicertidae, moreover, the disc is widened into a great plate-like extension, often beautifully lobed; and in many of the species a ciliated cup lies ventral to the lips, and is connected with the groove by a short ciliated channel on either side (Figs. 108, 4, and 116). Even the simpler wreath of Asplanchnidae is complicated by stronger lobes on either side bearing vibratile styles.
The most complex discs are found in Ploima, especially in {204}Brachionus, Hydatina, and Synchaeta, since the groove is replaced by a zone of lappets, as above mentioned. In Proales the whole face of the disc is strongly ciliated. The wreath is reduced in the parasitic genera Drilophagus, Albertia, Balatro, and the Seisonaceae; in Adineta and Taphrocampa it is only represented by a general but scanty ciliation of the disc.
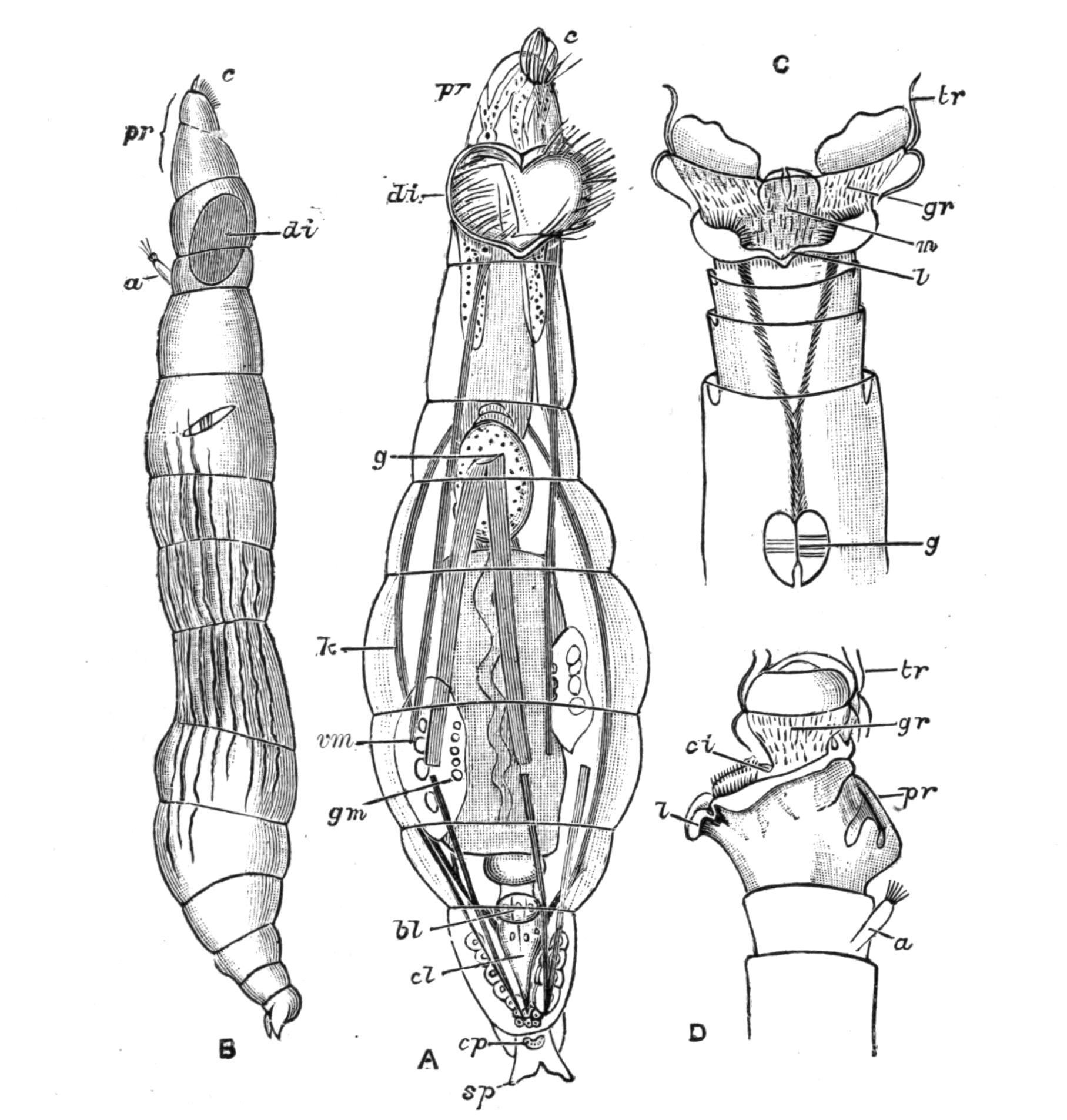
Fig. 109.—Callidina symbiotica. (After Zelinka.) A, Ventral view, with the disc half expanded, proboscis extended; B, lateral view, proboscis extended; C, ventral view of anterior segments with expanded disc; D, lateral view of same (proboscis retracted). a, Antenna; bl, bladder (enlargement of rectum); c, ciliated cup of the proboscis; ci, cingulum; cl, cloaca; cp, group of pores, the openings of cement glands; di, disc; g, gizzard; gm, germarium (that of the opposite side seen at a higher level); gr, ciliated groove; k, kidney; l, lip; m, mouth; pr, proboscis; sp, spurs of foot; tr, large cilia of trochus, showing vertical movements; vm, yolk-gland. The body muscles are represented by shaded bands.
The head is very frequently retractile, as a whole, by strong muscles. In Bdelloida the disc proper is retracted when the animal crawls, while the proboscis is exserted (Fig. 109). Ciliated patches occurring outside the region of the disc point to {205}a primitive condition when the whole surface of the body was ciliated, as does the partial ciliation of the foot in certain groups. Synchaeta and many Notommatidae possess a pair of lateral, hollow, ciliated pits on the body, which can be everted to serve as additional swimming organs; these are termed "auricles."
The cuticle varies much in texture. It may be smooth and flexible, dotted or shagreened, or in the Loricata firm and of definite shape, constituting a lorica, which may be more or less distinctly divided up into areas or separated into distinct pieces. In this case it resists decomposition, and several species are only known by this "skeleton." In Ploesoma it is much thickened and looks like a honeycomb. A regular alternation of harder and softer zones effects the annulation of the body in certain genera.
The hypoderm or protoplasmic layer of the skin has no cellular boundaries, though it contains large and distinct nuclei; it is usually somewhat granular. It forms the wall of the body-cavity, which contains a transparent liquid without corpuscles.
The principal external glands are the pedal or cement-glands, which secrete a viscid substance that sets in water and serves to anchor the animal. They are formed from an ingrowth of the hypoderm, are usually paired, and open by fine ducts on or near the apex of the toes, when these processes of the foot are present (Fig. 106, fg). These glands are mostly absent when there is no foot, as in most Asplanchnidae and in Anuraeidae, but in Asplanchna herrickii a small gland on the ventral side of the cloacal aperture appears to represent the last rudiment of the foot.
In addition to these, the ciliated ventral cup below the disc of many Melicertidae secretes a viscid substance (Fig. 116, p); and possibly the whole surface of the body is secretory in those species of this group, and of the Flosculariidae, whose tube (Fig. 115, A) is uniform and not made of pellets. In several other species belonging to Bdelloida and Ploima-Illoricata a viscid secretion of the surface of the body renders it "sordid" with adherent particles of dirt.
When the secretion takes the form of a tube, the body can be wholly withdrawn into it by the contraction of the foot. In Floscularia, Stephanoceros, and Conochilus the tube is hyaline and thin-walled; in Oecistes and Cephalosiphon it is more or less floccose; and in Limnias it is thin, firm, and annulated. In Melicerta and some species of Oecistes the tube thus secreted by {206}the body is only formed in a very young state. In M. janus and M. pilula it is increased by the successive deposition of ovoid faecal pellets on to the rim. In M. ringens (Fig. 116) and M. conifera pellets are formed of the excess of the food particles brought to the disc by the ciliary current; they are carried through the gutters on either side of the projecting ventral lip or "chin" into the ciliated glandular cup on that side of the head. Here, as they revolve, they are cemented together into a pellet which is spheroidal in the former species, cylindro-conoidal with a basal hollow like a rifle-bullet in the latter. After a pellet is completed the animal stoops down and deposits it on the edge of the tube. This may easily be verified by furnishing a young Melicerta with water containing solid particles of carmine. M. tubicolaria forms a thick tube which is laminated, the laminae being directed upwards and outwards, and having diatom shells, etc., between the layers. In this case we have observed that the faeces are pellucid, and sometimes are so ejected as to lie in a sheet against the funnel-shaped mouth of the tube, and we are inclined to believe that the tube itself is formed altogether in this way. A similar process probably occurs in Oecistes crystallinus and Oe. umbella.
The muscles are simple elongated fibres, usually having near the middle a mass of granular protoplasm containing a nucleus; they may be smooth or striated. The principal muscles of the body are conspicuously striated in many active free-swimming forms (Pedalion, Synchaeta, Pterodina, Triarthra).
The muscles of the body-wall are transverse and longitudinal. They are best seen in Bdelloida. The principal muscles of the body-cavity are longitudinal; the most conspicuous and constant are the retractors of the disc and of the foot, protraction of these organs being usually accomplished by the contraction of the transverse muscles. Special muscles effect the vigorous springing of the Triarthridae and Scirtopoda; in the former group the muscles only raise the spines, and their elastic recoil is the actual mechanism of progression; but in the latter (Fig. 117) special flexor muscles of the limbs are the effective agents of the leaping movements.
Movements.—The Rotifera vary very greatly in their movements. The cilia of the disc, and especially of the trochus, are the principal organs of prehension of food, and also of swimming when {207}the animal is not fixed by its foot. In some cases, as in Bdelloida, the cilia lash downwards successively in the longitudinal plane of the body (Fig. 109, C, D); this motion during fixation produces a hollow vortex ring, like the rings of a skilled cigarette-smoker, but when the animal is free it determines a simple forward progression through the water. In other cases the animal rotates on its long axis, or may even turn somersaults (Synchaeta). The appearance of the spokes of a wheel is a pure illusion due to the greater visibility of the cilia in their slow recovery than in their instantaneous down-lash. The finer cilia of the groove and cingulum play a very minor part in the act of swimming, and in the production of the great vortices at the edge of the disc when the animal is fixed; they serve to direct the particles brought by the vortices to the edge of the disc onwards towards the mouth. It is easy to see that the stream must be in opposite directions on opposite sides of the groove; its prolongation across the dorsal median line would be useless, which explains the existence of the dorsal median gap. At the ventral side we usually find a prominent ciliated lip, whose cilia work outwards, and carry off the excess of food particles as by an overflow spout. In many cases among the Notommatidae, Coluridae, etc., the disc serves as much for creeping over organic débris as for swimming.
We have already noticed the springing bristles and limbs of the Triarthridae and Scirtopoda respectively; the great foot of Scaridium is also used for leaping. The Bdelloida have the power of retracting their disc and progressing in loops like a leech or looper (Geometrid) caterpillar.
Baker, in a letter addressed to Martin Folkes, Esq., President of the Royal Society, dated London, 16th January 1744-5,[254] gives the following lively account of the aspect and movements of Philodina roseola belonging to this group, with figures, some of which we reproduce from the original copper-plate engraving:—"I call it a Water Animal, because its Appearance as a living Creature is only in that Element. I give it also for Distinction Sake the Name of Wheeler, Wheel Insect or Animal; from its being furnished with a Pair of Instruments, which in Figure and Motion appear much to resemble Wheels. It can, however, continue many Months out of Water, and dry as Dust; in which Condition its Shape is globular, its Bigness exceeds not a Grain of {208}Sand, and no Signs of Life appear. Notwithstanding, being put into Water, in the Space of Half an Hour a languid Motion begins, the Globule turns itself about, lengthens by slow Degrees, becomes in the Form of a lively Maggot, and most commonly in a few Minutes afterwards puts out its Wheels, and swims vigorously through the Water in Search of Food; or else, fixing by its Tail, works them in such a Manner as to bring its Food to it. But sometimes it will remain a long While in the Maggot Form and not shew its Wheels at all....
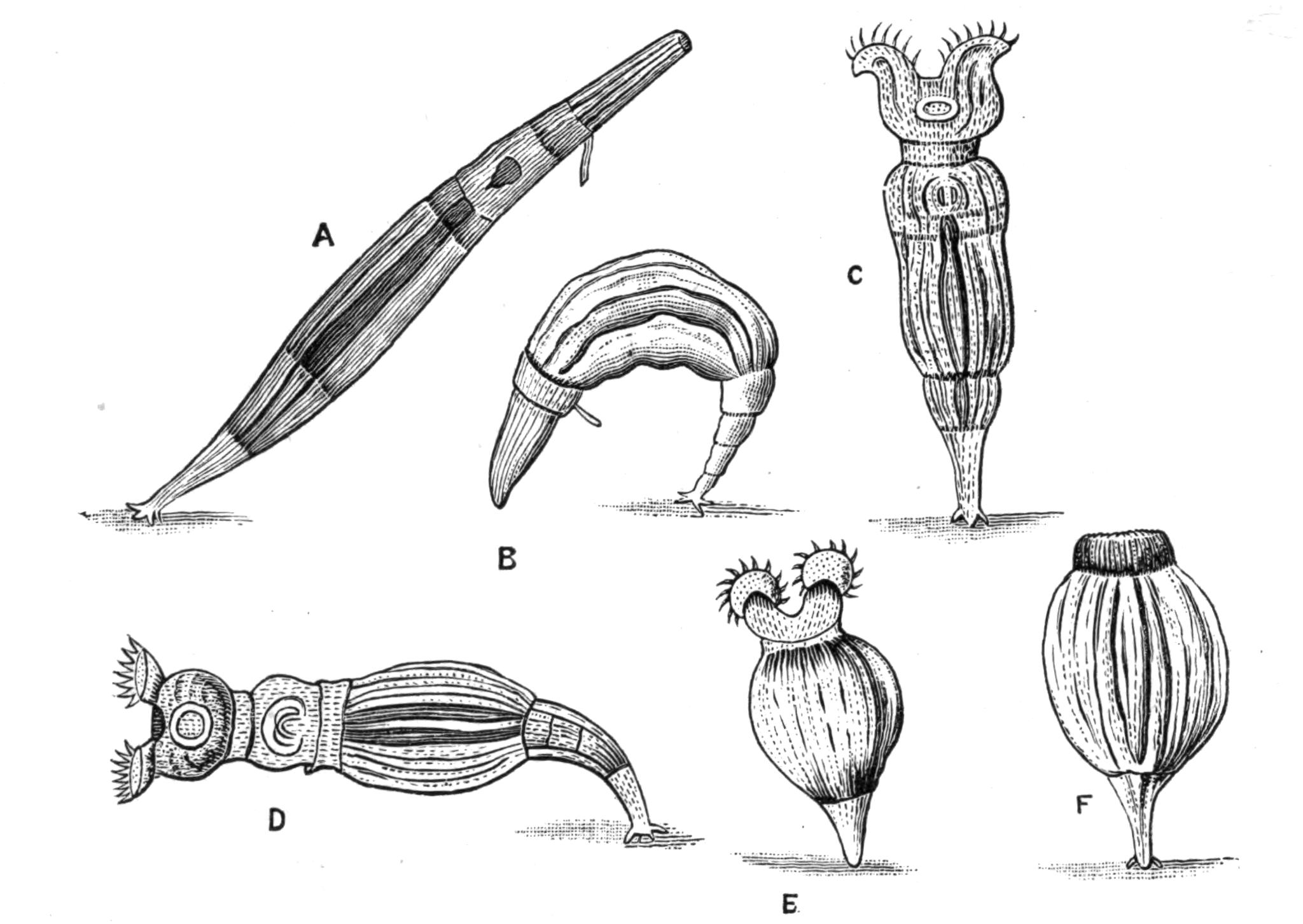
Fig. 110.—Philodina roseola. (After Baker.) A, B, Crawling, with extended proboscis, and showing antenna; C, D, E, attached, with "wheels" extended for catching food; F, attached, with anterior end retracted.
"If the Water standing in Gutters of Lead, or the slimy Sediment it leaves behind, has any Thing of a red Colour, one may be almost certain of finding them therein,[255] and, if in Summer, when all the Water is dried away, and nothing but Dust remains, that Dust appears red, or of a dark brown, one shall seldom fail, {209}on putting it into Water, to discover Multitudes of minute reddish Globules, which are indeed the Animals, and will soon change their Appearance, in the Manner just now mentioned....
"A Couple of circular Bodies, armed with small Teeth like those of the Balance-Wheel of a Watch, appear projecting forwards beyond the Head, and extending sideways somewhat wider than the Diameter thereof. They have very much the Similitude of Wheels, and seem to turn round with a considerable Degree of Velocity, by which Means a pretty rapid Current of Water is brought from a great Distance to the very Mouth of the Creature, who is thereby supplied with many little Animalcules and various Particles of Matter that the Waters are furnished with.
"As these Wheels (for so from their Appearance I shall beg Leave to call them) are every where excessively transparent, except about their circular Rim or Edge on which the Cogs or Teeth appear, it is very difficult to determine by what Contrivance they are turned about, or what their real Figure is, though they seem exactly to resemble Wheels moving round upon an Axis....
"As the Animal is capable of thrusting these Parts out, or drawing them in, somewhat in the Way that Snails do their Horns, the Figure of them is different in their several Degrees of Extension and Contraction, or according to their Position to the Eye of the Observer, whereby they not only appear in all the various Forms before represented, but seem at certain Times as if the circular Rim of the Wheel or Funnel were of some Thickness, and had two Rows of Cogs or Teeth, one above and the other below that Rim."
Digestive Organs.—The pharynx is usually a narrow ciliated tube, which varies in length from genus to genus, but in no other important point, save in Flosculariidae, where it assumes the form of a crop, into which the mouth hangs freely down as a narrow ciliated tube. At its lower end is an enlargement, the mastax or gizzard.[256] This is a strong muscular sac containing the trophi or hard chitinous chewing organs, with an {210}antero-ventral inlet from the pharynx, and a postero-dorsal outlet through which the food passes into the stomach either directly or through a slender gullet (Fig. 106, oe). In the ventral wall of the gizzard of most Ploima is a median piece, the fulcrum, from which run forwards and upwards two pieces, the rami, which are hinged on the fulcrum. The Y-shaped structure formed of these three pieces is called the incus (anvil). At either side of the gizzard and at a higher level is a paired piece, the malleus, so called from its resemblance to a hammer, of which the manubrium (handle) looks backwards, and is embedded in the side walls of the mastax, while the toothed claw or uncus looks forwards and inwards, and is hinged at its inner side with the tip of the ramus. As the unci and rami are usually strongly toothed, this gizzard forms a very efficient apparatus for chewing. In some cases, when the pharynx is short and dilatable, the points of the unci and rami may be protruded for biting, for clinging to the host (in the parasitic genera Albertia and Drilophagus), or for the prehension of food (Rattulidae, etc.).
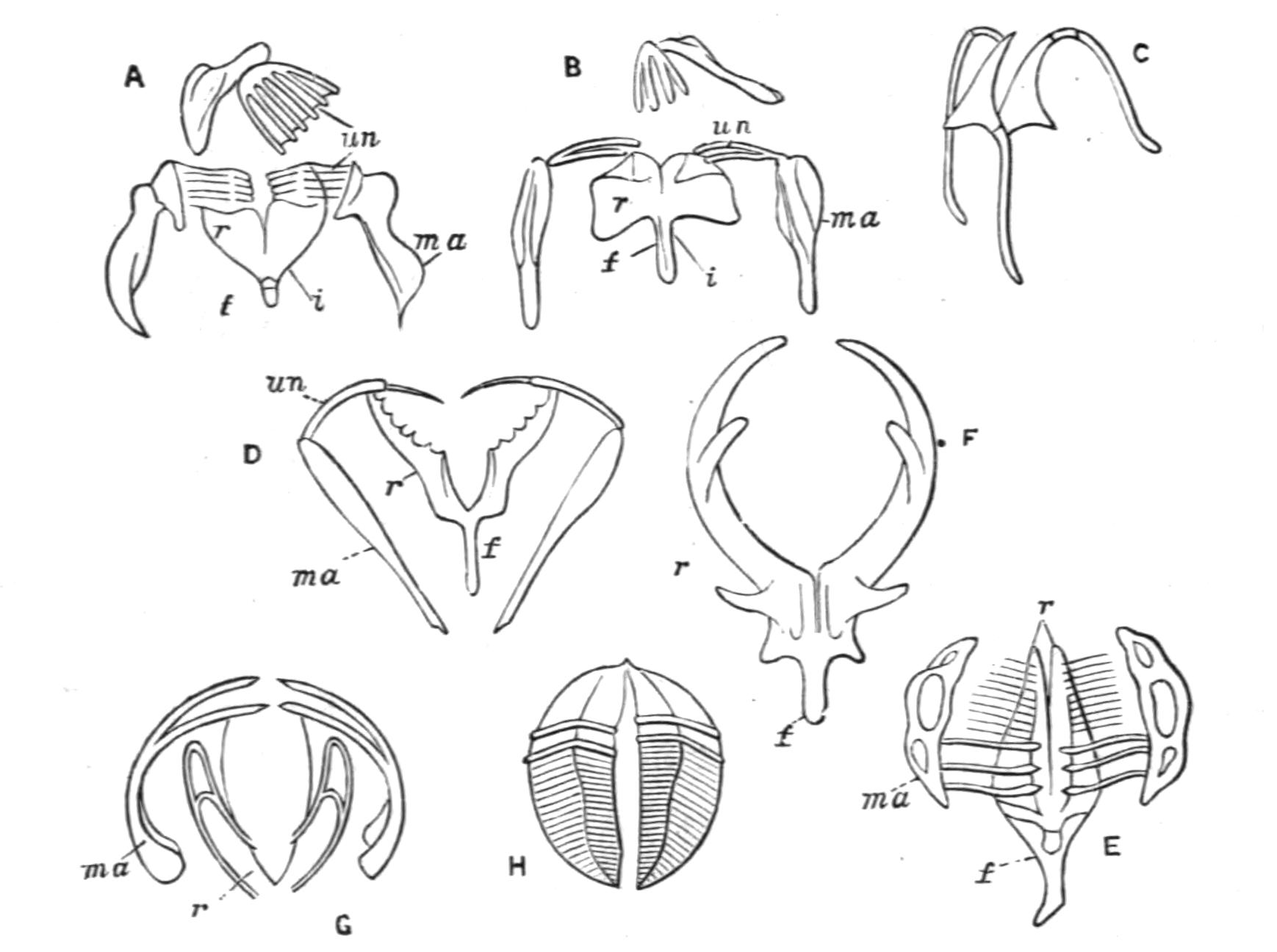
Fig. 111.—Diagram of trophi. (After Hudson.) A, Malleate; B, submalleate; C, virgate; D, forcipate; E, malleoramate (Melicerta); F, incudate (Asplanchna); G, uncinate (Stephanoceros); H, ramate (Rotifer). f, Fulcrum; i, incus; ma, manubrium (malleus in G); r, ramus; un, uncus.
The type we have just described is termed the "malleate" type (Fig. 111, A). If all the trophi are slender and scarcely toothed, we have the "virgate" type (C), which is frequently {211}asymmetrical. In the "submalleate" type (B) the mallei only are slender; in the "forcipate" type (D) both the unci and rami are slender and sharply pointed.[257] In the "malleoramate" type (E) the manubrium is a curious looped structure, while the uncus is formed of a number of parallel slender elongated teeth; this characterises the family Melicertidae, and the genera Triarthra, Pterodina, and Pedalion. In the "uncinate" type (G) the mallei are simply incurved hooks with a few teeth at the free end, the rami are simple or absent, and there is no fulcrum; this type occurs in Flosculariaceae only. In Asplanchnidae the rami are large and hooked, constituting the "incudate" mastax (F); but here reduced mallei are often present, and in Asplanchnopus they are almost as well developed as in Melicertidae, affording a transition to the malleoramate type. In this group too the mastax has a very peculiar form; it is divided into two chambers, dorsal and ventral. The dorsal chamber forms a great purse-like sac or crop, with a framework of four longitudinal bars: into this the gullet and pharynx open. The ventral pouch is much smaller, and in its base the large rami are inserted, so that they can be protruded into the crop. This ventral sac with the rami may even be everted through the crop and the mouth, to swallow the small Rotifers and Entomostraca which form the food of this group, or to eject the undigested remains of the food. Two lateral sacs open at the junction of the ventral pouch and the crop, but whether they play a part in the deglutition of food or in the disgorging of faeces is uncertain. The fact that the whole of this apparatus is lined by a non-ciliated chitinous cuticle justifies our view that it is simply an enlargement and specialisation of the mastax.
The trophi in Bdelloids also are only represented by the rami, which have the form of segments of a sphere, excavated on the curved sides for the attachment of muscles, and transversely ridged on the two flat sides; the gizzard is here called "ramate" (H).
It will be seen that the characters of the gizzard are very useful for classification, only breaking down indeed in the {212}Ploima; for though the majority of these present one or other of the four varieties of the malleate type, Triarthra and Pterodina (but not the other genera of their respective families) have the gizzard malleoramate.
The oesophagus is, when present, a contractile ciliated tube in which the food makes no sojourn on its way to the stomach.
The stomach may be nearly spherical, ovoid, or elongated and cylindrical. Its walls are formed of large cells, often granular and sometimes brownish, whence a hepatic function has been assigned to them. Its apertures are both surrounded by constricting muscular fibres. The intestine may be simple or divided by a similar constriction into intestine proper and rectum. The whole of the alimentary tract, with the exception of the mastax, is richly ciliated within. The rectum opens into the slender non-ciliated cloaca. The intestine is sharply bent upwards and towards the back in the tubicolous forms, but is nearly straight elsewhere; in Trochosphaera and Apsilus it is bent ventrally. In Asplanchnaceae and in Paraseison there is no rectum, the stomach being a blind sac.
The so-called salivary glands, usually two in number, open into the pharynx or mastax; and the paired gastric glands (Fig. 106, gg) open into the oesophagus or stomach. While the prehension of food is usually accomplished by the ciliary current of the disc and pharynx, we have seen that a more active swallowing action takes place in Flosculariaceae and Asplanchnidae, which devour whole Algae, Infusoria, and even other Rotifers, the long spines of Triarthra not availing as a protection. Many Ploima put out the tips of their trophi to nibble at débris, or, in the case of Diglena and Distemma, to attack Desmids, or the Infusorian Stentor. But this use of the trophi is most efficient in Ploesoma. Bilfinger[258] writes: "It has the courage to attack larger Rotifers; thus I was able to observe under the microscope how it fell upon a Rattulus but little smaller than itself and destroyed it. First it plunged the sharp prongs of its mastax deep into the tender frontal area of its unhappy victim; then followed a pumping action of the gizzard, and stroke by stroke the whole contents of the victim's body passed into the brigand's stomach." From this it is an easy transition to the ectoparasitism of Drilophagus, Balatro, and {213}some species of Albertia, which cling to their host by the exserted trophi.
Renal Organs.—The kidneys consist of a pair of convoluted tubes, formed of a succession of perforated, so-called "drainpipe" cells (Fig. 106, k); they open directly or indirectly into the cloaca. Their walls are thin in the straight parts, but thick and glandular in the coils which occur at intervals. These tubes bear little tag-like appendages, hanging freely into the body-cavity, often widening towards the free end, and flattened or circular in section (Fig. 106, ns). They show during life a peculiar flickering motion in their interior, like the equivalent "flame-cells" of many Platyhelminthes (see p. 25), and are in function the representatives of the multicellular renal funnels of Annelids. On one side, especially on the edge of the flattened tags, the appearance is as of a tapering whip-like lash, attached by its base to the free end of the tag and waving in its cavity; but the side view of the flattened tags shows an appearance of successive transverse or oblique waves. In many if not all cases the free end of the tag is closed by a vacuolated plug of protoplasm, which sometimes at least bears two flagella waving freely in the body-cavity. The probable explanation of the two distinct wave appearances within the tag is that the protoplasmic plug bears on its inner face a row or tuft of long cilia hanging down into the cavity of the tag. The tags probably keep up a current of liquid through the kidneys, while the contents of the body-cavity are constantly replenished by osmosis.
The two renal tubes may end blindly below the disc, or else join by a short transverse dorsal communication in front of the brain, as in Stephanoceros, Atrochus (Fig. 112, C), and Apsilus among Flosculariaceae, Lacinularia among Melicertidae, and Hydatina among the Illoricate Ploima (Fig. 106, rc). In some species of Asplanchna, if not all, a recurrent branch occurs opening at either end into the main tube of its own side.
The kidneys unite to discharge into the cloaca near its orifice, and on its distal (primitively ventral) side in many Melicertidae. In Bdelloida the common duct formed by their fusion opens into the ventral side of a dilated bladder-like section of the cloaca (Fig. 109, A, bl), which contracts rhythmically to discharge the liquid; while in the majority of the class they open singly or by a common duct into a separate contractile vesicle or bladder, which also discharges at regular intervals into the cloaca on its ventral or distal side (Figs. 106, bl and 112).
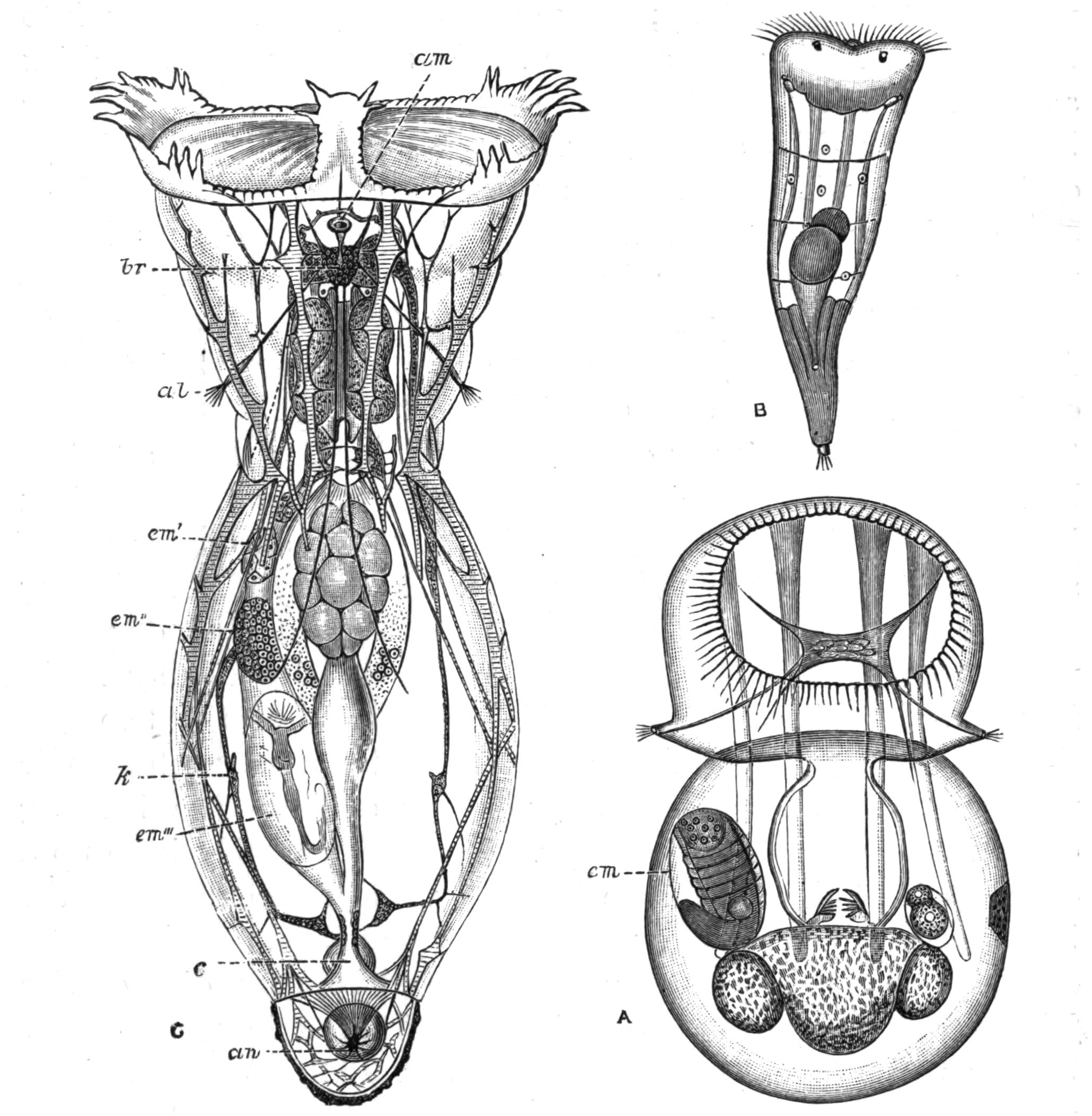
Fig. 112.—Apsilidae: A, Apsilus lentiformis, ♀, dorsal view (after Metschnikoff); the square brain is seen with nerves to the lateral antennae; B, larva of A. lentiformis (?), showing the paired eyes and ciliated cupped foot; C, adult of Atrochus appendiculatus, ♂ (after Wierzejski). al, Lateral antennae; am, median antenna (just in front is seen the renal commissure); an, anus; br, brain, below which the paired eyes are seen; c, cloaca; em, embryo; em', em', em''', three successive stages of embryos in the uterus of C; k, kidney. The coarser muscles are striated.
This bladder may reach when expanded one-third the diameter of the whole animal, and contract as often as three times per minute; so that in a period of nine minutes a bulk of water equal to that of the animal must have diffused through the body-wall, to be removed by the kidneys. It is obvious that while the function of the kidneys is primitively excretory, the passage of the water through the body must bring in the oxygen dissolved in the external {215}medium, and carry off the carbonic acid formed in the tissues, and so fulfil the act of respiration. This mechanism is physiologically comparable with that of the contractile vacuole of fresh-water Protozoa. In a few genera (Conochilus, Lacinularia, Pterodina) the kidneys open separately after a slight dilatation into the cloaca.
Nervous System.—The nervous centre of the Rotifera is the brain (Fig. 112, C, br), a ganglion lying dorsal to the pharynx; and when this is short it may be immediately below the surface of the disc (Microcodon). In Bdelloida a second ganglion is present below the pharynx, and is connected with the former by lateral cords which contain ganglion cells. From the brain, nerves are given off to the disc, to the muscles, and to the integument of the body, as well as to the sense organs. The largest nerves are two given off from the sides of the brain, each of which divides into a lateral and a ventral trunk, which run nearly the whole length of the animal.
The brain of several Notommatidae has a curious appendage, white by reflected light and very opaque; it is a sac full of chalky mineral matter, which dissolves readily in dilute acids.
Sense Organs.—The most widely diffused sense organs are the antennae or feelers, which may serve for touch or smell, or possibly both. Each antenna is a conical or tubular outgrowth of the skin; from its apex projects a fine pencil of sense hairs borne on a protoplasmic cushion, which receives a nerve. Often the antenna is elongated, and may then contain a muscle by which it is retractile (lateral antennae of Melicerta); sometimes it is reduced to a slight prominence bearing the setae (dorsal antenna of this genus). There are usually three antennae—a median dorsal (Figs. 109, B, a, and 112, C, am) and two lateral (Figs. 106, 112, C, and 115, A, al), often approximated towards the ventral surface, and sometimes all but fused on the middle line, or completely united (Conochilus dossuarius, Copeus caudatus).[259]
Most Rotifers possess an organ of sight. This in its simplest form is a refractive globule seated in a red pigmented cup through which the nerve passes; in other cases it lies directly on the brain. Very frequently the eye is paired (Figs. 112, B, and 115, A); and these paired eyes may lie on the brain, and then {216}are so close together that the pigment-cups have the shape of an x, or else they are seated in the dorsal region of the head behind the disc. In some cases they lie just under the ciliary wreath, or even within the region of the disc, and pass towards its ventral side in Pedalion (Fig. 117, A, e). In Rotifer they lie just under the dorsal side of the proboscis just below its apex. The median and two lateral eyes often exist together, as in Eosphora; and sometimes additional paired eyes exist. In Furcularia longiseta, var. grandis a pair of pigment spots (eyes?) occurs at the hinder end of the body just in front of the foot.
The active Ploima show a spontaneity of movement and marked power of avoiding obstacles, etc. This is still more marked in the very active Pedalion, which, as Rousselet notes, clearly avoids capture by the dropping tube, aided by its sense of sight, as he suggests, or by the tactile or olfactory powers of the antennae. They must rank as psychically high in the scale of creatures of simple organisation.
Reproductive Organs and Reproduction.—The most conspicuous organ in the female is the large yolk-gland or vitellarium (Figs. 106 and 109, A, vm), which was regarded as the ovary by all the older observers. It consists usually of eight cells, with conspicuous nuclei, lying on the ventral side of the stomach, and frequently displaced to one side; but in most Asplanchnidae it forms a broad transverse band of numerous cells. In Pterodina it is horseshoe-shaped, while in Seisonaceae and Bdelloida it is paired, either gland containing four or eight cells. The true ovary or germarium (Fig. 106, gm) lies more or less hidden between the yolk-gland and the stomach; it is composed of numerous minute rounded cells, of which the hindmost for the time being enlarges by nutrition from the yolk-gland, and finally receives a membranous shell. This true ovary is somewhat lateral in most Rotifers, but is median in Asplanchnidae, and paired in Pterodina, Bdelloida, and Seisonaceae. A membranous covering is common to the ovary and yolk-gland (paired when these are paired); it is continued into a thin-walled tube or oviduct, which opens into the cloaca on its ventral side beyond the bladder or common renal duct. In the viviparous species the mature ovum (Fig. 112, em) usually lies in the oviduct, dilating it into a sort of "uterus" until the birth of the young. The ordinary eggs or "summer eggs" are formed without any {217}fertilisation, and develop immediately; they are often hatched within the tube of the tubicolous species.
Under certain conditions the unfertilised females produce exclusively smaller eggs, which develop into males. Maupas[260] has demonstrated that a rise in temperature to a minimum of 26° C. (79° F.) is the efficient factor. But as Bergendal points out,[261] the critical temperature probably varies with the antecedent conditions of the race, since males occur in Greenland at a very much lower temperature; and it would seem probable that a temperature approaching that at which the pools habitually dry up is what is necessary for the production of males, as a provision for those fertilised eggs, which, having a hard shell often adorned with prickly prominences, and usually remaining for some time before development, are capable of withstanding drought; such eggs are termed "winter eggs," but a better term would be "resting eggs" (German, "Dauereier").[262]
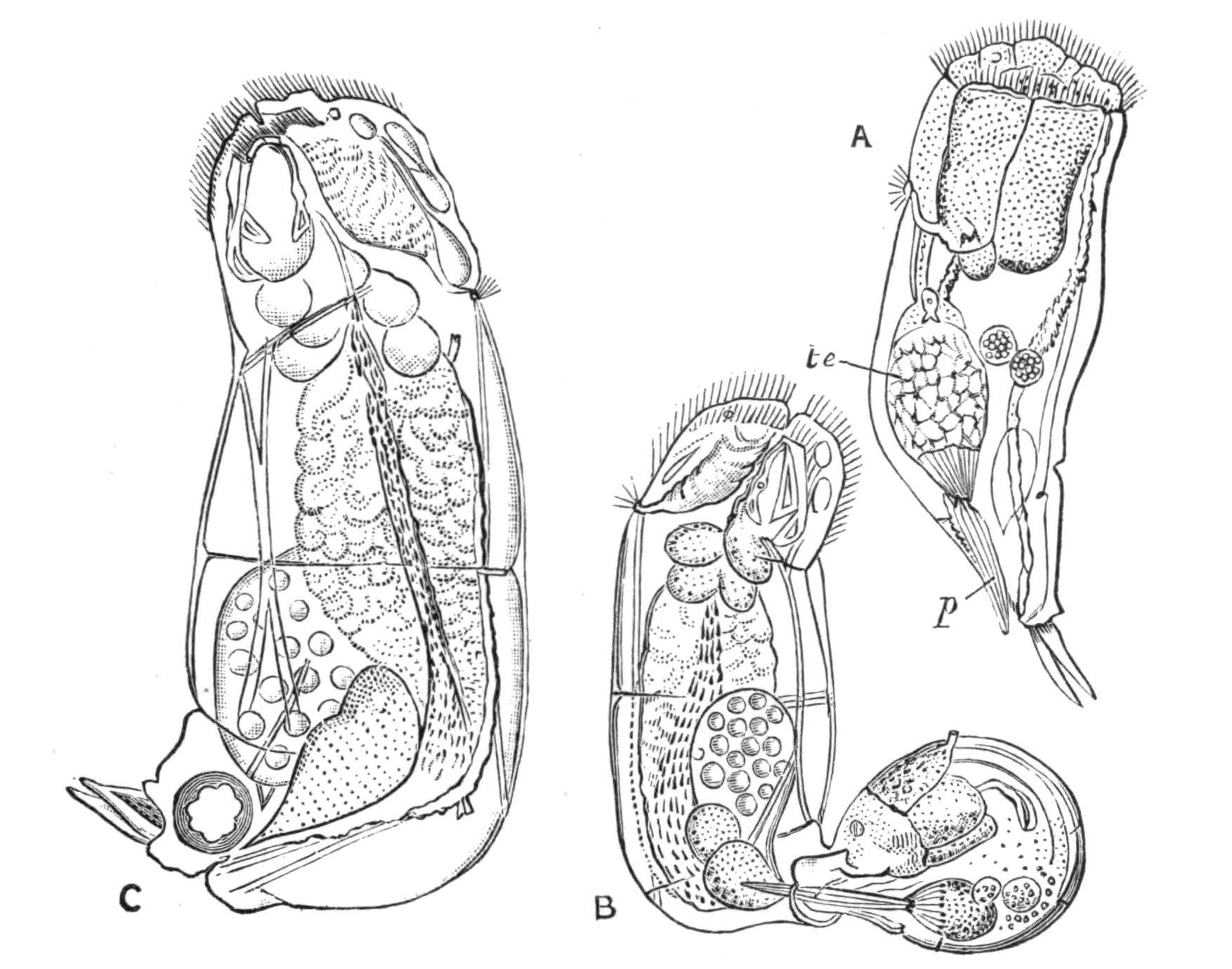
Fig. 113.—Diglena catellina. (After Weber.) A, Male; B, the pair in copula; C, female, p, Penis; te, testis.
The male organs consist of a testis (Fig. 113, A, te) with accessory glands, a large seminal vesicle, and a protrusible or projecting penis (p). In Notommata and Diglena true intromission at the cloaca (B) has been seen by many observers; but it {218}appears equally certain that in many cases the male bores into the body-wall of the female at any point, and deposits the spermatozoa in the body-cavity, so that they must pass through the wall of the oviduct to effect fertilisation. Maupas finds that the process of fertilisation is ineffective except upon such newly-hatched females as would otherwise be the parents of small male eggs; that fertilisation is inoperative even for these at a later age when their eggs have begun to mature; and that it is wholly useless for those that lay ordinary summer eggs. The parent of male or winter eggs would thus be comparable to the queen bee, which if not fertilised produces drones. These sexual relations find a close parallel in the Ostracod and Phyllopod Crustacea, as well as in many plant-lice (Homoptera).
Development.—This has only been fully studied in the summer egg; in Brachionus by Salensky,[263] in Melicerta by Joliet[264]; in Eosphora digitata and several other species by Tessin[265]; in Callidina and Melicerta by Zelinka,[266] the last two observers having utilised modern methods of research.[267] We shall base our account on Zelinka's observations. As in the case of most "parthenogenetic" eggs, the ovarian egg begins by a very uneven division to form two cells: the minute "first polar body" which undergoes no further development; and the definitive egg, which by its repeated divisions gives rise to the tissues and organs.
Segmentation is very unequal, and recalls that of Molluscs in several respects. The first division gives rise to a smaller and a larger cell. Both of these divide again, the latter unequally, so that now there are three smaller cells and one large one; and after repeated divisions of the small cells and unequal divisions of the larger one, a stage is reached where there are a number of small cells and one large one, which sinks in and is overgrown by the small ones. Just prior to this the large cell undergoes equal divisions; its cells are the "hypoblast" cells (Fig. 114, hyp), and give rise to the gullet, stomach, and intestine, with their appendages, and the generative organs; while the smaller cells constitute the "epiblast" (ep), which gives rise to the body-wall and muscles, to the cement glands, nervous system, pharynx and mastax, and probably to the kidneys.
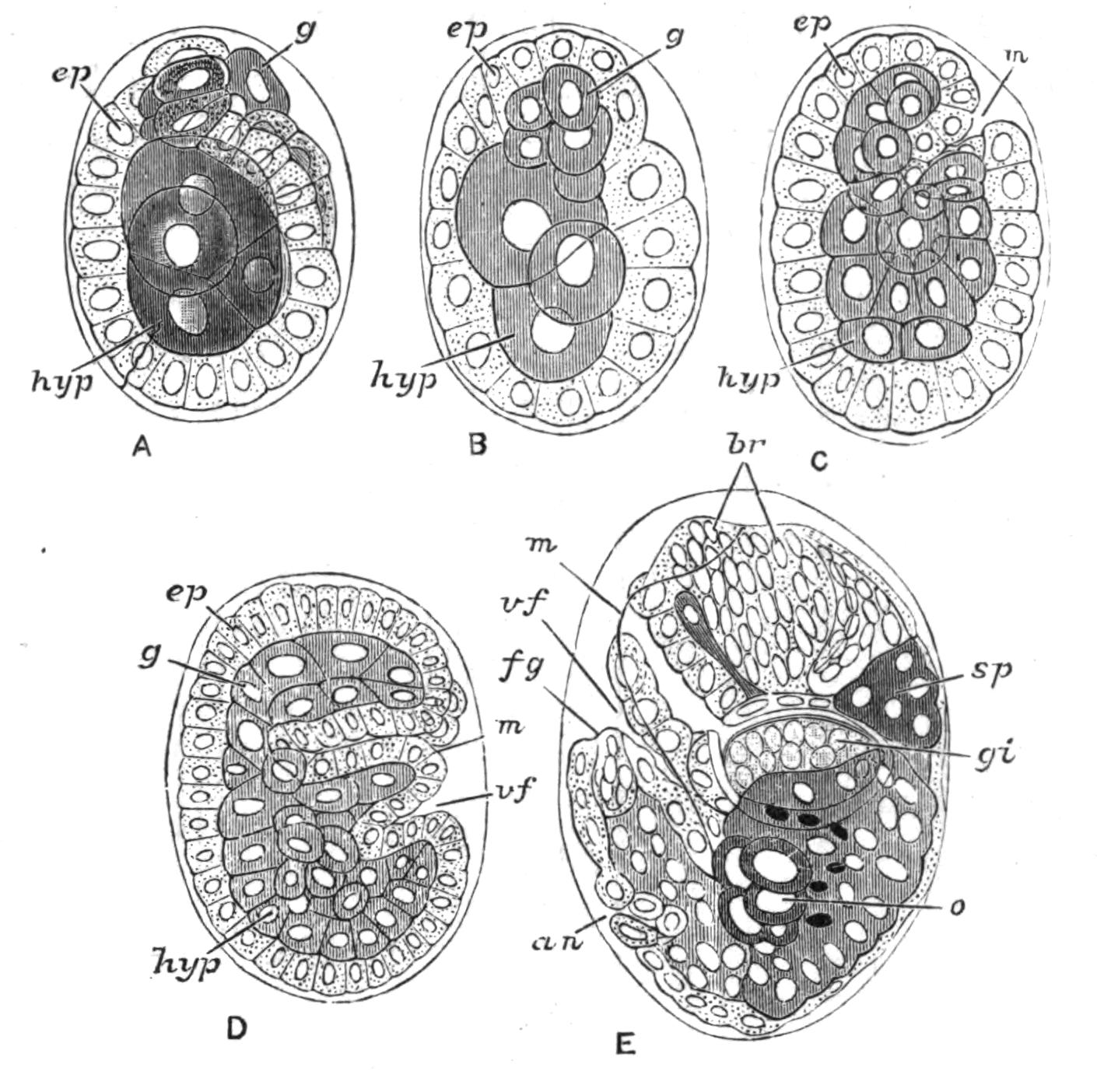
Fig. 114.—Development of Callidina. (After Zelinka.) A, Early stage showing involution of granular cells (g), to form the mastax or gizzard. B, Involution complete. C, Second involution of epiblast cells to form pharynx. D, The embryo bent on itself at ventral fold (vf). E, Showing ingrowth of epiblast to form brain (br): an, involution of epiblast to form cloaca; br, brain; ep, epiblast; fg, involution to form cement glands of foot; g, granular cells; gi, gizzard; hyp, hypoblast; m, mouth; o, ovary; sp, salivary glands; vf, limiting body from foot.
Owing to the elongation of the body within the narrow space of the egg the hinder part is bent up on the ventral surface (D, E); and this part, narrower than the rest, forms the foot, the centre of which is at first occupied by a column of hypoblast. The cloaca is now formed by a dorsal ingrowth of epiblast (the "proctodaeum") at the junction of the foot and the body (an). The hypoblast in the body anterior to the cloacal ingrowth forms the digestive apparatus; the part immediately behind forms the reproductive organs (o); and the hindmost part apparently disappears. An ingrowth of epiblast at the extreme tip of the foot gives rise to the cement glands (fg). The muscles arise from the epiblast cells. The disc arises from the modification of epiblast cells lateral to and behind the mouth, enclosing a so-called "polar area"; it is completed by the transformation of cells on the ventral side of the mouth. The brain (br) is formed by the multiplication of epiblast cells; and in Bdelloida a ventral ingrowth below the mouth forms the sub-oesophageal ganglion. The ciliated cup in Melicerta is formed as a ventral hollow, only later on united with the ciliated furrow of the wreath by the lateral grooves.[268] In Melicerta the two eyes are formed in the polar area. The young as hatched {220}differs from the adult in the greater simplicity of its ciliary wreath; and in the tubicolous forms the cupped end of the foot-gland is ciliated, and two eyes are present on the polar area, which later sink in, and often disappear more or less completely. It is stated that the young hatched from winter eggs do not pass through this larval state.
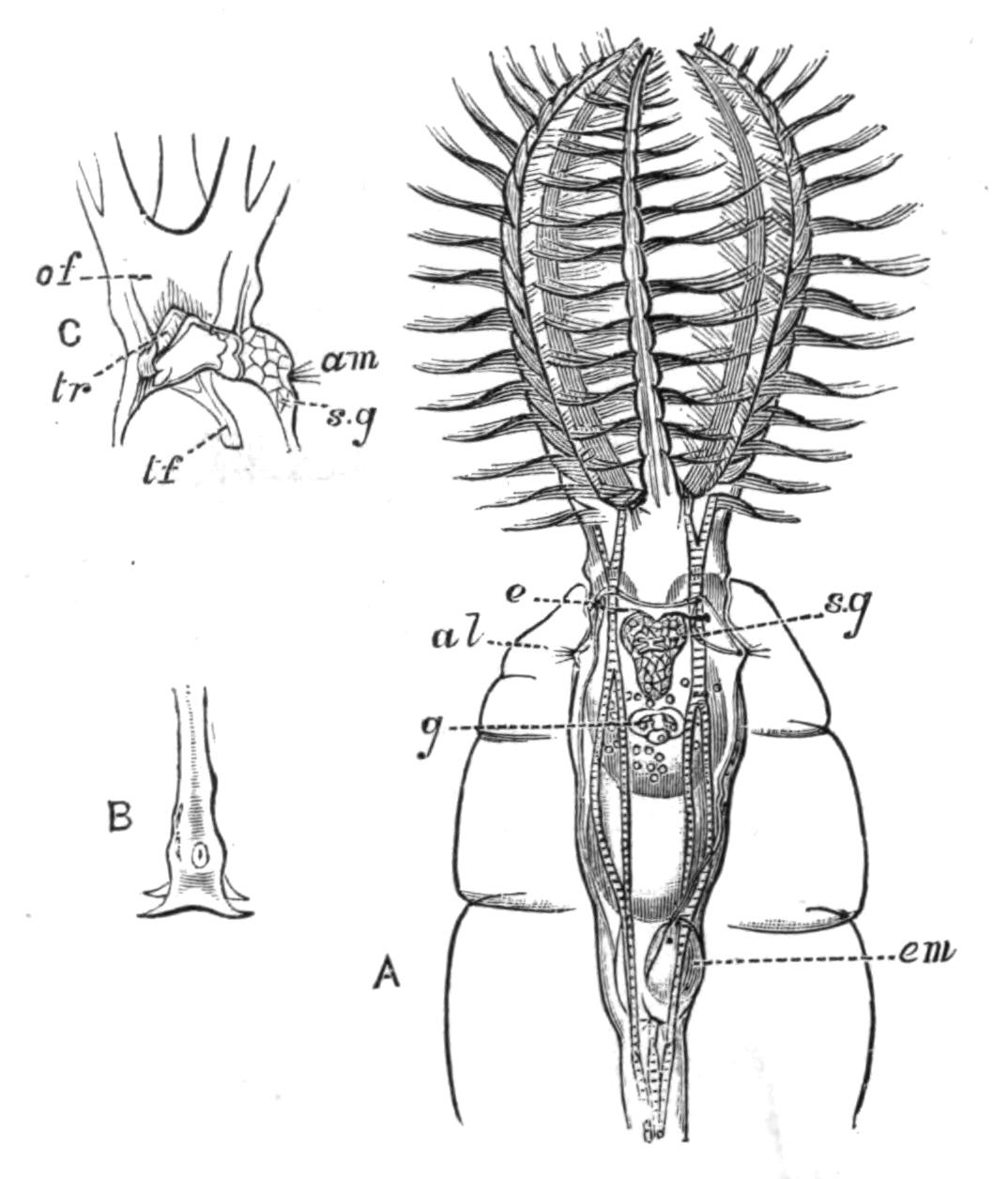
Fig. 115.—Stephanoceros eichhornii. (After Cubitt.) A, Dorsal view of the upper part in its tube: al, lateral antenna; e, eye; em, developing embryo in uterus; g, gizzard; s.g, median salivary gland. B, Extremity of foot. C, Lateral view of base of disc: am, median antenna; of, oral funnel; s.g, median salivary gland; tf, in the crop, indicates the ciliated tube prolonging the funnel; tr, horseshoe-shaped trochus.
Classification.[269]
Order I. Flosculariaceae.—Females mostly tubicolous, attached by a long contractile foot. Disc produced into a wide funnel-shaped contractile cup, produced into lobes with long setae (Floscularia) or coarse cilia (Stephanoceros), or entire (Apsilidae); an outer row of fine cilia rarely present; trochus a horseshoe, open behind. Oral funnel a slender tube hanging freely into a large pharyngeal crop; trophi uncinate projecting freely into the crop. Kidneys often united by an anterior cross-piece. Body-wall often containing a definite system of canals, filled with refractive granules, and serving by their contraction to {221}dilate the disc. Males (Fig. 107, 1) and larvae vermiform with a ciliated pedal cup, and a simple wreath, with two eyes on the disc.
Fam. 1. Flosculariidae: Floscularia Oken, Stephanoceros E. (Fig. 115).
Fam. 2. Apsilidae: Apsilus Metschnikoff (Fig. 112, A), Acyclus Leidy, Atrochus Wierzejski (Fig. 112, C).
The family Flosculariidae contains some most exquisite forms; Stephanoceros, the "Crown Animalcule," being probably the most lovely of the Class, and many of the Floscules coming not far behind. The Apsilidae are mostly mud-dwellers.
Order II. Melicertaceae.—Females (except in Trochosphaera) attached or tubicolous; tube variable. Disc with a dorsal gap (except Conochilus) often two-lobed or corolla-like; a ventral lip often separating off a ventral ciliated cup continuous by a pair of gutters with the ciliated groove; trochus of stronger cilia than the cingulum. Trophi malleoramate in a distinct mastax. Intestine much curved dorsally, cloaca long eversible (except Trochosphaera). Males and larvae as in Order I.
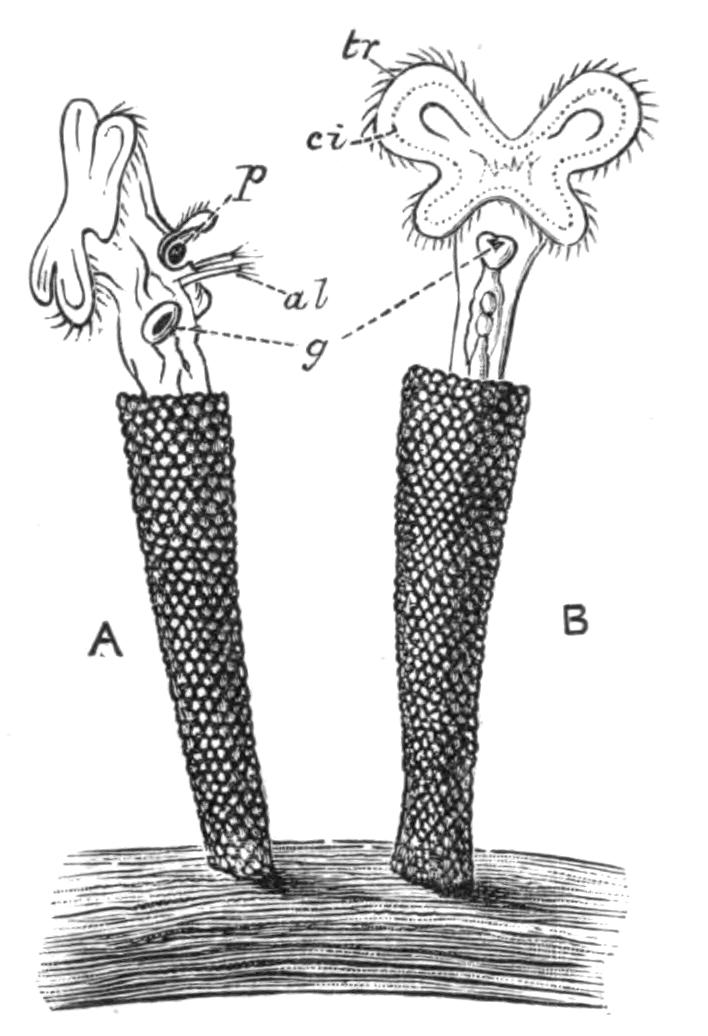
Fig. 116.—Melicerta ringens. (After Joliet). A, Side view; B, dorsal view. al, Lateral antennae; ci, cingulum seen by transparency; g, gizzard; p, pellet in ciliated cup, about to be deposited on edge of tube; tr, trochus.
Fam. 3. Melicertidae: Melicerta E. (Fig. 116), Limnias Schrank, Cephalosiphon E., Oecistes E., Lacinularia E., Megalotrocha E., Conochilus E., Octotrocha Thorpe.
Fam. 4. Trochosphaeridae: Trochosphaera Semper (Fig. 118, D).
The Melicertidae embrace a large number of tubicolous forms, many of which are social. This habit is especially noticeable in Lacinularia socialis, which forms a gelatinous incrustation easily seen by the naked eye; and in Conochilus volvox, which forms free-swimming globular aggregates, the young attaching themselves when hatched to the centre of the ball, and the ball splitting up into two as soon as undue pressure is exerted at the periphery by overcrowding. In this genus the eyes are very conspicuous in the adult, as they are in the similar free-swimming aggregates of Lacinularia racemovata.
Trochosphaera (Fig. 118, D) is remarkable for its peculiar {222}spherical shape, the absence of a foot, the limitation of the viscera to the lower hemisphere, and the dorsal position of the ovary. But a reference to the figure will show that the outgrowth of a foot in the quadrant between the mouth and anus and the flattening of the upper hemisphere would bring its organs on the whole into close correspondence with those of the rest of the Order. It is recorded from South China, the Philippines, and North-East Australia, and has only been seen by Semper, the founder of the genus, and by Thorpe, who saw the male of the first species, and described a second.[270]
Order III. Bdelloida.—Females creeping like a leech, as well as swimming (males unknown), susceptible of desiccation and revival ("anabiotic"). Body telescopic at both ends. Disc (except in Adineta) chiefly composed of two dorsal lobes like kettle-drums, wholly retractile; a dorsal proboscis or trunk-like prolongation of the body ends in a ciliated, sensory, and adhesive cup used in crawling, and overhung by a pair of membranous flaps. Trophi ramate; brain with a ventral ganglion, forming a complete ring. Eyes, two on the proboscis or brain, or absent. Bladder a mere dilatation of the rectum. Foot often possessing blind spurs, as well as two or three retractile perforated toes, or forming a terminal disc perforated by numerous pores of the cement glands, rarely ciliated.[271]
Fam. 5. Philodinidae: Philodina E. (Fig. 110), Rotifer Schrank, Actinurus E., Callidina E. (Fig. 109), Adineta H.
This group is remarkable for the great resisting powers of its members to drought and to heat and cold when dried, a fact which may explain the absence of males, though Janson records the occurrence of winter eggs in four species of Callidina and in Adineta vaga. The body is often strongly pigmented; red in Philodina roseola, Callidina scarlatina, and C. russeola, yellow in P. citrina, Rotifer citrinus, and Discopus synaptae. Most of the species are dust- or moss-dwellers; some, such as Rotifer vulgaris, are equally common in organic débris in infusions, pools, and ditches. Discopus adheres to the skin of the Holothurian Synapta.
Order IV. Asplanchnaceae.—Females ovoid, footless except in Asplanchnopus. Disc often bearing a pair of antennae; circular, often prolonged at the margin into two rounded lobes, interrupted {223}dorsally, depressed at the ventral side into a deep ventral funnel. Trophi incudate (virgate in Ascomorpha), mastax enlarged dorsally into a wide crop; stomach large, blind. Kidneys large, with a "recurrent duct" and numerous tags; bladder large. Brain large, with a median eye, and frequently paired smaller eyes at the base of the marginal processes of the disc; anterior antennae paired, relatively far back on dorsal surface. Males (Fig. 107, 5) relatively large, frequently found.
Fam. 6. Asplanchnidae: Asplanchna G., Asplanchnopus De Guerne, (?) Ascomorpha, Perty, (?) Dinops Western.
Order V. Scirtopoda.—Females of conical shape, with the body prolonged into hollow limb-like expansions (see p. 201) moved by strong muscles, and ending in branched setose fins like the limbs of Crustacea. Disc as in Bdelloids, but not retractile. Foot represented by two subventral toes, ciliated, inconstant or absent. Trophi malleoramate. Eyes two, latero-ventral, on the disc. Male (Fig. 107, 8) conical, with simple setae.
Order VI. Ploima.—Free-swimming forms, more rarely parasites, often adherent by their trophi to a host. Disc variable, often bearing within the cingulum a number of lobes fringed with coarse compound cilia. Foot rarely absent, marked off by a sharp constriction. Mastax variable, rarely malleoramate, never incudate or uncinate. Intestine not blind. Males small.[274]
Sub-Order A. Illoricata.—Ploima with a soft flexible integument; disc variable; ciliated auricles sometimes present (Synchaetidae, Notommatidae); foot rarely absent; trophi usually malleate.
Fam. 8. Microcodonidae: Microcodon E., Microcodides Bergendal.
Fam. 9. Rhinopidae: Rhinops H.
Fam. 10. Hydatinidae: Hydatina E. (Fig. 106), Notops H., Hudsonella Zach., Cyrtonia Rouss.
Fam. 11. Synchaetidae: Synchaeta E.
Fam. 12. Notommatidae: Notommata E., Pleurotrocha E., Copeus G., Proales G., Furcularia G., Eosphora G., Triophthalmus E., Diglena E. (Fig. 113), Distemma E., Triphylus E., Taphrocampa G., Albertia Duj., Balatro Clap.
Fam. 13. Drilophagidae: Drilophagus Vejdovsky.
Fam. 14. Triarthridae: Triarthra E., Polyarthra E., Pteroessa G., Pedetes G.
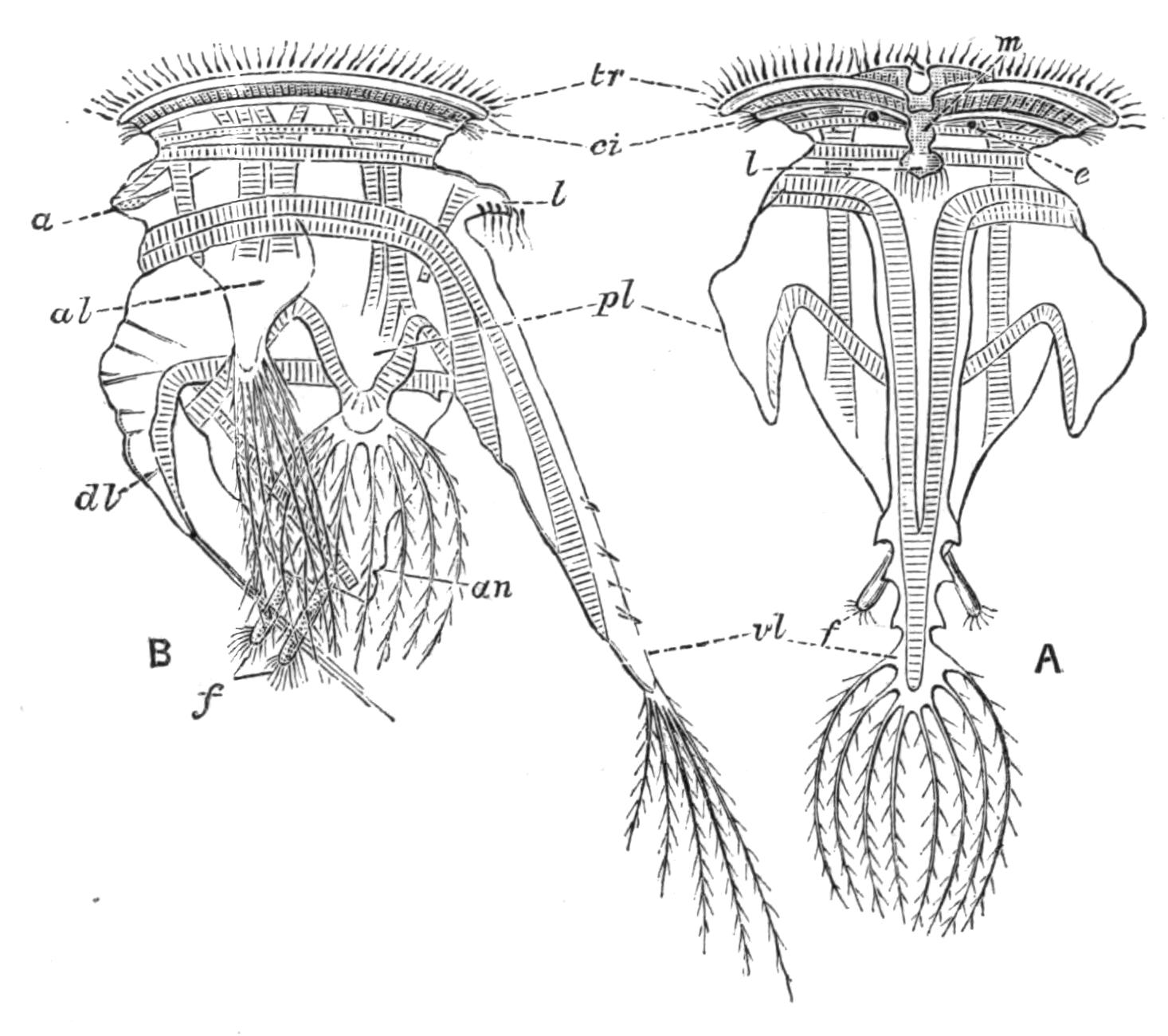
Fig. 117.—Pedalion mirum, female. (After Hudson.) A, Ventral view; B, side view. a, Median antenna; al, antero-lateral limb; an, anus; ci, cingulum; dl, dorso-median limb; e, eye; f, ciliated pedal processes; l, lip; m, mouth; pl, postero-lateral limb; tr, trochus; vl, ventro-median limb.
To this group belongs the eyeless Hydatina, a classical object of study, common in greenish pools, whose male was the first male Rotifer to be figured by Ehrenberg (1838), though he did not recognise its nature, and gave it the name of Enteroploea hydatina. Rhinops has the back of the corona curiously prolonged forwards into a sort of proboscis bearing two eyes. Some species of Notommata and Proales are distinctly annulated; in Taphrocampa the segmentation is so marked as to give the appearance of mesenteric septa extending inwards from the body-wall to the intestine. Microcodon has a wreath which is very peculiar in its extreme simplicity, with the mouth nearly central, and the eye lying just dorsal to the mouth. The Triarthridae, which resemble the Scirtopoda in having strong leaping spines fringed by fine bristles, should perhaps be placed in the next sub-Order.
Sub-Order B. Loricata.—Ploima with a firm elastic cuticle {225}of definite form, persistent after death, continuous, or divided by thinner strips into plates or shields, which again may be areolated. The cuticle may also be shagreened or embossed in various ways.
Fam. 15. Rattulidae: Rattulus E., Mastigocerca E., Coelopus G., Diurella (?) Eyfurth.
Fam. 16. Dinocharididae: Dinocharis E., Scaridium E., Stephanops E.
Fam. 17. Salpinidae: Salpina E., Diaschiza G., Ploesoma Herrick, Diplax G., Diplois G.
Fam. 18. Euchlanididae: Euchlanis E., Dapidia G., Apodoides Joseph.
Fam. 19. Cathypnidae: Cathypna G., Distyla Eckstein, Monostyla E.
Fam. 20. Coluridae: Colurus E., Metopidia E., Monura E., Mytilia G., Cochleare G., Dispinthera G.
Fam. 21. Pterodinidae: Pterodina E., Pompholyx G.
Fam. 22. Brachionidae: Brachionus E., Noteus E., Schizocerca Daday.
Fam. 23. Anuraeidae: Anuraea E., Notholca G., Eretmia G.
The group includes a number of very minute forms, besides others conspicuous both for size and beauty. A soft dorsal flap above the head occurs in Stephanops; also in Coluridae, a large family of minute species, where the flap is movable, and looks in profile like a hook overhanging the forehead. The genus Pterodina, like Pedalion and Triarthra, combines a Bdelloid disc with malleoramate trophi, while its exsertile wrinkled foot ends in a ciliated cup like that of a larval tubicolous species.
Brachionus, a large, often flat, transparent form, with a long wrinkled foot, is a very common genus, known to the earlier observers, and repeatedly figured by them. Pompholyx has a sack-like lorica, no foot, and carries its immense egg suspended by an elastic thread from the cloaca. The Anuraeidae lack the foot, and often have great spines or bristles projecting from the lorica, which no doubt facilitate floating. They are abundant in the "plankton" or floating fauna of large lakes far from the shore. Many marine species belong to this family.
Order VII. Seisonaceae.—Marine Rotifers parasitic on the Crustacean Nebalia; males resembling the females. Body elongated, with a slender retractile neck, a much reduced disc, an elongated foot with a terminal perforated disc as in Callidina. Trophi virgate exsertile. Genito-urinary cloaca opening at the base of the neck in the male, at the hinder end of the body in the female. Intestine complete (Seison) or blind (Paraseison).[275]
Fam. 24. Seisonidae: Seison Grube; Paraseison Plate; Saccobdella Van Beneden and Hesse.
Habits.—The habitat of Rotifers is well known to the student of pond life. Every dip from a greenish pool will give us a supply, if there be not an excessive contamination by manure; and such pools give us some of the largest and most beautiful forms, such as Hydatina and Brachionus, swimming about among the fibrous Algae and feeding on the organic débris among them. Almost any organic infusions freely exposed to the open air will yield Ploima shortly after the active putrefaction is completed. The finer water-weeds yield most of the beautiful tubicolous forms. A whole group of species and genera are quasi-pelagic in fresh and salt water, constituting a large proportion of the "plankton" or floating life near the surface; and some of these are found in deep water or in the depths of the lakes. Among them are the Asplanchnidae, Triarthridae, and Anuraeidae. A number of Loricates, such as Notholca and Eretmia, are armed with long spines, which doubtless render floating easier.
Among tubicolous forms Conochilus volvox and Lacinularia racemovata have this pelagic habit, forming floating globular or ovoid colonies, and two species of Floscularia also float freely in their tubes.
The following forms occur in salt or brackish water,[276] those marked with an asterisk (*) also occurring in fresh water:—
Floscularia campanulata.* Melicerta tubicolaria.* Rotifer citrinus.* Discopus synaptae. Synchaeta baltica, S. monopus, S. apus, S. tremula,* S. longipes, S. tavina. Asplanchna girodi.* Asplanchnopus syringoides. Hexarthra polyptera. Notommata naias, N. reinhardti. Proales decipiens. Furcularia forficula,* F. gracilis, F. reinhardti, F. marina, F. neapolitana. Diglena catellina,* D. suilla, D. putrida. Pleurotrocha leptura. Distemma raptor, D. marinum, D. platyceps.* Bothriocerca longicauda. Polyarthra platyptera.* Triarthra longiseta.* Rattulus calyptus. Diurella marina, D. brevidactylus, D. brevis. Diaschiza fretalis. Euchlanis luna. Monostyla quadridentata, M. lunaris. Colurus amblytelus, C. uncinatus,* C. dactylotus, C. coelopinus, C. pedatus, C. rotundatus, C. truncatus, C. caudatus.* Mytilia tavina. Pterodina clypeata. Brachionus bakeri,* B. mülleri. Anuraea valga,* A. biremis,* A. aculeata,* A. tecta,* A. cochlearis.* Notholca striata,* N. scapha,* N. thalassia, N. spinifera, N. inermis, N. jugosa, N. rhomboidea. Seison grubei, S. annulatus. Paraseison asplanchnus, P. nudus, P. proboscideus, P. ciliatus. Discobdella nebaliae.
Thus about seventy species are recorded as marine. Synchaeta baltica is truly pelagic, and contributes to the phosphorescence of the ocean.
Other forms again are parasitic. Proales werneckii is found in Vaucheria, a coarse, dark green, thread-like Alga found in fresh water; and the closely allied P. parasita is not uncommon in the beautiful floating green spheres of Volvox.[277] Albertia, Drilophagus, and Balatro are parasitic on or in fresh-water Oligochaetes; the curious Seisonaceae are parasitic on Nebalia, a small Crustacean easily obtained in masses of whelk's eggs; the aberrant Bdelloid Discopus attaches itself to the surface of the Holothurian Synapta. Similarly among this last Order Callidina parasitica attaches itself to the limbs of the fresh-water Crustacea Gammarus and Asellus. These are rather commensals than true parasites. The species of Brachionus often attach themselves temporarily to the common water-flea Daphnia.
Besides a few Ploima, the vast majority of the Bdelloids live in or among mosses and their roots. Many Callidina inhabit cup-like hollows in the leaves of the scale mosses (Jungermanniaceae), especially of the genus Frullania. Almost all the members of this Order are susceptible of desiccation and revival; certain species, such as Rotifer vulgaris, Philodina roseola, Adineta vaga, etc., can be readily obtained by moistening gutter dust. The mechanism of the process is as follows: when desiccation is gradual the animals close up their telescopic bodies and excrete gelatinous plugs at either end, which effectually seal them against further drying; if, however, they be dried on a slide without any débris, the process is too rapid for them to protect themselves, and they therefore die. This was dimly seen by others, and clearly demonstrated by H. Davis,[278] who records the following experiment:—The Rev. E. J. Holloway, having found Philodina roseola in gutters, placed strips of paper there in the rainy season, and succeeded in obtaining clean gatherings, taking dry groups of a hundred together, having a varnish-like covering all over; and being glued to one another, mostly in one plane, and to the paper, forming a pavement. In the dry condition they resist extremes of temperature; thus Zelinka found {228}Callidina revive after an exposure of -20° C. (-4° F. or 36° of frost), and immersion in hot water at 70° C. (158° F.). They will also resist deprivation of air in a vacuum of an ordinary air-pump, but not the all but perfect exhaustion of the Sprengel pump.
A very curious fact in relation to this Class is that often when a new form is once described from a single locality, fresh and widely distant stations for it rapidly become known.[279] Thus Pedalion mirum, first found at Clifton in 1872 by Hudson, was a few years after captured in a small pool above tide-marks on a rocky islet in Torres Straits. Since then it has been recorded from many different European stations, and a second closely allied species has been found in Finland. So a species of Ehrenberg's[280] was not seen again till within the last decade or so; but since then it has been independently found and described by six observers, who have given it as many distinct generic names. In the case of Pedalion it may well be that, as Hudson suggests, the species is of southern origin and has followed the flag, the winter egg being conveyed in dust by ships or travellers.
The above account of the habits gives the key to the collection of the various forms. The weed-loving species are collected with the weeds, and will keep with these in vessels if screened from direct sunlight and protected against dust. The free-swimming forms may be collected by sweeping with a net of fine gauze, with a bottle fixed in the bottom.
Except for their power of resisting desiccation, Rotifera are not very long-lived, and the males are especially short-lived; the most exact observations are those of Maupas on Hydatina. He found that the greatest age of the unfertilised female was thirteen days, during which it could produce some fifty eggs; the fertilised female lives for seven or eight days, producing about sixteen eggs; while the male dies in two or three days.
The preservation of Rotifers has been recently reduced to a fine art by Rousselet, who uses a solution consisting of cocaine hydrochlorate, 1 gramme; water, 50 cc.; and methylated spirit, 12 cc. This will keep without deterioration. When in use it {229}must be diluted in the proportion of two volumes to three of water. This solution is added cautiously to the capsule in which the Rotifers lie, and they are watched till their ciliary motions slacken; when this happens a drop or two of osmic acid solution (½ to 1 per cent) is added; the Rotifers are then sucked up by a capillary pipette, and transferred to fresh water; and then into a solution of "Formaline" diluted to contain 2½ per cent of formic aldehyde. In this solution they are transferred to shallow cells, ground out of the centre of an ordinary glass slide, covered with thin glass, and sealed.[281] Other methods of preparing Rotifers for minute study will be found in the papers of Plate, Tessin, and Zelinka.
The zoological affinities of the Rotifers have long been a subject of keen interest. As early as 1851 Huxley[282] suggested that they represent a primitive form, preserved, with modifications, in the larva of Molluscs, Annelids and other worms, and Echinoderms. Similar views were later maintained by Lankester,[283] who termed the larva of Polychaets, etc., a "trochosphere," for which "trochophore" has been substituted in order to avoid confusion with the Rotifer Trochosphaera; Balfour,[284] Hatschek,[285] Kleinenberg,[286] and others have developed these views. Serious difficulties, however, arise in the detailed comparison of Rotifers with this type; and the special students of this Class have found it practically impossible to agree in the identification of the various parts, a difficulty especially felt in the case of the Rotiferan genus Trochosphaera, though this is just the one which presents the closest superficial resemblance to the Trochophore larva. I have been induced to take a view of the structure of Rotifers that brings it into close relationship with the lower Platyhelminthes, and with the more primitive larva of the Nemertines termed Pilidium (Fig. 60, p. 113). This is hemispherical, ciliated all over, with the mouth in a ventral funnel lined by fine cilia; while the edge is fringed with two rows of strong cilia, separated by a finely ciliated groove, like those of the ciliary wreath of a Rotifer.
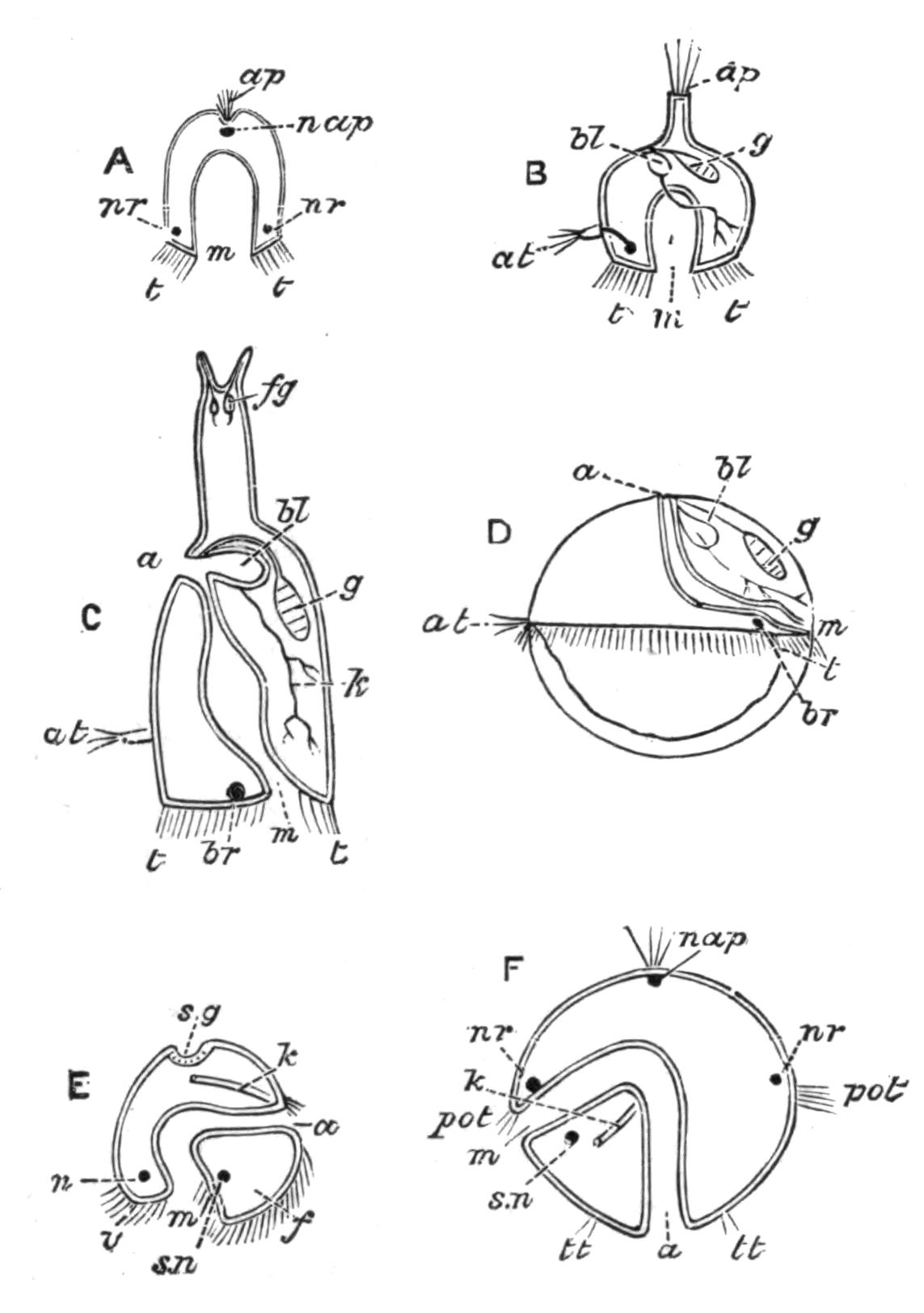
Fig. 118.—Diagram explaining the possible relations of Rotifers. A, Pilidium; B, hypothetical Rotifer modified from Asplanchnopus; C, a Ploimal Rotifer; D, Trochosphaera aequatorialis (modified from Semper, the extension of the ovary into the posterior ventral quadrant being omitted); E, Mollusc larva (Veliger); F, Trochophore larva of Annelid. a, Anus; ap, apical organ; at, median antenna (near which, in B, is a black spot, the brain); bl, bladder (receiving the ramified kidney in B, C, and D); br, brain; f, foot; fg, cement-glands, replacing apical organ; g, ovary; k, kidney; m, mouth; n, supra-oesophageal ganglion; nap, nerve of apical organ; nr, nerve-ring in section; pot, praeoral portion of trochus; s.g, shell-gland; s.n, sub-oesophageal ganglion; t, trochus or ciliary wreath; tt, posterior ciliated ring; v, velum, or expanded praeoral part of trochus.
The sides are produced on either side into lappets, which we do not take into account. A cup-shaped depression at the apical pole is lined by sense-cells, bearing long cilia which are probably sensory. A ring of nerve-cells passes within the ciliated rim of the hemisphere, and the stomach is a blind sac. If we compare this organism with a Rotifer, we find that the wreath corresponds in both, the funnel of the disc in such forms as Flosculariidae and Microcodon leading to the mouth of Pilidium, while the gut is blind in Asplanchnidae and in some of the highly developed Seisonidae. The circular nerve-ring of Pilidium is in many Rotifers only represented by its anterior part, the brain; though in Bdelloids a sub-oesophageal ganglion completes the ring. This leaves a difficulty with regard to the apical sense organ; but it is easy to understand that an organ of sensation should become an organ of fixation. In this case the foot with its glands would correspond to the sense organ of the Trochophore larva; and it retains its primitive ciliated character in the larvae and males of many Rotifera, and the adult female of Pterodina and Callidina tetraodon. Embryology tells {231}us that the anus of Rotifers cannot be homologous with that of Annelids, etc., for it is formed outside the area of the blastopore: it is an independent formation, probably due to the coalescence of the originally blind intestine at its extremity with the earlier genito-urinary cloaca. On this view we must change the orientation of the Rotifer, and place it, like a Cuttlefish, mouth downwards: for "anterior and posterior" we must substitute oral (or basal) and apical; for "dorsal" and "ventral" we must use anterior and posterior; while "right" and "left" are unchanged. And this correctly expresses the actual space-relations in those Ploima like Rattulus that swim with their disc in contact with the organic débris on which they feed, with the foot turned outwards and backwards. As these views are now published for the first time, I have thought it wiser to keep to the accepted relations in the general description, a course which has the advantage of avoiding difficulties in the study of the literature of the Class.
The supposed resemblance of Pedalion to the Crustacea is probably the result of convergence, not of consanguinity. The Polyzoa are a group of freely-budding organisms whose structure otherwise recalls in many respects that of the attached Rotifers; but a close investigation reveals so many differences in structure, orientation, and development, that we cannot regard the two groups as at all closely allied.
Thus the Rotifers may be regarded as a group apart, but probably representing an early offshoot from a free-swimming Platyhelminth, probably a Rhabdocoele; the modifications being the loss of the general ciliation of the surface, the arching of the back into an elongated vault, the conversion of the inner half of the pharynx into a gizzard, the change of position of the genital and urinary apertures to the antero-dorsal surface, and the opening of the intestine into the genito-urinary cloaca.
Gastrotricha.
This small and very homogeneous group consists of minute fresh-water organisms, closely resembling many Ciliate Infusoria in their movements, habit and habitat. They were first described in detail by Ehrenberg, and placed by him and Dujardin in the neighbourhood of Rotifers. In recent years A. C. Stokes[287] {232}in America and C. Zelinka[288] in Germany have contributed, the former a careful description of a number of new species and their habits, the latter a complete monograph of everything that is known of the Order.
The Gastrotricha dwell among filamentous Algae and organic débris, and are of frequent occurrence with Protozoa and Rotifera of similar habit. The largest known measures only 400 µ (1⁄60 in.) in length, and the smallest run as low as 74 µ (1⁄300 in.).
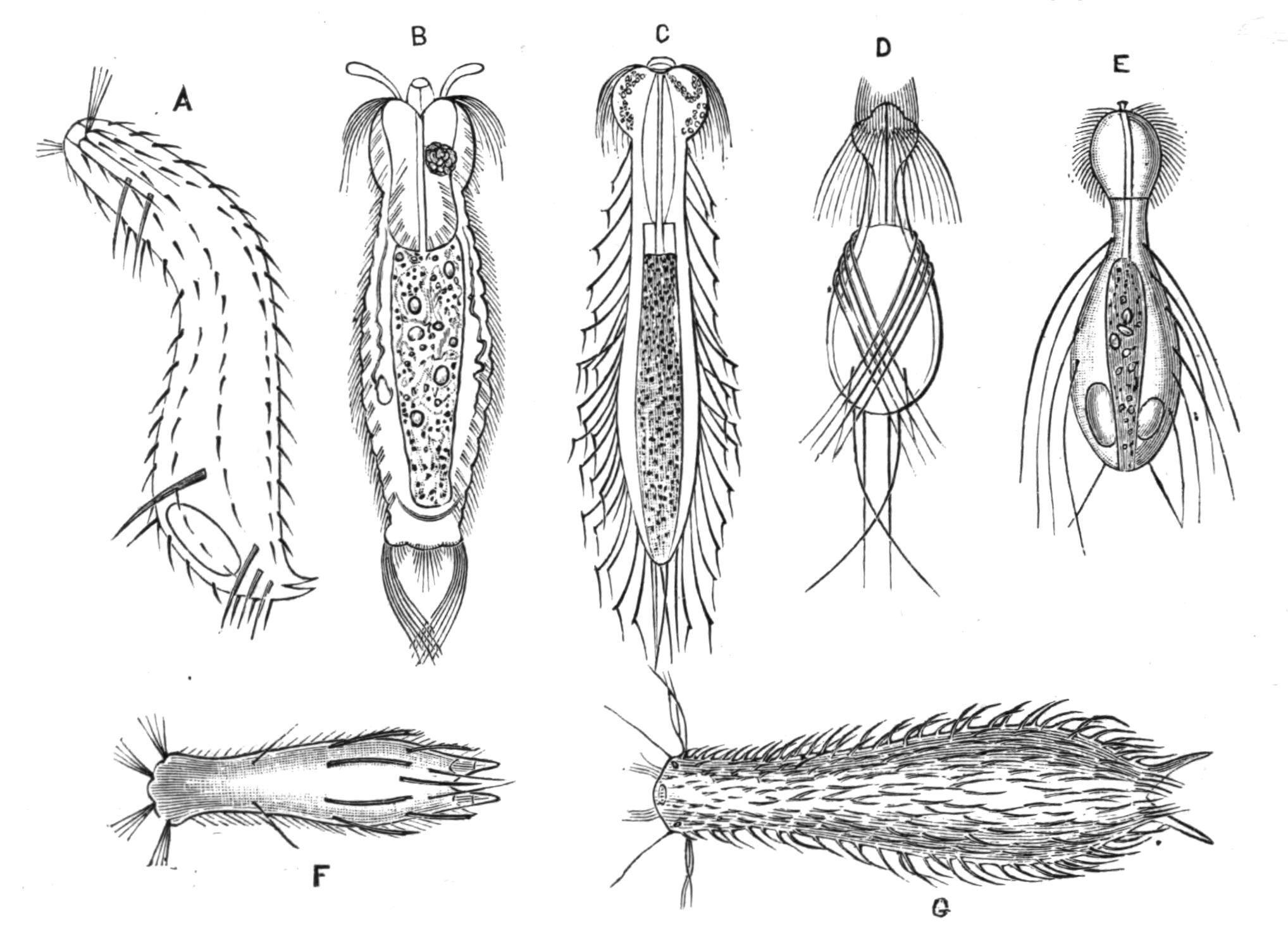
Fig. 119.—Gastrotricha. (From Zelinka.) A, Chaetonotus bogdanovii, side view (after Schimkewitsch); B, Gossea antenniger (after Gosse); C, Dasydetes goniathrix (after Gosse); D, Dasydetes saltitans (after Stokes); E, D. longisetosum (after Metschnikoff); F, Chaetonotus spinulosus (after Stokes); G, Chaetonotus schultzei (after Gosse and Bütschli). (Magnified.) B-F, × about 390; G, × about 125.
We shall follow Zelinka in his description of the common species Chaetonotus larus as a type. The body is nearly circular in section, flattened a little on the ventral side. The apertures are the terminal mouth; the anus, nearly terminal and slightly dorsal; the two kidney openings, ventral, nearly half-way down the trunk; besides the pore of a cement-gland on either terminal process. The short ventral and post-anal portion of the trunk with its processes therefore corresponds to the foot of a Rotifer. The integument of the body is a thin nucleated hypoderm, not {233}distinctly divided into cells, covered by a chitinised cuticle; it bears cilia, sensory hairs, and peculiar scale-like processes, sometimes produced into long bristles.
The cilia are chiefly arranged in two ventral bands, each extending nearly the whole length of the body, and composed of a series of transverse rows of single cilia; along these bands the hypoderm is thickened and more richly nucleated. The sides of the head also bear numerous long cilia.
The scales are hollow processes of the cuticle overlapping from before backwards. A ventral row lies between the ciliary bands; two series of alternating dorsal rows lie on the back and sides of the animal, and in the hirsute species it is these that are produced backward into bristles. A single large scale, the "frontal shield," protects the head above and behind, but does not extend down to the ventral surface. On either side of the head is a pair of flattened oval areas, the "lateral fields." From between these on either side springs a tuft of motile sensory hairs. Two pairs of similar tufts arise dorsally on the front margin of the frontal shield, and a fourth pair spring from the ventral surface a little behind the mouth. These hairs are distinguished from ordinary cilia by their length, and their insertion on large nucleated cells receiving nerves; two pairs of similar hairs lie farther back on the dorsal surface, one in the front of the neck, one near the base of the pedal processes.
The muscles lie some in the body-wall, and some traverse the body-cavity; only six pairs occur, simple, unstriated, and longitudinal. There are neither transverse nor circular muscles.
The alimentary canal is very simple and nearly straight from mouth to anus; it may be divided into pharynx, gullet, stomach, and rectum. The mouth is circular, and looks forwards and a little downwards. From the mouth opens the pharynx, a short chitinous tube, capable of eversion by being pushed forwards by the gullet; it bears half-way down a circlet of curved hooks, which open out when it is everted; within these are tooth-like thickenings.
The oesophagus or gullet is thick and muscular, extending through the whole of the neck of the animal; its cavity, as well as the opening from the pharynx, is triradiate like a leech-bite, but can be dilated by the action of the muscular walls, inserted into a firm external cuticle; the internal wall is also cuticulised, {234}not ciliated as in Rotifers. The hinder end of the gullet is produced into a short, wide, membranous funnel projecting freely into the midgut or stomach. The latter is elongated and oval, composed of four rows of hexagonal cells, with large nuclei. This is separated by a distinct constriction or sphincter from the short pear-shaped rectum, which opens by a minute anus on the back just in front of the pedal processes.
The food is chiefly organic débris; but Gastrotricha have been seen to attack large Infusoria by nibbling, and to swallow the protoplasm as it exudes from the wound in their prey.
The nervous system is chiefly composed of the large brain, a ganglion lying like a saddle above and on the sides of the gullet, and in direct continuity with the nerve-cells of the cephalic sense-hairs. A pair of dorsal nerve-trunks extend along the whole length of the gullet. The sense-hairs described with the general integument may be organs of external taste ("smell") or of touch. Eyes have been described in several species; and though Zelinka has failed to verify this, I have myself seen a pair of minute red eyes in the back of the head of an animal (probably a Chaetonotus), whose hasty escape into a mass of débris prevented my determining its species.
The kidneys are paired tubes lying at the sides of the front of the stomach, and sending a simple loop into the neck. Each tube is much convoluted, and ends at the one extremity in a long "flame-cell," like that of a Rotifer much drawn out, and at the other by a minute pore on the outer side of the ventral row of scales.
Reproductive Organs.—Only the female is with certainty known to occur; and the eggs, though recalling in their thick ornamented shell the fertilised winter eggs of Rotifers, are probably unfertilised and parthenogenetic like the summer eggs. The ovaries are two minute patches of cells lying at the junction of the stomach and rectum. The eggs, as they mature and enlarge, press against the side and back of the stomach, where they attain a length of one-third to one-half that of the mother. The extrusion of the egg has not been observed; but it is laid in the angles of weeds, the moulted shells of Entomostraca, etc., where its development may be studied. The sculpture of the shell serves to anchor it if laid among weeds. When hatched the head, trunk, and pedal processes are of the full adult size, all subsequent growth being limited to the neck.
The function of testis has been ascribed by Ludwig to a minute granular organ between the ovaries above the rectum; if this view be correct the Gastrotricha are hermaphrodite.
The movements of the Gastrotricha are very elegant, recalling those of the long-necked Ciliate Infusoria, like Amphileptus, Lacrymaria, etc., with the characteristic exception that they always swim forwards; the grace of their movements being due to the bending of the head and neck on the body. Those which are provided with long motile bristles like Dasydetes, alternate their gliding with leaps, like the springing Rotifers.
The Gastrotricha are divided into two sub-Orders—Euichthydina, with two pedal appendages, containing the genera Ichthydium Ehr., Lepidoderma Zel., Chaetonotus Ehr., and Chaetura Metsch.; and the Apodina, with no pedal appendages, comprising Dasydetes G. and Gossea Zel.
Their geographical distribution, like that of most microscopic fresh-water organisms, is cosmopolitan. Few observers have enumerated the members of this group; of their extra-temperate occurrence we have only the single observations of Ehrenberg, Schmarda, and Voeltzkow for Nubia, Ceylon, and Madagascar respectively.
Of the thirty-two species described, twelve are recorded by A. C. Stokes from Maine and New Jersey only, besides five others that occur also in Europe. In Europe nineteen species are recorded, one of which, Ichthydium podura, has also been found in Nubia and Ceylon. One species, Chaetonotus tabulatus Schmarda, has been recorded by its author from Colombia (in South America). As of the nineteen European species only seven have been recorded as British, we may expect to find that careful study will well repay the student in these islands.
The affinities of this group are probably with the Turbellarians and the Nematodes; they differ from the former in the highly developed alimentary canal, and from the latter in the possession of the ciliated ventral bands and wreath. The general chitinisation of the skin, the primitive body-cavity, the character of the alimentary canal, the ventral opening of the renal canals far in front of the anus are characters shared by the Nematodes, many of which possess bristles like this group. But their affinity must be rather to some hypothetical ancestral group than to any living Nematodes, which are destitute {236}of cilia. To the Rotifers the affinity, dwelt on by Zelinka, is less close.
Kinorhyncha.
This Class and Order comprises but one genus, Echinoderes (Fig. 120), founded in 1851 by Dujardin.[289] Reinhard's monograph[290] is the generally accepted authority on this subject, and contains a full bibliography, with diagnoses of the individual species, eighteen in number.
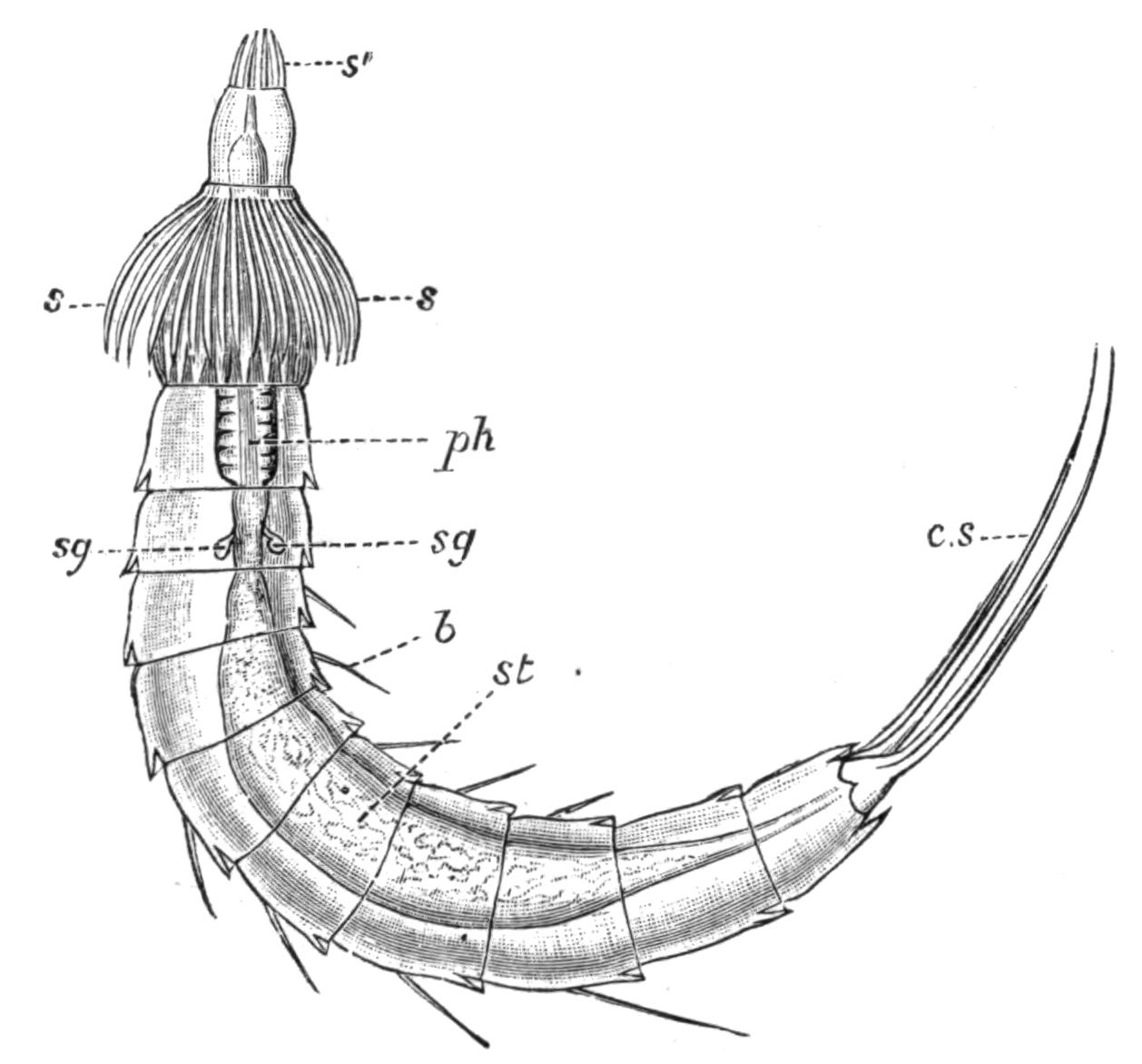
Fig. 120.—Echinoderes dujardinii (?), drawn from a preserved specimen taken at Worthing. × about 210. b, Bristle; c.s, caudal spine; ph, pharynx; s and s', the spines on the two segments of the proboscis; s.g, salivary glands; st, stomach.
The animals of this group are found in shallow seas with muddy bottom, below low-water mark, and feed on organic débris. They have been taken in the Black Sea, Mediterranean, British Channel, and North Sea, and off the Canary Islands (Lanzarote, Porto Pi, Palma di Mallorca). Their size varies from 0.86 mm. × 0.22 mm. in Echinoderes spinosus, to 0.14 mm. × 0.03 mm. in E. kowalevskii.[291]
The body is protected by a strong chitinous cuticle distinctly annulated, forming eleven rings, besides a retractile proboscis obscurely divided into two segments at the apex of which the mouth opens. The anus opens on the extreme end of the last segment, which is frequently retracted; the genital pores open right and left of the anus; and the renal pores lie on either side of the back of the ninth segment. The first ring may be undivided, or else distinctly divided into four plates, one dorsal, {237}two latero-ventral, and one ventral. In the remaining segments each ring has only three plates, one dorsal and two ventral, the two latter being sometimes more or less fused in the last or ventral segment. These plates all overlap from before backwards.
As the name Echinoderes implies (Thorn-skin), the cuticle is produced into points, bristles, or spines. The last segment frequently bears a large pair of these, which have been compared, on the flimsiest grounds, with the furcal processes of Crustacea and the perforated toes of Rotifers and Gastrotricha.
The proboscis when extruded has the form of a truncated cone, obscurely divided into two segments, a ring of strong spines marking the boundary between them, and a second double ring of spines surrounding the apex. The eversion is of the type termed by Lankester pleurembolic or acrecbolic, the sides being first withdrawn, the apex first extruded.
As in so many Invertebrata, the epidermis is not separated by boundaries into distinct cells. This layer sends out processes each of which lies in a hollow in the thick cuticle, and perforates it to end in a fine bristle. Minute orange pigment-granules occur at irregular intervals in this hypoderm.
The muscles of Echinoderes are simple striated bands. Numerous bands lie within and attached to the body-wall, extending its whole length; paired dorsi-ventral muscles separate the intestine from the reproductive organ on either side, and a complex system effect the movements of the proboscis.
Alimentary Canal.—The pore at the tip of the proboscis leads into a short thin-walled tube, which is rarely evaginated; into the base of this tube projects the short bluntly conical apex of the large ovoid muscular pharynx (or gullet?); this is lined by an epithelial layer of nucleated protoplasm, which secretes a strong cuticle. The stomach is a wide tube, somewhat dilated in each segment between the paired dorsi-ventral muscles, and tapering behind to end in the terminal anus. Four minute glands open at the junction of the pharynx and stomach.
Kidneys.—These are a pair of blind pear-shaped sacs, ciliated within (the only case of ciliation in Echinoderes), lying in the eighth segment, and opening by the taper ends right and left on the back of the ninth segment.
Nervous System.—All that has been clearly defined of this is a small brain or ganglion lying dorsally at the junction of the {238}pharynx and stomach. From two to eight eye-spots have been described by earlier writers, but Reinhard was unable to find them in the (distinct) species which he principally worked at, though he noted their existence in the solitary specimen of the original species, E. dujardini, which he obtained.
Reproductive Organs.—The sexes are distinct. The reproductive glands form a pair of tubular sacs, opening ventrally on either side of the anus, and extending forwards beside the gut as far forwards as the fifth to the second segment in the male, but only to the fourth at furthest in the female. The ova are large nucleated cells embedded in the protoplasmic lining of the ovarian sac, and acquiring a distinct shell as they approach its opening. Three-quarters of the testis sac is occupied with granular protoplasm containing a quantity of small nuclei; the lower part alone contains mature spermatozoa. Adjoining each external opening in the male are a pair of short hollowed spines, which may perhaps serve as organs of copulation; but nothing is really known of this process or of the development of the egg. It is almost certain, from the absence of developing eggs within Echinoderes, that the genus is not viviparous.
From the foregoing description it is obvious that Echinoderes approaches the Nematoda very closely: the two main points of difference are its ciliated kidneys and its bilaterally paired sexual organs. Possibly the study of such forms as Desmoscolex (Fig. 81, p. 159) may reveal closer affinities.
[Zelinka (Verh. D. Zool. Ges., 1894 and 1898), has given a preliminary account of a new research on this group. The principal addition is the discovery of a ventral nerve-cord, with a ganglionic dilatation in each segment, lying in the ectoderm of the body-wall, as indeed do the brain and nerve-collar. He divides the genus into two Orders according as the orifice of the retracted fore-part of the body is slit-like or circular. The former (Homalorhagae) retract the first two segments with the proboscis; they are mud-dwellers, sluggish, eyeless: the latter group (Cyclorhagae) only retract the first segment with the proboscis; they crawl among algae, and mostly have paired pigmented eye-spots, each with a lens, imbedded in the brain.—M. H., Jan. 1901.]
BY
W. BLAXLAND BENHAM, D.Sc. (Lond.), Hon. M.A. (Oxon.)
THE CHAETOPODOUS WORMS—THE ARCHIANNELIDA—ANATOMY OF NEREIS, AS TYPICAL OF THE POLYCHAETA
Those animals which possess lateral bundles of bristles (technically termed "chaetae") for use in locomotion constitute the group of "Bristle-worms," or Chaetopoda. The body of these animals is made up of a preoral lobe or prostomium, and a number of more or less distinct segments following one another in a line, and repeating one another in their internal and external structure. The Chaetopoda embrace the following smaller groups or Orders:—I. Archiannelida, II. Polychaeta, III. Myzostomaria, IV. Oligochaeta. The Archiannelida, although without the characteristic chaetae, are yet anatomically so similar to the true Chaetopoda that they must be included in the group, just as certain fishes are classed as "Vertebrata," although they do not possess vertebrae. The old term Annelida is sometimes used to include the above-mentioned groups, together with the Gephyrea[292] and the Hirudinea or leeches.
Order I. Archiannelida.
The Archiannelida are very simple worms, but simplicity may be, and very frequently is, the result of degeneration; and it is not always possible to determine whether a simple animal is primitively, i.e. ancestrally simple, or whether it is secondarily simplified. Hence the term Haplodrili has been employed by Professor Lankester as the name of the group; a term which does not prejudge the question as to whether or not the worms are {242}"primitive." It is quite possible, and even probable, that Dinophilus is ancestrally simple; whilst many features in Polygordius appear to be the result of simplification. For this reason it would be well to separate Dinophilus from the other two genera, on account of its much less elaborate and more generalised structure,—so generalised, in fact, that the worm is by some authorities placed amongst the Planarians; for the present, however, the group Archiannelida may be regarded as containing three genera: Dinophilus, Protodrilus, and Polygordius.[293]
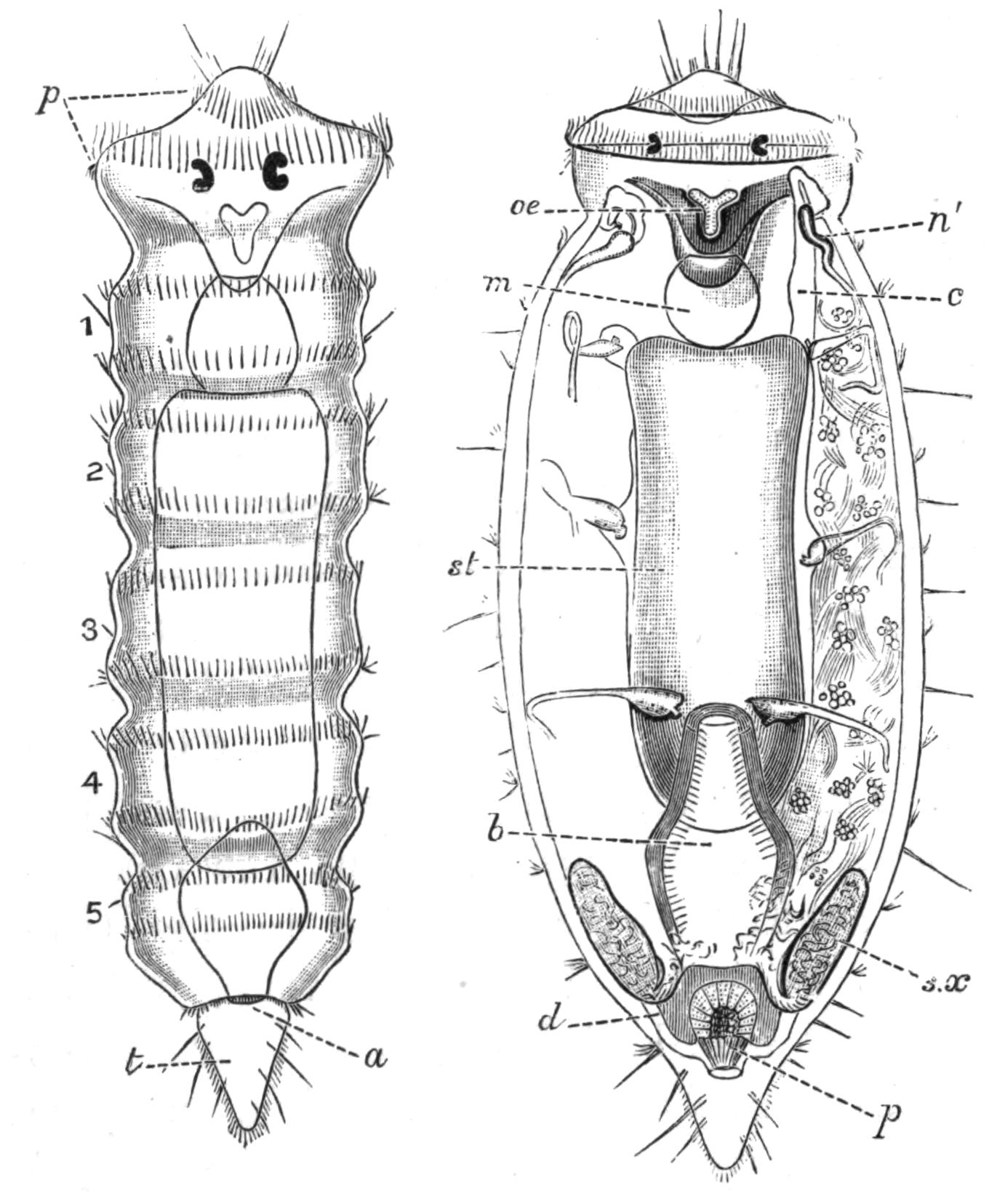
Fig. 121.—Dinophilus taeniatus. (From Harmer.) The left figure represents the dorsal surface of a young individual, × 76; the mouth and alimentary tract are seen by transparency: p, prostomium, with two bands of cilia and a pair of eyes; a, anus; t, tail; 1 to 5, the segments with ciliated bands. The right figure shows the anatomy of the male, × 38: b, rectum; c, body-cavity; d, vas deferens; m, muscular organ (pharynx); n', the first nephridium; oe, entrance to oesophagus; p, penis; st, intestine (stomach); s.x, seminal vesicle (5th nephridium).
Dinophilus is represented on our coasts by at least two species: D. gigas Weldon[294] and D. taeniatus Harmer.[295] The latter is about one-twelfth of an inch in length, bright orange in colour, and more or less abundant, at springtime, in the rock pools around Plymouth, where it may be found amongst green algae, or on the mud at the bottom of the pools.
The animal consists of a broad prostomium, with a pair of eyes; and of a body, distinctly constricted in immature specimens into five or six segments, followed by a short conical tail. There are neither chaetae nor tentacles; locomotion is chiefly effected by means of the bands of cilia which encircle the body in a regular fashion, two bands round the head, and two round each segment in D. taeniatus; in some species there is only a single band on each segment. The whole of the ventral surface is covered with cilia, by the aid of which the animal probably "creeps" along the weeds.
The alimentary canal is straight, and divisible into the regions shown in Fig. 121; a muscular protrusible organ, which is a ventral outgrowth of the foregut, is employed as a "sucker." The coelom is more or less obliterated (or ill developed). The excretory system in the genus is varied: in some species, as in D. gigas, it is stated to be constructed on the Planarian plan; in others, as in D. taeniatus, the organs are definite nephridia. Of these tubes there are five pairs, the last pair in the male serving as a seminal vesicle. Each nephridium is a ciliated tube, the internal end of which lies in the body-cavity and appears to be blocked by a ciliated tongue-shaped appendage. The first pair corresponds to the "larval nephridia" of Trochosphere larvae.
The nervous system, which is in contact with the epidermis, consists of a brain in the prostomium, and, on each side of the body, a ventral cord with five ganglia, connected by transverse commissures in as many segments.
The sexes are separate, and are usually similar; the male of D. gyrociliatus is, however, much smaller than the female. The generative organs occupy the greater part of the body-cavity; in the male the testes communicate, by means of the pair of seminal vesicles, with a median eversible apparatus. In the female the paired ovaries communicate with a median sac which serves as a spermatheca.
The development is simple:[296] the worm itself is more like a larval Polychaete than a full-grown worm. Dinophilus is an extremely interesting form, and it has been suggested that, while still possessing certain Planarian characteristics, it may be looked upon as closely resembling the ancestor from which the Chaetopoda have arisen.
Protodrilus and Polygordius are distinctly Annelidan in character. Protodrilus[297] is found in the mud of the "Pantano," an inlet of the sea near Messina; whilst of Polygordius[298] one species at least occurs on our shores, and several others in the Mediterranean and elsewhere. The worms are cylindrical, with many segments, but these segments are only indistinctly marked externally—by girdles of cilia in Protodrilus, or by faint grooves in Polygordius; but there are none of the characteristic Chaetopod bristles or chaetae. The small prostomium which overhangs the mouth is provided with a pair of ciliated pits, and carries a pair of tentacles, serving as sensory organs, which, in Protodrilus, are also respiratory. The anus is surrounded by glandular papillae in Polygordius, by means of which the animal can fix itself; these are represented in Protodrilus by a couple of processes.
The nervous system lies entirely in the epidermis. The body-cavity is regularly segmented by transverse septa passing from the body-wall to the intestinal wall. The foregut presents a slight eversible portion in Polygordius, whilst in Protodrilus it has a peculiar U-shaped muscular diverticulum on its ventral surface, corresponding with the similar apparatus in Dinophilus; it is capable of eversion, and aids the worm in burrowing, as well as in seizing and swallowing the mud. The vascular system is represented by a dorsal and a ventral vessel, neither of which, however, is contractile. In Protodrilus the dorsal vessel divides into two branches in the first segment, each of which passes to the tip of the tentacle, and returning, joins its fellow to form the ventral vessel. In some species of Polygordius there is a pair of vessels connecting the dorsal and ventral vessels in every segment, but no vessel to the tentacle. The blood is colourless in some species of Polygordius, but may be yellow (P. neapolitanus), red (P. lacteus), or green (P. erythrophthalmus). Paired nephridia, with distinct funnels, occur regularly throughout the body.
The sexes are separate in Polygordius, whilst Protodrilus is hermaphrodite, bearing ova in the first seven segments and testes in the remaining segments. The genital cells are produced from the body-wall in every segment; their mode of discharge is unknown in the male Polygordius, though probably the nephridia {245}convey the spermatozoa to the exterior; but in the female the body-wall ruptures to allow the ova to escape, and then the animal dies. The development of Polygordius has been made the subject of very careful study; the larva has long been known, and is a typical "trochosphere" of rather a depressed form. This "trochosphere" larva is of considerable importance, as it makes its appearance in sundry groups of animals in some form or another. Here, in Polygordius, it has the appearance of a couple of wide but low cones united together by their bases, which form the equator of the larva. This equator carries a double girdle of cilia, dividing the animal into a preoral and postoral region; for the mouth is placed on one side of the animal between the two girdles, while the anus lies at the apex of the postoral cone, and is surrounded by another girdle of cilia. The alimentary canal is divisible into three regions; it is separated from the body-wall by an extensive space, which contains cells destined to give rise to muscles and nephridia. A nervous system (apical plate) is present at the apex of the preoral cone. This little larva swims freely on the surface of the sea, moving, balancing, and feeding by means of the girdle of cilia. It soon increases in length by the active growth of the apex of the postoral cone, which becomes cylindrical and then segmented externally and internally. The greater part of the original larva remains of the same shape as before, and forms the head (prostomium and peristomium): small tentacles grow out of the preoral lobe, and after a gradual reduction in the relative size of the "head" by the growth of the segmented "body," the animal becomes worm-like and develops into a Polygordius.[299]
Order II. Polychaeta.
Anatomy of Nereis.—In order to obtain a general idea of a Polychaete worm, it is well to study a concrete example, and for this purpose the common Nereis serves excellently. Several species (see p. 315) occur more or less commonly on our coasts, and the general remarks will apply to one as well as to another.
Nereis pelagica Linnaeus reaches a length of 5 to 6 inches, and is about ¼ inch across. It is convex above, nearly flat below. Its {246}colour is brown or bronze. The worm, which is to be found in shallow water, is made up of a considerable number of rings or segments, constituting the "trunk" or "body," terminated at each end by modified segments known as "head" and "tail" (Fig. 122). The segments composing the trunk are all alike, except for small proportional differences, and it will be convenient to describe a "typical segment" before referring to the head or tail.
A typical body segment carries on each side a muscular lobed outgrowth, bearing bundles of bristles or "chaetae," and filamentous sensory organs known as "cirri." To this lateral locomotor organ Huxley gave the name "parapodium" (Fig. 124). Each parapodium or foot consists of a basal portion, supporting a dorsal and a ventral process, the "notopodium" (ntp) and "neuropodium" (nrp) respectively, each of which is bilobed. The lobes are very vascular and glandular, and probably serve as respiratory organs or "gills."
Fig. 123.—Chaetae of Nereis; enlarged. A, from neuropodium; B, from notopodium of N. diversicolor; C, swimming chaeta of Heteronereid stage of N. dumerilii.
The chaetae, or bristles, of each bundle project from the mouth of a great sac, the lips of which are particularly prominent in Nereis. Each chaeta arises from a single cell situated at the bottom of the sac. The chaetae of Nereis, as of many other Polychaetes, are of a kind usually termed compound or "jointed," each being composed of a long stalk and a small "appendix" articulated in a cup at its {247}extremity (Fig. 123). The shape of the cup varies; it is in some cases of equal height all round, or it is higher on one side than on the other. Further, the appendix may be short and curved, or more elongate and spear-like; it is generally notched or finely toothed on one side.
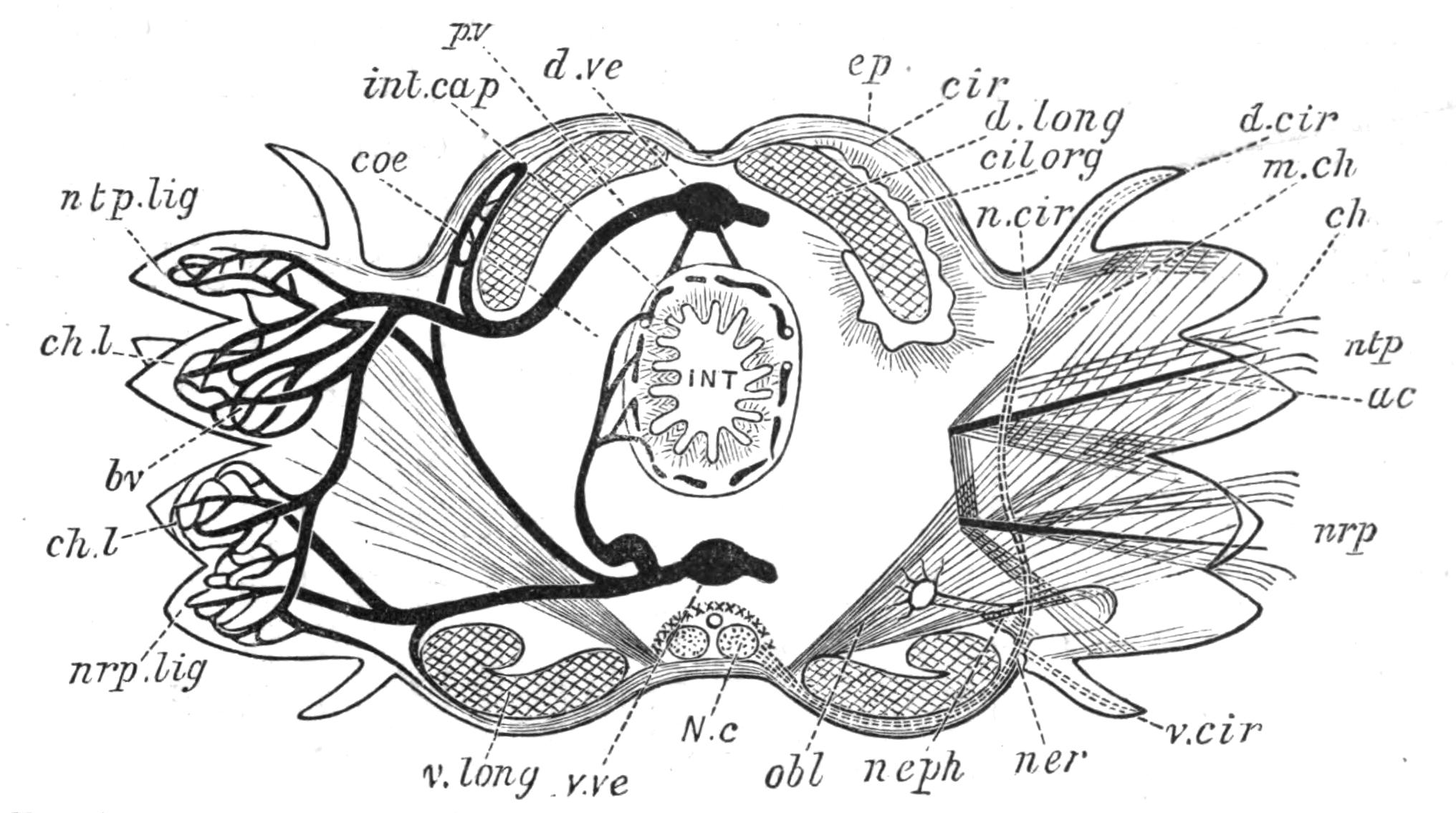
Fig. 124.—Nereis. Somewhat diagrammatic transverse section through the body. On the left the chief constituents of the vascular system are represented; on the right side the chaetae and their muscles, as well as the distribution of the lateral nerve, etc., are shown. ac, aciculum; bv, network of blood-vessels; ch, chaetae (only a few are shown) in two bundles; ch.l, lips of the chaetigerous sac; cil.org, dorsal ciliated organ; cir, circular muscular coat; coe, coelom; d.cir, dorsal cirrus; d.long, dorsal bundle of longitudinal muscles; d.ve, dorsal blood trunk; ep, epidermis; INT, intestine; int.cap, blood capillaries in its wall; m.ch, muscles which move the chaetae; N.c, ventral nerve cord; ner, lateral sensory nerve, dividing into a ventral branch entering the ventral cirrus, and a dorsal branch (n.cir) for the dorsal cirrus; neph, nephridium, seen through the oblique muscle through which its funnel passes; nrp, neuropodium; nrp.lig, neuropodial lobe or ligule; ntp, notopodium; ntp.lig, notopodial ligule; obl, oblique transverse muscle (muscle of the parapodium); pv, peripheral blood-vessel; v.cir, ventral cirrus; v.long, ventral bundle of longitudinal muscles; v.ve, ventral blood trunk.
In addition to these projecting locomotor chaetae, there is embedded in each of the two chaetigerous lobes a much stouter and dark-coloured, needle-shaped bristle known as an "aciculum," whose point only just projects beyond the surface. This aciculum extends into the interior of the body much farther than do the locomotor chaetae, and it is to it that the muscles serving to move the whole bundle of chaetae are attached. The acicula thus serve as an internal skeleton to the parapodium. The shape of the parapodium, the relative lengths of cirri and lobes, the shape and arrangement of the chaetae, are all employed as specific characters.
The head consists of a preoral portion above the mouth, the "prostomium," and a postoral region surrounding the mouth, the "peristomium" (Fig. 125). The prostomium varies in shape in different species of Nereis; but it always carries on its dorsal surface two pairs of eyes. From its narrower anterior end there arises a pair of short, somewhat conical, sensory processes known as the "prostomial tentacles." A second pair of processes springs from the under surface, and rather to the side of the prostomium; these are known as the "palps," and in Nereis are much more conspicuous than the tentacles; each is composed of two parts, a large basal piece and a smaller terminal joint, capable of being withdrawn into the former. The palps are highly muscular, and though they are sensory organs, act also as great lateral lips.
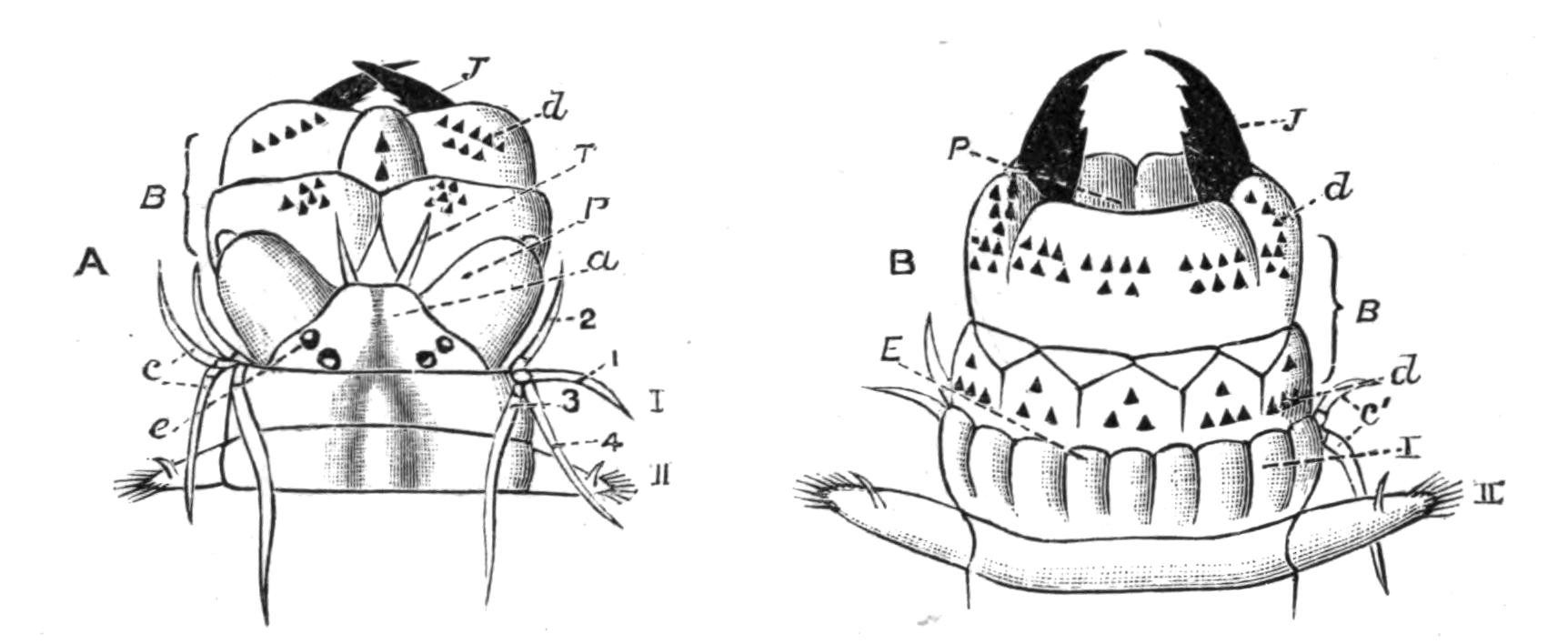
Fig. 125.—Nereis diversicolor Müll. × 4. Head, with buccal region everted. A, Dorsal view; B, ventral view. a, Prostomium; B, everted buccal region; c, c', peristomial cirri, 1, 2, 3, 4; d, denticles or paragnaths; e, eyes; E, lower lip; P, palp in A, entrance to pharynx in B; J, jaw; T, tentacle; I, peristomium; II, foot of apparent second segment.
The peristomium is in many species of Nereis (as in N. pelagica) considerably larger than the trunk segments; it carries at its anterior edge four filiform cirri on each side, which are directed forwards and used as feelers. They are arranged in couples; a more anterior couple of dorsal and ventral cirri, and a more posterior couple of dorsal and ventral cirri.
The Tail.—As the most anterior segment is perforated by the mouth, and is modified as described above, so the last or anal segment, which carries the anus, differs from the rest. It is more or less elongated, cylindrical, and without parapodia or chaetae. It retains, however, its pair of ventral cirri, which are very long.
Internal Anatomy.—In correspondence with the external {249}metamerism there is an internal repetition of parts. For, except in the anterior segments, where the powerful protrusible pharynx is situated, the body-cavity or "coelom" is divided into a series of chambers, by means of muscular septa inserted, on the one hand, into the body-wall at the level of the grooves between the external segments, and, on the other, into the wall of the alimentary canal. Each of these coelomic chambers contains a pair of nephridia, a portion of the intestine, of the vascular system, and of the nervous system, as will be seen in Fig. 124.
The epidermis, which forms the outer part of the body-wall, consists of a single layer of cells, covered externally by a thin, tough cuticle. The latter is usually stated to consist of the chemical substance known as chitin, but since the cuticle differs from true chitin by dissolving in caustic potash after a time, Eisig[300] has suggested that its substance is merely a stage in the formation of chitin. The epidermis contains gland-cells, which are especially abundant on the lobes of the parapodia. Below the epidermis lies the circular coat of muscles by whose contraction the worm diminishes its diameter: it is interrupted on each side at the junction of the parapodium with the body. Deeper still lie the longitudinal muscles, which form four great bundles, two dorsal, separated by the insertion of a small mesentery and dorsal blood-vessel, and two ventral bundles separated in the middle line by the nerve-cords. These longitudinal muscles, by their contraction, bend the worm from side to side, and are continuous from segment to segment. A very characteristic muscle, present in all the Polychaeta, is an obliquely transverse sheet of fibres passing from the body-wall at the side of the nerve-cord to the parapodium, where it spreads out and serves to move the parapodium (Fig. 124). All these muscles consist of smooth fibres, as in the earthworm.
The alimentary canal may be divided into the following four regions:—(1) buccal or eversible region, (2) pharynx, carrying the great jaws, (3) oesophagus, (4) intestine.
The first two regions constitute an "introvert" (Lankester[301]). When fully everted the whole of the buccal region is turned inside out, and the terminal aperture leads directly into the pharynx, which is not everted but merely protruded. {250}Throughout the following chapters the word "buccal" region is used for that part—if any—which is thus everted (Figs. 125, 126).
Both the buccal and pharyngeal regions are wrapped round by several coats of muscle, to form apparently a single muscular organ (Fig. 127, sh), which occupies about eight segments in a condition of complete introversion. The septa are absent from the anterior part of the body.
Fig. 126.—Diagrams to illustrate the action of the Chaetopodan "introvert." (From Lang.) A shows the apparatus at rest; the mouth leads into the buccal cavity (B), with paragnaths in its wall; P, the pharynx, with jaws at its anterior end; c, brain; p, protractor muscles; r, retractors. B, the pharynx has been brought forward or protruded by the eversion, or turning inside out, of the buccal region, so that the jaws (j) now lie some way in front of the head, which is represented by the brain.
The buccal region is lined with chitin, which is specially thickened at certain definite spots, forming small "denticles" or "paragnaths" (Fig. 125), which have a different arrangement in the various species.
The cavity of the pharynx is narrow and the walls thick and muscular; each side wall carries a large, dark, chitinous "jaw" (Fig. 127, J), which is hollow at the base, into which the muscles serving to move it are inserted, whilst the apex is solid, curved, and more or less notched. These two great jaws are used not only for tearing prey, but for seizing it; for when the pharynx is entirely protruded the two jaws are wide apart, and when retraction takes place they come together and grasp the prey.
Eversion of the apparatus is partly effected by protractor muscles (Fig. 126, A, p) and partly by the pressure of the coelomic fluid, compressed by the muscles of the body-wall; the eversion is stopped at a certain stage by a sheet of muscular tissue or "diaphragm" (Fig. 127, diaph) inserted round the buccal region and attached to the body-wall in the second segment. The introversion is effected partly by the contraction of this diaphragm and partly by the action of powerful retractor muscles (Fig. 126, r) inserted into the hinder end of the pharynx and passing to the body-wall (these are removed in Fig. 127). The {251}movement of the jaws themselves and of the wall of the apparatus is due to other muscles.
The oesophagus is quite short; into it opens a pair of sacculated diverticula or glands. Then follows the intestine, which extends through the rest of the body as a thin-walled tube, slightly dilated at the insertion of the septa.
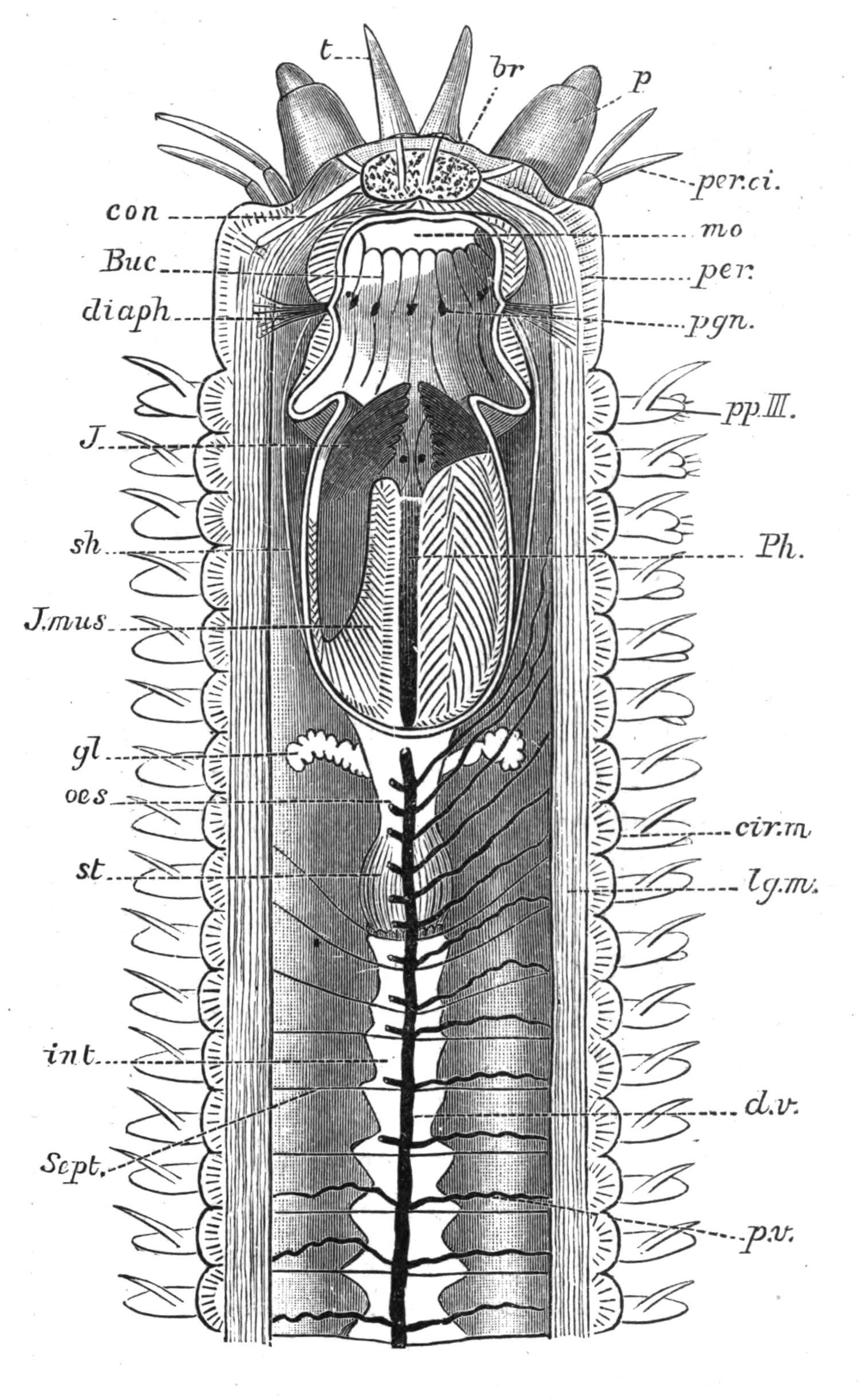
Fig. 127.—Nereis, laid open by removal of the dorsal body-wall. br, Cerebral ganglion, from which three pairs of nerves are represented as arising; a pair to the tentacles, a pair to the palps, and a pair (con) passing one on each side of the buccal region, to join the ventral nerve-cord; mo, mouth, exposed by removal of the dorsal wall of the buccal region (Buc); pgn, the paragnaths in its wall; Ph, pharynx; J, the large "jaws" embedded in its wall; J.mus, the muscles which work the jaws; sh, the muscular sheath; diaph, the "diaphragm"; oes, oesophagus; gl, its glands; st, stomach; int, intestine; Sept, septum; d.v, dorsal blood trunk; p.v, perivisceral branches, one pair in each segment; P, palp; t, tentacle; per, peristomium; per.ci, two of the four peristomial cirri; ppIII, the first parapodium which belongs to the third true, but second apparent, segment; cir.m, circular muscular coat; lg.m, longitudinal muscular coat of the body-wall.
The vascular system consists of a contractile dorsal vessel and of a non-contractile ventral vessel extending along the whole length of the body, from each of which paired and segmentally-arranged {252}vessels pass to the intestinal wall and to the body-wall, and here form extensive capillary networks (Fig. 124, p. 247). This type of vascular system is pretty generally adhered to throughout the Order, but in the Terebelliformia, Scoleciformia, and Cryptocephala the dorsal vessel and capillary plexus on the intestine are replaced by a continuous blood sinus, situated in the substance of the gut-wall. This "perienteric sinus" has the same relation to the segmental vessels as the dorsal vessel has in the Nereidiformia, and from it a tubular dorsal vessel arises anteriorly. In Arenicola the sinus is preceded in the young stage by a network the branches of which gradually enlarge, meet, and fuse to form the sinus.[302] Whether it is in all cases secondary is a moot point.
This system of vessels in the majority of Chaetopoda contains a respiratory fluid coloured red[303] by haemoglobin in solution; in it float a very few small oval nucleated non-amoeboid corpuscles. But the place of this red pigment is taken by a green one, named "chlorocruorin," in the Chlorhaemidae and many Sabelliformia;[304] whilst in Magelona[305] the blood is tinted madder-pink by a number of globules of "haemerythrin." The blood (or "haemal fluid") is driven forwards in the dorsal vessel, and passes backwards in the ventral vessel. Respiration in Nereis is carried on by the whole surface of the body, but naturally with greater activity in the surface of the parapodia, the lobes of which, with their extensive vascular plexus, may be termed "gills"; but it must be borne in mind that these organs have other functions as well.
The coelomic fluid, which fills the general body-cavity, is colourless, and contains amoeboid corpuscles or "leucocytes." It corresponds to the lymph of Vertebrates, being nutritive in function, in that it conveys absorbed material from the wall of the intestine to the organs of the body, and at the same time removes any waste substances from these organs; these waste substances contain nitrogen, and are ultimately removed by the nephridia. In Ophelia many of the corpuscles contain a curious dumb-bell-shaped rod of chitin, and it has been shown[306] that this substance {253}is a highly complex form of excretory material,—more complex than guanin, for instance, which exists in the corpuscles of the Capitelliformia.
In Glyceridae, Capitelliformia, and Polycirrus haematodes (a Terebellid), the vascular system is absent, and the coelomic corpuscles become coloured by haemoglobin, and in order that the coelomic fluid may be distributed to the organs of the body, the peritoneum is ciliated along certain definite tracts. The fluid in these "anangian" worms thus combines originally separate functions, and behaves like the "blood" of Vertebrates.
The excretory system is represented by a pair of nephridia in each segment, with the exception of a few anteriorly and a few posteriorly. The nephridium of Nereis differs from that of most other Polychaetes hitherto examined carefully, and rather resembles that of the Oligochaetous Enchytraeids. It consists of a compact gland-like organ, containing a much coiled tube, ciliated for the greater part of its length, but deprived of cilia in its last coils; this latter part—or duct—leaves the "gland" and pierces the body-wall, opening to the exterior at the base of the parapodium. The ciliated canal passes forwards into the next segment, where it opens by a funnel into the coelom. The lip of the funnel is extremely curious, for the cells constituting it are drawn out into very long, delicate processes covered with cilia.[307]
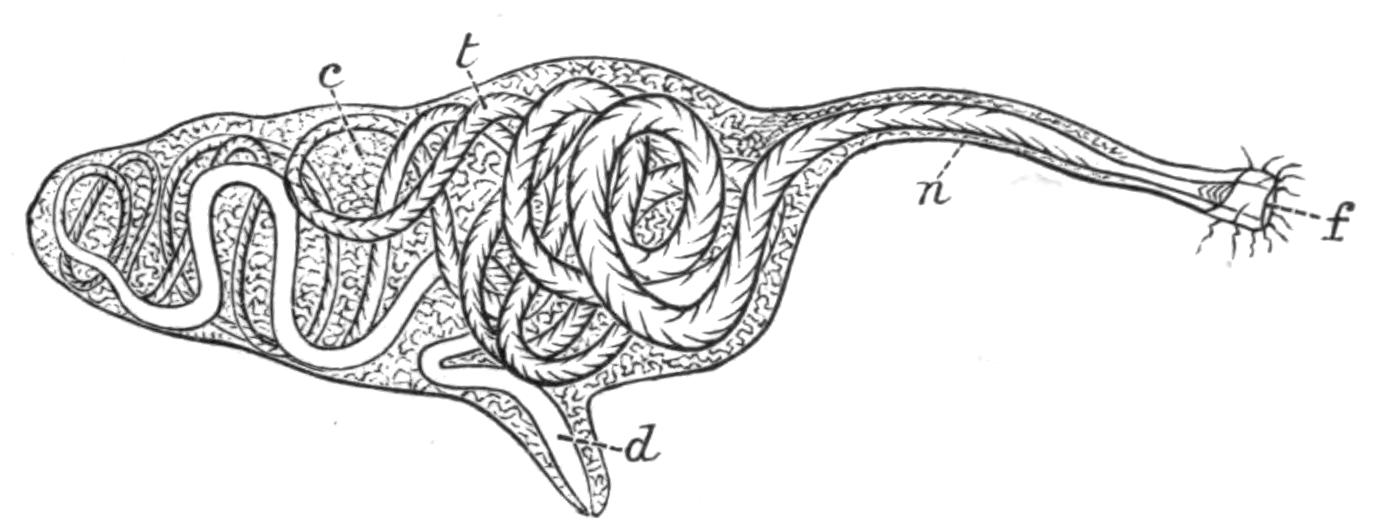
Fig. 128.—Nephridium of Nereis. (From Goodrich.) f, Funnel; n, neck, which passes through a septum; t, coiled tubule; c, connective tissue; d, duct.
In most Polychaetes the nephridium is a wide, sac-like tube as in Arenicola[308] (Fig. 129). Its walls are covered by a dense network of blood-vessels, and it not only acts as an excretory organ, but also as a genital duct (see p. 273).
Excretion, in the strict sense of the word, is carried out by {254}the cells forming the wall of the tube; they remove waste materials from the blood distributed over the surface of the organ. But, in addition, there is a removal from the coelom, by means of the funnel, of any dead or dying coelomic corpuscles which in their turn have eaten up or otherwise destroyed foreign bodies (such as Bacteria, etc.) that may have entered the animal.
In Nereis there is in each segment, in addition to the pair of nephridia, a pair of "dorsal ciliated organs" (Goodrich) (cil.org in Fig. 124). Each appears as a wide-mouthed funnel, greatly folded, and without any permanent outlet. But it is possible that these organs function as genital ducts, and that the external aperture will make its appearance temporarily at the period of maturity. This "dorsal ciliated organ" has not been met with in allied genera—such as Eunice, Nephthys, Polynoë, Glycera—where the nephridium is a wide tube, and serves as a genital duct.
Fig. 129.—Nephridium of Arenicola. (From Benham.) × 4. d, Dorsal lip of funnel; v, ventral lip of funnel; b, blood-vessel (all the black lines are blood-vessels); m, dilated bladder; x, part cut away from body-wall where the nephridium is passing to the exterior.
The nervous system, as in all Chaetopods, consists of a dorsal cerebral ganglion or "brain" (Fig. 127, br), connected by circum-buccal commissures with the anterior end of a ventral chain of ganglia. The brain occupies the prostomium,[309] and from it nerves pass away to the prostomial tentacles and palps. The circum-buccal commissures spring from the outer corner of the brain, and from each arises a nerve to the first pair of peristomial cirri. The first ventral ganglion lies in the third segment, and represents at least two ganglion-pairs fused together, for from it arise (1) a pair of nerves to the second pair of peristomial cirri and (2) a {255}pair to the first parapodium. In the remainder of the body there is a ganglion in each segment, whence nerves pass outwards to the parapodium and muscles of the segment (Fig. 124).
In Nereis the apparently single ganglion in each segment really consists of two halves, and the apparently single cord which traverses the whole length of the body consists of two closely apposed cords. In some worms, such as Serpulidae, the two cords are more or less widely separated, and the two ganglia of each segment are thus distinct, and connected by a transverse commissure. In Nereis, as well as in many other Polychaeta, the nerve-cords lie within the body-wall, but in other cases they lie in the epidermis, as they do in Archiannelida.
The visceral nervous system, supplying the muscles of the pharynx, is frequently highly developed. In Nereis it arises on each side by two roots, one from the brain, the second from the circum-buccal commissure.
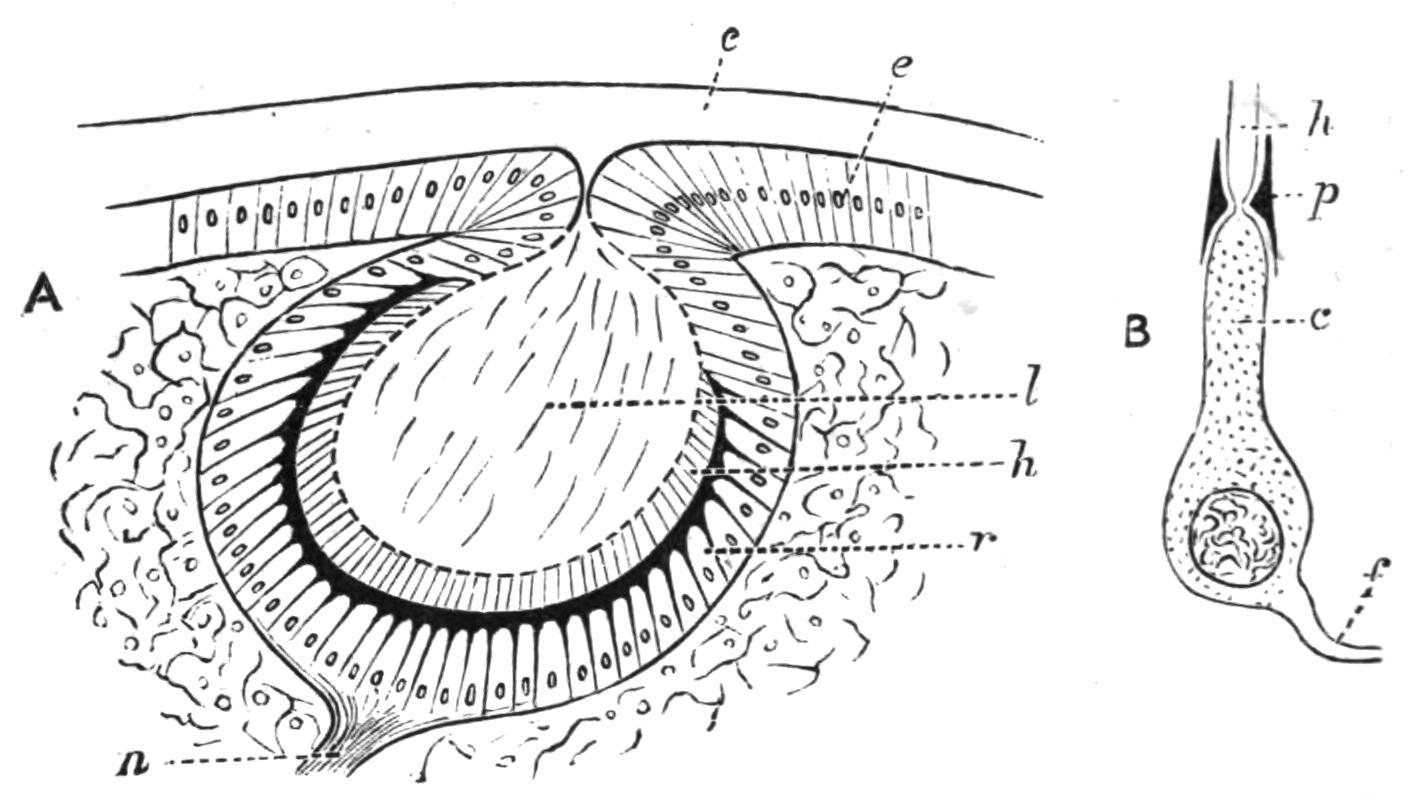
Fig. 130.—Eye of Nereis. (After Andrews.) × 150. A, Section through the entire eye; c, cuticle; e, epidermis; l, lens; h, rods; r, retina; n, optic nerve: B, isolated retinal element; c, cell; p, pigment; h, rod: f, nerve-fibre.
The organs of sense in Nereis are eyes, tentacles, palps, and cirri. The four eyes, which rest upon the brain, have the structure represented in Fig. 130. The retina consists of a single layer of cells containing pigment; each cell is drawn out peripherally into a nerve-fibre, whilst centrally it forms a cuticular product—the "rod" (h). The edges of the retina are continuous with the surrounding epidermis, and the cup thus formed remains widely opened to the cuticle in a few Polychaetes, e.g. Autolytus, and in the young of Nereis, but more usually it has the relations represented in the figure. The lens is produced by the retinal cells (according to Andrews[310]), and is in some cases (Eunice, Amphinome) continuous with the cuticle. It appears to be composed in other cases (Lepidonotus) of continuations of the retinal rods. The structure of the other sense organs {256}indicates their adaptation to a tactile function; in each case a nerve traverses the axis of the organ, and the nerve-fibrils terminate in sensory cells. Very probably the palps have a certain power of testing the food—a combination of the senses of taste and smell.
The Generative System.—In all the Polychaeta, with very few exceptions, the sexes are separate; and the reproductive cells—ova and spermatozoa—are produced at certain seasons of the year by the rapid proliferation and modification of coelomic epithelial cells surrounding the blood-vessels in the parapodium and its immediate neighbourhood. The sexual cells remain in the coelom till they are ripe.
The egg-cells become filled with yolk globules; a vitelline membrane is present, and an outer coat of albuminous material. It is doubtful by what means these sexual cells are discharged in Nereis. There is some evidence that the "dorsal ciliated organ" may act as a genital duct. In some other worms the nephridia serve this purpose, whilst in others a rupture of the body-wall allows the products to escape into the sea. According to Wistinghausen,[311] at the time of discharge the females of Nereis dumerilii become surrounded by a kind of gelatinous tube formed from a secretion of the parapodial glands, and into this tube the ova are discharged, and arranged in a single layer round its wall.
The common species Nereis diversicolor is viviparous. In a large number of species of Nereis the sexually-mature individuals undergo very marked changes in various parts of their body, so that they differ very greatly from the immature individuals.
These changes resulting in the "heteronereid" condition will be dealt with at some length in Chap. X. p. 276. The larvae of Polychaetes and other facts connected with reproduction are described in the same chapter.
CLASSIFICATION OF THE POLYCHAETA—SHAPE—HEAD—PARAPODIA—CHAETAE—GILLS—INTERNAL ORGANS—JAWS—SENSE ORGANS—REPRODUCTION—LARVAL FORMS—BUDDING—FISSION—BRANCHING—REGENERATION.
The Polychaeta are marine worms whose bodies are usually elongated and cylindrical; they either lead a free life, swimming in the open sea, or crawling along the bottom; or they pass their life in burrows or definite tubes of various kinds.
Each segment is normally provided on each side with a single or a couple of bundles of chaetae, by means of which locomotion is effected. These, in the free-living forms, are carried at the ends of lateral muscular outgrowths of the body, known as "parapodia," which are practically limbs.
The "head" of the worm generally carries eyes, and frequently more or less elongated tactile organs, the "tentacles" dorsally and "palps" ventrally. The foregut is frequently provided with a masticating apparatus in its anterior region, which is capable of protrusion; but this apparatus is absent in many burrowing and tubicolous forms. The sexes are separate, so that there is no such complicated system of generative organs as occurs in the Oligochaeta. The nephridia usually act as genital ducts. In the majority of cases the egg develops into a larva, the "Trochosphere," which leads a free life and undergoes a greater or less metamorphosis into the adult condition.
The classification of Polychaeta adopted in this work is as follows:[312]—
| Branch A. Phanerocephala. | |||||||||
| Sub-Order 1. Nereidiformia [= Errantia, auctt. + Ariciidae]. | |||||||||
| Family | 1. | Syllidae | see p. | 306 | Family | 8. | Amphinomidae | see p. | 318 |
| " | 2. | Hesionidae | " | 308 | " | 9. | Eunicidae | " | 318 |
| " | 3. | Aphroditidae | " | 309 | " | 10. | Glyceridae | " | 320 |
| " | 4. | Phyllodocidae | " | 313 | " | 11. | Sphaerodoridae | " | 320 |
| " | 5. | Tomopteridae | " | 315 | " | 12. | Ariciidae | " | 321 |
| " | 6. | Nereidae | " | 315 | " | 13. | Typhloscolecidae | " | 321 |
| " | 7. | Nephthydidae | " | 317 | |||||
| Sub-Order 2. Spioniformia. | |||||||||
| Family | 1. | Spionidae | see p. | 321 | Family | 4. | Magelonidae | see p. | 325 |
| " | 2. | Polydoridae | " | 323 | " | 5. | Ammocharidae | " | 325 |
| " | 3. | Chaetopteridae | " | 323 | |||||
| Sub-Order 3. Terebelliformia. | |||||||||
| Family | 1. | Cirratulidae | see p. | 325 | Family | 3. | Ampharetidae | see p. | 330 |
| " | 2. | Terebellidae | " | 327 | " | 4. | Amphictenidae | " | 330 |
| Sub-Order 4. Capitelliformia. | |||||||||
| Family. Capitellidae, see p. 331. | |||||||||
| Sub-Order 5. Scoleciformia. | |||||||||
| Family | 1. | Opheliidae | see p. | 331 | Family | 4. | Scalibregmidae | see p. | 334 |
| " | 2. | Maldanidae | " | 332 | " | 5. | Chlorhaemidae | " | 334 |
| " | 3. | Arenicolidae | " | 333 | " | 6. | Sternaspidae | " | 335 |
| Branch B. Cryptocephala. | |||||||||
| Sub-Order 1. Sabelliformia. | |||||||||
| Family | 1. | Sabellidae | see p. | 336 | Family | 3. | Amphicorinidae | see p. | 339 |
| " | 2. | Eriographidae | " | 338 | " | 4. | Serpulidae | " | 339 |
| Sub-Order 2. Hermelliformia. | |||||||||
| Family. Hermellidae, see p. 341. | |||||||||
Comparative Anatomy of the Polychaeta.
General Shape of the Body.—The majority of the Polychaeta have an elongated and very mobile body, like that of Nereis, consisting of an indefinite and usually of a considerable number of segments; a few, however, have a shorter body, with fewer segments, definite in number, for instance Aphrodite and Polynoë, which have thirty to forty segments; and some Hesionids, with only some seventeen to twenty segments.
In Aphroditidae and certain Amphinomidae the body is more or less oval in shape. In Lipobranchius and Sternaspis it is grub-like, short, and cylindrical, with rounded ends; in the former it is difficult to distinguish head and tail, or dorsal and ventral surfaces.
The segments composing the trunk may be all alike, or may constitute two more or less sharply marked regions, the thorax and abdomen, differing in the character of the chaetae, or in their arrangement, or in some other way, as in the Sabelliformia and the Capitelliformia.
As peculiar cuticular structures, the curious shields of Sternaspis, and of certain of the Maldanidae may be mentioned.
The posterior extremity is generally more or less narrowed, and most of the Nereidiformia are provided with special elongated cirri, borne by the anal segment. In the Maldanidae and others the body terminates in a funnel, at the bottom of which is placed the anus. Only in a few cases is the anus not terminal; in Notopygos and other Amphinomidae, as well as in some species of Polynoë, it is dorsal.[313] In Sabellaria and Pectinaria the hinder end of the body undergoes great degeneration; in the former it is achaetous, but cylindrical and bent forwards alongside the body (Fig. 131). In Pectinaria (Fig. 177), this region, which is called the "scapha," is leaf-like, and serves to close the narrower end of the tube in which the worm lives. Arenicola marina, and some Terebellids have no chaetae in the hinder, narrower part of the body.
The Head.—The prostomium is, in the majority of cases, rounded or conical, though it may be square (Nephthys) or elongated and jointed (Glycera), or even hammer-shaped (Tomopteris); or it may be fused with the peristomium, and apparently absent (Arenicola). In the great group Cryptocephala, the peristomium grows forwards so as to hide the prostomium entirely.
In a few of the Nereidiformia the prostomium is compressed, {260}and in the Amphinomidae it is provided with a dorsal ridge or "caruncle," which is a leaf-like process overlapping three or more segments. In many Aphroditidae (as well as in Polydora) there is a peculiar "frontal" ridge passing forwards from the prostomial tentacle, and downwards into the mouth (Figs. 132, c, and 133, A, x).
Fig. 132.—Aphrodite aculeata L. Ventral view of anterior region, × 6. a, Prostomium; c, frontal ridge on prostomium; d, neuropodial cirrus; l, lower lip; m, mouth; p, palp; s, intersegmental groove; t, tentacle; I, foot of peristomium, which has shifted forwards so as to lie in front of the mouth; II to V, successive feet.
In all the Nereidiformia, as well as in Sabelliformia and Chlorhaemidae, the prostomium bears sensory processes of two kinds, viz. dorsal tentacles and ventral palps. The latter are invariably two in number, and are particularly well developed in Aphroditidae, Nereidae, Syllidae, some of the Eunicidae, and in Chlorhaemidae. Even when they are apparently absent, as in Nephthys, it is possible that they are represented by certain lobes at the sides of the mouth, for in many Syllidae they are so fused with the prostomium as to be scarcely distinguishable. In the Chlorhaemids the palps[314] are grooved, and in the Sabelliformia they become considerably branched, and extend round the prostomium so as to nearly meet dorsally and ventrally. Each palp is, in this sub-Order, represented by a greater or smaller number of long, mobile filaments, arising from a common base; they are grooved along the inner side, ciliated, and provided with secondary processes. The crown of "gills," in fact, is nothing {261}more than the greatly subdivided and enormously elongated palps, as both Pruvot[315] and Meyer[316] have shown. In such forms as Haplobranchus and Amphicorine the process of subdivision (branching) has only gone a short way. In all the Sabelliformia each filament, in addition to its sensory function, aids in conveying food to the mouth by the action of the cilia, and has a blood-vessel within, thus acting as a respiratory organ. The filament may carry compound eyes (Fig. 143) either at its apex (Branchiomma) or at intervals along its course (Dasychone).
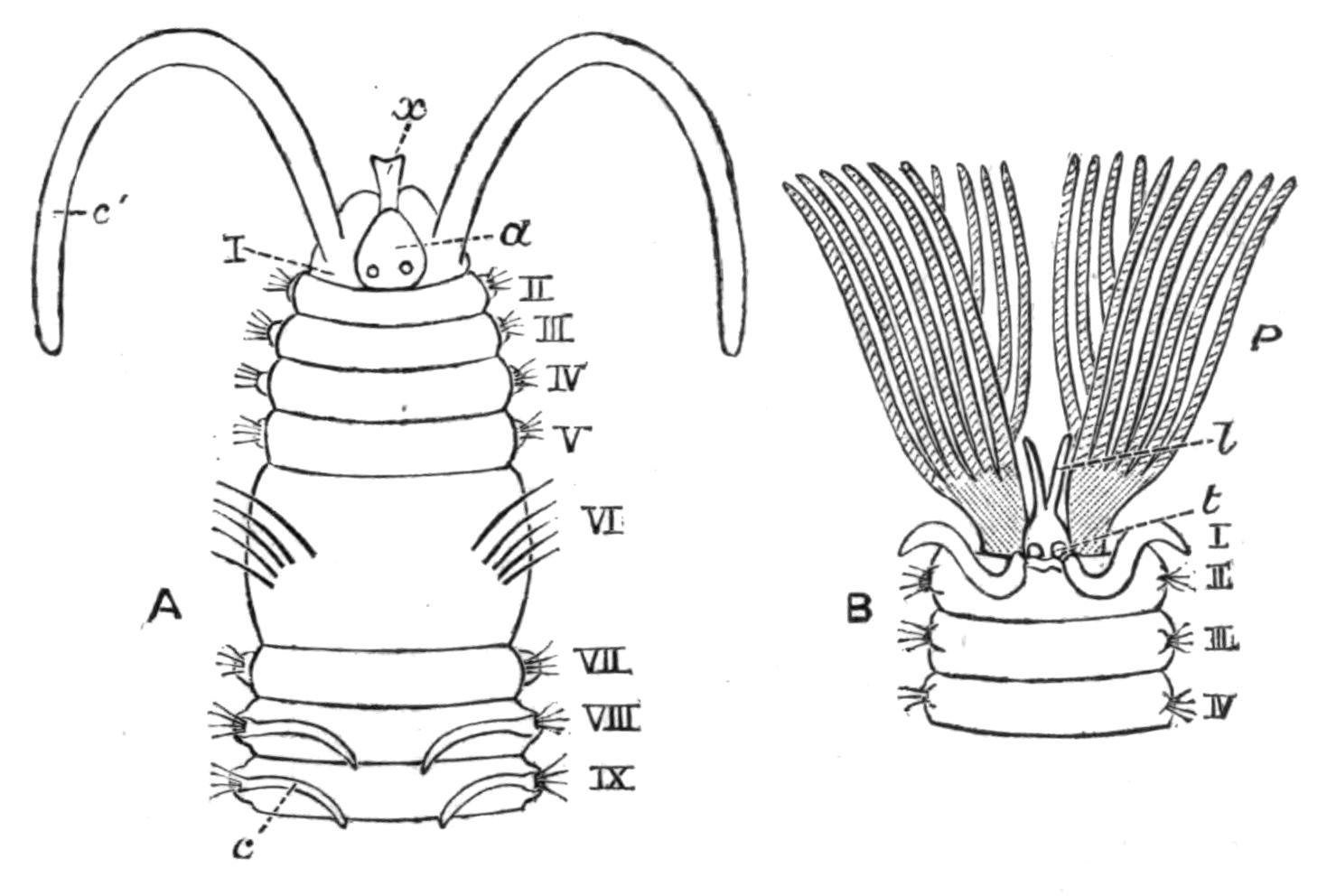
Fig. 133.—A, Anterior end of Polydora enlarged. a, Prostomium; x, frontal ridge; I, peristomium; c', its long cirrus; II, III, etc., the following segments; c, gill; B, head of Sabellid; P, palps (branchial crown); t, position of tentacles; l, processes of upper lip membrane; I, peristomium raised into a collar; II, III, IV, following segments.
In the family Serpulidae one (rarely two) of the most dorsally placed gill filaments is enlarged terminally, and acts as a stopper or "operculum," which closes the mouth of the tube when the animal withdraws into it. Further, in Spirorbis this operculum is grooved on one side, and serves as a brood pouch in which the eggs undergo development (Fig. 184, p. 341). It will be seen, therefore, that the palps may be very important organs for the life of the worm, and they are no less interesting to the comparative anatomist, serving as they do as an excellent illustration of the various uses which Nature finds for one and the same organ.
In the other sub-Orders the prostomium carries neither palps nor tentacles.
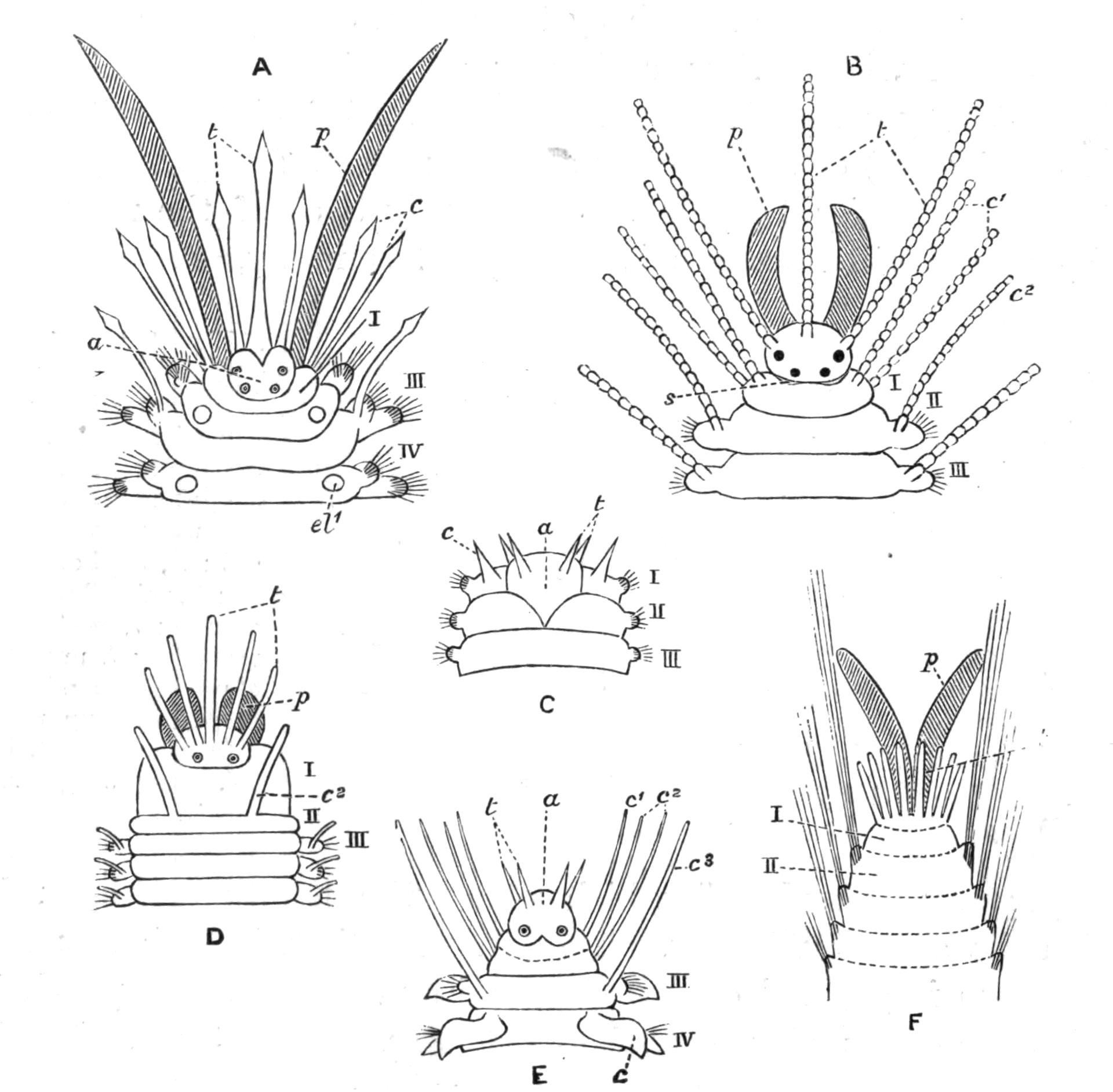
Fig. 134.—Heads of various Polychaeta (diagrammatic). A, Polynoid; B, Syllid; C, Nephthys; D, Eunice; E, Phyllodoce; F, Trophonia: a, prostomium; c, normal cirrus; c1, peristomial cirri; c2, cirrus of second segment; c3, cirrus of third segment; el1 point of attachment of elytron; p, palp: s, nuchal organ (ciliated pit); t, tentacle; I, peristomium; II, III, IV, segments.
The tentacles in the Nereidiformia present a wide variation in number; probably the typical number is three, one of which is median and two lateral—as in Polynoids, Syllidae, and some Eunicidae. Further, there is a certain amount of evidence in the nerve supply of the median tentacle to show that it was originally double. The presence of four tentacles, then, as in Nephthys, Phyllodoce, and Glycera, may be a primitive condition. By the disappearance of the paired lateral tentacles the worm possesses a single median one, as in Aphrodite and Amphinomids;[317] whilst a duplication of these lateral ones leads to the condition of Eunice and Hyalinoecia, which have five tentacles. In the Chlorhaemidae the number is further increased to five or more on each {263}side,[318] and in the Terebellidae these prostomial processes become very numerous.
In the Cryptocephala there is never more than a single pair of tentacles, and these are generally reduced to a group of sensory cells, though in Sabellaria they retain a considerable size.
In a few genera, such as Aphrodite, Nephthys, Capitella, the first postoral segment is distinguished from the succeeding segments only by its position with regard to the mouth (Fig. 132) and by its smaller size. But in the remainder of the Polychaeta, with here and there an exception, the peristomium is achaetous in the adult.[319]
Except in the Nereidiformia, peristomial or tentacular cirri are rare, being represented in the Spioniformia by the very long "tentacles." In the Nereidiformia one or more of the following segments may be added to the peristomium, and share in the "cephalisation," which is so characteristic a feature in this group. In Amphinomids the first three or four chaetigerous segments are incomplete ventrally, owing to the shifting of the mouth backwards; these segments form lateral lips, but they are not otherwise modified. In Phyllodoce, however, there are four cirri on each side of the mouth, and from the arrangement in the Alciopids we are justified in concluding that the segment which carries the four pairs of cirri is really made up of three segments (Fig. 134, E). Among the Hesionids there are four such "cephalised" achaetous segments with long cirri.
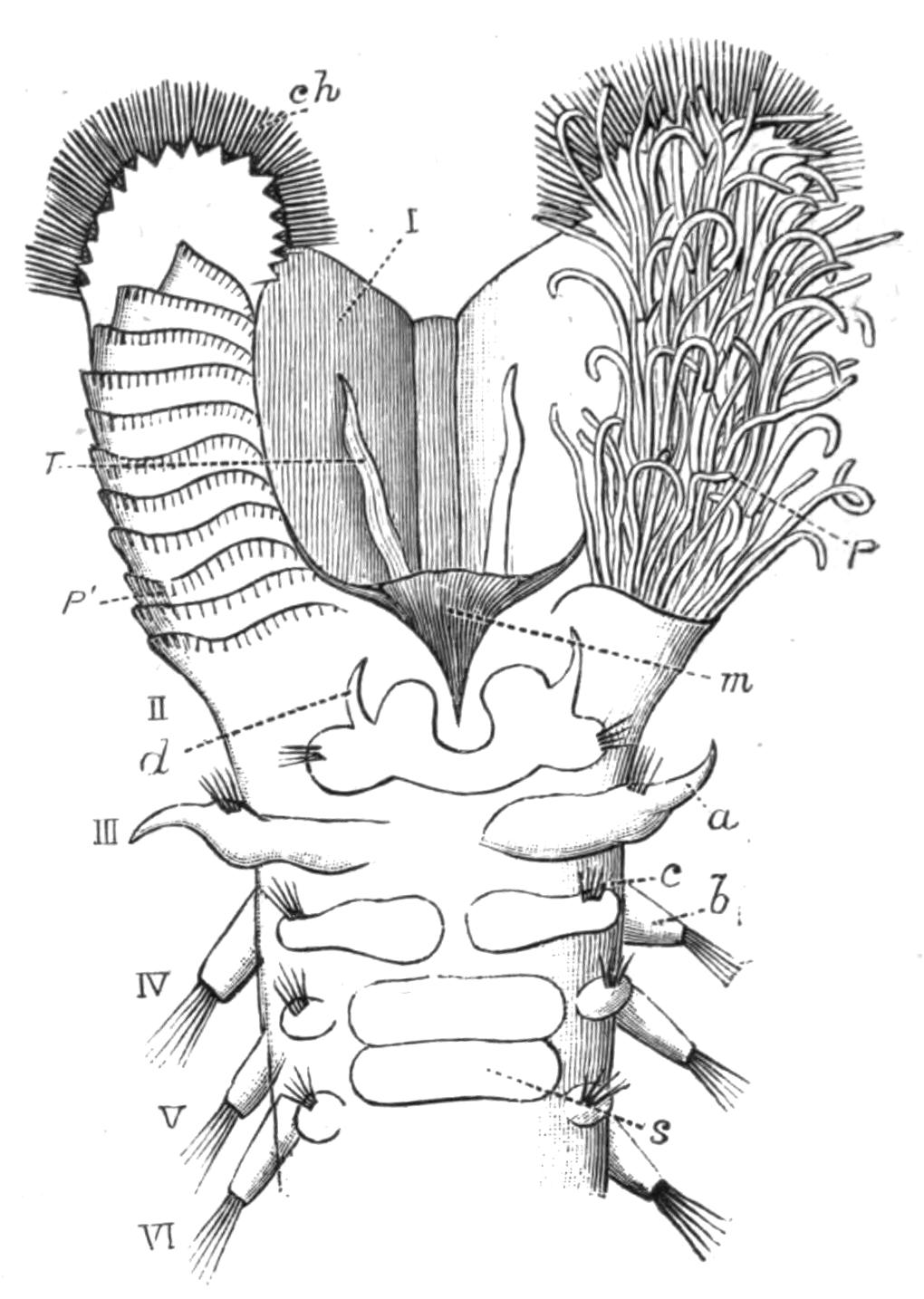
Fig. 135.—Sabellaria alveolata L. Ventral view of anterior region, × 10. a, Notopodial cirrus; b, notopodium; c, neuropodium; ch, peristomial chaetae; d, neuropodial cirrus; m, mouth; P, multifid palp (gill filaments); P', ridges after removal of gill filaments; s, ventral (tubiparous) gland shield; T, tentacle; I, hood formed by peristomium; II to VI, following segments.
In a few cases, such as the Chlorhaemids and Sternaspis, and to a slight degree in Arenicola, the "head" and even the anterior part of the worm is capable of being withdrawn into the body.
The Parapodia and Chaetae.—The typical parts of a parapodium have been described in the preceding chapter; here it is only necessary to refer to the series of diagrams (Figs. 136, 137) representing the parapodia of the more common Polychaetes, and to add a few remarks about them.
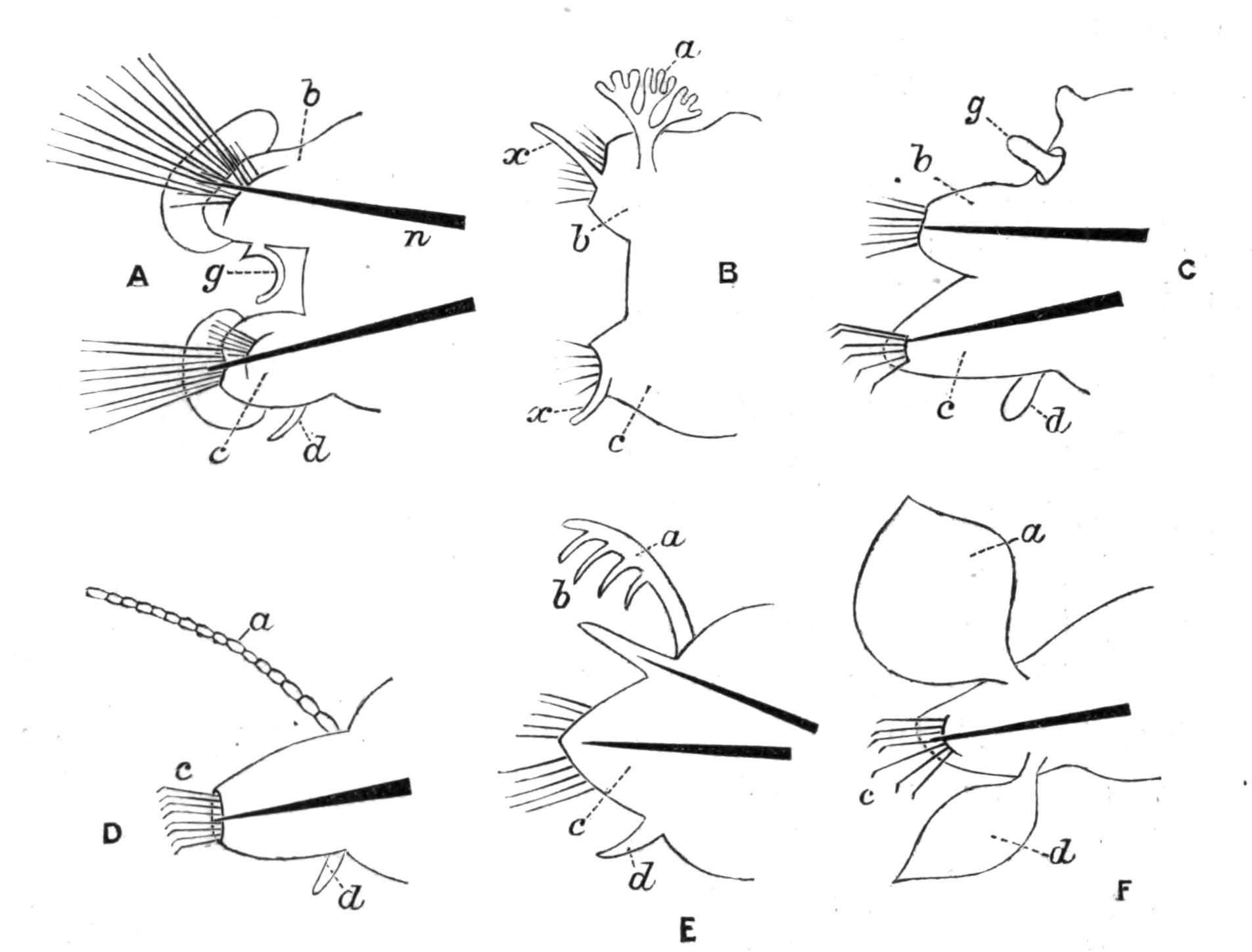
Fig. 136.—Parapodia. A, Nephthys; B, Amphinome; C, Glycera (the unlettered lobe above g is the notopodial cirrus); D, Syllis; E, Eunice; F, Phyllodoce. a, Notopodial cirrus; b, notopodium; c, neuropodium; d, neuropodial cirrus; g, special gill; n, aciculum (omitted in B); x, cirriform lip of chaetigerous sac.
In most Annelids the chaetae are in two bundles on each side, but there are certain families in which the dorsal bundle, and even the notopodium itself, is absent, as in the Eunicidae, Syllidae, and Phyllodocidae; or the dorsal bundle may be absent only in certain regions of the body, as in the hind-body of Terebellids. In some Amphinomidae and Aphroditidae the notopodium is scarcely distinct as a separate lobe, being a slight tubercle on the upper surface of the neuropodium; but the notopodial chaetae are present, and indeed particularly well developed in many cases.
But whilst, in the Nereidiformia, the parapodia, whether {265}consisting of two lobes or only one, are always well developed, and project to a more or less pronounced degree from the sides of the body, it is otherwise in the rest of the group, where the chaetigerous lobes are usually reduced to mere tubercles or ridges, no doubt in relation to their burrowing or tubicolous habits. In Sternaspis the chaetae issue directly from the body-wall.
Amongst the Nereidiformia we find examples in which the parapodia, instead of being more or less conical "legs," are flattened fore and aft so as to serve as efficient "fins," as in the active swimmers, Nereis virens and Nephthys caeca, and in the pelagic Phyllodocids, Alciopids, Typhloscolecids, and Tomopteris.
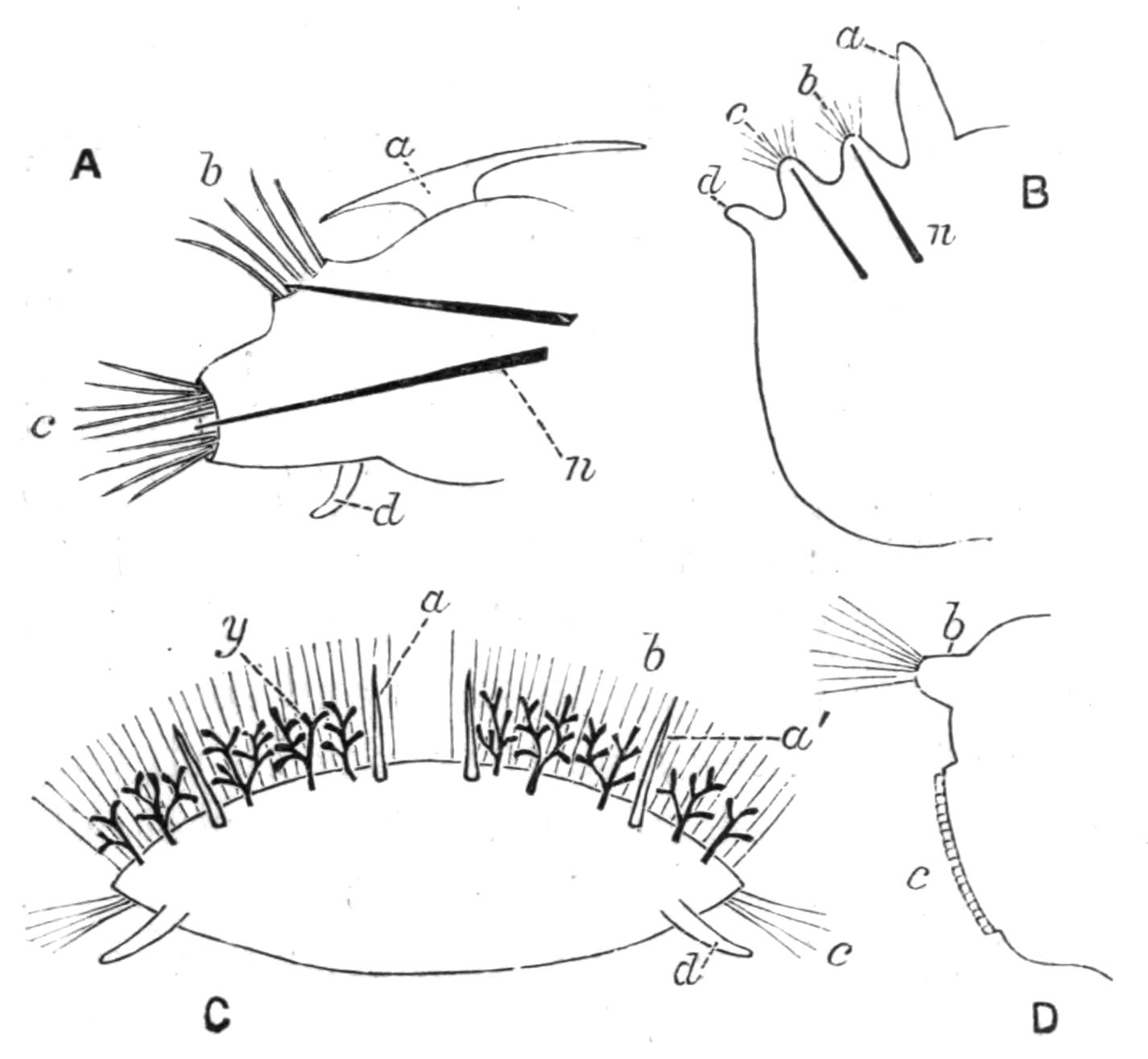
Fig. 137.—Parapodia. A, Polynoë; B, Scoloplos; C, Euphrosyne. (Transverse section of body.) a', Accessory cirrus; y, doubtful branchiae; D, Sabella (thoracic). a, Notopodial cirrus ("elytron" in A, "gill" in B); b, notopodium; c, neuropodium; d, neuropodial cirrus; n, aciculum (accidentally omitted in C).
Of the typical dorsal and ventral cirri, the ventral is only absent in some Amphinomids amongst the Nereidiformia; the dorsal is absent in Nephthys and degenerate in Glycera, whilst in a very large number of families of the other sub-Orders neither cirrus is present. These cirri, though originally filamentous and sensory, may, by virtue of special blood supply, become "gills," and this occurs in several families of different sub-Orders. Thus in Eunice this gill is comb-like; in Amphinome and in Arenicola (on certain segments) it is arborescent, as it is also in one to three segments in Terebellids; whilst in Ariciidae, Spioniformia, Cirratulidae, Opheliidae, and Sabellaria it remains more or less finger-shaped or filamentous. In the family Serpulidae the thoracic cirri, both dorsal and ventral, {266}become flattened and extended antero-posteriorly, and unite with one another to form the "thoracic membrane."[320] In Phyllodocidae the cirri are foliaceous and natatory, and they contain a great quantity of glands of a peculiar character. The Aphroditidae are distinguished from other Annelids by the possession of "elytra" or dorsal scales, which appear to be the dorso-ventrally flattened cirri, retaining their sensory nature, but adding to this function several others.[321]
The chaetae or bristles are mainly used in locomotion, but it is not unreasonable to believe that some of the stronger, serrated kinds may be used as weapons of offence and defence; certainly the Polynoids, bristling as they do with stiff chaetae along each side, must be rather unpleasant to their smaller enemies.
The various bristles may be placed in three chief groups, viz. (1) simple; (2) jointed; (3) uncini (see Fig. 138).
(1) The simple chaetae may be smooth and hair-shaped, i.e. "capillary," such as are present in nearly all families: or they may be forked (Amphinomidae), comb-shaped (Eunice), notched or serrated, or provided with a series of frills at right angles to their length, as in Aphroditidae; or fringed along one or both sides with a membranous expansion, as in Terebellids and Sabellids. The simple chaetae may also be short and spine-like, as in the ventral bundles of Arenicola; or they may be slightly curved at the end and notched, forming what are generally termed "crotchets," such as are common amongst Oligochaeta. These "crotchets" may be simple, or have numerous denticulations at the end (Maldanidae), or be provided with a membranous hood (Spioniformia, Capitelliformia). In Hermione peculiar sheathed, spear-like bristles occur (Fig. 138, N).
(2) Jointed chaetae have already been described (p. 246); they are confined to the sub-Order Nereidiformia, and occur only in certain families.
(3) The uncini are very short chaetae, which are simply embedded in the skin, and do not extend beyond the body-wall into the body-cavity. An uncinus is a sharply curved hook, which may have more or less numerous secondary teeth on it. They are characteristic of the Sabelliformia and the Terebelliformia.
The chaetae appear as solid, usually fibrillated structures, of a yellow or golden tint, transparent and refringent. Chemically {267}they consist of chitin, and each chaeta is the product of a single cell. The chaetae of Euphrosyne are hollow and calcareous, being peculiar in both characters.
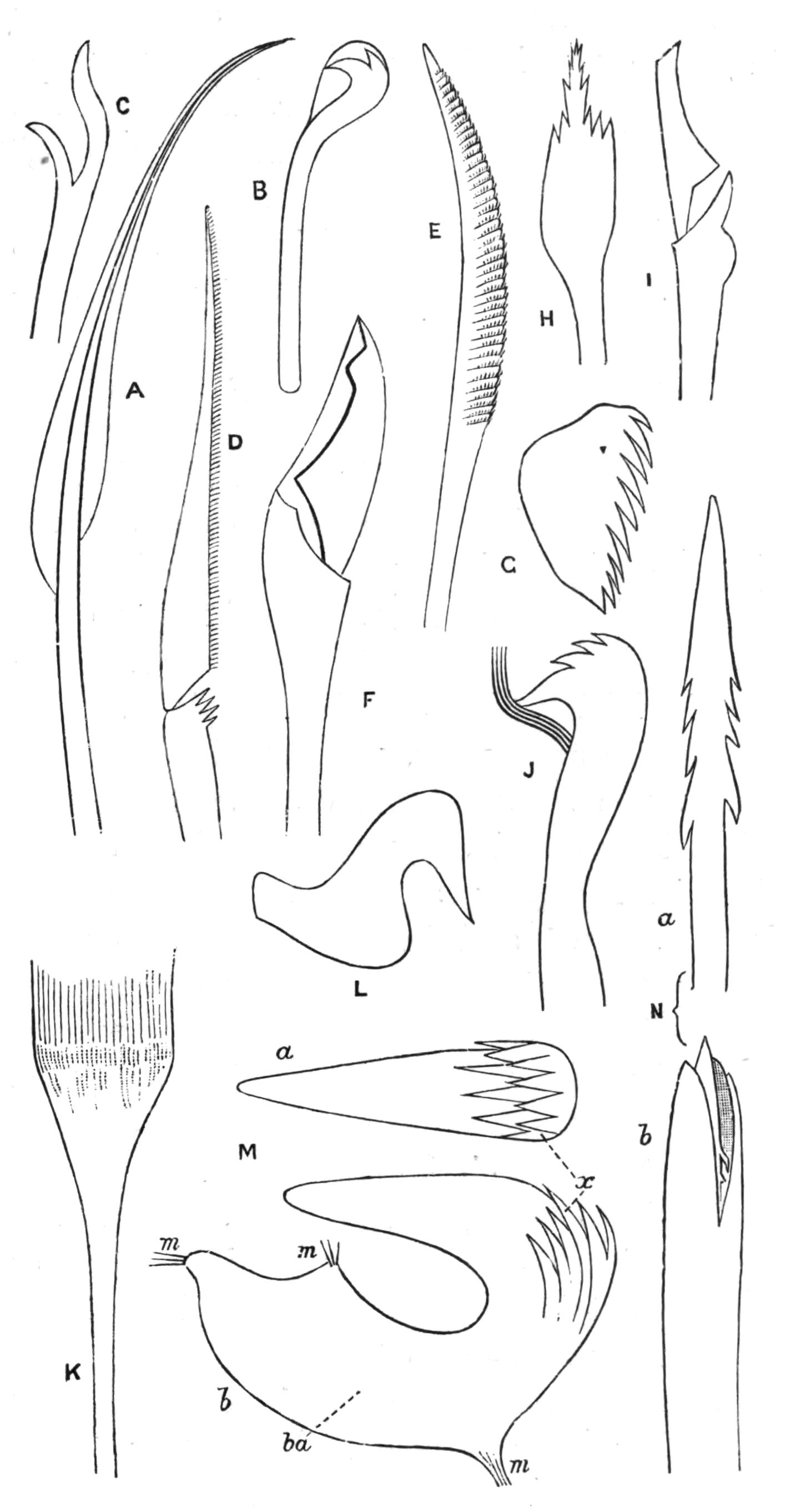
Fig. 138.—Chaetae of various Polychaetes (the magnification is not the same in all cases). A, Doubly-fringed capillary, from Terebellid; B, hooded crotchet, from Polydora; C, a fork, from Euphrosyne; D, jointed chaeta, from Phyllodoce; E, simple chaeta, with serrated ridges or frills, from a Polynoid; F, jointed chaeta, from Eunice; G, uncinus, from Pomatoceros (Serpulid); H, one of the outer series of paleae from the hood of Sabellaria spinulosa; I, jointed chaeta, from a Syllid; J, multidenticulate crotchet, from a Maldanid; K, comb-shaped chaeta, from Eunice; L, uncinus of a Sabellid; M, uncinus of Terebellid (Amphitrite Johnstoni); a, edgewise; b, side view; m, attachments of muscles into ba, basal plate; x, accessory teeth. N, Sheathed spear of Hermione; a, the spear-shaped capillary removed from its sheath; b, the same, with sheath.
Certain modifications of the chaetae presented by various worms deserve mention. In Polydora (Fig. 133, A) and in Chaetopterus (Fig. 173, p. 324) those of one segment are especially strong, but their significance is uncertain. In Capitella those {268}of the notopodium of the eighth and ninth segments are specially modified; they are analogous to the copulatory chaetae of Oligochaeta. In Aphrodite, in addition to the ordinary locomotor chaetae, there are brilliant, iridescent bristles and peculiar felting threads arising from the indistinct notopodium; these latter, however, are not true "chaetae," but are separate chitinous filaments similar to the constituent fibres of an ordinary chaeta.[322]
While the chaetae in the Nereidiformia and others are grouped in bundles, those of many other families are in vertical, transverse rows, as in Maldanidae and in Arenicola. The uncini are always embedded in such rows, usually slightly raised from the general level of the body surface, each being termed a "torus uncinigerus." These tori are usually limited to the sides of the body, but in Myxicola and in Notomastus they encroach upon the dorsal surface, and in Chaetozone, also upon the ventral, so as nearly to encircle the body, recalling the "perichaetous" condition of some earth-worms.
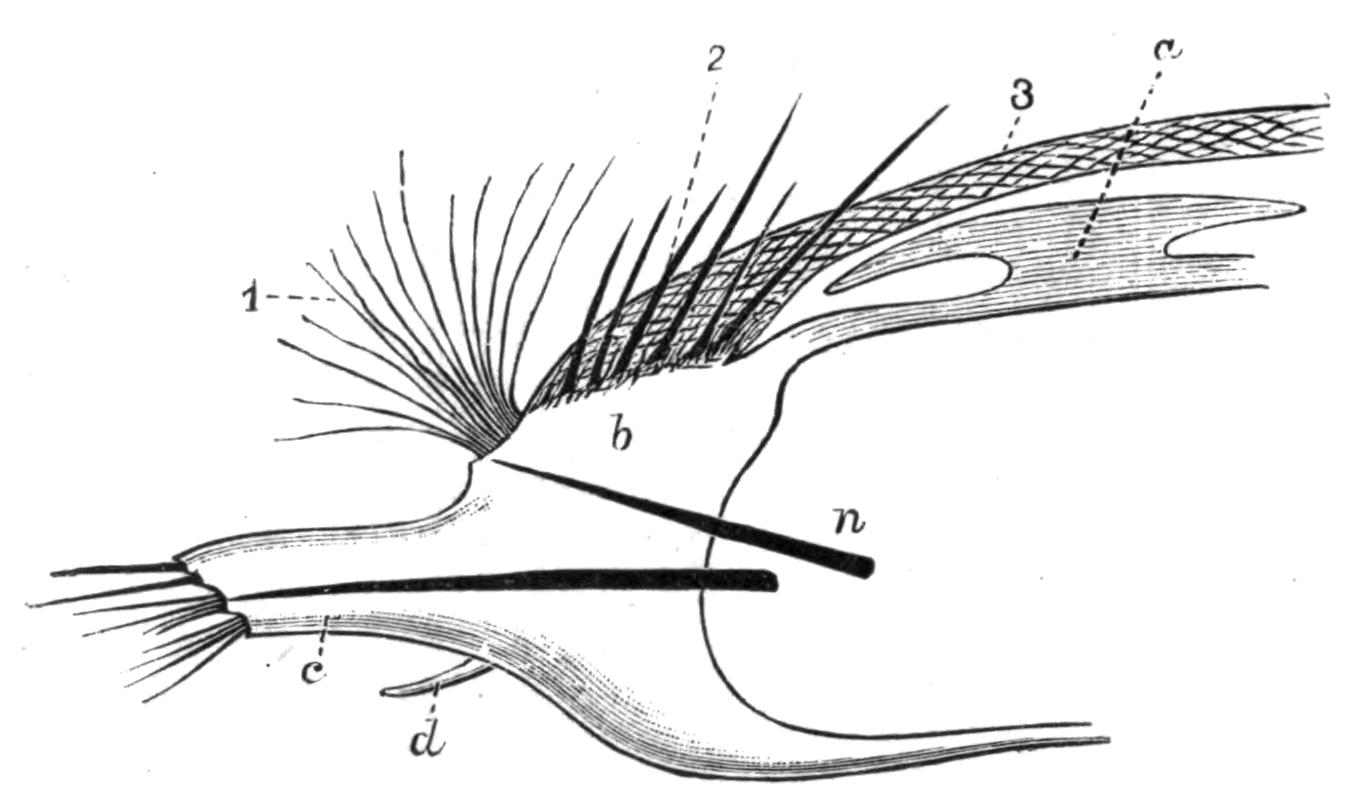
Fig. 139.—Aphrodite. Foot, × 2. a, Elytron; b, notopodium; c, neuropodium; d, neuropodial cirrus; n, aciculum; 1, iridescent bristles; 2, stiff chaetae; 3, felt.
Gills.—We have already seen that several different organs, e.g. the palps in Sabelliformia, the prostomial tentacles of Chlorhaemidae, and the notopodial cirri of sundry other Polychaetes, may take on a respiratory function. There are, however, certain "gills" developed either on the parapodium itself or elsewhere on the body which it is difficult to homologise. Such are the retractile gills on the parapodia of the Glyceridae (Fig. 136, C); those of Dasybranchus, near the abdominal neuropodia; those of Mastobranchus, near the notopodia. Nephthys has a sickle-shaped gill on the under surface of the notopodium. The long gill filaments at the posterior end of Sternaspis, again, are only doubtfully interpreted as the dorsal cirri of some of the posterior segments.
Since primitively the whole skin of the worm is respiratory, any part of the skin may become more or less specialised for this function, and chiefly, of course, on the more actively moving parapodia. The blood-vessels constituting the essential part of the "gill" may make use of any already existing outgrowth (such as a cirrus or a tentacle), or may push the body-wall out on their own account.
Internal Anatomy.
Probably those organs which have the greatest effect in modifying the shape of the body are the septa, for we find in the long, free-swimming worms that these are regularly present throughout the body, and external "segmentation" of the body is well marked. In burrowing and tubicolous forms the septa are frequently incompletely developed, or more or fewer may be absent; and the body becomes less distinctly segmented externally, tends to vary greatly in diameter during movement, or becomes plumper. With the disappearance of the septa there is also a diminution in the number of nephridia, as in Arenicola, with only six pairs. Further, there is frequently a dimorphism of these organs; instead of all of them serving equally as excretory organs and as genital ducts, some of the most anterior in the Sabelliformia and Terebelliformia become greatly enlarged, and take on practically the whole of the former function; whilst more or fewer of the posterior nephridia dwindle in size, and become genital ducts. The absence of septa allows a free communication between the successive segments, and thus a freer flow of coelomic fluid for the distension of the anterior end of the worm during burrowing.
The alimentary system presents certain modifications of a systematic value. In the Nereidiformia the muscular pharynx, which is always protrusible and is preceded by an eversible buccal region, frequently encloses thickened cuticular plates which serve as crushing and grasping organs. The form, number, and arrangement of these "jaws" vary in the different families. They form valuable fossil records of extinct worms.
In the Scoleciformia and Capitelliformia the buccal region exists, but there are no jaws. In the Sabelliformia and Terebelliformia eversion does not take place and jaws are absent.
Amongst the Nereidiformia the jaws are absent in the Phyllodocidae and Hesionidae; when present they are usually set in the direct course of the food. There may be one small tooth used for stabbing, as in some Syllids (Fig. 141, A); or a circle of such denticles (Autolytus, Fig. 140, D). To these are added powerful grasping jaws in Nereis (E); or the latter may alone be present, as in Glycera (F). In Polynoë the four jaws are carried by hard pieces, to which the muscles are attached (C and G). In Nephthys there is a dorsal and a ventral jaw.
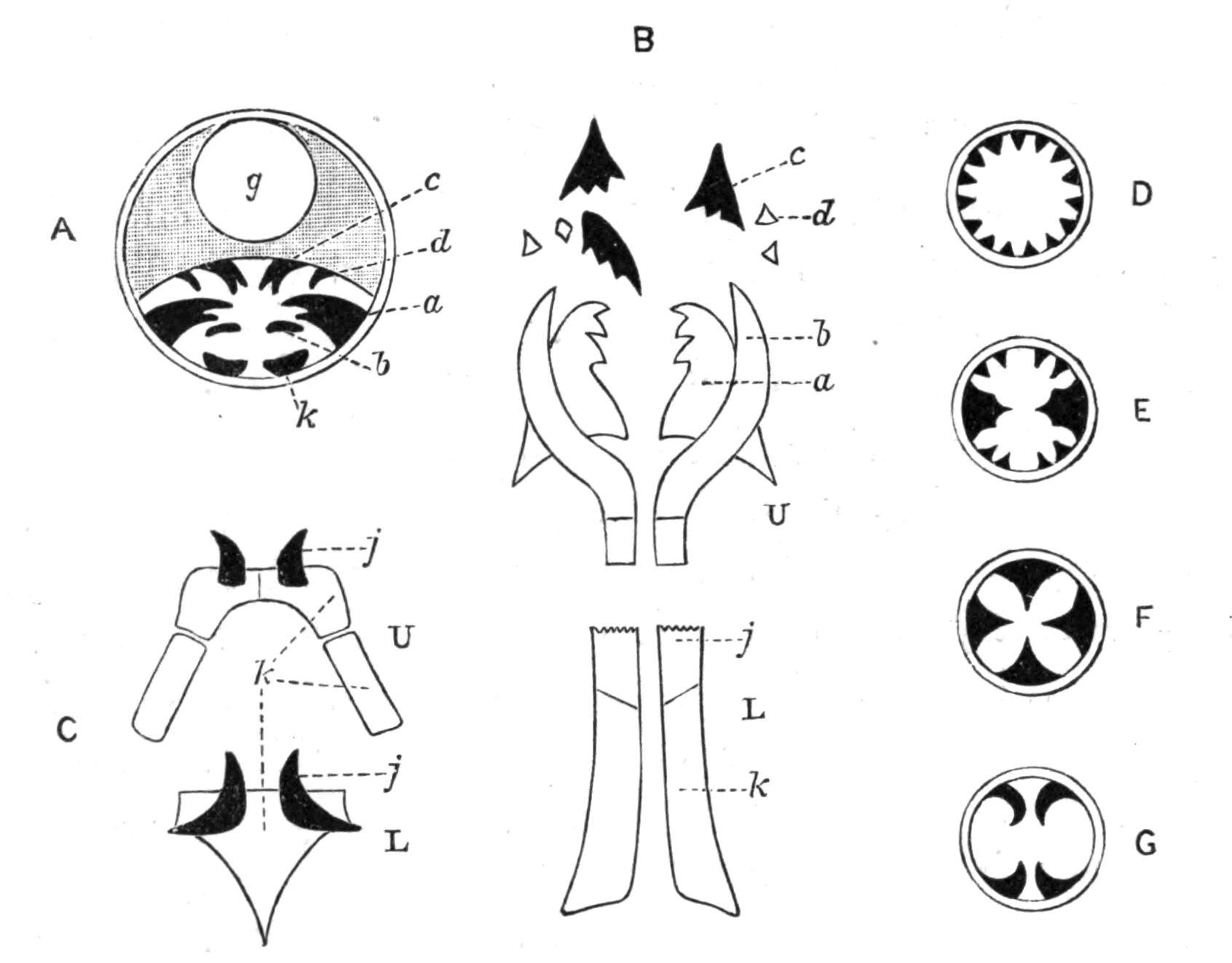
Fig. 140.—Jaws of various Chaetopods. A, Transverse section of the anterior end of Eunice; a, b, c, d, various parts of the upper series of denticles lying in a special chamber; g, oesophagus; k, lower jaw: B, the denticles of Eunice separated; U, upper series; a, grinder; b, forceps; c, rasping plates; d, grater; L, lower series; j, tooth; k, base into which muscles are inserted: C, Polynoid; U, upper, and L, lower jaws; j, tooth; k, base: D, Diagrammatic section across pharynx of Autolytus; E, of Nereis; F, of Glycera; G, of Polynoë.
In the Eunicidae, however, the numerous denticles are carried in a special pouch below the food tract, with which it communicates anteriorly.[323] They are arranged in an upper and lower series. The lower series (L) consists of a pair of flat plates (k) on each side partially embedded in and acted upon by muscles, with a harder enamelled piece—the actual lower "tooth" (j)—at its anterior end. The upper series (U) consists of several pieces, varying in shape and size in the various genera of this {271}family; but developmentally they result from modifications of two rows of small, similar pieces.[324]
The intestine is generally straight and cylindrical, and is usually constricted by the septa, if these are present. In the Polynoids the intervening sacculations become so long as to receive the name of "caeca," which, in Aphrodite, become enormously elongated (Fig. 142); there are eighteen pairs of them (c), each being a slender tube bent upon itself, giving off short branches and dilated distally, where it lies in the base of the parapodium.
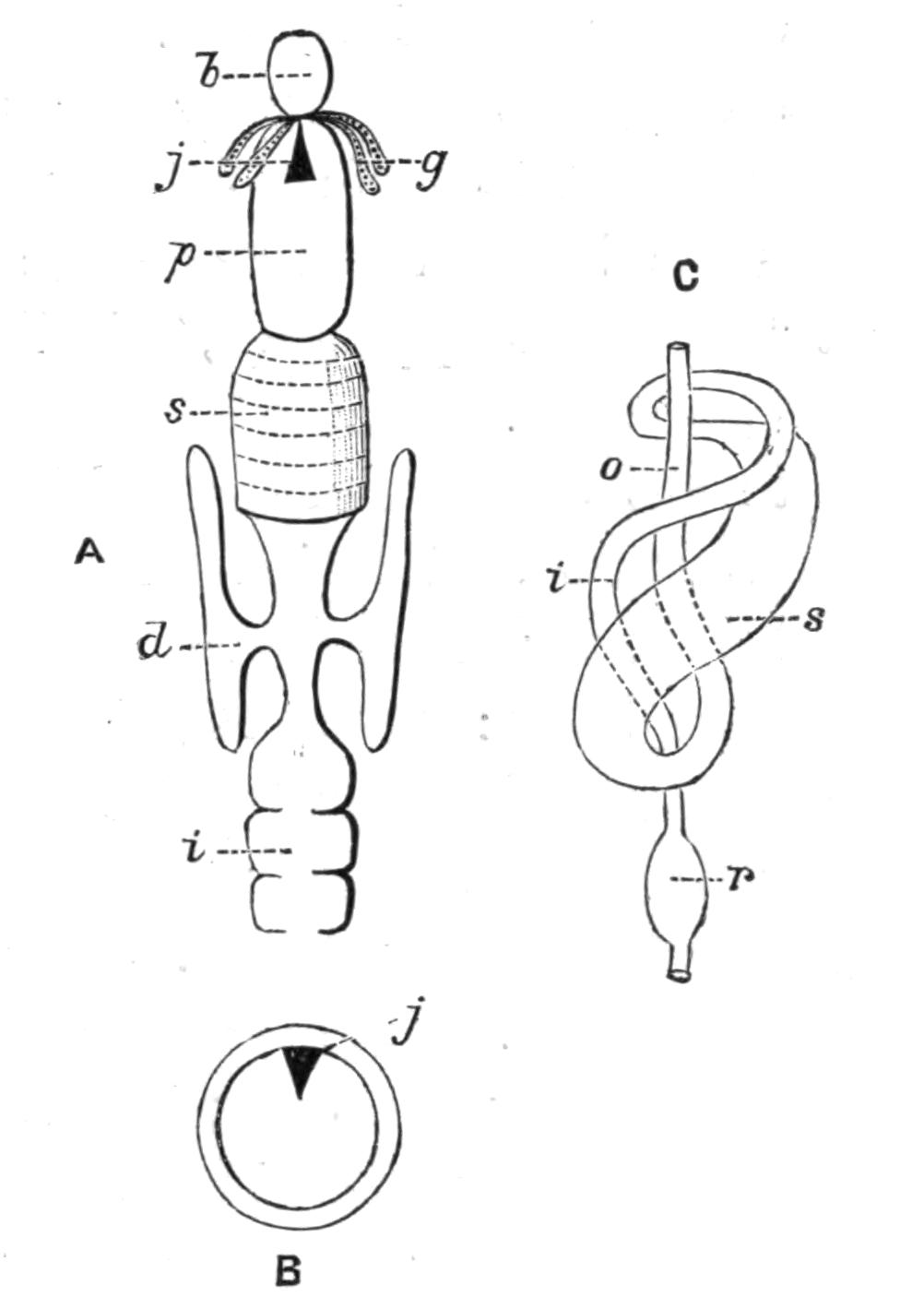
Fig. 141.—A, Alimentary canal of Syllid: B, transverse section of pharynx of the same; b, buccal region; d, oesophageal outgrowth; g, salivary glands; i, intestine; j, tooth; p, pharynx; s, gizzard: C, alimentary canal of Petta (after Wirén); i, intestine; o, oesophagus; r, rectum; s, stomach.
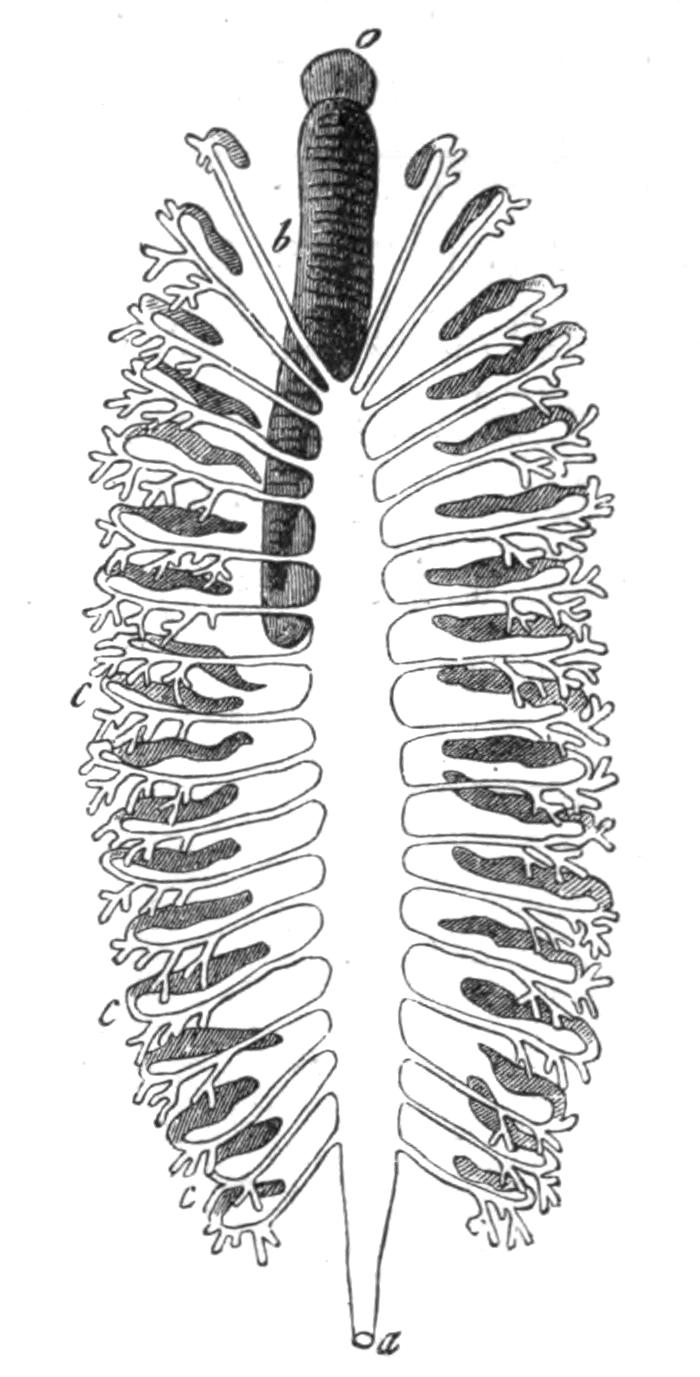
Fig. 142.—Alimentary canal of Aphrodite. × 1. (From Gegenbaur.) a, Anus; b, pharynx; c, caeca; o, mouth.
The intestine is looped in a few genera only, as in Trophonia, or coiled, as in Sternaspis, Petta (Amphictenid, Fig. 141, C), and Ammotrypane. In the course of the tube there may be a thick-walled muscular gizzard, with hard chitinous lining, as in certain Terebellids, where it appears to replace, in function, the pharynx of the Nereidiformia; in the Syllidae the gizzard is present in addition to the pharynx (Fig. 141, A).
Glandular appendages of the oesophagus are present in many {272}worms. Amongst the Nereidiformia, the Syllidae and Hesionidae possess oesophageal diverticula (Fig. 141, A, d), which are used, not for secreting a digestive fluid, but as reservoirs for water and air swallowed by the worms; and are provided with muscular walls, by which their contents can be driven out. They appear, in fact, to be used like the swim-bladder of fishes.[325] Many Chaetopods take in water by the anus—no doubt for respiratory purposes—and pass it forwards along the intestine. In the Capitelliformia a special groove conducts the water for some distance, then the groove becomes closed to form a canal, which, after a course forwards as a free tube below the intestine, again enters the latter, constituting a "siphonal apparatus," similar to that of the Echiuroids and the sea urchins.
Sense Organs.—In addition to the prostomial eyes, which are present in nearly all the Nereidiformia and Spioniformia, eyes may exist elsewhere on the body: thus Myxicola infundibulum and Fabricia possess a pair on the anal segment; in M. aesthetica Clap. there is a pair to every segment; in Branchiomma there is a compound eye near the tip of each gill filament (i.e. palp); whilst in Dasychone a series occurs along each gill filament. All these examples belong to the Cryptocephala, in which, owing to certain peculiar modes of life, these sense organs are required in correspondingly peculiar positions. It is usually stated that Polyophthalmus possesses, in addition to the usual prostomial eyes, twelve pairs on as many successive segments; but the minute structure of these organs points rather to their function as light-producing organs.
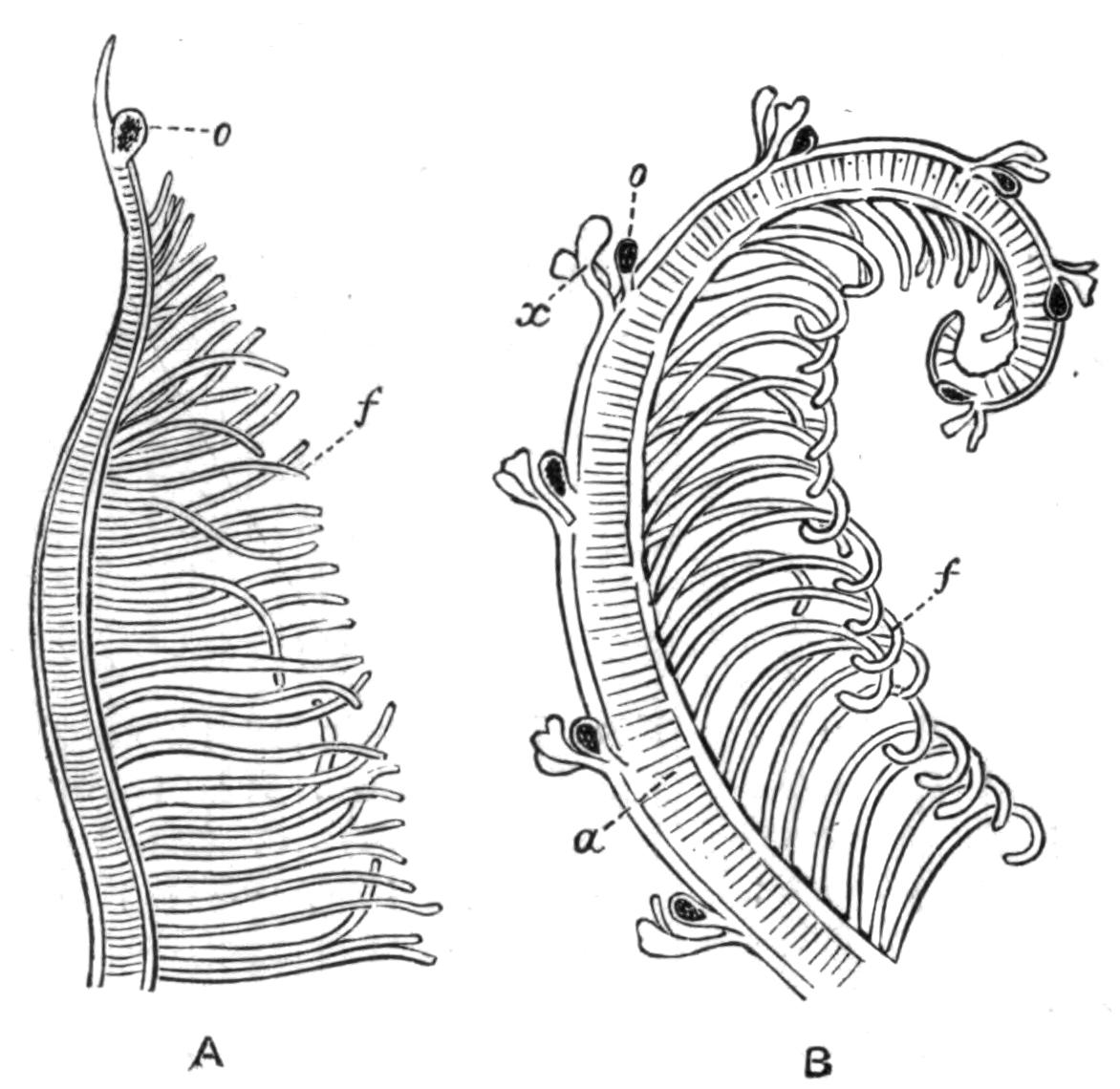
Fig. 143.—A gill filament, A, of Branchiomma, B, of Dasychone. a, Axis; f, secondary filaments; o, compound eye; x, lappets.
The Capitelliformia and Opheliidae possess a pair of peculiar "ciliated pits" or "nuchal organs" at the upper side of the head, {273}between the prostomium and peristomium, and capable of eversion (Fig. 144). They are most characteristically developed in the Capitelliformia, where each organ abuts upon a special lobe of the brain. The function of these "ciliated organs," which bear a great resemblance to those of the Nemertines, is a matter of speculation. Similar organs, in the form of simple pits or grooves, occur in many of the Nereidiformia, Terebelliformia, and others.[326]
Otocysts are rare. Arenicola possesses a pair at the base of the prostomium, each of which in some species retains an opening to the exterior.[327] They probably serve as "organs of direction" rather than of "hearing." Aricia and Polyophthalmus likewise have such organs on the prostomium; whilst Fabricia, Myxicola, Terebella, and a few others possess them in the peristomium, or in some other segment of the body.
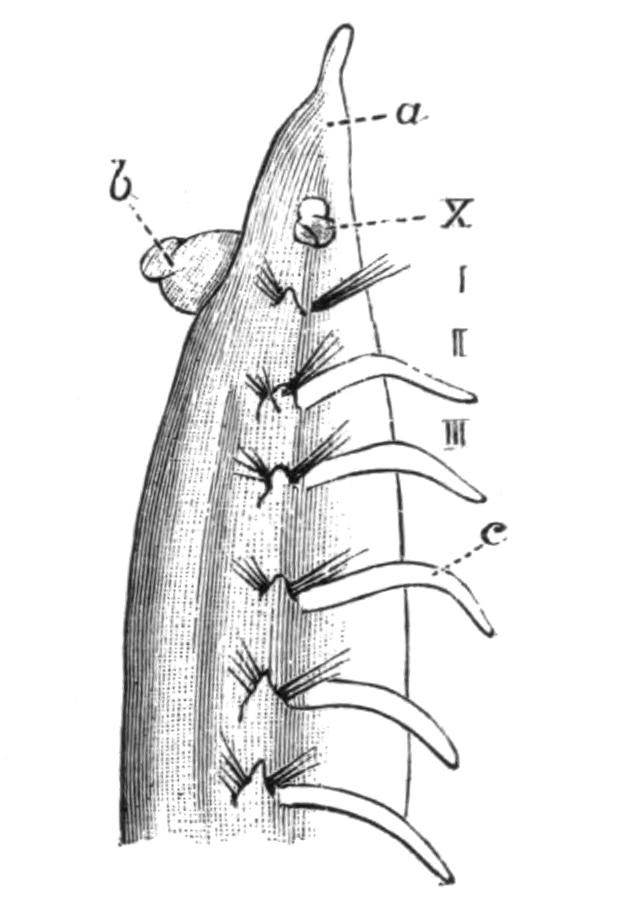
Fig. 144.—Ammotrypane aulogaster Rathke, enlarged. (From Cuningham.) Anterior end. a, Prostomium; b, everted buccal region; c, notopodial cirrus; X, ciliated organ everted; I, II, III, first three segments.
Reproductive Phenomena.—With a few exceptions mentioned below, the Polychaeta are unisexual. The sexual cells are developed in all cases from the lining epithelium of the body-cavity. The exact spot at which this occurs varies in different cases; it may be, though rarely, on the floor of the body-cavity; it is more usually on the wall of some blood-vessel, either the ventral vessel or on branches of it; or on the many blind blood-vessels of Aphrodite. The number of such genital organs is very great in most worms, but in those presenting two regions of the body they are confined to the posterior segments (Sabelliformia, Terebelliformia, Capitelliformia). The number is very limited in Arenicola and other worms presenting but few nephridia: in the former genus there being six pairs, in Trophonia only one pair.
The following genera are hermaphrodite:—Amphiglena, Salmacina, Protula, Spirorbis, belonging to the Sabelliformia, to {274}which must be added some Hesionidae. In this family ova and spermatozoa are developed around the same blood-vessel. But in the former group of worms (as also in Ophryotrocha) the two kinds of cells are produced in different regions of the body. Thus in Protula the anterior abdominal segments are male, the posterior ones female, while in Spirorbis the reverse arrangement holds; and in Syllis corruscans the anterior segments of the body contain eggs, whilst the posterior region contains spermatozoa, and this region separates and becomes a male worm.
The eggs and spermatozoa in the Polychaeta are discharged into the sea either by rupture of the body-wall or through the nephridia; the male and female elements unite, and the resulting fertilised eggs undergo development, either floating separately in the water, or embedded in jelly, or attached to the body or to the tube of the worm.
The result of the segmentation of the egg is a free-swimming larva known as a "Trochosphere," similar to that of Polygordius. The larvae of different species present various more or less marked departures from this type, for instead of the two girdles of cilia there may be only the anterior girdle, or there may be several complete or incomplete girdles between the two typical ones, or there may be (Chaetopterids) only a single girdle of cilia about the middle of the body, the two typical girdles being absent.[328] The postoral region, after elongation, generally becomes marked out into three segments, and these segments develop chaetae, which are usually temporary and specially long.
The little animal is thus equipped for an independent life: the provisional chaetae help in keeping it balanced; and in some cases (Spionidae) serve to protect the little soft creature, for when it is touched it curls up, and its chaetae stick out at the sides, so that it looks like a hairy caterpillar. But the larva is quite at the mercy of the sea, for it is carried hither and thither by currents, and in this way the species is disseminated. The larvae of the Polychaetes, like those of other animals, occur at certain periods of the year in large quantities at the surface of the sea, and serve as food for various larger animals.
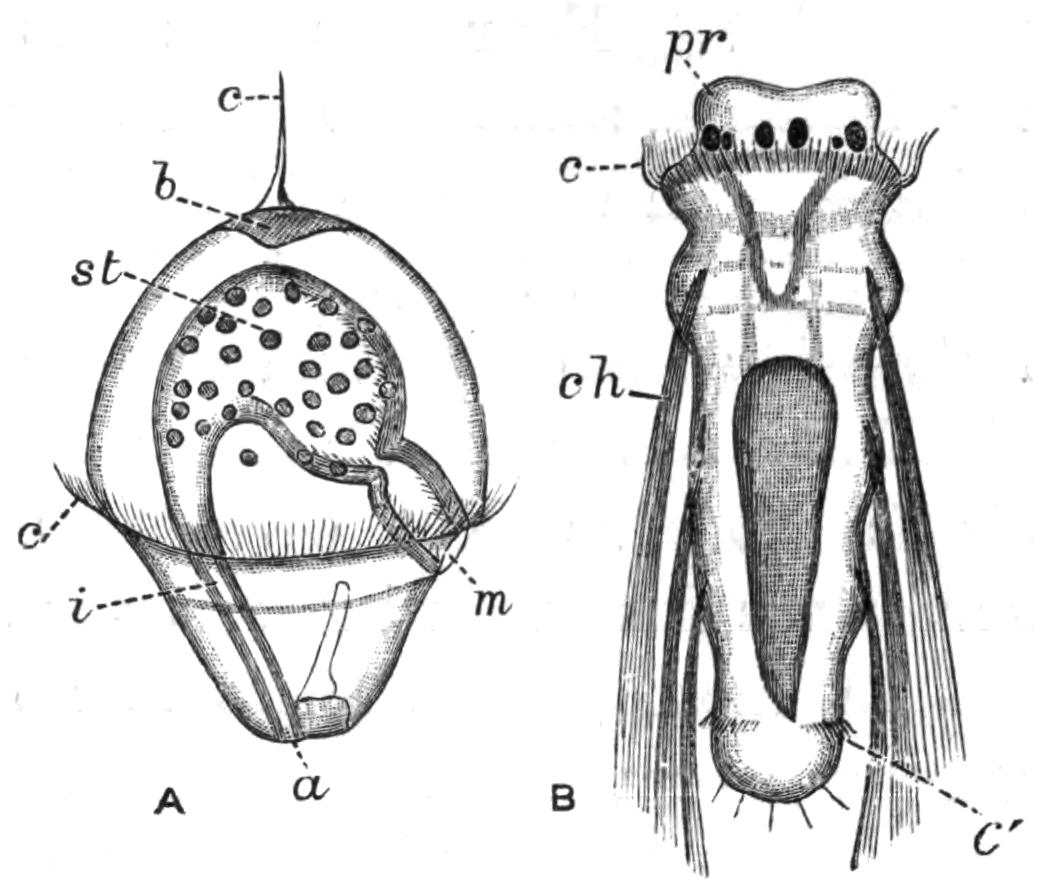
Fig. 145.—A, Trochosphere of Nephthys. × 65. a, Anus; b, apical plate (brain); c, apical tuft of cilia; c', girdle of cilia; i, intestine; m, mouth; st, stomach. B, Larva of Spio, with three segments, eight days old. × 100. c, Preoral girdle of cilia; c', preanal girdle; ch, long provisional chaetae; pr, prostomium with eyes. (From Claparède and Metschnikoff.)
These larvae are at first very different from the adult animal, and the necessary changes to be passed through are more or less great according to the species. It is not our intention to describe these changes in detail.[329] The larva increases in size, the permanent chaetae make their appearance in regular order, and the body exhibits segmentation, the new segments always appearing just in front of the anal segment. The internal organs gradually develop, and the prostomial and parapodial appendages grow out in their turn. In the Sabelliformia the multifilamentous "gills" arise by the continued branching of an at first simple process (the palp) arising from the latero-ventral surface of each side of the preoral lobe.[330] These gradually encroach dorsally and ventrally till the prostomium is more or less encircled; meanwhile the peristomium grows forwards so as to conceal the prostomium, which no longer increases at the same rate as does the rest of the body.
Although most worms appear to discharge their ova directly into the sea and take no further care of them, some make provision for their offspring either by laying the eggs in a jelly, which will serve as food for the young larvae—Aricia, Ophelia, Protula, Phyllodoce—or by attaching them to their body. In certain Polynoids the eggs are attached by means of a secretion to the back, under the elytra, where they undergo development up to a certain stage. In Exogone and some other Syllids they are attached to the ventral cirri, or in Grubea limbata, all over the back. In the female Autolytus (Sacconereis) a ventrally-placed brood sac is formed by the hardening of a {276}secretion; the eggs develop into embryos inside the brood sac, and then become free, with head appendages and three pairs of parapodia. Enormous numbers of such embryos may occur; for instance, some 300 were counted in a brood sac of Autolytus ebiensis. In the case of tubicolous worms, the eggs are frequently attached to the tube, either inside or outside. In Spirorbis and Salmacina the operculum serves as a brood pouch.
Only a very few species are known to be viviparous, viz. Syllis vivipara Kr., Cirratulus chrysoderma Clap., Marphysa sanguinea Mont., and Nereis diversicolor Müll.
In most genera there is no external difference between a mature worm filled with generative products and an immature one, except, it may be, in the colour; for the yolk of the eggs is frequently tinted yellow, or pink, or bluish, while the spermatozoa in mass are white; so that the normal colouring of the worm may be modified when filled with these elements. But in a few instances striking anatomical peculiarities are exhibited by the mature worm.[331] In many species of Nereis, for instance, those segments containing the generative products undergo more or less extensive changes, while the anterior ones remain unaltered. The body of the ripe Nereis is then distinguishable into an anterior non-sexual region and a posterior sexual region; and so great are these changes in certain species that the mature worms were for a long time believed to belong to a different genus, and received the name Heteronereis. But we now know their true relations, thanks to the work of Claparède and others. The males in the Heteronereid phase have fewer unaltered anterior segments than the females, so that there is a sexual dimorphism.
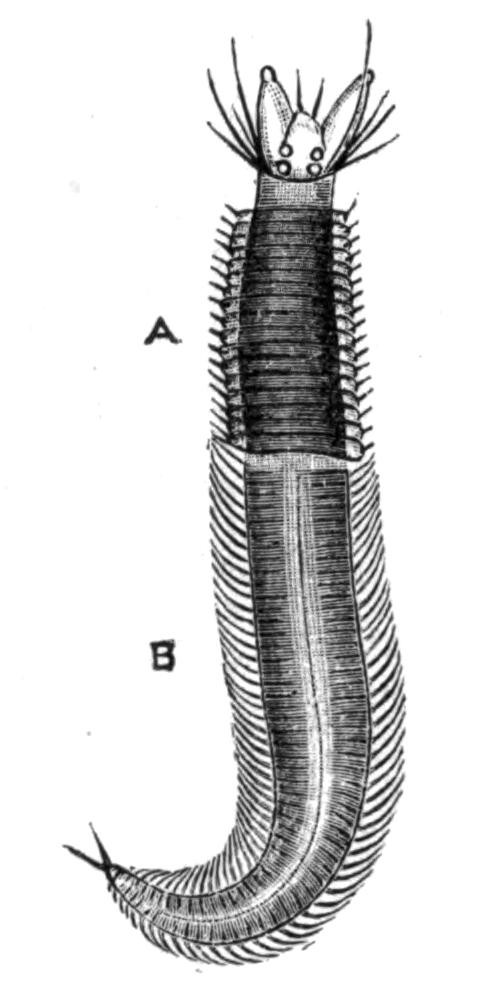
Fig. 146.—Male "Heteronereis" of N. pelagica L. × 1. A, Non-sexual region; B, sexual, modified region. (From Ehlers.)
The changes which Nereis undergoes in its transformation affect chiefly (a) the shape of the parapodia, and (b) the form of the chaetae of these parapodia. Other organs may also be affected, though less noticeably; thus the eyes become enlarged, the intestine may become so compressed by the generative {277}products as to be functionless, and the tail develops special sensory papillae.[332]
In the parapodia an increase in size and a sharper delineation of the various parts take place; then flattened foliaceous outgrowths (Fig. 147, x, y) arise from certain lobes of the feet, in which, too, the blood supply becomes greatly increased. The old chaetae are pushed out by the development of new ones of quite a different shape; these are jointed like the old ones, but the appendix is, in many species at least, flattened and oar-shaped (Fig. 123, C, p. 246); and the chaetae are arranged in a fan-like manner. Both these modifications are in evident relation to the free-swimming habit which the Heteronereid now adopts. The new foot serves as a swimming organ, the old one was a walking appendage.
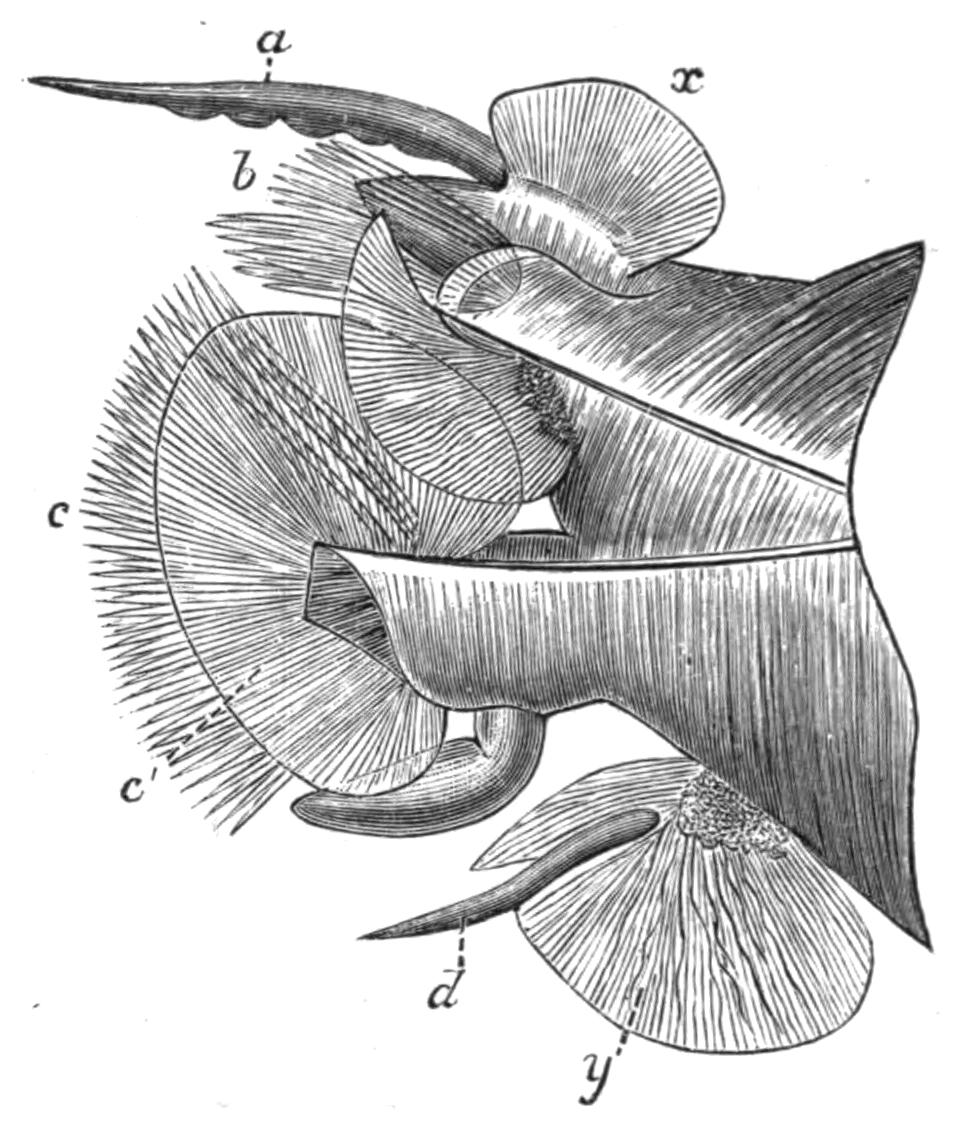
Fig. 147.—Parapodium of male "Heteronereis" of N. pelagica L. × 10. (From Ehlers.) a, Notopodial cirrus; b, notopodium; c, neuropodium with new chaetae; c', foliaceous outgrowth; d, neuropodial cirrus; x, y, foliaceous outgrowths.
Whilst some species, such as the common British N. diversicolor, undergo no change, and others become modified as just described, others, again, are polymorphic. Claparède was the first to show that N. dumerilii may occur in at least five different mature forms; these differ from one another in size, colour, mode of life, character of the eggs, etc. The immature forms may become ripe and lay eggs while still retaining the "Nereid" characteristics, or these immature forms may become "Heteronereids"[333] whilst the sexual elements are ripening. There are then three different kinds of males and of females in this one species, some being found at the bottom of the sea, as the large Heteronereid form, while the small Heteronereid swims on the surface. The relations of these various forms to one another, and the causes leading to the {278}assumption of a Heteronereid condition in some cases and not in others, are unknown.
A somewhat similar phenomenon is exhibited by members of the family Syllidae.[334] In this family sexual reproduction is frequently accompanied by the asexual modes of fission and gemmation. In some genera, such as Eusyllis, Odontosyllis, and Exogone, there occur changes quite similar to those characterising "Heteronereis"—that is, the posterior segments in which the genital organs exist become altered, so that the worm consists of two distinct regions, and is termed a "Heterosyllis." The most marked change is the appearance of a dorsal bundle of long capilliform chaetae in each of the genital segments (Fig. 148, I).
But in other genera the hinder genital region of the body becomes separated, on maturity, from the anterior non-sexual region. Various stages of this "schizogamy," or fission into a sexual and a non-sexual zooid, have been observed in different genera. In the genus Syllis the first segment of the sexual zooid, after its separation from the asexual zooid, proceeds to bud forth a head. The character of the head is alike in both sexes, though different species present heads of different shapes; and as the worms were originally described as distinct genera, the names then given are retained as descriptive terms. Thus the "Chaetosyllis" form has only two tentacles; the "Ioda" form has three tentacles and a pair of palps. One and the same species (e.g. S. hyalina) may successively pass through these stages.
With regard to the asexual portion, there is a regeneration of the tail segments after the sexual zooid has separated; and the number of segments so regenerated is usually equal to those that have become sexual. After a time these newly formed segments will produce generative organs, and take on the characteristic natatory chaetae, and this region will in its turn separate.
But in other genera, such as Autolytus, the regeneration of segments may commence before the separation of the sexual zooid; and the head of the sexual zooid becomes budded out before separation from the asexual portion. So that the animal now consists of two worms, each with its own head, separated by {279}a region or zone of proliferation (Fig. 148, IV). Moreover, in some species not only is the hinder part of the body converted into a sexual zooid, but the zone of proliferation becomes very active, and produces by gemmation a large number of segments, which become marked out, by the appearance of heads at intervals, into a number of zooids, in which genital organs will later make their appearance. A chain of as many as sixteen zooids may be formed in Autolytus (Fig. 148, V)—the hindermost by conversion of the hinder part of the body of the original "stock," the intervening zooids by gemmation.
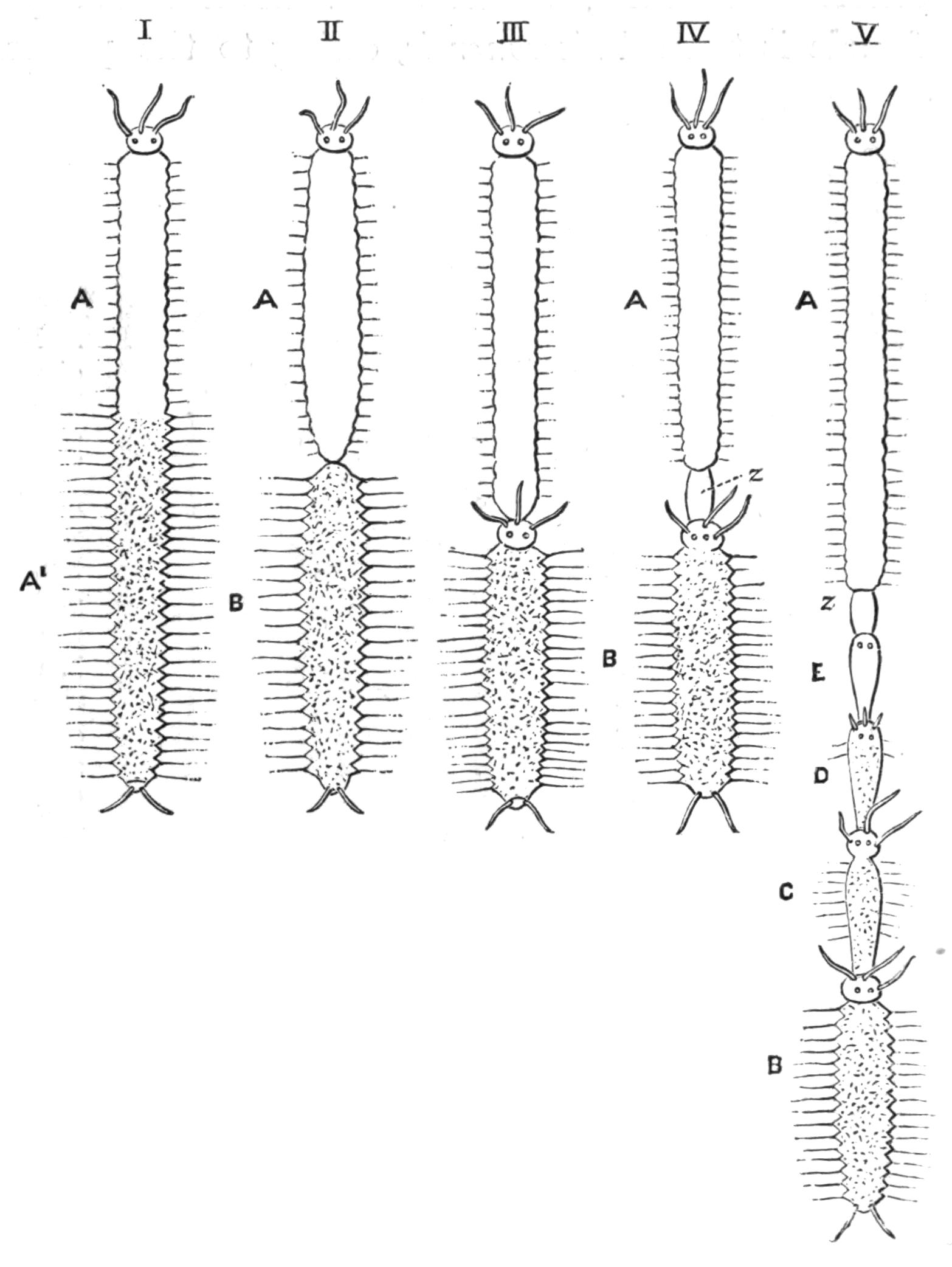
Fig. 148.—Diagrams illustrating the various stages in the asexual formation of a chain of zooids. (Modified from Malaquin.)
I, Heteronereid or Heterosyllid stage. A, Non-sexual; A', sexual region of the body, with modified parapodia.
II, Syllis. The hinder sexual region, B, is similarly modified, and will separate from the parent zooid, A, and become an independent zooid.
III, Autolytus. The hinder zooid, B, develops a head by budding before separation.
IV, Autolytus, etc. A zone of budding (z) makes its appearance in front of the head of B, and by its growth will give rise to a series of new segments in the middle of the body.
V, Myrianida, Autolytus, etc. From this zone of budding a very large number of segments have been formed, which have, further, become grouped so as to form three individuals, C, D, E; B is the hindmost zooid, which is either formed from the hinder segments of the parent zooid or is produced by budding, like C, D, E.
One original "stock," or asexual zooid, thus produces several sexual zooids, but these are only of one sex for a given stock. The males differ in several important characters from the females; so different, indeed, are the two sexes that before their history was {280}worked out by Agassiz[335] they were placed in different genera. The male zooid has thus come to be known as Polybostrichus (Fig. 149, B). It has three tentacles and two bifid palps; there are two pairs of peristomial cirri; the testes are confined to the four anterior segments, which are without natatory chaetae. The female is termed Sacconereis, owing to the possession of a great ventral brood sac; its head possesses no separate palps; the peristomium carries only one cirrus on each side; ova occur in every segment of the body, and may even extend into the hinder segments of the asexual zooid (Fig. 149, C).
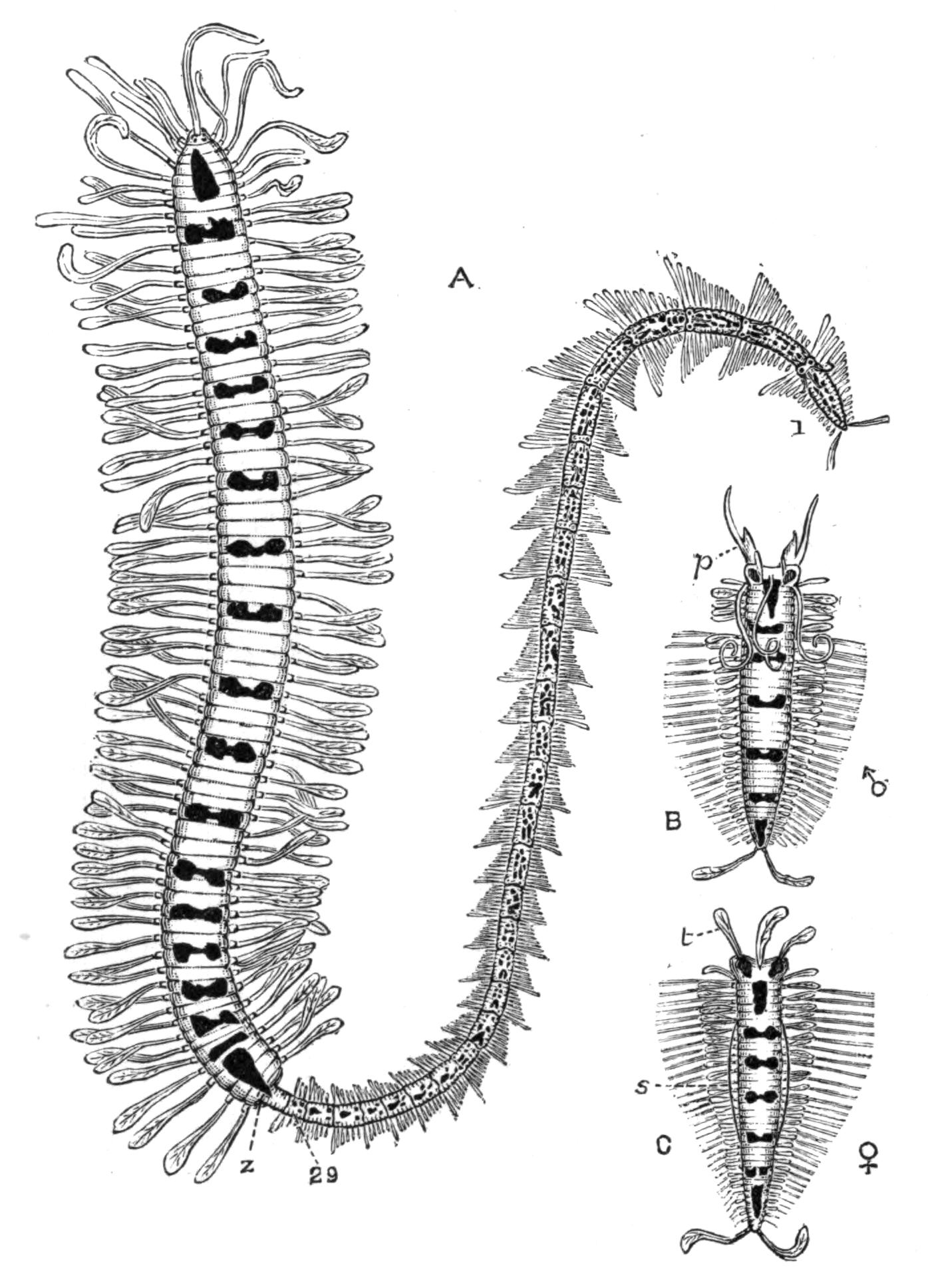
Fig. 149.—Myrianida fasciata. (From Malaquin.) The bright red markings of the living animal are here represented black. A, An asexual individual which has produced by budding from the zone (z) a chain of twenty-nine zooids, the oldest being labelled 1, the youngest 29. B, A ripe male zooid (Polybostrichus), with three tentacles and a pair of forked palps (p). There are five unaltered anterior segments. C, A ripe female zooid (Sacconereis) with the palps fused with the prostomium; s, the ventral brood pouch projecting on each side; t, tentacles.
A further development of this process of gemmiparity is exhibited by Myrianida. Here, there is no conversion of the hinder segments, but the normal preanal zone of proliferation gives rise to a large number of new segments. After a time the most anterior of these becomes a head, and thus a new zooid {281}is marked out. The zone of proliferation immediately in front of the new head now proceeds to form new segments, and a second zooid results. This process goes on till a considerable number of new worms have been formed at the tail of the original one, the oldest of these new ones being the most posterior, the youngest next the original "stock." In each zooid there is a zone of activity which adds to its number of segments, so that as we pass backwards the zooids increase in size. As many as twenty-nine such zooids may be formed in this way entirely by gemmation; and as each zooid becomes completed, genital organs make their appearance, and when these are ripe the zooid separates from the "colony" and leads an independent life. Here, as in Autolytus, the sexes are dimorphic, the male and female resembling those of that genus.
The process of gemmation, as seen in Autolytus, closely resembles that exhibited by certain Oligochaeta (Naididae), where there exists a definite alternation of generations; the production of new individuals by gemmation occurring throughout the greater part of the year, and sexual reproduction recurring only at certain intervals. In the Polychaeta such alternation exists in Myrianida; but it is only the terminal link of a series, which takes its starting-point in the process exhibited by the majority of Annelids, where no sexual character marks maturity. The next stage is presented by "epigamous" forms like Heteronereis and Heterosyllis; then "schizogamy" makes its appearance in certain Syllidae, resulting in the formation of two morphologically and physiologically distinct individuals which lead independent lives. The appearance of a head and of a zone of proliferation leading to the formation of a chain of sexual zooids is accompanied by a delay in the appearance of the genital organs, for in Autolytus these arise during the formation of the new individuals, as part of the general process of new formation; whilst in Myrianida the delay is prolonged, and the generative elements do not make their appearance till after the new individuals have reached some size.
More simple cases of the separation of the body into two parts, sexual and asexual, occur also in some of the Serpulidae. Thus in Filigrana and Salmacina the generative elements make their appearance in the hinder segments, as they do throughout the Sabelliformia; and this hinder part of the body separates {282}from the anterior region after the formation of a new head between the two regions.[336]
Another modification of the process of budding and fission is exhibited by Syllis ramosa, one of the most interesting forms of animal life which was obtained by the "Challenger." This worm consists of a main stem, whence arise a number of lateral branches, which may also branch so as to give rise to an arborescent colony (Fig. 150). The branches of the first and second and higher orders arise by budding from the sides of the original form or branches of lower order; and some of these branches develop generative products, and bud forth a head near the point of attachment. These sexual branches, no doubt, separate from the colony and distribute the ova. The worm lives in a Hexactinellid sponge, Crateromorpha meyeri, living in depths of 95 to 140 fathoms in the Eastern seas.[337]
Regeneration of lost Parts.—The process of budding and fission of the worm into two parts is merely an extension of that resulting in the formation of new segments when the worm is injured. In most of the Nereidiform Polychaetes the number of segments forming the body continues to increase throughout life by the formation of new segments between the anal segment and the one in front of it; that is to say, there is normally a process of budding taking place at this point. Now in many of the longer worms it may be noticed that the segments of the hinder end suddenly become smaller than the rest; these are segments newly formed to replace those lost by the worm. But this "regeneration," though the same in principle as ordinary growth {283}at the penultimate segment, is due to activity in a segment (any segment) further forwards; in other words, in the less modified worms every segment has the power of forming new tissues, just as each of the joints of a crab's leg has the power of forming the remaining joints when injured. It is not therefore surprising that a "zone of budding" arises in an uninjured worm at certain seasons, viz. that of reproduction; it is a property that each worm possesses, though generally it remains latent till injury provides the stimulus.
Moreover, not only can new segments arise at the hinder end, but a new head can be formed at the anterior end, as has been observed in worms belonging to many families—in the less modified Syllidae,[338] in others of the Nereidiformia, and even in Sabellids, where the greatly specialised gill filaments can be reproduced. Thus Sir J. Dalyell[339] noted in Dasychone that the crown of branchiae was regenerated in about a month in springtime, while in winter the process occupied 116 days. He cut a Dasychone into three pieces; the hindermost produced a head, the anterior piece developed an anus, and the middle portion formed both a head and tail!
These regenerated heads are of course at first smaller than the rest of the body, but soon grow to a normal size. Naturally this extensive power of regeneration is of extreme value to the Polychaetes, for if a fish or other enemy bites the head off a worm, a new one can form; and it is not difficult to see in this the origin of the reproduction by fission as a normal process.
NATURAL HISTORY OF POLYCHAETES—GENERAL HABITS—CHARACTER OF TUBE AND ITS FORMATION—COLOURING—PROTECTIVE AND MIMETIC DEVICES—PHOSPHORESCENCE—FOOD—USES—ASSOCIATED WORMS—WORMS AS HOSTS—DISTRIBUTION—FOSSIL REMAINS.
All the many hundreds of species of Polychaetes are marine, with a very few exceptions, which have been in recent years recorded from fresh (i.e. drinkable) water, viz. a species of Nereis from a lake in Mingrelia, another Nereis and a Lumbriconereis from running water in Trinidad,[340] a Sabellid, Manayunkia speciosa,[341] from Philadelphia; and another Sabellid, Coabangia,[342] from fresh water at Tonquin, which lives in borings in shells of Melania; and it is by no means improbable that other fresh-water Polychaetes exist in Lake Tanganyika in Africa, where a Medusa has recently been discovered.
In brackish water of various densities many Polychaetes live; Arenicola especially is regardless of the character of the medium, and Nereis diversicolor appears to withstand considerable admixture of fresh water.
The majority of the Polychaetes occur "inshore," that is, between tide-marks and in shallow water down to 20 fathoms; but they occur at all depths more or less abundantly, and some have been dredged from depths of more than 3000 fathoms.
The nature of the soil composing the shore has a good deal to do with the number of worms to be found there; thus in calcareous districts they are fewer than in places where harder rocks, {285}such as granite, form the shore line, for the chalk or limestone wears away more quickly, and exposes to destruction the worms which may have sheltered in its crevices: further, it does not give so permanent a place of attachment to seaweeds, on which many Polychaetes feed. The calcareous rocks, too, are more likely to be traversed by springs of fresh water, which is not to the taste of the worms. The sand resulting from the destruction of the rocks, whether hard or soft, is of itself unsuitable to the majority of worms, which are most abundant where mud containing decaying vegetable matter is mixed with the sand: this, which gives a firmer consistency to the soil, so that the burrows retain their form better, supplies food for the burrowers.
General Habits.—The division of the Polychaetes into the "Errantia" or free-swimming and wandering forms, and "Sedentaria" or tubicolous and sedentary forms, is a misleading mode of classification, for as a matter of fact only a comparatively few forms are really free-swimming throughout life; the majority, even if they do not form definite tubes, burrow galleries for themselves in the soil, and these burrows are in many cases only rarely left; this is true of both groups. Amongst the "errant" Polychaetes nearly all the Eunicidae secrete a parchment-like tube, and some Polynoids form mud tubes. Among the "sedentary worms" there are forms which merely burrow; while Myxicola readily leaves its gelatinous tube and swims freely; Pectinaria carries its house with it as it moves about, and Polycirrus, a Terebellid, does not form any tube at all.
Owing to their sedentary habits, quite a representative collection of genera may be made, especially at a spring tide, at any seaside place which is provided with a sandy shore, and with rocks and seaweed. The larger species, however, require to be dredged, and the best time is at night, for then many forms which during the day are concealed in their burrows, will be issuing forth to obtain food.
It may be useful to give instances of worms occurring in various situations between tide-marks. Throughout pretty well the whole of the area left uncovered by the tide, even up to nearly high-water mark in many parts of the coast, the cylindrical "castings" of sand and mud, forming little heaps, indicate the burrows of Arenicola, the common "lug-worm"; these "castings" have passed through the worm's body, having been {286}swallowed during the process of burrowing as well as for the purpose of obtaining food, as in the case of the earthworms. Rather nearer the water may be seen little tufts of sand-threads, about an inch high, springing from a short piece of cylindrical, sandy tube rising up out of the sand; this is the head end of the tube of Terebella conchilega (Fig. 153).
Amongst the rocks may be found loose stones of different sizes; on lifting them up, various kinds of worms may be brought to light, according to the locality, the time of year, the position with respect to the sea, and so on. Polynoë is pretty sure to be present somewhere near low-tide mark; the number of species is considerable, and their colouring very varied: but as the worms have a habit of remaining still on the under surface of the uplifted stone, the observer may easily overlook them.
Other worms occur below the stones, more or less buried in the sand or mud; for instance, a small Nereis may be lying in its temporary burrow immediately underneath, and will at once withdraw from the now injured part of the burrow; while deeper in the mud or sand, especially in rather highly-smelling mud, little red worms are abundant, such as Scoloplos, Nerine, Capitella, and others. By digging near low water one may find Nephthys, Glycera, and others burrowing or hiding in the soil.
In rock pools, or sandy stretches amongst rocks kept moist and cool by abundant Fucus, one may see under stones the red or yellow gill filaments of Cirratulus and of Terebellids protruding from their burrows and tubes, while other worms are to be met with in clefts of the rocks, and amongst the roots of Laminaria.
Still farther out, below low-water mark, where one must wade, can be seen the beautiful branchial crowns of various Sabellids protruding from their tubes; but care is necessary on approaching these worms, as eyes are, in many cases, present on the branchiae and a shadow is readily perceived; then the brightly-coloured tuft disappears, and only a piece of sandy or muddy cylindrical tubing remains to tell where the Sabella has withdrawn. In order to obtain the worms one must dig quickly and deeply before they have been disturbed; for the tube is of considerable length, and the inhabitant withdraws to the bottom of it. Some of these soft-skinned worms have the power of boring into hard rocks,[343] though by what means they do so is {287}uncertain.[344] Polydora ciliata makes a tube of mud projecting from the mouth of U-shaped galleries in chalk, limestone, shells, and even shale; it has no hard jaws or other structures sufficient to account for the holes, but it is possible that the specially strong chaetae on the sixth segment may be of some use in this work. Other lithodomous worms are Sabella saxicava and Dodecaceria concharum, which is a common little borer, forming galleries in oyster-shells, etc.
The tubes formed by these Polychaetes are very varied in constitution.[345] In some cases a mucus, which hardens to form a firm protective envelope, is secreted from special parts (e.g. the ventral gland shields of Terebellids and Sabelliformia), or from the greater part of the general surface of the body; in other cases the secretion serves to stick together particles of mud or sand, or shelly fragments, so as to form a more or less cylindrical tube (rarely branched), which is lined internally by the hardened "mucus," having the appearance of silk.
Fig. 151.—Clymene ebiensis in its tube (t) (from Règne Animal). a, Anterior, p, posterior end, which is, however, injured.
But the process of tube-making is not a simple one, for in many cases, at least, the worms exhibit definite powers of choice. Thus some species of Sabella choose only the very finest particles of mud; Terebella conchilega chooses fragments of shell and grains of sand; Onuphis conchylega employs small stones more or less of a size; Sabellaria makes use only of sand grains. Whilst some worms, like Terebella, Nicomache, and others, make a very irregular tube, Pectinaria builds a most remarkably neat house, open at each end, which it carries about with it, the narrow end uppermost (Fig. 152); the grains of sand are nearly all of the same size and only one layer in thickness, embedded in abundant "mucus," and with the outer surface quite smooth.
Sir J. Dalyell[346] made some most interesting observations on the method followed by sundry tube-formers in the building of {288}their tenements, and these observations, though made nearly half a century ago, have required very little addition or correction in modern times. In speaking of Sabella, he writes as follows:—

Fig. 152.—The tube of Pectinaria auricoma. × 3. (From M‘Intosh.) This is its natural position as carried about by the animal.
"Let a tall and ample crystal jar containing a Sabella be emptied of its contents and speedily replenished with sea-water; the animal, if in view, has retreated during the short interval; the orifice of the tube is closed, all is at rest. But soon after {289}replenishment it rises, to display its branchial plume still more vigorously than before, and remains stationary, as if enjoying the freshness of the renovated element, always so grateful—the harbinger of health and strength to those whose dwelling is there. The passing spectator would conclude that he now beholds only a beautiful flower, completely expanded, inclining towards the light like some of those ornaments of nature decorating our gardens. He pauses in admiration. But if a drop of liquid mud falls amidst the element from above, disturbing its purity, then, while the plume unfolds to its utmost capacity, does the animal commence a slow revolution, the body also passing around within the tube. Now are the thousands of cilia fringing the ribs [i.e. the secondary filaments] of the branchiae discovered to be in vigorous activity, and their office to be wondrous. A loose muddy mass is soon afterwards visibly accumulating in the bottom of the funnel; meantime the neck or first segment of the body, rising unusually high above the orifice of the tube, exhibits two trowels beating down the thin edge as they fold and clasp over the margin, like our fingers pressing a flattened cake against the palm of the hand. [This refers to the lappets of the peristomial collar.] During these operations muddy collections are seen descending between the roots of the fans [right and left gills] towards the trowels, while another organ, perhaps the mouth, is also occupied, it may be, in compounding the preparation with adhesive matter. Still does the partial or complete revolution of the plume above, and of the body within the tube, continue; the bulk of the muddy mass diminishes, activity abates; it is succeeded by repose, when the tube is found to have received evident prolongation."
Fig. 154.—Terebella conchilega Pall. Upper end of the tube (s) showing the anterior end of the worm. h, Its head; t, tentacles collecting sand grains (y) in their grooves; x, sand grains in mouth of worm; f, filamentous fringe of tube. (After Watson.)
The Terebellids use their numerous tentacles in searching {290}for particles of sand, etc.; each tentacle is grooved along its ventral surface, and the particle is conveyed along the furrow to the mouth. These particles are actually taken into the mouth, and mixed with some sort of secretion; on ejection again, each particle is placed by another tentacle in its position at the edge of the tube, and by means of its lower lip the Terebellid works it into place.[347]
But whereas the greater number of tubicolous worms make use of adventitious material wherewith to strengthen the wall of their tube, the Serpulidae secrete carbonate of lime from their tube-glands, and mould a tube of this substance. Amongst the Eunicidae the secreted substance is of itself strong enough to protect the animal; for in Hyalinoecia and species of Eunice the tube consists of a translucent, tough, parchment-like material.
Chemical analysis has been employed in a few cases to determine the substance composing the tube. In the case of Hyalinoecia (sometimes erroneously called Onuphis) the material consists of a phosphoric salt containing magnesia and a characteristic organic substance "onuphin"[348]; in Spirographis, a Sabellid, the name "spirographin" is given to its special secretion, whilst in Serpulids the organic base of the calcareous tube is "conchiolin."
Fig. 155.—Eunice tibiana Pourt. × ½. The branching tube (t) with the worm (w) protruding its head through one of several openings. (From Ehlers.)
The majority of worms are solitary, but there are a few instances of social worms—not that there is any co-operation or distribution of labour amongst the individuals, but they merely occur together in quantities; thus the sandy tubes of Sabellaria may form compact masses of several cubic feet, which, left uncovered by the receding tide, look like rocks upon the shore; as, for instance, at Paignton and Torquay. Filigrana implexa and Serpula uncinata similarly intertwine their calcareous tubes to form masses.
Whereas most worms live at the bottom of the sea, at various depths, a few are to be found at the surface. Purely pelagic habits are confined to a few families, viz. Tomopteridae, Typhloscolecidae, and the Alciopids and others amongst the Phyllodocidae; though Nectochaeta, one of the Polynoidae, and Ophryotrocha, one of the Eunicidae, are modified for this mode of life.[349] Several genera become pelagic during the breeding season. All these forms are excellent swimmers, and many of them are transparent.
The Colouring of Polychaetes.—The majority of Polychaetes quickly lose their colour in spirits, and become uniformly dull or light brown in museums. There are a few, however, which retain their brilliancy, like Aphrodite and Chloeia, but in both cases the coloration is due to the beautiful hair-like bristles ranged along each side of the animal; in the former the colours of the rainbow flash from specimens which have been kept in spirit for any length of time. The Polynoids, too, with their golden chaetae and pigmented scales, retain to some extent their characteristic colouring. But the colours of most Annelids are due to pigments in the skin, together with the haemoglobin of the blood, which are soluble, or otherwise changed, in alcohol; for instance, the bright greenish-blue tint of the common Phyllodoce of our coasts is changed to a rich chocolate brown; but such cases are rare, most worms becoming more or less decolorised.
The varied colouring in the Polychaetes, as in other animals, is due to a variety of causes. The red is in many cases due to haemoglobin of the vascular system showing through the transparent body; the green of the tentacles of the Sabellids and Chlorhaemids is similarly due to chlorocruorin. In other cases the contents of the intestine or the tint of the coelomic fluid may affect the colour of the worm. In Capitella the coloured excretory products are regained in the skin; in an Eunicid living in a yellow sponge, on which it feeds, the colouring matter is extracted and stored in the skin; in the same kind of way green caterpillars may owe their tint to feeding on green leaves. But many of the Polychaetes possess distinct pigments in the skin; thus in Arenicola the dark pigment {292}melanin has been recognised; in Cirratulus and Nereis certain lipochromes; whilst Eulalia viridis contains a pigment allied to bonellein. These various pigments yield different absorption bands when a solution is examined with the spectroscope; others, however, give no bands, but are distinguished by different chemical reactions.[350] The colour of the intestine of Chaetopterus has been stated to be due to "modified chlorophyll," but it is quite a different substance.
When seen in the living and healthy condition, however, these Polychaete worms vie with the very butterflies in their brilliant and beautiful colourings, and though our own worms are not lacking in beauty, many tropical and southern forms exceed them in gayness of tint. Bright reds, orange, yellows, greens, blues, rich violets, and sombre browns are all displayed.[351]
The handsome Terebella nebulosa of our own coasts is coloured bright red, sprinkled with white spots. Nicomache lumbricalis is pink, with red girdles. Eunicids are frequently red or brown, and the red gills along each side, together with a brilliant iridescence, render these worms very beautiful. Nereids present a great range of coloration, from light green to sundry tints of brown and red in various combinations. Amongst the Serpulids our common S. vermicularis is a very showy little worm, with its orange body, its red gills splashed with orange, and its orange operculum streaked with red; and a Southern form, Placostegus coeruleus, occurring at the Cape of Good Hope, is provided with beautiful lavender-blue gills. Our own Sabellids present examples of beautiful markings on the gills, in different colours or in different shades of the same colour. Amongst Polynoids, P. leucohyba, from the Antilles, has blue elytra; Hemilepidia erythrotaenia, a long worm from the Cape of Good Hope, has the anterior end of its body covered with light blue elytra, whilst the uncovered part is orange, with a broad magenta-red band along the dorsal surface.
The Phyllodocids are mostly very brightly coloured. The common P. lamelligera of our coast has a bluish-green body, with olive-green parapodia; but Lopadorhynchus erythrophyllum, {293}from Jamaica, has a blue body with red parapodia; whilst Notophyllum myriacyclum has a brown body with longitudinal dark-brown stripes and yellow parapodia. Both these worms live in coral reefs, where brilliancy of colour is one of the characteristic features of the fauna. Other worms are of various shades of green: the dark green Arenicola with red gills; the bright green Eulalia viridis; the deep green Amphinome smaragdina, from Jamaica; Gnathosyllis diplodonta, with its green and yellow body, serve as examples.
Patterns or "markings" may be exemplified by Lepidasthenia elegans (Fig. 156), and Myrianida fasciata, which has a bright red band on each segment (Fig. 149, p. 280). From this brief list of examples it will be seen that beautiful, and even brilliant, coloration is not confined to any particular mode of life; many of the most typically tubicolous forms, like the Terebellids and Serpulids, are as brilliantly coloured as the most typically free-swimming genera, like the Phyllodocids. Carnivorous forms like Amphinomids and Syllids present as wide a range of tint as the limivorous forms like Cirratulus, Sabella, or Maldanids. Shore-lovers, and deep-sea dwellers, and surface-swimmers, all exhibit equally bright or equally sombre tints; it is therefore difficult and rash to dogmatise on the "use" of these colourings to these animals, or to point to this worm as being protectively, to the other as being warningly, coloured; for we are too ignorant as to the habits of the worms.
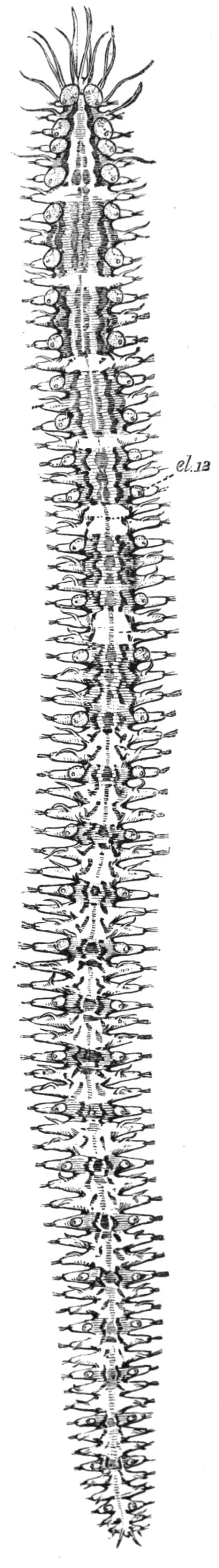
Fig. 156.—Lepidasthenia elegans Gr., × 2, to illustrate colour-markings: the dark bands in the anterior part of the body occupy two elytriferous, and the intermediate segments. In the hinder region, where the elytra are in every third segment, this one is dark. el.12, The twelfth elytron.
Protective and Mimetic Devices.—From the point of view of "protection" in the evolutionist's sense of the word, we can {294}say but little. Protective resemblance there is undoubtedly amongst the Polynoids, for the scales of these forms resemble more or less closely the stones or sand amongst which they live; in the same species there is great variety in coloration. This protective habit is carried still further in the case of Psammolyce by the attachment of sand grains to little cups on the elytra, so that the back of the animal is concealed. Certain commensals, such as Polynoë arenicolae, P. pentactes, are coloured so as to resemble their associates. In a few cases it is possible that the gills of Sabelliformia are protectively coloured; for in Sabella pavonia they vary from a light yellowish tint to a deep violet-brown, and the dark markings on them are therefore more or less distinct. Spread out as the gills are in life, they are in many cases difficult to recognise; it is rather their movement as they are withdrawn that attracts one's attention to them, as the tubes of these worms frequently serve for the attachment of brownish seaweeds, to which the gills bear resemblance. But, as a matter of fact, little work has been done in this direction, and speculation on the matter without evidence is worthless. Many pelagic forms, being transparent, such as Tomopteris and Alciopids, are no doubt protected by their lack of colour; yet these forms present brightly-coloured spots,—the light-producing organs in the parapodia of the former, and the large dark eyes of the latter.
Semper[352] mentions a case of possible mimicry in a species of Myxicola which lives in the clefts of a coral, Cladocora. The branchial funnel, when expanded, resembles very closely the expanded coral in size, colour, etc.; but he points out that the species occurs in other situations, where its colouring is not protective. Probably the "mimicry" is in other instances merely accidental.
No doubt many Polychaetes may be "warningly coloured," but experimental evidence is incomplete. Polycirrus aurantiacus is bright red, with orange tentacles; these worms were rejected by certain fish.[353] The animal has given up living in tubes as all its allies do, and it is the tentacles which appear to be distasteful to its enemies, for when irritated it coils itself up and wraps {295}itself round with its tentacles. Moreover, when the tentacles were cut off the fish did not reject the body of the worm. The tentacles are thus coloured in such a way that fish recognise them, and associate with the colour some distasteful property.
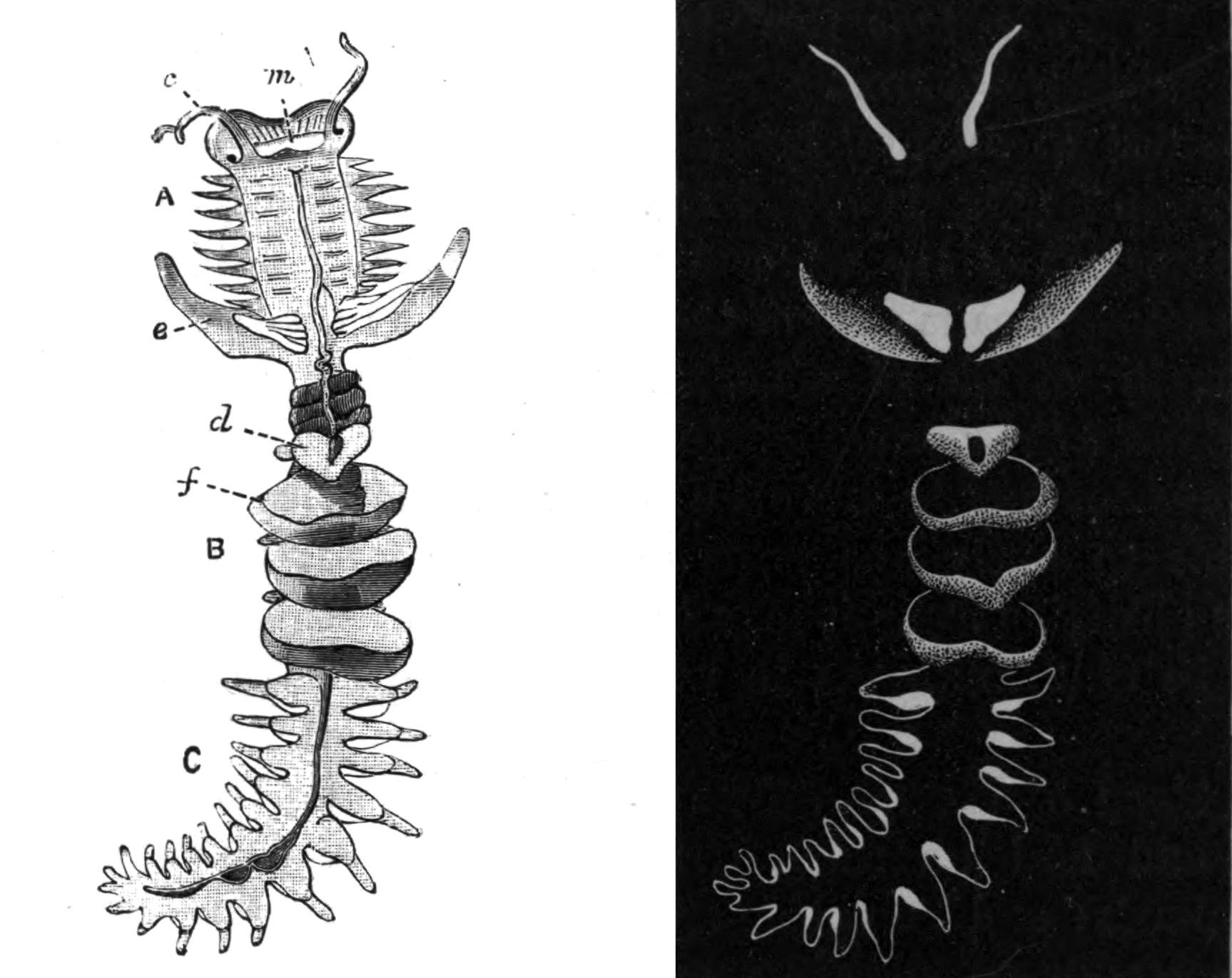
Fig. 157.—Chaetopterus variopedatus Ren. × ½. On the left the entire animal, with the three regions A, B, C. c, Peristomial cirrus; d, "sucker"; e, the great "wings"; f, "fan"; m, mouth. On the right the animal is represented in the dark, under stimulation, so as to exhibit the phosphorescent portions of the body. (From Panceri.)
Phosphorescence.—Many worms of very different habits have the power of emitting a light from some parts of the body, and they are then said to be "phosphorescent."[354] Probably Chaetopterus is most eminently photogenic; the base of the great "wings," the "fans," and other parts emit, on stimulation, an azure blue to greenish light, so bright that one may read one's watch by it. Several species of Polynoë exhibit a similar phenomenon, each elytron, with the exception of the area of attachment, being brilliantly illuminated. In these species the phosphorescent elytra are frequently thrown off by the animal, so that possibly they deceive enemies. Polycirrus aurantiacus produces a beautiful violet phosphorescence; usually its many tentacles alone show the light, but under strong stimulation the entire body takes {296}part in the display, and no doubt the phosphorescence has, like the colour, a "warning" purpose.
The production of the light in these various forms is apparently due to two different processes. In some cases, e.g. Chaetopterus, Syllids, Terebellids, it appears to be due to the oxidation of certain cell contents which are discharged more or less freely on irritation of the nerves; whilst in Polynoids the phenomenon is due to some purely nervous process, for the elytra have no glands, but are provided with ganglia and a nervous network.
In other worms, however, there are definite light-producing organs. In Tomopteris there is on each parapodium, above and below, a brightly-coloured spherical organ, which for a long time was regarded as an eye, but from its structure appears to be a "photogen" (Fig. 167, p. 315). The same is very likely the true explanation of the segmental "eyes" of Polyophthalmus, for their structure recalls that of the light-organs of deep-sea fishes.
As many of the phosphorescent Polynoids are commensals, while Chaetopterus inhabits tubes, and close allies of other phosphorescent worms have no power of emitting light, it is impossible to apply the same explanation of its purpose to all cases alike; in some it may be "accidental," though in others it may be of definite use in warning enemies or in attracting prey.
The Food of Worms.—The Nereidiformia are mostly carnivorous, and feed on small Crustacea, Mollusca, sponges, and other animals; and Polynoids are even said to eat one another. Many worms do not disdain various seaweeds, whilst the Spioniformia and Scoleciformia, which burrow in mud and sand, and are without biting organs, swallow the mud and digest what animal or vegetable débris it may contain. The Terebellids and Cryptocephala depend on minute organisms which may be driven into the mouth by the action of the cilia of the gills or tentacles.
In the case of deep-sea forms, it is an interesting fact that the intestines are not unfrequently crammed with Radiolaria and Foraminifera in a fairly fresh, uninjured condition, indicating that these Rhizopods do not merely sink to the bottom, but must actually live there.[355]
The economic purposes to which Polychaetes are put are few; they are used either as bait for fishes or as food for man.
One of the commonest baits used for certain fish, as all who have done any sea-fishing off the piers of our coasts know, is the common lug-worm (Arenicola marina), whilst Nephthys caeca and Nereis fucata are also used in some places; and for whiting Nereis cultrifera and N. diversicolor. Marphysa sanguinea, known to the fishermen in some parts as "varme," is less frequently used.
A peculiar worm—Palolo viridis—is used as food by the natives of Samoa and Fiji. The worm is similar to our Eunicid Lysidice ninetta, and lives in fissures among corals on the reefs, at a depth of about two fathoms. At certain days in October and November they leave the reefs and swim to the shores of the above islands, probably to spawn; and this occurs on two days in each of the above months—the day on which the moon is in her last quarter, and the day before. The natives, who call the worm "Mbalolo," give the name "Mbalolo lailai" (little) to October, and "Mbalolo levu" (large) to November, thereby indicating the relative abundance of the worms in these two months. The natives eat them either alive or baked, tied up in leaves; and they are esteemed so great a delicacy that presents of them are sent by the chiefs who live on shore to those living inland. A dark green-blue Phyllodocid, which is called "A'oon," occurs in abundance off Mota Island, amongst the New Hebrides, has similar habits, and is also eaten.[356]
Associated Worms.—A considerable number of worms live in association with other animals, either as commensals or as parasites, and it is not in every case possible to decide in what relation the two animals stand. Labrorostratus parasiticus, a Eunicid, is parasitic in the body-cavity of Odontosyllis ctenostomatus (Fig. 158); such an association between two members of the same group of animals is peculiar; but still more exceptional is the occurrence of Haematocleptes terebellides, as a parasite in Marphysa sanguinea, for both parasite and host are members of the same family, the Eunicidae. Another Eunicid, Oligognathus bonelliae, occurs in the body-cavity of the Gephyrean Bonellia.
The Polynoid Acholoe astericola and the Hesionid Ophiodromus flexuosus occur as ectoparasites (or perhaps commensals) in the ambulacral grooves of the starfish Astropecten aurantiacus. An Amphinomid is stated to live in the branchial chamber of {298}the barnacle, Lepas anatifera. Alciopina parasitica lives, during the early stages of its life-history, within Cydippe, and it is possible that most of the Alciopids thus make use of Ctenophores as their nurseries.
A considerable number of the Polynoids are ectoparasitic: P. castanea lodges in the peri-oral region of Spatangus purpureus, and in the ambulacral grooves of Astropecten; P. (Halosydna) bairdi lives between the mantle and foot of the mollusc Fissurella cratitia; P. pentactes is found on the body of the Holothurian Cucumaria pentactes, and appears to be protectively coloured. P. (Antinoë) parasitica lives under the elytra of another Polynoid, and P. acanellae on the coral Acanella normani.[357]
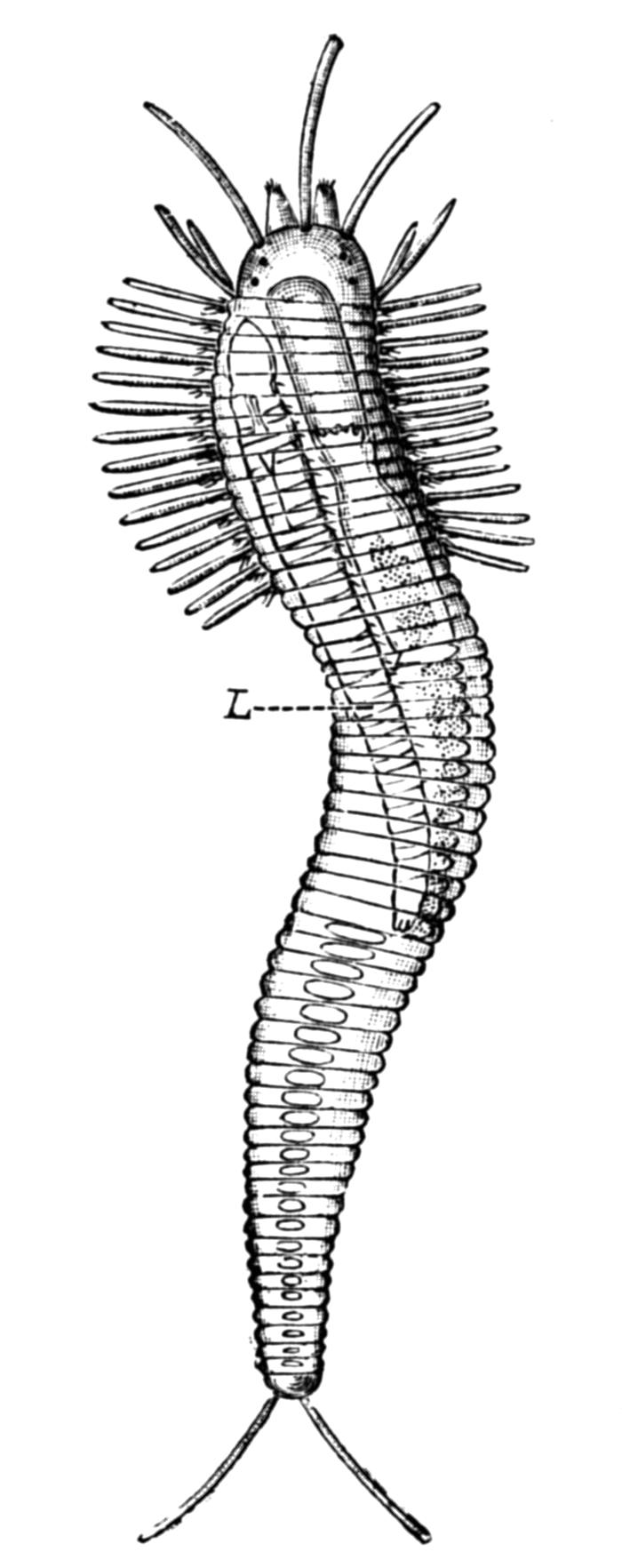
Fig. 158.—Odontosyllis ctenostomatus, with (L) Labrorostratus parasiticus in its body-cavity. The parapodia and cirri are omitted from the greater part of the body. (After St. Joseph.) × 4.
As commensals there may be mentioned Nereis fucata, which lives in the upper coil of whelk-shells which are inhabited by a hermit crab. The same shell usually bears a particular sea-anemone, so that there are three animals living together in or upon the cast-off house of a fourth. Siphonostoma is found in the "nests" made by the mollusc Lima. A Eunice is constantly associated with the coral Lophohelia prolifera, amongst the branches of which the worm twines its tube; whilst another Polychaete inhabits a tube formed by the interweaving of the fine branches of the coral Antipathes filix,[358] found in the West Indian seas. A species of Polydora forms its tube in Heliopora. The Polynoids present many instances of commensalism, a few of which may be here mentioned. P. johnstoni Marenz. is only found in the tubes of Terebella nebulosa; other species occur in the tubes of other Terebellids. P. marphysae lives in tubes of the Eunicid Marphysa sanguinea. Two species live in the tubes of Chaetopterus. P. extenuata has been found in tubes of Serpula vermicularis, while P. arenicolae occurs on {299}the body of the common lug-worm, with the colouring of which it closely harmonises.
Worms as Hosts.—The Polychaeta serve not only as food for fishes, Crustacea, and other predatory animals of larger size, but are also liable to be the hosts of parasites[359] such as Gregarines, and even, as we have seen, of other members of their own group. Sundry ectoparasitic Copepoda have been found attached to worms between the parapodia or to the sides of the feet, and an unnamed Copepod occurs attached, sometimes in considerable numbers, to the sides of Nereis cultrifera. The Polychaeta also act as protectors to other animals, for on the under surface of elytra of sundry Polynoids may very frequently be found specimens of Loxosoma, which may also be attached to gills of Eunicids; whilst below those of Aphrodite echidna and Hermadion pellucidum, Pedicellina belgica occurs. Under the felt of A. aculeata the Sabellid Branchiomma vigilans forms its tube, and Vorticellids may be found on chaetae, gills, or other parts of the body of sundry worms.
Distribution.—Very little can be said in a brief way of the geographical distribution of these worms, for many of the genera are cosmopolitan, although only a few species occur in all the great oceans, e.g. Polynoë imbricata, Hyalinoecia tubicola, Nerine (Scolecolepis) cirrata, and Terebellides stroemi.
As for species, it can be said generally that the different oceanic areas and even different coasts present different species, but we know practically nothing of variation amongst Polychaeta, and many so called species may be mere local varieties, for frequently the descriptions of "new species" are scarcely intelligible. At any rate we know that certain species occur at widely separated localities, for two or three species of Polynoids occur in Japan, and again at Dinard on the French coast. A considerable number of species are common to both sides of the North Atlantic ocean, having been obtained off Norway and in the Gulf of the St. Lawrence. A few of these which are common on our coasts may be enumerated:—Nereis pelagica, Nicomache lumbricalis, Glycera capitata, Thelepus cincinnatus, Scoloplos armiger, Sabella pavonia, Ophelia limacina, Aphrodite aculeata, Trophonia plumosa, Polynoë squamata, Capitella capitata, Sthenelais limicola.
As for bathymetrical distribution,[360] many genera occur at all depths, though Polychaetes appear to be most abundant, as far as we know at present, in "shallow water"—that is, down to twenty fathoms or so; but this may be due to the greater facility of collection on shore and in these slight depths, for the "Challenger" obtained considerable numbers of new species at greater depths.
The "deep-sea" forms are chiefly tubicolous, and since these tubes are fixed and partially embedded in the bottom, probably comparatively few are brought up. Some genera occur at very great depths; thus the Terebellid Leaena abyssorum and the Serpulid Placostegus benthalianus were brought up from 3125 fathoms—the greatest depth from which Polychaetes were obtained by H.M.S. "Challenger"; and it is interesting to note that species of each of these two genera occur in shallow water, the Serpulid being represented in our own coast fauna by P. tricuspidata.
Amongst our own fauna, a few examples may be given of the "replacement of species."[361] The littoral Sthenelais boa is represented by S. limicola in deeper water; Sabellaria alveolata by S. spinulosa; Polynoë imbricata by several deep-water species. Similarly with genera: the littoral Pomatoceros is replaced by Serpula in deeper water; and the Hesionid Psamathe by Castalia.
The limitation of species to certain regions, or to certain depths of an ocean, may appear at first sight peculiar, in view of the unrestricted communication between all its parts; but there are as efficient "barriers" there as on land, for generally a particular worm can live only in a certain temperature and at a certain pressure, and is dependent for its food on particular organisms, which in their turn depend on the depth and its accompaniments. It is, in fact, so much the more peculiar that certain species are more or less cosmopolitan, or occur at widely distant points. It is less peculiar, of course, to find different species of the same genus at different depths or in different areas, for any slight variation in a species advantageous to new conditions would readily be fixed, and give rise to a new species.
The distribution of the Polychaeta depends probably on the pelagic larvae, which are carried by currents from one part of an ocean to another. There can be little doubt that many {301}Polychaetes are very "plastic," and can adapt themselves to changed conditions of life with considerable ease; for Nereis diversicolor, Arenicola marina, and others live equally well in water of very different densities, and with a different food supply. The great variety in the "habitats," and presumably therefore in their food supply, etc., exhibited by many Polychaetes, as well as the great variation observable in some species of Polynoina, and the close affinity of the species and genera of this sub-family, lead us to the same conclusion.
Extinct Polychaetes.—The most numerous fossil records of the Polychaetes are calcareous tubes of various shapes and sizes; they are irregularly or spirally curved, and are very usually attached at one end, or by one surface, to stones or to fossils. These tubes belong to the Serpulidae, and are referred to the genera Serpula, Spirorbis, Ditrupa, and others.[362]
Spirorbis is the oldest unequivocal representative of the Polychaetes, as its tubes are found more or less abundantly in the Silurian and other Palaeozoic strata. In Palaeozoic times Serpula was rare, as it was too in the Trias and Lias, but in the Jurassic strata it becomes abundant. In the chalk, S. socialis may occur in masses like S. uncinata of the present day, forming "Serpulite chalk." In the older tertiaries the genus is represented by Spirulaea.
Terebella lapilloides occurs in the Lias as a cylindrical, more or less curved tube of sand-grains.
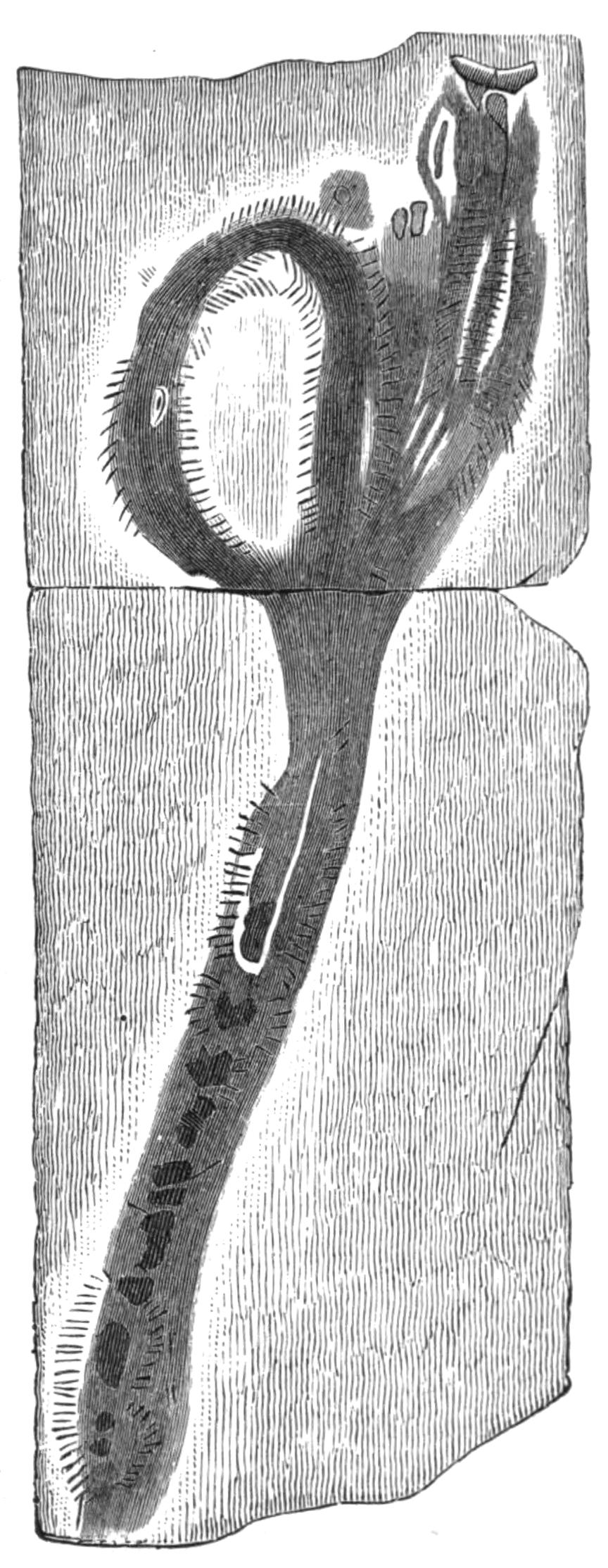
Fig. 159.—Eunicites avitus Ehl. A fossil worm from the lithographic slate of Solenhofen: the jaws are seen in front, and the acicula along each side. (From Ehlers.) Natural size.
Amongst the Nereidiformia the remains are fewer, but the {302}acicula and the hard jaws are preserved in certain rocks, and can be referred to existing families. Eunicites avitus[363] is represented by a double series of acicula, indicating the parapodia of the two sides; and by remains of both upper and lower jaws (Fig. 159). Four different species of the worm have been described from the lithographic slate of Bavaria, of Jurassic age; and several upper jaws of other Eunicids have been discovered in the Palaeozoic beds of Canada and Scotland, and have received the names Lumbriconereites, Oenonites, and Arabellites, in reference to their nearest allies amongst living genera.
There are, however, numerous remains, in the forms of tracks or casts, in the earlier rocks, which have been referred to the Polychaeta. The names Crossopodia, Myrianites, Nereites, Phyllodocites, have been given to some of these traces, though they are open to numerous other interpretations. Some of the "tracks" are similar to those made by living Crustacea in walking over wet sand; others appear to be the casts of some animals. Tubular burrows in rocks or fossils, some straight, others U-shaped, have received such names as Arenicolites, Scolithus, Histioderma; whilst under the name Lumbricaria certain cylindrical, coiled structures, resembling worm "castings," are met with in this same lithographic stone of Solenhofen. Many of the tubes referred to Polychaetes by the earlier palaeontologists have been transferred to other groups; thus Cornulites is now believed to be a Pteropod shell.
This very meagre geological record is quite insufficient to form any basis for a phylogeny of the group. And this poor supply of remains is not surprising, when we consider the soft nature of the tissues, the absence, in the majority of families, of skeleton and of other parts which could have been fossilised; yet we might have expected a greater abundance of fossilised jaws than is represented at present. But it must be borne in mind that the conditions of life of these soft-bodied animals are not conducive to their leaving abundant fossilised remains.
CHARACTERS OF THE SUB-ORDERS OF POLYCHAETES—CHARACTERS OF THE FAMILIES—DESCRIPTION OF BRITISH GENERA AND SPECIES—THE MYZOSTOMARIA.
Systematic.—The Order Polychaeta may be divided into two branches, in one of which, the Phanerocephala, the prostomium retains its ancestral condition as a lobe overhanging the mouth, and frequently carries, in addition to paired eyes, certain sensory processes of a simple structure, the tentacles and palps; the body-segments are more or less alike, and (except in some Spioniformia, some of the Terebelliformia, and the Capitelliformia) do not present two sharply marked regions, owing to the differential arrangement or character of the chaetae. In the second branch, the Cryptocephala, the peristomium grows forwards during development, so as to compress or even hide the prostomium, which thus becomes a very insignificant organ. The tentacles are reduced, but the palps become greatly developed and take on sundry new functions. The body in this group, by the character and arrangement of the chaetae, is distinguishable into a thorax and abdomen, presenting certain internal differences.
These two branches may be supposed to have arisen from a common ancestor having a general resemblance to a nereidiform worm, such as Syllis, possessing palps and tentacles on the prostomium, definite parapodia and cirri on the body, and internally, a well-marked and regular repetition of organs.
The branch Phanerocephala contains the following five sub-Orders, though it is possible that the Capitelliformia deserves a more important position in the system:—
Sub-Order 1.—The Nereidiformia have well-developed tentacles and palps; the peristomium almost invariably possesses {304}special cirri; the parapodia are well-marked locomotor organs, supported by acicula, and carry dorsal and ventral cirri. The chaetae are usually jointed, though unjointed ones may coexist with these; uncini are never present. An eversible buccal region leads into a muscular pharynx, which in the majority is armed with chitinous jaws; the septa and nephridia are regularly repeated throughout the body. The worms lead a predaceous life, and are mostly carnivorous; a few form tubes.
Sub-Order 2.—The Spioniformia possess neither tentacles nor palps; the peristomium usually carries a pair of long tentacular cirri, and extends forwards at the sides of the prostomium. The parapodia project only to a slight degree; the dorsal cirri may attain a considerable size, and act as gills throughout the greater part of the body. The chaetae are unjointed; uncini are only present in the aberrant Chaetopterus.[364] The body may present two regions more or less distinctly marked externally, but without corresponding internal differences. The buccal region may be eversible, but there are no jaws. Septa and nephridia are regularly developed. The worms are burrowers, or tubicolous.
Sub-Order 3. Terebelliformia.—The prostomium is a more or less prominent lobe (upper lip) with or without tentacles but without palps. The peristomium may carry cirri or "tentacular filaments."[365] The parapodia are feebly developed; there are no ventral cirri; the dorsal cirri may exist and function as gills on more or fewer of the anterior segments. The chaetae are unjointed, and uncini are usually present. The buccal region is not eversible; there are no jaws. The septa are usually incomplete, with the exception of one strongly-developed "diaphragm" anteriorly; the nephridia are dimorphic, those of the anterior (prediaphragmatic) segments are of large size and are excretory; the posterior series are mere funnels, and act as genital ducts. These worms are burrowers or tube-formers, and in the majority the tube-forming glands are grouped on the ventral surface of the anterior segments to form "gland-shields."
Sub-Order 4.—The Capitelliformia have no prostomial processes, but possess a pair of large retractile "ciliated organs." The parapodia do not project; the chaetae are unjointed, and are hair-like in the anterior segments and hooded "crotchets" posteriorly; this external division of the body does not correspond with definite internal differences. There are no cirri, though special "gills," often retractile, are frequently present. The buccal region is eversible; there is no armed pharynx. An "accessory gut" or "siphon" exists. The nephridia are small, and sometimes more than one pair in a segment; special genital funnels exist in more or fewer of the anterior segments of the hind body. There is no system of blood-vessels; the coelomic corpuscles are red. The worms are burrowers.
Sub-Order 5.—The Scoleciformia possess a prostomium, which rarely (Chlorhaemidae) carries any sensory processes; the peristomium is without cirri (except, perhaps, in the Chlorhaemidae). The parapodia are ill developed, and may be absent; only rarely are dorsal cirri present, acting as gills; ventral cirri are absent. The chaetae are unjointed; true uncini are not present. The buccal region is eversible, but there is no armed pharynx. The septa are not regularly developed, as more or fewer are absent, and the nephridia are considerably reduced in number, it may be to a single pair (Sternaspidae and some Chlorhaemidae), but they are all alike.[366] The worms are mostly burrowers.
The branch Cryptocephala contains two sub-Orders:—
Sub-Order 1. Sabelliformia.—The prostomium is entirely hidden by the forward extension of the peristomium; the tentacles are very small, being frequently represented merely by small knobs of sense-cells; the palps, on the other hand, are greatly developed, branched, and contain blood-vessels, acting as respiratory as well as sensory organs. The peristomium never carries cirri or chaetae, and it is usually raised up into a projecting collar, used in fashioning the lip of the animal's tube. The parapodia are but feebly developed; cirri are absent, except in the Serpulidae, where the dorsal and ventral cirri become united to form the "thoracic membrane" (Meyer). The chaetae are of two kinds—unjointed, hair-like, fringed bristles and "uncini." {306}By their arrangement the body is divided into a thorax of nine segments and an abdomen; in the former the capillary chaetae are dorsal, and in the latter ventral. The buccal region is not eversible; there is no pharynx. The septa are regularly developed in the abdomen, but are absent in the thorax; the nephridia are dimorphic; there are two large ones in the thorax opening by a median dorsal pore just above the brain; those of the abdomen are small funnels, and act as genital ducts. The worms are tubicolous; "gland-shields" are present on the thoracic segments.
Sub-Order 2. Hermelliformia.—The peristomium (Fig. 135) is enormously developed, and forms a bilobed hood capable of closing over the mouth; the truncated free end of each lobe carries three semicircles of peculiar chaetae, which act as an efficient protection when the worm is withdrawn into its tube. The prostomium is very small, but retains a pair of well-developed tentacles; the palps, which are subdivided as in the Sabelliformia, have become fused with the ventral edges of the peristomium, and appear as a series of ridges on each side, carrying numerous filaments. The thorax consists of five segments, the notopodia of three of which are well developed and bear strong chaetae; dorsal cirri are present along the greater part of the body, and act as gills. The arrangement of the chaetae and of the internal organs is as in the Sabelliformia. The worms form tubes of sand.
BRANCH A. PHANEROCEPHALA.
Sub-Order 1. Nereidiformia.[367]
Fam. 1. Syllidae.—These are small worms, the majority being less than an inch long, so that they are not easily observed. {307}The body consists of a fair number of segments.[368] In many genera a dorsal bundle of unjointed, natatory chaetae makes its appearance at maturity. The palps, which are grooved, are in some cases so united with one another and with the prostomium as to be scarcely recognisable. (For head see p. 262, and for feet see p. 264.) The pharynx is armed with one or more teeth. There is a special gizzard, following the pharynx, and provided with thick, muscular walls of peculiar structure. Following the gizzard, the oesophagus receives in many genera a pair of T-shaped diverticula, that are used for storing water, which is swallowed with food. These diverticula are absent in Autolytus and other free-swimming forms. The reproduction of the members of this family is interesting, and has already been described (p. 278).
Syllis.—The tentacles and cirri are moniliform; the palps large; there is a single dorsal tooth, which is provided with a poison gland, the duct of which opens near its apex; it is used rather for stabbing its prey than for grasping and tearing. S. krohnii Ehlers, is abundant under stones, and forms tubes of sand; it is nearly an inch long, and consists of some eighty-five to ninety-five segments marked with yellow bands. It may readily be identified by longer dorsal cirri, terminally dilated, alternating with shorter ones. S. cornuta Rathke, has a translucent green body, about half an inch long; no alternation {308}of cirri. Mediterranean, Atlantic, on the Norwegian coast, off Spitzbergen, and on the Madeira coast. S. armillaris Müll. is very common at low water; it is pale yellowish-brown, with a couple of dusky marks on each segment; and measures 2 inches. The dorsal cirri are quite short, consisting of only eight to ten joints. In Pionosyllis the tentacles and cirri are not moniliform; a single dorsal tooth. P. malmgreni M‘I. under stones. Sphaerosyllis.—The dorsal cirri are swollen at the base, and are not moniliform; the long palps are fused along nearly their whole extent. S. hystrix Clap. is only about one-eighth of an inch in length. Exogone Oerst. Grubea Qfg.
Autolytus.—The small palps are entirely fused with the prostomium; the pharynx, which is bent upon itself, is armed with a circle of denticles. Dorsal cirri somewhat foliaceous. There are no ventral cirri. The male and female differ from one another and from the asexual "stock" (see p. 279). A. pictus Ehl. is abundant under stones. It measures about two-thirds of an inch in length, is darkly coloured with a median lighter band; the anterior dorsal cirri are long. A. prolifer Müll. is common.
Myrianida fasciata Milne Edwards, with its foliaceous cirri, occurs off our coasts (see Fig. 149, p. 280). Atlantic, Mediterranean.
Fam. 2. Hesionidae.—The body is relatively short, with only a few segments (sixteen to fifty, according to the genus); in the larger forms it is cylindrical. The parapodium is usually uniramous; the dorsal cirri are long and multiarticulate; the chaetae are jointed. The prostomium carries, in addition to four eyes, two or three tentacles, and generally a pair of jointed palps. The peristomium and two or more of the following segments are achaetous, and carry long "peristomial" cirri. The pharynx is very long but unarmed.
Psamathe Johnston, has many segments; head with two tentacles and a pair of three-jointed palps. P. fusca Jnstn. occurs amongst coralline Algae, to which it bears some resemblance, which is heightened by the moniliform cirri. It is a small worm, less than an inch in length. Mediterranean. Castalia punctata Müll. is dirty green or brownish, with a narrow purplish band on each side. It occurs in deeper water than the preceding. In Ophiodromus the head has three tentacles; the palps are two-jointed; there are six pairs of peristomial cirri; the parapodia {309}are biramous. O. vittatus Sars is dredged in numbers off the Scotch coast, and is found also at low tides. It measures 2 inches in length. A closely allied species lives in the ambulacral grooves of the starfish Astropecten.
Fam. 3. Aphroditidae.[369]—The most characteristic feature of this family, and one by which its members are absolutely distinguished from all other Chaetopods, is the possession of scales or "elytra" on the back. These flattened dorsal cirri are of a somewhat horny texture, and are carried, generally, on alternate segments of the body; filamentous cirri occurring on the other segments. In the sub-families Hermionina and Polynoina the elytriferous segments are 2, 4, 5, 7, 9, etc., up to 23; then every third segment. The worms are usually short, with some thirty-five to forty-five segments, though Sthenelais and a few others have many more. (For head see p. 262, and for parapodium see pp. 265, 268.) The pharynx is very thick walled, and furnished with two pairs of jaws, which are, however, not hardened in the sub-family Hermionina. The intestine is provided with a number of paired longer or shorter caeca (Fig. 142). A considerable number of this family are commensal or parasitic (see p. 297). The family is well represented on our own coasts, so that only a few of the more readily distinguishable species can be here described.
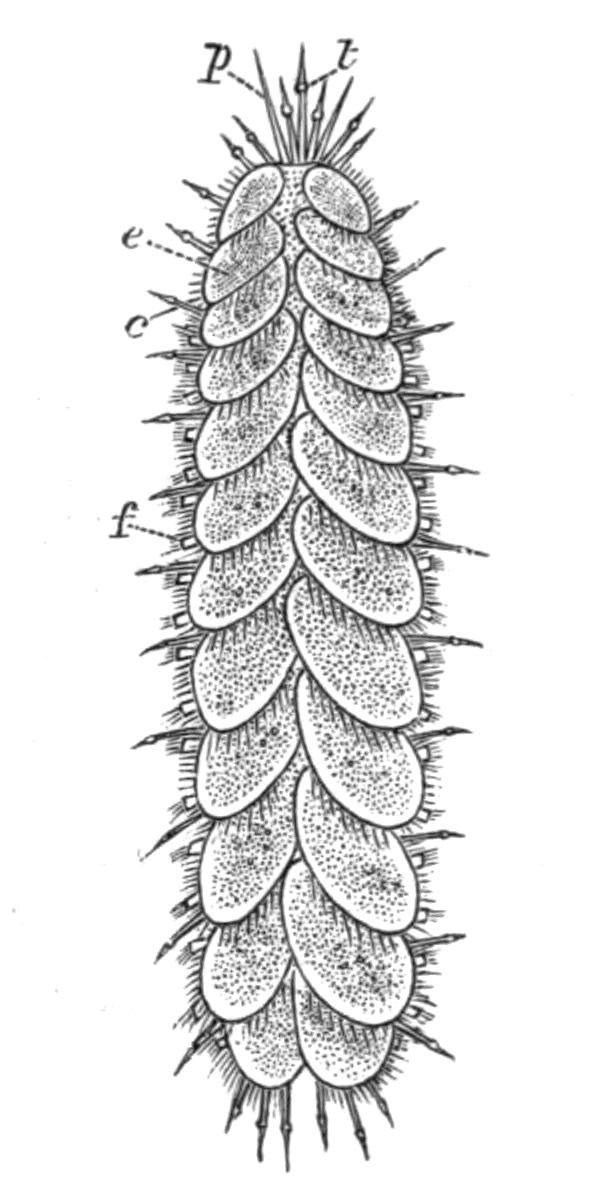
Fig. 161.—Polynoë squamata L. Nat. size. c, Notopodial cirrus; e, elytron; f, parapodium; p, palp; t, tentacle. (From Johnston.)
Sub-Fam. 1. Polynoina.—Body flattened, with nearly parallel sides, usually short, more rarely worm-like; three tentacles; peristomium with long dorsal and ventral cirri; the ventral cirri of the next segment are also elongated. Jaws are present. Elytra, usually twelve to eighteen pairs, the surface of which is more or less papillose, and may be "fringed" along the outer border, with long processes. The colouring of the elytra is characteristic in most cases, though liable to considerable variation in some species. The chaetae are generally strong, and of bright golden colour: they are all unjointed. The Polynoina are generally but feeble swimmers, {310}and are mostly found under stones at low tide. Some species have a very wide geographical range.
Polynoë.[370]—The body is short; none or only a few segments at the end of the body are uncovered by the elytra, except in the long body of P. johnstoni.
A. With twelve pairs of elytra.—In P. squamata Linn. the elytra entirely cover the body and conceal the head, each elytron overlapping the next posterior one, and those of the two sides overlapping. General colour sandy-brown, speckled, lighter or darker. The fringed elytra are very firmly fixed to the body. The notopodial chaetae scarcely project from below the elytra. The worm is common between tide-marks and in the coralline region, is about one to one and a half inches in length, and about one-third of an inch in width. Atlantic. P. clava Montagu, may attain a larger size, though it is generally smaller. The elytra are dark, usually grey, mottled with white or light grey, unfringed, and do not overlap to so great an extent as in P. squamata, so that the middle of the back and the hinder part of the body is more or less exposed. This is never the case in the preceding species, but even here it is subject to variation in extent, depending on the amount of food contained by the worm or on the ripeness of the genital products. It occurs in the Mediterranean.[371]
Fig. 162.—Elytra, A, of Polynoë squamata L.; B, of P. clava Mont. × 10. a, Area of attachment; e, external margin; f, fringe (the letter is at the posterior side of the elytron); i, internal margin. (From Bourne.)
B. With fifteen pairs of elytra.—P. imbricata L. is probably the commonest species of the genus, occurring nearly everywhere under stones at low tide. It is about an inch in length; the elytra are deciduous, and are very variously coloured and marked; sometimes uniformly grey or even black, sometimes {311}mottled with brown: in other specimens each elytron has its outer half pale or white, while its inner half is darker, usually some tint of brown or olive green, so that the worm appears to have a dark band along the middle of its back. Other patterns occur. The body is entirely covered by the elytra. The chaetae project considerably, and are nearly as long as half the width of the body; those of the notopodium are brown and are directed upwards, being nearly as long as the golden neuropodial chaetae. This species has a very wide range, occurring on both sides of the Atlantic, even on the shores of Nova Zembla, and reappearing again at Japan. P. semisculptus Leach is rather larger than the foregoing. The elytra are very readily detached: they are light in colour, without a fringe, but with large papillae near the margin. The notopodial chaetae are thicker than those of the neuropodium. Several other species are also common, but P. johnstoni v. Marenz.[372] differs from the rest in having an elongated body of some seventy segments, so that the posterior half is uncovered by the elytra, which are small, greenish-grey, speckled, and have no fringe. It is common and widely distributed, but appears to be only found in the tubes of Terebella nebulosa.
Fig. 163.—Elytron of Polynoë imbricata. a, Area of attachment to body; e, outer border; i, inner border.
C. With eighteen pairs of elytra.—P. gelatinosa Sars, may attain a length of 2 inches. The elytra are very faintly coloured, transparent and soft, attached by rather long peduncles. In spirit they become swollen and folded, giving the worm a very untidy appearance. The prostomium is partly overlapped by a peculiar collar-like fold of the peristomium.
D. With numerous pairs of elytra.—Lepidasthenia has a very long body, consisting of more than eighty segments. The elytra are quite small, and occur throughout the body on the usual segments. There are no notopodial chaetae. L. elegans Gr. is a very elegantly marked worm, which, however, has not been recorded from the British area; it occurs in the Mediterranean (see Fig. 156, p. 293).
Sub-Fam. 2. Hermionina.—The body is short, oval and {312}depressed; the particularly strong notopodial chaetae are directed upwards and backwards so as to protect the elytra. The neuropodial chaetae are also strong. The prostomium carries a single tentacle and two long palps; the prostomial ridge may be well developed. The peristomium is chaetigerous, with long cirri. The jaws are represented merely by thickened prominences.
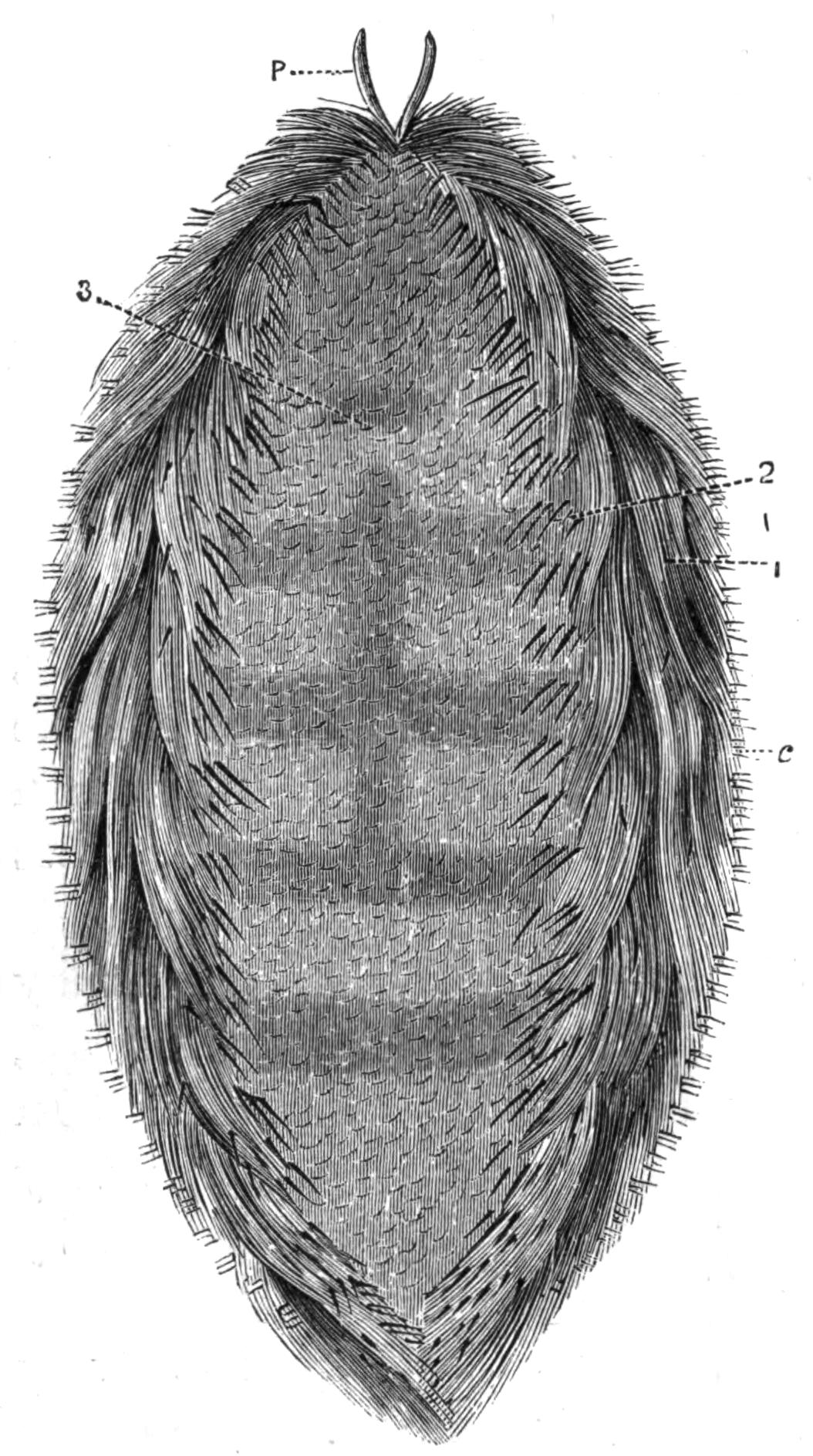
Fig. 164.—Aphrodite aculeata L. (from Règne Animal). Nat. size. c, Neuropodial chaetae; p, palps; 1, iridescent bristles; 2, stiff chaetae; 3, felting bristles of notopodium.
Aphrodite.—The fifteen pairs of elytra, arranged as in Polynoë, are concealed by a "felting" of hair-like chitinous threads arising from the notopodium (Fig. 139, p. 268). A. aculeata L.—The "sea-mouse" is one of the most beautiful of the Polychaetes. The small tentacle is very readily detached; the palps are very long; the parapodia of the peristomium are directed forwards so as to form lateral lips; and its cirri are not especially modified (see Fig. 132, p. 260). The body, which measures 3 to 6 inches, consists of thirty-five to forty segments, and is broadest in the middle, the last dozen segments being very small; the body terminates in a point. Some of the notopodial chaetae are brilliantly iridescent, and give the worm its characteristic coloration. It is fairly common in the coralline regions, and is frequently thrown ashore after storms. Atlantic and Mediterranean.
In Hermione the "felt" is absent, so that the elytra are exposed. H. hystrix Sav. occurs in ten to thirty fathoms of water all over the British area and Mediterranean. It resembles in its general appearance a fat Polynoid, with strong chaetae. Laetmonice filicornis Kinb. also occurs on our north-west coasts, and L. producta Gr. has been dredged in 500 fathoms off the {313}west coast of Ireland; it has been recorded also from Kerguelen and from Japan, so that it has a very wide distribution.[373]
Sub-Fam. 3. Acoetina.[374]—The long, vermiform body has some thirty-nine to ninety-three pairs of elytra, placed on every alternate segment throughout. It is represented in the British area by Panthalis from 75 fms., which forms a tube of black mud.
Sub-Fam. 4. Sigalionina.—This sub-family includes forms with a long, vermiform body; anteriorly the elytra are on alternate segments, up to the twenty-sixth, and posteriorly on every succeeding segment; "gills" here coexist with elytra; cirri are absent. The prostomium in Sthenelais Kinb. has a median tentacle, which is absent in Sigalion Aud. and Edw. Sth. boa Jnstn. is common off our coasts near low-water mark, where it burrows in the loose sand with rapidity. It is an elegant worm, and may attain a length of 8 inches, though it is generally smaller; it is narrow, flat, and only slightly tapering at each end; the elytra, which may be more than a hundred pairs, are greyish or slightly brownish, some being lighter than others; the margin is fringed with simple processes (which in Sigalion are pinnate). Atlantic and Mediterranean. In Psammolyce the elytra are covered with sand grains. British and Mediterranean.
Fam. 4. Phyllodocidae.—The members of this family make use of the foliaceous cirri (Fig. 136, F) in their very active movements. The rounded prostomium bears four or five tentacles; there are four long peristomial cirri on each side (see Fig. 134, E).
Sub-Fam. 1. Phyllodocina.—The body is elongated, with numerous segments; the eyes are small; the chaetae are jointed; the dorsal and ventral cirri are foliaceous; the pharynx is covered with papillae externally, but contains no "jaws."
Phyllodoce has a more or less depressed body; four prostomial tentacles; four pairs of peristomial cirri. P. lamelligera Jnstn.[375] (the "paddle-worm") may reach a length of 24 inches, but is usually 8 to 12 inches long and ½ inch across. The general colour is bright bluish-green or yellowish-green, with metallic iridescence; the parapodia olive-green or brown, the sensory {314}processes yellow. It lurks, during day, under stones and shells, etc., in the Laminarian zone. The green egg masses, so frequently referred to as belonging to Arenicola, are laid by Phyllodocids.[376]
In Eulalia an additional (fifth) tentacle arises from the middle of the back of the prostomium. E. viridis Müll. is a dark green worm smaller than the preceding; common between tide-marks, hiding in cavities and tunnels in limestone rocks, which have been bored by the mollusc Saxicava; it is rare where such rocks are absent. It might have been thought that its vivid colour would harmonise with its surroundings, but it is most abundant in regions where Fucus abounds and Ulva is absent. It is evident then that the colour is not protective; it may perhaps be of warning significance, for the mucus secreted in quantities by glands on the cirri of the Phyllodocids is probably objectionable to their enemies. Phalacrophorus Grf. and Pontodora Grf. may be mentioned as pelagic genera.
Sub-Fam. 2. Lopadorhynchina.—This includes small forms, Lopadorhynchus Gr., Pelagobia Grf., and other pelagic genera.
Sub-Fam. 3. Alciopina.—These are surface forms, and, like most pelagic animals, are colourless and transparent; the eyes, however, are very large, and, with certain brown spots in each segment,[377] are the only coloured parts in the body; in structure the eyes are much more complicated than {315}those of other Polychaetes. The prostomium has five tentacles; there are long peristomial cirri, and in general their anatomy agrees most closely with that of Phyllodocids. Alciope, Asterope, Vanadis, Nauphanta are genera of the family;[378] none have been recorded from the British area.
Fam. 5. Tomopteridae.[379]—This includes but one genus, Tomopteris, which is pelagic. The transparent, colourless body consists of only a few (eighteen to twenty) segments; the parapodia are as long as the body is wide, and carry no chaetae; each is bilobed, and fringed with a membrane; each of these lobes contains a yellow rosette-shaped photogenic organ. The only chaetae present in the worm are on the "head." The prostomium is hammer-shaped, and appears to carry a pair of short filaments ventrally (Fig. 167, x) each with a single chaeta within it; and a longer filament laterally (y), supported by a long, very delicate chaeta. The mouth is behind these, and they probably are the first pair of parapodia which have shifted forwards. T. onisciformis Eschscholtz is not unfrequently obtained off our shores in the tow-net.
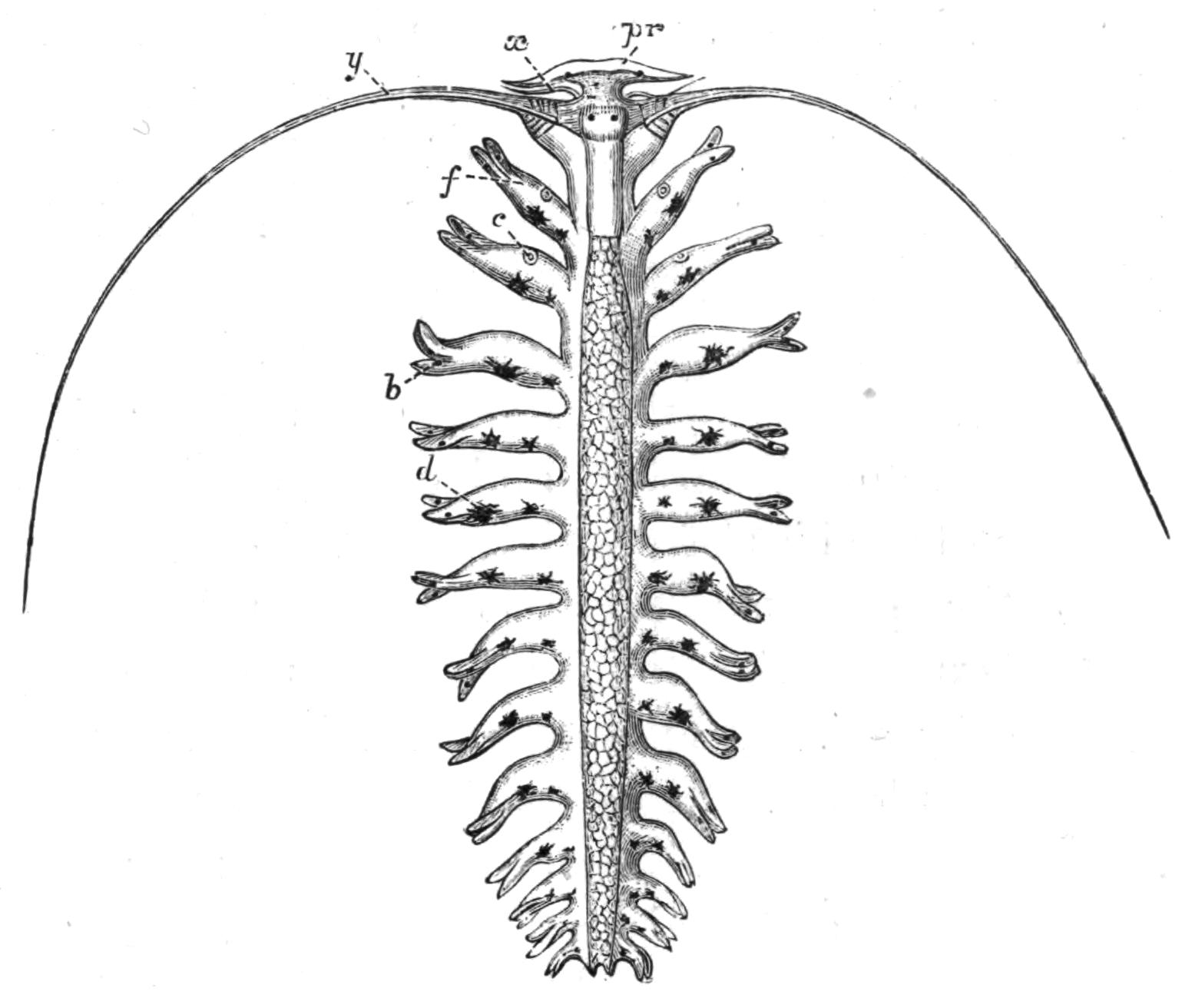
Fig. 167.—Tomopteris rolasi Grf. × 10. From Guinea Isles. pr, Hammer-shaped prostomium; x, first chaetigerous process; y, second chaetigerous process; c, rosette (photogenic) organ on first two parapodia; b, similar organ in the lobes of the following parapodia; d, pigment spots; f, parapodium. (From Greef.)
Fam. 6. Nereidae (Lycoridae).—This family contains a very large number of species, differing from one another in small and not readily recognisable characters, such as the relative lengths {316}of the various processes of the head, of the lobes of the feet, the arrangement of the "paragnaths" (see Fig. 125, d) and so forth. The general features of the family have been already described. The genus Nereis is represented by six fairly common species on our coast, which are almost world-wide in distribution.
N. diversicolor Müll. is about 3 to 4 inches in length, of a general fleshy-red colour, though tending in some cases to yellowish-brown or even greenish. It may be distinguished by two diverging brown bands, which start on the peristomium and pass backward one along each side of the body for several segments. The prostomium is broader than it is long. The worm burrows in mud or sand, all round our coast between tide-marks. It has a very wide distribution, being met with on this side of the Atlantic, and off the coast of Greenland, and off Japan. It is even found in brackish water at Bembridge, Isle of Wight.
N. cultrifera Gr. is green or greenish-grey, with a series of small rectangular light spots along the mid-dorsal surface, and oblique light lines at the sides of each segment. Posteriorly the greenish pigment becomes less and less till the hinder segments are flesh-coloured. The prostomium is as long as it is broad. This species attains a length of 6 inches. Southern coasts: locally known as "Red Cat."
N. dumerilii Aud. and Edw. is rather smaller and narrower than the two preceding species; it is reddish-violet in colour, marked with darker transverse lines in each segment. It is readily recognised by the two dark brown spots on the upper surface of the base of the notopodium in most of the segments, and by the great length of the peristomial cirri, the longest of which reaches the fifteenth segment. It is sometimes found enclosed in a cocoon-like tube of hardened grey mucus, more or less covered with foreign particles, such as sand grains. Atlantic, Mediterranean, Japan.
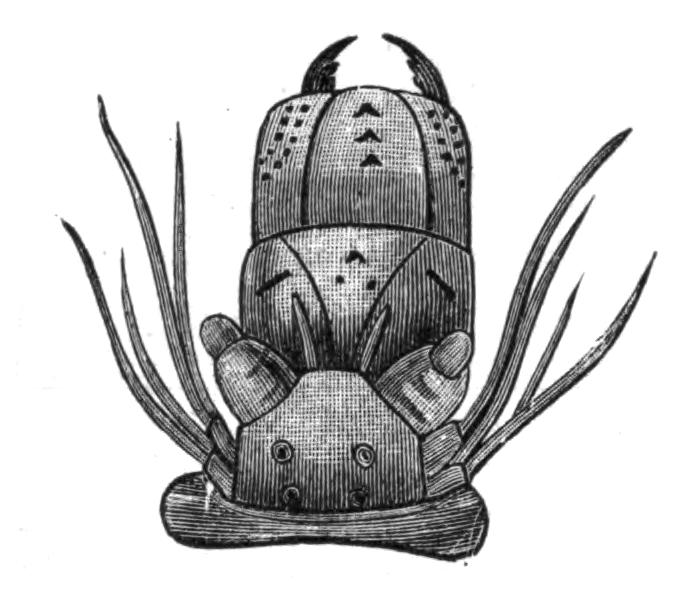
Fig. 168.—Nereis cultrifera Gr. × 6. Head with buccal region everted, to show the arrangement of the jaws. (From Ehlers.) Cf. N. diversicolor, Fig. 125, p. 248.
N. pelagica L. is red-brown or bronze in colour, and is generally larger than the other species, from which it is distinguished by being widest about the middle of the body (see Fig. 122, p. 246); whilst in the preceding species the greatest {317}breadth occurs at the segments immediately following the head. Further, the palps are long, the peristomium is twice as long as the next segment, and the back of the worm is strongly arched. At all depths on rocky and stony ground. Northern coasts.
N. (Nereilepas) fucata Sav. lives in the topmost whorls of empty whelk shells and in those occupied by hermit crabs. The ground colour is tile-red, with two milk-white bands along the dorsal surface. The dorsal lobe of the foot is slightly foliaceous, glandular, and vascular.
N. (Alitta) virens Sars. is a giant amongst Polychaetes, reaching a length of 18 inches. Its name suggests its colour; it is very plentiful at certain times at St. Andrews, and between tide-marks along the shore of the Mersey estuary, as well as elsewhere. It forms a burrow in the clay, etc., of the shore, and lines it with mucus, which is abundantly secreted by the great foliaceous lobes of the parapodia. These great leaf-like lobes of the foot recall the modification which the foot of many species of Nereis undergoes in transformation into Heteronereis: they are so greatly developed that, at first sight, the worm might be mistaken for a large Phyllodoce. The worm is known as the "Creeper," and is much esteemed as bait on some parts of our coast.
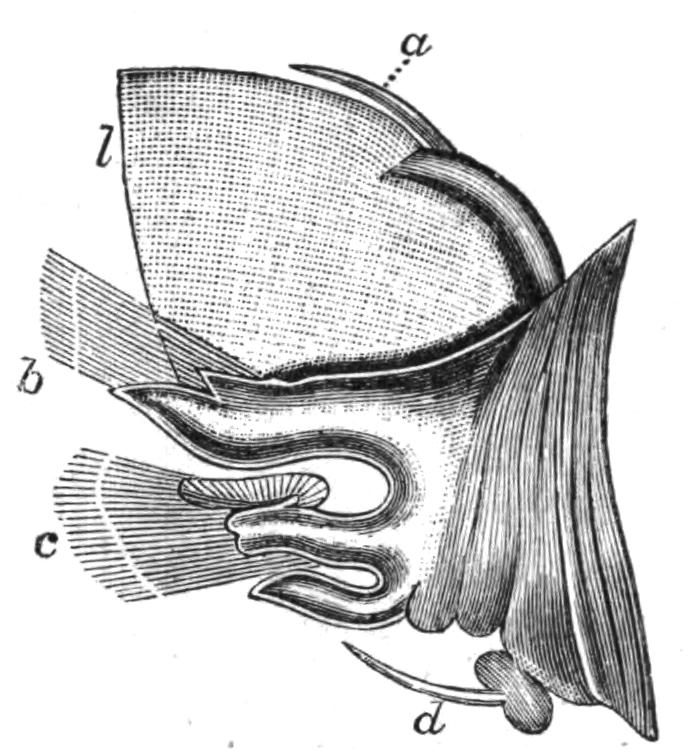
Fig. 169.—Parapodium of N. virens Sars. ×4. a, Notopodial cirrus; b, notopodium; c, neuropodium; d, neuropodial cirrus; l, foliaceous appendage. (From Ehlers.)
Fam. 7. Nephthydidae.—The elongated body is quadrangular in section, the dorsal and ventral surfaces being almost flat. (For head see p. 262, and for parapodium, p. 264.) The two lobes of the parapodium are widely separated, and each is fringed with a membrane, while a sickle-shaped "gill" hangs down from the under surface of the notopodium. The pharynx is enormous. Of the genus Nephthys two species, called the "Lurg" or "White Cat" by fishermen, occur on our coasts. Their active movements and beautiful mother-of-pearl tint are characteristic. N. hombergii Aud. and Edw. occurs on the shore, and down to 20 fathoms; it is 3 or 4 inches long, and may be found burrowing in the sand; the chaetae exceed in length those of N. caeca {318}Fabricius, which occurs less frequently and in deeper water, and is larger than the preceding. Both are Atlantic forms.
Fam. 8. Amphinomidae.—The body in this family is either vermiform, as in Eurythoe, or oval and flattened, as in Euphrosyne and Spinther. The head carries a peculiar sense organ, the "caruncle," consisting of a smooth axis with the sides folded so as to look like a number of lamellae. The parapodia carry gills. Most of the Amphinomids are tropical and Southern forms.
Eurythoe borealis Oerst. measuring 6 inches, occurs all round the British area, from the Shetlands, where it occurs in deep water, to the Channel Isles, where it lives on shore, under stones, etc. (For parapodium of Amphinome see p. 264.)
Euphrosyne.—The body is short, oval, and flattened. The parapodia are not distinct processes, but the chaetae extend from each side of each segment nearly to the middle dorsal line, and are absent ventrally (Fig. 137, C, p. 265). The dorsal and ventral cirri are more or less filiform, and there is an intermediate similar process on the back (? = lip of chaetigerous sac). Amongst the chaetae are a number of curious branched processes—usually called "gills."[380] The presence of these and of the chaetae give the upper surface of the body a fluffy appearance. E. foliosa Aud. and Edw. is fairly common under stones on our southern shores. It is about an inch in length and is of a cinnamon-red colour.
Fam. 9. Eunicidae.—The elongated body is provided with parapodial gills in more or fewer segments (except in Lumbriconereis). The "gills" may be cirriform (Hyalinoecia), pectinate (Eunice, Onuphis), or more complex (Diopatra). The notopodium is represented by a lobe (usually called "cirrus") into which an aciculum projects; in some cases it even contains a few chaetae; most of the neuropodial chaetae are jointed (Fig. 138, F). The prostomial tentacles vary in number; they may be three or five, or five and two short "frontal palps," or they may be absent. Peristomial cirri are absent, though in Eunice, Diopatra, and Onuphis "nuchal cirri" are present on the dorsal surface of the second segment (Fig. 134, D). One of the most characteristic features in the anatomy of the Eunicids is the peculiar jaw apparatus (see p. 270). The majority of the genera form permanent tubes of parchment-like consistency, {319}which may be further strengthened by the addition of grains of sand, small pebbles, etc.; the tubes may be branched.
Eunice has five tentacles, two great palps, and a pair of nuchal cirri; the gills are pectinate, and there are four anal cirri. E. harassii Aud. and Edw. is about 8 inches long. It is reddish-brown, with white spots down the back, one to each segment, and others at the sides. The gills begin at the sixth segment, and when fully developed have eleven branches. The dorsal cirrus is not longer than the gill. E. philocorallia Buch.[381] forms its tube amongst the branches of Lophohelia prolifera, in 200 fathoms, off the west coast of Ireland.
Marphysa resembles Eunice, but has no nuchal cirri. M. sanguinea Mont. is a fine bronze colour, with bright red gills, which commence on the twentieth segment, and have only four or five branches. The worm, which measures 12 to 18 inches, and is as thick as one's finger, hides in clefts in rocks and under stones below low water. Mediterranean. It is known as "Rockworm" in the Channel Islands.
Hyalinoecia Mgrn., in addition to the five prostomial tentacles and palps, possesses a pair of small "frontal palps" arising from the anterior border of the prostomium; there are no nuchal cirri, and the gills are simple filiform processes. H. tubicola Müll., about 3 inches long, is yellowish-brown, and forms a transparent, parchment-like tube. Atlantic and Mediterranean.
Fig. 170.—Ophryotrocha puerilis Clap., Metsch. × 25. ci, Bands of cilia; cp, ciliated pit (nuchal organ); J, jaws. (From Korschelt.)
Onuphis Oerst. has a head like the preceding, from which it differs in having pectinated gills and two nuchal cirri like Eunice. In making its tube it employs small pebbles, bits of shell, and even echinid spines, which it glues together with mucus, so that it bears a general resemblance {320}to its surroundings. O. conchylega Sars, has a flattened, scabbard-like tube, which can be carried about by its owner. Atlantic.
Lumbriconereis has a more or less conical prostomium, without any tentacles, but with large palps: segments without gills. L. fragilis Müll. is reddish or brownish, with a beautiful iridescence; it is cylindrical, very narrow, and some 5 or 6 inches long; L. tricolor Jnstn. is much larger.
Ophryotrocha (Fig. 170) is a small form often occurring in aquaria; it is chiefly remarkable for the possession of segmentally-arranged girdles of cilia—a permanent larval feature. Lysidice ninetta Aud. and Edw. belongs here.
Fam. 10. Glyceridae.—Elongated worms with numerous segments. The prostomium, though narrow, is long, conical, annulated, and carries at its apex four very small tentacles; at its base a pair of palps. Special retractile gills are present. The armed pharynx is very long, and when protruded appears wider than the animal. The members of this family are without any system of blood-vessels, but the coelomic corpuscles are coloured red. Glycera has four jaws, the parapodia are all alike (Fig. 136, C). G. capitata Oerst. is 2 or 3 inches in length, is yellowish in colour, with a dark-red median line. It may be found burrowing in sand. The setigerous lobes of each foot are coalesced to form one large lobe with pointed apex. The dorsal cirrus is a small wart above the base of the foot. Atlantic and Mediterranean. A second species, which is much larger and flesh-coloured, also occurs.
Goniada is distinguished from the preceding by the fact that the parapodia suddenly change in size and character at about one-third the length of the body. The pharynx has numerous paragnaths. G. maculata Oerst. occurs off our coasts.
Fam. 11. Sphaerodoridae.—The dorsal and ventral cirri of each segment are spherical. The chaetae are usually jointed, and there is an aciculum to each {321}parapodium. Ephesia Rthke. (E. gracilis R. = Sphaerodorum peripatus Jnstn.) is exceptional in having unjointed chaetae. North Sea, Arctic Ocean, and the Channel. The family, which is much modified, is allied in some respects to the Syllidae.
Fam. 12. Ariciidae.—These worms burrow in sand between tide-marks. The body consists of many short segments, and is nearly cylindrical. The prostomium is more or less pointed; the chaetae are all capillary; in the first few segments they project laterally but soon come to lie dorsally, and are carried by slight conical papillae (supported by acicula), which are longer in the middle of the body. Most of the segments carry filiform "gills," representing the dorsal cirri (Fig. 137, B).
Scoloplos armiger Müll. is extremely common on our coast. It is about an inch long, yellowish, with red gills, commencing about the twelfth segment. Each of the lobes of the parapodium possesses an aciculum, and the chaetae are bent in a peculiar way. The everted buccal region has the form of a six- or eight-rayed star. The spawn of this species may be found on the shore in spring as brown, pear-shaped, jelly-like masses, each with a long stalk, by which the mass is fixed to the sand. In the jelly are the eggs, which may be watched passing through the earlier stages of development. Atlantic on both shores, even off Spitzbergen, and Nova Zembla. Another representative is Theodisca mamillata Clap., which occurs amongst the roots of Laminaria.
Fam. 13. Typhloscolecidae.[382]—Pelagic, greatly modified forms, apparently related to the Phyllodocidae, but with very uncertain affinities. The prostomium is pointed and carries a pair of foliaceous tentacles; each of the first two segments bears a pair of foliaceous cirri; the remaining segments possess a dorsal and a ventral pair of foliaceous cirri, with a small bunch of chaetae and a single aciculum. All the cirri have peculiar rod-cells. Typhloscolex Busch, Sagitella Wagner, and Travisiopsis Uljanin: all small worms. North Sea Atlantic.
Sub-Order 2. Spioniformia.
Fam. 1. Spionidae.—Mostly small worms, with small ridge-like prostomium carrying a pair of eyes, but no tentacles or palps. The peristomium, which extends forwards on each side {322}of the prostomium, bears a pair of very long cirri (usually termed "tentacles") normally directed backwards, very mobile, and more or less coiled. They are readily thrown off by the animal. The notopodial cirri are long, finger-shaped, and curved over the back; they are vascular and ciliated, and function as "gills." The neuropodia project laterally. Both are usually provided with a "podal membrane" along their outer margin. There are no ventral cirri; the dorsal chaetae are fringed capillaries; the ventral are "crotchets." The buccal region is eversible. The worms burrow in mud and sand.
Spio seticornis Fabr. is a small worm less than an inch in length, colourless except for the red blood in its vessels. It builds long and flexible tubes of sand in the clefts of rocks and under stones in the upper part of the littoral zone. The prostomium is notched at the anterior margin. The gills commence on the twelfth segment, and do not extend to the end of the body. A membrane-like cirrus exists also on the second chaetigerous segment. The podal membrane is adnate to the gill throughout its extent. Four short anal cirri occur. Greenland and Scandinavia.
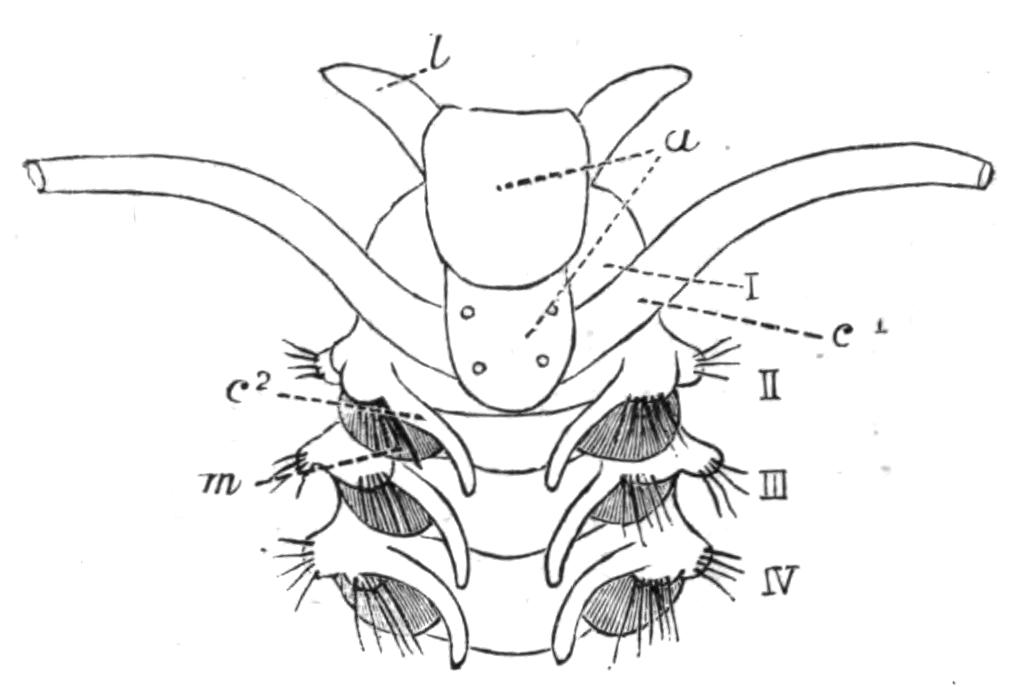
Fig. 172.—Nerine vulgaris Jnstn., enlarged. (From Cunningham.) a, Prostomium; c1, cirrus of peristomium; c2, "gill"; l, lobes; m, podal membrane; I, peristomium; II, III, IV, following segments.
Nerine is represented by two species, sometimes called "Ragworms." The genus is very similar to Spio, but the worms are of larger size. The prostomium is compressed by the forward growth of the peristomium, and appears as a ridge on the latter segment, extending downwards in front towards the mouth. The "gills" commence on the second segment, and are continued in every segment except the hindmost. Nerine (Scolecolepis) vulgaris Jnstn. is readily distinguished from other species by its somewhat T-shaped prostomium. It is an extremely common worm under stones and amongst seaweed at low water. It is some 3 or 4 inches in length and more slender than the following species. Its colour is yellowish-brown, and the red gills directed upwards and backwards give the appearance of oblique red lines. The podal membrane does not reach the tip of the gill. North Atlantic. It is {323}said to ascend rivers and live in brackish water. N. coniocephala Jnstn. is much the same colour, but reaches a length of 8 inches, and a diameter of ¼ inch. The prostomium is conical. The podal membrane reaches to the tip of the gill in the anterior segments. The worm burrows rather more deeply and nearer low-water mark than the preceding species.
Fam. 2. Polydoridae.—Polydora Bosc (= Leucodore Jnstn.) is readily distinguished from the other Spionids, and, indeed, from any other Polychaet (except Chaetopterus), by possessing specially strong chaetae in the enlarged fifth chaetigerous segment. The anterior segments differ from the rest in the absence of gills and in the character of the chaetae (Fig. 133, A, p. 261).
P. ciliata Jnstn. inhabits soft mud tubes near low water; it also makes U-shaped galleries in stones and shells, and the tube projects from each mouth. The worm is about ½ inch long, consists of some forty segments, and is yellowish or flesh-coloured. The prostomium resembles that of Spio; the peristomium is raised into a slight collar at each side. The anus is surrounded by an incomplete funnel. The species has almost a world-wide distribution, having been recorded from Iceland, Australia, the Philippine Islands, as well as from the European seas. P. coeca Oerst. often lives commensally with a sponge, having a protective odour.
Fam. 3. Chaetopteridae.—The family is represented on our coasts by Chaetopterus variopedatus Ren.,[383] which is found at the Channel Islands, the Scilly Isles, the Isle of Man, and the west Scottish coast, and probably at various other places, at low water and down to a depth of some 15 fathoms. It occurs in all European seas. The animal builds a long tube, the basis of which is a tough, parchment-like substance; this is coated externally with sand, small pebbles, and other débris: it is of considerable length and about ¾ inch in diameter, is U-shaped and open at both ends, the greater part of it being embedded in sand or in crevices of rocks. The animal, whose body-wall is thin and delicate, never leaves its tube. The body has a bizarre appearance; three regions are readily {324}distinguishable, which may be denoted by the letters A, B, and C. The most anterior region, A, is flattened, and carries nine pairs of conical lobes with delicate chaetae, though the fourth lobe possesses special stouter chaetae (as in Polydora). The anterior end of the body terminates in a wide funnel, the boundary of which is formed chiefly by the peristomium; on its dorsal surface is a pair of tentacle-like processes (peristomial cirri); the region between which represents the prostomium.
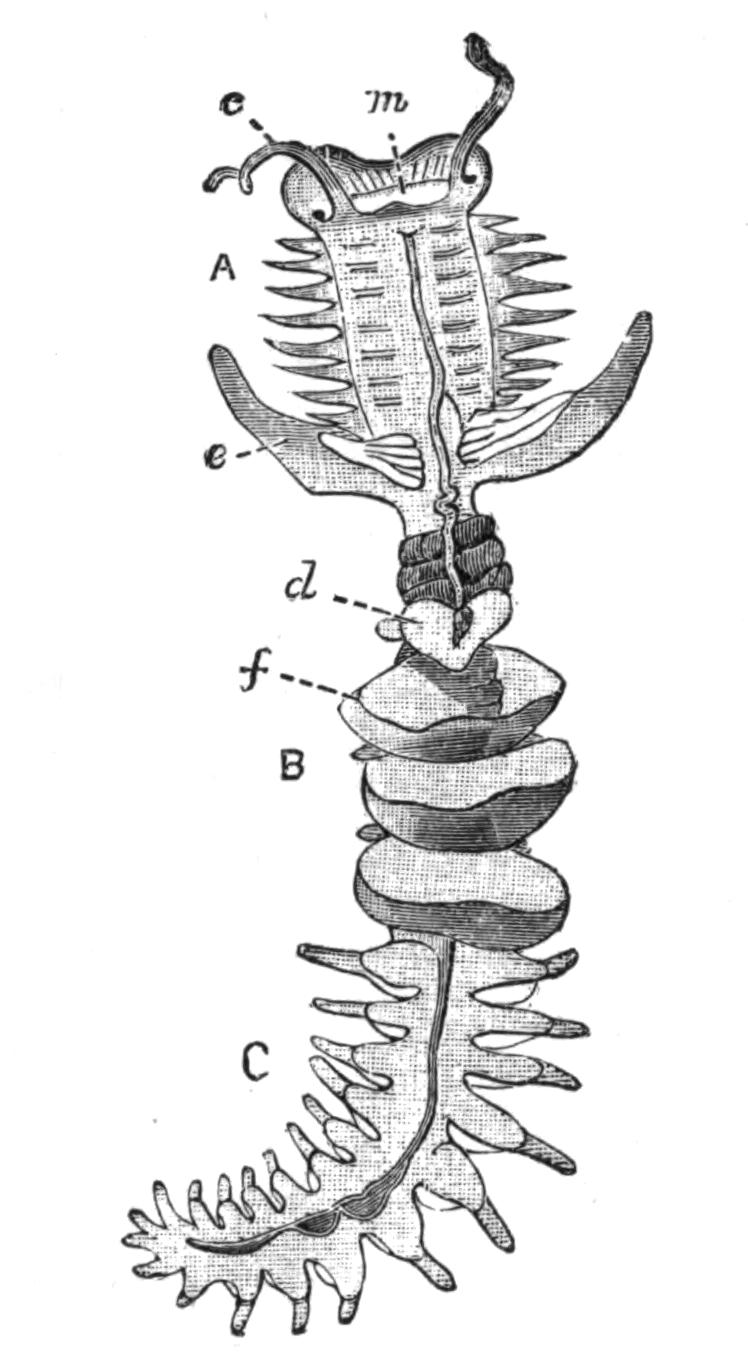
Fig. 173.—Chaetopterus. (From Panceri.) Natural size of a young specimen. A is the anterior region of the body; B, the middle region; C, the hinder region. c, Peristomial cirri; d, "sucker"; e, the great "wings"; f, the first of the three "fans"; m, mouth.
The second region, B, is very curiously modified; it is formed of five segments. The most anterior is produced on either side into a great wing-like process, which in life is directed forwards above the region A. Each is grooved on its inner side, the ciliated grooves being continuous with a median groove running forwards along the back of A; this apparatus serves to bring food to the great funnel-like mouth. The next segment (twelfth) carries a dorsal and ventral "sucker," representing the parapodia. Each of the segments 13, 14, 15 carries a membranous fold encircling the body. By the constant movement of these "fans," which have nearly the same diameter as the tube, a current of water is constantly washed over the animal. The fans represent the notopodia; the neuropodia are bilobed rounded knobs. The region C consists in the adult of about thirty segments, all alike, and less modified than the preceding. The animal is the most truly tubicolous of the Polychaetes, and is much modified on this account. No locomotor chaetae are present, though the great wings and notopodial processes of region C contains chitinous bristles, which, however, do not project;[384] the anterior region with its stiff chaetae, and the neuropodial uncinal plates of the rest of the body serve in its movements up and down the tube, while the "suckers" fix the worm temporarily to the wall of its house.
Chaetopterus is highly phosphorescent (see p. 295). It is further interesting on account of the green colouring-matter, which is extracted by alcohol. Two commensal Polynoids occur in the tube, viz. Polynoë glabra and P. cirrosa. The larva is "mesotrochal" (with a ciliated ring round its middle), that region of the body lying in front of the cilia giving rise to the region A, whilst the rest of the body gives rise to regions B and C.
Fam. 4. Magelonidae.—This family includes only the very peculiar worm, Magelona papillicornis Fr. Müll., which lives buried in sand, between tide-marks, in various parts of our coast and that of the United States. Its chief features are the large, flat, spoon-shaped prostomium; the long peristomial cirri, slightly expanded terminally, carrying papillae along one side; the enormous, eversible buccal region, which is an important respiratory organ. The blood is of a madder-pink colour, and the blood-vessels in the thorax are greatly dilated. The body of the worm is divisible into two well-marked regions, owing to differences in the chaetae.[385]
Fam. 5. Ammocharidae.—This family contains only one species, Owenia filiformis D. Ch. Some of the anterior segments are longer than the hinder ones, though the arrangement of chaetae is alike throughout. The mouth is wide, like that of Chaetopterus, and is surrounded, except ventrally, by a membrane, so deeply notched as to give rise to flattened filaments containing blood-vessels. These "gills" appear to belong to the peristomium. The small worm in its sandy tube is plentiful on our coasts in about 20 fathoms. Off Greenland and the Mediterranean.
Sub-Order 3. Terebelliformia.
Fam. 1. Cirratulidae.—These worms have a cylindrical body, more or less attenuated at each end; the segments are distinct, and similar throughout, with capillary chaetae on each side in two bundles, carried by small papillae. The prostomium is conical, the peristomium usually without cirri. On more or fewer segments the dorsal cirri are long and filamentous, and function as gills. There is a single pair of anterior nephridia: the septa and genital ducts are repeated throughout the hinder part of the body. The worms usually live in burrows.
Cirratulus.—The prostomium is long, sometimes annulated. In addition to the segmental filamentous "gills" there is a transverse row of long "tentacular filaments" across one of the anterior segments, and it has been suggested that they are really prostomial tentacles which have shifted backwards. They and the gills twist about in a very active fashion during life, and look like small independent worms, especially when they are detached. C. cirratus Müll, is a brown or dirty yellowish worm about 4 to 6 inches in length, usually to be found under stones, partially embedded in the mud or sand. The prostomium carries a pair of linear groups of "eye-spots"; the first chaetigerous segment carries a transverse row of tentacular filaments, and the red gill-filaments commence on the same segment. Common. C. tentaculatus Mont. is a larger worm, dark red in colour, and is distinguished from the preceding by the absence of eyes and by the fact that the tentacular filaments are on the seventh chaetigerous segment, while the gills commence more anteriorly. Atlantic and the Mediterranean.
Chaetozone setosa Mgrn. occurs in the North Atlantic. Dodecaceria concharum Oerst. is about an inch in length, olive-green or brownish in colour, and is not uncommon amongst roots of Laminaria. It is stated to live also in tortuous tubes bored in shells and stones, but whether it makes these tubes is uncertain. The worm has two thick tentacular filaments, and the thinner gills are only on four segments. Hekaterobranchus shrubsolii Buch.[386] is a small worm some ½ inch long, found at Sheerness, where it occurs at low tide in soft mud; here it forms a loosely coherent tube, though it also moves freely in the mud. Its chief features are (1) a pair of long, ciliated {327}"cephalic tentacles," probably peristomial, and similar to the "tentacular filaments" of Dodecaceria; (2) a pair of filamentous gills (dorsal cirri) on the first chaetigerous segment; (3) a pair of large green nephridia in the anterior segments. The describer placed it amongst the Spionids, but the above and other features point to Cirratulid affinities.
Fam. 2. Terebellidae.—The body is cylindrical, and generally larger in front than behind. The prostomium is generally flattened, and forms a mobile upper lip, which always carries a transverse series of many tentacles; it may bear "eye-spots," but never palps; the lower lip is formed by the peristomium. There are one to three pairs of gills, which are usually more or less branched, on as many segments.[387]
The chaetigerous lobes are small; the dorsal ones contain capillary chaetae, which are frequently confined to the anterior segments, whilst the ventral chaetae are uncini. The ventral surface of the anterior segments is thickened by glands which secrete the mucus employed in tube-building; the number of these "shields" and of the dorsal bundles of chaetae have to be noted in identifying the worms. There are one to three pairs of large anterior nephridia. A very strong "diaphragm"—usually more or less pouched—cuts off this anterior region of the body-cavity from the rest, and is the only complete septum in the body; from three to twelve pairs of small generative ducts occur behind it. The family is tubicolous, foreign materials being generally used in the formation of the tube.
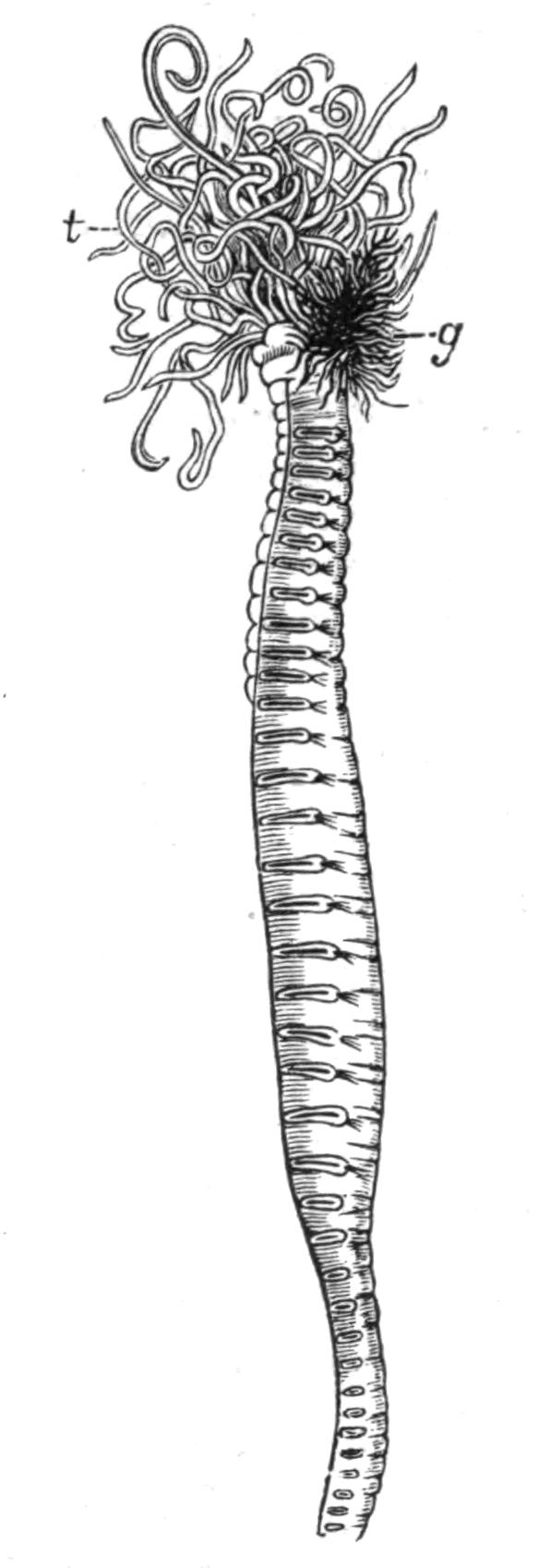
Fig. 175.—Amphitrite johnstoni (½ nat. size). g, Gills; t, prostomial tentacles. (From Cunningham and Ramage.)
There are six genera which are fairly common round our coast, and their identification may be facilitated by means of the following table[388]:—
| A. Capillary chaetae confined to the anterior part of body; commencing on the fourth segment. |
Gills ramose. |
3 pairs, which are | equal in size. |
Amphitrite. 24 notopodia. |
|
| unequal in size. |
Terebella. 17 notopodia. |
||||
|
2 pairs. 17 notopodia. |
Gills equal. Eye-spots. |
Nicolea. | |||
| Gills unequal and of peculiar shape. | Pista. | ||||
| B. Capillary chaetae throughout the body; commencing on the third segment. | Gills | arborescent; 3 pairs. | Leprea. | ||
| filiform; in transverse series in two segments. | Thelepus. | ||||
| A. Capillary chaetae confined to the anterior part of body; commencing on the fourth segment. | Gills ramose. |
 |
3 pairs, which are |  |
equal in size. | Amphitrite. 24 notopodia. |
|
| unequal in size. | Terebella. 17 notopodia. |
||||||
| 2 pairs. 17 notopodia. |
 |
Gills equal. Eye-spots. |
Nicolea. | ||||
| Gills unequal and of peculiar shape. | Pista. | ||||||
| B. Capillary chaetae throughout the body; commencing on the third segment. | Gills |  |
arborescent; 3 pairs. | Leprea. | |||
| filiform; in transverse series in two segments. | Thelepus. | ||||||
Amphitrite johnstoni Mgrn. (Fig. 175) is brown in colour, about 4 to 6 inches in length, and nearly ½ inch in breadth anteriorly. Each of the gills consists of a curved stem; from the convex side of which arise a number of branches, themselves dichotomously divided, the final branches being long (Fig. 176, A). There are twelve ventral "gland shields." The worm is fairly common between tide-marks, below stones in muddy places: the end of its tube of mud projects above the surface. Atlantic.
Terebella (Polymnia) nebulosa Mont. is distinguished by its bright red colour, spotted with white; it is 6 or 7 inches in length, and ½ inch across. Large specimens of this beautiful worm may be obtained at Weymouth and elsewhere on the south coast, where it lives in about 14 fathoms. Each gill appears much more arborescent than in the preceding (Fig. 176, B); it consists of a main stem, from which comparatively few branches arise; these subdivide frequently, and the terminal branchlets are quite short. The "gland shields" are fourteen to sixteen in number. The tube is of mud. North Sea and Mediterranean. T. (Leprea) lapidaria L. is 1 inch in length, orange-red in colour; and has 12 ventral shields. The tube, of fine mud, lies horizontally on the under surface of stones.
T. (Lanice) conchilega Pall. (the "sand mason") forms a very characteristic tube of sandy particles, small pebbles, and pieces of shell. It is buried in the sand, but a short portion protrudes, and bears, set round its edge, a fringe of branching sandy threads (Fig. 153) commonly seen on sandy shores between tide-marks. The worm may be distinguished from the preceding species by the fact that the series of fourteen to {329}seventeen gland shields are red, and continuous from segment to segment. The gill is shown in Fig. 176, C. North Sea, Atlantic, and Mediterranean.
Fig. 176.—Gills of various Terebellids. × 4. A, Amphitrite johnstoni Mgrn.; B, Terebella nebulosa Mont.; C, T. conchilega Pall.; D, Nicolea Mgrn. (the finer branches are not indicated); E, Pista Mgrn.; F, Terebellides Sars (after Malmgren). g, Gill; m, mouth; t, tentacles.
Nicolea venustula Mont. has only two pairs of equal, arborescent gills (Fig. 176, D); the tentacles are comparatively few. The animal, which is about an inch in length, is cinnamon-yellow with white spots, and has seventeen gland shields. 20 fathoms, North Sea and Mediterranean. Pista cristata Müll. is readily recognised by the shape of the gills (Fig. 176, E), of which there are only two pairs. Each consists of a long peduncle, bearing a number of dichotomously dividing, rather spirally-arranged branches, the whole having the appearance of a "bottle-brush." The worm is 2 to 4 inches long, of greyish-red to yellow colour. Atlantic east and west (even at the mouth of the Congo), and Mediterranean. Thelepus cincinnatus Fabr. is about the same length, pale red in colour, marked on its back with clear areas, giving the appearance of lacework. The gills are represented by numerous unbranched filaments arising separately in two transverse rows. The tube, which is adherent to shells, etc., along its whole length, is of thin, transparent, and flexible material like mica, covered with foreign bodies, and even with Polyzoa and Hydrozoa. 30 fathoms, Atlantic and Mediterranean.
Polycirrus aurantiacus Gr. is sometimes placed in a special sub-family, as it has no gills. The numerous tentacles are very long, and arise from a great hood over the mouth; the capillary chaetae commence in the first segment and extend for about half the length of the body; the uncini commence in the ninth segment. The ventral "shields" are paired. The animal is highly coloured; its phosphorescence and its distastefulness have already been mentioned on p. 294. In Terebellides stroemi Sars, four comb-like gills arise from a single common thick peduncle on the back of the second segment (Fig. 176, F). The ventral surface of the body bends upwards anteriorly so as to bring the mouth to the dorsal surface. 13 to 16 fathoms, muddy bottoms, North Sea and Mediterranean.
Fam. 3. Ampharetidae.—This family differs from the Terebellids chiefly in the shape of the head and in the presence of a bundle of strong chaetae (or paleae) on each side of the head in front of the gills, of which there are four on each side. Each gill is a simple filiform process, considerably longer than the tentacles, which are very few in number. Amphicteis gunneri Sars, Ampharete gracilis Mgrn., and Melinna cristata Sars, occur on our coasts.
Fig. 177.—Pectinaria belgica Pall. Slightly enlarged. a, Neuropodial chaetae; b, notopodium; ch, paleae; g, gills; sc, scapha; t, prostomial tentacles; I, peristomium. (From Malmgren.)
Fam. 4. Amphictenidae.—This contains the interesting genus Pectinaria, in which the head is protected by great golden chaetae on the second segment; they are flattened, curved, and pointed, and are arranged in a single transverse row on each side, serving as an operculum to the tube. The posterior end of the worm has undergone great degeneration, and is represented by a small leaf-like "scapha" which serves to close the tube posteriorly. The worm is 1½ inches in length and consists of only twenty segments, of which seventeen are chaetigerous. The tube is nearly cylindrical, but wider anteriorly than posteriorly (Fig. 152, p. 288); the sand grains are uniform in size, and are embedded in the secreted mucus in a very regular way, the surface being smooth both {331}inside and out. These tubes can be carried about by the worm, but may be found projecting from the sand at very low tides. P. belgica Pall. forms a straight tube, whilst in P. auricoma Müll. the tube is larger and slightly curved. The former species appears to be confined to the North Sea; the latter occurs in deeper water, and is also present in the Mediterranean.
Sub-Order 4. Capitelliformia.
Fam. Capitellidae.[389]—Capitella capitata v. Ben. occurs pretty frequently in the sand under stones near low tide-mark. It is a red worm, about 1½ to 2 inches long, greatly resembling a Tubificid Oligochaete. It may readily be distinguished from other Polychaeta by the strong genital chaetae in the male, which replace the notopodial chaetae of segments 8 and 9; those in the former segment are pointed backwards, and in the latter forwards. There is but a single pair of generative ducts in either sex in the eighth segment. North Sea, Mediterranean.
Notomastus latericeus Sars is a longer worm, living in shallow water, off our coast and in the Mediterranean. The anterior twelve segments are wider than the rest of the body. The notopodial chaetae of the anterior segments of the hind body form a ring. Dasybranchus caducus Gr., which occurs in the Mediterranean, but not on our coast, reaches a length of 2 or 3 feet. It has gills on the hinder segments above the neuropodia.
Mastobranchus Eis. is found in the Mediterranean.
Sub-Order 5. Scoleciformia.
Fam. 1. Opheliidae.—Comparatively short, rather ugly worms of a pearly colour, no prostomial processes: parapodia obscure. The family is represented in British waters by four species, occurring in shallow water.
Ammotrypane aulogaster Rathke, is about 2 inches long; the nearly cylindrical body has a ridge running along each side below the chaetae. The conical prostomium is tipped with a small knob, and carries at each side a ciliated pit (Fig. 144, p. 273). Every segment, except the first chaetigerous, is provided with a filamentous gill (dorsal cirrus). The segmentation {332}is very obscurely marked, for internally there are only three complete septa, placed far forwards. The intestine is bent upon itself. In Ophelia limacina Rthk. the gills commence in the eighth segment, and the longitudinal ridge does not extend in front of this segment. The worm is about 1½ inches long, and occurs between tide-marks. Travisia forbesi Jnstn., North Sea. Polyophthalmus pictus[390] Duj. is very abundant at some parts of the coast. There are two bundles of chaetae on each side of every segment; each bundle contains three chaetae, of which only one projects to any distance. Paired eye-like spots exist on the sides of twelve segments. The worm is about an inch in length.
Fam. 2. Maldanidae (= Clymenidae).—Represented on our coasts by four fairly common species. They form sandy tubes, which are embedded in the sand with a short portion projecting. In some places they are so abundant that at low water the sand has quite a rough appearance. The prostomium is frequently truncated and depressed, and is always fused with the peristomium. A horny plate may be developed on the upper surface of the head, and the skin at the side of the prostomium is frequently raised into a more or less prominent fold. The hinder end of the body carries a funnel surrounding the anus. There are no gills or sensory processes on the body. Some of the segments towards the middle of the body may be longer than the rest. Peculiar serrated hooks of characteristic shape constitute the neuropodial "torus." The buccal region is eversible.
Nicomache lumbricalis Fabr. is a rosy-pink worm with white spots anteriorly; the chaetigerous ridges are red. The worm consists of twenty-six segments, and measures 2 or 3 inches. It is very narrow and readily breaks in pieces. The prostomium is laterally compressed; the anal funnel is fringed with a number of short equal processes. Under stones in the Laminarian zone.
Axiothea catenata Mgrn., which may reach a length of 3 or 4 inches, resembles the above in general colour, though of a deeper tint. There are only eighteen chaetigerous segments. The head has a membranous fold of skin on each side, and the anal funnel is produced into longer and shorter processes. Both {333}these species are also found on the west side of the Atlantic. Clymene lumbricoides Qfg. is about 8 inches long; pink, with a light ring round each segment; the seventh segment is larger and reddish-brown. The prostomium is laterally compressed. Anus on a cone, which rises from the bottom of a funnel, the margin of which is entire. Atlantic.
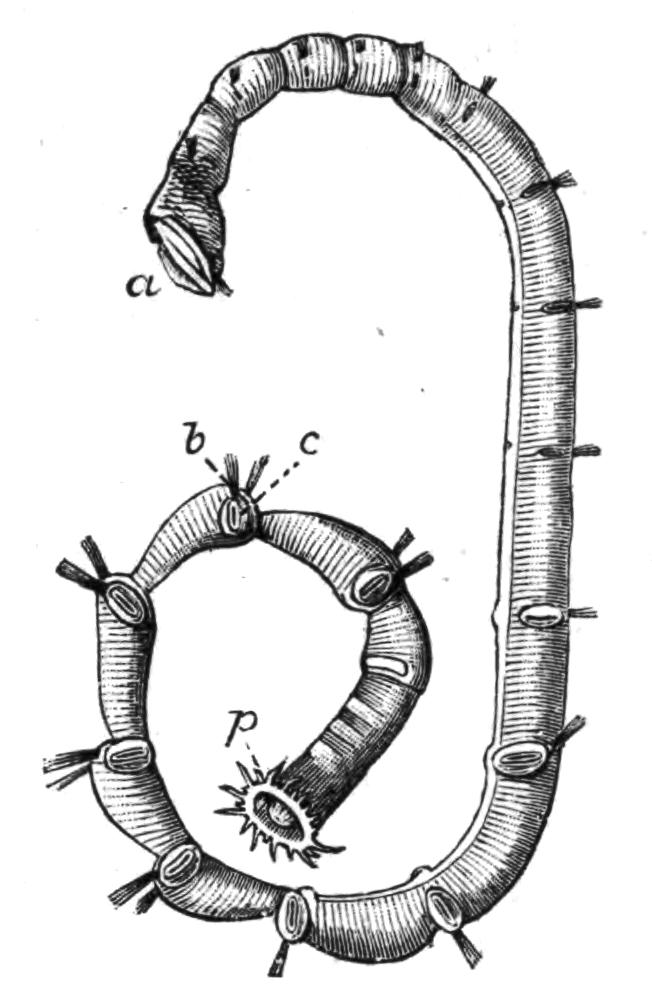
Fig. 179.—Axiothea catenata. × ½. a, Anterior end; b, notopodial and c, neuropodial chaetae; p, perianal funnel. (From Malmgren.)
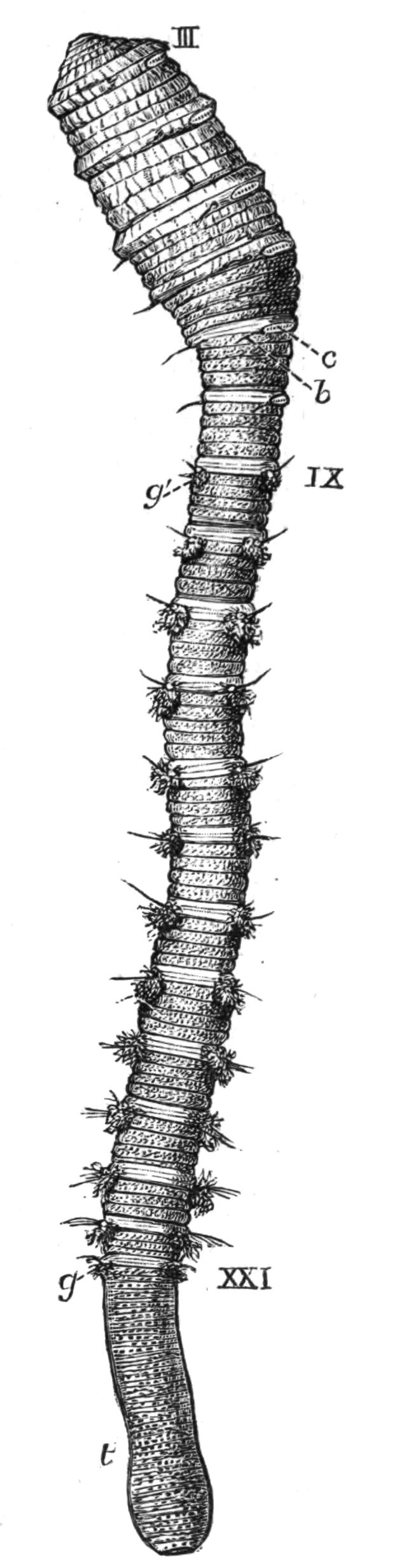
Fig. 180.—Arenicola marina. × 1. Dorsal view. The anterior end is seen partly from the side. III, The first chaetigerous segment; IX, the ninth chaetigerous, and first branchial segment; XXI, the last branchial segment; b, notopodial and c, neuropodial chaetae; g', g, the first and last gills; t, the non-chaetigerous tail.
Fam. 3. Arenicolidae.—Here belongs the common "lug-worm" Arenicola marina L., which occurs all round our coasts between tide-marks, and is so generally used as bait in fishing. The worm, which measures 5 to 8 inches, is of a dark tint, usually brownish-green. It burrows to a depth of some 18 inches or 2 feet, and throws up a considerable quantity of "castings" or "sand-ropes," which are noticeable on every shore consisting of mud or muddy sand. The body of the worm is cylindrical, thicker anteriorly; the segments are indistinct, owing to the secondary groovings and furrows on the skin. The prostomium is in the adult fused with the peristomium; this and the second segment are achaetous. Then follow twenty chaetigerous segments with dorsal bundles of capillary chaetae and ventral rows of short crotchets. The hinder region of the body is achaetous and narrower than the rest, forming a "tail." There are twelve (sometimes thirteen) pairs of arborescent red gills on segments 9 to 20 (21). Internally there are {334}only four complete septa, and six pairs of nephridia, which are of large size: the fore-gut is eversible. Atlantic and Mediterranean. A second and smaller species, A. ecaudata Jnstn., occurs on our southern coasts; it is readily distinguished by the absence of a "tail," the chaetae and gills being continued to the end of the body.
Fam. 4. Scalibregmidae.—Prostomium ill-marked, fringed with small processes. Parapodia represented by slight papillae; two bundles of chaetae; usually cirriform lobes above and below them. Lipobranchius jeffreysii M‘I. has a grub-like body pointed at each end; forms tubes of mud. Firth of Clyde and elsewhere in North Sea. Sclerocheilus Gr. in old oyster shells. Channel, Mediterranean. Eumenia crassa Oerst. has gills on first six segments. North Sea. Scalibregma inflata Rthke. has arborescent gills on segments 4 to 7. The anterior part of the body is dilated. North Sea.
Fam. 5. Chlorhaemidae.—The family derives its name from the green colour of the blood, due to chlorocruorin. The representatives are comparatively short worms, with capillary chaetae on all the segments, the limits of which are not evident. The prostomium carries a pair of long grooved yellowish processes, which are perhaps palps, and several green tentacles, acting as "gills," arranged in a transverse series above the mouth (Fig. 134, F, p. 262). The peristomium is achaetous; the whole "head" can be withdrawn into the body. The chaetae of the anterior segments are especially long, and directed forwards so as to form a "cage" for the head. The body-wall is covered with longer or shorter papillae. Internally, the chief points of interest are the presence of only two septa (Trophonia) or only one septum (Siphonostoma), situated somewhere in front of the middle of the body, and forming a great backwardly-directed pouch, which contains a part of the looped intestine, and the nephridia, of which there are only two or four.
Trophonia plumosa Müll. is about 2 to 4 inches long, yellowish-brown in colour, with a rough skin; the head is usually retracted. It lives in the mud amongst Laminarian roots down to 50 fathoms. North Atlantic. Siphonostoma (Flabelligera) diplochaitos Otto, has a transparent body-wall, so that the coloured viscera are visible. The skin carries long papillae, which traverse a thick jelly-like envelope secreted by it, in {335}which numerous diatoms live (symbiotically?); the surface is covered by particles of mud, etc. This species, which may be found under stones at low tide, occurs also in the Mediterranean.
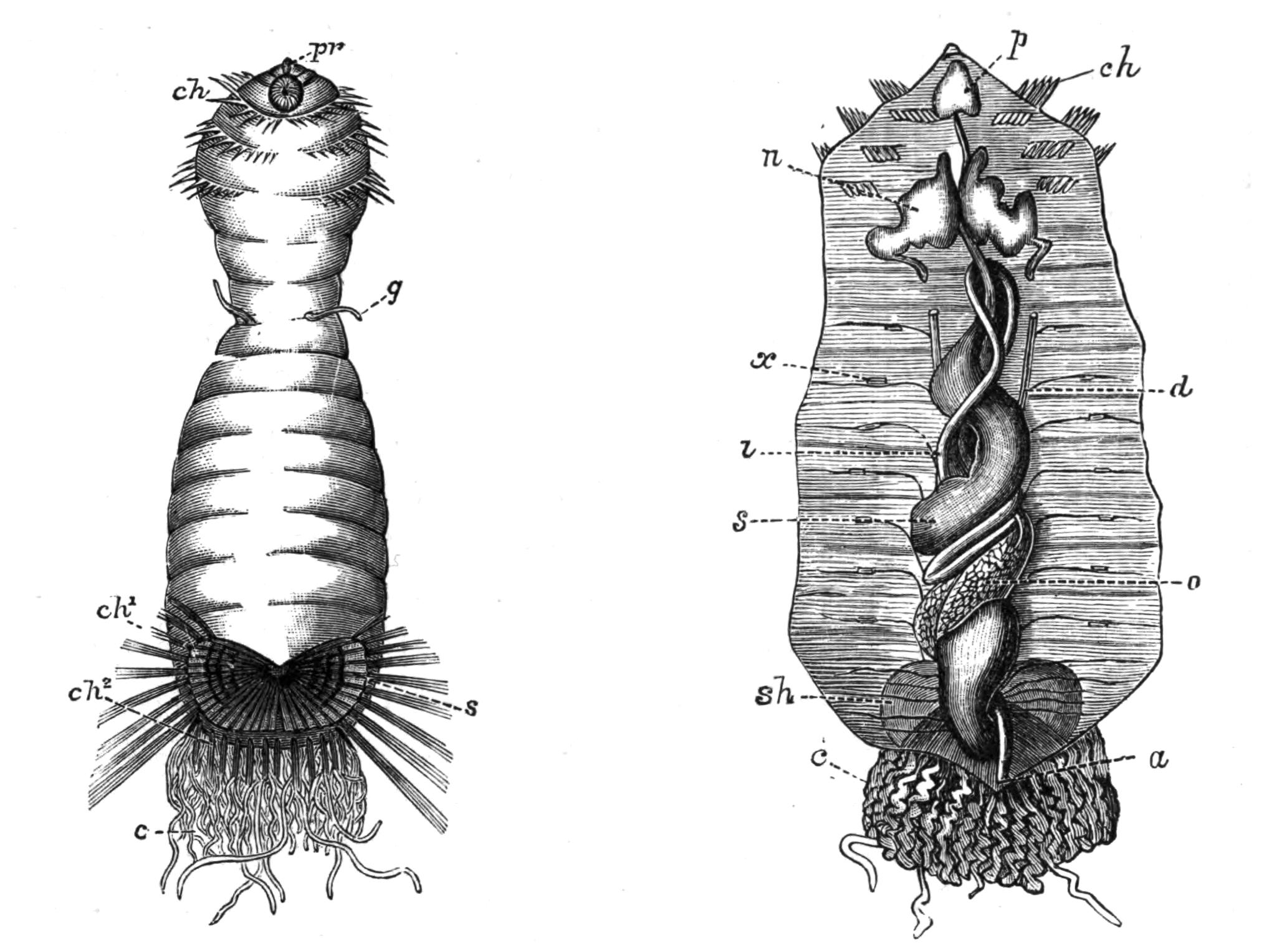
Fig. 181.—Sternaspis scutata Ranz. × 2. (From Vejdovsky.) The left figure shows the ventral surface; the right represents the internal organs as seen when the body-wall is pinned aside, having been slit up along its dorsal surface. a, Anus; c, gills; ch, anterior strong chaetae; ch1, bundles of chaetae along the lateral margin of the shield; ch2, the posterior marginal chaetae; d, oviduct; g, the external tube carrying genital pore; i, coiled intestine; n, nephridium ("brown tube"); o, ovary, amongst the coils of the alimentary canal; p, pharynx; pr, prostomium, with mouth just behind it; s, shield (on left figure); s, stomach (on right); sh, outline of shield seen through the ventral body-wall (in right figure); x, chaetae embedded in the body-wall, with nerves passing by them.
Fam. 6. Sternaspidae.—The single genus, Sternaspis, has not been recorded on our coasts, but is of so peculiar a structure as to deserve a description.[391] S. scutata Ranzani, occurring in the Mediterranean, is rather less than an inch in length, and derives its name from the possession of a pair of peculiar "horny" plates or shields on the ventral surface posteriorly. Around their margins are set about thirty bundles of long capillary chaetae. There are three half rings of stronger chaetae on each side near the anterior end of the body. The mouth is overhung by a very {336}small rounded knob (prostomium), which in S. spinosa Sluiter, is prolonged outwards on each side to form a grooved palp-like organ, recalling that of Bonellia. The anus is placed posteriorly; and in front of it, on the dorsal surface, are two bundles of many long thread-like gills. On the ventro-lateral surface, in front of the middle of the body, is a pair of finger-shaped processes containing the genital ducts. The anterior segments of the body can be withdrawn into the body, as in the Chlorhaemidae. Further examination leads to the conclusion that the body of Sternaspis consists of about thirty segments, most of them provided with paired bundles of capillary (neuropodial?) chaetae, distributed as follows:—Each of the segments 2, 3, 4 has a half ring of strong chaetae on each side; segments 5 to 7 are without chaetae; segments 8 to 14 have chaetae embedded in the body-wall, but not projecting. The shields cover the remaining segments; and along the outer edge of each are some ten bundles of chaetae, and along the hinder edge some five or six bundles, representing as many segments. Thus the worm consists of about thirty segments whose outlines are nearly obliterated (as in the Chlorhaemidae), and whose chaetae, except those which are specially developed, are disappearing: while posteriorly a great shortening of the body brings the bundles close together. A continuation of this process, involving a further disappearance of chaetae, leads readily to the condition met with in Echiurus, one of the chaetigerous Gephyrea. Internally, further evidence of the relation between Sternaspis and the Chlorhaemids with the Gephyrea is afforded by the absence of septa, by the coiled alimentary canal, and by the presence of a single pair of nephridia, which in the latter group act both as excretory organs and as genital ducts.
BRANCH B. CRYPTOCEPHALA.
Sub-Order 1. Sabelliformia.[392]
Fam. 1. Sabellidae.—The branchial crown consists of a usually considerable number of filaments arising from a semicircular base. The peristomium may be reflexed to form a collar, {337}which is frequently notched, so that a lateral and a ventral lobe on each side may be distinguished (Fig. 133, B, p. 261). The thorax consists of nine segments, and is provided with ventral "gland shields," which are continued along the abdomen, where they are subdivided into two by a ciliated "faecal groove," which sometimes bends to one side on reaching the thorax, and may extend forwards along the dorsal surface to the head: this groove serves to carry the faeces out of the tube. The gill filaments are always provided with secondary processes, and may be provided with compound eyes.[393] The tubes of the Sabellidae are always of fine mud or of sand.
Sabella pavonia Sav. is about 10 to 12 inches long and about ¼ inch across; the tube of fine mud is considerably longer and embedded deeply in the mud, with its free end projecting to some 2 or more inches, where it serves for the attachment of seaweed, Polyzoa, Hydrozoa, etc. The colour of the animal is orange-brown; the gills, which are about 1½ inches long, are green (due to contained blood) marked with more or less extensive brown or purple-brown spots, which may even hide the green tint. There is a pair of dark filaments arising between the dorsalmost gill filaments, which have been erroneously regarded as "prostomial tentacles"; they are, in fact, prolongations of a peculiar membrane or lip round the base of the gills, which bounds a groove leading to the mouth. These lip-processes (Fig. 133, B, l) occur in other Sabellids. Atlantic, North Sea, and Mediterranean.
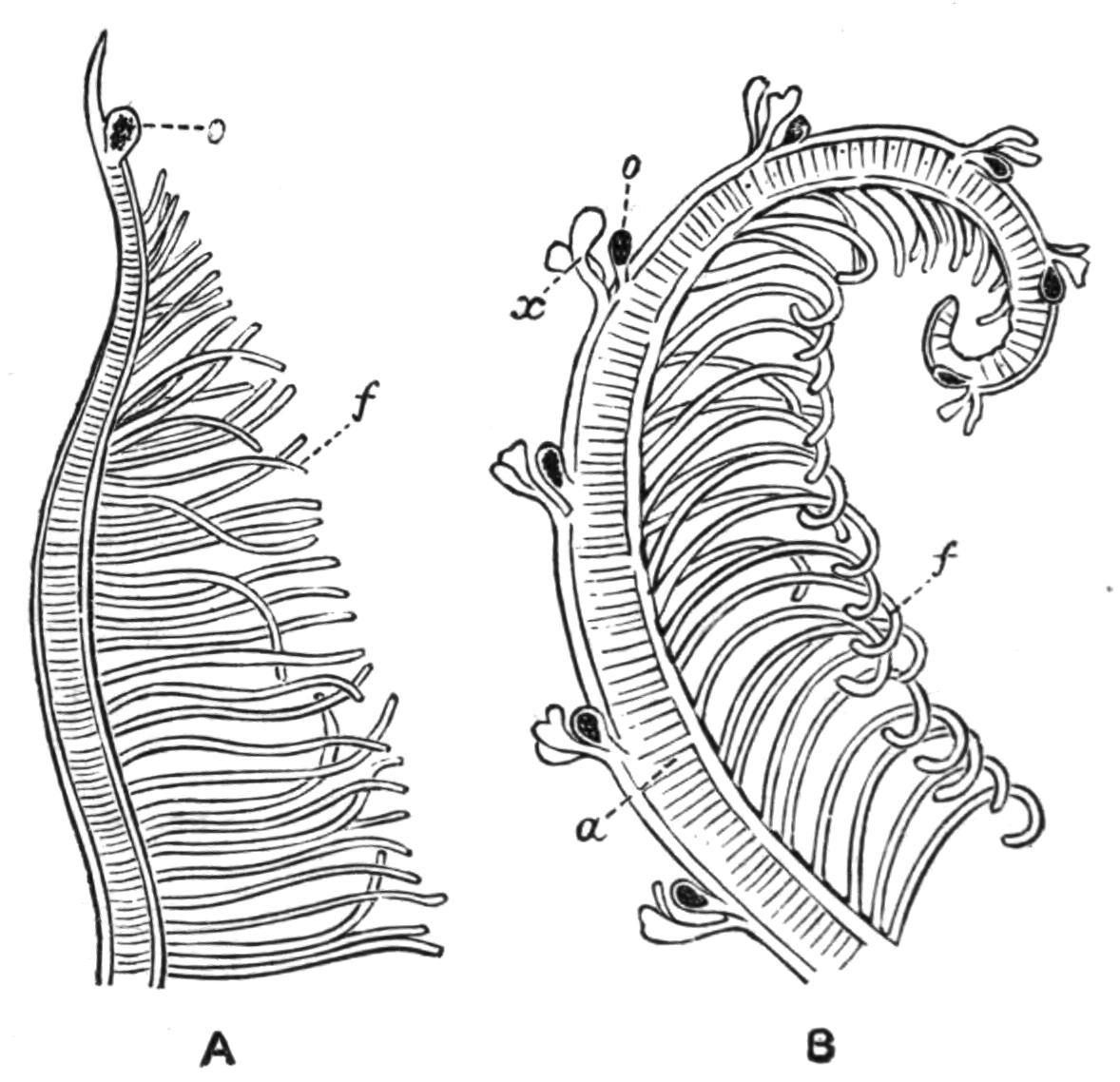
Fig. 182.—A, A gill filament of Branchiomma; B, of Dasychone. a, Axis; f, secondary filaments; o, eye; x, dorsal appendices.
Branchiomma vesiculosum Mont. forms a sandy tube near low tide-mark. The animal, measuring 6-7 inches, is rich brown, darker anteriorly, abundantly speckled with white; the ventral surface is pink; the gills are green or {338}olive-brown, marked with white bands in a fairly regular fashion. Each gill filament has, just below its tip, a compound eye, consisting of several lenses and retinae. North Sea and Mediterranean.
Dasychone bombyx Dalyl. is a short, comparatively stout worm usually 1 to 1½ inches long; reddish-brown in colour, with a darker spot on each side of every segment. The gills are lighter with greenish marks. This worm may readily be recognised, for each of the gill filaments carries some six to ten pairs of dark compound eyes at intervals along its length, and near to each pair there arise two short processes from the outer side of the filament, which are known as "dorsal appendices." The worm forms a tube of mud, more or less mixed with sand. It occurs at low water and to some depth round the coasts of the Atlantic, North Sea, and Mediterranean.
Chone infundibuliformis Kröyer may be recognised by the absence of lobes on the collar, the presence of a membrane connecting the gill filaments, and the passage of the faecal groove along the dorsal surface of the thorax. The worm is 6 inches long, with purple gills, spotted with yellowish-white. The tube is formed of yellowish membrane covered with sand, and is fixed to stones and other objects. Potamilla reniformis Müll. is about 3 inches long, with about twelve brown gill filaments, some of which have eyes near the base. The tube is transparent and horny, with sometimes a slight covering of sand. Found in old oyster shells. North Sea, Atlantic, Mediterranean.
The genus Spirographis contains one of the largest European Sabellids, S. spallanzanii Viv., which occurs off the Channel Islands and in the Mediterranean. The two gill plumes are unequal; the large rone forms an upright, spirally-coiled column.
Fam. 2. Eriographidae.—Myxicola infundibulum Mont. has its gill filaments connected by a membrane reaching nearly to their tips. Each gill plume forms a semicircle; there are no eyes; the peristomium does not form a collar; no gland shields. The worm requires neither of these structures, since it is practically a free-swimmer, envelopes itself in mucus, and moves tail first. The faecal groove is not well marked, though continued dorsally. In the abdomen the tori uncinigeri extend dorsally and ventrally {339}(beyond the neuropodial chaetae) and nearly encircle the body. The animal is 4 or 5 inches long, dull green, with purplish gills. Between tide-marks. North Sea and Mediterranean.
Amphiglena mediterranea Leyd. is only about ¼ inch long, hermaphrodite, and has eyes on the peristomium and on the anal segment. It is a very elegant little worm, and as a living object under the microscope, with the cilia on the gills, is very beautiful. The gills consist of six filaments on each side, provided with the usual double row of ciliated processes.
Fam. 3. Amphicorinidae.—Small hermaphrodite Sabellids in which each gill tuft contains only a few branching filaments. The simplest form is Haplobranchus aestuarinus Bourne,[394] which occurs in the rather foul mud at low tide in the estuaries of the Thames, the Liffey, and other rivers. The animal is about ¼ inch long, with four finger-shaped processes on each side, and a pair of larger, vascular processes on the ventral surface. These five branches are gills (palps), although, owing to the small size of the worm and simple vascular system, the four lateral filaments have no blood-vessels. The animal consists of only eleven chaetigerous segments, and lives in a tube made of mud particles.[395] Fabricia sabella Ehrenb. (Amphicora fabricia Müll.) has three gills on each side, each with a number of secondary branches of different sizes, but so arranged as all to reach the same level. It has eyes in its tail and swims backwards.
Fam. 4. Serpulidae.—The thorax is provided with an undulated membrane on each side, chiefly employed in smoothing the inside of the tube; it represents the dorsal and ventral cirri of these segments. The gland shields are confined to the thoracic segments. In many genera the dorsalmost gill filament on one or both sides is terminally dilated and serves as an operculum. The tube is calcareous, and attached to rocks, shells, etc., for a greater or smaller part of its extent.
Serpula vermicularis L. forms a pinkish tapering tube about 3 inches long; the narrower fixed end is coiled. It is marked at irregular intervals with encircling ridges, indicating cessation {340}in formation, and has a circular aperture. The worm itself is about 1½ inches long. The horny operculum is conical, with its base upwards, fringed with short processes. 20 fathoms in the North Sea and Mediterranean; also from 275 fathoms off the west coast of Ireland.
Pomatoceros triqueter L.—The white shell is adherent, with a distinct keel along its upper surface; the aperture is overhung by a spine. The tubes are abundant everywhere, attached to rocks, stones, shells, etc., between tide-marks and down to 18 fathoms. The animal is very handsome, the thorax being deep blue, the abdomen red in the female, whitish in the ripe male. The branchiae are barred and spotted with blue, orange, and white; the operculum is calcareous, and furnished with a couple of horn-like processes.
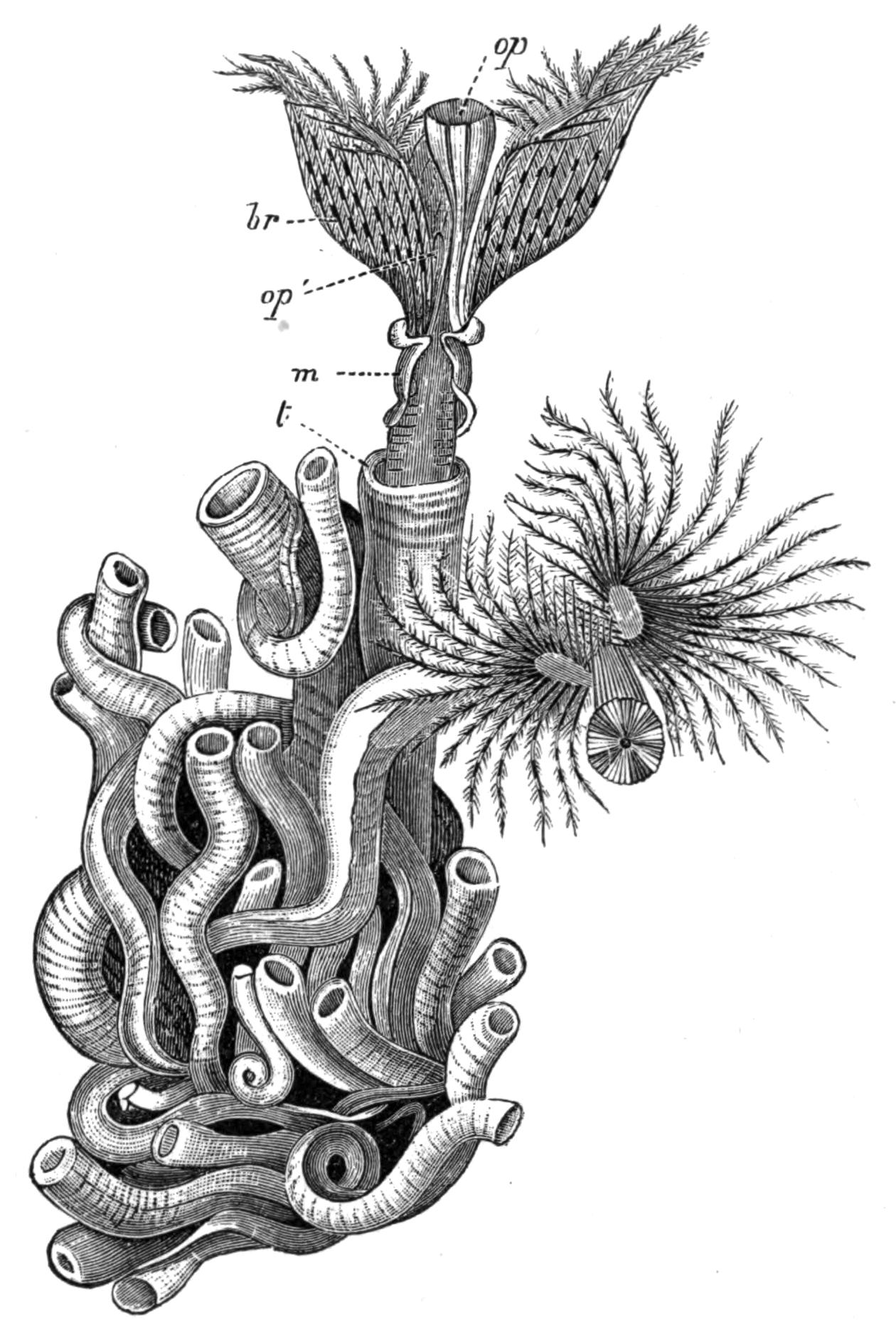
Fig. 183.—A group of tubes of Serpula vermicularis L., from the mouths of two of which the animals are protruding, that on the right being seen from above. br, The gill plume; m, thoracic membrane; op, operculum; op1, corresponding gill filament of the opposite side; t, tube. (From Cuvier's Règne Animal.) Nat. size.
Filigrana implexa Berkeley is a small worm, but the slender white tubes intertwine and adhere together in masses 3 or 4 inches high, occurring at low tide and down to 18 fathoms in the North Sea and Mediterranean. The animal has only eight gill filaments on each side, one of which on each side is slightly expanded to serve as an operculum. The worm multiplies by transverse division.
Spirorbis borealis Daudin is a still smaller worm, the tube of which is coiled in a flat spiral about 1⁄16 to ⅛ inch across; it is {341}common, adhering to Fucus, shells, and other objects. It is represented by fossils in the Palaeozoic rocks. Cosmopolitan.
Protula (Psygmobranchus) tubularia Mont. is a Serpulid without an operculum; it forms a straight or slightly and irregularly curved tube. Atlantic and Mediterranean. Salmacina dysteri Huxley has no operculum; it is a small worm incrusting seaweeds, or forming masses like Filigrana.
Sub-Order 2. Hermelliformia.
The single family Hermellidae is represented by two species—Sabellaria alveolata L., which is littoral, and S. spinulosa Leuck., occurring in 10 to 30 fathoms.
S. alveolata[396] is about an inch long; the thorax is purple, the abdomen yellow to red. The narrow caudal region is bent sharply forwards, so that the anus, situated at its tip, comes to lie at the orifice of the tube, which is irregular and sandy. Great numbers of the animals live together, so that the masses of their tubes may be 2 or 3 feet thick and several feet long. They are well seen on the shore, at Paignton, near Torquay, and on Hilbre Island, off the Cheshire coast. North Sea, Atlantic, Mediterranean.
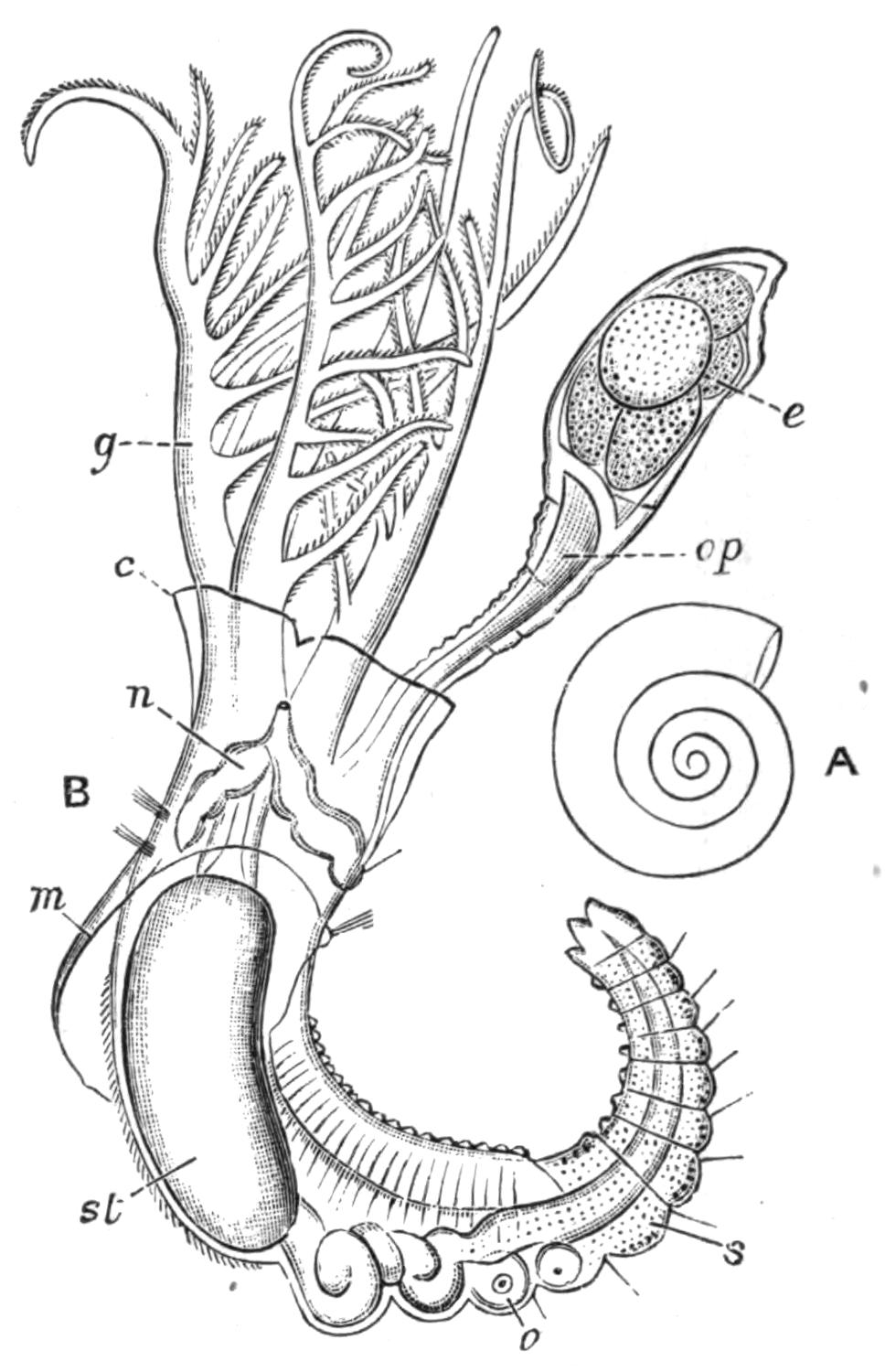
Fig. 184.—Spirorbis. A, the shell, enlarged. B, the animal, × 50. c, Peristomial collar; e, eggs in the brood pouch at the end of the operculum; g, gills; m, thoracic membrane (characteristic of Serpulidae); n, the single pair of thoracic nephridia opening by a median dorsal pore beneath the collar (common to all Sabelliformia); o, ova in the anterior abdominal segments; op, operculum; s, spermatozoa in the hinder abdominal segments; st, stomach. (From Claparède.)
Order III. Myzostomaria.[397]
These animals are parasitic on Crinoids or Asteroids.[398] The {342}single family, Myzostomatidae, contains but two genera, Myzostoma F. S. Lkt. and Stelechopus v. Gr.
Some of them move, more or less actively, on the surface of their hosts, others live in a sessile condition between the joints of the arms or pinnules, causing a greater or less malformation thereof, sometimes leading to the formation of a more or less globular cyst like a plant-gall, due to overgrowths of the joint, as in M. deformator v. Gr. and M. cysticola v. Gr.: while M. pulvinar v. Gr. is endoparasitic in the intestine. Two species occur on our common Antedon (Comatula) rosacea; one, M. cirriferum Lkt., creeps about the oral surface, especially along the food grooves of the disc and arms; the other, M. glabrum Lkt., lives close to the mouth of the Crinoid, so that its pharynx can be inserted into the oesophagus of the host; this species rarely moves from this position, and carries a young one on its back.
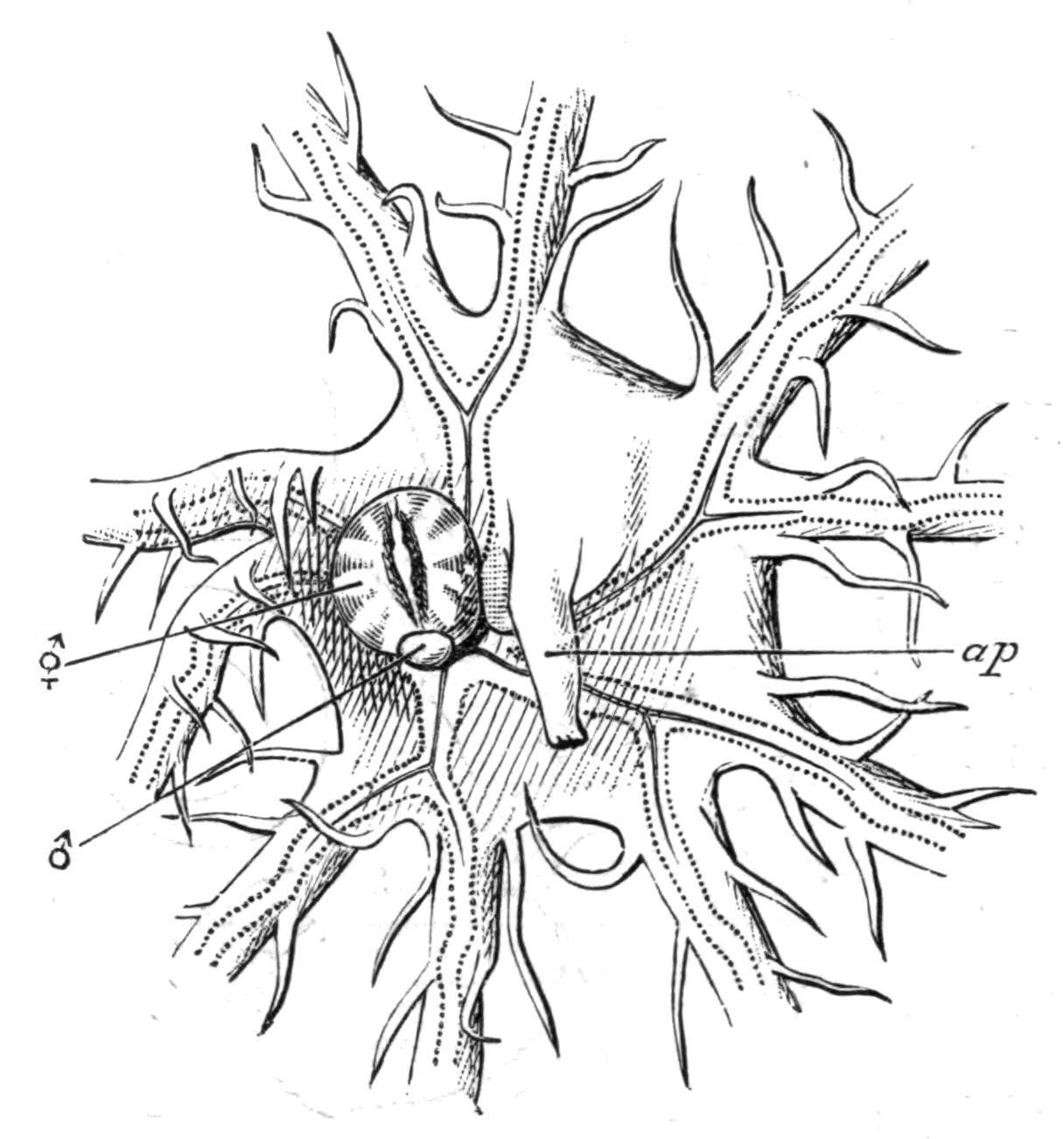
Fig. 185.—Myzostoma glabrum Lkt., on the disc of Antedon rosacea. The hermaphrodite individual (⚥) lies over the mouth of the Antedon, and carries on its back at the anterior end a young one (♂) with only male organs fully developed. ap, The anal papilla of Antedon. × 4.
The Myzostomaria are circular or oval, more or less markedly convex dorsally, flat ventrally; Stelechopus, however, which lives on Hyocrinus, is elongated. The margin of the body is provided with ten or more pairs of cirri, short (M. glabrum) or long (M. cirriferum), and the general appearance of the animal is greatly changed in some species by the great elongation of the hinder cirri, into which the viscera may extend (M. filicauda v. Gr.). On the ventral surface are five pairs of small conical "parapodia," arranged, like the internal organs, in a radiate manner. Each parapodium carries a couple of chaetae; one a hook, the other serving as a "guide" for this hook. The four "suckers" on each side are either glandular or sensory organs; and Wheeler considers them homologous with the lateral organs of Capitellids; they are usually little developed in those species which live inside cysts.
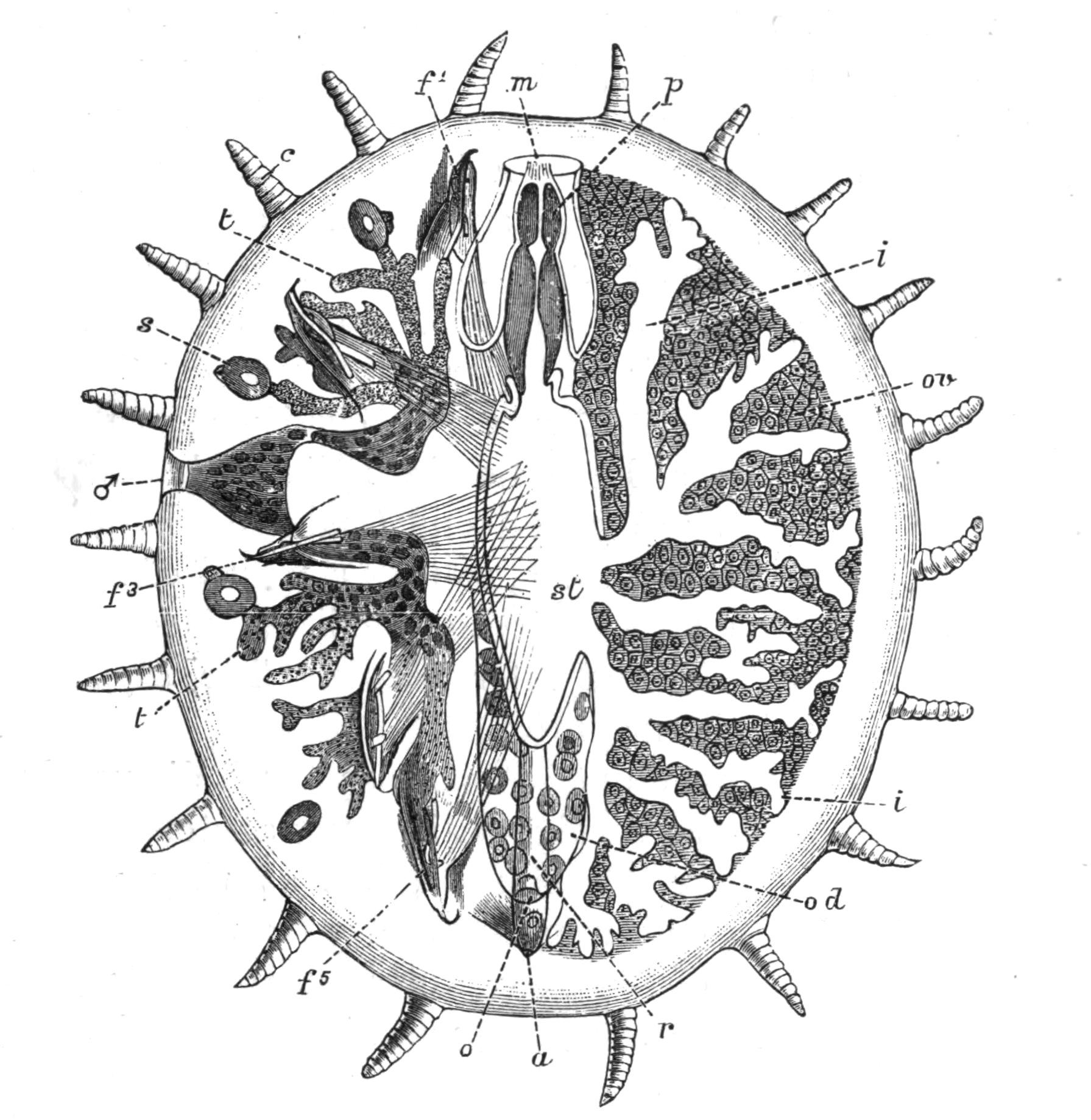
Fig. 186.—Myzostoma cirriferum. (After Lang and v. Graff.) The organs are supposed to be seen by transparency. On the right side the more dorsal organs are shown, and on the left, those lying more ventrally. a, Anus; c, ten pairs of marginal cirri; f1 to f5, the five parapodia of the left side, each with two chaetae; i, the branches of the intestine on the right side; m, mouth; o, the opening of the oviduct (od) into the rectum; ov, the uterus or coelom, filled with eggs, occupying the spaces between the lobes of the intestine; p, the pharynx (acrecbolic introvert) lying in the pharyngeal sac; r, rectum; s, the four "suckers" of the left side; these, like the parapodia, really lie on the ventral surface; st, stomach; t, the branching testis; ♂, the pore of the sperm-duct.
The mouth and anus are usually ventral; but in M. glabrum the anus is dorsal, and in a few species both apertures are carried on to the back by the great development of the ventral surface. The alimentary canal is provided with a protrusible pharynx; the intestine is branched; amongst its branches is the coelom, packed with eggs, and functioning as a uterus (usually called "ovary"). The true ovary is a small mass of cells on each side, a proliferation of the coelomic epithelium covering the intestinal wall. A median continuation of the {344}uterus passes backwards above the rectum, and opens either into it or by an independent pore dorsal to the anus. The "lateral oviducts" of Nansen are nephridia with ciliated funnels opening into the coelom (uterus), and with pores leading into the cloaca on its ventral surface; or, in M. belli Wheeler, opening to the exterior. The two testes are branched, and each sperm-duct opens laterally on a papilla, just outside the third parapodium of each side. Wheeler[399] has recently shown that in the young Myzostoma the spermatozoa ripen before the ova, so that it is functionally a male; before the spermatozoa are all discharged the ova mature, and the animal is for a time hermaphrodite; later on, however, when all the spermatozoa are used up, the worm is a female. Beard's "dwarf males" are therefore merely the young of hermaphrodite forms. In cysticolous species each cyst usually contains a large female individual and a small male. In these cases the young one (male) discharges all its spermatozoa before the ova ripen, so that a period of immaturity intervenes and a true hermaphrodite condition is omitted; the animal is at first male, and later female. The Myzostomaria are thus "protandric hermaphrodites."
The affinity of these animals has been much discussed; they superficially resemble the Tardigrada in many anatomical features, and differ greatly from Chaetopoda, but as they possess the characteristic chaetae or parapodia, and pass through a larval stage[400] similar to that of the Polychaetes, there is no doubt that they are closely allied to the group, and indeed may be regarded as degenerate Chaetopods. It has been suggested that they form a passage group between them and the Tardigrada; and von Graff forms a group Stelechopoda, to include the Myzostomaria, the Tardigrada, and the Linguatulida.
BY
F. E. BEDDARD, M.A. (Oxon.), F.R.S.
Prosector to the Zoological Society
OLIGOCHAETA (EARTHWORMS AND THEIR ALLIES)
INTRODUCTION—ANATOMY—REPRODUCTION—BIONOMICS—DISTRIBUTION—CLASSIFICATION—MICRODRILI AND MEGADRILI
The Oligochaeta form a well-marked branch of that exceedingly large assortment of animals vaguely spoken of as worms, and embracing a number of types many of which have no near relationship to each other. From this great and unnatural group, which has survived as "Vermes" even in some quite modern text-books, we can separate off those forms which show a plain segmentation or division of the body internally as well as externally into a series of more or less similar rings, as a Class Chaetopoda. This Class, consisting of the Orders mentioned on p. 241, includes the worms which form the subject of the present chapter—the Oligochaeta, as they were originally called by Grube, on account of the fewness of their chaetae as compared with the number possessed by the majority of the Polychaeta.
Our knowledge of this group, as of so many others, dates from Aristotle, who called the earthworms the "intestines of the earth." But it is only very recently that the numerous and remarkable genera of exotic earthworms have been anatomically investigated; indeed the common British species was not really well known before the publication of the memoirs of Lankester[401] and Claparède[402] in 1864 and 1868, in spite of the elaborate quarto devoted to it by Morren,[403] the botanist, in 1826. {348}Some of the aquatic species afforded material to Bonnet and Spallanzani for their experiments upon the powers of regeneration of the animals when cut into fragments, while the work of O. F. Müller[404] upon various Naids is a monument of careful anatomical description. Our knowledge of the aquatic Oligochaeta does not appear to have advanced so rapidly as has that of the earthworms.
External Characters.—The most salient external characteristic of this group of worms, which vary from 1 mm. to 2 metres in length, is of course the segmentation. The entire body is divided into a number of rings, which are for the most part similar to each other; a fragment of an earthworm's body could not be accurately replaced unless it had been cut from the anterior region. There is precisely the same regular segmentation in the aquatic representatives of the Order. At the anterior end of the body in the common earthworm (and in nearly all Oligochaeta) is a small unpaired lobe, which overhangs the mouth, and is usually termed the prostomium; the mouth itself is surrounded by the first segment of the body, which never bears any chaetae in any Oligochaete. The prostomium is occasionally greatly developed, and in such cases doubtless forms a tactile organ of importance. This is especially the case with the South American genus Rhinodrilus, where the lengthy prostomium can be retracted at will. The aquatic Nais lacustris (= Stylaria proboscidea) has also an exceedingly long prostomium, which cannot, however, be retracted, though it is contractile. At a certain distance from the anterior end of the body, fixed for the species, but varying greatly from genus to genus and from species to species, is the clitellum. This region of the body (popularly believed to mark the spot where a worm divided by the gardener's spade has come together again) is associated with the reproductive function, and serves to secrete the cocoon in which the creature's eggs are deposited. It has in the earthworm a thick glandular appearance. A more minute examination of the worm's body will show the orifices of the reproductive ducts and of the excretory organs which will be found described below. In addition to these, all British earthworms and a large percentage of the tropical forms have a row of pores along the back, which are between the successive segments in the median line. These "dorsal pores" {349}open directly into the body-cavity, and are mere perforations of the body-wall, not tubes lined by a special layer of cells. Professor Spencer of Melbourne[405] has observed a giant earthworm (Megascolides australis) of Gippsland which, when held in the hand, spurts out, to a height of several inches, the fluid of the body-cavity through its dorsal pores. The burrows, he remarks, are coated over with the same fluid, which is regarded by him as a lubricant. This, however, considering that the glandular cells of the epidermis can secrete a mucous fluid, seems to be an expensive use to which to put the important fluids of the interior of the body. It is more probable that the dorsal pores are a means of getting rid of waste products. Lim Boon Keng[406] suggests that the coelomic fluid possesses a bactericidal function. The dorsal pores are missing in many earthworms, and without exception in those Oligochaeta which live in water; but these latter worms have a pore upon the head, which appears to be wanting in the earthworms. Dr. Michaelsen has thought that the head-pore serves to relieve the brain from undue watery pressure—to act, in fact, as a kind of safety-valve for the liberation of superfluous fluid.
In some foreign worms the pores of the reproductive ducts are conspicuous external features (Fig. 197); even in our British species the turgescent male apertures upon the fifteenth segment are sometimes quite obvious.
Structure of the Body-Wall.—The body-wall consists in all Oligochaeta of three recognisable sheets of tissue. Outside is the epidermis, which always consists of a single layer of cells, except in the clitellar region of earthworms. It is a point of difference between the aquatic genera and the terrestrial forms that in the former the clitellum is only one cell thick, while in the higher Oligochaeta it is made up of more than one layer of cells. The epidermis is ciliated only in the genus Aeolosoma, and there only on the prostomium. It secretes a thin layer of chitin, which is defective opposite to the glandular cells, and becomes therefore perforated by numerous pores. The structure of the epidermis of Lumbricus has been studied by Cerfontaine, whose recent account[407] of the same is the fullest and most accurate that exists.
Underneath the epidermis comes a layer of circular muscle-fibres, {350}and underneath this again a layer of longitudinal muscles. In both layers the fibres have a softer core, outside which lies the radially striated muscular substance. The fibres are embedded in a granular matrix. It used to be considered at one time that such medullated fibres were distinctive of leeches as opposed to Oligochaeta. Their existence has been really known in the Oligochaeta since the researches of Ratzel; but Cerfontaine has fully described them, and emphasised the fact that the fibres of both circular and longitudinal coats are alike in this respect.
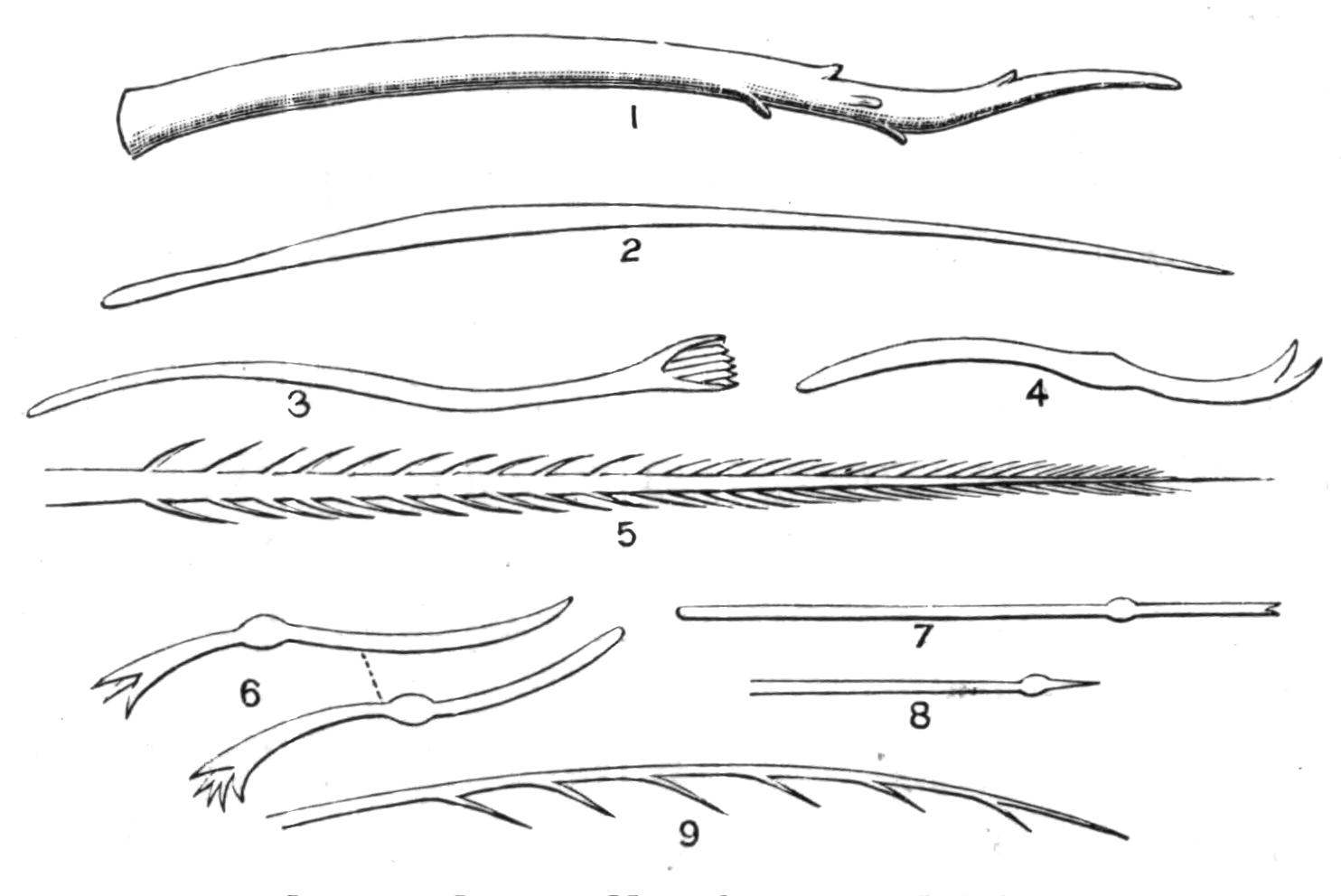
Fig. 187.—Chaetae of Oligochaeta. × 10. (After Michaelsen, Stolc, and Vejdovsky). 1, 2, Penial chaetae of Acanthodrilus georgianus; 3, Spirosperma; 4, Ilyodrilus; 5, Lophochaeta; 6, Tubifex; 7, 8, Nais; 9, Bohemilla. Figs. 3-9 are ordinary chaetae.
Chaetae.—The passive organs of locomotion in these animals are the chaetae, which are absent in only one family, Discodrilidae, and in one other genus, Anachaeta. In this latter worm the chaetae are represented by large glandular cells, which seem to correspond to the cells from which the chaetae arise in other forms. They are in this case, as in the others, cells of the epidermis. The chaetae of the Oligochaeta are not quite so variable in form as in the marine Polychaeta (see Fig. 138, p. 267). Figs. 187 and 188 illustrate some of the principal shapes which these bristles assume. The most prevalent form is an elongated S, which has been aptly compared to the mathematical sign ∫. This kind of chaeta is found in all earthworms, and in not a few aquatic genera such as the Lumbriculidae. In some of the latter and in the Tubificidae and Naids there is the same form of chaeta, which is cleft at the free end, and possibly enables the worm to grasp the leaves of aquatic plants, and otherwise facilitates progression in a laxer medium than the stiff soil frequented by the earthworms. Even earthworms, at any rate the genus Pontoscolex, have chaetae of this kind; some of the aquatic Oligochaeta have elongated and hair-like bristles, such as that of Tubifex.
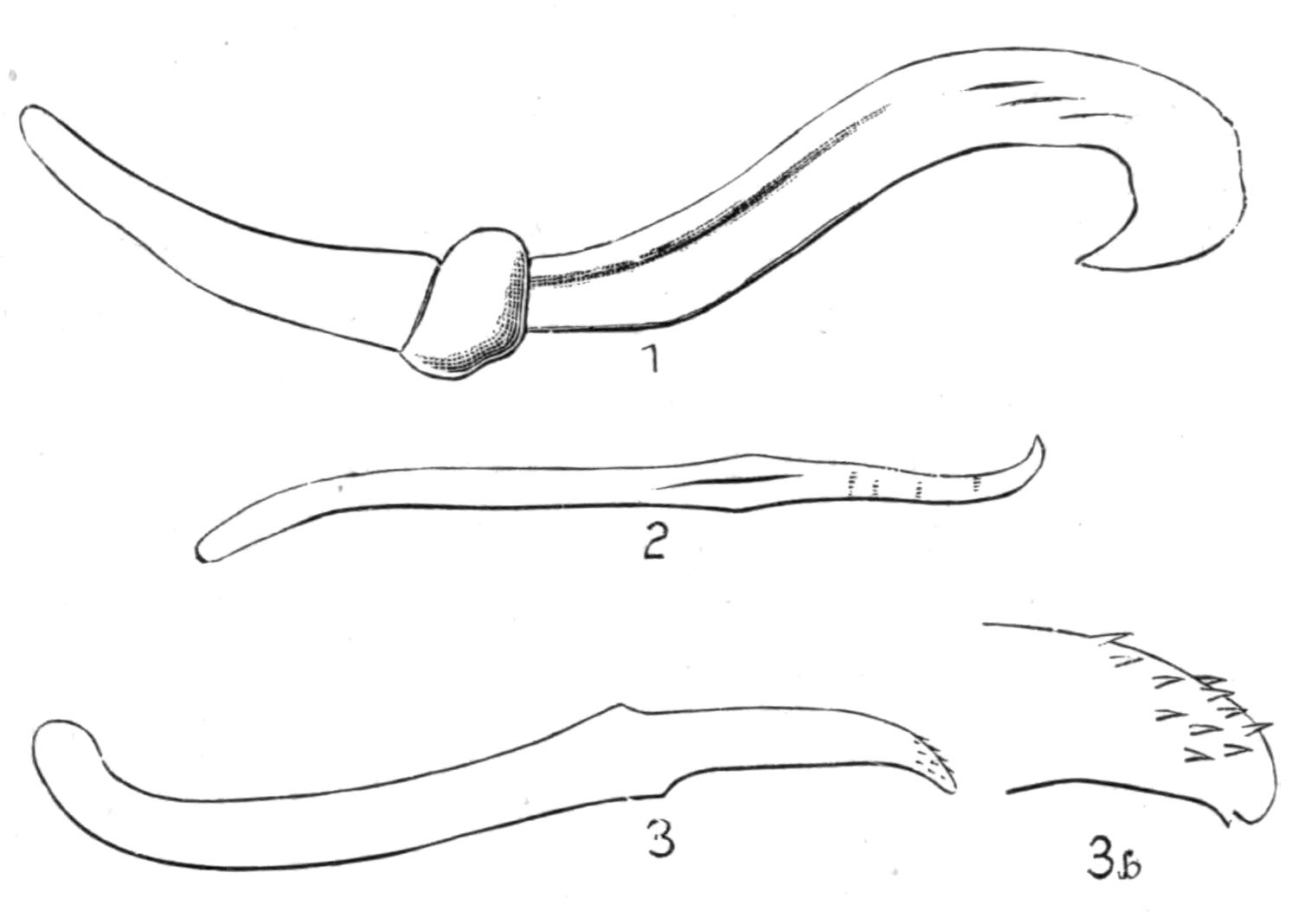
Fig. 188.—Chaetae. × 10. 1, Onychochaeta; 2, Pontoscolex; 3, Trichochaeta; 3b, the same, more highly magnified.
In the Tubificid Lophochaeta (Fig. 187, 5) the chaetae are ornamented on both sides with delicate processes, which give them the appearance of Crustacean hairs. Among earthworms the simple S-like form is sometimes complicated by the development of sinuous ridges upon the distal end. No doubt these bristles enable their possessor to get a firmer grip of adjacent objects; they are very commonly found, in the family Geoscolicidae, upon the segments of the clitellum, and permit of a firmer union during sexual congress. In no Oligochaeta are the chaetae borne upon parapodia, as is the case with the Polychaeta; but in many of the aquatic forms there are a considerable number to each bundle. In earthworms the number of chaetae varies greatly. The common earthworms of this country, belonging to the genera Lumbricus, Allolobophora, and Allurus, have only eight chaetae upon each segment of the body, and these are then, as a rule, arranged in pairs or rather couples, two of each on each side of the body. The genus Perichaeta and some of its allies have a much larger number of chaetae to each segment, disposed in a continuous row round the middle of the segment. The intermediate condition is to be seen in the genus Deinodrilus, where there are twelve in each segment, and in certain members of the genus Megascolex, where there are eight in each segment in the anterior region of the body, the number increasing in the posterior segments. The four bundles of chaetae in the Naids and Tubificids have been likened to the {352}notopodia and neuropodia of the Polychaetes; but it does not seem certain that this comparison is justifiable. It was at one time thought that the continuous circle of chaetae of the Perichaetidae was the primitive condition; but Professor Bourne has lately found that in Perichaeta the young embryos have not got this continuous circle; it is only acquired later.
Branchiae.—The Oligochaeta were called by Cuvier the "Annélides abranches sétigères." But the epithet "abranches" is now known to be inaccurate. In fact it really was so when Cuvier wrote; for naturalists were at that time well acquainted, chiefly through the elaborate work of O. F. Müller, with the little fresh-water Naid Dero, the posterior extremity of which is provided with a varying number of branchial processes. These are furnished with looped blood-vessels and are covered externally by cilia, so that the water containing oxygen is constantly renovated. The second instance of a gilled Oligochaete was discovered in the very same family. Professor Bourne[408] of Madras found in "tanks" a Naid which he named Chaetobranchus, in which the head segments, to the number of fifty or so, are provided with long ciliated processes, which as a rule enclose the dorsal chaetae of their segments, and in addition a capillary loop. Curiously enough, this very same worm made its appearance in the Victoria regia tank at the Botanical Gardens in the Regent's Park, whither it had in all probability been accidentally imported. Two members of the family Tubificidae were the next examples of gilled Oligochaeta made known to science; one of these, Branchiura sowerbyi,[409] appeared also in the Botanical Gardens, so that its native home is unknown. It differs from Chaetobranchus in that the gills are at the posterior end of the body, and are contractile; during the life of the worm they are in continual motion. A species of the South American genus Hesperodrilus,[410] H. branchiatus, is also gilled, and, so far as can be made out from a spirit-preserved specimen, the gills are precisely of the same pattern and contractility as those of its ally Branchiura. Possibly Branchiura ought to be included in the same genus with Hesperodrilus. A worm which was originally described by Grube as Alma nilotica, should really have been placed before the three last-mentioned {353}instances; but as this worm was only known from a fragment, and as the description was not by any means full, it was not thoroughly believed in; it was surmised that it might be a member of some marine genus, perhaps of the Capitellidae. Oddly enough, the same worm was independently described by a different name, Digitibranchus niloticus, a few years later by Levinsen. Quite recently Michaelsen has found by a reference to the original types that this worm is really gilled, and that it is specifically identical with a worm which had been given a totally different name, viz. Siphonogaster. The fact that the gills of the latter had been overlooked was readily explained by the circumstance that they are retractile, and not merely contractile. But all the species of the genus Siphonogaster, or Alma, as it ought really, following the rules of priority, to be called, have not got gills, as is the case too with the genus Hesperodrilus. The gills of Alma are branched, and there is therefore no longer any justification whatever for defining the Oligochaeta as a group of Annelids without gills. The simple gill-like processes of Chaetobranchus might have been held to be not accurately comparable to the more complex structures which we find in the marine worms.
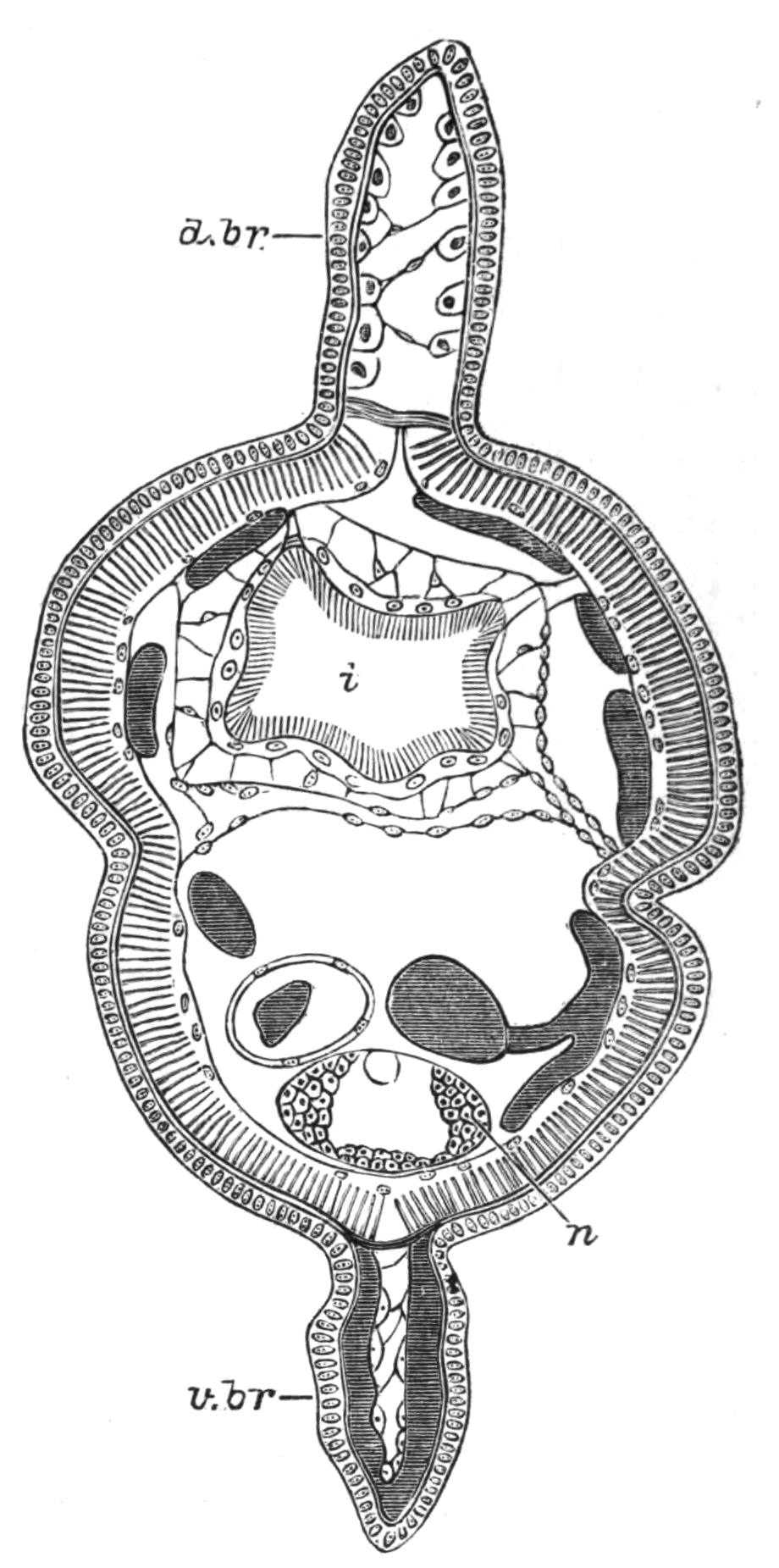
Fig. 189.—Transverse section through Branchiura sowerbyi. × 20. d.br, Dorsal branchia; i, intestine; n, nerve-cord; v.br, ventral branchia.
Nervous System.—The central nervous system of the Oligochaeta is very uniform in its structure in the entire group. The only family which is at all anomalous is that of the Aphaneura. In Aeolosoma there appears to be only a pair of cerebral ganglia, which retain the primitive position of these organs in being still in direct connexion with the epidermis. In all other Oligochaeta there are a pair of cerebral ganglia, {354}connected by a circumoesophageal commissure with a ventral ganglionated cord. From the cerebral ganglia arises a system of nerve-fibres and nerve-cells, which represents the stomatogastric nerves of other Invertebrates.
Senses and Sense-Organs.—The only organs that can be regarded with anything like probability as sense-organs are the pigmented eyes of certain Naids and the tactile cells of many worms. The latter are usually elongated cells provided at their free extremity with a stiff process; they occur associated in groups, and often these bundles of cells have a segmental arrangement. The head end of many of the lower Oligochaeta, for instance the genus Aeolosoma, has delicate processes projecting here and there; these appear to be also of a tactile nature, and are of course connected with cells of the epidermis. The eyes of certain Naids are little more than lenticular bodies embedded in a mass of pigment. In the genus Eudrilus and in many Eudrilidae are peculiar integumental bodies, which were independently discovered by Dr. Horst[411] and myself, and compared by us to the Pacinian bodies of Mammals. Whether these structures are connected with nerves or not is doubtful. In spite of the poor development and the simplicity of their sense organs, the higher Oligochaeta at any rate can feel, and can distinguish light from darkness. Darwin[412] came to the conclusion that "light affects worms by its intensity and its duration." And furthermore, it is only the anterior end of the body which is thus affected. Of the sense of hearing these animals appear to be utterly devoid. Some kept by Darwin "took not the least notice of the shrill notes from a metal whistle, which was repeatedly sounded near them; nor did they of the deepest and loudest tones of a bassoon." But it is always necessary to discriminate between sound and vibrations passing through any solid body, which would appeal rather to a sense of touch. Here worms are most sensitive. It is quite easy, by digging with some vigour, to arouse the worms in the neighbourhood, who will crawl to the surface and away from the scene of action; a proceeding on their part which is sometimes put down to a desire to escape from their enemy the mole.
Smell appears to be another sense which is somewhat deficient. {355}But worms are epicures, and exhibit a decided taste and preference for certain articles of diet. Like their fellow tiller of the soil, the agricultural labourer, worms have a keen relish for onions, which, however, they must recognise by the smell. They prefer green cabbage to red, celery to both, and raw meat appears to be the greatest delicacy that can be offered to them. It is only substances they are not likely to meet with, such as perfumes, tobacco, and paraffin, that produce no impression upon the worm's sense of smell.
Coelom and Vascular System.—When an earthworm is dissected the various organs are seen to lie in a fairly spacious cavity, which is interrupted and divided into a series of chambers by the mesenteries or septa which stretch across from wall to wall of the body, and correspond roughly in their position to the grooves which separate the body externally. This cavity, common to all the higher animals, is known as the coelom; it is lined by cells, which cover the intestines as well as the inside of the body-wall; and upon the intestine assume the form so characteristic of the group, namely, that of large yellow cells loaded with secreted matters, and called "chloragogen-cells" by Claparède. The coelom communicates with the exterior by means of the dorsal pores, the nephridia, and the ducts of the reproductive organs. As in all animals which possess a coelom, the reproductive tissues, ova and sperm, are developed on its walls.
Fig. 190.—Sparganophilus tamesis; general anatomy, × 3. (After Benham.) I-XVIII, segments. 1, 4, 6, Perivisceral vessels (6 is one of the hearts); 2, 3, 7, dorsal vessel; 5, spermatheca; 8, sperm sacs; 9, intestino-tegumentary vessels; 10, ovary; 11, 12, integumentary vessels.
The vascular system of the Oligochaeta forms a system of perfectly closed vessels, which ramify into fine capillary networks in the body-wall, in the coats of the alimentary canal, and upon {356}the other organs of the body. The main trunks are a dorsal and a ventral longitudinal, which communicate directly in the anterior end of the body by large transverse contractile trunks, the so-called hearts (see Fig. 190, 6). The dorsal vessel is also contractile, but not the ventral, or, when it occurs, the subnervian. The vascular system has many degrees of complexity in different families; it is simpler in the smaller aquatic forms. The blood is usually red, and the pigment which is suspended in the plasma is haemoglobin. The blood is corpusculated.
Excretory Organs.—There appears to be a great deal more variation in the structure of the excretory system than there is in many other groups. For a long time only Lumbricus and a few of the aquatic genera were known as regards their excretory systems. In these there is a pair of excretory organs or nephridia in nearly all the segments. These are much coiled tubes, in which it is always possible to recognise three divisions. The nephridium commences with an orifice of a funnel-like character, fringed with long cilia, and opening into the body-cavity; from this springs a tube, which immediately perforates the septum lying between the segment which contains the funnel and the following one; this tube has the peculiarity first pointed out by Claparède of being excavated in the substance of cells; the glandular part of the nephridium is a row of cells which are bored through by a continuous canal, the walls of which are here and there furnished with cilia. It often happens that the main canal gives off minute lateral ramifications, which may even form a kind of network round the principal canal. The terminal section of the nephridium is a muscular sac which opens on to the exterior by a pore, and from which the products of excretion are from time to time evacuated by contractions of its walls. This is a brief statement of the main facts in the structure of those Oligochaeta in which there is a single pair of nephridia to each segment of the body; small differences of more or less importance occur. In Chaetogaster, for example, there is no trace of a funnel; in some genera the terminal sac is much reduced or unusually extended, being even sometimes provided with a caecum of moderate dimensions. In Acanthodrilus novae-zelandiae and a few other species the point of opening of the nephridia varies from segment to segment, though it always bears some relation to the chaetae. In these {357}species the nephridia which open more dorsally are a little different in structure from those which open more ventrally. One set have a caecum, and the other have not.
The nephridia of the terrestrial forms are enveloped by a richly developed network of blood capillaries, which is absent in the smaller aquatic genera.
A very remarkable genus, Brachydrilus, has lately been described by Dr. Benham,[413] in which each segment has two pairs of nephridia instead of a single pair. More recently, certain Australian forms, which I propose to unite on this account into a genus Trinephrus, have been discovered which have no less than three distinct and separate pairs in each segment.[414]
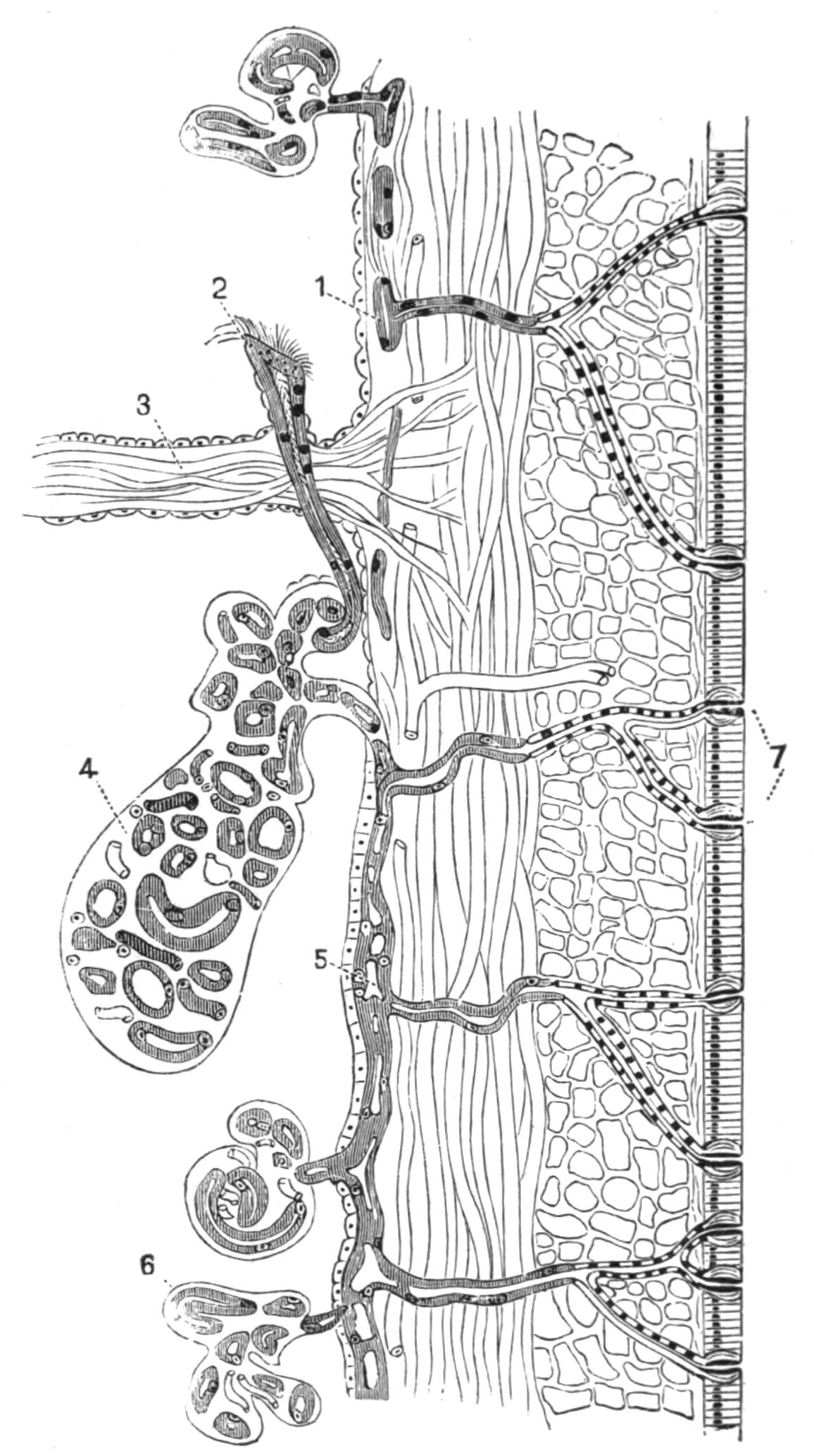
Fig. 191.—Section through body-wall of Megascolides australis, highly magnified. (After Spencer.) 1, 4, 5, 6, Coils of nephridia; 2, funnel; 3, septum; 7, external apertures.
In many Megascolicidae there is a nephridial system of a different character. In Perichaeta when dissected the nephridia appear, on account of their minute size, to be altogether absent. There is, however, in most Perichaetidae, in many Acanthodrilidae, and in many Cryptodrilidae a mass of minute tubules which cover the inside of the body-wall, and open on to the exterior by innumerable openings; there may be in a single segment one hundred or more of these external orifices, which are scattered about irregularly. It is at present uncertain whether these minute tubes are connected among {358}themselves, thus forming a network passing through the septum and from segment to segment, or whether each tube is isolated from its fellows, and forms a distinct nephridium, of which there are many in each segment and entirely separate. This is, however, certain, that the complex nephridial systems of at any rate Octochaetus and Megascolides are derived from the multiplication of a single pair of tubes which are alone present in the embryo. In Perichaeta the minute nephridia are furnished with coelomic funnels; in Octochaetus they are not, except in the case of certain nephridia which open into the terminal section of the intestine.
Both at the anterior and at the posterior end the nephridia occasionally open into the alimentary canal. In various genera the first pair of nephridia are larger than the others, and open into the buccal cavity; it seems likely that they serve as salivary glands. A somewhat similar condition of things exists in Peripatus (vol. v, p. 17). In Octochaetus multiporus, for example, there is a large tuft of nephridial tubes in the anterior region of the body, which opens by a long muscular duct into the buccal cavity. In the same species a good many of the nephridial tubes open into the posterior section of the intestine, reminding one of the anal vesicles of the Gephyrea (p. 436) and of the Malpighian tubes of the Arthropods.
In many Eudrilidae the ducts of the paired nephridia form a network in the body-wall, which opens on to the exterior by many pores.
Alimentary Canal.—The digestive tube is perfectly straight in nearly all Oligochaeta. Only in Plagiochaeta and a species of Digaster is it twisted in the intestinal region in a corkscrew-like fashion. The mouth is under the buccal lobe (where, as in the majority of cases, this is present); the anus is mostly terminal, or rarely, e.g. Criodrilus, a little in advance of the end of the body on the ventral side. In the simpler forms three regions can be distinguished, which are themselves simple in structure. The mouth leads into a buccal cavity, which in its turn opens into the pharynx; the latter is muscular, with thick walls. The narrower oesophagus opens into the wider intestine, which opens posteriorly, as already stated. In the earthworms there is as a rule some complication. The oesophagus bears certain glandular appendages, the calciferous glands; and a part of {359}it is modified into a gizzard. The gizzard is merely a portion of the oesophagus with very much thickened muscular walls and with a stout lining of chitin. It is not universally present among earthworms, and when present varies much in position. The rule is that one gizzard only is present. In Digaster, as is implied by the name, and in some other forms there are two in successive segments; in Trigaster, as the name also indicates, there are three gizzards; in Moniligaster and the Eudrilids Hyperiodrilus and Heliodrilus there are four to six; and a few other forms also have a considerable number of gizzards. The calciferous glands are diverticula of the oesophagus with folded and sometimes ciliated walls; their epithelium secretes calcareous particles, which are frequently of crystalline form. Darwin supposed that this secretion was provided in order to negative the humus-acids of the soil which is the food of earthworms. These organs are usually paired, but in the Eudrilidae there are unpaired as well as paired glands; the unpaired calciferous glands lie ventrally. These glands are totally wanting among the aquatic families, with the sole exception of the Enchytraeidae. In a few of these there are either paired or single glands of a very similar nature; Dr. Michaelsen has suggested that the function of these is rather absorptive than secretory. From the median unpaired gland of Buchholzia arises the dorsal vessel, which at first forms a sinus round the glandular epithelium; the epithelium, like that of the nephridia, is perforated by the ducts. In certain Oligochaeta there are some curious modifications of the calciferous glands. In Stuhlmannia and a few other Eudrilidae the oesophagus is beset with a larger number of paired structures than in any other genera of the family, where the calciferous glands are more limited in number. These glands consist of a short tube lined with epithelium opening into the oesophagus. Round this is a mass of cellular tissue, but the outlines of the constituent cells are lost; the whole is permeated with abundant blood-vessels. This layer seems to be peritoneal, and the entire gland seems to have lost its function as a secretory organ, and to have taken on some function in connexion with the vascular system. An analogous modification is to be found among the Enchytraeidae. In certain forms there is a structure known as the cardiac body; this is a chord of cells lying in the dorsal blood-vessel at the point where it springs from the intestine. It is tempting to regard this cellular rod as being {360}the altered dorsal glandular pouch already spoken of, which is surrounded by a blood sinus.
Reproductive Organs.—All the Oligochaeta are hermaphrodite animals. But, as is the case with other hermaphrodites, the male and female organs are in many cases mature at different times, thus leading to a practical unisexuality. Many of the aquatic forms appear to have fixed times for breeding, which may be in the winter or in the summer; but the earthworms are as a rule sexually mature the whole year round. Various accessory organs are developed in the majority of cases. In all, the reproductive glands lie in successive segments and are attached to the septa, from the peritoneal covering of which they originate. Their actual position differs greatly in different genera; the position is constant only in the earthworms, where the testes are in the tenth and eleventh segments and the ovaries in the thirteenth, in exactly corresponding situations. A few earthworms have only one pair of testes. The only exception, among terrestrial forms, to the position of the generative organs is in the family Moniligastridae, which show so many other affinities to the lower forms of Oligochaeta. In this family the ovaries have moved one or two segments forward. Among the fresh-water families the position of the testes and of the ovaries is not so uniform. They are generally more anterior than in the terrestrial genera, particularly the ovaries.
One of the chief differences between the Oligochaeta and the Polychaeta is that the reproductive organs of the former have special ducts to convey their products to the exterior. In Aeolosoma, the only exception to this rule, Dr. Stolc[415] has shown some reasons for believing that certain nephridia, but slightly altered in form, serve as the conduits of the spermatozoa, whilst the ova are extruded through a pore upon the ventral surface of the body. In the Enchytraeidae the same pore for the extrusion of the ova appears to exist; but a nearer examination shows that it is really not a mere perforation of the integument, like the dorsal pores, for example, but that its internal orifice is fringed with cells which seem to represent a rudimentary oviduct; perhaps Aeolosoma typifies a last stage in the reduction. Even so high in the scale as in the genus Nemertodrilus (Eudrilidae), there is an oviduct which can only be compared with that of the {361}Enchytraeidae. Elsewhere the oviducts are a pair of tubes with a wide, funnel-shaped, and ciliated mouth, which leads to the exterior by way of a ciliated tube of varying length.
The sperm-ducts are of an essentially similar structure; but they are commonly much longer, passing through a variable number of segments on their way to the exterior. In most earthworms there are, moreover, two of them on each side instead of only a single pair, as is the case with the oviducts. Among the Tubificidae, Naids, and other aquatic families there are only two sperm-ducts, one on each side of the body. But this is not a character of the aquatic families, for the Lumbriculidae have generally two pairs, as in the earthworms. It is, however, a rule with hardly an exception, that among the aquatic Oligochaets the sperm-ducts open, as do the oviducts in all Oligochaets, upon the segments following that which bears internally the ciliated funnel. It is only in the Moniligastridae among earthworms that the sperm-duct only traverses two segments in its course. But where it is short as regards the actual distance traversed between the two extremities, the tube itself is commonly long and coiled.
Sometimes, as in our common earthworms, the sperm-duct opens directly on to the exterior of the body, the lips of the external orifice being swollen by the development of cutaneous gland-cells. In the majority of cases the sperm-duct or ducts open near or into a glandular structure which in earthworms has been called "prostate"; in the aquatic forms, on the other hand, "atrium." As these terms are objectionable from the different way in which they have been used for structures of Vertebrates, I have suggested for both the term "spermiducal glands," indicating the identity of the structure in all Oligochaeta. The number of pairs of these glands varies, as does also their shape and size. The typical form is perhaps illustrated in the lower Oligochaeta, where there is but a single pair into which the sperm-duct or ducts of the same side open. The Naids, Tubificidae, Lumbriculidae, and Moniligastridae have a simple gland of this description on each side of the body. These glands may consist of a tuft of pear-shaped glandular cells attached to the organ at one side, as in most Tubificidae, or of a complete investment of gland-cells, as in Branchiura. Among earthworms it is only the Moniligastridae and the Eudrilidae in which the sperm-duct opens directly into {362}the end of the spermiducal gland; in the Perichaetidae the gland is differentiated into two sections; there is a muscular duct leading to the exterior, and a lobate glandular part, which is formed by a complicated branching of a single sac such as exists in the Tubificidae; in the Acanthodrilidae and in many Cryptodrilidae the spermiducal glands are of a tubular form and are not branched, though there is the same differentiation into a duct and a secreting portion. There are in the Acanthodrilidae two pairs of these, and as many as three pairs in Dichogaster; in the latter case in three successive segments. In the Acanthodrilidae the glands are upon the seventeenth and nineteenth segments. In most Cryptodrilidae the sperm-ducts do not open into the duct of the spermiducal gland, but on to the body-wall near to its orifice, the distance varying in different genera. In the Acanthodrilidae the male pore is on the eighteenth segment, removed therefore by the distance of a segment from the aperture of either of the glands. It may be that a large series of structures which exist in Microchaeta benhami[416] and in other Geoscolecids, and which have been termed copulatory glands, are the equivalents of the spermiducal glands.
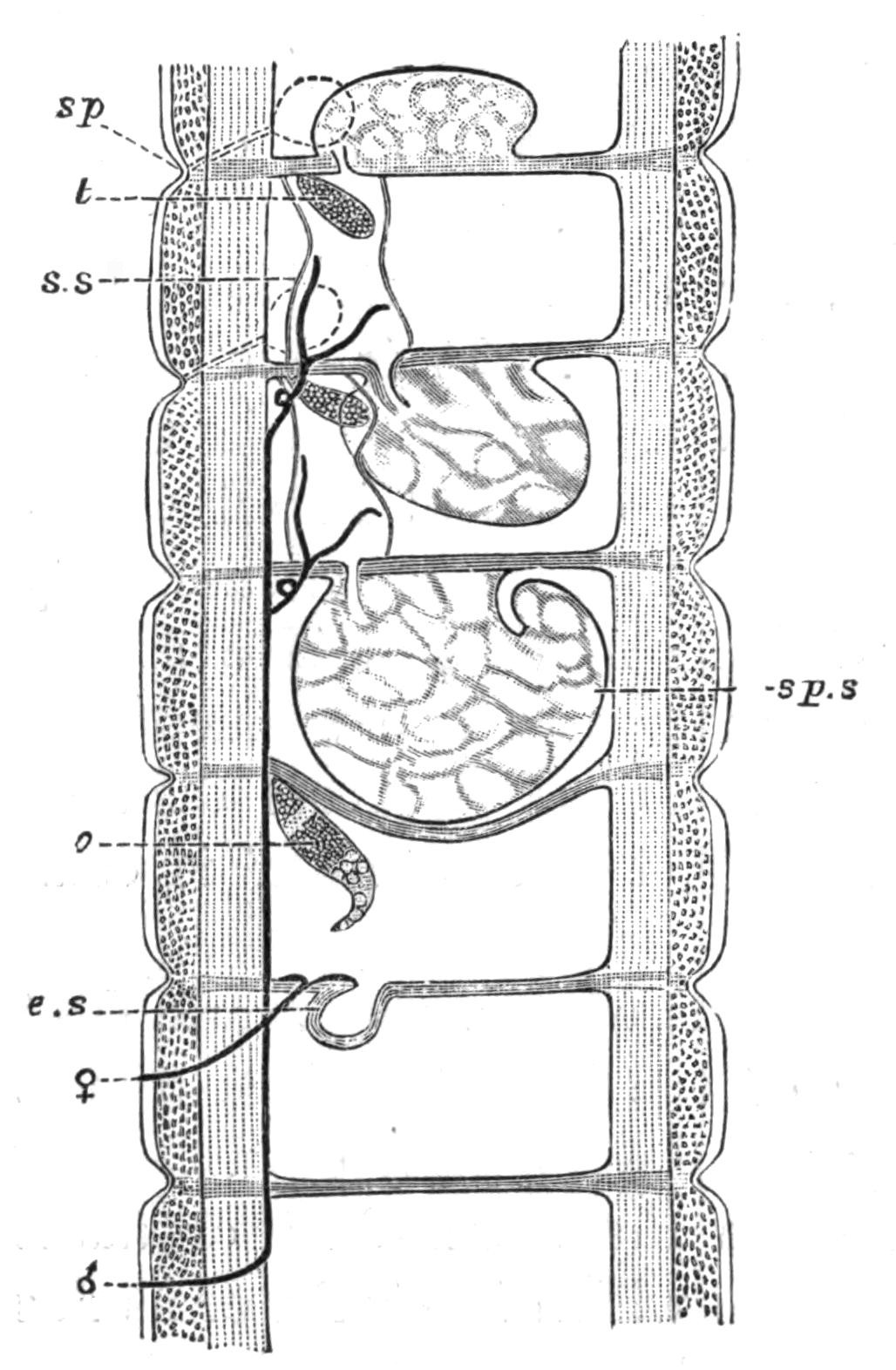
Fig. 192.—Diagrammatic longitudinal section of Lumbricus, showing the generative segments. × 3. (After Hesse.) sp, Spermathecal pore; t, testis; s.s, seminal sac; sp.s, sperm-sac; o, ovary; e.s, egg-sac; ♀, female pore; ♂, male pore.
In many earthworms there are, at the external opening of the male ducts, bundles of specially modified chaetae, which have been called, from their supposed function, penial chaetae; they are usually ornamented at the free end with spinelets or ridges, and frequently offer valuable specific characters. In the Lumbricidae and the Geoscolicidae there are modified chaetae upon the {363}clitellum; in a few forms, such as, for example, Acanthodrilus schmardae, the spermathecae have bundles of similar chaetae in their neighbourhood, often associated with glands not unlike the spermiducal glands.
In most, perhaps in all Oligochaeta the sperm is not matured in the testes, or even in the body-cavity; it is received into special sacs which are called sperm-sacs, and there ripens. These sacs, the vesiculae seminales, have been shown to be outgrowths of the septa; their cavity is thus a portion of the body-cavity shut off more or less completely from the general body-cavity.
The reproductive organs of the Eudrilidae, and particularly the female organs, are so divergent in many particulars from those of other Oligochaeta that it is convenient to treat them separately. The testes are normal, save that they are often adherent to the posterior wall of their segment, as, however, is the case with some other earthworms. In many Eudrilidae, for instance in the genus Hyperiodrilus, the funnels of the sperm-ducts are dependent from the anterior wall of the segment which contains them; the narrow tube which follows projects into the segment in front, and is there immediately dilated into a wide chamber, which again narrows, and bending round, re-traverses the same septum; the two ducts of each side (if there are two, which is not invariably the case) remain separate and open separately into the glandular part of the spermiducal gland. There is occasionally only a single median gland; and as a general rule the two glands open by a median unpaired orifice. Penial chaetae may or may not be present.
Fig. 193.—Female reproductive organs of Hyperiodrilus. XII-XV, Segments of the body; 1, spermathecal sac; 2, egg-sac; 3, spermatheca; 4, ovary.
The structure of the female organs differs considerably in detail in the different genera. But Hyperiodrilus may be taken as an instance of a genus in which these organs are as complicated as they are anywhere. The ovaries (Fig. 193, 4) are {364}perfectly normal in structure and in position. So also are the oviducts; but both are enclosed in sacs which communicate in rather an elaborate fashion. Each ovisac is somewhat rounded in form, and the two communicate by a narrow tube; from the ovisac also arises another narrow tube, which soon dilates into a chamber lying in the thirteenth segment; this contains the mouth of the oviduct and is continuous with the egg-sac; the latter is quite normal in position. Beyond the egg-sacs the two tubes unite round the intestine and open into a large median sac, which contains sperm and may be called the spermathecal sac (1). There is, however, a true spermatheca, single and median. This opens on to the exterior in the middle of the thirteenth segment, but lies chiefly in the right-hand sac behind the ovarian portion of the same. I never found this spermatheca to contain sperm. Dr. Rosa inferred on anatomical grounds, and I have been able to prove developmentally (in Libyodrilus), that these sacs which involve the ovaries and oviducts, and which also contain sperm, are derivatives of the septa; that in fact the spaces which they enclose are coelomic. In some Eudrilids these sacs are the only "spermathecae"; in others, as in Hyperiodrilus, there are in addition blind pouches lying within them which must be regarded as true spermathecae; these are smaller in some than in others. In fact there are various transitions in the entire replacement of true spermathecae apparently homologous with those of other earthworms by pouches which are derived from the septa, and which are therefore of an entirely different morphological significance; here is an excellent case of the substitution of organs, analogous to the replacement of the primitive notochord of the Vertebrate by the vertebral column.
So far as is known, all the Oligochaeta deposit their eggs in special chitinous cases, the cocoons. They share this peculiarity with the Hirudinea. The cocoons have long been known, but were originally mistaken for the eggs themselves. The cocoons contain several eggs and a variable quantity of albumen for the nutrition of the growing embryos. In the majority of earthworms they are more or less oval with projections at the two ends, and are of a brownish colour. In others the tint is rather to be described as green. The genera Criodrilus and Sparganophilus have a cocoon which is greatly elongated. These structures seem to be undoubtedly formed by the clitellum, the earlier {365}opinion of D'Udekem being that they were the product of certain glands developed in Lumbricus at the breeding season, which he thence called the capsulogenous glands. It is more probable that these glands, which have been up to the present but little investigated, are the seat of the formation of the albumen which is found within the cocoons. The cocoons are deposited at varying depths in the ground, or on the surface. Among the aquatic genera they are often attached to aquatic plants. The process of formation has been carefully watched by Vejdovsky[417] in the genus Rhynchelmis. The worm throws off the cocoon over its head, crawling backwards to free itself therefrom. The eggs, spermatozoa and albumen, reach the interior of the cocoon as it passes over the orifices of the respective ducts. Out of the numerous eggs which a single cocoon originally contains, only a few, sometimes only one, reaches to maturity. Among the Enchytraeidae, however, quite a large number of young emerge from a single cocoon. The development of all the Oligochaeta is direct, there being no free larval stage. It seems to be the rule for a process of fission to take place in the embryos of Allolobophora trapezoides[418] at least, according to the observations of Vejdovsky, in warm weather. In cold weather he found in each cocoon as a rule single embryos, and only 10 per cent of double embryos.
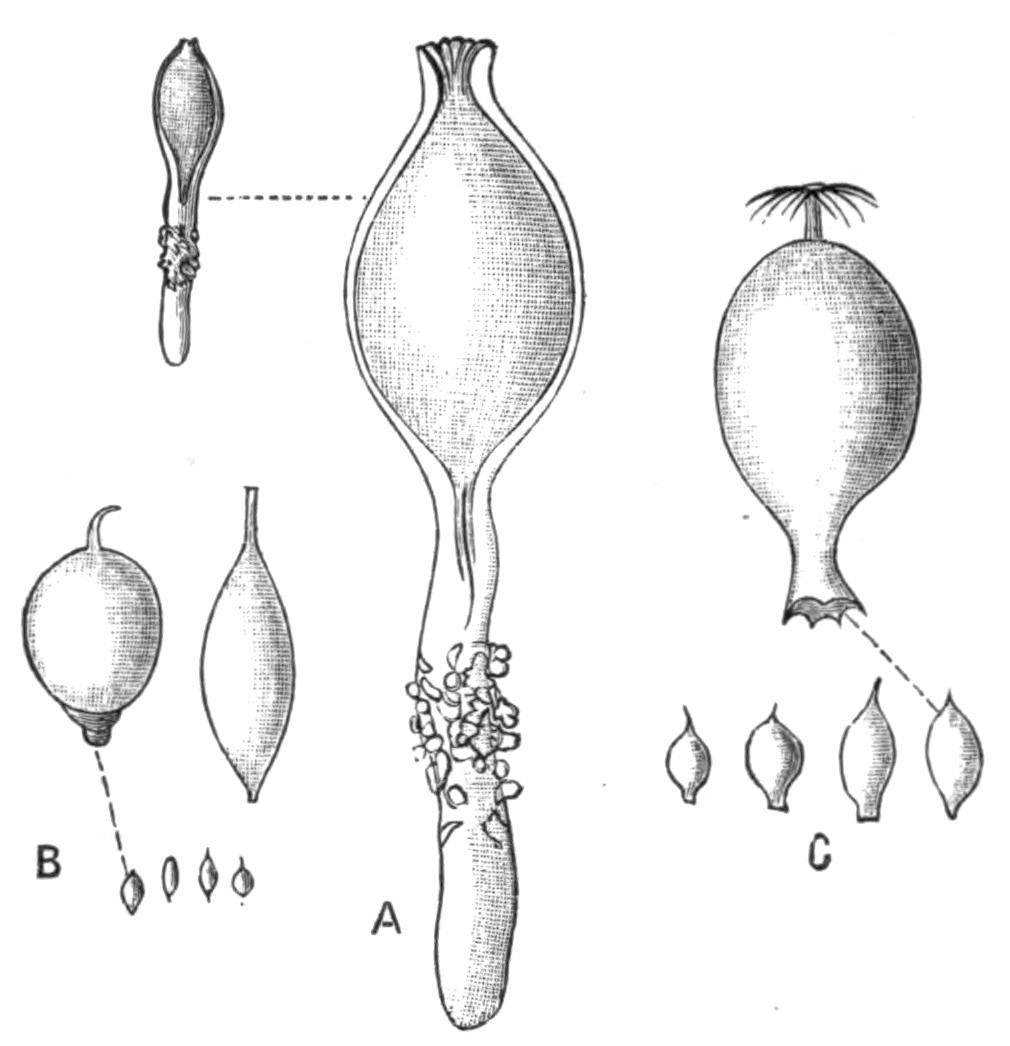
Fig. 194.—Cocoons of Lumbricidae. (After Vejdovsky.) A, Lumbricus rubellus, nat. size and × 3; B, Allurus, nat. size and × 6; C, Allolobophora foetida, nat. size and × 3.
Habitat.—Earthworms are found in almost every part of the world where they have been looked for. They occur far to the north, in Siberia and Nova Zembla,[419] while South Georgia and Kerguelen mark their southern limits. From arid tracts of country they are naturally absent, and also, which is more {366}curious, from certain districts of North America. In the tropics these animals seem to be on the whole less abundant than in more temperate climates. But this deficiency of individuals is counterbalanced by the greater variety of generic and specific types. From tropical Africa, little explored as it has been from this point of view, no less than thirty genera, including about ninety species, have been recorded; whereas in Great Britain only four genera and seventeen species occur, and in all probability but few remain to be discovered. The vertical range of these Annelids is also considerable. Several species have been met with in Europe and elsewhere at an altitude of 10,000 feet.
For the bulk of the species the term earthworm is an accurate description of their habitat. But there are not a few which occasionally or habitually prefer other localities. The genus Allurus is equally at home in soil or in water; I have taken it in the fast-flowing river Plym in Devonshire. The genus Acanthodrilus includes a few species which have at present only been met with in water; A. schmardae comes from fresh water in Queensland, A. stagnalis from ponds in South America; A. dalei is like Allurus in that it is to be found both on land and in streams and ponds. The Enchytraeidae are just as amphibious; Criodrilus and Sparganophilus appear to be purely aquatic. A more curious locality for a creature that is so characteristically terrestrial is the margin of the sea. For a long time a species belonging to a peculiar genus Pontodrilus has been known from the shores of the Mediterranean in the neighbourhood of Nice. It lives there among seaweed above high-water mark, but it must at least occasionally be splashed by the waves. Another species of the same genus occurs on the coast of Brazil and some of the West Indian islands; Pontoscolex corethrurus and Diachaeta littoralis were described by Schmarda[420] from the shores of Jamaica. The former species is one of the most widely distributed of earthworms, and, except in this particular part of the world, has been always taken on the land far from the sea. There are also partly marine forms among the Tubificidae; Clitellio arenarius is common on our coasts.
While there are several kinds of earthworms that are thus met with in fresh water, others will live for some time submerged {367}in water. Perrier found by experiment that various species could undergo with impunity a prolonged immersion in water, and I confirmed his experiments myself with a common species of Allolobophora. A correspondent of "Nature" stated that a certain number of species (not particularised) of earthworms in Ceylon could suffer with impunity the effects of sea-water. The importance of this fact will be again dealt with in considering the geographical distribution of the group.
Among the aquatic genera of Oligochaeta we do not as a rule meet with amphibious species. The Enchytraeidae however, as already mentioned, are an exception; so too appears to be the genus Phreoryctes, which in its structure is to some extent intermediate between the earthworms and the aquatic families.
Terrestrial and Aquatic Forms.—There are many obvious structural peculiarities which would prevent the normally aquatic worms from being thoroughly at home on dry land. The gills of Branchiura and the other gilled species would be injured, in all probability, by friction with the earth; the delicate and long chaetae of Naids and Tubifex are also most unsuited for progression through dry soil; and it is to be noted that those Oligochaeta, which, belonging to aquatic groups, are yet found away from water, have chaetae of the simple sigmoid pattern which characterises the earthworms.
There are other peculiarities found only in the aquatic species which have not so obvious a relation to their habitat. In no genus that is mainly aquatic in habit are the ova small and nearly unprovided with yolk as in Lumbricus; the ova of aquatic forms are invariably large and filled with abundant yolk.
The more delicate organisation of the aquatic Oligochaeta is not so hard to understand. The comparatively unresisting nature of the medium in which they live, water or fine mud, does not necessitate so strong a development of the layers of the body-wall as is essential to the earth-living forms, which have also thick septa in the anterior region, to protect the organs of reproduction as the strong muscular contractions of the body force the worm's way through the dense soil. With the weak structure of the integument are perhaps also correlated the simplicity of other organs of the body in the aquatic Oligochaeta. With thin body-walls, through which gases can diffuse with great ease, there would seem to be less need for the development of a system of {368}integumental blood capillaries. These are indeed for the most part absent in the aquatic forms, being only faintly developed in a few, an example possibly of degeneration.
Earthworms and the Soil.—Darwin has explained the enormous effects which these soft-bodied and small creatures have had upon the superficial structure of the earth. Their castings, brought up to the surface, are blown about by the wind when dry, and are thus spread over the ground in a fine layer. It has been calculated that in the space of an acre .2 of an inch in thickness of earth is annually brought to the surface. It is clear therefore that in a long period of years there would be a very large effect produced. On the sides of a hill this matter brought up from below would tend to roll down the slopes when dry, and would increase the débris carried away to the sea by streams and rivers, so that continents formerly deposited under the sea may owe no small proportion of their size to the continued work of earthworms in past ages.
Darwin has also pointed out the benefits to the agriculturist which accrue from the industry of these Annelids. The soil is thoroughly mixed and submitted to the action of the atmosphere. The secretions of the worms themselves cannot but have a good effect upon its fertility, while the burrows open up the deeper-lying layers to the rain. Mr. Alvan Millson,[421] in detailing the labours of the remarkable Yoruba worm (Siphonogaster millsoni Beddard), hints that they may serve as a check upon the fatal malaria of the west coast of Africa. By their incessant burrowings and ejecting of the undigested remains of their food many poisonous germs may be brought up from below, where they flourish in the absence of sunlight and oxygen, and submitted to the purifying influence of sun and air.
Phosphorescence.—Phosphorescence has been observed in several species of Oligochaeta. The most noteworthy instance of recent times is the discovery by Giard of the small worm which he called Photodrilus phosphoreus at Wimereux. During damp weather it was sufficient to disturb the gravel upon the walks of a certain garden to excite the luminosity of these Annelids. In all probability this species is identical with one whose luminosity had been noticed some years before (in 1837) by Dugès, and named by him Lumbricus phosphoreus. According {369}to Giard, the light is produced by a series of glands in the anterior region of the body debouching upon the exterior. This same worm has since been found in other localities, where it has been shown to be phosphorescent, by Moniez[422] and by Matzdorf[423]. It is remarkable that in some other cases the luminosity, though it exists, is very rarely seen. The exceedingly common Brandling (Allolobophora foetida) of dunghills has been observed on occasions to emit a phosphorescent light. This observation is due to Professor Vejdovsky,[424] and was made "upon a warm July night of 1881." He thinks that the seat of the light is in the secretion of the glandular cells of the epidermis, for when this and other worms are handled the phosphorescence clings to the fingers, as of course does the mucous secretion voided by the glands.
Phosphorescence has been observed also in some other families of Oligochaetes. The late Professor Allen Harker noticed a small worm in marshy ground in Northumberland which emitted a distinct light, and which was subsequently identified as a member of the family Enchytraeidae.
Geographical Distribution.[425]—In the succeeding pages some of the details of the geographical range of the Oligochaeta will be found. The present section deals with a few generalities, which appear to result from an examination of the facts.
As to the aquatic genera but little is known at present with regard to their range; they have not been widely collected in extra-European countries. What little is known points to the conclusion that while many parts of the world have their peculiar genera (such as Hesperodrilus in South America, Phreodrilus and Pelodrilus in New Zealand), some of the common European species are widely distributed. I have, for example, received Henlea ventriculosa from Kirghiz Tartary, and from New Zealand; and a New Zealand Tubifex appeared to me to be indistinguishable from the common T. rivulorum of our rivers and ponds. It is possible that these and similar instances may, at least in some cases, be due to accidental importation at the hands of man, a matter into which we shall enter later. But the aquatic genera have, many of them, facilities for extending their range in a natural fashion, which are greater than those possessed by earthworms. {370}It has been pointed out that the chaetae of the aquatic Oligochaeta are generally hooked at the extremity and bifid, which would give them a greater chance of holding on to the feet or feathers of aquatic birds; I am not myself disposed to lay much stress on the possibilities of migration by these means, since the tender bodies of the small worms would be liable to be soon dried up by wind while in the act of migration. More likely in every way is a migration when enclosed in the cocoon. The cocoons being small, and often deposited at the edges of ponds frequented by aquatic birds, there would be many chances of their being carried away with tolerable frequency; moreover, as Dr. Michaelsen has pointed out, the cocoons of some species, particularly among the Enchytraeidae, contain a large number of embryos; so that when such a cocoon reached a foreign shore there would be a better chance of the species establishing itself there. I have referred elsewhere[426] to the singular habit of forming a temporary cyst which characterises one species of the genus Aeolosoma; this would perhaps tend to facilitate its transference in the way indicated from one spot to another.
Earthworms, on the other hand, have not such easy means of travelling from country to country; the assistance which the cocoons in all probability give to the smaller aquatic Oligochaeta cannot be held to be of much importance in facilitating the migrations of the earthworms. In the first place, the animals themselves are of greater bulk, and their cocoons are naturally larger, and thus less easy of transportation. Secondly, they are deposited as a rule upon dry land, where the chances of their sticking to the feet of birds would be less; and thirdly, they are often deposited deep in the ground, which is a further bar to their being taken up. Another possible method by which earthworms could cross the sea is by the help of floating tree-trunks; it is, however, the case with many species that they are fatally injured by the contact of salt water. There are, it is true, a few species, such as Pontodrilus of the Mediterranean coast, which habitually live within reach of the waves; but with the majority any such passage across the sea seems to be impossible.[427] On the other hand, rivers and lakes are not a barrier to the dispersal of the group. There are a few species, such as Allurus tetragonurus, {371}which live indifferently on land and in fresh water; and even some habitually terrestrial species can be kept in water for many weeks with impunity. A desert, on the other hand, is a complete barrier; the animals are absolutely dependent upon moisture, and though in dry weather the worms of tropical countries bury themselves deep in the soil, and even make temporary cysts by the aid of their mucous secretions, this would be of no avail except in countries where there were at least occasional spells of wet weather.
The range of the existing genera and species is quite in keeping with the suggestions and facts already put forward. But in considering them we must first of all eliminate the direct influence of man. Every one who studies this group of animals knows perfectly well that importations of plants frequently contain accidentally-included earthworms; and there are other ways in which the transference of species from one country to another could be effected by man. There are various considerations which enable us to form a fair opinion as to the probability of a given species being really indigenous or imported. Oceanic islands afford one test. There are species of earthworms known from a good many, but with a few exceptions they are the same species as those which occur on the nearest mainland; in those cases where it is supposed that the animal inhabitants have reached an oceanic island by natural means of transit, it is a rule that the species are different, and even the genera are frequently different. That the bulk of them are the same seems to argue either frequent natural communication with the mainland or a great stability on the part of the species themselves. It is more probable that the identity is in this case to be ascribed to accidental transference.
Another argument comes from the distribution of the family Lumbricidae. This family forms the bulk of the earthworms of the European and North American continents. But they are also found all over the world. With one or two exceptions, such as Allolobophora moebii, from Madeira, the extra-north-temperate species are identical with those found within that region. Now, if the migration had been by natural means there would surely in the lapse of time been some differentiation of species. Furthermore, Dr. Michaelsen has pointed out that in South America the presumably European forms (i.e. Lumbricus and Allolobophora) are found upon the coast and in cultivated ground; it is inland that {372}the presumably indigenous species are met with. This again looks very like accidental transference.
A mapping of the world in regions indicative of the distribution of earthworms produces a result which is slightly different from the accepted division. North America, Europe, and Northern Asia so far as is known agree in having as their distinctive earthworms the family Lumbricidae, which is very nearly the only one represented in these parts of the world. The majority of the species are common to the two continents; there cannot, in fact, be a separation of Nearctic and Palaearctic; we must accept the Holarctic region of Professor Newton. The Ethiopian region, on the other hand, is quite as it is in other groups, being bounded to the north by the desert of Sahara. The Neotropical region is quite distinct, and includes Central as well as South America, and the West Indian islands, even the Bermudas. It is, however, a question whether the more southern portions of the continent should not be cut off from the rest and joined with New Zealand, to form an Antarctic region. In these two countries, and also in Kerguelen and Marion Islands, the prevailing genera are Acanthodrilus and Microscolex. In America Acanthodrilus is found nowhere but in the more southern regions of the southern continent, as well as in the Falklands and South Georgia. New Zealand is characterised by other genera of Acanthodrilids besides Acanthodrilus itself; but the bulk of the species belong to the latter genus. Acanthodrilus also occurs (three species only) in Queensland and at the Cape of Good Hope. Microscolex is rather more widely dispersed, being found in other parts of America and in Europe, the island of Madeira (? accidentally imported); but it is undoubtedly chiefly concentrated in South America and in New Zealand. Apart from New Zealand, which, as already said, can only be doubtfully referred to the Australian region, the latter appears to form one with the Oriental region (to which, on account of its Perichaetidae, Japan should be added) of other writers. There is, so far as earthworms are concerned, no "Wallace's line" at all. The characteristic genera Perichaeta and Megascolex range from one extremity of the Indo-Australian region to the other. It is true that Cryptodrilus and Megascolides are limited to Australia itself (with the apparent exception of a species or two in America, for I can hardly separate Argilophilus of Eisen from Megascolides); {373}but they are not at all well-defined genera, and indeed the generic distinctions of the whole family Cryptodrilidae are not in a satisfactory condition.
Classification.—The Oligochaeta do not shade into the Polychaeta so imperceptibly as might be inferred from the current schemes of classification. Apart from minor points, which are not universally characteristic of the two groups, though never found except in one or the other, the Oligochaeta are to be defined by the complicated reproductive system; although in a few undoubted Polychaets there is a faint approach to this in the specialisation of some of the nephridia as sperm-receptacles and even as sperm-ducts. But nowhere among the Polychaeta are there the diversified sperm-ducts and oviducts, spermathecae and sperm-sacs, that are universal among the Oligochaeta. Moreover, no Polychaet has a clitellum, which is so distinctive of the Oligochaeta, and of their near allies the Leeches. Dr. Eisig has compared the glandular modification of the integument at the mouths of the sperm-ducts in the Capitellidae to the beginnings of a clitellum. This may be the case, but it is, in my opinion, more comparable to the similar glandular spots at the male pores in earthworms. The reproductive glands in the Oligochaeta (save for a few apparently abnormal cases) are restricted to at most two pairs of each, which occur in the same individual; the Polychaeta being dioecious. There is, in short, no form known which cannot be definitely referred to either the Polychaeta or the Oligochaeta, excepting perhaps Ctenodrilus, the anatomy of whose reproductive organs is at present unknown.
It is a difficult task to classify the different families of the Oligochaeta; and to enter into the historical aspect of the matter would take too much space. I am myself disposed to divide them first of all into two main groups, for which I use Dr. Benham's[428] names of Microdrili and Megadrili.
The Microdrili are, as a rule, small and aquatic in habit; they have short sperm-ducts which open on to the exterior in the segment which immediately follows that which contains the internal aperture; the clitellum is only one cell thick; the egg-sacs are large; the epoch of sexual maturity is at a fixed period. This group, to my thinking, includes the Moniligastridae; although Professor Bourne has denied my statement with regard {374}to the clitellum, and in this case it is not so easy to decide their systematic position.
The Megadrili are characterised by the precisely opposite characters. The sperm-ducts are longer; the clitellum is composed of many layers of cells; the egg-sacs are rudimentary; sexual maturity appears to be more or less continuous.
There is, however, a substantial agreement about the families which I here adopt, which may be fairly taken to express our present knowledge of the Order. For fuller details the reader is referred to my Monograph of the Order Oligochaeta.[429]
I. Microdrili.
Fam. 1. Aphaneura.[430]—This name was originally given to the present family by Vejdovsky; the family contains a single genus, Aeolosoma, of which there are some seven species. The name is taken from, perhaps, the most important though not the most salient characteristic of the worms. The central nervous system appears in all of them to be reduced to the cerebral ganglia, which, moreover, retain the embryonic connexion with the epidermis. The worms of the genus are fairly common in fresh waters of this country, and they have been also met with in North and South America, and in Egypt, India, America, and tropical Africa. They are all small, generally minute (1 to 2 mm. long), and have a transparent body variously ornamented by brightly-coloured oil globules secreted by the {375}epidermis. These are reddish brown in A. quaternarium, bright green in A. variegatum and A. headleyi, in the latter even with a tinge of blue. In the largest species of the genus, A. tenebrarum they are olive green. In A. niveum the spots are colourless, and A. variegatum has colourless droplets mixed with the bright green ones. Fig. 195 shows very well the general appearance of the species of this genus. The body has less fixed outlines than in most worms, and the movement of the creatures is not unsuggestive of a Planarian. As the under side of the prostomium is ciliated, and as the movements of these cilia conduce towards the general movement of the body, the resemblance is intelligible. One species of Aeolosoma, at any rate, has a curious habit which is unique in the Order. At certain times, for some reason at present unknown, the worm secretes a chitinous capsule, inside which it moves about with considerable freedom; these capsules when first observed were mistaken for the cocoons of the worms; they are really homologous with the viscid secretion which the common earthworm throws off when in too dry soil, and with which it lines the chamber excavated in the earth in which it is lying. The worms of this genus multiply by fission; sexual reproduction has been but rarely observed.
Fam. 2. Enchytraeidae.[431]—This family consists at present of rather over fifty well-characterised species, which are distributed into eleven genera. It is common in this country and in Europe generally; it has been met with in Spitzbergen and the extreme north; it occurs in the American continent from the north to the extreme south; it is also an inhabitant of New Zealand. The worms of this family are nearly always of small size, sometimes minute; they never exceed an inch or so in length, and that is a rare occurrence. They are equally at home in water and in soil, some species being common to the two media; a few are marine or littoral in habit, while others are parasitic in vegetable tissues. Like most earthworms, and unlike the majority of aquatic worms, the chaetae are without a bifid termination; the body-wall, too, is comparatively thick. The perivisceral fluid is often (as in certain Naids) loaded with elliptical or rounded corpuscles. Resemblances to earthworms rather than to the aquatic families of Oligochaeta are suggested by the long distance which separates the {376}spermathecae from the male pores (segments 5 and 12), and by the paired or unpaired glands that have been already compared to the calciferous glands so universally present among earthworms. On the other hand, the male ducts are confined, as in the lower Oligochaeta, to two segments, upon one of which the internal, upon the other the external orifice is situated, and the oviduct is reduced to a simple pore, as in Naids; but this may be merely a matter of convergence by degeneration. Perhaps the most remarkable genus in the family is Anachaeta, which has no chaetae, but in their place a large cell projecting into the body-cavity, which appears to represent the formative cell of the chaeta. The integument of this genus contains true chlorophyll, according to Vejdovsky.
A singular character, found, however, also in Rhynchelmis and Sutroa among the Lumbriculidae, is the opening of the spermathecae into the alimentary canal. This was originally discovered by Dr. Michaelsen, but has been abundantly confirmed.
Stercutus is a singular genus which was originally found in manure, and has the peculiarity that the alimentary canal is often aborted; this degeneration seems to bear some relation to the food and conditions of life.
Fam. 3. Discodrilidae.[432]—This family consists of small parasitic forms which were at one time assigned to the Hirudinea; there seems, however, to be no doubt that they are rightly included in the present Order. Branchiobdella is found upon the gills of the Crayfish, Astacus fluviatilis; the American Bdellodrilus upon Cambarus. The chief reason for the former inclusion of these worms among the leeches was due to the absence of chaetae and to the presence of chitinous jaws and of suckers; apart from these structures there is nothing whatever leech-like about the worms. Bdellodrilus has two pairs of testes in segments 5 and 6; there are two pairs of sperm-ducts, all opening, however, by a common "atrium" on the sixth segment; on the fifth open a pair of spermathecae, likewise by a common pore. The ovaries are in segment 7, and the ova escape by a pair of pores apparently like the single pore of the Enchytraeidae. The entire worm consists of only eleven segments.
Fam. 4. Phreoryctidae.[433]—This family contains only two {377}genera, Phreoryctes and Pelodrilus. The former is widely spread, occurring in Europe, North America, and New Zealand. Pelodrilus is limited to New Zealand. Most species of Phreoryctes are distinguished by their extraordinary length and thinness, and there is frequently a tendency to the disappearance of the chaetae. The most important anatomical fact about Phreoryctes (at any rate P. smithii) is that there are two pairs of ovaries as well as two pairs of testes, and that the ducts of all are simple and very much alike. This seems to argue the low position of the family in the series.
Fam. 5. Naidomorpha.[434]—This family contains eight or nine genera, perhaps more; they are all of them aquatic and of small size, and they multiply by fission as well as sexually. The most noticeable peculiarity of the family is the "cephalisation" which occurs in the head segments. In some genera, in Pristina for example, there is no such cephalisation to be observed; but in others the dorsal bundles of chaetae commence a few segments farther back than the ventral, the segment where they commence being different and characteristic in the various genera. Thus in Dero the first four segments are without dorsal chaetae, and in Nais the first five are in this condition. There is thus a kind of "head" formed, whence the expression "cephalisation." Dero, Nais, and Pristina are commonly to be met with in ponds, lakes, etc., in this country. Bohemilla is rarer, and is to be distinguished by the remarkable serrated chaetae of the dorsal bundles. Of Dero and Nais there are a considerable number of different species; indeed it is usual perhaps to regard as distributable among three genera, Nais, Stylaria, and Slavina, the species which I am disposed to place in one genus, Nais. Stylaria is defined on this view by its extremely long prostomium, which has given rise to both its popular and technical names. "Die gezungelte Naide" was the term applied by one of its earliest investigators, and the name Stylaria proboscidea signifies the same peculiarity. But as the same inordinately long "proboscis" occurs in the South American Pristina proboscidea, belonging to a genus of which the other member does not possess so well developed a prostomium, it seems too variable a character upon which to differentiate a genus. Chaetogaster and {378}Amphichaeta have been placed by some systematists in a separate family. The first named contains four species which are fairly common. It is one of those worms in which the chaetae are not exactly related to the segmentation of other organs, which moreover sometimes show an independence in their segmentation; thus there are more nerve ganglia in the anterior segments of the body than there are septa.
Fam. 6. Tubificidae.—The worms belonging to this family are of small size, and are all inhabitants of fresh or salt water, or the margins of pools and the sea. They differ from the last family in that asexual reproduction never occurs, and that the reproductive organs are situated rather farther back in the body. The male pores are upon segment 11, and the oviduct-pores upon the following segment. This family differs from the Lumbriculidae in the fact that there are only a single pair of sperm-ducts.
The earliest known Tubificid was the common Tubifex rivulorum, so widely dispersed in this country and elsewhere; but with it was at first confounded the somewhat similar genus Limnodrilus, which only differs in that the chaetae are all of the cleft variety, and never capilliform, as in Tubifex. The genera are mainly distinguished by the characters of the chaetae and of the male ducts. At the base of the series perhaps lies Ilyodrilus, which has many points in common with the Naids. The form of the terminal chamber into which the sperm-duct opens has the same simplicity as in that group, and the intestine is surrounded with a network of blood-vessels as in the Naids, a structure which is otherwise wanting in the Tubificidae. The development of the ova also is upon a plan which is met with in the Naids. The atrium (see p. 361) becomes more complicated in other Tubificidae. The extremity also is as a rule modified into a retractile penis. The discrete "prostate," of which we have already spoken, marks out a considerable number of genera, such as Tubifex, Limnodrilus, Spirosperma, Hemitubifex. In the marine Clitellio there is no such structure at all, and it is also wanting in the South American Hesperodrilus. In Branchiura there is a complete prostatic investment of the atrium, and in Telmatodrilus a large number of separate aggregations forming as many distinct prostates. Vermiculus, a genus consisting of but one species, found by Mr. Goodrich on the sea-shore in the neighbourhood of Plymouth, is remarkable for the unpaired character of the {379}generative organs, a peculiarity which is shared by Stolc's genus Bothrioneuron. The gills upon the posterior segments of Branchiura sowerbyi and Hesperodrilus branchiatus have been already noticed above (p. 352). A very aberrant genus, perhaps not rightly referable to this family, is Phreodrilus,[435] from New Zealand, first collected in water from a subterranean spring. It differs from all other Tubificids except Hesperodrilus in that the spermathecae lie behind the male pores, a state of affairs which is met with in the Lumbriculidae. Another singularity of structure concerns the sperm-duct, which is wrapped in a thin-walled sac, which has every appearance of being simply the outer muscular wall of the duct. Within this are the complicated coils of the duct, and also a quantity of free spermatozoa, whose mode of ingress is difficult to understand. Many of the Tubificidae live in tubes fabricated by themselves, whence the tail end protrudes. The integument in more than one species is vascular. This integumental blood system, universal among the earthworms, appears to be restricted to the present group among the Limicolae of Claparède.
Fam. 7. Lumbriculidae.[436]—This family is not a large one, and is nearly limited in range to Europe and North America; indeed, if we omit the doubtful Alluroides, entirely to the Palaearctic region. There are only fourteen species, which are referred to eight genera. A number of dubious forms, as is the case with other families, may possibly ultimately swell this list. The type genus of the family, viz. Lumbriculus, upon which Bonnet made his experiments in section and subsequent regeneration, has only within the last year been thoroughly explored anatomically. But all the other genera are well known. The Lumbriculidae are of small or moderate size, and all of them aquatic in habitat. There are three characters which are nearly or quite universal in the genera of the family. In all of them the chaetae are only eight to each segment, arranged in couples, and are either cleft at the extremity or simple. As a rule which has but two exceptions, the genera Alluroides and Lumbriculus, there are two pairs of sperm-ducts, which, however, communicate with the exterior through a single terminal chamber on each side of the body.
The dorsal blood-vessel has in the Lumbriculidae a series of {380}contractile and blind appendages, which were at first mistaken for caeca of the intestine itself. There are two genera of this family in North America, which are not very different anatomically from their European representatives. The genera described by Eisen are Sutroa[437] and Eclipidrilus.[438] The latter lives in cold torrents at a great height in the mountains of the Sierra Nevada of California.
Fam. 8. Moniligastridae.—This family, terrestrial in habit, is probably Oriental in range; but I have described a single species from the Bahamas which may possibly be referable to the category of accidentally introduced specimens. Our knowledge of this family is conveniently summed up in Professor Bourne's paper[439] upon the genus Moniligaster. There are some eighteen species, which range in size from an inch or so in length (M. bahamensis) to about two feet; this last measurement is that of the huge M. grandis, of which, together with many others, Professor Bourne gives coloured drawings. There is a second genus, Desmogaster, which is mainly characterised by the doubling of the reproductive organs. This was described by Rosa from Burmah. The family is noteworthy on account of the fact that every species belonging to it has at least four distinct gizzards, sometimes more; but as this multiplication of the gizzards has been also found in Heliodrilus among the Eudrilidae, and indeed elsewhere, it is insufficient to define the family. More characteristic is the fact that the sperm-ducts open on to the next segment to, or even the same segment as, that which contains their funnels; consequently the apertures of the oviducts are behind instead of in front of them. These pores are also situated in a very anterior position, the male pores being upon the tenth segment or between the tenth and eleventh, and the oviducal pores upon the following one. In these features the family presents resemblance to the aquatic Oligochaeta, from which, however, its stoutly-built gizzards, and vascular nephridia differentiate it.
II. Megadrili.
Fam. 9. Perichaetidae.[440]—The Perichaetidae comprise a larger {381}number of species than any other family of earthworms; but it is a matter of considerable difficulty to divide the family satisfactorily into genera. The family as a whole may be defined as having numerous chaetae in most of the segments of the body.
There is no other definition which will distinguish this family from the next two families, and even this definition is not absolutely distinctive. There are Acanthodrilids which have a large number of chaetae in each segment. The only difference is that in this case—in the genus Plagiochaeta—the chaetae are implanted in twos; this is not the case in the Perichaetidae. In all Perichaetidae that are known the sperm-ducts open in common with the ducts of the spermiducal glands; they generally open into them at some distance from the common external pore. In Megascolex, Perichaeta, and Pleionogaster the nephridia are of the diffuse type so widely spread among these worms, and the spermiducal glands are lobate. Megascolex differs from the others in the fact that in addition to the small scattered nephridia there are a pair of large nephridia in each segment, and the chaetae do not form absolutely continuous circles, but are interrupted above and below. Pleionogaster has more than one gizzard but otherwise agrees with Perichaeta; it is confined to the East. Perichaeta is tropical and occurs—no doubt introduced—in Europe and America. Megascolex is Old World only, and, like Perichaeta, Australian as well as Oriental. But whereas Perichaeta is rare in the Australian region, Megascolex is common there. Perionyx and Diporochaeta are the other genera which it is possible to recognise. Both of them have paired nephridia, and neither of them have intestinal caeca, a peculiarity which they both share with Megascolex and Pleionogaster. Perionyx principally differs from Diporochaeta in that the spermiducal glands are lobate, whereas in the latter they are as in the Acanthodrilidae. Perionyx is Oriental; Diporochaeta occurs in Australia and New Zealand.
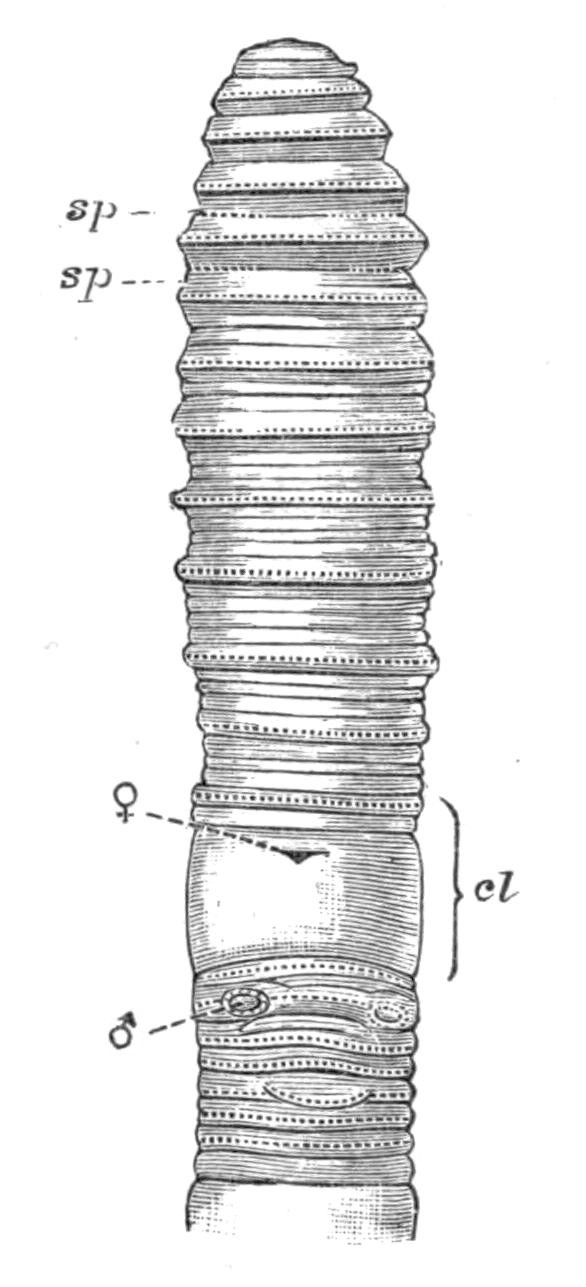
Fig. 196.—Perichaeta everetti F. E. B. × 1. sp, Spermathecal pores; cl, clitellum; ♀, female pore; ♂, male pore.
A very distinctive feature of Perichaeta—perhaps only of the {382}genus sensu stricto—is its exceeding activity. The first specimens ever noticed in this country, or at least of whose existence printed notice was taken, were exhibited by the late Dr. Baird of the British Museum, at a meeting of the Zoological Society. He remarked in that communication upon the agile fashion in which these tropical Annelids will spring off a table when touched or in any way interfered with. Numerous other observers have seen the same manifestations, and the name of "eel-worm" has been given to these Perichaeta by gardeners. It is worth putting on record here that in a species of Acanthodrilus (A. capensis) the same irritable behaviour is visible. When a Perichaeta moves it helps itself greatly by extending, or rather protruding, the buccal cavity, which serves as a sucker, and grips the ground in front until the rest of the body is brought forward. It is possibly on account of this extra facility for movement that the genus can climb trees with such ease. A species of Perichaeta has been recorded by Mr. Willey upon an epiphyte of a palm, and Dr. Benham has found that it is a new species, to which the name of Perichaeta willeyi has been given. The Lumbricid genus (if it be admitted as a genus), Dendrobaena, was so named on account of a similar habit of climbing trees. Very singular in its habit is the not inaptly-named Perichaeta musica of Java. It is a monster of its kind, several feet in length, and during the night makes "a sharp interrupted sound," apparently by the friction of the chaetae against stones. The species figured (p. 381) is, as are a few others, remarkable for the presence of twelve or seventeen spermathecae in segments 6 and 7.
Fam. 10. Cryptodrilidae.[441]—This family is one of the largest of the Oligochaeta; there are rather over 120 different species, which can be arranged in at least sixteen genera. They are found in most parts of the world, but abound principally in the tropics. Australia may be considered to be the headquarters of the family, which form its principal earthworm-inhabitants. Peculiar to this continent, or at least mainly confined to it, are the genera Megascolides, Cryptodrilus, Fletcherodrilus, Trinephrus, and Digaster. Microscolex, though occurring in many parts of the world, is characteristic of the more southern regions of South America and of New Zealand. Tropical Africa has the genera Nannodrilus and Millsonia limited to itself, and has besides nearly {383}all the species of the genus Gordiodrilus. This family is one which it is exceedingly difficult to define and to split up into different genera. It shades almost imperceptibly into the Perichaetidae on the one hand, and is very hard to differentiate from the Acanthodrilidae on the other. A Cryptodrilid, like any member of the genus Cryptodrilus, with complete circles of chaetae would be a Perichaetid; and as there are species of Perichaeta in which the anterior segments have only a few chaetae in each segment, it is perhaps wrong to separate the two families at all. Apart from the chaetae, there is no peculiarity in the organisation of the family Perichaetidae that is not also met with in the Cryptodrilidae. Even the highly characteristic intestinal caeca so distinctive of the genus Perichaeta itself, as contrasted with Megascolex and the other genera, occur, though more numerously, in the African Millsonia, where there are forty or fifty pairs of them. A fairly common feature in the family is the presence of two, or even three, pairs of gizzards, a character which is also met with in the genus Benhamia among the Acanthodrilidae, and occurs also in some other families. The names Digaster, Didymogaster, Perissogaster, and Dichogaster have been founded upon this character. The excretory organs may be paired (in Trinephrus there are three pairs to each segment) or of the diffuse kind. The male pores are usually upon the eighteenth segment, but not unfrequently upon the seventeenth, and are often armed with long and ornamented chaetae. Spermiducal glands are invariably present, and may be lobate or tubular. There are two groups of small-sized genera, which in their simplicity of organisation stand at the base of the series; but it is very possible that the simplification is rather due to degeneration than to primitive position. One of these groups includes the semi-marine genus Pontodrilus (with which I include the phosphorescent Photodrilus) and Microscolex. In these forms the gizzard has disappeared, or is represented by a rudimentary structure, and the male pores are upon the seventeenth segment. In the other group are the genera Ocnerodrilus,[442] Gordiodrilus,[443] and Nannodrilus, which are of even smaller size, and have in the same way the male pores upon the seventeenth segment. The species of this group are often aquatic, and there is not only no gizzard {384}(in most of the species), but the calciferous glands have been reduced to a single pair, which lie in the ninth segment. The latter character is also found in the Acanthodrilid Kerria, which has been associated with the above named. Gordiodrilus has the peculiarity that there are, as in Acanthodrilids, two pairs of tubular spermiducal glands.
Fam. 11. Acanthodrilidae.[444]—This family is only with difficulty to be distinguished from the last. The following definition applies to all the members of the family with one exception, and does not apply to any Cryptodrilid with, so far as is known, one exception only. There are two pairs of spermiducal glands, opening upon the segments in front of and behind that which bears the apertures of the sperm-ducts.
The one exception to this definition is the species Acanthodrilus monocystis, which I formerly placed in a distinct genus, Neodrilus. Microscolex modestus is the exception among the Cryptodrilidae; in that worm the male pores are upon the segment which follows that upon which the spermiducal glands open. The Acanthodrilidae show a considerable range of structural variation. This enables them to be separated into several well-marked genera. The type genus Acanthodrilus has a pair of nephridia in each segment. It contains thirty-five species, which are all from the southern hemisphere. These species show but little variation among themselves. Benhamia is a genus that differs from Acanthodrilus in the fact that the nephridia are of the complex type, so often met with in earthworms with many external pores. The segment that bears the male pores is entirely without any traces of the ventral chaetae. Here again there are a large number of species which are nearly confined to the continent of Africa. Dr. Michaelsen is indeed of opinion that the few species found in the East Indies and America are accidental importations. I have proposed to separate some of the New Zealand Acanthodrilids into a distinct genus, Octochaetus, which is somewhat intermediate between Acanthodrilus and Benhamia. They have multiple nephridia, but only a single gizzard. Plagiochaeta of Benham, from New Zealand, is in any case clearly a distinct form. It is mainly to be distinguished by the numerous chaetae in each segment. Trigaster Benham, is West Indian. Deinodrilus (New {385}Zealand) has twelve chaetae in each segment. Diplocardia, from North America, has the male pores on segments 18, 19, 20.
Fam. 12. Eudrilidae.[445]—This is perhaps the most remarkable family of terrestrial Oligochaeta. Its distribution is no less curious than its structure. Up to the present it is not known outside tropical Africa, with the exception of the genus Eudrilus itself, which is almost world-wide in range. As, however, but one species of Eudrilus is found out of Africa, and as that species is so common in gatherings from various tropical countries, it seems to be an instance of a species with large capacities for accidental transference from country to country. The type genus, Eudrilus, has been known since 1871, when it was originally described by M. Perrier.[446] Since that date nineteen other genera have been described from Africa by Dr. Michaelsen, Dr. Rosa, and myself. The most salient external character of the group, not universal but general, is the unpaired male and female orifices. The orifices are commonly very conspicuous (see Fig. 197).
The peculiarities of internal structure mainly concern the reproductive organs, the differences in which from genus to {386}genus are often very great. We have already referred to the remarkable branching of the nephridial duct in the body-wall, and to the much modified calciferous glands of Stuhlmannia and some other genera. These structural variations perhaps permit the family to be divided into two sub-families. In one there are calciferous glands of the normal type, though peculiar in that one or more are median and ventral in position, and are unpaired; there is no branching of the nephridium in the body-wall; there are always, so far as is known, the Pacinian-corpuscle-like bodies in the integument. In the other sub-family the calciferous glands, if present (they are absent, for instance, in Libyodrilus), have undergone much modification in structure; the nephridia, where they have been investigated, have been found to branch copiously in the body-wall; the peculiar integumental bodies hardly ever occur.
Fam. 13. Geoscolicidae.[447]—This family is essentially tropical, being found in South America and the West Indies, in tropical Africa, in India, and in some of the islands of the Malay Archipelago. But it also occurs (Sparganophilus and Criodrilus) in Europe and in America. A good many of the genera are aquatic. This is the case with the two already mentioned; the genera Glyphidrilus and Annadrilus of the Malay Archipelago can live in water. The family is easily definable if we take the more typical forms; but at one end of the series it fades into the next family, that of the Lumbricidae. Criodrilus is one of the genera which is difficult to place. As is the case with many Geoscolicidae, Criodrilus has ornamented chaetae not only upon the clitellum, but upon the other segments of the body. This character was until recently unknown among the Lumbricidae; it has been lately found in Allolobophora moebii and A. lonnbergi. The absence of spermathecae characterises Criodrilus as well as other Geoscolicidae; but here again the character is not by any means distinctive, for in Allolobophora constricta there is the same absence of these organs. In Criodrilus the male pores are upon segment 15, as in the Lumbricidae, but a species of Kynotus, which is certainly a Geoscolecid, has these pores upon precisely the same segment. The only point in which Criodrilus is definitely a Geoscolecid, or rather not a Lumbricid, is in the forward position of the clitellum, which begins upon the fifteenth segment, far earlier than it does {387}in any undoubted Lumbricid. The peculiar elongated cocoon, which much resembles that of Sparganophilus, is another character which favours its Geoscolecine affinities. Dr. Michaelsen has proposed to unite Criodrilus and Alma into a family intermediate between the Geoscolicidae and the Lumbricidae.
Perhaps the most remarkable genus in the whole family is Alma. One species lives in the Nile mud; another is the "Yoruba worm" of West Africa, whose habits have been described by Mr. Millson. The most marked character of this genus, apart from the branchiae (see p. 352) which apparently may be present or absent according to the species, is in the two enormous processes of the body-wall, which are illustrated in Fig. 198. These contain the sperm-ducts, which, however, open some way in front of the free end; they are provided on the ventral surface with a series of sucker-like structures and with peculiar chaetae. Another interesting genus is Pontoscolex, which was originally described from the sea-shore of Jamaica by Schmarda; there are only two species which are certainly characterised, though a variety from the Hawaian Islands may be a "good" species. It possesses the remarkable peculiarity that the chaetae at the end of the body are disposed in a perfectly irregular fashion, which earned for it the name of brush-tail at the hands of its discoverer, Fritz {388}Müller. This worm, which is universal, or nearly so, in its range, doubtless having been transferred accidentally from country to country, invariably shows a light spot not far from the tail; when this is examined with the microscope it is seen that the chaetae are here absent or very small, and that the muscular structure of the body-wall is slightly different; it was thought that this spot was a zone of growth where fresh segments could be added after the fashion of some of the aquatic Oligochaeta, to which, it may be remarked, the present genus shows a curious point of likeness in the bifid character of the chaetae. It seems, however, that there are really no grounds for the supposition, and it is possible that we have here a "weak" spot, such as that in the foot of certain land snails, which readily gives way when the worm is picked up by a bird, and allows the "better half" of the creature to escape. The Bermudian genus Onychochaeta offers a very strange peculiarity in that the chaetae on the hinder segments of the body are enormously larger than those in front, and end in strong hooks; it seems likely that their function is to maintain a tight hold of the ground while the worm is leaning out of its burrow, as every one has seen the common earthworms of this country do. Onychochaeta has the same irregular arrangement of the chaetae upon the greater part of the body, as has Pontoscolex. This family, like so many others, has its giants and its dwarfs. At one extreme is the great Antaeus of South America, several feet in length; at the other the inch-long Ilyogenia of Africa. The American Urobenus has a pair of intestinal caeca like those of Perichaeta, and placed in the same segment.
Fam. 14.9 Lumbricidae.[448]—This family is to be distinguished by the following assemblage of characters.
The male pores are usually upon segment 15, and never behind that segment; the clitellum commences some way behind the male pores. The gizzard, which is invariably single, is equally invariably at the end of the oesophagus. There are three pairs of calciferous glands. The nephridia are always paired. The spermathecae never have a diverticulum.
This family only contains three well-known genera, viz. {389}Lumbricus, Allolobophora, and Allurus. The American Bimastos may be distinct. Tetragonurus, not allowed by some, is at present unknown except as regards external characters; it differs from the other Lumbricidae in the fact that the male pores are upon the twelfth segment. In Allurus they are upon segment 13, and in the remaining genera upon the fifteenth. Lumbricus is to be distinguished from Allolobophora by its prostomium, which is continued by grooves on to the buccal segment, so as to cut the latter in half. It has also median sperm reservoirs, as well as the paired sperm sacs which are alone present in Allolobophora.
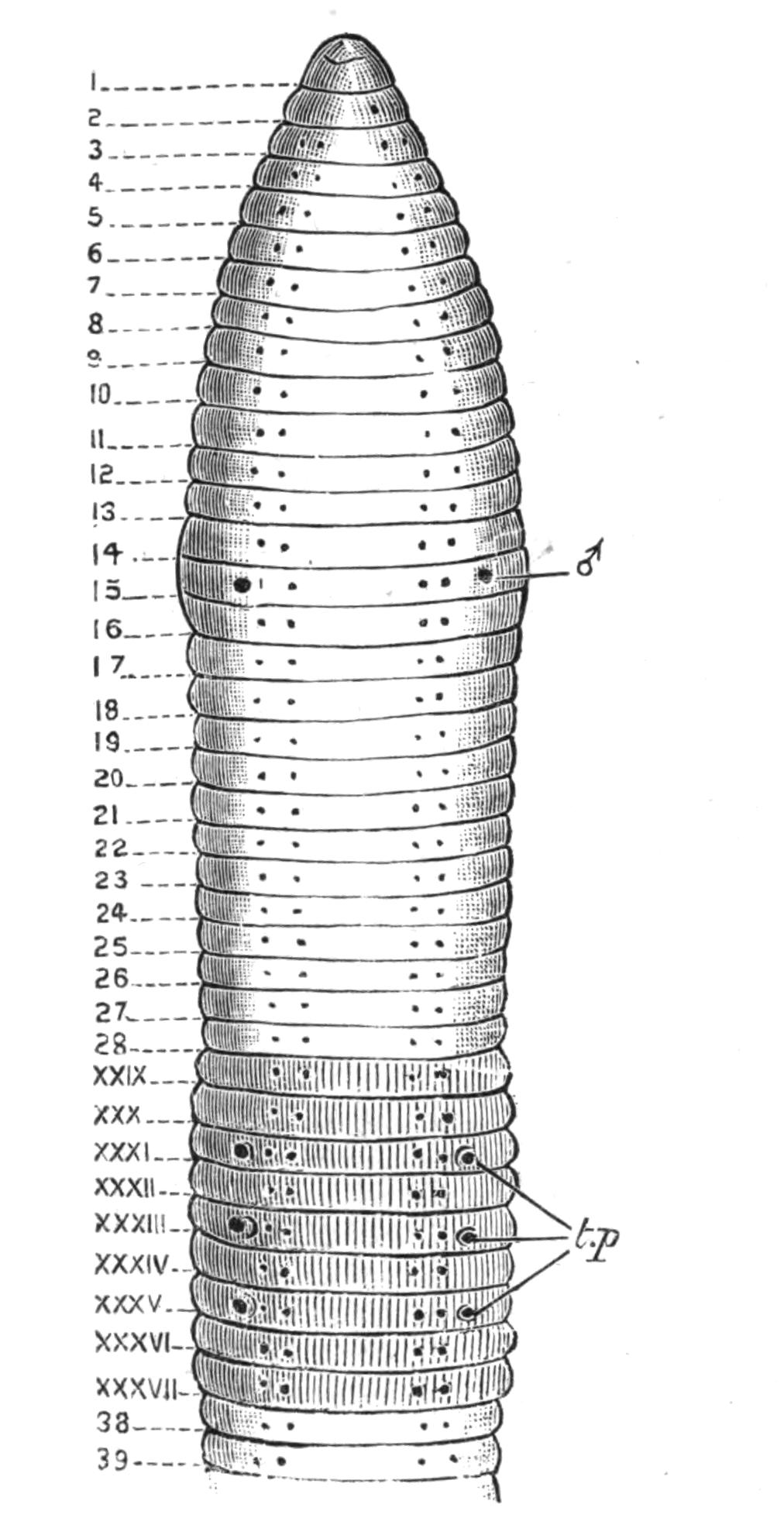
Fig. 199.—Allolobophora chlorotica Savigny. × 4. The clitellar segments are marked in Roman numerals. t.p, Tubercula pubertatis; ♂, male pore.
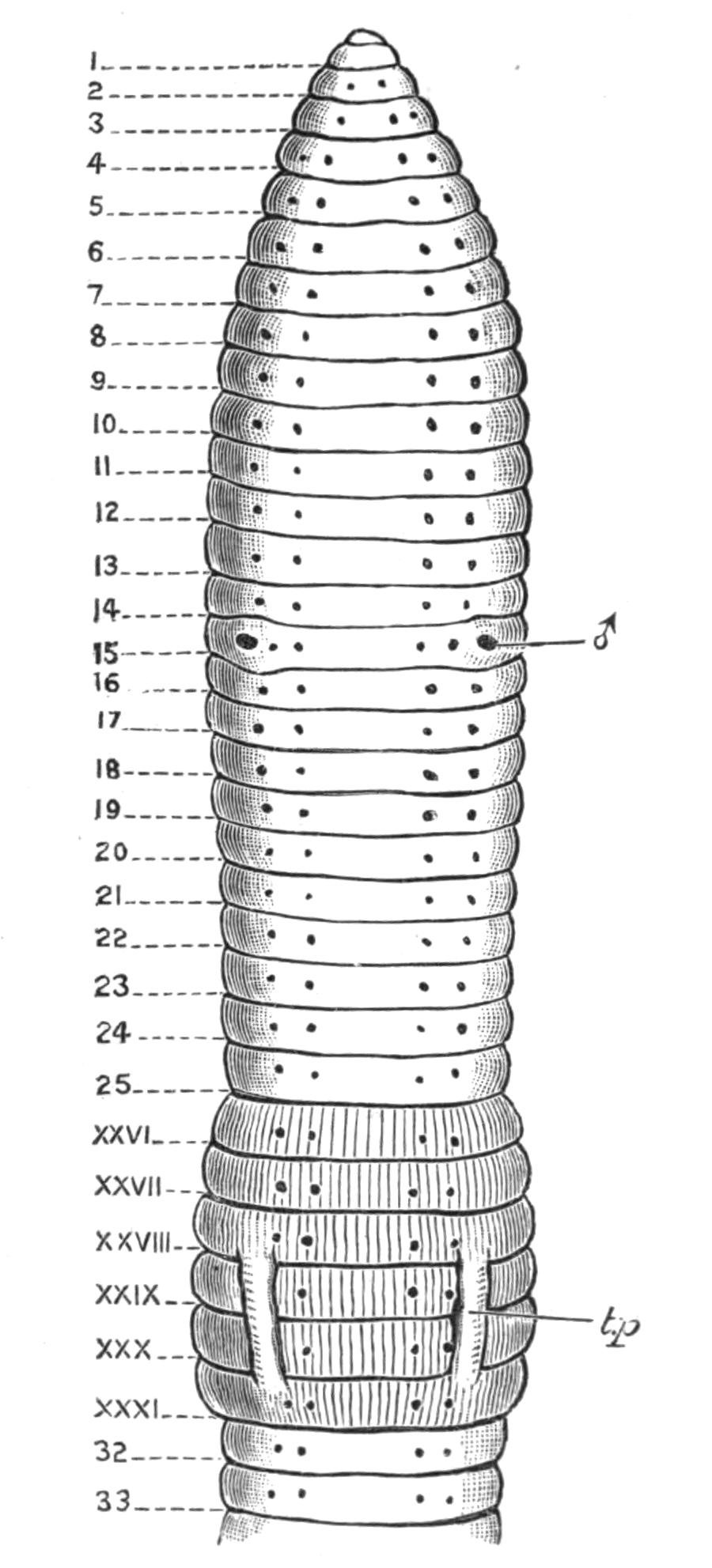
Fig. 200.—Allolobophora putris Vejd. × 5. Lettering as in Fig. 199. The black dots represent the chaetae in both figures.
This is the only family of earthworms which, so far as is known, can brave the ice and snow, and what is still more {390}difficult to understand, the perpetually frozen undersoil of the Arctic regions. Eisen has described a number of species from Spitzbergen, and Colonel Feilden recently sent me an example of Allolobophora octoedra from Kolguiev, where Mr. Trevor-Battye also saw another specimen. The family is characteristic of the Nearctic and Palaearctic regions, and though found beyond them, is probably elsewhere an accidental importation (see p. 371). There are at least fifteen species of this family found in England and Ireland, and probably more will be identified.
There does not exist at present any comprehensive account of the British species of earthworms, though all of them are included in Dr. Rosa's recent revision of the family. Most of the British forms belong to the genus Allolobophora, which may be divided into two series according to whether the chaetae are quite close together or further apart. The extent of the clitellum and the position of those swollen eminences which appear earlier than the clitellum, and are known as tubercula pubertatis, offer further characters. In the following tables, extracted from those of Rosa, the known British species of this genus are grouped according to these three characters. With the help of these tables and Figs. 199 and 200, any of the species ought to be easily identified.
With Chaetae Distant.[449]
| 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | |
| A. putris | ··· | — | — | — ··· |
— — |
— — |
— | ··· | ||||
| A. constricta | — | — | — | — | — | — | ||||||
| A. veneta | ··· | — | — | — | — — |
— — |
— | — | ||||
| A. octoedra | — | — | — |
— — |
— — |
··· | ||||||
| A. cyanea (subsp. profuga) |
— | — — |
— — |
— — |
— — |
— | ||||||
| A. rubida | — — |
— — |
— — |
— — |
— — |
— — |
||||||
| A. mammalis | — | — | — — |
— — |
— | — |
| 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | |
| A. putris | ······ | —— | —— | —— ······ |
—— —— |
—— —— |
—— | ······ | ||||
| A. constricta | —— | —— | —— | —— | —— | —— | ||||||
| A. veneta | ······ | —— | —— | —— | —— —— |
—— —— |
—— | —— | ||||
| A. octoedra | —— | —— | —— |
—— —— |
—— —— |
······ | ||||||
| A. cyanea (subsp. profuga) |
—— | —— —— |
—— —— |
—— —— |
—— —— |
—— | ||||||
| A. rubida | —— —— |
—— —— |
—— —— |
—— —— |
—— —— |
—— —— |
||||||
| A. mammalis | —— | —— | —— —— |
—— —— |
—— | —— |
With Chaetae Paired.
| 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | |
| A. rosea | ··· | — | — | — | — | — — |
— — |
— ··· |
— | |||||
| A. foetida | ··· | ··· | — | — | — — |
— — |
— — |
— ··· |
— | |||||
| A. eiseni | ··· | — | — | — | — | — | — | — | — | |||||
| A. caliginosa | ··· | — | — | — | — — |
— ··· |
— — |
— | ··· | |||||
| A. terrestris | ··· | — | — | — | — | — — |
— — |
— — |
— | |||||
| A. chlorotica | ··· | — | — | — — |
— | — — |
— | — — |
— | — | ||||
| A. georgii | ··· | — | — | — — |
— | — — |
— | — |
| 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | |
| A. rosea | ······ | —— | —— | —— | —— | —— —— |
—— —— |
—— ······ |
—— | —— | —— | |||||
| A. foetida | ······ | ······ | —— | —— | —— —— |
—— —— |
—— —— |
—— ······ |
—— | |||||||
| A. eiseni | ······ | —— | —— | —— | —— | —— | —— | —— | —— | |||||||
| A. caliginosa | ······ | —— | —— | —— | —— —— |
—— ······ |
—— —— |
—— | ······ | |||||||
| A. terrestris | ······ | —— | —— | —— | —— | —— —— |
—— —— |
—— —— |
—— | |||||||
| A. chlorotica | ······ | —— | —— | —— —— |
—— | —— —— |
—— | —— —— |
—— | —— | ||||||
| A. georgii | ······ | —— | —— | —— —— |
—— | —— —— |
—— | —— |
There are of course other points by which the different species can be distinguished. Colour in a few cases enables a species to be named at once without any further aid. One of the most striking of these cases is the Brandling, so common upon dunghills, and so dear to some anglers. This worm is ringed with brownish purple upon a yellowish ground. The greenish A. chlorotica is often found under stones, and curls itself round into nearly a complete circle when disturbed. A. cyanea, of a bluish grey colour, is one of the earthworms very commonly met with in the early morning in London and the neighbourhood. More generally, however, the colour is of a paler or darker red, verging towards and attaining brown, or even blackish brown; and is so variable that nothing in the way of identification can be attempted from the colour alone, even with the most elaborate description.
Lumbricus, as already mentioned, is distinguished from all Allolobophora except A. eiseni, by the complete dovetailing of the prostomium into the first segment. There are five species in this country which may be thus distinguished by the position of the tubercula pubertatis. The most familiar species is the common L. rubellus.
| L. rubellus Hoff. | tubercula pubertatis are on 28, 29, 30, 31 |
| L. castaneus Sav. | tube"cula pu"ertatis a"e on 28, 29, 30, 31, 32 |
| L. herculeus Sav. | tube"cula pu"ertatis a"e on 28, 29, 30, 31, 32 33, 34, 35, 36 |
| L. papillosus Friend | tube"cula pu"ertatis a"e on 28, 29, 30, 31, 32 33, 34, 35, 36, 37 |
| L. festivus Sav. | tube"cula pu"ertatis a"e on 28, 29, 30, 31, 32 33, 34, 35, 36, 37, 38. |
HIRUDINEA (LEECHES)
INTRODUCTION—ANATOMY—REPRODUCTION—CLASSIFICATION—RHYNCHOBDELLAE AND GNATHOBDELLAE
"The external appearance of the Hirudinea," remarks Professor Vaillant,[450] "permits us, save for rare exceptions, to recognise at once the animals which belong to that group." The leeches are distinguished as a rule by the possession of two suckers, one at each end of the body; their general shape usually differs from that of other Annelids by its oval contour and its dorso-ventral flattening. Cyclicobdella lumbricoides of Grube, which Blanchard has stated to be the same species as Nephelis tergestina, has, however, almost the form of an earthworm by reason of its cylindrical shape and the inconspicuousness of the suckers, while Lumbricobdella also resembles an earthworm and has no posterior sucker at all.[451] The Oligochaet family Discodrilidae (see p. 376) agree with the leeches in their parasitism, in their general shape, in the presence of two suckers, and, furthermore, in the existence of jaws, which are found in no other Oligochaet, but occur in a large number of the Hirudinea. These facts, indeed, though not perhaps important by themselves, are indications of the really close resemblance of the Hirudinea to the Oligochaeta, a group which they approach not merely in such habits as the formation of a cocoon in which the eggs are enclosed, but also in many important points of internal and external structure. Indeed, the fundamental differences between the two groups are not numerous, and are not of such importance as has been given them by some writers.
Leeches are to be found in most parts of the world, in {393}situations which are sufficiently damp for their comfort. But we do not at present possess enough knowledge to state much as to the facts of their distribution. The structure of leeches is not so well known as is that of the earthworms; for they have not been to so great an extent collected in extra-European countries. It would even be desirable to ascertain precisely the species which inhabit these islands, the most recent enumeration (1865) being that contained in the British Museum Catalogue of non-parasitical worms by the late Dr. George Johnston. For Italy this has been lately done by Dr. Blanchard, and a good many of the species are common to the two countries. Johnston enumerates altogether (after subtracting what are probably synonyms) twenty-one species, distributed among the genera Branchellion, Pontobdella, Piscicola, Nephelis, Trocheta, Haemopis, Hirudo, and Glossiphonia (= Clepsine), which number will be possibly still further reduced. The first two genera are marine, the remainder being fresh water or terrestrial; Trocheta has been probably introduced.
The use of Hirudo medicinalis is well known to many of us from personal experience. So extensively was this leech formerly made use of that it is now far from being a common species either in this country or in France. Those who desire full information as to Hirudiniculture should consult the work of Dr. Ebrard, published in 1857.[452] The former extensive use of the leech has led to the transfer of its name to the doctor who employs it, the authors of the sixteenth century constantly terming a physician a leech; it has been suggested, however, that the term was applied rather by way of analogy. The useful blood-sucking habits of the medicinal leech have been wrongly attributed to the innocent horseleech (Aulastomum)—innocent, that is to say, of the blood of Vertebrates, for it has been described as "a cruel and greedy worm," engulfing earthworms and even smaller specimens of its own species. {394}"Horsleches," said an old writer, "are wholesome to drawe foorthe foule blood, if thei are put into a hollowe rede, and one of their endes cutte of, whereby the blood maie run forthe." But it is clearly not easy for a creature destitute of jaws and teeth to bite, and the similarity of general aspect has doubtless led to a confusion with the savagely biting medicinal leech.
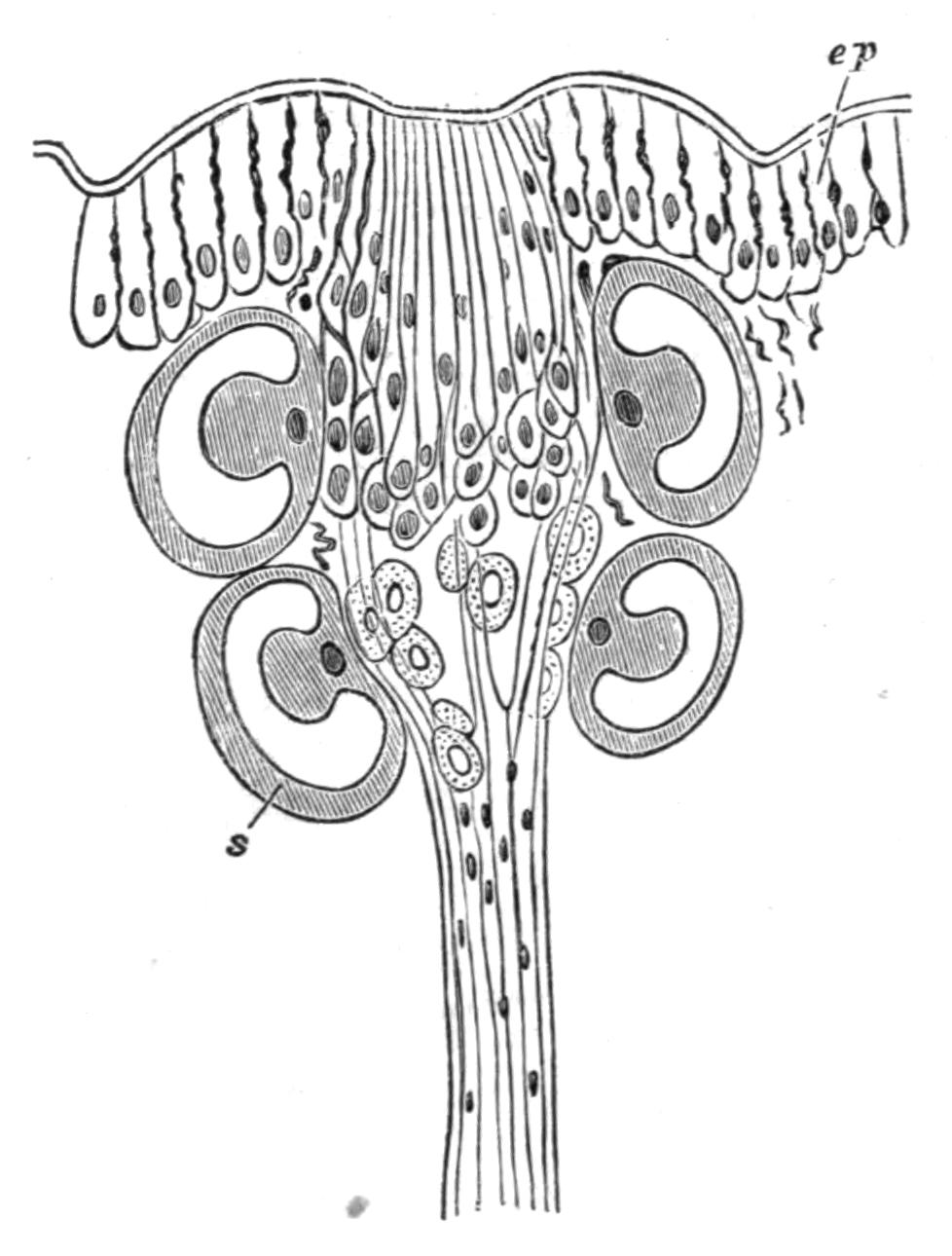
Fig. 202.—Sense body of Macrobdella sestertia. (After Whitman.) ep, Epidermis; s, clear cells. Highly magnified.
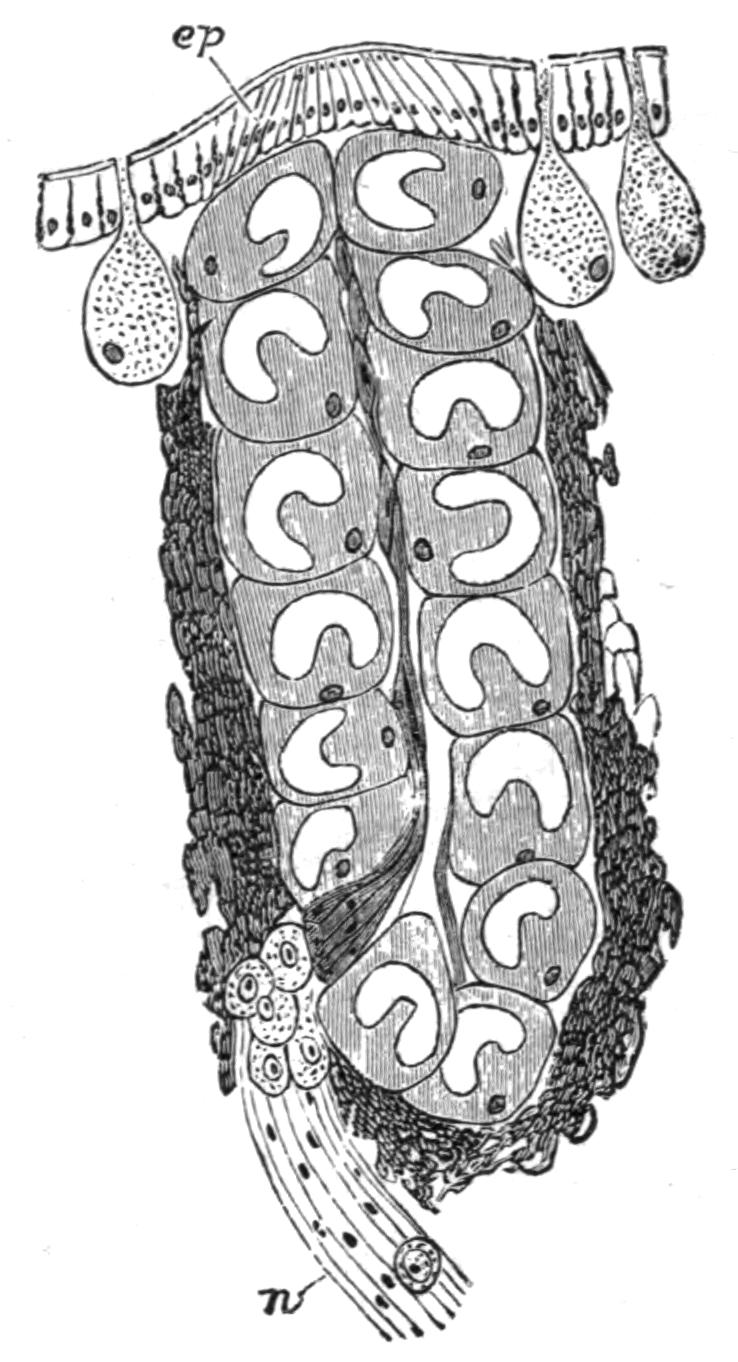
Fig. 203.—Section through eye of Haemadipsa japonica. (After Whitman.) ep, Epidermis; n, nerve. Highly magnified.
The Hirudinea are all distinctly segmented animals, but the segmentation differs from that of the Oligochaeta in two points. In the first place the number of segments is much smaller in a leech than in an Oligochaete, although the difference does not appear great at first sight.
A leech's body may seem to be composed of seventy, eighty, or one hundred segments, a number quite as great as is found, for example, in the genus Perichaeta among the earthworms; but the apparent number of segments in the leech is produced by a very marked annulation of the real segments; and this is indeed the second point of difference referred to above. But there are earthworms which show frequently a secondary annulation,—secondary because it appears late and does not affect other organs {395}of the body. A segment of an earthworm may indeed have five or six distinct annulations, but it will be bounded internally by two septa, and will bear only one set of chaetae externally. In the leech external clues to the definition of a segment were until recently wanting. They appear now to have been found in the sensory organs of the skin (Figs. 201 and 202), which are, according to Whitman,[453] disposed in a perfectly metameric fashion. Judged by this, and also by the nephridia and nerve-ganglia, the number of segments in a leech does not appear to exceed twenty-six, independently of the sucker, which may represent a few fused segments, seven (in the medicinal leech) according to Leuckart.
The eyes, which are so useful in the systematic arrangement of the group, appear to have been evolved from these sensory organs by a further exaggeration of their peculiarities. Figs. 202 and 203 show this point convincingly. The segmental sense organ is shown in Fig. 202; to the outside of certain sense cells, below which are a mass of ganglion cells, are certain peculiar transparent cells very similar to the clear cells found in the interior of the eye (Fig. 203). The segmental disposal of the sensory bodies and of the eyes is shown in Fig. 201.
Some Hirudinea are furnished with external branchiae; this is the case with Branchellion, in which genus the branchiae (Fig. 204) have an arborescent form; in Cystibranchus there are a series of paired simple vesicles which take the place of these more complicated respiratory organs of Branchellion. The Hirudinea do not, save for one exception (Acanthobdella), possess chaetae; but it must be borne in mind that the Discodrilidae and the genus Anachaeta among the Oligochaeta are in the same condition. In Acanthobdella[454] there are two pairs of chaetae upon each side of the anterior five segments of the body. According to the figure which Grube, the {396}original describer of the genus, gives of these chaetae, the part implanted in the body is straight, while the part extending freely beyond the body is sharply hooked.
The body of the leeches is never ciliated externally; there is, as in the higher Oligochaeta, a cuticle secreted by the underlying epidermis. The Hirudinea have, like the Oligochaeta, a clitellum which, as in some of the lower members of that group, is limited to a very few segments in the immediate neighbourhood of the generative openings. It occupies in Hirudo segments 9, 10, and 11. The epidermis gives rise to many unicellular glands which either remain in situ or get moved to a deeper position. In this the leeches exactly resemble the earthworms. There is generally a great deal of connective tissue in the body-wall. The muscles consist of circular, longitudinal, and radial series. The individual fibres have the same structure as those of the Oligochaeta, consisting of a soft and undifferentiated core, round which is a radially-striated sheath of contractile substance.
Alimentary Canal.—The leeches are divided into the Rhynchobdellae, which have a proboscis but no jaws, and the Gnathobdellae, which possess a series of jaws but have no proboscis. But the division is not a hard and fast one, for we have Whitman's genus Leptostoma, which should belong to the jawed division, but which has quite rudimentary jaws without the sharp denticles so characteristic of Hirudo. The pharynx is furnished with salivary glands. The oesophagus is followed by the proventriculus, which has a varying number of pairs of caeca; then comes the intestine and the rectum. The anus is, as a general rule, placed dorsally to the sucker, but there are a few rare exceptions where the anus is within the sucker. The caeca are totally absent in Trocheta.
Vascular System.—As will be seen from Fig. 205, the vascular system of the Hirudinea is constructed on a plan which closely resembles that of the Oligochaeta. The diagram represents Glossiphonia, one of the Rhynchobdellae, the group which comes nearer to the Oligochaeta in many particulars than the Gnathobdellae. We can recognise a dorsal and a ventral vessel, which are united in the anterior part of the body by three perioesophageal rings, the elongation of which, particularly of the last pair (v), from before backwards is very marked. In the region of the sucker the dorsal and ventral vessels are united by fourteen shorter loops, the number of which has an interesting {397}relation to the number of segments out of which this portion of the body is possibly formed. It will be observed also that the dorsal vessel is double in this region, a condition which obtains along its whole length in Branchellion—a repetition of what has been described in more than one species of Oligochaete. In the region of the last pair of digestive caeca the dorsal vessel has appended to it copious sinuses which embrace the intestine and supply its walls with blood. In Hirudo there are only a pair of lateral vessels, and neither dorsal nor ventral vessels; in this leech and in the Gnathobdellae generally there are intra-epidermic capillaries, a fact first discovered by Professor Lankester, and now known to occur also in the Oligochaeta.
The development of the blood-vessels shows that they have no relation whatever to the coelom, in spite of their subsequent connexion with it. The two longitudinal stems of Hirudo arise as cavities in the somatic layer of the mesoblast after the formation of the coelom. In Nephelis, but not in Hirudo, Dr. Bürger thinks that there is some reason for regarding the vascular system as the remains of the primitive segmentation-cavity of the embryo, an opinion which is held in respect of the vascular system of many other animals.
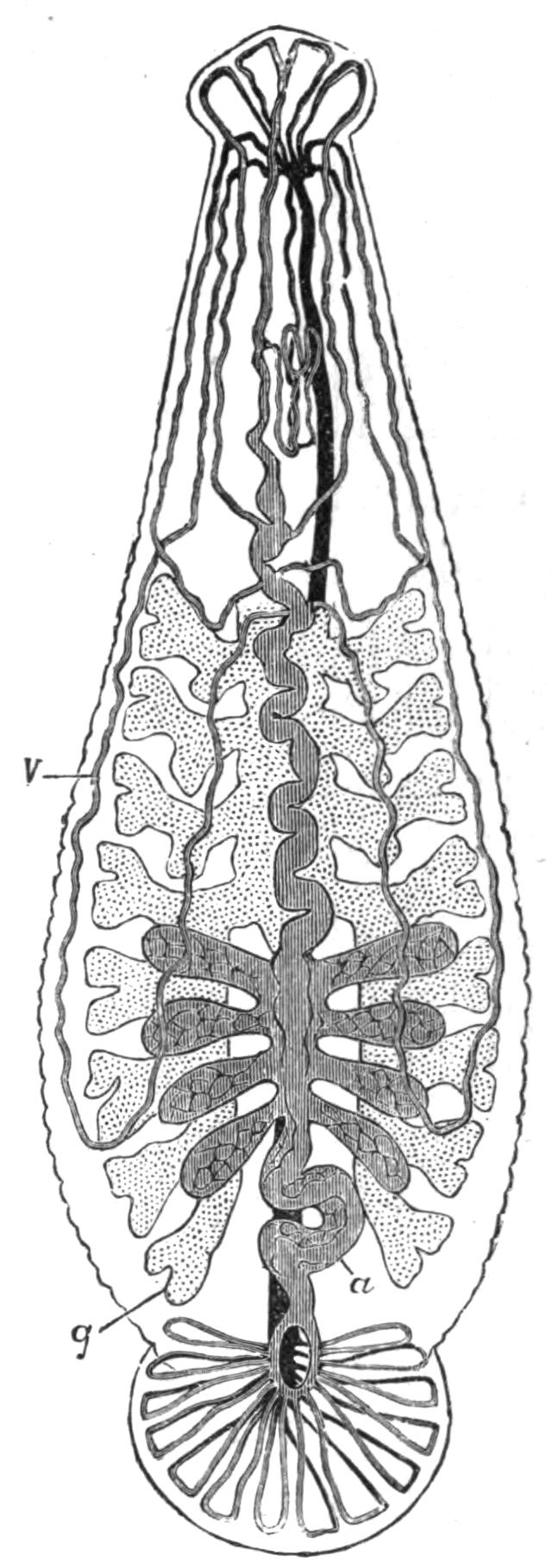
Fig. 205.—Glossiphonia marginata, vascular and alimentary system. ×4. (After Oka.) a, Dorsal vessel; g, intestinal caecum; v, one of the hearts.
Body-Cavity.—One of the most marked differences between the leeches and the Oligochaeta is in the body-cavity. In the latter there are a series of cavities corresponding to the segments, which are bounded in front and behind by the intersegmental septa, and in which all the viscera lie. In leeches such an arrangement is not recognisable save in Acanthobdella, where Kowalevsky[455] has quite recently described a typical coelom divided by septa into twenty segments. In transverse sections the body of other leeches appears at first sight to be solid, owing to the growth of the muscles and connective tissue. A more careful study, however, has revealed the {398}fact that there are considerable remains of the body-cavity or coelom which form a complicated system of spaces and channels. What has happened, in fact, in the leech is that the coelom has become gradually and partially obliterated by proliferation of the cells in the interior of the body, a process of obliteration which has already commenced in the Oligochaeta. In many of the latter, some of the principal blood-vessels have become surrounded by a space cut off from the general body-cavity, while in the majority a special cavity surrounds the testes and the funnels of the sperm-ducts. This process of the formation of separate cavities for the inclusion of the several viscera culminates in the leeches with the marked obliteration of the greater part of the coelom. This has become so much reduced to the condition of narrow tubes that there has been a tendency to confuse it with the vascular system, more especially perhaps in those forms in which the blood is tinged with haemoglobin, and in which there is a connexion between the two systems of spaces. This confusion has been further increased by the plan of injecting the vascular system, a method of investigation which must be employed with great care in delicately-organised creatures whose tissues can be easily ruptured, and so lead to a flow of the injecting fluid into places and in directions impossible during life.
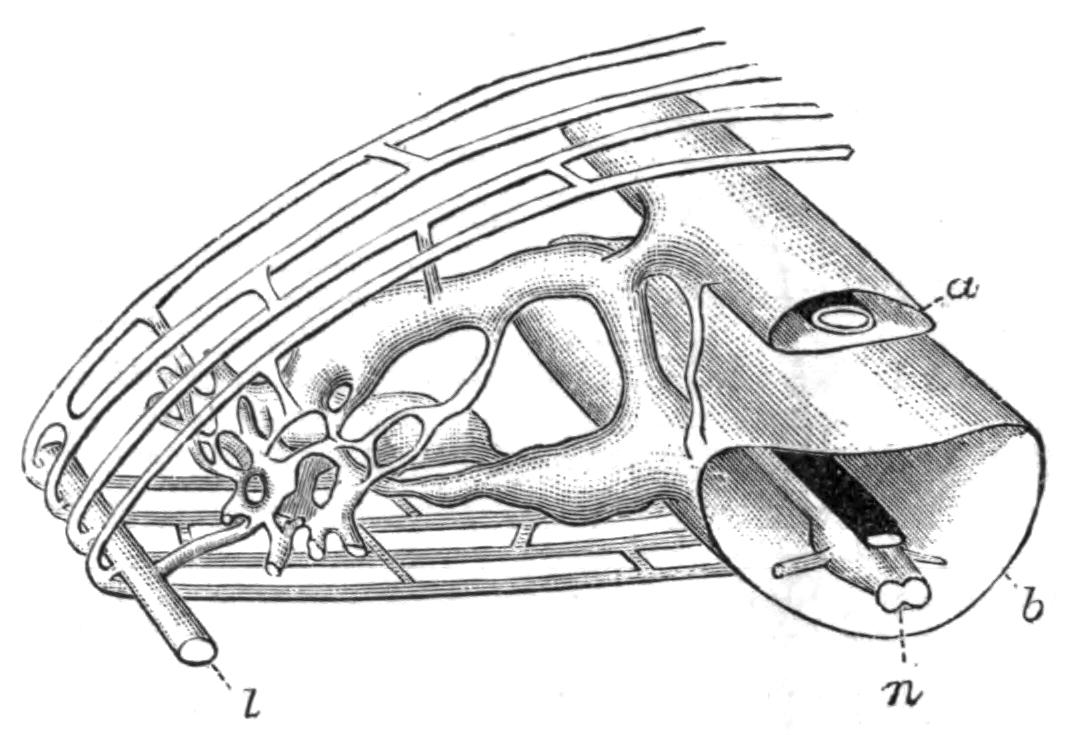
Fig. 206.—Coelomic canals of Glossiphonia complanata. × 10. (After Oka.) a, Dorsal canal containing dorsal blood-vessel; b, ventral canal containing ventral blood-vessel; l, lateral canal; n, nerve-cord.
In transverse sections of leeches it may be seen in successful preparations that the various organs of the body are enclosed in spaces. The funnels of the nephridia open into lacunae which could hardly in any case be regarded as blood spaces, while the blood-vessels themselves with their muscular walls cannot be confounded, at least in the case of the larger trunks, with the spaces not having muscular walls which surround them. Furthermore, it will be pointed out immediately that the reproductive organs are produced on the walls of spaces which are the commencement in the embryo of the reduced coelom of the adult worm. These spaces therefore conform in every particular to the general {399}conditions which have been laid down about the characters of a true coelom. As to the complexity of this system, attention may be directed, to the accompanying diagram (Fig. 206) of the coelom of a segment of Glossiphonia, which has been lately worked out in detail.[456] It will be observed that there are four main longitudinal sinuses which are connected by a complicated system of transverse tubes and spaces. In the anterior part of the body, before the point where the intestinal caeca arise, the dorsal and ventral lacunae fuse to form one larger so-called median lacuna. The cavity of this is interrupted, in correspondence with the segmentation of the body, by septa exactly comparable to those of Oligochaeta; but the septa in Glossiphonia are not present at every segment. So far our account of the coelom is chiefly derived from the genus Glossiphonia. In Hirudo, which is an example of the Gnathobdellae, the coelom is still further reduced; the lateral sinuses in them appear to be absent. But on the other hand there is formed a series of cavities in a form of connective tissue which has been termed botryoidal tissue. The cells of this tissue become hollowed out, and form channels which are in communication on the one hand with the remains of the coelom and on the other with the vascular system. This system has certain analogies with the lymphatic vessels of Vertebrates, and, like them, is an intermediary between the body-cavity and the blood. Originally, however, these botryoidal vessels have nothing whatever to do with either the vascular or the coelomic system; their connexion with both is a purely secondary affair, and only appears, comparatively speaking, late in life.
The development of the spaces here spoken of collectively as coelom confirms this interpretation of their nature. In the embryos of Hirudo, Aulastomum, and Nephelis there is a ventral space,[457] which includes the nerve-cord. Into this open a series of paired and segmentally-disposed lateral cavities, a pair to each segment. The ventral cavity itself is formed by fusion of the median parts of the lateral cavities. There is here clear evidence of a coelom, developed on a plan fundamentally identical with that of the Oligochaeta in that it is formed as a paired series of chambers corresponding to the segmentation of the body.
Nephridia.—The "segmental organs" or nephridia are seen in {400}their simplest form in such a type as Glossiphonia—the Rhynchobdellae, to which this genus belongs, being indeed in most particulars less specialised than the Gnathobdellae. Here we have a distinct funnel opening freely into the median or ventral coelomic space, which is immediately followed by a rounded swelling termed by Oka[458] the capsule; this is filled with cells, in the interstices of which the ductules are situated and meander. There is in this capsule a very strong likeness to the glandular brownish swelling which immediately follows the funnel in the nephridia of certain of the aquatic Oligochaeta, for example the Naids, where, as Vejdovsky has shown, there is a similar "rete mirabile" of the nephridial duct. After the capsule is a single row of cells which are disposed in a complicated coil. These cells are perforated by the duct, which is thus, as in the Oligochaeta, intracellular. In the first set of cells the duct is single, and gives off numerous branchlets into the interior of each cell, a condition which has also been observed in many Oligochaeta. Afterwards the cells are perforated by two, or even three, main ducts, for the duct returns upon itself and traverses the row of cells more than once; there are also branchlets developed from one or other of the main ducts. The terminal part of the nephridium is a short invagination from the exterior, which is lined by cells. There is clearly a close resemblance here with the nephridium of an Oligochaete. The nephridium, however, except for the funnel and the narrow tube immediately following it, does not appear to be ciliated.
There is, however, some difference of opinion as to the portions of the nephridium where there are two ducts in a single cell. Bourne[459] thinks that where there are two ducts there are two {401}cells, one lying inside the other, and that there is sometimes also a telescoping of cell within cell where the duct is single. In Hirudo the same writer has described the nephridial funnel, which has lost the simple character of that of Glossiphonia. The funnel is represented by a cabbage-head-like mass (Fig. 207, f) of ciliated cells with no single definite outlet to the exterior as in Glossiphonia. It appears to be an organ which has lost its proper function—a degeneration of the funnel being, as a matter of fact, not unknown in the Oligochaeta, where it may be carried to absolute extinction (Chaetogaster). In Branchellion and Pontobdella the simple metameric arrangement of the nephridia is to some extent lost, owing to the formation of a network continuous from segment to segment. It will be borne in mind that the Oligochaeta are the only other Chaetopods in which such a nephridial network has been stated to exist.
Male Reproductive Organs.—In Hirudo medicinalis there are nine, occasionally ten, pairs of testes, which are round white bodies arranged segmentally, i.e. a pair to each segment. From each arises a slender, somewhat sinuous tube, which enters the common collecting tube of its own side; each of these is much contorted at the upper end, the coiled portion being termed the epididymis. From this they enter a muscular penis which can be protruded. This is the arrangement met with in all leeches, save for the fact that the penis is absent in some; in Glossiphonia (see Fig. 208) this is the case. The number of pairs of testes also varies; and in Nephelis they are no longer arranged metamerically.
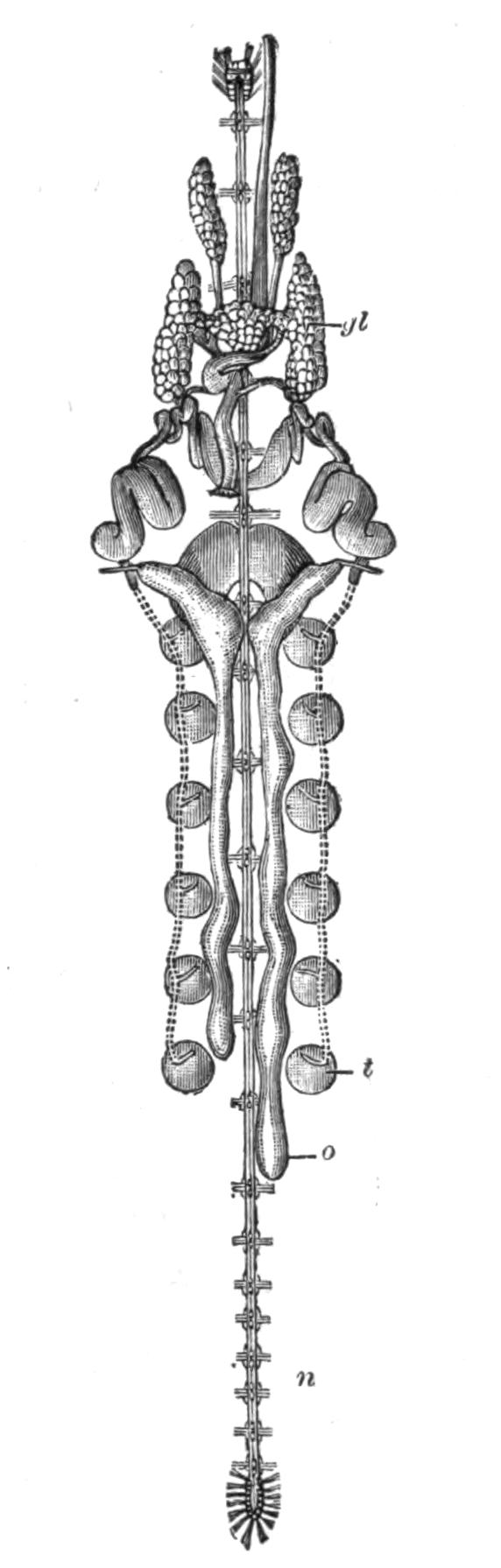
Fig. 208.—Nervous system and reproductive organs of Glossiphonia plana. × 2. (After Whitman.) gl, Prostate glands; n, nerve-cord; o, ovary; t, testes.
The testes arise as local proliferations of the epithelium of the lateral coelomic cavities, but from the somatic wall, not from the splanchnic, as in the case of the ovaries to be described later. A portion of the tissue which is to form the testis grows out laterally into a thin cord, which is to become the vas efferens of that {402}testis. Later both testis and duct become hollowed out with a common cavity. The main portion of the vas deferens of each side, as well as the terminal copulatory apparatus, is an ingrowth from the epidermis which meets the downgrowths from the testes.
That there are considerable differences between the reproductive organs of the leeches and those of Oligochaeta will be apparent from the above description. There are, however, to begin with, certain obvious similarities. In the first place, the origin of the reproductive glands is identical; in both groups also the efferent ducts consist of two portions—an invagination from the outside, and a formation of the proximal part of the ducts near to the glands. In Moniligaster, where—exceptionally—the testes develop on the posterior wall of their segment in close contact with the funnels of the sperm-ducts, there is no very hard and fast line to be observed between the tissues of the two. The hollowing out of the testis in the leech, and the continuity of the cavity thus formed with the duct, is a specialty of the leeches not found among the Oligochaeta.
Like many Oligochaeta, the leeches may form spermatophores in which the sperm is packed for its conveyance from one individual to another. Glossiphonia (Clepsine) plana, where the structure in question has been elaborately described by Whitman,[460] may be selected as an example. The spermatophore (Fig. 209) is about 8 mm. long, and is clearly formed of two halves, each of which is formed separately in one ductus ejaculatorius, the soldering together being effected in the common part of the male ducts, where also a basal portion with a single lumen is added. The spermatophore has a double wall. It is deposited not in the neighbourhood of the generative pores, but upon the back; and Whitman has discovered the extraordinary fact that the spermatozoa find their way through the body-wall of the leech into the interior of its body, where fertilisation presumably occurs.
Female Reproductive Organs.—The ovaries of the Hirudinea appear to differ from those of the Oligochaeta in that the ovaries are continuous with their ducts. In Hirudo, however, the real ovary of each side consists of masses of germinal tissue lying freely within a sac which communicates with a duct; the two ducts unite to form a much convoluted tube which opens into a thick-walled vagina, itself opening again on to the exterior by a median unpaired opening on the seventh segment. The muscular vagina is not always present.
The median unpaired female aperture offers now no particular difficulty, since in many earthworms, e.g. Perichaeta, this orifice is in the same condition; nor does the fusion of the oviducts and the so-called ovaries; for in Eudrilus, for example, and in many Eudrilidae, the ovary is contained in a sac into which the oviduct also opens. It will be noticed too that the existence of short oviducts as compared with the long sperm-ducts is a further point of likeness to at any rate the higher Oligochaeta. But a further comparison needs first to be based upon a consideration of the development of the different sections of the apparatus in the leech. The independence of the ovaries and their ducts has been proved by several observers; quite recently Bürger has dealt with the matter in Nephelis, Hirudo, and Aulastomum gulo.[461] He has found that the ovaries arise from the splanchnic wall of the lateral coelomic cavities; they are therefore proliferations of the coelomic epithelium, as in Oligochaeta and all Coelomates so far as is known. The peripheral layer of the mass of indifferent cells which constitutes the ovary becomes somewhat modified; its cells are flattened, and it at length separates itself and forms a capsule surrounding the other cells, which are in fact, or become, the ovary. This capsule meets and fuses with the ducts, which are invaginations from the exterior of the body.
There are clearly differences between the ovary of a leech and that of a typical Oligochaete like Lumbricus. The only point of agreement, in fact, is the origin of the reproductive gland itself from the walls of the body-cavity. In Lumbricus and allied forms, whatever may be held with regard to their homologies, the oviducts as a matter of fact appear first as funnels, which afterwards bore their way to the exterior. They are {404}purely mesoblastic structures, not—except perhaps the very extremity—derived from an ingrowth from the epidermis. We have, however, other Oligochaeta in which there are closer resemblances to what has just been described in the Hirudinea. In more than one point the aberrant family of the Eudrilidae come nearer to the Hirudinea than any other Oligochaeta, in spite of Branchiobdella with its jaws and sucker. Now in Eudrilus the ovary is enclosed in a capsule which becomes continuous with the duct of the great sperm-holding pouch, itself an invagination from the exterior; there is no reason in this Annelid why the ova should not reach the exterior by this system of ducts, although there is no actual experimental proof that they do so. In any case there is also an oviduct corresponding to that of Lumbricus, which opens into the opposite side of the duct of the spermathecal pouch on the one hand, and into the receptaculum ovorum on the other; it has no direct connexion whatever with the sac containing the ovary. If we were to cut off the receptaculum and the oviduct and reduce the spermathecal sac to its duct, the result would be much the same as we find it in the leech.
Cocoon.—The practice of forming a cocoon for the shelter of the eggs and of the developing young is shared with the Oligochaeta; but not all leeches deposit their eggs in this manner. Glossiphonia, for instance, carries its eggs upon the ventral face of the body, where the young remain for some time after they are hatched attached by the posterior sucker to their parent's body, and from which situation of safety they make short excursions. Other leeches deposit their eggs singly, but agglutinated together upon stones, etc. In the medicinal leech the cocoon is ovoid in shape, and from the end, which is closed by a temporary plug, the young when ready escape. This cocoon is deposited, as is that of an earthworm, in soil near to the borders of a marsh or pond, so that the young, while enjoying the requisite degree of moisture, may not be injured by a too wet environment. On the other hand Pontobdella and some other leeches lay their cocoons attached to bodies actually submerged in the water. The cocoon is secreted, as in Oligochaeta, by the clitellum, and as in them, is drawn off over the head, the ova and sperm probably flowing into it during the process. The elasticity of the slightly hardened mucus causes the two ends of the cocoon to {405}close up when it is free from the body; it is then whitish and soft, and the leech fixes it, and appears to polish the surface with its buccal sucker. In a few hours the cocoon becomes amber brown, a colour which characterises the cocoons of a great many earthworms and other Oligochaeta. The medicinal leech forms its cocoon in the same way as does Nephelis, to which the above description refers; but when the cocoon is formed the leech covers it with another layer of mucus, which Vaillant, from whose work the foregoing notes are extracted,[462] thinks may be produced from the so-called salivary glands.
Classification.—The number of different kinds of leeches is at present uncertain. Seeing that no less than sixty-four varieties of the common Hirudo medicinalis—colour varieties, it is true—are said to exist, it is not wonderful that the labours of some systematists have been severe, and have provoked much criticism and alteration on the part of others.[463] As to genera, Vaillant, in his recent continuation of de Quatrefages' "Annelés" in the Suites à Buffon, which includes the literature up to the year 1886, allows thirty-seven, some (three) of which, however, are admitted to be incertae sedis. Blanchard, who has paid a great deal of attention to the group, reduces these by six, which he considers to be synonyms; but on the other hand he has added or rescued from oblivion six or seven others, and Whitman has instituted several Japanese and Australian genera. Most of these generic types are, however, only imperfectly known, and from external characters only. It is quite problematical how many valid genera should be retained; in the meantime those that are fairly well known are divided by Blanchard[464] in the following way:—
Sub-Order 1. Rhynchobdellae.—Hirudinea with an exsertile proboscis, without jaws, and with colourless blood.
Fam. 1. Ichthyobdellidae.—Body formed of two regions, a narrower anterior portion and a wider "abdomen," both anterior and posterior suckers distinct from the body.
These leeches are parasites of fishes and of some other animals such as tortoises. The family contains a number of genera. Branchellion (Fig. 204) has a series of leaf-like branchiae on both sides; in Cystibranchus the respiratory organs are reduced to a series of round vesicles. The latter genus occurs in Europe and North America, and is parasitic upon marine and fresh-water fishes. Piscicola is a common leech which confines its attacks to the inhabitants of fresh water. Pontobdella (Fig. 210) is marine, and affects rays and sharks; the best known species, P. muricata, is usually of a green colour.
To the family Ichthyobdellidae also belongs the large Chilian leech Macrobdella valdiviana, of which there is or are also species in North America. Philippi's figure of this leech[465] shows the distinct neck; and as it has no jaws, it should be referred to the present family. It has got an undue reputation for size, 2½ feet[466] having been assigned to it. As a matter of fact Philippi's illustration depicts an Annelid of about 7 inches in length, with a greatest diameter of about an inch. But doubtless when extended in walking it would be longer—1½ feet, Philippi thinks. It has no eyes, a failing which is not unusual among the leeches.
Fam. 2. Glossiphoniidae.—Anterior sucker fused with the body, posterior sucker distinct. No cocoon.
The members of this family all inhabit fresh water; they have the habit of depositing the eggs separately, which are then fixed to the ventral surface of the body, and the young when hatched are {407}still protected by the parent, returning to its body for shelter. The type genus Glossiphonia ( = Clepsine) is common in Europe, and has many species. The Mexican and Amazonian Haementeria contains a number of species, of which the Mexican H. officinalis is used in medicine; but according to Miguel Jimenez, its use is apt to be attended with unpleasant symptoms. Drowsiness, a buzzing in the ears, and the development of a painful rash are some of the effects produced by its bite. It is disputed whether the animal's saliva or foreign matter introduced by it into the wound are the cause of the symptoms.
Mesobdella of Blanchard[467] is said to be intermediate between this family and the next. Each segment has three annuli, as in this family, but the leech has three jaws, as in Hirudo.
Sub-Order 2. Gnathobdellae.—Hirudinea without a proboscis, generally with jaws; the blood is red; the eggs are invariably deposited in cocoons.
Fam. 1. Gnathobdellidae.—Pharynx with three denticulate jaws.
This family as well as the next is terrestrial or fresh-water in habit. It contains a number of generic types, including the medicinal leech, Hirudo medicinalis, and the horseleech, Haemopis (Aulastomum) gulo. The former can be distinguished from the latter by its power of contracting itself into an oval olive-shaped form, which power is not possessed by the horseleech; the latter has, moreover, only two caeca, while the common leech has ten pairs of these appendages of the intestine. The genus Limnatis is called after the Greek word λιμνῆτις, which Theocritus applied to the leech. It is found in the Nile, and caused serious inconvenience to the army of Napoleon. His soldiers in drinking at pools sucked up the small leeches not thicker than a horse's hair, whose presence in the hinder part of the mouth cavity produced divers objectionable results, such as spitting of blood and hindered respiration.
Fam. 2. Herpobdellidae.—Pharynx without denticulate jaws, with three unarmed chitinous plates.
A characteristic genus of this family is Trocheta, which is so common at the Zoological Society's Gardens and in the Regent's Park, and which has been met with in other places near London; it is in this country an introduced species, but is found in many {408}parts of the continent. It is a land-leech, and lives upon earthworms.
The genus Haemadipsa, which M. Blanchard places in a special sub-family, contains a number of species which are for the most part land-leeches. Land-leeches occur in many parts of the world, but chiefly in the tropics—in India, Ceylon, Java, South America, etc. They lie in wait for their prey, upon the ground as a rule; but they may ascend herbs and shrubs to gain a better outlook when they are aware of an approaching footstep. A vivid account of the ferocity of these tiny Annelids in Ceylon can be read in Sir J. E. Tennent's Natural History of Ceylon. They have been said to be so pugnacious and so poisonous that persons surprised in their sleep by the pests have succumbed to their united efforts. A whole battalion of English soldiers decamped on one occasion from a wood which was overflowing with land-leeches. The familiar misquotation "lethalis hirudo" might well be applied to this species. Professor Whitman has written much upon the habits of the land-leech of Japan (Haemadipsa japonica), which bites so softly that its presence cannot be detected except for the stream of blood which trickles from the wound. While it is feeding it emits from the pores of the nephridia a clear fluid, which, as it appears, is used to keep the skin moist; when unduly dried the same phenomenon occurs. It is curious that in this and other leeches the nephridia should play a part which in the earthworm is played by the dorsal pores; in both animals the glands of the skin are also concerned with the same duty.
The purely aquatic leeches swim by undulations, and also crawl by the help of the two suckers, like a "Geometer" caterpillar. But when a land-leech is dropped into the water it at once sinks to the bottom and crawls out; it does not swim, but can survive immersion for a long period. In this it resembles the earthworms, which can also survive a prolonged immersion, and even in the case of some are indifferent to the medium, land or water, in which they live; the land-leech, however, is entirely dependent upon damp surroundings; a dry air is fatal to it. The land-leech of Japan leaves a slimy trail behind it as it crawls, in this respect recalling the land Planarian Bipalium kewense.
BY
ARTHUR E. SHIPLEY, M.A.
Fellow and Tutor of Christ's College, Cambridge
GEPHYREA
INTRODUCTION—ANATOMY—DEVELOPMENT—SIPUNCULOIDEA—PRIAPULOIDEA—ECHIUROIDEA—EPITHETOSOMATOIDEA—AFFINITIES OF THE GROUP.
The animals included in the above-named group were formerly associated with the Echinodermata. Delle Chiaje[468] states that Bohadsch of Prague in 1757 was the first to give an accurate description of Sipunculus under the name of Syrinx, but Linnaeus, who noted that in captivity the animal always kept its anus directed upwards, re-named it Sipunculus. Lamarck[469] placed the Gephyrea near the Holothurians; and Cuvier[470] also assigned them a position amongst the Echinoderms. He mentions Bonellia, Thalassema, Echiurus, Sternaspis, and three species of Sipunculus, one of which, S. edulis, "sert de nourriture aux Chinois qui habitent Java, et qui vont la chercher dans le sable au moyen de petits bambous préparés."
The name Gephyrea[471] was first used by Quatrefages, who regarded these animals as bridging the gulf between the Worms and the Echinoderms. He included in this group the genus Sternaspis (vide p. 335), now more usually classed with the Chaetopoda.
The Gephyrea are exclusively marine. They are subcylindrical animals, which can either retract the anterior end of their body—the introvert—carrying the mouth into the {412}interior; or are provided with a long flexible but non-retractile proboscis. The latter is easily cast off. They usually bear spines or hooks of a hard chitinous character, secreted by the epidermis or outermost layer of cells. The mouth is at the base of the proboscis or at the end of the protractile part, the anus is at the other end of the body or on the dorsal surface. The nervous system consists of a ring round the mouth and of a ventral nerve-cord. A vascular system is present as a rule. Nephridia are found which act as excretory organs, and in most cases also as ducts for the generative cells. The Gephyrea are bisexual, and the male is sometimes degenerate.
The group may be divided into four Orders:—(i.) Sipunculoidea; (ii.) Priapuloidea; (iii.) Echiuroidea; (iv.) Epithetosomatoidea; of these the first is by far the largest, both in number of genera and of species.
The Anatomy of Sipunculus nudus.
External Characters.—The body of S. nudus when fully extended may attain a length of a foot, or even a little more; in this condition it is seen to consist of two portions, the anterior of which is, however, retracted into the other when the animal is disturbed. The retractile portion is sometimes termed the proboscis, but as its nature is entirely different from that of the proboscis of the Echiuroidea, it is better to refer to it as the introvert. Special retractor muscles are attached on the one hand to the body-wall about half-way down the body, and on the other hand are fused into a muscular sheath which surrounds the gullet, just behind the mouth. When these muscles contract, they withdraw the introvert into the rest of the body or trunk in much the same way as the finger of a glove may be drawn into the hand, by a thread fastened to the inside of its apex. The introvert is protruded by the contraction of the circular muscles of the body-wall. These exert a pressure on the fluid which fills the body-cavity, and by this means the sides of the introvert are forced forward until finally the head is exposed.
The introvert occupies about one-sixth or one-fifth of the total body length. It is somewhat narrower than the trunk, and is covered by a number of small flattened papillae, some of which lie with their free ends directed backward, overlapping {413}one another like tiles on a roof. In some other genera, as Phymosoma, the introvert bears rows of horny hooks, which are apt to fall off as the animal grows old.
The trunk has from thirty to thirty-two longitudinal furrows, the elevations between which correspond with a similar number of muscles lying in the skin. This longitudinal marking is crossed at right angles by a circular marking of similar origin, the elevations of which correspond with the circular muscles in the skin. These two sets of markings thus divide the skin of the trunk into a number of small square areas, very regularly arranged (Fig. 212).
The outline of the trunk is more or less uniform, but it is capable of considerable change according to the state of contraction of its muscles. The circular muscles, for instance, may be contracted at one level, thus causing a constriction at this spot. The colour of S. nudus is a somewhat glistening greyish-white.
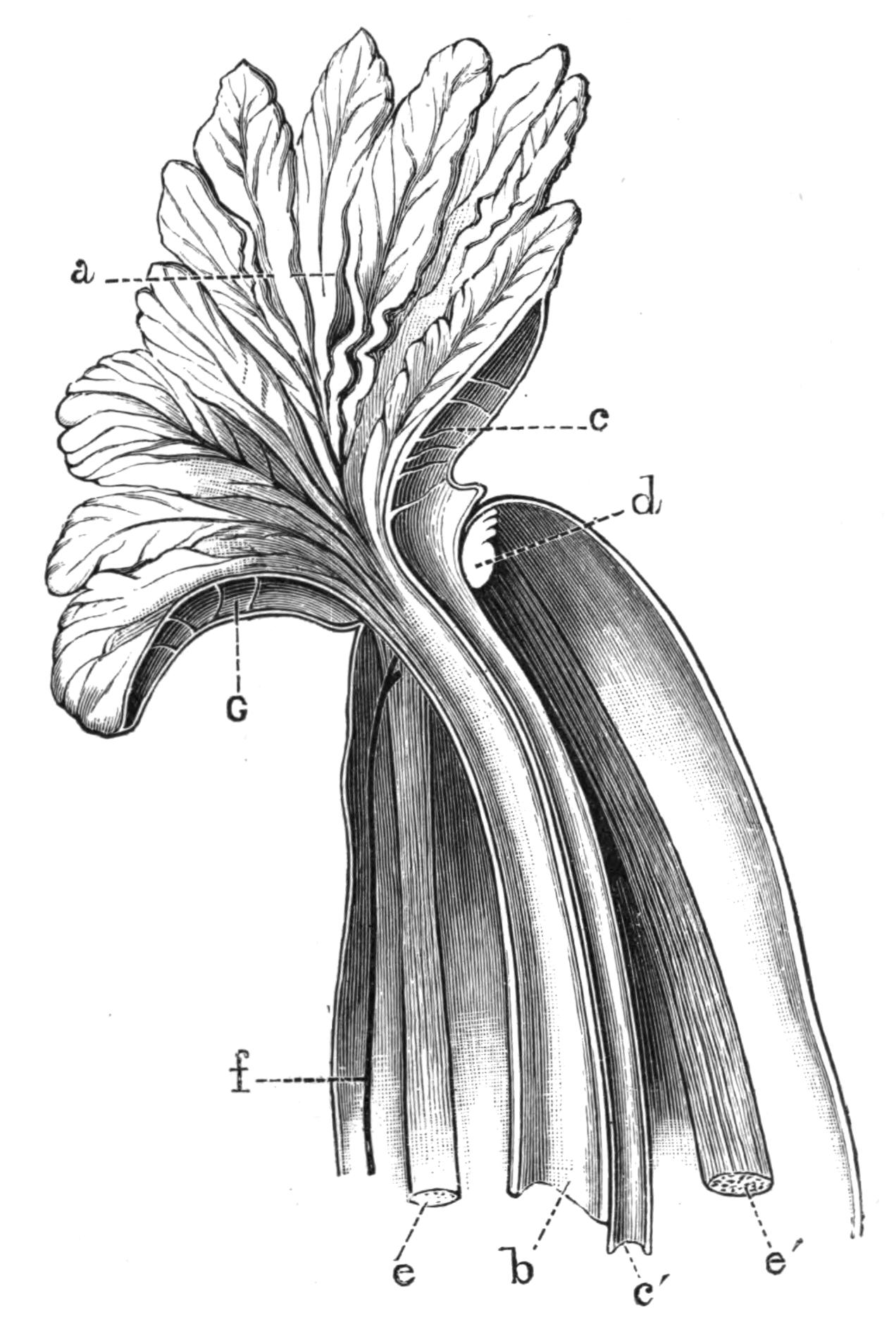
Fig. 211.—Right half of the anterior end of Sipunculus nudus L., seen from the inner side and magnified. a, Funnel-shaped grooved tentacular crown leading to the mouth; b, oesophagus; c, strands breaking up the cavity of the tentacular crown into vascular spaces; c', heart; d, brain; e, ventral, and e', dorsal retractor muscles; f, ventral nerve-cord; G, vascular spaces in tentacular crown.
The anterior end of the fully-expanded Sipunculus may be termed the head; here the skin is produced into a frayed fringe which stands up in the shape of a funnel round the mouth. This fringe is grooved on its internal surface with numerous little gutters, all of them lined with cilia, which by their constant motion keep up a current which sweeps food into the mouth. {414}The fringe may be in the form of a simple ring round the mouth, or the ring may be folded in at the dorsal side so as to take the form of a double horse-shoe (Figs. 211 and 212).
Body-wall.—The glistening appearance of Sipunculus is due to the cuticle, a chitinoid layer which is secreted by the external layer of cells, the epidermis. Beneath this lies a layer of connective tissue, which is not always present in other Gephyrea; within this lies a layer of circular muscles arranged in bundles, then comes a very thin sheath of oblique muscular fibres, then a thicker layer of longitudinal muscles, and finally a layer of peritoneal epithelial cells, which in Sipunculus are for the most part ciliated.
Scattered over the surface of the body, and opening by narrow tubes which pierce the cuticle, are a number of glandular bodies which may be either bi- or multi-cellular. The glandular cells are apparently enlarged and modified epidermal cells; they are arranged in a cup-shaped manner, with their apices directed towards the orifice. They are crowded with granules, which are presumably poured out over the cuticle, but the exact function of the secretion is entirely unknown. They have a well-developed nerve supply.
Digestive System.—The mouth lies in the centre of the fringe, and is not provided with any kind of jaw or biting armature; it leads directly into the thin-walled alimentary canal, the first part of which is ciliated. The alimentary canal is not marked out into definite regions, but passes as a thin-walled semi-transparent tube to the posterior end of the body, and then turns forward again and opens to the exterior by an anus situated about an inch below the junction of the introvert with the trunk, on the median dorsal line. The descending and ascending limbs of the alimentary canal are coiled together in a spiral, which may be more or less close in different individuals. The whole is supported by numerous fine muscular strands, which pass from the walls of the intestine to the skin, and by a spindle-muscle, which runs from the extreme posterior end of the trunk up the axis of the spiral and terminates in the skin close to the anus.
No glands open into the alimentary canal at any point of its course, but near the anus a simple diverticulum, or pocket, of unknown function arises. The size of this outgrowth differs {415}enormously in different individuals. The alimentary canal near the anus also bears two tuft-like organs, which, however, do not open into the intestine, but probably have some function in connexion with the fluid in the body-cavity.
Along the whole course of the alimentary canal there runs a ciliated groove, into which the food does not pass, but the cilia of which probably keep in motion a current of water whose function may be respiratory.
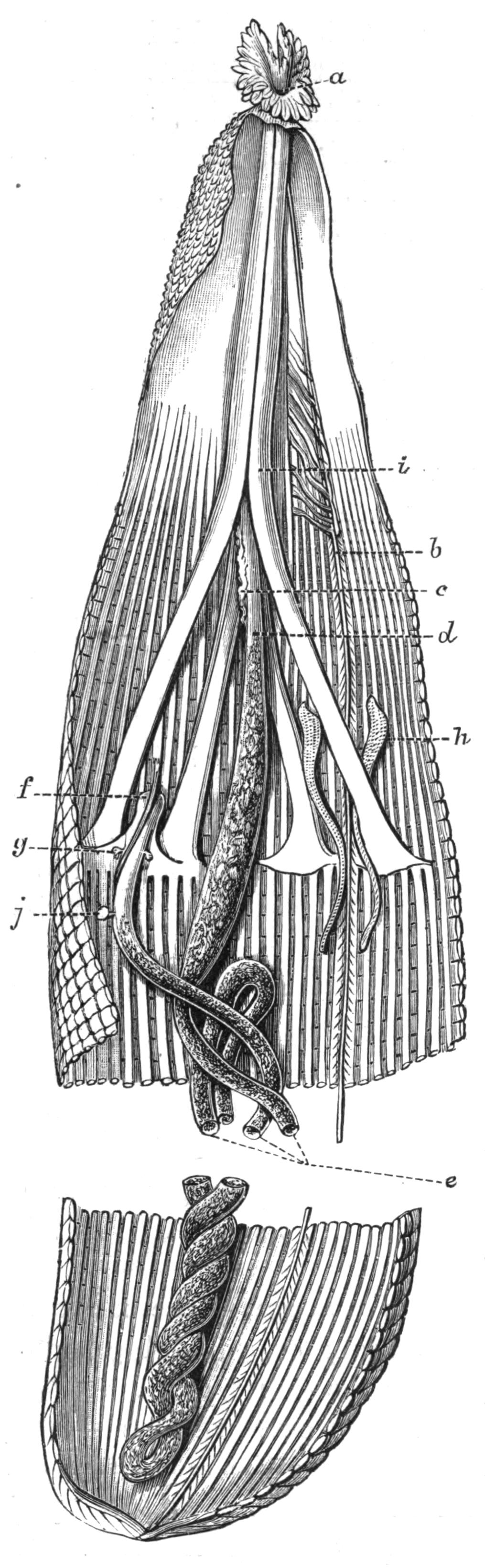
Fig. 212.—Sipunculus nudus L., with introvert and head fully extended, laid open by an incision along the right side to show the internal organs. × 2. a, Mouth; b, ventral nerve-cord; c, heart; d, oesophagus; e, intestine; f, position of anus; g, tuft-like organs; h, right nephridium; i, retractor muscles; j, diverticulum on rectum. The spindle-muscle is seen overlying the rectum.
Vascular System.—On the dorsal surface of the anterior end of the alimentary canal lies a contractile vessel, usually termed the heart. It is a tube about an inch long, ending blindly behind, but opening in front into a ring-shaped space surrounding the mouth and partially enveloping the brain. From this ring-like vessel numerous branches are given off which pass into the fringe round the mouth, and probably the chief function of the heart is by its contraction to force fluid into this fringe, and so to extend it. The heart contains a corpusculated fluid. {416}A similar but shorter tube is found on the ventral surface of the anterior end of the alimentary canal in the species in question; it also opens into the ring which surrounds the mouth.
Respiratory System.—There are no special respiratory organs, and it has long been a matter of dispute where the respiration of Gephyrea is carried on. The oxygenation of the blood probably takes place to some extent through the walls of the oral fringe, but the blood which receives its oxygen at this spot is limited in its distribution, and could only supply the brain and head. It seems probable that the remaining organs are supplied with oxygen by the fluid of the body-cavity, which bathes them on all sides. This might obtain its oxygen from the blood in the heart, or more probably, through the thin walls of the intestine, from the stream of water which is maintained by the ciliated groove described above. Quite recently a form—S. mundanus, var. branchiata—has been described[472] with thin-walled papillae covering parts of the skin. These papillae are full of corpuscles, and are regarded by their discoverer as branchiae.
Body-Cavity.—The pinkish fluid of the body-cavity contains numerous corpuscles, the products of the reproductive organs (either ova or spermatozoa), and some curious unicellular bodies known as "urns." The latter are shaped like a bowl with a ciliated rim, and are formed from the budding of certain cells on the walls of the dorsal blood-vessel.[473] Their function is unknown, but they resemble certain multicellular bodies found in the body-cavity of Phascolosoma. The generative cells found in the body-cavity are further considered below. The true corpuscles are either biconcave round corpuscles coloured with a chemical substance, the haemerythrin of Krukenberg, which apparently plays the same rôle as haemoglobin in other animals; or amoeboid corpuscles, which, though rare in Sipunculus, are very numerous in Phascolosoma.
Nervous System.—The nervous system of Sipunculus consists of a brain or cerebral ganglion, a circumoesophageal ring surrounding the gullet, and a ventral nerve-cord. The brain is a small bi-lobed nervous mass situated on the dorsal surface of the oesophagus, in the angle between the right and left dorsal retractor muscles close to their point of insertion. Numerous {417}nerves arise from it, and pass to the fringe surrounding the mouth and to neighbouring parts. At the sides, the brain is continued into two stout nerve-cords which encircle the oesophagus, and meeting, fuse together in the median ventral line to form the ventral nerve-cord (Fig. 211). The latter is of the same diameter throughout, and shows no signs of segmentation; it is oval in section, and consists of small ganglion cells heaped up on the ventral surface, i.e. next the skin, and of numerous fibres situated dorsally. The cord gives off many nerves, which usually arise in pairs. These pass into the skin, and forming rings, run round the body, and give off finer nerves as they go.
The nerve-cord is supported by numerous strands of muscle which pass to it from the skin. These are especially long in the region where the introvert joins the trunk, and thus allow free play to the nerve-cord when the former is being protruded or retracted.
Sipunculus is not well provided with sense-organs, but in an animal which lives buried in sand we should not expect to find these very highly developed. On the introvert there are certain patches of epithelium bearing long stout cilia, which have been regarded as tactile in function, and there is a tubular infolding reaching the brain, which almost certainly has some sensory function. Ward[474] has termed this "the cerebral organ." It consists of a duct lined with ciliated cells, which opens to the exterior in the middle dorsal line outside the tentacular fringe. The duct leads down to the brain, and expands at its lower end into a saucer-shaped space, covering that portion of the brain where its substance is continuous with the external epithelium. In Phymosoma this cavity is produced into two finger-shaped processes, which are sunk into the brain and are lined by cells crowded with a dense black pigment.[475] They are probably rudimentary eyes, perhaps distinguishing only between darkness and light. The pits appear to be absent in Sipunculus nudus, but Andrews states they are found, although without pigment, in S. gouldii.[476]
Excretory System.—The excretory organs or "brown tubes" are typical nephridia, that is to say, they consist of tubes {418}with glandular walls which open on the one side to the exterior, and on the other by means of a ciliated funnel-shaped opening into the body-cavity. In Gephyrea one wall of the tube is produced into a long diverticulum or sac which hangs down into the body-cavity, and is usually supported by muscle-fibres running to the body-wall. The lower end of the sac is broken up into a number of crypts or pits, lined by large glandular cells crowded with brown pigment. The pigment-granules are secreted into the cavity of the sac, and leave the body through the external opening; they probably consist of the nitrogenous excreta of the animal. The upper end of the sac, into which both the external and internal orifices open, is usually enlarged, and its walls are very muscular. As in so many other animals, the nephridia serve as ducts through which the reproductive cells leave the body of the parent.
Reproductive System.—The Gephyrea are bisexual. In Sipunculus the testes and ovaries are found in the same position in the two sexes, and are indistinguishable without microscopic investigation. They each consist of small ridges situated at the lower end of the ventral retractor muscles, just where the latter take their origin from the longitudinal muscles of the skin. At this level the cells which line the body-cavity on the inside of the skin are heaped up, and become modified in the one case into ova or eggs, and in the other into the mother-cells of the spermatozoa. This method of forming the reproductive organs from modified cells lining the body-cavity is very common in the higher animals; but it is seen in its simplest and least modified form in the Sipunculidae.
The eggs break away from the ovary in a very undeveloped condition, but whilst floating about in the body-cavity they increase in size and secrete a thick membrane around them. They have a well-marked nucleus, and are oval in outline.
The mother-cells of the spermatozoa also break away in an immature condition, and complete their development in the nutritive fluid of the body-cavity. They divide into a number of spermatozoa, usually eight or sixteen, which remain in contact. They each develop a tail, which projects outwards, and aids the cluster in swimming along. These clusters of spermatozoa are about the same size as the ova of the female, and, like them, make their way into the "brown tubes." The exact way in {419}which this is accomplished is not very clear, but the cilia on the funnel-shaped internal opening of the tube seem to have some power of selecting the generative cells when they come within their reach, and of passing them on, whilst they reject the much smaller corpuscles of the perivisceral fluid, which are never found in the nephridia.[477] Once inside the internal opening, the clusters break up and the spermatozoa escape singly into the sea. Here they meet with and fertilise the eggs which have escaped from the body of the female.
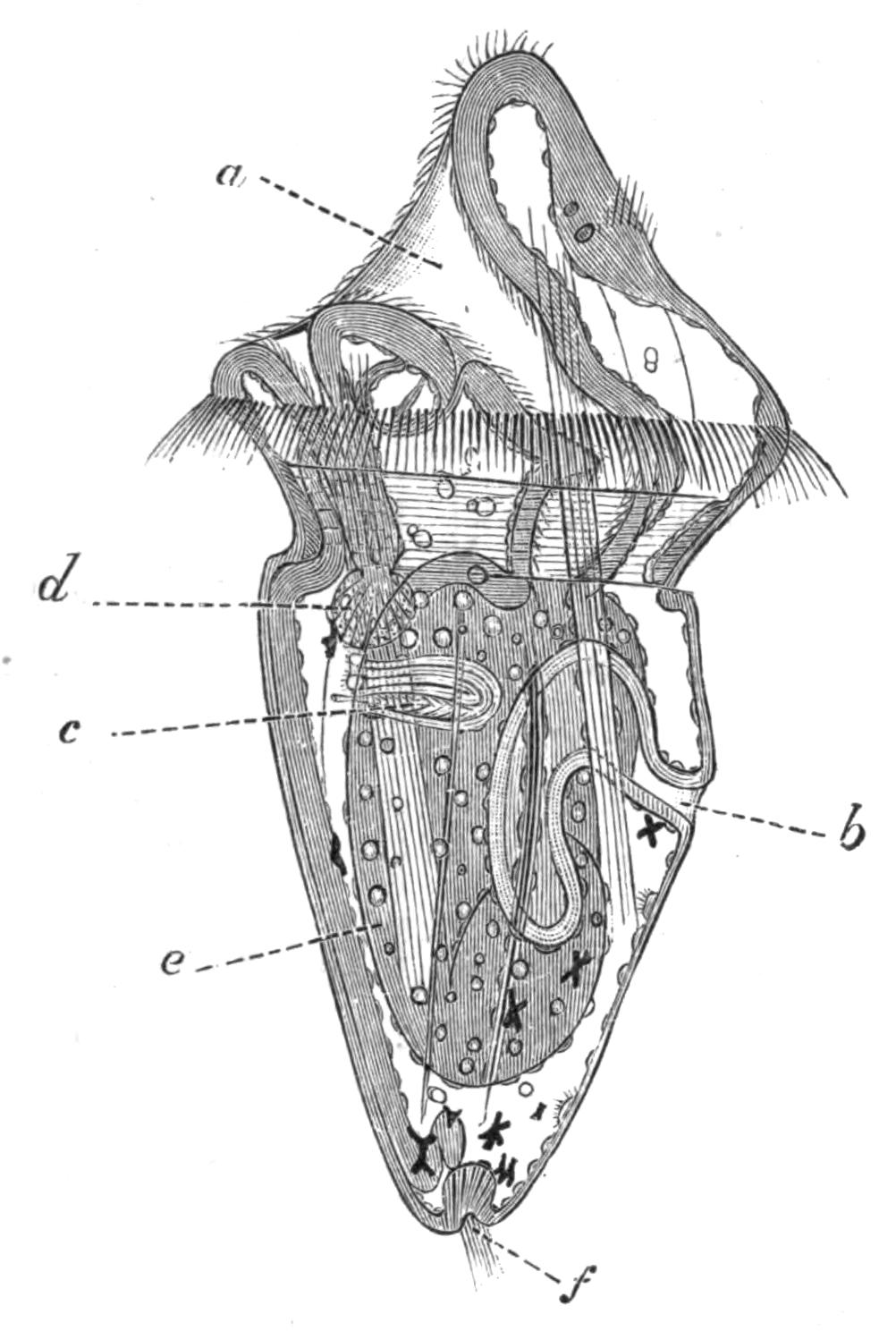
Fig. 213.—Larva of Sipunculus nudus L. × 150. (After Hatschek.) a, Mouth; b, anus; c, excretory organ; d, glandular appendage of oesophagus; e, wall of stomach over which the retractor muscle runs; f, invaginated sense-organ at aboral pole.
Development.—Hatschek,[478] who investigated the development of Sipunculus nudus at Pantano, an inlet of the sea near Messina, states that the spawning takes place during the night, and ceases about July 10. The rate of development depends upon the temperature, but the larvae usually free themselves from the egg-membrane during the third day. When hatched the embryos lengthen out a good deal, and take the form represented in Fig. 213. The larva swims actively by means of a ring of stout cilia, which encircle the body just behind the mouth. Other shorter cilia are found on the head, continuing into the lining of the mouth, and a little bunch of them is situated at the extreme posterior end. The alimentary canal is already formed, and is twisted, so that the anus lies dorsally, but not so far forward as it does in the adult. A glandular structure opens into the mouth, and another body of unknown function is connected with the oesophagus; both these disappear during larval life. A pair of excretory tubules, the {420}forerunners of the brown tubes, are found, and the chief muscle tracts are already established. The nervous system is still in close connexion with the skin, from the outer part of which it is derived; the cerebral thickening bears two eye-spots.
The fluid of the body-cavity contains corpuscles, which are kept in active circulation by the constant contractions of the body-wall, and by numerous tufts of cilia which are borne on the inner surface of the skin. The dorsal blood-vessel is one of the latest organs to arise.
The larva swims actively about for a month, during which time it increases greatly in size; it then undergoes a somewhat sudden metamorphosis. The ciliated ring and the structures related to the oesophagus begin to disappear, the distinction between the head and the rest of the body is obliterated, and the head becomes relatively small. The mouth changes its position, and becomes terminal instead of being somewhat ventral, and the tentacular membrane begins to appear. At the same time the larva relinquishes its free-swimming life, and sinks to the bottom; it begins creeping amongst the sand by protruding and retracting the anterior part of its body, and takes on all the characters and habits of the adult.
I. Order Sipunculoidea.
Besides the genus Sipunculus, the Order Sipunculoidea includes ten other genera. A key to these, taken for the most part from Selenka's admirable monograph, is given on page 424.
Phascolosoma contains, in comparison with Sipunculus, only small species, and it is easily distinguished by the fact that the longitudinal muscles are fused into a continuous sheath. As a rule the skin is smooth. A few species bear hooks, which are generally scattered irregularly and not arranged in transverse rows, as in Phymosoma (Fig. 214) and most of the other genera.
The fold which in S. nudus surrounds the mouth may be in the same species bent in so as to take the form of a double horse-shoe, the opening of which is always dorsal, just above the brain; in this case the mouth is crescentiform. In other genera the fold is broken up into discrete tentacles, and these are variously arranged; in Dendrostoma they are grouped together in four or six bundles round the mouth, but the more usual {421}arrangement is the horse-shoe-like row of tentacles which overhang the crescentiform mouth, as in Phymosoma and some species of Aspidosiphon.
The ventral side of each tentacle is grooved and ciliated, and the grooves are continued into the ciliated mouth. Their dorsal surface is pigmented, and in the hollow of the horse-shoe lies a deeply pigmented epithelium covering the brain.
A blood-vessel courses up each tentacle, and usually two channels return the blood to the vascular ring which surrounds the mouth. In those forms which possess tentacles on the dorsal side of the mouth only, the ventral part of the vascular ring lies in the lower lip, which is tumid and swollen. The brain supplies a nerve to each tentacle.
When the introvert is retracted the tentacular ring is withdrawn and to some extent collapsed; in this condition it would be almost touching the rough external surface of the introvert. In some species of Phymosoma the delicate appendages of the head are guarded from the hooks on the introvert by a thin membrane or collar,[479] which completely ensheaths the retracted head.
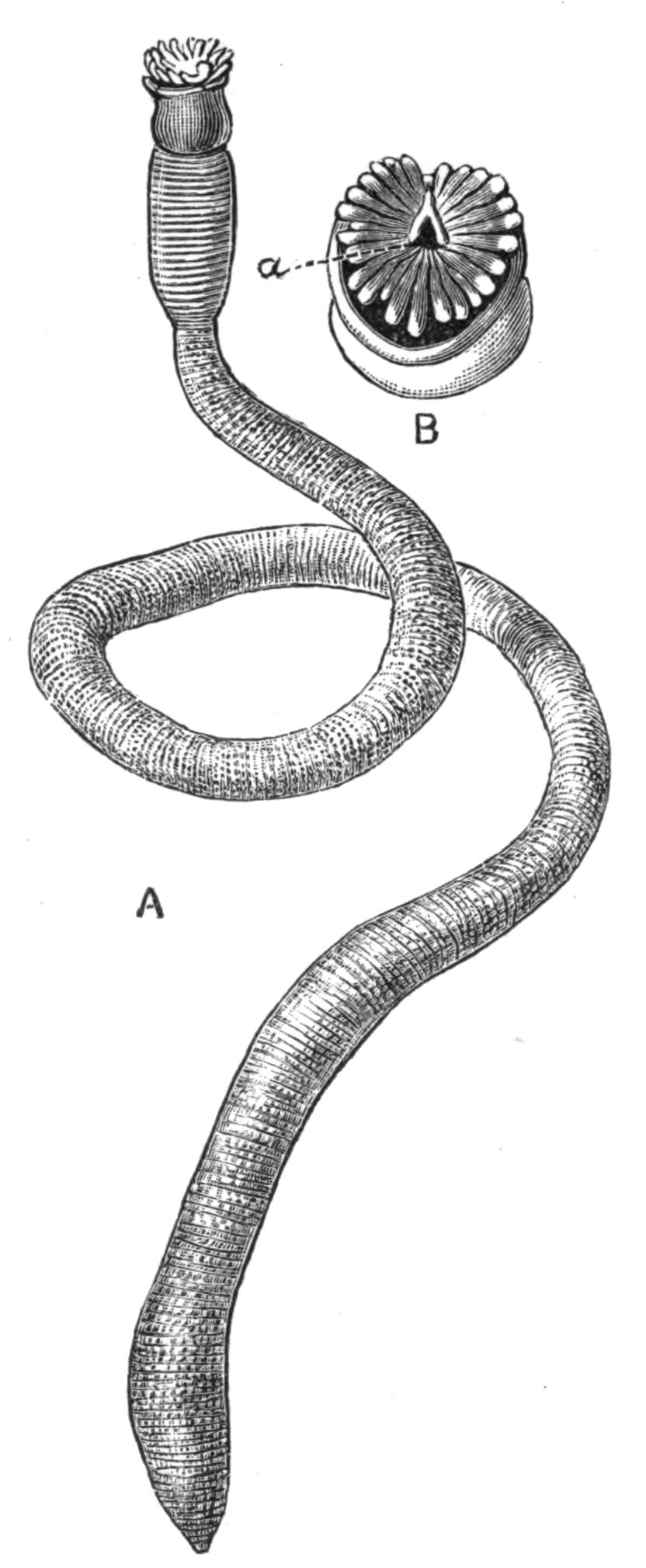
Fig. 214.—A, Phymosoma granulatum F. S. Leuck. × 2. B, Head of the same. × 4. a, Pigmented pit leading to brain. The crescentiform mouth on the lower side of the figure is overhung by the tentacles.
When the introvert is fully extended the dorsal blood-vessel contracts and sends its blood forward into the vascular ring, and thence into the tentacles or tentacular fold, which are thus erected. In several species of Sipunculus, as S. nudus, S. norvegicus, S. robustus, S. tesselatus, there is a ventral blind tube as well as a dorsal, into which the blood is withdrawn when the head is retracted. In many other species in various genera, such as Phymosoma {422}weldonii and Ph. asser, Dendrostoma signifer, S. vastus, the lumen of the dorsal vessel is increased by numerous hollow blind processes which it bears, hanging freely into the body-cavity. Three very small genera of Sipunculids—Onchnesoma, Petalostoma, and Tylosoma—are devoid of all trace of vascular system and of tentacles; the mouth opens in the centre of the anterior end of the introvert. In Onchnesoma the dorsal part of the lip is somewhat produced, so that the head has somewhat the shape of a Doge's cap, and in Petalostoma there are two leaf-like processes of the body-wall which guard the mouth.
The extent to which the intestine is coiled varies very much even in the same species; the axis of the coil is often supported by a spindle-muscle, but this is sometimes absent. The caecum, which opens into the rectum of S. nudus, is again a very variable structure, and when it is present varies remarkably in size.
The food of Sipunculids seems to consist almost entirely of sand, and their only nourishment must be such small microscopic organisms or particles of animal and vegetable débris as are to be found mixed with the sand. The alimentary canal is, as a rule, quite full of sand, and yet in spite of the tenuity of its walls they never seem to be ruptured. If the contents of the digestive tube be washed out with a pipette, it will be found that it requires considerable force to dislodge many of the sand-particles lying next the wall. These are more or less embedded in crypts or pockets of the wall, and as the sand passes along the intestine they probably serve as more or less fixed hard points, against which the sharp edges of the sand particles are worn off. Amongst the sand are usually to be found pieces of shell, sometimes with a diameter equal to that of the alimentary canal; these are usually rounded, but their angles may have been removed by attrition before they entered the mouth of the Sipunculid.
In S. tesselatus the sand is to some extent held together by a mucous deposit; in those cases where there is no sand in the intestine, there is always a coagulum of mucus, and the walls are contracted and thick; when full of sand the walls are tensely stretched and very thin. This thinness of the wall of the alimentary canal seems ill-adapted to a diet of sand, nevertheless it is also met with in other great sand-eating groups of animals, such as the Echinids and the Holothurians.
The enormous amount of sand and mud which passes through the bodies of the Sipunculids shows that they must take a considerable part in modifying the mineral substances which form the bottom of the sea. Just as earthworms, as shown by Darwin, play a considerable rôle in the formation of soil, so must these animals, in conjunction with Echinids and Holothurians, effect considerable modifications in the sand and mud which pass through their bodies. Mr. J. Y. Buchanan[480] is "led to believe that the principal agent in the comminution of the mineral matter found at the bottom of both deep and shallow seas and oceans, is the ground fauna of the sea, which depends for its subsistence on the organic matter which it can extract from the mud." The minerals at the bottom of the sea are exposed to a reducing process in passing through the bodies of the animals which eat them, and subsequently to an oxidising process due to the oxygen dissolved in the sea-water acting on the minerals extruded from the animals' bodies.
The rate at which the sand passes through the body of Sipunculus is unfortunately unknown, but that at any one moment a considerable quantity is contained in the intestine is shown by the fact that the average weight of five specimens of S. nudus from Naples, taken at random, was 19.08 grms., whilst the average weight of sand washed out of their alimentary canal was 10.03 grms. The sand contained in five other specimens of the same species measured respectively 6 c.c., 7 c.c., 6.5 c.c., 7.5 c.c., and 7.5 c.c., giving an average of 6.9 c.c. for each individual.
Onchnesoma and Tylosoma have only one retractor muscle; Aspidosiphon and Phascolion have, as a rule, two; Phymosoma and Sipunculus have four, and perhaps this is the more usual number.
Phascolion, Tylosoma, and Onchnesoma have but one "brown tube"; in Phascolion this is the right, in Onchnesoma it is sometimes the right and sometimes the left that persists. Most other genera retain two, but there are many exceptions; for instance, Phascolosoma squamatum has but one, and so has Aspidosiphon tortus, and in both cases it is that of the left side. No Sipunculid has more than two. It has been pointed out by Selenka that those species which have but one brown {424}tube are, as a rule, inhabitants of tubes or shells, and do not move actively about in the sand.
The eggs of all members of the family, with the exception of the genus Phymosoma, are spherical, but those of the last-named genus are elliptical. They are always surrounded by a thick membrane, the "zona radiata," pierced by numerous pores.
Aspidosiphon (Fig. 215) is easily recognised by the presence of two symmetrically-arranged cuticular shields, one at each end of the trunk. These are formed by the fusion of minute cuticular plates, such as exist in the skin of most Sipunculids. The posterior shield is radially symmetrical, but the anterior is somewhat like the shell of a Pecten, and symmetrical only about one plane. The introvert is protruded from the acute angle of the anterior shield, and when extended lies almost at right angles to the trunk, instead of being, as is usually the case, in the same straight line with it. In many specimens, and these seem as a rule to be the older ones, a deposit of calcium carbonate takes place over these shields, covering over and concealing their external markings.
Cloeosiphon (Echinosiphon) has a calcareous ring, consisting of four or five rows of lozenge-shaped calcareous bodies forming a close mosaic, arranged round the base of the introvert, which when extended is in the same straight line as the trunk. Each piece bears a brown spot, which is said to be the pore of a gland (Fig. 217). Golfingia Lankester, has a cylindrical horny thickening at the anterior end of the trunk and another at the posterior.
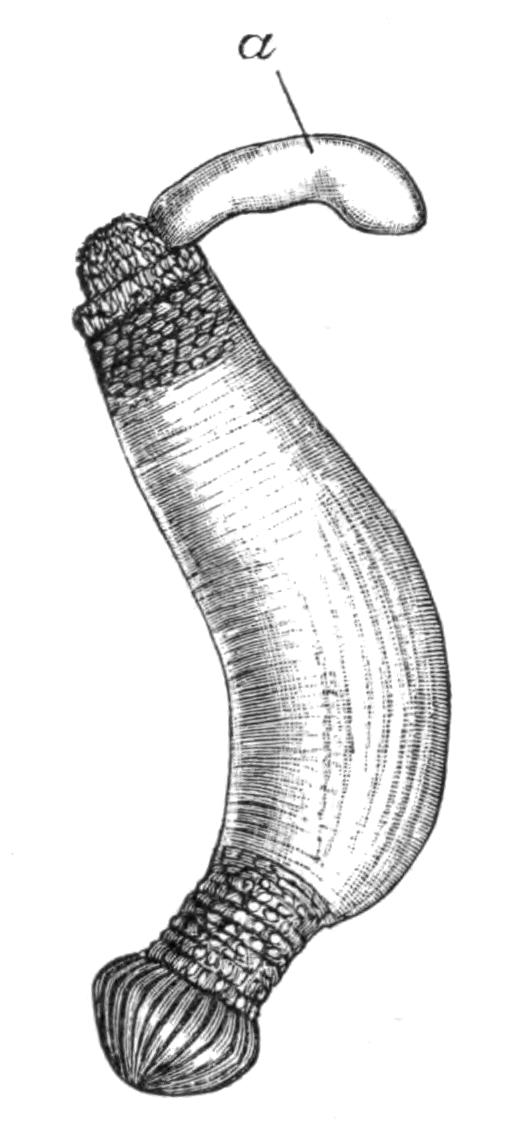
Fig. 215.—Aspidosiphon truncatus Kef. × 2. a, Introvert partially extended, but not sufficiently to show the head.
Key to the Genera of Sipunculoidea.[481]
I. The longitudinal muscles in the body-wall divided into 17-41 distinct bundles. Four retractor muscles.
A. Body covered with papillae. Numerous filiform tentacles which seldom (or never?) surround the mouth, but stand above and dorsal to it in a horse-shoe, with the opening dorsal. No rectal {425}caecum. Hooks usually present. Four retractors (in Ph. Rupellii only two?). Heart almost always without caeca. Eye-spots always present. Eggs oval, flat, reddish. Almost entirely small tropical species
1. Phymosoma
B. Body devoid of papillae. Tentacular membrane surrounds the mouth in a circlet. Rectum with one or more caeca (except S. edulis?). Hooks absent except in S. australis. Eggs spherical. The tentacular membrane contains a vascular network. A ventral contractile vessel usually present in addition to the heart. Mostly large forms. Found in all seas
2. Sipunculus
II. The longitudinal muscles in the body-wall form a continuous sheath, and are not split up into bundles.
A. Two brown tubes. Numerous tentacles form a wreath round the mouth. Alimentary canal forms a complete spiral, free behind except in Ph. Hanseni. Spindle-muscle usually present. One or more ligaments present, but only on the anterior convolutions of the intestine. Adhesive papillae always absent. Hooks very frequently absent. Eggs spherical. Found in all seas.
3. Phascolosoma
B. Two free brown tubes. Only four or six plumed tentacles. A complete intestinal spiral, not attached behind. Spindle-muscle always present. One or more ligaments present, but only on the anterior convolutions of the intestine. Hooks are present, but sometimes fall off early in life. Heart usually bears caeca. Found only in the tropics.
4. Dendrostoma
C. Only one brown tube, that of the right side, present; it is attached to the body-wall throughout its entire length. Numerous tentacles form a circle round the mouth. The alimentary canal forms no spiral, or an incomplete one. No spindle-muscle, but the intestine is attached to the body-wall throughout its length by numerous ligaments. Adhesive papillae often present. Not more than two retractors. Spherical eggs. Inhabits Mollusc shells or tubes. Found in all seas
5. Phascolion
III. At both ends of the trunk a distinct horny shield, or tube-like cornification, or a calcareous ring at the anterior end of the trunk. Hooks sometimes present. Longitudinal muscles continuous or split up into bundles.
A. A shield at both ends of the trunk. Introvert excentric, arising from the ventral side of the anterior shield. Tentacles small and few in number, arranged in a horse-shoe above the mouth. A spindle-muscle, which arises from the posterior end of the body, traverses the intestinal coil. Two retractors only, these are the ventral; they are frequently fused together from their point of origin.
6. Aspidosiphon
B. A calcareous ring surrounds the anterior end of the trunk, from the middle of which the introvert is extruded. Longitudinal muscles continuous. Hooks bifid. Tropical.
7. Cloeosiphon
C. A corneous ring, from which the introvert issues, surrounds the anterior end of the trunk, and the posterior end of the trunk is {426}produced into a corneous spike. Six pinnate tentacles encircle the mouth. Four retractors. Hooks present on the introvert. Longitudinal muscles continuous. Intestine not coiled throughout in a spiral nor fastened posteriorly. Spindle muscle present.
8. Golfingia
IV. No tentacles, but two leaf-like extensions of the body-wall guard the mouth. Four retractors. Few intestinal loops, quite free. No vascular system.
9. Petalostoma
V. No tentacles, no vascular system. One retractor, and one segmental organ.
A. Introvert long. Body small, pear-shaped.
10. Onchnesoma
B. No introvert (?). Body cylindrical, thickly covered with papillae, which are larger and more crowded at both ends of the trunk.
11. Tylosoma
Species of Sipunculoidea.—The genus Phymosoma (Fig. 214) contains more species than any other genus of Sipunculoidea, and they are all of fair size. Twenty-seven species are known, of which seventeen occur in the Malay Archipelago, thirteen being found there alone. Phymosoma affects shallow water, the deepest specimens being taken at a depth of about 50 fathoms; this may be due to the fact that they flourish only in comparatively warm water. With very few exceptions, they are found only in tropical seas, very often living in tubular excavations made in soft coral rock.
The genus Sipunculus contains sixteen species. They are the largest and the most conspicuous members of the group. They have a very wide distribution, some species, as S. nudus (Fig. 212) and S. australis, being almost cosmopolitan. They are most common in temperate and tropical seas, but S. norvegicus and S. priapuloides are found far north, but always at considerable depths, 100 to 200 fathoms.
The following account of the habits of Sipunculus gouldii is taken from Mr. Andrews'[482] paper on that species:—
"This Sipunculus is very abundant in certain small areas of compact, fine sand darkened by organic matter and not laid bare at ordinary low tide. In such places, only a few square metres in extent, they pierce the sand in all directions to a depth of more than half a metre, making burrows with persistent lumen running from the surface downward and then laterally, but with no regularity in direction.
"Kept in aquaria, the dependence of the animal upon the {427}nature of the sand and its method of locomotion may be readily observed. A vigorous individual buries itself in a few moments in the following manner: Running out the introvert to nearly its full extent, and applying it to the surface of the sand till some spot of less resistance is found, the animal still further expands the introvert so that it penetrates the sand, provided this is not too dense and firm, for then the body is merely shoved backward. When the introvert is inserted, the contraction of the longitudinal muscles of the body-wall brings the whole body forward somewhat, in case the introvert is fixed in the sand. In case soft ooze was present, this fixation did not take place, and the introvert was merely pulled out again, but when the sand was of the right consistency the introvert was fixed by becoming much swollen at the tip, and then constricted just posterior to this swollen area. This bulb-like area exerts lateral pressure on the sand, as could be seen by movements of the grains. The swelling of the anterior end of the introvert is brought about by the body-wall contracting elsewhere, and forcing in liquid to distend that end. Owing to the curved form assumed by the body in the normal contracted state when first removed from its burrow, the entrance of the introvert may often be nearly vertical, and hence the entire body is soon raised nearly upright in the water above the sand. If the body has thus been warped forward sufficiently to become somewhat fixed in the sand, the introvert is rolled in and again thrust forward from this new point of resistance, and so on till the animal is entirely buried. This locomotion increases in speed as the creature becomes more completely surrounded by sand, and is the only means of moving from place to place.
"On a smooth surface, or on one not presenting the right degree of resistance, the Sipunculus does not change its position, but remains till death finally occurs, rolling its introvert in and out and contracting its body-wall to no purpose.
"The essential factors in the mechanism bringing about this hydrostatic locomotion are an elongated contractile sac filled with liquid, and some means of definitely co-ordinating the contractions of the sac.
"In natural environment the animals are found with sometimes one, sometimes the other end nearer the surface of the sand: in the aquaria the same was observed, but when the {428}water became stagnant and impure the anterior end with expanded branchiae was often protruded somewhat above the surface of the sand."
The genus Phascolosoma contains at least twenty-five species, for the most part small. Ph. margaritaceum, however, measures[483] 10 cm. in length, and Ph. flagriferum, 13 cm. The latter is produced at the hinder end of its trunk into a long whip-like process, which recalls the horny spike of Golfingia. Most species live free, but a few inhabit the shells of dead Gasteropods or of Dentalium, or the abandoned tubes of worms. They occur in practically all seas.
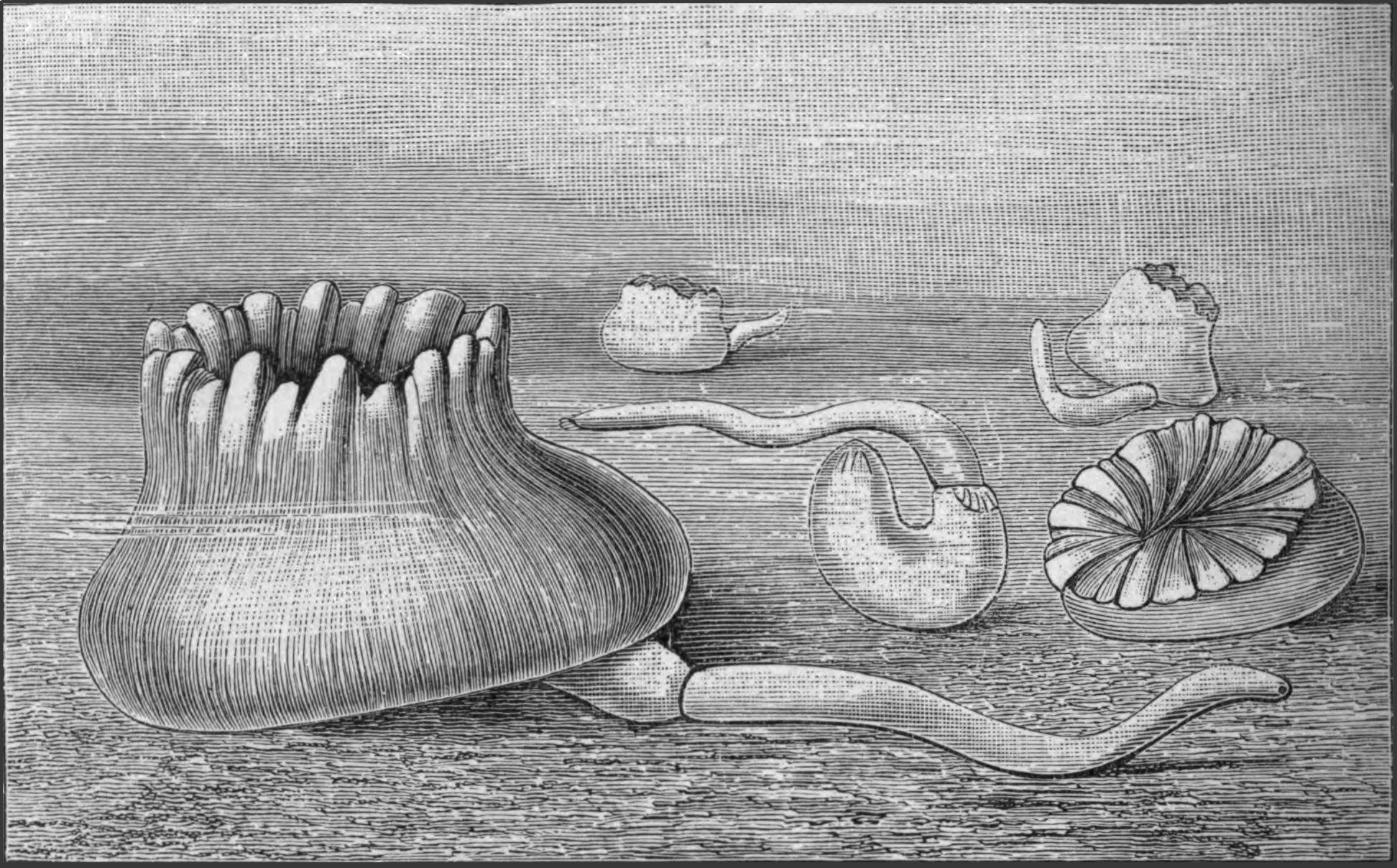
Fig. 216.—Specimens of the Coral Heteropsammia cochlea, with Aspidosiphon heteropsammiarum or A. michelini living in a state of commensalism with them. (From Bouvier.)
Dendrostoma contains but five species, which are all found within the tropics in the Pacific or in the West Atlantic. They are shallow-water forms, and some are found between tide-marks.
Phascolion is a smaller genus, containing but ten species, which may have been derived independently from different species of Phascolosoma, and in this case the genus should be broken up. The members of this genus live in Mollusc shells, such as Dentalium, Turritella, Buccinum, Chenopus (Aporrhais), Nassa, Strombus, and generally acquire the coiled shape of their host. They are usually attached to the shell by means of certain {429}adhesive papillae found on their posterior end. Ph. strombi fills its shell with mud, which must be kept together by some secretion of the animal. The body lies in a tube in this mud, and the introvert projects from the small round opening at the end of the tube, and explores the ground in every direction. They are found in all seas, but more especially in the colder waters.
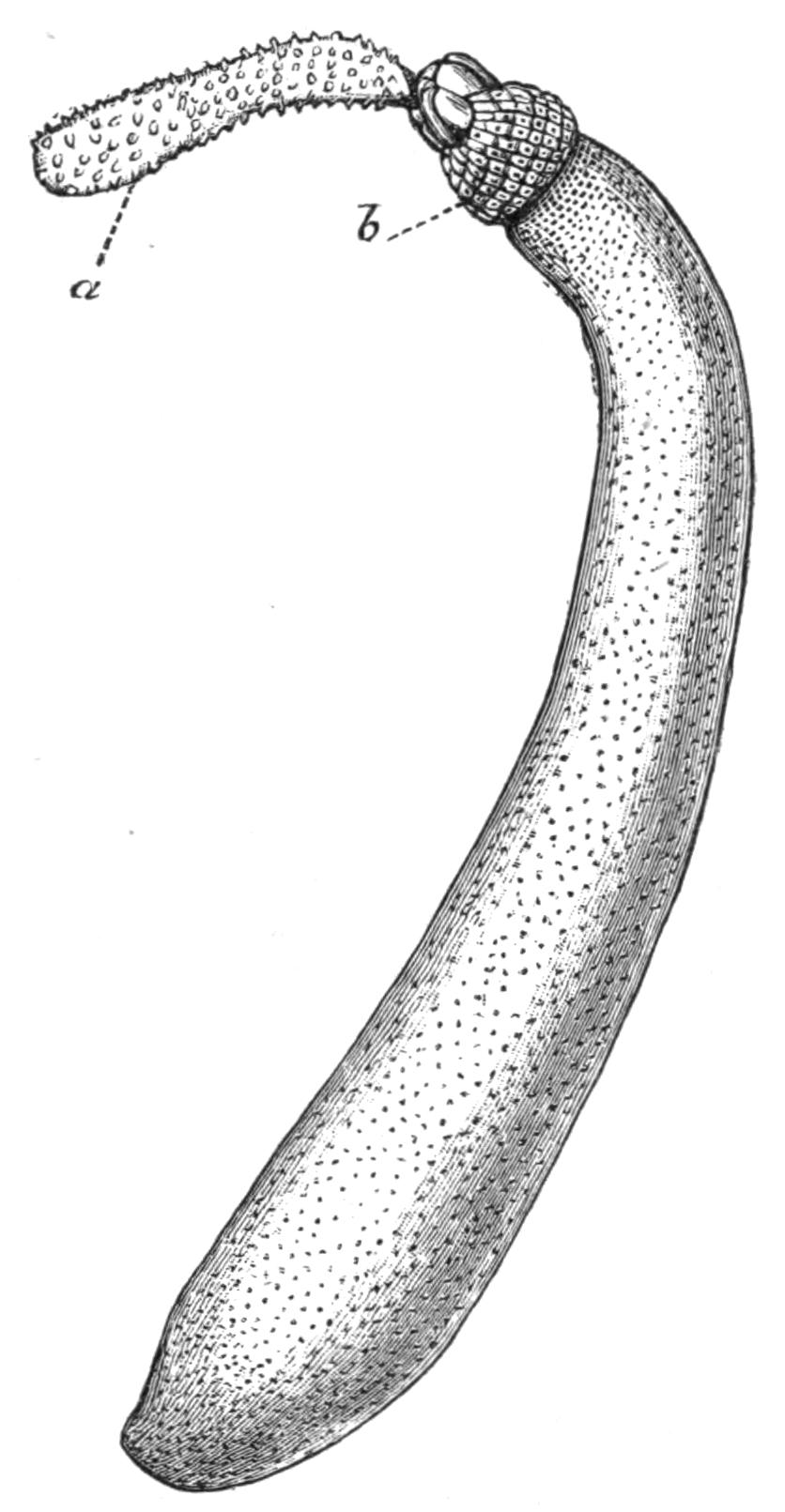
Fig. 217.—Cloeosiphon aspergillum Quatr. × ½. a, Introvert covered with spines and partially extended, but not sufficiently to show the head; b, calcareous plates surrounding the point of origin of the introvert.
The genus Aspidosiphon includes nineteen species, which are, with few exceptions, exclusively confined to the Indian Ocean and neighbouring seas, including the Red Sea. The exceptions are A. armatus from the Norwegian coast, and A. mülleri from the Mediterranean and Adriatic. A. truncatus is also stated to occur at Panama, the Bahamas, and at Mauritius. The remaining species almost all occur in the Malay Archipelago and neighbouring islands, and as was the case with Phymosoma, this part of the world seems to be the headquarters of the genus. A. mülleri lives in the interstices of rocks and stones, and occasionally in disused Mollusc shells.
Two species of Aspidosiphon have been described by Bouvier[484] living in a state of commensalism with two species of Madreporarian corals, Stephanoceris rousseaui and Heteropsammia cochlea, which live on and surrounding the shells of certain Molluscs at Aden (Fig. 216). Apparently the Gephyrean takes up its abode within its house at a tender age, and according to Bouvier, it provides for its increasing bulk by secreting a coiled calcareous tube, the outer surface of which affords space for the growth of the coral.
The genus Cloeosiphon, the Echinosiphon of Sluiter, includes three species: C. aspergillum (Fig. 217), C. molle, and C. javanicum. The first named occurs at Mauritius, the Malay Archipelago, and neighbouring islands; the others are {430}confined to the last-named area, which thus again forms the headquarters of a genus.
Golfingia, described by Lankester from a single specimen, was dredged in St. Andrews Bay, at the depth of 10 fathoms.
Petalostoma comprises but one species, P. minutum, which is found in the English Channel.
Onchnesoma comprises two species, O. steenstrupii and O. sarsii, both found off the coast of Norway at considerable depths between 200 and 300 fathoms.
Tylosoma comprises one species, T. lütkenii, also from the Norwegian coast. It is dredged from stony ground in 50 to 80 fathoms.
II. Order Priapuloidea.
Anatomy.—This Order consists of the two genera Priapulus and Halicryptus. Both are cylindrical animals with the mouth at one end and the anus at the other. The introvert is short, and is covered with rows of chitinous spines, which are continued to some extent over the body.
The skin is folded in a series of rings, and the body is usually somewhat swollen posteriorly. P. caudatus bears a curious caudal appendage, beset with a number of hollow lobes somewhat grape-like in appearance. This is situated ventral to the anus; its lumen is continuous with that of the body-cavity, but it can be separated from it by the action of a sphincter muscle. Two such appendages exist in P. bicaudatus.
There cannot be said to be any head in the Priapuloidea; they have no tentacles or tentacular fringe, no proboscis, and no distinct brain; simply a round aperture, the mouth, which is surrounded by a groove in the skin, at the bottom of which the circumoesophageal nerve-cord lies. The mouth leads into a very muscular pharynx lined with stout chitinous teeth; this passes into an intestine, which is as a rule straight, but in P. glandifer it has a single loop.
The Priapuloidea possess no vascular system and no brown tubes. Their skin has in the main the same structure as that of the Sipunculids, with spines, glandular bodies, and papillae with sensory hairs which resemble similar structures on Phymosoma varians. Retractor muscles arise from the longitudinal muscles {431}of the skin, and are inserted into the pharynx; they are short and not constant in number.
The nervous system has retained throughout its primitive connexion with the epidermis. In almost all animals the nervous system is formed from the epiblast or outermost cellular layer of the embryo; it usually, however, breaks away from this and sinks into the body. Thus in Sipunculus it lies within the body-cavity, and has retained its primitive connexion with the outer layers of the skin only in the region of the brain; but in the Priapulids the nervous system, which consists of a ring round the mouth and of a ventral cord, lies embedded in the skin, and the nerve cells are directly continuous with the cells of the epidermis. The nerve-ring lies at the base of a groove in the skin, which forms a kind of gutter round the mouth; the ventral nerve-cord is visible exteriorly as a light line which marks the ventral surface of the animal. In no place is the ring or cord differentiated in any way, and there cannot be said to be any brain or special sense-organs. Numerous nerves are given off from the ring to the pharynx and intestine, and from the cord to the body-wall.
The sexes are distinct, but they differ from the other Gephyrea in the nature of their reproductive organs. In mature specimens the ovaries or testes are easily recognisable, lying to the right and left of the alimentary canal. The reproductive glands are continuous with ducts, which act as oviducts and vasa deferentia respectively. Both glands and ducts are attached to the body-wall by a mesentery.
The excretory function is performed in the Priapuloidea by the ducts of the generative organs. These are primarily connected with a number of branching canals of small size which project into the body-cavity. According to Schauinsland,[485] one or more pear-shaped cells are found at the end of each branch, and each is {432}continued into a long cilium which hangs down into the lumen of the canal, and by its movement produces a flickering motion. Beyond the free end of the large cilium the canal is lined with ciliated cells. The remarkable resemblance this form of excretory organ presents to that of the Platyhelminthes (vide p. 25) and of certain Chaetopods is worthy of attention. In the young Priapuloidea the duct with its branching canals is not masked by the generative organs, but as the animals become mature, diverticula from the duct arise, and the cells covering these become modified into ova in the female, and into spermatozoa in the male. The presence of these follicles masks the excretory part of the gland. The ova and spermatozoa escape through the ciliated ducts which open to the exterior one on each side of the anus, and, contrary to what is the case with other Gephyrea, leave the body without having ever been in the body-cavity.
Nothing is known of the embryology of either member of this family, but both genera appear to be sexually mature from the end of May until October.
Classification.—The two genera which make up the Order Priapuloidea are characterised as follows:—
Priapulus.—The body is continued into one or two caudal appendages, beset with hollow papillae; these are ventral to the anus. The introvert forms ¼ to ⅓ of the total body-length; it is covered with spines in conspicuous longitudinal rows, the rest of the body being ringed. The retractor muscles are numerous, and are attached to the body-wall, some anteriorly and some posteriorly.
The genus includes the following five species:—
P. caudatus Lam. (Fig. 218). Hab. Coasts of Greenland, Norway, Great Britain, the North Sea, and the Baltic.
P. bicaudatus Dan. Hab. North Sea and Arctic Ocean.
P. glandifer Ehlers. Hab. Coast of Greenland, North Sea.
P. brevicaudatus Ehlers. Hab. North Sea and Baltic, from ten fathoms.
P. tuberculato-spinosus Baird. Hab. Falkland Islands.
Halicryptus.—No caudal appendages. Introvert ⅒ to 1⁄12 of the total body length, with numerous spines arranged in close circles. Retractors numerous and all attached to the body-wall anteriorly.
H. spinulosus v. Sieb. (Fig. 219). Hab. North Sea, Arctic Ocean, and Baltic, in from two to fifty fathoms.
It will be noticed that with the exception of P. tuberculato-spinosus, described by Baird from a single specimen, the whole family is confined to northern seas.
Habits.—The newly-captured specimens of both P. caudatus and H. spinulosus are of a flesh colour, with a somewhat metallic sheen. According to Apel, the latter lived in an aquarium for more than five months, whilst the former died during the first month. When first introduced into the aquarium they immediately began to busy themselves in the mud or sand at its bottom, and very seldom showed themselves above it. They forced their way into the sand by alternately contracting and extending their introvert, and the Priapulus arranged itself so that a portion, often a very small one, of its caudal appendage was exposed to the water; this fact supports the view that the appendage is respiratory in function. When the animal buries itself deeply, the appendage does not relinquish its position at the surface of the sand, but stretches itself until it in some cases surpasses the length of the body. On the other hand, Halicryptus (Fig. 219), according to the same observer, lies with the anterior end, the mouth, projecting from the surface of the sand, or else it curves itself, so that both ends project into the water.
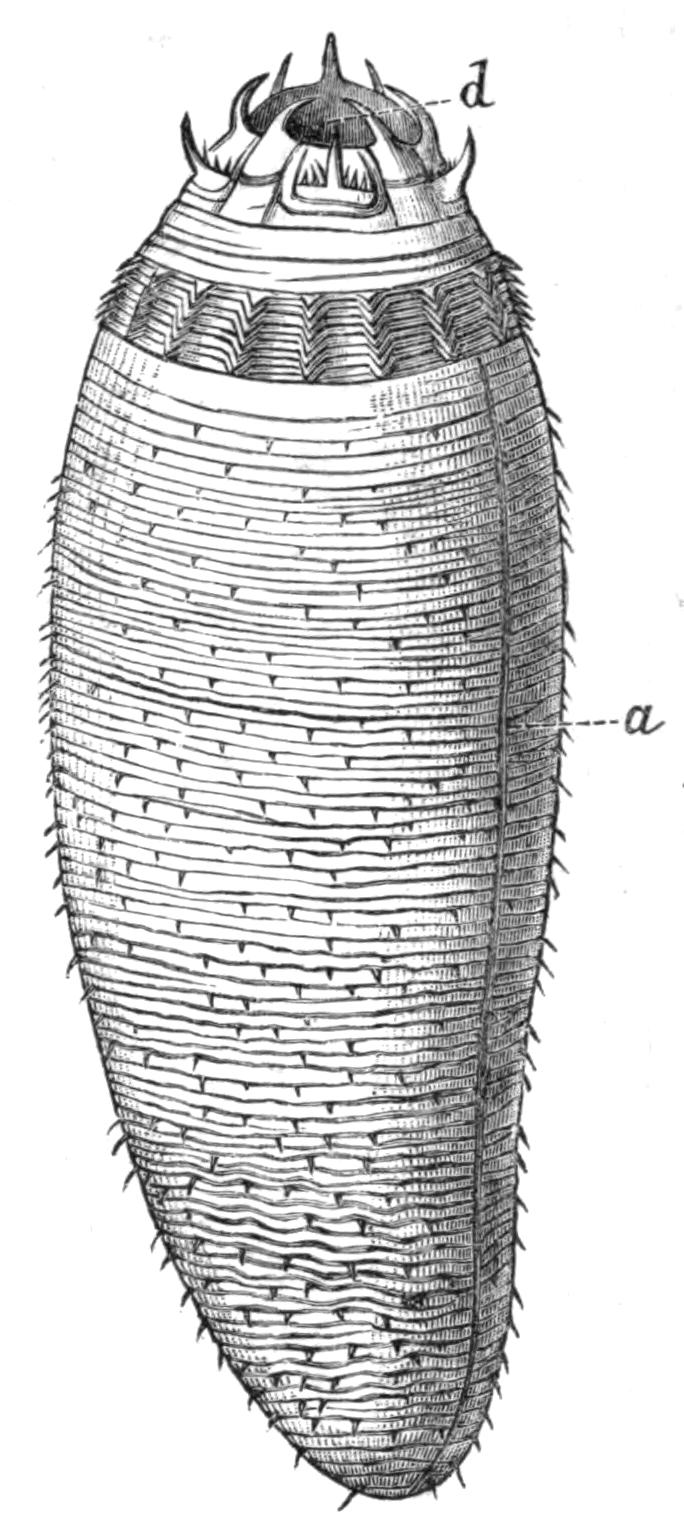
Fig. 219.—Halicryptus spinulosus v. Sieb. × 6. a, Dark line indicating the position of the ventral nerve-cord; d, mouth surrounded by spines.
Leckenby, who described specimens of P. caudatus which were found by fishermen searching for worms for bait in the outer harbour at Scarborough at half tide, states that they live in sandy clay in U-shaped tubes, at a depth of about 9 inches, the tubes opening at each end on to the surface of the sand. The fishermen of this district call them "sea mushrooms."
Halicryptus casts its cuticle in May and September; it becomes loose first at the hinder end, and the split between it and the skin grows forward until the animal lies free in a cuticular mantle. After some days this is split, and the animal frees itself from it; the cast-off cuticle includes for a short {434}distance the lining of the mouth, the anus, and the two generative pores.
III. Order Echiuroidea.
Anatomy.—The most striking peculiarity of the Echiuroidea, as opposed to the other two families of the Gephyrea, is the presence of a solid dorsal outgrowth of a portion of the head, forming the proboscis. The nature of this proboscis is something quite different from that of the introvert of the Sipunculoidea; it would appear to correspond to an extension, in the members of the last-named Order, of that part of the head which is dorsal to the mouth and is covered by a peculiar pigment-epithelium, often in continuity with the brain. In its outgrowth this portion of the body has carried with it the nerve-ring and the vascular ring, which both surround the mouth. The proboscis is found in all the genera with the exception of the aberrant genus Saccosoma.
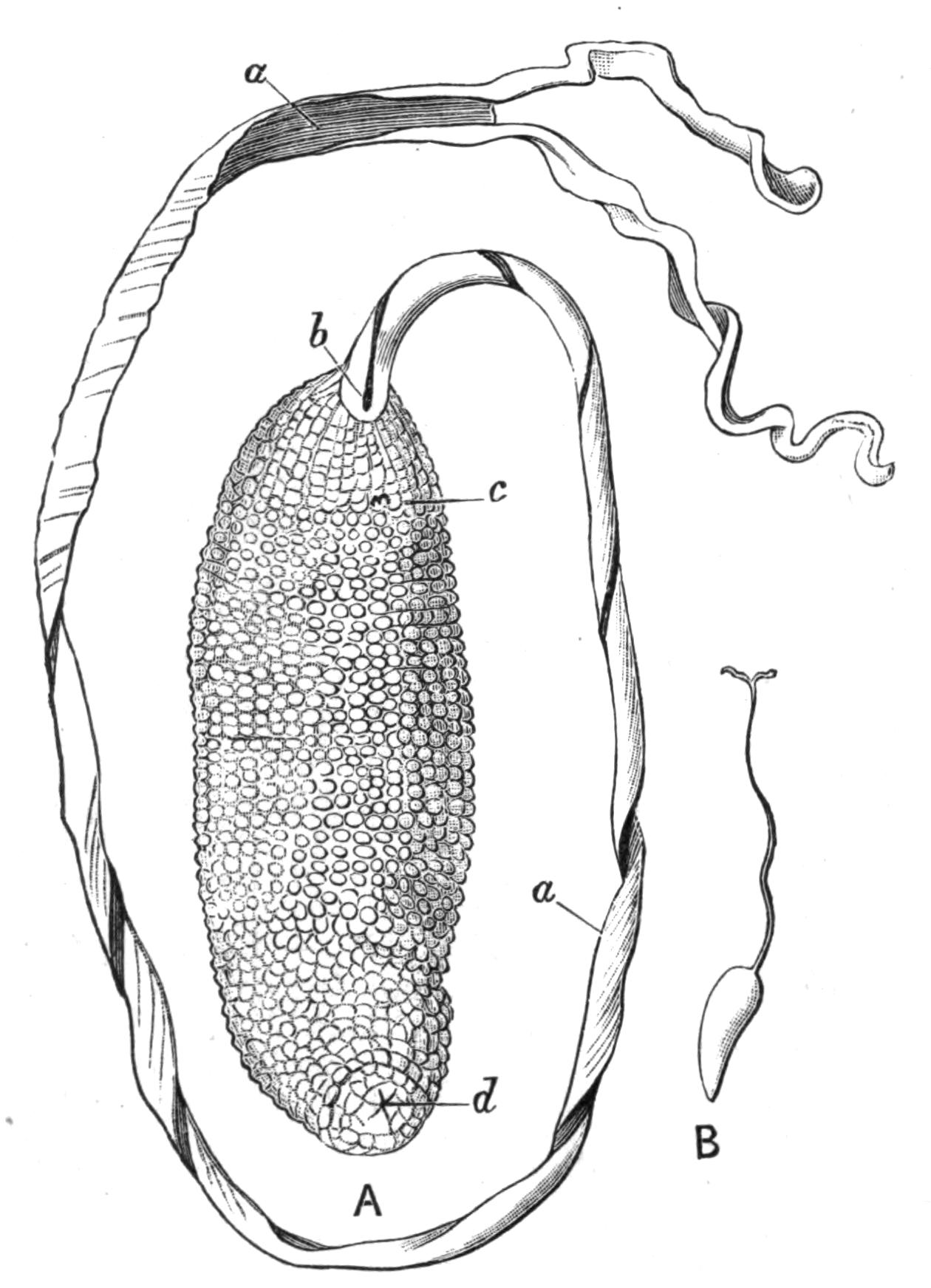
Fig. 220.—A, Bonellia viridis Rol., ♀; B, B. fuliginosa. Both nat. size. a, Grooved proboscis; b, mouth; c, ventral hooks; d, anus.
The body of the female Bonellia viridis, one of the best known species of Echiurids, is shaped like a small sausage, and is usually about 2 inches long. The proboscis arises from the anterior end, and is extremely extensible. At the distal end the proboscis splits into two short arms, which are often recurved; along the whole ventral surface runs a groove lined with cilia, which by the approximation of its edges can be converted into a tube. At the bottom of the proboscis the groove opens into the mouth. {435}Echiurus; Thalassema, and the female Hamingia have short proboscides, which do not bifurcate but otherwise resemble those of the female Bonellia.
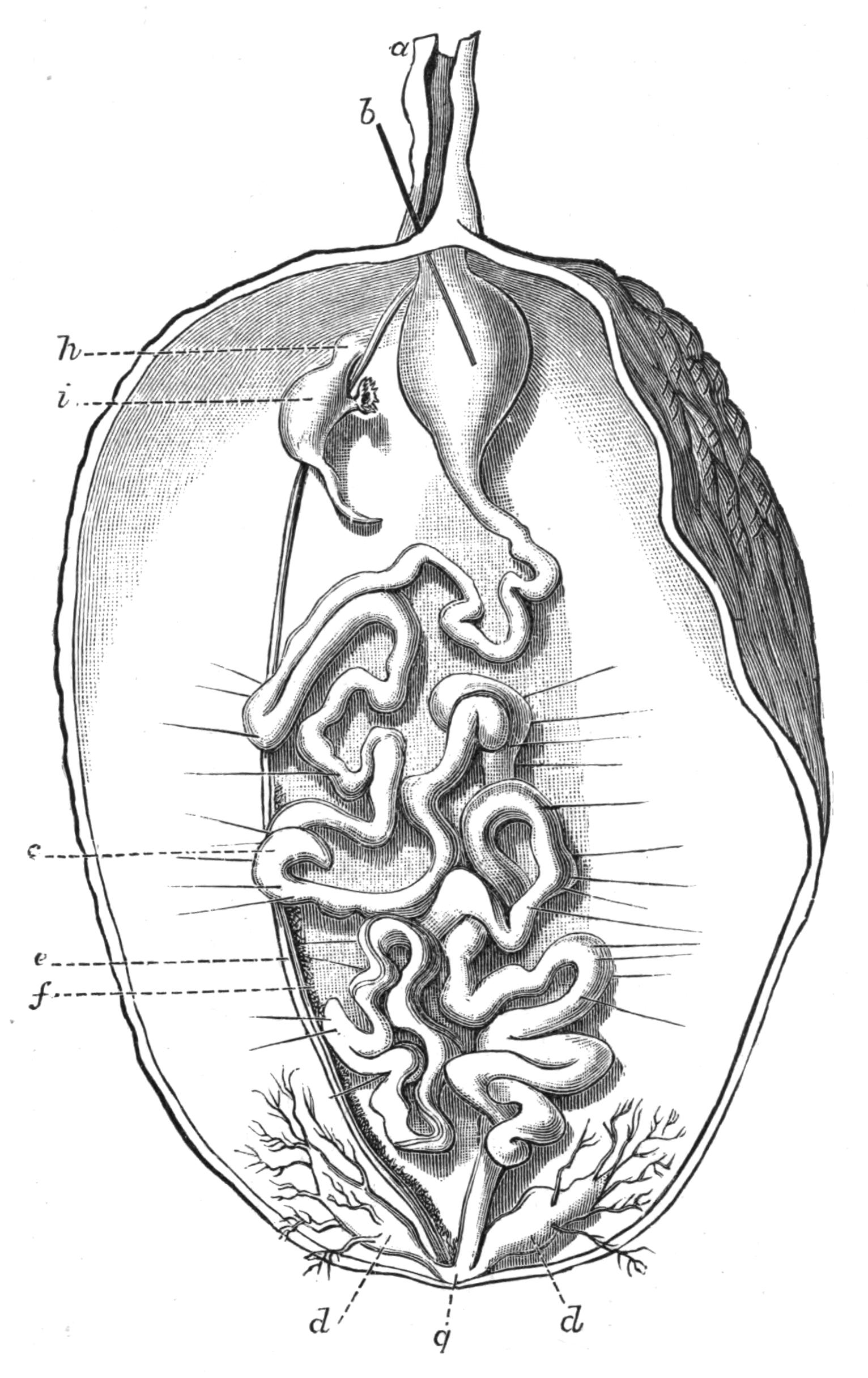
Fig. 221.—View of a female Bonellia viridis Rol., opened along the left side, × 2. a, Proboscis cut short; b, a bristle passed through the mouth into the pharynx; c, convoluted intestine; d, anal tufts or vesicles; e, ventral nerve-cord; f, ovary borne on ventral vessel running parallel with e; g, position of anus; h, points to position of external opening of nephridium; i, nephridium. This line is on a level with the internal funnel-shaped opening.
The green colour of B. viridis is due to a special pigment, "Bonellein," which at one time was thought to be identical with chlorophyll. A similar green colour is found in Hamingia arctica, Thalassema baronii, and the larvae of many forms.
A short distance behind the mouth, on the ventral surface, the female Bonellia and both sexes of Thalassema and Echiurus bear two incurved stout chitinous hooks; these gave the name {436}Gephyrea Armata to the above-mentioned genera. In addition to these, Echiurus has a row of chitinous bristles surrounding the posterior end of the body; the row is single in E. unicinctus, double in E. pallasii. These bristles are formed, like the hooks on the introvert of the Sipunculoidea, by epidermal cells; those of B. minor and of the posterior rings in Echiurus are said to arise each from a single cell, just as the bristles do in Chaetopods.
The skin consists of very much the same layers as does that of Sipunculus; the cuticle is thin, the epidermis is modified into numerous glandular cells, papillae, and pits, from which the bristles arise. A third layer of oblique or circular fibres is usually found inside the longitudinal muscle-layer. The proboscis is solid, and contains much connective-tissue and numerous muscle-fibres running in all directions; the ventral groove is ciliated.
The alimentary canal in the Echiuroidea consists of a long thin-walled tube with numerous convolutions; it is not coiled as in Sipunculids, but the loops are irregularly arranged, and are supported by numerous fine muscular strands which run from the skin. There is a ciliated groove running along one side of the intestine, as in the Sipunculids. The anus is terminal. The most striking peculiarity of the alimentary canal of the Echiurids is the existence of a collateral intestine or "siphon." This is a narrow tube which arises from the main canal not very far from the mouth, and re-enters it again lower down. A similar structure occurs in some Echinids, and in the Capitelliformia (pp. 272, 305). Its function is not certainly known.
Another characteristic feature of the Echiurids is the presence of "anal vesicles," branching structures which unite into a common stem opening into the intestine close to the anus. The free end of each of the branches terminates in a ciliated funnel-shaped opening. The function of these structures may be excretory, or they may control the amount of fluid in the body-cavity.
A closed vascular system exists in Echiurids, consisting of a contractile dorsal vessel running along the dorsal surface of the anterior end of the alimentary canal, and continued along the axis of the proboscis. At the tip of the proboscis it bifurcates, and each branch descends along the edge until it reaches the base where, having encircled the oesophagus, the two unite, and are continued as the ventral vessel which runs along the dorsal surface of the nerve-cord, and eventually ends blindly. There is also a vessel which {437}passes from the ventral vessel and encircles the intestine, opening into the posterior end of the dorsal vessel. In Echiurus the same vessel encircles a stout muscle which runs from the base of one of the ventral bristles to the other. In Thalassema Lankester states that the fluid within the vessels is colourless, and does not contain corpuscles similar to those in the body-cavity fluid.
The "brown tubes" or nephridia vary in number in the Echiurids. In the female Bonellia there is but one; in B. viridis the right, in B. minor the left usually persists. In shape, colour, contractility, and minute structure they closely resemble those of Sipunculus. Hamingia is said to have a pair of brown tubes; Echiurus has two pairs, except E. chilensis, which has three; their internal openings are produced into long coiled slits in some genera. Thalassema gigas has one pair; Th. neptuni, Th. baronii, Th. formosulum, and Th. exilii, two; whilst Th. vegrande, Th. moebii, Th. erythrogrammon, Th. caudex, and Th. sorbillans have three pairs.
The nervous system consists of a ventral cord lying in the body-cavity, as in the Sipunculoidea, but attached to the skin, and of a circumoesophageal ring. With the growth of the proboscis this ring is drawn out, and the two branches run along the sides of the proboscis and unite at the tip. There is no specialisation of brain, nor are any special sense organs present, but the ventral cord gives off paired nerves at regular intervals, which, uniting dorsally, form rings in the skin in some and probably in all species.
The perivisceral fluid is of a dark brown colour in Thalassema, containing numerous spherical corpuscles deeply impregnated, according to Lankester, with haemoglobin, and also containing granules of a brown pigment. Haemoglobin is also found in certain of the muscles and in part of the epithelial lining of the body-cavity. Lankester also describes the presence of haemoglobin in the corpuscles of the perivisceral fluid in Hamingia.
The genital glands are, like those of the Sipunculoidea, formed by a special development of the cells lining the body-cavity. These cells are massed together along the wall of the ventral blood-vessel. In Echiurus and in Thalassema the cells break off and float in the body-cavity, developing into ova and spermatozoa. In Bonellia each cell does not become an egg, but a mass of cells breaks off, one of which increases in size at the expense of the {438}others and forms the ovum. The mature sexual cells leave the body through the nephridia.
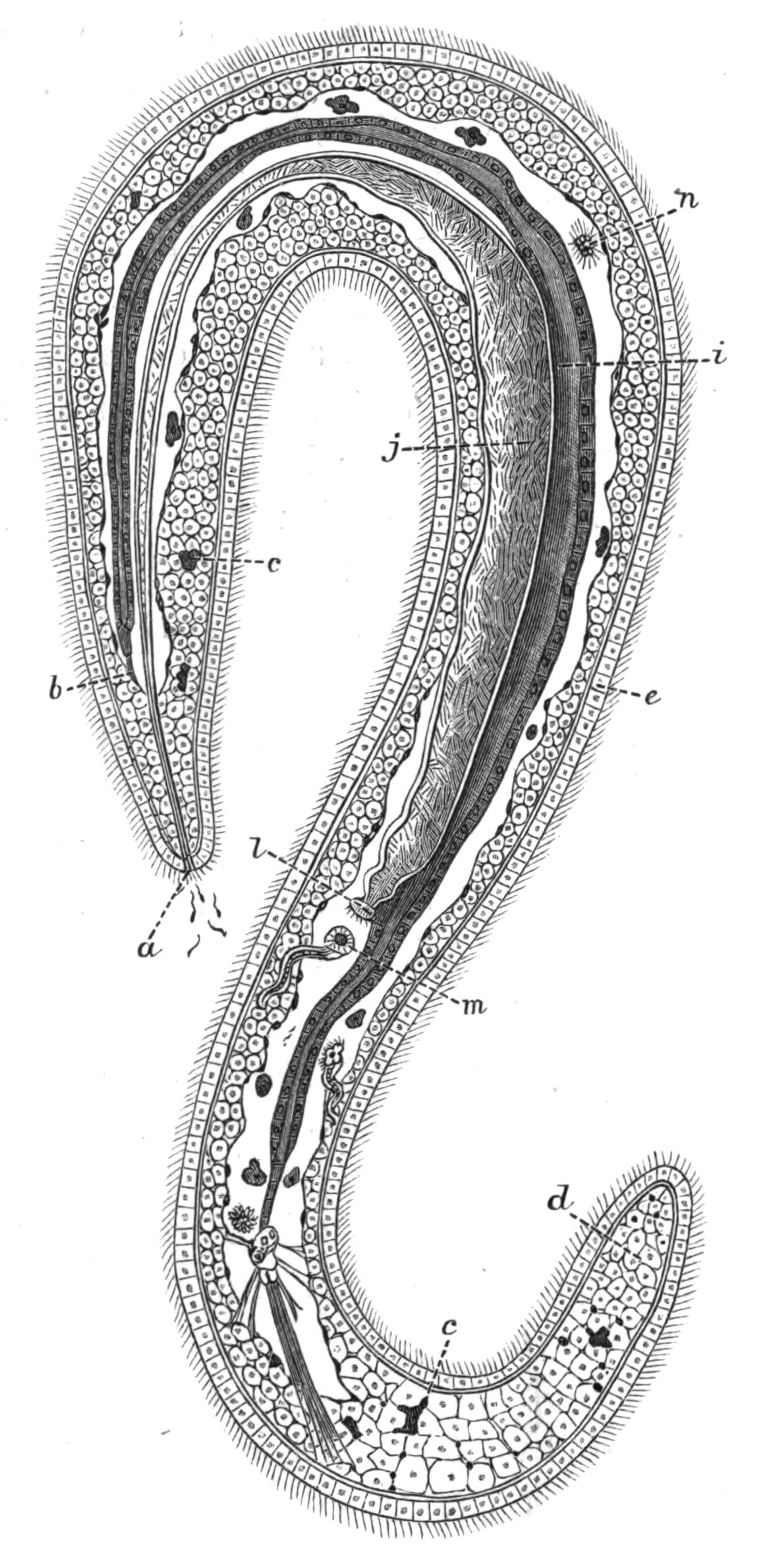
Fig. 222.—An adult male Bonellia viridis Rol. The original was 1.5 mm. long. The nervous system is not shown. (After Selenka.). a, Generative pore with spermatozoa coming out; b, anterior blind end of intestine attached to the parenchymatous tissue by muscular strands; c, green wandering cells containing chlorophyll; d, parenchymatous connective-tissue; e, epidermis; i, intestine; j, vas deferens; l, internal opening of vas deferens; m, the left anal vesicle; n, spermatozoa in the body-cavity.
Bonellia and Hamingia present very interesting cases of sexual dimorphism. In both genera the female is an animal of considerable size with the normal structure of the Echiuroidea, but the male (Fig. 222) is a microscopic Planarian-like animal, which lives in the mouth and in the nephridia of the female. Both in Bonellia[486] and in Hamingia the male is provided with a pair of hook-like ventral bristles; these are wanting in the female Hamingia. The surface of the male is ciliated, and the skin {439}contains circular and longitudinal muscle-fibres. The body-cavity is developed, but does not reach to either end of the body. The alimentary canal is closed, neither mouth nor anus existing; it is supported by regularly arranged dorso-ventral muscle strands. A nerve-ring and a ventral cord exist. There are also two rudimentary organs corresponding with the anal vesicles of the female, and a single nephridium which acts as a duct for the spermatozoa; the latter arise from modified cells lining the body-cavity.
In both sexes the larvae develop to a certain stage without showing any trace of sexual differentiation, but after this stage, the development of the male is to a certain extent arrested; in some respects, indeed, it undergoes retrogressive changes. At this time it is found clinging to the proboscis of the female, thence it makes its way to the mouth, where it undergoes its final change; and then creeping out, finds its way into the nephridium of the female, and spends the rest of its life there in a special recess cut off by a fold from the excretory part of this organ. In Hamingia, however, Lankester, who first described the male, did not find any in the nephridia, but found five specimens, each 1⁄12 inch long, within the dilated pharynx of the female.
Development.—In Bonellia and Hamingia it seems probable that the ova are fertilised in the nephridium of the female; in the other genera they are fertilised in the water after leaving the body of the mother.
In Thalassema and Echiurus the growth of the embryo results in the formation of a typical Trochosphere larva, a type widely spread in the animal kingdom, being found in the Chaetopoda (Fig. 145, A), Polyzoa (p. 510), and Mollusca. The large prae-oral lobe persists in the Echiuroidea as the proboscis; the mouth is ventral in position, with usually a ring of cilia encircling the body in front of and behind it; the anus is posterior and terminal. A pair of larval excretory organs are present, and a special nervous aggregation of cells at the apex of the prae-oral lobe is usually indicated by the presence of a bunch of long cilia.
The trunk of the Trochosphere is unsegmented, and in certain groups of animals it remains so, but in Chaetopods, and in Echiurus and Thalassema, it elongates and becomes divided {440}up into a series of somites or segments. Of these there are fifteen in Echiurus, and apparently eleven in Th. mellita; in this stage the Gephyrean larvae have again so close a resemblance to the segmenting Chaetopod larvae as to be easily mistaken for them. The segmentation is shown in the following way: (i.) the middle layer of cells or mesoblast is typically segmented, and forms septa, which separate each segment from its neighbours; (ii.) the ventral nerve-cord arises as segmentally-arranged thickenings of the epiblast, which fuse together, but retain their segmented appearance for some time; (iii.) the skin shows the segmentation of the body both by the arrangement of the pigment and by bands of cilia. The latter are replaced in the adult by rows of spines, and on the fourteenth and fifteenth segments in Echiurus pallasii by the two peri-anal circles of bristles. Each bristle, like those of Chaetopods, originates from a single cell.
The anal vesicles arise quite late in the development; when they have acquired their openings into the body-cavity, they seem to take in water. In Thalassema, as described by Conn, this is accompanied by remarkable changes, amounting almost to a metamorphosis. The body increases in bulk fourfold, the cilia of the prae-oral ring disappear, and the animal now moves only by means of its proboscis; the pigment is absorbed, and all traces of segmentation disappear. A similar intaking of water is described by Spengel in Bonellia. In this genus the larva, which is coloured bright green, and has two brown eye-spots, is not such a typical Trochosphere as is that of Echiurus and Thalassema.
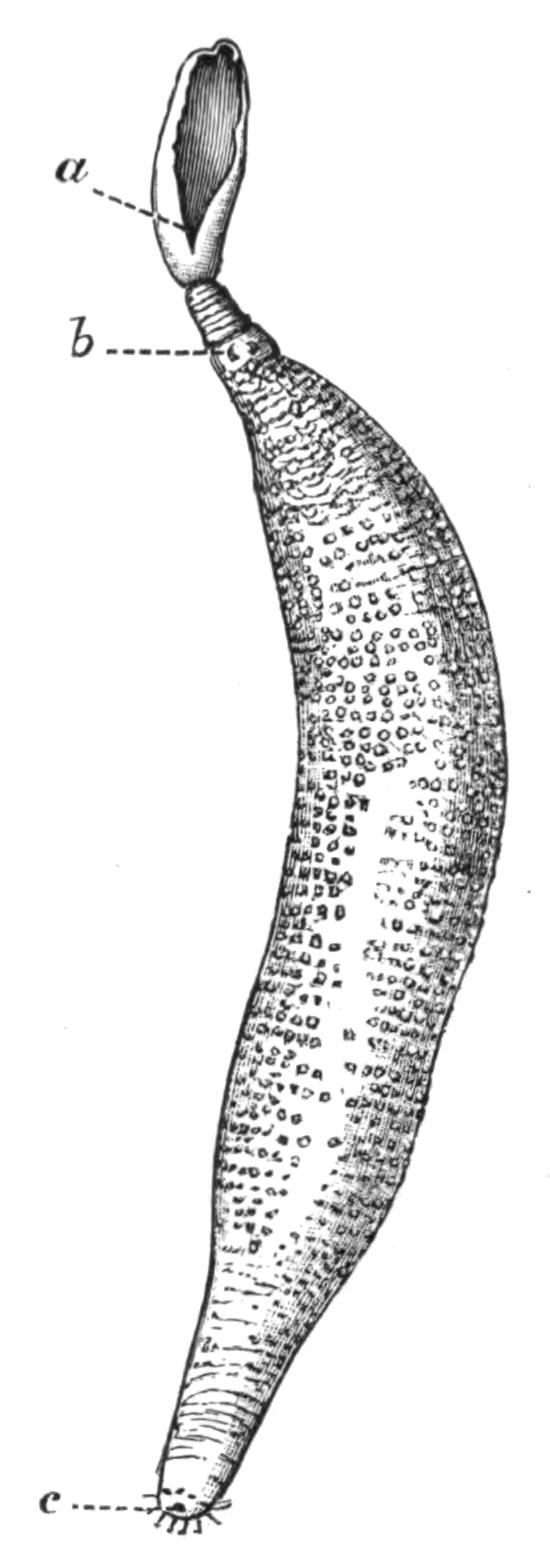
Fig. 223.—Echiurus pallasii Guér. × ½. a, Mouth at the end of the grooved proboscis; b, ventral hooks; c, anus.
Nothing is known of the development of Hamingia or of Saccosoma.
Species of Echiuroidea.—Echiurus. Proboscis not bifurcated at the end. Two ventral hooks and a single or double peri-anal ring of bristles. The body is to a varying extent marked {441}by rings bearing spines. Two or three (E. chilensis) pairs of nephridia, their external orifice often lengthened and spirally coiled. Both sexes alike.
Greef[487] mentions six species of Echiurus, viz. E. pallasii, E. forcipatus, E. sitchaensis, E. chilensis, E. carabaicus, and E. chrysacanthophorus; to these must be added E. unicinctus. It seems probable that E. forcipatus of Reinhardt is identical with E. pallasii, although bigger, whilst E. sitchaensis, E. carabaicus, and E. chrysacanthophorus are inadequately described. The distribution of the remaining three species is as follows:—
E. pallasii Guérin (Fig. 223). North Sea, Atlantic, English Channel.
E. unicinctus Drasche. Japan.
E. chilensis Max Müller. Chili.
Thalassema.—Proboscis rather pointed at the end, not bifurcated. No peri-anal bristles, but two ventral hook-like bristles placed anteriorly. One to three or four pairs of nephridia. The sexes resemble each other.
Greef mentions eight species of Thalassema and Rietsch thirteen; three of these, however, Th. grohmanni, Th. lessonii, and Th. pelzelnii, were not seen by either author, and their description is taken from Diesing. There is some reason for thinking that the two first-named species are identical with Th. neptuni. Conn has established a new species for the specimens whose embryology he worked out at Beaufort, Virginia, and Selenka described a new species from the Challenger material.
With the exception of the three doubtful species mentioned above, the list of species of Thalassema is as follows:—
Th. neptuni Gaertner (Fig. 224). English Channel (Devonshire coast), Concarneau, Mediterranean (Gulf of Marseilles), Irish coast (Dungarvan).
Th. gigas Max Müller. Trieste.
Th. vegrande Lampert. Philippine Islands.
Th. baronii Greef. Canary Islands (Lanzarote).
Th. formosulum Lampert. Shanghai and Philippine Islands.
Th. exilii Fr. Müller. Brazil (Desterro).
Th. moebii Greef. Mauritius.
Th. erythrogrammon Max Müller. Red Sea and East Indies (Billiton).
Th. caudex Lampert. Red Sea and Indian Ocean.
Th. sorbillans Lampert. Philippine Islands.
Th. mellita Conn. West Atlantic (Beaufort).
Th. faex Selenka. North of the Faroe Islands.
Bonellia.—Proboscis very extensible and bifurcated at the end. The body and proboscis are coloured a bright green. Two ventral hook-like bristles, but no peri-anal ring. A single nephridium. The above applies to the female; the males are degenerate, and live in the nephridium or pharynx of the female.
Three (or four?) species of this genus are known.
B. viridis Rolando (Fig. 220). Mediterranean, Adriatic, North Sea (Bergen).
B. minor Marion. Mediterranean (Gulf of Marseilles, Naples).
B. suhmii Selenka. Off Nova Scotia. Male not known.
B. fuliginosa Rolando? (Fig. 220). Mediterranean (Naples).
Hamingia.—Proboscis not bifurcated, about as long as body. No ventral hook-like bristles. One or two nephridia, which open at the apex of one or two well-marked papillae. The above applies to the female; as in the genus Bonellia, the male is minute and parasitic. It has two well-marked hook-like bristles situated behind the genital pore.
This genus was first described by Koren and Danielssen as H. arctica. Two specimens were afterwards described by Horst as H. glacialis. Later Lankester described two other specimens; he was the first to find the male in the pharynx of the female. He is of the opinion that all three descriptions apply to the same species, and for this the original name H. arctica must be retained.
Hamingia arctica K. and D. Two hundred miles north of North Cape and in the Hardanger Fjord.
Saccosoma.—No proboscis. The body is flask-shaped. The mouth and anus are terminal. The ovary is anterior, and there is only one nephridium. No bristles.
Our knowledge of this remarkable Gephyrean is very incomplete, but such as it is, it is due to the careful investigations of Koren and Danielssen, who had only a single specimen at their disposition.
Saccosoma vitreum K. and D. North of the Faroe Islands.
Habits of the Echiuroidea.—As a rule the members of this group conceal their bodies in clefts and fissures of rocks and stones, keeping up communication with the outer world by means of their proboscis. Rietsch[488] describes a specimen of Bonellia minor, which he placed in an aquarium, exploring with its {443}proboscis the nature of the bottom; when the animal had found a convenient crevice, it fixed its proboscis in it by means of the bifurcated end, and by its contraction drew the body up, and entered the hole, proboscis first. It then turned round, and during this operation doubtless the ventral hooks came into play; and then stretching out its proboscis, it began to explore the neighbourhood. The proboscis is evidently very sensitive, and in addition to being a locomotor organ, it is also used for the prehension of food. If cut off near the mouth, the animal does not long survive, but if a considerable portion is left the scar heals, and the lost part is probably regenerated. In captivity the animals frequently change their place of residence.
Eisig some years ago described the great extensibility of the proboscis of B. viridis when confined in the tanks of the Zoological Station at Naples. When contracted the proboscis was but a few inches long, but at times it was extended till it reached the length of 1½ metre, shining through the water as a transparent green thread. The body of the Bonellia was hidden under stones, but the proboscis could be seen seizing between its two ends the bodies of certain Ascidians which covered the inside of the tank, tearing them off the walls, and conveying them to the mouth along its grooved ventral surface.
The food of the Echiuroidea consists of organic matter, in the main of animal nature, but the group differs from the Sipunculoidea in not eating sand.
Rietsch describes Thalassema neptuni as being more active in its movements and less sedentary than B. minor. The proboscis is still the chief organ of locomotion, but the trunk plays a greater part in the movements of the animal than it does in the last-named species. Th. neptuni is found in cavities of stones or in the chambers worn out by the Mollusc Gastrochaena; when withdrawn from its house the body is found to be covered by a thick layer of tenacious viscid mucus.
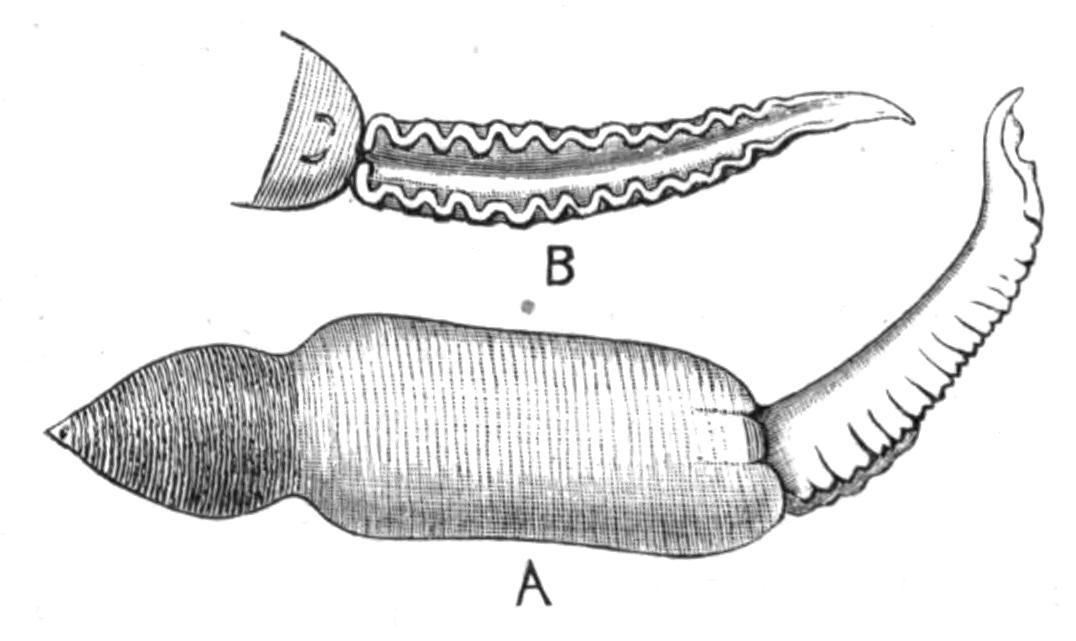
Fig. 224.—Thalassema neptuni Gaert. × 2. A, The animal lying on its ventral surface. B, Ventral view of the anterior end, showing the grooved proboscis ending behind in the mouth, and the ventral hooks.
Th. mellita was so named by Conn because it is found sheltering in the Echinid Mellita. "It enters the shell at the oral opening while yet very small, but once within its house it grows to its adult size, and is obliged therefore to remain during the rest of its life a prisoner." Each shell thus inhabited acquires a reddish brown horse-shoe-shaped marking, which affords a conspicuous signal that the shell contains a Thalassema.
Thalassema is seldom found living in sand, and Bonellia never, but Echiurus is almost always found in U-shaped tubes or passages in the sand, which it digs out for itself by the rapid contractions of its body-wall aided by its bristles. It, like the other two genera named above, does not long remain in the same hole, but frequently changes its home. As a rule the Echiurus sits near the mouth of its tube, which is often a foot or even two in depth, and sends out its proboscis in every direction; at the least sign of disturbance it withdraws into the deeper recesses. The walls of the tube are kept from falling in by a layer of mucus, which makes a smooth lining to the passage. The peri-anal bristles, which can be withdrawn or protruded at will, enable the animal to fix itself at any level in the tube.
The Echiuroidea are sometimes used by fishermen as bait. In Echiurus pallasii Greef found three parasites, all of them new species. One, a Gregarine, he named Conorhynchus gibbosus; the others were Platyhelminthes, and were named by him Distomum echiuri and Nemertoscolex parasiticus respectively.
IV. Order Epithetosomatoidea.
This Order includes the single Family Epithetosomatidae, which was established by Koren and Danielssen to contain the remarkable Gephyrean they described in 1881 under the name Epithetosoma norvegicum (Fig. 225).
Unfortunately only two specimens were at their disposition, and these were badly preserved, so that many details of their structure could not be made out. The animals are of an olive-green colour, and consist of a trunk about 12 mm. long, and of a proboscis 30 mm. in length; the latter differs essentially from the proboscis of the Echiuroidea inasmuch as it is hollow, and seems to be a whip-like tubular extension of the skin. Its lumen opens into the body-cavity. Ventral to the base {445}of the proboscis is the mouth; the intestine is straight, and terminates in the anus, which is posterior. The nervous system lies between the circular and the longitudinal muscles of the body-wall, and contains a tube, the nature of which is obscure. No vascular system is known. The ovary is attached to a mesentery ventral to the anterior part of the alimentary canal, and there is a single nephridium. No anal vesicles exist.
The most remarkable feature of the genus is a series of pore-like openings, which are stated to lead from the outside into the body-cavity (Fig. 225, a). These are arranged four on each side, at the bottom of two slit-like depressions in the skin, which lie one on each side of the base of the proboscis, slightly dorsal to it.
These remarkable structures are without parallel amongst the Gephyrea, and, together with the peculiar character of the proboscis, justify the Norwegian naturalists in adding a new family to the group.
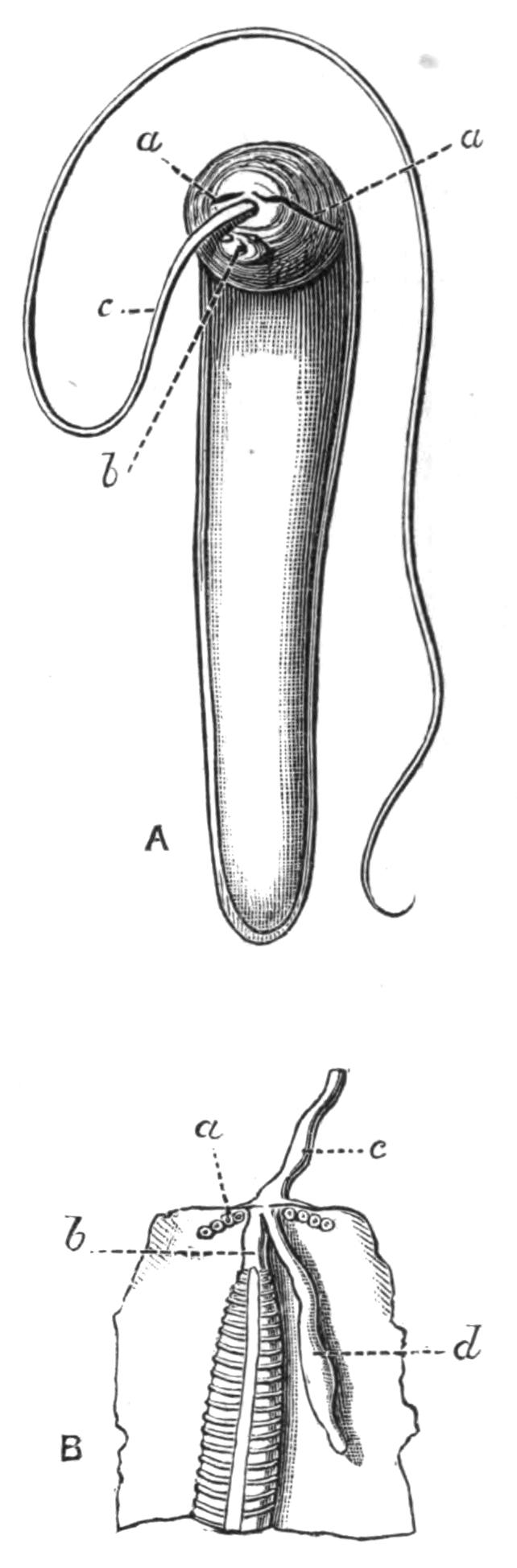
Fig. 225.—A, Epithetosoma norvegicum K. and D., magnified. a, a, Right and left slits leading to the pores; b, mouth; c, proboscis: B, the same animal opened dorsally; a, pores; b, oesophagus; c, proboscis; d, brown tube. (After Danielssen and Koren.)
Affinities of the Gephyrea.
Before considering to what other groups of animals the Gephyrea may be allied, it is advisable to discuss the relationship of the four Orders which compose the group.
Quatrefages, in the year 1865, divided the Gephyrea into I. Gephyrea Armata, with which he included the Echiuroidea and Sternaspis,[489] and II. Gephyrea Inermia or Sipunculoidea. The Gephyrea Inermia, sometimes called the Achaeta, have been extended to include the Order Priapuloidea, and opposed to the smaller sub-group the Gephyrea Armata or Chaetifera. In my opinion, however, these names now are no longer in accordance with our knowledge of the structure of the {446}animals they attempt to describe, and they should be given up. Both names had reference to the presence or absence of the two hook-like bristles described on the ventral surface of some of the Echiuroidea, but of the five genera of this family, two, Saccosoma and Hamingia (the latter in the female or normal form), are without these bristles, and can therefore be described neither as Armata nor as Chaetifera. On the other hand, hook-like chitinous bristles of somewhat the same nature, though smaller in size and varying in position, are very common on the introvert of Sipunculoidea and on the body of the Priapuloidea.
Again, the association of the two last-named Orders in one sub-group is, to my mind, an error. The Priapuloidea have little in common with the Sipunculoidea; almost the only real point of resemblance is the power of protruding the anterior part of the alimentary canal, and withdrawing it by the aid of retractor muscles. But in the Priapuloidea this power exists to a very small extent, and it is a power shared by very many animals besides the Gephyrea. The terminal anus of the former is a feature shared by the Echiuroidea and by Epithetosoma, but these have little else in common with the Priapuloidea. On the other hand, the entire absence of any head appendages, such as the proboscis of the Echiuroidea and the tentacles or tentacular membrane of the Sipunculoidea, the absence of a vascular system, of nephridia or anal vesicles, taken together with the straight intestine which occurs elsewhere only in Epithetosoma, the persistent connexion of the nervous system with the epidermis, the unique character of their excretory system and of the reproductive organs, are all features in which the Priapuloidea differ from the more normal members of the other three Orders. These constitute a list of peculiarities which are at least as important, and probably even more important, than those which characterise the Sipunculoidea and the Echiuroidea. Thus the Priapuloidea should, I think, be regarded as a distinct Order, which occupies a very isolated position in the group.
Until we know something about the development of Halicryptus and of Priapulus, it will be difficult to say whether the Order is more nearly allied to one or the other of the two great Orders of Gephyrea, whether it is very primitive or very specialised. The connexion of the entire nervous system with the epidermis and the absence of a vascular system are both {447}rather primitive features, and so is the Platyhelminthine character of the excretory organs. With regard to the vascular system, however, it should be pointed out that it arises very late in the larva of those Gephyrea whose development is known, and that it does not seem to correspond with the vascular system of other animals; it has no fine vessels or capillaries connected with it, and apparently does not act so much as the channel of the circulatory medium, but more as a mechanism for the expansion of the head appendages, the tentacles in the Sipunculoidea and the proboscis in the Echiuroidea; moreover, it is absent in some genera of the former, such as Onchnesoma, Tylosoma, and Petalostoma, where there are no tentacles.
The conclusion of the whole matter seems to be that the Priapuloidea are an isolated Order retaining many primitive features, and having no closer affinities to the Sipunculoidea than to the Echiuroidea.
Hatschek came to the conclusion, from his work on the development of Echiurus, that the Echiuroidea are true "Annelids," and from the presence and mode of formation of the bristles, that they are related to the Chaetopods. In this view he is confirmed by Conn, who worked out the development of Thalassema. This relationship is further confirmed by the discovery of Sluiter's that Sternaspis, the genus of Chaetopods which in other respects most nearly resembles the Gephyrea, has in one of its species (S. spinosa) a well-marked bifid proboscis, which, like that of the Echiuroidea, is thrown off at the least disturbance. Thus it seems fairly well established that the Echiuroidea are closely connected with the Chaetopoda, for although the only traces of segmentation they retain in the adult are the serially-repeated nephridia of Thalassema and Echiurus pallasii, and the two rows of peri-anal bristles in the latter, and possibly the circular nerves given off from the ventral cord, yet the larva is fully segmented, and in other respects is almost typically Chaetopodan.
The relationship of the Sipunculoidea to the Echiuroidea is a more doubtful point. Hatschek is inclined to separate them, and in this he is again supported by Conn. Embryology unfortunately does not help us much. The early stages and larvae of Sipunculus nudus and of Phascolosoma elongatum have been investigated by Hatschek and by Selenka respectively. In neither genus is there any trace of segmentation or of Annelid {448}features, with the possible exception of the bristles on the larval Phascolosoma. On the other hand, it must be remembered that the development of Sipunculus is remarkably abbreviated, and that such stages may have dropped out, the larvae hardly differing more from the Trochosphere of Echiurus and Thalassema than does that of Bonellia, an undoubted Echiurid. Still the facts that there is never a head-kidney present, that there is no trace of segmentation, and that at no stage is the anus terminal, must have a certain weight.
If we leave out of account the larval history, which, although pointing to a difference in the nature of the two families, is by no means decisive, and consider the adult structures, we find very considerable evidences of affinity. Taking firstly the main points of difference, we find these to be (i.) the nature of the cephalic appendages, either a proboscis or some modification of tentacles; (ii.) the position of the anus; (iii.) the presence of anal vesicles; (iv.) the number of the nephridia, never more than one pair in Sipunculids; and (v.) the difference in origin of the chaetae. Of these most undoubtedly the first is the most important. The Echiuroidea have retained the prae-oral lobe of the larva in the form of a solid outgrowth of the body, which outgrowth has carried with it the nerve-ring and vascular ring which surround the mouth. This has been lost in the Sipunculoidea, but is, I think, represented by a modified patch of epidermis which lies dorsal to the mouth and just above the brain. A solid extension of the skin in this region, which involved the nervous and vascular systems, would bring about the same relation of parts as is found in the Echiuroidea. The tentacular membrane or tentacles of the Sipunculoidea have such a variety of form and arrangement, whilst all subserving the same end, that I am inclined to believe that they have originated within the limits of the family.
The position of the anus in the Sipunculoidea is one common to very many animals which live embedded in sand or in tubular holes; it is probably not primitive, as in the larva of Sipunculus it is near the posterior end, and becomes more dorsal as the larva elongates.
The anal vesicles of the Echiuroidea probably have no representative in the Sipunculoidea. In appearance and position they are very like the little tufts which are found on the rectum of {449}Sipunculus, but since these open neither into the body-cavity nor into the alimentary canal, it is hardly fair to compare them.
The resemblances between the Orders seem to me, on the whole, to outweigh the differences. The general structure of the skin, the coiled alimentary canal, with its ciliated groove, supported by strands of muscles, the vascular system which gives off no capillaries, the structure of the brown tubes, the existence of chitinous hooks or bristles, the nervous system with its single unsegmented ventral cord, the formation of the generative organs, all point to a sufficiently close resemblance to justify us in classing the two Orders together. In addition to these there are considerable histological resemblances which cannot be discussed here, but which have a certain weight.
To sum up, it seems probable that the Echiuroidea are derived from the Chaetopoda, and that their nearest ally in this group is Sternaspis; and that the Sipunculoidea are allied to the Echiuroidea, but have further departed from the Annelid stock, and have lost even those traces of affinity with the parent group which have been preserved in the development of Echiurus and Thalassema.
So little is known of Epithetosoma that it is difficult to discuss its affinities. The presence of the hollow proboscis and the pores leading into the body-cavity undoubtedly justify its being placed in a separate Order, but beyond the presence of a terminal anus, in which it resembles the Echiuroidea, there is nothing in its structure which connects it more nearly with one than with the other of the three larger Orders of Gephyrea.
List of Gephyrea found in the British Area as defined by Canon Norman.
| Phascolosoma vulgare Blainv. | English Channel and North Sea. |
| Phasc"losoma elongatum Kef. | English Channel. |
| Phasc"losoma papillosum Thom. | English coast. |
| Phasc"losoma eremita Sars | North Sea. |
| Phasc"losoma procerum Moeb. | Bass Rock. |
| Phascolion strombi Mont. | English coast (Plymouth). |
| Sipunculus nudus L. | North Sea, English Channel (Paignton, Teignmouth). |
| Golfingia macintoshii Lank. | East coast of Scotland (St. Andrews Bay). |
| Petalostoma minutum Kef. | English Channel (Plymouth). |
| Priapulus caudatus Lam. | Scarborough, Outer Hebrides. |
| Echiurus pallasii Guérin | Coast of Scotland, English Channel. |
| Thalassema neptuni Gaertner | English Channel (Coast of Devonshire). |
PHORONIS
HISTORY—HABITS—STRUCTURE—REPRODUCTION—LARVA—METAMORPHOSIS—LIST OF SPECIES AND LOCALITIES—SYSTEMATIC POSITION.
This interesting genus was discovered and first described by Dr. Strethill Wright of Edinburgh, who in the year 1856 found specimens of it living on a stone with Caryophyllia sent to him from Ilfracombe. He christened the form Phoronis hippocrepia,[490] the generic name being apparently taken from an epithet applied to Io, the specific name having reference to the beautiful horseshoe shape of its tentacular crown. Two years later a closely allied or identical form was described by Professor P. J. van Beneden under the name of Crepina gracilis.[491]
Phoronis is a sedentary animal living in "colonies," but each member of the colony is distinct, and has no organic connexion with the others, from which it is isolated by the presence of a tube in which it lives, and into which it can be completely withdrawn. The tube is formed from a secretion which probably has its origin from the anterior end of the body-wall. The secretion hardens and forms at first a transparent coating, but it soon becomes opaque, and numerous sand particles, small pieces of shell, sponge spicules, and other marine objects adhere to the outside of the tubes, giving them a very characteristic appearance, and doubtless serving to protect the inhabitants from predatory animals.
What little we know about the habits of Phoronis is in the main due to the observations of Cori,[492] who studied Ph. psammophila at Faro, an inlet of the sea near Messina. The least disturbance causes the animal to withdraw its head with lightning rapidity into the tube, from which after a time it re-emerges very slowly, and does not expand its tentacular crown until its body is completely extended. Cori states that not unfrequently individuals are found either without the crown of tentacles or with the latter in process of regeneration. These may have been bitten off by fish, etc.; but, on the other hand, van Beneden describes in Crepina gracilis (Ph. hippocrepia) the throwing off and regeneration of the crown of tentacles; and Cori confirms his observation, at any rate as far as concerns those individuals kept in captivity, and whose surroundings were presumably somewhat unfavourable. He further observed the interesting fact that the cast-off crown of tentacles continued to live, and suggests that possibly it may develop a new body, in which case the phenomenon would be an interesting case of binary fission producing two new animals.
Fig. 226.—A piece of a matted colony of Ph. kowalevskii Cald. Slightly magnified. In most cases the tentacular head is protruding from the tube.
With regard to the habitation of Ph. australis, the largest species known, some discrepancies have crept into the literature of the genus, and to prevent their recurring again it may be worth while to quote the statements of its discoverer, Mr. Haswell.[493] He says: "Phoronis australis occurs in communities of twenty to thirty, in spaces in the substance of the wall of the tube inhabited and formed by a species of Cerianthus. Each worm has a tube of its own, very delicate and transparent, made up of several layers, the mouth opening on the outer surface of the tube of the Cerianthus. The Cerianthus tubes sometimes come up empty, as we should naturally expect, the animal having dropped out; but a sufficient number of {452}occupied tubes are found to show that, under ordinary circumstances, a living Cerianthus occupies the interior of the tube and a community of Phoronis live in its wall. This species of Phoronis is never found anywhere else, and the species of Cerianthus is very rarely found without the Phoronis."
Ph. australis is sluggish in its movements, but other species are capable of very active movement, and withdraw their heads in a moment at the approach of danger. A Neapolitan species, Ph. kowalevskii—known to the fishermen of that place as "Ficchetelli bianchi" or "Vermi di ceppa"—lives chiefly on submarine posts and piles; its tubes, closely interlacing, form a dense feltwork, upon which Ascidians and Sea-anemones often settle, and over which Ophiurids and Polychaets creep. The tubes of this species are rendered opaque by the excreta ejected from the body, and they do not attach foreign substances to the outside to anything like the same degree as Ph. psammophila, which live in sandy places, and are termed by the Sicilian fishermen "Tubi di sabbia." The feltwork of Ph. kowalevskii attains a thickness of 5 to 8 cm. In each case the tube is much longer than the animal it shelters, and is so entangled with its neighbours, to which it frequently adheres, that it is a matter of considerable difficulty to isolate it.
Fig. 227.—A piece of a colony of Ph. psammophila Cori. Slightly magnified. The tubes are covered by particles of sand, small shells, etc.
The various species of Phoronis differ a good deal in size; Cori gives the average length as varying from 1.5 to 7.9 mm. in Ph. hippocrepia and up to 127 mm. (6 inches) in Ph. australis. Probably the very short individuals of the first-named species had not attained their adult stature. Ph. australis has recently formed the subject of a memoir by Dr. W. B. Benham,[494] from whom the following account is mainly taken.
The length of the individuals varied from three to six inches, and their diameter, which is not very uniform, averaged one-eighth of an inch. At one end, which, since it bears the mouth, we may call the oral end, is the very characteristic tentacular {453}crown surrounding the mouth on all sides but one, where there is a slight break in its continuity. The crown of tentacles or lophophore is flattened, and the two ends drawn out, and each is coiled into a spiral (Fig. 229); between the bases of these two spirals three ridges can be seen, each ending in a pore; the median opening is the anus, the two lateral are the openings of the nephridia or kidneys, which also serve as ducts for the reproductive organs. The anus is thus approximated to the mouth, and since the continuity of the tentacular crown is broken at a spot just between the two, there would be nothing to separate these orifices if it were not for the presence of the epistome, a projection or flap of the body-wall which overhangs the mouth between it and part of the crown of tentacles.
The extent to which the ridge bearing the tentacles is incurved at each side varies in different species. In Ph. kowalevskii and Ph. psammophila the ends are only slightly turned in, so that the crown of tentacles is truly horse-shoe shaped; but in Ph. australis they are turned in and form three coils on each side. The number of tentacles also varies, being very numerous in Ph. australis and Ph. buskii—the latter having as many as 300, whilst the other species as a rule have from 60 to 90. The bases of the tentacles are fused for a short distance with one another, forming a thin membrane.
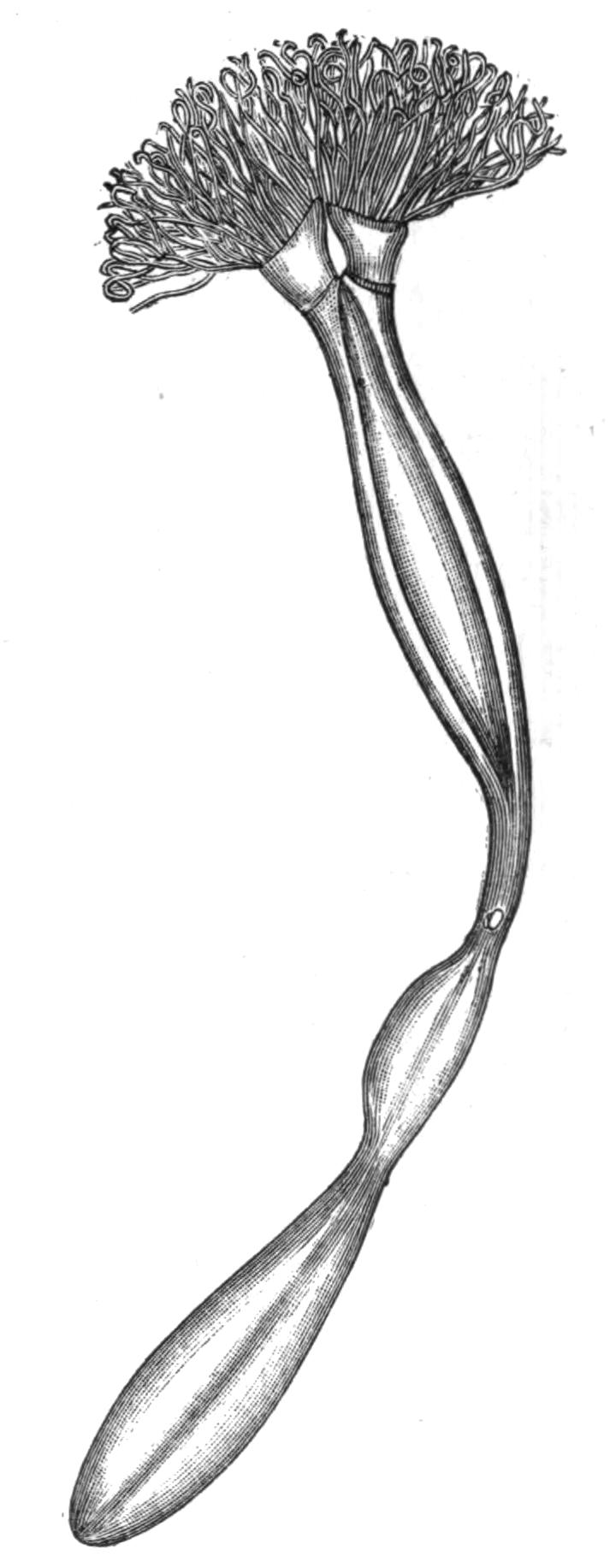
Fig. 228.—A specimen of Ph. buskii M‘Int. removed from its tube and seen from behind, × about 2. (After M‘Intosh.)
The rest of each tentacle is free, and its inner surface, or that turned towards the mouth, is covered with long cilia, which, by the currents they set up, doubtless serve to bring food to the mouth. The tentacles are hollow, and their cavity is kept open by a stiffening of the tissue, which almost resembles an internal skeleton; the cavity communicates with the anterior part of the general body-cavity, and up it runs a single blood-vessel containing red blood. A single nerve is also distributed to each tentacle.
At the base of the two spirals of the tentacular crown lie two ciliated pits, regarded by Caldwell and M‘Intosh[495] as sensory organs, but Benham looks upon them as glandular in structure and function. Perhaps they secrete the substance from which the tubes are formed.
The skin is covered by a delicate cuticle secreted by the underlying epidermis; within the latter is a well-marked basement membrane, and beneath this a layer of circular muscle fibres; these surround a layer of longitudinally-arranged fibres, which do not form a continuous sheet but are arranged in bundles. In both layers the fibres are unstriated. The longitudinal fibres are covered on their inner side by a layer of flat pavement cells, which line the general cavity of the body.
This space, the body-cavity, is divided into two parts by the presence of a diaphragm or septum which runs across from one side of the body to the other about the level of the ridge bearing the tentacular crown. The anterior space is continuous with the cavities of the tentacles and of the epistome. The partition is pierced by the blood-vessels and the oesophagus, but the rest of the alimentary canal, including the anus, the kidneys, and the reproductive organs, all lie in the posterior half of the body-cavity behind the diaphragm. This portion of the body-cavity is further subdivided by the presence of three longitudinal mesenteries supporting the alimentary canal and running between it and the body-wall. One of these mesenteries runs along the outside of the alimentary canal throughout its whole length, attaching both the descending and ascending limbs of the U-shaped tube to the body-wall. The other two are lateral mesenteries, which pass from the body-wall to the sides of the oesophagus. These mesenteries therefore divide the body-cavity into three spaces—one in which the rectum lies, which may be called the rectal, and two lateral; owing to the fact that the lateral mesenteries end before they reach the bend of the alimentary canal, the three chambers are in free communication one with another. The body-cavity is further traversed by irregular strands of tissue which run from the body-wall to the various organs. It contains a corpusculated fluid.
The alimentary canal (Fig. 230) consists of a U-shaped tube {455}which may be divided into four regions. The mouth (m) leads into the oesophagus (oe), which gradually enlarges into the stomach (st) situated just before the bend; a constriction just at the bend separates the stomach from the intestine (int), and this leads into the rectum (r), which terminates in the anus (an). The first three divisions of the alimentary canal are ciliated, but the rectum is not; the walls of the stomach also contain glandular cells, but there are no special glands opening into any part of the tract.
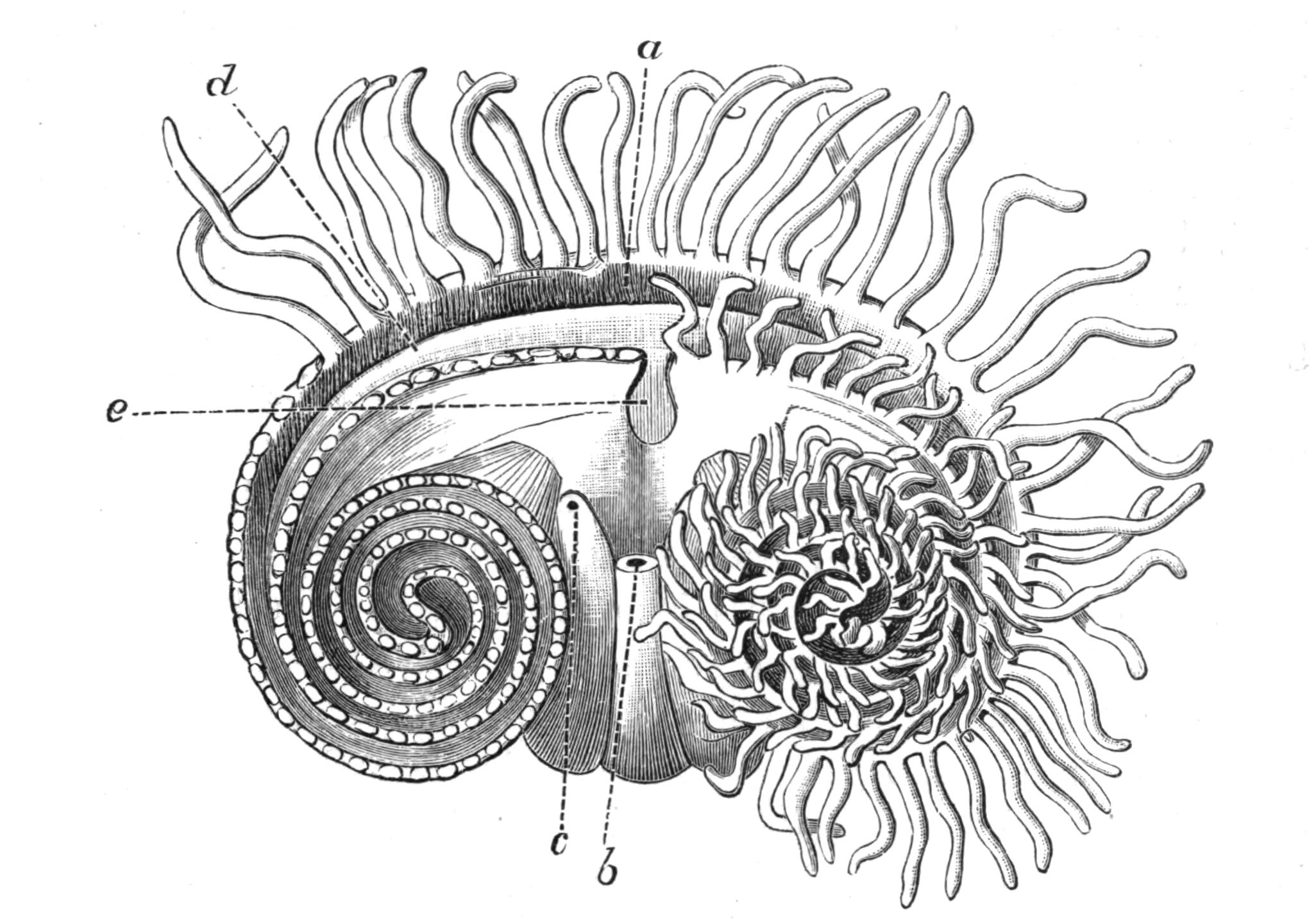
Fig. 229.—The dorsal surface of Ph. australis Has., looking down on the head. The tentacles are cut away on the left side, and the innermost are shortened on the right side to show the arrangement; in reality they are of the same length throughout. a, Mouth; b, anus; c, pore of left nephridium; d, epistome; e, break in the inner series of tentacles. The drawing is to some extent diagrammatic, and is considerably enlarged. (After Benham.)
One of the most interesting features of Phoronis is the presence of a closed system of blood-vessels containing red blood. There are two main blood-vessels; one, lying in the rectal chamber between the two limbs of the U-shaped alimentary canal, has been named the afferent vessel. Just below the diaphragm this splits into two, and each branch, after piercing this partition, runs in a spiral course along the base of the crown of tentacles, giving off a single blood-vessel into each tentacle. At its base each tentacular vessel opens not only into the above-mentioned {456}"distributing" vessel, but also into a "recipient" vessel which takes a course parallel with the former. The two recipient vessels pierce the diaphragm, and after running for some distance apart, fuse to form the efferent vessel, which continues down the body on the left side of the oesophagus. At the aboral end of the body the efferent vessel turns forward and becomes the afferent. Both the main vessels give off numerous blood diverticula, which are developed into plexiform sinuses on the walls of the stomach, and in this region they are covered with the reproductive cells. All the vessels are contractile, and Strethill Wright counted about fifteen pulsations a minute. The blood contains numerous nucleated, disc-shaped corpuscles differing in appearance from those of the fluid in the body-cavity. The corpuscles contain haemoglobin, which gives the red colour to the blood.
The two nephridia or kidneys are essentially tubes which open on the one side into the body-cavity, and on the other to the exterior. The position of the external pores has already been described, one being on each side of the anus. Each pore leads into a tube which passes into that part of the body-cavity situated below the diaphragm, where it divides, and each of the two branches terminates in a ciliated funnel-shaped opening. The smaller of these two funnels pierces the lateral mesentery and opens into the lateral chamber, whilst the larger, whose opening is very much drawn out longitudinally, opens into the rectal chamber. The whole organ is ciliated internally.
The nervous system lies in the skin immediately below the epidermis. This position is very primitive, and forms one of the most interesting anatomical peculiarities of the genus. The nervous tissue is probably diffused all over the body, but there is a special concentration or thickening in the form of a ring which surrounds the mouth, following the base of the tentacular spirals and giving off a nerve to each tentacle. The ring lies at the outside of the base of the tentacles, the anus is not included in it. Caldwell[496] has described in Ph. kowalevskii an asymmetrical nerve-cord given off from the ring and running along the left side of the body; associated with which is a tubular structure of unknown function. In Ph. australis Benham mentions two such tubes, one on each side of the body; their precise value is obscure.
The epithelium covering the nerve-ring is slightly modified in the neighbourhood of the kidney pore, and may have some special sensory function; no other organs of sense are known (but see p. 454).
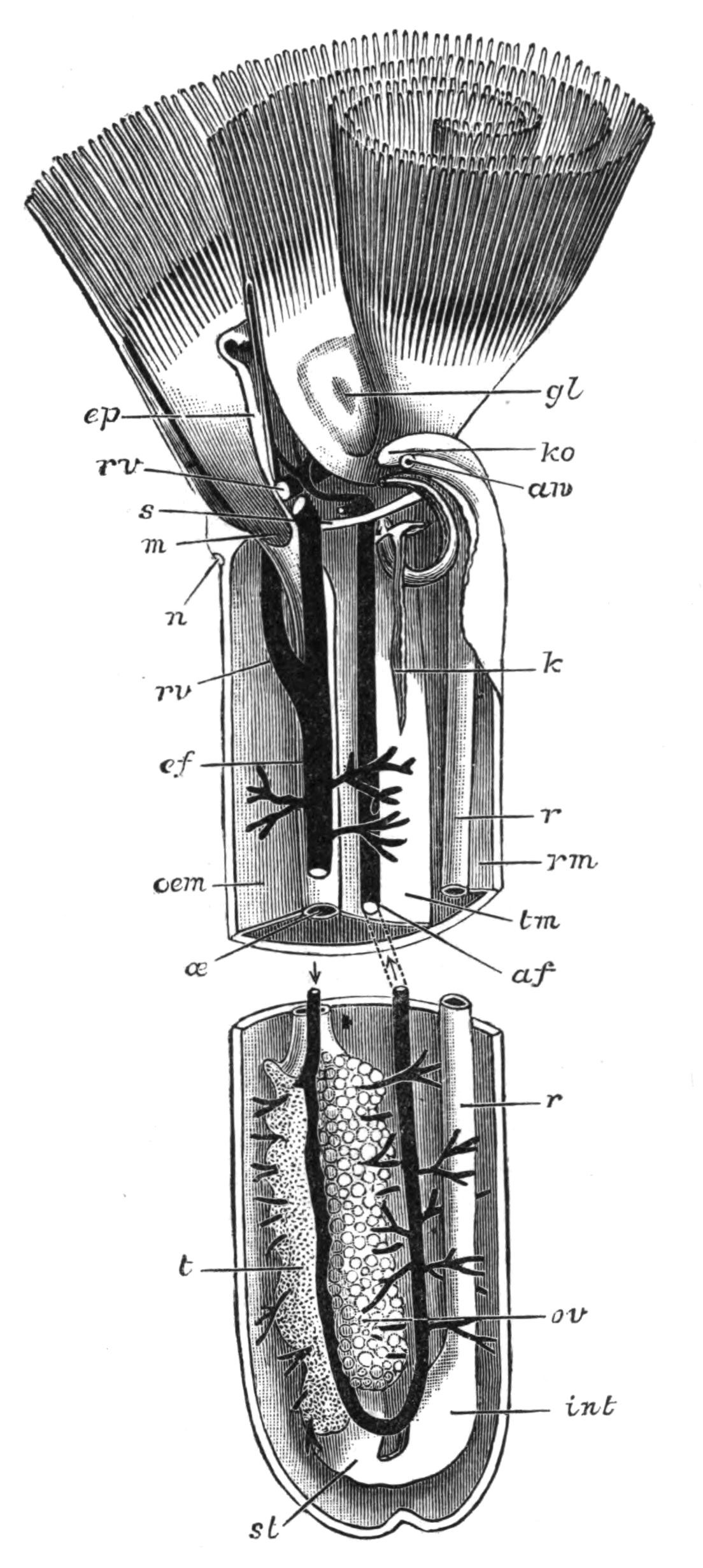
Fig. 230.—A schematic view of the interior of the body of Phoronis. The middle seven-eighths of the body are omitted, af, Afferent blood-vessel; an, anus; ef, efferent blood-vessel; ep, epistome; gl, glandular pit; int, intestine or "second stomach"; k, large funnel of the left nephridium; ko, opening of right nephridium, the opening of the left is seen immediately below in section; m, mouth; n, nerve concentration; oe, oesophagus; oem, oesophageal mesentery; ov, ovary; r, rectum; rm, rectal mesentery; rv, right recipient blood-vessel; s, septum; st, stomach; t, testis; tm, right lateral mesentery. (From Benham.)
Phoronis is hermaphrodite, male and female reproductive cells being formed in the same individual. The testes and ovaries form two white masses lying on the left side of the stomach, one on one side and the other on the other side of the efferent blood-vessel. The glands are traversed in all directions by the diverticula given off from this trunk, and are thus well supplied with blood; in fact both the ovary and the testis are formed by the {458}multiplication and growth of the epithelial cells which cover these diverticula. When ripe the ova and spermatozoa drop off into the body-cavity and make their way to the exterior through the duct of the kidney.
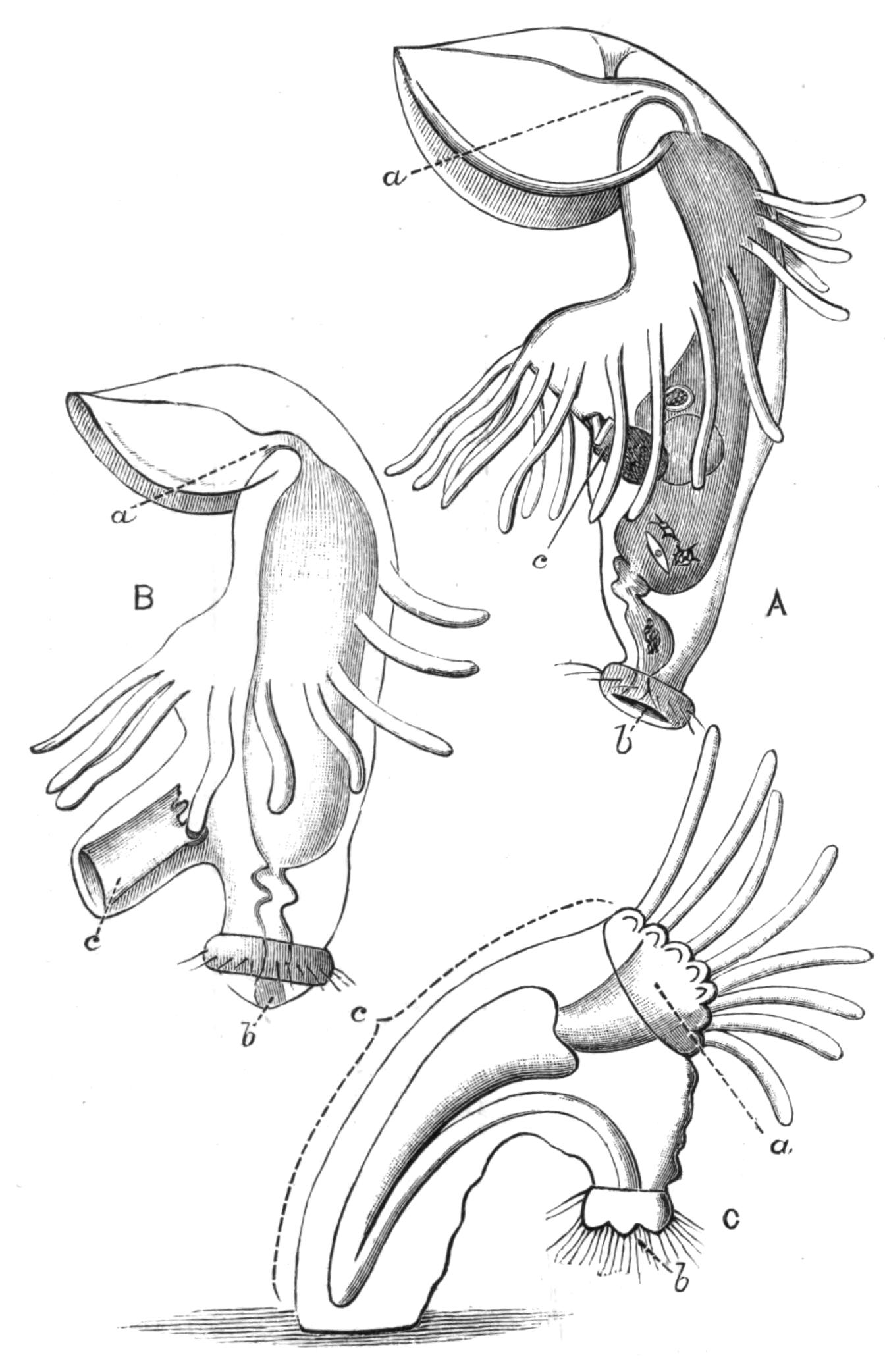
Fig. 231.—Three stages in the metamorphosis of the Actinotrocha into Phoronis. A, Actinotrocha larva with the invagination (c), which will form the trunk of the Phoronis larva beginning to appear. B, Stage with the invagination partly extruded. C, Stage when the extrusion is complete and the alimentary canal has passed into it. C is after Metschnikoff. a, Mouth; b, anus; c, invagination which ultimately forms the greater part of the body of the adult.
The ova are probably fertilised in the sea-water; they undergo the early stages of their development whilst entangled amongst the tentacles of the parent. The larval form to which they give rise was known long before its connexion with the adult was demonstrated by Kowalevsky.[497] It is known as the Actinotrocha (Fig. 231, A), and according to Caldwell has the following structure in Ph. kowalevskii. The mouth is anterior, and the anus terminal and posterior; the mouth is overhung by an immense prae-oral lobe, which bears a special larval nerve {459}ganglion, and in some species four eye-spots; at the base of this, but behind the mouth, is a ring of larval tentacles. The prae-oral lobe and the tentacles are ciliated; the margin of the lobe bears, however, specially long cilia, and there is also a ring of long cilia around the anus.
Before the Actinotrocha stage has been reached the larva has forsaken the shelter of its parent's tentacles, and swims actively about in the open sea. As it grows older a finger-like involution of the skin (c) arises just behind the tentacles on the ventral surface and grows into the body, increasing greatly in length and becoming much folded. The larva now sinks to the bottom of the sea, and after swimming round many times on its axis, undergoes a very astonishing metamorphosis (Fig. 231, B, C). The finger-like involution is suddenly turned inside out, and forms a large projection on the ventral surface, into which the alimentary canal passes, assuming a U-shape, as in the adult. This ventral process in fact forms all the body of the adult behind the line of tentacles, and subsequently contains, not only the alimentary canal, but the kidneys, the reproductive organs, and a large part of the vascular system. At the same time the prae-oral lobe breaks off, and, together with its ganglia and eye-spots, passes into the mouth and is digested in the stomach; the larval tentacles follow the prae-oral lobe, and are similarly digested. Their place is taken by a ring of adult tentacles which commence to appear just behind the larval tentacles before they fall off. The animal is now practically adult.[498]
It is obvious that this astonishing metamorphosis is accompanied by the rotation of the axes of the animal. The adult practically lives at right angles to the larva. In the latter the anus marked the posterior end, and the prae-oral lobe the anterior. The prae-oral lobe has disappeared in the adult, but its position is marked by the mouth. The ventral surface has enormously increased, and corresponds with the whole surface of the trunk. To be consistent we must therefore regard the mouth of the {460}adult as marking the anterior end of the animal, the anus the posterior. The short line between the mouth and anus across the centre of the tentacular crown marks the dorsal surface; and the line running all round the trunk from anus to mouth, the ventral. In fact, in its usual position in its tube Phoronis is lying on its ventral surface, its back faces upwards, and the anterior and posterior ends lie on one side or the other.
Species and Affinities.—In his exhaustive memoir on the anatomy and histology of Phoronis, Cori enumerates seven different species, and quotes the characters of each as enumerated by eight different authors. He, however, reserves his opinion as to the identity or distinctness of some of these species. Benham in his account of Ph. australis enumerates five species, including amongst them Ph. ovalis, which, however, he regards as probably a young form, an opinion in which Cori coincides. The latter regards it as possibly a young form of Ph. hippocrepia.
Without comparing specimens of each of the alleged species, it is difficult to come to any very satisfactory solution of the problem of how many distinct species are at present known, but it seems probable that there are at least six.
(i.) Phoronis hippocrepia Wright.—Under this name is included the first form, described and named by Wright in 1856; also Ph. ovalis, described two years later by the same observer as a distinct form, though it now seems probable that it is but a young form of Ph. hippocrepia. The Crepina gracilis of van Beneden is probably identical with this species.
This species occurs in membranous tubes embedded in limestone, corals, or oyster shells. Its length varies from 1.5 to 15 mm. The number of tentacles varies from 16 to 86. It has been found off the coast of Devonshire and in the Firth of Forth.
(ii.) Phoronis kowalevskii Caldwell.—This name is given by Benham to the species from Naples described by Caldwell, and replaces the name Ph. caespitosa, which was given by Cori. This species is found in the Bay of Naples, living in considerable colonies on submarine piles and posts. It is not firmly attached to its substratum. The tube may be coated with sand or other foreign particles. The length of the individuals varies from 3 to 39 mm. The lophophore is simple, with from 50 to 100 tentacles.
(iii.) Phoronis australis Haswell.—This is the giant of the genus, the length of the individuals being from 3 to 5 (76-127 mm.) or rarely 6 inches. It lives in delicate transparent tubes, interlacing the walls of the tube of a sea-anemone, Cerianthus. The arms of the lophophore coil into two spirals. The colour is reddish or purple. Found in Port Jackson.
(iv.) Phoronis buskii M‘Intosh.—This species was dredged by the Challenger {461}from a sandy bottom at a depth of 10 to 20 fathoms off the Philippines. Its tube is covered with particles of sand, sponge spicules, etc. Its length is 52 mm. or more (more than two inches). The anatomy of this species closely resembles that of Ph. australis, and Benham thinks that, in spite of the difference in their habitat, they may belong to the same species.
(v.) Phoronis architecta Andrews.—A species recently described by Andrews from Beaufort, N.C. Its distinctive features are: "the formation of isolated tubes covered by definite collections of sand grains; the presence of special prostomial organs, possibly of use in the formation of these tubes; the great development of the longitudinal muscles; the presence of a ciliated groove in the digestive tract; the apparent separation of the sexes."
(vi.) Phoronis psammophila Cori.—Found in Faro, near Messina. The tube is hyaline, and is covered by numerous grains of sand, some of considerable size. The length of the individuals is 25 to 50 mm. There are 60 to 90 tentacles. The colour is a fleshy red. A second species discovered by Haswell in Port Jackson had no points of importance to distinguish it from Ph. psammophila, except that no sand adheres to its tube and the number of tentacles is slightly greater.
In addition to the various species of Phoronis, several distinct forms of its larva, Actinotrocha, are known, and have been named without having been traced into their corresponding adult form.
The position of Phoronis in the animal kingdom has formed the matter of considerable divergence of opinion amongst the naturalists who have studied it. The earlier writers regarded Phoronis as allied to the Gephyrea, and it was for a long time classed with these animals, but placed in a separate sub-Order, the Gephyrea tubicola, which was opposed to the Gephyrea nuda, which comprised the true Gephyrea.
Caldwell referred Phoronis, the Brachiopoda, the Polyzoa, and the Gephyrea to the same type of body structure, and Lankester subsequently suggested the provisional name Podaxonia for this miscellaneous collection of animals. Lankester divided his phylum Podaxonia into three classes: (i.) the Sipunculoidea (Gephyrea), (ii.) the Brachiopoda, and (iii.) the Polyzoa. The last-named class he divided into three sections: (a) the Vermiformia, this includes the single genus Phoronis; (b) the Pterobranchia, including the forms Cephalodiscus and Rhabdopleura, whose affinities with Balanoglossus were subsequently demonstrated; and (c) the Eupolyzoa, including the forms treated as Polyzoa in the following pages.
Masterman's recent researches[499] on Phoronis seem to indicate {462}that the Vermiformia, like the Pterobranchia, must in future be grouped with the Hemichordata. He finds three well-defined coelomic spaces corresponding with the epistome, the collar, and the trunk, and also representatives of the collar pores, and is further inclined to believe that structures representing the notochord exist in Actinotrocha.
Should Masterman's researches be confirmed, Phoronis will be removed from its present isolated and enigmatical position, and placed with Cephalodiscus and Rhabdopleura amongst the Hemichordata, which will be described in Vol. VII. of this work.
BY
SIDNEY F. HARMER, M.A.
Fellow of King's College, Cambridge
POLYZOA
INTRODUCTION—GENERAL CHARACTERS AND TERMINOLOGY—BROWN BODIES—HISTORY—OUTLINES OF CLASSIFICATION—MARINE POLYZOA—OCCURRENCE—FORMS OF COLONY AND OF ZOOECIA—OVICELLS—AVICULARIA—VIBRACULA—ENTOPROCTA.
The following pages[500] deal with animals whose very existence is hardly known to those who are not professed naturalists. There are but few Polyzoa which have earned the distinction of possessing a popular name, and most of such names as do exist cannot be found outside treatises on Natural History. It is true that many of the members of this group have been vaguely termed "Zoophytes"; but this term implies no more than that they possess a superficial resemblance to certain plants, and it must be remembered that this habit of growth is assumed by many animals which have nothing to do with the Polyzoa. The term "Coralline" is sometimes applied to those calcareous Polyzoa which grow into coral-like forms; and the Tertiary deposit known as the "Coralline Crag" is so called from the large number of fossil Polyzoa which it contains.
The Polyzoa are none the less a most attractive group. Let any one examine a dry piece of a brown paper-like substance (Fig. 232, A), which may be found thrown up on the beach on many parts of our coasts. Of this species (Flustra foliacea), the {466}so-called "sea-mat," an old writer says: "For curiosity and beauty, I have not, among all the plants or vegetables I have yet observed, seen any one comparable to this seaweed."[501] Viewed with the microscope, the frond is seen to consist of two layers, placed back to back, of oblong chambers, each of which is the dried body-wall of a single individual. The whole is obviously a colony, and to this fact the term Polyzoa refers.
The chambers just noticed are termed "zooecia." Each is rounded at one end, near which is the "orifice," through which the tentacles of the living animal can be pushed out. Two short, stiff spines usually occur on each side of the orifice; and the symmetry of this forest of spines fully justifies the above-quoted remark.
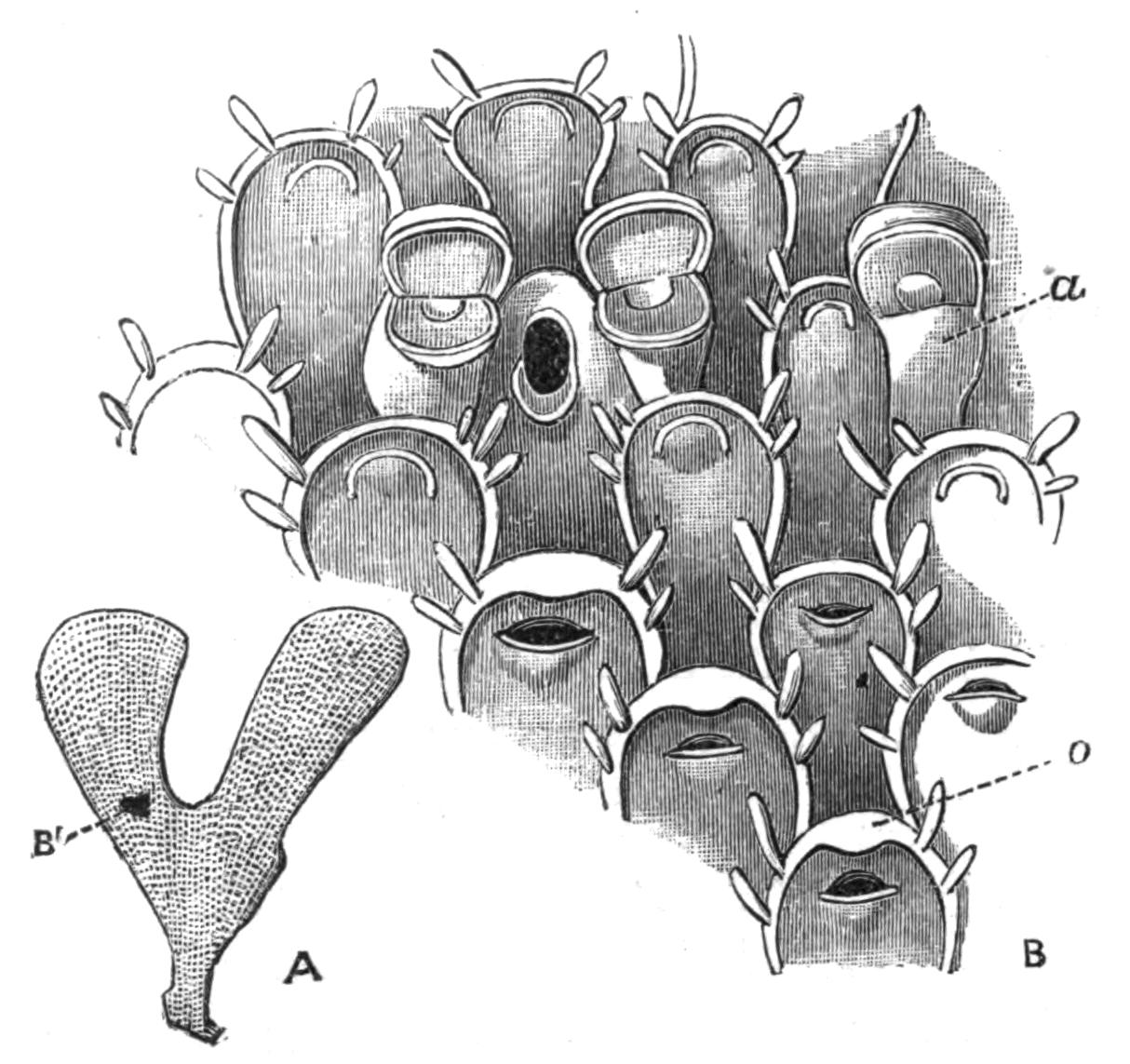
Fig. 232.—Flustra foliacea L., Cromer. A, Natural size, B' indicating the portion magnified in B (× 30): a, avicularium with closed mouth, to the left of which are seen two avicularia with open months; o, ovicell, forming the upper part of a zooecium. Ovicells are seen on three consecutive zooecia. The operculum, which closes the orifice of the zooecium, is seen in different positions in the individuals figured.
The upper part of some of the zooecia is somewhat swollen, these swellings representing the conspicuous "ovicells" of many other genera. In the early part of the year each ovicell protects an orange-coloured egg or embryo, and the larvae are readily liberated if the fresh colony be placed in clean sea-water. "At least ten thousand" were hatched out in three hours from a colony placed in a glass by Sir John Dalyell.[502] The larva swims freely in the water for a short time, and should it find a {467}suitable resting-place, it fixes itself and forms the starting-point of a colony, the number of whose individuals is continually increased by the production of buds at the growing edge. The "avicularia" of this species will be alluded to later (see p. 482).
F. foliacea has long been known to possess in the fresh state a remarkable odour, which is described, according to the fancy of the observer, as a strong odour of fish, or as the smell of violets after a shower. Others have compared it to that of the orange or verbena, or to that of a mixture of roses and geranium.
Flustrella hispida, another of our commonest Polyzoa, which may be found between tide-marks on the stalks of Fucus, consists of a softish brown encrustation, about one-sixteenth of an inch thick, covered by numerous spines. If examined undisturbed in a rock-pool, or transferred to a glass of sea-water, the brown mass will be seen to become surrounded by a delicate bluish halo, which is about as thick as the encrusting mass itself, and consists of the tentacles of the numerous individuals of the colony. The microscope shows that each individual is provided with a circlet of some thirty or more long, delicate tentacles, which together form a graceful funnel (as in Fig. 233). At the bottom of the funnel is the mouth, to which Diatoms or other minute organic particles are conveyed by the cilia which fringe the tentacles. If the tentacles be touched with a needle, the whole funnel is retracted with great rapidity, and in this retracted condition we see no more than the body-walls of the animals. After an interval the tips of the tentacles are cautiously protruded; the tentacles are gradually pushed out, at first in a close bundle, but finally separating from one another to form the funnel which we have already noticed.
There is hardly a more surprising spectacle in the whole animal kingdom than a living fragment of the genus Bugula. The colony grows in the shape of a small tree, whose height may amount to several inches; and is characterised, in many species, by a spiral arrangement of the branches, which makes the genus easy to recognise at first sight (Fig. 233, A). The stem and branches are composed of a single layer of zooecia, arranged two or more abreast. Each zooecium bears, on its outer side, a most singular body termed an avicularium, from its resemblance to a bird's head. Imagine a minute eagle's head attached by a short but flexible neck to the zooecium. Suppose further {468}that this structure moves backwards and forwards in a deliberate but determined fashion, its lower jaw usually widely open so as to be nearly 180° distant from its position when closed. Suppose that the lower jaw is moved by powerful muscles which can be distinctly seen inside the transparent head of the avicularium, and that every now and then it closes with a snap, seizing any unfortunate worm which may happen to be within reach with a grasp of iron. The above gives a very faint idea of the appearance of a living Bugula colony, with its hundreds of swaying avicularia, and with its tentacular funnels protruding from their zooecia, and withdrawing themselves capriciously from time to time.
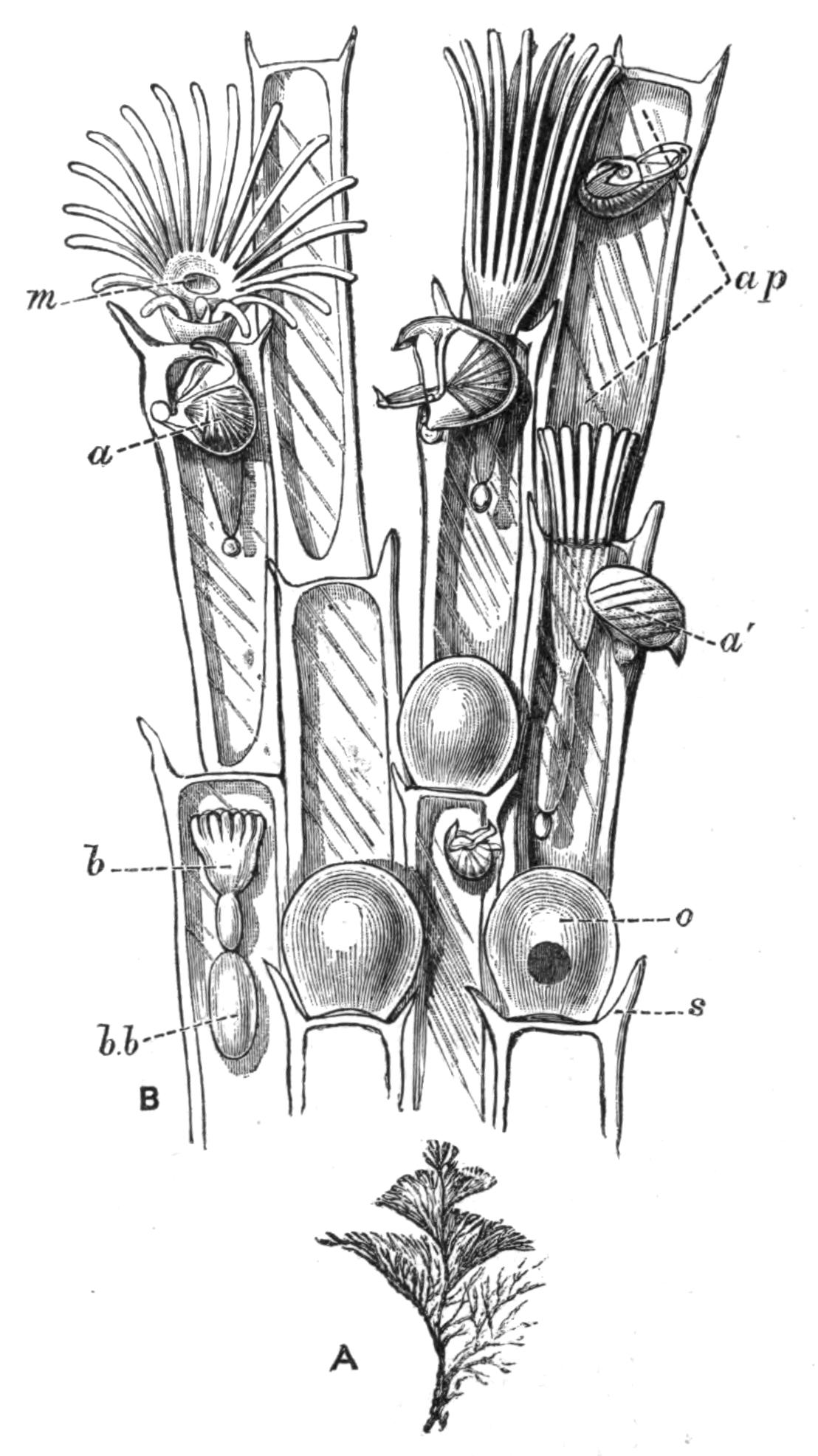
Fig. 233.—Bugula turbinata Alder, Plymouth. A, A small colony (natural size); B, portion of a branch (× 50): a, a', avicularia, in different positions; ap, "aperture" (see p. 524); b, polypide-bud, attached by its stomach to b.b, brown body; m, mouth, surrounded by the circle of tentacles; two individuals to the right show the tentacles partially expanded; o, ovicell; s, marginal spine. The avicularia of some of the zooecia have been omitted in B.
General Characters.—The Polyzoa are colonies, leaf-like or tree-like in form, and often strongly resembling seaweeds, or forming encrustations on the surface of stones and water-plants, or taking on other shapes. The units of the colony are complete individuals (Fig. 234). The zooecium or body-wall encloses a body-cavity, in which lies a digestive canal, with which are closely connected the central nervous system and the retractile, ciliated tentacles. The structures other than the zooecium constitute the "polypide." The mouth (m) leads into the ciliated pharynx (ph) which is followed by the oesophagus (oe) which again passes into the stomach (s), whose walls are coloured by a {469}characteristic yellowish pigment. The stomach gives off the intestine (in), which is lined by strong cilia, by means of which a rotatory movement is given to the faeces contained in it. This communicates by a narrow passage with the rectum (r), which opens by means of the anus (a).
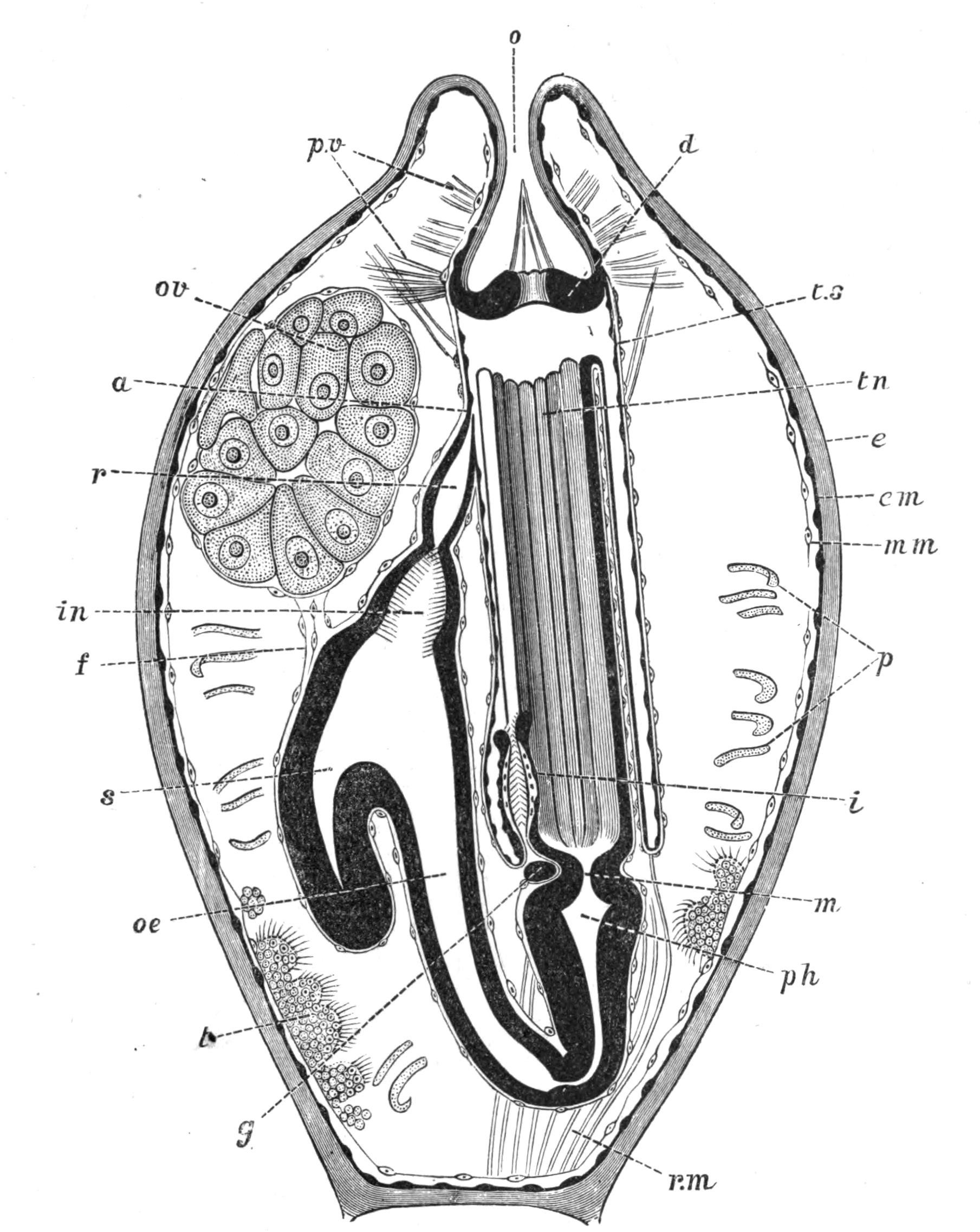
Fig. 234.—Alcyonidium albidum Alder, Banyuls-sur-Mer. Diagram showing the structure of a single zooecium with its polypide retracted: a, anus; d, diaphragm; e, ectocyst; em, ectoderm; f, funiculus; g, ganglion; i, intertentacular organ; in, intestine; m, mouth; mm, mesoderm of body-wall; o, orifice; oe, oesophagus; ov, ovary; p, parietal muscles; ph, pharynx; p.v, parieto-vaginal muscles; r, rectum; r.m, retractor muscles (contracted); s, stomach; t, testis; tn, tentacles; t.s, tentacle-sheath or kamptoderm. (After Prouho.[503])
In the retracted condition the tentacles (tn) lie in a cavity {470}which opens to the exterior by the orifice (o). The cavity is bounded by a thin membrane termed the "tentacle-sheath" (ts), and it is incompletely subdivided, near its upper end, by a diaphragm (d), perforated by a circular hole through which the tentacles can be protruded. The diaphragm bears the thin folded collar characteristic of the Ctenostomata, the group to which the species figured belongs (see p. 477).
Fig. 238, B, shows the tentacles of Bowerbankia in their fully expanded and partially expanded condition. Comparing this with Fig. 234, it will be clear that when protrusion is taking place, the tentacles are forced in a bundle, tips first, through the diaphragm and next through the orifice of the zooecium, the alimentary canal offering no resistance to this movement, owing to the length of the oesophagus. A moment's consideration will show that the bases of the tentacles, in passing through the orifice, will carry with them that part of the flexible tentacle-sheath to which they are attached; and it will further be clear that so much of the tentacle-sheath as is thus protruded will be turned inside out. This process of "evagination" continues until its further progress is stopped by the retractor-muscles (r.m), and by the parieto-vaginal muscles (p.v), which pass from the interior of the body-wall to the upper part of the tentacle-sheath. The latter has now become the delicate layer which connects the expanded tentacles with the zooecium; and the anus (Fig. 238, C, a) opens directly to the exterior. Since the name "tentacle-sheath" is thus descriptive of the condition of retraction only, the term "kamptoderm"[504] has been suggested as an alternative name.
The presence of a complete digestive canal and the ciliation of the tentacles in Polyzoa are conspicuous differences between these animals and the Hydroids, with some of which the Polyzoa may have a marked external similarity.
The outermost[505] layer of the body-wall is known as the "ectocyst" (Fig. 234, e). This may be densely calcareous, in which case the dried Polyzoon differs little in appearance from the living animal with its tentacles retracted; or it may be partially calcified, or it may consist entirely of a flexible cuticle, {471}as in Fig. 234. The ectocyst is prolonged through the orifice (o) as far as the diaphragm (d).
Forms with a calcareous ectocyst are commonly ornamented with ridges or other patterns, which are often of great beauty. The ectocyst in these cases is commonly interrupted at intervals by pores (Fig. 239, C), into which processes of the "endocyst"—the living, internal part of the body-wall—extend. These may appear as superficial pores, which apparently open to the exterior in the dried condition, or they may perforate the septa between adjacent individuals. This may be strikingly demonstrated by decalcifying a branch of Crisia (Fig. 237), in which the zooecia then appear connected by numerous strands of tissue. In many marine forms the communications between the individuals are in the form of small sieve-like plates known as "rosette-plates."
The endocyst may consist of definite layers of ectoderm (em) and mesoderm (mm), as in Fig. 234, but the mesoderm is commonly in the form of a loose network, some of which is attached to the body-wall, some to the alimentary canal, some forming connecting strands between these two layers, and other cells floating about freely in the body-cavity. These mesodermic structures are often spoken of as the "funicular tissue," since one or more strands of it commonly take on the form of a definite "funiculus" (f). This structure may bear the ovary (ov), while the testes (t) are found, commonly in the same zooecium, attached to various parts of the body-wall. The eggs and spermatozoa, when ripe, break off and float freely in the body-cavity.
The funicular tissue was at one time described as a "colonial nervous system." The idea expressed by this term must be considered erroneous from the fact that no nervous co-ordination of the individuals is known to exist, in the vast majority of cases. The actual nervous system consists of a ganglion (g) placed between the mouth and anus of each polypide, and lying in a small circular canal (not shown in Fig. 234) which immediately surrounds the oesophagus. This canal is developed in the bud as a part of the body-cavity, from which it becomes completely separated in marine forms. The Polyzoa have no vascular system.
Brown Bodies.—In the majority of cases, an extraordinary process of regeneration takes place periodically during the life of each zooecium. The tentacles, alimentary canal, and nervous system break down, and the tentacles cease to be capable of being {472}protruded (Fig. 235, 1). The degenerating organs become compacted into a rounded mass (Fig. 235, 2 and 3, b.b), known from its colour as the "brown body." This structure may readily be seen in a large proportion of the zooecia of transparent species. In active parts of the colony the body-wall next develops an internal bud-like structure (Fig. 235, 1, b), which rapidly acquires the form of a new polypide (Fig. 235, 2 and 3). This takes the place originally occupied by the old polypide, while the latter may either remain in the zooecium in the permanent form of a "brown body," or pass to the exterior. In Flustra the young polypide-bud becomes connected with the "brown body" by a funiculus (Fig. 235, 1, 2). The apex of the blind pouch or "caecum" of the young stomach is guided by this strand to the "brown body," which it partially surrounds (3). The "brown body" then breaks up, and its fragments pass into the cavity of the stomach, from which they reach the exterior by means of the anus.
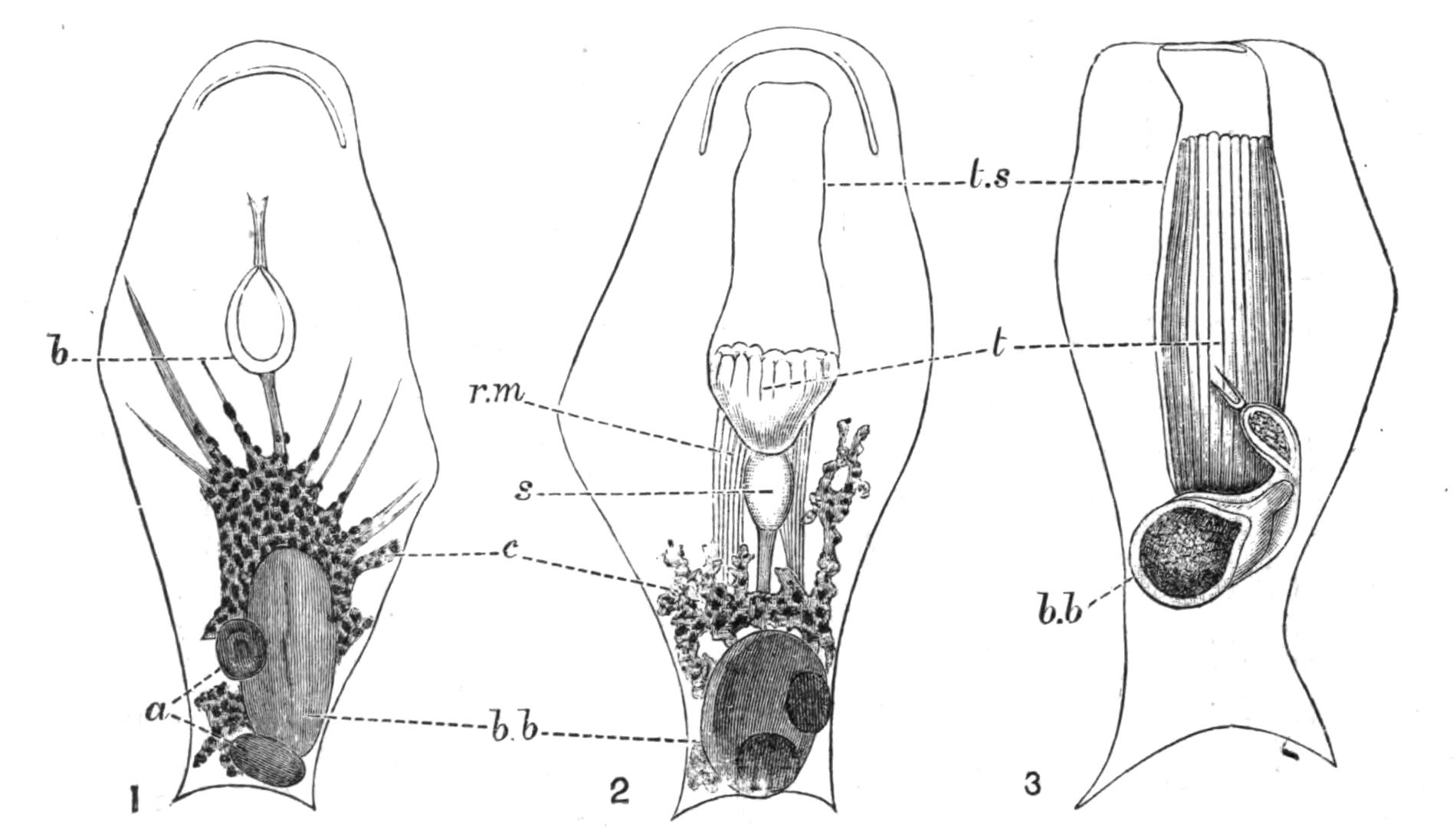
Fig. 235.—Flustra papyrea Pall. Naples. × 50. Illustrating the development of a new polypide after the formation of a "brown body." In 1, a, two masses formed from the alimentary canal; b, young polypide-bud; b.b, degenerating tentacles; c, connective tissue: 2, another zooecium, later stage; b.b, brown body; r.m, retractor muscles; s, stomach; t, tentacles of new polypide; t.s, tentacle-sheath: 3, the same zooecium, 191 hours later; letters as in 2. 1 and 2 are seen from the front, 3 from the back.[506]
There is some reason to believe[507] that these remarkable processes are connected with the removal of waste nitrogenous matters. The Marine Polyzoa are not known to be, in most cases, provided with definite excretory organs, although it is {473}possible that the intertentacular organ (Fig. 234, i) described on p. 508 may in some cases perform excretory functions. There can, however, be little doubt that some kind of excretion takes place in the Polyzoa; and in considering what organs could possibly perform this work, our attention is arrested by the alimentary canal. The digestive organs of the young bud are perfectly colourless. As growth proceeds, certain parts acquire a yellowish, and later a brown colour. The degeneration of the polypide is followed by the grouping of large numbers of the free cells of the body-cavity into a mass which closely surrounds the incipient "brown body." Under their action, the latter becomes considerably smaller, probably as the result of the absorption of matters of nutritive value into other tissues. The final result is the formation of the compact "brown body," whose colour is principally derived from the pigment formerly present in the alimentary canal. Experiments made by introducing into the tissues of the Polyzoa certain artificial pigments which are known to be excreted by the kidneys when injected into the bodies of other animals, have given some reason for believing that the appearance of the brown pigment in the wall of the digestive organs is, in part, a normal process of excretion; although that process is not entirely carried out by the organs in question.
Little is known with regard to the duration of life of a single polypide; but some information bearing on this question may be obtained from a set of observations made on Flustra papyrea.[508] The table gives the number of days from the time at which the polypides were noticed to commence their degeneration:—
| Days. | |
| 2 | "Brown body" partially formed, the parts of the polypide being still easily recognisable. |
| 5 | Tentacles still just recognisable: appearance of new polypide-bud. |
| 8 | Stage shown in Fig. 235, 2. |
| 11 | Union of apex of stomach with "brown body." |
| 16 | "Brown body" half surrounded by stomach, and preparing to break up (Fig. 235, 3). |
| 21 | "Brown body" broken up into numerous fragments, contained in the alimentary canal of the new polypide. |
| 35 | "Brown body" almost completely absorbed.[509] |
These results did not hold good for all the zooecia in a single colony. In some, the "brown body" was not completely got rid of at the end of sixty-eight days, the conclusion of the experiment.
So striking are the facts relating to the "brown bodies" that it has been believed[510] that what we have above described as the individual really consists of two kinds of individuals: firstly, the "polypide" or complex of tentacles and digestive organs; and secondly, the "zooecium," or house of the zooid or polypide, corresponding with what has been described above as the body-wall. The one individual, the zooecium, is on this view provided with successive generations of the second kind of individual, the polypide; and these latter function as the digestive organs of the two-fold organism. This view, though fascinating at first sight, is not borne out by an examination of all the facts of the case, especially when the Entoprocta are taken into account.
History.—The history of the Polyzoa, as far as 1856, has been fully treated by Allman in his great work on the Fresh-water Polyzoa;[511] but a few words may be said on this subject.
The Polyzoa attracted comparatively little attention before the beginning of the present century. Originally passed over as seaweeds, their real nature was established in connexion with the discovery of the animal nature of corals. So great a revolution could hardly be accepted without a struggle, and even Linnaeus went no further in this direction than to place them in a kind of half-way group of "zoophytes," whose nature was partly animal and partly vegetable. It is hardly necessary to point out that this view has now been abandoned by common consent; and indeed there is no more reason for regarding an animal as showing an approach to the plants because it grows in the external semblance of a seaweed than there would be for supposing a bee-orchid to be allied to the animal kingdom because of the form of its flowers.
But the claims of the Polyzoa to rank as a separate class were by no means admitted with the discovery that they were animals. They were still confounded with Hydroids, Alcyonarians, or Corals until their possession of a complete alimentary canal was recognised as a feature distinguishing them from those {475}animals. This was principally due to the observations of J. V. Thompson[512] in Ireland, who introduced the term Polyzoa; and of C. G. Ehrenberg[513] in Germany, who proposed the class-name Bryozoa, or moss-like animals.
It is impossible to avoid all mention of the controversy which has raged with regard to these two rival terms. The controversy is for the present at rest, the name Polyzoa being employed by the majority of English writers, amongst whom must be mentioned Allman, Busk, Hincks, and Norman, admittedly authorities of the first rank; while Bryozoa is employed by practically all the Continental writers.
The priority of Thompson's name is unquestioned. While Ehrenberg, however, definitely introduced Bryozoa as the name of a group, Thompson was less precise in this respect, although he states[514] that his discovery "must be the cause of extensive alterations and dismemberments in the class with which they [the Polyzoa] have hitherto been associated." Thompson, in fact, clearly understood that the Polyzoa could no longer rank with the Hydroids. The controversy has been summarised by Hincks, in his History of the British Marine Polyzoa,[515] where references to other papers on the same subject are given.
The Polyzoa were associated by H. Milne-Edwards with the Tunicata in the group Molluscoidea (Molluscoïdes[516]), to which the Brachiopoda were afterwards added by Huxley.[517] A knowledge of the development of the Tunicata has, however, shown that these animals must be withdrawn from any association with the other two groups; while there is little real evidence that even the Brachiopods have anything to do with the Polyzoa.
Classification.—The Polyzoa are divided into two sub-classes:—I, the Entoprocta; and II, the Ectoprocta.[518] Although the character referred to by these terms is merely the position of the anus with relation to the tentacles,[519] there can be no doubt that the two groups differ widely from one another in {476}many important respects. I do not, however, accept the view, maintained by some authors, that the Entoprocta and the Ectoprocta are two separate classes which are not nearly related.
The base from which the whole set of tentacles springs is known as the "lophophore."[520] In the Entoprocta (Fig. 236, 1) the lophophore is circular; the mouth is situated near the margin of the area surrounded by the tentacles; and the anus is found within the circlet, near the end opposite to the mouth.
In (2) and (3), representing the Ectoprocta, the anus is outside the series of tentacles. In the majority of cases, including all the marine Ectoprocta and one or two of the fresh-water forms, the lophophore is circular (2), the mouth occurring at the centre of the circle, and not being provided with a lip. These forms of Ectoprocta constitute the Order Gymnolaemata,[521] the dominant group of the Polyzoa in respect of number of genera and species. The remaining Ectoprocta belong to the exclusively fresh-water Order Phylactolaemata,[522] in which the mouth is protected by an overhanging lip or "epistome"; the ground-plan of the tentacles is, except in Fredericella, horse-shoe shaped (Fig. 236, 3), and the tentacles themselves are usually much more numerous than in the other cases.
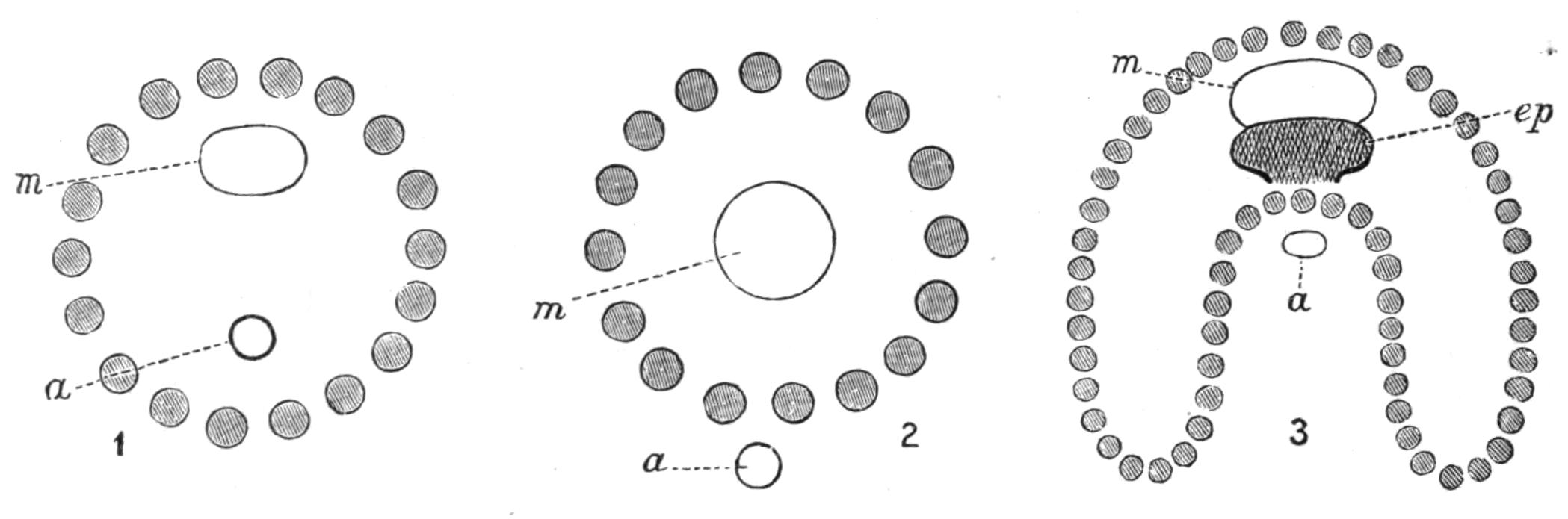
Fig. 236.—Ground-plan of the lophophore in (1) Entoprocta, (2) Gymnolaemata, (3) Phylactolaemata: a, anus; ep, epistome; m, mouth. The tentacles are represented by shaded circles.
The general characters of these divisions will be more easily understood by referring to the figures given of living representatives of the groups. The Entoprocta are illustrated by Figs. 243-245; the Gymnolaemata by Figs. 238, 240; and the Phylactolaemata by Figs. 247, 248.
The Gymnolaemata include three Sub-Orders:—
1. Cyclostomata.[523]—Body-wall densely calcareous, the zooecia being more or less tubular, usually with a circular orifice (Fig. 237).
2. Cheilostomata.[524]—Body-wall of varying consistency. The orifice is closed, in the retracted state of the polypide, by a chitinous lip or "operculum," which is more or less semicircular (Figs. 239, 241).
3. Ctenostomata.[525]—Body-wall always soft. The cavity into which the tentacles are retracted is closed by a frill-like membrane, the edges of whose folds have some resemblance to the teeth of a comb. This membrane, the "collar," is seen in different conditions of protrusion or retraction in Figs. 234, 238. The stomach may, in this group, be preceded by a muscular gizzard (Fig. 238, C, g).
Occurrence.—By far the larger number of the Polyzoa are inhabitants of the sea. A recently published catalogue[526] of marine Polyzoa includes nearly 1700 living species; and of these, the great majority belong to the Gymnolaemata. This group is further known to include an enormous number of fossil forms. Not only do we find that in living Polyzoa the members of a single Order largely outnumber the remainder of the Polyzoa, but we may further notice that the Cheilostomata, one of the sub-Orders of the dominant group, are at present largely in excess of the whole of the rest of the Polyzoa taken together.
Polyzoa may be collected with ease on almost any part of our coasts. The fronds of the "sea-mat" (Flustra foliacea) are thrown up by the waves in thousands in places where the bottom is shallow and sandy. The bases of the larger seaweeds growing on rocks between tide-marks are nearly always thickly covered with encrustations of Flustrella hispida or of species of Alcyonidium, in places where they are kept moist by being covered with a sufficiently thick layer of other algae. Rocks which are protected from the sun may be coated with calcareous Cheilostomes; and these are also found, in company with branching Polyzoa of various kinds, on the bases of the Laminaria thrown up by gales or exposed at spring tides. The graceful spirals of Bugula turbinata (Fig. 233, A) may be found hanging from the rocks at extreme low water; while colonies of Scrupocellaria, remarkable for their vibracula (see p. 484), are common in many places between tide-marks. Certain species affect the mouths of estuaries.
Membranipora membranacea commonly covers many square inches of the frond of Laminaria with its delicate lace-like encrustation. Nitsche[527] has shown that this species has its calcareous matter deposited in plates, separated by intervals of uncalcified ectocyst. The effect of this arrangement is to make the colony flexible, and to enable it to adapt its shape to the movements of the Laminaria, which is swayed to and fro by the action of the waves. Many of the calcareous forms growing on Laminaria have no special arrangement of this kind, and they accordingly grow in colonies whose area is so small that the greatest movements to which the seaweed is liable are not sufficient to crack or break the colony.
Many species show a decided, or even exclusive, preference for particular situations; as, for instance, species of Triticella, which are only found on certain Crustacea. Many encrusting forms prefer the inside of dead shells of Pecten, Cyprina, etc., to any other habitat. Terebripora[528] excavates tubular cavities in the substance of the shells of Molluscs. Hypophorella[529] inhabits passages which it forms in the walls of the tubes of the Polychaets, Lanice and Chaetopterus. Lepralia foliacea, one of the Cheilostomata, forms masses which may reach a circumference of several feet, simulating a small coral-reef. Its contorted plates are a regular museum of Polyzoa, so numerous are the species which delight to find shelter in the quiet interstices of the colony. The exquisite little colonies of Crisia eburnea are commonly found on red seaweeds, or on the branches of the Hydroid Sertularia.
The Polyzoa are found at all depths, certain Cheilostomes having been recorded from 3000 fathoms. The Cyclostomes dredged by the "Challenger" were all found in depths of 1600 fathoms or less, while the Ctenostomes are a distinctly shallow water group, most having been found at less than 40 fathoms, and only three at so great a depth as 150 fathoms.[530]
A few forms (Membranipora pilosa, Scrupocellaria reptans, etc.) are known to be phosphorescent;[531] but it is not known what is the purpose of this phenomenon.
External Form.—The Polyzoa may be roughly divided into (1) encrusting forms, usually calcareous, but sometimes soft; and (2) erect forms, which are either rigid or flexible. This flexibility can coexist with a highly calcified ectocyst, as in Crisia (Fig. 237), Cellaria, and others in which the branches are interrupted at intervals by chitinous joints. The coral-like forms may assume the most exquisite shapes, pre-eminent among which are the lovely net-like colonies of Retepora. Polyzoa of this type are seldom found between tide-marks, where their brittle branches would be liable to be snapped off by the waves. The erect species which occur in such positions are flexible, although flexible species are by no means restricted to the zone between tide-marks.
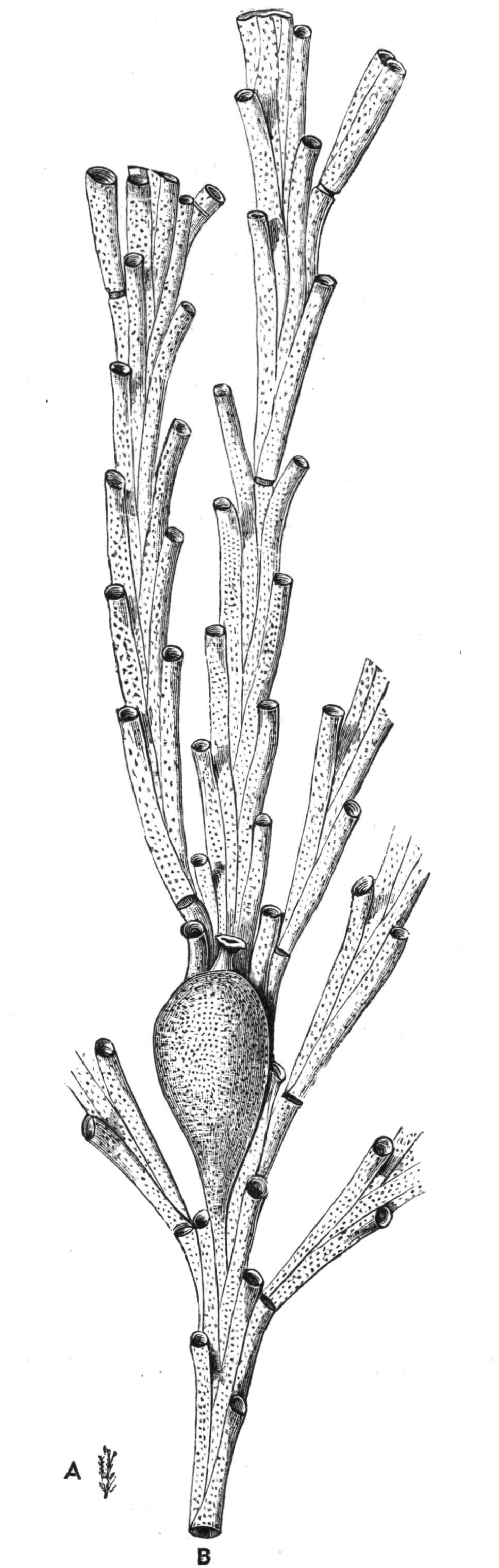
Fig. 237.—Crisia ramosa Harmer, Plymouth. A, End of a branch, × 1; B, another branch, × 20, showing the chitinous joints, the tubular zooecia characteristic of Cyclostomata, and the pear-shaped ovicell with a funnel-shaped orifice at its upper end.
Although the form of the colony is very different in different Polyzoa, a pocket-lens will usually show whether a given specimen belongs to the group or not. The surface is nearly always more or less distinctly composed of zooecia, or at least shows their orifices. The entire colony may be built up of these zooecia; and this is by far the commonest arrangement, both in encrusting and in erect forms. In certain genera, however, and particularly in some Ctenostomes (Fig. 238), and in most of the Entoprocta, the {480}individuals grow out at intervals from a cylindrical stem or "stolon" (st), which is not composed of zooecia.
The Cyclostomata may assume an erect or encrusting habit. Their zooecia are always more or less cylindrical; the upper ends being often completely free, although in many cases the whole zooecium is closely adnate to its neighbours. In the breeding season the forms which belong to this group are provided with curious "ovicells," which contain the embryos. These may either be pear-shaped swellings on the branches (Crisia, Fig. 237), or they may form inflations of the surface, between the zooecia. The mature ovicell is provided with one or more openings, through which the larvae escape.
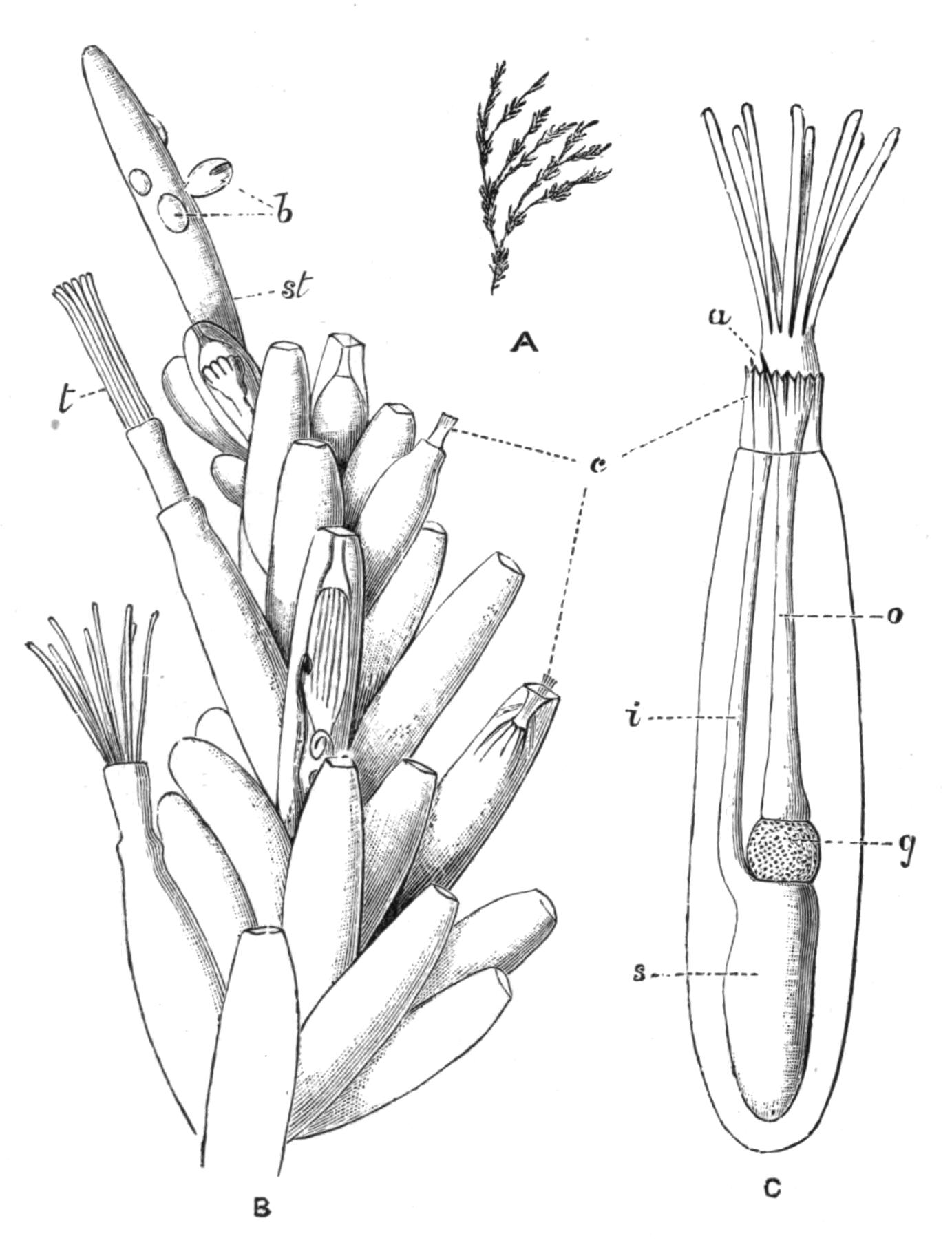
Fig. 238.—Bowerbankia pustulosa Ell. and Sol., Plymouth. A, Fragment of a colony, natural size, showing the branching stem, bearing tufts of zooecia: B, one of these tufts, with the growing apex of the stem (st), × 27; b, young zooecia (buds); c, the "collar" characteristic of Ctenostomata; t, tentacles; C, a single zooecium, with expanded tentacles, more highly magnified; a, anus; c, collar; g, gizzard; i, intestine; o, oesophagus; s, stomach.
The Ctenostomata rarely have even the slightest trace of calcareous matter. Alcyonidium and its allies form soft encrustations, or may even grow into erect masses six inches or more in height (A. gelatinosum). In this type the zooecia are often so closely united that it may be difficult or impossible to make out their limits in the living colony. Many of the dendritic or branching {481}Ctenostomes (Fig. 238) are characterised by an extreme delicacy of habit. The zooecia in these cases are sharply marked off from the stem. They are either cylindrical or ovoid, being commonly attached by a very narrow base, so that in some species they readily fall off, and may thus be completely absent in certain parts of the colony. In such forms as Vesicularia spinosa, it requires considerable experience to recognise a stem which has lost its zooecia as being part of a Polyzoon. In Mimosella the zooecia possess a remarkable power of movement on the stem, similar to that possessed by the leaflets of the Sensitive Plant.[532] In certain forms (Bowerbankia, Amathia) the zooecia occur in groups separated by intervals which are devoid of zooecia, but in other cases they may have a more irregular arrangement. The collar to which this group owes its name is by no means a conspicuous feature. Its position when retracted has been shown in Fig. 234, while Fig. 238 further illustrates its relations.
The Cheilostomata grow in a great variety of forms, and also show a wide range of character in their zooecia. The orifice is commonly surrounded by stiff spines (Fig. 257, p. 524), which perhaps have the function of protecting the delicate polypides from the sudden impact of foreign bodies. These spines may attain an enormous development, as in Bicellaria ciliata, and some forms of Electra (Membranipora) pilosa (Fig. 256, A).
The operculum is usually, though by no means always, a conspicuous feature of the Cheilostome zooecium. It is invariably of chitinous consistency, and is more or less semicircular in outline, the straight edge forming a hinge on which the operculum opens. In some cases the orifice is surrounded by a raised margin or "peristome" (Fig. 255, B, C); the operculum is then situated at the bottom of a depression of the surface, and may be concealed from view. In others, in which the front wall of the zooecium is membranous (Bugula, Fig. 233), the operculum is merely a part of this membrane, and so is quite inconspicuous; and in cases of this kind the membranous wall may be protected by an arched spine, the "fornix," developed from one side of the zooecium (Fig. 254, f). The ovicells are commonly a conspicuous feature of this group, although they are believed to differ fundamentally from those of Cyclostomata. They have the form of a helmet-like covering overhanging the orifice (Figs. 240, 241), {482}and may be either prominent or more or less concealed by the growth of adjacent parts of the zooecia. The presence of ovicells of this description is perfectly distinctive of the Cheilostomata.
Avicularia and Vibracula.—Most singular of the external appendages of the Cheilostomata are the extraordinary "avicularia" and "vibracula" of some genera.[533] By the comparison of a carefully selected series of genera, it has been established that the avicularium is a special modification of a zooecium. One of its least modified forms is found in Flustra foliacea (Fig. 232), where the avicularia (a) are small zooecia with a conspicuously large operculum ("mandible"). Avicularia of a similar type occur in Cellaria (Fig. 239, A), Schizotheca, etc., the avicularium occupying the place of an ordinary zooecium. These are the "vicarious" avicularia of Mr. Busk.[534]
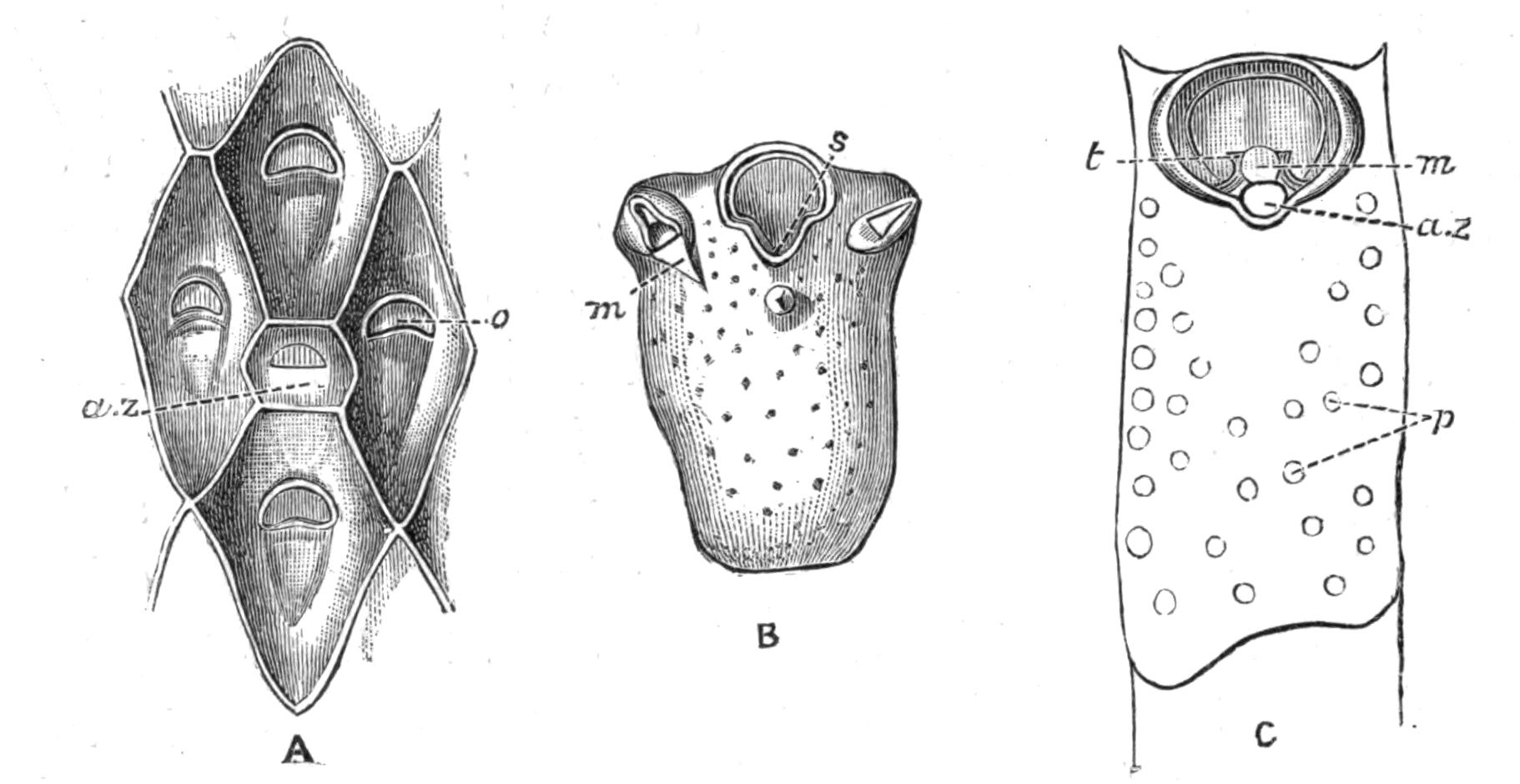
Fig. 239.—Forms of avicularia. A, Cellaria fistulosa L., Plymouth, × 43; a.z, avicularian zooecium, with closed mandible; o, operculum of zooecium: B, Schizoporella unicornis Johnst., Scilly Is., × 43; zooecium bearing two avicularia; m, opened mandible of avicularium; s, sinus of orifice: C, zooecium of Smittia landsborovii Johnst., Plymouth, × 43; the operculum is seen at the bottom of a depression surrounded by a thin collar or "peristome," in an emargination of which is seen an avicularian zooecium (a.z); m, mandible (opened); p, pores; t, tooth.
In the next stage (Figs. 239, B, 256, B) the avicularian zooecium is further reduced; it has in most cases lost its place in the series of individuals, and is found instead seated on some part of an ordinary zooecium ("adventitious" avicularia). The avicularium now consists of a much reduced zooecium, bearing the well-developed operculum or mandible.
Having arrived at this point, the avicularia seem to lose all sense of the propriety of remaining in the positions once occupied by zooecia. They have become degraded to the rank of appendages of the zooecia, and as such they may occur in an astonishing variety of positions. Sometimes one occurs on each zooecium in the middle line, or asymmetrically, or even on the top of the ovicell; in other cases the orifice is flanked by an avicularium on each side (Fig. 239, B). Sometimes (Cellepora) the avicularia are of more than one kind, some being large and some small, some having a pointed mandible and others a mandible with a rounded spoon-like end.
In the cases so far considered, the body of the avicularium is fixed. The highest differentiation acquired by these structures occurs in cases like Bugula, where they are borne on flexible stalks, which may even exceed the avicularia in length.[535]
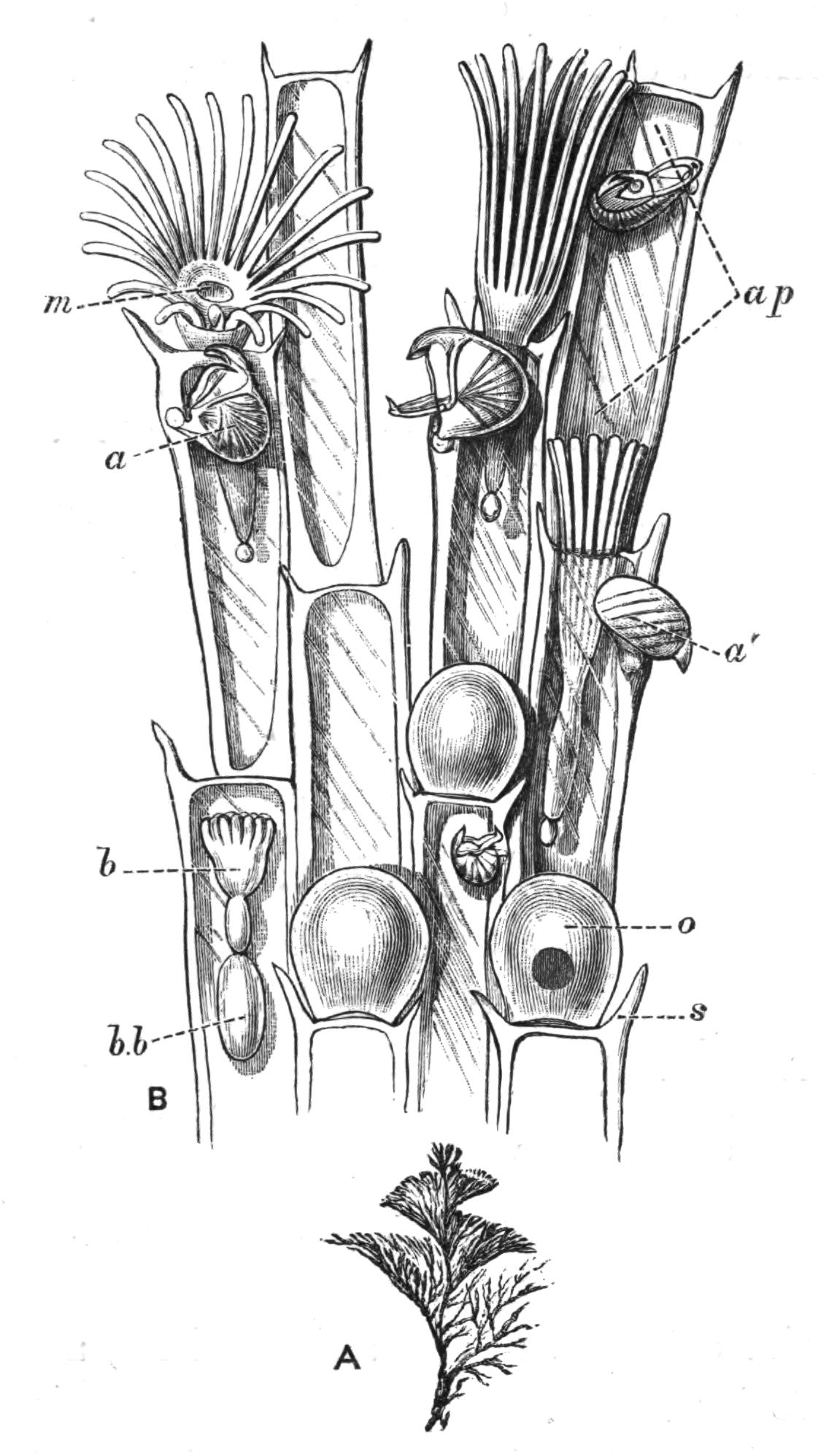
Fig. 240.—Bugula turbinata, showing avicularia (a, a'). The figure is explained on p. 468.
In Bugula turbinata (Fig. 240) each zooecium is provided with one of these appendages, attached to the base of the outer of the two spines which border its orifice. The avicularia of the two edges of the flattened branch are much larger than those of the more internal zooecia. The upper jaw is strengthened by a kind of buttress, or thickening of the ectocyst, which passes on each side across the avicularium to the hinge-line of its mandible. The upper part of the beak is strongly hooked, while the tip of the mandible bears a {484}prominent spike, which fits inside the upper beak when the jaw snaps. A great part of the head is filled with a strong muscle, whose fibres exhibit a distinct transverse striation, and converge into a median tendon. The latter is inserted into the middle of the mandible. The muscle serves to close the jaws, and is the representative of the muscles by which the operculum is closed in an ordinary zooecium. The lower jaw is opened by means of a pair of muscles which are situated immediately under the ectocyst of the avicularium, and pass into the mandible close to its hinge.
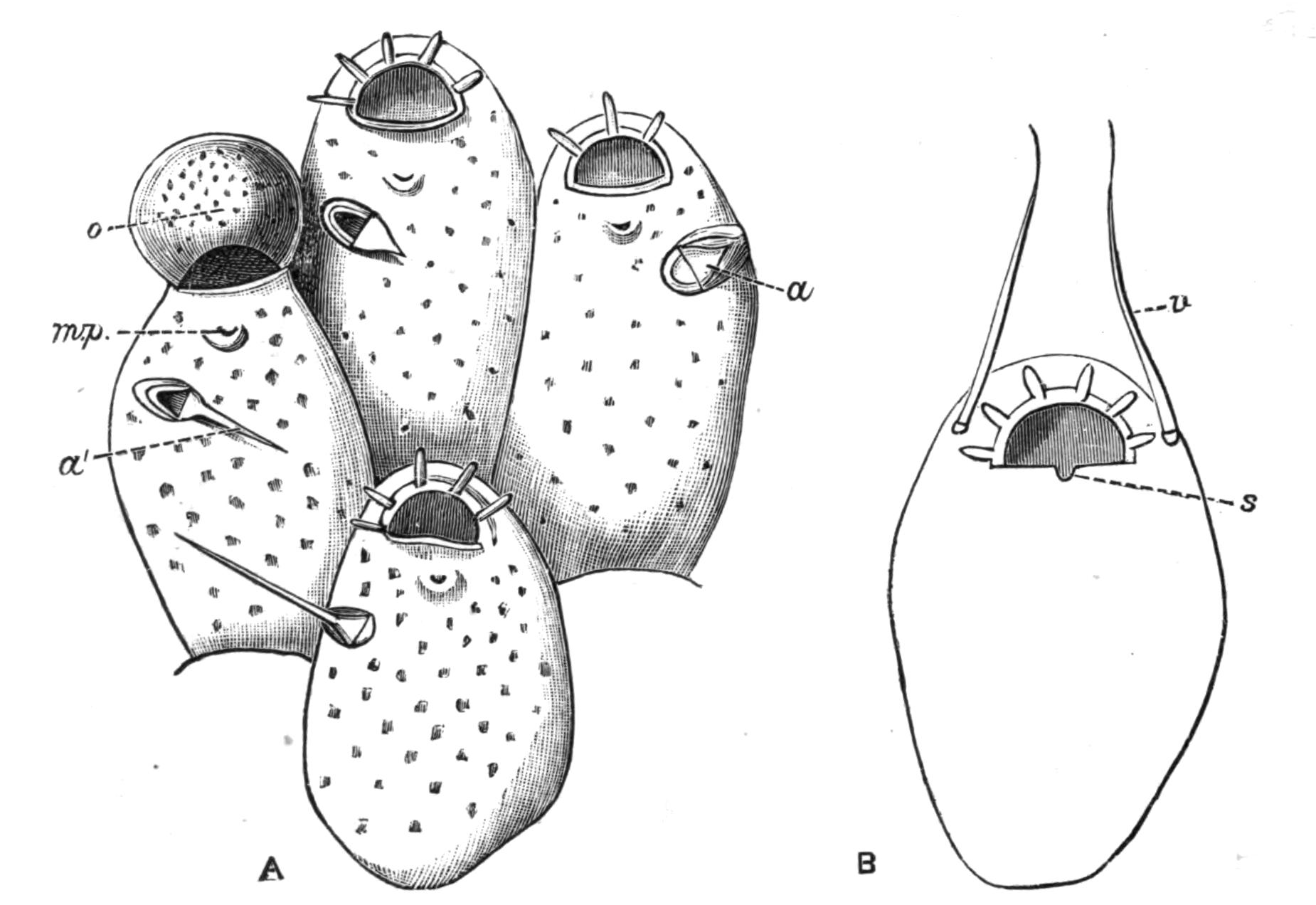
Fig. 241.—Illustrating the transition from avicularia to vibracula. A, Microporella ciliata Pall., Scilly Is., × 62; a, avicularium with short mandible (closed); a', avicularium with vibraculoid mandible (open); m.p, median pore; o, ovicell: B, Mastigophora dutertrei Aud., Shetland Is., × 47; s, sinus of orifice; v, seta of vibraculum (or vibraculoid avicularium).
Within the jaws, in the region which we may term the palate, is a rounded knob, which bears a tuft of delicate sensory hairs, which doubtless enable the avicularium to recognise the presence of any foreign body. The closure of the mouth may, indeed, be instantaneously induced by touching it with the point of a needle. It has been suggested that a small mass of cells which bears these hairs may represent the rudiment of the polypide.
The "vibraculum" (Fig. 242) is regarded as an avicularium in which the mandible has become elongated, so as to form a {485}thin, chitinous "seta," which from time to time moves through the water. The part of the vibraculum which represents the zooecium commonly bears a tubular rootlet, used for attaching the colony to the substance on which it is growing (Fig. 254, p. 517).
In Microporella ciliata (Fig. 241, A) the avicularia are very variable, and in some cases take on a "vibraculoid" character. But in the fully-developed vibraculum (Fig. 242) there is usually no such compromise of characters. It may, however, be noted that Scrupocellaria scabra (Fig. 254), which belongs to a genus characterised by its highly differentiated vibracula, possesses structures (v.z) which could hardly be distinguished from avicularia were it not for the presence of the rootlet (r).
In the course of some observations which I had the opportunity of making on Bugula calathus at Naples, a fine hair offered to a small colony was seized with such force by the avicularia that the entire colony was lifted out of the water by the hair. The same colony had captured (1) a small Nereis, which it held with several of its avicularia; (2) an Anisopod Crustacean, 2½ mm. long; and (3) a small Amphipod, which was held by one of its antennae. The Anisopod was held by the tip of one leg with one avicularium, and by the penultimate joint of one of its chelae with an avicularium of another branch. It was captured in such a way that its chela, the "hand" of which was about half as long as the avicularium, actually closed on to the avicularium without being able to effect its escape. A little later the other chela was caught by another avicularium. Curiously enough, however, an avicularium did not necessarily close even when part of a captured animal was actually in its mouth. The avicularia made no attempt to place themselves in an advantageous position for catching fresh parts of the Nereis, which they might easily have done. The avicularia which had captured prey remained motionless. The others moved backwards and forwards (cf. the various positions of the avicularia shown in Fig. 240) ten times in ¾ to 1 minute, snapping their jaws perhaps once in that time. The two Crustacea were still retained by the avicularia two days later. On the next day they had both disappeared; but the colony had again caught the Nereis, which had previously effected its escape with the loss of nearly all its tentacular cirri.
These observations, and others which have been recorded, do not, unfortunately, give any information as to the purpose of the {486}movements of the avicularia and vibracula. It is obvious that they may be defensive in character; and it cannot be doubted that the avicularia can prevent inquisitive worms from straying at will over the surface of the colony. There is no evidence to show that animals are discouraged from interfering with a Bugula owing to the presence of its defensive weapons.
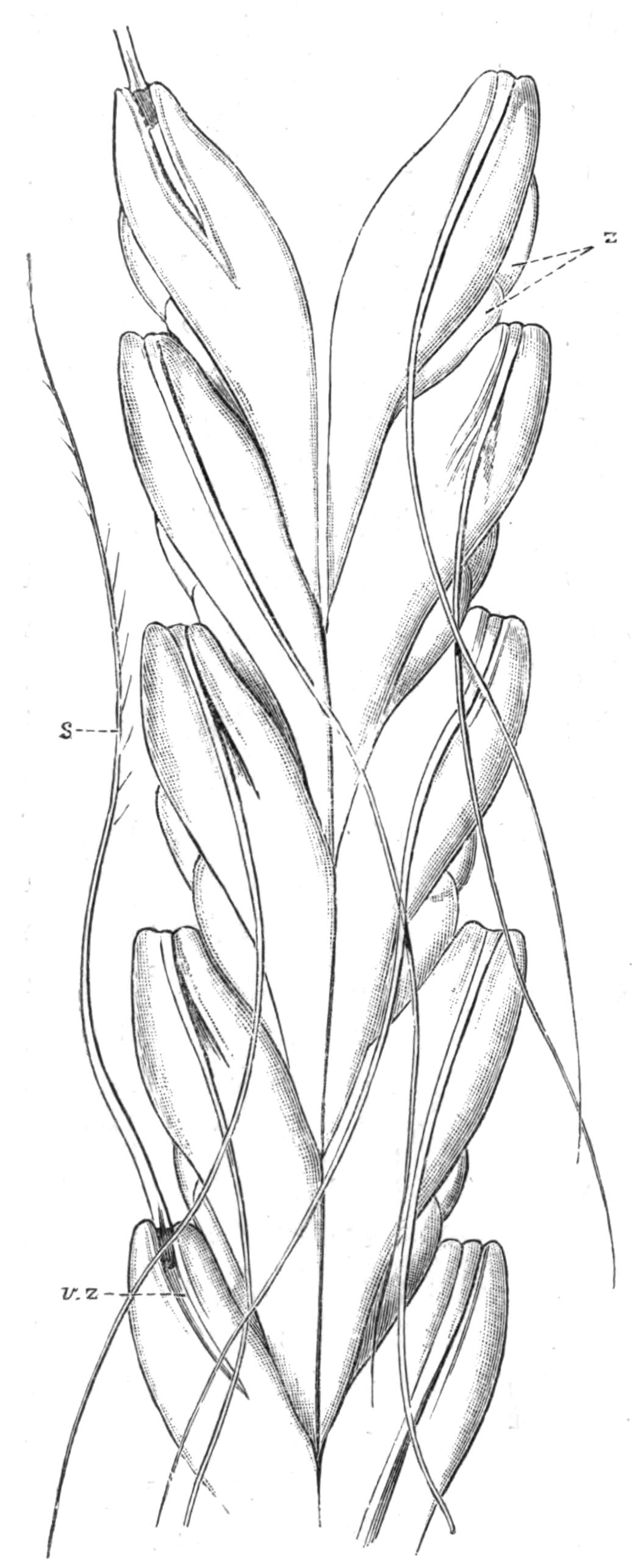
Fig. 242.—Caberea ellisii Flem., Norway. × 40. Back view of part of a branch. The large vibracular zooecia (v.z) occupy nearly the whole of the surface. s, Seta of vibraculum; z, zooecia.
It is not, indeed, certain what are the enemies against which the Polyzoa have specially to guard. Sea-urchins and certain Molluscs are known to browse on Polyzoa. Fresh-water Polyzoa, in which avicularia and vibracula are absent, are attacked by the larvae of Insects, and by Triclad Planarians. I have found the latter with their long pharynx everted and completely buried in a Cristatella colony. It is possible that some marine Cheilostomes may be saved from attacks of this kind owing to the existence of their armoury of avicularia and vibracula. It is also possible that these structures are of service by removing foreign particles which might otherwise settle on the colony, and tend to block up its orifices. It has further been suggested that animals seized by the avicularia may be held until they die, and that their disintegrating particles may then be carried to the mouths of the polypides by the ciliary currents of the tentacles; but proofs of this suggestion are {487}wanting, and it must be admitted that the subject needs further elucidation.
The vibracula ordinarily remain stationary for some little time, every now and then giving a sweep through the water. In the majority of cases these structures, like the avicularia, act perfectly independently of one another, so far as can be made out; but in Caberea (Fig. 242) the vibracula move in unison, the simultaneous action of the whole series, after a period of quiet, being described as "positively startling."[536]
It has been stated by Busk[537] that the entire colony in Selenaria and Lunulites may be moved from place to place by the large vibracula which these forms possess.
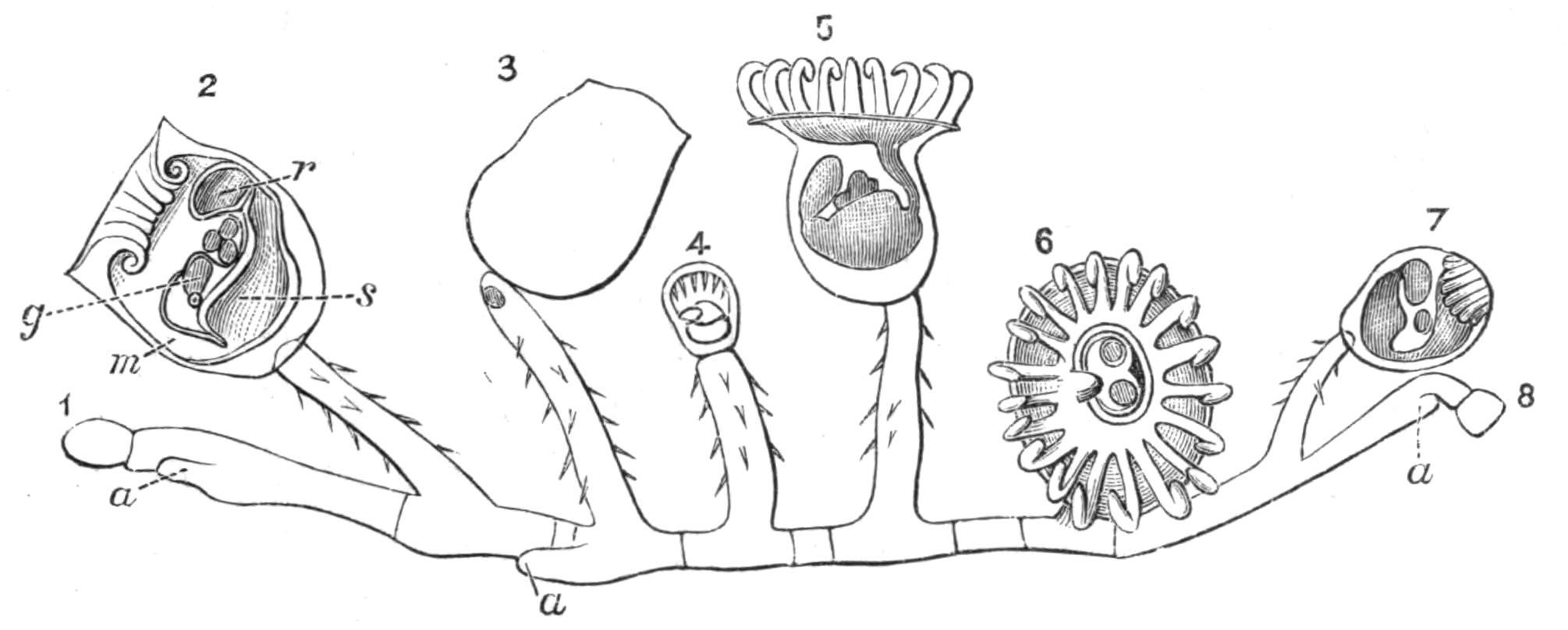
Fig. 243.—Pedicellina cernua Pall., Guernsey. Entire colony. × 27. The colony has three growing ends, a; 1-8, individuals of colony; 1 and 8 are quite immature; and 7 (tentacles retracted) is still young; 2, is seen in longitudinal section; g, generative organ, and below it the ganglion; m, mouth; r, rectum; s, stomach; between g and r are three embryos in the brood-pouch; the tentacles are retracted; in 5 and 6 the tentacles are expanded; in 6 two embryos are seen within the circle of the tentacles, to the left of them is the rectum, and to the right the mouth; 3 is in the act of losing its calyx, and has already developed the beginning of a new polypide-bud; in 4 the primary calyx has been lost, and the new calyx is clearly marked off from the stalk.
Entoprocta.—The Entoprocta, although a very small sub-class, deserve special consideration, if for no other reason, from the fact that many writers regard them as the most primitive group of Polyzoa, and consequently as the forms which show most affinity to other classes of animals.
Their most obvious characteristic is, as we have already seen,[538] the position of the anus within the circle of tentacles. The individuals formed by budding always remain more separate from one another than those of most Ectoprocta.
The commonest Entoproctous genus is Pedicellina, a graceful little animal, which occurs on many parts of our coast. It may often be discovered by looking carefully on the pink, jointed, calcareous alga, Corallina, which may be found growing at the edges of deep and cool rock-pools not too far above low-water mark. Its creeping stem or "stolon" is firmly attached to the surface of the seaweed, and sends off vertical stems here and there.[539] Each stem bears a "calyx," which is practically an individual of the colony. The stolon terminates, at one or both ends, in a growing-point (a), from which new individuals are budded off. The stalks bend from time to time in a curious spasmodic manner, by which means the calyces are moved about with an irritable and angry air. A good idea of the way in which the tentacles are folded away when the animal is disturbed may be obtained by putting the two wrists together, with the fingers spread out to represent the tentacles, the retraction of which would be represented by turning the tips of the fingers down into the space, the "vestibule," between the two palms. A delicate fold of skin growing from the edge of the calyx closes over the retracted tentacles, owing to the contraction of a sphincter muscle present in its circular edge. The body-wall is not separated from the alimentary canal by a definite body-cavity, so that there is no obvious distinction between the polypide and the zooecium. The existence of the Entoprocta is in fact a strong reason for refusing to admit that these two terms correspond with two different kinds of individuals.
Let us now imagine the condition we should have if a large and continuous cavity were developed between the alimentary canal and the body-wall. The body-wall would clearly have the general relations of a zooecium, while the alimentary canal and tentacles would obviously correspond with the polypide. The existence of the body-cavity would make it possible for the animal to retract its tentacles instead of merely turning them in. Regarded in this way, there is but little difficulty in comparing the Ectoprocta with the Entoprocta.
The calyces are deciduous, i.e. they are lost from time to time, the end of the stalk then producing a polypide-bud, which {489}forms the vestibule and alimentary canal of a new calyx. Hence the phenomenon which may so commonly be noticed in Pedicellina of a "young head on old shoulders." The loss of the calyces may have some relation to the formation of the "brown bodies" in the Ectoprocta.
Another Entoproct, Loxosoma (Fig. 245) is remarkable for being the only Polyzoon which is not colonial. The buds, which are formed in two lateral series, break off as soon as they are mature, and at once begin to lead an independent existence. Loxosoma is further remarkable for being almost invariably found commensally with other animals, where it may occur in enormous numbers. L. phascolosomatum, common in the Channel Islands, is only found on the tip of the tail of Phascolosoma (see p. 428), which inhabits the mud of Zostera-beds. Other species are found on the external surface of certain sponges (Tethya, Euspongia, Cacospongia); or on the outside of a compound Ascidian, Leptoclinum, which may itself be carried about as a detachable covering on the back of a crab (Dromia). Another species is found on the ventral surface of the Polychaet Aphrodite, and of its ally Hermione.
L. annelidicola, an interesting species recently investigated by Prouho,[540] was originally described in 1863 as a Trematode, under the name of Cyclatella. It escaped further notice until it was again found in the neighbourhood of Roscoff, in Brittany, on certain Polychaets belonging to the family Maldanidae (see p. 332). The calyx has a very flattened form, and is borne on a short stalk, which terminates in a large attaching disc, formerly mistaken for the sucker of a Trematode. The features in which this species differs from other members of the genus are shown by M. Prouho to be correlated with its mode of life. The animal has the habit of lying flat on its back, the disc at the end of its stalk being firmly attached to the skin of the worm, and its short stalk being bent round into a curve so {490}as to bring the calyx into a supine position, with its lophophore directed upwards. This habit, together with its flattened form, prevents it from being crushed between the worm and its tube. But without some further provision its position might be merely a source of danger. For supposing the calyx to be directed backwards in relation to the worm, a sudden backward movement of the latter into its tube might bring the Loxosoma into fatal contact with the inner surface of the tube. There would obviously not be sufficient room to turn round in a vertical plane, so as to bring the body into a position of safety, i.e. into a position in which it moves stalk first. But by a beautiful arrangement of the muscles of its stalk this movement is effected in a horizontal plane; on touching the Loxosoma with the point of a needle it would swing round in this way through 180° with "une rapidité qui étonne."
Urnatella[541] is a beautiful form with a segmented stalk, the stalks usually arising in pairs from a common base. It has at present only been found in fresh water in the United States.
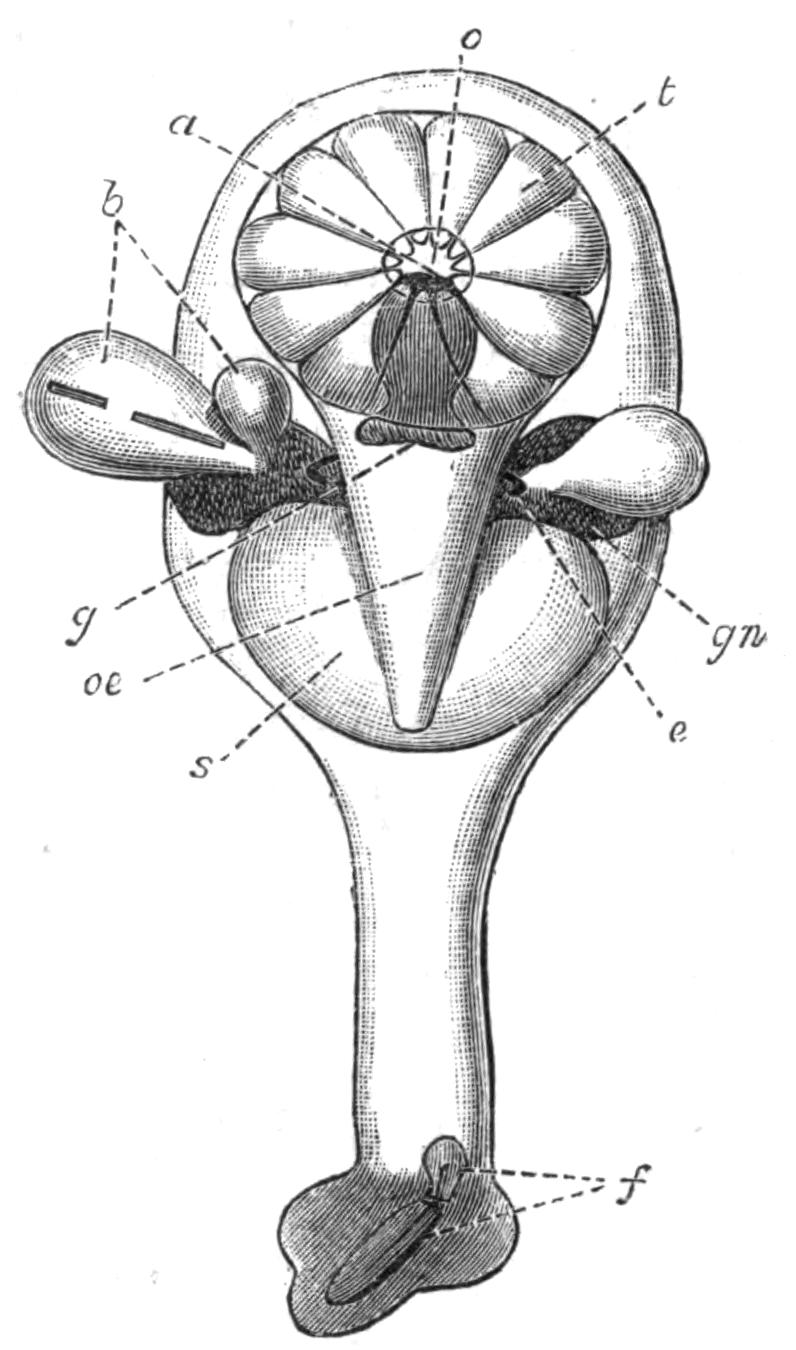
Fig. 245.—Diagram of the structure of Loxosoma, seen from the oesophageal side. × about 70. a, Anus; b, buds; e, excretory organ; f, foot-gland; g, ganglion; gn, generative organs; o, orifice of vestibule; oe, oesophagus; s, stomach; t, retracted tentacles.
In Pedicellina the plane of the lophophore is at right angles to the stalk, which is separated from its calyx by a marked constriction. In Loxosoma the lophophore is set obliquely,[542] and there is no constriction at the base of the calyx. In Urnatella we find an intermediate condition, the lophophore resembling that of Loxosoma, while the constriction at the base of the calyx is similar to that of Pedicellina. Since the latter is known to pass in its development[543] through a stage with an oblique lophophore, it may be presumed that Loxosoma is a more archaic form than Pedicellina. In other respects, the structure of the Entoprocta is very constant, whatever the genus.
A pair of ciliated excretory tubes open into the vestibule. These are similar in structure to the "head-kidneys" of the larvae of Polychaet worms, or to the excretory organs of adult Rotifers. Flame-cells have been described by Davenport in the stalk of Urnatella, but it is not known whether they are connected with the excretory tubes of the calyx. The animals are either hermaphrodite or have separate sexes, and the generative organs open by ducts of their own into the vestibule. The nervous system consists of a ganglion placed between the mouth and the anus, giving off a set of nerves, many of which end in delicate tactile hairs placed on the tentacles or other parts of the body.[544]
POLYZOA (continued)
FRESH-WATER POLYZOA—PHYLACTOLAEMATA—OCCURRENCE—STRUCTURE OF CRISTATELLA—DIVISION OF COLONY—MOVEMENTS OF COLONY—RETRACTION AND PROTRUSION OF POLYPIDES IN POLYZOA—STATOBLASTS—TABLE FOR DETERMINATION OF GENERA OF FRESH-WATER POLYZOA—REPRODUCTIVE PROCESSES OF POLYZOA—DEVELOPMENT—AFFINITIES—METAMORPHOSIS—BUDDING.
Fresh-water Polyzoa.—Although the Gymnolaemata are ordinarily marine animals, fresh-water examples from this Order are not altogether wanting. The Ctenostomata among the typically marine groups show the most tendency to stray into fresh-water.
Alcyonidium and Bowerbankia (Fig. 238) flourish in estuaries, while Victorella and Paludicella (Fig. 250) are only known as fresh or brackish water forms. Victorella was named after the Victoria Docks in London, where it was first found; more recently it has also been discovered in other parts of England and on the Continent.[545]
The systematic position of the genera Hislopia and Norodonia,[546] which have been described from fresh water of India and China respectively, is at present uncertain. The undoubted Cheilostome Membranipora has, however, a British representative (M. monostachys), which occurs in brackish water, in ditches on the coast of East Anglia. It is there known to form "friable, irregularly-shaped, sponge-like masses," which grow on water-plants.[547]
The Entoprocta, as we have seen, are represented in fresh water by the genus Urnatella.
The Phylactolaemata are an exclusively fresh-water group, and they are believed by Kraepelin[548] to have been derived from the Ctenostomata. Many of their special peculiarities can, with great probability, be regarded as adaptations to a fresh-water existence. This is particularly clear in the all but universal habit of dying down in the winter, and in the occurrence of the so-called statoblasts (Fig. 251), which are hard-shelled reproductive bodies, absolutely restricted to the Phylactolaemata, and capable of resisting the winter's cold and even a certain amount of drying up. Phylactolaemata have indeed been recorded from the tropics; but it is not yet sufficiently clear how they there behave in these respects. F. Müller[549] has found these animals in Brazil, where they are said to be more common at certain periods of the year than at others. Stuhlmann has found them in Tropical Africa (Victoria Nyanza, etc.);[550] and Meissner[551] has discovered the sessile statoblasts of Plumatella on the shells preserved in the Berlin Museum, of species of the Mollusc Aetheria from various localities in Africa. Fresh-water representatives of a considerable number of other groups of animals agree with the Phylactolaemata in the possession of reproductive bodies which are protected by hard coats. Such, for instance, are the ephippian ova of Daphnia—bodies which have an extraordinary external similarity to statoblasts—the gemmules of Spongillidae, the winter-eggs of Rhabdocoels and Rotifers, and the cysts of Protozoa. The evolution of these bodies in so many widely different cases may have been due to the selection of variations calculated to minimise the dangers attendant on the drying up of the water in summer, or on its freezing in winter.
The Phylactolaemata are by no means uncommon, although they can seldom be found without a careful search. Their presence may often be detected by taking advantage of the property of the free statoblasts of rising to the top of the water, where they can be discovered by skimming the surface with a fine hand-net.
The colonies themselves are usually found attached to water-plants, roots of trees or stones. Most of them flourish best in {494}a zone not more than two feet below the surface. Certain species show a preference for floating leaves, such as those of water-lilies, where they are not liable to be dried up by alterations in the level of the water. Some forms (e.g. Plumatella, Fig. 246) are, however, able to withstand being dried for some time. Most species prefer shady places, and accordingly settle on the lower sides of leaves and sticks. Others (e.g. Cristatella, Fig. 247) have no objection to the direct rays of the sun. Most forms prefer still water, but one or two are found in running water.
Fredericella is a common constituent of the deep-water fauna of Swiss Lakes (down to over forty fathoms); and reaches there a size considerably larger than the shallow-water form of the same species. Paludicella is common at thirteen fathoms. These two genera, with Plumatella, have been found in absolute darkness, under a pressure of 2½-5½ atmospheres, in the Hamburg aqueduct. The Polyzoa and other organisms growing in the water-supply of Hamburg were accused of being concerned in the spreading of cholera, during the recent epidemic, by choking up the water-pipes, and creating obstructions which formed a favourable nidus for the development of cholera-germs.
The colony may take the form of a series of delicate, branching tubes (Plumatella, Fredericella), of more massive aggregations of parallel tubes (as in the Alcyonelloid forms of Plumatella), or of gelatinous masses of varying size (Lophopus, Cristatella).
Fig. 246.—A, Plumatella (Alcyonella) fungosa Pall., Naples (fresh water), small part of a mass, natural size; B, Plumatella repens L., R. Yare, on the leaf of a water-lily, natural size.
Cristatella mucedo (Fig. 247) is remarkable for its power of moving from place to place; it consists of an elongated mass of greenish, gelatinous substance, which, in its fully developed state, may reach a length of eight inches or more, with a transverse diameter of three-eighths of an inch. It has a flattened sole on which it crawls, while the graceful plumes of its numerous polypides protrude as a delicate fringe from its upper side.
The tentacles are about eighty to ninety in number, and they are, as in other Phylactolaemata, united at their bases by a delicate web. The lophophore is horse-shoe-shaped (Fig. 236, 3) throughout the group, with the exception of Fredericella, in which genus it is circular.
In some Phylactolaemata the polypide has been observed to interlace its tentacles, so that the plume becomes a kind of cage, in which the more active Infusoria are imprisoned until their struggles have so far weakened them that they are swept into the mouth by the action of the cilia of the tentacles.[552]
Around the edge of the Cristatella is found a zone of budding tissue, which gives rise continuously to new individuals. Now, whereas in Gymnolaemata the growing edge gives rise to zooecia, whose cavities become completely cut off from that of the older ones; in Phylactolaemata the partitions between the zooecia are never completed. The body-cavity of Cristatella is thus a continuous space, interrupted at the margin only by vertical septa (see Fig. 247), which represent the partitions between the zooecia of other forms.
The body-wall consists of two epithelial layers of ectoderm and mesoderm, between which is a layer of muscular fibres. {496}Parts of the epithelium lining the body-cavity are ciliated. Into the common body-cavity hang the polypide-buds at the edge of the colony, and the mature polypides in the more central regions. There are usually three rows of polypides on either side of the middle line, in the neighbourhood of which is an area devoid of polypides, but containing "brown bodies" and statoblasts. The polypides nearest to the middle line pass in succession into the condition of "brown bodies," while young buds near the margin grow up coincidently to form new polypides.
The movement of the colony is in the direction of the long axis, although either end may go first. Sir John Dalyell records an observation[553] on a specimen (about one inch long) which was artificially divided into two halves. The two halves "receded from each other as if by common consent," and were nearly an inch apart in twenty hours.
An observation made at Cambridge on a small colony of about 7 mm. in greatest length gave the following results. The colony moved 13 mm. (nearly twice its own length) in 8¼ hours: in the next 40 hours it moved 20 mm. (⅘ inch); while in the following 24 hours it moved only 6 mm. Large colonies change their place only with reluctance.
The locomotive power possessed by Cristatella is not unique among Phylactolaemata. Lophopus, the first fresh-water Polyzoon of which any description was published, was originally described by Trembley in 1744 under the name of the "Polype à pannache." Trembley observed the spontaneous division of the colony, followed by the gradual separation from one another of the daughter-colonies.[554] The power of dividing spontaneously is also possessed by colonies of Cristatella and of Pectinatella.
The colonies of Lophopus are surrounded by an excessively hyaline ectocyst, and are usually triangular, as shown by Fig. 248. When division is about to occur, the base of the triangle becomes indented, and the indentation travels towards the apex in such a way as to bisect the triangle. The two halves diverge from one another during the process, so that before division is complete, they are looking, in some cases, in opposite directions. {497}After a time the narrow connection breaks, and two new colonies are formed.
Fig. 248 shows a colony shortly after division has taken place. The colony had moved forwards, in a direction away from its apex, for three days in a nearly straight line, the distances moved in each day being respectively 6, 8½, 8½ mm. These observations, for which I am indebted to Mr. Lister, show a considerably higher speed than in those recorded by Trembley, who observed no colony which moved more than half an inch (12.5 mm.) in eight days.
The genus Pectinatella also has some power of locomotion. This magnificent Polyzoon occurs in masses several feet in length (as much as six feet in P. gelatinosa from Japan[555]), and four to eight inches in thickness. The greater part of P. magnifica[556] consists of a thick, opaline, and gelatinous ectocyst, the upper surface of which is covered by hundreds of rosette-like colonies, which increase in number by division. The masses are thus aggregations of colonies, which secrete a common basal ectocyst. The latter decays in the autumn; and the separate rosettes, or groups of them, may thus be set free, being found as floating masses, which may again attach themselves to a solid object till the time of their death. Pectinatella has not yet been recorded in England, although, considering the ease with which statoblasts are transported, it is by no means improbable that it will eventually be recorded as a British genus. It is at present known to inhabit America, Japan, and Hamburg.
Fig. 248.—Lophopus crystallinus Pall., Cambridge, showing the rate of movement. The colony and the distances moved are × 2.
It is by no means certain what is the mechanism by which {498}movement takes place in the above cases. The ectocyst of Cristatella is confined to the base of the colony, and there forms a thin slimy film, which lubricates the surface over which the animal moves. It has been stated[557] that progression is produced in the following way. The polypides are withdrawn by means of retractor muscles, which originate from the septa and inner surface of the sole. Thus at each retraction of any polypide, the muscle pulls on a portion of the sole. Should the expanded polypides place themselves in a suitable position, the movement will be in the direction of the resultant of the forces due to the separate retractor muscles; while it is probable that their cilia assist in the onward movement. It should be noted that it is definitely stated that a colony in which all the polypides are retracted can alter its position,[558] although even then the retractor muscles might still contract to some extent.
The movement probably depends on several causes. It must probably be conceded that the sole itself has some effect on this process. Its outer cells are contractile, and have the power of raising themselves from the underlying ectocyst. They may then again attach themselves, and this new attachment does not always take place in exactly the same place as the former one. Any movement of the muscles of the sole, or of the retractor muscles, will thus shift the skin to a new place.[559]
Protrusion of the Polypide.—While it is perfectly clear that retraction is principally performed by the great retractor muscles acting directly on the polypide, it is less easy to explain the converse movement. There can, however, be little doubt that protrusion is effected by the pressure of the fluid of the body-cavity, caused in large part by contractions of the common body-wall.
Now since, in Cristatella, the body-cavity is a continuous space, any pressure on the fluid must act uniformly on all its contents. The cause which determines the protrusion of a polypide is thus to a large extent the relaxation of the sphincter-muscle which surrounds its orifice, aided by special muscles which dilate the orifice. Any polypide which is retracted while the pressure of the fluid in the body-cavity is sufficient to keep other polypides protruded, must therefore keep either its {499}retractor-muscles or its sphincter in a state of contraction in order to remain in that position. And as a matter of fact, Cristatella and Lophopus differ from most other Polyzoa in the readiness with which they expand their tentacles, after they have been induced to retract themselves by mechanical irritation.
Plumatella and other forms have a chitinous ectocyst, which, however, is sticky when it is first formed. By virtue of this property, the branches become attached to the leaf on which the colony is growing, and may have their natural transparency obscured by taking up foreign bodies. The stiffness of the ectocyst naturally involves some modification of the process by which the polypides are protruded. In some cases, this is effected by the separation of the endocyst from the ectocyst in the lower parts of the tube. The muscles of the body-wall can thus press on the fluid of the body-cavity without being restrained by the inflexible ectocyst. In other cases, the tube of ectocyst is rendered flexible by the presence of a thin line along one side where the chitin is deficient.
Fig. 249.—Plumatella repens L., R. Yare, × 30. a, Anus; b, polypide-bud; c, caecum of stomach; d, duplicature; e, epistome (see p. 476); f, funiculus; g, ganglion; m, retractor muscle; p, parieto-vaginal muscles; ph, pharynx; s, statoblasts attached to f.
The upper end of the retracted tentacle-sheath is connected with the body-wall by bands known as the parieto-vaginal muscles (Fig. 249, p). These {500}serve not only to dilate the orifice when protrusion is commencing, but also to prevent the polypide from being forced out too far. They are arranged in such a way that a circular fold, the duplicature (d), is never turned inside out, even in the state of complete protrusion of the polypide.
The mechanism of the protrusion of the polypide in the Gymnolaemata is in many cases obscure. The body-wall is not muscular in this group, in some forms of which, however, short strands known as the parietal muscles (Fig. 234, p) pass across the body-cavity from one point to another of the zooecium. As doubts have been thrown on the function of these muscles in causing protrusion, it will be worth while to refer to the detailed and convincing statements of Farre,[560] relating to this point.
Farre's observations were made on certain transparent Ctenostomes (Bowerbankia and Farrella). He states that the parietal muscles "were distinctly seen to contract whenever the protrusion of the animal took place, and to become relaxed again upon its retiring into its cell." Their contraction may indent the outline of the ectocyst, or may cause the separation of the endocyst from the ectocyst. The endocyst is then drawn into longitudinal lines at the origin and insertion of these fibres. It is further suggested that some part is played in the process by the muscular walls of the alimentary canal, which is a good deal bent in the retracted condition. The effort to straighten itself is believed to have some share in forcing out the polypide. The flexible, membranous character of the "aperture" (see p. 524) in Membranipora (Fig. 256, A) is said by Nitsche[561] to be an arrangement for the protrusion of the polypides; the parietal muscles passing from the lateral walls of the zooecium to the upper membranous wall, which is accordingly depressed by their contraction.
Although it is hardly possible to doubt the accuracy of Farre's observations, which have, moreover, been confirmed by Hincks, it is by no means certain that this is the whole explanation in all cases. Oka,[562] for instance, states that protrusion of the polypide in Phylactolaemata can be effected in a branch whose body-wall has been cut open. Pergens[563] believes that the diaphragm (Fig. {501}234, d) acts as a pump, introducing water from the tentacle-sheath into the body-cavity, into which it is said by him to open, and so forcing out the polypide. It is probable that many of the forms which have a stiff, unyielding ectocyst possess special arrangements for introducing water in some way into the space bounded by the ectocyst,[564] and so forcing out the polypide. Such, for instance, may be the median pore which occurs beneath the orifice in Microporella (Fig. 241, A, mp), and in certain other cases.
Reproduction of Phylactolaemata.—Sexual reproduction takes place in Cristatella from June to August. The spermatozoa are ordinarily produced on the funiculus. The ovaries usually occur on the inner side of the common wall of the colony, not far below the orifice of a polypide. Each ovary matures a single egg, which develops in situ, the free larva leaving the colony by the orifice of one of the degenerated polypides.
A second method of reproduction takes place by means of the statoblasts, which are developed on the funiculus (Fig. 249). According to Verworn,[565] each statoblast arises from a single cell of the funiculus; and on this view, the statoblast is, as supposed by the earlier observers, a special kind of winter-egg. According to more recent researches,[566] the funiculus consists of a central axis, formed from the ectoderm, and of an outer sheath of mesoderm-cells; the statoblast is developed from the two kinds of cells of which the funiculus is composed, and is consequently comparable in its mode of origin to an ordinary bud. Its special peculiarities are: its origin as an internal bud, its possession of a chitinous shell, and the fact that it is destined to leave the parent colony, and to develop, after a period of rest, into a new colony. Germination takes place by the formation of a polypide-bud inside the statoblast, which finally splits along its equator into two halves. The contents emerge as a young colony which possesses at least one fully-formed polypide.
Remarkable structures known as "hibernacula" occur in the fresh-water Ctenostomes, Paludicella and Victorella. These bodies are in the former (Fig. 250, B) specially modified external buds, which persist through the winter when the rest of the colony dies down. At the close of winter the shell splits into two {502}halves, exactly as takes place in the statoblasts, and a young colony emerges. It is possible that the statoblasts may have been evolved from a hibernaculum, which was at first produced externally, but has become modified in such a way as to acquire an internal mode of origin.[567]
The simplest known statoblast is that of Fredericella (Fig. 251, A), which differs from that of other Phylactolaemata in having no ring of air-cells. In Plumatella, the statoblast (Fig. 251, B) has a broad equatorial ring of air-cells, which enable it to float at the surface of the water on the decay of the parent tubes. In some species, certain statoblasts which are produced in the adherent parts of the colony remain attached to the substratum. These "sessile statoblasts" may have no trace of the ring of air-cells; but the fact that many sessile statoblasts have rudiments of this structure suggests that they are a secondary modification of the floating statoblast. In Lophopus (Fig. 251, C) the ring of air-cells is very broad, and is pointed at each end; while in Cristatella (Fig. 251, D) and in Pectinatella the statoblast is circular, and possesses an armature of hooked spines. That of Cristatella, measures about .75 mm. in its greatest length.
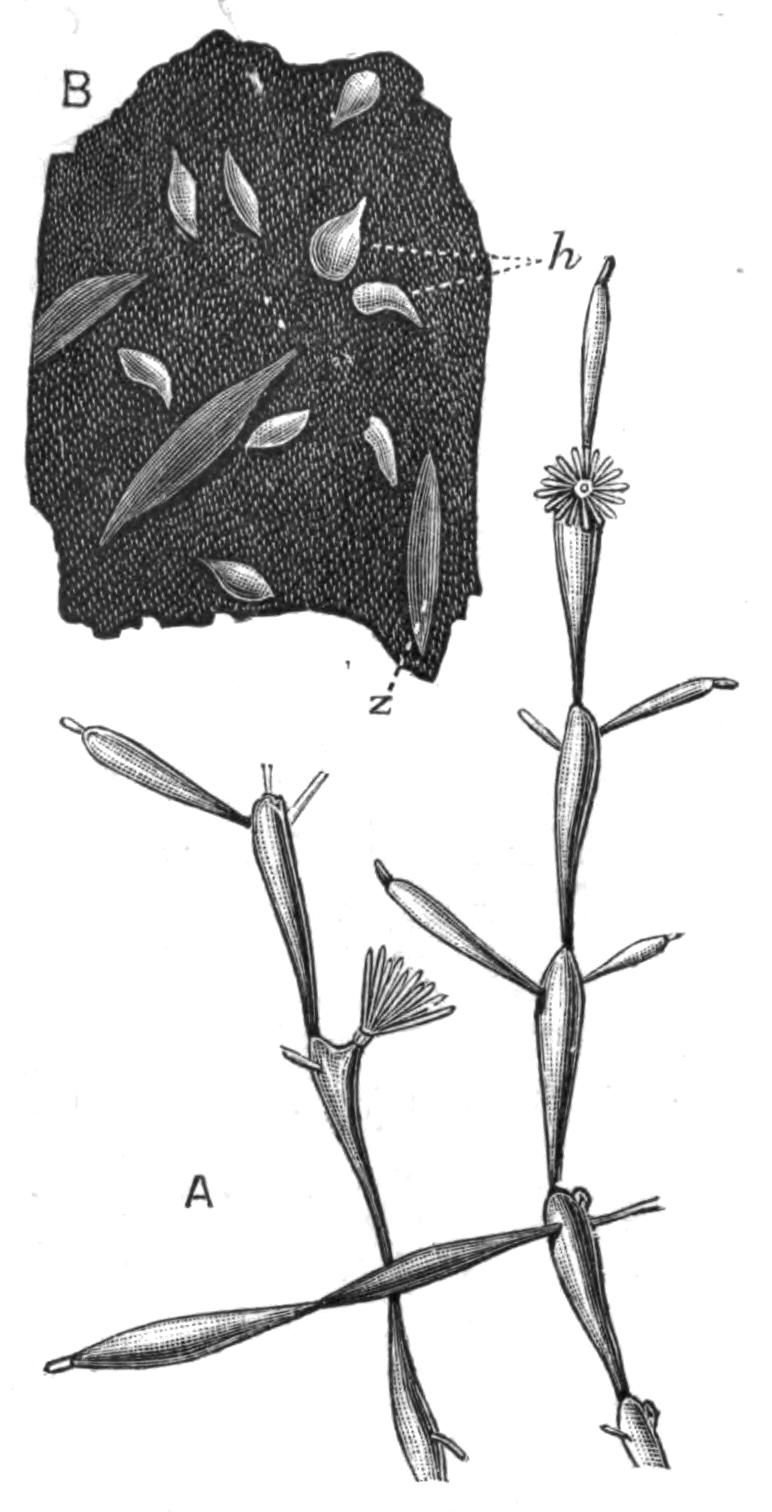
Fig. 250.—Paludicella ehrenbergi van Beneden, × about 3. A, Part of a colony with expanded polypides; B, remains of part of a colony which has produced hibernacula or winter-buds (h); z, zooecium. (From Kraepelin.)
Kraepelin has suggested that the above order of increasing complexity of the statoblasts corresponds with the order in which the genera to which they respectively belong would be placed, on the assumption that the Phylactolaemata have been derived from the Ctenostomata. Thus, in Fredericella, the form of the lophophore is circular, as in the Gymnolaemata. The number of the tentacles is comparatively small (20-24). The arborescent form of the colony resembles that of many Ctenostomes, and the zooecia are more or less cut off from one another by incomplete septa.
In Plumatella, the lophophore has become horse-shoe-shaped, and the tentacles are more numerous (38-60). In general form and in the arrangement of the septa this genus resembles Fredericella, with which it may easily be confused.
In Cristatella we have the most highly modified of all the Phylactolaemata. The individuality of the zooecium is here subordinated to that of the colony as a whole. The branched arrangement of the zooecia is greatly obscured. The body-cavities have become completely confluent, although rudiments of the septa still exist. The ectocyst has been lost, with the exception of the basal layer of the colony. The tentacles are more numerous (80-90); and in accordance with the increase in the elaboration of the genus, its statoblasts belong to the most complicated type known.
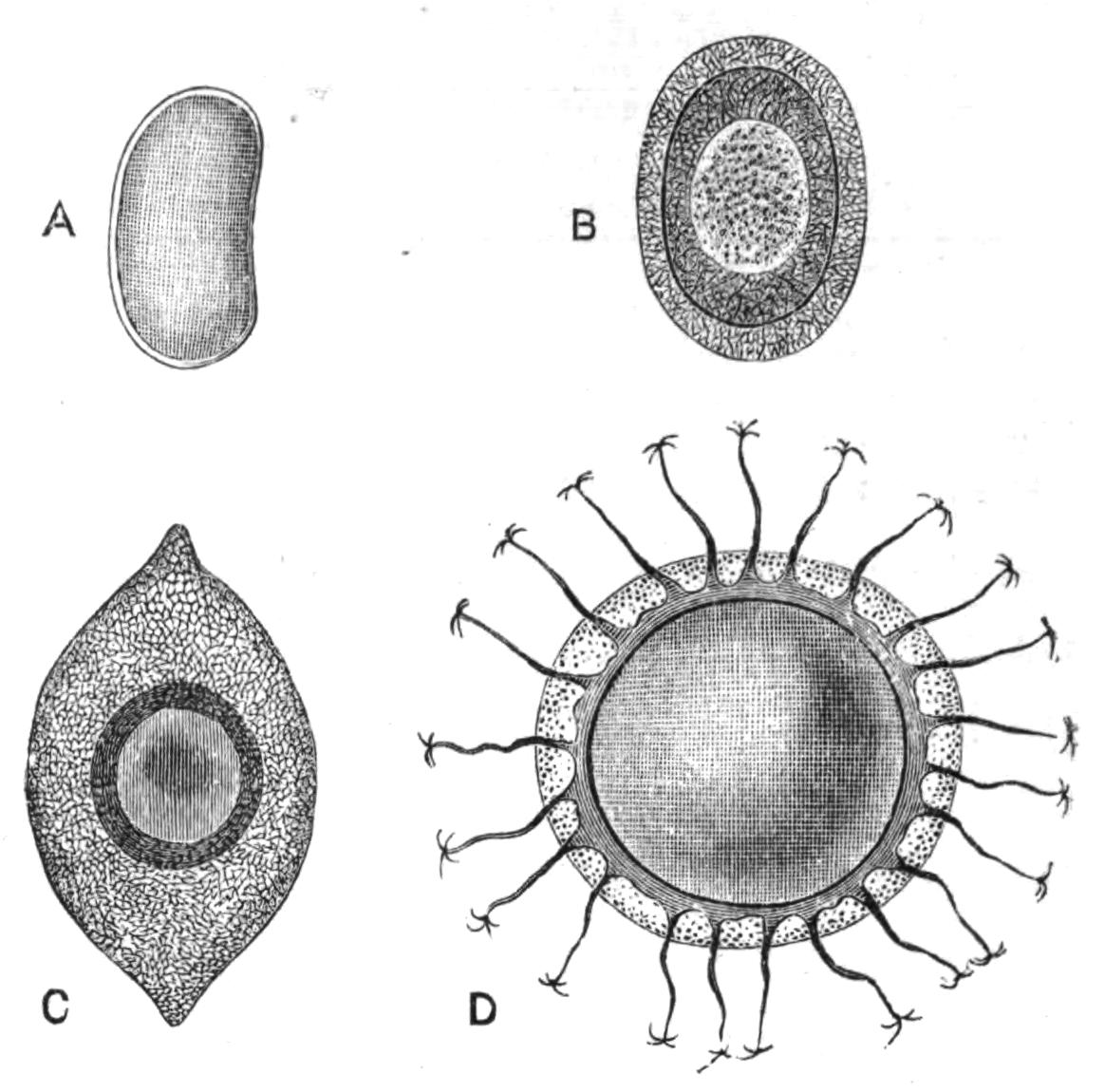
Fig. 251.—Statoblasts of Phylactolaemata. A, Fredericella sultana Blum., × 38; B, Plumatella repens L., × 38; C, Lophopus crystallinus Pall., × 28; D, Cristatella mucedo Cuv., × 28. (A, from Allman; B-D, from Kraepelin.)
The production of floating statoblasts may seem a strange adaptation to the conditions of fresh-water life, since it might be assumed, a priori, that these structures would be specially liable to be frozen during the winter. The following experiments made by Braem[568] show, however, that the germinating power of the statoblasts is improved by a certain amount of frost. A number of statoblasts were taken; half of these were placed in water, which was then frozen; and these were found to germinate readily when afterwards exposed to suitable conditions. The other half were not subjected to the action of frost; and these could not be made to germinate, even although the water had been cooled to a point slightly above the freezing point. It thus appears that the buoyancy, so far from being a risk, is a means of exposing the statoblast to the conditions which are most favourable to its later development.
Braem supposes that the beneficial action of frost is due to a lowering of the vital energy of the statoblast. As in the case of reproductive bodies known in many other fresh-water organisms, the statoblast germinates only after a period of rest. Although this period is often shortened by a lowering of the temperature, it can also be induced by the exclusion of air, as in an experiment during which the statoblasts were enclosed in airtight tubes. The respiratory processes were thereby lessened, and the germinating power was materially improved.
Since the development of the statoblasts depends largely on the temperature, the first warm weather in early spring will probably induce the germination of those which are floating; and the young colony, leaving the protection of the statoblast, will become susceptible to frost. But even if the first-formed colonies are killed off by a subsequent frost, other statoblasts which have remained in the mud during the winter are disentangled from time to time, and germinate on reaching the surface.
Distribution.—The protective value of the shell is also shown by the fact that the statoblast may be kept for some months in a dry condition without losing its power of germination. There can be little doubt that the capability of withstanding desiccation enables the species to enlarge its area of distribution. It is asserted that fresh-water Polyzoa decrease in abundance in proportion to the distance from the mouth of the river in which they are found. The current will naturally tend to bring together the statoblasts from the Polyzoa growing in the upper waters.
Nothing is more surprising than the wide geographical distribution of the Phylactolaemata. The European genera are all recorded from North America. Fredericella, Plumatella, and Lophopus are further recorded from Australia; while Plumatella is known to occur also in Malacca, the Philippine Islands, India, Japan, Africa, and South America, It is even stated that some of the Australian species are identical with those found in Europe.
Some of the fresh-water Polyzoa are extremely variable, and observers are by no means agreed in deciding whether certain well-known forms are to be regarded as varieties or as species. While certain genera, such as Cristatella and Lophopus, are comparatively constant in their form, Plumatella is excessively variable. Plumatella has a number of species greater than that of any other form, and the genus has a wider distribution than any {505}other. This greater variation of species of the dominant genus is in complete accordance with the general law enunciated by Darwin that "wide-ranging, much diffused, and common species vary most."
While the ordinary forms of Plumatella consist of branching colonies, which are either completely adherent to their substratum, or grow in a more or less erect manner, another habit which is assumed by this genus is so different from the first that it has been considered to mark a distinct genus, Alcyonella. The Alcyonelloid form (Fig. 246, A) consists of closely packed tubes which stand more or less at right angles to their substratum, which they may cover with a dense mass an inch thick, and with a superficial area of several square inches. But in spite of this difference, it is possible that A. fungosa is only a variety of an ordinary Plumatella form. Whether this is so or not, a typical Plumatella may in places take on an Alcyonelloid habit; and parts of an Alcyonella may become so lax in growth as to resemble a Plumatella.
The British genera of fresh-water Polyzoa may be distinguished from one another by means of the following table:—
| 1. |  |
Zooecia perfectly distinct from one another. Lophophore circular. Statoblasts absent 2 |
|
Colony formed of branching tubes composed of confluent zooecia 3 |
||
|
Colony gelatinous, not obviously formed of branching tubes. Lophophore horse-shoe shaped 4 |
||
| 2. |  |
Colony consisting of a stolon from which new zooecia originate. These may give rise to new stolons, or directly to new zooecia Victorella |
|
Branches composed entirely of club-shaped zooecia, each of which may give off two zooecia near its upper end Paludicella (Fig. 250) |
||
| 3. |  |
Tubes hyaline or opaque, usually containing numerous oval statoblasts (Fig. 251, B), most of which have a ring of air-cells. Lophophore horse-shoe shaped. |
|
||
|
(b) Tubes parallel with one another Alcyonella form of Plumatella (Fig. 246, A) |
||
|
Tubes cylindrical, usually dark brown. Statoblasts (Fig. 251, A) few, without air-cells. Lophophore circular Fredericella |
||
| 4. |  |
Colony hyaline, usually divided into three or four short lobes. Ectocyst thick. Statoblasts (Fig. 251, C) pointed at each end, with a broad ring of air-cells Lophopus (Fig. 248) |
|
Colony slug-shaped, crawling on a flattened sole. Ectocyst rudimentary. Statoblasts (Fig. 251, D) circular, with marginal hooks Cristatella (Fig. 247) |
||
|
Colonies consisting of small rosettes, many of which are attached to a thick basal layer of hyaline ectocyst. Statoblasts circular, with marginal hooks. (Not recorded as British) Pectinatella |
||
Reproductive Processes of Polyzoa in general.
In studying the reproductive processes of Polyzoa, we have to deal with two very distinct phenomena; firstly, with the development of eggs; and secondly, with the formation of buds.
The process of budding usually does no more than increase the number of individuals in a colony which already exists, and is seldom responsible for the commencement of a new colony. In Loxosoma, however, the buds break off and lead an independent existence; and in the Phylactolaemata a large proportion of the colonies have their origin in the statoblasts. In certain cases, again, new colonies may be formed by the detachment of parts of an old one, as by the fission of Cristatella and Lophopus, or by the breaking up of a richly-branched species into several colonies by the decay of the proximal parts.
We may then in the majority of cases look to an embryo for the foundation of a new colony. The embryo develops into a larva, which, after a period in which it swims freely, settles down, and is metamorphosed into the first zooecium. This primary individual forms the starting-point of a colony, and often differs to a considerable extent from the other zooecia which arise from it. In Cyclostomata, for instance, the proximal end of the primary zooecium permanently retains the disc-like shape assumed by the young larva when it first fixed itself. The primary zooecium may be recognised with equal ease in many Cheilostomata, and may differ from its successors by possessing a richer development of marginal spines, or in other respects.
Reproductive Organs.—Eggs and spermatozoa are commonly found in the same colony, either in different individuals, or else in the same zooecium (see Fig. 234, p. 469). In some cases, the zooecium first develops spermatozoa, and later eggs. The Entoprocta have a more marked separation of the sexes than obtains in other Polyzoa. The genus Loxosoma is perhaps always dioecious (i.e. with separate sexes). Pedicellina is sometimes found with ovaries and testes in the same individual, sometimes with these organs in different individuals; and it is not clear whether a given species always behaves alike in these respects.
The reproductive organs of the Entoprocta open by ducts of their own into the vestibule. In the Ectoprocta they are developed in the body-cavity, and they have no ducts.
The fate of the ripe egg differs widely in different cases. In the Entoprocta it develops in a kind of brood-pouch formed from part of the vestibule. The fact that in Pedicellina (Fig. 243) the embryos grow largely during their development, shows that nutritive material must be supplied to them from the parent. There is reason to believe that the epithelium of the brood-pouch is responsible for this process. The eggs are also known to develop at the expense of nutritive substances prepared by the parent in the ovicells of the Cyclostomata. In other cases, as in some species of Alcyonidium, the egg is large, and its copious yolk doubtless supplies a large part of the material required for development.
In the Ectoprocta, development takes place in a variety of places. In most Cheilostomata a single egg passes into the globular ovicell, which is formed above the orifice of many of the zooecia. In certain Ctenostomata,[569] Phylactolaemata,[570] and Cyclostomata,[571] the ripe egg is taken up by a rudimentary polypide-bud, which is specially formed for the purpose. In the Ctenostomata and in the fresh-water Polyzoa these buds, if present, are found in ordinary zooecia which do not become modified externally in any special way. In the Cyclostomata (Crisia), on the contrary, the formation of the polypide-bud is intimately bound up with the development of the ovicell. The number of the zooecia which produce eggs that are capable of development is greatly restricted in this group. The ovicell, which contains numerous embryos, is not merely a portion of a zooecium, as in the Cheilostomata; but it is probably to be regarded as a modification of the entire fertile zooecium or zooecia. These take on an appearance widely differing from that of the ordinary zooecia, and in course of time give rise to the ovicells (see Fig. 237).
In all these cases the egg develops inside the parent, and it was hardly known, before the publication of the interesting researches of M. Prouho,[572] that some of the Polyzoa lay eggs which develop externally. In these cases a considerable number of eggs are produced simultaneously by a single zooecium. {508}M. Prouho further throws light on a much contested subject; namely, the nature of the so-called "intertentacular organ" (i, Fig. 234, p. 469), described so long ago as 1837 by Farre,[573] but looked for in vain by the majority of later observers.
The failure to find this organ, even in species which possess it, in certain individuals, according to Farre's statements, is now satisfactorily explained by M. Prouho, who shows that while it is absent in a large number of polypides, it is normally present in those individuals which possess an ovary, and in those only; and that its primary function is that of an oviduct.
The intertentacular organ is an unpaired ciliated tube, which is situated between the two tentacles which are nearest to the ganglion. In the retracted condition of the polypide, it opens from the body-cavity into the tentacle-sheath; and in the expanded condition, directly to the exterior.
In the remarkable case of Alcyonidium duplex, each zooecium normally possesses two sexual polypides. The first of these produces a testis and then becomes a "brown body." The second is meanwhile developed, and produces an ovary and an intertentacular organ, a structure which was not present in the male polypide. The eggs pass through the intertentacular organ into the tentacle-sheath, and attach themselves to the diaphragm (d, Fig. 234), where they remain during their development.
Although the intertentacular organ has been found by Prouho in female polypides only, it would perhaps be going too far to assert that it is confined to polypides of that sex. Hincks[574] has observed the passage of spermatozoa in enormous numbers through the organ, although it may be noted that there is no sufficient proof that eggs were not present as well in these zooecia. It further appears that in some cases waste matters may be removed from the body-cavity through the same passage.
It may be presumed that the egg is normally fertilised by a spermatozoon, although this is at present largely a matter of inference. It is believed by Joliet[575] that fertilisation is reciprocal, although Prouho has come to the opposite conclusion. Joliet has, however, very justly pointed out that the enormous number of spermatozoa developed by a single individual would be disproportionately large, if their function were merely to fertilise the {509}ovum in the same zooecium. According to his view, the egg is fertilised by a spermatozoon after it has passed into the tentacle-sheath or ovicell, or some other place where it is in free communication with the outside water.
Development and Affinities.—Few parts of the history of the Polyzoa are more fascinating than that which deals with their development; and it is probable that no other is capable of giving so much insight into the affinities of the several groups to one another and to other groups of the animal kingdom.
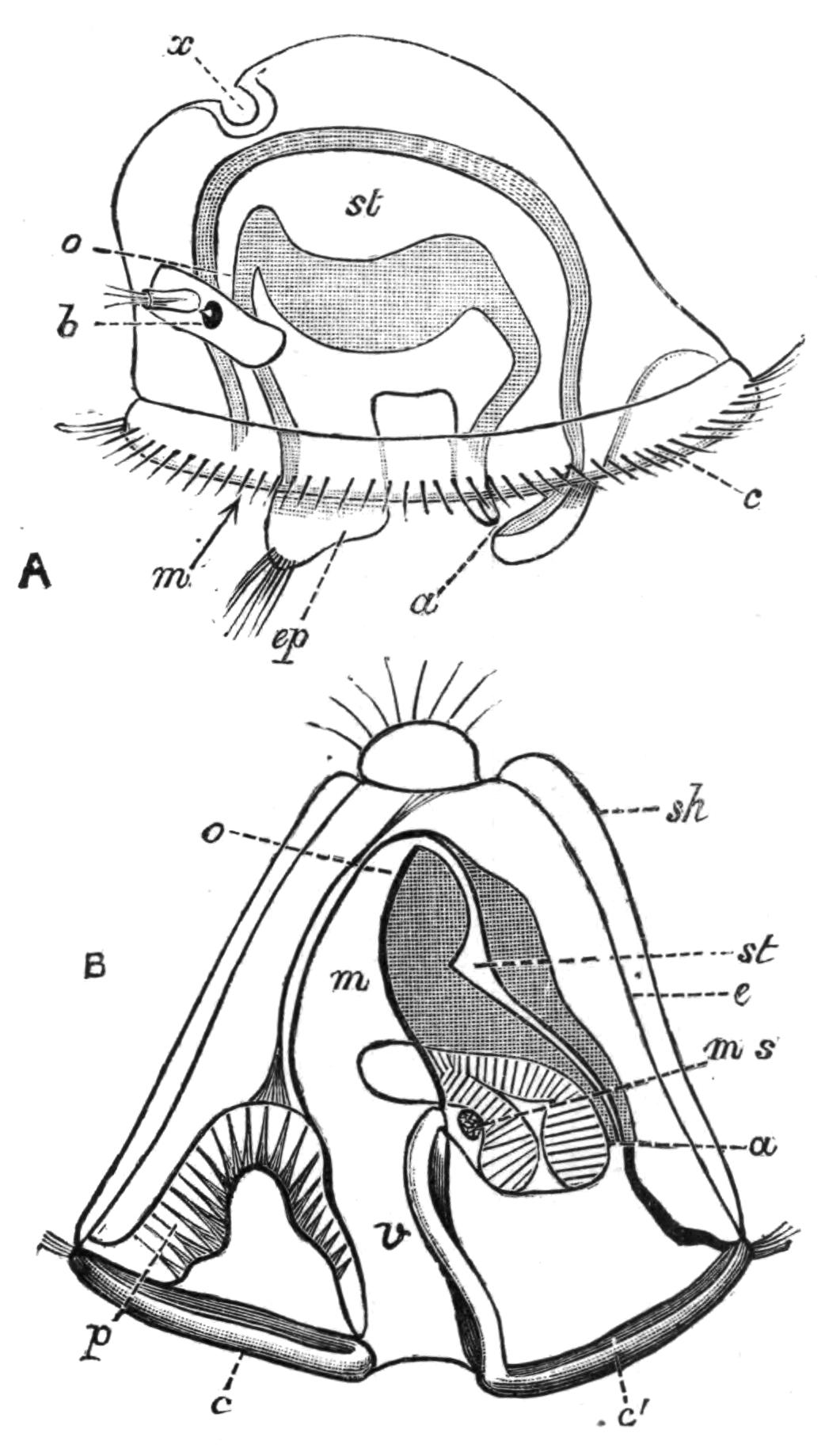
Fig. 252.—Diagrams of larvae. A, Loxosoma, × 208; a, anus; b, brain, with left eye and ciliated pit; c, ciliated ring; ep, epistome; m, mouth; o, oesophagus; st, stomach; x, aboral adhesive organ: B, Cyphonautes larva of Membranipora (Electra) pilosa, × about 90; a, m, o, st as in A; c, anterior part, and c', posterior part of the ciliated ring; e, epidermis; ms, adductor muscle of shells; p, pyriform organ, of unknown function; sh, shell; v, vestibule; the "internal sac" or sucker, by which fixation is effected, is seen between a and ms. (B, after Prouho.)
The comparative study of the larvae of the Polyzoa may be said to date from 1877, when J. Barrois published an elaborate Monograph[576] on this subject. Although some of Barrois' earlier opinions have been subsequently modified, this work still gives the best figures of the external form of the beautiful larvae of many genera. A detailed account of the larval forms of Polyzoa must be omitted from want of space; and the general conclusions only can be given.
The larvae of the Entoprocta (Fig. 252, A) resemble the so-called "Trochosphere" of Polychaeta (see p. 274). The common characters shared by the larvae of Chaetopoda, Echiuroid Gephyrea, Mollusca, and Polyzoa, and by adult Rotifera, may well point to the derivation of these groups from a common ancestor. On this assumption, it is possible that the Polyzoa have been derived from forms which existed long ages ago, which combined the common characters of these groups, and the structure of which we can picture to ourselves only so far as the "Trochosphere" larva can be taken to represent it in a much simplified condition. Such a view harmonises well with the great antiquity of the Polyzoa. Certain Ectoproct forms have a larva, known as Cyphonautes (Fig. 252, B), which closely resembles the larval form of the Entoprocta; and it is a fact which probably has considerable significance that this type of larva is known to occur only in those species of Membranipora (Electra), Alcyonidium, and Hypophorella, which lay eggs.[577] This may perhaps be regarded as a primitive form of development which has been lost in species in which development takes place inside the parent. Cyphonautes compressus (Fig. 252, B), one of the commonest objects taken in the surface-net off our own coasts, is the larva of Membranipora (Electra) pilosa. Whilst this larva is provided with a well-developed alimentary canal, those of most other Ectoprocta possess a mere rudiment of this structure, and depend for their nutrition either on yolk present in the egg or on material supplied by the parent. In most cases the mature larva has no recognisable trace of a digestive system; and, although it has a free-swimming period, it does not become truly pelagic.
The alimentary canal of the larva of Pedicellina is known to persist in the primary individual of the colony. In all other known cases, even in that of Cyphonautes, the larva at fixation loses practically all its internal organs, and becomes a mere body-wall containing a mass of degenerated larval tissues. It is in fact a zooecium containing a "brown body." A polypide-bud is now developed, the body-cavity appears as the result of the shrinkage of the "brown body," and the primary individual of the colony is thereby established.
The larvae of the Ectoprocta form a tolerably complete series, starting from Cyphonautes, itself allied to the larva of the {511}Entoprocta, and ending with the Phylactolaemata. Alcyonidium (Fig. 253, B) possesses a rudimentary alimentary canal,[578] although the most conspicuous structures are those connected with the fixation and other phenomena of larval life. The larvae of many of the encrusting Cheilostomes (Fig. 253, A) resemble that of Alcyonidium, while those of Bugula, Scrupocellaria, etc., belong to a type easily derivable from that of the encrusting forms. The branching Ctenostomes (Bowerbankia, etc.) have a larva which may be regarded as derived, along slightly different lines, from that of Alcyonidium. The Cyclostomata and the Phylactolaemata have the most modified forms of larva. That of the former group may owe some of its peculiarities to the occurrence of a remarkable process of embryonic fission, which takes place in the ovicell, and as the result of which each egg gives rise to a large number of larvae.[579] The Phylactolaemata have a larva which is not unlike that of Bowerbankia.
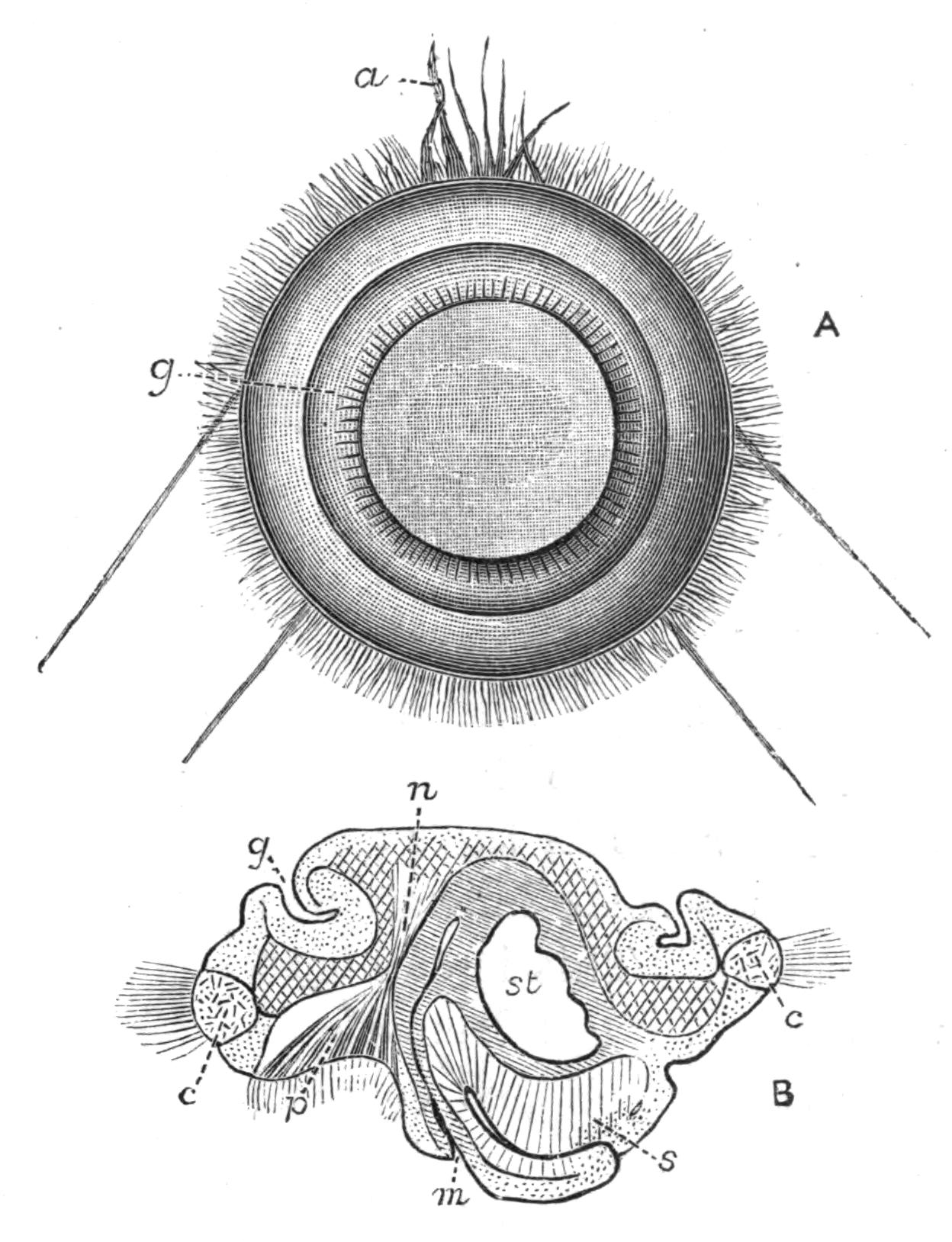
Fig. 253.—A, Aboral view of free larva of Lepralia foliacea Ell. and Sol.; a, long cilia of pyriform organ; g, aboral groove: B, longitudinal section of embryo of Alcyonidium, × 135; c, ciliated ring; g, aboral groove; m, mouth; n, nervous system; p, "pyriform organ," of unknown function; s, "internal sac" or "sucker," by which fixation is effected; st, stomach.
We have seen that the larva at fixation becomes a zooecium, {512}which in the Gymnolaemata forms a polypide-bud after fixation. The peculiarities of the Phylactolaematous larva may be explained by assuming that it becomes a zooecium while it is still free-swimming. Thus the larva of Plumatella develops one or sometimes two polypides, which actually reach maturity before fixation takes place. That of Cristatella develops from two to twenty[580] polypides or polypide-buds at the corresponding period, and it is in fact a young colony while still free-swimming.
Now in most colonial animals, such as Coelenterates and Ascidians, the larva metamorphoses itself into a temporarily solitary animal, which then gives rise to the remainder of the colony by budding. The majority of the Gymnolaemata behave in this way; while the Phylactolaemata may not only develop a multiplicity of polypides in their larval stage, but the individuality of the zooecia is then just as much obscured as in the adult state. These facts are more easily explained if we assume that Cristatella is the end-point in a series than if we suppose it to be a starting-point.
On the view maintained by many authorities, that the Polyzoa are related, through Phoronis, with the Gephyrea and the Brachiopoda, we should expect to find in those Polyzoa which most closely resemble Phoronis in their adult state—that is to say in the Phylactolaemata—some indications of affinity to that animal in their development. This is emphatically not the case. The hypothesis that the Phylactolaemata are related to Phoronis leads, moreover, to the improbable conclusion that the similarities between the Entoproct-larva and Cyphonautes, on the one hand, and the Trochosphere larva of Polychaeta, on the other hand, is entirely superficial and meaningless. In spite, therefore, of the similarity between Phoronis and a single individual of the Phylactolaemata, and in spite of the marked resemblance between its nephridia and structures which have been described in Cristatella[581] and Pectinatella[582] the comparative study of the development appears to indicate that the resemblances between Phoronis and the Phylactolaemata are the result of a coincidence rather than of any close relationship.
A few points connected with the metamorphosis of the {513}Polyzoa deserve more special notice. There is generally great difficulty in persuading larvae to fix themselves when kept in a small quantity of water, which becomes over-heated in the air of a laboratory. The difficulty may be surmounted by placing colonies containing embryos, together with some clean pieces of the seaweed on which the adults are habitually found, in a vessel closed by a piece of fine muslin, and by leaving the vessel attached to a buoy or in a deep tide-pool. The larvae being without an alimentary canal, fix themselves, after a very short free life, on the seaweed.
It is probable that a great struggle for existence normally takes place at the commencement of the metamorphosis. Any one who will examine, in June or July, rocks covered by Fucus on which Flustrella hispida is growing, will probably find numerous young fronds of Fucus, from half an inch to an inch or two in length, growing under the shelter of the older fronds. The bivalve larvae of Flustrella show a marked preference for fixing on these young fronds—perhaps in order that the duration of life of the colony may coincide with that of the Fucus—and these young fronds are commonly covered by very numerous recently-fixed larvae, and by young colonies of various ages. Or, it is easy to observe, by placing pregnant colonies of Bowerbankia in a vessel of water, that the larvae, which are hatched out in thousands, fix themselves in dense masses on certain parts of the wall of the vessel. It is clear that but a small proportion of these larvae will find room for further development.
Next with regard to the mode of fixation. Attachment always takes place by the surface on which the mouth or its rudiment is situated, and the permanent alimentary canal opens on the opposite surface. In Pedicellina, the one case in which the larval digestive organs are known to become those of the first adult individual, this presupposes a rotation of the alimentary canal, in order to bring it into its new position.
It is well known that the larvae of other fixed animals may undergo a somewhat similar change. Thus those of Ascidians and of Barnacles fix themselves by their anterior end, and ultimately reach their adult form by performing a kind of a somersault. The process may perhaps be explained by supposing that some part of the anterior end or of the oral surface is specially sensitive, and that the larva fixes itself by that portion of its {514}body which is best fitted for ascertaining which is the proper substance on which to fix.
Budding.—The formation of a new individual may take place by the outgrowth of part of the body-wall, as in Pedicellina (Fig. 243, p. 487) and in Bowerbankia (Fig. 238, p. 480). In Pedicellina a young stalk is formed by an outgrowth near one of the growing points, and the upper part of this outgrowth becomes constricted off to form the calyx. In other cases (cf. the growing ends of the branches in Fig. 237) a partition grows across the body-cavity at the growing edge of the colony, and so cuts off a part destined to become a new zooecium.
The zooecium formed in one of these ways acquires an alimentary canal by the formation of a polypide-bud, some stages in the growth of which are shown in Fig. 235 (p. 472). Contrary to what happens in Coelenterates and Tunicates, in which the endoderm takes part in the budding, there is good reason for believing that in Polyzoa the polypide-bud is developed entirely from ectoderm and mesoderm.[583] The bud is a two-layered vesicle, attached to the inner side of the body-wall. Its inner layer is derived from the ectoderm, which at first projects into the body-cavity in the form of a solid knob surrounded by mesoderm-cells. A cavity appears in the inner, ectodermic mass, and the upper part of the vesicle so developed becomes excessively thin, forming the tentacle-sheath, which is always developed in the condition of retraction. The lower part becomes thicker; its inner layer gives rise to the lining of the alimentary canal, to the nervous system, and to the outer epithelium of the tentacles, which grow out into the tentacle-sheath (cf. Fig. 235). The outer layer gives rise to the mesodermic structures, such as the muscles, connective tissue, and generative organs.
These processes are fundamentally similar, whether in the metamorphosed larva, in a young zooecium, in an old zooecium after the formation of a "brown body," or in the germinating statoblast of the Phylactolaemata.
POLYZOA (continued)
CLASSIFICATION—GEOGRAPHICAL DISTRIBUTION—PALAEONTOLOGY—METHODS FOR THE EXAMINATION OF SPECIFIC CHARACTERS—TERMINOLOGY—KEY FOR THE DETERMINATION OF THE GENERA OF BRITISH MARINE POLYZOA
Our account of the Polyzoa would be manifestly incomplete without some reference to the systematic arrangement of these animals. An outline of the principal groups has been given on p. 475. So far, the classification is easy, but it is otherwise when we attempt to subdivide most of the groups any further.
Systems of classification which depend exclusively upon the external characters of animals have been repeatedly shown to be unsatisfactory. Now with regard to the Polyzoa, not only is it the case that the great majority of forms are only known in their external characteristics, but current systems of classification cannot be regarded as final, because it is not yet certain which of the external features have most systematic value. Two obvious points can be at once selected—namely, the character of the zooecium and the character of the entire colony. One or two instances will serve to show what different results are obtained by depending exclusively on either of these characters by itself.
According to the older writers, the habit of the colony was taken as the most important generic character; and there can indeed be no doubt that this feature has great importance within certain limits. Any one who has examined different species of such genera as Flustra, Cellaria, Bugula, Retepora, etc., must feel that the form of the colony goes for a good deal. But a consideration of other cases shows that there is great risk in the {516}indiscriminate use of this method of arranging the Polyzoa. The old genus Eschara, composed of forms with an erect coral-like habit,[584] included species which are now placed in such different genera as Lepralia, Porella, Microporella, etc. The older works on Polyzoa include all encrusting forms of Cheilostomata, with a completely calcareous front wall, in the genus Lepralia, the members of which are now distributed in numerous widely separated genera.
As an instance of the converse arrangement—essential similarity of the zooecia with great differences of the general habit—may be mentioned the common Membranipora (Electra) pilosa.[585] Ordinarily growing in the form of close encrustations on seaweeds, this species may take on entirely different habits of growth. The zooecia are now dissociated, growing in single lines over the substratum; now forming erect tufts, composed of single lines of zooecia or of several rows. The erect, branching habit appears to be induced in the first instance by the character of the seaweed on which the colony begins life. Thus colonies which encrust the thin branches of Corallina may have impressed on them something of the mode of growth of the seaweed, so that when they extend beyond the tips of the branches of the Corallina, they continue to grow in delicate branches, which still retain more or less the same diameter as those which form their base. An extreme variation results in the beautiful form known as Electra verticillata, in which the zooecia are arranged with great regularity in whorls, which together form erect branches.[586] But with all these variations, the zooecia are so much alike that it is hardly possible to regard the extreme forms as more than varieties of a single species. A careful examination of this case would convince most observers that the characters of the zooecium are a more trustworthy guide to classification than those of the entire colony, a result which was first clearly stated by Smitt, and amply confirmed by Hincks.[587]
The avicularia of the Cheilostomata afford useful help in classifying this group; but while certain genera are always provided with avicularia, others include some species with these organs, and other species without them. Again, while the species {517}of some genera (e.g. Cellepora) possess a great variety of forms of avicularia, the same pattern of avicularium may characterise several widely different genera. Further, the position of the avicularium may be very different in species which are apparently closely related. Well-developed vibracula, although constant in their occurrence in such forms as Scrupocellaria (Fig. 254) and Caberea (Fig. 242), occur here and there in species of encrusting forms which are ordinarily placed in very different families.
Now although some of these discrepancies are perhaps due to errors in classification, whereby species which are really allied have been wrongly placed in distinct genera, this explanation would not prove satisfactory in all cases. Thus in Bugula, a genus which is specially characterised by the high development of its avicularia, these organs are normally absent in B. neritina. The fact that this species was rightly placed in the genus has been confirmed by the discovery made by Waters[588] that avicularia occur in specimens which are believed to be identical with that species.
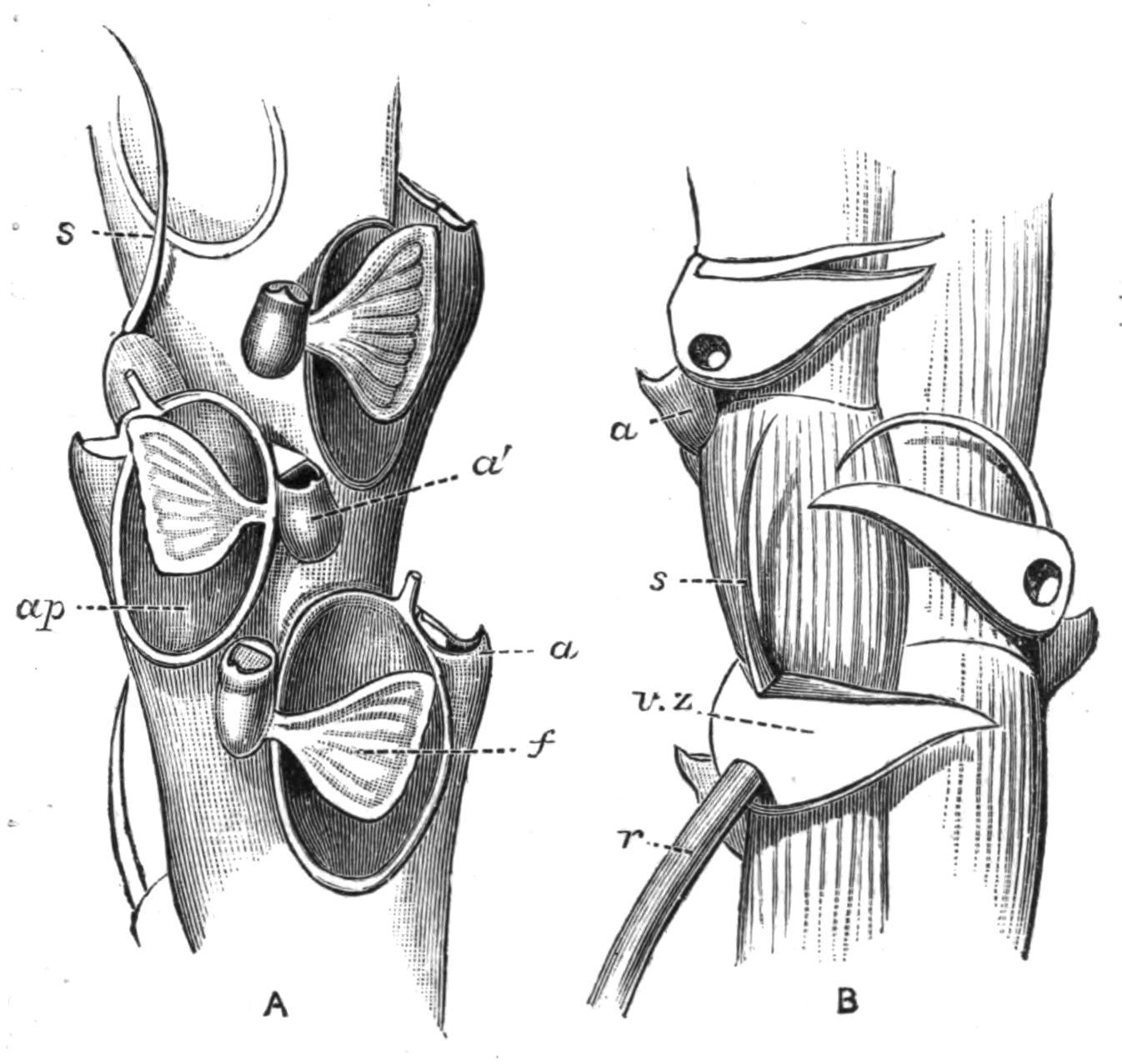
Fig. 254.—A, Front view, and B, back view of part of a branch of Scrupocellaria scabra, Van Ben., Durham Coast, × 43; a, lateral avicularium; a', smaller median avicularium; ap, membranous aperture; f, fornix; r, rootlet; s, seta of vibraculum; v.z, vibracular zooecium.
1. The Cyclostomata appear to fall naturally into two main groups, (A) the Articulata, including the Crisiidae (Fig. 237), distinguished by their erect branches, divided at intervals by chitinous joints; and (B) the Inarticulata, which include the remaining families, whether erect or encrusting, agreeing in the negative character of being unjointed.
2. The Cheilostomata consist of (A) the Cellularina, including the flexible, erect forms, such as Bugula (Fig. 233) and Scrupocellaria (Fig. 254); (B) the Flustrina, to which belong Flustra (Fig. 232), Membranipora (Fig. 256, A, B), Micropora (Fig. 256, C), and other forms in which the front wall of the zooecium is either membranous, or depressed and marked off by a ridge-like margin; (C) the Escharina, including the great majority of forms, in which no part of the front wall remains membranous, the wall of the zooecium being wholly calcified.
3. The Ctenostomata comprise (A) the Alcyonellea or encrusting forms; and (B) the Vesicularina or branching forms. The zooecia in the latter subdivision (Fig. 238) are given off from a tubular stem or stolon, which is usually erect and branching.
We thus have the following arrangement of recent forms. The genera mentioned are for the most part those which have already been alluded to in the preceding account:—
Sub-class I. Entoprocta.
Loxosoma, Pedicellina, Urnatella.
Sub-class II. Ectoprocta.
Order 1. Gymnolaemata.
Sub-order 1. Cyclostomata.
A. Articulata. Crisia.
B. Inarticulata. Hornera, Idmonea, Tubulipora, Stomatopora,
Diastopora, Entalophora, Lichenopora.
Sub-order 2. Cheilostomata.
A. Cellularina. Aetea, Eucratea,[589] Catenicella,
Cellularia, Gemellaria, Menipea, Scrupocellaria,
Caberea, Notamia (= Epistomia), Bicellaria, Bugula,
Beania.
B. Flustrina. Cellaria, Flustra, Membranipora, Electra,
Lunulites, Membraniporella, Cribrilina, Micropora,
Selenaria.
C. Escharina. Retepora, Microporella, Lepralia, Porella,
Smittia, Mucronella, Schizoporella, Schizotheca,
Mastigophora, Porina, Cellepora.
Sub-order 3. Ctenostomata.
A. Alcyonellea. Alcyonidium, Flustrella.
B. Vesicularina. Vesicularia, Amathia, Bowerbankia,
Farrella, Hypophorella, Triticella, Mimosella,
Victorella, Paludicella.
Order 2. Phylactolaemata.
Fredericella, Plumatella (including Alcyonella), Lophopus,
Cristatella, Pectinatella.
Even this classification, which deals only with the larger groups, must not be made use of without a word of warning. The division of the Cheilostomata is a matter of great difficulty; and no scheme which has yet been suggested can be regarded as more than tentative. The great number of forms included in this group makes its subdivision extremely desirable from the point of view of convenience; but a further knowledge of the anatomy and of the development of many of the forms of doubtful systematic position is probably necessary before any scheme which is likely to be permanent is put forward. Those who desire to make a further study of the classification of the Polyzoa should refer to the works of Hincks,[590] Busk,[591] MacGillivray,[592] and Gregory.[593]
The Polyzoa do not appear to lend any valuable assistance towards settling the disputed problems of Geographical Distribution. They are not in any case terrestrial, while the fresh-water species do not always respect the limits between the great zoogeographical regions. It has already been pointed out (p. 504) that Plumatella, Fredericella, and Lophopus are believed to occur in Australia, and the first-named genus is practically world-wide in its distribution.
Many marine forms also have a surprisingly wide distribution. Thus among the British species which are described by Mr. Hincks as occurring from Norway to New Zealand are Membranipora pilosa, Scrupocellaria scruposa, Cellaria fistulosa, Microporella ciliata, and M. malusii. Even if it should be proved that specific differences do exist between the southern forms and our own, there can be no doubt of the wide distribution of certain species. It was pointed out by D'Orbigny that Bugula neritina has the habit of attaching itself to the bottoms of ships, a fact which may possibly account for the wide distribution of this species; although it would not be safe to assume this explanation of the facts in all cases. Other Polyzoa, on the contrary, have a more restricted range. Thus Catenicella is specially characteristic of the Australian region.
It is perhaps surprising that marine Polyzoa should in so many cases have so wide a range. Even though it is the rule {520}for Polyzoa to have free larvae, the period during which these larvae are free-swimming is, so far as is known, a short one in most cases. Cyphonautes is a common pelagic form (see p. 510), and probably remains for a considerable period in the larval condition. Other Polyzoon-larvae appear to fix themselves very soon after their birth; and this would not appear to give much time for them to be carried to great distances by ocean-currents. It may, however, be suggested that it does not follow that because we know that a larva may, under favourable conditions fix itself a few minutes after it becomes free, we should be justified in assuming that that larva would not retain for a long period the power of undergoing a normal metamorphosis should it be drifted away from suitable fixing-grounds.
Palaeontology.[594]—The number of fossil Polyzoa is enormous. D'Orbigny devoted two hundred plates and more than a thousand octavo pages[595] to a Monograph on the Cretaceous Polyzoa of France. Many of the fossil forms are extraordinarily well preserved, and there is often no difficulty in recognising the identity between certain fossil species belonging to the more recent formations and living forms. It thus becomes necessary to consult Palaeontological memoirs in working at recent Polyzoa.
While the great majority of fossil Polyzoa do not differ in any essential particular from recent species, this is not altogether the case with the Palaeozoic forms. Leaving out of account the Stromatoporoids, which have been variously referred to the Sponges, Hydrozoa, and Foraminifera, as well as to the Polyzoa, the Palaeozoic strata contain large numbers of peculiar Cyclostomata, together with members of the Trepostomata, a fourth Sub-order of Gymnolaemata, allied to the Cyclostomata. The Trepostomata are for the most part Palaeozoic, but a few survived as late as the Jurassic period.[596] These, with the other Polyzoa from the same formations, are considered by Dr. Gregory in his recently published Catalogue of the Fossil Bryozoa in the British Museum (1896).
The number of Polyzoa recorded from the earlier secondary strata is small. The majority of the known Jurassic forms {521}belong to the Cyclostomata; and one or two Cheilostomes are recorded from the same period. Recent papers by Walford[597] on Jurassic Polyzoa contain the description of genera which are believed to be intermediate between the Cyclostomata and Cheilostomata, particularly with regard to the characters of their ovicells. Although it is not impossible there may be a connection between the ovicells of these two groups, it has yet to be proved that the two sets of structures are homologous.
The Cretaceous period marks the commencement of a large number of Cheilostome genera, although the Cyclostomes still remain numerous.
In the Tertiary formations the Cyclostomes gradually become less numerous, and although in earlier geological periods they far outnumbered the Cheilostomes, these relations are now reversed. Certain Tertiary strata, and particularly the Coralline Crag (Pliocene), are remarkable for the extremely large number of Polyzoa they contain. It will be noticed that no mention has been made of the Entoprocta, the Ctenostomata, and the Phylactolaemata. Their absence in the fossil condition[598] need not, however, be a matter for surprise, as none of these forms are so well suited for being fossilised as are the calcareous Cyclostomata and Cheilostomata. There is consequently no adequate reason for assuming that the absence of a palaeontological record implies that these groups have been recently evolved.
Determination of Genera of Marine Polyzoa.—The species to which a Polyzoon belongs can only be determined, in most cases, with the assistance of the low powers of a microscope. There are very great advantages in the use of a binocular instrument, by means of which a microscopic preparation appears with its parts standing up in proper relief.
In the case of the calcareous forms, the external characters may be more readily made out in a dry preparation than in any other way. For this purpose, the colony should be washed with fresh water, in order to remove the salts, which otherwise crystallise out on drying and obscure the surface. Preparations of this kind must be looked at with the aid of reflected light. Canada-balsam or glycerine preparations are also valuable, whether {522}stained or unstained; and are essential for the examination of the softer forms. In the case of erect species, both surfaces of the branch should be looked at. The opercula, avicularia, and rosette-plates afford important systematic characters in the case of the Cheilostomata.
It must not be forgotten to take account of the condition of the zooecia at different ages. The old zooecia often become entirely altered in form, by the deposition of additional calcareous matter, or by the loss of certain parts present in the younger zooecia. Thus the marginal spines may be entirely lost in the older individuals, while in those forms which develop a "peristome" (see Fig. 255 and p. 524), the characters of the orifice can often be determined in the young zooecia only. It is thus essential to examine the growing ends of the branches or the rim of the colony, as the case may be.
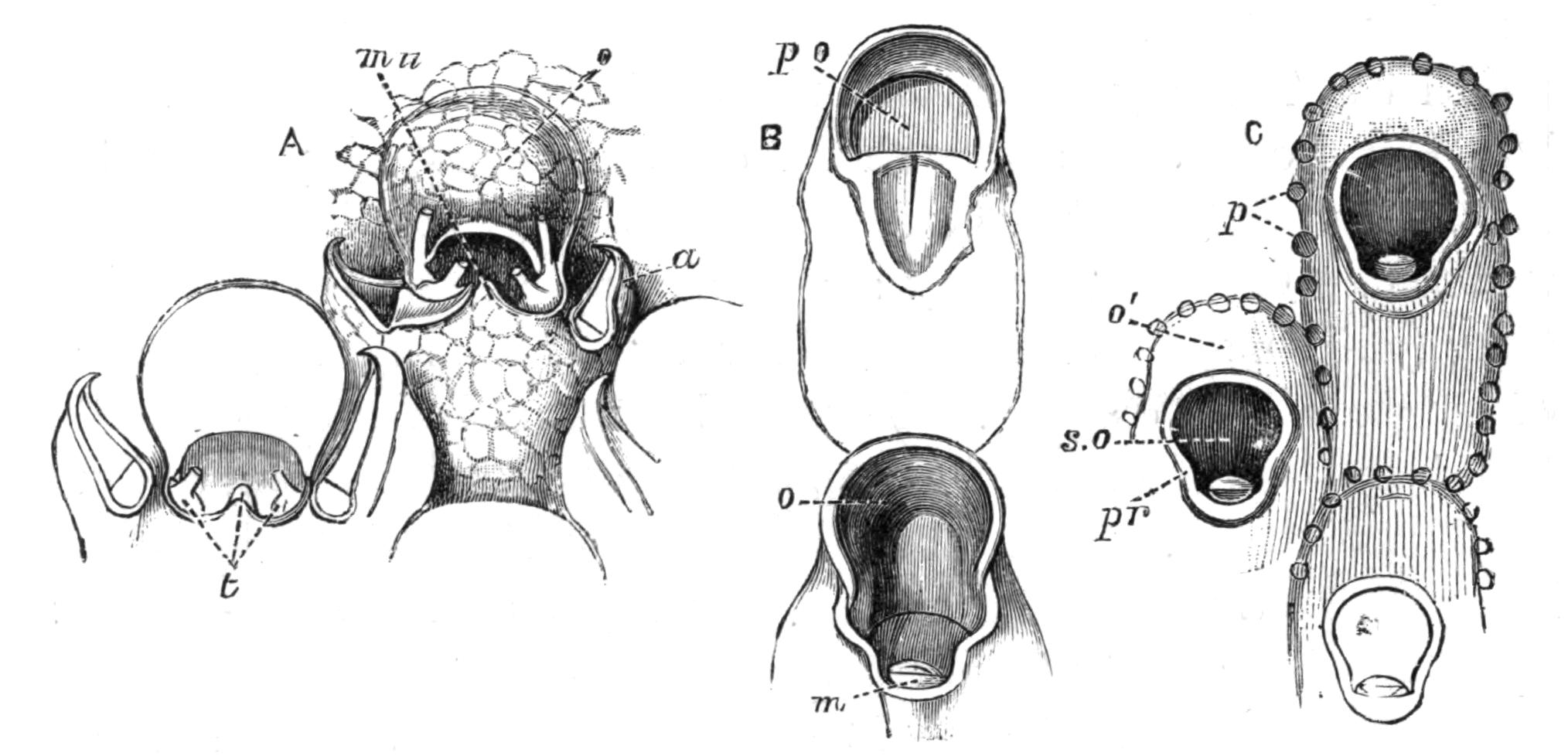
Fig. 255.—Illustrating the nature of a secondary orifice (Cheilostomata). A, Mucronella coccinea Abildg., Scilly Is., × 40. The ovicell (o) overhangs the primary orifice, which is concealed by the great development of the peristome, produced into the mucro (mu); t, the three teeth (denticles) within the secondary orifice; a, avicularium. B, Porella compressa Sowb., Norway, × 40; p.o. primary orifice, above which is a concave lamina, the beginning of the ovicell. In the lower zooecium the ovicell (o) is further grown. The primary orifice is still visible, but it is partially concealed by the growth of the peristome, which encloses a minute avicularium; m, mandible of avicularium. C, Older part of the same colony; pr, peristome; s.o, secondary orifice; o', adult ovicell; p, pores.
In order to make preparations with the tentacles expanded, hydrochlorate of cocaine, chloral hydrate or spirit should be added gradually to the water. When the animals are completely anaesthetised they may be killed by means of a 7-10 p.c. solution of sulphate of copper (best made in distilled water or in rain water). This method gives admirable results in the case of both {523}fresh-water and marine Polyzoa. The use of formaline (see p. 229) may be strongly recommended for the Vesicularina.
The only recent work dealing with all the marine British forms is Mr. Hincks' invaluable History of the British Marine Polyzoa.[599] As the use of this book, unaided by any artificial help, is by no means easy to the beginner, the following key has been compiled as an index to the genera. The Entoproct forms, Loxosoma and Pedicellina (see pp. 488-491), are not included in the table.
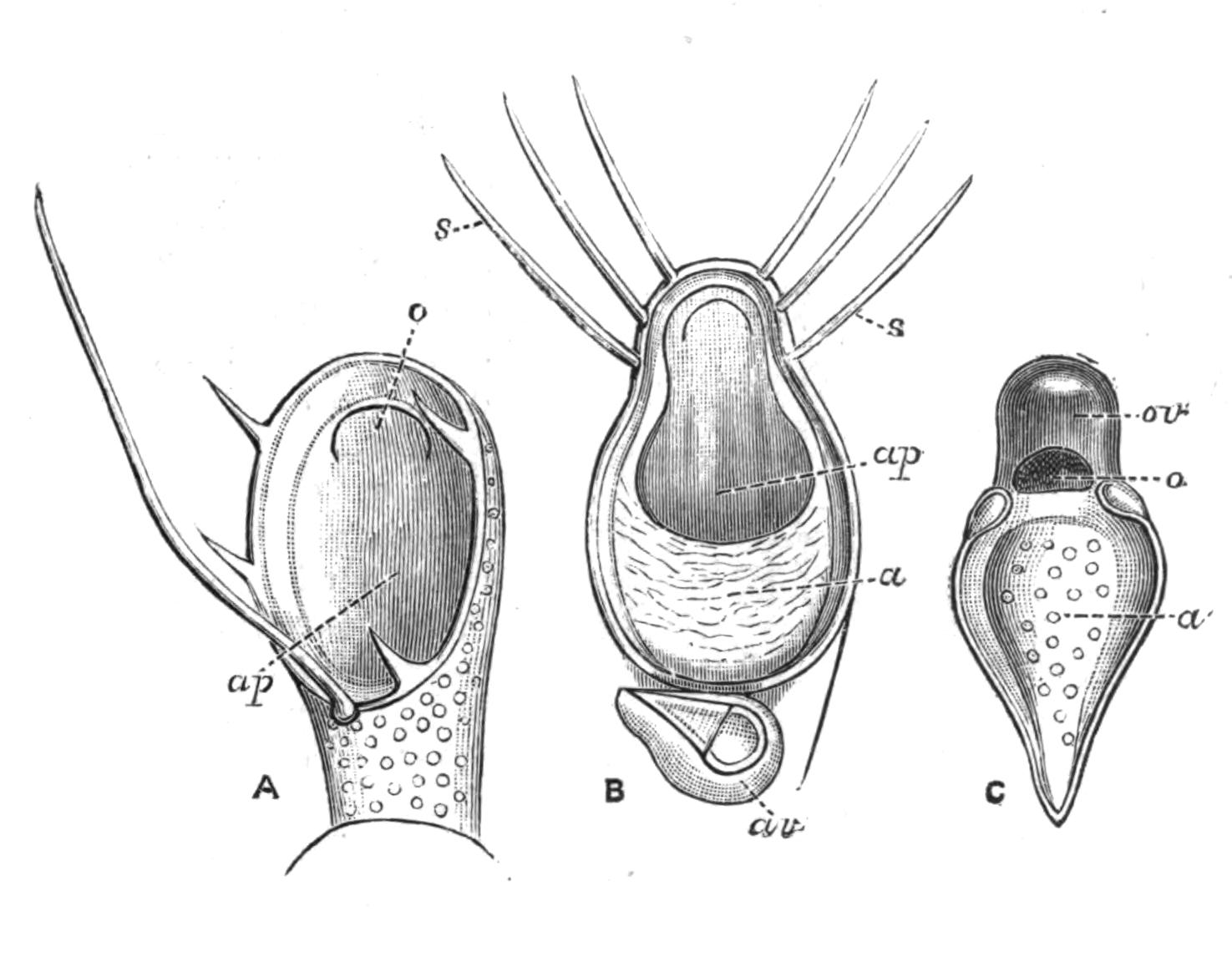
Fig. 256.—Illustrating the terminology of the front surface of the zooecium (Cheilostomata). A, Membranipora (Electra) pilosa L., Cromer, × 47; ap, the membranous "aperture;" o, orifice. B, Membranipora flemingii Busk, Plymouth, × 60; ap, the aperture, enclosed in a calcareous "area" (a); av, avicularium; s, marginal spines. C, Micropora coriacea Esper, Plymouth, × 43; a, area (calcareous); o, operculum; ov, ovicell.
In order to facilitate the use of the table here given in conjunction with Mr. Hincks' work, the nomenclature there adopted has been followed throughout. References to other descriptions of the species may be obtained by consulting Miss Jelly's admirable Synonymic Catalogue of the Recent Marine Bryozoa.[600]
Terminology.—A few technical terms must of necessity be employed. The colony is adherent when its zooecia are attached to the object on which the colony is growing. The zooecium is the body-wall of a single individual; and, except in transparent species, is the only part which can be seen from the outside in the retracted condition of the polypide or tentacles with the alimentary canal. The outermost layer of the zooecium is known as the ectocyst; it may be simply membranous, or calcified, or may be rendered opaque by foreign bodies; its surface in {524}calcareous forms is often marked by pores (Fig. 239, C, p), which are vacuities in the calcareous wall, closed externally by membrane. A special median pore (Fig. 241, A, m.p) may occur, and is in some cases at least a complete perforation through the body-wall.
The tentacles are protruded through the orifice, which in Cheilostomata is usually guarded by a movable chitinous lid, or operculum (Fig. 256, A, o). Should the ectocyst be thickened or raised into a ridge surrounding the orifice, a tubular passage results, known as the secondary orifice (Fig. 255), at the deeper end of which is the true orifice. The peristome (Fig. 255, C, pr) is the raised or thickened part which gives rise to the secondary orifice. Should the zooecium be outlined by a raised ridge, the part so enclosed is known as the area (Fig. 256, C, a), if calcareous. The aperture or opesia (Fig. 256, A, B, ap) is a membranous part of the front surface; and may consist of the whole or part of the area. The orifice or the aperture is commonly provided with spines (Fig. 256, B, s).
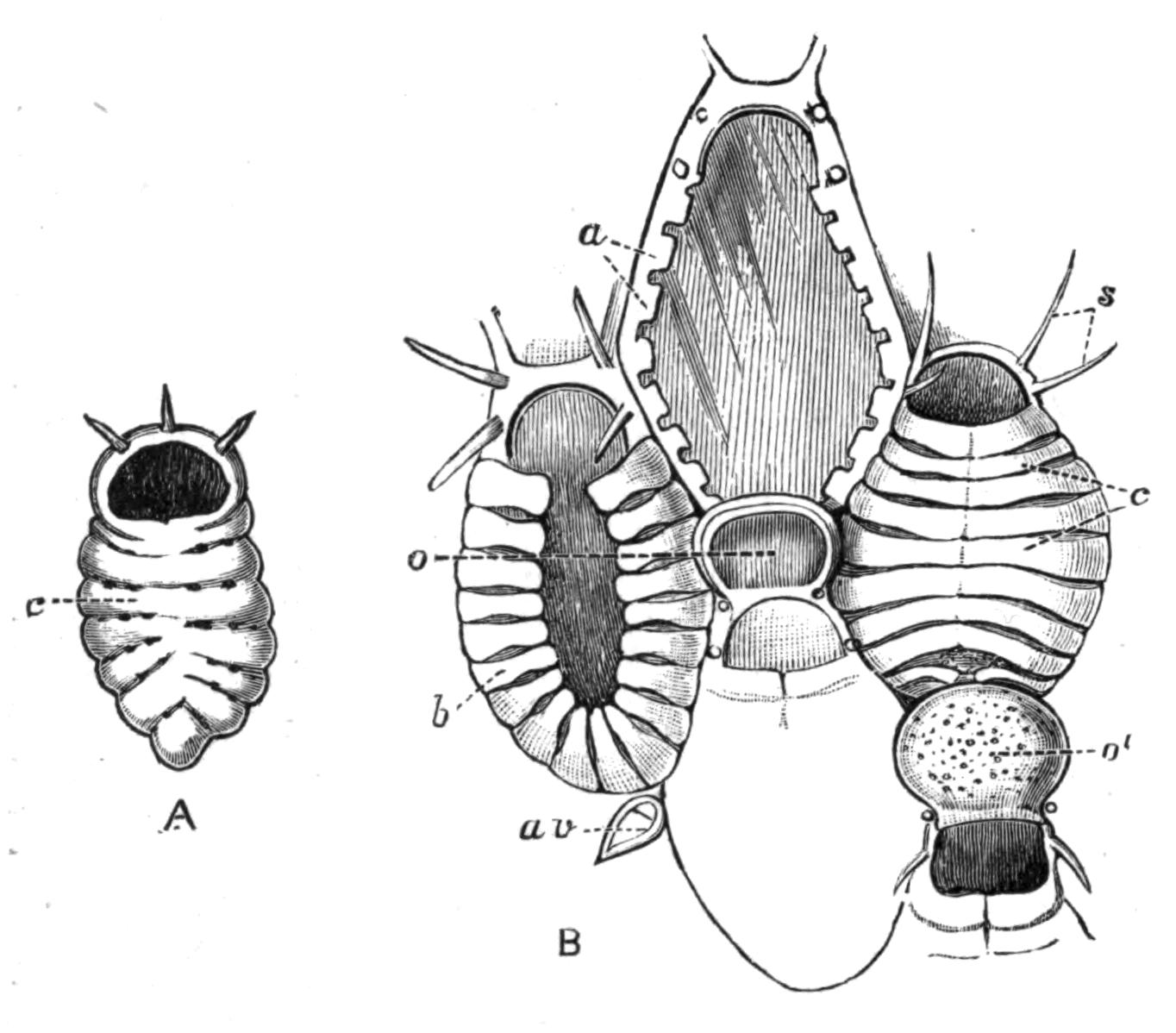
Fig. 257.—A, Cribrilina annulata Fabr., Norway, × 33; c, calcareous bars concealing the membranous aperture: B, Membraniporella nitida Johnst., Plymouth, × 45; a, calcareous bars growing up round the margin of the aperture; b, the same, further developed; c, the same, completely formed (as in A); av, avicularium; o, immature, and o', mature, ovicell; s, marginal spines.
The avicularium and the vibraculum are specially modified zooecia (see p. 482), which occur in a great variety of forms, in certain Cheilostomata only. The operculum of the ordinary zooecium is represented by the mandible (Fig. 239, B, m) in the avicularium, and by the seta (Fig. 242, s) in the vibraculum. The representative of the zooecium itself is known as the avicularian (Fig. 239, A, a.z) or vibracular zooecium (Fig. 242, v.z).
An ovicell is a swelling in which the embryo develops, in certain Cyclostomata (Fig. 237) and Cheilostomata (Fig. 241, A, o). A stolon (Fig. 238, B, st) is a stem, not formed of fused zooecia, from which new individuals originate. An internode, in a jointed colony, is the part between any two joints. The fornix or scutum (Fig. 254, A, f) is a modified spine which in some Cheilostomata overhangs the aperture. A mucro (Fig. 255, A, mu) is a spike or protuberance developed just below the orifice. A sinus (Fig. 239, B, s) is a slight bay on the lower margin of the orifice.
The orifice opens at the upper end of the zooecium, on its front surface. The length of the zooecium is the distance from the upper to the lower ends, and the width the distance between its sides.
| 1. |  |
One or more of the following characters: orifice provided with an operculum; avicularia or vibracula present; a globular ovicell above the orifice of certain zooecia (Cheilostomata) |
||
|
Opercula, avicularia, vibracula, and ovicells completely absent, or inconspicuous. Calcareous or non-calcareous. If calcareous, the orifice is not at the end of a free cylindrical portion |
||||
|
Calcareous; zooecia cylindrical, often united for the greater part of their length, but usually ending in a free cylindrical portion, which bears the terminal orifice. The zooecia may be much obscured by calcifications surrounding their basal parts |
||||
| 2. |  |
Zooecia long, tubular, with a lateral membranous region at the upper end, given off quite separately from a creeping stolon Aetea |
||
|
Zooecia more or less united to one another, orifice without chitinous operculum (Cyclostomata[601]) |
||||
| 3. |  |
Zooecia without marginal spines; arising from a branching axis, which is not formed of zooecia |
||
|
Colony adherent; or erect, fleshy and slightly branched; or erect, encrusted with earthy matter and repeatedly branched |
||||
|
||||
| 4. |  |
Zooecia minute, boat-shaped, united by a delicate tube. Aperture large, with marginal spines Beania mirabilis |
||
|
||||
| 5. |  |
|
||
|
Zooecia uniserial; with marginal spines. Branches arising from the top of a zooecium Brettia |
||||
| 6. |  |
Zooecia uniserial; branches arising just below the large aperture. An ovicell may be developed above the orifice of a modified zooecium Eucratea chelata |
||
|
Zooecia somewhat pear-shaped; orifice small, semicircular Huxleya fragilis |
||||
| {526}
|
||||
| 7. |  |
|
||
|
||||
|
Colony entirely adherent,[602] the zooecia usually in a single layer |
||||
Erect Cheilostomata.
| 8. |  |
Branches cylindrical, calcareous, divided by chitinous joints. Orifices arranged all round the branch Cellaria (Fig. 239, A) |
||
|
Branches flexible, jointed or unjointed. Orifices not arranged all round the branch. |
||||
|
||||
| 9. |  |
|
||
|
||||
| 10. |  |
Avicularia resembling birds' heads, movable Bugula (Fig. 233) |
||
|
Avicularia not resembling birds' heads, unstalked; or absent. Colony broadly leaf-shaped, composed of a single layer or of two layers of zooecia Flustra (Fig. 232) |
||||
| 11. |  |
|
||
|
||||
| 12. |  |
Branches numerous, straight. Zooecia back to back, with an oblique aperture. No avicularia Gemellaria loricata |
||
|
Branches delicate, curved. A pair of stalked avicularia between each two pairs of zooecia Notamia (= Epistomia) bursaria |
||||
| 13. |  |
|
||
|
||||
| 14. |  |
Avicularia resembling birds' heads, movable. Vibracula absent |
||
|
Avicularia large, unstalked. Vibracula present or absent |
||||
|
Avicularia inconspicuous. Setae of the vibracula large, very conspicuous, on oblique vibracular zooecia, which almost cover the backs of the branches Caberea (Fig. 242) |
||||
| 15. |  |
Zooecia in two series, alternate, with one or several conspicuously long marginal spines Bicellaria |
||
|
Zooecia in two or more series. Aperture occupying most of the front of the zooecium. Colony often spiral. Avicularia usually large Bugula (Fig. 233) |
||||
| 16. |  |
Zooecia long, narrow below, commonly in triplets, with two lateral avicularia to each triplet. Fornix present. Menipea ternata |
||
|
Zooecia biserial, a considerable number forming an internode separated by a joint (often inconspicuous) from the next internode. Lateral avicularia usually large. Vibracular zooecia on the back or sides of the branches Scrupocellaria (Fig. 254) |
||||
| 17. |  |
Characters as in Scrupocellaria (No. 16), but with inconspicuous avicularia. A branched fornix Scrupocellaria reptans |
||
|
||||
| 18. |  |
|
||
|
||||
| {527}
|
||||
| 19. |  |
|
||
|
Zooecia biserial. Aperture large, the semicircular orifice at its upper end, where there is commonly a short spine Cellularia peachii |
||||
|
Zooecia in one or two series. Branches originating from the backs of the zooecia, and facing in the opposite direction to the parent branch. Aperture small Scruparia clavata |
||||
| 20. |  |
One or more conspicuously long marginal spines. Avicularia present or absent Bicellaria |
||
|
||||
|
Zooecia biserial, in short internodes. An inconspicuous avicularium below the aperture. Fornix present Menipea jeffreysii |
||||
| 21. |  |
Colony consisting of a network of narrow branches, the zooecia opening only on one of their surfaces Retepora |
||
|
Colony large, brittle, composed of contorted plates, uniting irregularly, usually composed of two layers of zooecia. Orifice large, indented laterally Lepralia foliacea |
||||
|
||||
|
||||
| 22. |  |
|
||
|
Zooecia in more than four regular, longitudinal rows. Peristome raised, and, with the ovicell, forming a swelling on the surface of the branch Escharoides quincuncialis |
||||
| 23. |  |
Orifice circular. A row of pores round the margin of the zooecium. A median pore resembling a small orifice below the true orifice. Small lateral avicularia Porina borealis |
||
|
Orifice surrounded by a peristome, produced into a mucro beneath the orifice. No pores Palmicellaria elegans |
||||
| 24. |  |
|
||
|
Zooecia irregularly heaped, their long axes often perpendicular to the surface of the colony. Mucro largely developed, concealing the form of the orifice, and bearing an avicularium Cellepora |
||||
| 25. |  |
Orifice with a sinus; or peristome interrupted or extended below into a sinus-like outgrowth, which usually includes a small avicularium |
||
|
Neither median sinus nor interrupted or extended peristome |
||||
| 26. |  |
|
||
|
||||
| 27. |  |
Branches of various forms. Surface of the older parts very even. Secondary orifice rather long, usually wider above, enclosing a small avicularium below, and appearing as a hole in the even surface of the branch Porella (Fig. 255, B, C) |
||
|
A prominent tooth projects into the orifice from its lower side. Zooecia with thin walls Smittia landsborovii (Fig. 239, C) |
||||
|
No tooth in the orifice, at the side of which is a small avicularium. Old zooecia with thick walls. Colony composed of a short stem and flattened branches Escharoides rosacea |
||||
| {528}
|
||||
| 28. |  |
A tooth projects from the lower side into the large, subcircular orifice, on each side of which is a small oval avicularium (colony erect or encrusting) Mucronella pavonella |
||
|
||||
| 29. |  |
Branches cylindrical. Old zooecia with thick walls. Orifice in young zooecia longer than broad; beneath it a median pore, and in some cases a lateral avicularium with vibraculoid mandible Diporula verrucosa |
||
|
A distinct mucro, which may bear an avicularium above Palmicellaria |
||||
Encrusting Cheilostomata.
| 30. |  |
Usually growing on a small univalve shell. Orifice longer than broad, indented laterally. Mucro present Lepralia edax |
||
|
One or two conspicuous processes, each bearing an avicularium, near the orifice, which is often concealed. Avicularia in many cases found on other parts of the colony Cellepora |
||||
| 31. |  |
|
||
|
||||
| 32. |  |
|
||
|
||||
| 33. |  |
A tubular process below the aperture, in some cases: zooecia very narrow below Eucratea chelata, var. repens |
||
|
||||
| 34. |  |
|
||
|
||||
| 35. |  |
Zooecia minute, much narrowed below. Orifice small, usually with a sinus Hippothoa |
||
|
Zooecia not narrowed below. Orifice with a sinus Schizoporella |
||||
| 36. |  |
Zooecia partly separated by a thin calcareous crust. Colonies small |
||
|
||||
| 37. |  |
Zooecia pear-shaped. Orifice with a sinus Hippothoa expansa |
||
|
Zooecia ovoid. Orifice subcircular, with a tubular peristome Lagenipora socialis |
||||
| 38. |  |
Orifice close to the upper end of the zooecium (unless crowned by an ovicell). Front of the zooecium marked by transverse or radiating furrows or lines. The very young zooecium may possess a membranous area, which becomes roofed in by the union of two lateral series of converging bars (Fig. 257) |
||
|
||||
| 39. |  |
Furrows with uniserial rows of pores (often minute), which are rarely irregular Cribrilina (Fig. 257, A) |
||
|
No rows of pores. Distinct transverse lines or spaces and a median longitudinal suture between the bars Membraniporella (Fig. 257, B) |
||||
| {529}
|
||||
| 40. |  |
|
||
|
Zooecia irregularly[603] heaped together (cf. No. 30) Cellepora |
||||
| 41. |  |
Primary orifice conspicuous; with a sinus, or with a peristome extended or interrupted below, and sometimes simulating a sinus |
||
|
||||
|
Surface of the old zooecia much thickened, so that the secondary orifice does not project beyond the most prominent parts of the zooecium. Secondary orifice concealing the primary orifice, wider above, enclosing a small avicularium below Porella (Fig. 255, B, C) |
||||
| 42. |  |
|
||
|
A prominent tooth projects into the orifice from its lower side. Peristome interrupted or with a sinus. Surface of the old zooecia not much thickened Smittia |
||||
| 43. |  |
Orifice with a sinus and long spines. Peristome interrupted. Ovicell with a wedge-shaped or linear longitudinal fissure. Avicularia generally present, the avicularian zooecium conspicuous. Schizotheca |
||
|
Orifice semicircular. Vibracula present, near the orifice. Mastigophora (Fig. 241, B) |
||||
|
Orifice semicircular or subcircular. No vibracula; avicularia with vibraculoid mandibles may occur Schizoporella (Fig. 239, B) |
||||
| 44. |  |
Zooecium with a median pore; or completely tubular above |
||
|
Zooecium with no median pore. The orifice may be partially surrounded by a collar-like development of the peristome, but it is not completely tubular |
||||
| 45. |  |
Orifice not tubular. A median pore Microporella (Fig. 241, A) |
||
|
||||
| 46. |  |
|
||
|
||||
| 47. |  |
Orifice markedly tubular. Median pore conspicuous Porina tubulosa |
||
|
Colony very small. Zooecia irregularly arranged, with no median pore Celleporella |
||||
| 48. |  |
Zooecia very convex, with a granular surface; ovicells set far back. Orifice wider than long Mucronella microstoma |
||
|
Young zooecia with stellate pores. A minute avicularium, or merely a pore, on the upper and lower sides of the orifice in some zooecia. Anarthropora monodon |
||||
| 49. |  |
Front of zooecium with an elevated margin, enclosing an area |
||
|
||||
| 50. |  |
|
||
|
||||
| {530}
|
||||
| 51. |  |
Avicularian or vibracular zooecia replacing an ordinary zooecium, or at least situated between the zooecia |
||
|
Avicularia and vibracula absent, or if present not replacing a zooecium |
||||
| 52. |  |
Vibracula present. Colonies small. A pair of longitudinal slits within the area Setosella vulnerata |
||
|
Very large avicularia present. Ovicell closed by a movable lid. Orifice subcircular, with a minute lateral tooth on each side. Thalamoporella[605] (Steganoporella) smittii |
||||
| 53. |  |
Orifice semicircular, quite at the upper end of the zooecium; usually with a knob on each side Micropora coriacea[606] (Fig. 256, C) |
||
|
A transverse chitinous plate lies immediately below the operculum. A vibraculoid spine may occur Megapora ringens |
||||
| 54. |  |
Area entirely membranous, usually bordered by spines. Membranipora (including Electra[607]) (Fig. 256, A) |
||
|
Membranous portion reduced to a small portion, which may be variously lobed, enclosing the orifice. Membranipora (other species) (Fig. 256, B) |
||||
| 55. |  |
|
||
|
||||
| See also Mucronella pavonella, No. 28). | ||||
| 56. |  |
Peristome collar-like, much raised below and at the sides of the orifice, deficient above. No avicularia Phylactella |
||
|
Orifice large, longer than broad. Peristome not deficient above the orifice Lepralia |
||||
| 57. |  |
|
||
|
||||
| 58. |  |
A minute avicularium above the orifice, or where an ovicell is present, situated at the summit of that structure. Zooecia not quite contiguous. Mucro sometimes present. Chorizopora brongniartii |
||
|
No avicularia. Ovicells on rudimentary zooecia, lying in a plane superficial to that of the rest of the colony. Zooecia long. Schizoporella hyalina |
||||
| 59. |  |
|
||
|
Mucro rarely present. Orifice nearly always longer than broad, or nearly circular, usually large, and slightly indented laterally. Lepralia |
||||
| 60. |  |
|
||
|
||||
| {531}
|
||||
| 61. |  |
Colony glistening. Orifice much obscured by the mucro and by stout spines developed from the peristome. Tooth (concealed in old zooecia) large, strongly curved to one side. Rhynchopora (Rhynchozoon[608]) bispinosa |
||
|
Tooth of the lower margin of the orifice symmetrical, sometimes bifid. Avicularia may be present laterally, but are not developed on the mucro Mucronella (Fig. 255, A) |
||||
| 62. |  |
Orifice at least half the width of the zooecium, bordered below by a well-developed prominence or "umbo." Surface of the zooecium strongly areolated round the margin Umbonula[609] |
||
|
Orifice considerably less than half the width of the zooecium. Schizoporella (Fig. 239, B) |
||||
Encrusting Cyclostomata.
| 63. |  |
Colony erect. Branches of two or one series of zooecia, divided at intervals by chitinous joints. Ovicells pear-shaped. Crisia (Fig. 237) |
|
||
|
||
| 64. |  |
Colony more or less circular, discoidal or cup-shaped, sometimes forming secondary colonies by marginal budding |
|
||
| 65. |  |
Zooecia separated by calcified interspaces, which may contain large pores, often difficult to distinguish from the orifices |
|
No large pores as above. Orifices not spiny. Zooecia nearly always contiguous, except where an ovicell is developed |
||
| 66. |  |
Colony composed of one or more convex discs, bearing radial ridges, each composed of many zooecia Domopora |
|
Colony encircled by a thin calcareous lamina, which gives rise to new zooecia, its centre usually devoid of zooecia when adult, and often bearing the orifice(s) of the ovicell. Zooecial orifices often spiny. Lichenopora |
||
| 67. |  |
Zooecia with a long, tubular, free portion, in some cases curved in a horizontal plane. Colony fan-shaped until a late stage. Tubulipora flabellaris |
|
Tubular portion absent, or for the most part curved in a vertical plane. Some of the orifices may be closed by a calcareous plate. Colony circular or bluntly lobed Diastopora |
||
| {532}
|
||
| 68. |  |
Zooecia in one or few series, forming a linear or branched colony, which is closely adherent, but may give rise to short erect portions. Branches narrow, but often broadening at their ends. Zooecia usually with a free upper end Stomatopora |
|
Colony broadly lobed, some of the zooecia in transverse or oblique ridges composed of contiguous zooecia, arranged like a row of organ-pipes Idmonea serpens |
||
|
Colony broadly lobed, or fan-shaped; zooecia in many series, which are not arranged like organ-pipes Tubulipora |
||
| 69. |  |
Well branched. Orifices confined to one surface of the colony |
|
||
| 70. |  |
Zooecia in transverse rows, their upper ends united in the manner of a row of organ-pipes. Ovicell (when present) an inflation of the front of the branch Idmonea atlantica |
|
Zooecia not in regular transverse rows. Ovicell (when present) large, mostly on the back of the branch Hornera |
||
| 71. |  |
Branches cylindrical, their ends massive and raised into radial ridges, which carry the orifices Domopora stellata |
|
Ends of zooecia tubular, arranged all round the branch. Entalophora |
||
Encrusting Ctenostomata.
| 72. |  |
Colony entirely adherent, or forming thick, soft, erect lobes |
||
|
Colony erect, well-branched, dark and opaque, resembling seaweed. Zooecia with a long tubular free portion Anguinella palmata |
||||
| 73. |  |
Orifice large, with two distinct lips. A variable number of stout, brown spines. Encrusting Flustrella hispida |
||
|
Orifice small, rounded, borne by a more or less distinct papilla. Encrusting or erect. Zooecia crowded, rarely in single lines. Alcyonidium |
||||
|
Orifice small, rounded. Zooecia widely separated, connected by narrow tubes Arachnidium |
||||
| 74. |  |
|
||
|
||||
| 75. |  |
|
||
|
||||
| 76. |  |
|
||
|
Zooecia long, less regularly arranged. Polypide with a gizzard. Bowerbankia (Fig. 238) |
||||
| 77. |  |
Clusters of zooecia very regular, occurring immediately below a bifurcation of the axis. Zooecium with a broad base, not movable. Amathia lendigera |
||
|
Zooecia arranged like the pinnules of a leaf, with a constricted base, and movable on the branch Mimosella gracilis |
||||
| {533}
|
||||
| 78. |  |
Main stem zigzag. Branchlets delicate, many ending in sharp points. Zooecia small, ovoid Vesicularia spinosa |
||
|
Axis jointed. Zooecia small, in small clusters. Polypide without a gizzard Valkeria uva, var. cuscuta |
||||
|
Zooecia in whorls, attached to the axis by thread-like stalks, much longer than themselves Hippuraria egertoni |
||||
| 79. |  |
Zooecia pear-shaped, produced at the lower end into a distinct stalk. Gizzard absent |
||
|
Zooecia not distinctly stalked, although sometimes constricted at the base |
||||
| 80. |  |
Stalk long. Zooecium movable on its stalk, compressed, with a membranous area on one side. Twelve or more tentacles. Usually found on Crustacea Triticella |
||
|
Stalk variable. Zooecium very transparent; orifice bilabiate. Ten to sixteen tentacles Farrella repens |
||||
|
Zooecium very small, much elongated and narrow. Eight tentacles. Valkeria tremula |
||||
| (See also Arachnidium, No. 73). | ||||
| 81. |  |
Zooecia short, minute, with a few short spines on each side of its broadened base. Upper end tubular Buskia nitens |
||
|
||||
| 82. |  |
|
||
|
||||
| 83. |  |
Zooecia large (about 1⁄16 inch long), distant, constricted at the base, bearing scattered bristles. Usually found on Crabs or Hydroids. Avenella fusca |
||
|
Zooecia tall, cylindrical, not constricted at the base. Cylindroecium |
||||
| 84. |  |
Zooecia minute. Axis dilating at intervals into swellings, from which new zooecia originate. These may give rise to new stolons, or directly to new zooecia. No gizzard. Found in brackish or fresh water Victorella pavida |
||
|
||||
| 85. |  |
|
||
|
Zooecia long, scattered or in groups. Gizzard present. Bowerbankia (creeping forms) |
||||
It is highly probable that the Ctenostome genus Hypophorella[610] will before long be added to the British Fauna. The animal consists of delicate stolons, which give off small zooecia at intervals; and it is known to excavate passages in the substance of the tubes of certain Polychaet worms (Chaetopterus and Lanice).
ADDENDUM TO CHAETOGNATHA
Since the Chapter on the Chaetognatha was printed the following list[611] of "The Known Chaetognaths of American Waters" has appeared:—
1. Sagitta elegans Verr. This species resembles S. bipunctata (vide pp. 191 and 193), but differs in size, in the relative proportions of caudal and body segments, and in the presence of diverticula from the intestine.
2. Sagitta flaccida Con. This species resembles S. hexaptera (vide p. 193); it is, however, smaller (length, 1.3-1.8 cm.) and has more spines (anterior, 7-8, posterior, 10-12), and its tail segment is relatively smaller.
3. Sagitta tenuis Con. Length, 5.25 mm.; hooks, 7-8; anterior spines, 4-5; posterior spines, 7-10.
4. Sagitta hispida[612] Con. Length, 7-11 mm.; hooks, 8-9; anterior spines, 4-5; posterior spines, 8-15; tail segment one-third body length; intestine with two diverticula; sensory hairs very numerous.
5. Sagitta hexaptera (vide p. 193).
6. Krohnia hamata (vide p. 194).
7. Spadella maxima Con. Length, 5.2 cm.; hooks, 6; anterior spines, 3-5; posterior spines, 5-7; epidermal thickenings round the neck.
8. Spadella draco (vide p. 194).
9. Spadella schizoptera[612] Con. An opaque, yellowish-brown species living among algae. Length, 4 mm.; hooks, 8; anterior spines, 4-6; posterior spines wanting. Caudal segment occupies one-half the body length.
Professor Verrill states that the name S. gracilis (vide p. 191) was due to a clerical error, the species really referred to being S. elegans.
A. E. S.
Every reference is to the page: words in italics are names of genera or species; figures in italics indicate that the reference relates to systematic position; figures in thick type refer to an illustration; f. = and in following page or pages; n. = note.
Acanella, as host, 298
Acanthobdella, 395
Acanthocephala, 123, 124, 174 f.;
embryology, 179;
classification, 181
Acanthocotyle, 73
Acanthodrilidae, 357, 362, 381, 383, 384
Acanthodrilus, 356, 363, 366, 372, 382, 384;
chaetae, 350
Acanthozoon, 20
Accessory gut, of Polychaeta, 305
Aceros, 19
Achaeta, 445
Acholoe, 297
Acmostoma, 50
Acoela, 42;
occurrence and habits, 43;
reproduction, 47;
classification, 49
Acrorhynchus, 49;
occurrence, 44
Actinotrocha larva, 458
Acyclus, 221
Adaptation, of Trematodes, 52, 62;
of Cestodes, 74;
of Nematodes, 161
Adherent, 523
Adventitious avicularia, 482
Aeolosoma, 349, 353, 354, 360, 370, 374, 375
Agassiz, on Syllidae, 280
Alaurina claparedii, 49
Albertia, 204, 210, 213, 224, 227
Alciope, 315
Alciopids (Alciopina), 314;
head, 263;
parapodium, 265;
habit, 291;
light-organs, 294
Alciopina parasitica, 298
Alcyonellea, 518
Alcyonidium, 477, 480, 492, 518, 532;
structure of zooecium, 469;
Alimentary canal—see Digestive System
Alitta, 317
Allantonema, 131, 150, 151, 161
reproduction, 47;
classification, 50
Alloiogenesis, 66 n.
Allolobophora, 351, 367, 369, 371, 386, 389, 390 f.;
cocoons, 365
Allostoma pallidum, 50
Alluroides, 379
cocoons, 365
Alternation of generations, 66, 81, 281
intestine, 271
Ampharete, 330
Amphibia, Trematodes of, 55, 62, 71, 72;
Nematodes of, 163
Amphichaeta, 377
Amphicoerus, 49
Amphicora, 339
Amphicorine, gill, 261
Amphicteis, 330
Amphileptus, 235
Amphilina, 91
Amphinome, eye of, 255;
A. smaragdina, colour, 293
shape, 259;
parapodium, 264;
cirri, 265;
in Lepas, 297
British species, 110
Amphiptyches, 77
{536}Amphistomatidae, 73
A. hominis, 63
gill, 329
Ampullaria, Temnocephala with, 53
Anal, cirri, 259;
Anangian worms, 253
Anarthropora, 529
Andrews, on Sipunculus, 417, 426
Angiostomum, 134
Angler-fish, Trematodes of, 62, 72
A. tritici, 125;
A. diplogaster, 155
Anguinella, 532
Annadrilus, 386
Annelida, 241
Anocelis, 42
Anonymidae, 19
penes, 27
Anopla, 109
Anoplocephala, 91;
characters, 90:
A. mamillana, 90;
A. perfoliata, life-history, 83;
specific characters, 90:
A. plicata, specific characters, 90
Anoplodiscus, 73
Anoplodium, 50;
A. parasiticum, occurrence, 45
Antaeus, 388
Antedon, as host, 342
Antenna, of Rotifers, 215
Anthocotyle, 73
Antinoë, 298
Antipathes, as host, 298
Anuraeidae, 201, 205, 225, 226
A'oon, an edible worm, 297
Apel, on Priapuloidea, 433
Aperture, of zooecium, 468, 517, 523, 524
Aphanostoma, 49
Aphanostomatidae, 49
Aphrodite, 312;
shape, 258;
head, 260;
peristomium, 263;
chaetae, 268;
felting, 312;
intestine, 271;
genital cells, 273;
colour, 291:
A. aculeata, 312;
distribution, 299;
A. echidna, 299
frontal ridge, 260;
parapodium, 264;
chaetae, 266
Apical plate, of Trochosphere, 245
Apodina, 235
Apodoides, 225
Apogon, Scolex polymorphus in, 77
Apsilidae, 201, 203, 214, 220, 221
Apsilus, 201, 212, 213, 214, 221
Arabellites, 302
Arachnidium, 532
Archiannelida, 241;
anatomy, 243 f.;
nerve cords, 255;
significance of, 77
Arenicola, 333;
perienteric sinus, 252;
prostomium, 259;
body, 259;
head, 264;
gill, 265;
chaetae, 266 f.;
genital organs, 273;
otocyst, 273;
burrows, 285;
pigment, 291;
colour, 293;
A. marina, 333;
habits, 301;
in brackish water, 284;
as bait, 297;
eggs, 314
Argilophilus, 372
Arhynchidae, 185
Arhynchus hemignathi, 181, 185
Aricia, otocyst, 273;
eggs, 275
gill, 265
Aristotle, on Earthworms, 347
Arthropoda, absence of cilia in, 124
A. acus, 130;
A. alata, 140;
A. depressa, 141;
A. ferox, 141;
A. incurva, 141;
A. leptoptera, 141;
A. lumbricoides, 125, 134, 135, 139, 163;
A. megalocephala, 125, 127, 128, 131, 136, 140, 163;
A. mucronata, 141;
A. nigrovenosa, 155;
A. rubicunda, 141;
A. suillae, 139;
A. sulcata, 141;
Ascodictyon, 521 n.
Ascomorpha, 223
Ascopodaria, 488 n.
Asellus, Rotifers attached to, 227
Asexual reproduction, in Triclads, 40;
in Rhabdocoels, 44;
in Cestodes, 80;
in Trichoplax and Salinella, 96;
in Polychaeta, 278 f., 279, 280, 282, 340;
in Oligochaeta, 374, 375, 377;
Aspidobothridae, 73
Aspidocotyle, 73
Aspidocotylea, 73
Aspidosiphon, 421, 423, 424, 425, 428;
commensalism of, 429
Asplanchna, 200, 205, 210, 213, 215 n., 223, 226
Asplanchnaceae, 203, 212, 220, 222
Asplanchnidae, 200, 201, 203, 205, 211, 212, 216, 223, 226, 230
Asplanchnopus, 201, 211, 222, 223, 226, 230
Ass, parasites of, 140
Association, of Rhabdocoels with Lamellibranchs and Sea-urchins, 45;
of Monotus fuscus with littoral animals, 46;
significance, in Turbellaria, 51—see also Commensal and Parasitic
{537}Asteroids, as hosts, 341
Asterope, 315
Astropecten, as host, 297, 309
Atokous phase, 277 n.
Atractonema, 131, 150, 152, 153
Atrium (genital), in Planaria, 38, 39;
Auditory organs, of Turbellaria, 26;
of Nematoda, 128;
of Polychaeta, 273
Auricles, of Rotifers, 205
Autolytus, 308;
eye, 255;
denticles, 270;
sexual dimorphism, 281;
A. ebiensis, eggs, 276
Automolos, British species, 50
Avenella, 533
Avicularian zooecium, 482, 524
Avicularium, 466, 467, 468, 482 f., 482, 516, 517, 522 f., 524;
adventitious, 482;
vicarious, 482;
structure, 483;
movements, 485;
function, 486
Baely, on human parasites, 139
Baird, on Oligochaeta, 382
Bait, Polychaeta as, 297
Baker, on Rotifers, 197, 207; on Polyzoa,
496 n.
Balanoglossus, affinities of Nemertinea with, 120
Balfour, on Trochosphere, 229
Barentsia, 488 n.
Barrois, on Polyzoa, 509
Basement-membrane, of Leptoplana, 11, 12;
of Nemertines, 102, 103, 110 f.
Bathymetrical distribution, of Polychaeta, 300
Bdellodrilus, 376
Bdelloida, 201, 203 f., 211, 213, 215, 216, 222, 227
Beddard, on Tetrastemma aquarium dulcium, 118;
on Oligochaeta, 347 f.;
on Leeches, 392 f.
Bedwell, on Rotifers, 198
Beneden, van, on Cestodes, 76;
on Nematodes, 162;
on Phoronis, 450
Benham, on a fresh-water Tetrastemma, 118;
on Archiannelida, 241 f.;
on Polychaeta, 245 f.;
on Myzostomaria, 341 f.;
on Oligochaeta, 357, 373, 382;
on Phoronis, 452 f.
Benhamia, 383 f.
Bicellaria, 481, 518, 526, 527
Bilfinger, on Rotifers, 212
B. crassa, 70;
Bimastos, 389
Birds, Trematodes of, 62, 63, 64, 72;
Gordius of, 173;
Bisexual, Turbellaria, 44;
Trematodes, 70 f.
Bladder, of Rotifers, 214
Blanchard, on Cestoda, 91;
on Hirudinea, 392 f., 405, 408
Blastomeres, of egg of Distomum, 65
Blood, of Nemertinea, 108;
of Polygordius, 244;
of Chaetopoda, 252;
Blood-corpuscles, in Chaetopoda, 252
Body-cavity (including Coelom), of Nematoda, 130;
of Gordius, 166;
of Chaetognatha, 187;
of Polychaeta, 249;
of Myzostoma, 343;
of Oligochaeta, 355;
of Leeches, 397;
of Gephyrea, 416;
Body-wall, of Nemertinea, 102, 103;
of Nematodes, 125;
of Gordiidae, 165;
of Acanthocephala, 175;
of Chaetognatha, 187;
of Rotifers, 205;
of Nereis, 249;
of Oligochaeta, 349;
of Phoronis, 454;
Bohadsch, on Gephyrea, 411
Bohemilla, 377;
chaeta, 350
male, 438;
development, 439;
habits of, 442;
as host, 297
Bonnet, on Oligochaeta, 348, 379
Borlase, on Lineus marinus, 99
Borlasia elizabethae, 111, 114
Bothriocephalidae, 91
Bothriocephalinae, 91
Bothriocephalus, 91;
life-history, 84;
reproductive organs, 87 f.;
larva, 87;
B. mansoni (= B. liguloides), 81, 91
Bothriocerca, 226
Bothrioneuron, 379
Bothromesostoma personatum, 49
Bourne, on Oligochaeta, 352, 373, 377 n., 380;
on Leeches, 400
Bouvier, on commensal Gephyrea, 429
{538}Bowerbankia, 470, 480, 481, 492, 500, 518, 532, 533;
budding, 514
Brachionidae, 225
Brachionus, 200, 201, 204, 218, 225, 226, 227
Brachydrilus, 357
Brackish water, Rotifers, 226;
Polychaeta, 284;
Polyzoa, 492
Braem, on statoblasts, 503 f.
Brain—see Nervous System
Branchellion, 393, 395, 397, 401, 406
Branchial crown, 336;
regeneration of, 283
Branchiobdella, 376
Branchiomma, 337;
gills, 261;
eyes, 272;
B. vigilans on Aphrodite, 299
Branchiura, 352, 361, 367, 378 f.;
transverse section, 353
Braun, on Platyhelminthes, etc., 6 n., 55 n., 62, 94
Bristles = Chaetae, q.v.
Bristle-worms, 241
British, Polycladida, 19;
Tricladida, 42;
Rhabdocoelida, 49;
Polychaeta, 306 f.;
Earthworms, 390;
Leeches, 393;
Gephyrea, 449;
Brood-pouch, of Spirorbis, 261, 276, 341;
of Salmacina, 276;
Brood-sac, of Autolytus, 275;
of Myrianida, 280
Brown body, in Polyzoa, 468, 471 f., 472, 489, 496, 510, 514
Brown tubes (nephridia), of Sipunculoidea, 415, 417, 423, 425;
of Echiuroidea, 435, 437, 439, 441;
of Epithetosomatoidea, 445;
used as generative ducts, 418, 438;
absent in Priapuloidea, 430
Bryozoa, 475
Buccal region, in Polychaeta, 249, 250, 269;
of Nereis diversicolor, 248;
of N. cultrifera, 316
Buchanan, on marine muds, 423
Buchholzia, 359
Budding, in Syllidae, 279, 283 (see also Gemmation);
in Polyzoa, 467, 514 (see also Polypide-bud)
Bugula, 467, 468, 477, 481, 515, 517, 518, 519, 526;
larva, 511
Bunge, on respiration in Nematoda, 130
Bürger, on Nemertinea, 109, 112;
on Nectonema, 168;
Burrows, of Polychaeta, 285, 304;
of Cirratulus, 286;
of Arenicola, 333;
fossil, 302;
of Earthworms, 368;
of Sipunculus, 426
Bursa seminalis, in Rhabdocoels, 48
Busk, on Polyzoa, 465 n., 475, 487, 519
Buskia, 533
Bütschli, on Nematoda, 137
Byrsophlebs, occurrence, 44;
British species, 49
Caecum, in Polyzoa, 499
Calathus, host of Gordius, 172
Calceostominae, 73
Calceostomum, 73
Caldwell, on Phoronis, 454, 456, 461
Calicotyle, 73
Callidina, 201, 202, 204, 218, 219, 222, 225, 227, 230
larva, 77
Calotte, of Dicyemids, 93
Calyx, 488
Camerano, on development of Gordius, 170
Capitella, 331;
peristomium, 263;
habitat, 286;
colour, 291;
O. capitata, distribution, 299
guanin in, 253;
body, 259;
buccal region, 269;
siphon, 272;
genital organs, 273
Carabus, host of Gordius, 172
Carinella, 112;
British species, 112
Carinellidae, side organs of, 107
Caruncle, of Amphinomidae, 260, 273 n., 318
Caryophyllaeus, 91;
C. mutabilis, 77
Castalia, 308;
distribution of, 300
Castings, of Polychaeta, 285;
fossil, 302;
of Arenicola, 333
Cat, parasites of, 80, 125, 130, 140, 143, 144, 145
Catenula, 49
Cathypna, 225
Cathypnidae, 225
Cellaria, 479, 515, 518, 519, 526;
zooecia and avicularium, 482
Cellepora, 518, 527, 528, 529;
Celleporella, 529
Cellularina, 518
Cement-glands, of Rotifers, 205
Cephalic slits, of Nemertinea, 101, 104, 107, 111, 112
Cephalisation, in Polychaeta, 263;
in Oligochaeta, 377
Cephalodiscus, 461 f.
Cephalopods, parasites of, 78, 92;
list of, containing Dicyemids, 94
Cephalothrix, 112
{539}Cercaria, 13, 65, 67, 71 f.;
C. macrocerca, 72;
C. cystophora, 72
Cercyra, 42
Cerebral organ, of Nemertinea, 107;
of Gephyrea, 417
British species, 111
Cerfontaine, on Earthworms, 349, 350
Cestoda, characters of the group, 5, 74;
nature of, 76 f.;
occurrence, 77-82;
life-histories, 83;
structure and development, 84-89;
synoptic table of, 89 f.;
classification, 91
Cestodariidae (= Monozoa), 91
Cestoplanidae, 19
Chaetae, 241;
provisional, 274;
jointed, 246;
natatory, in sexual Syllid, 278, 307;
palmate, of Coabangia, 339 n.;
colour, 291;
genital, of Capitella, 331;
of Sternaspis, 336;
special, of Polydora, 261, 267;
of Myzostoma, 342;
of Oligochaeta, 347, 350, 351, 352;
penial, 362;
of Microdrili, 375 f.;
of Megadrili, 381 f.;
(= hooks), of Echiuroid Gephyrea, 434, 435, 438, 440 f., 446
Chaetobranchus, 352
anatomy, 186;
development, 189;
habits, 189;
classification, 191;
key to, 193;
American species, 534
Chaetopoda, 241 f.;
as food for Nemertinea, 115
anatomy, 323 f.;
special chaetae, 267;
pigment, 292;
Ch. variopedatus, 324
Chaetosoma, 158
Chaetosomatidae, 158
Chaetosyllis, form of head, 278
Chaetozone, 326;
uncini, 268
Chaetura, 235
Chalk, Serpulids of, 301
Charles, on male guinea-worm, 148
Cheilostomata, 477, 506, 518, 519, 525, 526 f.;
occurrence, 478;
external characters (see also Avicularium and Vibraculum), 481;
ovicells, 507;
reproduction, 507 f.;
larva, 511;
fossil, 521
Chiaje, Delle, on Gephyrea, 411
Chickoff, on Triclads, 41
Chironomus, host of Gordius, 172
in coelomic corpuscles, 252
Chloeia, colour, 291
Chlorhaemidae, 258, 305, 334, 336;
chlorocruorin in, 252;
palps, 260;
tentacles, 262
colour due to, 291
Chone, 338
Chorizopora, 530
Cilia, 3;
of Leptoplana, 10, 11, 12, 15;
of Müller's larva, 29;
of Land Planarians, 33;
of Planaria lactea, 35;
of Temnocephala, 53;
of Trematode-larvae, 3, 59, 60, 65;
of Cestode-larvae, 87;
absent in certain groups, 124, 396;
of Rotifers, 202 f.;
of Echiuroidea, 434;
of Phoronis, 453;
Ciliated, lappets, of Pterosyllis, 273 n.;
pits, of Polygordius, 244;
pits (= nuchal organs), of Polychaeta, 272 f.;
organs, of Capitelliformia, 305
Cingulum, in Rotifers, 202
gill, 265;
tentacular filaments, 304 n.
Cirratulus, 326;
burrows, 286;
pigment, 292;
colour, 293;
viviparous, 276;
C. tentaculatus, 326
Cirri, of Nereis, 246;
of Polychaeta, 265;
of Myzostoma, 342;
anal, 259;
nuchal, of Eunicidae, 318;
peristomial, of Nereis, 248;
nerves to, 254;
of Polychaeta, 263
Cladocora, with Myxicola, 294
Claparède, on Heteronereis, 276, 277;
Claus, on Nematoda, 138;
on Seisonaceae, 225 n.
Clepsine—see Glossiphonia
Clover sickness, 155
Clymene, 333;
C. ebiensis, tube of, 287
Clymenidae—see Maldanidae
Cobb, on Nematoda, 131 n., 138
Cobbold, on Nematoda, 140
Cobitis, host of Gordius, 173
Cochleare, 225
Cocoons, of Triclads, 40;
of Leeches, 404
Coelom—see Body-cavity
Coelomic fluid, of Polychaeta, 252;
as cause of colour, 291
Coelopus, 225
Cohn, on Rotifers, 198
Collar, peristomial, of Sabellidae, 336;
of Gephyrea, 421;
of Ctenostomata, 470, 477, 480, 481
Colonial nervous system, 471
{540}Colony, of Myrianida, 281;
of Syllis ramosa, 282;
of Polyzoa, 466
Colour of Polyclads, 20;
of Land Planarians, 33;
of Nemertinea, 102;
Comatula, as host, 342
Commensal, Polychaeta, 297 f., 323, 325;
Polyzoa, 489
Conn, on development of Gephyrea, 419 n., 441, 444, 447
Conoceros, 19
Conochilus, 202, 203, 205, 215, 221, 226
Conocyema, hosts of, 94
Convoluta, 45;
C. henseni, pelagic habit, 43;
C. roscoffensis, assimilating tissue, 43
Copepoda, on Polychaeta, 299
Coral reefs, Polychaeta in, 293
Corallina (= Coralline Alga), 14, 488, 516
Coralline, 465
Corallobothrium, 91
Corethra, host of Gordius, 172
Cori, on Phoronis, 451 f.
Cornulites, 302
Cotylogaster, 73
Cotyloplana, 35
Crateromorpha, as host, 282
Crayfish, Temnocephala associated with, 53
Creeper, 317
Crepina, 450
Crinoids, as hosts, 341
Crisia, 471, 478, 479, 480, 507, 518, 531
Crisiidae, 517
Crisp, on Parasites, 164
Cristatella, 494 f., 495, 499, 501, 503-505, 512, 518;
attacked by Planarians, 486;
larva, 512
Crossopodia, 302
Crustacea, parasites of, 174, 179, 182
vascular system, 252;
prostomium, 259;
tentacles, 263;
eyes, 272;
food, 296
Cryptodrilidae, 357, 362, 373, 382
Ctenodrilus, 373
Ctenophores, as hosts, 298
Ctenostomata, 470, 477, 479, 480, 518, 532;
occurrence, 478;
in fresh water, 492;
external characters, 480;
reproduction, 507;
larva, 511;
relation to Phylactolaemata, 493, 502 f.;
fossil, 521 n.
Cucumaria, as host, 298
Cuénot, on Gephyrea, 416 n.
Cuticle, of Nemathelminthes, 125, 165, 175;
of Rotifera, etc., 205, 233, 236;
of Polyzoa, 470—see also Epidermis
Cuvier, on Oligochaeta, 352;
on Gephyrea, 411
Cyclatella, 489
Cyclicobdella, 392
Cyclops, parasites of, 143, 148, 161
Cyclorhagae, 238
Cyclostomata, 477, 479, 506, 517, 518, 525, 531;
occurrence, 478;
external characters, 480;
ovicells, 507;
Cydippe, as host, 298
Cylindroecium, 533
Cylindrostoma, 46;
British species, 50
Cyphonautes, 509, 510, 512, 520
Cyprina, Malacobdella found on, 119
Cyrtonia, 224
Cyst, of Land-Planarians, 33;
(capsules), of Aeolosoma, 370, 375
Cysticercoid-larva, 83, 85, 88
list of, 83;
C. pisiformis, development, 81, 85, 89
Cysticolous, Myzostomaria, 344
Cystoidotaeninae, 91
Cystotaeninae, 91
Dactylogyrus, 73
Dalyell, on habits of Turbellaria, 6, 10, 20;
on regeneration in Polychaeta, 283;
on tubes of Polychaeta, 287;
on Hirudinea, 405 n.;
on larvae of Flustra, 466;
on Cristatella, 496
Danielssen and Koren, on Gephyrea, 442, 444
Daphnia, Rotifers attached to, 227
Dapidia, 225
Darwin, on Earthworms, 354, 359, 368
Dasybranchus, 331;
gill, 268
Dasychone, 338;
gills, 261;
eyes, 272;
regeneration, 283
Davaine, on Nematoda, 140, 145
Davainea, 91;
D. friedbergeri, 84;
D. proglottina, life-history, 84
Davenport, on Urnatella, 491
Davis, on Rotifers, 227
Deep-sea, Polychaeta, 300;
Polyzoa, 478
Delagia, 478 n.
{541}Dendrobaena, 382
Dendy, on Land Planarians, 33, 34, 38
Desmogaster, 380
Desmoscolecidae, 159
Desor, on Nemertine development, 99;
Type of, larva, 113
Development, of Polyclads, 28;
of Nematoda, 135;
of Gordius, 171;
of Acanthocephala, 179;
of Chaetognatha, 189;
of Rotifers, 218;
of Polychaeta, 274 f.;
of Oligochaeta, 365;
of Leeches, 399;
of Gephyrea, 419, 432, 439, 447;
of Phoronis, 458;
of Polyzoa, 506, 509—see also Life-history and Larva
Diachaeta, 366
Diaphragm, of Nereis introvert, 250, 251;
of Polyzoa, 469, 470, 500, 508
Diclidophora, 73
Dicranotaenia, 91;
D. coronula, life-history, 84
Dicyema, 93;
vermiform larva of, 92;
hosts of, 94
hosts of, 94
Dicyemidae, 92 f.
Didymogaster, 383
Didymozoon, 73;
D. thynni (= Monostomum bipartitum), 71
Didymozoontidae, 73
Digenea (Digenetic Trematodes), 5, 52, 62, 73;
occurrence and habits, 62;
Digestive system, of Leptoplana, 11, 12;
of Polyclads, 24;
of Rhabdocoelida, 42 f.;
of Polystomum, 57;
of Distomum, 62;
absence of, in Cestodes, 74;
of Nematoda, 130;
of Chaetognatha, 187;
of Rotifers, etc., 209, 233, 237;
of Archiannelida, 243;
of Oligochaeta, 358;
of Hirudinea, 396;
of Gephyrea, 414;
of Phoronis, 454;
Digitibranchus, 353
Digonopora, 16
Dimorphism, in Polystomum, 59;
sexual, of Trematodes, 70;
of Orthonectida, 95;
of Dinophilus, 243;
of Gephyrea, 438
Dinocharididae, 225
Dinocharis, 225
Dinophilus, 242;
D. taeniatus, 242;
D. gyrociliatus, sexual dimorphism, 243
Dinops, 223
Diopatra, gill, 318
Diphyllinae, 91
Diplax, 225
Diplectanum, 73
Diplobothrium, 73
Diplocardia, 385
Diplodiscus, 73;
D. (Amphistomum) subclavatum, life-history, 71
Diplogaster, 154
Diplois, 225
Diplostomum, 73
reproductive organs, 60
Diporochaeta, 381
Diporula, 528
specific characters, 90
Disc, in Rotifers, 200, 202 f., 202
Discharge, of genital cells;
Nemertinea, 116;
Archiannelida, 244;
Discobdella, 226
Discodrilidae, 350, 376, 392, 395
Dispinthera, 225
Distomatidae, 73
Distomum advena (= D. migrans), life-history, 71;
D. appendiculatum, life-history, 71;
D. ascidia, life-history, 71;
D. atriventre, life-history, 71;
D. brachysomum, life-history, 71;
D. buskii, 63;
D. caudatum, life-history, 71;
D. clavigerum, life-history, 72;
D. conjunctum, 63;
D. crassum, 63;
D. cygnoides, life-history, 72;
D. cylindraceum, 72;
D. dimorphum, 72;
D. echinatum, 63;
life-history, 72;
D. echiuri, 444;
D. endolobum, life-history, 72;
D. excavatum, 63;
D. ferox, 63;
D. globiporum, life-history, 72;
D. hians, 63;
D. hepaticum (liver-fluke), 3, 63, 67 f.,72;
D. heterophyes, 63;
D. hystrix, life-history, 72;
D. japonicum (= D. spathulatum), 63;
D. lanceolatum, 63;
D. luteum, excretory system of, 62;
D. macrostomum, life-history, 64, 65, 72;
D. magnum, 68;
D. militare, life-history, 72;
D. nodulosum, life-history, 72;
D. oculi-humani (= D. ophthalmobium), 63;
D. ovocaudatum, life-history, 72;
D. retusum, life-history, 72;
{542}D. ringeri (= D. pulmonale), 63;
D. signatum, life-history, 72;
D. sinense, 70; D. spathulatum, 63;
D. squamula, life-history, 72;
D. trigonocephalum, life-history, 72;
D. westermanni (= D. pulmonale), 63
Distyla, 225
Dithyridium, 91
Ditrupa, 301
Dochmius, 133, 135, 142, 160, 163;
D. cernua, 143;
D. stenocephala, 143;
D. trigonocephala, 143
Dog, parasites of, 80 f., 90, 125, 140, 142, 143, 145
Domestic animals, Trematodes of, 67, 68, 70, 72;
Acanthocephala of, 184
Dorsal ciliated organ, 247, 254, 256
Dorsal pores, 348
Drepanidotaenia, 91;
D. anatina, life-history, 84, 85;
D. gracilis, D. infundibuliformis, D. setigera, life-history, 84
Drepanophorus, excretory system, 108;
British species, 110
Drilophagidae, 224
Drilophagus, 204, 210, 212, 224, 227
on Oligochaeta, 368
Dujardin, on Rotifers, 198;
on Gastrotricha, 231;
on Kinorhyncha, 236
Duthiersia, 91
Dwarf males, of Myzostoma, 344;
of Gephyrea, 438
Ear-cockle, 155
senses, 354;
food, 359;
effect on the soil, 368;
distribution, 369 f.;
classification, 380 f.;
British, 390—see also Oligochaeta
Ebrard, on Hirudinea, 393
Echeneibothrium, 91
Echinella, 73
Echinobothrium, 74, 75, 85, 91
Echinocotyle, 91
Echinorhynchidae, 182
Echinorhynchus acus, 175, 179;
E. clavula, 183;
E. haeruca, 176;
E. linstowi, 183;
E. lutzii, 183;
E. moniliformis, 183;
E. proteus, 174, 180, 181, 182, 182
Echiuroidea, 241 n., 412, 434, 446;
anatomy, 434 f.;
classification, 440;
development, 439;
parasites, 444;
habits, 442
Echiurus, 336, 411, 435, 440, 441;
development, 439
Eckstein, on Rotifers, 198
Eclipidrilus, 380
Economic uses, of Polychaeta, 296, 297
of Phylactolaemata, 496 f., 503
Ectoparasitic Trematodes, 4, 52, 53
structure, 469;
lophophore, 476;
reproduction, 506 f.;
compared with Entoprocta, 488
Effects, of parasites on their hosts, 56, 68, 69, 80, 94, 162
of Polyclads, 28;
of Termatodes, 52;
of ectoparasitic Trematodes, 54, 58, 60, 61;
of endoparasitic Trematodes, 63, 69;
of Cestodes, 87;
of Orthonectidae, 95;
of Nemertinea, 116;
of Gordius, 171;
of Acanthocephala, 179;
of Chaetognatha, 189;
of Gastrotricha, 234;
of Nereis, 256;
of Phyllodocids, 314;
of Scoloplos, 321;
of Myzostoma, 343;
Ehrenberg, on Turbellaria, 3, 6;
on Rotifers, 198, 220 n., 228;
on Gastrotricha, 231;
on Polyzoa (Bryozoa), 475
Eichhorn, on Rotifers, 197
Eisen, on Oligochaeta, 380, 390
Eisig, on Capitellidae, 373;
on Bonellia, 443
variation, 516
Elytra, of Polynoids, 265, 292, 294, 298, 299, 309, 310, 311;
as brood-pouch, 275;
of Aphroditidae, 266;
arrangement of, 309;
of Sigalionina, 313
Enantiidae, 19
Enchelidium, 157
Enchytraeidae, 359, 360, 361, 366, 367, 370, 375
Enchytraeus, host of Gordius, 173
Encotylabe, 73;
eggs of E. pagelli, 58
Endocyst, 471
Endoparasitic Trematodes, 4, 52, 62
Enopla, 109
Enoplidae, 157
Enteroploea, 224
{543}Enterostoma, 46;
British species, 50
Entoprocta, 475, 479, 487 f., 518;
lophophore, 476;
reproduction, 506;
Ephemera, host of Gordius, 172, 173
Ephesia, 321
Epidermis, of Leptoplana, 11, 12;
of Trematodes, 56;
of Cestodes, 85;
of Nemertinea, 102;
of Nematoda, 125;
of Acanthocephala, 175;
of Nereis, 249;
of Oligochaeta, 349;
of Leeches, 396;
of Gephyrea, 414;
of Phoronis, 454—see also Hypodermis
Epigamous, phase, of Nereis, 277 n.;
worms, 281
Epistome, in Phoronis, 453, 455;
in Phylactolaemata, 476, 476, 499;
in larva of Loxosoma, 509
Epitokous, phase, of Nereis, 277 n.
Erpocotyle, 73
Eschara, 516
Escharina, 518
Escharoides, 527
Euchlanididae, 225
Eudrilidae, 359, 360, 380, 385, 403 f.
Euichthydina, 235
Eulalia viridis, 314;
pigment, 292;
colour, 293;
eggs, 314 n.
Eumenia, 334
nephridium, 254;
eye, 255;
head, 262;
parapodium, 264;
gill, 265;
jaws, 270;
substance of tube, 290;
commensal, 298;
E. tibiana, 290
palps, 260;
tentacles, 262;
jaws, 270;
parasitic, 297;
tubes containing Polynoids, 298;
Palaeozoic, 302
Euphrosyne, 318;
parapodium, 265;
chaetae, 267
Eupolia, 113
Eupolyzoa, 461
Eurycercus, shell of, inhabited by Rotifers, 227 n.
Eurylepta, 19
Euryleptidae, 19
Eurythoe, 318
Eustrongylus gigas, 142;
E. tubifex, 142
Eusyllis, reproduction of, 278
Excretion, as cause of colour, 291
Excretory system, of Leptoplana, 13;
of Polyclads, 25;
of Triclads, 41;
absent in Acoela, 42;
of Temnocephala, 54;
of Digenea, 62;
of Cestodes, 86;
of Nematodes, 133;
of Acanthocephala, 177;
of Gastrotricha, 234;
of Kinorhyncha, 237;
of Polychaeta, 253;
of Polyzoa, 472 f.—see also Nephridium
Exogone, 308;
attachment of eggs, 275;
reproduction, 278
development of, 30;
of larval Polystomatidae, 59, 60;
of Nematodes, 128;
of Gordius, 166;
of Chaetognatha, 188;
of Rotifera, 215;
of Gastrotricha, 234;
of Kinorhyncha, 238;
of Polychaeta, 255, 272, 314, 337, 339;
of Oligochaeta, 354;
of Gephyrea, 417;
of Polyzoon larva, 509
Fabricia, 339;
eyes, 272;
otocyst, 273
Faecal groove, of Sabellids, 337
Fans, of Chaetopterus, 295, 324
Faraday, on asexual reproduction of Planariae, 6, 40 n.
Fasciola, 67
Fecampia, life-history, 45
Fertilisation, of Nemertinea, 117;
of Nematodes, 135;
of Gordiidae, 171;
of Acanthocephala, 179;
of Chaetognatha, 188;
of Rotifers, 217
F. attenuata, 134;
F. cystica, 142;
F. denticulata, 125;
F. immitis, 148;
F. labiata, 163;
F. laticaudata, 125;
F. loa, 149;
F. sanguinis hominis, 149, 163;
F. papillosa, 163
Filariidae, 147
Filigrana, 340;
fission in, 281;
tubes, 290
Fimbria tenuis, 157
Fischer, on branchiae in Gephyrea, 416 n.
Fishes, Trematodes of, 4, 53, 55, 62, 64, 71, 72;
Nemathelminthes of, 142, 143, 149, 163, 173, 182
Fission, in Bipalium, 34;
in Planariae, 40;
in Trichoplax, 96;
in Salinella, 96;
in Polychaeta, 278 f., 279, 280, 282, 340;
in Oligochaeta, 374, 375, 377;
in Phylactolaemata, 496;
Fissurella, as host, 298
Flabelligera, 334
Flame-cells, in Leptoplana, 13;
in Thysanozoon, 25;
in Triclads, 41;
in Temnocephala, 54;
in Polystomatidae and Tristomatidae, 56;
in Distomum luteum, 62;
{544}in Cestodes, 86;
in Rotifers, 213;
in Urnatella, 491
Fletcherodrilus, 382
Floscularia, 200, 203, 205, 220, 221, 226
Flosculariaceae, 202, 211, 213, 220
Flosculariidae, 201, 203, 205, 220 n., 221, 230
Flukes, 51
Flustra, 465, 466, 467, 472, 473, 477, 515, 518, 526;
avicularia, 482
Flustrella, 467, 477, 518, 532;
larva, 513
Flustrina, 518
Food, of Turbellaria, 4;
of Leptoplana, 10;
of Polyclads, 24;
of Triclads, 37;
of Acoela, 43 f.;
of Rhabdocoela, 45;
of Temnocephala, 53;
of Cestoda, 5;
of Nemertinea, 115;
of Nematodes, 131;
of Acanthocephala, 177;
of Chaetognatha, 190;
of Gastrotricha, 234;
of Polychaeta, 296;
of Earthworms, 359;
of Polyzoa, 467
Foraminifera, as food of Polychaeta, 296
Forceps, of Eunice, 270
Formula, for Nematoda, 138
Fossil, Polychaeta, 301;
Polyzoa, 520;
Nemertinea, absence of, 119
Fredericella, 476, 494, 502-505, 518, 519;
lophophore, 495;
Frenzel, on Trematoda, 62;
on Salinella, 96
Fresh-water, Turbellaria, compared with marine, 46;
Polychaeta, 284;
Polyzoa, 492
Friend, on Oligochaeta, 388 n.
Frogs, Trematodes of, 55, 58, 62, 71 f.;
Nematodes of, 140, 142, 160, 173
Frontal organ, of Nemertinea, 107
Frontal palps, of Eunicidae, 318 f.
Frontal ridge, 260
Frullania, inhabited by Rotifers, 227
Fulcrum, in Rotifers, 210
Funicular tissue, 471
Funiculus, 469, 471, 472, 499, 501
Gamble, on Platyhelminthes, 3 f.;
on Mesozoa, 92 f.
Gammarus, Rotifers attached to, 227
Gapes, cause of, 144
Gardiner, on development of Acoela, 44 n.
Gasterostomatidae, 73
Gasterostomum, 73;
G. fimbriatum, life-history, 72;
G. gracilescens, 72
Gastrocotyle, 73
Gastrodiscus, 73
Gastrothylax, 73
Gastrotricha, 231 f.
Gegenbaur, on Nemertine development, 99
Gemmation, 281;
Geobia, 37
Geographical distribution, of Turbellaria, 32 f.;
of Nemertinea, 117;
of Gastrotricha, 235;
of Polychaeta, 299 f.;
of Oligochaeta, 369 f.;
of Leeches, 405;
of Gephyrea, 426 f., 432, 441 f.;
of Phoronis, 460;
Geonemertes, 101;
description of G. chalicophora, 117
Gephyrea, 411 f.;
history, 411;
external characters, 412, 420 f., 430 f., 434 f., 444;
digestive system, 414, 422, 430, 436, 445;
vascular system, 415, 421, 436;
respiratory system, 416;
nervous system, 416, 431, 437, 445;
excretory system, 417, 423, 431, 437, 445;
reproductive system, 418, 431, 437, 445;
commensalism, 429;
affinities, 241 n., 336, 445 f., 512;
British, 449
Germarium, of Rotifers, 216
Germ-yolk-gland, 47
Giard, on Oligochaeta, 368
Gid, induced by Coenurus, 82
Gigantorhynchidae, 183
Gigantorhynchus gigas, 174, 177, 184;
G. echinodiscus, G. spira, G. taenioides, 184
Gills, of Polychaeta, 252, 265, 268 f.;
of Arenicola, 333;
of Chlorhaemidae, 334;
of Cirratulus, 326;
of Eunicidae, 318;
of Nereis, 246;
development of, 275;
of Sabella, 286;
colour of, 294;
of Sabellaria, 263;
of Serpulidae, 261;
of Sternaspis, 336;
of Sigalionina, 313;
of Terebellidae, 329;
of Leeches, 395;
of Gephyrea, 416
Gizzard, in Rotifers, 199, 210;
in Oligochaeta, 358;
Glands, on parapodia, 249;
of Phyllodocids, 314;
of Sabellids, 337;
of Terebellids, 327
Glandular papillae, of Polygordius, 244
{545}Glossiphonia (= Clepsine), 393, 396, 399, 404, 407;
spermatophore, 402
Glossiphoniidae, 406
Glycera, cirrus, 265;
habitat, 286;
jaws, 270;
nephridium, 254;
parapodium, 264;
prostomium, 259;
tentacles, 262;
G. capitata, distribution, 299;
G. meckelii, 320
gills, 268;
coelomic corpuscles, 253
Glyphidrilus, 386
Gnathobdellidae, 407
Gnathosyllis, colour of, 293
Goniada, 320
Goodrich, on Oligochaeta, 378
Gordiodrilus, 383 f.
Gosse, on Rotifers, 198, 209 n., 220
Goujon, on Trichina, 146
Gourret, on Chaetognatha, 187
Graff, von, on Rhabdocoelida, 44;
Graffilla, 50;
occurrence, 45
Grassi, on Cestoda, 80 n., 89;
Grater, of Eunicidae, 270
Greef, on Echiuroidea, 441, 444
Gregarines, in Polychaeta, 299;
in Gephyrea, 444
Grinder, of Eunicidae, 270
Grube, on Oligochaeta, 347, 352;
on Hirudinea, 395
Grubea, 308;
attachment of eggs, 275
Guanin, in coelomic corpuscles, 253
Guinea-worm, 147
Gundidae, 42
lophophore, 476;
in fresh water, 492
Gyrator hermaphroditus, 49
Gyrocotyle (= Amphiptyches), 91;
G. rugosa, 77;
G. urna, 77
Gyrodactylinae, 73
Haberlandt, on Convoluta, 43
Habits, of Platyhelminthes, 3, 7, 21, 35, 43;
of Nemertinea, 114;
of Gordiidae, 170 f.;
of Chaetognatha, 189;
of Gastrotricha, 232, 234, 235;
of Polychaeta, 285;
of Oligochaeta, 366;
of Phoronis, 451
Haemadipsa, 408;
eye, 394
Haemal fluid, 252
Haematocleptes, parasitic, 297
Haementeria, 407
Haemerythrin, 416;
in Magelona, 252
Haemoglobin, in Nemertinea, 107, 108;
in Chaetopoda, 252, 253, 291, 356;
in Gephyrea, 437;
in Phoronis, 456
anatomy, 430
Hallez, on Turbellaria, 7, 21, 40
Halosydna, 298
Hamann, on Nematoda, 131, 133, 136 n.;
males of, 438
Haplobranchus, 339;
gills, 261
Haplodrili, 241
Harker, on Oligochaeta, 369
Harmer, on Polyzoa, 465 f.
Harris, on Rotifera, 197
Hartog, on Rotifera, 197 f.;
on Gastrotricha, 231 f.;
on Kinorhyncha, 236 f.
Haswell, on Temnocephalidae, 53;
on Phoronis, 451
Hatschek, on affinities of Polyclads, 28 n.;
on nature of Cestodes, 76;
on Trochophore, 229;
on development of Gephyrea, 419, 447
of Polychaeta, 259 f.;
regeneration of, 283;
of Tomopteris, 315;
of Aphrodite, 260;
of Chlorhaemidae, 334;
of Eunice, 262;
of Nephthys, 262;
of Phyllodoce, 262;
of Polydora, 261;
of Polynoid, 262;
of Sabella, 261;
of Sabellaria, 263;
of Syllid, 262;
of Trophonia, 262
Hedruris, 163
Hekaterobranchus, 326
Heliopora, containing Polydora, 298
Heller, on human parasites, 139
Hemichordata, 462
Hemilepidia, colour of, 292
Hemistomum, 73
Hemitubifex, 378
Henlea, 369
Hermadion, 299
Hermaphrodite, Nemertinea, 109;
Polychaeta, 273 f.
Hermione, 312;
Herpobdellidae, 407
Hertwig, O., on Chaetognatha, 187 n.
segments, 258;
head, 263;
swim-bladder, 272;
genital organs, 274;
parasitic, 297
Hesperodrilus, 352 f., 369, 378 f.
Hesse, on nervous system of Nematoda, 127
Heterocotylea, 73
Heterogamy, 66 n.
Heteronemertini, 113
Heteronereid phase, 276 f.;
Heteronereis, 276, 281—see Heteronereid
Heteropora, 520 n.
Hexacotyle, 73
Hincks, on Polyzoa, 475, 500, 508, 516, 519, 523
Hippothoa, 528
Hippuraria, 533
external characters, 392;
British species, 393;
eyes, 395;
branchiae, 395;
alimentary canal, 396;
vascular system, 396;
body-cavity, 397;
nephridia, 399;
reproductive organs, 401;
cocoons, 404;
classification, 405
Hirudiniculture, 393
Hirudo, 393, 396 f., 399, 403, 405, 407;
nephridium, 400
Hislopia, 492
Histioderma, 302
Histriobdella, 242 n.
Histriodrilus, 242 n.
Holloway, on Rotifers, 227
Holomyarii, 137
Holostomum, 73;
H. excavatum, 63
Holothurian, as host, 298
Homalogaster, 73
Hooks, of Trematodes, 53, 56, 57;
taxonomic value in Cestodes, 90 f.
British species, 110;
characters, 110;
development, 113;
proboscis, 110
Horse, parasites of, 68, 83, 90, 125, 140, 142, 163
Horse-leeches, 393 f.
Horst, on Oligochaeta, 354
Hubrecht on Nemertinea, affinities, 120;
classification, 109;
excretory system, 108;
nervous system, 105
Hudson, on Rotifera, 197, 198, 215 n., 220 n., 223 n., 228
Hudsonella, 224
Huxley, on Rotifers, 198, 229;
on Polychaeta, 246;
on Molluscoidea, 475
tentacles, 262;
composition of tube of, 290
Hydatina, 199, 200, 202, 204, 213, 224, 226, 228
Hydatinidae, 224
Hymenolepis, 91;
generic characters, 91;
H. diminuta (= Taenia flavopunctata), life-history, 83;
specific characters, 91;
H. murina (= Taenia murina), 70;
H. nana, 80;
life-history, 83;
specific characters, 91
Hyocrinus, as host, 342
Hyperiodrilus, 363 f.;
reproductive organs, 363
Hypodermic impregnation, 27, 218
Hypodermis (= Epidermis) of Gordius, 165;
larva, 510
Hyporhynchus, 49
Ichthydium, 235
Ichthyobdellidae, 406
Ichthyonema, 131, 147, 149, 163
Ichthyotaenia, 91
Iguana, parasites of, 142
Iijima, on yolk-glands, 38 n.
Illoricata, 223
Ilyodrilus, 378;
chaeta, 350
Ilyogenia, 388
Imogine, 19
Incus, in Rotifers, 210
Inermia, 445
Infusoriform embryos, 93
Insects, parasites of, 142, 150, 152, 153, 154, 160, 163, 179, 183, 184, 185
Internode, 525
Interproglottidal glands, 90
Intertentacular organ, 469, 473, 508
Introvert, in Polychaeta, 249, 250;
Investing membrane, in ectoparasitic Trematodes, 57;
in Cestodes, 85
Ioda, form of head, 278
Isacis, 163
Jammes, on skin of Nematoda, 126
Jaws, of Nereis, 248, 250, 270;
of Polychaeta, 269 f.;
of Eunicidae, 270;
of Polynoid, 270;
of Autolytus, 270;
fossil, 302;
of Glycera, 270
Jelly, Miss, on Polyzoa, 523
Jensenia, 50
Jimenez, on Leeches, 407
Joblot, on Rotifers, 197
Johnston, on Hirudinea, 393
Joliet, on development of Rotifers, 218;
on Polyzoa, 508
{547}Jungermanniaceae, inhabited by Rotifers, 227
Jurassic, Serpulids, 301;
Polyzoa, 520 f.
Kaiser, on Acanthocephala, 177
Keferstein, on Polycladida, 7, 10
Kennel, von, on Nemertinea, 108;
Kerria, 384
Kinorhyncha, 236 f.
Kleinenberg, on Trochophore, 229
Koellikeria, 73
Kölliker, on Distomum okenii, 71
Koren and Danielssen, on Gephyrea, 442, 443
Kowalevsky, on Hirudinea, 397;
on Phoronis, 458
Kraepelin, on Polyzoa, 493, 502
Krause, on parasites, 163
Krohn, on Nemertine development, 99
Krohnia, 186;
American species, 534
Krukenberg, on haemerythrin, 416
Kynotus, 386
Labrorostratus, 297
Lacinularia, 200, 213, 215, 221, 226
Lacrymaria, 235
Laetmonice, 312
Lagenipora, 528
Lamarck, on Gephyrea, 411
Land, Planarians, 4, 30, 33, 34, 36;
Oligochaeta, 347 f.;
Leeches, 408
Lang, on Polyclads, 7, 17, 21 f., 27, 28 n.;
on nature of Cestodes, 76
Lanice, 328;
Hypophorella in tubes of, 478, 533
Lankester, on Trochosphere, 229;
on Earthworms, 347;
on Hirudinea, 397;
on Podaxonia, 461
Larva, Müller's, 29;
of Polystomum, 59;
of Diplozoon, 60;
of Gyrodactylus, 61;
hosts of larvae of digenetic Trematodes, 71 f.;
of Calliobothrium, 77;
of Cestodes, 79 f.;
table of Cestode larvae, 83, 85, 87;
of Schistocephalus, 84;
of Bothriocephalus, 87;
of Dipylidium (Cysticercoid), 83, 88 f.;
of Nemertinea, 113;
Trochosphere, 229, 274, 439, 510;
of Polygordius, 245;
of Chaetopterus, 325;
of Myzostoma, 344;
of Phoronis, 458;
of Polyzoa, 466, 509, 511, 520
Lateral organs, of Capitellidae, 343—see also Ciliated pits
Laurer's Canal (= Laurer-Stieda canal), 57, 87
Leckenby, on Priapulus, 433
Leeches, 241, 392 f.—see also Hirudinea
Leeuwenhoek, on Rotifera, 197
Legrain, on Nematodes in deserts, 156
Legros, on Trichina, 146
Leidy, on Rotifers, 198
Lemnisci, 176
Lepas, as host, 298
Lepidoderma, 235
Lepidonotus (subgenus of Polynoe), eye, 255
Lepralia, 516, 518, 528, 530, 531 n.;
larva, 511
Leprea, 328
Leptoplana, 7, 8, 9, 11, 14, 17;
British species, 19;
habits, 8 f.;
anatomy, 11 f.
Leptoplanidae, 19
Leptostoma, 396
Lesson, on Polyclads, 24
Leuciscus, parasites of, 173, 182
Leuckart, on Platyhelminthes, 6, 64 f., 70, 76;
on Nemertinea, 99;
on Nematoda, 136, 139, 140, 142, 146, 163;
on Hirudinea, 395
Leucocytes, of Polychaeta, 252
Leucodore—see Polydora
Leydig, on Rotifers, 198
Lias, Serpulids in, 301
Life-history, of Trematodes, 4;
of Polystomatidae (Polystomum, Diplozoon, Gyrodactylus), 58 f.;
of digenetic Trematodes, 63 f.;
table of, 71;
table of life-histories of Cestodes, 83;
of Dipylidium, 88;
of Dicyemidae, 93;
of Nematoda, 159;
of Gordius, 170;
of Acanthocephala, 179—see also Development
Ligula, 91;
occurrence, 85
Ligulinae, 91
Lim Boon Keng, on Earthworms, 349
Lima, nests of, 298
Lime, secreted by Serpulidae, 290
Limnaea truncatula, host of larvae of Distomum hepaticum, 67, 72
Limnatis, 407
early description of, 197
Limnodrilus, 378
Lineus, 111;
L. marinus (= L. longissimus), 99, 100, 111, 114;
Borlase on, 99;
size of, 100;
L. gesserensis (= L. obscurus = L. sanguineus), recuperative powers of, 116
{548}Linguatulida, affinities of, 344
Linnaeus, on Cestodes, 78;
on Gephyrea, 411;
on Polyzoa, 474
Linstow, von, on classification of Nematodes, 137;
on life-history of Ascaris lumbricoides, 140;
on parasitism, 162;
Lip, lip-membrane, lip-processes, of Sabellidae, 261, 337
Lipobranchius, 334;
shape of body, 259
Lipochromes, in Polychaeta, 292
Lister, on Polyzoa, 497
Lithodomous, Polychaeta, 287
Lithographic slate, fossil Polychaeta from, 301, 302
Liver, -fluke, 51;
-rot, 68
Locomotion, of Planarians, 9, 10, 36;
of Polyclads, 22 f.;
of Chaetognatha, 190;
of Dinophilus, 243;
of Phylactolaemata, 496
Looss, on Trematoda, 62 n., 66, 70
Lopadorhynchina, 314
Lopadorhynchus, 314;
L. erythrophyllum, colour of, 292
Lophochaeta, 351;
chaeta, 350
Lophohelia, with Eunice, 298, 319
Lophophore, in Phoronis, 453;
Lophopus, 494, 499, 504, 505, 518, 519;
Lorica, in Rotifers, 205
Lota, parasites of, 182
Loxosoma, 489 f., 489, 490, 506, 518;
on other animals, 489;
larva, 509
Lug-worm, castings of, 285;
as bait, 297
Lumbricaria, 302
Lumbricobdella, 392
from fresh water, 284
Lumbriconereites (misprinted in text), 302
Lumbriculus, 379;
as host of Rotifer, 211 n.
Lumbricus, 349, 351, 356, 367, 368, 371, 389 f., 403 f.;
generative organs, 362;
cocoon, 365
Lunulites, 518;
vibracula, 487
Lurg, 317
Lycoridae, 315
MacGillivray, on Polyzoa, 519
M‘Intosh, on Nemertinea, 110 n., 115, 116;
on Phoronis, 454
Macraspis, 73
sense-body, 394
British species, 49
British species, 49
Macrostomatidae, 49
Magelona, 325;
haemerythrin in, 252
Main-gut, of Leptoplana, 8, 13;
of Polyclads, 17
Malacobdella, 101;
description of, 119;
excretory system of, 108
Malacocotylea (= Digenea), 73
Maldanidae (= Clymenidae), 258, 332;
shields, 259;
anal funnel, 259;
colour, 293
Male, of Rotifers, 199, 200, 217, 223 n.;
of Echiuroids, 438
Malleate, 210
Malleus, 210
Mammals, Trematodes of, 62, 63, 67-70, 71 f.;
Nematodes of, 163;
Man, Trematode-parasites of, 63, 68-70;
Cestodes of, 74, 78-81, 83, 89 f.;
Nematodes of, 125, 139, 140, 143, 145, 147, 163;
Gordius of, 173;
Echinorhynchus of, 183
Man, De, on free-living Nematodes, 157
Mandible, of avicularium, 482, 524
Manson, on Filaria, 149
Manubrium, 210
Marginal groove, of Leptoplana, 15
Marine, Rotifers, 226;
Oligochaeta, 366;
Leeches, 406
as bait, 297;
as host, 297
Masterman, on Chaetognatha, 190;
on Phoronis, 461
Mastigocerca, 225
vibraculum, 484
Mastobranchus, gill of, 268, 331
Matzdorf, on Leeches, 369
Maupas, on Rotifers, 217
Mbalolo, 297
Meckelia asulcata, 111
Mecynostoma, 49
Median pore, in Cheilostomata, 484, 524
Megalotrocha, 221
Megapora, 530
Megascolex, 351, 372, 381, 383
Megascolides, 349, 358, 372, 382;
body-wall and nephridia, 357
Mégnin, on parasites, 164
Meissner, on Polyzoa, 493
Melania, Coabangia in shell of, 284
Melanin, 292
{549}Melicerta, 202, 205, 206, 210, 215, 218, 219, 221, 226;
early description of, 197
Melicertidae, 201, 203, 205, 211, 213, 221
Melinna, 330
Membranipora, 481, 492, 518, 519, 523, 528, 530;
phosphorescence, 478;
function of aperture, 500;
variation, 516
Membraniporella, 518, 524, 528
Mesobdella, 407
Mesocestoides, 91;
M. lineatus, life-history, 84;
specific characters, 90
Mesonemertini, 112
reproduction, 48;
British species, 49
Mesostomatidae, 49
Mesotrochal larva, 325
Mesozoa, 92 f.
Metamerism, 249
Metamorphosis, in Polycladida, 16, 28;
in Cestodes, 5, 74, 76, 87-89;
in Phoronis, 459;
in Polyzoa, 512
Metanemertini, 112
Metastatica (= Holostomatidae), 73;
life-histories, 64
Metschnikoff, on Orthonectida, 94;
on Nemertines, 99;
on Chaetosomatidae, 158
Meyer, on Polychaeta, 261
Michaelsen, on Earthworms, 349, 353, 359, 370 f., 375, 385
Microchaeta, 362
Microcodides, 224
Microcodon, 202, 215, 224, 230
Microcodonidae, 202, 220 n., 224
Microcotyle, 73;
eggs of M. labracis, 58
Microcotylinae, 73
zooecium, 523
Microporella, 516, 518, 519, 529;
median pore, 501
Microscolex, 372, 382, 383, 384
Microstoma, 44;
asexual reproduction, 44;
sexual organs, 47;
British species, 49
Microstomatidae, 49
British species, 111
Migrations, of Trematode-larvae, 5, 52, 63 f.;
of Cestode-larvae, 5, 74, 83, 87
Millson, on Oligochaeta, 368, 387
Millsonia, 382 f.
Milne-Edwards, on Polyzoa, 475
Mimicry, in Polychaeta, 293 f.
movement of zooecia, 481
Molluscoidea, 475
Molops, host of Gordius, 172
Monhystera, 160
Moniez, on Cestodes, 84 n., 85;
on Earthworms, 369
characters of genus, 90;
M. alba, M. benedeni, M. denticulata, 90;
M. neumani, M. oblongiceps, M. planissima, M. trigonophora, 90
Moniligastridae, 361, 373, 380
Monkey, parasites of, 145
Monocotyle, 73
Monocotylinae, 73
Monogenea (Monogenetic Trematodes), 5, 53;
classification, 73
Mononchus, 154
Monoophorum striatum, 50
Monopora vivipara, 117
Monoporus rubropunctatus, 49;
reproductive organs of, 47
Monostomatidae, 73
life-history, 72
Monotidae, 50
Monotus, 36;
British species, 50;
M. fuscus, 45;
habits, 46
Monozoa, 91
Montgomery, on Stichostemma eilhardii, 118
Monura, 225
Morren, on Earthworms, 347
Moseley, on Land Planarians, 7, 35 n., 37;
Movements, in Rotifers, etc., 206, 235
Moxon, on Rotifers, 198
Mucronella, 518, 522, 528, 529, 530, 531
Müller, F., on Triclads, 37;
on Polyzoa, 493
Müller, O. F., on Turbellaria, 6;
on Rotifers, 197;
Muscles, of Leptoplana, 12;
of intestine in Polyclads, 24;
of Nematoda, 128;
of Gordiidae, 165;
of Chaetognatha, 187;
of Rotifers, etc., 206, 233, 237;
of Polyzoa, 469, 470, 472, 499, 500—see also Body-wall
Musculo-glandular organ, in Triclads, 39, 40
markings, 293
Myrianites, 302
{550}Myxicola, 338;
tori uncinigeri, 268;
eyes, 272;
otocyst, 273;
tube, 285;
supposed mimicry, 294
Myzostoma, anatomy, 342 f.;
M. cirriferum, 343;
M. glabrum, 342
Myzostomaria, 241;
structure and affinities, 342 f.
Myzostomatidae, 342
Naidomorpha (= Naididae and Naids), 348, 352, 375 f., 377, 378, 400;
reproductive organs, 361;
asexual reproduction, 281, 377
chaetae, 350
Nannodrilus, 382 f.
Natatory chaetae, of Nereidae, 277;
Nathusius, on parasites, 63, 163
Nebalia, Rotifers parasitic on, 225
Nectochaeta, 291
Nectonema agile, 164, 168, 169, 173
Nematoda, 123;
anatomy, 124 f.;
embryology, 135;
classification, 136;
life-history of, 159 f.
Nematogen, 94;
Secondary Nematogen, 94
Nematozoa, 137
Nemertes, British species, 110;
deposition of ova and spermatozoa in, 116
Nemertinea, affinities, 119;
anatomy, 102;
body-wall, 102;
breeding, 116;
cephalic slits and cerebral organs, 107, 273;
classification, 109 f.;
colour, 102;
contractility, 102;
development, 113;
diagram, 104;
digestive system, 103;
external characters, 101;
food, 115;
generative organs, 109;
geographical distribution, 117;
habitat, 100;
habits, 114;
proboscis, 100, 101 f., 103, 104, 110, 115;
regeneration, 115;
size, 100
Nemertodrilus, 360
Nemertoscolex, 444
Neodrilus, 384
Neorhynchidae, 184
Neorhynchus clavaeceps, 178, 181, 184;
N. agilis, 184
Nephelis, 392 f., 397, 399, 401, 403, 405
Nephridium, of Archiannelida, 243, 244;
of Nereis, 253;
of Polychaeta, 253;
dimorphism of, in Polychaeta, 269;
thoracic, of Sabelliformia, 306;
of Terebellidae, 327;
of Chlorhaemidae, 334;
of Myzostoma, 344;
of Oligochaeta, 356 f., 381 f., 400;
of Leeches, 399;
of Gephyrea—see Brown tubes;
of Phoronis, 456
Nephthys, 317;
nephridium, 254;
head, 262;
tentacles, 262;
peristomium, 263;
cirrus, 265;
gill, 268;
jaws, 270;
habitat, 286;
as bait, 297
palps, 260;
colour, 292
vascular system, 252;
anal cirri, 259;
tentacles, 262;
peristomial cirri, 263;
parapodium, 264 f.;
cirri, 265;
chaetae, 266;
jaws, 269;
eyes, 272;
ciliated organ, 273;
regeneration in, 283;
food, 296;
fossil, 301 f.
Nereilepas, 317
Nereis, 246, 299, 301, 316 f.;
transverse section, 247;
parapodium, 246, 247, 265, 317;
chaetae, 246;
alimentary canal, 249 f., 251;
eye, 255;
dorsal ciliated organ, 247, 254, 256;
nephridium, 253;
reproduction, 256;
sexual dimorphism and Heteroneid phase, 276 f.;
epitokous (= epigamous) and atokous phase, 277 n.;
from fresh water, 284;
burrow, 286;
pigment, 292;
as bait, 297;
commensalism, 298;
as host, 299;
British species, 316 f.
Nereites, 302
habitat, 286;
N. vulgaris, head, 322
Nerve plexus of Nemertinea, 103, 105
Nervous system, of Leptoplana, 13, 14;
of Planaria lactea, 39;
reduced in parasitic Rhabdocoels, 45;
of Temnocephala, 54;
of Polystomatidae, 56;
of Nematoda, 127;
of Gordiidae, 166;
of Acanthocephala, 177;
of Chaetognatha, 187;
of Rotifera, etc., 215, 234, 237;
of Polychaeta, 254;
visceral, of Polychaeta, 255;
of Gephyrea, 416, 431, 437, 440, 445;
of Phoronis, 456;
of Polyzoa, 471
Neumann, on parasites, 164
Neuropodium, 246, 247, 264, 266, 268
Newton, on zoo-geographical regions, 372
Nicolea, 328;
gill, 329
Nicomache, 332;
tail, 332;
tube, 287;
N. lumbricalis, colour, 292;
distribution, 299
Nitsche, on Polyzoa, 475 n., 478, 500
Norman, on Polyzoa, 475
Norodonia, 492
Noteus, 225
Notocotyle, 73
Notomastus, 331;
chaetae, 268
Notommatidae, 200, 205, 207, 215, 223, 224
Notophyllum, colour, 293
Notopodium, 246, 247, 264, 265, 268
Notopygos, 259
Nuchal cirri of Eunicidae, 318;
Nuchal organ—see Ciliated pits
Ocnerodrilus, 383
Octocotylinae, 73
Octotrocha, 221
Odontosyllis, reproduction of, 278;
as host, 297.
Oenonites, 302
Ogmogaster, 73
Oka, on Hirudinea, 399 f.;
on Polyzoa, 500
Olfactory pits, in Polyclads, 26;
in Triclads, 36
Oligocelis, 42
external characters, 348;
body-wall, 349;
branchiae, 352;
nervous system, 353;
sense-organs, 354;
coelom and vascular system, 355;
excretory organs, 356;
alimentary canal, 358;
reproductive organs, 360;
habitat, 365;
phosphorescence, 368;
distribution, 369;
classification, 373;
Rotifers parasitic on, 227
Oligognathus, 297
Ollulanus, 142;
Omalostoma, 49
Onchnesoma, 422, 423, 426, 430, 447
Onchocotyle, 73
Oncholaimus, 157
Onchosphaera-larva of Cestodes, 87, 88
Onuphin, 290
O. conchylega, tube, 287
Onychochaeta, 388;
chaeta, 351
Onyx, 131
Ootype, in Polystomum, 59.
Operculum, of Serpulidae, 261, 276, 339;
of Spirorbis, as brood-pouch, 261, 276;
of Cheilostomata, 466, 477, 481, 482, 522, 524
Opesia, 524
eggs, 275;
coelomic corpuscles, 252
gill, 265;
ciliated pits, 272
Ophiodromus, 308;
O. flexuosus, parasitic, 297
pelagic, 291;
genital organs, 274
Opisthotrema, 73
Opistoma, 50
Orbigny, D', on Polyzoa, 519, 520
Orifice, of zooecium, 466, 469, 470, 524;
Örley, on classification of Nematodes, 137
Otocyst (and Otolith), of Turbellaria, 26;
of Polychaeta, 273
Otoplana, 50
Oudemans, on Nemertinea, 108
Ovary (and Oviduct), of Leptoplana, 11, 14, 16;
of Polyclads, 27;
of Rhabdocoelida, 47;
of Temnocephala, 54;
of Polystomatidae, 57;
of Diplozoon, 60;
of Gyrodactylus, 61;
of Distomum macrostomum, 65;
of Calliobothrium, 75;
of Archigetes, 76;
of Schistocephalus, 86;
of Rhopalura, 95;
of Myzostoma, 343—see also Reproductive organs
Ovicell, in Cheilostomata, 466, 466, 468, 481, 482, 484, 522 f., 525;
in Cyclostomata, 479, 480, 521, 525
Oviduct—see Ovary
Owenia, 325
Ox, parasites of, 79, 83, 125, 139, 140, 143
Oxyuris, 129, 131, 135, 139, 141, 160;
O. ambigua, 141;
O. blattae, O. blatticola, O. hydrophili, O. megatyphlon, O. spirotheca, 142
Paddle worm, 313
Paedogenesis, 151
Pagenstecher, on Nemertinea, 99
Palaemonetes, host of Nectonema, 174
characters, 111;
development, 113
Palaeozoic, Serpulidae, 301;
Eunicidae, 302;
Polyzoa, 520
Paleae, of Sabellaria, 267
Palolo viridis, as food, 297
nerves to, 254;
of Polychaeta, 260 f.;
development of, in Sabelliformia, 275;
of Hermelliformia, 306;
of Syllidae, 307
Paludicella, 492, 494, 501, 502, 505, 518
Panthalis, 313
Parapodium, of Nereis, 246, 247;
of Polychaeta, 264 f.;
muscles of, 247;
of Myzostoma, 342
Parasitic, Turbellaria, 51;
Polyclads, 22;
Triclads, 32;
Polychaeta, 297;
Leeches, 406
Parasitism, effect on the parasite, 161, 177;
effects on the host, 162
Parenchyma, in Leptoplana, 11, 12;
of Müller's larva, 29;
in Triclads, 41;
in Acoela, 42;
Parovaria, of Phagocata, 38 n.
Parthenogenesis, amongst Rotifers, 200
Pectinaria, 330;
body, 259;
P. auricoma, tube, 288;
P. belgica, 330
Pectinatella, 496, 497, 505, 512, 518;
statoblast, 502
Pedalion, 200, 201, 206, 211, 216, 223, 224, 225, 228, 230
Pedalionidae, 223
Pedetes, 224
Pedicellina, 487, 488, 490, 506, 507, 518;
budding, 514;
on Polychaeta, 299
Pelagic, Nemertinea, 101, 114;
Chaetognatha, 189; Rotifera, 226;
larvae of Polychaeta, 300, of Polyzoa, 520
Pelagobia, 314
Pelodytes, 134
of Polyclads, 27;
of Rhabdocoelida, 47;
of Temnocephala, 54;
of Calliobothrium, 75;
of Schistocephalus (cirrus-sac), 86
Pennant, on Hirudinea, 406 n.
Pereyaslawzewa, on Acoela, 44 n.
Pergens, on Polyzoa, 500
Perichaeta, 351, 357, 358, 372, 381, 381 f., 388, 394, 403
Perienteric, blood-sinus, 252
Perionyx, 381
Perissogaster, 383
Peristomial (tentacular) cirri, of Nereis, 248;
of Polychaeta, 263;
nerves to, 254
Peristomium, of Nereis, 248;
of Polychaeta, 263;
of Sabellidae, 336
Perrier, on Oligochaeta, 367, 385
Petalostoma, 422, 426, 430, 447
Petromyzon, host of Gordius, 173
Petta, intestine of, 271
Phalacrophorus, 314
Pharynx, 4;
of Discocelis, 23;
development of, in Polyclads, 29, 30;
of Polystomatidae, 56;
of Polychaeta, 269
Phascolosoma, 416, 420, 423, 425, 428, 447;
as host of Loxosoma, 489
Philippi, on Hirudinea, 406
Philodinidae, 222
Phoronis, 450 f., 451, 452, 453, 455;
habits, 451;
species, 460;
Phosphorescence (and light-producing organs), in Rotifers, 226;
in Oligochaeta, 368;
in Polyzoa, 478
Photogen (light-producing organ), of Polyophthalmus, 272;
Phreoryctidae, 376
Phylactolaemata, 476, 493 f., 518;
occurrence, 493;
affinities, 512
Phyllacanthinae, 91
Phyllobothrinae, 91
Phyllocotyle, 73
parapodium, 264;
chaeta, 267
parapodial cirri, 266;
as food, 297;
eggs, 314;
Phyllodocites, 302
Phyllonella, 73
Phymosoma, 413, 420, 421, 423 f., 425, 426
Physaloptera, 163
Pig, parasites of, 68, 79, 139, 147, 184
Pigments, of Polychaeta, 291 f.;
of Gephyrea, 435
Pike, Trematode of, 62;
Pilidium larva, 113, 113, 229, 230
Pionosyllis, 308
Pista, 328;
gill of, 329
Pits, ciliated, of Polychaeta, 272, 273
{553}Placostegus, colour, 292;
from deep sea, 300
Placunella, 73
Plagiostoma, 46;
British species, 50
Plagiostomatidae, 50
British species, 42
Dinophilus, compared with, 242, 243
Planariidae, 42
Planctoplana, 19
Plankton, Rotifers in, 225
Planorbis, host of Gordius, 173
Plants, parasites on, 154, 155, 157, 160
Plasmodium, nature of, in Orthonectids, 94
Plate, on Rotifers, 198, 225 n.
Platyaspis, 73
Platycotyle, 73
Platyhelminthes, 3 f.;
Nemertinea classed with, 119
Plectanocotyle, 73
Plectus, 160
Pleionogaster, 381
Plerocercoid larva, 84
Plessis, du, on Tetrastemma lacustre, 101 n., 118
Pleurocotyle, 73
Ploima, 202, 203, 212, 213, 216, 220, 223, 226, 227
Plumatella, 493, 494, 499, 503-505, 518, 519;
protrusion of polypides, 499;
larva, 512
Podal membrane, of Spionidae, 322
Podaxonia, 461
Polybostrichus, 280
classification, 257, 258, 303 f,;
coelomic fluid, 252;
nervous system, 254;
ciliated pits, 272;
alimentary canal, with pharynx, 249, 250, 251, 269, 270, 271;
oesophageal glands, 271 f.;
nephridium, 253, 254, 269, 274;
hermaphrodite, 273;
habits, 285;
carnivorous, 304;
distribution, 299;
from fresh water, 284;
from deep sea, 300;
boring, 287;
tubes, 287;
pigments, 291;
colours, 291 f.;
warning colours, 294;
protective devices and mimicry, 293;
phosphorescent, 295;
as food for man, 297;
commensalism, 297 f.;
parasitic, 297 f.;
as hosts, 299;
provisional chaetae, 274
Polychoerus, 49;
development of, 44 n.
Polycirrus, 330;
habits of, 285;
P. aurantiacus, warning colours, 294;
phosphorescence, 295;
P. haematodes, coelomic corpuscles, 253
Polycladida (Polyclads), 4 f., 7;
classification, 16 f.;
development, 28 f.;
Polycladus, 42
Polycotyle, 73
Polydora (= Leucodore), 323;
frontal ridge, 260;
head, 261;
special chaetae, 267;
with Heliopora, 298;
P. ciliata, borings, 287
development, 245
Polymnia, 328
Polymorphism, of Nereis, 277
Polynoe 310;
segments 258;
parapodium, 265;
jaws, 270;
anus, 259;
nephridium, 254;
habits, 286;
as ectoparasites or commensals, 294, 298, 325;
P. squamata, 309;
elytron, 310;
P. clava, elytron, 310;
P. imbricata, elytron, 311
Polynoina (= Polynoids), 309;
head, 262;
jaw, 270;
sexual dimorphism, 276 n.;
tubes, 285;
protective resemblance, 294;
food, 296;
parasitic and commensal, 297, 325;
elytra, 275, 294, 295, 299, 309 f.
Polyodontes, 313 n.
Polyophthalmus, 332;
otocyst, 273
Polype à pannache, 496
Polypide, 468, 469, 474, 488, 523;
retraction and protrusion, 498 f.
Polypide-bud, 468, 472, 487, 496, 499, 501, 510;
connected with reproduction, 507
Polypostia, 19;
penes, 27
Polystomatinae, 73
external characters, 465 f., 479 f.;
history, 474 f.;
classification, 475 f., 515, 517 f.;
occurrence, 477 f.;
avicularia and vibracula, 482;
enemies, 486;
Entoprocta, 487;
fresh water, 492 f.;
development, 509;
metamorphosis, 512;
budding, 514;
palaeontology, 520;
terminology, 523;
determination of British genera, 505, 521, 525
{554}Pomatoceros, habitat, 300, 340
Pontobdella, 393, 401, 404, 406
Pontodora, 314
Pontoscolex, 350, 366, 387 f.;
chaeta, 351
Pore, in Polyzoa, 471, 482, 522, 524;
dorsal, 348
Porella, 516, 518, 522, 527, 529
Potamilla, 338
Praeoral lobe (= Prostomium), 245, 439
Predaceous worms, 304
anatomy, 430;
classification, 432;
habits, 433
anatomy, 430 f.
Pristina, 377
Proboscidae, 49;
occurrence, 44
Proboscis, of Nemertinea, 100, 101 f., 103 f.;
severance of, 116;
of Acanthocephala, 174 f.;
of Rotifers, 203;
of Kinorhyncha, 237;
of Echiuroidea, 434
Proboscis-pore of Nemertinea, 102, 103
Proboscis-sheath of Nemertinea, 103, 103 f.
Procerodes, 42
Procerodidae, 42
Procerus, host of Gordius, 172
Promesostoma, occurrence, 44;
British species, 49
Proporidae, 49
Proporus venenosus, 49
Prorhynchidae, 49
Prorhynchus sphyrocephalus, terrestrial habit, 44;
P. stagnalis, 49
Prosorhochmus claparedii, 110, 114, 117
Prostate-gland, of Leptoplana, 16;
of Polyclads, 30;
of Planaria, 39;
of Rhabdocoela, 47;
of Oligochaeta, 361
spermatophores, 27
Prosthiostomatidae, 19
Prosthiostomum, 17, 18, 19, 24
Prostomial tentacles, 248, 262
Prostomium, 241;
of Dinophilus, 243;
of Polygordius, 244;
of Trochosphere, 245;
of Nereis, 248;
of Polychaeta, 259;
of Glyceridae, 320;
of Terebellidae, 327;
of Oligochaeta, 348
Protonemertini, 112
Protula, 341;
eggs, 275
Prouho, on Polyzoa, 489, 507 f.
Provisional chaetae, 274
Provortex, British species, 50
Proxenetes, 44;
British species, 49
Pruvot, on Polychaeta, 261
Psammolyce, 313;
Pseudaxine, 73
Pseudocotyle, 73
Pseudorhynchus bifidus, 49
Psygmobranchus, 341
Pterobranchia, 461
Pterodina, 200, 201, 203, 206, 211, 215, 216, 225, 226, 230
Pteroessa, 224
Pteronella, 73
Pterostichus niger, infested by Gordius, 170, 170, 172
Pterosyllis, ciliated lappets, 273 n.
Quatrefages, on Gephyrea, 411, 445
Rabbit, parasites of, 141, 145
Ragworm, 322
Railliet, on Cestodes, 91
Rami, in Rotifers, 210
Rasping plate, of Eunicidae, 270
Ratzel, on Earthworms, 350
Red Cat, 316
Regeneration of lost parts, in Polyclads, 26;
in Triclads, 40;
in Cestodes, 77;
in Nemertinea, 115;
Repetition of parts, 249
Replacement of species, 300
Reproduction (and Reproductive organs), of Leptoplana, 14 f.;
of Temnocephala, 54;
of Polystomatidae, 57 f.;
of Diplozoon, 60;
of Digenea, 65;
of Calliobothrium, 75;
of Schistocephalus, 86;
of Mesozoa, 93 f.;
of Nemertinea, 102, 103, 104, 109;
of Nematoda, 134;
of Acanthocephala, 178;
of Chaetognatha, 188;
of Rotifera, etc., 216, 234, 238;
of Archiannelida, 243 f.;
of Polychaeta, 253, 254, 256, 269, 273;
of Myzostoma, 343;
of Oligochaeta, 360;
of Leeches, 401;
of Phoronis, 457;
of Polyzoa, 471, 490, 501, 506—see also Ovary and Asexual reproduction
Reptiles, parasites of, 163
Respiration, in Nereis, 252;
in Chaetopoda, 272;
in Gephyrea, 416
Rhabdites (rods), of Leptoplana, 11, 12;
in Polyclads, 29;
{555}in Triclads, 37;
absent in parasitic Rhabdocoela, 45;
Rhabditiformae, 137
Rhabdocoelida, 4, 7, 36, 42 f.;
occurrence and habits, 43;
parasitic forms, 44;
reproduction, 47;
classification, 49;
Rhabdogaster, 158
Rhabdonema nigrovenosum, 134, 136, 140, 151, 160, 161
Rhabdopleura, 461 f.
Rhinodrilus, 348
Rhinops, 224;
male of, 223 n.
Rhizopoda, as food for Polychaeta, 296
Rhizota, 220 n.
Rhombogen (form of Dicyemid), 93
Rhopalonaria, 521 n.
Rhopalophorus, 73
Rhopalura giardii, occurrence and structure, 94, 95;
R. intoshii, 94
Rhynchopora, 531
Riches, on British Nemertinea, 110;
on Malacobdella, 119
Rietsch, on Gephyrea, 443
Rockworm, 319
Rods—see Rhabdites
Rohde, on muscles of Nematoda, 128 f.
Rosa, on Oligochaeta, 364, 380, 385, 390
Rotifer, 201, 202, 210, 216, 222, 226, 227
Rotifera, 197 f.;
distribution, 200;
parasitic, 204;
digestive organs, 209;
renal organs, 213;
nervous system and sense organs, 215;
reproduction and development, 216;
classification, 220;
habits 226;
preservation, 228;
affinities, 229
Rousselet, on Rotifers, 198, 216, 228
Sabella, 299, 337; parapodium, 265;
habitat, 286;
tube, 287;
tube-building, 288;
S. saxicava, habits of, 287
Sabellaria, 341;
body, 259;
cirri, 265.;
S. spinulosa, paleae, 267, 300
head, 261;
regeneration, 283;
from fresh water, 284;
colour, 292
chlorocruorin in, 252; body, 259;
genital organs, 273;
development of gills, 275;
gland shields, 287
Saccobdella, 226
Sagitella, 321
development, 189;
habits, 190;
American species, 534
Salensky, on development of Nemertinea, 99;
of Rotifers, 218
Salivary glands, in Polyclads, 10, 24;
in Leeches, 396
brood-pouch, 276;
fission, 281
Salpinidae, 225
Sandmason, 328
Saxicava, Eulalia in borings of, 314
Scales, of Gastrotricha, 233
Scalibregma, 334
reproductive organs, 86;
larva, 84;
Schizocerca, 225
Schizogamy, in Syllidae, 278, 279, 281
Schizonemertea, 109;
characters, 111;
development, 113;
transverse section, 103
Schizoporella, 518, 527, 528, 529, 530, 531;
zooecium and avicularia, 482;
Leptoplana on, 22
Schmarda, on Oligochaeta, 366, 387
Schmidt, on Rhabdocoels, 6
Schneider, on life-history of certain Mesostoma, 48;
on classification of Nematoda, 129;
on oesophageal glands, 131;
on Strongylidae, 142
Schultze, on Polyclads, 13, 26;
Schultzia, 50
Schulze, F. E., Stichostemma found by, 118;
Trichoplax found by, 96
Scirtopoda, 200, 201, 203, 206, 207, 223
Sclerocheilus, 334
Sclerostomum, 163
Scoleciformia, 258, 305, 331 f.;
vascular system, 252;
buccal region, 269;
food of, 296
S. polymorphus, 77;
of Taenia solium, 79
Scolithus, 302
parapodium, 265;
habitat, 286
Scruparia, 527
Scrupocellaria, 517, 518, 519, 526;
phosphorescence, 478;
larva, 511
{556}Scutum, 525
Sea-mouse, 312
Sedentaria, 285
Segment, 241;
Seison, 226
Seisonaceae, 204, 216, 220 n., 225, 227
Seisonidae, 226
Selenaria, 518;
vibracula, 487
Selenka, on Sipunculids, 424 n., 447
Self-fertilisation, in certain Mesostoma, 48;
in Cestodes, 86
Semper, on excretory system of Nemertinea, 108;
on Geonemertes palaensis, 101 n., 117;
on mimicry in Polychaeta, 294
Sense-organs, of Leptoplana, 13;
of Polyclads, 26;
of Triclads, 36;
of Cestodes, 86;
of Nemertinea, 106;
of Nematoda, 128;
of Gordius, 166;
of Acanthocephala, 178;
of Chaetognatha, 188;
of Rotifera, etc., 215, 233, 234;
of Oligochaeta, 354;
of Leeches, 395;
of Gephyrea, 417;
of Phoronis, 457
Septum, of Archiannelida, 244;
of Polychaeta, 269;
of Chlorhaemidae, 334;
of Oligochaeta, 355;
of Gephyrea, 440
fossil, 301;
commensal with Polynoid, 298;
colour, 292
nerve cords, 255;
gills, 261;
operculum, 261;
cirri, 265;
thoracic membrane, 266;
uncinus, 267;
fission, 281;
tube, 290;
from great depth, 300;
fossil, 301
Serpulite chalk, 301
Seta, of vibraculum, 484, 485, 486, 517, 524
Setosella, 530
Sharks, Trematodes of, 62, 72;
Cestodes of, 78
Sheep, parasites of, 67, 81, 82, 83
Sheldon, Miss, on Nemertinea, 99 f.
Shell-gland, of Leptoplana, 8, 9, 14, 16;
of Polyclads, 28;
of Trematodes, 59;
of Cestodes, 86
Shield, cuticular, of Polychaeta, 259;
of Sternaspis, 335;
glandular—see Gland shields.
Shipley, on Bipalium, 37;
on Nemathelminthes, 123 f.;
on Gephyrea, 411 f.;
on Phoronis, 450 f.
Sialis lutaria, host of Gordius, 171, 172;
host of Acanthocephala, 185
Side organs, of Carinellidae, 107
Siebold, von, on Tape-worms, 76
Sigalion, 313
Silliman, on Nemertinea, 101, 109, 118
Silurian, Polychaeta, 301
Sinus, in Polyzoa, 482, 484, 525
Siphon, of Capitelliformia, 272, 305;
of Gephyrea, 436
Siphonostoma, 334;
commensal, 298
species, 426
Sipunculus, 425;
history, 411;
species, 426;
food, 422;
habits, 426
Size, of Cestodes, 5;
of Polyclads, 20;
of Land Planarians, 33;
of Cestodes, 75;
of Nemertinea, 100
Slavina, 377
Sluiter, on Gephyrea, 429, 447
Smitt, on Polyzoa, 516
zooecium and avicularium, 482
Snakes, parasites of, 142
Solenopharyngidae, 50
Solenopharynx, 50
Solenophorinae, 91
Solenophorus, 91
Sorocelis, 42
anatomy, 186 f.;
eggs, 189;
habits, 190;
American species, 534
Spallanzani, on Oligochaeta, 348
anatomy, 355.
Spatangus, as host, 298
Spencer, on Land-Planarians, 34;
Spengel, on Gephyrea, 440
Spermatheca, of Dinophilus, 243;
Spermiducal gland, 361
Sphaerodoridae, 320
Sphaerodorum, 321
Sphaerosyllis, 308
Sphaerularia, 150, 153, 160, 161
Sphyranura, 73;
setae in, 56
Spine, of Polyzoa, 481, 523 f., 524
Spinther, 318
Spio, 322
peristomial cirri, 263;
gill, 265;
eyes, 272;
food, 296
Spirographin, 290
Spirographis, 338;
substance of tube, 290
S. reticulata, 149;
S. obtusa, 161;
S. alata, 163
Spirosperma, 378;
chaeta, 350
Spirulaea, 301
{557}Sporocysts, 92;
of Distomum macrostomum, 64, 65;
of D. hepaticum, 67;
hosts of, 71
Staggers, induced by Coenurus, 82
Statoblast, 493, 499, 501 f., 506;
sessile, 502;
resemblance to ephippian ova, 493
Steenstrup, on Tape-worms, 76
Steganoporella, 530
Stelechopoda, 344
Stelechopus, 342
asexual reproduction, 44
Stephanoceros, 202, 205, 210, 213, 220, 221
Stephanops, 225
Stercutus, 376
nephridia of, 305
shape, 259;
shield, 259;
head, 264;
chaetae, 265;
gills, 268;
intestine, 271;
compared with Gephyrea, 336, 447, 449
Sthenelais, 299, 300, 309, 313
Stichostemma eilhardii, 118
Stilesia, 91;
generic characters, 90;
S. centripunctata, 91;
S. globipunctata, 91
Stock, asexual, of Autolytus, 279;
of Myrianida, 281
Stolc, on Oligochaeta, 360
Stolonata, 518 n.
Strobilation, 76
Strodtmann, on Chaetognatha, 191
Stromatoporoids, 520
Strongylus, 129, 142, 143, 160, 163;
S. filaria, 132;
S. tetracanthus, 163
Stuhlmann, on Polyzoa, 493
Stylets of Nemertine proboscis, 104, 110
Stylochus, 19;
development, 28
Succinea putris, infested by larvae of Distomum macrostomum, 64, 66
Sucker, of Leptoplana, 8, 16 n.;
of Dinophilus, 243;
of Chaetopterus, 324;
of Myzostoma, 342;
Summer-eggs, of Mesostoma, 48;
of Rotifera, 216
Swim-bladder, of Syllidae, 272
Swimming, of Leptoplana, 9, 10;
of Polyclads, 23;
palps, 260;
tentacles, 262;
head, 262;
parapodium, 264;
alimentary tract, 271;
swim-bladder, 272;
asexual reproduction, 278 f., 279;
colours, 293;
phosphorescence, 296;
ancestral, 303
development, 278;
S. armillaris, 307;
S. ramosa, 282;
S. vivipara, 276
Synapta, bearing Rotifers, 222, 227
Synchaeta, 200, 204 f., 224, 226
Syncytium, 125
Syngamus trachealis, 130, 142, 144, 161, 163, 164
Syrinx, 411
life-histories of species of, 83;
table of species, 89;
T. (Cysticercus) acanthotrias, 80;
life-history, 83;
specific characters, 90;
T. crassicollis, life-history, 78, 83;
specific characters, 89;
T. echinococcus, 80;
life-history, 83;
specific characters, 90;
T. krabbei, 81;
T. marginata, 81;
life-history, 83;
specific characters, 90;
T. (Hymenolepis) murina, 70, 80 n., 89;
specific characters, 91;
T. perfoliata, 163;
T. saginata (= T. mediocanellata), 78, 79;
life-history, 83;
specific characters, 89;
T. serialis, 82;
life-history, 83;
specific characters, 90;
T. serrata, 81;
specific characters, 90;
T. solium, 79;
specific characters, 89
Taeniasis, 82
Taeniidae, 91
Tail, of Arenicola, 333;
regeneration of, 283
Tanypus, host of Gordius, 172
Tardigrada, affinities, 344
Telmatodrilus, 378
habits and structure, 53 f.;
affinities, 54
Tennent, on land-leeches, 408
Tentacles, in Polyclads, 15, 26;
in Trematodes, 53;
(peristomial), of Spionidae, 322;
(prostomial), 255, 260, 262 f.;
of Nereis, 248;
of Polygordius, 244;
of Terebellids, use of, 289;
nerves to, 254
{558}Tentacle-sheath, in Polyzoa, 470
Tentacular cirri = Peristomial cirri, q.v.
Tentacular filaments, 304;
Terebella, 328;
otocyst, 273;
fossil, 301;
T. conchilega, tube, 286, 287, 288;
gill, 329;
T. nebulosa, colour, 292;
as host, 311;
gill, 329
shape, 259;
tentacles, 263;
gill, 265;
gizzard, 271;
tube, 286;
use of tentacles, 289;
colour, 293;
phosphorescence, 296;
food of, 296;
tube containing Polynoid, 298
gill, 329
definition, 304;
genital organs, 273;
gland shields, 287;
nephridia, 269;
nuchal organs, 273;
vascular system, 252
Terebripora, 478
Tertiary, Polyzoa, 521
Testes, of Leptoplana, 14, 15;
of Acoela and Alloeocoela, 47;
of Temnocephala, 54;
of Polystomum, 57;
of Cestodes, 75, 76, 86—see also Reproductive organs
Tetragonurus, 389
Tetraonchus, 73
Tetraphyllidae, 91
Tetrarhynchidae, 91
Tetrarhynchus, 75, 76 n., 85, 91
Tetrastemma, British species, 110;
habits, 114;
hermaphrodite species, 109;
viviparous species, 117
Thalamoporella, 530
Thalassema, 411, 435 f., 441, 443;
development, 439;
habits, 443
Theodisca, 321
Thompson, J. V., on term Polyzoa, 475
Thorax, of Polychaeta, 259, 306, 337
Thysanosoma, 91;
generic characters, 90;
T. fimbriata, life-history, 83;
specific characters, 90;
T. giardii, specific characters, 90
Tomopteris, 315;
colour, 294;
light-producing organ, 296;
prostomium, 259;
T. rolasi, 315
Tortoise, Temnocephala associated with, 53
Torus uncinigerus, 268
Tracks, fossil, 302
Travisia, 332
Travisiopsis, 321
life-histories, 71;
classification, 73
Trembley, on Turbellaria, 6;
Trepostomata, 520
Triaenophorus (= Tricuspidaria), 91;
excretory system, 84
Triarthra, 201, 203, 206, 211, 224, 225, 226
Triarthridae, 200, 201, 202, 206, 207, 224, 226
Trias, Serpulid in, 301
Trichocephalus, 131, 135, 136, 144, 160, 163;
species of, 145;
T. dispar, 145
Trichochaeta, chaeta, 351
Trichoderma, 159
species of, 145
Trichotrachelidae, 144
habits, 35 f.;
sexual reproduction, 38;
asexual reproduction, 40;
classification, 42;
British species, 31, 32, 34, 42
Tricoma cincta, 157
Tricuspidaria, 91
Triophthalmus, 224
Triphylus, 224
Tristicochaeta, 158
Tristomatinae, 73
Tristomum, 73
Trochophore, 229
Trochopus, 73
Trochosphaera, 200, 201, 221, 229, 230
Trochosphaeridae, 221
Trochosphere, of Archiannelida, 243, 245;
of Polychaeta, 274, 275, 510, 512;
of Polyzoa, 510
Trochus, in Rotifers, 202, 204
genital organs, 273;
head, 262;
intestine, 271
Trunk, of Nereis, 246;
of Polychaeta, 259;
of Gephyrea, 412 f.
Tube, of Rotifers, 205;
of Polychaeta, 287 f.; composition of, 290;
of Chaetopterus, 323;
of Clymene, 287;
of Dodecaceria, 326;
of Eunice tibiana, 290;
of Haplobranchus, 339;
of Hekaterobranchus, 326;
of Maldanidae, 332;
of Nereis, 316;
of Nicomache, 287;
of Owenia, 325;
{559}of Polydora, 323;
of Polynoids, 285;
of Panthalis, 313;
of Sabella, 287 f.;
of Sabellaria, 287;
of Sabellidae, 337;
of Serpulidae, 290, 339 f., 340;
of Terebellidae, 286, 287, 288, 289, 327 f.;
of Priapuloidea, 433;
of Echiurus, 444
Tube-forming glands, 304
Tube-making, of Polychaeta, 287 f.
Tubicolous Polychaeta, 285, 300, 304, 306
chaetae, 350
Tubificidae, 350, 361, 366, 378
Turbellaria, 3 f.
Turtles, parasites of, 142
Tylenchus, 131, 154, 155, 157, 160, 163
Tylosoma, 422, 423, 426, 430, 447
Typhloscolecidae, 258, 291, 321;
nuchal organ, 273 n.
Typhloscolex, 321
Typosyllis, regeneration of head, 283 n.
Udekem, D', on Oligochaeta, 365
Udonella, 73;
U. caligorum, 55;
U. pollachii, eggs of, 58
Udonellinae, 73
Umbonula, 531
Uncinaria, 143
Uncini, of Polychaeta, 266, 267, 304, 305
Uncus, 210
Urobenus, 388
Uteriporus, 42
of Triclads, 40;
of Rhabdocoela, 48;
of Temnocephala, 54;
of Diplozoon, 60;
of Didymozoon, 71;
of Calliobothrium, 75;
of Taenia, 79;
of Schistocephalus, 86;
in Bothriidae, 87;
of Rotifers, 216
Vagina, of Leptoplana, 16;
in ectoparasitic Trematodes, 57 f.;
Vaillant, on Hirudinea, 392, 405
Valencinia, 113;
V. lineformis, 112
Valkeria, 533
Vallentin, on Rotifers, 198
Vallisnia, 73
Vanadis, 315
Varme, 297
Vasa deferentia, of Leptoplana, 14, 15;
of Acoela and Alloeocoela, 47;
of Diplozoon, 60;
of Schistocephalus, 86
Vasa efferentia, of Leptoplana, 14, 15;
of Triclads, 38
Vascular System, of Nemertinea, 106, 107;
of Archiannelida, 244;
of Nereis, 251 f.;
of Polychaeta, 251 f.;
of Cryptocephala, 252;
of Scoleciformia, 252;
of Terebelliformia, 252;
absence of, in certain Polychaeta, 253;
of Oligochaeta, 355;
of Leeches, 396;
of Phoronis, 455
Vaucheria, Rotifers in, 227
Vejdovsky, on Rhabdocoels, 46;
on Oligochaeta, 365, 369, 374, 400
Vermes, 347
Vermiculus, 378
Vermiformia, 461
Verrill, on Chaetognatha, 534
Vertebrates, parasites of, 163, 174, 179, 183
Verworn, on statoblasts, 501
Vesicula seminalis, of Planaria, 39
of larva, 509
Vibracular zooecium, 485, 486, 517, 524
Vibraculum, 477, 484, 485, 517, 524;
movements, 487;
function, 486
Vicarious avicularia, 482
Victorella, 492, 501, 505, 518, 533
Villot, on life-history of Gordius, 172
Vinella, 521 n.
Virgate, 210
Visceral nervous system, of Nereis, 255
Vitellarium = Yolk-gland, q.v.
Vitello-intestinal canal, in Polystomatidae, 57
Viviparous, Nemertinea, 109, 117;
Volvox, Rotifers in, 227
Vortex, 44;
British species, 50;
body-cavity, 43
Vorticellids, on Polychaeta, 299
Vorticeros, British species, 45, 46, 50
Vorticidae, 50
Vuillemin (misprinted in text), on Nematodes in deserts, 156
Walford, on Polyzoa, 521
Ward, on Nectonema, 168;
on Sipunculus, 417
Warning colours, in Polychaeta, 294, 314
Waters, on Polyzoa, 517
Wheeler, on Myzostomaria, 344
Whelk, shell occupied by Nereis, 298
White Cat, 317
Whitman, on Dicyemidae, 94;
on Hirudinea, 395 f., 402, 405 f.
Willemoes-Suhm, von, on Tetrastemma agricola, 101, 115, 117, 118
Willey, on affinities of Nemertinea, 120 n.;
on Oligochaeta, 382
Wings, of Chaetopterus, 295, 324
Winter-eggs, of Mesostoma, 48;
of Rotifers, 217;
compared with statoblasts, 493
Woodworth, on yolk-glands, 38 n.
Wreath, in Rotifers, 200
{560}Yellow-cells, in Leptoplana, 13
Yolk-gland, in Planaria, 38, 39;
in Rhabdocoelida, 47;
in Temnocephala, 54;
in Polystomatidae, 57;
in Calliobothrium, 75;
in Schistocephalus, 86;
Youatt, on Coenurus, 82
Zebra, parasites of, 140
Zelinka, on Rotifers, 198, 215 n., 218, 219, 227, 229;
on Gastrotricha, 232
Zooecium, 466, 469, 474, 488, 523;
of Phylactolaemata, 495;
loss of zooecia, 481, (= calyces), 488;
primary, 506;
alterations with age, 522
Zooid, sexual and asexual, 278 f.
END OF VOL. II
PREVIOUS VOLUMES OF
THE CAMBRIDGE NATURAL HISTORY
Edited by S. F. Harmer, M.A., Fellow of King's College, Cambridge, Superintendent of the University Museum of Zoology; and A. E. Shipley, M.A., Fellow of Christ's College, Cambridge, University Lecturer on the Morphology of Invertebrates.
Volume III. Molluscs and Brachiopods. By the Rev. A. H. Cooke, M.A., Fellow and Tutor of King's College, Cambridge; A. E. Shipley, M.A., Fellow of Christ's College, Cambridge; and F. R. C. Reed, M.A., Trinity College, Cambridge. Illustrated. Medium 8vo. 17s. net.
TIMES.—"There are very many, not only among educated people who take an interest in science, but even among specialists, who will welcome a work of reasonable compass and handy form containing a trustworthy treatment of the various departments of Natural History by men who are familiar with, and competent to deal with, the latest results of scientific research.... Altogether, to judge from this first volume, The Cambridge Natural History promises to fulfil all the expectations that its prospectus holds out."
DAILY CHRONICLE.—"It can be read with profit by the zoologist, and there is a vast amount of matter which is interesting to those who like the tit-bits of science; but do not care so much for the more serious aspects of the subject."
NATURE NOTES.—"The work as a whole is thoroughly well got up, and we cordially recommend it to our readers."
FIELD.—"The work is really an admirable introduction to the study of molluscs, treating of their position in the animal kingdom, the habits and general economy of land and fresh-water species, their structure, general distribution over the earth's surface (which is illustrated by some very valuable maps), their growth and development, their uses to man, the cultivation for food of such species as the oyster, the mussel, and the snail, and the utilisation of their shells for money and ornament.... We know of no book available to the general reader which affords such a vast fund of information on the structure and habits of molluscs."
Volume V. Peripatus. By Adam Sedgwick, M.A., F.R.S. Myriapods. By F. G. Sinclair, M.A. Insects, Part I. By David Sharp, M.A. Cantab., M.B. Edin., F.R.S. Fully Illustrated. Medium 8vo. 17s. net.
Prof. Raphael Meldola, F.R.S., F.C.S., in his Presidential Address to the Entomological Society of London, said:—"The authors of this volume are certainly to be congratulated upon having furnished such a valuable contribution to our literature. When its successor appears, and I will venture to express the hope that this will be at no very distant period, we shall be in possession of a treatise on the natural history of insects which, from the point of view of the general reader, will compare most favourably with any similar work that has been published in the English language."
ENTOMOLOGISTS' MONTHLY MAGAZINE.—"We venture to think the work will be found indispensable to all who seek to extend their general knowledge beyond the narrowing influence of exclusive attention to certain orders or groups, and that it will take a high position in The Cambridge Natural History series."
MACMILLAN AND CO., Ltd., LONDON.
BOOKS FOR
STUDENTS OF NATURAL HISTORY
A HANDBOOK OF BRITISH LEPIDOPTERA. By Edward Meyrick, B.A., F.L.S., F.E.S. Extra Crown 8vo. 10s. 6d. net.
ENTOMOLOGISTS' MONTHLY MAGAZINE.—"This book cannot fail to afford great assistance to the student who desires to recognise and identify his specimens without the necessity of comparing them with named examples. It is without exception the best class-book that has yet appeared for imparting real sound knowledge of structure, evolution, and classification."
NATURAL HISTORY AND ANTIQUITIES OF SELBORNE. By Gilbert White. With Notes by Frank Buckland, a Chapter on Antiquities by Lord Selborne, and New Letters, Illustrated by P. H. Delamotte. New and Cheaper Edition. Crown 8vo. 6s. Also an Edition in Two Vols., with Introduction by John Burroughs, and Illustrations by Clifton Johnson. Crown 8vo. 10s. 6d.
FORTY YEARS IN A MOORLAND PARISH. Reminiscences and Researches in Danby-in-Cleveland. By Rev. J. C. Atkinson, D.C.L., Canon of York and Incumbent of the Parish, Author of "A History of Cleveland," "A Glossary of the Cleveland Dialect." With Maps and Illustrations. Extra Crown 8vo. 5s. net.
THE SCENERY OF SWITZERLAND AND THE CAUSES TO WHICH IT IS DUE. By the Right Hon. Sir John Lubbock, Bart., M.P., F.R.S., etc. Crown 8vo. 6s.
DAILY TELEGRAPH.—"By his 'Scenery of Switzerland,' he will enhance the pleasure of many a rambler, and insensibly teach, by practical example, the great lessons that are decipherable on the face of nature."
AN INTRODUCTION TO THE STUDY OF SEAWEEDS. By George Murray, F.L.S., Keeper of Botany in the Natural History Department of the British Museum. Illustrated. Crown 8vo. 7s. 6d.
A SKETCH OF THE NATURAL HISTORY OF AUSTRALIA, WITH SOME NOTES ON SPORT. By Frederick G. Aflalo, F.R.G.S., F.Z.S., etc. Illustrated by F. Seth. Crown 8vo. 6s.
HANDBOOK OF FIELD AND GENERAL ORNITHOLOGY. A Manual of the Structure and Classification of Birds. With Instructions for Collecting and Preserving Specimens. By Elliott Coues. Profusely Illustrated. 8vo. 10s. net.
STRUCTURE AND LIFE OF BIRDS. By F. W. Headley, Assistant Master in Haileybury College. Illustrated. 8vo. 7s. 6d.
TALES OF THE BIRDS. By W. Warde Fowler, M.A. With Illustrations by Bryan Hook. Second Edition. Crown 8vo. 8s. 6d.
A YEAR WITH THE BIRDS. By W. Warde Fowler, M.A. With Illustrations by Bryan Hook. Third Edition, enlarged. Crown 8vo. 3s. 6d.
SUMMER STUDIES OF BIRDS AND BOOKS. By W. Warde Fowler, M.A. Crown 8vo. 6s.
THE NATURAL HISTORY OF AQUATIC INSECTS. By L. C. Miall, F.R.S., Professor of Biology in the Yorkshire College, Leeds. Illustrated. Crown 8vo. 6s.
ROMANCE OF THE INSECT WORLD. By L. N. Badenoch. With Illustrations by Margaret J. D. Badenoch and others. Crown 8vo. 6s.
THE DEPTHS OF THE SEA. An Account of the General Results of the Dredging Cruises of H.M.SS. Porcupine and Lightning during the Summers of 1868, 1869, and 1870, under the Scientific Direction of Dr. Carpenter, F.R.S.; J. Gwyn Jeffreys, F.R.S. and Dr. Wyville Thomson, F.R.S. By Sir C. Wyville Thomson, F.R.S. With numerous Illustrations and Maps. Second Edition. Medium 8vo. 31s. 6d.
THE VOYAGE OF THE CHALLENGER. THE ATLANTIC. A Preliminary Account of the General Results of the Exploring Voyage of H.M.S. Challenger during the Year 1873 and the Early Part of the Year 1876. With Portrait, Plates, and Maps. In two Vols. Published by Authority of the Lords Commissioner of the Admiralty. Medium 8vo. 45s.
OBSERVATIONS ON THE GEOLOGY AND ZOOLOGY OF ABYSSINIA, made during the Progress of the British Expedition to that Country in 1867-1868. By W. T. Blanford, F.R.S. With Illustrations and Geological Map. 8vo. 21s.
WORKS BY A. R. WALLACE, F.R.S.
DARWINISM: An Exposition of the Theory of Natural Selection, with Some of its Applications. With Maps and Illustrations. Extra Crown 8vo. 9s.
NATURAL SELECTION AND TROPICAL NATURE. Essays on Descriptive and Theoretical Biology. Extra Crown 8vo. 6s.
THE MALAY ARCHIPELAGO: The Land of the Orang Utan and the Bird of Paradise. A Narrative of Travel. With Studies of Man and Nature. With Maps and Illustrations. Fourth Edition. Extra Crown 8vo. 6s.
ISLAND LIFE: or the Phenomena and Causes of Insular Faunas and Floras. Including a Revision and Attempted Solution of the Problem of Geological Climates. With Illustrations and Maps. Second Edition. Extra Crown 8vo. 6s.
THE GEOGRAPHICAL DISTRIBUTION OF ANIMALS; with a Study of the Relations of Living and Extinct Faunas as Elucidating the Past Changes of the Earth's Surface. With Maps and Illustrations. In Two Vols. Medium 8vo. 42s.
* * * * *
AN INTRODUCTION TO THE OSTEOLOGY OF THE MAMMALIA: being the substance of the course of lectures delivered at the Royal College of Surgeons of England in 1870. By Sir W. H. Flower, F.R.S., F.R.C.S., Director of the Natural History Museum. Illustrated. Third Edition. Revised with the assistance of Hans Gadow, Ph.D. Crown 8vo. 10s. 6d.
WILD BEASTS AND THEIR WAYS. Reminiscences of Europe, Asia, Africa, and America, from 1845-1888. By Sir Samuel W. Baker, Pacha, F.R.S. With Illustrations. New and Cheaper Edition. Extra Crown 8vo. 12s. 6d.
WANDERINGS IN SOUTH AMERICA, THE NORTH-EAST OF THE UNITED STATES, AND THE ANTILLES, IN THE YEARS 1812, 1816, 1820, and 1824. With Original Instructions for the perfect Preservation of Birds, etc., for Cabinets of Natural History. By Charles Waterton, Esq. Edited by the Rev. J. G. Wood. With 100 Illustrations. Crown 8vo. 6s.
FROM A NEW ENGLAND HILLSIDE. Notes from Underledge. By William Potts. Pott 8vo. 3s.
THE FRIENDSHIP OF NATURE. A New England Chronicle of Birds and Flowers. By Mabel Osgood Wright. 16mo. 3s.
MACMILLAN AND CO., Ltd., LONDON.
WORKS ON
BIOLOGY AND ZOOLOGY
A TEXT-BOOK OF COMPARATIVE ANATOMY. By Dr. Arnold Lang, Professor of Zoology in the University of Zurich, formerly Ritter Professor of Phylogeny in the University of Jena. With Preface to the English Translation by Professor Dr. Ernst Haeckel. Translated into English by Henry M. Bernard, M.A., Cantab., and Matilda Bernard. Part I. 8vo. Part II. (completing the Anatomy of the Invertebrates) 17s. net. each.
AN ATLAS OF PRACTICAL ELEMENTARY BIOLOGY. By G. B. Howes, Assistant Professor of Zoology. With a preface by Professor Huxley, F.R.S. Med. 4to. 14s.
MATERIALS FOR THE STUDY OF VARIATION. Treated with especial regard to Discontinuity in the Origin of Species. By William Bateson, M.A., Fellow of St. John's College, Cambridge. 8vo. 21s. net.
AMPHIOXUS AND THE ANCESTRY OF THE VERTEBRATES. By Arthur Willey, B.Sc., Tutor in Biology, Columbia College; Balfour Student of the University of Cambridge. With a preface by Henry Fairfield Osborn. 8vo. 10s. 6d. net.
THE MYOLOGY OF THE RAVEN (Corvus corax Sinuatus). A Guide to the Study of the Muscular System in Birds. By R. W. Shufeldt, of the Smithsonian Institute, Washington, U.S.A. With Illustrations. 8vo. 13s. net.
ORGANIC EVOLUTION AS THE RESULT OF THE INHERITANCE OF ACQUIRED CHARACTERS ACCORDING TO THE LAWS OF ORGANIC GROWTH. By Dr. G. H. Theodor Eimer, Professor of Zoology and Comparative Anatomy in Tübingen. Translated by J. T. Cunningham, M.A., F.R.S.E., late Fellow of University College, Oxford. 8vo. 12s. 6d.
FROM THE GREEKS TO DARWIN. An Outline of the Development of the Evolution Idea. By Henry Fairfield Osborn, Sc.D., Da Costa Professor of Biology in Columbia College, Curator in the American Museum of Natural History. 8vo. 9s. net.
LESSONS IN ELEMENTARY BIOLOGY. By T. Jeffrey Parker, B.Sc., F.R.S., Professor of Biology in the University of Otago, New Zealand. Illustrated. Second Edition. Crown 8vo. 10s. 6d.
KEY TO NORTH AMERICAN BIRDS. Containing a concise account of every species of living and fossil bird at present known from the continent north of the Mexican and United States boundary, inclusive of Greenland. Second Edition, with which are incorporated General Ornithology—an outline of the structure and classification of birds, and Field Ornithology—a manual of collecting, preparing, and preserving birds. By Elliott Coues, M.A., M.D., Ph.D., Member of the National Academy of Sciences. Profusely illustrated. Super Royal 8vo. 42s.
MONOGRAPH OF THE BRITISH CICADÆ OR TETTIGIIDÆ (Froghoppers and Grassflies). By George Bowdler Buckton, F.R.S., Corr. Memb. Acad. Nat. Hist, of Philadelphia, Memb. de la Soc. Ent. de France. Illustrated by more than 400 Coloured Drawings. Two Vols. Demy 8vo. 33s. 6d. each net. Also in Eight Parts. 8s. each net.
THE FOSSIL INSECTS OF NORTH AMERICA. With Notes on some European Species. By Samuel H. Scudder. Vol. I. The Pretertiary Insects. With 34 Plates. Vol. II. The Tertiary Insects. With a Map and 28 Plates. 4to. Paper Boards. 90s. net.
MACMILLAN AND CO., Ltd., LONDON.
NOTES
Hemprich and Ehrenberg, Symbolae physicae, Berlin, fol. 1831.
Τρῆμα, a hole; referring to the orifices of the suckers.
Mémoires pour servir à l'histoire d. Polypes d'eau douce, Leyden, 1744.
Die Parasiten des Menschen, 1879——. Engl. Transl. by W. E. Hoyle, i. 1886.
Band 4, by M. Braun. (Mesozoa and Trematoda completed; Cestoda in progress.)
Verm. terr. et fluv. ... succincta historia, 1773; Zool. Danica, 1777.
Observations on Planariae, Edinburgh, 1813.
M. Faraday, "On the Planariae," Medical Gazette, Feb. 1832; and in Edinburgh New Philosoph. Journal, vol. xiv. 1833, pp. 183-189.
Nov. Act. Acad. Caes. Leop.-Carol. tom. xiii. 1827.
Ann. Sci. Nat. (Zool.) I. tom. xv. 1828.; ibid. tom. xxi. 1830.
Mém. Acad. St. Pétersbourg, 5th ser. tom. ii. 1832.
Die rhabdocoelen Turbellarien des Süsswassers. Jena 1848.
Monographie d. Turbellarien. I. Rhabdocoelida, 1882. Die Acoela, Leipzig, 1892.
"Die Polycladen," Fauna u. Flora d. Golfes v. Neapel, Monogr. XI. 1884.
Phil. Trans. 1874, p. 105.
Since no food, but only the pharynx, passes through this "mouth," the term is unfortunate. Moreover the true mouth is the aperture placing the stomach in communication with the pharynx (Fig. 5, gm).
Ann. Sci. Nat. 1 sér. tom. xv. 1828, p. 146. "La Planaire trémellaire ... peut parcourir ... en faisant battre rapidement ses parties latérales à la manière des larges nageoires des Raies."
Observations on Planariae. Edinburgh, 1813, p. 12.
"Zur Anat. u. Entwickl. einiger Seeplanarien v. St. Malo," Abh. K. Gesellschaft d. Wiss. Göttingen, 1868.
The roof of the peripharyngeal chamber is hence known as the "diaphragm."
See Brandt, Fauna u. Flora d. Golfes v. Neapel, Monogr. XIII. 1885, p. 65.
Verhandlungen d. med. Gesellschaft zu Würzburg, iv. 1854, p. 223.
Enantia spinifera Grff. Mittheil. d. Naturwiss. Verein. f. Steiermark, 1889.
The sucker of Leptoplana tremellaris probably does not correspond with that of the Cotylea.
Collingwood, Trans. Linn. Soc. 2 ser. vol. i. pt. 3, 1876, p. 83.
Von Stummer-Traunfels, Zeitschr. f. wiss. Zool. Bd. lx. 1895, p. 689.
Planocera pellucida Mertens, P. simrothi v. Grff., P. grubei Grff., Stylochoplana sargassicola Mertens, S. californica Woodworth, Planctoplana challengeri Grff., all belonging to the Planoceridae. See v. Graff, "Pelagische Polycladen," Zeitschrift f. wiss. Zoologie, Bd. lv. 1892, p. 190.
Cambridge Natural History, vol. iii. p. 74.
Lang, "Polycladen," p. 629.
Wheeler, Journal of Morphology, vol. ix. part 2, 1894, p. 195.
Many Nudibranchiate Mollusca undergo this change of habitat. See Garstang, Journal of the Marine Biological Assoc. n.s. i. No. 4, 1890, p. 447.
Chun, "Ctenophoren," Fauna u. Flora G. v. Neapel, Monogr. I. 1880, p. 180.
See Lang, "Polycladen," p. 607.
Lang, "Polycladen," Pl. 30, Fig. 8.
Kongl. Fysiograf. Sällskapets Handlingar, Bd. iv. Lund, 1892-93.
Whitman, Journal of Morphology, vol. iv. 1890, p. 361.
A full account of Polyclad development is contained in Lang's "Polycladen," with references to the literature of the subject. Since the date of that work (1884) the embryology of Ctenophora has become better known, but, though the segmentation of the egg and early stages of development are very similar in both cases, the elaborate investigations of E. B. Wilson (Journ. Morphology, vol. vi. p. 361) show that the segmentation of Polychaet worms is again similar. The question of the affinities of the Polycladida is also discussed by Lang ("Polycladen" p. 642 et seq.). The work of the last decade has neither proved nor disproved his suggestion that the Ctenophores and Polyclads have been derived from common ancestors. On this subject the remarks made by Hatschek (Lehrbuch d. Zoologie, p. 319) are some of the weightiest that have appeared.
Hallez, Revue Biologique du Nord de la France, tom. ii. 1889-90.
Voigt, Zool. Anz. xv. p. 238.
Grube, Archiv f. Naturgeschichte, 38 Jahrg. Bd. i. 1872, p. 273.
Vejdovsky, Zeitschr. f. wiss. Zoologie, Bd. lx. 1895, p. 200.
Woodworth, Bulletin Mus. Comp. Zoology, Harvard, vol. xxi. No. 1, 1891.
Mitth. Zool. Stat. Neapel, 1882, p. 187.
Wheeler, Journal of Morphology, vol. ix. 1894, p. 167.
Dendy, Trans. Roy. Soc. Victoria 1890, p. 65; Id. Austral. Assoc. Brisbane, 1895, "Presid. Add. to Sect. D," p. 15.
Darwin, Ann. and Mag. Nat. Hist. vol. xiv. 1844, p. 241.
Shipley, Proc. Camb. Phil. Soc. vol. vii. pt. 4, 1891 (with literature).
Trans. Roy. Soc. Victoria from 1889 onwards. Trans. New Zealand Institute, 1894-95.
Moseley, Phil. Trans. 1874, p. 105; Id. Quart. Journ. Micr. Sci. vol. xlvii. 1877, p. 273; Loman, Bijdrag tot d. Dierkunde, Aflev. 14, 1887, p. 71; Id. Zool. Ergeb. ein. Reise in Nieder-Ost-Indien, Hft. 1, p. 131; Beddard, Zoogeography, 1895, p. 53.
Beobachtungen ü. Anat. u. Entwickel. an der Küste von Normandie, 1863, p. 18.
Archiv f. Naturgeschichte, 57 Jahrg. Bd. i. Hft. 3, 1891, p. 308.
Dendy, Proc. Roy. Soc. Victoria, vol. iv. n.s. i. 1892.
Schmarda, Neue wirbellose Thiere, Leipzig, 1859, I. i. p. 30.
Abhandl. d. Naturf. Gesell. zu Halle, Bd. iv. 1857, p. 33.
Arb. Zool.-Zoot. Instit. Würzburg, Bd. v. 1882, p. 120.
Woodworth (loc. cit. p. 38) states that in Phagocata the yolk-glands arise by proliferation from two parovaria, placed just in front of the ordinary ovaries. Iijima, however (Zeitschr. f. wiss. Zool. Bd. xl. 1883, p. 454), regarded them as derivatives of the parenchyma.
The extensive literature on this subject is fairly completely summarised by Voigt in Biol. Centralblatt, vol. xiv. Nos. 20, 21, 1894. Faraday's observations (cf. p. 6, note 8) have been generally overlooked.
Archives d. Biologie, tom. xii. 1892, p. 437.
Mitth. Zool. Stat. Neapel, Bd. iii. 1882, p. 187.
Böhmig, Ergebnisse d. Plankton Expedition, Bd. ii. H. g. 1895.
von Graff, Die Acoela, Leipzig, 1892. Appendix.
The development of the Acoela has been worked out recently by Mdlle. Pereyaslawzewa (Zapiski Novoross. Obshch. Odessa, 17 Bd. 1892) and Gardiner (Journal of Morphology, xi. No. 1, 1895, p. 155) with conflicting results. The former finds four endoderm cells, which give rise to a larval intestine. The Acoela are for her, Pseudacoela. Gardiner, on the other hand, finds no trace of an endoderm at any stage of the development of Polychoerus caudatus.
Tijdschr. Nederland. Dierk. Ver. Deel ii. 1875.
Von Graff, Monographie d. Turbellarien: I. Rhabdocoeliden, 1882. Gamble, Quart. Journ. Microscop. Science, vol. xxxiv. 1893, p. 433.
Zeitschr. f. wiss. Zoologie, Bd. lx. 1895, p. 163.
See von Graffs Monographie, pl. ix.; and Jensen, Turbellaria ad Litora Norvegiae, Bergen, 1878, pl. iv.
For the reproductive organs of Rhabdocoelida, consult von Graff, Monographie, "Die Acoela"; and Böhmig, Zeitschr. f. wiss. Zool. Bd. li. 1891, p. 167.
Untersuchungen ü. Platyhelminthen, Giessen, 1873, p. 101.
Haswell, Monograph of the Temnocephaleae. Macleay Memorial Volume. Mem. iii. 1893.
Braun, in Bronn's Klassen u. Ordn. d. Thierreichs, vol. iv. p. 407, gives a valuable summary of our knowledge of this group. For figures, see van Beneden and Hesse, Mémoires de l'Acad. roy. de Belgique, tom. xxxiv. 1864, pp. 1-169. A valuable paper (with synoptic tables) on Japanese Monogenea, by Goto, Journ. Coll. Sci. Japan, vol. viii. pt. 1, 1894, has recently appeared.
See Leuckart, "Parasiten" Bd. ii. p. 238.
Zeller, Zeitschr. f. wiss. Zool. xxii. 1872, pp. 1, 168; also Bd. xxvii. 1876, p. 238; xlvi. 1888, p. 233.
An excellent and beautifully illustrated account, by Looss, of the Distomatidae of Frogs and Fishes may be found in Leuckart and Chun's Bibliotheca Zoologica, Heft 16, 1894.
Leuckart, Parasiten d. Menschen, "Trematoden," 1892-94; R. Blanchard, Traité d. Zool. médicale, i. 1889; H. B. Ward, Report for 1894 of Nebraska State Board of Agric. Lincoln, U.S.A. 1895, p. 225.
Huxley, Anat. of Invert. Animals, 1877, p. 194.
Braun, Bronn's Thierreichs, Bd. iv. p. 792; Leuckart, Parasiten d. Menschen, 11 Abth. p. 158; Brandes, in Spengels Zool. Jahrb. Syst. Abtheil. Bd. v. 1890, p. 849; v. Nordmann, Mikr. Beitr. i. Berlin, 1832.
Heckert, Bibliotheca Zoologica (Leuckart and Chun), Heft 4, 1889. I am not aware that Leucochloridium has been noticed in England.
"Heterogamy" usually means the alternation of bisexual and unisexual generations (e.g. Rhabdonema nigrovenosum), but is, unfortunately, also used in the sense of Alloiogenesis, as defined above. See Grobben, Arbeit. Zool. zoot. Ints. Wien, Bd. iv. 1881, p. 201.
Parasiten, Bd. i. Abth. II. p. 152.
Festschrift f. Leuckart, Leipzig, 1892, p. 167.
Quart. Journ. Micros. Sci. vol. xxiii. 1883, p. 90.
The intermediate host in the Sandwich Islands is said to be Limnaea peregra. See Lutz, Centralbl. f. Bakter. xi. 1892, p. 783.
The mortality in wet years, however, is said to be largely due to pulmonary inflammation. This and other causes of death are not always discriminated in the returns.
See Thomas, Quart. Journ. Micros. Science, xxiii. 1883. Neumann, Parasites of Domesticated Animals, translated by Fleming, 1892.
Leuckart, loc. cit.; Looss, Archiv f. mikroskop. Anatomie, Bd. xlvi. 1895, p. 1.
In Leuckart, Die Parasiten d. Menschen, pp. 521-528, 1894.
See Braun. Bronn's Klassen u. Ordnungen d. Thierreichs, vol. iv. p. 572.
Braun, loc. cit. p. 573.
Taken largely from Braun, Ibid. pp. 864-866, where the literature of the subject is referred to fully.
Festschr. f. Leuckart, 1892, p. 134.
Arbeit. Inst. Wien, iii. 1881, p. 163; see also ibid. ix. 1890, p. 57.
For figures of various scolices see van Beneden, Mémoire sur les vers Intestinaux, 1861; Braun in Bronn's Thierreich, Cestoda (in progress), Bd. iv. Pl. xxxviii.-xlv.
The mature proglottis of Calliobothrium eschrichti is 8-9 mm. long, whereas the strobila only measures 4-5 mm. in length. Species of Phylliobothrium, Anthobothrium, and Tetrarhynchus show a similar but not an equal contrast between the size of the parent and proglottis (P. J. van Beneden, "Les Vers Cestoides," Nouv. Mém. de l'Acad. Roy. d. Belgique, tom. xxv. 1850).
The difficult question of the nature of the Cestode body and Cestode larvae is adequately discussed by Braun, loc. cit. p. 1167.
Leuckart, Die Parasiten d. Menschen [English trans. by W. E. Hoyle]; Blanchard, Traité de Zoologie médicale, 1893.
For a full account of the history of this subject see Leuckart, Parasiten d. Menschen, p. 28; Braun, loc. cit. Bd. iv. p. 929 et seq.; Huxley, Collected Essays, vol. viii. p. 229.
By Grassi this form is considered identical with T. murina. The latter species is known, from this author's researches, to develop in rats without migration into an intermediate host. Should Grassi's synonymy prove correct, the presence of large numbers of this tape-worm in man would readily receive its explanation.
Leuckart, loc. cit. p. 752 et seq.
For description of the Cercocystis-larva see Villot, Ann. Sci. Nat. (Zool.) (6), xv. 1883, Art 4; and compare Leuckart's criticism of this paper, "Parasiten," p. 979.
Moniez, "Sur les Cysticerques," Paris, 1880; Id. "Sur les Cestodes," 1881; Zschokke, "Recherches sur la structure anatomique et histologique d. Cestodes," Genève, 1888.
Schmidt, Archiv f. Naturgeschichte, Jahrg. lx. Bd. 1, 1894, p. 65.
For example, the genitalia in Dipylidium caninum are duplicated in each proglottis. Other differences are noted in the following table (pp. 89-90).
Taken from Neumann, Parasites of Domesticated Animals, 1892, p. 448.
µ = 1⁄1000 millimetre.
For a description of these glands, and for further diagnostic details and literature, see Stiles and Hassall, U. S. Department of Agriculture, Bureau of Animal Industry, Bulletin 4, 1893.
Ed. van Beneden, Bull. Acad. Roy. Belgique, 1876, p. 35.
Whitman, Mittheil. Zool. Stat. Neapel, Bd. iv.; see also Braun, in Bronn's Thierreich, Bd. iv. p. 253.
Braun, loc. cit. p. 281 (with literature).
Giard, "La Castration parasitaire," Bull. Sci. d. France et de Belgique, 3 sér. i. 1888, p. 12.
Schulze, Abh. Akad. Berlin, 1891, p. 1.
Arch. Naturg. lviii. 1891, p. 66.
P. Boston Soc. vol. vi. 1848.
Zeitschr. wiss. Zool. Bd. v. 1854, p. 344.
Arch. Anat. 1858, p. 289.
Ibid. 1858, p. 558.
Mem. Ac. St. Petersb. ser. vii. tom. xiv. 1869.
Zeitschr. wiss. Zool. Bd. xliii. 1886, p. 481.
R. von Willemoes-Suhm, Ann. Nat. Hist. ser. iv. xiii. 1874, p. 409.
Semper, Zeitschr. wiss. Zool. Bd. xiii. 1863, p. 558.
L. von Graff, Morphol. Jahrb. Bd. v. 1879, p. 430.
W. A. Silliman, Zeitschr. wiss. Zool. Bd. xli. 1885, p. 48.
du Plessis, Zool. Anz. vol. xv. 1892, p. 64.
J. von Kennel, Arb. Inst. Würzburg, Bd. iv. 1877-78, p. 305.
H. N. Moseley, Ann. Nat. Hist. ser. iv. vol. xv. 1875, p. 165.
See Hubrecht, in Verh. Ak. Amsterdam, vol. xx. 1880; and in Quart. J. Micr. Sci. vol. xx. 1880, p. 431.
"Nemertinen," Fauna und Flora G. von Neapel, 22 Monogr. 1895.
Beiträge zur Naturgeschichte der Turbellarien, Griefswald, 1851.
Arb. Inst. Würzburg, Bd. iii. 1876, p. 115.
Ibid. Bd. iv. 1877, p. 305.
Zool. Anz. vol. viii. 1885, p. 51.
Quart. J. Micr. Sci. vol. xxv. 1885, suppl. p. 1.
Zeitschr. wiss. Zool. Bd. xli. 1885, p. 48.
Ibid. Bd. liii. 1892, p. 322, and Fauna und Flora G. von Neapel, 22 Monogr. 1895.
Ann. Sci. Nat. (5) vol. xvii. 1873.
Zeitschr. wiss. Zool. Bd. iv. 1853, p. 178.
Our knowledge of British species is mainly due to M‘Intosh (British Annelids, Ray Society, 4to, 1873) and Riches (Journ. Mar. Biol. Ass. vol. iii. 1893-1895, p. 1).
Fauna und Flora G. von Neapel, 22 Monogr. 1895.
See M‘Intosh, British Annelids, Ray Society, 4to, 1873.
Loc. cit.
Zitschr. wiss. Zool. Bd. xli. 1885, p. 48.
Nature, vol. xlvi. 1892, p. 611.
Zool. Anz. vol. xv. 1892, p. 64.
Zeitschr. wiss. Zool. Bd. lix. 1895, p. 83.
Arb. Inst. Würzburg, Bd. iv. 1877-1878, p. 305.
Journ. Mar. Biol. Ass. vol. iii. 1893-1895, p. 22.
Quart. J. Micr. Sci. vol. xxiii. 1883, p. 349; Ibid. vol. xxvii. 1887, p. 605.
Cf. Willey, Amphioxus and the Ancestry of the Vertebrates, Macmillan, 1894.
Ann. Sci. nat. 7, sér. vol. xiii. 1892, p. 321.
E. Rohde, SB. Ak. Berlin, 1892, p. 515.
R. Hesse, Zeitschr. wiss. Zool. Bd. liv. 1892, p. 548.
E. Rohde, Zool. Beitr. Bd. i. 1885, p. 11.
E. Rohde, Zool. Anz. xvii. 1894, p. 38.
Monographie der Nematoden, 4to, Berlin, 1866.
Zeit. Physiol. Chem. vol. xiv. 1890, p. 318.
N. A. Cobb, P. Linn. Soc. N.S. Wales, 2nd ser. vol. vi. 1891, p. 143.
Monographie der Nematoden, Berlin, 1866, p. 192.
Zool. Anz. vol. xvi. 1893, p. 432.
Arch. Naturg. 60 Jahrg. Bd. i. 1894, p. 255.
SB. Ak. Berlin, 1891, p. 57.
[Hamann subsequently withdrew these statements.]
Leuckart, The Parasites of Man, English Trans. by W. E. Hoyle, Edinburgh, 1886, p. 56.
O. Bütschli, Zeitschr. wiss. Zool. Bd. xxvi. 1876, p. 103.
O. Hamann, Centrlb. Bakter. vol. xi. 1892, p. 501.
Zeitschr. wiss. Zool. vol. xxiii. 1873, p. 402.
Ibid. vol. xlii. 1885, p. 708.
Ann. Nat. Hist. 5th ser. vol. ix. 1882, p. 301.
Macleay Memorial Volume, Sydney, 1893, p. 252; and Proc. Linn. Soc. N.S.W. 2nd ser. vol. v. 1890, p. 449.
4th edition, 1880.
Compendium der Helminthologie, Hannover, 1878, and Nachtrag, 1889.
A. Heller, "Darmschmarotzen" in v. Liemssen's Handb. d. sp. Path. u. Ther. vol. vii.
Cobbold's Parasites, London, 1879, p. 246.
Arch. Zool. exper. 1 sér. tom. vii. 1878, p. 283.
Arch. Zool. exper. 1 sér. tom. vii. 1878, p. 283.
Balbiani, Anat. Physiol. 7th year, 1870-71, p. 180.
A Treatise on Parasites and Parasitic Diseases. English Trans. by G. Fleming, London, 1892.
Journ. Roy. Agric. Soc. 3rd series, vol. iv. 1893.
Sci. Mem. Medic. Officers, Army of India, vol. vii. 1892, p. 51.
Shipley, Proc. Phil. Soc. Camb. vol. viii. 1892-95, p. 211.
"The Distribution, etc., of Filaria sanguinis hominis," Trans. of 7th Inter. Congress of Hygiene, vol. i. 1892, p. 79.
v. Linstow, Arch. mikr. Anat. vol. xl. 1892, p. 498.
zur Strassen, Zeitschr. wiss. Zool. vol. liv. 1892, p. 655.
Rud. Leuckart, Abh. Sachs. Ges. vol. xiii. 1887, p. 567.
Macleay Memorial Vol. Sydney, 1893, p. 253.
Centrbl. Bakter. vol. viii. 1890, p. 489.
J. Percival, Nat. Sci. vol. vi. 1895, p. 187.
A. Strubell, Bibl. Zool. Bd. i. Heft 2, 1888, p. 1.
C. R. Ac. Sci. cxviii. 1894, p. 549.
Bihang Svenska Ak. Handl. viii. No. 11, 1883.
Anat. Untersuch. ü. freilebende Nordsee-Nematoden, Leipzig, 1886.
Cobb, P. Linn. Soc. N. S. Wales, 2nd ser. viii. 1893, p. 389.
Zeitschr. wiss. Zool. Bd. xvii. 1867, p. 539.
Panceri, Atti Acc. Napoli, vii. 1878, No. 10.
Panceri, Atti Acc. Napoli, vii. 1878, No. 10.
Arch. Naturg. 35 (i.), 1869, p. 112.
Zeitschr. wiss. Zool. Bd. xlii. 1885, p. 708.
Arch. Naturg. Jahrg. iii. Bd. i. 1837, p. 52; and van Beneden, Animal Parasites, p. 91. International Sci. Series.
F. Vejdovsky, Zeitschr. wiss. Zool. Bd. xliii. 1886, p. 369; Zeitschr. wiss. Zool. Bd. xlix. 1888, p. 188.
Arch. mikr. Anat. Bd. xxxvii. 1891, p. 239.
Arch. mikr. Anat. Bd. xxxiv. 1889, p. 248.
A. E. Verrill, P. U. S. Mus. vol. ii. 1879, p. 165.
O. Bürger, Zool. Jahrb. Anat. Bd. iv. 1891, p. 631.
H. B. Ward, Bull. Mus. Harvard, vol. xxiii. 1892-93, p. 135.
Mem. Acc. Torino, 2nd ser. vol. xl. 1890, p. 1.
Centrlb. Bakter. Bd. ix. 1891, p. 760.
Zeitschr. wiss. Zool. Bd. vii. 1856, p. 1.
Zool. Anz. vol. x. 1887, p. 602.
Von Linstow, Hannover, 1878, and Nachtrag, 1889.
H. B. Ward, P. Amer. Ac. new ser. vol. xix. 1892, p. 260.
Jen. Zeitschr. Bd. xxv. 1891, p. 113.
Bibl. Zool. Bd. ii. Heft 7. 1893.
Jen. Zeitschr. Bd. xxv. 1891, p. 113.
Zool. Anz. Bd. xv. 1892, p. 195.
Zool. Anz. vol. xv. 1892, p. 52.
Zool. Anz. vol. viii. 1885, p. 19.
Shipley, Quart. J. Micr. Sci. vol. xxxix. 1896.
O. Hertwig, Jen. Zeitschr. Bd. xiv. 1880, p. 196.
P. Gourret, Ann. Mus. Marseille, tom. ii. Mem. 2, 1884, p. 103.
Bibl. Zool. vol. i. 1888-89, p. 1.
Scott, Annals of Scottish Natural History, 1892 and 1893.
E. Béraneck, Rev. Zool. Suisse, vol. iii. 1895, p. 137.
Ann. Mag. Nat. Hist. 6th ser. vol. xiii. 1894, p. 440.
Archiv Naturg. 58 Jahrg. Bd. i. 1892, p. 333.
I Chetognati, Flora u. Fauna d. Golfes von Neapel, Mon. v. 1883.
loc. cit.
loc. cit.
The Rotifera, two vols, and supplt. London, 1886-89.
Phil. Trans. vol. xix. No. 220, p. 254 (abridged ed. vol. iii. 1705, p. 651).
Ibid. vol. xxiii. No. 283, p. 1304 (abridged ed. vol. v. p. 6).
Ibid. vol. xxiii. No. 295, p. 1784 (abridged ed. vol. v. p. 175).
Ibid. No. 337, vol. xxviii. 1714, p. 160.
Employment for the Microscope. London, 1785.
Paris, 1841.
Quart. Journ. Micr. Sci. vol. i. 1853, pp. 3-8, 65-76.
Trans. Micr. Soc. London, vol. i. (n.s.), 1853, pp. 1-19.
Verh. Ges. Würzb. vol. iv. 1854; Zeitschr. wiss. Zool. vols. iii. vi. 1851-55.
Zeitschr. wiss. Zool. vols. vii. ix. xii. 1856-58-63.
London, 1861.
Zeitschr. wiss. Zool. vol. xxxix. 1883.
Ann. Nat. Hist. ser. 6, vol. v. 1890, p. 1; viii. 1891, p. 34.
Jen. Zeitschr. Nat. vol. xix. 1886; and Zeitschr. wiss. Zool. vols. xliii. xlix. 1886-90.
Zeitschr. wiss. Zool. vol. xliv. 1886, p. 273.
Ibid. vol. xliv. p. 396; xlvii. 1888, p. 353; liii. 1892, p. 1.
For additions see Rousselet, J. Roy. Micr. Soc. 1893 and 1897.
Quart. Journ. Micr. Soc. (n.s.) vol. xxiv. 1884, p. 352.
The definition of the Orders and systematic position of the genera and species referred to under this head will be found in a following section (pp. 220 f.).
Reprinted in Baker's Employment for the Microscope, 1785, pp. 267 f.
"Wheel Animals, though found with most Certainty in Leaden Gutters, etc. are often discovered in the Waters of some Ditches, and likewise in Water that has stood a considerable Time even in the House; for I have often met with them, in sufficient Plenty, in a Sort of slimy Matter that is apt to be produced on the Sides of Glasses and other Vessels, that are kept long with the Infusions of Hay or other Vegetables; and probably they are wafted thither by the Air, when in the Condition of little dry Globules."
Gosse's account of the "Structure, Functions, and Homologies of the Manducatory Organs in the Class Rotifera" (in Phil. Trans. 1856) remains as the most complete anatomical account we have, though his attempt to identify these parts with the modified limbs of the Arthropod mouth has met with no support from subsequent workers. Gosse rendered these parts clearly visible by the use of dilute caustic alkali.
A modification of this type is seen in the parasite Drilophagus, where the unci and rami are two-pronged at the end, but the trophi are not movable on one another, but protrusible as a whole to serve as an organ of attachment to the Oligochaete Lumbriculus, to which this Rotifer attaches itself. See Vejdovsky, "Ueb. Drilophaga bucephalus," etc., in SB. Böhm. Ges. Jahrg. 1882 (1883), p. 390.
"Zur Rotatorien Württemburgs," in Jahresb. Ver. Würt. vol. l. 1894, p. 57.
Similarly Hudson and Zelinka both regard the dorsal antenna as formed by the coalescence of two antennae. These retain their distinctness in Asplanchna; in some Bdelloida the single antenna is supplied by a pair of nerves.
C. R. Ac. Sci. cxi. 1890, p. 310; cxiii. 1891, p. 388.
Acta Univ. Lund. xxviii. 1891-92.
[See, however, Calman, Natural Science, xiii. 1898, p. 43.]
Zeitschr. wiss. Zool. xxii. 1872, p. 455.
Arch. Zool. Exp. sér. 2, i. 1883, p. 131.
Zeitschr. wiss. Zool. xliv. 1886, p. 273.
Ibid. liii. 1892, p. 1.
[See further Jennings, Bull. Mus. Harvard, xxx. 1896, p. 1; Erlanger and Lauterborn, Zool. Anz. xx. 1897, p, 452; and Lenssen, Zool. Anz. xxi. 1898, p. 617.]
It does not appear to us that Zelinka is justified by his account of the development in regarding this cup as other than a part of the disc.
The classification we have adopted is a modification of that made by Hudson and Gosse; we have divided up their first Order Rhizota into two, and split off from Flosculariidae the family Apsilidae; removed the Asplanchnaceae from the admittedly heterogeneous Order Ploima, made distinct families in the Ploima for Microcodonidae and Rhinopidae, and created a third new Order for the Seisonaceae. Ehrenberg, Gosse, and Hudson, being the authors of most of the genera, are designated by their initials only.
This second species has also been found in the Northern United States.
This Order has been monographed recently by Janson in Abh. Ver. Brem. Bd. xii. Beilage, 1893, p. 1.
See Hudson in Month. Micr. Journ. vol. vi. 1871, pp. 121, 215, and Quart. Journ. Micr. Sci. (n.s.) xii. 1872, p. 333; Lankester, ibid. p. 338; Levander in Act. Soc. Faun. Fenn. xi. 1894.
In Denk. Ak. Wien, vol. vii. 1854, 2 Abth., p. 15. As has been suggested by Deby and by Daday, it is not impossible that Hexarthra is identical with Pedalion (and in this case the latter name, as newer, should be suppressed in favour of the former); but we must suppose that Schmarda's figure of the front view is a combination, more or less from memory or notes, of two sketches or notes taken some time before publication; the one a side view somewhat obliquely flattened, showing the two eyes as in Levander's Fig. 3; the other a front view, showing the two pairs of lateral limbs in their correct positions under pressure.
The male of Rhinops vitrea is exceptional in possessing a complete, functional alimentary canal, with mastax, stomach, and intestine (Rousselet). That of Proales werneckii has a mastax, but no intestine (Rothert).
For a full account of this group see Claus in Festschr. Z.-B. Ges. Wien, 1876, p. 75; and Plate in Mt. Stat. Neapel, vol. vii. 1886-87, p. 234; Ann. Nat. Hist. ser. 6, vol. ii., 1888, p. 86.
[Eighteen more have since been recorded.]
I have recently found a large species of this genus dwelling in the shell of the large Cladoceran Crustacean, Eurycercus lamellatus. It is remarkable for its power of completely telescoping its extremities within the middle segments, and for its immense foot-glands, both characters being doubtless correlated with its habitat. Rousselet identifies it with P. petromyzon.
Month. Micr. Journ. vol. ix. 1873, p. 287; Journ. Quekett Club, ser. 2, vol. ii. 1884-86, p. 231.
See Dr. Hudson's very suggestive presidential addresses to the Royal Microscopical Society, published in their Journal, vols. ix.-xi. 1889-91.
Euchlanis lynceus.—This is clearly not an Euchlanis, and of the six names referred to—Ploesoma, Gomphogaster, Gastropus, Gastroschiza, Bipalpus, and Dictyoderma—the first has priority, and the other five drop by the laws of zoological nomenclature.
Journ. Quekett Club, ser. 2, vol. v. 1892-94, p. 205.
Trans. Micr. Soc. (n.s.) i. 1853, p. 18 (read Dec. 31, 1851): "We may say, therefore, that the Rotifera are organized upon the plan of an Annelid larva.... I do not hesitate to draw the conclusion ... that the Rotifera are the permanent forms of Echinoderm larvae, and hold the same relation to the Echinoderms that the Hydriform Polypi hold to the Medusae, or that Appendicularia holds to the Ascidians."
Quart. Journ. Micr. Sci. (n.s.) vol. xvii. 1877, p. 399.
Ibid. (n.s.) vol. xx. 1880, p. 381.
Arb. Z. Inst. Wien, vols. i. iii. v. 1878-84; Lehrbuch der Zoologie, part iii. 1891.
Zeitschr. wiss. Zool. vol. xliv. 1886, p. 1.
The Microscope (Detroit), 1887-88.
Zeitschr. wiss. Zool. xlix. 1890, p. 209.
Ann. Sci. Nat. ser. 3, vol. xv. 1851, p. 158.
Zeitschr. wiss. Zool. vol. xlv. 1887, pp. 401-467, t. xx-xxii.
The breadth of the latter is estimated from Reinhard's figure.
The Echiuroid Gephyrea (see p. 434) are by some authorities considered to be a division of the Chaetopoda.
Another worm, Histriobdella (Histriodrilus) homari, which is parasitic on the eggs of the lobster, and which occurs on our coast, has been placed amongst the Archiannelida. It is a minute form, with peculiarities in its anatomy which render its affinities uncertain.
Quart. J. Micr. Sci. xxvii. 1887, p. 109.
J. Mar. Biol. Assoc. vol. i. (n.s.) 1889-90, p. 119.
Schimkewitsch, Zeitschr. f. wiss. Zool. lix. 1895, p. 46.
Hatschek, Arb. Zool. Inst. Wien, iii. 1881, p. 79.
Fraipont, "Le Genre Polygordius," Fauna u. Flora des Golfes v. Neapel, Monogr. xiv. 1887.
T. J. Parker, Lessons in Elementary Biology, London, 1891, p. 267, gives a full account of the anatomy and development of Polygordius.
"Die Capitelliden," Fauna u. Flora d. Golfes v. Neapel, Monogr. xvi. 1887, p. 350.
Encyclopaedia Britannica, 9th ed., Art. "Mollusca," p. 652.
Benham, "The Post-Larval Stage of Arenicola," J. Mar. Biol. Assoc. iii. (n.s.) 1893, p. 48.
The blood is colourless in Syllidae and Nephthydidae.
Ehlers states that some Eunicidae have green blood.
Benham, Quart. J. Micr. Sci. xxxix. 1896, p. 1.
Schaeppi, Jena. Zeit. xxviii. 1894, p. 217.
Goodrich, Quart. J. Micr. Sci. xxxiv. 1893, p. 387.
Benham, Quart. J. Micr. Sci. xxxii. 1891, p. 325. See also Bourne (nephridium of Polynoë), Tr. Linn. Soc. (Zool.), ii. 1883, p. 357; Meyer, for nephridium of Terebellidae, Sabellidae, and Cirratulidae, in Mt. Zool. Stat. Neapel, vii. 1887, p. 592.
It is worthy of note that in Aeolosoma alone amongst the Oligochaeta does the brain lie in the prostomium in the adult.
Andrews, "The Eyes of Polychaetes," J. Morph. vii. 1892, p. 169.
Wistinghausen, "Entwick. v. N. dumerilii," Mt. Zool. Stat. Neapel, x. 1891, p. 41.
This is a modification of the classification proposed by me at the meeting of the British Association at Oxford, 1894 (see Report, p. 696). For further characteristics of these Orders and sub-Orders see below Chap. XII. Ehlers, "Die Borstenwürmer," 1864, gives a historical survey of the group, and enumerates the earlier classifications.
It is doubtful whether these organs are palps or only lateral lips.
Pruvot traced the nerve supply to these organs, and thus established their homology. Arch. d. Zool. Expér. (ser. 2) iii. 1885, p. 211.
Meyer, "Stud. ub. d. Körperbau der Anneliden," Mt. Zool. Stat. Neapel, vii. 1887, p. 592; viii. 1888, p. 462. In this work a great number of important and interesting anatomical facts are recorded with respect to the Terebelliformia and Sabelliformia, as well as certain details as to the structure and development of the nephridia.
In some of the members of this family paired lateral tentacles appear to exist.
It is possible that some of these may be peristomial.
Individual cases in which chaetae are present have been recorded.
Meyer, loc. cit.
Haswell, P. Linn. Soc. N.S. Wales, vii. 1883, p. 251.
Eisig, "Die Capitelliden," Fauna u. Flora G. v. Neapel, Monogr. xvi. 1887, p. 331.
Compare with this the muscular organ of Dinophilus, p. 243, Protodrilus, and a similar structure which occurs in Terebellids.
Korschelt, "Über Ophryotrocha puerilis," Zeitschr. f. wiss. Zool. lv. 1893, p. 224.
Eisig, Mt. Zool. Stat. Neapel, ii. 1881, p. 255.
They are specially large also in the Typhloscolecidae; while Racovitza (Ann. Mag. N. H. (ser. 6), xv. 1895, p. 279) has recently suggested that the caruncle of Amphinomidae belongs to the category of nuchal organs, and compares it with the ciliated lappets of Pterosyllis.
Ehlers, Zeitschr. f. wiss. Zool. liii. 1892, p. 217.
See Claparède and Metschnikoff, "Beit. zur Kennt. d. Entwick der Chaetopoden," Zeitschr. f. wiss. Zool. xix. 1869, p. 163; and Fewkes, "On the Development of certain Worm Larvae," Bulletin Mus. Harvard, xi. 1883, p. 167.
For an account of the anatomy and development of a Trochosphere, see Hatschek, on Eupomatus, in Arbeit. Zool. Inst. Wien, vi. 1885. Also Meyer, Mt. Zool. Stat. Neapel, viii. 1888, p. 462; and for Polynoid larva see Häcker, Zool. Jahrb. Abth. Anat. viii. 1895, p. 245.
Many of the Polynoids are sexually dimorphic.
Claparède, "Annélides Chétopodes du Golfe de Naples," Supplement, 1870; and Wistinghausen, Mt. Zool. Stat. Neapel, x. 1891, p. 41.
Claparède used the term "epigamous" for this phase; Ehlers employed the term "epitokous," whilst he called the "Nereid" phase "atokous," under the impression that the worm did not become mature in this condition.
Malaquin gives a detailed account of the asexual reproduction in Syllidae in Recherches sur les Syllidiens, Lille, 1893, and in Revue Biol. d. Nord de la France, iii. 1891. See also St. Joseph, "Les annelides polychétes des côtes de Dinard," Ann. Sci. Nat. Zool. (7th ser.) i. 1886, p. 134.
Alex. Agassiz, Boston J. Nat. Hist. vii. 1863, p. 384.
Huxley, Edinb. New Philosoph. Journ. 1855, i. p. 113.
"Challenger" Reports, vol. xii. 1885, "Polychaeta," p. 198; and Oka, Zoolog. Centralbl. ii. 1895, p. 591.
Two new heads have been observed in Typosyllis variegata by Langerhans, and two new tails in another Syllis.
Dalyell, The Powers of the Creator revealed, etc., vol. ii. 1853, p. 225 et seq.
von Kennel, Arb. Zool. Instit. Würzburg, vi. 1883, p. 259.
Leidy, Proc. Acad. Nat. Hist. Philadelphia, 1883, p. 204.
Giard, C. R. Soc. Biol. v. 1893, p. 473.
See M‘Intosh, Ann. Mag. Nat. Hist. (ser. 4) ii. 1868, p. 276.
Lankester has suggested that a strong acid is secreted for the purpose, see Ann. Mag. Nat. Hist. (ser. 4) i. 1868, p. 233.
M‘Intosh, Ann. Mag. Nat. Hist. (ser. 6) xiii. 1894, p. 1.
Dalyell, The Powers of the Creator revealed, ii. 1853, p. 217.
Watson, Journ. R. Mic. Soc. 1890, p. 685; see also Dalyell, loc. cit. ii. p. 195.
Schmiedeberg, Mt. Zool. Stat. Neapel, iii. 1882, p. 373.
For pelagic forms, see Camille Viguier, Arch. de Zool. Expér. (ser. 2) iv. 1886, p. 347; also Reibisch, Die pelag. Phyllodociden u. Typhloscoleciden d. Plankton Exped. 1895.
Lankester, Journ. Anat. and Physiol. 1868, p. 114; and 1870, p. 119; see also MacMunn, "On the Chromatology of the Blood in some Invertebrates," Quart. J. Micr. Sci. xxv. 1885, p. 469.
For coloured pictures of worms consult Schmarda, "Neue wirbellose Thiere," 2nd part, 1861; Milne Edwards in Cuvier's "Règne Animal" (Ed. Disciples de Cuvier).
Semper, Animal Life, "Internat. Sci. Series," 1881, p. 401.
The experiments were made by Mr. Garstang at the Laboratory of the Marine Biological Association, and are recorded by Poulton in The Colours of Animals, "Internat. Sci. Series," 1890, p. 201.
Panceri, Atti Acad. Sci. Napoli, vii. 1875.
M‘Intosh, H.M.S. "Challenger" Reports, "Polychaeta," vol. xii. p. ix.
For an account of these worms see M‘Intosh, loc. cit. p. 257.
For a list of parasitic Polychaetes see St. Joseph, Ann. Sci. Nat. (ser. 7) v. 1888, p. 141.
Semper, loc. cit. p. 340.
See "Challenger Reports," and St. Joseph, loc. cit.
"Challenger" Reports, loc. cit. p. xxx.
See Hornell, Fauna of Liverpool Bay, Report III. 1892, p. 126.
Zittel, Handbuch d. Palaeontologic (Palaeozoologie), i. 1876-80, p. 562.
Ehlers, Zeitschr. f. wiss. Zool. xviii. 1868, p. 241.
The Chaetopteridae may have to be placed elsewhere in the system, as they are peculiarly modified, and present features recalling the Cryptocephala, from which it is possible they have descended.
Meyer (Mt. Zool. Stat. Neapel, vii. 1887, p. 669, note) suggests that the tentacular filaments of Cirratulids are really prostomial, but have shifted back on to the peristomium, or even farther.
It is probable that the genital ducts of Sternaspis and Chlorhaemids are modified nephridia.
The character of head and parapodium in each family will be gathered from the figures accompanying the general description in Chap. X., so that detailed description is unnecessary. In all cases the chaetae form valuable specific characters.
The examples of the various families are British, unless the opposite is expressly stated; but most of them are not confined to our shores, and the foreign localities are usually given. No attempt is made to enumerate all the British species.
The following books may be found useful for identifying the worms:—
Claparède, Recherches anat. sur les Annélides observées dans les Hebrides, 1861; Annélides Chétopodes du golfe de Naples, 1868, and Suppl., 1870.
Cunningham and Ramage, "Polychaeta Sedentaria of the Firth of Forth," Trans. Roy. Soc. Edinburgh, xxxiii. 1888, p. 635.
Ehlers, Die Borstenwürmer, 1868.
Johnston, "British Museum Catalogue of Non-Parasitical Worms," 1865.
M‘Intosh, "British Annelida," Trans. Zool. Soc. ix. 1877, p. 371; "Invert. Marine Fauna of St. Andrews; Annelida," Ann. Mag. Nat. Hist. (4) xiv. 1874, p. 144.
Malmgren, "Nordiska Hafs-Annulater," Öfversigt af K. Vet.-Akad. Förhandlingar, 1865, pp. 51, 181, 355; and "Annulata Polychaeta," ibid. 1867, p. 127.
St. Joseph, "Les Annélides Polychétes des côtes de Dinard," Ann. Sci. Nat. (Zool.) (7) vol. i. 1886, p. 127; v. 1888, p. 141; xvii. 1894, p. 1; xx. 1895, p. 185.
Malaquin, Recherches sur les Syllidiens, 1893; for structure of the gizzard, see also Haswell, Quart. J. Micr. Sci. xxvi. 1886, p. 471; and xxx. 1889, p. 31.
See M‘Intosh's Memoirs, loc. cit.
Herein are included the various genera formed by Kinberg, Malmgren, and others.
It appears to be the same as P. grubiana Clap.
Marenzeller has shown that Johnston's P. scolopendrina is not identical with that of Savigny, and suggests the above name for it.
F. Buchanan, "Report on Polychaetes, Part I." Sci. Proc. Roy. Dublin Soc. vii. (n.s.) 1893, p. 169.
Polyodontes Ran. deserves mention as being a large, rare form with peculiar pedal gland; cf. Eisig (ref. on p. 268), p. 324; and Buchanan, Quart. J. Micr. Sc. xxxv. 1894, p. 433.
Many authorities regard this species as synonymous with Savigny's P. laminosa.
According to a verbal communication from Mr. J. Hornell of Jersey, they belong to P. maculata Müll., while Mr. Garstang believes them to belong to Eulalia viridis.
These segmentally-arranged brown spots may perhaps be photogenic.
Greef, Acta Ac. German., xxxix. 1877.
Greef, Zeitschr. f. wiss. Zool. xlii. 1885, p. 432.
Buchanan, Quart. J. Micr. Sci. xxxv. 1894, p. 445.
Buchanan, Sci. Proc. R. Dublin Soc. viii. (n.s.) 1893, p. 169.
Reibisch, Phyllodociden u. Typhloscoleciden d. Plankton Exped. 1895.
The British species is usually referred to as C. insignis Baird, but Joyeux Laffuie (Arch. Zool. Exp. (ser. 2) viii. 1890, p. 244) has shown that there is only one European species. It is possible that there is a closer affinity with the Sabelliformia than is at present supposed.
Compare Sternaspis, p. 336.
For literature, see Benham, Quart. J. Micr. Sci. xxxix. part 1, 1896, p. 1.
F. Buchanan, Quart. J. Micr. Sci. xxxi. 1890, p. 175.
In some genera there are no gills, e.g. Leaena.
These characters are not necessarily generic.
Eisig, "Die Capitelliden," Fauna u. Flora G. v. Neapel, Monogr. xvi. 1887.
Ed. Meyer., Arch. mikr. Anat. xxi. 1882, p. 769.
Vejdovsky, Denk. Akad. Wien, xliii. 1882, part 2, p. 33; and Rietsch, Ann. Sci. Nat. (Zool.) ser. 6, xiii. 1882, art. 5.
For anatomy see Meyer, Mt. Zool. Stat. Neapel, vii. 1887.
Andrews, Journ. Morph. v. 1891, p. 271.
A. G. Bourne, Quart. J. Micr. Sci. xxiii. 1883, p. 168.
Closely allied is Manayunkia Leidy, which occurs in fresh-water lakes of America. Another fresh-water genus is Coabangia Giard, which perhaps deserves the creation of a special family. The anus is ventral and anterior. The chaetae are peculiarly arranged, dorsal uncini being present only on four segments. The first body segment carries a ventral bundle of five great "palmate" chaetae.
For the anatomy see Meyer, Mt. Stat. Neapel, vii. 1887; see also above, p. 306.
von Graff, "Myzostomida," "Challenger" Reports, part 27, vol. x. 1884; and "Supplement," part 61, vol. xx. 1887.
Marenzeller, Anz. Akad. Wien, xxxii. p. 192.
Mt. Zool. Stat. Neapel, xii. 1896, p. 227; where, too, see literature.
Beard, Mt. Zool. St. Neap. v. 1884, p. 544.
Quart. J. Micr. Sci. (n.s.) vol. iv. 1864, p. 258; and v. pp. 7, 99.
Zeitschr. wiss. Zool. xix. 1869, p. 563.
De Lumbrici terrestris Historia naturali, Brussels, 1829.
Naturg. ein. Wurm-Arten d. süssen u. salzigen Wasser, Copenhagen, 1771.
Trans. Roy. Soc. Victoria, vol. i. 1888, p. 1.
Phil. Trans. clxxxvi. 1895, A, p. 383.
Mém. cour. Ac. Belg. lii. 1890-93.
Quart. J. Micr. Sci. xxxi. 1890, p. 83.
Beddard, Ibid. xxxiii. 1892, p. 325.
Beddard, Ann. Mag. Nat. Hist. (6) xiii. 1894, p. 205.
Mém. Soc. Zool. France, iii. 1890, p. 223.
Vegetable Mould and Earthworms, London, 1881.
Zool. Anz. xi. 1888, p. 72.
See Fletcher, P. Linn. Soc. N.S.W. (2) iii. 1889, p. 1542.
In Sitzungs-Ber. Böhm. Ges. 1889, p. 183.
See Dr. Rosa in Ann. Hofmus. Wien, vi. 1891, p. 379.
Entwickelungsgeschichtliche Untersuchungen, Prag, Heft i. 1888, p. 33.
See Kleinenberg, Quart. J. Micr. Sci. xix., 1879, p. 206.
Both Col. Feilden and Mr. Trevor-Battye found specimens in Kolguiev.
Neue wirbellose Thiere, Leipzig, ii. 1861, p. 11.
Kew Bull. Misc. Information, No. 46, 1890.
Rev. Biol. Nord France, i. 1889, p. 197.
SB. Ges. naturf. Berlin, 1893, p. 19.
System u. Morph. d. Oligochaeten, Prag, 1884.
See my text-book of Zoogeography (Cambridge, 1895) for fuller treatment.
Ann. Mag. Nat. Hist. (6) ix. 1892, p. 12.
Darwin, Vegetable Mould and Earthworms, p. 121.
"An Attempt to classify Earthworms," Quart. J. Micr. Sci. xxxi. 1890, p. 201.
Oxford, 1895.
See especially Vejdovsky, Syst. u. Morph. Olig. Prag, 1884.
Vejdovsky, Monographie der Enchytraeiden, Prag, 1879. Michaelsen, "Synopsis der Enchytraiden," Abh. Ver. Hamburg, xi. 1889, p. 1.
J. P. Moore, "The Anatomy of Bdellodrilus," J. Morphol. x. 1895, p. 497.
Beddard, Trans. Roy. Soc. Edin. xxxv. 1890, p. 629, and xxxvi. 1892, p. 1.
A. G. Bourne, "On the Naidiform Oligochaeta," Quart. J. Micr. Sci. xxxii. 1891, p. 335.
F. E. Beddard, Trans. Roy. Soc. Edin. xxxvi. 1892, p. 273.
Vejdovsky, System u. Morph. d. Oligochaeten, Prag, 1884.
"Anatomical Notes on Sutroa," Zoe. ii. 1892, p. 321.
"Pacific Coast Oligochaeta," Mem. California Acad. Sci. vol. ii.
Quart. J. Micr. Sci. xxxvi. 1894, p. 307.
See Spencer, Proc. Roy. Soc. Vict. v. 1893, and Fletcher, P. Linn. Soc. N.S.W. 1886-1888, for Australian forms; Rosa, Ann. Mus. civ. Genova, vi. 1886, x. 1890, and xii. 1892, for Oriental species, etc.
See Fletcher and Spencer, already quoted, for Australian species.
Eisen, "Anat. Studies on Ocnerodrilus," Proc. Calif. Acad. (2) iii. 1892, p. 228.
Beddard, Ann. Mag. Nat. Hist. (6) x. 1892, p. 74.
Beddard, P. Z. S. 1885 and 1895, for Antarctic Acanthodrilids; Michaelsen, in Jahrb. Hamburg. Anst. 1888-95, for Benhamia.
For a general account of the Eudrilidae, see my Monograph of the Order Oligochaeta, Oxford, 1895.
Nouv. Arch. Mus. Paris, viii. 1872, p. 5.
The scattered literature of this family is due to Benham, Michaelsen, Perrier, Rosa, and others.
Rosa, "Revisione dei Lumbricidae," Mem. Acc. Torino (2), xliii. 1893, p. 399; also the Rev. H. Friend's numerous and useful papers, and especially "A New Species of Earthworms," Proc. Roy. Irish Ac. (3) ii. 1891-93, p. 402; and "The Earthworms of Ireland," Irish Nat. v. 1896, p. 69, etc.
In the tables the figures refer to the segments of the body. Opposite the name of each species are two sets of lines; the upper series indicate the segments occupied by the clitellum; the lower series those occupied by the tubercula pubertatis. The dots indicate the occasional extension of the clitellum or of the tubercula.
"Annelés," vol. iii. 1889-90, p. 477, in the Suites à Buffon.
See v. Kennel, Zool. Jahrb. ii. 1887, p. 37.
Nouvelle Monographie des Sangsues médicinales. Paris, 1857.
Quart. J. Micr. Sci. xxvi. 1886, p. 317.
See Grube, "Annulaten" of Middendorff's Sibirische Reise, Zoology, 1851, p. 20; and Kowalevsky, Bull. Ac. St. Petersb. v. June 1896.
Asajiro Oka, Zeitschr. wiss. Zool. lviii. 1894, p. 79.
Loc. cit.
Quart. J. Micr. Sci. xxiv. 1884, p. 419; see also ibid. xxxiv. 1893, p. 545, which is mainly a criticism of Bolsius' additions to the very considerable literature upon the Leech nephridium.
"Spermatophores as a Means of Hypodermic Impregnation," J. Morphol. iv. 1891, p. 361.
Zeitschr. wiss. Zool. lviii. 1894, p. 440; and Zool. Jahrb. Anat. iv. 1891, p. 697.
"Annelés," vol. iii. 1889-90, p. 493, in the Suites à Buffon.
Whitman quotes with regretful approval (Proc. Americ. Acad. xx. 1884-85, p. 76) Sir J. Dalyell's remark, "It does not appear that the history of the leech has advanced in proportion to the number of literati who have rendered it the subject of discussion," and adds on his own account the following severe indictment of his predecessors: "As a considerable share of the work done in this direction is purely systematic, it is somewhat surprising that not a single description of any Hirudo has been given with sufficient accuracy and completeness for a close comparison of even its more important external characters with those of other species."
"Hirudinées de l'Italie," etc., Boll. Mus. Zool. Torino, vol. ix. 1894, No. 192. See also Apathy, "Süsswasser-Hirudineen," Zool. Jahrb. Syst. iii. 1888, p. 725.
Zeitschr. f. die gesammt. Naturwiss. vi. 1872, p. 422.
But Pennant in his British Zoology has referred to a leech which is even larger. Upon the huge Basking shark (Selache) the fishermen sometimes observe a leech, which invariably drops off when the fish is brought to the surface, "of a reddish colour and about 2 feet in length"; this may be a Pontobdella.
Ann. Mag. Nat. Hist. (6) xii. 1893, p. 75.
Memorie sulla Storia e Notomia degli Animali senza Vertebre, 1823.
Histoire Naturelle des Animaux sans Vertèbres, vol. iii. 1816, p. 76.
Le Règne Animal, 2nd ed. 1830.
γέφῦρα = a bridge, Ann. Sci. Nat. (3), vol. vii. 1847, p. 340.
Fischer, Abh. Ver. Hamburg, Bd. xiii. 1895, p. 1.
Cuénot, Arch. Zool. exp. (2) ix. 1891, p. 593.
Bull. Mus. Harvard, vol. xxi. 1891, p. 143.
Shipley, Quart. J. Micr. Sci. vol. xxxi. 1890, p. 1.
Stud. Johns Hopkins Univ. vol. iv. 1887-90, p. 389.
Conn, Stud. Johns Hopkins Univ. vol. iii. 1884-87, p. 351.
Arb. Instit. Wien, Bd. v. 1884, p. 61.
Shipley, Quart. J. Micr. Sci. vol. xxxii. 1891, p. 111.
Proc. Roy. Soc. Edin. xviii. 1892, p. 17.
Selenka, Die Sipunculiden. Semper's Reisen im Archipel d. Philippinen, vol. iv. 1883.
Stud. Johns Hopkins Univ. vol. iv. 1887-90, p. 389.
Selenka, Challenger Reports, vol. xiii. 1885.
Ann. Sci. nat. (7) vol. xx. 1895, p. 1.
Zool. Anz. ix. 1886, p. 574.
This is not true of all species.
Acta Ac. German, Halle, xli. Part II. No. 1, 1879.
Recueil Zool. Suisse, iii. 1886, p. 313.
P. Phys. Soc. Edinb. vol. i. 1856, p. 165; and Edinb. New Phil. Journ. vol. iv. (n.s.) 1856, p. 313; Ann. Sci. Nat. 4th ser. vol. xi. 1859, p. 150; and F. D. Dyster, Tr. Linn. Soc. London, vol. xxii. 1859, p. 251.
Ann. Sci. Nat. 4th ser. vol. x. 1858, p. 11.
"Beiträge zur Anatomie der Phoronis," Inaug. Dissert. Prag. 1889, and Zeitschr. wiss. Zool. vol. li. 1891, p. 480.
P. Linn. Soc. N. S. Wales, 1st ser. vol. vii. 1883, p. 606; and 2nd ser. vol. vii. 1893, p. 340.
Quart. J. Micr. Sci. vol. xxx. 1890, p. 125.
Challenger Reports, vol. xxvii. 1888; and Proc. Roy. Soc. Edinb. vol. xi. 1882, p. 211.
Proc. Roy. Soc. London, vol. xxxiv. 1883, p. 371.
Zapiski Acad. St. Petersb. vol. xi. No. 1, 1867 (Russian). Abstract in Arch. Naturg. Jahrg. xxxiii. 1867, Bd. ii. p. 235.
Caldwell, loc. cit. Foettinger, Arch. Biol. vol. iii. 1882, p. 679; Gegenbaur, Zeitschr. wiss. Zool. vol. v. 1854, p. 345; Krohn, Arch. Anat. Jahrgang 1858, p. 289; Metschnikoff, Nachricht. k. Ges. Wiss. Göttingen, No. 12, 1869, p. 227, and Zeitschr. wiss. Zool. vol. xxi. 1871, p. 233; J. Müller, Arch. Anat. Jahrgang 1846, p. 101; Schneider, Monatsber. Ak. wiss. Berlin, 1861, p. 934, and Arch. Anat. Jahrgang 1862, p. 47; Wagener, Arch. Anat. Jahrgang 1847, p. 202; Wilson, Amer. Natural. vol. xiv. 1880.
Proc. Roy. Soc. Edinb. vol. xxi. 1896, p. 59; and Zool. Anz. xix. 1896, p. 266.
The account given in the following pages has been deliberately restricted, for the most part, to British species. Our own fauna contains an assemblage of Polyzoa which is so representative that it has seemed better to do some justice to the British forms than to attempt to cover the whole ground in the limited number of pages devoted to this group. Those who desire to make a wider study of the subject should refer, for marine forms, to Busk's Catalogue of Marine Polyzoa in the Collection of the British Museum, Parts I.-III. 1852, 1854, 1875; to the Challenger Reports on Polyzoa, Parts 30 (1884), 50 (1886), and 79 (1888); for references and lists of species, to Vine's Report on Recent Marine Polyzoa, Cheilostomata and Cyclostomata (Report, 55th meeting Brit. Ass. Aberdeen, 1885, pp. 481-680); [and to Nickles and Bassler, Synopsis Amer. Foss. Bryozoa incl. Bibliography (Bull. U.S. Geol. Survey, No. 173, 1900)]. References to the literature of the fresh-water forms will be found below, in Chap. XVIII.
Hooker, quoted by Landsborough, Hist. Brit. Zoophytes, 1852, p. 346.
Rare and Remarkable Animals of Scotland, ii. 1848, p. 15.
Arch. Zool. Exp. 2 sér. x. 1892.
Kraepelin, Abh. Ver. Hamburg, x. 1887, No. ix. p. 19; κάμπτειν, to bend; δέρμα, skin.
Parts of the ectocyst of some calcareous forms are covered by an external investment of cells, which give rise to secondary thickenings, ridges, and other growths.
From the Quart. J. Micr. Sci. xxxiii. 1892.
Ibid. p. 123.
Quart. J. Micr. Sci. xxxiii. 1892, p. 147. The experiment was conducted in a laboratory, and the results may not be perfectly normal with regard to the time occupied.
See also Joliet, Arch. Zool. Exp. vi. 1877, p. 202, and explanation of plate viii. for another series of observations.
See especially G. J. Allman, Monograph of the Fresh-water Polyzoa, Ray Society, 1856, p. 41; and H. Nitsche, Zeitschr. wiss. Zool. xxi. 1871, p. 479.
Ray Society, 4to, 1856.
Zoological Researches and Illustrations, v. "On Polyzoa." Cork, 1830.
"Symbolae Physicae," 1831, and Abh. Ak. Berlin, 1832, i. p. 377, etc.
T. cit. p. 92.
Vol. i. 1880, Introduction, p. cxxxi.
Élémens de Zoologie, 2nd ed. Animaux sans Vertèbres, 1843, pp. 238, 312. Prof. A. Milne-Edwards has kindly written to me, informing me that he believes this to have been the first occasion on which the term was thus used.
Phil. Trans. vol. cxliii, 1853, p. 62.
Nitsche, Zeitschr. wiss. Zool. xx. 1870, p. 34.
πρωκτός, anus; ἐντός, within; ἐκτός, without.
λοφός, crest or tuft.
γυμνός, naked; λαιμός, throat.
φυλάσσω, I guard.
κύκλος, circle; στόμα, mouth.
χεῖλος, lip.
κτείς, κτενός, comb.
Miss E. C. Jelly, Synonymic Cat. Recent Marine Bryozoa, London, 1889.
Zeitschr. wiss. Zool. xxi. 1871, p. 421.
Fischer, Nouv. Arch. Mus. Paris, ii. 1866, p. 293.
Ehlers, Abh. Ges. Göttingen, xxi. 1876, p. 3, and Joyeux-Laffuie, (as Delagia) Arch. Zool. Exp. 2 sér. vi. 1888, p. 135.
Busk, "Challenger" Reports, Parts 30 and 50.
Hincks, Brit. Marine Polyzoa, Introduction, p. cxxxv.
Hincks, Brit. Mar. Polyzoa, i. p. 558.
See Hincks, Brit. Mar. Polyzoa, i. p. lxiv.; and Busk, Cat. of Marine Polyzoa in the British Museum, part ii. 1854, p. 103.
J. Linn. Soc. xv. 1881, p. 359.
"Challenger" Report, part xxx. 1884, pl. ix.
Hincks, Brit. Mar. Polyzoa, i. p. 58.
Brit. Mus. Cat. part ii. 1854, p. 106; Hincks, t. cit. p. 181 n.
Barentsia Hincks (= Ascopodaria Busk) differs from Pedicellina in that each stem has a muscular swelling at its base. The genus is represented by two British species, B. gracilis Sars and B. nodosa Lomas.
Arch. Zool. Exp. 2 sér. ix. 1891, p. 91.
For structure, see Davenport, Bull. Mus. Harvard, xxiv. 1893, p. 1.
λοξός, oblique; σῶμα, body.
Quart. J. Micr. Sci. xxvii. 1887, pl. xxi. Fig. 10.
For a recent account of the Entoprocta, see Ehlers, "Zur Kenntniss d. Pedicellineen," Abh. Ges. Göttingen, xxxvi. 1890, No. iii.
[An important account of the structure of marine Ectoprocta is given by Calvet, "Contribution à l'Histoire Naturelle des Bryozoaires Ectoproctes Marins," Trav. Inst. Zool. Montpellier, N.S., Mém. No. 8, 1900.]
Kraepelin, K., "Die deutschen Süsswasser-Bryozoen."—Abh. Ver. Hamburg, x. 1887, No. 9, p. 95.
Jullien, Bull. Soc. Zool. France, x. 1885, p. 92.
Hincks, Brit. Marine Polyzoa, i. p. 132.
T. cit., p. 167.
Quoted by Kraepelin, t. cit., p. 83.
Kraepelin, Abh. Ver. Hamburg, xii. 1893, No. 2, p. 65.
Zool. Anz., xvi. 1893 (1894), p. 385.
Hyatt, Proc. Essex Institute (U.S.A.) (reprint from vols. iv., v. 1866-1868), p. 9.
Rare and Remarkable Animals of Scotland, ii. 1848, p. 93.
Trembley, Mém. Hist. Polypes, 1744; iii. Mém., p. 217. The same processes are described by Baker, Employment for the Microscope, new ed. 1785, p. 311.
Oka, J. Coll. Japan, iv. 1891, p. 90.
Hyatt, t. cit. p. 99.
Verworn, Zeitschr. wiss. Zool. xlvi. 1888, p. 119.
Dalyell, t. cit. p. 94.
Kraepelin, Abh. Ver. Hamburg, x. 1887, No. 9, p. 141.
Phil. Trans. 1837, p. 396.
Zeitschr. wiss. Zool. xxi. 1871, p. 426.
J. Coll. Japan, iv. 1891, p. 113.
Zool. Anz. xii. 1889, p. 508. This paper contains references to M. Jullien's writings on the mechanism of protrusion.
[See P. Cambridge Soc. vol. xi. Part 1, 1901.]
Zeitschr. wiss. Zool. xlvi. 1888, p. 124.
Kraepelin, Abh. Ver. Hamburg, xii. 1893, No. 2, p. 47; Braem, Bibl. Zool. (Bd. ii.) Heft 6, 1890, pp. 66 f.
Cf. Kraepelin, Abh. Ver. Hamburg, x. 1887, No. 9, pp. 154 f.
T. cit. p. 83.
Joliet, Arch. Zool. Exp. vi. 1877, p. 262.
Kraepelin, Abh. Ver. Hamburg, xii. 1893, No. 2, p. 22.
Harmer, Quart. J. Micr. Sci. xxxiv. 1893, p. 211.
Arch. Zool. Exp. 2 sér. x. 1892, p. 557.
Phil. Trans. 1837, p. 408.
Brit. Marine Polyzoa, Introduction, pp. lxxxvi, xc.
Arch. Zool. Exp. vi. 1877, p. 261.
Recherches sur l'Embryologie des Bryozoaires, 4to Lille, 1877.
Prouho, loc. cit.
Arch. Zool. Exp. 2 ser. v. 1887, p. 446.
Quart. J. Micr. Sci. xxxiv. 1893, p. 199; xxxix. part i. 1896, p. 71.
Jullien, Mém. Soc. Zool. France, iii. 1890, p. 381.
Cori, Zeitschr. wiss. Zool. lv. 1893, p. 626.
Oka, J. Coll. Japan, iv. 1891, p. 109; viii. 1895, p. 339.
Cf. Seeliger, Zeitschr. wiss. Zool. xlix. 1890, p. 168; and l. 1890, p. 560.
Cf. Milne-Edwards (H.), Ann. Sci. Nat. 2 ser. vi. 1836, pp. 5, 321.
See Norman, Ann. Nat. Hist. ser. 6, xiii. 1894, p. 114.
See Holdsworth, P. Zool. Soc. pt. xxvi. 1858, p. 306.
Brit. Mar. Polyzoa, Introduction, p. cxxii.
Ann. Nat. Hist. ser. 5, xx. 1887, p. 91.
Aetea, Eucratea, and certain other forms were separated off by Mr. Busk as a distinct division, the Stolonata.
Most of the writings of this author are referred to on pp. 277, 278 of Miss Jelly's Synonymic Catalogue, referred to on p. 523.
Catalogue of Marine Polyzoa in the Collection of the British Museum, parts i.-iii. 1852-1875; and Challenger Reports, Parts 30 (1884) and 50 (1886).
Trans. and Proc. R. Soc. Victoria, xxiii. 1887, p. 187, and Tr. R. Soc. Victoria, iv. 1895, p. 1.
Tr. Zool. Soc. xiii. 1895, p. 223.
Zittel, Text Book of Palaeontology (Eng. Trans.), 1900, p. 257 (Bryozoa, by E. O. Ulrich).
Paléontologie Française. Terrains Crétacés, tome v., Bryozoaires, 8vo. Paris, 1850-1851. This great work refers, however, to recent as well as to fossil species.
Heteropora, of which recent species exist, is placed by Dr. Gregory in the Trepostomata.
Quart. J. Geol. Soc. l. 1894, pp. 72, 79.
See, however, Vine, Ann. Nat. Hist. ser. 5. xiv. 1884, pp. 87, 88, and P. Yorksh. Geol. Soc. xii. 1891, p. 74, for possible Palaeozoic Ctenostomes (Ascodictyon, Rhopalonaria, and Vinella).
Two vols. 8vo. London (Van Voorst), 1880.
8vo. London (Dulau), 1889.
One or two genera of Cheilostomata may be mistaken for Cyclostomata. In case of doubt, 7 et seq. must be worked through.
Certain varieties of adherent species occasionally assume an erect form.
For Celleporella (colony minute: orifice tubular), see 41 et seq.
Rhynchozoon (see No. 61), in which the primary orifice becomes much obscured by the development of a large mucro, is placed in this section.
Hincks, J. Linn. Soc. xxi. 1889, p. 123.
Micropora complanata, Norman, should be placed in the genus Lepralia. See Hincks, Ann. Nat. Hist. 5 ser. xix. 1887, p. 304.
See Norman, Ann. Nat. Hist. ser. 6, xiii. 1894, p. 113.
Hincks, "Marine Polyzoa" (reprints from Ann. Nat. Hist. 1880-91), Index, p. v. note. (Replacing Rhynchopora, preoccupied for a Brachiopod.)
A form of Lepralia pallasiana, in which a mucro is developed, may be mistaken for Umbonula (see characters given for Lepralia under No. 59).
See Arch. Zool. Exp. 2 ser. vi. 1888, p. 135 (as Delagia), and ibid. x. 1892, p. 594. [See also J. Mar. Biol. Ass. v., 1897-99, p. 51.]
F. S. Conant, Johns Hopkins Univ. Circ. vol. xv. 1896, p. 82.
Ibid. vol. xiv. 1896, p. 77.